User login
Molecular insights suggest novel therapies for hidradenitis suppurativa
at the virtual annual congress of the European Academy of Dermatology and Venereology.
He presented highlights of a multicenter translational study, which utilized whole transcriptome analysis of lesional and nonlesional skin from patients with HS and normal controls along with quantitative real-time PCR and immunohistochemistry. The purpose was to further define the molecular taxonomy of this inflammatory disease. And while this objective was achieved, the results also underscored a truism regarding the painful and scarring disease: “HS is characterized by an ever-growing complexity, which translates into multiple potential mechanistic drivers,” observed Dr. da Costa, head of immunology precision medicine at AstraZeneca in Gothenburg, Sweden.
Indeed, the study identified a panel of immune-related drivers in HS that influence innate immunity and cell differentiation in follicular and epidermal keratinocytes. The research by Dr. da Costa and coinvestigators identified a broad array of promising novel therapeutic targets in HS.
“Our findings provide evidence of an inflammatory process coupled with impaired barrier function, altered epidermal cell differentiation, and possibly abnormal microbiome activity which can be seen at the follicular and epidermal keratinocytes and also to a minor degree at the level of the skin glands,” Dr. da Costa said.
There is a huge unmet need for new therapies for HS, since at present adalimumab (Humira) is the only approved medication for this debilitating inflammatory disease. Some good news that emerged from this translational study is that some of the novel molecular mediators implicated in HS are targeted by multiple Food and Drug Administration–approved therapies that have other indications. From a drug development standpoint, repurposing a commercially available drug for a novel indication is a much more efficient and less costly endeavor than is necessary to establish the safety and efficacy of an unproven new agent.
The translational work demonstrated that the proteins calgranulin-A and -B and serpin-B4 were strongly expressed in the hair root sheaths of patients with HS. Connexin-32 and koebnerisin were present in stratum granulosum, matrix metallopeptidase-9 was strongly expressed in resident monocytes, small prolin-rich protein 3 in apocrine sweat glands and ducts as well as in sebaceous glands and ducts, and transcobalamin-1 was prominent in stratum spinosum.
Of the 19 key molecular mediators of HS identified in the study, FDA-approved agents are already available that target 12 of them. For example, apremilast (Otezla) targets interferon-gamma and tumor necrosis factor–alpha. Gentamicin targets growth arrest-specific 6 (GAS6) and interleukin-17 (IL-17). Secukinumab (Cosentyx) and ixekizumab (Taltz) target IL-17A, and brodalumab (Siliq) more broadly targets IL-17A as well as all the other IL-17 receptors. Thalidomide targets hepatocyte growth factor (HGF) and TNF-alpha. Spironolactone targets androgen receptor (AR) and TNF-alpha. Colchicine targets tubulin. Anakinra (Kineret) homes in on the IL-1 receptor. And prednisone targets NFxB.
Other key molecular mediators of HS, which are targeted by commercially available drugs, include epidermal growth factor (EGF), macrophage colony-stimulating factor (MCSF), epiregulin (EREG), fibroblast growth factor 1 (FGF1), FGF2, insulin-like growth factor 2 (IGF2), and IL-6, according to Dr. da Costa.
In addition, clinical trials are underway in HS involving totally investigational agents, including several Janus kinase inhibitors and tyrosine kinase 2 inhibitors.
The work described by Dr. da Costa had multiple funding sources, including the European Hidradenitis Suppurativa Foundation, the University of Copenhagen, the Icahn School of Medicine at Mount Sinai, AstraZeneca, and the German Federal Ministry of Education and Research. Dr. da Costa is an employee of AstraZeneca, Gothenburg, Sweden.
at the virtual annual congress of the European Academy of Dermatology and Venereology.
He presented highlights of a multicenter translational study, which utilized whole transcriptome analysis of lesional and nonlesional skin from patients with HS and normal controls along with quantitative real-time PCR and immunohistochemistry. The purpose was to further define the molecular taxonomy of this inflammatory disease. And while this objective was achieved, the results also underscored a truism regarding the painful and scarring disease: “HS is characterized by an ever-growing complexity, which translates into multiple potential mechanistic drivers,” observed Dr. da Costa, head of immunology precision medicine at AstraZeneca in Gothenburg, Sweden.
Indeed, the study identified a panel of immune-related drivers in HS that influence innate immunity and cell differentiation in follicular and epidermal keratinocytes. The research by Dr. da Costa and coinvestigators identified a broad array of promising novel therapeutic targets in HS.
“Our findings provide evidence of an inflammatory process coupled with impaired barrier function, altered epidermal cell differentiation, and possibly abnormal microbiome activity which can be seen at the follicular and epidermal keratinocytes and also to a minor degree at the level of the skin glands,” Dr. da Costa said.
There is a huge unmet need for new therapies for HS, since at present adalimumab (Humira) is the only approved medication for this debilitating inflammatory disease. Some good news that emerged from this translational study is that some of the novel molecular mediators implicated in HS are targeted by multiple Food and Drug Administration–approved therapies that have other indications. From a drug development standpoint, repurposing a commercially available drug for a novel indication is a much more efficient and less costly endeavor than is necessary to establish the safety and efficacy of an unproven new agent.
The translational work demonstrated that the proteins calgranulin-A and -B and serpin-B4 were strongly expressed in the hair root sheaths of patients with HS. Connexin-32 and koebnerisin were present in stratum granulosum, matrix metallopeptidase-9 was strongly expressed in resident monocytes, small prolin-rich protein 3 in apocrine sweat glands and ducts as well as in sebaceous glands and ducts, and transcobalamin-1 was prominent in stratum spinosum.
Of the 19 key molecular mediators of HS identified in the study, FDA-approved agents are already available that target 12 of them. For example, apremilast (Otezla) targets interferon-gamma and tumor necrosis factor–alpha. Gentamicin targets growth arrest-specific 6 (GAS6) and interleukin-17 (IL-17). Secukinumab (Cosentyx) and ixekizumab (Taltz) target IL-17A, and brodalumab (Siliq) more broadly targets IL-17A as well as all the other IL-17 receptors. Thalidomide targets hepatocyte growth factor (HGF) and TNF-alpha. Spironolactone targets androgen receptor (AR) and TNF-alpha. Colchicine targets tubulin. Anakinra (Kineret) homes in on the IL-1 receptor. And prednisone targets NFxB.
Other key molecular mediators of HS, which are targeted by commercially available drugs, include epidermal growth factor (EGF), macrophage colony-stimulating factor (MCSF), epiregulin (EREG), fibroblast growth factor 1 (FGF1), FGF2, insulin-like growth factor 2 (IGF2), and IL-6, according to Dr. da Costa.
In addition, clinical trials are underway in HS involving totally investigational agents, including several Janus kinase inhibitors and tyrosine kinase 2 inhibitors.
The work described by Dr. da Costa had multiple funding sources, including the European Hidradenitis Suppurativa Foundation, the University of Copenhagen, the Icahn School of Medicine at Mount Sinai, AstraZeneca, and the German Federal Ministry of Education and Research. Dr. da Costa is an employee of AstraZeneca, Gothenburg, Sweden.
at the virtual annual congress of the European Academy of Dermatology and Venereology.
He presented highlights of a multicenter translational study, which utilized whole transcriptome analysis of lesional and nonlesional skin from patients with HS and normal controls along with quantitative real-time PCR and immunohistochemistry. The purpose was to further define the molecular taxonomy of this inflammatory disease. And while this objective was achieved, the results also underscored a truism regarding the painful and scarring disease: “HS is characterized by an ever-growing complexity, which translates into multiple potential mechanistic drivers,” observed Dr. da Costa, head of immunology precision medicine at AstraZeneca in Gothenburg, Sweden.
Indeed, the study identified a panel of immune-related drivers in HS that influence innate immunity and cell differentiation in follicular and epidermal keratinocytes. The research by Dr. da Costa and coinvestigators identified a broad array of promising novel therapeutic targets in HS.
“Our findings provide evidence of an inflammatory process coupled with impaired barrier function, altered epidermal cell differentiation, and possibly abnormal microbiome activity which can be seen at the follicular and epidermal keratinocytes and also to a minor degree at the level of the skin glands,” Dr. da Costa said.
There is a huge unmet need for new therapies for HS, since at present adalimumab (Humira) is the only approved medication for this debilitating inflammatory disease. Some good news that emerged from this translational study is that some of the novel molecular mediators implicated in HS are targeted by multiple Food and Drug Administration–approved therapies that have other indications. From a drug development standpoint, repurposing a commercially available drug for a novel indication is a much more efficient and less costly endeavor than is necessary to establish the safety and efficacy of an unproven new agent.
The translational work demonstrated that the proteins calgranulin-A and -B and serpin-B4 were strongly expressed in the hair root sheaths of patients with HS. Connexin-32 and koebnerisin were present in stratum granulosum, matrix metallopeptidase-9 was strongly expressed in resident monocytes, small prolin-rich protein 3 in apocrine sweat glands and ducts as well as in sebaceous glands and ducts, and transcobalamin-1 was prominent in stratum spinosum.
Of the 19 key molecular mediators of HS identified in the study, FDA-approved agents are already available that target 12 of them. For example, apremilast (Otezla) targets interferon-gamma and tumor necrosis factor–alpha. Gentamicin targets growth arrest-specific 6 (GAS6) and interleukin-17 (IL-17). Secukinumab (Cosentyx) and ixekizumab (Taltz) target IL-17A, and brodalumab (Siliq) more broadly targets IL-17A as well as all the other IL-17 receptors. Thalidomide targets hepatocyte growth factor (HGF) and TNF-alpha. Spironolactone targets androgen receptor (AR) and TNF-alpha. Colchicine targets tubulin. Anakinra (Kineret) homes in on the IL-1 receptor. And prednisone targets NFxB.
Other key molecular mediators of HS, which are targeted by commercially available drugs, include epidermal growth factor (EGF), macrophage colony-stimulating factor (MCSF), epiregulin (EREG), fibroblast growth factor 1 (FGF1), FGF2, insulin-like growth factor 2 (IGF2), and IL-6, according to Dr. da Costa.
In addition, clinical trials are underway in HS involving totally investigational agents, including several Janus kinase inhibitors and tyrosine kinase 2 inhibitors.
The work described by Dr. da Costa had multiple funding sources, including the European Hidradenitis Suppurativa Foundation, the University of Copenhagen, the Icahn School of Medicine at Mount Sinai, AstraZeneca, and the German Federal Ministry of Education and Research. Dr. da Costa is an employee of AstraZeneca, Gothenburg, Sweden.
FROM THE EADV CONGRESS
Adalimumab enhances primary wound closure after HS surgery
, a pilot study suggests.
“Our experience suggests that under the effects of treatment with adalimumab, wound healing disorders with primary wound closure occur less often. And primary wound closure offers advantages over secondary wound healing: shorter length of inpatient stay, lower morbidity, fewer functional problems, and better quality of life,” Gefion Girbig, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
She noted that primary wound closure following surgery for HS is controversial. For example, current German guidelines recommend complete surgical excision of HS lesions, followed by secondary wound healing; the guidelines advise against primary wound closure. But those guidelines were issued back in 2012, years before adalimumab (Humira) achieved regulatory approval as the first and to date only medication indicated for treatment of HS.
Experts agree that while adalimumab has been a difference maker for many patients with HS, surgery is still often necessary. And many surgeons prefer secondary wound healing in HS. That’s because healing by first intention has historically often resulted in complications involving wound healing disorders and infection. These complications necessitate loosening of the primary closure to permit further wound healing by second intention, with a resultant prolonged healing time, explained Dr. Girbig, of the Institute for Health Sciences Research in Dermatology and Nursing at University Medical Center Hamburg-Eppendorf (Germany).
She and her coinvestigators hypothesized that the disordered wound healing is a consequence of the underlying inflammatory disease that lies at the core of HS, and that quelling the inflammation with adalimumab for at least 6 months before performing surgery with primary closure while the anti-TNF therapy continues would reduce the incidence of wound healing disorders.
This was borne out in the group’s small observational pilot study. It included 10 patients with HS who underwent surgery only after at least 6 months on adalimumab. Six had surgery for axillary HS and four for inguinal disease. Only 2 of the 10 developed a wound healing disorder. Both had surgical reconstruction in the inguinal area. Neither case involved infection. Surgical management entailed opening part of the suture to allow simultaneous secondary wound closure.
This 20% incidence of disordered wound healing when primary closure was carried out while systemic inflammation was controlled via adalimumab is markedly lower than rates reported using primary closure without adalimumab. Dr. Girbig and her coinvestigators are now conducting a larger controlled study to confirm their findings.
She reported having no financial conflicts regarding her study.
, a pilot study suggests.
“Our experience suggests that under the effects of treatment with adalimumab, wound healing disorders with primary wound closure occur less often. And primary wound closure offers advantages over secondary wound healing: shorter length of inpatient stay, lower morbidity, fewer functional problems, and better quality of life,” Gefion Girbig, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
She noted that primary wound closure following surgery for HS is controversial. For example, current German guidelines recommend complete surgical excision of HS lesions, followed by secondary wound healing; the guidelines advise against primary wound closure. But those guidelines were issued back in 2012, years before adalimumab (Humira) achieved regulatory approval as the first and to date only medication indicated for treatment of HS.
Experts agree that while adalimumab has been a difference maker for many patients with HS, surgery is still often necessary. And many surgeons prefer secondary wound healing in HS. That’s because healing by first intention has historically often resulted in complications involving wound healing disorders and infection. These complications necessitate loosening of the primary closure to permit further wound healing by second intention, with a resultant prolonged healing time, explained Dr. Girbig, of the Institute for Health Sciences Research in Dermatology and Nursing at University Medical Center Hamburg-Eppendorf (Germany).
She and her coinvestigators hypothesized that the disordered wound healing is a consequence of the underlying inflammatory disease that lies at the core of HS, and that quelling the inflammation with adalimumab for at least 6 months before performing surgery with primary closure while the anti-TNF therapy continues would reduce the incidence of wound healing disorders.
This was borne out in the group’s small observational pilot study. It included 10 patients with HS who underwent surgery only after at least 6 months on adalimumab. Six had surgery for axillary HS and four for inguinal disease. Only 2 of the 10 developed a wound healing disorder. Both had surgical reconstruction in the inguinal area. Neither case involved infection. Surgical management entailed opening part of the suture to allow simultaneous secondary wound closure.
This 20% incidence of disordered wound healing when primary closure was carried out while systemic inflammation was controlled via adalimumab is markedly lower than rates reported using primary closure without adalimumab. Dr. Girbig and her coinvestigators are now conducting a larger controlled study to confirm their findings.
She reported having no financial conflicts regarding her study.
, a pilot study suggests.
“Our experience suggests that under the effects of treatment with adalimumab, wound healing disorders with primary wound closure occur less often. And primary wound closure offers advantages over secondary wound healing: shorter length of inpatient stay, lower morbidity, fewer functional problems, and better quality of life,” Gefion Girbig, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
She noted that primary wound closure following surgery for HS is controversial. For example, current German guidelines recommend complete surgical excision of HS lesions, followed by secondary wound healing; the guidelines advise against primary wound closure. But those guidelines were issued back in 2012, years before adalimumab (Humira) achieved regulatory approval as the first and to date only medication indicated for treatment of HS.
Experts agree that while adalimumab has been a difference maker for many patients with HS, surgery is still often necessary. And many surgeons prefer secondary wound healing in HS. That’s because healing by first intention has historically often resulted in complications involving wound healing disorders and infection. These complications necessitate loosening of the primary closure to permit further wound healing by second intention, with a resultant prolonged healing time, explained Dr. Girbig, of the Institute for Health Sciences Research in Dermatology and Nursing at University Medical Center Hamburg-Eppendorf (Germany).
She and her coinvestigators hypothesized that the disordered wound healing is a consequence of the underlying inflammatory disease that lies at the core of HS, and that quelling the inflammation with adalimumab for at least 6 months before performing surgery with primary closure while the anti-TNF therapy continues would reduce the incidence of wound healing disorders.
This was borne out in the group’s small observational pilot study. It included 10 patients with HS who underwent surgery only after at least 6 months on adalimumab. Six had surgery for axillary HS and four for inguinal disease. Only 2 of the 10 developed a wound healing disorder. Both had surgical reconstruction in the inguinal area. Neither case involved infection. Surgical management entailed opening part of the suture to allow simultaneous secondary wound closure.
This 20% incidence of disordered wound healing when primary closure was carried out while systemic inflammation was controlled via adalimumab is markedly lower than rates reported using primary closure without adalimumab. Dr. Girbig and her coinvestigators are now conducting a larger controlled study to confirm their findings.
She reported having no financial conflicts regarding her study.
FROM THE EADV CONGRESS
Expert highlights advances in DRESS
Mounting evidence suggests , Sarah Walsh, MD, said at the virtual annual congress of the European Academy of Dermatology and Venereology.
The standard dictum has been that diagnosis of this severe T-cell-mediated drug reaction requires more than a 2-week delay in symptom onset following initial drug intake. But this can steer physicians in the wrong direction and lead to stopping an innocent drug while the true culprit medication remains on board. This adversely affects patient prognosis, since a longer duration of drug exposure after symptom onset is associated with increased hospital length of stay and greater mortality risk, explained Dr. Walsh, clinical lead for dermatology at King’s College Hospital, London.
In addition to . These include clues provided by rash morphology and histopathology, HLA testing, and a novel scoring system to assess DRESS severity and the risk of potentially fatal cytomegalovirus reactivation.
Short-delay DRESS onset
In a retrospective study of 41 patients with a first episode of DRESS in three French dermatology departments, 14 (34%) had onset within 15 days or less of initial exposure to the causative drug. In 6 of 14 patients in the rapid-onset group the offending drug was an antibiotic, while in another 5 the culprit was iodinated contrast media. In the delayed-onset DRESS group, the chief sensitizers were allopurinol in 8 patients, lamotrigine in 6, carbamazepine in 4, and sulfasalazine in 2; of note, none of these 4 delayed-onset DRESS drugs were implicated in any cases of rapid-onset DRESS. There were no differences in the clinical manifestations of DRESS between the rapid- and delayed-onset groups.
Similarly, dermatologists at Government Medical College in Kerala, India, reported in a retrospective study of 100 consecutive patients with DRESS, the drug reaction emerged within 2 weeks after starting the culprit medication in 36% of cases. Indeed, 11 patients became symptomatic within 3-7 days after beginning the medication; in 10 of the 11 cases, the offending agent was an antibiotic, and in 1 patient it was terbinafine. In the 25 cases of DRESS that arose on day 8-14 of drug therapy, the culprit was phenytoin in 14, antibiotics in 6, and 1 each for clopidogrel, hydroxychloroquine, sodium valproate, lamotrigine, and vitamin D3.
Both groups of investigators concluded that a short time lag between starting a drug and development of symptoms of a drug reaction shouldn’t rule out DRESS as a possibility provided other criteria consistent with the diagnosis are present. Hallmarks of DRESS include an acute extensive rash, fever greater than 38 degrees C, enlarged lymph nodes at two or more sites, internal organ involvement, a low platelet count, elevated eosinophils, and abnormal lymphocyte levels.
Rash morphology and histology as prognostic indicators
Dr. Walsh was the lead investigator in a study that identified four distinct patterns of skin involvement in patients with DRESS. The most common type of rash in this single-center retrospective study of 27 consecutive patients was an urticated papular exanthem, present in 13 of the 27 patients. An erythema multiforme-like reaction was present in 8, exfoliative erythroderma in 3, and a morbilliform erythema in 3 others. The worst prognosis was in the subgroup with an erythema multiforme-like rash.
All 27 patients had hepatic involvement, which was severe in 9 cases. Six of the 9 with severe liver impairment had an erythema multiforme-like rash, compared with just 2 of the 18 with mild or moderate liver involvement; thus, an erythema multiforme-like skin eruption was associated with a fivefold increased likelihood of severe hepatic involvement.
“It is a clinical sign that we take seriously at presentation if atypical target lesions are present,” the dermatologist said.
Separately, Taiwanese investigators compared clinical and histopathologic features in a study of 32 patients with DRESS and 17 with maculopapular exanthem. Interface vacuolization, which was present in 29 of the 32 patients with DRESS, was far more prominent than in the comparator group. Moreover, severe dyskeratosis was significantly associated with more severe liver impairment in the DRESS group.
HLA testing
Testing for HLA haplotypes associated with severe drug reactions has a useful role as a screening tool prior to prescribing selected high-risk drugs, Dr. Walsh said. For example, it’s known that 6.8% of individuals of European ancestry carry HLA-A*32:01, an allele that was strongly associated with an increased rate of vancomycin-associated DRESS in a case-control study at Vanderbilt University, Nashville, Tenn. Indeed, 19 of 23 individuals with vancomycin-associated DRESS were HLA-A*32:01 positive, compared with none of 46 vancomycin-tolerant controls. Nineteen percent of HLA-A*32:01-positive patients developed DRESS during treatment with vancomycin, and the drug reaction occurred within 4 weeks.
The investigators noted that testing for HLA-A*32:01 is also useful in DRESS occurring in patients on vancomycin and multiple other drugs because the test’s high negative predictive value may safely allow continued therapy with this potent antibiotic for Gram-positive infections.
A DRESS prognostic scoring system
Japanese researchers have developed a scoring system for DRESS for use in monitoring severity of the drug reaction, predicting prognosis, and estimating the risk of developing cytomegalovirus disease and its potentially fatal complications. The scoring system incorporates patient factors, including age, duration of drug exposure after symptom onset; rash characteristics, such as percentage of body surface area involved and presence or absence of erythroderma; appetite loss; and laboratory values.
“It yields a prognostic score that can be used to determine treatment choices, such as immediate intervention with anti-CMV agents. It’s a very useful tool,” Dr. Walsh said.
She reported having no financial conflicts regarding her presentation.
Mounting evidence suggests , Sarah Walsh, MD, said at the virtual annual congress of the European Academy of Dermatology and Venereology.
The standard dictum has been that diagnosis of this severe T-cell-mediated drug reaction requires more than a 2-week delay in symptom onset following initial drug intake. But this can steer physicians in the wrong direction and lead to stopping an innocent drug while the true culprit medication remains on board. This adversely affects patient prognosis, since a longer duration of drug exposure after symptom onset is associated with increased hospital length of stay and greater mortality risk, explained Dr. Walsh, clinical lead for dermatology at King’s College Hospital, London.
In addition to . These include clues provided by rash morphology and histopathology, HLA testing, and a novel scoring system to assess DRESS severity and the risk of potentially fatal cytomegalovirus reactivation.
Short-delay DRESS onset
In a retrospective study of 41 patients with a first episode of DRESS in three French dermatology departments, 14 (34%) had onset within 15 days or less of initial exposure to the causative drug. In 6 of 14 patients in the rapid-onset group the offending drug was an antibiotic, while in another 5 the culprit was iodinated contrast media. In the delayed-onset DRESS group, the chief sensitizers were allopurinol in 8 patients, lamotrigine in 6, carbamazepine in 4, and sulfasalazine in 2; of note, none of these 4 delayed-onset DRESS drugs were implicated in any cases of rapid-onset DRESS. There were no differences in the clinical manifestations of DRESS between the rapid- and delayed-onset groups.
Similarly, dermatologists at Government Medical College in Kerala, India, reported in a retrospective study of 100 consecutive patients with DRESS, the drug reaction emerged within 2 weeks after starting the culprit medication in 36% of cases. Indeed, 11 patients became symptomatic within 3-7 days after beginning the medication; in 10 of the 11 cases, the offending agent was an antibiotic, and in 1 patient it was terbinafine. In the 25 cases of DRESS that arose on day 8-14 of drug therapy, the culprit was phenytoin in 14, antibiotics in 6, and 1 each for clopidogrel, hydroxychloroquine, sodium valproate, lamotrigine, and vitamin D3.
Both groups of investigators concluded that a short time lag between starting a drug and development of symptoms of a drug reaction shouldn’t rule out DRESS as a possibility provided other criteria consistent with the diagnosis are present. Hallmarks of DRESS include an acute extensive rash, fever greater than 38 degrees C, enlarged lymph nodes at two or more sites, internal organ involvement, a low platelet count, elevated eosinophils, and abnormal lymphocyte levels.
Rash morphology and histology as prognostic indicators
Dr. Walsh was the lead investigator in a study that identified four distinct patterns of skin involvement in patients with DRESS. The most common type of rash in this single-center retrospective study of 27 consecutive patients was an urticated papular exanthem, present in 13 of the 27 patients. An erythema multiforme-like reaction was present in 8, exfoliative erythroderma in 3, and a morbilliform erythema in 3 others. The worst prognosis was in the subgroup with an erythema multiforme-like rash.
All 27 patients had hepatic involvement, which was severe in 9 cases. Six of the 9 with severe liver impairment had an erythema multiforme-like rash, compared with just 2 of the 18 with mild or moderate liver involvement; thus, an erythema multiforme-like skin eruption was associated with a fivefold increased likelihood of severe hepatic involvement.
“It is a clinical sign that we take seriously at presentation if atypical target lesions are present,” the dermatologist said.
Separately, Taiwanese investigators compared clinical and histopathologic features in a study of 32 patients with DRESS and 17 with maculopapular exanthem. Interface vacuolization, which was present in 29 of the 32 patients with DRESS, was far more prominent than in the comparator group. Moreover, severe dyskeratosis was significantly associated with more severe liver impairment in the DRESS group.
HLA testing
Testing for HLA haplotypes associated with severe drug reactions has a useful role as a screening tool prior to prescribing selected high-risk drugs, Dr. Walsh said. For example, it’s known that 6.8% of individuals of European ancestry carry HLA-A*32:01, an allele that was strongly associated with an increased rate of vancomycin-associated DRESS in a case-control study at Vanderbilt University, Nashville, Tenn. Indeed, 19 of 23 individuals with vancomycin-associated DRESS were HLA-A*32:01 positive, compared with none of 46 vancomycin-tolerant controls. Nineteen percent of HLA-A*32:01-positive patients developed DRESS during treatment with vancomycin, and the drug reaction occurred within 4 weeks.
The investigators noted that testing for HLA-A*32:01 is also useful in DRESS occurring in patients on vancomycin and multiple other drugs because the test’s high negative predictive value may safely allow continued therapy with this potent antibiotic for Gram-positive infections.
A DRESS prognostic scoring system
Japanese researchers have developed a scoring system for DRESS for use in monitoring severity of the drug reaction, predicting prognosis, and estimating the risk of developing cytomegalovirus disease and its potentially fatal complications. The scoring system incorporates patient factors, including age, duration of drug exposure after symptom onset; rash characteristics, such as percentage of body surface area involved and presence or absence of erythroderma; appetite loss; and laboratory values.
“It yields a prognostic score that can be used to determine treatment choices, such as immediate intervention with anti-CMV agents. It’s a very useful tool,” Dr. Walsh said.
She reported having no financial conflicts regarding her presentation.
Mounting evidence suggests , Sarah Walsh, MD, said at the virtual annual congress of the European Academy of Dermatology and Venereology.
The standard dictum has been that diagnosis of this severe T-cell-mediated drug reaction requires more than a 2-week delay in symptom onset following initial drug intake. But this can steer physicians in the wrong direction and lead to stopping an innocent drug while the true culprit medication remains on board. This adversely affects patient prognosis, since a longer duration of drug exposure after symptom onset is associated with increased hospital length of stay and greater mortality risk, explained Dr. Walsh, clinical lead for dermatology at King’s College Hospital, London.
In addition to . These include clues provided by rash morphology and histopathology, HLA testing, and a novel scoring system to assess DRESS severity and the risk of potentially fatal cytomegalovirus reactivation.
Short-delay DRESS onset
In a retrospective study of 41 patients with a first episode of DRESS in three French dermatology departments, 14 (34%) had onset within 15 days or less of initial exposure to the causative drug. In 6 of 14 patients in the rapid-onset group the offending drug was an antibiotic, while in another 5 the culprit was iodinated contrast media. In the delayed-onset DRESS group, the chief sensitizers were allopurinol in 8 patients, lamotrigine in 6, carbamazepine in 4, and sulfasalazine in 2; of note, none of these 4 delayed-onset DRESS drugs were implicated in any cases of rapid-onset DRESS. There were no differences in the clinical manifestations of DRESS between the rapid- and delayed-onset groups.
Similarly, dermatologists at Government Medical College in Kerala, India, reported in a retrospective study of 100 consecutive patients with DRESS, the drug reaction emerged within 2 weeks after starting the culprit medication in 36% of cases. Indeed, 11 patients became symptomatic within 3-7 days after beginning the medication; in 10 of the 11 cases, the offending agent was an antibiotic, and in 1 patient it was terbinafine. In the 25 cases of DRESS that arose on day 8-14 of drug therapy, the culprit was phenytoin in 14, antibiotics in 6, and 1 each for clopidogrel, hydroxychloroquine, sodium valproate, lamotrigine, and vitamin D3.
Both groups of investigators concluded that a short time lag between starting a drug and development of symptoms of a drug reaction shouldn’t rule out DRESS as a possibility provided other criteria consistent with the diagnosis are present. Hallmarks of DRESS include an acute extensive rash, fever greater than 38 degrees C, enlarged lymph nodes at two or more sites, internal organ involvement, a low platelet count, elevated eosinophils, and abnormal lymphocyte levels.
Rash morphology and histology as prognostic indicators
Dr. Walsh was the lead investigator in a study that identified four distinct patterns of skin involvement in patients with DRESS. The most common type of rash in this single-center retrospective study of 27 consecutive patients was an urticated papular exanthem, present in 13 of the 27 patients. An erythema multiforme-like reaction was present in 8, exfoliative erythroderma in 3, and a morbilliform erythema in 3 others. The worst prognosis was in the subgroup with an erythema multiforme-like rash.
All 27 patients had hepatic involvement, which was severe in 9 cases. Six of the 9 with severe liver impairment had an erythema multiforme-like rash, compared with just 2 of the 18 with mild or moderate liver involvement; thus, an erythema multiforme-like skin eruption was associated with a fivefold increased likelihood of severe hepatic involvement.
“It is a clinical sign that we take seriously at presentation if atypical target lesions are present,” the dermatologist said.
Separately, Taiwanese investigators compared clinical and histopathologic features in a study of 32 patients with DRESS and 17 with maculopapular exanthem. Interface vacuolization, which was present in 29 of the 32 patients with DRESS, was far more prominent than in the comparator group. Moreover, severe dyskeratosis was significantly associated with more severe liver impairment in the DRESS group.
HLA testing
Testing for HLA haplotypes associated with severe drug reactions has a useful role as a screening tool prior to prescribing selected high-risk drugs, Dr. Walsh said. For example, it’s known that 6.8% of individuals of European ancestry carry HLA-A*32:01, an allele that was strongly associated with an increased rate of vancomycin-associated DRESS in a case-control study at Vanderbilt University, Nashville, Tenn. Indeed, 19 of 23 individuals with vancomycin-associated DRESS were HLA-A*32:01 positive, compared with none of 46 vancomycin-tolerant controls. Nineteen percent of HLA-A*32:01-positive patients developed DRESS during treatment with vancomycin, and the drug reaction occurred within 4 weeks.
The investigators noted that testing for HLA-A*32:01 is also useful in DRESS occurring in patients on vancomycin and multiple other drugs because the test’s high negative predictive value may safely allow continued therapy with this potent antibiotic for Gram-positive infections.
A DRESS prognostic scoring system
Japanese researchers have developed a scoring system for DRESS for use in monitoring severity of the drug reaction, predicting prognosis, and estimating the risk of developing cytomegalovirus disease and its potentially fatal complications. The scoring system incorporates patient factors, including age, duration of drug exposure after symptom onset; rash characteristics, such as percentage of body surface area involved and presence or absence of erythroderma; appetite loss; and laboratory values.
“It yields a prognostic score that can be used to determine treatment choices, such as immediate intervention with anti-CMV agents. It’s a very useful tool,” Dr. Walsh said.
She reported having no financial conflicts regarding her presentation.
FROM THE EADV CONGRESS
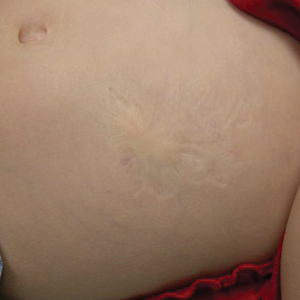
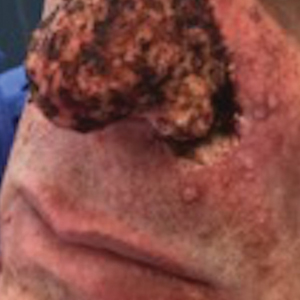
Atrophic Lesion on the Abdomen
The Diagnosis: Anetoderma of Prematurity
Anetoderma is a rare benign cutaneous disorder characterized by atrophic patches of skin due to dermal thinning. The term anetoderma is derived from the Greek words anetos (relaxed) and derma (skin).1 The physical appearance of the skin is associated with a reduction or loss of elastic tissue in the dermal layer, as seen on histolopathology.2
Two forms of anetoderma have been described. Primary anetoderma is an idiopathic form with no preceding inflammatory lesions. Secondary anetoderma is a reactive process linked to a known preceding inflammatory, infectious, autoimmune, or drug-induced condition.3 On histopathology, both primary and secondary anetoderma are characterized by a loss of elastic tissue or elastin fibers in the superficial to mid dermis.2
Anetoderma of prematurity was first described in 1996 by Prizant et al4 in 9 extremely premature (24-29 weeks' gestation) infants in neonatal intensive care units (NICUs). Although the exact mechanism behind anetoderma of prematurity is still unknown, Prizant et al4 and other investigators5 postulated that application of adhesive monitoring leads in the NICU played a role in the development of the lesions.
Iatrogenic anetoderma of prematurity is clinically characterized by circumscribed areas of either wrinkled macular depression or pouchlike herniations, ranging from flesh-colored to violaceous hues. Lesion size varies from a few millimeters to several centimeters in diameter, and they often are oval or round in shape.2 Although not common, it is possible for the atrophic patches to be preceded by an area of ecchymosis without necrosis or atrophy and, if present, they usually evolve within a few days to the characteristic appearance of anetoderma.3 They are found at discrete sites where monitoring leads or other medical devices are commonly placed, such as the forehead, abdomen, chest, and proximal limbs.
Lesions of anetoderma of prematurity are not present at birth, which distinguishes them from congenital anetoderma.6 It is unclear if the lesions are associated with the degree of prematurity, extremely low birth weight, or other associated factors of preterm birth. Although often clinically diagnosed, the diagnosis can be confirmed by a loss of elastic fibers on histopathology when stained with Verhoeff-van Gieson stain.1 Over time, the atrophic patches have the potential to evolve into herniated forms of anetoderma. Self-healing or improvement of the lesions often does not occur. Although the lesion is benign, it often requires surgical correction later in life for cosmesis.
Infants in the NICU are at risk for iatrogenic cutaneous injuries, which rarely may include anetoderma. Anetoderma of prematurity has been linked to the use of monitoring leads, adhesive tape, and other medical devices placed on the skin. Prizant et al4 postulated that the cause of anetoderma in these infants was irritants such as skin cleansers, urine, or sweat that may be trapped under the electrodes. Other hypotheses include local hypoxemia due to prolonged pressure from the electrodes on immature skin or excessive traction used when removing adhesive tape from the skin.7,8 Premature infants may be more susceptible to these lesions because of the reduced epidermal thickness of premature skin; immaturity of skin structure; or functional immaturity of elastin deposition regulators, such as elastase, lysyl oxidase, the complement system, and decay-accelerating factor.3 The diagnosis should be differentiated from congenital anetoderma, which also has been described in premature neonates but is characterized by lesions that are present at birth. Its origins are still unclear, despite having histopathologic features similar to iatrogenic anetoderma.9
Focal dermal hypoplasia (FDH) is the hallmark cutaneous finding in Goltz syndrome, a rare set of congenital abnormalities of the skin, oral structures, musculoskeletal system, and central nervous system. Similar to congenital anetoderma, FDH also is characterized by atrophic cutaneous lesions; however, the cutaneous lesions in FDH appear as linear, streaky atrophic lesions often with telangiectasias that follow Blaschko lines.10 The cutaneous lesions in FDH often are associated with other noncutaneous signs such as polydactyly or asymmetric limbs.10 Cutis laxa is caused by an abnormality in the elastic tissue resulting in a loose sagging appearance of the skin and frequently results in an aged facial appearance. There are both acquired and inherited forms that can be either solely cutaneous or present with extracutaneous features, such as cardiac abnormalities or emphysema.11
In contrast to the atrophic appearance of anetodermas, connective tissue nevi and nevus lipomatosus superficialis present as hamartomas that either can be present at birth or arise in infancy. Connective tissue nevi are hamartomas of dermal connective tissue that consist of excessive production of collagen, elastin, or glycosaminoglycans and appear as slightly elevated, flesh-colored to yellow nodules or plaques.12 Connective tissue nevi often are described in association with other diseases, most commonly tuberous sclerosis (shagreen patches) or familial cutaneous collagenoma. Nevus lipomatosus superficialis is an asymptomatic connective tissue hamartoma composed of mature adipocytes in the dermis. The lesions consist of clusters of flesh-colored to yellow, soft, rubbery papules or nodules with a smooth or verrucoid surface that do not cross the midline and may follow Blaschko lines.11
With advances in neonatal infant medical care, survival of extremely premature infants is increasing, and it is possible that this rare cutaneous disorder may become more prevalent. Care should be taken to avoid unnecessary pressure on surfaces where electrodes are placed and tightly applied adhesive tape. When electrodes are placed on the ventral side, the child should be placed supine; similarly, place electrodes on the dorsal side when the child is lying prone.5 A diagnosis of anetoderma of prematurity later in childhood may be difficult, so knowledge and awareness can help guide pediatricians and dermatologists to a correct diagnosis and prevent unnecessary evaluations and/or concerns.
- Misch KJ, Rhodes EL, Allen J, et al. Anetoderma of Jadassohn. J R Soc Med.1988;81:734-736.
- Venencie PY, Winkelmann RK. Histopathologic findings in anetoderma. Arch Dermatol. 1984;120:1040-1044.
- Maffeis L, Pugni L, Pietrasanta C, et al. Case report iatrogenic anetoderma of prematurity: a case report and review of the literature. 2014;2014:781493.
- Prizant TL, Lucky AW, Frieden IJ, et al. Spontaneous atrophic patches in extremely premature infants: anetoderma of prematurity. Arch Dermatol. 1996;132:671-674.
- Goujon E, Beer F, Gay S, et al. Anetoderma of prematurity: an iatrogenic consequence of neonatal intensive care anetoderma of prematurity from NICU. Arch Dermatol. 2010;146:565-567.
- Wain EM, Mellerio JE, Robson A, et al. Congenital anetoderma in a preterm infant. Pediatr Dermatol. 2008;25:626-629.
- Colditz PB, Dunster KR, Joy GJ, et al. Anetoderma of prematurity in association with electrocardiographic electrodes. J Am Acad Dermatol. 1999;41:479-481.
- Goujan E, Beer F, Gay S, et al. Study supervision. Arch Dermatol. 2010;146:565-567.
- Aberer E, Weissenbacher G. Congenital anetoderma induced by intrauterine infection? Arch Dermatol. 1997;133:526-527.
- Mallory SB, Krafchik BR, Moore DJ, et al. Goltz syndrome. Pediatr Dermatol. 1989;6:251-253.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. Elsevier Saunders; 2017.
- Uitto J, Santa Cruz DJ, Eisen AZ. Connective tissue nevi of the skin. clinical, genetic, and histopathologic classification of hamartomas of the collagen, elastin, and proteoglycan type. J Am Acad Dermatol. 1980;3:441-461.
The Diagnosis: Anetoderma of Prematurity
Anetoderma is a rare benign cutaneous disorder characterized by atrophic patches of skin due to dermal thinning. The term anetoderma is derived from the Greek words anetos (relaxed) and derma (skin).1 The physical appearance of the skin is associated with a reduction or loss of elastic tissue in the dermal layer, as seen on histolopathology.2
Two forms of anetoderma have been described. Primary anetoderma is an idiopathic form with no preceding inflammatory lesions. Secondary anetoderma is a reactive process linked to a known preceding inflammatory, infectious, autoimmune, or drug-induced condition.3 On histopathology, both primary and secondary anetoderma are characterized by a loss of elastic tissue or elastin fibers in the superficial to mid dermis.2
Anetoderma of prematurity was first described in 1996 by Prizant et al4 in 9 extremely premature (24-29 weeks' gestation) infants in neonatal intensive care units (NICUs). Although the exact mechanism behind anetoderma of prematurity is still unknown, Prizant et al4 and other investigators5 postulated that application of adhesive monitoring leads in the NICU played a role in the development of the lesions.
Iatrogenic anetoderma of prematurity is clinically characterized by circumscribed areas of either wrinkled macular depression or pouchlike herniations, ranging from flesh-colored to violaceous hues. Lesion size varies from a few millimeters to several centimeters in diameter, and they often are oval or round in shape.2 Although not common, it is possible for the atrophic patches to be preceded by an area of ecchymosis without necrosis or atrophy and, if present, they usually evolve within a few days to the characteristic appearance of anetoderma.3 They are found at discrete sites where monitoring leads or other medical devices are commonly placed, such as the forehead, abdomen, chest, and proximal limbs.
Lesions of anetoderma of prematurity are not present at birth, which distinguishes them from congenital anetoderma.6 It is unclear if the lesions are associated with the degree of prematurity, extremely low birth weight, or other associated factors of preterm birth. Although often clinically diagnosed, the diagnosis can be confirmed by a loss of elastic fibers on histopathology when stained with Verhoeff-van Gieson stain.1 Over time, the atrophic patches have the potential to evolve into herniated forms of anetoderma. Self-healing or improvement of the lesions often does not occur. Although the lesion is benign, it often requires surgical correction later in life for cosmesis.
Infants in the NICU are at risk for iatrogenic cutaneous injuries, which rarely may include anetoderma. Anetoderma of prematurity has been linked to the use of monitoring leads, adhesive tape, and other medical devices placed on the skin. Prizant et al4 postulated that the cause of anetoderma in these infants was irritants such as skin cleansers, urine, or sweat that may be trapped under the electrodes. Other hypotheses include local hypoxemia due to prolonged pressure from the electrodes on immature skin or excessive traction used when removing adhesive tape from the skin.7,8 Premature infants may be more susceptible to these lesions because of the reduced epidermal thickness of premature skin; immaturity of skin structure; or functional immaturity of elastin deposition regulators, such as elastase, lysyl oxidase, the complement system, and decay-accelerating factor.3 The diagnosis should be differentiated from congenital anetoderma, which also has been described in premature neonates but is characterized by lesions that are present at birth. Its origins are still unclear, despite having histopathologic features similar to iatrogenic anetoderma.9
Focal dermal hypoplasia (FDH) is the hallmark cutaneous finding in Goltz syndrome, a rare set of congenital abnormalities of the skin, oral structures, musculoskeletal system, and central nervous system. Similar to congenital anetoderma, FDH also is characterized by atrophic cutaneous lesions; however, the cutaneous lesions in FDH appear as linear, streaky atrophic lesions often with telangiectasias that follow Blaschko lines.10 The cutaneous lesions in FDH often are associated with other noncutaneous signs such as polydactyly or asymmetric limbs.10 Cutis laxa is caused by an abnormality in the elastic tissue resulting in a loose sagging appearance of the skin and frequently results in an aged facial appearance. There are both acquired and inherited forms that can be either solely cutaneous or present with extracutaneous features, such as cardiac abnormalities or emphysema.11
In contrast to the atrophic appearance of anetodermas, connective tissue nevi and nevus lipomatosus superficialis present as hamartomas that either can be present at birth or arise in infancy. Connective tissue nevi are hamartomas of dermal connective tissue that consist of excessive production of collagen, elastin, or glycosaminoglycans and appear as slightly elevated, flesh-colored to yellow nodules or plaques.12 Connective tissue nevi often are described in association with other diseases, most commonly tuberous sclerosis (shagreen patches) or familial cutaneous collagenoma. Nevus lipomatosus superficialis is an asymptomatic connective tissue hamartoma composed of mature adipocytes in the dermis. The lesions consist of clusters of flesh-colored to yellow, soft, rubbery papules or nodules with a smooth or verrucoid surface that do not cross the midline and may follow Blaschko lines.11
With advances in neonatal infant medical care, survival of extremely premature infants is increasing, and it is possible that this rare cutaneous disorder may become more prevalent. Care should be taken to avoid unnecessary pressure on surfaces where electrodes are placed and tightly applied adhesive tape. When electrodes are placed on the ventral side, the child should be placed supine; similarly, place electrodes on the dorsal side when the child is lying prone.5 A diagnosis of anetoderma of prematurity later in childhood may be difficult, so knowledge and awareness can help guide pediatricians and dermatologists to a correct diagnosis and prevent unnecessary evaluations and/or concerns.
The Diagnosis: Anetoderma of Prematurity
Anetoderma is a rare benign cutaneous disorder characterized by atrophic patches of skin due to dermal thinning. The term anetoderma is derived from the Greek words anetos (relaxed) and derma (skin).1 The physical appearance of the skin is associated with a reduction or loss of elastic tissue in the dermal layer, as seen on histolopathology.2
Two forms of anetoderma have been described. Primary anetoderma is an idiopathic form with no preceding inflammatory lesions. Secondary anetoderma is a reactive process linked to a known preceding inflammatory, infectious, autoimmune, or drug-induced condition.3 On histopathology, both primary and secondary anetoderma are characterized by a loss of elastic tissue or elastin fibers in the superficial to mid dermis.2
Anetoderma of prematurity was first described in 1996 by Prizant et al4 in 9 extremely premature (24-29 weeks' gestation) infants in neonatal intensive care units (NICUs). Although the exact mechanism behind anetoderma of prematurity is still unknown, Prizant et al4 and other investigators5 postulated that application of adhesive monitoring leads in the NICU played a role in the development of the lesions.
Iatrogenic anetoderma of prematurity is clinically characterized by circumscribed areas of either wrinkled macular depression or pouchlike herniations, ranging from flesh-colored to violaceous hues. Lesion size varies from a few millimeters to several centimeters in diameter, and they often are oval or round in shape.2 Although not common, it is possible for the atrophic patches to be preceded by an area of ecchymosis without necrosis or atrophy and, if present, they usually evolve within a few days to the characteristic appearance of anetoderma.3 They are found at discrete sites where monitoring leads or other medical devices are commonly placed, such as the forehead, abdomen, chest, and proximal limbs.
Lesions of anetoderma of prematurity are not present at birth, which distinguishes them from congenital anetoderma.6 It is unclear if the lesions are associated with the degree of prematurity, extremely low birth weight, or other associated factors of preterm birth. Although often clinically diagnosed, the diagnosis can be confirmed by a loss of elastic fibers on histopathology when stained with Verhoeff-van Gieson stain.1 Over time, the atrophic patches have the potential to evolve into herniated forms of anetoderma. Self-healing or improvement of the lesions often does not occur. Although the lesion is benign, it often requires surgical correction later in life for cosmesis.
Infants in the NICU are at risk for iatrogenic cutaneous injuries, which rarely may include anetoderma. Anetoderma of prematurity has been linked to the use of monitoring leads, adhesive tape, and other medical devices placed on the skin. Prizant et al4 postulated that the cause of anetoderma in these infants was irritants such as skin cleansers, urine, or sweat that may be trapped under the electrodes. Other hypotheses include local hypoxemia due to prolonged pressure from the electrodes on immature skin or excessive traction used when removing adhesive tape from the skin.7,8 Premature infants may be more susceptible to these lesions because of the reduced epidermal thickness of premature skin; immaturity of skin structure; or functional immaturity of elastin deposition regulators, such as elastase, lysyl oxidase, the complement system, and decay-accelerating factor.3 The diagnosis should be differentiated from congenital anetoderma, which also has been described in premature neonates but is characterized by lesions that are present at birth. Its origins are still unclear, despite having histopathologic features similar to iatrogenic anetoderma.9
Focal dermal hypoplasia (FDH) is the hallmark cutaneous finding in Goltz syndrome, a rare set of congenital abnormalities of the skin, oral structures, musculoskeletal system, and central nervous system. Similar to congenital anetoderma, FDH also is characterized by atrophic cutaneous lesions; however, the cutaneous lesions in FDH appear as linear, streaky atrophic lesions often with telangiectasias that follow Blaschko lines.10 The cutaneous lesions in FDH often are associated with other noncutaneous signs such as polydactyly or asymmetric limbs.10 Cutis laxa is caused by an abnormality in the elastic tissue resulting in a loose sagging appearance of the skin and frequently results in an aged facial appearance. There are both acquired and inherited forms that can be either solely cutaneous or present with extracutaneous features, such as cardiac abnormalities or emphysema.11
In contrast to the atrophic appearance of anetodermas, connective tissue nevi and nevus lipomatosus superficialis present as hamartomas that either can be present at birth or arise in infancy. Connective tissue nevi are hamartomas of dermal connective tissue that consist of excessive production of collagen, elastin, or glycosaminoglycans and appear as slightly elevated, flesh-colored to yellow nodules or plaques.12 Connective tissue nevi often are described in association with other diseases, most commonly tuberous sclerosis (shagreen patches) or familial cutaneous collagenoma. Nevus lipomatosus superficialis is an asymptomatic connective tissue hamartoma composed of mature adipocytes in the dermis. The lesions consist of clusters of flesh-colored to yellow, soft, rubbery papules or nodules with a smooth or verrucoid surface that do not cross the midline and may follow Blaschko lines.11
With advances in neonatal infant medical care, survival of extremely premature infants is increasing, and it is possible that this rare cutaneous disorder may become more prevalent. Care should be taken to avoid unnecessary pressure on surfaces where electrodes are placed and tightly applied adhesive tape. When electrodes are placed on the ventral side, the child should be placed supine; similarly, place electrodes on the dorsal side when the child is lying prone.5 A diagnosis of anetoderma of prematurity later in childhood may be difficult, so knowledge and awareness can help guide pediatricians and dermatologists to a correct diagnosis and prevent unnecessary evaluations and/or concerns.
- Misch KJ, Rhodes EL, Allen J, et al. Anetoderma of Jadassohn. J R Soc Med.1988;81:734-736.
- Venencie PY, Winkelmann RK. Histopathologic findings in anetoderma. Arch Dermatol. 1984;120:1040-1044.
- Maffeis L, Pugni L, Pietrasanta C, et al. Case report iatrogenic anetoderma of prematurity: a case report and review of the literature. 2014;2014:781493.
- Prizant TL, Lucky AW, Frieden IJ, et al. Spontaneous atrophic patches in extremely premature infants: anetoderma of prematurity. Arch Dermatol. 1996;132:671-674.
- Goujon E, Beer F, Gay S, et al. Anetoderma of prematurity: an iatrogenic consequence of neonatal intensive care anetoderma of prematurity from NICU. Arch Dermatol. 2010;146:565-567.
- Wain EM, Mellerio JE, Robson A, et al. Congenital anetoderma in a preterm infant. Pediatr Dermatol. 2008;25:626-629.
- Colditz PB, Dunster KR, Joy GJ, et al. Anetoderma of prematurity in association with electrocardiographic electrodes. J Am Acad Dermatol. 1999;41:479-481.
- Goujan E, Beer F, Gay S, et al. Study supervision. Arch Dermatol. 2010;146:565-567.
- Aberer E, Weissenbacher G. Congenital anetoderma induced by intrauterine infection? Arch Dermatol. 1997;133:526-527.
- Mallory SB, Krafchik BR, Moore DJ, et al. Goltz syndrome. Pediatr Dermatol. 1989;6:251-253.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. Elsevier Saunders; 2017.
- Uitto J, Santa Cruz DJ, Eisen AZ. Connective tissue nevi of the skin. clinical, genetic, and histopathologic classification of hamartomas of the collagen, elastin, and proteoglycan type. J Am Acad Dermatol. 1980;3:441-461.
- Misch KJ, Rhodes EL, Allen J, et al. Anetoderma of Jadassohn. J R Soc Med.1988;81:734-736.
- Venencie PY, Winkelmann RK. Histopathologic findings in anetoderma. Arch Dermatol. 1984;120:1040-1044.
- Maffeis L, Pugni L, Pietrasanta C, et al. Case report iatrogenic anetoderma of prematurity: a case report and review of the literature. 2014;2014:781493.
- Prizant TL, Lucky AW, Frieden IJ, et al. Spontaneous atrophic patches in extremely premature infants: anetoderma of prematurity. Arch Dermatol. 1996;132:671-674.
- Goujon E, Beer F, Gay S, et al. Anetoderma of prematurity: an iatrogenic consequence of neonatal intensive care anetoderma of prematurity from NICU. Arch Dermatol. 2010;146:565-567.
- Wain EM, Mellerio JE, Robson A, et al. Congenital anetoderma in a preterm infant. Pediatr Dermatol. 2008;25:626-629.
- Colditz PB, Dunster KR, Joy GJ, et al. Anetoderma of prematurity in association with electrocardiographic electrodes. J Am Acad Dermatol. 1999;41:479-481.
- Goujan E, Beer F, Gay S, et al. Study supervision. Arch Dermatol. 2010;146:565-567.
- Aberer E, Weissenbacher G. Congenital anetoderma induced by intrauterine infection? Arch Dermatol. 1997;133:526-527.
- Mallory SB, Krafchik BR, Moore DJ, et al. Goltz syndrome. Pediatr Dermatol. 1989;6:251-253.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. Elsevier Saunders; 2017.
- Uitto J, Santa Cruz DJ, Eisen AZ. Connective tissue nevi of the skin. clinical, genetic, and histopathologic classification of hamartomas of the collagen, elastin, and proteoglycan type. J Am Acad Dermatol. 1980;3:441-461.
An 18-month-old child presented with a 4-cm, atrophic, flesh-colored plaque on the left lateral aspect of the abdomen with overlying wrinkling of the skin. There was no outpouching of the skin or pain associated with the lesion. No other skin abnormalities were noted. The child was born premature at 30 weeks’ gestation (birth weight, 1400 g). The postnatal course was complicated by respiratory distress syndrome requiring prolonged ventilator support. The infant was in the neonatal intensive care unit for 5 months. The atrophic lesion first developed at 5 months of life and remained stable. Although the lesion was not present at birth, the parents noted that it was preceded by an ecchymotic lesion without necrosis that was first noticed at 2 months of life while the patient was in the neonatal intensive care unit.
Expert offers clinical pearls on leg ulcer therapy
Elena Conde Montero, MD, PhD, asserted at the virtual annual congress of the European Academy of Dermatology and Venereology.
In addition to delving into the finer points of compression therapy, she offered other clinical pearls for the treatment of chronic leg ulcers. These included the use of autologous punch grafting to reduce pain as well as promote healing, when to employ adjunctive negative pressure therapy, and the benefits of liquid sevoflurane for highly effective topical analgesia during wound cleansing and debridement.
Compression therapy
“If no contraindications exist, compression therapy is the best antihypertensive and anti-inflammatory treatment for all leg ulcers, not only venous leg ulcers,” according to Dr. Conde, a dermatologist at Infanta Leonor University Hospital in Madrid.
The list of absolute contraindications to compression treatment is brief, as highlighted in a recent international consensus statement. The expert writing panel named only four: severe peripheral artery disease, the presence of an epifascial arterial bypass, severe cardiac insufficiency, and true allergy to compression material.
Compression therapy provides multiple salutary effects. These include reduced capillary filtration of fluids to tissue, decreased swelling, enhanced tissue remodeling, better lymphatic drainage, reduced inflammatory cell counts, and increased arterial flow.
“This means that people with mild arterial disease will benefit from active compression because perfusion will improve,” Dr. Conde said.
Similarly, leg ulcers secondary to pyoderma gangrenosum will benefit from the anti-inflammatory effects of compression therapy in conjunction with standard immunotherapy, added the dermatologist, who coauthored a recent publication by the European Wound Management Association entitled “Atypical Wounds: Best Clinical Practices and Challenges.”
Four broad types of compression therapy are available: compression stockings, short-stretch bandages, multicomponent bandage systems, and self-adjusting compression wrap devices. The best clinical outcomes are achieved by individualized selection of a compression method based upon patient characteristics.
Short-stretch, low-elasticity bandages – such as the classic Unna boot loaded with zinc paste and topical corticosteroids – are well suited for patients with large leg ulcers. These bandages feature high working pressures during muscle contraction. They also provide low resting pressures, which is advantageous in patients with peripheral artery disease. The major disadvantage of short-stretch bandages is the need for frequent dressing changes by a nurse or other trained professional, since the compression is quickly lost as an unwanted consequence of the welcome reduction in swelling.
Multicomponent bandage systems feature two to four layers of bandages of differing stiffness, as well as padding material and in many cases pressure indicators. These bandages can often be worn for up to a week without needing to be changed, since they maintain adequate pressure long term. “These are very easy to use by nonexperts,” Dr. Conde noted.
A caveat regarding both short-stretch bandages and the multicomponent bandage systems: before applying them, it’s important to pad at-risk areas against injury caused by high pressures. These high-risk areas include the Achilles tendon, the pretibial region, and the lateral foot.
Self-adjusting compression systems are comprised of strips of short-stretch, low-elasticity fabric, which wrap around the leg and are fixed with Velcro closures. Dr. Conde hailed these devices as “a great innovation in compression therapy, without doubt.” Their major advantage is ease of application and removal by the patient. They are best-suited for treatment of small ulcers in patients who find it difficult to use compression stockings because of obesity or osteoarthritis, in patients who can’t tolerate such stockings because they have peripheral artery disease and the stockings’ high resting pressure is uncomfortable, or in individuals ill-suited for compression bandages because they lack adequate access to nursing care for the required frequent dressing changes.
Compression stockings are a good option for small ulcers. It’s easier for patients to wear shoes with compression stockings and thereby engage in normal everyday activities than with short-stretch bandages. A tip: Many patients find it arduous to don and remove a high-compression stocking that achieves the recommended pressure of 30-40 mm Hg at the point of transition between the Achilles tendon and the calf muscle, but the same effect can be achieved by overlapping two easier-to-use lower-compression stockings.
Punch grafting
This simple, cost-effective outpatient procedure was first described as a means of enhancing wound healing 150 years ago. The method involves utilizing a scalpel, curette, or punch to obtain a series of thin split-thickness skin grafts that contain epidermis and dermis down to the superficial papillary dermis. The grafts, usually harvested from the anterior thigh, are placed on the wound. This is followed by at least 5 days of local pressure and rest to promote graft uptake.
Sequential punch grafting is an excellent option for particularly challenging chronic ulcers, including Martorell hypertensive ischemic leg ulcers and other arteriolopathic ulcers in the elderly.
“Sequential punch grafting of wounds is very common in our clinics, especially for wounds that lack perfect grafting conditions,” Dr. Conde said.
She considers Martorell hypertensive ischemic leg ulcers to be underdiagnosed and undertreated. The Martorell leg ulcer is an exceedingly painful, rapidly progressive ischemic lesion, or bilateral lesions, with inflamed irregular margins. The disorder is caused by obstruction of subcutaneous arterioles in the absence of signs of vasculitis, and generally occurs in older individuals who have had well-controlled hypertension for many years. Diabetes, obesity, dyslipidemia, and peripheral artery disease are common comorbid conditions. The most common form of treatment – bioactive dressings in a moist environment – produces unsatisfactory results because it doesn’t address the inflammatory process.
Dr. Conde and coworkers have published the full details of how they achieved complete healing of Martorell hypertensive ischemic leg ulcers 3-8 weeks after punch grafting in three affected patients, all of whom presented with pain scores of 10/10 refractory even to opioid analgesics. The punch grafting was preceded by 15 days of topical corticosteroids and low-elasticity compression bandages in order to create adequate granulation tissue in the wound bed, which had the added benefit of achieving a 2- to 3-point reduction in pain scores even before the surgical procedure.
The pain-reducing effect of punch grafting isn’t as well appreciated as the wound-healing effect. Dr. Conde was first author of a recent study in which investigators systematically measured pain reduction in 136 patients with hard-to-heal leg ulcers of various etiologies treated with punch grafting. Nearly three-quarters of those who presented with painful ulcers were pain free after punch grafting, and the rest experienced greater than 70% pain reduction.
Pain suppression wasn’t dependent upon the percentage of graft uptake in this study. That’s because, as long as the wound isn’t overcleaned during dressing changes, even grafts that haven’t attached to the wound will release growth factors that promote wound healing, Dr. Conde explained.
Adjunctive negative pressure therapy
Portable vacuum-based negative pressure therapy devices are easy to use as a means to promote punch graft uptake. Negative pressure is best employed as an adjunct to punch grafting in suboptimal wound beds, longstanding ulcers, in patients with previous graft failure, or in challenging anatomic locations, such as the Achilles tendon or ankle. Dr. Conde has found the combination of punch grafting and negative pressure therapy especially helpful in patients with clinically inactive pyoderma gangrenosum.
Topical sevoflurane for analgesia
Most of the literature on topical sevoflurane for ulcer care has been published by Spanish researchers, but this form of analgesia deserves much more widespread use, according to Dr. Conde.
Sevoflurane is most often used as a gas in general anesthesia. In liquid form, however, it not only has a rapid, long-lasting analgesic effect when applied to painful leg ulcers, it also promotes healing because it is both antibacterial and a vasodilator. So before performing a potentially painful ulcer or wound cleaning, Dr. Conde recommended protecting perilesional skin with petroleum jelly, then irrigating the ulcer site with liquid sevoflurane. After that, it’s advisable to wait just 5-10 minutes before proceeding.
“It takes effect in much less time than EMLA cream,” she noted.
In one study of 30 adults aged over age 65 years with painful chronic venous ulcers refractory to conventional analgesics who underwent ulcer cleaning supported by topical sevoflurane at a dose of roughly 1 mL/cm2 of ulcer area every 2 days for a month, Spanish investigators documented onset of analgesic effect in 2-7 minutes, with a duration of 8-18 hours. The researchers found that the use of backup conventional analgesics ranging from acetaminophen to opioids was diminished. Side effects were limited to mild, transient itching and redness.
Dr. Conde reported having no financial conflicts of interest regarding her presentation.
Elena Conde Montero, MD, PhD, asserted at the virtual annual congress of the European Academy of Dermatology and Venereology.
In addition to delving into the finer points of compression therapy, she offered other clinical pearls for the treatment of chronic leg ulcers. These included the use of autologous punch grafting to reduce pain as well as promote healing, when to employ adjunctive negative pressure therapy, and the benefits of liquid sevoflurane for highly effective topical analgesia during wound cleansing and debridement.
Compression therapy
“If no contraindications exist, compression therapy is the best antihypertensive and anti-inflammatory treatment for all leg ulcers, not only venous leg ulcers,” according to Dr. Conde, a dermatologist at Infanta Leonor University Hospital in Madrid.
The list of absolute contraindications to compression treatment is brief, as highlighted in a recent international consensus statement. The expert writing panel named only four: severe peripheral artery disease, the presence of an epifascial arterial bypass, severe cardiac insufficiency, and true allergy to compression material.
Compression therapy provides multiple salutary effects. These include reduced capillary filtration of fluids to tissue, decreased swelling, enhanced tissue remodeling, better lymphatic drainage, reduced inflammatory cell counts, and increased arterial flow.
“This means that people with mild arterial disease will benefit from active compression because perfusion will improve,” Dr. Conde said.
Similarly, leg ulcers secondary to pyoderma gangrenosum will benefit from the anti-inflammatory effects of compression therapy in conjunction with standard immunotherapy, added the dermatologist, who coauthored a recent publication by the European Wound Management Association entitled “Atypical Wounds: Best Clinical Practices and Challenges.”
Four broad types of compression therapy are available: compression stockings, short-stretch bandages, multicomponent bandage systems, and self-adjusting compression wrap devices. The best clinical outcomes are achieved by individualized selection of a compression method based upon patient characteristics.
Short-stretch, low-elasticity bandages – such as the classic Unna boot loaded with zinc paste and topical corticosteroids – are well suited for patients with large leg ulcers. These bandages feature high working pressures during muscle contraction. They also provide low resting pressures, which is advantageous in patients with peripheral artery disease. The major disadvantage of short-stretch bandages is the need for frequent dressing changes by a nurse or other trained professional, since the compression is quickly lost as an unwanted consequence of the welcome reduction in swelling.
Multicomponent bandage systems feature two to four layers of bandages of differing stiffness, as well as padding material and in many cases pressure indicators. These bandages can often be worn for up to a week without needing to be changed, since they maintain adequate pressure long term. “These are very easy to use by nonexperts,” Dr. Conde noted.
A caveat regarding both short-stretch bandages and the multicomponent bandage systems: before applying them, it’s important to pad at-risk areas against injury caused by high pressures. These high-risk areas include the Achilles tendon, the pretibial region, and the lateral foot.
Self-adjusting compression systems are comprised of strips of short-stretch, low-elasticity fabric, which wrap around the leg and are fixed with Velcro closures. Dr. Conde hailed these devices as “a great innovation in compression therapy, without doubt.” Their major advantage is ease of application and removal by the patient. They are best-suited for treatment of small ulcers in patients who find it difficult to use compression stockings because of obesity or osteoarthritis, in patients who can’t tolerate such stockings because they have peripheral artery disease and the stockings’ high resting pressure is uncomfortable, or in individuals ill-suited for compression bandages because they lack adequate access to nursing care for the required frequent dressing changes.
Compression stockings are a good option for small ulcers. It’s easier for patients to wear shoes with compression stockings and thereby engage in normal everyday activities than with short-stretch bandages. A tip: Many patients find it arduous to don and remove a high-compression stocking that achieves the recommended pressure of 30-40 mm Hg at the point of transition between the Achilles tendon and the calf muscle, but the same effect can be achieved by overlapping two easier-to-use lower-compression stockings.
Punch grafting
This simple, cost-effective outpatient procedure was first described as a means of enhancing wound healing 150 years ago. The method involves utilizing a scalpel, curette, or punch to obtain a series of thin split-thickness skin grafts that contain epidermis and dermis down to the superficial papillary dermis. The grafts, usually harvested from the anterior thigh, are placed on the wound. This is followed by at least 5 days of local pressure and rest to promote graft uptake.
Sequential punch grafting is an excellent option for particularly challenging chronic ulcers, including Martorell hypertensive ischemic leg ulcers and other arteriolopathic ulcers in the elderly.
“Sequential punch grafting of wounds is very common in our clinics, especially for wounds that lack perfect grafting conditions,” Dr. Conde said.
She considers Martorell hypertensive ischemic leg ulcers to be underdiagnosed and undertreated. The Martorell leg ulcer is an exceedingly painful, rapidly progressive ischemic lesion, or bilateral lesions, with inflamed irregular margins. The disorder is caused by obstruction of subcutaneous arterioles in the absence of signs of vasculitis, and generally occurs in older individuals who have had well-controlled hypertension for many years. Diabetes, obesity, dyslipidemia, and peripheral artery disease are common comorbid conditions. The most common form of treatment – bioactive dressings in a moist environment – produces unsatisfactory results because it doesn’t address the inflammatory process.
Dr. Conde and coworkers have published the full details of how they achieved complete healing of Martorell hypertensive ischemic leg ulcers 3-8 weeks after punch grafting in three affected patients, all of whom presented with pain scores of 10/10 refractory even to opioid analgesics. The punch grafting was preceded by 15 days of topical corticosteroids and low-elasticity compression bandages in order to create adequate granulation tissue in the wound bed, which had the added benefit of achieving a 2- to 3-point reduction in pain scores even before the surgical procedure.
The pain-reducing effect of punch grafting isn’t as well appreciated as the wound-healing effect. Dr. Conde was first author of a recent study in which investigators systematically measured pain reduction in 136 patients with hard-to-heal leg ulcers of various etiologies treated with punch grafting. Nearly three-quarters of those who presented with painful ulcers were pain free after punch grafting, and the rest experienced greater than 70% pain reduction.
Pain suppression wasn’t dependent upon the percentage of graft uptake in this study. That’s because, as long as the wound isn’t overcleaned during dressing changes, even grafts that haven’t attached to the wound will release growth factors that promote wound healing, Dr. Conde explained.
Adjunctive negative pressure therapy
Portable vacuum-based negative pressure therapy devices are easy to use as a means to promote punch graft uptake. Negative pressure is best employed as an adjunct to punch grafting in suboptimal wound beds, longstanding ulcers, in patients with previous graft failure, or in challenging anatomic locations, such as the Achilles tendon or ankle. Dr. Conde has found the combination of punch grafting and negative pressure therapy especially helpful in patients with clinically inactive pyoderma gangrenosum.
Topical sevoflurane for analgesia
Most of the literature on topical sevoflurane for ulcer care has been published by Spanish researchers, but this form of analgesia deserves much more widespread use, according to Dr. Conde.
Sevoflurane is most often used as a gas in general anesthesia. In liquid form, however, it not only has a rapid, long-lasting analgesic effect when applied to painful leg ulcers, it also promotes healing because it is both antibacterial and a vasodilator. So before performing a potentially painful ulcer or wound cleaning, Dr. Conde recommended protecting perilesional skin with petroleum jelly, then irrigating the ulcer site with liquid sevoflurane. After that, it’s advisable to wait just 5-10 minutes before proceeding.
“It takes effect in much less time than EMLA cream,” she noted.
In one study of 30 adults aged over age 65 years with painful chronic venous ulcers refractory to conventional analgesics who underwent ulcer cleaning supported by topical sevoflurane at a dose of roughly 1 mL/cm2 of ulcer area every 2 days for a month, Spanish investigators documented onset of analgesic effect in 2-7 minutes, with a duration of 8-18 hours. The researchers found that the use of backup conventional analgesics ranging from acetaminophen to opioids was diminished. Side effects were limited to mild, transient itching and redness.
Dr. Conde reported having no financial conflicts of interest regarding her presentation.
Elena Conde Montero, MD, PhD, asserted at the virtual annual congress of the European Academy of Dermatology and Venereology.
In addition to delving into the finer points of compression therapy, she offered other clinical pearls for the treatment of chronic leg ulcers. These included the use of autologous punch grafting to reduce pain as well as promote healing, when to employ adjunctive negative pressure therapy, and the benefits of liquid sevoflurane for highly effective topical analgesia during wound cleansing and debridement.
Compression therapy
“If no contraindications exist, compression therapy is the best antihypertensive and anti-inflammatory treatment for all leg ulcers, not only venous leg ulcers,” according to Dr. Conde, a dermatologist at Infanta Leonor University Hospital in Madrid.
The list of absolute contraindications to compression treatment is brief, as highlighted in a recent international consensus statement. The expert writing panel named only four: severe peripheral artery disease, the presence of an epifascial arterial bypass, severe cardiac insufficiency, and true allergy to compression material.
Compression therapy provides multiple salutary effects. These include reduced capillary filtration of fluids to tissue, decreased swelling, enhanced tissue remodeling, better lymphatic drainage, reduced inflammatory cell counts, and increased arterial flow.
“This means that people with mild arterial disease will benefit from active compression because perfusion will improve,” Dr. Conde said.
Similarly, leg ulcers secondary to pyoderma gangrenosum will benefit from the anti-inflammatory effects of compression therapy in conjunction with standard immunotherapy, added the dermatologist, who coauthored a recent publication by the European Wound Management Association entitled “Atypical Wounds: Best Clinical Practices and Challenges.”
Four broad types of compression therapy are available: compression stockings, short-stretch bandages, multicomponent bandage systems, and self-adjusting compression wrap devices. The best clinical outcomes are achieved by individualized selection of a compression method based upon patient characteristics.
Short-stretch, low-elasticity bandages – such as the classic Unna boot loaded with zinc paste and topical corticosteroids – are well suited for patients with large leg ulcers. These bandages feature high working pressures during muscle contraction. They also provide low resting pressures, which is advantageous in patients with peripheral artery disease. The major disadvantage of short-stretch bandages is the need for frequent dressing changes by a nurse or other trained professional, since the compression is quickly lost as an unwanted consequence of the welcome reduction in swelling.
Multicomponent bandage systems feature two to four layers of bandages of differing stiffness, as well as padding material and in many cases pressure indicators. These bandages can often be worn for up to a week without needing to be changed, since they maintain adequate pressure long term. “These are very easy to use by nonexperts,” Dr. Conde noted.
A caveat regarding both short-stretch bandages and the multicomponent bandage systems: before applying them, it’s important to pad at-risk areas against injury caused by high pressures. These high-risk areas include the Achilles tendon, the pretibial region, and the lateral foot.
Self-adjusting compression systems are comprised of strips of short-stretch, low-elasticity fabric, which wrap around the leg and are fixed with Velcro closures. Dr. Conde hailed these devices as “a great innovation in compression therapy, without doubt.” Their major advantage is ease of application and removal by the patient. They are best-suited for treatment of small ulcers in patients who find it difficult to use compression stockings because of obesity or osteoarthritis, in patients who can’t tolerate such stockings because they have peripheral artery disease and the stockings’ high resting pressure is uncomfortable, or in individuals ill-suited for compression bandages because they lack adequate access to nursing care for the required frequent dressing changes.
Compression stockings are a good option for small ulcers. It’s easier for patients to wear shoes with compression stockings and thereby engage in normal everyday activities than with short-stretch bandages. A tip: Many patients find it arduous to don and remove a high-compression stocking that achieves the recommended pressure of 30-40 mm Hg at the point of transition between the Achilles tendon and the calf muscle, but the same effect can be achieved by overlapping two easier-to-use lower-compression stockings.
Punch grafting
This simple, cost-effective outpatient procedure was first described as a means of enhancing wound healing 150 years ago. The method involves utilizing a scalpel, curette, or punch to obtain a series of thin split-thickness skin grafts that contain epidermis and dermis down to the superficial papillary dermis. The grafts, usually harvested from the anterior thigh, are placed on the wound. This is followed by at least 5 days of local pressure and rest to promote graft uptake.
Sequential punch grafting is an excellent option for particularly challenging chronic ulcers, including Martorell hypertensive ischemic leg ulcers and other arteriolopathic ulcers in the elderly.
“Sequential punch grafting of wounds is very common in our clinics, especially for wounds that lack perfect grafting conditions,” Dr. Conde said.
She considers Martorell hypertensive ischemic leg ulcers to be underdiagnosed and undertreated. The Martorell leg ulcer is an exceedingly painful, rapidly progressive ischemic lesion, or bilateral lesions, with inflamed irregular margins. The disorder is caused by obstruction of subcutaneous arterioles in the absence of signs of vasculitis, and generally occurs in older individuals who have had well-controlled hypertension for many years. Diabetes, obesity, dyslipidemia, and peripheral artery disease are common comorbid conditions. The most common form of treatment – bioactive dressings in a moist environment – produces unsatisfactory results because it doesn’t address the inflammatory process.
Dr. Conde and coworkers have published the full details of how they achieved complete healing of Martorell hypertensive ischemic leg ulcers 3-8 weeks after punch grafting in three affected patients, all of whom presented with pain scores of 10/10 refractory even to opioid analgesics. The punch grafting was preceded by 15 days of topical corticosteroids and low-elasticity compression bandages in order to create adequate granulation tissue in the wound bed, which had the added benefit of achieving a 2- to 3-point reduction in pain scores even before the surgical procedure.
The pain-reducing effect of punch grafting isn’t as well appreciated as the wound-healing effect. Dr. Conde was first author of a recent study in which investigators systematically measured pain reduction in 136 patients with hard-to-heal leg ulcers of various etiologies treated with punch grafting. Nearly three-quarters of those who presented with painful ulcers were pain free after punch grafting, and the rest experienced greater than 70% pain reduction.
Pain suppression wasn’t dependent upon the percentage of graft uptake in this study. That’s because, as long as the wound isn’t overcleaned during dressing changes, even grafts that haven’t attached to the wound will release growth factors that promote wound healing, Dr. Conde explained.
Adjunctive negative pressure therapy
Portable vacuum-based negative pressure therapy devices are easy to use as a means to promote punch graft uptake. Negative pressure is best employed as an adjunct to punch grafting in suboptimal wound beds, longstanding ulcers, in patients with previous graft failure, or in challenging anatomic locations, such as the Achilles tendon or ankle. Dr. Conde has found the combination of punch grafting and negative pressure therapy especially helpful in patients with clinically inactive pyoderma gangrenosum.
Topical sevoflurane for analgesia
Most of the literature on topical sevoflurane for ulcer care has been published by Spanish researchers, but this form of analgesia deserves much more widespread use, according to Dr. Conde.
Sevoflurane is most often used as a gas in general anesthesia. In liquid form, however, it not only has a rapid, long-lasting analgesic effect when applied to painful leg ulcers, it also promotes healing because it is both antibacterial and a vasodilator. So before performing a potentially painful ulcer or wound cleaning, Dr. Conde recommended protecting perilesional skin with petroleum jelly, then irrigating the ulcer site with liquid sevoflurane. After that, it’s advisable to wait just 5-10 minutes before proceeding.
“It takes effect in much less time than EMLA cream,” she noted.
In one study of 30 adults aged over age 65 years with painful chronic venous ulcers refractory to conventional analgesics who underwent ulcer cleaning supported by topical sevoflurane at a dose of roughly 1 mL/cm2 of ulcer area every 2 days for a month, Spanish investigators documented onset of analgesic effect in 2-7 minutes, with a duration of 8-18 hours. The researchers found that the use of backup conventional analgesics ranging from acetaminophen to opioids was diminished. Side effects were limited to mild, transient itching and redness.
Dr. Conde reported having no financial conflicts of interest regarding her presentation.
FROM THE EADV CONGRESS
Optimizing Patient Positioning During Dermatologic Surgery
Practice Gap
Practical patient positioning is a commonly overlooked method of tension control during excision and repair that allows for easier closure.1 Although positioning is a basic step in dermatologic surgery, it often is difficult and awkward for both the patient and physician. Here, we describe basic principles in patient positioning that increase tension across the surgical site during excision and reduce tension during closure. By reducing the amount of work required for excision and closure, procedures are completed more quickly, which increases efficiency. These techniques should be considered during dermatologic surgery at sites that are subject to both high tension and repetitive motion, such as the upper back and lower extremities.
Technique: Upper Back Procedures
When removing lesions on the upper back, lying completely prone is uncomfortable for the patient and leaves the shoulders hyperextended.2 Instead, position the patient with the arms extended anteriorly, hugging a pillow, while lying prone or on one side (Figure 1). In this position, excision of the lesion is facilitated by increased tension across the upper back. In addition, this position is notably more comfortable for the patient. During closure, the patient should lie on the side contralateral to the surgical site, with the elbow resting at the hip and the ipsilateral arm lying parallel to the torso (Figure 2).
Following procedures on the upper back and shoulders, we typically recommend that the patient wear an arm sling on the ipsilateral side for 1 week. Doing so reliably limits mobility postoperatively and does not require the patient to constantly monitor their movement.
Technique: Lower Extremity Procedures
Anterior Lower Extremity
During excision of a lesion on the anterior lower extremity, we recommend that the patient be positioned with their knee bent and heel resting on the examination table. Ideally, the knee is flexed at approximately a 45° angle (Figure 3).3 In this position, excision of the lesion is facilitated by increased tension across the anterior lower extremity. During closure of these lesions, the patient should lie supine with the knee fully extended and the leg resting on the surgical bed or a pillow.
Posterior Lower Extremity
During excision of lesions on the posterior lower extremity, the patient should be positioned lying prone, with the knee fully extended, resting on the surgical bed or a pillow, which facilitates excision of the lesion by increasing tension across the site. During closure of these lesions, the patient should lie on the side contralateral to the surgical site, with the leg fully extended for support. The surgical leg should be flexed at the knee at approximately a 45° angle (Figure 4).
Practice Implications
Despite being an important step, patient positioning is an often-overlooked component of dermatologic surgery. Positioning becomes even more important in areas of high tension and repetitive motion, such as the upper back and lower extremities, where the risk of wound dehiscence and poor scar cosmesis is increased.1 Experienced dermatologic surgeons should utilize patient positioning, taking advantage of tension instead of working against it.
We have found that these 2 simple principles can aid in simplifying the excision and repair processes. Increasing tension across the surgical site during excision reduces the work required by the surgeon to reach the appropriate depth. Conversely, decreased tension across the surgical site decreases the work required for closure. These principles should be considered prior to the procedure; the patient should then be positioned in a way that maximizes tension across the surgical site during excision and minimizes tension across the surgical site during closure.
Incorporating these techniques, especially at sites that are subject to both high tension and repetitive motion, such as the upper back and lower extremities, not only increases efficiency but may also reduce the risk for wound dehiscence once the patient returns home and maintains their normal level of physical activity.
- Rohrer TE, Cook JL, Kaufman AJ. Flaps and Grafts in Dermatologic Surgery. 2nd ed. Elsevier; 2007.
- Kantor J. Atlas of Suturing Techniques: Approaches to Surgical Wound, Laceration, and Cosmetic Repair. 2nd ed. McGraw-Hill Education; 2016.
- Kiwanuka E, Cruz AP. Multistep approach for improved aesthetic and functional outcomes for lower extremity wound closure after Mohs micrographic surgery. Dermatol Surg. 2017;43:704-707.
Practice Gap
Practical patient positioning is a commonly overlooked method of tension control during excision and repair that allows for easier closure.1 Although positioning is a basic step in dermatologic surgery, it often is difficult and awkward for both the patient and physician. Here, we describe basic principles in patient positioning that increase tension across the surgical site during excision and reduce tension during closure. By reducing the amount of work required for excision and closure, procedures are completed more quickly, which increases efficiency. These techniques should be considered during dermatologic surgery at sites that are subject to both high tension and repetitive motion, such as the upper back and lower extremities.
Technique: Upper Back Procedures
When removing lesions on the upper back, lying completely prone is uncomfortable for the patient and leaves the shoulders hyperextended.2 Instead, position the patient with the arms extended anteriorly, hugging a pillow, while lying prone or on one side (Figure 1). In this position, excision of the lesion is facilitated by increased tension across the upper back. In addition, this position is notably more comfortable for the patient. During closure, the patient should lie on the side contralateral to the surgical site, with the elbow resting at the hip and the ipsilateral arm lying parallel to the torso (Figure 2).
Following procedures on the upper back and shoulders, we typically recommend that the patient wear an arm sling on the ipsilateral side for 1 week. Doing so reliably limits mobility postoperatively and does not require the patient to constantly monitor their movement.
Technique: Lower Extremity Procedures
Anterior Lower Extremity
During excision of a lesion on the anterior lower extremity, we recommend that the patient be positioned with their knee bent and heel resting on the examination table. Ideally, the knee is flexed at approximately a 45° angle (Figure 3).3 In this position, excision of the lesion is facilitated by increased tension across the anterior lower extremity. During closure of these lesions, the patient should lie supine with the knee fully extended and the leg resting on the surgical bed or a pillow.
Posterior Lower Extremity
During excision of lesions on the posterior lower extremity, the patient should be positioned lying prone, with the knee fully extended, resting on the surgical bed or a pillow, which facilitates excision of the lesion by increasing tension across the site. During closure of these lesions, the patient should lie on the side contralateral to the surgical site, with the leg fully extended for support. The surgical leg should be flexed at the knee at approximately a 45° angle (Figure 4).
Practice Implications
Despite being an important step, patient positioning is an often-overlooked component of dermatologic surgery. Positioning becomes even more important in areas of high tension and repetitive motion, such as the upper back and lower extremities, where the risk of wound dehiscence and poor scar cosmesis is increased.1 Experienced dermatologic surgeons should utilize patient positioning, taking advantage of tension instead of working against it.
We have found that these 2 simple principles can aid in simplifying the excision and repair processes. Increasing tension across the surgical site during excision reduces the work required by the surgeon to reach the appropriate depth. Conversely, decreased tension across the surgical site decreases the work required for closure. These principles should be considered prior to the procedure; the patient should then be positioned in a way that maximizes tension across the surgical site during excision and minimizes tension across the surgical site during closure.
Incorporating these techniques, especially at sites that are subject to both high tension and repetitive motion, such as the upper back and lower extremities, not only increases efficiency but may also reduce the risk for wound dehiscence once the patient returns home and maintains their normal level of physical activity.
Practice Gap
Practical patient positioning is a commonly overlooked method of tension control during excision and repair that allows for easier closure.1 Although positioning is a basic step in dermatologic surgery, it often is difficult and awkward for both the patient and physician. Here, we describe basic principles in patient positioning that increase tension across the surgical site during excision and reduce tension during closure. By reducing the amount of work required for excision and closure, procedures are completed more quickly, which increases efficiency. These techniques should be considered during dermatologic surgery at sites that are subject to both high tension and repetitive motion, such as the upper back and lower extremities.
Technique: Upper Back Procedures
When removing lesions on the upper back, lying completely prone is uncomfortable for the patient and leaves the shoulders hyperextended.2 Instead, position the patient with the arms extended anteriorly, hugging a pillow, while lying prone or on one side (Figure 1). In this position, excision of the lesion is facilitated by increased tension across the upper back. In addition, this position is notably more comfortable for the patient. During closure, the patient should lie on the side contralateral to the surgical site, with the elbow resting at the hip and the ipsilateral arm lying parallel to the torso (Figure 2).
Following procedures on the upper back and shoulders, we typically recommend that the patient wear an arm sling on the ipsilateral side for 1 week. Doing so reliably limits mobility postoperatively and does not require the patient to constantly monitor their movement.
Technique: Lower Extremity Procedures
Anterior Lower Extremity
During excision of a lesion on the anterior lower extremity, we recommend that the patient be positioned with their knee bent and heel resting on the examination table. Ideally, the knee is flexed at approximately a 45° angle (Figure 3).3 In this position, excision of the lesion is facilitated by increased tension across the anterior lower extremity. During closure of these lesions, the patient should lie supine with the knee fully extended and the leg resting on the surgical bed or a pillow.
Posterior Lower Extremity
During excision of lesions on the posterior lower extremity, the patient should be positioned lying prone, with the knee fully extended, resting on the surgical bed or a pillow, which facilitates excision of the lesion by increasing tension across the site. During closure of these lesions, the patient should lie on the side contralateral to the surgical site, with the leg fully extended for support. The surgical leg should be flexed at the knee at approximately a 45° angle (Figure 4).
Practice Implications
Despite being an important step, patient positioning is an often-overlooked component of dermatologic surgery. Positioning becomes even more important in areas of high tension and repetitive motion, such as the upper back and lower extremities, where the risk of wound dehiscence and poor scar cosmesis is increased.1 Experienced dermatologic surgeons should utilize patient positioning, taking advantage of tension instead of working against it.
We have found that these 2 simple principles can aid in simplifying the excision and repair processes. Increasing tension across the surgical site during excision reduces the work required by the surgeon to reach the appropriate depth. Conversely, decreased tension across the surgical site decreases the work required for closure. These principles should be considered prior to the procedure; the patient should then be positioned in a way that maximizes tension across the surgical site during excision and minimizes tension across the surgical site during closure.
Incorporating these techniques, especially at sites that are subject to both high tension and repetitive motion, such as the upper back and lower extremities, not only increases efficiency but may also reduce the risk for wound dehiscence once the patient returns home and maintains their normal level of physical activity.
- Rohrer TE, Cook JL, Kaufman AJ. Flaps and Grafts in Dermatologic Surgery. 2nd ed. Elsevier; 2007.
- Kantor J. Atlas of Suturing Techniques: Approaches to Surgical Wound, Laceration, and Cosmetic Repair. 2nd ed. McGraw-Hill Education; 2016.
- Kiwanuka E, Cruz AP. Multistep approach for improved aesthetic and functional outcomes for lower extremity wound closure after Mohs micrographic surgery. Dermatol Surg. 2017;43:704-707.
- Rohrer TE, Cook JL, Kaufman AJ. Flaps and Grafts in Dermatologic Surgery. 2nd ed. Elsevier; 2007.
- Kantor J. Atlas of Suturing Techniques: Approaches to Surgical Wound, Laceration, and Cosmetic Repair. 2nd ed. McGraw-Hill Education; 2016.
- Kiwanuka E, Cruz AP. Multistep approach for improved aesthetic and functional outcomes for lower extremity wound closure after Mohs micrographic surgery. Dermatol Surg. 2017;43:704-707.
Aquatic Antagonists: Sponge Dermatitis
Sponges are among the oldest animals on earth, appearing more than 640 million years ago before the Cambrian explosion, a period when most major animal phyla appeared in the fossil records.1 More than 10,000 species of sponges have been identified worldwide and are distributed from polar to tropical regions in both marine (Figure 1) and freshwater (Figure 2) environments. They inhabit both shallow waters as well as depths of more than 2800 m, with shallower sponges tending to be more vibrantly colored than their deeper counterparts. The wide-ranging habitats of sponges have led to size variations from as small as 0.05 mm to more than 3 m in height.2 Their taxonomic phylum, Porifera (meaning pore bearers), is derived from the millions of pores lining the surface of the sponge that are used to filter planktonic organisms.3 Flagellated epithelioid cells called choanocytes line the internal chambers of sponges, creating a water current that promotes filter feeding as well as nutrient absorption across their microvilli.4 The body walls of many sponges consist of a collagenous skeleton made up of spongin and spicules of silicon dioxide (silica) or calcium carbonate embedded in the spongin connective tissue matrix.5 Bath sponges lack silica spicules.
Sponges have been used in medicine for centuries. The first use in Western culture was recorded in 405
Mechanisms and Symptoms of Injury
Bathing sponges (silk sponges) derived from Spongia officinalis are harmless. Other sponges can exert their damaging effects through a variety of mechanisms that lead to dermatologic manifestations (eTable). Some species of sponges produce and secrete toxic metabolites (eg, crinotoxins) onto the body surface or into the surrounding water. They also are capable of synthesizing a mucous slime that can be irritating to human skin. Direct trauma also can be caused by fragments of the silica or calcium carbonate sponge skeleton penetrating the skin. Stinging members of the phylum Cnidaria can colonize the sponge, leading to injury when a human handles the sponge.25-27
Sponge dermatitis can be divided into 2 major categories: an initial pruritic dermatitis (Figure 3) that occurs within 20 minutes to a few hours after contact and a delayed irritant dermatitis caused by penetration of the spicules and chemical agents into skin.28 Importantly, different species can lead to varying manifestations.
The initial pruritic dermatitis is characterized by itching and burning that progresses to local edema, vesiculation, joint swelling, and stiffness. Because most contact with sponges occurs with handling, joint immobility may ensue within 24 hours of the encounter. Rarely, larger areas of the skin are affected, and fever, chills, malaise, dizziness, nausea, purulent bullae, muscle cramps, and formication may occur.28 Anaphylactic reactions have been described in a small subset of patients. There have even been reports of delayed (ie, 1–2 weeks following exposure) erythema multiforme, livedo reticularis, purpura, and dyshidrotic eczema.16,20,29 The irritant dermatitis caused by spicule trauma is due to a foreign body reaction that can be exacerbated by toxins entering the skin. In severe cases, desquamation, recurrent eczema, and arthralgia can occur.30 In general, more mild cases should self-resolve within 3 to 7 days. Dermatologic conditions also can be caused by organisms that inhabit sponges and as a result produce a dermatitis when the sponge is handled, including sponge divers disease (maladie des plongeurs), a necrotic dermatitis caused by stinging Cnidaria species.31 Dogger Bank itch, first described as a dermatitis caused by sensitization to (2-hydroxyethyl) dimethylsulfoxonium chloride, initially was isolated from the sea chervil (a type of Bryozoan); however, that same chemical also was later found in sponges, producing the same dermatitis after handling the sponge.32 Freshwater sponges also have been reported to be injurious and exist worldwide. In contrast to marine sponges, lesions from freshwater sponges are disseminated pruritic erythematous papules with ulcerations, crusts, and secondary infections.22 The disseminated nature of the dermatitis caused by freshwater sponges is due to contact with the spicules of dead sponges that are dispersed throughout the water rather than from direct handling. Sponge dermatitis occurs mostly in sponge collectors, divers, trawlers, and biology students and has been reported extensively in the United States, Caribbean Islands, Australia, New Zealand, and Brazil.18,27,33,34
Management
Treatment should consist of an initial decontamination; the skin should be dried, and adhesive tape or rubber cement should be utilized to remove any spicules embedded in the skin. Diluted vinegar soaks should be initiated for 10 to 30 minutes on the affected area(s) 3 or 4 times daily.19 The initial decontamination should occur immediately, as delay may lead to persistent purulent bullae that may take months to heal. Topical steroids may be used following the initial decontamination to help relieve inflammation. Antihistamines and nonsteroidal anti-inflammatory drugs may be used to alleviate pruritus and pain, respectively. Severe cases may require systemic glucocorticoids. Additionally, immunization status against tetanus toxoid should be assessed.35 In the event of an anaphylactic reaction, it is important to maintain a patent airway and normalized blood pressure through the use of intramuscular epinephrine.36 Frequent follow-up is warranted, as serious secondary infections can develop.37 Patients also should be counseled on the potential for delayed dermatologic reactions, including erythema multiforme. Contact between humans and coastal environments has been increasing in the last few decades; therefore, an increase in contact with sponges is to be expected.22
- Gold DA, Grabenstatter J, de Mendoza A, et al. Sterol and genomic analyses validate the sponge biomarker hypothesis. Proc Natl Acad Sci U S A. 2016;113:2684-2689.
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by sponges. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology. 2nd ed. Springer; 2016:121-126.
- Marsh LM, Slack-Smith S, Gurry DL. Field Guide to Sea Stingers and Other Venomous and Poisonous Marine Invertebrates. 2nd ed. Western Australian Museum; 2010.
- Eid E, Al-Tawaha M. A Guide to Harmful and Toxic Creatures in the Gulf of Aqaba Jordan. The Royal Marine Conservation Society of Jordan; 2016.
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131.
- Dormandy TL. Trace element analysis of hair. Br Med J (Clin Res Ed). 1986;293:975-976.
- Voultsiadou E. Sponges: an historical survey of their knowledge in Greek antiquity. J Mar Biol Assoc UK. 2007;87:1757-1763.
- Senthilkumar K, Kim SK. Marine invertebrate natural products for anti-inflammatory and chronic diseases [published online December 31, 2013]. Evid Based Complement Alternat Med. doi:10.1155/2013/572859
- Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8:2619-2638.
- Usagawa T, Nishimura M, Itoh Y, et al. Preparation of monoclonal antibodies against okadaic acid prepared from the sponge Halichondria okadai. Toxicon. 1989;27:1323-1330.
- Elston DM. Aquatic antagonists: sponge dermatitis. Cutis. 2007;80:279-280.
- Parra-Velandia FJ, Zea S, Van Soest RW. Reef sponges of the genus Agelas (Porifera: Demospongiae) from the Greater Caribbean. Zootaxa. 2014;3794:301-343.
- Hooper JN, Capon RJ, Hodder RA. A new species of toxic marine sponge (Porifera: Demospongiae: Poecilosclerida) from northwest Australia. The Beagle, Records of the Northern Territory Museum of Arts and sciences. 1991;8:27-36.
- Burnett JW, Calton GJ, Morgan RJ. Dermatitis due to stinging sponges. Cutis. 1987;39:476.
- Kizer KW. Marine envenomations. J Toxicol Clin Toxicol. 1983;21:527-555.
- Isbister GK, Hooper JN. Clinical effects of stings by sponges of the genus Tedania and a review of sponge stings worldwide. Toxicon. 2005;46:782-785.
- Fromont J, Abdo DA. New species of Haliclona (Demospongiae: Haplosclerida: Chalinidae) from Western Australia. Zootaxa. 2014;3835:97-109.
- Flachsenberger W, Holmes NJ, Leigh C, et al. Properties of the extract and spicules of the dermatitis inducing sponge Neofibularia mordens Hartman. J Toxicol Clin Toxicol. 1987;25:255-272.
- Southcott RV, Coulter JR. The effects of the southern Australian marine stinging sponges, Neofibularia mordens and Lissodendoryx sp. Med J Aust. 1971;2:895-901.
- Yaffee HS, Stargardter F. Erythema multiforme from Tedania ignis. report of a case and an experimental study of the mechanism of cutaneous irritation from the fire sponge. Arch Dermatol. 1963;87:601-604.
- Yaffee HS. Irritation from red sponge. N Engl J Med. 1970;282:51.
- Haddad V Jr. Environmental dermatology: skin manifestations of injuries caused by invertebrate aquatic animals. An Bras Dermatol. 2013;88:496-506.
- Volkmer-Ribeiro C, Lenzi HL, Orefice F, et al. Freshwater sponge spicules: a new agent of ocular pathology. Mem Inst Oswaldo Cruz. 2006;101:899-903.
- Cruz AA, Alencar VM, Medina NH, et al. Dangerous waters: outbreak of eye lesions caused by fresh water sponge spicules. Eye (Lond). 2013;27:398-402.
- Haddad V Jr. Clinical and therapeutic aspects of envenomations caused by sponges and jellyfish. In: Gopalakrishnakone P, Haddad V Jr, Kem WR, et al, eds. Marine and Freshwater Toxins. Springer; 2016:317-325.
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-750.
- Gaastra MT. Aquatic skin disorders. In: Faber WR, Hay RJ, Naafs B, eds. Imported Skin Diseases. 2nd ed. Wiley; 2012:283-292.
- Auerbach P. Envenomation by aquatic invertebrates. In: Auerbach P, ed. Wilderness Medicine. 6th ed. Elsevier Mosby; 2011;1596-1627.
- Sims JK, Irei MY. Human Hawaiian marine sponge poisoning. Hawaii Med J. 1979;38:263-270.
- Haddad V Jr. Aquatic animals of medical importance in Brazil. Rev Soc Bras Med Trop. 2003;36:591-597.
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Warabi K, Nakao Y, Matsunaga S, et al. Dogger Bank itch revisited: isolation of (2-hydroxyethyl) dimethylsulfoxonium chloride as a cytotoxic constituent from the marine sponge Theonella aff. mirabilis. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:27-30.
- Southcott R. Human injuries from invertebrate animals in the Australian seas. Clin Toxicol. 1970;3:617-636.
- Russell FE. Sponge injury—traumatic, toxic or allergic? N Engl J Med. 1970;282:753-754.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026-1045.
- Kizer K, Auerbach P, Dwyer B. Marine envenomations: not just a problem of the tropics. Emerg Med Rep. 1985;6:129-135.
Sponges are among the oldest animals on earth, appearing more than 640 million years ago before the Cambrian explosion, a period when most major animal phyla appeared in the fossil records.1 More than 10,000 species of sponges have been identified worldwide and are distributed from polar to tropical regions in both marine (Figure 1) and freshwater (Figure 2) environments. They inhabit both shallow waters as well as depths of more than 2800 m, with shallower sponges tending to be more vibrantly colored than their deeper counterparts. The wide-ranging habitats of sponges have led to size variations from as small as 0.05 mm to more than 3 m in height.2 Their taxonomic phylum, Porifera (meaning pore bearers), is derived from the millions of pores lining the surface of the sponge that are used to filter planktonic organisms.3 Flagellated epithelioid cells called choanocytes line the internal chambers of sponges, creating a water current that promotes filter feeding as well as nutrient absorption across their microvilli.4 The body walls of many sponges consist of a collagenous skeleton made up of spongin and spicules of silicon dioxide (silica) or calcium carbonate embedded in the spongin connective tissue matrix.5 Bath sponges lack silica spicules.

Sponges have been used in medicine for centuries. The first use in Western culture was recorded in 405
Mechanisms and Symptoms of Injury
Bathing sponges (silk sponges) derived from Spongia officinalis are harmless. Other sponges can exert their damaging effects through a variety of mechanisms that lead to dermatologic manifestations (eTable). Some species of sponges produce and secrete toxic metabolites (eg, crinotoxins) onto the body surface or into the surrounding water. They also are capable of synthesizing a mucous slime that can be irritating to human skin. Direct trauma also can be caused by fragments of the silica or calcium carbonate sponge skeleton penetrating the skin. Stinging members of the phylum Cnidaria can colonize the sponge, leading to injury when a human handles the sponge.25-27
Sponge dermatitis can be divided into 2 major categories: an initial pruritic dermatitis (Figure 3) that occurs within 20 minutes to a few hours after contact and a delayed irritant dermatitis caused by penetration of the spicules and chemical agents into skin.28 Importantly, different species can lead to varying manifestations.
The initial pruritic dermatitis is characterized by itching and burning that progresses to local edema, vesiculation, joint swelling, and stiffness. Because most contact with sponges occurs with handling, joint immobility may ensue within 24 hours of the encounter. Rarely, larger areas of the skin are affected, and fever, chills, malaise, dizziness, nausea, purulent bullae, muscle cramps, and formication may occur.28 Anaphylactic reactions have been described in a small subset of patients. There have even been reports of delayed (ie, 1–2 weeks following exposure) erythema multiforme, livedo reticularis, purpura, and dyshidrotic eczema.16,20,29 The irritant dermatitis caused by spicule trauma is due to a foreign body reaction that can be exacerbated by toxins entering the skin. In severe cases, desquamation, recurrent eczema, and arthralgia can occur.30 In general, more mild cases should self-resolve within 3 to 7 days. Dermatologic conditions also can be caused by organisms that inhabit sponges and as a result produce a dermatitis when the sponge is handled, including sponge divers disease (maladie des plongeurs), a necrotic dermatitis caused by stinging Cnidaria species.31 Dogger Bank itch, first described as a dermatitis caused by sensitization to (2-hydroxyethyl) dimethylsulfoxonium chloride, initially was isolated from the sea chervil (a type of Bryozoan); however, that same chemical also was later found in sponges, producing the same dermatitis after handling the sponge.32 Freshwater sponges also have been reported to be injurious and exist worldwide. In contrast to marine sponges, lesions from freshwater sponges are disseminated pruritic erythematous papules with ulcerations, crusts, and secondary infections.22 The disseminated nature of the dermatitis caused by freshwater sponges is due to contact with the spicules of dead sponges that are dispersed throughout the water rather than from direct handling. Sponge dermatitis occurs mostly in sponge collectors, divers, trawlers, and biology students and has been reported extensively in the United States, Caribbean Islands, Australia, New Zealand, and Brazil.18,27,33,34
Management
Treatment should consist of an initial decontamination; the skin should be dried, and adhesive tape or rubber cement should be utilized to remove any spicules embedded in the skin. Diluted vinegar soaks should be initiated for 10 to 30 minutes on the affected area(s) 3 or 4 times daily.19 The initial decontamination should occur immediately, as delay may lead to persistent purulent bullae that may take months to heal. Topical steroids may be used following the initial decontamination to help relieve inflammation. Antihistamines and nonsteroidal anti-inflammatory drugs may be used to alleviate pruritus and pain, respectively. Severe cases may require systemic glucocorticoids. Additionally, immunization status against tetanus toxoid should be assessed.35 In the event of an anaphylactic reaction, it is important to maintain a patent airway and normalized blood pressure through the use of intramuscular epinephrine.36 Frequent follow-up is warranted, as serious secondary infections can develop.37 Patients also should be counseled on the potential for delayed dermatologic reactions, including erythema multiforme. Contact between humans and coastal environments has been increasing in the last few decades; therefore, an increase in contact with sponges is to be expected.22
Sponges are among the oldest animals on earth, appearing more than 640 million years ago before the Cambrian explosion, a period when most major animal phyla appeared in the fossil records.1 More than 10,000 species of sponges have been identified worldwide and are distributed from polar to tropical regions in both marine (Figure 1) and freshwater (Figure 2) environments. They inhabit both shallow waters as well as depths of more than 2800 m, with shallower sponges tending to be more vibrantly colored than their deeper counterparts. The wide-ranging habitats of sponges have led to size variations from as small as 0.05 mm to more than 3 m in height.2 Their taxonomic phylum, Porifera (meaning pore bearers), is derived from the millions of pores lining the surface of the sponge that are used to filter planktonic organisms.3 Flagellated epithelioid cells called choanocytes line the internal chambers of sponges, creating a water current that promotes filter feeding as well as nutrient absorption across their microvilli.4 The body walls of many sponges consist of a collagenous skeleton made up of spongin and spicules of silicon dioxide (silica) or calcium carbonate embedded in the spongin connective tissue matrix.5 Bath sponges lack silica spicules.

Sponges have been used in medicine for centuries. The first use in Western culture was recorded in 405
Mechanisms and Symptoms of Injury
Bathing sponges (silk sponges) derived from Spongia officinalis are harmless. Other sponges can exert their damaging effects through a variety of mechanisms that lead to dermatologic manifestations (eTable). Some species of sponges produce and secrete toxic metabolites (eg, crinotoxins) onto the body surface or into the surrounding water. They also are capable of synthesizing a mucous slime that can be irritating to human skin. Direct trauma also can be caused by fragments of the silica or calcium carbonate sponge skeleton penetrating the skin. Stinging members of the phylum Cnidaria can colonize the sponge, leading to injury when a human handles the sponge.25-27
Sponge dermatitis can be divided into 2 major categories: an initial pruritic dermatitis (Figure 3) that occurs within 20 minutes to a few hours after contact and a delayed irritant dermatitis caused by penetration of the spicules and chemical agents into skin.28 Importantly, different species can lead to varying manifestations.
The initial pruritic dermatitis is characterized by itching and burning that progresses to local edema, vesiculation, joint swelling, and stiffness. Because most contact with sponges occurs with handling, joint immobility may ensue within 24 hours of the encounter. Rarely, larger areas of the skin are affected, and fever, chills, malaise, dizziness, nausea, purulent bullae, muscle cramps, and formication may occur.28 Anaphylactic reactions have been described in a small subset of patients. There have even been reports of delayed (ie, 1–2 weeks following exposure) erythema multiforme, livedo reticularis, purpura, and dyshidrotic eczema.16,20,29 The irritant dermatitis caused by spicule trauma is due to a foreign body reaction that can be exacerbated by toxins entering the skin. In severe cases, desquamation, recurrent eczema, and arthralgia can occur.30 In general, more mild cases should self-resolve within 3 to 7 days. Dermatologic conditions also can be caused by organisms that inhabit sponges and as a result produce a dermatitis when the sponge is handled, including sponge divers disease (maladie des plongeurs), a necrotic dermatitis caused by stinging Cnidaria species.31 Dogger Bank itch, first described as a dermatitis caused by sensitization to (2-hydroxyethyl) dimethylsulfoxonium chloride, initially was isolated from the sea chervil (a type of Bryozoan); however, that same chemical also was later found in sponges, producing the same dermatitis after handling the sponge.32 Freshwater sponges also have been reported to be injurious and exist worldwide. In contrast to marine sponges, lesions from freshwater sponges are disseminated pruritic erythematous papules with ulcerations, crusts, and secondary infections.22 The disseminated nature of the dermatitis caused by freshwater sponges is due to contact with the spicules of dead sponges that are dispersed throughout the water rather than from direct handling. Sponge dermatitis occurs mostly in sponge collectors, divers, trawlers, and biology students and has been reported extensively in the United States, Caribbean Islands, Australia, New Zealand, and Brazil.18,27,33,34
Management
Treatment should consist of an initial decontamination; the skin should be dried, and adhesive tape or rubber cement should be utilized to remove any spicules embedded in the skin. Diluted vinegar soaks should be initiated for 10 to 30 minutes on the affected area(s) 3 or 4 times daily.19 The initial decontamination should occur immediately, as delay may lead to persistent purulent bullae that may take months to heal. Topical steroids may be used following the initial decontamination to help relieve inflammation. Antihistamines and nonsteroidal anti-inflammatory drugs may be used to alleviate pruritus and pain, respectively. Severe cases may require systemic glucocorticoids. Additionally, immunization status against tetanus toxoid should be assessed.35 In the event of an anaphylactic reaction, it is important to maintain a patent airway and normalized blood pressure through the use of intramuscular epinephrine.36 Frequent follow-up is warranted, as serious secondary infections can develop.37 Patients also should be counseled on the potential for delayed dermatologic reactions, including erythema multiforme. Contact between humans and coastal environments has been increasing in the last few decades; therefore, an increase in contact with sponges is to be expected.22
- Gold DA, Grabenstatter J, de Mendoza A, et al. Sterol and genomic analyses validate the sponge biomarker hypothesis. Proc Natl Acad Sci U S A. 2016;113:2684-2689.
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by sponges. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology. 2nd ed. Springer; 2016:121-126.
- Marsh LM, Slack-Smith S, Gurry DL. Field Guide to Sea Stingers and Other Venomous and Poisonous Marine Invertebrates. 2nd ed. Western Australian Museum; 2010.
- Eid E, Al-Tawaha M. A Guide to Harmful and Toxic Creatures in the Gulf of Aqaba Jordan. The Royal Marine Conservation Society of Jordan; 2016.
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131.
- Dormandy TL. Trace element analysis of hair. Br Med J (Clin Res Ed). 1986;293:975-976.
- Voultsiadou E. Sponges: an historical survey of their knowledge in Greek antiquity. J Mar Biol Assoc UK. 2007;87:1757-1763.
- Senthilkumar K, Kim SK. Marine invertebrate natural products for anti-inflammatory and chronic diseases [published online December 31, 2013]. Evid Based Complement Alternat Med. doi:10.1155/2013/572859
- Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8:2619-2638.
- Usagawa T, Nishimura M, Itoh Y, et al. Preparation of monoclonal antibodies against okadaic acid prepared from the sponge Halichondria okadai. Toxicon. 1989;27:1323-1330.
- Elston DM. Aquatic antagonists: sponge dermatitis. Cutis. 2007;80:279-280.
- Parra-Velandia FJ, Zea S, Van Soest RW. Reef sponges of the genus Agelas (Porifera: Demospongiae) from the Greater Caribbean. Zootaxa. 2014;3794:301-343.
- Hooper JN, Capon RJ, Hodder RA. A new species of toxic marine sponge (Porifera: Demospongiae: Poecilosclerida) from northwest Australia. The Beagle, Records of the Northern Territory Museum of Arts and sciences. 1991;8:27-36.
- Burnett JW, Calton GJ, Morgan RJ. Dermatitis due to stinging sponges. Cutis. 1987;39:476.
- Kizer KW. Marine envenomations. J Toxicol Clin Toxicol. 1983;21:527-555.
- Isbister GK, Hooper JN. Clinical effects of stings by sponges of the genus Tedania and a review of sponge stings worldwide. Toxicon. 2005;46:782-785.
- Fromont J, Abdo DA. New species of Haliclona (Demospongiae: Haplosclerida: Chalinidae) from Western Australia. Zootaxa. 2014;3835:97-109.
- Flachsenberger W, Holmes NJ, Leigh C, et al. Properties of the extract and spicules of the dermatitis inducing sponge Neofibularia mordens Hartman. J Toxicol Clin Toxicol. 1987;25:255-272.
- Southcott RV, Coulter JR. The effects of the southern Australian marine stinging sponges, Neofibularia mordens and Lissodendoryx sp. Med J Aust. 1971;2:895-901.
- Yaffee HS, Stargardter F. Erythema multiforme from Tedania ignis. report of a case and an experimental study of the mechanism of cutaneous irritation from the fire sponge. Arch Dermatol. 1963;87:601-604.
- Yaffee HS. Irritation from red sponge. N Engl J Med. 1970;282:51.
- Haddad V Jr. Environmental dermatology: skin manifestations of injuries caused by invertebrate aquatic animals. An Bras Dermatol. 2013;88:496-506.
- Volkmer-Ribeiro C, Lenzi HL, Orefice F, et al. Freshwater sponge spicules: a new agent of ocular pathology. Mem Inst Oswaldo Cruz. 2006;101:899-903.
- Cruz AA, Alencar VM, Medina NH, et al. Dangerous waters: outbreak of eye lesions caused by fresh water sponge spicules. Eye (Lond). 2013;27:398-402.
- Haddad V Jr. Clinical and therapeutic aspects of envenomations caused by sponges and jellyfish. In: Gopalakrishnakone P, Haddad V Jr, Kem WR, et al, eds. Marine and Freshwater Toxins. Springer; 2016:317-325.
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-750.
- Gaastra MT. Aquatic skin disorders. In: Faber WR, Hay RJ, Naafs B, eds. Imported Skin Diseases. 2nd ed. Wiley; 2012:283-292.
- Auerbach P. Envenomation by aquatic invertebrates. In: Auerbach P, ed. Wilderness Medicine. 6th ed. Elsevier Mosby; 2011;1596-1627.
- Sims JK, Irei MY. Human Hawaiian marine sponge poisoning. Hawaii Med J. 1979;38:263-270.
- Haddad V Jr. Aquatic animals of medical importance in Brazil. Rev Soc Bras Med Trop. 2003;36:591-597.
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Warabi K, Nakao Y, Matsunaga S, et al. Dogger Bank itch revisited: isolation of (2-hydroxyethyl) dimethylsulfoxonium chloride as a cytotoxic constituent from the marine sponge Theonella aff. mirabilis. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:27-30.
- Southcott R. Human injuries from invertebrate animals in the Australian seas. Clin Toxicol. 1970;3:617-636.
- Russell FE. Sponge injury—traumatic, toxic or allergic? N Engl J Med. 1970;282:753-754.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026-1045.
- Kizer K, Auerbach P, Dwyer B. Marine envenomations: not just a problem of the tropics. Emerg Med Rep. 1985;6:129-135.
- Gold DA, Grabenstatter J, de Mendoza A, et al. Sterol and genomic analyses validate the sponge biomarker hypothesis. Proc Natl Acad Sci U S A. 2016;113:2684-2689.
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by sponges. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology. 2nd ed. Springer; 2016:121-126.
- Marsh LM, Slack-Smith S, Gurry DL. Field Guide to Sea Stingers and Other Venomous and Poisonous Marine Invertebrates. 2nd ed. Western Australian Museum; 2010.
- Eid E, Al-Tawaha M. A Guide to Harmful and Toxic Creatures in the Gulf of Aqaba Jordan. The Royal Marine Conservation Society of Jordan; 2016.
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131.
- Dormandy TL. Trace element analysis of hair. Br Med J (Clin Res Ed). 1986;293:975-976.
- Voultsiadou E. Sponges: an historical survey of their knowledge in Greek antiquity. J Mar Biol Assoc UK. 2007;87:1757-1763.
- Senthilkumar K, Kim SK. Marine invertebrate natural products for anti-inflammatory and chronic diseases [published online December 31, 2013]. Evid Based Complement Alternat Med. doi:10.1155/2013/572859
- Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8:2619-2638.
- Usagawa T, Nishimura M, Itoh Y, et al. Preparation of monoclonal antibodies against okadaic acid prepared from the sponge Halichondria okadai. Toxicon. 1989;27:1323-1330.
- Elston DM. Aquatic antagonists: sponge dermatitis. Cutis. 2007;80:279-280.
- Parra-Velandia FJ, Zea S, Van Soest RW. Reef sponges of the genus Agelas (Porifera: Demospongiae) from the Greater Caribbean. Zootaxa. 2014;3794:301-343.
- Hooper JN, Capon RJ, Hodder RA. A new species of toxic marine sponge (Porifera: Demospongiae: Poecilosclerida) from northwest Australia. The Beagle, Records of the Northern Territory Museum of Arts and sciences. 1991;8:27-36.
- Burnett JW, Calton GJ, Morgan RJ. Dermatitis due to stinging sponges. Cutis. 1987;39:476.
- Kizer KW. Marine envenomations. J Toxicol Clin Toxicol. 1983;21:527-555.
- Isbister GK, Hooper JN. Clinical effects of stings by sponges of the genus Tedania and a review of sponge stings worldwide. Toxicon. 2005;46:782-785.
- Fromont J, Abdo DA. New species of Haliclona (Demospongiae: Haplosclerida: Chalinidae) from Western Australia. Zootaxa. 2014;3835:97-109.
- Flachsenberger W, Holmes NJ, Leigh C, et al. Properties of the extract and spicules of the dermatitis inducing sponge Neofibularia mordens Hartman. J Toxicol Clin Toxicol. 1987;25:255-272.
- Southcott RV, Coulter JR. The effects of the southern Australian marine stinging sponges, Neofibularia mordens and Lissodendoryx sp. Med J Aust. 1971;2:895-901.
- Yaffee HS, Stargardter F. Erythema multiforme from Tedania ignis. report of a case and an experimental study of the mechanism of cutaneous irritation from the fire sponge. Arch Dermatol. 1963;87:601-604.
- Yaffee HS. Irritation from red sponge. N Engl J Med. 1970;282:51.
- Haddad V Jr. Environmental dermatology: skin manifestations of injuries caused by invertebrate aquatic animals. An Bras Dermatol. 2013;88:496-506.
- Volkmer-Ribeiro C, Lenzi HL, Orefice F, et al. Freshwater sponge spicules: a new agent of ocular pathology. Mem Inst Oswaldo Cruz. 2006;101:899-903.
- Cruz AA, Alencar VM, Medina NH, et al. Dangerous waters: outbreak of eye lesions caused by fresh water sponge spicules. Eye (Lond). 2013;27:398-402.
- Haddad V Jr. Clinical and therapeutic aspects of envenomations caused by sponges and jellyfish. In: Gopalakrishnakone P, Haddad V Jr, Kem WR, et al, eds. Marine and Freshwater Toxins. Springer; 2016:317-325.
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-750.
- Gaastra MT. Aquatic skin disorders. In: Faber WR, Hay RJ, Naafs B, eds. Imported Skin Diseases. 2nd ed. Wiley; 2012:283-292.
- Auerbach P. Envenomation by aquatic invertebrates. In: Auerbach P, ed. Wilderness Medicine. 6th ed. Elsevier Mosby; 2011;1596-1627.
- Sims JK, Irei MY. Human Hawaiian marine sponge poisoning. Hawaii Med J. 1979;38:263-270.
- Haddad V Jr. Aquatic animals of medical importance in Brazil. Rev Soc Bras Med Trop. 2003;36:591-597.
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Warabi K, Nakao Y, Matsunaga S, et al. Dogger Bank itch revisited: isolation of (2-hydroxyethyl) dimethylsulfoxonium chloride as a cytotoxic constituent from the marine sponge Theonella aff. mirabilis. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:27-30.
- Southcott R. Human injuries from invertebrate animals in the Australian seas. Clin Toxicol. 1970;3:617-636.
- Russell FE. Sponge injury—traumatic, toxic or allergic? N Engl J Med. 1970;282:753-754.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026-1045.
- Kizer K, Auerbach P, Dwyer B. Marine envenomations: not just a problem of the tropics. Emerg Med Rep. 1985;6:129-135.
Practice Points
- Sponges exist in both marine and freshwater environments throughout the world.
- Immediate management of sponge dermatitis should include decontamination by removing the sponge spicules with tape or rubber cement followed by dilute vinegar soaks.
- Topical steroids may be used only after initial decontamination. Use of oral steroids may be needed for more severe reactions.
Analysis characterizes common wound microbes in epidermolysis bullosa
– in a retrospective analysis of over 700 wound cultures from 158 patients across the United States and Canada.
The findings from the EB Clinical Characterization and Outcomes Database speak to the value of surveillance cultures with routine testing for microbial resistance – including mupirocin resistance – and to the importance of antibiotic stewardship not only for oral antibiotics but for topicals as well, according to Laura E. Levin, MD, and Kimberly D. Morel, MD, of the departments of dermatology and pediatrics, Columbia University Irving Medical Center, New York, the lead and senior authors, respectively, of the paper recently published in Pediatric Dermatology.
Almost all of the 158 patients with at least one wound culture recorded in the database from the period of 2001-2018 had one or more positive culture results. Of 152 patients with positive cultures, 131 (86%) were positive for SA and 56 (37%) and 34 (22%) were positive for PA and GAS, respectively. Other bacteria isolated included Corynebacterium spp and Proteus spp. Nearly half (47%) of patients with SA-positive cultures had methicillin-resistant SA, and 68% had methicillin-susceptible SA. (Some patients grew both MSSA and MRSA at different points in time.)
Mupirocin-susceptibility testing was performed at only some of the 13 participating centers. Of 15 patients whose cultures had recorded SA mupirocin-susceptibility testing, 11 had cultures positive for mupirocin-susceptible SA and 6 (40%) had mupirocin-resistant SA isolates (2 patients grew both). Of these six patients, half had isolates that were also methicillin-resistant.
Mupirocin, a topical antibiotic, has been a cornerstone of decolonization regimens for MSSA and MRSA, but resistance has been demonstrated in other research as well and is not specific to EB, wrote Dr. Levin, Dr. Morel, and coauthors.
“Pediatric dermatologists often rely on topical antimicrobials in the treatment of patients’ open wounds to both prevent and treat infection, depending on the clinical scenario,” and surveillance cultures with routine testing for mupirocin resistance can help guide antibiotic choice and management strategies, Dr. Levin said in an interview.
More broadly, she added, “it’s helpful to know what bacteria are routinely colonizing wounds, not causing infection, versus those that are more likely to be associated with infection, chronic wounds, or the risk of developing skin cancer ... [to know] which wounds need to be treated more aggressively.”
A subset of patients with EB have been known to be at risk for squamous cell carcinoma, and research is implicating certain bacteria “as contributing to wound inflammation,” Dr. Morel said in an interview.
SCC was reported in 23 out of 717 patients in the database – but fewer than half of the patients with SCC had recorded wound cultures. The small numbers precluded the identification of microbes that may confer significant risk.
Correlating particular microbes with clinical features also will take more research. About half (57%) of the patients with recorded wound cultures had wounds with purulent exudate or other features of clinical infection. However, the presence or absence of clinical signs of infection was not temporally correlated with culture results in the database.
The 158 patients with recorded wound cultures had a mean age of 12.8 years and represented a range of EB subtypes.
PA was present in the wounds of patients as young as 1 month old, the authors noted. Investigators are “looking to further study PA and characterize clinical features ... to understand more about this microbe and its impact on patients with EB,” Dr. Morel said.
In the meantime, the analysis reaffirms the importance of antibiotic stewardship. Mupirocin is labeled to be used three times a day for a short period of time, but “tends to be prescribed and used less judiciously than intended,” Dr. Morel said. “It’s important [not to overuse it]. We have seen that patients’ culture results become sensitive to mupirocin again in the future when they avoid it for a period of time.”
The work was supported by the EB Research Partnership and EB Medical Research Foundation, as well as an NIH/NCATS grant. No investigator disclosures were listed.
SOURCE: Pediatr Dermatol. 2020 Nov 28. doi: 10.1111/pde.14444.
– in a retrospective analysis of over 700 wound cultures from 158 patients across the United States and Canada.
The findings from the EB Clinical Characterization and Outcomes Database speak to the value of surveillance cultures with routine testing for microbial resistance – including mupirocin resistance – and to the importance of antibiotic stewardship not only for oral antibiotics but for topicals as well, according to Laura E. Levin, MD, and Kimberly D. Morel, MD, of the departments of dermatology and pediatrics, Columbia University Irving Medical Center, New York, the lead and senior authors, respectively, of the paper recently published in Pediatric Dermatology.
Almost all of the 158 patients with at least one wound culture recorded in the database from the period of 2001-2018 had one or more positive culture results. Of 152 patients with positive cultures, 131 (86%) were positive for SA and 56 (37%) and 34 (22%) were positive for PA and GAS, respectively. Other bacteria isolated included Corynebacterium spp and Proteus spp. Nearly half (47%) of patients with SA-positive cultures had methicillin-resistant SA, and 68% had methicillin-susceptible SA. (Some patients grew both MSSA and MRSA at different points in time.)
Mupirocin-susceptibility testing was performed at only some of the 13 participating centers. Of 15 patients whose cultures had recorded SA mupirocin-susceptibility testing, 11 had cultures positive for mupirocin-susceptible SA and 6 (40%) had mupirocin-resistant SA isolates (2 patients grew both). Of these six patients, half had isolates that were also methicillin-resistant.
Mupirocin, a topical antibiotic, has been a cornerstone of decolonization regimens for MSSA and MRSA, but resistance has been demonstrated in other research as well and is not specific to EB, wrote Dr. Levin, Dr. Morel, and coauthors.
“Pediatric dermatologists often rely on topical antimicrobials in the treatment of patients’ open wounds to both prevent and treat infection, depending on the clinical scenario,” and surveillance cultures with routine testing for mupirocin resistance can help guide antibiotic choice and management strategies, Dr. Levin said in an interview.
More broadly, she added, “it’s helpful to know what bacteria are routinely colonizing wounds, not causing infection, versus those that are more likely to be associated with infection, chronic wounds, or the risk of developing skin cancer ... [to know] which wounds need to be treated more aggressively.”
A subset of patients with EB have been known to be at risk for squamous cell carcinoma, and research is implicating certain bacteria “as contributing to wound inflammation,” Dr. Morel said in an interview.
SCC was reported in 23 out of 717 patients in the database – but fewer than half of the patients with SCC had recorded wound cultures. The small numbers precluded the identification of microbes that may confer significant risk.
Correlating particular microbes with clinical features also will take more research. About half (57%) of the patients with recorded wound cultures had wounds with purulent exudate or other features of clinical infection. However, the presence or absence of clinical signs of infection was not temporally correlated with culture results in the database.
The 158 patients with recorded wound cultures had a mean age of 12.8 years and represented a range of EB subtypes.
PA was present in the wounds of patients as young as 1 month old, the authors noted. Investigators are “looking to further study PA and characterize clinical features ... to understand more about this microbe and its impact on patients with EB,” Dr. Morel said.
In the meantime, the analysis reaffirms the importance of antibiotic stewardship. Mupirocin is labeled to be used three times a day for a short period of time, but “tends to be prescribed and used less judiciously than intended,” Dr. Morel said. “It’s important [not to overuse it]. We have seen that patients’ culture results become sensitive to mupirocin again in the future when they avoid it for a period of time.”
The work was supported by the EB Research Partnership and EB Medical Research Foundation, as well as an NIH/NCATS grant. No investigator disclosures were listed.
SOURCE: Pediatr Dermatol. 2020 Nov 28. doi: 10.1111/pde.14444.
– in a retrospective analysis of over 700 wound cultures from 158 patients across the United States and Canada.
The findings from the EB Clinical Characterization and Outcomes Database speak to the value of surveillance cultures with routine testing for microbial resistance – including mupirocin resistance – and to the importance of antibiotic stewardship not only for oral antibiotics but for topicals as well, according to Laura E. Levin, MD, and Kimberly D. Morel, MD, of the departments of dermatology and pediatrics, Columbia University Irving Medical Center, New York, the lead and senior authors, respectively, of the paper recently published in Pediatric Dermatology.
Almost all of the 158 patients with at least one wound culture recorded in the database from the period of 2001-2018 had one or more positive culture results. Of 152 patients with positive cultures, 131 (86%) were positive for SA and 56 (37%) and 34 (22%) were positive for PA and GAS, respectively. Other bacteria isolated included Corynebacterium spp and Proteus spp. Nearly half (47%) of patients with SA-positive cultures had methicillin-resistant SA, and 68% had methicillin-susceptible SA. (Some patients grew both MSSA and MRSA at different points in time.)
Mupirocin-susceptibility testing was performed at only some of the 13 participating centers. Of 15 patients whose cultures had recorded SA mupirocin-susceptibility testing, 11 had cultures positive for mupirocin-susceptible SA and 6 (40%) had mupirocin-resistant SA isolates (2 patients grew both). Of these six patients, half had isolates that were also methicillin-resistant.
Mupirocin, a topical antibiotic, has been a cornerstone of decolonization regimens for MSSA and MRSA, but resistance has been demonstrated in other research as well and is not specific to EB, wrote Dr. Levin, Dr. Morel, and coauthors.
“Pediatric dermatologists often rely on topical antimicrobials in the treatment of patients’ open wounds to both prevent and treat infection, depending on the clinical scenario,” and surveillance cultures with routine testing for mupirocin resistance can help guide antibiotic choice and management strategies, Dr. Levin said in an interview.
More broadly, she added, “it’s helpful to know what bacteria are routinely colonizing wounds, not causing infection, versus those that are more likely to be associated with infection, chronic wounds, or the risk of developing skin cancer ... [to know] which wounds need to be treated more aggressively.”
A subset of patients with EB have been known to be at risk for squamous cell carcinoma, and research is implicating certain bacteria “as contributing to wound inflammation,” Dr. Morel said in an interview.
SCC was reported in 23 out of 717 patients in the database – but fewer than half of the patients with SCC had recorded wound cultures. The small numbers precluded the identification of microbes that may confer significant risk.
Correlating particular microbes with clinical features also will take more research. About half (57%) of the patients with recorded wound cultures had wounds with purulent exudate or other features of clinical infection. However, the presence or absence of clinical signs of infection was not temporally correlated with culture results in the database.
The 158 patients with recorded wound cultures had a mean age of 12.8 years and represented a range of EB subtypes.
PA was present in the wounds of patients as young as 1 month old, the authors noted. Investigators are “looking to further study PA and characterize clinical features ... to understand more about this microbe and its impact on patients with EB,” Dr. Morel said.
In the meantime, the analysis reaffirms the importance of antibiotic stewardship. Mupirocin is labeled to be used three times a day for a short period of time, but “tends to be prescribed and used less judiciously than intended,” Dr. Morel said. “It’s important [not to overuse it]. We have seen that patients’ culture results become sensitive to mupirocin again in the future when they avoid it for a period of time.”
The work was supported by the EB Research Partnership and EB Medical Research Foundation, as well as an NIH/NCATS grant. No investigator disclosures were listed.
SOURCE: Pediatr Dermatol. 2020 Nov 28. doi: 10.1111/pde.14444.
FROM PEDIATRIC DERMATOLOGY
Combined Treatment of Disfiguring Facial Angiofibromas in Tuberous Sclerosis Complex With Surgical Debulking and Topical Sirolimus
Practice Gap
Tuberous sclerosis complex (TSC) is an autosomal-dominant genetic disorder resulting in loss-of-function mutations in the TSC1 and TSC2 genes. These mutations lead to constitutive activation of the mitogenic mTOR pathway and release of lymphangiogenic growth factors, causing the formation of hamartomatous tumors throughout multiple organ systems.1 Facial angiofibromas (FAs) are a common cutaneous manifestation of TSC, affecting up to 80% of patients worldwide.2 Aesthetic disfigurement, vision obstruction, and breathing impairment often are associated with FAs. They frequently arise in children with TSC and impose a psychosocial burden that can affect the patient’s overall quality of life.
Cutaneous stigmata of TSC pose a significant therapeutic challenge. Topical sirolimus has become a first-line treatment of FAs by inhibiting the mitogenic mTOR pathway1; however, thicker, more extensive lesions are less responsive to topical therapy. The entire dermis is involved in TSC, and topical sirolimus alone often is ineffective for large fibrous FAs.3 Likewise, oral mTOR inhibition has shown only 25% to 50% improvement in FAs and has potential side effects that can limit patients’ tolerance and compliance.4
The Technique
A 46-year-old man with TSC was referred to dermatology for treatment of numerous facial papules and plaques that had been present since childhood and were consistent with FAs (Figure 1A). The lesions were tender, impaired the patient’s breathing, and caused emotional distress. Dermabrasion was attempted 20 years prior with minimal improvement and subsequent progression of the FAs. Other stigmata of TSC were present, including cutaneous hypopigmented macules and shagreen patches as well as seizures and renal angiomyolipomas. Due to multiorgan involvement, the patient was started on once-daily oral everolimus 2.5 mg; however, the FAs were progressive despite the systemic mTOR inhibition. Furthermore, it was presumed that topical sirolimus monotherapy would be ineffective due to thickness and extent of FAs; therefore, we proposed a novel treatment approach combining initial surgical debulking with subsequent longitudinal use of topical sirolimus to reduce the risk of recurrence.
Local anesthesia with lidocaine 1% and epinephrine 1:100,000 was administered. Larger FAs were removed at the base with a sterile surgical blade. Nasal recontouring subsequently was performed using a combination of shave biopsy and curettage. Extensive electrocautery was performed for hemostasis and destruction of residual FAs. Figure 1B shows the immediate postoperative result.
One month postoperatively, the patient stopped the oral everolimus at his oncologist’s recommendation due to abdominal pain and peripheral edema. Once the abraded skin showed evidence of wound healing, the patient was instructed to initiate sirolimus ointment 1% twice daily to reduce the risk of recurrence.1,5,6 At 8-week follow-up, the patient was noted to have cosmetic improvement and resolution of breathing impairment (Figure 2A). He continued to show excellent cosmetic results at 1-year follow-up using topical sirolimus monotherapy (Figure 2B).
Practical Implications
Surgical debulking combined with longitudinal use of sirolimus ointment 1% can achieve an optimal therapeutic response for disfiguring phymatous presentation of FAs in the setting of TSC. We believe it is an effective approach for thick disfiguring FAs that are unlikely to respond to mTOR inhibition alone.
- Wataya-Kaneda M, Nakamura A, Tanaka M, et al. Efficacy and safety of topical sirolimus therapy for facial angiofibromas in the tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2017;153:39‐48.
- Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Drugs R D. 2012;12:121-126.
- Wataya-Kaneda M, Ohno Y, Fujita Y, et al. Sirolimus gel treatment vs placebo for facial angiofibromas in patients with tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2018;154:781-788.
- Nathan N, Wang JA, Li S, et al. Improvement of tuberous sclerosis complex (TSC) skin tumors during long-term treatment with oral sirolimus. J Am Acad Dermatol. 2015;73:802-808.
- Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando). 2014;28:126-133.
- Haemel AK, O’Brian AL, Teng JM. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Arch Dermatol. 2010;146:1538-3652.
Practice Gap
Tuberous sclerosis complex (TSC) is an autosomal-dominant genetic disorder resulting in loss-of-function mutations in the TSC1 and TSC2 genes. These mutations lead to constitutive activation of the mitogenic mTOR pathway and release of lymphangiogenic growth factors, causing the formation of hamartomatous tumors throughout multiple organ systems.1 Facial angiofibromas (FAs) are a common cutaneous manifestation of TSC, affecting up to 80% of patients worldwide.2 Aesthetic disfigurement, vision obstruction, and breathing impairment often are associated with FAs. They frequently arise in children with TSC and impose a psychosocial burden that can affect the patient’s overall quality of life.
Cutaneous stigmata of TSC pose a significant therapeutic challenge. Topical sirolimus has become a first-line treatment of FAs by inhibiting the mitogenic mTOR pathway1; however, thicker, more extensive lesions are less responsive to topical therapy. The entire dermis is involved in TSC, and topical sirolimus alone often is ineffective for large fibrous FAs.3 Likewise, oral mTOR inhibition has shown only 25% to 50% improvement in FAs and has potential side effects that can limit patients’ tolerance and compliance.4
The Technique
A 46-year-old man with TSC was referred to dermatology for treatment of numerous facial papules and plaques that had been present since childhood and were consistent with FAs (Figure 1A). The lesions were tender, impaired the patient’s breathing, and caused emotional distress. Dermabrasion was attempted 20 years prior with minimal improvement and subsequent progression of the FAs. Other stigmata of TSC were present, including cutaneous hypopigmented macules and shagreen patches as well as seizures and renal angiomyolipomas. Due to multiorgan involvement, the patient was started on once-daily oral everolimus 2.5 mg; however, the FAs were progressive despite the systemic mTOR inhibition. Furthermore, it was presumed that topical sirolimus monotherapy would be ineffective due to thickness and extent of FAs; therefore, we proposed a novel treatment approach combining initial surgical debulking with subsequent longitudinal use of topical sirolimus to reduce the risk of recurrence.
Local anesthesia with lidocaine 1% and epinephrine 1:100,000 was administered. Larger FAs were removed at the base with a sterile surgical blade. Nasal recontouring subsequently was performed using a combination of shave biopsy and curettage. Extensive electrocautery was performed for hemostasis and destruction of residual FAs. Figure 1B shows the immediate postoperative result.
One month postoperatively, the patient stopped the oral everolimus at his oncologist’s recommendation due to abdominal pain and peripheral edema. Once the abraded skin showed evidence of wound healing, the patient was instructed to initiate sirolimus ointment 1% twice daily to reduce the risk of recurrence.1,5,6 At 8-week follow-up, the patient was noted to have cosmetic improvement and resolution of breathing impairment (Figure 2A). He continued to show excellent cosmetic results at 1-year follow-up using topical sirolimus monotherapy (Figure 2B).
Practical Implications
Surgical debulking combined with longitudinal use of sirolimus ointment 1% can achieve an optimal therapeutic response for disfiguring phymatous presentation of FAs in the setting of TSC. We believe it is an effective approach for thick disfiguring FAs that are unlikely to respond to mTOR inhibition alone.
Practice Gap
Tuberous sclerosis complex (TSC) is an autosomal-dominant genetic disorder resulting in loss-of-function mutations in the TSC1 and TSC2 genes. These mutations lead to constitutive activation of the mitogenic mTOR pathway and release of lymphangiogenic growth factors, causing the formation of hamartomatous tumors throughout multiple organ systems.1 Facial angiofibromas (FAs) are a common cutaneous manifestation of TSC, affecting up to 80% of patients worldwide.2 Aesthetic disfigurement, vision obstruction, and breathing impairment often are associated with FAs. They frequently arise in children with TSC and impose a psychosocial burden that can affect the patient’s overall quality of life.
Cutaneous stigmata of TSC pose a significant therapeutic challenge. Topical sirolimus has become a first-line treatment of FAs by inhibiting the mitogenic mTOR pathway1; however, thicker, more extensive lesions are less responsive to topical therapy. The entire dermis is involved in TSC, and topical sirolimus alone often is ineffective for large fibrous FAs.3 Likewise, oral mTOR inhibition has shown only 25% to 50% improvement in FAs and has potential side effects that can limit patients’ tolerance and compliance.4
The Technique
A 46-year-old man with TSC was referred to dermatology for treatment of numerous facial papules and plaques that had been present since childhood and were consistent with FAs (Figure 1A). The lesions were tender, impaired the patient’s breathing, and caused emotional distress. Dermabrasion was attempted 20 years prior with minimal improvement and subsequent progression of the FAs. Other stigmata of TSC were present, including cutaneous hypopigmented macules and shagreen patches as well as seizures and renal angiomyolipomas. Due to multiorgan involvement, the patient was started on once-daily oral everolimus 2.5 mg; however, the FAs were progressive despite the systemic mTOR inhibition. Furthermore, it was presumed that topical sirolimus monotherapy would be ineffective due to thickness and extent of FAs; therefore, we proposed a novel treatment approach combining initial surgical debulking with subsequent longitudinal use of topical sirolimus to reduce the risk of recurrence.
Local anesthesia with lidocaine 1% and epinephrine 1:100,000 was administered. Larger FAs were removed at the base with a sterile surgical blade. Nasal recontouring subsequently was performed using a combination of shave biopsy and curettage. Extensive electrocautery was performed for hemostasis and destruction of residual FAs. Figure 1B shows the immediate postoperative result.
One month postoperatively, the patient stopped the oral everolimus at his oncologist’s recommendation due to abdominal pain and peripheral edema. Once the abraded skin showed evidence of wound healing, the patient was instructed to initiate sirolimus ointment 1% twice daily to reduce the risk of recurrence.1,5,6 At 8-week follow-up, the patient was noted to have cosmetic improvement and resolution of breathing impairment (Figure 2A). He continued to show excellent cosmetic results at 1-year follow-up using topical sirolimus monotherapy (Figure 2B).
Practical Implications
Surgical debulking combined with longitudinal use of sirolimus ointment 1% can achieve an optimal therapeutic response for disfiguring phymatous presentation of FAs in the setting of TSC. We believe it is an effective approach for thick disfiguring FAs that are unlikely to respond to mTOR inhibition alone.
- Wataya-Kaneda M, Nakamura A, Tanaka M, et al. Efficacy and safety of topical sirolimus therapy for facial angiofibromas in the tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2017;153:39‐48.
- Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Drugs R D. 2012;12:121-126.
- Wataya-Kaneda M, Ohno Y, Fujita Y, et al. Sirolimus gel treatment vs placebo for facial angiofibromas in patients with tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2018;154:781-788.
- Nathan N, Wang JA, Li S, et al. Improvement of tuberous sclerosis complex (TSC) skin tumors during long-term treatment with oral sirolimus. J Am Acad Dermatol. 2015;73:802-808.
- Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando). 2014;28:126-133.
- Haemel AK, O’Brian AL, Teng JM. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Arch Dermatol. 2010;146:1538-3652.
- Wataya-Kaneda M, Nakamura A, Tanaka M, et al. Efficacy and safety of topical sirolimus therapy for facial angiofibromas in the tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2017;153:39‐48.
- Koenig MK, Hebert AA, Roberson J, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Drugs R D. 2012;12:121-126.
- Wataya-Kaneda M, Ohno Y, Fujita Y, et al. Sirolimus gel treatment vs placebo for facial angiofibromas in patients with tuberous sclerosis complex: a randomized clinical trial. JAMA Dermatol. 2018;154:781-788.
- Nathan N, Wang JA, Li S, et al. Improvement of tuberous sclerosis complex (TSC) skin tumors during long-term treatment with oral sirolimus. J Am Acad Dermatol. 2015;73:802-808.
- Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando). 2014;28:126-133.
- Haemel AK, O’Brian AL, Teng JM. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex. Arch Dermatol. 2010;146:1538-3652.
Doctor in a Bottle: Examining the Increase in Essential Oil Use
What Are Essential Oils?
Essential oils are aromatic volatile oils produced by medicinal plants that give them their distinct flavors and aromas. They are extracted using a variety of different techniques, such as microwave-assisted extraction, headspace extraction, and the most commonly employed hydrodistillation.1 Different parts of the plant are used for the specific oils; the shoots and leaves of Origanum vulgare are used for oregano oil, whereas the skins of Citrus limonum are used for lemon oil.2 Historically, essential oils have been used for cooking, food preservation, perfume, and medicine.3,4
Historical Uses for Essential Oils
Essential oils and their intact medicinal plants were among the first medicines widely available to the ancient world. The Ancient Greeks used topical and oral oregano as a cure-all for ailments including wounds, sore muscles, and diarrhea. Because of its use as a cure-all medicine, it remains a popular folk remedy in parts of Europe today.3 Lavender also has a long history of being a cure-all plant and oil. Some of the many claims behind this flower include treatment of burns, insect bites, parasites, muscle spasms, nausea, and anxiety/depression.5 With an extensive list of historical uses, many essential oils are being researched to determine if their acclaimed qualities have quantifiable properties.
Science Behind the Belief
In vitro experiments with oregano (O vulgare) have demonstrated notable antifungal and antimicrobial effects.6 Gas chromatographic analysis of the oil shows much of it is composed of phenolic monoterpenes, such as thymol and carvacrol. They exhibit strong antifungal effects with a slightly stronger effect on the dermatophyte Trichophyton rubrum over other yeast species such as Candida.7,8 The full effect of the monoterpenes on fungi is not completely understood, but early data show it has a strong affinity for the ergosterol used in the cell-wall synthesis. Other effects demonstrated in in vitro studies include the ability to block drug efflux pumps, biofilm formation, cellular communication among bacteria, and mycotoxin production.9
A double-blind, randomized trial by Akhondzadeh et al10 demonstrated lavender (Lavandula officinalis) to have a mild antidepressant quality but a noticeably more potent effect when combined with imipramine. The effects of the lavender with imipramine were stronger and provided earlier improvement than imipramine alone for treatment of mild to moderate depression. The team concluded that lavender may be an effective adjunct therapy in treating depression.10
In a study by Mori et al,11 full-thickness circular wounds were made in rats and treated with either lavender oil (L officinalis), nothing, or a control oil. With the lavender oil being at only 1% solution, the wounds treated with lavender oil demonstrated earlier closure than the other 2 groups of wounds, where no major difference was noted. On cellular analysis, it was seen that the lavender had increased the rate of granulation as well as expression of types I and III collagen. The most striking result was the large expression of transforming growth factor β seen in the lavender group compared to the others. The final thoughts on this experiment were that lavender may provide new approaches to wound care in the future.11
Potential Problems With Purity
One major concern raised about essential oils is their purity and the fidelity of their chemical composition. The specific aromatic chemicals in each essential oil are maintained for each species, but the proportions of each change even with the time of year.12 Gas chromatograph analysis of the same oil distilled with different techniques showed that the proportions of aromatic chemicals varied with technique. However, the major constituents of the oil remained present in large quantities, just at different percentages.1 Even using the same distillation technique for different time periods can greatly affect the yield and composition of the oil. Although the percentage of each aromatic compound can be affected by distillation times, the antioxidant and antimicrobial effects of the oil remain constant regardless of these variables.2 There is clearly a lack in standardization in essential oil production, which may not be an issue for its use in complementary medicine if its properties are maintained regardless.
Safety Concerns and Regulations
With essential oils being a natural cure for everyday ailments, some people are turning first to oils for every cut and bruise. The danger in these natural cures is that essential oils can cause several types of dermatitis and allergic reactions. The development of allergies to essential oils is at an even higher risk, considering people frequently put them on wounds and rashes where the skin barrier is already weakened. Many essential oils fall into the fragrance category in patch tests, negating the widely circulating blogger and online reports that essential oils cannot cause allergies.
Some of the oils, although regarded safe by the US Food and Drug Administration for consumption, can cause dermatitis from simple contact or with sun exposure.13 Members of the citrus family are notorious for the phytophotodermatitis reaction, which can leave hyperpigmented scarring after exposure of the oils to sunlight.14 Most companies that sell essential oils are aware of this reaction and include it in the warning labels.
The legal problem with selling and classifying essential oils is that the US Food and Drug Administration requires products intended for treatment to be labeled as drugs, which hinders their sales on the open market.13 It all boils down to intended use, so some companies sell the oils under a food or fragrance classification with vague instructions on how to use said oil for medicinal purposes, which leads to lack of supervision, anecdotal cures, and false health claims. One company claims in their safety guide for topical applications of their oils that “[i]f a rash occurs, this may be a sign of detoxification.”15 If essential oils had only minimal absorption topically, their safety would be less concerning, but this does not appear to be the case.
Absorption and Systemics
The effects of essential oils on the skin is one aspect of their use to be studied; another is the more systemic effects from absorption through the skin. Most essential oils used in small quantities for fragrance in over-the-counter lotions prove only to be an issue for allergens in sensitive patient groups. However, topical applications of essential oils in their pure concentrated form get absorbed into the skin faster than if used with a carrier oil, emulsion, or solvent.16 For most minor uses of essential oils, the body can detoxify absorbed chemicals the same way it does when a person eats the plants the oils came from (eg, basil essential oils leaching from the leaves into a tomato sauce). A possible danger of the oils’ systemic properties lies in the pregnant patient population who use essential oils thinking that natural is safe.
Many essential oils, such as lavender (L officinalis), exhibit hormonal mimicry with phytoestrogens and can produce emmenagogue (increasing menstrual flow) effects in women. Other oils, such as those of nutmeg (Myristica fragrans) and myrrh (Commiphora myrrha), can have abortifacient effects. These natural essential oils can lead to unintended health risks for mother and baby.17 With implications this serious, many essential oil companies put pregnancy warnings on most if not all of their products, but pregnant patients may not always note the risk.
Conclusion
Essential oils are not the newest medical fad. They outdate every drug on the market and were used by some of the first physicians in history. It is important to continue research into the antimicrobial effects of essential oils, as they may hold the secret to treatment options with the continued rise of multidrug-resistant organisms. The danger of these oils lies not in their hidden potential but in the belief that natural things are safe. A few animal studies have been performed, but little is known about the full effects of essential oils in humans. Patients need to be educated that these are not panaceas with freedom from side effects and that treatment options backed by the scientific method should be their first choice under the supervision of trained physicians. The Table outlines the uses and side effects of the essential oils discussed here.
- Fan S, Chang J, Zong Y, et al. GC-MS analysis of the composition of the essential oil from Dendranthema indicum var. aromaticum using three extraction methods and two columns. Molecules. 2018;23:576.
- Zheljazkov VD, Astatkie T, Schlegel V. Distillation time changes oregano essential oil yields and composition but not the antioxidant or antimicrobial activities. HortScience. 2012;47:777-784.
- Singletary K. Oregano: overview of the literature on health benefits. Nutr Today. 2010;45:129-138.
- Cortés-Rojas DF, de Souza CRF, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 2014;4:90-96.
- Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:681304.
- Cleff MB, Meinerz AR, Xavier M, et al. In vitro activity of Origanum vulgare essential oil against Candida species. Brazilian J Microbiol. 2010;41:116-123.
- Adam K, Sivropoulou A, Kokkini S, et al. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa essential oils against human pathogenic fungi. J Agric Food Chem. 1998;46:1739-1745.
- Miron D, Battisti F, Silva FK, et al. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Brazil J Pharmacognosy. 2014;24:660-667.
- Nazzaro F, Fratianni F, Coppola R, et al. Essential oils and antifungal activity. Pharmaceuticals (Basel). 2017;10:86.
- Akhondzadeh S, Kashani L, Fotouhi A, et al. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: a double-blind, randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:123-127.
- Mori H-M, Kawanami H, Kawahata H, et al. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement Altern Med. 2016;16:144.
- Vekiari SA, Protopapadakis EE, Papadopoulou P, et al. Composition and seasonal variation of the essential oil from leaves and peel of a cretan lemon variety. J Agric Food Chem. 2002;50:147-153.
- Aromatherapy. US Food & Drug Administration website. https://www.fda.gov/cosmetics/productsingredients/products/ucm127054.htm. Accessed October 14, 2020.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.25090.
- Essential Oil Safety Guide. Young Living Essential Oils website. https://www.youngliving.com/en_US/discover/essential-oil-safety. Accessed October 14, 2020.
- Cal K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Medica. 2006;72:311-316.
- Ernst E. Herbal medicinal products during pregnancy: are they safe? BJOG. 2002;109:227-235.
- Hsouna AB, Halima NB, Smaoui S, et al. Citrus lemon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017;16:146.
- Chen Y, Zhou C, Ge Z, et al. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol Lett. 2013;6:1140-1146.
- Zhang WK, Tao S-S, Li T-T, et al. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food Nutr Res. 2016;60:30849.
- Glodde N, Jakobs M, Bald T, et al. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci. 2015;138:35-40.
What Are Essential Oils?
Essential oils are aromatic volatile oils produced by medicinal plants that give them their distinct flavors and aromas. They are extracted using a variety of different techniques, such as microwave-assisted extraction, headspace extraction, and the most commonly employed hydrodistillation.1 Different parts of the plant are used for the specific oils; the shoots and leaves of Origanum vulgare are used for oregano oil, whereas the skins of Citrus limonum are used for lemon oil.2 Historically, essential oils have been used for cooking, food preservation, perfume, and medicine.3,4
Historical Uses for Essential Oils
Essential oils and their intact medicinal plants were among the first medicines widely available to the ancient world. The Ancient Greeks used topical and oral oregano as a cure-all for ailments including wounds, sore muscles, and diarrhea. Because of its use as a cure-all medicine, it remains a popular folk remedy in parts of Europe today.3 Lavender also has a long history of being a cure-all plant and oil. Some of the many claims behind this flower include treatment of burns, insect bites, parasites, muscle spasms, nausea, and anxiety/depression.5 With an extensive list of historical uses, many essential oils are being researched to determine if their acclaimed qualities have quantifiable properties.
Science Behind the Belief
In vitro experiments with oregano (O vulgare) have demonstrated notable antifungal and antimicrobial effects.6 Gas chromatographic analysis of the oil shows much of it is composed of phenolic monoterpenes, such as thymol and carvacrol. They exhibit strong antifungal effects with a slightly stronger effect on the dermatophyte Trichophyton rubrum over other yeast species such as Candida.7,8 The full effect of the monoterpenes on fungi is not completely understood, but early data show it has a strong affinity for the ergosterol used in the cell-wall synthesis. Other effects demonstrated in in vitro studies include the ability to block drug efflux pumps, biofilm formation, cellular communication among bacteria, and mycotoxin production.9
A double-blind, randomized trial by Akhondzadeh et al10 demonstrated lavender (Lavandula officinalis) to have a mild antidepressant quality but a noticeably more potent effect when combined with imipramine. The effects of the lavender with imipramine were stronger and provided earlier improvement than imipramine alone for treatment of mild to moderate depression. The team concluded that lavender may be an effective adjunct therapy in treating depression.10
In a study by Mori et al,11 full-thickness circular wounds were made in rats and treated with either lavender oil (L officinalis), nothing, or a control oil. With the lavender oil being at only 1% solution, the wounds treated with lavender oil demonstrated earlier closure than the other 2 groups of wounds, where no major difference was noted. On cellular analysis, it was seen that the lavender had increased the rate of granulation as well as expression of types I and III collagen. The most striking result was the large expression of transforming growth factor β seen in the lavender group compared to the others. The final thoughts on this experiment were that lavender may provide new approaches to wound care in the future.11
Potential Problems With Purity
One major concern raised about essential oils is their purity and the fidelity of their chemical composition. The specific aromatic chemicals in each essential oil are maintained for each species, but the proportions of each change even with the time of year.12 Gas chromatograph analysis of the same oil distilled with different techniques showed that the proportions of aromatic chemicals varied with technique. However, the major constituents of the oil remained present in large quantities, just at different percentages.1 Even using the same distillation technique for different time periods can greatly affect the yield and composition of the oil. Although the percentage of each aromatic compound can be affected by distillation times, the antioxidant and antimicrobial effects of the oil remain constant regardless of these variables.2 There is clearly a lack in standardization in essential oil production, which may not be an issue for its use in complementary medicine if its properties are maintained regardless.
Safety Concerns and Regulations
With essential oils being a natural cure for everyday ailments, some people are turning first to oils for every cut and bruise. The danger in these natural cures is that essential oils can cause several types of dermatitis and allergic reactions. The development of allergies to essential oils is at an even higher risk, considering people frequently put them on wounds and rashes where the skin barrier is already weakened. Many essential oils fall into the fragrance category in patch tests, negating the widely circulating blogger and online reports that essential oils cannot cause allergies.
Some of the oils, although regarded safe by the US Food and Drug Administration for consumption, can cause dermatitis from simple contact or with sun exposure.13 Members of the citrus family are notorious for the phytophotodermatitis reaction, which can leave hyperpigmented scarring after exposure of the oils to sunlight.14 Most companies that sell essential oils are aware of this reaction and include it in the warning labels.
The legal problem with selling and classifying essential oils is that the US Food and Drug Administration requires products intended for treatment to be labeled as drugs, which hinders their sales on the open market.13 It all boils down to intended use, so some companies sell the oils under a food or fragrance classification with vague instructions on how to use said oil for medicinal purposes, which leads to lack of supervision, anecdotal cures, and false health claims. One company claims in their safety guide for topical applications of their oils that “[i]f a rash occurs, this may be a sign of detoxification.”15 If essential oils had only minimal absorption topically, their safety would be less concerning, but this does not appear to be the case.
Absorption and Systemics
The effects of essential oils on the skin is one aspect of their use to be studied; another is the more systemic effects from absorption through the skin. Most essential oils used in small quantities for fragrance in over-the-counter lotions prove only to be an issue for allergens in sensitive patient groups. However, topical applications of essential oils in their pure concentrated form get absorbed into the skin faster than if used with a carrier oil, emulsion, or solvent.16 For most minor uses of essential oils, the body can detoxify absorbed chemicals the same way it does when a person eats the plants the oils came from (eg, basil essential oils leaching from the leaves into a tomato sauce). A possible danger of the oils’ systemic properties lies in the pregnant patient population who use essential oils thinking that natural is safe.
Many essential oils, such as lavender (L officinalis), exhibit hormonal mimicry with phytoestrogens and can produce emmenagogue (increasing menstrual flow) effects in women. Other oils, such as those of nutmeg (Myristica fragrans) and myrrh (Commiphora myrrha), can have abortifacient effects. These natural essential oils can lead to unintended health risks for mother and baby.17 With implications this serious, many essential oil companies put pregnancy warnings on most if not all of their products, but pregnant patients may not always note the risk.
Conclusion
Essential oils are not the newest medical fad. They outdate every drug on the market and were used by some of the first physicians in history. It is important to continue research into the antimicrobial effects of essential oils, as they may hold the secret to treatment options with the continued rise of multidrug-resistant organisms. The danger of these oils lies not in their hidden potential but in the belief that natural things are safe. A few animal studies have been performed, but little is known about the full effects of essential oils in humans. Patients need to be educated that these are not panaceas with freedom from side effects and that treatment options backed by the scientific method should be their first choice under the supervision of trained physicians. The Table outlines the uses and side effects of the essential oils discussed here.
What Are Essential Oils?
Essential oils are aromatic volatile oils produced by medicinal plants that give them their distinct flavors and aromas. They are extracted using a variety of different techniques, such as microwave-assisted extraction, headspace extraction, and the most commonly employed hydrodistillation.1 Different parts of the plant are used for the specific oils; the shoots and leaves of Origanum vulgare are used for oregano oil, whereas the skins of Citrus limonum are used for lemon oil.2 Historically, essential oils have been used for cooking, food preservation, perfume, and medicine.3,4
Historical Uses for Essential Oils
Essential oils and their intact medicinal plants were among the first medicines widely available to the ancient world. The Ancient Greeks used topical and oral oregano as a cure-all for ailments including wounds, sore muscles, and diarrhea. Because of its use as a cure-all medicine, it remains a popular folk remedy in parts of Europe today.3 Lavender also has a long history of being a cure-all plant and oil. Some of the many claims behind this flower include treatment of burns, insect bites, parasites, muscle spasms, nausea, and anxiety/depression.5 With an extensive list of historical uses, many essential oils are being researched to determine if their acclaimed qualities have quantifiable properties.
Science Behind the Belief
In vitro experiments with oregano (O vulgare) have demonstrated notable antifungal and antimicrobial effects.6 Gas chromatographic analysis of the oil shows much of it is composed of phenolic monoterpenes, such as thymol and carvacrol. They exhibit strong antifungal effects with a slightly stronger effect on the dermatophyte Trichophyton rubrum over other yeast species such as Candida.7,8 The full effect of the monoterpenes on fungi is not completely understood, but early data show it has a strong affinity for the ergosterol used in the cell-wall synthesis. Other effects demonstrated in in vitro studies include the ability to block drug efflux pumps, biofilm formation, cellular communication among bacteria, and mycotoxin production.9
A double-blind, randomized trial by Akhondzadeh et al10 demonstrated lavender (Lavandula officinalis) to have a mild antidepressant quality but a noticeably more potent effect when combined with imipramine. The effects of the lavender with imipramine were stronger and provided earlier improvement than imipramine alone for treatment of mild to moderate depression. The team concluded that lavender may be an effective adjunct therapy in treating depression.10
In a study by Mori et al,11 full-thickness circular wounds were made in rats and treated with either lavender oil (L officinalis), nothing, or a control oil. With the lavender oil being at only 1% solution, the wounds treated with lavender oil demonstrated earlier closure than the other 2 groups of wounds, where no major difference was noted. On cellular analysis, it was seen that the lavender had increased the rate of granulation as well as expression of types I and III collagen. The most striking result was the large expression of transforming growth factor β seen in the lavender group compared to the others. The final thoughts on this experiment were that lavender may provide new approaches to wound care in the future.11
Potential Problems With Purity
One major concern raised about essential oils is their purity and the fidelity of their chemical composition. The specific aromatic chemicals in each essential oil are maintained for each species, but the proportions of each change even with the time of year.12 Gas chromatograph analysis of the same oil distilled with different techniques showed that the proportions of aromatic chemicals varied with technique. However, the major constituents of the oil remained present in large quantities, just at different percentages.1 Even using the same distillation technique for different time periods can greatly affect the yield and composition of the oil. Although the percentage of each aromatic compound can be affected by distillation times, the antioxidant and antimicrobial effects of the oil remain constant regardless of these variables.2 There is clearly a lack in standardization in essential oil production, which may not be an issue for its use in complementary medicine if its properties are maintained regardless.
Safety Concerns and Regulations
With essential oils being a natural cure for everyday ailments, some people are turning first to oils for every cut and bruise. The danger in these natural cures is that essential oils can cause several types of dermatitis and allergic reactions. The development of allergies to essential oils is at an even higher risk, considering people frequently put them on wounds and rashes where the skin barrier is already weakened. Many essential oils fall into the fragrance category in patch tests, negating the widely circulating blogger and online reports that essential oils cannot cause allergies.
Some of the oils, although regarded safe by the US Food and Drug Administration for consumption, can cause dermatitis from simple contact or with sun exposure.13 Members of the citrus family are notorious for the phytophotodermatitis reaction, which can leave hyperpigmented scarring after exposure of the oils to sunlight.14 Most companies that sell essential oils are aware of this reaction and include it in the warning labels.
The legal problem with selling and classifying essential oils is that the US Food and Drug Administration requires products intended for treatment to be labeled as drugs, which hinders their sales on the open market.13 It all boils down to intended use, so some companies sell the oils under a food or fragrance classification with vague instructions on how to use said oil for medicinal purposes, which leads to lack of supervision, anecdotal cures, and false health claims. One company claims in their safety guide for topical applications of their oils that “[i]f a rash occurs, this may be a sign of detoxification.”15 If essential oils had only minimal absorption topically, their safety would be less concerning, but this does not appear to be the case.
Absorption and Systemics
The effects of essential oils on the skin is one aspect of their use to be studied; another is the more systemic effects from absorption through the skin. Most essential oils used in small quantities for fragrance in over-the-counter lotions prove only to be an issue for allergens in sensitive patient groups. However, topical applications of essential oils in their pure concentrated form get absorbed into the skin faster than if used with a carrier oil, emulsion, or solvent.16 For most minor uses of essential oils, the body can detoxify absorbed chemicals the same way it does when a person eats the plants the oils came from (eg, basil essential oils leaching from the leaves into a tomato sauce). A possible danger of the oils’ systemic properties lies in the pregnant patient population who use essential oils thinking that natural is safe.
Many essential oils, such as lavender (L officinalis), exhibit hormonal mimicry with phytoestrogens and can produce emmenagogue (increasing menstrual flow) effects in women. Other oils, such as those of nutmeg (Myristica fragrans) and myrrh (Commiphora myrrha), can have abortifacient effects. These natural essential oils can lead to unintended health risks for mother and baby.17 With implications this serious, many essential oil companies put pregnancy warnings on most if not all of their products, but pregnant patients may not always note the risk.
Conclusion
Essential oils are not the newest medical fad. They outdate every drug on the market and were used by some of the first physicians in history. It is important to continue research into the antimicrobial effects of essential oils, as they may hold the secret to treatment options with the continued rise of multidrug-resistant organisms. The danger of these oils lies not in their hidden potential but in the belief that natural things are safe. A few animal studies have been performed, but little is known about the full effects of essential oils in humans. Patients need to be educated that these are not panaceas with freedom from side effects and that treatment options backed by the scientific method should be their first choice under the supervision of trained physicians. The Table outlines the uses and side effects of the essential oils discussed here.
- Fan S, Chang J, Zong Y, et al. GC-MS analysis of the composition of the essential oil from Dendranthema indicum var. aromaticum using three extraction methods and two columns. Molecules. 2018;23:576.
- Zheljazkov VD, Astatkie T, Schlegel V. Distillation time changes oregano essential oil yields and composition but not the antioxidant or antimicrobial activities. HortScience. 2012;47:777-784.
- Singletary K. Oregano: overview of the literature on health benefits. Nutr Today. 2010;45:129-138.
- Cortés-Rojas DF, de Souza CRF, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 2014;4:90-96.
- Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:681304.
- Cleff MB, Meinerz AR, Xavier M, et al. In vitro activity of Origanum vulgare essential oil against Candida species. Brazilian J Microbiol. 2010;41:116-123.
- Adam K, Sivropoulou A, Kokkini S, et al. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa essential oils against human pathogenic fungi. J Agric Food Chem. 1998;46:1739-1745.
- Miron D, Battisti F, Silva FK, et al. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Brazil J Pharmacognosy. 2014;24:660-667.
- Nazzaro F, Fratianni F, Coppola R, et al. Essential oils and antifungal activity. Pharmaceuticals (Basel). 2017;10:86.
- Akhondzadeh S, Kashani L, Fotouhi A, et al. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: a double-blind, randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:123-127.
- Mori H-M, Kawanami H, Kawahata H, et al. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement Altern Med. 2016;16:144.
- Vekiari SA, Protopapadakis EE, Papadopoulou P, et al. Composition and seasonal variation of the essential oil from leaves and peel of a cretan lemon variety. J Agric Food Chem. 2002;50:147-153.
- Aromatherapy. US Food & Drug Administration website. https://www.fda.gov/cosmetics/productsingredients/products/ucm127054.htm. Accessed October 14, 2020.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.25090.
- Essential Oil Safety Guide. Young Living Essential Oils website. https://www.youngliving.com/en_US/discover/essential-oil-safety. Accessed October 14, 2020.
- Cal K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Medica. 2006;72:311-316.
- Ernst E. Herbal medicinal products during pregnancy: are they safe? BJOG. 2002;109:227-235.
- Hsouna AB, Halima NB, Smaoui S, et al. Citrus lemon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017;16:146.
- Chen Y, Zhou C, Ge Z, et al. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol Lett. 2013;6:1140-1146.
- Zhang WK, Tao S-S, Li T-T, et al. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food Nutr Res. 2016;60:30849.
- Glodde N, Jakobs M, Bald T, et al. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci. 2015;138:35-40.
- Fan S, Chang J, Zong Y, et al. GC-MS analysis of the composition of the essential oil from Dendranthema indicum var. aromaticum using three extraction methods and two columns. Molecules. 2018;23:576.
- Zheljazkov VD, Astatkie T, Schlegel V. Distillation time changes oregano essential oil yields and composition but not the antioxidant or antimicrobial activities. HortScience. 2012;47:777-784.
- Singletary K. Oregano: overview of the literature on health benefits. Nutr Today. 2010;45:129-138.
- Cortés-Rojas DF, de Souza CRF, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed. 2014;4:90-96.
- Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:681304.
- Cleff MB, Meinerz AR, Xavier M, et al. In vitro activity of Origanum vulgare essential oil against Candida species. Brazilian J Microbiol. 2010;41:116-123.
- Adam K, Sivropoulou A, Kokkini S, et al. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa essential oils against human pathogenic fungi. J Agric Food Chem. 1998;46:1739-1745.
- Miron D, Battisti F, Silva FK, et al. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Brazil J Pharmacognosy. 2014;24:660-667.
- Nazzaro F, Fratianni F, Coppola R, et al. Essential oils and antifungal activity. Pharmaceuticals (Basel). 2017;10:86.
- Akhondzadeh S, Kashani L, Fotouhi A, et al. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: a double-blind, randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:123-127.
- Mori H-M, Kawanami H, Kawahata H, et al. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement Altern Med. 2016;16:144.
- Vekiari SA, Protopapadakis EE, Papadopoulou P, et al. Composition and seasonal variation of the essential oil from leaves and peel of a cretan lemon variety. J Agric Food Chem. 2002;50:147-153.
- Aromatherapy. US Food & Drug Administration website. https://www.fda.gov/cosmetics/productsingredients/products/ucm127054.htm. Accessed October 14, 2020.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.25090.
- Essential Oil Safety Guide. Young Living Essential Oils website. https://www.youngliving.com/en_US/discover/essential-oil-safety. Accessed October 14, 2020.
- Cal K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Medica. 2006;72:311-316.
- Ernst E. Herbal medicinal products during pregnancy: are they safe? BJOG. 2002;109:227-235.
- Hsouna AB, Halima NB, Smaoui S, et al. Citrus lemon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017;16:146.
- Chen Y, Zhou C, Ge Z, et al. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol Lett. 2013;6:1140-1146.
- Zhang WK, Tao S-S, Li T-T, et al. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food Nutr Res. 2016;60:30849.
- Glodde N, Jakobs M, Bald T, et al. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci. 2015;138:35-40.
Practice Points
- Essential oils are a rising trend of nonprescribed topical supplements used by patients to self-treat.
- Research into historically medicinal essential oils may unlock treatment opportunities in the near future.
- Keeping an open-minded line of communication is critical for divulgence of potential home remedies that could be causing patients harm.
- Understanding the mindset of the essential oil–using community is key to building trust and treating these patients who are often distrusting of Western medicine.