User login
Death of pig heart transplant patient is more a beginning than an end
The genetically altered pig’s heart “worked like a rock star, beautifully functioning,” the surgeon who performed the pioneering Jan. 7 xenotransplant procedure said in a press statement on the death of the patient, David Bennett Sr.
“He wasn’t able to overcome what turned out to be devastating – the debilitation from his previous period of heart failure, which was extreme,” said Bartley P. Griffith, MD, clinical director of the cardiac xenotransplantation program at the University of Maryland, Baltimore.
Representatives of the institution aren’t offering many details on the cause of Mr. Bennett’s death on March 8, 60 days after his operation, but said they will elaborate when their findings are formally published. But their comments seem to downplay the unique nature of the implanted heart itself as a culprit and instead implicate the patient’s diminished overall clinical condition and what grew into an ongoing battle with infections.
The 57-year-old Bennett, bedridden with end-stage heart failure, judged a poor candidate for a ventricular assist device, and on extracorporeal membrane oxygenation (ECMO), reportedly was offered the extraordinary surgery after being turned down for a conventional transplant at several major centers.
“Until day 45 or 50, he was doing very well,” Muhammad M. Mohiuddin, MD, the xenotransplantation program’s scientific director, observed in the statement. But infections soon took advantage of his hobbled immune system.
Given his “preexisting condition and how frail his body was,” Dr. Mohiuddin said, “we were having difficulty maintaining a balance between his immunosuppression and controlling his infection.” Mr. Bennett went into multiple organ failure and “I think that resulted in his passing away.”
Beyond wildest dreams
The surgeons confidently framed Mr. Bennett’s experience as a milestone for heart xenotransplantation. “The demonstration that it was possible, beyond the wildest dreams of most people in the field, even, at this point – that we were able to take a genetically engineered organ and watch it function flawlessly for 9 weeks – is pretty positive in terms of the potential of this therapy,” Dr. Griffith said.
But enough questions linger that others were more circumspect, even as they praised the accomplishment. “There’s no question that this is a historic event,” Mandeep R. Mehra, MD, of Harvard Medical School, and director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital, both in Boston, said in an interview.
Still, “I don’t think we should just conclude that it was the patient’s frailty or death from infection,” Dr. Mehra said. With so few details available, “I would be very careful in prematurely concluding that the problem did not reside with the heart but with the patient. We cannot be sure.”
For example, he noted, “6 to 8 weeks is right around the time when some cardiac complications, like accelerated forms of vasculopathy, could become evident.” Immune-mediated cardiac allograft vasculopathy is a common cause of heart transplant failure.
Or, “it could as easily have been the fact that immunosuppression was modified at 6 to 7 weeks in response to potential infection, which could have led to a cardiac compromise,” Dr. Mehra said. “We just don’t know.”
“It’s really important that this be reported in a scientifically accurate way, because we will all learn from this,” Lori J. West, MD, DPhil, said in an interview.
Little seems to be known for sure about the actual cause of death, “but the fact there was not hyperacute rejection is itself a big step forward. And we know, at least from the limited information we have, that it did not occur,” observed Dr. West, who directs the Alberta Transplant Institute, Edmonton, and the Canadian Donation and Transplantation Research Program. She is a professor of pediatrics with adjunct positions in the departments of surgery and microbiology/immunology.
Dr. West also sees Mr. Bennett’s struggle with infections and adjustments to his unique immunosuppressive regimen, at least as characterized by his care team, as in line with the experience of many heart transplant recipients facing the same threat.
“We already walk this tightrope with every transplant patient,” she said. Typically, they’re put on a somewhat standardized immunosuppressant regimen, “and then we modify it a bit, either increasing or decreasing it, depending on the posttransplant course.” The regimen can become especially intense in response to new signs of rejection, “and you know that that’s going to have an impact on susceptibility to all kinds of infections.”
Full circle
The porcine heart was protected along two fronts against assault from Mr. Bennett’s immune system and other inhospitable aspects of his physiology, either of which could also have been obstacles to success: Genetic modification (Revivicor) of the pig that provided the heart, and a singularly aggressive antirejection drug regimen for the patient.
The knockout of three genes targeting specific porcine cell-surface carbohydrates that provoke a strong human antibody response reportedly averted a hyperacute rejection response that would have caused the graft to fail almost immediately.
Other genetic manipulations, some using CRISPR technology, silenced genes encoded for porcine endogenous retroviruses. Others were aimed at controlling myocardial growth and stemming graft microangiopathy.
Mr. Bennett himself was treated with powerful immunosuppressants, including an investigational anti-CD40 monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals) that, according to UMSOM, inhibits a well-recognized pathway critical to B-cell proliferation, T-cell activation, and antibody production.
“I suspect the patient may not have had rejection, but unfortunately, that intense immunosuppression really set him up – even if he had been half that age – for a very difficult time,” David A. Baran, MD, a cardiologist from Sentara Advanced Heart Failure Center, Norfolk, Va., who studies transplant immunology, said in an interview.
“This is in some ways like the original heart transplant in 1967, when the ability to do the surgery evolved before understanding of the immunosuppression needed. Four or 5 years later, heart transplantation almost died out, before the development of better immunosuppressants like cyclosporine and later tacrolimus,” Dr. Baran said.
“The current age, when we use less immunosuppression than ever, is based on 30 years of progressive success,” he noted. This landmark xenotransplantation “basically turns back the clock to a time when the intensity of immunosuppression by definition had to be extremely high, because we really didn’t know what to expect.”
Emerging role of xeno-organs
Xenotransplantation has been touted as potential strategy for expanding the pool of organs available for transplantation. Mr. Bennett’s “breakthrough surgery” takes the world “one step closer to solving the organ shortage crisis,” his surgeon, Dr. Griffith, announced soon after the procedure. “There are simply not enough donor human hearts available to meet the long list of potential recipients.”
But it’s not the only proposed approach. Measures could be taken, for example, to make more efficient use of the human organs that become available, partly by opening the field to additional less-than-ideal hearts and loosening regulatory mandates for projected graft survival.
“Every year, more than two-thirds of donor organs in the United States are discarded. So it’s not actually that we don’t have enough organs, it’s that we don’t have enough organs that people are willing to take,” Dr. Baran said. Still, it’s important to pursue all promising avenues, and “the genetic manipulation pathway is remarkable.”
But “honestly, organs such as kidneys probably make the most sense” for early study of xenotransplantation from pigs, he said. “The waiting list for kidneys is also very long, but if the kidney graft were to fail, the patient wouldn’t die. It would allow us to work out the immunosuppression without putting patients’ lives at risk.”
Often overlooked in assessments of organ demand, Dr. West said, is that “a lot of patients who could benefit from a transplant will never even be listed for a transplant.” It’s not clear why; perhaps they have multiple comorbidities, live too far from a transplant center, “or they’re too big or too small. Even if there were unlimited organs, you could never meet the needs of people who could benefit from transplantation.”
So even if more available donor organs were used, she said, there would still be a gap that xenotransplantation could help fill. “I’m very much in favor of research that allows us to continue to try to find a pathway to xenotransplantation. I think it’s critically important.”
Unquestionably, “we now need to have a dialogue to entertain how a technology like this, using modern medicine with gene editing, is really going to be utilized,” Dr. Mehra said. The Bennett case “does open up the field, but it also raises caution.” There should be broad participation to move the field forward, “coordinated through either societies or nationally allocated advisory committees that oversee the movement of this technology, to the next step.”
Ideally, that next step “would be to do a safety clinical trial in the right patient,” he said. “And the right patient, by definition, would be one who does not have a life-prolonging option, either mechanical circulatory support or allograft transplantation. That would be the goal.”
Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board NuPulseCV, Leviticus, and FineHeart. Dr. Baran disclosed consulting for Getinge and LivaNova; speaking for Pfizer; and serving on trial steering committees for CareDx and Procyrion, all unrelated to xenotransplantation. Dr. West has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
The genetically altered pig’s heart “worked like a rock star, beautifully functioning,” the surgeon who performed the pioneering Jan. 7 xenotransplant procedure said in a press statement on the death of the patient, David Bennett Sr.
“He wasn’t able to overcome what turned out to be devastating – the debilitation from his previous period of heart failure, which was extreme,” said Bartley P. Griffith, MD, clinical director of the cardiac xenotransplantation program at the University of Maryland, Baltimore.
Representatives of the institution aren’t offering many details on the cause of Mr. Bennett’s death on March 8, 60 days after his operation, but said they will elaborate when their findings are formally published. But their comments seem to downplay the unique nature of the implanted heart itself as a culprit and instead implicate the patient’s diminished overall clinical condition and what grew into an ongoing battle with infections.
The 57-year-old Bennett, bedridden with end-stage heart failure, judged a poor candidate for a ventricular assist device, and on extracorporeal membrane oxygenation (ECMO), reportedly was offered the extraordinary surgery after being turned down for a conventional transplant at several major centers.
“Until day 45 or 50, he was doing very well,” Muhammad M. Mohiuddin, MD, the xenotransplantation program’s scientific director, observed in the statement. But infections soon took advantage of his hobbled immune system.
Given his “preexisting condition and how frail his body was,” Dr. Mohiuddin said, “we were having difficulty maintaining a balance between his immunosuppression and controlling his infection.” Mr. Bennett went into multiple organ failure and “I think that resulted in his passing away.”
Beyond wildest dreams
The surgeons confidently framed Mr. Bennett’s experience as a milestone for heart xenotransplantation. “The demonstration that it was possible, beyond the wildest dreams of most people in the field, even, at this point – that we were able to take a genetically engineered organ and watch it function flawlessly for 9 weeks – is pretty positive in terms of the potential of this therapy,” Dr. Griffith said.
But enough questions linger that others were more circumspect, even as they praised the accomplishment. “There’s no question that this is a historic event,” Mandeep R. Mehra, MD, of Harvard Medical School, and director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital, both in Boston, said in an interview.
Still, “I don’t think we should just conclude that it was the patient’s frailty or death from infection,” Dr. Mehra said. With so few details available, “I would be very careful in prematurely concluding that the problem did not reside with the heart but with the patient. We cannot be sure.”
For example, he noted, “6 to 8 weeks is right around the time when some cardiac complications, like accelerated forms of vasculopathy, could become evident.” Immune-mediated cardiac allograft vasculopathy is a common cause of heart transplant failure.
Or, “it could as easily have been the fact that immunosuppression was modified at 6 to 7 weeks in response to potential infection, which could have led to a cardiac compromise,” Dr. Mehra said. “We just don’t know.”
“It’s really important that this be reported in a scientifically accurate way, because we will all learn from this,” Lori J. West, MD, DPhil, said in an interview.
Little seems to be known for sure about the actual cause of death, “but the fact there was not hyperacute rejection is itself a big step forward. And we know, at least from the limited information we have, that it did not occur,” observed Dr. West, who directs the Alberta Transplant Institute, Edmonton, and the Canadian Donation and Transplantation Research Program. She is a professor of pediatrics with adjunct positions in the departments of surgery and microbiology/immunology.
Dr. West also sees Mr. Bennett’s struggle with infections and adjustments to his unique immunosuppressive regimen, at least as characterized by his care team, as in line with the experience of many heart transplant recipients facing the same threat.
“We already walk this tightrope with every transplant patient,” she said. Typically, they’re put on a somewhat standardized immunosuppressant regimen, “and then we modify it a bit, either increasing or decreasing it, depending on the posttransplant course.” The regimen can become especially intense in response to new signs of rejection, “and you know that that’s going to have an impact on susceptibility to all kinds of infections.”
Full circle
The porcine heart was protected along two fronts against assault from Mr. Bennett’s immune system and other inhospitable aspects of his physiology, either of which could also have been obstacles to success: Genetic modification (Revivicor) of the pig that provided the heart, and a singularly aggressive antirejection drug regimen for the patient.
The knockout of three genes targeting specific porcine cell-surface carbohydrates that provoke a strong human antibody response reportedly averted a hyperacute rejection response that would have caused the graft to fail almost immediately.
Other genetic manipulations, some using CRISPR technology, silenced genes encoded for porcine endogenous retroviruses. Others were aimed at controlling myocardial growth and stemming graft microangiopathy.
Mr. Bennett himself was treated with powerful immunosuppressants, including an investigational anti-CD40 monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals) that, according to UMSOM, inhibits a well-recognized pathway critical to B-cell proliferation, T-cell activation, and antibody production.
“I suspect the patient may not have had rejection, but unfortunately, that intense immunosuppression really set him up – even if he had been half that age – for a very difficult time,” David A. Baran, MD, a cardiologist from Sentara Advanced Heart Failure Center, Norfolk, Va., who studies transplant immunology, said in an interview.
“This is in some ways like the original heart transplant in 1967, when the ability to do the surgery evolved before understanding of the immunosuppression needed. Four or 5 years later, heart transplantation almost died out, before the development of better immunosuppressants like cyclosporine and later tacrolimus,” Dr. Baran said.
“The current age, when we use less immunosuppression than ever, is based on 30 years of progressive success,” he noted. This landmark xenotransplantation “basically turns back the clock to a time when the intensity of immunosuppression by definition had to be extremely high, because we really didn’t know what to expect.”
Emerging role of xeno-organs
Xenotransplantation has been touted as potential strategy for expanding the pool of organs available for transplantation. Mr. Bennett’s “breakthrough surgery” takes the world “one step closer to solving the organ shortage crisis,” his surgeon, Dr. Griffith, announced soon after the procedure. “There are simply not enough donor human hearts available to meet the long list of potential recipients.”
But it’s not the only proposed approach. Measures could be taken, for example, to make more efficient use of the human organs that become available, partly by opening the field to additional less-than-ideal hearts and loosening regulatory mandates for projected graft survival.
“Every year, more than two-thirds of donor organs in the United States are discarded. So it’s not actually that we don’t have enough organs, it’s that we don’t have enough organs that people are willing to take,” Dr. Baran said. Still, it’s important to pursue all promising avenues, and “the genetic manipulation pathway is remarkable.”
But “honestly, organs such as kidneys probably make the most sense” for early study of xenotransplantation from pigs, he said. “The waiting list for kidneys is also very long, but if the kidney graft were to fail, the patient wouldn’t die. It would allow us to work out the immunosuppression without putting patients’ lives at risk.”
Often overlooked in assessments of organ demand, Dr. West said, is that “a lot of patients who could benefit from a transplant will never even be listed for a transplant.” It’s not clear why; perhaps they have multiple comorbidities, live too far from a transplant center, “or they’re too big or too small. Even if there were unlimited organs, you could never meet the needs of people who could benefit from transplantation.”
So even if more available donor organs were used, she said, there would still be a gap that xenotransplantation could help fill. “I’m very much in favor of research that allows us to continue to try to find a pathway to xenotransplantation. I think it’s critically important.”
Unquestionably, “we now need to have a dialogue to entertain how a technology like this, using modern medicine with gene editing, is really going to be utilized,” Dr. Mehra said. The Bennett case “does open up the field, but it also raises caution.” There should be broad participation to move the field forward, “coordinated through either societies or nationally allocated advisory committees that oversee the movement of this technology, to the next step.”
Ideally, that next step “would be to do a safety clinical trial in the right patient,” he said. “And the right patient, by definition, would be one who does not have a life-prolonging option, either mechanical circulatory support or allograft transplantation. That would be the goal.”
Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board NuPulseCV, Leviticus, and FineHeart. Dr. Baran disclosed consulting for Getinge and LivaNova; speaking for Pfizer; and serving on trial steering committees for CareDx and Procyrion, all unrelated to xenotransplantation. Dr. West has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
The genetically altered pig’s heart “worked like a rock star, beautifully functioning,” the surgeon who performed the pioneering Jan. 7 xenotransplant procedure said in a press statement on the death of the patient, David Bennett Sr.
“He wasn’t able to overcome what turned out to be devastating – the debilitation from his previous period of heart failure, which was extreme,” said Bartley P. Griffith, MD, clinical director of the cardiac xenotransplantation program at the University of Maryland, Baltimore.
Representatives of the institution aren’t offering many details on the cause of Mr. Bennett’s death on March 8, 60 days after his operation, but said they will elaborate when their findings are formally published. But their comments seem to downplay the unique nature of the implanted heart itself as a culprit and instead implicate the patient’s diminished overall clinical condition and what grew into an ongoing battle with infections.
The 57-year-old Bennett, bedridden with end-stage heart failure, judged a poor candidate for a ventricular assist device, and on extracorporeal membrane oxygenation (ECMO), reportedly was offered the extraordinary surgery after being turned down for a conventional transplant at several major centers.
“Until day 45 or 50, he was doing very well,” Muhammad M. Mohiuddin, MD, the xenotransplantation program’s scientific director, observed in the statement. But infections soon took advantage of his hobbled immune system.
Given his “preexisting condition and how frail his body was,” Dr. Mohiuddin said, “we were having difficulty maintaining a balance between his immunosuppression and controlling his infection.” Mr. Bennett went into multiple organ failure and “I think that resulted in his passing away.”
Beyond wildest dreams
The surgeons confidently framed Mr. Bennett’s experience as a milestone for heart xenotransplantation. “The demonstration that it was possible, beyond the wildest dreams of most people in the field, even, at this point – that we were able to take a genetically engineered organ and watch it function flawlessly for 9 weeks – is pretty positive in terms of the potential of this therapy,” Dr. Griffith said.
But enough questions linger that others were more circumspect, even as they praised the accomplishment. “There’s no question that this is a historic event,” Mandeep R. Mehra, MD, of Harvard Medical School, and director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital, both in Boston, said in an interview.
Still, “I don’t think we should just conclude that it was the patient’s frailty or death from infection,” Dr. Mehra said. With so few details available, “I would be very careful in prematurely concluding that the problem did not reside with the heart but with the patient. We cannot be sure.”
For example, he noted, “6 to 8 weeks is right around the time when some cardiac complications, like accelerated forms of vasculopathy, could become evident.” Immune-mediated cardiac allograft vasculopathy is a common cause of heart transplant failure.
Or, “it could as easily have been the fact that immunosuppression was modified at 6 to 7 weeks in response to potential infection, which could have led to a cardiac compromise,” Dr. Mehra said. “We just don’t know.”
“It’s really important that this be reported in a scientifically accurate way, because we will all learn from this,” Lori J. West, MD, DPhil, said in an interview.
Little seems to be known for sure about the actual cause of death, “but the fact there was not hyperacute rejection is itself a big step forward. And we know, at least from the limited information we have, that it did not occur,” observed Dr. West, who directs the Alberta Transplant Institute, Edmonton, and the Canadian Donation and Transplantation Research Program. She is a professor of pediatrics with adjunct positions in the departments of surgery and microbiology/immunology.
Dr. West also sees Mr. Bennett’s struggle with infections and adjustments to his unique immunosuppressive regimen, at least as characterized by his care team, as in line with the experience of many heart transplant recipients facing the same threat.
“We already walk this tightrope with every transplant patient,” she said. Typically, they’re put on a somewhat standardized immunosuppressant regimen, “and then we modify it a bit, either increasing or decreasing it, depending on the posttransplant course.” The regimen can become especially intense in response to new signs of rejection, “and you know that that’s going to have an impact on susceptibility to all kinds of infections.”
Full circle
The porcine heart was protected along two fronts against assault from Mr. Bennett’s immune system and other inhospitable aspects of his physiology, either of which could also have been obstacles to success: Genetic modification (Revivicor) of the pig that provided the heart, and a singularly aggressive antirejection drug regimen for the patient.
The knockout of three genes targeting specific porcine cell-surface carbohydrates that provoke a strong human antibody response reportedly averted a hyperacute rejection response that would have caused the graft to fail almost immediately.
Other genetic manipulations, some using CRISPR technology, silenced genes encoded for porcine endogenous retroviruses. Others were aimed at controlling myocardial growth and stemming graft microangiopathy.
Mr. Bennett himself was treated with powerful immunosuppressants, including an investigational anti-CD40 monoclonal antibody (KPL-404, Kiniksa Pharmaceuticals) that, according to UMSOM, inhibits a well-recognized pathway critical to B-cell proliferation, T-cell activation, and antibody production.
“I suspect the patient may not have had rejection, but unfortunately, that intense immunosuppression really set him up – even if he had been half that age – for a very difficult time,” David A. Baran, MD, a cardiologist from Sentara Advanced Heart Failure Center, Norfolk, Va., who studies transplant immunology, said in an interview.
“This is in some ways like the original heart transplant in 1967, when the ability to do the surgery evolved before understanding of the immunosuppression needed. Four or 5 years later, heart transplantation almost died out, before the development of better immunosuppressants like cyclosporine and later tacrolimus,” Dr. Baran said.
“The current age, when we use less immunosuppression than ever, is based on 30 years of progressive success,” he noted. This landmark xenotransplantation “basically turns back the clock to a time when the intensity of immunosuppression by definition had to be extremely high, because we really didn’t know what to expect.”
Emerging role of xeno-organs
Xenotransplantation has been touted as potential strategy for expanding the pool of organs available for transplantation. Mr. Bennett’s “breakthrough surgery” takes the world “one step closer to solving the organ shortage crisis,” his surgeon, Dr. Griffith, announced soon after the procedure. “There are simply not enough donor human hearts available to meet the long list of potential recipients.”
But it’s not the only proposed approach. Measures could be taken, for example, to make more efficient use of the human organs that become available, partly by opening the field to additional less-than-ideal hearts and loosening regulatory mandates for projected graft survival.
“Every year, more than two-thirds of donor organs in the United States are discarded. So it’s not actually that we don’t have enough organs, it’s that we don’t have enough organs that people are willing to take,” Dr. Baran said. Still, it’s important to pursue all promising avenues, and “the genetic manipulation pathway is remarkable.”
But “honestly, organs such as kidneys probably make the most sense” for early study of xenotransplantation from pigs, he said. “The waiting list for kidneys is also very long, but if the kidney graft were to fail, the patient wouldn’t die. It would allow us to work out the immunosuppression without putting patients’ lives at risk.”
Often overlooked in assessments of organ demand, Dr. West said, is that “a lot of patients who could benefit from a transplant will never even be listed for a transplant.” It’s not clear why; perhaps they have multiple comorbidities, live too far from a transplant center, “or they’re too big or too small. Even if there were unlimited organs, you could never meet the needs of people who could benefit from transplantation.”
So even if more available donor organs were used, she said, there would still be a gap that xenotransplantation could help fill. “I’m very much in favor of research that allows us to continue to try to find a pathway to xenotransplantation. I think it’s critically important.”
Unquestionably, “we now need to have a dialogue to entertain how a technology like this, using modern medicine with gene editing, is really going to be utilized,” Dr. Mehra said. The Bennett case “does open up the field, but it also raises caution.” There should be broad participation to move the field forward, “coordinated through either societies or nationally allocated advisory committees that oversee the movement of this technology, to the next step.”
Ideally, that next step “would be to do a safety clinical trial in the right patient,” he said. “And the right patient, by definition, would be one who does not have a life-prolonging option, either mechanical circulatory support or allograft transplantation. That would be the goal.”
Dr. Mehra has reported receiving payments to his institution from Abbott for consulting; consulting fees from Janssen, Mesoblast, Broadview Ventures, Natera, Paragonix, Moderna, and the Baim Institute for Clinical Research; and serving on a scientific advisory board NuPulseCV, Leviticus, and FineHeart. Dr. Baran disclosed consulting for Getinge and LivaNova; speaking for Pfizer; and serving on trial steering committees for CareDx and Procyrion, all unrelated to xenotransplantation. Dr. West has declared no relevant conflicts.
A version of this article first appeared on Medscape.com.
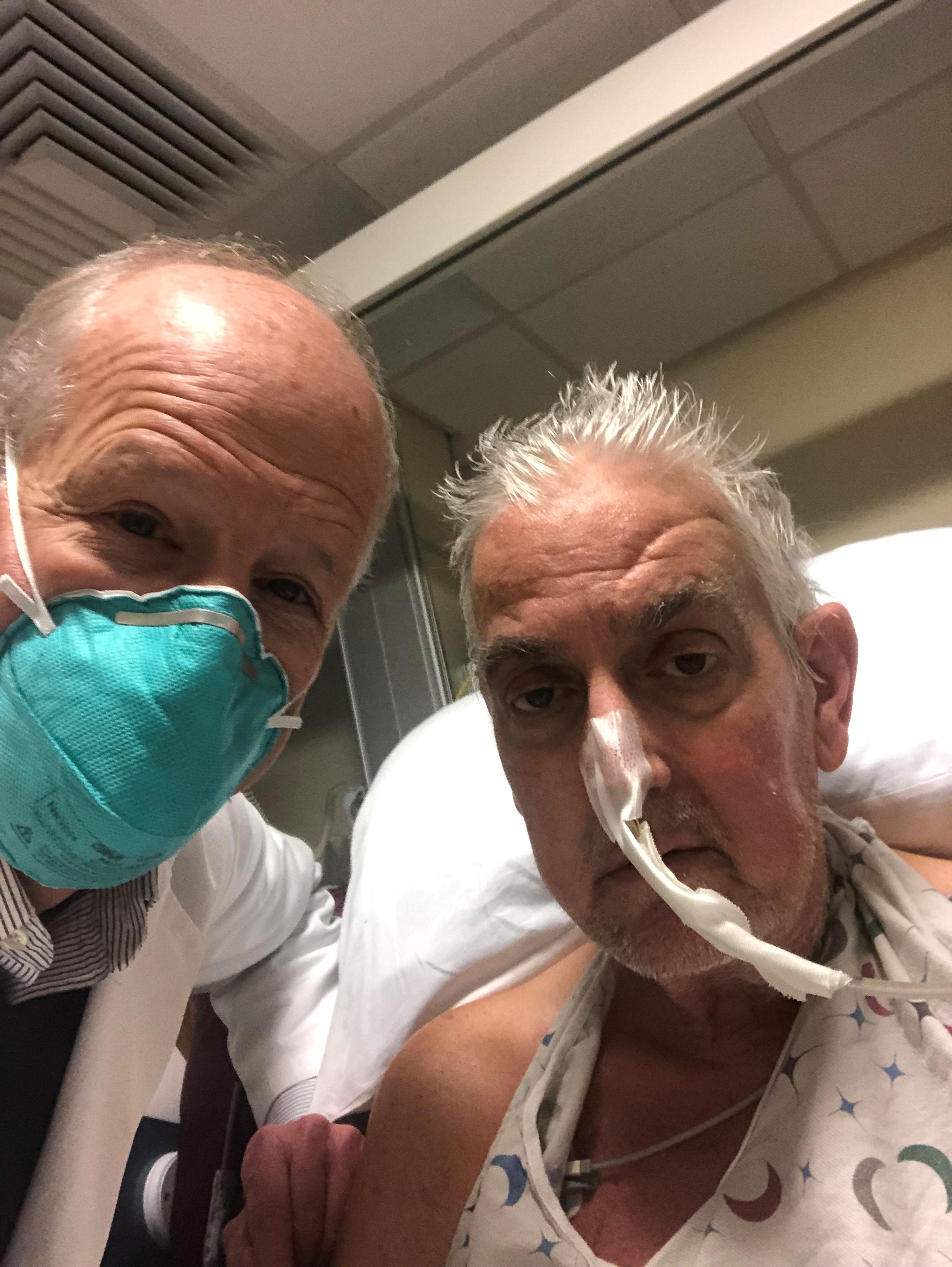
Man who received first modified pig heart transplant dies
He passed away March 8, according to a statement from the University of Maryland Medical Center (UMMC), Baltimore, where the transplant was performed.
Mr. Bennett received the transplant on January 7 and lived for 2 months following the surgery.
Although not providing the exact cause of his death, UMMC said Mr. Bennett’s condition began deteriorating several days before his death.
When it became clear that he would not recover, he was given compassionate palliative care and was able to communicate with his family during his final hours.
“We are devastated by the loss of Mr. Bennett. He proved to be a brave and noble patient who fought all the way to the end. We extend our sincerest condolences to his family,” Bartley P. Griffith, MD, who performed the transplant, said in the statement.
“We are grateful to Mr. Bennett for his unique and historic role in helping to contribute to a vast array of knowledge to the field of xenotransplantation,” added Muhammad M. Mohiuddin, MD, director of the cardiac xenotransplantation program at University of Maryland School of Medicine.
Before receiving the genetically modified pig heart, Mr. Bennett had required mechanical circulatory support to stay alive but was rejected for standard heart transplantation at UMMC and other centers. He was ineligible for an implanted ventricular assist device due to ventricular arrhythmias.
Following surgery, the transplanted pig heart performed well for several weeks without any signs of rejection. The patient was able to spend time with his family and participate in physical therapy to help regain strength.
“This organ transplant demonstrated for the first time that a genetically modified animal heart can function like a human heart without immediate rejection by the body,” UMMC said in a statement issued 3 days after the surgery.
Thanks to Mr. Bennett, “we have gained invaluable insights learning that the genetically modified pig heart can function well within the human body while the immune system is adequately suppressed,” said Dr. Mohiuddin. “We remain optimistic and plan on continuing our work in future clinical trials.”
The patient’s son, David Bennett Jr, said the family is “profoundly grateful for the life-extending opportunity” provided to his father by the “stellar team” at the University of Maryland School of Medicine and the University of Maryland Medical Center.
“We were able to spend some precious weeks together while he recovered from the transplant surgery, weeks we would not have had without this miraculous effort,” he said.
“We also hope that what was learned from his surgery will benefit future patients and hopefully, one day, end the organ shortage that costs so many lives each year,” he added.
A version of this article first appeared on Medscape.com.
He passed away March 8, according to a statement from the University of Maryland Medical Center (UMMC), Baltimore, where the transplant was performed.
Mr. Bennett received the transplant on January 7 and lived for 2 months following the surgery.
Although not providing the exact cause of his death, UMMC said Mr. Bennett’s condition began deteriorating several days before his death.
When it became clear that he would not recover, he was given compassionate palliative care and was able to communicate with his family during his final hours.
“We are devastated by the loss of Mr. Bennett. He proved to be a brave and noble patient who fought all the way to the end. We extend our sincerest condolences to his family,” Bartley P. Griffith, MD, who performed the transplant, said in the statement.
“We are grateful to Mr. Bennett for his unique and historic role in helping to contribute to a vast array of knowledge to the field of xenotransplantation,” added Muhammad M. Mohiuddin, MD, director of the cardiac xenotransplantation program at University of Maryland School of Medicine.
Before receiving the genetically modified pig heart, Mr. Bennett had required mechanical circulatory support to stay alive but was rejected for standard heart transplantation at UMMC and other centers. He was ineligible for an implanted ventricular assist device due to ventricular arrhythmias.
Following surgery, the transplanted pig heart performed well for several weeks without any signs of rejection. The patient was able to spend time with his family and participate in physical therapy to help regain strength.
“This organ transplant demonstrated for the first time that a genetically modified animal heart can function like a human heart without immediate rejection by the body,” UMMC said in a statement issued 3 days after the surgery.
Thanks to Mr. Bennett, “we have gained invaluable insights learning that the genetically modified pig heart can function well within the human body while the immune system is adequately suppressed,” said Dr. Mohiuddin. “We remain optimistic and plan on continuing our work in future clinical trials.”
The patient’s son, David Bennett Jr, said the family is “profoundly grateful for the life-extending opportunity” provided to his father by the “stellar team” at the University of Maryland School of Medicine and the University of Maryland Medical Center.
“We were able to spend some precious weeks together while he recovered from the transplant surgery, weeks we would not have had without this miraculous effort,” he said.
“We also hope that what was learned from his surgery will benefit future patients and hopefully, one day, end the organ shortage that costs so many lives each year,” he added.
A version of this article first appeared on Medscape.com.
He passed away March 8, according to a statement from the University of Maryland Medical Center (UMMC), Baltimore, where the transplant was performed.
Mr. Bennett received the transplant on January 7 and lived for 2 months following the surgery.
Although not providing the exact cause of his death, UMMC said Mr. Bennett’s condition began deteriorating several days before his death.
When it became clear that he would not recover, he was given compassionate palliative care and was able to communicate with his family during his final hours.
“We are devastated by the loss of Mr. Bennett. He proved to be a brave and noble patient who fought all the way to the end. We extend our sincerest condolences to his family,” Bartley P. Griffith, MD, who performed the transplant, said in the statement.
“We are grateful to Mr. Bennett for his unique and historic role in helping to contribute to a vast array of knowledge to the field of xenotransplantation,” added Muhammad M. Mohiuddin, MD, director of the cardiac xenotransplantation program at University of Maryland School of Medicine.
Before receiving the genetically modified pig heart, Mr. Bennett had required mechanical circulatory support to stay alive but was rejected for standard heart transplantation at UMMC and other centers. He was ineligible for an implanted ventricular assist device due to ventricular arrhythmias.
Following surgery, the transplanted pig heart performed well for several weeks without any signs of rejection. The patient was able to spend time with his family and participate in physical therapy to help regain strength.
“This organ transplant demonstrated for the first time that a genetically modified animal heart can function like a human heart without immediate rejection by the body,” UMMC said in a statement issued 3 days after the surgery.
Thanks to Mr. Bennett, “we have gained invaluable insights learning that the genetically modified pig heart can function well within the human body while the immune system is adequately suppressed,” said Dr. Mohiuddin. “We remain optimistic and plan on continuing our work in future clinical trials.”
The patient’s son, David Bennett Jr, said the family is “profoundly grateful for the life-extending opportunity” provided to his father by the “stellar team” at the University of Maryland School of Medicine and the University of Maryland Medical Center.
“We were able to spend some precious weeks together while he recovered from the transplant surgery, weeks we would not have had without this miraculous effort,” he said.
“We also hope that what was learned from his surgery will benefit future patients and hopefully, one day, end the organ shortage that costs so many lives each year,” he added.
A version of this article first appeared on Medscape.com.
Organ transplantation: Unvaccinated need not apply
I agree with most advice given by the affable TV character Ted Lasso. “Every choice is a chance,” he said. Pandemic-era physicians must now consider whether a politically motivated choice to decline COVID-19 vaccination should negatively affect the chance to receive an organ donation.
And in confronting these choices, we have a chance to educate the public on the complexities of the organ allocation process.
A well-informed patient’s personal choice should be honored, even if clinicians disagree, if it does not affect the well-being of others. For example, I once had a patient in acute leukemic crisis who declined blood products because she was a Jehovah’s Witness. She died. Her choice affected her longevity only.
Compare that decision with awarding an organ to an individual who has declined readily available protection of that organ. Weigh that choice against the fact that said protection is against an infectious disease that has killed over 5.5 million worldwide.
Some institutions stand strong, others hedge their bets
Admirably, Loyola University Health System understands that difference. They published a firm stand on transplant candidacy and COVID-19 vaccination status in the Journal of Heart and Lung Transplant. Daniel Dilling, MD, medical director of the lung transplantation program , and Mark Kuczewski, PhD, a professor of medical ethics at Loyola University Chicago, Maywood, Ill., wrote that: “We believe that requiring vaccination against COVID-19 should not be controversial when we focus strictly on established frameworks and practices surrounding eligibility for wait-listing to receive a solid organ transplant.”
The Cleveland Clinic apparently agrees. In October 2021, they denied a liver transplant to Michelle Vitullo of Ohio, whose daughter had been deemed “a perfect match.” Her daughter, also unvaccinated, stated: “Being denied for a nonmedical reason for someone’s beliefs that are different to yours, I mean that’s not how that should be.”
But vaccination status is a medical reason, given well-established data regarding increased mortality among the immunosuppressed. Ms. Vitullo then said: “We are trying to get to UPMC [University of Pittsburgh Medical Center] as they don’t require a vaccination.”
The public information page on transplant candidacy from UPMC reads (my italics): It is recommended that all transplant candidates, transplant recipients, and their household members receive COVID-19 vaccination when the vaccine is available to them. It is preferred that transplant candidates are vaccinated more than 2 weeks before transplantation.
I reached out to UPMC for clarification and was told by email that “we do not have a policy regarding COVID-19 vaccination requirement for current transplant candidates.” Houston Methodist shares the same agnostic stance.
Compare these opinions with Brigham and Women’s Hospital, where the requirements are resolute: “Like most other transplant programs across the country, the COVID-19 vaccine is one of several vaccines and lifestyle behaviors that are required for patients awaiting solid organ transplant.”
They add that “transplant candidates must also receive the seasonal influenza and hepatitis B vaccines, follow other healthy behaviors, and demonstrate they can commit to taking the required medications following transplant.”
In January 2022, Brigham and Women’s Hospital declared 31-year-old D.J. Ferguson ineligible for a heart transplant because he declined to be vaccinated against COVID-19. According to the New York Post and ABC News, his physicians resorted to left ventricular assist device support. His mother, Tracy Ferguson, is quoted as saying: “He’s not an antivaxxer. He has all of his vaccines.” I’ll just leave that right there.
Unfortunately, Michelle Vitullo’s obituary was published in December 2021. Regardless of whether she received her liver transplant, the outcome is tragic, and whatever you think of this family’s battle playing out in the glare of the national spotlight, their loss is no less devastating.
The directed-donation aspect of this case poses an interesting question. A news anchor asked the mother and daughter: “If you both accept the risks, why doesn’t the hospital just let you try?” The answers are obvious to us clinicians. Performing a transplantation in an unvaccinated patient could lead to their early death if they became infected because of their immunocompromised state, would open the door for transplantation of any patient who is unvaccinated for anything, including influenza and hepatitis B, which could result in the preventable waste of organs, and puts other vulnerable hospitalized patients at risk during the initial transplant stay and follow-up.
That’s not to mention the potential legal suit. Never has a consent form dissuaded any party from lodging an accusation of wrongful death or medical malpractice. In the face of strong data on higher mortality in unvaccinated, immunocompromised patients, a good lawyer could charge that the institution and transplant surgeons should have known better, regardless of the donor and recipient’s willingness to accept the risks.
The Vitullo and Ferguson cases are among many similar dilemmas surrounding transplant candidacy across the United States.
University of Virginia Health in Charlottesville denied 42-year-old Shamgar Connors a kidney transplant because he is unvaccinated, despite a previous COVID-19 infection. In October 2021, Leilani Lutali of Colorado was denied a kidney by UCHealth because she declined vaccination.
As Ted Lasso says: “There’s a bunch of crazy stuff on Twitter.”
Predictably, social media is full of public outcry. “Some cold-hearted people on here” tweeted one. “What if it was one of your loved ones who needed a transplant?” Another tweeted the Hippocratic oath with the comment that “They all swore under this noble ‘oat’, but I guess it’s been forgotten.” (This was followed with a photo of a box of Quaker Oats in a failed attempt at humor.) These discussions among the Twitterati highlight the depths of misunderstanding on organ transplantation.
To be fair, unless you have been personally involved in the decision-making process for transplant candidacy, there is little opportunity to be educated. I explain to my anxious patients and their families that a donor organ is like a fumbled football. There may be well over 100 patients at all levels of transplant status in many geographic locations diving for that same organ.
The transplant team is tasked with finding the best match, determining who is the sickest, assessing time for transport of that organ, and, above all, who will be the best steward of that organ.
Take heart transplantation, for instance. Approximately 3,500 patients in the United States are awaiting one each year. Instead of facing an almost certain death within 5 years, a transplant recipient has a chance at a median survival of 12-13 years. The cost of a heart transplant is approximately $1.38 million, according to Milliman, a consulting firm. This is “an incredibly resource intensive procedure,” including expenditures for transportation, antirejection medication, office visits, physician fees, ICU stays, rejection surveillance, and acute rejection therapies.
Transplant denial is nothing new
People get turned down for organ transplants all the time. My patient with end-stage dilated cardiomyopathy was denied a heart transplant when it was discovered that he had scores of outstanding parking tickets. This was seen as a surrogate for an inability to afford his antirejection medication.
Another patient swore that her positive cotinine levels were caused by endless hours at the bingo hall where second-hand smoke swirled. She was also denied. Many potential candidates who are in acute decline hold precariously to newfound sobriety. They are denied. A patient’s boyfriend told the transplant team that he couldn’t be relied upon to drive her to her appointments. She was denied.
Many people who engage in antisocial behaviors have no idea that these actions may result in the denial of an organ transplant should their future selves need one. These are hard lines, but everyone should agree that the odds of survival are heavily in favor of the consistently adherent.
We should take this opportunity to educate the public on how complicated obtaining an organ transplant can be. More than 6,000 people die each year waiting for an organ because of the supply-and-demand disparities in the transplantation arena. I’m willing to bet that many of the loudest protesters in favor of unvaccinated transplant recipients have not signed the organ donor box on the back of their driver’s license. This conversation is an opportunity to change that and remind people that organ donation may be their only opportunity to save a fellow human’s life.
Again, to quote Ted Lasso: “If you care about someone and you got a little love in your heart, there ain’t nothing you can’t get through together.” That philosophy should apply to the tasks of selecting the best organ donors as well as the best organ recipients.
And every organ should go to the one who will honor their donor and their donor’s family by taking the best care of that ultimate gift of life, including being vaccinated against COVID-19.
Dr. Walton-Shirley is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
I agree with most advice given by the affable TV character Ted Lasso. “Every choice is a chance,” he said. Pandemic-era physicians must now consider whether a politically motivated choice to decline COVID-19 vaccination should negatively affect the chance to receive an organ donation.
And in confronting these choices, we have a chance to educate the public on the complexities of the organ allocation process.
A well-informed patient’s personal choice should be honored, even if clinicians disagree, if it does not affect the well-being of others. For example, I once had a patient in acute leukemic crisis who declined blood products because she was a Jehovah’s Witness. She died. Her choice affected her longevity only.
Compare that decision with awarding an organ to an individual who has declined readily available protection of that organ. Weigh that choice against the fact that said protection is against an infectious disease that has killed over 5.5 million worldwide.
Some institutions stand strong, others hedge their bets
Admirably, Loyola University Health System understands that difference. They published a firm stand on transplant candidacy and COVID-19 vaccination status in the Journal of Heart and Lung Transplant. Daniel Dilling, MD, medical director of the lung transplantation program , and Mark Kuczewski, PhD, a professor of medical ethics at Loyola University Chicago, Maywood, Ill., wrote that: “We believe that requiring vaccination against COVID-19 should not be controversial when we focus strictly on established frameworks and practices surrounding eligibility for wait-listing to receive a solid organ transplant.”
The Cleveland Clinic apparently agrees. In October 2021, they denied a liver transplant to Michelle Vitullo of Ohio, whose daughter had been deemed “a perfect match.” Her daughter, also unvaccinated, stated: “Being denied for a nonmedical reason for someone’s beliefs that are different to yours, I mean that’s not how that should be.”
But vaccination status is a medical reason, given well-established data regarding increased mortality among the immunosuppressed. Ms. Vitullo then said: “We are trying to get to UPMC [University of Pittsburgh Medical Center] as they don’t require a vaccination.”
The public information page on transplant candidacy from UPMC reads (my italics): It is recommended that all transplant candidates, transplant recipients, and their household members receive COVID-19 vaccination when the vaccine is available to them. It is preferred that transplant candidates are vaccinated more than 2 weeks before transplantation.
I reached out to UPMC for clarification and was told by email that “we do not have a policy regarding COVID-19 vaccination requirement for current transplant candidates.” Houston Methodist shares the same agnostic stance.
Compare these opinions with Brigham and Women’s Hospital, where the requirements are resolute: “Like most other transplant programs across the country, the COVID-19 vaccine is one of several vaccines and lifestyle behaviors that are required for patients awaiting solid organ transplant.”
They add that “transplant candidates must also receive the seasonal influenza and hepatitis B vaccines, follow other healthy behaviors, and demonstrate they can commit to taking the required medications following transplant.”
In January 2022, Brigham and Women’s Hospital declared 31-year-old D.J. Ferguson ineligible for a heart transplant because he declined to be vaccinated against COVID-19. According to the New York Post and ABC News, his physicians resorted to left ventricular assist device support. His mother, Tracy Ferguson, is quoted as saying: “He’s not an antivaxxer. He has all of his vaccines.” I’ll just leave that right there.
Unfortunately, Michelle Vitullo’s obituary was published in December 2021. Regardless of whether she received her liver transplant, the outcome is tragic, and whatever you think of this family’s battle playing out in the glare of the national spotlight, their loss is no less devastating.
The directed-donation aspect of this case poses an interesting question. A news anchor asked the mother and daughter: “If you both accept the risks, why doesn’t the hospital just let you try?” The answers are obvious to us clinicians. Performing a transplantation in an unvaccinated patient could lead to their early death if they became infected because of their immunocompromised state, would open the door for transplantation of any patient who is unvaccinated for anything, including influenza and hepatitis B, which could result in the preventable waste of organs, and puts other vulnerable hospitalized patients at risk during the initial transplant stay and follow-up.
That’s not to mention the potential legal suit. Never has a consent form dissuaded any party from lodging an accusation of wrongful death or medical malpractice. In the face of strong data on higher mortality in unvaccinated, immunocompromised patients, a good lawyer could charge that the institution and transplant surgeons should have known better, regardless of the donor and recipient’s willingness to accept the risks.
The Vitullo and Ferguson cases are among many similar dilemmas surrounding transplant candidacy across the United States.
University of Virginia Health in Charlottesville denied 42-year-old Shamgar Connors a kidney transplant because he is unvaccinated, despite a previous COVID-19 infection. In October 2021, Leilani Lutali of Colorado was denied a kidney by UCHealth because she declined vaccination.
As Ted Lasso says: “There’s a bunch of crazy stuff on Twitter.”
Predictably, social media is full of public outcry. “Some cold-hearted people on here” tweeted one. “What if it was one of your loved ones who needed a transplant?” Another tweeted the Hippocratic oath with the comment that “They all swore under this noble ‘oat’, but I guess it’s been forgotten.” (This was followed with a photo of a box of Quaker Oats in a failed attempt at humor.) These discussions among the Twitterati highlight the depths of misunderstanding on organ transplantation.
To be fair, unless you have been personally involved in the decision-making process for transplant candidacy, there is little opportunity to be educated. I explain to my anxious patients and their families that a donor organ is like a fumbled football. There may be well over 100 patients at all levels of transplant status in many geographic locations diving for that same organ.
The transplant team is tasked with finding the best match, determining who is the sickest, assessing time for transport of that organ, and, above all, who will be the best steward of that organ.
Take heart transplantation, for instance. Approximately 3,500 patients in the United States are awaiting one each year. Instead of facing an almost certain death within 5 years, a transplant recipient has a chance at a median survival of 12-13 years. The cost of a heart transplant is approximately $1.38 million, according to Milliman, a consulting firm. This is “an incredibly resource intensive procedure,” including expenditures for transportation, antirejection medication, office visits, physician fees, ICU stays, rejection surveillance, and acute rejection therapies.
Transplant denial is nothing new
People get turned down for organ transplants all the time. My patient with end-stage dilated cardiomyopathy was denied a heart transplant when it was discovered that he had scores of outstanding parking tickets. This was seen as a surrogate for an inability to afford his antirejection medication.
Another patient swore that her positive cotinine levels were caused by endless hours at the bingo hall where second-hand smoke swirled. She was also denied. Many potential candidates who are in acute decline hold precariously to newfound sobriety. They are denied. A patient’s boyfriend told the transplant team that he couldn’t be relied upon to drive her to her appointments. She was denied.
Many people who engage in antisocial behaviors have no idea that these actions may result in the denial of an organ transplant should their future selves need one. These are hard lines, but everyone should agree that the odds of survival are heavily in favor of the consistently adherent.
We should take this opportunity to educate the public on how complicated obtaining an organ transplant can be. More than 6,000 people die each year waiting for an organ because of the supply-and-demand disparities in the transplantation arena. I’m willing to bet that many of the loudest protesters in favor of unvaccinated transplant recipients have not signed the organ donor box on the back of their driver’s license. This conversation is an opportunity to change that and remind people that organ donation may be their only opportunity to save a fellow human’s life.
Again, to quote Ted Lasso: “If you care about someone and you got a little love in your heart, there ain’t nothing you can’t get through together.” That philosophy should apply to the tasks of selecting the best organ donors as well as the best organ recipients.
And every organ should go to the one who will honor their donor and their donor’s family by taking the best care of that ultimate gift of life, including being vaccinated against COVID-19.
Dr. Walton-Shirley is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
I agree with most advice given by the affable TV character Ted Lasso. “Every choice is a chance,” he said. Pandemic-era physicians must now consider whether a politically motivated choice to decline COVID-19 vaccination should negatively affect the chance to receive an organ donation.
And in confronting these choices, we have a chance to educate the public on the complexities of the organ allocation process.
A well-informed patient’s personal choice should be honored, even if clinicians disagree, if it does not affect the well-being of others. For example, I once had a patient in acute leukemic crisis who declined blood products because she was a Jehovah’s Witness. She died. Her choice affected her longevity only.
Compare that decision with awarding an organ to an individual who has declined readily available protection of that organ. Weigh that choice against the fact that said protection is against an infectious disease that has killed over 5.5 million worldwide.
Some institutions stand strong, others hedge their bets
Admirably, Loyola University Health System understands that difference. They published a firm stand on transplant candidacy and COVID-19 vaccination status in the Journal of Heart and Lung Transplant. Daniel Dilling, MD, medical director of the lung transplantation program , and Mark Kuczewski, PhD, a professor of medical ethics at Loyola University Chicago, Maywood, Ill., wrote that: “We believe that requiring vaccination against COVID-19 should not be controversial when we focus strictly on established frameworks and practices surrounding eligibility for wait-listing to receive a solid organ transplant.”
The Cleveland Clinic apparently agrees. In October 2021, they denied a liver transplant to Michelle Vitullo of Ohio, whose daughter had been deemed “a perfect match.” Her daughter, also unvaccinated, stated: “Being denied for a nonmedical reason for someone’s beliefs that are different to yours, I mean that’s not how that should be.”
But vaccination status is a medical reason, given well-established data regarding increased mortality among the immunosuppressed. Ms. Vitullo then said: “We are trying to get to UPMC [University of Pittsburgh Medical Center] as they don’t require a vaccination.”
The public information page on transplant candidacy from UPMC reads (my italics): It is recommended that all transplant candidates, transplant recipients, and their household members receive COVID-19 vaccination when the vaccine is available to them. It is preferred that transplant candidates are vaccinated more than 2 weeks before transplantation.
I reached out to UPMC for clarification and was told by email that “we do not have a policy regarding COVID-19 vaccination requirement for current transplant candidates.” Houston Methodist shares the same agnostic stance.
Compare these opinions with Brigham and Women’s Hospital, where the requirements are resolute: “Like most other transplant programs across the country, the COVID-19 vaccine is one of several vaccines and lifestyle behaviors that are required for patients awaiting solid organ transplant.”
They add that “transplant candidates must also receive the seasonal influenza and hepatitis B vaccines, follow other healthy behaviors, and demonstrate they can commit to taking the required medications following transplant.”
In January 2022, Brigham and Women’s Hospital declared 31-year-old D.J. Ferguson ineligible for a heart transplant because he declined to be vaccinated against COVID-19. According to the New York Post and ABC News, his physicians resorted to left ventricular assist device support. His mother, Tracy Ferguson, is quoted as saying: “He’s not an antivaxxer. He has all of his vaccines.” I’ll just leave that right there.
Unfortunately, Michelle Vitullo’s obituary was published in December 2021. Regardless of whether she received her liver transplant, the outcome is tragic, and whatever you think of this family’s battle playing out in the glare of the national spotlight, their loss is no less devastating.
The directed-donation aspect of this case poses an interesting question. A news anchor asked the mother and daughter: “If you both accept the risks, why doesn’t the hospital just let you try?” The answers are obvious to us clinicians. Performing a transplantation in an unvaccinated patient could lead to their early death if they became infected because of their immunocompromised state, would open the door for transplantation of any patient who is unvaccinated for anything, including influenza and hepatitis B, which could result in the preventable waste of organs, and puts other vulnerable hospitalized patients at risk during the initial transplant stay and follow-up.
That’s not to mention the potential legal suit. Never has a consent form dissuaded any party from lodging an accusation of wrongful death or medical malpractice. In the face of strong data on higher mortality in unvaccinated, immunocompromised patients, a good lawyer could charge that the institution and transplant surgeons should have known better, regardless of the donor and recipient’s willingness to accept the risks.
The Vitullo and Ferguson cases are among many similar dilemmas surrounding transplant candidacy across the United States.
University of Virginia Health in Charlottesville denied 42-year-old Shamgar Connors a kidney transplant because he is unvaccinated, despite a previous COVID-19 infection. In October 2021, Leilani Lutali of Colorado was denied a kidney by UCHealth because she declined vaccination.
As Ted Lasso says: “There’s a bunch of crazy stuff on Twitter.”
Predictably, social media is full of public outcry. “Some cold-hearted people on here” tweeted one. “What if it was one of your loved ones who needed a transplant?” Another tweeted the Hippocratic oath with the comment that “They all swore under this noble ‘oat’, but I guess it’s been forgotten.” (This was followed with a photo of a box of Quaker Oats in a failed attempt at humor.) These discussions among the Twitterati highlight the depths of misunderstanding on organ transplantation.
To be fair, unless you have been personally involved in the decision-making process for transplant candidacy, there is little opportunity to be educated. I explain to my anxious patients and their families that a donor organ is like a fumbled football. There may be well over 100 patients at all levels of transplant status in many geographic locations diving for that same organ.
The transplant team is tasked with finding the best match, determining who is the sickest, assessing time for transport of that organ, and, above all, who will be the best steward of that organ.
Take heart transplantation, for instance. Approximately 3,500 patients in the United States are awaiting one each year. Instead of facing an almost certain death within 5 years, a transplant recipient has a chance at a median survival of 12-13 years. The cost of a heart transplant is approximately $1.38 million, according to Milliman, a consulting firm. This is “an incredibly resource intensive procedure,” including expenditures for transportation, antirejection medication, office visits, physician fees, ICU stays, rejection surveillance, and acute rejection therapies.
Transplant denial is nothing new
People get turned down for organ transplants all the time. My patient with end-stage dilated cardiomyopathy was denied a heart transplant when it was discovered that he had scores of outstanding parking tickets. This was seen as a surrogate for an inability to afford his antirejection medication.
Another patient swore that her positive cotinine levels were caused by endless hours at the bingo hall where second-hand smoke swirled. She was also denied. Many potential candidates who are in acute decline hold precariously to newfound sobriety. They are denied. A patient’s boyfriend told the transplant team that he couldn’t be relied upon to drive her to her appointments. She was denied.
Many people who engage in antisocial behaviors have no idea that these actions may result in the denial of an organ transplant should their future selves need one. These are hard lines, but everyone should agree that the odds of survival are heavily in favor of the consistently adherent.
We should take this opportunity to educate the public on how complicated obtaining an organ transplant can be. More than 6,000 people die each year waiting for an organ because of the supply-and-demand disparities in the transplantation arena. I’m willing to bet that many of the loudest protesters in favor of unvaccinated transplant recipients have not signed the organ donor box on the back of their driver’s license. This conversation is an opportunity to change that and remind people that organ donation may be their only opportunity to save a fellow human’s life.
Again, to quote Ted Lasso: “If you care about someone and you got a little love in your heart, there ain’t nothing you can’t get through together.” That philosophy should apply to the tasks of selecting the best organ donors as well as the best organ recipients.
And every organ should go to the one who will honor their donor and their donor’s family by taking the best care of that ultimate gift of life, including being vaccinated against COVID-19.
Dr. Walton-Shirley is a native Kentuckian who retired from full-time invasive cardiology. She enjoys locums work in Montana and is a champion of physician rights and patient safety. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
Real-world data reinforce stem cell transplant for progressive systemic sclerosis
Current selection criteria for autologous hematopoietic stem cell transplant (AHSCT) in patients with rapidly progressing systemic sclerosis were validated in a study presented at the annual meeting of the Canadian Rheumatology Association.
The study, which associated AHSCT with improvement in overall survival and an acceptable risk of adverse events, “provides valuable real-world, long-term data pertaining to key clinical outcomes to support the use of AHSCT in patients with rapidly progressing systemic sclerosis,” reported Nancy Maltez, MD, a rheumatologist and clinical investigator who is on the faculty of the University of Ottawa.
The prospective study enrolled 85 patients in Canada and 41 patients in France with rapidly progressing systemic sclerosis. The patients in both countries were enrolled with the same eligibility criteria for AHSCT, but patients in France underwent AHSCT while the patients in Canada were treated with conventional therapies, such as cyclophosphamide.
On the primary outcome of overall survival, the Kaplan-Meier curve split almost immediately in favor of AHSCT. At 4 years, more than 25% of patients in the conventional therapy group had died versus less than 5% of those who underwent AHSCT. Although the mortality curve did slope downwards in the AHSCT group over the subsequent 6 years of follow-up, it largely paralleled and remained superior to convention therapy.
About 50% survival advantage seen for AHSCT
In this nonrandomized study, the statistical survival advantage of AHSCT was not provided, but the survival graph showed about 75% survival at 8 years of follow-up in the AHSCT group, compared with about 50% survival in the conventional-therapy group.
Many of the secondary outcomes, including those evaluating skin involvement, preservation of lung function, and absence of renal complications also favored AHSCT, according to Dr. Maltez.
On the modified Rodnan skin score, a significant difference (P < .001) observed at 12 months was sustained at 36 months, when the score was 4.48 points lower among patients treated with AHSCT. The difference in forced vital capacity (FVC) was about 10% higher (P < .0001) in the AHSCT group.
Over long-term follow-up, the incidence of scleroderma renal crisis per 100 person-years was 6.02 cases in the conventional therapy group versus 0.58 cases (P < .001) in the AHSCT group. There was no significant difference in the proportion of patients in the two groups receiving a pacemaker over the course of follow-up, but the rate of new malignancies per 100 person-years was 3.71 in the conventional care group versus 0.58 (P < .001) in the AHSCT group.
Significant complications attributed to AHSCT were uncommon. This is important, because AHSCT was not uniformly well tolerated in the initial trials. The first of three randomized trials with AHSCT in progressive systemic sclerosis was published more than 10 years ago after a series of promising early phase trials. Each associated AHSCT with benefit, but patient selection appeared to be important.
In the ASSIST trial of 2011, AHSCT was associated with significant reductions in skin involvement and improvements in pulmonary function relative to cyclophosphamide, but enrollment was stopped after only 19 patients, and follow-up extended to only 2 years.
Substantial AHSCT-related mortality in ASTIS
In the second trial, called ASTIS, AHSCT was associated with a higher rate of mortality than cyclophosphamide after 1 year of follow-up, although there was a significantly greater long-term event-free survival for AHSCT when patients were followed out to 4 years. This study reinforced the need for cardiac screening because of because of concern that severe cardiac compromise contributed to the increased risk of AHSCT-related mortality.
The SCOT trial employed a high-intensity myeloablative conditioning regimen and total body irradiation prior to AHSCT. It is not clear that these contributed to improved survival, particularly because of the risk for irradiation to exacerbate complications in the lung and kidney, but AHSCT-related mortality was only 3% at 54 months. Patient enrollment criteria in this trial were also suspected of having played a role in the favorable results.
In the Canadian-French collaborative study, patients were considered eligible for AHSCT if they met the enrollment criteria used in the ASTIS trial, according to Dr. Maltez. She attributed the low rates of early mortality and relative absence of transplant-related death to the lessons learned in the published trials.
Overall, the data support the routine but selective use of AHSCT in rapidly progressing systemic sclerosis, Dr. Maltez concluded.
Maria Carolina Oliveira, MD, of the department of internal medicine at the University of São Paulo, generally agreed. A coauthor of a recent review of AHSCT for systemic sclerosis, Dr. Oliveira emphasized that patient selection is critical.
“AHSCT for systemic sclerosis has very specific inclusion criteria. Indeed, it is indicated for patients with severe and progressive disease but under two specific conditions: severe and progressive diffuse skin involvement and/or progressive interstitial lung disease,” she said in an interview.
Because of the thin line between benefit and risk according to disease subtypes and comorbidities, she said that it is important to be aware of relative contraindications and to recognize the risks of AHSCT.
At this time, and in the absence of better biomarkers to identify those most likely to benefit, “patients with other forms of severe scleroderma, such as those with pulmonary hypertension, scleroderma renal crisis, or severe cardiac involvement, for example, are not eligible,” she said.
Dr. Maltez and Dr. Oliveira reported having no potential conflicts of interest.
Current selection criteria for autologous hematopoietic stem cell transplant (AHSCT) in patients with rapidly progressing systemic sclerosis were validated in a study presented at the annual meeting of the Canadian Rheumatology Association.
The study, which associated AHSCT with improvement in overall survival and an acceptable risk of adverse events, “provides valuable real-world, long-term data pertaining to key clinical outcomes to support the use of AHSCT in patients with rapidly progressing systemic sclerosis,” reported Nancy Maltez, MD, a rheumatologist and clinical investigator who is on the faculty of the University of Ottawa.
The prospective study enrolled 85 patients in Canada and 41 patients in France with rapidly progressing systemic sclerosis. The patients in both countries were enrolled with the same eligibility criteria for AHSCT, but patients in France underwent AHSCT while the patients in Canada were treated with conventional therapies, such as cyclophosphamide.
On the primary outcome of overall survival, the Kaplan-Meier curve split almost immediately in favor of AHSCT. At 4 years, more than 25% of patients in the conventional therapy group had died versus less than 5% of those who underwent AHSCT. Although the mortality curve did slope downwards in the AHSCT group over the subsequent 6 years of follow-up, it largely paralleled and remained superior to convention therapy.
About 50% survival advantage seen for AHSCT
In this nonrandomized study, the statistical survival advantage of AHSCT was not provided, but the survival graph showed about 75% survival at 8 years of follow-up in the AHSCT group, compared with about 50% survival in the conventional-therapy group.
Many of the secondary outcomes, including those evaluating skin involvement, preservation of lung function, and absence of renal complications also favored AHSCT, according to Dr. Maltez.
On the modified Rodnan skin score, a significant difference (P < .001) observed at 12 months was sustained at 36 months, when the score was 4.48 points lower among patients treated with AHSCT. The difference in forced vital capacity (FVC) was about 10% higher (P < .0001) in the AHSCT group.
Over long-term follow-up, the incidence of scleroderma renal crisis per 100 person-years was 6.02 cases in the conventional therapy group versus 0.58 cases (P < .001) in the AHSCT group. There was no significant difference in the proportion of patients in the two groups receiving a pacemaker over the course of follow-up, but the rate of new malignancies per 100 person-years was 3.71 in the conventional care group versus 0.58 (P < .001) in the AHSCT group.
Significant complications attributed to AHSCT were uncommon. This is important, because AHSCT was not uniformly well tolerated in the initial trials. The first of three randomized trials with AHSCT in progressive systemic sclerosis was published more than 10 years ago after a series of promising early phase trials. Each associated AHSCT with benefit, but patient selection appeared to be important.
In the ASSIST trial of 2011, AHSCT was associated with significant reductions in skin involvement and improvements in pulmonary function relative to cyclophosphamide, but enrollment was stopped after only 19 patients, and follow-up extended to only 2 years.
Substantial AHSCT-related mortality in ASTIS
In the second trial, called ASTIS, AHSCT was associated with a higher rate of mortality than cyclophosphamide after 1 year of follow-up, although there was a significantly greater long-term event-free survival for AHSCT when patients were followed out to 4 years. This study reinforced the need for cardiac screening because of because of concern that severe cardiac compromise contributed to the increased risk of AHSCT-related mortality.
The SCOT trial employed a high-intensity myeloablative conditioning regimen and total body irradiation prior to AHSCT. It is not clear that these contributed to improved survival, particularly because of the risk for irradiation to exacerbate complications in the lung and kidney, but AHSCT-related mortality was only 3% at 54 months. Patient enrollment criteria in this trial were also suspected of having played a role in the favorable results.
In the Canadian-French collaborative study, patients were considered eligible for AHSCT if they met the enrollment criteria used in the ASTIS trial, according to Dr. Maltez. She attributed the low rates of early mortality and relative absence of transplant-related death to the lessons learned in the published trials.
Overall, the data support the routine but selective use of AHSCT in rapidly progressing systemic sclerosis, Dr. Maltez concluded.
Maria Carolina Oliveira, MD, of the department of internal medicine at the University of São Paulo, generally agreed. A coauthor of a recent review of AHSCT for systemic sclerosis, Dr. Oliveira emphasized that patient selection is critical.
“AHSCT for systemic sclerosis has very specific inclusion criteria. Indeed, it is indicated for patients with severe and progressive disease but under two specific conditions: severe and progressive diffuse skin involvement and/or progressive interstitial lung disease,” she said in an interview.
Because of the thin line between benefit and risk according to disease subtypes and comorbidities, she said that it is important to be aware of relative contraindications and to recognize the risks of AHSCT.
At this time, and in the absence of better biomarkers to identify those most likely to benefit, “patients with other forms of severe scleroderma, such as those with pulmonary hypertension, scleroderma renal crisis, or severe cardiac involvement, for example, are not eligible,” she said.
Dr. Maltez and Dr. Oliveira reported having no potential conflicts of interest.
Current selection criteria for autologous hematopoietic stem cell transplant (AHSCT) in patients with rapidly progressing systemic sclerosis were validated in a study presented at the annual meeting of the Canadian Rheumatology Association.
The study, which associated AHSCT with improvement in overall survival and an acceptable risk of adverse events, “provides valuable real-world, long-term data pertaining to key clinical outcomes to support the use of AHSCT in patients with rapidly progressing systemic sclerosis,” reported Nancy Maltez, MD, a rheumatologist and clinical investigator who is on the faculty of the University of Ottawa.
The prospective study enrolled 85 patients in Canada and 41 patients in France with rapidly progressing systemic sclerosis. The patients in both countries were enrolled with the same eligibility criteria for AHSCT, but patients in France underwent AHSCT while the patients in Canada were treated with conventional therapies, such as cyclophosphamide.
On the primary outcome of overall survival, the Kaplan-Meier curve split almost immediately in favor of AHSCT. At 4 years, more than 25% of patients in the conventional therapy group had died versus less than 5% of those who underwent AHSCT. Although the mortality curve did slope downwards in the AHSCT group over the subsequent 6 years of follow-up, it largely paralleled and remained superior to convention therapy.
About 50% survival advantage seen for AHSCT
In this nonrandomized study, the statistical survival advantage of AHSCT was not provided, but the survival graph showed about 75% survival at 8 years of follow-up in the AHSCT group, compared with about 50% survival in the conventional-therapy group.
Many of the secondary outcomes, including those evaluating skin involvement, preservation of lung function, and absence of renal complications also favored AHSCT, according to Dr. Maltez.
On the modified Rodnan skin score, a significant difference (P < .001) observed at 12 months was sustained at 36 months, when the score was 4.48 points lower among patients treated with AHSCT. The difference in forced vital capacity (FVC) was about 10% higher (P < .0001) in the AHSCT group.
Over long-term follow-up, the incidence of scleroderma renal crisis per 100 person-years was 6.02 cases in the conventional therapy group versus 0.58 cases (P < .001) in the AHSCT group. There was no significant difference in the proportion of patients in the two groups receiving a pacemaker over the course of follow-up, but the rate of new malignancies per 100 person-years was 3.71 in the conventional care group versus 0.58 (P < .001) in the AHSCT group.
Significant complications attributed to AHSCT were uncommon. This is important, because AHSCT was not uniformly well tolerated in the initial trials. The first of three randomized trials with AHSCT in progressive systemic sclerosis was published more than 10 years ago after a series of promising early phase trials. Each associated AHSCT with benefit, but patient selection appeared to be important.
In the ASSIST trial of 2011, AHSCT was associated with significant reductions in skin involvement and improvements in pulmonary function relative to cyclophosphamide, but enrollment was stopped after only 19 patients, and follow-up extended to only 2 years.
Substantial AHSCT-related mortality in ASTIS
In the second trial, called ASTIS, AHSCT was associated with a higher rate of mortality than cyclophosphamide after 1 year of follow-up, although there was a significantly greater long-term event-free survival for AHSCT when patients were followed out to 4 years. This study reinforced the need for cardiac screening because of because of concern that severe cardiac compromise contributed to the increased risk of AHSCT-related mortality.
The SCOT trial employed a high-intensity myeloablative conditioning regimen and total body irradiation prior to AHSCT. It is not clear that these contributed to improved survival, particularly because of the risk for irradiation to exacerbate complications in the lung and kidney, but AHSCT-related mortality was only 3% at 54 months. Patient enrollment criteria in this trial were also suspected of having played a role in the favorable results.
In the Canadian-French collaborative study, patients were considered eligible for AHSCT if they met the enrollment criteria used in the ASTIS trial, according to Dr. Maltez. She attributed the low rates of early mortality and relative absence of transplant-related death to the lessons learned in the published trials.
Overall, the data support the routine but selective use of AHSCT in rapidly progressing systemic sclerosis, Dr. Maltez concluded.
Maria Carolina Oliveira, MD, of the department of internal medicine at the University of São Paulo, generally agreed. A coauthor of a recent review of AHSCT for systemic sclerosis, Dr. Oliveira emphasized that patient selection is critical.
“AHSCT for systemic sclerosis has very specific inclusion criteria. Indeed, it is indicated for patients with severe and progressive disease but under two specific conditions: severe and progressive diffuse skin involvement and/or progressive interstitial lung disease,” she said in an interview.
Because of the thin line between benefit and risk according to disease subtypes and comorbidities, she said that it is important to be aware of relative contraindications and to recognize the risks of AHSCT.
At this time, and in the absence of better biomarkers to identify those most likely to benefit, “patients with other forms of severe scleroderma, such as those with pulmonary hypertension, scleroderma renal crisis, or severe cardiac involvement, for example, are not eligible,” she said.
Dr. Maltez and Dr. Oliveira reported having no potential conflicts of interest.
FROM THE ANNUAL MEETING OF THE CANADIAN RHEUMATOLOGY ASSOCIATION
No COVID vax, no transplant: Unfair or good medicine?
Right now, more than 106,600 people in the United States are on the national transplant waiting list, each hoping to hear soon that a lung, kidney, heart, or other vital organ has been found for them. It’s the promise not just of a new organ, but a new life.
Well before they are placed on that list, transplant candidates, as they’re known, are evaluated with a battery of tests and exams to be sure they are infection free, their other organs are healthy, and that all their vaccinations are up to date.
In January, a 31-year-old Boston father of two declined to get the COVID-19 vaccine, and Brigham and Women’s Hospital officials removed him from the heart transplant waiting list. And in North Carolina, a 38-year-old man in need of a kidney transplant said he, too, was denied the organ when he declined to get the vaccination.
Those are just two of the most recent cases. The decisions by the transplant centers to remove the candidates from the waiting list have set off a national debate among ethicists, family members, doctors, patients, and others.
On social media and in conversation, the question persists: Is removing them from the list unfair and cruel, or simply business as usual to keep the patient as healthy as possible and the transplant as successful as possible?
Two recent tweets sum up the debate.
“The people responsible for this should be charged with attempted homicide,” one Twitter user said, while another suggested that the more accurate way to headline the news about a transplant candidate refusing the COVID-19 vaccine would be: “Patient voluntarily forfeits donor organ.”
Doctors and ethics experts, as well as other patients on the waiting list, say it’s simply good medicine to require the COVID vaccine, along with a host of other pretransplant requirements.
Transplant protocols
“Transplant medicine has always been a strong promoter of vaccination,” said Silas Prescod Norman, MD, a clinical associate professor of nephrology and internal medicine at the University of Michigan, Ann Arbor. He is a kidney specialist who works in the university’s transplant clinic.
Requiring the COVID vaccine is in line with requirements to get numerous other vaccines, he said.“Promoting the COVID vaccine among our transplant candidates and recipients is just an extension of our usual practice.
“In transplantation, first and foremost is patient safety,” Dr. Norman said. “And we know that solid organ transplant patients are at substantially higher risk of contracting COVID than nontransplant patients.”
After the transplant, they are placed on immunosuppressant drugs, that weaken the immune system while also decreasing the body’s ability to reject the new organ.
“We know now, because there is good data about the vaccine to show that people who are on transplant medications are less likely to make detectable antibodies after vaccination,” said Dr. Norman, who’s also a medical adviser for the American Kidney Fund, a nonprofit that provides kidney health information and financial assistance for dialysis.
And this is not a surprise because of the immunosuppressive effects, he said. “So it only makes sense to get people vaccinated before transplantation.”
Researchers compared the cases of more than 17,000 people who had received organ transplants and were hospitalized from April to November 2020, either for COVID (1,682 of them) or other health issues. Those who had COVID were more likely to have complications and to die in the hospital than those who did not have it.
Vaccination guidelines, policies
Federal COVID-19 treatment guidelines from the National Institutes of Health state that transplant patients on immunosuppressant drugs used after the procedure should be considered at a higher risk of getting severe COVID if infected.
In a joint statement from the American Society of Transplant Surgeons, the American Society of Transplantation, and the International Society for Heart and Lung Transplantation, the organizations say they “strongly recommend that all eligible children and adult transplant candidates and recipients be vaccinated with a COVID-19 vaccine [and booster] that is approved or authorized in their jurisdiction. Whenever possible, vaccination should occur prior to transplantation.” Ideally, it should be completed at least 2 weeks before the transplant.
The organizations also “support the development of institutional policies regarding pretransplant vaccination. We believe that this is in the best interest of the transplant candidate, optimizing their chances of getting through the perioperative and posttransplant periods without severe COVID-19 disease, especially at times of greater infection prevalence.”
Officials at Brigham and Women’s Hospital, where the 31-year-old father was removed from the list, issued a statement that reads, in part: “Our Mass General Brigham health care system requires several [Centers for Disease Control and Prevention]-recommended vaccines, including the COVID-19 vaccine, and lifestyle behaviors for transplant candidates to create both the best chance for a successful operation and to optimize the patient’s survival after transplantation, given that their immune system is drastically suppressed. Patients are not active on the wait list without this.”
Ethics amid organ shortage
“Organs are scarce,” said Arthur L. Caplan, PhD, director of the division of medical ethics at New York University Langone Medical Center. That makes the goal of choosing the very best candidates for success even more crucial.
“You try to maximize the chance the organ will work,” he said. Pretransplant vaccination is one way.
The shortage is most severe for kidney transplants. In 2020, according to federal statistics, more than 91,000 kidney transplants were needed, but fewer than 23,000 were received. During 2021, 41,354 transplants were done, an increase of nearly 6% over the previous year. The total includes kidneys, hearts, lungs, and other organs, with kidneys accounting for more than 24,000 of the total.
Even with the rise in transplant numbers, supply does not meet demand. According to federal statistics, 17 people in the United States die each day waiting for an organ transplant. Every 9 minutes, someone is added to the waiting list.
“This isn’t and it shouldn’t be a fight about the COVID vaccine,” Dr. Caplan said. “This isn’t an issue about punishing non-COVID vaccinators. It’s deciding who is going to get a scarce organ.”
“A lot of people [opposed to removing the nonvaccinated from the list] think: ‘Oh, they are just killing those people who won’t take a COVID vaccine.’ That’s not what is going on.”
The transplant candidate must be in the best possible shape overall, Dr. Caplan and doctors agreed. Someone who is smoking, drinking heavily, or abusing drugs isn’t going to the top of the list either. And for other procedures, such as bariatric surgery or knee surgery, some patients are told first to lose weight before a surgeon will operate.
The worry about side effects from the vaccine, which some patients have cited as a concern, is misplaced, Dr. Caplan said. What transplant candidates who refuse the COVID vaccine may not be thinking about is that they are facing a serious operation and will be on numerous anti-rejection drugs, with side effects, after the surgery.
“So to be worried about the side effects of a COVID vaccine is irrational,” he said.
Transplants: The process
The patients who were recently removed from the transplant list could seek care and a transplant at an alternate center, said Anne Paschke, a spokesperson for the United Network for Organ Sharing, a nonprofit group that is under contract with the federal government and operates the national Organ Procurement and Transplantation Network (OPTN).
“Transplant hospitals decide which patients to add to the wait list based on their own criteria and medical judgment to create the best chance for a positive transplant outcome,” she said. That’s done with the understanding that patients will help with their medical care.
So, if one program won’t accept a patient, another may. But, if a patient turned down at one center due to refusing to get the COVID vaccine tries another center, the requirements at that hospital may be the same, she said.
OPTN maintains a list of transplant centers. As of Jan. 28, there were 251 transplant centers, according to UNOS, which manages the waiting list, matches donors and recipients, and strives for equity, among other duties.
Pretransplant refusers not typical
“The cases we are seeing are outliers,” Dr. Caplan said of the handful of known candidates who have refused the vaccine. Most ask their doctor exactly what they need to do to live and follow those instructions.
Dr. Norman agreed. Most of the kidney patients he cares for who are hoping for a transplant have been on dialysis, “which they do not like. They are doing whatever they can to make sure they don’t go back on dialysis. As a group, they tend to be very adherent, very safety conscious because they understand their risk and they understand the gift they have received [or will receive] through transplantation. They want to do everything they can to respect and protect that gift.”
Not surprisingly, some on the transplant list who are vaccinated have strong opinions about those who refuse to get the vaccine. Dana J. Ufkes, 61, a Seattle realtor, has been on the kidney transplant list – this time – since 2003, hoping for her third transplant. When asked if potential recipients should be removed from the list if they refuse the COVID vaccine, her answer was immediate: “Absolutely.”
At age 17, Ms. Ufkes got a serious kidney infection that went undiagnosed and untreated. Her kidney health worsened, and she needed a transplant. She got her first one in 1986, then again in 1992.
“They last longer than they used to,” she said. But not forever. (According to the American Kidney Fund, transplants from a living kidney donor last about 15-20 years; from a deceased donor, 10-15.)
The decision to decline the vaccine is, of course, each person’s choice, Ms. Ufkes said. But “if they don’t want to be vaccinated [and still want to be on the list], I think that’s BS.”
Citing the lack of organs, “it’s not like they are handing these out like jellybeans.”
A version of this article first appeared on WebMD.com.
Right now, more than 106,600 people in the United States are on the national transplant waiting list, each hoping to hear soon that a lung, kidney, heart, or other vital organ has been found for them. It’s the promise not just of a new organ, but a new life.
Well before they are placed on that list, transplant candidates, as they’re known, are evaluated with a battery of tests and exams to be sure they are infection free, their other organs are healthy, and that all their vaccinations are up to date.
In January, a 31-year-old Boston father of two declined to get the COVID-19 vaccine, and Brigham and Women’s Hospital officials removed him from the heart transplant waiting list. And in North Carolina, a 38-year-old man in need of a kidney transplant said he, too, was denied the organ when he declined to get the vaccination.
Those are just two of the most recent cases. The decisions by the transplant centers to remove the candidates from the waiting list have set off a national debate among ethicists, family members, doctors, patients, and others.
On social media and in conversation, the question persists: Is removing them from the list unfair and cruel, or simply business as usual to keep the patient as healthy as possible and the transplant as successful as possible?
Two recent tweets sum up the debate.
“The people responsible for this should be charged with attempted homicide,” one Twitter user said, while another suggested that the more accurate way to headline the news about a transplant candidate refusing the COVID-19 vaccine would be: “Patient voluntarily forfeits donor organ.”
Doctors and ethics experts, as well as other patients on the waiting list, say it’s simply good medicine to require the COVID vaccine, along with a host of other pretransplant requirements.
Transplant protocols
“Transplant medicine has always been a strong promoter of vaccination,” said Silas Prescod Norman, MD, a clinical associate professor of nephrology and internal medicine at the University of Michigan, Ann Arbor. He is a kidney specialist who works in the university’s transplant clinic.
Requiring the COVID vaccine is in line with requirements to get numerous other vaccines, he said.“Promoting the COVID vaccine among our transplant candidates and recipients is just an extension of our usual practice.
“In transplantation, first and foremost is patient safety,” Dr. Norman said. “And we know that solid organ transplant patients are at substantially higher risk of contracting COVID than nontransplant patients.”
After the transplant, they are placed on immunosuppressant drugs, that weaken the immune system while also decreasing the body’s ability to reject the new organ.
“We know now, because there is good data about the vaccine to show that people who are on transplant medications are less likely to make detectable antibodies after vaccination,” said Dr. Norman, who’s also a medical adviser for the American Kidney Fund, a nonprofit that provides kidney health information and financial assistance for dialysis.
And this is not a surprise because of the immunosuppressive effects, he said. “So it only makes sense to get people vaccinated before transplantation.”
Researchers compared the cases of more than 17,000 people who had received organ transplants and were hospitalized from April to November 2020, either for COVID (1,682 of them) or other health issues. Those who had COVID were more likely to have complications and to die in the hospital than those who did not have it.
Vaccination guidelines, policies
Federal COVID-19 treatment guidelines from the National Institutes of Health state that transplant patients on immunosuppressant drugs used after the procedure should be considered at a higher risk of getting severe COVID if infected.
In a joint statement from the American Society of Transplant Surgeons, the American Society of Transplantation, and the International Society for Heart and Lung Transplantation, the organizations say they “strongly recommend that all eligible children and adult transplant candidates and recipients be vaccinated with a COVID-19 vaccine [and booster] that is approved or authorized in their jurisdiction. Whenever possible, vaccination should occur prior to transplantation.” Ideally, it should be completed at least 2 weeks before the transplant.
The organizations also “support the development of institutional policies regarding pretransplant vaccination. We believe that this is in the best interest of the transplant candidate, optimizing their chances of getting through the perioperative and posttransplant periods without severe COVID-19 disease, especially at times of greater infection prevalence.”
Officials at Brigham and Women’s Hospital, where the 31-year-old father was removed from the list, issued a statement that reads, in part: “Our Mass General Brigham health care system requires several [Centers for Disease Control and Prevention]-recommended vaccines, including the COVID-19 vaccine, and lifestyle behaviors for transplant candidates to create both the best chance for a successful operation and to optimize the patient’s survival after transplantation, given that their immune system is drastically suppressed. Patients are not active on the wait list without this.”
Ethics amid organ shortage
“Organs are scarce,” said Arthur L. Caplan, PhD, director of the division of medical ethics at New York University Langone Medical Center. That makes the goal of choosing the very best candidates for success even more crucial.
“You try to maximize the chance the organ will work,” he said. Pretransplant vaccination is one way.
The shortage is most severe for kidney transplants. In 2020, according to federal statistics, more than 91,000 kidney transplants were needed, but fewer than 23,000 were received. During 2021, 41,354 transplants were done, an increase of nearly 6% over the previous year. The total includes kidneys, hearts, lungs, and other organs, with kidneys accounting for more than 24,000 of the total.
Even with the rise in transplant numbers, supply does not meet demand. According to federal statistics, 17 people in the United States die each day waiting for an organ transplant. Every 9 minutes, someone is added to the waiting list.
“This isn’t and it shouldn’t be a fight about the COVID vaccine,” Dr. Caplan said. “This isn’t an issue about punishing non-COVID vaccinators. It’s deciding who is going to get a scarce organ.”
“A lot of people [opposed to removing the nonvaccinated from the list] think: ‘Oh, they are just killing those people who won’t take a COVID vaccine.’ That’s not what is going on.”
The transplant candidate must be in the best possible shape overall, Dr. Caplan and doctors agreed. Someone who is smoking, drinking heavily, or abusing drugs isn’t going to the top of the list either. And for other procedures, such as bariatric surgery or knee surgery, some patients are told first to lose weight before a surgeon will operate.
The worry about side effects from the vaccine, which some patients have cited as a concern, is misplaced, Dr. Caplan said. What transplant candidates who refuse the COVID vaccine may not be thinking about is that they are facing a serious operation and will be on numerous anti-rejection drugs, with side effects, after the surgery.
“So to be worried about the side effects of a COVID vaccine is irrational,” he said.
Transplants: The process
The patients who were recently removed from the transplant list could seek care and a transplant at an alternate center, said Anne Paschke, a spokesperson for the United Network for Organ Sharing, a nonprofit group that is under contract with the federal government and operates the national Organ Procurement and Transplantation Network (OPTN).
“Transplant hospitals decide which patients to add to the wait list based on their own criteria and medical judgment to create the best chance for a positive transplant outcome,” she said. That’s done with the understanding that patients will help with their medical care.
So, if one program won’t accept a patient, another may. But, if a patient turned down at one center due to refusing to get the COVID vaccine tries another center, the requirements at that hospital may be the same, she said.
OPTN maintains a list of transplant centers. As of Jan. 28, there were 251 transplant centers, according to UNOS, which manages the waiting list, matches donors and recipients, and strives for equity, among other duties.
Pretransplant refusers not typical
“The cases we are seeing are outliers,” Dr. Caplan said of the handful of known candidates who have refused the vaccine. Most ask their doctor exactly what they need to do to live and follow those instructions.
Dr. Norman agreed. Most of the kidney patients he cares for who are hoping for a transplant have been on dialysis, “which they do not like. They are doing whatever they can to make sure they don’t go back on dialysis. As a group, they tend to be very adherent, very safety conscious because they understand their risk and they understand the gift they have received [or will receive] through transplantation. They want to do everything they can to respect and protect that gift.”
Not surprisingly, some on the transplant list who are vaccinated have strong opinions about those who refuse to get the vaccine. Dana J. Ufkes, 61, a Seattle realtor, has been on the kidney transplant list – this time – since 2003, hoping for her third transplant. When asked if potential recipients should be removed from the list if they refuse the COVID vaccine, her answer was immediate: “Absolutely.”
At age 17, Ms. Ufkes got a serious kidney infection that went undiagnosed and untreated. Her kidney health worsened, and she needed a transplant. She got her first one in 1986, then again in 1992.
“They last longer than they used to,” she said. But not forever. (According to the American Kidney Fund, transplants from a living kidney donor last about 15-20 years; from a deceased donor, 10-15.)
The decision to decline the vaccine is, of course, each person’s choice, Ms. Ufkes said. But “if they don’t want to be vaccinated [and still want to be on the list], I think that’s BS.”
Citing the lack of organs, “it’s not like they are handing these out like jellybeans.”
A version of this article first appeared on WebMD.com.
Right now, more than 106,600 people in the United States are on the national transplant waiting list, each hoping to hear soon that a lung, kidney, heart, or other vital organ has been found for them. It’s the promise not just of a new organ, but a new life.
Well before they are placed on that list, transplant candidates, as they’re known, are evaluated with a battery of tests and exams to be sure they are infection free, their other organs are healthy, and that all their vaccinations are up to date.
In January, a 31-year-old Boston father of two declined to get the COVID-19 vaccine, and Brigham and Women’s Hospital officials removed him from the heart transplant waiting list. And in North Carolina, a 38-year-old man in need of a kidney transplant said he, too, was denied the organ when he declined to get the vaccination.
Those are just two of the most recent cases. The decisions by the transplant centers to remove the candidates from the waiting list have set off a national debate among ethicists, family members, doctors, patients, and others.
On social media and in conversation, the question persists: Is removing them from the list unfair and cruel, or simply business as usual to keep the patient as healthy as possible and the transplant as successful as possible?
Two recent tweets sum up the debate.
“The people responsible for this should be charged with attempted homicide,” one Twitter user said, while another suggested that the more accurate way to headline the news about a transplant candidate refusing the COVID-19 vaccine would be: “Patient voluntarily forfeits donor organ.”
Doctors and ethics experts, as well as other patients on the waiting list, say it’s simply good medicine to require the COVID vaccine, along with a host of other pretransplant requirements.
Transplant protocols
“Transplant medicine has always been a strong promoter of vaccination,” said Silas Prescod Norman, MD, a clinical associate professor of nephrology and internal medicine at the University of Michigan, Ann Arbor. He is a kidney specialist who works in the university’s transplant clinic.
Requiring the COVID vaccine is in line with requirements to get numerous other vaccines, he said.“Promoting the COVID vaccine among our transplant candidates and recipients is just an extension of our usual practice.
“In transplantation, first and foremost is patient safety,” Dr. Norman said. “And we know that solid organ transplant patients are at substantially higher risk of contracting COVID than nontransplant patients.”
After the transplant, they are placed on immunosuppressant drugs, that weaken the immune system while also decreasing the body’s ability to reject the new organ.
“We know now, because there is good data about the vaccine to show that people who are on transplant medications are less likely to make detectable antibodies after vaccination,” said Dr. Norman, who’s also a medical adviser for the American Kidney Fund, a nonprofit that provides kidney health information and financial assistance for dialysis.
And this is not a surprise because of the immunosuppressive effects, he said. “So it only makes sense to get people vaccinated before transplantation.”
Researchers compared the cases of more than 17,000 people who had received organ transplants and were hospitalized from April to November 2020, either for COVID (1,682 of them) or other health issues. Those who had COVID were more likely to have complications and to die in the hospital than those who did not have it.
Vaccination guidelines, policies
Federal COVID-19 treatment guidelines from the National Institutes of Health state that transplant patients on immunosuppressant drugs used after the procedure should be considered at a higher risk of getting severe COVID if infected.
In a joint statement from the American Society of Transplant Surgeons, the American Society of Transplantation, and the International Society for Heart and Lung Transplantation, the organizations say they “strongly recommend that all eligible children and adult transplant candidates and recipients be vaccinated with a COVID-19 vaccine [and booster] that is approved or authorized in their jurisdiction. Whenever possible, vaccination should occur prior to transplantation.” Ideally, it should be completed at least 2 weeks before the transplant.
The organizations also “support the development of institutional policies regarding pretransplant vaccination. We believe that this is in the best interest of the transplant candidate, optimizing their chances of getting through the perioperative and posttransplant periods without severe COVID-19 disease, especially at times of greater infection prevalence.”
Officials at Brigham and Women’s Hospital, where the 31-year-old father was removed from the list, issued a statement that reads, in part: “Our Mass General Brigham health care system requires several [Centers for Disease Control and Prevention]-recommended vaccines, including the COVID-19 vaccine, and lifestyle behaviors for transplant candidates to create both the best chance for a successful operation and to optimize the patient’s survival after transplantation, given that their immune system is drastically suppressed. Patients are not active on the wait list without this.”
Ethics amid organ shortage
“Organs are scarce,” said Arthur L. Caplan, PhD, director of the division of medical ethics at New York University Langone Medical Center. That makes the goal of choosing the very best candidates for success even more crucial.
“You try to maximize the chance the organ will work,” he said. Pretransplant vaccination is one way.
The shortage is most severe for kidney transplants. In 2020, according to federal statistics, more than 91,000 kidney transplants were needed, but fewer than 23,000 were received. During 2021, 41,354 transplants were done, an increase of nearly 6% over the previous year. The total includes kidneys, hearts, lungs, and other organs, with kidneys accounting for more than 24,000 of the total.
Even with the rise in transplant numbers, supply does not meet demand. According to federal statistics, 17 people in the United States die each day waiting for an organ transplant. Every 9 minutes, someone is added to the waiting list.
“This isn’t and it shouldn’t be a fight about the COVID vaccine,” Dr. Caplan said. “This isn’t an issue about punishing non-COVID vaccinators. It’s deciding who is going to get a scarce organ.”
“A lot of people [opposed to removing the nonvaccinated from the list] think: ‘Oh, they are just killing those people who won’t take a COVID vaccine.’ That’s not what is going on.”
The transplant candidate must be in the best possible shape overall, Dr. Caplan and doctors agreed. Someone who is smoking, drinking heavily, or abusing drugs isn’t going to the top of the list either. And for other procedures, such as bariatric surgery or knee surgery, some patients are told first to lose weight before a surgeon will operate.
The worry about side effects from the vaccine, which some patients have cited as a concern, is misplaced, Dr. Caplan said. What transplant candidates who refuse the COVID vaccine may not be thinking about is that they are facing a serious operation and will be on numerous anti-rejection drugs, with side effects, after the surgery.
“So to be worried about the side effects of a COVID vaccine is irrational,” he said.
Transplants: The process
The patients who were recently removed from the transplant list could seek care and a transplant at an alternate center, said Anne Paschke, a spokesperson for the United Network for Organ Sharing, a nonprofit group that is under contract with the federal government and operates the national Organ Procurement and Transplantation Network (OPTN).
“Transplant hospitals decide which patients to add to the wait list based on their own criteria and medical judgment to create the best chance for a positive transplant outcome,” she said. That’s done with the understanding that patients will help with their medical care.
So, if one program won’t accept a patient, another may. But, if a patient turned down at one center due to refusing to get the COVID vaccine tries another center, the requirements at that hospital may be the same, she said.
OPTN maintains a list of transplant centers. As of Jan. 28, there were 251 transplant centers, according to UNOS, which manages the waiting list, matches donors and recipients, and strives for equity, among other duties.
Pretransplant refusers not typical
“The cases we are seeing are outliers,” Dr. Caplan said of the handful of known candidates who have refused the vaccine. Most ask their doctor exactly what they need to do to live and follow those instructions.
Dr. Norman agreed. Most of the kidney patients he cares for who are hoping for a transplant have been on dialysis, “which they do not like. They are doing whatever they can to make sure they don’t go back on dialysis. As a group, they tend to be very adherent, very safety conscious because they understand their risk and they understand the gift they have received [or will receive] through transplantation. They want to do everything they can to respect and protect that gift.”
Not surprisingly, some on the transplant list who are vaccinated have strong opinions about those who refuse to get the vaccine. Dana J. Ufkes, 61, a Seattle realtor, has been on the kidney transplant list – this time – since 2003, hoping for her third transplant. When asked if potential recipients should be removed from the list if they refuse the COVID vaccine, her answer was immediate: “Absolutely.”
At age 17, Ms. Ufkes got a serious kidney infection that went undiagnosed and untreated. Her kidney health worsened, and she needed a transplant. She got her first one in 1986, then again in 1992.
“They last longer than they used to,” she said. But not forever. (According to the American Kidney Fund, transplants from a living kidney donor last about 15-20 years; from a deceased donor, 10-15.)
The decision to decline the vaccine is, of course, each person’s choice, Ms. Ufkes said. But “if they don’t want to be vaccinated [and still want to be on the list], I think that’s BS.”
Citing the lack of organs, “it’s not like they are handing these out like jellybeans.”
A version of this article first appeared on WebMD.com.
100 coauthored papers, 10 years: Cancer transplant pioneers model 'team science'
On July 29, 2021, Sergio Giralt, MD, deputy division head of the division of hematologic malignancies and Miguel-Angel Perales, MD, chief of the adult bone marrow transplant service at MSKCC, published their 100th peer-reviewed paper as coauthors. Listing hundreds of such articles on a CV is standard for top-tier physicians, but the pair had gone one better: 100 publications written together in 10 years.
Their centenary article hit scientific newsstands almost exactly a decade after their first joint paper, which appeared in September 2011, not long after they met.
Born in Cuba, Dr. Giralt grew up in Venezuela. From the age of 14, he knew that medicine was his path, and in 1984 he earned a medical degree from the Universidad Central de Venezuela, Caracas. Next came a research position at Harvard Medical School, a residency at the Good Samaritan Hospital, Cincinnati, and a fellowship at the University of Texas MD Anderson Cancer Center, Houston. Dr. Giralt arrived at MSKCC in 2010 as the new chief of the adult bone marrow transplant service. There he was introduced to a new colleague, Dr. Perales. They soon learned that in addition to expertise in hematology, they had second language in common: Spanish.
Dr. Giralt said: “We both have a Spanish background and in a certain sense, there was an affinity there. ... We both have shared experiences.”
Dr. Perales was brought up in Belgium, a European nation with three official languages: French, Dutch, and German. He speaks five tongues in all and learned Spanish from his father, who came from Spain.
Fluency in Spanish enables both physicians to take care of the many New Yorkers who are more comfortable in that language – especially when navigating cancer treatment. However, both Dr. Giralt and Dr. Perales said that a second language is more than a professional tool. They described the enjoyable change of persona that happens when they switch to Spanish.
“People who are multilingual have different roles [as much as] different languages,” said Dr. Perales. “When I’m in Spanish, part of my brain is [thinking back to] summer vacations and hanging out with my cousins.”
When it comes to clinical science, however, English is the language of choice.
Global leaders in HSCT
Dr. Giralt and Dr. Perales are known worldwide in the field of allogeneic HSCT, a potentially curative treatment for an elongating list of both malignant and nonmalignant diseases.
In 1973, MSKCC conducted the first bone-marrow transplant from an unrelated donor. Fifty years on, medical oncologists in the United States conduct approximately 8,500 allogeneic transplants each year, 72% to treat acute leukemias or myelodysplastic syndrome (MDS).
However, stripping the immune system with intensive chemotherapy ‘conditioning,’ then rebuilding it with non-diseased donor hematopoietic cells is a hazardous undertaking. Older patients are less likely to survive the intensive conditioning, so historically have missed out. Also, even with a good human leukocyte antigen (HLA) match, the recipient needs often brutal immunosuppression.
Since Dr. Giralt and Dr. Perales began their partnership in 2010, the goals of their work have not changed: to develop safer, lower-intensity transplantation suitable for older, more vulnerable patients and reduce fearsome posttransplant sequelae such as graft-versus-host disease (GVHD).
Dr. Giralt’s publication list spans more than 600 peer-reviewed papers, articles and book chapters, almost exclusively on HSCT. Dr. Perales has more than 300 publication credits on the topic.
The two paired up on their first paper just months after Dr. Giralt arrived at MSKCC. That article, published in Biology of Blood and Marrow Transplantation, compared umbilical cord blood for HSCT with donor blood in 367 people with a variety of hematologic malignancies, including acute and chronic leukemias, MDS, and lymphoma.
The MSKCC team found that transplant-related mortality in the first 180 days was higher for the cord blood (21%), but thereafter mortality and relapse were much lower than for donated blood, with the result that 2-year progression-free survival of 55% was similar. Dr. Perales, Dr. Giralt and their coauthors concluded that the data provided “strong support” for further work on cord blood as an alternative stem-cell source.
During their first decade of collaboration, Dr. Giralt and Dr. Perales worked on any promising avenue that could improve outcomes and the experience of HSCT recipients, including reduced-intensity conditioning regimens to allow older adults to benefit from curative HSCT and donor T-cell depletion by CD34 selection, to reduce graft-versus-host disease (GVHD).
The CD34 protein is typically found on the surface of early stage and highly active stem cell types. Selecting these cell types using a range of techniques can eliminate many other potentially interfering or inactive cells. This enriches the transplant population with the most effective cells and can lower the risk of GVHD.
The 100th paper on which Dr. Giralt and Dr. Perales were coauthors was published in Blood Advances on July 27, 2021. The retrospective study examined the fate of 58 MSKCC patients with a rare form of chronic lymphocytic leukemia, CLL with Richter’s transformation (CLL-RT). It was the largest such study to date of this rare disease.
M.D. Anderson Cancer Center had shown in 2006 that, despite chemotherapy, overall survival in patients with CLL-RT was approximately 8 months. HSCT improved survival dramatically (75% at 3 years; n = 7). However, with the advent of novel targeted drugs for CLL such as ibrutinib (Imbruvica), venetoclax (Venclexta), or idelalisib (Zydelig), the MSKCC team asked themselves: What was the role of reduced-intensive conditioning HSCT? Was it even safe? Among other findings, Dr. Giralt and Dr. Perales’ 100th paper showed that reduced-intensity HSCT remained a viable alternative after a CLL-RT patient progressed on a novel agent.
Impact of the pandemic
When COVID-19 hit, the team lost many research staff and developed a huge backlog, said Dr. Giralt. He and Dr. Perales realized that they needed to be “thoughtful and careful” about which studies to continue. “For example, the CD-34 selection trials we did not close because these are our workhorse trials,” Dr. Giralt said. “We have people we need to treat, and some of the patients that we need to treat can only be treated on trial.”
The team was also able to pivot some of their work into COVID 19 itself, and they collected crucial information on HSCT in recovered COVID-19 patients, as an example.
“We were living through a critical time, but that doesn’t mean we [aren’t] obligated to continue our mission, our research mission,” said Dr. Giralt. “It really is team science. The way we look at it ... there’s a common thread: We both like to do allogeneic transplant, and we both believe in trying to make CD-34 selection better. So we’re both very much [working on] how can we improve what we call ‘the Memorial way’ of doing transplants. Where we separate is, Miguel does primarily lymphoma. He doesn’t do myeloma [like me]. So in those two areas, we’re helping develop the junior faculty in a different way.”
Something more in common
Right from the start, Dr. Perales and Dr. Giralt also shared a commitment to mentoring. Since 2010, Dr. Perales has mentored 22 up-and-coming junior faculty, including 10 from Europe (8 from Spain) and 2 from Latin America.
“[It makes] the research enterprise much more productive but [these young scientists] really increase the visibility of the program,” said Dr. Giralt.
He cited Dr. Perales’ track record of mentoring as one of the reasons for his promotion to chief of the adult bone marrow transplant service. In March 2020, Dr. Perales seamlessly stepped into Dr. Giralt’s shoes, while Dr. Giralt moved on to his present role as deputy division head of the division of hematologic malignancies.
Dr. Perales said: “The key aspect [of these promotions] is the fantastic working relationship that we’ve had over the years. ... I consider Sergio my mentor, but also a good friend and colleague. And so I think it’s this ability that we’ve had to work together and that relationship of trust, which has been key.”
“Sergio is somebody who lifts people up,” Dr. Perales added. “Many people will tell you that Sergio has helped them in their career. ... And I think that’s a lesson I’ve learned from him: training the next generation. And [that’s] not just in the U.S., but outside. I think that’s a key role that we have. And our responsibility.”
Asked to comment on their 100th-paper milestone, Dr. Perales firmly turned the spotlight from himself and Dr. Giralt to the junior investigators who have passed through the doors of the bone-marrow transplant program: “This body of work represents not just our collaboration but also the many contributions of our team at MSK ... and beyond MSK.”
This article was updated 1/26/22.
On July 29, 2021, Sergio Giralt, MD, deputy division head of the division of hematologic malignancies and Miguel-Angel Perales, MD, chief of the adult bone marrow transplant service at MSKCC, published their 100th peer-reviewed paper as coauthors. Listing hundreds of such articles on a CV is standard for top-tier physicians, but the pair had gone one better: 100 publications written together in 10 years.
Their centenary article hit scientific newsstands almost exactly a decade after their first joint paper, which appeared in September 2011, not long after they met.
Born in Cuba, Dr. Giralt grew up in Venezuela. From the age of 14, he knew that medicine was his path, and in 1984 he earned a medical degree from the Universidad Central de Venezuela, Caracas. Next came a research position at Harvard Medical School, a residency at the Good Samaritan Hospital, Cincinnati, and a fellowship at the University of Texas MD Anderson Cancer Center, Houston. Dr. Giralt arrived at MSKCC in 2010 as the new chief of the adult bone marrow transplant service. There he was introduced to a new colleague, Dr. Perales. They soon learned that in addition to expertise in hematology, they had second language in common: Spanish.
Dr. Giralt said: “We both have a Spanish background and in a certain sense, there was an affinity there. ... We both have shared experiences.”
Dr. Perales was brought up in Belgium, a European nation with three official languages: French, Dutch, and German. He speaks five tongues in all and learned Spanish from his father, who came from Spain.
Fluency in Spanish enables both physicians to take care of the many New Yorkers who are more comfortable in that language – especially when navigating cancer treatment. However, both Dr. Giralt and Dr. Perales said that a second language is more than a professional tool. They described the enjoyable change of persona that happens when they switch to Spanish.
“People who are multilingual have different roles [as much as] different languages,” said Dr. Perales. “When I’m in Spanish, part of my brain is [thinking back to] summer vacations and hanging out with my cousins.”
When it comes to clinical science, however, English is the language of choice.
Global leaders in HSCT
Dr. Giralt and Dr. Perales are known worldwide in the field of allogeneic HSCT, a potentially curative treatment for an elongating list of both malignant and nonmalignant diseases.
In 1973, MSKCC conducted the first bone-marrow transplant from an unrelated donor. Fifty years on, medical oncologists in the United States conduct approximately 8,500 allogeneic transplants each year, 72% to treat acute leukemias or myelodysplastic syndrome (MDS).
However, stripping the immune system with intensive chemotherapy ‘conditioning,’ then rebuilding it with non-diseased donor hematopoietic cells is a hazardous undertaking. Older patients are less likely to survive the intensive conditioning, so historically have missed out. Also, even with a good human leukocyte antigen (HLA) match, the recipient needs often brutal immunosuppression.
Since Dr. Giralt and Dr. Perales began their partnership in 2010, the goals of their work have not changed: to develop safer, lower-intensity transplantation suitable for older, more vulnerable patients and reduce fearsome posttransplant sequelae such as graft-versus-host disease (GVHD).
Dr. Giralt’s publication list spans more than 600 peer-reviewed papers, articles and book chapters, almost exclusively on HSCT. Dr. Perales has more than 300 publication credits on the topic.
The two paired up on their first paper just months after Dr. Giralt arrived at MSKCC. That article, published in Biology of Blood and Marrow Transplantation, compared umbilical cord blood for HSCT with donor blood in 367 people with a variety of hematologic malignancies, including acute and chronic leukemias, MDS, and lymphoma.
The MSKCC team found that transplant-related mortality in the first 180 days was higher for the cord blood (21%), but thereafter mortality and relapse were much lower than for donated blood, with the result that 2-year progression-free survival of 55% was similar. Dr. Perales, Dr. Giralt and their coauthors concluded that the data provided “strong support” for further work on cord blood as an alternative stem-cell source.
During their first decade of collaboration, Dr. Giralt and Dr. Perales worked on any promising avenue that could improve outcomes and the experience of HSCT recipients, including reduced-intensity conditioning regimens to allow older adults to benefit from curative HSCT and donor T-cell depletion by CD34 selection, to reduce graft-versus-host disease (GVHD).
The CD34 protein is typically found on the surface of early stage and highly active stem cell types. Selecting these cell types using a range of techniques can eliminate many other potentially interfering or inactive cells. This enriches the transplant population with the most effective cells and can lower the risk of GVHD.
The 100th paper on which Dr. Giralt and Dr. Perales were coauthors was published in Blood Advances on July 27, 2021. The retrospective study examined the fate of 58 MSKCC patients with a rare form of chronic lymphocytic leukemia, CLL with Richter’s transformation (CLL-RT). It was the largest such study to date of this rare disease.
M.D. Anderson Cancer Center had shown in 2006 that, despite chemotherapy, overall survival in patients with CLL-RT was approximately 8 months. HSCT improved survival dramatically (75% at 3 years; n = 7). However, with the advent of novel targeted drugs for CLL such as ibrutinib (Imbruvica), venetoclax (Venclexta), or idelalisib (Zydelig), the MSKCC team asked themselves: What was the role of reduced-intensive conditioning HSCT? Was it even safe? Among other findings, Dr. Giralt and Dr. Perales’ 100th paper showed that reduced-intensity HSCT remained a viable alternative after a CLL-RT patient progressed on a novel agent.
Impact of the pandemic
When COVID-19 hit, the team lost many research staff and developed a huge backlog, said Dr. Giralt. He and Dr. Perales realized that they needed to be “thoughtful and careful” about which studies to continue. “For example, the CD-34 selection trials we did not close because these are our workhorse trials,” Dr. Giralt said. “We have people we need to treat, and some of the patients that we need to treat can only be treated on trial.”
The team was also able to pivot some of their work into COVID 19 itself, and they collected crucial information on HSCT in recovered COVID-19 patients, as an example.
“We were living through a critical time, but that doesn’t mean we [aren’t] obligated to continue our mission, our research mission,” said Dr. Giralt. “It really is team science. The way we look at it ... there’s a common thread: We both like to do allogeneic transplant, and we both believe in trying to make CD-34 selection better. So we’re both very much [working on] how can we improve what we call ‘the Memorial way’ of doing transplants. Where we separate is, Miguel does primarily lymphoma. He doesn’t do myeloma [like me]. So in those two areas, we’re helping develop the junior faculty in a different way.”
Something more in common
Right from the start, Dr. Perales and Dr. Giralt also shared a commitment to mentoring. Since 2010, Dr. Perales has mentored 22 up-and-coming junior faculty, including 10 from Europe (8 from Spain) and 2 from Latin America.
“[It makes] the research enterprise much more productive but [these young scientists] really increase the visibility of the program,” said Dr. Giralt.
He cited Dr. Perales’ track record of mentoring as one of the reasons for his promotion to chief of the adult bone marrow transplant service. In March 2020, Dr. Perales seamlessly stepped into Dr. Giralt’s shoes, while Dr. Giralt moved on to his present role as deputy division head of the division of hematologic malignancies.
Dr. Perales said: “The key aspect [of these promotions] is the fantastic working relationship that we’ve had over the years. ... I consider Sergio my mentor, but also a good friend and colleague. And so I think it’s this ability that we’ve had to work together and that relationship of trust, which has been key.”
“Sergio is somebody who lifts people up,” Dr. Perales added. “Many people will tell you that Sergio has helped them in their career. ... And I think that’s a lesson I’ve learned from him: training the next generation. And [that’s] not just in the U.S., but outside. I think that’s a key role that we have. And our responsibility.”
Asked to comment on their 100th-paper milestone, Dr. Perales firmly turned the spotlight from himself and Dr. Giralt to the junior investigators who have passed through the doors of the bone-marrow transplant program: “This body of work represents not just our collaboration but also the many contributions of our team at MSK ... and beyond MSK.”
This article was updated 1/26/22.
On July 29, 2021, Sergio Giralt, MD, deputy division head of the division of hematologic malignancies and Miguel-Angel Perales, MD, chief of the adult bone marrow transplant service at MSKCC, published their 100th peer-reviewed paper as coauthors. Listing hundreds of such articles on a CV is standard for top-tier physicians, but the pair had gone one better: 100 publications written together in 10 years.
Their centenary article hit scientific newsstands almost exactly a decade after their first joint paper, which appeared in September 2011, not long after they met.
Born in Cuba, Dr. Giralt grew up in Venezuela. From the age of 14, he knew that medicine was his path, and in 1984 he earned a medical degree from the Universidad Central de Venezuela, Caracas. Next came a research position at Harvard Medical School, a residency at the Good Samaritan Hospital, Cincinnati, and a fellowship at the University of Texas MD Anderson Cancer Center, Houston. Dr. Giralt arrived at MSKCC in 2010 as the new chief of the adult bone marrow transplant service. There he was introduced to a new colleague, Dr. Perales. They soon learned that in addition to expertise in hematology, they had second language in common: Spanish.
Dr. Giralt said: “We both have a Spanish background and in a certain sense, there was an affinity there. ... We both have shared experiences.”
Dr. Perales was brought up in Belgium, a European nation with three official languages: French, Dutch, and German. He speaks five tongues in all and learned Spanish from his father, who came from Spain.
Fluency in Spanish enables both physicians to take care of the many New Yorkers who are more comfortable in that language – especially when navigating cancer treatment. However, both Dr. Giralt and Dr. Perales said that a second language is more than a professional tool. They described the enjoyable change of persona that happens when they switch to Spanish.
“People who are multilingual have different roles [as much as] different languages,” said Dr. Perales. “When I’m in Spanish, part of my brain is [thinking back to] summer vacations and hanging out with my cousins.”
When it comes to clinical science, however, English is the language of choice.
Global leaders in HSCT
Dr. Giralt and Dr. Perales are known worldwide in the field of allogeneic HSCT, a potentially curative treatment for an elongating list of both malignant and nonmalignant diseases.
In 1973, MSKCC conducted the first bone-marrow transplant from an unrelated donor. Fifty years on, medical oncologists in the United States conduct approximately 8,500 allogeneic transplants each year, 72% to treat acute leukemias or myelodysplastic syndrome (MDS).
However, stripping the immune system with intensive chemotherapy ‘conditioning,’ then rebuilding it with non-diseased donor hematopoietic cells is a hazardous undertaking. Older patients are less likely to survive the intensive conditioning, so historically have missed out. Also, even with a good human leukocyte antigen (HLA) match, the recipient needs often brutal immunosuppression.
Since Dr. Giralt and Dr. Perales began their partnership in 2010, the goals of their work have not changed: to develop safer, lower-intensity transplantation suitable for older, more vulnerable patients and reduce fearsome posttransplant sequelae such as graft-versus-host disease (GVHD).
Dr. Giralt’s publication list spans more than 600 peer-reviewed papers, articles and book chapters, almost exclusively on HSCT. Dr. Perales has more than 300 publication credits on the topic.
The two paired up on their first paper just months after Dr. Giralt arrived at MSKCC. That article, published in Biology of Blood and Marrow Transplantation, compared umbilical cord blood for HSCT with donor blood in 367 people with a variety of hematologic malignancies, including acute and chronic leukemias, MDS, and lymphoma.
The MSKCC team found that transplant-related mortality in the first 180 days was higher for the cord blood (21%), but thereafter mortality and relapse were much lower than for donated blood, with the result that 2-year progression-free survival of 55% was similar. Dr. Perales, Dr. Giralt and their coauthors concluded that the data provided “strong support” for further work on cord blood as an alternative stem-cell source.
During their first decade of collaboration, Dr. Giralt and Dr. Perales worked on any promising avenue that could improve outcomes and the experience of HSCT recipients, including reduced-intensity conditioning regimens to allow older adults to benefit from curative HSCT and donor T-cell depletion by CD34 selection, to reduce graft-versus-host disease (GVHD).
The CD34 protein is typically found on the surface of early stage and highly active stem cell types. Selecting these cell types using a range of techniques can eliminate many other potentially interfering or inactive cells. This enriches the transplant population with the most effective cells and can lower the risk of GVHD.
The 100th paper on which Dr. Giralt and Dr. Perales were coauthors was published in Blood Advances on July 27, 2021. The retrospective study examined the fate of 58 MSKCC patients with a rare form of chronic lymphocytic leukemia, CLL with Richter’s transformation (CLL-RT). It was the largest such study to date of this rare disease.
M.D. Anderson Cancer Center had shown in 2006 that, despite chemotherapy, overall survival in patients with CLL-RT was approximately 8 months. HSCT improved survival dramatically (75% at 3 years; n = 7). However, with the advent of novel targeted drugs for CLL such as ibrutinib (Imbruvica), venetoclax (Venclexta), or idelalisib (Zydelig), the MSKCC team asked themselves: What was the role of reduced-intensive conditioning HSCT? Was it even safe? Among other findings, Dr. Giralt and Dr. Perales’ 100th paper showed that reduced-intensity HSCT remained a viable alternative after a CLL-RT patient progressed on a novel agent.
Impact of the pandemic
When COVID-19 hit, the team lost many research staff and developed a huge backlog, said Dr. Giralt. He and Dr. Perales realized that they needed to be “thoughtful and careful” about which studies to continue. “For example, the CD-34 selection trials we did not close because these are our workhorse trials,” Dr. Giralt said. “We have people we need to treat, and some of the patients that we need to treat can only be treated on trial.”
The team was also able to pivot some of their work into COVID 19 itself, and they collected crucial information on HSCT in recovered COVID-19 patients, as an example.
“We were living through a critical time, but that doesn’t mean we [aren’t] obligated to continue our mission, our research mission,” said Dr. Giralt. “It really is team science. The way we look at it ... there’s a common thread: We both like to do allogeneic transplant, and we both believe in trying to make CD-34 selection better. So we’re both very much [working on] how can we improve what we call ‘the Memorial way’ of doing transplants. Where we separate is, Miguel does primarily lymphoma. He doesn’t do myeloma [like me]. So in those two areas, we’re helping develop the junior faculty in a different way.”
Something more in common
Right from the start, Dr. Perales and Dr. Giralt also shared a commitment to mentoring. Since 2010, Dr. Perales has mentored 22 up-and-coming junior faculty, including 10 from Europe (8 from Spain) and 2 from Latin America.
“[It makes] the research enterprise much more productive but [these young scientists] really increase the visibility of the program,” said Dr. Giralt.
He cited Dr. Perales’ track record of mentoring as one of the reasons for his promotion to chief of the adult bone marrow transplant service. In March 2020, Dr. Perales seamlessly stepped into Dr. Giralt’s shoes, while Dr. Giralt moved on to his present role as deputy division head of the division of hematologic malignancies.
Dr. Perales said: “The key aspect [of these promotions] is the fantastic working relationship that we’ve had over the years. ... I consider Sergio my mentor, but also a good friend and colleague. And so I think it’s this ability that we’ve had to work together and that relationship of trust, which has been key.”
“Sergio is somebody who lifts people up,” Dr. Perales added. “Many people will tell you that Sergio has helped them in their career. ... And I think that’s a lesson I’ve learned from him: training the next generation. And [that’s] not just in the U.S., but outside. I think that’s a key role that we have. And our responsibility.”
Asked to comment on their 100th-paper milestone, Dr. Perales firmly turned the spotlight from himself and Dr. Giralt to the junior investigators who have passed through the doors of the bone-marrow transplant program: “This body of work represents not just our collaboration but also the many contributions of our team at MSK ... and beyond MSK.”
This article was updated 1/26/22.
DKMS: Small nonprofit to world’s largest stem cell donor registry
When Mechtild Harf was diagnosed with acute leukemia in 1990, physicians told her and her husband Peter that a bone marrow transplant was her best hope for survival. Back then, her native Germany had only 3,000 registered donors, and none was a match.
“My dad just went crazy, you know, to save his wife,” recalled Katharina Harf, who was a young teen at the time of her mother’s diagnosis.
In the course of 1 year, the Harfs recruited more than 68,000 potential bone marrow donors, but their heroic efforts couldn’t save Mechtild.
“She unfortunately didn’t make it. She died because of leukemia,” Katharina said.
Although Mechtild Harf did not survive, her legacy lives on in the bone marrow and stem cell donor recruitment organization DKMS (Deutsche Knochenmarkspenderdatei, or German Bone Marrow Donor Center).
In May of 1991, Peter Harf and Gerhard Ehninger, MD, the hematologist who treated Mechtild, founded DKMS with the mission, as its website states, “to provide as many blood cancer patients as possible with a second chance at life.”
From its German roots, the nonprofit organization has extended its mission to the United States (where it was initially known as Delete Blood Cancer DKMS), Poland, the United Kingdom, Chile, and in 2021, to South Africa.
Three decades after her mother’s death, Katharina Harf serves as Executive Chairwoman of DKMS U.S., based in New York.
World’s largest registry
“DKMS has the largest number of unrelated donors of any organization in the world,” noted Richard E. Champlin, MD, chair of the department of stem cell transplantation and cellular therapy at the University of Texas MD Anderson Cancer Center in Houston.
“In a large fraction of our donor searches, we find matches that are in the DKMS registry,” he said in an interview,
Alexander Schmidt, MD, PhD, global chief medical officer for DKMS, said that approximately 25% of all registered donors worldwide were recruited by his organization, and 39% of all unrelated donor transplants are made with peripheral blood stem cell or bone marrow products, donated by volunteers who are recruited by DKMS.
Since its founding, DKMS has registered 7.1 million potential donors in Germany, who made a total of 80,000 stem cell donations. DKMS U.S., which began operations in 2004, has registered 1.1 million donors and enabled 4,700 donations.
Global partners
DKMS partners with donor centers and recruitment organizations in each country where it operates. In the United States, DKMS works with the National Marrow Donor Program (NMDP) and its “Be The Match” donor registry.
“DKMS donors, both those from DKMS in Germany and those from DKMS in the United States are also listed in the NMDP registry, to make it easier for US search coordinators to accept these donors,” Dr. Schmidt explained in an interview.
The international cooperation and coordination makes it possible for a donor in the UK, for example, to save a life of a patient in Germany, the U.S., Chile, India, or many other parts of the world – anywhere that can be reached in time for a patient in need to receive a stem cell donation.
Pandemic affects donations
But, as with just about every aspect of life, the COVID-19 pandemic has created enormous challenges for recruiters, donor centers, and stem cell transplant centers.
Dr. Schmidt said that decline in donations during the pandemic was less severe than initially feared, with a decrease of just 3.5% in 2020, compared with the prepandemic year of 2019. In contrast, though, the average annual growth rate for donations prior to the pandemic was about 4%.
“Nevertheless, at the beginning of the pandemic in March 2020, for a few days things looked quite terrible, because all the borders were closed and flights were canceled, and about 50% of all stem cell products go abroad, and between 20% and 25% go intercontinental,” Dr. Schmidt said.
However, close cooperation and coordination between donor centers and national health authorities soon resolved the problem and helped insure that the flow of life-saving donations could continue with minimal disruption, he noted.
“I don’t think we had any product that could not be delivered at the end of the day, due to the pandemic,” he told this news organization.
Workforce and clinical problems
Although the flow of donations within and between nations has continued, the COVID-19 pandemic has had profound negative effects on transplant centers, particularly during the wave of infections caused by the Omicron variant, according to a transplant expert.
“With this most recent strain and how transmissible it is, what we’re dealing with is mass workforce shortages,” said Yi-Bin Chen, MD, director of the bone marrow transplant program at Massachusetts General Hospital in Boston.
“On top of a short-staffed hospital, you then take a very transmissible variant and deplete it even more due to the need to quarantine,” he said in an interview.
Both Dr. Champlin and Dr. Chen said that on-again, off-again pandemic travel bans and donor illnesses have necessitated first obtaining products and cryopreserving them before starting the recipient on a conditioning regimen for the transplant.
“The problem is that, while you can preserve peripheral blood stem cells pretty reliably, cryopreserving bone marrow is a bit more difficult,” Dr. Chen said.
In addition, evidence from recent studies comparing stem cell sources suggest that outcomes are less good with cryopreserved products than with fresh products, and with peripheral blood stem cells compared with bone marrow.
“But you’ve got to make do. A transplant with a cryopreserved product is better than no transplant,” Dr. Chen said.
To make things even more frustrating, as the pandemic waxed and waned throughout 2020 and 2021, the recommendations from donor centers seesawed between using fresh or cryopreserved product, making it difficult to plan a transplant for an individual patient.
The Omicron wave has also resulted in a much higher rate of donor dropout than anticipated, making it that much harder to schedule a transplant, Dr. Chen noted.
‘Every patient saved’
The pandemic will eventually subside, however, while the need for stem cell transplantation to treat hematologic malignancies will continue.
DKMS recently launched special aid programs to improve access to stem cell transplants in developing nations by offering financial support, free HLA typing, and other services.
In addition to its core mission of recruiting donors, DKMS is dedicated to improving the quality and efficiency of stem cell transplants. For example, in 2017 scientists in DKMS’ Life Science Lab created an antibody test for donor cytomegalovirus (CMV) infection, using a simple buccal swab rather than a more invasive blood sample. CMV infections can compromise the integrity of stem cell grafts and could be fatal to immunocompromised transplant recipients.
The last word goes to Mechtild Harf’s daughter Katharina.
“My big dream is that every patient will be saved from blood cancer,” she said in a video posted on the DKMS website. “When they get sick, we have a solution for them, whether it’s because they need a donor, with research, building hospitals, providing them with the best medical care we can. I will just keep fighting and keep spreading the word, recruiting donors, raising money – all the things that it takes for us to delete blood cancer.”
“I have to believe that this dream will come true because otherwise, why dream, right?” she said.
Dr. Champlin was the recipient of a Mechtild Harf Science Award and is a member of the board of DKMS U.S. Dr. Schmidt is employed by DKMS. Dr. Chen reported having no relevant disclosures.
When Mechtild Harf was diagnosed with acute leukemia in 1990, physicians told her and her husband Peter that a bone marrow transplant was her best hope for survival. Back then, her native Germany had only 3,000 registered donors, and none was a match.
“My dad just went crazy, you know, to save his wife,” recalled Katharina Harf, who was a young teen at the time of her mother’s diagnosis.
In the course of 1 year, the Harfs recruited more than 68,000 potential bone marrow donors, but their heroic efforts couldn’t save Mechtild.
“She unfortunately didn’t make it. She died because of leukemia,” Katharina said.
Although Mechtild Harf did not survive, her legacy lives on in the bone marrow and stem cell donor recruitment organization DKMS (Deutsche Knochenmarkspenderdatei, or German Bone Marrow Donor Center).
In May of 1991, Peter Harf and Gerhard Ehninger, MD, the hematologist who treated Mechtild, founded DKMS with the mission, as its website states, “to provide as many blood cancer patients as possible with a second chance at life.”
From its German roots, the nonprofit organization has extended its mission to the United States (where it was initially known as Delete Blood Cancer DKMS), Poland, the United Kingdom, Chile, and in 2021, to South Africa.
Three decades after her mother’s death, Katharina Harf serves as Executive Chairwoman of DKMS U.S., based in New York.
World’s largest registry
“DKMS has the largest number of unrelated donors of any organization in the world,” noted Richard E. Champlin, MD, chair of the department of stem cell transplantation and cellular therapy at the University of Texas MD Anderson Cancer Center in Houston.
“In a large fraction of our donor searches, we find matches that are in the DKMS registry,” he said in an interview,
Alexander Schmidt, MD, PhD, global chief medical officer for DKMS, said that approximately 25% of all registered donors worldwide were recruited by his organization, and 39% of all unrelated donor transplants are made with peripheral blood stem cell or bone marrow products, donated by volunteers who are recruited by DKMS.
Since its founding, DKMS has registered 7.1 million potential donors in Germany, who made a total of 80,000 stem cell donations. DKMS U.S., which began operations in 2004, has registered 1.1 million donors and enabled 4,700 donations.
Global partners
DKMS partners with donor centers and recruitment organizations in each country where it operates. In the United States, DKMS works with the National Marrow Donor Program (NMDP) and its “Be The Match” donor registry.
“DKMS donors, both those from DKMS in Germany and those from DKMS in the United States are also listed in the NMDP registry, to make it easier for US search coordinators to accept these donors,” Dr. Schmidt explained in an interview.
The international cooperation and coordination makes it possible for a donor in the UK, for example, to save a life of a patient in Germany, the U.S., Chile, India, or many other parts of the world – anywhere that can be reached in time for a patient in need to receive a stem cell donation.
Pandemic affects donations
But, as with just about every aspect of life, the COVID-19 pandemic has created enormous challenges for recruiters, donor centers, and stem cell transplant centers.
Dr. Schmidt said that decline in donations during the pandemic was less severe than initially feared, with a decrease of just 3.5% in 2020, compared with the prepandemic year of 2019. In contrast, though, the average annual growth rate for donations prior to the pandemic was about 4%.
“Nevertheless, at the beginning of the pandemic in March 2020, for a few days things looked quite terrible, because all the borders were closed and flights were canceled, and about 50% of all stem cell products go abroad, and between 20% and 25% go intercontinental,” Dr. Schmidt said.
However, close cooperation and coordination between donor centers and national health authorities soon resolved the problem and helped insure that the flow of life-saving donations could continue with minimal disruption, he noted.
“I don’t think we had any product that could not be delivered at the end of the day, due to the pandemic,” he told this news organization.
Workforce and clinical problems
Although the flow of donations within and between nations has continued, the COVID-19 pandemic has had profound negative effects on transplant centers, particularly during the wave of infections caused by the Omicron variant, according to a transplant expert.
“With this most recent strain and how transmissible it is, what we’re dealing with is mass workforce shortages,” said Yi-Bin Chen, MD, director of the bone marrow transplant program at Massachusetts General Hospital in Boston.
“On top of a short-staffed hospital, you then take a very transmissible variant and deplete it even more due to the need to quarantine,” he said in an interview.
Both Dr. Champlin and Dr. Chen said that on-again, off-again pandemic travel bans and donor illnesses have necessitated first obtaining products and cryopreserving them before starting the recipient on a conditioning regimen for the transplant.
“The problem is that, while you can preserve peripheral blood stem cells pretty reliably, cryopreserving bone marrow is a bit more difficult,” Dr. Chen said.
In addition, evidence from recent studies comparing stem cell sources suggest that outcomes are less good with cryopreserved products than with fresh products, and with peripheral blood stem cells compared with bone marrow.
“But you’ve got to make do. A transplant with a cryopreserved product is better than no transplant,” Dr. Chen said.
To make things even more frustrating, as the pandemic waxed and waned throughout 2020 and 2021, the recommendations from donor centers seesawed between using fresh or cryopreserved product, making it difficult to plan a transplant for an individual patient.
The Omicron wave has also resulted in a much higher rate of donor dropout than anticipated, making it that much harder to schedule a transplant, Dr. Chen noted.
‘Every patient saved’
The pandemic will eventually subside, however, while the need for stem cell transplantation to treat hematologic malignancies will continue.
DKMS recently launched special aid programs to improve access to stem cell transplants in developing nations by offering financial support, free HLA typing, and other services.
In addition to its core mission of recruiting donors, DKMS is dedicated to improving the quality and efficiency of stem cell transplants. For example, in 2017 scientists in DKMS’ Life Science Lab created an antibody test for donor cytomegalovirus (CMV) infection, using a simple buccal swab rather than a more invasive blood sample. CMV infections can compromise the integrity of stem cell grafts and could be fatal to immunocompromised transplant recipients.
The last word goes to Mechtild Harf’s daughter Katharina.
“My big dream is that every patient will be saved from blood cancer,” she said in a video posted on the DKMS website. “When they get sick, we have a solution for them, whether it’s because they need a donor, with research, building hospitals, providing them with the best medical care we can. I will just keep fighting and keep spreading the word, recruiting donors, raising money – all the things that it takes for us to delete blood cancer.”
“I have to believe that this dream will come true because otherwise, why dream, right?” she said.
Dr. Champlin was the recipient of a Mechtild Harf Science Award and is a member of the board of DKMS U.S. Dr. Schmidt is employed by DKMS. Dr. Chen reported having no relevant disclosures.
When Mechtild Harf was diagnosed with acute leukemia in 1990, physicians told her and her husband Peter that a bone marrow transplant was her best hope for survival. Back then, her native Germany had only 3,000 registered donors, and none was a match.
“My dad just went crazy, you know, to save his wife,” recalled Katharina Harf, who was a young teen at the time of her mother’s diagnosis.
In the course of 1 year, the Harfs recruited more than 68,000 potential bone marrow donors, but their heroic efforts couldn’t save Mechtild.
“She unfortunately didn’t make it. She died because of leukemia,” Katharina said.
Although Mechtild Harf did not survive, her legacy lives on in the bone marrow and stem cell donor recruitment organization DKMS (Deutsche Knochenmarkspenderdatei, or German Bone Marrow Donor Center).
In May of 1991, Peter Harf and Gerhard Ehninger, MD, the hematologist who treated Mechtild, founded DKMS with the mission, as its website states, “to provide as many blood cancer patients as possible with a second chance at life.”
From its German roots, the nonprofit organization has extended its mission to the United States (where it was initially known as Delete Blood Cancer DKMS), Poland, the United Kingdom, Chile, and in 2021, to South Africa.
Three decades after her mother’s death, Katharina Harf serves as Executive Chairwoman of DKMS U.S., based in New York.
World’s largest registry
“DKMS has the largest number of unrelated donors of any organization in the world,” noted Richard E. Champlin, MD, chair of the department of stem cell transplantation and cellular therapy at the University of Texas MD Anderson Cancer Center in Houston.
“In a large fraction of our donor searches, we find matches that are in the DKMS registry,” he said in an interview,
Alexander Schmidt, MD, PhD, global chief medical officer for DKMS, said that approximately 25% of all registered donors worldwide were recruited by his organization, and 39% of all unrelated donor transplants are made with peripheral blood stem cell or bone marrow products, donated by volunteers who are recruited by DKMS.
Since its founding, DKMS has registered 7.1 million potential donors in Germany, who made a total of 80,000 stem cell donations. DKMS U.S., which began operations in 2004, has registered 1.1 million donors and enabled 4,700 donations.
Global partners
DKMS partners with donor centers and recruitment organizations in each country where it operates. In the United States, DKMS works with the National Marrow Donor Program (NMDP) and its “Be The Match” donor registry.
“DKMS donors, both those from DKMS in Germany and those from DKMS in the United States are also listed in the NMDP registry, to make it easier for US search coordinators to accept these donors,” Dr. Schmidt explained in an interview.
The international cooperation and coordination makes it possible for a donor in the UK, for example, to save a life of a patient in Germany, the U.S., Chile, India, or many other parts of the world – anywhere that can be reached in time for a patient in need to receive a stem cell donation.
Pandemic affects donations
But, as with just about every aspect of life, the COVID-19 pandemic has created enormous challenges for recruiters, donor centers, and stem cell transplant centers.
Dr. Schmidt said that decline in donations during the pandemic was less severe than initially feared, with a decrease of just 3.5% in 2020, compared with the prepandemic year of 2019. In contrast, though, the average annual growth rate for donations prior to the pandemic was about 4%.
“Nevertheless, at the beginning of the pandemic in March 2020, for a few days things looked quite terrible, because all the borders were closed and flights were canceled, and about 50% of all stem cell products go abroad, and between 20% and 25% go intercontinental,” Dr. Schmidt said.
However, close cooperation and coordination between donor centers and national health authorities soon resolved the problem and helped insure that the flow of life-saving donations could continue with minimal disruption, he noted.
“I don’t think we had any product that could not be delivered at the end of the day, due to the pandemic,” he told this news organization.
Workforce and clinical problems
Although the flow of donations within and between nations has continued, the COVID-19 pandemic has had profound negative effects on transplant centers, particularly during the wave of infections caused by the Omicron variant, according to a transplant expert.
“With this most recent strain and how transmissible it is, what we’re dealing with is mass workforce shortages,” said Yi-Bin Chen, MD, director of the bone marrow transplant program at Massachusetts General Hospital in Boston.
“On top of a short-staffed hospital, you then take a very transmissible variant and deplete it even more due to the need to quarantine,” he said in an interview.
Both Dr. Champlin and Dr. Chen said that on-again, off-again pandemic travel bans and donor illnesses have necessitated first obtaining products and cryopreserving them before starting the recipient on a conditioning regimen for the transplant.
“The problem is that, while you can preserve peripheral blood stem cells pretty reliably, cryopreserving bone marrow is a bit more difficult,” Dr. Chen said.
In addition, evidence from recent studies comparing stem cell sources suggest that outcomes are less good with cryopreserved products than with fresh products, and with peripheral blood stem cells compared with bone marrow.
“But you’ve got to make do. A transplant with a cryopreserved product is better than no transplant,” Dr. Chen said.
To make things even more frustrating, as the pandemic waxed and waned throughout 2020 and 2021, the recommendations from donor centers seesawed between using fresh or cryopreserved product, making it difficult to plan a transplant for an individual patient.
The Omicron wave has also resulted in a much higher rate of donor dropout than anticipated, making it that much harder to schedule a transplant, Dr. Chen noted.
‘Every patient saved’
The pandemic will eventually subside, however, while the need for stem cell transplantation to treat hematologic malignancies will continue.
DKMS recently launched special aid programs to improve access to stem cell transplants in developing nations by offering financial support, free HLA typing, and other services.
In addition to its core mission of recruiting donors, DKMS is dedicated to improving the quality and efficiency of stem cell transplants. For example, in 2017 scientists in DKMS’ Life Science Lab created an antibody test for donor cytomegalovirus (CMV) infection, using a simple buccal swab rather than a more invasive blood sample. CMV infections can compromise the integrity of stem cell grafts and could be fatal to immunocompromised transplant recipients.
The last word goes to Mechtild Harf’s daughter Katharina.
“My big dream is that every patient will be saved from blood cancer,” she said in a video posted on the DKMS website. “When they get sick, we have a solution for them, whether it’s because they need a donor, with research, building hospitals, providing them with the best medical care we can. I will just keep fighting and keep spreading the word, recruiting donors, raising money – all the things that it takes for us to delete blood cancer.”
“I have to believe that this dream will come true because otherwise, why dream, right?” she said.
Dr. Champlin was the recipient of a Mechtild Harf Science Award and is a member of the board of DKMS U.S. Dr. Schmidt is employed by DKMS. Dr. Chen reported having no relevant disclosures.
HIV+ patients get good outcomes after kidney or liver transplant
in new research that represents some of the longest follow-up on these patients to date.
The findings further support the inclusion of people with HIV in transplant resource allocation, say the researchers.
“Overall, the excellent outcomes following liver and kidney transplant recipients in HIV-infected recipients justify the utilization of a scarce resource,” senior author Peter G. Stock, MD, PhD, surgical director of the Kidney and Pancreas Transplant Program and surgical director of the Pediatric Renal Transplant Program at the University of California, San Francisco (UCSF), said in an interview.
“Many centers still view HIV as a strict contraindication [for transplantation]. This data shows it is not,” he emphasized.
The study, published in JAMA Surgery, involved HIV-positive patients who received kidney or liver transplants between 2000 and 2019 at UCSF, which has unique access to some of the longest-term data on those outcomes.
“UCSF was the first U.S. center to do transplants routinely in people with HIV, and based on the large volume of transplants that are performed, we were able to use propensity matching to address the comparison of HIV-positive and negative liver and kidney transplant recipients at a single center,” Dr. Stock explained.
“To the best of our knowledge, there are no long-term reports [greater than 10 years] on [transplant] outcomes in the HIV-positive population.”
Commenting on the study, David Klassen, MD, chief medical officer of the United Network for Organ Sharing (UNOS), noted that the findings “confirm previous research done at UCSF and reported in the New England Journal of Medicine” in 2010. “It extends the previous findings.”
“The take-home message is that these HIV-positive patients can be successfully transplanted with expected good outcomes and will derive substantial benefit from transplantation,” Dr. Klassen said.
Kidney transplant patient survival lower, graft survival similar
For the kidney transplant analysis, 119 HIV-positive recipients were propensity matched with 655 recipients who were HIV-negative, with the patients’ mean age about 52 and approximately 70% male.
At 15-years post-transplant, patient survival was 53.6% among the HIV-positive patients versus 79.6% for HIV-negative (P = .03).
Graft survival among the kidney transplant patients was proportionally higher among HIV-positive patients after 15 years (75% vs. 57%); however, the difference was not statistically significant (P = .77).
First author Arya Zarinsefat, MD, of the Department of Surgery at UCSF, speculated that the lower long-term patient survival among HIV-positive kidney transplant recipients may reflect known cardiovascular risks among those patients.
“We postulated that part of this may be due to the fact that HIV-positive patients certainly have additional comorbidities, specifically cardiovascular” ones, he told this news organization.
“When looking at the survival curve, survival was nearly identical at 5 years and only started to diverge at 10 years post-transplant,” he noted.
A further evaluation of patients with HIV who were co-infected with hepatitis C (HCV) showed that those with HIV-HCV co-infection prior to the center’s introduction of anti-HCV direct-acting antiviral (DAA) medications in 2014 had the lowest survival rate of all subgroups, at 57.1% at 5 years post-transplant (P = .045 vs. those treated after 2014).
Liver transplant patient survival similar
In terms of liver transplant outcomes, among 83 HIV-positive recipients who were propensity-matched with 468 HIV-negative recipients, the mean age was about 53 and about 66% were male.
The patient survival rates at 15 years were not significantly different between the groups, at 70% for HIV-positive and 75.7% for HIV-negative, (P = .12).
Similar to the kidney transplant recipients, the worst survival among all liver transplant subgroups was among HIV-HCV co-infected patients prior to access to HCV direct-acting antivirals in 2014, with a 5-year survival of 59.5% (P = .04).
“Since the advent of HCV direct-acting antivirals, liver transplant outcomes in HCV mono-infected patients are comparable to HCV/HIV co-infected recipients,” Dr. Stock said.
Acute rejection rates higher with HIV-positivity versus national averages
The rates of acute rejection at 1 year in the kidney and liver transplant, HIV-positive groups – at about 20% and 30%, respectively – were, however, higher than national average incidence rates of about 10% at 1 year.
Long-term data on those patients showed the acute rejection affected graft survival outcomes with kidney transplant recipients: HIV-positive kidney transplant recipients who had at least one episode of acute rejection had a graft survival of just 52.8% at 15 years post-transplant, compared with 91.8% among recipients without acute rejection.
Such differences were not observed among HIV-positive liver transplant recipients.
The authors note that the increased risk of acute rejection in HIV-positive kidney transplant patients is consistent with previous studies, with causes that may be multifactorial.
Top theories include drug interactions with protease inhibitors, resulting in some centers transitioning HIV-infected patients from those regimens to integrase-based regimens prior to transplant.
“The management and prevention of acute rejection in HIV-positive kidney transplant [patients] will therefore continue to be a key component in the care of these patients,” the authors note in their study.
The study was supported in part by the National Institutes of Health. The study authors and Dr. Klassen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in new research that represents some of the longest follow-up on these patients to date.
The findings further support the inclusion of people with HIV in transplant resource allocation, say the researchers.
“Overall, the excellent outcomes following liver and kidney transplant recipients in HIV-infected recipients justify the utilization of a scarce resource,” senior author Peter G. Stock, MD, PhD, surgical director of the Kidney and Pancreas Transplant Program and surgical director of the Pediatric Renal Transplant Program at the University of California, San Francisco (UCSF), said in an interview.
“Many centers still view HIV as a strict contraindication [for transplantation]. This data shows it is not,” he emphasized.
The study, published in JAMA Surgery, involved HIV-positive patients who received kidney or liver transplants between 2000 and 2019 at UCSF, which has unique access to some of the longest-term data on those outcomes.
“UCSF was the first U.S. center to do transplants routinely in people with HIV, and based on the large volume of transplants that are performed, we were able to use propensity matching to address the comparison of HIV-positive and negative liver and kidney transplant recipients at a single center,” Dr. Stock explained.
“To the best of our knowledge, there are no long-term reports [greater than 10 years] on [transplant] outcomes in the HIV-positive population.”
Commenting on the study, David Klassen, MD, chief medical officer of the United Network for Organ Sharing (UNOS), noted that the findings “confirm previous research done at UCSF and reported in the New England Journal of Medicine” in 2010. “It extends the previous findings.”
“The take-home message is that these HIV-positive patients can be successfully transplanted with expected good outcomes and will derive substantial benefit from transplantation,” Dr. Klassen said.
Kidney transplant patient survival lower, graft survival similar
For the kidney transplant analysis, 119 HIV-positive recipients were propensity matched with 655 recipients who were HIV-negative, with the patients’ mean age about 52 and approximately 70% male.
At 15-years post-transplant, patient survival was 53.6% among the HIV-positive patients versus 79.6% for HIV-negative (P = .03).
Graft survival among the kidney transplant patients was proportionally higher among HIV-positive patients after 15 years (75% vs. 57%); however, the difference was not statistically significant (P = .77).
First author Arya Zarinsefat, MD, of the Department of Surgery at UCSF, speculated that the lower long-term patient survival among HIV-positive kidney transplant recipients may reflect known cardiovascular risks among those patients.
“We postulated that part of this may be due to the fact that HIV-positive patients certainly have additional comorbidities, specifically cardiovascular” ones, he told this news organization.
“When looking at the survival curve, survival was nearly identical at 5 years and only started to diverge at 10 years post-transplant,” he noted.
A further evaluation of patients with HIV who were co-infected with hepatitis C (HCV) showed that those with HIV-HCV co-infection prior to the center’s introduction of anti-HCV direct-acting antiviral (DAA) medications in 2014 had the lowest survival rate of all subgroups, at 57.1% at 5 years post-transplant (P = .045 vs. those treated after 2014).
Liver transplant patient survival similar
In terms of liver transplant outcomes, among 83 HIV-positive recipients who were propensity-matched with 468 HIV-negative recipients, the mean age was about 53 and about 66% were male.
The patient survival rates at 15 years were not significantly different between the groups, at 70% for HIV-positive and 75.7% for HIV-negative, (P = .12).
Similar to the kidney transplant recipients, the worst survival among all liver transplant subgroups was among HIV-HCV co-infected patients prior to access to HCV direct-acting antivirals in 2014, with a 5-year survival of 59.5% (P = .04).
“Since the advent of HCV direct-acting antivirals, liver transplant outcomes in HCV mono-infected patients are comparable to HCV/HIV co-infected recipients,” Dr. Stock said.
Acute rejection rates higher with HIV-positivity versus national averages
The rates of acute rejection at 1 year in the kidney and liver transplant, HIV-positive groups – at about 20% and 30%, respectively – were, however, higher than national average incidence rates of about 10% at 1 year.
Long-term data on those patients showed the acute rejection affected graft survival outcomes with kidney transplant recipients: HIV-positive kidney transplant recipients who had at least one episode of acute rejection had a graft survival of just 52.8% at 15 years post-transplant, compared with 91.8% among recipients without acute rejection.
Such differences were not observed among HIV-positive liver transplant recipients.
The authors note that the increased risk of acute rejection in HIV-positive kidney transplant patients is consistent with previous studies, with causes that may be multifactorial.
Top theories include drug interactions with protease inhibitors, resulting in some centers transitioning HIV-infected patients from those regimens to integrase-based regimens prior to transplant.
“The management and prevention of acute rejection in HIV-positive kidney transplant [patients] will therefore continue to be a key component in the care of these patients,” the authors note in their study.
The study was supported in part by the National Institutes of Health. The study authors and Dr. Klassen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in new research that represents some of the longest follow-up on these patients to date.
The findings further support the inclusion of people with HIV in transplant resource allocation, say the researchers.
“Overall, the excellent outcomes following liver and kidney transplant recipients in HIV-infected recipients justify the utilization of a scarce resource,” senior author Peter G. Stock, MD, PhD, surgical director of the Kidney and Pancreas Transplant Program and surgical director of the Pediatric Renal Transplant Program at the University of California, San Francisco (UCSF), said in an interview.
“Many centers still view HIV as a strict contraindication [for transplantation]. This data shows it is not,” he emphasized.
The study, published in JAMA Surgery, involved HIV-positive patients who received kidney or liver transplants between 2000 and 2019 at UCSF, which has unique access to some of the longest-term data on those outcomes.
“UCSF was the first U.S. center to do transplants routinely in people with HIV, and based on the large volume of transplants that are performed, we were able to use propensity matching to address the comparison of HIV-positive and negative liver and kidney transplant recipients at a single center,” Dr. Stock explained.
“To the best of our knowledge, there are no long-term reports [greater than 10 years] on [transplant] outcomes in the HIV-positive population.”
Commenting on the study, David Klassen, MD, chief medical officer of the United Network for Organ Sharing (UNOS), noted that the findings “confirm previous research done at UCSF and reported in the New England Journal of Medicine” in 2010. “It extends the previous findings.”
“The take-home message is that these HIV-positive patients can be successfully transplanted with expected good outcomes and will derive substantial benefit from transplantation,” Dr. Klassen said.
Kidney transplant patient survival lower, graft survival similar
For the kidney transplant analysis, 119 HIV-positive recipients were propensity matched with 655 recipients who were HIV-negative, with the patients’ mean age about 52 and approximately 70% male.
At 15-years post-transplant, patient survival was 53.6% among the HIV-positive patients versus 79.6% for HIV-negative (P = .03).
Graft survival among the kidney transplant patients was proportionally higher among HIV-positive patients after 15 years (75% vs. 57%); however, the difference was not statistically significant (P = .77).
First author Arya Zarinsefat, MD, of the Department of Surgery at UCSF, speculated that the lower long-term patient survival among HIV-positive kidney transplant recipients may reflect known cardiovascular risks among those patients.
“We postulated that part of this may be due to the fact that HIV-positive patients certainly have additional comorbidities, specifically cardiovascular” ones, he told this news organization.
“When looking at the survival curve, survival was nearly identical at 5 years and only started to diverge at 10 years post-transplant,” he noted.
A further evaluation of patients with HIV who were co-infected with hepatitis C (HCV) showed that those with HIV-HCV co-infection prior to the center’s introduction of anti-HCV direct-acting antiviral (DAA) medications in 2014 had the lowest survival rate of all subgroups, at 57.1% at 5 years post-transplant (P = .045 vs. those treated after 2014).
Liver transplant patient survival similar
In terms of liver transplant outcomes, among 83 HIV-positive recipients who were propensity-matched with 468 HIV-negative recipients, the mean age was about 53 and about 66% were male.
The patient survival rates at 15 years were not significantly different between the groups, at 70% for HIV-positive and 75.7% for HIV-negative, (P = .12).
Similar to the kidney transplant recipients, the worst survival among all liver transplant subgroups was among HIV-HCV co-infected patients prior to access to HCV direct-acting antivirals in 2014, with a 5-year survival of 59.5% (P = .04).
“Since the advent of HCV direct-acting antivirals, liver transplant outcomes in HCV mono-infected patients are comparable to HCV/HIV co-infected recipients,” Dr. Stock said.
Acute rejection rates higher with HIV-positivity versus national averages
The rates of acute rejection at 1 year in the kidney and liver transplant, HIV-positive groups – at about 20% and 30%, respectively – were, however, higher than national average incidence rates of about 10% at 1 year.
Long-term data on those patients showed the acute rejection affected graft survival outcomes with kidney transplant recipients: HIV-positive kidney transplant recipients who had at least one episode of acute rejection had a graft survival of just 52.8% at 15 years post-transplant, compared with 91.8% among recipients without acute rejection.
Such differences were not observed among HIV-positive liver transplant recipients.
The authors note that the increased risk of acute rejection in HIV-positive kidney transplant patients is consistent with previous studies, with causes that may be multifactorial.
Top theories include drug interactions with protease inhibitors, resulting in some centers transitioning HIV-infected patients from those regimens to integrase-based regimens prior to transplant.
“The management and prevention of acute rejection in HIV-positive kidney transplant [patients] will therefore continue to be a key component in the care of these patients,” the authors note in their study.
The study was supported in part by the National Institutes of Health. The study authors and Dr. Klassen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Lung transplantation in the era of COVID-19: New issues and paradigms
Data is sparse thus far, but there is concern in lung transplant medicine about the long-term risk of chronic lung allograft dysfunction (CLAD) and a potentially shortened longevity of transplanted lungs in recipients who become ill with COVID-19.
“My fear is that we’re potentially sitting on this iceberg worth of people who, come 6 months or a year from [the acute phase of] their COVID illness, will in fact have earlier and progressive, chronic rejection,” said Cameron R. Wolfe, MBBS, MPH, associate professor of medicine in transplant infectious disease at Duke University, Durham, N.C.
Lower respiratory viral infections have long been concerning for lung transplant recipients given their propensity to cause scarring, a decline in lung function, and a heightened risk of allograft rejection. Time will tell whether lung transplant recipients who survive COVID-19 follow a similar path, or one that is worse, he said.
Short-term data
Outcomes beyond hospitalization and acute illness for lung transplant recipients affected by COVID-19 have been reported in the literature by only a few lung transplant programs. These reports – as well as anecdotal experiences being informally shared among transplant programs – have raised the specter of more severe dysfunction following the acute phase and more early CLAD, said Tathagat Narula, MD, assistant professor of medicine at the Mayo Medical School, Rochester, Minn., and a consultant in lung transplantation at the Mayo Clinic’s Jacksonville program.
“The available data cover only 3-6 months out. We don’t know what will happen in the next 6 months and beyond,” Dr. Narula said in an interview.
The risks of COVID-19 in already-transplanted patients and issues relating to the inadequate antibody responses to vaccination are just some of the challenges of lung transplant medicine in the era of SARS-CoV-2. “COVID-19,” said Dr. Narula, “has completely changed the way we practice lung transplant medicine – the way we’re looking both at our recipients and our donors.”
Potential donors are being evaluated with lower respiratory SARS-CoV-2 testing and an abundance of caution. And patients with severe COVID-19 affecting their own lungs are roundly expected to drive up lung transplant volume in the near future. “The whole paradigm has changed,” Dr. Narula said.
Post-acute trajectories
A chart review study published in October by the lung transplant team at the University of Texas Southwestern Medical Center, Dallas, covered 44 consecutive survivors at a median follow-up of 4.5 months from hospital discharge or acute illness (the survival rate was 83.3%). Patients had significantly impaired functional status, and 18 of the 44 (40.9%) had a significant and persistent loss of forced vital capacity or forced expiratory volume in 1 second (>10% from pre–COVID-19 baseline).
Three patients met the criteria for new CLAD after COVID-19 infection, with all three classified as restrictive allograft syndrome (RAS) phenotype.
Moreover, the majority of COVID-19 survivors who had CT chest scans (22 of 28) showed persistent parenchymal opacities – a finding that, regardless of symptomatology, suggests persistent allograft injury, said Amit Banga, MD, associate professor of medicine and medical director of the ex vivo lung perfusion program in UT Southwestern’s lung transplant program.
“The implication is that there may be long-term consequences of COVID-19, perhaps related to some degree of ongoing inflammation and damage,” said Dr. Banga, a coauthor of the postinfection outcomes paper.
The UT Southwestern lung transplant program, which normally performs 60-80 transplants a year, began routine CT scanning 4-5 months into the pandemic, after “stumbling into a few patients who had no symptoms indicative of COVID pneumonia and no changes on an x-ray but significant involvement on a CT,” he said.
Without routine scanning in the general population of COVID-19 patients, Dr. Banga noted, “we’re limited in convincingly saying that COVID is uniquely doing this to lung transplant recipients.” Nor can they conclude that SARS-CoV-2 is unique from other respiratory viruses such as respiratory syncytial virus (RSV) in this regard. (The program has added CT scanning to its protocol for lung transplant recipients afflicted with other respiratory viruses to learn more.)
However, in the big picture, COVID-19 has proven to be far worse for lung transplant recipients than illness with other respiratory viruses, including RSV. “Patients have more frequent and greater loss of lung function, and worse debility from the acute illness,” Dr. Banga said.
“The cornerstones of treatment of both these viruses are very similar, but both the in-hospital course and the postdischarge outcomes are significantly different.”
In an initial paper published in September 2021, Dr. Banga and colleagues compared their first 25 lung transplant patients testing positive for SARS-CoV-2 with a historical cohort of 36 patients with RSV treated during 2016-2018.
Patients with COVID-19 had significantly worse morbidity and mortality, including worse postinfection lung function loss, functional decline, and 3-month survival.
More time, he said, will shed light on the risks of CLAD and the long-term potential for recovery of lung function. Currently, at UT Southwestern, it appears that patients who survive acute illness and the “first 3-6 months after COVID-19, when we’re seeing all the postinfection morbidity, may [enter] a period of stability,” Dr. Banga said.
Overall, he said, patients in their initial cohort are “holding steady” without unusual morbidity, readmissions, or “other setbacks to their allografts.”
At the Mayo Clinic in Jacksonville, which normally performs 40-50 lung transplants a year, transplant physicians have similarly observed significant declines in lung function beyond the acute phase of COVID-19. “Anecdotally, we’re seeing that some patients are beginning to recover some of their lung function, while others have not,” said Dr. Narula. “And we don’t have predictors as to who will progress to CLAD. It’s a big knowledge gap.”
Dr. Narula noted that patients with restrictive allograft syndrome, such as those reported by the UT Southwestern team, “have scarring of the lung and a much worse prognosis than the obstructive type of chronic rejection.” Whether there’s a role for antifibrotic therapy is a question worthy of research.
In UT Southwestern’s analysis, persistently lower absolute lymphocyte counts (< 600/dL) and higher ferritin levels (>150 ng/mL) at the time of hospital discharge were independently associated with significant lung function loss. This finding, reported in their October paper, has helped guide their management practices, Dr. Banga said.
“Persistently elevated ferritin may indicate ongoing inflammation at the allograft level,” he said. “We now send [such patients] home on a longer course of oral corticosteroids.”
At the front end of care for infected lung transplant recipients, Dr. Banga said that his team and physicians at other lung transplant programs are holding the cell-cycle inhibitor component of patients’ maintenance immunosuppression therapy (commonly mycophenolate or azathioprine) once infection is diagnosed to maximize chances of a better outcome.
“There may be variation on how long [the regimens are adjusted],” he said. “We changed our duration from 4 weeks to 2 due to patients developing a rebound worsening in the third and fourth week of acute illness.”
There is significant variation from institution to institution in how viral infections are managed in lung transplant recipients, he and Dr. Narula said. “Our numbers are so small in lung transplant, and we don’t have standardized protocols – it’s one of the biggest challenges in our field,” said Dr. Narula.
Vaccination issues, evaluation of donors
Whether or not immunosuppression regimens should be adjusted prior to vaccination is a controversial question, but is “an absolutely valid one” and is currently being studied in at least one National Institutes of Health–funded trial involving solid organ transplant recipients, said Dr. Wolfe.
“Some have jumped to the conclusion [based on some earlier data] that they should reduce immunosuppression regimens for everyone at the time of vaccination ... but I don’t know the answer yet,” he said. “Balancing staying rejection free with potentially gaining more immune response is complicated ... and it may depend on where the pandemic is going in your area and other factors.”
Reductions aside, Dr. Wolfe tells lung transplant recipients that, based on his approximation of a number of different studies in solid organ transplant recipients, approximately 40%-50% of patients who are immunized with two doses of the COVID-19 mRNA vaccines will develop meaningful antibody levels – and that this rises to 50%-60% after a third dose.
It is difficult to glean from available studies the level of vaccine response for lung transplant recipients specifically. But given that their level of maintenance immunosuppression is higher than for recipients of other donor organs, “as a broad sweep, lung transplant recipients tend to be lower in the pecking order of response,” he said.
Still, “there’s a lot to gain,” he said, pointing to a recent study from the Morbidity and Mortality Weekly Report (2021 Nov 5. doi: 10.15585/mmwr.mm7044e3) showing that effectiveness of mRNA vaccination against COVID-19–associated hospitalization was 77% among immunocompromised adults (compared with 90% in immunocompetent adults).
“This is good vindication to keep vaccinating,” he said, “and perhaps speaks to how difficult it is to assess the vaccine response [through measurement of antibody only].”
Neither Duke University’s transplant program, which performed 100-120 lung transplants a year pre-COVID, nor the programs at UT Southwestern or the Mayo Clinic in Jacksonville require that solid organ transplant candidates be vaccinated against SARS-CoV-2 in order to receive transplants, as some other transplant programs have done. (When asked about the issue, Dr. Banga and Dr. Narula each said that they have had no or little trouble convincing patients awaiting lung transplants of the need for COVID-19 vaccination.)
In an August statement, the American Society of Transplantation recommended vaccination for all solid organ transplant recipients, preferably prior to transplantation, and said that it “support[s] the development of institutional policies regarding pretransplant vaccination.”
The Society is not tracking centers’ vaccination policies. But Kaiser Health News reported in October that a growing number of transplant programs, such as UCHealth in Denver and UW Medicine in Seattle, have decided to either bar patients who refuse to be vaccinated from receiving transplants or give them lower priority on waitlists.
Potential lung donors, meanwhile, must be evaluated with lower respiratory COVID-19 testing, with results available prior to transplantation, according to policy developed by the Organ Procurement and Transplantation Network and effective in May 2021. The policy followed three published cases of donor-derived COVID-19 in lung transplant recipients, said Dr. Wolfe, who wrote about use of COVID-positive donors in an editorial published in October.
In each case, the donor had a negative COVID-19 nasopharyngeal swab at the time of organ procurement but was later found to have the virus on bronchoalveolar lavage, he said.
(The use of other organs from COVID-positive donors is appearing thus far to be safe, Dr. Wolfe noted. In the editorial, he references 13 cases of solid organ transplantation from SARS-CoV-2–infected donors into noninfected recipients; none of the 13 transplant recipients developed COVID-19).
Some questions remain, such as how many lower respiratory tests should be run, and how donors should be evaluated in cases of discordant results. Dr. Banga shared the case of a donor with one positive lower respiratory test result followed by two negative results. After internal debate, and consideration of potential false positives and other issues, the team at UT Southwestern decided to decline the donor, Dr. Banga said.
Other programs are likely making similar, appropriately cautious decisions, said Dr. Wolfe. “There’s no way in real-time donor evaluation to know whether the positive test is active virus that could infect the recipient and replicate ... or whether it’s [picking up] inactive or dead fragments of virus that was there several weeks ago. Our tests don’t differentiate that.”
Transplants in COVID-19 patients
Decision-making about lung transplant candidacy among patients with COVID-19 acute respiratory distress syndrome is complex and in need of a new paradigm.
“Some of these patients have the potential to recover, and they’re going to recover way later than what we’re used to,” said Dr. Banga. “We can’t extrapolate for COVID ARDS what we’ve learned for any other virus-related ARDS.”
Dr. Narula also has recently seen at least one COVID-19 patient on ECMO and under evaluation for transplantation recover. “We do not want to transplant too early,” he said, noting that there is consensus that lung transplant should be pursued only when the damage is deemed irreversible clinically and radiologically in the best judgment of the team. Still, “for many of these patients the only exit route will be lung transplants. For the next 12-24 months, a significant proportion of our lung transplant patients will have had post-COVID–related lung damage.”
As of October 2021, 233 lung transplants had been performed in the United States in recipients whose primary diagnosis was reported as COVID related, said Anne Paschke, media relations specialist with the United Network for Organ Sharing.
Dr. Banga, Dr. Wolfe, and Dr. Narula reported that they have no relevant disclosures.
Data is sparse thus far, but there is concern in lung transplant medicine about the long-term risk of chronic lung allograft dysfunction (CLAD) and a potentially shortened longevity of transplanted lungs in recipients who become ill with COVID-19.
“My fear is that we’re potentially sitting on this iceberg worth of people who, come 6 months or a year from [the acute phase of] their COVID illness, will in fact have earlier and progressive, chronic rejection,” said Cameron R. Wolfe, MBBS, MPH, associate professor of medicine in transplant infectious disease at Duke University, Durham, N.C.
Lower respiratory viral infections have long been concerning for lung transplant recipients given their propensity to cause scarring, a decline in lung function, and a heightened risk of allograft rejection. Time will tell whether lung transplant recipients who survive COVID-19 follow a similar path, or one that is worse, he said.
Short-term data
Outcomes beyond hospitalization and acute illness for lung transplant recipients affected by COVID-19 have been reported in the literature by only a few lung transplant programs. These reports – as well as anecdotal experiences being informally shared among transplant programs – have raised the specter of more severe dysfunction following the acute phase and more early CLAD, said Tathagat Narula, MD, assistant professor of medicine at the Mayo Medical School, Rochester, Minn., and a consultant in lung transplantation at the Mayo Clinic’s Jacksonville program.
“The available data cover only 3-6 months out. We don’t know what will happen in the next 6 months and beyond,” Dr. Narula said in an interview.
The risks of COVID-19 in already-transplanted patients and issues relating to the inadequate antibody responses to vaccination are just some of the challenges of lung transplant medicine in the era of SARS-CoV-2. “COVID-19,” said Dr. Narula, “has completely changed the way we practice lung transplant medicine – the way we’re looking both at our recipients and our donors.”
Potential donors are being evaluated with lower respiratory SARS-CoV-2 testing and an abundance of caution. And patients with severe COVID-19 affecting their own lungs are roundly expected to drive up lung transplant volume in the near future. “The whole paradigm has changed,” Dr. Narula said.
Post-acute trajectories
A chart review study published in October by the lung transplant team at the University of Texas Southwestern Medical Center, Dallas, covered 44 consecutive survivors at a median follow-up of 4.5 months from hospital discharge or acute illness (the survival rate was 83.3%). Patients had significantly impaired functional status, and 18 of the 44 (40.9%) had a significant and persistent loss of forced vital capacity or forced expiratory volume in 1 second (>10% from pre–COVID-19 baseline).
Three patients met the criteria for new CLAD after COVID-19 infection, with all three classified as restrictive allograft syndrome (RAS) phenotype.
Moreover, the majority of COVID-19 survivors who had CT chest scans (22 of 28) showed persistent parenchymal opacities – a finding that, regardless of symptomatology, suggests persistent allograft injury, said Amit Banga, MD, associate professor of medicine and medical director of the ex vivo lung perfusion program in UT Southwestern’s lung transplant program.
“The implication is that there may be long-term consequences of COVID-19, perhaps related to some degree of ongoing inflammation and damage,” said Dr. Banga, a coauthor of the postinfection outcomes paper.
The UT Southwestern lung transplant program, which normally performs 60-80 transplants a year, began routine CT scanning 4-5 months into the pandemic, after “stumbling into a few patients who had no symptoms indicative of COVID pneumonia and no changes on an x-ray but significant involvement on a CT,” he said.
Without routine scanning in the general population of COVID-19 patients, Dr. Banga noted, “we’re limited in convincingly saying that COVID is uniquely doing this to lung transplant recipients.” Nor can they conclude that SARS-CoV-2 is unique from other respiratory viruses such as respiratory syncytial virus (RSV) in this regard. (The program has added CT scanning to its protocol for lung transplant recipients afflicted with other respiratory viruses to learn more.)
However, in the big picture, COVID-19 has proven to be far worse for lung transplant recipients than illness with other respiratory viruses, including RSV. “Patients have more frequent and greater loss of lung function, and worse debility from the acute illness,” Dr. Banga said.
“The cornerstones of treatment of both these viruses are very similar, but both the in-hospital course and the postdischarge outcomes are significantly different.”
In an initial paper published in September 2021, Dr. Banga and colleagues compared their first 25 lung transplant patients testing positive for SARS-CoV-2 with a historical cohort of 36 patients with RSV treated during 2016-2018.
Patients with COVID-19 had significantly worse morbidity and mortality, including worse postinfection lung function loss, functional decline, and 3-month survival.
More time, he said, will shed light on the risks of CLAD and the long-term potential for recovery of lung function. Currently, at UT Southwestern, it appears that patients who survive acute illness and the “first 3-6 months after COVID-19, when we’re seeing all the postinfection morbidity, may [enter] a period of stability,” Dr. Banga said.
Overall, he said, patients in their initial cohort are “holding steady” without unusual morbidity, readmissions, or “other setbacks to their allografts.”
At the Mayo Clinic in Jacksonville, which normally performs 40-50 lung transplants a year, transplant physicians have similarly observed significant declines in lung function beyond the acute phase of COVID-19. “Anecdotally, we’re seeing that some patients are beginning to recover some of their lung function, while others have not,” said Dr. Narula. “And we don’t have predictors as to who will progress to CLAD. It’s a big knowledge gap.”
Dr. Narula noted that patients with restrictive allograft syndrome, such as those reported by the UT Southwestern team, “have scarring of the lung and a much worse prognosis than the obstructive type of chronic rejection.” Whether there’s a role for antifibrotic therapy is a question worthy of research.
In UT Southwestern’s analysis, persistently lower absolute lymphocyte counts (< 600/dL) and higher ferritin levels (>150 ng/mL) at the time of hospital discharge were independently associated with significant lung function loss. This finding, reported in their October paper, has helped guide their management practices, Dr. Banga said.
“Persistently elevated ferritin may indicate ongoing inflammation at the allograft level,” he said. “We now send [such patients] home on a longer course of oral corticosteroids.”
At the front end of care for infected lung transplant recipients, Dr. Banga said that his team and physicians at other lung transplant programs are holding the cell-cycle inhibitor component of patients’ maintenance immunosuppression therapy (commonly mycophenolate or azathioprine) once infection is diagnosed to maximize chances of a better outcome.
“There may be variation on how long [the regimens are adjusted],” he said. “We changed our duration from 4 weeks to 2 due to patients developing a rebound worsening in the third and fourth week of acute illness.”
There is significant variation from institution to institution in how viral infections are managed in lung transplant recipients, he and Dr. Narula said. “Our numbers are so small in lung transplant, and we don’t have standardized protocols – it’s one of the biggest challenges in our field,” said Dr. Narula.
Vaccination issues, evaluation of donors
Whether or not immunosuppression regimens should be adjusted prior to vaccination is a controversial question, but is “an absolutely valid one” and is currently being studied in at least one National Institutes of Health–funded trial involving solid organ transplant recipients, said Dr. Wolfe.
“Some have jumped to the conclusion [based on some earlier data] that they should reduce immunosuppression regimens for everyone at the time of vaccination ... but I don’t know the answer yet,” he said. “Balancing staying rejection free with potentially gaining more immune response is complicated ... and it may depend on where the pandemic is going in your area and other factors.”
Reductions aside, Dr. Wolfe tells lung transplant recipients that, based on his approximation of a number of different studies in solid organ transplant recipients, approximately 40%-50% of patients who are immunized with two doses of the COVID-19 mRNA vaccines will develop meaningful antibody levels – and that this rises to 50%-60% after a third dose.
It is difficult to glean from available studies the level of vaccine response for lung transplant recipients specifically. But given that their level of maintenance immunosuppression is higher than for recipients of other donor organs, “as a broad sweep, lung transplant recipients tend to be lower in the pecking order of response,” he said.
Still, “there’s a lot to gain,” he said, pointing to a recent study from the Morbidity and Mortality Weekly Report (2021 Nov 5. doi: 10.15585/mmwr.mm7044e3) showing that effectiveness of mRNA vaccination against COVID-19–associated hospitalization was 77% among immunocompromised adults (compared with 90% in immunocompetent adults).
“This is good vindication to keep vaccinating,” he said, “and perhaps speaks to how difficult it is to assess the vaccine response [through measurement of antibody only].”
Neither Duke University’s transplant program, which performed 100-120 lung transplants a year pre-COVID, nor the programs at UT Southwestern or the Mayo Clinic in Jacksonville require that solid organ transplant candidates be vaccinated against SARS-CoV-2 in order to receive transplants, as some other transplant programs have done. (When asked about the issue, Dr. Banga and Dr. Narula each said that they have had no or little trouble convincing patients awaiting lung transplants of the need for COVID-19 vaccination.)
In an August statement, the American Society of Transplantation recommended vaccination for all solid organ transplant recipients, preferably prior to transplantation, and said that it “support[s] the development of institutional policies regarding pretransplant vaccination.”
The Society is not tracking centers’ vaccination policies. But Kaiser Health News reported in October that a growing number of transplant programs, such as UCHealth in Denver and UW Medicine in Seattle, have decided to either bar patients who refuse to be vaccinated from receiving transplants or give them lower priority on waitlists.
Potential lung donors, meanwhile, must be evaluated with lower respiratory COVID-19 testing, with results available prior to transplantation, according to policy developed by the Organ Procurement and Transplantation Network and effective in May 2021. The policy followed three published cases of donor-derived COVID-19 in lung transplant recipients, said Dr. Wolfe, who wrote about use of COVID-positive donors in an editorial published in October.
In each case, the donor had a negative COVID-19 nasopharyngeal swab at the time of organ procurement but was later found to have the virus on bronchoalveolar lavage, he said.
(The use of other organs from COVID-positive donors is appearing thus far to be safe, Dr. Wolfe noted. In the editorial, he references 13 cases of solid organ transplantation from SARS-CoV-2–infected donors into noninfected recipients; none of the 13 transplant recipients developed COVID-19).
Some questions remain, such as how many lower respiratory tests should be run, and how donors should be evaluated in cases of discordant results. Dr. Banga shared the case of a donor with one positive lower respiratory test result followed by two negative results. After internal debate, and consideration of potential false positives and other issues, the team at UT Southwestern decided to decline the donor, Dr. Banga said.
Other programs are likely making similar, appropriately cautious decisions, said Dr. Wolfe. “There’s no way in real-time donor evaluation to know whether the positive test is active virus that could infect the recipient and replicate ... or whether it’s [picking up] inactive or dead fragments of virus that was there several weeks ago. Our tests don’t differentiate that.”
Transplants in COVID-19 patients
Decision-making about lung transplant candidacy among patients with COVID-19 acute respiratory distress syndrome is complex and in need of a new paradigm.
“Some of these patients have the potential to recover, and they’re going to recover way later than what we’re used to,” said Dr. Banga. “We can’t extrapolate for COVID ARDS what we’ve learned for any other virus-related ARDS.”
Dr. Narula also has recently seen at least one COVID-19 patient on ECMO and under evaluation for transplantation recover. “We do not want to transplant too early,” he said, noting that there is consensus that lung transplant should be pursued only when the damage is deemed irreversible clinically and radiologically in the best judgment of the team. Still, “for many of these patients the only exit route will be lung transplants. For the next 12-24 months, a significant proportion of our lung transplant patients will have had post-COVID–related lung damage.”
As of October 2021, 233 lung transplants had been performed in the United States in recipients whose primary diagnosis was reported as COVID related, said Anne Paschke, media relations specialist with the United Network for Organ Sharing.
Dr. Banga, Dr. Wolfe, and Dr. Narula reported that they have no relevant disclosures.
Data is sparse thus far, but there is concern in lung transplant medicine about the long-term risk of chronic lung allograft dysfunction (CLAD) and a potentially shortened longevity of transplanted lungs in recipients who become ill with COVID-19.
“My fear is that we’re potentially sitting on this iceberg worth of people who, come 6 months or a year from [the acute phase of] their COVID illness, will in fact have earlier and progressive, chronic rejection,” said Cameron R. Wolfe, MBBS, MPH, associate professor of medicine in transplant infectious disease at Duke University, Durham, N.C.
Lower respiratory viral infections have long been concerning for lung transplant recipients given their propensity to cause scarring, a decline in lung function, and a heightened risk of allograft rejection. Time will tell whether lung transplant recipients who survive COVID-19 follow a similar path, or one that is worse, he said.
Short-term data
Outcomes beyond hospitalization and acute illness for lung transplant recipients affected by COVID-19 have been reported in the literature by only a few lung transplant programs. These reports – as well as anecdotal experiences being informally shared among transplant programs – have raised the specter of more severe dysfunction following the acute phase and more early CLAD, said Tathagat Narula, MD, assistant professor of medicine at the Mayo Medical School, Rochester, Minn., and a consultant in lung transplantation at the Mayo Clinic’s Jacksonville program.
“The available data cover only 3-6 months out. We don’t know what will happen in the next 6 months and beyond,” Dr. Narula said in an interview.
The risks of COVID-19 in already-transplanted patients and issues relating to the inadequate antibody responses to vaccination are just some of the challenges of lung transplant medicine in the era of SARS-CoV-2. “COVID-19,” said Dr. Narula, “has completely changed the way we practice lung transplant medicine – the way we’re looking both at our recipients and our donors.”
Potential donors are being evaluated with lower respiratory SARS-CoV-2 testing and an abundance of caution. And patients with severe COVID-19 affecting their own lungs are roundly expected to drive up lung transplant volume in the near future. “The whole paradigm has changed,” Dr. Narula said.
Post-acute trajectories
A chart review study published in October by the lung transplant team at the University of Texas Southwestern Medical Center, Dallas, covered 44 consecutive survivors at a median follow-up of 4.5 months from hospital discharge or acute illness (the survival rate was 83.3%). Patients had significantly impaired functional status, and 18 of the 44 (40.9%) had a significant and persistent loss of forced vital capacity or forced expiratory volume in 1 second (>10% from pre–COVID-19 baseline).
Three patients met the criteria for new CLAD after COVID-19 infection, with all three classified as restrictive allograft syndrome (RAS) phenotype.
Moreover, the majority of COVID-19 survivors who had CT chest scans (22 of 28) showed persistent parenchymal opacities – a finding that, regardless of symptomatology, suggests persistent allograft injury, said Amit Banga, MD, associate professor of medicine and medical director of the ex vivo lung perfusion program in UT Southwestern’s lung transplant program.
“The implication is that there may be long-term consequences of COVID-19, perhaps related to some degree of ongoing inflammation and damage,” said Dr. Banga, a coauthor of the postinfection outcomes paper.
The UT Southwestern lung transplant program, which normally performs 60-80 transplants a year, began routine CT scanning 4-5 months into the pandemic, after “stumbling into a few patients who had no symptoms indicative of COVID pneumonia and no changes on an x-ray but significant involvement on a CT,” he said.
Without routine scanning in the general population of COVID-19 patients, Dr. Banga noted, “we’re limited in convincingly saying that COVID is uniquely doing this to lung transplant recipients.” Nor can they conclude that SARS-CoV-2 is unique from other respiratory viruses such as respiratory syncytial virus (RSV) in this regard. (The program has added CT scanning to its protocol for lung transplant recipients afflicted with other respiratory viruses to learn more.)
However, in the big picture, COVID-19 has proven to be far worse for lung transplant recipients than illness with other respiratory viruses, including RSV. “Patients have more frequent and greater loss of lung function, and worse debility from the acute illness,” Dr. Banga said.
“The cornerstones of treatment of both these viruses are very similar, but both the in-hospital course and the postdischarge outcomes are significantly different.”
In an initial paper published in September 2021, Dr. Banga and colleagues compared their first 25 lung transplant patients testing positive for SARS-CoV-2 with a historical cohort of 36 patients with RSV treated during 2016-2018.
Patients with COVID-19 had significantly worse morbidity and mortality, including worse postinfection lung function loss, functional decline, and 3-month survival.
More time, he said, will shed light on the risks of CLAD and the long-term potential for recovery of lung function. Currently, at UT Southwestern, it appears that patients who survive acute illness and the “first 3-6 months after COVID-19, when we’re seeing all the postinfection morbidity, may [enter] a period of stability,” Dr. Banga said.
Overall, he said, patients in their initial cohort are “holding steady” without unusual morbidity, readmissions, or “other setbacks to their allografts.”
At the Mayo Clinic in Jacksonville, which normally performs 40-50 lung transplants a year, transplant physicians have similarly observed significant declines in lung function beyond the acute phase of COVID-19. “Anecdotally, we’re seeing that some patients are beginning to recover some of their lung function, while others have not,” said Dr. Narula. “And we don’t have predictors as to who will progress to CLAD. It’s a big knowledge gap.”
Dr. Narula noted that patients with restrictive allograft syndrome, such as those reported by the UT Southwestern team, “have scarring of the lung and a much worse prognosis than the obstructive type of chronic rejection.” Whether there’s a role for antifibrotic therapy is a question worthy of research.
In UT Southwestern’s analysis, persistently lower absolute lymphocyte counts (< 600/dL) and higher ferritin levels (>150 ng/mL) at the time of hospital discharge were independently associated with significant lung function loss. This finding, reported in their October paper, has helped guide their management practices, Dr. Banga said.
“Persistently elevated ferritin may indicate ongoing inflammation at the allograft level,” he said. “We now send [such patients] home on a longer course of oral corticosteroids.”
At the front end of care for infected lung transplant recipients, Dr. Banga said that his team and physicians at other lung transplant programs are holding the cell-cycle inhibitor component of patients’ maintenance immunosuppression therapy (commonly mycophenolate or azathioprine) once infection is diagnosed to maximize chances of a better outcome.
“There may be variation on how long [the regimens are adjusted],” he said. “We changed our duration from 4 weeks to 2 due to patients developing a rebound worsening in the third and fourth week of acute illness.”
There is significant variation from institution to institution in how viral infections are managed in lung transplant recipients, he and Dr. Narula said. “Our numbers are so small in lung transplant, and we don’t have standardized protocols – it’s one of the biggest challenges in our field,” said Dr. Narula.
Vaccination issues, evaluation of donors
Whether or not immunosuppression regimens should be adjusted prior to vaccination is a controversial question, but is “an absolutely valid one” and is currently being studied in at least one National Institutes of Health–funded trial involving solid organ transplant recipients, said Dr. Wolfe.
“Some have jumped to the conclusion [based on some earlier data] that they should reduce immunosuppression regimens for everyone at the time of vaccination ... but I don’t know the answer yet,” he said. “Balancing staying rejection free with potentially gaining more immune response is complicated ... and it may depend on where the pandemic is going in your area and other factors.”
Reductions aside, Dr. Wolfe tells lung transplant recipients that, based on his approximation of a number of different studies in solid organ transplant recipients, approximately 40%-50% of patients who are immunized with two doses of the COVID-19 mRNA vaccines will develop meaningful antibody levels – and that this rises to 50%-60% after a third dose.
It is difficult to glean from available studies the level of vaccine response for lung transplant recipients specifically. But given that their level of maintenance immunosuppression is higher than for recipients of other donor organs, “as a broad sweep, lung transplant recipients tend to be lower in the pecking order of response,” he said.
Still, “there’s a lot to gain,” he said, pointing to a recent study from the Morbidity and Mortality Weekly Report (2021 Nov 5. doi: 10.15585/mmwr.mm7044e3) showing that effectiveness of mRNA vaccination against COVID-19–associated hospitalization was 77% among immunocompromised adults (compared with 90% in immunocompetent adults).
“This is good vindication to keep vaccinating,” he said, “and perhaps speaks to how difficult it is to assess the vaccine response [through measurement of antibody only].”
Neither Duke University’s transplant program, which performed 100-120 lung transplants a year pre-COVID, nor the programs at UT Southwestern or the Mayo Clinic in Jacksonville require that solid organ transplant candidates be vaccinated against SARS-CoV-2 in order to receive transplants, as some other transplant programs have done. (When asked about the issue, Dr. Banga and Dr. Narula each said that they have had no or little trouble convincing patients awaiting lung transplants of the need for COVID-19 vaccination.)
In an August statement, the American Society of Transplantation recommended vaccination for all solid organ transplant recipients, preferably prior to transplantation, and said that it “support[s] the development of institutional policies regarding pretransplant vaccination.”
The Society is not tracking centers’ vaccination policies. But Kaiser Health News reported in October that a growing number of transplant programs, such as UCHealth in Denver and UW Medicine in Seattle, have decided to either bar patients who refuse to be vaccinated from receiving transplants or give them lower priority on waitlists.
Potential lung donors, meanwhile, must be evaluated with lower respiratory COVID-19 testing, with results available prior to transplantation, according to policy developed by the Organ Procurement and Transplantation Network and effective in May 2021. The policy followed three published cases of donor-derived COVID-19 in lung transplant recipients, said Dr. Wolfe, who wrote about use of COVID-positive donors in an editorial published in October.
In each case, the donor had a negative COVID-19 nasopharyngeal swab at the time of organ procurement but was later found to have the virus on bronchoalveolar lavage, he said.
(The use of other organs from COVID-positive donors is appearing thus far to be safe, Dr. Wolfe noted. In the editorial, he references 13 cases of solid organ transplantation from SARS-CoV-2–infected donors into noninfected recipients; none of the 13 transplant recipients developed COVID-19).
Some questions remain, such as how many lower respiratory tests should be run, and how donors should be evaluated in cases of discordant results. Dr. Banga shared the case of a donor with one positive lower respiratory test result followed by two negative results. After internal debate, and consideration of potential false positives and other issues, the team at UT Southwestern decided to decline the donor, Dr. Banga said.
Other programs are likely making similar, appropriately cautious decisions, said Dr. Wolfe. “There’s no way in real-time donor evaluation to know whether the positive test is active virus that could infect the recipient and replicate ... or whether it’s [picking up] inactive or dead fragments of virus that was there several weeks ago. Our tests don’t differentiate that.”
Transplants in COVID-19 patients
Decision-making about lung transplant candidacy among patients with COVID-19 acute respiratory distress syndrome is complex and in need of a new paradigm.
“Some of these patients have the potential to recover, and they’re going to recover way later than what we’re used to,” said Dr. Banga. “We can’t extrapolate for COVID ARDS what we’ve learned for any other virus-related ARDS.”
Dr. Narula also has recently seen at least one COVID-19 patient on ECMO and under evaluation for transplantation recover. “We do not want to transplant too early,” he said, noting that there is consensus that lung transplant should be pursued only when the damage is deemed irreversible clinically and radiologically in the best judgment of the team. Still, “for many of these patients the only exit route will be lung transplants. For the next 12-24 months, a significant proportion of our lung transplant patients will have had post-COVID–related lung damage.”
As of October 2021, 233 lung transplants had been performed in the United States in recipients whose primary diagnosis was reported as COVID related, said Anne Paschke, media relations specialist with the United Network for Organ Sharing.
Dr. Banga, Dr. Wolfe, and Dr. Narula reported that they have no relevant disclosures.
U.S. kidney transplants grow in number and success
During 2016-2019, U.S. centers performed kidney transplants in nearly 77,000 patients, a jump of almost 25% compared with 4-year averages of about 62,000 patients throughout 2004-2015. That works out to about 15,000 more patients receiving donor kidneys, Sundaram Hariharan, MD, and associates reported in the New England Journal of Medicine in a review of all U.S. renal transplantations performed during 1996-2019.
Coupled with the volume uptick during this 24-year period were new lows in graft losses and patient deaths. By 2018, mortality during the first year following transplantation occurred at about a 1% rate among patients who had received a kidney from a living donor, and at about a 3% rate when the organ came from a deceased donor, nearly half the rate of 2 decades earlier, in 1996. Rates of first-year graft loss during 2017 were also about half of what they had been in 1996, occurring in about 2% of patients who received a living donor organ and in about 6% of those who got a kidney from a deceased donor during 2017.
“Twenty years ago, kidney transplantation was the preferred option compared with dialysis, and even more so now,” summed up Dr. Hariharan, a senior transplant nephrologist and professor of medicine and surgery at the University of Pittsburgh Medical Center and first author of the report. Kidney transplantation survival at U.S. centers “improved steadily over the past 24 years, despite patient variables becoming worse,” he said in an interview.
Kidney recipients are older, more obese, and have more prevalent diabetes
During the period studied, kidney transplant recipients became on average older and more obese, and had a higher prevalence of diabetes; the age of organ donors grew as well. The prevalence of diabetes among patients who received a kidney from a deceased donor increased from 24% during 1996-1999 to 36% during 2016-2019, while diabetes prevalence among recipients of an organ from a living donor rose from 25% in 1996-1999 to 29% during 2016-2019.
The improved graft and patient survival numbers “are very encouraging trends,” said Michelle A. Josephson, MD, professor and medical director of kidney transplantation at the University of Chicago, who was not involved with the report. “We have been hearing for a number of years that short-term graft survival had improved, but I’m thrilled to learn that long-term survival has also improved.”
The report documented 10-year survival of graft recipients during 2008-2011 of 67%, up from 61% during 1996-1999, and a 10-year overall graft survival rate of 54% in the 2008-2011 cohort, an improvement from the 42% rate in patients who received their organs in 1996-1999, changes Dr. Hariharan characterized as “modest.”
These improvements in long-term graft and patient survival are “meaningful, and particularly notable that outcomes improved despite increased complexity of the transplant population,” said Krista L. Lentine, MD, PhD, professor and medical director of living donation at Saint Louis University. But “despite these improvements, long-term graft survival remains limited,” she cautioned, especially because of risks for substantial complications from chronic immunosuppressive treatment including infection, cancer, glucose intolerance, and dyslipidemia.
The analysis reported by Dr. Hariharan and his associates used data collected by the Scientific Registry of Transplant Patients, run under contract with the U.S. Department of Health and Human Services, which has tracked all patients who have had kidney transplants at U.S. centers since the late 1980s, said Dr. Hariharan. The database included just over 362,000 total transplants during the 24-year period studied, with 36% of all transplants involving organs from living donors with the remaining patients receiving kidneys from deceased donors.
Living donations still stagnant; deceased-donor kidneys rise
The data showed that the rate of transplants from living donors was stagnant for 2 decades, with 22,525 patients transplanted during 2000-2003, and 23,746 transplanted during 2016-2019, with very similar rates during the intervening years. The recent spurt in transplants during 2016-2019 compared with the preceding decade depended almost entirely on kidneys from deceased donors. This rate jumped from the steady, slow rise it showed during 1996-2015, when deceased-donor transplants rose from about 30,000 during 1996-1999 to about 41,000 during 2012-2015, to a more dramatic increase of about 12,000 additional transplants during the most recent period, adding up to a total of more than 53,000 transplants from deceased donors during 2016-2019.
“I strongly recommend organs from living donors” when feasible, said Dr. Hariharan. “At some centers, a high proportion of transplants use living donors, but not at other centers,” he said.
It’s unknown why transplants using organs from deceased donors has shown this growth, but Dr. Hariharan suggested a multifactorial explanation. Those factors include growth in the number of patients with end-stage renal disease who require dialysis, increased numbers of patients listed for kidney transplant, new approaches that allow organs from older donors and those infected with pathogens such as hepatitis C virus or HIV, greater numbers of people and families agreeing to donate organs, and possibly the opioid crisis that may have led to increased organ donation. The number of U.S. centers performing kidney transplants rose from fewer than 200 about a quarter of a century ago to about 250 today, he added.
‘Immuno Bill’ guarantees Medicare coverage for immunosuppression
Dr. Hariharan voiced optimism that graft and patient survival rates will continue to improve going forward. One factor will likely be the passage in late 2020 of the “Immuno Bill” by the U.S. Congress, which among other things mandated ongoing coverage starting in 2023 for immunosuppressive drugs for all Medicare beneficiaries with a kidney transplant. Until then, Medicare provides coverage for only 36 months, a time limit that has resulted in nearly 400 kidney recipients annually losing coverage of their immunosuppression medications.
Dr. Hariharan and coauthors called the existing potential for discontinuation of immunosuppressive drug an “unnecessary impediment to long-term survival for which patients and society paid a heavy price.”
“Kidney transplantation, especially from living donors, offers patients with kidney failure the best chance for long-term survival and improved quality of life, with lower cost to the health care system,” Dr. Lentine said in an interview. Despite the many positive trends detailed in the report from Dr. Hariharan and coauthors, “the vast majority of the more than 700,000 people in the United States with kidney failure will not have an opportunity to receive a transplant due to limitations in organ supply.” And many patients who receive a kidney transplant eventually must resume dialysis because of “limited long-term graft survival resulting from allograft nephropathy, recurrent native disease, medication nonadherence, or other causes.” Plus many potentially transplantable organs go unused.
Dr. Lentine cited a position statement issued in July 2021 by the National Kidney Foundation that made several recommendations on how to improve access to kidney transplants and improve outcomes. “Expanding opportunities for safe living donation, eliminating racial disparities in living-donor access, improving wait-list access and transport readiness, maximizing use of deceased-donor organs, and extending graft longevity are critical priorities,” said Dr. Lentine, lead author on the statement.
“For many or even most patients with kidney failure transplantation is the optimal form of renal replacement. The better recent outcomes and evolving management strategies make transplantation an even more attractive option,” said Dr. Josephson. Improved outcomes among U.S. transplant patients also highlights the “importance of increasing access to kidney transplantation” for all people with kidney failure who could benefit from this treatment, she added.
Dr. Hariharan and Dr. Lentine had no relevant disclosures. Dr. Josephson has been a consultant to UCB and has an ownership interest in Seagen.
During 2016-2019, U.S. centers performed kidney transplants in nearly 77,000 patients, a jump of almost 25% compared with 4-year averages of about 62,000 patients throughout 2004-2015. That works out to about 15,000 more patients receiving donor kidneys, Sundaram Hariharan, MD, and associates reported in the New England Journal of Medicine in a review of all U.S. renal transplantations performed during 1996-2019.
Coupled with the volume uptick during this 24-year period were new lows in graft losses and patient deaths. By 2018, mortality during the first year following transplantation occurred at about a 1% rate among patients who had received a kidney from a living donor, and at about a 3% rate when the organ came from a deceased donor, nearly half the rate of 2 decades earlier, in 1996. Rates of first-year graft loss during 2017 were also about half of what they had been in 1996, occurring in about 2% of patients who received a living donor organ and in about 6% of those who got a kidney from a deceased donor during 2017.
“Twenty years ago, kidney transplantation was the preferred option compared with dialysis, and even more so now,” summed up Dr. Hariharan, a senior transplant nephrologist and professor of medicine and surgery at the University of Pittsburgh Medical Center and first author of the report. Kidney transplantation survival at U.S. centers “improved steadily over the past 24 years, despite patient variables becoming worse,” he said in an interview.
Kidney recipients are older, more obese, and have more prevalent diabetes
During the period studied, kidney transplant recipients became on average older and more obese, and had a higher prevalence of diabetes; the age of organ donors grew as well. The prevalence of diabetes among patients who received a kidney from a deceased donor increased from 24% during 1996-1999 to 36% during 2016-2019, while diabetes prevalence among recipients of an organ from a living donor rose from 25% in 1996-1999 to 29% during 2016-2019.
The improved graft and patient survival numbers “are very encouraging trends,” said Michelle A. Josephson, MD, professor and medical director of kidney transplantation at the University of Chicago, who was not involved with the report. “We have been hearing for a number of years that short-term graft survival had improved, but I’m thrilled to learn that long-term survival has also improved.”
The report documented 10-year survival of graft recipients during 2008-2011 of 67%, up from 61% during 1996-1999, and a 10-year overall graft survival rate of 54% in the 2008-2011 cohort, an improvement from the 42% rate in patients who received their organs in 1996-1999, changes Dr. Hariharan characterized as “modest.”
These improvements in long-term graft and patient survival are “meaningful, and particularly notable that outcomes improved despite increased complexity of the transplant population,” said Krista L. Lentine, MD, PhD, professor and medical director of living donation at Saint Louis University. But “despite these improvements, long-term graft survival remains limited,” she cautioned, especially because of risks for substantial complications from chronic immunosuppressive treatment including infection, cancer, glucose intolerance, and dyslipidemia.
The analysis reported by Dr. Hariharan and his associates used data collected by the Scientific Registry of Transplant Patients, run under contract with the U.S. Department of Health and Human Services, which has tracked all patients who have had kidney transplants at U.S. centers since the late 1980s, said Dr. Hariharan. The database included just over 362,000 total transplants during the 24-year period studied, with 36% of all transplants involving organs from living donors with the remaining patients receiving kidneys from deceased donors.
Living donations still stagnant; deceased-donor kidneys rise
The data showed that the rate of transplants from living donors was stagnant for 2 decades, with 22,525 patients transplanted during 2000-2003, and 23,746 transplanted during 2016-2019, with very similar rates during the intervening years. The recent spurt in transplants during 2016-2019 compared with the preceding decade depended almost entirely on kidneys from deceased donors. This rate jumped from the steady, slow rise it showed during 1996-2015, when deceased-donor transplants rose from about 30,000 during 1996-1999 to about 41,000 during 2012-2015, to a more dramatic increase of about 12,000 additional transplants during the most recent period, adding up to a total of more than 53,000 transplants from deceased donors during 2016-2019.
“I strongly recommend organs from living donors” when feasible, said Dr. Hariharan. “At some centers, a high proportion of transplants use living donors, but not at other centers,” he said.
It’s unknown why transplants using organs from deceased donors has shown this growth, but Dr. Hariharan suggested a multifactorial explanation. Those factors include growth in the number of patients with end-stage renal disease who require dialysis, increased numbers of patients listed for kidney transplant, new approaches that allow organs from older donors and those infected with pathogens such as hepatitis C virus or HIV, greater numbers of people and families agreeing to donate organs, and possibly the opioid crisis that may have led to increased organ donation. The number of U.S. centers performing kidney transplants rose from fewer than 200 about a quarter of a century ago to about 250 today, he added.
‘Immuno Bill’ guarantees Medicare coverage for immunosuppression
Dr. Hariharan voiced optimism that graft and patient survival rates will continue to improve going forward. One factor will likely be the passage in late 2020 of the “Immuno Bill” by the U.S. Congress, which among other things mandated ongoing coverage starting in 2023 for immunosuppressive drugs for all Medicare beneficiaries with a kidney transplant. Until then, Medicare provides coverage for only 36 months, a time limit that has resulted in nearly 400 kidney recipients annually losing coverage of their immunosuppression medications.
Dr. Hariharan and coauthors called the existing potential for discontinuation of immunosuppressive drug an “unnecessary impediment to long-term survival for which patients and society paid a heavy price.”
“Kidney transplantation, especially from living donors, offers patients with kidney failure the best chance for long-term survival and improved quality of life, with lower cost to the health care system,” Dr. Lentine said in an interview. Despite the many positive trends detailed in the report from Dr. Hariharan and coauthors, “the vast majority of the more than 700,000 people in the United States with kidney failure will not have an opportunity to receive a transplant due to limitations in organ supply.” And many patients who receive a kidney transplant eventually must resume dialysis because of “limited long-term graft survival resulting from allograft nephropathy, recurrent native disease, medication nonadherence, or other causes.” Plus many potentially transplantable organs go unused.
Dr. Lentine cited a position statement issued in July 2021 by the National Kidney Foundation that made several recommendations on how to improve access to kidney transplants and improve outcomes. “Expanding opportunities for safe living donation, eliminating racial disparities in living-donor access, improving wait-list access and transport readiness, maximizing use of deceased-donor organs, and extending graft longevity are critical priorities,” said Dr. Lentine, lead author on the statement.
“For many or even most patients with kidney failure transplantation is the optimal form of renal replacement. The better recent outcomes and evolving management strategies make transplantation an even more attractive option,” said Dr. Josephson. Improved outcomes among U.S. transplant patients also highlights the “importance of increasing access to kidney transplantation” for all people with kidney failure who could benefit from this treatment, she added.
Dr. Hariharan and Dr. Lentine had no relevant disclosures. Dr. Josephson has been a consultant to UCB and has an ownership interest in Seagen.
During 2016-2019, U.S. centers performed kidney transplants in nearly 77,000 patients, a jump of almost 25% compared with 4-year averages of about 62,000 patients throughout 2004-2015. That works out to about 15,000 more patients receiving donor kidneys, Sundaram Hariharan, MD, and associates reported in the New England Journal of Medicine in a review of all U.S. renal transplantations performed during 1996-2019.
Coupled with the volume uptick during this 24-year period were new lows in graft losses and patient deaths. By 2018, mortality during the first year following transplantation occurred at about a 1% rate among patients who had received a kidney from a living donor, and at about a 3% rate when the organ came from a deceased donor, nearly half the rate of 2 decades earlier, in 1996. Rates of first-year graft loss during 2017 were also about half of what they had been in 1996, occurring in about 2% of patients who received a living donor organ and in about 6% of those who got a kidney from a deceased donor during 2017.
“Twenty years ago, kidney transplantation was the preferred option compared with dialysis, and even more so now,” summed up Dr. Hariharan, a senior transplant nephrologist and professor of medicine and surgery at the University of Pittsburgh Medical Center and first author of the report. Kidney transplantation survival at U.S. centers “improved steadily over the past 24 years, despite patient variables becoming worse,” he said in an interview.
Kidney recipients are older, more obese, and have more prevalent diabetes
During the period studied, kidney transplant recipients became on average older and more obese, and had a higher prevalence of diabetes; the age of organ donors grew as well. The prevalence of diabetes among patients who received a kidney from a deceased donor increased from 24% during 1996-1999 to 36% during 2016-2019, while diabetes prevalence among recipients of an organ from a living donor rose from 25% in 1996-1999 to 29% during 2016-2019.
The improved graft and patient survival numbers “are very encouraging trends,” said Michelle A. Josephson, MD, professor and medical director of kidney transplantation at the University of Chicago, who was not involved with the report. “We have been hearing for a number of years that short-term graft survival had improved, but I’m thrilled to learn that long-term survival has also improved.”
The report documented 10-year survival of graft recipients during 2008-2011 of 67%, up from 61% during 1996-1999, and a 10-year overall graft survival rate of 54% in the 2008-2011 cohort, an improvement from the 42% rate in patients who received their organs in 1996-1999, changes Dr. Hariharan characterized as “modest.”
These improvements in long-term graft and patient survival are “meaningful, and particularly notable that outcomes improved despite increased complexity of the transplant population,” said Krista L. Lentine, MD, PhD, professor and medical director of living donation at Saint Louis University. But “despite these improvements, long-term graft survival remains limited,” she cautioned, especially because of risks for substantial complications from chronic immunosuppressive treatment including infection, cancer, glucose intolerance, and dyslipidemia.
The analysis reported by Dr. Hariharan and his associates used data collected by the Scientific Registry of Transplant Patients, run under contract with the U.S. Department of Health and Human Services, which has tracked all patients who have had kidney transplants at U.S. centers since the late 1980s, said Dr. Hariharan. The database included just over 362,000 total transplants during the 24-year period studied, with 36% of all transplants involving organs from living donors with the remaining patients receiving kidneys from deceased donors.
Living donations still stagnant; deceased-donor kidneys rise
The data showed that the rate of transplants from living donors was stagnant for 2 decades, with 22,525 patients transplanted during 2000-2003, and 23,746 transplanted during 2016-2019, with very similar rates during the intervening years. The recent spurt in transplants during 2016-2019 compared with the preceding decade depended almost entirely on kidneys from deceased donors. This rate jumped from the steady, slow rise it showed during 1996-2015, when deceased-donor transplants rose from about 30,000 during 1996-1999 to about 41,000 during 2012-2015, to a more dramatic increase of about 12,000 additional transplants during the most recent period, adding up to a total of more than 53,000 transplants from deceased donors during 2016-2019.
“I strongly recommend organs from living donors” when feasible, said Dr. Hariharan. “At some centers, a high proportion of transplants use living donors, but not at other centers,” he said.
It’s unknown why transplants using organs from deceased donors has shown this growth, but Dr. Hariharan suggested a multifactorial explanation. Those factors include growth in the number of patients with end-stage renal disease who require dialysis, increased numbers of patients listed for kidney transplant, new approaches that allow organs from older donors and those infected with pathogens such as hepatitis C virus or HIV, greater numbers of people and families agreeing to donate organs, and possibly the opioid crisis that may have led to increased organ donation. The number of U.S. centers performing kidney transplants rose from fewer than 200 about a quarter of a century ago to about 250 today, he added.
‘Immuno Bill’ guarantees Medicare coverage for immunosuppression
Dr. Hariharan voiced optimism that graft and patient survival rates will continue to improve going forward. One factor will likely be the passage in late 2020 of the “Immuno Bill” by the U.S. Congress, which among other things mandated ongoing coverage starting in 2023 for immunosuppressive drugs for all Medicare beneficiaries with a kidney transplant. Until then, Medicare provides coverage for only 36 months, a time limit that has resulted in nearly 400 kidney recipients annually losing coverage of their immunosuppression medications.
Dr. Hariharan and coauthors called the existing potential for discontinuation of immunosuppressive drug an “unnecessary impediment to long-term survival for which patients and society paid a heavy price.”
“Kidney transplantation, especially from living donors, offers patients with kidney failure the best chance for long-term survival and improved quality of life, with lower cost to the health care system,” Dr. Lentine said in an interview. Despite the many positive trends detailed in the report from Dr. Hariharan and coauthors, “the vast majority of the more than 700,000 people in the United States with kidney failure will not have an opportunity to receive a transplant due to limitations in organ supply.” And many patients who receive a kidney transplant eventually must resume dialysis because of “limited long-term graft survival resulting from allograft nephropathy, recurrent native disease, medication nonadherence, or other causes.” Plus many potentially transplantable organs go unused.
Dr. Lentine cited a position statement issued in July 2021 by the National Kidney Foundation that made several recommendations on how to improve access to kidney transplants and improve outcomes. “Expanding opportunities for safe living donation, eliminating racial disparities in living-donor access, improving wait-list access and transport readiness, maximizing use of deceased-donor organs, and extending graft longevity are critical priorities,” said Dr. Lentine, lead author on the statement.
“For many or even most patients with kidney failure transplantation is the optimal form of renal replacement. The better recent outcomes and evolving management strategies make transplantation an even more attractive option,” said Dr. Josephson. Improved outcomes among U.S. transplant patients also highlights the “importance of increasing access to kidney transplantation” for all people with kidney failure who could benefit from this treatment, she added.
Dr. Hariharan and Dr. Lentine had no relevant disclosures. Dr. Josephson has been a consultant to UCB and has an ownership interest in Seagen.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE