User login
Photodermatoses: Exploring Clinical Presentations, Causative Factors, Differential Diagnoses, and Treatment Strategies
Photodermatoses: Exploring Clinical Presentations, Causative Factors, Differential Diagnoses, and Treatment Strategies
Photosensitivity refers to clinical manifestations arising from exposure to sunlight. Photodermatoses encompass a group of skin diseases caused by varying degrees of radiation exposure, including UV radiation and visible light. Photodermatoses can be categorized into 5 main types: primary, exogenous, photoexacerbated, metabolic, and genetic.1 The clinical features of photodermatoses vary depending on the underlying cause but often include pruritic flares, wheals, or dermatitis on sun-exposed areas of the skin.2 While photodermatoses typically are not life threatening, they can greatly impact patients’ quality of life. It is crucial to emphasize the importance of photoprotection and sunlight avoidance to patients as preventive measures against the manifestations of these skin diseases. Furthermore, we present a case of photocontact dermatitis (PCD) and discuss common causative agents, diagnostic mimickers, and treatment options.
Case Report
A 51-year-old woman with no relevant medical history presented to the dermatology clinic with a rash on the neck and under the eyes of 6 days’ duration. The rash was intermittently pruritic but otherwise asymptomatic. The patient reported that she had spent extensive time on the golf course the day of the rash onset and noted that a similar rash had occurred one other time 2 to 3 months prior, also following a prolonged period on the golf course. She had been using over-the-counter fexofenadine 180 mg and over-the-counter lidocaine spray for symptom relief.
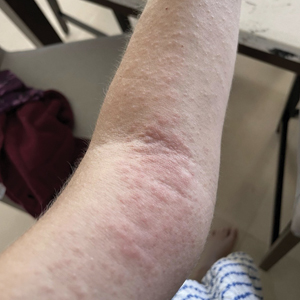
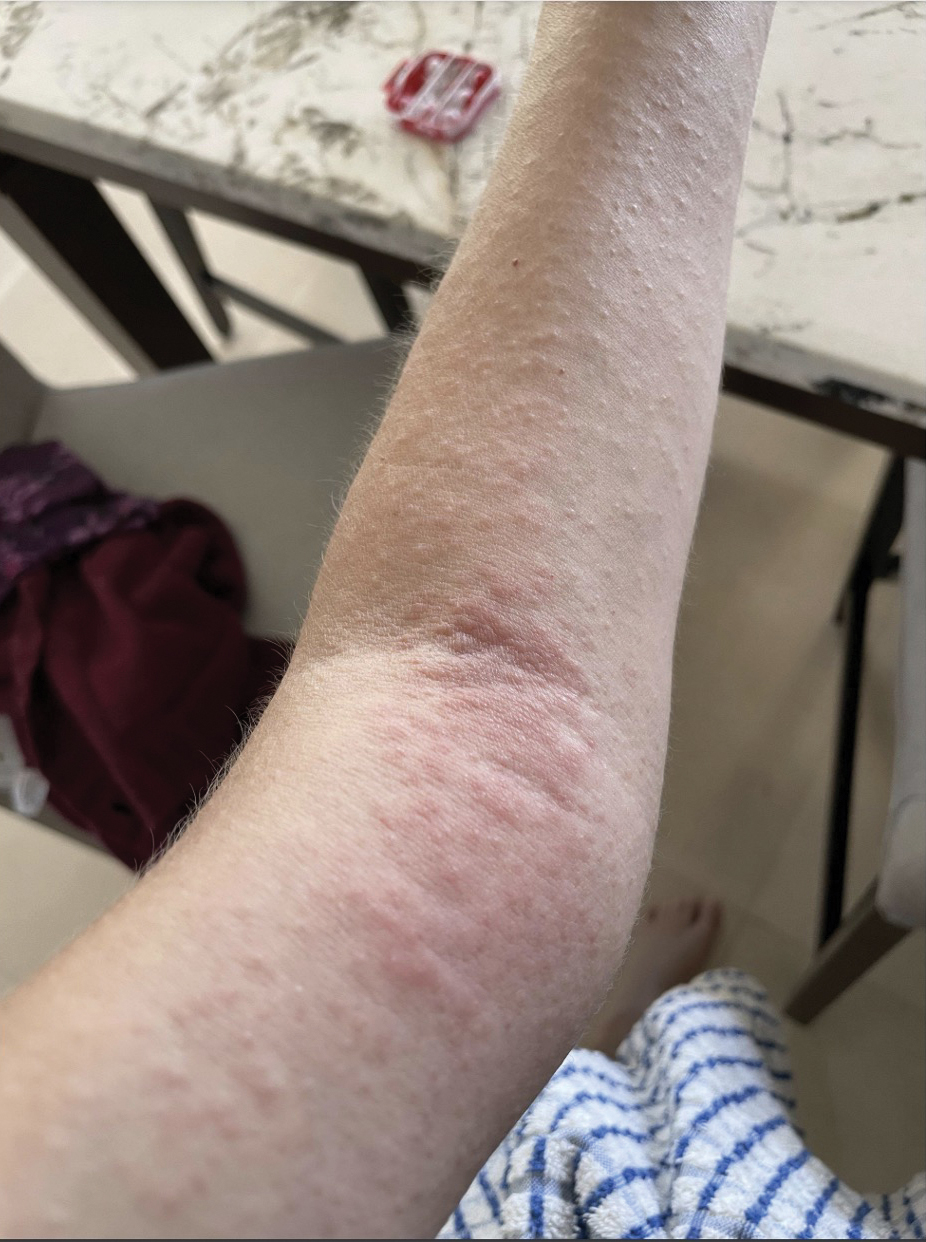
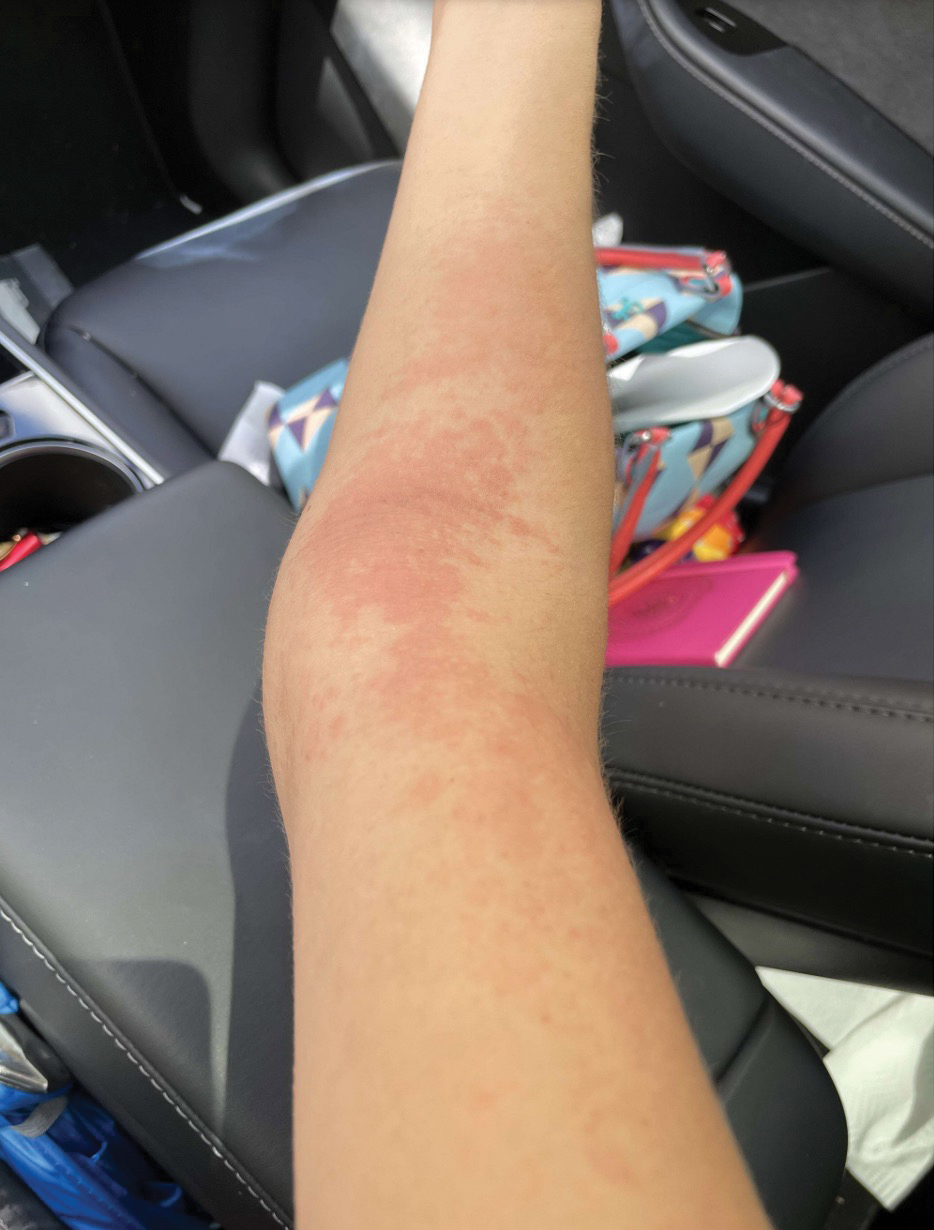
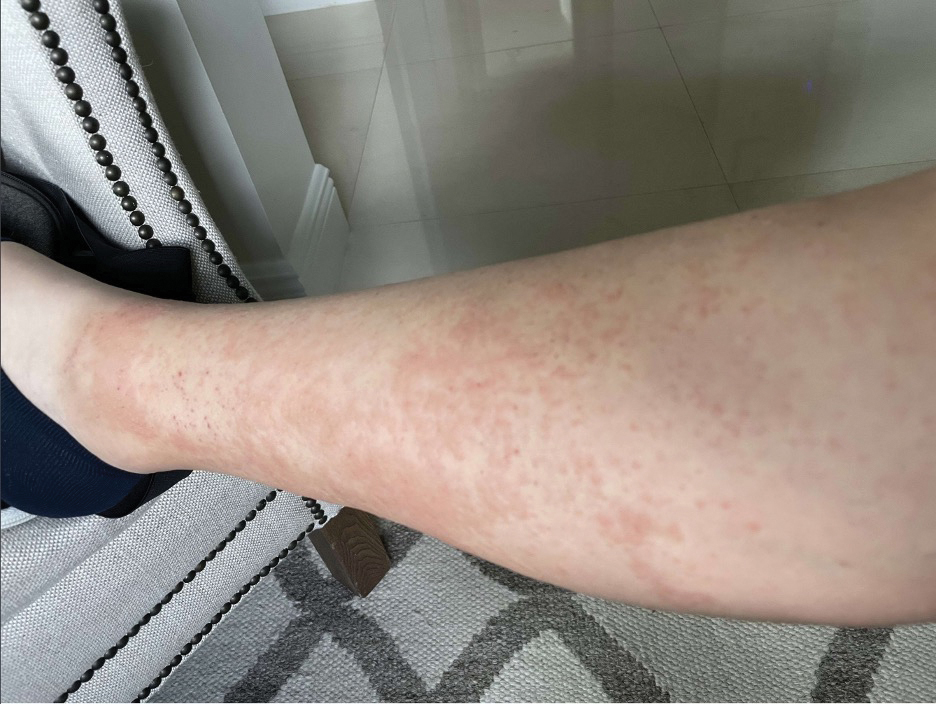
Upon physical examination, erythematous patches were appreciated in a photodistributed pattern on the arms, legs, neck, face, and chest—areas that were not covered by clothing (Figures 1-3). Due to the distribution and morphology of the erythematous patches along with clinical course of onset following exposure to various environmental agents including pesticides, herbicides, oak, and pollen, a diagnosis of PCD was made. The patient was prescribed hydrocortisone cream 2.5%, fluticasone propionate cream 0.05%, and methylprednisolone in addition to the antihistamine. Improvement was noted after 3 days with complete resolution of the skin manifestations. She was counseled on wearing clothing with a universal protection factor rating of 50+ when on the golf course and when sun exposure is expected for an extended period of time.
Causative Agents
Photodermatoses are caused by antigenic substances that lead to photosensitization acquired by either contact or oral ingestion with subsequent sensitization to UV radiation. Halogenated salicylanilide, fenticlor, hexachlorophene, bithionol and, in rare cases, sunscreens, have been reported as triggers.3 In a study performed in 2010, sunscreens, antimicrobial agents, medications, fragrances, plants/plant derivatives, and pesticides were the most commonly reported offending agents listed from highest to lowest frequency. Of the antimicrobial agents, fenticlor, a topical antimicrobial and antifungal that is now mostly used in veterinary medicine, was the most common culprit, causing 60% of cases.4,5
Clinical Manifestations
Clinical manifestations of photodermatoses vary depending upon the specific type of reaction. Examples of primary photodermatoses include polymorphous light eruption (PMLE) and solar urticaria. The cardinal symptoms of PMLE consist of severely pruritic skin lesions that can have macular, papular, papulovesicular, urticarial, multiformelike, and plaquelike variants that develop hours to days after sun exposure.3 Conversely, solar urticaria commonly develops more abruptly, with indurated plaques and wheals appearing on the arms and neck within 30 minutes of sun exposure. The lesions typically resolve within 24 hours.1
Examples of the exogenous subtype include drug-induced photosensitivity, PCD, and pseudoporphyria, with the common clinical presentation of eruption following contact with the causative agent. Drug-induced photosensitivity primarily manifests as a severe sunburnlike rash commonly caused by systemic drugs such as tetracyclines. Photocontact dermatitis is limited to sun-exposed areas of the skin and is caused by a reactive irritant such as chemicals or topical creams. Pseudoporphyria, usually caused by nonsteroidal anti-inflammatory drugs, can manifest with skin fragility and subepidermal blisters.6
Photoexacerbated photodermatoses encompass a variety of conditions ranging from hyperpigmentation disorders such as melasma to autoimmune conditions such as systemic lupus erythematosus (SLE) and dermatomyositis (DM). Common clinical features of these diseases include photodistributed erythema, often involving the cheeks, upper back, and anterior neck. Photo-exposed areas of the dorsal hands also are commonplace for both SLE and DM. Clinical manifestations of PCD are limited to sun-exposed areas of the body, specifically those that come into contact with photoallergic triggers.3 Manifestations of PCD can include pruritic eczematous eruptions resembling those of contact dermatitis 1 to 2 days after sun exposure.1
Photocontact dermatitis represents a specific sensitization via contact or oral ingestion acquired prior to sunlight exposure. It can be broken down into 2 distinct subtypes: photoallergic and photoirritant dermatitis, dependent on whether an allergic or irritant reaction is invoked.2 Plants are known to be a common trigger of photoirritant reactions, while extrinsic triggers include psoralens and medications such as tetracycline antibiotics or sulfonamides. Photoallergic reactions commonly can be caused by topical application of sunscreen or medications, namely nonsteroidal anti-inflammatory drugs.2 Clinical manifestations that may point to photoirritant dermatitis include a photodistributed eruption and classic morphology showing erythema and edema with bullae present in severe cases. These can be contrasted with the clinical manifestations of photoallergic reactions, which usually do not correlate to sun-exposed areas and consist of a monomorphous distribution pattern similar to that of eczema. Although there are distinguishing features of both subtypes of PCD, the overlapping clinical features can mimic those of solar urticaria, PMLE, cutaneous lupus erythematosus, and more systemic conditions such as SLE and DM.7
Systemic lupus erythematosus is associated with a broad range of cutaneous manifestations.8 Exposure to UV radiation is a common trigger for lupus and has the propensity to cause a malar (butterfly) rash that covers the cheeks and nasal bridge but classically spares the nasolabial folds. The rash may display confluent reddish-purple discoloration with papules and/or edema and typically is present at diagnosis in 40% to 52% of patients with SLE.8 Discoid lupus erythematosus, one of the most common cutaneous forms of lupus, manifests with various-sized coin-shaped plaques with adherent follicular hyperkeratosis and plugging. These lesions usually develop on the face, scalp, and ears but also may appear in non–sun-exposed areas.8 Dermatomyositis can manifest with photodistributed erythema affecting classic areas such as the upper back (shawl sign), anterior neck and upper chest (V-sign), and a malar rash similar to that seen in lupus, though DM classically does not spare the nasolabial folds.8,9
Because SLE and DM manifest with photodistributed rashes, it can be difficult to distinguish them from the classic symptoms of photoirritant dermatitis.9 Thus, it is imperative that providers have a high clinical index of suspicion when dealing with patients of similar presentations, as the treatment regimens vastly differ. Approaching the patient with a thorough medical history review, review of systems, biopsy (including immunofluorescence), and appropriate laboratory workup may aid in excluding more complex differential diagnoses such as SLE and DM.
Metabolic and genetic photodermatoses are more rare but can include conditions such as porphyria cutanea tarda and xeroderma pigmentosum, both of which demonstrate fragile skin, slow wound healing, and bullae on photo-exposed skin.1 Although the manifestations can be similar in these systemic conditions, they are caused by very different mechanisms. Porphyria cutanea tarda is caused by deficiencies in enzymes involved in the heme synthesis pathway, whereas xeroderma pigmentosum is caused by an alteration in DNA repair mechanisms.7
Prevalence and the Need for Standardized Testing
Most practicing dermatologists see cases of PCD due to its multiple causative agents; however, little is known about its overall prevalence. The incidence of PCD is fairly low in the general population, but this may be due to its clinical diagnosis, which excludes diagnostic testing such as phototesting and photopatch testing.10 While the incidence of photoallergic contact dermatitis also is fairly unknown, the inception of testing modalities has allowed statistics to be drawn. Research conducted in the United States has disclosed that the incidence of photoallergic contact dermatitis in individuals with a history of a prior photosensitivity eruption is approximately 10% to 20%.10 The development of guidelines and a registry for photopatch testing would aid in a greater understanding of the incidence of PCD and overall consistency of diagnosis.7 Regardless of this lack of consensus, these conditions can be properly managed and prevented if recognized clinically, while newer testing modalities would allow for confirmation of the diagnosis. It is important that any patient presenting with a history of photosensitivity be seen as a candidate for photopatch testing, especially today, as the general population is increasingly exposed to new chemicals entering the market and new social trends.7,10
Diagnosis and Treatment
It is important to consider a detailed history, including the timing, location, duration, family history, and seasonal variation of suspected photodermatoses. A thorough skin examination that takes note of the specific areas affected, morphology, and involvement of the rash or lesions can be helpful.1 Further diagnostic testing such as phototesting and photopatch testing can be employed and is especially important when distinguishing photoallergy from phototoxicity.11 Phototesting involves exposing the patient’s skin to different doses of UVA, UVB, and visible light, followed by an immediate clinical reading of the results and then a delayed reading conducted after 24 hours.1 Photopatch testing involves the application of 2 sets of identical photoallergens to prepped skin (typically cleansed with isopropyl alcohol), with one being irradiated with UVA after 24 hours and one serving as the control. A clinical assessment is conducted at 24 hours and repeated 7 days later.1 In photodermatoses, a visible reaction can be appreciated on the treatment arm while the control arm remains clear. When both sides reveal a visible reaction, this is more indicative of a light-independent allergic contact dermatitis.1
Photodermatoses occur only if there has been a specific sensitization, and therefore it is important to work with the patient to discover any new products that have been introduced into their regimen. Though many photosensitizers in personal care products (eg, antiseptics in soap and topical creams) have been discontinued, certain allergenic ingredients may remain.12 It also is important to note that sensitization to a substance that previously was not a known allergen for a particular patient can occur later in life. Avoiding further sun exposure can rapidly improve the dermatitis, and it is possible for spontaneous remission without further intervention; however, as photoallergic reactions can cause severely pruritic skin lesions, the mainstay of symptomatic treatment consists of topical corticosteroids. Oral and topical antihistamines may help alleviate the pruritus but should not be heavily relied on as this can lead to medication resistance and diminishing efficacy.3 Use of short-term oral steroids also may be considered for rapid improvement of symptoms when the patient is in moderate distress and there are no contraindications. By identifying a temporal association between the introduction of new products and the emergence of dermatitis, it may be possible to identify the causative agent. The patient should promptly discontinue the suspected agent and remain under close observation by the clinician for any further eruptions, especially following additional sun exposure.
Prevention Strategies
In the case of PCD, prevention is key. As PCD indicates a photoallergy, it is important to inform patients that the allergy will persist for a lifetime, much like in contact dermatitis; therefore, the causative agent should be avoided indefinitely.3 Patients with PCD should make intentional efforts to read ingredient lists when purchasing new personal care products to ensure they do not contain the specific causative allergen if one has been identified. Further steps should be taken to ensure proper photoprotection, including use of dense clothing and sunscreen with UVA and UVB filters (broad spectrum).3 It has also been suggested that utilizing sunscreen with ectoin, an amino acid–derived molecule, may result in increased protection against UVA-induced photodermatoses.13
Final Thoughts
Photodermatoses are a group of skin diseases caused by exposure to UV radiation. Photocontact dermatitis/photoallergy is a form of allergic contact dermatitis that results from exposure to an allergen, whether topical, oral, or environmental. The allergen is activated by exposure to UV radiation to sensitize the allergic response, resulting in a rash characterized by confluent erythematous patches or plaques, papular vesicles, and rarely blisters.3 Photocontact dermatitis, although rare, is an important differential diagnosis to consider when the presenting rash is restricted to sun-exposed areas of the skin such as the arms, legs, neck, and face. Diagnosis remains a challenge; however, new testing modalities such as photopatch testing may open the door for further confirmation and aid in proper diagnosis leading to earlier treatment times for patients. It is recommended that the clinician and patient work together to identify the possible causative agent to prevent further eruptions.
- Santoro FA, Lim HW. Update on photodermatoses. Semin Cutan Med Surg. 2011;30:229-238.
- Gimenez-Arnau A, Maurer M, De La Cuadra J, et al. Immediate contact skin reactions, an update of contact urticaria, contact urticaria syndrome and protein contact dermatitis—“a never ending story.” Eur J Dermatol. 2010;20:555-562.
- Lehmann P, Schwarz T. Photodermatoses: diagnosis and treatment. Dtsch Arztebl Int. 2011;108:135-141.
- Victor FC, Cohen DE, Soter NA. A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. J Am Acad Dermatol. 2010;62:605-610.
- Fenticlor (Code 65671). National Cancer Institute EVS Explore. Accessed October 28, 2025. https://ncithesaurus.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCIThesaurus&ns=ncit&code=C65671
- Elmets CA. Photosensitivity disorders (photodermatoses): clinical manifestations, diagnosis, and treatment. UptoDate. Updated February 23, 2023. Accessed October 28, 2025. https://www.uptodate.com/contents/photosensitivity-disorders-photodermatoses-clinical-manifestations-diagnosis-and-treatment
- Snyder M, Turrentine JE, Cruz PD Jr. Photocontact dermatitis and its clinical mimics: an overview for the allergist. Clin Rev Allergy Immunol. 2019;56:32-40.
- Cooper EE, Pisano CE, Shapiro SC. Cutaneous manifestations of “lupus”: systemic lupus erythematosus and beyond. Int J Rheumatol. 2021;2021:6610509.
- Christopher-Stine L, Amato AA, Vleugels RA. Diagnosis and differential diagnosis of dermatomyositis and polymyositis in adults. UptoDate. Updated March 3, 2025. Accessed October 28, 2025. https://www.uptodate.com/contents/diagnosis-and-differential-diagnosis-of-dermatomyositis-and-polymyositis-in-adults?search=Diagnosis%20and%20differential%20diagnosis%20of%20dermatomyositis%20and%20polymyositis%20in%20adults&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Deleo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- Gonçalo M. Photopatch testing. In: Johansen J, Frosch P, Lepoittevin JP, eds. Contact Dermatitis. Springer; 2011:519-531.
- Enta T. Dermacase. Contact photodermatitis. Can Fam Physician. 1995;41:577,586-587.
- Duteil L, Queille-Roussel C, Aladren S, et al. Prevention of polymophic light eruption afforded by a very high broad-spectrum protection sunscreen containing ectoin. Dermatol Ther (Heidelb). 2022;12:1603-1613.
Photosensitivity refers to clinical manifestations arising from exposure to sunlight. Photodermatoses encompass a group of skin diseases caused by varying degrees of radiation exposure, including UV radiation and visible light. Photodermatoses can be categorized into 5 main types: primary, exogenous, photoexacerbated, metabolic, and genetic.1 The clinical features of photodermatoses vary depending on the underlying cause but often include pruritic flares, wheals, or dermatitis on sun-exposed areas of the skin.2 While photodermatoses typically are not life threatening, they can greatly impact patients’ quality of life. It is crucial to emphasize the importance of photoprotection and sunlight avoidance to patients as preventive measures against the manifestations of these skin diseases. Furthermore, we present a case of photocontact dermatitis (PCD) and discuss common causative agents, diagnostic mimickers, and treatment options.
Case Report
A 51-year-old woman with no relevant medical history presented to the dermatology clinic with a rash on the neck and under the eyes of 6 days’ duration. The rash was intermittently pruritic but otherwise asymptomatic. The patient reported that she had spent extensive time on the golf course the day of the rash onset and noted that a similar rash had occurred one other time 2 to 3 months prior, also following a prolonged period on the golf course. She had been using over-the-counter fexofenadine 180 mg and over-the-counter lidocaine spray for symptom relief.
Upon physical examination, erythematous patches were appreciated in a photodistributed pattern on the arms, legs, neck, face, and chest—areas that were not covered by clothing (Figures 1-3). Due to the distribution and morphology of the erythematous patches along with clinical course of onset following exposure to various environmental agents including pesticides, herbicides, oak, and pollen, a diagnosis of PCD was made. The patient was prescribed hydrocortisone cream 2.5%, fluticasone propionate cream 0.05%, and methylprednisolone in addition to the antihistamine. Improvement was noted after 3 days with complete resolution of the skin manifestations. She was counseled on wearing clothing with a universal protection factor rating of 50+ when on the golf course and when sun exposure is expected for an extended period of time.



Causative Agents
Photodermatoses are caused by antigenic substances that lead to photosensitization acquired by either contact or oral ingestion with subsequent sensitization to UV radiation. Halogenated salicylanilide, fenticlor, hexachlorophene, bithionol and, in rare cases, sunscreens, have been reported as triggers.3 In a study performed in 2010, sunscreens, antimicrobial agents, medications, fragrances, plants/plant derivatives, and pesticides were the most commonly reported offending agents listed from highest to lowest frequency. Of the antimicrobial agents, fenticlor, a topical antimicrobial and antifungal that is now mostly used in veterinary medicine, was the most common culprit, causing 60% of cases.4,5
Clinical Manifestations
Clinical manifestations of photodermatoses vary depending upon the specific type of reaction. Examples of primary photodermatoses include polymorphous light eruption (PMLE) and solar urticaria. The cardinal symptoms of PMLE consist of severely pruritic skin lesions that can have macular, papular, papulovesicular, urticarial, multiformelike, and plaquelike variants that develop hours to days after sun exposure.3 Conversely, solar urticaria commonly develops more abruptly, with indurated plaques and wheals appearing on the arms and neck within 30 minutes of sun exposure. The lesions typically resolve within 24 hours.1
Examples of the exogenous subtype include drug-induced photosensitivity, PCD, and pseudoporphyria, with the common clinical presentation of eruption following contact with the causative agent. Drug-induced photosensitivity primarily manifests as a severe sunburnlike rash commonly caused by systemic drugs such as tetracyclines. Photocontact dermatitis is limited to sun-exposed areas of the skin and is caused by a reactive irritant such as chemicals or topical creams. Pseudoporphyria, usually caused by nonsteroidal anti-inflammatory drugs, can manifest with skin fragility and subepidermal blisters.6
Photoexacerbated photodermatoses encompass a variety of conditions ranging from hyperpigmentation disorders such as melasma to autoimmune conditions such as systemic lupus erythematosus (SLE) and dermatomyositis (DM). Common clinical features of these diseases include photodistributed erythema, often involving the cheeks, upper back, and anterior neck. Photo-exposed areas of the dorsal hands also are commonplace for both SLE and DM. Clinical manifestations of PCD are limited to sun-exposed areas of the body, specifically those that come into contact with photoallergic triggers.3 Manifestations of PCD can include pruritic eczematous eruptions resembling those of contact dermatitis 1 to 2 days after sun exposure.1
Photocontact dermatitis represents a specific sensitization via contact or oral ingestion acquired prior to sunlight exposure. It can be broken down into 2 distinct subtypes: photoallergic and photoirritant dermatitis, dependent on whether an allergic or irritant reaction is invoked.2 Plants are known to be a common trigger of photoirritant reactions, while extrinsic triggers include psoralens and medications such as tetracycline antibiotics or sulfonamides. Photoallergic reactions commonly can be caused by topical application of sunscreen or medications, namely nonsteroidal anti-inflammatory drugs.2 Clinical manifestations that may point to photoirritant dermatitis include a photodistributed eruption and classic morphology showing erythema and edema with bullae present in severe cases. These can be contrasted with the clinical manifestations of photoallergic reactions, which usually do not correlate to sun-exposed areas and consist of a monomorphous distribution pattern similar to that of eczema. Although there are distinguishing features of both subtypes of PCD, the overlapping clinical features can mimic those of solar urticaria, PMLE, cutaneous lupus erythematosus, and more systemic conditions such as SLE and DM.7
Systemic lupus erythematosus is associated with a broad range of cutaneous manifestations.8 Exposure to UV radiation is a common trigger for lupus and has the propensity to cause a malar (butterfly) rash that covers the cheeks and nasal bridge but classically spares the nasolabial folds. The rash may display confluent reddish-purple discoloration with papules and/or edema and typically is present at diagnosis in 40% to 52% of patients with SLE.8 Discoid lupus erythematosus, one of the most common cutaneous forms of lupus, manifests with various-sized coin-shaped plaques with adherent follicular hyperkeratosis and plugging. These lesions usually develop on the face, scalp, and ears but also may appear in non–sun-exposed areas.8 Dermatomyositis can manifest with photodistributed erythema affecting classic areas such as the upper back (shawl sign), anterior neck and upper chest (V-sign), and a malar rash similar to that seen in lupus, though DM classically does not spare the nasolabial folds.8,9
Because SLE and DM manifest with photodistributed rashes, it can be difficult to distinguish them from the classic symptoms of photoirritant dermatitis.9 Thus, it is imperative that providers have a high clinical index of suspicion when dealing with patients of similar presentations, as the treatment regimens vastly differ. Approaching the patient with a thorough medical history review, review of systems, biopsy (including immunofluorescence), and appropriate laboratory workup may aid in excluding more complex differential diagnoses such as SLE and DM.
Metabolic and genetic photodermatoses are more rare but can include conditions such as porphyria cutanea tarda and xeroderma pigmentosum, both of which demonstrate fragile skin, slow wound healing, and bullae on photo-exposed skin.1 Although the manifestations can be similar in these systemic conditions, they are caused by very different mechanisms. Porphyria cutanea tarda is caused by deficiencies in enzymes involved in the heme synthesis pathway, whereas xeroderma pigmentosum is caused by an alteration in DNA repair mechanisms.7
Prevalence and the Need for Standardized Testing
Most practicing dermatologists see cases of PCD due to its multiple causative agents; however, little is known about its overall prevalence. The incidence of PCD is fairly low in the general population, but this may be due to its clinical diagnosis, which excludes diagnostic testing such as phototesting and photopatch testing.10 While the incidence of photoallergic contact dermatitis also is fairly unknown, the inception of testing modalities has allowed statistics to be drawn. Research conducted in the United States has disclosed that the incidence of photoallergic contact dermatitis in individuals with a history of a prior photosensitivity eruption is approximately 10% to 20%.10 The development of guidelines and a registry for photopatch testing would aid in a greater understanding of the incidence of PCD and overall consistency of diagnosis.7 Regardless of this lack of consensus, these conditions can be properly managed and prevented if recognized clinically, while newer testing modalities would allow for confirmation of the diagnosis. It is important that any patient presenting with a history of photosensitivity be seen as a candidate for photopatch testing, especially today, as the general population is increasingly exposed to new chemicals entering the market and new social trends.7,10
Diagnosis and Treatment
It is important to consider a detailed history, including the timing, location, duration, family history, and seasonal variation of suspected photodermatoses. A thorough skin examination that takes note of the specific areas affected, morphology, and involvement of the rash or lesions can be helpful.1 Further diagnostic testing such as phototesting and photopatch testing can be employed and is especially important when distinguishing photoallergy from phototoxicity.11 Phototesting involves exposing the patient’s skin to different doses of UVA, UVB, and visible light, followed by an immediate clinical reading of the results and then a delayed reading conducted after 24 hours.1 Photopatch testing involves the application of 2 sets of identical photoallergens to prepped skin (typically cleansed with isopropyl alcohol), with one being irradiated with UVA after 24 hours and one serving as the control. A clinical assessment is conducted at 24 hours and repeated 7 days later.1 In photodermatoses, a visible reaction can be appreciated on the treatment arm while the control arm remains clear. When both sides reveal a visible reaction, this is more indicative of a light-independent allergic contact dermatitis.1
Photodermatoses occur only if there has been a specific sensitization, and therefore it is important to work with the patient to discover any new products that have been introduced into their regimen. Though many photosensitizers in personal care products (eg, antiseptics in soap and topical creams) have been discontinued, certain allergenic ingredients may remain.12 It also is important to note that sensitization to a substance that previously was not a known allergen for a particular patient can occur later in life. Avoiding further sun exposure can rapidly improve the dermatitis, and it is possible for spontaneous remission without further intervention; however, as photoallergic reactions can cause severely pruritic skin lesions, the mainstay of symptomatic treatment consists of topical corticosteroids. Oral and topical antihistamines may help alleviate the pruritus but should not be heavily relied on as this can lead to medication resistance and diminishing efficacy.3 Use of short-term oral steroids also may be considered for rapid improvement of symptoms when the patient is in moderate distress and there are no contraindications. By identifying a temporal association between the introduction of new products and the emergence of dermatitis, it may be possible to identify the causative agent. The patient should promptly discontinue the suspected agent and remain under close observation by the clinician for any further eruptions, especially following additional sun exposure.
Prevention Strategies
In the case of PCD, prevention is key. As PCD indicates a photoallergy, it is important to inform patients that the allergy will persist for a lifetime, much like in contact dermatitis; therefore, the causative agent should be avoided indefinitely.3 Patients with PCD should make intentional efforts to read ingredient lists when purchasing new personal care products to ensure they do not contain the specific causative allergen if one has been identified. Further steps should be taken to ensure proper photoprotection, including use of dense clothing and sunscreen with UVA and UVB filters (broad spectrum).3 It has also been suggested that utilizing sunscreen with ectoin, an amino acid–derived molecule, may result in increased protection against UVA-induced photodermatoses.13
Final Thoughts
Photodermatoses are a group of skin diseases caused by exposure to UV radiation. Photocontact dermatitis/photoallergy is a form of allergic contact dermatitis that results from exposure to an allergen, whether topical, oral, or environmental. The allergen is activated by exposure to UV radiation to sensitize the allergic response, resulting in a rash characterized by confluent erythematous patches or plaques, papular vesicles, and rarely blisters.3 Photocontact dermatitis, although rare, is an important differential diagnosis to consider when the presenting rash is restricted to sun-exposed areas of the skin such as the arms, legs, neck, and face. Diagnosis remains a challenge; however, new testing modalities such as photopatch testing may open the door for further confirmation and aid in proper diagnosis leading to earlier treatment times for patients. It is recommended that the clinician and patient work together to identify the possible causative agent to prevent further eruptions.
Photosensitivity refers to clinical manifestations arising from exposure to sunlight. Photodermatoses encompass a group of skin diseases caused by varying degrees of radiation exposure, including UV radiation and visible light. Photodermatoses can be categorized into 5 main types: primary, exogenous, photoexacerbated, metabolic, and genetic.1 The clinical features of photodermatoses vary depending on the underlying cause but often include pruritic flares, wheals, or dermatitis on sun-exposed areas of the skin.2 While photodermatoses typically are not life threatening, they can greatly impact patients’ quality of life. It is crucial to emphasize the importance of photoprotection and sunlight avoidance to patients as preventive measures against the manifestations of these skin diseases. Furthermore, we present a case of photocontact dermatitis (PCD) and discuss common causative agents, diagnostic mimickers, and treatment options.
Case Report
A 51-year-old woman with no relevant medical history presented to the dermatology clinic with a rash on the neck and under the eyes of 6 days’ duration. The rash was intermittently pruritic but otherwise asymptomatic. The patient reported that she had spent extensive time on the golf course the day of the rash onset and noted that a similar rash had occurred one other time 2 to 3 months prior, also following a prolonged period on the golf course. She had been using over-the-counter fexofenadine 180 mg and over-the-counter lidocaine spray for symptom relief.
Upon physical examination, erythematous patches were appreciated in a photodistributed pattern on the arms, legs, neck, face, and chest—areas that were not covered by clothing (Figures 1-3). Due to the distribution and morphology of the erythematous patches along with clinical course of onset following exposure to various environmental agents including pesticides, herbicides, oak, and pollen, a diagnosis of PCD was made. The patient was prescribed hydrocortisone cream 2.5%, fluticasone propionate cream 0.05%, and methylprednisolone in addition to the antihistamine. Improvement was noted after 3 days with complete resolution of the skin manifestations. She was counseled on wearing clothing with a universal protection factor rating of 50+ when on the golf course and when sun exposure is expected for an extended period of time.



Causative Agents
Photodermatoses are caused by antigenic substances that lead to photosensitization acquired by either contact or oral ingestion with subsequent sensitization to UV radiation. Halogenated salicylanilide, fenticlor, hexachlorophene, bithionol and, in rare cases, sunscreens, have been reported as triggers.3 In a study performed in 2010, sunscreens, antimicrobial agents, medications, fragrances, plants/plant derivatives, and pesticides were the most commonly reported offending agents listed from highest to lowest frequency. Of the antimicrobial agents, fenticlor, a topical antimicrobial and antifungal that is now mostly used in veterinary medicine, was the most common culprit, causing 60% of cases.4,5
Clinical Manifestations
Clinical manifestations of photodermatoses vary depending upon the specific type of reaction. Examples of primary photodermatoses include polymorphous light eruption (PMLE) and solar urticaria. The cardinal symptoms of PMLE consist of severely pruritic skin lesions that can have macular, papular, papulovesicular, urticarial, multiformelike, and plaquelike variants that develop hours to days after sun exposure.3 Conversely, solar urticaria commonly develops more abruptly, with indurated plaques and wheals appearing on the arms and neck within 30 minutes of sun exposure. The lesions typically resolve within 24 hours.1
Examples of the exogenous subtype include drug-induced photosensitivity, PCD, and pseudoporphyria, with the common clinical presentation of eruption following contact with the causative agent. Drug-induced photosensitivity primarily manifests as a severe sunburnlike rash commonly caused by systemic drugs such as tetracyclines. Photocontact dermatitis is limited to sun-exposed areas of the skin and is caused by a reactive irritant such as chemicals or topical creams. Pseudoporphyria, usually caused by nonsteroidal anti-inflammatory drugs, can manifest with skin fragility and subepidermal blisters.6
Photoexacerbated photodermatoses encompass a variety of conditions ranging from hyperpigmentation disorders such as melasma to autoimmune conditions such as systemic lupus erythematosus (SLE) and dermatomyositis (DM). Common clinical features of these diseases include photodistributed erythema, often involving the cheeks, upper back, and anterior neck. Photo-exposed areas of the dorsal hands also are commonplace for both SLE and DM. Clinical manifestations of PCD are limited to sun-exposed areas of the body, specifically those that come into contact with photoallergic triggers.3 Manifestations of PCD can include pruritic eczematous eruptions resembling those of contact dermatitis 1 to 2 days after sun exposure.1
Photocontact dermatitis represents a specific sensitization via contact or oral ingestion acquired prior to sunlight exposure. It can be broken down into 2 distinct subtypes: photoallergic and photoirritant dermatitis, dependent on whether an allergic or irritant reaction is invoked.2 Plants are known to be a common trigger of photoirritant reactions, while extrinsic triggers include psoralens and medications such as tetracycline antibiotics or sulfonamides. Photoallergic reactions commonly can be caused by topical application of sunscreen or medications, namely nonsteroidal anti-inflammatory drugs.2 Clinical manifestations that may point to photoirritant dermatitis include a photodistributed eruption and classic morphology showing erythema and edema with bullae present in severe cases. These can be contrasted with the clinical manifestations of photoallergic reactions, which usually do not correlate to sun-exposed areas and consist of a monomorphous distribution pattern similar to that of eczema. Although there are distinguishing features of both subtypes of PCD, the overlapping clinical features can mimic those of solar urticaria, PMLE, cutaneous lupus erythematosus, and more systemic conditions such as SLE and DM.7
Systemic lupus erythematosus is associated with a broad range of cutaneous manifestations.8 Exposure to UV radiation is a common trigger for lupus and has the propensity to cause a malar (butterfly) rash that covers the cheeks and nasal bridge but classically spares the nasolabial folds. The rash may display confluent reddish-purple discoloration with papules and/or edema and typically is present at diagnosis in 40% to 52% of patients with SLE.8 Discoid lupus erythematosus, one of the most common cutaneous forms of lupus, manifests with various-sized coin-shaped plaques with adherent follicular hyperkeratosis and plugging. These lesions usually develop on the face, scalp, and ears but also may appear in non–sun-exposed areas.8 Dermatomyositis can manifest with photodistributed erythema affecting classic areas such as the upper back (shawl sign), anterior neck and upper chest (V-sign), and a malar rash similar to that seen in lupus, though DM classically does not spare the nasolabial folds.8,9
Because SLE and DM manifest with photodistributed rashes, it can be difficult to distinguish them from the classic symptoms of photoirritant dermatitis.9 Thus, it is imperative that providers have a high clinical index of suspicion when dealing with patients of similar presentations, as the treatment regimens vastly differ. Approaching the patient with a thorough medical history review, review of systems, biopsy (including immunofluorescence), and appropriate laboratory workup may aid in excluding more complex differential diagnoses such as SLE and DM.
Metabolic and genetic photodermatoses are more rare but can include conditions such as porphyria cutanea tarda and xeroderma pigmentosum, both of which demonstrate fragile skin, slow wound healing, and bullae on photo-exposed skin.1 Although the manifestations can be similar in these systemic conditions, they are caused by very different mechanisms. Porphyria cutanea tarda is caused by deficiencies in enzymes involved in the heme synthesis pathway, whereas xeroderma pigmentosum is caused by an alteration in DNA repair mechanisms.7
Prevalence and the Need for Standardized Testing
Most practicing dermatologists see cases of PCD due to its multiple causative agents; however, little is known about its overall prevalence. The incidence of PCD is fairly low in the general population, but this may be due to its clinical diagnosis, which excludes diagnostic testing such as phototesting and photopatch testing.10 While the incidence of photoallergic contact dermatitis also is fairly unknown, the inception of testing modalities has allowed statistics to be drawn. Research conducted in the United States has disclosed that the incidence of photoallergic contact dermatitis in individuals with a history of a prior photosensitivity eruption is approximately 10% to 20%.10 The development of guidelines and a registry for photopatch testing would aid in a greater understanding of the incidence of PCD and overall consistency of diagnosis.7 Regardless of this lack of consensus, these conditions can be properly managed and prevented if recognized clinically, while newer testing modalities would allow for confirmation of the diagnosis. It is important that any patient presenting with a history of photosensitivity be seen as a candidate for photopatch testing, especially today, as the general population is increasingly exposed to new chemicals entering the market and new social trends.7,10
Diagnosis and Treatment
It is important to consider a detailed history, including the timing, location, duration, family history, and seasonal variation of suspected photodermatoses. A thorough skin examination that takes note of the specific areas affected, morphology, and involvement of the rash or lesions can be helpful.1 Further diagnostic testing such as phototesting and photopatch testing can be employed and is especially important when distinguishing photoallergy from phototoxicity.11 Phototesting involves exposing the patient’s skin to different doses of UVA, UVB, and visible light, followed by an immediate clinical reading of the results and then a delayed reading conducted after 24 hours.1 Photopatch testing involves the application of 2 sets of identical photoallergens to prepped skin (typically cleansed with isopropyl alcohol), with one being irradiated with UVA after 24 hours and one serving as the control. A clinical assessment is conducted at 24 hours and repeated 7 days later.1 In photodermatoses, a visible reaction can be appreciated on the treatment arm while the control arm remains clear. When both sides reveal a visible reaction, this is more indicative of a light-independent allergic contact dermatitis.1
Photodermatoses occur only if there has been a specific sensitization, and therefore it is important to work with the patient to discover any new products that have been introduced into their regimen. Though many photosensitizers in personal care products (eg, antiseptics in soap and topical creams) have been discontinued, certain allergenic ingredients may remain.12 It also is important to note that sensitization to a substance that previously was not a known allergen for a particular patient can occur later in life. Avoiding further sun exposure can rapidly improve the dermatitis, and it is possible for spontaneous remission without further intervention; however, as photoallergic reactions can cause severely pruritic skin lesions, the mainstay of symptomatic treatment consists of topical corticosteroids. Oral and topical antihistamines may help alleviate the pruritus but should not be heavily relied on as this can lead to medication resistance and diminishing efficacy.3 Use of short-term oral steroids also may be considered for rapid improvement of symptoms when the patient is in moderate distress and there are no contraindications. By identifying a temporal association between the introduction of new products and the emergence of dermatitis, it may be possible to identify the causative agent. The patient should promptly discontinue the suspected agent and remain under close observation by the clinician for any further eruptions, especially following additional sun exposure.
Prevention Strategies
In the case of PCD, prevention is key. As PCD indicates a photoallergy, it is important to inform patients that the allergy will persist for a lifetime, much like in contact dermatitis; therefore, the causative agent should be avoided indefinitely.3 Patients with PCD should make intentional efforts to read ingredient lists when purchasing new personal care products to ensure they do not contain the specific causative allergen if one has been identified. Further steps should be taken to ensure proper photoprotection, including use of dense clothing and sunscreen with UVA and UVB filters (broad spectrum).3 It has also been suggested that utilizing sunscreen with ectoin, an amino acid–derived molecule, may result in increased protection against UVA-induced photodermatoses.13
Final Thoughts
Photodermatoses are a group of skin diseases caused by exposure to UV radiation. Photocontact dermatitis/photoallergy is a form of allergic contact dermatitis that results from exposure to an allergen, whether topical, oral, or environmental. The allergen is activated by exposure to UV radiation to sensitize the allergic response, resulting in a rash characterized by confluent erythematous patches or plaques, papular vesicles, and rarely blisters.3 Photocontact dermatitis, although rare, is an important differential diagnosis to consider when the presenting rash is restricted to sun-exposed areas of the skin such as the arms, legs, neck, and face. Diagnosis remains a challenge; however, new testing modalities such as photopatch testing may open the door for further confirmation and aid in proper diagnosis leading to earlier treatment times for patients. It is recommended that the clinician and patient work together to identify the possible causative agent to prevent further eruptions.
- Santoro FA, Lim HW. Update on photodermatoses. Semin Cutan Med Surg. 2011;30:229-238.
- Gimenez-Arnau A, Maurer M, De La Cuadra J, et al. Immediate contact skin reactions, an update of contact urticaria, contact urticaria syndrome and protein contact dermatitis—“a never ending story.” Eur J Dermatol. 2010;20:555-562.
- Lehmann P, Schwarz T. Photodermatoses: diagnosis and treatment. Dtsch Arztebl Int. 2011;108:135-141.
- Victor FC, Cohen DE, Soter NA. A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. J Am Acad Dermatol. 2010;62:605-610.
- Fenticlor (Code 65671). National Cancer Institute EVS Explore. Accessed October 28, 2025. https://ncithesaurus.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCIThesaurus&ns=ncit&code=C65671
- Elmets CA. Photosensitivity disorders (photodermatoses): clinical manifestations, diagnosis, and treatment. UptoDate. Updated February 23, 2023. Accessed October 28, 2025. https://www.uptodate.com/contents/photosensitivity-disorders-photodermatoses-clinical-manifestations-diagnosis-and-treatment
- Snyder M, Turrentine JE, Cruz PD Jr. Photocontact dermatitis and its clinical mimics: an overview for the allergist. Clin Rev Allergy Immunol. 2019;56:32-40.
- Cooper EE, Pisano CE, Shapiro SC. Cutaneous manifestations of “lupus”: systemic lupus erythematosus and beyond. Int J Rheumatol. 2021;2021:6610509.
- Christopher-Stine L, Amato AA, Vleugels RA. Diagnosis and differential diagnosis of dermatomyositis and polymyositis in adults. UptoDate. Updated March 3, 2025. Accessed October 28, 2025. https://www.uptodate.com/contents/diagnosis-and-differential-diagnosis-of-dermatomyositis-and-polymyositis-in-adults?search=Diagnosis%20and%20differential%20diagnosis%20of%20dermatomyositis%20and%20polymyositis%20in%20adults&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Deleo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- Gonçalo M. Photopatch testing. In: Johansen J, Frosch P, Lepoittevin JP, eds. Contact Dermatitis. Springer; 2011:519-531.
- Enta T. Dermacase. Contact photodermatitis. Can Fam Physician. 1995;41:577,586-587.
- Duteil L, Queille-Roussel C, Aladren S, et al. Prevention of polymophic light eruption afforded by a very high broad-spectrum protection sunscreen containing ectoin. Dermatol Ther (Heidelb). 2022;12:1603-1613.
- Santoro FA, Lim HW. Update on photodermatoses. Semin Cutan Med Surg. 2011;30:229-238.
- Gimenez-Arnau A, Maurer M, De La Cuadra J, et al. Immediate contact skin reactions, an update of contact urticaria, contact urticaria syndrome and protein contact dermatitis—“a never ending story.” Eur J Dermatol. 2010;20:555-562.
- Lehmann P, Schwarz T. Photodermatoses: diagnosis and treatment. Dtsch Arztebl Int. 2011;108:135-141.
- Victor FC, Cohen DE, Soter NA. A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. J Am Acad Dermatol. 2010;62:605-610.
- Fenticlor (Code 65671). National Cancer Institute EVS Explore. Accessed October 28, 2025. https://ncithesaurus.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCIThesaurus&ns=ncit&code=C65671
- Elmets CA. Photosensitivity disorders (photodermatoses): clinical manifestations, diagnosis, and treatment. UptoDate. Updated February 23, 2023. Accessed October 28, 2025. https://www.uptodate.com/contents/photosensitivity-disorders-photodermatoses-clinical-manifestations-diagnosis-and-treatment
- Snyder M, Turrentine JE, Cruz PD Jr. Photocontact dermatitis and its clinical mimics: an overview for the allergist. Clin Rev Allergy Immunol. 2019;56:32-40.
- Cooper EE, Pisano CE, Shapiro SC. Cutaneous manifestations of “lupus”: systemic lupus erythematosus and beyond. Int J Rheumatol. 2021;2021:6610509.
- Christopher-Stine L, Amato AA, Vleugels RA. Diagnosis and differential diagnosis of dermatomyositis and polymyositis in adults. UptoDate. Updated March 3, 2025. Accessed October 28, 2025. https://www.uptodate.com/contents/diagnosis-and-differential-diagnosis-of-dermatomyositis-and-polymyositis-in-adults?search=Diagnosis%20and%20differential%20diagnosis%20of%20dermatomyositis%20and%20polymyositis%20in%20adults&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Deleo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- Gonçalo M. Photopatch testing. In: Johansen J, Frosch P, Lepoittevin JP, eds. Contact Dermatitis. Springer; 2011:519-531.
- Enta T. Dermacase. Contact photodermatitis. Can Fam Physician. 1995;41:577,586-587.
- Duteil L, Queille-Roussel C, Aladren S, et al. Prevention of polymophic light eruption afforded by a very high broad-spectrum protection sunscreen containing ectoin. Dermatol Ther (Heidelb). 2022;12:1603-1613.
Photodermatoses: Exploring Clinical Presentations, Causative Factors, Differential Diagnoses, and Treatment Strategies
Photodermatoses: Exploring Clinical Presentations, Causative Factors, Differential Diagnoses, and Treatment Strategies
Practice Points
- It is important to consider photodermatoses in patients presenting with a rash that is restricted to light-exposed areas of the skin, such as the arms, legs, neck, and face.
- The mainstay of treatment consists of topical corticosteroids. Oral antihistamines should not be heavily relied on, but short-term oral steroids may be considered for rapid improvement if symptoms are severe.
- It is important to note that, much like in contact dermatitis, the underlying photoallergy causing photocontact dermatitis will persist for a lifetime.
Spreading Ulcerations and Lymphadenopathy in a Traveler Returning from Costa Rica
Spreading Ulcerations and Lymphadenopathy in a Traveler Returning from Costa Rica
THE DIAGNOSIS: Cutaneous Leishmaniasis
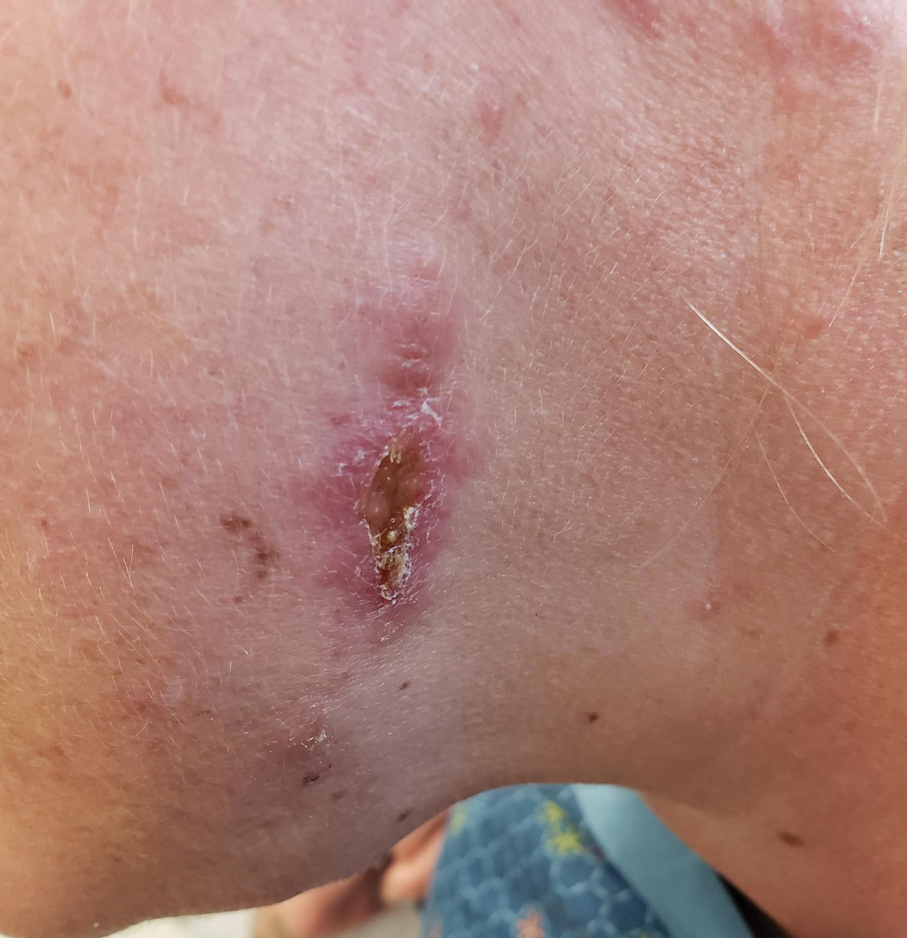
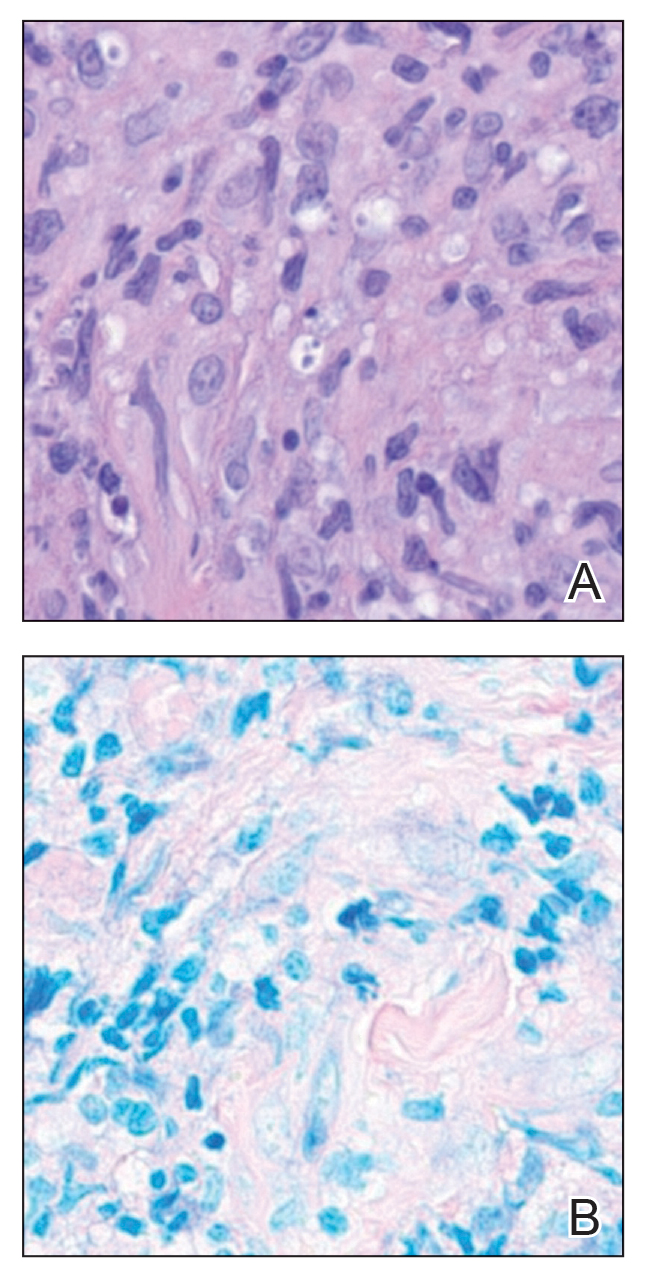
The biopsy results revealed amastigotes at the periphery of parasitized histiocytes, consistent with a diagnosis of cutaneous leishmaniasis. Polymerase chain reaction analysis revealed Leishmania guyanensis species complex, which includes both L guyanensis and Leishmania panamensis. In this case of disseminated cutaneous leishmaniasis (Figure 1), our patient received a prolonged course of systemic therapy with oral miltefosine 50 mg 3 times daily. At the most recent follow-up appointment, she showed ongoing resolution of ulcerations, subcutaneous plaques, and lymphadenopathy on the trunk and face, but development of subcutaneous nodules continued on the arms and legs. At the next follow-up, physical examination revealed that the lesions slowly started to fade.
Leishmania species are parasites transmitted by bites of female sand flies, which belong to the genera Phlebotomus (Old World, Eastern Hemisphere) and Lutzomyia (New World, Western Hemisphere) genera.1 Leishmania species have a complex life cycle, propagating within human macrophages, ultimately leading to cutaneous, mucocutaneous, and visceral disease manifestations.2 Cutaneous leishmaniasis manifests classically as scattered, painless, slow-healing ulcers.3 A biopsy taken from the edge of a cutaneous ulcer for hematoxylin and eosin processing is recommended for initial diagnosis, and subsequent polymerase chain reaction of the sample is required for speciation, which guides therapeutic options.4,5 Classic hematoxylin and eosin and Giemsa stain findings include amastigotes lining the edges of parasitized histiocytes (Figure 2).
Systemic treatment options include sodium stibogluconate, amphotericin B, pentamidine, paromomycin, miltefosine, and azole antifungals.2,5 Geography often plays a critical role in selecting treatment options due to resistance rates of individual Leishmania species; for example, paromomycin compounds are more effective for cutaneous disease caused by Leishmania major than Leishmania tropica. Miltefosine is not effective for treating Leishmania braziliensis which can be acquired outside Guatemala, and higher doses of amphotericin B are recommended for visceral disease from East Africa.2,5 In patients with cutaneous leishmaniasis caused by L guyanensis, miltefosine remains a first-line option due to its oral formulation and long half-life within organisms, though there is a risk for teratogenicity.2 Amphotericin B remains the most effective treatment for visceral leishmaniasis and can be used off label to treat mucocutaneous disease or when cutaneous disease is refractory to other treatment options.3
Given the potential of L guyanensis to progress to mucocutaneous disease, monitoring for mucosal involvement should be performed at regular intervals for 6 months to 1 year.2 Treatment may be considered efficacious if no new skin lesions occur after 4 to 6 weeks of therapy; existing skin lesions should be re-epithelializing and reduced by 50% in size, with most cutaneous disease adequately controlled after 3 months of therapy.2
- Olivier M, Minguez-Menendez A, Fernandez-Prada C. Leishmania viannia guyanensis. Trends Parasitol. 2019;35:1018-1019. doi:10.1016 /j.pt.2019.06.008
- Singh R, Kashif M, Srivastava P, et al. Recent advances in chemotherapeutics for leishmaniasis: importance of the cellular biochemistry of the parasite and its molecular interaction with the host. Pathogens. 2023;12:706. doi:10.3390/pathogens12050706
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63: 1539-1557. doi:10.1093/cid/ciw742
- Specimen Collection Guide for Laboratory Diagnosis of Leishmaniasis. Centers for Disease Control and Prevention. Accessed October 14, 2025. https://www.cdc.gov/dpdx/diagnosticprocedures /other/leish.html
- Aronson NE, Joya CA. Cutaneous leishmaniasis: updates in diagnosis and management. Infect Dis Clin North Am. 2019;33:101-117. doi:10.1016/j.idc.2018.10.004
THE DIAGNOSIS: Cutaneous Leishmaniasis
The biopsy results revealed amastigotes at the periphery of parasitized histiocytes, consistent with a diagnosis of cutaneous leishmaniasis. Polymerase chain reaction analysis revealed Leishmania guyanensis species complex, which includes both L guyanensis and Leishmania panamensis. In this case of disseminated cutaneous leishmaniasis (Figure 1), our patient received a prolonged course of systemic therapy with oral miltefosine 50 mg 3 times daily. At the most recent follow-up appointment, she showed ongoing resolution of ulcerations, subcutaneous plaques, and lymphadenopathy on the trunk and face, but development of subcutaneous nodules continued on the arms and legs. At the next follow-up, physical examination revealed that the lesions slowly started to fade.

Leishmania species are parasites transmitted by bites of female sand flies, which belong to the genera Phlebotomus (Old World, Eastern Hemisphere) and Lutzomyia (New World, Western Hemisphere) genera.1 Leishmania species have a complex life cycle, propagating within human macrophages, ultimately leading to cutaneous, mucocutaneous, and visceral disease manifestations.2 Cutaneous leishmaniasis manifests classically as scattered, painless, slow-healing ulcers.3 A biopsy taken from the edge of a cutaneous ulcer for hematoxylin and eosin processing is recommended for initial diagnosis, and subsequent polymerase chain reaction of the sample is required for speciation, which guides therapeutic options.4,5 Classic hematoxylin and eosin and Giemsa stain findings include amastigotes lining the edges of parasitized histiocytes (Figure 2).

Systemic treatment options include sodium stibogluconate, amphotericin B, pentamidine, paromomycin, miltefosine, and azole antifungals.2,5 Geography often plays a critical role in selecting treatment options due to resistance rates of individual Leishmania species; for example, paromomycin compounds are more effective for cutaneous disease caused by Leishmania major than Leishmania tropica. Miltefosine is not effective for treating Leishmania braziliensis which can be acquired outside Guatemala, and higher doses of amphotericin B are recommended for visceral disease from East Africa.2,5 In patients with cutaneous leishmaniasis caused by L guyanensis, miltefosine remains a first-line option due to its oral formulation and long half-life within organisms, though there is a risk for teratogenicity.2 Amphotericin B remains the most effective treatment for visceral leishmaniasis and can be used off label to treat mucocutaneous disease or when cutaneous disease is refractory to other treatment options.3
Given the potential of L guyanensis to progress to mucocutaneous disease, monitoring for mucosal involvement should be performed at regular intervals for 6 months to 1 year.2 Treatment may be considered efficacious if no new skin lesions occur after 4 to 6 weeks of therapy; existing skin lesions should be re-epithelializing and reduced by 50% in size, with most cutaneous disease adequately controlled after 3 months of therapy.2
THE DIAGNOSIS: Cutaneous Leishmaniasis
The biopsy results revealed amastigotes at the periphery of parasitized histiocytes, consistent with a diagnosis of cutaneous leishmaniasis. Polymerase chain reaction analysis revealed Leishmania guyanensis species complex, which includes both L guyanensis and Leishmania panamensis. In this case of disseminated cutaneous leishmaniasis (Figure 1), our patient received a prolonged course of systemic therapy with oral miltefosine 50 mg 3 times daily. At the most recent follow-up appointment, she showed ongoing resolution of ulcerations, subcutaneous plaques, and lymphadenopathy on the trunk and face, but development of subcutaneous nodules continued on the arms and legs. At the next follow-up, physical examination revealed that the lesions slowly started to fade.

Leishmania species are parasites transmitted by bites of female sand flies, which belong to the genera Phlebotomus (Old World, Eastern Hemisphere) and Lutzomyia (New World, Western Hemisphere) genera.1 Leishmania species have a complex life cycle, propagating within human macrophages, ultimately leading to cutaneous, mucocutaneous, and visceral disease manifestations.2 Cutaneous leishmaniasis manifests classically as scattered, painless, slow-healing ulcers.3 A biopsy taken from the edge of a cutaneous ulcer for hematoxylin and eosin processing is recommended for initial diagnosis, and subsequent polymerase chain reaction of the sample is required for speciation, which guides therapeutic options.4,5 Classic hematoxylin and eosin and Giemsa stain findings include amastigotes lining the edges of parasitized histiocytes (Figure 2).

Systemic treatment options include sodium stibogluconate, amphotericin B, pentamidine, paromomycin, miltefosine, and azole antifungals.2,5 Geography often plays a critical role in selecting treatment options due to resistance rates of individual Leishmania species; for example, paromomycin compounds are more effective for cutaneous disease caused by Leishmania major than Leishmania tropica. Miltefosine is not effective for treating Leishmania braziliensis which can be acquired outside Guatemala, and higher doses of amphotericin B are recommended for visceral disease from East Africa.2,5 In patients with cutaneous leishmaniasis caused by L guyanensis, miltefosine remains a first-line option due to its oral formulation and long half-life within organisms, though there is a risk for teratogenicity.2 Amphotericin B remains the most effective treatment for visceral leishmaniasis and can be used off label to treat mucocutaneous disease or when cutaneous disease is refractory to other treatment options.3
Given the potential of L guyanensis to progress to mucocutaneous disease, monitoring for mucosal involvement should be performed at regular intervals for 6 months to 1 year.2 Treatment may be considered efficacious if no new skin lesions occur after 4 to 6 weeks of therapy; existing skin lesions should be re-epithelializing and reduced by 50% in size, with most cutaneous disease adequately controlled after 3 months of therapy.2
- Olivier M, Minguez-Menendez A, Fernandez-Prada C. Leishmania viannia guyanensis. Trends Parasitol. 2019;35:1018-1019. doi:10.1016 /j.pt.2019.06.008
- Singh R, Kashif M, Srivastava P, et al. Recent advances in chemotherapeutics for leishmaniasis: importance of the cellular biochemistry of the parasite and its molecular interaction with the host. Pathogens. 2023;12:706. doi:10.3390/pathogens12050706
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63: 1539-1557. doi:10.1093/cid/ciw742
- Specimen Collection Guide for Laboratory Diagnosis of Leishmaniasis. Centers for Disease Control and Prevention. Accessed October 14, 2025. https://www.cdc.gov/dpdx/diagnosticprocedures /other/leish.html
- Aronson NE, Joya CA. Cutaneous leishmaniasis: updates in diagnosis and management. Infect Dis Clin North Am. 2019;33:101-117. doi:10.1016/j.idc.2018.10.004
- Olivier M, Minguez-Menendez A, Fernandez-Prada C. Leishmania viannia guyanensis. Trends Parasitol. 2019;35:1018-1019. doi:10.1016 /j.pt.2019.06.008
- Singh R, Kashif M, Srivastava P, et al. Recent advances in chemotherapeutics for leishmaniasis: importance of the cellular biochemistry of the parasite and its molecular interaction with the host. Pathogens. 2023;12:706. doi:10.3390/pathogens12050706
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63: 1539-1557. doi:10.1093/cid/ciw742
- Specimen Collection Guide for Laboratory Diagnosis of Leishmaniasis. Centers for Disease Control and Prevention. Accessed October 14, 2025. https://www.cdc.gov/dpdx/diagnosticprocedures /other/leish.html
- Aronson NE, Joya CA. Cutaneous leishmaniasis: updates in diagnosis and management. Infect Dis Clin North Am. 2019;33:101-117. doi:10.1016/j.idc.2018.10.004
Spreading Ulcerations and Lymphadenopathy in a Traveler Returning from Costa Rica
Spreading Ulcerations and Lymphadenopathy in a Traveler Returning from Costa Rica
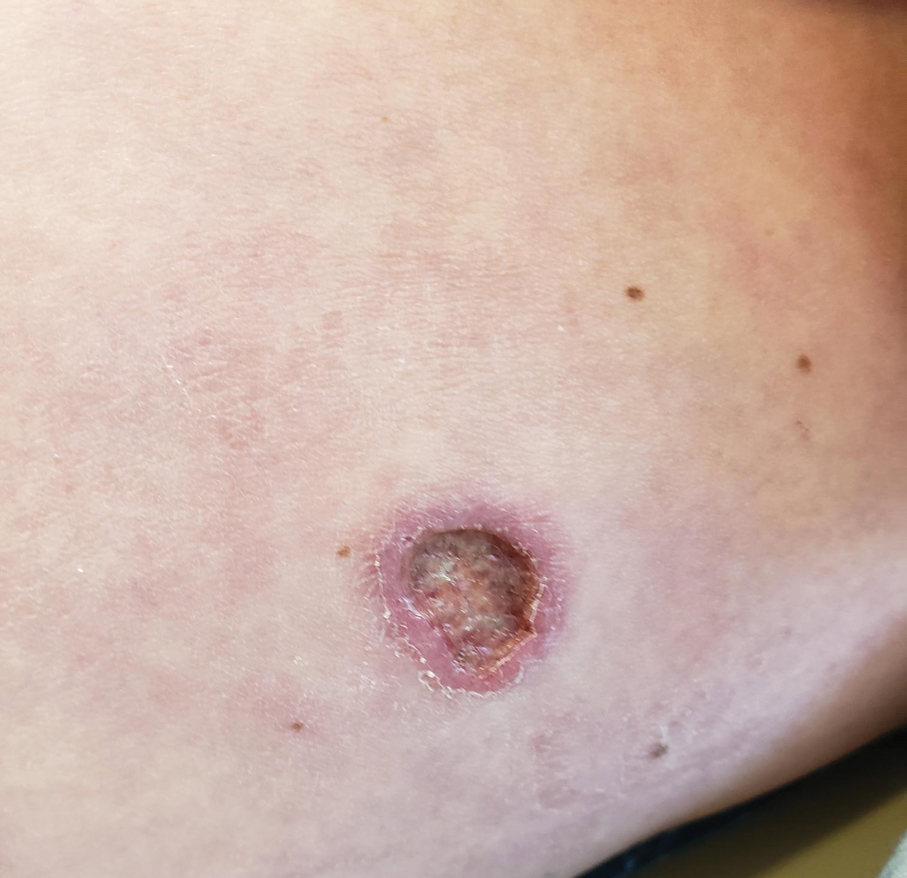
A 43-year-old woman presented to the dermatology clinic with widespread scaly plaques and ulcerations of 2 months’ duration. Her medical history was otherwise unremarkable. The patient reported that the eruption began after returning from a vacation to Costa Rica, during which she spent time on the beach and white-water rafting. She noted that she had been exposed to numerous insects during her trip, and that her roommate, who had accompanied her, had similar exposure history and lesions. The plaques were refractory to multiple oral antibiotics previously prescribed by primary care. Physical examination revealed submental lymphadenopathy and painless ulcerations with indurated borders without purulent drainage alongside scattered scaly papules and plaques on the face, neck, arms, and legs. A biopsy was taken from an ulceration edge on the left thigh.
Crusted Lesion at the Implantation Site of a Pacemaker
Crusted Lesion at the Implantation Site of a Pacemaker
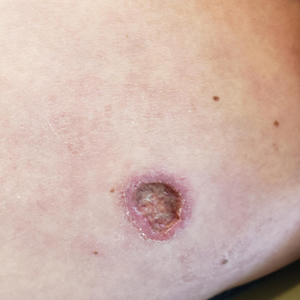
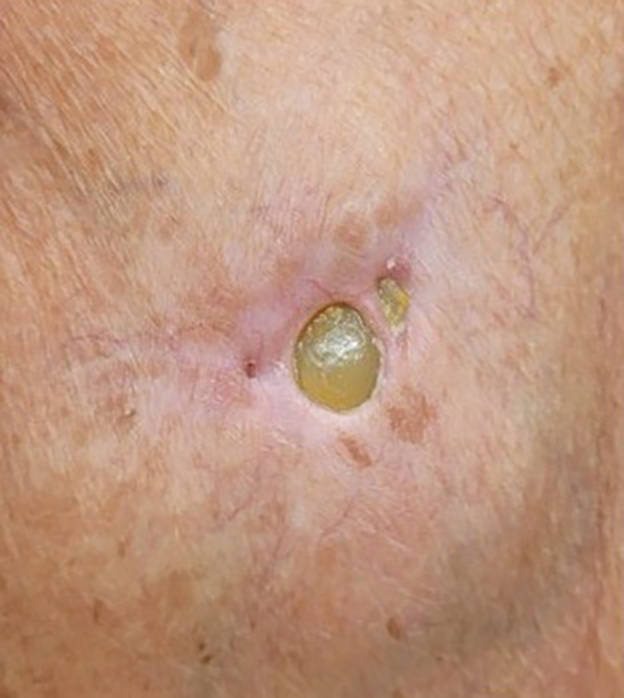
THE DIAGNOSIS: Pacemaker Extrusion
The lesion crust was easily scraped away to reveal extrusion of the permanent pacemaker (PPM) through the skin with a visible overlying gelatinous biofilm (Figure). The patient subsequently completed a 2-week course of clindamycin 300 mg 3 times daily followed by generator and lead removal, with reimplantation of the PPM into the right chest, as is the standard of care in the treatment of pacemaker extrusion.1
Ours is the first known reported case of pacemaker extrusion referred to dermatology with a primary concern for cutaneous malignancy. Pacemaker extrusion through the skin is not common, but it is the most common complication of PPM implantation, followed by infection.1 Pacemaker extrusion results from pressure necrosis and occurs when the PPM emerges through erythematous skin.1,2 Pacemaker extrusions generally are diagnosed by cardiology; however, it is important for dermatologists to recognize this phenomenon and differentiate it from other cutaneous pathologies, as the morphology of skin changes related to pacemaker extrusion through the skin can mimic cutaneous malignancy or other primary skin disease, especially if the outer layer of a biofilm that forms around the PPM hardens to form a crust. Our case emphasizes the importance of removing crusts when evaluating lesions.3
- Harcombe AA, Newell SA, Ludman PF, et al. Late complications following permanent pacemaker implantation or elective unit replacement. Heart. 1998;80:240-244. doi:10.1136/hrt.80.3.240
- Sanderson A, Hahn B. Pacemaker extrusion. Ann Emerg Med. 2013;62:648. doi:10.1016/j.annemergmed.2013.04.022
- Andrade AC, Hayashida MZ, Enokihara MMSES, et al. Dermoscopy of crusted lesion: diagnostic challenge and choice of technique for the analysis. An Bras Dermatol. 2021;96:387-388. doi:10.1016/j.abd.2020.06.016
THE DIAGNOSIS: Pacemaker Extrusion
The lesion crust was easily scraped away to reveal extrusion of the permanent pacemaker (PPM) through the skin with a visible overlying gelatinous biofilm (Figure). The patient subsequently completed a 2-week course of clindamycin 300 mg 3 times daily followed by generator and lead removal, with reimplantation of the PPM into the right chest, as is the standard of care in the treatment of pacemaker extrusion.1

Ours is the first known reported case of pacemaker extrusion referred to dermatology with a primary concern for cutaneous malignancy. Pacemaker extrusion through the skin is not common, but it is the most common complication of PPM implantation, followed by infection.1 Pacemaker extrusion results from pressure necrosis and occurs when the PPM emerges through erythematous skin.1,2 Pacemaker extrusions generally are diagnosed by cardiology; however, it is important for dermatologists to recognize this phenomenon and differentiate it from other cutaneous pathologies, as the morphology of skin changes related to pacemaker extrusion through the skin can mimic cutaneous malignancy or other primary skin disease, especially if the outer layer of a biofilm that forms around the PPM hardens to form a crust. Our case emphasizes the importance of removing crusts when evaluating lesions.3
THE DIAGNOSIS: Pacemaker Extrusion
The lesion crust was easily scraped away to reveal extrusion of the permanent pacemaker (PPM) through the skin with a visible overlying gelatinous biofilm (Figure). The patient subsequently completed a 2-week course of clindamycin 300 mg 3 times daily followed by generator and lead removal, with reimplantation of the PPM into the right chest, as is the standard of care in the treatment of pacemaker extrusion.1

Ours is the first known reported case of pacemaker extrusion referred to dermatology with a primary concern for cutaneous malignancy. Pacemaker extrusion through the skin is not common, but it is the most common complication of PPM implantation, followed by infection.1 Pacemaker extrusion results from pressure necrosis and occurs when the PPM emerges through erythematous skin.1,2 Pacemaker extrusions generally are diagnosed by cardiology; however, it is important for dermatologists to recognize this phenomenon and differentiate it from other cutaneous pathologies, as the morphology of skin changes related to pacemaker extrusion through the skin can mimic cutaneous malignancy or other primary skin disease, especially if the outer layer of a biofilm that forms around the PPM hardens to form a crust. Our case emphasizes the importance of removing crusts when evaluating lesions.3
- Harcombe AA, Newell SA, Ludman PF, et al. Late complications following permanent pacemaker implantation or elective unit replacement. Heart. 1998;80:240-244. doi:10.1136/hrt.80.3.240
- Sanderson A, Hahn B. Pacemaker extrusion. Ann Emerg Med. 2013;62:648. doi:10.1016/j.annemergmed.2013.04.022
- Andrade AC, Hayashida MZ, Enokihara MMSES, et al. Dermoscopy of crusted lesion: diagnostic challenge and choice of technique for the analysis. An Bras Dermatol. 2021;96:387-388. doi:10.1016/j.abd.2020.06.016
- Harcombe AA, Newell SA, Ludman PF, et al. Late complications following permanent pacemaker implantation or elective unit replacement. Heart. 1998;80:240-244. doi:10.1136/hrt.80.3.240
- Sanderson A, Hahn B. Pacemaker extrusion. Ann Emerg Med. 2013;62:648. doi:10.1016/j.annemergmed.2013.04.022
- Andrade AC, Hayashida MZ, Enokihara MMSES, et al. Dermoscopy of crusted lesion: diagnostic challenge and choice of technique for the analysis. An Bras Dermatol. 2021;96:387-388. doi:10.1016/j.abd.2020.06.016
Crusted Lesion at the Implantation Site of a Pacemaker
Crusted Lesion at the Implantation Site of a Pacemaker
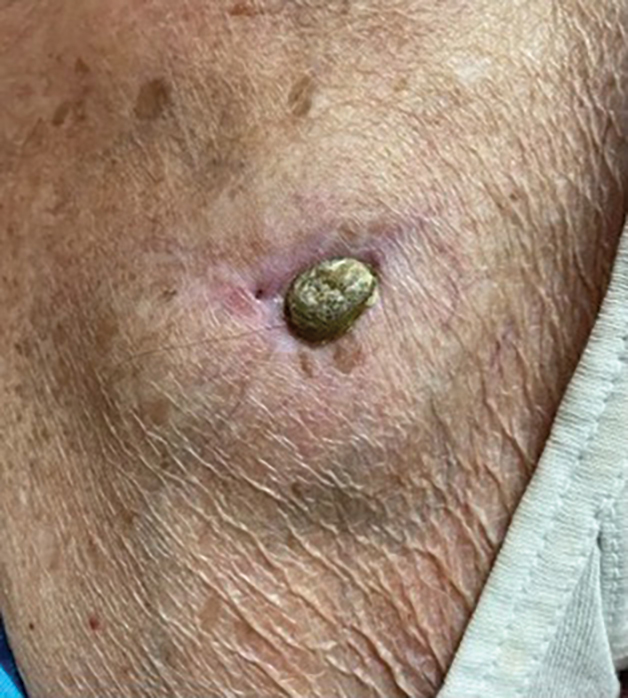
A 78-year-old woman was referred to dermatology from the cardiology clinic with concerns of a nonhealing, scablike lesion on the left chest over the implantation site of a dual-chamber permanent pacemaker (PPM). Eight months prior, the patient underwent successful PPM implantation for symptomatic bradycardia and second-degree atrioventricular block. Her cardiologists subsequently noticed an oozing crusting scab at the site of implantation and eventually referred her to dermatology with concerns for squamous cell carcinoma. Physical examination at the current presentation revealed an exophytic serous crust overlying the PPM implantation site on the left chest.
Intralesional Methotrexate: A Cost-Effective, High-Efficacy Alternative to Surgery for Cutaneous Squamous Cell Carcinoma
Intralesional Methotrexate: A Cost-Effective, High-Efficacy Alternative to Surgery for Cutaneous Squamous Cell Carcinoma
Squamous cell carcinoma (SCC) is the malignant proliferation of keratinocytes in the epidermis of the skin. Most SCCs are caused by UV light exposure, with sex and increased age acting as the primary known risk factors: SCCs are nearly twice as prevalent in men vs women, and the average age of presentation is the middle of the seventh decade of life.1 In the United States, there are an estimated 1.8 million new SCC cases annually.2 Although not usually life threatening, if left untreated, SCC can metastasize, thereby reducing the 10-year survival rate from above 90% with treatment to 16%.3-6
Most invasive SCC lesions are treated surgically, but intralesional methotrexate (IL-MTX) has emerged as an alternative treatment for cutaneous SCC. It offers the potential for lower-cost, efficacious outpatient treatment.7-12 Methotrexate competitively inhibits the enzyme dihydrofolate reductase, which converts dihydrofolate into tetrahydrofolate.13 In doing so, MTX indirectly prevents the synthesis of thymine, a nucleotide required for DNA synthesis. Thus, MTX can halt DNA synthesis and consequently, cell division. Intralesional MTX has been shown to successfully treat keratoacanthomas, lymphomas, and various inflammatory dermatologic conditions.8-12
Surgical options include standard excision, Mohs micrographic surgery, or electrodesiccation and curettage. Surgical treatment has high (92% to 99%) cure rates and typically requires only 1 or 2 appointments.14,15 Although costs can vary, one 2012 study using Medicare fee schedules found that total costs (including primary procedure, biopsy, follow-up appointments through 2 months, and other associated costs) for cutaneous SCC were $475 for electrodesiccation and curettage, $1302.92 for excision, and $2093.14 for Mohs micrographic surgery.16 For some patients, surgery is not an ideal option due to the tumor location, poor wound healing, anticoagulation, and cost. In these patients, photodynamic therapy, topical therapy with 5-fluorouracil or imiquimod, radiation, and cryotherapy are options listed in the American Academy of Dermatology guidelines.15 Compared with surgery, radiation is more demanding on the patient, often requiring multiple visits a week and including common undesirable adverse effects such as radiation dermatitis and prolonged wounds on the lower legs.17 Radiation also can be costly, with one study reporting costs between $2559 and $3431 for SCC of the forearm.18 Furthermore, in young patients, radiotherapy can increase the risk for developing nonmelanoma skin cancer later in life.16
Intralesional MTX is a localized treatment option that avoids the high costs of surgery, the side effects of radiotherapy, prolonged healing, and the systemic effects of chemotherapy. Treatment with IL-MTX can vary depending on the number of treatments necessary but usually only costs a few hundred dollars, rarely costing more than $1000.7 Although IL-MTX is less expensive, it typically requires several follow-up visits, whereas surgical removal may only require 1 visit.
Prior research has noted the efficacy of IL-MTX as a neoadjuvant therapy, with one study finding that IL-MTX can reduce the size of SCC lesions by an average of 0.52 cm2 prior to surgery.19 Several case studies also have documented the effectiveness of IL-MTX as a treatment for SCC.20-22 However, larger studies involving multiple patients to evaluate the efficacy of IL-MTX as a sole treatment for SCC are lacking. Gualdi et al23 looked at the outcomes (complete resolution, partial response, or no response) for SCC treated with IL-MTX and found that 62% (13/21) of patients experienced improvement, with 48% (10/21) experiencing at least 50% improvement. Although these results are promising, further research is needed.
Our study sought to examine IL-MTX efficacy as well as evaluate the dosage and number of appointments/sessions needed to achieve resolution of the lesions.
Methods
We conducted a retrospective chart review of patients who received only IL-MTX for clinically evident or biopsy-proven SCC at US Dermatology Partners clinics in Phoenix, Arizona, from January 1, 2022, to June 30, 2023. Patients aged 18 to 89 years were included, and they had not received other treatment for their SCC lesions such as radiation or systemic chemotherapy. Each patient received at least 1 dose of IL-MTX, beginning with a concentration of 12.5 mg/mL and with all subsequent doses at a concentration of 25 mg/mL (low dose vs high dose). Lesion resolution was categorized as no gross clinical tumor on follow-up. Patients received additional doses of IL-MTX based on the clinical appearance of their lesion(s).
Patient-level descriptive statistics are reported as mean (SD) or median (interquartile range [IQR]) for continuous variables as well as frequency and percentage for categorical variables. To account for the correlation of multiple lesions within individual patients, marginal Cox proportional hazard models were used. Time as well as cumulative dose to lesion resolution were evaluated and presented via the cumulative hazard function, while differences in resolution were estimated using separate Cox models for age, sex, and initial dose.
Results
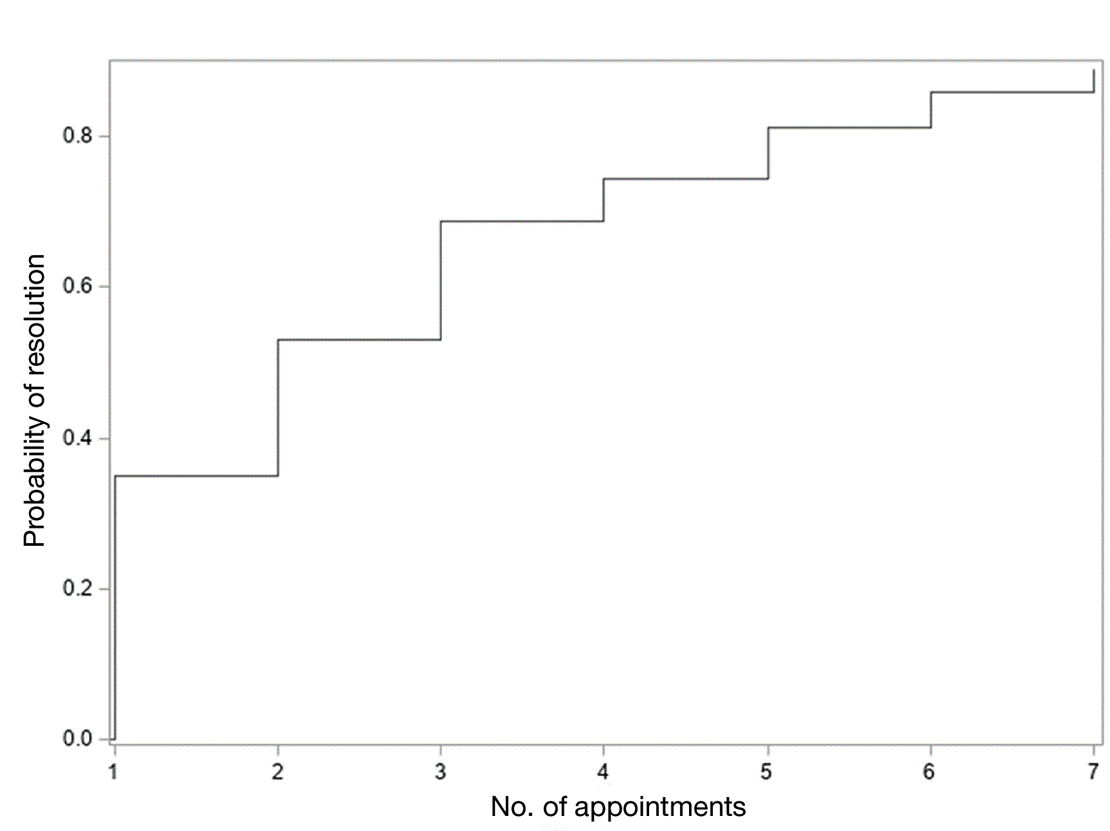
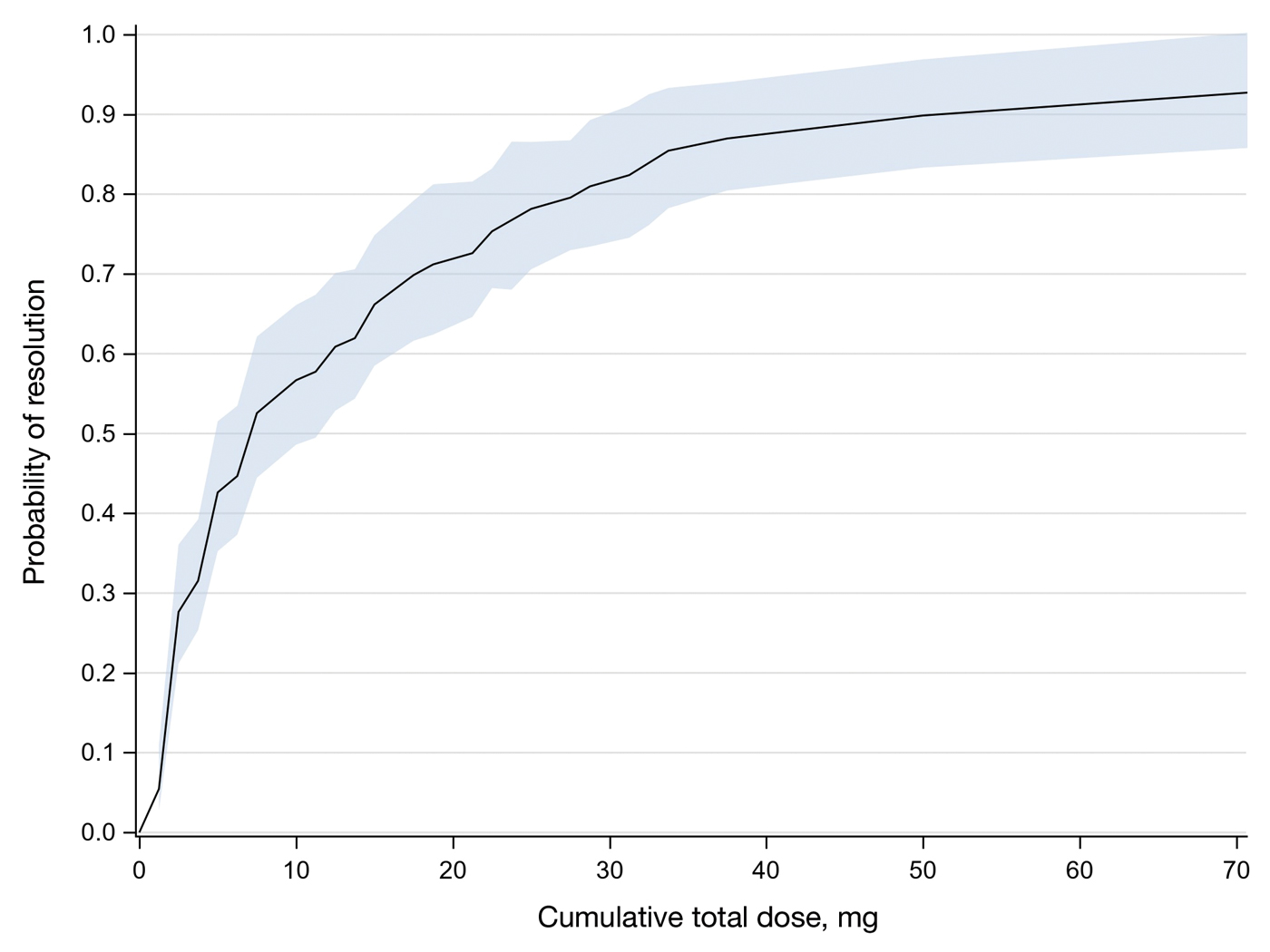
In total, 107 different lesions from 21 patients were included in the analysis. The median number of lesions was 4 per patient (range, 1-15; IQR, 2-7), with a mean (SD) age of 80 (6) years. Patients were primarily female (81% [17/21]). From the data provided, the majority of lesions (83% [89/107]) resolved with IL-MTX. Of the 18 unresolved lesions, 5 (5%) were referred for a different procedure, and the remaining 13 (12%) were censored (lost to follow-up). Figure 1 provides the cumulative incidence function for lesion resolution. Approximately 50% of patient lesions resolved by the second appointment. Similarly, Figure 2 provides the cumulative dose function for lesion resolution; the median cumulative total dose for resolution was 5 mg (IQR, 2.5–12.5). Finally, concerning the ratio for case resolution, no difference in hazard ratio (HR) was observed for age (female vs male, HR: 1.01; 95% CI: 0.96-1.06), biological sex (HR, 1.01; 95% CI, 0.63-1.63), or initial dose (high vs low, HR: 1.13; 95% CI: 0.77-1.65).
Comment
Results of this study demonstrate the efficacy of IL-MTX for the treatment of cutaneous SCC. More than 80% of the lesions resolved by IL-MTX alone. This treatment approach is more cost-effective with fewer adverse effects when compared to other options. In our study, treatment with IL-MTX also proved to be reasonable in terms of the number of appointments and total dose required, with more than 50% of lesions resolving within 2 appointments and a median cumulative total dose of 5 mg. Intralesional MTX appears to be similarly efficacious in men and women, and the concentration of the initial dose (12.5 mg/mL vs 25 mg/mL) does not change the treatment outcome.
Although these data are encouraging for the use of IL-MTX in the treatment of SCC, future work should consider the relationships between lesion characteristics (such as size and location) and case resolution with IL-MTX as well as recurrence rates with lesions treated by IL-MTX compared to other treatment options.
Conclusion
This study demonstrated the efficacy of IL-MTX as a treatment for SCC that is cost-effective, avoids bothersome side effects, and can be accomplished in relatively few appointments. However, more data are needed to characterize the lesion type best suited to this treatment.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- The Skin Cancer Foundation. Skin cancer facts & statistics: what you need to know. Updated January 2026. Accessed January 20, 2026. https://www.skincancer.org/skin-cancer-information/skin-cancer-facts
- Rees JR, Zens MS, Celaya MO, et al. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878-884.
- Weinberg A, Ogle C, Shin E. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007;33:885-899.
- Varra V, Woody NM, Reddy C, et al. Suboptimal outcomes in cutaneous squamous cell cancer of the head and neck with nodal metastases. Anticancer Res. 2018;38:5825-5830. doi:10.21873/anticanres.12923
- Epstein E, Epstein NN, Bragg K, et al. Metastases from squamous cell carcinomas of the skin. Arch Dermatol. 1968;97:245-251.
- Chitwood K, Etzkorn J, Cohen G. Topical and intralesional treatment of nonmelanoma skin cancer: efficacy and cost comparisons. Dermatol Surg. 2013;39:1306-1316
- Scalvenzi M, Patrì A, Costa C, et al. Intralesional methotrexate for the treatment of keratoacanthoma: the Neapolitan experience. Dermatol Ther. 2019;9:369-372.
- Patel NP, Cervino AL. Treatment of keratoacanthoma: is intralesional methotrexate an option? Can J Plast Surg. 2011;19:E15-E18.
- Smith C, Srivastava D, Nijhawan RI. Intralesional methotrexate for keratoacanthomas: a retrospective cohort study. JAAD Int. 2020;83:904-905.
- Blume JE, Stoll HL, Cheney RT. Treatment of primary cutaneous CD30+ anaplastic large cell lymphoma with intralesional methotrexate. J Am Acad Dermatol. 2006;54(5 Suppl):S229-S230.
- Nedelcu RI, Balaban M, Turcu G, et al. Efficacy of methotrexate as anti‑inflammatory and anti‑proliferative drug in dermatology: three case reports. Exp Ther Med. 2019;18:905-910.
- Lester RS. Methotrexate. Clin Dermatol. 1989;7:128-135.
- Roenigk RK, Roenigk HH. Current surgical management of skin cancer in dermatology. J Dermatol Surg Oncol. 1990;16:136-151.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
- Wilson LS, Pregenzer M, Basu R, et al. Fee comparisons of treatments for nonmelanoma skin cancer in a private practice academic setting. Dermatol Surg. 2012;38:570-584.
- DeConti RC. Chemotherapy of squamous cell carcinoma of the skin. Semin Oncol. 2012;39:145-149.
- Rogers HW, Coldiron BM. A relative value unit–based cost comparison of treatment modalities for nonmelanoma skin cancer: effect of the loss of the Mohs multiple surgery reduction exemption. J Am Acad Dermatol. 2009;61:96-103.
- Salido-Vallejo R, Cuevas-Asencio I, Garnacho-Sucedo G, et al. Neoadjuvant intralesional methotrexate in cutaneous squamous cell carcinoma: a comparative cohort study. J Eur Acad Dermatol Venereol. 2016;30:1120-1124.
- Salido-Vallejo R, Garnacho-Saucedo G, Sánchez-Arca M, et al. Neoadjuvant intralesional methotrexate before surgical treatment of invasive squamous cell carcinoma of the lower lip. Dermatol Surg. 2012;38:1849-1850.
- Vega-González LG, Morales-Pérez MI, Molina-Pérez T, et al. Successful treatment of squamous cell carcinoma with intralesional methotrexate. JAAD Case Rep. 2022;24:68-70.
- Moye MS, Clark AH, Legler AA, et al. Intralesional methotrexate for treatment of invasive squamous cell carcinomas in a patient taking vemurafenib for treatment of metastatic melanoma. J Clin Oncol. 2016;34:E134-E136.
- Gualdi G, Caravello S, Frasci F, et al. Intralesional methotrexate for the treatment of advanced keratinocytic tumors: a multi-center retrospective study. Dermatol Ther (Heidelb). 2020;10:769-777.
Squamous cell carcinoma (SCC) is the malignant proliferation of keratinocytes in the epidermis of the skin. Most SCCs are caused by UV light exposure, with sex and increased age acting as the primary known risk factors: SCCs are nearly twice as prevalent in men vs women, and the average age of presentation is the middle of the seventh decade of life.1 In the United States, there are an estimated 1.8 million new SCC cases annually.2 Although not usually life threatening, if left untreated, SCC can metastasize, thereby reducing the 10-year survival rate from above 90% with treatment to 16%.3-6
Most invasive SCC lesions are treated surgically, but intralesional methotrexate (IL-MTX) has emerged as an alternative treatment for cutaneous SCC. It offers the potential for lower-cost, efficacious outpatient treatment.7-12 Methotrexate competitively inhibits the enzyme dihydrofolate reductase, which converts dihydrofolate into tetrahydrofolate.13 In doing so, MTX indirectly prevents the synthesis of thymine, a nucleotide required for DNA synthesis. Thus, MTX can halt DNA synthesis and consequently, cell division. Intralesional MTX has been shown to successfully treat keratoacanthomas, lymphomas, and various inflammatory dermatologic conditions.8-12
Surgical options include standard excision, Mohs micrographic surgery, or electrodesiccation and curettage. Surgical treatment has high (92% to 99%) cure rates and typically requires only 1 or 2 appointments.14,15 Although costs can vary, one 2012 study using Medicare fee schedules found that total costs (including primary procedure, biopsy, follow-up appointments through 2 months, and other associated costs) for cutaneous SCC were $475 for electrodesiccation and curettage, $1302.92 for excision, and $2093.14 for Mohs micrographic surgery.16 For some patients, surgery is not an ideal option due to the tumor location, poor wound healing, anticoagulation, and cost. In these patients, photodynamic therapy, topical therapy with 5-fluorouracil or imiquimod, radiation, and cryotherapy are options listed in the American Academy of Dermatology guidelines.15 Compared with surgery, radiation is more demanding on the patient, often requiring multiple visits a week and including common undesirable adverse effects such as radiation dermatitis and prolonged wounds on the lower legs.17 Radiation also can be costly, with one study reporting costs between $2559 and $3431 for SCC of the forearm.18 Furthermore, in young patients, radiotherapy can increase the risk for developing nonmelanoma skin cancer later in life.16
Intralesional MTX is a localized treatment option that avoids the high costs of surgery, the side effects of radiotherapy, prolonged healing, and the systemic effects of chemotherapy. Treatment with IL-MTX can vary depending on the number of treatments necessary but usually only costs a few hundred dollars, rarely costing more than $1000.7 Although IL-MTX is less expensive, it typically requires several follow-up visits, whereas surgical removal may only require 1 visit.
Prior research has noted the efficacy of IL-MTX as a neoadjuvant therapy, with one study finding that IL-MTX can reduce the size of SCC lesions by an average of 0.52 cm2 prior to surgery.19 Several case studies also have documented the effectiveness of IL-MTX as a treatment for SCC.20-22 However, larger studies involving multiple patients to evaluate the efficacy of IL-MTX as a sole treatment for SCC are lacking. Gualdi et al23 looked at the outcomes (complete resolution, partial response, or no response) for SCC treated with IL-MTX and found that 62% (13/21) of patients experienced improvement, with 48% (10/21) experiencing at least 50% improvement. Although these results are promising, further research is needed.
Our study sought to examine IL-MTX efficacy as well as evaluate the dosage and number of appointments/sessions needed to achieve resolution of the lesions.
Methods
We conducted a retrospective chart review of patients who received only IL-MTX for clinically evident or biopsy-proven SCC at US Dermatology Partners clinics in Phoenix, Arizona, from January 1, 2022, to June 30, 2023. Patients aged 18 to 89 years were included, and they had not received other treatment for their SCC lesions such as radiation or systemic chemotherapy. Each patient received at least 1 dose of IL-MTX, beginning with a concentration of 12.5 mg/mL and with all subsequent doses at a concentration of 25 mg/mL (low dose vs high dose). Lesion resolution was categorized as no gross clinical tumor on follow-up. Patients received additional doses of IL-MTX based on the clinical appearance of their lesion(s).
Patient-level descriptive statistics are reported as mean (SD) or median (interquartile range [IQR]) for continuous variables as well as frequency and percentage for categorical variables. To account for the correlation of multiple lesions within individual patients, marginal Cox proportional hazard models were used. Time as well as cumulative dose to lesion resolution were evaluated and presented via the cumulative hazard function, while differences in resolution were estimated using separate Cox models for age, sex, and initial dose.
Results
In total, 107 different lesions from 21 patients were included in the analysis. The median number of lesions was 4 per patient (range, 1-15; IQR, 2-7), with a mean (SD) age of 80 (6) years. Patients were primarily female (81% [17/21]). From the data provided, the majority of lesions (83% [89/107]) resolved with IL-MTX. Of the 18 unresolved lesions, 5 (5%) were referred for a different procedure, and the remaining 13 (12%) were censored (lost to follow-up). Figure 1 provides the cumulative incidence function for lesion resolution. Approximately 50% of patient lesions resolved by the second appointment. Similarly, Figure 2 provides the cumulative dose function for lesion resolution; the median cumulative total dose for resolution was 5 mg (IQR, 2.5–12.5). Finally, concerning the ratio for case resolution, no difference in hazard ratio (HR) was observed for age (female vs male, HR: 1.01; 95% CI: 0.96-1.06), biological sex (HR, 1.01; 95% CI, 0.63-1.63), or initial dose (high vs low, HR: 1.13; 95% CI: 0.77-1.65).


Comment
Results of this study demonstrate the efficacy of IL-MTX for the treatment of cutaneous SCC. More than 80% of the lesions resolved by IL-MTX alone. This treatment approach is more cost-effective with fewer adverse effects when compared to other options. In our study, treatment with IL-MTX also proved to be reasonable in terms of the number of appointments and total dose required, with more than 50% of lesions resolving within 2 appointments and a median cumulative total dose of 5 mg. Intralesional MTX appears to be similarly efficacious in men and women, and the concentration of the initial dose (12.5 mg/mL vs 25 mg/mL) does not change the treatment outcome.
Although these data are encouraging for the use of IL-MTX in the treatment of SCC, future work should consider the relationships between lesion characteristics (such as size and location) and case resolution with IL-MTX as well as recurrence rates with lesions treated by IL-MTX compared to other treatment options.
Conclusion
This study demonstrated the efficacy of IL-MTX as a treatment for SCC that is cost-effective, avoids bothersome side effects, and can be accomplished in relatively few appointments. However, more data are needed to characterize the lesion type best suited to this treatment.
Squamous cell carcinoma (SCC) is the malignant proliferation of keratinocytes in the epidermis of the skin. Most SCCs are caused by UV light exposure, with sex and increased age acting as the primary known risk factors: SCCs are nearly twice as prevalent in men vs women, and the average age of presentation is the middle of the seventh decade of life.1 In the United States, there are an estimated 1.8 million new SCC cases annually.2 Although not usually life threatening, if left untreated, SCC can metastasize, thereby reducing the 10-year survival rate from above 90% with treatment to 16%.3-6
Most invasive SCC lesions are treated surgically, but intralesional methotrexate (IL-MTX) has emerged as an alternative treatment for cutaneous SCC. It offers the potential for lower-cost, efficacious outpatient treatment.7-12 Methotrexate competitively inhibits the enzyme dihydrofolate reductase, which converts dihydrofolate into tetrahydrofolate.13 In doing so, MTX indirectly prevents the synthesis of thymine, a nucleotide required for DNA synthesis. Thus, MTX can halt DNA synthesis and consequently, cell division. Intralesional MTX has been shown to successfully treat keratoacanthomas, lymphomas, and various inflammatory dermatologic conditions.8-12
Surgical options include standard excision, Mohs micrographic surgery, or electrodesiccation and curettage. Surgical treatment has high (92% to 99%) cure rates and typically requires only 1 or 2 appointments.14,15 Although costs can vary, one 2012 study using Medicare fee schedules found that total costs (including primary procedure, biopsy, follow-up appointments through 2 months, and other associated costs) for cutaneous SCC were $475 for electrodesiccation and curettage, $1302.92 for excision, and $2093.14 for Mohs micrographic surgery.16 For some patients, surgery is not an ideal option due to the tumor location, poor wound healing, anticoagulation, and cost. In these patients, photodynamic therapy, topical therapy with 5-fluorouracil or imiquimod, radiation, and cryotherapy are options listed in the American Academy of Dermatology guidelines.15 Compared with surgery, radiation is more demanding on the patient, often requiring multiple visits a week and including common undesirable adverse effects such as radiation dermatitis and prolonged wounds on the lower legs.17 Radiation also can be costly, with one study reporting costs between $2559 and $3431 for SCC of the forearm.18 Furthermore, in young patients, radiotherapy can increase the risk for developing nonmelanoma skin cancer later in life.16
Intralesional MTX is a localized treatment option that avoids the high costs of surgery, the side effects of radiotherapy, prolonged healing, and the systemic effects of chemotherapy. Treatment with IL-MTX can vary depending on the number of treatments necessary but usually only costs a few hundred dollars, rarely costing more than $1000.7 Although IL-MTX is less expensive, it typically requires several follow-up visits, whereas surgical removal may only require 1 visit.
Prior research has noted the efficacy of IL-MTX as a neoadjuvant therapy, with one study finding that IL-MTX can reduce the size of SCC lesions by an average of 0.52 cm2 prior to surgery.19 Several case studies also have documented the effectiveness of IL-MTX as a treatment for SCC.20-22 However, larger studies involving multiple patients to evaluate the efficacy of IL-MTX as a sole treatment for SCC are lacking. Gualdi et al23 looked at the outcomes (complete resolution, partial response, or no response) for SCC treated with IL-MTX and found that 62% (13/21) of patients experienced improvement, with 48% (10/21) experiencing at least 50% improvement. Although these results are promising, further research is needed.
Our study sought to examine IL-MTX efficacy as well as evaluate the dosage and number of appointments/sessions needed to achieve resolution of the lesions.
Methods
We conducted a retrospective chart review of patients who received only IL-MTX for clinically evident or biopsy-proven SCC at US Dermatology Partners clinics in Phoenix, Arizona, from January 1, 2022, to June 30, 2023. Patients aged 18 to 89 years were included, and they had not received other treatment for their SCC lesions such as radiation or systemic chemotherapy. Each patient received at least 1 dose of IL-MTX, beginning with a concentration of 12.5 mg/mL and with all subsequent doses at a concentration of 25 mg/mL (low dose vs high dose). Lesion resolution was categorized as no gross clinical tumor on follow-up. Patients received additional doses of IL-MTX based on the clinical appearance of their lesion(s).
Patient-level descriptive statistics are reported as mean (SD) or median (interquartile range [IQR]) for continuous variables as well as frequency and percentage for categorical variables. To account for the correlation of multiple lesions within individual patients, marginal Cox proportional hazard models were used. Time as well as cumulative dose to lesion resolution were evaluated and presented via the cumulative hazard function, while differences in resolution were estimated using separate Cox models for age, sex, and initial dose.
Results
In total, 107 different lesions from 21 patients were included in the analysis. The median number of lesions was 4 per patient (range, 1-15; IQR, 2-7), with a mean (SD) age of 80 (6) years. Patients were primarily female (81% [17/21]). From the data provided, the majority of lesions (83% [89/107]) resolved with IL-MTX. Of the 18 unresolved lesions, 5 (5%) were referred for a different procedure, and the remaining 13 (12%) were censored (lost to follow-up). Figure 1 provides the cumulative incidence function for lesion resolution. Approximately 50% of patient lesions resolved by the second appointment. Similarly, Figure 2 provides the cumulative dose function for lesion resolution; the median cumulative total dose for resolution was 5 mg (IQR, 2.5–12.5). Finally, concerning the ratio for case resolution, no difference in hazard ratio (HR) was observed for age (female vs male, HR: 1.01; 95% CI: 0.96-1.06), biological sex (HR, 1.01; 95% CI, 0.63-1.63), or initial dose (high vs low, HR: 1.13; 95% CI: 0.77-1.65).


Comment
Results of this study demonstrate the efficacy of IL-MTX for the treatment of cutaneous SCC. More than 80% of the lesions resolved by IL-MTX alone. This treatment approach is more cost-effective with fewer adverse effects when compared to other options. In our study, treatment with IL-MTX also proved to be reasonable in terms of the number of appointments and total dose required, with more than 50% of lesions resolving within 2 appointments and a median cumulative total dose of 5 mg. Intralesional MTX appears to be similarly efficacious in men and women, and the concentration of the initial dose (12.5 mg/mL vs 25 mg/mL) does not change the treatment outcome.
Although these data are encouraging for the use of IL-MTX in the treatment of SCC, future work should consider the relationships between lesion characteristics (such as size and location) and case resolution with IL-MTX as well as recurrence rates with lesions treated by IL-MTX compared to other treatment options.
Conclusion
This study demonstrated the efficacy of IL-MTX as a treatment for SCC that is cost-effective, avoids bothersome side effects, and can be accomplished in relatively few appointments. However, more data are needed to characterize the lesion type best suited to this treatment.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- The Skin Cancer Foundation. Skin cancer facts & statistics: what you need to know. Updated January 2026. Accessed January 20, 2026. https://www.skincancer.org/skin-cancer-information/skin-cancer-facts
- Rees JR, Zens MS, Celaya MO, et al. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878-884.
- Weinberg A, Ogle C, Shin E. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007;33:885-899.
- Varra V, Woody NM, Reddy C, et al. Suboptimal outcomes in cutaneous squamous cell cancer of the head and neck with nodal metastases. Anticancer Res. 2018;38:5825-5830. doi:10.21873/anticanres.12923
- Epstein E, Epstein NN, Bragg K, et al. Metastases from squamous cell carcinomas of the skin. Arch Dermatol. 1968;97:245-251.
- Chitwood K, Etzkorn J, Cohen G. Topical and intralesional treatment of nonmelanoma skin cancer: efficacy and cost comparisons. Dermatol Surg. 2013;39:1306-1316
- Scalvenzi M, Patrì A, Costa C, et al. Intralesional methotrexate for the treatment of keratoacanthoma: the Neapolitan experience. Dermatol Ther. 2019;9:369-372.
- Patel NP, Cervino AL. Treatment of keratoacanthoma: is intralesional methotrexate an option? Can J Plast Surg. 2011;19:E15-E18.
- Smith C, Srivastava D, Nijhawan RI. Intralesional methotrexate for keratoacanthomas: a retrospective cohort study. JAAD Int. 2020;83:904-905.
- Blume JE, Stoll HL, Cheney RT. Treatment of primary cutaneous CD30+ anaplastic large cell lymphoma with intralesional methotrexate. J Am Acad Dermatol. 2006;54(5 Suppl):S229-S230.
- Nedelcu RI, Balaban M, Turcu G, et al. Efficacy of methotrexate as anti‑inflammatory and anti‑proliferative drug in dermatology: three case reports. Exp Ther Med. 2019;18:905-910.
- Lester RS. Methotrexate. Clin Dermatol. 1989;7:128-135.
- Roenigk RK, Roenigk HH. Current surgical management of skin cancer in dermatology. J Dermatol Surg Oncol. 1990;16:136-151.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
- Wilson LS, Pregenzer M, Basu R, et al. Fee comparisons of treatments for nonmelanoma skin cancer in a private practice academic setting. Dermatol Surg. 2012;38:570-584.
- DeConti RC. Chemotherapy of squamous cell carcinoma of the skin. Semin Oncol. 2012;39:145-149.
- Rogers HW, Coldiron BM. A relative value unit–based cost comparison of treatment modalities for nonmelanoma skin cancer: effect of the loss of the Mohs multiple surgery reduction exemption. J Am Acad Dermatol. 2009;61:96-103.
- Salido-Vallejo R, Cuevas-Asencio I, Garnacho-Sucedo G, et al. Neoadjuvant intralesional methotrexate in cutaneous squamous cell carcinoma: a comparative cohort study. J Eur Acad Dermatol Venereol. 2016;30:1120-1124.
- Salido-Vallejo R, Garnacho-Saucedo G, Sánchez-Arca M, et al. Neoadjuvant intralesional methotrexate before surgical treatment of invasive squamous cell carcinoma of the lower lip. Dermatol Surg. 2012;38:1849-1850.
- Vega-González LG, Morales-Pérez MI, Molina-Pérez T, et al. Successful treatment of squamous cell carcinoma with intralesional methotrexate. JAAD Case Rep. 2022;24:68-70.
- Moye MS, Clark AH, Legler AA, et al. Intralesional methotrexate for treatment of invasive squamous cell carcinomas in a patient taking vemurafenib for treatment of metastatic melanoma. J Clin Oncol. 2016;34:E134-E136.
- Gualdi G, Caravello S, Frasci F, et al. Intralesional methotrexate for the treatment of advanced keratinocytic tumors: a multi-center retrospective study. Dermatol Ther (Heidelb). 2020;10:769-777.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- The Skin Cancer Foundation. Skin cancer facts & statistics: what you need to know. Updated January 2026. Accessed January 20, 2026. https://www.skincancer.org/skin-cancer-information/skin-cancer-facts
- Rees JR, Zens MS, Celaya MO, et al. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878-884.
- Weinberg A, Ogle C, Shin E. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007;33:885-899.
- Varra V, Woody NM, Reddy C, et al. Suboptimal outcomes in cutaneous squamous cell cancer of the head and neck with nodal metastases. Anticancer Res. 2018;38:5825-5830. doi:10.21873/anticanres.12923
- Epstein E, Epstein NN, Bragg K, et al. Metastases from squamous cell carcinomas of the skin. Arch Dermatol. 1968;97:245-251.
- Chitwood K, Etzkorn J, Cohen G. Topical and intralesional treatment of nonmelanoma skin cancer: efficacy and cost comparisons. Dermatol Surg. 2013;39:1306-1316
- Scalvenzi M, Patrì A, Costa C, et al. Intralesional methotrexate for the treatment of keratoacanthoma: the Neapolitan experience. Dermatol Ther. 2019;9:369-372.
- Patel NP, Cervino AL. Treatment of keratoacanthoma: is intralesional methotrexate an option? Can J Plast Surg. 2011;19:E15-E18.
- Smith C, Srivastava D, Nijhawan RI. Intralesional methotrexate for keratoacanthomas: a retrospective cohort study. JAAD Int. 2020;83:904-905.
- Blume JE, Stoll HL, Cheney RT. Treatment of primary cutaneous CD30+ anaplastic large cell lymphoma with intralesional methotrexate. J Am Acad Dermatol. 2006;54(5 Suppl):S229-S230.
- Nedelcu RI, Balaban M, Turcu G, et al. Efficacy of methotrexate as anti‑inflammatory and anti‑proliferative drug in dermatology: three case reports. Exp Ther Med. 2019;18:905-910.
- Lester RS. Methotrexate. Clin Dermatol. 1989;7:128-135.
- Roenigk RK, Roenigk HH. Current surgical management of skin cancer in dermatology. J Dermatol Surg Oncol. 1990;16:136-151.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
- Wilson LS, Pregenzer M, Basu R, et al. Fee comparisons of treatments for nonmelanoma skin cancer in a private practice academic setting. Dermatol Surg. 2012;38:570-584.
- DeConti RC. Chemotherapy of squamous cell carcinoma of the skin. Semin Oncol. 2012;39:145-149.
- Rogers HW, Coldiron BM. A relative value unit–based cost comparison of treatment modalities for nonmelanoma skin cancer: effect of the loss of the Mohs multiple surgery reduction exemption. J Am Acad Dermatol. 2009;61:96-103.
- Salido-Vallejo R, Cuevas-Asencio I, Garnacho-Sucedo G, et al. Neoadjuvant intralesional methotrexate in cutaneous squamous cell carcinoma: a comparative cohort study. J Eur Acad Dermatol Venereol. 2016;30:1120-1124.
- Salido-Vallejo R, Garnacho-Saucedo G, Sánchez-Arca M, et al. Neoadjuvant intralesional methotrexate before surgical treatment of invasive squamous cell carcinoma of the lower lip. Dermatol Surg. 2012;38:1849-1850.
- Vega-González LG, Morales-Pérez MI, Molina-Pérez T, et al. Successful treatment of squamous cell carcinoma with intralesional methotrexate. JAAD Case Rep. 2022;24:68-70.
- Moye MS, Clark AH, Legler AA, et al. Intralesional methotrexate for treatment of invasive squamous cell carcinomas in a patient taking vemurafenib for treatment of metastatic melanoma. J Clin Oncol. 2016;34:E134-E136.
- Gualdi G, Caravello S, Frasci F, et al. Intralesional methotrexate for the treatment of advanced keratinocytic tumors: a multi-center retrospective study. Dermatol Ther (Heidelb). 2020;10:769-777.
Intralesional Methotrexate: A Cost-Effective, High-Efficacy Alternative to Surgery for Cutaneous Squamous Cell Carcinoma
Intralesional Methotrexate: A Cost-Effective, High-Efficacy Alternative to Surgery for Cutaneous Squamous Cell Carcinoma
PRACTICE POINTS
- Intralesional methotrexate (IL-MTX) is an efficacious treatment option for cutaneous squamous cell carcinoma lesions in patients who are not good candidates for surgical excision.
- The starting concentration of the initial IL-MTX dose did not substantially impact outcomes; however, a 25 mg/mL concentration is standard for subsequent treatments to maintain efficacy.
What Dermatology Residents Need to Know About Joining Group Practices
What Dermatology Residents Need to Know About Joining Group Practices
Choosing your first job out of residency can be overwhelming. The things you need to consider go way beyond the job itself: things like geography, work/life balance, and practice focus (eg, skin cancer, cosmetics, medical dermatology, pediatrics) are all relatively independent factors from the specific practice you join. About 1 in 6 dermatologists change practices every year, with even higher rates for new graduates.1
Drawing from my 20 years of experience as a dermatologist (10 in academia and 10 in 3 different private group practice settings—one that was independently owned and 2 with private equity owners, of which I am one), I have seen firsthand what matters and what does not when it comes to joining a group practice, both in my own career and in watching the careers of many young dermatologists. I will do my best to summarize that experience into useful advice.
Important Factors to Consider When Choosing a Practice
As a second- or third-year resident, you likely are excited but nervous about leaping into practice. To approach it with confidence, allow me to outline certain factors that apply to all dermatology practices that you may consider joining as you start your career and beyond.
- Every Practice Owner Has to Prioritize Profit. Independent owners need to build the value of their main asset, academics need to fund research and teaching, and private equity owners need to drive returns for their investors. In other words, there are lots of negative situations in academic and independently owned groups, although private equity gets all the bad press.2-5 Nothing inherently makes one type of practice setting better; it depends on the specific organization. Owners do care about other things beyond just profit (eg, providing quality care, performing cutting-edge research), but when the rubber meets the road, if a practice is providing amazing quality care but losing money doing so, in a short time it won’t be providing any care at all.
- There Is No Free Money. Your long-term compensation will 100% be determined by how much revenue you generate minus the overhead. There is no magic fund to boost your pay long term, no matter how badly the practice needs or wants you. Be clear and even blunt: ask how the practice is going to profit from you. Ask how they plan to make back any signing bonus or guaranteed salary. If they are paying you a higher percentage of collections, ask them how they are able to pay more than competitors. If they say it is because they are more efficient with lower overhead, make sure that increased efficiency does not translate into less support.
- Percent of Collections Is Irrelevant. OK, perhaps not completely irrelevant, but it is one of the least important aspects in determining how much you will make or how happy you will be. Percent of collections is the percentage of the money that the practice actually collects that is paid out to you as compensation. For example, if your percentage of collections is 40%, that means that if the practice collects $1,000,000 for the care you deliver, you will be compensated $400,000. Read on to find out why it is not as important as it seems.
- Don’t Get Too Hung Up on the Details of the Contract. I have seen so many young dermatologists spend enormous amounts of time and money on attorneys and negotiating the fine points of the contract, but not a single one has ever said later that because of all that negotiation they were protected or treated well when things got contentious. What it comes down to is that, if the practice wants to treat you well, they will. If they want to treat you badly, no contract on earth can protect you from all the ways they can do so. And if you leave, no matter what the contract says, they can do whatever they want unless you are willing to spend hundreds of thousands of dollars to fight them in court. So review the contract with an attorney and know what it says, but don’t sweat every period and clause. It isn’t worth it.
- Your Day-to-Day Is Everything. The practice you join may be the best-run practice in the world in every way, except that the office you happen to be going to work in is the one office in the practice that has 2 providers who are jerks and everyone dreads coming to work every morning. There are so many other examples of ways one location can be a disaster even in a great practice—and unfortunately, even great locations can change. The best you can do is to make sure you know where you will be working and with whom. Go and visit the actual office and spend a day shadowing to feel what the vibe is.
What Really Matters
If factors like the percentage of collections you keep are not the big things, what are? The good and bad news is that there isn’t a single answer to this question. Rather, the fundamental question is whether the practice’s plan to maximize profit includes having satisfied, motivated, and engaged long-term providers. Obviously every group practice says this is fundamental to them, but often it isn’t true. Your real job is to find out whether or not it is. The second fundamental question is whether or not the leadership and members of a group practice are competent. It doesn’t matter if they want and intend to do everything right; if the practice is not competent at getting it done, your life practicing dermatology there is not going to be good.
As a dermatologist who has practiced for 20 years in multiple settings, here are some of the questions I would ask when assessing a practice setting I might consider joining. The practice should be able to easily answer all of these questions. If they won’t, can’t, or don’t—or if they answer but don’t give you clear, concrete responses—it is a huge red flag. It could be that they know you won’t like the answers or it could be that they don’t know the answers, but either reason indicates a big problem.
- How do the contracted rates compare to other practices? Pick 5 to 10 Current Procedural Terminology codes you expect to bill the most and ask the practice to tell you the contracted rates for each of those codes with their top 5 payers. Get the same information from all the practices you are considering joining and compare them. The variation between 2 practices in the same market can be as high as 30%. That means that for doing the same work at Practice A you could collect $800,000 and at Practice B in the same market you could collect more than $1,000,000. Getting 45% of your collection from Practice A is a losing proposition compared to getting 40% at Practice B.
- What is the collection rate? If the practice has great contracted rates but terrible revenue cycle management operations, the rates don’t matter. For example, maybe their contracted rate for a given code is $150, compared to another practice whose contracted rate is $125. But if their collection rate is only 60% and the other practice has a collection rate of 80%, they are only getting $90 while the practice with the lower contracted rate is getting $100.
- What billing and coding support does the practice offer? Are you expected to know and keep up with all the procedure codes, modifiers, etc, and use them correctly yourself or do they have professional coders who review every visit? Do they appeal every denied claim? Will you get reports on what charges get denied and why so that you can adjust your practices to avoid further denials?
- How do they train and assign medical assistants (MAs)? The single biggest determinant of your day-to-day productivity and happiness will be your MAs. Having 3 experienced, efficient MAs will allow you to see 50 patients per day with less effort and more fun than seeing 30 patients per day with 2 inexperienced, inefficient MAs. Seeing 50 patients per day at 40% of collections leads to you earning a lot more than seeing 30 per day at 50% of collections. Beyond the basic question of how many MAs you will have, also ask: Will you be expected to train them yourself, or does the practice have a formal training program? Who assesses how well they are performing? Will you have the same MAs every day? When more senior providers have MAs call off sick or leave the practice, will your MAs be pulled to cover their clinics? If that happens, will you be compensated in some way? Get the answers in writing.
- What is the “feel” of the office you will be working in? Ideally you will go and spend a day seeing patients in the office with one of the existing providers to get a sense of whether it’s a place you will be excited to come to every morning. Do you like the other providers? Is there someone who could act as a mentor for you? Does the staff seem happy? Will the physical layout and square footage accommodate the way you imagine practicing? Are the sociodemographics of the patients a fit for what you want?
- Who will be the office manager responsible for your personal practice? Some practices have an on-site manager for every location; others have district or regional managers who are split between multiple practices. Some have both. All can work, but having a competent, supportive office manager with whom you get along with whom and who “gets it” is crucial. You should ask about office manager turnover (high rates are bad, of course) and should ask to meet and interview the office manager who will be the boots on the ground for the practice in your location.
- How much demand for services is there and how are new patients scheduled? If the new hire gets all the hair loss, acne, and eczema cases and the established providers get all the skin cancer/Medicare patients, you are not going to have a balanced patient mix, and you are not going to meet productivity goals because you won’t be doing enough procedures. If there is not enough demand to fill your schedule, what kind of marketing support does the practice offer and what other approaches might they take? If you want to do cosmetics, how are they going to help you grow in that area? Are there other providers who don’t do cosmetics and will refer to you? Is there already someone in the practice who all the referrals go to? Is your percentage of collections based on total collections or on collections after the cost of injectables is deducted?
- What educational support does the practice offer? Do they have an annual meeting for networking and continuing medical education (CME)? If so, will you be expected to use your CME budget to pay to attend? Are there restrictions on what you can use your CME budget for? Is your CME budget considered part of your percentage of collections? Are there experts in the practice you can go to if you have a challenging case or difficult situation?
- Are physician associates and nurse practitioners a big part of the practice? Will you have the opportunity to increase your compensation by supervising them? If so, what is expected of supervising physicians and how are they compensated (flat fee vs percentage of collections vs another model)?
- What does the noncompete say? Obviously the shorter the time period and the smaller the distance, the better. For most practices, a noncompete is nonnegotiable. But there are some nuances to consider: Is the restricted distance from any location that the practice has in the market, or from any location(s) in which you personally have practiced, or from the primary location(s) in which you have practiced? If it is from the primary location(s), get details on how this term is defined: How much do you have to be at a location before it is considered primary? If you stop going to a location, after what period of time is it no longer considered a primary location? Additional questions to ask about noncompetes include: Is there a nonsolicitation clause for employees or patients? Will the practice include a buy-out clause in which you can pay them a set amount to waive the noncompete? Will they make the noncompete time dependent? In other words, if it is a terrible fit and you want to leave in the first year, there is very little justification for them to enforce a noncompete—but unless it is in your contract that they won’t enforce it if you leave before a certain amount of time, they will enforce it.
- Is there a path to having equity in the practice? In academia, this obviously is not a possibility. In independent practices it generally is referred to as an ownership stake or becoming a partner, and in private-equity groups it is literally referred to as “equity,” but they mean essentially the same thing for our purposes. It benefits the practice if you have equity because it gives you a reason to work to help increase the value of the practice. It benefits you to have equity because it means you have more input into decisions that will affect you (and the influence is proportional to how much equity you have) and the equity is an asset that can become very valuable.
The primary advice I have when it comes to being promised an opportunity to become an owner/partner in an independent group is to get the timing and conditions under which you can become an owner in the contract and strongly advocate for a clause that states that if you are not offered the opportunity as defined in the contract that you will be compensated. Also consider what happens if the current owner(s) sell the practice before you become an owner.
In private-equity groups, ask how many of the current providers have equity and ask how the equity is currently divided (what percentage is held by the private equity group, what percentage is held by the CEO and other executives, and what percentage is held by providers). The more equity held by the executive leadership and providers the better, as that means more people are on the same team of trying to increase the value of the practice. Find out how and when you will be able to buy in and try to get this in the contract or at least in writing. Also ask for a guarantee that your equity will not decrease in value. There are instances in which the practice loses value over time due to mismanagement, and the legal structure typically prioritizes the equity of the private-equity owners over the equity of providers. This is called an equity waterfall. Equity that providers were told was worth millions can literally be worth nothing.
One Key Thing You Need to Know
More important than the formal interviews and meetings that will provide you with answers to the questions outlined here, you need to know if you can trust the answers and you need to know the overall culture of the organization. Are they truly pro-provider, and do they believe that engaged and supported providers are the best route to long-term profit maximization? Or do they see providers primarily as replaceable adversaries who need to be placated and managed in order to minimize overhead? The only way to find out is to talk to providers already working there.
If you ask the practice for contact information for providers you can talk to, they likely will put you in touch with those who they know are going to talk about the practice in the best possible light. Be aware that providers may speak positively about a practice for a few different reasons other than that they are actually happy. Maybe the provider has an ownership stake in the practice and will benefit financially if you join. Keep in mind that, if a friend or colleague introduced you to the practice, they are almost certainly getting a substantial referral bonus if you join, so they may not be unbiased; however, if they are an actual friend, the last thing they want is for you to join and be unhappy in the practice because they didn’t tell the truth.
To learn about the experiences of others in your situation who have joined the practice, go to the website and look through the list of providers. Ideally, look for people who are in their first 3 years out of residency who have been there long enough to know the ins and outs but who still are considered newbies and almost certainly don’t have a meaningful ownership stake or strong allegiance to the practice. If it is a geographically widespread practice, focus on people in the region you will be in, but also talk to at least one person from a distant site.
Next, go to the American Academy of Dermatology’s website to find the email addresses for the providers you want to contact in the member directory. Send them an email explaining that you are thinking about joining the practice and that you would like to have an off-the-record phone conversation with them about their experiences. If they decline or don’t respond, it could be a red flag that likely means they don’t think they can speak positively about the practice. If they do agree to speak with you, you can reiterate at that time that the conversation is off the record and that you won’t relay your discussion to anyone at the practice.
Here is a sample email you can use to reach out to providers from a practice you are considering joining:
Subject: Advice on Joining [Practice Name]
Dear Dr. [Name],
I’m a dermatology resident considering joining [Practice Name] and came across your profile. Would you be willing to have a brief (5 to 10 minutes), off-the-record call about your experience? I’d value your perspective and won’t share our conversation with the practice. Thank you!
Best, [Your Name]
Start the conversation with open-ended questions and see where it goes. Some things to ask might be, are you glad you joined the practice? Was there anything that surprised you after you joined? Is there anything you wish you would have asked or known before you joined? I would recommend not asking specifically about their compensation, as it likely will be different from what you are being offered due to variations in location and current market situations.
Final Thoughts
There is no perfect dermatology practice, but the approach outlined here—rooted in first principles and real-world experience—will help you find one that is right for you. Ask tough questions, talk to other providers, and trust your instincts.
- Cwalina TB, Mazmudar RS, Bordeaux JS, et al. Dermatologist workforce mobility: recent trends and characteristics. JAMA Dermatol. 2022;158:323-325. doi:10.1001/jamadermatol.2021.5862
- Oscherwitz ME, Godinich BM, Patel RH, et al. Effects of private equity on dermatologic quality of patient care. J Eur Acad Dermatol Venereol. 2025;39:E100-E102. doi:10.1111/jdv.20191
- Walsh S, Seaton E. Private equity in dermatology: a cloud on the horizon of quality care? J Eur Acad Dermatol Venereol. 2025;39:9-10. doi:10.1111/jdv.20272
- Konda S, Patel S, Francis J. Private equity: the bad and the ugly. Dermatol Clin. 2023;41:597-610. doi:10.1016/j.det.2023.04.004
- Novice T, Portney D, Eshaq M. Dermatology resident perspectives on practice ownership structures and private equity-backed group practices. Clin Dermatol. 2020;38:296-302. doi:10.1016/j.clindermatol.2020.02.008
Choosing your first job out of residency can be overwhelming. The things you need to consider go way beyond the job itself: things like geography, work/life balance, and practice focus (eg, skin cancer, cosmetics, medical dermatology, pediatrics) are all relatively independent factors from the specific practice you join. About 1 in 6 dermatologists change practices every year, with even higher rates for new graduates.1
Drawing from my 20 years of experience as a dermatologist (10 in academia and 10 in 3 different private group practice settings—one that was independently owned and 2 with private equity owners, of which I am one), I have seen firsthand what matters and what does not when it comes to joining a group practice, both in my own career and in watching the careers of many young dermatologists. I will do my best to summarize that experience into useful advice.
Important Factors to Consider When Choosing a Practice
As a second- or third-year resident, you likely are excited but nervous about leaping into practice. To approach it with confidence, allow me to outline certain factors that apply to all dermatology practices that you may consider joining as you start your career and beyond.
- Every Practice Owner Has to Prioritize Profit. Independent owners need to build the value of their main asset, academics need to fund research and teaching, and private equity owners need to drive returns for their investors. In other words, there are lots of negative situations in academic and independently owned groups, although private equity gets all the bad press.2-5 Nothing inherently makes one type of practice setting better; it depends on the specific organization. Owners do care about other things beyond just profit (eg, providing quality care, performing cutting-edge research), but when the rubber meets the road, if a practice is providing amazing quality care but losing money doing so, in a short time it won’t be providing any care at all.
- There Is No Free Money. Your long-term compensation will 100% be determined by how much revenue you generate minus the overhead. There is no magic fund to boost your pay long term, no matter how badly the practice needs or wants you. Be clear and even blunt: ask how the practice is going to profit from you. Ask how they plan to make back any signing bonus or guaranteed salary. If they are paying you a higher percentage of collections, ask them how they are able to pay more than competitors. If they say it is because they are more efficient with lower overhead, make sure that increased efficiency does not translate into less support.
- Percent of Collections Is Irrelevant. OK, perhaps not completely irrelevant, but it is one of the least important aspects in determining how much you will make or how happy you will be. Percent of collections is the percentage of the money that the practice actually collects that is paid out to you as compensation. For example, if your percentage of collections is 40%, that means that if the practice collects $1,000,000 for the care you deliver, you will be compensated $400,000. Read on to find out why it is not as important as it seems.
- Don’t Get Too Hung Up on the Details of the Contract. I have seen so many young dermatologists spend enormous amounts of time and money on attorneys and negotiating the fine points of the contract, but not a single one has ever said later that because of all that negotiation they were protected or treated well when things got contentious. What it comes down to is that, if the practice wants to treat you well, they will. If they want to treat you badly, no contract on earth can protect you from all the ways they can do so. And if you leave, no matter what the contract says, they can do whatever they want unless you are willing to spend hundreds of thousands of dollars to fight them in court. So review the contract with an attorney and know what it says, but don’t sweat every period and clause. It isn’t worth it.
- Your Day-to-Day Is Everything. The practice you join may be the best-run practice in the world in every way, except that the office you happen to be going to work in is the one office in the practice that has 2 providers who are jerks and everyone dreads coming to work every morning. There are so many other examples of ways one location can be a disaster even in a great practice—and unfortunately, even great locations can change. The best you can do is to make sure you know where you will be working and with whom. Go and visit the actual office and spend a day shadowing to feel what the vibe is.
What Really Matters
If factors like the percentage of collections you keep are not the big things, what are? The good and bad news is that there isn’t a single answer to this question. Rather, the fundamental question is whether the practice’s plan to maximize profit includes having satisfied, motivated, and engaged long-term providers. Obviously every group practice says this is fundamental to them, but often it isn’t true. Your real job is to find out whether or not it is. The second fundamental question is whether or not the leadership and members of a group practice are competent. It doesn’t matter if they want and intend to do everything right; if the practice is not competent at getting it done, your life practicing dermatology there is not going to be good.
As a dermatologist who has practiced for 20 years in multiple settings, here are some of the questions I would ask when assessing a practice setting I might consider joining. The practice should be able to easily answer all of these questions. If they won’t, can’t, or don’t—or if they answer but don’t give you clear, concrete responses—it is a huge red flag. It could be that they know you won’t like the answers or it could be that they don’t know the answers, but either reason indicates a big problem.
- How do the contracted rates compare to other practices? Pick 5 to 10 Current Procedural Terminology codes you expect to bill the most and ask the practice to tell you the contracted rates for each of those codes with their top 5 payers. Get the same information from all the practices you are considering joining and compare them. The variation between 2 practices in the same market can be as high as 30%. That means that for doing the same work at Practice A you could collect $800,000 and at Practice B in the same market you could collect more than $1,000,000. Getting 45% of your collection from Practice A is a losing proposition compared to getting 40% at Practice B.
- What is the collection rate? If the practice has great contracted rates but terrible revenue cycle management operations, the rates don’t matter. For example, maybe their contracted rate for a given code is $150, compared to another practice whose contracted rate is $125. But if their collection rate is only 60% and the other practice has a collection rate of 80%, they are only getting $90 while the practice with the lower contracted rate is getting $100.
- What billing and coding support does the practice offer? Are you expected to know and keep up with all the procedure codes, modifiers, etc, and use them correctly yourself or do they have professional coders who review every visit? Do they appeal every denied claim? Will you get reports on what charges get denied and why so that you can adjust your practices to avoid further denials?
- How do they train and assign medical assistants (MAs)? The single biggest determinant of your day-to-day productivity and happiness will be your MAs. Having 3 experienced, efficient MAs will allow you to see 50 patients per day with less effort and more fun than seeing 30 patients per day with 2 inexperienced, inefficient MAs. Seeing 50 patients per day at 40% of collections leads to you earning a lot more than seeing 30 per day at 50% of collections. Beyond the basic question of how many MAs you will have, also ask: Will you be expected to train them yourself, or does the practice have a formal training program? Who assesses how well they are performing? Will you have the same MAs every day? When more senior providers have MAs call off sick or leave the practice, will your MAs be pulled to cover their clinics? If that happens, will you be compensated in some way? Get the answers in writing.
- What is the “feel” of the office you will be working in? Ideally you will go and spend a day seeing patients in the office with one of the existing providers to get a sense of whether it’s a place you will be excited to come to every morning. Do you like the other providers? Is there someone who could act as a mentor for you? Does the staff seem happy? Will the physical layout and square footage accommodate the way you imagine practicing? Are the sociodemographics of the patients a fit for what you want?
- Who will be the office manager responsible for your personal practice? Some practices have an on-site manager for every location; others have district or regional managers who are split between multiple practices. Some have both. All can work, but having a competent, supportive office manager with whom you get along with whom and who “gets it” is crucial. You should ask about office manager turnover (high rates are bad, of course) and should ask to meet and interview the office manager who will be the boots on the ground for the practice in your location.
- How much demand for services is there and how are new patients scheduled? If the new hire gets all the hair loss, acne, and eczema cases and the established providers get all the skin cancer/Medicare patients, you are not going to have a balanced patient mix, and you are not going to meet productivity goals because you won’t be doing enough procedures. If there is not enough demand to fill your schedule, what kind of marketing support does the practice offer and what other approaches might they take? If you want to do cosmetics, how are they going to help you grow in that area? Are there other providers who don’t do cosmetics and will refer to you? Is there already someone in the practice who all the referrals go to? Is your percentage of collections based on total collections or on collections after the cost of injectables is deducted?
- What educational support does the practice offer? Do they have an annual meeting for networking and continuing medical education (CME)? If so, will you be expected to use your CME budget to pay to attend? Are there restrictions on what you can use your CME budget for? Is your CME budget considered part of your percentage of collections? Are there experts in the practice you can go to if you have a challenging case or difficult situation?
- Are physician associates and nurse practitioners a big part of the practice? Will you have the opportunity to increase your compensation by supervising them? If so, what is expected of supervising physicians and how are they compensated (flat fee vs percentage of collections vs another model)?
- What does the noncompete say? Obviously the shorter the time period and the smaller the distance, the better. For most practices, a noncompete is nonnegotiable. But there are some nuances to consider: Is the restricted distance from any location that the practice has in the market, or from any location(s) in which you personally have practiced, or from the primary location(s) in which you have practiced? If it is from the primary location(s), get details on how this term is defined: How much do you have to be at a location before it is considered primary? If you stop going to a location, after what period of time is it no longer considered a primary location? Additional questions to ask about noncompetes include: Is there a nonsolicitation clause for employees or patients? Will the practice include a buy-out clause in which you can pay them a set amount to waive the noncompete? Will they make the noncompete time dependent? In other words, if it is a terrible fit and you want to leave in the first year, there is very little justification for them to enforce a noncompete—but unless it is in your contract that they won’t enforce it if you leave before a certain amount of time, they will enforce it.
- Is there a path to having equity in the practice? In academia, this obviously is not a possibility. In independent practices it generally is referred to as an ownership stake or becoming a partner, and in private-equity groups it is literally referred to as “equity,” but they mean essentially the same thing for our purposes. It benefits the practice if you have equity because it gives you a reason to work to help increase the value of the practice. It benefits you to have equity because it means you have more input into decisions that will affect you (and the influence is proportional to how much equity you have) and the equity is an asset that can become very valuable.
The primary advice I have when it comes to being promised an opportunity to become an owner/partner in an independent group is to get the timing and conditions under which you can become an owner in the contract and strongly advocate for a clause that states that if you are not offered the opportunity as defined in the contract that you will be compensated. Also consider what happens if the current owner(s) sell the practice before you become an owner.
In private-equity groups, ask how many of the current providers have equity and ask how the equity is currently divided (what percentage is held by the private equity group, what percentage is held by the CEO and other executives, and what percentage is held by providers). The more equity held by the executive leadership and providers the better, as that means more people are on the same team of trying to increase the value of the practice. Find out how and when you will be able to buy in and try to get this in the contract or at least in writing. Also ask for a guarantee that your equity will not decrease in value. There are instances in which the practice loses value over time due to mismanagement, and the legal structure typically prioritizes the equity of the private-equity owners over the equity of providers. This is called an equity waterfall. Equity that providers were told was worth millions can literally be worth nothing.
One Key Thing You Need to Know
More important than the formal interviews and meetings that will provide you with answers to the questions outlined here, you need to know if you can trust the answers and you need to know the overall culture of the organization. Are they truly pro-provider, and do they believe that engaged and supported providers are the best route to long-term profit maximization? Or do they see providers primarily as replaceable adversaries who need to be placated and managed in order to minimize overhead? The only way to find out is to talk to providers already working there.
If you ask the practice for contact information for providers you can talk to, they likely will put you in touch with those who they know are going to talk about the practice in the best possible light. Be aware that providers may speak positively about a practice for a few different reasons other than that they are actually happy. Maybe the provider has an ownership stake in the practice and will benefit financially if you join. Keep in mind that, if a friend or colleague introduced you to the practice, they are almost certainly getting a substantial referral bonus if you join, so they may not be unbiased; however, if they are an actual friend, the last thing they want is for you to join and be unhappy in the practice because they didn’t tell the truth.
To learn about the experiences of others in your situation who have joined the practice, go to the website and look through the list of providers. Ideally, look for people who are in their first 3 years out of residency who have been there long enough to know the ins and outs but who still are considered newbies and almost certainly don’t have a meaningful ownership stake or strong allegiance to the practice. If it is a geographically widespread practice, focus on people in the region you will be in, but also talk to at least one person from a distant site.
Next, go to the American Academy of Dermatology’s website to find the email addresses for the providers you want to contact in the member directory. Send them an email explaining that you are thinking about joining the practice and that you would like to have an off-the-record phone conversation with them about their experiences. If they decline or don’t respond, it could be a red flag that likely means they don’t think they can speak positively about the practice. If they do agree to speak with you, you can reiterate at that time that the conversation is off the record and that you won’t relay your discussion to anyone at the practice.
Here is a sample email you can use to reach out to providers from a practice you are considering joining:
Subject: Advice on Joining [Practice Name]
Dear Dr. [Name],
I’m a dermatology resident considering joining [Practice Name] and came across your profile. Would you be willing to have a brief (5 to 10 minutes), off-the-record call about your experience? I’d value your perspective and won’t share our conversation with the practice. Thank you!
Best, [Your Name]
Start the conversation with open-ended questions and see where it goes. Some things to ask might be, are you glad you joined the practice? Was there anything that surprised you after you joined? Is there anything you wish you would have asked or known before you joined? I would recommend not asking specifically about their compensation, as it likely will be different from what you are being offered due to variations in location and current market situations.
Final Thoughts
There is no perfect dermatology practice, but the approach outlined here—rooted in first principles and real-world experience—will help you find one that is right for you. Ask tough questions, talk to other providers, and trust your instincts.
Choosing your first job out of residency can be overwhelming. The things you need to consider go way beyond the job itself: things like geography, work/life balance, and practice focus (eg, skin cancer, cosmetics, medical dermatology, pediatrics) are all relatively independent factors from the specific practice you join. About 1 in 6 dermatologists change practices every year, with even higher rates for new graduates.1
Drawing from my 20 years of experience as a dermatologist (10 in academia and 10 in 3 different private group practice settings—one that was independently owned and 2 with private equity owners, of which I am one), I have seen firsthand what matters and what does not when it comes to joining a group practice, both in my own career and in watching the careers of many young dermatologists. I will do my best to summarize that experience into useful advice.
Important Factors to Consider When Choosing a Practice
As a second- or third-year resident, you likely are excited but nervous about leaping into practice. To approach it with confidence, allow me to outline certain factors that apply to all dermatology practices that you may consider joining as you start your career and beyond.
- Every Practice Owner Has to Prioritize Profit. Independent owners need to build the value of their main asset, academics need to fund research and teaching, and private equity owners need to drive returns for their investors. In other words, there are lots of negative situations in academic and independently owned groups, although private equity gets all the bad press.2-5 Nothing inherently makes one type of practice setting better; it depends on the specific organization. Owners do care about other things beyond just profit (eg, providing quality care, performing cutting-edge research), but when the rubber meets the road, if a practice is providing amazing quality care but losing money doing so, in a short time it won’t be providing any care at all.
- There Is No Free Money. Your long-term compensation will 100% be determined by how much revenue you generate minus the overhead. There is no magic fund to boost your pay long term, no matter how badly the practice needs or wants you. Be clear and even blunt: ask how the practice is going to profit from you. Ask how they plan to make back any signing bonus or guaranteed salary. If they are paying you a higher percentage of collections, ask them how they are able to pay more than competitors. If they say it is because they are more efficient with lower overhead, make sure that increased efficiency does not translate into less support.
- Percent of Collections Is Irrelevant. OK, perhaps not completely irrelevant, but it is one of the least important aspects in determining how much you will make or how happy you will be. Percent of collections is the percentage of the money that the practice actually collects that is paid out to you as compensation. For example, if your percentage of collections is 40%, that means that if the practice collects $1,000,000 for the care you deliver, you will be compensated $400,000. Read on to find out why it is not as important as it seems.
- Don’t Get Too Hung Up on the Details of the Contract. I have seen so many young dermatologists spend enormous amounts of time and money on attorneys and negotiating the fine points of the contract, but not a single one has ever said later that because of all that negotiation they were protected or treated well when things got contentious. What it comes down to is that, if the practice wants to treat you well, they will. If they want to treat you badly, no contract on earth can protect you from all the ways they can do so. And if you leave, no matter what the contract says, they can do whatever they want unless you are willing to spend hundreds of thousands of dollars to fight them in court. So review the contract with an attorney and know what it says, but don’t sweat every period and clause. It isn’t worth it.
- Your Day-to-Day Is Everything. The practice you join may be the best-run practice in the world in every way, except that the office you happen to be going to work in is the one office in the practice that has 2 providers who are jerks and everyone dreads coming to work every morning. There are so many other examples of ways one location can be a disaster even in a great practice—and unfortunately, even great locations can change. The best you can do is to make sure you know where you will be working and with whom. Go and visit the actual office and spend a day shadowing to feel what the vibe is.
What Really Matters
If factors like the percentage of collections you keep are not the big things, what are? The good and bad news is that there isn’t a single answer to this question. Rather, the fundamental question is whether the practice’s plan to maximize profit includes having satisfied, motivated, and engaged long-term providers. Obviously every group practice says this is fundamental to them, but often it isn’t true. Your real job is to find out whether or not it is. The second fundamental question is whether or not the leadership and members of a group practice are competent. It doesn’t matter if they want and intend to do everything right; if the practice is not competent at getting it done, your life practicing dermatology there is not going to be good.
As a dermatologist who has practiced for 20 years in multiple settings, here are some of the questions I would ask when assessing a practice setting I might consider joining. The practice should be able to easily answer all of these questions. If they won’t, can’t, or don’t—or if they answer but don’t give you clear, concrete responses—it is a huge red flag. It could be that they know you won’t like the answers or it could be that they don’t know the answers, but either reason indicates a big problem.
- How do the contracted rates compare to other practices? Pick 5 to 10 Current Procedural Terminology codes you expect to bill the most and ask the practice to tell you the contracted rates for each of those codes with their top 5 payers. Get the same information from all the practices you are considering joining and compare them. The variation between 2 practices in the same market can be as high as 30%. That means that for doing the same work at Practice A you could collect $800,000 and at Practice B in the same market you could collect more than $1,000,000. Getting 45% of your collection from Practice A is a losing proposition compared to getting 40% at Practice B.
- What is the collection rate? If the practice has great contracted rates but terrible revenue cycle management operations, the rates don’t matter. For example, maybe their contracted rate for a given code is $150, compared to another practice whose contracted rate is $125. But if their collection rate is only 60% and the other practice has a collection rate of 80%, they are only getting $90 while the practice with the lower contracted rate is getting $100.
- What billing and coding support does the practice offer? Are you expected to know and keep up with all the procedure codes, modifiers, etc, and use them correctly yourself or do they have professional coders who review every visit? Do they appeal every denied claim? Will you get reports on what charges get denied and why so that you can adjust your practices to avoid further denials?
- How do they train and assign medical assistants (MAs)? The single biggest determinant of your day-to-day productivity and happiness will be your MAs. Having 3 experienced, efficient MAs will allow you to see 50 patients per day with less effort and more fun than seeing 30 patients per day with 2 inexperienced, inefficient MAs. Seeing 50 patients per day at 40% of collections leads to you earning a lot more than seeing 30 per day at 50% of collections. Beyond the basic question of how many MAs you will have, also ask: Will you be expected to train them yourself, or does the practice have a formal training program? Who assesses how well they are performing? Will you have the same MAs every day? When more senior providers have MAs call off sick or leave the practice, will your MAs be pulled to cover their clinics? If that happens, will you be compensated in some way? Get the answers in writing.
- What is the “feel” of the office you will be working in? Ideally you will go and spend a day seeing patients in the office with one of the existing providers to get a sense of whether it’s a place you will be excited to come to every morning. Do you like the other providers? Is there someone who could act as a mentor for you? Does the staff seem happy? Will the physical layout and square footage accommodate the way you imagine practicing? Are the sociodemographics of the patients a fit for what you want?
- Who will be the office manager responsible for your personal practice? Some practices have an on-site manager for every location; others have district or regional managers who are split between multiple practices. Some have both. All can work, but having a competent, supportive office manager with whom you get along with whom and who “gets it” is crucial. You should ask about office manager turnover (high rates are bad, of course) and should ask to meet and interview the office manager who will be the boots on the ground for the practice in your location.
- How much demand for services is there and how are new patients scheduled? If the new hire gets all the hair loss, acne, and eczema cases and the established providers get all the skin cancer/Medicare patients, you are not going to have a balanced patient mix, and you are not going to meet productivity goals because you won’t be doing enough procedures. If there is not enough demand to fill your schedule, what kind of marketing support does the practice offer and what other approaches might they take? If you want to do cosmetics, how are they going to help you grow in that area? Are there other providers who don’t do cosmetics and will refer to you? Is there already someone in the practice who all the referrals go to? Is your percentage of collections based on total collections or on collections after the cost of injectables is deducted?
- What educational support does the practice offer? Do they have an annual meeting for networking and continuing medical education (CME)? If so, will you be expected to use your CME budget to pay to attend? Are there restrictions on what you can use your CME budget for? Is your CME budget considered part of your percentage of collections? Are there experts in the practice you can go to if you have a challenging case or difficult situation?
- Are physician associates and nurse practitioners a big part of the practice? Will you have the opportunity to increase your compensation by supervising them? If so, what is expected of supervising physicians and how are they compensated (flat fee vs percentage of collections vs another model)?
- What does the noncompete say? Obviously the shorter the time period and the smaller the distance, the better. For most practices, a noncompete is nonnegotiable. But there are some nuances to consider: Is the restricted distance from any location that the practice has in the market, or from any location(s) in which you personally have practiced, or from the primary location(s) in which you have practiced? If it is from the primary location(s), get details on how this term is defined: How much do you have to be at a location before it is considered primary? If you stop going to a location, after what period of time is it no longer considered a primary location? Additional questions to ask about noncompetes include: Is there a nonsolicitation clause for employees or patients? Will the practice include a buy-out clause in which you can pay them a set amount to waive the noncompete? Will they make the noncompete time dependent? In other words, if it is a terrible fit and you want to leave in the first year, there is very little justification for them to enforce a noncompete—but unless it is in your contract that they won’t enforce it if you leave before a certain amount of time, they will enforce it.
- Is there a path to having equity in the practice? In academia, this obviously is not a possibility. In independent practices it generally is referred to as an ownership stake or becoming a partner, and in private-equity groups it is literally referred to as “equity,” but they mean essentially the same thing for our purposes. It benefits the practice if you have equity because it gives you a reason to work to help increase the value of the practice. It benefits you to have equity because it means you have more input into decisions that will affect you (and the influence is proportional to how much equity you have) and the equity is an asset that can become very valuable.
The primary advice I have when it comes to being promised an opportunity to become an owner/partner in an independent group is to get the timing and conditions under which you can become an owner in the contract and strongly advocate for a clause that states that if you are not offered the opportunity as defined in the contract that you will be compensated. Also consider what happens if the current owner(s) sell the practice before you become an owner.
In private-equity groups, ask how many of the current providers have equity and ask how the equity is currently divided (what percentage is held by the private equity group, what percentage is held by the CEO and other executives, and what percentage is held by providers). The more equity held by the executive leadership and providers the better, as that means more people are on the same team of trying to increase the value of the practice. Find out how and when you will be able to buy in and try to get this in the contract or at least in writing. Also ask for a guarantee that your equity will not decrease in value. There are instances in which the practice loses value over time due to mismanagement, and the legal structure typically prioritizes the equity of the private-equity owners over the equity of providers. This is called an equity waterfall. Equity that providers were told was worth millions can literally be worth nothing.
One Key Thing You Need to Know
More important than the formal interviews and meetings that will provide you with answers to the questions outlined here, you need to know if you can trust the answers and you need to know the overall culture of the organization. Are they truly pro-provider, and do they believe that engaged and supported providers are the best route to long-term profit maximization? Or do they see providers primarily as replaceable adversaries who need to be placated and managed in order to minimize overhead? The only way to find out is to talk to providers already working there.
If you ask the practice for contact information for providers you can talk to, they likely will put you in touch with those who they know are going to talk about the practice in the best possible light. Be aware that providers may speak positively about a practice for a few different reasons other than that they are actually happy. Maybe the provider has an ownership stake in the practice and will benefit financially if you join. Keep in mind that, if a friend or colleague introduced you to the practice, they are almost certainly getting a substantial referral bonus if you join, so they may not be unbiased; however, if they are an actual friend, the last thing they want is for you to join and be unhappy in the practice because they didn’t tell the truth.
To learn about the experiences of others in your situation who have joined the practice, go to the website and look through the list of providers. Ideally, look for people who are in their first 3 years out of residency who have been there long enough to know the ins and outs but who still are considered newbies and almost certainly don’t have a meaningful ownership stake or strong allegiance to the practice. If it is a geographically widespread practice, focus on people in the region you will be in, but also talk to at least one person from a distant site.
Next, go to the American Academy of Dermatology’s website to find the email addresses for the providers you want to contact in the member directory. Send them an email explaining that you are thinking about joining the practice and that you would like to have an off-the-record phone conversation with them about their experiences. If they decline or don’t respond, it could be a red flag that likely means they don’t think they can speak positively about the practice. If they do agree to speak with you, you can reiterate at that time that the conversation is off the record and that you won’t relay your discussion to anyone at the practice.
Here is a sample email you can use to reach out to providers from a practice you are considering joining:
Subject: Advice on Joining [Practice Name]
Dear Dr. [Name],
I’m a dermatology resident considering joining [Practice Name] and came across your profile. Would you be willing to have a brief (5 to 10 minutes), off-the-record call about your experience? I’d value your perspective and won’t share our conversation with the practice. Thank you!
Best, [Your Name]
Start the conversation with open-ended questions and see where it goes. Some things to ask might be, are you glad you joined the practice? Was there anything that surprised you after you joined? Is there anything you wish you would have asked or known before you joined? I would recommend not asking specifically about their compensation, as it likely will be different from what you are being offered due to variations in location and current market situations.
Final Thoughts
There is no perfect dermatology practice, but the approach outlined here—rooted in first principles and real-world experience—will help you find one that is right for you. Ask tough questions, talk to other providers, and trust your instincts.
- Cwalina TB, Mazmudar RS, Bordeaux JS, et al. Dermatologist workforce mobility: recent trends and characteristics. JAMA Dermatol. 2022;158:323-325. doi:10.1001/jamadermatol.2021.5862
- Oscherwitz ME, Godinich BM, Patel RH, et al. Effects of private equity on dermatologic quality of patient care. J Eur Acad Dermatol Venereol. 2025;39:E100-E102. doi:10.1111/jdv.20191
- Walsh S, Seaton E. Private equity in dermatology: a cloud on the horizon of quality care? J Eur Acad Dermatol Venereol. 2025;39:9-10. doi:10.1111/jdv.20272
- Konda S, Patel S, Francis J. Private equity: the bad and the ugly. Dermatol Clin. 2023;41:597-610. doi:10.1016/j.det.2023.04.004
- Novice T, Portney D, Eshaq M. Dermatology resident perspectives on practice ownership structures and private equity-backed group practices. Clin Dermatol. 2020;38:296-302. doi:10.1016/j.clindermatol.2020.02.008
- Cwalina TB, Mazmudar RS, Bordeaux JS, et al. Dermatologist workforce mobility: recent trends and characteristics. JAMA Dermatol. 2022;158:323-325. doi:10.1001/jamadermatol.2021.5862
- Oscherwitz ME, Godinich BM, Patel RH, et al. Effects of private equity on dermatologic quality of patient care. J Eur Acad Dermatol Venereol. 2025;39:E100-E102. doi:10.1111/jdv.20191
- Walsh S, Seaton E. Private equity in dermatology: a cloud on the horizon of quality care? J Eur Acad Dermatol Venereol. 2025;39:9-10. doi:10.1111/jdv.20272
- Konda S, Patel S, Francis J. Private equity: the bad and the ugly. Dermatol Clin. 2023;41:597-610. doi:10.1016/j.det.2023.04.004
- Novice T, Portney D, Eshaq M. Dermatology resident perspectives on practice ownership structures and private equity-backed group practices. Clin Dermatol. 2020;38:296-302. doi:10.1016/j.clindermatol.2020.02.008
What Dermatology Residents Need to Know About Joining Group Practices
What Dermatology Residents Need to Know About Joining Group Practices
PRACTICE POINTS
- Finding the right fit in the first position out of dermatology residency can be difficult and feel overwhelming.
- Leaving one practice and joining another is especially common in the first 10 years after residency.
- Asking the right questions can increase the probability of finding the right practice for you and receiving fair compensation.
Median Income and Clinical Outcomes of Hospitalized Persons With COVID-19 at an Urban Veterans Affairs Medical Center
Median Income and Clinical Outcomes of Hospitalized Persons With COVID-19 at an Urban Veterans Affairs Medical Center
Large epidemiologic studies have shown disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status (SES). Racial and ethnic minorities and individuals of lower SES have experienced disproportionately higher rates of intensive care unit (ICU) admission and death. In Washington, DC, Black individuals (47% of the population) accounted for 51% of COVID-19 cases and 75% of deaths. In comparison, White individuals (41% of the population) accounted for 21% of cases and 11% of deaths.1 Place of residence, such as living in socially vulnerable communities, has also been shown to be associated with higher rates of COVID-19 mortality and lower vaccination rates.2-4 Social and structural inequities, such as limited access to health care services and mistrust of the health care system, may explain some of the observed disparities.5 However, data are limited regarding COVID-19 outcomes for individuals with equal access to care.
The Veterans Health Administration (VHA) is the largest integrated US health care system and operates 123 acute care hospitals. Previous research has demonstrated that disparities in outcomes for other diseases are attenuated or erased among veterans receiving VHA care.6,7 Based on literature from the pandemic, markers of health care inequity relating to SES (eg, place of residence, median income) are expected to impact the outcomes of patients acutely hospitalized with COVID-19.4 We hypothesized that the impact on clinical outcomes of infection would be mitigated for veterans receiving VHA care.
This retrospective cohort study included veterans who presented to Washington Veterans Affairs Medical Center (WVAMC) with the goal of determining whether place of residence as a marker of SES, health care access, and median income were predictive of COVID-19 disease severity.
Methods
The WVAMC serves about 125,000 veterans across the metropolitan area, including parts of Maryland and Virginia. It is a high-complexity hospital with 164 acute care beds, 30 psychosocial residential rehabilitation beds, and an adjacent 120-bed community living center providing long-term, hospice, and palliative care.8
The WVAMC developed a dashboard that tracked patients with COVID-19 through on-site testing by admission date, ward, and other key demographics (PowerBi, Corporate Data Warehouse). All patients admitted to WVAMC with a diagnosis of COVID-19 between March 1, 2020, and June 30, 2021, were included in this retrospective review. Using the Computerized Patient Record System (CPRS) and the dashboard, we collected demographic information, baseline clinical diagnoses, laboratory results, and clinical interventions for all patients with documented COVID-19 infection as established by laboratory testing methods available at the time of diagnosis. Veterans treated exclusively outside the WVAMC were excluded. Hospitalization was defined as any acute inpatient admission or transfer recorded within 5 days before and 30 days after the laboratory collection of a positive COVID-19 test. Home testing kits were not widely available during the study period. An ICU stay was defined as any inpatient admission or transfer recorded within 5 days before or 30 days after the laboratory collection of a positive COVID-19 test for which the ward location had the specialty of medical or surgical ICU. Death due to COVID-19 was defined as occurring within 42 days (6 weeks) of a positive COVID-19 test.9 This definition assumed that during the peak of the pandemic, COVID-19 was the attributable cause of death, despite the possible contribution of underlying health conditions.
Patients’ admission periods were based on US Centers for Disease Control and Prevention (CDC) national data and classified as early 2020 (January 2020–April 2020), mid-2020 (May 2020–August 2020), late 2020 (September 2020–December 2020), and early 2021 (January 2021–April 2021).10 We chose to use these time periods as surrogates for the frequent changes in circulating COVID-19 variants, surges in case numbers, therapies and interventions available during the pandemic. The dominant COVID-19 variant during the study period was Alpha (B.1.17). Beta (B.1.351) variants were circulating infrequently, and Delta and Omicron appeared after the study period.11 Treatment strategies evolved rapidly with emerging evidence, including the use of dexamethasone, beginning in June 2020.12 WVAMC followed the Advisory Committee on Immunization Practices guidance on vaccination rollout beginning in December 2020.13
Patients' income was estimated by the median household income of the zip code residence based on US Census Bureau 2021 estimates and was assessed as both a continuous and categorical variable.14 The Charlson Comorbidity Index (CCI) was included in models as a continuous variable.15 Variables contributing to the CCI include myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, hemiplegia or paraplegia, ulcer disease, hepatic disease, diabetes (with or without end-organ damage), chronic obstructive pulmonary disease (COPD), connective tissue disease, leukemia, lymphoma, moderate or severe renal disease, solid tumor (with or without metastases), and HIV/AIDS. The WVAMC Institutional Review Board approved this study (IRB #1573071).
Variables
This study assessed 3 primary outcomes as indicators of disease severity during hospitalization: need for high-flow oxygen (HFO), intubation, and presumed mortality at any time during hospitalization. The following variables were collected as potential social determinants or clinical risk-adjustment predictors of disease severity outcomes: age; sex; race and ethnicity; median income for patient’s zip code residence, state, and county; wards within Washington, DC; comorbidities, CCI; tobacco use; and body mass index.15 Although medications at baseline, treatments during hospitalization for COVID-19, and laboratory parameters during hospitalization are shown in eAppendices 1 and 2, they are beyond the scope of this analysis.
Statistical Analysis
Three types of logistic regression models were calculated for predicting the disease severity outcomes: (1) simple unadjusted models; (2) models predicting from single variables plus age (age-adjusted); and (3) multivariable models using all nonredundant potential predictors with adequate sample sizes (multivariable). Variables were considered to have inadequate sample sizes if there was nontrivial missing data or small numbers within categories, (eg, AIDS, connective tissue disease). Potential predictors for the multivariable model included age, sex, race, median income by zip code residence, CCI, CDC admission period, obesity, hypertension, chronic kidney disease, obstructive sleep apnea (OSA), diabetes, COPD or asthma, liver disease, antibiotics, and acute kidney injury.
For the multivariable models, the following modifications were made to avoid unreliable parameter estimation and computation problems (quasi-separation): age and CCI were included as continuous rather than categorical variables. Race was recoded as a 2-category variable (Black vs other [White, Hispanic, American Indian, Alaska Native, Asian, Native Hawaiian, and Pacific Islander]), and ethnicity was excluded because of the small number of patients in this group (n = 16). Admission period was included. Predicted probability plots were generated for each outcome with continuous independent predictors (income and CCI), both unadjusted and adjusted for age as a continuous covariate. All analyses were performed using SAS version 9.4.
Heat Maps
Heat maps were generated to visualize the geospatial distribution of COVID-19 cases and median incomes across zip codes in the greater Washington, DC area. Patient case data and median income, aggregated by zip code, were imported using ArcGIS Online. A zip code boundary layer from Esri (United States Zip Code Boundaries) was used to spatially align the case data. Data were joined by matching zip codes or median incomes in the patient dataset to those in the boundary layer. The resulting polygon layer was styled using the Counts and Amounts (Color) symbology in ArcGIS Online, with case counts or median income determining the intensity of the color gradient.
Results
Between March 1, 2020, and June 30, 2021, 348 patients were hospitalized with COVID-19 (Table 1). The mean (SD) age was 68.4 (13.9) years, 313 patients (90.2%) were male, 281 patients (83.4%) were Black, 47 patients (13.6%) were White, and 16 patients (4.8%) were Hispanic. One hundred forty patients (40.2%) resided in Washington, DC, 151 (43.4%) in Maryland, and 19 (5.5%) in Virginia. HFO was received by 86 patients (24.7%), 33 (9.5%) required intubation and mechanical ventilation, and 57 (16.4%) died. All intubations and deaths occurred among patients aged > 50 years, with death occurring in 17.8% of patients aged > 50 years.
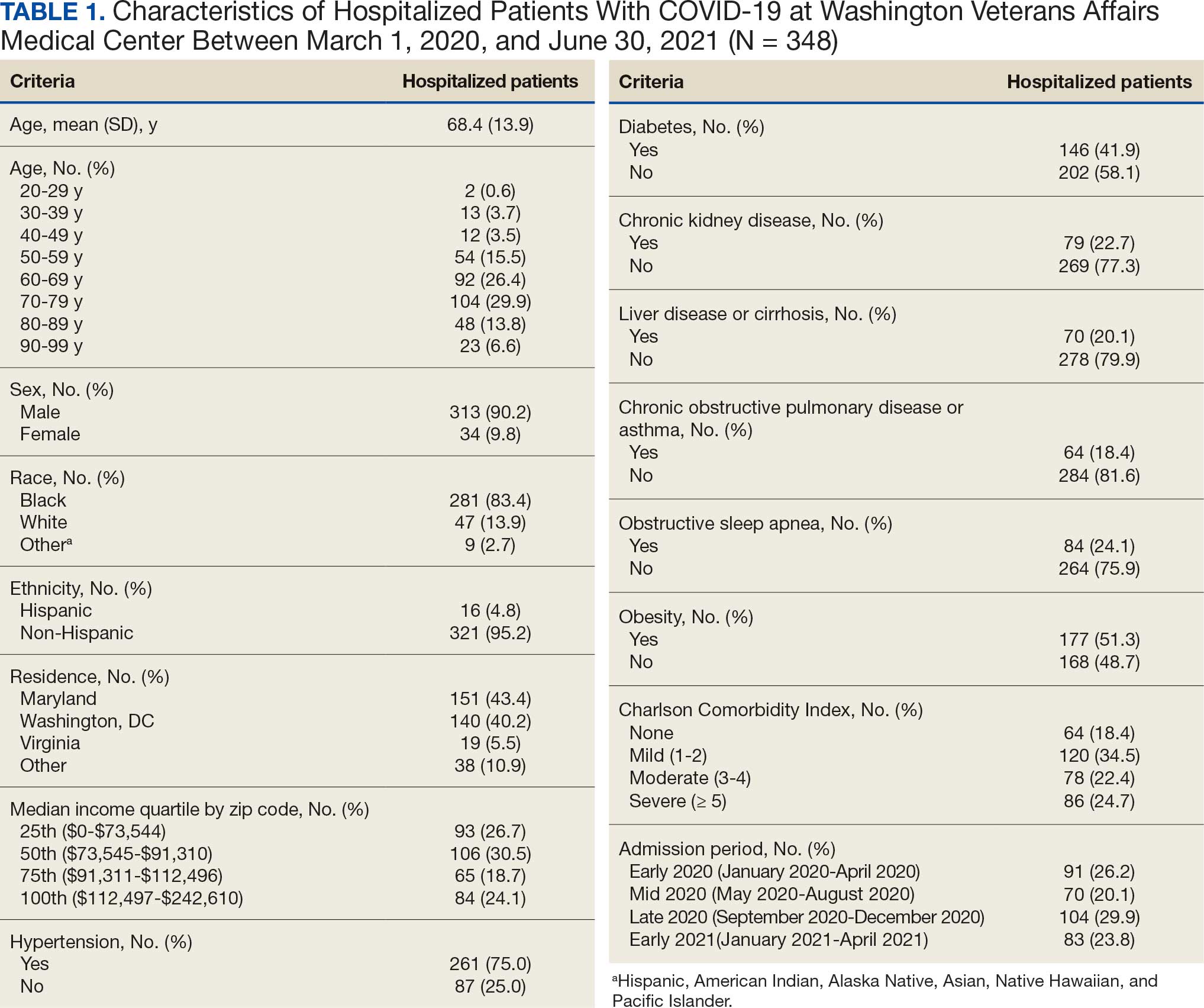
Demographic characteristics and baseline comorbidities associated with COVID-19 disease severity can be found in eAppendix 2. In unadjusted analyses, age was significantly associated with the risk of HFO, with a mean (SD) age of 72.5 (11.7) years among those requiring HFO and 67.1 (14.4) years among patients without HFO (odds ratio [OR], 1.03; 95% CI, 1.01-1.05; P = .002). Although age was not associated with the risk of intubation, it was significantly associated with mortality. Patients who died had a mean (SD) age of 76.8 (11.8) years compared with 66.8 (13.7) years among survivors (OR, 1.06; 95% CI, 1.04-1.09; P < .001).
Compared with patients with no comorbidities, CCI categories of mild, moderate, and severe were associated with increased risk of requiring HFO (eAppendix 3). The adjusted OR (aOR) was highest among patients with severe CCI (aOR, 7.00; 95% CI, 2.42-20.32; P = .0007). In age-adjusted analyses, CCI was not associated with intubation or mortality.
Geospatial Analyses
State of residence, county of residence, and geographic area (including Washington, DC wards, and geographic divisions within counties of residence in Maryland and Virginia) were not associated with the clinical outcomes studied (eAppendix 4). However, zip code-based median income, analyzed as a continuous variable, was associated with a reduced likelihood of receiving HFO (aOR, 0.91; 95% CI, 0.84-0.99; P = .03). Income was not significantly associated with intubation or mortality.
The majority of patients hospitalized for COVID-19 at WVAMC resided in zip codes in eastern Washington, DC, inclusive of wards 7 and 8, and Prince George’s County, Maryland (Figure 1). These areas also corresponded to the lowest median household income by zip code (Figure 2).
Code
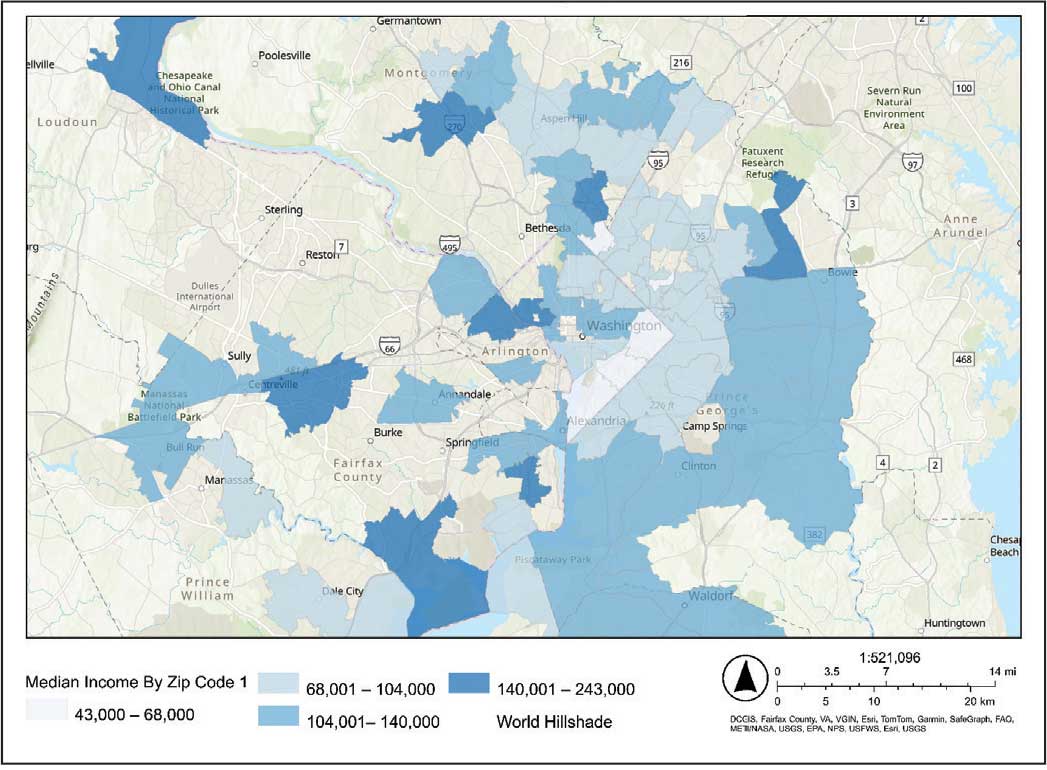
Code
Multivariable Analysis
Significant predictors of HFO requirement included comorbid diabetes (OR, 2.42; 95% CI, 1.27-4.61; P = .006) and liver disease or cirrhosis (OR, 2.19; 95% CI, 1.09-4.39; P = .02) (Table 2). CDC admission period was also associated with HFO need. Patients admitted after early 2020 had lower odds of receiving HFO. Race and median income based on zip code residence were not associated with HFO requirement.
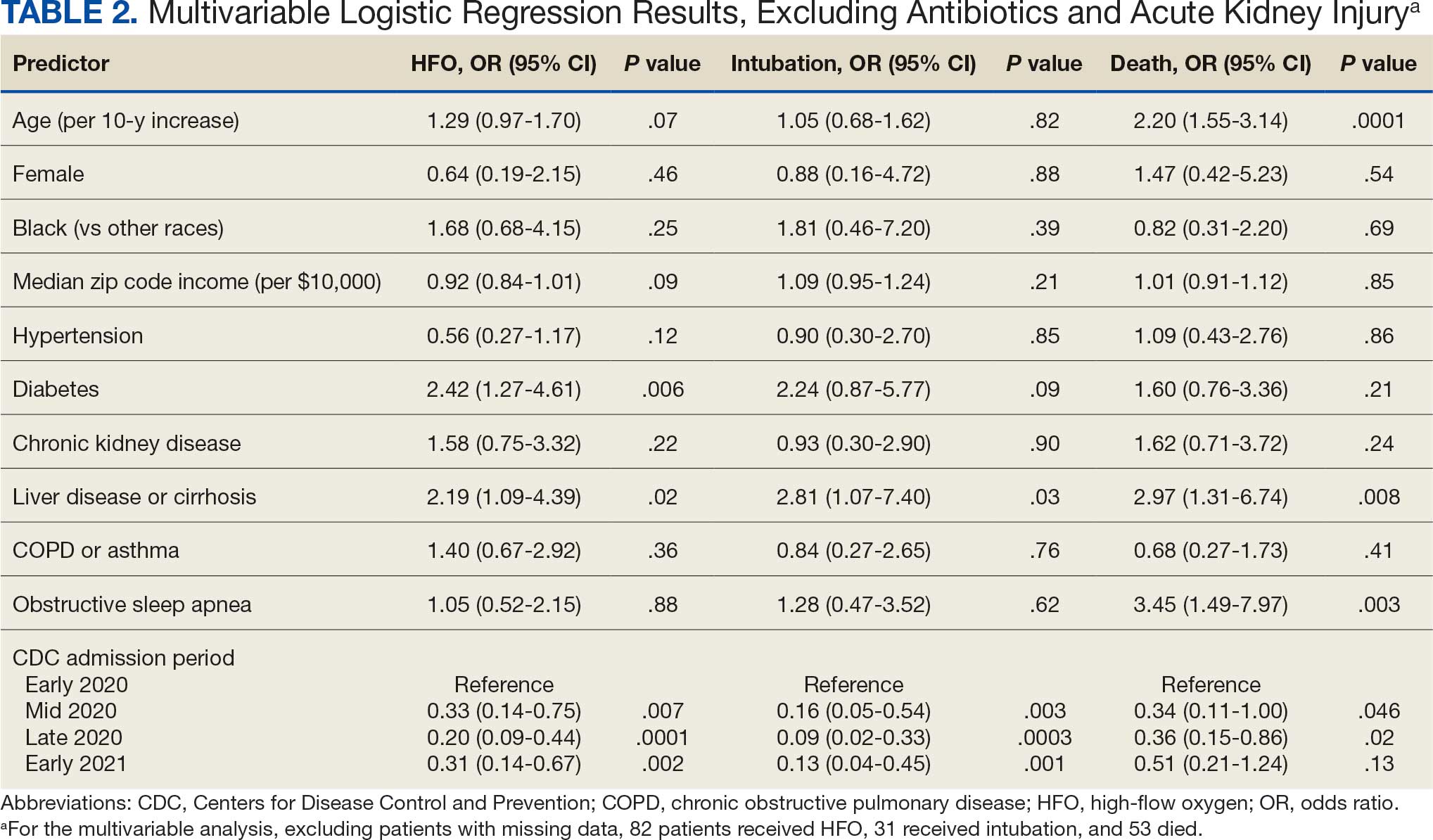
Comorbid liver disease or cirrhosis was a significant predictor of intubation (OR, 2.81; 95% CI, 1.07-7.40; P = .03). CDC admission period was associated with intubation with lower odds of intubation for patients admitted after early 2020. Race and median income by zip code were not associated with intubation.
Significant predictors of mortality included age (OR, 2.20; 95% CI, 1.55-3.14; P = .0001), comorbid liver disease or cirrhosis (OR, 2.97; 95% CI, 1.31-6.74; P = .008), and OSA (OR, 3.45; 95% CI, 1.49-7.97; P = .003). CDC admission period was associated with mortality, with lower odds of intubation for patients admitted in mid- and late 2020. Race and median income by zip code residence were not associated with intubation.
Discussion
In this study of COVID-19 disease severity at a large integrated health care system that provides equal access to care, race, ethnicity, and geographic location were not associated with the need for HFO, intubation, or presumed mortality. Median income by zip code residence was associated with reduced HFO use in univariable analyses but not in multivariable models.
These findings support existing literature suggesting that race and ethnicity alone do not explain disparities in COVID-19 outcomes. Multiple studies have demonstrated that disparities in health outcomes have been reduced for patients receiving VHA care.6,16-19 However, even within a health care system with assumed equal access, the finding of an association between income and need for HFO in the univariable analysis may reflect a greater likelihood of delays in care due to structural barriers. Multiple studies suggest low SES may be an independent risk factor for severe COVID-19 disease. Individuals with low SES have higher rates of chronic diseases of obesity, diabetes, heart disease, and lung disease; thus, they are also at greater risk of serious illness with COVID-19.20-24 Socioeconomic disadvantage may also have limited individuals’ ability to engage in protective behaviors to reduce COVID-19 infection risk, including food stockpiling, social distancing, avoidance of public transportation, and refraining from working in “essential jobs.”21
Beyond SES, place of residence also influences health outcomes. Prior literature supports using zip codes to assess area-based SES status and monitor health disparities.25 The Social Vulnerability Index incorporates SES factors for communities and measures social determinates of health at a zip code level exclusive of race and ethnicity.26 Socially vulnerable communities are known to have higher rates of chronic diseases, COVID-19 mortality, and lower vaccination rates.3 Within a defined geographic area, an individual’s outcome for COVID-19 can be influenced by individual resources such as access to care and median income. Disposable income may mitigate COVID-19 risk by facilitating timely care, reducing occupational exposure, improving housing stability, and supporting health-promoting behaviors.21
Limitations
Due to the evolving nature of the COVID-19 pandemic, variants, treatments, and interventions varied throughout the study period and are not included in this analysis. In late December 2020, COVID-19 vaccination was approved with a tiered allocation for at-risk patients and direct health care professionals. Three of the 4 study periods analyzed in this study were prior to vaccine rollout and therefore vaccination history was not assessed. However, we tried to capture the evolving changes in COVID-19 variants, treatments and interventions, and skill in treating the disease through use of CDC-defined time frames. Another limitation is that some studies have shown that use of median income by zip code residence can underestimate mortality.27 Also, shared resources and access to other sources of disposable income can impact the immediate attainment of social needs. For example, during the COVID-19 pandemic, health care systems in Washington, DC assisted vulnerable individuals by providing food, housing, and other resources.28,29 Finally, the modest sample size limits generalizability and power to detect differences for certain variables, including Hispanic ethnicity.
Conclusions
There have been widely described disparities in disease severity and death during the COVID-19 pandemic. In this urban veteran cohort of hospitalized patients, there was no difference in the need for intubation or mortality associated with race. The findings suggest that a lower median income by zip code residence may be associated with greater disease severity at presentation, but do not predict severe outcomes and mortality overall. VHA care, which provides equal access to care, may mitigate the disparities seen in the private sector.
- District of Columbia: All Race & Ethnicity Data. The COVID Tracking Project. Accessed December 10, 2025. https://covidtracking.com/data/state/district-of-columbia/race-ethnicity
- Freese KE, Vega A, Lawrence JJ, et al. Social vulnerability is associated with risk of COVID-19 related mortality in U.S. counties with confirmed cases. J Health Care Poor Underserved. 2021;32:245-257. doi:10.1353/hpu.2021.0022
- Saulsberry L, Bhargava A, Zeng S, et al. The social vulnerability metric (SVM) as a new tool for public health. Health Serv Res. 2023;58:873-881. doi:10.1111/1475-6773.14102
- Romano SD, Blackstock AJ, Taylor EV, et al. Trends in racial and ethnic disparities in COVID-19 hospitalizations, by region - United States, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021;70:560-565. doi:10.15585/mmwr.mm7015e2
- Kullar R, Marcelin JR, Swartz TH, et al. Racial disparity of coronavirus disease 2019 in African American communities. J Infect Dis. 2020;222:890-893. doi:10.1093/infdis/jiaa372
- Riviere P, Luterstein E, Kumar A, et al. Survival of African American and non-Hispanic White men with prostate cancer in an equal-access health care system. Cancer. 2020;126:1683-1690. doi:10.1002/cncr.32666
- Ohl ME, Richardson Miell K, Beck BF, et al. Mortality among US veterans admitted to community vs Veterans Health Administration hospitals for COVID-19. JAMA Netw Open. 2023;6:e2315902. doi:10.1001/jamanetworkopen.2023.15902
- US Department of Veterans Affairs. VA Washington DC Health Care. Accessed January 16, 2026. https://www.va.gov/washington-dc-health-care/about-us/
- Trottier C, La J, Li LL, et al. Maintaining the utility of coronavirus disease 2019 pandemic severity surveillance: evaluation of trends in attributable deaths and development and validation of a measurement tool. Clin Infect Dis. 2023;77:1247-1256. doi:10.1093/cid/ciad381
- Centers for Disease Control and Prevention. CDC Museum COVID-19 Timeline. Updated July 8, 2024. Accessed January 16, 2026. https://www.cdc.gov/museum/timeline/covid19.html#Early-2020
- Centers for Disease Control and Prevention. Covid-surveillance and data analytics. September 5, 2025. Accessed January 16, 2026. cdc.gov/covid/php/surveillance/index.html12.
- RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
- Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660. doi:10.15585/mmwr.mm695152e2
- US Census Bureau. Explore census data. Accessed December 10, 2025. https://data.census.gov/profile?q=Income%20by%20Zip%20code%20tabulation%20area
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
- Zullig LL, Carpenter WR, Provenzale D, Weinberger M, Reeve BB, Jackson GL. Examining potential colorectal cancer care disparities in the Veterans Affairs health care system. J Clin Oncol. 2013;31:3579-3584. doi:10.1200/JCO.2013.50.4753
- Grubaugh AL, Slagle DM, Long M, Frueh BC, Magruder KM. Racial disparities in trauma exposure, psychiatric symptoms, and service use among female patients in Veterans Affairs primary care clinics. Womens Health Issues. 2008;18:433-441. doi:10.1016/j.whi.2008.08.001
- Bosworth HB, Parsey KS, Butterfield MI, et al. Racial variation in wanting and obtaining mental health services among women veterans in a primary care clinic. J Natl Med Assoc. 2000;92:231-236.
- Luo J, Rosales M, Wei G, et al. Hospitalization, mechanical ventilation, and case-fatality outcomes in US veterans with COVID-19 disease between years 2020-2021. Ann Epidemiol. 2022;70:37-44. doi:10.1016/j.annepidem.2022.04.003
- Kondo K, Low A, Everson T, et al. Health disparities in veterans: a map of the evidence. Med Care. 2017;55 Suppl 9 Suppl 2:S9-S15. doi:10.1097/MLR.0000000000000756
- Grosicki GJ, Bunsawat K, Jeong S, Robinson AT. Racial and ethnic disparities in cardiometabolic disease and COVID-19 outcomes in White, Black/African American, and Latinx populations: Social determinants of health. Prog Cardiovasc Dis. 2022;71:4-10. doi:10.1016/j.pcad.2022.04.004
- National Center for Immunization and Respiratory Diseases (U.S.). Division of Viral Diseases. Coronavirus Disease 2019 (COVID-19): COVID-19 in Racial and Ethnic Minority Groups: June 4, 2020. CDC Stacks. June 4, 2020. Accessed January 14, 2026. https://stacks.cdc.gov/view/cdc/88770
- Yancy CW. COVID-19 and African Americans. JAMA. 2020;323:1891-1892. doi:10.1001/jama.2020.6548
- Magesh S, John D, Li WT, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis. JAMA Netw Open. 2021;4:e2134147. doi:10.1001/jamanetworkopen.2021.34147
- Berkowitz SA, Traore CY, Singer DE, Atlas SJ. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417. doi:10.1111/1475-6773.12229
- Social Vulnerability Index. Agency for Toxicity and Disease Registry. July 22, 2024. Accessed January 14, 2026. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
- Moss JL, Johnson NJ, Yu M, Altekruse SF, Cronin KA. Comparisons of individual- and area-level socioeconomic status as proxies for individual-level measures: evidence from the Mortality Disparities in American Communities study. Popul Health Metr. 2021;19:1. doi:10.1186/s12963-020-00244-x
- DC Department of Human Services. Response to COVID-19. Accessed January 14, 2026. https://dhs.dc.gov/page/responsetocovid19
- Wang PG, Brisbon NM, Hubbell H, et al. Is the Gap Closing? Comparison of sociodemographic cisparities in COVID-19 hospitalizations and outcomes between two temporal waves of admissions. J Racial Ethn Health Disparities. 2023;10:593-602. doi:10.1007/s40615-022-01249-y
Large epidemiologic studies have shown disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status (SES). Racial and ethnic minorities and individuals of lower SES have experienced disproportionately higher rates of intensive care unit (ICU) admission and death. In Washington, DC, Black individuals (47% of the population) accounted for 51% of COVID-19 cases and 75% of deaths. In comparison, White individuals (41% of the population) accounted for 21% of cases and 11% of deaths.1 Place of residence, such as living in socially vulnerable communities, has also been shown to be associated with higher rates of COVID-19 mortality and lower vaccination rates.2-4 Social and structural inequities, such as limited access to health care services and mistrust of the health care system, may explain some of the observed disparities.5 However, data are limited regarding COVID-19 outcomes for individuals with equal access to care.
The Veterans Health Administration (VHA) is the largest integrated US health care system and operates 123 acute care hospitals. Previous research has demonstrated that disparities in outcomes for other diseases are attenuated or erased among veterans receiving VHA care.6,7 Based on literature from the pandemic, markers of health care inequity relating to SES (eg, place of residence, median income) are expected to impact the outcomes of patients acutely hospitalized with COVID-19.4 We hypothesized that the impact on clinical outcomes of infection would be mitigated for veterans receiving VHA care.
This retrospective cohort study included veterans who presented to Washington Veterans Affairs Medical Center (WVAMC) with the goal of determining whether place of residence as a marker of SES, health care access, and median income were predictive of COVID-19 disease severity.
Methods
The WVAMC serves about 125,000 veterans across the metropolitan area, including parts of Maryland and Virginia. It is a high-complexity hospital with 164 acute care beds, 30 psychosocial residential rehabilitation beds, and an adjacent 120-bed community living center providing long-term, hospice, and palliative care.8
The WVAMC developed a dashboard that tracked patients with COVID-19 through on-site testing by admission date, ward, and other key demographics (PowerBi, Corporate Data Warehouse). All patients admitted to WVAMC with a diagnosis of COVID-19 between March 1, 2020, and June 30, 2021, were included in this retrospective review. Using the Computerized Patient Record System (CPRS) and the dashboard, we collected demographic information, baseline clinical diagnoses, laboratory results, and clinical interventions for all patients with documented COVID-19 infection as established by laboratory testing methods available at the time of diagnosis. Veterans treated exclusively outside the WVAMC were excluded. Hospitalization was defined as any acute inpatient admission or transfer recorded within 5 days before and 30 days after the laboratory collection of a positive COVID-19 test. Home testing kits were not widely available during the study period. An ICU stay was defined as any inpatient admission or transfer recorded within 5 days before or 30 days after the laboratory collection of a positive COVID-19 test for which the ward location had the specialty of medical or surgical ICU. Death due to COVID-19 was defined as occurring within 42 days (6 weeks) of a positive COVID-19 test.9 This definition assumed that during the peak of the pandemic, COVID-19 was the attributable cause of death, despite the possible contribution of underlying health conditions.
Patients’ admission periods were based on US Centers for Disease Control and Prevention (CDC) national data and classified as early 2020 (January 2020–April 2020), mid-2020 (May 2020–August 2020), late 2020 (September 2020–December 2020), and early 2021 (January 2021–April 2021).10 We chose to use these time periods as surrogates for the frequent changes in circulating COVID-19 variants, surges in case numbers, therapies and interventions available during the pandemic. The dominant COVID-19 variant during the study period was Alpha (B.1.17). Beta (B.1.351) variants were circulating infrequently, and Delta and Omicron appeared after the study period.11 Treatment strategies evolved rapidly with emerging evidence, including the use of dexamethasone, beginning in June 2020.12 WVAMC followed the Advisory Committee on Immunization Practices guidance on vaccination rollout beginning in December 2020.13
Patients' income was estimated by the median household income of the zip code residence based on US Census Bureau 2021 estimates and was assessed as both a continuous and categorical variable.14 The Charlson Comorbidity Index (CCI) was included in models as a continuous variable.15 Variables contributing to the CCI include myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, hemiplegia or paraplegia, ulcer disease, hepatic disease, diabetes (with or without end-organ damage), chronic obstructive pulmonary disease (COPD), connective tissue disease, leukemia, lymphoma, moderate or severe renal disease, solid tumor (with or without metastases), and HIV/AIDS. The WVAMC Institutional Review Board approved this study (IRB #1573071).
Variables
This study assessed 3 primary outcomes as indicators of disease severity during hospitalization: need for high-flow oxygen (HFO), intubation, and presumed mortality at any time during hospitalization. The following variables were collected as potential social determinants or clinical risk-adjustment predictors of disease severity outcomes: age; sex; race and ethnicity; median income for patient’s zip code residence, state, and county; wards within Washington, DC; comorbidities, CCI; tobacco use; and body mass index.15 Although medications at baseline, treatments during hospitalization for COVID-19, and laboratory parameters during hospitalization are shown in eAppendices 1 and 2, they are beyond the scope of this analysis.
Statistical Analysis
Three types of logistic regression models were calculated for predicting the disease severity outcomes: (1) simple unadjusted models; (2) models predicting from single variables plus age (age-adjusted); and (3) multivariable models using all nonredundant potential predictors with adequate sample sizes (multivariable). Variables were considered to have inadequate sample sizes if there was nontrivial missing data or small numbers within categories, (eg, AIDS, connective tissue disease). Potential predictors for the multivariable model included age, sex, race, median income by zip code residence, CCI, CDC admission period, obesity, hypertension, chronic kidney disease, obstructive sleep apnea (OSA), diabetes, COPD or asthma, liver disease, antibiotics, and acute kidney injury.
For the multivariable models, the following modifications were made to avoid unreliable parameter estimation and computation problems (quasi-separation): age and CCI were included as continuous rather than categorical variables. Race was recoded as a 2-category variable (Black vs other [White, Hispanic, American Indian, Alaska Native, Asian, Native Hawaiian, and Pacific Islander]), and ethnicity was excluded because of the small number of patients in this group (n = 16). Admission period was included. Predicted probability plots were generated for each outcome with continuous independent predictors (income and CCI), both unadjusted and adjusted for age as a continuous covariate. All analyses were performed using SAS version 9.4.
Heat Maps
Heat maps were generated to visualize the geospatial distribution of COVID-19 cases and median incomes across zip codes in the greater Washington, DC area. Patient case data and median income, aggregated by zip code, were imported using ArcGIS Online. A zip code boundary layer from Esri (United States Zip Code Boundaries) was used to spatially align the case data. Data were joined by matching zip codes or median incomes in the patient dataset to those in the boundary layer. The resulting polygon layer was styled using the Counts and Amounts (Color) symbology in ArcGIS Online, with case counts or median income determining the intensity of the color gradient.
Results
Between March 1, 2020, and June 30, 2021, 348 patients were hospitalized with COVID-19 (Table 1). The mean (SD) age was 68.4 (13.9) years, 313 patients (90.2%) were male, 281 patients (83.4%) were Black, 47 patients (13.6%) were White, and 16 patients (4.8%) were Hispanic. One hundred forty patients (40.2%) resided in Washington, DC, 151 (43.4%) in Maryland, and 19 (5.5%) in Virginia. HFO was received by 86 patients (24.7%), 33 (9.5%) required intubation and mechanical ventilation, and 57 (16.4%) died. All intubations and deaths occurred among patients aged > 50 years, with death occurring in 17.8% of patients aged > 50 years.

Demographic characteristics and baseline comorbidities associated with COVID-19 disease severity can be found in eAppendix 2. In unadjusted analyses, age was significantly associated with the risk of HFO, with a mean (SD) age of 72.5 (11.7) years among those requiring HFO and 67.1 (14.4) years among patients without HFO (odds ratio [OR], 1.03; 95% CI, 1.01-1.05; P = .002). Although age was not associated with the risk of intubation, it was significantly associated with mortality. Patients who died had a mean (SD) age of 76.8 (11.8) years compared with 66.8 (13.7) years among survivors (OR, 1.06; 95% CI, 1.04-1.09; P < .001).
Compared with patients with no comorbidities, CCI categories of mild, moderate, and severe were associated with increased risk of requiring HFO (eAppendix 3). The adjusted OR (aOR) was highest among patients with severe CCI (aOR, 7.00; 95% CI, 2.42-20.32; P = .0007). In age-adjusted analyses, CCI was not associated with intubation or mortality.
Geospatial Analyses
State of residence, county of residence, and geographic area (including Washington, DC wards, and geographic divisions within counties of residence in Maryland and Virginia) were not associated with the clinical outcomes studied (eAppendix 4). However, zip code-based median income, analyzed as a continuous variable, was associated with a reduced likelihood of receiving HFO (aOR, 0.91; 95% CI, 0.84-0.99; P = .03). Income was not significantly associated with intubation or mortality.
The majority of patients hospitalized for COVID-19 at WVAMC resided in zip codes in eastern Washington, DC, inclusive of wards 7 and 8, and Prince George’s County, Maryland (Figure 1). These areas also corresponded to the lowest median household income by zip code (Figure 2).

Code

Code
Multivariable Analysis
Significant predictors of HFO requirement included comorbid diabetes (OR, 2.42; 95% CI, 1.27-4.61; P = .006) and liver disease or cirrhosis (OR, 2.19; 95% CI, 1.09-4.39; P = .02) (Table 2). CDC admission period was also associated with HFO need. Patients admitted after early 2020 had lower odds of receiving HFO. Race and median income based on zip code residence were not associated with HFO requirement.

Comorbid liver disease or cirrhosis was a significant predictor of intubation (OR, 2.81; 95% CI, 1.07-7.40; P = .03). CDC admission period was associated with intubation with lower odds of intubation for patients admitted after early 2020. Race and median income by zip code were not associated with intubation.
Significant predictors of mortality included age (OR, 2.20; 95% CI, 1.55-3.14; P = .0001), comorbid liver disease or cirrhosis (OR, 2.97; 95% CI, 1.31-6.74; P = .008), and OSA (OR, 3.45; 95% CI, 1.49-7.97; P = .003). CDC admission period was associated with mortality, with lower odds of intubation for patients admitted in mid- and late 2020. Race and median income by zip code residence were not associated with intubation.
Discussion
In this study of COVID-19 disease severity at a large integrated health care system that provides equal access to care, race, ethnicity, and geographic location were not associated with the need for HFO, intubation, or presumed mortality. Median income by zip code residence was associated with reduced HFO use in univariable analyses but not in multivariable models.
These findings support existing literature suggesting that race and ethnicity alone do not explain disparities in COVID-19 outcomes. Multiple studies have demonstrated that disparities in health outcomes have been reduced for patients receiving VHA care.6,16-19 However, even within a health care system with assumed equal access, the finding of an association between income and need for HFO in the univariable analysis may reflect a greater likelihood of delays in care due to structural barriers. Multiple studies suggest low SES may be an independent risk factor for severe COVID-19 disease. Individuals with low SES have higher rates of chronic diseases of obesity, diabetes, heart disease, and lung disease; thus, they are also at greater risk of serious illness with COVID-19.20-24 Socioeconomic disadvantage may also have limited individuals’ ability to engage in protective behaviors to reduce COVID-19 infection risk, including food stockpiling, social distancing, avoidance of public transportation, and refraining from working in “essential jobs.”21
Beyond SES, place of residence also influences health outcomes. Prior literature supports using zip codes to assess area-based SES status and monitor health disparities.25 The Social Vulnerability Index incorporates SES factors for communities and measures social determinates of health at a zip code level exclusive of race and ethnicity.26 Socially vulnerable communities are known to have higher rates of chronic diseases, COVID-19 mortality, and lower vaccination rates.3 Within a defined geographic area, an individual’s outcome for COVID-19 can be influenced by individual resources such as access to care and median income. Disposable income may mitigate COVID-19 risk by facilitating timely care, reducing occupational exposure, improving housing stability, and supporting health-promoting behaviors.21
Limitations
Due to the evolving nature of the COVID-19 pandemic, variants, treatments, and interventions varied throughout the study period and are not included in this analysis. In late December 2020, COVID-19 vaccination was approved with a tiered allocation for at-risk patients and direct health care professionals. Three of the 4 study periods analyzed in this study were prior to vaccine rollout and therefore vaccination history was not assessed. However, we tried to capture the evolving changes in COVID-19 variants, treatments and interventions, and skill in treating the disease through use of CDC-defined time frames. Another limitation is that some studies have shown that use of median income by zip code residence can underestimate mortality.27 Also, shared resources and access to other sources of disposable income can impact the immediate attainment of social needs. For example, during the COVID-19 pandemic, health care systems in Washington, DC assisted vulnerable individuals by providing food, housing, and other resources.28,29 Finally, the modest sample size limits generalizability and power to detect differences for certain variables, including Hispanic ethnicity.
Conclusions
There have been widely described disparities in disease severity and death during the COVID-19 pandemic. In this urban veteran cohort of hospitalized patients, there was no difference in the need for intubation or mortality associated with race. The findings suggest that a lower median income by zip code residence may be associated with greater disease severity at presentation, but do not predict severe outcomes and mortality overall. VHA care, which provides equal access to care, may mitigate the disparities seen in the private sector.
Large epidemiologic studies have shown disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status (SES). Racial and ethnic minorities and individuals of lower SES have experienced disproportionately higher rates of intensive care unit (ICU) admission and death. In Washington, DC, Black individuals (47% of the population) accounted for 51% of COVID-19 cases and 75% of deaths. In comparison, White individuals (41% of the population) accounted for 21% of cases and 11% of deaths.1 Place of residence, such as living in socially vulnerable communities, has also been shown to be associated with higher rates of COVID-19 mortality and lower vaccination rates.2-4 Social and structural inequities, such as limited access to health care services and mistrust of the health care system, may explain some of the observed disparities.5 However, data are limited regarding COVID-19 outcomes for individuals with equal access to care.
The Veterans Health Administration (VHA) is the largest integrated US health care system and operates 123 acute care hospitals. Previous research has demonstrated that disparities in outcomes for other diseases are attenuated or erased among veterans receiving VHA care.6,7 Based on literature from the pandemic, markers of health care inequity relating to SES (eg, place of residence, median income) are expected to impact the outcomes of patients acutely hospitalized with COVID-19.4 We hypothesized that the impact on clinical outcomes of infection would be mitigated for veterans receiving VHA care.
This retrospective cohort study included veterans who presented to Washington Veterans Affairs Medical Center (WVAMC) with the goal of determining whether place of residence as a marker of SES, health care access, and median income were predictive of COVID-19 disease severity.
Methods
The WVAMC serves about 125,000 veterans across the metropolitan area, including parts of Maryland and Virginia. It is a high-complexity hospital with 164 acute care beds, 30 psychosocial residential rehabilitation beds, and an adjacent 120-bed community living center providing long-term, hospice, and palliative care.8
The WVAMC developed a dashboard that tracked patients with COVID-19 through on-site testing by admission date, ward, and other key demographics (PowerBi, Corporate Data Warehouse). All patients admitted to WVAMC with a diagnosis of COVID-19 between March 1, 2020, and June 30, 2021, were included in this retrospective review. Using the Computerized Patient Record System (CPRS) and the dashboard, we collected demographic information, baseline clinical diagnoses, laboratory results, and clinical interventions for all patients with documented COVID-19 infection as established by laboratory testing methods available at the time of diagnosis. Veterans treated exclusively outside the WVAMC were excluded. Hospitalization was defined as any acute inpatient admission or transfer recorded within 5 days before and 30 days after the laboratory collection of a positive COVID-19 test. Home testing kits were not widely available during the study period. An ICU stay was defined as any inpatient admission or transfer recorded within 5 days before or 30 days after the laboratory collection of a positive COVID-19 test for which the ward location had the specialty of medical or surgical ICU. Death due to COVID-19 was defined as occurring within 42 days (6 weeks) of a positive COVID-19 test.9 This definition assumed that during the peak of the pandemic, COVID-19 was the attributable cause of death, despite the possible contribution of underlying health conditions.
Patients’ admission periods were based on US Centers for Disease Control and Prevention (CDC) national data and classified as early 2020 (January 2020–April 2020), mid-2020 (May 2020–August 2020), late 2020 (September 2020–December 2020), and early 2021 (January 2021–April 2021).10 We chose to use these time periods as surrogates for the frequent changes in circulating COVID-19 variants, surges in case numbers, therapies and interventions available during the pandemic. The dominant COVID-19 variant during the study period was Alpha (B.1.17). Beta (B.1.351) variants were circulating infrequently, and Delta and Omicron appeared after the study period.11 Treatment strategies evolved rapidly with emerging evidence, including the use of dexamethasone, beginning in June 2020.12 WVAMC followed the Advisory Committee on Immunization Practices guidance on vaccination rollout beginning in December 2020.13
Patients' income was estimated by the median household income of the zip code residence based on US Census Bureau 2021 estimates and was assessed as both a continuous and categorical variable.14 The Charlson Comorbidity Index (CCI) was included in models as a continuous variable.15 Variables contributing to the CCI include myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, hemiplegia or paraplegia, ulcer disease, hepatic disease, diabetes (with or without end-organ damage), chronic obstructive pulmonary disease (COPD), connective tissue disease, leukemia, lymphoma, moderate or severe renal disease, solid tumor (with or without metastases), and HIV/AIDS. The WVAMC Institutional Review Board approved this study (IRB #1573071).
Variables
This study assessed 3 primary outcomes as indicators of disease severity during hospitalization: need for high-flow oxygen (HFO), intubation, and presumed mortality at any time during hospitalization. The following variables were collected as potential social determinants or clinical risk-adjustment predictors of disease severity outcomes: age; sex; race and ethnicity; median income for patient’s zip code residence, state, and county; wards within Washington, DC; comorbidities, CCI; tobacco use; and body mass index.15 Although medications at baseline, treatments during hospitalization for COVID-19, and laboratory parameters during hospitalization are shown in eAppendices 1 and 2, they are beyond the scope of this analysis.
Statistical Analysis
Three types of logistic regression models were calculated for predicting the disease severity outcomes: (1) simple unadjusted models; (2) models predicting from single variables plus age (age-adjusted); and (3) multivariable models using all nonredundant potential predictors with adequate sample sizes (multivariable). Variables were considered to have inadequate sample sizes if there was nontrivial missing data or small numbers within categories, (eg, AIDS, connective tissue disease). Potential predictors for the multivariable model included age, sex, race, median income by zip code residence, CCI, CDC admission period, obesity, hypertension, chronic kidney disease, obstructive sleep apnea (OSA), diabetes, COPD or asthma, liver disease, antibiotics, and acute kidney injury.
For the multivariable models, the following modifications were made to avoid unreliable parameter estimation and computation problems (quasi-separation): age and CCI were included as continuous rather than categorical variables. Race was recoded as a 2-category variable (Black vs other [White, Hispanic, American Indian, Alaska Native, Asian, Native Hawaiian, and Pacific Islander]), and ethnicity was excluded because of the small number of patients in this group (n = 16). Admission period was included. Predicted probability plots were generated for each outcome with continuous independent predictors (income and CCI), both unadjusted and adjusted for age as a continuous covariate. All analyses were performed using SAS version 9.4.
Heat Maps
Heat maps were generated to visualize the geospatial distribution of COVID-19 cases and median incomes across zip codes in the greater Washington, DC area. Patient case data and median income, aggregated by zip code, were imported using ArcGIS Online. A zip code boundary layer from Esri (United States Zip Code Boundaries) was used to spatially align the case data. Data were joined by matching zip codes or median incomes in the patient dataset to those in the boundary layer. The resulting polygon layer was styled using the Counts and Amounts (Color) symbology in ArcGIS Online, with case counts or median income determining the intensity of the color gradient.
Results
Between March 1, 2020, and June 30, 2021, 348 patients were hospitalized with COVID-19 (Table 1). The mean (SD) age was 68.4 (13.9) years, 313 patients (90.2%) were male, 281 patients (83.4%) were Black, 47 patients (13.6%) were White, and 16 patients (4.8%) were Hispanic. One hundred forty patients (40.2%) resided in Washington, DC, 151 (43.4%) in Maryland, and 19 (5.5%) in Virginia. HFO was received by 86 patients (24.7%), 33 (9.5%) required intubation and mechanical ventilation, and 57 (16.4%) died. All intubations and deaths occurred among patients aged > 50 years, with death occurring in 17.8% of patients aged > 50 years.

Demographic characteristics and baseline comorbidities associated with COVID-19 disease severity can be found in eAppendix 2. In unadjusted analyses, age was significantly associated with the risk of HFO, with a mean (SD) age of 72.5 (11.7) years among those requiring HFO and 67.1 (14.4) years among patients without HFO (odds ratio [OR], 1.03; 95% CI, 1.01-1.05; P = .002). Although age was not associated with the risk of intubation, it was significantly associated with mortality. Patients who died had a mean (SD) age of 76.8 (11.8) years compared with 66.8 (13.7) years among survivors (OR, 1.06; 95% CI, 1.04-1.09; P < .001).
Compared with patients with no comorbidities, CCI categories of mild, moderate, and severe were associated with increased risk of requiring HFO (eAppendix 3). The adjusted OR (aOR) was highest among patients with severe CCI (aOR, 7.00; 95% CI, 2.42-20.32; P = .0007). In age-adjusted analyses, CCI was not associated with intubation or mortality.
Geospatial Analyses
State of residence, county of residence, and geographic area (including Washington, DC wards, and geographic divisions within counties of residence in Maryland and Virginia) were not associated with the clinical outcomes studied (eAppendix 4). However, zip code-based median income, analyzed as a continuous variable, was associated with a reduced likelihood of receiving HFO (aOR, 0.91; 95% CI, 0.84-0.99; P = .03). Income was not significantly associated with intubation or mortality.
The majority of patients hospitalized for COVID-19 at WVAMC resided in zip codes in eastern Washington, DC, inclusive of wards 7 and 8, and Prince George’s County, Maryland (Figure 1). These areas also corresponded to the lowest median household income by zip code (Figure 2).

Code

Code
Multivariable Analysis
Significant predictors of HFO requirement included comorbid diabetes (OR, 2.42; 95% CI, 1.27-4.61; P = .006) and liver disease or cirrhosis (OR, 2.19; 95% CI, 1.09-4.39; P = .02) (Table 2). CDC admission period was also associated with HFO need. Patients admitted after early 2020 had lower odds of receiving HFO. Race and median income based on zip code residence were not associated with HFO requirement.

Comorbid liver disease or cirrhosis was a significant predictor of intubation (OR, 2.81; 95% CI, 1.07-7.40; P = .03). CDC admission period was associated with intubation with lower odds of intubation for patients admitted after early 2020. Race and median income by zip code were not associated with intubation.
Significant predictors of mortality included age (OR, 2.20; 95% CI, 1.55-3.14; P = .0001), comorbid liver disease or cirrhosis (OR, 2.97; 95% CI, 1.31-6.74; P = .008), and OSA (OR, 3.45; 95% CI, 1.49-7.97; P = .003). CDC admission period was associated with mortality, with lower odds of intubation for patients admitted in mid- and late 2020. Race and median income by zip code residence were not associated with intubation.
Discussion
In this study of COVID-19 disease severity at a large integrated health care system that provides equal access to care, race, ethnicity, and geographic location were not associated with the need for HFO, intubation, or presumed mortality. Median income by zip code residence was associated with reduced HFO use in univariable analyses but not in multivariable models.
These findings support existing literature suggesting that race and ethnicity alone do not explain disparities in COVID-19 outcomes. Multiple studies have demonstrated that disparities in health outcomes have been reduced for patients receiving VHA care.6,16-19 However, even within a health care system with assumed equal access, the finding of an association between income and need for HFO in the univariable analysis may reflect a greater likelihood of delays in care due to structural barriers. Multiple studies suggest low SES may be an independent risk factor for severe COVID-19 disease. Individuals with low SES have higher rates of chronic diseases of obesity, diabetes, heart disease, and lung disease; thus, they are also at greater risk of serious illness with COVID-19.20-24 Socioeconomic disadvantage may also have limited individuals’ ability to engage in protective behaviors to reduce COVID-19 infection risk, including food stockpiling, social distancing, avoidance of public transportation, and refraining from working in “essential jobs.”21
Beyond SES, place of residence also influences health outcomes. Prior literature supports using zip codes to assess area-based SES status and monitor health disparities.25 The Social Vulnerability Index incorporates SES factors for communities and measures social determinates of health at a zip code level exclusive of race and ethnicity.26 Socially vulnerable communities are known to have higher rates of chronic diseases, COVID-19 mortality, and lower vaccination rates.3 Within a defined geographic area, an individual’s outcome for COVID-19 can be influenced by individual resources such as access to care and median income. Disposable income may mitigate COVID-19 risk by facilitating timely care, reducing occupational exposure, improving housing stability, and supporting health-promoting behaviors.21
Limitations
Due to the evolving nature of the COVID-19 pandemic, variants, treatments, and interventions varied throughout the study period and are not included in this analysis. In late December 2020, COVID-19 vaccination was approved with a tiered allocation for at-risk patients and direct health care professionals. Three of the 4 study periods analyzed in this study were prior to vaccine rollout and therefore vaccination history was not assessed. However, we tried to capture the evolving changes in COVID-19 variants, treatments and interventions, and skill in treating the disease through use of CDC-defined time frames. Another limitation is that some studies have shown that use of median income by zip code residence can underestimate mortality.27 Also, shared resources and access to other sources of disposable income can impact the immediate attainment of social needs. For example, during the COVID-19 pandemic, health care systems in Washington, DC assisted vulnerable individuals by providing food, housing, and other resources.28,29 Finally, the modest sample size limits generalizability and power to detect differences for certain variables, including Hispanic ethnicity.
Conclusions
There have been widely described disparities in disease severity and death during the COVID-19 pandemic. In this urban veteran cohort of hospitalized patients, there was no difference in the need for intubation or mortality associated with race. The findings suggest that a lower median income by zip code residence may be associated with greater disease severity at presentation, but do not predict severe outcomes and mortality overall. VHA care, which provides equal access to care, may mitigate the disparities seen in the private sector.
- District of Columbia: All Race & Ethnicity Data. The COVID Tracking Project. Accessed December 10, 2025. https://covidtracking.com/data/state/district-of-columbia/race-ethnicity
- Freese KE, Vega A, Lawrence JJ, et al. Social vulnerability is associated with risk of COVID-19 related mortality in U.S. counties with confirmed cases. J Health Care Poor Underserved. 2021;32:245-257. doi:10.1353/hpu.2021.0022
- Saulsberry L, Bhargava A, Zeng S, et al. The social vulnerability metric (SVM) as a new tool for public health. Health Serv Res. 2023;58:873-881. doi:10.1111/1475-6773.14102
- Romano SD, Blackstock AJ, Taylor EV, et al. Trends in racial and ethnic disparities in COVID-19 hospitalizations, by region - United States, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021;70:560-565. doi:10.15585/mmwr.mm7015e2
- Kullar R, Marcelin JR, Swartz TH, et al. Racial disparity of coronavirus disease 2019 in African American communities. J Infect Dis. 2020;222:890-893. doi:10.1093/infdis/jiaa372
- Riviere P, Luterstein E, Kumar A, et al. Survival of African American and non-Hispanic White men with prostate cancer in an equal-access health care system. Cancer. 2020;126:1683-1690. doi:10.1002/cncr.32666
- Ohl ME, Richardson Miell K, Beck BF, et al. Mortality among US veterans admitted to community vs Veterans Health Administration hospitals for COVID-19. JAMA Netw Open. 2023;6:e2315902. doi:10.1001/jamanetworkopen.2023.15902
- US Department of Veterans Affairs. VA Washington DC Health Care. Accessed January 16, 2026. https://www.va.gov/washington-dc-health-care/about-us/
- Trottier C, La J, Li LL, et al. Maintaining the utility of coronavirus disease 2019 pandemic severity surveillance: evaluation of trends in attributable deaths and development and validation of a measurement tool. Clin Infect Dis. 2023;77:1247-1256. doi:10.1093/cid/ciad381
- Centers for Disease Control and Prevention. CDC Museum COVID-19 Timeline. Updated July 8, 2024. Accessed January 16, 2026. https://www.cdc.gov/museum/timeline/covid19.html#Early-2020
- Centers for Disease Control and Prevention. Covid-surveillance and data analytics. September 5, 2025. Accessed January 16, 2026. cdc.gov/covid/php/surveillance/index.html12.
- RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
- Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660. doi:10.15585/mmwr.mm695152e2
- US Census Bureau. Explore census data. Accessed December 10, 2025. https://data.census.gov/profile?q=Income%20by%20Zip%20code%20tabulation%20area
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
- Zullig LL, Carpenter WR, Provenzale D, Weinberger M, Reeve BB, Jackson GL. Examining potential colorectal cancer care disparities in the Veterans Affairs health care system. J Clin Oncol. 2013;31:3579-3584. doi:10.1200/JCO.2013.50.4753
- Grubaugh AL, Slagle DM, Long M, Frueh BC, Magruder KM. Racial disparities in trauma exposure, psychiatric symptoms, and service use among female patients in Veterans Affairs primary care clinics. Womens Health Issues. 2008;18:433-441. doi:10.1016/j.whi.2008.08.001
- Bosworth HB, Parsey KS, Butterfield MI, et al. Racial variation in wanting and obtaining mental health services among women veterans in a primary care clinic. J Natl Med Assoc. 2000;92:231-236.
- Luo J, Rosales M, Wei G, et al. Hospitalization, mechanical ventilation, and case-fatality outcomes in US veterans with COVID-19 disease between years 2020-2021. Ann Epidemiol. 2022;70:37-44. doi:10.1016/j.annepidem.2022.04.003
- Kondo K, Low A, Everson T, et al. Health disparities in veterans: a map of the evidence. Med Care. 2017;55 Suppl 9 Suppl 2:S9-S15. doi:10.1097/MLR.0000000000000756
- Grosicki GJ, Bunsawat K, Jeong S, Robinson AT. Racial and ethnic disparities in cardiometabolic disease and COVID-19 outcomes in White, Black/African American, and Latinx populations: Social determinants of health. Prog Cardiovasc Dis. 2022;71:4-10. doi:10.1016/j.pcad.2022.04.004
- National Center for Immunization and Respiratory Diseases (U.S.). Division of Viral Diseases. Coronavirus Disease 2019 (COVID-19): COVID-19 in Racial and Ethnic Minority Groups: June 4, 2020. CDC Stacks. June 4, 2020. Accessed January 14, 2026. https://stacks.cdc.gov/view/cdc/88770
- Yancy CW. COVID-19 and African Americans. JAMA. 2020;323:1891-1892. doi:10.1001/jama.2020.6548
- Magesh S, John D, Li WT, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis. JAMA Netw Open. 2021;4:e2134147. doi:10.1001/jamanetworkopen.2021.34147
- Berkowitz SA, Traore CY, Singer DE, Atlas SJ. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417. doi:10.1111/1475-6773.12229
- Social Vulnerability Index. Agency for Toxicity and Disease Registry. July 22, 2024. Accessed January 14, 2026. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
- Moss JL, Johnson NJ, Yu M, Altekruse SF, Cronin KA. Comparisons of individual- and area-level socioeconomic status as proxies for individual-level measures: evidence from the Mortality Disparities in American Communities study. Popul Health Metr. 2021;19:1. doi:10.1186/s12963-020-00244-x
- DC Department of Human Services. Response to COVID-19. Accessed January 14, 2026. https://dhs.dc.gov/page/responsetocovid19
- Wang PG, Brisbon NM, Hubbell H, et al. Is the Gap Closing? Comparison of sociodemographic cisparities in COVID-19 hospitalizations and outcomes between two temporal waves of admissions. J Racial Ethn Health Disparities. 2023;10:593-602. doi:10.1007/s40615-022-01249-y
- District of Columbia: All Race & Ethnicity Data. The COVID Tracking Project. Accessed December 10, 2025. https://covidtracking.com/data/state/district-of-columbia/race-ethnicity
- Freese KE, Vega A, Lawrence JJ, et al. Social vulnerability is associated with risk of COVID-19 related mortality in U.S. counties with confirmed cases. J Health Care Poor Underserved. 2021;32:245-257. doi:10.1353/hpu.2021.0022
- Saulsberry L, Bhargava A, Zeng S, et al. The social vulnerability metric (SVM) as a new tool for public health. Health Serv Res. 2023;58:873-881. doi:10.1111/1475-6773.14102
- Romano SD, Blackstock AJ, Taylor EV, et al. Trends in racial and ethnic disparities in COVID-19 hospitalizations, by region - United States, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021;70:560-565. doi:10.15585/mmwr.mm7015e2
- Kullar R, Marcelin JR, Swartz TH, et al. Racial disparity of coronavirus disease 2019 in African American communities. J Infect Dis. 2020;222:890-893. doi:10.1093/infdis/jiaa372
- Riviere P, Luterstein E, Kumar A, et al. Survival of African American and non-Hispanic White men with prostate cancer in an equal-access health care system. Cancer. 2020;126:1683-1690. doi:10.1002/cncr.32666
- Ohl ME, Richardson Miell K, Beck BF, et al. Mortality among US veterans admitted to community vs Veterans Health Administration hospitals for COVID-19. JAMA Netw Open. 2023;6:e2315902. doi:10.1001/jamanetworkopen.2023.15902
- US Department of Veterans Affairs. VA Washington DC Health Care. Accessed January 16, 2026. https://www.va.gov/washington-dc-health-care/about-us/
- Trottier C, La J, Li LL, et al. Maintaining the utility of coronavirus disease 2019 pandemic severity surveillance: evaluation of trends in attributable deaths and development and validation of a measurement tool. Clin Infect Dis. 2023;77:1247-1256. doi:10.1093/cid/ciad381
- Centers for Disease Control and Prevention. CDC Museum COVID-19 Timeline. Updated July 8, 2024. Accessed January 16, 2026. https://www.cdc.gov/museum/timeline/covid19.html#Early-2020
- Centers for Disease Control and Prevention. Covid-surveillance and data analytics. September 5, 2025. Accessed January 16, 2026. cdc.gov/covid/php/surveillance/index.html12.
- RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
- Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660. doi:10.15585/mmwr.mm695152e2
- US Census Bureau. Explore census data. Accessed December 10, 2025. https://data.census.gov/profile?q=Income%20by%20Zip%20code%20tabulation%20area
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
- Zullig LL, Carpenter WR, Provenzale D, Weinberger M, Reeve BB, Jackson GL. Examining potential colorectal cancer care disparities in the Veterans Affairs health care system. J Clin Oncol. 2013;31:3579-3584. doi:10.1200/JCO.2013.50.4753
- Grubaugh AL, Slagle DM, Long M, Frueh BC, Magruder KM. Racial disparities in trauma exposure, psychiatric symptoms, and service use among female patients in Veterans Affairs primary care clinics. Womens Health Issues. 2008;18:433-441. doi:10.1016/j.whi.2008.08.001
- Bosworth HB, Parsey KS, Butterfield MI, et al. Racial variation in wanting and obtaining mental health services among women veterans in a primary care clinic. J Natl Med Assoc. 2000;92:231-236.
- Luo J, Rosales M, Wei G, et al. Hospitalization, mechanical ventilation, and case-fatality outcomes in US veterans with COVID-19 disease between years 2020-2021. Ann Epidemiol. 2022;70:37-44. doi:10.1016/j.annepidem.2022.04.003
- Kondo K, Low A, Everson T, et al. Health disparities in veterans: a map of the evidence. Med Care. 2017;55 Suppl 9 Suppl 2:S9-S15. doi:10.1097/MLR.0000000000000756
- Grosicki GJ, Bunsawat K, Jeong S, Robinson AT. Racial and ethnic disparities in cardiometabolic disease and COVID-19 outcomes in White, Black/African American, and Latinx populations: Social determinants of health. Prog Cardiovasc Dis. 2022;71:4-10. doi:10.1016/j.pcad.2022.04.004
- National Center for Immunization and Respiratory Diseases (U.S.). Division of Viral Diseases. Coronavirus Disease 2019 (COVID-19): COVID-19 in Racial and Ethnic Minority Groups: June 4, 2020. CDC Stacks. June 4, 2020. Accessed January 14, 2026. https://stacks.cdc.gov/view/cdc/88770
- Yancy CW. COVID-19 and African Americans. JAMA. 2020;323:1891-1892. doi:10.1001/jama.2020.6548
- Magesh S, John D, Li WT, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis. JAMA Netw Open. 2021;4:e2134147. doi:10.1001/jamanetworkopen.2021.34147
- Berkowitz SA, Traore CY, Singer DE, Atlas SJ. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417. doi:10.1111/1475-6773.12229
- Social Vulnerability Index. Agency for Toxicity and Disease Registry. July 22, 2024. Accessed January 14, 2026. https://www.atsdr.cdc.gov/placeandhealth/svi/index.html
- Moss JL, Johnson NJ, Yu M, Altekruse SF, Cronin KA. Comparisons of individual- and area-level socioeconomic status as proxies for individual-level measures: evidence from the Mortality Disparities in American Communities study. Popul Health Metr. 2021;19:1. doi:10.1186/s12963-020-00244-x
- DC Department of Human Services. Response to COVID-19. Accessed January 14, 2026. https://dhs.dc.gov/page/responsetocovid19
- Wang PG, Brisbon NM, Hubbell H, et al. Is the Gap Closing? Comparison of sociodemographic cisparities in COVID-19 hospitalizations and outcomes between two temporal waves of admissions. J Racial Ethn Health Disparities. 2023;10:593-602. doi:10.1007/s40615-022-01249-y
Median Income and Clinical Outcomes of Hospitalized Persons With COVID-19 at an Urban Veterans Affairs Medical Center
Median Income and Clinical Outcomes of Hospitalized Persons With COVID-19 at an Urban Veterans Affairs Medical Center
Cross-Sectional Analysis of Biologic Use in the Treatment of Veterans With Hidradenitis Suppurativa
Cross-Sectional Analysis of Biologic Use in the Treatment of Veterans With Hidradenitis Suppurativa
Hidradenitis suppurativa (HS) is a chronic, inflammatory skin disorder characterized by painful nodules, abscesses, and tunnels predominantly affecting intertriginous areas of the body.1,2 The condition poses significant challenges in terms of diagnosis, treatment, and quality of life for affected individuals. Various systemic therapies have been explored to manage this debilitating condition, with the emergence of biologic agents offering hope for improved outcomes. In 2015, adalimumab (ADA) was the first biologic approved by the US Food and Drug Administration (FDA) for the treatment of HS, followed by secukinumab in 2023 and bimekizumab in 2024. However, the off-label use of other biologics and/or tumor necrosis factor inhibitors such as infliximab (IFX) has become common practice.3
Although these therapies have demonstrated promising results in the treatment of HS, their widespread use may be hindered by accessibility and cost barriers. Orenstein et al analyzed data from the IBM Explorys platform from 2015 to 2020 and found that only 1.8% of patients diagnosed with HS had been prescribed ADA or IFX.4 More recently, Garg et al examined IBM MarketScan and IBM US Medicaid data from 2015 to 2018 to evaluate trends in clinical care and treatment. The prevalence of ADA and IFX prescriptions among patients with HS ranged from 2.3% to 8.0% (ADA) and 0.7% to 0.9% (IFX) for patients with commercial insurance, and 1.4% to 4.8% (ADA) and 0.5% to 0.7% (IFX) for patients with Medicaid.5 Biologics are often expensive, and the high cost associated with these therapies has been identified as a significant barrier to access for patients with HS, particularly those who lack adequate insurance coverage or face financial constraints.6
Furthermore, these barriers, particularly the financial barriers, are potentially compounded by the demographics of patients most notably affected by HS. In the US, a disproportionate incidence of HS has been noted in specific groups and age ranges, including women, individuals aged 18 to 29 years, and Black individuals.4 Orenstein et al found a statistically significant difference in use of ADA and IFX biologics based on age, sex, and race.4
The aim of this study was to examine the use of 2 biologics (ADA and IFX) in the Veterans Health Administration (VHA), a unique population in which financial barriers are reduced due to the single-payer government health care system structure. This design allowed for improved isolation and evaluation of variation in ADA and/or IFX prescription rates by demographics and health-related factors among patients with HS. To our knowledge, no studies have analyzed these metrics within the VHA.
Methods
This retrospective, cross-sectional analysis of VHA patients used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a data repository that provides access to longitudinal national electronic health record data for all veterans receiving care through VHA facilities. This study received ethical approval from institutional review boards at the Minneapolis Veterans Affairs Health Care System and VA Salt Lake City Healthcare System. Patient information was deidentified, and patient consent was not required.
Patients with HS were identified using ≥ 1 International Classification of Diseases (ICD) diagnostic code: (ICD-9 [705.83] or ICD-10 [L73.2]) between January 1, 2011, and December 31, 2021. The study included patients aged ≥ 18 years as of January 1, 2011, with ≥ 2 patient encounters during the postdiagnosis follow-up period, and with ≥ 1 encounter 6 months postindex. Patients with a biologic prescription prior to HS diagnosis were excluded. For this study, the term biologics refers to ADA and/or IFX prescriptions, unless otherwise specified. Only ADA and IFX were included in this analysis because ADA, a tumor necrosis factor (TNF)-á inhibitor, was the only FDA-approved medication at the time of the search, and IFX is another common TNF-α inhibitor used for the treatment of HS.
Statistical Analysis
We calculated logistic regression using SAS 9.4 (SAS Institute, Cary, NC). For each variable, the univariate relationship with biologic prescriptions was examined first, followed by the multivariate relationship controlling for all other variables. The following variables were controlled for in the multivariate models and were chosen a priori: sex, age, race, ethnicity, US region, hospital setting, current or previous tobacco use, obesity (defined as body mass index [BMI] ≥ 30), and Charlson Comorbidity Index (CCI).7
Results
Using ICD codes, we identified 29,483 individuals with ≥ 1 HS diagnosis (Figure 1). Of those identified, 1537 patients (5.21%) had been prescribed ≥ 1 biologic. The cohort was predominantly White (60.56%), male (75.27%), obese (59.34%), and had a history of current or previous tobacco use (73.47%) (Table 1). There were significant adjusted differences in prescription rates among veterans with HS based on age, race, and BMI. Notably, there was an age-dependent reduction in the odds of being prescribed a biologic in patients with HS. Compared with patients aged 18 to 44 years, patients aged 45 to 64 years (adjusted odds ratio [aOR], 0.63; 95% CI, 0.54–0.74; P < .001) and patients aged ≥ 65 years (aOR, 0.36; 95% CI, 0.27–0.48; P < .001) had significantly lower odds of receiving a biologic prescription (Table 2). Compared with White patients with HS, Native Hawaiian (NH) or Pacific Islander (PI) patients were less likely to be prescribed a biologic (aOR, 0.23; 95% CI, 0.06–0.92; P = .04). Patients with obesity had significantly higher odds of receiving a biologic prescription compared with patients without obesity (aOR, 1.47; 95% CI, 1.27– 1.71; P < .001).
Included in Analysis.
After adjusting for the variables listed in Table 1, there were no significant differences in biologic prescription rates for men compared with women (aOR, 0.97; 95% CI, 0.83-1.12; P = .68). We observed slight variations in biologic prescriptions between US regions (Midwest 5.0%, East 4.2%, South 5.8%, West 4.6%), none of which were significantly different in the fully adjusted model. No statistically significant differences were found in biologic prescriptions between urban and rural VA settings (5.4% vs 4.8%; aOR, 1.06; 95% CI, 0.90–1.24; P = .47). Tobacco use was not associated with the rate of biologic prescription receipt (aOR, 1.14; 95% CI, 0.97–1.34; P = .11). After adjusting for other variables (as outlined in Table 2), no significant differences were found between CCI of 0 and 1 (aOR, 0.97; 95% CI, 0.82–1.16; P = .77) or between CCI of 0 and 2 (aOR, 0.89; 95% CI, 0.74–1.07; P = .22).7
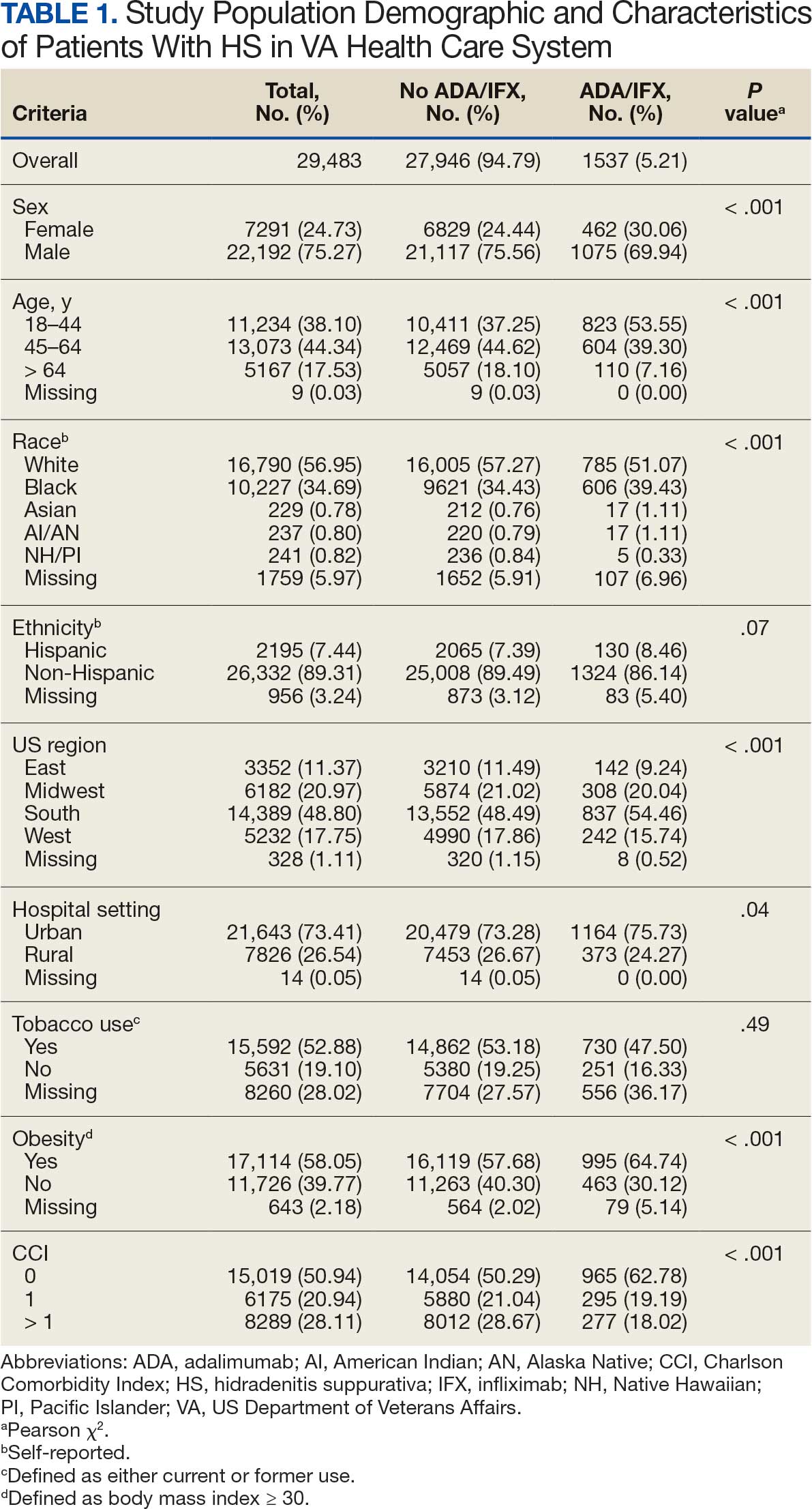
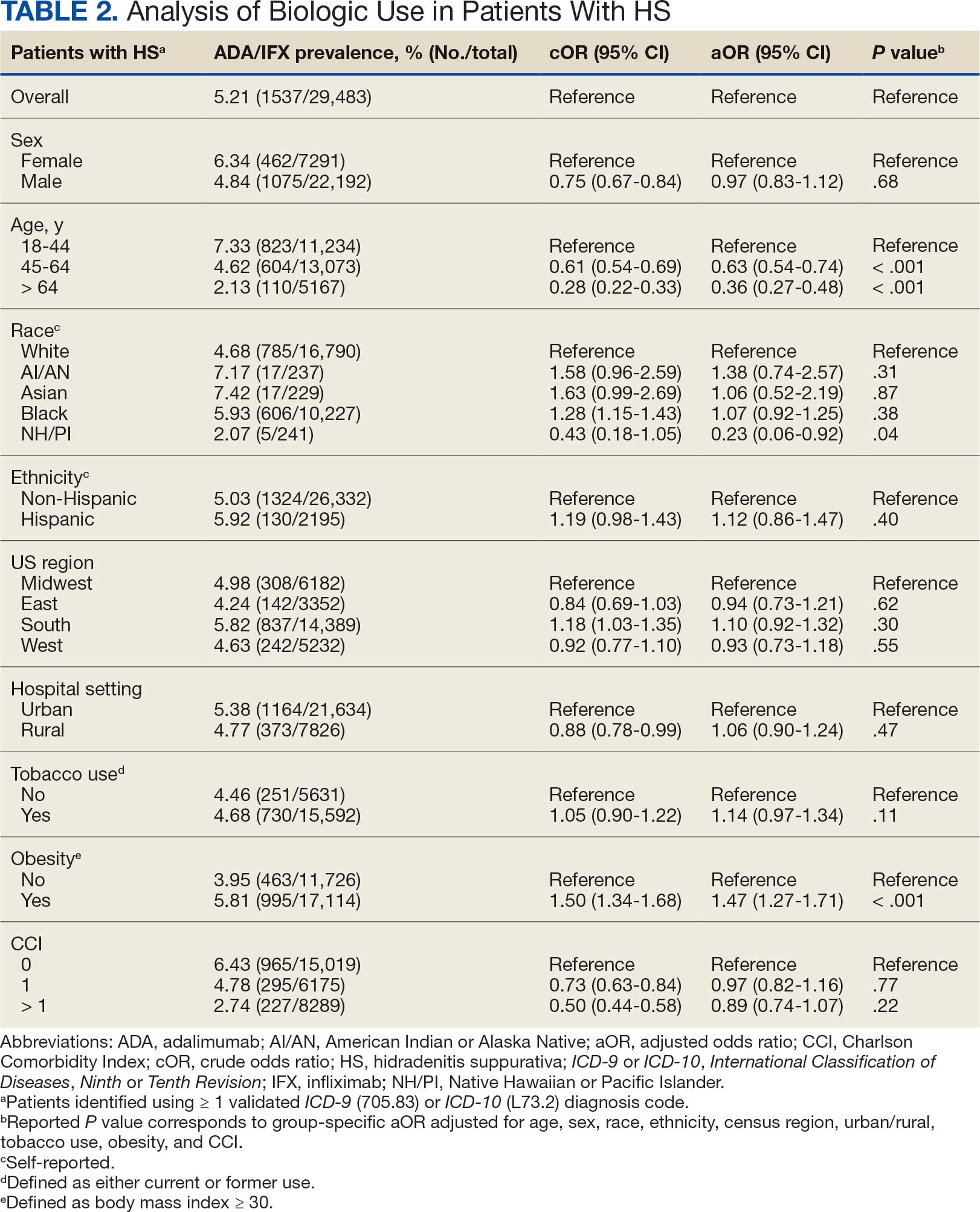
Discussion
The aim of the study was to ascertain potential discrepancies in biologic prescription patterns among patients with HS in the VHA by demographic and lifestyle behavior modifiers. Veteran cohorts are unique in composition, consisting predominantly of older White men within a single-payer health care system. The prevalence of biologic prescriptions in this population was low (5.2%), consistent with prior studies (1.8%–8.9%).4,5
We found a significant difference in ADA/IFX prescription patterns between White patients and NH/PI patients (aOR, 0.23; 95% CI, 0.06-0.92; P = .04). Further replication of this result is needed due to the small number of NH/PI patients included in the study (n = 241). Notably, we did not find a significant difference in the odds of Black patients being prescribed a biologic compared with White patients (aOR, 1.07; 95% CI, 0.92–1.25; P = .38), consistent with prior studies.4
In line with prior studies, age was associated with the likelihood of receiving a biologic prescription.4 Using the multivariate model adjusting for variables listed in Table 1, including CCI, patients aged 45 to 64 years and > 64 years were less likely to be prescribed a biologic than patients aged 18 to 44 years. HS disease activity could be a potential confounding variable, as HS severity may subside in some people with increasing age or menopause.8
Because different regions in the US have different sociopolitical ideologies and governing legislation, we hypothesized that there may be dissimilarities in the prevalence rates of biologic prescribing across various US regions. However, no significant differences were found in prescription patterns among US regions or between rural and urban settings. Previous research has demonstrated discernible disparities in both dermatologic care and clinical outcomes based on hospital setting (ie, urban vs rural).9-11
Tobacco use has been demonstrated to be associated with the development of HS.12 In a large retrospective analysis, Garg et al reported increased odds of receiving a new HS diagnosis in known tobacco users (aOR, 1.9; 95% CI, 1.8–2.0).13 The extent to which tobacco use affects HS severity is less understood. While some studies have found an association between smoking and HS severity, other analyses have failed to find this association.14,15 The effects of smoking cessation on the disease course of HS are unknown.16 This analysis, found no significant difference in prescriptions for biologics among patients with HS comparing current or previous tobacco users with nonusers.
There is a known positive correlation between increasing BMI and HS prevalence and severity that may be explained by the downstream effects of adipose tissue secretion of proinflammatory mediators and insulin resistance in the setting of chronic inflammation.12 This analysis found that patients with HS and obesity were 1.47 times more likely to be prescribed a biologic than patients with HS without obesity, which may be confounded by increased HS severity among patients with obesity. The initial concern when analyzing tobacco use and obesity was that clinician bias may result in a decrease in the prevalence of biologic use in these demographics, which was not supported in this study.
Although we identified few disparities, the results demonstrated a substantial underutilization of biologic therapies (5.2%), similar to the other US civilian studies (1.8-8.9%).4,5 While there is no current universal, standardized severity scoring system to evaluate HS (it is difficult to objectively define moderate to severe HS), estimates have shown that 40.3% to 65.8% of patients with HS have Hurley stage II or III.17-19 Therefore, only a small percentage of patients with moderate to severe disease were prescribed the only FDA-approved medication during this time period. The persistence of this underutilization within a medical system that reduces financial barriers suggests that nonfinancial barriers have a notable role in the underutilization of biologics.
For instance, risk of adverse events, particularly lymphoma and infection, has been cited by patients as a reason to avoid biologics. Additionally, treatment fatigue reduced some patients’ willingness to try new treatments, as did lack of knowledge about treatment options.6,20 Other reported barriers included the frequency of injections and fear of needles.6 Additionally, within the VA, ADA may require prior authorization at the local facility level.21 An established relationship with a dermatologist has been shown to significantly increase the odds of being prescribed a biologic medication in the face of these barriers.4 Future system-wide quality improvement initiatives could be implemented to identify patients with HS not followed by dermatology, with the goal of establishing care with a dermatologist.
Limitations
Limitations to this study include an inability to categorize HS disease severity and assess the degree to which disease severity confounded study findings, particularly in relation to tobacco use and obesity. The generalizability of this study is also limited because of the demographic characteristics of the veteran patient population, which is predominantly older, White, and male, whereas HS disproportionately affects younger, Black, and female individuals in the US.22 Despite these limitations, this study contributes valuable insights into the use of biologic therapies for veteran populations with HS using a national dataset.
Conclusions
This study was performed within a single-payer government medical system, likely reducing or removing the financial barriers that some patient populations may face when pursuing biologics for HS treatment. However, the prevalence of biologic use in this population was low overall (5.2%), suggesting that other factors play a role in the underutilization of biologics in HS. Consistent with previous studies, younger individuals were more likely to be prescribed a biologic, and no difference in prescription rates between Black and White patients was observed. Unlike previous studies, no significant difference in prescription rates between men and women was observed.
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-1058. doi:10.1016/j.jaad.2019.08.090
- Tchero H, Herlin C, Bekara F, et al. Hidradenitis suppurativa: a systematic review and meta-analysis of therapeutic interventions. Indian J Dermatol Venereol Leprol. 2019;85:248-257. doi:10.4103/ijdvl.IJDVL_69_18
- Shih T, Lee K, Grogan T, et al. Infliximab in hidradenitis suppurativa: a systematic review and meta-analysis. Dermatol Ther. 2022;35:e15691. doi:10.1111/dth.15691
- Orenstein LAV, Wright S, Strunk A, et al. Low prescription of tumor necrosis alpha inhibitors in hidradenitis suppurativa: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1399-1401. doi:10.1016/j.jaad.2020.07.108
- Garg A, Naik HB, Alavi A, et al. Real-world findings on the characteristics and treatment exposures of patients with hidradenitis suppurativa from US claims data. Dermatol Ther (Heidelb). 2023;13:581-594. doi:10.1007/s13555-022-00872-1
- De DR, Shih T, Fixsen D, et al. Biologic use in hidradenitis suppurativa: patient perspectives and barriers. J Dermatolog Treat. 2022;33:3060-3062. doi:10.1080/09546634.2022.2089336
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373- 383. doi:10.1016/0021-9681(87)90171-8
- von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14:389-392. doi:10.1046/j.1468-3083.2000.00087.x
- Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126:417-428.e2. doi:10.1016/j.anai.2020.12.020
- Wu YP, Parsons B, Jo Y, et al. Outdoor activities and sunburn among urban and rural families in a Western region of the US: implications for skin cancer prevention. Prev Med Rep. 2022;29:101914. doi:10.1016/j.pmedr.2022.101914
- Mannschreck DB, Li X, Okoye G. Rural melanoma patients in Maryland do not present with more advanced disease than urban patients. Dermatol Online J. 2021;27. doi:10.5070/D327553607
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population- based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Sartorius K, Emtestam L, Jemec GBE, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831- 839. doi:10.1111/j.1365-2133.2009.09198.x
- Canoui-Poitrine F, Revuz JE, Wolkenstein P, et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol. 2009;61:51-57. doi:10.1016/j.jaad.2009.02.013
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216- 221. doi:10.1136/postgradmedj-2013-131994
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population- based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Vanlaerhoven AMJD, Ardon CB, van Straalen KR, et al. Hurley III hidradenitis suppurativa has an aggressive disease course. Dermatology. 2018;234:232-233. doi:10.1159/000491547
- Shahi V, Alikhan A, Vazquez BG, et al. Prevalence of hidradenitis suppurativa: a population-based study in Olmsted County, Minnesota. Dermatology. 2014;229:154-158. doi:10.1159/000363381
- Salame N, Sow YN, Siira MR, et al. Factors affecting treatment selection among patients with hidradenitis suppurativa. JAMA Dermatol. 2024;160:179. doi:10.1001/jamadermatol.2023.5425
- VA Formulary Advisor: ADALIMUMAB-BWWD INJ,SOLN. US Department of Veterans Affairs. Updated December 17, 2025. Accessed January 15, 2026. https://www.va.gov/formularyadvisor/drugs/4042383-ADALIMUMAB-BWWD-INJ-SOLN
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118- 122. doi:10.1016/j.jaad.2017.02.005
Hidradenitis suppurativa (HS) is a chronic, inflammatory skin disorder characterized by painful nodules, abscesses, and tunnels predominantly affecting intertriginous areas of the body.1,2 The condition poses significant challenges in terms of diagnosis, treatment, and quality of life for affected individuals. Various systemic therapies have been explored to manage this debilitating condition, with the emergence of biologic agents offering hope for improved outcomes. In 2015, adalimumab (ADA) was the first biologic approved by the US Food and Drug Administration (FDA) for the treatment of HS, followed by secukinumab in 2023 and bimekizumab in 2024. However, the off-label use of other biologics and/or tumor necrosis factor inhibitors such as infliximab (IFX) has become common practice.3
Although these therapies have demonstrated promising results in the treatment of HS, their widespread use may be hindered by accessibility and cost barriers. Orenstein et al analyzed data from the IBM Explorys platform from 2015 to 2020 and found that only 1.8% of patients diagnosed with HS had been prescribed ADA or IFX.4 More recently, Garg et al examined IBM MarketScan and IBM US Medicaid data from 2015 to 2018 to evaluate trends in clinical care and treatment. The prevalence of ADA and IFX prescriptions among patients with HS ranged from 2.3% to 8.0% (ADA) and 0.7% to 0.9% (IFX) for patients with commercial insurance, and 1.4% to 4.8% (ADA) and 0.5% to 0.7% (IFX) for patients with Medicaid.5 Biologics are often expensive, and the high cost associated with these therapies has been identified as a significant barrier to access for patients with HS, particularly those who lack adequate insurance coverage or face financial constraints.6
Furthermore, these barriers, particularly the financial barriers, are potentially compounded by the demographics of patients most notably affected by HS. In the US, a disproportionate incidence of HS has been noted in specific groups and age ranges, including women, individuals aged 18 to 29 years, and Black individuals.4 Orenstein et al found a statistically significant difference in use of ADA and IFX biologics based on age, sex, and race.4
The aim of this study was to examine the use of 2 biologics (ADA and IFX) in the Veterans Health Administration (VHA), a unique population in which financial barriers are reduced due to the single-payer government health care system structure. This design allowed for improved isolation and evaluation of variation in ADA and/or IFX prescription rates by demographics and health-related factors among patients with HS. To our knowledge, no studies have analyzed these metrics within the VHA.
Methods
This retrospective, cross-sectional analysis of VHA patients used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a data repository that provides access to longitudinal national electronic health record data for all veterans receiving care through VHA facilities. This study received ethical approval from institutional review boards at the Minneapolis Veterans Affairs Health Care System and VA Salt Lake City Healthcare System. Patient information was deidentified, and patient consent was not required.
Patients with HS were identified using ≥ 1 International Classification of Diseases (ICD) diagnostic code: (ICD-9 [705.83] or ICD-10 [L73.2]) between January 1, 2011, and December 31, 2021. The study included patients aged ≥ 18 years as of January 1, 2011, with ≥ 2 patient encounters during the postdiagnosis follow-up period, and with ≥ 1 encounter 6 months postindex. Patients with a biologic prescription prior to HS diagnosis were excluded. For this study, the term biologics refers to ADA and/or IFX prescriptions, unless otherwise specified. Only ADA and IFX were included in this analysis because ADA, a tumor necrosis factor (TNF)-á inhibitor, was the only FDA-approved medication at the time of the search, and IFX is another common TNF-α inhibitor used for the treatment of HS.
Statistical Analysis
We calculated logistic regression using SAS 9.4 (SAS Institute, Cary, NC). For each variable, the univariate relationship with biologic prescriptions was examined first, followed by the multivariate relationship controlling for all other variables. The following variables were controlled for in the multivariate models and were chosen a priori: sex, age, race, ethnicity, US region, hospital setting, current or previous tobacco use, obesity (defined as body mass index [BMI] ≥ 30), and Charlson Comorbidity Index (CCI).7
Results
Using ICD codes, we identified 29,483 individuals with ≥ 1 HS diagnosis (Figure 1). Of those identified, 1537 patients (5.21%) had been prescribed ≥ 1 biologic. The cohort was predominantly White (60.56%), male (75.27%), obese (59.34%), and had a history of current or previous tobacco use (73.47%) (Table 1). There were significant adjusted differences in prescription rates among veterans with HS based on age, race, and BMI. Notably, there was an age-dependent reduction in the odds of being prescribed a biologic in patients with HS. Compared with patients aged 18 to 44 years, patients aged 45 to 64 years (adjusted odds ratio [aOR], 0.63; 95% CI, 0.54–0.74; P < .001) and patients aged ≥ 65 years (aOR, 0.36; 95% CI, 0.27–0.48; P < .001) had significantly lower odds of receiving a biologic prescription (Table 2). Compared with White patients with HS, Native Hawaiian (NH) or Pacific Islander (PI) patients were less likely to be prescribed a biologic (aOR, 0.23; 95% CI, 0.06–0.92; P = .04). Patients with obesity had significantly higher odds of receiving a biologic prescription compared with patients without obesity (aOR, 1.47; 95% CI, 1.27– 1.71; P < .001).

Included in Analysis.
After adjusting for the variables listed in Table 1, there were no significant differences in biologic prescription rates for men compared with women (aOR, 0.97; 95% CI, 0.83-1.12; P = .68). We observed slight variations in biologic prescriptions between US regions (Midwest 5.0%, East 4.2%, South 5.8%, West 4.6%), none of which were significantly different in the fully adjusted model. No statistically significant differences were found in biologic prescriptions between urban and rural VA settings (5.4% vs 4.8%; aOR, 1.06; 95% CI, 0.90–1.24; P = .47). Tobacco use was not associated with the rate of biologic prescription receipt (aOR, 1.14; 95% CI, 0.97–1.34; P = .11). After adjusting for other variables (as outlined in Table 2), no significant differences were found between CCI of 0 and 1 (aOR, 0.97; 95% CI, 0.82–1.16; P = .77) or between CCI of 0 and 2 (aOR, 0.89; 95% CI, 0.74–1.07; P = .22).7


Discussion
The aim of the study was to ascertain potential discrepancies in biologic prescription patterns among patients with HS in the VHA by demographic and lifestyle behavior modifiers. Veteran cohorts are unique in composition, consisting predominantly of older White men within a single-payer health care system. The prevalence of biologic prescriptions in this population was low (5.2%), consistent with prior studies (1.8%–8.9%).4,5
We found a significant difference in ADA/IFX prescription patterns between White patients and NH/PI patients (aOR, 0.23; 95% CI, 0.06-0.92; P = .04). Further replication of this result is needed due to the small number of NH/PI patients included in the study (n = 241). Notably, we did not find a significant difference in the odds of Black patients being prescribed a biologic compared with White patients (aOR, 1.07; 95% CI, 0.92–1.25; P = .38), consistent with prior studies.4
In line with prior studies, age was associated with the likelihood of receiving a biologic prescription.4 Using the multivariate model adjusting for variables listed in Table 1, including CCI, patients aged 45 to 64 years and > 64 years were less likely to be prescribed a biologic than patients aged 18 to 44 years. HS disease activity could be a potential confounding variable, as HS severity may subside in some people with increasing age or menopause.8
Because different regions in the US have different sociopolitical ideologies and governing legislation, we hypothesized that there may be dissimilarities in the prevalence rates of biologic prescribing across various US regions. However, no significant differences were found in prescription patterns among US regions or between rural and urban settings. Previous research has demonstrated discernible disparities in both dermatologic care and clinical outcomes based on hospital setting (ie, urban vs rural).9-11
Tobacco use has been demonstrated to be associated with the development of HS.12 In a large retrospective analysis, Garg et al reported increased odds of receiving a new HS diagnosis in known tobacco users (aOR, 1.9; 95% CI, 1.8–2.0).13 The extent to which tobacco use affects HS severity is less understood. While some studies have found an association between smoking and HS severity, other analyses have failed to find this association.14,15 The effects of smoking cessation on the disease course of HS are unknown.16 This analysis, found no significant difference in prescriptions for biologics among patients with HS comparing current or previous tobacco users with nonusers.
There is a known positive correlation between increasing BMI and HS prevalence and severity that may be explained by the downstream effects of adipose tissue secretion of proinflammatory mediators and insulin resistance in the setting of chronic inflammation.12 This analysis found that patients with HS and obesity were 1.47 times more likely to be prescribed a biologic than patients with HS without obesity, which may be confounded by increased HS severity among patients with obesity. The initial concern when analyzing tobacco use and obesity was that clinician bias may result in a decrease in the prevalence of biologic use in these demographics, which was not supported in this study.
Although we identified few disparities, the results demonstrated a substantial underutilization of biologic therapies (5.2%), similar to the other US civilian studies (1.8-8.9%).4,5 While there is no current universal, standardized severity scoring system to evaluate HS (it is difficult to objectively define moderate to severe HS), estimates have shown that 40.3% to 65.8% of patients with HS have Hurley stage II or III.17-19 Therefore, only a small percentage of patients with moderate to severe disease were prescribed the only FDA-approved medication during this time period. The persistence of this underutilization within a medical system that reduces financial barriers suggests that nonfinancial barriers have a notable role in the underutilization of biologics.
For instance, risk of adverse events, particularly lymphoma and infection, has been cited by patients as a reason to avoid biologics. Additionally, treatment fatigue reduced some patients’ willingness to try new treatments, as did lack of knowledge about treatment options.6,20 Other reported barriers included the frequency of injections and fear of needles.6 Additionally, within the VA, ADA may require prior authorization at the local facility level.21 An established relationship with a dermatologist has been shown to significantly increase the odds of being prescribed a biologic medication in the face of these barriers.4 Future system-wide quality improvement initiatives could be implemented to identify patients with HS not followed by dermatology, with the goal of establishing care with a dermatologist.
Limitations
Limitations to this study include an inability to categorize HS disease severity and assess the degree to which disease severity confounded study findings, particularly in relation to tobacco use and obesity. The generalizability of this study is also limited because of the demographic characteristics of the veteran patient population, which is predominantly older, White, and male, whereas HS disproportionately affects younger, Black, and female individuals in the US.22 Despite these limitations, this study contributes valuable insights into the use of biologic therapies for veteran populations with HS using a national dataset.
Conclusions
This study was performed within a single-payer government medical system, likely reducing or removing the financial barriers that some patient populations may face when pursuing biologics for HS treatment. However, the prevalence of biologic use in this population was low overall (5.2%), suggesting that other factors play a role in the underutilization of biologics in HS. Consistent with previous studies, younger individuals were more likely to be prescribed a biologic, and no difference in prescription rates between Black and White patients was observed. Unlike previous studies, no significant difference in prescription rates between men and women was observed.
Hidradenitis suppurativa (HS) is a chronic, inflammatory skin disorder characterized by painful nodules, abscesses, and tunnels predominantly affecting intertriginous areas of the body.1,2 The condition poses significant challenges in terms of diagnosis, treatment, and quality of life for affected individuals. Various systemic therapies have been explored to manage this debilitating condition, with the emergence of biologic agents offering hope for improved outcomes. In 2015, adalimumab (ADA) was the first biologic approved by the US Food and Drug Administration (FDA) for the treatment of HS, followed by secukinumab in 2023 and bimekizumab in 2024. However, the off-label use of other biologics and/or tumor necrosis factor inhibitors such as infliximab (IFX) has become common practice.3
Although these therapies have demonstrated promising results in the treatment of HS, their widespread use may be hindered by accessibility and cost barriers. Orenstein et al analyzed data from the IBM Explorys platform from 2015 to 2020 and found that only 1.8% of patients diagnosed with HS had been prescribed ADA or IFX.4 More recently, Garg et al examined IBM MarketScan and IBM US Medicaid data from 2015 to 2018 to evaluate trends in clinical care and treatment. The prevalence of ADA and IFX prescriptions among patients with HS ranged from 2.3% to 8.0% (ADA) and 0.7% to 0.9% (IFX) for patients with commercial insurance, and 1.4% to 4.8% (ADA) and 0.5% to 0.7% (IFX) for patients with Medicaid.5 Biologics are often expensive, and the high cost associated with these therapies has been identified as a significant barrier to access for patients with HS, particularly those who lack adequate insurance coverage or face financial constraints.6
Furthermore, these barriers, particularly the financial barriers, are potentially compounded by the demographics of patients most notably affected by HS. In the US, a disproportionate incidence of HS has been noted in specific groups and age ranges, including women, individuals aged 18 to 29 years, and Black individuals.4 Orenstein et al found a statistically significant difference in use of ADA and IFX biologics based on age, sex, and race.4
The aim of this study was to examine the use of 2 biologics (ADA and IFX) in the Veterans Health Administration (VHA), a unique population in which financial barriers are reduced due to the single-payer government health care system structure. This design allowed for improved isolation and evaluation of variation in ADA and/or IFX prescription rates by demographics and health-related factors among patients with HS. To our knowledge, no studies have analyzed these metrics within the VHA.
Methods
This retrospective, cross-sectional analysis of VHA patients used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a data repository that provides access to longitudinal national electronic health record data for all veterans receiving care through VHA facilities. This study received ethical approval from institutional review boards at the Minneapolis Veterans Affairs Health Care System and VA Salt Lake City Healthcare System. Patient information was deidentified, and patient consent was not required.
Patients with HS were identified using ≥ 1 International Classification of Diseases (ICD) diagnostic code: (ICD-9 [705.83] or ICD-10 [L73.2]) between January 1, 2011, and December 31, 2021. The study included patients aged ≥ 18 years as of January 1, 2011, with ≥ 2 patient encounters during the postdiagnosis follow-up period, and with ≥ 1 encounter 6 months postindex. Patients with a biologic prescription prior to HS diagnosis were excluded. For this study, the term biologics refers to ADA and/or IFX prescriptions, unless otherwise specified. Only ADA and IFX were included in this analysis because ADA, a tumor necrosis factor (TNF)-á inhibitor, was the only FDA-approved medication at the time of the search, and IFX is another common TNF-α inhibitor used for the treatment of HS.
Statistical Analysis
We calculated logistic regression using SAS 9.4 (SAS Institute, Cary, NC). For each variable, the univariate relationship with biologic prescriptions was examined first, followed by the multivariate relationship controlling for all other variables. The following variables were controlled for in the multivariate models and were chosen a priori: sex, age, race, ethnicity, US region, hospital setting, current or previous tobacco use, obesity (defined as body mass index [BMI] ≥ 30), and Charlson Comorbidity Index (CCI).7
Results
Using ICD codes, we identified 29,483 individuals with ≥ 1 HS diagnosis (Figure 1). Of those identified, 1537 patients (5.21%) had been prescribed ≥ 1 biologic. The cohort was predominantly White (60.56%), male (75.27%), obese (59.34%), and had a history of current or previous tobacco use (73.47%) (Table 1). There were significant adjusted differences in prescription rates among veterans with HS based on age, race, and BMI. Notably, there was an age-dependent reduction in the odds of being prescribed a biologic in patients with HS. Compared with patients aged 18 to 44 years, patients aged 45 to 64 years (adjusted odds ratio [aOR], 0.63; 95% CI, 0.54–0.74; P < .001) and patients aged ≥ 65 years (aOR, 0.36; 95% CI, 0.27–0.48; P < .001) had significantly lower odds of receiving a biologic prescription (Table 2). Compared with White patients with HS, Native Hawaiian (NH) or Pacific Islander (PI) patients were less likely to be prescribed a biologic (aOR, 0.23; 95% CI, 0.06–0.92; P = .04). Patients with obesity had significantly higher odds of receiving a biologic prescription compared with patients without obesity (aOR, 1.47; 95% CI, 1.27– 1.71; P < .001).

Included in Analysis.
After adjusting for the variables listed in Table 1, there were no significant differences in biologic prescription rates for men compared with women (aOR, 0.97; 95% CI, 0.83-1.12; P = .68). We observed slight variations in biologic prescriptions between US regions (Midwest 5.0%, East 4.2%, South 5.8%, West 4.6%), none of which were significantly different in the fully adjusted model. No statistically significant differences were found in biologic prescriptions between urban and rural VA settings (5.4% vs 4.8%; aOR, 1.06; 95% CI, 0.90–1.24; P = .47). Tobacco use was not associated with the rate of biologic prescription receipt (aOR, 1.14; 95% CI, 0.97–1.34; P = .11). After adjusting for other variables (as outlined in Table 2), no significant differences were found between CCI of 0 and 1 (aOR, 0.97; 95% CI, 0.82–1.16; P = .77) or between CCI of 0 and 2 (aOR, 0.89; 95% CI, 0.74–1.07; P = .22).7


Discussion
The aim of the study was to ascertain potential discrepancies in biologic prescription patterns among patients with HS in the VHA by demographic and lifestyle behavior modifiers. Veteran cohorts are unique in composition, consisting predominantly of older White men within a single-payer health care system. The prevalence of biologic prescriptions in this population was low (5.2%), consistent with prior studies (1.8%–8.9%).4,5
We found a significant difference in ADA/IFX prescription patterns between White patients and NH/PI patients (aOR, 0.23; 95% CI, 0.06-0.92; P = .04). Further replication of this result is needed due to the small number of NH/PI patients included in the study (n = 241). Notably, we did not find a significant difference in the odds of Black patients being prescribed a biologic compared with White patients (aOR, 1.07; 95% CI, 0.92–1.25; P = .38), consistent with prior studies.4
In line with prior studies, age was associated with the likelihood of receiving a biologic prescription.4 Using the multivariate model adjusting for variables listed in Table 1, including CCI, patients aged 45 to 64 years and > 64 years were less likely to be prescribed a biologic than patients aged 18 to 44 years. HS disease activity could be a potential confounding variable, as HS severity may subside in some people with increasing age or menopause.8
Because different regions in the US have different sociopolitical ideologies and governing legislation, we hypothesized that there may be dissimilarities in the prevalence rates of biologic prescribing across various US regions. However, no significant differences were found in prescription patterns among US regions or between rural and urban settings. Previous research has demonstrated discernible disparities in both dermatologic care and clinical outcomes based on hospital setting (ie, urban vs rural).9-11
Tobacco use has been demonstrated to be associated with the development of HS.12 In a large retrospective analysis, Garg et al reported increased odds of receiving a new HS diagnosis in known tobacco users (aOR, 1.9; 95% CI, 1.8–2.0).13 The extent to which tobacco use affects HS severity is less understood. While some studies have found an association between smoking and HS severity, other analyses have failed to find this association.14,15 The effects of smoking cessation on the disease course of HS are unknown.16 This analysis, found no significant difference in prescriptions for biologics among patients with HS comparing current or previous tobacco users with nonusers.
There is a known positive correlation between increasing BMI and HS prevalence and severity that may be explained by the downstream effects of adipose tissue secretion of proinflammatory mediators and insulin resistance in the setting of chronic inflammation.12 This analysis found that patients with HS and obesity were 1.47 times more likely to be prescribed a biologic than patients with HS without obesity, which may be confounded by increased HS severity among patients with obesity. The initial concern when analyzing tobacco use and obesity was that clinician bias may result in a decrease in the prevalence of biologic use in these demographics, which was not supported in this study.
Although we identified few disparities, the results demonstrated a substantial underutilization of biologic therapies (5.2%), similar to the other US civilian studies (1.8-8.9%).4,5 While there is no current universal, standardized severity scoring system to evaluate HS (it is difficult to objectively define moderate to severe HS), estimates have shown that 40.3% to 65.8% of patients with HS have Hurley stage II or III.17-19 Therefore, only a small percentage of patients with moderate to severe disease were prescribed the only FDA-approved medication during this time period. The persistence of this underutilization within a medical system that reduces financial barriers suggests that nonfinancial barriers have a notable role in the underutilization of biologics.
For instance, risk of adverse events, particularly lymphoma and infection, has been cited by patients as a reason to avoid biologics. Additionally, treatment fatigue reduced some patients’ willingness to try new treatments, as did lack of knowledge about treatment options.6,20 Other reported barriers included the frequency of injections and fear of needles.6 Additionally, within the VA, ADA may require prior authorization at the local facility level.21 An established relationship with a dermatologist has been shown to significantly increase the odds of being prescribed a biologic medication in the face of these barriers.4 Future system-wide quality improvement initiatives could be implemented to identify patients with HS not followed by dermatology, with the goal of establishing care with a dermatologist.
Limitations
Limitations to this study include an inability to categorize HS disease severity and assess the degree to which disease severity confounded study findings, particularly in relation to tobacco use and obesity. The generalizability of this study is also limited because of the demographic characteristics of the veteran patient population, which is predominantly older, White, and male, whereas HS disproportionately affects younger, Black, and female individuals in the US.22 Despite these limitations, this study contributes valuable insights into the use of biologic therapies for veteran populations with HS using a national dataset.
Conclusions
This study was performed within a single-payer government medical system, likely reducing or removing the financial barriers that some patient populations may face when pursuing biologics for HS treatment. However, the prevalence of biologic use in this population was low overall (5.2%), suggesting that other factors play a role in the underutilization of biologics in HS. Consistent with previous studies, younger individuals were more likely to be prescribed a biologic, and no difference in prescription rates between Black and White patients was observed. Unlike previous studies, no significant difference in prescription rates between men and women was observed.
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-1058. doi:10.1016/j.jaad.2019.08.090
- Tchero H, Herlin C, Bekara F, et al. Hidradenitis suppurativa: a systematic review and meta-analysis of therapeutic interventions. Indian J Dermatol Venereol Leprol. 2019;85:248-257. doi:10.4103/ijdvl.IJDVL_69_18
- Shih T, Lee K, Grogan T, et al. Infliximab in hidradenitis suppurativa: a systematic review and meta-analysis. Dermatol Ther. 2022;35:e15691. doi:10.1111/dth.15691
- Orenstein LAV, Wright S, Strunk A, et al. Low prescription of tumor necrosis alpha inhibitors in hidradenitis suppurativa: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1399-1401. doi:10.1016/j.jaad.2020.07.108
- Garg A, Naik HB, Alavi A, et al. Real-world findings on the characteristics and treatment exposures of patients with hidradenitis suppurativa from US claims data. Dermatol Ther (Heidelb). 2023;13:581-594. doi:10.1007/s13555-022-00872-1
- De DR, Shih T, Fixsen D, et al. Biologic use in hidradenitis suppurativa: patient perspectives and barriers. J Dermatolog Treat. 2022;33:3060-3062. doi:10.1080/09546634.2022.2089336
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373- 383. doi:10.1016/0021-9681(87)90171-8
- von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14:389-392. doi:10.1046/j.1468-3083.2000.00087.x
- Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126:417-428.e2. doi:10.1016/j.anai.2020.12.020
- Wu YP, Parsons B, Jo Y, et al. Outdoor activities and sunburn among urban and rural families in a Western region of the US: implications for skin cancer prevention. Prev Med Rep. 2022;29:101914. doi:10.1016/j.pmedr.2022.101914
- Mannschreck DB, Li X, Okoye G. Rural melanoma patients in Maryland do not present with more advanced disease than urban patients. Dermatol Online J. 2021;27. doi:10.5070/D327553607
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population- based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Sartorius K, Emtestam L, Jemec GBE, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831- 839. doi:10.1111/j.1365-2133.2009.09198.x
- Canoui-Poitrine F, Revuz JE, Wolkenstein P, et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol. 2009;61:51-57. doi:10.1016/j.jaad.2009.02.013
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216- 221. doi:10.1136/postgradmedj-2013-131994
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population- based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Vanlaerhoven AMJD, Ardon CB, van Straalen KR, et al. Hurley III hidradenitis suppurativa has an aggressive disease course. Dermatology. 2018;234:232-233. doi:10.1159/000491547
- Shahi V, Alikhan A, Vazquez BG, et al. Prevalence of hidradenitis suppurativa: a population-based study in Olmsted County, Minnesota. Dermatology. 2014;229:154-158. doi:10.1159/000363381
- Salame N, Sow YN, Siira MR, et al. Factors affecting treatment selection among patients with hidradenitis suppurativa. JAMA Dermatol. 2024;160:179. doi:10.1001/jamadermatol.2023.5425
- VA Formulary Advisor: ADALIMUMAB-BWWD INJ,SOLN. US Department of Veterans Affairs. Updated December 17, 2025. Accessed January 15, 2026. https://www.va.gov/formularyadvisor/drugs/4042383-ADALIMUMAB-BWWD-INJ-SOLN
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118- 122. doi:10.1016/j.jaad.2017.02.005
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-1058. doi:10.1016/j.jaad.2019.08.090
- Tchero H, Herlin C, Bekara F, et al. Hidradenitis suppurativa: a systematic review and meta-analysis of therapeutic interventions. Indian J Dermatol Venereol Leprol. 2019;85:248-257. doi:10.4103/ijdvl.IJDVL_69_18
- Shih T, Lee K, Grogan T, et al. Infliximab in hidradenitis suppurativa: a systematic review and meta-analysis. Dermatol Ther. 2022;35:e15691. doi:10.1111/dth.15691
- Orenstein LAV, Wright S, Strunk A, et al. Low prescription of tumor necrosis alpha inhibitors in hidradenitis suppurativa: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1399-1401. doi:10.1016/j.jaad.2020.07.108
- Garg A, Naik HB, Alavi A, et al. Real-world findings on the characteristics and treatment exposures of patients with hidradenitis suppurativa from US claims data. Dermatol Ther (Heidelb). 2023;13:581-594. doi:10.1007/s13555-022-00872-1
- De DR, Shih T, Fixsen D, et al. Biologic use in hidradenitis suppurativa: patient perspectives and barriers. J Dermatolog Treat. 2022;33:3060-3062. doi:10.1080/09546634.2022.2089336
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373- 383. doi:10.1016/0021-9681(87)90171-8
- von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14:389-392. doi:10.1046/j.1468-3083.2000.00087.x
- Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126:417-428.e2. doi:10.1016/j.anai.2020.12.020
- Wu YP, Parsons B, Jo Y, et al. Outdoor activities and sunburn among urban and rural families in a Western region of the US: implications for skin cancer prevention. Prev Med Rep. 2022;29:101914. doi:10.1016/j.pmedr.2022.101914
- Mannschreck DB, Li X, Okoye G. Rural melanoma patients in Maryland do not present with more advanced disease than urban patients. Dermatol Online J. 2021;27. doi:10.5070/D327553607
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population- based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Sartorius K, Emtestam L, Jemec GBE, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831- 839. doi:10.1111/j.1365-2133.2009.09198.x
- Canoui-Poitrine F, Revuz JE, Wolkenstein P, et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol. 2009;61:51-57. doi:10.1016/j.jaad.2009.02.013
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216- 221. doi:10.1136/postgradmedj-2013-131994
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population- based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Vanlaerhoven AMJD, Ardon CB, van Straalen KR, et al. Hurley III hidradenitis suppurativa has an aggressive disease course. Dermatology. 2018;234:232-233. doi:10.1159/000491547
- Shahi V, Alikhan A, Vazquez BG, et al. Prevalence of hidradenitis suppurativa: a population-based study in Olmsted County, Minnesota. Dermatology. 2014;229:154-158. doi:10.1159/000363381
- Salame N, Sow YN, Siira MR, et al. Factors affecting treatment selection among patients with hidradenitis suppurativa. JAMA Dermatol. 2024;160:179. doi:10.1001/jamadermatol.2023.5425
- VA Formulary Advisor: ADALIMUMAB-BWWD INJ,SOLN. US Department of Veterans Affairs. Updated December 17, 2025. Accessed January 15, 2026. https://www.va.gov/formularyadvisor/drugs/4042383-ADALIMUMAB-BWWD-INJ-SOLN
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118- 122. doi:10.1016/j.jaad.2017.02.005
Cross-Sectional Analysis of Biologic Use in the Treatment of Veterans With Hidradenitis Suppurativa
Cross-Sectional Analysis of Biologic Use in the Treatment of Veterans With Hidradenitis Suppurativa
Managing Resistance to Change Along the Journey to High Reliability
Managing Resistance to Change Along the Journey to High Reliability
To improve safety performance, many health care organizations have embarked on the journey to becoming high reliability organizations (HROs). HROs operate in complex, high-risk, constantly changing environments and avoid catastrophic events despite the inherent risks.1 HROs maintain high levels of safety and reliability by adhering to core principles, foundational practices, rigorous processes, a strong organizational culture, and continuous learning and process improvement.1-3
Becoming an HRO requires understanding what makes systems safer for patients and staff at all levels by taking ownership of 5 principles: (1) sensitivity to operations (increased awareness of the current status of systems); (2) reluctance to simplify (avoiding oversimplification of the cause[s] of problems); (3) preoccupation with failure (anticipating risks that might be symptomatic of a larger problem); (4) deference to expertise (relying on the most qualified individuals to make decisions); and (5) commitment to resilience (planning for potential failure and being prepared to respond).1,2,4 In addition to these, the Veterans Health Administration has identified 3 pillars of HROs: leadership commitment (safety and reliability are central to leadership vision, decision-making, and action-oriented behaviors), safety culture (across the organization, safety values are key to preventing harm and learning from mistakes), and continuous process improvement (promoting constant learning and improvement with evidence-based tools and methodologies).5
Implementing these principles is not enough to achieve high reliability. This transition requires significant change, which can be met with resistance. Without attending to organizational change, implementation of HRO principles can be superficial, scattered, and isolated.6 Large organizations often struggle with change as it conflicts with the fundamental human need for stability and security.7 Consequently, the journey to becoming an HRO requires an understanding of the reasons for resistance to change (RtC) as well as evidence-based strategies.
REASONS FOR RESISTANCE TO CHANGE
RtC is the informal and covert behavior of an individual or group to a particular change. RtC is commonly recognized as the failure of employees to do anything requested by managers and is a main reason change initiatives fail.8 While some staff see change as opportunities for learning and growth, others resist based on uncertainty about how the changes will impact their current work situation, or fear, frustration, confusion, and distrust.8,9 Resistance can overtly manifest with some staff publicly expressing their discontent in public without offering solutions, or covertly by ignoring the change or avoiding participation in any aspect of the change process. Both forms of RtC are equally detrimental.8
Frequent changes in organizations can also cause cynicism. Employees will view the change as something initially popular, but will only last until another change comes along.8,9 Resistance can result in the failure to achieve desired objectives, wasted time, effort, and resources, decreased momentum, and loss of confidence and trust in leaders to effectively manage the change process.9 To understand RtC, 3 main factors must be considered: individual, interpersonal, and organizational.
Individual
An individual’s personality can be an important indicator for how they will respond to change. Some individuals welcome and thrive on change while others resist in preference for the status quo.8,10 Individuals will also resist change if they believe their position, power, or prestige within the organization are in jeopardy or that the change is contrary to current personal or organizational values, principles, and objectives.8-12 Resistance can also be the result of uncertainty about what the change means, lack of information regarding the change, or questioning motives for the change.9
Interpersonal
Another influence on RtC is the interpersonal factors of employees. The personal satisfaction individuals receive from their work and the type of interactions they experience with colleagues can impact RtC. When communication with colleagues is lacking before and during change implementation, negative reactions to the change can fuel resistance.11 Cross-functional and bidirectional communication is vital; its absence can leave staff feeling inadequately informed and less supportive of the change.8 Employees’ understanding of changes through communication between other members of the organization is critical to success.11
Organizational
How organizational leaders introduce change affects the extent to which staff respond.10 RtC can emerge if staff feel change is imposed on them. Change is better received when people are actively engaged in the process and adopt a sense of ownership that will ultimately affect them and their role within the organization.12,13 Organizations are also better equipped to address potential RtC when leadership is respected and have a genuine concern for the overall well-being of staff members. Organizational leaders who mainly focus on the bottom line and have little regard for staff are more likely to be perceived as untrustworthy, which contributes to RtC.9,13 Lack of proper education and guidance from organizational leaders, as well as poor communication, can lead to RtC.8,13
MANAGING RESISTANCE TO CHANGE
RtC can be a significant factor in the success or failure of the change process. Poorly managed change can exponentially increase resistance, necessitating a multifaceted approach to managing RtC, while well-managed change can result in a high success rate. Evidence-based strategies to counter RtC focus on communication, employee participation, education and training, and engaging managers.8
Communication
Open and effective communication is critical to managing RtC, as uncertainty often exaggerates the negative aspects of change. Effective communication involves active listening, with leadership and management addressing employee concerns in a clear and concise manner. A psychologically safe culture for open dialogue is essential when addressing RtC.9,14,15 Psychological safety empowers staff to speak up, ask questions, and offer ideas, forming a solid basis for open and effective communication and participation. Leaders and managers should create opportunities for open dialogue for all members of the organization throughout the process. This can be accomplished with one-on-one meetings, open forums, town hall meetings, electronic mail, newsletters, and social media. Topics should cover the reasons for change, details of what is changing, the individual, organizational, and patient risks of not changing, as well as the benefits of changing.9 Encouraging staff to ask questions and provide feedback to promote bidirectional and closed-loop communication is essential to avoid misunderstandings.9,15 While open communication is essential, leaders must carefully plan what information to share, how much to share, and how to avoid information overload. Information about the change should be timely, adequate, applicable, and informative.15 The HRO practice of leader rounding for high reliability can be instrumental to ensure effective, bidirectional communication and collaboration among all disciplines across a health care organization through improving leadership visibility during times of change and enhancing interactions and communication with staff.3
Employee Participation
Involving staff in the change process significantly reduces RtC. Engagement fosters ownership in the change process, increasing the likelihood employees will support and even champion it. Health care professionals welcome opportunities to be involved in helping with aspects of organizational change, especially when invited to participate in the change early in the process and throughout the course of change.7,14,15
Leaders should encourage staff to provide feedback to understand the impact the change is having on them and their roles and responsibilities within the organization. This exemplifies the HRO principle of deference to expertise as the employee often has the most in-depth knowledge of their work setting. Employee perspectives can significantly influence the success of change initatives.7,14 Participation is impactful in providing employees with a sense of agency facilitating acceptance and improving desire to adopt the change.14
Tiered safety huddles and visual management systems (VMSs) also can engage staff. Tiered safety huddles provide a forum for transparent communication, increasing situational awareness, and improving a health care organization’s ability to appropriately respond to staff questions, suggestions, and concerns. VMSs display the status and progress toward organizational goals during the change process, and are highly effective in creating environments where staff feel empowered to voice concerns related to the change process.3
Education and Training
Educating employees on the value of change is crucial to overcome RtC. RtC often stems from employees not feeling prepared to adapt or adopt new processes. Health care professionals who do not receive information about change are less likely to support it.7,12,15 Staff are more likely to accept change when they understand why it is needed and how it impacts the organization’s long-term mission.11,15 Timely, compelling, and informative education on how to adapt to the change will promote more positive appraisal of the change and reduce RtC.8,15 Employees must feel confident they will receive the appropriate training, resources, and support to successfully adapt to the change. This requires leaders and managers taking time to clarify expectations, conduct a gap analysis to identify the skills and knowledge needed to support the planned change, and provide sufficient educational opportunities to fill those gaps.8 For example, the US Department of Veterans Affairs offers classes to employees on the Prosci ADKAR (Awareness, Desire, Knowledge, Ability, and Reinforcement) Model. This training provides individuals with the information and skills needed for change to be successful.16
Safety forums can be influential and allow leadership to educate staff on updates related to change processes and promote bidirectional communication.3 In safety forums, staff have an opportunity to ask questions, especially as they relate to learning about available resources to become more informed about the organizational changes.
Engaging Managers
Managers are pivotal to the successful implementation of organizational change.8 They serve as the bridge between senior leadership and frontline employees and are positioned to influence the adoption and success of change initiatives. Often the first point of contact for employees, managers can effectively communicate the need for change, and act as the liaison to align it with individual employee motivations. Since they are often the first to encounter resistance among employees, managers serve as advocates through the process. Through a coaching role, managers can help employees develop the knowledge and ability to be successful and thrive in the new environment. The Table summarizes the evidence-based strategies.
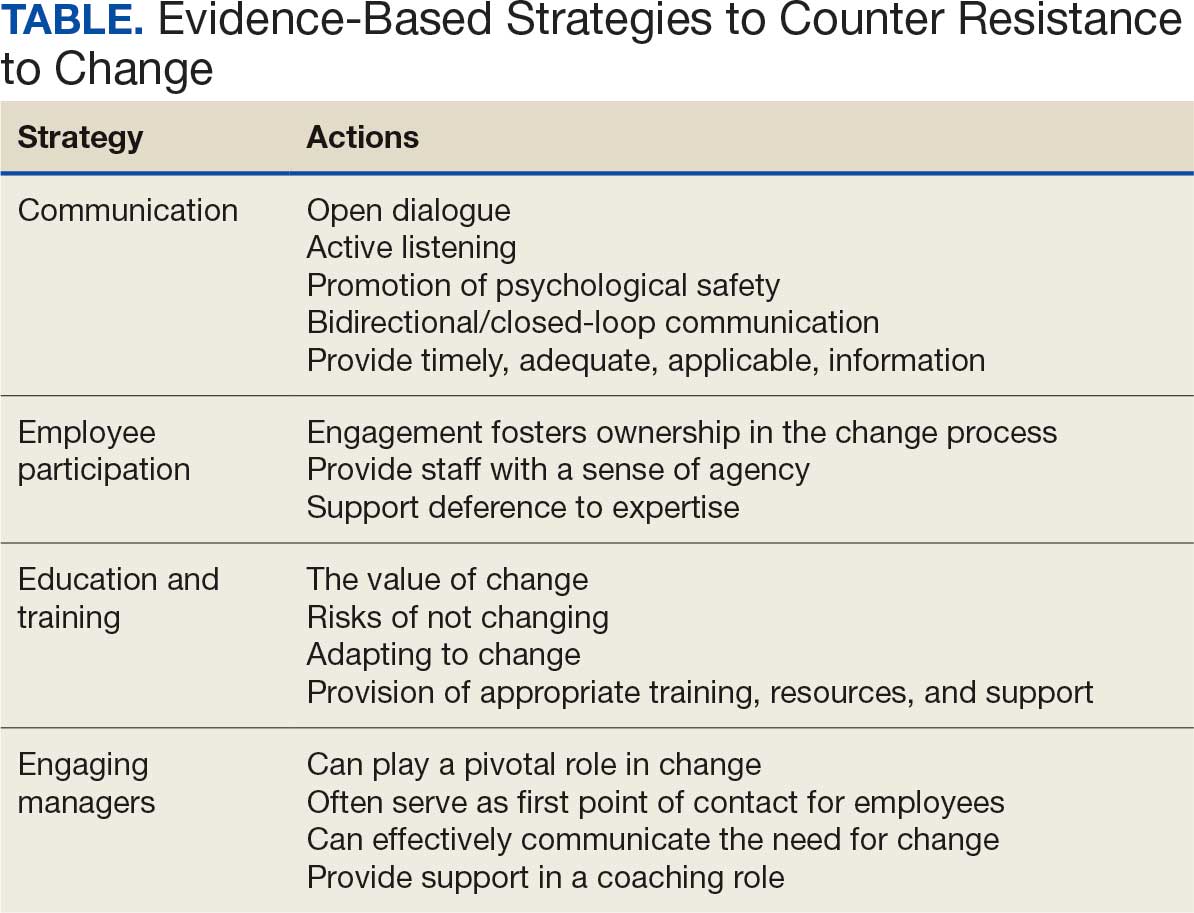
CONCLUSIONS
Implementing change in health care organizations can be challenging, especially on the journey to high reliability. RtC is the result of factors at the individual, interpersonal, and organizational levels that leaders must address to increase chances for success. Organizational changes in health care are more likely to succeed when staff understand why the change is needed through open and continuous communication, can influence the change by sharing their own perspectives, and have the knowledge, skills, and resources to prepare for and participate in the process.
- Merchant NB, O’Neal J, Dealing-Perez C, et al. A high-reliability organization mindset. Am J Med Qual. 2022;37:504-510. doi:10.1097/JMQ.0000000000000086
- Veazie S, Peterson K, Bourne D, et al. Implementing high-reliability organization principles into practice: a rapid evidence review. J Patient Saf. 2022;18:e320-e328. doi:10.1097/PTS.0000000000000768
- Murray JS, Baghdadi A, Dannenberg W, et al. The role of high reliability organization foundational practices in building a culture of safety. Fed Pract. 2024;41:214-221. doi:10.12788/fp.0486
- Ford J, Isaacks DB, Anderson T. Creating, executing and sustaining a high-reliability organization in health care. The Learning Organization: An International Journal. 2024;31:817-833. doi:10.1108/TLO-03-2023-0048
- Cox GR, Starr LM. VHA’s movement for change: implementing high-reliability principles and practices. J Healthc Manag. 2023;68:151-157. doi:10.1097/JHM-D-00056
- Myers CG, Sutcliffe KM. High reliability organising in healthcare: still a long way left to go. BMJ Qual Saf. 2022;31:845-848. doi:10.1136/bmjqs-2021-014141
- Nilsen P, Seing I, Ericsson C, et al. Characteristics of successful changes in health care organizations: an interview study with physicians, registered nurses and assistant nurses. BMC Health Serv Res. 2020;20:147. doi:10.1186/s12913-020-4999-8
- Cheraghi R, Ebrahimi H, Kheibar N, et al. Reasons for resistance to change in nursing: an integrative review. BMC Nurs. 2023;22:310. doi:10/1186/s12912-023-01460-0
- Warrick DD. Revisiting resistance to change and how to manage it: what has been learned and what organizations need to do. Bus Horiz. 2023;66:433-441. doi:10.1016/j.bushor.2022.09.001
- Sverdlik N, Oreg S. Beyond the individual-level conceptualization of dispositional resistance to change: multilevel effects on the response to organizational change. J Organ Behav. 2023;44:1066-1077. doi:10.1002/job.2678
- Khaw KW, Alnoor A, Al-Abrrow H, et al. Reactions towards organizational change: a systematic literature review. Curr Psychol. 2022;13:1-24. doi:10.1007/s12144-022-03070-6
- Pomare C, Churruca K, Long JC, et al. Organisational change in hospitals: a qualitative case-study of staff perspectives. BMC Health Serv Res. 2019;19:840. doi:10.1186/s12913-019-4704-y
- DuBose BM, Mayo AM. RtC: a concept analysis. Nurs Forum. 2020;55:631-636. doi:10.1111/nuf.12479
- Sahay S, Goldthwaite C. Participatory practices during organizational change: rethinking participation and resistance. Manag Commun Q. 2024;38(2):279-306. doi:10.1177/08933189231187883
- Damawan AH, Azizah S. Resistance to change: causes and strategies as an organizational challenge. ASSEHR. 2020;395(2020):49-53. doi:10.2991/assehr.k.200120.010
- Wong Q, Lacombe M, Keller R, et al. Leading change with ADKAR. Nurs Manage. 2019;50:28-35. doi:10.1097/01.NUMA.0000554341.70508.75
To improve safety performance, many health care organizations have embarked on the journey to becoming high reliability organizations (HROs). HROs operate in complex, high-risk, constantly changing environments and avoid catastrophic events despite the inherent risks.1 HROs maintain high levels of safety and reliability by adhering to core principles, foundational practices, rigorous processes, a strong organizational culture, and continuous learning and process improvement.1-3
Becoming an HRO requires understanding what makes systems safer for patients and staff at all levels by taking ownership of 5 principles: (1) sensitivity to operations (increased awareness of the current status of systems); (2) reluctance to simplify (avoiding oversimplification of the cause[s] of problems); (3) preoccupation with failure (anticipating risks that might be symptomatic of a larger problem); (4) deference to expertise (relying on the most qualified individuals to make decisions); and (5) commitment to resilience (planning for potential failure and being prepared to respond).1,2,4 In addition to these, the Veterans Health Administration has identified 3 pillars of HROs: leadership commitment (safety and reliability are central to leadership vision, decision-making, and action-oriented behaviors), safety culture (across the organization, safety values are key to preventing harm and learning from mistakes), and continuous process improvement (promoting constant learning and improvement with evidence-based tools and methodologies).5
Implementing these principles is not enough to achieve high reliability. This transition requires significant change, which can be met with resistance. Without attending to organizational change, implementation of HRO principles can be superficial, scattered, and isolated.6 Large organizations often struggle with change as it conflicts with the fundamental human need for stability and security.7 Consequently, the journey to becoming an HRO requires an understanding of the reasons for resistance to change (RtC) as well as evidence-based strategies.
REASONS FOR RESISTANCE TO CHANGE
RtC is the informal and covert behavior of an individual or group to a particular change. RtC is commonly recognized as the failure of employees to do anything requested by managers and is a main reason change initiatives fail.8 While some staff see change as opportunities for learning and growth, others resist based on uncertainty about how the changes will impact their current work situation, or fear, frustration, confusion, and distrust.8,9 Resistance can overtly manifest with some staff publicly expressing their discontent in public without offering solutions, or covertly by ignoring the change or avoiding participation in any aspect of the change process. Both forms of RtC are equally detrimental.8
Frequent changes in organizations can also cause cynicism. Employees will view the change as something initially popular, but will only last until another change comes along.8,9 Resistance can result in the failure to achieve desired objectives, wasted time, effort, and resources, decreased momentum, and loss of confidence and trust in leaders to effectively manage the change process.9 To understand RtC, 3 main factors must be considered: individual, interpersonal, and organizational.
Individual
An individual’s personality can be an important indicator for how they will respond to change. Some individuals welcome and thrive on change while others resist in preference for the status quo.8,10 Individuals will also resist change if they believe their position, power, or prestige within the organization are in jeopardy or that the change is contrary to current personal or organizational values, principles, and objectives.8-12 Resistance can also be the result of uncertainty about what the change means, lack of information regarding the change, or questioning motives for the change.9
Interpersonal
Another influence on RtC is the interpersonal factors of employees. The personal satisfaction individuals receive from their work and the type of interactions they experience with colleagues can impact RtC. When communication with colleagues is lacking before and during change implementation, negative reactions to the change can fuel resistance.11 Cross-functional and bidirectional communication is vital; its absence can leave staff feeling inadequately informed and less supportive of the change.8 Employees’ understanding of changes through communication between other members of the organization is critical to success.11
Organizational
How organizational leaders introduce change affects the extent to which staff respond.10 RtC can emerge if staff feel change is imposed on them. Change is better received when people are actively engaged in the process and adopt a sense of ownership that will ultimately affect them and their role within the organization.12,13 Organizations are also better equipped to address potential RtC when leadership is respected and have a genuine concern for the overall well-being of staff members. Organizational leaders who mainly focus on the bottom line and have little regard for staff are more likely to be perceived as untrustworthy, which contributes to RtC.9,13 Lack of proper education and guidance from organizational leaders, as well as poor communication, can lead to RtC.8,13
MANAGING RESISTANCE TO CHANGE
RtC can be a significant factor in the success or failure of the change process. Poorly managed change can exponentially increase resistance, necessitating a multifaceted approach to managing RtC, while well-managed change can result in a high success rate. Evidence-based strategies to counter RtC focus on communication, employee participation, education and training, and engaging managers.8
Communication
Open and effective communication is critical to managing RtC, as uncertainty often exaggerates the negative aspects of change. Effective communication involves active listening, with leadership and management addressing employee concerns in a clear and concise manner. A psychologically safe culture for open dialogue is essential when addressing RtC.9,14,15 Psychological safety empowers staff to speak up, ask questions, and offer ideas, forming a solid basis for open and effective communication and participation. Leaders and managers should create opportunities for open dialogue for all members of the organization throughout the process. This can be accomplished with one-on-one meetings, open forums, town hall meetings, electronic mail, newsletters, and social media. Topics should cover the reasons for change, details of what is changing, the individual, organizational, and patient risks of not changing, as well as the benefits of changing.9 Encouraging staff to ask questions and provide feedback to promote bidirectional and closed-loop communication is essential to avoid misunderstandings.9,15 While open communication is essential, leaders must carefully plan what information to share, how much to share, and how to avoid information overload. Information about the change should be timely, adequate, applicable, and informative.15 The HRO practice of leader rounding for high reliability can be instrumental to ensure effective, bidirectional communication and collaboration among all disciplines across a health care organization through improving leadership visibility during times of change and enhancing interactions and communication with staff.3
Employee Participation
Involving staff in the change process significantly reduces RtC. Engagement fosters ownership in the change process, increasing the likelihood employees will support and even champion it. Health care professionals welcome opportunities to be involved in helping with aspects of organizational change, especially when invited to participate in the change early in the process and throughout the course of change.7,14,15
Leaders should encourage staff to provide feedback to understand the impact the change is having on them and their roles and responsibilities within the organization. This exemplifies the HRO principle of deference to expertise as the employee often has the most in-depth knowledge of their work setting. Employee perspectives can significantly influence the success of change initatives.7,14 Participation is impactful in providing employees with a sense of agency facilitating acceptance and improving desire to adopt the change.14
Tiered safety huddles and visual management systems (VMSs) also can engage staff. Tiered safety huddles provide a forum for transparent communication, increasing situational awareness, and improving a health care organization’s ability to appropriately respond to staff questions, suggestions, and concerns. VMSs display the status and progress toward organizational goals during the change process, and are highly effective in creating environments where staff feel empowered to voice concerns related to the change process.3
Education and Training
Educating employees on the value of change is crucial to overcome RtC. RtC often stems from employees not feeling prepared to adapt or adopt new processes. Health care professionals who do not receive information about change are less likely to support it.7,12,15 Staff are more likely to accept change when they understand why it is needed and how it impacts the organization’s long-term mission.11,15 Timely, compelling, and informative education on how to adapt to the change will promote more positive appraisal of the change and reduce RtC.8,15 Employees must feel confident they will receive the appropriate training, resources, and support to successfully adapt to the change. This requires leaders and managers taking time to clarify expectations, conduct a gap analysis to identify the skills and knowledge needed to support the planned change, and provide sufficient educational opportunities to fill those gaps.8 For example, the US Department of Veterans Affairs offers classes to employees on the Prosci ADKAR (Awareness, Desire, Knowledge, Ability, and Reinforcement) Model. This training provides individuals with the information and skills needed for change to be successful.16
Safety forums can be influential and allow leadership to educate staff on updates related to change processes and promote bidirectional communication.3 In safety forums, staff have an opportunity to ask questions, especially as they relate to learning about available resources to become more informed about the organizational changes.
Engaging Managers
Managers are pivotal to the successful implementation of organizational change.8 They serve as the bridge between senior leadership and frontline employees and are positioned to influence the adoption and success of change initiatives. Often the first point of contact for employees, managers can effectively communicate the need for change, and act as the liaison to align it with individual employee motivations. Since they are often the first to encounter resistance among employees, managers serve as advocates through the process. Through a coaching role, managers can help employees develop the knowledge and ability to be successful and thrive in the new environment. The Table summarizes the evidence-based strategies.

CONCLUSIONS
Implementing change in health care organizations can be challenging, especially on the journey to high reliability. RtC is the result of factors at the individual, interpersonal, and organizational levels that leaders must address to increase chances for success. Organizational changes in health care are more likely to succeed when staff understand why the change is needed through open and continuous communication, can influence the change by sharing their own perspectives, and have the knowledge, skills, and resources to prepare for and participate in the process.
To improve safety performance, many health care organizations have embarked on the journey to becoming high reliability organizations (HROs). HROs operate in complex, high-risk, constantly changing environments and avoid catastrophic events despite the inherent risks.1 HROs maintain high levels of safety and reliability by adhering to core principles, foundational practices, rigorous processes, a strong organizational culture, and continuous learning and process improvement.1-3
Becoming an HRO requires understanding what makes systems safer for patients and staff at all levels by taking ownership of 5 principles: (1) sensitivity to operations (increased awareness of the current status of systems); (2) reluctance to simplify (avoiding oversimplification of the cause[s] of problems); (3) preoccupation with failure (anticipating risks that might be symptomatic of a larger problem); (4) deference to expertise (relying on the most qualified individuals to make decisions); and (5) commitment to resilience (planning for potential failure and being prepared to respond).1,2,4 In addition to these, the Veterans Health Administration has identified 3 pillars of HROs: leadership commitment (safety and reliability are central to leadership vision, decision-making, and action-oriented behaviors), safety culture (across the organization, safety values are key to preventing harm and learning from mistakes), and continuous process improvement (promoting constant learning and improvement with evidence-based tools and methodologies).5
Implementing these principles is not enough to achieve high reliability. This transition requires significant change, which can be met with resistance. Without attending to organizational change, implementation of HRO principles can be superficial, scattered, and isolated.6 Large organizations often struggle with change as it conflicts with the fundamental human need for stability and security.7 Consequently, the journey to becoming an HRO requires an understanding of the reasons for resistance to change (RtC) as well as evidence-based strategies.
REASONS FOR RESISTANCE TO CHANGE
RtC is the informal and covert behavior of an individual or group to a particular change. RtC is commonly recognized as the failure of employees to do anything requested by managers and is a main reason change initiatives fail.8 While some staff see change as opportunities for learning and growth, others resist based on uncertainty about how the changes will impact their current work situation, or fear, frustration, confusion, and distrust.8,9 Resistance can overtly manifest with some staff publicly expressing their discontent in public without offering solutions, or covertly by ignoring the change or avoiding participation in any aspect of the change process. Both forms of RtC are equally detrimental.8
Frequent changes in organizations can also cause cynicism. Employees will view the change as something initially popular, but will only last until another change comes along.8,9 Resistance can result in the failure to achieve desired objectives, wasted time, effort, and resources, decreased momentum, and loss of confidence and trust in leaders to effectively manage the change process.9 To understand RtC, 3 main factors must be considered: individual, interpersonal, and organizational.
Individual
An individual’s personality can be an important indicator for how they will respond to change. Some individuals welcome and thrive on change while others resist in preference for the status quo.8,10 Individuals will also resist change if they believe their position, power, or prestige within the organization are in jeopardy or that the change is contrary to current personal or organizational values, principles, and objectives.8-12 Resistance can also be the result of uncertainty about what the change means, lack of information regarding the change, or questioning motives for the change.9
Interpersonal
Another influence on RtC is the interpersonal factors of employees. The personal satisfaction individuals receive from their work and the type of interactions they experience with colleagues can impact RtC. When communication with colleagues is lacking before and during change implementation, negative reactions to the change can fuel resistance.11 Cross-functional and bidirectional communication is vital; its absence can leave staff feeling inadequately informed and less supportive of the change.8 Employees’ understanding of changes through communication between other members of the organization is critical to success.11
Organizational
How organizational leaders introduce change affects the extent to which staff respond.10 RtC can emerge if staff feel change is imposed on them. Change is better received when people are actively engaged in the process and adopt a sense of ownership that will ultimately affect them and their role within the organization.12,13 Organizations are also better equipped to address potential RtC when leadership is respected and have a genuine concern for the overall well-being of staff members. Organizational leaders who mainly focus on the bottom line and have little regard for staff are more likely to be perceived as untrustworthy, which contributes to RtC.9,13 Lack of proper education and guidance from organizational leaders, as well as poor communication, can lead to RtC.8,13
MANAGING RESISTANCE TO CHANGE
RtC can be a significant factor in the success or failure of the change process. Poorly managed change can exponentially increase resistance, necessitating a multifaceted approach to managing RtC, while well-managed change can result in a high success rate. Evidence-based strategies to counter RtC focus on communication, employee participation, education and training, and engaging managers.8
Communication
Open and effective communication is critical to managing RtC, as uncertainty often exaggerates the negative aspects of change. Effective communication involves active listening, with leadership and management addressing employee concerns in a clear and concise manner. A psychologically safe culture for open dialogue is essential when addressing RtC.9,14,15 Psychological safety empowers staff to speak up, ask questions, and offer ideas, forming a solid basis for open and effective communication and participation. Leaders and managers should create opportunities for open dialogue for all members of the organization throughout the process. This can be accomplished with one-on-one meetings, open forums, town hall meetings, electronic mail, newsletters, and social media. Topics should cover the reasons for change, details of what is changing, the individual, organizational, and patient risks of not changing, as well as the benefits of changing.9 Encouraging staff to ask questions and provide feedback to promote bidirectional and closed-loop communication is essential to avoid misunderstandings.9,15 While open communication is essential, leaders must carefully plan what information to share, how much to share, and how to avoid information overload. Information about the change should be timely, adequate, applicable, and informative.15 The HRO practice of leader rounding for high reliability can be instrumental to ensure effective, bidirectional communication and collaboration among all disciplines across a health care organization through improving leadership visibility during times of change and enhancing interactions and communication with staff.3
Employee Participation
Involving staff in the change process significantly reduces RtC. Engagement fosters ownership in the change process, increasing the likelihood employees will support and even champion it. Health care professionals welcome opportunities to be involved in helping with aspects of organizational change, especially when invited to participate in the change early in the process and throughout the course of change.7,14,15
Leaders should encourage staff to provide feedback to understand the impact the change is having on them and their roles and responsibilities within the organization. This exemplifies the HRO principle of deference to expertise as the employee often has the most in-depth knowledge of their work setting. Employee perspectives can significantly influence the success of change initatives.7,14 Participation is impactful in providing employees with a sense of agency facilitating acceptance and improving desire to adopt the change.14
Tiered safety huddles and visual management systems (VMSs) also can engage staff. Tiered safety huddles provide a forum for transparent communication, increasing situational awareness, and improving a health care organization’s ability to appropriately respond to staff questions, suggestions, and concerns. VMSs display the status and progress toward organizational goals during the change process, and are highly effective in creating environments where staff feel empowered to voice concerns related to the change process.3
Education and Training
Educating employees on the value of change is crucial to overcome RtC. RtC often stems from employees not feeling prepared to adapt or adopt new processes. Health care professionals who do not receive information about change are less likely to support it.7,12,15 Staff are more likely to accept change when they understand why it is needed and how it impacts the organization’s long-term mission.11,15 Timely, compelling, and informative education on how to adapt to the change will promote more positive appraisal of the change and reduce RtC.8,15 Employees must feel confident they will receive the appropriate training, resources, and support to successfully adapt to the change. This requires leaders and managers taking time to clarify expectations, conduct a gap analysis to identify the skills and knowledge needed to support the planned change, and provide sufficient educational opportunities to fill those gaps.8 For example, the US Department of Veterans Affairs offers classes to employees on the Prosci ADKAR (Awareness, Desire, Knowledge, Ability, and Reinforcement) Model. This training provides individuals with the information and skills needed for change to be successful.16
Safety forums can be influential and allow leadership to educate staff on updates related to change processes and promote bidirectional communication.3 In safety forums, staff have an opportunity to ask questions, especially as they relate to learning about available resources to become more informed about the organizational changes.
Engaging Managers
Managers are pivotal to the successful implementation of organizational change.8 They serve as the bridge between senior leadership and frontline employees and are positioned to influence the adoption and success of change initiatives. Often the first point of contact for employees, managers can effectively communicate the need for change, and act as the liaison to align it with individual employee motivations. Since they are often the first to encounter resistance among employees, managers serve as advocates through the process. Through a coaching role, managers can help employees develop the knowledge and ability to be successful and thrive in the new environment. The Table summarizes the evidence-based strategies.

CONCLUSIONS
Implementing change in health care organizations can be challenging, especially on the journey to high reliability. RtC is the result of factors at the individual, interpersonal, and organizational levels that leaders must address to increase chances for success. Organizational changes in health care are more likely to succeed when staff understand why the change is needed through open and continuous communication, can influence the change by sharing their own perspectives, and have the knowledge, skills, and resources to prepare for and participate in the process.
- Merchant NB, O’Neal J, Dealing-Perez C, et al. A high-reliability organization mindset. Am J Med Qual. 2022;37:504-510. doi:10.1097/JMQ.0000000000000086
- Veazie S, Peterson K, Bourne D, et al. Implementing high-reliability organization principles into practice: a rapid evidence review. J Patient Saf. 2022;18:e320-e328. doi:10.1097/PTS.0000000000000768
- Murray JS, Baghdadi A, Dannenberg W, et al. The role of high reliability organization foundational practices in building a culture of safety. Fed Pract. 2024;41:214-221. doi:10.12788/fp.0486
- Ford J, Isaacks DB, Anderson T. Creating, executing and sustaining a high-reliability organization in health care. The Learning Organization: An International Journal. 2024;31:817-833. doi:10.1108/TLO-03-2023-0048
- Cox GR, Starr LM. VHA’s movement for change: implementing high-reliability principles and practices. J Healthc Manag. 2023;68:151-157. doi:10.1097/JHM-D-00056
- Myers CG, Sutcliffe KM. High reliability organising in healthcare: still a long way left to go. BMJ Qual Saf. 2022;31:845-848. doi:10.1136/bmjqs-2021-014141
- Nilsen P, Seing I, Ericsson C, et al. Characteristics of successful changes in health care organizations: an interview study with physicians, registered nurses and assistant nurses. BMC Health Serv Res. 2020;20:147. doi:10.1186/s12913-020-4999-8
- Cheraghi R, Ebrahimi H, Kheibar N, et al. Reasons for resistance to change in nursing: an integrative review. BMC Nurs. 2023;22:310. doi:10/1186/s12912-023-01460-0
- Warrick DD. Revisiting resistance to change and how to manage it: what has been learned and what organizations need to do. Bus Horiz. 2023;66:433-441. doi:10.1016/j.bushor.2022.09.001
- Sverdlik N, Oreg S. Beyond the individual-level conceptualization of dispositional resistance to change: multilevel effects on the response to organizational change. J Organ Behav. 2023;44:1066-1077. doi:10.1002/job.2678
- Khaw KW, Alnoor A, Al-Abrrow H, et al. Reactions towards organizational change: a systematic literature review. Curr Psychol. 2022;13:1-24. doi:10.1007/s12144-022-03070-6
- Pomare C, Churruca K, Long JC, et al. Organisational change in hospitals: a qualitative case-study of staff perspectives. BMC Health Serv Res. 2019;19:840. doi:10.1186/s12913-019-4704-y
- DuBose BM, Mayo AM. RtC: a concept analysis. Nurs Forum. 2020;55:631-636. doi:10.1111/nuf.12479
- Sahay S, Goldthwaite C. Participatory practices during organizational change: rethinking participation and resistance. Manag Commun Q. 2024;38(2):279-306. doi:10.1177/08933189231187883
- Damawan AH, Azizah S. Resistance to change: causes and strategies as an organizational challenge. ASSEHR. 2020;395(2020):49-53. doi:10.2991/assehr.k.200120.010
- Wong Q, Lacombe M, Keller R, et al. Leading change with ADKAR. Nurs Manage. 2019;50:28-35. doi:10.1097/01.NUMA.0000554341.70508.75
- Merchant NB, O’Neal J, Dealing-Perez C, et al. A high-reliability organization mindset. Am J Med Qual. 2022;37:504-510. doi:10.1097/JMQ.0000000000000086
- Veazie S, Peterson K, Bourne D, et al. Implementing high-reliability organization principles into practice: a rapid evidence review. J Patient Saf. 2022;18:e320-e328. doi:10.1097/PTS.0000000000000768
- Murray JS, Baghdadi A, Dannenberg W, et al. The role of high reliability organization foundational practices in building a culture of safety. Fed Pract. 2024;41:214-221. doi:10.12788/fp.0486
- Ford J, Isaacks DB, Anderson T. Creating, executing and sustaining a high-reliability organization in health care. The Learning Organization: An International Journal. 2024;31:817-833. doi:10.1108/TLO-03-2023-0048
- Cox GR, Starr LM. VHA’s movement for change: implementing high-reliability principles and practices. J Healthc Manag. 2023;68:151-157. doi:10.1097/JHM-D-00056
- Myers CG, Sutcliffe KM. High reliability organising in healthcare: still a long way left to go. BMJ Qual Saf. 2022;31:845-848. doi:10.1136/bmjqs-2021-014141
- Nilsen P, Seing I, Ericsson C, et al. Characteristics of successful changes in health care organizations: an interview study with physicians, registered nurses and assistant nurses. BMC Health Serv Res. 2020;20:147. doi:10.1186/s12913-020-4999-8
- Cheraghi R, Ebrahimi H, Kheibar N, et al. Reasons for resistance to change in nursing: an integrative review. BMC Nurs. 2023;22:310. doi:10/1186/s12912-023-01460-0
- Warrick DD. Revisiting resistance to change and how to manage it: what has been learned and what organizations need to do. Bus Horiz. 2023;66:433-441. doi:10.1016/j.bushor.2022.09.001
- Sverdlik N, Oreg S. Beyond the individual-level conceptualization of dispositional resistance to change: multilevel effects on the response to organizational change. J Organ Behav. 2023;44:1066-1077. doi:10.1002/job.2678
- Khaw KW, Alnoor A, Al-Abrrow H, et al. Reactions towards organizational change: a systematic literature review. Curr Psychol. 2022;13:1-24. doi:10.1007/s12144-022-03070-6
- Pomare C, Churruca K, Long JC, et al. Organisational change in hospitals: a qualitative case-study of staff perspectives. BMC Health Serv Res. 2019;19:840. doi:10.1186/s12913-019-4704-y
- DuBose BM, Mayo AM. RtC: a concept analysis. Nurs Forum. 2020;55:631-636. doi:10.1111/nuf.12479
- Sahay S, Goldthwaite C. Participatory practices during organizational change: rethinking participation and resistance. Manag Commun Q. 2024;38(2):279-306. doi:10.1177/08933189231187883
- Damawan AH, Azizah S. Resistance to change: causes and strategies as an organizational challenge. ASSEHR. 2020;395(2020):49-53. doi:10.2991/assehr.k.200120.010
- Wong Q, Lacombe M, Keller R, et al. Leading change with ADKAR. Nurs Manage. 2019;50:28-35. doi:10.1097/01.NUMA.0000554341.70508.75
Managing Resistance to Change Along the Journey to High Reliability
Managing Resistance to Change Along the Journey to High Reliability
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
Candida is a common commensal organism of human skin and mucous membranes. Candidiasis of the skin and nails is caused by overgrowth of Candida species due to excess skin moisture, skin barrier disruption, or immunosuppression. Candidiasis of the skin manifests as red, moist, itchy patches that develop particularly in skin folds. Nail involvement is associated with onycholysis (separation of the nail plate from the nail bed) and subungual debris.1 Data on the prevalence of candidiasis of the skin and nails in the United States are scarce. In this study, we evaluated the prevalence, characteristics, and treatment practices of candidiasis of the skin and nails using data from 2 large US health insurance claims databases.
Methods
We used the 2023 Merative MarketScan Commercial, Medicare Supplemental, and Multi-State Medicaid Databases (https://www.merative.com/documents/merative-marketscan-research-databases) to identify outpatients with the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code B37.2 for candidiasis of the skin and nails. The Commercial and Medicare Supplemental databases include health insurance claims data submitted by large employers and health plans for more than 19 million patients throughout the United States, and the Multi-State Medicaid database includes similar data from more than 5 million patients across several geographically dispersed states. The index date for each patient corresponded with their first qualifying diagnosis of skin and nail candidiasis during January 1, 2023, to December 31, 2023. Inclusion in the study required continuous insurance enrollment from 30 days prior to 7 days after the index date, resulting in exclusion of 7% of commercial/Medicare patients and 8% of Medicaid patients. Prevalence per 1000 outpatients was calculated, with stratification by demographic characteristics.
We examined selected diagnoses made on or within 30 days before the index date, diagnostic testing performed within the 7 days before or after the index date after using specific Current Procedural Terminology codes, and outpatient antifungal and combination antifungal-corticosteroid prescriptions made within 7 days before or after the index date (Table). Race/ethnicity data are unavailable in the commercial/Medicare database, and geographic data are unavailable in the Medicaid database.
Results
The prevalence of skin and nail candidiasis was 3.7 per 1000 commercial/Medicare outpatients and 7.8 per 1000 Medicaid outpatients (eTable 1). Prevalence was highest among patients aged 0 to 3 years (commercial/Medicare, 30.3 per 1000; Medicaid, 43.6 per 1000), followed by patients 65 years or older (commercial/Medicare, 7.4 per 1000; Medicaid, 7.5 per 1000). Prevalence was higher among females compared with males (commercial/Medicare, 4.8 vs 2.4 per 1000, respectively; Medicaid, 8.8 vs 6.4 per 1000, respectively). Among Medicaid patients, prevalence was highest among those of other race, non-Hispanic (8.9 per 1000) and White non-Hispanic patients (7.5 per 1000). In the commercial/Medicare dataset, prevalence was highest in patients residing in the Midwest (4.4 per 1000) and the South (4.0 per 1000).
Diaper dermatitis was listed as a concurrent diagnosis among 51% of patients aged 0 to 3 years in both datasets (eTable 2). Diabetes (commercial/Medicare, 32%; Medicaid, 36%) and immunosuppressive conditions (commercial/Medicare, 10%; Medicaid, 7%) were most frequent among patients aged 65 years or older. Obesity was most commonly listed as a concurrent diagnosis among patients aged 35 to 64 years (commercial/Medicare, 17%; Medicaid, 23%).

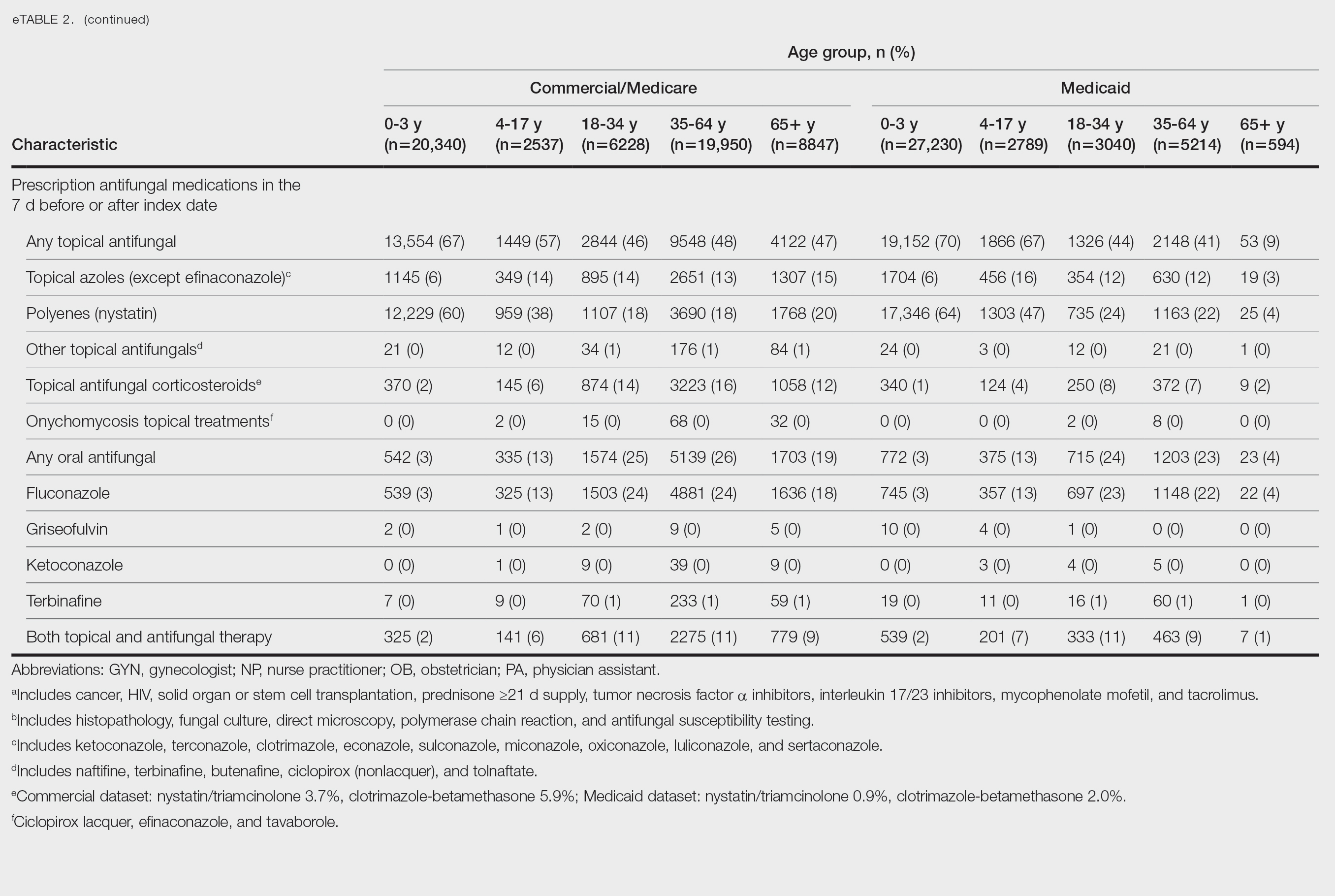
Patients aged 18 to 34 years had the highest rates of diagnostic testing in the 7 days before or after the index date (commercial/Medicare, 9%; Medicaid, 10%). Topical antifungal medications (primarily nystatin) were most frequently prescribed for patients aged 0 to 3 years (commercial/Medicare, 67%; Medicaid, 70%). Topical combination antifungal-corticosteroid medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (16%) and for patients aged 18 to 34 years in the Medicaid dataset (8%). Topical onychomycosis treatments were prescribed for fewer than 1% of patients in both datasets. Oral antifungal medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (26%) and for patients aged 18 to 34 years in the Medicaid dataset (24%). Fewer than 11% of patients across all age groups in both datasets were prescribed both topical and oral antifungal medications.
Comment
Our analysis provides preliminary insight into the prevalence of skin and nail candidiasis in the United States based on health insurance claims data. Higher prevalence of skin and nail candidiasis among patients with Medicaid compared with those with commercial/Medicare health insurance is consistent with previous studies showing increased rates of other superficial fungal infections (eg, dermatophytosis) among patients of lower socioeconomic status.2 This finding could reflect differences in underlying health status or reduced access to health care, which could delay treatment or follow-up care and potentially lead to prolonged exposure to conditions favoring the development of candidiasis.
In both the commercial/Medicare health insurance and Medicaid datasets, prevalence of diagnosis codes for candidiasis of the skin and nails was highest among infants and toddlers. Diaper dermatitis also was observed in more than half of patients aged 0 to 3 years; this is a well-established risk factor for cutaneous candidiasis, as immature skin barrier function and prolonged exposure to moisture and occlusion facilitate fungal overgrowth.3 In adults, diabetes and obesity were among the most frequent comorbidities observed; both conditions are recognized risk factors for superficial candidiasis due to their impact on immune function and skin integrity.4
In both study cohorts, diagnostic testing in the 7 days before or after the index date was infrequent (≤10%), consistent with most cases being diagnosed clinically.5 Topical antifungals, especially nystatin, were most frequently prescribed for young children, while oral antifungals were more frequently prescribed for adults; nystatin is one of the most well-studied topical treatments for cutaneous candidiasis, and oral fluconazole is the primary systemic treatment for cutaneous candidiasis.1 In our study, the ICD-10-CM code B37.2 appeared to be used primarily for diagnosis of skin rather than nail infections based on the low proportions of patients who received treatment that was onychomycosis specific.
Our study was limited by potential misclassification inherent to data based on diagnosis codes; incomplete capture of underlying conditions given the short continuous enrollment criteria; and lack of information about affected body site(s) and laboratory results, including data identifying the Candida species. A previous study found that Candida parapsilosis and Candida albicans were the most common species involved in candidiasis of the skin and nails and that one-third of isolates exhibited low sensitivity to commonly used antifungals.6 For nails, Candida species are sometimes contaminants rather than pathogens.
Conclusion
Our findings provide a baseline understanding of the epidemiology of candidiasis of the skin and nails in the United States. The growing threat of antifungal resistance, particularly among non-albicans Candida species, underscores the need for appropriate use of antifungals.7 Future epidemiologic studies about laboratory-confirmed candidiasis of the skin and nails to understand causative species and drug resistance would be useful, as would further investigation into disparities.
- Taudorf EH, Jemec GBE, Hay RJ, et al. Cutaneous candidiasis—an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019;33:1863-1873. doi:10.1111/jdv.15782
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. eClinicalMedicine. 2023;66:102325. doi:10.1016/j.eclinm.2023.102325
- Benitez Ojeda AB, Mendez MD. Diaper dermatitis. StatPearls [Internet]. Updated July 3, 2023. Accessed January 14, 2026. https://www.ncbi.nlm.nih.gov/books/NBK559067/
- Shahabudin S, Azmi NS, Lani MN, et al. Candida albicans skin infection in diabetic patients: an updated review of pathogenesis and management. Mycoses. 2024;67:E13753. doi:10.1111/myc.13753
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Ranđelovic M, Ignjatovic A, Đorđevic M, et al. Superficial candidiasis: cluster analysis of species distribution and their antifungal susceptibility in vitro. J Fungi (Basel). 2025;11:338.
- Hay R. Therapy of skin, hair and nail fungal infections. J Fungi (Basel). 2018;4:99. doi:10.3390/jof4030099
Candida is a common commensal organism of human skin and mucous membranes. Candidiasis of the skin and nails is caused by overgrowth of Candida species due to excess skin moisture, skin barrier disruption, or immunosuppression. Candidiasis of the skin manifests as red, moist, itchy patches that develop particularly in skin folds. Nail involvement is associated with onycholysis (separation of the nail plate from the nail bed) and subungual debris.1 Data on the prevalence of candidiasis of the skin and nails in the United States are scarce. In this study, we evaluated the prevalence, characteristics, and treatment practices of candidiasis of the skin and nails using data from 2 large US health insurance claims databases.
Methods
We used the 2023 Merative MarketScan Commercial, Medicare Supplemental, and Multi-State Medicaid Databases (https://www.merative.com/documents/merative-marketscan-research-databases) to identify outpatients with the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code B37.2 for candidiasis of the skin and nails. The Commercial and Medicare Supplemental databases include health insurance claims data submitted by large employers and health plans for more than 19 million patients throughout the United States, and the Multi-State Medicaid database includes similar data from more than 5 million patients across several geographically dispersed states. The index date for each patient corresponded with their first qualifying diagnosis of skin and nail candidiasis during January 1, 2023, to December 31, 2023. Inclusion in the study required continuous insurance enrollment from 30 days prior to 7 days after the index date, resulting in exclusion of 7% of commercial/Medicare patients and 8% of Medicaid patients. Prevalence per 1000 outpatients was calculated, with stratification by demographic characteristics.
We examined selected diagnoses made on or within 30 days before the index date, diagnostic testing performed within the 7 days before or after the index date after using specific Current Procedural Terminology codes, and outpatient antifungal and combination antifungal-corticosteroid prescriptions made within 7 days before or after the index date (Table). Race/ethnicity data are unavailable in the commercial/Medicare database, and geographic data are unavailable in the Medicaid database.

Results
The prevalence of skin and nail candidiasis was 3.7 per 1000 commercial/Medicare outpatients and 7.8 per 1000 Medicaid outpatients (eTable 1). Prevalence was highest among patients aged 0 to 3 years (commercial/Medicare, 30.3 per 1000; Medicaid, 43.6 per 1000), followed by patients 65 years or older (commercial/Medicare, 7.4 per 1000; Medicaid, 7.5 per 1000). Prevalence was higher among females compared with males (commercial/Medicare, 4.8 vs 2.4 per 1000, respectively; Medicaid, 8.8 vs 6.4 per 1000, respectively). Among Medicaid patients, prevalence was highest among those of other race, non-Hispanic (8.9 per 1000) and White non-Hispanic patients (7.5 per 1000). In the commercial/Medicare dataset, prevalence was highest in patients residing in the Midwest (4.4 per 1000) and the South (4.0 per 1000).

Diaper dermatitis was listed as a concurrent diagnosis among 51% of patients aged 0 to 3 years in both datasets (eTable 2). Diabetes (commercial/Medicare, 32%; Medicaid, 36%) and immunosuppressive conditions (commercial/Medicare, 10%; Medicaid, 7%) were most frequent among patients aged 65 years or older. Obesity was most commonly listed as a concurrent diagnosis among patients aged 35 to 64 years (commercial/Medicare, 17%; Medicaid, 23%).


Patients aged 18 to 34 years had the highest rates of diagnostic testing in the 7 days before or after the index date (commercial/Medicare, 9%; Medicaid, 10%). Topical antifungal medications (primarily nystatin) were most frequently prescribed for patients aged 0 to 3 years (commercial/Medicare, 67%; Medicaid, 70%). Topical combination antifungal-corticosteroid medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (16%) and for patients aged 18 to 34 years in the Medicaid dataset (8%). Topical onychomycosis treatments were prescribed for fewer than 1% of patients in both datasets. Oral antifungal medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (26%) and for patients aged 18 to 34 years in the Medicaid dataset (24%). Fewer than 11% of patients across all age groups in both datasets were prescribed both topical and oral antifungal medications.
Comment
Our analysis provides preliminary insight into the prevalence of skin and nail candidiasis in the United States based on health insurance claims data. Higher prevalence of skin and nail candidiasis among patients with Medicaid compared with those with commercial/Medicare health insurance is consistent with previous studies showing increased rates of other superficial fungal infections (eg, dermatophytosis) among patients of lower socioeconomic status.2 This finding could reflect differences in underlying health status or reduced access to health care, which could delay treatment or follow-up care and potentially lead to prolonged exposure to conditions favoring the development of candidiasis.
In both the commercial/Medicare health insurance and Medicaid datasets, prevalence of diagnosis codes for candidiasis of the skin and nails was highest among infants and toddlers. Diaper dermatitis also was observed in more than half of patients aged 0 to 3 years; this is a well-established risk factor for cutaneous candidiasis, as immature skin barrier function and prolonged exposure to moisture and occlusion facilitate fungal overgrowth.3 In adults, diabetes and obesity were among the most frequent comorbidities observed; both conditions are recognized risk factors for superficial candidiasis due to their impact on immune function and skin integrity.4
In both study cohorts, diagnostic testing in the 7 days before or after the index date was infrequent (≤10%), consistent with most cases being diagnosed clinically.5 Topical antifungals, especially nystatin, were most frequently prescribed for young children, while oral antifungals were more frequently prescribed for adults; nystatin is one of the most well-studied topical treatments for cutaneous candidiasis, and oral fluconazole is the primary systemic treatment for cutaneous candidiasis.1 In our study, the ICD-10-CM code B37.2 appeared to be used primarily for diagnosis of skin rather than nail infections based on the low proportions of patients who received treatment that was onychomycosis specific.
Our study was limited by potential misclassification inherent to data based on diagnosis codes; incomplete capture of underlying conditions given the short continuous enrollment criteria; and lack of information about affected body site(s) and laboratory results, including data identifying the Candida species. A previous study found that Candida parapsilosis and Candida albicans were the most common species involved in candidiasis of the skin and nails and that one-third of isolates exhibited low sensitivity to commonly used antifungals.6 For nails, Candida species are sometimes contaminants rather than pathogens.
Conclusion
Our findings provide a baseline understanding of the epidemiology of candidiasis of the skin and nails in the United States. The growing threat of antifungal resistance, particularly among non-albicans Candida species, underscores the need for appropriate use of antifungals.7 Future epidemiologic studies about laboratory-confirmed candidiasis of the skin and nails to understand causative species and drug resistance would be useful, as would further investigation into disparities.
Candida is a common commensal organism of human skin and mucous membranes. Candidiasis of the skin and nails is caused by overgrowth of Candida species due to excess skin moisture, skin barrier disruption, or immunosuppression. Candidiasis of the skin manifests as red, moist, itchy patches that develop particularly in skin folds. Nail involvement is associated with onycholysis (separation of the nail plate from the nail bed) and subungual debris.1 Data on the prevalence of candidiasis of the skin and nails in the United States are scarce. In this study, we evaluated the prevalence, characteristics, and treatment practices of candidiasis of the skin and nails using data from 2 large US health insurance claims databases.
Methods
We used the 2023 Merative MarketScan Commercial, Medicare Supplemental, and Multi-State Medicaid Databases (https://www.merative.com/documents/merative-marketscan-research-databases) to identify outpatients with the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code B37.2 for candidiasis of the skin and nails. The Commercial and Medicare Supplemental databases include health insurance claims data submitted by large employers and health plans for more than 19 million patients throughout the United States, and the Multi-State Medicaid database includes similar data from more than 5 million patients across several geographically dispersed states. The index date for each patient corresponded with their first qualifying diagnosis of skin and nail candidiasis during January 1, 2023, to December 31, 2023. Inclusion in the study required continuous insurance enrollment from 30 days prior to 7 days after the index date, resulting in exclusion of 7% of commercial/Medicare patients and 8% of Medicaid patients. Prevalence per 1000 outpatients was calculated, with stratification by demographic characteristics.
We examined selected diagnoses made on or within 30 days before the index date, diagnostic testing performed within the 7 days before or after the index date after using specific Current Procedural Terminology codes, and outpatient antifungal and combination antifungal-corticosteroid prescriptions made within 7 days before or after the index date (Table). Race/ethnicity data are unavailable in the commercial/Medicare database, and geographic data are unavailable in the Medicaid database.

Results
The prevalence of skin and nail candidiasis was 3.7 per 1000 commercial/Medicare outpatients and 7.8 per 1000 Medicaid outpatients (eTable 1). Prevalence was highest among patients aged 0 to 3 years (commercial/Medicare, 30.3 per 1000; Medicaid, 43.6 per 1000), followed by patients 65 years or older (commercial/Medicare, 7.4 per 1000; Medicaid, 7.5 per 1000). Prevalence was higher among females compared with males (commercial/Medicare, 4.8 vs 2.4 per 1000, respectively; Medicaid, 8.8 vs 6.4 per 1000, respectively). Among Medicaid patients, prevalence was highest among those of other race, non-Hispanic (8.9 per 1000) and White non-Hispanic patients (7.5 per 1000). In the commercial/Medicare dataset, prevalence was highest in patients residing in the Midwest (4.4 per 1000) and the South (4.0 per 1000).

Diaper dermatitis was listed as a concurrent diagnosis among 51% of patients aged 0 to 3 years in both datasets (eTable 2). Diabetes (commercial/Medicare, 32%; Medicaid, 36%) and immunosuppressive conditions (commercial/Medicare, 10%; Medicaid, 7%) were most frequent among patients aged 65 years or older. Obesity was most commonly listed as a concurrent diagnosis among patients aged 35 to 64 years (commercial/Medicare, 17%; Medicaid, 23%).


Patients aged 18 to 34 years had the highest rates of diagnostic testing in the 7 days before or after the index date (commercial/Medicare, 9%; Medicaid, 10%). Topical antifungal medications (primarily nystatin) were most frequently prescribed for patients aged 0 to 3 years (commercial/Medicare, 67%; Medicaid, 70%). Topical combination antifungal-corticosteroid medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (16%) and for patients aged 18 to 34 years in the Medicaid dataset (8%). Topical onychomycosis treatments were prescribed for fewer than 1% of patients in both datasets. Oral antifungal medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (26%) and for patients aged 18 to 34 years in the Medicaid dataset (24%). Fewer than 11% of patients across all age groups in both datasets were prescribed both topical and oral antifungal medications.
Comment
Our analysis provides preliminary insight into the prevalence of skin and nail candidiasis in the United States based on health insurance claims data. Higher prevalence of skin and nail candidiasis among patients with Medicaid compared with those with commercial/Medicare health insurance is consistent with previous studies showing increased rates of other superficial fungal infections (eg, dermatophytosis) among patients of lower socioeconomic status.2 This finding could reflect differences in underlying health status or reduced access to health care, which could delay treatment or follow-up care and potentially lead to prolonged exposure to conditions favoring the development of candidiasis.
In both the commercial/Medicare health insurance and Medicaid datasets, prevalence of diagnosis codes for candidiasis of the skin and nails was highest among infants and toddlers. Diaper dermatitis also was observed in more than half of patients aged 0 to 3 years; this is a well-established risk factor for cutaneous candidiasis, as immature skin barrier function and prolonged exposure to moisture and occlusion facilitate fungal overgrowth.3 In adults, diabetes and obesity were among the most frequent comorbidities observed; both conditions are recognized risk factors for superficial candidiasis due to their impact on immune function and skin integrity.4
In both study cohorts, diagnostic testing in the 7 days before or after the index date was infrequent (≤10%), consistent with most cases being diagnosed clinically.5 Topical antifungals, especially nystatin, were most frequently prescribed for young children, while oral antifungals were more frequently prescribed for adults; nystatin is one of the most well-studied topical treatments for cutaneous candidiasis, and oral fluconazole is the primary systemic treatment for cutaneous candidiasis.1 In our study, the ICD-10-CM code B37.2 appeared to be used primarily for diagnosis of skin rather than nail infections based on the low proportions of patients who received treatment that was onychomycosis specific.
Our study was limited by potential misclassification inherent to data based on diagnosis codes; incomplete capture of underlying conditions given the short continuous enrollment criteria; and lack of information about affected body site(s) and laboratory results, including data identifying the Candida species. A previous study found that Candida parapsilosis and Candida albicans were the most common species involved in candidiasis of the skin and nails and that one-third of isolates exhibited low sensitivity to commonly used antifungals.6 For nails, Candida species are sometimes contaminants rather than pathogens.
Conclusion
Our findings provide a baseline understanding of the epidemiology of candidiasis of the skin and nails in the United States. The growing threat of antifungal resistance, particularly among non-albicans Candida species, underscores the need for appropriate use of antifungals.7 Future epidemiologic studies about laboratory-confirmed candidiasis of the skin and nails to understand causative species and drug resistance would be useful, as would further investigation into disparities.
- Taudorf EH, Jemec GBE, Hay RJ, et al. Cutaneous candidiasis—an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019;33:1863-1873. doi:10.1111/jdv.15782
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. eClinicalMedicine. 2023;66:102325. doi:10.1016/j.eclinm.2023.102325
- Benitez Ojeda AB, Mendez MD. Diaper dermatitis. StatPearls [Internet]. Updated July 3, 2023. Accessed January 14, 2026. https://www.ncbi.nlm.nih.gov/books/NBK559067/
- Shahabudin S, Azmi NS, Lani MN, et al. Candida albicans skin infection in diabetic patients: an updated review of pathogenesis and management. Mycoses. 2024;67:E13753. doi:10.1111/myc.13753
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Ranđelovic M, Ignjatovic A, Đorđevic M, et al. Superficial candidiasis: cluster analysis of species distribution and their antifungal susceptibility in vitro. J Fungi (Basel). 2025;11:338.
- Hay R. Therapy of skin, hair and nail fungal infections. J Fungi (Basel). 2018;4:99. doi:10.3390/jof4030099
- Taudorf EH, Jemec GBE, Hay RJ, et al. Cutaneous candidiasis—an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019;33:1863-1873. doi:10.1111/jdv.15782
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. eClinicalMedicine. 2023;66:102325. doi:10.1016/j.eclinm.2023.102325
- Benitez Ojeda AB, Mendez MD. Diaper dermatitis. StatPearls [Internet]. Updated July 3, 2023. Accessed January 14, 2026. https://www.ncbi.nlm.nih.gov/books/NBK559067/
- Shahabudin S, Azmi NS, Lani MN, et al. Candida albicans skin infection in diabetic patients: an updated review of pathogenesis and management. Mycoses. 2024;67:E13753. doi:10.1111/myc.13753
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Ranđelovic M, Ignjatovic A, Đorđevic M, et al. Superficial candidiasis: cluster analysis of species distribution and their antifungal susceptibility in vitro. J Fungi (Basel). 2025;11:338.
- Hay R. Therapy of skin, hair and nail fungal infections. J Fungi (Basel). 2018;4:99. doi:10.3390/jof4030099
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
Practice Points
- Candidiasis of the skin or nails is a common outpatient condition that is most frequently diagnosed in infants, toddlers, and adults aged 65 years or older.
- Most cases are diagnosed clinically without diagnostic testing and treated with topical antifungals, but increased attention to formal diagnosis and treatment may be warranted given the emergence of antifungal-resistant Candida species.
Dermatologic Implications of Prickly Pear Cacti (Opuntia)
Dermatologic Implications of Prickly Pear Cacti (Opuntia)
The genus of flowering plants commonly known as prickly pear cacti (Opuntia) or sabra are native to the Americas but are naturalized in many parts of the world, particularly southwest Asia and Sicily, Italy, where they are grown commercially and commonly are seen growing on rocky hillsides. (Figure 1). A prickly pear cactus has paddles that represent modified stems, and the spines are modified leaves (Figure 2). Its bright red or yellow flowers, dark-red fruit, low water requirement, and adaptability to poor-quality soil make it an attractive plant for landscaping and an important agricultural crop in many parts of the world, including the United States, Mexico, and Southern Europe. The prickly pear fruit is tasty but loaded with seeds and often is eaten fresh or used to make jam. The paddles are sometimes cut into strips, breaded or battered, and fried. The spines are easily embedded in skin and are an important cause of dermatitis.


Identifying Features
Opuntia species are found in both warm and temperate zones and grow well in arid climates. Like other cacti, they are distinguished by their water-hoarding stems and glochids (needlelike modified leaves). In prickly pears, the stems flatten to leaflike paddles that alternate in direction. Photosynthesis occurs in the stem tissues, while modified leaves (spines) are purely for defense against predators and unsuspecting humans. Opuntia species are easily identified by their broad flattened stems and dark-red fruits, both of which bear glochids (Figures 3-5).


Dermatologic Implications of Prickly Pear Injury
Prickly pear spines are very small, sharp, and difficult to see. They embed in the skin in great numbers when the plant or its fruit are handled by unsuspecting humans and have a tendency to burrow into soft tissue and underlying structures. It is very difficult to remove prickly pear spines with forceps, and attempts to do so often drive them deeper into the skin.1 Better results are obtained by tape stripping or using water-activated cosmetic pore strips.
Cactus spine injuries may lead to mucoceles of the oral mucosa and sinuses, especially in individuals who attempt to bite into the fruit without first scorching the spines with a blow torch.2 Inflammatory responses to the embedded spines are common and often result in prolonged erythematous inflammatory papules at sites of injury. Recalcitrant dermatitis and edema of underlying tissues typically occur near the point of entry of a prickly pear spine and extend to areas where the spine migrates.3,4 Individuals who casually brush up against the plant may not be aware that they have been inoculated with the spines and may not relate the prior accidental contact with the onset of erythematous papules and edema that occurs days later. Biopsy may reveal the prickly pear spines or a granulomatous reaction pattern within the dermis. Linear patterns of necrosis surrounded by palisading histiocytes may be noted, representing the tract of the inoculation injury.
If identified in tissue, glochids are variably refractile and measure 40 to 70 µm in diameter. Glochids initiate a delayed-type hypersensitivity and foreign body response. A T-helper 1 cytokine signal is typical, and there may be a secondary influx of neutrophils, but tissue eosinophilia is uncommon. Systemic inflammation also has been reported, including eosinophilic cholangitis without biliary stricture5 and septic and aseptic arthritis near the site of leaf puncture and at distant sites.6,7 Allergic contact dermatitis has been reported due to contact with the fruit of the plant and can be confirmed by patch testing.8,9
Potential Medicinal Benefits
Prickly pear cacti have shown potential medicinal properties. While the spines may produce intense inflammation when embedded in the skin, extracts of the fruit and leaf juices have shown anti-inflammatory properties. Various vesicle and polysaccharide extracts of Opuntia cacti have been shown to reduce environmental and chemical stressors associated with open wounds.10-12 Preclinical studies also have suggested that they could be helpful in speeding the wound-healing process when applied topically. Opuntia species also have shown promise in reducing hyperpigmentation after topical application.13 Preliminary data in animals also have suggested that oral administration of the fruit may slow kidney deterioration in patients with diabetes.14 Following tissue penetration by the spines, Opuntia extracts have demonstrated the ability to prevent calcium deposition in soft tissue.15 Similar preliminary data also have suggested that Opuntia extracts may reduce toxicity from cadmium, chromium, methotrexate, and acetaminophen.16-19 Extracts from the peel of the red pitaya (Hylocereus polyrhizus), a closely related cactus, have been studied for their potential to prevent the advance of alcohol-associated liver disease, suggesting that studies evaluating the benefits of prickly pear cacti and related species may be worth pursuing.20
Final Thoughts
Prickly pear cacti have the potential to act as both friend and foe. The flowers and fruit are beautiful, and the plant is well adapted to xeriscape gardens in areas under perpetual water restriction. The fruit and flesh are edible if handled properly, and prickly pear jam is delicious. While the spines are capable of inflicting local injury and migrating to internal sites, causing arthritis and other deep tissue injury, extracts of the fruit and stems have potential uses for their anti-inflammatory effects and ability to protect against toxic injury. Further studies are needed to evaluate the therapeutic potential of Opuntia and related species.
- Ford AM, Haywood ST, Gallo DR. Novel method for removing embedded cactus spines in the emergency department. Case Rep Emerg Med. 2019;2019:6062531.
- Patel D, Clarkson J, Amirapu S. Frontal sinus post-traumatic mucocele secondary to a cactus spine. N Z Med J. 2020;133:112-115.
- Magro C, Lipner S. Sabra dermatitis: combined features of delayed hypersensitivity and foreign body reaction to implanted glochidia. Dermatol Online J. 2020;26:13030/qt2157f9g0.
- Ruini C, von Braunmühl T, Ruzicka T, et al. Granulomatous reaction after cholla cactus spine injury. Cutis. 2020;105:143-145;E2.
- Kitagawa S, Okamura K, Ichihara S, et al. Eosinophilic cholangitis without biliary stricture after cactus spine injury. Am J Gastroenterol. 2022;117:1731.
- Ontiveros ST, Minns AB. Accidental arthrotomy causing aseptic monoarthritis due to agave sap: a case report. Clin Pract Cases Emerg Med. 2021;5:246-248.
- Kim S, Baradia H, Sambasivan A. The use of ultrasonography in expediting septic joint identification and treatment: a case report. Am J Phys Med Rehabil. 2020;99:449-451.
- Yoon HJ, Won CH, Moon SE. Allergic contact dermatitis due to Opuntia ficus-indica var. saboten. Contact Dermatitis. 2004;51:311-312.
- Bonamonte D, Foti C, Gullo G, et al. Plant contact dermatitis. In: Angelini G, Bonamonte D, Foti C, eds. Clinical Contact Dermatitis. 2021; Springer, Cham. doi:10.1007/978-3-030-49332-5_16
- Valentino A, Conte R, Bousta D, et al. Extracellular vesicles derived from Opuntia ficus-indica fruit (OFI-EVs) speed up the normal wound healing processes by modulating cellular responses. Int J Mol Sci. 2024;25:7103.
- Das IJ, Bal T. Evaluation of Opuntia-carrageenan superporous hydrogel (OPM-CRG SPH) as an effective biomaterial for drug release and tissue scaffold. Int J Biol Macromol. 2024;256(Pt 2):128503.
- Adjafre BL, Lima IC, Alves APNN, et al. Anti-inflammatory and healing effect of the polysaccharidic extract of Opuntia ficus-indica cladodes in cutaneous excisional wounds in rats. Int J Exp Pathol. 2024;105:33-44.
- Chiu CS, Cheng YT, Chan YJ, et al. Mechanism and inhibitory effects of cactus (Opuntia dillenii) extract on melanocytes and its potential application for whitening cosmetics. Sci Rep. 2023;13:501.
- Sutariya B, Saraf M. Betanin, isolated from fruits of Opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-β pathway. J Ethnopharmacol. 2017;198:432-443.
- Partovi N, Ebadzadeh MR, Fatemi SJ, et al. Effect of fruit extract on renal stone formation and kidney injury in rats. Nat Prod Res. 2018;32:1180-1183.
- Zhu X, Athmouni K. HPLC analysis and the antioxidant and preventive actions of Opuntia stricta juice extract against hepato-nephrotoxicity and testicular injury induced by cadmium exposure. Molecules. 2022;27:4972.
- Akacha A, Badraoui R, Rebai T, et al. Effect of Opuntia ficus indica extract on methotrexate-induced testicular injury: a biochemical, docking and histological study. J Biomol Struct Dyn. 2022;40:4341-4351.
- González-Ponce HA, Martínez-Saldaña MC, Tepper PG, et al. Betacyanins, major components in Opuntia red-purple fruits, protect against acetaminophen-induced acute liver failure. Food Res Int. 2020;137:109461.
- Akacha A, Rebai T, Zourgui L, et al. Preventive effect of ethanolic extract of cactus (Opuntia ficus-indica) cladodes on methotrexate-induced oxidative damage of the small intestine in Wistar rats. J Cancer Res Ther. 2018;14(Suppl):S779-S784.
- Yeh WJ, Tsai CC, Ko J, et al. Hylocereus polyrhizus peel extract retards alcoholic liver disease progression by modulating oxidative stress and inflammatory responses in C57BL/6 mice. Nutrients. 2020;12:3884.
The genus of flowering plants commonly known as prickly pear cacti (Opuntia) or sabra are native to the Americas but are naturalized in many parts of the world, particularly southwest Asia and Sicily, Italy, where they are grown commercially and commonly are seen growing on rocky hillsides. (Figure 1). A prickly pear cactus has paddles that represent modified stems, and the spines are modified leaves (Figure 2). Its bright red or yellow flowers, dark-red fruit, low water requirement, and adaptability to poor-quality soil make it an attractive plant for landscaping and an important agricultural crop in many parts of the world, including the United States, Mexico, and Southern Europe. The prickly pear fruit is tasty but loaded with seeds and often is eaten fresh or used to make jam. The paddles are sometimes cut into strips, breaded or battered, and fried. The spines are easily embedded in skin and are an important cause of dermatitis.


Identifying Features
Opuntia species are found in both warm and temperate zones and grow well in arid climates. Like other cacti, they are distinguished by their water-hoarding stems and glochids (needlelike modified leaves). In prickly pears, the stems flatten to leaflike paddles that alternate in direction. Photosynthesis occurs in the stem tissues, while modified leaves (spines) are purely for defense against predators and unsuspecting humans. Opuntia species are easily identified by their broad flattened stems and dark-red fruits, both of which bear glochids (Figures 3-5).



Dermatologic Implications of Prickly Pear Injury
Prickly pear spines are very small, sharp, and difficult to see. They embed in the skin in great numbers when the plant or its fruit are handled by unsuspecting humans and have a tendency to burrow into soft tissue and underlying structures. It is very difficult to remove prickly pear spines with forceps, and attempts to do so often drive them deeper into the skin.1 Better results are obtained by tape stripping or using water-activated cosmetic pore strips.
Cactus spine injuries may lead to mucoceles of the oral mucosa and sinuses, especially in individuals who attempt to bite into the fruit without first scorching the spines with a blow torch.2 Inflammatory responses to the embedded spines are common and often result in prolonged erythematous inflammatory papules at sites of injury. Recalcitrant dermatitis and edema of underlying tissues typically occur near the point of entry of a prickly pear spine and extend to areas where the spine migrates.3,4 Individuals who casually brush up against the plant may not be aware that they have been inoculated with the spines and may not relate the prior accidental contact with the onset of erythematous papules and edema that occurs days later. Biopsy may reveal the prickly pear spines or a granulomatous reaction pattern within the dermis. Linear patterns of necrosis surrounded by palisading histiocytes may be noted, representing the tract of the inoculation injury.
If identified in tissue, glochids are variably refractile and measure 40 to 70 µm in diameter. Glochids initiate a delayed-type hypersensitivity and foreign body response. A T-helper 1 cytokine signal is typical, and there may be a secondary influx of neutrophils, but tissue eosinophilia is uncommon. Systemic inflammation also has been reported, including eosinophilic cholangitis without biliary stricture5 and septic and aseptic arthritis near the site of leaf puncture and at distant sites.6,7 Allergic contact dermatitis has been reported due to contact with the fruit of the plant and can be confirmed by patch testing.8,9
Potential Medicinal Benefits
Prickly pear cacti have shown potential medicinal properties. While the spines may produce intense inflammation when embedded in the skin, extracts of the fruit and leaf juices have shown anti-inflammatory properties. Various vesicle and polysaccharide extracts of Opuntia cacti have been shown to reduce environmental and chemical stressors associated with open wounds.10-12 Preclinical studies also have suggested that they could be helpful in speeding the wound-healing process when applied topically. Opuntia species also have shown promise in reducing hyperpigmentation after topical application.13 Preliminary data in animals also have suggested that oral administration of the fruit may slow kidney deterioration in patients with diabetes.14 Following tissue penetration by the spines, Opuntia extracts have demonstrated the ability to prevent calcium deposition in soft tissue.15 Similar preliminary data also have suggested that Opuntia extracts may reduce toxicity from cadmium, chromium, methotrexate, and acetaminophen.16-19 Extracts from the peel of the red pitaya (Hylocereus polyrhizus), a closely related cactus, have been studied for their potential to prevent the advance of alcohol-associated liver disease, suggesting that studies evaluating the benefits of prickly pear cacti and related species may be worth pursuing.20
Final Thoughts
Prickly pear cacti have the potential to act as both friend and foe. The flowers and fruit are beautiful, and the plant is well adapted to xeriscape gardens in areas under perpetual water restriction. The fruit and flesh are edible if handled properly, and prickly pear jam is delicious. While the spines are capable of inflicting local injury and migrating to internal sites, causing arthritis and other deep tissue injury, extracts of the fruit and stems have potential uses for their anti-inflammatory effects and ability to protect against toxic injury. Further studies are needed to evaluate the therapeutic potential of Opuntia and related species.
The genus of flowering plants commonly known as prickly pear cacti (Opuntia) or sabra are native to the Americas but are naturalized in many parts of the world, particularly southwest Asia and Sicily, Italy, where they are grown commercially and commonly are seen growing on rocky hillsides. (Figure 1). A prickly pear cactus has paddles that represent modified stems, and the spines are modified leaves (Figure 2). Its bright red or yellow flowers, dark-red fruit, low water requirement, and adaptability to poor-quality soil make it an attractive plant for landscaping and an important agricultural crop in many parts of the world, including the United States, Mexico, and Southern Europe. The prickly pear fruit is tasty but loaded with seeds and often is eaten fresh or used to make jam. The paddles are sometimes cut into strips, breaded or battered, and fried. The spines are easily embedded in skin and are an important cause of dermatitis.


Identifying Features
Opuntia species are found in both warm and temperate zones and grow well in arid climates. Like other cacti, they are distinguished by their water-hoarding stems and glochids (needlelike modified leaves). In prickly pears, the stems flatten to leaflike paddles that alternate in direction. Photosynthesis occurs in the stem tissues, while modified leaves (spines) are purely for defense against predators and unsuspecting humans. Opuntia species are easily identified by their broad flattened stems and dark-red fruits, both of which bear glochids (Figures 3-5).



Dermatologic Implications of Prickly Pear Injury
Prickly pear spines are very small, sharp, and difficult to see. They embed in the skin in great numbers when the plant or its fruit are handled by unsuspecting humans and have a tendency to burrow into soft tissue and underlying structures. It is very difficult to remove prickly pear spines with forceps, and attempts to do so often drive them deeper into the skin.1 Better results are obtained by tape stripping or using water-activated cosmetic pore strips.
Cactus spine injuries may lead to mucoceles of the oral mucosa and sinuses, especially in individuals who attempt to bite into the fruit without first scorching the spines with a blow torch.2 Inflammatory responses to the embedded spines are common and often result in prolonged erythematous inflammatory papules at sites of injury. Recalcitrant dermatitis and edema of underlying tissues typically occur near the point of entry of a prickly pear spine and extend to areas where the spine migrates.3,4 Individuals who casually brush up against the plant may not be aware that they have been inoculated with the spines and may not relate the prior accidental contact with the onset of erythematous papules and edema that occurs days later. Biopsy may reveal the prickly pear spines or a granulomatous reaction pattern within the dermis. Linear patterns of necrosis surrounded by palisading histiocytes may be noted, representing the tract of the inoculation injury.
If identified in tissue, glochids are variably refractile and measure 40 to 70 µm in diameter. Glochids initiate a delayed-type hypersensitivity and foreign body response. A T-helper 1 cytokine signal is typical, and there may be a secondary influx of neutrophils, but tissue eosinophilia is uncommon. Systemic inflammation also has been reported, including eosinophilic cholangitis without biliary stricture5 and septic and aseptic arthritis near the site of leaf puncture and at distant sites.6,7 Allergic contact dermatitis has been reported due to contact with the fruit of the plant and can be confirmed by patch testing.8,9
Potential Medicinal Benefits
Prickly pear cacti have shown potential medicinal properties. While the spines may produce intense inflammation when embedded in the skin, extracts of the fruit and leaf juices have shown anti-inflammatory properties. Various vesicle and polysaccharide extracts of Opuntia cacti have been shown to reduce environmental and chemical stressors associated with open wounds.10-12 Preclinical studies also have suggested that they could be helpful in speeding the wound-healing process when applied topically. Opuntia species also have shown promise in reducing hyperpigmentation after topical application.13 Preliminary data in animals also have suggested that oral administration of the fruit may slow kidney deterioration in patients with diabetes.14 Following tissue penetration by the spines, Opuntia extracts have demonstrated the ability to prevent calcium deposition in soft tissue.15 Similar preliminary data also have suggested that Opuntia extracts may reduce toxicity from cadmium, chromium, methotrexate, and acetaminophen.16-19 Extracts from the peel of the red pitaya (Hylocereus polyrhizus), a closely related cactus, have been studied for their potential to prevent the advance of alcohol-associated liver disease, suggesting that studies evaluating the benefits of prickly pear cacti and related species may be worth pursuing.20
Final Thoughts
Prickly pear cacti have the potential to act as both friend and foe. The flowers and fruit are beautiful, and the plant is well adapted to xeriscape gardens in areas under perpetual water restriction. The fruit and flesh are edible if handled properly, and prickly pear jam is delicious. While the spines are capable of inflicting local injury and migrating to internal sites, causing arthritis and other deep tissue injury, extracts of the fruit and stems have potential uses for their anti-inflammatory effects and ability to protect against toxic injury. Further studies are needed to evaluate the therapeutic potential of Opuntia and related species.
- Ford AM, Haywood ST, Gallo DR. Novel method for removing embedded cactus spines in the emergency department. Case Rep Emerg Med. 2019;2019:6062531.
- Patel D, Clarkson J, Amirapu S. Frontal sinus post-traumatic mucocele secondary to a cactus spine. N Z Med J. 2020;133:112-115.
- Magro C, Lipner S. Sabra dermatitis: combined features of delayed hypersensitivity and foreign body reaction to implanted glochidia. Dermatol Online J. 2020;26:13030/qt2157f9g0.
- Ruini C, von Braunmühl T, Ruzicka T, et al. Granulomatous reaction after cholla cactus spine injury. Cutis. 2020;105:143-145;E2.
- Kitagawa S, Okamura K, Ichihara S, et al. Eosinophilic cholangitis without biliary stricture after cactus spine injury. Am J Gastroenterol. 2022;117:1731.
- Ontiveros ST, Minns AB. Accidental arthrotomy causing aseptic monoarthritis due to agave sap: a case report. Clin Pract Cases Emerg Med. 2021;5:246-248.
- Kim S, Baradia H, Sambasivan A. The use of ultrasonography in expediting septic joint identification and treatment: a case report. Am J Phys Med Rehabil. 2020;99:449-451.
- Yoon HJ, Won CH, Moon SE. Allergic contact dermatitis due to Opuntia ficus-indica var. saboten. Contact Dermatitis. 2004;51:311-312.
- Bonamonte D, Foti C, Gullo G, et al. Plant contact dermatitis. In: Angelini G, Bonamonte D, Foti C, eds. Clinical Contact Dermatitis. 2021; Springer, Cham. doi:10.1007/978-3-030-49332-5_16
- Valentino A, Conte R, Bousta D, et al. Extracellular vesicles derived from Opuntia ficus-indica fruit (OFI-EVs) speed up the normal wound healing processes by modulating cellular responses. Int J Mol Sci. 2024;25:7103.
- Das IJ, Bal T. Evaluation of Opuntia-carrageenan superporous hydrogel (OPM-CRG SPH) as an effective biomaterial for drug release and tissue scaffold. Int J Biol Macromol. 2024;256(Pt 2):128503.
- Adjafre BL, Lima IC, Alves APNN, et al. Anti-inflammatory and healing effect of the polysaccharidic extract of Opuntia ficus-indica cladodes in cutaneous excisional wounds in rats. Int J Exp Pathol. 2024;105:33-44.
- Chiu CS, Cheng YT, Chan YJ, et al. Mechanism and inhibitory effects of cactus (Opuntia dillenii) extract on melanocytes and its potential application for whitening cosmetics. Sci Rep. 2023;13:501.
- Sutariya B, Saraf M. Betanin, isolated from fruits of Opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-β pathway. J Ethnopharmacol. 2017;198:432-443.
- Partovi N, Ebadzadeh MR, Fatemi SJ, et al. Effect of fruit extract on renal stone formation and kidney injury in rats. Nat Prod Res. 2018;32:1180-1183.
- Zhu X, Athmouni K. HPLC analysis and the antioxidant and preventive actions of Opuntia stricta juice extract against hepato-nephrotoxicity and testicular injury induced by cadmium exposure. Molecules. 2022;27:4972.
- Akacha A, Badraoui R, Rebai T, et al. Effect of Opuntia ficus indica extract on methotrexate-induced testicular injury: a biochemical, docking and histological study. J Biomol Struct Dyn. 2022;40:4341-4351.
- González-Ponce HA, Martínez-Saldaña MC, Tepper PG, et al. Betacyanins, major components in Opuntia red-purple fruits, protect against acetaminophen-induced acute liver failure. Food Res Int. 2020;137:109461.
- Akacha A, Rebai T, Zourgui L, et al. Preventive effect of ethanolic extract of cactus (Opuntia ficus-indica) cladodes on methotrexate-induced oxidative damage of the small intestine in Wistar rats. J Cancer Res Ther. 2018;14(Suppl):S779-S784.
- Yeh WJ, Tsai CC, Ko J, et al. Hylocereus polyrhizus peel extract retards alcoholic liver disease progression by modulating oxidative stress and inflammatory responses in C57BL/6 mice. Nutrients. 2020;12:3884.
- Ford AM, Haywood ST, Gallo DR. Novel method for removing embedded cactus spines in the emergency department. Case Rep Emerg Med. 2019;2019:6062531.
- Patel D, Clarkson J, Amirapu S. Frontal sinus post-traumatic mucocele secondary to a cactus spine. N Z Med J. 2020;133:112-115.
- Magro C, Lipner S. Sabra dermatitis: combined features of delayed hypersensitivity and foreign body reaction to implanted glochidia. Dermatol Online J. 2020;26:13030/qt2157f9g0.
- Ruini C, von Braunmühl T, Ruzicka T, et al. Granulomatous reaction after cholla cactus spine injury. Cutis. 2020;105:143-145;E2.
- Kitagawa S, Okamura K, Ichihara S, et al. Eosinophilic cholangitis without biliary stricture after cactus spine injury. Am J Gastroenterol. 2022;117:1731.
- Ontiveros ST, Minns AB. Accidental arthrotomy causing aseptic monoarthritis due to agave sap: a case report. Clin Pract Cases Emerg Med. 2021;5:246-248.
- Kim S, Baradia H, Sambasivan A. The use of ultrasonography in expediting septic joint identification and treatment: a case report. Am J Phys Med Rehabil. 2020;99:449-451.
- Yoon HJ, Won CH, Moon SE. Allergic contact dermatitis due to Opuntia ficus-indica var. saboten. Contact Dermatitis. 2004;51:311-312.
- Bonamonte D, Foti C, Gullo G, et al. Plant contact dermatitis. In: Angelini G, Bonamonte D, Foti C, eds. Clinical Contact Dermatitis. 2021; Springer, Cham. doi:10.1007/978-3-030-49332-5_16
- Valentino A, Conte R, Bousta D, et al. Extracellular vesicles derived from Opuntia ficus-indica fruit (OFI-EVs) speed up the normal wound healing processes by modulating cellular responses. Int J Mol Sci. 2024;25:7103.
- Das IJ, Bal T. Evaluation of Opuntia-carrageenan superporous hydrogel (OPM-CRG SPH) as an effective biomaterial for drug release and tissue scaffold. Int J Biol Macromol. 2024;256(Pt 2):128503.
- Adjafre BL, Lima IC, Alves APNN, et al. Anti-inflammatory and healing effect of the polysaccharidic extract of Opuntia ficus-indica cladodes in cutaneous excisional wounds in rats. Int J Exp Pathol. 2024;105:33-44.
- Chiu CS, Cheng YT, Chan YJ, et al. Mechanism and inhibitory effects of cactus (Opuntia dillenii) extract on melanocytes and its potential application for whitening cosmetics. Sci Rep. 2023;13:501.
- Sutariya B, Saraf M. Betanin, isolated from fruits of Opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-β pathway. J Ethnopharmacol. 2017;198:432-443.
- Partovi N, Ebadzadeh MR, Fatemi SJ, et al. Effect of fruit extract on renal stone formation and kidney injury in rats. Nat Prod Res. 2018;32:1180-1183.
- Zhu X, Athmouni K. HPLC analysis and the antioxidant and preventive actions of Opuntia stricta juice extract against hepato-nephrotoxicity and testicular injury induced by cadmium exposure. Molecules. 2022;27:4972.
- Akacha A, Badraoui R, Rebai T, et al. Effect of Opuntia ficus indica extract on methotrexate-induced testicular injury: a biochemical, docking and histological study. J Biomol Struct Dyn. 2022;40:4341-4351.
- González-Ponce HA, Martínez-Saldaña MC, Tepper PG, et al. Betacyanins, major components in Opuntia red-purple fruits, protect against acetaminophen-induced acute liver failure. Food Res Int. 2020;137:109461.
- Akacha A, Rebai T, Zourgui L, et al. Preventive effect of ethanolic extract of cactus (Opuntia ficus-indica) cladodes on methotrexate-induced oxidative damage of the small intestine in Wistar rats. J Cancer Res Ther. 2018;14(Suppl):S779-S784.
- Yeh WJ, Tsai CC, Ko J, et al. Hylocereus polyrhizus peel extract retards alcoholic liver disease progression by modulating oxidative stress and inflammatory responses in C57BL/6 mice. Nutrients. 2020;12:3884.
Dermatologic Implications of Prickly Pear Cacti (Opuntia)
Dermatologic Implications of Prickly Pear Cacti (Opuntia)
Practice Points
- Prickly pear cacti have fine spines that must be removed via scorching or mechanical means before the fruit can be handled safely.
- Prickly pear spines that become embedded in the skin are associated with local and systemic inflammatory conditions as well as allergic contact dermatitis.
- Preclinical studies have suggested that extracts of the prickly pear cactus could be used in medicine for their anti-inflammatory effects.