User login
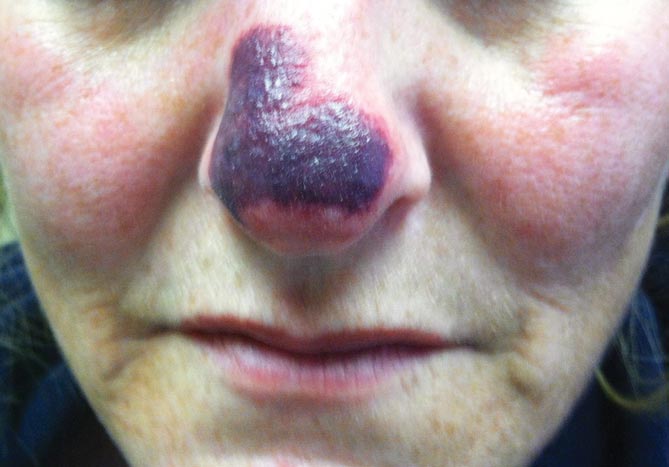
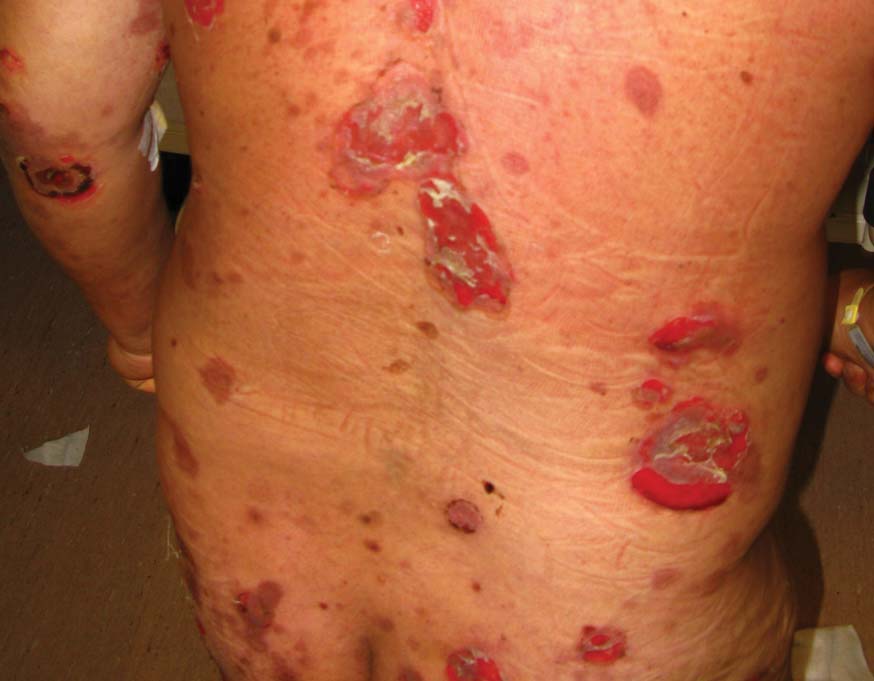
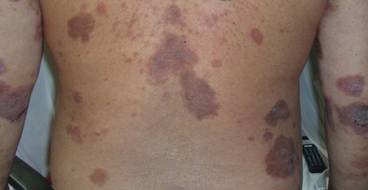
Diabetic foot ulcer: Early closure post debridement best
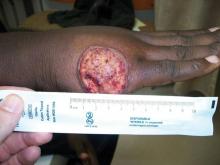
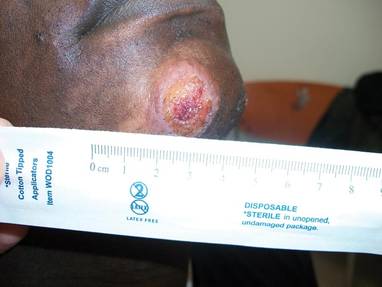
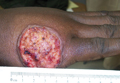
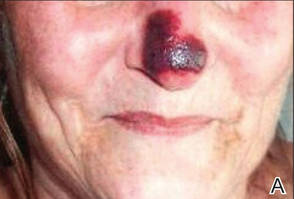
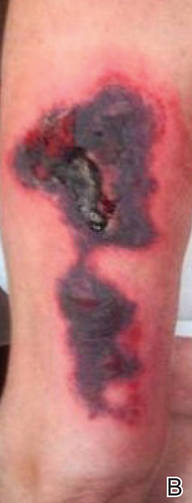
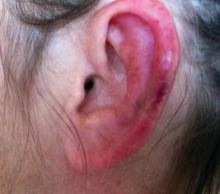
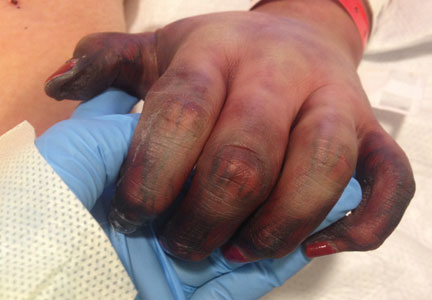
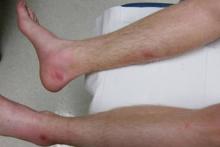
SAN DIEGO – Early wound closure prior to hospital discharge after surgical debridement of infected diabetic foot ulcers yields higher ulcer healing rates and a shorter time to healing, compared with various nonclosure wound management methods, according to a propensity-matched study.
How best to manage the open wound following nonamputative surgery of infected diabetic foot ulcers has been controversial. But early wound closure during the index hospitalization was the clear winner in this comparative study, Dr. Shey-Ying Chen reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
He presented a retrospective comparison between 179 diabetic foot ulcer (DFU) patients with early wound closure after surgical debridement and an equal number of matched controls treated with various nonclosure techniques, including negative pressure wound therapy and the repeated application of moist dressings. The two study groups were matched first on the basis of DFU location – toe, forefoot, midfoot, or rear foot – and then further propensity matched based on demographics, comorbid conditions, the presence of neuropathy, ulcer status by Wagner classification, infection severity, revascularization procedures, and other variables.
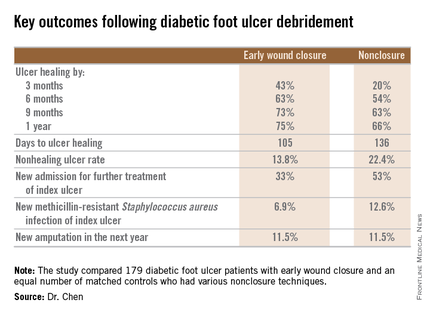
During 1 year of follow-up post discharge, ulcer healing occurred in 75% of the early wound closure group, compared with 66% of the nonclosure patients. Readmission for further treatment of the index ulcer occurred in 33% of the early closure group and 52% of the nonclosure group. Other outcomes were also superior in the early wound closure group, noted Dr. Chen of Beth Israel Deaconess Medical Center, Boston.
Two independent predictors of DFU healing during the follow-up period emerged from a Cox regression analysis: early wound closure, with an adjusted odds ratio of 1.63, and acute as opposed to chronic DFU, with an OR of 1.35.
Ulcer healing was significantly less likely in patients with peripheral vascular disease, with an OR of 0.62; neuropathy, with an OR of 0.53; and methicillin-resistant Staphylococcus aureus wound infection, with an OR of 0.59, he continued.
Underscoring the longer-term difficulties faced by patients with DFUs, it’s noteworthy that 11.5% of patients in both study arms underwent new amputations during the year of follow-up. Moreover, a new diagnosis of osteomyelitis was made in 20% of the early wound closure group and 26% of the nonclosure group, a nonsignificant difference.
Dr. Adolf W. Karchmer, Dr. Chen’s senior coinvestigator, said the outcome data are too new to be able to gauge how vascular, orthopedic, and podiatric surgeons will react.
The investigators reported having no financial conflicts with regard to this study, conducted without commercial sponsorship.
SAN DIEGO – Early wound closure prior to hospital discharge after surgical debridement of infected diabetic foot ulcers yields higher ulcer healing rates and a shorter time to healing, compared with various nonclosure wound management methods, according to a propensity-matched study.
How best to manage the open wound following nonamputative surgery of infected diabetic foot ulcers has been controversial. But early wound closure during the index hospitalization was the clear winner in this comparative study, Dr. Shey-Ying Chen reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.

He presented a retrospective comparison between 179 diabetic foot ulcer (DFU) patients with early wound closure after surgical debridement and an equal number of matched controls treated with various nonclosure techniques, including negative pressure wound therapy and the repeated application of moist dressings. The two study groups were matched first on the basis of DFU location – toe, forefoot, midfoot, or rear foot – and then further propensity matched based on demographics, comorbid conditions, the presence of neuropathy, ulcer status by Wagner classification, infection severity, revascularization procedures, and other variables.
During 1 year of follow-up post discharge, ulcer healing occurred in 75% of the early wound closure group, compared with 66% of the nonclosure patients. Readmission for further treatment of the index ulcer occurred in 33% of the early closure group and 52% of the nonclosure group. Other outcomes were also superior in the early wound closure group, noted Dr. Chen of Beth Israel Deaconess Medical Center, Boston.
Two independent predictors of DFU healing during the follow-up period emerged from a Cox regression analysis: early wound closure, with an adjusted odds ratio of 1.63, and acute as opposed to chronic DFU, with an OR of 1.35.
Ulcer healing was significantly less likely in patients with peripheral vascular disease, with an OR of 0.62; neuropathy, with an OR of 0.53; and methicillin-resistant Staphylococcus aureus wound infection, with an OR of 0.59, he continued.
Underscoring the longer-term difficulties faced by patients with DFUs, it’s noteworthy that 11.5% of patients in both study arms underwent new amputations during the year of follow-up. Moreover, a new diagnosis of osteomyelitis was made in 20% of the early wound closure group and 26% of the nonclosure group, a nonsignificant difference.
Dr. Adolf W. Karchmer, Dr. Chen’s senior coinvestigator, said the outcome data are too new to be able to gauge how vascular, orthopedic, and podiatric surgeons will react.
The investigators reported having no financial conflicts with regard to this study, conducted without commercial sponsorship.
SAN DIEGO – Early wound closure prior to hospital discharge after surgical debridement of infected diabetic foot ulcers yields higher ulcer healing rates and a shorter time to healing, compared with various nonclosure wound management methods, according to a propensity-matched study.
How best to manage the open wound following nonamputative surgery of infected diabetic foot ulcers has been controversial. But early wound closure during the index hospitalization was the clear winner in this comparative study, Dr. Shey-Ying Chen reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.

He presented a retrospective comparison between 179 diabetic foot ulcer (DFU) patients with early wound closure after surgical debridement and an equal number of matched controls treated with various nonclosure techniques, including negative pressure wound therapy and the repeated application of moist dressings. The two study groups were matched first on the basis of DFU location – toe, forefoot, midfoot, or rear foot – and then further propensity matched based on demographics, comorbid conditions, the presence of neuropathy, ulcer status by Wagner classification, infection severity, revascularization procedures, and other variables.
During 1 year of follow-up post discharge, ulcer healing occurred in 75% of the early wound closure group, compared with 66% of the nonclosure patients. Readmission for further treatment of the index ulcer occurred in 33% of the early closure group and 52% of the nonclosure group. Other outcomes were also superior in the early wound closure group, noted Dr. Chen of Beth Israel Deaconess Medical Center, Boston.
Two independent predictors of DFU healing during the follow-up period emerged from a Cox regression analysis: early wound closure, with an adjusted odds ratio of 1.63, and acute as opposed to chronic DFU, with an OR of 1.35.
Ulcer healing was significantly less likely in patients with peripheral vascular disease, with an OR of 0.62; neuropathy, with an OR of 0.53; and methicillin-resistant Staphylococcus aureus wound infection, with an OR of 0.59, he continued.
Underscoring the longer-term difficulties faced by patients with DFUs, it’s noteworthy that 11.5% of patients in both study arms underwent new amputations during the year of follow-up. Moreover, a new diagnosis of osteomyelitis was made in 20% of the early wound closure group and 26% of the nonclosure group, a nonsignificant difference.
Dr. Adolf W. Karchmer, Dr. Chen’s senior coinvestigator, said the outcome data are too new to be able to gauge how vascular, orthopedic, and podiatric surgeons will react.
The investigators reported having no financial conflicts with regard to this study, conducted without commercial sponsorship.
AT ICAAC 2015
Key clinical point: Diabetic foot ulcers are more likely to heal with early wound closure following surgical debridement than with nonclosure techniques.
Major finding: Healing of diabetic foot ulcers after surgical debridement took an average of 105 days in patients who underwent early wound closure prior to hospital discharge, compared with 136 days in those whose wounds were managed with nonclosure techniques.
Data source: A retrospective, nonrandomized study featuring two propensity score–matched groups, with 179 patients in each, who were followed for 1 year post discharge for surgical debridement of a diabetic foot ulcer.
Disclosures: The presenter reported having no financial conflicts regarding this study, conducted free of commercial support.
Dermatologists should be central to wound care, expert says
The way Dr. Adam Friedman sees it, dermatologists deserve a prominent place at the table when it comes to the treatment of acute and chronic wounds.
“As masters of the integument, we should be central to wound care, whether it be for research, in terms of developing better technologies, medications, approaches, diagnostics, but also in terms of managing these wounds, given the rich breadth of pathophysiology and biology we learn during our residency and maintain during our continuing education as practicing dermatologists,” said Dr. Friedman of the department of dermatology at George Washington University, Washington.
When the Journal of Drugs in Dermatology invited Dr. Friedman to serve as guest editor for a special feature section on wound care for its July 2015 issue, he jumped at the chance “to give the dermatology community a small taste of what’s going on in the wound healing world.”
Currently, he said, there is wide variability in the types of clinicians leading wound care centers in the United States, with dermatologists often sitting on the sidelines. “At one institution, it may be the vascular surgery service, at others it may be the family medicine service or even the emergency medicine department,” said Dr. Friedman, who is an editorial advisor to Dermatology News.
“That’s a big problem, in that there’s no uniformity from one center to the next in terms of who is expected to and should be taking responsibility for the wound healing service at their institutions. The reality is, it should be an interdisciplinary team, which not only involves dermatology but vascular surgery, nutrition, internal medicine, subspecialties of medicine like rheumatology, and rehab medicine. However, what is happening more often than not is that you’re getting just one or two of these elements, which cannot be as effective because you miss out on a broader, holistic view.”
There are two chief reasons why dermatologists aren’t more involved in wound care management, he continued. One stems from a lack of training on the topic. In one of the abstracts from the special JDD wound care section, researchers led by Dr. Emily Stamell Ruiz conducted an online survey of dermatology residents in the United States, to ask them about their preparedness to care for wounds and to assess the amount and type of training devoted to wound care during residency. Of the 175 respondents, 78% and 85% did not feel prepared to manage acute and chronic wounds, respectively, while 77% felt that more education is needed during their residency (J Drugs Dermatol. 2015;14[7]:716-20). “Residents felt that there was a clinical as well as a didactical gap, so they felt that they needed more training both through lectures as well as in clinics,” said Dr. Ruiz of the department of dermatology at Brigham and Women’s Hospital, Boston. “It’s not just a focal problem, it really is a universal curriculum problem. Future reforms to the current dermatology curriculum to include wound care training could help close the gap in wound care training.”
Another reason why dermatologists aren’t more involved in wound care management is the time commitment, said Dr. Friedman, who is also director of translational research at George Washington. The treatment of chronic wounds is “physically and financially burdensome,” he said. “It takes not only yourself being comfortable with managing the whole patient which includes the wound[s] with a side order of comorbidities, but your support staff as well – having nurses who know how to use the different wound dressings and how to help you with debridement. You need the right infrastructure. It also costs a lot on the provider side to manage wounds. You need a setup where you can get these patients in, have support staff to help with the wound dressings once you’ve identified what’s necessary, and be able to move on to the next patient.”
In another manuscript contained in the JDD special section, Dr. Friedman and his associates retrospectively reviewed the characteristics of 51 patients with burn injuries who were seen by seven different dermatologists at the Einstein-Montefiore division of dermatology from April 2010 to July 2014 (J Drugs Dermatol. 2015;14[7]:721-4). It found that the main mechanism of injury was burn from hot metal (22%), followed by contact with hot liquids (18%). It also found that silver sulfadiazine was the most commonly prescribed treatment, “even though there are considerable data illustrating that its use will delay wound closure and healing (J Invest Dermatol. 2015 May;135[5]:1459-62),” Dr. Friedman said. He went on to note that for patients who suffer an acute burn, “the ability to access a dermatologist is somewhat limited because their schedules are heavily booked well in advance, and the format doesn’t allow for these types of emergencies. More often than not they go to the ED or to primary care. That might not necessarily be the right decision because these are physicians who may not have the necessary training in terms of not only proper burn care, but skin care overall.”
Another manuscript in the special section describes a method in which partial thickness wounds were induced by cryosurgery to create wounds that could facilitate wound healing research and development. For the study, researchers led by Dr. Robert Kirsner, interim chairman of the department of dermatology and cutaneous surgery at the University of Miami, used liquid nitrogen spray to induce freeze injuries on the forearms of eight healthy adult volunteers (J. Drugs Dermatol. 2015;14[7]: 734-8). They delivered the spray onto a target area of a 1-cm circular opening at a distance from the cryodevice to the skin of 0.5-1 cm and implemented several freeze-thaw time cycles by administering pulses that ranged from 3-12 seconds.
After a 24-hour follow-up, Dr. Kirsner and his associates observed that freeze times exceeding 5 seconds caused a majority of study participants to develop blisters, while freeze times exceeding 8 seconds caused uniform blister formation. Time to healing among subjects in the 8-second freeze time group was 12-13 days, while time to healing among those in the 12-second time freeze group was 21 days.
“Cryo-induced wound healing is a little bit slower than you’d expect with a scalpel, but that wasn’t really surprising,” Dr. Kirsner said. “The fact that it healed a little bit slower was a pretty good thing because if everything healed too fast then it couldn’t serve as a model to speed or slow epithelialization. We were quite pleased.” He noted that the model “could be used as a safety test for chronic wound treatment and as an efficacy test for acute wound treatment. It’s relatively inexpensive and a relatively simple technique. If you’re developing a product for widespread use, it’s probably a minor cost in the whole development process.”
Other manuscripts in the JDD special section include a preclinical study using a murine multithermal burn model which found that N-acetylcysteine S-nitrosothiol nanoparticles prevent wound expansion and accelerate burn closure, and a practical, systematic approach to using wound dressings for the wound care novice. Dr. Friedman hopes that the special section not only stimulates further interest in wound care, but that it serves as “a call for action. We really need to be more involved in wound care from the acute and chronic perspective,” he said. “Wound centers around the country should be involving dermatologists. We have so much to offer from bench to bedside because the skin is our thing. I hope this is a reminder that we should be part of this picture.”
Dr. Friedman disclosed that he serves as a consultant for Galderma, Biogen, Aveeno, Intraderm, Puracore, La Roche-Posay, Amgen, Pfizer, PHD Skin Care. He also serves as an advisory board member for Nerium International, Valeant, Nano BioMed, MicroCures, and Novartis, and has received research grants from Valeant. Dr. Ruiz and Dr. Kirsner reported no financial disclosures.
The way Dr. Adam Friedman sees it, dermatologists deserve a prominent place at the table when it comes to the treatment of acute and chronic wounds.
“As masters of the integument, we should be central to wound care, whether it be for research, in terms of developing better technologies, medications, approaches, diagnostics, but also in terms of managing these wounds, given the rich breadth of pathophysiology and biology we learn during our residency and maintain during our continuing education as practicing dermatologists,” said Dr. Friedman of the department of dermatology at George Washington University, Washington.
When the Journal of Drugs in Dermatology invited Dr. Friedman to serve as guest editor for a special feature section on wound care for its July 2015 issue, he jumped at the chance “to give the dermatology community a small taste of what’s going on in the wound healing world.”
Currently, he said, there is wide variability in the types of clinicians leading wound care centers in the United States, with dermatologists often sitting on the sidelines. “At one institution, it may be the vascular surgery service, at others it may be the family medicine service or even the emergency medicine department,” said Dr. Friedman, who is an editorial advisor to Dermatology News.
“That’s a big problem, in that there’s no uniformity from one center to the next in terms of who is expected to and should be taking responsibility for the wound healing service at their institutions. The reality is, it should be an interdisciplinary team, which not only involves dermatology but vascular surgery, nutrition, internal medicine, subspecialties of medicine like rheumatology, and rehab medicine. However, what is happening more often than not is that you’re getting just one or two of these elements, which cannot be as effective because you miss out on a broader, holistic view.”
There are two chief reasons why dermatologists aren’t more involved in wound care management, he continued. One stems from a lack of training on the topic. In one of the abstracts from the special JDD wound care section, researchers led by Dr. Emily Stamell Ruiz conducted an online survey of dermatology residents in the United States, to ask them about their preparedness to care for wounds and to assess the amount and type of training devoted to wound care during residency. Of the 175 respondents, 78% and 85% did not feel prepared to manage acute and chronic wounds, respectively, while 77% felt that more education is needed during their residency (J Drugs Dermatol. 2015;14[7]:716-20). “Residents felt that there was a clinical as well as a didactical gap, so they felt that they needed more training both through lectures as well as in clinics,” said Dr. Ruiz of the department of dermatology at Brigham and Women’s Hospital, Boston. “It’s not just a focal problem, it really is a universal curriculum problem. Future reforms to the current dermatology curriculum to include wound care training could help close the gap in wound care training.”
Another reason why dermatologists aren’t more involved in wound care management is the time commitment, said Dr. Friedman, who is also director of translational research at George Washington. The treatment of chronic wounds is “physically and financially burdensome,” he said. “It takes not only yourself being comfortable with managing the whole patient which includes the wound[s] with a side order of comorbidities, but your support staff as well – having nurses who know how to use the different wound dressings and how to help you with debridement. You need the right infrastructure. It also costs a lot on the provider side to manage wounds. You need a setup where you can get these patients in, have support staff to help with the wound dressings once you’ve identified what’s necessary, and be able to move on to the next patient.”
In another manuscript contained in the JDD special section, Dr. Friedman and his associates retrospectively reviewed the characteristics of 51 patients with burn injuries who were seen by seven different dermatologists at the Einstein-Montefiore division of dermatology from April 2010 to July 2014 (J Drugs Dermatol. 2015;14[7]:721-4). It found that the main mechanism of injury was burn from hot metal (22%), followed by contact with hot liquids (18%). It also found that silver sulfadiazine was the most commonly prescribed treatment, “even though there are considerable data illustrating that its use will delay wound closure and healing (J Invest Dermatol. 2015 May;135[5]:1459-62),” Dr. Friedman said. He went on to note that for patients who suffer an acute burn, “the ability to access a dermatologist is somewhat limited because their schedules are heavily booked well in advance, and the format doesn’t allow for these types of emergencies. More often than not they go to the ED or to primary care. That might not necessarily be the right decision because these are physicians who may not have the necessary training in terms of not only proper burn care, but skin care overall.”
Another manuscript in the special section describes a method in which partial thickness wounds were induced by cryosurgery to create wounds that could facilitate wound healing research and development. For the study, researchers led by Dr. Robert Kirsner, interim chairman of the department of dermatology and cutaneous surgery at the University of Miami, used liquid nitrogen spray to induce freeze injuries on the forearms of eight healthy adult volunteers (J. Drugs Dermatol. 2015;14[7]: 734-8). They delivered the spray onto a target area of a 1-cm circular opening at a distance from the cryodevice to the skin of 0.5-1 cm and implemented several freeze-thaw time cycles by administering pulses that ranged from 3-12 seconds.
After a 24-hour follow-up, Dr. Kirsner and his associates observed that freeze times exceeding 5 seconds caused a majority of study participants to develop blisters, while freeze times exceeding 8 seconds caused uniform blister formation. Time to healing among subjects in the 8-second freeze time group was 12-13 days, while time to healing among those in the 12-second time freeze group was 21 days.
“Cryo-induced wound healing is a little bit slower than you’d expect with a scalpel, but that wasn’t really surprising,” Dr. Kirsner said. “The fact that it healed a little bit slower was a pretty good thing because if everything healed too fast then it couldn’t serve as a model to speed or slow epithelialization. We were quite pleased.” He noted that the model “could be used as a safety test for chronic wound treatment and as an efficacy test for acute wound treatment. It’s relatively inexpensive and a relatively simple technique. If you’re developing a product for widespread use, it’s probably a minor cost in the whole development process.”
Other manuscripts in the JDD special section include a preclinical study using a murine multithermal burn model which found that N-acetylcysteine S-nitrosothiol nanoparticles prevent wound expansion and accelerate burn closure, and a practical, systematic approach to using wound dressings for the wound care novice. Dr. Friedman hopes that the special section not only stimulates further interest in wound care, but that it serves as “a call for action. We really need to be more involved in wound care from the acute and chronic perspective,” he said. “Wound centers around the country should be involving dermatologists. We have so much to offer from bench to bedside because the skin is our thing. I hope this is a reminder that we should be part of this picture.”
Dr. Friedman disclosed that he serves as a consultant for Galderma, Biogen, Aveeno, Intraderm, Puracore, La Roche-Posay, Amgen, Pfizer, PHD Skin Care. He also serves as an advisory board member for Nerium International, Valeant, Nano BioMed, MicroCures, and Novartis, and has received research grants from Valeant. Dr. Ruiz and Dr. Kirsner reported no financial disclosures.
The way Dr. Adam Friedman sees it, dermatologists deserve a prominent place at the table when it comes to the treatment of acute and chronic wounds.
“As masters of the integument, we should be central to wound care, whether it be for research, in terms of developing better technologies, medications, approaches, diagnostics, but also in terms of managing these wounds, given the rich breadth of pathophysiology and biology we learn during our residency and maintain during our continuing education as practicing dermatologists,” said Dr. Friedman of the department of dermatology at George Washington University, Washington.
When the Journal of Drugs in Dermatology invited Dr. Friedman to serve as guest editor for a special feature section on wound care for its July 2015 issue, he jumped at the chance “to give the dermatology community a small taste of what’s going on in the wound healing world.”
Currently, he said, there is wide variability in the types of clinicians leading wound care centers in the United States, with dermatologists often sitting on the sidelines. “At one institution, it may be the vascular surgery service, at others it may be the family medicine service or even the emergency medicine department,” said Dr. Friedman, who is an editorial advisor to Dermatology News.
“That’s a big problem, in that there’s no uniformity from one center to the next in terms of who is expected to and should be taking responsibility for the wound healing service at their institutions. The reality is, it should be an interdisciplinary team, which not only involves dermatology but vascular surgery, nutrition, internal medicine, subspecialties of medicine like rheumatology, and rehab medicine. However, what is happening more often than not is that you’re getting just one or two of these elements, which cannot be as effective because you miss out on a broader, holistic view.”
There are two chief reasons why dermatologists aren’t more involved in wound care management, he continued. One stems from a lack of training on the topic. In one of the abstracts from the special JDD wound care section, researchers led by Dr. Emily Stamell Ruiz conducted an online survey of dermatology residents in the United States, to ask them about their preparedness to care for wounds and to assess the amount and type of training devoted to wound care during residency. Of the 175 respondents, 78% and 85% did not feel prepared to manage acute and chronic wounds, respectively, while 77% felt that more education is needed during their residency (J Drugs Dermatol. 2015;14[7]:716-20). “Residents felt that there was a clinical as well as a didactical gap, so they felt that they needed more training both through lectures as well as in clinics,” said Dr. Ruiz of the department of dermatology at Brigham and Women’s Hospital, Boston. “It’s not just a focal problem, it really is a universal curriculum problem. Future reforms to the current dermatology curriculum to include wound care training could help close the gap in wound care training.”
Another reason why dermatologists aren’t more involved in wound care management is the time commitment, said Dr. Friedman, who is also director of translational research at George Washington. The treatment of chronic wounds is “physically and financially burdensome,” he said. “It takes not only yourself being comfortable with managing the whole patient which includes the wound[s] with a side order of comorbidities, but your support staff as well – having nurses who know how to use the different wound dressings and how to help you with debridement. You need the right infrastructure. It also costs a lot on the provider side to manage wounds. You need a setup where you can get these patients in, have support staff to help with the wound dressings once you’ve identified what’s necessary, and be able to move on to the next patient.”
In another manuscript contained in the JDD special section, Dr. Friedman and his associates retrospectively reviewed the characteristics of 51 patients with burn injuries who were seen by seven different dermatologists at the Einstein-Montefiore division of dermatology from April 2010 to July 2014 (J Drugs Dermatol. 2015;14[7]:721-4). It found that the main mechanism of injury was burn from hot metal (22%), followed by contact with hot liquids (18%). It also found that silver sulfadiazine was the most commonly prescribed treatment, “even though there are considerable data illustrating that its use will delay wound closure and healing (J Invest Dermatol. 2015 May;135[5]:1459-62),” Dr. Friedman said. He went on to note that for patients who suffer an acute burn, “the ability to access a dermatologist is somewhat limited because their schedules are heavily booked well in advance, and the format doesn’t allow for these types of emergencies. More often than not they go to the ED or to primary care. That might not necessarily be the right decision because these are physicians who may not have the necessary training in terms of not only proper burn care, but skin care overall.”
Another manuscript in the special section describes a method in which partial thickness wounds were induced by cryosurgery to create wounds that could facilitate wound healing research and development. For the study, researchers led by Dr. Robert Kirsner, interim chairman of the department of dermatology and cutaneous surgery at the University of Miami, used liquid nitrogen spray to induce freeze injuries on the forearms of eight healthy adult volunteers (J. Drugs Dermatol. 2015;14[7]: 734-8). They delivered the spray onto a target area of a 1-cm circular opening at a distance from the cryodevice to the skin of 0.5-1 cm and implemented several freeze-thaw time cycles by administering pulses that ranged from 3-12 seconds.
After a 24-hour follow-up, Dr. Kirsner and his associates observed that freeze times exceeding 5 seconds caused a majority of study participants to develop blisters, while freeze times exceeding 8 seconds caused uniform blister formation. Time to healing among subjects in the 8-second freeze time group was 12-13 days, while time to healing among those in the 12-second time freeze group was 21 days.
“Cryo-induced wound healing is a little bit slower than you’d expect with a scalpel, but that wasn’t really surprising,” Dr. Kirsner said. “The fact that it healed a little bit slower was a pretty good thing because if everything healed too fast then it couldn’t serve as a model to speed or slow epithelialization. We were quite pleased.” He noted that the model “could be used as a safety test for chronic wound treatment and as an efficacy test for acute wound treatment. It’s relatively inexpensive and a relatively simple technique. If you’re developing a product for widespread use, it’s probably a minor cost in the whole development process.”
Other manuscripts in the JDD special section include a preclinical study using a murine multithermal burn model which found that N-acetylcysteine S-nitrosothiol nanoparticles prevent wound expansion and accelerate burn closure, and a practical, systematic approach to using wound dressings for the wound care novice. Dr. Friedman hopes that the special section not only stimulates further interest in wound care, but that it serves as “a call for action. We really need to be more involved in wound care from the acute and chronic perspective,” he said. “Wound centers around the country should be involving dermatologists. We have so much to offer from bench to bedside because the skin is our thing. I hope this is a reminder that we should be part of this picture.”
Dr. Friedman disclosed that he serves as a consultant for Galderma, Biogen, Aveeno, Intraderm, Puracore, La Roche-Posay, Amgen, Pfizer, PHD Skin Care. He also serves as an advisory board member for Nerium International, Valeant, Nano BioMed, MicroCures, and Novartis, and has received research grants from Valeant. Dr. Ruiz and Dr. Kirsner reported no financial disclosures.
How to Teach the Potassium Hydroxide Preparation: A Disappearing Clinical Art Form
Potassium hydroxide (KOH) preparations remain an important bedside test for prompt and accurate diagnosis of superficial fungal infections known as dermatophytoses. This tool has been used for at least 100 years, with early terminology referring to it as potash; for the last century, it has largely been a technique passed down as a skill from master technician to learning apprentice. The original pioneer of the KOH preparation remains a mystery.1
Variations on techniques for performing the KOH preparation exist, and tips and tricks on the use of this test are a hot topic among dermatologists.2 Although primary care and dermatology-specific publications espouse the importance of the KOH preparation,3,4 it has unfortunately been identified and labeled as one of the forgotten diagnostic tools.5
It is incumbent on dermatologists to educate medical students and residents using a simple and specific method to ensure that this simple and effective technique, with sensitivity reported between 87% and 91% depending on the expertise of the examiner,6 remains part of the clinical armamentarium. One concern in the instruction of large groups of students and clinicians is the ready accessibility or availability of viable skin samples. This article describes a method of collecting and storing skin samples that will allow educators to train large groups of students on performing KOH preparations without having to repeatedly seek skin samples or patients with superficial skin infections. A detailed description of the pedagogy used to teach the preparation and interpretation of KOH slides to a large group of students also is reviewed.
Specimen Collection
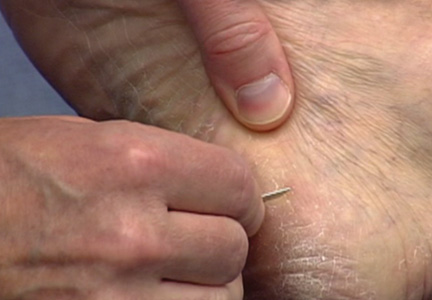
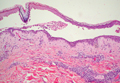
The first step in teaching the KOH preparation to a large group is the collection of a suitable number of skin scrapings from patients with a superficial fungal skin infection (eg, tinea corporis, tinea versicolor). A common technique for obtaining skin samples is to use a no. 15 scalpel blade (Figure 1) to scrape the scale of the lesion at its scaly border once the area is moistened with an alcohol pad or soap and water.7 The moisture from the alcohol pad allows the scale to stick to the no. 15 blade, facilitating collection. Once a suitable amount of scale is collected, it is placed on a glass microscope slide by smearing the scale from the blade onto the slide. This process has been modified to facilitate a larger quantity of specimen as follows: dermatophyte-infected plaques with scale are rubbed with the no. 15 blade and the free scale drops into a standard urine specimen cup. This process is repeated multiple times from different sites to capture the displaced scale with the dermatophyte. We have found that as long as the specimen cups are sealed tightly and stored in a relatively dry and cool environment (room temperature), the samples can be used to construct KOH teaching slides for at least 3 years. We have not used them beyond 3 years but suspect that they would continue to be viable after this time.
Preparation of Slides
Given that time for teaching often is limited, it is beneficial to fix many skin scrapings on a large number of glass slides prior to the session, which enables students to simply add KOH to the slides on the teaching day. To prepare the slides in advance, it is necessary to gather the following materials: a specimen cup with skin samples, glass slides, pickups or tweezers, a small pipette, a cup of water, protective gloves, and a pencil. After donning protective gloves, the pickups or tweezers are used to retrieve a few flakes of scale from the specimen cup and place them on the center of a glass slide. Using the pipette, 1 or 2 drops of water are added to the scale, and the slide is then allowed to dry. The slides are marked with the pencil to indicate the “up” side to prevent the students from applying KOH solution to the wrong side of the slide. The skin scale is fixed in place on the slide as the water evaporates and may be stored until needed for use in a standard slide box or folder.
Performing the KOH Preparation
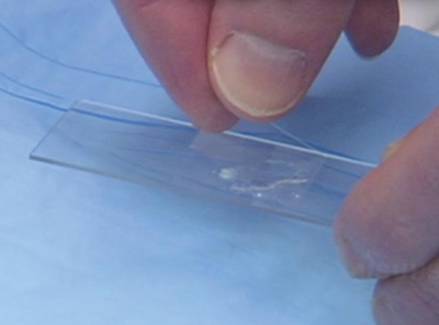
On the day of teaching, it is helpful to engage the entire group of students with an introductory lecture on the purpose and use of the KOH preparation. Upon completion, students move to a workstation with all of the materials needed to prepare the slide. Additional items needed at this time are 10% KOH solution, coverslips, and a heating device (eg, lighter, Bunsen burner, match)(optional). Students are instructed to place 1 or 2 skin scales onto a glass slide or retrieve a slide with skin scales already fixed, and then add 1 drop of 10% KOH solution directly to the sample (Figure 2). Next, they should place a slide coverslip onto the KOH drop and skin sample using a side-to-side technique that will move the scale into a thin layer within the KOH solution and push away any excess solution to the periphery (Figure 3). Large amounts of excess KOH solution should be cleared away with a paper towel, lens paper, or tissue. The heat source can be used to gently heat the underside of the glass slide (Figure 4), but it often is sufficient to simply wait 3 to 5 minutes for the KOH solution to take effect. The heat accelerates the maceration of the scale and makes it easier to see the hyphae among the keratinocytes. Some physicians advocate the use of dimethyl sulfoxide in lieu of heating,8 but this solution may not be available in all primary care settings.

| 
|
Microscopic Examination
Prior to examining the slides under the microscope, students may complete a self-guided tutorial (eg, digital or paper slide show) on the various features seen through the microscope that are indicative of dermatophytes, including branching hyphae and yeast buds. They also should be educated about the common appearance of artifacts that may resemble hyphae. Once the students have completed the tutorial, they may proceed to microscopic examination.
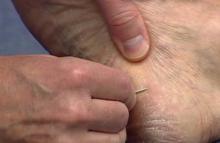
While the students are viewing their slides under the microscope, we find it helpful to have at least 1 experienced faculty member for every group of 10 students. This instructor should encourage the students to lower the microscope condenser all the way to facilitate better observation. Students should start with low power (×4 or red band) and scan for areas that are rich in skin scale. Once a collection of scale is found, the student can switch to higher power (×10 or yellow band) and start scanning for hyphae. Students should be reminded to search for filamentous and branching tubes that are refractile. The term refractile may be confusing to some students, so we explain that shifting the focus up or down will show the hyphae to change in brightness and may reveal a greenish tint. Another helpful indicator to point out is the feature that hyphae will cross the border of epidermal skin cells, whereas artifacts will not (Figure 5). Once the students have identified evidence of a dermatophyte infection, they must call the instructor to their station to verify the presence of hyphae or yeast buds, which helps confirm their understanding of the procedure. Once the student accurately identifies these items, the session is complete.
Comment
The use of a KOH preparation is a fast, simple, accurate, and cost-effective way to diagnose superficial fungal infections; however, because of insufficient familiarity with this tool, the technique often is replaced by initiation of empiric antifungal therapy in patients with suspected dermatophytosis. This empiric treatment has the potential to delay appropriate diagnosis and treatment (eg, in a patient with nummular dermatitis, which can clinically mimic tinea corporis). One way to encourage the use of the KOH preparation in the primary care and dermatologic setting is to educate large groups of next-generation physicians while in medical training. This article describes a teaching technique that allows for long-term storage of positive skin samples and a detailed description of the pedagogy used to train and educate a large group of students in a relatively short period of time.
All KOH preparations fall under the US federal government’s Clinical Laboratory Improvement Amendments and require proficiency testing.9 Although the teaching method presented here is designed for teaching medical students, it may be utilized to educate or refamiliarize experienced physicians with the procedure in an effort to improve proficiency in point-of-care testing programs used in many health care systems to comply with the Clinical Laboratories Improvement Amendments. Future analyses could assess whether the method described here improves provider performance on such proficiency measures and whether it ultimately helps ensure quality patient care.
1. Dasgupta T, Sahu J. Origins of the KOH technique. Clin Dermatol. 2012;2:238-242.
2. Stone S. Editor’s commentary. Clin Dermatol. 2012;2:241-242.
3. Monroe JR. The diagnostic value of a KOH. JAAPA. 2001;4:50-51.
4. Hainer BL. Dermatophyte infections. Am Fam Physician. 2003;1:101-109.
5. Ponka D, Baddar F. Microscopic potassium hydroxide preparation. Can Fam Physician. 2014;60:57.
6. Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;4:620-626.
7. Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. New York, NY: Elsevier Saunders; 2012.
8. James WD, Berger T, Elston D. Andrew’s Diseases of the Skin: Clinical Dermatology. 11th ed. New York, NY: Elsevier Saunders; 2011.
9. Clinical Laboratory Improvement Amendments (CLIA). Centers for Medicare & Medicaid Services Web site. https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/index.html?redirect=/clia/. Updated June 6, 2015. Accessed July 21, 2015.
Potassium hydroxide (KOH) preparations remain an important bedside test for prompt and accurate diagnosis of superficial fungal infections known as dermatophytoses. This tool has been used for at least 100 years, with early terminology referring to it as potash; for the last century, it has largely been a technique passed down as a skill from master technician to learning apprentice. The original pioneer of the KOH preparation remains a mystery.1
Variations on techniques for performing the KOH preparation exist, and tips and tricks on the use of this test are a hot topic among dermatologists.2 Although primary care and dermatology-specific publications espouse the importance of the KOH preparation,3,4 it has unfortunately been identified and labeled as one of the forgotten diagnostic tools.5
It is incumbent on dermatologists to educate medical students and residents using a simple and specific method to ensure that this simple and effective technique, with sensitivity reported between 87% and 91% depending on the expertise of the examiner,6 remains part of the clinical armamentarium. One concern in the instruction of large groups of students and clinicians is the ready accessibility or availability of viable skin samples. This article describes a method of collecting and storing skin samples that will allow educators to train large groups of students on performing KOH preparations without having to repeatedly seek skin samples or patients with superficial skin infections. A detailed description of the pedagogy used to teach the preparation and interpretation of KOH slides to a large group of students also is reviewed.
Specimen Collection
The first step in teaching the KOH preparation to a large group is the collection of a suitable number of skin scrapings from patients with a superficial fungal skin infection (eg, tinea corporis, tinea versicolor). A common technique for obtaining skin samples is to use a no. 15 scalpel blade (Figure 1) to scrape the scale of the lesion at its scaly border once the area is moistened with an alcohol pad or soap and water.7 The moisture from the alcohol pad allows the scale to stick to the no. 15 blade, facilitating collection. Once a suitable amount of scale is collected, it is placed on a glass microscope slide by smearing the scale from the blade onto the slide. This process has been modified to facilitate a larger quantity of specimen as follows: dermatophyte-infected plaques with scale are rubbed with the no. 15 blade and the free scale drops into a standard urine specimen cup. This process is repeated multiple times from different sites to capture the displaced scale with the dermatophyte. We have found that as long as the specimen cups are sealed tightly and stored in a relatively dry and cool environment (room temperature), the samples can be used to construct KOH teaching slides for at least 3 years. We have not used them beyond 3 years but suspect that they would continue to be viable after this time.
Preparation of Slides
Given that time for teaching often is limited, it is beneficial to fix many skin scrapings on a large number of glass slides prior to the session, which enables students to simply add KOH to the slides on the teaching day. To prepare the slides in advance, it is necessary to gather the following materials: a specimen cup with skin samples, glass slides, pickups or tweezers, a small pipette, a cup of water, protective gloves, and a pencil. After donning protective gloves, the pickups or tweezers are used to retrieve a few flakes of scale from the specimen cup and place them on the center of a glass slide. Using the pipette, 1 or 2 drops of water are added to the scale, and the slide is then allowed to dry. The slides are marked with the pencil to indicate the “up” side to prevent the students from applying KOH solution to the wrong side of the slide. The skin scale is fixed in place on the slide as the water evaporates and may be stored until needed for use in a standard slide box or folder.
Performing the KOH Preparation
On the day of teaching, it is helpful to engage the entire group of students with an introductory lecture on the purpose and use of the KOH preparation. Upon completion, students move to a workstation with all of the materials needed to prepare the slide. Additional items needed at this time are 10% KOH solution, coverslips, and a heating device (eg, lighter, Bunsen burner, match)(optional). Students are instructed to place 1 or 2 skin scales onto a glass slide or retrieve a slide with skin scales already fixed, and then add 1 drop of 10% KOH solution directly to the sample (Figure 2). Next, they should place a slide coverslip onto the KOH drop and skin sample using a side-to-side technique that will move the scale into a thin layer within the KOH solution and push away any excess solution to the periphery (Figure 3). Large amounts of excess KOH solution should be cleared away with a paper towel, lens paper, or tissue. The heat source can be used to gently heat the underside of the glass slide (Figure 4), but it often is sufficient to simply wait 3 to 5 minutes for the KOH solution to take effect. The heat accelerates the maceration of the scale and makes it easier to see the hyphae among the keratinocytes. Some physicians advocate the use of dimethyl sulfoxide in lieu of heating,8 but this solution may not be available in all primary care settings.

| 
|
Microscopic Examination
Prior to examining the slides under the microscope, students may complete a self-guided tutorial (eg, digital or paper slide show) on the various features seen through the microscope that are indicative of dermatophytes, including branching hyphae and yeast buds. They also should be educated about the common appearance of artifacts that may resemble hyphae. Once the students have completed the tutorial, they may proceed to microscopic examination.
While the students are viewing their slides under the microscope, we find it helpful to have at least 1 experienced faculty member for every group of 10 students. This instructor should encourage the students to lower the microscope condenser all the way to facilitate better observation. Students should start with low power (×4 or red band) and scan for areas that are rich in skin scale. Once a collection of scale is found, the student can switch to higher power (×10 or yellow band) and start scanning for hyphae. Students should be reminded to search for filamentous and branching tubes that are refractile. The term refractile may be confusing to some students, so we explain that shifting the focus up or down will show the hyphae to change in brightness and may reveal a greenish tint. Another helpful indicator to point out is the feature that hyphae will cross the border of epidermal skin cells, whereas artifacts will not (Figure 5). Once the students have identified evidence of a dermatophyte infection, they must call the instructor to their station to verify the presence of hyphae or yeast buds, which helps confirm their understanding of the procedure. Once the student accurately identifies these items, the session is complete.
Comment
The use of a KOH preparation is a fast, simple, accurate, and cost-effective way to diagnose superficial fungal infections; however, because of insufficient familiarity with this tool, the technique often is replaced by initiation of empiric antifungal therapy in patients with suspected dermatophytosis. This empiric treatment has the potential to delay appropriate diagnosis and treatment (eg, in a patient with nummular dermatitis, which can clinically mimic tinea corporis). One way to encourage the use of the KOH preparation in the primary care and dermatologic setting is to educate large groups of next-generation physicians while in medical training. This article describes a teaching technique that allows for long-term storage of positive skin samples and a detailed description of the pedagogy used to train and educate a large group of students in a relatively short period of time.
All KOH preparations fall under the US federal government’s Clinical Laboratory Improvement Amendments and require proficiency testing.9 Although the teaching method presented here is designed for teaching medical students, it may be utilized to educate or refamiliarize experienced physicians with the procedure in an effort to improve proficiency in point-of-care testing programs used in many health care systems to comply with the Clinical Laboratories Improvement Amendments. Future analyses could assess whether the method described here improves provider performance on such proficiency measures and whether it ultimately helps ensure quality patient care.
Potassium hydroxide (KOH) preparations remain an important bedside test for prompt and accurate diagnosis of superficial fungal infections known as dermatophytoses. This tool has been used for at least 100 years, with early terminology referring to it as potash; for the last century, it has largely been a technique passed down as a skill from master technician to learning apprentice. The original pioneer of the KOH preparation remains a mystery.1
Variations on techniques for performing the KOH preparation exist, and tips and tricks on the use of this test are a hot topic among dermatologists.2 Although primary care and dermatology-specific publications espouse the importance of the KOH preparation,3,4 it has unfortunately been identified and labeled as one of the forgotten diagnostic tools.5
It is incumbent on dermatologists to educate medical students and residents using a simple and specific method to ensure that this simple and effective technique, with sensitivity reported between 87% and 91% depending on the expertise of the examiner,6 remains part of the clinical armamentarium. One concern in the instruction of large groups of students and clinicians is the ready accessibility or availability of viable skin samples. This article describes a method of collecting and storing skin samples that will allow educators to train large groups of students on performing KOH preparations without having to repeatedly seek skin samples or patients with superficial skin infections. A detailed description of the pedagogy used to teach the preparation and interpretation of KOH slides to a large group of students also is reviewed.
Specimen Collection
The first step in teaching the KOH preparation to a large group is the collection of a suitable number of skin scrapings from patients with a superficial fungal skin infection (eg, tinea corporis, tinea versicolor). A common technique for obtaining skin samples is to use a no. 15 scalpel blade (Figure 1) to scrape the scale of the lesion at its scaly border once the area is moistened with an alcohol pad or soap and water.7 The moisture from the alcohol pad allows the scale to stick to the no. 15 blade, facilitating collection. Once a suitable amount of scale is collected, it is placed on a glass microscope slide by smearing the scale from the blade onto the slide. This process has been modified to facilitate a larger quantity of specimen as follows: dermatophyte-infected plaques with scale are rubbed with the no. 15 blade and the free scale drops into a standard urine specimen cup. This process is repeated multiple times from different sites to capture the displaced scale with the dermatophyte. We have found that as long as the specimen cups are sealed tightly and stored in a relatively dry and cool environment (room temperature), the samples can be used to construct KOH teaching slides for at least 3 years. We have not used them beyond 3 years but suspect that they would continue to be viable after this time.
Preparation of Slides
Given that time for teaching often is limited, it is beneficial to fix many skin scrapings on a large number of glass slides prior to the session, which enables students to simply add KOH to the slides on the teaching day. To prepare the slides in advance, it is necessary to gather the following materials: a specimen cup with skin samples, glass slides, pickups or tweezers, a small pipette, a cup of water, protective gloves, and a pencil. After donning protective gloves, the pickups or tweezers are used to retrieve a few flakes of scale from the specimen cup and place them on the center of a glass slide. Using the pipette, 1 or 2 drops of water are added to the scale, and the slide is then allowed to dry. The slides are marked with the pencil to indicate the “up” side to prevent the students from applying KOH solution to the wrong side of the slide. The skin scale is fixed in place on the slide as the water evaporates and may be stored until needed for use in a standard slide box or folder.
Performing the KOH Preparation
On the day of teaching, it is helpful to engage the entire group of students with an introductory lecture on the purpose and use of the KOH preparation. Upon completion, students move to a workstation with all of the materials needed to prepare the slide. Additional items needed at this time are 10% KOH solution, coverslips, and a heating device (eg, lighter, Bunsen burner, match)(optional). Students are instructed to place 1 or 2 skin scales onto a glass slide or retrieve a slide with skin scales already fixed, and then add 1 drop of 10% KOH solution directly to the sample (Figure 2). Next, they should place a slide coverslip onto the KOH drop and skin sample using a side-to-side technique that will move the scale into a thin layer within the KOH solution and push away any excess solution to the periphery (Figure 3). Large amounts of excess KOH solution should be cleared away with a paper towel, lens paper, or tissue. The heat source can be used to gently heat the underside of the glass slide (Figure 4), but it often is sufficient to simply wait 3 to 5 minutes for the KOH solution to take effect. The heat accelerates the maceration of the scale and makes it easier to see the hyphae among the keratinocytes. Some physicians advocate the use of dimethyl sulfoxide in lieu of heating,8 but this solution may not be available in all primary care settings.

| 
|
Microscopic Examination
Prior to examining the slides under the microscope, students may complete a self-guided tutorial (eg, digital or paper slide show) on the various features seen through the microscope that are indicative of dermatophytes, including branching hyphae and yeast buds. They also should be educated about the common appearance of artifacts that may resemble hyphae. Once the students have completed the tutorial, they may proceed to microscopic examination.
While the students are viewing their slides under the microscope, we find it helpful to have at least 1 experienced faculty member for every group of 10 students. This instructor should encourage the students to lower the microscope condenser all the way to facilitate better observation. Students should start with low power (×4 or red band) and scan for areas that are rich in skin scale. Once a collection of scale is found, the student can switch to higher power (×10 or yellow band) and start scanning for hyphae. Students should be reminded to search for filamentous and branching tubes that are refractile. The term refractile may be confusing to some students, so we explain that shifting the focus up or down will show the hyphae to change in brightness and may reveal a greenish tint. Another helpful indicator to point out is the feature that hyphae will cross the border of epidermal skin cells, whereas artifacts will not (Figure 5). Once the students have identified evidence of a dermatophyte infection, they must call the instructor to their station to verify the presence of hyphae or yeast buds, which helps confirm their understanding of the procedure. Once the student accurately identifies these items, the session is complete.
Comment
The use of a KOH preparation is a fast, simple, accurate, and cost-effective way to diagnose superficial fungal infections; however, because of insufficient familiarity with this tool, the technique often is replaced by initiation of empiric antifungal therapy in patients with suspected dermatophytosis. This empiric treatment has the potential to delay appropriate diagnosis and treatment (eg, in a patient with nummular dermatitis, which can clinically mimic tinea corporis). One way to encourage the use of the KOH preparation in the primary care and dermatologic setting is to educate large groups of next-generation physicians while in medical training. This article describes a teaching technique that allows for long-term storage of positive skin samples and a detailed description of the pedagogy used to train and educate a large group of students in a relatively short period of time.
All KOH preparations fall under the US federal government’s Clinical Laboratory Improvement Amendments and require proficiency testing.9 Although the teaching method presented here is designed for teaching medical students, it may be utilized to educate or refamiliarize experienced physicians with the procedure in an effort to improve proficiency in point-of-care testing programs used in many health care systems to comply with the Clinical Laboratories Improvement Amendments. Future analyses could assess whether the method described here improves provider performance on such proficiency measures and whether it ultimately helps ensure quality patient care.
1. Dasgupta T, Sahu J. Origins of the KOH technique. Clin Dermatol. 2012;2:238-242.
2. Stone S. Editor’s commentary. Clin Dermatol. 2012;2:241-242.
3. Monroe JR. The diagnostic value of a KOH. JAAPA. 2001;4:50-51.
4. Hainer BL. Dermatophyte infections. Am Fam Physician. 2003;1:101-109.
5. Ponka D, Baddar F. Microscopic potassium hydroxide preparation. Can Fam Physician. 2014;60:57.
6. Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;4:620-626.
7. Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. New York, NY: Elsevier Saunders; 2012.
8. James WD, Berger T, Elston D. Andrew’s Diseases of the Skin: Clinical Dermatology. 11th ed. New York, NY: Elsevier Saunders; 2011.
9. Clinical Laboratory Improvement Amendments (CLIA). Centers for Medicare & Medicaid Services Web site. https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/index.html?redirect=/clia/. Updated June 6, 2015. Accessed July 21, 2015.
1. Dasgupta T, Sahu J. Origins of the KOH technique. Clin Dermatol. 2012;2:238-242.
2. Stone S. Editor’s commentary. Clin Dermatol. 2012;2:241-242.
3. Monroe JR. The diagnostic value of a KOH. JAAPA. 2001;4:50-51.
4. Hainer BL. Dermatophyte infections. Am Fam Physician. 2003;1:101-109.
5. Ponka D, Baddar F. Microscopic potassium hydroxide preparation. Can Fam Physician. 2014;60:57.
6. Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;4:620-626.
7. Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. New York, NY: Elsevier Saunders; 2012.
8. James WD, Berger T, Elston D. Andrew’s Diseases of the Skin: Clinical Dermatology. 11th ed. New York, NY: Elsevier Saunders; 2011.
9. Clinical Laboratory Improvement Amendments (CLIA). Centers for Medicare & Medicaid Services Web site. https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/index.html?redirect=/clia/. Updated June 6, 2015. Accessed July 21, 2015.
Practice Points
- Potassium hydroxide (KOH) preparations can lead to diagnostic confidence and direct appropriate therapy.
- Refreshing the basics of this simple technique can lead to better patient outcomes in the primary care setting and in the dermatology specialty clinic.
- Teaching the KOH preparation to the next generation of physicians will ensure its longevity and assure future benefit to patients.
Yoga for Dermatologic Conditions
Regardless of its spiritual origins, yoga has become a popular way of reaching mind and body well-being with nearly 30 million people practicing regularly worldwide.1 Yoga, which is the combination of physical postures, controlled breathing, and meditation or mindfulness, has long been used in complementary and alternative medicine around the world and recently has gained popularity as a therapeutic practice, with nearly 14 million Americans reporting that yoga was recommended to them by a physician or therapist.2,3 Studies suggest that people who participate in even brief yoga programs may see improvements in anxiety, somatic stress and discomfort, health-related quality of life, and self-rated sleep quality, all benefits that can help medical conditions, especially those that are dermatologic in nature.4,5
Stress and Dermatologic Conditions
The interaction between the mind, skin, and body is well known. Research in psychoneuroimmunology, the interaction between psychological processes and the nervous and immune systems, has examined the role of neuropeptides, hormones, and neurotransmitters in psychodermatological disorders. The correlation between neuroimmunological pathways and skin inflammation is now well recognized, specifically the interactions between the brain and skin underlying many dermatological diseases (eg, acne, alopecia areata, various types of eczema and dermatitis, oral and genital herpes, hyperhidrosis, pruritus, psoriasis, rosacea, urticaria, warts, breaking or ridging of the nails).6-9
Two biological systems are known to be affected by the systemic stress response: (1) the hypothalamic-pituitary-adrenal axis, which regulates the release of adrenocorticotropin, ß-endorphin, and cortisol, and (2) the sympathoadrenal medullary system, which regulates the release of catecholamines (eg, epinephrine, norepinephrine).7 Cortisol and catecholamines have been shown to have potent effects on the immune system as well as the inflammatory response.9 Additionally, it has been shown that cutaneous sensory nerve terminals release neuropeptides, including calcitonin gene-related peptide and substance P, both of which have different effects on the local inflammatory response.10,11
Psychological stress is well known to trigger many dermatologic conditions, but it also may lead to abnormal skin barrier function.12 The mechanism in which skin barrier function is affected appears to involve a stress-induced increase of endogenous glucocorticoids, which may consequently disrupt skin barrier function and recovery rates, stratum corneum cohesion, and epidermal antimicrobial function.13,14
Atopic dermatitis, for example, is classified as a psychophysiological disorder. Although it is not caused by stress, atopic dermatitis has been described to be precipitated or exacerbated by stress in patients.15 In fact, it was found that stressful life events preceded the onset of itching in more than 70% of patients with atopic dermatitis,16 which is especially relevant, as there is no cure and patients often experience a lifelong struggle with the condition. Additionally, stress mediates the degranulation of mast cells via corticotropin-releasing hormone and neuropeptides, and the upregulation of mast cell corticotropin-releasing hormone receptors supporting its putative role in the pathogenesis of urticaria.9,17 Furthermore, the increase in cortisol also has been described in the exacerbation of acne during times of stress.18
Psychological factors affect the management of skin conditions in more than one-third of reported dermatology patients; therefore, it is important to consider these factors in the treatment of chronic dermatological conditions, especially when they are inquired by the patient.19,20
Yoga Benefits in the Literature
The therapeutic potential of yoga has been explored in a growing number of randomized controlled trials to date.21 A recently published bibliometric analysis provided a comprehensive review of the characteristics of the randomized yoga trials available in the literature.22 The review included 366 full-text articles, with the 2 earliest studies published in 1975 and nearly 90% published within the last decade. In addition to healthy patients, it was found these randomized controlled yoga trials most commonly enrolled patients with breast cancer, depression, asthma, and type 2 diabetes mellitus.22 Another study examined psychological (eg, self-rated stress and stress behavior, anger, exhaustion, quality of life) and physiological (eg, blood pressure, heart rate, urinary catecholamines, salivary cortisol) measurements obtained before and after a 10-session yoga program that participants completed over a 4-month period, with results showing significant improvements (P<.05) on almost all stress-related subjective and physiological variables. Results were comparable with cognitive behavioral therapy.23
Not only has it been shown that yoga helps patients on a psychological level, but a recent study reported that 90-minute sessions of mindfulness meditation and gentle Hatha yoga over an 8-week period led to observable benefits on a cellular level, as telomere length was maintained in distressed breast cancer survivors compared to decreases in telomere length in the control group with patients who solely participated in a stress management seminar.24 To date, there are no known studies examining the effects of yoga on patients with skin cancer. However, a few studies have specifically examined the effect of yoga in managing non–cancer-related dermatologic issues. Specifically, one small study of psoriasis patients found that those who listened to mindfulness meditation tapes while undergoing standard phototherapy (psoralen plus UVA) healed faster than those who underwent phototherapy treatment alone.25
Because some dermatologic problems have comorbidities and increased risk factors of other medical problems, such as psoriasis with psoriatic arthritis and metabolic diseases (eg, abdominal obesity, diabetes, nonalcoholic fatty liver disease, dyslipidemia, metabolic syndrome, chronic kidney disease), it is even more pertinent to recommend approaches for healthy mind and body well-being as a supplement to medical care.26
Final Thoughts
With accurate diagnosis by a dermatologist, appropriate conventional treatments can improve dermatologic problems. These treatments alone can reduce patients’ stress and improve skin, hair, and nail conditions; however, if it is clear that stress is interfering with a patient’s overall well-being and ability to cope with his/her dermatologic condition, concurrent stress management interventions may be warranted. In some instances, recommending yoga sessions, mindful meditation, or breathing exercises may help, while in others referral to a mental health professional may be necessary.
Beyond the direct physiological effects of stress, it also is worth mentioning that patients who deal with stress also tend to scratch, pick, or irritate their skin more and often lack the motivation to adhere to skin care regimens or treatments, again supporting the idea that our approach in managing these patients must be multifaceted. As dermatologists in training, residents should be cognizant of the potential psychological sequelae of some dermatologic problems and be aware of the possible use of supplemental interventions by our patients.
1. Dangerfield A. Yoga wars. BBC News. http://news.bbc.co.uk/1/hi/7844691.stm. Published January 23, 2009. Accessed March 25, 2015.
2. Yoga Journal releases 2012 yoga in America market study [press release]. San Francisco, CA: Yoga Journal; December 6, 2012.
3. De Michaelis E. A History of Modern Yoga: Patanjali and Western Esotericism. London, United Kingdom: A&C Black; 2005.
4. Telles S, Singh N, Yadav A, et al. Effect of yoga on different aspects of mental health. Indian J Physiol Pharmacol. 2012;56:245-254.
5. Rodriguez-Vallecillo E, Woodbury-Fariña MA. Dermatological manifestations of stress in normal and psychiatric populations. Psychiatr Clin North Am. 2014;37:625-651.
6. Stander S, Raap U, Weisshaar E, et al. Pathogenesis of pruritus. J Dtsch Dermatol Ges. 2011;9:456-463.
7. Arck PC, Slominski A, Theoharides TC, et al. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 2006;126:1697-1704.
8. Recognizing the mind-skin connection. Harvard Health Publications Web site. http://www.health.harvard.edu/newsletter_article/Recognizing_the_mind-skin_connection. Published November 1, 2006. Accessed March 31, 2015.
9. Tausk F, Elenkov I, Moynihan J. Psychoneuroimmunology. Dermatol Ther. 2008;21:22-31.
10. Pavlovic S, Liezmann C, Blois SM, et al. Substance P is a key mediator of stress-induced protection from allergic sensitization via modified antigen presentation. J Immunol. 2011;186:848-855.
11. Toyoda M, Nakamura M, Makino T, et al. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br J Dermatol. 2002;147:71-79.
12. Koo JYM, Lee CS. General approach to evaluating psychodermatological disorders. In: Koo JYM, Lee CS, eds. Psychocutaneous Medicine. New York, NY: Marcel Dekker; 2003:1-29.
13. Garg A, Chren MM, Sands LP, et al. Psychological stress perturbs epidermal permeability barrier homeostasis: implications for the pathogenesis of stress-associated skin disorders. Arch Dermatol. 2001;137:53-59.
14. Elias PM, Sun R, Eder AR, et al. Treating atopic dermatitis at the source: corrective barrier repair therapy based upon new pathogenic insights. Expert Rev Dermatol. 2013;8:27-36.
15. Morren MA, Przybilla B, Bamelis M, et al. Atopic dermatitis: triggering factors. J Am Acad Dermatol. 1994;31:467-473.
16. Faulstich ME, Williamson DA. An overview of atopic dermatitis: toward a bio-behavioural integration. J Psychosom Res. 1985;29:647-654.
17. Theoharides TC, Donelan JM, Papadopoulou N, et al. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563-568.
18. Suh DH, Kwon HH. What’s new in the physiopathology of acne [published online ahead of print Jan 24, 2015]? Br J Dermatol. doi:10.1111/bjd.13634.
19. Picardi A, Mazzotti E, Pasquini P. Prevalence and correlates of suicidal ideation among patients with skin disease. J Am Acad Dermatol. 2006;54:420-426.
20. Ponarovsky B, Amital D, Lazarov A, et al. Anxiety and depression in patients with allergic and non-allergic cutaneous disorders. Int J Dermatol. 2011;50:1217-1222.
21. Khalsa SB. Yoga as a therapeutic intervention: a bibliometric analysis of published research studies. Indian J Physiol Pharmacol. 2004;48:269-285.
22. Cramer H, Lauche R, Dobos G. Characteristics of randomized controlled trials of yoga: a bibliometric analysis. BMC Complement Altern Med. 2014;14:328.
23. Granath J, Ingvarsson S, von Thiele U, et al. Stress management: a randomized study of cognitive behavioural therapy and yoga. Cogn Behav Ther. 2006;35:3-10.
24. Carlson LE, Beattie TL, Giese-Davis J, et al. Mindfulness-based cancer recovery and supportive-expressive therapy maintain telomere length relative to controls in distressed breast cancer survivors. Cancer. 2015;121:476-484.
25. Kabat-Zinn J, Wheeler E, Light T, et al. Influence of a mindfulness meditation-based stress reduction intervention on rates of skin clearing in patients with moderate to severe psoriasis undergoing phototherapy (UVB) and photochemotherapy (PUVA). Psychosom Med. 1998;60:625-632.
26. Gisondi P, Galvan A, Idolazzi L, et al. Management of moderate to severe psoriasis in patients with metabolic comorbidities. Front Med (Lausanne). 2015;2:1.
Regardless of its spiritual origins, yoga has become a popular way of reaching mind and body well-being with nearly 30 million people practicing regularly worldwide.1 Yoga, which is the combination of physical postures, controlled breathing, and meditation or mindfulness, has long been used in complementary and alternative medicine around the world and recently has gained popularity as a therapeutic practice, with nearly 14 million Americans reporting that yoga was recommended to them by a physician or therapist.2,3 Studies suggest that people who participate in even brief yoga programs may see improvements in anxiety, somatic stress and discomfort, health-related quality of life, and self-rated sleep quality, all benefits that can help medical conditions, especially those that are dermatologic in nature.4,5
Stress and Dermatologic Conditions
The interaction between the mind, skin, and body is well known. Research in psychoneuroimmunology, the interaction between psychological processes and the nervous and immune systems, has examined the role of neuropeptides, hormones, and neurotransmitters in psychodermatological disorders. The correlation between neuroimmunological pathways and skin inflammation is now well recognized, specifically the interactions between the brain and skin underlying many dermatological diseases (eg, acne, alopecia areata, various types of eczema and dermatitis, oral and genital herpes, hyperhidrosis, pruritus, psoriasis, rosacea, urticaria, warts, breaking or ridging of the nails).6-9
Two biological systems are known to be affected by the systemic stress response: (1) the hypothalamic-pituitary-adrenal axis, which regulates the release of adrenocorticotropin, ß-endorphin, and cortisol, and (2) the sympathoadrenal medullary system, which regulates the release of catecholamines (eg, epinephrine, norepinephrine).7 Cortisol and catecholamines have been shown to have potent effects on the immune system as well as the inflammatory response.9 Additionally, it has been shown that cutaneous sensory nerve terminals release neuropeptides, including calcitonin gene-related peptide and substance P, both of which have different effects on the local inflammatory response.10,11
Psychological stress is well known to trigger many dermatologic conditions, but it also may lead to abnormal skin barrier function.12 The mechanism in which skin barrier function is affected appears to involve a stress-induced increase of endogenous glucocorticoids, which may consequently disrupt skin barrier function and recovery rates, stratum corneum cohesion, and epidermal antimicrobial function.13,14
Atopic dermatitis, for example, is classified as a psychophysiological disorder. Although it is not caused by stress, atopic dermatitis has been described to be precipitated or exacerbated by stress in patients.15 In fact, it was found that stressful life events preceded the onset of itching in more than 70% of patients with atopic dermatitis,16 which is especially relevant, as there is no cure and patients often experience a lifelong struggle with the condition. Additionally, stress mediates the degranulation of mast cells via corticotropin-releasing hormone and neuropeptides, and the upregulation of mast cell corticotropin-releasing hormone receptors supporting its putative role in the pathogenesis of urticaria.9,17 Furthermore, the increase in cortisol also has been described in the exacerbation of acne during times of stress.18
Psychological factors affect the management of skin conditions in more than one-third of reported dermatology patients; therefore, it is important to consider these factors in the treatment of chronic dermatological conditions, especially when they are inquired by the patient.19,20
Yoga Benefits in the Literature
The therapeutic potential of yoga has been explored in a growing number of randomized controlled trials to date.21 A recently published bibliometric analysis provided a comprehensive review of the characteristics of the randomized yoga trials available in the literature.22 The review included 366 full-text articles, with the 2 earliest studies published in 1975 and nearly 90% published within the last decade. In addition to healthy patients, it was found these randomized controlled yoga trials most commonly enrolled patients with breast cancer, depression, asthma, and type 2 diabetes mellitus.22 Another study examined psychological (eg, self-rated stress and stress behavior, anger, exhaustion, quality of life) and physiological (eg, blood pressure, heart rate, urinary catecholamines, salivary cortisol) measurements obtained before and after a 10-session yoga program that participants completed over a 4-month period, with results showing significant improvements (P<.05) on almost all stress-related subjective and physiological variables. Results were comparable with cognitive behavioral therapy.23
Not only has it been shown that yoga helps patients on a psychological level, but a recent study reported that 90-minute sessions of mindfulness meditation and gentle Hatha yoga over an 8-week period led to observable benefits on a cellular level, as telomere length was maintained in distressed breast cancer survivors compared to decreases in telomere length in the control group with patients who solely participated in a stress management seminar.24 To date, there are no known studies examining the effects of yoga on patients with skin cancer. However, a few studies have specifically examined the effect of yoga in managing non–cancer-related dermatologic issues. Specifically, one small study of psoriasis patients found that those who listened to mindfulness meditation tapes while undergoing standard phototherapy (psoralen plus UVA) healed faster than those who underwent phototherapy treatment alone.25
Because some dermatologic problems have comorbidities and increased risk factors of other medical problems, such as psoriasis with psoriatic arthritis and metabolic diseases (eg, abdominal obesity, diabetes, nonalcoholic fatty liver disease, dyslipidemia, metabolic syndrome, chronic kidney disease), it is even more pertinent to recommend approaches for healthy mind and body well-being as a supplement to medical care.26
Final Thoughts
With accurate diagnosis by a dermatologist, appropriate conventional treatments can improve dermatologic problems. These treatments alone can reduce patients’ stress and improve skin, hair, and nail conditions; however, if it is clear that stress is interfering with a patient’s overall well-being and ability to cope with his/her dermatologic condition, concurrent stress management interventions may be warranted. In some instances, recommending yoga sessions, mindful meditation, or breathing exercises may help, while in others referral to a mental health professional may be necessary.
Beyond the direct physiological effects of stress, it also is worth mentioning that patients who deal with stress also tend to scratch, pick, or irritate their skin more and often lack the motivation to adhere to skin care regimens or treatments, again supporting the idea that our approach in managing these patients must be multifaceted. As dermatologists in training, residents should be cognizant of the potential psychological sequelae of some dermatologic problems and be aware of the possible use of supplemental interventions by our patients.
Regardless of its spiritual origins, yoga has become a popular way of reaching mind and body well-being with nearly 30 million people practicing regularly worldwide.1 Yoga, which is the combination of physical postures, controlled breathing, and meditation or mindfulness, has long been used in complementary and alternative medicine around the world and recently has gained popularity as a therapeutic practice, with nearly 14 million Americans reporting that yoga was recommended to them by a physician or therapist.2,3 Studies suggest that people who participate in even brief yoga programs may see improvements in anxiety, somatic stress and discomfort, health-related quality of life, and self-rated sleep quality, all benefits that can help medical conditions, especially those that are dermatologic in nature.4,5
Stress and Dermatologic Conditions
The interaction between the mind, skin, and body is well known. Research in psychoneuroimmunology, the interaction between psychological processes and the nervous and immune systems, has examined the role of neuropeptides, hormones, and neurotransmitters in psychodermatological disorders. The correlation between neuroimmunological pathways and skin inflammation is now well recognized, specifically the interactions between the brain and skin underlying many dermatological diseases (eg, acne, alopecia areata, various types of eczema and dermatitis, oral and genital herpes, hyperhidrosis, pruritus, psoriasis, rosacea, urticaria, warts, breaking or ridging of the nails).6-9
Two biological systems are known to be affected by the systemic stress response: (1) the hypothalamic-pituitary-adrenal axis, which regulates the release of adrenocorticotropin, ß-endorphin, and cortisol, and (2) the sympathoadrenal medullary system, which regulates the release of catecholamines (eg, epinephrine, norepinephrine).7 Cortisol and catecholamines have been shown to have potent effects on the immune system as well as the inflammatory response.9 Additionally, it has been shown that cutaneous sensory nerve terminals release neuropeptides, including calcitonin gene-related peptide and substance P, both of which have different effects on the local inflammatory response.10,11
Psychological stress is well known to trigger many dermatologic conditions, but it also may lead to abnormal skin barrier function.12 The mechanism in which skin barrier function is affected appears to involve a stress-induced increase of endogenous glucocorticoids, which may consequently disrupt skin barrier function and recovery rates, stratum corneum cohesion, and epidermal antimicrobial function.13,14
Atopic dermatitis, for example, is classified as a psychophysiological disorder. Although it is not caused by stress, atopic dermatitis has been described to be precipitated or exacerbated by stress in patients.15 In fact, it was found that stressful life events preceded the onset of itching in more than 70% of patients with atopic dermatitis,16 which is especially relevant, as there is no cure and patients often experience a lifelong struggle with the condition. Additionally, stress mediates the degranulation of mast cells via corticotropin-releasing hormone and neuropeptides, and the upregulation of mast cell corticotropin-releasing hormone receptors supporting its putative role in the pathogenesis of urticaria.9,17 Furthermore, the increase in cortisol also has been described in the exacerbation of acne during times of stress.18
Psychological factors affect the management of skin conditions in more than one-third of reported dermatology patients; therefore, it is important to consider these factors in the treatment of chronic dermatological conditions, especially when they are inquired by the patient.19,20
Yoga Benefits in the Literature
The therapeutic potential of yoga has been explored in a growing number of randomized controlled trials to date.21 A recently published bibliometric analysis provided a comprehensive review of the characteristics of the randomized yoga trials available in the literature.22 The review included 366 full-text articles, with the 2 earliest studies published in 1975 and nearly 90% published within the last decade. In addition to healthy patients, it was found these randomized controlled yoga trials most commonly enrolled patients with breast cancer, depression, asthma, and type 2 diabetes mellitus.22 Another study examined psychological (eg, self-rated stress and stress behavior, anger, exhaustion, quality of life) and physiological (eg, blood pressure, heart rate, urinary catecholamines, salivary cortisol) measurements obtained before and after a 10-session yoga program that participants completed over a 4-month period, with results showing significant improvements (P<.05) on almost all stress-related subjective and physiological variables. Results were comparable with cognitive behavioral therapy.23
Not only has it been shown that yoga helps patients on a psychological level, but a recent study reported that 90-minute sessions of mindfulness meditation and gentle Hatha yoga over an 8-week period led to observable benefits on a cellular level, as telomere length was maintained in distressed breast cancer survivors compared to decreases in telomere length in the control group with patients who solely participated in a stress management seminar.24 To date, there are no known studies examining the effects of yoga on patients with skin cancer. However, a few studies have specifically examined the effect of yoga in managing non–cancer-related dermatologic issues. Specifically, one small study of psoriasis patients found that those who listened to mindfulness meditation tapes while undergoing standard phototherapy (psoralen plus UVA) healed faster than those who underwent phototherapy treatment alone.25
Because some dermatologic problems have comorbidities and increased risk factors of other medical problems, such as psoriasis with psoriatic arthritis and metabolic diseases (eg, abdominal obesity, diabetes, nonalcoholic fatty liver disease, dyslipidemia, metabolic syndrome, chronic kidney disease), it is even more pertinent to recommend approaches for healthy mind and body well-being as a supplement to medical care.26
Final Thoughts
With accurate diagnosis by a dermatologist, appropriate conventional treatments can improve dermatologic problems. These treatments alone can reduce patients’ stress and improve skin, hair, and nail conditions; however, if it is clear that stress is interfering with a patient’s overall well-being and ability to cope with his/her dermatologic condition, concurrent stress management interventions may be warranted. In some instances, recommending yoga sessions, mindful meditation, or breathing exercises may help, while in others referral to a mental health professional may be necessary.
Beyond the direct physiological effects of stress, it also is worth mentioning that patients who deal with stress also tend to scratch, pick, or irritate their skin more and often lack the motivation to adhere to skin care regimens or treatments, again supporting the idea that our approach in managing these patients must be multifaceted. As dermatologists in training, residents should be cognizant of the potential psychological sequelae of some dermatologic problems and be aware of the possible use of supplemental interventions by our patients.
1. Dangerfield A. Yoga wars. BBC News. http://news.bbc.co.uk/1/hi/7844691.stm. Published January 23, 2009. Accessed March 25, 2015.
2. Yoga Journal releases 2012 yoga in America market study [press release]. San Francisco, CA: Yoga Journal; December 6, 2012.
3. De Michaelis E. A History of Modern Yoga: Patanjali and Western Esotericism. London, United Kingdom: A&C Black; 2005.
4. Telles S, Singh N, Yadav A, et al. Effect of yoga on different aspects of mental health. Indian J Physiol Pharmacol. 2012;56:245-254.
5. Rodriguez-Vallecillo E, Woodbury-Fariña MA. Dermatological manifestations of stress in normal and psychiatric populations. Psychiatr Clin North Am. 2014;37:625-651.
6. Stander S, Raap U, Weisshaar E, et al. Pathogenesis of pruritus. J Dtsch Dermatol Ges. 2011;9:456-463.
7. Arck PC, Slominski A, Theoharides TC, et al. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 2006;126:1697-1704.
8. Recognizing the mind-skin connection. Harvard Health Publications Web site. http://www.health.harvard.edu/newsletter_article/Recognizing_the_mind-skin_connection. Published November 1, 2006. Accessed March 31, 2015.
9. Tausk F, Elenkov I, Moynihan J. Psychoneuroimmunology. Dermatol Ther. 2008;21:22-31.
10. Pavlovic S, Liezmann C, Blois SM, et al. Substance P is a key mediator of stress-induced protection from allergic sensitization via modified antigen presentation. J Immunol. 2011;186:848-855.
11. Toyoda M, Nakamura M, Makino T, et al. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br J Dermatol. 2002;147:71-79.
12. Koo JYM, Lee CS. General approach to evaluating psychodermatological disorders. In: Koo JYM, Lee CS, eds. Psychocutaneous Medicine. New York, NY: Marcel Dekker; 2003:1-29.
13. Garg A, Chren MM, Sands LP, et al. Psychological stress perturbs epidermal permeability barrier homeostasis: implications for the pathogenesis of stress-associated skin disorders. Arch Dermatol. 2001;137:53-59.
14. Elias PM, Sun R, Eder AR, et al. Treating atopic dermatitis at the source: corrective barrier repair therapy based upon new pathogenic insights. Expert Rev Dermatol. 2013;8:27-36.
15. Morren MA, Przybilla B, Bamelis M, et al. Atopic dermatitis: triggering factors. J Am Acad Dermatol. 1994;31:467-473.
16. Faulstich ME, Williamson DA. An overview of atopic dermatitis: toward a bio-behavioural integration. J Psychosom Res. 1985;29:647-654.
17. Theoharides TC, Donelan JM, Papadopoulou N, et al. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563-568.
18. Suh DH, Kwon HH. What’s new in the physiopathology of acne [published online ahead of print Jan 24, 2015]? Br J Dermatol. doi:10.1111/bjd.13634.
19. Picardi A, Mazzotti E, Pasquini P. Prevalence and correlates of suicidal ideation among patients with skin disease. J Am Acad Dermatol. 2006;54:420-426.
20. Ponarovsky B, Amital D, Lazarov A, et al. Anxiety and depression in patients with allergic and non-allergic cutaneous disorders. Int J Dermatol. 2011;50:1217-1222.
21. Khalsa SB. Yoga as a therapeutic intervention: a bibliometric analysis of published research studies. Indian J Physiol Pharmacol. 2004;48:269-285.
22. Cramer H, Lauche R, Dobos G. Characteristics of randomized controlled trials of yoga: a bibliometric analysis. BMC Complement Altern Med. 2014;14:328.
23. Granath J, Ingvarsson S, von Thiele U, et al. Stress management: a randomized study of cognitive behavioural therapy and yoga. Cogn Behav Ther. 2006;35:3-10.
24. Carlson LE, Beattie TL, Giese-Davis J, et al. Mindfulness-based cancer recovery and supportive-expressive therapy maintain telomere length relative to controls in distressed breast cancer survivors. Cancer. 2015;121:476-484.
25. Kabat-Zinn J, Wheeler E, Light T, et al. Influence of a mindfulness meditation-based stress reduction intervention on rates of skin clearing in patients with moderate to severe psoriasis undergoing phototherapy (UVB) and photochemotherapy (PUVA). Psychosom Med. 1998;60:625-632.
26. Gisondi P, Galvan A, Idolazzi L, et al. Management of moderate to severe psoriasis in patients with metabolic comorbidities. Front Med (Lausanne). 2015;2:1.
1. Dangerfield A. Yoga wars. BBC News. http://news.bbc.co.uk/1/hi/7844691.stm. Published January 23, 2009. Accessed March 25, 2015.
2. Yoga Journal releases 2012 yoga in America market study [press release]. San Francisco, CA: Yoga Journal; December 6, 2012.
3. De Michaelis E. A History of Modern Yoga: Patanjali and Western Esotericism. London, United Kingdom: A&C Black; 2005.
4. Telles S, Singh N, Yadav A, et al. Effect of yoga on different aspects of mental health. Indian J Physiol Pharmacol. 2012;56:245-254.
5. Rodriguez-Vallecillo E, Woodbury-Fariña MA. Dermatological manifestations of stress in normal and psychiatric populations. Psychiatr Clin North Am. 2014;37:625-651.
6. Stander S, Raap U, Weisshaar E, et al. Pathogenesis of pruritus. J Dtsch Dermatol Ges. 2011;9:456-463.
7. Arck PC, Slominski A, Theoharides TC, et al. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 2006;126:1697-1704.
8. Recognizing the mind-skin connection. Harvard Health Publications Web site. http://www.health.harvard.edu/newsletter_article/Recognizing_the_mind-skin_connection. Published November 1, 2006. Accessed March 31, 2015.
9. Tausk F, Elenkov I, Moynihan J. Psychoneuroimmunology. Dermatol Ther. 2008;21:22-31.
10. Pavlovic S, Liezmann C, Blois SM, et al. Substance P is a key mediator of stress-induced protection from allergic sensitization via modified antigen presentation. J Immunol. 2011;186:848-855.
11. Toyoda M, Nakamura M, Makino T, et al. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br J Dermatol. 2002;147:71-79.
12. Koo JYM, Lee CS. General approach to evaluating psychodermatological disorders. In: Koo JYM, Lee CS, eds. Psychocutaneous Medicine. New York, NY: Marcel Dekker; 2003:1-29.
13. Garg A, Chren MM, Sands LP, et al. Psychological stress perturbs epidermal permeability barrier homeostasis: implications for the pathogenesis of stress-associated skin disorders. Arch Dermatol. 2001;137:53-59.
14. Elias PM, Sun R, Eder AR, et al. Treating atopic dermatitis at the source: corrective barrier repair therapy based upon new pathogenic insights. Expert Rev Dermatol. 2013;8:27-36.
15. Morren MA, Przybilla B, Bamelis M, et al. Atopic dermatitis: triggering factors. J Am Acad Dermatol. 1994;31:467-473.
16. Faulstich ME, Williamson DA. An overview of atopic dermatitis: toward a bio-behavioural integration. J Psychosom Res. 1985;29:647-654.
17. Theoharides TC, Donelan JM, Papadopoulou N, et al. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563-568.
18. Suh DH, Kwon HH. What’s new in the physiopathology of acne [published online ahead of print Jan 24, 2015]? Br J Dermatol. doi:10.1111/bjd.13634.
19. Picardi A, Mazzotti E, Pasquini P. Prevalence and correlates of suicidal ideation among patients with skin disease. J Am Acad Dermatol. 2006;54:420-426.
20. Ponarovsky B, Amital D, Lazarov A, et al. Anxiety and depression in patients with allergic and non-allergic cutaneous disorders. Int J Dermatol. 2011;50:1217-1222.
21. Khalsa SB. Yoga as a therapeutic intervention: a bibliometric analysis of published research studies. Indian J Physiol Pharmacol. 2004;48:269-285.
22. Cramer H, Lauche R, Dobos G. Characteristics of randomized controlled trials of yoga: a bibliometric analysis. BMC Complement Altern Med. 2014;14:328.
23. Granath J, Ingvarsson S, von Thiele U, et al. Stress management: a randomized study of cognitive behavioural therapy and yoga. Cogn Behav Ther. 2006;35:3-10.
24. Carlson LE, Beattie TL, Giese-Davis J, et al. Mindfulness-based cancer recovery and supportive-expressive therapy maintain telomere length relative to controls in distressed breast cancer survivors. Cancer. 2015;121:476-484.
25. Kabat-Zinn J, Wheeler E, Light T, et al. Influence of a mindfulness meditation-based stress reduction intervention on rates of skin clearing in patients with moderate to severe psoriasis undergoing phototherapy (UVB) and photochemotherapy (PUVA). Psychosom Med. 1998;60:625-632.
26. Gisondi P, Galvan A, Idolazzi L, et al. Management of moderate to severe psoriasis in patients with metabolic comorbidities. Front Med (Lausanne). 2015;2:1.
What Is Your Diagnosis? New World Cutaneous Leishmaniasis
A 40-year-old man presented with a nonhealing ulcer on the right hand of 2 months’ duration. The lesion had started as a pruritic papule while he was visiting Guyana 2 months prior. The area had slowly enlarged with progressive ulceration. He denied any systemic signs including fever, chills, or weight loss, and his medical history was unremarkable. Physical examination revealed a 4-cm fungating ulceration with heaped-up borders on the dorsal aspect of the right hand.
The Diagnosis: New World Cutaneous Leishmaniasis
In addition to the ulceration on the right hand, a 2-cm ulcerated plaque also had developed on the right side of the chin a few days later (Figure 1). A biopsy was obtained from the lesion on the hand. Histopathologic examination revealed granulomatous inflammation with numerous histiocytes containing intracellular organisms (Figure 2). The microorganisms had pale pink nuclei with basophilic kinetoplasts (Figure 3). Tissue culture showed a mixed growth of gram-positive and gram-negative organisms but no predominant organism. Fungal and mycobacterial cultures were negative. A diagnosis of New World cutaneous leishmaniasis (CL) was made due to visualization of intracellular microorganisms containing basophilic kinetoplasts. Polymerase chain reaction on the tissue block confirmed the presence of Leishmania guyanensis.
|
Cutaneous leishmaniasis is caused by protozoa from the Leishmania species and is transmitted by the bite of the female sandfly. There are 2 classifications for the disease: Old World and New World. Old World CL is transmitted by the sandfly of the genus Phlebotomus, which is endemic in Asia, Africa, the Mediterranean, and the Middle East. New World CL is transmitted by the sandflies of the genus Lutzomyia, which are endemic in Mexico, Central America, and South America. There have been occasional cases of autochthonous transmission reported in Texas and Oklahoma,1 but there has been no known transmission of CL in Australia or the Pacific Islands.2 Human infection can be transmitted by 21 species of Leishmania and can be speciated by designated laboratories such as the US Centers for Disease Control and Prevention (CDC) and the Walter Reed Army Institute of Research (Silver Spring, Maryland) using tissue culture and polymerase chain reaction.1 The CDC can assist with the collection of specimens and supply of culture medium for cases occurring in the United States. Identification of the species is important because there are associated implications for treatment and prognosis.
There are 3 major forms of leishmaniasis: cutaneous, mucocutaneous, and visceral. Cutaneous leishmaniasis is the most common form. There are approximately 1.5 million new cases of CL each year worldwide,3 and more than 90% of these cases occur in Afghanistan, Algeria, Iran, Iraq, Saudi Arabia, Syria, Brazil, and Peru.1 New World CL is caused by 2 species complexes: Leishmania mexicana and Leishmania viannia, including the subspecies L guyanensis, which was seen in our patient.
Cutaneous leishmaniasis usually begins as a small, well-defined papule at the site of the insect bite that then enlarges and becomes a nodule or plaque. Next, the lesion becomes ulcerated with raised borders (Figure 1). The ulcer typically is painless unless there is secondary bacterial or fungal infection.3,4 The incubation period usually is 2 to 8 weeks and multiple lesions may be present, as seen in our patient.4
Old World CL can resolve without treatment, but New World CL is less likely to spontaneously resolve. Additionally, there is a greater risk for spread of the infection to mucous membranes or for systemic dissemination if New World CL is left untreated.2,5 Patients with multiple lesions (ie, ≥3); large lesions (ie, >2.5 cm); lesions on the face, hands, feet, or joints; and those who are immunocompromised should be treated promptly.2 Pentavalent antimonials (eg, meglumine antimoniate, sodium stibogluconate) are the treatment of choice for New World CL, except for infections caused by L guyanensis. The most common pentavalent antimonial agent used in the United States is sodium stibogluconate and is given at a standard intravenous dose of 20 mg antimony/kg daily for 20 days.2 The drug is only available through the CDC’s Drug Service under an Investigational New Drug protocol.1
Intramuscular pentamidine (3 mg/kg daily every other day for 4 doses) is the first-line treatment of CL caused by L guyanensis because systemic antimony usually is not effective.2,6,7 Intralesional injection with pentavalent antimonials usually is not recommended for treatment of New World CL because of the possibility of disseminated disease.2 Liposomal amphotericin B has mainly been used to treat visceral and mucosal leishmaniasis, but there have been some small studies and case reports that have showed it to be successful in treating CL.8-10 Larger controlled studies need to be performed. Oral antifungal drugs (eg, fluconazole, ketoconazole, itraconazole) also have been used to treat CL with variable results depending on the Leishmania species.11
There currently are no vaccines or drugs available to prevent against leishmaniasis. Preventive measures such as avoiding outdoor activities from dusk to dawn when sandflies are the most active, wearing protective clothing, and applying insect repellent that contains DEET (diethyltoluamide) can help reduce a traveler’s risk for becoming infected. Mosquito nets also should be treated with permethrin, which acts as an insect repellent, as sandflies are so small that they can penetrate mosquito nets.1,3,11
Acknowledgements—We would like to thank Francis Steurer, MS, and Barbara Herwaldt, MD, MPH, at the CDC in Atlanta, Georgia, for their help with the identification of the Leishmania species.
1. Herwaldt BL, Magill AJ. Infectious diseases related to travel: leishmaniasis, cutaneous. Centers for Disease Control and Prevention Web site. http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-5/cutaneous-leish maniasis.htm. Published August 1, 2013. Accessed March 5, 2015.
2. Mitropolos P, Konidas P, Durkin-Konidas M. New World cutaneous leishmaniasis: updated review of current and future diagnosis and treatment. J Am Acad Dermatol. 2010;63:309-322.
3. Hepburn NC. Cutaneous leishmaniasis: an overview. J Postgrad Med. 2003;49:50-54.
4. Markle WH, Makhoul K. Cutaneous leishmaniasis: recognition and treatment. Am Fam Physician. 2004;69:1455-1460.
5. Couppié P, Clyti E, Sainte Marie D, et al. Disseminated cutaneous leishmaniasis due to Leishmania guyanensis: case of a patient with 425 lesions. Am J Trop Med Hyg. 2004;71:558-560.
6. Nacher M, Carme B, Sainte Marie D, et al. Influence of clinical presentation on the efficacy of a short course of pentamidine in the treatment of cutaneous leishmaniasis in French Guiana. Ann Trop Med Parasitol. 2001;95:331-336.
7. Minodier P, Parola P. Cutaneous leishmaniasis treatment. Trav Med Inf Dis. 2007;5:150-158.
8. Solomon M, Baum S, Barzilai A, et al. Liposomal amphotericin B in comparison to sodium stibogluconate for cutaneous infection due to Leishmania braziliensis. J Am Acad Dermatol. 2007;56:612-616.
9. Konecny P, Stark DJ. An Australian case of New World cutaneous leishmaniasis. Med J Aust. 2007;186:315-317.
10. Brown M, Noursadeghi M, Boyle J, et al. Successful liposomal amphotericin B treatment of Leishmania braziliensis cutaneous leishmaniasis. Br J Dermatol. 2005;153:203-205.
11. Parasites: leishmaniasis. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/parasites/leishmaniasis/index.html. Updated January 10, 2013. Accessed March 5, 2015.
A 40-year-old man presented with a nonhealing ulcer on the right hand of 2 months’ duration. The lesion had started as a pruritic papule while he was visiting Guyana 2 months prior. The area had slowly enlarged with progressive ulceration. He denied any systemic signs including fever, chills, or weight loss, and his medical history was unremarkable. Physical examination revealed a 4-cm fungating ulceration with heaped-up borders on the dorsal aspect of the right hand.
The Diagnosis: New World Cutaneous Leishmaniasis
In addition to the ulceration on the right hand, a 2-cm ulcerated plaque also had developed on the right side of the chin a few days later (Figure 1). A biopsy was obtained from the lesion on the hand. Histopathologic examination revealed granulomatous inflammation with numerous histiocytes containing intracellular organisms (Figure 2). The microorganisms had pale pink nuclei with basophilic kinetoplasts (Figure 3). Tissue culture showed a mixed growth of gram-positive and gram-negative organisms but no predominant organism. Fungal and mycobacterial cultures were negative. A diagnosis of New World cutaneous leishmaniasis (CL) was made due to visualization of intracellular microorganisms containing basophilic kinetoplasts. Polymerase chain reaction on the tissue block confirmed the presence of Leishmania guyanensis.
|
Cutaneous leishmaniasis is caused by protozoa from the Leishmania species and is transmitted by the bite of the female sandfly. There are 2 classifications for the disease: Old World and New World. Old World CL is transmitted by the sandfly of the genus Phlebotomus, which is endemic in Asia, Africa, the Mediterranean, and the Middle East. New World CL is transmitted by the sandflies of the genus Lutzomyia, which are endemic in Mexico, Central America, and South America. There have been occasional cases of autochthonous transmission reported in Texas and Oklahoma,1 but there has been no known transmission of CL in Australia or the Pacific Islands.2 Human infection can be transmitted by 21 species of Leishmania and can be speciated by designated laboratories such as the US Centers for Disease Control and Prevention (CDC) and the Walter Reed Army Institute of Research (Silver Spring, Maryland) using tissue culture and polymerase chain reaction.1 The CDC can assist with the collection of specimens and supply of culture medium for cases occurring in the United States. Identification of the species is important because there are associated implications for treatment and prognosis.
There are 3 major forms of leishmaniasis: cutaneous, mucocutaneous, and visceral. Cutaneous leishmaniasis is the most common form. There are approximately 1.5 million new cases of CL each year worldwide,3 and more than 90% of these cases occur in Afghanistan, Algeria, Iran, Iraq, Saudi Arabia, Syria, Brazil, and Peru.1 New World CL is caused by 2 species complexes: Leishmania mexicana and Leishmania viannia, including the subspecies L guyanensis, which was seen in our patient.
Cutaneous leishmaniasis usually begins as a small, well-defined papule at the site of the insect bite that then enlarges and becomes a nodule or plaque. Next, the lesion becomes ulcerated with raised borders (Figure 1). The ulcer typically is painless unless there is secondary bacterial or fungal infection.3,4 The incubation period usually is 2 to 8 weeks and multiple lesions may be present, as seen in our patient.4
Old World CL can resolve without treatment, but New World CL is less likely to spontaneously resolve. Additionally, there is a greater risk for spread of the infection to mucous membranes or for systemic dissemination if New World CL is left untreated.2,5 Patients with multiple lesions (ie, ≥3); large lesions (ie, >2.5 cm); lesions on the face, hands, feet, or joints; and those who are immunocompromised should be treated promptly.2 Pentavalent antimonials (eg, meglumine antimoniate, sodium stibogluconate) are the treatment of choice for New World CL, except for infections caused by L guyanensis. The most common pentavalent antimonial agent used in the United States is sodium stibogluconate and is given at a standard intravenous dose of 20 mg antimony/kg daily for 20 days.2 The drug is only available through the CDC’s Drug Service under an Investigational New Drug protocol.1
Intramuscular pentamidine (3 mg/kg daily every other day for 4 doses) is the first-line treatment of CL caused by L guyanensis because systemic antimony usually is not effective.2,6,7 Intralesional injection with pentavalent antimonials usually is not recommended for treatment of New World CL because of the possibility of disseminated disease.2 Liposomal amphotericin B has mainly been used to treat visceral and mucosal leishmaniasis, but there have been some small studies and case reports that have showed it to be successful in treating CL.8-10 Larger controlled studies need to be performed. Oral antifungal drugs (eg, fluconazole, ketoconazole, itraconazole) also have been used to treat CL with variable results depending on the Leishmania species.11
There currently are no vaccines or drugs available to prevent against leishmaniasis. Preventive measures such as avoiding outdoor activities from dusk to dawn when sandflies are the most active, wearing protective clothing, and applying insect repellent that contains DEET (diethyltoluamide) can help reduce a traveler’s risk for becoming infected. Mosquito nets also should be treated with permethrin, which acts as an insect repellent, as sandflies are so small that they can penetrate mosquito nets.1,3,11
Acknowledgements—We would like to thank Francis Steurer, MS, and Barbara Herwaldt, MD, MPH, at the CDC in Atlanta, Georgia, for their help with the identification of the Leishmania species.
A 40-year-old man presented with a nonhealing ulcer on the right hand of 2 months’ duration. The lesion had started as a pruritic papule while he was visiting Guyana 2 months prior. The area had slowly enlarged with progressive ulceration. He denied any systemic signs including fever, chills, or weight loss, and his medical history was unremarkable. Physical examination revealed a 4-cm fungating ulceration with heaped-up borders on the dorsal aspect of the right hand.
The Diagnosis: New World Cutaneous Leishmaniasis
In addition to the ulceration on the right hand, a 2-cm ulcerated plaque also had developed on the right side of the chin a few days later (Figure 1). A biopsy was obtained from the lesion on the hand. Histopathologic examination revealed granulomatous inflammation with numerous histiocytes containing intracellular organisms (Figure 2). The microorganisms had pale pink nuclei with basophilic kinetoplasts (Figure 3). Tissue culture showed a mixed growth of gram-positive and gram-negative organisms but no predominant organism. Fungal and mycobacterial cultures were negative. A diagnosis of New World cutaneous leishmaniasis (CL) was made due to visualization of intracellular microorganisms containing basophilic kinetoplasts. Polymerase chain reaction on the tissue block confirmed the presence of Leishmania guyanensis.
|
Cutaneous leishmaniasis is caused by protozoa from the Leishmania species and is transmitted by the bite of the female sandfly. There are 2 classifications for the disease: Old World and New World. Old World CL is transmitted by the sandfly of the genus Phlebotomus, which is endemic in Asia, Africa, the Mediterranean, and the Middle East. New World CL is transmitted by the sandflies of the genus Lutzomyia, which are endemic in Mexico, Central America, and South America. There have been occasional cases of autochthonous transmission reported in Texas and Oklahoma,1 but there has been no known transmission of CL in Australia or the Pacific Islands.2 Human infection can be transmitted by 21 species of Leishmania and can be speciated by designated laboratories such as the US Centers for Disease Control and Prevention (CDC) and the Walter Reed Army Institute of Research (Silver Spring, Maryland) using tissue culture and polymerase chain reaction.1 The CDC can assist with the collection of specimens and supply of culture medium for cases occurring in the United States. Identification of the species is important because there are associated implications for treatment and prognosis.
There are 3 major forms of leishmaniasis: cutaneous, mucocutaneous, and visceral. Cutaneous leishmaniasis is the most common form. There are approximately 1.5 million new cases of CL each year worldwide,3 and more than 90% of these cases occur in Afghanistan, Algeria, Iran, Iraq, Saudi Arabia, Syria, Brazil, and Peru.1 New World CL is caused by 2 species complexes: Leishmania mexicana and Leishmania viannia, including the subspecies L guyanensis, which was seen in our patient.
Cutaneous leishmaniasis usually begins as a small, well-defined papule at the site of the insect bite that then enlarges and becomes a nodule or plaque. Next, the lesion becomes ulcerated with raised borders (Figure 1). The ulcer typically is painless unless there is secondary bacterial or fungal infection.3,4 The incubation period usually is 2 to 8 weeks and multiple lesions may be present, as seen in our patient.4
Old World CL can resolve without treatment, but New World CL is less likely to spontaneously resolve. Additionally, there is a greater risk for spread of the infection to mucous membranes or for systemic dissemination if New World CL is left untreated.2,5 Patients with multiple lesions (ie, ≥3); large lesions (ie, >2.5 cm); lesions on the face, hands, feet, or joints; and those who are immunocompromised should be treated promptly.2 Pentavalent antimonials (eg, meglumine antimoniate, sodium stibogluconate) are the treatment of choice for New World CL, except for infections caused by L guyanensis. The most common pentavalent antimonial agent used in the United States is sodium stibogluconate and is given at a standard intravenous dose of 20 mg antimony/kg daily for 20 days.2 The drug is only available through the CDC’s Drug Service under an Investigational New Drug protocol.1
Intramuscular pentamidine (3 mg/kg daily every other day for 4 doses) is the first-line treatment of CL caused by L guyanensis because systemic antimony usually is not effective.2,6,7 Intralesional injection with pentavalent antimonials usually is not recommended for treatment of New World CL because of the possibility of disseminated disease.2 Liposomal amphotericin B has mainly been used to treat visceral and mucosal leishmaniasis, but there have been some small studies and case reports that have showed it to be successful in treating CL.8-10 Larger controlled studies need to be performed. Oral antifungal drugs (eg, fluconazole, ketoconazole, itraconazole) also have been used to treat CL with variable results depending on the Leishmania species.11
There currently are no vaccines or drugs available to prevent against leishmaniasis. Preventive measures such as avoiding outdoor activities from dusk to dawn when sandflies are the most active, wearing protective clothing, and applying insect repellent that contains DEET (diethyltoluamide) can help reduce a traveler’s risk for becoming infected. Mosquito nets also should be treated with permethrin, which acts as an insect repellent, as sandflies are so small that they can penetrate mosquito nets.1,3,11
Acknowledgements—We would like to thank Francis Steurer, MS, and Barbara Herwaldt, MD, MPH, at the CDC in Atlanta, Georgia, for their help with the identification of the Leishmania species.
1. Herwaldt BL, Magill AJ. Infectious diseases related to travel: leishmaniasis, cutaneous. Centers for Disease Control and Prevention Web site. http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-5/cutaneous-leish maniasis.htm. Published August 1, 2013. Accessed March 5, 2015.
2. Mitropolos P, Konidas P, Durkin-Konidas M. New World cutaneous leishmaniasis: updated review of current and future diagnosis and treatment. J Am Acad Dermatol. 2010;63:309-322.
3. Hepburn NC. Cutaneous leishmaniasis: an overview. J Postgrad Med. 2003;49:50-54.
4. Markle WH, Makhoul K. Cutaneous leishmaniasis: recognition and treatment. Am Fam Physician. 2004;69:1455-1460.
5. Couppié P, Clyti E, Sainte Marie D, et al. Disseminated cutaneous leishmaniasis due to Leishmania guyanensis: case of a patient with 425 lesions. Am J Trop Med Hyg. 2004;71:558-560.
6. Nacher M, Carme B, Sainte Marie D, et al. Influence of clinical presentation on the efficacy of a short course of pentamidine in the treatment of cutaneous leishmaniasis in French Guiana. Ann Trop Med Parasitol. 2001;95:331-336.
7. Minodier P, Parola P. Cutaneous leishmaniasis treatment. Trav Med Inf Dis. 2007;5:150-158.
8. Solomon M, Baum S, Barzilai A, et al. Liposomal amphotericin B in comparison to sodium stibogluconate for cutaneous infection due to Leishmania braziliensis. J Am Acad Dermatol. 2007;56:612-616.
9. Konecny P, Stark DJ. An Australian case of New World cutaneous leishmaniasis. Med J Aust. 2007;186:315-317.
10. Brown M, Noursadeghi M, Boyle J, et al. Successful liposomal amphotericin B treatment of Leishmania braziliensis cutaneous leishmaniasis. Br J Dermatol. 2005;153:203-205.
11. Parasites: leishmaniasis. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/parasites/leishmaniasis/index.html. Updated January 10, 2013. Accessed March 5, 2015.
1. Herwaldt BL, Magill AJ. Infectious diseases related to travel: leishmaniasis, cutaneous. Centers for Disease Control and Prevention Web site. http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-5/cutaneous-leish maniasis.htm. Published August 1, 2013. Accessed March 5, 2015.
2. Mitropolos P, Konidas P, Durkin-Konidas M. New World cutaneous leishmaniasis: updated review of current and future diagnosis and treatment. J Am Acad Dermatol. 2010;63:309-322.
3. Hepburn NC. Cutaneous leishmaniasis: an overview. J Postgrad Med. 2003;49:50-54.
4. Markle WH, Makhoul K. Cutaneous leishmaniasis: recognition and treatment. Am Fam Physician. 2004;69:1455-1460.
5. Couppié P, Clyti E, Sainte Marie D, et al. Disseminated cutaneous leishmaniasis due to Leishmania guyanensis: case of a patient with 425 lesions. Am J Trop Med Hyg. 2004;71:558-560.
6. Nacher M, Carme B, Sainte Marie D, et al. Influence of clinical presentation on the efficacy of a short course of pentamidine in the treatment of cutaneous leishmaniasis in French Guiana. Ann Trop Med Parasitol. 2001;95:331-336.
7. Minodier P, Parola P. Cutaneous leishmaniasis treatment. Trav Med Inf Dis. 2007;5:150-158.
8. Solomon M, Baum S, Barzilai A, et al. Liposomal amphotericin B in comparison to sodium stibogluconate for cutaneous infection due to Leishmania braziliensis. J Am Acad Dermatol. 2007;56:612-616.
9. Konecny P, Stark DJ. An Australian case of New World cutaneous leishmaniasis. Med J Aust. 2007;186:315-317.
10. Brown M, Noursadeghi M, Boyle J, et al. Successful liposomal amphotericin B treatment of Leishmania braziliensis cutaneous leishmaniasis. Br J Dermatol. 2005;153:203-205.
11. Parasites: leishmaniasis. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/parasites/leishmaniasis/index.html. Updated January 10, 2013. Accessed March 5, 2015.
Painful Purpura and Cutaneous Necrosis
The Diagnosis: Levamisole-Contaminated Cocaine
Physical examination revealed scattered palpable purpura including the nasal tip and large necrotizing skin lesions with bullae (Figure 1). Retiform purpura was noted on the patient’s trunk and legs. Purpuric plaques in various stages of necrosis were identified on the arms, trunk, breasts, and thighs, with an early lesion involving the left ear (Figure 2). Vitals revealed a blood pressure of 151/79 mm Hg and a temperature of 37.3°C. Although the patient initially denied illicit drug use, she later admitted to smoking crack cocaine prior to the skin eruption.
|
Levamisole, an agent used in the veterinary setting to deworm cattle and pigs, is a common additive found in nearly 77% of the seized cocaine supplies in the United States.1 Because of its immunomodulator effects, the agent was once used in humans to treat conditions such as colon cancer, rheumatoid arthritis, and nephritic syndrome. Therapeutic levamisole use has been associated with purpura on the external ears, cheeks, and nasal tip. The predilection for purpura on the ears was first described in children using levam-isole as treatment of nephritic syndrome.2 Antinuclear cytoplasmic antibodies (ANCA) and antiphospholipid antibodies also were associated with therapeutic use.3 Reported effects of levamisole in cocaine users include fever, agranulocytosis, and infection. The mechanism is currently unknown but is thought to be an immunological process as evidenced by the presence of positive autoantibodies.4 Characteristic purpura in a cocaine abuser should alert the physician to consider levamisole as the culprit.
The diagnosis is largely a clinical one and other serious etiologies must be ruled out. The differential should include purpura fulminans, a rare hemorrhagic condition caused by severe infection, such as meningococcemia or deficiency of the vitamin K–dependent anticoagulants protein C and protein S. Given our patient’s history of hepatitis, purpura fulminans could have been likely. Purpura fulminans is rapidly progressive and is usually accompanied by disseminated intravascular coagulation. Coagulation studies and evidence of a consumptive coagulopathy such as thrombocytopenia, prolonged partial thromboplastin time, and activated partial thromboplastin time would favor this diagnosis. These studies as well as protein C and protein S were normal in our patient. Mixed cryoglobulinemia also should be suspected in a patient with hepatitis C virus who presents with vasculitis and arthralgia. An undetectable viral load in addition to a negative assay for cryoglobulins and normal complement (C3 and C4) levels make this diagnosis unlikely. Further, the purpura in cryoglobulinemia is typically confined to the lower extremities.5 Warfarin-induced skin necrosis also should be considered in patients who are taking warfarin.
Laboratory tests can be used to confirm the presence of infection, agranulocytosis, and coagulopathy. Although urine drug screen can confirm exposure to cocaine, levamisole is not detected in routine toxicology screening. Its half-life is between 5 and 6 hours; therefore, detection in blood or urine should be done within 48 hours of exposure.3,4 Unfortunately, our patient presented 2 weeks after cocaine use, making detection of levamisole unfeasible. Rheumatologic screening also is appropriate in cases of suspected vasculitis. Our patient had a negative antinuclear antibody but tested positive for lupus anticoagulant and perinuclear ANCA. IgA and IgG anticardiolipin were negative with an indeterminate IgM anticardiolipin level. The literature supports these findings, as ANCA antibodies occurred in more than 90% of reported cases.3 Further, IgM anticardiolipin antibodies and lupus anticoagulant were positive in 65% and 51% of cases, respectively.3 Leukocyte and neutrophil count were normal in our patient.
Management of these patients is mainly supportive. Complete avoidance of the offending agent is absolutely essential for resolution and avoidance of recurrence. Provided that the patient abstains from cocaine, the skin findings typically resolve in 2 to 3 weeks.3,6 The antibodies, however, may be present for up to 14 months.2,3,6 Treatment with steroids and other immunosuppressants has been reported, but evidence is lacking on their role in the resolution of the lesions.3 In patients who develop a temporary antiphospholipid syndrome, aspirin may be recommended as a preventative measure.6 Pain management, treatment of secondary infection especially in situations with agranulocytosis, and surgical debridement of wounds are all potential problems that can arise.
Our patient had complete resolution of the purpura involving the nose and ear over the course of 3 weeks. She did, however, have to undergo surgical debridement of the nonhealing full-thickness necrotic lesions on her legs and arms.
The 2013 National Survey on Drug Use and Health estimates 1.5 million individuals aged 12 years or older were current (within the last month) users of cocaine.7 With levamisole becoming more prevalent in cocaine supplies, clinicians should be aware of this emerging condition. Rapid recognition can spare the patient unnecessary testing and inappropriate treatment regimens. Because testing for levamisole is difficult and not routinely performed, this case highlights the importance of clinical clues and an accurate social history in clinching the diagnosis.
1. National drug threat assessment: 2011. National Drug Intelligence Center Web site. http://www.justice.gov/archive/ndic/pubs44/44849/44849p.pdf. Published August 2011. Accessed March 9, 2015.
2. Rongioletti F, Gio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;40:948-951.
3. Gulati S, Donato AA. Lupus anticoagulant and ANCA associated thrombotic vasculopathy due to cocaine contaminated with levamisole: a case report and review of the literature. J Thromb Thrombolysis. 2012;34:7-10.
4. de la Hera I, Sanz V, Cullen D, et al. Necrosis of ears after cocaine probably adulterated with levamisole. Dermatology. 2011;223:25-28.
5. Seo P. Immune complex-mediated vasculitis. In: Klippel JH. Primer on the Rheumatic Diseases. 13th ed. New York, NY: Springer Science+Business Media, LLC; 2008:427-443.
6. Ching JA, Smith DS. Levamisole-induced necrosis of skin, soft tissue, and bone: case report and review of literature. J Burn Care Res. 2012;33:e1-e5.
7. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
The Diagnosis: Levamisole-Contaminated Cocaine
Physical examination revealed scattered palpable purpura including the nasal tip and large necrotizing skin lesions with bullae (Figure 1). Retiform purpura was noted on the patient’s trunk and legs. Purpuric plaques in various stages of necrosis were identified on the arms, trunk, breasts, and thighs, with an early lesion involving the left ear (Figure 2). Vitals revealed a blood pressure of 151/79 mm Hg and a temperature of 37.3°C. Although the patient initially denied illicit drug use, she later admitted to smoking crack cocaine prior to the skin eruption.
|
Levamisole, an agent used in the veterinary setting to deworm cattle and pigs, is a common additive found in nearly 77% of the seized cocaine supplies in the United States.1 Because of its immunomodulator effects, the agent was once used in humans to treat conditions such as colon cancer, rheumatoid arthritis, and nephritic syndrome. Therapeutic levamisole use has been associated with purpura on the external ears, cheeks, and nasal tip. The predilection for purpura on the ears was first described in children using levam-isole as treatment of nephritic syndrome.2 Antinuclear cytoplasmic antibodies (ANCA) and antiphospholipid antibodies also were associated with therapeutic use.3 Reported effects of levamisole in cocaine users include fever, agranulocytosis, and infection. The mechanism is currently unknown but is thought to be an immunological process as evidenced by the presence of positive autoantibodies.4 Characteristic purpura in a cocaine abuser should alert the physician to consider levamisole as the culprit.
The diagnosis is largely a clinical one and other serious etiologies must be ruled out. The differential should include purpura fulminans, a rare hemorrhagic condition caused by severe infection, such as meningococcemia or deficiency of the vitamin K–dependent anticoagulants protein C and protein S. Given our patient’s history of hepatitis, purpura fulminans could have been likely. Purpura fulminans is rapidly progressive and is usually accompanied by disseminated intravascular coagulation. Coagulation studies and evidence of a consumptive coagulopathy such as thrombocytopenia, prolonged partial thromboplastin time, and activated partial thromboplastin time would favor this diagnosis. These studies as well as protein C and protein S were normal in our patient. Mixed cryoglobulinemia also should be suspected in a patient with hepatitis C virus who presents with vasculitis and arthralgia. An undetectable viral load in addition to a negative assay for cryoglobulins and normal complement (C3 and C4) levels make this diagnosis unlikely. Further, the purpura in cryoglobulinemia is typically confined to the lower extremities.5 Warfarin-induced skin necrosis also should be considered in patients who are taking warfarin.
Laboratory tests can be used to confirm the presence of infection, agranulocytosis, and coagulopathy. Although urine drug screen can confirm exposure to cocaine, levamisole is not detected in routine toxicology screening. Its half-life is between 5 and 6 hours; therefore, detection in blood or urine should be done within 48 hours of exposure.3,4 Unfortunately, our patient presented 2 weeks after cocaine use, making detection of levamisole unfeasible. Rheumatologic screening also is appropriate in cases of suspected vasculitis. Our patient had a negative antinuclear antibody but tested positive for lupus anticoagulant and perinuclear ANCA. IgA and IgG anticardiolipin were negative with an indeterminate IgM anticardiolipin level. The literature supports these findings, as ANCA antibodies occurred in more than 90% of reported cases.3 Further, IgM anticardiolipin antibodies and lupus anticoagulant were positive in 65% and 51% of cases, respectively.3 Leukocyte and neutrophil count were normal in our patient.
Management of these patients is mainly supportive. Complete avoidance of the offending agent is absolutely essential for resolution and avoidance of recurrence. Provided that the patient abstains from cocaine, the skin findings typically resolve in 2 to 3 weeks.3,6 The antibodies, however, may be present for up to 14 months.2,3,6 Treatment with steroids and other immunosuppressants has been reported, but evidence is lacking on their role in the resolution of the lesions.3 In patients who develop a temporary antiphospholipid syndrome, aspirin may be recommended as a preventative measure.6 Pain management, treatment of secondary infection especially in situations with agranulocytosis, and surgical debridement of wounds are all potential problems that can arise.
Our patient had complete resolution of the purpura involving the nose and ear over the course of 3 weeks. She did, however, have to undergo surgical debridement of the nonhealing full-thickness necrotic lesions on her legs and arms.
The 2013 National Survey on Drug Use and Health estimates 1.5 million individuals aged 12 years or older were current (within the last month) users of cocaine.7 With levamisole becoming more prevalent in cocaine supplies, clinicians should be aware of this emerging condition. Rapid recognition can spare the patient unnecessary testing and inappropriate treatment regimens. Because testing for levamisole is difficult and not routinely performed, this case highlights the importance of clinical clues and an accurate social history in clinching the diagnosis.
The Diagnosis: Levamisole-Contaminated Cocaine
Physical examination revealed scattered palpable purpura including the nasal tip and large necrotizing skin lesions with bullae (Figure 1). Retiform purpura was noted on the patient’s trunk and legs. Purpuric plaques in various stages of necrosis were identified on the arms, trunk, breasts, and thighs, with an early lesion involving the left ear (Figure 2). Vitals revealed a blood pressure of 151/79 mm Hg and a temperature of 37.3°C. Although the patient initially denied illicit drug use, she later admitted to smoking crack cocaine prior to the skin eruption.
|
Levamisole, an agent used in the veterinary setting to deworm cattle and pigs, is a common additive found in nearly 77% of the seized cocaine supplies in the United States.1 Because of its immunomodulator effects, the agent was once used in humans to treat conditions such as colon cancer, rheumatoid arthritis, and nephritic syndrome. Therapeutic levamisole use has been associated with purpura on the external ears, cheeks, and nasal tip. The predilection for purpura on the ears was first described in children using levam-isole as treatment of nephritic syndrome.2 Antinuclear cytoplasmic antibodies (ANCA) and antiphospholipid antibodies also were associated with therapeutic use.3 Reported effects of levamisole in cocaine users include fever, agranulocytosis, and infection. The mechanism is currently unknown but is thought to be an immunological process as evidenced by the presence of positive autoantibodies.4 Characteristic purpura in a cocaine abuser should alert the physician to consider levamisole as the culprit.
The diagnosis is largely a clinical one and other serious etiologies must be ruled out. The differential should include purpura fulminans, a rare hemorrhagic condition caused by severe infection, such as meningococcemia or deficiency of the vitamin K–dependent anticoagulants protein C and protein S. Given our patient’s history of hepatitis, purpura fulminans could have been likely. Purpura fulminans is rapidly progressive and is usually accompanied by disseminated intravascular coagulation. Coagulation studies and evidence of a consumptive coagulopathy such as thrombocytopenia, prolonged partial thromboplastin time, and activated partial thromboplastin time would favor this diagnosis. These studies as well as protein C and protein S were normal in our patient. Mixed cryoglobulinemia also should be suspected in a patient with hepatitis C virus who presents with vasculitis and arthralgia. An undetectable viral load in addition to a negative assay for cryoglobulins and normal complement (C3 and C4) levels make this diagnosis unlikely. Further, the purpura in cryoglobulinemia is typically confined to the lower extremities.5 Warfarin-induced skin necrosis also should be considered in patients who are taking warfarin.
Laboratory tests can be used to confirm the presence of infection, agranulocytosis, and coagulopathy. Although urine drug screen can confirm exposure to cocaine, levamisole is not detected in routine toxicology screening. Its half-life is between 5 and 6 hours; therefore, detection in blood or urine should be done within 48 hours of exposure.3,4 Unfortunately, our patient presented 2 weeks after cocaine use, making detection of levamisole unfeasible. Rheumatologic screening also is appropriate in cases of suspected vasculitis. Our patient had a negative antinuclear antibody but tested positive for lupus anticoagulant and perinuclear ANCA. IgA and IgG anticardiolipin were negative with an indeterminate IgM anticardiolipin level. The literature supports these findings, as ANCA antibodies occurred in more than 90% of reported cases.3 Further, IgM anticardiolipin antibodies and lupus anticoagulant were positive in 65% and 51% of cases, respectively.3 Leukocyte and neutrophil count were normal in our patient.
Management of these patients is mainly supportive. Complete avoidance of the offending agent is absolutely essential for resolution and avoidance of recurrence. Provided that the patient abstains from cocaine, the skin findings typically resolve in 2 to 3 weeks.3,6 The antibodies, however, may be present for up to 14 months.2,3,6 Treatment with steroids and other immunosuppressants has been reported, but evidence is lacking on their role in the resolution of the lesions.3 In patients who develop a temporary antiphospholipid syndrome, aspirin may be recommended as a preventative measure.6 Pain management, treatment of secondary infection especially in situations with agranulocytosis, and surgical debridement of wounds are all potential problems that can arise.
Our patient had complete resolution of the purpura involving the nose and ear over the course of 3 weeks. She did, however, have to undergo surgical debridement of the nonhealing full-thickness necrotic lesions on her legs and arms.
The 2013 National Survey on Drug Use and Health estimates 1.5 million individuals aged 12 years or older were current (within the last month) users of cocaine.7 With levamisole becoming more prevalent in cocaine supplies, clinicians should be aware of this emerging condition. Rapid recognition can spare the patient unnecessary testing and inappropriate treatment regimens. Because testing for levamisole is difficult and not routinely performed, this case highlights the importance of clinical clues and an accurate social history in clinching the diagnosis.
1. National drug threat assessment: 2011. National Drug Intelligence Center Web site. http://www.justice.gov/archive/ndic/pubs44/44849/44849p.pdf. Published August 2011. Accessed March 9, 2015.
2. Rongioletti F, Gio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;40:948-951.
3. Gulati S, Donato AA. Lupus anticoagulant and ANCA associated thrombotic vasculopathy due to cocaine contaminated with levamisole: a case report and review of the literature. J Thromb Thrombolysis. 2012;34:7-10.
4. de la Hera I, Sanz V, Cullen D, et al. Necrosis of ears after cocaine probably adulterated with levamisole. Dermatology. 2011;223:25-28.
5. Seo P. Immune complex-mediated vasculitis. In: Klippel JH. Primer on the Rheumatic Diseases. 13th ed. New York, NY: Springer Science+Business Media, LLC; 2008:427-443.
6. Ching JA, Smith DS. Levamisole-induced necrosis of skin, soft tissue, and bone: case report and review of literature. J Burn Care Res. 2012;33:e1-e5.
7. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
1. National drug threat assessment: 2011. National Drug Intelligence Center Web site. http://www.justice.gov/archive/ndic/pubs44/44849/44849p.pdf. Published August 2011. Accessed March 9, 2015.
2. Rongioletti F, Gio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;40:948-951.
3. Gulati S, Donato AA. Lupus anticoagulant and ANCA associated thrombotic vasculopathy due to cocaine contaminated with levamisole: a case report and review of the literature. J Thromb Thrombolysis. 2012;34:7-10.
4. de la Hera I, Sanz V, Cullen D, et al. Necrosis of ears after cocaine probably adulterated with levamisole. Dermatology. 2011;223:25-28.
5. Seo P. Immune complex-mediated vasculitis. In: Klippel JH. Primer on the Rheumatic Diseases. 13th ed. New York, NY: Springer Science+Business Media, LLC; 2008:427-443.
6. Ching JA, Smith DS. Levamisole-induced necrosis of skin, soft tissue, and bone: case report and review of literature. J Burn Care Res. 2012;33:e1-e5.
7. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
A 44-year-old woman with a history of hepatitis C virus and cocaine abuse presented with painful bruising and skin breakdown. Three weeks prior she was treated at an urgent care clinic with oral clindamycin for a recurrent Staphylococcus infection of her finger. One week later she started developing purpura and painful skin necrosis and ulceration. Most notable was the purpura on the tip of the nose. She also reported arthralgia and low-grade fever.
Plasmapheresis in Refractory Pemphigus Vulgaris: Revisiting an Old Treatment Modality Used in Synchrony With Pulse Cyclophosphamide
To the Editor:
Pemphigus vulgaris is an uncommon autoimmune blistering dermatosis characterized by painful mucocutaneous erosions. It can be a life-threatening condition if left untreated. The autoimmune process is mediated by autoantibodies against the keratinocyte surface antigens desmoglein 1 and 3.1 Therapy is directed at lowering autoantibody levels with systemic corticosteroids and immunosuppressive agents. Use of these agents often is limited by collateral adverse effects.2 Refractory disease may occur despite the use of high-dose corticosteroids or a combination of other immunosuppressants. The level of these pathogenic autoantibodies generally parallels the extent of disease activity, and removing them with plasmapheresis followed by immunosuppression should result in therapeutic response.3 We report a case of refractory pemphigus vulgaris that was controlled with plasmapheresis used in synchrony with pulse cyclophosphamide.
A 48-year-old Chinese man first presented with mucocutaneous erosions 2 years ago, and a diagnosis of pemphigus vulgaris was confirmed based on typical histologic and immunofluorescence features. Histologic features included suprabasal acantholysis with an intraepidermal blister as well as basal keratinocytes attached to the dermal papillae and present along the entire dermoepidermal junction (Figure 1). Direct immunofluorescence demonstrated intercellular deposits of IgG and complements in the lower epidermis, and indirect immunofluorescence showed the presence of the pathogenic pemphigus autoantibodies. The patient was initially treated with prednisolone (up to 1 mg/kg daily) and mycophenolate mofetil (1 g twice daily) for 6 months with moderate disease response. Two months later he experienced a disease flare that was triggered by sun exposure and concomitant herpes simplex virus infection. He achieved moderate disease control with acyclovir, 3 days of intravenous immunoglobulin, and combination prednisolone and azathioprine. There was no other relevant medical history. For the last year, the patient received continuous prednisolone (varying doses 0.5–1 mg/kg daily), concomitant azathioprine (up to 3 mg/kg daily), and long-term prophylactic acyclovir, but he continued to have residual crusted erosions over the scalp and face (best score of 25 points based on the autoimmune bullous skin disorder intensity score [ABSIS] ranging from 0–150 points4). He was admitted at the current presentation with another, more severe disease flare with extensive painful erosions over the trunk, arms, legs, face, and scalp (80% body surface area involvement and ABSIS score of 120 points)(Figure 2)4 that occurred after azathioprine was temporarily ceased for 1 week due to transaminitis, and despite a temporary increment in prednisolone dose. There was, however, no significant oral mucosal involvement. The desmoglein 1 and 3 antibody levels were elevated at more than 300 U/mL and 186 U/mL, respectively (>20 U/mL indicates positivity). A 3-day course of pulse intravenous methylprednisolone (10 mg/kg) failed to achieve clinical improvement or reduction of antibody titers. The use of various immunosuppressive agents was limited by persistent transaminitis and transient leukopenia.
|
Because of remarkable morbidity, the patient underwent interim plasmapheresis for rapid disease control. Plasmapheresis was carried out through a pheresible central venous catheter. One plasma volume exchange was done each session, which was 5 L for the patient’s body weight and hematocrit. Equal volume of colloid comprising 2.5 L of fresh frozen plasma and 2.5 L of 5% albumin was used for replacement. Plasma exchange was performed with a cell separator by discontinuous flow centrifugation with 4% acid citrate dextrose as an anticoagulant. For each session of plasmapheresis, 16 cycles of exchange (each processing approximately 300 mL of blood) was carried out, the entire process lasting for 4 hours. The coagulation and biochemical profile was checked after each session of plasmapheresis and corrected when necessary. The patient underwent 9 sessions of plasmapheresis over a 3-week period, synchronized with pulse intravenous cyclophosphamide (15 mg/kg) immediately after completion of the plasmapheresis sessions, resulting in a remarkable decrease in pathogenic antibody titers to near undetectable levels and clinical improvement (Figure 3). The extensive erosions gradually healed with good reepithelialization, and there was a notable reduction in the ABSIS score to 12 points. He received 3 more monthly treatments with pulse intravenous cyclophosphamide (15 mg/kg) and is currently maintained on oral cyclophosphamide (2 mg/kg daily) and low-dose prednisolone (0.3 mg/kg daily). There was no subsequent disease relapse at 6-month follow-up, with the ABSIS score maintained at 5 points, and no increase in pathogenic autoantibody titers. The patient subsequently was lost to follow-up.
Patients with severe disease or refractory cases of pemphigus vulgaris that have been maintained on unacceptably high doses of corticosteroids or immunosuppressants that cannot be tapered without a disease flare may develop remarkable adverse effects, both from medications and from long-term immunosuppression.2 Our case illustrates the short-term benefit of plasmapheresis combined with immunosuppressants resulting in rapid disease control.
Plasmapheresis involves the selective removal of pathogenic materials from the circulation to achieve therapeutic effect, followed by appropriate replacement fluids. Treating pemphigus vulgaris with plasmapheresis was introduced in 1978 based on the rationale of removing pathogenic autoantibodies from the circulation.3,5 Using desmoglein enzyme-linked immunosorbent assay, it has been shown that one centrifugal plasmapheresis procedure eliminates approximately 15% of the IgG autoantibodies from the whole body.6 An average of 5 plasmapheresis sessions on alternate days usually is required to deplete the levels of pathogenic autoantibodies to near undetectable levels.7 Our case required 9 plasmapheresis sessions over 3 weeks to achieve good therapeutic response.
It seems that using plasmapheresis to treat pemphigus vulgaris has fallen out of favor due to its inability to prevent the antibody rebound occurring during weeks 1 and 2 posttreatment. Because of a feedback mechanism, a massive antibody depletion by plasmapheresis triggers a rebound synthesis of more autoantibodies by pathogenic B cells to titers comparable to or higher than those before plasmapheresis.8 The use of plasmapheresis should be supported by immunosuppressive therapy to prevent antibody feedback rebound. Due to the advent of available immunosuppressive agents in recent years, there is a resurgence in the successful use of this old treatment modality combined with immunosuppressive therapy in managing refractory pemphigus vulgaris.7,8 At present there is no clear data to support the use of one immunosuppressant versus another, but our case supports the use of pulse intravenous cyclophosphamide, as documented in other reports.7,9 The success of immunosuppressive agents at reducing antibody levels depends on the timing (immediately after plasmapheresis) as well as individual responsiveness to the immunosuppressant.7
Our armamentarium of therapies for refractory pemphigus vulgaris continues to evolve. A more selective method of removing antibodies by extracorporeal immunoadsorption has the benefit of higher removal rates and reduced inadvertent loss of other plasma components.10 The combination of protein A immunoadsorption with rituximab, a monoclonal anti-CD20 antibody that induces B-cell depletion, also has been shown to induce rapid and durable remission in refractory cases.11
Our case shows that plasmapheresis can be a useful alternative or adjunctive intervention in pemphigus vulgaris that is not responding to conventional therapy or in cases when steroids or immunosuppressants are contraindicated. There is a definite role for such therapeutic plasma exchanges in the rapid control of potentially life-threatening disease. Its benefits are optimized when used in synchrony with immunosuppressants immediately following plasmapheresis to prevent rebound effect of antibody depletion.
1. Udey MC, Stanley JR. Pemphigus–disease of antidesmosomal autoimmunity. JAMA. 1999;282:572-576.
2. Huilgol SC, Black MM. Management of the immunobullous disorders. II. pemphigus. Clin Exp Dermatol. 1995;20:283-293.
3. Cotterill JA, Barker DJ, Millard LG. Plasma exchange in the treatment of pemphigus vulgaris. Br J Dermatol. 1978;98:243.
4. Pfutze M, Niedermeier A, Hertl M, et al. Introducing a novel Autoimmune Bullous Skin Disorder Intensity Score (ABSIS) in pemphigus [published online ahead of print February 27, 2007]. Eur J Dermatol. 2007;17:4-11.
5. Ruocco V, Rossi A, Argenziano G, et al. Pathogenicity of the intercellular antibodies of pemphigus their periodic removal from the circulation by plasmapheresis. Br J Dermatol. 1978;98:237-241.
6. Nagasaka T, Fujii Y, Ishida A, et al. Evaluating efficacy of plasmapheresis for patients with pemphigus using desmoglein enzyme-linked immunosorbent assay [published online ahead of print January 30, 2008]. Br J Dermatol. 2008;158:685-690.
7. Turner MS, Sutton D, Sauder DN. The use of plasmapheresis and immunosuppression in the treatment of pemphigus vulgaris. J Am Acad Dermatol. 2000;43:1058-1064.
8. Roujeau JC, Andre C, Joneau Fabre M, et al. Plasma exchange in pemphigus. uncontrolled study of ten patients. Arch Dermatol. 1983;119:215-221.
9. Euler HH, Löffler H, Christophers E. Synchronization of plasmapheresis and pulse cyclophosphamide therapy in pemphigus vulgaris. Arch Dermatol. 1987;123:1205-1210.
10. Lüftl M, Stauber A, Mainka A, et al. Successful removal of pathogenic autoantibodies in pemphigus by immunoadsorption with a tryptophan-linked polyvinylalcohol adsorber. Br J Dermatol. 2003;149:598-605.
11. Shimanovich I, Nitschke M, Rose C, et al. Treatment of severe pemphigus with protein A immunoadsorption, rituximab and intravenous immunoglobulins. Br J Dermatol. 2008;158:382-388.
To the Editor:
Pemphigus vulgaris is an uncommon autoimmune blistering dermatosis characterized by painful mucocutaneous erosions. It can be a life-threatening condition if left untreated. The autoimmune process is mediated by autoantibodies against the keratinocyte surface antigens desmoglein 1 and 3.1 Therapy is directed at lowering autoantibody levels with systemic corticosteroids and immunosuppressive agents. Use of these agents often is limited by collateral adverse effects.2 Refractory disease may occur despite the use of high-dose corticosteroids or a combination of other immunosuppressants. The level of these pathogenic autoantibodies generally parallels the extent of disease activity, and removing them with plasmapheresis followed by immunosuppression should result in therapeutic response.3 We report a case of refractory pemphigus vulgaris that was controlled with plasmapheresis used in synchrony with pulse cyclophosphamide.
A 48-year-old Chinese man first presented with mucocutaneous erosions 2 years ago, and a diagnosis of pemphigus vulgaris was confirmed based on typical histologic and immunofluorescence features. Histologic features included suprabasal acantholysis with an intraepidermal blister as well as basal keratinocytes attached to the dermal papillae and present along the entire dermoepidermal junction (Figure 1). Direct immunofluorescence demonstrated intercellular deposits of IgG and complements in the lower epidermis, and indirect immunofluorescence showed the presence of the pathogenic pemphigus autoantibodies. The patient was initially treated with prednisolone (up to 1 mg/kg daily) and mycophenolate mofetil (1 g twice daily) for 6 months with moderate disease response. Two months later he experienced a disease flare that was triggered by sun exposure and concomitant herpes simplex virus infection. He achieved moderate disease control with acyclovir, 3 days of intravenous immunoglobulin, and combination prednisolone and azathioprine. There was no other relevant medical history. For the last year, the patient received continuous prednisolone (varying doses 0.5–1 mg/kg daily), concomitant azathioprine (up to 3 mg/kg daily), and long-term prophylactic acyclovir, but he continued to have residual crusted erosions over the scalp and face (best score of 25 points based on the autoimmune bullous skin disorder intensity score [ABSIS] ranging from 0–150 points4). He was admitted at the current presentation with another, more severe disease flare with extensive painful erosions over the trunk, arms, legs, face, and scalp (80% body surface area involvement and ABSIS score of 120 points)(Figure 2)4 that occurred after azathioprine was temporarily ceased for 1 week due to transaminitis, and despite a temporary increment in prednisolone dose. There was, however, no significant oral mucosal involvement. The desmoglein 1 and 3 antibody levels were elevated at more than 300 U/mL and 186 U/mL, respectively (>20 U/mL indicates positivity). A 3-day course of pulse intravenous methylprednisolone (10 mg/kg) failed to achieve clinical improvement or reduction of antibody titers. The use of various immunosuppressive agents was limited by persistent transaminitis and transient leukopenia.
|
Because of remarkable morbidity, the patient underwent interim plasmapheresis for rapid disease control. Plasmapheresis was carried out through a pheresible central venous catheter. One plasma volume exchange was done each session, which was 5 L for the patient’s body weight and hematocrit. Equal volume of colloid comprising 2.5 L of fresh frozen plasma and 2.5 L of 5% albumin was used for replacement. Plasma exchange was performed with a cell separator by discontinuous flow centrifugation with 4% acid citrate dextrose as an anticoagulant. For each session of plasmapheresis, 16 cycles of exchange (each processing approximately 300 mL of blood) was carried out, the entire process lasting for 4 hours. The coagulation and biochemical profile was checked after each session of plasmapheresis and corrected when necessary. The patient underwent 9 sessions of plasmapheresis over a 3-week period, synchronized with pulse intravenous cyclophosphamide (15 mg/kg) immediately after completion of the plasmapheresis sessions, resulting in a remarkable decrease in pathogenic antibody titers to near undetectable levels and clinical improvement (Figure 3). The extensive erosions gradually healed with good reepithelialization, and there was a notable reduction in the ABSIS score to 12 points. He received 3 more monthly treatments with pulse intravenous cyclophosphamide (15 mg/kg) and is currently maintained on oral cyclophosphamide (2 mg/kg daily) and low-dose prednisolone (0.3 mg/kg daily). There was no subsequent disease relapse at 6-month follow-up, with the ABSIS score maintained at 5 points, and no increase in pathogenic autoantibody titers. The patient subsequently was lost to follow-up.
Patients with severe disease or refractory cases of pemphigus vulgaris that have been maintained on unacceptably high doses of corticosteroids or immunosuppressants that cannot be tapered without a disease flare may develop remarkable adverse effects, both from medications and from long-term immunosuppression.2 Our case illustrates the short-term benefit of plasmapheresis combined with immunosuppressants resulting in rapid disease control.
Plasmapheresis involves the selective removal of pathogenic materials from the circulation to achieve therapeutic effect, followed by appropriate replacement fluids. Treating pemphigus vulgaris with plasmapheresis was introduced in 1978 based on the rationale of removing pathogenic autoantibodies from the circulation.3,5 Using desmoglein enzyme-linked immunosorbent assay, it has been shown that one centrifugal plasmapheresis procedure eliminates approximately 15% of the IgG autoantibodies from the whole body.6 An average of 5 plasmapheresis sessions on alternate days usually is required to deplete the levels of pathogenic autoantibodies to near undetectable levels.7 Our case required 9 plasmapheresis sessions over 3 weeks to achieve good therapeutic response.
It seems that using plasmapheresis to treat pemphigus vulgaris has fallen out of favor due to its inability to prevent the antibody rebound occurring during weeks 1 and 2 posttreatment. Because of a feedback mechanism, a massive antibody depletion by plasmapheresis triggers a rebound synthesis of more autoantibodies by pathogenic B cells to titers comparable to or higher than those before plasmapheresis.8 The use of plasmapheresis should be supported by immunosuppressive therapy to prevent antibody feedback rebound. Due to the advent of available immunosuppressive agents in recent years, there is a resurgence in the successful use of this old treatment modality combined with immunosuppressive therapy in managing refractory pemphigus vulgaris.7,8 At present there is no clear data to support the use of one immunosuppressant versus another, but our case supports the use of pulse intravenous cyclophosphamide, as documented in other reports.7,9 The success of immunosuppressive agents at reducing antibody levels depends on the timing (immediately after plasmapheresis) as well as individual responsiveness to the immunosuppressant.7
Our armamentarium of therapies for refractory pemphigus vulgaris continues to evolve. A more selective method of removing antibodies by extracorporeal immunoadsorption has the benefit of higher removal rates and reduced inadvertent loss of other plasma components.10 The combination of protein A immunoadsorption with rituximab, a monoclonal anti-CD20 antibody that induces B-cell depletion, also has been shown to induce rapid and durable remission in refractory cases.11
Our case shows that plasmapheresis can be a useful alternative or adjunctive intervention in pemphigus vulgaris that is not responding to conventional therapy or in cases when steroids or immunosuppressants are contraindicated. There is a definite role for such therapeutic plasma exchanges in the rapid control of potentially life-threatening disease. Its benefits are optimized when used in synchrony with immunosuppressants immediately following plasmapheresis to prevent rebound effect of antibody depletion.
To the Editor:
Pemphigus vulgaris is an uncommon autoimmune blistering dermatosis characterized by painful mucocutaneous erosions. It can be a life-threatening condition if left untreated. The autoimmune process is mediated by autoantibodies against the keratinocyte surface antigens desmoglein 1 and 3.1 Therapy is directed at lowering autoantibody levels with systemic corticosteroids and immunosuppressive agents. Use of these agents often is limited by collateral adverse effects.2 Refractory disease may occur despite the use of high-dose corticosteroids or a combination of other immunosuppressants. The level of these pathogenic autoantibodies generally parallels the extent of disease activity, and removing them with plasmapheresis followed by immunosuppression should result in therapeutic response.3 We report a case of refractory pemphigus vulgaris that was controlled with plasmapheresis used in synchrony with pulse cyclophosphamide.
A 48-year-old Chinese man first presented with mucocutaneous erosions 2 years ago, and a diagnosis of pemphigus vulgaris was confirmed based on typical histologic and immunofluorescence features. Histologic features included suprabasal acantholysis with an intraepidermal blister as well as basal keratinocytes attached to the dermal papillae and present along the entire dermoepidermal junction (Figure 1). Direct immunofluorescence demonstrated intercellular deposits of IgG and complements in the lower epidermis, and indirect immunofluorescence showed the presence of the pathogenic pemphigus autoantibodies. The patient was initially treated with prednisolone (up to 1 mg/kg daily) and mycophenolate mofetil (1 g twice daily) for 6 months with moderate disease response. Two months later he experienced a disease flare that was triggered by sun exposure and concomitant herpes simplex virus infection. He achieved moderate disease control with acyclovir, 3 days of intravenous immunoglobulin, and combination prednisolone and azathioprine. There was no other relevant medical history. For the last year, the patient received continuous prednisolone (varying doses 0.5–1 mg/kg daily), concomitant azathioprine (up to 3 mg/kg daily), and long-term prophylactic acyclovir, but he continued to have residual crusted erosions over the scalp and face (best score of 25 points based on the autoimmune bullous skin disorder intensity score [ABSIS] ranging from 0–150 points4). He was admitted at the current presentation with another, more severe disease flare with extensive painful erosions over the trunk, arms, legs, face, and scalp (80% body surface area involvement and ABSIS score of 120 points)(Figure 2)4 that occurred after azathioprine was temporarily ceased for 1 week due to transaminitis, and despite a temporary increment in prednisolone dose. There was, however, no significant oral mucosal involvement. The desmoglein 1 and 3 antibody levels were elevated at more than 300 U/mL and 186 U/mL, respectively (>20 U/mL indicates positivity). A 3-day course of pulse intravenous methylprednisolone (10 mg/kg) failed to achieve clinical improvement or reduction of antibody titers. The use of various immunosuppressive agents was limited by persistent transaminitis and transient leukopenia.
|
Because of remarkable morbidity, the patient underwent interim plasmapheresis for rapid disease control. Plasmapheresis was carried out through a pheresible central venous catheter. One plasma volume exchange was done each session, which was 5 L for the patient’s body weight and hematocrit. Equal volume of colloid comprising 2.5 L of fresh frozen plasma and 2.5 L of 5% albumin was used for replacement. Plasma exchange was performed with a cell separator by discontinuous flow centrifugation with 4% acid citrate dextrose as an anticoagulant. For each session of plasmapheresis, 16 cycles of exchange (each processing approximately 300 mL of blood) was carried out, the entire process lasting for 4 hours. The coagulation and biochemical profile was checked after each session of plasmapheresis and corrected when necessary. The patient underwent 9 sessions of plasmapheresis over a 3-week period, synchronized with pulse intravenous cyclophosphamide (15 mg/kg) immediately after completion of the plasmapheresis sessions, resulting in a remarkable decrease in pathogenic antibody titers to near undetectable levels and clinical improvement (Figure 3). The extensive erosions gradually healed with good reepithelialization, and there was a notable reduction in the ABSIS score to 12 points. He received 3 more monthly treatments with pulse intravenous cyclophosphamide (15 mg/kg) and is currently maintained on oral cyclophosphamide (2 mg/kg daily) and low-dose prednisolone (0.3 mg/kg daily). There was no subsequent disease relapse at 6-month follow-up, with the ABSIS score maintained at 5 points, and no increase in pathogenic autoantibody titers. The patient subsequently was lost to follow-up.
Patients with severe disease or refractory cases of pemphigus vulgaris that have been maintained on unacceptably high doses of corticosteroids or immunosuppressants that cannot be tapered without a disease flare may develop remarkable adverse effects, both from medications and from long-term immunosuppression.2 Our case illustrates the short-term benefit of plasmapheresis combined with immunosuppressants resulting in rapid disease control.
Plasmapheresis involves the selective removal of pathogenic materials from the circulation to achieve therapeutic effect, followed by appropriate replacement fluids. Treating pemphigus vulgaris with plasmapheresis was introduced in 1978 based on the rationale of removing pathogenic autoantibodies from the circulation.3,5 Using desmoglein enzyme-linked immunosorbent assay, it has been shown that one centrifugal plasmapheresis procedure eliminates approximately 15% of the IgG autoantibodies from the whole body.6 An average of 5 plasmapheresis sessions on alternate days usually is required to deplete the levels of pathogenic autoantibodies to near undetectable levels.7 Our case required 9 plasmapheresis sessions over 3 weeks to achieve good therapeutic response.
It seems that using plasmapheresis to treat pemphigus vulgaris has fallen out of favor due to its inability to prevent the antibody rebound occurring during weeks 1 and 2 posttreatment. Because of a feedback mechanism, a massive antibody depletion by plasmapheresis triggers a rebound synthesis of more autoantibodies by pathogenic B cells to titers comparable to or higher than those before plasmapheresis.8 The use of plasmapheresis should be supported by immunosuppressive therapy to prevent antibody feedback rebound. Due to the advent of available immunosuppressive agents in recent years, there is a resurgence in the successful use of this old treatment modality combined with immunosuppressive therapy in managing refractory pemphigus vulgaris.7,8 At present there is no clear data to support the use of one immunosuppressant versus another, but our case supports the use of pulse intravenous cyclophosphamide, as documented in other reports.7,9 The success of immunosuppressive agents at reducing antibody levels depends on the timing (immediately after plasmapheresis) as well as individual responsiveness to the immunosuppressant.7
Our armamentarium of therapies for refractory pemphigus vulgaris continues to evolve. A more selective method of removing antibodies by extracorporeal immunoadsorption has the benefit of higher removal rates and reduced inadvertent loss of other plasma components.10 The combination of protein A immunoadsorption with rituximab, a monoclonal anti-CD20 antibody that induces B-cell depletion, also has been shown to induce rapid and durable remission in refractory cases.11
Our case shows that plasmapheresis can be a useful alternative or adjunctive intervention in pemphigus vulgaris that is not responding to conventional therapy or in cases when steroids or immunosuppressants are contraindicated. There is a definite role for such therapeutic plasma exchanges in the rapid control of potentially life-threatening disease. Its benefits are optimized when used in synchrony with immunosuppressants immediately following plasmapheresis to prevent rebound effect of antibody depletion.
1. Udey MC, Stanley JR. Pemphigus–disease of antidesmosomal autoimmunity. JAMA. 1999;282:572-576.
2. Huilgol SC, Black MM. Management of the immunobullous disorders. II. pemphigus. Clin Exp Dermatol. 1995;20:283-293.
3. Cotterill JA, Barker DJ, Millard LG. Plasma exchange in the treatment of pemphigus vulgaris. Br J Dermatol. 1978;98:243.
4. Pfutze M, Niedermeier A, Hertl M, et al. Introducing a novel Autoimmune Bullous Skin Disorder Intensity Score (ABSIS) in pemphigus [published online ahead of print February 27, 2007]. Eur J Dermatol. 2007;17:4-11.
5. Ruocco V, Rossi A, Argenziano G, et al. Pathogenicity of the intercellular antibodies of pemphigus their periodic removal from the circulation by plasmapheresis. Br J Dermatol. 1978;98:237-241.
6. Nagasaka T, Fujii Y, Ishida A, et al. Evaluating efficacy of plasmapheresis for patients with pemphigus using desmoglein enzyme-linked immunosorbent assay [published online ahead of print January 30, 2008]. Br J Dermatol. 2008;158:685-690.
7. Turner MS, Sutton D, Sauder DN. The use of plasmapheresis and immunosuppression in the treatment of pemphigus vulgaris. J Am Acad Dermatol. 2000;43:1058-1064.
8. Roujeau JC, Andre C, Joneau Fabre M, et al. Plasma exchange in pemphigus. uncontrolled study of ten patients. Arch Dermatol. 1983;119:215-221.
9. Euler HH, Löffler H, Christophers E. Synchronization of plasmapheresis and pulse cyclophosphamide therapy in pemphigus vulgaris. Arch Dermatol. 1987;123:1205-1210.
10. Lüftl M, Stauber A, Mainka A, et al. Successful removal of pathogenic autoantibodies in pemphigus by immunoadsorption with a tryptophan-linked polyvinylalcohol adsorber. Br J Dermatol. 2003;149:598-605.
11. Shimanovich I, Nitschke M, Rose C, et al. Treatment of severe pemphigus with protein A immunoadsorption, rituximab and intravenous immunoglobulins. Br J Dermatol. 2008;158:382-388.
1. Udey MC, Stanley JR. Pemphigus–disease of antidesmosomal autoimmunity. JAMA. 1999;282:572-576.
2. Huilgol SC, Black MM. Management of the immunobullous disorders. II. pemphigus. Clin Exp Dermatol. 1995;20:283-293.
3. Cotterill JA, Barker DJ, Millard LG. Plasma exchange in the treatment of pemphigus vulgaris. Br J Dermatol. 1978;98:243.
4. Pfutze M, Niedermeier A, Hertl M, et al. Introducing a novel Autoimmune Bullous Skin Disorder Intensity Score (ABSIS) in pemphigus [published online ahead of print February 27, 2007]. Eur J Dermatol. 2007;17:4-11.
5. Ruocco V, Rossi A, Argenziano G, et al. Pathogenicity of the intercellular antibodies of pemphigus their periodic removal from the circulation by plasmapheresis. Br J Dermatol. 1978;98:237-241.
6. Nagasaka T, Fujii Y, Ishida A, et al. Evaluating efficacy of plasmapheresis for patients with pemphigus using desmoglein enzyme-linked immunosorbent assay [published online ahead of print January 30, 2008]. Br J Dermatol. 2008;158:685-690.
7. Turner MS, Sutton D, Sauder DN. The use of plasmapheresis and immunosuppression in the treatment of pemphigus vulgaris. J Am Acad Dermatol. 2000;43:1058-1064.
8. Roujeau JC, Andre C, Joneau Fabre M, et al. Plasma exchange in pemphigus. uncontrolled study of ten patients. Arch Dermatol. 1983;119:215-221.
9. Euler HH, Löffler H, Christophers E. Synchronization of plasmapheresis and pulse cyclophosphamide therapy in pemphigus vulgaris. Arch Dermatol. 1987;123:1205-1210.
10. Lüftl M, Stauber A, Mainka A, et al. Successful removal of pathogenic autoantibodies in pemphigus by immunoadsorption with a tryptophan-linked polyvinylalcohol adsorber. Br J Dermatol. 2003;149:598-605.
11. Shimanovich I, Nitschke M, Rose C, et al. Treatment of severe pemphigus with protein A immunoadsorption, rituximab and intravenous immunoglobulins. Br J Dermatol. 2008;158:382-388.
Dermatologic Emergencies
Dermatologic emergency may sound like an oxymoron, but there are many emergencies that dermatology residents may encounter in their careers. In some instances the skin is the primary organ that is affected, while in others cutaneous symptoms and life-threatening signs are important diagnostic clues for what may lie beneath the skin.
As residents who are occasionally on call or on consultation services, it is important for us to recognize dermatologic emergencies quickly because some of these conditions can acutely evolve and become lethal if a diagnosis is not made early in the disease course with the appropriate treatment administered. Dermatologic emergencies can range from severe drug reactions, infections, autoimmune exacerbations, and inflammatory conditions (eg, erythroderma) to environmental insults such as burns (Figure 1) and child abuse.1
Critical Infections
Some dermatologic emergencies are infectious in origin, and although these infections are most commonly bacterial (eg, necrotizing fasciitis), they also can range from viral to fungal (eg, mucormycosis) in nature. Some areas with large populations of immunocompromised patients (eg, human immunodeficiency virus–positive patients, organ transplant recipients) may warrant a high index of suspicion for possible zebras (rare conditions) and opportunistic infections that may quickly escalate to life-threatening situations.
Although few cutaneous manifestations in emergent infections are pathognomonic, they sometimes can be categorized according to the appearance of the primary lesion: erythrodermic (eg, staphylococcal scalded skin syndrome), maculopapular (eg, Lyme disease), purpuric/petechial (eg, Rocky Mountain spotted fever), pustular (eg, disseminated candidiasis), or vesicular (eg, neonatal herpes simplex virus)(Table). On consultations, dermatology residents frequently get called to evaluate hemorrhagic and ischemic lesions in inpatients (Figure 2). Aside from infectious causes, the differential diagnosis may include coagulation abnormalities (eg, concurrent anticoagulant therapies), vasculitides, poisoning, vascular disease, or Stevens-Johnson syndrome and toxic epidermal necrolysis, which can occasionally present with hemorrhagic lesions.1,2
Necrotizing Fasciitis
Dermatology residents may frequently encounter necrotizing fasciitis, either in clinic or on the wards (Figure 3). Recognition of the skin signs in this condition is essential to patient survival. As an intern, I once had an attending teach me that patients with necrotizing fasciitis only have a couple of hours to live. The rapid unfolding of this flesh-eating disease and its high morbidity and mortality has led to recent attention in the press and media.
Although necrotizing fasciitis may be caused by several different bacterial organisms (eg, gram positive, gram negative, polymicrobial), it usually is rapidly progressive, destroying muscle and subcutaneous tissues in a matter of hours.3 Bacteria usually enter through a traumatic or present wound and quickly move along fascial planes, destroying blood vessels and whatever subcutaneous tissues happen to be in the way. Within the first few hours, the involved area that was initially erythematous becomes indurated, woody, extremely painful, and dusky, indicating a lack of circulation to the area. Extensive debridement is required until reaching noninfected tissue that is no longer purulent, necrotic, or woody to the touch. If necrotizing fasciitis is not diagnosed and treated early, patients may lose one or several limbs and death may occur.
Key findings of necrotizing fasciitis include systemic toxicity, localized painful induration, well-defined dusky blue discoloration, and a lack of bleeding or purulent discharge on incision and squeezing of the affected tissue. Crepitation or a crackling sensation can occasionally be felt when palpating the area secondary to gas formation in the tissue, though it is not always present. Patients with necrotizing fasciitis often initially present to dermatology clinics because the first manifestation happens to be in the skin. The role of dermatologists in treating this critical condition may prompt recognition and collaboration with other specialists to reach a viable outcome for the patient.3
Drug Reactions
Cutaneous drug eruptions usually are relatively benign, consisting of a morbilliform eruption often without any other accompanying symptoms. However, sometimes these reactions can present as exfoliative dermatitis or red man syndrome in which patients can develop total body erythema with diffuse scaling and pruritus.4 Aside from drug reactions, other causes of exfoliative dermatitis such as psoriasis, atopic and seborrheic dermatitis, mycosis fungoides, and lymphoma should be ruled out. Other drug eruptions that can be classified as dermatologic emergencies include leukocytoclastic vasculitis, severe urticaria or angioedema, erythema multiforme, or Stevens-Johnson syndrome and toxic epidermal necrolysis.
Severe Acne
If not treated promptly, serious cases of acne can lead to severe scarring and psychologic problems. Acne fulminans is characterized by a rapid eruption of suppurative and large, highly inflamed nodules, plaques, and cysts that result in ragged ulcerations and cicatrization of the chest, back, and occasionally the face. Systemic symptoms of fever, arthralgia, leukocytosis, and myalgia suggest an upregulation of the immune system in affected patients.
Final Comment
In summary, dermatologic emergencies do exist and some may present with characteristic skin findings. In almost all cases, collaboration with other departments such as trauma, burn, internal medicine, rheumatology, and infectious diseases is extremely helpful in diagnosing and treating these medical emergencies. Collaboration can provide insight into how brainstorming through different approaches can lead to a better outcome whether it be solving the cause of a puzzling rash in a patient with multiple comorbidities or surgically removing a bullet from a trauma patient (Figure 4). Recognition of specific cutaneous manifestations and early diagnosis of dermatologic emergencies can be lifesaving.
1. McQueen A, Martin SA, Lio PA. Derm emergencies: detecting early signs of trouble. J Fam Pract. 2012;61:71-78.
2. Bennion S. Dermatologic emergencies. In: Fitzpatrick J, Morelli J, eds. Dermatology Secrets Plus. 4th ed. Philadelphia, PA: Mosby; 2011:442-452.
3. Sarani B, Strong M, Pascual J, et al. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208:279-288.
4. Wolf R, Orion E, Marcos B, et al. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171-181.
Dermatologic emergency may sound like an oxymoron, but there are many emergencies that dermatology residents may encounter in their careers. In some instances the skin is the primary organ that is affected, while in others cutaneous symptoms and life-threatening signs are important diagnostic clues for what may lie beneath the skin.
As residents who are occasionally on call or on consultation services, it is important for us to recognize dermatologic emergencies quickly because some of these conditions can acutely evolve and become lethal if a diagnosis is not made early in the disease course with the appropriate treatment administered. Dermatologic emergencies can range from severe drug reactions, infections, autoimmune exacerbations, and inflammatory conditions (eg, erythroderma) to environmental insults such as burns (Figure 1) and child abuse.1
Critical Infections
Some dermatologic emergencies are infectious in origin, and although these infections are most commonly bacterial (eg, necrotizing fasciitis), they also can range from viral to fungal (eg, mucormycosis) in nature. Some areas with large populations of immunocompromised patients (eg, human immunodeficiency virus–positive patients, organ transplant recipients) may warrant a high index of suspicion for possible zebras (rare conditions) and opportunistic infections that may quickly escalate to life-threatening situations.
Although few cutaneous manifestations in emergent infections are pathognomonic, they sometimes can be categorized according to the appearance of the primary lesion: erythrodermic (eg, staphylococcal scalded skin syndrome), maculopapular (eg, Lyme disease), purpuric/petechial (eg, Rocky Mountain spotted fever), pustular (eg, disseminated candidiasis), or vesicular (eg, neonatal herpes simplex virus)(Table). On consultations, dermatology residents frequently get called to evaluate hemorrhagic and ischemic lesions in inpatients (Figure 2). Aside from infectious causes, the differential diagnosis may include coagulation abnormalities (eg, concurrent anticoagulant therapies), vasculitides, poisoning, vascular disease, or Stevens-Johnson syndrome and toxic epidermal necrolysis, which can occasionally present with hemorrhagic lesions.1,2
Necrotizing Fasciitis
Dermatology residents may frequently encounter necrotizing fasciitis, either in clinic or on the wards (Figure 3). Recognition of the skin signs in this condition is essential to patient survival. As an intern, I once had an attending teach me that patients with necrotizing fasciitis only have a couple of hours to live. The rapid unfolding of this flesh-eating disease and its high morbidity and mortality has led to recent attention in the press and media.
Although necrotizing fasciitis may be caused by several different bacterial organisms (eg, gram positive, gram negative, polymicrobial), it usually is rapidly progressive, destroying muscle and subcutaneous tissues in a matter of hours.3 Bacteria usually enter through a traumatic or present wound and quickly move along fascial planes, destroying blood vessels and whatever subcutaneous tissues happen to be in the way. Within the first few hours, the involved area that was initially erythematous becomes indurated, woody, extremely painful, and dusky, indicating a lack of circulation to the area. Extensive debridement is required until reaching noninfected tissue that is no longer purulent, necrotic, or woody to the touch. If necrotizing fasciitis is not diagnosed and treated early, patients may lose one or several limbs and death may occur.
Key findings of necrotizing fasciitis include systemic toxicity, localized painful induration, well-defined dusky blue discoloration, and a lack of bleeding or purulent discharge on incision and squeezing of the affected tissue. Crepitation or a crackling sensation can occasionally be felt when palpating the area secondary to gas formation in the tissue, though it is not always present. Patients with necrotizing fasciitis often initially present to dermatology clinics because the first manifestation happens to be in the skin. The role of dermatologists in treating this critical condition may prompt recognition and collaboration with other specialists to reach a viable outcome for the patient.3
Drug Reactions
Cutaneous drug eruptions usually are relatively benign, consisting of a morbilliform eruption often without any other accompanying symptoms. However, sometimes these reactions can present as exfoliative dermatitis or red man syndrome in which patients can develop total body erythema with diffuse scaling and pruritus.4 Aside from drug reactions, other causes of exfoliative dermatitis such as psoriasis, atopic and seborrheic dermatitis, mycosis fungoides, and lymphoma should be ruled out. Other drug eruptions that can be classified as dermatologic emergencies include leukocytoclastic vasculitis, severe urticaria or angioedema, erythema multiforme, or Stevens-Johnson syndrome and toxic epidermal necrolysis.
Severe Acne
If not treated promptly, serious cases of acne can lead to severe scarring and psychologic problems. Acne fulminans is characterized by a rapid eruption of suppurative and large, highly inflamed nodules, plaques, and cysts that result in ragged ulcerations and cicatrization of the chest, back, and occasionally the face. Systemic symptoms of fever, arthralgia, leukocytosis, and myalgia suggest an upregulation of the immune system in affected patients.
Final Comment
In summary, dermatologic emergencies do exist and some may present with characteristic skin findings. In almost all cases, collaboration with other departments such as trauma, burn, internal medicine, rheumatology, and infectious diseases is extremely helpful in diagnosing and treating these medical emergencies. Collaboration can provide insight into how brainstorming through different approaches can lead to a better outcome whether it be solving the cause of a puzzling rash in a patient with multiple comorbidities or surgically removing a bullet from a trauma patient (Figure 4). Recognition of specific cutaneous manifestations and early diagnosis of dermatologic emergencies can be lifesaving.
Dermatologic emergency may sound like an oxymoron, but there are many emergencies that dermatology residents may encounter in their careers. In some instances the skin is the primary organ that is affected, while in others cutaneous symptoms and life-threatening signs are important diagnostic clues for what may lie beneath the skin.
As residents who are occasionally on call or on consultation services, it is important for us to recognize dermatologic emergencies quickly because some of these conditions can acutely evolve and become lethal if a diagnosis is not made early in the disease course with the appropriate treatment administered. Dermatologic emergencies can range from severe drug reactions, infections, autoimmune exacerbations, and inflammatory conditions (eg, erythroderma) to environmental insults such as burns (Figure 1) and child abuse.1
Critical Infections
Some dermatologic emergencies are infectious in origin, and although these infections are most commonly bacterial (eg, necrotizing fasciitis), they also can range from viral to fungal (eg, mucormycosis) in nature. Some areas with large populations of immunocompromised patients (eg, human immunodeficiency virus–positive patients, organ transplant recipients) may warrant a high index of suspicion for possible zebras (rare conditions) and opportunistic infections that may quickly escalate to life-threatening situations.
Although few cutaneous manifestations in emergent infections are pathognomonic, they sometimes can be categorized according to the appearance of the primary lesion: erythrodermic (eg, staphylococcal scalded skin syndrome), maculopapular (eg, Lyme disease), purpuric/petechial (eg, Rocky Mountain spotted fever), pustular (eg, disseminated candidiasis), or vesicular (eg, neonatal herpes simplex virus)(Table). On consultations, dermatology residents frequently get called to evaluate hemorrhagic and ischemic lesions in inpatients (Figure 2). Aside from infectious causes, the differential diagnosis may include coagulation abnormalities (eg, concurrent anticoagulant therapies), vasculitides, poisoning, vascular disease, or Stevens-Johnson syndrome and toxic epidermal necrolysis, which can occasionally present with hemorrhagic lesions.1,2
Necrotizing Fasciitis
Dermatology residents may frequently encounter necrotizing fasciitis, either in clinic or on the wards (Figure 3). Recognition of the skin signs in this condition is essential to patient survival. As an intern, I once had an attending teach me that patients with necrotizing fasciitis only have a couple of hours to live. The rapid unfolding of this flesh-eating disease and its high morbidity and mortality has led to recent attention in the press and media.
Although necrotizing fasciitis may be caused by several different bacterial organisms (eg, gram positive, gram negative, polymicrobial), it usually is rapidly progressive, destroying muscle and subcutaneous tissues in a matter of hours.3 Bacteria usually enter through a traumatic or present wound and quickly move along fascial planes, destroying blood vessels and whatever subcutaneous tissues happen to be in the way. Within the first few hours, the involved area that was initially erythematous becomes indurated, woody, extremely painful, and dusky, indicating a lack of circulation to the area. Extensive debridement is required until reaching noninfected tissue that is no longer purulent, necrotic, or woody to the touch. If necrotizing fasciitis is not diagnosed and treated early, patients may lose one or several limbs and death may occur.
Key findings of necrotizing fasciitis include systemic toxicity, localized painful induration, well-defined dusky blue discoloration, and a lack of bleeding or purulent discharge on incision and squeezing of the affected tissue. Crepitation or a crackling sensation can occasionally be felt when palpating the area secondary to gas formation in the tissue, though it is not always present. Patients with necrotizing fasciitis often initially present to dermatology clinics because the first manifestation happens to be in the skin. The role of dermatologists in treating this critical condition may prompt recognition and collaboration with other specialists to reach a viable outcome for the patient.3
Drug Reactions
Cutaneous drug eruptions usually are relatively benign, consisting of a morbilliform eruption often without any other accompanying symptoms. However, sometimes these reactions can present as exfoliative dermatitis or red man syndrome in which patients can develop total body erythema with diffuse scaling and pruritus.4 Aside from drug reactions, other causes of exfoliative dermatitis such as psoriasis, atopic and seborrheic dermatitis, mycosis fungoides, and lymphoma should be ruled out. Other drug eruptions that can be classified as dermatologic emergencies include leukocytoclastic vasculitis, severe urticaria or angioedema, erythema multiforme, or Stevens-Johnson syndrome and toxic epidermal necrolysis.
Severe Acne
If not treated promptly, serious cases of acne can lead to severe scarring and psychologic problems. Acne fulminans is characterized by a rapid eruption of suppurative and large, highly inflamed nodules, plaques, and cysts that result in ragged ulcerations and cicatrization of the chest, back, and occasionally the face. Systemic symptoms of fever, arthralgia, leukocytosis, and myalgia suggest an upregulation of the immune system in affected patients.
Final Comment
In summary, dermatologic emergencies do exist and some may present with characteristic skin findings. In almost all cases, collaboration with other departments such as trauma, burn, internal medicine, rheumatology, and infectious diseases is extremely helpful in diagnosing and treating these medical emergencies. Collaboration can provide insight into how brainstorming through different approaches can lead to a better outcome whether it be solving the cause of a puzzling rash in a patient with multiple comorbidities or surgically removing a bullet from a trauma patient (Figure 4). Recognition of specific cutaneous manifestations and early diagnosis of dermatologic emergencies can be lifesaving.
1. McQueen A, Martin SA, Lio PA. Derm emergencies: detecting early signs of trouble. J Fam Pract. 2012;61:71-78.
2. Bennion S. Dermatologic emergencies. In: Fitzpatrick J, Morelli J, eds. Dermatology Secrets Plus. 4th ed. Philadelphia, PA: Mosby; 2011:442-452.
3. Sarani B, Strong M, Pascual J, et al. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208:279-288.
4. Wolf R, Orion E, Marcos B, et al. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171-181.
1. McQueen A, Martin SA, Lio PA. Derm emergencies: detecting early signs of trouble. J Fam Pract. 2012;61:71-78.
2. Bennion S. Dermatologic emergencies. In: Fitzpatrick J, Morelli J, eds. Dermatology Secrets Plus. 4th ed. Philadelphia, PA: Mosby; 2011:442-452.
3. Sarani B, Strong M, Pascual J, et al. Necrotizing fasciitis: current concepts and review of the literature. J Am Coll Surg. 2009;208:279-288.
4. Wolf R, Orion E, Marcos B, et al. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171-181.
Furuncular Myiasis in 2 American Travelers Returning From Senegal
Case Reports
Patient 1
A 16-year-old adolescent boy presented to the emergency department with painful, pruritic, erythematous nodules on the bilateral legs of 1 week’s duration. The lesions had developed 1 week after returning from a monthlong trip to Senegal with a volunteer youth group. He did not recall sustaining any painful insect bites or illnesses while traveling in Africa and only noticed the erythematous papules on the legs when he returned home to the United States. After consulting with his primary care physician and a local dermatologist, the patient began taking oral cephalexin for suspected bacterial furunculosis with no considerable improvement. Over the course of 1 week, the lesions became increasingly painful and pruritic, prompting a visit to the emergency department. Prior to his arrival, the patient reported squeezing a live worm from one of the lesions on the right ankle.
On presentation, the patient was afebrile (temperature, 36.7°C) and his vital signs revealed no abnormalities. Physical examination revealed tender erythematous nodules on the bilateral heels, ankles, and shins with pinpoint puncta noted at the center of many of the lesions (Figure 1). The nodules were warm and indurated and no pulsatile movement was appreciated. The legs appeared to be well perfused with intact sensation and motor function. The patient brought in the live mobile larva that he extruded from the lesion on the right ankle. Both the departments of infectious diseases and dermatology were consulted and a preliminary diagnosis of furuncular myiasis was made.
The lesions were occluded with petroleum jelly and the patient was instructed to follow-up with the dermatology department later that same day. On follow-up in the dermatology clinic, the tips of intact larvae were appreciated at the central puncta of some of the lesions (Figure 2). Lidocaine adrenaline tetracaine gel was applied to lesions on the legs for 40 minutes, then lidocaine gel 1% was injected into each lesion. On injection, immobile larvae were ejected from the central puncta of most of the lesions; the remaining lesions were treated via 3-mm punch biopsy as a means of extraction. Each nodule contained only a single larva, all of which were dead at the time of removal (Figure 3). The wounds were left open and the patient was instructed to continue treatment with cephalexin with leg elevation and rest. Pathologic examination of deep dermal skin sections revealed larval fragments encased by a thick chitinous cuticle with spines that were consistent with furuncular myiasis (Figures 4 and 5). Given the patient’s recent history of travel to Africa along with the morphology of the extracted specimens, the larvae were identified as Cordylobia anthropophaga, a common cause of furuncular myiasis in that region.
Patient 2
The next week, a 17-year-old adolescent girl who had been on the same trip to Senegal as patient 1 presented with 2 similar erythematous nodules with central crusts on the left inner thigh and buttock. On noticing the lesions approximately 3 days prior to presentation, the patient applied topical antibiotic ointment to each nodule, which incited the evacuation of white tube-shaped structures that were presented for examination. On presentation, the nodules were healing well. Given the patient’s travel history and physical examination, a presumptive diagnosis of furuncular myiasis from C anthropophaga also was made.

|
Comment
The term myiasis stems from the Greek term for fly and is used to describe the infestation of fly larvae in living vertebrates.1 Myiasis has many classifications, the 3 most common being furuncular, migratory, and wound myiasis, which are differentiated by the different fly species found in distinct regions of the world. Furuncular myiasis is the most benign form, usually affecting only a localized region of the skin; migratory myiasis is characterized by larvae traveling substantial distances from one anatomic site to another within the lower layers of the epidermis; and wound myiasis involves rapid reproduction of larvae in necrotic tissue with subsequent tissue destruction.2
The clinical presentation of the lesions noted in our patients suggested a diagnosis of furuncular myiasis, which commonly is caused by Dermatobia hominis, C anthropophaga, Cuterebra species, Wohlfahrtia vigil, and Wohlfahrtia opaca larvae.3Dermatobia hominis is the most common cause of furuncular myiasis and usually is found in Central and South America. Our patients likely developed an infestation of C anthropophaga (also known as the tumbu fly), a yellow-brown, 7- to 12-mm blowfly commonly found throughout tropical Africa.3 Although C anthropophaga is historically limited to sub-Saharan Africa, there has been a report of a case acquired in Portugal.4
In a review of the literature, C anthropophaga myiasis was documented in Italian travelers returning from Senegal5-7; our cases are unique because they represent North American travelers returning from Senegal with furuncular myiasis. Furuncular myiasis from C anthropophaga has been reported in travelers returning to North America from other African countries, including Angola,8 Tanzania,9-11 Kenya,9 Sierra Leone,12 and Ivory Coast.13 Several cases of ocular myiasis from D hominis and Oestrus ovis have been reported in European travelers returning from Tunisia.14,15
Tumbu fly infestations typically affect dogs and rodents but can arise in human hosts.3 Children may be affected by C anthropophaga furuncular myiasis more often than adults because they have thinner skin and less immunity to the larvae.2
|
There are 2 mechanisms by which infestation of human hosts by C anthropophaga can occur. Most commonly, female flies lay eggs in shady areas in soil that is contaminated by feces or urine. The hatched larvae can survive in the ground for up to 2 weeks and later attach to a host when prompted by heat or movement.3 Therefore, clothing set out to dry may be contaminated by this soil. Alternatively, female flies can lay eggs directly onto clothing that is contaminated by feces or urine and the larvae subsequently hatch outside the soil with easy access to human skin once the clothing is worn.2
Common penetration sites are the head, neck, and back, as well as areas covered by contaminated or infested clothing.2,3 Penetration of the human skin occurs instantly and is a painless process that is rarely noticed by the human host.3 The larvae burrow into the skin for 8 to 12 days, resulting in a furuncle that occasionally secretes a serous fluid.2 Within the first 2 days of infestation, the host may experience symptoms ranging from local pruritus to severe pain. Six days following initial onset, an intense inflammatory response may result in local lymphadenopathy along with fever and fatigue.2 The larvae use their posterior spiracles to create openings in the skin to create air holes that allow them to breathe.3 On physical examination, the spiracles generally appear as 1- to 3-mm dark linear streaks within furuncles, which is important in the diagnosis of C anthropophaga furuncular myiasis.1,3 If spiracles are not appreciated on initial examination, diagnosis can be made by submerging the affected areas in water or saliva to look for air bubbles arising from the central puncta of the lesions.1
All causes of furuncular myiasis are characterized by a ratio of 1 larva to 1 furuncle.16 Although most of these types of larvae that can cause furuncular myiasis result in single lesions, C anthropophaga infestation often produces several furuncles that may coalesce into plaques.1,2 The differential diagnosis for C anthropophaga furuncular myiasis includes pyoderma, impetigo, staphylococcal furunculosis, cutaneous leishmaniasis, infected cyst, retained foreign body, and facticial disease.2,3 Dracunculiasis also may be considered, which occurs after ingestion of contaminated water.2 Ultrasonography may be helpful for the diagnosis of furuncular myiasis, as it can facilitate identification of foreign bodies, abscesses, and even larvae in some cases.17 Definitive diagnosis of any type of myiasis involves extraction of the larva and identification of the family, genus, and species by a parasitologist.1 Some experts suggest rearing preserved live larvae with raw meat after extraction because adult specimens are more reliable than larvae for species diagnosis.1
Treatment of furuncular myiasis involves occlusion and extraction of the larvae from the skin. Suffocation of the larvae by occlusion of air holes with petroleum jelly, paraffin oil, bacon fat, glue, and other obstructing substances forces the larvae to emerge in search of oxygen, though immature larvae may be more reluctant than mature ones.2,3 Definitive treatment involves the direct removal of the larvae by surgery or expulsion by pressure, though it is recommended that lesions are pretreated with occlusive techniques.1,3 Other reported methods of extraction include injection of lidocaine and the use of a commercial venom extractor.1 It should be noted that rupture and incomplete extraction of larvae can lead to secondary infections and allergic reactions. Lesions can be pretreated with lidocaine gel prior to extraction, and antibiotics should be used in cases of secondary bacterial infection. Ivermectin also has been reported as a treatment of furuncular myiasis and other types of myiasis.1 Prevention of infestation by C anthropophaga includes avoidance of endemic areas, maintaining good hygiene, and ironing clothing or drying it in sunny locations.1,2 Overall, furuncular myiasis has a good prognosis with rapid recovery and a low incidence of complications.1
Conclusion
We present 2 cases of travelers returning to North America from Senegal with C anthropophaga furuncular myiasis. Careful review of travel history, physical examination, and identification of fly larvae are important for diagnosis. Individuals traveling to sub-Saharan Africa should avoid drying clothes in shady places and lying on the ground. They also are urged to iron their clothing before wearing it.
1. Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
2. McGraw TA, Turiansky GW. Cutaneous myiasis. J Am Acad Dermatol. 2008;58:907-926.
3. Robbins K, Khachemoune A. Cutaneous myiasis: a review of the common types of myiasis. Int J Dermatol. 2010;49:1092-1098.
4. Curtis SJ, Edwards C, Athulathmuda C, et al. Case of the month: cutaneous myiasis in a returning traveller from the Algarve: first report of tumbu maggots, Cordylobia anthropophaga, acquired in Portugal. Emerg Med J. 2006;23:236-237.
5. Veraldi S, Brusasco A, Süss L. Cutaneous myiasis caused by larvae of Cordylobia anthropophaga (Blanchard). Int J Dermatol. 1993;32:184-187.
6. Cultrera R, Dettori G, Calderaro A, et al. Cutaneous myiasis caused by Cordylobia anthropophaga (Blanchard 1872): description of 5 cases from costal regions of Senegal [in Italian]. Parassitologia. 1993;35:47-49.
7. Fusco FM, Nardiello S, Brancaccio G, et al. Cutaneous myiasis from Cordylobia anthropophaga in a traveller returning from Senegal: a case study [in Italian]. Infez Med. 2005;13:109-111.
8. Lee EJ, Robinson F. Furuncular myiasis of the face caused by larva of the tumbu fly (Cordylobia anthropophaga)[published online ahead of print July 21, 2006]. Eye (Lond). 2007;21:268-269.
9. Rice PL, Gleason N. Two cases of myiasis in the United States by the African tumbu fly, Cordylobia anthropophaga (Diptera, Calliphoridae). Am J Trop Med Hyg. 1972;21:62-65.
10. March CH. A case of “ver du Cayor” in Manhattan. Arch Dermatol. 1964;90:32-33.
11. Schorr WF. Tumbu-fly myiasis in Marshfield, Wis. Arch Dermatol. 1967;95:61-62.
12. Potter TS, Dorman MA, Ghaemi M, et al. Inflammatory papules on the back of a traveling businessman. tumbu
fly myiasis. Arch Dermatol. 1995;131:951, 954.
13. Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
14. Kaouech E, Kallel K, Belhadj S, et al. Dermatobia hominis furuncular myiasis in a man returning from Latin America: first imported case in Tunisia [in French]. Med Trop (Mars). 2010;70:135-136.
15. Zayani A, Chaabouni M, Gouiaa R, et al. Conjuctival myiasis. 23 cases in the Tunisian Sahel [in French]. Arch Inst Pasteur Tunis. 1989;66:289-292.
16. Latorre M, Ullate JV, Sanchez J, et al. A case of myiasis due to Dermatobia hominis. Eur J Clin Microbiol Infect Dis. 1993;12:968-969.
17. Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominis: a case of human botfly infestation [published online ahead of print February 1, 2010]. J Emerg Med. 2012;43:618-621.
Case Reports
Patient 1
A 16-year-old adolescent boy presented to the emergency department with painful, pruritic, erythematous nodules on the bilateral legs of 1 week’s duration. The lesions had developed 1 week after returning from a monthlong trip to Senegal with a volunteer youth group. He did not recall sustaining any painful insect bites or illnesses while traveling in Africa and only noticed the erythematous papules on the legs when he returned home to the United States. After consulting with his primary care physician and a local dermatologist, the patient began taking oral cephalexin for suspected bacterial furunculosis with no considerable improvement. Over the course of 1 week, the lesions became increasingly painful and pruritic, prompting a visit to the emergency department. Prior to his arrival, the patient reported squeezing a live worm from one of the lesions on the right ankle.
On presentation, the patient was afebrile (temperature, 36.7°C) and his vital signs revealed no abnormalities. Physical examination revealed tender erythematous nodules on the bilateral heels, ankles, and shins with pinpoint puncta noted at the center of many of the lesions (Figure 1). The nodules were warm and indurated and no pulsatile movement was appreciated. The legs appeared to be well perfused with intact sensation and motor function. The patient brought in the live mobile larva that he extruded from the lesion on the right ankle. Both the departments of infectious diseases and dermatology were consulted and a preliminary diagnosis of furuncular myiasis was made.
The lesions were occluded with petroleum jelly and the patient was instructed to follow-up with the dermatology department later that same day. On follow-up in the dermatology clinic, the tips of intact larvae were appreciated at the central puncta of some of the lesions (Figure 2). Lidocaine adrenaline tetracaine gel was applied to lesions on the legs for 40 minutes, then lidocaine gel 1% was injected into each lesion. On injection, immobile larvae were ejected from the central puncta of most of the lesions; the remaining lesions were treated via 3-mm punch biopsy as a means of extraction. Each nodule contained only a single larva, all of which were dead at the time of removal (Figure 3). The wounds were left open and the patient was instructed to continue treatment with cephalexin with leg elevation and rest. Pathologic examination of deep dermal skin sections revealed larval fragments encased by a thick chitinous cuticle with spines that were consistent with furuncular myiasis (Figures 4 and 5). Given the patient’s recent history of travel to Africa along with the morphology of the extracted specimens, the larvae were identified as Cordylobia anthropophaga, a common cause of furuncular myiasis in that region.
Patient 2
The next week, a 17-year-old adolescent girl who had been on the same trip to Senegal as patient 1 presented with 2 similar erythematous nodules with central crusts on the left inner thigh and buttock. On noticing the lesions approximately 3 days prior to presentation, the patient applied topical antibiotic ointment to each nodule, which incited the evacuation of white tube-shaped structures that were presented for examination. On presentation, the nodules were healing well. Given the patient’s travel history and physical examination, a presumptive diagnosis of furuncular myiasis from C anthropophaga also was made.


|
Comment
The term myiasis stems from the Greek term for fly and is used to describe the infestation of fly larvae in living vertebrates.1 Myiasis has many classifications, the 3 most common being furuncular, migratory, and wound myiasis, which are differentiated by the different fly species found in distinct regions of the world. Furuncular myiasis is the most benign form, usually affecting only a localized region of the skin; migratory myiasis is characterized by larvae traveling substantial distances from one anatomic site to another within the lower layers of the epidermis; and wound myiasis involves rapid reproduction of larvae in necrotic tissue with subsequent tissue destruction.2
The clinical presentation of the lesions noted in our patients suggested a diagnosis of furuncular myiasis, which commonly is caused by Dermatobia hominis, C anthropophaga, Cuterebra species, Wohlfahrtia vigil, and Wohlfahrtia opaca larvae.3Dermatobia hominis is the most common cause of furuncular myiasis and usually is found in Central and South America. Our patients likely developed an infestation of C anthropophaga (also known as the tumbu fly), a yellow-brown, 7- to 12-mm blowfly commonly found throughout tropical Africa.3 Although C anthropophaga is historically limited to sub-Saharan Africa, there has been a report of a case acquired in Portugal.4
In a review of the literature, C anthropophaga myiasis was documented in Italian travelers returning from Senegal5-7; our cases are unique because they represent North American travelers returning from Senegal with furuncular myiasis. Furuncular myiasis from C anthropophaga has been reported in travelers returning to North America from other African countries, including Angola,8 Tanzania,9-11 Kenya,9 Sierra Leone,12 and Ivory Coast.13 Several cases of ocular myiasis from D hominis and Oestrus ovis have been reported in European travelers returning from Tunisia.14,15
Tumbu fly infestations typically affect dogs and rodents but can arise in human hosts.3 Children may be affected by C anthropophaga furuncular myiasis more often than adults because they have thinner skin and less immunity to the larvae.2
|
There are 2 mechanisms by which infestation of human hosts by C anthropophaga can occur. Most commonly, female flies lay eggs in shady areas in soil that is contaminated by feces or urine. The hatched larvae can survive in the ground for up to 2 weeks and later attach to a host when prompted by heat or movement.3 Therefore, clothing set out to dry may be contaminated by this soil. Alternatively, female flies can lay eggs directly onto clothing that is contaminated by feces or urine and the larvae subsequently hatch outside the soil with easy access to human skin once the clothing is worn.2
Common penetration sites are the head, neck, and back, as well as areas covered by contaminated or infested clothing.2,3 Penetration of the human skin occurs instantly and is a painless process that is rarely noticed by the human host.3 The larvae burrow into the skin for 8 to 12 days, resulting in a furuncle that occasionally secretes a serous fluid.2 Within the first 2 days of infestation, the host may experience symptoms ranging from local pruritus to severe pain. Six days following initial onset, an intense inflammatory response may result in local lymphadenopathy along with fever and fatigue.2 The larvae use their posterior spiracles to create openings in the skin to create air holes that allow them to breathe.3 On physical examination, the spiracles generally appear as 1- to 3-mm dark linear streaks within furuncles, which is important in the diagnosis of C anthropophaga furuncular myiasis.1,3 If spiracles are not appreciated on initial examination, diagnosis can be made by submerging the affected areas in water or saliva to look for air bubbles arising from the central puncta of the lesions.1
All causes of furuncular myiasis are characterized by a ratio of 1 larva to 1 furuncle.16 Although most of these types of larvae that can cause furuncular myiasis result in single lesions, C anthropophaga infestation often produces several furuncles that may coalesce into plaques.1,2 The differential diagnosis for C anthropophaga furuncular myiasis includes pyoderma, impetigo, staphylococcal furunculosis, cutaneous leishmaniasis, infected cyst, retained foreign body, and facticial disease.2,3 Dracunculiasis also may be considered, which occurs after ingestion of contaminated water.2 Ultrasonography may be helpful for the diagnosis of furuncular myiasis, as it can facilitate identification of foreign bodies, abscesses, and even larvae in some cases.17 Definitive diagnosis of any type of myiasis involves extraction of the larva and identification of the family, genus, and species by a parasitologist.1 Some experts suggest rearing preserved live larvae with raw meat after extraction because adult specimens are more reliable than larvae for species diagnosis.1
Treatment of furuncular myiasis involves occlusion and extraction of the larvae from the skin. Suffocation of the larvae by occlusion of air holes with petroleum jelly, paraffin oil, bacon fat, glue, and other obstructing substances forces the larvae to emerge in search of oxygen, though immature larvae may be more reluctant than mature ones.2,3 Definitive treatment involves the direct removal of the larvae by surgery or expulsion by pressure, though it is recommended that lesions are pretreated with occlusive techniques.1,3 Other reported methods of extraction include injection of lidocaine and the use of a commercial venom extractor.1 It should be noted that rupture and incomplete extraction of larvae can lead to secondary infections and allergic reactions. Lesions can be pretreated with lidocaine gel prior to extraction, and antibiotics should be used in cases of secondary bacterial infection. Ivermectin also has been reported as a treatment of furuncular myiasis and other types of myiasis.1 Prevention of infestation by C anthropophaga includes avoidance of endemic areas, maintaining good hygiene, and ironing clothing or drying it in sunny locations.1,2 Overall, furuncular myiasis has a good prognosis with rapid recovery and a low incidence of complications.1
Conclusion
We present 2 cases of travelers returning to North America from Senegal with C anthropophaga furuncular myiasis. Careful review of travel history, physical examination, and identification of fly larvae are important for diagnosis. Individuals traveling to sub-Saharan Africa should avoid drying clothes in shady places and lying on the ground. They also are urged to iron their clothing before wearing it.
Case Reports
Patient 1
A 16-year-old adolescent boy presented to the emergency department with painful, pruritic, erythematous nodules on the bilateral legs of 1 week’s duration. The lesions had developed 1 week after returning from a monthlong trip to Senegal with a volunteer youth group. He did not recall sustaining any painful insect bites or illnesses while traveling in Africa and only noticed the erythematous papules on the legs when he returned home to the United States. After consulting with his primary care physician and a local dermatologist, the patient began taking oral cephalexin for suspected bacterial furunculosis with no considerable improvement. Over the course of 1 week, the lesions became increasingly painful and pruritic, prompting a visit to the emergency department. Prior to his arrival, the patient reported squeezing a live worm from one of the lesions on the right ankle.
On presentation, the patient was afebrile (temperature, 36.7°C) and his vital signs revealed no abnormalities. Physical examination revealed tender erythematous nodules on the bilateral heels, ankles, and shins with pinpoint puncta noted at the center of many of the lesions (Figure 1). The nodules were warm and indurated and no pulsatile movement was appreciated. The legs appeared to be well perfused with intact sensation and motor function. The patient brought in the live mobile larva that he extruded from the lesion on the right ankle. Both the departments of infectious diseases and dermatology were consulted and a preliminary diagnosis of furuncular myiasis was made.
The lesions were occluded with petroleum jelly and the patient was instructed to follow-up with the dermatology department later that same day. On follow-up in the dermatology clinic, the tips of intact larvae were appreciated at the central puncta of some of the lesions (Figure 2). Lidocaine adrenaline tetracaine gel was applied to lesions on the legs for 40 minutes, then lidocaine gel 1% was injected into each lesion. On injection, immobile larvae were ejected from the central puncta of most of the lesions; the remaining lesions were treated via 3-mm punch biopsy as a means of extraction. Each nodule contained only a single larva, all of which were dead at the time of removal (Figure 3). The wounds were left open and the patient was instructed to continue treatment with cephalexin with leg elevation and rest. Pathologic examination of deep dermal skin sections revealed larval fragments encased by a thick chitinous cuticle with spines that were consistent with furuncular myiasis (Figures 4 and 5). Given the patient’s recent history of travel to Africa along with the morphology of the extracted specimens, the larvae were identified as Cordylobia anthropophaga, a common cause of furuncular myiasis in that region.
Patient 2
The next week, a 17-year-old adolescent girl who had been on the same trip to Senegal as patient 1 presented with 2 similar erythematous nodules with central crusts on the left inner thigh and buttock. On noticing the lesions approximately 3 days prior to presentation, the patient applied topical antibiotic ointment to each nodule, which incited the evacuation of white tube-shaped structures that were presented for examination. On presentation, the nodules were healing well. Given the patient’s travel history and physical examination, a presumptive diagnosis of furuncular myiasis from C anthropophaga also was made.


|
Comment
The term myiasis stems from the Greek term for fly and is used to describe the infestation of fly larvae in living vertebrates.1 Myiasis has many classifications, the 3 most common being furuncular, migratory, and wound myiasis, which are differentiated by the different fly species found in distinct regions of the world. Furuncular myiasis is the most benign form, usually affecting only a localized region of the skin; migratory myiasis is characterized by larvae traveling substantial distances from one anatomic site to another within the lower layers of the epidermis; and wound myiasis involves rapid reproduction of larvae in necrotic tissue with subsequent tissue destruction.2
The clinical presentation of the lesions noted in our patients suggested a diagnosis of furuncular myiasis, which commonly is caused by Dermatobia hominis, C anthropophaga, Cuterebra species, Wohlfahrtia vigil, and Wohlfahrtia opaca larvae.3Dermatobia hominis is the most common cause of furuncular myiasis and usually is found in Central and South America. Our patients likely developed an infestation of C anthropophaga (also known as the tumbu fly), a yellow-brown, 7- to 12-mm blowfly commonly found throughout tropical Africa.3 Although C anthropophaga is historically limited to sub-Saharan Africa, there has been a report of a case acquired in Portugal.4
In a review of the literature, C anthropophaga myiasis was documented in Italian travelers returning from Senegal5-7; our cases are unique because they represent North American travelers returning from Senegal with furuncular myiasis. Furuncular myiasis from C anthropophaga has been reported in travelers returning to North America from other African countries, including Angola,8 Tanzania,9-11 Kenya,9 Sierra Leone,12 and Ivory Coast.13 Several cases of ocular myiasis from D hominis and Oestrus ovis have been reported in European travelers returning from Tunisia.14,15
Tumbu fly infestations typically affect dogs and rodents but can arise in human hosts.3 Children may be affected by C anthropophaga furuncular myiasis more often than adults because they have thinner skin and less immunity to the larvae.2
|
There are 2 mechanisms by which infestation of human hosts by C anthropophaga can occur. Most commonly, female flies lay eggs in shady areas in soil that is contaminated by feces or urine. The hatched larvae can survive in the ground for up to 2 weeks and later attach to a host when prompted by heat or movement.3 Therefore, clothing set out to dry may be contaminated by this soil. Alternatively, female flies can lay eggs directly onto clothing that is contaminated by feces or urine and the larvae subsequently hatch outside the soil with easy access to human skin once the clothing is worn.2
Common penetration sites are the head, neck, and back, as well as areas covered by contaminated or infested clothing.2,3 Penetration of the human skin occurs instantly and is a painless process that is rarely noticed by the human host.3 The larvae burrow into the skin for 8 to 12 days, resulting in a furuncle that occasionally secretes a serous fluid.2 Within the first 2 days of infestation, the host may experience symptoms ranging from local pruritus to severe pain. Six days following initial onset, an intense inflammatory response may result in local lymphadenopathy along with fever and fatigue.2 The larvae use their posterior spiracles to create openings in the skin to create air holes that allow them to breathe.3 On physical examination, the spiracles generally appear as 1- to 3-mm dark linear streaks within furuncles, which is important in the diagnosis of C anthropophaga furuncular myiasis.1,3 If spiracles are not appreciated on initial examination, diagnosis can be made by submerging the affected areas in water or saliva to look for air bubbles arising from the central puncta of the lesions.1
All causes of furuncular myiasis are characterized by a ratio of 1 larva to 1 furuncle.16 Although most of these types of larvae that can cause furuncular myiasis result in single lesions, C anthropophaga infestation often produces several furuncles that may coalesce into plaques.1,2 The differential diagnosis for C anthropophaga furuncular myiasis includes pyoderma, impetigo, staphylococcal furunculosis, cutaneous leishmaniasis, infected cyst, retained foreign body, and facticial disease.2,3 Dracunculiasis also may be considered, which occurs after ingestion of contaminated water.2 Ultrasonography may be helpful for the diagnosis of furuncular myiasis, as it can facilitate identification of foreign bodies, abscesses, and even larvae in some cases.17 Definitive diagnosis of any type of myiasis involves extraction of the larva and identification of the family, genus, and species by a parasitologist.1 Some experts suggest rearing preserved live larvae with raw meat after extraction because adult specimens are more reliable than larvae for species diagnosis.1
Treatment of furuncular myiasis involves occlusion and extraction of the larvae from the skin. Suffocation of the larvae by occlusion of air holes with petroleum jelly, paraffin oil, bacon fat, glue, and other obstructing substances forces the larvae to emerge in search of oxygen, though immature larvae may be more reluctant than mature ones.2,3 Definitive treatment involves the direct removal of the larvae by surgery or expulsion by pressure, though it is recommended that lesions are pretreated with occlusive techniques.1,3 Other reported methods of extraction include injection of lidocaine and the use of a commercial venom extractor.1 It should be noted that rupture and incomplete extraction of larvae can lead to secondary infections and allergic reactions. Lesions can be pretreated with lidocaine gel prior to extraction, and antibiotics should be used in cases of secondary bacterial infection. Ivermectin also has been reported as a treatment of furuncular myiasis and other types of myiasis.1 Prevention of infestation by C anthropophaga includes avoidance of endemic areas, maintaining good hygiene, and ironing clothing or drying it in sunny locations.1,2 Overall, furuncular myiasis has a good prognosis with rapid recovery and a low incidence of complications.1
Conclusion
We present 2 cases of travelers returning to North America from Senegal with C anthropophaga furuncular myiasis. Careful review of travel history, physical examination, and identification of fly larvae are important for diagnosis. Individuals traveling to sub-Saharan Africa should avoid drying clothes in shady places and lying on the ground. They also are urged to iron their clothing before wearing it.
1. Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
2. McGraw TA, Turiansky GW. Cutaneous myiasis. J Am Acad Dermatol. 2008;58:907-926.
3. Robbins K, Khachemoune A. Cutaneous myiasis: a review of the common types of myiasis. Int J Dermatol. 2010;49:1092-1098.
4. Curtis SJ, Edwards C, Athulathmuda C, et al. Case of the month: cutaneous myiasis in a returning traveller from the Algarve: first report of tumbu maggots, Cordylobia anthropophaga, acquired in Portugal. Emerg Med J. 2006;23:236-237.
5. Veraldi S, Brusasco A, Süss L. Cutaneous myiasis caused by larvae of Cordylobia anthropophaga (Blanchard). Int J Dermatol. 1993;32:184-187.
6. Cultrera R, Dettori G, Calderaro A, et al. Cutaneous myiasis caused by Cordylobia anthropophaga (Blanchard 1872): description of 5 cases from costal regions of Senegal [in Italian]. Parassitologia. 1993;35:47-49.
7. Fusco FM, Nardiello S, Brancaccio G, et al. Cutaneous myiasis from Cordylobia anthropophaga in a traveller returning from Senegal: a case study [in Italian]. Infez Med. 2005;13:109-111.
8. Lee EJ, Robinson F. Furuncular myiasis of the face caused by larva of the tumbu fly (Cordylobia anthropophaga)[published online ahead of print July 21, 2006]. Eye (Lond). 2007;21:268-269.
9. Rice PL, Gleason N. Two cases of myiasis in the United States by the African tumbu fly, Cordylobia anthropophaga (Diptera, Calliphoridae). Am J Trop Med Hyg. 1972;21:62-65.
10. March CH. A case of “ver du Cayor” in Manhattan. Arch Dermatol. 1964;90:32-33.
11. Schorr WF. Tumbu-fly myiasis in Marshfield, Wis. Arch Dermatol. 1967;95:61-62.
12. Potter TS, Dorman MA, Ghaemi M, et al. Inflammatory papules on the back of a traveling businessman. tumbu
fly myiasis. Arch Dermatol. 1995;131:951, 954.
13. Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
14. Kaouech E, Kallel K, Belhadj S, et al. Dermatobia hominis furuncular myiasis in a man returning from Latin America: first imported case in Tunisia [in French]. Med Trop (Mars). 2010;70:135-136.
15. Zayani A, Chaabouni M, Gouiaa R, et al. Conjuctival myiasis. 23 cases in the Tunisian Sahel [in French]. Arch Inst Pasteur Tunis. 1989;66:289-292.
16. Latorre M, Ullate JV, Sanchez J, et al. A case of myiasis due to Dermatobia hominis. Eur J Clin Microbiol Infect Dis. 1993;12:968-969.
17. Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominis: a case of human botfly infestation [published online ahead of print February 1, 2010]. J Emerg Med. 2012;43:618-621.
1. Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
2. McGraw TA, Turiansky GW. Cutaneous myiasis. J Am Acad Dermatol. 2008;58:907-926.
3. Robbins K, Khachemoune A. Cutaneous myiasis: a review of the common types of myiasis. Int J Dermatol. 2010;49:1092-1098.
4. Curtis SJ, Edwards C, Athulathmuda C, et al. Case of the month: cutaneous myiasis in a returning traveller from the Algarve: first report of tumbu maggots, Cordylobia anthropophaga, acquired in Portugal. Emerg Med J. 2006;23:236-237.
5. Veraldi S, Brusasco A, Süss L. Cutaneous myiasis caused by larvae of Cordylobia anthropophaga (Blanchard). Int J Dermatol. 1993;32:184-187.
6. Cultrera R, Dettori G, Calderaro A, et al. Cutaneous myiasis caused by Cordylobia anthropophaga (Blanchard 1872): description of 5 cases from costal regions of Senegal [in Italian]. Parassitologia. 1993;35:47-49.
7. Fusco FM, Nardiello S, Brancaccio G, et al. Cutaneous myiasis from Cordylobia anthropophaga in a traveller returning from Senegal: a case study [in Italian]. Infez Med. 2005;13:109-111.
8. Lee EJ, Robinson F. Furuncular myiasis of the face caused by larva of the tumbu fly (Cordylobia anthropophaga)[published online ahead of print July 21, 2006]. Eye (Lond). 2007;21:268-269.
9. Rice PL, Gleason N. Two cases of myiasis in the United States by the African tumbu fly, Cordylobia anthropophaga (Diptera, Calliphoridae). Am J Trop Med Hyg. 1972;21:62-65.
10. March CH. A case of “ver du Cayor” in Manhattan. Arch Dermatol. 1964;90:32-33.
11. Schorr WF. Tumbu-fly myiasis in Marshfield, Wis. Arch Dermatol. 1967;95:61-62.
12. Potter TS, Dorman MA, Ghaemi M, et al. Inflammatory papules on the back of a traveling businessman. tumbu
fly myiasis. Arch Dermatol. 1995;131:951, 954.
13. Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
14. Kaouech E, Kallel K, Belhadj S, et al. Dermatobia hominis furuncular myiasis in a man returning from Latin America: first imported case in Tunisia [in French]. Med Trop (Mars). 2010;70:135-136.
15. Zayani A, Chaabouni M, Gouiaa R, et al. Conjuctival myiasis. 23 cases in the Tunisian Sahel [in French]. Arch Inst Pasteur Tunis. 1989;66:289-292.
16. Latorre M, Ullate JV, Sanchez J, et al. A case of myiasis due to Dermatobia hominis. Eur J Clin Microbiol Infect Dis. 1993;12:968-969.
17. Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominis: a case of human botfly infestation [published online ahead of print February 1, 2010]. J Emerg Med. 2012;43:618-621.
Practice Points
- Cutaneous myiasis is caused by an infestation of fly larvae and can present as furuncles (furuncular myiasis), migratory inflammatory linear plaques (migratory myiasis), and worsening tissue destruction in existing wounds (wound myiasis).
- Furuncular myiasis should be included in the differential diagnosis in patients with furuncular skin lesions who have recently traveled to Central America, South America, or sub-Saharan Africa.
- Furuncular myiasis may be treated by both occlusive and extraction techniques.
Hemorrhagic Bullous Lesions Due to Bacillus cereus in a Cirrhotic Patient
To the Editor:
A 42-year-old man with hypertension, hypothyroidism, and alcohol-related cirrhosis was admitted for evaluation of rapidly deteriorating mental status. He was referred from a rehabilitation facility where he had been admitted 4 days earlier after a hospitalization for hepatorenal syndrome and pneumonia. He was alert and ambulating until the day of the current admission. On arrival he was hypotensive(54/42 mm Hg); hypothermic (35°C, rectally); and unresponsive, except to painful stimuli. Jaundice, hepatosplenomegaly, ascites, and bilateral lower extremity edema were noted. There were multiple tense and flaccid bullous lesions containing serosanguineous fluid over both tibias and calves, without crepitus (Figure 1).

|
|
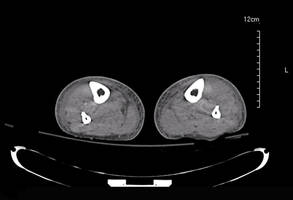
|
Laboratory test results revealed leukocytosis (total leukocytes, 10,900/mm3 [reference range, 4500–10,800/mm3), hypoglycemia (glucose, <20 mg/dL [reference range, 74–106 mg/dL]), renal insufficiency (serum creatinine, 2.5 mg/dL [reference range, 0.66–1.25 mg/dL]), metabolic acidosis (pH, 7.1 [reference range, 7.35–7.45]; bicarbonate, 13 mmol/L [reference range, 22–30 mmol/L]; lactic acid, 11.9 mmol/L [reference range, 0.7–2.1 mmol/L]), liver dysfunction (aspartate aminotransferase, 576 IU/L [reference range, 15–46 IU/L]), and coagulopathy with evidence of diffuse intravascular coagulation (total platelets, 75,000/mm3 [reference range, 150,000–450,000/mm3]; international normalized ratio, 9.5 [reference range, 0.8–1.2]; partial thromboplastin time, 108 seconds [reference range 23.0–35.0 seconds]; fibrinogen, 145 mg/dL [reference range, 228–501 mg/dL]; D-dimer, >20 µg/mL [reference range, 0.01–0.58 μg/mL]). Computed tomography of the pelvis and legs showed ascites, extensive subcutaneous edema, and cutaneous blisterlike lesions superior to the level of the ankles bilaterally. No gas, foreign bodies, collections, asymmetric facial thickening, or evidence of infection across tissue planes was present (Figures 2 and 3).
Specimens of blood and aspirates from the bullae at multiple lower leg sites were sent for microbiologic evaluation. The blood specimens were inoculated at bedside into aerobic and anaerobic blood culture bottles and incubated in an automated blood culture system. The aspirate samples were inoculated to trypticase soy agar with 5% sheep blood, Columbia-nalidixic acid agar, chocolate agar, MacConkey agar, and thioglycollate broth, which were incubated at 37ºC in air supplemented with 5% CO2, and to CDC anaerobic blood agar, which was incubated under anaerobic conditions. Gram-stained smears of the aspirates from the bullae demonstrated few granulocytes and numerous large gram-positive bacilli (Figure 4). By the next day, growth of large gram-positive bacilli was detected in both aerobic and anaerobic blood culture bottles and in pure culture from all the bullae samples. The bacterial colonies on sheep blood agar were opaque and white-gray in color, with a rough surface, undulate margins, and surrounding β hemolysis. The isolate was a motile, catalase-positive, arginine-positive, salicin-positive, lecithinase-positive, and penicillin-resistant organism that was identified as Bacillus cereus.
Antimicrobial susceptibility testing for B cereus has not been standardized, but evaluation by broth microdilution suggested decreased susceptibility to penicillin (minimum inhibitory concentration [MIC], 2 µg/mL) and clindamycin (MIC, 2 µg/mL), but retained susceptibility to ciprofloxacin (MIC, ≤0.25 µg/mL), tetracycline (MIC, ≤1 µg/mL), rifampin (MIC, ≤1 µg/mL), and vancomycin (MIC, ≤2 µg/mL).
The patient was admitted to the intensive care unit and was treated initially with fluid resuscitation; transfusions; ventilatory support; and intravenous vancomycin, clindamycin, and imipenem. This regimen was changed to vancomycin and ciprofloxacin when culture and susceptibility results became available to complete a 14-day course. Signs of sepsis resolved and the mental status and skin lesions improved. Ultimately, the patient died due to complications of hepatic failure.
Bacillus cereus is a rod-shaped, gram-positive, facultative, aerobic organism that is widely distributed in the environment.1 Spore formation makes B cereus resistant to most physical and chemical disinfection methods; as a consequence, it is a frequent contaminant in materials (eg, plants, dust, soil, sediment), foodstuffs, and clinical specimens.1
Traditionally considered in the context of foodborne illness, B cereus is recognized increasingly as a cause of systemic and local infections in both immunosuppressed and immunocompetent patients. Nongastrointestinal infections reported include fulminant bacteremia, pneumonia, meningitis, brain abscesses, endophthalmitis, necrotizing fasciitis, and central line catheter–related and cutaneous infections.1,2
Cutaneous lesions may have a variety of forms and appearance at initial presentation, including small papules or vesicles that progress into a rapidly spreading cellulitis1,2 with a characteristic serosanguineous draining fluid,2 single necrotic bullae,3 and gas-gangrenelike infections with extensive soft tissue involvement resembling clostridial myonecrosis.1,4 Single or multiple papulovesicular lesions can even mimic cutaneous anthrax.1-4 Necrotic or hemorrhagic bullous lesions,3 such as those observed in our patient, are rare.
Exposed areas such as extremities and digits are most often affected, presumably due to entrance of spores from soil, water, decaying organic material, or fomites through skin microabrasions or trauma-induced wounds.1 Once in the tissue, the crystalline surface protein layer (S-layer) of the bacilli promotes adhesion to human epithelial cells and neutrophils,5 followed by release of virulence factors including proteases, collagenases, lecithinaselike enzymes, necrotizing exotoxinlike hemolysins, phospholipases, and most importantly a dermonecrotic vascular permeability factor.1,5 Toxins produced by B cereus are similar to those closely related to Bacillus anthracis, the agent of anthrax.1,2
When large gram-positive bacilli are observed in tissue or wound specimens, initial therapy should address both aerobic (Bacillus species) and anaerobic (Clostridium species) organisms.1,4,6 Once B cereus is recovered, treatment should rely on susceptibility testing of the isolate. Bacillus cereus produces ß-lactamase, thus penicillin and cephalosporin should be avoided.1 Vancomycin, clindamycin, aminoglycosides, and fluoroquinolones are the drugs of choice.1,3,4,6 Daptomycin and linezolid also are active in vitro,1 but clinical experience with these agents is limited. Necrotic infection or deep tissue involvement requires surgical intervention.
Numerous other organisms can cause cellulitis and soft tissue infections with hemorrhagic bullae.1,3,6 Streptococci, particularly Streptococcus pyogenes, and occasionally staphylococci are the primary consideration in normal hosts without trauma.3,6 In immunocompromised patients, including those with cirrhosis, diabetes mellitus, and malignancy, Clostridium perfringens and gram-negative organisms such as Escherichia coli, other enteric bacteria including Pseudomonas aeruginosa, Aeromonas, and halophilic Vibrio species are more frequent.3,6
We describe a patient with underlying cirrhosis who developed bilateral lower extremity hemorrhagic bullous lesions and sepsis due to infection with B cereus, an emerging cause of serious infections in patients with underlying immunocompromising conditions such as cirrhosis, diabetes mellitus, and malignancy. Hemorrhagic bullae in immunocompromised patients are associated with sepsis and rapidly progressive illness, and rapid treatment is essential. Bacillus cereus should be included as a consideration in the differential diagnosis and management of patients presenting with bullous cellulitis and sepsis.
1. Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev. 2010;23:382-398.
2. Henrickson KJ. A second species of bacillus causing primary cutaneous disease. Int J Dermatol. 1990;29:19-20.
3. Liu BM, Hsiao CT, Chung KJ, et al. Hemorrhagic bullae represent an ominous sign for cirrhotic patients [published online ahead of print November 5, 2007]. J Emer Med. 2008;34:277-281.
4. Meredith FT, Fowler VG, Gautier M, et al. Bacillus cereus necrotizing cellulitis mimicking clostridial myonecrosis: case report and review of the literature. Scand J Infect Dis. 1997;29:528-529.
5. Kotiranta A, Lounatmaa K, Haapasalo M. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2000;2:189-198.
6. Lee CC, Chi CH, Lee NY, et al. Necrotizing fasciitis in patients with liver cirrhosis: predominance of monomicrobial gram-negative bacillary infections [published online ahead of print July 23, 2008]. Diagn Microbiol Infect Dis. 2008;62:219-225.
To the Editor:
A 42-year-old man with hypertension, hypothyroidism, and alcohol-related cirrhosis was admitted for evaluation of rapidly deteriorating mental status. He was referred from a rehabilitation facility where he had been admitted 4 days earlier after a hospitalization for hepatorenal syndrome and pneumonia. He was alert and ambulating until the day of the current admission. On arrival he was hypotensive(54/42 mm Hg); hypothermic (35°C, rectally); and unresponsive, except to painful stimuli. Jaundice, hepatosplenomegaly, ascites, and bilateral lower extremity edema were noted. There were multiple tense and flaccid bullous lesions containing serosanguineous fluid over both tibias and calves, without crepitus (Figure 1).

|

|

|
Laboratory test results revealed leukocytosis (total leukocytes, 10,900/mm3 [reference range, 4500–10,800/mm3), hypoglycemia (glucose, <20 mg/dL [reference range, 74–106 mg/dL]), renal insufficiency (serum creatinine, 2.5 mg/dL [reference range, 0.66–1.25 mg/dL]), metabolic acidosis (pH, 7.1 [reference range, 7.35–7.45]; bicarbonate, 13 mmol/L [reference range, 22–30 mmol/L]; lactic acid, 11.9 mmol/L [reference range, 0.7–2.1 mmol/L]), liver dysfunction (aspartate aminotransferase, 576 IU/L [reference range, 15–46 IU/L]), and coagulopathy with evidence of diffuse intravascular coagulation (total platelets, 75,000/mm3 [reference range, 150,000–450,000/mm3]; international normalized ratio, 9.5 [reference range, 0.8–1.2]; partial thromboplastin time, 108 seconds [reference range 23.0–35.0 seconds]; fibrinogen, 145 mg/dL [reference range, 228–501 mg/dL]; D-dimer, >20 µg/mL [reference range, 0.01–0.58 μg/mL]). Computed tomography of the pelvis and legs showed ascites, extensive subcutaneous edema, and cutaneous blisterlike lesions superior to the level of the ankles bilaterally. No gas, foreign bodies, collections, asymmetric facial thickening, or evidence of infection across tissue planes was present (Figures 2 and 3).
Specimens of blood and aspirates from the bullae at multiple lower leg sites were sent for microbiologic evaluation. The blood specimens were inoculated at bedside into aerobic and anaerobic blood culture bottles and incubated in an automated blood culture system. The aspirate samples were inoculated to trypticase soy agar with 5% sheep blood, Columbia-nalidixic acid agar, chocolate agar, MacConkey agar, and thioglycollate broth, which were incubated at 37ºC in air supplemented with 5% CO2, and to CDC anaerobic blood agar, which was incubated under anaerobic conditions. Gram-stained smears of the aspirates from the bullae demonstrated few granulocytes and numerous large gram-positive bacilli (Figure 4). By the next day, growth of large gram-positive bacilli was detected in both aerobic and anaerobic blood culture bottles and in pure culture from all the bullae samples. The bacterial colonies on sheep blood agar were opaque and white-gray in color, with a rough surface, undulate margins, and surrounding β hemolysis. The isolate was a motile, catalase-positive, arginine-positive, salicin-positive, lecithinase-positive, and penicillin-resistant organism that was identified as Bacillus cereus.
Antimicrobial susceptibility testing for B cereus has not been standardized, but evaluation by broth microdilution suggested decreased susceptibility to penicillin (minimum inhibitory concentration [MIC], 2 µg/mL) and clindamycin (MIC, 2 µg/mL), but retained susceptibility to ciprofloxacin (MIC, ≤0.25 µg/mL), tetracycline (MIC, ≤1 µg/mL), rifampin (MIC, ≤1 µg/mL), and vancomycin (MIC, ≤2 µg/mL).
The patient was admitted to the intensive care unit and was treated initially with fluid resuscitation; transfusions; ventilatory support; and intravenous vancomycin, clindamycin, and imipenem. This regimen was changed to vancomycin and ciprofloxacin when culture and susceptibility results became available to complete a 14-day course. Signs of sepsis resolved and the mental status and skin lesions improved. Ultimately, the patient died due to complications of hepatic failure.
Bacillus cereus is a rod-shaped, gram-positive, facultative, aerobic organism that is widely distributed in the environment.1 Spore formation makes B cereus resistant to most physical and chemical disinfection methods; as a consequence, it is a frequent contaminant in materials (eg, plants, dust, soil, sediment), foodstuffs, and clinical specimens.1
Traditionally considered in the context of foodborne illness, B cereus is recognized increasingly as a cause of systemic and local infections in both immunosuppressed and immunocompetent patients. Nongastrointestinal infections reported include fulminant bacteremia, pneumonia, meningitis, brain abscesses, endophthalmitis, necrotizing fasciitis, and central line catheter–related and cutaneous infections.1,2
Cutaneous lesions may have a variety of forms and appearance at initial presentation, including small papules or vesicles that progress into a rapidly spreading cellulitis1,2 with a characteristic serosanguineous draining fluid,2 single necrotic bullae,3 and gas-gangrenelike infections with extensive soft tissue involvement resembling clostridial myonecrosis.1,4 Single or multiple papulovesicular lesions can even mimic cutaneous anthrax.1-4 Necrotic or hemorrhagic bullous lesions,3 such as those observed in our patient, are rare.
Exposed areas such as extremities and digits are most often affected, presumably due to entrance of spores from soil, water, decaying organic material, or fomites through skin microabrasions or trauma-induced wounds.1 Once in the tissue, the crystalline surface protein layer (S-layer) of the bacilli promotes adhesion to human epithelial cells and neutrophils,5 followed by release of virulence factors including proteases, collagenases, lecithinaselike enzymes, necrotizing exotoxinlike hemolysins, phospholipases, and most importantly a dermonecrotic vascular permeability factor.1,5 Toxins produced by B cereus are similar to those closely related to Bacillus anthracis, the agent of anthrax.1,2
When large gram-positive bacilli are observed in tissue or wound specimens, initial therapy should address both aerobic (Bacillus species) and anaerobic (Clostridium species) organisms.1,4,6 Once B cereus is recovered, treatment should rely on susceptibility testing of the isolate. Bacillus cereus produces ß-lactamase, thus penicillin and cephalosporin should be avoided.1 Vancomycin, clindamycin, aminoglycosides, and fluoroquinolones are the drugs of choice.1,3,4,6 Daptomycin and linezolid also are active in vitro,1 but clinical experience with these agents is limited. Necrotic infection or deep tissue involvement requires surgical intervention.
Numerous other organisms can cause cellulitis and soft tissue infections with hemorrhagic bullae.1,3,6 Streptococci, particularly Streptococcus pyogenes, and occasionally staphylococci are the primary consideration in normal hosts without trauma.3,6 In immunocompromised patients, including those with cirrhosis, diabetes mellitus, and malignancy, Clostridium perfringens and gram-negative organisms such as Escherichia coli, other enteric bacteria including Pseudomonas aeruginosa, Aeromonas, and halophilic Vibrio species are more frequent.3,6
We describe a patient with underlying cirrhosis who developed bilateral lower extremity hemorrhagic bullous lesions and sepsis due to infection with B cereus, an emerging cause of serious infections in patients with underlying immunocompromising conditions such as cirrhosis, diabetes mellitus, and malignancy. Hemorrhagic bullae in immunocompromised patients are associated with sepsis and rapidly progressive illness, and rapid treatment is essential. Bacillus cereus should be included as a consideration in the differential diagnosis and management of patients presenting with bullous cellulitis and sepsis.
To the Editor:
A 42-year-old man with hypertension, hypothyroidism, and alcohol-related cirrhosis was admitted for evaluation of rapidly deteriorating mental status. He was referred from a rehabilitation facility where he had been admitted 4 days earlier after a hospitalization for hepatorenal syndrome and pneumonia. He was alert and ambulating until the day of the current admission. On arrival he was hypotensive(54/42 mm Hg); hypothermic (35°C, rectally); and unresponsive, except to painful stimuli. Jaundice, hepatosplenomegaly, ascites, and bilateral lower extremity edema were noted. There were multiple tense and flaccid bullous lesions containing serosanguineous fluid over both tibias and calves, without crepitus (Figure 1).

|

|

|
Laboratory test results revealed leukocytosis (total leukocytes, 10,900/mm3 [reference range, 4500–10,800/mm3), hypoglycemia (glucose, <20 mg/dL [reference range, 74–106 mg/dL]), renal insufficiency (serum creatinine, 2.5 mg/dL [reference range, 0.66–1.25 mg/dL]), metabolic acidosis (pH, 7.1 [reference range, 7.35–7.45]; bicarbonate, 13 mmol/L [reference range, 22–30 mmol/L]; lactic acid, 11.9 mmol/L [reference range, 0.7–2.1 mmol/L]), liver dysfunction (aspartate aminotransferase, 576 IU/L [reference range, 15–46 IU/L]), and coagulopathy with evidence of diffuse intravascular coagulation (total platelets, 75,000/mm3 [reference range, 150,000–450,000/mm3]; international normalized ratio, 9.5 [reference range, 0.8–1.2]; partial thromboplastin time, 108 seconds [reference range 23.0–35.0 seconds]; fibrinogen, 145 mg/dL [reference range, 228–501 mg/dL]; D-dimer, >20 µg/mL [reference range, 0.01–0.58 μg/mL]). Computed tomography of the pelvis and legs showed ascites, extensive subcutaneous edema, and cutaneous blisterlike lesions superior to the level of the ankles bilaterally. No gas, foreign bodies, collections, asymmetric facial thickening, or evidence of infection across tissue planes was present (Figures 2 and 3).
Specimens of blood and aspirates from the bullae at multiple lower leg sites were sent for microbiologic evaluation. The blood specimens were inoculated at bedside into aerobic and anaerobic blood culture bottles and incubated in an automated blood culture system. The aspirate samples were inoculated to trypticase soy agar with 5% sheep blood, Columbia-nalidixic acid agar, chocolate agar, MacConkey agar, and thioglycollate broth, which were incubated at 37ºC in air supplemented with 5% CO2, and to CDC anaerobic blood agar, which was incubated under anaerobic conditions. Gram-stained smears of the aspirates from the bullae demonstrated few granulocytes and numerous large gram-positive bacilli (Figure 4). By the next day, growth of large gram-positive bacilli was detected in both aerobic and anaerobic blood culture bottles and in pure culture from all the bullae samples. The bacterial colonies on sheep blood agar were opaque and white-gray in color, with a rough surface, undulate margins, and surrounding β hemolysis. The isolate was a motile, catalase-positive, arginine-positive, salicin-positive, lecithinase-positive, and penicillin-resistant organism that was identified as Bacillus cereus.
Antimicrobial susceptibility testing for B cereus has not been standardized, but evaluation by broth microdilution suggested decreased susceptibility to penicillin (minimum inhibitory concentration [MIC], 2 µg/mL) and clindamycin (MIC, 2 µg/mL), but retained susceptibility to ciprofloxacin (MIC, ≤0.25 µg/mL), tetracycline (MIC, ≤1 µg/mL), rifampin (MIC, ≤1 µg/mL), and vancomycin (MIC, ≤2 µg/mL).
The patient was admitted to the intensive care unit and was treated initially with fluid resuscitation; transfusions; ventilatory support; and intravenous vancomycin, clindamycin, and imipenem. This regimen was changed to vancomycin and ciprofloxacin when culture and susceptibility results became available to complete a 14-day course. Signs of sepsis resolved and the mental status and skin lesions improved. Ultimately, the patient died due to complications of hepatic failure.
Bacillus cereus is a rod-shaped, gram-positive, facultative, aerobic organism that is widely distributed in the environment.1 Spore formation makes B cereus resistant to most physical and chemical disinfection methods; as a consequence, it is a frequent contaminant in materials (eg, plants, dust, soil, sediment), foodstuffs, and clinical specimens.1
Traditionally considered in the context of foodborne illness, B cereus is recognized increasingly as a cause of systemic and local infections in both immunosuppressed and immunocompetent patients. Nongastrointestinal infections reported include fulminant bacteremia, pneumonia, meningitis, brain abscesses, endophthalmitis, necrotizing fasciitis, and central line catheter–related and cutaneous infections.1,2
Cutaneous lesions may have a variety of forms and appearance at initial presentation, including small papules or vesicles that progress into a rapidly spreading cellulitis1,2 with a characteristic serosanguineous draining fluid,2 single necrotic bullae,3 and gas-gangrenelike infections with extensive soft tissue involvement resembling clostridial myonecrosis.1,4 Single or multiple papulovesicular lesions can even mimic cutaneous anthrax.1-4 Necrotic or hemorrhagic bullous lesions,3 such as those observed in our patient, are rare.
Exposed areas such as extremities and digits are most often affected, presumably due to entrance of spores from soil, water, decaying organic material, or fomites through skin microabrasions or trauma-induced wounds.1 Once in the tissue, the crystalline surface protein layer (S-layer) of the bacilli promotes adhesion to human epithelial cells and neutrophils,5 followed by release of virulence factors including proteases, collagenases, lecithinaselike enzymes, necrotizing exotoxinlike hemolysins, phospholipases, and most importantly a dermonecrotic vascular permeability factor.1,5 Toxins produced by B cereus are similar to those closely related to Bacillus anthracis, the agent of anthrax.1,2
When large gram-positive bacilli are observed in tissue or wound specimens, initial therapy should address both aerobic (Bacillus species) and anaerobic (Clostridium species) organisms.1,4,6 Once B cereus is recovered, treatment should rely on susceptibility testing of the isolate. Bacillus cereus produces ß-lactamase, thus penicillin and cephalosporin should be avoided.1 Vancomycin, clindamycin, aminoglycosides, and fluoroquinolones are the drugs of choice.1,3,4,6 Daptomycin and linezolid also are active in vitro,1 but clinical experience with these agents is limited. Necrotic infection or deep tissue involvement requires surgical intervention.
Numerous other organisms can cause cellulitis and soft tissue infections with hemorrhagic bullae.1,3,6 Streptococci, particularly Streptococcus pyogenes, and occasionally staphylococci are the primary consideration in normal hosts without trauma.3,6 In immunocompromised patients, including those with cirrhosis, diabetes mellitus, and malignancy, Clostridium perfringens and gram-negative organisms such as Escherichia coli, other enteric bacteria including Pseudomonas aeruginosa, Aeromonas, and halophilic Vibrio species are more frequent.3,6
We describe a patient with underlying cirrhosis who developed bilateral lower extremity hemorrhagic bullous lesions and sepsis due to infection with B cereus, an emerging cause of serious infections in patients with underlying immunocompromising conditions such as cirrhosis, diabetes mellitus, and malignancy. Hemorrhagic bullae in immunocompromised patients are associated with sepsis and rapidly progressive illness, and rapid treatment is essential. Bacillus cereus should be included as a consideration in the differential diagnosis and management of patients presenting with bullous cellulitis and sepsis.
1. Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev. 2010;23:382-398.
2. Henrickson KJ. A second species of bacillus causing primary cutaneous disease. Int J Dermatol. 1990;29:19-20.
3. Liu BM, Hsiao CT, Chung KJ, et al. Hemorrhagic bullae represent an ominous sign for cirrhotic patients [published online ahead of print November 5, 2007]. J Emer Med. 2008;34:277-281.
4. Meredith FT, Fowler VG, Gautier M, et al. Bacillus cereus necrotizing cellulitis mimicking clostridial myonecrosis: case report and review of the literature. Scand J Infect Dis. 1997;29:528-529.
5. Kotiranta A, Lounatmaa K, Haapasalo M. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2000;2:189-198.
6. Lee CC, Chi CH, Lee NY, et al. Necrotizing fasciitis in patients with liver cirrhosis: predominance of monomicrobial gram-negative bacillary infections [published online ahead of print July 23, 2008]. Diagn Microbiol Infect Dis. 2008;62:219-225.
1. Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev. 2010;23:382-398.
2. Henrickson KJ. A second species of bacillus causing primary cutaneous disease. Int J Dermatol. 1990;29:19-20.
3. Liu BM, Hsiao CT, Chung KJ, et al. Hemorrhagic bullae represent an ominous sign for cirrhotic patients [published online ahead of print November 5, 2007]. J Emer Med. 2008;34:277-281.
4. Meredith FT, Fowler VG, Gautier M, et al. Bacillus cereus necrotizing cellulitis mimicking clostridial myonecrosis: case report and review of the literature. Scand J Infect Dis. 1997;29:528-529.
5. Kotiranta A, Lounatmaa K, Haapasalo M. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2000;2:189-198.
6. Lee CC, Chi CH, Lee NY, et al. Necrotizing fasciitis in patients with liver cirrhosis: predominance of monomicrobial gram-negative bacillary infections [published online ahead of print July 23, 2008]. Diagn Microbiol Infect Dis. 2008;62:219-225.