User login
More Mobile Clinics Are Bringing Long-Acting Birth Control to Rural Areas
Twice a month, a 40-foot-long truck transformed into a mobile clinic travels the Rio Grande Valley to provide rural Texans with women’s health care, including birth control.
The clinic, called the UniMóvil, is part of the Healthy Mujeres program at the University of Texas Rio Grande Valley School of Medicine in Edinburg.
The United States has about 3000 mobile health programs. But Saul Rivas, an ob.gyn., said he wasn’t aware of any that shared the specific mission of Healthy Mujeres when he helped launch the initiative in 2017. “Mujeres” means “women” in Spanish.
It’s now part of a small but growing number of mobile programs aimed at increasing rural access to women’s health services, including long-acting reversible contraception.
There are two kinds of these highly effective methods: intrauterine devices, known as IUDs, and hormonal implants inserted into the upper arm. These birth control options can be especially difficult to obtain — or have removed — in rural areas.
“Women who want to prevent an unintended pregnancy should have whatever works best for them,” said Kelly Conroy, senior director of mobile and maternal health programs at the University of Arkansas for Medical Sciences, Little Rock.
The school is launching a mobile women’s health and contraception program in rural parts of the state in October.
Rural areas have disproportionately fewer doctors, including ob.gyns., than urban areas. And rural providers may not be able to afford to stock long-acting birth control devices or may not be trained in administering them, program leaders say.
Mobile clinics help shrink that gap in rural care, but they can be challenging to operate, said Elizabeth Jones, a senior director at the National Family Planning & Reproductive Health Association.
Money is the greatest obstacle, Jones said. The Texas program costs up to $400,000 a year. A 2020 study of 173 mobile clinics found they cost an average of more than $630,000 a year. Mobile dental programs were the most expensive, averaging more than $1 million.
While many programs launch with the help of grants, they can be difficult to sustain, especially with over a decade of decreased or stagnant funding to Title X, a federal money stream that helps low-income people receive family planning services.
For example, a mobile contraception program serving rural Pennsylvania lasted less than 3 years before closing in 2023. It shut down after losing federal funding, said a spokesperson for the clinic that ran it.
Rural mobile programs aren’t as efficient or profitable as brick-and-mortar clinics. That’s because staff members may have to make hours-long trips to reach towns where they’ll probably see fewer patients than they would at a traditional site, Jones said.
She said organizations that can’t afford mobile programs can consider setting up “pop-up clinics” at existing health and community sites in rural areas.
Maria Briones is a patient who has benefited from the Healthy Mujeres program in southern Texas. The 41-year-old day care worker was concerned because she wasn’t getting her menstrual period with her IUD.
She considered going to Mexico to have the device removed because few doctors take her insurance on the US side of the Rio Grande Valley.
But Briones learned that the UniMóvil was visiting a small Texas city about 20 minutes from her home. She told the staff there that she doesn’t want more kids but was worried about the IUD.
Briones decided to keep the device after learning it’s safe and normal not to have periods while using an IUD. She won’t get billed for her appointment with the mobile clinic, even though the university health system doesn’t take her insurance.
“They have a lot of patience, and they answered all the questions that I had,” Briones said.
IUDs and hormonal implants are highly effective and can last up to 10 years. But they’re also expensive — devices can cost more than $1,000 without insurance — and inserting an IUD can be painful.
Patient-rights advocates are also concerned that some providers pressure people to use these devices.
They say ethical birth control programs aim to empower patients to choose the contraceptive method — if any — that is best for them, instead of promoting long-acting methods in an attempt to lower birth and poverty rates. They point to the history of eugenics-inspired sterilization and even more recent incidents.
For example, an investigation by Time magazine found doctors are more likely to push Black, Latina, young, and low-income women than other patients to use long-acting birth control — and to refuse to remove the devices.
Rivas said Healthy Mujeres staffers are trained on this issue.
“Our goal isn’t necessarily to place IUDs and implants,” he said. It’s to “provide education and help patients make the best decisions for themselves.”
David Wise, a spokesperson for the University of Arkansas for Medical Sciences, said staff members with the university’s mobile program will ask patients if they want to get pregnant in the next year, and will support their choice. The Arkansas and Texas programs also remove IUDs and hormonal arm implants if patients aren’t happy with them.
The Arkansas initiative will visit 14 rural counties with four vehicles the size of food trucks that were used in previous mobile health efforts. Staffing and equipment will be covered by a 2-year, $431,000 grant from an anonymous donor, Wise said.
In addition to contraception, faculty and medical residents staffing the vehicles will offer women’s health screenings, vaccinations, prenatal care, and testing and treatment for sexually transmitted infections.
Rivas said the Texas program was inspired by a study that found that, 6 months after giving birth, 34% of surveyed Texas mothers said long-acting contraception is their preferred birth control option — but only 13% were using that method.
“We started thinking about ways to address that gap,” Rivas said.
Healthy Mujeres, which is funded through multiple grants, started with a focus on contraception. It later expanded to services such as pregnancy ultrasounds, cervical cancer screenings, and testing for sexually transmitted infections.
While the Texas and Arkansas programs can bill insurance, they also have funding to help uninsured and underinsured patients afford their services. Both use community health workers — called promotoras in largely Spanish-speaking communities like the Rio Grande Valley — to connect patients with food, transportation, additional medical services, and other needs.
They partner with organizations that locals trust, such as food pantries and community colleges, which let the mobile units set up in their parking lots. And to further increase the availability of long-acting contraception in rural areas, the universities are training their students and local providers on how to insert, remove, and get reimbursed for the devices.
One difference between the programs is dictated by state laws. The Arkansas program can provide birth control to minors without a parent or guardian’s consent. But in Texas, most minors need consent before receiving health care, including contraception.
Advocates say these initiatives might help lower the rates of unintended and teen pregnancies in both states, which are higher than the national average.
Rivas and Conroy said their programs haven’t received much pushback. But Rivas said some churches that had asked the UniMóvil to visit their congregations changed their minds after learning the services included birth control.
Catherine Phillips, director of the Respect Life Office at Arkansas’ Catholic diocese, said the diocese supports efforts to achieve health care equity and she’s personally interested in mobile programs that visit rural areas such as where she lives.
But Phillips said the Arkansas program’s focus on birth control, especially long-acting methods, violates the teachings of the Catholic Church. Offering these services to minors without parental consent “makes it more egregious,” she said.
Jones said that, while these programs have hefty costs and other challenges, they also have benefits that can’t be measured in numbers.
“Building community trust and making an impact in the communities most impacted by health inequities — that’s invaluable,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Twice a month, a 40-foot-long truck transformed into a mobile clinic travels the Rio Grande Valley to provide rural Texans with women’s health care, including birth control.
The clinic, called the UniMóvil, is part of the Healthy Mujeres program at the University of Texas Rio Grande Valley School of Medicine in Edinburg.
The United States has about 3000 mobile health programs. But Saul Rivas, an ob.gyn., said he wasn’t aware of any that shared the specific mission of Healthy Mujeres when he helped launch the initiative in 2017. “Mujeres” means “women” in Spanish.
It’s now part of a small but growing number of mobile programs aimed at increasing rural access to women’s health services, including long-acting reversible contraception.
There are two kinds of these highly effective methods: intrauterine devices, known as IUDs, and hormonal implants inserted into the upper arm. These birth control options can be especially difficult to obtain — or have removed — in rural areas.
“Women who want to prevent an unintended pregnancy should have whatever works best for them,” said Kelly Conroy, senior director of mobile and maternal health programs at the University of Arkansas for Medical Sciences, Little Rock.
The school is launching a mobile women’s health and contraception program in rural parts of the state in October.
Rural areas have disproportionately fewer doctors, including ob.gyns., than urban areas. And rural providers may not be able to afford to stock long-acting birth control devices or may not be trained in administering them, program leaders say.
Mobile clinics help shrink that gap in rural care, but they can be challenging to operate, said Elizabeth Jones, a senior director at the National Family Planning & Reproductive Health Association.
Money is the greatest obstacle, Jones said. The Texas program costs up to $400,000 a year. A 2020 study of 173 mobile clinics found they cost an average of more than $630,000 a year. Mobile dental programs were the most expensive, averaging more than $1 million.
While many programs launch with the help of grants, they can be difficult to sustain, especially with over a decade of decreased or stagnant funding to Title X, a federal money stream that helps low-income people receive family planning services.
For example, a mobile contraception program serving rural Pennsylvania lasted less than 3 years before closing in 2023. It shut down after losing federal funding, said a spokesperson for the clinic that ran it.
Rural mobile programs aren’t as efficient or profitable as brick-and-mortar clinics. That’s because staff members may have to make hours-long trips to reach towns where they’ll probably see fewer patients than they would at a traditional site, Jones said.
She said organizations that can’t afford mobile programs can consider setting up “pop-up clinics” at existing health and community sites in rural areas.
Maria Briones is a patient who has benefited from the Healthy Mujeres program in southern Texas. The 41-year-old day care worker was concerned because she wasn’t getting her menstrual period with her IUD.
She considered going to Mexico to have the device removed because few doctors take her insurance on the US side of the Rio Grande Valley.
But Briones learned that the UniMóvil was visiting a small Texas city about 20 minutes from her home. She told the staff there that she doesn’t want more kids but was worried about the IUD.
Briones decided to keep the device after learning it’s safe and normal not to have periods while using an IUD. She won’t get billed for her appointment with the mobile clinic, even though the university health system doesn’t take her insurance.
“They have a lot of patience, and they answered all the questions that I had,” Briones said.
IUDs and hormonal implants are highly effective and can last up to 10 years. But they’re also expensive — devices can cost more than $1,000 without insurance — and inserting an IUD can be painful.
Patient-rights advocates are also concerned that some providers pressure people to use these devices.
They say ethical birth control programs aim to empower patients to choose the contraceptive method — if any — that is best for them, instead of promoting long-acting methods in an attempt to lower birth and poverty rates. They point to the history of eugenics-inspired sterilization and even more recent incidents.
For example, an investigation by Time magazine found doctors are more likely to push Black, Latina, young, and low-income women than other patients to use long-acting birth control — and to refuse to remove the devices.
Rivas said Healthy Mujeres staffers are trained on this issue.
“Our goal isn’t necessarily to place IUDs and implants,” he said. It’s to “provide education and help patients make the best decisions for themselves.”
David Wise, a spokesperson for the University of Arkansas for Medical Sciences, said staff members with the university’s mobile program will ask patients if they want to get pregnant in the next year, and will support their choice. The Arkansas and Texas programs also remove IUDs and hormonal arm implants if patients aren’t happy with them.
The Arkansas initiative will visit 14 rural counties with four vehicles the size of food trucks that were used in previous mobile health efforts. Staffing and equipment will be covered by a 2-year, $431,000 grant from an anonymous donor, Wise said.
In addition to contraception, faculty and medical residents staffing the vehicles will offer women’s health screenings, vaccinations, prenatal care, and testing and treatment for sexually transmitted infections.
Rivas said the Texas program was inspired by a study that found that, 6 months after giving birth, 34% of surveyed Texas mothers said long-acting contraception is their preferred birth control option — but only 13% were using that method.
“We started thinking about ways to address that gap,” Rivas said.
Healthy Mujeres, which is funded through multiple grants, started with a focus on contraception. It later expanded to services such as pregnancy ultrasounds, cervical cancer screenings, and testing for sexually transmitted infections.
While the Texas and Arkansas programs can bill insurance, they also have funding to help uninsured and underinsured patients afford their services. Both use community health workers — called promotoras in largely Spanish-speaking communities like the Rio Grande Valley — to connect patients with food, transportation, additional medical services, and other needs.
They partner with organizations that locals trust, such as food pantries and community colleges, which let the mobile units set up in their parking lots. And to further increase the availability of long-acting contraception in rural areas, the universities are training their students and local providers on how to insert, remove, and get reimbursed for the devices.
One difference between the programs is dictated by state laws. The Arkansas program can provide birth control to minors without a parent or guardian’s consent. But in Texas, most minors need consent before receiving health care, including contraception.
Advocates say these initiatives might help lower the rates of unintended and teen pregnancies in both states, which are higher than the national average.
Rivas and Conroy said their programs haven’t received much pushback. But Rivas said some churches that had asked the UniMóvil to visit their congregations changed their minds after learning the services included birth control.
Catherine Phillips, director of the Respect Life Office at Arkansas’ Catholic diocese, said the diocese supports efforts to achieve health care equity and she’s personally interested in mobile programs that visit rural areas such as where she lives.
But Phillips said the Arkansas program’s focus on birth control, especially long-acting methods, violates the teachings of the Catholic Church. Offering these services to minors without parental consent “makes it more egregious,” she said.
Jones said that, while these programs have hefty costs and other challenges, they also have benefits that can’t be measured in numbers.
“Building community trust and making an impact in the communities most impacted by health inequities — that’s invaluable,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Twice a month, a 40-foot-long truck transformed into a mobile clinic travels the Rio Grande Valley to provide rural Texans with women’s health care, including birth control.
The clinic, called the UniMóvil, is part of the Healthy Mujeres program at the University of Texas Rio Grande Valley School of Medicine in Edinburg.
The United States has about 3000 mobile health programs. But Saul Rivas, an ob.gyn., said he wasn’t aware of any that shared the specific mission of Healthy Mujeres when he helped launch the initiative in 2017. “Mujeres” means “women” in Spanish.
It’s now part of a small but growing number of mobile programs aimed at increasing rural access to women’s health services, including long-acting reversible contraception.
There are two kinds of these highly effective methods: intrauterine devices, known as IUDs, and hormonal implants inserted into the upper arm. These birth control options can be especially difficult to obtain — or have removed — in rural areas.
“Women who want to prevent an unintended pregnancy should have whatever works best for them,” said Kelly Conroy, senior director of mobile and maternal health programs at the University of Arkansas for Medical Sciences, Little Rock.
The school is launching a mobile women’s health and contraception program in rural parts of the state in October.
Rural areas have disproportionately fewer doctors, including ob.gyns., than urban areas. And rural providers may not be able to afford to stock long-acting birth control devices or may not be trained in administering them, program leaders say.
Mobile clinics help shrink that gap in rural care, but they can be challenging to operate, said Elizabeth Jones, a senior director at the National Family Planning & Reproductive Health Association.
Money is the greatest obstacle, Jones said. The Texas program costs up to $400,000 a year. A 2020 study of 173 mobile clinics found they cost an average of more than $630,000 a year. Mobile dental programs were the most expensive, averaging more than $1 million.
While many programs launch with the help of grants, they can be difficult to sustain, especially with over a decade of decreased or stagnant funding to Title X, a federal money stream that helps low-income people receive family planning services.
For example, a mobile contraception program serving rural Pennsylvania lasted less than 3 years before closing in 2023. It shut down after losing federal funding, said a spokesperson for the clinic that ran it.
Rural mobile programs aren’t as efficient or profitable as brick-and-mortar clinics. That’s because staff members may have to make hours-long trips to reach towns where they’ll probably see fewer patients than they would at a traditional site, Jones said.
She said organizations that can’t afford mobile programs can consider setting up “pop-up clinics” at existing health and community sites in rural areas.
Maria Briones is a patient who has benefited from the Healthy Mujeres program in southern Texas. The 41-year-old day care worker was concerned because she wasn’t getting her menstrual period with her IUD.
She considered going to Mexico to have the device removed because few doctors take her insurance on the US side of the Rio Grande Valley.
But Briones learned that the UniMóvil was visiting a small Texas city about 20 minutes from her home. She told the staff there that she doesn’t want more kids but was worried about the IUD.
Briones decided to keep the device after learning it’s safe and normal not to have periods while using an IUD. She won’t get billed for her appointment with the mobile clinic, even though the university health system doesn’t take her insurance.
“They have a lot of patience, and they answered all the questions that I had,” Briones said.
IUDs and hormonal implants are highly effective and can last up to 10 years. But they’re also expensive — devices can cost more than $1,000 without insurance — and inserting an IUD can be painful.
Patient-rights advocates are also concerned that some providers pressure people to use these devices.
They say ethical birth control programs aim to empower patients to choose the contraceptive method — if any — that is best for them, instead of promoting long-acting methods in an attempt to lower birth and poverty rates. They point to the history of eugenics-inspired sterilization and even more recent incidents.
For example, an investigation by Time magazine found doctors are more likely to push Black, Latina, young, and low-income women than other patients to use long-acting birth control — and to refuse to remove the devices.
Rivas said Healthy Mujeres staffers are trained on this issue.
“Our goal isn’t necessarily to place IUDs and implants,” he said. It’s to “provide education and help patients make the best decisions for themselves.”
David Wise, a spokesperson for the University of Arkansas for Medical Sciences, said staff members with the university’s mobile program will ask patients if they want to get pregnant in the next year, and will support their choice. The Arkansas and Texas programs also remove IUDs and hormonal arm implants if patients aren’t happy with them.
The Arkansas initiative will visit 14 rural counties with four vehicles the size of food trucks that were used in previous mobile health efforts. Staffing and equipment will be covered by a 2-year, $431,000 grant from an anonymous donor, Wise said.
In addition to contraception, faculty and medical residents staffing the vehicles will offer women’s health screenings, vaccinations, prenatal care, and testing and treatment for sexually transmitted infections.
Rivas said the Texas program was inspired by a study that found that, 6 months after giving birth, 34% of surveyed Texas mothers said long-acting contraception is their preferred birth control option — but only 13% were using that method.
“We started thinking about ways to address that gap,” Rivas said.
Healthy Mujeres, which is funded through multiple grants, started with a focus on contraception. It later expanded to services such as pregnancy ultrasounds, cervical cancer screenings, and testing for sexually transmitted infections.
While the Texas and Arkansas programs can bill insurance, they also have funding to help uninsured and underinsured patients afford their services. Both use community health workers — called promotoras in largely Spanish-speaking communities like the Rio Grande Valley — to connect patients with food, transportation, additional medical services, and other needs.
They partner with organizations that locals trust, such as food pantries and community colleges, which let the mobile units set up in their parking lots. And to further increase the availability of long-acting contraception in rural areas, the universities are training their students and local providers on how to insert, remove, and get reimbursed for the devices.
One difference between the programs is dictated by state laws. The Arkansas program can provide birth control to minors without a parent or guardian’s consent. But in Texas, most minors need consent before receiving health care, including contraception.
Advocates say these initiatives might help lower the rates of unintended and teen pregnancies in both states, which are higher than the national average.
Rivas and Conroy said their programs haven’t received much pushback. But Rivas said some churches that had asked the UniMóvil to visit their congregations changed their minds after learning the services included birth control.
Catherine Phillips, director of the Respect Life Office at Arkansas’ Catholic diocese, said the diocese supports efforts to achieve health care equity and she’s personally interested in mobile programs that visit rural areas such as where she lives.
But Phillips said the Arkansas program’s focus on birth control, especially long-acting methods, violates the teachings of the Catholic Church. Offering these services to minors without parental consent “makes it more egregious,” she said.
Jones said that, while these programs have hefty costs and other challenges, they also have benefits that can’t be measured in numbers.
“Building community trust and making an impact in the communities most impacted by health inequities — that’s invaluable,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
The Heavy Physical and Psychological Burden of Premenstrual Dysphoric Disorder
Premenstrual disorders (PMDs), including premenstrual dysphoric disorder (PMDD), adversely affect the lives of millions of women worldwide. Most girls and women — as many as 80%-90%— will experience some premenstrual discomfort such as irritability, depressed mood, food or alcohol cravings, bloating, body aches, breast pain, constipation, or fatigue.
Diagnosable menstrual disorders include, collectively, premenstrual syndrome (PMS); PMDD, formerly called late luteal phase dysphoric disorder; and premenstrual worsening of another medical condition.
The most debilitating of these is PMDD, which has an estimated prevalence of about 4%-8% in women of reproductive age, according to obstetrician/gynecologist Hoosna Haque, MD, assistant professor of medicine at Columbia University Irving Medical Center in New York City.
“It’s difficult to be sure because this condition is underreported,” said Luu D. Ireland, MD, MPH, assistant professor of obstetrics and gynecology at UMass Memorial Medical Center in Worcester, Massachusetts. “But more women are coming forward, and there’s more discussion and media coverage of this condition.”
Occurring in the same post-follicular timeframe as PMS, PMDD takes cyclical discomfort to a more intense level, with a trifecta of affective comorbidities, somatic manifestations, and behavioral changes, all of which can seriously impair daily functioning, including work, physical activities, and personal relationships. Romantic and marital relationships can be particularly impaired.
Although recent cost figures are lacking, PMDs exact a considerable economic toll with increased direct healthcare costs from doctor visits and pharmaceuticals. A 2010 study found that US women with PMS were more likely to accrue in excess of $500 in healthcare visit costs over 2 years, and the figure would likely be higher today. PMDs also increase work/school absenteeism and reduce productivity.
Etiology
Brain areas that regulate emotion and behavior contain receptors for estrogen, progesterone, and other sex hormones, which affect the functioning of neurotransmitter systems influencing mood and thinking. Although the precise pathophysiology remains unclear, PMDD is likely multifactorial and results in a heightened sensitivity to normal fluctuations in estrogen and progesterone during the luteal phase of the menstrual cycle and dysfunction of the serotonin and gamma-aminobutyric acid neurotransmitter systems.
Patients with PMDD have lower levels of cortisol and beta-endorphins during both the follicular and luteal phases, suggesting abnormalities in the hypothalamic-pituitary-gonadal axis (HPGA), which is consistent with dysregulation in mood disorders.
Risk Factors
These include family history, past traumatic events, smoking, chronic pain syndrome, and obesity. There may be a genetic component as recent studies have suggested the involvement of the gene that codes for the serotonergic 5HT1A receptor and allelic variants of ESR1 in the development of PMS/PMDD.
A particularly concerning aspect of PMDs of any sort is their possible association with a higher risk for death from non-natural causes. In a recent Swedish study, which did not distinguish between PMDs in general and PMDD in particular, patients had an almost 60% greater risk for death from non-natural causes and nearly twice the risk for death by suicide compared with women without PMDs.
Those diagnosed with a PMD at an early age showed excess mortality, and the risk for suicide was elevated regardless of age. “These findings support the need for careful follow-up for young women with PMDs and the need for suicide prevention strategies,” wrote lead author Marion Opatowski, PhD, a medical epidemiologist at Karolinska Institutet in Stockholm, Sweden. “Women with severe PMDD should definitely be monitored for suicidal thoughts or behavior and they should have an emergency outreach plan in place,” Haque added.
Diagnosis
Although the somatic manifestations of PMDD resemble those of PMS, they are more severe and associated psychological symptoms are greater. “In my experience, PMDD symptoms can last the whole 2 weeks of the luteal phase, whereas PMS might occur a couple of days before menstruation,” said Ireland.
Symptoms include labile mood, nervousness, hopelessness, anger and aggressiveness, as well as tension and irritability. Those affected may have suicidal thoughts or even behaviors. In addition to a lethargic loss of interest in normal activities, patients with PMDD may feel paranoid, confused, exhausted, or out of control and experience insomnia or hypersomnia. They may have trouble concentrating or remembering. Some patients with PMDD may already be prone to attention-deficit/hyperactivity disorder and non–cycle-related depression, anxiety, and panic attacks.
Diagnosis is based on the presence of any five of the typical affective, somatic, or behavioral symptoms outlined above in the week before onset of menses.
“It’s important to do a careful diagnosis for PMDD and rule out other underlying conditions such as existing depressive or anxiety disorders,” said Haque. “Symptoms tend to be more intense in periods of high hormonal fluctuation such as in the postpartum and perimenopause periods. Women with PMDD should be monitored for postpartum depression.”
PMDD is considered both a gynecologic-genitourinary disorder and an affective condition.
In 2013, it was controversially included as a depressive disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Strongly advocated by some patients, psychiatrists, and pharmaceutical companies, its inclusion was criticized by psychologists and generalists, who feared it would lead to overdiagnosis and pathologization of normal female hormonal changes. Women’s advocates protested that this inclusion would stigmatize female biology and harm their advance in society and the workplace, while some doctors continued to dismiss PMDD as not a serious concern.
Treatments
In its latest clinical practice guideline on PMDs, the American College of Obstetricians and Gynecologists (ACOG), for which Ireland served as the lead author, recommends that most patients with PMDD get medical treatment and outlines the following therapies, based on varying degrees of evidence strength.
Antidepressants. These may benefit patients with strong affective symptoms. Selective serotonin reuptake inhibitors such as sertraline (Zoloft), citalopram (Celexa), escitalopram (Lexapro), or fluoxetine (Prozac) are first choices.
Antidepressants may interrupt aberrant signaling in the HPGA, the circuit linking brain and ovaries and regulating the reproductive cycle. Serotonin norepinephrine reuptake inhibitor venlafaxine (Effexor) may also improve symptoms, but other types of antidepressants have not proven effective.
“The response to these well-tolerated drugs is rapid and can happen in the first 2 days,” said Ireland. The drugs may be taken either just in the luteal period or over the month, especially by patients with chronic depression or anxiety.
Hormonal therapy. ACOG recommends the use of combined oral contraceptives (COCs), gonadotropin-releasing hormone (GnRH) agonists to induce anovulation (with combined add-back hormones), progestin-only methods, and noncontraceptive continuous estrogen formulations. It notes, however, that COCs have not been more effective than placebo in reducing depressive symptom scores.
If symptoms do not improve over two to three cycles, an alternate therapy should be considered. Haque recommends an assessment after three cycles and then yearly.
Some women in her practice take both antidepressant and hormone therapy. “Unfortunately, there are no new pharmaceutical treatments on the horizon, but we have good ones already and we would love for patients to utilize them more often,” Ireland said.
Nonsteroidal anti-inflammatory drugs. Limited evidence shows these may reduce physical symptoms such as abdominal cramps, headaches, and general body aches, as well as some mood-related symptoms, which may be an indirect effect of pain alleviation.
Surgery. For women with the most severe intractable symptoms, bilateral oophorectomy with or without hysterectomy may be a last-resort option when medical management has failed. A trial period of GnRH agonist therapy (with or without adjunctive estrogen add-back treatment) is advised before surgery to predict a patient’s response to surgical management.
Acupuncture. ACOG suggests that acupuncture may help manage physical and affective premenstrual symptoms.
Diet. The usual dietary advice for premenstrual symptoms — such as consuming less caffeine, sugar, or alcohol and eating smaller, more frequent meals — is unlikely to help women with PMDD.
Exercise. Although it has not been well studied for PMDD, aerobic exercises such as walking, swimming, and biking tend to improve mood and energy levels in general. Exercise may reduce symptoms through several pathways, including effects on beta-endorphin, cortisol, and ovarian hormone levels.
Supplements. Vitamin B6, calcium and magnesium supplements, and herbal remedies are not supported by consistent or compelling evidence of efficacy. ACOG conditionally recommends calcium supplementation of 100-200 mg/d in adults to help manage physical and affective symptoms.
A small study suggested that supplemental zinc may improve both physical and psychological symptoms.
Cognitive-behavioral therapy. This treatment aims to interrupt negative and irrational thought patterns and may include awareness and education, as well as relaxation techniques, problem-solving and coping skills, and stress management. It has been associated with small to moderate improvement in anxiety and depression, said Ireland.
Peer support. Patients should consider joining a support group. The International Association for Premenstrual Disorders can help patients connect and develop coping skills.
The bottom line is that people with strong symptomatic evidence of PMDD should have medical intervention — to the benefit of their health and quality of life. Screening for PMDD should be part of women’s wellness examinations, said Ireland. “The impact of PMDD should not be minimized or dismissed,” said Haque. “And patients need to know there are very effective treatments.”
Ireland and Haque had no competing interests with regard to their comments.
A version of this article first appeared on Medscape.com.
Premenstrual disorders (PMDs), including premenstrual dysphoric disorder (PMDD), adversely affect the lives of millions of women worldwide. Most girls and women — as many as 80%-90%— will experience some premenstrual discomfort such as irritability, depressed mood, food or alcohol cravings, bloating, body aches, breast pain, constipation, or fatigue.
Diagnosable menstrual disorders include, collectively, premenstrual syndrome (PMS); PMDD, formerly called late luteal phase dysphoric disorder; and premenstrual worsening of another medical condition.
The most debilitating of these is PMDD, which has an estimated prevalence of about 4%-8% in women of reproductive age, according to obstetrician/gynecologist Hoosna Haque, MD, assistant professor of medicine at Columbia University Irving Medical Center in New York City.
“It’s difficult to be sure because this condition is underreported,” said Luu D. Ireland, MD, MPH, assistant professor of obstetrics and gynecology at UMass Memorial Medical Center in Worcester, Massachusetts. “But more women are coming forward, and there’s more discussion and media coverage of this condition.”
Occurring in the same post-follicular timeframe as PMS, PMDD takes cyclical discomfort to a more intense level, with a trifecta of affective comorbidities, somatic manifestations, and behavioral changes, all of which can seriously impair daily functioning, including work, physical activities, and personal relationships. Romantic and marital relationships can be particularly impaired.
Although recent cost figures are lacking, PMDs exact a considerable economic toll with increased direct healthcare costs from doctor visits and pharmaceuticals. A 2010 study found that US women with PMS were more likely to accrue in excess of $500 in healthcare visit costs over 2 years, and the figure would likely be higher today. PMDs also increase work/school absenteeism and reduce productivity.
Etiology
Brain areas that regulate emotion and behavior contain receptors for estrogen, progesterone, and other sex hormones, which affect the functioning of neurotransmitter systems influencing mood and thinking. Although the precise pathophysiology remains unclear, PMDD is likely multifactorial and results in a heightened sensitivity to normal fluctuations in estrogen and progesterone during the luteal phase of the menstrual cycle and dysfunction of the serotonin and gamma-aminobutyric acid neurotransmitter systems.
Patients with PMDD have lower levels of cortisol and beta-endorphins during both the follicular and luteal phases, suggesting abnormalities in the hypothalamic-pituitary-gonadal axis (HPGA), which is consistent with dysregulation in mood disorders.
Risk Factors
These include family history, past traumatic events, smoking, chronic pain syndrome, and obesity. There may be a genetic component as recent studies have suggested the involvement of the gene that codes for the serotonergic 5HT1A receptor and allelic variants of ESR1 in the development of PMS/PMDD.
A particularly concerning aspect of PMDs of any sort is their possible association with a higher risk for death from non-natural causes. In a recent Swedish study, which did not distinguish between PMDs in general and PMDD in particular, patients had an almost 60% greater risk for death from non-natural causes and nearly twice the risk for death by suicide compared with women without PMDs.
Those diagnosed with a PMD at an early age showed excess mortality, and the risk for suicide was elevated regardless of age. “These findings support the need for careful follow-up for young women with PMDs and the need for suicide prevention strategies,” wrote lead author Marion Opatowski, PhD, a medical epidemiologist at Karolinska Institutet in Stockholm, Sweden. “Women with severe PMDD should definitely be monitored for suicidal thoughts or behavior and they should have an emergency outreach plan in place,” Haque added.
Diagnosis
Although the somatic manifestations of PMDD resemble those of PMS, they are more severe and associated psychological symptoms are greater. “In my experience, PMDD symptoms can last the whole 2 weeks of the luteal phase, whereas PMS might occur a couple of days before menstruation,” said Ireland.
Symptoms include labile mood, nervousness, hopelessness, anger and aggressiveness, as well as tension and irritability. Those affected may have suicidal thoughts or even behaviors. In addition to a lethargic loss of interest in normal activities, patients with PMDD may feel paranoid, confused, exhausted, or out of control and experience insomnia or hypersomnia. They may have trouble concentrating or remembering. Some patients with PMDD may already be prone to attention-deficit/hyperactivity disorder and non–cycle-related depression, anxiety, and panic attacks.
Diagnosis is based on the presence of any five of the typical affective, somatic, or behavioral symptoms outlined above in the week before onset of menses.
“It’s important to do a careful diagnosis for PMDD and rule out other underlying conditions such as existing depressive or anxiety disorders,” said Haque. “Symptoms tend to be more intense in periods of high hormonal fluctuation such as in the postpartum and perimenopause periods. Women with PMDD should be monitored for postpartum depression.”
PMDD is considered both a gynecologic-genitourinary disorder and an affective condition.
In 2013, it was controversially included as a depressive disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Strongly advocated by some patients, psychiatrists, and pharmaceutical companies, its inclusion was criticized by psychologists and generalists, who feared it would lead to overdiagnosis and pathologization of normal female hormonal changes. Women’s advocates protested that this inclusion would stigmatize female biology and harm their advance in society and the workplace, while some doctors continued to dismiss PMDD as not a serious concern.
Treatments
In its latest clinical practice guideline on PMDs, the American College of Obstetricians and Gynecologists (ACOG), for which Ireland served as the lead author, recommends that most patients with PMDD get medical treatment and outlines the following therapies, based on varying degrees of evidence strength.
Antidepressants. These may benefit patients with strong affective symptoms. Selective serotonin reuptake inhibitors such as sertraline (Zoloft), citalopram (Celexa), escitalopram (Lexapro), or fluoxetine (Prozac) are first choices.
Antidepressants may interrupt aberrant signaling in the HPGA, the circuit linking brain and ovaries and regulating the reproductive cycle. Serotonin norepinephrine reuptake inhibitor venlafaxine (Effexor) may also improve symptoms, but other types of antidepressants have not proven effective.
“The response to these well-tolerated drugs is rapid and can happen in the first 2 days,” said Ireland. The drugs may be taken either just in the luteal period or over the month, especially by patients with chronic depression or anxiety.
Hormonal therapy. ACOG recommends the use of combined oral contraceptives (COCs), gonadotropin-releasing hormone (GnRH) agonists to induce anovulation (with combined add-back hormones), progestin-only methods, and noncontraceptive continuous estrogen formulations. It notes, however, that COCs have not been more effective than placebo in reducing depressive symptom scores.
If symptoms do not improve over two to three cycles, an alternate therapy should be considered. Haque recommends an assessment after three cycles and then yearly.
Some women in her practice take both antidepressant and hormone therapy. “Unfortunately, there are no new pharmaceutical treatments on the horizon, but we have good ones already and we would love for patients to utilize them more often,” Ireland said.
Nonsteroidal anti-inflammatory drugs. Limited evidence shows these may reduce physical symptoms such as abdominal cramps, headaches, and general body aches, as well as some mood-related symptoms, which may be an indirect effect of pain alleviation.
Surgery. For women with the most severe intractable symptoms, bilateral oophorectomy with or without hysterectomy may be a last-resort option when medical management has failed. A trial period of GnRH agonist therapy (with or without adjunctive estrogen add-back treatment) is advised before surgery to predict a patient’s response to surgical management.
Acupuncture. ACOG suggests that acupuncture may help manage physical and affective premenstrual symptoms.
Diet. The usual dietary advice for premenstrual symptoms — such as consuming less caffeine, sugar, or alcohol and eating smaller, more frequent meals — is unlikely to help women with PMDD.
Exercise. Although it has not been well studied for PMDD, aerobic exercises such as walking, swimming, and biking tend to improve mood and energy levels in general. Exercise may reduce symptoms through several pathways, including effects on beta-endorphin, cortisol, and ovarian hormone levels.
Supplements. Vitamin B6, calcium and magnesium supplements, and herbal remedies are not supported by consistent or compelling evidence of efficacy. ACOG conditionally recommends calcium supplementation of 100-200 mg/d in adults to help manage physical and affective symptoms.
A small study suggested that supplemental zinc may improve both physical and psychological symptoms.
Cognitive-behavioral therapy. This treatment aims to interrupt negative and irrational thought patterns and may include awareness and education, as well as relaxation techniques, problem-solving and coping skills, and stress management. It has been associated with small to moderate improvement in anxiety and depression, said Ireland.
Peer support. Patients should consider joining a support group. The International Association for Premenstrual Disorders can help patients connect and develop coping skills.
The bottom line is that people with strong symptomatic evidence of PMDD should have medical intervention — to the benefit of their health and quality of life. Screening for PMDD should be part of women’s wellness examinations, said Ireland. “The impact of PMDD should not be minimized or dismissed,” said Haque. “And patients need to know there are very effective treatments.”
Ireland and Haque had no competing interests with regard to their comments.
A version of this article first appeared on Medscape.com.
Premenstrual disorders (PMDs), including premenstrual dysphoric disorder (PMDD), adversely affect the lives of millions of women worldwide. Most girls and women — as many as 80%-90%— will experience some premenstrual discomfort such as irritability, depressed mood, food or alcohol cravings, bloating, body aches, breast pain, constipation, or fatigue.
Diagnosable menstrual disorders include, collectively, premenstrual syndrome (PMS); PMDD, formerly called late luteal phase dysphoric disorder; and premenstrual worsening of another medical condition.
The most debilitating of these is PMDD, which has an estimated prevalence of about 4%-8% in women of reproductive age, according to obstetrician/gynecologist Hoosna Haque, MD, assistant professor of medicine at Columbia University Irving Medical Center in New York City.
“It’s difficult to be sure because this condition is underreported,” said Luu D. Ireland, MD, MPH, assistant professor of obstetrics and gynecology at UMass Memorial Medical Center in Worcester, Massachusetts. “But more women are coming forward, and there’s more discussion and media coverage of this condition.”
Occurring in the same post-follicular timeframe as PMS, PMDD takes cyclical discomfort to a more intense level, with a trifecta of affective comorbidities, somatic manifestations, and behavioral changes, all of which can seriously impair daily functioning, including work, physical activities, and personal relationships. Romantic and marital relationships can be particularly impaired.
Although recent cost figures are lacking, PMDs exact a considerable economic toll with increased direct healthcare costs from doctor visits and pharmaceuticals. A 2010 study found that US women with PMS were more likely to accrue in excess of $500 in healthcare visit costs over 2 years, and the figure would likely be higher today. PMDs also increase work/school absenteeism and reduce productivity.
Etiology
Brain areas that regulate emotion and behavior contain receptors for estrogen, progesterone, and other sex hormones, which affect the functioning of neurotransmitter systems influencing mood and thinking. Although the precise pathophysiology remains unclear, PMDD is likely multifactorial and results in a heightened sensitivity to normal fluctuations in estrogen and progesterone during the luteal phase of the menstrual cycle and dysfunction of the serotonin and gamma-aminobutyric acid neurotransmitter systems.
Patients with PMDD have lower levels of cortisol and beta-endorphins during both the follicular and luteal phases, suggesting abnormalities in the hypothalamic-pituitary-gonadal axis (HPGA), which is consistent with dysregulation in mood disorders.
Risk Factors
These include family history, past traumatic events, smoking, chronic pain syndrome, and obesity. There may be a genetic component as recent studies have suggested the involvement of the gene that codes for the serotonergic 5HT1A receptor and allelic variants of ESR1 in the development of PMS/PMDD.
A particularly concerning aspect of PMDs of any sort is their possible association with a higher risk for death from non-natural causes. In a recent Swedish study, which did not distinguish between PMDs in general and PMDD in particular, patients had an almost 60% greater risk for death from non-natural causes and nearly twice the risk for death by suicide compared with women without PMDs.
Those diagnosed with a PMD at an early age showed excess mortality, and the risk for suicide was elevated regardless of age. “These findings support the need for careful follow-up for young women with PMDs and the need for suicide prevention strategies,” wrote lead author Marion Opatowski, PhD, a medical epidemiologist at Karolinska Institutet in Stockholm, Sweden. “Women with severe PMDD should definitely be monitored for suicidal thoughts or behavior and they should have an emergency outreach plan in place,” Haque added.
Diagnosis
Although the somatic manifestations of PMDD resemble those of PMS, they are more severe and associated psychological symptoms are greater. “In my experience, PMDD symptoms can last the whole 2 weeks of the luteal phase, whereas PMS might occur a couple of days before menstruation,” said Ireland.
Symptoms include labile mood, nervousness, hopelessness, anger and aggressiveness, as well as tension and irritability. Those affected may have suicidal thoughts or even behaviors. In addition to a lethargic loss of interest in normal activities, patients with PMDD may feel paranoid, confused, exhausted, or out of control and experience insomnia or hypersomnia. They may have trouble concentrating or remembering. Some patients with PMDD may already be prone to attention-deficit/hyperactivity disorder and non–cycle-related depression, anxiety, and panic attacks.
Diagnosis is based on the presence of any five of the typical affective, somatic, or behavioral symptoms outlined above in the week before onset of menses.
“It’s important to do a careful diagnosis for PMDD and rule out other underlying conditions such as existing depressive or anxiety disorders,” said Haque. “Symptoms tend to be more intense in periods of high hormonal fluctuation such as in the postpartum and perimenopause periods. Women with PMDD should be monitored for postpartum depression.”
PMDD is considered both a gynecologic-genitourinary disorder and an affective condition.
In 2013, it was controversially included as a depressive disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Strongly advocated by some patients, psychiatrists, and pharmaceutical companies, its inclusion was criticized by psychologists and generalists, who feared it would lead to overdiagnosis and pathologization of normal female hormonal changes. Women’s advocates protested that this inclusion would stigmatize female biology and harm their advance in society and the workplace, while some doctors continued to dismiss PMDD as not a serious concern.
Treatments
In its latest clinical practice guideline on PMDs, the American College of Obstetricians and Gynecologists (ACOG), for which Ireland served as the lead author, recommends that most patients with PMDD get medical treatment and outlines the following therapies, based on varying degrees of evidence strength.
Antidepressants. These may benefit patients with strong affective symptoms. Selective serotonin reuptake inhibitors such as sertraline (Zoloft), citalopram (Celexa), escitalopram (Lexapro), or fluoxetine (Prozac) are first choices.
Antidepressants may interrupt aberrant signaling in the HPGA, the circuit linking brain and ovaries and regulating the reproductive cycle. Serotonin norepinephrine reuptake inhibitor venlafaxine (Effexor) may also improve symptoms, but other types of antidepressants have not proven effective.
“The response to these well-tolerated drugs is rapid and can happen in the first 2 days,” said Ireland. The drugs may be taken either just in the luteal period or over the month, especially by patients with chronic depression or anxiety.
Hormonal therapy. ACOG recommends the use of combined oral contraceptives (COCs), gonadotropin-releasing hormone (GnRH) agonists to induce anovulation (with combined add-back hormones), progestin-only methods, and noncontraceptive continuous estrogen formulations. It notes, however, that COCs have not been more effective than placebo in reducing depressive symptom scores.
If symptoms do not improve over two to three cycles, an alternate therapy should be considered. Haque recommends an assessment after three cycles and then yearly.
Some women in her practice take both antidepressant and hormone therapy. “Unfortunately, there are no new pharmaceutical treatments on the horizon, but we have good ones already and we would love for patients to utilize them more often,” Ireland said.
Nonsteroidal anti-inflammatory drugs. Limited evidence shows these may reduce physical symptoms such as abdominal cramps, headaches, and general body aches, as well as some mood-related symptoms, which may be an indirect effect of pain alleviation.
Surgery. For women with the most severe intractable symptoms, bilateral oophorectomy with or without hysterectomy may be a last-resort option when medical management has failed. A trial period of GnRH agonist therapy (with or without adjunctive estrogen add-back treatment) is advised before surgery to predict a patient’s response to surgical management.
Acupuncture. ACOG suggests that acupuncture may help manage physical and affective premenstrual symptoms.
Diet. The usual dietary advice for premenstrual symptoms — such as consuming less caffeine, sugar, or alcohol and eating smaller, more frequent meals — is unlikely to help women with PMDD.
Exercise. Although it has not been well studied for PMDD, aerobic exercises such as walking, swimming, and biking tend to improve mood and energy levels in general. Exercise may reduce symptoms through several pathways, including effects on beta-endorphin, cortisol, and ovarian hormone levels.
Supplements. Vitamin B6, calcium and magnesium supplements, and herbal remedies are not supported by consistent or compelling evidence of efficacy. ACOG conditionally recommends calcium supplementation of 100-200 mg/d in adults to help manage physical and affective symptoms.
A small study suggested that supplemental zinc may improve both physical and psychological symptoms.
Cognitive-behavioral therapy. This treatment aims to interrupt negative and irrational thought patterns and may include awareness and education, as well as relaxation techniques, problem-solving and coping skills, and stress management. It has been associated with small to moderate improvement in anxiety and depression, said Ireland.
Peer support. Patients should consider joining a support group. The International Association for Premenstrual Disorders can help patients connect and develop coping skills.
The bottom line is that people with strong symptomatic evidence of PMDD should have medical intervention — to the benefit of their health and quality of life. Screening for PMDD should be part of women’s wellness examinations, said Ireland. “The impact of PMDD should not be minimized or dismissed,” said Haque. “And patients need to know there are very effective treatments.”
Ireland and Haque had no competing interests with regard to their comments.
A version of this article first appeared on Medscape.com.
Topical JAK Inhibitor Shows Benefits in Small Frontal Fibrosing Alopecia Study
AMSTERDAM —
“This is an exciting avenue for FFA if the data are recapitulated in a larger population. It could be an important new treatment option,” said Maryanne Senna, MD, director at Lahey Hospital & Medical Center’s Hair Loss Center of Excellence, Burlington, Massachusetts, and assistant dermatology professor at Harvard Medical School, Boston, Massachusetts.
In a design characterized as “exploratory,” the trial had two parts: a randomized, double-blind, vehicle-controlled intervention for 12 weeks, followed by an open-label extension of topical delgocitinib for all participants for another 12 weeks.
The primary efficacy endpoint was change in the molecular signature of FFA inflammation at 12 weeks. Clinical improvement was monitored with both trichoscopic images capturing the numbers of hairs and follicular units at 12 weeks and clinical severity scores through week 24. In a topical cream formulation, the Janus kinase inhibitor (JAKi) delgocitinib was associated with favorable activity for both.
Some Hair Regrowth for All
“At 24 weeks, all patients achieved some degree of hair regrowth and a stabilization of disease based on hairline measurements,” Senna reported in a late-breaking news session at the 2024 European Academy of Dermatology and Venereology (EADV) Congress.
On the clinical endpoints, Senna noted an upward trajectory in clinical improvement at the completion of the study.
The 30 participants were randomly assigned in a 1:1 ratio to receive delgocitinib cream in a concentration of 20 mg/g or vehicle cream applied twice daily for 12 weeks. At the end of this double-blind period, patients on vehicle were crossed over to the active therapy, and all patients were monitored for another 12 weeks in an open-label extension.
The change from baseline in FFA biomarkers was selected as the primary endpoint based on previous work showing up-regulation in the expression of the Th1 biomarkers CXCL9, CXCL10, and interferon gamma in lesional vs nonlesional scalp in patients with FFA.
When biopsies at the end of 12 weeks in the double-blind phase of the study were compared with the baseline biopsies, researchers found a decrease in expression of the three local inflammation markers in all patients receiving the JAKi, but not in those receiving the vehicle cream. In this small patient sample, only the reduction in expression of CXCL9, a cytokine known for differentiation and promotion of leukocytes, reached statistical significance (P < .05).
But in an analysis involving the expression of multiple genes, “lesions treated with delgocitinib had a 4% improvement in normalization toward a nonlesional transcriptomic profile, while patients treated with vehicle had a 33% worsening,” Senna reported. The difference was highly significant (P < .001).
Furthermore, the decrease in total Lichen Planopilaris Activity Index and FFA severity scores were numerically and statistically greater (P = .023) in the active-treatment arm than in the vehicle arm by the end of the double-blind part of the trial, she said.
On trichoscopy, there was an increased number of hairs and follicular units at 12 weeks relative to baseline among those treated with topical delgocitinib but a reduction in those treated with vehicle.
JAKi Patients Gained Hair, Vehicle Patients Lost Hair
On the basis of hair count per square centimeter from baseline, delgocitinib-treated patients gained on average of seven hairs whereas vehicle recipients lost an average of 11 hairs at 24 weeks, Senna reported.
Patients originally treated with vehicle did improve in most outcome measures in the open-label extension of the experimental treatment after crossover, but they did not catch up to those initially randomized to delgocitinib because of further accrual of favorable changes in the active-treatment group over time.
“There were no adverse events associated with active therapy or vehicle, including application-site reactions,” Senna said. The one between-group difference was a higher rate of COVID-19, but this was greater in the control arm.
All 30 of the participants in this study were women, and all had moderate to severe disease at enrollment. The median age was 64 years. Because of the predominant population at the hair loss center, all but one of the participants were White, and one participant was Asian.
Characterizing FFA as “devastating and disfiguring,” Senna, who specializes in the care of alopecia, noted that this a difficult disease to control with the off-label strategies that are now used. The slow progress to identify treatments for FFA is illustrated by the fact that only one other double-blind and randomized trial has ever been conducted in FFA, she said.
Exploratory Study Supports Anecdotal Experience
On the basis of prior anecdotal experience with JAKi treatment for FFA, Senna said, “I do think that it is possible to get largely clear skin with this therapy.” However, she is now hoping for definitive trials to better characterize the efficacy and safety of oral and topical therapies, perhaps used sequentially to maintain clinical improvement.
In light of the limited current options, Menno de Rie, MD, PhD, professor of dermatology at the University of Amsterdam in the Netherlands, called these data “very inspiring and hopeful.” He suggested the promise of this therapy was reinforced by the upward trajectory of the biomarkers and clinical improvement over the study period.
“Any improvement in treatment options would be welcome, because we do not [have] any reliable therapies for this condition,” de Rie, who was not an investigator, said in an interview after the presentation.
Ultimately, Senna said, once effective therapy is established, the goal will be to start as early as possible in the disease process. She noted that there is evidence that prompt therapy can reverse the disorder, not just prevent progression.
“If you can get to the hair follicles before the point of no return, there is [a] chance [of] follicular rescue,” she said.
Delgocitinib cream (Anzupgo) was approved in Europe for treating chronic hand eczema in late September and is under review for the same indication in the United States.
Senna has financial relationships with Arena, Concert, Eli Lilly, Pfizer, and Leo Pharma, which provided funding for this study. de Rie reported no potential conflicts of interest.
A version of this article appeared on Medscape.com.
AMSTERDAM —
“This is an exciting avenue for FFA if the data are recapitulated in a larger population. It could be an important new treatment option,” said Maryanne Senna, MD, director at Lahey Hospital & Medical Center’s Hair Loss Center of Excellence, Burlington, Massachusetts, and assistant dermatology professor at Harvard Medical School, Boston, Massachusetts.
In a design characterized as “exploratory,” the trial had two parts: a randomized, double-blind, vehicle-controlled intervention for 12 weeks, followed by an open-label extension of topical delgocitinib for all participants for another 12 weeks.
The primary efficacy endpoint was change in the molecular signature of FFA inflammation at 12 weeks. Clinical improvement was monitored with both trichoscopic images capturing the numbers of hairs and follicular units at 12 weeks and clinical severity scores through week 24. In a topical cream formulation, the Janus kinase inhibitor (JAKi) delgocitinib was associated with favorable activity for both.
Some Hair Regrowth for All
“At 24 weeks, all patients achieved some degree of hair regrowth and a stabilization of disease based on hairline measurements,” Senna reported in a late-breaking news session at the 2024 European Academy of Dermatology and Venereology (EADV) Congress.
On the clinical endpoints, Senna noted an upward trajectory in clinical improvement at the completion of the study.
The 30 participants were randomly assigned in a 1:1 ratio to receive delgocitinib cream in a concentration of 20 mg/g or vehicle cream applied twice daily for 12 weeks. At the end of this double-blind period, patients on vehicle were crossed over to the active therapy, and all patients were monitored for another 12 weeks in an open-label extension.
The change from baseline in FFA biomarkers was selected as the primary endpoint based on previous work showing up-regulation in the expression of the Th1 biomarkers CXCL9, CXCL10, and interferon gamma in lesional vs nonlesional scalp in patients with FFA.
When biopsies at the end of 12 weeks in the double-blind phase of the study were compared with the baseline biopsies, researchers found a decrease in expression of the three local inflammation markers in all patients receiving the JAKi, but not in those receiving the vehicle cream. In this small patient sample, only the reduction in expression of CXCL9, a cytokine known for differentiation and promotion of leukocytes, reached statistical significance (P < .05).
But in an analysis involving the expression of multiple genes, “lesions treated with delgocitinib had a 4% improvement in normalization toward a nonlesional transcriptomic profile, while patients treated with vehicle had a 33% worsening,” Senna reported. The difference was highly significant (P < .001).
Furthermore, the decrease in total Lichen Planopilaris Activity Index and FFA severity scores were numerically and statistically greater (P = .023) in the active-treatment arm than in the vehicle arm by the end of the double-blind part of the trial, she said.
On trichoscopy, there was an increased number of hairs and follicular units at 12 weeks relative to baseline among those treated with topical delgocitinib but a reduction in those treated with vehicle.
JAKi Patients Gained Hair, Vehicle Patients Lost Hair
On the basis of hair count per square centimeter from baseline, delgocitinib-treated patients gained on average of seven hairs whereas vehicle recipients lost an average of 11 hairs at 24 weeks, Senna reported.
Patients originally treated with vehicle did improve in most outcome measures in the open-label extension of the experimental treatment after crossover, but they did not catch up to those initially randomized to delgocitinib because of further accrual of favorable changes in the active-treatment group over time.
“There were no adverse events associated with active therapy or vehicle, including application-site reactions,” Senna said. The one between-group difference was a higher rate of COVID-19, but this was greater in the control arm.
All 30 of the participants in this study were women, and all had moderate to severe disease at enrollment. The median age was 64 years. Because of the predominant population at the hair loss center, all but one of the participants were White, and one participant was Asian.
Characterizing FFA as “devastating and disfiguring,” Senna, who specializes in the care of alopecia, noted that this a difficult disease to control with the off-label strategies that are now used. The slow progress to identify treatments for FFA is illustrated by the fact that only one other double-blind and randomized trial has ever been conducted in FFA, she said.
Exploratory Study Supports Anecdotal Experience
On the basis of prior anecdotal experience with JAKi treatment for FFA, Senna said, “I do think that it is possible to get largely clear skin with this therapy.” However, she is now hoping for definitive trials to better characterize the efficacy and safety of oral and topical therapies, perhaps used sequentially to maintain clinical improvement.
In light of the limited current options, Menno de Rie, MD, PhD, professor of dermatology at the University of Amsterdam in the Netherlands, called these data “very inspiring and hopeful.” He suggested the promise of this therapy was reinforced by the upward trajectory of the biomarkers and clinical improvement over the study period.
“Any improvement in treatment options would be welcome, because we do not [have] any reliable therapies for this condition,” de Rie, who was not an investigator, said in an interview after the presentation.
Ultimately, Senna said, once effective therapy is established, the goal will be to start as early as possible in the disease process. She noted that there is evidence that prompt therapy can reverse the disorder, not just prevent progression.
“If you can get to the hair follicles before the point of no return, there is [a] chance [of] follicular rescue,” she said.
Delgocitinib cream (Anzupgo) was approved in Europe for treating chronic hand eczema in late September and is under review for the same indication in the United States.
Senna has financial relationships with Arena, Concert, Eli Lilly, Pfizer, and Leo Pharma, which provided funding for this study. de Rie reported no potential conflicts of interest.
A version of this article appeared on Medscape.com.
AMSTERDAM —
“This is an exciting avenue for FFA if the data are recapitulated in a larger population. It could be an important new treatment option,” said Maryanne Senna, MD, director at Lahey Hospital & Medical Center’s Hair Loss Center of Excellence, Burlington, Massachusetts, and assistant dermatology professor at Harvard Medical School, Boston, Massachusetts.
In a design characterized as “exploratory,” the trial had two parts: a randomized, double-blind, vehicle-controlled intervention for 12 weeks, followed by an open-label extension of topical delgocitinib for all participants for another 12 weeks.
The primary efficacy endpoint was change in the molecular signature of FFA inflammation at 12 weeks. Clinical improvement was monitored with both trichoscopic images capturing the numbers of hairs and follicular units at 12 weeks and clinical severity scores through week 24. In a topical cream formulation, the Janus kinase inhibitor (JAKi) delgocitinib was associated with favorable activity for both.
Some Hair Regrowth for All
“At 24 weeks, all patients achieved some degree of hair regrowth and a stabilization of disease based on hairline measurements,” Senna reported in a late-breaking news session at the 2024 European Academy of Dermatology and Venereology (EADV) Congress.
On the clinical endpoints, Senna noted an upward trajectory in clinical improvement at the completion of the study.
The 30 participants were randomly assigned in a 1:1 ratio to receive delgocitinib cream in a concentration of 20 mg/g or vehicle cream applied twice daily for 12 weeks. At the end of this double-blind period, patients on vehicle were crossed over to the active therapy, and all patients were monitored for another 12 weeks in an open-label extension.
The change from baseline in FFA biomarkers was selected as the primary endpoint based on previous work showing up-regulation in the expression of the Th1 biomarkers CXCL9, CXCL10, and interferon gamma in lesional vs nonlesional scalp in patients with FFA.
When biopsies at the end of 12 weeks in the double-blind phase of the study were compared with the baseline biopsies, researchers found a decrease in expression of the three local inflammation markers in all patients receiving the JAKi, but not in those receiving the vehicle cream. In this small patient sample, only the reduction in expression of CXCL9, a cytokine known for differentiation and promotion of leukocytes, reached statistical significance (P < .05).
But in an analysis involving the expression of multiple genes, “lesions treated with delgocitinib had a 4% improvement in normalization toward a nonlesional transcriptomic profile, while patients treated with vehicle had a 33% worsening,” Senna reported. The difference was highly significant (P < .001).
Furthermore, the decrease in total Lichen Planopilaris Activity Index and FFA severity scores were numerically and statistically greater (P = .023) in the active-treatment arm than in the vehicle arm by the end of the double-blind part of the trial, she said.
On trichoscopy, there was an increased number of hairs and follicular units at 12 weeks relative to baseline among those treated with topical delgocitinib but a reduction in those treated with vehicle.
JAKi Patients Gained Hair, Vehicle Patients Lost Hair
On the basis of hair count per square centimeter from baseline, delgocitinib-treated patients gained on average of seven hairs whereas vehicle recipients lost an average of 11 hairs at 24 weeks, Senna reported.
Patients originally treated with vehicle did improve in most outcome measures in the open-label extension of the experimental treatment after crossover, but they did not catch up to those initially randomized to delgocitinib because of further accrual of favorable changes in the active-treatment group over time.
“There were no adverse events associated with active therapy or vehicle, including application-site reactions,” Senna said. The one between-group difference was a higher rate of COVID-19, but this was greater in the control arm.
All 30 of the participants in this study were women, and all had moderate to severe disease at enrollment. The median age was 64 years. Because of the predominant population at the hair loss center, all but one of the participants were White, and one participant was Asian.
Characterizing FFA as “devastating and disfiguring,” Senna, who specializes in the care of alopecia, noted that this a difficult disease to control with the off-label strategies that are now used. The slow progress to identify treatments for FFA is illustrated by the fact that only one other double-blind and randomized trial has ever been conducted in FFA, she said.
Exploratory Study Supports Anecdotal Experience
On the basis of prior anecdotal experience with JAKi treatment for FFA, Senna said, “I do think that it is possible to get largely clear skin with this therapy.” However, she is now hoping for definitive trials to better characterize the efficacy and safety of oral and topical therapies, perhaps used sequentially to maintain clinical improvement.
In light of the limited current options, Menno de Rie, MD, PhD, professor of dermatology at the University of Amsterdam in the Netherlands, called these data “very inspiring and hopeful.” He suggested the promise of this therapy was reinforced by the upward trajectory of the biomarkers and clinical improvement over the study period.
“Any improvement in treatment options would be welcome, because we do not [have] any reliable therapies for this condition,” de Rie, who was not an investigator, said in an interview after the presentation.
Ultimately, Senna said, once effective therapy is established, the goal will be to start as early as possible in the disease process. She noted that there is evidence that prompt therapy can reverse the disorder, not just prevent progression.
“If you can get to the hair follicles before the point of no return, there is [a] chance [of] follicular rescue,” she said.
Delgocitinib cream (Anzupgo) was approved in Europe for treating chronic hand eczema in late September and is under review for the same indication in the United States.
Senna has financial relationships with Arena, Concert, Eli Lilly, Pfizer, and Leo Pharma, which provided funding for this study. de Rie reported no potential conflicts of interest.
A version of this article appeared on Medscape.com.
FROM EADV 2024
ACOG Updates Breast Cancer Screening Guidelines
The American College of Obstetricians and Gynecologists (ACOG) has updated its breast cancer screening guidelines, recommending that individuals at an average risk for breast cancer initiate mammography screening at age 40. This change reflects evolving evidence that starting earlier screening yields greater net benefits in reducing breast cancer mortality, particularly for certain racial groups with higher risk factors.
Breast cancer is the second leading cause of cancer deaths in American women overall and the leading cause of cancer deaths among Black and Hispanic women. Although mammography has long been recognized as a life-saving tool by detecting cancer early, there has been debate on when screening should begin due to concerns about overdiagnosis, false positives, and potential harms such as unnecessary biopsies.
Recent evidence has prompted ACOG to revise its recommendation for individuals assigned female at birth, including cisgender women, transgender men, and nonbinary individuals. This updated guidance includes individuals with dense breast tissue or a family history of breast cancer but excludes those with higher risk factors, such as a personal history of breast cancer or previous high-risk lesion on a breast biopsy, genetic mutations linked to higher cancer risk, or a history of high-dose radiation therapy to their chest at a young age.
Under the new guidelines, routine screening mammography should start at age 40 and can be performed annually or every 2 years, based on an informed, shared decision-making process that considers the benefits and potential harms of frequent screening.
Previously, ACOG recommended initiating screening between ages 40 and 50, depending on individual risk factors and preferences, with screening required by age 50 at the latest. However, several factors, including an increasing incidence of breast cancer in younger women, have influenced the decision to lower the recommended starting age.
Increasing Incidence Among Younger Women
Between 2015 and 2019, the incidence of invasive breast cancer in women aged 40-49 years increased by approximately 2% per year.
“There has been a concerning trend of increasing breast cancer diagnoses among women in their 40s, and new data shows that earlier screening could make a significant difference in decreasing breast cancer deaths,” said Eve Zaritsky, MD, FACOG, coauthor of the clinical practice update. “While screening can sometimes cause anxiety for people and even unnecessary follow-up, the benefits of diagnosing breast cancer earlier outweigh those risks enough to warrant starting to get mammograms at age 40.”
Studies commissioned by the US Preventive Services Task Force (USPSTF) show that starting mammography at age 40 provides a greater overall benefit than beginning at age 50. Early screening reduces the number of breast cancer deaths and increases life years gained when weighed against the harms of false positives, overdiagnosis, and benign biopsies.
Addressing Health Inequities
The benefits of earlier screening are expected to be particularly significant for Black women, who have disproportionately high mortality rates from breast cancer. Even though Black women have a lower overall incidence of breast cancer than White women, they have a 40% higher 5-year age-adjusted mortality rate from the disease and a 45% increased incidence of invasive breast cancer before age 50. Black women are also more likely to be diagnosed with aggressive subtypes, such as triple-negative breast cancer, which is harder to detect and treat and occurs at younger ages.
Racial disparities in breast cancer outcomes are deeply rooted in inequities in social determinants of health, such as access to care, housing, and environmental conditions. Black women are also less likely to receive timely or comprehensive treatment than White women, which contributes to worse survival rates even after adjusting for socioeconomic factors and insurance status.
“Our updated recommendation addresses important inequities in breast cancer diagnosis, treatment, and death, and we hope that the earlier initiation of mammography screening across the board will have a great net benefit in outcomes for Black women especially, who have been shown to have the poorest outcomes when it comes to breast cancer, in part because of long-standing inequities in social determinants of health,” added coauthor Cherie C. Hill, MD, FACOG.
ACOG’s updated recommendation aligns with that of other leading organizations, including the USPSTF, the National Comprehensive Cancer Network, the American College of Radiology, and the Society of Breast Imaging. This growing consensus among experts is expected to reduce confusion among clinicians and patients regarding when to begin screening, thus improving screening rates in individuals in the 40- to 49-year age group.
Zaritsky and Hill reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
The American College of Obstetricians and Gynecologists (ACOG) has updated its breast cancer screening guidelines, recommending that individuals at an average risk for breast cancer initiate mammography screening at age 40. This change reflects evolving evidence that starting earlier screening yields greater net benefits in reducing breast cancer mortality, particularly for certain racial groups with higher risk factors.
Breast cancer is the second leading cause of cancer deaths in American women overall and the leading cause of cancer deaths among Black and Hispanic women. Although mammography has long been recognized as a life-saving tool by detecting cancer early, there has been debate on when screening should begin due to concerns about overdiagnosis, false positives, and potential harms such as unnecessary biopsies.
Recent evidence has prompted ACOG to revise its recommendation for individuals assigned female at birth, including cisgender women, transgender men, and nonbinary individuals. This updated guidance includes individuals with dense breast tissue or a family history of breast cancer but excludes those with higher risk factors, such as a personal history of breast cancer or previous high-risk lesion on a breast biopsy, genetic mutations linked to higher cancer risk, or a history of high-dose radiation therapy to their chest at a young age.
Under the new guidelines, routine screening mammography should start at age 40 and can be performed annually or every 2 years, based on an informed, shared decision-making process that considers the benefits and potential harms of frequent screening.
Previously, ACOG recommended initiating screening between ages 40 and 50, depending on individual risk factors and preferences, with screening required by age 50 at the latest. However, several factors, including an increasing incidence of breast cancer in younger women, have influenced the decision to lower the recommended starting age.
Increasing Incidence Among Younger Women
Between 2015 and 2019, the incidence of invasive breast cancer in women aged 40-49 years increased by approximately 2% per year.
“There has been a concerning trend of increasing breast cancer diagnoses among women in their 40s, and new data shows that earlier screening could make a significant difference in decreasing breast cancer deaths,” said Eve Zaritsky, MD, FACOG, coauthor of the clinical practice update. “While screening can sometimes cause anxiety for people and even unnecessary follow-up, the benefits of diagnosing breast cancer earlier outweigh those risks enough to warrant starting to get mammograms at age 40.”
Studies commissioned by the US Preventive Services Task Force (USPSTF) show that starting mammography at age 40 provides a greater overall benefit than beginning at age 50. Early screening reduces the number of breast cancer deaths and increases life years gained when weighed against the harms of false positives, overdiagnosis, and benign biopsies.
Addressing Health Inequities
The benefits of earlier screening are expected to be particularly significant for Black women, who have disproportionately high mortality rates from breast cancer. Even though Black women have a lower overall incidence of breast cancer than White women, they have a 40% higher 5-year age-adjusted mortality rate from the disease and a 45% increased incidence of invasive breast cancer before age 50. Black women are also more likely to be diagnosed with aggressive subtypes, such as triple-negative breast cancer, which is harder to detect and treat and occurs at younger ages.
Racial disparities in breast cancer outcomes are deeply rooted in inequities in social determinants of health, such as access to care, housing, and environmental conditions. Black women are also less likely to receive timely or comprehensive treatment than White women, which contributes to worse survival rates even after adjusting for socioeconomic factors and insurance status.
“Our updated recommendation addresses important inequities in breast cancer diagnosis, treatment, and death, and we hope that the earlier initiation of mammography screening across the board will have a great net benefit in outcomes for Black women especially, who have been shown to have the poorest outcomes when it comes to breast cancer, in part because of long-standing inequities in social determinants of health,” added coauthor Cherie C. Hill, MD, FACOG.
ACOG’s updated recommendation aligns with that of other leading organizations, including the USPSTF, the National Comprehensive Cancer Network, the American College of Radiology, and the Society of Breast Imaging. This growing consensus among experts is expected to reduce confusion among clinicians and patients regarding when to begin screening, thus improving screening rates in individuals in the 40- to 49-year age group.
Zaritsky and Hill reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
The American College of Obstetricians and Gynecologists (ACOG) has updated its breast cancer screening guidelines, recommending that individuals at an average risk for breast cancer initiate mammography screening at age 40. This change reflects evolving evidence that starting earlier screening yields greater net benefits in reducing breast cancer mortality, particularly for certain racial groups with higher risk factors.
Breast cancer is the second leading cause of cancer deaths in American women overall and the leading cause of cancer deaths among Black and Hispanic women. Although mammography has long been recognized as a life-saving tool by detecting cancer early, there has been debate on when screening should begin due to concerns about overdiagnosis, false positives, and potential harms such as unnecessary biopsies.
Recent evidence has prompted ACOG to revise its recommendation for individuals assigned female at birth, including cisgender women, transgender men, and nonbinary individuals. This updated guidance includes individuals with dense breast tissue or a family history of breast cancer but excludes those with higher risk factors, such as a personal history of breast cancer or previous high-risk lesion on a breast biopsy, genetic mutations linked to higher cancer risk, or a history of high-dose radiation therapy to their chest at a young age.
Under the new guidelines, routine screening mammography should start at age 40 and can be performed annually or every 2 years, based on an informed, shared decision-making process that considers the benefits and potential harms of frequent screening.
Previously, ACOG recommended initiating screening between ages 40 and 50, depending on individual risk factors and preferences, with screening required by age 50 at the latest. However, several factors, including an increasing incidence of breast cancer in younger women, have influenced the decision to lower the recommended starting age.
Increasing Incidence Among Younger Women
Between 2015 and 2019, the incidence of invasive breast cancer in women aged 40-49 years increased by approximately 2% per year.
“There has been a concerning trend of increasing breast cancer diagnoses among women in their 40s, and new data shows that earlier screening could make a significant difference in decreasing breast cancer deaths,” said Eve Zaritsky, MD, FACOG, coauthor of the clinical practice update. “While screening can sometimes cause anxiety for people and even unnecessary follow-up, the benefits of diagnosing breast cancer earlier outweigh those risks enough to warrant starting to get mammograms at age 40.”
Studies commissioned by the US Preventive Services Task Force (USPSTF) show that starting mammography at age 40 provides a greater overall benefit than beginning at age 50. Early screening reduces the number of breast cancer deaths and increases life years gained when weighed against the harms of false positives, overdiagnosis, and benign biopsies.
Addressing Health Inequities
The benefits of earlier screening are expected to be particularly significant for Black women, who have disproportionately high mortality rates from breast cancer. Even though Black women have a lower overall incidence of breast cancer than White women, they have a 40% higher 5-year age-adjusted mortality rate from the disease and a 45% increased incidence of invasive breast cancer before age 50. Black women are also more likely to be diagnosed with aggressive subtypes, such as triple-negative breast cancer, which is harder to detect and treat and occurs at younger ages.
Racial disparities in breast cancer outcomes are deeply rooted in inequities in social determinants of health, such as access to care, housing, and environmental conditions. Black women are also less likely to receive timely or comprehensive treatment than White women, which contributes to worse survival rates even after adjusting for socioeconomic factors and insurance status.
“Our updated recommendation addresses important inequities in breast cancer diagnosis, treatment, and death, and we hope that the earlier initiation of mammography screening across the board will have a great net benefit in outcomes for Black women especially, who have been shown to have the poorest outcomes when it comes to breast cancer, in part because of long-standing inequities in social determinants of health,” added coauthor Cherie C. Hill, MD, FACOG.
ACOG’s updated recommendation aligns with that of other leading organizations, including the USPSTF, the National Comprehensive Cancer Network, the American College of Radiology, and the Society of Breast Imaging. This growing consensus among experts is expected to reduce confusion among clinicians and patients regarding when to begin screening, thus improving screening rates in individuals in the 40- to 49-year age group.
Zaritsky and Hill reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Does the Road to Treating Endometriosis Start in the Gut?
Researchers may be on track to develop a much-needed tool for studying endometriosis: A noninvasive stool test that could replace the current gold standard of laparoscopy.
Their approach, which focuses on the link between the gut microbiome and endometriosis, also identified a bacterial metabolite they said might be developed as an oral medication for the condition, which affects at least 11% of women.
In previous research, Rama Kommagani, PhD, an associate professor in the Department of Pathology & Immunology at Baylor College of Medicine in Houston, Texas, worked with a mouse model in which endometrial tissue from donor rodents was injected into the peritoneal space of healthy rodents to induce the disorder.
Transfer of fecal microbiota from mice with endometriosis to those without the condition induced the trademark lesions, suggesting the microbiome influences the development of endometriosis. Treating the animals with the antibiotic metronidazole inhibited the progression of endometrial lesions.
Kommagani speculated the microbes release metabolites that stimulate the growth of the endometrial lesions. “Bad bacteria release metabolites, you know, which actually promote the disease,” Kommagani said. “But the good bacteria might release some protective metabolites such as short-chain fatty acids.”
In a new study, Kommagani and his colleagues sought to identify a unique profile of bacteria-derived metabolites that could reliably diagnose endometriosis. Using stool specimens from 18 women with the condition and 31 without the disease, his team conducted whole metabolic profiling of the gut microbiota.
After identifying hundreds of metabolites in the samples, further analysis revealed a subset of 12 metabolites that consistently differentiated women with and without endometriosis.
These findings led to more questions. “If a metabolite is lower in women with endometriosis, does it have any functional relevance?” Kommagani said.
One candidate was 4-hydroxyindole (4HI), which was found in lower levels in the stool of patients. This substance is a little-understood derivative of its parent compound, indole, which occurs naturally in plants and has a wide range of therapeutic uses.
Using a mouse model, Kommagani’s lab demonstrated that feeding mice 4HI before receiving an endometrial transplant prevented the development of lesions typical of endometriosis. Mice given 4HI after they had developed endometriosis showed regression of lesions and decreased response to painful stimuli.
“In a nutshell, we found a specific set of bacterial metabolites in stool, which could be used towards a noninvasive diagnostic test,” Kommagani said. “But we also found this distinct, specific metabolite that could be used as a therapeutic molecule.”
Tatnai Burnett, MD, an assistant professor of obstetrics and gynecology at Mayo Clinic in Rochester, Minnesota, who is not associated with the study, said a noninvasive test would have several advantages over the current methods of diagnosing endometriosis. Clinicians could detect and treat women earlier in the course of their disease. Secondly, a test with sufficient negative predictive value would be helpful in deciding whether to initiate treatment with hormones or other oral medications or go straight to surgery. “I would choose not to do a surgery if I knew with enough certainty that I wasn’t going to find anything,” said Burnett.
Lastly, a test that was quantitative and showed a response to treatment could be used as a disease activity marker to monitor the course of someone’s treatment.
But Burnett said more data on the approach are necessary. “This is a fairly small study, as it goes, for developing a screening test,” he said. “We need to see what its positive predictive value, negative predictive value, sensitivity, and specificity are in a bigger group.”
The road to a cure is even longer than the path to developing a screening test. Kommagani’s lab is now conducting more studies in mice to elucidate the pharmacokinetics and toxicology of 4HI before human trials can be attempted.
And as Burnett pointed out, although mouse models are great for experimentation and generating hypotheses, “We’ve seen way too many times in the past where something’s really exciting in a mouse model or a rat model or a monkey model, and it just doesn’t pan out in humans.”
Kommagani received funding from National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development grants (R01HD102680, R01HD104813) and a Research Scholar Grant from the American Cancer Society. Burnett reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Researchers may be on track to develop a much-needed tool for studying endometriosis: A noninvasive stool test that could replace the current gold standard of laparoscopy.
Their approach, which focuses on the link between the gut microbiome and endometriosis, also identified a bacterial metabolite they said might be developed as an oral medication for the condition, which affects at least 11% of women.
In previous research, Rama Kommagani, PhD, an associate professor in the Department of Pathology & Immunology at Baylor College of Medicine in Houston, Texas, worked with a mouse model in which endometrial tissue from donor rodents was injected into the peritoneal space of healthy rodents to induce the disorder.
Transfer of fecal microbiota from mice with endometriosis to those without the condition induced the trademark lesions, suggesting the microbiome influences the development of endometriosis. Treating the animals with the antibiotic metronidazole inhibited the progression of endometrial lesions.
Kommagani speculated the microbes release metabolites that stimulate the growth of the endometrial lesions. “Bad bacteria release metabolites, you know, which actually promote the disease,” Kommagani said. “But the good bacteria might release some protective metabolites such as short-chain fatty acids.”
In a new study, Kommagani and his colleagues sought to identify a unique profile of bacteria-derived metabolites that could reliably diagnose endometriosis. Using stool specimens from 18 women with the condition and 31 without the disease, his team conducted whole metabolic profiling of the gut microbiota.
After identifying hundreds of metabolites in the samples, further analysis revealed a subset of 12 metabolites that consistently differentiated women with and without endometriosis.
These findings led to more questions. “If a metabolite is lower in women with endometriosis, does it have any functional relevance?” Kommagani said.
One candidate was 4-hydroxyindole (4HI), which was found in lower levels in the stool of patients. This substance is a little-understood derivative of its parent compound, indole, which occurs naturally in plants and has a wide range of therapeutic uses.
Using a mouse model, Kommagani’s lab demonstrated that feeding mice 4HI before receiving an endometrial transplant prevented the development of lesions typical of endometriosis. Mice given 4HI after they had developed endometriosis showed regression of lesions and decreased response to painful stimuli.
“In a nutshell, we found a specific set of bacterial metabolites in stool, which could be used towards a noninvasive diagnostic test,” Kommagani said. “But we also found this distinct, specific metabolite that could be used as a therapeutic molecule.”
Tatnai Burnett, MD, an assistant professor of obstetrics and gynecology at Mayo Clinic in Rochester, Minnesota, who is not associated with the study, said a noninvasive test would have several advantages over the current methods of diagnosing endometriosis. Clinicians could detect and treat women earlier in the course of their disease. Secondly, a test with sufficient negative predictive value would be helpful in deciding whether to initiate treatment with hormones or other oral medications or go straight to surgery. “I would choose not to do a surgery if I knew with enough certainty that I wasn’t going to find anything,” said Burnett.
Lastly, a test that was quantitative and showed a response to treatment could be used as a disease activity marker to monitor the course of someone’s treatment.
But Burnett said more data on the approach are necessary. “This is a fairly small study, as it goes, for developing a screening test,” he said. “We need to see what its positive predictive value, negative predictive value, sensitivity, and specificity are in a bigger group.”
The road to a cure is even longer than the path to developing a screening test. Kommagani’s lab is now conducting more studies in mice to elucidate the pharmacokinetics and toxicology of 4HI before human trials can be attempted.
And as Burnett pointed out, although mouse models are great for experimentation and generating hypotheses, “We’ve seen way too many times in the past where something’s really exciting in a mouse model or a rat model or a monkey model, and it just doesn’t pan out in humans.”
Kommagani received funding from National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development grants (R01HD102680, R01HD104813) and a Research Scholar Grant from the American Cancer Society. Burnett reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Researchers may be on track to develop a much-needed tool for studying endometriosis: A noninvasive stool test that could replace the current gold standard of laparoscopy.
Their approach, which focuses on the link between the gut microbiome and endometriosis, also identified a bacterial metabolite they said might be developed as an oral medication for the condition, which affects at least 11% of women.
In previous research, Rama Kommagani, PhD, an associate professor in the Department of Pathology & Immunology at Baylor College of Medicine in Houston, Texas, worked with a mouse model in which endometrial tissue from donor rodents was injected into the peritoneal space of healthy rodents to induce the disorder.
Transfer of fecal microbiota from mice with endometriosis to those without the condition induced the trademark lesions, suggesting the microbiome influences the development of endometriosis. Treating the animals with the antibiotic metronidazole inhibited the progression of endometrial lesions.
Kommagani speculated the microbes release metabolites that stimulate the growth of the endometrial lesions. “Bad bacteria release metabolites, you know, which actually promote the disease,” Kommagani said. “But the good bacteria might release some protective metabolites such as short-chain fatty acids.”
In a new study, Kommagani and his colleagues sought to identify a unique profile of bacteria-derived metabolites that could reliably diagnose endometriosis. Using stool specimens from 18 women with the condition and 31 without the disease, his team conducted whole metabolic profiling of the gut microbiota.
After identifying hundreds of metabolites in the samples, further analysis revealed a subset of 12 metabolites that consistently differentiated women with and without endometriosis.
These findings led to more questions. “If a metabolite is lower in women with endometriosis, does it have any functional relevance?” Kommagani said.
One candidate was 4-hydroxyindole (4HI), which was found in lower levels in the stool of patients. This substance is a little-understood derivative of its parent compound, indole, which occurs naturally in plants and has a wide range of therapeutic uses.
Using a mouse model, Kommagani’s lab demonstrated that feeding mice 4HI before receiving an endometrial transplant prevented the development of lesions typical of endometriosis. Mice given 4HI after they had developed endometriosis showed regression of lesions and decreased response to painful stimuli.
“In a nutshell, we found a specific set of bacterial metabolites in stool, which could be used towards a noninvasive diagnostic test,” Kommagani said. “But we also found this distinct, specific metabolite that could be used as a therapeutic molecule.”
Tatnai Burnett, MD, an assistant professor of obstetrics and gynecology at Mayo Clinic in Rochester, Minnesota, who is not associated with the study, said a noninvasive test would have several advantages over the current methods of diagnosing endometriosis. Clinicians could detect and treat women earlier in the course of their disease. Secondly, a test with sufficient negative predictive value would be helpful in deciding whether to initiate treatment with hormones or other oral medications or go straight to surgery. “I would choose not to do a surgery if I knew with enough certainty that I wasn’t going to find anything,” said Burnett.
Lastly, a test that was quantitative and showed a response to treatment could be used as a disease activity marker to monitor the course of someone’s treatment.
But Burnett said more data on the approach are necessary. “This is a fairly small study, as it goes, for developing a screening test,” he said. “We need to see what its positive predictive value, negative predictive value, sensitivity, and specificity are in a bigger group.”
The road to a cure is even longer than the path to developing a screening test. Kommagani’s lab is now conducting more studies in mice to elucidate the pharmacokinetics and toxicology of 4HI before human trials can be attempted.
And as Burnett pointed out, although mouse models are great for experimentation and generating hypotheses, “We’ve seen way too many times in the past where something’s really exciting in a mouse model or a rat model or a monkey model, and it just doesn’t pan out in humans.”
Kommagani received funding from National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development grants (R01HD102680, R01HD104813) and a Research Scholar Grant from the American Cancer Society. Burnett reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Is BMI Underestimating Breast Cancer Risk in Postmenopausal Women?
TOPLINE:
Accurate body fat measures are crucial for effective cancer prevention.
METHODOLOGY:
- Researchers conducted a case-control study including 1033 breast cancer cases and 1143 postmenopausal population controls from the MCC-Spain study.
- Participants were aged 20-85 years. BMI was calculated as the ratio of weight to height squared and categorized using World Health Organization standards: < 25, 25-29.9, 30-34.9, and ≥ 35.
- CUN-BAE was calculated using a specific equation and categorized according to the estimated percentage of body fat: < 35%, 35%-39.9%, 40%-44.9%, and ≥ 45%.
- Odds ratios (ORs) were estimated with 95% CIs for both measures (BMI and CUN-BAE) for breast cancer cases using unconditional logistic regression.
TAKEAWAY:
- Excess body weight attributable to the risk for breast cancer was 23% when assessed using a BMI value > 30 and 38% when assessed using a CUN-BAE value > 40% body fat.
- Hormone receptor stratification showed that these differences in population-attributable fractions were only observed in hormone receptor–positive cases, with an estimated burden of 19.9% for BMI and 41.9% for CUN-BAE.
- The highest categories of CUN-BAE showed an increase in the risk for postmenopausal breast cancer (OR, 2.13 for body fat ≥ 45% compared with the reference category < 35%).
- No similar trend was observed for BMI, as the gradient declined after a BMI ≥ 35.
IN PRACTICE:
“The results of our study indicate that excess body fat is a significant risk factor for hormone receptor–positive breast cancer in postmenopausal women. Our findings suggest that the population impact could be underestimated when using traditional BMI estimates, and that more accurate measures of body fat, such as CUN-BAE, should be considered,” the authors of the study wrote.
SOURCE:
This study was led by Verónica Dávila-Batista, University of Las Palmas de Gran Canaria in Las Palmas de Gran Canaria, Spain. It was published online in Journal of Epidemiology and Community Health.
LIMITATIONS:
The case-control design of the study may have limited the ability to establish causal relationships. BMI was self-reported at the time of the interview for controls and 1 year before diagnosis for cancer cases, which may have introduced recall bias. The formula for CUN-BAE was calculated from a sedentary convenience sample, which may not have been representative of the general population. The small sample size of cases that did not express hormone receptors was another limitation. The study’s findings may not be generalizable to non-White populations as non-White participants were excluded.
DISCLOSURES:
Dávila-Batista disclosed receiving grants from the Carlos III Health Institute. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Accurate body fat measures are crucial for effective cancer prevention.
METHODOLOGY:
- Researchers conducted a case-control study including 1033 breast cancer cases and 1143 postmenopausal population controls from the MCC-Spain study.
- Participants were aged 20-85 years. BMI was calculated as the ratio of weight to height squared and categorized using World Health Organization standards: < 25, 25-29.9, 30-34.9, and ≥ 35.
- CUN-BAE was calculated using a specific equation and categorized according to the estimated percentage of body fat: < 35%, 35%-39.9%, 40%-44.9%, and ≥ 45%.
- Odds ratios (ORs) were estimated with 95% CIs for both measures (BMI and CUN-BAE) for breast cancer cases using unconditional logistic regression.
TAKEAWAY:
- Excess body weight attributable to the risk for breast cancer was 23% when assessed using a BMI value > 30 and 38% when assessed using a CUN-BAE value > 40% body fat.
- Hormone receptor stratification showed that these differences in population-attributable fractions were only observed in hormone receptor–positive cases, with an estimated burden of 19.9% for BMI and 41.9% for CUN-BAE.
- The highest categories of CUN-BAE showed an increase in the risk for postmenopausal breast cancer (OR, 2.13 for body fat ≥ 45% compared with the reference category < 35%).
- No similar trend was observed for BMI, as the gradient declined after a BMI ≥ 35.
IN PRACTICE:
“The results of our study indicate that excess body fat is a significant risk factor for hormone receptor–positive breast cancer in postmenopausal women. Our findings suggest that the population impact could be underestimated when using traditional BMI estimates, and that more accurate measures of body fat, such as CUN-BAE, should be considered,” the authors of the study wrote.
SOURCE:
This study was led by Verónica Dávila-Batista, University of Las Palmas de Gran Canaria in Las Palmas de Gran Canaria, Spain. It was published online in Journal of Epidemiology and Community Health.
LIMITATIONS:
The case-control design of the study may have limited the ability to establish causal relationships. BMI was self-reported at the time of the interview for controls and 1 year before diagnosis for cancer cases, which may have introduced recall bias. The formula for CUN-BAE was calculated from a sedentary convenience sample, which may not have been representative of the general population. The small sample size of cases that did not express hormone receptors was another limitation. The study’s findings may not be generalizable to non-White populations as non-White participants were excluded.
DISCLOSURES:
Dávila-Batista disclosed receiving grants from the Carlos III Health Institute. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Accurate body fat measures are crucial for effective cancer prevention.
METHODOLOGY:
- Researchers conducted a case-control study including 1033 breast cancer cases and 1143 postmenopausal population controls from the MCC-Spain study.
- Participants were aged 20-85 years. BMI was calculated as the ratio of weight to height squared and categorized using World Health Organization standards: < 25, 25-29.9, 30-34.9, and ≥ 35.
- CUN-BAE was calculated using a specific equation and categorized according to the estimated percentage of body fat: < 35%, 35%-39.9%, 40%-44.9%, and ≥ 45%.
- Odds ratios (ORs) were estimated with 95% CIs for both measures (BMI and CUN-BAE) for breast cancer cases using unconditional logistic regression.
TAKEAWAY:
- Excess body weight attributable to the risk for breast cancer was 23% when assessed using a BMI value > 30 and 38% when assessed using a CUN-BAE value > 40% body fat.
- Hormone receptor stratification showed that these differences in population-attributable fractions were only observed in hormone receptor–positive cases, with an estimated burden of 19.9% for BMI and 41.9% for CUN-BAE.
- The highest categories of CUN-BAE showed an increase in the risk for postmenopausal breast cancer (OR, 2.13 for body fat ≥ 45% compared with the reference category < 35%).
- No similar trend was observed for BMI, as the gradient declined after a BMI ≥ 35.
IN PRACTICE:
“The results of our study indicate that excess body fat is a significant risk factor for hormone receptor–positive breast cancer in postmenopausal women. Our findings suggest that the population impact could be underestimated when using traditional BMI estimates, and that more accurate measures of body fat, such as CUN-BAE, should be considered,” the authors of the study wrote.
SOURCE:
This study was led by Verónica Dávila-Batista, University of Las Palmas de Gran Canaria in Las Palmas de Gran Canaria, Spain. It was published online in Journal of Epidemiology and Community Health.
LIMITATIONS:
The case-control design of the study may have limited the ability to establish causal relationships. BMI was self-reported at the time of the interview for controls and 1 year before diagnosis for cancer cases, which may have introduced recall bias. The formula for CUN-BAE was calculated from a sedentary convenience sample, which may not have been representative of the general population. The small sample size of cases that did not express hormone receptors was another limitation. The study’s findings may not be generalizable to non-White populations as non-White participants were excluded.
DISCLOSURES:
Dávila-Batista disclosed receiving grants from the Carlos III Health Institute. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Eggs: A Weighty Matter for Postmenopausal Women?
TOPLINE:
such as processed and red meat, French fries, sweets, and deserts. Genetic predisposition for a high body mass index (BMI) also influences weight gain with higher egg intake.
METHODOLOGY:
- Egg consumption and elevated body weight are each linked to an increased risk for serious chronic diseases; however, whether elevated body weight mediates the association between egg intake and an elevated risk for serious chronic diseases needs further assessment.
- To investigate the association between eating eggs and weight gain, as well as the role of genetic susceptibility to an elevated BMI, researchers conducted a prospective study including 4439 postmenopausal women of European descent from the Women’s Health Initiative (WHI).
- They measured the participants’ consumption of egg and egg nutrients using a self-administered food frequency questionnaire.
- Changes in the consumption of eggs and egg nutrients such as cholesterol, choline, and betaine were assessed between baseline and follow-up visits at 1, 3, 6, and 9 years.
- The primary outcome was the change in body weight between baseline and each follow-up visit. Furthermore, an exploratory analysis evaluated how eating Western foods and genetic predisposition for a high BMI (assessed through a polygenic score) influenced weight change.
TAKEAWAY:
- An increased consumption of eggs was associated with weight gain, showing a positive linear trend at 1, 2, 3, and 6 years. By the third year, women whose egg consumption had increased by two eggs per week gained 0.70 kg more weight (P = .0002) than women whose egg consumption was reduced by 2.4 eggs per week (P-linear < .0001).
- An increase in the consumption of nutrients obtained from eggs, including cholesterol (P-linear < .0001) and choline (P-linear < .02), was positively associated with weight gain.
- Women with a higher consumption of Western foods showed significant associations between changes in egg, cholesterol, and choline intake and weight gain.
- A significant association was found between the BMI polygenic score and changes in body weight, with women most genetically predisposed to a higher BMI showing greater weight gain when their egg consumption increased by an average of 3.5 eggs per week.
IN PRACTICE:
“These results suggest that even relatively small increases or decreases in egg consumption could cause increases or decreases, respectively, in body weight among postmenopausal women, unless there are adequate compensating changes in factors such as dietary energy intake or physical activity,” the authors wrote. “Our results require confirmation,” they added.
SOURCE:
This study, led by James A. Greenberg, Department of Health and Nutrition Sciences, Brooklyn College, The City University of New York, was published online in Clinical Nutrition.
LIMITATIONS:
This observational study was susceptible to residual confounding, which suggests that the observed associations may not have established causality. Additionally, the results were according to data from a group of postmenopausal American women of European descent, which limited the generalizability to other populations.
DISCLOSURES:
The WHI program was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health & Human Services. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
such as processed and red meat, French fries, sweets, and deserts. Genetic predisposition for a high body mass index (BMI) also influences weight gain with higher egg intake.
METHODOLOGY:
- Egg consumption and elevated body weight are each linked to an increased risk for serious chronic diseases; however, whether elevated body weight mediates the association between egg intake and an elevated risk for serious chronic diseases needs further assessment.
- To investigate the association between eating eggs and weight gain, as well as the role of genetic susceptibility to an elevated BMI, researchers conducted a prospective study including 4439 postmenopausal women of European descent from the Women’s Health Initiative (WHI).
- They measured the participants’ consumption of egg and egg nutrients using a self-administered food frequency questionnaire.
- Changes in the consumption of eggs and egg nutrients such as cholesterol, choline, and betaine were assessed between baseline and follow-up visits at 1, 3, 6, and 9 years.
- The primary outcome was the change in body weight between baseline and each follow-up visit. Furthermore, an exploratory analysis evaluated how eating Western foods and genetic predisposition for a high BMI (assessed through a polygenic score) influenced weight change.
TAKEAWAY:
- An increased consumption of eggs was associated with weight gain, showing a positive linear trend at 1, 2, 3, and 6 years. By the third year, women whose egg consumption had increased by two eggs per week gained 0.70 kg more weight (P = .0002) than women whose egg consumption was reduced by 2.4 eggs per week (P-linear < .0001).
- An increase in the consumption of nutrients obtained from eggs, including cholesterol (P-linear < .0001) and choline (P-linear < .02), was positively associated with weight gain.
- Women with a higher consumption of Western foods showed significant associations between changes in egg, cholesterol, and choline intake and weight gain.
- A significant association was found between the BMI polygenic score and changes in body weight, with women most genetically predisposed to a higher BMI showing greater weight gain when their egg consumption increased by an average of 3.5 eggs per week.
IN PRACTICE:
“These results suggest that even relatively small increases or decreases in egg consumption could cause increases or decreases, respectively, in body weight among postmenopausal women, unless there are adequate compensating changes in factors such as dietary energy intake or physical activity,” the authors wrote. “Our results require confirmation,” they added.
SOURCE:
This study, led by James A. Greenberg, Department of Health and Nutrition Sciences, Brooklyn College, The City University of New York, was published online in Clinical Nutrition.
LIMITATIONS:
This observational study was susceptible to residual confounding, which suggests that the observed associations may not have established causality. Additionally, the results were according to data from a group of postmenopausal American women of European descent, which limited the generalizability to other populations.
DISCLOSURES:
The WHI program was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health & Human Services. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
such as processed and red meat, French fries, sweets, and deserts. Genetic predisposition for a high body mass index (BMI) also influences weight gain with higher egg intake.
METHODOLOGY:
- Egg consumption and elevated body weight are each linked to an increased risk for serious chronic diseases; however, whether elevated body weight mediates the association between egg intake and an elevated risk for serious chronic diseases needs further assessment.
- To investigate the association between eating eggs and weight gain, as well as the role of genetic susceptibility to an elevated BMI, researchers conducted a prospective study including 4439 postmenopausal women of European descent from the Women’s Health Initiative (WHI).
- They measured the participants’ consumption of egg and egg nutrients using a self-administered food frequency questionnaire.
- Changes in the consumption of eggs and egg nutrients such as cholesterol, choline, and betaine were assessed between baseline and follow-up visits at 1, 3, 6, and 9 years.
- The primary outcome was the change in body weight between baseline and each follow-up visit. Furthermore, an exploratory analysis evaluated how eating Western foods and genetic predisposition for a high BMI (assessed through a polygenic score) influenced weight change.
TAKEAWAY:
- An increased consumption of eggs was associated with weight gain, showing a positive linear trend at 1, 2, 3, and 6 years. By the third year, women whose egg consumption had increased by two eggs per week gained 0.70 kg more weight (P = .0002) than women whose egg consumption was reduced by 2.4 eggs per week (P-linear < .0001).
- An increase in the consumption of nutrients obtained from eggs, including cholesterol (P-linear < .0001) and choline (P-linear < .02), was positively associated with weight gain.
- Women with a higher consumption of Western foods showed significant associations between changes in egg, cholesterol, and choline intake and weight gain.
- A significant association was found between the BMI polygenic score and changes in body weight, with women most genetically predisposed to a higher BMI showing greater weight gain when their egg consumption increased by an average of 3.5 eggs per week.
IN PRACTICE:
“These results suggest that even relatively small increases or decreases in egg consumption could cause increases or decreases, respectively, in body weight among postmenopausal women, unless there are adequate compensating changes in factors such as dietary energy intake or physical activity,” the authors wrote. “Our results require confirmation,” they added.
SOURCE:
This study, led by James A. Greenberg, Department of Health and Nutrition Sciences, Brooklyn College, The City University of New York, was published online in Clinical Nutrition.
LIMITATIONS:
This observational study was susceptible to residual confounding, which suggests that the observed associations may not have established causality. Additionally, the results were according to data from a group of postmenopausal American women of European descent, which limited the generalizability to other populations.
DISCLOSURES:
The WHI program was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health & Human Services. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Increasing Non–Candida albicans Yeasts in Vulvovaginal Candidiasis and Fluconazole Resistance in Leeds
TOPLINE:
Fluconazole resistance in yeast isolates from women with recurrent vulvovaginal candidiasis in Leeds, England, increased from 3.5% to 9.6% over 3 years. Non–Candida albicans yeasts also rose from 6.0% to 12.6% during the same period.
METHODOLOGY:
- Researchers conducted a retrospective data search of vaginal cultures from adult women in Leeds, England, between April 2018 and March 2021.
- A total of 5461 vaginal samples from women with clinical information indicating complicated/recurrent vulvovaginal candidiasis were included.
- Samples were processed on the WASPLAB automated platform, and species identification and antifungal susceptibility testing were performed in the Mycology Reference Centre by Matrix-assisted laser desorption ionization–time-of-flight mass spectrometry.
- Susceptibility to fluconazole was determined using disc diffusion and the Sensititre YeastOne microbroth dilution assay.
TAKEAWAY:
According to the authors, the prevalence of non–C albicans yeasts increased from 6.0% in 2018-2019 to 12.6% in 2020-2021 (P = .0003).
Fluconazole-sensitive (dose-dependent) and fluconazole-resistant isolates increased from 3.5% in 2018-2019 to 9.6% in 2020-2021 (P = .0001).
Most fluconazole resistance was observed in C albicans, with other species such as Nakaseomyces glabrata and Pichia kudriavzevii also showing resistance.
The authors state that the increase in fluconazole resistance and non–C albicans yeasts may be linked to a policy change encouraging empirical treatment of vulvovaginal candidiasis in primary care.
IN PRACTICE:
“This study shows that the rates of non–Candida albicans and fluconazole-resistant C albicans have increased year on year in the 3 years studied. The exact reasons for this increase remain unclear, but it follows the introduction of restricted access to fungal cultures for the diagnosis of vulvovaginal candidiasis by those working in primary care. A clinical diagnosis, followed by empirical treatment, has been recommended instead. Consequently, we believe this policy of encouraging empirical vaginitis treatment based on nonspecific symptoms and signs needs revisiting,” the authors wrote.
SOURCE:
The study was led by Jennifer C. Ratner, Leeds Teaching Hospitals NHS Trust, England. It was published online in Sexually Transmitted Infections.
LIMITATIONS:
The study’s limitations included a potential bias introduced by the reduced number of samples received from specialist sexual health clinics during the COVID-19 pandemic. Additionally, the study could not distinguish between cases of recurrent vulvovaginal candidiasis with complete resolution of symptoms and those with persistent symptoms despite treatment.
DISCLOSURES:
One coauthor disclosed receiving fees from Pfizer for contributing to webinar presentations in 2023. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Fluconazole resistance in yeast isolates from women with recurrent vulvovaginal candidiasis in Leeds, England, increased from 3.5% to 9.6% over 3 years. Non–Candida albicans yeasts also rose from 6.0% to 12.6% during the same period.
METHODOLOGY:
- Researchers conducted a retrospective data search of vaginal cultures from adult women in Leeds, England, between April 2018 and March 2021.
- A total of 5461 vaginal samples from women with clinical information indicating complicated/recurrent vulvovaginal candidiasis were included.
- Samples were processed on the WASPLAB automated platform, and species identification and antifungal susceptibility testing were performed in the Mycology Reference Centre by Matrix-assisted laser desorption ionization–time-of-flight mass spectrometry.
- Susceptibility to fluconazole was determined using disc diffusion and the Sensititre YeastOne microbroth dilution assay.
TAKEAWAY:
According to the authors, the prevalence of non–C albicans yeasts increased from 6.0% in 2018-2019 to 12.6% in 2020-2021 (P = .0003).
Fluconazole-sensitive (dose-dependent) and fluconazole-resistant isolates increased from 3.5% in 2018-2019 to 9.6% in 2020-2021 (P = .0001).
Most fluconazole resistance was observed in C albicans, with other species such as Nakaseomyces glabrata and Pichia kudriavzevii also showing resistance.
The authors state that the increase in fluconazole resistance and non–C albicans yeasts may be linked to a policy change encouraging empirical treatment of vulvovaginal candidiasis in primary care.
IN PRACTICE:
“This study shows that the rates of non–Candida albicans and fluconazole-resistant C albicans have increased year on year in the 3 years studied. The exact reasons for this increase remain unclear, but it follows the introduction of restricted access to fungal cultures for the diagnosis of vulvovaginal candidiasis by those working in primary care. A clinical diagnosis, followed by empirical treatment, has been recommended instead. Consequently, we believe this policy of encouraging empirical vaginitis treatment based on nonspecific symptoms and signs needs revisiting,” the authors wrote.
SOURCE:
The study was led by Jennifer C. Ratner, Leeds Teaching Hospitals NHS Trust, England. It was published online in Sexually Transmitted Infections.
LIMITATIONS:
The study’s limitations included a potential bias introduced by the reduced number of samples received from specialist sexual health clinics during the COVID-19 pandemic. Additionally, the study could not distinguish between cases of recurrent vulvovaginal candidiasis with complete resolution of symptoms and those with persistent symptoms despite treatment.
DISCLOSURES:
One coauthor disclosed receiving fees from Pfizer for contributing to webinar presentations in 2023. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Fluconazole resistance in yeast isolates from women with recurrent vulvovaginal candidiasis in Leeds, England, increased from 3.5% to 9.6% over 3 years. Non–Candida albicans yeasts also rose from 6.0% to 12.6% during the same period.
METHODOLOGY:
- Researchers conducted a retrospective data search of vaginal cultures from adult women in Leeds, England, between April 2018 and March 2021.
- A total of 5461 vaginal samples from women with clinical information indicating complicated/recurrent vulvovaginal candidiasis were included.
- Samples were processed on the WASPLAB automated platform, and species identification and antifungal susceptibility testing were performed in the Mycology Reference Centre by Matrix-assisted laser desorption ionization–time-of-flight mass spectrometry.
- Susceptibility to fluconazole was determined using disc diffusion and the Sensititre YeastOne microbroth dilution assay.
TAKEAWAY:
According to the authors, the prevalence of non–C albicans yeasts increased from 6.0% in 2018-2019 to 12.6% in 2020-2021 (P = .0003).
Fluconazole-sensitive (dose-dependent) and fluconazole-resistant isolates increased from 3.5% in 2018-2019 to 9.6% in 2020-2021 (P = .0001).
Most fluconazole resistance was observed in C albicans, with other species such as Nakaseomyces glabrata and Pichia kudriavzevii also showing resistance.
The authors state that the increase in fluconazole resistance and non–C albicans yeasts may be linked to a policy change encouraging empirical treatment of vulvovaginal candidiasis in primary care.
IN PRACTICE:
“This study shows that the rates of non–Candida albicans and fluconazole-resistant C albicans have increased year on year in the 3 years studied. The exact reasons for this increase remain unclear, but it follows the introduction of restricted access to fungal cultures for the diagnosis of vulvovaginal candidiasis by those working in primary care. A clinical diagnosis, followed by empirical treatment, has been recommended instead. Consequently, we believe this policy of encouraging empirical vaginitis treatment based on nonspecific symptoms and signs needs revisiting,” the authors wrote.
SOURCE:
The study was led by Jennifer C. Ratner, Leeds Teaching Hospitals NHS Trust, England. It was published online in Sexually Transmitted Infections.
LIMITATIONS:
The study’s limitations included a potential bias introduced by the reduced number of samples received from specialist sexual health clinics during the COVID-19 pandemic. Additionally, the study could not distinguish between cases of recurrent vulvovaginal candidiasis with complete resolution of symptoms and those with persistent symptoms despite treatment.
DISCLOSURES:
One coauthor disclosed receiving fees from Pfizer for contributing to webinar presentations in 2023. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Underutilized Mifepristone Shows Promise in Care of Early Pregnancy Loss
TOPLINE:
Mifepristone plus misoprostol reduces the need for subsequent uterine aspiration and emergency department visits in the management of early pregnancy loss. Despite its effectiveness, mifepristone remains underutilized, with 8.6% of patients receiving it in 2022.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using national insurance claims data of US patients with commercial insurance.
- More than 31,000 pregnant women (mean age, 32.7 years) with a diagnosis of early pregnancy loss between 2015 and 2022 were included.
- The diagnosis of patients included having a missed abortion (72.3%), spontaneous abortion (26.9%), or both (0.8%).
- Researchers compared the outcomes of individuals who received a combination of mifepristone and misoprostol vs those who received misoprostol alone. The outcome measures included the need for subsequent procedural management (uterine aspiration), return visits to the emergency department or an outpatient clinic, hospitalizations, and complications within 6 weeks of initial diagnosis.
TAKEAWAY:
- The use of mifepristone was more common in outpatient clinics than in emergency departments (3.4% vs 0.9%; P < .001).
- The use of mifepristone plus misoprostol vs misoprostol alone was linked to a lower incidence of subsequent procedural management (10.5% vs 14.0%; P = .002) and fewer emergency department visits (3.5% vs 7.9%; P < .001).
- The multivariable analysis showed that the use of mifepristone was linked to decreased odds of subsequent procedural management (adjusted odds ratio, 0.71; 95% CI, 0.57-0.87).
- Despite its effectiveness, mifepristone was used in only 8.6% of those receiving medication management for early pregnancy loss in 2022.
IN PRACTICE:
“Continued efforts are needed to reduce barriers to mifepristone use for medication management of EPL,” the authors wrote.
“Any practitioner who cares for patients experiencing early pregnancy loss should consider mifepristone pretreatment to misoprostol to be the standard of care for medication management. Provision of the evidence-based standard of care with the use of mifepristone for early pregnancy loss is an opportunity to advocate for an essential strategy in improving sexual and reproductive health in the US,” wrote Sarita Sonalkar, MD, MPH, and Rachel McKean, MD, MPH, of the Perelman School of Medicine at the University of Pennsylvania, Philadelphia, in an invited commentary.
SOURCE:
The study was led by Lyndsey S. Benson, MD, MS, of the University of Washington School of Medicine, Seattle, and was published online in JAMA Network Open.
LIMITATIONS:
The study was limited by the accuracy of the diagnosis of early pregnancy loss and procedure codes because claims data are intended for billing purposes and may be incomplete or inaccurate. The use of de-identified data meant that specific gestational durations, exact dosing, or routes of misoprostol administration could not be determined. The findings may not be generalizable to those with public insurance or no insurance.
DISCLOSURES:
The study was supported in part by a grant from a Women’s Reproductive Health Research grant from the National Institutes of Health Eunice Kennedy Shriver National Institute for Child Health and Human Development. One author reported serving as an adviser and investigator, while another reported receiving personal fees and serving as an expert witness, contributing editor, and course instructor outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Mifepristone plus misoprostol reduces the need for subsequent uterine aspiration and emergency department visits in the management of early pregnancy loss. Despite its effectiveness, mifepristone remains underutilized, with 8.6% of patients receiving it in 2022.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using national insurance claims data of US patients with commercial insurance.
- More than 31,000 pregnant women (mean age, 32.7 years) with a diagnosis of early pregnancy loss between 2015 and 2022 were included.
- The diagnosis of patients included having a missed abortion (72.3%), spontaneous abortion (26.9%), or both (0.8%).
- Researchers compared the outcomes of individuals who received a combination of mifepristone and misoprostol vs those who received misoprostol alone. The outcome measures included the need for subsequent procedural management (uterine aspiration), return visits to the emergency department or an outpatient clinic, hospitalizations, and complications within 6 weeks of initial diagnosis.
TAKEAWAY:
- The use of mifepristone was more common in outpatient clinics than in emergency departments (3.4% vs 0.9%; P < .001).
- The use of mifepristone plus misoprostol vs misoprostol alone was linked to a lower incidence of subsequent procedural management (10.5% vs 14.0%; P = .002) and fewer emergency department visits (3.5% vs 7.9%; P < .001).
- The multivariable analysis showed that the use of mifepristone was linked to decreased odds of subsequent procedural management (adjusted odds ratio, 0.71; 95% CI, 0.57-0.87).
- Despite its effectiveness, mifepristone was used in only 8.6% of those receiving medication management for early pregnancy loss in 2022.
IN PRACTICE:
“Continued efforts are needed to reduce barriers to mifepristone use for medication management of EPL,” the authors wrote.
“Any practitioner who cares for patients experiencing early pregnancy loss should consider mifepristone pretreatment to misoprostol to be the standard of care for medication management. Provision of the evidence-based standard of care with the use of mifepristone for early pregnancy loss is an opportunity to advocate for an essential strategy in improving sexual and reproductive health in the US,” wrote Sarita Sonalkar, MD, MPH, and Rachel McKean, MD, MPH, of the Perelman School of Medicine at the University of Pennsylvania, Philadelphia, in an invited commentary.
SOURCE:
The study was led by Lyndsey S. Benson, MD, MS, of the University of Washington School of Medicine, Seattle, and was published online in JAMA Network Open.
LIMITATIONS:
The study was limited by the accuracy of the diagnosis of early pregnancy loss and procedure codes because claims data are intended for billing purposes and may be incomplete or inaccurate. The use of de-identified data meant that specific gestational durations, exact dosing, or routes of misoprostol administration could not be determined. The findings may not be generalizable to those with public insurance or no insurance.
DISCLOSURES:
The study was supported in part by a grant from a Women’s Reproductive Health Research grant from the National Institutes of Health Eunice Kennedy Shriver National Institute for Child Health and Human Development. One author reported serving as an adviser and investigator, while another reported receiving personal fees and serving as an expert witness, contributing editor, and course instructor outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Mifepristone plus misoprostol reduces the need for subsequent uterine aspiration and emergency department visits in the management of early pregnancy loss. Despite its effectiveness, mifepristone remains underutilized, with 8.6% of patients receiving it in 2022.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using national insurance claims data of US patients with commercial insurance.
- More than 31,000 pregnant women (mean age, 32.7 years) with a diagnosis of early pregnancy loss between 2015 and 2022 were included.
- The diagnosis of patients included having a missed abortion (72.3%), spontaneous abortion (26.9%), or both (0.8%).
- Researchers compared the outcomes of individuals who received a combination of mifepristone and misoprostol vs those who received misoprostol alone. The outcome measures included the need for subsequent procedural management (uterine aspiration), return visits to the emergency department or an outpatient clinic, hospitalizations, and complications within 6 weeks of initial diagnosis.
TAKEAWAY:
- The use of mifepristone was more common in outpatient clinics than in emergency departments (3.4% vs 0.9%; P < .001).
- The use of mifepristone plus misoprostol vs misoprostol alone was linked to a lower incidence of subsequent procedural management (10.5% vs 14.0%; P = .002) and fewer emergency department visits (3.5% vs 7.9%; P < .001).
- The multivariable analysis showed that the use of mifepristone was linked to decreased odds of subsequent procedural management (adjusted odds ratio, 0.71; 95% CI, 0.57-0.87).
- Despite its effectiveness, mifepristone was used in only 8.6% of those receiving medication management for early pregnancy loss in 2022.
IN PRACTICE:
“Continued efforts are needed to reduce barriers to mifepristone use for medication management of EPL,” the authors wrote.
“Any practitioner who cares for patients experiencing early pregnancy loss should consider mifepristone pretreatment to misoprostol to be the standard of care for medication management. Provision of the evidence-based standard of care with the use of mifepristone for early pregnancy loss is an opportunity to advocate for an essential strategy in improving sexual and reproductive health in the US,” wrote Sarita Sonalkar, MD, MPH, and Rachel McKean, MD, MPH, of the Perelman School of Medicine at the University of Pennsylvania, Philadelphia, in an invited commentary.
SOURCE:
The study was led by Lyndsey S. Benson, MD, MS, of the University of Washington School of Medicine, Seattle, and was published online in JAMA Network Open.
LIMITATIONS:
The study was limited by the accuracy of the diagnosis of early pregnancy loss and procedure codes because claims data are intended for billing purposes and may be incomplete or inaccurate. The use of de-identified data meant that specific gestational durations, exact dosing, or routes of misoprostol administration could not be determined. The findings may not be generalizable to those with public insurance or no insurance.
DISCLOSURES:
The study was supported in part by a grant from a Women’s Reproductive Health Research grant from the National Institutes of Health Eunice Kennedy Shriver National Institute for Child Health and Human Development. One author reported serving as an adviser and investigator, while another reported receiving personal fees and serving as an expert witness, contributing editor, and course instructor outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Maternal Immunization to Prevent Serious Respiratory Illness
Editor’s Note: Sadly, this is the last column in the Master Class Obstetrics series. This award-winning column has been part of Ob.Gyn. News for 20 years. The deep discussion of cutting-edge topics in obstetrics by specialists and researchers will be missed as will the leadership and curation of topics by Dr. E. Albert Reece.
Introduction: The Need for Increased Vigilance About Maternal Immunization
Viruses are becoming increasingly prevalent in our world and the consequences of viral infections are implicated in a growing number of disease states. It is well established that certain cancers are caused by viruses and it is increasingly evident that viral infections can trigger the development of chronic illness. In pregnant women, viruses such as cytomegalovirus can cause infection in utero and lead to long-term impairments for the baby.
Likewise, it appears that the virulence of viruses is increasing, whether it be the respiratory syncytial virus (RSV) in children or the severe acute respiratory syndrome (SARS) coronaviruses in adults. Clearly, our environment is changing, with increases in population growth and urbanization, for instance, and an intensification of climate change and its effects. Viruses are part of this changing background.
Vaccines are our most powerful tool to protect people of all ages against viral threats, and fortunately, we benefit from increasing expertise in vaccinology. Since 1974, the University of Maryland School of Medicine has a Center for Vaccine Development and Global Health that has conducted research on vaccines to defend against the Zika virus, H1N1, Ebola, and SARS-CoV-2.
We’re not alone. Other vaccinology centers across the country — as well as the National Institutes of Health at the national level, through its National Institute of Allergy and Infectious Diseases — are doing research and developing vaccines to combat viral diseases.
In this column, we are focused on viral diseases in pregnancy and the role that vaccines can play in preventing serious respiratory illness in mothers and their newborns. I have invited Laura E. Riley, MD, the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine, to address the importance of maternal immunization and how we can best counsel our patients and improve immunization rates.
As Dr. Riley explains, we are in a new era, and it behooves us all to be more vigilant about recommending vaccines, combating misperceptions, addressing patients’ knowledge gaps, and administering vaccines whenever possible.
Dr. Reece is the former Dean of Medicine & University Executive VP, and The Distinguished University and Endowed Professor & Director of the Center for Advanced Research Training and Innovation (CARTI) at the University of Maryland School of Medicine, as well as senior scientist at the Center for Birth Defects Research.
The alarming decline in maternal immunization rates that occurred in the wake of the COVID-19 pandemic means that, now more than ever, we must fully embrace our responsibility to recommend immunizations in pregnancy and to communicate what is known about their efficacy and safety. Data show that vaccination rates drop when we do not offer vaccines in our offices, so whenever possible, we should administer them as well.
The ob.gyn. is the patient’s most trusted person in pregnancy. When patients decline or express hesitancy about vaccines, it is incumbent upon us to ask why. Oftentimes, we can identify areas in which patients lack knowledge or have misperceptions and we can successfully educate the patient or change their perspective or misunderstanding concerning the importance of vaccination for themselves and their babies. (See Table 1.) We can also successfully address concerns about safety.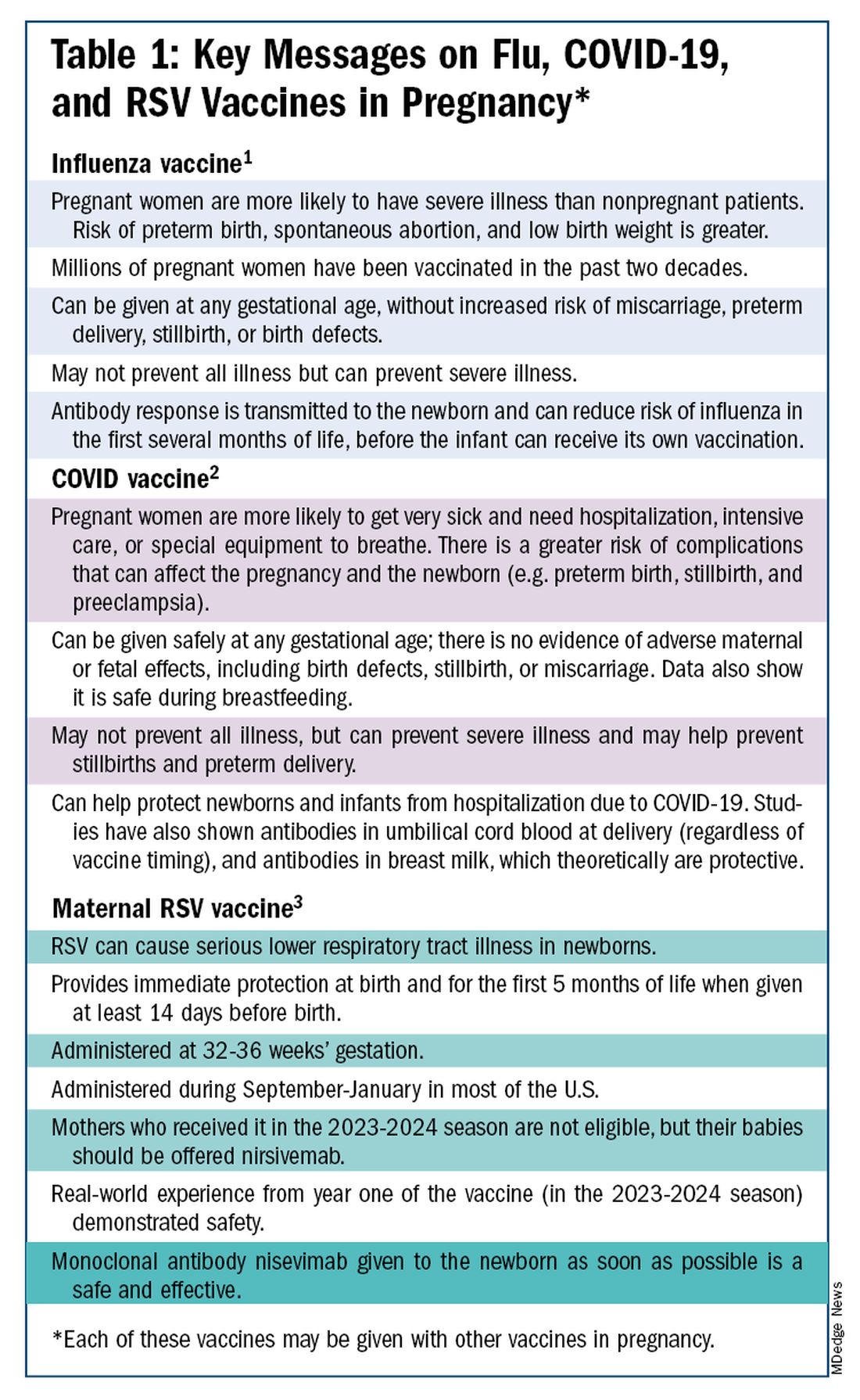
The safety of COVID-19 vaccinations in pregnancy is now backed by several years of data from multiple studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
Data also show that pregnant patients are more likely than patients who are not pregnant to need hospitalization and intensive care when infected with SARS-CoV-2 and are at risk of having complications that can affect pregnancy and the newborn, including preterm birth and stillbirth. Vaccination has been shown to reduce the risk of severe illness and the risk of such adverse obstetrical outcomes, in addition to providing protection for the infant early on.
Similarly, influenza has long been more likely to be severe in pregnant patients, with an increased risk of poor obstetrical outcomes. Vaccines similarly provide “two for one protection,” protecting both mother and baby, and are, of course, backed by many years of safety and efficacy data.
With the new maternal respiratory syncytial virus (RSV) vaccine, now in its second year of availability, the goal is to protect the baby from RSV-caused serious lower respiratory tract illness. The illness has contributed to tens of thousands of annual hospitalizations and up to several hundred deaths every year in children younger than 5 years — particularly in those under age 6 months.
The RSV monoclonal antibody nirsevimab is available for the newborn as an alternative to maternal immunization but the maternal vaccine is optimal in that it will provide immediate rather than delayed protection for the newborn. The maternal vaccine is recommended during weeks 32-36 of pregnancy in mothers who were not vaccinated during last year’s RSV season. With real-world experience from year one, the available safety data are reassuring.
Counseling About Influenza and COVID-19 Vaccination
The COVID-19 pandemic took a toll on vaccination interest/receptivity broadly in pregnant and nonpregnant people. Among pregnant individuals, influenza vaccination coverage declined from 71% in the 2019-2020 influenza season to 56% in the 2021-2022 season, according to data from the Centers for Disease Control and Prevention’s Vaccine Safety Datalink.4 Coverage for the 2022-2023 and 2023-2024 influenza seasons was even worse: well under 50%.5
Fewer pregnant women have received updated COVID-19 vaccines. Only 13% of pregnant persons overall received the updated 2023-2024 COVID-19 booster vaccine (through March 30, 2024), according to the CDC.6
Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), and flu vaccines have been given to millions of pregnant women, but the H1N1 pandemic of 2009 reinforced its value as a priority for prenatal care. Most of the women who became severely ill from the H1N1 virus were young and healthy, without co-existing conditions known to increase risk.7
It became clearer during the H1N1 pandemic that pregnancy itself — which is associated with physiologic changes such as decreased lung capacity, increased nasal congestion and changes in the immune system – is its own significant risk factor for severe illness from the influenza virus. This increased risk applies to COVID-19 as well.
As COVID-19 has become endemic, with hospitalizations and deaths not reaching the levels of previous surges — and with mask-wearing and other preventive measures having declined — patients understandably have become more complacent. Some patients are vaccine deniers, but in my practice, these patients are a much smaller group than those who believe COVID-19 “is no big deal,” especially if they have had infections recently.
This is why it’s important to actively listen to concerns and to ask patients who decline a vaccination why they are hesitant. Blanket messages about vaccine efficacy and safety are the first step, but individualized, more pointed conversations based on the patient’s personal experiences and beliefs have become increasingly important.
I routinely tell pregnant patients about the risks of COVID-19 and I explain that it has been difficult to predict who will develop severe illness. Sometimes more conversation is needed. For those who are still hesitant or who tell me they feel protected by a recent infection, for instance, I provide more detail on the unique risks of pregnancy — the fact that “pregnancy is different” — and that natural immunity wanes while the protection afforded by immunization is believed to last longer. Many women are also concerned about the safety of the COVID-19 vaccine, so having safety data at your fingertips is helpful. (See Table 2.)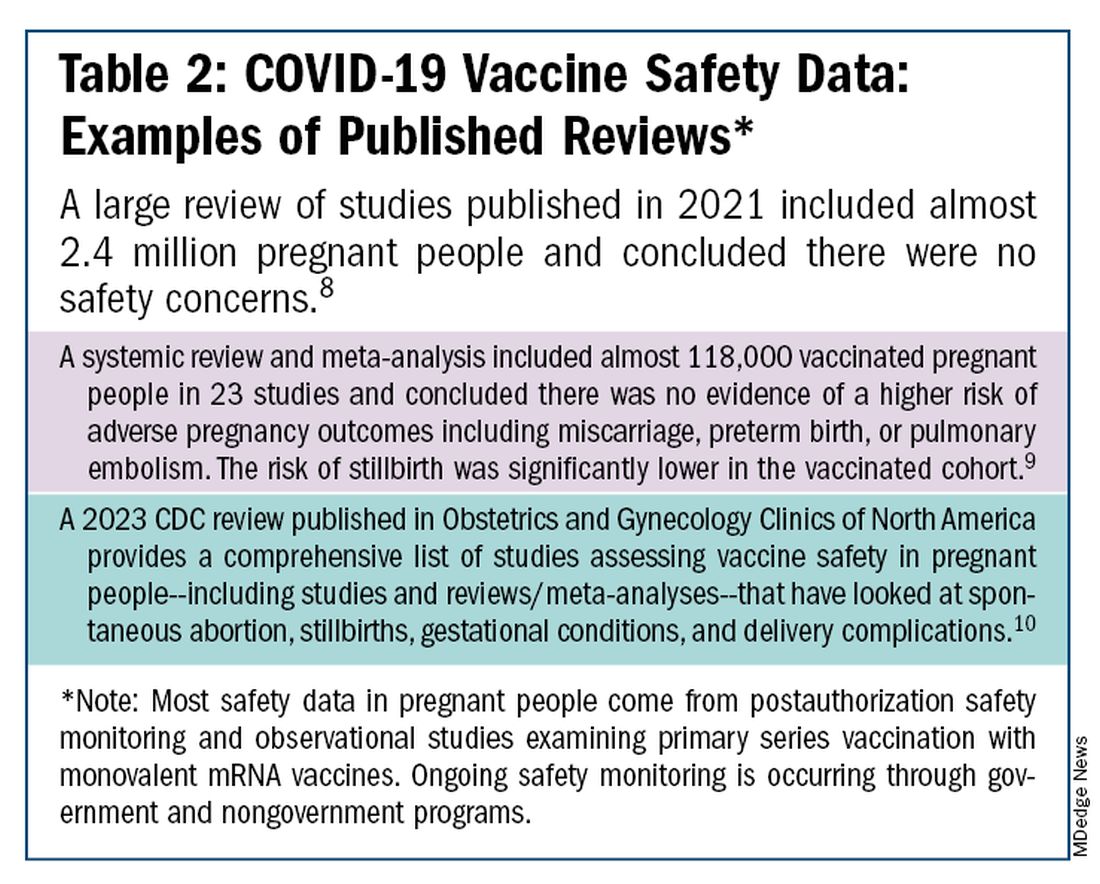
The fact that influenza and COVID-19 vaccination protect the newborn as well as the mother is something that I find is underappreciated by many patients. Explaining that infants likely benefit from the passage of antibodies across the placenta should be part of patient counseling.
Counseling About RSV Vaccination
Importantly, for the 2024-2025 RSV season, the maternal RSV vaccine (Abrysvo, Pfizer) is recommended only for pregnant women who did not receive the vaccine during the 2023-2024 season. When more research is done and more data are obtained showing how long the immune response persists post vaccination, it may be that the US Food and Drug Administration (FDA) will approve the maternal RSV vaccine for use in every pregnancy.
The later timing of the vaccination recommendation — 32-36 weeks’ gestation — reflects a conservative approach taken by the FDA in response to data from one of the pivotal trials showing a numerical trend toward more preterm deliveries among vaccinated compared with unvaccinated patients. This imbalance in the original trial, which administered the vaccine during 24-36 weeks of gestation, was seen only in low-income countries with no temporal association, however.
In our experience at two Weill Cornell Medical College–associated hospitals we did not see this trend. Our cohort study of almost 3000 pregnant patients who delivered at 32 weeks’ gestation or later found no increased risk of preterm birth among the 35% of patients who received the RSV vaccine during the 2023-2024 RSV season. We also did not see any difference in preeclampsia, in contrast with original trial data that showed a signal for increased risk.11
When fewer than 2 weeks have elapsed between maternal vaccination and delivery, the monoclonal antibody nirsevimab is recommended for the newborn — ideally before the newborn leaves the hospital. Nirsevimab is also recommended for newborns of mothers who decline vaccination or were not candidates (e.g. vaccinated in a previous pregnancy), or when there is concern about the adequacy of the maternal immune response to the vaccine (e.g. in cases of immunosuppression).
While there was a limited supply of the monoclonal antibody last year, limitations are not expected this year, especially after October.
The ultimate goal is that patients choose the vaccine or the immunoglobulin, given the severity of RSV disease. Patient preferences should be considered. However, given that it takes 2 weeks after vaccination for protection to build up, I stress to patients that if they’ve vaccinated themselves, their newborn will leave the hospital with protection. If nirsevimab is relied upon, I explain, their newborn may not be protected for some period of time.
Take-home Messages
- When patients decline or are hesitant about vaccines, ask why. Listen actively, and work to correct misperceptions and knowledge gaps.
- Whenever possible, offer vaccines in your practice. Vaccination rates drop when this does not occur.
- COVID-vaccine safety is backed by many studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
- Pregnant women are more likely to have severe illness from the influenza and SARS-CoV-2 viruses. Vaccines can prevent severe illness and can protect the newborn as well as the mother.
- Recommend/administer the maternal RSV vaccine at 32-36 weeks’ gestation in women who did not receive the vaccine in the 2023-2024 season. If mothers aren’t eligible their babies should be offered nirsevimab.
Dr. Riley is the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine and the obstetrician and gynecologist-in-chief at New York Presbyterian Hospital. She disclosed that she has provided one-time consultations to Pfizer (Abrysvo RSV vaccine) and GSK (cytomegalovirus vaccine), and is providing consultant education on CMV for Moderna. She is chair of ACOG’s task force on immunization and emerging infectious diseases, serves on the medical advisory board for MAVEN, and serves as an editor or editorial board member for several medical publications.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131(6):e214-e217.
2. Centers for Disease Control and Prevention. COVID-19 Vaccination for People Who are Pregnant or Breastfeeding. https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html.
3. ACOG Practice Advisory on Maternal Respiratory Syncytial Virus Vaccination, September 2023. (Updated August 2024).4. Irving S et al. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.1002.
5. Flu Vaccination Dashboard, CDC, National Center for Immunization and Respiratory Diseases.
6. Weekly COVID-19 Vaccination Dashboard, CDC. https://www.cdc.gov/covidvaxview/weekly-dashboard/index.html
7. Louie JK et al. N Engl J Med. 2010;362:27-35. 8. Ciapponi A et al. Vaccine. 2021;39(40):5891-908.
9. Prasad S et al. Nature Communications. 2022;13:2414. 10. Fleming-Dutra KE et al. Obstet Gynecol Clin North Am 2023;50(2):279-97. 11. Mouen S et al. JAMA Network Open 2024;7(7):e2419268.
Editor’s Note: Sadly, this is the last column in the Master Class Obstetrics series. This award-winning column has been part of Ob.Gyn. News for 20 years. The deep discussion of cutting-edge topics in obstetrics by specialists and researchers will be missed as will the leadership and curation of topics by Dr. E. Albert Reece.
Introduction: The Need for Increased Vigilance About Maternal Immunization
Viruses are becoming increasingly prevalent in our world and the consequences of viral infections are implicated in a growing number of disease states. It is well established that certain cancers are caused by viruses and it is increasingly evident that viral infections can trigger the development of chronic illness. In pregnant women, viruses such as cytomegalovirus can cause infection in utero and lead to long-term impairments for the baby.
Likewise, it appears that the virulence of viruses is increasing, whether it be the respiratory syncytial virus (RSV) in children or the severe acute respiratory syndrome (SARS) coronaviruses in adults. Clearly, our environment is changing, with increases in population growth and urbanization, for instance, and an intensification of climate change and its effects. Viruses are part of this changing background.
Vaccines are our most powerful tool to protect people of all ages against viral threats, and fortunately, we benefit from increasing expertise in vaccinology. Since 1974, the University of Maryland School of Medicine has a Center for Vaccine Development and Global Health that has conducted research on vaccines to defend against the Zika virus, H1N1, Ebola, and SARS-CoV-2.
We’re not alone. Other vaccinology centers across the country — as well as the National Institutes of Health at the national level, through its National Institute of Allergy and Infectious Diseases — are doing research and developing vaccines to combat viral diseases.
In this column, we are focused on viral diseases in pregnancy and the role that vaccines can play in preventing serious respiratory illness in mothers and their newborns. I have invited Laura E. Riley, MD, the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine, to address the importance of maternal immunization and how we can best counsel our patients and improve immunization rates.
As Dr. Riley explains, we are in a new era, and it behooves us all to be more vigilant about recommending vaccines, combating misperceptions, addressing patients’ knowledge gaps, and administering vaccines whenever possible.
Dr. Reece is the former Dean of Medicine & University Executive VP, and The Distinguished University and Endowed Professor & Director of the Center for Advanced Research Training and Innovation (CARTI) at the University of Maryland School of Medicine, as well as senior scientist at the Center for Birth Defects Research.
The alarming decline in maternal immunization rates that occurred in the wake of the COVID-19 pandemic means that, now more than ever, we must fully embrace our responsibility to recommend immunizations in pregnancy and to communicate what is known about their efficacy and safety. Data show that vaccination rates drop when we do not offer vaccines in our offices, so whenever possible, we should administer them as well.
The ob.gyn. is the patient’s most trusted person in pregnancy. When patients decline or express hesitancy about vaccines, it is incumbent upon us to ask why. Oftentimes, we can identify areas in which patients lack knowledge or have misperceptions and we can successfully educate the patient or change their perspective or misunderstanding concerning the importance of vaccination for themselves and their babies. (See Table 1.) We can also successfully address concerns about safety.
The safety of COVID-19 vaccinations in pregnancy is now backed by several years of data from multiple studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
Data also show that pregnant patients are more likely than patients who are not pregnant to need hospitalization and intensive care when infected with SARS-CoV-2 and are at risk of having complications that can affect pregnancy and the newborn, including preterm birth and stillbirth. Vaccination has been shown to reduce the risk of severe illness and the risk of such adverse obstetrical outcomes, in addition to providing protection for the infant early on.
Similarly, influenza has long been more likely to be severe in pregnant patients, with an increased risk of poor obstetrical outcomes. Vaccines similarly provide “two for one protection,” protecting both mother and baby, and are, of course, backed by many years of safety and efficacy data.
With the new maternal respiratory syncytial virus (RSV) vaccine, now in its second year of availability, the goal is to protect the baby from RSV-caused serious lower respiratory tract illness. The illness has contributed to tens of thousands of annual hospitalizations and up to several hundred deaths every year in children younger than 5 years — particularly in those under age 6 months.
The RSV monoclonal antibody nirsevimab is available for the newborn as an alternative to maternal immunization but the maternal vaccine is optimal in that it will provide immediate rather than delayed protection for the newborn. The maternal vaccine is recommended during weeks 32-36 of pregnancy in mothers who were not vaccinated during last year’s RSV season. With real-world experience from year one, the available safety data are reassuring.
Counseling About Influenza and COVID-19 Vaccination
The COVID-19 pandemic took a toll on vaccination interest/receptivity broadly in pregnant and nonpregnant people. Among pregnant individuals, influenza vaccination coverage declined from 71% in the 2019-2020 influenza season to 56% in the 2021-2022 season, according to data from the Centers for Disease Control and Prevention’s Vaccine Safety Datalink.4 Coverage for the 2022-2023 and 2023-2024 influenza seasons was even worse: well under 50%.5
Fewer pregnant women have received updated COVID-19 vaccines. Only 13% of pregnant persons overall received the updated 2023-2024 COVID-19 booster vaccine (through March 30, 2024), according to the CDC.6
Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), and flu vaccines have been given to millions of pregnant women, but the H1N1 pandemic of 2009 reinforced its value as a priority for prenatal care. Most of the women who became severely ill from the H1N1 virus were young and healthy, without co-existing conditions known to increase risk.7
It became clearer during the H1N1 pandemic that pregnancy itself — which is associated with physiologic changes such as decreased lung capacity, increased nasal congestion and changes in the immune system – is its own significant risk factor for severe illness from the influenza virus. This increased risk applies to COVID-19 as well.
As COVID-19 has become endemic, with hospitalizations and deaths not reaching the levels of previous surges — and with mask-wearing and other preventive measures having declined — patients understandably have become more complacent. Some patients are vaccine deniers, but in my practice, these patients are a much smaller group than those who believe COVID-19 “is no big deal,” especially if they have had infections recently.
This is why it’s important to actively listen to concerns and to ask patients who decline a vaccination why they are hesitant. Blanket messages about vaccine efficacy and safety are the first step, but individualized, more pointed conversations based on the patient’s personal experiences and beliefs have become increasingly important.
I routinely tell pregnant patients about the risks of COVID-19 and I explain that it has been difficult to predict who will develop severe illness. Sometimes more conversation is needed. For those who are still hesitant or who tell me they feel protected by a recent infection, for instance, I provide more detail on the unique risks of pregnancy — the fact that “pregnancy is different” — and that natural immunity wanes while the protection afforded by immunization is believed to last longer. Many women are also concerned about the safety of the COVID-19 vaccine, so having safety data at your fingertips is helpful. (See Table 2.)
The fact that influenza and COVID-19 vaccination protect the newborn as well as the mother is something that I find is underappreciated by many patients. Explaining that infants likely benefit from the passage of antibodies across the placenta should be part of patient counseling.
Counseling About RSV Vaccination
Importantly, for the 2024-2025 RSV season, the maternal RSV vaccine (Abrysvo, Pfizer) is recommended only for pregnant women who did not receive the vaccine during the 2023-2024 season. When more research is done and more data are obtained showing how long the immune response persists post vaccination, it may be that the US Food and Drug Administration (FDA) will approve the maternal RSV vaccine for use in every pregnancy.
The later timing of the vaccination recommendation — 32-36 weeks’ gestation — reflects a conservative approach taken by the FDA in response to data from one of the pivotal trials showing a numerical trend toward more preterm deliveries among vaccinated compared with unvaccinated patients. This imbalance in the original trial, which administered the vaccine during 24-36 weeks of gestation, was seen only in low-income countries with no temporal association, however.
In our experience at two Weill Cornell Medical College–associated hospitals we did not see this trend. Our cohort study of almost 3000 pregnant patients who delivered at 32 weeks’ gestation or later found no increased risk of preterm birth among the 35% of patients who received the RSV vaccine during the 2023-2024 RSV season. We also did not see any difference in preeclampsia, in contrast with original trial data that showed a signal for increased risk.11
When fewer than 2 weeks have elapsed between maternal vaccination and delivery, the monoclonal antibody nirsevimab is recommended for the newborn — ideally before the newborn leaves the hospital. Nirsevimab is also recommended for newborns of mothers who decline vaccination or were not candidates (e.g. vaccinated in a previous pregnancy), or when there is concern about the adequacy of the maternal immune response to the vaccine (e.g. in cases of immunosuppression).
While there was a limited supply of the monoclonal antibody last year, limitations are not expected this year, especially after October.
The ultimate goal is that patients choose the vaccine or the immunoglobulin, given the severity of RSV disease. Patient preferences should be considered. However, given that it takes 2 weeks after vaccination for protection to build up, I stress to patients that if they’ve vaccinated themselves, their newborn will leave the hospital with protection. If nirsevimab is relied upon, I explain, their newborn may not be protected for some period of time.
Take-home Messages
- When patients decline or are hesitant about vaccines, ask why. Listen actively, and work to correct misperceptions and knowledge gaps.
- Whenever possible, offer vaccines in your practice. Vaccination rates drop when this does not occur.
- COVID-vaccine safety is backed by many studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
- Pregnant women are more likely to have severe illness from the influenza and SARS-CoV-2 viruses. Vaccines can prevent severe illness and can protect the newborn as well as the mother.
- Recommend/administer the maternal RSV vaccine at 32-36 weeks’ gestation in women who did not receive the vaccine in the 2023-2024 season. If mothers aren’t eligible their babies should be offered nirsevimab.
Dr. Riley is the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine and the obstetrician and gynecologist-in-chief at New York Presbyterian Hospital. She disclosed that she has provided one-time consultations to Pfizer (Abrysvo RSV vaccine) and GSK (cytomegalovirus vaccine), and is providing consultant education on CMV for Moderna. She is chair of ACOG’s task force on immunization and emerging infectious diseases, serves on the medical advisory board for MAVEN, and serves as an editor or editorial board member for several medical publications.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131(6):e214-e217.
2. Centers for Disease Control and Prevention. COVID-19 Vaccination for People Who are Pregnant or Breastfeeding. https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html.
3. ACOG Practice Advisory on Maternal Respiratory Syncytial Virus Vaccination, September 2023. (Updated August 2024).4. Irving S et al. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.1002.
5. Flu Vaccination Dashboard, CDC, National Center for Immunization and Respiratory Diseases.
6. Weekly COVID-19 Vaccination Dashboard, CDC. https://www.cdc.gov/covidvaxview/weekly-dashboard/index.html
7. Louie JK et al. N Engl J Med. 2010;362:27-35. 8. Ciapponi A et al. Vaccine. 2021;39(40):5891-908.
9. Prasad S et al. Nature Communications. 2022;13:2414. 10. Fleming-Dutra KE et al. Obstet Gynecol Clin North Am 2023;50(2):279-97. 11. Mouen S et al. JAMA Network Open 2024;7(7):e2419268.
Editor’s Note: Sadly, this is the last column in the Master Class Obstetrics series. This award-winning column has been part of Ob.Gyn. News for 20 years. The deep discussion of cutting-edge topics in obstetrics by specialists and researchers will be missed as will the leadership and curation of topics by Dr. E. Albert Reece.
Introduction: The Need for Increased Vigilance About Maternal Immunization
Viruses are becoming increasingly prevalent in our world and the consequences of viral infections are implicated in a growing number of disease states. It is well established that certain cancers are caused by viruses and it is increasingly evident that viral infections can trigger the development of chronic illness. In pregnant women, viruses such as cytomegalovirus can cause infection in utero and lead to long-term impairments for the baby.
Likewise, it appears that the virulence of viruses is increasing, whether it be the respiratory syncytial virus (RSV) in children or the severe acute respiratory syndrome (SARS) coronaviruses in adults. Clearly, our environment is changing, with increases in population growth and urbanization, for instance, and an intensification of climate change and its effects. Viruses are part of this changing background.
Vaccines are our most powerful tool to protect people of all ages against viral threats, and fortunately, we benefit from increasing expertise in vaccinology. Since 1974, the University of Maryland School of Medicine has a Center for Vaccine Development and Global Health that has conducted research on vaccines to defend against the Zika virus, H1N1, Ebola, and SARS-CoV-2.
We’re not alone. Other vaccinology centers across the country — as well as the National Institutes of Health at the national level, through its National Institute of Allergy and Infectious Diseases — are doing research and developing vaccines to combat viral diseases.
In this column, we are focused on viral diseases in pregnancy and the role that vaccines can play in preventing serious respiratory illness in mothers and their newborns. I have invited Laura E. Riley, MD, the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine, to address the importance of maternal immunization and how we can best counsel our patients and improve immunization rates.
As Dr. Riley explains, we are in a new era, and it behooves us all to be more vigilant about recommending vaccines, combating misperceptions, addressing patients’ knowledge gaps, and administering vaccines whenever possible.
Dr. Reece is the former Dean of Medicine & University Executive VP, and The Distinguished University and Endowed Professor & Director of the Center for Advanced Research Training and Innovation (CARTI) at the University of Maryland School of Medicine, as well as senior scientist at the Center for Birth Defects Research.
The alarming decline in maternal immunization rates that occurred in the wake of the COVID-19 pandemic means that, now more than ever, we must fully embrace our responsibility to recommend immunizations in pregnancy and to communicate what is known about their efficacy and safety. Data show that vaccination rates drop when we do not offer vaccines in our offices, so whenever possible, we should administer them as well.
The ob.gyn. is the patient’s most trusted person in pregnancy. When patients decline or express hesitancy about vaccines, it is incumbent upon us to ask why. Oftentimes, we can identify areas in which patients lack knowledge or have misperceptions and we can successfully educate the patient or change their perspective or misunderstanding concerning the importance of vaccination for themselves and their babies. (See Table 1.) We can also successfully address concerns about safety.
The safety of COVID-19 vaccinations in pregnancy is now backed by several years of data from multiple studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
Data also show that pregnant patients are more likely than patients who are not pregnant to need hospitalization and intensive care when infected with SARS-CoV-2 and are at risk of having complications that can affect pregnancy and the newborn, including preterm birth and stillbirth. Vaccination has been shown to reduce the risk of severe illness and the risk of such adverse obstetrical outcomes, in addition to providing protection for the infant early on.
Similarly, influenza has long been more likely to be severe in pregnant patients, with an increased risk of poor obstetrical outcomes. Vaccines similarly provide “two for one protection,” protecting both mother and baby, and are, of course, backed by many years of safety and efficacy data.
With the new maternal respiratory syncytial virus (RSV) vaccine, now in its second year of availability, the goal is to protect the baby from RSV-caused serious lower respiratory tract illness. The illness has contributed to tens of thousands of annual hospitalizations and up to several hundred deaths every year in children younger than 5 years — particularly in those under age 6 months.
The RSV monoclonal antibody nirsevimab is available for the newborn as an alternative to maternal immunization but the maternal vaccine is optimal in that it will provide immediate rather than delayed protection for the newborn. The maternal vaccine is recommended during weeks 32-36 of pregnancy in mothers who were not vaccinated during last year’s RSV season. With real-world experience from year one, the available safety data are reassuring.
Counseling About Influenza and COVID-19 Vaccination
The COVID-19 pandemic took a toll on vaccination interest/receptivity broadly in pregnant and nonpregnant people. Among pregnant individuals, influenza vaccination coverage declined from 71% in the 2019-2020 influenza season to 56% in the 2021-2022 season, according to data from the Centers for Disease Control and Prevention’s Vaccine Safety Datalink.4 Coverage for the 2022-2023 and 2023-2024 influenza seasons was even worse: well under 50%.5
Fewer pregnant women have received updated COVID-19 vaccines. Only 13% of pregnant persons overall received the updated 2023-2024 COVID-19 booster vaccine (through March 30, 2024), according to the CDC.6
Maternal immunization for influenza has been recommended in the United States since 2004 (part of the recommendation that everyone over the age of 6 months receive an annual flu vaccine), and flu vaccines have been given to millions of pregnant women, but the H1N1 pandemic of 2009 reinforced its value as a priority for prenatal care. Most of the women who became severely ill from the H1N1 virus were young and healthy, without co-existing conditions known to increase risk.7
It became clearer during the H1N1 pandemic that pregnancy itself — which is associated with physiologic changes such as decreased lung capacity, increased nasal congestion and changes in the immune system – is its own significant risk factor for severe illness from the influenza virus. This increased risk applies to COVID-19 as well.
As COVID-19 has become endemic, with hospitalizations and deaths not reaching the levels of previous surges — and with mask-wearing and other preventive measures having declined — patients understandably have become more complacent. Some patients are vaccine deniers, but in my practice, these patients are a much smaller group than those who believe COVID-19 “is no big deal,” especially if they have had infections recently.
This is why it’s important to actively listen to concerns and to ask patients who decline a vaccination why they are hesitant. Blanket messages about vaccine efficacy and safety are the first step, but individualized, more pointed conversations based on the patient’s personal experiences and beliefs have become increasingly important.
I routinely tell pregnant patients about the risks of COVID-19 and I explain that it has been difficult to predict who will develop severe illness. Sometimes more conversation is needed. For those who are still hesitant or who tell me they feel protected by a recent infection, for instance, I provide more detail on the unique risks of pregnancy — the fact that “pregnancy is different” — and that natural immunity wanes while the protection afforded by immunization is believed to last longer. Many women are also concerned about the safety of the COVID-19 vaccine, so having safety data at your fingertips is helpful. (See Table 2.)
The fact that influenza and COVID-19 vaccination protect the newborn as well as the mother is something that I find is underappreciated by many patients. Explaining that infants likely benefit from the passage of antibodies across the placenta should be part of patient counseling.
Counseling About RSV Vaccination
Importantly, for the 2024-2025 RSV season, the maternal RSV vaccine (Abrysvo, Pfizer) is recommended only for pregnant women who did not receive the vaccine during the 2023-2024 season. When more research is done and more data are obtained showing how long the immune response persists post vaccination, it may be that the US Food and Drug Administration (FDA) will approve the maternal RSV vaccine for use in every pregnancy.
The later timing of the vaccination recommendation — 32-36 weeks’ gestation — reflects a conservative approach taken by the FDA in response to data from one of the pivotal trials showing a numerical trend toward more preterm deliveries among vaccinated compared with unvaccinated patients. This imbalance in the original trial, which administered the vaccine during 24-36 weeks of gestation, was seen only in low-income countries with no temporal association, however.
In our experience at two Weill Cornell Medical College–associated hospitals we did not see this trend. Our cohort study of almost 3000 pregnant patients who delivered at 32 weeks’ gestation or later found no increased risk of preterm birth among the 35% of patients who received the RSV vaccine during the 2023-2024 RSV season. We also did not see any difference in preeclampsia, in contrast with original trial data that showed a signal for increased risk.11
When fewer than 2 weeks have elapsed between maternal vaccination and delivery, the monoclonal antibody nirsevimab is recommended for the newborn — ideally before the newborn leaves the hospital. Nirsevimab is also recommended for newborns of mothers who decline vaccination or were not candidates (e.g. vaccinated in a previous pregnancy), or when there is concern about the adequacy of the maternal immune response to the vaccine (e.g. in cases of immunosuppression).
While there was a limited supply of the monoclonal antibody last year, limitations are not expected this year, especially after October.
The ultimate goal is that patients choose the vaccine or the immunoglobulin, given the severity of RSV disease. Patient preferences should be considered. However, given that it takes 2 weeks after vaccination for protection to build up, I stress to patients that if they’ve vaccinated themselves, their newborn will leave the hospital with protection. If nirsevimab is relied upon, I explain, their newborn may not be protected for some period of time.
Take-home Messages
- When patients decline or are hesitant about vaccines, ask why. Listen actively, and work to correct misperceptions and knowledge gaps.
- Whenever possible, offer vaccines in your practice. Vaccination rates drop when this does not occur.
- COVID-vaccine safety is backed by many studies showing no increase in birth defects, preterm delivery, miscarriage, or stillbirth.
- Pregnant women are more likely to have severe illness from the influenza and SARS-CoV-2 viruses. Vaccines can prevent severe illness and can protect the newborn as well as the mother.
- Recommend/administer the maternal RSV vaccine at 32-36 weeks’ gestation in women who did not receive the vaccine in the 2023-2024 season. If mothers aren’t eligible their babies should be offered nirsevimab.
Dr. Riley is the Given Foundation Professor and Chair of Obstetrics and Gynecology at Weill Cornell Medicine and the obstetrician and gynecologist-in-chief at New York Presbyterian Hospital. She disclosed that she has provided one-time consultations to Pfizer (Abrysvo RSV vaccine) and GSK (cytomegalovirus vaccine), and is providing consultant education on CMV for Moderna. She is chair of ACOG’s task force on immunization and emerging infectious diseases, serves on the medical advisory board for MAVEN, and serves as an editor or editorial board member for several medical publications.
References
1. ACOG Committee Opinion No. 741: Maternal Immunization. Obstet Gynecol. 2018;131(6):e214-e217.
2. Centers for Disease Control and Prevention. COVID-19 Vaccination for People Who are Pregnant or Breastfeeding. https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html.
3. ACOG Practice Advisory on Maternal Respiratory Syncytial Virus Vaccination, September 2023. (Updated August 2024).4. Irving S et al. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.1002.
5. Flu Vaccination Dashboard, CDC, National Center for Immunization and Respiratory Diseases.
6. Weekly COVID-19 Vaccination Dashboard, CDC. https://www.cdc.gov/covidvaxview/weekly-dashboard/index.html
7. Louie JK et al. N Engl J Med. 2010;362:27-35. 8. Ciapponi A et al. Vaccine. 2021;39(40):5891-908.
9. Prasad S et al. Nature Communications. 2022;13:2414. 10. Fleming-Dutra KE et al. Obstet Gynecol Clin North Am 2023;50(2):279-97. 11. Mouen S et al. JAMA Network Open 2024;7(7):e2419268.


