User login
Official Newspaper of the American College of Surgeons
SRS beats surgery in early control of brain mets, advantage fades with time
Stereotactic radiosurgery (SRS) provides better early local control of brain metastases than complete surgical resection, but this advantage fades with time, according to investigators.
By 6 months, lower risks associated with SRS shifted in favor of those who had surgical resection, reported lead author Thomas Churilla, MD, of Fox Chase Cancer Center in Philadelphia and his colleagues.
“Outside recognized indications for surgery such as establishing diagnosis or relieving mass effect, little evidence is available to guide the therapeutic choice of SRS vs. surgical resection in the treatment of patients with limited brain metastases,” the investigators wrote in JAMA Oncology.
The investigators performed an exploratory analysis of data from the European Organization for the Research and Treatment of Cancer (EORTC) 22952-26001 phase 3 trial, which was designed to evaluate whole-brain radiotherapy for patients with one to three brain metastases who had undergone SRS or complete surgical resection. The present analysis involved 268 patients, of whom 154 had SRS and 114 had complete surgical resection.
Primary tumors included lung, breast, colorectum, kidney, and melanoma. Initial analysis showed that patients undergoing surgical resection, compared with those who had SRS, typically had larger brain metastases (median, 28 mm vs. 20 mm) and more often had 1 brain metastasis (98.2% vs. 74.0%). Mass locality also differed between groups; compared with patients receiving SRS, surgical patients more often had metastases in the posterior fossa (26.3% vs. 7.8%) and less often in the parietal lobe (18.4% vs. 39.6%).
After median follow-up of 39.9 months, risks of local recurrence were similar between surgical and SRS groups (hazard ratio, 1.15). Stratifying by interval, however, showed that surgical patients were at much higher risk of local recurrence in the first 3 months following treatment (HR for 0-3 months, 5.94). Of note, this risk faded with time (HR for 3-6 months, 1.37; HR for 6-9 months, 0.75; HR for 9 months or longer, 0.36). From the 6-9 months interval onward, surgical patients had lower risk of recurrence, compared with SRS patients, and the risk even decreased after the 6-9 month interval.
“Prospective controlled trials are warranted to direct the optimal local approach for patients with brain metastases and to define whether any population may benefit from escalation in local therapy,” the investigators concluded.
The study was funded by the National Cancer Institute, National Institutes of Health, and Fonds Cancer in Belgium. One author reported receiving financial compensation from Pfizer via her institution.
SOURCE: Churilla T et al. JAMA Onc. 2018. doi: 10.1001/jamaoncol.2018.4610.
Stereotactic radiosurgery (SRS) provides better early local control of brain metastases than complete surgical resection, but this advantage fades with time, according to investigators.
By 6 months, lower risks associated with SRS shifted in favor of those who had surgical resection, reported lead author Thomas Churilla, MD, of Fox Chase Cancer Center in Philadelphia and his colleagues.
“Outside recognized indications for surgery such as establishing diagnosis or relieving mass effect, little evidence is available to guide the therapeutic choice of SRS vs. surgical resection in the treatment of patients with limited brain metastases,” the investigators wrote in JAMA Oncology.
The investigators performed an exploratory analysis of data from the European Organization for the Research and Treatment of Cancer (EORTC) 22952-26001 phase 3 trial, which was designed to evaluate whole-brain radiotherapy for patients with one to three brain metastases who had undergone SRS or complete surgical resection. The present analysis involved 268 patients, of whom 154 had SRS and 114 had complete surgical resection.
Primary tumors included lung, breast, colorectum, kidney, and melanoma. Initial analysis showed that patients undergoing surgical resection, compared with those who had SRS, typically had larger brain metastases (median, 28 mm vs. 20 mm) and more often had 1 brain metastasis (98.2% vs. 74.0%). Mass locality also differed between groups; compared with patients receiving SRS, surgical patients more often had metastases in the posterior fossa (26.3% vs. 7.8%) and less often in the parietal lobe (18.4% vs. 39.6%).
After median follow-up of 39.9 months, risks of local recurrence were similar between surgical and SRS groups (hazard ratio, 1.15). Stratifying by interval, however, showed that surgical patients were at much higher risk of local recurrence in the first 3 months following treatment (HR for 0-3 months, 5.94). Of note, this risk faded with time (HR for 3-6 months, 1.37; HR for 6-9 months, 0.75; HR for 9 months or longer, 0.36). From the 6-9 months interval onward, surgical patients had lower risk of recurrence, compared with SRS patients, and the risk even decreased after the 6-9 month interval.
“Prospective controlled trials are warranted to direct the optimal local approach for patients with brain metastases and to define whether any population may benefit from escalation in local therapy,” the investigators concluded.
The study was funded by the National Cancer Institute, National Institutes of Health, and Fonds Cancer in Belgium. One author reported receiving financial compensation from Pfizer via her institution.
SOURCE: Churilla T et al. JAMA Onc. 2018. doi: 10.1001/jamaoncol.2018.4610.
Stereotactic radiosurgery (SRS) provides better early local control of brain metastases than complete surgical resection, but this advantage fades with time, according to investigators.
By 6 months, lower risks associated with SRS shifted in favor of those who had surgical resection, reported lead author Thomas Churilla, MD, of Fox Chase Cancer Center in Philadelphia and his colleagues.
“Outside recognized indications for surgery such as establishing diagnosis or relieving mass effect, little evidence is available to guide the therapeutic choice of SRS vs. surgical resection in the treatment of patients with limited brain metastases,” the investigators wrote in JAMA Oncology.
The investigators performed an exploratory analysis of data from the European Organization for the Research and Treatment of Cancer (EORTC) 22952-26001 phase 3 trial, which was designed to evaluate whole-brain radiotherapy for patients with one to three brain metastases who had undergone SRS or complete surgical resection. The present analysis involved 268 patients, of whom 154 had SRS and 114 had complete surgical resection.
Primary tumors included lung, breast, colorectum, kidney, and melanoma. Initial analysis showed that patients undergoing surgical resection, compared with those who had SRS, typically had larger brain metastases (median, 28 mm vs. 20 mm) and more often had 1 brain metastasis (98.2% vs. 74.0%). Mass locality also differed between groups; compared with patients receiving SRS, surgical patients more often had metastases in the posterior fossa (26.3% vs. 7.8%) and less often in the parietal lobe (18.4% vs. 39.6%).
After median follow-up of 39.9 months, risks of local recurrence were similar between surgical and SRS groups (hazard ratio, 1.15). Stratifying by interval, however, showed that surgical patients were at much higher risk of local recurrence in the first 3 months following treatment (HR for 0-3 months, 5.94). Of note, this risk faded with time (HR for 3-6 months, 1.37; HR for 6-9 months, 0.75; HR for 9 months or longer, 0.36). From the 6-9 months interval onward, surgical patients had lower risk of recurrence, compared with SRS patients, and the risk even decreased after the 6-9 month interval.
“Prospective controlled trials are warranted to direct the optimal local approach for patients with brain metastases and to define whether any population may benefit from escalation in local therapy,” the investigators concluded.
The study was funded by the National Cancer Institute, National Institutes of Health, and Fonds Cancer in Belgium. One author reported receiving financial compensation from Pfizer via her institution.
SOURCE: Churilla T et al. JAMA Onc. 2018. doi: 10.1001/jamaoncol.2018.4610.
FROM JAMA ONCOLOGY
Key clinical point: Stereotactic radiosurgery (SRS) provides better early local control of brain metastases than surgical resection, but this advantage fades with time.
Major finding: Patients treated with surgery were more likely to have local recurrence in the first 3 months following treatment, compared with patients treated with SRS (hazard ratio, 5.94).
Study details: An exploratory analysis of data from the European Organization for the Research and Treatment of Cancer (EORTC) 22952-26001 phase 3 trial. Analysis involved 268 patients with one to three brain metastases who underwent whole-brain radiotherapy or observation after SRS (n = 154) or complete surgical resection (n = 114).
Disclosures: The study was funded by the National Cancer Institute, National Institutes of Health, and Fonds Cancer in Belgium. Dr. Handorf reported financial compensation from Pfizer, via her institution.
Source: Churilla T et al. JAMA Onc. 2018. doi: 10.1001/jamaoncol.2018.4610.
Midterm election boosts Medicaid expansion, but challenges remain
Medicaid – which has been a political football between Washington and state capitols during the past decade – scored big in the Nov. 6 election.
Three deep-red states passed ballot measures expanding their programs and two other states elected governors who have said they will accept expansion bills from their legislatures.
Supporters were so excited by the victories they said they will start planning for more voter referendums in 2020.
Medicaid proponents also were celebrating the Democrats’ takeover of the House, which would impede any Republican efforts to repeal the ACA and make major cuts to the federal-state health insurance program for low-income people.
“Tuesday was huge for the Medicaid program,” said Katherine Howitt, associate director of policy at Community Catalyst, a Boston-based advocacy group. “The overall message is that the electorate does not see this as a Democrat or GOP issue but as an issue of basic fairness, access to care, and pocketbook issue. Medicaid is working and is something Americans want to protect.”
But health experts caution that GOP opposition won’t fade away.
David K. Jones, PhD, of the department of health law, policy and management at Boston University School of Public Health, said ballot organizers now have a blueprint on how to expand Medicaid in states that have resisted. “I see this as a turning point in ACA politics,” he said. Still, he added‚ “it’s not inevitable.”
Medicaid is the largest government health program, insuring at least 73 million low-income Americans. Half of them are children. To date, 32 states and the District of Columbia have expanded it under the ACA. Before that law, Medicaid was generally limited to children, sometimes their parents, pregnant women, and people with disabilities.
The ACA encouraged states to open the program to all Americans earning up to 138% of the poverty level ($16,753 for an individual in 2018). The federal government is paying the bulk of the cost: 94% this year, but gradually dropping to 90% in 2020. States pay the rest.
GOP opposition has left about 4.2 million low-income Americans without coverage in various states.
“It’s not over until it’s over is the story of Medicaid expansion and the Affordable Care Act as the politics never ends and the opportunity for obstruction never ends,” said Dr. Jones. “But the trend overall has been to increasing implementation and increasing coverage.”
Montana fails to endorse funding
Two years after President Donald Trump carried Idaho, Nebraska, and Utah by double-digit margins with a message that included repeal of the ACA, voters in those states approved the ballot referendums on Nov. 6. Together, the states have about 300,000 uninsured adults who would be eligible for the program.
In addition, Democrats secured the governor’s offices in Kansas and Maine, which will increase the likelihood those states will pursue expansion. Legislatures in both states have previously voted to expand, only to have GOP governors block the bills. Maine voters also passed a referendum in 2017 endorsing expansion, but Republican Gov. Paul LePage again refused to accept it.
Current and incoming Republican governors in Utah and Idaho said they wouldn’t block implementation of the effort if voters approved it. Nebraska Gov. Pete Ricketts (R) said on Nov. 7 he would follow the will of the voters but would not support paying for it with a tax increase.
It wasn’t a clean sweep, however, for Medicaid.
In preliminary results, a ballot issue to fund Montana’s Medicaid expansion – which is already in place and slated to expire next July – was failing. Tobacco companies had mounted a campaign to stop the measure, which would have partially financed the expansion with taxes on tobacco products.
The Montana legislature and the Democratic governor are expected to address the issue in the session that starts in January. No state has reversed its Medicaid expansion, even though GOP governors in Kansas and Arkansas have threatened to do so.
Nearly 100,000 Montana residents have received Medicaid since its expansion, twice as many as expected.
Nancy Ballance, the Republican chairwoman of the Montana House Appropriations Committee who opposed the bill that expanded Medicaid in 2015, said she is confident the state legislature will extend the program past July. But she expects the legislature to put some limits on the program, such as adding an asset test and work requirements.
“There are some people in the state who may not have disabilities but need some help to access coverage,” she said. “I think we can pass something without people having a gap in coverage. … That will be a priority.”
“It was never our intent to simply sunset the expansion and have it go away,” she said. Rather, the legislature put the sunset provision in to revisit the provision to make any changes.
Chris Jacobs, a conservative health policy analyst in Washington, said the Montana results showed that when voters are given a choice of having to pay for Medicaid expansion through a new tax, they were not willing to go along.
But in Utah, voters did agree to fund their state plan by adding 0.15% to the state’s sales tax, just over a penny for a $10 purchase.
Fernando Wilson, acting director of the Center for Health Policy at the University of Nebraska Medical Center in Omaha, said the vote on the state’s ballot question indicated many people wanted to help 80,000 uninsured Nebraskans gain coverage.
“I think it showed there was a clear need for it,” he said. The legislature likely won’t block the expansion, Wilson said, though it may try to add a conservative twist such as adding premiums or other steps.
Sheila Burke, a lecturer in health policy at Harvard Kennedy School in Cambridge, Mass., said voters approved Medicaid expansion not just because it would help improve health coverage for their residents but to help stabilize their hospitals, particularly those in rural areas. Hospitals have said this step helps their bottom lines because it cuts down on uninsured patients and uncompensated care.
“The broad population does see the value of Medicaid,” she said. “They saw it as a loss by their states not to accept the federal funds,” she said.
Despite the victories, Ms. Burke said, advocates should not assume other states such as Florida, Texas, and Tennessee will follow suit.
“I don’t see a radical shift, but it moves us closer,” she said.
‘Fertile ground’ for more referendums
If advocates press for more referendums, Florida might be a tempting target. More than 700,000 adults there could become eligible, but the campaign would likely also be very costly.
Jonathan Schleifer, executive director of the Fairness Project, which financed the ballot initiatives in Maine in 2017 and the four states this year, refused to say which states would be targeted next.
The group is funded by the Service Employees International Union–United Healthcare Workers West, a California health care workers union.
“The GOP has been bashing the ACA for nearly a decade, and voters in the reddest states in the country just rejected that message,” Mr. Schleifer said. “It’s a repudiation and a tectonic shift in health care in this country.”
“There is fertile ground” for more such ballot votes, said Topher Spiro, vice president for health policy at the Center for American Progress, a liberal think tank. “It is clear that public opinion is on the side of Medicaid expansion and the election results merely confirm that.”
“This will build momentum for expansion in other states,” he added.
The election results also could have consequences on efforts by states to implement work requirements for Medicaid enrollees.
New Hampshire and Michigan — which expanded the program but recently won federal approval to add controversial work requirements — could revisit that additional mandate as a result of Democrats winning control over both houses of the legislature in New Hampshire and the governor’s office in Michigan.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Medicaid – which has been a political football between Washington and state capitols during the past decade – scored big in the Nov. 6 election.
Three deep-red states passed ballot measures expanding their programs and two other states elected governors who have said they will accept expansion bills from their legislatures.
Supporters were so excited by the victories they said they will start planning for more voter referendums in 2020.
Medicaid proponents also were celebrating the Democrats’ takeover of the House, which would impede any Republican efforts to repeal the ACA and make major cuts to the federal-state health insurance program for low-income people.
“Tuesday was huge for the Medicaid program,” said Katherine Howitt, associate director of policy at Community Catalyst, a Boston-based advocacy group. “The overall message is that the electorate does not see this as a Democrat or GOP issue but as an issue of basic fairness, access to care, and pocketbook issue. Medicaid is working and is something Americans want to protect.”
But health experts caution that GOP opposition won’t fade away.
David K. Jones, PhD, of the department of health law, policy and management at Boston University School of Public Health, said ballot organizers now have a blueprint on how to expand Medicaid in states that have resisted. “I see this as a turning point in ACA politics,” he said. Still, he added‚ “it’s not inevitable.”
Medicaid is the largest government health program, insuring at least 73 million low-income Americans. Half of them are children. To date, 32 states and the District of Columbia have expanded it under the ACA. Before that law, Medicaid was generally limited to children, sometimes their parents, pregnant women, and people with disabilities.
The ACA encouraged states to open the program to all Americans earning up to 138% of the poverty level ($16,753 for an individual in 2018). The federal government is paying the bulk of the cost: 94% this year, but gradually dropping to 90% in 2020. States pay the rest.
GOP opposition has left about 4.2 million low-income Americans without coverage in various states.
“It’s not over until it’s over is the story of Medicaid expansion and the Affordable Care Act as the politics never ends and the opportunity for obstruction never ends,” said Dr. Jones. “But the trend overall has been to increasing implementation and increasing coverage.”
Montana fails to endorse funding
Two years after President Donald Trump carried Idaho, Nebraska, and Utah by double-digit margins with a message that included repeal of the ACA, voters in those states approved the ballot referendums on Nov. 6. Together, the states have about 300,000 uninsured adults who would be eligible for the program.
In addition, Democrats secured the governor’s offices in Kansas and Maine, which will increase the likelihood those states will pursue expansion. Legislatures in both states have previously voted to expand, only to have GOP governors block the bills. Maine voters also passed a referendum in 2017 endorsing expansion, but Republican Gov. Paul LePage again refused to accept it.
Current and incoming Republican governors in Utah and Idaho said they wouldn’t block implementation of the effort if voters approved it. Nebraska Gov. Pete Ricketts (R) said on Nov. 7 he would follow the will of the voters but would not support paying for it with a tax increase.
It wasn’t a clean sweep, however, for Medicaid.
In preliminary results, a ballot issue to fund Montana’s Medicaid expansion – which is already in place and slated to expire next July – was failing. Tobacco companies had mounted a campaign to stop the measure, which would have partially financed the expansion with taxes on tobacco products.
The Montana legislature and the Democratic governor are expected to address the issue in the session that starts in January. No state has reversed its Medicaid expansion, even though GOP governors in Kansas and Arkansas have threatened to do so.
Nearly 100,000 Montana residents have received Medicaid since its expansion, twice as many as expected.
Nancy Ballance, the Republican chairwoman of the Montana House Appropriations Committee who opposed the bill that expanded Medicaid in 2015, said she is confident the state legislature will extend the program past July. But she expects the legislature to put some limits on the program, such as adding an asset test and work requirements.
“There are some people in the state who may not have disabilities but need some help to access coverage,” she said. “I think we can pass something without people having a gap in coverage. … That will be a priority.”
“It was never our intent to simply sunset the expansion and have it go away,” she said. Rather, the legislature put the sunset provision in to revisit the provision to make any changes.
Chris Jacobs, a conservative health policy analyst in Washington, said the Montana results showed that when voters are given a choice of having to pay for Medicaid expansion through a new tax, they were not willing to go along.
But in Utah, voters did agree to fund their state plan by adding 0.15% to the state’s sales tax, just over a penny for a $10 purchase.
Fernando Wilson, acting director of the Center for Health Policy at the University of Nebraska Medical Center in Omaha, said the vote on the state’s ballot question indicated many people wanted to help 80,000 uninsured Nebraskans gain coverage.
“I think it showed there was a clear need for it,” he said. The legislature likely won’t block the expansion, Wilson said, though it may try to add a conservative twist such as adding premiums or other steps.
Sheila Burke, a lecturer in health policy at Harvard Kennedy School in Cambridge, Mass., said voters approved Medicaid expansion not just because it would help improve health coverage for their residents but to help stabilize their hospitals, particularly those in rural areas. Hospitals have said this step helps their bottom lines because it cuts down on uninsured patients and uncompensated care.
“The broad population does see the value of Medicaid,” she said. “They saw it as a loss by their states not to accept the federal funds,” she said.
Despite the victories, Ms. Burke said, advocates should not assume other states such as Florida, Texas, and Tennessee will follow suit.
“I don’t see a radical shift, but it moves us closer,” she said.
‘Fertile ground’ for more referendums
If advocates press for more referendums, Florida might be a tempting target. More than 700,000 adults there could become eligible, but the campaign would likely also be very costly.
Jonathan Schleifer, executive director of the Fairness Project, which financed the ballot initiatives in Maine in 2017 and the four states this year, refused to say which states would be targeted next.
The group is funded by the Service Employees International Union–United Healthcare Workers West, a California health care workers union.
“The GOP has been bashing the ACA for nearly a decade, and voters in the reddest states in the country just rejected that message,” Mr. Schleifer said. “It’s a repudiation and a tectonic shift in health care in this country.”
“There is fertile ground” for more such ballot votes, said Topher Spiro, vice president for health policy at the Center for American Progress, a liberal think tank. “It is clear that public opinion is on the side of Medicaid expansion and the election results merely confirm that.”
“This will build momentum for expansion in other states,” he added.
The election results also could have consequences on efforts by states to implement work requirements for Medicaid enrollees.
New Hampshire and Michigan — which expanded the program but recently won federal approval to add controversial work requirements — could revisit that additional mandate as a result of Democrats winning control over both houses of the legislature in New Hampshire and the governor’s office in Michigan.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Medicaid – which has been a political football between Washington and state capitols during the past decade – scored big in the Nov. 6 election.
Three deep-red states passed ballot measures expanding their programs and two other states elected governors who have said they will accept expansion bills from their legislatures.
Supporters were so excited by the victories they said they will start planning for more voter referendums in 2020.
Medicaid proponents also were celebrating the Democrats’ takeover of the House, which would impede any Republican efforts to repeal the ACA and make major cuts to the federal-state health insurance program for low-income people.
“Tuesday was huge for the Medicaid program,” said Katherine Howitt, associate director of policy at Community Catalyst, a Boston-based advocacy group. “The overall message is that the electorate does not see this as a Democrat or GOP issue but as an issue of basic fairness, access to care, and pocketbook issue. Medicaid is working and is something Americans want to protect.”
But health experts caution that GOP opposition won’t fade away.
David K. Jones, PhD, of the department of health law, policy and management at Boston University School of Public Health, said ballot organizers now have a blueprint on how to expand Medicaid in states that have resisted. “I see this as a turning point in ACA politics,” he said. Still, he added‚ “it’s not inevitable.”
Medicaid is the largest government health program, insuring at least 73 million low-income Americans. Half of them are children. To date, 32 states and the District of Columbia have expanded it under the ACA. Before that law, Medicaid was generally limited to children, sometimes their parents, pregnant women, and people with disabilities.
The ACA encouraged states to open the program to all Americans earning up to 138% of the poverty level ($16,753 for an individual in 2018). The federal government is paying the bulk of the cost: 94% this year, but gradually dropping to 90% in 2020. States pay the rest.
GOP opposition has left about 4.2 million low-income Americans without coverage in various states.
“It’s not over until it’s over is the story of Medicaid expansion and the Affordable Care Act as the politics never ends and the opportunity for obstruction never ends,” said Dr. Jones. “But the trend overall has been to increasing implementation and increasing coverage.”
Montana fails to endorse funding
Two years after President Donald Trump carried Idaho, Nebraska, and Utah by double-digit margins with a message that included repeal of the ACA, voters in those states approved the ballot referendums on Nov. 6. Together, the states have about 300,000 uninsured adults who would be eligible for the program.
In addition, Democrats secured the governor’s offices in Kansas and Maine, which will increase the likelihood those states will pursue expansion. Legislatures in both states have previously voted to expand, only to have GOP governors block the bills. Maine voters also passed a referendum in 2017 endorsing expansion, but Republican Gov. Paul LePage again refused to accept it.
Current and incoming Republican governors in Utah and Idaho said they wouldn’t block implementation of the effort if voters approved it. Nebraska Gov. Pete Ricketts (R) said on Nov. 7 he would follow the will of the voters but would not support paying for it with a tax increase.
It wasn’t a clean sweep, however, for Medicaid.
In preliminary results, a ballot issue to fund Montana’s Medicaid expansion – which is already in place and slated to expire next July – was failing. Tobacco companies had mounted a campaign to stop the measure, which would have partially financed the expansion with taxes on tobacco products.
The Montana legislature and the Democratic governor are expected to address the issue in the session that starts in January. No state has reversed its Medicaid expansion, even though GOP governors in Kansas and Arkansas have threatened to do so.
Nearly 100,000 Montana residents have received Medicaid since its expansion, twice as many as expected.
Nancy Ballance, the Republican chairwoman of the Montana House Appropriations Committee who opposed the bill that expanded Medicaid in 2015, said she is confident the state legislature will extend the program past July. But she expects the legislature to put some limits on the program, such as adding an asset test and work requirements.
“There are some people in the state who may not have disabilities but need some help to access coverage,” she said. “I think we can pass something without people having a gap in coverage. … That will be a priority.”
“It was never our intent to simply sunset the expansion and have it go away,” she said. Rather, the legislature put the sunset provision in to revisit the provision to make any changes.
Chris Jacobs, a conservative health policy analyst in Washington, said the Montana results showed that when voters are given a choice of having to pay for Medicaid expansion through a new tax, they were not willing to go along.
But in Utah, voters did agree to fund their state plan by adding 0.15% to the state’s sales tax, just over a penny for a $10 purchase.
Fernando Wilson, acting director of the Center for Health Policy at the University of Nebraska Medical Center in Omaha, said the vote on the state’s ballot question indicated many people wanted to help 80,000 uninsured Nebraskans gain coverage.
“I think it showed there was a clear need for it,” he said. The legislature likely won’t block the expansion, Wilson said, though it may try to add a conservative twist such as adding premiums or other steps.
Sheila Burke, a lecturer in health policy at Harvard Kennedy School in Cambridge, Mass., said voters approved Medicaid expansion not just because it would help improve health coverage for their residents but to help stabilize their hospitals, particularly those in rural areas. Hospitals have said this step helps their bottom lines because it cuts down on uninsured patients and uncompensated care.
“The broad population does see the value of Medicaid,” she said. “They saw it as a loss by their states not to accept the federal funds,” she said.
Despite the victories, Ms. Burke said, advocates should not assume other states such as Florida, Texas, and Tennessee will follow suit.
“I don’t see a radical shift, but it moves us closer,” she said.
‘Fertile ground’ for more referendums
If advocates press for more referendums, Florida might be a tempting target. More than 700,000 adults there could become eligible, but the campaign would likely also be very costly.
Jonathan Schleifer, executive director of the Fairness Project, which financed the ballot initiatives in Maine in 2017 and the four states this year, refused to say which states would be targeted next.
The group is funded by the Service Employees International Union–United Healthcare Workers West, a California health care workers union.
“The GOP has been bashing the ACA for nearly a decade, and voters in the reddest states in the country just rejected that message,” Mr. Schleifer said. “It’s a repudiation and a tectonic shift in health care in this country.”
“There is fertile ground” for more such ballot votes, said Topher Spiro, vice president for health policy at the Center for American Progress, a liberal think tank. “It is clear that public opinion is on the side of Medicaid expansion and the election results merely confirm that.”
“This will build momentum for expansion in other states,” he added.
The election results also could have consequences on efforts by states to implement work requirements for Medicaid enrollees.
New Hampshire and Michigan — which expanded the program but recently won federal approval to add controversial work requirements — could revisit that additional mandate as a result of Democrats winning control over both houses of the legislature in New Hampshire and the governor’s office in Michigan.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Open enrollment: Slow first week at HealthCare.gov
About 372,000 people selected a health insurance plan during the first week of Affordable Care Act open enrollment for 2019, according to the Centers for Medicare & Medicaid Services. The first week was short, with only 3 days to select coverage.
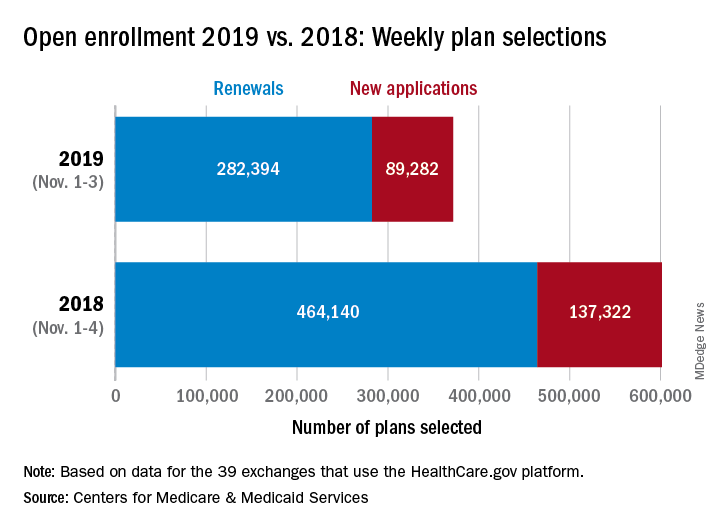
For Nov. 1-3, the exact number of plans selected was 371,676, which is about 38% less than last year’s first week, which was 4 days long, so the average number of plans selected per day was down by a little less than 18%, CMS data show.
“The final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations,” CMS officials said, adding that the weekly data release “only reports new plan selections and active plan renewals and does not report the number of consumers who have paid premiums to effectuate their enrollment.”
Open enrollment will continue for another 6 weeks, with Dec. 15 being the final day to enroll for 2019 coverage on the 39 state exchanges that use the HealthCare.gov platform.
About 372,000 people selected a health insurance plan during the first week of Affordable Care Act open enrollment for 2019, according to the Centers for Medicare & Medicaid Services. The first week was short, with only 3 days to select coverage.

For Nov. 1-3, the exact number of plans selected was 371,676, which is about 38% less than last year’s first week, which was 4 days long, so the average number of plans selected per day was down by a little less than 18%, CMS data show.
“The final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations,” CMS officials said, adding that the weekly data release “only reports new plan selections and active plan renewals and does not report the number of consumers who have paid premiums to effectuate their enrollment.”
Open enrollment will continue for another 6 weeks, with Dec. 15 being the final day to enroll for 2019 coverage on the 39 state exchanges that use the HealthCare.gov platform.
About 372,000 people selected a health insurance plan during the first week of Affordable Care Act open enrollment for 2019, according to the Centers for Medicare & Medicaid Services. The first week was short, with only 3 days to select coverage.

For Nov. 1-3, the exact number of plans selected was 371,676, which is about 38% less than last year’s first week, which was 4 days long, so the average number of plans selected per day was down by a little less than 18%, CMS data show.
“The final number of plan selections associated with enrollment activity during a reporting period may change due to plan modifications or cancellations,” CMS officials said, adding that the weekly data release “only reports new plan selections and active plan renewals and does not report the number of consumers who have paid premiums to effectuate their enrollment.”
Open enrollment will continue for another 6 weeks, with Dec. 15 being the final day to enroll for 2019 coverage on the 39 state exchanges that use the HealthCare.gov platform.
Two novel approaches for infected ventral hernia mesh
BOSTON – Deep according to Cleveland Clinic investigators.
When infected mesh is removed, however, there’s a novel approach that avoids the pitfalls of both immediate and staged abdominal wall reconstruction, according to a second team from the Georgetown University, Washington.
The two approaches were offered at the annual clinical congress of the American College of Surgery as alternatives to usual care. Infected ventral hernia mesh is a well-known headache for general surgeons, and management isn’t standardized. Surgeons are keenly alert for new approaches to improve outcomes; the presenters said they hoped their talks would help.
The work “is really pushing this forward, and giving us new data to manage a really vexing problem,” said an audience member.
Almost 80% salvageable
Infected meshes are usually removed, but the Cleveland Clinic investigators found that that’s often not necessary.
They reviewed 905 elective ventral hernia repairs at the clinic with synthetic sublay mesh in the retrorectus space. The median hernia width was about 15 cm, and the implanted mesh – usually medium- or heavy-weight polypropylene – had a mean area of 900 cm2, “so these were big hernias with a lot of mesh. [Patients] often come to us as a last resort because they’ve been told no elsewhere,” said lead investigator Dominykas Burneikis, MD.
Twenty-four patients (2.7%) developed deep surgical site infections below the anterior rectus fascia. Instead of returning to the OR for new mesh, the team opened, drained, and debrided the wounds, and patients received antibiotics plus negative pressure wound therapy.
Those measures were enough for all but one patient. Mesh was generally found to be granulating well into surrounding tissue, so it was left completely intact in 19 cases (79%), and just trimmed a bit in four others. One man had an excision after his skin flap died and the hernia recurred. At 8 months, 11 patients were completely healed, and 12 had granulating wounds with no visible mesh. There were no cutaneous fistulas.
In short, “we had an 80% mesh salvage rate at 8 months, [which] led us to conclude that most synthetic mesh infections after retrorectus sublay repair do not require explanation,” Dr. Burneikis said.
A hybrid approach
When infected mesh does need to come out, abdominal wall reconstruction is either done in the same procedure or months later. Immediate reconstruction generally means operating in a contaminated field, with subsequent rates of wound infection of up to 48%. Delayed closure, meanwhile, means long-term wound care and temporary hernia recurrence, among other problems.
The Georgetown team reported good outcomes with a hybrid approach that combines the benefits of both procedures while avoiding their pitfalls. In the first step, mesh is removed, the abdominal wall debrided, fistulas taken down, and cultures obtained, explained lead investigator and surgery resident Kieranjeet Nijhar, MD.
The wound is temporarily closed with a sterile plastic liner under negative pressure, and patients are taken to the floor for IV antibiotics based on culture results. Three days later, after the infection has been knocked down, the patient is returned to the OR for debridement to healthy tissue and definitive reconstruction with biologic mesh. It’s all done during the same hospitalization.
Dr. Nijhar reviewed 53 cases at Georgetown since 2009. Patients were a mean age of 58 years, with an average body mass index of 35.1 kg/m2. Infected mesh was most commonly underlain or retrorectus; mean defect size was 206 cm2. Patients spent an average of 11 days in the hospital.
During a mean follow-up of about 9 months, 17 patients (32%) had surgical site problems – infection, dehiscence, hematoma, or seroma – and hernia recurred in six (11.3%); the results compare favorably with especially immediate reconstruction. As in prior studies, higher age and bridge repair were associated with recurrence and methicillin-resistant Staphylococcus aureus (MRSA) infection with surgical site problems.
“We propose this as a potential alternative for” repairs of ventral hernias with infected mesh, Dr. Nijhar said.
Dr. Nijhar and Dr. Burneikis had no relevant disclosures. There was no external funding for the work.
BOSTON – Deep according to Cleveland Clinic investigators.
When infected mesh is removed, however, there’s a novel approach that avoids the pitfalls of both immediate and staged abdominal wall reconstruction, according to a second team from the Georgetown University, Washington.
The two approaches were offered at the annual clinical congress of the American College of Surgery as alternatives to usual care. Infected ventral hernia mesh is a well-known headache for general surgeons, and management isn’t standardized. Surgeons are keenly alert for new approaches to improve outcomes; the presenters said they hoped their talks would help.
The work “is really pushing this forward, and giving us new data to manage a really vexing problem,” said an audience member.
Almost 80% salvageable
Infected meshes are usually removed, but the Cleveland Clinic investigators found that that’s often not necessary.
They reviewed 905 elective ventral hernia repairs at the clinic with synthetic sublay mesh in the retrorectus space. The median hernia width was about 15 cm, and the implanted mesh – usually medium- or heavy-weight polypropylene – had a mean area of 900 cm2, “so these were big hernias with a lot of mesh. [Patients] often come to us as a last resort because they’ve been told no elsewhere,” said lead investigator Dominykas Burneikis, MD.
Twenty-four patients (2.7%) developed deep surgical site infections below the anterior rectus fascia. Instead of returning to the OR for new mesh, the team opened, drained, and debrided the wounds, and patients received antibiotics plus negative pressure wound therapy.
Those measures were enough for all but one patient. Mesh was generally found to be granulating well into surrounding tissue, so it was left completely intact in 19 cases (79%), and just trimmed a bit in four others. One man had an excision after his skin flap died and the hernia recurred. At 8 months, 11 patients were completely healed, and 12 had granulating wounds with no visible mesh. There were no cutaneous fistulas.
In short, “we had an 80% mesh salvage rate at 8 months, [which] led us to conclude that most synthetic mesh infections after retrorectus sublay repair do not require explanation,” Dr. Burneikis said.
A hybrid approach
When infected mesh does need to come out, abdominal wall reconstruction is either done in the same procedure or months later. Immediate reconstruction generally means operating in a contaminated field, with subsequent rates of wound infection of up to 48%. Delayed closure, meanwhile, means long-term wound care and temporary hernia recurrence, among other problems.
The Georgetown team reported good outcomes with a hybrid approach that combines the benefits of both procedures while avoiding their pitfalls. In the first step, mesh is removed, the abdominal wall debrided, fistulas taken down, and cultures obtained, explained lead investigator and surgery resident Kieranjeet Nijhar, MD.
The wound is temporarily closed with a sterile plastic liner under negative pressure, and patients are taken to the floor for IV antibiotics based on culture results. Three days later, after the infection has been knocked down, the patient is returned to the OR for debridement to healthy tissue and definitive reconstruction with biologic mesh. It’s all done during the same hospitalization.
Dr. Nijhar reviewed 53 cases at Georgetown since 2009. Patients were a mean age of 58 years, with an average body mass index of 35.1 kg/m2. Infected mesh was most commonly underlain or retrorectus; mean defect size was 206 cm2. Patients spent an average of 11 days in the hospital.
During a mean follow-up of about 9 months, 17 patients (32%) had surgical site problems – infection, dehiscence, hematoma, or seroma – and hernia recurred in six (11.3%); the results compare favorably with especially immediate reconstruction. As in prior studies, higher age and bridge repair were associated with recurrence and methicillin-resistant Staphylococcus aureus (MRSA) infection with surgical site problems.
“We propose this as a potential alternative for” repairs of ventral hernias with infected mesh, Dr. Nijhar said.
Dr. Nijhar and Dr. Burneikis had no relevant disclosures. There was no external funding for the work.
BOSTON – Deep according to Cleveland Clinic investigators.
When infected mesh is removed, however, there’s a novel approach that avoids the pitfalls of both immediate and staged abdominal wall reconstruction, according to a second team from the Georgetown University, Washington.
The two approaches were offered at the annual clinical congress of the American College of Surgery as alternatives to usual care. Infected ventral hernia mesh is a well-known headache for general surgeons, and management isn’t standardized. Surgeons are keenly alert for new approaches to improve outcomes; the presenters said they hoped their talks would help.
The work “is really pushing this forward, and giving us new data to manage a really vexing problem,” said an audience member.
Almost 80% salvageable
Infected meshes are usually removed, but the Cleveland Clinic investigators found that that’s often not necessary.
They reviewed 905 elective ventral hernia repairs at the clinic with synthetic sublay mesh in the retrorectus space. The median hernia width was about 15 cm, and the implanted mesh – usually medium- or heavy-weight polypropylene – had a mean area of 900 cm2, “so these were big hernias with a lot of mesh. [Patients] often come to us as a last resort because they’ve been told no elsewhere,” said lead investigator Dominykas Burneikis, MD.
Twenty-four patients (2.7%) developed deep surgical site infections below the anterior rectus fascia. Instead of returning to the OR for new mesh, the team opened, drained, and debrided the wounds, and patients received antibiotics plus negative pressure wound therapy.
Those measures were enough for all but one patient. Mesh was generally found to be granulating well into surrounding tissue, so it was left completely intact in 19 cases (79%), and just trimmed a bit in four others. One man had an excision after his skin flap died and the hernia recurred. At 8 months, 11 patients were completely healed, and 12 had granulating wounds with no visible mesh. There were no cutaneous fistulas.
In short, “we had an 80% mesh salvage rate at 8 months, [which] led us to conclude that most synthetic mesh infections after retrorectus sublay repair do not require explanation,” Dr. Burneikis said.
A hybrid approach
When infected mesh does need to come out, abdominal wall reconstruction is either done in the same procedure or months later. Immediate reconstruction generally means operating in a contaminated field, with subsequent rates of wound infection of up to 48%. Delayed closure, meanwhile, means long-term wound care and temporary hernia recurrence, among other problems.
The Georgetown team reported good outcomes with a hybrid approach that combines the benefits of both procedures while avoiding their pitfalls. In the first step, mesh is removed, the abdominal wall debrided, fistulas taken down, and cultures obtained, explained lead investigator and surgery resident Kieranjeet Nijhar, MD.
The wound is temporarily closed with a sterile plastic liner under negative pressure, and patients are taken to the floor for IV antibiotics based on culture results. Three days later, after the infection has been knocked down, the patient is returned to the OR for debridement to healthy tissue and definitive reconstruction with biologic mesh. It’s all done during the same hospitalization.
Dr. Nijhar reviewed 53 cases at Georgetown since 2009. Patients were a mean age of 58 years, with an average body mass index of 35.1 kg/m2. Infected mesh was most commonly underlain or retrorectus; mean defect size was 206 cm2. Patients spent an average of 11 days in the hospital.
During a mean follow-up of about 9 months, 17 patients (32%) had surgical site problems – infection, dehiscence, hematoma, or seroma – and hernia recurred in six (11.3%); the results compare favorably with especially immediate reconstruction. As in prior studies, higher age and bridge repair were associated with recurrence and methicillin-resistant Staphylococcus aureus (MRSA) infection with surgical site problems.
“We propose this as a potential alternative for” repairs of ventral hernias with infected mesh, Dr. Nijhar said.
Dr. Nijhar and Dr. Burneikis had no relevant disclosures. There was no external funding for the work.
REPORTING FROM THE ACS CLINICAL CONGRESS
Key clinical point: Infected mesh can sometimes be left in place, and a new surgical approach splits the difference between immediate and staged reconstruction.
Major finding: The salvage rate for infected ventral hernia mesh was almost 80% at 8 months, and the recurrence rate was 11.3% with hybrid reconstruction at 9 months.
Study details: Reviews of 24 infected mesh cases and 53 hybrid repairs
Disclosures: The study leads didn’t have any disclosures, and there was no external funding.
Needle aspiration comes first for most breast abscesses
BOSTON – When surgeon Wendy R. Greene, MD, FACS, director of acute and critical care surgery at Emory University, Atlanta, asked a room of about 300 general surgeons at the annual clinical congress of the American College of Surgeons how many use needle aspiration first for breast abscesses, and how many use a scalpel, it was about a 50-50 split.
This divided response is why Dr. Greene addressed in her presentation the right approach to the problem of breast abscesses. In short, “for run-of-the-mill abscesses less than 5 cm, don’t get out the scalpel; get out the needle first,” she said.
New mothers over age 30 years are most at risk, especially if they are past 40 weeks’ gestation.
There certainly are indications for the scalpel first. If the skin overlaying the abscess is dead, shiny, sloughing off, or leaking pus, or if the abscess is larger than 5 cm on ultrasound, a small stab incision is in order, and it should be made at the maximum point of fluctuation, after numbing the surrounding tissue. Put a wipe in place to catch the pus, debride as necessary, and “irrigate, irrigate, irrigate,” Dr. Greene said.
She uses suction to make sure all the pus is out, then injects a lidocaine into the cavity for pain control and lets it rest a few minutes before another round of suction.
Septic, deteriorating patients, and the immunocompromised, need a larger incision and drainage, with IV antibiotics in the hospital, but even in those cases, “avoid placing percutaneous drains; there’s rarely a role for them in modern management of breast abscesses.” Women will have poorer results and poorer cosmesis, Dr. Greene said.
Aggressive drainage isn’t necessary most of the time, and it can destroy healthy tissue and leave new mothers with breastfeeding problems and milk fistulas. There’s also a risk for scarring, deformity, and loss of the ability to lactate.
An 18-21 gauge needle with local anesthetic is usually enough. The lesion should be obvious on ultrasound, and it’s useful to guide the needle and ensure the cavity collapses on aspiration.
Dr. Greene said it also is important to culture milk in new mothers, and culture her infant’s nose and mouth, because cracked skin on the breast can let germs from nursing into the milk ducts.
Women are sent home after aspiration with antibiotics for 7-10 days, ones that are safe for nursing infants. They might be back with another abscess in a few weeks, so it’s important to be patient and ready for ongoing treatment.
The ultimate worry with recurrent cases is that a breast mass is blocking a milk duct, so mammography is often in order for repeat patients, especially with a family history of breast cancer. Wait until the acute infection has died down; a mammograph can be too painful otherwise, Dr. Greene said.
In the meantime, let infants nurse. They “are a great way to help drain the breast,” she said.
Dr. Greene had no relevant disclosures.
BOSTON – When surgeon Wendy R. Greene, MD, FACS, director of acute and critical care surgery at Emory University, Atlanta, asked a room of about 300 general surgeons at the annual clinical congress of the American College of Surgeons how many use needle aspiration first for breast abscesses, and how many use a scalpel, it was about a 50-50 split.
This divided response is why Dr. Greene addressed in her presentation the right approach to the problem of breast abscesses. In short, “for run-of-the-mill abscesses less than 5 cm, don’t get out the scalpel; get out the needle first,” she said.
New mothers over age 30 years are most at risk, especially if they are past 40 weeks’ gestation.
There certainly are indications for the scalpel first. If the skin overlaying the abscess is dead, shiny, sloughing off, or leaking pus, or if the abscess is larger than 5 cm on ultrasound, a small stab incision is in order, and it should be made at the maximum point of fluctuation, after numbing the surrounding tissue. Put a wipe in place to catch the pus, debride as necessary, and “irrigate, irrigate, irrigate,” Dr. Greene said.
She uses suction to make sure all the pus is out, then injects a lidocaine into the cavity for pain control and lets it rest a few minutes before another round of suction.
Septic, deteriorating patients, and the immunocompromised, need a larger incision and drainage, with IV antibiotics in the hospital, but even in those cases, “avoid placing percutaneous drains; there’s rarely a role for them in modern management of breast abscesses.” Women will have poorer results and poorer cosmesis, Dr. Greene said.
Aggressive drainage isn’t necessary most of the time, and it can destroy healthy tissue and leave new mothers with breastfeeding problems and milk fistulas. There’s also a risk for scarring, deformity, and loss of the ability to lactate.
An 18-21 gauge needle with local anesthetic is usually enough. The lesion should be obvious on ultrasound, and it’s useful to guide the needle and ensure the cavity collapses on aspiration.
Dr. Greene said it also is important to culture milk in new mothers, and culture her infant’s nose and mouth, because cracked skin on the breast can let germs from nursing into the milk ducts.
Women are sent home after aspiration with antibiotics for 7-10 days, ones that are safe for nursing infants. They might be back with another abscess in a few weeks, so it’s important to be patient and ready for ongoing treatment.
The ultimate worry with recurrent cases is that a breast mass is blocking a milk duct, so mammography is often in order for repeat patients, especially with a family history of breast cancer. Wait until the acute infection has died down; a mammograph can be too painful otherwise, Dr. Greene said.
In the meantime, let infants nurse. They “are a great way to help drain the breast,” she said.
Dr. Greene had no relevant disclosures.
BOSTON – When surgeon Wendy R. Greene, MD, FACS, director of acute and critical care surgery at Emory University, Atlanta, asked a room of about 300 general surgeons at the annual clinical congress of the American College of Surgeons how many use needle aspiration first for breast abscesses, and how many use a scalpel, it was about a 50-50 split.
This divided response is why Dr. Greene addressed in her presentation the right approach to the problem of breast abscesses. In short, “for run-of-the-mill abscesses less than 5 cm, don’t get out the scalpel; get out the needle first,” she said.
New mothers over age 30 years are most at risk, especially if they are past 40 weeks’ gestation.
There certainly are indications for the scalpel first. If the skin overlaying the abscess is dead, shiny, sloughing off, or leaking pus, or if the abscess is larger than 5 cm on ultrasound, a small stab incision is in order, and it should be made at the maximum point of fluctuation, after numbing the surrounding tissue. Put a wipe in place to catch the pus, debride as necessary, and “irrigate, irrigate, irrigate,” Dr. Greene said.
She uses suction to make sure all the pus is out, then injects a lidocaine into the cavity for pain control and lets it rest a few minutes before another round of suction.
Septic, deteriorating patients, and the immunocompromised, need a larger incision and drainage, with IV antibiotics in the hospital, but even in those cases, “avoid placing percutaneous drains; there’s rarely a role for them in modern management of breast abscesses.” Women will have poorer results and poorer cosmesis, Dr. Greene said.
Aggressive drainage isn’t necessary most of the time, and it can destroy healthy tissue and leave new mothers with breastfeeding problems and milk fistulas. There’s also a risk for scarring, deformity, and loss of the ability to lactate.
An 18-21 gauge needle with local anesthetic is usually enough. The lesion should be obvious on ultrasound, and it’s useful to guide the needle and ensure the cavity collapses on aspiration.
Dr. Greene said it also is important to culture milk in new mothers, and culture her infant’s nose and mouth, because cracked skin on the breast can let germs from nursing into the milk ducts.
Women are sent home after aspiration with antibiotics for 7-10 days, ones that are safe for nursing infants. They might be back with another abscess in a few weeks, so it’s important to be patient and ready for ongoing treatment.
The ultimate worry with recurrent cases is that a breast mass is blocking a milk duct, so mammography is often in order for repeat patients, especially with a family history of breast cancer. Wait until the acute infection has died down; a mammograph can be too painful otherwise, Dr. Greene said.
In the meantime, let infants nurse. They “are a great way to help drain the breast,” she said.
Dr. Greene had no relevant disclosures.
EXPERT ANALYSIS FROM THE ACS CLINICAL CONGRESS
FDA approval of powerful opioid tinged with irony
The timing of the Food and Drug Administration’s Nov. 2 approval of the medication Dsuvia, a sublingual formulation of the synthetic opioid sufentanil, is interesting – to say the least. Dsuvia is a powerful pain medication, said to be 10 times more potent than fentanyl and 1,000 times more potent than morphine. The medication, developed by AcelRx Pharmaceuticals for use in medically supervised settings, has an indication for moderate to severe pain, and is packaged in single-dose applicators.
The chairperson of the FDA’s Anesthetic and Analgesics Drug Product Advisory Committee, Raeford E. Brown Jr., MD, a professor of pediatric anesthesia at the University of Kentucky, Lexington, could not be present Oct. 12 at the committee vote recommending approval. With the consumer advocacy group Public Citizen, Dr. Brown wrote a letter to FDA leaders detailing concerns about the new formulation of sufentanil.
“It is my observation,” Dr. Brown wrote, “that once the FDA approves an opioid compound, there are no safeguards as to the population that will be exposed, the postmarketing analysis of prescribing behavior, or the ongoing analysis of the risks of the drug to the general population relative to its benefit to the public health. Briefly stated, for all of the opioids that have been marketed in the last 10 years, there has not been sufficient demonstration of safety, nor has there been postmarketing assessment of who is taking the drug, how often prescribing is inappropriate, and whether there was ever a reason to risk the health of the general population by having one more opioid on the market.”
Dr. Brown went on to detail his concerns about sufentanil. In the intravenous formulation, the medication has been in use for more than two decades.
“It is so potent that abusers of this intravenous formulation often die when they inject the first dose; I have witnessed this in resuscitating physicians, medical students, technicians, and other health care providers, some successfully, as a part of my duties as a clinician in a major academic medical center. Because it is so potent, the dosing volume, whether in the IV formulation or the sublingual form, can be quite small. It is thus an extremely divertible drug, and I predict that we will encounter diversion, abuse, and death within the early months of its availability on the market.”
The letter finishes by criticizing the fact that the full Drug Safety and Risk Management Advisory Committee was not invited to the Oct. 12 meeting, and finally, about the ease of diversion among health care professionals – and anesthesiologists in particular.
Meanwhile, Scott Gottlieb, MD, commissioner of the FDA, posted a lengthy explanation on the organization’s website on Nov. 2, after the vote. In his statement on the agency’s approval of Dsuvia and the FDA’s future consideration of new opioids, Dr. Gottlieb explains: “To address concerns about the potential risks associated with Dsuvia, this product will have strong limitations on its use. It can’t be dispensed to patients for home use and should not be used for more than 72 hours. And it should only be administered by a health care provider using a single-dose applicator. That means it won’t be available at retail pharmacies for patients to take home. These measures to restrict the use of this product only within a supervised health care setting, and not for home use, are important steps to help prevent misuse and abuse of Dsuvia, as well reduce the potential for diversion. Because of the risks of addiction, abuse, and misuse with opioids, Dsuvia also is to be reserved for use in patients for whom alternative pain treatment options have not been tolerated, or are not expected to be tolerated, where existing treatment options have not provided adequate analgesia, or where these alternatives are not expected to provide adequate analgesia.”
In addition to the statement posted on the FDA’s website, Dr. Gottlieb made the approval of Dsuvia the topic of his weekly #SundayTweetorial on Nov. 4. In this venue, Dr. Gottlieb posts tweets on a single topic. On both Twitter and the FDA website, he noted that a major factor in the approval of Dsuvia was advantages it might convey for pain control to soldiers on the battlefield, where oral medications might take time to work and intravenous access might not be possible.
One tweet read: “Whether there’s a need for another powerful opioid in the throes of a massive crisis of addiction is a critical question. As a public health agency, we have an obligation to address this question for patients with pain, for the addiction crisis, for innovators, for all Americans.”
Another tweet stated, “While Dsuvia brings another highly potent opioid to market it fulfills a limited, unmet medical need in treating our nation’s soldiers on the battlefield. That’s why the Pentagon worked closely with the sponsor on developing Dsuvia. FDA committed to prioritize needs of our troops.”
in possible deaths from misdirected use of a very potent agent. And while the new opioid may have been geared toward unmet military needs, Dsuvia will be available for use in civilian medical facilities as well.
There is some irony to the idea that a pharmaceutical company would continue to develop opioids when there is so much need for nonaddictive agents for pain control and so much pressure on physicians to limit access of opiates to pain patients. We are left to stand by and watch as yet another potent opioid preparation is introduced.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016), and assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore.
The timing of the Food and Drug Administration’s Nov. 2 approval of the medication Dsuvia, a sublingual formulation of the synthetic opioid sufentanil, is interesting – to say the least. Dsuvia is a powerful pain medication, said to be 10 times more potent than fentanyl and 1,000 times more potent than morphine. The medication, developed by AcelRx Pharmaceuticals for use in medically supervised settings, has an indication for moderate to severe pain, and is packaged in single-dose applicators.
The chairperson of the FDA’s Anesthetic and Analgesics Drug Product Advisory Committee, Raeford E. Brown Jr., MD, a professor of pediatric anesthesia at the University of Kentucky, Lexington, could not be present Oct. 12 at the committee vote recommending approval. With the consumer advocacy group Public Citizen, Dr. Brown wrote a letter to FDA leaders detailing concerns about the new formulation of sufentanil.
“It is my observation,” Dr. Brown wrote, “that once the FDA approves an opioid compound, there are no safeguards as to the population that will be exposed, the postmarketing analysis of prescribing behavior, or the ongoing analysis of the risks of the drug to the general population relative to its benefit to the public health. Briefly stated, for all of the opioids that have been marketed in the last 10 years, there has not been sufficient demonstration of safety, nor has there been postmarketing assessment of who is taking the drug, how often prescribing is inappropriate, and whether there was ever a reason to risk the health of the general population by having one more opioid on the market.”
Dr. Brown went on to detail his concerns about sufentanil. In the intravenous formulation, the medication has been in use for more than two decades.
“It is so potent that abusers of this intravenous formulation often die when they inject the first dose; I have witnessed this in resuscitating physicians, medical students, technicians, and other health care providers, some successfully, as a part of my duties as a clinician in a major academic medical center. Because it is so potent, the dosing volume, whether in the IV formulation or the sublingual form, can be quite small. It is thus an extremely divertible drug, and I predict that we will encounter diversion, abuse, and death within the early months of its availability on the market.”
The letter finishes by criticizing the fact that the full Drug Safety and Risk Management Advisory Committee was not invited to the Oct. 12 meeting, and finally, about the ease of diversion among health care professionals – and anesthesiologists in particular.
Meanwhile, Scott Gottlieb, MD, commissioner of the FDA, posted a lengthy explanation on the organization’s website on Nov. 2, after the vote. In his statement on the agency’s approval of Dsuvia and the FDA’s future consideration of new opioids, Dr. Gottlieb explains: “To address concerns about the potential risks associated with Dsuvia, this product will have strong limitations on its use. It can’t be dispensed to patients for home use and should not be used for more than 72 hours. And it should only be administered by a health care provider using a single-dose applicator. That means it won’t be available at retail pharmacies for patients to take home. These measures to restrict the use of this product only within a supervised health care setting, and not for home use, are important steps to help prevent misuse and abuse of Dsuvia, as well reduce the potential for diversion. Because of the risks of addiction, abuse, and misuse with opioids, Dsuvia also is to be reserved for use in patients for whom alternative pain treatment options have not been tolerated, or are not expected to be tolerated, where existing treatment options have not provided adequate analgesia, or where these alternatives are not expected to provide adequate analgesia.”
In addition to the statement posted on the FDA’s website, Dr. Gottlieb made the approval of Dsuvia the topic of his weekly #SundayTweetorial on Nov. 4. In this venue, Dr. Gottlieb posts tweets on a single topic. On both Twitter and the FDA website, he noted that a major factor in the approval of Dsuvia was advantages it might convey for pain control to soldiers on the battlefield, where oral medications might take time to work and intravenous access might not be possible.
One tweet read: “Whether there’s a need for another powerful opioid in the throes of a massive crisis of addiction is a critical question. As a public health agency, we have an obligation to address this question for patients with pain, for the addiction crisis, for innovators, for all Americans.”
Another tweet stated, “While Dsuvia brings another highly potent opioid to market it fulfills a limited, unmet medical need in treating our nation’s soldiers on the battlefield. That’s why the Pentagon worked closely with the sponsor on developing Dsuvia. FDA committed to prioritize needs of our troops.”
in possible deaths from misdirected use of a very potent agent. And while the new opioid may have been geared toward unmet military needs, Dsuvia will be available for use in civilian medical facilities as well.
There is some irony to the idea that a pharmaceutical company would continue to develop opioids when there is so much need for nonaddictive agents for pain control and so much pressure on physicians to limit access of opiates to pain patients. We are left to stand by and watch as yet another potent opioid preparation is introduced.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016), and assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore.
The timing of the Food and Drug Administration’s Nov. 2 approval of the medication Dsuvia, a sublingual formulation of the synthetic opioid sufentanil, is interesting – to say the least. Dsuvia is a powerful pain medication, said to be 10 times more potent than fentanyl and 1,000 times more potent than morphine. The medication, developed by AcelRx Pharmaceuticals for use in medically supervised settings, has an indication for moderate to severe pain, and is packaged in single-dose applicators.
The chairperson of the FDA’s Anesthetic and Analgesics Drug Product Advisory Committee, Raeford E. Brown Jr., MD, a professor of pediatric anesthesia at the University of Kentucky, Lexington, could not be present Oct. 12 at the committee vote recommending approval. With the consumer advocacy group Public Citizen, Dr. Brown wrote a letter to FDA leaders detailing concerns about the new formulation of sufentanil.
“It is my observation,” Dr. Brown wrote, “that once the FDA approves an opioid compound, there are no safeguards as to the population that will be exposed, the postmarketing analysis of prescribing behavior, or the ongoing analysis of the risks of the drug to the general population relative to its benefit to the public health. Briefly stated, for all of the opioids that have been marketed in the last 10 years, there has not been sufficient demonstration of safety, nor has there been postmarketing assessment of who is taking the drug, how often prescribing is inappropriate, and whether there was ever a reason to risk the health of the general population by having one more opioid on the market.”
Dr. Brown went on to detail his concerns about sufentanil. In the intravenous formulation, the medication has been in use for more than two decades.
“It is so potent that abusers of this intravenous formulation often die when they inject the first dose; I have witnessed this in resuscitating physicians, medical students, technicians, and other health care providers, some successfully, as a part of my duties as a clinician in a major academic medical center. Because it is so potent, the dosing volume, whether in the IV formulation or the sublingual form, can be quite small. It is thus an extremely divertible drug, and I predict that we will encounter diversion, abuse, and death within the early months of its availability on the market.”
The letter finishes by criticizing the fact that the full Drug Safety and Risk Management Advisory Committee was not invited to the Oct. 12 meeting, and finally, about the ease of diversion among health care professionals – and anesthesiologists in particular.
Meanwhile, Scott Gottlieb, MD, commissioner of the FDA, posted a lengthy explanation on the organization’s website on Nov. 2, after the vote. In his statement on the agency’s approval of Dsuvia and the FDA’s future consideration of new opioids, Dr. Gottlieb explains: “To address concerns about the potential risks associated with Dsuvia, this product will have strong limitations on its use. It can’t be dispensed to patients for home use and should not be used for more than 72 hours. And it should only be administered by a health care provider using a single-dose applicator. That means it won’t be available at retail pharmacies for patients to take home. These measures to restrict the use of this product only within a supervised health care setting, and not for home use, are important steps to help prevent misuse and abuse of Dsuvia, as well reduce the potential for diversion. Because of the risks of addiction, abuse, and misuse with opioids, Dsuvia also is to be reserved for use in patients for whom alternative pain treatment options have not been tolerated, or are not expected to be tolerated, where existing treatment options have not provided adequate analgesia, or where these alternatives are not expected to provide adequate analgesia.”
In addition to the statement posted on the FDA’s website, Dr. Gottlieb made the approval of Dsuvia the topic of his weekly #SundayTweetorial on Nov. 4. In this venue, Dr. Gottlieb posts tweets on a single topic. On both Twitter and the FDA website, he noted that a major factor in the approval of Dsuvia was advantages it might convey for pain control to soldiers on the battlefield, where oral medications might take time to work and intravenous access might not be possible.
One tweet read: “Whether there’s a need for another powerful opioid in the throes of a massive crisis of addiction is a critical question. As a public health agency, we have an obligation to address this question for patients with pain, for the addiction crisis, for innovators, for all Americans.”
Another tweet stated, “While Dsuvia brings another highly potent opioid to market it fulfills a limited, unmet medical need in treating our nation’s soldiers on the battlefield. That’s why the Pentagon worked closely with the sponsor on developing Dsuvia. FDA committed to prioritize needs of our troops.”
in possible deaths from misdirected use of a very potent agent. And while the new opioid may have been geared toward unmet military needs, Dsuvia will be available for use in civilian medical facilities as well.
There is some irony to the idea that a pharmaceutical company would continue to develop opioids when there is so much need for nonaddictive agents for pain control and so much pressure on physicians to limit access of opiates to pain patients. We are left to stand by and watch as yet another potent opioid preparation is introduced.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016), and assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore.
Complications cluster in inflammatory arthritis patients after total knee replacement
CHICAGO – Patients with an inflammatory arthritis had significantly higher rates of infections, transfusions, and readmissions following total knee replacement than did patients without inflammatory arthritis in a study of more than 137,000 Americans who underwent this surgery.
A sampling of U.S. patients who underwent total knee arthroplasty (TKA) during 2007-2016 showed that among the small percentage of these patients who had an inflammatory arthritis (IA), the rate of periprosthetic joint or wound infection while hospitalized or out to 30 days after surgery was a statistically significant 64% higher relative to patients without inflammatory arthritis, after adjustment for several demographic and clinical confounders, including recent glucocorticoid treatment, Susan M. Goodman, MD, said at the annual meeting of the American College of Rheumatology. The analysis also showed a statistically significant 46% higher relative rate of hospital readmission for any cause during the 90 days after surgery, and a significant 39% relative increase in blood transfusions during the 30 days after TKA in the IA patients.
“These results have important implications for evolving bundled payment models” for TKA, said Dr. Goodman, a rheumatologist at the Hospital for Special Surgery in New York. “Hospitals should receive commensurate resources to maintain access to total TKA for patients with IA.”
For this analysis, Dr. Goodman and her associates classified IA as a patient with a recorded diagnosis of rheumatoid arthritis, spondyloarthritis, or systemic lupus erythematosus if the patient had also received treatment during the year before surgery with a disease-modifying antirheumatic drug, a biologic agent, or a drug that treats systemic lupus erythematosus.
Complications following TKA became a particular concern to hospitals starting in 2013 when the Centers for Medicare & Medicaid Services began a program that penalized hospitals for outcomes such as excessive readmissions following selected types of hospitalizations and also with recent steps to bundle TKA reimbursement with related 90-day outcomes.
“My concern is to ensure that patients with IA aren’t penalized and can maintain access” to TKA despite recent policy moves by the CMS. Faced with potential disincentives to treat patients with an IA, “hospitals might cherry pick patients,” Dr. Goodman said in an interview. The new findings “are a reason for administrators to argue for patients with IA to come out of the cost bundle.”
Dr. Goodman expressed hope that future policies will better reflect the higher levels of risk faced by patients with an IA undergoing TKA. CMS “is pretty responsive,” she said.
The study used data collected by Humana for about 25 million American health insurance beneficiaries during 2007-2016, which included 137,550 people who underwent a TKA. Of these, 3,067 (2%) met the study’s definition for IA, and 134,483 did not. Most of those who did not meet the definition likely had osteoarthritis, Dr. Goodman said. This low percentage of U.S. TKA patients with IA was consistent with numbers in prior reports.
The researchers calculated the relative risk of the IA patients, compared with all the others, for nine potential complications, including acute MI, pneumonia, sepsis, pulmonary embolism, and death. The complications with significantly higher rates among the IA patients after confounder adjustment were 30-day infections, 30-day transfusions, and 90-day readmissions.
Dr. Goodman had no relevant disclosures.
SOURCE: Richardson S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1932.
CHICAGO – Patients with an inflammatory arthritis had significantly higher rates of infections, transfusions, and readmissions following total knee replacement than did patients without inflammatory arthritis in a study of more than 137,000 Americans who underwent this surgery.
A sampling of U.S. patients who underwent total knee arthroplasty (TKA) during 2007-2016 showed that among the small percentage of these patients who had an inflammatory arthritis (IA), the rate of periprosthetic joint or wound infection while hospitalized or out to 30 days after surgery was a statistically significant 64% higher relative to patients without inflammatory arthritis, after adjustment for several demographic and clinical confounders, including recent glucocorticoid treatment, Susan M. Goodman, MD, said at the annual meeting of the American College of Rheumatology. The analysis also showed a statistically significant 46% higher relative rate of hospital readmission for any cause during the 90 days after surgery, and a significant 39% relative increase in blood transfusions during the 30 days after TKA in the IA patients.
“These results have important implications for evolving bundled payment models” for TKA, said Dr. Goodman, a rheumatologist at the Hospital for Special Surgery in New York. “Hospitals should receive commensurate resources to maintain access to total TKA for patients with IA.”
For this analysis, Dr. Goodman and her associates classified IA as a patient with a recorded diagnosis of rheumatoid arthritis, spondyloarthritis, or systemic lupus erythematosus if the patient had also received treatment during the year before surgery with a disease-modifying antirheumatic drug, a biologic agent, or a drug that treats systemic lupus erythematosus.
Complications following TKA became a particular concern to hospitals starting in 2013 when the Centers for Medicare & Medicaid Services began a program that penalized hospitals for outcomes such as excessive readmissions following selected types of hospitalizations and also with recent steps to bundle TKA reimbursement with related 90-day outcomes.
“My concern is to ensure that patients with IA aren’t penalized and can maintain access” to TKA despite recent policy moves by the CMS. Faced with potential disincentives to treat patients with an IA, “hospitals might cherry pick patients,” Dr. Goodman said in an interview. The new findings “are a reason for administrators to argue for patients with IA to come out of the cost bundle.”
Dr. Goodman expressed hope that future policies will better reflect the higher levels of risk faced by patients with an IA undergoing TKA. CMS “is pretty responsive,” she said.
The study used data collected by Humana for about 25 million American health insurance beneficiaries during 2007-2016, which included 137,550 people who underwent a TKA. Of these, 3,067 (2%) met the study’s definition for IA, and 134,483 did not. Most of those who did not meet the definition likely had osteoarthritis, Dr. Goodman said. This low percentage of U.S. TKA patients with IA was consistent with numbers in prior reports.
The researchers calculated the relative risk of the IA patients, compared with all the others, for nine potential complications, including acute MI, pneumonia, sepsis, pulmonary embolism, and death. The complications with significantly higher rates among the IA patients after confounder adjustment were 30-day infections, 30-day transfusions, and 90-day readmissions.
Dr. Goodman had no relevant disclosures.
SOURCE: Richardson S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1932.
CHICAGO – Patients with an inflammatory arthritis had significantly higher rates of infections, transfusions, and readmissions following total knee replacement than did patients without inflammatory arthritis in a study of more than 137,000 Americans who underwent this surgery.
A sampling of U.S. patients who underwent total knee arthroplasty (TKA) during 2007-2016 showed that among the small percentage of these patients who had an inflammatory arthritis (IA), the rate of periprosthetic joint or wound infection while hospitalized or out to 30 days after surgery was a statistically significant 64% higher relative to patients without inflammatory arthritis, after adjustment for several demographic and clinical confounders, including recent glucocorticoid treatment, Susan M. Goodman, MD, said at the annual meeting of the American College of Rheumatology. The analysis also showed a statistically significant 46% higher relative rate of hospital readmission for any cause during the 90 days after surgery, and a significant 39% relative increase in blood transfusions during the 30 days after TKA in the IA patients.
“These results have important implications for evolving bundled payment models” for TKA, said Dr. Goodman, a rheumatologist at the Hospital for Special Surgery in New York. “Hospitals should receive commensurate resources to maintain access to total TKA for patients with IA.”
For this analysis, Dr. Goodman and her associates classified IA as a patient with a recorded diagnosis of rheumatoid arthritis, spondyloarthritis, or systemic lupus erythematosus if the patient had also received treatment during the year before surgery with a disease-modifying antirheumatic drug, a biologic agent, or a drug that treats systemic lupus erythematosus.
Complications following TKA became a particular concern to hospitals starting in 2013 when the Centers for Medicare & Medicaid Services began a program that penalized hospitals for outcomes such as excessive readmissions following selected types of hospitalizations and also with recent steps to bundle TKA reimbursement with related 90-day outcomes.
“My concern is to ensure that patients with IA aren’t penalized and can maintain access” to TKA despite recent policy moves by the CMS. Faced with potential disincentives to treat patients with an IA, “hospitals might cherry pick patients,” Dr. Goodman said in an interview. The new findings “are a reason for administrators to argue for patients with IA to come out of the cost bundle.”
Dr. Goodman expressed hope that future policies will better reflect the higher levels of risk faced by patients with an IA undergoing TKA. CMS “is pretty responsive,” she said.
The study used data collected by Humana for about 25 million American health insurance beneficiaries during 2007-2016, which included 137,550 people who underwent a TKA. Of these, 3,067 (2%) met the study’s definition for IA, and 134,483 did not. Most of those who did not meet the definition likely had osteoarthritis, Dr. Goodman said. This low percentage of U.S. TKA patients with IA was consistent with numbers in prior reports.
The researchers calculated the relative risk of the IA patients, compared with all the others, for nine potential complications, including acute MI, pneumonia, sepsis, pulmonary embolism, and death. The complications with significantly higher rates among the IA patients after confounder adjustment were 30-day infections, 30-day transfusions, and 90-day readmissions.
Dr. Goodman had no relevant disclosures.
SOURCE: Richardson S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1932.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Complications were more common after total knee arthroplasty in patients with an inflammatory arthritis.
Major finding: Inflammatory arthritis patients had a 64% higher rate of infections after total knee arthroplasty, compared with patients without inflammatory arthritis.
Study details: Data analysis for 137,550 Americans who underwent total knee arthroplasty during 2007-2016.
Disclosures: Dr. Goodman had no relevant disclosures.
Source: Richardson S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1932.
Ultrasound excels for assessing shoulder dislocation
SAN DIEGO – Point-of-care ultrasound should be the go-to approach for the routine assessment of suspected shoulder dislocations in the ED, based on data from a prospective, multicenter study presented at the annual meeting of the American College of Emergency Physicians.
In the observational study, the average time needed to diagnose shoulder dislocation using ultrasound was 18 seconds, far faster than time from triage to x-ray, according to Michael Secko, MD, director of the emergency ultrasound division at Stony Brook University (NY).
The results from this study, called MUDDS (Musculoskeletal Ultrasound to Diagnose Dislocated Shoulders), support point-of-care ultrasound as a faster and more readily performed alternative to x-ray. Of the 46 adult patients enrolled so far in the ongoing study, ultrasound’s sensitivity has been 96% and its specificity 100% when validated by x-ray findings.
In the study, adults presenting to the ED are evaluated with point-of-care ultrasound from a posterior approach using either a curvilinear or linear transducer in the transverse plane. About half of the patients enrolled so far had injuries caused by falls, and many of the others had a shoulder complaint related to being pulled. Slightly more than one-third had a previous shoulder dislocation.
When evaluated with point-of-care ultrasound and x-ray, 23 of the 42 evaluable patients had a dislocation. The time from triage to ultrasound evaluation averaged 60 minutes, 40 minutes faster than the average of 100 minutes from triage to x-ray. Both tests were ordered at the same time.
Ultrasound performed less well for the diagnosis of a fracture, with a sensitivity of only 53%. Dr. Secko said the anterior approach would not be expected to provide a comprehensive assessment for fracture. He noted, for example, that there was no attempt in this study to evaluate patients for the presence of Bankart lesions. However, in those found to have a fracture on point-of-care ultrasound, the specificity of this imaging tool was 96%.
Ultimately, a major goal of this study was to determine whether a posterior point-of-care ultrasound could provide a quick answer to the question, “is it in or out?” Although patients are still being enrolled, Dr. Secko believed there is already good evidence that ultrasound is fast and effective for diagnosing dislocations.
Others have addressed this same question. Citing a meta-analysis published last year, Dr. Secko reported that all but one of four studies evaluating ultrasound for shoulder dislocations found a sensitivity and specificity of 100% (Gottlieb M et al. West J Emerg Med. 2017 Aug;18[5]:937-942).
Many centers have already switched to ultrasound for the evaluation of shoulder dislocations, according to Andrew S. Liteplo, MD, who moderated the ACEP session in which Dr. Secko presented his data. “If you are not already doing this for suspected shoulder dislocation, start right away because it is easy and awesome,” said Dr. Liteplo, who is chief of the division of ultrasound in emergency medicine at Massachusetts General Hospital, Boston. He also advised that ultrasound can also can be performed after reduction to confirm the efficacy of treatment.
Dr. Secko reported no financial relationships relevant to this study.
SAN DIEGO – Point-of-care ultrasound should be the go-to approach for the routine assessment of suspected shoulder dislocations in the ED, based on data from a prospective, multicenter study presented at the annual meeting of the American College of Emergency Physicians.
In the observational study, the average time needed to diagnose shoulder dislocation using ultrasound was 18 seconds, far faster than time from triage to x-ray, according to Michael Secko, MD, director of the emergency ultrasound division at Stony Brook University (NY).
The results from this study, called MUDDS (Musculoskeletal Ultrasound to Diagnose Dislocated Shoulders), support point-of-care ultrasound as a faster and more readily performed alternative to x-ray. Of the 46 adult patients enrolled so far in the ongoing study, ultrasound’s sensitivity has been 96% and its specificity 100% when validated by x-ray findings.
In the study, adults presenting to the ED are evaluated with point-of-care ultrasound from a posterior approach using either a curvilinear or linear transducer in the transverse plane. About half of the patients enrolled so far had injuries caused by falls, and many of the others had a shoulder complaint related to being pulled. Slightly more than one-third had a previous shoulder dislocation.
When evaluated with point-of-care ultrasound and x-ray, 23 of the 42 evaluable patients had a dislocation. The time from triage to ultrasound evaluation averaged 60 minutes, 40 minutes faster than the average of 100 minutes from triage to x-ray. Both tests were ordered at the same time.
Ultrasound performed less well for the diagnosis of a fracture, with a sensitivity of only 53%. Dr. Secko said the anterior approach would not be expected to provide a comprehensive assessment for fracture. He noted, for example, that there was no attempt in this study to evaluate patients for the presence of Bankart lesions. However, in those found to have a fracture on point-of-care ultrasound, the specificity of this imaging tool was 96%.
Ultimately, a major goal of this study was to determine whether a posterior point-of-care ultrasound could provide a quick answer to the question, “is it in or out?” Although patients are still being enrolled, Dr. Secko believed there is already good evidence that ultrasound is fast and effective for diagnosing dislocations.
Others have addressed this same question. Citing a meta-analysis published last year, Dr. Secko reported that all but one of four studies evaluating ultrasound for shoulder dislocations found a sensitivity and specificity of 100% (Gottlieb M et al. West J Emerg Med. 2017 Aug;18[5]:937-942).
Many centers have already switched to ultrasound for the evaluation of shoulder dislocations, according to Andrew S. Liteplo, MD, who moderated the ACEP session in which Dr. Secko presented his data. “If you are not already doing this for suspected shoulder dislocation, start right away because it is easy and awesome,” said Dr. Liteplo, who is chief of the division of ultrasound in emergency medicine at Massachusetts General Hospital, Boston. He also advised that ultrasound can also can be performed after reduction to confirm the efficacy of treatment.
Dr. Secko reported no financial relationships relevant to this study.
SAN DIEGO – Point-of-care ultrasound should be the go-to approach for the routine assessment of suspected shoulder dislocations in the ED, based on data from a prospective, multicenter study presented at the annual meeting of the American College of Emergency Physicians.
In the observational study, the average time needed to diagnose shoulder dislocation using ultrasound was 18 seconds, far faster than time from triage to x-ray, according to Michael Secko, MD, director of the emergency ultrasound division at Stony Brook University (NY).
The results from this study, called MUDDS (Musculoskeletal Ultrasound to Diagnose Dislocated Shoulders), support point-of-care ultrasound as a faster and more readily performed alternative to x-ray. Of the 46 adult patients enrolled so far in the ongoing study, ultrasound’s sensitivity has been 96% and its specificity 100% when validated by x-ray findings.
In the study, adults presenting to the ED are evaluated with point-of-care ultrasound from a posterior approach using either a curvilinear or linear transducer in the transverse plane. About half of the patients enrolled so far had injuries caused by falls, and many of the others had a shoulder complaint related to being pulled. Slightly more than one-third had a previous shoulder dislocation.
When evaluated with point-of-care ultrasound and x-ray, 23 of the 42 evaluable patients had a dislocation. The time from triage to ultrasound evaluation averaged 60 minutes, 40 minutes faster than the average of 100 minutes from triage to x-ray. Both tests were ordered at the same time.
Ultrasound performed less well for the diagnosis of a fracture, with a sensitivity of only 53%. Dr. Secko said the anterior approach would not be expected to provide a comprehensive assessment for fracture. He noted, for example, that there was no attempt in this study to evaluate patients for the presence of Bankart lesions. However, in those found to have a fracture on point-of-care ultrasound, the specificity of this imaging tool was 96%.
Ultimately, a major goal of this study was to determine whether a posterior point-of-care ultrasound could provide a quick answer to the question, “is it in or out?” Although patients are still being enrolled, Dr. Secko believed there is already good evidence that ultrasound is fast and effective for diagnosing dislocations.
Others have addressed this same question. Citing a meta-analysis published last year, Dr. Secko reported that all but one of four studies evaluating ultrasound for shoulder dislocations found a sensitivity and specificity of 100% (Gottlieb M et al. West J Emerg Med. 2017 Aug;18[5]:937-942).
Many centers have already switched to ultrasound for the evaluation of shoulder dislocations, according to Andrew S. Liteplo, MD, who moderated the ACEP session in which Dr. Secko presented his data. “If you are not already doing this for suspected shoulder dislocation, start right away because it is easy and awesome,” said Dr. Liteplo, who is chief of the division of ultrasound in emergency medicine at Massachusetts General Hospital, Boston. He also advised that ultrasound can also can be performed after reduction to confirm the efficacy of treatment.
Dr. Secko reported no financial relationships relevant to this study.
REPORTING FROM ACEP18
Key clinical point: Point-of-care ultrasound is accurate, simple, and fast, relative to x-ray, for the evaluation of shoulder dislocation.
Major finding: Based on results from 42 patients, time from triage to ultrasound, which had a sensitivity of 96% and specificity of 100%, was 60 minutes versus 100 minutes for x-ray.
Study details: An ongoing prospective, multicenter, observational study.
Disclosures: Dr. Secko reported no financial relationships relevant to this study.
Nipple-sparing mastectomy safe in older patients
BOSTON – For women undergoing results of recent studies suggest.
The procedure was “surgically safe” in older patients, with complication rates comparable to those seen in younger patients, Solange E. Cox, MD, of MedStar Georgetown University Hospital, Washington, said in a presentation of one those two retrospective analyses at the annual clinical congress of the American College of Surgeons.
“From this, we think that eligible older patients should be offered a nipple-sparing mastectomy as a surgical option for breast cancer, and age alone should not be used as criteria to exclude these patients from the option,” she said.
The second retrospective study showed that patients undergoing neoadjuvant chemotherapy had a rate of surgical complications and unintended reoperations comparable to what was seen in women undergoing primary surgery.
“Our big-picture takeaway from this study is that receipt of neoadjuvant chemotherapy is not a contraindication for nipple-sparing mastectomy,” said investigator Alex J. Bartholomew, MS, also of Medstar Georgetown University Hospital.
Mr. Bartholomew’s conclusion was based on an analysis of the nipple-sparing mastectomy registry of the American Society of Breast Surgeons that included a total of 3,125 breasts. Neoadjuvant chemotherapy was used in 528, or 16.9%, while primary surgery was performed in 2,597, or 83.1%.
The overall rate of complications was 11%, with nonsignificant differences between the neoadjuvant chemotherapy and primary surgery groups at 12.7% and 10.7%, respectively.
The rate of unintended reoperation, at 4.9%, was not significantly different in the neoadjuvant chemotherapy and primary surgery groups, at 5.2% and 4.8%, Mr. Bartholomew said. Similarly, he found that the rate of nipple areolar complex loss of 1% overall was not different between groups.
Advanced age was likewise not associated with increased complications in the study presented by Dr. Cox, which was a retrospective review of data for patients undergoing nipple-sparing mastectomy from 1998 to 2015 at a single institution. That cohort included 38 patients age 60 years or older, and 358 younger patients.
The rate of complications was 15.5% for patients over age 60 years, and similarly, 13.0% for their younger counterparts (P = .590), Dr. Cox reported. Likewise, the rate of unintended operations was 13.3% and 15.3% for older and younger patients, respectively (P = .274).
These findings are important because advancing age has been associated with a decrease in the likelihood of nipple-sparing mastectomy, according to Dr. Cox.
For mastectomies in general, advanced age has been implicated as a potential risk factor for necrosis, technical complications, and poor outcomes with mastectomies. However, no prior studies had been done specifically to evaluate nipple-sparing mastectomies in older breast cancer patients, Dr. Cox said.
Nipple-sparing mastectomy provides both cosmetic and psychosocial benefits to patients, according to the researchers, because the procedure spares the nipple-areolar complex.
The researchers who had no relevant disclosures.
SOURCES: Cox S et al. SF310 abstract; Bartholomew AJ et al. SF310 abstract ACS Clinical Congress 2018
BOSTON – For women undergoing results of recent studies suggest.
The procedure was “surgically safe” in older patients, with complication rates comparable to those seen in younger patients, Solange E. Cox, MD, of MedStar Georgetown University Hospital, Washington, said in a presentation of one those two retrospective analyses at the annual clinical congress of the American College of Surgeons.
“From this, we think that eligible older patients should be offered a nipple-sparing mastectomy as a surgical option for breast cancer, and age alone should not be used as criteria to exclude these patients from the option,” she said.
The second retrospective study showed that patients undergoing neoadjuvant chemotherapy had a rate of surgical complications and unintended reoperations comparable to what was seen in women undergoing primary surgery.
“Our big-picture takeaway from this study is that receipt of neoadjuvant chemotherapy is not a contraindication for nipple-sparing mastectomy,” said investigator Alex J. Bartholomew, MS, also of Medstar Georgetown University Hospital.
Mr. Bartholomew’s conclusion was based on an analysis of the nipple-sparing mastectomy registry of the American Society of Breast Surgeons that included a total of 3,125 breasts. Neoadjuvant chemotherapy was used in 528, or 16.9%, while primary surgery was performed in 2,597, or 83.1%.
The overall rate of complications was 11%, with nonsignificant differences between the neoadjuvant chemotherapy and primary surgery groups at 12.7% and 10.7%, respectively.
The rate of unintended reoperation, at 4.9%, was not significantly different in the neoadjuvant chemotherapy and primary surgery groups, at 5.2% and 4.8%, Mr. Bartholomew said. Similarly, he found that the rate of nipple areolar complex loss of 1% overall was not different between groups.
Advanced age was likewise not associated with increased complications in the study presented by Dr. Cox, which was a retrospective review of data for patients undergoing nipple-sparing mastectomy from 1998 to 2015 at a single institution. That cohort included 38 patients age 60 years or older, and 358 younger patients.
The rate of complications was 15.5% for patients over age 60 years, and similarly, 13.0% for their younger counterparts (P = .590), Dr. Cox reported. Likewise, the rate of unintended operations was 13.3% and 15.3% for older and younger patients, respectively (P = .274).
These findings are important because advancing age has been associated with a decrease in the likelihood of nipple-sparing mastectomy, according to Dr. Cox.
For mastectomies in general, advanced age has been implicated as a potential risk factor for necrosis, technical complications, and poor outcomes with mastectomies. However, no prior studies had been done specifically to evaluate nipple-sparing mastectomies in older breast cancer patients, Dr. Cox said.
Nipple-sparing mastectomy provides both cosmetic and psychosocial benefits to patients, according to the researchers, because the procedure spares the nipple-areolar complex.
The researchers who had no relevant disclosures.
SOURCES: Cox S et al. SF310 abstract; Bartholomew AJ et al. SF310 abstract ACS Clinical Congress 2018
BOSTON – For women undergoing results of recent studies suggest.
The procedure was “surgically safe” in older patients, with complication rates comparable to those seen in younger patients, Solange E. Cox, MD, of MedStar Georgetown University Hospital, Washington, said in a presentation of one those two retrospective analyses at the annual clinical congress of the American College of Surgeons.
“From this, we think that eligible older patients should be offered a nipple-sparing mastectomy as a surgical option for breast cancer, and age alone should not be used as criteria to exclude these patients from the option,” she said.
The second retrospective study showed that patients undergoing neoadjuvant chemotherapy had a rate of surgical complications and unintended reoperations comparable to what was seen in women undergoing primary surgery.
“Our big-picture takeaway from this study is that receipt of neoadjuvant chemotherapy is not a contraindication for nipple-sparing mastectomy,” said investigator Alex J. Bartholomew, MS, also of Medstar Georgetown University Hospital.
Mr. Bartholomew’s conclusion was based on an analysis of the nipple-sparing mastectomy registry of the American Society of Breast Surgeons that included a total of 3,125 breasts. Neoadjuvant chemotherapy was used in 528, or 16.9%, while primary surgery was performed in 2,597, or 83.1%.
The overall rate of complications was 11%, with nonsignificant differences between the neoadjuvant chemotherapy and primary surgery groups at 12.7% and 10.7%, respectively.
The rate of unintended reoperation, at 4.9%, was not significantly different in the neoadjuvant chemotherapy and primary surgery groups, at 5.2% and 4.8%, Mr. Bartholomew said. Similarly, he found that the rate of nipple areolar complex loss of 1% overall was not different between groups.
Advanced age was likewise not associated with increased complications in the study presented by Dr. Cox, which was a retrospective review of data for patients undergoing nipple-sparing mastectomy from 1998 to 2015 at a single institution. That cohort included 38 patients age 60 years or older, and 358 younger patients.
The rate of complications was 15.5% for patients over age 60 years, and similarly, 13.0% for their younger counterparts (P = .590), Dr. Cox reported. Likewise, the rate of unintended operations was 13.3% and 15.3% for older and younger patients, respectively (P = .274).
These findings are important because advancing age has been associated with a decrease in the likelihood of nipple-sparing mastectomy, according to Dr. Cox.
For mastectomies in general, advanced age has been implicated as a potential risk factor for necrosis, technical complications, and poor outcomes with mastectomies. However, no prior studies had been done specifically to evaluate nipple-sparing mastectomies in older breast cancer patients, Dr. Cox said.
Nipple-sparing mastectomy provides both cosmetic and psychosocial benefits to patients, according to the researchers, because the procedure spares the nipple-areolar complex.
The researchers who had no relevant disclosures.
SOURCES: Cox S et al. SF310 abstract; Bartholomew AJ et al. SF310 abstract ACS Clinical Congress 2018
REPORTING FROM THE ACS CLINICAL CONGRESS
Key clinical point: Neoadjuvant chemotherapy and advancing age were not associated with increased rates of complications in women undergoing nipple-sparing mastectomy in two recent studies.
Major finding: The overall rate of complications was not significantly different for neoadjuvant chemotherapy vs. primary surgery (12.7% vs. 10.7%) or for age over 60 years vs. younger age (15.5% vs. 13.0%).
Study details: Retrospective studies of a nipple-sparing mastectomy registry including more than 3,000 breasts (neoadjuvant chemotherapy vs. primary surgery study) and a single-institution study of nearly 400 patients (older vs. younger study).
Disclosures: The authors reported no conflicts of interest.
Source: Cox S et al. and Bartholomew AJ et al. Session SF310 ACS Clinical Congress 2018.
FDA approves sufentanil for adults with acute pain
The Food and Drug Administration on Nov. 2 approved sufentanil (Dsuvia) for managing acute pain in adult patients in certified, medically supervised health care settings.
Sufentanil, an opioid analgesic manufactured by AcelRx Pharmaceuticals, was approved as a 30-mcg sublingual tablet. The efficacy of Dsuvia was shown in a randomized, clinical trial where patients who received the drug demonstrated significantly greater pain relief after both 15 minutes and 12 hours, compared with placebo.
“As a single-dose, noninvasive medication with a rapid reduction in pain intensity, Dsuvia represents an important alternative for health care providers to offer patients for acute pain management,” David Leiman, MD, of the department of surgery at the University of Texas, Houston, said in the AcelRx press statement.
FDA Commissioner Scott Gottlieb, MD, commented on the approval amid concerns expressed by some, such as the advocacy group Public Citizen, that the drug is “more than 1,000 times more potent than morphine,” and that approval could lead to diversion and abuse – particularly in light of the U.S. opioid epidemic.
In his statement, Dr. Gottlieb identified one broad, significant issue. “Why do we need an oral formulation of sufentanil – a more potent form of fentanyl that’s been approved for intravenous and epidural use in the U.S. since 1984 – on the market?”
In particular, he focused on the needs of the military. The Department of Defense has taken interest in sufentanil as it fulfills a small but specific battlefield need, namely as a means of pain relief in battlefield situations where soldiers cannot swallow oral medication and access to intravenous medication is limited.
Dr. Gottlieb made clear that sufentanil was meant only to be taken in controlled settings and will have strong limitations on its use. It cannot be prescribed for home use, and treatment should be limited to 72 hours. It can only be delivered by health care professionals using a single-dose applicator and will not be available in pharmacies. It is only to be used in patients who have not tolerated or are expected not to tolerate alternative methods of pain management.
“The FDA has implemented a REMS [Risk Evaluation and Mitigation Strategy] that reflects the potential risks associated with this product and mandates that Dsuvia will only be made available for use in a certified medically supervised heath care setting, including its use on the battlefield,” Dr. Gottlieb said.
However, he recognized that the debate runs deeper than how the FDA should mitigate risk over a new drug, and “as a public health agency, we have an obligation to address this question openly and directly. As a physician and regulator, I won’t bypass legitimate questions and concerns related to our role in addressing the opioid crisis,” he said.
Find Dr. Gottlieb’s full statement on the FDA website.
The Food and Drug Administration on Nov. 2 approved sufentanil (Dsuvia) for managing acute pain in adult patients in certified, medically supervised health care settings.
Sufentanil, an opioid analgesic manufactured by AcelRx Pharmaceuticals, was approved as a 30-mcg sublingual tablet. The efficacy of Dsuvia was shown in a randomized, clinical trial where patients who received the drug demonstrated significantly greater pain relief after both 15 minutes and 12 hours, compared with placebo.
“As a single-dose, noninvasive medication with a rapid reduction in pain intensity, Dsuvia represents an important alternative for health care providers to offer patients for acute pain management,” David Leiman, MD, of the department of surgery at the University of Texas, Houston, said in the AcelRx press statement.
FDA Commissioner Scott Gottlieb, MD, commented on the approval amid concerns expressed by some, such as the advocacy group Public Citizen, that the drug is “more than 1,000 times more potent than morphine,” and that approval could lead to diversion and abuse – particularly in light of the U.S. opioid epidemic.
In his statement, Dr. Gottlieb identified one broad, significant issue. “Why do we need an oral formulation of sufentanil – a more potent form of fentanyl that’s been approved for intravenous and epidural use in the U.S. since 1984 – on the market?”
In particular, he focused on the needs of the military. The Department of Defense has taken interest in sufentanil as it fulfills a small but specific battlefield need, namely as a means of pain relief in battlefield situations where soldiers cannot swallow oral medication and access to intravenous medication is limited.
Dr. Gottlieb made clear that sufentanil was meant only to be taken in controlled settings and will have strong limitations on its use. It cannot be prescribed for home use, and treatment should be limited to 72 hours. It can only be delivered by health care professionals using a single-dose applicator and will not be available in pharmacies. It is only to be used in patients who have not tolerated or are expected not to tolerate alternative methods of pain management.
“The FDA has implemented a REMS [Risk Evaluation and Mitigation Strategy] that reflects the potential risks associated with this product and mandates that Dsuvia will only be made available for use in a certified medically supervised heath care setting, including its use on the battlefield,” Dr. Gottlieb said.
However, he recognized that the debate runs deeper than how the FDA should mitigate risk over a new drug, and “as a public health agency, we have an obligation to address this question openly and directly. As a physician and regulator, I won’t bypass legitimate questions and concerns related to our role in addressing the opioid crisis,” he said.
Find Dr. Gottlieb’s full statement on the FDA website.
The Food and Drug Administration on Nov. 2 approved sufentanil (Dsuvia) for managing acute pain in adult patients in certified, medically supervised health care settings.
Sufentanil, an opioid analgesic manufactured by AcelRx Pharmaceuticals, was approved as a 30-mcg sublingual tablet. The efficacy of Dsuvia was shown in a randomized, clinical trial where patients who received the drug demonstrated significantly greater pain relief after both 15 minutes and 12 hours, compared with placebo.
“As a single-dose, noninvasive medication with a rapid reduction in pain intensity, Dsuvia represents an important alternative for health care providers to offer patients for acute pain management,” David Leiman, MD, of the department of surgery at the University of Texas, Houston, said in the AcelRx press statement.
FDA Commissioner Scott Gottlieb, MD, commented on the approval amid concerns expressed by some, such as the advocacy group Public Citizen, that the drug is “more than 1,000 times more potent than morphine,” and that approval could lead to diversion and abuse – particularly in light of the U.S. opioid epidemic.
In his statement, Dr. Gottlieb identified one broad, significant issue. “Why do we need an oral formulation of sufentanil – a more potent form of fentanyl that’s been approved for intravenous and epidural use in the U.S. since 1984 – on the market?”
In particular, he focused on the needs of the military. The Department of Defense has taken interest in sufentanil as it fulfills a small but specific battlefield need, namely as a means of pain relief in battlefield situations where soldiers cannot swallow oral medication and access to intravenous medication is limited.
Dr. Gottlieb made clear that sufentanil was meant only to be taken in controlled settings and will have strong limitations on its use. It cannot be prescribed for home use, and treatment should be limited to 72 hours. It can only be delivered by health care professionals using a single-dose applicator and will not be available in pharmacies. It is only to be used in patients who have not tolerated or are expected not to tolerate alternative methods of pain management.
“The FDA has implemented a REMS [Risk Evaluation and Mitigation Strategy] that reflects the potential risks associated with this product and mandates that Dsuvia will only be made available for use in a certified medically supervised heath care setting, including its use on the battlefield,” Dr. Gottlieb said.
However, he recognized that the debate runs deeper than how the FDA should mitigate risk over a new drug, and “as a public health agency, we have an obligation to address this question openly and directly. As a physician and regulator, I won’t bypass legitimate questions and concerns related to our role in addressing the opioid crisis,” he said.
Find Dr. Gottlieb’s full statement on the FDA website.













