User login
The antimicrobial peptide that even Pharma can love
Fastest peptide north, south, east, aaaaand west of the Pecos
Bacterial infections are supposed to be simple. You get infected, you get an antibiotic to treat it. Easy. Some bacteria, though, don’t play by the rules. Those antibiotics may kill 99.9% of germs, but what about the 0.1% that gets left behind? With their fallen comrades out of the way, the accidentally drug resistant species are free to inherit the Earth.
Antibiotic resistance is thus a major concern for the medical community. Naturally, anything that prevents doctors from successfully curing sick people is a priority. Unless you’re a major pharmaceutical company that has been loath to develop new drugs that can beat antibiotic-resistant bacteria. Blah blah, time and money, blah blah, long time between development and market application, blah blah, no profit. We all know the story with pharmaceutical companies.
Research from other sources has continued, however, and Brazilian scientists recently published research involving a peptide known as plantaricin 149. This peptide, derived from the bacterium Lactobacillus plantarum, has been known for nearly 30 years to have antibacterial properties. Pln149 in its natural state, though, is not particularly efficient at bacteria-killing. Fortunately, we have science and technology on our side.
The researchers synthesized 20 analogs of Pln149, of which Pln149-PEP20 had the best results. The elegantly named compound is less than half the size of the original peptide, less toxic, and far better at killing any and all drug-resistant bacteria the researchers threw at it. How much better? Pln149-PEP20 started killing bacteria less than an hour after being introduced in lab trials.
The research is just in its early days – just because something is less toxic doesn’t necessarily mean you want to go and help yourself to it – but we can only hope that those lovely pharmaceutical companies deign to look down upon us and actually develop a drug utilizing Pln149-PEP20 to, you know, actually help sick people, instead of trying to build monopolies or avoiding paying billions in taxes. Yeah, we couldn’t keep a straight face through that last sentence either.
Speed healing: The wavy wound gets the swirl
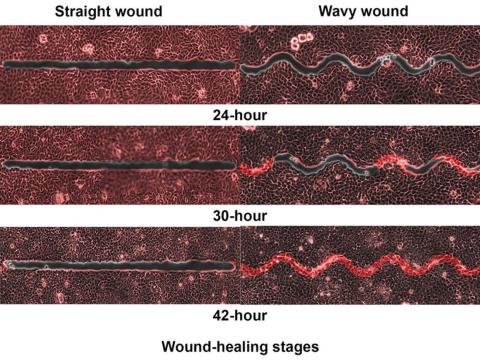
Did you know that wavy wounds heal faster than straight wounds? Well, we didn’t, but apparently quite a few people did, because somebody has been trying to figure out why wavy wounds heal faster than straight ones. Do the surgeons know about this? How about you dermatologists? Wavy over straight? We’re the media. We’re supposed to report this kind of stuff. Maybe hit us with a tweet next time you do something important, or push a TikTok our way, okay?
You could be more like the investigators at Nanyang Technological University in Singapore, who figured out the why and then released a statement about it.
They created synthetic wounds – some straight, some wavy – in micropatterned hydrogel substrates that mimicked human skin. Then they used an advanced optical technique known as particle image velocimetry to measure fluid flow and learn how cells moved to close the wound gaps.
The wavy wounds “induced more complex collective cell movements, such as a swirly, vortex-like motion,” according to the written statement from NTU Singapore. In the straight wounds, cell movements paralleled the wound front, “moving in straight lines like a marching band,” they pointed out, unlike some researchers who never call us unless they need money.
Complex epithelial cell movements are better, it turns out. Over an observation period of 64 hours the NTU team found that the healing efficiency of wavy gaps – measured by the area covered by the cells over time – is nearly five times faster than straight gaps.
The complex motion “enabled cells to quickly connect with similar cells on the opposite site of the wound edge, forming a bridge and closing the wavy wound gaps faster than straight gaps,” explained lead author Xu Hongmei, a doctoral student at NTU’s School of Mechanical and Aerospace Engineering, who seems to have time to toss out a tumblr or two to keep the press informed.
As for the rest of you, would it kill you to pick up a phone once in a while? Maybe let a journalist know that you’re still alive? We have feelings too, you know, and we worry.
A little Jekyll, a little Hyde, and a little shop of horrors
More “Little Shop of Horrors” references are coming, so be prepared.
We begin with Triphyophyllum peltatum. This woody vine is of great interest to medical and pharmaceutical researchers because its constituents have shown promise against pancreatic cancer and leukemia cells, among others, along with the pathogens that cause malaria and other diseases. There is another side, however. T. peltatum also has a tendency to turn into a realistic Audrey II when deprived.
No, of course they’re not craving human flesh, but it does become … carnivorous in its appetite.
T. peltatum, native to the West African tropics and not found in a New York florist shop, has the unique ability to change its diet and development based on the environmental circumstances. For some unknown reason, the leaves would develop adhesive traps in the form of sticky drops that capture insect prey. The plant is notoriously hard to grow, however, so no one could study the transformation under lab conditions. Until now.
A group of German scientists “exposed the plant to different stress factors, including deficiencies of various nutrients, and studied how it responded to each,” said Dr. Traud Winkelmann of Leibniz University Hannover. “Only in one case were we able to observe the formation of traps: in the case of a lack of phosphorus.”
Well, there you have it: phosphorus. We need it for healthy bones and teeth, which this plant doesn’t have to worry about, unlike its Tony Award–nominated counterpart. The investigators hope that their findings could lead to “future molecular analyses that will help understand the origins of carnivory,” but we’re guessing that a certain singing alien species will be left out of that research.
Fastest peptide north, south, east, aaaaand west of the Pecos
Bacterial infections are supposed to be simple. You get infected, you get an antibiotic to treat it. Easy. Some bacteria, though, don’t play by the rules. Those antibiotics may kill 99.9% of germs, but what about the 0.1% that gets left behind? With their fallen comrades out of the way, the accidentally drug resistant species are free to inherit the Earth.
Antibiotic resistance is thus a major concern for the medical community. Naturally, anything that prevents doctors from successfully curing sick people is a priority. Unless you’re a major pharmaceutical company that has been loath to develop new drugs that can beat antibiotic-resistant bacteria. Blah blah, time and money, blah blah, long time between development and market application, blah blah, no profit. We all know the story with pharmaceutical companies.
Research from other sources has continued, however, and Brazilian scientists recently published research involving a peptide known as plantaricin 149. This peptide, derived from the bacterium Lactobacillus plantarum, has been known for nearly 30 years to have antibacterial properties. Pln149 in its natural state, though, is not particularly efficient at bacteria-killing. Fortunately, we have science and technology on our side.
The researchers synthesized 20 analogs of Pln149, of which Pln149-PEP20 had the best results. The elegantly named compound is less than half the size of the original peptide, less toxic, and far better at killing any and all drug-resistant bacteria the researchers threw at it. How much better? Pln149-PEP20 started killing bacteria less than an hour after being introduced in lab trials.
The research is just in its early days – just because something is less toxic doesn’t necessarily mean you want to go and help yourself to it – but we can only hope that those lovely pharmaceutical companies deign to look down upon us and actually develop a drug utilizing Pln149-PEP20 to, you know, actually help sick people, instead of trying to build monopolies or avoiding paying billions in taxes. Yeah, we couldn’t keep a straight face through that last sentence either.
Speed healing: The wavy wound gets the swirl
Did you know that wavy wounds heal faster than straight wounds? Well, we didn’t, but apparently quite a few people did, because somebody has been trying to figure out why wavy wounds heal faster than straight ones. Do the surgeons know about this? How about you dermatologists? Wavy over straight? We’re the media. We’re supposed to report this kind of stuff. Maybe hit us with a tweet next time you do something important, or push a TikTok our way, okay?
You could be more like the investigators at Nanyang Technological University in Singapore, who figured out the why and then released a statement about it.
They created synthetic wounds – some straight, some wavy – in micropatterned hydrogel substrates that mimicked human skin. Then they used an advanced optical technique known as particle image velocimetry to measure fluid flow and learn how cells moved to close the wound gaps.
The wavy wounds “induced more complex collective cell movements, such as a swirly, vortex-like motion,” according to the written statement from NTU Singapore. In the straight wounds, cell movements paralleled the wound front, “moving in straight lines like a marching band,” they pointed out, unlike some researchers who never call us unless they need money.
Complex epithelial cell movements are better, it turns out. Over an observation period of 64 hours the NTU team found that the healing efficiency of wavy gaps – measured by the area covered by the cells over time – is nearly five times faster than straight gaps.
The complex motion “enabled cells to quickly connect with similar cells on the opposite site of the wound edge, forming a bridge and closing the wavy wound gaps faster than straight gaps,” explained lead author Xu Hongmei, a doctoral student at NTU’s School of Mechanical and Aerospace Engineering, who seems to have time to toss out a tumblr or two to keep the press informed.
As for the rest of you, would it kill you to pick up a phone once in a while? Maybe let a journalist know that you’re still alive? We have feelings too, you know, and we worry.
A little Jekyll, a little Hyde, and a little shop of horrors
More “Little Shop of Horrors” references are coming, so be prepared.
We begin with Triphyophyllum peltatum. This woody vine is of great interest to medical and pharmaceutical researchers because its constituents have shown promise against pancreatic cancer and leukemia cells, among others, along with the pathogens that cause malaria and other diseases. There is another side, however. T. peltatum also has a tendency to turn into a realistic Audrey II when deprived.
No, of course they’re not craving human flesh, but it does become … carnivorous in its appetite.
T. peltatum, native to the West African tropics and not found in a New York florist shop, has the unique ability to change its diet and development based on the environmental circumstances. For some unknown reason, the leaves would develop adhesive traps in the form of sticky drops that capture insect prey. The plant is notoriously hard to grow, however, so no one could study the transformation under lab conditions. Until now.
A group of German scientists “exposed the plant to different stress factors, including deficiencies of various nutrients, and studied how it responded to each,” said Dr. Traud Winkelmann of Leibniz University Hannover. “Only in one case were we able to observe the formation of traps: in the case of a lack of phosphorus.”
Well, there you have it: phosphorus. We need it for healthy bones and teeth, which this plant doesn’t have to worry about, unlike its Tony Award–nominated counterpart. The investigators hope that their findings could lead to “future molecular analyses that will help understand the origins of carnivory,” but we’re guessing that a certain singing alien species will be left out of that research.
Fastest peptide north, south, east, aaaaand west of the Pecos
Bacterial infections are supposed to be simple. You get infected, you get an antibiotic to treat it. Easy. Some bacteria, though, don’t play by the rules. Those antibiotics may kill 99.9% of germs, but what about the 0.1% that gets left behind? With their fallen comrades out of the way, the accidentally drug resistant species are free to inherit the Earth.
Antibiotic resistance is thus a major concern for the medical community. Naturally, anything that prevents doctors from successfully curing sick people is a priority. Unless you’re a major pharmaceutical company that has been loath to develop new drugs that can beat antibiotic-resistant bacteria. Blah blah, time and money, blah blah, long time between development and market application, blah blah, no profit. We all know the story with pharmaceutical companies.
Research from other sources has continued, however, and Brazilian scientists recently published research involving a peptide known as plantaricin 149. This peptide, derived from the bacterium Lactobacillus plantarum, has been known for nearly 30 years to have antibacterial properties. Pln149 in its natural state, though, is not particularly efficient at bacteria-killing. Fortunately, we have science and technology on our side.
The researchers synthesized 20 analogs of Pln149, of which Pln149-PEP20 had the best results. The elegantly named compound is less than half the size of the original peptide, less toxic, and far better at killing any and all drug-resistant bacteria the researchers threw at it. How much better? Pln149-PEP20 started killing bacteria less than an hour after being introduced in lab trials.
The research is just in its early days – just because something is less toxic doesn’t necessarily mean you want to go and help yourself to it – but we can only hope that those lovely pharmaceutical companies deign to look down upon us and actually develop a drug utilizing Pln149-PEP20 to, you know, actually help sick people, instead of trying to build monopolies or avoiding paying billions in taxes. Yeah, we couldn’t keep a straight face through that last sentence either.
Speed healing: The wavy wound gets the swirl
Did you know that wavy wounds heal faster than straight wounds? Well, we didn’t, but apparently quite a few people did, because somebody has been trying to figure out why wavy wounds heal faster than straight ones. Do the surgeons know about this? How about you dermatologists? Wavy over straight? We’re the media. We’re supposed to report this kind of stuff. Maybe hit us with a tweet next time you do something important, or push a TikTok our way, okay?
You could be more like the investigators at Nanyang Technological University in Singapore, who figured out the why and then released a statement about it.
They created synthetic wounds – some straight, some wavy – in micropatterned hydrogel substrates that mimicked human skin. Then they used an advanced optical technique known as particle image velocimetry to measure fluid flow and learn how cells moved to close the wound gaps.
The wavy wounds “induced more complex collective cell movements, such as a swirly, vortex-like motion,” according to the written statement from NTU Singapore. In the straight wounds, cell movements paralleled the wound front, “moving in straight lines like a marching band,” they pointed out, unlike some researchers who never call us unless they need money.
Complex epithelial cell movements are better, it turns out. Over an observation period of 64 hours the NTU team found that the healing efficiency of wavy gaps – measured by the area covered by the cells over time – is nearly five times faster than straight gaps.
The complex motion “enabled cells to quickly connect with similar cells on the opposite site of the wound edge, forming a bridge and closing the wavy wound gaps faster than straight gaps,” explained lead author Xu Hongmei, a doctoral student at NTU’s School of Mechanical and Aerospace Engineering, who seems to have time to toss out a tumblr or two to keep the press informed.
As for the rest of you, would it kill you to pick up a phone once in a while? Maybe let a journalist know that you’re still alive? We have feelings too, you know, and we worry.
A little Jekyll, a little Hyde, and a little shop of horrors
More “Little Shop of Horrors” references are coming, so be prepared.
We begin with Triphyophyllum peltatum. This woody vine is of great interest to medical and pharmaceutical researchers because its constituents have shown promise against pancreatic cancer and leukemia cells, among others, along with the pathogens that cause malaria and other diseases. There is another side, however. T. peltatum also has a tendency to turn into a realistic Audrey II when deprived.
No, of course they’re not craving human flesh, but it does become … carnivorous in its appetite.
T. peltatum, native to the West African tropics and not found in a New York florist shop, has the unique ability to change its diet and development based on the environmental circumstances. For some unknown reason, the leaves would develop adhesive traps in the form of sticky drops that capture insect prey. The plant is notoriously hard to grow, however, so no one could study the transformation under lab conditions. Until now.
A group of German scientists “exposed the plant to different stress factors, including deficiencies of various nutrients, and studied how it responded to each,” said Dr. Traud Winkelmann of Leibniz University Hannover. “Only in one case were we able to observe the formation of traps: in the case of a lack of phosphorus.”
Well, there you have it: phosphorus. We need it for healthy bones and teeth, which this plant doesn’t have to worry about, unlike its Tony Award–nominated counterpart. The investigators hope that their findings could lead to “future molecular analyses that will help understand the origins of carnivory,” but we’re guessing that a certain singing alien species will be left out of that research.
Acral Necrosis After PD-L1 Immune Checkpoint Inhibitor Therapy
To the Editor:
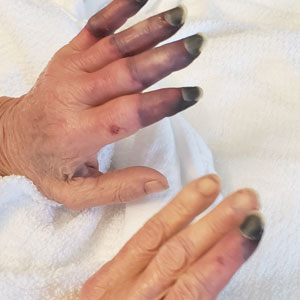
A 67-year-old woman presented to the hospital with painful hands and feet. Two weeks prior, the patient experienced a few days of intermittent purple discoloration of the fingers, followed by black discoloration of the fingers, toes, and nose with notable pain. She reported no illness preceding the presenting symptoms, and there was no progression of symptoms in the days preceding presentation.
The patient had a history of smoking. She had a medical history of chronic obstructive pulmonary disease as well as recurrent non–small cell lung cancer that was treated most recently with a 1-year course of the programmed death-ligand 1 (PD-L1) immune checkpoint inhibitor durvalumab (last treatment was 4 months prior to the current presentation).
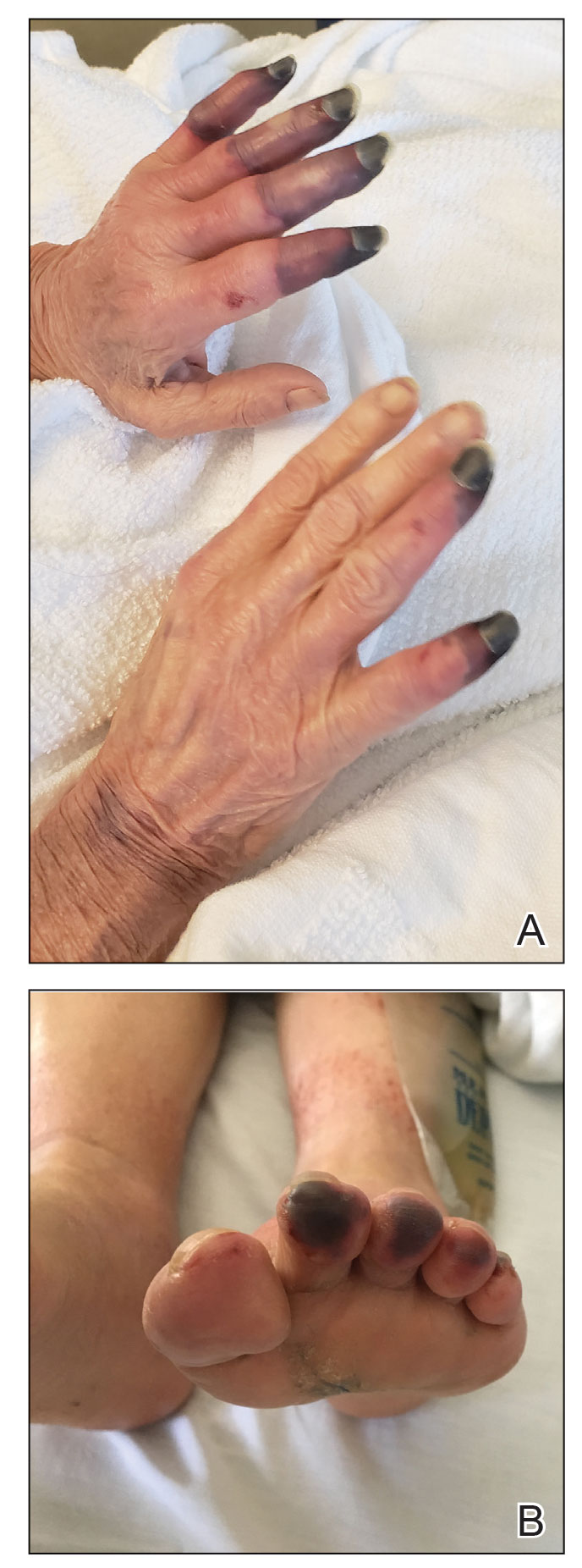
Physical examination revealed necrosis of the tips of the second, third, and fourth fingers of the left hand, as well as the tips of the third and fourth fingers of the right hand, progressing to purpura proximally on all involved fingers (Figure, A); scattered purpura and necrotic papules on the toe pads (Figure, B); and a 2- to 3-cm black plaque on the nasal tip. The patient was afebrile.
An embolic and vascular workup was performed. Transthoracic echocardiography was negative for thrombi, ankle brachial indices were within reference range, and computed tomography angiography revealed a few nonocclusive coronary plaques. Conventional angiography was not performed.
Laboratory testing revealed a mildly elevated level of cryofibrinogens (cryocrit, 2.5%); cold agglutinins (1:32); mild monoclonal κ IgG gammopathy (0.1 g/dL); and elevated inflammatory markers (C-reactive protein, 76 mg/L [reference range, 0–10 mg/L]; erythrocyte sedimentation rate, 38 mm/h [reference range, 0–20 mm/h]; fibrinogen, 571 mg/dL [reference range, 150–450 mg/dL]; and ferritin, 394 ng/mL [reference range, 10–180 ng/mL]). Additional laboratory studies were negative or within reference range, including tests of anti-RNA polymerase antibody, rheumatoid factor, antinuclear antibody, anticardiolipin antibody, anti-β2 glycoprotein antibody, antineutrophil cytoplasmic antibodies (myeloperoxidase and proteinase-3), cryoglobulins, and complement; human immunodeficiency virus and hepatitis B and C virus serologic studies; prothrombin time, partial thromboplastin time, and lupus anticoagulant; and a heparin-induced thrombocytopenia panel.
A skin biopsy adjacent to an area of necrosis on the finger showed thickened walls of dermal vessels, sparse leukocytoclastic debris, and evidence of recanalizing medium-sized vessels. Direct immunofluorescence studies were negative.
Based on the clinical history and histologic findings showing an absence of vasculitis, a diagnosis of acral necrosis associated with the PD-L1 immune checkpoint inhibitor durvalumab—a delayed immune-related event (DIRE)—was favored. The calcium channel blocker amlodipine was started at a dosage of 2.5 mg/d orally. Necrosis of the toes resolved over the course of 1 week; however, necrosis of the fingers remained unchanged. After 1 week of hospitalization, the patient was discharged at her request.
Acral necrosis following immune checkpoint inhibitor therapy has been reported as a rare and recalcitrant immune-related adverse event (AE).1-4 However, our patient’s symptoms occurred months after treatment was discontinued, which is consistent with a DIRE.5 The course of acral necrosis begins with acrocyanosis (a Raynaud disease–like phenomenon) of the fingers that progresses to necrosis. A history of Raynaud disease or other autoimmune disorder generally is absent.1 Our patient’s history indicated actively smoking at the time of presentation, similar to a case described by Khaddour et al.1 Similarly, in a case presented by Comont et al,3 the patient also had a history of smoking. In a recent study of acute vascular events associated with immune checkpoint inhibitors, 16 of 31 patients had a history of smoking.6
No definitive diagnostic laboratory or pathologic findings are associated with acral necrosis following immune checkpoint inhibitor therapy. Histopathologic analysis does not demonstrate vasculitis or other overt vascular pathology.2,3
The optimal treatment of immune checkpoint inhibitor–associated digital necrosis is unclear. Corticosteroids and discontinuation of the immune checkpoint inhibitor generally are employed,1-4 though treatment response has been variable. Other therapies such as calcium channel blockers (as in our case), sympathectomy,1 epoprostenol, botulinum injection, rituximab,2 and alprostadil4 have been attempted without clear effect.
We considered a diagnosis of paraneoplastic acral vascular syndrome in our patient, which was ruled out because the syndrome typically occurs in the setting of a worsening underlying malignancy7; our patient’s cancer was stable to improved. Thromboangiitis obliterans was ruled out by the absence of a characteristic thrombus on biopsy, the patient’s older age, and involvement of the nose.
We report an unusual case of acral necrosis occurring as a DIRE in response to administration of an immune checkpoint inhibitor. Further description is needed to clarify the diagnostic criteria for and treatment of this rare autoimmune phenomenon.
- Khaddour K, Singh V, Shayuk M. Acral vascular necrosis associated with immune-check point inhibitors: case report with literature review. BMC Cancer. 2019;19:449. doi:10.1186/s12885-019-5661-x
- Padda A, Schiopu E, Sovich J, et al. Ipilimumab induced digital vasculitis. J Immunother Cancer. 2018;6:12. doi:10.1186/s40425-018-0321-2
- Comont T, Sibaud V, Mourey L, et al. Immune checkpoint inhibitor-related acral vasculitis. J Immunother Cancer. 2018;6:120. doi:10.1186/s40425-018-0443-6
- Gambichler T, Strutzmann S, Tannapfel A, et al. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer. 2017;17:327. doi:10.1186/s12885-017-3313-6
- Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer. 2019;7:165. doi:10.1186/s40425-019-0645-6
- Bar J, Markel G, Gottfried T, et al. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer. 2019;120:122-131. doi:10.1016/j.ejca.2019.06.021
- Poszepczynska-Guigné E, Viguier M, Chosidow O, et al. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol. 2002;47:47-52. doi:10.1067/mjd.2002.120474
To the Editor:
A 67-year-old woman presented to the hospital with painful hands and feet. Two weeks prior, the patient experienced a few days of intermittent purple discoloration of the fingers, followed by black discoloration of the fingers, toes, and nose with notable pain. She reported no illness preceding the presenting symptoms, and there was no progression of symptoms in the days preceding presentation.
The patient had a history of smoking. She had a medical history of chronic obstructive pulmonary disease as well as recurrent non–small cell lung cancer that was treated most recently with a 1-year course of the programmed death-ligand 1 (PD-L1) immune checkpoint inhibitor durvalumab (last treatment was 4 months prior to the current presentation).
Physical examination revealed necrosis of the tips of the second, third, and fourth fingers of the left hand, as well as the tips of the third and fourth fingers of the right hand, progressing to purpura proximally on all involved fingers (Figure, A); scattered purpura and necrotic papules on the toe pads (Figure, B); and a 2- to 3-cm black plaque on the nasal tip. The patient was afebrile.

An embolic and vascular workup was performed. Transthoracic echocardiography was negative for thrombi, ankle brachial indices were within reference range, and computed tomography angiography revealed a few nonocclusive coronary plaques. Conventional angiography was not performed.
Laboratory testing revealed a mildly elevated level of cryofibrinogens (cryocrit, 2.5%); cold agglutinins (1:32); mild monoclonal κ IgG gammopathy (0.1 g/dL); and elevated inflammatory markers (C-reactive protein, 76 mg/L [reference range, 0–10 mg/L]; erythrocyte sedimentation rate, 38 mm/h [reference range, 0–20 mm/h]; fibrinogen, 571 mg/dL [reference range, 150–450 mg/dL]; and ferritin, 394 ng/mL [reference range, 10–180 ng/mL]). Additional laboratory studies were negative or within reference range, including tests of anti-RNA polymerase antibody, rheumatoid factor, antinuclear antibody, anticardiolipin antibody, anti-β2 glycoprotein antibody, antineutrophil cytoplasmic antibodies (myeloperoxidase and proteinase-3), cryoglobulins, and complement; human immunodeficiency virus and hepatitis B and C virus serologic studies; prothrombin time, partial thromboplastin time, and lupus anticoagulant; and a heparin-induced thrombocytopenia panel.
A skin biopsy adjacent to an area of necrosis on the finger showed thickened walls of dermal vessels, sparse leukocytoclastic debris, and evidence of recanalizing medium-sized vessels. Direct immunofluorescence studies were negative.
Based on the clinical history and histologic findings showing an absence of vasculitis, a diagnosis of acral necrosis associated with the PD-L1 immune checkpoint inhibitor durvalumab—a delayed immune-related event (DIRE)—was favored. The calcium channel blocker amlodipine was started at a dosage of 2.5 mg/d orally. Necrosis of the toes resolved over the course of 1 week; however, necrosis of the fingers remained unchanged. After 1 week of hospitalization, the patient was discharged at her request.
Acral necrosis following immune checkpoint inhibitor therapy has been reported as a rare and recalcitrant immune-related adverse event (AE).1-4 However, our patient’s symptoms occurred months after treatment was discontinued, which is consistent with a DIRE.5 The course of acral necrosis begins with acrocyanosis (a Raynaud disease–like phenomenon) of the fingers that progresses to necrosis. A history of Raynaud disease or other autoimmune disorder generally is absent.1 Our patient’s history indicated actively smoking at the time of presentation, similar to a case described by Khaddour et al.1 Similarly, in a case presented by Comont et al,3 the patient also had a history of smoking. In a recent study of acute vascular events associated with immune checkpoint inhibitors, 16 of 31 patients had a history of smoking.6
No definitive diagnostic laboratory or pathologic findings are associated with acral necrosis following immune checkpoint inhibitor therapy. Histopathologic analysis does not demonstrate vasculitis or other overt vascular pathology.2,3
The optimal treatment of immune checkpoint inhibitor–associated digital necrosis is unclear. Corticosteroids and discontinuation of the immune checkpoint inhibitor generally are employed,1-4 though treatment response has been variable. Other therapies such as calcium channel blockers (as in our case), sympathectomy,1 epoprostenol, botulinum injection, rituximab,2 and alprostadil4 have been attempted without clear effect.
We considered a diagnosis of paraneoplastic acral vascular syndrome in our patient, which was ruled out because the syndrome typically occurs in the setting of a worsening underlying malignancy7; our patient’s cancer was stable to improved. Thromboangiitis obliterans was ruled out by the absence of a characteristic thrombus on biopsy, the patient’s older age, and involvement of the nose.
We report an unusual case of acral necrosis occurring as a DIRE in response to administration of an immune checkpoint inhibitor. Further description is needed to clarify the diagnostic criteria for and treatment of this rare autoimmune phenomenon.
To the Editor:
A 67-year-old woman presented to the hospital with painful hands and feet. Two weeks prior, the patient experienced a few days of intermittent purple discoloration of the fingers, followed by black discoloration of the fingers, toes, and nose with notable pain. She reported no illness preceding the presenting symptoms, and there was no progression of symptoms in the days preceding presentation.
The patient had a history of smoking. She had a medical history of chronic obstructive pulmonary disease as well as recurrent non–small cell lung cancer that was treated most recently with a 1-year course of the programmed death-ligand 1 (PD-L1) immune checkpoint inhibitor durvalumab (last treatment was 4 months prior to the current presentation).
Physical examination revealed necrosis of the tips of the second, third, and fourth fingers of the left hand, as well as the tips of the third and fourth fingers of the right hand, progressing to purpura proximally on all involved fingers (Figure, A); scattered purpura and necrotic papules on the toe pads (Figure, B); and a 2- to 3-cm black plaque on the nasal tip. The patient was afebrile.

An embolic and vascular workup was performed. Transthoracic echocardiography was negative for thrombi, ankle brachial indices were within reference range, and computed tomography angiography revealed a few nonocclusive coronary plaques. Conventional angiography was not performed.
Laboratory testing revealed a mildly elevated level of cryofibrinogens (cryocrit, 2.5%); cold agglutinins (1:32); mild monoclonal κ IgG gammopathy (0.1 g/dL); and elevated inflammatory markers (C-reactive protein, 76 mg/L [reference range, 0–10 mg/L]; erythrocyte sedimentation rate, 38 mm/h [reference range, 0–20 mm/h]; fibrinogen, 571 mg/dL [reference range, 150–450 mg/dL]; and ferritin, 394 ng/mL [reference range, 10–180 ng/mL]). Additional laboratory studies were negative or within reference range, including tests of anti-RNA polymerase antibody, rheumatoid factor, antinuclear antibody, anticardiolipin antibody, anti-β2 glycoprotein antibody, antineutrophil cytoplasmic antibodies (myeloperoxidase and proteinase-3), cryoglobulins, and complement; human immunodeficiency virus and hepatitis B and C virus serologic studies; prothrombin time, partial thromboplastin time, and lupus anticoagulant; and a heparin-induced thrombocytopenia panel.
A skin biopsy adjacent to an area of necrosis on the finger showed thickened walls of dermal vessels, sparse leukocytoclastic debris, and evidence of recanalizing medium-sized vessels. Direct immunofluorescence studies were negative.
Based on the clinical history and histologic findings showing an absence of vasculitis, a diagnosis of acral necrosis associated with the PD-L1 immune checkpoint inhibitor durvalumab—a delayed immune-related event (DIRE)—was favored. The calcium channel blocker amlodipine was started at a dosage of 2.5 mg/d orally. Necrosis of the toes resolved over the course of 1 week; however, necrosis of the fingers remained unchanged. After 1 week of hospitalization, the patient was discharged at her request.
Acral necrosis following immune checkpoint inhibitor therapy has been reported as a rare and recalcitrant immune-related adverse event (AE).1-4 However, our patient’s symptoms occurred months after treatment was discontinued, which is consistent with a DIRE.5 The course of acral necrosis begins with acrocyanosis (a Raynaud disease–like phenomenon) of the fingers that progresses to necrosis. A history of Raynaud disease or other autoimmune disorder generally is absent.1 Our patient’s history indicated actively smoking at the time of presentation, similar to a case described by Khaddour et al.1 Similarly, in a case presented by Comont et al,3 the patient also had a history of smoking. In a recent study of acute vascular events associated with immune checkpoint inhibitors, 16 of 31 patients had a history of smoking.6
No definitive diagnostic laboratory or pathologic findings are associated with acral necrosis following immune checkpoint inhibitor therapy. Histopathologic analysis does not demonstrate vasculitis or other overt vascular pathology.2,3
The optimal treatment of immune checkpoint inhibitor–associated digital necrosis is unclear. Corticosteroids and discontinuation of the immune checkpoint inhibitor generally are employed,1-4 though treatment response has been variable. Other therapies such as calcium channel blockers (as in our case), sympathectomy,1 epoprostenol, botulinum injection, rituximab,2 and alprostadil4 have been attempted without clear effect.
We considered a diagnosis of paraneoplastic acral vascular syndrome in our patient, which was ruled out because the syndrome typically occurs in the setting of a worsening underlying malignancy7; our patient’s cancer was stable to improved. Thromboangiitis obliterans was ruled out by the absence of a characteristic thrombus on biopsy, the patient’s older age, and involvement of the nose.
We report an unusual case of acral necrosis occurring as a DIRE in response to administration of an immune checkpoint inhibitor. Further description is needed to clarify the diagnostic criteria for and treatment of this rare autoimmune phenomenon.
- Khaddour K, Singh V, Shayuk M. Acral vascular necrosis associated with immune-check point inhibitors: case report with literature review. BMC Cancer. 2019;19:449. doi:10.1186/s12885-019-5661-x
- Padda A, Schiopu E, Sovich J, et al. Ipilimumab induced digital vasculitis. J Immunother Cancer. 2018;6:12. doi:10.1186/s40425-018-0321-2
- Comont T, Sibaud V, Mourey L, et al. Immune checkpoint inhibitor-related acral vasculitis. J Immunother Cancer. 2018;6:120. doi:10.1186/s40425-018-0443-6
- Gambichler T, Strutzmann S, Tannapfel A, et al. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer. 2017;17:327. doi:10.1186/s12885-017-3313-6
- Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer. 2019;7:165. doi:10.1186/s40425-019-0645-6
- Bar J, Markel G, Gottfried T, et al. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer. 2019;120:122-131. doi:10.1016/j.ejca.2019.06.021
- Poszepczynska-Guigné E, Viguier M, Chosidow O, et al. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol. 2002;47:47-52. doi:10.1067/mjd.2002.120474
- Khaddour K, Singh V, Shayuk M. Acral vascular necrosis associated with immune-check point inhibitors: case report with literature review. BMC Cancer. 2019;19:449. doi:10.1186/s12885-019-5661-x
- Padda A, Schiopu E, Sovich J, et al. Ipilimumab induced digital vasculitis. J Immunother Cancer. 2018;6:12. doi:10.1186/s40425-018-0321-2
- Comont T, Sibaud V, Mourey L, et al. Immune checkpoint inhibitor-related acral vasculitis. J Immunother Cancer. 2018;6:120. doi:10.1186/s40425-018-0443-6
- Gambichler T, Strutzmann S, Tannapfel A, et al. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer. 2017;17:327. doi:10.1186/s12885-017-3313-6
- Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (DIRE) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer. 2019;7:165. doi:10.1186/s40425-019-0645-6
- Bar J, Markel G, Gottfried T, et al. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer. 2019;120:122-131. doi:10.1016/j.ejca.2019.06.021
- Poszepczynska-Guigné E, Viguier M, Chosidow O, et al. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol. 2002;47:47-52. doi:10.1067/mjd.2002.120474
Practice Points
- Dermatologists should be aware of acral necrosis as a rare adverse event of treatment with an immune checkpoint inhibitor.
- Delayed immune-related events are sequelae of immune checkpoint inhibitors that can occur months after treatment is discontinued.
Persistent Wounds Refractory to Broad-Spectrum Antibiotics
The Diagnosis: PASH (Pyoderma Gangrenosum, Acne, Hidradenitis Suppurativa) Syndrome
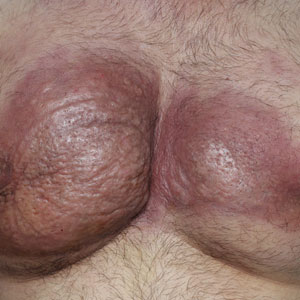
Obtaining our patient’s history of hidradenitis suppurativa (HS), a hallmark sterile neutrophilic dermatosis, was key to making the correct diagnosis of PASH (pyoderma gangrenosum, acne, HS) syndrome. In our patient, the history of HS increased the consideration of pyoderma gangrenosum (PG) due to the persistent breast and leg wounds. Additionally, it was important to consider a diagnosis of PG in lesions that were not responding to broad-spectrum antimicrobial treatment. In our patient, the concurrent presentation of draining abscesses in the axillae (Figure, A) and inflammatory nodulocystic facial acne (Figure, B) were additional diagnostic clues that suggested the triad of PASH syndrome.
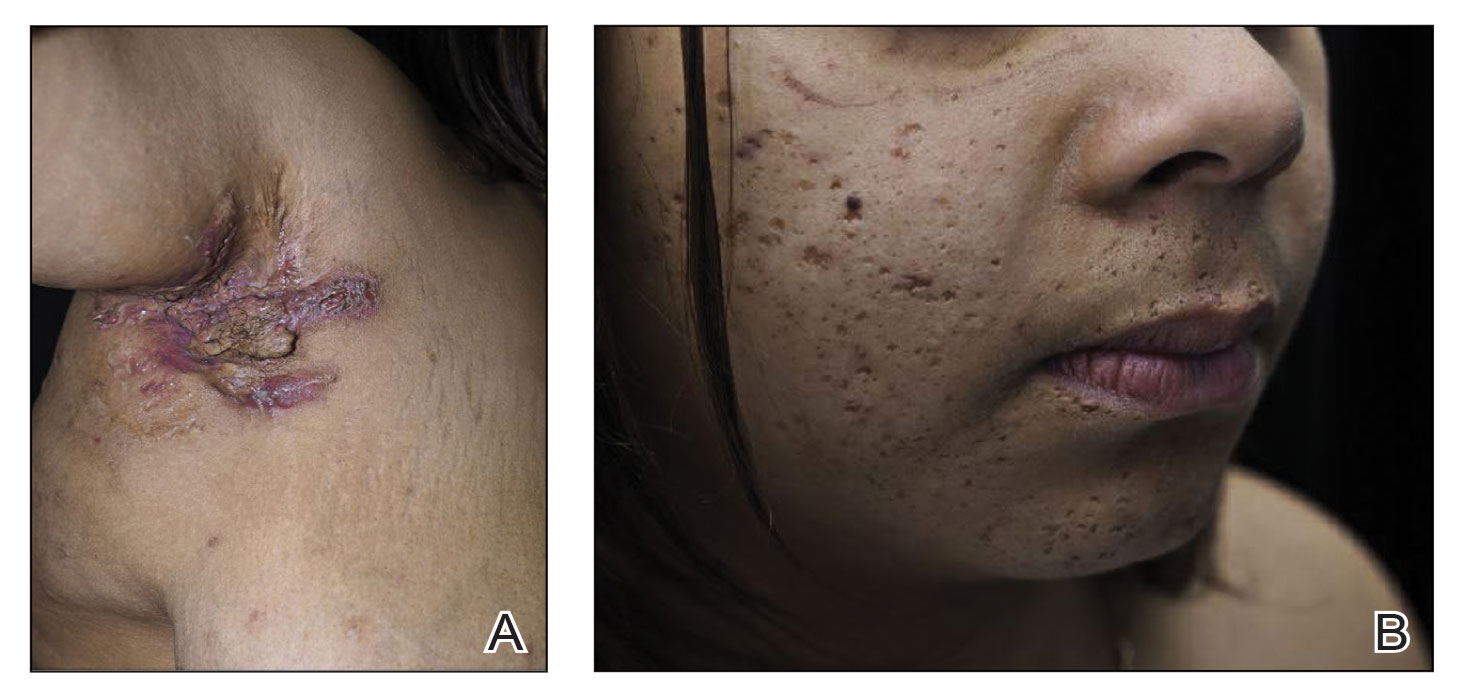
Although SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome also can present with cutaneous features of acne and HS, the lack of bone and joint involvement in our patient made this diagnosis less likely. Calciphylaxis can present as ulcerations on the lower extremities, but it usually presents with a livedolike pattern with overlying black eschar and is unlikely in the absence of underlying metabolic or renal disease. PAPA (pyogenic arthritis, PG, acne) syndrome is characterized by recurrent joint involvement and lacks features of HS. Lastly, our patient was immunocompetent with no risk factors for mycobacterial infection.
PASH syndrome is a rare inherited syndrome, but its constituent inflammatory conditions are ubiquitous. They share a common underlying mechanism consisting of overactivation of the innate immune systems driven by increased production of the inflammatory cytokines IL-1, IL-17, and tumor necrosis factor α, resulting in sterile neutrophilic dermatoses.1 The diagnosis is based on the clinical presentation, as laboratory investigations are nondiagnostic. Biopsies and cultures can be performed to rule out infectious etiologies. Additionally, PASH syndrome is considered part of a larger spectrum of syndromes including PAPA and PAPASH (pyogenic arthritis, acne, PG, HS) syndromes. The absence of pyogenic arthritis distinguishes PASH syndrome from PAPA and PAPASH syndromes.2 Clinically, PASH syndrome and the related sterile neutrophilic dermatoses share the characteristic of pronounced cutaneous involvement that substantially alters the patient’s quality of life. Cigarette smoking is an exacerbating factor and has a well-established association with HS.3 Therefore, smoking cessation should be encouraged in these patients to avoid exacerbation of the disease process.
Maintaining adequate immunosuppression is key to managing the underlying disease processes. Classic immunosuppressive agents such as systemic glucocorticoids and methotrexate may fail to satisfactorily control the disease.4 Treatment options currently are somewhat limited and are aimed at targeting the inflammatory cytokines that propagate the disease. The most consistent responses have been observed with anti–tumor necrosis factor α antagonists such as adalimumab, infliximab, and etanercept.5 Additionally, there is varied response to anakinra, suggesting the importance of selectively targeting IL-1β.6 Unfortunately, misdiagnosis for an infectious etiology is common, and antibiotics and debridement are of limited use for the underlying pathophysiology of PASH syndrome. Importantly, biopsy and debridement often are discouraged due to the risk of pathergy.7
Our case demonstrates the importance of maintaining a high clinical suspicion for immune-mediated lesions that are refractory to antimicrobial agents. Additionally, prior history of multiple neutrophilic dermatoses should prompt consideration for the PASH/PAPA/PAPASH disease spectrum. Early and accurate identification of neutrophilic dermatoses such as PG and HS are crucial to initiating proper cytokine-targeting treatment and achieving disease remission.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Genovese G, Moltrasio C, Garcovich S, et al. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155:542-550.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261-264.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Saint-Georges V, Peternel S, Kaštelan M, et al. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26:173-178.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Patel DK, Locke M, Jarrett P. Pyoderma gangrenosum with pathergy: a potentially significant complication following breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70:884-892.
The Diagnosis: PASH (Pyoderma Gangrenosum, Acne, Hidradenitis Suppurativa) Syndrome
Obtaining our patient’s history of hidradenitis suppurativa (HS), a hallmark sterile neutrophilic dermatosis, was key to making the correct diagnosis of PASH (pyoderma gangrenosum, acne, HS) syndrome. In our patient, the history of HS increased the consideration of pyoderma gangrenosum (PG) due to the persistent breast and leg wounds. Additionally, it was important to consider a diagnosis of PG in lesions that were not responding to broad-spectrum antimicrobial treatment. In our patient, the concurrent presentation of draining abscesses in the axillae (Figure, A) and inflammatory nodulocystic facial acne (Figure, B) were additional diagnostic clues that suggested the triad of PASH syndrome.

Although SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome also can present with cutaneous features of acne and HS, the lack of bone and joint involvement in our patient made this diagnosis less likely. Calciphylaxis can present as ulcerations on the lower extremities, but it usually presents with a livedolike pattern with overlying black eschar and is unlikely in the absence of underlying metabolic or renal disease. PAPA (pyogenic arthritis, PG, acne) syndrome is characterized by recurrent joint involvement and lacks features of HS. Lastly, our patient was immunocompetent with no risk factors for mycobacterial infection.
PASH syndrome is a rare inherited syndrome, but its constituent inflammatory conditions are ubiquitous. They share a common underlying mechanism consisting of overactivation of the innate immune systems driven by increased production of the inflammatory cytokines IL-1, IL-17, and tumor necrosis factor α, resulting in sterile neutrophilic dermatoses.1 The diagnosis is based on the clinical presentation, as laboratory investigations are nondiagnostic. Biopsies and cultures can be performed to rule out infectious etiologies. Additionally, PASH syndrome is considered part of a larger spectrum of syndromes including PAPA and PAPASH (pyogenic arthritis, acne, PG, HS) syndromes. The absence of pyogenic arthritis distinguishes PASH syndrome from PAPA and PAPASH syndromes.2 Clinically, PASH syndrome and the related sterile neutrophilic dermatoses share the characteristic of pronounced cutaneous involvement that substantially alters the patient’s quality of life. Cigarette smoking is an exacerbating factor and has a well-established association with HS.3 Therefore, smoking cessation should be encouraged in these patients to avoid exacerbation of the disease process.
Maintaining adequate immunosuppression is key to managing the underlying disease processes. Classic immunosuppressive agents such as systemic glucocorticoids and methotrexate may fail to satisfactorily control the disease.4 Treatment options currently are somewhat limited and are aimed at targeting the inflammatory cytokines that propagate the disease. The most consistent responses have been observed with anti–tumor necrosis factor α antagonists such as adalimumab, infliximab, and etanercept.5 Additionally, there is varied response to anakinra, suggesting the importance of selectively targeting IL-1β.6 Unfortunately, misdiagnosis for an infectious etiology is common, and antibiotics and debridement are of limited use for the underlying pathophysiology of PASH syndrome. Importantly, biopsy and debridement often are discouraged due to the risk of pathergy.7
Our case demonstrates the importance of maintaining a high clinical suspicion for immune-mediated lesions that are refractory to antimicrobial agents. Additionally, prior history of multiple neutrophilic dermatoses should prompt consideration for the PASH/PAPA/PAPASH disease spectrum. Early and accurate identification of neutrophilic dermatoses such as PG and HS are crucial to initiating proper cytokine-targeting treatment and achieving disease remission.
The Diagnosis: PASH (Pyoderma Gangrenosum, Acne, Hidradenitis Suppurativa) Syndrome
Obtaining our patient’s history of hidradenitis suppurativa (HS), a hallmark sterile neutrophilic dermatosis, was key to making the correct diagnosis of PASH (pyoderma gangrenosum, acne, HS) syndrome. In our patient, the history of HS increased the consideration of pyoderma gangrenosum (PG) due to the persistent breast and leg wounds. Additionally, it was important to consider a diagnosis of PG in lesions that were not responding to broad-spectrum antimicrobial treatment. In our patient, the concurrent presentation of draining abscesses in the axillae (Figure, A) and inflammatory nodulocystic facial acne (Figure, B) were additional diagnostic clues that suggested the triad of PASH syndrome.

Although SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome also can present with cutaneous features of acne and HS, the lack of bone and joint involvement in our patient made this diagnosis less likely. Calciphylaxis can present as ulcerations on the lower extremities, but it usually presents with a livedolike pattern with overlying black eschar and is unlikely in the absence of underlying metabolic or renal disease. PAPA (pyogenic arthritis, PG, acne) syndrome is characterized by recurrent joint involvement and lacks features of HS. Lastly, our patient was immunocompetent with no risk factors for mycobacterial infection.
PASH syndrome is a rare inherited syndrome, but its constituent inflammatory conditions are ubiquitous. They share a common underlying mechanism consisting of overactivation of the innate immune systems driven by increased production of the inflammatory cytokines IL-1, IL-17, and tumor necrosis factor α, resulting in sterile neutrophilic dermatoses.1 The diagnosis is based on the clinical presentation, as laboratory investigations are nondiagnostic. Biopsies and cultures can be performed to rule out infectious etiologies. Additionally, PASH syndrome is considered part of a larger spectrum of syndromes including PAPA and PAPASH (pyogenic arthritis, acne, PG, HS) syndromes. The absence of pyogenic arthritis distinguishes PASH syndrome from PAPA and PAPASH syndromes.2 Clinically, PASH syndrome and the related sterile neutrophilic dermatoses share the characteristic of pronounced cutaneous involvement that substantially alters the patient’s quality of life. Cigarette smoking is an exacerbating factor and has a well-established association with HS.3 Therefore, smoking cessation should be encouraged in these patients to avoid exacerbation of the disease process.
Maintaining adequate immunosuppression is key to managing the underlying disease processes. Classic immunosuppressive agents such as systemic glucocorticoids and methotrexate may fail to satisfactorily control the disease.4 Treatment options currently are somewhat limited and are aimed at targeting the inflammatory cytokines that propagate the disease. The most consistent responses have been observed with anti–tumor necrosis factor α antagonists such as adalimumab, infliximab, and etanercept.5 Additionally, there is varied response to anakinra, suggesting the importance of selectively targeting IL-1β.6 Unfortunately, misdiagnosis for an infectious etiology is common, and antibiotics and debridement are of limited use for the underlying pathophysiology of PASH syndrome. Importantly, biopsy and debridement often are discouraged due to the risk of pathergy.7
Our case demonstrates the importance of maintaining a high clinical suspicion for immune-mediated lesions that are refractory to antimicrobial agents. Additionally, prior history of multiple neutrophilic dermatoses should prompt consideration for the PASH/PAPA/PAPASH disease spectrum. Early and accurate identification of neutrophilic dermatoses such as PG and HS are crucial to initiating proper cytokine-targeting treatment and achieving disease remission.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Genovese G, Moltrasio C, Garcovich S, et al. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155:542-550.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261-264.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Saint-Georges V, Peternel S, Kaštelan M, et al. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26:173-178.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Patel DK, Locke M, Jarrett P. Pyoderma gangrenosum with pathergy: a potentially significant complication following breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70:884-892.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Genovese G, Moltrasio C, Garcovich S, et al. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155:542-550.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261-264.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Saint-Georges V, Peternel S, Kaštelan M, et al. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26:173-178.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Patel DK, Locke M, Jarrett P. Pyoderma gangrenosum with pathergy: a potentially significant complication following breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70:884-892.
A 28-year-old Black woman presented to the hospital for evaluation of worsening leg wounds as well as a similar eroding plaque on the left breast of 1 month’s duration. Broad-spectrum antibiotics prescribed during a prior emergency department visit resulted in no improvement. Her medical history was notable for hidradenitis suppurativa that previously was well controlled on adalimumab prior to discontinuation 1 year prior. A review of systems was negative for fever, chills, shortness of breath, chest pain, night sweats, and arthralgia. The patient had discontinued the antibiotics and was not taking any other medications at the time of presentation. She reported a history of smoking cigarettes (5 pack years). Physical examination revealed hyperkeratotic eroded plaques with violaceous borders circumferentially around the left breast (top) and legs with notable undermining (bottom). Inflammatory nodulocystic acne of the face as well as sinus tract formation with purulent drainage in the axillae also were present. Laboratory workup revealed an elevated erythrocyte sedimentation rate (116 mm/h [reference range, <20 mm/h]). Computed tomography of the leg wound was negative for soft-tissue infection. Aerobic and anaerobic tissue cultures demonstrated no growth.

Foot ulcers red flag for eye disease in diabetes
Sores on the feet can signal problems with the eyes in patients with diabetes.
Prior research and anecdotal experience show that diabetic foot ulcers and diabetic retinopathy frequently co-occur.
David J. Ramsey, MD, PhD, MPH, director of ophthalmic research at Lahey Hospital & Medical Center, Burlington, Mass., said when clinicians detect either condition, they should involve a team that can intervene to help protect a patient’s vision and mobility.
For example, they should ensure patients receive comprehensive eye and foot evaluations and help them optimize diabetes management.
The new study, presented at the annual meeting of the Association for Research in Vision and Ophthalmology, “adds an important dimension” to understanding the association between the conditions, said Dr. Ramsey, who recently reviewed correlations between diabetic foot ulcers and diabetic retinopathy and their underlying causes.
“Patients with diabetic foot ulcers appear to receive less attention to their diabetic retinopathy and may receive fewer treatments with eye injections targeting vascular endothelial growth factor (VEGF), an important driver of progression of diabetic retinopathy,” said Dr. Ramsey, who is also an associate professor of ophthalmology at Tufts University School of Medicine, Boston. He was not involved in the study presented at ARVO 2023.
In the new study, Christopher T. Zhu, a medical student at UT Health San Antonio, and colleagues analyzed data from 426 eyes of 213 patients with type 2 diabetes who had had at least two eye exams between 2012 and 2022; 72 of the patients had diabetic foot ulcers. Patients were followed for about 4 years on average.
Patients with diabetic foot ulcers had a higher percentage of eyes with macular edema on their initial exam (32.6% vs. 28%). By the final exam, the percentage of eyes with macular edema was significantly greater in the group with diabetic foot ulcers (64.6% vs. 37.6%; P < .0001), Mr. Zhu’s group reported.
Eyes with nonproliferative diabetic retinopathy progressed to proliferative diabetic retinopathy, the worst grade, at a higher rate in the group with foot ulcers (50.6% vs. 35.6%; P = .03). In addition, patients with foot ulcers were more likely to experience vitreous hemorrhage (55.6% vs. 38.7%), the researchers found.
Despite patients with foot ulcers tending to have worse disease, they received fewer treatments for retinopathy. Those without ulcers received an average of 6.9 anti-VEGF injections per eye, while those with ulcers averaged 4.3.
Foot ulcers may hinder the ability of patients to get to appointments to receive the injections, Mr. Zhu and colleagues wrote. “For many patients in our part of the country [South Texas], a lack of transportation is a particular barrier to health care access,” Mr. Zhu told this news organization.
Mr. Zhu’s team conducted their study after noticing that patients with diabetes and foot ulcers who presented to their eye clinics “appeared to progress faster to worse grades of retinopathy” than patients with diabetes who did not have ulcers.
“Similar to how foot ulcers develop due to a severe disruption in blood flow [vascular] and a loss of sensation [neurologic], diabetic retinopathy may have a relation to microvascular disease, neurologic degeneration, and inflammation,” he said.
The findings confirm “that poor perfusion of the eye and foot are linked and can cause ischemic retinopathy leading to the development of proliferative diabetic retinopathy and vitreous hemorrhages, both serious, vision-threatening conditions,” Dr. Ramsey said.
To some extent, fewer treatments with anti-VEGF agents may account for why patients with foot ulcers have more eye complications, Dr. Ramsey added. “Additional research needs to be done to further dissect the cause and the effect, but it’s a very important finding that we need to increase awareness about,” he said.
Dr. Ramsey and Mr. Zhu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sores on the feet can signal problems with the eyes in patients with diabetes.
Prior research and anecdotal experience show that diabetic foot ulcers and diabetic retinopathy frequently co-occur.
David J. Ramsey, MD, PhD, MPH, director of ophthalmic research at Lahey Hospital & Medical Center, Burlington, Mass., said when clinicians detect either condition, they should involve a team that can intervene to help protect a patient’s vision and mobility.
For example, they should ensure patients receive comprehensive eye and foot evaluations and help them optimize diabetes management.
The new study, presented at the annual meeting of the Association for Research in Vision and Ophthalmology, “adds an important dimension” to understanding the association between the conditions, said Dr. Ramsey, who recently reviewed correlations between diabetic foot ulcers and diabetic retinopathy and their underlying causes.
“Patients with diabetic foot ulcers appear to receive less attention to their diabetic retinopathy and may receive fewer treatments with eye injections targeting vascular endothelial growth factor (VEGF), an important driver of progression of diabetic retinopathy,” said Dr. Ramsey, who is also an associate professor of ophthalmology at Tufts University School of Medicine, Boston. He was not involved in the study presented at ARVO 2023.
In the new study, Christopher T. Zhu, a medical student at UT Health San Antonio, and colleagues analyzed data from 426 eyes of 213 patients with type 2 diabetes who had had at least two eye exams between 2012 and 2022; 72 of the patients had diabetic foot ulcers. Patients were followed for about 4 years on average.
Patients with diabetic foot ulcers had a higher percentage of eyes with macular edema on their initial exam (32.6% vs. 28%). By the final exam, the percentage of eyes with macular edema was significantly greater in the group with diabetic foot ulcers (64.6% vs. 37.6%; P < .0001), Mr. Zhu’s group reported.
Eyes with nonproliferative diabetic retinopathy progressed to proliferative diabetic retinopathy, the worst grade, at a higher rate in the group with foot ulcers (50.6% vs. 35.6%; P = .03). In addition, patients with foot ulcers were more likely to experience vitreous hemorrhage (55.6% vs. 38.7%), the researchers found.
Despite patients with foot ulcers tending to have worse disease, they received fewer treatments for retinopathy. Those without ulcers received an average of 6.9 anti-VEGF injections per eye, while those with ulcers averaged 4.3.
Foot ulcers may hinder the ability of patients to get to appointments to receive the injections, Mr. Zhu and colleagues wrote. “For many patients in our part of the country [South Texas], a lack of transportation is a particular barrier to health care access,” Mr. Zhu told this news organization.
Mr. Zhu’s team conducted their study after noticing that patients with diabetes and foot ulcers who presented to their eye clinics “appeared to progress faster to worse grades of retinopathy” than patients with diabetes who did not have ulcers.
“Similar to how foot ulcers develop due to a severe disruption in blood flow [vascular] and a loss of sensation [neurologic], diabetic retinopathy may have a relation to microvascular disease, neurologic degeneration, and inflammation,” he said.
The findings confirm “that poor perfusion of the eye and foot are linked and can cause ischemic retinopathy leading to the development of proliferative diabetic retinopathy and vitreous hemorrhages, both serious, vision-threatening conditions,” Dr. Ramsey said.
To some extent, fewer treatments with anti-VEGF agents may account for why patients with foot ulcers have more eye complications, Dr. Ramsey added. “Additional research needs to be done to further dissect the cause and the effect, but it’s a very important finding that we need to increase awareness about,” he said.
Dr. Ramsey and Mr. Zhu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sores on the feet can signal problems with the eyes in patients with diabetes.
Prior research and anecdotal experience show that diabetic foot ulcers and diabetic retinopathy frequently co-occur.
David J. Ramsey, MD, PhD, MPH, director of ophthalmic research at Lahey Hospital & Medical Center, Burlington, Mass., said when clinicians detect either condition, they should involve a team that can intervene to help protect a patient’s vision and mobility.
For example, they should ensure patients receive comprehensive eye and foot evaluations and help them optimize diabetes management.
The new study, presented at the annual meeting of the Association for Research in Vision and Ophthalmology, “adds an important dimension” to understanding the association between the conditions, said Dr. Ramsey, who recently reviewed correlations between diabetic foot ulcers and diabetic retinopathy and their underlying causes.
“Patients with diabetic foot ulcers appear to receive less attention to their diabetic retinopathy and may receive fewer treatments with eye injections targeting vascular endothelial growth factor (VEGF), an important driver of progression of diabetic retinopathy,” said Dr. Ramsey, who is also an associate professor of ophthalmology at Tufts University School of Medicine, Boston. He was not involved in the study presented at ARVO 2023.
In the new study, Christopher T. Zhu, a medical student at UT Health San Antonio, and colleagues analyzed data from 426 eyes of 213 patients with type 2 diabetes who had had at least two eye exams between 2012 and 2022; 72 of the patients had diabetic foot ulcers. Patients were followed for about 4 years on average.
Patients with diabetic foot ulcers had a higher percentage of eyes with macular edema on their initial exam (32.6% vs. 28%). By the final exam, the percentage of eyes with macular edema was significantly greater in the group with diabetic foot ulcers (64.6% vs. 37.6%; P < .0001), Mr. Zhu’s group reported.
Eyes with nonproliferative diabetic retinopathy progressed to proliferative diabetic retinopathy, the worst grade, at a higher rate in the group with foot ulcers (50.6% vs. 35.6%; P = .03). In addition, patients with foot ulcers were more likely to experience vitreous hemorrhage (55.6% vs. 38.7%), the researchers found.
Despite patients with foot ulcers tending to have worse disease, they received fewer treatments for retinopathy. Those without ulcers received an average of 6.9 anti-VEGF injections per eye, while those with ulcers averaged 4.3.
Foot ulcers may hinder the ability of patients to get to appointments to receive the injections, Mr. Zhu and colleagues wrote. “For many patients in our part of the country [South Texas], a lack of transportation is a particular barrier to health care access,” Mr. Zhu told this news organization.
Mr. Zhu’s team conducted their study after noticing that patients with diabetes and foot ulcers who presented to their eye clinics “appeared to progress faster to worse grades of retinopathy” than patients with diabetes who did not have ulcers.
“Similar to how foot ulcers develop due to a severe disruption in blood flow [vascular] and a loss of sensation [neurologic], diabetic retinopathy may have a relation to microvascular disease, neurologic degeneration, and inflammation,” he said.
The findings confirm “that poor perfusion of the eye and foot are linked and can cause ischemic retinopathy leading to the development of proliferative diabetic retinopathy and vitreous hemorrhages, both serious, vision-threatening conditions,” Dr. Ramsey said.
To some extent, fewer treatments with anti-VEGF agents may account for why patients with foot ulcers have more eye complications, Dr. Ramsey added. “Additional research needs to be done to further dissect the cause and the effect, but it’s a very important finding that we need to increase awareness about,” he said.
Dr. Ramsey and Mr. Zhu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARVO 2023
Fat Necrosis of the Breast Mimicking Breast Cancer in a Male Patient Following Wax Hair Removal
To the Editor:
Fat necrosis of the breast is a benign inflammatory disease of adipose tissue commonly observed after trauma in the female breast during the perimenopausal period.1 Fat necrosis of the male breast is rare, first described by Silverstone2 in 1949; the condition usually presents with unilateral, painful or asymptomatic, firm nodules, which in rare cases are observed as skin retraction and thickening, ecchymosis, erythematous plaque–like cellulitis, local depression, and/or discoloration of the breast skin.3-5
Diagnosis of fat necrosis of the male breast may need to be confirmed via biopsy in conjunction with clinical and radiologic findings because the condition can mimic breast cancer.1 We report a case of bilateral fat necrosis of the breast mimicking breast cancer following wax hair removal.
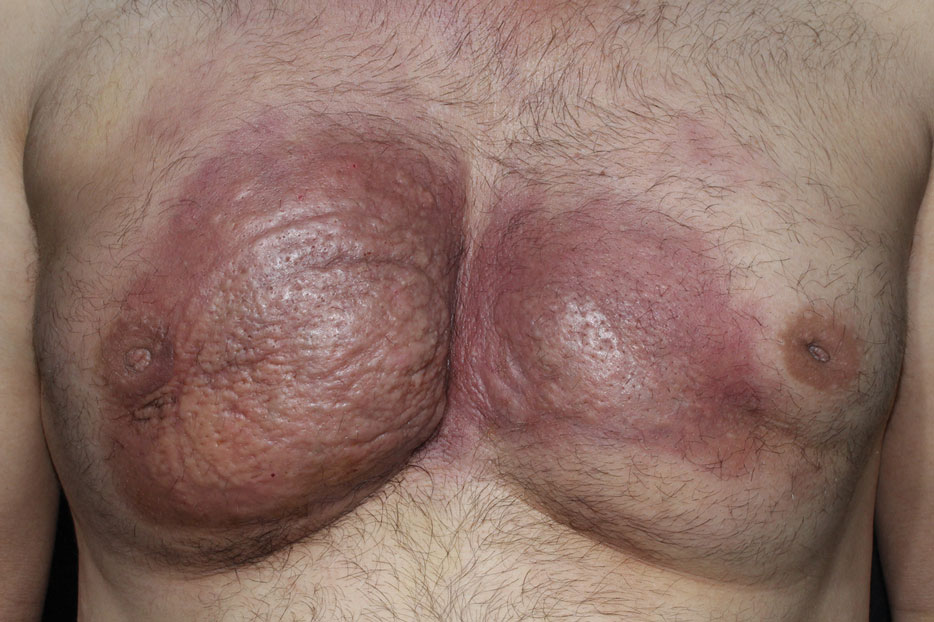
A 42-year-old man presented to our outpatient dermatology clinic for evaluation of redness, swelling, and hardness of the skin of both breasts of 3 weeks’ duration. The patient had a history of wax hair removal of the entire anterior aspect of the body. He reported an erythematous, edematous, warm plaque that developed on the breasts 2 days after waxing. The plaque did not respond to antibiotics. The swelling and induration progressed over the 2 weeks after the patient was waxed. The patient had no family history of breast cancer. He had a standing diagnosis of gynecomastia. He denied any history of fat or filler injection in the affected area.
Dermatologic examination revealed erythematous, edematous, indurated, asymptomatic plaques with a peau d’orange appearance on the bilateral pectoral and presternal region. Minimal retraction of the right areola was noted (Figure 1). The bilateral axillary lymph nodes were palpable.
Laboratory results including erythrocyte sedimentation rate (108 mm/h [reference range, 2–20 mm/h]), C-reactive protein (9.2 mg/dL [reference range, >0.5 mg/dL]), and ferritin levels (645
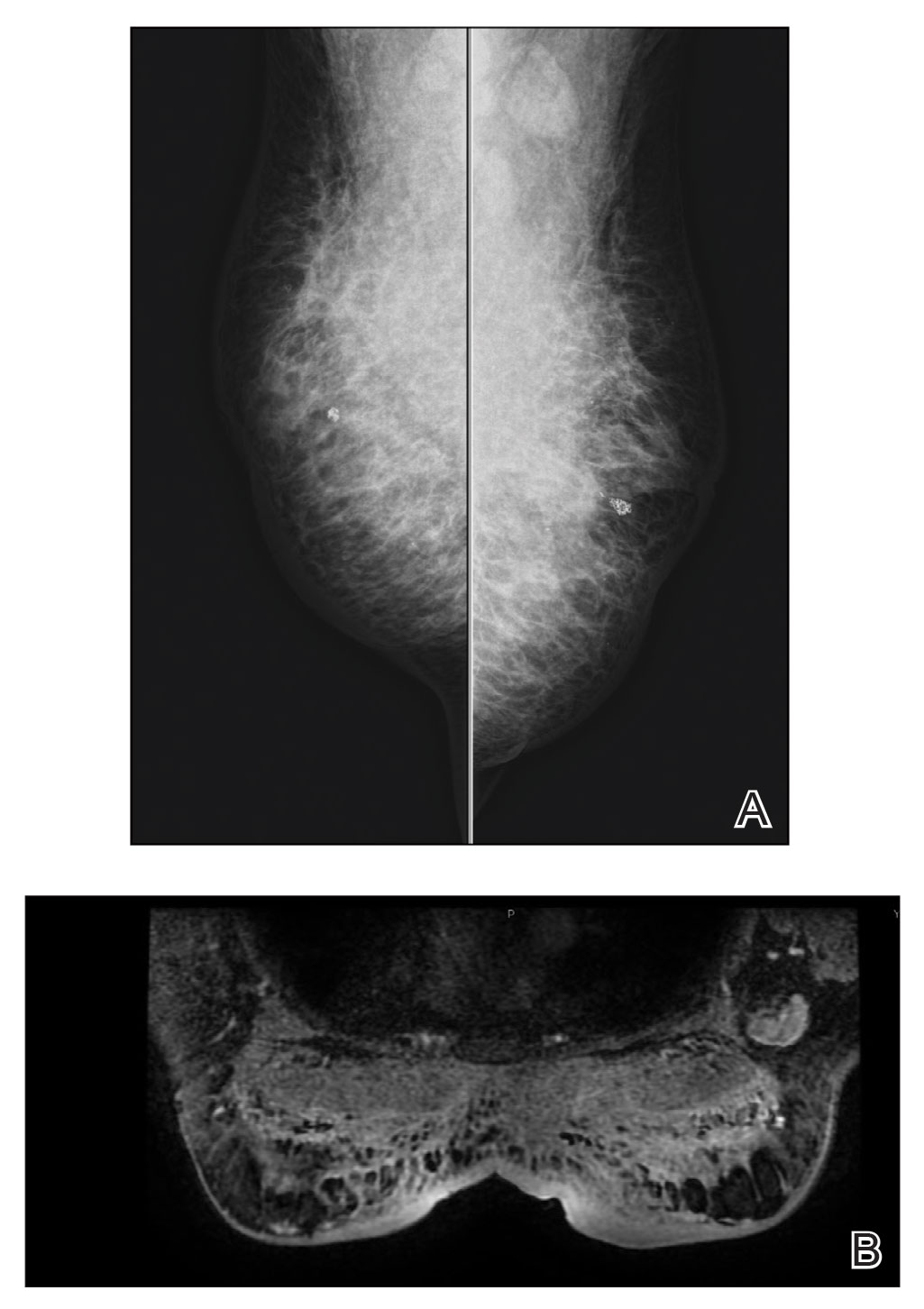
Mammography of both breasts revealed a Breast Imaging Reporting and Data System (BI-RADS) score of 4 with a suspicious abnormality (ie, diffuse edema of the breast, multiple calcifications in a nonspecific pattern, oil cysts with calcifications, and bilateral axillary lymphadenopathy with a diameter of 2.5 cm and a thick and irregular cortex)(Figure 2A). Ultrasonography of both breasts revealed an inflammatory breast. Magnetic resonance imaging showed similar findings with diffuse edema and a heterogeneous appearance. Contrast-enhanced magnetic resonance imaging showed diffuse contrast enhancement in both breasts extending to the pectoral muscles and axillary regions, consistent with inflammatory changes (Figure 2B).
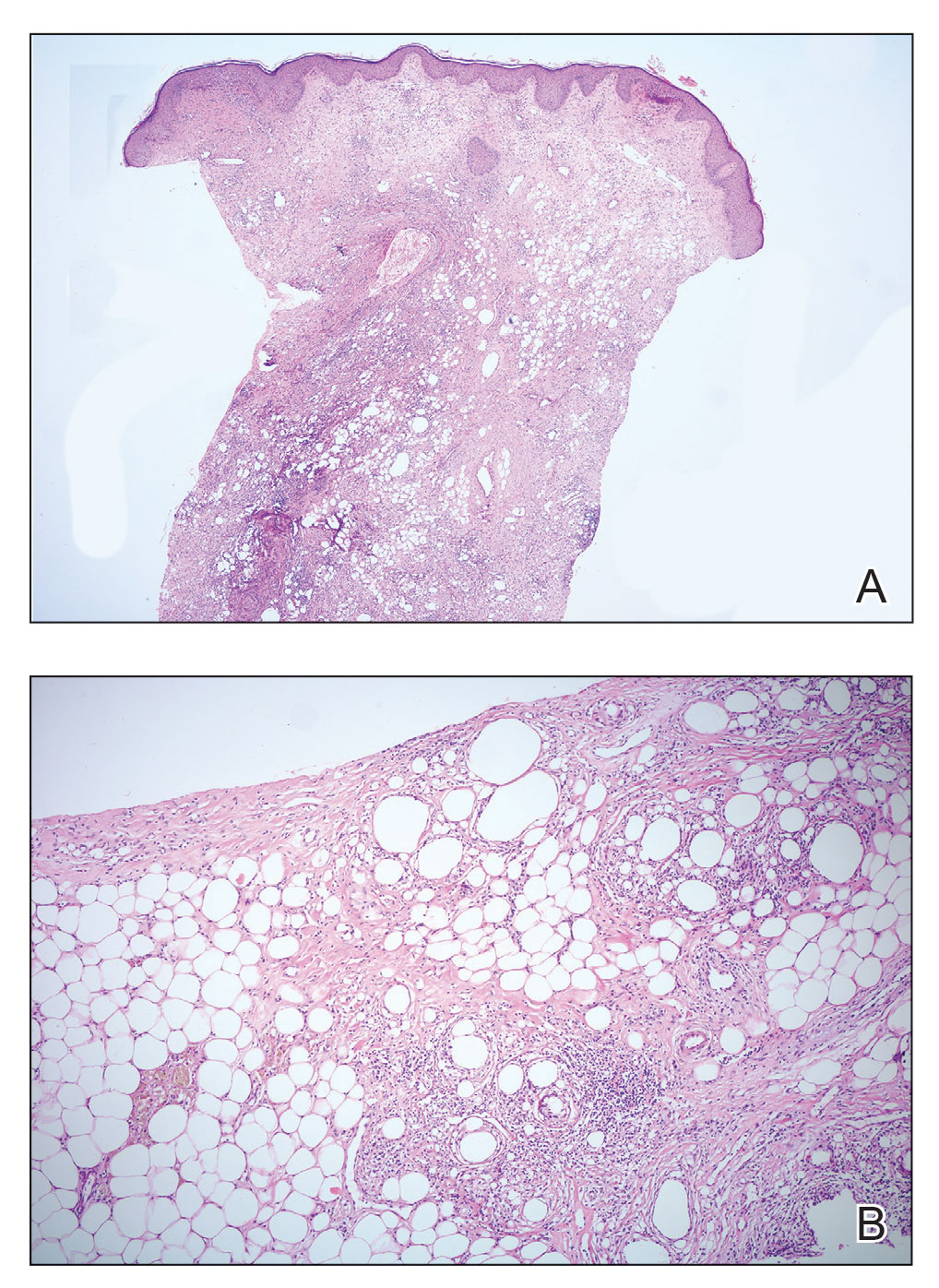
Because of difficulty differentiating inflammation and an infiltrating tumor, histopathologic examination was recommended by radiology. Results from a 5-mm punch biopsy from the right breast yielded the following differential diagnoses: cellulitis, panniculitis, inflammatory breast cancer, subcutaneous fat necrosis, and paraffinoma. Histopathologic examination of the skin revealed a normal epidermis and a dense inflammatory cell infiltrate comprising lymphocytes and monocytes in the dermis and subcutaneous tissue. Marked fibrosis also was noted in the dermis and subcutaneous tissue. Lipophagic fat necrosis accompanied by a variable inflammatory cell infiltrate consisted of histiocytes and neutrophils (Figure 3A). Pankeratin immunostaining was negative. Fat necrosis was present in a biopsy specimen obtained from the right breast; no signs of malignancy were present (Figure 3B). Fine-needle aspiration of the axillary lymph nodes was benign. Given these histopathologic findings, malignancy was excluded from the differential diagnosis. Paraffinoma also was ruled out because the patient insistently denied any history of fat or filler injection.
Based on the clinical, histopathologic, and radiologic findings, as well as the history of minor trauma due to wax hair removal, a diagnosis of fat necrosis of the breast was made. Intervention was not recommended by the plastic surgeons who subsequently evaluated the patient, because the additional trauma may aggravate the lesion. He was treated with nonsteroidal anti-inflammatory drugs.
At 6-month follow-up, there was marked reduction in the erythema and edema but no notable improvement of the induration. A potent topical steroid was added to the treatment, but only slight regression of the induration was observed.
The normal male breast is comprised of fat and a few secretory ducts.6 Gynecomastia and breast cancer are the 2 most common conditions of the male breast; fat necrosis of the male breast is rare. In a study of 236 male patients with breast disease, only 5 had fat necrosis.7
Fat necrosis of the breast can be observed with various clinical and radiological presentations. Subcutaneous nodules, skin retraction and thickening, local skin depression, and ecchymosis are the more common presentations of fat necrosis.3-5 In our case, the first symptoms of disease were similar to those seen in cellulitis. The presentation of fat necrosis–like cellulitis has been described only rarely in the medical literature. Haikin et al5 reported a case of fat necrosis of the leg in a child that presented with cellulitis followed by induration, which did not respond to antibiotics, as was the case with our patient.5
Blunt trauma, breast reduction surgery, and breast augmentation surgery can cause fat necrosis of the breast1,4; in some cases, the cause cannot be determined.8 The only pertinent history in our patient was wax hair removal. Fat necrosis was an unexpected complication, but hair removal can be considered minor trauma; however, this is not commonly reported in the literature following hair removal with wax. In a study that reviewed diseases of the male breast, the investigators observed that all male patients with fat necrosis had pseudogynecomastia (adipomastia).7 Although our patient’s entire anterior trunk was epilated, only the breast was affected. This situation might be explained by underlying gynecomastia because fat necrosis is common in areas of the body where subcutaneous fat tissue is dense.
Fat necrosis of the breast can be mistaken—both clinically and radiologically—for malignancy, such as in our case. Diagnosis of fat necrosis of the breast should be a diagnosis of exclusion; therefore, histopathologic confirmation of the lesion is imperative.9
In conclusion, fat necrosis of the male breast is rare. The condition can present as cellulitis. Hair removal with wax might be a cause of fat necrosis. Because breast cancer and fat necrosis can exhibit clinical and radiologic similarities, the diagnosis of fat necrosis should be confirmed by histopathologic analysis in conjunction with clinical and radiologic findings.
- Tan PH, Lai LM, Carrington EV, et al. Fat necrosis of the breast—a review. Breast. 2006;15:313-318. doi:10.1016/j.breast.2005.07.003
- Silverstone M. Fat necrosis of the breast with report of a case in a male. Br J Surg. 1949;37:49-52. doi:10.1002/bjs.18003714508
- Akyol M, Kayali A, Yildirim N. Traumatic fat necrosis of male breast. Clin Imaging. 2013;37:954-956. doi:10.1016/j.clinimag.2013.05.009
- Crawford EA, King JJ, Fox EJ, et al. Symptomatic fat necrosis and lipoatrophy of the posterior pelvis following trauma. Orthopedics. 2009;32:444. doi:10.3928/01477447-20090511-25
- Haikin Herzberger E, Aviner S, Cherniavsky E. Posttraumatic fat necrosis presented as cellulitis of the leg. Case Rep Pediatr. 2012;2012:672397. doi:10.1155/2012/672397
- Michels LG, Gold RH, Arndt RD. Radiography of gynecomastia and other disorders of the male breast. Radiology. 1977;122:117-122. doi:10.1148/122.1.117
- Günhan-Bilgen I, Bozkaya H, Ustün E, et al. Male breast disease: clinical, mammographic, and ultrasonographic features. Eur J Radiol. 2002;43:246-255. doi:10.1016/s0720-048x(01)00483-1
- Chala LF, de Barros N, de Camargo Moraes P, et al. Fat necrosis of the breast: mammographic, sonographic, computed tomography, and magnetic resonance imaging findings. Curr Probl Diagn Radiol. 2004;33:106-126. doi:10.1067/j.cpradiol.2004.01.001
- Pullyblank AM, Davies JD, Basten J, et al. Fat necrosis of the female breast—Hadfield re-visited. Breast. 2001;10:388-391. doi:10.1054/brst.2000.0287
To the Editor:
Fat necrosis of the breast is a benign inflammatory disease of adipose tissue commonly observed after trauma in the female breast during the perimenopausal period.1 Fat necrosis of the male breast is rare, first described by Silverstone2 in 1949; the condition usually presents with unilateral, painful or asymptomatic, firm nodules, which in rare cases are observed as skin retraction and thickening, ecchymosis, erythematous plaque–like cellulitis, local depression, and/or discoloration of the breast skin.3-5
Diagnosis of fat necrosis of the male breast may need to be confirmed via biopsy in conjunction with clinical and radiologic findings because the condition can mimic breast cancer.1 We report a case of bilateral fat necrosis of the breast mimicking breast cancer following wax hair removal.
A 42-year-old man presented to our outpatient dermatology clinic for evaluation of redness, swelling, and hardness of the skin of both breasts of 3 weeks’ duration. The patient had a history of wax hair removal of the entire anterior aspect of the body. He reported an erythematous, edematous, warm plaque that developed on the breasts 2 days after waxing. The plaque did not respond to antibiotics. The swelling and induration progressed over the 2 weeks after the patient was waxed. The patient had no family history of breast cancer. He had a standing diagnosis of gynecomastia. He denied any history of fat or filler injection in the affected area.
Dermatologic examination revealed erythematous, edematous, indurated, asymptomatic plaques with a peau d’orange appearance on the bilateral pectoral and presternal region. Minimal retraction of the right areola was noted (Figure 1). The bilateral axillary lymph nodes were palpable.

Laboratory results including erythrocyte sedimentation rate (108 mm/h [reference range, 2–20 mm/h]), C-reactive protein (9.2 mg/dL [reference range, >0.5 mg/dL]), and ferritin levels (645
Mammography of both breasts revealed a Breast Imaging Reporting and Data System (BI-RADS) score of 4 with a suspicious abnormality (ie, diffuse edema of the breast, multiple calcifications in a nonspecific pattern, oil cysts with calcifications, and bilateral axillary lymphadenopathy with a diameter of 2.5 cm and a thick and irregular cortex)(Figure 2A). Ultrasonography of both breasts revealed an inflammatory breast. Magnetic resonance imaging showed similar findings with diffuse edema and a heterogeneous appearance. Contrast-enhanced magnetic resonance imaging showed diffuse contrast enhancement in both breasts extending to the pectoral muscles and axillary regions, consistent with inflammatory changes (Figure 2B).

Because of difficulty differentiating inflammation and an infiltrating tumor, histopathologic examination was recommended by radiology. Results from a 5-mm punch biopsy from the right breast yielded the following differential diagnoses: cellulitis, panniculitis, inflammatory breast cancer, subcutaneous fat necrosis, and paraffinoma. Histopathologic examination of the skin revealed a normal epidermis and a dense inflammatory cell infiltrate comprising lymphocytes and monocytes in the dermis and subcutaneous tissue. Marked fibrosis also was noted in the dermis and subcutaneous tissue. Lipophagic fat necrosis accompanied by a variable inflammatory cell infiltrate consisted of histiocytes and neutrophils (Figure 3A). Pankeratin immunostaining was negative. Fat necrosis was present in a biopsy specimen obtained from the right breast; no signs of malignancy were present (Figure 3B). Fine-needle aspiration of the axillary lymph nodes was benign. Given these histopathologic findings, malignancy was excluded from the differential diagnosis. Paraffinoma also was ruled out because the patient insistently denied any history of fat or filler injection.

Based on the clinical, histopathologic, and radiologic findings, as well as the history of minor trauma due to wax hair removal, a diagnosis of fat necrosis of the breast was made. Intervention was not recommended by the plastic surgeons who subsequently evaluated the patient, because the additional trauma may aggravate the lesion. He was treated with nonsteroidal anti-inflammatory drugs.
At 6-month follow-up, there was marked reduction in the erythema and edema but no notable improvement of the induration. A potent topical steroid was added to the treatment, but only slight regression of the induration was observed.
The normal male breast is comprised of fat and a few secretory ducts.6 Gynecomastia and breast cancer are the 2 most common conditions of the male breast; fat necrosis of the male breast is rare. In a study of 236 male patients with breast disease, only 5 had fat necrosis.7
Fat necrosis of the breast can be observed with various clinical and radiological presentations. Subcutaneous nodules, skin retraction and thickening, local skin depression, and ecchymosis are the more common presentations of fat necrosis.3-5 In our case, the first symptoms of disease were similar to those seen in cellulitis. The presentation of fat necrosis–like cellulitis has been described only rarely in the medical literature. Haikin et al5 reported a case of fat necrosis of the leg in a child that presented with cellulitis followed by induration, which did not respond to antibiotics, as was the case with our patient.5
Blunt trauma, breast reduction surgery, and breast augmentation surgery can cause fat necrosis of the breast1,4; in some cases, the cause cannot be determined.8 The only pertinent history in our patient was wax hair removal. Fat necrosis was an unexpected complication, but hair removal can be considered minor trauma; however, this is not commonly reported in the literature following hair removal with wax. In a study that reviewed diseases of the male breast, the investigators observed that all male patients with fat necrosis had pseudogynecomastia (adipomastia).7 Although our patient’s entire anterior trunk was epilated, only the breast was affected. This situation might be explained by underlying gynecomastia because fat necrosis is common in areas of the body where subcutaneous fat tissue is dense.
Fat necrosis of the breast can be mistaken—both clinically and radiologically—for malignancy, such as in our case. Diagnosis of fat necrosis of the breast should be a diagnosis of exclusion; therefore, histopathologic confirmation of the lesion is imperative.9
In conclusion, fat necrosis of the male breast is rare. The condition can present as cellulitis. Hair removal with wax might be a cause of fat necrosis. Because breast cancer and fat necrosis can exhibit clinical and radiologic similarities, the diagnosis of fat necrosis should be confirmed by histopathologic analysis in conjunction with clinical and radiologic findings.
To the Editor:
Fat necrosis of the breast is a benign inflammatory disease of adipose tissue commonly observed after trauma in the female breast during the perimenopausal period.1 Fat necrosis of the male breast is rare, first described by Silverstone2 in 1949; the condition usually presents with unilateral, painful or asymptomatic, firm nodules, which in rare cases are observed as skin retraction and thickening, ecchymosis, erythematous plaque–like cellulitis, local depression, and/or discoloration of the breast skin.3-5
Diagnosis of fat necrosis of the male breast may need to be confirmed via biopsy in conjunction with clinical and radiologic findings because the condition can mimic breast cancer.1 We report a case of bilateral fat necrosis of the breast mimicking breast cancer following wax hair removal.
A 42-year-old man presented to our outpatient dermatology clinic for evaluation of redness, swelling, and hardness of the skin of both breasts of 3 weeks’ duration. The patient had a history of wax hair removal of the entire anterior aspect of the body. He reported an erythematous, edematous, warm plaque that developed on the breasts 2 days after waxing. The plaque did not respond to antibiotics. The swelling and induration progressed over the 2 weeks after the patient was waxed. The patient had no family history of breast cancer. He had a standing diagnosis of gynecomastia. He denied any history of fat or filler injection in the affected area.
Dermatologic examination revealed erythematous, edematous, indurated, asymptomatic plaques with a peau d’orange appearance on the bilateral pectoral and presternal region. Minimal retraction of the right areola was noted (Figure 1). The bilateral axillary lymph nodes were palpable.

Laboratory results including erythrocyte sedimentation rate (108 mm/h [reference range, 2–20 mm/h]), C-reactive protein (9.2 mg/dL [reference range, >0.5 mg/dL]), and ferritin levels (645
Mammography of both breasts revealed a Breast Imaging Reporting and Data System (BI-RADS) score of 4 with a suspicious abnormality (ie, diffuse edema of the breast, multiple calcifications in a nonspecific pattern, oil cysts with calcifications, and bilateral axillary lymphadenopathy with a diameter of 2.5 cm and a thick and irregular cortex)(Figure 2A). Ultrasonography of both breasts revealed an inflammatory breast. Magnetic resonance imaging showed similar findings with diffuse edema and a heterogeneous appearance. Contrast-enhanced magnetic resonance imaging showed diffuse contrast enhancement in both breasts extending to the pectoral muscles and axillary regions, consistent with inflammatory changes (Figure 2B).

Because of difficulty differentiating inflammation and an infiltrating tumor, histopathologic examination was recommended by radiology. Results from a 5-mm punch biopsy from the right breast yielded the following differential diagnoses: cellulitis, panniculitis, inflammatory breast cancer, subcutaneous fat necrosis, and paraffinoma. Histopathologic examination of the skin revealed a normal epidermis and a dense inflammatory cell infiltrate comprising lymphocytes and monocytes in the dermis and subcutaneous tissue. Marked fibrosis also was noted in the dermis and subcutaneous tissue. Lipophagic fat necrosis accompanied by a variable inflammatory cell infiltrate consisted of histiocytes and neutrophils (Figure 3A). Pankeratin immunostaining was negative. Fat necrosis was present in a biopsy specimen obtained from the right breast; no signs of malignancy were present (Figure 3B). Fine-needle aspiration of the axillary lymph nodes was benign. Given these histopathologic findings, malignancy was excluded from the differential diagnosis. Paraffinoma also was ruled out because the patient insistently denied any history of fat or filler injection.

Based on the clinical, histopathologic, and radiologic findings, as well as the history of minor trauma due to wax hair removal, a diagnosis of fat necrosis of the breast was made. Intervention was not recommended by the plastic surgeons who subsequently evaluated the patient, because the additional trauma may aggravate the lesion. He was treated with nonsteroidal anti-inflammatory drugs.
At 6-month follow-up, there was marked reduction in the erythema and edema but no notable improvement of the induration. A potent topical steroid was added to the treatment, but only slight regression of the induration was observed.
The normal male breast is comprised of fat and a few secretory ducts.6 Gynecomastia and breast cancer are the 2 most common conditions of the male breast; fat necrosis of the male breast is rare. In a study of 236 male patients with breast disease, only 5 had fat necrosis.7
Fat necrosis of the breast can be observed with various clinical and radiological presentations. Subcutaneous nodules, skin retraction and thickening, local skin depression, and ecchymosis are the more common presentations of fat necrosis.3-5 In our case, the first symptoms of disease were similar to those seen in cellulitis. The presentation of fat necrosis–like cellulitis has been described only rarely in the medical literature. Haikin et al5 reported a case of fat necrosis of the leg in a child that presented with cellulitis followed by induration, which did not respond to antibiotics, as was the case with our patient.5
Blunt trauma, breast reduction surgery, and breast augmentation surgery can cause fat necrosis of the breast1,4; in some cases, the cause cannot be determined.8 The only pertinent history in our patient was wax hair removal. Fat necrosis was an unexpected complication, but hair removal can be considered minor trauma; however, this is not commonly reported in the literature following hair removal with wax. In a study that reviewed diseases of the male breast, the investigators observed that all male patients with fat necrosis had pseudogynecomastia (adipomastia).7 Although our patient’s entire anterior trunk was epilated, only the breast was affected. This situation might be explained by underlying gynecomastia because fat necrosis is common in areas of the body where subcutaneous fat tissue is dense.
Fat necrosis of the breast can be mistaken—both clinically and radiologically—for malignancy, such as in our case. Diagnosis of fat necrosis of the breast should be a diagnosis of exclusion; therefore, histopathologic confirmation of the lesion is imperative.9
In conclusion, fat necrosis of the male breast is rare. The condition can present as cellulitis. Hair removal with wax might be a cause of fat necrosis. Because breast cancer and fat necrosis can exhibit clinical and radiologic similarities, the diagnosis of fat necrosis should be confirmed by histopathologic analysis in conjunction with clinical and radiologic findings.
- Tan PH, Lai LM, Carrington EV, et al. Fat necrosis of the breast—a review. Breast. 2006;15:313-318. doi:10.1016/j.breast.2005.07.003
- Silverstone M. Fat necrosis of the breast with report of a case in a male. Br J Surg. 1949;37:49-52. doi:10.1002/bjs.18003714508
- Akyol M, Kayali A, Yildirim N. Traumatic fat necrosis of male breast. Clin Imaging. 2013;37:954-956. doi:10.1016/j.clinimag.2013.05.009
- Crawford EA, King JJ, Fox EJ, et al. Symptomatic fat necrosis and lipoatrophy of the posterior pelvis following trauma. Orthopedics. 2009;32:444. doi:10.3928/01477447-20090511-25
- Haikin Herzberger E, Aviner S, Cherniavsky E. Posttraumatic fat necrosis presented as cellulitis of the leg. Case Rep Pediatr. 2012;2012:672397. doi:10.1155/2012/672397
- Michels LG, Gold RH, Arndt RD. Radiography of gynecomastia and other disorders of the male breast. Radiology. 1977;122:117-122. doi:10.1148/122.1.117
- Günhan-Bilgen I, Bozkaya H, Ustün E, et al. Male breast disease: clinical, mammographic, and ultrasonographic features. Eur J Radiol. 2002;43:246-255. doi:10.1016/s0720-048x(01)00483-1
- Chala LF, de Barros N, de Camargo Moraes P, et al. Fat necrosis of the breast: mammographic, sonographic, computed tomography, and magnetic resonance imaging findings. Curr Probl Diagn Radiol. 2004;33:106-126. doi:10.1067/j.cpradiol.2004.01.001
- Pullyblank AM, Davies JD, Basten J, et al. Fat necrosis of the female breast—Hadfield re-visited. Breast. 2001;10:388-391. doi:10.1054/brst.2000.0287
- Tan PH, Lai LM, Carrington EV, et al. Fat necrosis of the breast—a review. Breast. 2006;15:313-318. doi:10.1016/j.breast.2005.07.003
- Silverstone M. Fat necrosis of the breast with report of a case in a male. Br J Surg. 1949;37:49-52. doi:10.1002/bjs.18003714508
- Akyol M, Kayali A, Yildirim N. Traumatic fat necrosis of male breast. Clin Imaging. 2013;37:954-956. doi:10.1016/j.clinimag.2013.05.009
- Crawford EA, King JJ, Fox EJ, et al. Symptomatic fat necrosis and lipoatrophy of the posterior pelvis following trauma. Orthopedics. 2009;32:444. doi:10.3928/01477447-20090511-25
- Haikin Herzberger E, Aviner S, Cherniavsky E. Posttraumatic fat necrosis presented as cellulitis of the leg. Case Rep Pediatr. 2012;2012:672397. doi:10.1155/2012/672397
- Michels LG, Gold RH, Arndt RD. Radiography of gynecomastia and other disorders of the male breast. Radiology. 1977;122:117-122. doi:10.1148/122.1.117
- Günhan-Bilgen I, Bozkaya H, Ustün E, et al. Male breast disease: clinical, mammographic, and ultrasonographic features. Eur J Radiol. 2002;43:246-255. doi:10.1016/s0720-048x(01)00483-1
- Chala LF, de Barros N, de Camargo Moraes P, et al. Fat necrosis of the breast: mammographic, sonographic, computed tomography, and magnetic resonance imaging findings. Curr Probl Diagn Radiol. 2004;33:106-126. doi:10.1067/j.cpradiol.2004.01.001
- Pullyblank AM, Davies JD, Basten J, et al. Fat necrosis of the female breast—Hadfield re-visited. Breast. 2001;10:388-391. doi:10.1054/brst.2000.0287
Practice Points
- Fat necrosis of the breast can be mistaken—both clinically and radiologically—for malignancy; therefore, diagnosis should be confirmed by histopathology in conjunction with clinical and radiologic findings.
- Fat necrosis of the male breast is rare, and hair removal with wax may be a rare cause of the disease.
Dermatologic Implications of Sleep Deprivation in the US Military
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
Sleep deprivation can increase emotional distress and mood disorders; reduce quality of life; and lead to cognitive, memory, and performance deficits.1 Military service predisposes members to disordered sleep due to the rigors of deployments and field training, such as long shifts, shift changes, stressful work environments, and time zone changes. Evidence shows that sleep deprivation is associated with cardiovascular disease, gastrointestinal disease, and some cancers.2 We explore multiple mechanisms by which sleep deprivation may affect the skin. We also review the potential impacts of sleep deprivation on specific topics in dermatology, including atopic dermatitis (AD), psoriasis, alopecia areata, physical attractiveness, wound healing, and skin cancer.
Sleep and Military Service
Approximately 35.2% of Americans experience short sleep duration, which the Centers for Disease Control and Prevention defines as sleeping fewer than 7 hours per 24-hour period.3 Short sleep duration is even more common among individuals working in protective services and the military (50.4%).4 United States military service members experience multiple contributors to disordered sleep, including combat operations, shift work, psychiatric disorders such as posttraumatic stress disorder, and traumatic brain injury.5 Bramoweth and Germain6 described the case of a 27-year-old man who served 2 combat tours as an infantryman in Afghanistan, during which time he routinely remained awake for more than 24 hours at a time due to night missions and extended operations. Even when he was not directly involved in combat operations, he was rarely able to keep a regular sleep schedule.6 Service members returning from deployment also report decreased sleep. In one study (N=2717), 43% of respondents reported short sleep duration (<7 hours of sleep per night) and 29% reported very short sleep duration (<6 hours of sleep per night).7 Even stateside, service members experience acute sleep deprivation during training.8
Sleep and Skin
The idea that skin conditions can affect quality of sleep is not controversial. Pruritus, pain, and emotional distress associated with different dermatologic conditions have all been implicated in adversely affecting sleep.9 Given the effects of sleep deprivation on other organ systems, it also can affect the skin. Possible mechanisms of action include negative effects of sleep deprivation on the hypothalamic-pituitary-adrenal (HPA) axis, cutaneous barrier function, and immune function. First, the HPA axis activity follows a circadian rhythm.10 Activation outside of the bounds of this normal rhythm can have adverse effects on sleep. Alternatively, sleep deprivation and decreased sleep quality can negatively affect the HPA axis.10 These changes can adversely affect cutaneous barrier and immune function.11 Cutaneous barrier function is vitally important in the context of inflammatory dermatologic conditions. Transepidermal water loss, a measurement used to estimate cutaneous barrier function, is increased by sleep deprivation.12 Finally, the cutaneous immune system is an important component of inflammatory dermatologic conditions, cancer immune surveillance, and wound healing, and it also is negatively impacted by sleep deprivation.13 This framework of sleep deprivation affecting the HPA axis, cutaneous barrier function, and cutaneous immune function will help to guide the following discussion on the effects of decreased sleep on specific dermatologic conditions.
Atopic Dermatitis—Individuals with AD are at higher odds of having insomnia, fatigue, and overall poorer health status, including more sick days and increased visits to a physician.14 Additionally, it is possible that the relationship between AD and sleep is not unidirectional. Chang and Chiang15 discussed the possibility of sleep disturbances contributing to AD flares and listed 3 possible mechanisms by which sleep disturbance could potentially flare AD: exacerbation of the itch-scratch cycle; changes in the immune system, including a possible shift to helper T cell (TH2) dominance; and worsening of chronic stress in patients with AD. These changes may lead to a vicious cycle of impaired sleep and AD exacerbations. It may be helpful to view sleep impairment and AD as comorbid conditions requiring co-management for optimal outcomes. This perspective has military relevance because even without considering sleep deprivation, deployment and field conditions are known to increase the risk for AD flares.16
Psoriasis—Psoriasis also may have a bidirectional relationship with sleep. A study utilizing data from the Nurses’ Health Study showed that working a night shift increased the risk for psoriasis.17 Importantly, this connection is associative and not causative. It is possible that other factors in those who worked night shifts such as probable decreased UV exposure or reported increased body mass index played a role. Studies using psoriasis mice models have shown increased inflammation with sleep deprivation.18 Another possible connection is the effect of sleep deprivation on the gut microbiome. Sleep dysfunction is associated with altered gut bacteria ratios, and similar gut bacteria ratios were found in patients with psoriasis, which may indicate an association between sleep deprivation and psoriasis disease progression.19 There also is an increased association of obstructive sleep apnea in patients with psoriasis compared to the general population.20 Fortunately, the rate of consultations for psoriasis in deployed soldiers in the last several conflicts has been quite low, making up only 2.1% of diagnosed dermatologic conditions,21 which is because service members with moderate to severe psoriasis likely will not be deployed.
Alopecia Areata—Alopecia areata also may be associated with sleep deprivation. A large retrospective cohort study looking at the risk for alopecia in patients with sleep disorders showed that a sleep disorder was an independent risk factor for alopecia areata.22 The impact of sleep on the HPA axis portrays a possible mechanism for the negative effects of sleep deprivation on the immune system. Interestingly, in this study, the association was strongest for the 0- to 24-year-old age group. According to the 2020 demographics profile of the military community, 45% of active-duty personnel are 25 years or younger.23 Fortunately, although alopecia areata can be a distressing condition, it should not have much effect on military readiness, as most individuals with this diagnosis are still deployable.
Physical Appearance—
Wound Healing—Wound healing is of particular importance to the health of military members. Research is suggestive but not definitive of the relationship between sleep and wound healing. One intriguing study looked at the healing of blisters induced via suction in well-rested and sleep-deprived individuals. The results showed a difference, with the sleep-deprived individuals taking approximately 1 day longer to heal.13 This has some specific relevance to the military, as friction blisters can be common.30 A cross-sectional survey looking at a group of service members deployed in Iraq showed a prevalence of foot friction blisters of 33%, with 11% of individuals requiring medical care.31 Although this is an interesting example, it is not necessarily applicable to full-thickness wounds. A study utilizing rat models did not identify any differences between sleep-deprived and well-rested models in the healing of punch biopsy sites.32
Skin Cancer—Altered circadian rhythms resulting in changes in melatonin levels, changes in circadian rhythm–related gene pathways, and immunologic changes have been proposed as possible contributing mechanisms for the observed increased risk for skin cancers in military and civilian pilots.33,34 One study showed that UV-related erythema resolved quicker in well-rested individuals compared with those with short sleep duration, which could represent more efficient DNA repair given the relationship between UV-associated erythema and DNA damage and repair.35 Another study looking at circadian changes in the repair of UV-related DNA damage showed that mice exposed to UV radiation in the early morning had higher rates of squamous cell carcinoma than those exposed in the afternoon.36 However, a large cohort study using data from the Nurses’ Health Study II did not support a positive connection between short sleep duration and skin cancer; rather, it showed that a short sleep duration was associated with a decreased risk for melanoma and basal cell carcinoma, with no effect noted for squamous cell carcinoma.37 This does not support a positive association between short sleep duration and skin cancer and in some cases actually suggests a negative association.
Final Thoughts
Although more research is needed, there is evidence that sleep deprivation can negatively affect the skin. Randomized controlled trials looking at groups of individuals with specific dermatologic conditions with a very short sleep duration group (<6 hours of sleep per night), short sleep duration group (<7 hours of sleep per night), and a well-rested group (>7 hours of sleep per night) could be very helpful in this endeavor. Possible mechanisms include the HPA axis, immune system, and skin barrier function that are associated with sleep deprivation. Specific dermatologic conditions that may be affected by sleep deprivation include AD, psoriasis, alopecia areata, physical appearance, wound healing, and skin cancer. The impact of sleep deprivation on dermatologic conditions is particularly relevant to the military, as service members are at an increased risk for short sleep duration. It is possible that improving sleep may lead to better disease control for many dermatologic conditions.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
- Carskadon M, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107-113.
- Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;19;9:151-161.
- Sleep and sleep disorders. Centers for Disease Control and Prevention website. Reviewed September 12, 2022. Accessed February 17, 2023. https://www.cdc.gov/sleep/data_statistics.html
- Khubchandani J, Price JH. Short sleep duration in working American adults, 2010-2018. J Community Health. 2020;45:219-227.
- Good CH, Brager AJ, Capaldi VF, et al. Sleep in the United States military. Neuropsychopharmacology. 2020;45:176-191.
- Bramoweth AD, Germain A. Deployment-related insomnia in military personnel and veterans. Curr Psychiatry Rep. 2013;15:401.
- Luxton DD, Greenburg D, Ryan J, et al. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep. 2011;34:1189-1195.
- Crowley SK, Wilkinson LL, Burroughs EL, et al. Sleep during basic combat training: a qualitative study. Mil Med. 2012;177:823-828.
- Spindler M, Przybyłowicz K, Hawro M, et al. Sleep disturbance in adult dermatologic patients: a cross-sectional study on prevalence, burden, and associated factors. J Am Acad Dermatol. 2021;85:910-922.
- Guyon A, Balbo M, Morselli LL, et al. Adverse effects of two nights of sleep restriction on the hypothalamic-pituitary-adrenal axis in healthy men. J Clin Endocrinol Metab. 2014;99:2861-2868.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131.
- Pinnagoda J, Tupker RA, Agner T, et al. Guidelines for transepidermal water loss (TEWL) measurement. a report from theStandardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22:164-178.
- Smith TJ, Wilson MA, Karl JP, et al. Impact of sleep restriction on local immune response and skin barrier restoration with and without “multinutrient” nutrition intervention. J Appl Physiol (1985). 2018;124:190-200.
- Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135:56-66.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018;142:1033-1040.
- Riegleman KL, Farnsworth GS, Wong EB. Atopic dermatitis in the US military. Cutis. 2019;104:144-147.
- Li WQ, Qureshi AA, Schernhammer ES, et al. Rotating night-shift work and risk of psoriasis in US women. J Invest Dermatol. 2013;133:565-567.
- Hirotsu C, Rydlewski M, Araújo MS, et al. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS One. 2012;7:E51183.
- Myers B, Vidhatha R, Nicholas B, et al. Sleep and the gut microbiome in psoriasis: clinical implications for disease progression and the development of cardiometabolic comorbidities. J Psoriasis Psoriatic Arthritis. 2021;6:27-37.
- Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: a systematic review. Sleep Med Rev. 2016;29:63-75.
- Gelman AB, Norton SA, Valdes-Rodriguez R, et al. A review of skin conditions in modern warfare and peacekeeping operations. Mil Med. 2015;180:32-37.
- Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41:10.1093/sleep/zsy111.
- Department of Defense. 2020 Demographics: Profile of the Military Community. Military One Source website. Accessed February 17, 2023. https://download.militaryonesource.mil/12038/MOS/Reports/2020-demographics-report.pdf
- Sundelin T, Lekander M, Kecklund G, et al. Cues of fatigue: effects of sleep deprivation on facial appearance. Sleep. 2013;36:1355-1360.
- Sundelin T, Lekander M, Sorjonen K, et a. Negative effects of restricted sleep on facial appearance and social appeal. R Soc Open Sci. 2017;4:160918.
- Holding BC, Sundelin T, Cairns P, et al. The effect of sleep deprivation on objective and subjective measures of facial appearance. J Sleep Res. 2019;28:E12860.
- Léger D, Gauriau C, Etzi C, et al. “You look sleepy…” the impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep Med. 2022;89:97-103.
- Talamas SN, Mavor KI, Perrett DI. Blinded by beauty: attractiveness bias and accurate perceptions of academic performance. PLoS One. 2016;11:E0148284.
- Department of the Army. Enlisted Promotions and Reductions. Army Publishing Directorate website. Published May 16, 2019. Accessed February 17, 2023. https://armypubs.army.mil/epubs/DR_pubs/DR_a/pdf/web/ARN17424_R600_8_19_Admin_FINAL.pdf
- Levy PD, Hile DC, Hile LM, et al. A prospective analysis of the treatment of friction blisters with 2-octylcyanoacrylate. J Am Podiatr Med Assoc. 2006;96:232-237.
- Brennan FH Jr, Jackson CR, Olsen C, et al. Blisters on the battlefield: the prevalence of and factors associated with foot friction blisters during Operation Iraqi Freedom I. Mil Med. 2012;177:157-162.
- Mostaghimi L, Obermeyer WH, Ballamudi B, et al. Effects of sleep deprivation on wound healing. J Sleep Res. 2005;14:213-219.
- Wilkison BD, Wong EB. Skin cancer in military pilots: a special population with special risk factors. Cutis. 2017;100:218-220.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Shiftwork. World Health Organization International Agency for Research on Cancer; 2010. Accessed February 20, 2023. https://www.ncbi.nlm.nih.gov/books/NBK326814/
- Oyetakin-White P, Suggs A, Koo B, et al. Does poor sleep quality affect skin ageing? Clin Exp Dermatol. 2015;40:17-22.
- Gaddameedhi S, Selby CP, Kaufmann WK, et al. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790-18795.
- Heckman CJ, Kloss JD, Feskanich D, et al. Associations among rotating night shift work, sleep and skin cancer in Nurses’ Health Study II participants. Occup Environ Med. 2017;74:169-175.
Practice Points
- Sleep deprivation may have negative effects on skin function and worsen dermatologic conditions.
- Proposed mechanisms of action for these negative effects include dysregulation of the hypothalamic-pituitary-adrenal axis, impairment of cutaneous barrier function, and alteration of cutaneous immune function.
- Members of the US Military are at an increased risk for sleep deprivation, especially during training and overseas deployments.
Characterization of Blood-borne Pathogen Exposures During Dermatologic Procedures: The Mayo Clinic Experience
Dermatology providers are at an increased risk for blood-borne pathogen (BBP) exposures during procedures in clinical practice.1-3 Current data regarding the characterization of these exposures are limited. Prior studies are based on surveys that result in low response rates and potential for selection bias. Donnelly et al1 reported a 26% response rate in a national survey-based study evaluating BBP exposures in resident physicians, fellows, and practicing dermatologists, with 85% of respondents reporting at least 1 injury. Similarly, Goulart et al2 reported a 35% response rate in a survey evaluating sharps injuries in residents and medical students, with 85% reporting a sharps injury. In addition, there are conflicting data regarding characteristics of these exposures, including common implicated instruments and procedures.1-3 Prior studies also have not evaluated exposures in all members of dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff.
To make appropriate quality improvements in dermatologic procedures, a more comprehensive understanding of BBP exposures is needed. We conducted a retrospective review of BBP incidence reports to identify the incidence of BBP events among all dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff. We further investigated the type of exposure, the type of procedure associated with each exposure, anatomic locations of exposures, and instruments involved in each exposure.
Methods
Data on BBP exposures in the dermatology departments were obtained from the occupational health departments at each of 3 Mayo Clinic sites—Scottsdale, Arizona; Jacksonville, Florida; and Rochester, Minnesota—from March 2010 through January 2021. The institutional review board at Mayo Clinic, Scottsdale, Arizona, granted approval of this study (IRB #20-012625). A retrospective review of each exposure was conducted to identify the incidence of BBP exposures. Occupational BBP exposure was defined as
Statistical Analysis—Variables were summarized using counts and percentages. The 3 most common categories for each variable were then compared among occupational groups using the Fisher exact test. All other categories were grouped for analysis purposes. Medical staff were categorized into 3 occupational groups: practicing dermatologists; resident physicians; and ancillary staff, including nurse/medical assistants, physician assistants, and clinical laboratory technologists. All analyses were 2 sided and considered statistically significant at P<.05. Analyses were performed using SAS 9.4 (SAS Institute Inc).
Results
Type of Exposure—A total of 222 BBP exposures were identified through the trisite retrospective review from March 2010 through January 2021. One hundred ninety-nine (89.6%) of 222 exposures were attributed to needlesticks and medical sharps, while 23 (10.4%) of 222 exposures were attributed to splash incidents (Table).
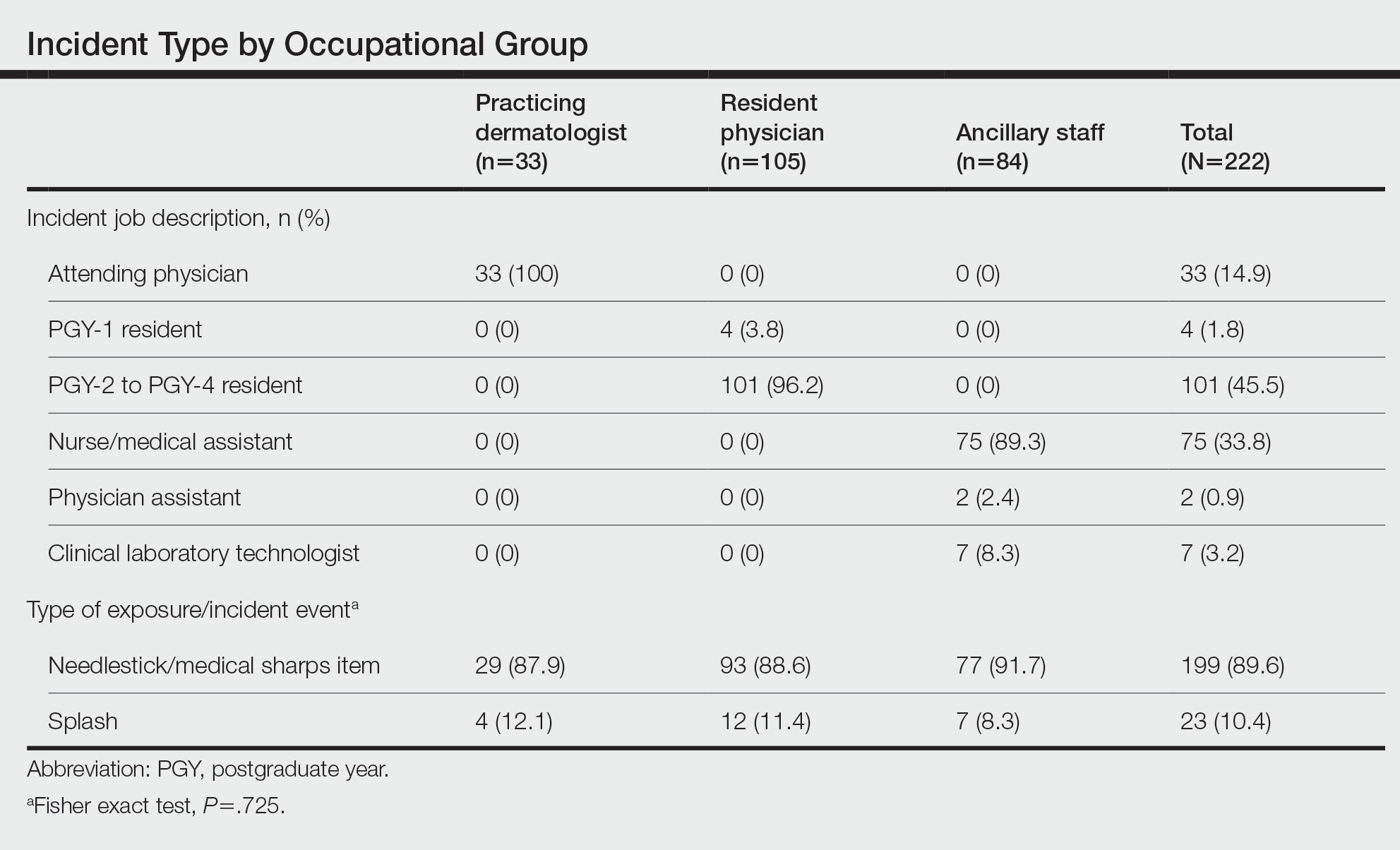
Anatomic Sites Affected—The anatomic location most frequently involved was the thumb (130/217 events [59.9%]), followed by the hand (39/217 events [18.0%]) and finger (22/217 events [10.1%]). The arm, face, and knee were affected with the lowest frequency, with only 1 event reported at each anatomic site (0.5%)(eTable). Five incidents were excluded from the analysis of anatomic location because of insufficient details of events.
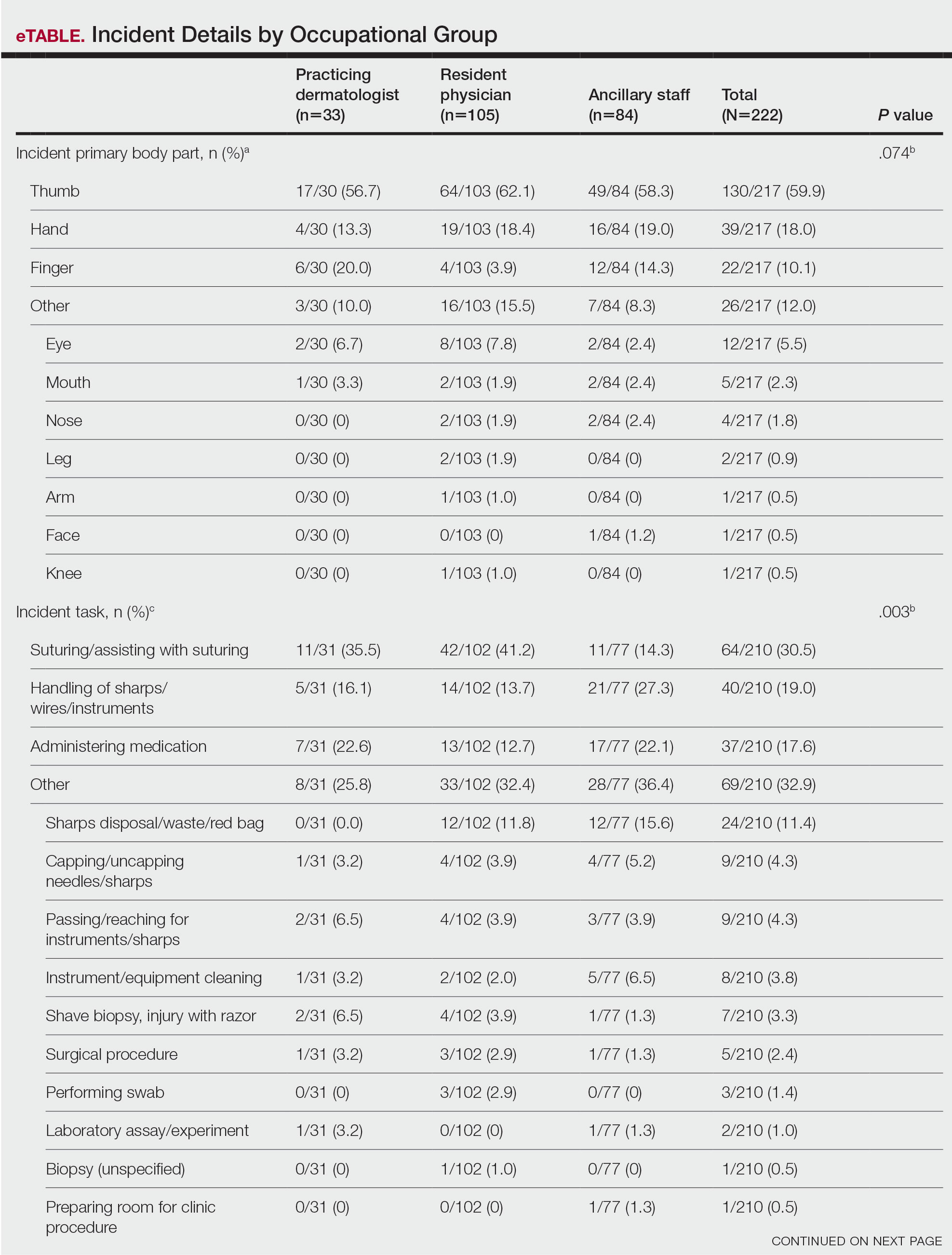
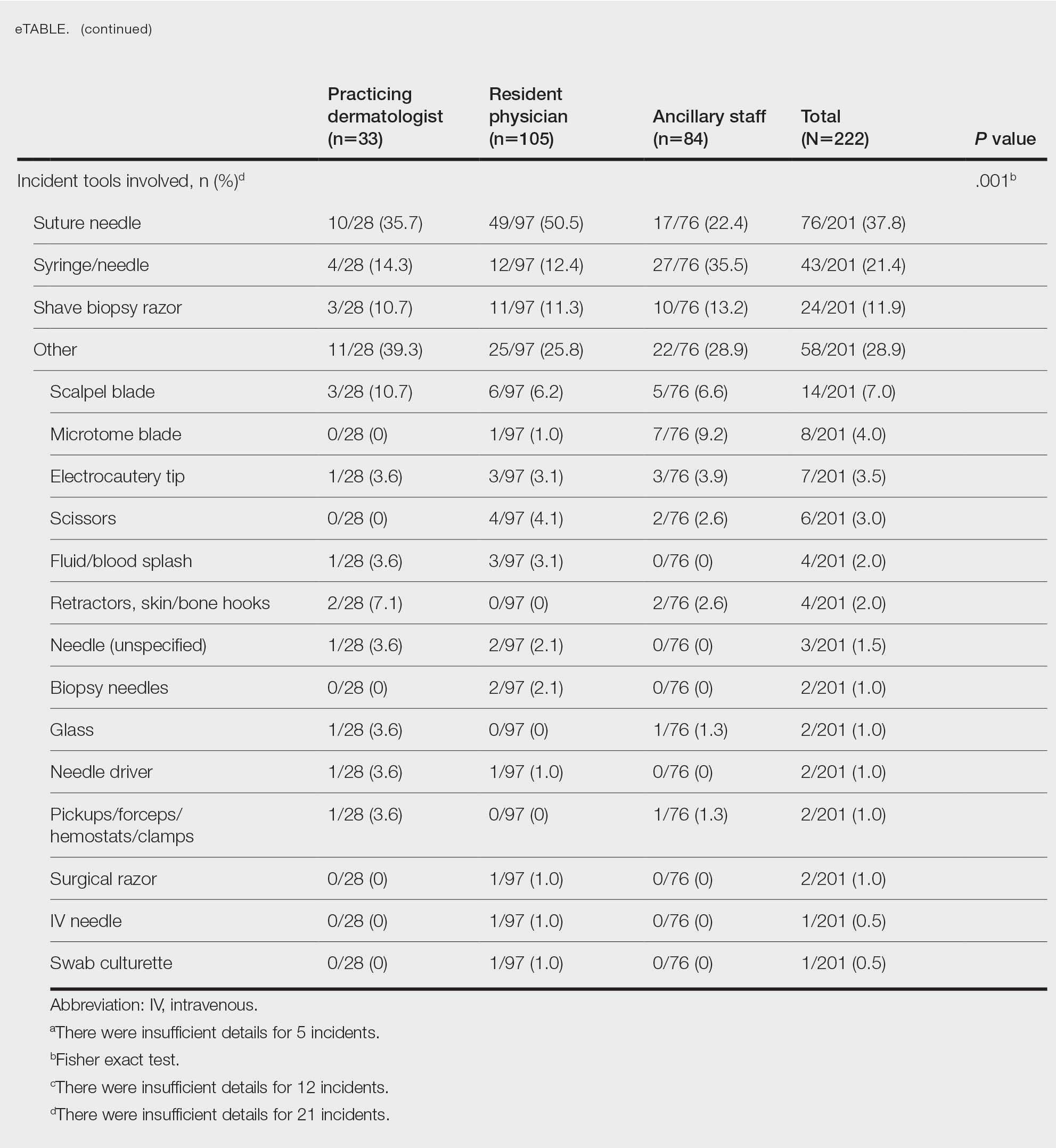
Incident Tasks and Tools—Most BBP exposures occurred during suturing or assisting with suturing (64/210 events [30.5%]), followed by handling of sharps, wires, or instruments (40/210 events [19.0%]) and medication administration (37/210 events [17.6%])(eTable). Twelve incidents were excluded from the analysis of implicated tasks because of insufficient details of events.
The tools involved in exposure events with the greatest prevalence included the suture needle (76/201 events [37.8%]), injection syringe/needle (43/201 events [21.4%]), and shave biopsy razor (24/201 events [11.9%])(eTable). Twenty-one incidents were excluded from the analysis of implicated instruments because of insufficient details of events.
Providers Affected by BBP Exposures—Resident physicians experienced the greatest number of BBP exposures (105/222 events [47.3%]), followed by ancillary providers (84/222 events [37.8%]) and practicing dermatologists (33/222 events [14.9%]). All occupational groups experienced more BBP exposures through needlesticks/medical sharps compared with splash incidents (resident physicians, 88.6%; ancillary staff, 91.7%; practicing dermatologists, 87.9%; P=.725)(Table).
Among resident physicians, practicing dermatologists, and ancillary staff, the most frequent site of injury was the thumb. Suturing/assisting with suturing was the most common task leading to injury, and the suture needle was the most common instrument of injury for both resident physicians and practicing dermatologists. Handling of sharps, wires, or instruments was the most common task leading to injury for ancillary staff, and the injection syringe/needle was the most common instrument of injury in this cohort.
Resident physicians experienced the lowest rate of BBP exposures during administration of medications (12.7%; P=.003). Ancillary staff experienced the highest rate of BBP exposures with an injection needle (35.5%; P=.001). There were no statistically significant differences among occupational groups for the anatomic location of injury (P=.074)(eTable).
Comment
In the year 2000, the annual global incidence of occupational BBP exposures among health care workers worldwide for hepatitis B virus, hepatitis C virus, and HIV was estimated at 2.1 million, 926,000, and 327,000, respectively. Most of these exposures were due to sharps injuries.4 Dermatologists are particularly at risk for BBP exposures given their reliance on frequent procedures in practice. During an 11-year period, 222 BBP exposures were documented in the dermatology departments at 3 Mayo Clinic institutions. Most exposures were due to needlestick/sharps across all occupational groups compared with splash injuries. Prior survey studies confirm that sharps injuries are frequently implicated, with 75% to 94% of residents and practicing dermatologists reporting at least 1 sharps injury.1
Among occupational groups, resident physicians had the highest rate of BBP exposures, followed by nurse/medical assistants and practicing dermatologists, which may be secondary to lack of training or experience. Data from other surgical fields, including general surgery, support that resident physicians have the highest rate of sharps injuries.5 In a survey study (N=452), 51% of residents reported that extra training in safe techniques would be beneficial.2 Safety training may be beneficial in reducing the incidence of BBP exposures in residency programs.
The most common implicated task in resident physicians and practicing dermatologists was suturing or assisting with suturing, and the most common implicated instrument was the suture needle. Prior studies showed conflicting data regarding common implicated tasks and instruments in this cohort.1,2 The task of suturing and the suture needle also were the most implicated means of injury among other surgical specialties.6 Ancillary staff experienced most BBP exposures during handling of sharps, wires, or instruments, as well as the use of an injection needle. The designation of tasks among dermatologic staff likely explains the difference among occupational groups. This new information may provide the opportunity to improve safety measures among all members of the dermatologic team.
Limitations—There are several limitations to this study. This retrospective review was conducted at a single health system at 3 institutions. Hence, similar safety protocols likely were in place across all sites, which may reduce the generalizability of the results. In addition, there is risk of nonreporting bias among staff, as only documented incidence reports were evaluated. Prior studies demonstrated a nonreporting prevalence of 33% to 64% among dermatology staff.1-3 We also did not evaluate whether injuries resulted in BBP exposure or transmission. The rates of postexposure prophylaxis also were not studied. This information was not available for review because of concerns for privacy. Demographic features, such as gender or years of training, also were not evaluated.
Conclusion
This study provides additional insight on the incidence of BBP exposures in dermatology, as well as the implicated tasks, instruments, and anatomic locations of injury. Studies show that implementing formal education regarding the risks of BBP exposure may result in reduction of sharps injuries.7 Formal education in residency programs may be needed in the field of dermatology to reduce BBP exposures. Quality improvement measures should focus on identified risk factors among occupational groups to reduce BBP exposures in the workplace.
- Donnelly AF, Chang Y-HH, Nemeth-Ochoa SA. Sharps injuries and reporting practices of U.S. dermatologists [published online November 14, 2013]. Dermatol Surg. 2013;39:1813-1821.
- Goulart J, Oliveria S, Levitt J. Safety during dermatologic procedures and surgeries: a survey of resident injuries and prevention strategies. J Am Acad Dermatol. 2011;65:648-650.
- Ken K, Golda N. Contaminated sharps injuries: a survey among dermatology residents. J Am Acad Dermatol. 2019;80:1786-1788.
- Pruss-Ustun A, Rapiti E, Hutin Y. Estimation of global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med. 2005;48:482-490.
- Choi L, Torres R, Syed S, et al. Sharps and needlestick injuries among medical students, surgical residents, faculty, and operating room staff at a single academic institution. J Surg Educ. 2017;74:131-136.
- Bakaeen F, Awad S, Albo D, et al. Epidemiology of exposure to blood borne pathogens on a surgical service. Am J Surg. 2006;192:E18-E21.
- Li WJ, Zhang M, Shi CL, et al. Study on intervention of bloodborne pathogen exposure in a general hospital [in Chinese]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2017;35:34-41.
Dermatology providers are at an increased risk for blood-borne pathogen (BBP) exposures during procedures in clinical practice.1-3 Current data regarding the characterization of these exposures are limited. Prior studies are based on surveys that result in low response rates and potential for selection bias. Donnelly et al1 reported a 26% response rate in a national survey-based study evaluating BBP exposures in resident physicians, fellows, and practicing dermatologists, with 85% of respondents reporting at least 1 injury. Similarly, Goulart et al2 reported a 35% response rate in a survey evaluating sharps injuries in residents and medical students, with 85% reporting a sharps injury. In addition, there are conflicting data regarding characteristics of these exposures, including common implicated instruments and procedures.1-3 Prior studies also have not evaluated exposures in all members of dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff.
To make appropriate quality improvements in dermatologic procedures, a more comprehensive understanding of BBP exposures is needed. We conducted a retrospective review of BBP incidence reports to identify the incidence of BBP events among all dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff. We further investigated the type of exposure, the type of procedure associated with each exposure, anatomic locations of exposures, and instruments involved in each exposure.
Methods
Data on BBP exposures in the dermatology departments were obtained from the occupational health departments at each of 3 Mayo Clinic sites—Scottsdale, Arizona; Jacksonville, Florida; and Rochester, Minnesota—from March 2010 through January 2021. The institutional review board at Mayo Clinic, Scottsdale, Arizona, granted approval of this study (IRB #20-012625). A retrospective review of each exposure was conducted to identify the incidence of BBP exposures. Occupational BBP exposure was defined as
Statistical Analysis—Variables were summarized using counts and percentages. The 3 most common categories for each variable were then compared among occupational groups using the Fisher exact test. All other categories were grouped for analysis purposes. Medical staff were categorized into 3 occupational groups: practicing dermatologists; resident physicians; and ancillary staff, including nurse/medical assistants, physician assistants, and clinical laboratory technologists. All analyses were 2 sided and considered statistically significant at P<.05. Analyses were performed using SAS 9.4 (SAS Institute Inc).
Results
Type of Exposure—A total of 222 BBP exposures were identified through the trisite retrospective review from March 2010 through January 2021. One hundred ninety-nine (89.6%) of 222 exposures were attributed to needlesticks and medical sharps, while 23 (10.4%) of 222 exposures were attributed to splash incidents (Table).

Anatomic Sites Affected—The anatomic location most frequently involved was the thumb (130/217 events [59.9%]), followed by the hand (39/217 events [18.0%]) and finger (22/217 events [10.1%]). The arm, face, and knee were affected with the lowest frequency, with only 1 event reported at each anatomic site (0.5%)(eTable). Five incidents were excluded from the analysis of anatomic location because of insufficient details of events.


Incident Tasks and Tools—Most BBP exposures occurred during suturing or assisting with suturing (64/210 events [30.5%]), followed by handling of sharps, wires, or instruments (40/210 events [19.0%]) and medication administration (37/210 events [17.6%])(eTable). Twelve incidents were excluded from the analysis of implicated tasks because of insufficient details of events.
The tools involved in exposure events with the greatest prevalence included the suture needle (76/201 events [37.8%]), injection syringe/needle (43/201 events [21.4%]), and shave biopsy razor (24/201 events [11.9%])(eTable). Twenty-one incidents were excluded from the analysis of implicated instruments because of insufficient details of events.
Providers Affected by BBP Exposures—Resident physicians experienced the greatest number of BBP exposures (105/222 events [47.3%]), followed by ancillary providers (84/222 events [37.8%]) and practicing dermatologists (33/222 events [14.9%]). All occupational groups experienced more BBP exposures through needlesticks/medical sharps compared with splash incidents (resident physicians, 88.6%; ancillary staff, 91.7%; practicing dermatologists, 87.9%; P=.725)(Table).
Among resident physicians, practicing dermatologists, and ancillary staff, the most frequent site of injury was the thumb. Suturing/assisting with suturing was the most common task leading to injury, and the suture needle was the most common instrument of injury for both resident physicians and practicing dermatologists. Handling of sharps, wires, or instruments was the most common task leading to injury for ancillary staff, and the injection syringe/needle was the most common instrument of injury in this cohort.
Resident physicians experienced the lowest rate of BBP exposures during administration of medications (12.7%; P=.003). Ancillary staff experienced the highest rate of BBP exposures with an injection needle (35.5%; P=.001). There were no statistically significant differences among occupational groups for the anatomic location of injury (P=.074)(eTable).
Comment
In the year 2000, the annual global incidence of occupational BBP exposures among health care workers worldwide for hepatitis B virus, hepatitis C virus, and HIV was estimated at 2.1 million, 926,000, and 327,000, respectively. Most of these exposures were due to sharps injuries.4 Dermatologists are particularly at risk for BBP exposures given their reliance on frequent procedures in practice. During an 11-year period, 222 BBP exposures were documented in the dermatology departments at 3 Mayo Clinic institutions. Most exposures were due to needlestick/sharps across all occupational groups compared with splash injuries. Prior survey studies confirm that sharps injuries are frequently implicated, with 75% to 94% of residents and practicing dermatologists reporting at least 1 sharps injury.1
Among occupational groups, resident physicians had the highest rate of BBP exposures, followed by nurse/medical assistants and practicing dermatologists, which may be secondary to lack of training or experience. Data from other surgical fields, including general surgery, support that resident physicians have the highest rate of sharps injuries.5 In a survey study (N=452), 51% of residents reported that extra training in safe techniques would be beneficial.2 Safety training may be beneficial in reducing the incidence of BBP exposures in residency programs.
The most common implicated task in resident physicians and practicing dermatologists was suturing or assisting with suturing, and the most common implicated instrument was the suture needle. Prior studies showed conflicting data regarding common implicated tasks and instruments in this cohort.1,2 The task of suturing and the suture needle also were the most implicated means of injury among other surgical specialties.6 Ancillary staff experienced most BBP exposures during handling of sharps, wires, or instruments, as well as the use of an injection needle. The designation of tasks among dermatologic staff likely explains the difference among occupational groups. This new information may provide the opportunity to improve safety measures among all members of the dermatologic team.
Limitations—There are several limitations to this study. This retrospective review was conducted at a single health system at 3 institutions. Hence, similar safety protocols likely were in place across all sites, which may reduce the generalizability of the results. In addition, there is risk of nonreporting bias among staff, as only documented incidence reports were evaluated. Prior studies demonstrated a nonreporting prevalence of 33% to 64% among dermatology staff.1-3 We also did not evaluate whether injuries resulted in BBP exposure or transmission. The rates of postexposure prophylaxis also were not studied. This information was not available for review because of concerns for privacy. Demographic features, such as gender or years of training, also were not evaluated.
Conclusion
This study provides additional insight on the incidence of BBP exposures in dermatology, as well as the implicated tasks, instruments, and anatomic locations of injury. Studies show that implementing formal education regarding the risks of BBP exposure may result in reduction of sharps injuries.7 Formal education in residency programs may be needed in the field of dermatology to reduce BBP exposures. Quality improvement measures should focus on identified risk factors among occupational groups to reduce BBP exposures in the workplace.
Dermatology providers are at an increased risk for blood-borne pathogen (BBP) exposures during procedures in clinical practice.1-3 Current data regarding the characterization of these exposures are limited. Prior studies are based on surveys that result in low response rates and potential for selection bias. Donnelly et al1 reported a 26% response rate in a national survey-based study evaluating BBP exposures in resident physicians, fellows, and practicing dermatologists, with 85% of respondents reporting at least 1 injury. Similarly, Goulart et al2 reported a 35% response rate in a survey evaluating sharps injuries in residents and medical students, with 85% reporting a sharps injury. In addition, there are conflicting data regarding characteristics of these exposures, including common implicated instruments and procedures.1-3 Prior studies also have not evaluated exposures in all members of dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff.
To make appropriate quality improvements in dermatologic procedures, a more comprehensive understanding of BBP exposures is needed. We conducted a retrospective review of BBP incidence reports to identify the incidence of BBP events among all dermatologic staff, including resident physicians, practicing dermatologists, and ancillary staff. We further investigated the type of exposure, the type of procedure associated with each exposure, anatomic locations of exposures, and instruments involved in each exposure.
Methods
Data on BBP exposures in the dermatology departments were obtained from the occupational health departments at each of 3 Mayo Clinic sites—Scottsdale, Arizona; Jacksonville, Florida; and Rochester, Minnesota—from March 2010 through January 2021. The institutional review board at Mayo Clinic, Scottsdale, Arizona, granted approval of this study (IRB #20-012625). A retrospective review of each exposure was conducted to identify the incidence of BBP exposures. Occupational BBP exposure was defined as
Statistical Analysis—Variables were summarized using counts and percentages. The 3 most common categories for each variable were then compared among occupational groups using the Fisher exact test. All other categories were grouped for analysis purposes. Medical staff were categorized into 3 occupational groups: practicing dermatologists; resident physicians; and ancillary staff, including nurse/medical assistants, physician assistants, and clinical laboratory technologists. All analyses were 2 sided and considered statistically significant at P<.05. Analyses were performed using SAS 9.4 (SAS Institute Inc).
Results
Type of Exposure—A total of 222 BBP exposures were identified through the trisite retrospective review from March 2010 through January 2021. One hundred ninety-nine (89.6%) of 222 exposures were attributed to needlesticks and medical sharps, while 23 (10.4%) of 222 exposures were attributed to splash incidents (Table).

Anatomic Sites Affected—The anatomic location most frequently involved was the thumb (130/217 events [59.9%]), followed by the hand (39/217 events [18.0%]) and finger (22/217 events [10.1%]). The arm, face, and knee were affected with the lowest frequency, with only 1 event reported at each anatomic site (0.5%)(eTable). Five incidents were excluded from the analysis of anatomic location because of insufficient details of events.


Incident Tasks and Tools—Most BBP exposures occurred during suturing or assisting with suturing (64/210 events [30.5%]), followed by handling of sharps, wires, or instruments (40/210 events [19.0%]) and medication administration (37/210 events [17.6%])(eTable). Twelve incidents were excluded from the analysis of implicated tasks because of insufficient details of events.
The tools involved in exposure events with the greatest prevalence included the suture needle (76/201 events [37.8%]), injection syringe/needle (43/201 events [21.4%]), and shave biopsy razor (24/201 events [11.9%])(eTable). Twenty-one incidents were excluded from the analysis of implicated instruments because of insufficient details of events.
Providers Affected by BBP Exposures—Resident physicians experienced the greatest number of BBP exposures (105/222 events [47.3%]), followed by ancillary providers (84/222 events [37.8%]) and practicing dermatologists (33/222 events [14.9%]). All occupational groups experienced more BBP exposures through needlesticks/medical sharps compared with splash incidents (resident physicians, 88.6%; ancillary staff, 91.7%; practicing dermatologists, 87.9%; P=.725)(Table).
Among resident physicians, practicing dermatologists, and ancillary staff, the most frequent site of injury was the thumb. Suturing/assisting with suturing was the most common task leading to injury, and the suture needle was the most common instrument of injury for both resident physicians and practicing dermatologists. Handling of sharps, wires, or instruments was the most common task leading to injury for ancillary staff, and the injection syringe/needle was the most common instrument of injury in this cohort.
Resident physicians experienced the lowest rate of BBP exposures during administration of medications (12.7%; P=.003). Ancillary staff experienced the highest rate of BBP exposures with an injection needle (35.5%; P=.001). There were no statistically significant differences among occupational groups for the anatomic location of injury (P=.074)(eTable).
Comment
In the year 2000, the annual global incidence of occupational BBP exposures among health care workers worldwide for hepatitis B virus, hepatitis C virus, and HIV was estimated at 2.1 million, 926,000, and 327,000, respectively. Most of these exposures were due to sharps injuries.4 Dermatologists are particularly at risk for BBP exposures given their reliance on frequent procedures in practice. During an 11-year period, 222 BBP exposures were documented in the dermatology departments at 3 Mayo Clinic institutions. Most exposures were due to needlestick/sharps across all occupational groups compared with splash injuries. Prior survey studies confirm that sharps injuries are frequently implicated, with 75% to 94% of residents and practicing dermatologists reporting at least 1 sharps injury.1
Among occupational groups, resident physicians had the highest rate of BBP exposures, followed by nurse/medical assistants and practicing dermatologists, which may be secondary to lack of training or experience. Data from other surgical fields, including general surgery, support that resident physicians have the highest rate of sharps injuries.5 In a survey study (N=452), 51% of residents reported that extra training in safe techniques would be beneficial.2 Safety training may be beneficial in reducing the incidence of BBP exposures in residency programs.
The most common implicated task in resident physicians and practicing dermatologists was suturing or assisting with suturing, and the most common implicated instrument was the suture needle. Prior studies showed conflicting data regarding common implicated tasks and instruments in this cohort.1,2 The task of suturing and the suture needle also were the most implicated means of injury among other surgical specialties.6 Ancillary staff experienced most BBP exposures during handling of sharps, wires, or instruments, as well as the use of an injection needle. The designation of tasks among dermatologic staff likely explains the difference among occupational groups. This new information may provide the opportunity to improve safety measures among all members of the dermatologic team.
Limitations—There are several limitations to this study. This retrospective review was conducted at a single health system at 3 institutions. Hence, similar safety protocols likely were in place across all sites, which may reduce the generalizability of the results. In addition, there is risk of nonreporting bias among staff, as only documented incidence reports were evaluated. Prior studies demonstrated a nonreporting prevalence of 33% to 64% among dermatology staff.1-3 We also did not evaluate whether injuries resulted in BBP exposure or transmission. The rates of postexposure prophylaxis also were not studied. This information was not available for review because of concerns for privacy. Demographic features, such as gender or years of training, also were not evaluated.
Conclusion
This study provides additional insight on the incidence of BBP exposures in dermatology, as well as the implicated tasks, instruments, and anatomic locations of injury. Studies show that implementing formal education regarding the risks of BBP exposure may result in reduction of sharps injuries.7 Formal education in residency programs may be needed in the field of dermatology to reduce BBP exposures. Quality improvement measures should focus on identified risk factors among occupational groups to reduce BBP exposures in the workplace.
- Donnelly AF, Chang Y-HH, Nemeth-Ochoa SA. Sharps injuries and reporting practices of U.S. dermatologists [published online November 14, 2013]. Dermatol Surg. 2013;39:1813-1821.
- Goulart J, Oliveria S, Levitt J. Safety during dermatologic procedures and surgeries: a survey of resident injuries and prevention strategies. J Am Acad Dermatol. 2011;65:648-650.
- Ken K, Golda N. Contaminated sharps injuries: a survey among dermatology residents. J Am Acad Dermatol. 2019;80:1786-1788.
- Pruss-Ustun A, Rapiti E, Hutin Y. Estimation of global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med. 2005;48:482-490.
- Choi L, Torres R, Syed S, et al. Sharps and needlestick injuries among medical students, surgical residents, faculty, and operating room staff at a single academic institution. J Surg Educ. 2017;74:131-136.
- Bakaeen F, Awad S, Albo D, et al. Epidemiology of exposure to blood borne pathogens on a surgical service. Am J Surg. 2006;192:E18-E21.
- Li WJ, Zhang M, Shi CL, et al. Study on intervention of bloodborne pathogen exposure in a general hospital [in Chinese]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2017;35:34-41.
- Donnelly AF, Chang Y-HH, Nemeth-Ochoa SA. Sharps injuries and reporting practices of U.S. dermatologists [published online November 14, 2013]. Dermatol Surg. 2013;39:1813-1821.
- Goulart J, Oliveria S, Levitt J. Safety during dermatologic procedures and surgeries: a survey of resident injuries and prevention strategies. J Am Acad Dermatol. 2011;65:648-650.
- Ken K, Golda N. Contaminated sharps injuries: a survey among dermatology residents. J Am Acad Dermatol. 2019;80:1786-1788.
- Pruss-Ustun A, Rapiti E, Hutin Y. Estimation of global burden of disease attributable to contaminated sharps injuries among health-care workers. Am J Ind Med. 2005;48:482-490.
- Choi L, Torres R, Syed S, et al. Sharps and needlestick injuries among medical students, surgical residents, faculty, and operating room staff at a single academic institution. J Surg Educ. 2017;74:131-136.
- Bakaeen F, Awad S, Albo D, et al. Epidemiology of exposure to blood borne pathogens on a surgical service. Am J Surg. 2006;192:E18-E21.
- Li WJ, Zhang M, Shi CL, et al. Study on intervention of bloodborne pathogen exposure in a general hospital [in Chinese]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2017;35:34-41.
Practice Points
- Most blood-borne pathogen (BBP) exposures in dermatologic staff occur due to medical sharps as opposed to splash incidents.
- The most common implicated task in resident physicians and practicing dermatologists is suturing or assisting with suturing, and the most commonly associated instrument is the suture needle. In contrast, ancillary staff experience most BBP exposures during handling of sharps, wires, or instruments, and the injection syringe/needle is the most common instrument of injury.
- Quality improvement measures are needed in prevention of BBP exposures and should focus on identified risk factors among occupational groups in the workplace.
Camellia japonica
The various Camellia species originated in Eastern Asia and are believed to have been introduced in northwestern Spain in the 18th century. Camellia japonica, a flowering evergreen tree with various medical and cosmetic applications, is found throughout Galicia, Spain, where it is cultivated as an ornamental plant, and is native to Japan, South Korea, and China.1-4 The flowers and seeds of C. japonica have been used in traditional medicine and cosmetics in East Asia, with the oil of C. japonica used there to restore skin elasticity and to enhance skin health.4-6
While the use of C. sinensis in traditional and modern medicine is much better researched, understood, and characterized, C. japonica is now being considered for various health benefits. This column will focus on the bioactivity and scientific support for dermatologic applications of C. japonica. It is worth noting that a dry oil known as tsubaki oil, derived from C. japonica and rich in oleic acid, polyphenols, as well as vitamins A, C, D, and E, is used for skin and hair care in moisturizers produced primarily in Japan.
Antioxidant activity
In 2005, Lee and colleagues determined that C. japonica leaf and flower extracts display antioxidant, antifungal, and antibacterial activities (with the latter showing greater gram-positive than gram-negative activity).8 Investigating the antioxidant characteristics of the ethanol extract of the C. japonica flower in 2011, Piao and colleagues reported that the botanical exerted scavenging activity against reactive oxygen species in human HaCaT keratinocytes and enhanced protein expression and function of the antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxidase.9
Less than a decade later, Yoon and colleagues determined that C. japonica leaf extract contains high concentrations of vitamin E and rutin as well as other active constituents and that it exhibits antioxidant and antihyperuricemic activity in vitro and in vivo.4
Since then, Kim and colleagues have demonstrated, using cultured normal human dermal fibroblasts, that C. japonica flower extract effectively hindered urban air pollutants–induced reactive oxygen species synthesis. In ex vivo results, the investigators showed that the botanical agent suppressed matrix metalloproteinase (MMP)-1 expression, fostered collagen production, and decreased levels of pollutants-induced malondialdehyde. The authors concluded that C. japonica flower extract shows promise as a protective agent against pollutant-induced cutaneous damage.10
Anti-inflammatory and wound-healing activity
In 2012, Kim and colleagues found that C. japonica oil imparts anti-inflammatory activity via down-regulation of iNOS and COX-2 gene expression by suppressing of NF-KB and AP-1 signaling.6
Jeon and colleagues determined, in a 2018 investigation of 3,695 native plant extracts, that extracts from C. japonica fruit and stems improved induced pluripotent stem cell (iPSC) generation in mouse and human skin and enhanced wound healing in an in vivo mouse wound model. They suggested that their findings may point toward more effective approaches to developing clinical-grade iPSCs and wound-healing therapies.11
Cosmeceutical potential
Among the important bioactive ingredients present in C. japonica are phenolic compounds, terpenoids, and fatty acids, which are thought to account for the anti-inflammatory, antioxidant, antimicrobial, and anticancer activity associated with the plant.1 The high concentration of polyphenolic substances, in particular, is thought to at least partly account for the inclusion of C. japonica leaf extracts in antiaging cosmetics and cosmeceuticals.12 Specifically, some of the antioxidant substances found in C. japonica extracts include quercetin, quercetin-3-O-glucoside, quercitrin, and kaempferol.9
Wrinkle reduction and moisturization
In 2007, Jung and colleagues found that C. japonica oil activated collagen 1A2 promotion in human dermal fibroblast cells in a concentration-dependent fashion. The oil also suppressed MMP-1 functions and spurred the production of human type I procollagen. On human skin, C. japonica oil was tested on the upper back of 30 volunteers and failed to provoke any adverse reactions. The oil also diminished transepidermal water loss on the forearm. The researchers concluded that C. japonica oil merits consideration as an antiwrinkle ingredient in topical formulations.13
More recently, Choi and colleagues showed that ceramide nanoparticles developed through the use of natural oils derived from Korean traditional plants (including C. japonica, along with Panax ginseng, C. sinensis, Glycine max napjakong, and Glycine max seoritae) improve skin carrier functions and promote gene expressions needed for epidermal homeostasis. The expressions of the FLG, CASP14, and INV genes were notably enhanced by the tested formulation. The researchers observed from in vivo human studies that the application of the ceramide nanoparticles yielded more rapid recovery in impaired skin barriers than the control formulation. Amelioration of stratum corneum cohesion was also noted. The investigators concluded that this and other natural oil–derived ceramide nanoparticle formulations may represent the potential for developing better moisturizers for enhancing skin barrier function.14
Hair-growth promotion and skin-whitening activity
Early in 2021, Cho and colleagues demonstrated that C. japonica phytoplacenta extract spurred the up-regulation of the expression of hair growth–marker genes in human follicle dermal papilla cells in vitro. In clinical tests with 42 adult female volunteers, a solution with 0.5% C. japonica placenta extract raised moisture content of the scalp and reduced sebum levels, dead scalp keratin, and redness. The researchers concluded that C. japonica phytoplacenta extract displays promise as a scalp treatment and hair growth–promoting agent.2
Later that year, Ha and colleagues reported on their findings regarding the tyrosinase inhibitory activity of the essential oil of C. japonica seeds. They identified hexamethylcyclotrisiloxane (42.36%) and octamethylcyclotetrasiloxane (23.28%) as the main constituents of the oil, which demonstrated comparable inhibitory activity to arbutin (positive control) against mushroom tyrosinase. Melanogenesis was also significantly suppressed by C. japonica seed essential oil in B16F10 melanoma cells. The investigators concluded that the essential oil of C. japonica seeds exhibits robust antityrosinase activity and, therefore, warrants consideration as a skin-whitening agent.15
Conclusion
C. japonica is not as popular or well researched as another Camellia species, C. sinensis (the primary tea plant consumed globally and highly touted and appreciated for its multitude of health benefits), but it has its own history of traditional uses for medical and cosmetic purposes and is a subject of increasing research interest along with popular applications. Its antioxidant and anti-inflammatory properties are thought to be central in conferring the ability to protect the skin from aging. Its effects on the skin barrier help skin hydration. More research is necessary to elucidate the apparently widespread potential of this botanical agent that is already found in some over-the-counter products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Pereira AG et al. Food Chem X. 2022 Feb 17;13:100258.
2. Cho WK et al. FEBS Open Bio. 2021 Mar;11(3):633-51.
3. Chung MY et al. Evolution. 2003 Jan;57(1):62-73.
4. Yoon IS et al. Int J Mol Med. 2017 Jun;39(6):1613-20.
5. Lee HH et al. Evid Based Complement Alternat Med. 2016;2016:9679867.
6. Kim S et al. BMB Rep. 2012 Mar;45(3):177-82.
7. Majumder S et al. Bull Nat Res Cen. 2020 Dec;44(1):1-4.
8. Lee SY et al. Korean Journal of Medicinal Crop Science. 2005;13(3):93-100.
9. Piao MJ et al. Int J Mol Sci. 2011;12(4):2618-30.
10. Kim M et al. BMC Complement Altern Med. 2019 Jan 28;19(1):30.
11. Jeon H et al. J Clin Med. 2018 Nov 20;7(11):449.
12. Mizutani T, Masaki H. Exp Dermatol. 2014 Oct;23 Suppl 1:23-6.
13. Jung E et al. J Ethnopharmacol. 2007 May 30;112(1):127-31.
14. Choi HK et al. J Cosmet Dermatol. 2022 Oct;21(10):4931-41.
15. Ha SY et al. Evid Based Complement Alternat Med. 2021 Nov 16;2021:6328767.
The various Camellia species originated in Eastern Asia and are believed to have been introduced in northwestern Spain in the 18th century. Camellia japonica, a flowering evergreen tree with various medical and cosmetic applications, is found throughout Galicia, Spain, where it is cultivated as an ornamental plant, and is native to Japan, South Korea, and China.1-4 The flowers and seeds of C. japonica have been used in traditional medicine and cosmetics in East Asia, with the oil of C. japonica used there to restore skin elasticity and to enhance skin health.4-6
While the use of C. sinensis in traditional and modern medicine is much better researched, understood, and characterized, C. japonica is now being considered for various health benefits. This column will focus on the bioactivity and scientific support for dermatologic applications of C. japonica. It is worth noting that a dry oil known as tsubaki oil, derived from C. japonica and rich in oleic acid, polyphenols, as well as vitamins A, C, D, and E, is used for skin and hair care in moisturizers produced primarily in Japan.
Antioxidant activity
In 2005, Lee and colleagues determined that C. japonica leaf and flower extracts display antioxidant, antifungal, and antibacterial activities (with the latter showing greater gram-positive than gram-negative activity).8 Investigating the antioxidant characteristics of the ethanol extract of the C. japonica flower in 2011, Piao and colleagues reported that the botanical exerted scavenging activity against reactive oxygen species in human HaCaT keratinocytes and enhanced protein expression and function of the antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxidase.9
Less than a decade later, Yoon and colleagues determined that C. japonica leaf extract contains high concentrations of vitamin E and rutin as well as other active constituents and that it exhibits antioxidant and antihyperuricemic activity in vitro and in vivo.4
Since then, Kim and colleagues have demonstrated, using cultured normal human dermal fibroblasts, that C. japonica flower extract effectively hindered urban air pollutants–induced reactive oxygen species synthesis. In ex vivo results, the investigators showed that the botanical agent suppressed matrix metalloproteinase (MMP)-1 expression, fostered collagen production, and decreased levels of pollutants-induced malondialdehyde. The authors concluded that C. japonica flower extract shows promise as a protective agent against pollutant-induced cutaneous damage.10
Anti-inflammatory and wound-healing activity
In 2012, Kim and colleagues found that C. japonica oil imparts anti-inflammatory activity via down-regulation of iNOS and COX-2 gene expression by suppressing of NF-KB and AP-1 signaling.6
Jeon and colleagues determined, in a 2018 investigation of 3,695 native plant extracts, that extracts from C. japonica fruit and stems improved induced pluripotent stem cell (iPSC) generation in mouse and human skin and enhanced wound healing in an in vivo mouse wound model. They suggested that their findings may point toward more effective approaches to developing clinical-grade iPSCs and wound-healing therapies.11
Cosmeceutical potential
Among the important bioactive ingredients present in C. japonica are phenolic compounds, terpenoids, and fatty acids, which are thought to account for the anti-inflammatory, antioxidant, antimicrobial, and anticancer activity associated with the plant.1 The high concentration of polyphenolic substances, in particular, is thought to at least partly account for the inclusion of C. japonica leaf extracts in antiaging cosmetics and cosmeceuticals.12 Specifically, some of the antioxidant substances found in C. japonica extracts include quercetin, quercetin-3-O-glucoside, quercitrin, and kaempferol.9
Wrinkle reduction and moisturization
In 2007, Jung and colleagues found that C. japonica oil activated collagen 1A2 promotion in human dermal fibroblast cells in a concentration-dependent fashion. The oil also suppressed MMP-1 functions and spurred the production of human type I procollagen. On human skin, C. japonica oil was tested on the upper back of 30 volunteers and failed to provoke any adverse reactions. The oil also diminished transepidermal water loss on the forearm. The researchers concluded that C. japonica oil merits consideration as an antiwrinkle ingredient in topical formulations.13
More recently, Choi and colleagues showed that ceramide nanoparticles developed through the use of natural oils derived from Korean traditional plants (including C. japonica, along with Panax ginseng, C. sinensis, Glycine max napjakong, and Glycine max seoritae) improve skin carrier functions and promote gene expressions needed for epidermal homeostasis. The expressions of the FLG, CASP14, and INV genes were notably enhanced by the tested formulation. The researchers observed from in vivo human studies that the application of the ceramide nanoparticles yielded more rapid recovery in impaired skin barriers than the control formulation. Amelioration of stratum corneum cohesion was also noted. The investigators concluded that this and other natural oil–derived ceramide nanoparticle formulations may represent the potential for developing better moisturizers for enhancing skin barrier function.14
Hair-growth promotion and skin-whitening activity
Early in 2021, Cho and colleagues demonstrated that C. japonica phytoplacenta extract spurred the up-regulation of the expression of hair growth–marker genes in human follicle dermal papilla cells in vitro. In clinical tests with 42 adult female volunteers, a solution with 0.5% C. japonica placenta extract raised moisture content of the scalp and reduced sebum levels, dead scalp keratin, and redness. The researchers concluded that C. japonica phytoplacenta extract displays promise as a scalp treatment and hair growth–promoting agent.2
Later that year, Ha and colleagues reported on their findings regarding the tyrosinase inhibitory activity of the essential oil of C. japonica seeds. They identified hexamethylcyclotrisiloxane (42.36%) and octamethylcyclotetrasiloxane (23.28%) as the main constituents of the oil, which demonstrated comparable inhibitory activity to arbutin (positive control) against mushroom tyrosinase. Melanogenesis was also significantly suppressed by C. japonica seed essential oil in B16F10 melanoma cells. The investigators concluded that the essential oil of C. japonica seeds exhibits robust antityrosinase activity and, therefore, warrants consideration as a skin-whitening agent.15
Conclusion
C. japonica is not as popular or well researched as another Camellia species, C. sinensis (the primary tea plant consumed globally and highly touted and appreciated for its multitude of health benefits), but it has its own history of traditional uses for medical and cosmetic purposes and is a subject of increasing research interest along with popular applications. Its antioxidant and anti-inflammatory properties are thought to be central in conferring the ability to protect the skin from aging. Its effects on the skin barrier help skin hydration. More research is necessary to elucidate the apparently widespread potential of this botanical agent that is already found in some over-the-counter products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Pereira AG et al. Food Chem X. 2022 Feb 17;13:100258.
2. Cho WK et al. FEBS Open Bio. 2021 Mar;11(3):633-51.
3. Chung MY et al. Evolution. 2003 Jan;57(1):62-73.
4. Yoon IS et al. Int J Mol Med. 2017 Jun;39(6):1613-20.
5. Lee HH et al. Evid Based Complement Alternat Med. 2016;2016:9679867.
6. Kim S et al. BMB Rep. 2012 Mar;45(3):177-82.
7. Majumder S et al. Bull Nat Res Cen. 2020 Dec;44(1):1-4.
8. Lee SY et al. Korean Journal of Medicinal Crop Science. 2005;13(3):93-100.
9. Piao MJ et al. Int J Mol Sci. 2011;12(4):2618-30.
10. Kim M et al. BMC Complement Altern Med. 2019 Jan 28;19(1):30.
11. Jeon H et al. J Clin Med. 2018 Nov 20;7(11):449.
12. Mizutani T, Masaki H. Exp Dermatol. 2014 Oct;23 Suppl 1:23-6.
13. Jung E et al. J Ethnopharmacol. 2007 May 30;112(1):127-31.
14. Choi HK et al. J Cosmet Dermatol. 2022 Oct;21(10):4931-41.
15. Ha SY et al. Evid Based Complement Alternat Med. 2021 Nov 16;2021:6328767.
The various Camellia species originated in Eastern Asia and are believed to have been introduced in northwestern Spain in the 18th century. Camellia japonica, a flowering evergreen tree with various medical and cosmetic applications, is found throughout Galicia, Spain, where it is cultivated as an ornamental plant, and is native to Japan, South Korea, and China.1-4 The flowers and seeds of C. japonica have been used in traditional medicine and cosmetics in East Asia, with the oil of C. japonica used there to restore skin elasticity and to enhance skin health.4-6
While the use of C. sinensis in traditional and modern medicine is much better researched, understood, and characterized, C. japonica is now being considered for various health benefits. This column will focus on the bioactivity and scientific support for dermatologic applications of C. japonica. It is worth noting that a dry oil known as tsubaki oil, derived from C. japonica and rich in oleic acid, polyphenols, as well as vitamins A, C, D, and E, is used for skin and hair care in moisturizers produced primarily in Japan.
Antioxidant activity
In 2005, Lee and colleagues determined that C. japonica leaf and flower extracts display antioxidant, antifungal, and antibacterial activities (with the latter showing greater gram-positive than gram-negative activity).8 Investigating the antioxidant characteristics of the ethanol extract of the C. japonica flower in 2011, Piao and colleagues reported that the botanical exerted scavenging activity against reactive oxygen species in human HaCaT keratinocytes and enhanced protein expression and function of the antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxidase.9
Less than a decade later, Yoon and colleagues determined that C. japonica leaf extract contains high concentrations of vitamin E and rutin as well as other active constituents and that it exhibits antioxidant and antihyperuricemic activity in vitro and in vivo.4
Since then, Kim and colleagues have demonstrated, using cultured normal human dermal fibroblasts, that C. japonica flower extract effectively hindered urban air pollutants–induced reactive oxygen species synthesis. In ex vivo results, the investigators showed that the botanical agent suppressed matrix metalloproteinase (MMP)-1 expression, fostered collagen production, and decreased levels of pollutants-induced malondialdehyde. The authors concluded that C. japonica flower extract shows promise as a protective agent against pollutant-induced cutaneous damage.10
Anti-inflammatory and wound-healing activity
In 2012, Kim and colleagues found that C. japonica oil imparts anti-inflammatory activity via down-regulation of iNOS and COX-2 gene expression by suppressing of NF-KB and AP-1 signaling.6
Jeon and colleagues determined, in a 2018 investigation of 3,695 native plant extracts, that extracts from C. japonica fruit and stems improved induced pluripotent stem cell (iPSC) generation in mouse and human skin and enhanced wound healing in an in vivo mouse wound model. They suggested that their findings may point toward more effective approaches to developing clinical-grade iPSCs and wound-healing therapies.11
Cosmeceutical potential
Among the important bioactive ingredients present in C. japonica are phenolic compounds, terpenoids, and fatty acids, which are thought to account for the anti-inflammatory, antioxidant, antimicrobial, and anticancer activity associated with the plant.1 The high concentration of polyphenolic substances, in particular, is thought to at least partly account for the inclusion of C. japonica leaf extracts in antiaging cosmetics and cosmeceuticals.12 Specifically, some of the antioxidant substances found in C. japonica extracts include quercetin, quercetin-3-O-glucoside, quercitrin, and kaempferol.9
Wrinkle reduction and moisturization
In 2007, Jung and colleagues found that C. japonica oil activated collagen 1A2 promotion in human dermal fibroblast cells in a concentration-dependent fashion. The oil also suppressed MMP-1 functions and spurred the production of human type I procollagen. On human skin, C. japonica oil was tested on the upper back of 30 volunteers and failed to provoke any adverse reactions. The oil also diminished transepidermal water loss on the forearm. The researchers concluded that C. japonica oil merits consideration as an antiwrinkle ingredient in topical formulations.13
More recently, Choi and colleagues showed that ceramide nanoparticles developed through the use of natural oils derived from Korean traditional plants (including C. japonica, along with Panax ginseng, C. sinensis, Glycine max napjakong, and Glycine max seoritae) improve skin carrier functions and promote gene expressions needed for epidermal homeostasis. The expressions of the FLG, CASP14, and INV genes were notably enhanced by the tested formulation. The researchers observed from in vivo human studies that the application of the ceramide nanoparticles yielded more rapid recovery in impaired skin barriers than the control formulation. Amelioration of stratum corneum cohesion was also noted. The investigators concluded that this and other natural oil–derived ceramide nanoparticle formulations may represent the potential for developing better moisturizers for enhancing skin barrier function.14
Hair-growth promotion and skin-whitening activity
Early in 2021, Cho and colleagues demonstrated that C. japonica phytoplacenta extract spurred the up-regulation of the expression of hair growth–marker genes in human follicle dermal papilla cells in vitro. In clinical tests with 42 adult female volunteers, a solution with 0.5% C. japonica placenta extract raised moisture content of the scalp and reduced sebum levels, dead scalp keratin, and redness. The researchers concluded that C. japonica phytoplacenta extract displays promise as a scalp treatment and hair growth–promoting agent.2
Later that year, Ha and colleagues reported on their findings regarding the tyrosinase inhibitory activity of the essential oil of C. japonica seeds. They identified hexamethylcyclotrisiloxane (42.36%) and octamethylcyclotetrasiloxane (23.28%) as the main constituents of the oil, which demonstrated comparable inhibitory activity to arbutin (positive control) against mushroom tyrosinase. Melanogenesis was also significantly suppressed by C. japonica seed essential oil in B16F10 melanoma cells. The investigators concluded that the essential oil of C. japonica seeds exhibits robust antityrosinase activity and, therefore, warrants consideration as a skin-whitening agent.15
Conclusion
C. japonica is not as popular or well researched as another Camellia species, C. sinensis (the primary tea plant consumed globally and highly touted and appreciated for its multitude of health benefits), but it has its own history of traditional uses for medical and cosmetic purposes and is a subject of increasing research interest along with popular applications. Its antioxidant and anti-inflammatory properties are thought to be central in conferring the ability to protect the skin from aging. Its effects on the skin barrier help skin hydration. More research is necessary to elucidate the apparently widespread potential of this botanical agent that is already found in some over-the-counter products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Pereira AG et al. Food Chem X. 2022 Feb 17;13:100258.
2. Cho WK et al. FEBS Open Bio. 2021 Mar;11(3):633-51.
3. Chung MY et al. Evolution. 2003 Jan;57(1):62-73.
4. Yoon IS et al. Int J Mol Med. 2017 Jun;39(6):1613-20.
5. Lee HH et al. Evid Based Complement Alternat Med. 2016;2016:9679867.
6. Kim S et al. BMB Rep. 2012 Mar;45(3):177-82.
7. Majumder S et al. Bull Nat Res Cen. 2020 Dec;44(1):1-4.
8. Lee SY et al. Korean Journal of Medicinal Crop Science. 2005;13(3):93-100.
9. Piao MJ et al. Int J Mol Sci. 2011;12(4):2618-30.
10. Kim M et al. BMC Complement Altern Med. 2019 Jan 28;19(1):30.
11. Jeon H et al. J Clin Med. 2018 Nov 20;7(11):449.
12. Mizutani T, Masaki H. Exp Dermatol. 2014 Oct;23 Suppl 1:23-6.
13. Jung E et al. J Ethnopharmacol. 2007 May 30;112(1):127-31.
14. Choi HK et al. J Cosmet Dermatol. 2022 Oct;21(10):4931-41.
15. Ha SY et al. Evid Based Complement Alternat Med. 2021 Nov 16;2021:6328767.
Topical gene therapy heals dystrophic epidermolysis bullosa wounds
.
In a phase 3 study of patients with DEB, “we found that repeated topical application of B-VEC [beremagene geperpavec], an HSV-1–based gene therapy, resulted in a greater likelihood of complete wound healing than the topical application of placebo at up to 6 months,” the authors wrote. The study was published in The New England Journal of Medicine. “Longer and larger trials are warranted to determine the durability of effect and risks of this approach,” the authors noted.
“The results prove that B-VEC, the first topical in vivo gene therapy to reach late-stage development, can heal DEB,” senior author M. Peter Marinkovich, MD, associate professor of dermatology at Stanford University, Redwood City, Calif., said in an interview.
“In the past, DEB was a very specialized disease that only a handful of dermatologists would see but could not do much to treat,” he said. “With gene therapy, many more dermatologists who may not be familiar with DEB will be able to treat these patients in their offices.” It is expected that nurses will be able to administer the treatment to patients at home, he added.
Rare, life-threatening, genetic blistering disease
DEB, a rare disease that affects one to three persons per million in the United States, is caused by mutations in the COL7A1 gene that encodes the alpha-1 chain of collagen type VII (C7) protein. C7 forms the anchoring fibrils that attach the epidermis to the underlying dermal connective tissue.
COL71A mutations that lead to defective, decreased, or absent C7 can make the skin so fragile it tears with the slightest touch. This has led to patients being called “butterfly children.” Epithelial tissues blister and scar, causing esophageal and genitourinary strictures, adhesion of digits, malnutrition, anemia, infection, and bothersome itch and pain. Morbidity and mortality are high. The leading cause of death in adults is chronic wounds leading to aggressive squamous cell cancers.
The first therapy for DEB, under FDA review
B-VEC restores C7 protein by using an engineered replication-defective herpes simplex virus type 1 (HSV-1) vector to deliver the COL7A1 gene directly to skin cells to restore functional C7 protein fibrils that stabilize the skin structure.
On the basis of manufacturing information submitted to the FDA in December 2022, the agency extended the date for a decision on approval by 3 months, to May 19, 2023, according to a statement from Krystal Biotech, the developer of B-VEC and the sponsor of the NEJM study.
Dr. Marinkovich and his colleagues conducted the double-blind, randomized, controlled GEM-3 trial of B-VEC at three sites in the United States. The 31 study participants ranged in age from 1 to 44 years (median age, 16 years) and had genetically confirmed DEB (30 with the recessive form and 1 with the dominant form).
For each participant, a pair of wounds was chosen that were matched in size, region, and appearance. The wounds within each pair were randomly allocated to receive weekly applications of either B-VEC or placebo gel for 26 weeks.
The results of the study included the following:
- Complete healing at 6 months occurred in 67% of the wounds treated with B-VEC (including a wound in the patient with dominant DEB), vs. 22% of those who received placebo (95% confidence interval [CI], 24-68; P = .002).
- Complete healing at 3 months occurred in 71% of the wounds treated with B-VEC, vs. 20% of those who received placebo (95% CI, 29-73; P < .001).
- The mean change from baseline to week 22 in pain severity during wound-dressing changes for patients aged 6 years and older, as determined on the basis of a visual analogue scale, was –0.88 with B-VEC, vs. –0.71 with placebo (adjusted least-squares mean difference, –0.61; 95% CI, –1.10 to –0.13); similar mean changes were seen at weeks 24 and 26.
- Among all patients, 58% had at least one adverse event. Most adverse events were mild or moderate. The most common were pruritus, chills, and squamous cell carcinoma (SCC), which were reported in three patients each (SCC cases occurred at wound sites that had not been exposed to B-VEC or placebo). Serious adverse events, which were unrelated to the treatment, occurred in three patients: diarrhea, anemia, cellulitis, and a positive blood culture related to a hemodialysis catheter.
“With the ability to treat patients with topical gene therapy, dermatology is entering a new age of treatment possibilities,” Dr. Marinkovich said in the interview.
The researchers were surprised that the redosable in vivo gene therapy worked so well, he added. In vivo gene therapy has been plagued by the occurrence of immune reactions against the viral vectors used, Dr. Marinkovich explained. But because the herpes simplex virus has evolved to evade the immune system, his team could use the viral vector every week for 6 months without inflammatory reactions.
“The immune system’s inability to fight off or get rid of the herpes simplex vector makes it bad as a disease, but as a gene therapy vector, it provides a huge advantage,” he added.
Asked to comment on the results, Christen Ebens, MD, MPH, assistant professor in the department of pediatrics at the University of Minnesota, Minneapolis, whose clinical and research interests include EB, called the results exciting for patients, families, and doctors.
“Side effects were minimal, and importantly, use of the replication-incompetent HSV vector means that the payload gene does not integrate into the patient’s DNA,” Dr. Ebens, who was not involved in the study, said in an interview. “B-VEC is not a lifelong cure but potentially an effective maintenance therapy requiring repeated doses,” she added.
Although the researchers found no clinically important immune reactions to B-VEC, Dr. Ebens said she would like to see results from longer studies of the treatment. “We will want to see that patients do not produce neutralizing antibodies against B-VEC or its components, as such antibodies may yield the treatment ineffective or cause significant side effects.”
In an interview, Vanessa R. Holland, MD, associate clinical professor in the division of dermatology at UCLA Health, Burbank, Calif., who was not involved in the study, said that “topical replication-defective HSV-1 is a brilliant vector to deliver the depleted collagen.” She added that “such a vehicle may significantly alter management of these disorders and improve or extend lives by minimizing potentially fatal complications.”
Paras P. Vakharia, MD, PharmD, assistant professor of dermatology at Northwestern University, Chicago, who was not involved in the study, was surprised by the high percentage of healed wounds and wounds that remained healed over time.
In an interview, Dr. Vakharia said that he’d like to know whether patients develop antibodies against HSV and C7 with long-term treatment and whether problems will arise related to drug availability.
B-VEC for treating other conditions
Dr. Marinkovich noted that an ongoing phase 1 clinical trial, also sponsored by Krystal Biotech, is using the HSV-1 vector to deliver a different biologic (KB105) to establish dose and safety in the treatment of ichthyosis. He added that he would like to explore the use of B-VEC to treat DEB at mucosal surfaces, including inside the mouth, the eye, and the esophagus.
Authors of two editorials that accompanied the study also referred to other conditions B-VEC might treat.
This study “highlights potential future investigations,” David V. Schaffer, PhD, professor of chemical and biomolecular engineering, bioengineering, and molecular and cell biology at the University of California, Berkeley, wrotes in one of the editorials.
Important considerations he mentioned include the likelihood of the treatment becoming lifelong; the inability of HSV to penetrate intact skin, making B-VEC unsuitable for preventing the development of new wounds; and the inability of this treatment to treat EB lesions along the digestive tract. “This important trial builds on and extends gene-therapy successes to new targets and vectors, an advance for patients,” he added.
In the second editorial, Aimee S. Payne, MD, PhD, professor of dermatology at the University of Pennsylvania, Philadelphia, raised the question of whether B-VEC’s clinical success for treating DEB can translate to other genetic diseases.
“Formulations for ophthalmic, oral-gastrointestinal, or respiratory delivery would be of great value to address the extracutaneous manifestations of epidermolysis bullosa and other genetic diseases,” she wrote.
Referring to an ongoing trial of a topical gene therapy for cystic fibrosis that is delivered by a nebulizer, Dr. Payne noted, “Ultimately, the completion of clinical trials such as this one will be required to determine whether HSV-1–mediated gene delivery can go more than skin deep.”
Earlier data and more details of the study were presented in a poster at the annual meeting of the Society for Pediatric Dermatology in July 2022.
Dr. Marinkovich has disclosed no relevant financial relationships. Several coauthors are employees of or have other financial relationships with Krystal Biotech, the study’s sponsor and the developer of beremagene geperpavec. Dr. Schaffer and Dr. Payne have financial relationships with the pharmaceutical industry. Dr. Ebens, Dr. Holland, and Dr. Vakharia have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
.
In a phase 3 study of patients with DEB, “we found that repeated topical application of B-VEC [beremagene geperpavec], an HSV-1–based gene therapy, resulted in a greater likelihood of complete wound healing than the topical application of placebo at up to 6 months,” the authors wrote. The study was published in The New England Journal of Medicine. “Longer and larger trials are warranted to determine the durability of effect and risks of this approach,” the authors noted.
“The results prove that B-VEC, the first topical in vivo gene therapy to reach late-stage development, can heal DEB,” senior author M. Peter Marinkovich, MD, associate professor of dermatology at Stanford University, Redwood City, Calif., said in an interview.
“In the past, DEB was a very specialized disease that only a handful of dermatologists would see but could not do much to treat,” he said. “With gene therapy, many more dermatologists who may not be familiar with DEB will be able to treat these patients in their offices.” It is expected that nurses will be able to administer the treatment to patients at home, he added.
Rare, life-threatening, genetic blistering disease
DEB, a rare disease that affects one to three persons per million in the United States, is caused by mutations in the COL7A1 gene that encodes the alpha-1 chain of collagen type VII (C7) protein. C7 forms the anchoring fibrils that attach the epidermis to the underlying dermal connective tissue.
COL71A mutations that lead to defective, decreased, or absent C7 can make the skin so fragile it tears with the slightest touch. This has led to patients being called “butterfly children.” Epithelial tissues blister and scar, causing esophageal and genitourinary strictures, adhesion of digits, malnutrition, anemia, infection, and bothersome itch and pain. Morbidity and mortality are high. The leading cause of death in adults is chronic wounds leading to aggressive squamous cell cancers.
The first therapy for DEB, under FDA review
B-VEC restores C7 protein by using an engineered replication-defective herpes simplex virus type 1 (HSV-1) vector to deliver the COL7A1 gene directly to skin cells to restore functional C7 protein fibrils that stabilize the skin structure.
On the basis of manufacturing information submitted to the FDA in December 2022, the agency extended the date for a decision on approval by 3 months, to May 19, 2023, according to a statement from Krystal Biotech, the developer of B-VEC and the sponsor of the NEJM study.
Dr. Marinkovich and his colleagues conducted the double-blind, randomized, controlled GEM-3 trial of B-VEC at three sites in the United States. The 31 study participants ranged in age from 1 to 44 years (median age, 16 years) and had genetically confirmed DEB (30 with the recessive form and 1 with the dominant form).
For each participant, a pair of wounds was chosen that were matched in size, region, and appearance. The wounds within each pair were randomly allocated to receive weekly applications of either B-VEC or placebo gel for 26 weeks.
The results of the study included the following:
- Complete healing at 6 months occurred in 67% of the wounds treated with B-VEC (including a wound in the patient with dominant DEB), vs. 22% of those who received placebo (95% confidence interval [CI], 24-68; P = .002).
- Complete healing at 3 months occurred in 71% of the wounds treated with B-VEC, vs. 20% of those who received placebo (95% CI, 29-73; P < .001).
- The mean change from baseline to week 22 in pain severity during wound-dressing changes for patients aged 6 years and older, as determined on the basis of a visual analogue scale, was –0.88 with B-VEC, vs. –0.71 with placebo (adjusted least-squares mean difference, –0.61; 95% CI, –1.10 to –0.13); similar mean changes were seen at weeks 24 and 26.
- Among all patients, 58% had at least one adverse event. Most adverse events were mild or moderate. The most common were pruritus, chills, and squamous cell carcinoma (SCC), which were reported in three patients each (SCC cases occurred at wound sites that had not been exposed to B-VEC or placebo). Serious adverse events, which were unrelated to the treatment, occurred in three patients: diarrhea, anemia, cellulitis, and a positive blood culture related to a hemodialysis catheter.
“With the ability to treat patients with topical gene therapy, dermatology is entering a new age of treatment possibilities,” Dr. Marinkovich said in the interview.
The researchers were surprised that the redosable in vivo gene therapy worked so well, he added. In vivo gene therapy has been plagued by the occurrence of immune reactions against the viral vectors used, Dr. Marinkovich explained. But because the herpes simplex virus has evolved to evade the immune system, his team could use the viral vector every week for 6 months without inflammatory reactions.
“The immune system’s inability to fight off or get rid of the herpes simplex vector makes it bad as a disease, but as a gene therapy vector, it provides a huge advantage,” he added.
Asked to comment on the results, Christen Ebens, MD, MPH, assistant professor in the department of pediatrics at the University of Minnesota, Minneapolis, whose clinical and research interests include EB, called the results exciting for patients, families, and doctors.
“Side effects were minimal, and importantly, use of the replication-incompetent HSV vector means that the payload gene does not integrate into the patient’s DNA,” Dr. Ebens, who was not involved in the study, said in an interview. “B-VEC is not a lifelong cure but potentially an effective maintenance therapy requiring repeated doses,” she added.
Although the researchers found no clinically important immune reactions to B-VEC, Dr. Ebens said she would like to see results from longer studies of the treatment. “We will want to see that patients do not produce neutralizing antibodies against B-VEC or its components, as such antibodies may yield the treatment ineffective or cause significant side effects.”
In an interview, Vanessa R. Holland, MD, associate clinical professor in the division of dermatology at UCLA Health, Burbank, Calif., who was not involved in the study, said that “topical replication-defective HSV-1 is a brilliant vector to deliver the depleted collagen.” She added that “such a vehicle may significantly alter management of these disorders and improve or extend lives by minimizing potentially fatal complications.”
Paras P. Vakharia, MD, PharmD, assistant professor of dermatology at Northwestern University, Chicago, who was not involved in the study, was surprised by the high percentage of healed wounds and wounds that remained healed over time.
In an interview, Dr. Vakharia said that he’d like to know whether patients develop antibodies against HSV and C7 with long-term treatment and whether problems will arise related to drug availability.
B-VEC for treating other conditions
Dr. Marinkovich noted that an ongoing phase 1 clinical trial, also sponsored by Krystal Biotech, is using the HSV-1 vector to deliver a different biologic (KB105) to establish dose and safety in the treatment of ichthyosis. He added that he would like to explore the use of B-VEC to treat DEB at mucosal surfaces, including inside the mouth, the eye, and the esophagus.
Authors of two editorials that accompanied the study also referred to other conditions B-VEC might treat.
This study “highlights potential future investigations,” David V. Schaffer, PhD, professor of chemical and biomolecular engineering, bioengineering, and molecular and cell biology at the University of California, Berkeley, wrotes in one of the editorials.
Important considerations he mentioned include the likelihood of the treatment becoming lifelong; the inability of HSV to penetrate intact skin, making B-VEC unsuitable for preventing the development of new wounds; and the inability of this treatment to treat EB lesions along the digestive tract. “This important trial builds on and extends gene-therapy successes to new targets and vectors, an advance for patients,” he added.
In the second editorial, Aimee S. Payne, MD, PhD, professor of dermatology at the University of Pennsylvania, Philadelphia, raised the question of whether B-VEC’s clinical success for treating DEB can translate to other genetic diseases.
“Formulations for ophthalmic, oral-gastrointestinal, or respiratory delivery would be of great value to address the extracutaneous manifestations of epidermolysis bullosa and other genetic diseases,” she wrote.
Referring to an ongoing trial of a topical gene therapy for cystic fibrosis that is delivered by a nebulizer, Dr. Payne noted, “Ultimately, the completion of clinical trials such as this one will be required to determine whether HSV-1–mediated gene delivery can go more than skin deep.”
Earlier data and more details of the study were presented in a poster at the annual meeting of the Society for Pediatric Dermatology in July 2022.
Dr. Marinkovich has disclosed no relevant financial relationships. Several coauthors are employees of or have other financial relationships with Krystal Biotech, the study’s sponsor and the developer of beremagene geperpavec. Dr. Schaffer and Dr. Payne have financial relationships with the pharmaceutical industry. Dr. Ebens, Dr. Holland, and Dr. Vakharia have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
.
In a phase 3 study of patients with DEB, “we found that repeated topical application of B-VEC [beremagene geperpavec], an HSV-1–based gene therapy, resulted in a greater likelihood of complete wound healing than the topical application of placebo at up to 6 months,” the authors wrote. The study was published in The New England Journal of Medicine. “Longer and larger trials are warranted to determine the durability of effect and risks of this approach,” the authors noted.
“The results prove that B-VEC, the first topical in vivo gene therapy to reach late-stage development, can heal DEB,” senior author M. Peter Marinkovich, MD, associate professor of dermatology at Stanford University, Redwood City, Calif., said in an interview.
“In the past, DEB was a very specialized disease that only a handful of dermatologists would see but could not do much to treat,” he said. “With gene therapy, many more dermatologists who may not be familiar with DEB will be able to treat these patients in their offices.” It is expected that nurses will be able to administer the treatment to patients at home, he added.
Rare, life-threatening, genetic blistering disease
DEB, a rare disease that affects one to three persons per million in the United States, is caused by mutations in the COL7A1 gene that encodes the alpha-1 chain of collagen type VII (C7) protein. C7 forms the anchoring fibrils that attach the epidermis to the underlying dermal connective tissue.
COL71A mutations that lead to defective, decreased, or absent C7 can make the skin so fragile it tears with the slightest touch. This has led to patients being called “butterfly children.” Epithelial tissues blister and scar, causing esophageal and genitourinary strictures, adhesion of digits, malnutrition, anemia, infection, and bothersome itch and pain. Morbidity and mortality are high. The leading cause of death in adults is chronic wounds leading to aggressive squamous cell cancers.
The first therapy for DEB, under FDA review
B-VEC restores C7 protein by using an engineered replication-defective herpes simplex virus type 1 (HSV-1) vector to deliver the COL7A1 gene directly to skin cells to restore functional C7 protein fibrils that stabilize the skin structure.
On the basis of manufacturing information submitted to the FDA in December 2022, the agency extended the date for a decision on approval by 3 months, to May 19, 2023, according to a statement from Krystal Biotech, the developer of B-VEC and the sponsor of the NEJM study.
Dr. Marinkovich and his colleagues conducted the double-blind, randomized, controlled GEM-3 trial of B-VEC at three sites in the United States. The 31 study participants ranged in age from 1 to 44 years (median age, 16 years) and had genetically confirmed DEB (30 with the recessive form and 1 with the dominant form).
For each participant, a pair of wounds was chosen that were matched in size, region, and appearance. The wounds within each pair were randomly allocated to receive weekly applications of either B-VEC or placebo gel for 26 weeks.
The results of the study included the following:
- Complete healing at 6 months occurred in 67% of the wounds treated with B-VEC (including a wound in the patient with dominant DEB), vs. 22% of those who received placebo (95% confidence interval [CI], 24-68; P = .002).
- Complete healing at 3 months occurred in 71% of the wounds treated with B-VEC, vs. 20% of those who received placebo (95% CI, 29-73; P < .001).
- The mean change from baseline to week 22 in pain severity during wound-dressing changes for patients aged 6 years and older, as determined on the basis of a visual analogue scale, was –0.88 with B-VEC, vs. –0.71 with placebo (adjusted least-squares mean difference, –0.61; 95% CI, –1.10 to –0.13); similar mean changes were seen at weeks 24 and 26.
- Among all patients, 58% had at least one adverse event. Most adverse events were mild or moderate. The most common were pruritus, chills, and squamous cell carcinoma (SCC), which were reported in three patients each (SCC cases occurred at wound sites that had not been exposed to B-VEC or placebo). Serious adverse events, which were unrelated to the treatment, occurred in three patients: diarrhea, anemia, cellulitis, and a positive blood culture related to a hemodialysis catheter.
“With the ability to treat patients with topical gene therapy, dermatology is entering a new age of treatment possibilities,” Dr. Marinkovich said in the interview.
The researchers were surprised that the redosable in vivo gene therapy worked so well, he added. In vivo gene therapy has been plagued by the occurrence of immune reactions against the viral vectors used, Dr. Marinkovich explained. But because the herpes simplex virus has evolved to evade the immune system, his team could use the viral vector every week for 6 months without inflammatory reactions.
“The immune system’s inability to fight off or get rid of the herpes simplex vector makes it bad as a disease, but as a gene therapy vector, it provides a huge advantage,” he added.
Asked to comment on the results, Christen Ebens, MD, MPH, assistant professor in the department of pediatrics at the University of Minnesota, Minneapolis, whose clinical and research interests include EB, called the results exciting for patients, families, and doctors.
“Side effects were minimal, and importantly, use of the replication-incompetent HSV vector means that the payload gene does not integrate into the patient’s DNA,” Dr. Ebens, who was not involved in the study, said in an interview. “B-VEC is not a lifelong cure but potentially an effective maintenance therapy requiring repeated doses,” she added.
Although the researchers found no clinically important immune reactions to B-VEC, Dr. Ebens said she would like to see results from longer studies of the treatment. “We will want to see that patients do not produce neutralizing antibodies against B-VEC or its components, as such antibodies may yield the treatment ineffective or cause significant side effects.”
In an interview, Vanessa R. Holland, MD, associate clinical professor in the division of dermatology at UCLA Health, Burbank, Calif., who was not involved in the study, said that “topical replication-defective HSV-1 is a brilliant vector to deliver the depleted collagen.” She added that “such a vehicle may significantly alter management of these disorders and improve or extend lives by minimizing potentially fatal complications.”
Paras P. Vakharia, MD, PharmD, assistant professor of dermatology at Northwestern University, Chicago, who was not involved in the study, was surprised by the high percentage of healed wounds and wounds that remained healed over time.
In an interview, Dr. Vakharia said that he’d like to know whether patients develop antibodies against HSV and C7 with long-term treatment and whether problems will arise related to drug availability.
B-VEC for treating other conditions
Dr. Marinkovich noted that an ongoing phase 1 clinical trial, also sponsored by Krystal Biotech, is using the HSV-1 vector to deliver a different biologic (KB105) to establish dose and safety in the treatment of ichthyosis. He added that he would like to explore the use of B-VEC to treat DEB at mucosal surfaces, including inside the mouth, the eye, and the esophagus.
Authors of two editorials that accompanied the study also referred to other conditions B-VEC might treat.
This study “highlights potential future investigations,” David V. Schaffer, PhD, professor of chemical and biomolecular engineering, bioengineering, and molecular and cell biology at the University of California, Berkeley, wrotes in one of the editorials.
Important considerations he mentioned include the likelihood of the treatment becoming lifelong; the inability of HSV to penetrate intact skin, making B-VEC unsuitable for preventing the development of new wounds; and the inability of this treatment to treat EB lesions along the digestive tract. “This important trial builds on and extends gene-therapy successes to new targets and vectors, an advance for patients,” he added.
In the second editorial, Aimee S. Payne, MD, PhD, professor of dermatology at the University of Pennsylvania, Philadelphia, raised the question of whether B-VEC’s clinical success for treating DEB can translate to other genetic diseases.
“Formulations for ophthalmic, oral-gastrointestinal, or respiratory delivery would be of great value to address the extracutaneous manifestations of epidermolysis bullosa and other genetic diseases,” she wrote.
Referring to an ongoing trial of a topical gene therapy for cystic fibrosis that is delivered by a nebulizer, Dr. Payne noted, “Ultimately, the completion of clinical trials such as this one will be required to determine whether HSV-1–mediated gene delivery can go more than skin deep.”
Earlier data and more details of the study were presented in a poster at the annual meeting of the Society for Pediatric Dermatology in July 2022.
Dr. Marinkovich has disclosed no relevant financial relationships. Several coauthors are employees of or have other financial relationships with Krystal Biotech, the study’s sponsor and the developer of beremagene geperpavec. Dr. Schaffer and Dr. Payne have financial relationships with the pharmaceutical industry. Dr. Ebens, Dr. Holland, and Dr. Vakharia have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Bone Wax as a Physical Hemostatic Agent
Practice Gap
Hemostasis after cutaneous surgery typically can be aided by mechanical occlusion with petrolatum and gauze known as a pressure bandage. However, in certain scenarios such as bone bleeding or irregularly shaped areas (eg, conchal bowl), difficulty applying a pressure bandage necessitates alternative hemostatic measures.1 In those instances, physical hemostatic agents, such as gelatin, oxidized cellulose, microporous polysaccharide spheres, hydrophilic polymers with potassium salts, microfibrillar collagen, and chitin, also can be used.2 However, those agents are expensive and often adhere to wound edges, inducing repeat trauma with removal. To avoid such concerns, we propose the use of bone wax as an effective hemostatic technique.
The Technique
When secondary intention healing is chosen or a temporary bandage needs to be placed, we offer the use of bone wax as an alternative to help achieve hemostasis. Bone wax—a combination of beeswax, isopropyl palmitate, and a stabilizing agent such as almond oils or sterilized salicylic acid3—helps achieve hemostasis by purely mechanical means. It is malleable and can be easily adapted to the architecture of the surgical site (Figure 1). The bone wax can be applied immediately following surgery and removed during bandage change.

Practice Implications
Use of bone wax as a physical hemostatic agent provides a practical alternative to other options commonly used in dermatologic surgery for deep wounds or irregular surfaces. It offers several advantages.
Bone wax is not absorbed and does not adhere to wound surfaces, which makes removal easy and painless. Furthermore, bone wax allows for excellent growth of granulation tissue2 (Figure 2), most likely due to the healing and emollient properties of the beeswax and the moist occlusive environment created by the bone wax.
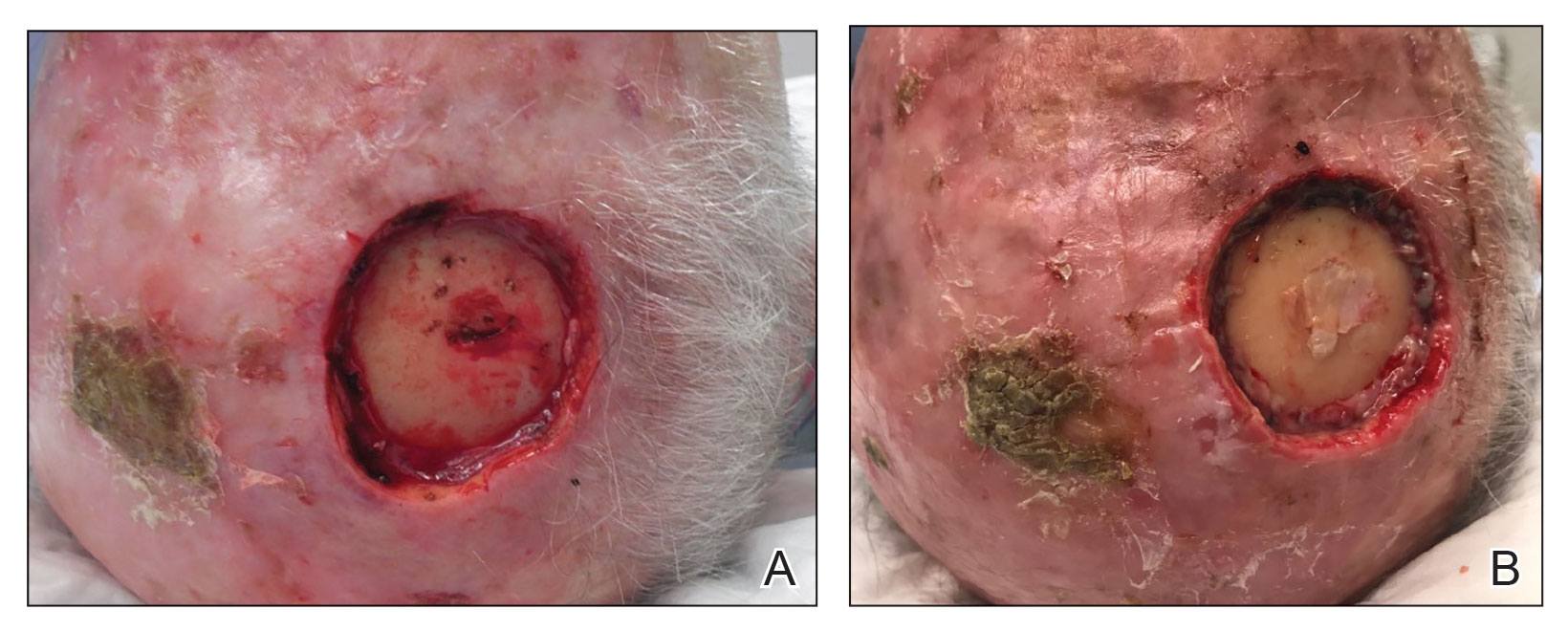
Additional advantages are its low cost, especially compared to other hemostatic agents, and long shelf-life (approximately 5 years).2 Furthermore, in scenarios when cutaneous tumors extend into the calvarium, bone wax can prevent air emboli from entering noncollapsible emissary veins.4
When bone wax is used as a temporary measure in a dermatologic setting, complications inherent to its use in bone healing (eg, granulomatous reaction, infection)—for which it is left in place indefinitely—are avoided.
- Perandones-González H, Fernández-Canga P, Rodríguez-Prieto MA. Bone wax as an ideal dressing for auricle concha. J Am Acad Dermatol. 2021;84:e75-e76. doi:10.1016/j.jaad.2019.08.002
- Palm MD, Altman JS. Topical hemostatic agents: a review. Dermatol Surg. 2008;34:431-445. doi:10.1111/j.1524-4725.2007.34090.x
- Alegre M, Garcés JR, Puig L. Bone wax in dermatologic surgery. Actas Dermosifiliogr. 2013;104:299-303. doi:10.1016/j.adengl.2013.03.001
- Goldman G, Altmayer S, Sambandan P, et al. Development of cerebral air emboli during Mohs micrographic surgery. Dermatol Surg. 2009;35:1414-1421. doi:10.1111/j.1524-4725.2009.01250.x
Practice Gap
Hemostasis after cutaneous surgery typically can be aided by mechanical occlusion with petrolatum and gauze known as a pressure bandage. However, in certain scenarios such as bone bleeding or irregularly shaped areas (eg, conchal bowl), difficulty applying a pressure bandage necessitates alternative hemostatic measures.1 In those instances, physical hemostatic agents, such as gelatin, oxidized cellulose, microporous polysaccharide spheres, hydrophilic polymers with potassium salts, microfibrillar collagen, and chitin, also can be used.2 However, those agents are expensive and often adhere to wound edges, inducing repeat trauma with removal. To avoid such concerns, we propose the use of bone wax as an effective hemostatic technique.
The Technique
When secondary intention healing is chosen or a temporary bandage needs to be placed, we offer the use of bone wax as an alternative to help achieve hemostasis. Bone wax—a combination of beeswax, isopropyl palmitate, and a stabilizing agent such as almond oils or sterilized salicylic acid3—helps achieve hemostasis by purely mechanical means. It is malleable and can be easily adapted to the architecture of the surgical site (Figure 1). The bone wax can be applied immediately following surgery and removed during bandage change.

Practice Implications
Use of bone wax as a physical hemostatic agent provides a practical alternative to other options commonly used in dermatologic surgery for deep wounds or irregular surfaces. It offers several advantages.
Bone wax is not absorbed and does not adhere to wound surfaces, which makes removal easy and painless. Furthermore, bone wax allows for excellent growth of granulation tissue2 (Figure 2), most likely due to the healing and emollient properties of the beeswax and the moist occlusive environment created by the bone wax.

Additional advantages are its low cost, especially compared to other hemostatic agents, and long shelf-life (approximately 5 years).2 Furthermore, in scenarios when cutaneous tumors extend into the calvarium, bone wax can prevent air emboli from entering noncollapsible emissary veins.4
When bone wax is used as a temporary measure in a dermatologic setting, complications inherent to its use in bone healing (eg, granulomatous reaction, infection)—for which it is left in place indefinitely—are avoided.
Practice Gap
Hemostasis after cutaneous surgery typically can be aided by mechanical occlusion with petrolatum and gauze known as a pressure bandage. However, in certain scenarios such as bone bleeding or irregularly shaped areas (eg, conchal bowl), difficulty applying a pressure bandage necessitates alternative hemostatic measures.1 In those instances, physical hemostatic agents, such as gelatin, oxidized cellulose, microporous polysaccharide spheres, hydrophilic polymers with potassium salts, microfibrillar collagen, and chitin, also can be used.2 However, those agents are expensive and often adhere to wound edges, inducing repeat trauma with removal. To avoid such concerns, we propose the use of bone wax as an effective hemostatic technique.
The Technique
When secondary intention healing is chosen or a temporary bandage needs to be placed, we offer the use of bone wax as an alternative to help achieve hemostasis. Bone wax—a combination of beeswax, isopropyl palmitate, and a stabilizing agent such as almond oils or sterilized salicylic acid3—helps achieve hemostasis by purely mechanical means. It is malleable and can be easily adapted to the architecture of the surgical site (Figure 1). The bone wax can be applied immediately following surgery and removed during bandage change.

Practice Implications
Use of bone wax as a physical hemostatic agent provides a practical alternative to other options commonly used in dermatologic surgery for deep wounds or irregular surfaces. It offers several advantages.
Bone wax is not absorbed and does not adhere to wound surfaces, which makes removal easy and painless. Furthermore, bone wax allows for excellent growth of granulation tissue2 (Figure 2), most likely due to the healing and emollient properties of the beeswax and the moist occlusive environment created by the bone wax.

Additional advantages are its low cost, especially compared to other hemostatic agents, and long shelf-life (approximately 5 years).2 Furthermore, in scenarios when cutaneous tumors extend into the calvarium, bone wax can prevent air emboli from entering noncollapsible emissary veins.4
When bone wax is used as a temporary measure in a dermatologic setting, complications inherent to its use in bone healing (eg, granulomatous reaction, infection)—for which it is left in place indefinitely—are avoided.
- Perandones-González H, Fernández-Canga P, Rodríguez-Prieto MA. Bone wax as an ideal dressing for auricle concha. J Am Acad Dermatol. 2021;84:e75-e76. doi:10.1016/j.jaad.2019.08.002
- Palm MD, Altman JS. Topical hemostatic agents: a review. Dermatol Surg. 2008;34:431-445. doi:10.1111/j.1524-4725.2007.34090.x
- Alegre M, Garcés JR, Puig L. Bone wax in dermatologic surgery. Actas Dermosifiliogr. 2013;104:299-303. doi:10.1016/j.adengl.2013.03.001
- Goldman G, Altmayer S, Sambandan P, et al. Development of cerebral air emboli during Mohs micrographic surgery. Dermatol Surg. 2009;35:1414-1421. doi:10.1111/j.1524-4725.2009.01250.x
- Perandones-González H, Fernández-Canga P, Rodríguez-Prieto MA. Bone wax as an ideal dressing for auricle concha. J Am Acad Dermatol. 2021;84:e75-e76. doi:10.1016/j.jaad.2019.08.002
- Palm MD, Altman JS. Topical hemostatic agents: a review. Dermatol Surg. 2008;34:431-445. doi:10.1111/j.1524-4725.2007.34090.x
- Alegre M, Garcés JR, Puig L. Bone wax in dermatologic surgery. Actas Dermosifiliogr. 2013;104:299-303. doi:10.1016/j.adengl.2013.03.001
- Goldman G, Altmayer S, Sambandan P, et al. Development of cerebral air emboli during Mohs micrographic surgery. Dermatol Surg. 2009;35:1414-1421. doi:10.1111/j.1524-4725.2009.01250.x