User login
Identifying and Managing Abscess Formation Related to Soft-Tissue Fillers
Injectable soft-tissue fillers continue to be popular in the cosmetic arena. In the United States there are many fillers currently on the market and many more coming through the pipeline. A multitude of products are available outside the United States. As with any procedure, the more fillers we inject, the more complications we are bound to see.
Conrad et al (Modern Plastic Surgery. 2015;5:14-18) performed a retrospective analysis of patients treated over a 10-year period with soft-tissue injections (1559 patients) looking for cases complicated by abscess formation. Four patients were identified (0.3% of total patients). The authors discussed the 4 cases, the patients’ medical history and experience with other injectable agents, and the management of each complication.
Case 1 was a 52-year-old woman with systemic lupus erythematosus on a low-dose steroid who presented with an inflammatory response in the lower lip 7 days following injection with a hyaluronic acid (HA)–based gel filler in 2011. Her history was notable for prior HA filler in 2008 and polyacrylamide filler in 2009 and 2010. She was treated with 4 sessions of incision and drainage (I&D) and systemic clindamycin. Most of the cultures were negative, but one showed streptococci.
Case 2 was a 56-year-old woman treated in the nasolabial fold with HA in 2009. She developed inflammation shortly after and an abscess at the site a month later. She was treated with clindamycin both times, though cultures were negative. Furthermore, the abscess was treated with I&D and an intralesional steroid. She was a smoker and had been treated with a polymethyl methacrylate filler in 2002 and subsequently in 2013 with no issues.
Case 3 was a 39-year-old woman injected with an HA filler in the upper and lower lips in 2011. One month later she developed abscesses in both areas that were treated twice with I&D. Cultures were negative. She had a history of polyacrylamide injections of the nasolabial fold in 2009. The patient’s medical history was notable for scleroderma.
Case 4 was a 58-year-old woman injected with an HA filler in 2009 in the prejowl sulcus and nasolabial fold. She developed recurrent sterile abscesses in the areas 8 months after treatment that were managed by drainage of the areas and intralesional steroid injections over the ensuing 6 months. The scars were then excised, lasered 6 weeks later, and then filled in with expanded polytetrafluoroethylene implants, followed by 1 more session of laser resurfacing. She had a history of polymethyl methacrylate filler in 2002.
All patients eventually recovered. The authors stressed 3 important factors in managing dermal filler complications: (1) identifying the causative pathogen, (2) choosing the appropriate treatment of delayed-onset abscess formation, and (3) identifying the risk factors for patients at risk for abscess formation.
The issue of biofilms complicates the ability to identify the bacterial agent, yet biofilms are becoming recognized as the causative factors in what were previously thought of as sterile abscesses. The authors suggested using a peptide nucleic acid fluorescent in situ hybridization test to identify the biofilm bacteria. Conrad et el also discussed the development of slippery liquid-infused porous surfaces technology to coat the inside of syringes to help prevent biofilm formation.
The management of these patients is tricky because it is difficult to differentiate between a biofilm abscess and a hypersensitivity reaction. For this reason, the authors advocated using hyaluronidase versus intralesional steroids in the initial management to make the area more susceptible to antibiotics and to avoid promoting the growth of bacteria with the use of steroids. For patient risk factors, the authors focused on the fact that 2 of 4 patients had concomitant autoimmune disorders—scleroderma and systemic lupus erythematosus—that may have predisposed them to infection. Lastly, 2 patients had prior polyacrylamide injections and the authors also speculated if the positive charge of this filler attracted bacteria.
What’s the issue?
The use of fillers will continue to increase as there are more fillers with novel properties entering the market. As with new technology, only time will tell if we will see any particular type of reaction or risk for infection with them. The issue of biofilm bacterial contamination is real. It is recognized as one of the causes of capsular contraction following breast implant surgery. The etiology may not be from contamination during production but from contamination of the filler after injection due to any transient bacteremia that the patient may experience. A concern is that dental manipulation (eg, dental cleaning, filling of dental caries, periodontal surgery) during the 2- to 4-week postfiller period may “seed” bacteria into the area and cause the bacteria to settle and grow on the foreign substance. For patients who have semipermanent or permanent fillers such as polyacrylamide, polymethyl methacrylate beads, or poly-L-lactic acid, biofilm risk is greater and can occur months to years after the procedure. I have personally seen 2 cases of poly-L-lactic acid filler develop red, tender, sterile abscesses 1 year after placement in the tissue. Both cases responded to prolonged clarithromycin use (2 months). However, these cases highlight the fact that the fillers persist long after we place them, and any bacteremia, even mild, can cause an unsightly reaction.
Have you seen delayed soft-tissue filler reactions in your practice? Given this information, will you change the way you advise patients on dental procedures in the 2- to 4-week postfiller period?
Injectable soft-tissue fillers continue to be popular in the cosmetic arena. In the United States there are many fillers currently on the market and many more coming through the pipeline. A multitude of products are available outside the United States. As with any procedure, the more fillers we inject, the more complications we are bound to see.
Conrad et al (Modern Plastic Surgery. 2015;5:14-18) performed a retrospective analysis of patients treated over a 10-year period with soft-tissue injections (1559 patients) looking for cases complicated by abscess formation. Four patients were identified (0.3% of total patients). The authors discussed the 4 cases, the patients’ medical history and experience with other injectable agents, and the management of each complication.
Case 1 was a 52-year-old woman with systemic lupus erythematosus on a low-dose steroid who presented with an inflammatory response in the lower lip 7 days following injection with a hyaluronic acid (HA)–based gel filler in 2011. Her history was notable for prior HA filler in 2008 and polyacrylamide filler in 2009 and 2010. She was treated with 4 sessions of incision and drainage (I&D) and systemic clindamycin. Most of the cultures were negative, but one showed streptococci.
Case 2 was a 56-year-old woman treated in the nasolabial fold with HA in 2009. She developed inflammation shortly after and an abscess at the site a month later. She was treated with clindamycin both times, though cultures were negative. Furthermore, the abscess was treated with I&D and an intralesional steroid. She was a smoker and had been treated with a polymethyl methacrylate filler in 2002 and subsequently in 2013 with no issues.
Case 3 was a 39-year-old woman injected with an HA filler in the upper and lower lips in 2011. One month later she developed abscesses in both areas that were treated twice with I&D. Cultures were negative. She had a history of polyacrylamide injections of the nasolabial fold in 2009. The patient’s medical history was notable for scleroderma.
Case 4 was a 58-year-old woman injected with an HA filler in 2009 in the prejowl sulcus and nasolabial fold. She developed recurrent sterile abscesses in the areas 8 months after treatment that were managed by drainage of the areas and intralesional steroid injections over the ensuing 6 months. The scars were then excised, lasered 6 weeks later, and then filled in with expanded polytetrafluoroethylene implants, followed by 1 more session of laser resurfacing. She had a history of polymethyl methacrylate filler in 2002.
All patients eventually recovered. The authors stressed 3 important factors in managing dermal filler complications: (1) identifying the causative pathogen, (2) choosing the appropriate treatment of delayed-onset abscess formation, and (3) identifying the risk factors for patients at risk for abscess formation.
The issue of biofilms complicates the ability to identify the bacterial agent, yet biofilms are becoming recognized as the causative factors in what were previously thought of as sterile abscesses. The authors suggested using a peptide nucleic acid fluorescent in situ hybridization test to identify the biofilm bacteria. Conrad et el also discussed the development of slippery liquid-infused porous surfaces technology to coat the inside of syringes to help prevent biofilm formation.
The management of these patients is tricky because it is difficult to differentiate between a biofilm abscess and a hypersensitivity reaction. For this reason, the authors advocated using hyaluronidase versus intralesional steroids in the initial management to make the area more susceptible to antibiotics and to avoid promoting the growth of bacteria with the use of steroids. For patient risk factors, the authors focused on the fact that 2 of 4 patients had concomitant autoimmune disorders—scleroderma and systemic lupus erythematosus—that may have predisposed them to infection. Lastly, 2 patients had prior polyacrylamide injections and the authors also speculated if the positive charge of this filler attracted bacteria.
What’s the issue?
The use of fillers will continue to increase as there are more fillers with novel properties entering the market. As with new technology, only time will tell if we will see any particular type of reaction or risk for infection with them. The issue of biofilm bacterial contamination is real. It is recognized as one of the causes of capsular contraction following breast implant surgery. The etiology may not be from contamination during production but from contamination of the filler after injection due to any transient bacteremia that the patient may experience. A concern is that dental manipulation (eg, dental cleaning, filling of dental caries, periodontal surgery) during the 2- to 4-week postfiller period may “seed” bacteria into the area and cause the bacteria to settle and grow on the foreign substance. For patients who have semipermanent or permanent fillers such as polyacrylamide, polymethyl methacrylate beads, or poly-L-lactic acid, biofilm risk is greater and can occur months to years after the procedure. I have personally seen 2 cases of poly-L-lactic acid filler develop red, tender, sterile abscesses 1 year after placement in the tissue. Both cases responded to prolonged clarithromycin use (2 months). However, these cases highlight the fact that the fillers persist long after we place them, and any bacteremia, even mild, can cause an unsightly reaction.
Have you seen delayed soft-tissue filler reactions in your practice? Given this information, will you change the way you advise patients on dental procedures in the 2- to 4-week postfiller period?
Injectable soft-tissue fillers continue to be popular in the cosmetic arena. In the United States there are many fillers currently on the market and many more coming through the pipeline. A multitude of products are available outside the United States. As with any procedure, the more fillers we inject, the more complications we are bound to see.
Conrad et al (Modern Plastic Surgery. 2015;5:14-18) performed a retrospective analysis of patients treated over a 10-year period with soft-tissue injections (1559 patients) looking for cases complicated by abscess formation. Four patients were identified (0.3% of total patients). The authors discussed the 4 cases, the patients’ medical history and experience with other injectable agents, and the management of each complication.
Case 1 was a 52-year-old woman with systemic lupus erythematosus on a low-dose steroid who presented with an inflammatory response in the lower lip 7 days following injection with a hyaluronic acid (HA)–based gel filler in 2011. Her history was notable for prior HA filler in 2008 and polyacrylamide filler in 2009 and 2010. She was treated with 4 sessions of incision and drainage (I&D) and systemic clindamycin. Most of the cultures were negative, but one showed streptococci.
Case 2 was a 56-year-old woman treated in the nasolabial fold with HA in 2009. She developed inflammation shortly after and an abscess at the site a month later. She was treated with clindamycin both times, though cultures were negative. Furthermore, the abscess was treated with I&D and an intralesional steroid. She was a smoker and had been treated with a polymethyl methacrylate filler in 2002 and subsequently in 2013 with no issues.
Case 3 was a 39-year-old woman injected with an HA filler in the upper and lower lips in 2011. One month later she developed abscesses in both areas that were treated twice with I&D. Cultures were negative. She had a history of polyacrylamide injections of the nasolabial fold in 2009. The patient’s medical history was notable for scleroderma.
Case 4 was a 58-year-old woman injected with an HA filler in 2009 in the prejowl sulcus and nasolabial fold. She developed recurrent sterile abscesses in the areas 8 months after treatment that were managed by drainage of the areas and intralesional steroid injections over the ensuing 6 months. The scars were then excised, lasered 6 weeks later, and then filled in with expanded polytetrafluoroethylene implants, followed by 1 more session of laser resurfacing. She had a history of polymethyl methacrylate filler in 2002.
All patients eventually recovered. The authors stressed 3 important factors in managing dermal filler complications: (1) identifying the causative pathogen, (2) choosing the appropriate treatment of delayed-onset abscess formation, and (3) identifying the risk factors for patients at risk for abscess formation.
The issue of biofilms complicates the ability to identify the bacterial agent, yet biofilms are becoming recognized as the causative factors in what were previously thought of as sterile abscesses. The authors suggested using a peptide nucleic acid fluorescent in situ hybridization test to identify the biofilm bacteria. Conrad et el also discussed the development of slippery liquid-infused porous surfaces technology to coat the inside of syringes to help prevent biofilm formation.
The management of these patients is tricky because it is difficult to differentiate between a biofilm abscess and a hypersensitivity reaction. For this reason, the authors advocated using hyaluronidase versus intralesional steroids in the initial management to make the area more susceptible to antibiotics and to avoid promoting the growth of bacteria with the use of steroids. For patient risk factors, the authors focused on the fact that 2 of 4 patients had concomitant autoimmune disorders—scleroderma and systemic lupus erythematosus—that may have predisposed them to infection. Lastly, 2 patients had prior polyacrylamide injections and the authors also speculated if the positive charge of this filler attracted bacteria.
What’s the issue?
The use of fillers will continue to increase as there are more fillers with novel properties entering the market. As with new technology, only time will tell if we will see any particular type of reaction or risk for infection with them. The issue of biofilm bacterial contamination is real. It is recognized as one of the causes of capsular contraction following breast implant surgery. The etiology may not be from contamination during production but from contamination of the filler after injection due to any transient bacteremia that the patient may experience. A concern is that dental manipulation (eg, dental cleaning, filling of dental caries, periodontal surgery) during the 2- to 4-week postfiller period may “seed” bacteria into the area and cause the bacteria to settle and grow on the foreign substance. For patients who have semipermanent or permanent fillers such as polyacrylamide, polymethyl methacrylate beads, or poly-L-lactic acid, biofilm risk is greater and can occur months to years after the procedure. I have personally seen 2 cases of poly-L-lactic acid filler develop red, tender, sterile abscesses 1 year after placement in the tissue. Both cases responded to prolonged clarithromycin use (2 months). However, these cases highlight the fact that the fillers persist long after we place them, and any bacteremia, even mild, can cause an unsightly reaction.
Have you seen delayed soft-tissue filler reactions in your practice? Given this information, will you change the way you advise patients on dental procedures in the 2- to 4-week postfiller period?
Don’t Get Hung Up on Fishhooks: A Guide to Fishhook Removal
Fishing is one of the world’s most beloved activities, enjoyed as a sport or a leisure activity. However, a common injury from fishing is embedment of the fishhook in the cutaneous tissue. Barbed fishhooks are used for their effectiveness in maintaining the fish on the hook once it is caught, but when implanted in the hand of a fisherman or fisherwoman, barbs can pose problems for removal without exacerbating internal tissue injury. Nevertheless, dermatologists should not shy away from removal of barbed fishhooks, as there are several simple methods that can be easily utilized in the outpatient setting.
Case Report
A 68-year-old man presented to an outpatient dermatology clinic after sustaining a barbed fishhook injury while fishing. The fishhook was firmly inserted into the ventral side of the third digit of the right hand (Figure 1).
Prior to presenting to dermatology, the patient went to 2 urgent care clinics the same day seeking treatment. He reported that practitioners at the first clinic were not able to remove the fishhook because they did not have pliers in stock. At the second clinic he was told the fishhook might be embedded in deeper tissues and was advised to go to the emergency department at the local hospital. When he arrived at the emergency department, a 6-hour wait time prompted him to see a local dermatologist instead.
To remove the fishhook, the area was cleaned and prepared first; lidocaine 2% was administered for local anesthesia. An 18-gauge needle was then advanced through the puncture site parallel to the fishhook’s inner shaft on the same side as the barb, which could be successfully palpated using the tip of the 18-gauge needle. The tip of the needle was then used to cap the barb beneath the skin. This technique allowed for the hook to be easily extracted in a retrograde manner without causing further destruction to the surrounding tissue. The patient then was started on prophylaxis cephalexin 500 mg 3 times daily for 3 days.
Comment
The hand is the most common site of fishhook injury, followed closely by the head and eyes.1 Barbless fishhooks usually can be removed by pushing the hook in a retrograde manner along the path of insertion. This method is simple and rarely results in complications. However, there are no guidelines for removal of barbed fishhooks. Furthermore, removing a barbed fishhook in the same retrograde manner would result in extensive internal tissue destruction and increased complications. Due to the popularity of the sport of fishing, fishhook injuries, depending on geographical location, are not uncommon.2 For this reason, trauma and emergency practitioners have become well versed in safe methods for barbed fishhook removal. However, patients are not always able or willing to seek medical care in emergency departments and may opt to seek treatment in outpatient settings, such as in our case. As a result, dermatologists should familiarize themselves with safe and effective fishhook removal methods, as they are not time consuming and do not require complex equipment. Failure to treat the patient may lead to further patient discomfort and increased risk for complications. Additionally, many of the techniques for removal may be useful with other foreign bodies embedded in cutaneous tissue (eg, splinters).
There are a number of safe and effective techniques for removing barbed fishhooks from cutaneous tissue, including the advance-and-cut method, the cut-it-out technique, the string-pull method, and the needle cover technique.1-3 The method chosen to remove the fishhook is dependent on a variety of factors, such as anatomic location, tissue depth, and provider comfort.
With the advance-and-cut method (Figure 2), the affected area is anesthetized and a small incision in the skin is created to expose the barb. The fishhook is then advanced through the incision, providing visibility of the barb and thus allowing the practitioner to cut the barbed tip without creating further damage to the surrounding tissue. The shaft of the fishhook can subsequently be removed in a retrograde fashion. The advantages of this technique include that it may be successfully used in all types of barbed fishhooks and it provides the practitioner with direct visibility of the barb, thus minimizing risk for neurovascular injury during removal.1 However, the primary disadvantage is that a second cutaneous wound is created in exposing the barb.
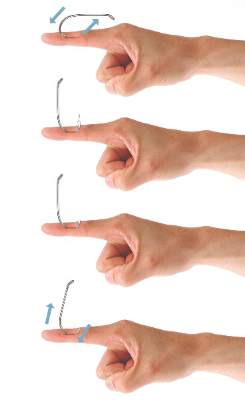
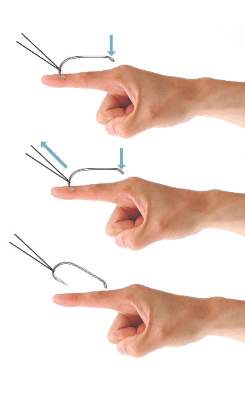
| 
| |
| Figure 2. The advance-and-cut method for fishhook removal. | Figure 3. The cut-it-out method for fishhook removal. |
|
The cut-it-out technique (Figure 3) is similar to the advance-and-cut method in that they both require anesthesia along with creating an incision. With this method, a scalpel is used to create a small linear incision originating at the fishhook entrance site and ending at the approximated location of the fishhook’s tip. The fishhook then is simply lifted superiorly in a retrograde fashion.
|
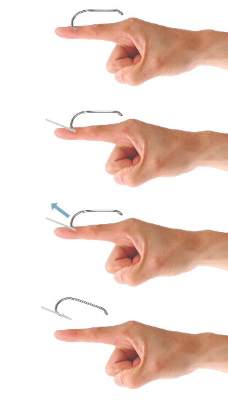
The string-pull method (Figure 4) has been credited to fishermen in South Australia and was first described by Cooke2 in 1961. This method is relatively painless, does not require anesthesia, and has a high success rate when properly administered. However, it does require rapid and confident motions (ie, without hesitation) by the practitioner and should not be performed on free-moving areas of the body (eg, earlobe).3 With this technique, a sturdy piece of suture (eg, 2/0 or 3/0 strength silk) is looped around the hook and is extended away from the practitioner at a 30° angle. The free end of the suture is then securely fastened around the index finger of the practitioner’s dominant hand. The index finger of the nondominant hand should apply a downward pressure to the hook shaft to disengage the barb from the tissue. Simultaneously and rather quickly and forcefully the practitioner must pull the dominant index finger with the string attached in a superior and lateral direction, as depicted by the long arrow in Figure 4. If successful, the barbed hook will pull out of the entrance site. The use of string in pulling the fishhook parallel to the site of injury is helpful for smaller fishhooks that may be difficult to grab with fingers alone. However, with larger fishhooks, the string may not be required so long as the practitioner is able to obtain a secure grasp on the fishhook shaft. The string-pull method becomes particularly useful when anesthesia is unavailable or when the barb of the hook is embedded too deeply for safe advancement through tissue to visualize and cut the barb.
|
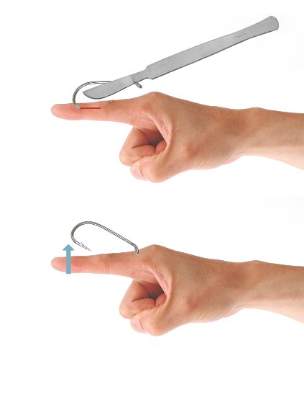
Lastly, the needle cover technique (Figure 5) is another simple method that does not require the creation of a secondary wound. An 18-gauge needle is simply inserted parallel to the fishhook curvature into the site of entry. By using the needle to slide along the fishhook’s curve, the practitioner is able to follow its pathway while in the tissue. The tip of the 18-gauge needle is then used to cap or cover the barb, thus allowing the fishhook to be removed in a retrograde fashion from the wound. In an outpatient setting, this technique does not require the creation of additional tissue damage and practitioners who are inexperienced with fishhook removal may proceed through the motions more slowly and methodically than the string-pull method permits.
Wound care following fishhook removal should involve adequate flushing of the wound with normal saline along with the application of topical antibiotics and a simple dressing and adhesive bandage. Oral prophylactic antibiotics typically are not required for shallow cutaneous injuries unless the fishhook is dirty, the patient is immunocompromised, or the patient has a condition lending to poor wound healing (eg, diabetes mellitus, peripheral vascular disease).3 When deciding on antibiotics, it is important to note that fishhook injuries while saltwater fishing are associated with Vibrio infection, while injuries sustained during freshwater fishing are associated with gram-negative bacteria (eg, Pseudomonas and Aeromonas species).3 Lastly, it is essential to find out the immunization status of the patient, and tetanus immune globulin should be provided if necessary.
| 
| |
| Figure 4. The string-pull method for fishhook removal. | Figure 5. The needle cover technique for fishhook removal. |
Conclusion
Although guidelines for barbed fishhook removal are not available, outpatient physicians, including dermatologists, should not fear removal procedures. There are many safe and effective fishhook removal methods that are not time consuming and do not require complex equipment. Furthermore, familiarization with these same techniques may be useful for removal of other foreign bodies embedded in cutaneous tissue.
1. Khan HA, Kamal Y, Lone AU. Fish hook injury: removal by “push through and cut off” technique: a case report and brief literature review [published online March 24, 2014]. Trauma Mon. 2014;19:e17728.
2. Cooke T. How to remove fish-hooks with a bit of string. Med J Aust. 1961;48:815-816.
3. Thommasen HV, Thommasen A. The occasional removal of an embedded fish hook. Can J Rural Med. 2005;10:255-259.
Fishing is one of the world’s most beloved activities, enjoyed as a sport or a leisure activity. However, a common injury from fishing is embedment of the fishhook in the cutaneous tissue. Barbed fishhooks are used for their effectiveness in maintaining the fish on the hook once it is caught, but when implanted in the hand of a fisherman or fisherwoman, barbs can pose problems for removal without exacerbating internal tissue injury. Nevertheless, dermatologists should not shy away from removal of barbed fishhooks, as there are several simple methods that can be easily utilized in the outpatient setting.
Case Report
A 68-year-old man presented to an outpatient dermatology clinic after sustaining a barbed fishhook injury while fishing. The fishhook was firmly inserted into the ventral side of the third digit of the right hand (Figure 1).
Prior to presenting to dermatology, the patient went to 2 urgent care clinics the same day seeking treatment. He reported that practitioners at the first clinic were not able to remove the fishhook because they did not have pliers in stock. At the second clinic he was told the fishhook might be embedded in deeper tissues and was advised to go to the emergency department at the local hospital. When he arrived at the emergency department, a 6-hour wait time prompted him to see a local dermatologist instead.
To remove the fishhook, the area was cleaned and prepared first; lidocaine 2% was administered for local anesthesia. An 18-gauge needle was then advanced through the puncture site parallel to the fishhook’s inner shaft on the same side as the barb, which could be successfully palpated using the tip of the 18-gauge needle. The tip of the needle was then used to cap the barb beneath the skin. This technique allowed for the hook to be easily extracted in a retrograde manner without causing further destruction to the surrounding tissue. The patient then was started on prophylaxis cephalexin 500 mg 3 times daily for 3 days.
Comment
The hand is the most common site of fishhook injury, followed closely by the head and eyes.1 Barbless fishhooks usually can be removed by pushing the hook in a retrograde manner along the path of insertion. This method is simple and rarely results in complications. However, there are no guidelines for removal of barbed fishhooks. Furthermore, removing a barbed fishhook in the same retrograde manner would result in extensive internal tissue destruction and increased complications. Due to the popularity of the sport of fishing, fishhook injuries, depending on geographical location, are not uncommon.2 For this reason, trauma and emergency practitioners have become well versed in safe methods for barbed fishhook removal. However, patients are not always able or willing to seek medical care in emergency departments and may opt to seek treatment in outpatient settings, such as in our case. As a result, dermatologists should familiarize themselves with safe and effective fishhook removal methods, as they are not time consuming and do not require complex equipment. Failure to treat the patient may lead to further patient discomfort and increased risk for complications. Additionally, many of the techniques for removal may be useful with other foreign bodies embedded in cutaneous tissue (eg, splinters).
There are a number of safe and effective techniques for removing barbed fishhooks from cutaneous tissue, including the advance-and-cut method, the cut-it-out technique, the string-pull method, and the needle cover technique.1-3 The method chosen to remove the fishhook is dependent on a variety of factors, such as anatomic location, tissue depth, and provider comfort.
With the advance-and-cut method (Figure 2), the affected area is anesthetized and a small incision in the skin is created to expose the barb. The fishhook is then advanced through the incision, providing visibility of the barb and thus allowing the practitioner to cut the barbed tip without creating further damage to the surrounding tissue. The shaft of the fishhook can subsequently be removed in a retrograde fashion. The advantages of this technique include that it may be successfully used in all types of barbed fishhooks and it provides the practitioner with direct visibility of the barb, thus minimizing risk for neurovascular injury during removal.1 However, the primary disadvantage is that a second cutaneous wound is created in exposing the barb.

| 
| |
| Figure 2. The advance-and-cut method for fishhook removal. | Figure 3. The cut-it-out method for fishhook removal. |
|
The cut-it-out technique (Figure 3) is similar to the advance-and-cut method in that they both require anesthesia along with creating an incision. With this method, a scalpel is used to create a small linear incision originating at the fishhook entrance site and ending at the approximated location of the fishhook’s tip. The fishhook then is simply lifted superiorly in a retrograde fashion.
|
The string-pull method (Figure 4) has been credited to fishermen in South Australia and was first described by Cooke2 in 1961. This method is relatively painless, does not require anesthesia, and has a high success rate when properly administered. However, it does require rapid and confident motions (ie, without hesitation) by the practitioner and should not be performed on free-moving areas of the body (eg, earlobe).3 With this technique, a sturdy piece of suture (eg, 2/0 or 3/0 strength silk) is looped around the hook and is extended away from the practitioner at a 30° angle. The free end of the suture is then securely fastened around the index finger of the practitioner’s dominant hand. The index finger of the nondominant hand should apply a downward pressure to the hook shaft to disengage the barb from the tissue. Simultaneously and rather quickly and forcefully the practitioner must pull the dominant index finger with the string attached in a superior and lateral direction, as depicted by the long arrow in Figure 4. If successful, the barbed hook will pull out of the entrance site. The use of string in pulling the fishhook parallel to the site of injury is helpful for smaller fishhooks that may be difficult to grab with fingers alone. However, with larger fishhooks, the string may not be required so long as the practitioner is able to obtain a secure grasp on the fishhook shaft. The string-pull method becomes particularly useful when anesthesia is unavailable or when the barb of the hook is embedded too deeply for safe advancement through tissue to visualize and cut the barb.
|
Lastly, the needle cover technique (Figure 5) is another simple method that does not require the creation of a secondary wound. An 18-gauge needle is simply inserted parallel to the fishhook curvature into the site of entry. By using the needle to slide along the fishhook’s curve, the practitioner is able to follow its pathway while in the tissue. The tip of the 18-gauge needle is then used to cap or cover the barb, thus allowing the fishhook to be removed in a retrograde fashion from the wound. In an outpatient setting, this technique does not require the creation of additional tissue damage and practitioners who are inexperienced with fishhook removal may proceed through the motions more slowly and methodically than the string-pull method permits.
Wound care following fishhook removal should involve adequate flushing of the wound with normal saline along with the application of topical antibiotics and a simple dressing and adhesive bandage. Oral prophylactic antibiotics typically are not required for shallow cutaneous injuries unless the fishhook is dirty, the patient is immunocompromised, or the patient has a condition lending to poor wound healing (eg, diabetes mellitus, peripheral vascular disease).3 When deciding on antibiotics, it is important to note that fishhook injuries while saltwater fishing are associated with Vibrio infection, while injuries sustained during freshwater fishing are associated with gram-negative bacteria (eg, Pseudomonas and Aeromonas species).3 Lastly, it is essential to find out the immunization status of the patient, and tetanus immune globulin should be provided if necessary.

| 
| |
| Figure 4. The string-pull method for fishhook removal. | Figure 5. The needle cover technique for fishhook removal. |
Conclusion
Although guidelines for barbed fishhook removal are not available, outpatient physicians, including dermatologists, should not fear removal procedures. There are many safe and effective fishhook removal methods that are not time consuming and do not require complex equipment. Furthermore, familiarization with these same techniques may be useful for removal of other foreign bodies embedded in cutaneous tissue.
Fishing is one of the world’s most beloved activities, enjoyed as a sport or a leisure activity. However, a common injury from fishing is embedment of the fishhook in the cutaneous tissue. Barbed fishhooks are used for their effectiveness in maintaining the fish on the hook once it is caught, but when implanted in the hand of a fisherman or fisherwoman, barbs can pose problems for removal without exacerbating internal tissue injury. Nevertheless, dermatologists should not shy away from removal of barbed fishhooks, as there are several simple methods that can be easily utilized in the outpatient setting.
Case Report
A 68-year-old man presented to an outpatient dermatology clinic after sustaining a barbed fishhook injury while fishing. The fishhook was firmly inserted into the ventral side of the third digit of the right hand (Figure 1).
Prior to presenting to dermatology, the patient went to 2 urgent care clinics the same day seeking treatment. He reported that practitioners at the first clinic were not able to remove the fishhook because they did not have pliers in stock. At the second clinic he was told the fishhook might be embedded in deeper tissues and was advised to go to the emergency department at the local hospital. When he arrived at the emergency department, a 6-hour wait time prompted him to see a local dermatologist instead.
To remove the fishhook, the area was cleaned and prepared first; lidocaine 2% was administered for local anesthesia. An 18-gauge needle was then advanced through the puncture site parallel to the fishhook’s inner shaft on the same side as the barb, which could be successfully palpated using the tip of the 18-gauge needle. The tip of the needle was then used to cap the barb beneath the skin. This technique allowed for the hook to be easily extracted in a retrograde manner without causing further destruction to the surrounding tissue. The patient then was started on prophylaxis cephalexin 500 mg 3 times daily for 3 days.
Comment
The hand is the most common site of fishhook injury, followed closely by the head and eyes.1 Barbless fishhooks usually can be removed by pushing the hook in a retrograde manner along the path of insertion. This method is simple and rarely results in complications. However, there are no guidelines for removal of barbed fishhooks. Furthermore, removing a barbed fishhook in the same retrograde manner would result in extensive internal tissue destruction and increased complications. Due to the popularity of the sport of fishing, fishhook injuries, depending on geographical location, are not uncommon.2 For this reason, trauma and emergency practitioners have become well versed in safe methods for barbed fishhook removal. However, patients are not always able or willing to seek medical care in emergency departments and may opt to seek treatment in outpatient settings, such as in our case. As a result, dermatologists should familiarize themselves with safe and effective fishhook removal methods, as they are not time consuming and do not require complex equipment. Failure to treat the patient may lead to further patient discomfort and increased risk for complications. Additionally, many of the techniques for removal may be useful with other foreign bodies embedded in cutaneous tissue (eg, splinters).
There are a number of safe and effective techniques for removing barbed fishhooks from cutaneous tissue, including the advance-and-cut method, the cut-it-out technique, the string-pull method, and the needle cover technique.1-3 The method chosen to remove the fishhook is dependent on a variety of factors, such as anatomic location, tissue depth, and provider comfort.
With the advance-and-cut method (Figure 2), the affected area is anesthetized and a small incision in the skin is created to expose the barb. The fishhook is then advanced through the incision, providing visibility of the barb and thus allowing the practitioner to cut the barbed tip without creating further damage to the surrounding tissue. The shaft of the fishhook can subsequently be removed in a retrograde fashion. The advantages of this technique include that it may be successfully used in all types of barbed fishhooks and it provides the practitioner with direct visibility of the barb, thus minimizing risk for neurovascular injury during removal.1 However, the primary disadvantage is that a second cutaneous wound is created in exposing the barb.

| 
| |
| Figure 2. The advance-and-cut method for fishhook removal. | Figure 3. The cut-it-out method for fishhook removal. |
|
The cut-it-out technique (Figure 3) is similar to the advance-and-cut method in that they both require anesthesia along with creating an incision. With this method, a scalpel is used to create a small linear incision originating at the fishhook entrance site and ending at the approximated location of the fishhook’s tip. The fishhook then is simply lifted superiorly in a retrograde fashion.
|
The string-pull method (Figure 4) has been credited to fishermen in South Australia and was first described by Cooke2 in 1961. This method is relatively painless, does not require anesthesia, and has a high success rate when properly administered. However, it does require rapid and confident motions (ie, without hesitation) by the practitioner and should not be performed on free-moving areas of the body (eg, earlobe).3 With this technique, a sturdy piece of suture (eg, 2/0 or 3/0 strength silk) is looped around the hook and is extended away from the practitioner at a 30° angle. The free end of the suture is then securely fastened around the index finger of the practitioner’s dominant hand. The index finger of the nondominant hand should apply a downward pressure to the hook shaft to disengage the barb from the tissue. Simultaneously and rather quickly and forcefully the practitioner must pull the dominant index finger with the string attached in a superior and lateral direction, as depicted by the long arrow in Figure 4. If successful, the barbed hook will pull out of the entrance site. The use of string in pulling the fishhook parallel to the site of injury is helpful for smaller fishhooks that may be difficult to grab with fingers alone. However, with larger fishhooks, the string may not be required so long as the practitioner is able to obtain a secure grasp on the fishhook shaft. The string-pull method becomes particularly useful when anesthesia is unavailable or when the barb of the hook is embedded too deeply for safe advancement through tissue to visualize and cut the barb.
|
Lastly, the needle cover technique (Figure 5) is another simple method that does not require the creation of a secondary wound. An 18-gauge needle is simply inserted parallel to the fishhook curvature into the site of entry. By using the needle to slide along the fishhook’s curve, the practitioner is able to follow its pathway while in the tissue. The tip of the 18-gauge needle is then used to cap or cover the barb, thus allowing the fishhook to be removed in a retrograde fashion from the wound. In an outpatient setting, this technique does not require the creation of additional tissue damage and practitioners who are inexperienced with fishhook removal may proceed through the motions more slowly and methodically than the string-pull method permits.
Wound care following fishhook removal should involve adequate flushing of the wound with normal saline along with the application of topical antibiotics and a simple dressing and adhesive bandage. Oral prophylactic antibiotics typically are not required for shallow cutaneous injuries unless the fishhook is dirty, the patient is immunocompromised, or the patient has a condition lending to poor wound healing (eg, diabetes mellitus, peripheral vascular disease).3 When deciding on antibiotics, it is important to note that fishhook injuries while saltwater fishing are associated with Vibrio infection, while injuries sustained during freshwater fishing are associated with gram-negative bacteria (eg, Pseudomonas and Aeromonas species).3 Lastly, it is essential to find out the immunization status of the patient, and tetanus immune globulin should be provided if necessary.

| 
| |
| Figure 4. The string-pull method for fishhook removal. | Figure 5. The needle cover technique for fishhook removal. |
Conclusion
Although guidelines for barbed fishhook removal are not available, outpatient physicians, including dermatologists, should not fear removal procedures. There are many safe and effective fishhook removal methods that are not time consuming and do not require complex equipment. Furthermore, familiarization with these same techniques may be useful for removal of other foreign bodies embedded in cutaneous tissue.
1. Khan HA, Kamal Y, Lone AU. Fish hook injury: removal by “push through and cut off” technique: a case report and brief literature review [published online March 24, 2014]. Trauma Mon. 2014;19:e17728.
2. Cooke T. How to remove fish-hooks with a bit of string. Med J Aust. 1961;48:815-816.
3. Thommasen HV, Thommasen A. The occasional removal of an embedded fish hook. Can J Rural Med. 2005;10:255-259.
1. Khan HA, Kamal Y, Lone AU. Fish hook injury: removal by “push through and cut off” technique: a case report and brief literature review [published online March 24, 2014]. Trauma Mon. 2014;19:e17728.
2. Cooke T. How to remove fish-hooks with a bit of string. Med J Aust. 1961;48:815-816.
3. Thommasen HV, Thommasen A. The occasional removal of an embedded fish hook. Can J Rural Med. 2005;10:255-259.
Practice Points
- Barbed fishhooks should never be removed by pushing the hook in a retrograde manner along the path of insertion, as this method may result in extensive internal tissue destruction and increased complications.
- There are a number of safe and effective techniques for removing barbed fishhooks from cutaneous tissue that also may be applicable in removing other foreign bodies embedded in cutaneous tissue (eg, splinters).
Erythematous Eruption on the Left Leg
The Diagnosis: Bullous Henoch-Schönlein Purpura
Laboratory tests in this patient showed no abnormalities for complete blood cell count, immunoglobulins, anti–double-stranded DNA, antinuclear antibody, p–antineutrophil cytoplasmic antibodies, lupus anticoagulant, Sjögren antibodies, liver enzymes, and erythrocyte sedimentation rate. Urinalysis was normal. Punch biopsies were obtained and a histologic examination showed an intense inflammatory infiltrate of neutrophils around blood vessels within the dermis (Figure). These blood vessels showed swollen endothelium and narrowing of the vessel lumina with leukocytoclasia. Direct immunofluorescence revealed granular IgA, C3, fibrin, and weak IgM deposits in blood vessels in the papillary dermis consistent with Henoch-Schönlein purpura (HSP).
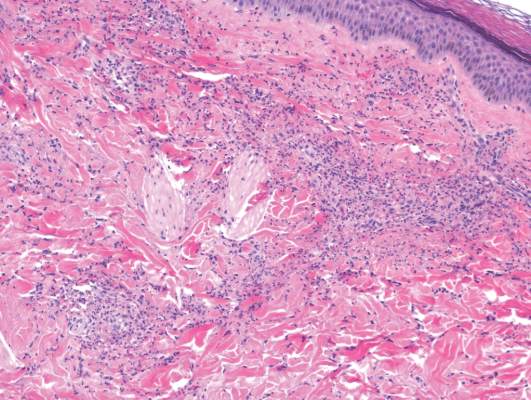
Henoch-Schönlein purpura is the most common vasculitis in children.1-6 However, its bullous variant is rare, with few pediatric cases reported. Bullous HSP affects arterioles through an IgA-mediated pathway.1-6 It is believed that the bullae are formed secondary to neutrophilic release of matrix metalloproteinase 9 (MMP-9), which degrades extracellular collagen.2 Additionally, bullous fluid from HSP has been noted to have markedly elevated levels of soluble CD23, a form of the CD23 B-cell surface receptor used in antibody feedback regulation and B-cell recruitment, which also has been found to be elevated in the fluid of bullous pemphigoid, suggesting a similar pathogenesis of exaggerated humoral immunity.3
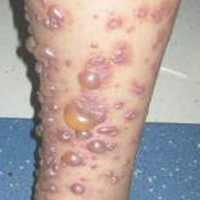
The most common sign of HSP is palpable purpura; however, other cutaneous findings can be present including targetoid plaques, macules, papules, petechiae, and bullae that may become hemorrhagic, ulcerated, necrotic, or scarred.1-6 Bullae appear in the most dependent parts of the body, such as the feet and lower legs. Hydrostatic pressure may play a role in the pathogenesis of this phenomenon.1 When other classic signs of HSP are absent, the presence of bullae clouds the diagnosis and creates controversy regarding treatment, as there is a dearth of literature on proper therapy for severe cutaneous manifestations of HSP.6
Our patient was treated with morphine for pain management along with topical mupirocin and nonadherent dressings for wound care. She also received pulse intravenous methylprednisolone 2 mg/kg daily for 3 days and then was transitioned to oral prednisone 1 mg/kg daily, which was tapered over 3 weeks after discharge. This regimen resulted in resolution of symptoms with rapid regression of bullae and subsequent postinflammatory hyperpigmentation. Prior reports have noted that the presence of bullae does not alter the prognosis or predict probability of renal involvement of this self-limited disease, leading to controversy in determining if treatment offers more favorable outcomes.1,3 One study suggested that steroids only improve symptoms, arthralgia, and abdominal pain, but they do not aid in the resolution of cutaneous lesions or prevent the progression of renal disease.3 Contrarily, others have suggested that the presence of bullae and renal disease is an indication to start treatment.6 This claim is based on the mechanistic finding that immunosuppression with corticosteroids decreases inflammation by inhibiting activator protein 1, a transcription factor for MMP-9, thereby reducing MMP-9 activity and the formation of bullae.4 Clinical anecdotes, including our own, that demonstrate dramatic improvement of hemorrhagic bullae with the administration of corticosteroids substantiate this mechanism. Through the inhibition of neutrophil interactions and IgA production, other anti-inflammatory and immunosuppressive medications such as colchicine, dapsone, and azathioprine also have been reported to aid in resolution of the cutaneous lesions.1,5,6 Although there is a clear drawback to the lack of controlled trials and prospective studies regarding the treatment of bullous HSP, it is nearly impossible to expect such studies to be carried out given the rare and unpredictable nature of the disease. For now, claims derived from case series and case reports guide our understanding of treatment efficacy.
Acknowledgment—Quiz photograph courtesy of Steve Taylor, BS, Phoenix, Arizona.
- Trapani S, Mariotti P, Resti M, et al. Severe hemorrhagic bullous lesions in Henoch Schönlein purpura: three pediatric cases and review of the literature [published online July 16, 2009]. Rheumatol Int. 2010;30:1355-1359. doi:10.1007/s00296-009-1055-8.
- Kobayashi T, Sakuraoka K, Iwamoto M, et al. A case of anaphylactoid purpura with multiple blister formation: possible pathophysiologic role of gelatinate (MMP-9). Dermatology. 1990;197:62-64.
- Bansal AS, Dwivedi N, Adsett M. Serum and blister fluid cytokines and complement proteins in a patient with Henoch Schönlein purpura associated with a bullous skin rash. Australas J Dermatol. 1997;38:190-192.
- Aljada A, Ghanim H, Mohanty P, et al. Hydrocortisone suppresses intranuclear activator-protein-1 (AP-1) binding activity in mononuclear cells and plasma matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9). J Clin Endocrinol Metab. 2001;86:5988-5991.
- Iqbal H, Evans A. Dapsone therapy for Henoch-Schönlein purpura: a case series. Arch Dis Child. 2005;90:985-986.
- den Boer SL, Pasmans SG, Wulffraat NM, et al. Bullous lesions in Henoch Schönlein purpura as indication to start systemic prednisone [published online January 5, 2009]. Acta Paediatr. 2010;99:781-783. doi:10.1111/j.1651-2227.2009.01650.x.
The Diagnosis: Bullous Henoch-Schönlein Purpura
Laboratory tests in this patient showed no abnormalities for complete blood cell count, immunoglobulins, anti–double-stranded DNA, antinuclear antibody, p–antineutrophil cytoplasmic antibodies, lupus anticoagulant, Sjögren antibodies, liver enzymes, and erythrocyte sedimentation rate. Urinalysis was normal. Punch biopsies were obtained and a histologic examination showed an intense inflammatory infiltrate of neutrophils around blood vessels within the dermis (Figure). These blood vessels showed swollen endothelium and narrowing of the vessel lumina with leukocytoclasia. Direct immunofluorescence revealed granular IgA, C3, fibrin, and weak IgM deposits in blood vessels in the papillary dermis consistent with Henoch-Schönlein purpura (HSP).

Henoch-Schönlein purpura is the most common vasculitis in children.1-6 However, its bullous variant is rare, with few pediatric cases reported. Bullous HSP affects arterioles through an IgA-mediated pathway.1-6 It is believed that the bullae are formed secondary to neutrophilic release of matrix metalloproteinase 9 (MMP-9), which degrades extracellular collagen.2 Additionally, bullous fluid from HSP has been noted to have markedly elevated levels of soluble CD23, a form of the CD23 B-cell surface receptor used in antibody feedback regulation and B-cell recruitment, which also has been found to be elevated in the fluid of bullous pemphigoid, suggesting a similar pathogenesis of exaggerated humoral immunity.3
The most common sign of HSP is palpable purpura; however, other cutaneous findings can be present including targetoid plaques, macules, papules, petechiae, and bullae that may become hemorrhagic, ulcerated, necrotic, or scarred.1-6 Bullae appear in the most dependent parts of the body, such as the feet and lower legs. Hydrostatic pressure may play a role in the pathogenesis of this phenomenon.1 When other classic signs of HSP are absent, the presence of bullae clouds the diagnosis and creates controversy regarding treatment, as there is a dearth of literature on proper therapy for severe cutaneous manifestations of HSP.6
Our patient was treated with morphine for pain management along with topical mupirocin and nonadherent dressings for wound care. She also received pulse intravenous methylprednisolone 2 mg/kg daily for 3 days and then was transitioned to oral prednisone 1 mg/kg daily, which was tapered over 3 weeks after discharge. This regimen resulted in resolution of symptoms with rapid regression of bullae and subsequent postinflammatory hyperpigmentation. Prior reports have noted that the presence of bullae does not alter the prognosis or predict probability of renal involvement of this self-limited disease, leading to controversy in determining if treatment offers more favorable outcomes.1,3 One study suggested that steroids only improve symptoms, arthralgia, and abdominal pain, but they do not aid in the resolution of cutaneous lesions or prevent the progression of renal disease.3 Contrarily, others have suggested that the presence of bullae and renal disease is an indication to start treatment.6 This claim is based on the mechanistic finding that immunosuppression with corticosteroids decreases inflammation by inhibiting activator protein 1, a transcription factor for MMP-9, thereby reducing MMP-9 activity and the formation of bullae.4 Clinical anecdotes, including our own, that demonstrate dramatic improvement of hemorrhagic bullae with the administration of corticosteroids substantiate this mechanism. Through the inhibition of neutrophil interactions and IgA production, other anti-inflammatory and immunosuppressive medications such as colchicine, dapsone, and azathioprine also have been reported to aid in resolution of the cutaneous lesions.1,5,6 Although there is a clear drawback to the lack of controlled trials and prospective studies regarding the treatment of bullous HSP, it is nearly impossible to expect such studies to be carried out given the rare and unpredictable nature of the disease. For now, claims derived from case series and case reports guide our understanding of treatment efficacy.
Acknowledgment—Quiz photograph courtesy of Steve Taylor, BS, Phoenix, Arizona.
The Diagnosis: Bullous Henoch-Schönlein Purpura
Laboratory tests in this patient showed no abnormalities for complete blood cell count, immunoglobulins, anti–double-stranded DNA, antinuclear antibody, p–antineutrophil cytoplasmic antibodies, lupus anticoagulant, Sjögren antibodies, liver enzymes, and erythrocyte sedimentation rate. Urinalysis was normal. Punch biopsies were obtained and a histologic examination showed an intense inflammatory infiltrate of neutrophils around blood vessels within the dermis (Figure). These blood vessels showed swollen endothelium and narrowing of the vessel lumina with leukocytoclasia. Direct immunofluorescence revealed granular IgA, C3, fibrin, and weak IgM deposits in blood vessels in the papillary dermis consistent with Henoch-Schönlein purpura (HSP).

Henoch-Schönlein purpura is the most common vasculitis in children.1-6 However, its bullous variant is rare, with few pediatric cases reported. Bullous HSP affects arterioles through an IgA-mediated pathway.1-6 It is believed that the bullae are formed secondary to neutrophilic release of matrix metalloproteinase 9 (MMP-9), which degrades extracellular collagen.2 Additionally, bullous fluid from HSP has been noted to have markedly elevated levels of soluble CD23, a form of the CD23 B-cell surface receptor used in antibody feedback regulation and B-cell recruitment, which also has been found to be elevated in the fluid of bullous pemphigoid, suggesting a similar pathogenesis of exaggerated humoral immunity.3
The most common sign of HSP is palpable purpura; however, other cutaneous findings can be present including targetoid plaques, macules, papules, petechiae, and bullae that may become hemorrhagic, ulcerated, necrotic, or scarred.1-6 Bullae appear in the most dependent parts of the body, such as the feet and lower legs. Hydrostatic pressure may play a role in the pathogenesis of this phenomenon.1 When other classic signs of HSP are absent, the presence of bullae clouds the diagnosis and creates controversy regarding treatment, as there is a dearth of literature on proper therapy for severe cutaneous manifestations of HSP.6
Our patient was treated with morphine for pain management along with topical mupirocin and nonadherent dressings for wound care. She also received pulse intravenous methylprednisolone 2 mg/kg daily for 3 days and then was transitioned to oral prednisone 1 mg/kg daily, which was tapered over 3 weeks after discharge. This regimen resulted in resolution of symptoms with rapid regression of bullae and subsequent postinflammatory hyperpigmentation. Prior reports have noted that the presence of bullae does not alter the prognosis or predict probability of renal involvement of this self-limited disease, leading to controversy in determining if treatment offers more favorable outcomes.1,3 One study suggested that steroids only improve symptoms, arthralgia, and abdominal pain, but they do not aid in the resolution of cutaneous lesions or prevent the progression of renal disease.3 Contrarily, others have suggested that the presence of bullae and renal disease is an indication to start treatment.6 This claim is based on the mechanistic finding that immunosuppression with corticosteroids decreases inflammation by inhibiting activator protein 1, a transcription factor for MMP-9, thereby reducing MMP-9 activity and the formation of bullae.4 Clinical anecdotes, including our own, that demonstrate dramatic improvement of hemorrhagic bullae with the administration of corticosteroids substantiate this mechanism. Through the inhibition of neutrophil interactions and IgA production, other anti-inflammatory and immunosuppressive medications such as colchicine, dapsone, and azathioprine also have been reported to aid in resolution of the cutaneous lesions.1,5,6 Although there is a clear drawback to the lack of controlled trials and prospective studies regarding the treatment of bullous HSP, it is nearly impossible to expect such studies to be carried out given the rare and unpredictable nature of the disease. For now, claims derived from case series and case reports guide our understanding of treatment efficacy.
Acknowledgment—Quiz photograph courtesy of Steve Taylor, BS, Phoenix, Arizona.
- Trapani S, Mariotti P, Resti M, et al. Severe hemorrhagic bullous lesions in Henoch Schönlein purpura: three pediatric cases and review of the literature [published online July 16, 2009]. Rheumatol Int. 2010;30:1355-1359. doi:10.1007/s00296-009-1055-8.
- Kobayashi T, Sakuraoka K, Iwamoto M, et al. A case of anaphylactoid purpura with multiple blister formation: possible pathophysiologic role of gelatinate (MMP-9). Dermatology. 1990;197:62-64.
- Bansal AS, Dwivedi N, Adsett M. Serum and blister fluid cytokines and complement proteins in a patient with Henoch Schönlein purpura associated with a bullous skin rash. Australas J Dermatol. 1997;38:190-192.
- Aljada A, Ghanim H, Mohanty P, et al. Hydrocortisone suppresses intranuclear activator-protein-1 (AP-1) binding activity in mononuclear cells and plasma matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9). J Clin Endocrinol Metab. 2001;86:5988-5991.
- Iqbal H, Evans A. Dapsone therapy for Henoch-Schönlein purpura: a case series. Arch Dis Child. 2005;90:985-986.
- den Boer SL, Pasmans SG, Wulffraat NM, et al. Bullous lesions in Henoch Schönlein purpura as indication to start systemic prednisone [published online January 5, 2009]. Acta Paediatr. 2010;99:781-783. doi:10.1111/j.1651-2227.2009.01650.x.
- Trapani S, Mariotti P, Resti M, et al. Severe hemorrhagic bullous lesions in Henoch Schönlein purpura: three pediatric cases and review of the literature [published online July 16, 2009]. Rheumatol Int. 2010;30:1355-1359. doi:10.1007/s00296-009-1055-8.
- Kobayashi T, Sakuraoka K, Iwamoto M, et al. A case of anaphylactoid purpura with multiple blister formation: possible pathophysiologic role of gelatinate (MMP-9). Dermatology. 1990;197:62-64.
- Bansal AS, Dwivedi N, Adsett M. Serum and blister fluid cytokines and complement proteins in a patient with Henoch Schönlein purpura associated with a bullous skin rash. Australas J Dermatol. 1997;38:190-192.
- Aljada A, Ghanim H, Mohanty P, et al. Hydrocortisone suppresses intranuclear activator-protein-1 (AP-1) binding activity in mononuclear cells and plasma matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9). J Clin Endocrinol Metab. 2001;86:5988-5991.
- Iqbal H, Evans A. Dapsone therapy for Henoch-Schönlein purpura: a case series. Arch Dis Child. 2005;90:985-986.
- den Boer SL, Pasmans SG, Wulffraat NM, et al. Bullous lesions in Henoch Schönlein purpura as indication to start systemic prednisone [published online January 5, 2009]. Acta Paediatr. 2010;99:781-783. doi:10.1111/j.1651-2227.2009.01650.x.

A 12-year-old girl presented with an erythematous eruption that had started on the left leg approximately 1 week prior with subsequent spread to the abdomen and arms. She had associated knee pain, myalgia, abdominal pain, nausea, and nonbloody and nonbilious emesis. Her medical history was notable for methicillin-resistant Staphylococcus aureus abscesses, the most recent of which was treated with trimethoprim-sulfamethoxazole; treatment was completed 5 days before the onset of the rash. Family history was notable for her paternal aunt who died of systemic lupus erythematosus. Physical examination showed erythematous macules and purpuric papules with central vesiculation extending up the thighs and lower abdomen associated with edema of the lower extremities and pain after palpation. Tense bullae also were present.
Wound-healing template approved for diabetic foot ulcers
A bilayer matrix used for dermal regeneration and first approved in 1996 as a treatment for third-degree burns is now approved as a treatment for diabetic foot ulcers.
The Integra Dermal Regeneration Template was approved for the new indication based on a study that showed that the matrix device “improved ulcer healing compared to standard diabetic foot ulcer care,” according to a Food and Drug Administration statement announcing the approval on Jan. 7. Specifically, the new indication is for treating “partial and full-thickness neuropathic diabetic foot ulcers that are greater than 6 weeks in duration, with no capsule, tendon or bone exposed, when used in conjunction with standard diabetic ulcer care.”
The product is a dermal-replacement layer that “consists of a porous, three-dimensional matrix, comprised of bovine collagen and chondroitin-6-sulfate,” with a temporary epidermal silicone layer “to provide immediate wound coverage and control moisture loss. … [It] provides an environment for new skin and tissue to regenerate and heal the wound,” according to the agency’s approval summary.
In a multicenter, randomized controlled study, 307 patients were first treated with 0.9% sodium chloride gel, a secondary dressing, and an offloading device for 2 weeks and were then randomized to a treatment or a control group that received continued treatment with the gel. After 16 weeks, 51% of those treated with the device and 32% of those in the control group had healed completely (P = .001). Among those whose wounds healed, the median time to healing was 43 days in the treatment group and 78 days in the control group.
More patients in the control group had severe adverse events (26.8% vs. 15.6%) and moderate adverse events (42.5% vs. 31.8%).The results of the study, funded and sponsored by the manufacturer, were recently published (Wound Repair Regen. 2015;23[6]:891-900).
The product is contraindicated in patients with bovine or chondroitin allergies and in patients with infected wounds.
The manufacturer, Integra LifeSciences, is marketing the device as Integra Omnigraft Dermal Regeneration Matrix for the diabetic foot ulcer indication.
A bilayer matrix used for dermal regeneration and first approved in 1996 as a treatment for third-degree burns is now approved as a treatment for diabetic foot ulcers.
The Integra Dermal Regeneration Template was approved for the new indication based on a study that showed that the matrix device “improved ulcer healing compared to standard diabetic foot ulcer care,” according to a Food and Drug Administration statement announcing the approval on Jan. 7. Specifically, the new indication is for treating “partial and full-thickness neuropathic diabetic foot ulcers that are greater than 6 weeks in duration, with no capsule, tendon or bone exposed, when used in conjunction with standard diabetic ulcer care.”
The product is a dermal-replacement layer that “consists of a porous, three-dimensional matrix, comprised of bovine collagen and chondroitin-6-sulfate,” with a temporary epidermal silicone layer “to provide immediate wound coverage and control moisture loss. … [It] provides an environment for new skin and tissue to regenerate and heal the wound,” according to the agency’s approval summary.
In a multicenter, randomized controlled study, 307 patients were first treated with 0.9% sodium chloride gel, a secondary dressing, and an offloading device for 2 weeks and were then randomized to a treatment or a control group that received continued treatment with the gel. After 16 weeks, 51% of those treated with the device and 32% of those in the control group had healed completely (P = .001). Among those whose wounds healed, the median time to healing was 43 days in the treatment group and 78 days in the control group.
More patients in the control group had severe adverse events (26.8% vs. 15.6%) and moderate adverse events (42.5% vs. 31.8%).The results of the study, funded and sponsored by the manufacturer, were recently published (Wound Repair Regen. 2015;23[6]:891-900).
The product is contraindicated in patients with bovine or chondroitin allergies and in patients with infected wounds.
The manufacturer, Integra LifeSciences, is marketing the device as Integra Omnigraft Dermal Regeneration Matrix for the diabetic foot ulcer indication.
A bilayer matrix used for dermal regeneration and first approved in 1996 as a treatment for third-degree burns is now approved as a treatment for diabetic foot ulcers.
The Integra Dermal Regeneration Template was approved for the new indication based on a study that showed that the matrix device “improved ulcer healing compared to standard diabetic foot ulcer care,” according to a Food and Drug Administration statement announcing the approval on Jan. 7. Specifically, the new indication is for treating “partial and full-thickness neuropathic diabetic foot ulcers that are greater than 6 weeks in duration, with no capsule, tendon or bone exposed, when used in conjunction with standard diabetic ulcer care.”
The product is a dermal-replacement layer that “consists of a porous, three-dimensional matrix, comprised of bovine collagen and chondroitin-6-sulfate,” with a temporary epidermal silicone layer “to provide immediate wound coverage and control moisture loss. … [It] provides an environment for new skin and tissue to regenerate and heal the wound,” according to the agency’s approval summary.
In a multicenter, randomized controlled study, 307 patients were first treated with 0.9% sodium chloride gel, a secondary dressing, and an offloading device for 2 weeks and were then randomized to a treatment or a control group that received continued treatment with the gel. After 16 weeks, 51% of those treated with the device and 32% of those in the control group had healed completely (P = .001). Among those whose wounds healed, the median time to healing was 43 days in the treatment group and 78 days in the control group.
More patients in the control group had severe adverse events (26.8% vs. 15.6%) and moderate adverse events (42.5% vs. 31.8%).The results of the study, funded and sponsored by the manufacturer, were recently published (Wound Repair Regen. 2015;23[6]:891-900).
The product is contraindicated in patients with bovine or chondroitin allergies and in patients with infected wounds.
The manufacturer, Integra LifeSciences, is marketing the device as Integra Omnigraft Dermal Regeneration Matrix for the diabetic foot ulcer indication.
Consider pyoderma gangrenosum for nonhealing wounds
LAS VEGAS – Though pyoderma gangrenosum and other neutrophilic skin disorders are rare, clinicians should include them in their differential, especially for nonhealing surgical wounds or skin “infections.”
Since these painful areas of ulceration need corticosteroid treatment, not antibiotics, for resolution, accurate diagnosis is critical for healing, Dr. J. Mark Jackson said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In a review of pyoderma gangrenosum (PG) and its cousins at the meeting, Dr. Jackson noted that the etiology of PG is unknown, but disordered neutrophilic chemotaxis is thought to be a factor. The many different manifestations of this disease are now collectively called the “neutrophilic dermatoses,” he said.
“Pyoderma gangrenosum is a very important diagnosis to consider in the differential diagnosis for nonhealing ulcerations, as suspicion and early recognition of this debilitating condition can prevent long-term sequelae such as pain, scarring, and long-term immunosuppressive medications,” said Dr. Jackson of the department of dermatology at the University of Louisville (Ky.).
The diagnosis should be suspected in the setting of a painful cutaneous ulcer with necrolysis. The border is typically irregular, violaceous, and undermined, he said, adding that this classic undermined border is caused by the sheets of neutrophils that characterize the disease.
Noting that half of patients with PG have underlying associated conditions such as Crohn’s disease, ulcerative colitis, rheumatoid arthritis, and hematologic malignancies, Dr. Jackson emphasized that systemic disease associated with PG should heighten suspicion. “Histopathologic findings may be consistent with but not diagnostic of PG,” and can include a sterile dermal neutrophilia, with or without mixed inflammation and a lymphocytic vasculitis.
“Where you biopsy is important,” he continued, emphasizing that the biopsy must capture the margin of ulceration, where the sheets of neutrophils characteristic of PG will be seen on pathology.
Therapy consists of corticosteroids, with or without an immunosuppressive agent, and cessation of treatments that may continue to provoke pathergy.
Other diseases should also be considered in the differential diagnosis, including dangerous infectious causes, such as atypical mycobacteria, deep fungal infections, and staphylococcal and streptococcal infections. Squamous cell carcinoma, lymphoma, and leukemia may also present with similar lesions, as may metastatic Crohn’s disease, Dr. Jackson said. Several vasculitic and vasculopathic inflammatory conditions can also have similar appearances, including Wegener’s granulomatosis and vasoocclusive disorders such as peripheral vascular disease and cryoglobulinemia.
Classically, PG presents as painful ulcerated areas, most often on the lower extremities, that have a typical undermined border, caused by the sheets of neutrophils that characterize PG, he pointed out. PG may be mistaken for venous stasis ulcers, pressure ulcers, and cellulitis, but it doesn’t improve with antibiotics and mechanical manipulation from exfoliative dressings – and debridement may worsen the condition.
For susceptible individuals, surgery may provoke a pathergic response and trigger PG at the site of the surgical wound, and dogged attempts at conventional wound care may cause continued pathergy and begin a vicious cycle, Dr. Jackson said.
Peristomal pyoderma gangrenosum is a disease subcategory that may be seen in patients whose inflammatory bowel disease has been surgically treated and who have a stoma. Patients will have ulcerating lesions around their stoma site that are often misdiagnosed and treated as infections. Some wound care therapies, such as debridement, may continue to provoke the pathergic response and worsen peristomal PG, he said.
Though associated disease is seen in up to 50% of individuals with PG, there’s no predictable timeline linking the development of PG with the course of the associated disorder. In classic PG, usually occurring on the legs, autoimmune diseases such as inflammatory bowel disease, rheumatoid arthritis or another inflammatory arthritis, and paraproteinemia may be seen. Atypical PG, occurring more commonly on the upper extremities and face, is associated with myelogenous leukemia and preleukemic states, Dr. Jackson said.
Pyoderma gangrenosum lesions improve with corticosteroid administration. Depending on disease severity and location, topical, intralesional, or systemic steroids may be used.
Adjunctive treatments for PG and other neutrophilic dermatoses can include antibiotics with anti-inflammatory properties, such as minocycline or doxycycline, dapsone, and metronidazole. Immunosuppressives such as cyclosporine, azathioprine, and mycophenolate mofetil may also help speed resolution. In some cases, skin grafts may be necessary.
PG patients with Crohn’s disease or rheumatoid arthritis who are prescribed tumor necrosis factor–alpha (TNF-alpha) inhibitors for their systemic disease may also see improvement in PG lesions, Dr. Jackson said.
Other rare categories of neutrophilic dermatoses include Sweet’s syndrome, an acute febrile neutrophilic dermatosis, and neutrophilic dermatosis of the dorsum of the hand.
Neutrophilic invasion can also occur in other organs. “These extracutaneous lesions are also ‘sterile’ neutrophilic abscesses, which are often misdiagnosed as infections,” Dr. Jackson said. The most common site of extracutaneous neutrophilic infiltration is the lungs, though any organ system may be affected.
Dr. Jackson disclosed that he has received research support, honoraria, consulting fees, and other support from Abbvie, Amgen, Celgene, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and Top MD.
The Skin Disease Education Foundation and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Though pyoderma gangrenosum and other neutrophilic skin disorders are rare, clinicians should include them in their differential, especially for nonhealing surgical wounds or skin “infections.”
Since these painful areas of ulceration need corticosteroid treatment, not antibiotics, for resolution, accurate diagnosis is critical for healing, Dr. J. Mark Jackson said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In a review of pyoderma gangrenosum (PG) and its cousins at the meeting, Dr. Jackson noted that the etiology of PG is unknown, but disordered neutrophilic chemotaxis is thought to be a factor. The many different manifestations of this disease are now collectively called the “neutrophilic dermatoses,” he said.
“Pyoderma gangrenosum is a very important diagnosis to consider in the differential diagnosis for nonhealing ulcerations, as suspicion and early recognition of this debilitating condition can prevent long-term sequelae such as pain, scarring, and long-term immunosuppressive medications,” said Dr. Jackson of the department of dermatology at the University of Louisville (Ky.).
The diagnosis should be suspected in the setting of a painful cutaneous ulcer with necrolysis. The border is typically irregular, violaceous, and undermined, he said, adding that this classic undermined border is caused by the sheets of neutrophils that characterize the disease.
Noting that half of patients with PG have underlying associated conditions such as Crohn’s disease, ulcerative colitis, rheumatoid arthritis, and hematologic malignancies, Dr. Jackson emphasized that systemic disease associated with PG should heighten suspicion. “Histopathologic findings may be consistent with but not diagnostic of PG,” and can include a sterile dermal neutrophilia, with or without mixed inflammation and a lymphocytic vasculitis.
“Where you biopsy is important,” he continued, emphasizing that the biopsy must capture the margin of ulceration, where the sheets of neutrophils characteristic of PG will be seen on pathology.
Therapy consists of corticosteroids, with or without an immunosuppressive agent, and cessation of treatments that may continue to provoke pathergy.
Other diseases should also be considered in the differential diagnosis, including dangerous infectious causes, such as atypical mycobacteria, deep fungal infections, and staphylococcal and streptococcal infections. Squamous cell carcinoma, lymphoma, and leukemia may also present with similar lesions, as may metastatic Crohn’s disease, Dr. Jackson said. Several vasculitic and vasculopathic inflammatory conditions can also have similar appearances, including Wegener’s granulomatosis and vasoocclusive disorders such as peripheral vascular disease and cryoglobulinemia.
Classically, PG presents as painful ulcerated areas, most often on the lower extremities, that have a typical undermined border, caused by the sheets of neutrophils that characterize PG, he pointed out. PG may be mistaken for venous stasis ulcers, pressure ulcers, and cellulitis, but it doesn’t improve with antibiotics and mechanical manipulation from exfoliative dressings – and debridement may worsen the condition.
For susceptible individuals, surgery may provoke a pathergic response and trigger PG at the site of the surgical wound, and dogged attempts at conventional wound care may cause continued pathergy and begin a vicious cycle, Dr. Jackson said.
Peristomal pyoderma gangrenosum is a disease subcategory that may be seen in patients whose inflammatory bowel disease has been surgically treated and who have a stoma. Patients will have ulcerating lesions around their stoma site that are often misdiagnosed and treated as infections. Some wound care therapies, such as debridement, may continue to provoke the pathergic response and worsen peristomal PG, he said.
Though associated disease is seen in up to 50% of individuals with PG, there’s no predictable timeline linking the development of PG with the course of the associated disorder. In classic PG, usually occurring on the legs, autoimmune diseases such as inflammatory bowel disease, rheumatoid arthritis or another inflammatory arthritis, and paraproteinemia may be seen. Atypical PG, occurring more commonly on the upper extremities and face, is associated with myelogenous leukemia and preleukemic states, Dr. Jackson said.
Pyoderma gangrenosum lesions improve with corticosteroid administration. Depending on disease severity and location, topical, intralesional, or systemic steroids may be used.
Adjunctive treatments for PG and other neutrophilic dermatoses can include antibiotics with anti-inflammatory properties, such as minocycline or doxycycline, dapsone, and metronidazole. Immunosuppressives such as cyclosporine, azathioprine, and mycophenolate mofetil may also help speed resolution. In some cases, skin grafts may be necessary.
PG patients with Crohn’s disease or rheumatoid arthritis who are prescribed tumor necrosis factor–alpha (TNF-alpha) inhibitors for their systemic disease may also see improvement in PG lesions, Dr. Jackson said.
Other rare categories of neutrophilic dermatoses include Sweet’s syndrome, an acute febrile neutrophilic dermatosis, and neutrophilic dermatosis of the dorsum of the hand.
Neutrophilic invasion can also occur in other organs. “These extracutaneous lesions are also ‘sterile’ neutrophilic abscesses, which are often misdiagnosed as infections,” Dr. Jackson said. The most common site of extracutaneous neutrophilic infiltration is the lungs, though any organ system may be affected.
Dr. Jackson disclosed that he has received research support, honoraria, consulting fees, and other support from Abbvie, Amgen, Celgene, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and Top MD.
The Skin Disease Education Foundation and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Though pyoderma gangrenosum and other neutrophilic skin disorders are rare, clinicians should include them in their differential, especially for nonhealing surgical wounds or skin “infections.”
Since these painful areas of ulceration need corticosteroid treatment, not antibiotics, for resolution, accurate diagnosis is critical for healing, Dr. J. Mark Jackson said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
In a review of pyoderma gangrenosum (PG) and its cousins at the meeting, Dr. Jackson noted that the etiology of PG is unknown, but disordered neutrophilic chemotaxis is thought to be a factor. The many different manifestations of this disease are now collectively called the “neutrophilic dermatoses,” he said.
“Pyoderma gangrenosum is a very important diagnosis to consider in the differential diagnosis for nonhealing ulcerations, as suspicion and early recognition of this debilitating condition can prevent long-term sequelae such as pain, scarring, and long-term immunosuppressive medications,” said Dr. Jackson of the department of dermatology at the University of Louisville (Ky.).
The diagnosis should be suspected in the setting of a painful cutaneous ulcer with necrolysis. The border is typically irregular, violaceous, and undermined, he said, adding that this classic undermined border is caused by the sheets of neutrophils that characterize the disease.
Noting that half of patients with PG have underlying associated conditions such as Crohn’s disease, ulcerative colitis, rheumatoid arthritis, and hematologic malignancies, Dr. Jackson emphasized that systemic disease associated with PG should heighten suspicion. “Histopathologic findings may be consistent with but not diagnostic of PG,” and can include a sterile dermal neutrophilia, with or without mixed inflammation and a lymphocytic vasculitis.
“Where you biopsy is important,” he continued, emphasizing that the biopsy must capture the margin of ulceration, where the sheets of neutrophils characteristic of PG will be seen on pathology.
Therapy consists of corticosteroids, with or without an immunosuppressive agent, and cessation of treatments that may continue to provoke pathergy.
Other diseases should also be considered in the differential diagnosis, including dangerous infectious causes, such as atypical mycobacteria, deep fungal infections, and staphylococcal and streptococcal infections. Squamous cell carcinoma, lymphoma, and leukemia may also present with similar lesions, as may metastatic Crohn’s disease, Dr. Jackson said. Several vasculitic and vasculopathic inflammatory conditions can also have similar appearances, including Wegener’s granulomatosis and vasoocclusive disorders such as peripheral vascular disease and cryoglobulinemia.
Classically, PG presents as painful ulcerated areas, most often on the lower extremities, that have a typical undermined border, caused by the sheets of neutrophils that characterize PG, he pointed out. PG may be mistaken for venous stasis ulcers, pressure ulcers, and cellulitis, but it doesn’t improve with antibiotics and mechanical manipulation from exfoliative dressings – and debridement may worsen the condition.
For susceptible individuals, surgery may provoke a pathergic response and trigger PG at the site of the surgical wound, and dogged attempts at conventional wound care may cause continued pathergy and begin a vicious cycle, Dr. Jackson said.
Peristomal pyoderma gangrenosum is a disease subcategory that may be seen in patients whose inflammatory bowel disease has been surgically treated and who have a stoma. Patients will have ulcerating lesions around their stoma site that are often misdiagnosed and treated as infections. Some wound care therapies, such as debridement, may continue to provoke the pathergic response and worsen peristomal PG, he said.
Though associated disease is seen in up to 50% of individuals with PG, there’s no predictable timeline linking the development of PG with the course of the associated disorder. In classic PG, usually occurring on the legs, autoimmune diseases such as inflammatory bowel disease, rheumatoid arthritis or another inflammatory arthritis, and paraproteinemia may be seen. Atypical PG, occurring more commonly on the upper extremities and face, is associated with myelogenous leukemia and preleukemic states, Dr. Jackson said.
Pyoderma gangrenosum lesions improve with corticosteroid administration. Depending on disease severity and location, topical, intralesional, or systemic steroids may be used.
Adjunctive treatments for PG and other neutrophilic dermatoses can include antibiotics with anti-inflammatory properties, such as minocycline or doxycycline, dapsone, and metronidazole. Immunosuppressives such as cyclosporine, azathioprine, and mycophenolate mofetil may also help speed resolution. In some cases, skin grafts may be necessary.
PG patients with Crohn’s disease or rheumatoid arthritis who are prescribed tumor necrosis factor–alpha (TNF-alpha) inhibitors for their systemic disease may also see improvement in PG lesions, Dr. Jackson said.
Other rare categories of neutrophilic dermatoses include Sweet’s syndrome, an acute febrile neutrophilic dermatosis, and neutrophilic dermatosis of the dorsum of the hand.
Neutrophilic invasion can also occur in other organs. “These extracutaneous lesions are also ‘sterile’ neutrophilic abscesses, which are often misdiagnosed as infections,” Dr. Jackson said. The most common site of extracutaneous neutrophilic infiltration is the lungs, though any organ system may be affected.
Dr. Jackson disclosed that he has received research support, honoraria, consulting fees, and other support from Abbvie, Amgen, Celgene, Dermira, Galderma, Genentech, Janssen, Lilly, Medimetriks, Merck, Novartis, Pfizer, Promius, and Top MD.
The Skin Disease Education Foundation and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
EADV: Venous leg ulcers herald increased cancer risk
COPENHAGEN – Patients with venous leg ulcers have an increased risk of occult cancer – especially hematologic and immune-related malignancies, according to a Danish nationwide cohort study.
The risk of newly detected cancer was greatest during the first 89 days after diagnosis of a venous leg ulcer. Indeed, during that initial period the risk of being diagnosed with a hematologic cancer was 3.48-fold greater than expected based upon Danish Cancer Registry data, Dr. Sigrun Alba Johannesdottir Schmidt reported at the annual congress of the European Academy of Dermatology and Venereology.
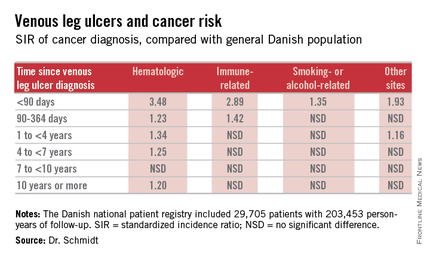
It’s reasonable to assume that the vast majority of cancers identified within a year following diagnosis of a venous leg ulcer were probably present at the time when the ulcer was first diagnosed, meaning venous leg ulcers can serve as a red flag for occult cancer.
However, it’s also worth noting that the increased risk of hematologic malignancies persisted, albeit to a lesser degree, for up to 10 years. This suggests that venous ulceration could also have a carcinogenic effect. It’s biologically plausible that a venous leg ulcer could promote development of cancer through a variety of mechanisms, including inflammation, alteration of plasma viscosity and the adhesive properties of blood cells, and disruptions of venous pressure that encourage direct neoplastic invasion, according to Dr. Schmidt of Aarhus (Denmark) University.
She presented a Danish national patient registry study, which included all 29,705 patients with a first-time inpatient, outpatient, or emergency department diagnosis of a venous leg ulcer during 1982-2010. Fifty-five percent of them were age 70 years or older at the time of ulcer diagnosis. And 42% had moderate to very severe comorbid conditions based upon their Charlson Comorbidity Index score. During a median of 5.1 years of follow-up, or a total of 203,453 person-years, their overall risk of a first-time cancer diagnosis was significantly increased by 11%,compared with the general Danish population.
The malignancy risk was strongly time-dependent . However, the absolute risk of cancer was relatively low: less than 1% within the first 90 days after diagnosis of a venous leg ulcer. The number of patients who would need to be examined for a possible malignancy at the time of diagnosis of a venous leg ulcer in order to diagnosis one excess cancer was 146.
Dr. Schmidt indicated she would defer to experts in cost-benefit analysis as to whether an extensive work-up for occult malignancy is worthwhile in patients with a newly diagnosed venous leg ulcer, given the low absolute risk of cancer.
She reported having no financial conflicts of interest regarding her study, which was conducted with Danish institutional research funds.
COPENHAGEN – Patients with venous leg ulcers have an increased risk of occult cancer – especially hematologic and immune-related malignancies, according to a Danish nationwide cohort study.
The risk of newly detected cancer was greatest during the first 89 days after diagnosis of a venous leg ulcer. Indeed, during that initial period the risk of being diagnosed with a hematologic cancer was 3.48-fold greater than expected based upon Danish Cancer Registry data, Dr. Sigrun Alba Johannesdottir Schmidt reported at the annual congress of the European Academy of Dermatology and Venereology.

It’s reasonable to assume that the vast majority of cancers identified within a year following diagnosis of a venous leg ulcer were probably present at the time when the ulcer was first diagnosed, meaning venous leg ulcers can serve as a red flag for occult cancer.
However, it’s also worth noting that the increased risk of hematologic malignancies persisted, albeit to a lesser degree, for up to 10 years. This suggests that venous ulceration could also have a carcinogenic effect. It’s biologically plausible that a venous leg ulcer could promote development of cancer through a variety of mechanisms, including inflammation, alteration of plasma viscosity and the adhesive properties of blood cells, and disruptions of venous pressure that encourage direct neoplastic invasion, according to Dr. Schmidt of Aarhus (Denmark) University.
She presented a Danish national patient registry study, which included all 29,705 patients with a first-time inpatient, outpatient, or emergency department diagnosis of a venous leg ulcer during 1982-2010. Fifty-five percent of them were age 70 years or older at the time of ulcer diagnosis. And 42% had moderate to very severe comorbid conditions based upon their Charlson Comorbidity Index score. During a median of 5.1 years of follow-up, or a total of 203,453 person-years, their overall risk of a first-time cancer diagnosis was significantly increased by 11%,compared with the general Danish population.
The malignancy risk was strongly time-dependent . However, the absolute risk of cancer was relatively low: less than 1% within the first 90 days after diagnosis of a venous leg ulcer. The number of patients who would need to be examined for a possible malignancy at the time of diagnosis of a venous leg ulcer in order to diagnosis one excess cancer was 146.
Dr. Schmidt indicated she would defer to experts in cost-benefit analysis as to whether an extensive work-up for occult malignancy is worthwhile in patients with a newly diagnosed venous leg ulcer, given the low absolute risk of cancer.
She reported having no financial conflicts of interest regarding her study, which was conducted with Danish institutional research funds.
COPENHAGEN – Patients with venous leg ulcers have an increased risk of occult cancer – especially hematologic and immune-related malignancies, according to a Danish nationwide cohort study.
The risk of newly detected cancer was greatest during the first 89 days after diagnosis of a venous leg ulcer. Indeed, during that initial period the risk of being diagnosed with a hematologic cancer was 3.48-fold greater than expected based upon Danish Cancer Registry data, Dr. Sigrun Alba Johannesdottir Schmidt reported at the annual congress of the European Academy of Dermatology and Venereology.

It’s reasonable to assume that the vast majority of cancers identified within a year following diagnosis of a venous leg ulcer were probably present at the time when the ulcer was first diagnosed, meaning venous leg ulcers can serve as a red flag for occult cancer.
However, it’s also worth noting that the increased risk of hematologic malignancies persisted, albeit to a lesser degree, for up to 10 years. This suggests that venous ulceration could also have a carcinogenic effect. It’s biologically plausible that a venous leg ulcer could promote development of cancer through a variety of mechanisms, including inflammation, alteration of plasma viscosity and the adhesive properties of blood cells, and disruptions of venous pressure that encourage direct neoplastic invasion, according to Dr. Schmidt of Aarhus (Denmark) University.
She presented a Danish national patient registry study, which included all 29,705 patients with a first-time inpatient, outpatient, or emergency department diagnosis of a venous leg ulcer during 1982-2010. Fifty-five percent of them were age 70 years or older at the time of ulcer diagnosis. And 42% had moderate to very severe comorbid conditions based upon their Charlson Comorbidity Index score. During a median of 5.1 years of follow-up, or a total of 203,453 person-years, their overall risk of a first-time cancer diagnosis was significantly increased by 11%,compared with the general Danish population.
The malignancy risk was strongly time-dependent . However, the absolute risk of cancer was relatively low: less than 1% within the first 90 days after diagnosis of a venous leg ulcer. The number of patients who would need to be examined for a possible malignancy at the time of diagnosis of a venous leg ulcer in order to diagnosis one excess cancer was 146.
Dr. Schmidt indicated she would defer to experts in cost-benefit analysis as to whether an extensive work-up for occult malignancy is worthwhile in patients with a newly diagnosed venous leg ulcer, given the low absolute risk of cancer.
She reported having no financial conflicts of interest regarding her study, which was conducted with Danish institutional research funds.
AT THE EADV CONGRESS
Key clinical point: Venous leg ulceration appears to be a red flag for occult malignancy.
Major finding: During the first 89 days following diagnosis of a venous leg ulcer, affected patients are at a 3.48-fold increased risk of being diagnosed with a hematologic malignancy and a 2.89-fold greater risk of immune-related cancer compared with the general population.
Data source: This Danish nationwide cohort study compared standardized incidence ratios for various types of cancer during more than 200,000 person-years of follow-up in 29,705 patients with a venous leg ulcer vs. the general population.
Disclosures: The presenter reported having no financial conflicts of interest regarding her study, which was supported by Danish institutional funds.
A Novel Method of Skin Closure for Aging or Fragile Skin
Patients who have been on steroids, aspirin, or anticoagulants or who are elderly may have a fragile outer skin layer that is similar to parchment paper, which may be challenging for surgeons. In these patients, the epidermal layer is thin and translucent; when a surgeon cuts through this thin layer, the tissue beneath shows minimal dermis and poor-quality fat with weakened tissue support. When undergoing excisional surgery, there is no strong tissue to help the closure sutures remain intact. Surgeons may struggle with skin tears around the sutures and dehiscence on suture removal.
This article describes a novel approach to skin closure in patients with aging or thin skin using a polyethylene film with an acrylate adhesive in the excision area to aid in maintaining skin integrity throughout the healing process following surgery.
Closure Technique
First, the skin area is cleansed with a sterilizing soap preparation. A sterile marking pen then is used to outline the excision area. A 10×12-cm layer of polyethylene film is then attached to the excision site. Excision of the tumor is performed by cutting through the film in the marked area (Figure 1A), and closure is performed by suturing the wound edges through the polyethylene film while the area is still covered with the film (Figure 1B). The sutures can be left in for 2 weeks or longer if necessary. The patient should be instructed not to remove the film or perform any extensive cleansing of the treatment area. Antibiotics should be administered, as the polyethylene film maintains its sterile integrity for 7 days only. Because sutures are on the surface of the film, they are easily accessed for removal. Figure 1C shows the excision site after removal of the sutures and polyethylene film on the left tibia of a 95-year-old woman. Adhesive butterfly closures can be applied to strengthen the excision area after suture removal and prevent dehiscence.
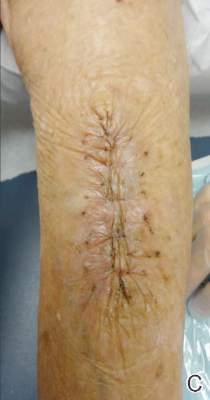
|
Figure 1. The excision site was marked after polyethylene adhesive film was applied to a squamous cell carcinoma on the left tibia of 95-year-old woman (A). Closure was performed by suturing the wound edges through the polyethylene film (B). The excision site appeared to have no dehiscence or signs of infection after removal of the sutures and polyethylene film (C). |
Case Reports
Twelve procedures for skin cancer excision were conducted in 10 patients using polyethylene adhesive film as a surgical aid due to extremely poor quality of the epidermis. The tumors were all squamous cell carcinomas and were located on the arms and legs. Patients were aged 73 to 95 years. Figure 2 demonstrates an example of excision of a squamous cell carcinoma on the left tibia of an 82-year-old man with prior dehiscence and infection after leg surgeries. Good results were achieved using the closure technique described here, along with prophylactic antibiotics.
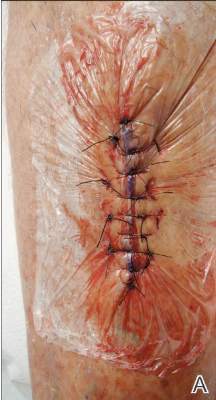
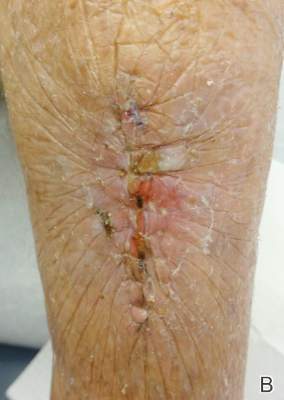
|
Figure 2. A squamous cell carcinoma excision site on the left tibia of an 82-year-old man that had been covered with polyethylene adhesive film prior to excision (A) and 17 days following removal of the sutures and film (B). |
One patient had complications from a Staphylococcus infection because antibiotics were not administered. The patient had prior infections with other surgeries. Antibiotics were given 4 days after surgery. The infection was cleared and the polyethylene film was retained for a total of 12 days.
Sutures were removed after 14 days for excision sites on the arms and 17 days for excision sites on the legs. All excision sites healed without dehiscence with a cosmetically acceptable scar. Figure 3A shows a completed excision on the left hand of a 92-year-old man, and Figure 3B is the result 5 weeks after excision.
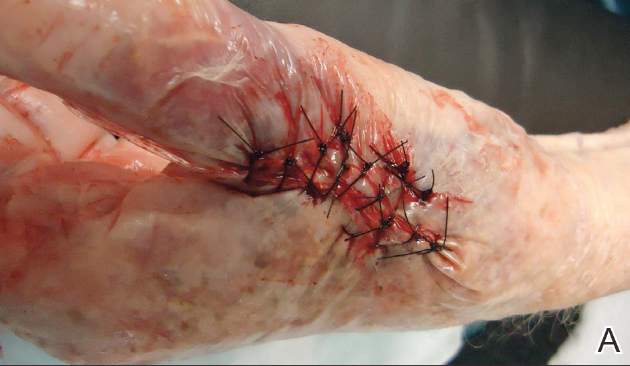
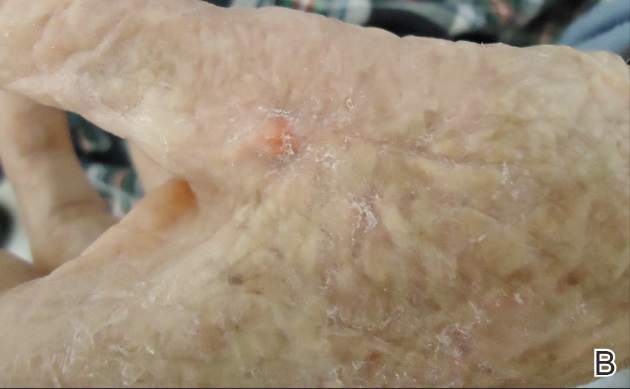
|
Figure 3. A squamous cell carcinoma excision site on the left thumb of a 92-year-old man that had been covered with polyethylene adhesive film prior to exci- sion (A). No visible scarring or dehiscence was noted 5 weeks after excision, following removal of the sutures and film (B). |
None of the patients reported discomfort from the polyethylene film remaining on the skin following surgery, though postoperative care required extra caution when dressing so as not to disturb or compromise the film. Patients were advised about postoperative care and were instructed not to remove the dressing. They were all given antibiotics as a necessary adjunct to maintain a lessened bacteria burden imposed by an impervious layer of acrylate adhesive. Complications resulted from failure to immediately provide antibiotics to 1 patient. The polyethylene film did not hinder healing or postoperative results.
Comment
Various techniques for handling fragile skin during surgery have been described in the literature. Fomon et al1 discussed aging skin as it relates to plastic surgery. Foster and Chan2 described a skin support technique for closing elliptical incisions in patients with fragile skin. Mazzurco and Krach3 discussed the use of a hydrocolloid dressing to aid in the closure of surgical wounds in patients with fragile skin.
The closure method described here was found to be particularly helpful when used as an adjunct to surgery in patients with fragile skin that lacked a suitable dermis. The polyethylene adhesive film helped to hold the sutures more securely. This method is cost-effective and is associated with a high level of patient satisfaction. For the surgeon, this technique may aid in dealing with difficult surgical situations and helps prevent wound complications in elderly patients or those with fragile skin.
1. Fomon S, Bell JW, Schattner A. Aging skin, a surgical challenge. AMA Arch Otolaryngol. 1955;61:554-562.
2. Foster RS, Chan J. The Fixomull skin support method for wound closure in patients with fragile skin. Australas J Dermatol. 2011;52:209-211.
3. Mazzurco JD, Krach KJ. Use of a hydrocolloid dressing to aid in the closure of surgical wounds in patients with fragile skin. J Am Acad Dermatol. 2012;66:335-336.
Patients who have been on steroids, aspirin, or anticoagulants or who are elderly may have a fragile outer skin layer that is similar to parchment paper, which may be challenging for surgeons. In these patients, the epidermal layer is thin and translucent; when a surgeon cuts through this thin layer, the tissue beneath shows minimal dermis and poor-quality fat with weakened tissue support. When undergoing excisional surgery, there is no strong tissue to help the closure sutures remain intact. Surgeons may struggle with skin tears around the sutures and dehiscence on suture removal.
This article describes a novel approach to skin closure in patients with aging or thin skin using a polyethylene film with an acrylate adhesive in the excision area to aid in maintaining skin integrity throughout the healing process following surgery.
Closure Technique
First, the skin area is cleansed with a sterilizing soap preparation. A sterile marking pen then is used to outline the excision area. A 10×12-cm layer of polyethylene film is then attached to the excision site. Excision of the tumor is performed by cutting through the film in the marked area (Figure 1A), and closure is performed by suturing the wound edges through the polyethylene film while the area is still covered with the film (Figure 1B). The sutures can be left in for 2 weeks or longer if necessary. The patient should be instructed not to remove the film or perform any extensive cleansing of the treatment area. Antibiotics should be administered, as the polyethylene film maintains its sterile integrity for 7 days only. Because sutures are on the surface of the film, they are easily accessed for removal. Figure 1C shows the excision site after removal of the sutures and polyethylene film on the left tibia of a 95-year-old woman. Adhesive butterfly closures can be applied to strengthen the excision area after suture removal and prevent dehiscence.



|
Figure 1. The excision site was marked after polyethylene adhesive film was applied to a squamous cell carcinoma on the left tibia of 95-year-old woman (A). Closure was performed by suturing the wound edges through the polyethylene film (B). The excision site appeared to have no dehiscence or signs of infection after removal of the sutures and polyethylene film (C). |
Case Reports
Twelve procedures for skin cancer excision were conducted in 10 patients using polyethylene adhesive film as a surgical aid due to extremely poor quality of the epidermis. The tumors were all squamous cell carcinomas and were located on the arms and legs. Patients were aged 73 to 95 years. Figure 2 demonstrates an example of excision of a squamous cell carcinoma on the left tibia of an 82-year-old man with prior dehiscence and infection after leg surgeries. Good results were achieved using the closure technique described here, along with prophylactic antibiotics.


|
Figure 2. A squamous cell carcinoma excision site on the left tibia of an 82-year-old man that had been covered with polyethylene adhesive film prior to excision (A) and 17 days following removal of the sutures and film (B). |
One patient had complications from a Staphylococcus infection because antibiotics were not administered. The patient had prior infections with other surgeries. Antibiotics were given 4 days after surgery. The infection was cleared and the polyethylene film was retained for a total of 12 days.
Sutures were removed after 14 days for excision sites on the arms and 17 days for excision sites on the legs. All excision sites healed without dehiscence with a cosmetically acceptable scar. Figure 3A shows a completed excision on the left hand of a 92-year-old man, and Figure 3B is the result 5 weeks after excision.


|
Figure 3. A squamous cell carcinoma excision site on the left thumb of a 92-year-old man that had been covered with polyethylene adhesive film prior to exci- sion (A). No visible scarring or dehiscence was noted 5 weeks after excision, following removal of the sutures and film (B). |
None of the patients reported discomfort from the polyethylene film remaining on the skin following surgery, though postoperative care required extra caution when dressing so as not to disturb or compromise the film. Patients were advised about postoperative care and were instructed not to remove the dressing. They were all given antibiotics as a necessary adjunct to maintain a lessened bacteria burden imposed by an impervious layer of acrylate adhesive. Complications resulted from failure to immediately provide antibiotics to 1 patient. The polyethylene film did not hinder healing or postoperative results.
Comment
Various techniques for handling fragile skin during surgery have been described in the literature. Fomon et al1 discussed aging skin as it relates to plastic surgery. Foster and Chan2 described a skin support technique for closing elliptical incisions in patients with fragile skin. Mazzurco and Krach3 discussed the use of a hydrocolloid dressing to aid in the closure of surgical wounds in patients with fragile skin.
The closure method described here was found to be particularly helpful when used as an adjunct to surgery in patients with fragile skin that lacked a suitable dermis. The polyethylene adhesive film helped to hold the sutures more securely. This method is cost-effective and is associated with a high level of patient satisfaction. For the surgeon, this technique may aid in dealing with difficult surgical situations and helps prevent wound complications in elderly patients or those with fragile skin.
Patients who have been on steroids, aspirin, or anticoagulants or who are elderly may have a fragile outer skin layer that is similar to parchment paper, which may be challenging for surgeons. In these patients, the epidermal layer is thin and translucent; when a surgeon cuts through this thin layer, the tissue beneath shows minimal dermis and poor-quality fat with weakened tissue support. When undergoing excisional surgery, there is no strong tissue to help the closure sutures remain intact. Surgeons may struggle with skin tears around the sutures and dehiscence on suture removal.
This article describes a novel approach to skin closure in patients with aging or thin skin using a polyethylene film with an acrylate adhesive in the excision area to aid in maintaining skin integrity throughout the healing process following surgery.
Closure Technique
First, the skin area is cleansed with a sterilizing soap preparation. A sterile marking pen then is used to outline the excision area. A 10×12-cm layer of polyethylene film is then attached to the excision site. Excision of the tumor is performed by cutting through the film in the marked area (Figure 1A), and closure is performed by suturing the wound edges through the polyethylene film while the area is still covered with the film (Figure 1B). The sutures can be left in for 2 weeks or longer if necessary. The patient should be instructed not to remove the film or perform any extensive cleansing of the treatment area. Antibiotics should be administered, as the polyethylene film maintains its sterile integrity for 7 days only. Because sutures are on the surface of the film, they are easily accessed for removal. Figure 1C shows the excision site after removal of the sutures and polyethylene film on the left tibia of a 95-year-old woman. Adhesive butterfly closures can be applied to strengthen the excision area after suture removal and prevent dehiscence.



|
Figure 1. The excision site was marked after polyethylene adhesive film was applied to a squamous cell carcinoma on the left tibia of 95-year-old woman (A). Closure was performed by suturing the wound edges through the polyethylene film (B). The excision site appeared to have no dehiscence or signs of infection after removal of the sutures and polyethylene film (C). |
Case Reports
Twelve procedures for skin cancer excision were conducted in 10 patients using polyethylene adhesive film as a surgical aid due to extremely poor quality of the epidermis. The tumors were all squamous cell carcinomas and were located on the arms and legs. Patients were aged 73 to 95 years. Figure 2 demonstrates an example of excision of a squamous cell carcinoma on the left tibia of an 82-year-old man with prior dehiscence and infection after leg surgeries. Good results were achieved using the closure technique described here, along with prophylactic antibiotics.


|
Figure 2. A squamous cell carcinoma excision site on the left tibia of an 82-year-old man that had been covered with polyethylene adhesive film prior to excision (A) and 17 days following removal of the sutures and film (B). |
One patient had complications from a Staphylococcus infection because antibiotics were not administered. The patient had prior infections with other surgeries. Antibiotics were given 4 days after surgery. The infection was cleared and the polyethylene film was retained for a total of 12 days.
Sutures were removed after 14 days for excision sites on the arms and 17 days for excision sites on the legs. All excision sites healed without dehiscence with a cosmetically acceptable scar. Figure 3A shows a completed excision on the left hand of a 92-year-old man, and Figure 3B is the result 5 weeks after excision.


|
Figure 3. A squamous cell carcinoma excision site on the left thumb of a 92-year-old man that had been covered with polyethylene adhesive film prior to exci- sion (A). No visible scarring or dehiscence was noted 5 weeks after excision, following removal of the sutures and film (B). |
None of the patients reported discomfort from the polyethylene film remaining on the skin following surgery, though postoperative care required extra caution when dressing so as not to disturb or compromise the film. Patients were advised about postoperative care and were instructed not to remove the dressing. They were all given antibiotics as a necessary adjunct to maintain a lessened bacteria burden imposed by an impervious layer of acrylate adhesive. Complications resulted from failure to immediately provide antibiotics to 1 patient. The polyethylene film did not hinder healing or postoperative results.
Comment
Various techniques for handling fragile skin during surgery have been described in the literature. Fomon et al1 discussed aging skin as it relates to plastic surgery. Foster and Chan2 described a skin support technique for closing elliptical incisions in patients with fragile skin. Mazzurco and Krach3 discussed the use of a hydrocolloid dressing to aid in the closure of surgical wounds in patients with fragile skin.
The closure method described here was found to be particularly helpful when used as an adjunct to surgery in patients with fragile skin that lacked a suitable dermis. The polyethylene adhesive film helped to hold the sutures more securely. This method is cost-effective and is associated with a high level of patient satisfaction. For the surgeon, this technique may aid in dealing with difficult surgical situations and helps prevent wound complications in elderly patients or those with fragile skin.
1. Fomon S, Bell JW, Schattner A. Aging skin, a surgical challenge. AMA Arch Otolaryngol. 1955;61:554-562.
2. Foster RS, Chan J. The Fixomull skin support method for wound closure in patients with fragile skin. Australas J Dermatol. 2011;52:209-211.
3. Mazzurco JD, Krach KJ. Use of a hydrocolloid dressing to aid in the closure of surgical wounds in patients with fragile skin. J Am Acad Dermatol. 2012;66:335-336.
1. Fomon S, Bell JW, Schattner A. Aging skin, a surgical challenge. AMA Arch Otolaryngol. 1955;61:554-562.
2. Foster RS, Chan J. The Fixomull skin support method for wound closure in patients with fragile skin. Australas J Dermatol. 2011;52:209-211.
3. Mazzurco JD, Krach KJ. Use of a hydrocolloid dressing to aid in the closure of surgical wounds in patients with fragile skin. J Am Acad Dermatol. 2012;66:335-336.
Practice Points
- A novel method of skin closure using a polyethylene film with an acrylate adhesive can aid in strengthening suture integrity and preventing skin tears.
- Dehiscence of excision sites in patients with aging or fragile skin can be prevented.
- This closure technique promotes healing and efficient scar formation.
What Is Your Diagnosis? Fixed Cutaneous Sporotrichosis
The Diagnosis: Fixed Cutaneous Sporotrichosis
On further questioning at our dermatology clinic, the patient reported having landed face-first into rocks and gravel during the all-terrain vehicle accident. After his medical history was noted and a physical examination was completed, bacterial and fungal cultures of the wound were taken. The fungal culture was positive for Sporothrix schenckii. The patient was prescribed itraconazole 200 mg 3 times daily for 3 days, then 200 mg twice daily for an additional 4 weeks after the lesions completely resolved. An ophthalmologist was immediately consulted to rule out sinus and periorbital involvement. After computed tomography revealed possible preseptal cellulitis with frontal sinus involvement, the patient was admitted and intravenous amphotericin B was administered. Following consultations with infectious disease specialists and radiologists, amphotericin B was discontinued and the patient was discharged on itraconazole 200 mg twice daily with close monitoring. At 3-month follow-up, the sporotrichosis infection had completely cleared (Figure).
Deep fungal infections comprise 2 distinct groups: systemic and subcutaneous mycoses. Individuals with subcutaneous mycoses present with skin involvement as the primary feature. Sporotrichosis is the most common cause of this type of mycosis1 and is caused by the dimorphic fungus S schenckii, an environmental saprophyte often residing in soil. Sporothrix schenckii exists as mold in a natural environment but exists as yeast in host tissue, thus causing ensuing infection.
Epidemiology
Sporotrichosis occurs worldwide but most frequently in temperate tropical and subtropical regions. The majority of cases are reported in Mexico and Central and South America1; however, cases have been seen in the southern United States, Japan, and Australia.2 In the United States, sporotrichosis is most commonly found in river valleys of the Midwest.
Sporothrix schenckii is most commonly isolated in hay, sphagnum moss, thorny plants, and soil, but it also has been described in other manifold host environments. Unusual origins of inoculation include an old and rust-stained camping tent in Mexico,3 crawl space joists of a house in Indiana,4 and hay bales used as props in a haunted house in Oklahoma.5
The incidence of infection is primarily sporadic; however, outbreaks among individuals who share a common environment favorable for the growth of S schenckii are at risk. Those identified to be at risk include rose gardeners, berry pickers, those who work in tree nurseries, horticulturists, landscapers, and miners.
Pathogenesis
As a dimorphic fungus, infection occurs when a conidium in the mold phase is introduced into the skin, usually by traumatic skin injury, and is converted to the yeast form in vivo. Distribution of infection by this organism is most commonly localized to the cutaneous, subcutaneous, and lymphocutaneous regions in healthy hosts but can involve visceral and osteoarticular structures in immunocompromised hosts.1,6 Pulmonary and disseminated forms are rare but can occur when S schenckii conidia are inhaled. Zoonotic transmission of the fungus also can occur with exposure to infected animals. Sporothrix schenckii has been reported to occur in cats, dogs, horses, donkeys, squirrels, armadillos, and dolphins.7-11
Pathology
Sporothrix schenckii is typically not visualized on microscopic examination due to the small number of microorganisms present; however, cultures grow rapidly (3–5 days) on Sabouraud agar. The fungus most commonly develops as white or off-white compact colonies that progressively darken with age, transitioning to gray and then black.1 Microscopically, the hyphae produce oval or pyriform conidia, which are assembled in a typical bouquetlike manner. Conversion of the organism to yeast on enriched medium such as brain-heart infusion agar or blood-cysteine-glucose agar confirms the diagnosis.
Acute lesions typically show a nonspecific mixed infiltrate, but established lesions may reveal granulomatous formation and neutrophilic microabscesses.1,2 Asteroid bodies, which are cigar-shaped yeasts surrounded by eosinophilic coronae radiata, may be found. Organisms are sparsely distributed within the lesions, necessitating a thorough examination of the culture for identification.
Clinical Features
Sporotrichosis has 3 main classifications: lymphocutaneous, fixed cutaneous, and disseminated. Lymphocutaneous sporotrichosis is the most common form of the infection.2 The disease presents with a small indurated papule occurring approximately 7 to 30 days after inoculation into the skin. The papule slowly enlarges, forms a nodule, and then frequently ulcerates. Over time, draining lymphatics become edematous and inflammatory, and a chain of secondary nodules begins to appear proximal to the initial lesion. The primary and secondary nodules may continue to ulcerate; alternately, they may heal or become chronic.
In fixed cutaneous sporotrichosis, the infection remains localized to one region and a granuloma may develop, which also may ulcerate. Satellite nodules may appear along the periphery of the lesion. Lymphatic spread is not observed in this form of the disease.
The disseminated form is a result of hematogenous spread from the primary inoculation site and typically occurs in an immunocompromised host. This form can present as pulmonary disease, sinusitis, and meningitis.1
Differential Diagnosis
The differential diagnosis for sporotrichosis includes atypical mycobacteria, nocardiosis, blastomycosis, pyogenic bacteria, leishmaniasis, tularemia, and tuberculosis.
Treatment
Treatment of sporotrichosis is always required. A saturated solution of potassium iodide has classically been used; however, it is frequently associated with side effects and can be problematic to administer.12 Given its low cost and traditional efficacy, it may still be used in some parts of the world.
Currently, the treatment of choice for fixed cutaneous and lymphocutaneous sporotrichosis is itraconazole 100 to 200 mg once daily for 3 to 6 months.1 The recommended treatment of osteoarticular sporotrichosis is itraconazole, but prolonged therapy is required.
Heat therapy is an alternative treatment option, as certain strains of S schenckii do not grow at temperatures higher than 35°C. Hot compresses must be used for at least 1 hour a day for several months, which may affect patient compliance.
Immunocompromised patients often have disseminated infection and require lifelong suppressive therapy with itraconazole and may require initial treatment with amphotericin B.13
Conclusion
Subcutaneous sporotrichosis can develop in patients with a traumatic injury involving vegetation, soil, or animals. Although some patients may develop more invasive disease, most infections in immunocompetent patients will resolve after 3 to 6 months of itraconazole 100 to 200 mg once daily.1
- De Araujo T, Marques AC, Kerdel F. Sporotrichosis. Int J Dermatol. 2001;40:737-742.
- Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 2. 6th ed. New York, NY: McGraw-Hill; 2003.
- Campos P, Arenas R, Coronado H. Epidemic cutaneous sporotrichosis. Int J Dermatol. 1994;33:38-41.
- Dillon GP, Lehmann PF, Talanin NY. Handyperson’s hazard: crawl space sporotrichosis. JAMA. 1995;274: 1673-1674.
- Dooley DP, Bostic PS, Beckius ML. Spook house sporotrichosis: a point-source outbreak of sporotrichosis associated with hay bale props in a Halloween haunted house. Arch Int Med. 1997;157:1885-1887.
- Kauffman CA. Sporotrichosis. Clin Infect Dis. 1999;29:231-236.
- Migaki G, Font RL, Kaplan W, et al. Sporotrichosis in a Pacific white-sided dolphin (Lagenorhynchus obliquidens). Am J Vet Res. 1978;39:1916-1919.
- Crothers SL, White SD, Ihrke PJ, et al. Sporotrichosis: a retrospective evaluation of 23 cases seen in northern California (1987-2007). Vet Dermatol. 2009;20:249-259.
- Saravanakumar PS, Eslami P, Zar FA. Lymphocutaneous sporotrichosis associated with a squirrel bite: case reports and review. Clin Infect Dis. 1996;23:647-648.
- Wenker CJ, Kaufman L, Bacciarini LN, et al. Sporotrichosis in a nine-banded armadillo (Dasypus novemcinctus). J Zoo Wildl Med. 1998;29:474-478.
- Barros MB, Schubach Ade O, do Valle AC, et al. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis. 2004;38:529-535.
- Kauffman CA. Old and new therapies for sporotrichosis. Clin Infect Dis. 1995;21:981-985.
- Kauffman CA, Hajjeh R, Chapman SW. Practice guidelines for the managements of patients with sporotrichosis. Clin Infect Dis. 2000;30:684-687.
The Diagnosis: Fixed Cutaneous Sporotrichosis
On further questioning at our dermatology clinic, the patient reported having landed face-first into rocks and gravel during the all-terrain vehicle accident. After his medical history was noted and a physical examination was completed, bacterial and fungal cultures of the wound were taken. The fungal culture was positive for Sporothrix schenckii. The patient was prescribed itraconazole 200 mg 3 times daily for 3 days, then 200 mg twice daily for an additional 4 weeks after the lesions completely resolved. An ophthalmologist was immediately consulted to rule out sinus and periorbital involvement. After computed tomography revealed possible preseptal cellulitis with frontal sinus involvement, the patient was admitted and intravenous amphotericin B was administered. Following consultations with infectious disease specialists and radiologists, amphotericin B was discontinued and the patient was discharged on itraconazole 200 mg twice daily with close monitoring. At 3-month follow-up, the sporotrichosis infection had completely cleared (Figure).
Deep fungal infections comprise 2 distinct groups: systemic and subcutaneous mycoses. Individuals with subcutaneous mycoses present with skin involvement as the primary feature. Sporotrichosis is the most common cause of this type of mycosis1 and is caused by the dimorphic fungus S schenckii, an environmental saprophyte often residing in soil. Sporothrix schenckii exists as mold in a natural environment but exists as yeast in host tissue, thus causing ensuing infection.
Epidemiology
Sporotrichosis occurs worldwide but most frequently in temperate tropical and subtropical regions. The majority of cases are reported in Mexico and Central and South America1; however, cases have been seen in the southern United States, Japan, and Australia.2 In the United States, sporotrichosis is most commonly found in river valleys of the Midwest.
Sporothrix schenckii is most commonly isolated in hay, sphagnum moss, thorny plants, and soil, but it also has been described in other manifold host environments. Unusual origins of inoculation include an old and rust-stained camping tent in Mexico,3 crawl space joists of a house in Indiana,4 and hay bales used as props in a haunted house in Oklahoma.5
The incidence of infection is primarily sporadic; however, outbreaks among individuals who share a common environment favorable for the growth of S schenckii are at risk. Those identified to be at risk include rose gardeners, berry pickers, those who work in tree nurseries, horticulturists, landscapers, and miners.
Pathogenesis
As a dimorphic fungus, infection occurs when a conidium in the mold phase is introduced into the skin, usually by traumatic skin injury, and is converted to the yeast form in vivo. Distribution of infection by this organism is most commonly localized to the cutaneous, subcutaneous, and lymphocutaneous regions in healthy hosts but can involve visceral and osteoarticular structures in immunocompromised hosts.1,6 Pulmonary and disseminated forms are rare but can occur when S schenckii conidia are inhaled. Zoonotic transmission of the fungus also can occur with exposure to infected animals. Sporothrix schenckii has been reported to occur in cats, dogs, horses, donkeys, squirrels, armadillos, and dolphins.7-11
Pathology
Sporothrix schenckii is typically not visualized on microscopic examination due to the small number of microorganisms present; however, cultures grow rapidly (3–5 days) on Sabouraud agar. The fungus most commonly develops as white or off-white compact colonies that progressively darken with age, transitioning to gray and then black.1 Microscopically, the hyphae produce oval or pyriform conidia, which are assembled in a typical bouquetlike manner. Conversion of the organism to yeast on enriched medium such as brain-heart infusion agar or blood-cysteine-glucose agar confirms the diagnosis.
Acute lesions typically show a nonspecific mixed infiltrate, but established lesions may reveal granulomatous formation and neutrophilic microabscesses.1,2 Asteroid bodies, which are cigar-shaped yeasts surrounded by eosinophilic coronae radiata, may be found. Organisms are sparsely distributed within the lesions, necessitating a thorough examination of the culture for identification.
Clinical Features
Sporotrichosis has 3 main classifications: lymphocutaneous, fixed cutaneous, and disseminated. Lymphocutaneous sporotrichosis is the most common form of the infection.2 The disease presents with a small indurated papule occurring approximately 7 to 30 days after inoculation into the skin. The papule slowly enlarges, forms a nodule, and then frequently ulcerates. Over time, draining lymphatics become edematous and inflammatory, and a chain of secondary nodules begins to appear proximal to the initial lesion. The primary and secondary nodules may continue to ulcerate; alternately, they may heal or become chronic.
In fixed cutaneous sporotrichosis, the infection remains localized to one region and a granuloma may develop, which also may ulcerate. Satellite nodules may appear along the periphery of the lesion. Lymphatic spread is not observed in this form of the disease.
The disseminated form is a result of hematogenous spread from the primary inoculation site and typically occurs in an immunocompromised host. This form can present as pulmonary disease, sinusitis, and meningitis.1
Differential Diagnosis
The differential diagnosis for sporotrichosis includes atypical mycobacteria, nocardiosis, blastomycosis, pyogenic bacteria, leishmaniasis, tularemia, and tuberculosis.
Treatment
Treatment of sporotrichosis is always required. A saturated solution of potassium iodide has classically been used; however, it is frequently associated with side effects and can be problematic to administer.12 Given its low cost and traditional efficacy, it may still be used in some parts of the world.
Currently, the treatment of choice for fixed cutaneous and lymphocutaneous sporotrichosis is itraconazole 100 to 200 mg once daily for 3 to 6 months.1 The recommended treatment of osteoarticular sporotrichosis is itraconazole, but prolonged therapy is required.
Heat therapy is an alternative treatment option, as certain strains of S schenckii do not grow at temperatures higher than 35°C. Hot compresses must be used for at least 1 hour a day for several months, which may affect patient compliance.
Immunocompromised patients often have disseminated infection and require lifelong suppressive therapy with itraconazole and may require initial treatment with amphotericin B.13
Conclusion
Subcutaneous sporotrichosis can develop in patients with a traumatic injury involving vegetation, soil, or animals. Although some patients may develop more invasive disease, most infections in immunocompetent patients will resolve after 3 to 6 months of itraconazole 100 to 200 mg once daily.1
The Diagnosis: Fixed Cutaneous Sporotrichosis
On further questioning at our dermatology clinic, the patient reported having landed face-first into rocks and gravel during the all-terrain vehicle accident. After his medical history was noted and a physical examination was completed, bacterial and fungal cultures of the wound were taken. The fungal culture was positive for Sporothrix schenckii. The patient was prescribed itraconazole 200 mg 3 times daily for 3 days, then 200 mg twice daily for an additional 4 weeks after the lesions completely resolved. An ophthalmologist was immediately consulted to rule out sinus and periorbital involvement. After computed tomography revealed possible preseptal cellulitis with frontal sinus involvement, the patient was admitted and intravenous amphotericin B was administered. Following consultations with infectious disease specialists and radiologists, amphotericin B was discontinued and the patient was discharged on itraconazole 200 mg twice daily with close monitoring. At 3-month follow-up, the sporotrichosis infection had completely cleared (Figure).
Deep fungal infections comprise 2 distinct groups: systemic and subcutaneous mycoses. Individuals with subcutaneous mycoses present with skin involvement as the primary feature. Sporotrichosis is the most common cause of this type of mycosis1 and is caused by the dimorphic fungus S schenckii, an environmental saprophyte often residing in soil. Sporothrix schenckii exists as mold in a natural environment but exists as yeast in host tissue, thus causing ensuing infection.
Epidemiology
Sporotrichosis occurs worldwide but most frequently in temperate tropical and subtropical regions. The majority of cases are reported in Mexico and Central and South America1; however, cases have been seen in the southern United States, Japan, and Australia.2 In the United States, sporotrichosis is most commonly found in river valleys of the Midwest.
Sporothrix schenckii is most commonly isolated in hay, sphagnum moss, thorny plants, and soil, but it also has been described in other manifold host environments. Unusual origins of inoculation include an old and rust-stained camping tent in Mexico,3 crawl space joists of a house in Indiana,4 and hay bales used as props in a haunted house in Oklahoma.5
The incidence of infection is primarily sporadic; however, outbreaks among individuals who share a common environment favorable for the growth of S schenckii are at risk. Those identified to be at risk include rose gardeners, berry pickers, those who work in tree nurseries, horticulturists, landscapers, and miners.
Pathogenesis
As a dimorphic fungus, infection occurs when a conidium in the mold phase is introduced into the skin, usually by traumatic skin injury, and is converted to the yeast form in vivo. Distribution of infection by this organism is most commonly localized to the cutaneous, subcutaneous, and lymphocutaneous regions in healthy hosts but can involve visceral and osteoarticular structures in immunocompromised hosts.1,6 Pulmonary and disseminated forms are rare but can occur when S schenckii conidia are inhaled. Zoonotic transmission of the fungus also can occur with exposure to infected animals. Sporothrix schenckii has been reported to occur in cats, dogs, horses, donkeys, squirrels, armadillos, and dolphins.7-11
Pathology
Sporothrix schenckii is typically not visualized on microscopic examination due to the small number of microorganisms present; however, cultures grow rapidly (3–5 days) on Sabouraud agar. The fungus most commonly develops as white or off-white compact colonies that progressively darken with age, transitioning to gray and then black.1 Microscopically, the hyphae produce oval or pyriform conidia, which are assembled in a typical bouquetlike manner. Conversion of the organism to yeast on enriched medium such as brain-heart infusion agar or blood-cysteine-glucose agar confirms the diagnosis.
Acute lesions typically show a nonspecific mixed infiltrate, but established lesions may reveal granulomatous formation and neutrophilic microabscesses.1,2 Asteroid bodies, which are cigar-shaped yeasts surrounded by eosinophilic coronae radiata, may be found. Organisms are sparsely distributed within the lesions, necessitating a thorough examination of the culture for identification.
Clinical Features
Sporotrichosis has 3 main classifications: lymphocutaneous, fixed cutaneous, and disseminated. Lymphocutaneous sporotrichosis is the most common form of the infection.2 The disease presents with a small indurated papule occurring approximately 7 to 30 days after inoculation into the skin. The papule slowly enlarges, forms a nodule, and then frequently ulcerates. Over time, draining lymphatics become edematous and inflammatory, and a chain of secondary nodules begins to appear proximal to the initial lesion. The primary and secondary nodules may continue to ulcerate; alternately, they may heal or become chronic.
In fixed cutaneous sporotrichosis, the infection remains localized to one region and a granuloma may develop, which also may ulcerate. Satellite nodules may appear along the periphery of the lesion. Lymphatic spread is not observed in this form of the disease.
The disseminated form is a result of hematogenous spread from the primary inoculation site and typically occurs in an immunocompromised host. This form can present as pulmonary disease, sinusitis, and meningitis.1
Differential Diagnosis
The differential diagnosis for sporotrichosis includes atypical mycobacteria, nocardiosis, blastomycosis, pyogenic bacteria, leishmaniasis, tularemia, and tuberculosis.
Treatment
Treatment of sporotrichosis is always required. A saturated solution of potassium iodide has classically been used; however, it is frequently associated with side effects and can be problematic to administer.12 Given its low cost and traditional efficacy, it may still be used in some parts of the world.
Currently, the treatment of choice for fixed cutaneous and lymphocutaneous sporotrichosis is itraconazole 100 to 200 mg once daily for 3 to 6 months.1 The recommended treatment of osteoarticular sporotrichosis is itraconazole, but prolonged therapy is required.
Heat therapy is an alternative treatment option, as certain strains of S schenckii do not grow at temperatures higher than 35°C. Hot compresses must be used for at least 1 hour a day for several months, which may affect patient compliance.
Immunocompromised patients often have disseminated infection and require lifelong suppressive therapy with itraconazole and may require initial treatment with amphotericin B.13
Conclusion
Subcutaneous sporotrichosis can develop in patients with a traumatic injury involving vegetation, soil, or animals. Although some patients may develop more invasive disease, most infections in immunocompetent patients will resolve after 3 to 6 months of itraconazole 100 to 200 mg once daily.1
- De Araujo T, Marques AC, Kerdel F. Sporotrichosis. Int J Dermatol. 2001;40:737-742.
- Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 2. 6th ed. New York, NY: McGraw-Hill; 2003.
- Campos P, Arenas R, Coronado H. Epidemic cutaneous sporotrichosis. Int J Dermatol. 1994;33:38-41.
- Dillon GP, Lehmann PF, Talanin NY. Handyperson’s hazard: crawl space sporotrichosis. JAMA. 1995;274: 1673-1674.
- Dooley DP, Bostic PS, Beckius ML. Spook house sporotrichosis: a point-source outbreak of sporotrichosis associated with hay bale props in a Halloween haunted house. Arch Int Med. 1997;157:1885-1887.
- Kauffman CA. Sporotrichosis. Clin Infect Dis. 1999;29:231-236.
- Migaki G, Font RL, Kaplan W, et al. Sporotrichosis in a Pacific white-sided dolphin (Lagenorhynchus obliquidens). Am J Vet Res. 1978;39:1916-1919.
- Crothers SL, White SD, Ihrke PJ, et al. Sporotrichosis: a retrospective evaluation of 23 cases seen in northern California (1987-2007). Vet Dermatol. 2009;20:249-259.
- Saravanakumar PS, Eslami P, Zar FA. Lymphocutaneous sporotrichosis associated with a squirrel bite: case reports and review. Clin Infect Dis. 1996;23:647-648.
- Wenker CJ, Kaufman L, Bacciarini LN, et al. Sporotrichosis in a nine-banded armadillo (Dasypus novemcinctus). J Zoo Wildl Med. 1998;29:474-478.
- Barros MB, Schubach Ade O, do Valle AC, et al. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis. 2004;38:529-535.
- Kauffman CA. Old and new therapies for sporotrichosis. Clin Infect Dis. 1995;21:981-985.
- Kauffman CA, Hajjeh R, Chapman SW. Practice guidelines for the managements of patients with sporotrichosis. Clin Infect Dis. 2000;30:684-687.
- De Araujo T, Marques AC, Kerdel F. Sporotrichosis. Int J Dermatol. 2001;40:737-742.
- Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 2. 6th ed. New York, NY: McGraw-Hill; 2003.
- Campos P, Arenas R, Coronado H. Epidemic cutaneous sporotrichosis. Int J Dermatol. 1994;33:38-41.
- Dillon GP, Lehmann PF, Talanin NY. Handyperson’s hazard: crawl space sporotrichosis. JAMA. 1995;274: 1673-1674.
- Dooley DP, Bostic PS, Beckius ML. Spook house sporotrichosis: a point-source outbreak of sporotrichosis associated with hay bale props in a Halloween haunted house. Arch Int Med. 1997;157:1885-1887.
- Kauffman CA. Sporotrichosis. Clin Infect Dis. 1999;29:231-236.
- Migaki G, Font RL, Kaplan W, et al. Sporotrichosis in a Pacific white-sided dolphin (Lagenorhynchus obliquidens). Am J Vet Res. 1978;39:1916-1919.
- Crothers SL, White SD, Ihrke PJ, et al. Sporotrichosis: a retrospective evaluation of 23 cases seen in northern California (1987-2007). Vet Dermatol. 2009;20:249-259.
- Saravanakumar PS, Eslami P, Zar FA. Lymphocutaneous sporotrichosis associated with a squirrel bite: case reports and review. Clin Infect Dis. 1996;23:647-648.
- Wenker CJ, Kaufman L, Bacciarini LN, et al. Sporotrichosis in a nine-banded armadillo (Dasypus novemcinctus). J Zoo Wildl Med. 1998;29:474-478.
- Barros MB, Schubach Ade O, do Valle AC, et al. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis. 2004;38:529-535.
- Kauffman CA. Old and new therapies for sporotrichosis. Clin Infect Dis. 1995;21:981-985.
- Kauffman CA, Hajjeh R, Chapman SW. Practice guidelines for the managements of patients with sporotrichosis. Clin Infect Dis. 2000;30:684-687.
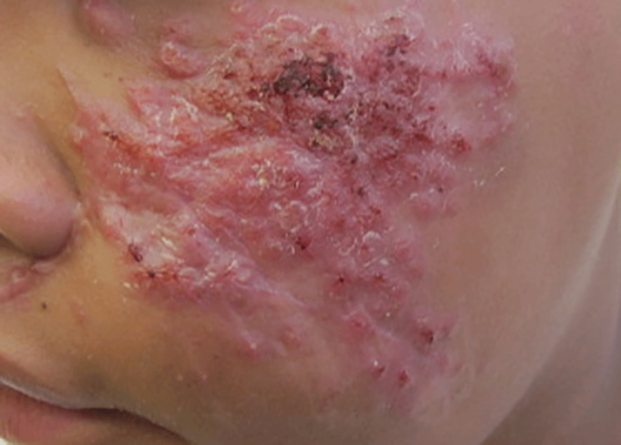
A 13-year-old adolescent boy presented with erythematous, tender, scaly, indurated nodules coalescing into plaques on the left cheek and periocular region. He denied any vision changes, the extraocular muscles were intact, and he was afebrile. Two weeks prior to presentation, the patient was hospitalized after an all-terrain vehicle accident that resulted in an extensive midfacial avulsion of the left cheek. The wound was cleaned and repaired by an otorhinolaryngologist. Three days later, he developed swelling and erythema of the left cheek, which was treated by his primary care provider with oral cephalexin, then trimethoprim-sulfamethoxazole for postsurgical wound infection. After completing his antibiotic course, he noticed continued worsening of the wound with increased edema, erythema, and tenderness. He was then referred to our clinic for further evaluation.
Pearls on the Use of Tape in Dermatology: Report From the AAD Meeting
At the recent Summer Meeting of the American Academy of Dermatology, Dr. Stone discussed the use of tapes in dermatology. He provides highlights from this session, including the diagnosis of tinea versicolor with plastic tape, suturing in patients with fragile skin to add strength to skin, and the diagnosis of scabies.
At the recent Summer Meeting of the American Academy of Dermatology, Dr. Stone discussed the use of tapes in dermatology. He provides highlights from this session, including the diagnosis of tinea versicolor with plastic tape, suturing in patients with fragile skin to add strength to skin, and the diagnosis of scabies.
At the recent Summer Meeting of the American Academy of Dermatology, Dr. Stone discussed the use of tapes in dermatology. He provides highlights from this session, including the diagnosis of tinea versicolor with plastic tape, suturing in patients with fragile skin to add strength to skin, and the diagnosis of scabies.
ICAAC: 2015 brings three major systematic reviews of diabetic foot infection therapy
SAN DIEGO – This has been a banner year for various expert panels to weigh in on the treatment of diabetic foot infections, with three major organizations each releasing systematic reviews. And all three in-depth reports reached the same conclusion regarding the antimicrobials of choice: it really doesn’t matter.
“In general, there are no significant differences in outcomes in studies comparing different groups of antibiotics,” Dr. Edgar J.G. Peters declared at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“You all want to know what is the magic bullet – what should we give our patients? Unfortunately, I can’t tell you. It depends on your local situation. But that doesn’t mean we should be fatalistic about diabetic foot infections. We now have a lot of data to support that we can use a lot of different antibiotics successfully. And in our review, we noticed that the quality level of the more recent studies, especially those in the last 5 years, has improved a lot,” said Dr. Peters of VU University Medical Center, Amsterdam, who was lead author of the systematic review by the International Working Group on the Diabetic Foot (Diabetes Metab Res Rev. 2015 Sep 7. doi: 10.1002/dmrr.2706. [Epub ahead of print]).
That review noted one potential exception to the all-antibiotics-are-similarly-effective principle: a randomized phase-III study that found tigecycline to be inferior to ertapenem with or without vancoymycin (Diagn Microbiol Infect Dis. 2014 Apr;78(4):469-80). This study was also cited in the 2015 systematic reviews by the Cochrane Collaboration and the UK National Institute for Health and Care Excellence. The finding was particularly noteworthy because the maker of tigecycline sponsored the study.
The systematic reviews followed somewhat different methodologies in reaching the same conclusion: The relative efficacy of different antibiotics used in the treatment of diabetic foot infections is unclear, largely due to low-quality evidence, flawed study designs, and bias. However, the Cochrane group found the literature does permit some reliable conclusions to be drawn as to the relative safety of the various antimicrobials. The evidence indicates that carbapenems have fewer adverse effects than anti-pseudomonal penicillins, daptomycin causes fewer complications than do semisynthetic penicillins, broad spectrum penicillins have fewer side effects than does linezolid, and ertapenem with or without vancomycin has fewer adverse events than does tigecycline.
Most side effects involved relatively mild nausea and diarrhea. The exception was linezolid, which was associated with an increased risk of anemia.
The International Working Group led by Dr. Peters looked beyond antimicrobials at evidence for other types of therapy for diabetic foot infections. The reviewers concluded that hyperbaric oxygen therapy has no effect on infection as an outcome, and that granulocyte-colony stimulating factor therapy showed mixed and inconclusive results based upon five studies. Two cohort studies suggest that early surgical debridement leads to a reduction in major amputations. And in patients with diabetic skin and soft tissue infection plus osteomyelitis, outcomes are improved if a bone biopsy is performed and antibiotics are targeted to the findings.
Dr. Peters pointed out that the studies of antimicrobial therapy for combined diabetic skin and soft tissue infection and osteomyelitis featured 6-28 days of treatment. That’s a surprisingly short course.
“I think 28 days is a pretty odd number,” he commented. “I don’t know about you, but we usually give antibiotics to those patients for a lot longer than 28 days.”
The internist shared several personal opinions derived from his in-depth review of the field of diabetic foot infection treatment.
“If antimicrobial therapies are equally effective, why not choose a cheap and narrow-spectrum one?” he suggested.
He recommended two high-quality sources useful in choosing a specific regimen: the International Working Group’s supplementary guidance document (Diabetes Metab Res Rev. 2015 Sep 19. doi: 10.1002/dmrr.2699. [Epub ahead of print] that accompanies the systematic review, and the Infectious Diseases Society of America 2012 guidelines, which Dr. Peters coauthored.
“Are IV antibiotics always necessary? I would say, probably not. Consider oral small-spectrum antibiotics for milder infections. It’s probably best to go broader-spectrum if you have a more severe infection because the stakes are higher in that case,” Dr. Peters said.
His in-depth examination of the evidence has taught him several other things. For one, 20-year-old studies are probably not terribly relevant to contemporary management of diabetic foot infections, given the considerable changes that have occurred in antimicrobial susceptibility and the organization of health care systems. And pathogen eradication is probably not a valid study endpoint.
Moreover, the available evidence does not support the popular notion that empiric coverage for Pseudomonas improves outcomes, he added.
Dr. Peters reported having no financial conflicts regarding his presentation.
SAN DIEGO – This has been a banner year for various expert panels to weigh in on the treatment of diabetic foot infections, with three major organizations each releasing systematic reviews. And all three in-depth reports reached the same conclusion regarding the antimicrobials of choice: it really doesn’t matter.
“In general, there are no significant differences in outcomes in studies comparing different groups of antibiotics,” Dr. Edgar J.G. Peters declared at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“You all want to know what is the magic bullet – what should we give our patients? Unfortunately, I can’t tell you. It depends on your local situation. But that doesn’t mean we should be fatalistic about diabetic foot infections. We now have a lot of data to support that we can use a lot of different antibiotics successfully. And in our review, we noticed that the quality level of the more recent studies, especially those in the last 5 years, has improved a lot,” said Dr. Peters of VU University Medical Center, Amsterdam, who was lead author of the systematic review by the International Working Group on the Diabetic Foot (Diabetes Metab Res Rev. 2015 Sep 7. doi: 10.1002/dmrr.2706. [Epub ahead of print]).
That review noted one potential exception to the all-antibiotics-are-similarly-effective principle: a randomized phase-III study that found tigecycline to be inferior to ertapenem with or without vancoymycin (Diagn Microbiol Infect Dis. 2014 Apr;78(4):469-80). This study was also cited in the 2015 systematic reviews by the Cochrane Collaboration and the UK National Institute for Health and Care Excellence. The finding was particularly noteworthy because the maker of tigecycline sponsored the study.
The systematic reviews followed somewhat different methodologies in reaching the same conclusion: The relative efficacy of different antibiotics used in the treatment of diabetic foot infections is unclear, largely due to low-quality evidence, flawed study designs, and bias. However, the Cochrane group found the literature does permit some reliable conclusions to be drawn as to the relative safety of the various antimicrobials. The evidence indicates that carbapenems have fewer adverse effects than anti-pseudomonal penicillins, daptomycin causes fewer complications than do semisynthetic penicillins, broad spectrum penicillins have fewer side effects than does linezolid, and ertapenem with or without vancomycin has fewer adverse events than does tigecycline.
Most side effects involved relatively mild nausea and diarrhea. The exception was linezolid, which was associated with an increased risk of anemia.
The International Working Group led by Dr. Peters looked beyond antimicrobials at evidence for other types of therapy for diabetic foot infections. The reviewers concluded that hyperbaric oxygen therapy has no effect on infection as an outcome, and that granulocyte-colony stimulating factor therapy showed mixed and inconclusive results based upon five studies. Two cohort studies suggest that early surgical debridement leads to a reduction in major amputations. And in patients with diabetic skin and soft tissue infection plus osteomyelitis, outcomes are improved if a bone biopsy is performed and antibiotics are targeted to the findings.
Dr. Peters pointed out that the studies of antimicrobial therapy for combined diabetic skin and soft tissue infection and osteomyelitis featured 6-28 days of treatment. That’s a surprisingly short course.
“I think 28 days is a pretty odd number,” he commented. “I don’t know about you, but we usually give antibiotics to those patients for a lot longer than 28 days.”
The internist shared several personal opinions derived from his in-depth review of the field of diabetic foot infection treatment.
“If antimicrobial therapies are equally effective, why not choose a cheap and narrow-spectrum one?” he suggested.
He recommended two high-quality sources useful in choosing a specific regimen: the International Working Group’s supplementary guidance document (Diabetes Metab Res Rev. 2015 Sep 19. doi: 10.1002/dmrr.2699. [Epub ahead of print] that accompanies the systematic review, and the Infectious Diseases Society of America 2012 guidelines, which Dr. Peters coauthored.
“Are IV antibiotics always necessary? I would say, probably not. Consider oral small-spectrum antibiotics for milder infections. It’s probably best to go broader-spectrum if you have a more severe infection because the stakes are higher in that case,” Dr. Peters said.
His in-depth examination of the evidence has taught him several other things. For one, 20-year-old studies are probably not terribly relevant to contemporary management of diabetic foot infections, given the considerable changes that have occurred in antimicrobial susceptibility and the organization of health care systems. And pathogen eradication is probably not a valid study endpoint.
Moreover, the available evidence does not support the popular notion that empiric coverage for Pseudomonas improves outcomes, he added.
Dr. Peters reported having no financial conflicts regarding his presentation.
SAN DIEGO – This has been a banner year for various expert panels to weigh in on the treatment of diabetic foot infections, with three major organizations each releasing systematic reviews. And all three in-depth reports reached the same conclusion regarding the antimicrobials of choice: it really doesn’t matter.
“In general, there are no significant differences in outcomes in studies comparing different groups of antibiotics,” Dr. Edgar J.G. Peters declared at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“You all want to know what is the magic bullet – what should we give our patients? Unfortunately, I can’t tell you. It depends on your local situation. But that doesn’t mean we should be fatalistic about diabetic foot infections. We now have a lot of data to support that we can use a lot of different antibiotics successfully. And in our review, we noticed that the quality level of the more recent studies, especially those in the last 5 years, has improved a lot,” said Dr. Peters of VU University Medical Center, Amsterdam, who was lead author of the systematic review by the International Working Group on the Diabetic Foot (Diabetes Metab Res Rev. 2015 Sep 7. doi: 10.1002/dmrr.2706. [Epub ahead of print]).
That review noted one potential exception to the all-antibiotics-are-similarly-effective principle: a randomized phase-III study that found tigecycline to be inferior to ertapenem with or without vancoymycin (Diagn Microbiol Infect Dis. 2014 Apr;78(4):469-80). This study was also cited in the 2015 systematic reviews by the Cochrane Collaboration and the UK National Institute for Health and Care Excellence. The finding was particularly noteworthy because the maker of tigecycline sponsored the study.
The systematic reviews followed somewhat different methodologies in reaching the same conclusion: The relative efficacy of different antibiotics used in the treatment of diabetic foot infections is unclear, largely due to low-quality evidence, flawed study designs, and bias. However, the Cochrane group found the literature does permit some reliable conclusions to be drawn as to the relative safety of the various antimicrobials. The evidence indicates that carbapenems have fewer adverse effects than anti-pseudomonal penicillins, daptomycin causes fewer complications than do semisynthetic penicillins, broad spectrum penicillins have fewer side effects than does linezolid, and ertapenem with or without vancomycin has fewer adverse events than does tigecycline.
Most side effects involved relatively mild nausea and diarrhea. The exception was linezolid, which was associated with an increased risk of anemia.
The International Working Group led by Dr. Peters looked beyond antimicrobials at evidence for other types of therapy for diabetic foot infections. The reviewers concluded that hyperbaric oxygen therapy has no effect on infection as an outcome, and that granulocyte-colony stimulating factor therapy showed mixed and inconclusive results based upon five studies. Two cohort studies suggest that early surgical debridement leads to a reduction in major amputations. And in patients with diabetic skin and soft tissue infection plus osteomyelitis, outcomes are improved if a bone biopsy is performed and antibiotics are targeted to the findings.
Dr. Peters pointed out that the studies of antimicrobial therapy for combined diabetic skin and soft tissue infection and osteomyelitis featured 6-28 days of treatment. That’s a surprisingly short course.
“I think 28 days is a pretty odd number,” he commented. “I don’t know about you, but we usually give antibiotics to those patients for a lot longer than 28 days.”
The internist shared several personal opinions derived from his in-depth review of the field of diabetic foot infection treatment.
“If antimicrobial therapies are equally effective, why not choose a cheap and narrow-spectrum one?” he suggested.
He recommended two high-quality sources useful in choosing a specific regimen: the International Working Group’s supplementary guidance document (Diabetes Metab Res Rev. 2015 Sep 19. doi: 10.1002/dmrr.2699. [Epub ahead of print] that accompanies the systematic review, and the Infectious Diseases Society of America 2012 guidelines, which Dr. Peters coauthored.
“Are IV antibiotics always necessary? I would say, probably not. Consider oral small-spectrum antibiotics for milder infections. It’s probably best to go broader-spectrum if you have a more severe infection because the stakes are higher in that case,” Dr. Peters said.
His in-depth examination of the evidence has taught him several other things. For one, 20-year-old studies are probably not terribly relevant to contemporary management of diabetic foot infections, given the considerable changes that have occurred in antimicrobial susceptibility and the organization of health care systems. And pathogen eradication is probably not a valid study endpoint.
Moreover, the available evidence does not support the popular notion that empiric coverage for Pseudomonas improves outcomes, he added.
Dr. Peters reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM ICAAC 2015