User login
Quality care is a team effort
Everyone in community oncology practice has a role to play in the delivery of quality care. If you are looking to implement a quality program, each member of the practice, patients included, should be involved in the planning, implementing, reporting, and revising processes. Once those processes are in place, the practice can set up partnerships with payers based on standardized measures, costs, and outcomes, with appropriate payments.
Click on the PDF icon at the top of this introduction to read the full article.
Everyone in community oncology practice has a role to play in the delivery of quality care. If you are looking to implement a quality program, each member of the practice, patients included, should be involved in the planning, implementing, reporting, and revising processes. Once those processes are in place, the practice can set up partnerships with payers based on standardized measures, costs, and outcomes, with appropriate payments.
Click on the PDF icon at the top of this introduction to read the full article.
Everyone in community oncology practice has a role to play in the delivery of quality care. If you are looking to implement a quality program, each member of the practice, patients included, should be involved in the planning, implementing, reporting, and revising processes. Once those processes are in place, the practice can set up partnerships with payers based on standardized measures, costs, and outcomes, with appropriate payments.
Click on the PDF icon at the top of this introduction to read the full article.
Personality disorders in the clinical setting
Review
Walter F. Baile MD![]()
![]()
Available online 2 April 2011.
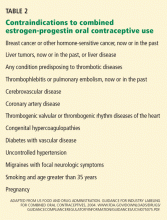
In many cases the stresses of the cancer illness are responsible for amplification of traits, such as passivity in the person with a dependent personality or exaggerated attention to details exhibited by the obsessive compulsive personality. Recognition of these traits can allow the clinician to adjust his or her behavior to the patient's needs. For example, persons with narcissistic traits (Table 2) may be particularly prone to loss of self-esteem and depression when they undergo disfiguring surgery. Acknowledging the challenge the illness presents to patients and praising them for their perseverance may be a useful strategy. The authors also point out that it is important for busy clinicians not to be annoyed with patients who require more time or patience.
A more serious problem is represented by the 5%–8% of the population affected by a personality disorder such as antisocial behavior or borderline personality. These patients are often challenging in the oncology setting because their behaviors may be more disruptive than that of patients with exaggerated personality traits. Acting out in the form of aggressive behavior or unexpected anger at staff can be particularly troublesome. In the case of the borderline disorder, patients may pit staff against one another or engage in other behaviors, as outlined by the authors (Table 2). In my experience, the clinic and especially the inpatient staff have great difficulty in distinguishing these two situations. Patients are allowed to seriously act out before help in managing the individual is requested. It is important to pay attention to clues that might suggest a more serious disorder. For example, substance abuse revealed through a patient's personal history would be a clue for a borderline or antisocial personality. When serious disruptive behavior does occur, early consultation by mental health professionals can help define the diagnosis and provide management strategies for the treatment team and support for the staff, who often feel frustrated with their ability to manage such problems.
Vitae
Dr. Baile is affiliated with the Program in Interpersonal Communication and Relationship Enhancement at the University of Texas MD Anderson Cancer Center, Houston, Texas.
Review
Walter F. Baile MD![]()
![]()
Available online 2 April 2011.
In many cases the stresses of the cancer illness are responsible for amplification of traits, such as passivity in the person with a dependent personality or exaggerated attention to details exhibited by the obsessive compulsive personality. Recognition of these traits can allow the clinician to adjust his or her behavior to the patient's needs. For example, persons with narcissistic traits (Table 2) may be particularly prone to loss of self-esteem and depression when they undergo disfiguring surgery. Acknowledging the challenge the illness presents to patients and praising them for their perseverance may be a useful strategy. The authors also point out that it is important for busy clinicians not to be annoyed with patients who require more time or patience.
A more serious problem is represented by the 5%–8% of the population affected by a personality disorder such as antisocial behavior or borderline personality. These patients are often challenging in the oncology setting because their behaviors may be more disruptive than that of patients with exaggerated personality traits. Acting out in the form of aggressive behavior or unexpected anger at staff can be particularly troublesome. In the case of the borderline disorder, patients may pit staff against one another or engage in other behaviors, as outlined by the authors (Table 2). In my experience, the clinic and especially the inpatient staff have great difficulty in distinguishing these two situations. Patients are allowed to seriously act out before help in managing the individual is requested. It is important to pay attention to clues that might suggest a more serious disorder. For example, substance abuse revealed through a patient's personal history would be a clue for a borderline or antisocial personality. When serious disruptive behavior does occur, early consultation by mental health professionals can help define the diagnosis and provide management strategies for the treatment team and support for the staff, who often feel frustrated with their ability to manage such problems.
Vitae
Dr. Baile is affiliated with the Program in Interpersonal Communication and Relationship Enhancement at the University of Texas MD Anderson Cancer Center, Houston, Texas.
Review
Walter F. Baile MD![]()
![]()
Available online 2 April 2011.
In many cases the stresses of the cancer illness are responsible for amplification of traits, such as passivity in the person with a dependent personality or exaggerated attention to details exhibited by the obsessive compulsive personality. Recognition of these traits can allow the clinician to adjust his or her behavior to the patient's needs. For example, persons with narcissistic traits (Table 2) may be particularly prone to loss of self-esteem and depression when they undergo disfiguring surgery. Acknowledging the challenge the illness presents to patients and praising them for their perseverance may be a useful strategy. The authors also point out that it is important for busy clinicians not to be annoyed with patients who require more time or patience.
A more serious problem is represented by the 5%–8% of the population affected by a personality disorder such as antisocial behavior or borderline personality. These patients are often challenging in the oncology setting because their behaviors may be more disruptive than that of patients with exaggerated personality traits. Acting out in the form of aggressive behavior or unexpected anger at staff can be particularly troublesome. In the case of the borderline disorder, patients may pit staff against one another or engage in other behaviors, as outlined by the authors (Table 2). In my experience, the clinic and especially the inpatient staff have great difficulty in distinguishing these two situations. Patients are allowed to seriously act out before help in managing the individual is requested. It is important to pay attention to clues that might suggest a more serious disorder. For example, substance abuse revealed through a patient's personal history would be a clue for a borderline or antisocial personality. When serious disruptive behavior does occur, early consultation by mental health professionals can help define the diagnosis and provide management strategies for the treatment team and support for the staff, who often feel frustrated with their ability to manage such problems.
Vitae
Dr. Baile is affiliated with the Program in Interpersonal Communication and Relationship Enhancement at the University of Texas MD Anderson Cancer Center, Houston, Texas.
A protocol to triage preoperative assessments to either nurses or nurse practitioners/physician assistants
Traumatic subdural hematoma: An update on morbidity
Menstrual manipulation: Options for suppressing the cycle
If they wish, women can have more control over when and if they menstruate. By using hormonal contraceptives in extended or continuous regimens, they can have their period less often, a practice called menstrual manipulation or menstrual suppression.
Actually, with the help of their clinicians, women have been doing this for years. But now that several products have been approved by the US Food and Drug Administration (FDA) specifically for use in extended or continuous regimens, the practice has become more widely accepted.
Reasons for suppressing menstrual flow range from avoiding bleeding during a particular event (eg, a wedding, graduation, or sports competition) to finding relief from dysmenorrhea or reducing or eliminating menstruation in the treatment of endometriosis, migraine, and other medical conditions exacerbated by hormonal changes around the time of menses.1 Alternatively, some women may practice menstrual manipulation for no other reason than to simply avoid menstruation.
MENSTRUAL DISORDERS ARE TROUBLESOME, COMMON
Each year in the United States, menstrual disorders such as dysmenorrhea (painful menstruation), menorrhagia (excessive or frequent menstruation), metrorrhagia (irregular menstruation), menometrorrhagia (excessive and irregular menstruation), and premenstrual syndrome affect nearly 2.5 million women age 18 to 50 years.2 Menstrual disorders are the leading cause of gynecologic morbidity in the United States, outnumbering adnexal masses (the second most common cause) by a factor of three.2 In addition, these disorders extend into the workplace, costing US industry about 8% of its total wage bill.3
A BRIEF HISTORY OF CONTRACEPTIVE DEVELOPMENT
The idea of using progestins for birth control was first advanced in the 1950s by Dr. Gregory Pincus, who proposed a regimen of 21 days of active drug followed by 7 drug-free days to allow withdrawal bleeding, mimicking the natural cycle.4 This “21/7” regimen was designed to follow the lunar cycle in the hope it would be, in the words of Dr. John Rock, “a morally permissible variant of the rhythm method,”5 thereby making it acceptable to women, clinicians, and the Catholic Church.
In 1977, Loudon et al6 reported the results of a study in which women took active pills for 84 days instead of 21 days, which reduced the frequency of menstruation to every 3 months. Since then, extending the active pills beyond 21 days to avoid menses and other hormone-withdrawal symptoms has become popular in clinical practice, and many studies have investigated the extended or continuous use of oral and other forms of contraception to delay menses.7–18
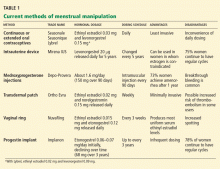
CURRENT METHODS OF MENSTRUAL MANIPULATION
A variety of available products prevent conception by altering the menstrual cycle:
- Oral estrogen-progestin contraceptive pills
- A drug-releasing intrauterine device
- Depot medroxyprogesterone acetate injections
- A contraceptive vaginal ring
- An implantable etonogestrel contraceptive.
Their use in menstrual manipulation is summarized in Table 1.
Oral contraceptive pills
The most common way to manipulate the menstrual cycle is to extend the time between hormone-free weeks in an oral contraceptive regimen.
If the patient is young, you can prescribe a monophasic 21/7 oral contraceptive and tell her to take one active pill every day for 21 days and then start a new pack and keep taking active pills for up to 84 consecutive days, skipping the placebo pills until she wants to have her menstrual period. She can choose which week to have it: if the scheduled 12th week of an extended-cycle oral contraceptive regimen is inconvenient, she can plan it for week 10, or week 9, or whichever week is convenient.
The rationale for using an 84-day (12-week) cycle is that it still provides four periods per year, alleviating fears of hypertrophic endometrium.19
In this scenario, unscheduled or breakthrough bleeding can be managed by taking a “double-up pill” from a spare pack on any day breakthrough bleeding occurs and until it resolves. Menstrual periods should not be planned for intervals shorter than 21 days, owing to the risk of ovulation. Missed days of pills or use of placebo pills should also not exceed 7 days to prevent escape ovulation. 20
In some women with endometriosis and other medical reasons, continuous oral contraception with no placebo week can be prescribed.
Unfortunately, the downside to suppressing withdrawal bleeding is unscheduled or “breakthrough” bleeding. The best way to treat this unscheduled bleeding is not known. Patients who are not sexually active can be reassured that the goal of an atrophic endometrium can still be achieved, with resultant pill amenorrhea (particularly useful for those with severe dysmenorrhea or other reasons to want to avoid flow). Patients could also try to manage flow by periodically taking a 3- to 5-day break from hormone-containing pills to allow flow. They can also try switching to another oral contraceptive that has a different progestin that would spiral the arterioles of the endometrium more tightly and thus more aggressively induce atrophy.13,17,21 For instance, levonorgestrel is 10 to 20 times more potent than norethindrone. Choosing a pill with a higher monophasic dosing of levonorgestrel or a similar progestin may minimize unscheduled bleeding.
Currently, several oral contraceptives are approved for use in an extended regimen.
Seasonale was the first oral contraceptive marketed in the United States with an extended active regimen.22 It comes in a pack of 84 pills containing ethinyl estradiol 0.03 mg and levonorgestrel 0.15 mg, plus 7 placebo pills.
Seasonique is similar to Seasonale, but instead of placebo pills it has seven pills that contain ethinyl estradiol 0.010 mg.
Lybrel is a low-dose combination containing ethinyl estradiol 0.02 mg and levonorgestrel 0.09 mg. Packaged as an entire year’s worth of active pills to be taken continuously for 365 days without a placebo phase or pillfree interval,23 it is the only FDA-approved continuous oral contraceptive available in the United States.
An intrauterine device
Intrauterine devices were originally developed as contraceptives. The addition of a progestin to these devices has been shown to reduce heavy menstrual bleeding by up to 90%.24,25
Mirena IUS, a levonorgestrel-releasing device, is the only medicated intrauterine device that is currently available in the United States. (“IUS” stands for “intrauterine system.”) It was recently approved by the FDA to treat heavy menstrual bleeding in women who use intrauterine contraception as their method of pregnancy prevention.26 About 50% of women who use this device develop amenorrhea within 6 months of insertion, while 25% report oligomenorrhea.27
The Mirena device can be left in the uterus for up to 5 years. It may be a good choice for inducing amenorrhea in women with hemostatic disorders or in whom estrogen either is contraindicated or causes health concerns.18 The copper intrauterine device (Paragard; Duramed Pharmaceuticals Inc., Pomona, NY) remains a viable option for those who cannot or do not tolerate hormonal therapy. However, Mirena may provide less unscheduled bleeding than the copper intrauterine device.
Depot medroxyprogesterone acetate injections
Depo-Provera (depot medroxyprogesterone acetate) injections are given at 90-day intervals. 28 This contraceptive method inhibits ovulation and decidualizes the endometrium, thereby reducing or eliminating uterine bleeding. 29
While new users may initially experience excessive prolonged bleeding (10 or more days) while shedding their existing lining, the rate of amenorrhea has been shown to increase over time as the lining atrophies.30 Thus, prolonged use of this agent reduces the frequency of menstruation as well as menstruation-related symptoms.
Depot medroxyprogesterone acetate is ideal for patients whose menstrual periods pose a significant hygiene problem (eg, developmentally challenged girls). In our experience, the injections can be given at shorter intervals to induce atrophy of the endometrium quickly. In this scenario, the clinician might give an injection every 4 to 6 weeks for two or three doses to induce amenorrhea and then return to every-12-week dosing.
The main risk when using medroxyprogesterone injections to induce amenorrhea is the potential for bone loss. Users of this method have been shown to have lower mean bone mineral density31–33 and significantly higher levels of biomarkers of bone formation and resorption32,34 than nonusers. However, these changes are similar to those seen in breastfeeding women,35 are reversible with cessation, 36 and are not associated with increased fracture risk.37 In adolescent girls, pregnancy poses similar risks to the bones, with longerterm consequences.
Medroxyprogesterone can also stimulate appetite, causing 10 to 20 kg of weight gain in adolescents and women who are already obese and have trouble with appetite regulation.38 Slender users tend not to gain weight, however.
Given this information, depot medroxyprogesterone acetate appears to be a cost-effective contraceptive option that should be considered in the context of the clinical situation and preference of each patient.
Transdermal contraceptive patch
Ortho Evra, a transdermal patch, is designed to deliver ethinyl estradiol 0.02 mg and norelgestromin 0.150 mg daily.39 It is usually applied weekly for 3 weeks, followed by a patch-free week to induce regular monthly withdrawal bleeding.
Extended use of the patch to manipulate menstruation is an off-label use. In the only trial evaluating extended use of the patch, amenorrhea occurred in 12% of users, but unscheduled bleeding and spotting were common. 16
Although there is some evidence that the long-term use of the patch may increase the risk of venous thromboembolism,40,41 the risk in women who use the patch has been found to be similar to that in women using an oral contraceptive.42 However, serum ethinyl estradiol levels have been found to be higher with the use of the weekly patch than with oral contraceptives or the contraceptive vaginal ring39; as a result, many physicians are hesitant to recommend its continuous use.
Pending further data about the safety profile of this contraceptive, the World Health Organization Medical Eligibility Criteria for Contraceptive Use suggest that the same guidelines for the prescription of combination oral contraceptives should also apply to the patch.43
Contraceptive vaginal ring
NuvaRing, a contraceptive vaginal ring, releases a daily dose of ethinyl estradiol 0.015 mg and etonogestrel 0.12 mg.10 It is inserted, left in for 21 days, and then removed and left out for 7 days, during which withdrawal bleeding occurs.10
Vaginal administration has been shown to allow low, continuous dosing, which results in more stable serum concentrations than with the patch or oral contraceptives.39 In the only trial comparing an extended vaginal ring regimen and the traditional 28-day regimen, extended use resulted in fewer overall days of bleeding than monthly use, but with more unscheduled spotting.15
The most common side effects include headache, vaginitis, and leukorrhea,44 but there is no evidence of bacteriologic or cytologic changes in the cervicovaginal epithelium, even with extended use.45,46
Etonogestrel implantable contraceptive
Implanon, a single-rod progestin implant, is available in the United States and elsewhere. It is placed subdermally in the inner upper arm and provides contraception for as long as 3 years.
Implanon contains 68 mg of the progestin etonogestrel, which it slowly releases over time, initially at 0.06 to 0.07 mg/day, decreasing to 0.035 to 0.045 mg/day at the end of the first year, to 0.03 to 0.04 mg/day at the end of the second year, and then to 0.025 to 0.03 mg/day at the end of the third year.47
The amount of vaginal bleeding associated with the use of the implant is generally modest, but the pattern tends to be unpredictable. 48 In addition, because amenorrhea is reported as a side effect in only 22% of women during the first 2 years of its use,48 the progestin implant is a less satisfactory means of menstrual suppression than the other methods discussed above.
BENEFITS OF MENSTRUAL MANIPULATION
Menstrual manipulation has a number of benefits in terms of both overall health and lifestyle.
For most women, using a long-acting hormonal contraceptive carries low risks and substantial health benefits. Women who take oral contraceptives are less likely to develop osteoporosis, ovarian or endometrial cancer, benign breast changes, or pelvic inflammatory disease. 49 Long-term use of an oral contraceptive can also preserve fertility by reducing and delaying the incidence of endometriosis,50 and is effective at treating acne vulgaris, which tends to be common among patients with polycystic ovary syndrome.51,52 In addition, this practice can be used to reduce overall blood loss, an application that is particularly important in women with a bleeding diathesis such as von Willebrand disease, who frequently suffer from menorrhagia.53
Reduced menstruation may also prove more convenient during particular occasions, such as vacations and athletic activities. Specifically, it may be useful to women serving in the military. In a study by Schneider et al,54 a cohort of 83 female cadets reported a significant perceived impact of premenstrual and menstrual-related symptoms on academic, physical, and military activities, as well as difficulties in obtaining, changing, and disposing of menstrual materials in a military setting. Likewise, reduced menstrual frequency or amenorrhea may play an important role in female athletes, who reportedly use oral contraceptives to control premenstrual symptoms, to protect bone health, and to manipulate the menstrual cycle in order to maximize performance.55
Adolescent girls are another group who may benefit from reduced or absent menses, once they have reached near-final height. By practicing menstrual suppression, girls can avoid dysmenorrhea and the inconvenience of menstruation during the school day, when their access to painkillers, sanitary pads or tampons, and a change of clothes may be limited. 56 Clinicians who discuss with teenage patients the benefits of innovative hormonal contraceptive schedules that reduce menstrual frequency may be able to improve the quality of life for these young women.
In a very short girl just after menarche, care must be taken not to start a hormonal method too early so as not to prematurely close epiphyses and stunt final height; after menarche, most girls still have 1 to 4 inches of potential growth. For a young lady 4 feet 11 inches tall, that extra inch may be important.
Finally, menstrual manipulation may also find a niche among the developmentally challenged. Women with cognitive impairment and physical disabilities may have difficulty with hygienic practice around menses. For a number of years, contraceptives have been used to manage menstrual hygiene in patients with catamenial (ie, menstrual) epilepsy, and to address caregiver concerns in women with severe mental retardation, with improved behavior noted in some patients.57–59 In this setting, an agent that suppresses menses and also provides contraception, especially for those girls and women at risk of abuse, may offer substantial benefits.
DISADVANTAGES OF MENSTRUAL MANIPULATION
Rates of adverse events and of discontinuation of extended and continuous oral contraceptive regimens are comparable with those reported for cyclic regimens, except for higher rates of breakthrough bleeding.
In a trial of continuous oral contraceptive use in more than 2,000 patients, 396 (18.5%) withdrew from the study as a result of bothersome uterine bleeding.60 However, while breakthrough bleeding often occurs during the first few months of extended oral contraceptive use, it usually decreases with each successive cycle of therapy and is comparable to that reported by patients on the conventional oral contraceptive regimen by the fourth extended cycle.12
CONTRACEPTIVE EFFICACY
The efficacy of extended and continuous oral contraceptive regimens is comparable with that of cyclic regimens.12,60,61 One reason for this may be better adherence to continuous regimens: women using this regimen have been shown to miss fewer pills than those on a cyclic regimen, especially during the critical first week of the pill pack.21
Several studies have shown that some women ovulate during the standard 21/7 oral contraceptive regimen even if they do not miss any pills or take pills off-schedule, putting them at greater risk of pregnancy.62 Large studies evaluating the efficacy of an extendedcycle regimen have shown a pregnancy rate during the 1-year study period that was either comparable with61 or lower than12,60 rates with standard regimens.
Heterosexual couples need to be advised to use condoms to further reduce the already low failure rate and to prevent sexually transmitted diseases.
ACCEPTABILITY OF MENSTRUAL MANIPULATION
Ever since the earliest trial of an extended oral contraceptive regimen, participants have expressed a favorable response to the resulting decrease in menstrual frequency; in the 1977 study by Loudon et al,6 patients on the extended regimen cited infrequent periods (82%), fewer menstrual problems (20%), and easier pill-taking (19%) as favorable features.
In 1999, den Tonkelaar and Oddens63 surveyed 1,300 Dutch women about their preferred frequency of menstruation and found that about 70% between the ages of 15 and 49 preferred a frequency of between every 3 months and never. A similar survey in the United States indicated that 58% preferred a bleeding frequency of either every 3 months or never to more frequent periods.64
While patients find menstrual manipulation generally acceptable, clinician approval has been more varied. Loudon et al reported that “the doctors and nurses on the clinic staff were less enthusiastic about this regimen than the volunteers themselves.”6 In a survey of 222 clinicians,65 90% of responders reported ever having prescribed extended or continuous dosing regimens to adolescents, and 33% reported that extended cycles made up more than 10% of their total oral contraceptive prescriptions.
Myths and misperceptions about menstrual manipulation abound. Many clinicians believe that routine use of an extended or continuous oral contraceptive regimen is inadvisable, despite the lack of evidence to support this notion.66 Therefore, many care providers need more education about the practice and benefits of menstrual manipulation.
THE RIGHT METHOD FOR THE RIGHT PATIENT
Manipulation and suppression of menstruation through continuous or extended use of oral contraceptives or by other means may have a number of advantages to women, including fewer menstrual-related syndromes, reduced absenteeism from work or school, and greater overall satisfaction.
For women whose goal is to reduce but not necessarily to eliminate monthly bleeding, the cyclic use of estrogen-progestin contraception (rather than progestins alone or continuous use of combined hormonal preparations) is suggested.
Clinicians should not overestimate the risks of oral contraceptives and other hormonal methods, but rather educate themselves so that they can utilize menstrual manipulation safely to match the individual patient’s needs.
- Association of Reproductive Health Professionals. Extended and continuous use of contraceptives to reduce menstruation. September 2004. http://www.arhp.org/publications-and-resources/clinical-proceedings/reduce-menses. Accessed May 17, 2010.
- Kjerulff KH, Erickson BA, Langenberg PW. Chronic gynecological conditions reported by US women: findings from the National Health Interview Survey, 1984 to 1992. Am J Public Health 1996; 86:195–199.
- Thomas SL, Ellertson C. Nuisance or natural and healthy: should monthly menstruation be optional for women? Lancet 2000; 355:922–924.
- Connell EB. Contraception in the prepill era. Contraception 1999; 59(suppl 1):7S–10S.
- Marks LV. Sexual chemistry: a history of the contraceptive pill. New Haven, CT: Yale University Press, 2001.
- Loudon NB, Foxwell M, Potts DM, Guild AL, Short RV. Acceptability of an oral contraceptive that reduces the frequency of menstruation: the tri-cycle pill regimen. Br Med J 1977; 2:487–490.
- Sulak PJ, Cressman BE, Waldrop E, Holleman S, Kuehl TJ. Extending the duration of active oral contraceptive pills to manage hormone withdrawal symptoms. Obstet Gynecol 1997; 89:179–183.
- Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception 1997; 56:341–352.
- Miller L, Notter KM. Menstrual reduction with extended use of combination oral contraceptive pills: randomized controlled trial. Obstet Gynecol 2001; 98:771–778.
- Mulders TM, Dieben TO. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertil Steril 2001; 75:865–870.
- Stanford JB, Mikolajczyk RT. Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects. Am J Obstet Gynecol 2002; 187:1699–1708.
- Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception 2003; 68:89–96.
- Miller L, Hughes JP. Continuous combination oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstet Gynecol 2003; 101:653–661.
- Sillem M, Schneidereit R, Heithecker R, Mueck AO. Use of an oral contraceptive containing drospirenone in an extended regimen. Eur J Contracept Reprod Health Care 2003; 8:162–169.
- Miller L, Verhoeven CH, Hout J. Extended regimens of the contraceptive vaginal ring: a randomized trial. Obstet Gynecol 2005; 106:473–482.
- Stewart FH, Kaunitz AM, Laguardia KD, Karvois DL, Fisher AC, Friedman AJ. Extended use of transdermal norelgestromin/ethinyl estradiol: a randomized trial. Obstet Gynecol 2005; 105:1389–1396.
- Sulak PJ, Kuehl TJ, Coffee A, Willis S. Prospective analysis of occurrence and management of breakthrough bleeding during an extended oral contraceptive regimen. Am J Obstet Gynecol 2006; 195:935–941.
- Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel– releasing intrauterine system in women with hemostatic disorders. Fertil Steril 2008; 90:673–677.
- Anderson FD, Feldman R, Reape KZ. Endometrial effects of a 91-day extended-regimen oral contraceptive with low-dose estrogen in place of placebo. Contraception 2008; 77:91–96.
- Wright KP, Johnson JV. Evaluation of extended and continuous use oral contraceptives. Ther Clin Risk Manag 2008; 4:905–911.
- Edelman AB, Gallo MF, Jensen JT, Nichols MD, Schulz KF, Grimes DA. Continuous or extended cycle vs. cyclic use of combined oral contraceptives for contraception. Cochrane Database Syst Rev 2005; 3:CD004695.
- Sulak PJ, Kuehl TJ, Ortiz M, Shull BL. Acceptance of altering the standard 21-day/7-day oral contraceptive regimen to delay menses and reduce hormone withdrawal symptoms. Am J Obstet Gynecol 2002; 186:1142–1149.
- Turok D. The quest for better contraception: future methods. Obstet Gynecol Clin North Am 2007; 34:137–166.
- Bergqvist A, Rybo G. Treatment of menorrhagia with intrauterine release of progesterone. Br J Obstet Gynaecol 1983; 90:255–258.
- Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception 1994; 49:56–72.
- US Food and Drug Administration. FDA Approves Additional Use for IUD Mirena to Treat Heavy Menstrual Bleeding in IUD Users. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm184747.htm. Accessed May 17, 2010.
- Hidalgo M, Bahamondes L, Perrotti M, Diaz J, Dantas-Monteiro C, Petta C. Bleeding patterns and clinical performance of the levonorgestrel-releasing intrauterine system (Mirena) up to two years. Contraception 2002; 65:129–132.
- Schwallie PC, Assenzo JR. Contraceptive use—efficacy study utilizing medroxyprogesterone acetate administered as an intramuscular injection once every 90 days. Fertil Steril 1973; 24:331–339.
- Kaunitz AM. Injectable contraception. New and existing options. Obstet Gynecol Clin North Am 2000; 27:741–780.
- Mainwaring R, Hales HA, Stevenson K, et al. Metabolic parameter, bleeding, and weight changes in US women using progestin only contraceptives. Contraception 1995; 51:149–153.
- Curtis KM, Martins SL. Progestogen-only contraception and bone mineral density: a systematic review. Contraception 2006; 73:470–487.
- Shaarawy M, El-Mallah SY, Seoudi S, Hassan M, Mohsen IA. Effects of the long-term use of depot medroxyprogesterone acetate as hormonal contraceptive on bone mineral density and biochemical markers of bone remodeling. Contraception 2006; 74:297–302.
- Cromer BA, Bonny AE, Stager M, et al. Bone mineral density in adolescent females using injectable or oral contraceptives: a 24-month prospective study. Fertil Steril 2008; 90:2060–2067.
- Rome E, Ziegler J, Secic M, et al. Bone biochemical markers in adolescent girls using either depot medroxyprogesterone acetate or an oral contraceptive. J Pediatr Adolesc Gynecol 2004; 17:373–377.
- More C, Bettembuk P, Bhattoa HP, Balogh A. The effects of pregnancy and lactation on bone mineral density. Osteoporos Int 2001; 12:732–737.
- Kaunitz AM, Miller PD, Rice VM, Ross D, McClung MR. Bone mineral density in women aged 25-35 years receiving depot medroxyprogesterone acetate: recovery following discontinuation. Contraception 2006; 74:90–99.
- Guilbert ER, Brown JP, Kaunitz AM, et al. The use of depot-medroxyprogesterone acetate in contraception and its potential impact on skeletal health. Contraception 2009; 79:167–177.
- Bonny AE, Ziegler J, Harvey R, Debanne SM, Secic M, Cromer BA. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch Pediatr Adolesc Med 2006; 160:40–45.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Douketis JD, Ginsberg JS, Holbrook A, Crowther M, Duku EK, Burrows RF. A reevaluation of the risk for venous thromboembolism with the use of oral contraceptives and hormone replacement therapy. Arch Intern Med 1997; 157:1522–1530.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 3rd ed. Geneva: Reproductive Health and Research, World Health Organization; 2004.
- Dieben TO, Roumen FJ, Apter D. Efficacy, cycle control, and user acceptability of a novel combined contraceptive vaginal ring. Obstet Gynecol 2002; 100:585–593.
- Davies GC, Feng LX, Newton JR, Dieben TO, Coelingh-Bennink HJ. The effects of a combined contraceptive vaginal ring releasing ethinyloestradiol and 3-ketodesogestrel on vaginal flora. Contraception 1992; 45:511–518.
- Roumen FJ, Boon ME, van Velzen D, Dieben TO, Coelingh Bennink HJ. The cervico-vaginal epithelium during 20 cycles’ use of a combined contraceptive vaginal ring. Hum Reprod 1996; 11:2443–2448.
- Wenzl R, van Beek A, Schnabel P, Huber J. Pharmacokinetics of etonogestrel released from the contraceptive implant Implanon. Contraception 1998; 58:283–288.
- Darney P, Patel A, Rosen K, Shapiro LS, Kaunitz AM. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril 2009; 91:1646–1653.
- Jensen JT, Speroff L. Health benefits of oral contraceptives. Obstet Gynecol Clin North Am 2000; 27:705–721.
- Seracchioli R, Mabrouk M, Frascà C, et al. Long-term cyclic and continuous oral contraceptive therapy and endometrioma recurrence: a randomized controlled trial. Fertil Steril 2010; 93:52–56.
- Falsetti L, Dordoni D, Gastaldi C, Gastaldi A. A new association of ethinylestradiol (0.035 mg) cyproterone acetate (2 mg) in the therapy of polycystic ovary syndrome. Acta Eur Fertil 1986; 17:19–25.
- Koltun W, Lucky AW, Thiboutot D, et al. Efficacy and safety of 3 mg drospirenone/20 mcg ethinylestradiol oral contraceptive administered in 24/4 regimen in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled trial. Contraception 2008; 77:249–256.
- Kadir RA, Sabin CA, Pollard D, Lee CA, Economides DL. Quality of life during menstruation in patients with inherited bleeding disorders. Haemophilia 1998; 4:836–841.
- Schneider MB, Fisher M, Friedman SB, Bijur PE, Toffler AP. Menstrual and premenstrual issues in female military cadets: a unique population with significant concerns. J Pediatr Adolesc Gynecol 1999; 12:195–201.
- Bennell K, White S, Crossley K. The oral contraceptive pill: a revolution for sportswomen? Br J Sports Med 1999; 33:231–238.
- Kaplowitz PB, Oberfield SE. Reexamination of the age limit for defining when puberty is precocious in girls in the United States: implications for evaluation and treatment. Drug and Therapeutics and Executive Committees of the Lawson Wilkins Pediatric Endocrine Society. Pediatrics 1999; 104:936–941.
- Roxburgh DR, West MJ. The use of norethisterone to suppress menstruation in the intellectually severely retarded woman. Med J Aust 1973; 2:310–313.
- Egan TM, Siegert RJ, Fairley NA. Use of hormonal contraceptives in an institutional setting: reasons for use, consent and safety in women with psychiatric and intellectual disabilities. N Z Med J 1993; 106:338–341.
- Pillai M, O’Brien K, Hill E. The levonorgestrel intrauterine system (Mirena) for the treatment of menstrual problems in adolescents with medical disorders, or physical or learning disabilities. BJOG 2010; 117:216–221.
- Archer DF, Jensen JT, Johnson JV, Borisute H, Grubb GS, Constantine GD. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception 2006; 74:439–445.
- Kroll R, Reape KZ, Margolis M. The efficacy and safety of a low-dose, 91-day, extended-regimen oral contraceptive with continuous ethinyl estradiol. Contraception 2010; 81:41–48.
- Archer DF. Menstrual-cycle-related symptoms: a review of the rationale for continuous use of oral contraceptives. Contraception 2006; 74:359–366.
- den Tonkelaar I, Oddens BJ. Preferred frequency and characteristics of menstrual bleeding in relation to reproductive status, oral contraceptive use, and hormone replacement therapy use. Contraception 1999; 59:357–362.
- Edelman A, Lew R, Cwiak C, Nichols M, Jensen J. Acceptability of contraceptive-induced amenorrhea in a racially diverse group of US women. Contraception 2007; 75:450–453.
- Gerschultz KL, Sucato GS, Hennon TR, Murray PJ, Gold MA. Extended cycling of combined hormonal contraceptives in adolescents: physician views and prescribing practices. J Adolesc Health 2007; 40:151–157.
- Frankovich RJ, Lebrun CM. Menstrual cycle, contraception, and performance. Clin Sports Med 2000; 19:251–271.
- Speroff L, Darney PD. A Clinical Guide for Contraception. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
- Kaunitz AM. Long-acting contraceptive options. Int J Fertil Menopausal Stud 1996; 41:69–76.
- US Food and Drug Administration. Guidance for Industry Labeling for Combined Oral Contraceptives, 2004. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm075075.pdfAccessed May 17, 2010.
If they wish, women can have more control over when and if they menstruate. By using hormonal contraceptives in extended or continuous regimens, they can have their period less often, a practice called menstrual manipulation or menstrual suppression.
Actually, with the help of their clinicians, women have been doing this for years. But now that several products have been approved by the US Food and Drug Administration (FDA) specifically for use in extended or continuous regimens, the practice has become more widely accepted.
Reasons for suppressing menstrual flow range from avoiding bleeding during a particular event (eg, a wedding, graduation, or sports competition) to finding relief from dysmenorrhea or reducing or eliminating menstruation in the treatment of endometriosis, migraine, and other medical conditions exacerbated by hormonal changes around the time of menses.1 Alternatively, some women may practice menstrual manipulation for no other reason than to simply avoid menstruation.
MENSTRUAL DISORDERS ARE TROUBLESOME, COMMON
Each year in the United States, menstrual disorders such as dysmenorrhea (painful menstruation), menorrhagia (excessive or frequent menstruation), metrorrhagia (irregular menstruation), menometrorrhagia (excessive and irregular menstruation), and premenstrual syndrome affect nearly 2.5 million women age 18 to 50 years.2 Menstrual disorders are the leading cause of gynecologic morbidity in the United States, outnumbering adnexal masses (the second most common cause) by a factor of three.2 In addition, these disorders extend into the workplace, costing US industry about 8% of its total wage bill.3
A BRIEF HISTORY OF CONTRACEPTIVE DEVELOPMENT
The idea of using progestins for birth control was first advanced in the 1950s by Dr. Gregory Pincus, who proposed a regimen of 21 days of active drug followed by 7 drug-free days to allow withdrawal bleeding, mimicking the natural cycle.4 This “21/7” regimen was designed to follow the lunar cycle in the hope it would be, in the words of Dr. John Rock, “a morally permissible variant of the rhythm method,”5 thereby making it acceptable to women, clinicians, and the Catholic Church.
In 1977, Loudon et al6 reported the results of a study in which women took active pills for 84 days instead of 21 days, which reduced the frequency of menstruation to every 3 months. Since then, extending the active pills beyond 21 days to avoid menses and other hormone-withdrawal symptoms has become popular in clinical practice, and many studies have investigated the extended or continuous use of oral and other forms of contraception to delay menses.7–18
CURRENT METHODS OF MENSTRUAL MANIPULATION
A variety of available products prevent conception by altering the menstrual cycle:
- Oral estrogen-progestin contraceptive pills
- A drug-releasing intrauterine device
- Depot medroxyprogesterone acetate injections
- A contraceptive vaginal ring
- An implantable etonogestrel contraceptive.
Their use in menstrual manipulation is summarized in Table 1.
Oral contraceptive pills
The most common way to manipulate the menstrual cycle is to extend the time between hormone-free weeks in an oral contraceptive regimen.
If the patient is young, you can prescribe a monophasic 21/7 oral contraceptive and tell her to take one active pill every day for 21 days and then start a new pack and keep taking active pills for up to 84 consecutive days, skipping the placebo pills until she wants to have her menstrual period. She can choose which week to have it: if the scheduled 12th week of an extended-cycle oral contraceptive regimen is inconvenient, she can plan it for week 10, or week 9, or whichever week is convenient.
The rationale for using an 84-day (12-week) cycle is that it still provides four periods per year, alleviating fears of hypertrophic endometrium.19
In this scenario, unscheduled or breakthrough bleeding can be managed by taking a “double-up pill” from a spare pack on any day breakthrough bleeding occurs and until it resolves. Menstrual periods should not be planned for intervals shorter than 21 days, owing to the risk of ovulation. Missed days of pills or use of placebo pills should also not exceed 7 days to prevent escape ovulation. 20
In some women with endometriosis and other medical reasons, continuous oral contraception with no placebo week can be prescribed.
Unfortunately, the downside to suppressing withdrawal bleeding is unscheduled or “breakthrough” bleeding. The best way to treat this unscheduled bleeding is not known. Patients who are not sexually active can be reassured that the goal of an atrophic endometrium can still be achieved, with resultant pill amenorrhea (particularly useful for those with severe dysmenorrhea or other reasons to want to avoid flow). Patients could also try to manage flow by periodically taking a 3- to 5-day break from hormone-containing pills to allow flow. They can also try switching to another oral contraceptive that has a different progestin that would spiral the arterioles of the endometrium more tightly and thus more aggressively induce atrophy.13,17,21 For instance, levonorgestrel is 10 to 20 times more potent than norethindrone. Choosing a pill with a higher monophasic dosing of levonorgestrel or a similar progestin may minimize unscheduled bleeding.
Currently, several oral contraceptives are approved for use in an extended regimen.
Seasonale was the first oral contraceptive marketed in the United States with an extended active regimen.22 It comes in a pack of 84 pills containing ethinyl estradiol 0.03 mg and levonorgestrel 0.15 mg, plus 7 placebo pills.
Seasonique is similar to Seasonale, but instead of placebo pills it has seven pills that contain ethinyl estradiol 0.010 mg.
Lybrel is a low-dose combination containing ethinyl estradiol 0.02 mg and levonorgestrel 0.09 mg. Packaged as an entire year’s worth of active pills to be taken continuously for 365 days without a placebo phase or pillfree interval,23 it is the only FDA-approved continuous oral contraceptive available in the United States.
An intrauterine device
Intrauterine devices were originally developed as contraceptives. The addition of a progestin to these devices has been shown to reduce heavy menstrual bleeding by up to 90%.24,25
Mirena IUS, a levonorgestrel-releasing device, is the only medicated intrauterine device that is currently available in the United States. (“IUS” stands for “intrauterine system.”) It was recently approved by the FDA to treat heavy menstrual bleeding in women who use intrauterine contraception as their method of pregnancy prevention.26 About 50% of women who use this device develop amenorrhea within 6 months of insertion, while 25% report oligomenorrhea.27
The Mirena device can be left in the uterus for up to 5 years. It may be a good choice for inducing amenorrhea in women with hemostatic disorders or in whom estrogen either is contraindicated or causes health concerns.18 The copper intrauterine device (Paragard; Duramed Pharmaceuticals Inc., Pomona, NY) remains a viable option for those who cannot or do not tolerate hormonal therapy. However, Mirena may provide less unscheduled bleeding than the copper intrauterine device.
Depot medroxyprogesterone acetate injections
Depo-Provera (depot medroxyprogesterone acetate) injections are given at 90-day intervals. 28 This contraceptive method inhibits ovulation and decidualizes the endometrium, thereby reducing or eliminating uterine bleeding. 29
While new users may initially experience excessive prolonged bleeding (10 or more days) while shedding their existing lining, the rate of amenorrhea has been shown to increase over time as the lining atrophies.30 Thus, prolonged use of this agent reduces the frequency of menstruation as well as menstruation-related symptoms.
Depot medroxyprogesterone acetate is ideal for patients whose menstrual periods pose a significant hygiene problem (eg, developmentally challenged girls). In our experience, the injections can be given at shorter intervals to induce atrophy of the endometrium quickly. In this scenario, the clinician might give an injection every 4 to 6 weeks for two or three doses to induce amenorrhea and then return to every-12-week dosing.
The main risk when using medroxyprogesterone injections to induce amenorrhea is the potential for bone loss. Users of this method have been shown to have lower mean bone mineral density31–33 and significantly higher levels of biomarkers of bone formation and resorption32,34 than nonusers. However, these changes are similar to those seen in breastfeeding women,35 are reversible with cessation, 36 and are not associated with increased fracture risk.37 In adolescent girls, pregnancy poses similar risks to the bones, with longerterm consequences.
Medroxyprogesterone can also stimulate appetite, causing 10 to 20 kg of weight gain in adolescents and women who are already obese and have trouble with appetite regulation.38 Slender users tend not to gain weight, however.
Given this information, depot medroxyprogesterone acetate appears to be a cost-effective contraceptive option that should be considered in the context of the clinical situation and preference of each patient.
Transdermal contraceptive patch
Ortho Evra, a transdermal patch, is designed to deliver ethinyl estradiol 0.02 mg and norelgestromin 0.150 mg daily.39 It is usually applied weekly for 3 weeks, followed by a patch-free week to induce regular monthly withdrawal bleeding.
Extended use of the patch to manipulate menstruation is an off-label use. In the only trial evaluating extended use of the patch, amenorrhea occurred in 12% of users, but unscheduled bleeding and spotting were common. 16
Although there is some evidence that the long-term use of the patch may increase the risk of venous thromboembolism,40,41 the risk in women who use the patch has been found to be similar to that in women using an oral contraceptive.42 However, serum ethinyl estradiol levels have been found to be higher with the use of the weekly patch than with oral contraceptives or the contraceptive vaginal ring39; as a result, many physicians are hesitant to recommend its continuous use.
Pending further data about the safety profile of this contraceptive, the World Health Organization Medical Eligibility Criteria for Contraceptive Use suggest that the same guidelines for the prescription of combination oral contraceptives should also apply to the patch.43
Contraceptive vaginal ring
NuvaRing, a contraceptive vaginal ring, releases a daily dose of ethinyl estradiol 0.015 mg and etonogestrel 0.12 mg.10 It is inserted, left in for 21 days, and then removed and left out for 7 days, during which withdrawal bleeding occurs.10
Vaginal administration has been shown to allow low, continuous dosing, which results in more stable serum concentrations than with the patch or oral contraceptives.39 In the only trial comparing an extended vaginal ring regimen and the traditional 28-day regimen, extended use resulted in fewer overall days of bleeding than monthly use, but with more unscheduled spotting.15
The most common side effects include headache, vaginitis, and leukorrhea,44 but there is no evidence of bacteriologic or cytologic changes in the cervicovaginal epithelium, even with extended use.45,46
Etonogestrel implantable contraceptive
Implanon, a single-rod progestin implant, is available in the United States and elsewhere. It is placed subdermally in the inner upper arm and provides contraception for as long as 3 years.
Implanon contains 68 mg of the progestin etonogestrel, which it slowly releases over time, initially at 0.06 to 0.07 mg/day, decreasing to 0.035 to 0.045 mg/day at the end of the first year, to 0.03 to 0.04 mg/day at the end of the second year, and then to 0.025 to 0.03 mg/day at the end of the third year.47
The amount of vaginal bleeding associated with the use of the implant is generally modest, but the pattern tends to be unpredictable. 48 In addition, because amenorrhea is reported as a side effect in only 22% of women during the first 2 years of its use,48 the progestin implant is a less satisfactory means of menstrual suppression than the other methods discussed above.
BENEFITS OF MENSTRUAL MANIPULATION
Menstrual manipulation has a number of benefits in terms of both overall health and lifestyle.
For most women, using a long-acting hormonal contraceptive carries low risks and substantial health benefits. Women who take oral contraceptives are less likely to develop osteoporosis, ovarian or endometrial cancer, benign breast changes, or pelvic inflammatory disease. 49 Long-term use of an oral contraceptive can also preserve fertility by reducing and delaying the incidence of endometriosis,50 and is effective at treating acne vulgaris, which tends to be common among patients with polycystic ovary syndrome.51,52 In addition, this practice can be used to reduce overall blood loss, an application that is particularly important in women with a bleeding diathesis such as von Willebrand disease, who frequently suffer from menorrhagia.53
Reduced menstruation may also prove more convenient during particular occasions, such as vacations and athletic activities. Specifically, it may be useful to women serving in the military. In a study by Schneider et al,54 a cohort of 83 female cadets reported a significant perceived impact of premenstrual and menstrual-related symptoms on academic, physical, and military activities, as well as difficulties in obtaining, changing, and disposing of menstrual materials in a military setting. Likewise, reduced menstrual frequency or amenorrhea may play an important role in female athletes, who reportedly use oral contraceptives to control premenstrual symptoms, to protect bone health, and to manipulate the menstrual cycle in order to maximize performance.55
Adolescent girls are another group who may benefit from reduced or absent menses, once they have reached near-final height. By practicing menstrual suppression, girls can avoid dysmenorrhea and the inconvenience of menstruation during the school day, when their access to painkillers, sanitary pads or tampons, and a change of clothes may be limited. 56 Clinicians who discuss with teenage patients the benefits of innovative hormonal contraceptive schedules that reduce menstrual frequency may be able to improve the quality of life for these young women.
In a very short girl just after menarche, care must be taken not to start a hormonal method too early so as not to prematurely close epiphyses and stunt final height; after menarche, most girls still have 1 to 4 inches of potential growth. For a young lady 4 feet 11 inches tall, that extra inch may be important.
Finally, menstrual manipulation may also find a niche among the developmentally challenged. Women with cognitive impairment and physical disabilities may have difficulty with hygienic practice around menses. For a number of years, contraceptives have been used to manage menstrual hygiene in patients with catamenial (ie, menstrual) epilepsy, and to address caregiver concerns in women with severe mental retardation, with improved behavior noted in some patients.57–59 In this setting, an agent that suppresses menses and also provides contraception, especially for those girls and women at risk of abuse, may offer substantial benefits.
DISADVANTAGES OF MENSTRUAL MANIPULATION
Rates of adverse events and of discontinuation of extended and continuous oral contraceptive regimens are comparable with those reported for cyclic regimens, except for higher rates of breakthrough bleeding.
In a trial of continuous oral contraceptive use in more than 2,000 patients, 396 (18.5%) withdrew from the study as a result of bothersome uterine bleeding.60 However, while breakthrough bleeding often occurs during the first few months of extended oral contraceptive use, it usually decreases with each successive cycle of therapy and is comparable to that reported by patients on the conventional oral contraceptive regimen by the fourth extended cycle.12
CONTRACEPTIVE EFFICACY
The efficacy of extended and continuous oral contraceptive regimens is comparable with that of cyclic regimens.12,60,61 One reason for this may be better adherence to continuous regimens: women using this regimen have been shown to miss fewer pills than those on a cyclic regimen, especially during the critical first week of the pill pack.21
Several studies have shown that some women ovulate during the standard 21/7 oral contraceptive regimen even if they do not miss any pills or take pills off-schedule, putting them at greater risk of pregnancy.62 Large studies evaluating the efficacy of an extendedcycle regimen have shown a pregnancy rate during the 1-year study period that was either comparable with61 or lower than12,60 rates with standard regimens.
Heterosexual couples need to be advised to use condoms to further reduce the already low failure rate and to prevent sexually transmitted diseases.
ACCEPTABILITY OF MENSTRUAL MANIPULATION
Ever since the earliest trial of an extended oral contraceptive regimen, participants have expressed a favorable response to the resulting decrease in menstrual frequency; in the 1977 study by Loudon et al,6 patients on the extended regimen cited infrequent periods (82%), fewer menstrual problems (20%), and easier pill-taking (19%) as favorable features.
In 1999, den Tonkelaar and Oddens63 surveyed 1,300 Dutch women about their preferred frequency of menstruation and found that about 70% between the ages of 15 and 49 preferred a frequency of between every 3 months and never. A similar survey in the United States indicated that 58% preferred a bleeding frequency of either every 3 months or never to more frequent periods.64
While patients find menstrual manipulation generally acceptable, clinician approval has been more varied. Loudon et al reported that “the doctors and nurses on the clinic staff were less enthusiastic about this regimen than the volunteers themselves.”6 In a survey of 222 clinicians,65 90% of responders reported ever having prescribed extended or continuous dosing regimens to adolescents, and 33% reported that extended cycles made up more than 10% of their total oral contraceptive prescriptions.
Myths and misperceptions about menstrual manipulation abound. Many clinicians believe that routine use of an extended or continuous oral contraceptive regimen is inadvisable, despite the lack of evidence to support this notion.66 Therefore, many care providers need more education about the practice and benefits of menstrual manipulation.
THE RIGHT METHOD FOR THE RIGHT PATIENT
Manipulation and suppression of menstruation through continuous or extended use of oral contraceptives or by other means may have a number of advantages to women, including fewer menstrual-related syndromes, reduced absenteeism from work or school, and greater overall satisfaction.
For women whose goal is to reduce but not necessarily to eliminate monthly bleeding, the cyclic use of estrogen-progestin contraception (rather than progestins alone or continuous use of combined hormonal preparations) is suggested.
Clinicians should not overestimate the risks of oral contraceptives and other hormonal methods, but rather educate themselves so that they can utilize menstrual manipulation safely to match the individual patient’s needs.
If they wish, women can have more control over when and if they menstruate. By using hormonal contraceptives in extended or continuous regimens, they can have their period less often, a practice called menstrual manipulation or menstrual suppression.
Actually, with the help of their clinicians, women have been doing this for years. But now that several products have been approved by the US Food and Drug Administration (FDA) specifically for use in extended or continuous regimens, the practice has become more widely accepted.
Reasons for suppressing menstrual flow range from avoiding bleeding during a particular event (eg, a wedding, graduation, or sports competition) to finding relief from dysmenorrhea or reducing or eliminating menstruation in the treatment of endometriosis, migraine, and other medical conditions exacerbated by hormonal changes around the time of menses.1 Alternatively, some women may practice menstrual manipulation for no other reason than to simply avoid menstruation.
MENSTRUAL DISORDERS ARE TROUBLESOME, COMMON
Each year in the United States, menstrual disorders such as dysmenorrhea (painful menstruation), menorrhagia (excessive or frequent menstruation), metrorrhagia (irregular menstruation), menometrorrhagia (excessive and irregular menstruation), and premenstrual syndrome affect nearly 2.5 million women age 18 to 50 years.2 Menstrual disorders are the leading cause of gynecologic morbidity in the United States, outnumbering adnexal masses (the second most common cause) by a factor of three.2 In addition, these disorders extend into the workplace, costing US industry about 8% of its total wage bill.3
A BRIEF HISTORY OF CONTRACEPTIVE DEVELOPMENT
The idea of using progestins for birth control was first advanced in the 1950s by Dr. Gregory Pincus, who proposed a regimen of 21 days of active drug followed by 7 drug-free days to allow withdrawal bleeding, mimicking the natural cycle.4 This “21/7” regimen was designed to follow the lunar cycle in the hope it would be, in the words of Dr. John Rock, “a morally permissible variant of the rhythm method,”5 thereby making it acceptable to women, clinicians, and the Catholic Church.
In 1977, Loudon et al6 reported the results of a study in which women took active pills for 84 days instead of 21 days, which reduced the frequency of menstruation to every 3 months. Since then, extending the active pills beyond 21 days to avoid menses and other hormone-withdrawal symptoms has become popular in clinical practice, and many studies have investigated the extended or continuous use of oral and other forms of contraception to delay menses.7–18
CURRENT METHODS OF MENSTRUAL MANIPULATION
A variety of available products prevent conception by altering the menstrual cycle:
- Oral estrogen-progestin contraceptive pills
- A drug-releasing intrauterine device
- Depot medroxyprogesterone acetate injections
- A contraceptive vaginal ring
- An implantable etonogestrel contraceptive.
Their use in menstrual manipulation is summarized in Table 1.
Oral contraceptive pills
The most common way to manipulate the menstrual cycle is to extend the time between hormone-free weeks in an oral contraceptive regimen.
If the patient is young, you can prescribe a monophasic 21/7 oral contraceptive and tell her to take one active pill every day for 21 days and then start a new pack and keep taking active pills for up to 84 consecutive days, skipping the placebo pills until she wants to have her menstrual period. She can choose which week to have it: if the scheduled 12th week of an extended-cycle oral contraceptive regimen is inconvenient, she can plan it for week 10, or week 9, or whichever week is convenient.
The rationale for using an 84-day (12-week) cycle is that it still provides four periods per year, alleviating fears of hypertrophic endometrium.19
In this scenario, unscheduled or breakthrough bleeding can be managed by taking a “double-up pill” from a spare pack on any day breakthrough bleeding occurs and until it resolves. Menstrual periods should not be planned for intervals shorter than 21 days, owing to the risk of ovulation. Missed days of pills or use of placebo pills should also not exceed 7 days to prevent escape ovulation. 20
In some women with endometriosis and other medical reasons, continuous oral contraception with no placebo week can be prescribed.
Unfortunately, the downside to suppressing withdrawal bleeding is unscheduled or “breakthrough” bleeding. The best way to treat this unscheduled bleeding is not known. Patients who are not sexually active can be reassured that the goal of an atrophic endometrium can still be achieved, with resultant pill amenorrhea (particularly useful for those with severe dysmenorrhea or other reasons to want to avoid flow). Patients could also try to manage flow by periodically taking a 3- to 5-day break from hormone-containing pills to allow flow. They can also try switching to another oral contraceptive that has a different progestin that would spiral the arterioles of the endometrium more tightly and thus more aggressively induce atrophy.13,17,21 For instance, levonorgestrel is 10 to 20 times more potent than norethindrone. Choosing a pill with a higher monophasic dosing of levonorgestrel or a similar progestin may minimize unscheduled bleeding.
Currently, several oral contraceptives are approved for use in an extended regimen.
Seasonale was the first oral contraceptive marketed in the United States with an extended active regimen.22 It comes in a pack of 84 pills containing ethinyl estradiol 0.03 mg and levonorgestrel 0.15 mg, plus 7 placebo pills.
Seasonique is similar to Seasonale, but instead of placebo pills it has seven pills that contain ethinyl estradiol 0.010 mg.
Lybrel is a low-dose combination containing ethinyl estradiol 0.02 mg and levonorgestrel 0.09 mg. Packaged as an entire year’s worth of active pills to be taken continuously for 365 days without a placebo phase or pillfree interval,23 it is the only FDA-approved continuous oral contraceptive available in the United States.
An intrauterine device
Intrauterine devices were originally developed as contraceptives. The addition of a progestin to these devices has been shown to reduce heavy menstrual bleeding by up to 90%.24,25
Mirena IUS, a levonorgestrel-releasing device, is the only medicated intrauterine device that is currently available in the United States. (“IUS” stands for “intrauterine system.”) It was recently approved by the FDA to treat heavy menstrual bleeding in women who use intrauterine contraception as their method of pregnancy prevention.26 About 50% of women who use this device develop amenorrhea within 6 months of insertion, while 25% report oligomenorrhea.27
The Mirena device can be left in the uterus for up to 5 years. It may be a good choice for inducing amenorrhea in women with hemostatic disorders or in whom estrogen either is contraindicated or causes health concerns.18 The copper intrauterine device (Paragard; Duramed Pharmaceuticals Inc., Pomona, NY) remains a viable option for those who cannot or do not tolerate hormonal therapy. However, Mirena may provide less unscheduled bleeding than the copper intrauterine device.
Depot medroxyprogesterone acetate injections
Depo-Provera (depot medroxyprogesterone acetate) injections are given at 90-day intervals. 28 This contraceptive method inhibits ovulation and decidualizes the endometrium, thereby reducing or eliminating uterine bleeding. 29
While new users may initially experience excessive prolonged bleeding (10 or more days) while shedding their existing lining, the rate of amenorrhea has been shown to increase over time as the lining atrophies.30 Thus, prolonged use of this agent reduces the frequency of menstruation as well as menstruation-related symptoms.
Depot medroxyprogesterone acetate is ideal for patients whose menstrual periods pose a significant hygiene problem (eg, developmentally challenged girls). In our experience, the injections can be given at shorter intervals to induce atrophy of the endometrium quickly. In this scenario, the clinician might give an injection every 4 to 6 weeks for two or three doses to induce amenorrhea and then return to every-12-week dosing.
The main risk when using medroxyprogesterone injections to induce amenorrhea is the potential for bone loss. Users of this method have been shown to have lower mean bone mineral density31–33 and significantly higher levels of biomarkers of bone formation and resorption32,34 than nonusers. However, these changes are similar to those seen in breastfeeding women,35 are reversible with cessation, 36 and are not associated with increased fracture risk.37 In adolescent girls, pregnancy poses similar risks to the bones, with longerterm consequences.
Medroxyprogesterone can also stimulate appetite, causing 10 to 20 kg of weight gain in adolescents and women who are already obese and have trouble with appetite regulation.38 Slender users tend not to gain weight, however.
Given this information, depot medroxyprogesterone acetate appears to be a cost-effective contraceptive option that should be considered in the context of the clinical situation and preference of each patient.
Transdermal contraceptive patch
Ortho Evra, a transdermal patch, is designed to deliver ethinyl estradiol 0.02 mg and norelgestromin 0.150 mg daily.39 It is usually applied weekly for 3 weeks, followed by a patch-free week to induce regular monthly withdrawal bleeding.
Extended use of the patch to manipulate menstruation is an off-label use. In the only trial evaluating extended use of the patch, amenorrhea occurred in 12% of users, but unscheduled bleeding and spotting were common. 16
Although there is some evidence that the long-term use of the patch may increase the risk of venous thromboembolism,40,41 the risk in women who use the patch has been found to be similar to that in women using an oral contraceptive.42 However, serum ethinyl estradiol levels have been found to be higher with the use of the weekly patch than with oral contraceptives or the contraceptive vaginal ring39; as a result, many physicians are hesitant to recommend its continuous use.
Pending further data about the safety profile of this contraceptive, the World Health Organization Medical Eligibility Criteria for Contraceptive Use suggest that the same guidelines for the prescription of combination oral contraceptives should also apply to the patch.43
Contraceptive vaginal ring
NuvaRing, a contraceptive vaginal ring, releases a daily dose of ethinyl estradiol 0.015 mg and etonogestrel 0.12 mg.10 It is inserted, left in for 21 days, and then removed and left out for 7 days, during which withdrawal bleeding occurs.10
Vaginal administration has been shown to allow low, continuous dosing, which results in more stable serum concentrations than with the patch or oral contraceptives.39 In the only trial comparing an extended vaginal ring regimen and the traditional 28-day regimen, extended use resulted in fewer overall days of bleeding than monthly use, but with more unscheduled spotting.15
The most common side effects include headache, vaginitis, and leukorrhea,44 but there is no evidence of bacteriologic or cytologic changes in the cervicovaginal epithelium, even with extended use.45,46
Etonogestrel implantable contraceptive
Implanon, a single-rod progestin implant, is available in the United States and elsewhere. It is placed subdermally in the inner upper arm and provides contraception for as long as 3 years.
Implanon contains 68 mg of the progestin etonogestrel, which it slowly releases over time, initially at 0.06 to 0.07 mg/day, decreasing to 0.035 to 0.045 mg/day at the end of the first year, to 0.03 to 0.04 mg/day at the end of the second year, and then to 0.025 to 0.03 mg/day at the end of the third year.47
The amount of vaginal bleeding associated with the use of the implant is generally modest, but the pattern tends to be unpredictable. 48 In addition, because amenorrhea is reported as a side effect in only 22% of women during the first 2 years of its use,48 the progestin implant is a less satisfactory means of menstrual suppression than the other methods discussed above.
BENEFITS OF MENSTRUAL MANIPULATION
Menstrual manipulation has a number of benefits in terms of both overall health and lifestyle.
For most women, using a long-acting hormonal contraceptive carries low risks and substantial health benefits. Women who take oral contraceptives are less likely to develop osteoporosis, ovarian or endometrial cancer, benign breast changes, or pelvic inflammatory disease. 49 Long-term use of an oral contraceptive can also preserve fertility by reducing and delaying the incidence of endometriosis,50 and is effective at treating acne vulgaris, which tends to be common among patients with polycystic ovary syndrome.51,52 In addition, this practice can be used to reduce overall blood loss, an application that is particularly important in women with a bleeding diathesis such as von Willebrand disease, who frequently suffer from menorrhagia.53
Reduced menstruation may also prove more convenient during particular occasions, such as vacations and athletic activities. Specifically, it may be useful to women serving in the military. In a study by Schneider et al,54 a cohort of 83 female cadets reported a significant perceived impact of premenstrual and menstrual-related symptoms on academic, physical, and military activities, as well as difficulties in obtaining, changing, and disposing of menstrual materials in a military setting. Likewise, reduced menstrual frequency or amenorrhea may play an important role in female athletes, who reportedly use oral contraceptives to control premenstrual symptoms, to protect bone health, and to manipulate the menstrual cycle in order to maximize performance.55
Adolescent girls are another group who may benefit from reduced or absent menses, once they have reached near-final height. By practicing menstrual suppression, girls can avoid dysmenorrhea and the inconvenience of menstruation during the school day, when their access to painkillers, sanitary pads or tampons, and a change of clothes may be limited. 56 Clinicians who discuss with teenage patients the benefits of innovative hormonal contraceptive schedules that reduce menstrual frequency may be able to improve the quality of life for these young women.
In a very short girl just after menarche, care must be taken not to start a hormonal method too early so as not to prematurely close epiphyses and stunt final height; after menarche, most girls still have 1 to 4 inches of potential growth. For a young lady 4 feet 11 inches tall, that extra inch may be important.
Finally, menstrual manipulation may also find a niche among the developmentally challenged. Women with cognitive impairment and physical disabilities may have difficulty with hygienic practice around menses. For a number of years, contraceptives have been used to manage menstrual hygiene in patients with catamenial (ie, menstrual) epilepsy, and to address caregiver concerns in women with severe mental retardation, with improved behavior noted in some patients.57–59 In this setting, an agent that suppresses menses and also provides contraception, especially for those girls and women at risk of abuse, may offer substantial benefits.
DISADVANTAGES OF MENSTRUAL MANIPULATION
Rates of adverse events and of discontinuation of extended and continuous oral contraceptive regimens are comparable with those reported for cyclic regimens, except for higher rates of breakthrough bleeding.
In a trial of continuous oral contraceptive use in more than 2,000 patients, 396 (18.5%) withdrew from the study as a result of bothersome uterine bleeding.60 However, while breakthrough bleeding often occurs during the first few months of extended oral contraceptive use, it usually decreases with each successive cycle of therapy and is comparable to that reported by patients on the conventional oral contraceptive regimen by the fourth extended cycle.12
CONTRACEPTIVE EFFICACY
The efficacy of extended and continuous oral contraceptive regimens is comparable with that of cyclic regimens.12,60,61 One reason for this may be better adherence to continuous regimens: women using this regimen have been shown to miss fewer pills than those on a cyclic regimen, especially during the critical first week of the pill pack.21
Several studies have shown that some women ovulate during the standard 21/7 oral contraceptive regimen even if they do not miss any pills or take pills off-schedule, putting them at greater risk of pregnancy.62 Large studies evaluating the efficacy of an extendedcycle regimen have shown a pregnancy rate during the 1-year study period that was either comparable with61 or lower than12,60 rates with standard regimens.
Heterosexual couples need to be advised to use condoms to further reduce the already low failure rate and to prevent sexually transmitted diseases.
ACCEPTABILITY OF MENSTRUAL MANIPULATION
Ever since the earliest trial of an extended oral contraceptive regimen, participants have expressed a favorable response to the resulting decrease in menstrual frequency; in the 1977 study by Loudon et al,6 patients on the extended regimen cited infrequent periods (82%), fewer menstrual problems (20%), and easier pill-taking (19%) as favorable features.
In 1999, den Tonkelaar and Oddens63 surveyed 1,300 Dutch women about their preferred frequency of menstruation and found that about 70% between the ages of 15 and 49 preferred a frequency of between every 3 months and never. A similar survey in the United States indicated that 58% preferred a bleeding frequency of either every 3 months or never to more frequent periods.64
While patients find menstrual manipulation generally acceptable, clinician approval has been more varied. Loudon et al reported that “the doctors and nurses on the clinic staff were less enthusiastic about this regimen than the volunteers themselves.”6 In a survey of 222 clinicians,65 90% of responders reported ever having prescribed extended or continuous dosing regimens to adolescents, and 33% reported that extended cycles made up more than 10% of their total oral contraceptive prescriptions.
Myths and misperceptions about menstrual manipulation abound. Many clinicians believe that routine use of an extended or continuous oral contraceptive regimen is inadvisable, despite the lack of evidence to support this notion.66 Therefore, many care providers need more education about the practice and benefits of menstrual manipulation.
THE RIGHT METHOD FOR THE RIGHT PATIENT
Manipulation and suppression of menstruation through continuous or extended use of oral contraceptives or by other means may have a number of advantages to women, including fewer menstrual-related syndromes, reduced absenteeism from work or school, and greater overall satisfaction.
For women whose goal is to reduce but not necessarily to eliminate monthly bleeding, the cyclic use of estrogen-progestin contraception (rather than progestins alone or continuous use of combined hormonal preparations) is suggested.
Clinicians should not overestimate the risks of oral contraceptives and other hormonal methods, but rather educate themselves so that they can utilize menstrual manipulation safely to match the individual patient’s needs.
- Association of Reproductive Health Professionals. Extended and continuous use of contraceptives to reduce menstruation. September 2004. http://www.arhp.org/publications-and-resources/clinical-proceedings/reduce-menses. Accessed May 17, 2010.
- Kjerulff KH, Erickson BA, Langenberg PW. Chronic gynecological conditions reported by US women: findings from the National Health Interview Survey, 1984 to 1992. Am J Public Health 1996; 86:195–199.
- Thomas SL, Ellertson C. Nuisance or natural and healthy: should monthly menstruation be optional for women? Lancet 2000; 355:922–924.
- Connell EB. Contraception in the prepill era. Contraception 1999; 59(suppl 1):7S–10S.
- Marks LV. Sexual chemistry: a history of the contraceptive pill. New Haven, CT: Yale University Press, 2001.
- Loudon NB, Foxwell M, Potts DM, Guild AL, Short RV. Acceptability of an oral contraceptive that reduces the frequency of menstruation: the tri-cycle pill regimen. Br Med J 1977; 2:487–490.
- Sulak PJ, Cressman BE, Waldrop E, Holleman S, Kuehl TJ. Extending the duration of active oral contraceptive pills to manage hormone withdrawal symptoms. Obstet Gynecol 1997; 89:179–183.
- Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception 1997; 56:341–352.
- Miller L, Notter KM. Menstrual reduction with extended use of combination oral contraceptive pills: randomized controlled trial. Obstet Gynecol 2001; 98:771–778.
- Mulders TM, Dieben TO. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertil Steril 2001; 75:865–870.
- Stanford JB, Mikolajczyk RT. Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects. Am J Obstet Gynecol 2002; 187:1699–1708.
- Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception 2003; 68:89–96.
- Miller L, Hughes JP. Continuous combination oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstet Gynecol 2003; 101:653–661.
- Sillem M, Schneidereit R, Heithecker R, Mueck AO. Use of an oral contraceptive containing drospirenone in an extended regimen. Eur J Contracept Reprod Health Care 2003; 8:162–169.
- Miller L, Verhoeven CH, Hout J. Extended regimens of the contraceptive vaginal ring: a randomized trial. Obstet Gynecol 2005; 106:473–482.
- Stewart FH, Kaunitz AM, Laguardia KD, Karvois DL, Fisher AC, Friedman AJ. Extended use of transdermal norelgestromin/ethinyl estradiol: a randomized trial. Obstet Gynecol 2005; 105:1389–1396.
- Sulak PJ, Kuehl TJ, Coffee A, Willis S. Prospective analysis of occurrence and management of breakthrough bleeding during an extended oral contraceptive regimen. Am J Obstet Gynecol 2006; 195:935–941.
- Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel– releasing intrauterine system in women with hemostatic disorders. Fertil Steril 2008; 90:673–677.
- Anderson FD, Feldman R, Reape KZ. Endometrial effects of a 91-day extended-regimen oral contraceptive with low-dose estrogen in place of placebo. Contraception 2008; 77:91–96.
- Wright KP, Johnson JV. Evaluation of extended and continuous use oral contraceptives. Ther Clin Risk Manag 2008; 4:905–911.
- Edelman AB, Gallo MF, Jensen JT, Nichols MD, Schulz KF, Grimes DA. Continuous or extended cycle vs. cyclic use of combined oral contraceptives for contraception. Cochrane Database Syst Rev 2005; 3:CD004695.
- Sulak PJ, Kuehl TJ, Ortiz M, Shull BL. Acceptance of altering the standard 21-day/7-day oral contraceptive regimen to delay menses and reduce hormone withdrawal symptoms. Am J Obstet Gynecol 2002; 186:1142–1149.
- Turok D. The quest for better contraception: future methods. Obstet Gynecol Clin North Am 2007; 34:137–166.
- Bergqvist A, Rybo G. Treatment of menorrhagia with intrauterine release of progesterone. Br J Obstet Gynaecol 1983; 90:255–258.
- Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception 1994; 49:56–72.
- US Food and Drug Administration. FDA Approves Additional Use for IUD Mirena to Treat Heavy Menstrual Bleeding in IUD Users. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm184747.htm. Accessed May 17, 2010.
- Hidalgo M, Bahamondes L, Perrotti M, Diaz J, Dantas-Monteiro C, Petta C. Bleeding patterns and clinical performance of the levonorgestrel-releasing intrauterine system (Mirena) up to two years. Contraception 2002; 65:129–132.
- Schwallie PC, Assenzo JR. Contraceptive use—efficacy study utilizing medroxyprogesterone acetate administered as an intramuscular injection once every 90 days. Fertil Steril 1973; 24:331–339.
- Kaunitz AM. Injectable contraception. New and existing options. Obstet Gynecol Clin North Am 2000; 27:741–780.
- Mainwaring R, Hales HA, Stevenson K, et al. Metabolic parameter, bleeding, and weight changes in US women using progestin only contraceptives. Contraception 1995; 51:149–153.
- Curtis KM, Martins SL. Progestogen-only contraception and bone mineral density: a systematic review. Contraception 2006; 73:470–487.
- Shaarawy M, El-Mallah SY, Seoudi S, Hassan M, Mohsen IA. Effects of the long-term use of depot medroxyprogesterone acetate as hormonal contraceptive on bone mineral density and biochemical markers of bone remodeling. Contraception 2006; 74:297–302.
- Cromer BA, Bonny AE, Stager M, et al. Bone mineral density in adolescent females using injectable or oral contraceptives: a 24-month prospective study. Fertil Steril 2008; 90:2060–2067.
- Rome E, Ziegler J, Secic M, et al. Bone biochemical markers in adolescent girls using either depot medroxyprogesterone acetate or an oral contraceptive. J Pediatr Adolesc Gynecol 2004; 17:373–377.
- More C, Bettembuk P, Bhattoa HP, Balogh A. The effects of pregnancy and lactation on bone mineral density. Osteoporos Int 2001; 12:732–737.
- Kaunitz AM, Miller PD, Rice VM, Ross D, McClung MR. Bone mineral density in women aged 25-35 years receiving depot medroxyprogesterone acetate: recovery following discontinuation. Contraception 2006; 74:90–99.
- Guilbert ER, Brown JP, Kaunitz AM, et al. The use of depot-medroxyprogesterone acetate in contraception and its potential impact on skeletal health. Contraception 2009; 79:167–177.
- Bonny AE, Ziegler J, Harvey R, Debanne SM, Secic M, Cromer BA. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch Pediatr Adolesc Med 2006; 160:40–45.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Douketis JD, Ginsberg JS, Holbrook A, Crowther M, Duku EK, Burrows RF. A reevaluation of the risk for venous thromboembolism with the use of oral contraceptives and hormone replacement therapy. Arch Intern Med 1997; 157:1522–1530.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 3rd ed. Geneva: Reproductive Health and Research, World Health Organization; 2004.
- Dieben TO, Roumen FJ, Apter D. Efficacy, cycle control, and user acceptability of a novel combined contraceptive vaginal ring. Obstet Gynecol 2002; 100:585–593.
- Davies GC, Feng LX, Newton JR, Dieben TO, Coelingh-Bennink HJ. The effects of a combined contraceptive vaginal ring releasing ethinyloestradiol and 3-ketodesogestrel on vaginal flora. Contraception 1992; 45:511–518.
- Roumen FJ, Boon ME, van Velzen D, Dieben TO, Coelingh Bennink HJ. The cervico-vaginal epithelium during 20 cycles’ use of a combined contraceptive vaginal ring. Hum Reprod 1996; 11:2443–2448.
- Wenzl R, van Beek A, Schnabel P, Huber J. Pharmacokinetics of etonogestrel released from the contraceptive implant Implanon. Contraception 1998; 58:283–288.
- Darney P, Patel A, Rosen K, Shapiro LS, Kaunitz AM. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril 2009; 91:1646–1653.
- Jensen JT, Speroff L. Health benefits of oral contraceptives. Obstet Gynecol Clin North Am 2000; 27:705–721.
- Seracchioli R, Mabrouk M, Frascà C, et al. Long-term cyclic and continuous oral contraceptive therapy and endometrioma recurrence: a randomized controlled trial. Fertil Steril 2010; 93:52–56.
- Falsetti L, Dordoni D, Gastaldi C, Gastaldi A. A new association of ethinylestradiol (0.035 mg) cyproterone acetate (2 mg) in the therapy of polycystic ovary syndrome. Acta Eur Fertil 1986; 17:19–25.
- Koltun W, Lucky AW, Thiboutot D, et al. Efficacy and safety of 3 mg drospirenone/20 mcg ethinylestradiol oral contraceptive administered in 24/4 regimen in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled trial. Contraception 2008; 77:249–256.
- Kadir RA, Sabin CA, Pollard D, Lee CA, Economides DL. Quality of life during menstruation in patients with inherited bleeding disorders. Haemophilia 1998; 4:836–841.
- Schneider MB, Fisher M, Friedman SB, Bijur PE, Toffler AP. Menstrual and premenstrual issues in female military cadets: a unique population with significant concerns. J Pediatr Adolesc Gynecol 1999; 12:195–201.
- Bennell K, White S, Crossley K. The oral contraceptive pill: a revolution for sportswomen? Br J Sports Med 1999; 33:231–238.
- Kaplowitz PB, Oberfield SE. Reexamination of the age limit for defining when puberty is precocious in girls in the United States: implications for evaluation and treatment. Drug and Therapeutics and Executive Committees of the Lawson Wilkins Pediatric Endocrine Society. Pediatrics 1999; 104:936–941.
- Roxburgh DR, West MJ. The use of norethisterone to suppress menstruation in the intellectually severely retarded woman. Med J Aust 1973; 2:310–313.
- Egan TM, Siegert RJ, Fairley NA. Use of hormonal contraceptives in an institutional setting: reasons for use, consent and safety in women with psychiatric and intellectual disabilities. N Z Med J 1993; 106:338–341.
- Pillai M, O’Brien K, Hill E. The levonorgestrel intrauterine system (Mirena) for the treatment of menstrual problems in adolescents with medical disorders, or physical or learning disabilities. BJOG 2010; 117:216–221.
- Archer DF, Jensen JT, Johnson JV, Borisute H, Grubb GS, Constantine GD. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception 2006; 74:439–445.
- Kroll R, Reape KZ, Margolis M. The efficacy and safety of a low-dose, 91-day, extended-regimen oral contraceptive with continuous ethinyl estradiol. Contraception 2010; 81:41–48.
- Archer DF. Menstrual-cycle-related symptoms: a review of the rationale for continuous use of oral contraceptives. Contraception 2006; 74:359–366.
- den Tonkelaar I, Oddens BJ. Preferred frequency and characteristics of menstrual bleeding in relation to reproductive status, oral contraceptive use, and hormone replacement therapy use. Contraception 1999; 59:357–362.
- Edelman A, Lew R, Cwiak C, Nichols M, Jensen J. Acceptability of contraceptive-induced amenorrhea in a racially diverse group of US women. Contraception 2007; 75:450–453.
- Gerschultz KL, Sucato GS, Hennon TR, Murray PJ, Gold MA. Extended cycling of combined hormonal contraceptives in adolescents: physician views and prescribing practices. J Adolesc Health 2007; 40:151–157.
- Frankovich RJ, Lebrun CM. Menstrual cycle, contraception, and performance. Clin Sports Med 2000; 19:251–271.
- Speroff L, Darney PD. A Clinical Guide for Contraception. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
- Kaunitz AM. Long-acting contraceptive options. Int J Fertil Menopausal Stud 1996; 41:69–76.
- US Food and Drug Administration. Guidance for Industry Labeling for Combined Oral Contraceptives, 2004. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm075075.pdfAccessed May 17, 2010.
- Association of Reproductive Health Professionals. Extended and continuous use of contraceptives to reduce menstruation. September 2004. http://www.arhp.org/publications-and-resources/clinical-proceedings/reduce-menses. Accessed May 17, 2010.
- Kjerulff KH, Erickson BA, Langenberg PW. Chronic gynecological conditions reported by US women: findings from the National Health Interview Survey, 1984 to 1992. Am J Public Health 1996; 86:195–199.
- Thomas SL, Ellertson C. Nuisance or natural and healthy: should monthly menstruation be optional for women? Lancet 2000; 355:922–924.
- Connell EB. Contraception in the prepill era. Contraception 1999; 59(suppl 1):7S–10S.
- Marks LV. Sexual chemistry: a history of the contraceptive pill. New Haven, CT: Yale University Press, 2001.
- Loudon NB, Foxwell M, Potts DM, Guild AL, Short RV. Acceptability of an oral contraceptive that reduces the frequency of menstruation: the tri-cycle pill regimen. Br Med J 1977; 2:487–490.
- Sulak PJ, Cressman BE, Waldrop E, Holleman S, Kuehl TJ. Extending the duration of active oral contraceptive pills to manage hormone withdrawal symptoms. Obstet Gynecol 1997; 89:179–183.
- Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C. Contraception 1997; 56:341–352.
- Miller L, Notter KM. Menstrual reduction with extended use of combination oral contraceptive pills: randomized controlled trial. Obstet Gynecol 2001; 98:771–778.
- Mulders TM, Dieben TO. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertil Steril 2001; 75:865–870.
- Stanford JB, Mikolajczyk RT. Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects. Am J Obstet Gynecol 2002; 187:1699–1708.
- Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception 2003; 68:89–96.
- Miller L, Hughes JP. Continuous combination oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstet Gynecol 2003; 101:653–661.
- Sillem M, Schneidereit R, Heithecker R, Mueck AO. Use of an oral contraceptive containing drospirenone in an extended regimen. Eur J Contracept Reprod Health Care 2003; 8:162–169.
- Miller L, Verhoeven CH, Hout J. Extended regimens of the contraceptive vaginal ring: a randomized trial. Obstet Gynecol 2005; 106:473–482.
- Stewart FH, Kaunitz AM, Laguardia KD, Karvois DL, Fisher AC, Friedman AJ. Extended use of transdermal norelgestromin/ethinyl estradiol: a randomized trial. Obstet Gynecol 2005; 105:1389–1396.
- Sulak PJ, Kuehl TJ, Coffee A, Willis S. Prospective analysis of occurrence and management of breakthrough bleeding during an extended oral contraceptive regimen. Am J Obstet Gynecol 2006; 195:935–941.
- Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel– releasing intrauterine system in women with hemostatic disorders. Fertil Steril 2008; 90:673–677.
- Anderson FD, Feldman R, Reape KZ. Endometrial effects of a 91-day extended-regimen oral contraceptive with low-dose estrogen in place of placebo. Contraception 2008; 77:91–96.
- Wright KP, Johnson JV. Evaluation of extended and continuous use oral contraceptives. Ther Clin Risk Manag 2008; 4:905–911.
- Edelman AB, Gallo MF, Jensen JT, Nichols MD, Schulz KF, Grimes DA. Continuous or extended cycle vs. cyclic use of combined oral contraceptives for contraception. Cochrane Database Syst Rev 2005; 3:CD004695.
- Sulak PJ, Kuehl TJ, Ortiz M, Shull BL. Acceptance of altering the standard 21-day/7-day oral contraceptive regimen to delay menses and reduce hormone withdrawal symptoms. Am J Obstet Gynecol 2002; 186:1142–1149.
- Turok D. The quest for better contraception: future methods. Obstet Gynecol Clin North Am 2007; 34:137–166.
- Bergqvist A, Rybo G. Treatment of menorrhagia with intrauterine release of progesterone. Br J Obstet Gynaecol 1983; 90:255–258.
- Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception 1994; 49:56–72.
- US Food and Drug Administration. FDA Approves Additional Use for IUD Mirena to Treat Heavy Menstrual Bleeding in IUD Users. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm184747.htm. Accessed May 17, 2010.
- Hidalgo M, Bahamondes L, Perrotti M, Diaz J, Dantas-Monteiro C, Petta C. Bleeding patterns and clinical performance of the levonorgestrel-releasing intrauterine system (Mirena) up to two years. Contraception 2002; 65:129–132.
- Schwallie PC, Assenzo JR. Contraceptive use—efficacy study utilizing medroxyprogesterone acetate administered as an intramuscular injection once every 90 days. Fertil Steril 1973; 24:331–339.
- Kaunitz AM. Injectable contraception. New and existing options. Obstet Gynecol Clin North Am 2000; 27:741–780.
- Mainwaring R, Hales HA, Stevenson K, et al. Metabolic parameter, bleeding, and weight changes in US women using progestin only contraceptives. Contraception 1995; 51:149–153.
- Curtis KM, Martins SL. Progestogen-only contraception and bone mineral density: a systematic review. Contraception 2006; 73:470–487.
- Shaarawy M, El-Mallah SY, Seoudi S, Hassan M, Mohsen IA. Effects of the long-term use of depot medroxyprogesterone acetate as hormonal contraceptive on bone mineral density and biochemical markers of bone remodeling. Contraception 2006; 74:297–302.
- Cromer BA, Bonny AE, Stager M, et al. Bone mineral density in adolescent females using injectable or oral contraceptives: a 24-month prospective study. Fertil Steril 2008; 90:2060–2067.
- Rome E, Ziegler J, Secic M, et al. Bone biochemical markers in adolescent girls using either depot medroxyprogesterone acetate or an oral contraceptive. J Pediatr Adolesc Gynecol 2004; 17:373–377.
- More C, Bettembuk P, Bhattoa HP, Balogh A. The effects of pregnancy and lactation on bone mineral density. Osteoporos Int 2001; 12:732–737.
- Kaunitz AM, Miller PD, Rice VM, Ross D, McClung MR. Bone mineral density in women aged 25-35 years receiving depot medroxyprogesterone acetate: recovery following discontinuation. Contraception 2006; 74:90–99.
- Guilbert ER, Brown JP, Kaunitz AM, et al. The use of depot-medroxyprogesterone acetate in contraception and its potential impact on skeletal health. Contraception 2009; 79:167–177.
- Bonny AE, Ziegler J, Harvey R, Debanne SM, Secic M, Cromer BA. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch Pediatr Adolesc Med 2006; 160:40–45.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, Kaptein MC. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception 2005; 72:168–174.
- Douketis JD, Ginsberg JS, Holbrook A, Crowther M, Duku EK, Burrows RF. A reevaluation of the risk for venous thromboembolism with the use of oral contraceptives and hormone replacement therapy. Arch Intern Med 1997; 157:1522–1530.
- Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol 2007; 109:339–346.
- Jick S, Kaye JA, Li L, Jick H. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception 2007; 76:4–7.
- World Health Organization. Medical Eligibility Criteria for Contraceptive Use. 3rd ed. Geneva: Reproductive Health and Research, World Health Organization; 2004.
- Dieben TO, Roumen FJ, Apter D. Efficacy, cycle control, and user acceptability of a novel combined contraceptive vaginal ring. Obstet Gynecol 2002; 100:585–593.
- Davies GC, Feng LX, Newton JR, Dieben TO, Coelingh-Bennink HJ. The effects of a combined contraceptive vaginal ring releasing ethinyloestradiol and 3-ketodesogestrel on vaginal flora. Contraception 1992; 45:511–518.
- Roumen FJ, Boon ME, van Velzen D, Dieben TO, Coelingh Bennink HJ. The cervico-vaginal epithelium during 20 cycles’ use of a combined contraceptive vaginal ring. Hum Reprod 1996; 11:2443–2448.
- Wenzl R, van Beek A, Schnabel P, Huber J. Pharmacokinetics of etonogestrel released from the contraceptive implant Implanon. Contraception 1998; 58:283–288.
- Darney P, Patel A, Rosen K, Shapiro LS, Kaunitz AM. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril 2009; 91:1646–1653.
- Jensen JT, Speroff L. Health benefits of oral contraceptives. Obstet Gynecol Clin North Am 2000; 27:705–721.
- Seracchioli R, Mabrouk M, Frascà C, et al. Long-term cyclic and continuous oral contraceptive therapy and endometrioma recurrence: a randomized controlled trial. Fertil Steril 2010; 93:52–56.
- Falsetti L, Dordoni D, Gastaldi C, Gastaldi A. A new association of ethinylestradiol (0.035 mg) cyproterone acetate (2 mg) in the therapy of polycystic ovary syndrome. Acta Eur Fertil 1986; 17:19–25.
- Koltun W, Lucky AW, Thiboutot D, et al. Efficacy and safety of 3 mg drospirenone/20 mcg ethinylestradiol oral contraceptive administered in 24/4 regimen in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled trial. Contraception 2008; 77:249–256.
- Kadir RA, Sabin CA, Pollard D, Lee CA, Economides DL. Quality of life during menstruation in patients with inherited bleeding disorders. Haemophilia 1998; 4:836–841.
- Schneider MB, Fisher M, Friedman SB, Bijur PE, Toffler AP. Menstrual and premenstrual issues in female military cadets: a unique population with significant concerns. J Pediatr Adolesc Gynecol 1999; 12:195–201.
- Bennell K, White S, Crossley K. The oral contraceptive pill: a revolution for sportswomen? Br J Sports Med 1999; 33:231–238.
- Kaplowitz PB, Oberfield SE. Reexamination of the age limit for defining when puberty is precocious in girls in the United States: implications for evaluation and treatment. Drug and Therapeutics and Executive Committees of the Lawson Wilkins Pediatric Endocrine Society. Pediatrics 1999; 104:936–941.
- Roxburgh DR, West MJ. The use of norethisterone to suppress menstruation in the intellectually severely retarded woman. Med J Aust 1973; 2:310–313.
- Egan TM, Siegert RJ, Fairley NA. Use of hormonal contraceptives in an institutional setting: reasons for use, consent and safety in women with psychiatric and intellectual disabilities. N Z Med J 1993; 106:338–341.
- Pillai M, O’Brien K, Hill E. The levonorgestrel intrauterine system (Mirena) for the treatment of menstrual problems in adolescents with medical disorders, or physical or learning disabilities. BJOG 2010; 117:216–221.
- Archer DF, Jensen JT, Johnson JV, Borisute H, Grubb GS, Constantine GD. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception 2006; 74:439–445.
- Kroll R, Reape KZ, Margolis M. The efficacy and safety of a low-dose, 91-day, extended-regimen oral contraceptive with continuous ethinyl estradiol. Contraception 2010; 81:41–48.
- Archer DF. Menstrual-cycle-related symptoms: a review of the rationale for continuous use of oral contraceptives. Contraception 2006; 74:359–366.
- den Tonkelaar I, Oddens BJ. Preferred frequency and characteristics of menstrual bleeding in relation to reproductive status, oral contraceptive use, and hormone replacement therapy use. Contraception 1999; 59:357–362.
- Edelman A, Lew R, Cwiak C, Nichols M, Jensen J. Acceptability of contraceptive-induced amenorrhea in a racially diverse group of US women. Contraception 2007; 75:450–453.
- Gerschultz KL, Sucato GS, Hennon TR, Murray PJ, Gold MA. Extended cycling of combined hormonal contraceptives in adolescents: physician views and prescribing practices. J Adolesc Health 2007; 40:151–157.
- Frankovich RJ, Lebrun CM. Menstrual cycle, contraception, and performance. Clin Sports Med 2000; 19:251–271.
- Speroff L, Darney PD. A Clinical Guide for Contraception. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.
- Kaunitz AM. Long-acting contraceptive options. Int J Fertil Menopausal Stud 1996; 41:69–76.
- US Food and Drug Administration. Guidance for Industry Labeling for Combined Oral Contraceptives, 2004. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm075075.pdfAccessed May 17, 2010.
KEY POINTS
- The options for menstrual manipulation are extended or continuous regimens of oral, transdermal, or vaginal hormonal contraceptives; a levonorgestrel-releasing intrauterine device; a progestin implant; and depot medroxyprogesterone injections.
- Benefits include fewer menstrual-related syndromes, less absenteeism from work or school, and greater overall satisfaction. Medical indications for it are conditions exacerbated by hormonal changes around the time of menses.
- The main disadvantage is a higher rate of breakthrough bleeding.
- Myths and misperceptions about menstrual manipulation persist; some physicians believe it is somehow inadvisable.
A 40-year-old man with spells of generalized weakness and paresthesias
A 40-year-old man who works as a roofer began, 1 week ago, to experience episodes of generalized weakness, perioral numbness, and diffuse paresthesias. In the past he has had recurring nosebleeds but no history of other medical conditions.
His recent “spells” come on abruptly and spontaneously, without warning, and last about 15 minutes. He never loses consciousness, but he reports a feeling of derealization or an out-of-body experience—he can hear the people around him talking during the spells, but he feels that everything is far away. He has been having about three episodes per day. They typically occur after mild exertion or heavy lifting, and each episode resolves with complete rest. He has had no nausea, vomiting, loss of bowel or bladder control, fever, chills, or traumatic brain injury.
The patient first reported to the emergency department of a local hospital for evaluation. There, he underwent computed tomography (CT) of the head without contrast, which showed nothing abnormal. However, he had an episode while in the emergency department, which prompted his physician to admit him to the hospital.
In the hospital, he underwent an extensive medical evaluation. CT angiography revealed no evidence of vasculitis or occlusive disease. Results of electroencephalography during these spells were normal. Results of magnetic resonance imaging of the cervical and lumbar spine were also normal.
A neurologist was consulted. Concerned that the spells were due to paradoxical emboli coming through a patent foramen ovale, the neurologist recommended transthoracic echocardiography with agitated saline. This study showed a normal ejection fraction and a right-to-left shunt through a left pulmonary arteriovenous malformation (AVM). Unfortunately, the shunt fraction could not be estimated because the patient had another episode during the procedure, and so the procedure was cut short. CT of the chest confirmed a large AVM in the upper lobe of the left lung (Figure 1).
The patient is transferred
The patient’s physician requested that he be transferred to Mayo Clinic for further evaluation.
When he arrived, we performed a complete physical examination, in which we noted scattered erythematous maculopapular telangiectases in the lower lips and significant digital clubbing (Figure 2). He could not recall any family members having rheumatologic or cardiovascular diseases, but he recalled that his father has oral telangiectases and recurrent epistaxis.
His examination was interrupted by yet another spell, during which his oxygen saturation fell to 85%. We immediately started giving him oxygen by nasal cannula, which raised his oxygen saturation to 96%, and the spell promptly ended.
After his physical examination was completed and his records from the other hospital were reviewed, a diagnosis was made. No further diagnostic studies were pursued.
WHICH IS THE MOST LIKELY DIAGNOSIS?
1. Based on the information available, which of the following is the most likely diagnosis?
- Generalized tonic-clonic seizures
- Osler-Weber-Rendu disease
- Subarachnoid hemorrhage
- Conversion disorder
- Atrial septal defect
Generalized tonic-clonic seizures begin with abrupt loss of consciousness, followed by stiffening of the body and extremities. This is the tonic phase, which may last for 1 minute. The clonic phase follows, characterized by abnormal jerking and teeth-clenching (raising the concern that the patient will bite his or her tongue). The clonic phase lasts 1 to 2 minutes. After a seizure, confusion and headache are common. On electroencephalography, epileptiform abnormalities are documented in about 23% of patients with a first documented seizure.1
Our patient’s history of remaining fully conscious and of having normal electroencephalographic findings during his spells does not suggest generalized tonic-clonic seizures.
Osler-Weber-Rendu disease is also known as hereditary hemorrhagic telangiectasia (HHT). Its pathophysiology is complex, and it is believed to be related to mutations in an endothelial protein2 that lead to abnormal vascular structures. The estimated prevalence in European studies is 1 in 5,000; in Japanese studies it is 1 in 8,000.3–4
The diagnosis of HHT is based on four clinical criteria:
- Spontaneous and recurrent epistaxis
- Multiple mucocutaneous telangiectases
- Pulmonary, cerebral, or gastrointestinal AVMs
- A first-degree relative with the disease.
The presence of three or four of these criteria establishes a “definite” diagnosis, while fewer than two makes it “unlikely.”5 Since the spectrum of this disease is wide, varying from mild epistaxis to iron-deficiency anemia, its diagnosis is often missed.6
Our patient meets at least three of the criteria—recurrent epistaxis, oral telangiectases, and a CT-documented pulmonary AVM. His father has a history of oral telangiectases and epistaxis but was never formally diagnosed with HHT. The patient presented with spells of weakness and paresthesias from worsening hypoxemia due to an enlarged pulmonary AVM. Thus, based on these features, HHT is the most likely diagnosis.
Subarachnoid hemorrhage is commonly from a ruptured cerebral aneurysm. Common symptoms include sudden, severe headaches with focal neurologic deficits, a stiff neck, brief loss of consciousness, nausea, and vomiting.7
Our patient’s CT scan showed no intracranial bleeding, and CT angiography showed no evidence of aneurysm. Thus, he has neither clinical nor radiographic features of subarachnoid hemorrhage.
Conversion disorder is typically associated with psychological stressors.8 It is characterized by the sudden onset of neurologic deficits such as blindness, paralysis, and numbness that cannot be explained by a general medical condition.
Our patient has a known pulmonary AVM with clinical and laboratory findings of hypoxemia that explain his spells. Therefore, the diagnosis of conversion disorder cannot be made.
A right-to-left intracardiac shunt can be present in patients with patent foramen ovale, atrial septal defects with shunt reversal, Eisenmenger syndrome, or tetralogy of Fallot (even in adults). It can present with hypoxemia and neurologic weakness.
Our patient’s echocardiogram ruled out these conditions.
MANIFESTATIONS OF HEREDITARY HEMORRHAGIC TELANGIECTASIA
2. Which is the most common clinical manifestation of HHT?
- Epistaxis
- Mucocutaneous telangiectases
- Hematochezia
- Dyspnea
Epistaxis is the most common presentation, occurring in more than 90% of patients.9 Many patients experience only mild occasional nosebleeds that are not frequent or severe enough to cause anemia or to lead to medical treatment or consultation. Others, however, have heavy, frequent bleeding that requires invasive interventions.10
Mucocutaneous telangiectases are the second most common clinical manifestation, documented in about 75% of patients. They are cosmetically unpleasant but rarely bleed. They occur most commonly on the face, lips, tongue, and fingertips, and they increase in size and number with age.11
Gastrointestinal bleeding, sometimes manifesting as hematochezia, occurs in one-third of people with HHT. It most commonly presents with iron-deficiency anemia in patients over age 40.12
Dyspnea. Pulmonary AVMs occur in 30% to 50% of affected people, but interestingly, most patients with pulmonary AVMs have no respiratory symptoms, including dyspnea.
In pulmonary AVMs, abnormal vessels replace normal capillary beds, creating a capillary-free communication between the pulmonary and systemic circulations. This abnormal connection prevents blood from the pulmonary arterial system from being oxygenated, resulting in hypoxemia and secondary polycythemia, as in our patient. One-third of patients have evidence of right-to-left shunting, such as the clubbing in our patient.9,13
Other, less common complications of HHT include seizures or hemorrhage from cerebral AVMs and stroke and brain abscesses from paradoxical embolization due to the loss of the capillary filter in the pulmonary vascular bed. Hepatic involvement may result in portal hypertension and hepatic encephalopathy.14
Back to our patient
As mentioned above, during one of the patient’s spells of paresthesia and weakness, we noted his oxygen saturation by oximetry was 85%. At that time, his arterial Po2 was also low at 50 mm Hg (normal 70–100). With oxygen supplementation, his spell completely resolved and his Po2 improved to 80 mm Hg. Though the shunt fraction of his pulmonary AVM was never measured, it was likely less than 30% of the cardiac output, as his hypoxemia improved with oxygen supplementation alone.15 When he was taken off oxygen supplementation, his spells recurred, but with oxygen support he remained clinically stable.
MANAGEMENT
3. Which is the next logical step in our patient’s management?
- Consult a surgeon for lobectomy
- Consult an interventional radiologist for embolization therapy
- Transfer to the intensive care unit for elective intubation
- Observe with close follow-up
Untreated pulmonary AVMs enlarge at an estimated rate of 0.3 mm/year. The estimated death rate is up to 15.8% per year, with most deaths resulting from stroke, cerebral abscess, hemoptysis, and hemothorax.16–18 Common indications for treatment are progressively enlarging lesions, symptomatic hypoxemia, and paradoxical embolization.19 Pulmonary AVMs in which the feeding artery is 3 mm or more in diameter require treatment.
Embolization therapy, in which the AVM is occluded angiographically, is considered a first-line treatment for pulmonary AVM, with a procedural success rate (defined as involution of the AVM) of 97%.20 Embolization therapy allows patients to avoid major surgery, with its potential complications, and it has a shorter recovery time.
Surgical procedures such as excision, vascular ligation, or lobectomy can be considered if the lesion cannot be treated by embolization or if the patient has an anaphylactic allergy to contrast dyes.
This patient had no clinical signs of impending respiratory failure requiring elective intubation.
Since he was experiencing symptoms, there is no role for observation in this case.
Back to our patient
An interventional radiologist was consulted, and the patient underwent bilateral pulmonary artery angiography with successful coil embolization of his large left-upper-lobe AVM. He was weaned off oxygen and had no further spells of generalized weakness and paresthesias.
Given his father’s history of recurrent epistaxis and oral telangiectases, the patient asks about the risk of his children acquiring this disease.
GENETICS OF HEREDITARY HEMORRHAGIC TELANGIECTASIA
4. Which of the following is the inheritance pattern for HHT?
- Autosomal dominant
- Autosomal recessive
- Maternal inheritance
- X-linked recessive
The inheritance pattern is autosomal dominant with variable expression and penetrance. At least four different mutations have been identified in genes on chromosomes 9 and 12 that result in abnormal vascular malformations.21–24 The other modes of inheritance have not been described in HHT.
RECOMMENDATIONS FOR OUR PATIENT
5. Which of the following is not recommended for our patient?
- Consideration of genetic testing
- Consideration of screening of first-degree relatives
- Dental prophylaxis
- Scuba diving
Genetic testing. The molecular diagnosis of HHT is primarily based on sequencing of the entire coding regions of the ENG and ALK1 genes on chromosomes 9 and 12, respectively. The interpretation of these results is quite complex. The clinical genetics laboratories in North America that currently offer molecular diagnostic testing for HHT recommend that testing be coordinated and ordered through a center that specializes in this disease or by a genetics professional. Testing of the index case is performed to confirm the clinical diagnosis and also to determine if genetic testing will be possible in at-risk relatives. Further genetic testing should be pursued in at-risk family members only if the index case has a positive result.25
Screening of relatives. Given that HHT is an autosomal dominant disease, the current practice is to offer molecular genetic screening early in life for first-degree relatives.25,26 The external signs such as telangiectases and nosebleeds may not manifest until the second or third decade of life. However, AVMs in the brain, spinal cord, lungs, and liver are usually congenital and may present suddenly and with serious complications, even in childhood.
Dental prophylaxis. People with HHT and a pulmonary AVM are at risk of bacteremia and consequent brain abscesses after dental procedures. Antibiotic prophylaxis is therefore highly recommended.27
One sport to avoid. There have been several case reports of paradoxical air emboli occurring in patients with HHT complicated by a pulmonary AVM. Hsu et al28 reported a 31-year-old man with an undiagnosed large pulmonary AVM and HHT who became comatose with diffuse bilateral hemispheric brain swelling on head CT after scuba diving, due to air embolism.
The HHT Foundation International recommends that people with this disease avoid scuba diving (the only sport to be avoided) owing to the risk of air emboli from small lung AVMs. It also recommends that patients alert health care providers about their risk of air embolism whenever intravenous access is being established.
Back to our patient
The patient met with a geneticist, and blood was collected for genetic testing before he was sent home. Additionally, the need to screen his first-degree relatives was thoroughly discussed. Four days after discharge he returned to work, and his spells have not recurred. He has a follow-up appointment scheduled with a pulmonologist specializing in this disease for the results of genetic testing and for continued management.
TAKE-HOME POINTS
- The diagnosis of HHT is based on the following four clinical criteria: spontaneous or recurrent epistaxis, multiple mucocutaneous telangiectases, visceral involvement (eg, cerebral, pulmonary, or gastrointestinal AVM), and a first-degree relative with this disease.
- The diagnosis may be confirmed with genetic testing.
- The diagnosis may be underreported, given the wide spectrum of disease presentation, from inconsequential epistaxis to massive gastrointestinal bleeding.
- HHT is autosomal dominant, and therefore all first-degree relatives should be screened.
- Krumholz A, Wiebe S, Gronseth G, et al. Practice parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2007; 69:1996–2007.
- Bourdeau A, Dumont DJ, Letarte M. A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest 1999; 104:1343–1351.
- Dakeishi M, Shioya T, Wada Y, et al. Genetic epidemiology of hereditary hemorrhagic telangiectasia in a local community in the northern part of Japan. Hum Mutat 2002; 19:140–148.
- Guttmacher AE, Marchuk DA, White RI. Hereditary hemorrhagic telangiectasia. N Engl J Med 1995; 333:918–924.
- Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 2000; 91:66–67.
- Gallitelli M, Pasculli G, Fiore T, Carella A, Sabbà C. Emergencies in hereditary haemorrhagic telangiectasia. QJM 2006; 99:15–22.
- Gorelick PB, Hier DB, Caplan LR, Langenberg P. Headache in acute cerebrovascular disease. Neurology 1986; 36:1445–1450.
- Stonnington CM, Barry JJ, Fisher RS. Conversion disorder. Am J Psychiatry 2006; 163:1510–1517.
- Shovlin CL, Letarte M. Hereditary hemorrhagic telangiectasia and pulmonary arteriovenous malformations: issues in clinical management and review of pathogenic mechanisms. Thorax 1999; 54:714–729.
- AAssar OS, Friedman CM, White RI. The natural history of epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope 1991; 101:977–980.
- McAllister KA, Lennon F, Bowles-Biesecker B, et al. Genetic heterogenicity in hereditary haemorrhagic telangiectasia: possible correlation with clinical phenotype. J Med Genet 1994; 31:927–932.
- Plauchu H, de Chadarevian JP, Bideau A, Robert JM. Age-related clinical profile of hereditary hemorrhagic telangiectasia in an epidemiologically recruited population. Am J Med Genet 1989; 32:291–297.
- Shovlin CL, Jaskson JE, Bamford KB, et al. Primary determinants of ischaemic stroke/brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax 2008; 63:259–266.
- Garcia-Tsao G, Korzenik JR, Young L, et al. Liver disease in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 2000; 343:931–936.
- Kolleft MH, Micek ST. Critical care. In:Cooper DH, Krainik AJ, Lubner SJ, Reno HEL, editors. Washington Manual of Medical Therapeutics. 32nd ed. Philadelphia: Lippincott Williams & Wilkins, 2007:224–230.
- Swanson KL, Prakash UB, Stanson AW. Pulmonary arteriovenous fistulas: Mayo Clinic experience: 1872–1997. Mayo Clin Proc 1999; 74:671–680.
- Dines DE, Arms RA, Bernatz PE, Gomes MR. Pulmonary arteriovenous fistulas. Mayo Clinic Proc 1974; 49:460–465.
- Sluiter-Eringa H, Orie NG, Sluiter HJ. Pulmonary arteriovenous fistula: diagnosis and prognosis in noncompliant patients. Am Rev Respir Dis 1969; 100:177–188.
- Dines DE, Seward JB, Bernatz PE. Pulmonary arteriovenous fistula. Mayo Clin Proc 1983; 58:176–181.
- Pollak JS, Saluja S, Thabet A, Henderson KJ, Denbow N, White RI. Clinical and anatomic outcomes after embolotherapy of pulmonary arteriovenous malformations. J Vasc Interv Radio 2006; 17:35–44.
- Berg JN, Gallion CJ, Stenzel TT, et al. The activin receptor-like kinase 1 gene: genomic structure and mutations in hereditary hemorrhagic telangiectasia type 2. Am J Hum Genet 1997; 61:60–67.
- McAllister KA, Grogg KM, Johnson DW, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary hemorrhagic telangiectasias type 1. Nat Genet 1994; 8:345–351.
- Johnson DW, Berg JN, Baldwin MA, et al. Mutations in the activin receptor-like kinase gene in hereditary haemorrhagic telangeictasia type 2. Nat Genet 1996; 13:189–195.
- Abdalla SA, Letarte M. Hereditary haemorrhagic telangiectasia: current views on genetics and mechanisms of disease. J Med Genet 2006; 43:97–110.
- Bayrak-Toydemir P, Mao R, Lewin S, McDonald J. Hereditary hemorrhagic telangiectasia: an overview of diagnosis and management in the molecular era for clinicians. Genet Med 2004; 6:175–191.
- Cohen JH, Faughnan ME, Letarte M, Vandezande K, Kennedy SJ, Krahn MD. Cost comparison of genetic and clinical screening in families with hereditary hemorrhagic telangiectasia. Am J Med Genet A 2005; 137:153–160.
- Shovlin C, Bamfort K, Wray D. Post-NICE 2008: Antibiotic prophylaxis prior to dental procedures for patients with pulmonary arteriovenous malformations (PAVMs) and hereditary haemorrhagic telangiectasia. Br Dent J 2008; 205:531–533.
- Hsu YL, Wang HC, Yang PC. Desbaric air embolism during diving: an unusual complication of Osler-Weber-Rendu disease. Br J Sports Med 2004; 38:E6.
A 40-year-old man who works as a roofer began, 1 week ago, to experience episodes of generalized weakness, perioral numbness, and diffuse paresthesias. In the past he has had recurring nosebleeds but no history of other medical conditions.
His recent “spells” come on abruptly and spontaneously, without warning, and last about 15 minutes. He never loses consciousness, but he reports a feeling of derealization or an out-of-body experience—he can hear the people around him talking during the spells, but he feels that everything is far away. He has been having about three episodes per day. They typically occur after mild exertion or heavy lifting, and each episode resolves with complete rest. He has had no nausea, vomiting, loss of bowel or bladder control, fever, chills, or traumatic brain injury.
The patient first reported to the emergency department of a local hospital for evaluation. There, he underwent computed tomography (CT) of the head without contrast, which showed nothing abnormal. However, he had an episode while in the emergency department, which prompted his physician to admit him to the hospital.
In the hospital, he underwent an extensive medical evaluation. CT angiography revealed no evidence of vasculitis or occlusive disease. Results of electroencephalography during these spells were normal. Results of magnetic resonance imaging of the cervical and lumbar spine were also normal.
A neurologist was consulted. Concerned that the spells were due to paradoxical emboli coming through a patent foramen ovale, the neurologist recommended transthoracic echocardiography with agitated saline. This study showed a normal ejection fraction and a right-to-left shunt through a left pulmonary arteriovenous malformation (AVM). Unfortunately, the shunt fraction could not be estimated because the patient had another episode during the procedure, and so the procedure was cut short. CT of the chest confirmed a large AVM in the upper lobe of the left lung (Figure 1).
The patient is transferred
The patient’s physician requested that he be transferred to Mayo Clinic for further evaluation.
When he arrived, we performed a complete physical examination, in which we noted scattered erythematous maculopapular telangiectases in the lower lips and significant digital clubbing (Figure 2). He could not recall any family members having rheumatologic or cardiovascular diseases, but he recalled that his father has oral telangiectases and recurrent epistaxis.
His examination was interrupted by yet another spell, during which his oxygen saturation fell to 85%. We immediately started giving him oxygen by nasal cannula, which raised his oxygen saturation to 96%, and the spell promptly ended.
After his physical examination was completed and his records from the other hospital were reviewed, a diagnosis was made. No further diagnostic studies were pursued.
WHICH IS THE MOST LIKELY DIAGNOSIS?
1. Based on the information available, which of the following is the most likely diagnosis?
- Generalized tonic-clonic seizures
- Osler-Weber-Rendu disease
- Subarachnoid hemorrhage
- Conversion disorder
- Atrial septal defect
Generalized tonic-clonic seizures begin with abrupt loss of consciousness, followed by stiffening of the body and extremities. This is the tonic phase, which may last for 1 minute. The clonic phase follows, characterized by abnormal jerking and teeth-clenching (raising the concern that the patient will bite his or her tongue). The clonic phase lasts 1 to 2 minutes. After a seizure, confusion and headache are common. On electroencephalography, epileptiform abnormalities are documented in about 23% of patients with a first documented seizure.1
Our patient’s history of remaining fully conscious and of having normal electroencephalographic findings during his spells does not suggest generalized tonic-clonic seizures.
Osler-Weber-Rendu disease is also known as hereditary hemorrhagic telangiectasia (HHT). Its pathophysiology is complex, and it is believed to be related to mutations in an endothelial protein2 that lead to abnormal vascular structures. The estimated prevalence in European studies is 1 in 5,000; in Japanese studies it is 1 in 8,000.3–4
The diagnosis of HHT is based on four clinical criteria:
- Spontaneous and recurrent epistaxis
- Multiple mucocutaneous telangiectases
- Pulmonary, cerebral, or gastrointestinal AVMs
- A first-degree relative with the disease.
The presence of three or four of these criteria establishes a “definite” diagnosis, while fewer than two makes it “unlikely.”5 Since the spectrum of this disease is wide, varying from mild epistaxis to iron-deficiency anemia, its diagnosis is often missed.6
Our patient meets at least three of the criteria—recurrent epistaxis, oral telangiectases, and a CT-documented pulmonary AVM. His father has a history of oral telangiectases and epistaxis but was never formally diagnosed with HHT. The patient presented with spells of weakness and paresthesias from worsening hypoxemia due to an enlarged pulmonary AVM. Thus, based on these features, HHT is the most likely diagnosis.
Subarachnoid hemorrhage is commonly from a ruptured cerebral aneurysm. Common symptoms include sudden, severe headaches with focal neurologic deficits, a stiff neck, brief loss of consciousness, nausea, and vomiting.7
Our patient’s CT scan showed no intracranial bleeding, and CT angiography showed no evidence of aneurysm. Thus, he has neither clinical nor radiographic features of subarachnoid hemorrhage.
Conversion disorder is typically associated with psychological stressors.8 It is characterized by the sudden onset of neurologic deficits such as blindness, paralysis, and numbness that cannot be explained by a general medical condition.
Our patient has a known pulmonary AVM with clinical and laboratory findings of hypoxemia that explain his spells. Therefore, the diagnosis of conversion disorder cannot be made.
A right-to-left intracardiac shunt can be present in patients with patent foramen ovale, atrial septal defects with shunt reversal, Eisenmenger syndrome, or tetralogy of Fallot (even in adults). It can present with hypoxemia and neurologic weakness.
Our patient’s echocardiogram ruled out these conditions.
MANIFESTATIONS OF HEREDITARY HEMORRHAGIC TELANGIECTASIA
2. Which is the most common clinical manifestation of HHT?
- Epistaxis
- Mucocutaneous telangiectases
- Hematochezia
- Dyspnea
Epistaxis is the most common presentation, occurring in more than 90% of patients.9 Many patients experience only mild occasional nosebleeds that are not frequent or severe enough to cause anemia or to lead to medical treatment or consultation. Others, however, have heavy, frequent bleeding that requires invasive interventions.10
Mucocutaneous telangiectases are the second most common clinical manifestation, documented in about 75% of patients. They are cosmetically unpleasant but rarely bleed. They occur most commonly on the face, lips, tongue, and fingertips, and they increase in size and number with age.11
Gastrointestinal bleeding, sometimes manifesting as hematochezia, occurs in one-third of people with HHT. It most commonly presents with iron-deficiency anemia in patients over age 40.12
Dyspnea. Pulmonary AVMs occur in 30% to 50% of affected people, but interestingly, most patients with pulmonary AVMs have no respiratory symptoms, including dyspnea.
In pulmonary AVMs, abnormal vessels replace normal capillary beds, creating a capillary-free communication between the pulmonary and systemic circulations. This abnormal connection prevents blood from the pulmonary arterial system from being oxygenated, resulting in hypoxemia and secondary polycythemia, as in our patient. One-third of patients have evidence of right-to-left shunting, such as the clubbing in our patient.9,13
Other, less common complications of HHT include seizures or hemorrhage from cerebral AVMs and stroke and brain abscesses from paradoxical embolization due to the loss of the capillary filter in the pulmonary vascular bed. Hepatic involvement may result in portal hypertension and hepatic encephalopathy.14
Back to our patient
As mentioned above, during one of the patient’s spells of paresthesia and weakness, we noted his oxygen saturation by oximetry was 85%. At that time, his arterial Po2 was also low at 50 mm Hg (normal 70–100). With oxygen supplementation, his spell completely resolved and his Po2 improved to 80 mm Hg. Though the shunt fraction of his pulmonary AVM was never measured, it was likely less than 30% of the cardiac output, as his hypoxemia improved with oxygen supplementation alone.15 When he was taken off oxygen supplementation, his spells recurred, but with oxygen support he remained clinically stable.
MANAGEMENT
3. Which is the next logical step in our patient’s management?
- Consult a surgeon for lobectomy
- Consult an interventional radiologist for embolization therapy
- Transfer to the intensive care unit for elective intubation
- Observe with close follow-up
Untreated pulmonary AVMs enlarge at an estimated rate of 0.3 mm/year. The estimated death rate is up to 15.8% per year, with most deaths resulting from stroke, cerebral abscess, hemoptysis, and hemothorax.16–18 Common indications for treatment are progressively enlarging lesions, symptomatic hypoxemia, and paradoxical embolization.19 Pulmonary AVMs in which the feeding artery is 3 mm or more in diameter require treatment.
Embolization therapy, in which the AVM is occluded angiographically, is considered a first-line treatment for pulmonary AVM, with a procedural success rate (defined as involution of the AVM) of 97%.20 Embolization therapy allows patients to avoid major surgery, with its potential complications, and it has a shorter recovery time.
Surgical procedures such as excision, vascular ligation, or lobectomy can be considered if the lesion cannot be treated by embolization or if the patient has an anaphylactic allergy to contrast dyes.
This patient had no clinical signs of impending respiratory failure requiring elective intubation.
Since he was experiencing symptoms, there is no role for observation in this case.
Back to our patient
An interventional radiologist was consulted, and the patient underwent bilateral pulmonary artery angiography with successful coil embolization of his large left-upper-lobe AVM. He was weaned off oxygen and had no further spells of generalized weakness and paresthesias.
Given his father’s history of recurrent epistaxis and oral telangiectases, the patient asks about the risk of his children acquiring this disease.
GENETICS OF HEREDITARY HEMORRHAGIC TELANGIECTASIA
4. Which of the following is the inheritance pattern for HHT?
- Autosomal dominant
- Autosomal recessive
- Maternal inheritance
- X-linked recessive
The inheritance pattern is autosomal dominant with variable expression and penetrance. At least four different mutations have been identified in genes on chromosomes 9 and 12 that result in abnormal vascular malformations.21–24 The other modes of inheritance have not been described in HHT.
RECOMMENDATIONS FOR OUR PATIENT
5. Which of the following is not recommended for our patient?
- Consideration of genetic testing
- Consideration of screening of first-degree relatives
- Dental prophylaxis
- Scuba diving
Genetic testing. The molecular diagnosis of HHT is primarily based on sequencing of the entire coding regions of the ENG and ALK1 genes on chromosomes 9 and 12, respectively. The interpretation of these results is quite complex. The clinical genetics laboratories in North America that currently offer molecular diagnostic testing for HHT recommend that testing be coordinated and ordered through a center that specializes in this disease or by a genetics professional. Testing of the index case is performed to confirm the clinical diagnosis and also to determine if genetic testing will be possible in at-risk relatives. Further genetic testing should be pursued in at-risk family members only if the index case has a positive result.25
Screening of relatives. Given that HHT is an autosomal dominant disease, the current practice is to offer molecular genetic screening early in life for first-degree relatives.25,26 The external signs such as telangiectases and nosebleeds may not manifest until the second or third decade of life. However, AVMs in the brain, spinal cord, lungs, and liver are usually congenital and may present suddenly and with serious complications, even in childhood.
Dental prophylaxis. People with HHT and a pulmonary AVM are at risk of bacteremia and consequent brain abscesses after dental procedures. Antibiotic prophylaxis is therefore highly recommended.27
One sport to avoid. There have been several case reports of paradoxical air emboli occurring in patients with HHT complicated by a pulmonary AVM. Hsu et al28 reported a 31-year-old man with an undiagnosed large pulmonary AVM and HHT who became comatose with diffuse bilateral hemispheric brain swelling on head CT after scuba diving, due to air embolism.
The HHT Foundation International recommends that people with this disease avoid scuba diving (the only sport to be avoided) owing to the risk of air emboli from small lung AVMs. It also recommends that patients alert health care providers about their risk of air embolism whenever intravenous access is being established.
Back to our patient
The patient met with a geneticist, and blood was collected for genetic testing before he was sent home. Additionally, the need to screen his first-degree relatives was thoroughly discussed. Four days after discharge he returned to work, and his spells have not recurred. He has a follow-up appointment scheduled with a pulmonologist specializing in this disease for the results of genetic testing and for continued management.
TAKE-HOME POINTS
- The diagnosis of HHT is based on the following four clinical criteria: spontaneous or recurrent epistaxis, multiple mucocutaneous telangiectases, visceral involvement (eg, cerebral, pulmonary, or gastrointestinal AVM), and a first-degree relative with this disease.
- The diagnosis may be confirmed with genetic testing.
- The diagnosis may be underreported, given the wide spectrum of disease presentation, from inconsequential epistaxis to massive gastrointestinal bleeding.
- HHT is autosomal dominant, and therefore all first-degree relatives should be screened.
A 40-year-old man who works as a roofer began, 1 week ago, to experience episodes of generalized weakness, perioral numbness, and diffuse paresthesias. In the past he has had recurring nosebleeds but no history of other medical conditions.
His recent “spells” come on abruptly and spontaneously, without warning, and last about 15 minutes. He never loses consciousness, but he reports a feeling of derealization or an out-of-body experience—he can hear the people around him talking during the spells, but he feels that everything is far away. He has been having about three episodes per day. They typically occur after mild exertion or heavy lifting, and each episode resolves with complete rest. He has had no nausea, vomiting, loss of bowel or bladder control, fever, chills, or traumatic brain injury.
The patient first reported to the emergency department of a local hospital for evaluation. There, he underwent computed tomography (CT) of the head without contrast, which showed nothing abnormal. However, he had an episode while in the emergency department, which prompted his physician to admit him to the hospital.
In the hospital, he underwent an extensive medical evaluation. CT angiography revealed no evidence of vasculitis or occlusive disease. Results of electroencephalography during these spells were normal. Results of magnetic resonance imaging of the cervical and lumbar spine were also normal.
A neurologist was consulted. Concerned that the spells were due to paradoxical emboli coming through a patent foramen ovale, the neurologist recommended transthoracic echocardiography with agitated saline. This study showed a normal ejection fraction and a right-to-left shunt through a left pulmonary arteriovenous malformation (AVM). Unfortunately, the shunt fraction could not be estimated because the patient had another episode during the procedure, and so the procedure was cut short. CT of the chest confirmed a large AVM in the upper lobe of the left lung (Figure 1).
The patient is transferred
The patient’s physician requested that he be transferred to Mayo Clinic for further evaluation.
When he arrived, we performed a complete physical examination, in which we noted scattered erythematous maculopapular telangiectases in the lower lips and significant digital clubbing (Figure 2). He could not recall any family members having rheumatologic or cardiovascular diseases, but he recalled that his father has oral telangiectases and recurrent epistaxis.
His examination was interrupted by yet another spell, during which his oxygen saturation fell to 85%. We immediately started giving him oxygen by nasal cannula, which raised his oxygen saturation to 96%, and the spell promptly ended.
After his physical examination was completed and his records from the other hospital were reviewed, a diagnosis was made. No further diagnostic studies were pursued.
WHICH IS THE MOST LIKELY DIAGNOSIS?
1. Based on the information available, which of the following is the most likely diagnosis?
- Generalized tonic-clonic seizures
- Osler-Weber-Rendu disease
- Subarachnoid hemorrhage
- Conversion disorder
- Atrial septal defect
Generalized tonic-clonic seizures begin with abrupt loss of consciousness, followed by stiffening of the body and extremities. This is the tonic phase, which may last for 1 minute. The clonic phase follows, characterized by abnormal jerking and teeth-clenching (raising the concern that the patient will bite his or her tongue). The clonic phase lasts 1 to 2 minutes. After a seizure, confusion and headache are common. On electroencephalography, epileptiform abnormalities are documented in about 23% of patients with a first documented seizure.1
Our patient’s history of remaining fully conscious and of having normal electroencephalographic findings during his spells does not suggest generalized tonic-clonic seizures.
Osler-Weber-Rendu disease is also known as hereditary hemorrhagic telangiectasia (HHT). Its pathophysiology is complex, and it is believed to be related to mutations in an endothelial protein2 that lead to abnormal vascular structures. The estimated prevalence in European studies is 1 in 5,000; in Japanese studies it is 1 in 8,000.3–4
The diagnosis of HHT is based on four clinical criteria:
- Spontaneous and recurrent epistaxis
- Multiple mucocutaneous telangiectases
- Pulmonary, cerebral, or gastrointestinal AVMs
- A first-degree relative with the disease.
The presence of three or four of these criteria establishes a “definite” diagnosis, while fewer than two makes it “unlikely.”5 Since the spectrum of this disease is wide, varying from mild epistaxis to iron-deficiency anemia, its diagnosis is often missed.6
Our patient meets at least three of the criteria—recurrent epistaxis, oral telangiectases, and a CT-documented pulmonary AVM. His father has a history of oral telangiectases and epistaxis but was never formally diagnosed with HHT. The patient presented with spells of weakness and paresthesias from worsening hypoxemia due to an enlarged pulmonary AVM. Thus, based on these features, HHT is the most likely diagnosis.
Subarachnoid hemorrhage is commonly from a ruptured cerebral aneurysm. Common symptoms include sudden, severe headaches with focal neurologic deficits, a stiff neck, brief loss of consciousness, nausea, and vomiting.7
Our patient’s CT scan showed no intracranial bleeding, and CT angiography showed no evidence of aneurysm. Thus, he has neither clinical nor radiographic features of subarachnoid hemorrhage.
Conversion disorder is typically associated with psychological stressors.8 It is characterized by the sudden onset of neurologic deficits such as blindness, paralysis, and numbness that cannot be explained by a general medical condition.
Our patient has a known pulmonary AVM with clinical and laboratory findings of hypoxemia that explain his spells. Therefore, the diagnosis of conversion disorder cannot be made.
A right-to-left intracardiac shunt can be present in patients with patent foramen ovale, atrial septal defects with shunt reversal, Eisenmenger syndrome, or tetralogy of Fallot (even in adults). It can present with hypoxemia and neurologic weakness.
Our patient’s echocardiogram ruled out these conditions.
MANIFESTATIONS OF HEREDITARY HEMORRHAGIC TELANGIECTASIA
2. Which is the most common clinical manifestation of HHT?
- Epistaxis
- Mucocutaneous telangiectases
- Hematochezia
- Dyspnea
Epistaxis is the most common presentation, occurring in more than 90% of patients.9 Many patients experience only mild occasional nosebleeds that are not frequent or severe enough to cause anemia or to lead to medical treatment or consultation. Others, however, have heavy, frequent bleeding that requires invasive interventions.10
Mucocutaneous telangiectases are the second most common clinical manifestation, documented in about 75% of patients. They are cosmetically unpleasant but rarely bleed. They occur most commonly on the face, lips, tongue, and fingertips, and they increase in size and number with age.11
Gastrointestinal bleeding, sometimes manifesting as hematochezia, occurs in one-third of people with HHT. It most commonly presents with iron-deficiency anemia in patients over age 40.12
Dyspnea. Pulmonary AVMs occur in 30% to 50% of affected people, but interestingly, most patients with pulmonary AVMs have no respiratory symptoms, including dyspnea.
In pulmonary AVMs, abnormal vessels replace normal capillary beds, creating a capillary-free communication between the pulmonary and systemic circulations. This abnormal connection prevents blood from the pulmonary arterial system from being oxygenated, resulting in hypoxemia and secondary polycythemia, as in our patient. One-third of patients have evidence of right-to-left shunting, such as the clubbing in our patient.9,13
Other, less common complications of HHT include seizures or hemorrhage from cerebral AVMs and stroke and brain abscesses from paradoxical embolization due to the loss of the capillary filter in the pulmonary vascular bed. Hepatic involvement may result in portal hypertension and hepatic encephalopathy.14
Back to our patient
As mentioned above, during one of the patient’s spells of paresthesia and weakness, we noted his oxygen saturation by oximetry was 85%. At that time, his arterial Po2 was also low at 50 mm Hg (normal 70–100). With oxygen supplementation, his spell completely resolved and his Po2 improved to 80 mm Hg. Though the shunt fraction of his pulmonary AVM was never measured, it was likely less than 30% of the cardiac output, as his hypoxemia improved with oxygen supplementation alone.15 When he was taken off oxygen supplementation, his spells recurred, but with oxygen support he remained clinically stable.
MANAGEMENT
3. Which is the next logical step in our patient’s management?
- Consult a surgeon for lobectomy
- Consult an interventional radiologist for embolization therapy
- Transfer to the intensive care unit for elective intubation
- Observe with close follow-up
Untreated pulmonary AVMs enlarge at an estimated rate of 0.3 mm/year. The estimated death rate is up to 15.8% per year, with most deaths resulting from stroke, cerebral abscess, hemoptysis, and hemothorax.16–18 Common indications for treatment are progressively enlarging lesions, symptomatic hypoxemia, and paradoxical embolization.19 Pulmonary AVMs in which the feeding artery is 3 mm or more in diameter require treatment.
Embolization therapy, in which the AVM is occluded angiographically, is considered a first-line treatment for pulmonary AVM, with a procedural success rate (defined as involution of the AVM) of 97%.20 Embolization therapy allows patients to avoid major surgery, with its potential complications, and it has a shorter recovery time.
Surgical procedures such as excision, vascular ligation, or lobectomy can be considered if the lesion cannot be treated by embolization or if the patient has an anaphylactic allergy to contrast dyes.
This patient had no clinical signs of impending respiratory failure requiring elective intubation.
Since he was experiencing symptoms, there is no role for observation in this case.
Back to our patient
An interventional radiologist was consulted, and the patient underwent bilateral pulmonary artery angiography with successful coil embolization of his large left-upper-lobe AVM. He was weaned off oxygen and had no further spells of generalized weakness and paresthesias.
Given his father’s history of recurrent epistaxis and oral telangiectases, the patient asks about the risk of his children acquiring this disease.
GENETICS OF HEREDITARY HEMORRHAGIC TELANGIECTASIA
4. Which of the following is the inheritance pattern for HHT?
- Autosomal dominant
- Autosomal recessive
- Maternal inheritance
- X-linked recessive
The inheritance pattern is autosomal dominant with variable expression and penetrance. At least four different mutations have been identified in genes on chromosomes 9 and 12 that result in abnormal vascular malformations.21–24 The other modes of inheritance have not been described in HHT.
RECOMMENDATIONS FOR OUR PATIENT
5. Which of the following is not recommended for our patient?
- Consideration of genetic testing
- Consideration of screening of first-degree relatives
- Dental prophylaxis
- Scuba diving
Genetic testing. The molecular diagnosis of HHT is primarily based on sequencing of the entire coding regions of the ENG and ALK1 genes on chromosomes 9 and 12, respectively. The interpretation of these results is quite complex. The clinical genetics laboratories in North America that currently offer molecular diagnostic testing for HHT recommend that testing be coordinated and ordered through a center that specializes in this disease or by a genetics professional. Testing of the index case is performed to confirm the clinical diagnosis and also to determine if genetic testing will be possible in at-risk relatives. Further genetic testing should be pursued in at-risk family members only if the index case has a positive result.25
Screening of relatives. Given that HHT is an autosomal dominant disease, the current practice is to offer molecular genetic screening early in life for first-degree relatives.25,26 The external signs such as telangiectases and nosebleeds may not manifest until the second or third decade of life. However, AVMs in the brain, spinal cord, lungs, and liver are usually congenital and may present suddenly and with serious complications, even in childhood.
Dental prophylaxis. People with HHT and a pulmonary AVM are at risk of bacteremia and consequent brain abscesses after dental procedures. Antibiotic prophylaxis is therefore highly recommended.27
One sport to avoid. There have been several case reports of paradoxical air emboli occurring in patients with HHT complicated by a pulmonary AVM. Hsu et al28 reported a 31-year-old man with an undiagnosed large pulmonary AVM and HHT who became comatose with diffuse bilateral hemispheric brain swelling on head CT after scuba diving, due to air embolism.
The HHT Foundation International recommends that people with this disease avoid scuba diving (the only sport to be avoided) owing to the risk of air emboli from small lung AVMs. It also recommends that patients alert health care providers about their risk of air embolism whenever intravenous access is being established.
Back to our patient
The patient met with a geneticist, and blood was collected for genetic testing before he was sent home. Additionally, the need to screen his first-degree relatives was thoroughly discussed. Four days after discharge he returned to work, and his spells have not recurred. He has a follow-up appointment scheduled with a pulmonologist specializing in this disease for the results of genetic testing and for continued management.
TAKE-HOME POINTS
- The diagnosis of HHT is based on the following four clinical criteria: spontaneous or recurrent epistaxis, multiple mucocutaneous telangiectases, visceral involvement (eg, cerebral, pulmonary, or gastrointestinal AVM), and a first-degree relative with this disease.
- The diagnosis may be confirmed with genetic testing.
- The diagnosis may be underreported, given the wide spectrum of disease presentation, from inconsequential epistaxis to massive gastrointestinal bleeding.
- HHT is autosomal dominant, and therefore all first-degree relatives should be screened.
- Krumholz A, Wiebe S, Gronseth G, et al. Practice parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2007; 69:1996–2007.
- Bourdeau A, Dumont DJ, Letarte M. A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest 1999; 104:1343–1351.
- Dakeishi M, Shioya T, Wada Y, et al. Genetic epidemiology of hereditary hemorrhagic telangiectasia in a local community in the northern part of Japan. Hum Mutat 2002; 19:140–148.
- Guttmacher AE, Marchuk DA, White RI. Hereditary hemorrhagic telangiectasia. N Engl J Med 1995; 333:918–924.
- Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 2000; 91:66–67.
- Gallitelli M, Pasculli G, Fiore T, Carella A, Sabbà C. Emergencies in hereditary haemorrhagic telangiectasia. QJM 2006; 99:15–22.
- Gorelick PB, Hier DB, Caplan LR, Langenberg P. Headache in acute cerebrovascular disease. Neurology 1986; 36:1445–1450.
- Stonnington CM, Barry JJ, Fisher RS. Conversion disorder. Am J Psychiatry 2006; 163:1510–1517.
- Shovlin CL, Letarte M. Hereditary hemorrhagic telangiectasia and pulmonary arteriovenous malformations: issues in clinical management and review of pathogenic mechanisms. Thorax 1999; 54:714–729.
- AAssar OS, Friedman CM, White RI. The natural history of epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope 1991; 101:977–980.
- McAllister KA, Lennon F, Bowles-Biesecker B, et al. Genetic heterogenicity in hereditary haemorrhagic telangiectasia: possible correlation with clinical phenotype. J Med Genet 1994; 31:927–932.
- Plauchu H, de Chadarevian JP, Bideau A, Robert JM. Age-related clinical profile of hereditary hemorrhagic telangiectasia in an epidemiologically recruited population. Am J Med Genet 1989; 32:291–297.
- Shovlin CL, Jaskson JE, Bamford KB, et al. Primary determinants of ischaemic stroke/brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax 2008; 63:259–266.
- Garcia-Tsao G, Korzenik JR, Young L, et al. Liver disease in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 2000; 343:931–936.
- Kolleft MH, Micek ST. Critical care. In:Cooper DH, Krainik AJ, Lubner SJ, Reno HEL, editors. Washington Manual of Medical Therapeutics. 32nd ed. Philadelphia: Lippincott Williams & Wilkins, 2007:224–230.
- Swanson KL, Prakash UB, Stanson AW. Pulmonary arteriovenous fistulas: Mayo Clinic experience: 1872–1997. Mayo Clin Proc 1999; 74:671–680.
- Dines DE, Arms RA, Bernatz PE, Gomes MR. Pulmonary arteriovenous fistulas. Mayo Clinic Proc 1974; 49:460–465.
- Sluiter-Eringa H, Orie NG, Sluiter HJ. Pulmonary arteriovenous fistula: diagnosis and prognosis in noncompliant patients. Am Rev Respir Dis 1969; 100:177–188.
- Dines DE, Seward JB, Bernatz PE. Pulmonary arteriovenous fistula. Mayo Clin Proc 1983; 58:176–181.
- Pollak JS, Saluja S, Thabet A, Henderson KJ, Denbow N, White RI. Clinical and anatomic outcomes after embolotherapy of pulmonary arteriovenous malformations. J Vasc Interv Radio 2006; 17:35–44.
- Berg JN, Gallion CJ, Stenzel TT, et al. The activin receptor-like kinase 1 gene: genomic structure and mutations in hereditary hemorrhagic telangiectasia type 2. Am J Hum Genet 1997; 61:60–67.
- McAllister KA, Grogg KM, Johnson DW, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary hemorrhagic telangiectasias type 1. Nat Genet 1994; 8:345–351.
- Johnson DW, Berg JN, Baldwin MA, et al. Mutations in the activin receptor-like kinase gene in hereditary haemorrhagic telangeictasia type 2. Nat Genet 1996; 13:189–195.
- Abdalla SA, Letarte M. Hereditary haemorrhagic telangiectasia: current views on genetics and mechanisms of disease. J Med Genet 2006; 43:97–110.
- Bayrak-Toydemir P, Mao R, Lewin S, McDonald J. Hereditary hemorrhagic telangiectasia: an overview of diagnosis and management in the molecular era for clinicians. Genet Med 2004; 6:175–191.
- Cohen JH, Faughnan ME, Letarte M, Vandezande K, Kennedy SJ, Krahn MD. Cost comparison of genetic and clinical screening in families with hereditary hemorrhagic telangiectasia. Am J Med Genet A 2005; 137:153–160.
- Shovlin C, Bamfort K, Wray D. Post-NICE 2008: Antibiotic prophylaxis prior to dental procedures for patients with pulmonary arteriovenous malformations (PAVMs) and hereditary haemorrhagic telangiectasia. Br Dent J 2008; 205:531–533.
- Hsu YL, Wang HC, Yang PC. Desbaric air embolism during diving: an unusual complication of Osler-Weber-Rendu disease. Br J Sports Med 2004; 38:E6.
- Krumholz A, Wiebe S, Gronseth G, et al. Practice parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2007; 69:1996–2007.
- Bourdeau A, Dumont DJ, Letarte M. A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest 1999; 104:1343–1351.
- Dakeishi M, Shioya T, Wada Y, et al. Genetic epidemiology of hereditary hemorrhagic telangiectasia in a local community in the northern part of Japan. Hum Mutat 2002; 19:140–148.
- Guttmacher AE, Marchuk DA, White RI. Hereditary hemorrhagic telangiectasia. N Engl J Med 1995; 333:918–924.
- Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 2000; 91:66–67.
- Gallitelli M, Pasculli G, Fiore T, Carella A, Sabbà C. Emergencies in hereditary haemorrhagic telangiectasia. QJM 2006; 99:15–22.
- Gorelick PB, Hier DB, Caplan LR, Langenberg P. Headache in acute cerebrovascular disease. Neurology 1986; 36:1445–1450.
- Stonnington CM, Barry JJ, Fisher RS. Conversion disorder. Am J Psychiatry 2006; 163:1510–1517.
- Shovlin CL, Letarte M. Hereditary hemorrhagic telangiectasia and pulmonary arteriovenous malformations: issues in clinical management and review of pathogenic mechanisms. Thorax 1999; 54:714–729.
- AAssar OS, Friedman CM, White RI. The natural history of epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope 1991; 101:977–980.
- McAllister KA, Lennon F, Bowles-Biesecker B, et al. Genetic heterogenicity in hereditary haemorrhagic telangiectasia: possible correlation with clinical phenotype. J Med Genet 1994; 31:927–932.
- Plauchu H, de Chadarevian JP, Bideau A, Robert JM. Age-related clinical profile of hereditary hemorrhagic telangiectasia in an epidemiologically recruited population. Am J Med Genet 1989; 32:291–297.
- Shovlin CL, Jaskson JE, Bamford KB, et al. Primary determinants of ischaemic stroke/brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax 2008; 63:259–266.
- Garcia-Tsao G, Korzenik JR, Young L, et al. Liver disease in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 2000; 343:931–936.
- Kolleft MH, Micek ST. Critical care. In:Cooper DH, Krainik AJ, Lubner SJ, Reno HEL, editors. Washington Manual of Medical Therapeutics. 32nd ed. Philadelphia: Lippincott Williams & Wilkins, 2007:224–230.
- Swanson KL, Prakash UB, Stanson AW. Pulmonary arteriovenous fistulas: Mayo Clinic experience: 1872–1997. Mayo Clin Proc 1999; 74:671–680.
- Dines DE, Arms RA, Bernatz PE, Gomes MR. Pulmonary arteriovenous fistulas. Mayo Clinic Proc 1974; 49:460–465.
- Sluiter-Eringa H, Orie NG, Sluiter HJ. Pulmonary arteriovenous fistula: diagnosis and prognosis in noncompliant patients. Am Rev Respir Dis 1969; 100:177–188.
- Dines DE, Seward JB, Bernatz PE. Pulmonary arteriovenous fistula. Mayo Clin Proc 1983; 58:176–181.
- Pollak JS, Saluja S, Thabet A, Henderson KJ, Denbow N, White RI. Clinical and anatomic outcomes after embolotherapy of pulmonary arteriovenous malformations. J Vasc Interv Radio 2006; 17:35–44.
- Berg JN, Gallion CJ, Stenzel TT, et al. The activin receptor-like kinase 1 gene: genomic structure and mutations in hereditary hemorrhagic telangiectasia type 2. Am J Hum Genet 1997; 61:60–67.
- McAllister KA, Grogg KM, Johnson DW, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary hemorrhagic telangiectasias type 1. Nat Genet 1994; 8:345–351.
- Johnson DW, Berg JN, Baldwin MA, et al. Mutations in the activin receptor-like kinase gene in hereditary haemorrhagic telangeictasia type 2. Nat Genet 1996; 13:189–195.
- Abdalla SA, Letarte M. Hereditary haemorrhagic telangiectasia: current views on genetics and mechanisms of disease. J Med Genet 2006; 43:97–110.
- Bayrak-Toydemir P, Mao R, Lewin S, McDonald J. Hereditary hemorrhagic telangiectasia: an overview of diagnosis and management in the molecular era for clinicians. Genet Med 2004; 6:175–191.
- Cohen JH, Faughnan ME, Letarte M, Vandezande K, Kennedy SJ, Krahn MD. Cost comparison of genetic and clinical screening in families with hereditary hemorrhagic telangiectasia. Am J Med Genet A 2005; 137:153–160.
- Shovlin C, Bamfort K, Wray D. Post-NICE 2008: Antibiotic prophylaxis prior to dental procedures for patients with pulmonary arteriovenous malformations (PAVMs) and hereditary haemorrhagic telangiectasia. Br Dent J 2008; 205:531–533.
- Hsu YL, Wang HC, Yang PC. Desbaric air embolism during diving: an unusual complication of Osler-Weber-Rendu disease. Br J Sports Med 2004; 38:E6.
Sorting through the recent controversies in breast cancer screening
Editor’s Note: This commentary, written by members of the Cleveland Clinic Breast Cancer Screening Task Force, was not independently peer-reviewed.
In November 2009, the US Preventive Services Task Force (USPSTF) announced its new guidelines for breast cancer screening—and created an instant controversy by suggesting that fewer screening tests be done.1
The November 2009 update recommended that most women wait until age 50 to get their first screening mammogram instead of getting it at age 40, that they get a mammogram every other year instead of every year, and that physicians not teach their patients breast self-examination anymore. However, on December 4, 2009, the USPSTF members voted to modify the recommendation for women under age 50, stating that the decision to start screening mammography every 2 years should be individualized, taking into account the patient’s preferences after being apprised of the possible benefits and harms.2
Various professional and advocacy groups have reacted differently to the new guidelines, and as a result, women are unsure about the optimal screening for breast cancer.
NEW GUIDELINES ARE BASED ON TWO STUDIES
The USPSTF commissioned two studies, which it used to formulate the new recommendations.3,4 Its goal was to evaluate the current evidence for the efficacy of several screening tests and schedules in reducing breast cancer mortality rates.
An updated systematic review
Nelson et al3 performed a systematic review of studies of the benefit and harm of screening with mammography, clinical breast examination, and breast self-examination.
Screening mammography continued to demonstrate a reduction in deaths due to breast cancer. The risk reduction ranged from 14% to 32% in women age 50 to 69. Similarly, it was calculated to reduce the incidence of deaths due to breast cancer by 15% in women age 39 to 49. However, this younger age group has a relatively low incidence of breast cancer, and therefore, according to this analysis, 556 women need to undergo one round of screening to detect one case of invasive breast cancer, and 1,904 women need to be offered screening (over several rounds, which varied by trial) to prevent one breast cancer death.3
Most of the harm of screening in the 39-to-49-year age category was due to false-positive results, which were more common in this group than in older women. The authors calculated that after every round of screening mammography, about 84 of every 1,000 women in the younger age category need additional imaging and about 9 need a biopsy. The issue of overdiagnosis (detection of cancers that would have never been a problem in one’s lifetime) was not specifically addressed for this age category, and in different studies, estimates of overdiagnosis rates for all age groups varied widely, from less than 1% to 30%.
Beyond age 70, the authors reported the data insufficient for evaluating the benefit and harm of screening mammography.
Breast self-examination was found to offer no benefit, based largely on two randomized studies, one in St. Petersburg, Russia,5 and the other in Shanghai, China,6 both places where screening mammography was not routinely offered. These studies and one observational study in the United States7 failed to show a reduction in breast cancer mortality rates with breast self-examination.
Clinical breast examination (ie, by a health care provider) lacked sufficient data to draw conclusions.
A study based on statistical models of mammography
Mandelblatt et al4 used statistical modeling to estimate the effect of mammographic screening at various ages and at different intervals.
The authors used six statistical models previously shown to give similar qualitative estimates of the contribution of screening in reducing breast cancer mortality rates. They estimated the number of mammograms required relative to the number of cancers detected, the number of breast cancer deaths prevented, and the harms (false-positive mammograms, unnecessary biopsies, and overdiagnosis) incurred with 20 different screening strategies, ie, screening with different starting and stopping ages and at intervals of either 1 or 2 years.
They estimated that screening every other year would achieve most of the benefit of screening every year, with less harm. Looking at the different strategies and models, on average, biennial screening would, by their calculations, achieve about 81% of the mortality reduction achieved with annual screening. Compared with screening women ages 50 to 69 only, extending screening to women age 40 to 49 would reduce the cancer mortality rate by 3% more, while extending it up to age 79 would reduce it by another 7% to 8%.
In terms of harm, the models predicted more false-positive studies if screening were started before age 50 and if it were done annually rather than biennially. They also predicted that more unnecessary biopsies would be done with annual screening than with screening every 2 years. The models suggested that the risk for overdiagnosis was higher in older age groups because of higher rates of death from causes other than breast cancer, and that the overdiagnosis rate was also somewhat higher with annual than with 2-year screening.
WHAT WOULD LESS SCREENING MEAN?
Our practice has been to initiate annual screening with mammography at age 40 and to continue as long as the patient’s life expectancy is at least 10 years.
According to the models used by Mandelblatt et al,4 screening 1,000 women every year, starting at age 40 and continuing until age 84, would result in 177 to 227 life-years gained compared with no screening. In contrast, screening only women age 50 to 74 and only every other year (as advocated in the new guidelines) would entail about one-third the number of mammograms but would result in fewer life-years gained per 1,000 women screened: between 96 and 128. If we take the mean of the estimates from the six models, adherence to the new screening guidelines would be estimated to result in about 79 fewer life-years gained for every 1,000 women screened. On the other hand, each woman screened would need to undergo about 25 fewer screening mammograms in her lifetime.4
KEY POINTS ABOUT BREAST CANCER SCREENING
Together, these studies demonstrate several points about breast cancer screening.
Importantly, randomized controlled trials and model analyses continue to show that screening mammography reduces the breast cancer mortality rate.
The studies and models also reinforce the concept that those at greatest risk get the most benefit from screening. Because the incidence of breast cancer rises with age, the probability of a true-positive result is higher in women over age 50 than it is in younger women, and, therefore, the screening test performs better.
On the other hand, women at high risk of dying of other causes, such as those over age 75, achieve less benefit from screening, as some of the cancers detected in this manner may not contribute to their death even if they are not detected early.
Screening is therefore best targeted at people who are healthy but who are at sufficient risk for the disease in question to justify the screening.
CLEVELAND CLINIC’S POSITION
In December 2009, the Cleveland Clinic Breast Cancer Screening Task Force, a multidisciplinary panel of breast cancer experts, breast radiologists, and primary care providers, convened to review the literature and set forth institutional recommendations for breast cancer screening for healthy women. The authors of this paper are members of this task force. Our consensus recommendations:
- We continue to recommend annual mammography for most healthy women over age 40.
- Screening every other year is an option for older postmenopausal women, as they are likely to achieve most of the benefit of annual screening with this schedule.
- We agree with the USPSTF finding that there are insufficient data to provide evidenced-based recommendations regarding the benefits and harms of clinical breast examination. However, breast examination was done as part of the screening in many of the randomized trials of mammography and cannot easily be separated from mammography. Therefore, we believe that careful examination of the breasts remains an important consideration in the general physical examination.
- The USPSTF recommendation not to teach breast self-examination was based on studies that probably do not apply to the US population. Therefore, we continue to recommend that women be familiar with their breasts and report any changes to their physicians.
How we reached these conclusions
The task force discussions focused heavily on at what age mammography should be started and how often it should be done. In addition to an in-depth review of the studies on which the USPSTF recommendations were based, we considered a review posted on the Society of Breast Imaging (SBI) Web site.8
A key point from the SBI’s review is that although breast cancer occurs less often in women under age 50, approximately 1 in 69 women are diagnosed with invasive cancer when in their 40s. Some—probably a minority— have a family history of breast cancer and thus warrant earlier screening on that basis.
Breast cancer is, therefore, an important public health concern for women ages 40 to 49. While mammography is an imperfect test, it has a demonstrated ability to find cancers at an earlier stage in this age group. The SBI statement also summarized data suggesting that the 40-to-49-year age group would experience significantly fewer lives saved by screening if the mammography interval were increased from once a year to every other year (ie, by approximately one-half—from 36% of deaths prevented with annual screening to 18% deaths prevented with screening every other year).
Screening every other year is also expected to result in fewer lives saved in women ages 50 to 69 (39% of deaths prevented by biennial screening instead of 44% to 46% with annual screening). However, this proportion of deaths prevented with more frequent (ie, annual) screening is smaller than in the younger age group. Breast cancers that arise before menopause are considered biologically more aggressive, so the longer the interval between screening tests, the lower the likelihood of detecting some of these potentially more lethal cancers.
We believe, for several reasons, that the randomized trials may have underestimated the benefit of mammography. The trials included in the USPSTF studies did not use modern mammographic techniques such as digital mammography. Some of the trials used single-view mammography, which may be less sensitive. Also, the rate of compliance with screening in these randomized trials was only about 70%, which would lead to an underestimation of the number of lives saved with mammography screening. Yet in spite of these limitations, the data continue to show a reduction in breast cancer deaths in all age categories studied.
Other issues the task force considered
Harms of screening are acceptable. We agree that the need for additional imaging or possibly breast biopsy is an acceptable consequence of screening for most women, especially when weighed against the potential benefit of improving survival. Nelson et al3 briefly discussed the risk of inducing other cancers through radiation exposure, and any such risk appears to be low enough that it is overshadowed by the reduction in the breast cancer mortality rate achieved from screening.
The USPSTF studies did not address the issue of cost, which is another potential harm of screening. However, screening mammography is relatively inexpensive compared with other potentially life-saving screening tests.
Our position differs from that of the American College of Physicians (ACP), which has endorsed the USPSTF recommendation for reduced breast cancer screening. The USPSTF has been a leading group in providing practice recommendations based on high-level evidence predominantly from randomized controlled clinical trials, and its recommendations have been consistently followed by the ACP and many of its members, including Cleveland Clinic physicians. It is, therefore, not without considerable discussion that we have come to our consensus.
Evidence for less screening was not compelling. One of our concerns about the new USPSTF recommendations is that the changes are based largely on a model analysis of the efficiency of different screening strategies rather than on randomized controlled trials comparing different strategies. We did not find this level of present evidence to be sufficiently compelling to make a change in our practice that may result in loss of lives from breast cancer.
Screening guidelines will continue to change over time as technology improves and new data are introduced. In the future, risk-assessment strategies such as incorporating genetic profiles may allow us to use factors more predictive than age to target our screening population.
While we continue to strive for better means of early detection and cancer prevention, the Cleveland Clinic task force is currently recommending yearly screening with mammography and breast examination for most women, starting at age 40.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:716–726.
- US Preventive Services Task Force (USPSTF). Recommendation statement from USPSTF: screening for breast cancer. Medscape. http://www.medscape.com/viewarticle/714016. Accessed 12/28/2009.
- Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med 2009; 151:727–737.
- Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med 2009; 151:738–747.
- Semiglazov VF, Manikhas AG, Moiseenko VM, et al. Results of a prospective randomized investigation [Russia (St. Petersburg)/WHO] to evaluate the significance of self-examination for the early detection of breast cancer [in Russian]. Vopr Onkol 2003; 49:434–441. Cited by Nelson et al (see reference 3, above).
- Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst 2002; 94:1445–1457. Cited by Nelson et al (see reference 3, above).
- Tu SP, Reisch LM, Taplin SH, Kreuter W, Elmore JG. Breast self-examination: self-reported frequency, quality, and associated outcomes. J Cancer Educ 2006; 21:175–181. Cited by Nelson et al (see reference 3, above).
- Berg WA, Hendrick E, Kopans DB, Smith RA. Frequently asked questions about mammography and the USPSTF recommendations: a guide for practitioners. Society of Breast Imaging. http://www.sbi-online.org/associations/8199/files/Detailed_Response_to_USPSTF_Guidelines-12-11-09-Berg.pdf. Accessed 12/28/2009.
Editor’s Note: This commentary, written by members of the Cleveland Clinic Breast Cancer Screening Task Force, was not independently peer-reviewed.
In November 2009, the US Preventive Services Task Force (USPSTF) announced its new guidelines for breast cancer screening—and created an instant controversy by suggesting that fewer screening tests be done.1
The November 2009 update recommended that most women wait until age 50 to get their first screening mammogram instead of getting it at age 40, that they get a mammogram every other year instead of every year, and that physicians not teach their patients breast self-examination anymore. However, on December 4, 2009, the USPSTF members voted to modify the recommendation for women under age 50, stating that the decision to start screening mammography every 2 years should be individualized, taking into account the patient’s preferences after being apprised of the possible benefits and harms.2
Various professional and advocacy groups have reacted differently to the new guidelines, and as a result, women are unsure about the optimal screening for breast cancer.
NEW GUIDELINES ARE BASED ON TWO STUDIES
The USPSTF commissioned two studies, which it used to formulate the new recommendations.3,4 Its goal was to evaluate the current evidence for the efficacy of several screening tests and schedules in reducing breast cancer mortality rates.
An updated systematic review
Nelson et al3 performed a systematic review of studies of the benefit and harm of screening with mammography, clinical breast examination, and breast self-examination.
Screening mammography continued to demonstrate a reduction in deaths due to breast cancer. The risk reduction ranged from 14% to 32% in women age 50 to 69. Similarly, it was calculated to reduce the incidence of deaths due to breast cancer by 15% in women age 39 to 49. However, this younger age group has a relatively low incidence of breast cancer, and therefore, according to this analysis, 556 women need to undergo one round of screening to detect one case of invasive breast cancer, and 1,904 women need to be offered screening (over several rounds, which varied by trial) to prevent one breast cancer death.3
Most of the harm of screening in the 39-to-49-year age category was due to false-positive results, which were more common in this group than in older women. The authors calculated that after every round of screening mammography, about 84 of every 1,000 women in the younger age category need additional imaging and about 9 need a biopsy. The issue of overdiagnosis (detection of cancers that would have never been a problem in one’s lifetime) was not specifically addressed for this age category, and in different studies, estimates of overdiagnosis rates for all age groups varied widely, from less than 1% to 30%.
Beyond age 70, the authors reported the data insufficient for evaluating the benefit and harm of screening mammography.
Breast self-examination was found to offer no benefit, based largely on two randomized studies, one in St. Petersburg, Russia,5 and the other in Shanghai, China,6 both places where screening mammography was not routinely offered. These studies and one observational study in the United States7 failed to show a reduction in breast cancer mortality rates with breast self-examination.
Clinical breast examination (ie, by a health care provider) lacked sufficient data to draw conclusions.
A study based on statistical models of mammography
Mandelblatt et al4 used statistical modeling to estimate the effect of mammographic screening at various ages and at different intervals.
The authors used six statistical models previously shown to give similar qualitative estimates of the contribution of screening in reducing breast cancer mortality rates. They estimated the number of mammograms required relative to the number of cancers detected, the number of breast cancer deaths prevented, and the harms (false-positive mammograms, unnecessary biopsies, and overdiagnosis) incurred with 20 different screening strategies, ie, screening with different starting and stopping ages and at intervals of either 1 or 2 years.
They estimated that screening every other year would achieve most of the benefit of screening every year, with less harm. Looking at the different strategies and models, on average, biennial screening would, by their calculations, achieve about 81% of the mortality reduction achieved with annual screening. Compared with screening women ages 50 to 69 only, extending screening to women age 40 to 49 would reduce the cancer mortality rate by 3% more, while extending it up to age 79 would reduce it by another 7% to 8%.
In terms of harm, the models predicted more false-positive studies if screening were started before age 50 and if it were done annually rather than biennially. They also predicted that more unnecessary biopsies would be done with annual screening than with screening every 2 years. The models suggested that the risk for overdiagnosis was higher in older age groups because of higher rates of death from causes other than breast cancer, and that the overdiagnosis rate was also somewhat higher with annual than with 2-year screening.
WHAT WOULD LESS SCREENING MEAN?
Our practice has been to initiate annual screening with mammography at age 40 and to continue as long as the patient’s life expectancy is at least 10 years.
According to the models used by Mandelblatt et al,4 screening 1,000 women every year, starting at age 40 and continuing until age 84, would result in 177 to 227 life-years gained compared with no screening. In contrast, screening only women age 50 to 74 and only every other year (as advocated in the new guidelines) would entail about one-third the number of mammograms but would result in fewer life-years gained per 1,000 women screened: between 96 and 128. If we take the mean of the estimates from the six models, adherence to the new screening guidelines would be estimated to result in about 79 fewer life-years gained for every 1,000 women screened. On the other hand, each woman screened would need to undergo about 25 fewer screening mammograms in her lifetime.4
KEY POINTS ABOUT BREAST CANCER SCREENING
Together, these studies demonstrate several points about breast cancer screening.
Importantly, randomized controlled trials and model analyses continue to show that screening mammography reduces the breast cancer mortality rate.
The studies and models also reinforce the concept that those at greatest risk get the most benefit from screening. Because the incidence of breast cancer rises with age, the probability of a true-positive result is higher in women over age 50 than it is in younger women, and, therefore, the screening test performs better.
On the other hand, women at high risk of dying of other causes, such as those over age 75, achieve less benefit from screening, as some of the cancers detected in this manner may not contribute to their death even if they are not detected early.
Screening is therefore best targeted at people who are healthy but who are at sufficient risk for the disease in question to justify the screening.
CLEVELAND CLINIC’S POSITION
In December 2009, the Cleveland Clinic Breast Cancer Screening Task Force, a multidisciplinary panel of breast cancer experts, breast radiologists, and primary care providers, convened to review the literature and set forth institutional recommendations for breast cancer screening for healthy women. The authors of this paper are members of this task force. Our consensus recommendations:
- We continue to recommend annual mammography for most healthy women over age 40.
- Screening every other year is an option for older postmenopausal women, as they are likely to achieve most of the benefit of annual screening with this schedule.
- We agree with the USPSTF finding that there are insufficient data to provide evidenced-based recommendations regarding the benefits and harms of clinical breast examination. However, breast examination was done as part of the screening in many of the randomized trials of mammography and cannot easily be separated from mammography. Therefore, we believe that careful examination of the breasts remains an important consideration in the general physical examination.
- The USPSTF recommendation not to teach breast self-examination was based on studies that probably do not apply to the US population. Therefore, we continue to recommend that women be familiar with their breasts and report any changes to their physicians.
How we reached these conclusions
The task force discussions focused heavily on at what age mammography should be started and how often it should be done. In addition to an in-depth review of the studies on which the USPSTF recommendations were based, we considered a review posted on the Society of Breast Imaging (SBI) Web site.8
A key point from the SBI’s review is that although breast cancer occurs less often in women under age 50, approximately 1 in 69 women are diagnosed with invasive cancer when in their 40s. Some—probably a minority— have a family history of breast cancer and thus warrant earlier screening on that basis.
Breast cancer is, therefore, an important public health concern for women ages 40 to 49. While mammography is an imperfect test, it has a demonstrated ability to find cancers at an earlier stage in this age group. The SBI statement also summarized data suggesting that the 40-to-49-year age group would experience significantly fewer lives saved by screening if the mammography interval were increased from once a year to every other year (ie, by approximately one-half—from 36% of deaths prevented with annual screening to 18% deaths prevented with screening every other year).
Screening every other year is also expected to result in fewer lives saved in women ages 50 to 69 (39% of deaths prevented by biennial screening instead of 44% to 46% with annual screening). However, this proportion of deaths prevented with more frequent (ie, annual) screening is smaller than in the younger age group. Breast cancers that arise before menopause are considered biologically more aggressive, so the longer the interval between screening tests, the lower the likelihood of detecting some of these potentially more lethal cancers.
We believe, for several reasons, that the randomized trials may have underestimated the benefit of mammography. The trials included in the USPSTF studies did not use modern mammographic techniques such as digital mammography. Some of the trials used single-view mammography, which may be less sensitive. Also, the rate of compliance with screening in these randomized trials was only about 70%, which would lead to an underestimation of the number of lives saved with mammography screening. Yet in spite of these limitations, the data continue to show a reduction in breast cancer deaths in all age categories studied.
Other issues the task force considered
Harms of screening are acceptable. We agree that the need for additional imaging or possibly breast biopsy is an acceptable consequence of screening for most women, especially when weighed against the potential benefit of improving survival. Nelson et al3 briefly discussed the risk of inducing other cancers through radiation exposure, and any such risk appears to be low enough that it is overshadowed by the reduction in the breast cancer mortality rate achieved from screening.
The USPSTF studies did not address the issue of cost, which is another potential harm of screening. However, screening mammography is relatively inexpensive compared with other potentially life-saving screening tests.
Our position differs from that of the American College of Physicians (ACP), which has endorsed the USPSTF recommendation for reduced breast cancer screening. The USPSTF has been a leading group in providing practice recommendations based on high-level evidence predominantly from randomized controlled clinical trials, and its recommendations have been consistently followed by the ACP and many of its members, including Cleveland Clinic physicians. It is, therefore, not without considerable discussion that we have come to our consensus.
Evidence for less screening was not compelling. One of our concerns about the new USPSTF recommendations is that the changes are based largely on a model analysis of the efficiency of different screening strategies rather than on randomized controlled trials comparing different strategies. We did not find this level of present evidence to be sufficiently compelling to make a change in our practice that may result in loss of lives from breast cancer.
Screening guidelines will continue to change over time as technology improves and new data are introduced. In the future, risk-assessment strategies such as incorporating genetic profiles may allow us to use factors more predictive than age to target our screening population.
While we continue to strive for better means of early detection and cancer prevention, the Cleveland Clinic task force is currently recommending yearly screening with mammography and breast examination for most women, starting at age 40.
Editor’s Note: This commentary, written by members of the Cleveland Clinic Breast Cancer Screening Task Force, was not independently peer-reviewed.
In November 2009, the US Preventive Services Task Force (USPSTF) announced its new guidelines for breast cancer screening—and created an instant controversy by suggesting that fewer screening tests be done.1
The November 2009 update recommended that most women wait until age 50 to get their first screening mammogram instead of getting it at age 40, that they get a mammogram every other year instead of every year, and that physicians not teach their patients breast self-examination anymore. However, on December 4, 2009, the USPSTF members voted to modify the recommendation for women under age 50, stating that the decision to start screening mammography every 2 years should be individualized, taking into account the patient’s preferences after being apprised of the possible benefits and harms.2
Various professional and advocacy groups have reacted differently to the new guidelines, and as a result, women are unsure about the optimal screening for breast cancer.
NEW GUIDELINES ARE BASED ON TWO STUDIES
The USPSTF commissioned two studies, which it used to formulate the new recommendations.3,4 Its goal was to evaluate the current evidence for the efficacy of several screening tests and schedules in reducing breast cancer mortality rates.
An updated systematic review
Nelson et al3 performed a systematic review of studies of the benefit and harm of screening with mammography, clinical breast examination, and breast self-examination.
Screening mammography continued to demonstrate a reduction in deaths due to breast cancer. The risk reduction ranged from 14% to 32% in women age 50 to 69. Similarly, it was calculated to reduce the incidence of deaths due to breast cancer by 15% in women age 39 to 49. However, this younger age group has a relatively low incidence of breast cancer, and therefore, according to this analysis, 556 women need to undergo one round of screening to detect one case of invasive breast cancer, and 1,904 women need to be offered screening (over several rounds, which varied by trial) to prevent one breast cancer death.3
Most of the harm of screening in the 39-to-49-year age category was due to false-positive results, which were more common in this group than in older women. The authors calculated that after every round of screening mammography, about 84 of every 1,000 women in the younger age category need additional imaging and about 9 need a biopsy. The issue of overdiagnosis (detection of cancers that would have never been a problem in one’s lifetime) was not specifically addressed for this age category, and in different studies, estimates of overdiagnosis rates for all age groups varied widely, from less than 1% to 30%.
Beyond age 70, the authors reported the data insufficient for evaluating the benefit and harm of screening mammography.
Breast self-examination was found to offer no benefit, based largely on two randomized studies, one in St. Petersburg, Russia,5 and the other in Shanghai, China,6 both places where screening mammography was not routinely offered. These studies and one observational study in the United States7 failed to show a reduction in breast cancer mortality rates with breast self-examination.
Clinical breast examination (ie, by a health care provider) lacked sufficient data to draw conclusions.
A study based on statistical models of mammography
Mandelblatt et al4 used statistical modeling to estimate the effect of mammographic screening at various ages and at different intervals.
The authors used six statistical models previously shown to give similar qualitative estimates of the contribution of screening in reducing breast cancer mortality rates. They estimated the number of mammograms required relative to the number of cancers detected, the number of breast cancer deaths prevented, and the harms (false-positive mammograms, unnecessary biopsies, and overdiagnosis) incurred with 20 different screening strategies, ie, screening with different starting and stopping ages and at intervals of either 1 or 2 years.
They estimated that screening every other year would achieve most of the benefit of screening every year, with less harm. Looking at the different strategies and models, on average, biennial screening would, by their calculations, achieve about 81% of the mortality reduction achieved with annual screening. Compared with screening women ages 50 to 69 only, extending screening to women age 40 to 49 would reduce the cancer mortality rate by 3% more, while extending it up to age 79 would reduce it by another 7% to 8%.
In terms of harm, the models predicted more false-positive studies if screening were started before age 50 and if it were done annually rather than biennially. They also predicted that more unnecessary biopsies would be done with annual screening than with screening every 2 years. The models suggested that the risk for overdiagnosis was higher in older age groups because of higher rates of death from causes other than breast cancer, and that the overdiagnosis rate was also somewhat higher with annual than with 2-year screening.
WHAT WOULD LESS SCREENING MEAN?
Our practice has been to initiate annual screening with mammography at age 40 and to continue as long as the patient’s life expectancy is at least 10 years.
According to the models used by Mandelblatt et al,4 screening 1,000 women every year, starting at age 40 and continuing until age 84, would result in 177 to 227 life-years gained compared with no screening. In contrast, screening only women age 50 to 74 and only every other year (as advocated in the new guidelines) would entail about one-third the number of mammograms but would result in fewer life-years gained per 1,000 women screened: between 96 and 128. If we take the mean of the estimates from the six models, adherence to the new screening guidelines would be estimated to result in about 79 fewer life-years gained for every 1,000 women screened. On the other hand, each woman screened would need to undergo about 25 fewer screening mammograms in her lifetime.4
KEY POINTS ABOUT BREAST CANCER SCREENING
Together, these studies demonstrate several points about breast cancer screening.
Importantly, randomized controlled trials and model analyses continue to show that screening mammography reduces the breast cancer mortality rate.
The studies and models also reinforce the concept that those at greatest risk get the most benefit from screening. Because the incidence of breast cancer rises with age, the probability of a true-positive result is higher in women over age 50 than it is in younger women, and, therefore, the screening test performs better.
On the other hand, women at high risk of dying of other causes, such as those over age 75, achieve less benefit from screening, as some of the cancers detected in this manner may not contribute to their death even if they are not detected early.
Screening is therefore best targeted at people who are healthy but who are at sufficient risk for the disease in question to justify the screening.
CLEVELAND CLINIC’S POSITION
In December 2009, the Cleveland Clinic Breast Cancer Screening Task Force, a multidisciplinary panel of breast cancer experts, breast radiologists, and primary care providers, convened to review the literature and set forth institutional recommendations for breast cancer screening for healthy women. The authors of this paper are members of this task force. Our consensus recommendations:
- We continue to recommend annual mammography for most healthy women over age 40.
- Screening every other year is an option for older postmenopausal women, as they are likely to achieve most of the benefit of annual screening with this schedule.
- We agree with the USPSTF finding that there are insufficient data to provide evidenced-based recommendations regarding the benefits and harms of clinical breast examination. However, breast examination was done as part of the screening in many of the randomized trials of mammography and cannot easily be separated from mammography. Therefore, we believe that careful examination of the breasts remains an important consideration in the general physical examination.
- The USPSTF recommendation not to teach breast self-examination was based on studies that probably do not apply to the US population. Therefore, we continue to recommend that women be familiar with their breasts and report any changes to their physicians.
How we reached these conclusions
The task force discussions focused heavily on at what age mammography should be started and how often it should be done. In addition to an in-depth review of the studies on which the USPSTF recommendations were based, we considered a review posted on the Society of Breast Imaging (SBI) Web site.8
A key point from the SBI’s review is that although breast cancer occurs less often in women under age 50, approximately 1 in 69 women are diagnosed with invasive cancer when in their 40s. Some—probably a minority— have a family history of breast cancer and thus warrant earlier screening on that basis.
Breast cancer is, therefore, an important public health concern for women ages 40 to 49. While mammography is an imperfect test, it has a demonstrated ability to find cancers at an earlier stage in this age group. The SBI statement also summarized data suggesting that the 40-to-49-year age group would experience significantly fewer lives saved by screening if the mammography interval were increased from once a year to every other year (ie, by approximately one-half—from 36% of deaths prevented with annual screening to 18% deaths prevented with screening every other year).
Screening every other year is also expected to result in fewer lives saved in women ages 50 to 69 (39% of deaths prevented by biennial screening instead of 44% to 46% with annual screening). However, this proportion of deaths prevented with more frequent (ie, annual) screening is smaller than in the younger age group. Breast cancers that arise before menopause are considered biologically more aggressive, so the longer the interval between screening tests, the lower the likelihood of detecting some of these potentially more lethal cancers.
We believe, for several reasons, that the randomized trials may have underestimated the benefit of mammography. The trials included in the USPSTF studies did not use modern mammographic techniques such as digital mammography. Some of the trials used single-view mammography, which may be less sensitive. Also, the rate of compliance with screening in these randomized trials was only about 70%, which would lead to an underestimation of the number of lives saved with mammography screening. Yet in spite of these limitations, the data continue to show a reduction in breast cancer deaths in all age categories studied.
Other issues the task force considered
Harms of screening are acceptable. We agree that the need for additional imaging or possibly breast biopsy is an acceptable consequence of screening for most women, especially when weighed against the potential benefit of improving survival. Nelson et al3 briefly discussed the risk of inducing other cancers through radiation exposure, and any such risk appears to be low enough that it is overshadowed by the reduction in the breast cancer mortality rate achieved from screening.
The USPSTF studies did not address the issue of cost, which is another potential harm of screening. However, screening mammography is relatively inexpensive compared with other potentially life-saving screening tests.
Our position differs from that of the American College of Physicians (ACP), which has endorsed the USPSTF recommendation for reduced breast cancer screening. The USPSTF has been a leading group in providing practice recommendations based on high-level evidence predominantly from randomized controlled clinical trials, and its recommendations have been consistently followed by the ACP and many of its members, including Cleveland Clinic physicians. It is, therefore, not without considerable discussion that we have come to our consensus.
Evidence for less screening was not compelling. One of our concerns about the new USPSTF recommendations is that the changes are based largely on a model analysis of the efficiency of different screening strategies rather than on randomized controlled trials comparing different strategies. We did not find this level of present evidence to be sufficiently compelling to make a change in our practice that may result in loss of lives from breast cancer.
Screening guidelines will continue to change over time as technology improves and new data are introduced. In the future, risk-assessment strategies such as incorporating genetic profiles may allow us to use factors more predictive than age to target our screening population.
While we continue to strive for better means of early detection and cancer prevention, the Cleveland Clinic task force is currently recommending yearly screening with mammography and breast examination for most women, starting at age 40.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:716–726.
- US Preventive Services Task Force (USPSTF). Recommendation statement from USPSTF: screening for breast cancer. Medscape. http://www.medscape.com/viewarticle/714016. Accessed 12/28/2009.
- Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med 2009; 151:727–737.
- Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med 2009; 151:738–747.
- Semiglazov VF, Manikhas AG, Moiseenko VM, et al. Results of a prospective randomized investigation [Russia (St. Petersburg)/WHO] to evaluate the significance of self-examination for the early detection of breast cancer [in Russian]. Vopr Onkol 2003; 49:434–441. Cited by Nelson et al (see reference 3, above).
- Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst 2002; 94:1445–1457. Cited by Nelson et al (see reference 3, above).
- Tu SP, Reisch LM, Taplin SH, Kreuter W, Elmore JG. Breast self-examination: self-reported frequency, quality, and associated outcomes. J Cancer Educ 2006; 21:175–181. Cited by Nelson et al (see reference 3, above).
- Berg WA, Hendrick E, Kopans DB, Smith RA. Frequently asked questions about mammography and the USPSTF recommendations: a guide for practitioners. Society of Breast Imaging. http://www.sbi-online.org/associations/8199/files/Detailed_Response_to_USPSTF_Guidelines-12-11-09-Berg.pdf. Accessed 12/28/2009.
- US Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 151:716–726.
- US Preventive Services Task Force (USPSTF). Recommendation statement from USPSTF: screening for breast cancer. Medscape. http://www.medscape.com/viewarticle/714016. Accessed 12/28/2009.
- Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med 2009; 151:727–737.
- Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med 2009; 151:738–747.
- Semiglazov VF, Manikhas AG, Moiseenko VM, et al. Results of a prospective randomized investigation [Russia (St. Petersburg)/WHO] to evaluate the significance of self-examination for the early detection of breast cancer [in Russian]. Vopr Onkol 2003; 49:434–441. Cited by Nelson et al (see reference 3, above).
- Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst 2002; 94:1445–1457. Cited by Nelson et al (see reference 3, above).
- Tu SP, Reisch LM, Taplin SH, Kreuter W, Elmore JG. Breast self-examination: self-reported frequency, quality, and associated outcomes. J Cancer Educ 2006; 21:175–181. Cited by Nelson et al (see reference 3, above).
- Berg WA, Hendrick E, Kopans DB, Smith RA. Frequently asked questions about mammography and the USPSTF recommendations: a guide for practitioners. Society of Breast Imaging. http://www.sbi-online.org/associations/8199/files/Detailed_Response_to_USPSTF_Guidelines-12-11-09-Berg.pdf. Accessed 12/28/2009.
Abdominal pain in a 20-year-old woman
A 20-year-old woman presents to the emergency department with postprandial epigastric and right-upper-quadrant pain, sometimes associated with nausea. She has been having six to eight loose bowel movements every day, with no blood or mucus, and she has lost about 20 lb despite a good appetite. The diarrhea did not improve when she tried omitting milk products and carbohydrates.
Her symptoms began several months ago, but she says that 3 days ago the pain worsened steadily, radiating to the middle of her back, with associated episodes of nonbloody, nonbilious emesis. She cannot keep down liquids or solids. She says she has never had such episodes in the past.
She reports no oral ulcers, urinary symptoms, skin rashes, musculoskeletal pain, or neurologic symptoms, and she denies being anxious or depressed.
She has no history of serious illness, surgery, or hospitalization. She smokes a half pack of cigarettes a day, drinks alcohol occasionally, and smokes marijuana occasionally. She is employed as a certified nursing assistant.
She is taking ethinyl estradiol-levonorgestrel pills for birth control and takes calcium carbonate as needed for abdominal discomfort. She is taking no other medications, including nonsteroidal anti-inflammatory drugs (NSAIDs).
Her maternal uncle died of colon cancer at age 32, and her mother had colon polyps on colonoscopy. There is no family history of inflammatory bowel disease or celiac sprue. Her father committed suicide.
Her laboratory values
- White blood cell count 10.2 × 109/L (normal range 4–11)
- Red blood cell count 4.71 × 1012/L (3.9–5.5)
- Hemoglobin 14.4 g/dL (12–16)
- Hematocrit 42.4% (37%–47%)
- Mean corpuscular volume 90 fL (83–99)
- Mean corpuscular hemoglobin 30.6 pg (27–33)
- Platelet count 230 × 109/L (150–400)
- Red cell distribution width 13.3% (11.5%–14.5%)
- Sodium 140 mmol/L (132–148)
- Potassium 3.3 mmol/L (3.5–5.0)
- Chloride 104 mmol/L (98–111)
- Bicarbonate 28 mmol/L (23–32)
- Blood urea nitrogen 9 mg/dL (8–25)
- Creatinine 0.8 mg/dL (0.7–1.4)
- Glucose 87 mg/dL (65–100)
- Alanine aminotransferase 26 U/L (0–45)
- Aspartate aminotransferase 21 U/L (7–40)
- Alkaline phosphatase 101 U/L (40–150)
- Total bilirubin 0.8 mg/dL (0–1.5)
- Albumin 3.5 g/dL (3.5–5)
- Pregnancy screen negative
- Urine toxicology screen negative.
Physical examination
The patient is very thin and appears quite uncomfortable. Her temperature is 99.7°F (37.6°C), pulse rate 101, respiratory rate 18, blood pressure 111/67 mm Hg, and oxygen saturation 96% on room air. Her skin is warm and dry. Her height is 66 inches, weight 116 lb, and body mass index 18.7.
Examination of the head and neck shows normal dentition, dry mucus membranes, and no oral exudates. The thyroid is normal, and no masses or lymphadenopathy are noted.
Heart sounds and rhythm are normal, and the lungs are clear with no crackles or rubs. The abdomen is scaphoid and soft, with no distention. She has epigastric tenderness but no rebound, guarding, rigidity, palpable mass, or costovertebral angle tenderness. Bowel sounds are normal. The neurologic examination is normal.
NARROWING THE DIAGNOSIS
1. Given the history and findings so far, which is the least likely cause of her symptoms?
- Lactose intolerance
- Celiac disease
- Crohn disease
- Duodenal ulcer
- Eating disorder
This young woman’s presentation has some features found in all of these conditions. However, the least likely is lactose intolerance.
Lactose intolerance results from a shortage of the enzyme lactase, which is normally produced by the cells that line the small intestine. Close to 50 million American adults have lactose intolerance. Common symptoms include nausea, cramps, bloating, gas, and diarrhea, which begin about 30 minutes to 2 hours after eating or drinking foods containing lactose.
Since the patient’s symptoms did not improve when she tried omitting milk products, and since lactose intolerance is rarely associated with pain radiating to the back and with severe vomiting, this is the least likely cause of her symptoms.
Celiac disease presents with a myriad of symptoms—sometimes without gastrointestinal (GI) symptoms. Anemia is the most common laboratory finding, due most often to iron deficiency, but also due to deficiencies of vitamin B12 and folate as a result of malabsorption.1
Our patient’s laboratory values—especially her red cell indices—do not confirm this finding. One must also remember, however, that hemoglobin tends to be falsely elevated in patients who are dehydrated.
Crohn disease often presents with occult blood loss, low-grade fever, weight loss, and anemia. Though the condition is most often ileocolic, it can affect any part of the gastrointestinal tract. Nevertheless, most patients with gastroduodenal involvement have previously been diagnosed with ileocolic disease, and gastroduodenal involvement manifests later. Nonradiating epigastric pain is very common. Obstructive symptoms due to gastroduodenal strictures (eg, postprandial vomiting, epigastric pain, weight loss, bloating) are also common. 2
Duodenal ulcer. The most important factors responsible for duodenal ulcers are NSAID use and Helicobacter pylori infection.3 Duodenal ulcers have a variety of clinical presentations, ranging from no symptoms to severe pain. Epigastric pain can be sharp, dull, burning, or penetrating. Many patients complain of a feeling of hunger and weight gain—as opposed to gastric ulcer, in which patients experience anorexia and weight loss. Abdominal pain generally occurs several hours after meals and often awakens the patient at night. Pain is often relieved by food, but this phenomenon is present in only 20% to 60% of patients and probably is not specific for duodenal ulcer.
Our patient does not use NSAIDs, but some of her symptoms, such as postprandial pain, epigastric pain radiating to the back, and nausea and vomiting are seen with duodenal ulcer.
Eating disorders. The two main types of eating disorders—anorexia nervosa and bulimia nervosa—have a significant diagnostic overlap,4 and a third type, binge-eating disorder, is currently being investigated and defined. Girls and women are 10 times as likely as boys and men to develop an eating disorder.
People with anorexia have a distorted view of their bodies. Even when they are extremely thin, they see themselves as too fat.
Bulimia is characterized by binge-eating, purging, and overexercising to compensate for the excess calories. Patients are often close to normal weight.
Binge-eating disorder involves the consumption of very large amounts of food in a short period of time. About 2% of all young adults in the United States struggle with bingeeating. They are either overweight or obese.
These disorders tend to be associated with other psychiatric disorders such as depression or obsessive-compulsive disorder. Our patient sought medical attention and was appropriately concerned about her weight loss, which make an eating disorder unlikely.
CASE CONTINUED: SHE UNDERGOES CT
2. Which of the following is the most likely diagnosis at this point?
- SMA syndrome
- Chronic mesenteric ischemia involving the SMA
- Megaduodenum due to a connective tissue disorder
SMA syndrome is the most likely diagnosis. Despite its name, this syndrome is not a vascular condition. It is an uncommon cause of proximal intestinal obstruction in which the duodenum is compressed between the SMA and the aorta. First described in 1861, it has also been known as cast syndrome, Wilkie syndrome, and arteriomesenteric duodenal obstruction.5
To date, more than 400 cases of this syndrome have been reported, twice as many in women as in men. Most patients are between 20 and 40 years of age at the time of diagnosis. Common presenting symptoms include postprandial abdominal pain, nausea, vomiting, and weight loss, which may further reduce the angle between the SMA and the aorta. Diarrhea is not generally associated with this syndrome, and in our patient’s case the diarrhea was thought to be unrelated to the SMA syndrome, since it subsided spontaneously.
Conditions and events that cause, contribute to, or worsen SMA syndrome include:
- Rapid weight loss (as in cancer or burns) or lean body habitus
- Prolonged bed rest
- Use of a body cast
- Malabsorption
- Spinal disease, deformity, or trauma
- Scoliosis surgery
- Rapid linear growth without compensatory weight gain
- Abnormally high and fixed position of the ligament of Treitz
- Abdominal surgery
- Cardiac cachexia
- Unusually low origin of the SMA.7
More common causes of mechanical smallbowel obstruction are adhesions, hernias, and tumors.8 Hyperactive, high-pitched peristalsis with rushes coinciding with cramps is typical. Abdominal cramps are centered around the umbilicus or in the epigastrium and are associated with vomiting; obstipation develops in patients with complete obstruction. Patients with partial obstruction may develop diarrhea. Paralytic ileus secondary to hypokalemia is an important consideration in partial obstruction. However, abdominal radiography and CT did not confirm an obstruction, and her symptoms persisted despite correction of the potassium level.
Chronic mesenteric ischemia can be caused by vasculitis, nonocclusive conditions that cause prolonged vasoconstriction (eg, cocaine ingestion), or reduced cardiac output.9 Symptoms are due to the gradual reduction in blood flow to the intestine that occurs during eating. Our patient’s toxicology report did not suggest cocaine abuse, and her history and the workup thus far do not suggest heart failure. A workup for vasculitis was negative.
Megaduodenum, SMA-like syndrome. In rare cases, dilation of the duodenum at the level of the SMA may be part of a generalized duodenal dilation caused by something other than obstruction due to mechanical compression. There are conditions, as described below, that cause an SMA-like syndrome.
A compression defect of the duodenum at the site where the SMA crossed the duodenum was found in a series of 11 cases of systemic sclerosis.10 These patients had definite dilation of the duodenum, but it was a result of atrophy of the muscle layers and replacement by collagenous tissue, changes that result in diminished peristalsis, loss of muscle tone, and dilation. The duodenum yields to pressure in its third portion under the SMA.
Several pathologic conditions, particularly connective tissue disorders, may predispose to the development of a megaduodenum that may result in an imprint on the duodenum at the level of the SMA. The most noteworthy of these conditions is scleroderma. Other conditions that can cause reduced duodenal peristalsis include diabetes, pancreatitis, dermatomyositis, lupus erythematosus, myxedema, and amyloidosis.11
It is important to distinguish SMA syndrome from SMA-like syndromes for several reasons.12 SMA-like syndromes result in loss of normal peristalsis. Further, the conditions have different outcomes, even though they are managed similarly initially, ie, with rehydration and parenteral nutrition. Surgery is to be avoided if possible in conditions that affect widespread areas of the intestine, such as scleroderma or diabetic neuropathy.
3. Which of the following is helpful in confirming SMA syndrome?
- CT of the abdomen
- Upper GI radiography series
- Upper GI endoscopy
All three can help confirm the diagnosis.
CT of the abdomen is a convenient, safe, rapid, readily available, and relatively noninvasive way to evaluate the aortomesenteric angle and to view retroperitoneal and mesenteric fat.13 Rehydration before injecting intravenous dye is important to avoid precipitating renal failure. In this patient, CT findings that helped make the diagnosis included a narrow aortomesenteric angle, compression of the duodenum, and a paucity of fat around the SMA.
An upper GI series can reveal dilation of the first and second portions of the duodenum and abrupt compression of the duodenal mucosal folds. Other findings can include a delay of 4 to 6 hours in gastroduodenal transit and relief of the obstruction when the patient is in the left lateral decubitus position. The Hayes maneuver refers to the disappearance of these radiologic features in the knee-chest position on cinefluoroscopy.14 The findings mentioned above are best noted in the supine position on both radiography and CT.
Endoscopy is necessary to rule out mechanical causes of duodenal obstruction. A pulsatile extrinsic compression suggests this condition but is found only occasionally.
Other imaging studies, such as ultrasonography, arteriography, and hypotonic duodenography, are used less often.
4. At this time, which of the following would be the most appropriate initial treatment in this patient?
- Conservative treatment
- Narcotics
- Duodenojejunostomy
Conservative treatment is indicated initially in all cases of SMA syndrome.15 This involves reversing precipitating factors and replacing fluid, electrolytes, and nutrition via total parenteral nutrition and nasogastric decompression.
To avoid keeping the patient on intravenous therapy for a prolonged time, it is important to start enteral feeding once the pain has subsided and the patient can tolerate it. A double-lumen nasojejunal tube is passed distal to the obstruction under fluoroscopic guidance. During feedings, the patient should be in the modified knee-chest, prone, or leftside-down position, all of which increase the aortomesenteric angle.
Delaying the treatment of SMA syndrome can increase the risk of morbidity and mortality by progressive malnutrition, dehydration, oliguria, electrolyte abnormalities (eg, hypokalemia), or intestinal perforation from prolonged ischemia.16,17
Narcotics and other drugs known to slow gut motility should be avoided.
Symptoms typically improve after restoration of normal body weight. If conservative treatment fails, or if the case is severe or chronic, surgery is required.18 Fortunately, this is not required often.
Duodenojejunostomy is the most common surgical treatment and involves creation of an alternate route between the duodenum and the jejunum, bypassing the compression between the aorta and the SMA. Other procedures include gastrojejunostomy, laparoscopic duodenojejunostomy, 19 a Roux-en-Y procedure, robotically assisted duodenojejunostomy, and anterior transposition of the third portion of the duodenum. Cleavage of the ligament of Treitz is another option, enabling the duodenum to drop away from the apex of the sharpened aortomesenteric angle.
WHEN TO CONSIDER SMA SYNDROME
The SMA syndrome is an uncommon cause of a very common presenting symptom, ie, abdominal pain. Nevertheless, it should be considered in the differential diagnosis of abdominal pain, especially in patients who have conditions that cause significant weight loss, such as anorexia nervosa, malabsorption, or hypercatabolic states such as burns, major surgery, severe injuries, or malignancies. The diagnosis is based on a thorough history and on supportive findings from CT and endoscopy.
In our patient, weight loss began with nonspecific diarrhea but perpetuated itself as SMA syndrome occurred.
Appropriate management consists of interrupting the cycle of weight loss and secondary upper gut obstruction. For patients in whom more definitive therapy is not feasible, a gastrostomy tube for decompression with a jejunal extension available for feeding appears to be a reasonable and safe treatment option. Duodenojejunostomy is considered the procedure of choice in severe cases.
CASE CONCLUDED
Fortunately, our patient responded well to conservative management. She was treated with intravenous hydration and correction of electrolytes and 10 days later was able to tolerate a soft diet. She was discharged in stable condition. At a follow-up visit 2 weeks later, she reported minimal abdominal discomfort, was able to tolerate meals, and had gained a few pounds. She continues to do well.
- Iovino P, Ciacci C, Sabbatini F, Acioli DM, D'Argenio G, Mazzacca G. Esophageal impairment in adult celiac disease with steatorrhea. Am J Gastroenterol 1998; 93:1243–1249.
- Loftus EV. Upper gastrointestinal tract Crohn’s disease. Clin Perspect Gastroenterol 2002; 5:188–191.
- Zapata-Colindres JC, Zepeda-Gómez S, Montaño-Loza A, Vázquez-Ballesteros E, de Jesús Villalobos J, Valdovinos-Andraca F. The association of Helicobacter pylori infection and nonsteroidal antiinflammatory drugs in peptic ulcer disease. Can J Gastroenterol 2006; 20:277–280.
- Milos G, Spindler A, Schnyder U, Fairburn CG. Instability of eating disorder diagnoses: prospective study. Br J Psychiatry 2005; 187:573–578.
- Wilkie DP. Chronic duodenal ileus. Br J Surg 1921; 9:204–214.
- Ozkurt H, Cenker MM, Bas N, Erturk SM, Basak M. Measurement of the distance and angle between the aorta and superior mesenteric artery: normal values in different BMI categories. Surg Radiol Anat 2007; 29:595–599.
- Lippl F, Hannig C, Weiss W, Allescher HD, Classen M, Kurjak M. Superior mesenteric artery syndrome: diagnosis and treatment from the gastroenterologist's view. J Gastroenterol 2002; 37:640–643.
- Balthazar EJ. George W. Holmes Lecture. CT of small-bowel obstruction. AJR Am J Roentgenol 1994; 162:255–261.
- Chang JB, Stein TA. Mesenteric ischemia: acute and chronic. Ann Vasc Surg 2003; 17:323–328.
- Gondos B. Duodenal compression defect and the “superior mesenteric artery syndrome” 1. Radiology 1977; 123:575–580.
- Cohen LB, Field SP, Sachar DB. The superior mesenteric artery syndrome. The disease that isn't, or is it? J Clin Gastroenterol 1985; 7:113–716.
- Ahmed AR, Taylor I. Superior mesenteric artery syndrome. Postgrad Med J 1997; 73:776–778.
- Santer R, Young C, Rossi T, Riddlesberger MM. Computed tomography in superior mesenteric artery syndrome. Pediatr Radiol 1991; 21:154–155.
- Lukes PJ, Rolny P, Nilson AE, Gamklou R, Darle N, Dotevall G. Diagnostic value of hypotonic duodenography in superior mesenteric artery syndrome. Acta Chir Scand 1978; 144:39–43.
- Dietz UA, Debus ES, Heuko-Valiati L, et al. Aorto-mesenteric artery compression syndrome. Chirurg 2000; 71:1345–1351.
- Lim JE, Duke GL, Eachempati SR. Superior mesenteric artery syndrome presenting with acute massive gastric dilatation, gastric wall pneumatosis, and portal venous gas. Surgery 2003; 134:840–843.
- Fuhrman MA, Felig DM, Tanchel ME. Superior mesenteric artery syndrome with obstructing duodenal bezoar. Gastrointest Endosc 2003; 57:387.
- Hines JR, Gore RM, Ballantyne GH. Superior mesenteric artery syndrome. Diagnostic criteria and therapeutic approaches. Am J Surg 1984; 148:630–632.
- Gersin KS, Heniford BT. Laparoscopic duodenojejunostomy for treatment of superior mesenteric artery syndrome. JSLS 1998; 2:281–284.
A 20-year-old woman presents to the emergency department with postprandial epigastric and right-upper-quadrant pain, sometimes associated with nausea. She has been having six to eight loose bowel movements every day, with no blood or mucus, and she has lost about 20 lb despite a good appetite. The diarrhea did not improve when she tried omitting milk products and carbohydrates.
Her symptoms began several months ago, but she says that 3 days ago the pain worsened steadily, radiating to the middle of her back, with associated episodes of nonbloody, nonbilious emesis. She cannot keep down liquids or solids. She says she has never had such episodes in the past.
She reports no oral ulcers, urinary symptoms, skin rashes, musculoskeletal pain, or neurologic symptoms, and she denies being anxious or depressed.
She has no history of serious illness, surgery, or hospitalization. She smokes a half pack of cigarettes a day, drinks alcohol occasionally, and smokes marijuana occasionally. She is employed as a certified nursing assistant.
She is taking ethinyl estradiol-levonorgestrel pills for birth control and takes calcium carbonate as needed for abdominal discomfort. She is taking no other medications, including nonsteroidal anti-inflammatory drugs (NSAIDs).
Her maternal uncle died of colon cancer at age 32, and her mother had colon polyps on colonoscopy. There is no family history of inflammatory bowel disease or celiac sprue. Her father committed suicide.
Her laboratory values
- White blood cell count 10.2 × 109/L (normal range 4–11)
- Red blood cell count 4.71 × 1012/L (3.9–5.5)
- Hemoglobin 14.4 g/dL (12–16)
- Hematocrit 42.4% (37%–47%)
- Mean corpuscular volume 90 fL (83–99)
- Mean corpuscular hemoglobin 30.6 pg (27–33)
- Platelet count 230 × 109/L (150–400)
- Red cell distribution width 13.3% (11.5%–14.5%)
- Sodium 140 mmol/L (132–148)
- Potassium 3.3 mmol/L (3.5–5.0)
- Chloride 104 mmol/L (98–111)
- Bicarbonate 28 mmol/L (23–32)
- Blood urea nitrogen 9 mg/dL (8–25)
- Creatinine 0.8 mg/dL (0.7–1.4)
- Glucose 87 mg/dL (65–100)
- Alanine aminotransferase 26 U/L (0–45)
- Aspartate aminotransferase 21 U/L (7–40)
- Alkaline phosphatase 101 U/L (40–150)
- Total bilirubin 0.8 mg/dL (0–1.5)
- Albumin 3.5 g/dL (3.5–5)
- Pregnancy screen negative
- Urine toxicology screen negative.
Physical examination
The patient is very thin and appears quite uncomfortable. Her temperature is 99.7°F (37.6°C), pulse rate 101, respiratory rate 18, blood pressure 111/67 mm Hg, and oxygen saturation 96% on room air. Her skin is warm and dry. Her height is 66 inches, weight 116 lb, and body mass index 18.7.
Examination of the head and neck shows normal dentition, dry mucus membranes, and no oral exudates. The thyroid is normal, and no masses or lymphadenopathy are noted.
Heart sounds and rhythm are normal, and the lungs are clear with no crackles or rubs. The abdomen is scaphoid and soft, with no distention. She has epigastric tenderness but no rebound, guarding, rigidity, palpable mass, or costovertebral angle tenderness. Bowel sounds are normal. The neurologic examination is normal.
NARROWING THE DIAGNOSIS
1. Given the history and findings so far, which is the least likely cause of her symptoms?
- Lactose intolerance
- Celiac disease
- Crohn disease
- Duodenal ulcer
- Eating disorder
This young woman’s presentation has some features found in all of these conditions. However, the least likely is lactose intolerance.
Lactose intolerance results from a shortage of the enzyme lactase, which is normally produced by the cells that line the small intestine. Close to 50 million American adults have lactose intolerance. Common symptoms include nausea, cramps, bloating, gas, and diarrhea, which begin about 30 minutes to 2 hours after eating or drinking foods containing lactose.
Since the patient’s symptoms did not improve when she tried omitting milk products, and since lactose intolerance is rarely associated with pain radiating to the back and with severe vomiting, this is the least likely cause of her symptoms.
Celiac disease presents with a myriad of symptoms—sometimes without gastrointestinal (GI) symptoms. Anemia is the most common laboratory finding, due most often to iron deficiency, but also due to deficiencies of vitamin B12 and folate as a result of malabsorption.1
Our patient’s laboratory values—especially her red cell indices—do not confirm this finding. One must also remember, however, that hemoglobin tends to be falsely elevated in patients who are dehydrated.
Crohn disease often presents with occult blood loss, low-grade fever, weight loss, and anemia. Though the condition is most often ileocolic, it can affect any part of the gastrointestinal tract. Nevertheless, most patients with gastroduodenal involvement have previously been diagnosed with ileocolic disease, and gastroduodenal involvement manifests later. Nonradiating epigastric pain is very common. Obstructive symptoms due to gastroduodenal strictures (eg, postprandial vomiting, epigastric pain, weight loss, bloating) are also common. 2
Duodenal ulcer. The most important factors responsible for duodenal ulcers are NSAID use and Helicobacter pylori infection.3 Duodenal ulcers have a variety of clinical presentations, ranging from no symptoms to severe pain. Epigastric pain can be sharp, dull, burning, or penetrating. Many patients complain of a feeling of hunger and weight gain—as opposed to gastric ulcer, in which patients experience anorexia and weight loss. Abdominal pain generally occurs several hours after meals and often awakens the patient at night. Pain is often relieved by food, but this phenomenon is present in only 20% to 60% of patients and probably is not specific for duodenal ulcer.
Our patient does not use NSAIDs, but some of her symptoms, such as postprandial pain, epigastric pain radiating to the back, and nausea and vomiting are seen with duodenal ulcer.
Eating disorders. The two main types of eating disorders—anorexia nervosa and bulimia nervosa—have a significant diagnostic overlap,4 and a third type, binge-eating disorder, is currently being investigated and defined. Girls and women are 10 times as likely as boys and men to develop an eating disorder.
People with anorexia have a distorted view of their bodies. Even when they are extremely thin, they see themselves as too fat.
Bulimia is characterized by binge-eating, purging, and overexercising to compensate for the excess calories. Patients are often close to normal weight.
Binge-eating disorder involves the consumption of very large amounts of food in a short period of time. About 2% of all young adults in the United States struggle with bingeeating. They are either overweight or obese.
These disorders tend to be associated with other psychiatric disorders such as depression or obsessive-compulsive disorder. Our patient sought medical attention and was appropriately concerned about her weight loss, which make an eating disorder unlikely.
CASE CONTINUED: SHE UNDERGOES CT
2. Which of the following is the most likely diagnosis at this point?
- SMA syndrome
- Chronic mesenteric ischemia involving the SMA
- Megaduodenum due to a connective tissue disorder
SMA syndrome is the most likely diagnosis. Despite its name, this syndrome is not a vascular condition. It is an uncommon cause of proximal intestinal obstruction in which the duodenum is compressed between the SMA and the aorta. First described in 1861, it has also been known as cast syndrome, Wilkie syndrome, and arteriomesenteric duodenal obstruction.5
To date, more than 400 cases of this syndrome have been reported, twice as many in women as in men. Most patients are between 20 and 40 years of age at the time of diagnosis. Common presenting symptoms include postprandial abdominal pain, nausea, vomiting, and weight loss, which may further reduce the angle between the SMA and the aorta. Diarrhea is not generally associated with this syndrome, and in our patient’s case the diarrhea was thought to be unrelated to the SMA syndrome, since it subsided spontaneously.
Conditions and events that cause, contribute to, or worsen SMA syndrome include:
- Rapid weight loss (as in cancer or burns) or lean body habitus
- Prolonged bed rest
- Use of a body cast
- Malabsorption
- Spinal disease, deformity, or trauma
- Scoliosis surgery
- Rapid linear growth without compensatory weight gain
- Abnormally high and fixed position of the ligament of Treitz
- Abdominal surgery
- Cardiac cachexia
- Unusually low origin of the SMA.7
More common causes of mechanical smallbowel obstruction are adhesions, hernias, and tumors.8 Hyperactive, high-pitched peristalsis with rushes coinciding with cramps is typical. Abdominal cramps are centered around the umbilicus or in the epigastrium and are associated with vomiting; obstipation develops in patients with complete obstruction. Patients with partial obstruction may develop diarrhea. Paralytic ileus secondary to hypokalemia is an important consideration in partial obstruction. However, abdominal radiography and CT did not confirm an obstruction, and her symptoms persisted despite correction of the potassium level.
Chronic mesenteric ischemia can be caused by vasculitis, nonocclusive conditions that cause prolonged vasoconstriction (eg, cocaine ingestion), or reduced cardiac output.9 Symptoms are due to the gradual reduction in blood flow to the intestine that occurs during eating. Our patient’s toxicology report did not suggest cocaine abuse, and her history and the workup thus far do not suggest heart failure. A workup for vasculitis was negative.
Megaduodenum, SMA-like syndrome. In rare cases, dilation of the duodenum at the level of the SMA may be part of a generalized duodenal dilation caused by something other than obstruction due to mechanical compression. There are conditions, as described below, that cause an SMA-like syndrome.
A compression defect of the duodenum at the site where the SMA crossed the duodenum was found in a series of 11 cases of systemic sclerosis.10 These patients had definite dilation of the duodenum, but it was a result of atrophy of the muscle layers and replacement by collagenous tissue, changes that result in diminished peristalsis, loss of muscle tone, and dilation. The duodenum yields to pressure in its third portion under the SMA.
Several pathologic conditions, particularly connective tissue disorders, may predispose to the development of a megaduodenum that may result in an imprint on the duodenum at the level of the SMA. The most noteworthy of these conditions is scleroderma. Other conditions that can cause reduced duodenal peristalsis include diabetes, pancreatitis, dermatomyositis, lupus erythematosus, myxedema, and amyloidosis.11
It is important to distinguish SMA syndrome from SMA-like syndromes for several reasons.12 SMA-like syndromes result in loss of normal peristalsis. Further, the conditions have different outcomes, even though they are managed similarly initially, ie, with rehydration and parenteral nutrition. Surgery is to be avoided if possible in conditions that affect widespread areas of the intestine, such as scleroderma or diabetic neuropathy.
3. Which of the following is helpful in confirming SMA syndrome?
- CT of the abdomen
- Upper GI radiography series
- Upper GI endoscopy
All three can help confirm the diagnosis.
CT of the abdomen is a convenient, safe, rapid, readily available, and relatively noninvasive way to evaluate the aortomesenteric angle and to view retroperitoneal and mesenteric fat.13 Rehydration before injecting intravenous dye is important to avoid precipitating renal failure. In this patient, CT findings that helped make the diagnosis included a narrow aortomesenteric angle, compression of the duodenum, and a paucity of fat around the SMA.
An upper GI series can reveal dilation of the first and second portions of the duodenum and abrupt compression of the duodenal mucosal folds. Other findings can include a delay of 4 to 6 hours in gastroduodenal transit and relief of the obstruction when the patient is in the left lateral decubitus position. The Hayes maneuver refers to the disappearance of these radiologic features in the knee-chest position on cinefluoroscopy.14 The findings mentioned above are best noted in the supine position on both radiography and CT.
Endoscopy is necessary to rule out mechanical causes of duodenal obstruction. A pulsatile extrinsic compression suggests this condition but is found only occasionally.
Other imaging studies, such as ultrasonography, arteriography, and hypotonic duodenography, are used less often.
4. At this time, which of the following would be the most appropriate initial treatment in this patient?
- Conservative treatment
- Narcotics
- Duodenojejunostomy
Conservative treatment is indicated initially in all cases of SMA syndrome.15 This involves reversing precipitating factors and replacing fluid, electrolytes, and nutrition via total parenteral nutrition and nasogastric decompression.
To avoid keeping the patient on intravenous therapy for a prolonged time, it is important to start enteral feeding once the pain has subsided and the patient can tolerate it. A double-lumen nasojejunal tube is passed distal to the obstruction under fluoroscopic guidance. During feedings, the patient should be in the modified knee-chest, prone, or leftside-down position, all of which increase the aortomesenteric angle.
Delaying the treatment of SMA syndrome can increase the risk of morbidity and mortality by progressive malnutrition, dehydration, oliguria, electrolyte abnormalities (eg, hypokalemia), or intestinal perforation from prolonged ischemia.16,17
Narcotics and other drugs known to slow gut motility should be avoided.
Symptoms typically improve after restoration of normal body weight. If conservative treatment fails, or if the case is severe or chronic, surgery is required.18 Fortunately, this is not required often.
Duodenojejunostomy is the most common surgical treatment and involves creation of an alternate route between the duodenum and the jejunum, bypassing the compression between the aorta and the SMA. Other procedures include gastrojejunostomy, laparoscopic duodenojejunostomy, 19 a Roux-en-Y procedure, robotically assisted duodenojejunostomy, and anterior transposition of the third portion of the duodenum. Cleavage of the ligament of Treitz is another option, enabling the duodenum to drop away from the apex of the sharpened aortomesenteric angle.
WHEN TO CONSIDER SMA SYNDROME
The SMA syndrome is an uncommon cause of a very common presenting symptom, ie, abdominal pain. Nevertheless, it should be considered in the differential diagnosis of abdominal pain, especially in patients who have conditions that cause significant weight loss, such as anorexia nervosa, malabsorption, or hypercatabolic states such as burns, major surgery, severe injuries, or malignancies. The diagnosis is based on a thorough history and on supportive findings from CT and endoscopy.
In our patient, weight loss began with nonspecific diarrhea but perpetuated itself as SMA syndrome occurred.
Appropriate management consists of interrupting the cycle of weight loss and secondary upper gut obstruction. For patients in whom more definitive therapy is not feasible, a gastrostomy tube for decompression with a jejunal extension available for feeding appears to be a reasonable and safe treatment option. Duodenojejunostomy is considered the procedure of choice in severe cases.
CASE CONCLUDED
Fortunately, our patient responded well to conservative management. She was treated with intravenous hydration and correction of electrolytes and 10 days later was able to tolerate a soft diet. She was discharged in stable condition. At a follow-up visit 2 weeks later, she reported minimal abdominal discomfort, was able to tolerate meals, and had gained a few pounds. She continues to do well.
A 20-year-old woman presents to the emergency department with postprandial epigastric and right-upper-quadrant pain, sometimes associated with nausea. She has been having six to eight loose bowel movements every day, with no blood or mucus, and she has lost about 20 lb despite a good appetite. The diarrhea did not improve when she tried omitting milk products and carbohydrates.
Her symptoms began several months ago, but she says that 3 days ago the pain worsened steadily, radiating to the middle of her back, with associated episodes of nonbloody, nonbilious emesis. She cannot keep down liquids or solids. She says she has never had such episodes in the past.
She reports no oral ulcers, urinary symptoms, skin rashes, musculoskeletal pain, or neurologic symptoms, and she denies being anxious or depressed.
She has no history of serious illness, surgery, or hospitalization. She smokes a half pack of cigarettes a day, drinks alcohol occasionally, and smokes marijuana occasionally. She is employed as a certified nursing assistant.
She is taking ethinyl estradiol-levonorgestrel pills for birth control and takes calcium carbonate as needed for abdominal discomfort. She is taking no other medications, including nonsteroidal anti-inflammatory drugs (NSAIDs).
Her maternal uncle died of colon cancer at age 32, and her mother had colon polyps on colonoscopy. There is no family history of inflammatory bowel disease or celiac sprue. Her father committed suicide.
Her laboratory values
- White blood cell count 10.2 × 109/L (normal range 4–11)
- Red blood cell count 4.71 × 1012/L (3.9–5.5)
- Hemoglobin 14.4 g/dL (12–16)
- Hematocrit 42.4% (37%–47%)
- Mean corpuscular volume 90 fL (83–99)
- Mean corpuscular hemoglobin 30.6 pg (27–33)
- Platelet count 230 × 109/L (150–400)
- Red cell distribution width 13.3% (11.5%–14.5%)
- Sodium 140 mmol/L (132–148)
- Potassium 3.3 mmol/L (3.5–5.0)
- Chloride 104 mmol/L (98–111)
- Bicarbonate 28 mmol/L (23–32)
- Blood urea nitrogen 9 mg/dL (8–25)
- Creatinine 0.8 mg/dL (0.7–1.4)
- Glucose 87 mg/dL (65–100)
- Alanine aminotransferase 26 U/L (0–45)
- Aspartate aminotransferase 21 U/L (7–40)
- Alkaline phosphatase 101 U/L (40–150)
- Total bilirubin 0.8 mg/dL (0–1.5)
- Albumin 3.5 g/dL (3.5–5)
- Pregnancy screen negative
- Urine toxicology screen negative.
Physical examination
The patient is very thin and appears quite uncomfortable. Her temperature is 99.7°F (37.6°C), pulse rate 101, respiratory rate 18, blood pressure 111/67 mm Hg, and oxygen saturation 96% on room air. Her skin is warm and dry. Her height is 66 inches, weight 116 lb, and body mass index 18.7.
Examination of the head and neck shows normal dentition, dry mucus membranes, and no oral exudates. The thyroid is normal, and no masses or lymphadenopathy are noted.
Heart sounds and rhythm are normal, and the lungs are clear with no crackles or rubs. The abdomen is scaphoid and soft, with no distention. She has epigastric tenderness but no rebound, guarding, rigidity, palpable mass, or costovertebral angle tenderness. Bowel sounds are normal. The neurologic examination is normal.
NARROWING THE DIAGNOSIS
1. Given the history and findings so far, which is the least likely cause of her symptoms?
- Lactose intolerance
- Celiac disease
- Crohn disease
- Duodenal ulcer
- Eating disorder
This young woman’s presentation has some features found in all of these conditions. However, the least likely is lactose intolerance.
Lactose intolerance results from a shortage of the enzyme lactase, which is normally produced by the cells that line the small intestine. Close to 50 million American adults have lactose intolerance. Common symptoms include nausea, cramps, bloating, gas, and diarrhea, which begin about 30 minutes to 2 hours after eating or drinking foods containing lactose.
Since the patient’s symptoms did not improve when she tried omitting milk products, and since lactose intolerance is rarely associated with pain radiating to the back and with severe vomiting, this is the least likely cause of her symptoms.
Celiac disease presents with a myriad of symptoms—sometimes without gastrointestinal (GI) symptoms. Anemia is the most common laboratory finding, due most often to iron deficiency, but also due to deficiencies of vitamin B12 and folate as a result of malabsorption.1
Our patient’s laboratory values—especially her red cell indices—do not confirm this finding. One must also remember, however, that hemoglobin tends to be falsely elevated in patients who are dehydrated.
Crohn disease often presents with occult blood loss, low-grade fever, weight loss, and anemia. Though the condition is most often ileocolic, it can affect any part of the gastrointestinal tract. Nevertheless, most patients with gastroduodenal involvement have previously been diagnosed with ileocolic disease, and gastroduodenal involvement manifests later. Nonradiating epigastric pain is very common. Obstructive symptoms due to gastroduodenal strictures (eg, postprandial vomiting, epigastric pain, weight loss, bloating) are also common. 2
Duodenal ulcer. The most important factors responsible for duodenal ulcers are NSAID use and Helicobacter pylori infection.3 Duodenal ulcers have a variety of clinical presentations, ranging from no symptoms to severe pain. Epigastric pain can be sharp, dull, burning, or penetrating. Many patients complain of a feeling of hunger and weight gain—as opposed to gastric ulcer, in which patients experience anorexia and weight loss. Abdominal pain generally occurs several hours after meals and often awakens the patient at night. Pain is often relieved by food, but this phenomenon is present in only 20% to 60% of patients and probably is not specific for duodenal ulcer.
Our patient does not use NSAIDs, but some of her symptoms, such as postprandial pain, epigastric pain radiating to the back, and nausea and vomiting are seen with duodenal ulcer.
Eating disorders. The two main types of eating disorders—anorexia nervosa and bulimia nervosa—have a significant diagnostic overlap,4 and a third type, binge-eating disorder, is currently being investigated and defined. Girls and women are 10 times as likely as boys and men to develop an eating disorder.
People with anorexia have a distorted view of their bodies. Even when they are extremely thin, they see themselves as too fat.
Bulimia is characterized by binge-eating, purging, and overexercising to compensate for the excess calories. Patients are often close to normal weight.
Binge-eating disorder involves the consumption of very large amounts of food in a short period of time. About 2% of all young adults in the United States struggle with bingeeating. They are either overweight or obese.
These disorders tend to be associated with other psychiatric disorders such as depression or obsessive-compulsive disorder. Our patient sought medical attention and was appropriately concerned about her weight loss, which make an eating disorder unlikely.
CASE CONTINUED: SHE UNDERGOES CT
2. Which of the following is the most likely diagnosis at this point?
- SMA syndrome
- Chronic mesenteric ischemia involving the SMA
- Megaduodenum due to a connective tissue disorder
SMA syndrome is the most likely diagnosis. Despite its name, this syndrome is not a vascular condition. It is an uncommon cause of proximal intestinal obstruction in which the duodenum is compressed between the SMA and the aorta. First described in 1861, it has also been known as cast syndrome, Wilkie syndrome, and arteriomesenteric duodenal obstruction.5
To date, more than 400 cases of this syndrome have been reported, twice as many in women as in men. Most patients are between 20 and 40 years of age at the time of diagnosis. Common presenting symptoms include postprandial abdominal pain, nausea, vomiting, and weight loss, which may further reduce the angle between the SMA and the aorta. Diarrhea is not generally associated with this syndrome, and in our patient’s case the diarrhea was thought to be unrelated to the SMA syndrome, since it subsided spontaneously.
Conditions and events that cause, contribute to, or worsen SMA syndrome include:
- Rapid weight loss (as in cancer or burns) or lean body habitus
- Prolonged bed rest
- Use of a body cast
- Malabsorption
- Spinal disease, deformity, or trauma
- Scoliosis surgery
- Rapid linear growth without compensatory weight gain
- Abnormally high and fixed position of the ligament of Treitz
- Abdominal surgery
- Cardiac cachexia
- Unusually low origin of the SMA.7
More common causes of mechanical smallbowel obstruction are adhesions, hernias, and tumors.8 Hyperactive, high-pitched peristalsis with rushes coinciding with cramps is typical. Abdominal cramps are centered around the umbilicus or in the epigastrium and are associated with vomiting; obstipation develops in patients with complete obstruction. Patients with partial obstruction may develop diarrhea. Paralytic ileus secondary to hypokalemia is an important consideration in partial obstruction. However, abdominal radiography and CT did not confirm an obstruction, and her symptoms persisted despite correction of the potassium level.
Chronic mesenteric ischemia can be caused by vasculitis, nonocclusive conditions that cause prolonged vasoconstriction (eg, cocaine ingestion), or reduced cardiac output.9 Symptoms are due to the gradual reduction in blood flow to the intestine that occurs during eating. Our patient’s toxicology report did not suggest cocaine abuse, and her history and the workup thus far do not suggest heart failure. A workup for vasculitis was negative.
Megaduodenum, SMA-like syndrome. In rare cases, dilation of the duodenum at the level of the SMA may be part of a generalized duodenal dilation caused by something other than obstruction due to mechanical compression. There are conditions, as described below, that cause an SMA-like syndrome.
A compression defect of the duodenum at the site where the SMA crossed the duodenum was found in a series of 11 cases of systemic sclerosis.10 These patients had definite dilation of the duodenum, but it was a result of atrophy of the muscle layers and replacement by collagenous tissue, changes that result in diminished peristalsis, loss of muscle tone, and dilation. The duodenum yields to pressure in its third portion under the SMA.
Several pathologic conditions, particularly connective tissue disorders, may predispose to the development of a megaduodenum that may result in an imprint on the duodenum at the level of the SMA. The most noteworthy of these conditions is scleroderma. Other conditions that can cause reduced duodenal peristalsis include diabetes, pancreatitis, dermatomyositis, lupus erythematosus, myxedema, and amyloidosis.11
It is important to distinguish SMA syndrome from SMA-like syndromes for several reasons.12 SMA-like syndromes result in loss of normal peristalsis. Further, the conditions have different outcomes, even though they are managed similarly initially, ie, with rehydration and parenteral nutrition. Surgery is to be avoided if possible in conditions that affect widespread areas of the intestine, such as scleroderma or diabetic neuropathy.
3. Which of the following is helpful in confirming SMA syndrome?
- CT of the abdomen
- Upper GI radiography series
- Upper GI endoscopy
All three can help confirm the diagnosis.
CT of the abdomen is a convenient, safe, rapid, readily available, and relatively noninvasive way to evaluate the aortomesenteric angle and to view retroperitoneal and mesenteric fat.13 Rehydration before injecting intravenous dye is important to avoid precipitating renal failure. In this patient, CT findings that helped make the diagnosis included a narrow aortomesenteric angle, compression of the duodenum, and a paucity of fat around the SMA.
An upper GI series can reveal dilation of the first and second portions of the duodenum and abrupt compression of the duodenal mucosal folds. Other findings can include a delay of 4 to 6 hours in gastroduodenal transit and relief of the obstruction when the patient is in the left lateral decubitus position. The Hayes maneuver refers to the disappearance of these radiologic features in the knee-chest position on cinefluoroscopy.14 The findings mentioned above are best noted in the supine position on both radiography and CT.
Endoscopy is necessary to rule out mechanical causes of duodenal obstruction. A pulsatile extrinsic compression suggests this condition but is found only occasionally.
Other imaging studies, such as ultrasonography, arteriography, and hypotonic duodenography, are used less often.
4. At this time, which of the following would be the most appropriate initial treatment in this patient?
- Conservative treatment
- Narcotics
- Duodenojejunostomy
Conservative treatment is indicated initially in all cases of SMA syndrome.15 This involves reversing precipitating factors and replacing fluid, electrolytes, and nutrition via total parenteral nutrition and nasogastric decompression.
To avoid keeping the patient on intravenous therapy for a prolonged time, it is important to start enteral feeding once the pain has subsided and the patient can tolerate it. A double-lumen nasojejunal tube is passed distal to the obstruction under fluoroscopic guidance. During feedings, the patient should be in the modified knee-chest, prone, or leftside-down position, all of which increase the aortomesenteric angle.
Delaying the treatment of SMA syndrome can increase the risk of morbidity and mortality by progressive malnutrition, dehydration, oliguria, electrolyte abnormalities (eg, hypokalemia), or intestinal perforation from prolonged ischemia.16,17
Narcotics and other drugs known to slow gut motility should be avoided.
Symptoms typically improve after restoration of normal body weight. If conservative treatment fails, or if the case is severe or chronic, surgery is required.18 Fortunately, this is not required often.
Duodenojejunostomy is the most common surgical treatment and involves creation of an alternate route between the duodenum and the jejunum, bypassing the compression between the aorta and the SMA. Other procedures include gastrojejunostomy, laparoscopic duodenojejunostomy, 19 a Roux-en-Y procedure, robotically assisted duodenojejunostomy, and anterior transposition of the third portion of the duodenum. Cleavage of the ligament of Treitz is another option, enabling the duodenum to drop away from the apex of the sharpened aortomesenteric angle.
WHEN TO CONSIDER SMA SYNDROME
The SMA syndrome is an uncommon cause of a very common presenting symptom, ie, abdominal pain. Nevertheless, it should be considered in the differential diagnosis of abdominal pain, especially in patients who have conditions that cause significant weight loss, such as anorexia nervosa, malabsorption, or hypercatabolic states such as burns, major surgery, severe injuries, or malignancies. The diagnosis is based on a thorough history and on supportive findings from CT and endoscopy.
In our patient, weight loss began with nonspecific diarrhea but perpetuated itself as SMA syndrome occurred.
Appropriate management consists of interrupting the cycle of weight loss and secondary upper gut obstruction. For patients in whom more definitive therapy is not feasible, a gastrostomy tube for decompression with a jejunal extension available for feeding appears to be a reasonable and safe treatment option. Duodenojejunostomy is considered the procedure of choice in severe cases.
CASE CONCLUDED
Fortunately, our patient responded well to conservative management. She was treated with intravenous hydration and correction of electrolytes and 10 days later was able to tolerate a soft diet. She was discharged in stable condition. At a follow-up visit 2 weeks later, she reported minimal abdominal discomfort, was able to tolerate meals, and had gained a few pounds. She continues to do well.
- Iovino P, Ciacci C, Sabbatini F, Acioli DM, D'Argenio G, Mazzacca G. Esophageal impairment in adult celiac disease with steatorrhea. Am J Gastroenterol 1998; 93:1243–1249.
- Loftus EV. Upper gastrointestinal tract Crohn’s disease. Clin Perspect Gastroenterol 2002; 5:188–191.
- Zapata-Colindres JC, Zepeda-Gómez S, Montaño-Loza A, Vázquez-Ballesteros E, de Jesús Villalobos J, Valdovinos-Andraca F. The association of Helicobacter pylori infection and nonsteroidal antiinflammatory drugs in peptic ulcer disease. Can J Gastroenterol 2006; 20:277–280.
- Milos G, Spindler A, Schnyder U, Fairburn CG. Instability of eating disorder diagnoses: prospective study. Br J Psychiatry 2005; 187:573–578.
- Wilkie DP. Chronic duodenal ileus. Br J Surg 1921; 9:204–214.
- Ozkurt H, Cenker MM, Bas N, Erturk SM, Basak M. Measurement of the distance and angle between the aorta and superior mesenteric artery: normal values in different BMI categories. Surg Radiol Anat 2007; 29:595–599.
- Lippl F, Hannig C, Weiss W, Allescher HD, Classen M, Kurjak M. Superior mesenteric artery syndrome: diagnosis and treatment from the gastroenterologist's view. J Gastroenterol 2002; 37:640–643.
- Balthazar EJ. George W. Holmes Lecture. CT of small-bowel obstruction. AJR Am J Roentgenol 1994; 162:255–261.
- Chang JB, Stein TA. Mesenteric ischemia: acute and chronic. Ann Vasc Surg 2003; 17:323–328.
- Gondos B. Duodenal compression defect and the “superior mesenteric artery syndrome” 1. Radiology 1977; 123:575–580.
- Cohen LB, Field SP, Sachar DB. The superior mesenteric artery syndrome. The disease that isn't, or is it? J Clin Gastroenterol 1985; 7:113–716.
- Ahmed AR, Taylor I. Superior mesenteric artery syndrome. Postgrad Med J 1997; 73:776–778.
- Santer R, Young C, Rossi T, Riddlesberger MM. Computed tomography in superior mesenteric artery syndrome. Pediatr Radiol 1991; 21:154–155.
- Lukes PJ, Rolny P, Nilson AE, Gamklou R, Darle N, Dotevall G. Diagnostic value of hypotonic duodenography in superior mesenteric artery syndrome. Acta Chir Scand 1978; 144:39–43.
- Dietz UA, Debus ES, Heuko-Valiati L, et al. Aorto-mesenteric artery compression syndrome. Chirurg 2000; 71:1345–1351.
- Lim JE, Duke GL, Eachempati SR. Superior mesenteric artery syndrome presenting with acute massive gastric dilatation, gastric wall pneumatosis, and portal venous gas. Surgery 2003; 134:840–843.
- Fuhrman MA, Felig DM, Tanchel ME. Superior mesenteric artery syndrome with obstructing duodenal bezoar. Gastrointest Endosc 2003; 57:387.
- Hines JR, Gore RM, Ballantyne GH. Superior mesenteric artery syndrome. Diagnostic criteria and therapeutic approaches. Am J Surg 1984; 148:630–632.
- Gersin KS, Heniford BT. Laparoscopic duodenojejunostomy for treatment of superior mesenteric artery syndrome. JSLS 1998; 2:281–284.
- Iovino P, Ciacci C, Sabbatini F, Acioli DM, D'Argenio G, Mazzacca G. Esophageal impairment in adult celiac disease with steatorrhea. Am J Gastroenterol 1998; 93:1243–1249.
- Loftus EV. Upper gastrointestinal tract Crohn’s disease. Clin Perspect Gastroenterol 2002; 5:188–191.
- Zapata-Colindres JC, Zepeda-Gómez S, Montaño-Loza A, Vázquez-Ballesteros E, de Jesús Villalobos J, Valdovinos-Andraca F. The association of Helicobacter pylori infection and nonsteroidal antiinflammatory drugs in peptic ulcer disease. Can J Gastroenterol 2006; 20:277–280.
- Milos G, Spindler A, Schnyder U, Fairburn CG. Instability of eating disorder diagnoses: prospective study. Br J Psychiatry 2005; 187:573–578.
- Wilkie DP. Chronic duodenal ileus. Br J Surg 1921; 9:204–214.
- Ozkurt H, Cenker MM, Bas N, Erturk SM, Basak M. Measurement of the distance and angle between the aorta and superior mesenteric artery: normal values in different BMI categories. Surg Radiol Anat 2007; 29:595–599.
- Lippl F, Hannig C, Weiss W, Allescher HD, Classen M, Kurjak M. Superior mesenteric artery syndrome: diagnosis and treatment from the gastroenterologist's view. J Gastroenterol 2002; 37:640–643.
- Balthazar EJ. George W. Holmes Lecture. CT of small-bowel obstruction. AJR Am J Roentgenol 1994; 162:255–261.
- Chang JB, Stein TA. Mesenteric ischemia: acute and chronic. Ann Vasc Surg 2003; 17:323–328.
- Gondos B. Duodenal compression defect and the “superior mesenteric artery syndrome” 1. Radiology 1977; 123:575–580.
- Cohen LB, Field SP, Sachar DB. The superior mesenteric artery syndrome. The disease that isn't, or is it? J Clin Gastroenterol 1985; 7:113–716.
- Ahmed AR, Taylor I. Superior mesenteric artery syndrome. Postgrad Med J 1997; 73:776–778.
- Santer R, Young C, Rossi T, Riddlesberger MM. Computed tomography in superior mesenteric artery syndrome. Pediatr Radiol 1991; 21:154–155.
- Lukes PJ, Rolny P, Nilson AE, Gamklou R, Darle N, Dotevall G. Diagnostic value of hypotonic duodenography in superior mesenteric artery syndrome. Acta Chir Scand 1978; 144:39–43.
- Dietz UA, Debus ES, Heuko-Valiati L, et al. Aorto-mesenteric artery compression syndrome. Chirurg 2000; 71:1345–1351.
- Lim JE, Duke GL, Eachempati SR. Superior mesenteric artery syndrome presenting with acute massive gastric dilatation, gastric wall pneumatosis, and portal venous gas. Surgery 2003; 134:840–843.
- Fuhrman MA, Felig DM, Tanchel ME. Superior mesenteric artery syndrome with obstructing duodenal bezoar. Gastrointest Endosc 2003; 57:387.
- Hines JR, Gore RM, Ballantyne GH. Superior mesenteric artery syndrome. Diagnostic criteria and therapeutic approaches. Am J Surg 1984; 148:630–632.
- Gersin KS, Heniford BT. Laparoscopic duodenojejunostomy for treatment of superior mesenteric artery syndrome. JSLS 1998; 2:281–284.
Clobetasol Propionate Shampoo 0.05% Is Efficacious and Safe for Long-term Control of Scalp Psoriasis
Standard Management Options for Rosacea, Part 1: Overview and Broad Spectrum of Care
Rosacea is well established as a chronic typology or syndrome, primarily affecting the convexities of the central face (ie, cheeks, nose, chin, forehead) and often affecting the eyes. In 2002, the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea reported on a standard classification system that identified primary and secondary features of rosacea and described 4 common patterns of signs and symptoms designated as subtypes.1 In 2004, the committee published a standard grading system for assessing the relative severity of rosacea to enhance the utility of the classification system for researchers and clinicians.2
Developed and reviewed by 21 experts worldwide, these standard systems are essential to perform research; analyze results and compare data from different sources; and provide a common terminology and reference for the diagnosis, treatment, and assessment of results in clinical practice. Because present scientific knowledge of the etiology of rosacea is limited, these systems are considered provisional and are based on morphologic characteristics alone to avoid assumptions about pathogenesis and progression. They are intended to facilitate communication and ultimately the development of a research-based understanding of the disorder.
As a final step, the committee has developed standard management options based on these standard criteria to assist in providing optimal patient care. Because it is fundamental in the management of rosacea to consider the broad spectrum of potential therapies, the consensus committee and review panel have been expanded to include leading experts in dermatology, laser therapy, skin care, and ophthalmology. As with the standard classification and grading systems, the standard management options are considered provisional and may be expanded and updated as scientific knowledge increases and additional therapies become available.
Although rosacea encompasses various combinations of signs and symptoms, in most cases, some rather than all of these features appear in any given patient and often are characterized by remissions and exacerbations. Therefore, it is important to define the roles of respective treatment modalities as well as lifestyle management and skin care within the context of specific potential manifestations. In this way, an optimal management approach may be tailored for each individual patient.3,4
The standard management options are intended to serve as a menu of options rather than a treatment protocol. Although there is no cure for rosacea, its various signs and symptoms may be reduced or controlled with a range of therapeutic modalities, even though their actions may not be fully defined by clinical data.5 It should be noted that clinical trials are rarely a reflection of clinical practice because they are typically intended to discern only the contribution of a specific treatment.6 In practice, clinicians rarely rely on a single mode of care alone, and in the case of rosacea, factors such as proper skin care and avoidance of exacerbating factors may substantially improve results. Thus, patients often may experience better outcomes than might be suggested by clinical studies designed to isolate the effect of a single therapy.
Part 1 of this 2-part series will review the patient evaluation process and respective modalities of care.
Medical History
In addition to clinical observation of potential primary and secondary features of rosacea (Table 1, PLEASE REFER TO THE PDF TO VIEW THE TABLE), a medical history is needed to identify features that may not be visually evident or present at the time of the patient visit, to rule out alternative diagnoses, and to help identify potential environmental and lifestyle triggers. There is no laboratory test for rosacea and a biopsy is warranted only to rule out alternative diagnoses.
It may be difficult to clinically distinguish between the effects of chronic actinic damage on sun-sensitive skin (heliodermatitis) and subtype 1 (erythematotelangiectactic) rosacea. In some individuals, there may be overlapping features. A medical history may be especially useful in differentiating between erythematotelangiectactic rosacea and isolated photodamage. For example, any patient whose occupation or lifestyle has involved extensive sun exposure may experience chronic actinic damage, whereas patients with a history of flushing alone may be more likely to have rosacea. In addition, in the case of rosacea, erythema and telangiectasia tend to present with a central facial distribution.7 Other differential diagnoses include seborrheic dermatitis, lupus erythematosus, polycythemia vera, and carcinoid syndrome, with flushing mimicking rosacea.
A medical history also may be relevant for treatment purposes in distinguishing between dry flushing, which often is caused by exogenous or endogenous vasoactive agents, and wet flushing, which is accompanied by sweating that is regulated by the autonomic nervous system. Flushing can be further divided according to causes such as physical exertion, heat, or emotional reaction.8
Importantly, a medical history can uncover ocular involvement that may not be currently present or readily apparent from clinical observation as well as identify physical discomfort such as burning or stinging that may substantially affect quality of life for many patients.
Because rosacea affects facial appearance, its presence also may have considerable impact on an individual patient’s psychologic well-being and ability to interact socially or professionally. An assessment can help guide the physician toward providing an appropriate level of care.
Drug Therapy
The papules and pustules of rosacea, as well as nodules, plaques, or perilesional erythema, can be effectively treated in most patients with drugs that have been extensively studied in clinical trials and approved by the US Food and Drug Administration for rosacea, such as topical metronidazole, topical azelaic acid, and oral controlled-release doxycycline 40 mg, all approved for the treatment of inflammatory lesions of rosacea. In addition, the efficacy of topical sodium
sulfacetamide–sulfur is supported by many years of clinical experience in treating rosacea, though it was allowed to be marketed for rosacea prior to more stringent modern requirements for clinical studies and US Food and Drug Administration review. Options for the use of approved medications as well as off-label use of other medications such as oral tetracycline are reviewed in detail in part 2 of this series.9
Several oral antibiotics commonly are prescribed on an off-label basis for subtype 4 (ocular) rosacea. Moreover, when appropriate, the off-label use of other medical therapies may be administered to treat flushing and background erythema, which will be discussed in detail in part 2 of this series.9 The committee encourages further drug research aimed to improve the treatment of background erythema, which represents a great unmet clinical need in rosacea therapy.10
In all cases, physicians should review the package insert for prescribing information. This document is not intended to suggest the monitoring and actual dosing practices for drugs.
Laser and Light Therapy
The efficacy of laser therapy for the treatment of telangiectasia has been well established in clinical practice,11-16 and limited studies also have suggested that it may reduce erythema and flushing.11,15,17 Most lasers used to treat vascular components of rosacea have wavelengths in the 500 to 600 nm range and are known as nonablative (they do not destroy tissue). Recent developments using long-pulsed pulsed dye lasers,13 a technique of stacking pulses,18 or 532-nm potassium-titanyl-phosphate lasers19 may produce excellent improvement in erythema and telangiectasia without purpura.13
Polychromatic light-emitting devices such as intense pulsed light devices (515–1200 nm) also have been found to be effective in reducing erythema and telangiectasia.15,20
Ablative lasers, such as the 2.94-nm erbium:YAG or 10,600-nm CO2 lasers, destroy tissue and may be used to treat subtype 3 (phymatous) rosacea.3,12,21
Lifestyle Management
Signs and symptoms of rosacea often appear to be triggered by environmental or lifestyle factors, most related to flushing. Some of the most common rosacea triggers include sun exposure, emotional stress, hot or cold weather, wind, heavy exercise, alcohol consumption, hot baths, spicy foods, humidity, indoor heat, certain skin care products, heated beverages, certain cosmetics, medications, medical conditions, and certain foods (Table 2, PLEASE REFER TO THE PDF TO VIEW THE TABLE).22 However, triggers that may affect one patient may not affect another, and avoidance of every potential factor may be unnecessary as well as impractical.
An appropriate management strategy identifies and avoids only those lifestyle factors that trigger or exacerbate rosacea symptoms in each individual patient. To help identify a patient’s individual rosacea triggers, the patient can record daily contact with the most common rosacea triggers and other possible factors and then match them to flare-ups of signs and symptoms. In unscientific surveys of patients with rosacea who identified and avoided their personal rosacea triggers, more than 90% reported that their condition had improved in varying degrees.23
Adjunctive Care
Skin Care Products
Because patients with rosacea often have skin that is sensitive and easily irritated, causing redness, inflammation, and stinging, skin care is an important component of rosacea management.21,24 The goal of everyday skin care for patients with rosacea is to maintain the integrity of the skin barrier while avoiding agents that cause inflammation or flushing.
Complicating skin care is the typical heightened neurosensory response in many patients with rosacea who may experience stinging and burning from minor irritants more frequently than the general population. Patients may therefore be advised to select cleansers and moisturizers that do not irritate their skin.
Sunscreens or sunblocks effective against the full spectrum of UVA and UVB radiation can be especially important for patients with rosacea whose facial skin may be particularly susceptible to actinic damage and consequent rosacea flare-ups. A sun protection factor of 15 or higher is recommended, and physical blocks utilizing zinc or titanium dioxide may be effective if chemical sunscreens cause irritation.
A useful rule of thumb may be to select products for patients with rosacea that contain no sensory provoking ingredients, no volatile substances, no minor irritants or allergens, minimal botanical agents, and no unnecessary ingredients.
Cleansing Regimen
Patients should be informed that compliance with instructions on facial cleansing and topical medication application may be critical to avoiding irritation, burning, and stinging. They may be advised to wash the face gently with a nonirritating cleanser, avoiding the use of abrasive materials such as washcloths and loofahs. They also may be advised to blot, not rub, the face dry with a soft towel and wait up to 30 minutes for the face to completely dry before applying topical medication or other products, as stinging most often occurs when the skin is wet.6,8
After this routine is established and the face is not irritated, the patient can reduce the amount of time waiting to dry by 5 minutes every day to determine the shortest waiting time necessary for the individual patient.
Cosmetics
Cosmetics, especially those with a green or yellow tint, may be effective in reducing the appearance of redness. However, as with skin cleansers and moisturizers, care should be taken to minimize irritation.
Patients should be advised to avoid any products that cause burning, stinging, itching, or other discomfort. They also may be advised that waterproof cosmetics may be difficult to remove, requiring the use of harsh agents that may induce irritation.
New cosmetics should be regularly purchased to minimize microbial contamination and degradation. Brushes are preferred over sponges to avoid abrasion and because brushes can be easily cleaned to decrease bacterial contamination.24
Conclusion
Managing the various potential signs and symptoms of rosacea calls for consideration of a broad spectrum of care, and a more precise selection of therapeutic options may become increasingly possible as their mechanisms of action are more definitively established. Until the etiology and pathogenesis are more completely understood, however, the classification of rosacea by its morphologic features and grading by severity may serve as an appropriate guide for its effective management.
As with the standard classification and grading systems, the options described here are provisional and subject to modification with the development of new therapies, increase in scientific knowledge, and testing of their relevance and applicability by investigators and clinicians. Also, as with any consensus document, these options do not necessarily reflect the views of any single individual and not all comments were incorporated.
Acknowledgments
The committee thanks the following individuals who reviewed and contributed to this document: Joel Bamford, MD, Duluth, Minnesota; Mats Berg, MD, Uppsala, Sweden; James Del Rosso, DO, Las Vegas, Nevada; Roy Geronemus, MD, New York, New York; David Goldberg, MD, JD, Hackensack, New Jersey; Richard Granstein, MD, New York, New York; William James, MD, Philadelphia, Pennsylvania; Albert Kligman, MD, PhD, Philadelphia, Pennsylvania; Mark Mannis, MD, Davis, California; Ronald Marks, MD, Cardiff, United Kingdom; Michelle Pelle, MD, San Diego, California; Noah Scheinfeld, MD, JD, New York, New York; Bryan Sires, MD, PhD, Kirkland, Washington; Helen Torok, MD, Medina, Ohio; John Wolf, MD, Houston, Texas; and Mina Yaar, MD, Boston, Massachusetts.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Wilkin J, Dahl M, Detmar M, et al; National Rosacea Society Expert Committee. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Powell FC. Rosacea. N Engl J Med. 2005;352:793-803.
- Wilkin JK. Rosacea: pathophysiology and treatment. Arch Dermatol. 1994;130:359-362.
- van Zuuren EJ, Graber MA, Hollis S, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2005;(3):CD003262.
- Wilkin JK. Use of topical products for maintaining remission in rosacea. Arch Dermatol. 1999;135:79-80.
- Odom R. Rosacea, acne rosacea, and actinic telangiectasia: in reply. J Am Acad Dermatol. 2005;53:1103-1104.
- Wilkin JK. The red face: flushing disorders. Clin Dermatol. 1993;11:211-223.
- Odom R, Dahl M, Dover K, et al; National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. Standard management options for rosacea, part 2: options according to subtype. Cutis. In press.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective a1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Goldberg DJ. Lasers and light sources for rosacea. Cutis. 2005;75(suppl 3):22-26, 33-36.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-685.
- Schroeter CA, Haaf-von Below S, Neumann HA. Effective treatment of rosacea using intense pulsed light systems. Dermatol Surg. 2005;31:1285-1289.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med. 2002;17:26-33.
- Alster T, Anderson RR, Bank DE, et al. The use of photodynamic therapy in dermatology: results of a consensus conference. J Drugs Dermatol. 2006;5:140-154.
- Tan SR, Tope WD. Pulsed dye laser treatment of rosacea improves erythema, symptomatology, and quality of life. J Am Acad Dermatol. 2004;51:592-599.
- Rohrer TE, Chatrath V, Iyengar V. Does pulse stacking improve the results of treatment with variable-pulse pulsed-dye lasers? Dermatol Surg. 2004;30:163-167.
- Railan D, Parlette EC, Uebelhoer NS, et al. Laser treatment of vascular lesions. Clin Dermatol. 2006;24:8-15.
- Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95-100.
- Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512.
- Drake L, ed; National Rosacea Society. New survey pinpoints leading factors that trigger symptoms. Rosacea Review. Summer 2002. http://www.rosacea.org/rr/2002/summer/article_3.php. Accessed June 15, 2009.
- Drake L, ed; National Rosacea Society. Survey shows lifestyle changes help control rosacea flare-ups. Rosacea Review. Winter 1998. http://www.rosacea.org/rr/1998/winter/article_3.php. Accessed June 15, 2009.
- Draelos ZD. Cosmetics in acne and rosacea. Semin Cutan Med Surg. 2001;20:209-214.
Rosacea is well established as a chronic typology or syndrome, primarily affecting the convexities of the central face (ie, cheeks, nose, chin, forehead) and often affecting the eyes. In 2002, the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea reported on a standard classification system that identified primary and secondary features of rosacea and described 4 common patterns of signs and symptoms designated as subtypes.1 In 2004, the committee published a standard grading system for assessing the relative severity of rosacea to enhance the utility of the classification system for researchers and clinicians.2
Developed and reviewed by 21 experts worldwide, these standard systems are essential to perform research; analyze results and compare data from different sources; and provide a common terminology and reference for the diagnosis, treatment, and assessment of results in clinical practice. Because present scientific knowledge of the etiology of rosacea is limited, these systems are considered provisional and are based on morphologic characteristics alone to avoid assumptions about pathogenesis and progression. They are intended to facilitate communication and ultimately the development of a research-based understanding of the disorder.
As a final step, the committee has developed standard management options based on these standard criteria to assist in providing optimal patient care. Because it is fundamental in the management of rosacea to consider the broad spectrum of potential therapies, the consensus committee and review panel have been expanded to include leading experts in dermatology, laser therapy, skin care, and ophthalmology. As with the standard classification and grading systems, the standard management options are considered provisional and may be expanded and updated as scientific knowledge increases and additional therapies become available.
Although rosacea encompasses various combinations of signs and symptoms, in most cases, some rather than all of these features appear in any given patient and often are characterized by remissions and exacerbations. Therefore, it is important to define the roles of respective treatment modalities as well as lifestyle management and skin care within the context of specific potential manifestations. In this way, an optimal management approach may be tailored for each individual patient.3,4
The standard management options are intended to serve as a menu of options rather than a treatment protocol. Although there is no cure for rosacea, its various signs and symptoms may be reduced or controlled with a range of therapeutic modalities, even though their actions may not be fully defined by clinical data.5 It should be noted that clinical trials are rarely a reflection of clinical practice because they are typically intended to discern only the contribution of a specific treatment.6 In practice, clinicians rarely rely on a single mode of care alone, and in the case of rosacea, factors such as proper skin care and avoidance of exacerbating factors may substantially improve results. Thus, patients often may experience better outcomes than might be suggested by clinical studies designed to isolate the effect of a single therapy.
Part 1 of this 2-part series will review the patient evaluation process and respective modalities of care.
Medical History
In addition to clinical observation of potential primary and secondary features of rosacea (Table 1, PLEASE REFER TO THE PDF TO VIEW THE TABLE), a medical history is needed to identify features that may not be visually evident or present at the time of the patient visit, to rule out alternative diagnoses, and to help identify potential environmental and lifestyle triggers. There is no laboratory test for rosacea and a biopsy is warranted only to rule out alternative diagnoses.
It may be difficult to clinically distinguish between the effects of chronic actinic damage on sun-sensitive skin (heliodermatitis) and subtype 1 (erythematotelangiectactic) rosacea. In some individuals, there may be overlapping features. A medical history may be especially useful in differentiating between erythematotelangiectactic rosacea and isolated photodamage. For example, any patient whose occupation or lifestyle has involved extensive sun exposure may experience chronic actinic damage, whereas patients with a history of flushing alone may be more likely to have rosacea. In addition, in the case of rosacea, erythema and telangiectasia tend to present with a central facial distribution.7 Other differential diagnoses include seborrheic dermatitis, lupus erythematosus, polycythemia vera, and carcinoid syndrome, with flushing mimicking rosacea.
A medical history also may be relevant for treatment purposes in distinguishing between dry flushing, which often is caused by exogenous or endogenous vasoactive agents, and wet flushing, which is accompanied by sweating that is regulated by the autonomic nervous system. Flushing can be further divided according to causes such as physical exertion, heat, or emotional reaction.8
Importantly, a medical history can uncover ocular involvement that may not be currently present or readily apparent from clinical observation as well as identify physical discomfort such as burning or stinging that may substantially affect quality of life for many patients.
Because rosacea affects facial appearance, its presence also may have considerable impact on an individual patient’s psychologic well-being and ability to interact socially or professionally. An assessment can help guide the physician toward providing an appropriate level of care.
Drug Therapy
The papules and pustules of rosacea, as well as nodules, plaques, or perilesional erythema, can be effectively treated in most patients with drugs that have been extensively studied in clinical trials and approved by the US Food and Drug Administration for rosacea, such as topical metronidazole, topical azelaic acid, and oral controlled-release doxycycline 40 mg, all approved for the treatment of inflammatory lesions of rosacea. In addition, the efficacy of topical sodium
sulfacetamide–sulfur is supported by many years of clinical experience in treating rosacea, though it was allowed to be marketed for rosacea prior to more stringent modern requirements for clinical studies and US Food and Drug Administration review. Options for the use of approved medications as well as off-label use of other medications such as oral tetracycline are reviewed in detail in part 2 of this series.9
Several oral antibiotics commonly are prescribed on an off-label basis for subtype 4 (ocular) rosacea. Moreover, when appropriate, the off-label use of other medical therapies may be administered to treat flushing and background erythema, which will be discussed in detail in part 2 of this series.9 The committee encourages further drug research aimed to improve the treatment of background erythema, which represents a great unmet clinical need in rosacea therapy.10
In all cases, physicians should review the package insert for prescribing information. This document is not intended to suggest the monitoring and actual dosing practices for drugs.
Laser and Light Therapy
The efficacy of laser therapy for the treatment of telangiectasia has been well established in clinical practice,11-16 and limited studies also have suggested that it may reduce erythema and flushing.11,15,17 Most lasers used to treat vascular components of rosacea have wavelengths in the 500 to 600 nm range and are known as nonablative (they do not destroy tissue). Recent developments using long-pulsed pulsed dye lasers,13 a technique of stacking pulses,18 or 532-nm potassium-titanyl-phosphate lasers19 may produce excellent improvement in erythema and telangiectasia without purpura.13
Polychromatic light-emitting devices such as intense pulsed light devices (515–1200 nm) also have been found to be effective in reducing erythema and telangiectasia.15,20
Ablative lasers, such as the 2.94-nm erbium:YAG or 10,600-nm CO2 lasers, destroy tissue and may be used to treat subtype 3 (phymatous) rosacea.3,12,21
Lifestyle Management
Signs and symptoms of rosacea often appear to be triggered by environmental or lifestyle factors, most related to flushing. Some of the most common rosacea triggers include sun exposure, emotional stress, hot or cold weather, wind, heavy exercise, alcohol consumption, hot baths, spicy foods, humidity, indoor heat, certain skin care products, heated beverages, certain cosmetics, medications, medical conditions, and certain foods (Table 2, PLEASE REFER TO THE PDF TO VIEW THE TABLE).22 However, triggers that may affect one patient may not affect another, and avoidance of every potential factor may be unnecessary as well as impractical.
An appropriate management strategy identifies and avoids only those lifestyle factors that trigger or exacerbate rosacea symptoms in each individual patient. To help identify a patient’s individual rosacea triggers, the patient can record daily contact with the most common rosacea triggers and other possible factors and then match them to flare-ups of signs and symptoms. In unscientific surveys of patients with rosacea who identified and avoided their personal rosacea triggers, more than 90% reported that their condition had improved in varying degrees.23
Adjunctive Care
Skin Care Products
Because patients with rosacea often have skin that is sensitive and easily irritated, causing redness, inflammation, and stinging, skin care is an important component of rosacea management.21,24 The goal of everyday skin care for patients with rosacea is to maintain the integrity of the skin barrier while avoiding agents that cause inflammation or flushing.
Complicating skin care is the typical heightened neurosensory response in many patients with rosacea who may experience stinging and burning from minor irritants more frequently than the general population. Patients may therefore be advised to select cleansers and moisturizers that do not irritate their skin.
Sunscreens or sunblocks effective against the full spectrum of UVA and UVB radiation can be especially important for patients with rosacea whose facial skin may be particularly susceptible to actinic damage and consequent rosacea flare-ups. A sun protection factor of 15 or higher is recommended, and physical blocks utilizing zinc or titanium dioxide may be effective if chemical sunscreens cause irritation.
A useful rule of thumb may be to select products for patients with rosacea that contain no sensory provoking ingredients, no volatile substances, no minor irritants or allergens, minimal botanical agents, and no unnecessary ingredients.
Cleansing Regimen
Patients should be informed that compliance with instructions on facial cleansing and topical medication application may be critical to avoiding irritation, burning, and stinging. They may be advised to wash the face gently with a nonirritating cleanser, avoiding the use of abrasive materials such as washcloths and loofahs. They also may be advised to blot, not rub, the face dry with a soft towel and wait up to 30 minutes for the face to completely dry before applying topical medication or other products, as stinging most often occurs when the skin is wet.6,8
After this routine is established and the face is not irritated, the patient can reduce the amount of time waiting to dry by 5 minutes every day to determine the shortest waiting time necessary for the individual patient.
Cosmetics
Cosmetics, especially those with a green or yellow tint, may be effective in reducing the appearance of redness. However, as with skin cleansers and moisturizers, care should be taken to minimize irritation.
Patients should be advised to avoid any products that cause burning, stinging, itching, or other discomfort. They also may be advised that waterproof cosmetics may be difficult to remove, requiring the use of harsh agents that may induce irritation.
New cosmetics should be regularly purchased to minimize microbial contamination and degradation. Brushes are preferred over sponges to avoid abrasion and because brushes can be easily cleaned to decrease bacterial contamination.24
Conclusion
Managing the various potential signs and symptoms of rosacea calls for consideration of a broad spectrum of care, and a more precise selection of therapeutic options may become increasingly possible as their mechanisms of action are more definitively established. Until the etiology and pathogenesis are more completely understood, however, the classification of rosacea by its morphologic features and grading by severity may serve as an appropriate guide for its effective management.
As with the standard classification and grading systems, the options described here are provisional and subject to modification with the development of new therapies, increase in scientific knowledge, and testing of their relevance and applicability by investigators and clinicians. Also, as with any consensus document, these options do not necessarily reflect the views of any single individual and not all comments were incorporated.
Acknowledgments
The committee thanks the following individuals who reviewed and contributed to this document: Joel Bamford, MD, Duluth, Minnesota; Mats Berg, MD, Uppsala, Sweden; James Del Rosso, DO, Las Vegas, Nevada; Roy Geronemus, MD, New York, New York; David Goldberg, MD, JD, Hackensack, New Jersey; Richard Granstein, MD, New York, New York; William James, MD, Philadelphia, Pennsylvania; Albert Kligman, MD, PhD, Philadelphia, Pennsylvania; Mark Mannis, MD, Davis, California; Ronald Marks, MD, Cardiff, United Kingdom; Michelle Pelle, MD, San Diego, California; Noah Scheinfeld, MD, JD, New York, New York; Bryan Sires, MD, PhD, Kirkland, Washington; Helen Torok, MD, Medina, Ohio; John Wolf, MD, Houston, Texas; and Mina Yaar, MD, Boston, Massachusetts.
Rosacea is well established as a chronic typology or syndrome, primarily affecting the convexities of the central face (ie, cheeks, nose, chin, forehead) and often affecting the eyes. In 2002, the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea reported on a standard classification system that identified primary and secondary features of rosacea and described 4 common patterns of signs and symptoms designated as subtypes.1 In 2004, the committee published a standard grading system for assessing the relative severity of rosacea to enhance the utility of the classification system for researchers and clinicians.2
Developed and reviewed by 21 experts worldwide, these standard systems are essential to perform research; analyze results and compare data from different sources; and provide a common terminology and reference for the diagnosis, treatment, and assessment of results in clinical practice. Because present scientific knowledge of the etiology of rosacea is limited, these systems are considered provisional and are based on morphologic characteristics alone to avoid assumptions about pathogenesis and progression. They are intended to facilitate communication and ultimately the development of a research-based understanding of the disorder.
As a final step, the committee has developed standard management options based on these standard criteria to assist in providing optimal patient care. Because it is fundamental in the management of rosacea to consider the broad spectrum of potential therapies, the consensus committee and review panel have been expanded to include leading experts in dermatology, laser therapy, skin care, and ophthalmology. As with the standard classification and grading systems, the standard management options are considered provisional and may be expanded and updated as scientific knowledge increases and additional therapies become available.
Although rosacea encompasses various combinations of signs and symptoms, in most cases, some rather than all of these features appear in any given patient and often are characterized by remissions and exacerbations. Therefore, it is important to define the roles of respective treatment modalities as well as lifestyle management and skin care within the context of specific potential manifestations. In this way, an optimal management approach may be tailored for each individual patient.3,4
The standard management options are intended to serve as a menu of options rather than a treatment protocol. Although there is no cure for rosacea, its various signs and symptoms may be reduced or controlled with a range of therapeutic modalities, even though their actions may not be fully defined by clinical data.5 It should be noted that clinical trials are rarely a reflection of clinical practice because they are typically intended to discern only the contribution of a specific treatment.6 In practice, clinicians rarely rely on a single mode of care alone, and in the case of rosacea, factors such as proper skin care and avoidance of exacerbating factors may substantially improve results. Thus, patients often may experience better outcomes than might be suggested by clinical studies designed to isolate the effect of a single therapy.
Part 1 of this 2-part series will review the patient evaluation process and respective modalities of care.
Medical History
In addition to clinical observation of potential primary and secondary features of rosacea (Table 1, PLEASE REFER TO THE PDF TO VIEW THE TABLE), a medical history is needed to identify features that may not be visually evident or present at the time of the patient visit, to rule out alternative diagnoses, and to help identify potential environmental and lifestyle triggers. There is no laboratory test for rosacea and a biopsy is warranted only to rule out alternative diagnoses.
It may be difficult to clinically distinguish between the effects of chronic actinic damage on sun-sensitive skin (heliodermatitis) and subtype 1 (erythematotelangiectactic) rosacea. In some individuals, there may be overlapping features. A medical history may be especially useful in differentiating between erythematotelangiectactic rosacea and isolated photodamage. For example, any patient whose occupation or lifestyle has involved extensive sun exposure may experience chronic actinic damage, whereas patients with a history of flushing alone may be more likely to have rosacea. In addition, in the case of rosacea, erythema and telangiectasia tend to present with a central facial distribution.7 Other differential diagnoses include seborrheic dermatitis, lupus erythematosus, polycythemia vera, and carcinoid syndrome, with flushing mimicking rosacea.
A medical history also may be relevant for treatment purposes in distinguishing between dry flushing, which often is caused by exogenous or endogenous vasoactive agents, and wet flushing, which is accompanied by sweating that is regulated by the autonomic nervous system. Flushing can be further divided according to causes such as physical exertion, heat, or emotional reaction.8
Importantly, a medical history can uncover ocular involvement that may not be currently present or readily apparent from clinical observation as well as identify physical discomfort such as burning or stinging that may substantially affect quality of life for many patients.
Because rosacea affects facial appearance, its presence also may have considerable impact on an individual patient’s psychologic well-being and ability to interact socially or professionally. An assessment can help guide the physician toward providing an appropriate level of care.
Drug Therapy
The papules and pustules of rosacea, as well as nodules, plaques, or perilesional erythema, can be effectively treated in most patients with drugs that have been extensively studied in clinical trials and approved by the US Food and Drug Administration for rosacea, such as topical metronidazole, topical azelaic acid, and oral controlled-release doxycycline 40 mg, all approved for the treatment of inflammatory lesions of rosacea. In addition, the efficacy of topical sodium
sulfacetamide–sulfur is supported by many years of clinical experience in treating rosacea, though it was allowed to be marketed for rosacea prior to more stringent modern requirements for clinical studies and US Food and Drug Administration review. Options for the use of approved medications as well as off-label use of other medications such as oral tetracycline are reviewed in detail in part 2 of this series.9
Several oral antibiotics commonly are prescribed on an off-label basis for subtype 4 (ocular) rosacea. Moreover, when appropriate, the off-label use of other medical therapies may be administered to treat flushing and background erythema, which will be discussed in detail in part 2 of this series.9 The committee encourages further drug research aimed to improve the treatment of background erythema, which represents a great unmet clinical need in rosacea therapy.10
In all cases, physicians should review the package insert for prescribing information. This document is not intended to suggest the monitoring and actual dosing practices for drugs.
Laser and Light Therapy
The efficacy of laser therapy for the treatment of telangiectasia has been well established in clinical practice,11-16 and limited studies also have suggested that it may reduce erythema and flushing.11,15,17 Most lasers used to treat vascular components of rosacea have wavelengths in the 500 to 600 nm range and are known as nonablative (they do not destroy tissue). Recent developments using long-pulsed pulsed dye lasers,13 a technique of stacking pulses,18 or 532-nm potassium-titanyl-phosphate lasers19 may produce excellent improvement in erythema and telangiectasia without purpura.13
Polychromatic light-emitting devices such as intense pulsed light devices (515–1200 nm) also have been found to be effective in reducing erythema and telangiectasia.15,20
Ablative lasers, such as the 2.94-nm erbium:YAG or 10,600-nm CO2 lasers, destroy tissue and may be used to treat subtype 3 (phymatous) rosacea.3,12,21
Lifestyle Management
Signs and symptoms of rosacea often appear to be triggered by environmental or lifestyle factors, most related to flushing. Some of the most common rosacea triggers include sun exposure, emotional stress, hot or cold weather, wind, heavy exercise, alcohol consumption, hot baths, spicy foods, humidity, indoor heat, certain skin care products, heated beverages, certain cosmetics, medications, medical conditions, and certain foods (Table 2, PLEASE REFER TO THE PDF TO VIEW THE TABLE).22 However, triggers that may affect one patient may not affect another, and avoidance of every potential factor may be unnecessary as well as impractical.
An appropriate management strategy identifies and avoids only those lifestyle factors that trigger or exacerbate rosacea symptoms in each individual patient. To help identify a patient’s individual rosacea triggers, the patient can record daily contact with the most common rosacea triggers and other possible factors and then match them to flare-ups of signs and symptoms. In unscientific surveys of patients with rosacea who identified and avoided their personal rosacea triggers, more than 90% reported that their condition had improved in varying degrees.23
Adjunctive Care
Skin Care Products
Because patients with rosacea often have skin that is sensitive and easily irritated, causing redness, inflammation, and stinging, skin care is an important component of rosacea management.21,24 The goal of everyday skin care for patients with rosacea is to maintain the integrity of the skin barrier while avoiding agents that cause inflammation or flushing.
Complicating skin care is the typical heightened neurosensory response in many patients with rosacea who may experience stinging and burning from minor irritants more frequently than the general population. Patients may therefore be advised to select cleansers and moisturizers that do not irritate their skin.
Sunscreens or sunblocks effective against the full spectrum of UVA and UVB radiation can be especially important for patients with rosacea whose facial skin may be particularly susceptible to actinic damage and consequent rosacea flare-ups. A sun protection factor of 15 or higher is recommended, and physical blocks utilizing zinc or titanium dioxide may be effective if chemical sunscreens cause irritation.
A useful rule of thumb may be to select products for patients with rosacea that contain no sensory provoking ingredients, no volatile substances, no minor irritants or allergens, minimal botanical agents, and no unnecessary ingredients.
Cleansing Regimen
Patients should be informed that compliance with instructions on facial cleansing and topical medication application may be critical to avoiding irritation, burning, and stinging. They may be advised to wash the face gently with a nonirritating cleanser, avoiding the use of abrasive materials such as washcloths and loofahs. They also may be advised to blot, not rub, the face dry with a soft towel and wait up to 30 minutes for the face to completely dry before applying topical medication or other products, as stinging most often occurs when the skin is wet.6,8
After this routine is established and the face is not irritated, the patient can reduce the amount of time waiting to dry by 5 minutes every day to determine the shortest waiting time necessary for the individual patient.
Cosmetics
Cosmetics, especially those with a green or yellow tint, may be effective in reducing the appearance of redness. However, as with skin cleansers and moisturizers, care should be taken to minimize irritation.
Patients should be advised to avoid any products that cause burning, stinging, itching, or other discomfort. They also may be advised that waterproof cosmetics may be difficult to remove, requiring the use of harsh agents that may induce irritation.
New cosmetics should be regularly purchased to minimize microbial contamination and degradation. Brushes are preferred over sponges to avoid abrasion and because brushes can be easily cleaned to decrease bacterial contamination.24
Conclusion
Managing the various potential signs and symptoms of rosacea calls for consideration of a broad spectrum of care, and a more precise selection of therapeutic options may become increasingly possible as their mechanisms of action are more definitively established. Until the etiology and pathogenesis are more completely understood, however, the classification of rosacea by its morphologic features and grading by severity may serve as an appropriate guide for its effective management.
As with the standard classification and grading systems, the options described here are provisional and subject to modification with the development of new therapies, increase in scientific knowledge, and testing of their relevance and applicability by investigators and clinicians. Also, as with any consensus document, these options do not necessarily reflect the views of any single individual and not all comments were incorporated.
Acknowledgments
The committee thanks the following individuals who reviewed and contributed to this document: Joel Bamford, MD, Duluth, Minnesota; Mats Berg, MD, Uppsala, Sweden; James Del Rosso, DO, Las Vegas, Nevada; Roy Geronemus, MD, New York, New York; David Goldberg, MD, JD, Hackensack, New Jersey; Richard Granstein, MD, New York, New York; William James, MD, Philadelphia, Pennsylvania; Albert Kligman, MD, PhD, Philadelphia, Pennsylvania; Mark Mannis, MD, Davis, California; Ronald Marks, MD, Cardiff, United Kingdom; Michelle Pelle, MD, San Diego, California; Noah Scheinfeld, MD, JD, New York, New York; Bryan Sires, MD, PhD, Kirkland, Washington; Helen Torok, MD, Medina, Ohio; John Wolf, MD, Houston, Texas; and Mina Yaar, MD, Boston, Massachusetts.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Wilkin J, Dahl M, Detmar M, et al; National Rosacea Society Expert Committee. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Powell FC. Rosacea. N Engl J Med. 2005;352:793-803.
- Wilkin JK. Rosacea: pathophysiology and treatment. Arch Dermatol. 1994;130:359-362.
- van Zuuren EJ, Graber MA, Hollis S, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2005;(3):CD003262.
- Wilkin JK. Use of topical products for maintaining remission in rosacea. Arch Dermatol. 1999;135:79-80.
- Odom R. Rosacea, acne rosacea, and actinic telangiectasia: in reply. J Am Acad Dermatol. 2005;53:1103-1104.
- Wilkin JK. The red face: flushing disorders. Clin Dermatol. 1993;11:211-223.
- Odom R, Dahl M, Dover K, et al; National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. Standard management options for rosacea, part 2: options according to subtype. Cutis. In press.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective a1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Goldberg DJ. Lasers and light sources for rosacea. Cutis. 2005;75(suppl 3):22-26, 33-36.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-685.
- Schroeter CA, Haaf-von Below S, Neumann HA. Effective treatment of rosacea using intense pulsed light systems. Dermatol Surg. 2005;31:1285-1289.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med. 2002;17:26-33.
- Alster T, Anderson RR, Bank DE, et al. The use of photodynamic therapy in dermatology: results of a consensus conference. J Drugs Dermatol. 2006;5:140-154.
- Tan SR, Tope WD. Pulsed dye laser treatment of rosacea improves erythema, symptomatology, and quality of life. J Am Acad Dermatol. 2004;51:592-599.
- Rohrer TE, Chatrath V, Iyengar V. Does pulse stacking improve the results of treatment with variable-pulse pulsed-dye lasers? Dermatol Surg. 2004;30:163-167.
- Railan D, Parlette EC, Uebelhoer NS, et al. Laser treatment of vascular lesions. Clin Dermatol. 2006;24:8-15.
- Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95-100.
- Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512.
- Drake L, ed; National Rosacea Society. New survey pinpoints leading factors that trigger symptoms. Rosacea Review. Summer 2002. http://www.rosacea.org/rr/2002/summer/article_3.php. Accessed June 15, 2009.
- Drake L, ed; National Rosacea Society. Survey shows lifestyle changes help control rosacea flare-ups. Rosacea Review. Winter 1998. http://www.rosacea.org/rr/1998/winter/article_3.php. Accessed June 15, 2009.
- Draelos ZD. Cosmetics in acne and rosacea. Semin Cutan Med Surg. 2001;20:209-214.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Wilkin J, Dahl M, Detmar M, et al; National Rosacea Society Expert Committee. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Powell FC. Rosacea. N Engl J Med. 2005;352:793-803.
- Wilkin JK. Rosacea: pathophysiology and treatment. Arch Dermatol. 1994;130:359-362.
- van Zuuren EJ, Graber MA, Hollis S, et al. Interventions for rosacea. Cochrane Database Syst Rev. 2005;(3):CD003262.
- Wilkin JK. Use of topical products for maintaining remission in rosacea. Arch Dermatol. 1999;135:79-80.
- Odom R. Rosacea, acne rosacea, and actinic telangiectasia: in reply. J Am Acad Dermatol. 2005;53:1103-1104.
- Wilkin JK. The red face: flushing disorders. Clin Dermatol. 1993;11:211-223.
- Odom R, Dahl M, Dover K, et al; National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. Standard management options for rosacea, part 2: options according to subtype. Cutis. In press.
- Shanler SD, Ondo AL. Successful treatment of the erythema and flushing of rosacea using a topically applied selective a1-adrenergic receptor agonist, oxymetazoline. Arch Dermatol. 2007;143:1369-1371.
- Goldberg DJ. Lasers and light sources for rosacea. Cutis. 2005;75(suppl 3):22-26, 33-36.
- Jasim ZF, Woo WK, Handley JM. Long-pulsed (6-ms) pulsed dye laser treatment of rosacea-associated telangiectasia using subpurpuric clinical threshold. Dermatol Surg. 2004;30:37-40.
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg. 2003;29:681-685.
- Schroeter CA, Haaf-von Below S, Neumann HA. Effective treatment of rosacea using intense pulsed light systems. Dermatol Surg. 2005;31:1285-1289.
- Clark SM, Lanigan SW, Marks R. Laser treatment of erythema and telangiectasia associated with rosacea. Lasers Med. 2002;17:26-33.
- Alster T, Anderson RR, Bank DE, et al. The use of photodynamic therapy in dermatology: results of a consensus conference. J Drugs Dermatol. 2006;5:140-154.
- Tan SR, Tope WD. Pulsed dye laser treatment of rosacea improves erythema, symptomatology, and quality of life. J Am Acad Dermatol. 2004;51:592-599.
- Rohrer TE, Chatrath V, Iyengar V. Does pulse stacking improve the results of treatment with variable-pulse pulsed-dye lasers? Dermatol Surg. 2004;30:163-167.
- Railan D, Parlette EC, Uebelhoer NS, et al. Laser treatment of vascular lesions. Clin Dermatol. 2006;24:8-15.
- Angermeier MC. Treatment of facial vascular lesions with intense pulsed light. J Cutan Laser Ther. 1999;1:95-100.
- Pelle MT, Crawford GH, James WD. Rosacea: II. therapy. J Am Acad Dermatol. 2004;51:499-512.
- Drake L, ed; National Rosacea Society. New survey pinpoints leading factors that trigger symptoms. Rosacea Review. Summer 2002. http://www.rosacea.org/rr/2002/summer/article_3.php. Accessed June 15, 2009.
- Drake L, ed; National Rosacea Society. Survey shows lifestyle changes help control rosacea flare-ups. Rosacea Review. Winter 1998. http://www.rosacea.org/rr/1998/winter/article_3.php. Accessed June 15, 2009.
- Draelos ZD. Cosmetics in acne and rosacea. Semin Cutan Med Surg. 2001;20:209-214.