User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Quitting Smoking Boosts Life Expectancy at Any Age
TOPLINE:
Quitting smoking at any age increases life expectancy, with the most significant increases observed in younger individuals. But people who quit over age 65 can extend life expectancy.
METHODOLOGY:
- Researchers analyzed the detrimental effects of smoking and the positive impacts of cessation on life expectancy in individuals aged 35-75 years.
- Age-specific death rates by smoking status were calculated using the relative risks for all-cause mortality derived from the Cancer Prevention Study II data, 2018 National Health Interview Survey smoking prevalence data, and 2018 all-cause mortality rates.
- Life tables were constructed to obtain information on the life expectancies of people who never smoked, those who currently smoked, and those who previously smoked but quit at various ages.
- Estimates of years lost due to smoking and years gained by quitting smoking were calculated for people starting at age 35 and over 10-year increments.
TAKEAWAY:
- Compared with people who never smoked, those who smoked at ages 35, 45, 55, 65, and 75 years and continued smoking throughout their lives would lose 9.1, 8.3, 7.3, 5.9, and 4.4 years, respectively.
- People who quit smoking at ages 35, 45, 55, 65, and 75 years would have life expectancies that are shorter by 1.2, 2.7, 3.9, 4.2, and 3.7 years, respectively, than those of same-age individuals who never smoked.
- Individuals who quit smoking at ages 35, 45, 55, 65, and 75 years would experience an additional 8.0, 5.6, 3.4, 1.7, and 0.7 years of life expectancy compared with those who continued smoking.
- People who quit at ages 65 and 75 years would have a 23.4% and 14.2% chance of gaining at least 1 additional year of life.
IN PRACTICE:
“This cessation benefit is not limited to young- and middle-aged adults who smoke; this study demonstrates its applicability to seniors as well. These findings may be valuable for clinicians seeking scientific evidence to motivate their patients who smoke to quit,” the authors wrote.
SOURCE:
The study was led by Thuy T.T. Le, PhD, from the Department of Health Management and Policy at the University of Michigan School of Public Health in Ann Arbor and published online in the American Journal of Preventive Medicine.
LIMITATIONS:
The study’s estimates were according to data from 2018 and may not reflect current trends. The estimates also did not account for variability in smoking intensity among individuals.
DISCLOSURES:
The study was supported by grants from the National Cancer Institute of the US National Institutes of Health and the US Food and Drug Administration Center for Tobacco Products. The authors declared that they had no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Quitting smoking at any age increases life expectancy, with the most significant increases observed in younger individuals. But people who quit over age 65 can extend life expectancy.
METHODOLOGY:
- Researchers analyzed the detrimental effects of smoking and the positive impacts of cessation on life expectancy in individuals aged 35-75 years.
- Age-specific death rates by smoking status were calculated using the relative risks for all-cause mortality derived from the Cancer Prevention Study II data, 2018 National Health Interview Survey smoking prevalence data, and 2018 all-cause mortality rates.
- Life tables were constructed to obtain information on the life expectancies of people who never smoked, those who currently smoked, and those who previously smoked but quit at various ages.
- Estimates of years lost due to smoking and years gained by quitting smoking were calculated for people starting at age 35 and over 10-year increments.
TAKEAWAY:
- Compared with people who never smoked, those who smoked at ages 35, 45, 55, 65, and 75 years and continued smoking throughout their lives would lose 9.1, 8.3, 7.3, 5.9, and 4.4 years, respectively.
- People who quit smoking at ages 35, 45, 55, 65, and 75 years would have life expectancies that are shorter by 1.2, 2.7, 3.9, 4.2, and 3.7 years, respectively, than those of same-age individuals who never smoked.
- Individuals who quit smoking at ages 35, 45, 55, 65, and 75 years would experience an additional 8.0, 5.6, 3.4, 1.7, and 0.7 years of life expectancy compared with those who continued smoking.
- People who quit at ages 65 and 75 years would have a 23.4% and 14.2% chance of gaining at least 1 additional year of life.
IN PRACTICE:
“This cessation benefit is not limited to young- and middle-aged adults who smoke; this study demonstrates its applicability to seniors as well. These findings may be valuable for clinicians seeking scientific evidence to motivate their patients who smoke to quit,” the authors wrote.
SOURCE:
The study was led by Thuy T.T. Le, PhD, from the Department of Health Management and Policy at the University of Michigan School of Public Health in Ann Arbor and published online in the American Journal of Preventive Medicine.
LIMITATIONS:
The study’s estimates were according to data from 2018 and may not reflect current trends. The estimates also did not account for variability in smoking intensity among individuals.
DISCLOSURES:
The study was supported by grants from the National Cancer Institute of the US National Institutes of Health and the US Food and Drug Administration Center for Tobacco Products. The authors declared that they had no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Quitting smoking at any age increases life expectancy, with the most significant increases observed in younger individuals. But people who quit over age 65 can extend life expectancy.
METHODOLOGY:
- Researchers analyzed the detrimental effects of smoking and the positive impacts of cessation on life expectancy in individuals aged 35-75 years.
- Age-specific death rates by smoking status were calculated using the relative risks for all-cause mortality derived from the Cancer Prevention Study II data, 2018 National Health Interview Survey smoking prevalence data, and 2018 all-cause mortality rates.
- Life tables were constructed to obtain information on the life expectancies of people who never smoked, those who currently smoked, and those who previously smoked but quit at various ages.
- Estimates of years lost due to smoking and years gained by quitting smoking were calculated for people starting at age 35 and over 10-year increments.
TAKEAWAY:
- Compared with people who never smoked, those who smoked at ages 35, 45, 55, 65, and 75 years and continued smoking throughout their lives would lose 9.1, 8.3, 7.3, 5.9, and 4.4 years, respectively.
- People who quit smoking at ages 35, 45, 55, 65, and 75 years would have life expectancies that are shorter by 1.2, 2.7, 3.9, 4.2, and 3.7 years, respectively, than those of same-age individuals who never smoked.
- Individuals who quit smoking at ages 35, 45, 55, 65, and 75 years would experience an additional 8.0, 5.6, 3.4, 1.7, and 0.7 years of life expectancy compared with those who continued smoking.
- People who quit at ages 65 and 75 years would have a 23.4% and 14.2% chance of gaining at least 1 additional year of life.
IN PRACTICE:
“This cessation benefit is not limited to young- and middle-aged adults who smoke; this study demonstrates its applicability to seniors as well. These findings may be valuable for clinicians seeking scientific evidence to motivate their patients who smoke to quit,” the authors wrote.
SOURCE:
The study was led by Thuy T.T. Le, PhD, from the Department of Health Management and Policy at the University of Michigan School of Public Health in Ann Arbor and published online in the American Journal of Preventive Medicine.
LIMITATIONS:
The study’s estimates were according to data from 2018 and may not reflect current trends. The estimates also did not account for variability in smoking intensity among individuals.
DISCLOSURES:
The study was supported by grants from the National Cancer Institute of the US National Institutes of Health and the US Food and Drug Administration Center for Tobacco Products. The authors declared that they had no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Specific Antipsychotics Linked to Increased Pneumonia Risk
TOPLINE:
High-dose antipsychotics, particularly quetiapine, clozapine, and olanzapine, are linked to increased pneumonia risk in patients with schizophrenia, new data show. Monotherapy with high anticholinergic burden also raises pneumonia risk.
METHODOLOGY:
- Using several nationwide data registers, investigators pulled data on individuals who received inpatient care for schizophrenia or schizoaffective disorder (n = 61,889) between 1972 and 2014.
- Data on drug use were gathered from a prescription register and included dispensing dates, cost, dose, package size, and drug formulation. Data on dates and causes of death were obtained from the Causes of Death register.
- After entering the cohort, follow-up started in January 1996 or after the first diagnosis of schizophrenia for those diagnosed between 1996 and 2014.
- The primary outcome was hospitalization caused by pneumonia as the main diagnosis for hospital admission.
TAKEAWAY:
- During 22 years of follow-up, 8917 patients (14.4%) had one or more hospitalizations for pneumonia, and 1137 (12.8%) died within 30 days of admission.
- Pneumonia risk was the highest with the use of high-dose (> 440 mg/d) quetiapine (P = .003), followed by high- (≥ 330 mg/d) and medium-dose (180 to < 330 mg/d) clozapine (both P < .001) and high-dose (≥ 11 mg/d) olanzapine (P = .02).
- Compared with no antipsychotic use, antipsychotic monotherapy was associated with an increased pneumonia risk (P = .03), whereas antipsychotic polytherapy was not.
- Only the use of antipsychotics with high anticholinergic potency was associated with pneumonia risk (P < .001).
IN PRACTICE:
“Identification of antipsychotic drugs that are associated with pneumonia risk may better inform prevention programs (eg, vaccinations),” the researchers noted. “Second, the availability of pneumonia risk estimates for individual antipsychotics and for groups of antipsychotics may foster personalized prescribing guidelines.”
SOURCE:
The study was led by Jurjen Luykx, MD, Amsterdam University Medical Center, Amsterdam, the Netherlands. It was published online in JAMA Psychiatry.
LIMITATIONS:
The investigators could not correct for all possible risk factors that may increase pneumonia risk in individuals with schizophrenia, such as smoking and lifestyle habits. Also, cases of pneumonia that didn’t require hospital admission couldn’t be included in the analysis, so the findings may generalize only to cases of severe pneumonia.
DISCLOSURES:
The study was funded by the Finnish Ministry of Social Affairs and Health.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
High-dose antipsychotics, particularly quetiapine, clozapine, and olanzapine, are linked to increased pneumonia risk in patients with schizophrenia, new data show. Monotherapy with high anticholinergic burden also raises pneumonia risk.
METHODOLOGY:
- Using several nationwide data registers, investigators pulled data on individuals who received inpatient care for schizophrenia or schizoaffective disorder (n = 61,889) between 1972 and 2014.
- Data on drug use were gathered from a prescription register and included dispensing dates, cost, dose, package size, and drug formulation. Data on dates and causes of death were obtained from the Causes of Death register.
- After entering the cohort, follow-up started in January 1996 or after the first diagnosis of schizophrenia for those diagnosed between 1996 and 2014.
- The primary outcome was hospitalization caused by pneumonia as the main diagnosis for hospital admission.
TAKEAWAY:
- During 22 years of follow-up, 8917 patients (14.4%) had one or more hospitalizations for pneumonia, and 1137 (12.8%) died within 30 days of admission.
- Pneumonia risk was the highest with the use of high-dose (> 440 mg/d) quetiapine (P = .003), followed by high- (≥ 330 mg/d) and medium-dose (180 to < 330 mg/d) clozapine (both P < .001) and high-dose (≥ 11 mg/d) olanzapine (P = .02).
- Compared with no antipsychotic use, antipsychotic monotherapy was associated with an increased pneumonia risk (P = .03), whereas antipsychotic polytherapy was not.
- Only the use of antipsychotics with high anticholinergic potency was associated with pneumonia risk (P < .001).
IN PRACTICE:
“Identification of antipsychotic drugs that are associated with pneumonia risk may better inform prevention programs (eg, vaccinations),” the researchers noted. “Second, the availability of pneumonia risk estimates for individual antipsychotics and for groups of antipsychotics may foster personalized prescribing guidelines.”
SOURCE:
The study was led by Jurjen Luykx, MD, Amsterdam University Medical Center, Amsterdam, the Netherlands. It was published online in JAMA Psychiatry.
LIMITATIONS:
The investigators could not correct for all possible risk factors that may increase pneumonia risk in individuals with schizophrenia, such as smoking and lifestyle habits. Also, cases of pneumonia that didn’t require hospital admission couldn’t be included in the analysis, so the findings may generalize only to cases of severe pneumonia.
DISCLOSURES:
The study was funded by the Finnish Ministry of Social Affairs and Health.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
High-dose antipsychotics, particularly quetiapine, clozapine, and olanzapine, are linked to increased pneumonia risk in patients with schizophrenia, new data show. Monotherapy with high anticholinergic burden also raises pneumonia risk.
METHODOLOGY:
- Using several nationwide data registers, investigators pulled data on individuals who received inpatient care for schizophrenia or schizoaffective disorder (n = 61,889) between 1972 and 2014.
- Data on drug use were gathered from a prescription register and included dispensing dates, cost, dose, package size, and drug formulation. Data on dates and causes of death were obtained from the Causes of Death register.
- After entering the cohort, follow-up started in January 1996 or after the first diagnosis of schizophrenia for those diagnosed between 1996 and 2014.
- The primary outcome was hospitalization caused by pneumonia as the main diagnosis for hospital admission.
TAKEAWAY:
- During 22 years of follow-up, 8917 patients (14.4%) had one or more hospitalizations for pneumonia, and 1137 (12.8%) died within 30 days of admission.
- Pneumonia risk was the highest with the use of high-dose (> 440 mg/d) quetiapine (P = .003), followed by high- (≥ 330 mg/d) and medium-dose (180 to < 330 mg/d) clozapine (both P < .001) and high-dose (≥ 11 mg/d) olanzapine (P = .02).
- Compared with no antipsychotic use, antipsychotic monotherapy was associated with an increased pneumonia risk (P = .03), whereas antipsychotic polytherapy was not.
- Only the use of antipsychotics with high anticholinergic potency was associated with pneumonia risk (P < .001).
IN PRACTICE:
“Identification of antipsychotic drugs that are associated with pneumonia risk may better inform prevention programs (eg, vaccinations),” the researchers noted. “Second, the availability of pneumonia risk estimates for individual antipsychotics and for groups of antipsychotics may foster personalized prescribing guidelines.”
SOURCE:
The study was led by Jurjen Luykx, MD, Amsterdam University Medical Center, Amsterdam, the Netherlands. It was published online in JAMA Psychiatry.
LIMITATIONS:
The investigators could not correct for all possible risk factors that may increase pneumonia risk in individuals with schizophrenia, such as smoking and lifestyle habits. Also, cases of pneumonia that didn’t require hospital admission couldn’t be included in the analysis, so the findings may generalize only to cases of severe pneumonia.
DISCLOSURES:
The study was funded by the Finnish Ministry of Social Affairs and Health.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Weight Loss Drugs Cut Cancer Risk in Diabetes Patients
Recent research on popular weight loss drugs has uncovered surprising benefits beyond their intended use, like lowering the risk of fatal heart attacks. And now there may be another unforeseen advantage:
That’s according to a study published July 5 in JAMA Network Open where researchers studied glucagon-like peptide receptor agonists (known as GLP-1RAs), a class of drugs used to treat diabetes and obesity. Ozempic, Wegovy, Mounjaro, and Zepbound, which have become well-known recently because they are linked to rapid weight loss, contain GLP-1RAs.
For the study, they looked at electronic health records of 1.7 million patients who had type 2 diabetes, no prior diagnosis of obesity-related cancers, and had been prescribed GLP-1RAs, insulins, or metformin from March 2005 to November 2018.
The scientists found that compared to patients who took insulin, people who took GLP-1RAs had a “significant risk reduction” in 10 of 13 obesity-related cancers. Those 10 cancers were esophageal, colorectal, endometrial, gallbladder, kidney, liver, ovarian, and pancreatic cancers, as well as meningioma and multiple myeloma.
Compared with patients taking insulin, patients taking GLP-1RAs showed no statistically significant reduction in stomach cancer and no reduced risk of breast and thyroid cancers, the study said.
But the study found no decrease in cancer risk with GLP-1RAs compared with metformin.
While the study results suggest that these drugs may reduce the risk of certain obesity-related cancers better than insulins, more research is needed, they said.
A version of this article appeared on WebMD.com.
Recent research on popular weight loss drugs has uncovered surprising benefits beyond their intended use, like lowering the risk of fatal heart attacks. And now there may be another unforeseen advantage:
That’s according to a study published July 5 in JAMA Network Open where researchers studied glucagon-like peptide receptor agonists (known as GLP-1RAs), a class of drugs used to treat diabetes and obesity. Ozempic, Wegovy, Mounjaro, and Zepbound, which have become well-known recently because they are linked to rapid weight loss, contain GLP-1RAs.
For the study, they looked at electronic health records of 1.7 million patients who had type 2 diabetes, no prior diagnosis of obesity-related cancers, and had been prescribed GLP-1RAs, insulins, or metformin from March 2005 to November 2018.
The scientists found that compared to patients who took insulin, people who took GLP-1RAs had a “significant risk reduction” in 10 of 13 obesity-related cancers. Those 10 cancers were esophageal, colorectal, endometrial, gallbladder, kidney, liver, ovarian, and pancreatic cancers, as well as meningioma and multiple myeloma.
Compared with patients taking insulin, patients taking GLP-1RAs showed no statistically significant reduction in stomach cancer and no reduced risk of breast and thyroid cancers, the study said.
But the study found no decrease in cancer risk with GLP-1RAs compared with metformin.
While the study results suggest that these drugs may reduce the risk of certain obesity-related cancers better than insulins, more research is needed, they said.
A version of this article appeared on WebMD.com.
Recent research on popular weight loss drugs has uncovered surprising benefits beyond their intended use, like lowering the risk of fatal heart attacks. And now there may be another unforeseen advantage:
That’s according to a study published July 5 in JAMA Network Open where researchers studied glucagon-like peptide receptor agonists (known as GLP-1RAs), a class of drugs used to treat diabetes and obesity. Ozempic, Wegovy, Mounjaro, and Zepbound, which have become well-known recently because they are linked to rapid weight loss, contain GLP-1RAs.
For the study, they looked at electronic health records of 1.7 million patients who had type 2 diabetes, no prior diagnosis of obesity-related cancers, and had been prescribed GLP-1RAs, insulins, or metformin from March 2005 to November 2018.
The scientists found that compared to patients who took insulin, people who took GLP-1RAs had a “significant risk reduction” in 10 of 13 obesity-related cancers. Those 10 cancers were esophageal, colorectal, endometrial, gallbladder, kidney, liver, ovarian, and pancreatic cancers, as well as meningioma and multiple myeloma.
Compared with patients taking insulin, patients taking GLP-1RAs showed no statistically significant reduction in stomach cancer and no reduced risk of breast and thyroid cancers, the study said.
But the study found no decrease in cancer risk with GLP-1RAs compared with metformin.
While the study results suggest that these drugs may reduce the risk of certain obesity-related cancers better than insulins, more research is needed, they said.
A version of this article appeared on WebMD.com.
Feds May End Hospital System’s Noncompete Contract for Part-Time Docs
Mount Sinai Health System in New York City is forcing part-time physicians to sign employment contracts that violate their labor rights, according to a June 2024 complaint by the National Labor Relations Board (NLRB).
The complaint stems from no-poaching and confidentiality clauses in the agreements required as a condition of employment, NLRB officials alleged.
according to a copy of the terms included in NLRB’s June 18 complaint.
By requiring the agreements, NLRB officials claimed, Mount Sinai is “interfering with, restraining, and coercing employees” in violation of the National Labor Relations Act. The health system’s “unfair labor practices” affects commerce as outlined under the law, according to the NLRB. The Act bans employers from burdening or obstructing commerce or the free flow of commerce.
Mount Sinai did not respond to requests for comment.
The NLRB’s complaint follows a landmark decision by the Federal Trade Commission (FTC) to ban noncompete agreements nationwide. In April 2024, the FTC voted to prohibit noncompetes indefinitely in an effort to protect workers.
“Noncompete clauses keep wages low, suppress new ideas, and rob the American economy of dynamism, including from the more than 8500 new startups that would be created a year once noncompetes are banned,” FTC Chair Lina M. Khan said in a statement. “The FTC’s final rule to ban noncompetes will ensure Americans have the freedom to pursue a new job, start a new business, or bring a new idea to market.”
Business groups and agencies have since sued to challenge against the ban, including the Chamber of Commerce. The Chamber and other business groups argue that noncompete agreements are important for companies to protect trade secrets, shield recruiting investments, and hide confidential information. The lawsuits are ongoing.
A Physician Blows the Whistle
An anonymous physician first alerted the NLRB to the contract language in November 2023. The doctor was required the sign the hospital system’s agreement for part-time physicians. The complaint does not say if the employee is still employed by the hospital system.
To remedy the unfair labor practices alleged, the NLRB seeks an order requiring the health system to rescind the contract language, stop any actions against current or former employees to enforce the provisions, and make whole any employees who suffered financial losses related to the contract terms.
The allegation against Mount Sinai is among a rising number of grievances filed with the NLRB that claim unfair labor practices. During the first 6 months of fiscal year 2024, unfair labor practice charges filed across the NLRB’s field offices increased 7% — from 9612 in 2023 to 10,278 in 2024, according to a news release.
NLRB, meanwhile has been cracking down on anticompetitive labor practices and confidentiality provisions that prevent employees from speaking out.
In a February 2023 decision for instance, NLRB ruled that an employer violates the National Labor Relations Act by offering severance agreements to workers that include restrictive confidentiality and nondisparagement terms. In 2022, the NLRB and the Federal Trade Commission forged a partnership to more widely combat unfair, anticompetitive, and deceptive business practices.
“Noncompete provisions reasonably tend to chill employees in the exercise of Section 7 rights when the provisions could reasonably be construed by employees to deny them the ability to quit or change jobs by cutting off their access to other employment opportunities that they are qualified for,” NLRB General Counsel Jennifer Abruzzo said in a 2023 release.
Ms. Abruzzo stressed in a memo that NLR Act is committed to an interagency approach to restrictions on the exercise of employee rights, “including limits to workers’ job mobility, information sharing, and referrals to other agencies.”
Mount Sinai Health System must respond to the NLRB’s complaint by July 16, and an administrative law judge is scheduled to hear the case on September 24.
A version of this article first appeared on Medscape.com.
Mount Sinai Health System in New York City is forcing part-time physicians to sign employment contracts that violate their labor rights, according to a June 2024 complaint by the National Labor Relations Board (NLRB).
The complaint stems from no-poaching and confidentiality clauses in the agreements required as a condition of employment, NLRB officials alleged.
according to a copy of the terms included in NLRB’s June 18 complaint.
By requiring the agreements, NLRB officials claimed, Mount Sinai is “interfering with, restraining, and coercing employees” in violation of the National Labor Relations Act. The health system’s “unfair labor practices” affects commerce as outlined under the law, according to the NLRB. The Act bans employers from burdening or obstructing commerce or the free flow of commerce.
Mount Sinai did not respond to requests for comment.
The NLRB’s complaint follows a landmark decision by the Federal Trade Commission (FTC) to ban noncompete agreements nationwide. In April 2024, the FTC voted to prohibit noncompetes indefinitely in an effort to protect workers.
“Noncompete clauses keep wages low, suppress new ideas, and rob the American economy of dynamism, including from the more than 8500 new startups that would be created a year once noncompetes are banned,” FTC Chair Lina M. Khan said in a statement. “The FTC’s final rule to ban noncompetes will ensure Americans have the freedom to pursue a new job, start a new business, or bring a new idea to market.”
Business groups and agencies have since sued to challenge against the ban, including the Chamber of Commerce. The Chamber and other business groups argue that noncompete agreements are important for companies to protect trade secrets, shield recruiting investments, and hide confidential information. The lawsuits are ongoing.
A Physician Blows the Whistle
An anonymous physician first alerted the NLRB to the contract language in November 2023. The doctor was required the sign the hospital system’s agreement for part-time physicians. The complaint does not say if the employee is still employed by the hospital system.
To remedy the unfair labor practices alleged, the NLRB seeks an order requiring the health system to rescind the contract language, stop any actions against current or former employees to enforce the provisions, and make whole any employees who suffered financial losses related to the contract terms.
The allegation against Mount Sinai is among a rising number of grievances filed with the NLRB that claim unfair labor practices. During the first 6 months of fiscal year 2024, unfair labor practice charges filed across the NLRB’s field offices increased 7% — from 9612 in 2023 to 10,278 in 2024, according to a news release.
NLRB, meanwhile has been cracking down on anticompetitive labor practices and confidentiality provisions that prevent employees from speaking out.
In a February 2023 decision for instance, NLRB ruled that an employer violates the National Labor Relations Act by offering severance agreements to workers that include restrictive confidentiality and nondisparagement terms. In 2022, the NLRB and the Federal Trade Commission forged a partnership to more widely combat unfair, anticompetitive, and deceptive business practices.
“Noncompete provisions reasonably tend to chill employees in the exercise of Section 7 rights when the provisions could reasonably be construed by employees to deny them the ability to quit or change jobs by cutting off their access to other employment opportunities that they are qualified for,” NLRB General Counsel Jennifer Abruzzo said in a 2023 release.
Ms. Abruzzo stressed in a memo that NLR Act is committed to an interagency approach to restrictions on the exercise of employee rights, “including limits to workers’ job mobility, information sharing, and referrals to other agencies.”
Mount Sinai Health System must respond to the NLRB’s complaint by July 16, and an administrative law judge is scheduled to hear the case on September 24.
A version of this article first appeared on Medscape.com.
Mount Sinai Health System in New York City is forcing part-time physicians to sign employment contracts that violate their labor rights, according to a June 2024 complaint by the National Labor Relations Board (NLRB).
The complaint stems from no-poaching and confidentiality clauses in the agreements required as a condition of employment, NLRB officials alleged.
according to a copy of the terms included in NLRB’s June 18 complaint.
By requiring the agreements, NLRB officials claimed, Mount Sinai is “interfering with, restraining, and coercing employees” in violation of the National Labor Relations Act. The health system’s “unfair labor practices” affects commerce as outlined under the law, according to the NLRB. The Act bans employers from burdening or obstructing commerce or the free flow of commerce.
Mount Sinai did not respond to requests for comment.
The NLRB’s complaint follows a landmark decision by the Federal Trade Commission (FTC) to ban noncompete agreements nationwide. In April 2024, the FTC voted to prohibit noncompetes indefinitely in an effort to protect workers.
“Noncompete clauses keep wages low, suppress new ideas, and rob the American economy of dynamism, including from the more than 8500 new startups that would be created a year once noncompetes are banned,” FTC Chair Lina M. Khan said in a statement. “The FTC’s final rule to ban noncompetes will ensure Americans have the freedom to pursue a new job, start a new business, or bring a new idea to market.”
Business groups and agencies have since sued to challenge against the ban, including the Chamber of Commerce. The Chamber and other business groups argue that noncompete agreements are important for companies to protect trade secrets, shield recruiting investments, and hide confidential information. The lawsuits are ongoing.
A Physician Blows the Whistle
An anonymous physician first alerted the NLRB to the contract language in November 2023. The doctor was required the sign the hospital system’s agreement for part-time physicians. The complaint does not say if the employee is still employed by the hospital system.
To remedy the unfair labor practices alleged, the NLRB seeks an order requiring the health system to rescind the contract language, stop any actions against current or former employees to enforce the provisions, and make whole any employees who suffered financial losses related to the contract terms.
The allegation against Mount Sinai is among a rising number of grievances filed with the NLRB that claim unfair labor practices. During the first 6 months of fiscal year 2024, unfair labor practice charges filed across the NLRB’s field offices increased 7% — from 9612 in 2023 to 10,278 in 2024, according to a news release.
NLRB, meanwhile has been cracking down on anticompetitive labor practices and confidentiality provisions that prevent employees from speaking out.
In a February 2023 decision for instance, NLRB ruled that an employer violates the National Labor Relations Act by offering severance agreements to workers that include restrictive confidentiality and nondisparagement terms. In 2022, the NLRB and the Federal Trade Commission forged a partnership to more widely combat unfair, anticompetitive, and deceptive business practices.
“Noncompete provisions reasonably tend to chill employees in the exercise of Section 7 rights when the provisions could reasonably be construed by employees to deny them the ability to quit or change jobs by cutting off their access to other employment opportunities that they are qualified for,” NLRB General Counsel Jennifer Abruzzo said in a 2023 release.
Ms. Abruzzo stressed in a memo that NLR Act is committed to an interagency approach to restrictions on the exercise of employee rights, “including limits to workers’ job mobility, information sharing, and referrals to other agencies.”
Mount Sinai Health System must respond to the NLRB’s complaint by July 16, and an administrative law judge is scheduled to hear the case on September 24.
A version of this article first appeared on Medscape.com.
Cold or Flu Virus May Trigger Relapse of Long COVID
researchers have found.
In some cases, they may be experiencing what researchers call viral interference, something also experienced by people with HIV and other infections associated with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
Clinical studies on the issue are limited, but patients, doctors, and researchers report many people who previously had long COVID have developed recurring symptoms after consequent viral infections.
Viral persistence — where bits of virus linger in the body — and viral reactivation remain two of the leading suspects for Yale researchers. Viral activation occurs when the immune system responds to an infection by triggering a dormant virus.
Anecdotally, these flare-ups occur more commonly in patients with long COVID with autonomic dysfunction — severe dizziness when standing up — and other symptoms of ME/CFS, said Alba Azola, MD, a Johns Hopkins Medicine rehabilitation specialist in Baltimore, Maryland, who works with patients with long COVID and other “fatiguing illnesses.”
At last count, about 18% of those surveyed by the Centers for Disease Control and Prevention said they had experienced long COVID. Nearly 60% of those surveyed said they had contracted COVID-19 at least once.
Dr. Azola said that very afternoon she had seen a patient with the flu and a recurrence of previous long COVID symptoms. Not much data exist about cases like this.
“I can’t say there is a specific study looking at this, but anecdotally, we see it all the time,” Dr. Azola said.
She has not seen completely different symptoms; more commonly, she sees a flare-up of previously existing symptoms.
David Putrino, PhD, is director of rehabilitation innovation for the Mount Sinai Health System in New York City. He treats and studies patients with long COVID and echoes what others have seen.
Patients can “recover (or feel recovered) from long COVID until the next immune challenge — another COVID infection, flu infection, pregnancy, food poisoning (all examples we have seen in the clinic) — and experience a significant flare-up of your initial COVID infection,” he said.
“Relapse” is a better term than reinfection, said Jeffrey Parsonnet, MD, an infectious diseases specialist and director of the Dartmouth Hitchcock Post-Acute COVID Syndrome Clinic, Lebanon, New Hampshire.
“We see patients who had COVID-19 followed by long COVID who then get better — either completely or mostly better. Then they’ve gotten COVID again, and this is followed by recurrence of long COVID symptoms,” he said.
“Every patient looks different in terms of what gets better and how quickly. And again, some patients are not better (or even minimally so) after a couple of years,” he said.
Patients Tell Their Stories
On the COVID-19 Long Haulers Support Facebook group, many of the 100,000 followers ask about viral reactivation. Delainne “Laney” Bond, RN, who has battled postinfection chronic illness herself, runs the Facebook group. From what she sees, “each time a person is infected or reinfected with SARS-CoV-2, they have a risk of developing long COVID or experiencing worse long COVID. Multiple infections can lead to progressive health complications.”
The posts on her site include many queries about reinfections. A post from December included nearly 80 comments with people describing the full range of symptoms. Some stories relayed how the reinfection symptoms were short lived. Some report returning to their baseline — not completely symptom free but improved.
Doctors and patients say long COVID comes and goes — relapsing-remitting — and shares many features with other complex multisystem chronic conditions, according to a new National Academy of Sciences report. Those include ME/CFS and the Epstein-Barr virus.
As far as how to treat, Dr. Putrino is one of the clinical researchers testing antivirals. One is Paxlovid; the others are drugs developed for the AIDS virus.
“A plausible mechanism for long COVID is persistence of the SARS-CoV-2 virus in tissue and/or the reactivation of latent pathogens,” according to an explanation of the research on the PolyBio Institute website, which is involved with the research.
In the meantime, “long COVID appears to be a chronic condition with few patients achieving full remission,” according to a new Academy of Sciences report. The report concludes that long COVID recovery can plateau at 6-12 months. They also note that 18%-22% of people who have long COVID symptoms at 5 months are still ill at 1 year.
A version of this article first appeared on Medscape.com.
researchers have found.
In some cases, they may be experiencing what researchers call viral interference, something also experienced by people with HIV and other infections associated with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
Clinical studies on the issue are limited, but patients, doctors, and researchers report many people who previously had long COVID have developed recurring symptoms after consequent viral infections.
Viral persistence — where bits of virus linger in the body — and viral reactivation remain two of the leading suspects for Yale researchers. Viral activation occurs when the immune system responds to an infection by triggering a dormant virus.
Anecdotally, these flare-ups occur more commonly in patients with long COVID with autonomic dysfunction — severe dizziness when standing up — and other symptoms of ME/CFS, said Alba Azola, MD, a Johns Hopkins Medicine rehabilitation specialist in Baltimore, Maryland, who works with patients with long COVID and other “fatiguing illnesses.”
At last count, about 18% of those surveyed by the Centers for Disease Control and Prevention said they had experienced long COVID. Nearly 60% of those surveyed said they had contracted COVID-19 at least once.
Dr. Azola said that very afternoon she had seen a patient with the flu and a recurrence of previous long COVID symptoms. Not much data exist about cases like this.
“I can’t say there is a specific study looking at this, but anecdotally, we see it all the time,” Dr. Azola said.
She has not seen completely different symptoms; more commonly, she sees a flare-up of previously existing symptoms.
David Putrino, PhD, is director of rehabilitation innovation for the Mount Sinai Health System in New York City. He treats and studies patients with long COVID and echoes what others have seen.
Patients can “recover (or feel recovered) from long COVID until the next immune challenge — another COVID infection, flu infection, pregnancy, food poisoning (all examples we have seen in the clinic) — and experience a significant flare-up of your initial COVID infection,” he said.
“Relapse” is a better term than reinfection, said Jeffrey Parsonnet, MD, an infectious diseases specialist and director of the Dartmouth Hitchcock Post-Acute COVID Syndrome Clinic, Lebanon, New Hampshire.
“We see patients who had COVID-19 followed by long COVID who then get better — either completely or mostly better. Then they’ve gotten COVID again, and this is followed by recurrence of long COVID symptoms,” he said.
“Every patient looks different in terms of what gets better and how quickly. And again, some patients are not better (or even minimally so) after a couple of years,” he said.
Patients Tell Their Stories
On the COVID-19 Long Haulers Support Facebook group, many of the 100,000 followers ask about viral reactivation. Delainne “Laney” Bond, RN, who has battled postinfection chronic illness herself, runs the Facebook group. From what she sees, “each time a person is infected or reinfected with SARS-CoV-2, they have a risk of developing long COVID or experiencing worse long COVID. Multiple infections can lead to progressive health complications.”
The posts on her site include many queries about reinfections. A post from December included nearly 80 comments with people describing the full range of symptoms. Some stories relayed how the reinfection symptoms were short lived. Some report returning to their baseline — not completely symptom free but improved.
Doctors and patients say long COVID comes and goes — relapsing-remitting — and shares many features with other complex multisystem chronic conditions, according to a new National Academy of Sciences report. Those include ME/CFS and the Epstein-Barr virus.
As far as how to treat, Dr. Putrino is one of the clinical researchers testing antivirals. One is Paxlovid; the others are drugs developed for the AIDS virus.
“A plausible mechanism for long COVID is persistence of the SARS-CoV-2 virus in tissue and/or the reactivation of latent pathogens,” according to an explanation of the research on the PolyBio Institute website, which is involved with the research.
In the meantime, “long COVID appears to be a chronic condition with few patients achieving full remission,” according to a new Academy of Sciences report. The report concludes that long COVID recovery can plateau at 6-12 months. They also note that 18%-22% of people who have long COVID symptoms at 5 months are still ill at 1 year.
A version of this article first appeared on Medscape.com.
researchers have found.
In some cases, they may be experiencing what researchers call viral interference, something also experienced by people with HIV and other infections associated with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).
Clinical studies on the issue are limited, but patients, doctors, and researchers report many people who previously had long COVID have developed recurring symptoms after consequent viral infections.
Viral persistence — where bits of virus linger in the body — and viral reactivation remain two of the leading suspects for Yale researchers. Viral activation occurs when the immune system responds to an infection by triggering a dormant virus.
Anecdotally, these flare-ups occur more commonly in patients with long COVID with autonomic dysfunction — severe dizziness when standing up — and other symptoms of ME/CFS, said Alba Azola, MD, a Johns Hopkins Medicine rehabilitation specialist in Baltimore, Maryland, who works with patients with long COVID and other “fatiguing illnesses.”
At last count, about 18% of those surveyed by the Centers for Disease Control and Prevention said they had experienced long COVID. Nearly 60% of those surveyed said they had contracted COVID-19 at least once.
Dr. Azola said that very afternoon she had seen a patient with the flu and a recurrence of previous long COVID symptoms. Not much data exist about cases like this.
“I can’t say there is a specific study looking at this, but anecdotally, we see it all the time,” Dr. Azola said.
She has not seen completely different symptoms; more commonly, she sees a flare-up of previously existing symptoms.
David Putrino, PhD, is director of rehabilitation innovation for the Mount Sinai Health System in New York City. He treats and studies patients with long COVID and echoes what others have seen.
Patients can “recover (or feel recovered) from long COVID until the next immune challenge — another COVID infection, flu infection, pregnancy, food poisoning (all examples we have seen in the clinic) — and experience a significant flare-up of your initial COVID infection,” he said.
“Relapse” is a better term than reinfection, said Jeffrey Parsonnet, MD, an infectious diseases specialist and director of the Dartmouth Hitchcock Post-Acute COVID Syndrome Clinic, Lebanon, New Hampshire.
“We see patients who had COVID-19 followed by long COVID who then get better — either completely or mostly better. Then they’ve gotten COVID again, and this is followed by recurrence of long COVID symptoms,” he said.
“Every patient looks different in terms of what gets better and how quickly. And again, some patients are not better (or even minimally so) after a couple of years,” he said.
Patients Tell Their Stories
On the COVID-19 Long Haulers Support Facebook group, many of the 100,000 followers ask about viral reactivation. Delainne “Laney” Bond, RN, who has battled postinfection chronic illness herself, runs the Facebook group. From what she sees, “each time a person is infected or reinfected with SARS-CoV-2, they have a risk of developing long COVID or experiencing worse long COVID. Multiple infections can lead to progressive health complications.”
The posts on her site include many queries about reinfections. A post from December included nearly 80 comments with people describing the full range of symptoms. Some stories relayed how the reinfection symptoms were short lived. Some report returning to their baseline — not completely symptom free but improved.
Doctors and patients say long COVID comes and goes — relapsing-remitting — and shares many features with other complex multisystem chronic conditions, according to a new National Academy of Sciences report. Those include ME/CFS and the Epstein-Barr virus.
As far as how to treat, Dr. Putrino is one of the clinical researchers testing antivirals. One is Paxlovid; the others are drugs developed for the AIDS virus.
“A plausible mechanism for long COVID is persistence of the SARS-CoV-2 virus in tissue and/or the reactivation of latent pathogens,” according to an explanation of the research on the PolyBio Institute website, which is involved with the research.
In the meantime, “long COVID appears to be a chronic condition with few patients achieving full remission,” according to a new Academy of Sciences report. The report concludes that long COVID recovery can plateau at 6-12 months. They also note that 18%-22% of people who have long COVID symptoms at 5 months are still ill at 1 year.
A version of this article first appeared on Medscape.com.
Pediatric Studies Produce Mixed Messages on Relationship Between COVID and Asthma
In one of several recently published studies on the relationship between COVID-19 infection and asthma, according to data drawn from the National Survey of Children’s Health (NSCH).
The inverse correlation between symptoms and vaccination was strong and statistically significant, according to investigators led by Matthew M. Davis, MD, Physician in Chief and Chief Scientific Officer, Nemours Children’s Health, Wilmington, Delaware.
“With each increase of 10 percentage points in COVID-19 vaccination coverage, the parent-reported child asthma symptoms prevalence decreased by 0.36 percentage points (P < .05),” Dr. Davis and his coinvestigators reported in a research letter published in JAMA Network Open.
Studies Explore Relationship of COVID and Asthma
The reduced risk of asthma symptoms with COVID-19 vaccination in children at the population level is just one of several recently published studies exploring the interaction between COVID-19 infection and asthma, but two studies that posed the same question did not reach the same conclusion.
In one, COVID-19 infection in children was not found to be a trigger for new-onset asthma, but the second found that it was. In a third study, the preponderance of evidence from a meta-analysis found that patients with asthma – whether children or adults – did not necessarily experience a more severe course of COVID-19 infection than in those without asthma.
The NSCH database study calculated state-level change in scores for patient-reported childhood asthma symptoms in the years in the years 2018-2019, which preceded the pandemic and the years 2020-2021, when the pandemic began. The hypothesis was that the proportion of the population 5 years of age or older who completed the COVID-19 primary vaccination would be inversely related to asthma symptom prevalence.
Relative to the 2018-2019 years, the mean rate of parent-reported asthma symptoms was 0.85% lower (6.93% vs 7.77%; P < .001) in 2020-2021, when the mean primary series COVID-19 vaccination rate was 72.3%.
The study was not able to evaluate the impact of COVID-19 vaccination specifically in children with asthma, because history of asthma is not captured in the NSCH data, but Dr. Davis contended that the reduction in symptomatic asthma among children with increased vaccination offers validation for the state-level findings.
“Moreover, the absence of an association of COVID-19 vaccination administered predominantly in 2021 with population-level COVID-19 mortality in 2020 serves as a negative control,” he and his colleagues wrote in their research letter.
Protection from Respiratory Viruses Seen for Asthma Patients
In an interview, Dr. Davis reported that these data are consistent with previous evidence that immunization against influenza also reduces risk of asthma symptoms. In a meta-analysis published in 2017, it was estimated that live vaccines reduced risk of influenza by 81% and prevented 59%-72% of asthma attacks leading to hospitalizations or emergency room visits.
“The similarity of our findings regarding COVID-19 vaccination to prior data regarding influenza vaccination underscores the importance of preventing viral illnesses in individuals with a history of asthma,” Dr. Davis said. It is not yet clear if this is true of respiratory syncytial virus (RSV). Because of the short time that the RSV vaccine has been available, it is too soon to conduct an analysis.
One message from this study is that “clinicians should continue to encourage COVID-19 vaccination for children because of its general benefits in preventing coronavirus-related illness and the apparent specific benefits for children with a history of asthma,” he said.
While vaccination appears to reduce asthmatic symptoms related to COVID-19 infection, one study suggests that COVID-19 does not trigger new-onset asthma. In a retrospective study published in Pediatrics, no association between COVID-19 infection and new-onset asthma could be made in an analysis of 27,423 children (ages, 1-16 years) from the Children’s Hospital of Philadelphia (CHOP) Care Network.
Across all the pediatric age groups evaluated, the consistent finding was “SARS-CoV-2 positivity does not confer an additional risk for asthma diagnosis at least within the first 18 months after a [polymerase chain reaction] test,” concluded the investigators, led by David A. Hill, MD, PhD, Division of Allergy and Immunology, CHOP, Philadelphia, Pennsylvania.
Risk of Asthma Doubled After COVID-19 Infection
However, the opposite conclusion was reached by investigators evaluating data from two cohorts of children ages 5-18 drawn from the TriNetX database, a global health research network with data on more than 250 million individuals. Cohort 1 included more than 250,000 children. These children had never received COVID-19 vaccination. The 50,000 patients in cohort 2 had all received COVID19 vaccination.
To compare the impact of COVID-19 infection on new-onset asthma, the patients who were infected with COVID-19 were compared with those who were not infected after propensity score matching over 18 months of follow-up.
In cohort 1, the rate of new onset asthma was more than twofold greater among those with COVID-19 infection (4.7% vs 2.0%). The hazard ratio (HR) of 2.25 had tight confidence intervals (95% CI, 2.158-2.367).
In cohort 2, the risk of new-onset asthma at 18 months among those who had a COVID-19 infection relative to those without was even greater (8.3% vs 3.1%). The relative risk approached a 3-fold increase (HR 2.745; 95% CI, 2.521-2.99).
The conclusion of these investigators, led by Chia-Chi Lung, PhD, Department of Public Health, Chung Shan Medical University, Taichung City, Taiwan, was that there is “a critical need for ongoing monitoring and customized healthcare strategies to mitigate the long-term respiratory impacts of COVID-19 in children.”
These health risks might not be as significant as once feared. In the recently published study from Environmental Health Insights, the goal of a meta-analysis was to determine if patients with asthma relative to those without asthma face a higher risk of serious disease from COVID-19 infection. The meta-analysis included studies of children and adults. The answer, according an in-depth analysis of 21 articles in a “scoping review,” was a qualified no.
Of the 21 articles, 4 concluded that asthma is a risk factor for serious COVID-19 infection, but 17 did not, according to Chukwudi S. Ubah, PhD, Department of Public Health, Brody School of Medicine, East Caroline University, Greenville, North Carolina.
None of These Questions are Fully Resolved
However, given the disparity in the results and the fact that many of the studies included in this analysis had small sample sizes, Dr. Ubah called for larger studies and studies with better controls. He noted, for example, that the studies did not consistently evaluate mitigating factors, such as used of inhaled or oral corticosteroids, which might affect risk of the severity of a COVID-19 infection.
Rather, “our findings pointed out that the type of medication prescribed for asthma may have implications for the severity of COVID-19 infection in these patients,” Dr. Ubah said in an interview.
Overall, the data do not support a major interaction between asthma and COVID-19, even if the data are not conclusive. Each of the senior authors of these studies called for larger and better investigations to further explore whether COVID-19 infection and preexisting asthma interact. So far, the data indicate that if COVID-19 infection poses a risk of precipitating new-onset asthma or inducing a more severe infection in children with asthma, it is low, but the degree of risk, if any, remains unresolved in subgroups defined by asthma treatment or asthma severity.
Dr. Davis, Dr. Hill, Dr. Lung, and Dr. Ubah reported no potential conflicts of interest. None of these studies received funding from commercial interests.
In one of several recently published studies on the relationship between COVID-19 infection and asthma, according to data drawn from the National Survey of Children’s Health (NSCH).
The inverse correlation between symptoms and vaccination was strong and statistically significant, according to investigators led by Matthew M. Davis, MD, Physician in Chief and Chief Scientific Officer, Nemours Children’s Health, Wilmington, Delaware.
“With each increase of 10 percentage points in COVID-19 vaccination coverage, the parent-reported child asthma symptoms prevalence decreased by 0.36 percentage points (P < .05),” Dr. Davis and his coinvestigators reported in a research letter published in JAMA Network Open.
Studies Explore Relationship of COVID and Asthma
The reduced risk of asthma symptoms with COVID-19 vaccination in children at the population level is just one of several recently published studies exploring the interaction between COVID-19 infection and asthma, but two studies that posed the same question did not reach the same conclusion.
In one, COVID-19 infection in children was not found to be a trigger for new-onset asthma, but the second found that it was. In a third study, the preponderance of evidence from a meta-analysis found that patients with asthma – whether children or adults – did not necessarily experience a more severe course of COVID-19 infection than in those without asthma.
The NSCH database study calculated state-level change in scores for patient-reported childhood asthma symptoms in the years in the years 2018-2019, which preceded the pandemic and the years 2020-2021, when the pandemic began. The hypothesis was that the proportion of the population 5 years of age or older who completed the COVID-19 primary vaccination would be inversely related to asthma symptom prevalence.
Relative to the 2018-2019 years, the mean rate of parent-reported asthma symptoms was 0.85% lower (6.93% vs 7.77%; P < .001) in 2020-2021, when the mean primary series COVID-19 vaccination rate was 72.3%.
The study was not able to evaluate the impact of COVID-19 vaccination specifically in children with asthma, because history of asthma is not captured in the NSCH data, but Dr. Davis contended that the reduction in symptomatic asthma among children with increased vaccination offers validation for the state-level findings.
“Moreover, the absence of an association of COVID-19 vaccination administered predominantly in 2021 with population-level COVID-19 mortality in 2020 serves as a negative control,” he and his colleagues wrote in their research letter.
Protection from Respiratory Viruses Seen for Asthma Patients
In an interview, Dr. Davis reported that these data are consistent with previous evidence that immunization against influenza also reduces risk of asthma symptoms. In a meta-analysis published in 2017, it was estimated that live vaccines reduced risk of influenza by 81% and prevented 59%-72% of asthma attacks leading to hospitalizations or emergency room visits.
“The similarity of our findings regarding COVID-19 vaccination to prior data regarding influenza vaccination underscores the importance of preventing viral illnesses in individuals with a history of asthma,” Dr. Davis said. It is not yet clear if this is true of respiratory syncytial virus (RSV). Because of the short time that the RSV vaccine has been available, it is too soon to conduct an analysis.
One message from this study is that “clinicians should continue to encourage COVID-19 vaccination for children because of its general benefits in preventing coronavirus-related illness and the apparent specific benefits for children with a history of asthma,” he said.
While vaccination appears to reduce asthmatic symptoms related to COVID-19 infection, one study suggests that COVID-19 does not trigger new-onset asthma. In a retrospective study published in Pediatrics, no association between COVID-19 infection and new-onset asthma could be made in an analysis of 27,423 children (ages, 1-16 years) from the Children’s Hospital of Philadelphia (CHOP) Care Network.
Across all the pediatric age groups evaluated, the consistent finding was “SARS-CoV-2 positivity does not confer an additional risk for asthma diagnosis at least within the first 18 months after a [polymerase chain reaction] test,” concluded the investigators, led by David A. Hill, MD, PhD, Division of Allergy and Immunology, CHOP, Philadelphia, Pennsylvania.
Risk of Asthma Doubled After COVID-19 Infection
However, the opposite conclusion was reached by investigators evaluating data from two cohorts of children ages 5-18 drawn from the TriNetX database, a global health research network with data on more than 250 million individuals. Cohort 1 included more than 250,000 children. These children had never received COVID-19 vaccination. The 50,000 patients in cohort 2 had all received COVID19 vaccination.
To compare the impact of COVID-19 infection on new-onset asthma, the patients who were infected with COVID-19 were compared with those who were not infected after propensity score matching over 18 months of follow-up.
In cohort 1, the rate of new onset asthma was more than twofold greater among those with COVID-19 infection (4.7% vs 2.0%). The hazard ratio (HR) of 2.25 had tight confidence intervals (95% CI, 2.158-2.367).
In cohort 2, the risk of new-onset asthma at 18 months among those who had a COVID-19 infection relative to those without was even greater (8.3% vs 3.1%). The relative risk approached a 3-fold increase (HR 2.745; 95% CI, 2.521-2.99).
The conclusion of these investigators, led by Chia-Chi Lung, PhD, Department of Public Health, Chung Shan Medical University, Taichung City, Taiwan, was that there is “a critical need for ongoing monitoring and customized healthcare strategies to mitigate the long-term respiratory impacts of COVID-19 in children.”
These health risks might not be as significant as once feared. In the recently published study from Environmental Health Insights, the goal of a meta-analysis was to determine if patients with asthma relative to those without asthma face a higher risk of serious disease from COVID-19 infection. The meta-analysis included studies of children and adults. The answer, according an in-depth analysis of 21 articles in a “scoping review,” was a qualified no.
Of the 21 articles, 4 concluded that asthma is a risk factor for serious COVID-19 infection, but 17 did not, according to Chukwudi S. Ubah, PhD, Department of Public Health, Brody School of Medicine, East Caroline University, Greenville, North Carolina.
None of These Questions are Fully Resolved
However, given the disparity in the results and the fact that many of the studies included in this analysis had small sample sizes, Dr. Ubah called for larger studies and studies with better controls. He noted, for example, that the studies did not consistently evaluate mitigating factors, such as used of inhaled or oral corticosteroids, which might affect risk of the severity of a COVID-19 infection.
Rather, “our findings pointed out that the type of medication prescribed for asthma may have implications for the severity of COVID-19 infection in these patients,” Dr. Ubah said in an interview.
Overall, the data do not support a major interaction between asthma and COVID-19, even if the data are not conclusive. Each of the senior authors of these studies called for larger and better investigations to further explore whether COVID-19 infection and preexisting asthma interact. So far, the data indicate that if COVID-19 infection poses a risk of precipitating new-onset asthma or inducing a more severe infection in children with asthma, it is low, but the degree of risk, if any, remains unresolved in subgroups defined by asthma treatment or asthma severity.
Dr. Davis, Dr. Hill, Dr. Lung, and Dr. Ubah reported no potential conflicts of interest. None of these studies received funding from commercial interests.
In one of several recently published studies on the relationship between COVID-19 infection and asthma, according to data drawn from the National Survey of Children’s Health (NSCH).
The inverse correlation between symptoms and vaccination was strong and statistically significant, according to investigators led by Matthew M. Davis, MD, Physician in Chief and Chief Scientific Officer, Nemours Children’s Health, Wilmington, Delaware.
“With each increase of 10 percentage points in COVID-19 vaccination coverage, the parent-reported child asthma symptoms prevalence decreased by 0.36 percentage points (P < .05),” Dr. Davis and his coinvestigators reported in a research letter published in JAMA Network Open.
Studies Explore Relationship of COVID and Asthma
The reduced risk of asthma symptoms with COVID-19 vaccination in children at the population level is just one of several recently published studies exploring the interaction between COVID-19 infection and asthma, but two studies that posed the same question did not reach the same conclusion.
In one, COVID-19 infection in children was not found to be a trigger for new-onset asthma, but the second found that it was. In a third study, the preponderance of evidence from a meta-analysis found that patients with asthma – whether children or adults – did not necessarily experience a more severe course of COVID-19 infection than in those without asthma.
The NSCH database study calculated state-level change in scores for patient-reported childhood asthma symptoms in the years in the years 2018-2019, which preceded the pandemic and the years 2020-2021, when the pandemic began. The hypothesis was that the proportion of the population 5 years of age or older who completed the COVID-19 primary vaccination would be inversely related to asthma symptom prevalence.
Relative to the 2018-2019 years, the mean rate of parent-reported asthma symptoms was 0.85% lower (6.93% vs 7.77%; P < .001) in 2020-2021, when the mean primary series COVID-19 vaccination rate was 72.3%.
The study was not able to evaluate the impact of COVID-19 vaccination specifically in children with asthma, because history of asthma is not captured in the NSCH data, but Dr. Davis contended that the reduction in symptomatic asthma among children with increased vaccination offers validation for the state-level findings.
“Moreover, the absence of an association of COVID-19 vaccination administered predominantly in 2021 with population-level COVID-19 mortality in 2020 serves as a negative control,” he and his colleagues wrote in their research letter.
Protection from Respiratory Viruses Seen for Asthma Patients
In an interview, Dr. Davis reported that these data are consistent with previous evidence that immunization against influenza also reduces risk of asthma symptoms. In a meta-analysis published in 2017, it was estimated that live vaccines reduced risk of influenza by 81% and prevented 59%-72% of asthma attacks leading to hospitalizations or emergency room visits.
“The similarity of our findings regarding COVID-19 vaccination to prior data regarding influenza vaccination underscores the importance of preventing viral illnesses in individuals with a history of asthma,” Dr. Davis said. It is not yet clear if this is true of respiratory syncytial virus (RSV). Because of the short time that the RSV vaccine has been available, it is too soon to conduct an analysis.
One message from this study is that “clinicians should continue to encourage COVID-19 vaccination for children because of its general benefits in preventing coronavirus-related illness and the apparent specific benefits for children with a history of asthma,” he said.
While vaccination appears to reduce asthmatic symptoms related to COVID-19 infection, one study suggests that COVID-19 does not trigger new-onset asthma. In a retrospective study published in Pediatrics, no association between COVID-19 infection and new-onset asthma could be made in an analysis of 27,423 children (ages, 1-16 years) from the Children’s Hospital of Philadelphia (CHOP) Care Network.
Across all the pediatric age groups evaluated, the consistent finding was “SARS-CoV-2 positivity does not confer an additional risk for asthma diagnosis at least within the first 18 months after a [polymerase chain reaction] test,” concluded the investigators, led by David A. Hill, MD, PhD, Division of Allergy and Immunology, CHOP, Philadelphia, Pennsylvania.
Risk of Asthma Doubled After COVID-19 Infection
However, the opposite conclusion was reached by investigators evaluating data from two cohorts of children ages 5-18 drawn from the TriNetX database, a global health research network with data on more than 250 million individuals. Cohort 1 included more than 250,000 children. These children had never received COVID-19 vaccination. The 50,000 patients in cohort 2 had all received COVID19 vaccination.
To compare the impact of COVID-19 infection on new-onset asthma, the patients who were infected with COVID-19 were compared with those who were not infected after propensity score matching over 18 months of follow-up.
In cohort 1, the rate of new onset asthma was more than twofold greater among those with COVID-19 infection (4.7% vs 2.0%). The hazard ratio (HR) of 2.25 had tight confidence intervals (95% CI, 2.158-2.367).
In cohort 2, the risk of new-onset asthma at 18 months among those who had a COVID-19 infection relative to those without was even greater (8.3% vs 3.1%). The relative risk approached a 3-fold increase (HR 2.745; 95% CI, 2.521-2.99).
The conclusion of these investigators, led by Chia-Chi Lung, PhD, Department of Public Health, Chung Shan Medical University, Taichung City, Taiwan, was that there is “a critical need for ongoing monitoring and customized healthcare strategies to mitigate the long-term respiratory impacts of COVID-19 in children.”
These health risks might not be as significant as once feared. In the recently published study from Environmental Health Insights, the goal of a meta-analysis was to determine if patients with asthma relative to those without asthma face a higher risk of serious disease from COVID-19 infection. The meta-analysis included studies of children and adults. The answer, according an in-depth analysis of 21 articles in a “scoping review,” was a qualified no.
Of the 21 articles, 4 concluded that asthma is a risk factor for serious COVID-19 infection, but 17 did not, according to Chukwudi S. Ubah, PhD, Department of Public Health, Brody School of Medicine, East Caroline University, Greenville, North Carolina.
None of These Questions are Fully Resolved
However, given the disparity in the results and the fact that many of the studies included in this analysis had small sample sizes, Dr. Ubah called for larger studies and studies with better controls. He noted, for example, that the studies did not consistently evaluate mitigating factors, such as used of inhaled or oral corticosteroids, which might affect risk of the severity of a COVID-19 infection.
Rather, “our findings pointed out that the type of medication prescribed for asthma may have implications for the severity of COVID-19 infection in these patients,” Dr. Ubah said in an interview.
Overall, the data do not support a major interaction between asthma and COVID-19, even if the data are not conclusive. Each of the senior authors of these studies called for larger and better investigations to further explore whether COVID-19 infection and preexisting asthma interact. So far, the data indicate that if COVID-19 infection poses a risk of precipitating new-onset asthma or inducing a more severe infection in children with asthma, it is low, but the degree of risk, if any, remains unresolved in subgroups defined by asthma treatment or asthma severity.
Dr. Davis, Dr. Hill, Dr. Lung, and Dr. Ubah reported no potential conflicts of interest. None of these studies received funding from commercial interests.
FROM JAMA NETWORK OPEN
Urticaria Linked to Higher Cancer Risk, Study Finds
TOPLINE:
which decreased to 6% in subsequent years, in a cohort study using Danish healthcare databases.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from Danish healthcare registries and compared the incident cancer risk between patients with urticaria and the risk in the general population.
- They identified 87,507 patients (58% women) with a primary or secondary first-time hospital outpatient clinic, emergency room, or inpatient diagnosis of urticaria between 1980 and 2022, who were followed for a median of 10.1 years.
- Incident cancers, including nonmelanoma skin cancer, were identified using the Danish Cancer Registry and classified by the extent of spread at the time of diagnosis.
- This study computed the absolute cancer risk within the first year of an urticaria diagnosis and standardized incidence ratios (SIRs), with 95% CIs standardized to Danish national cancer rates.
TAKEAWAY:
- For the first year of follow-up, the absolute risk for all cancer types was 0.7%, and it was 29.5% for subsequent years. The overall SIR for all types of cancer was 1.09 (95% CI, 1.06-1.11), which was based on 7788 observed cancer cases compared with 7161 cases expected over the entire follow-up period.
- Within the first year of follow-up, 588 patients with urticaria were diagnosed with cancer, for an SIR of 1.49 (95% CI, 1.38-1.62) for all cancer types.
- After the first year, the SIR for all cancer sites decreased and stabilized at 1.06 (95% CI, 1.04-1.09), with 7200 observed cancer cases.
- The risk was highest for hematological cancers in the first year, particularly Hodgkin lymphoma (SIR, 5.35; 95% CI, 2.56-9.85).
IN PRACTICE:
“Our study suggests that urticaria may be a marker of occult cancer and that it is associated with a slightly increased long-term cancer risk,” the authors wrote.
SOURCE:
The study was led by Sissel B.T. Sørensen, departments of dermatology and rheumatology, Aarhus University Hospital, Aarhus, Denmark. It was published online on June 27, 2024, in the British Journal of Dermatology.
LIMITATIONS:
The study is limited by its observational design and reliance on registry data, which may be subject to misclassification or incomplete information. In addition, the study could not assess individual patient factors such as lifestyle or genetic predispositions that may influence cancer risk, and the results may not be generalizable to other populations. Finally, the exact biologic mechanisms linking urticaria and cancer remain unclear, warranting further investigation.
DISCLOSURES:
The study did not receive any funding. The authors reported that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
which decreased to 6% in subsequent years, in a cohort study using Danish healthcare databases.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from Danish healthcare registries and compared the incident cancer risk between patients with urticaria and the risk in the general population.
- They identified 87,507 patients (58% women) with a primary or secondary first-time hospital outpatient clinic, emergency room, or inpatient diagnosis of urticaria between 1980 and 2022, who were followed for a median of 10.1 years.
- Incident cancers, including nonmelanoma skin cancer, were identified using the Danish Cancer Registry and classified by the extent of spread at the time of diagnosis.
- This study computed the absolute cancer risk within the first year of an urticaria diagnosis and standardized incidence ratios (SIRs), with 95% CIs standardized to Danish national cancer rates.
TAKEAWAY:
- For the first year of follow-up, the absolute risk for all cancer types was 0.7%, and it was 29.5% for subsequent years. The overall SIR for all types of cancer was 1.09 (95% CI, 1.06-1.11), which was based on 7788 observed cancer cases compared with 7161 cases expected over the entire follow-up period.
- Within the first year of follow-up, 588 patients with urticaria were diagnosed with cancer, for an SIR of 1.49 (95% CI, 1.38-1.62) for all cancer types.
- After the first year, the SIR for all cancer sites decreased and stabilized at 1.06 (95% CI, 1.04-1.09), with 7200 observed cancer cases.
- The risk was highest for hematological cancers in the first year, particularly Hodgkin lymphoma (SIR, 5.35; 95% CI, 2.56-9.85).
IN PRACTICE:
“Our study suggests that urticaria may be a marker of occult cancer and that it is associated with a slightly increased long-term cancer risk,” the authors wrote.
SOURCE:
The study was led by Sissel B.T. Sørensen, departments of dermatology and rheumatology, Aarhus University Hospital, Aarhus, Denmark. It was published online on June 27, 2024, in the British Journal of Dermatology.
LIMITATIONS:
The study is limited by its observational design and reliance on registry data, which may be subject to misclassification or incomplete information. In addition, the study could not assess individual patient factors such as lifestyle or genetic predispositions that may influence cancer risk, and the results may not be generalizable to other populations. Finally, the exact biologic mechanisms linking urticaria and cancer remain unclear, warranting further investigation.
DISCLOSURES:
The study did not receive any funding. The authors reported that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
which decreased to 6% in subsequent years, in a cohort study using Danish healthcare databases.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from Danish healthcare registries and compared the incident cancer risk between patients with urticaria and the risk in the general population.
- They identified 87,507 patients (58% women) with a primary or secondary first-time hospital outpatient clinic, emergency room, or inpatient diagnosis of urticaria between 1980 and 2022, who were followed for a median of 10.1 years.
- Incident cancers, including nonmelanoma skin cancer, were identified using the Danish Cancer Registry and classified by the extent of spread at the time of diagnosis.
- This study computed the absolute cancer risk within the first year of an urticaria diagnosis and standardized incidence ratios (SIRs), with 95% CIs standardized to Danish national cancer rates.
TAKEAWAY:
- For the first year of follow-up, the absolute risk for all cancer types was 0.7%, and it was 29.5% for subsequent years. The overall SIR for all types of cancer was 1.09 (95% CI, 1.06-1.11), which was based on 7788 observed cancer cases compared with 7161 cases expected over the entire follow-up period.
- Within the first year of follow-up, 588 patients with urticaria were diagnosed with cancer, for an SIR of 1.49 (95% CI, 1.38-1.62) for all cancer types.
- After the first year, the SIR for all cancer sites decreased and stabilized at 1.06 (95% CI, 1.04-1.09), with 7200 observed cancer cases.
- The risk was highest for hematological cancers in the first year, particularly Hodgkin lymphoma (SIR, 5.35; 95% CI, 2.56-9.85).
IN PRACTICE:
“Our study suggests that urticaria may be a marker of occult cancer and that it is associated with a slightly increased long-term cancer risk,” the authors wrote.
SOURCE:
The study was led by Sissel B.T. Sørensen, departments of dermatology and rheumatology, Aarhus University Hospital, Aarhus, Denmark. It was published online on June 27, 2024, in the British Journal of Dermatology.
LIMITATIONS:
The study is limited by its observational design and reliance on registry data, which may be subject to misclassification or incomplete information. In addition, the study could not assess individual patient factors such as lifestyle or genetic predispositions that may influence cancer risk, and the results may not be generalizable to other populations. Finally, the exact biologic mechanisms linking urticaria and cancer remain unclear, warranting further investigation.
DISCLOSURES:
The study did not receive any funding. The authors reported that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Does An Elevated Lp(a) Call for Low-dose Aspirin?
Should a patient with high lipoprotein (a), or Lp(a), be started on low-dose aspirin?
This is the conundrum facing many physicians and patients, but even getting to that point will require more availability and coverage of tests and a greater appreciation of the risk associated with Lp(a), said cardiologists.
Lp(a): The Silent Risk
On Lp(a) Awareness Day, C. Michael Gibson, MD, MA, CEO of the Baim Institute for Clinical Research, Boston, Massachusetts, and PERFUSE took the opportunity to talk about his experiences with testing on X.
The professor of medicine at Harvard Medical School, also in Boston, said he was surprised to find that he had a very high calcium score, despite a low-density lipoprotein (LDL) cholesterol level of just 70 mg/dL. Eventually, he found out that he had a “very, very high Lp(a),” which was particularly concerning because his grandfather died of a heart attack at 45 years of age.
But how much risk does that represent?
A 2022 consensus statement from the European Atherosclerosis Society (EAS) highlighted that epidemiologic and genetic studies “strongly support a causal and continuous association between Lp(a) concentration and cardiovascular outcomes,” even at very low LDL cholesterol levels.
This is because Lp(a) has proinflammatory and proatherosclerotic properties, and high levels are associated with both micro- and macrocalcification of the aortic valve. Findings from a US registry study also suggest the threshold related to increased cardiovascular risk may differ for primary and secondary prevention populations (J Am Coll Cardiol. 2024 Mar 5;83[9]:873-886).
Lp(a) is, however, genetically determined, and there are no drugs available that directly lower levels, although some are on the horizon. In the meantime, the experts behind the consensus statement recommend that all adults be tested at least once in their lifetime.
Testing Cost and Availability
This recommendation has been translated into guidelines in “many, many” countries, said lead author Florian Kronenberg, MD, MAE, Institute of Genetic Epidemiology, Medical University of Innsbruck, Innsbruck, Austria, but “we are far away from reaching that goal.”
“We’ve got a real problem,” added Stephen Nicholls, MD, PhD, director of the Victorian Heart Institute and a professor of cardiology at Monash University, Melbourne, Australia, as there is “not a country in the world where there’s good access to Lp(a) testing.”
Dr. Kronenberg said that the consensus statement “created a kind of momentum” toward universal testing.
Ulrich Laufs, MD, PhD, professor and chair, Department of Cardiology, University Hospital Leipzig, Leipzig, Germany, agreed, saying that, overall, Lp(a) testing has “increased dramatically,” albeit from “extremely low levels.”
Dr. Kronenberg believes that “we have to be really patient.” He cited a lack of knowledge among physicians as one of the biggest barriers to greater uptake of testing.
“There is still no appreciation of the role of Lp(a),” agreed Alberico L. Catapano, MD, PhD, director of Cardiovascular Research and of the Lipoproteins and Atherosclerosis Laboratory of IRCCS Multimedica, Milan, Italy, and past president of the EAS.
“That’s why it’s not mentioned” to patients, he said. “What is really needed is to inform physician colleagues that Lp(a) is not only a risk factor but is the cause” of atherosclerotic cardiovascular disease (ASCVD).
Dr. Kronenberg said that the pressure for testing can often come from the patient themselves.
Physicians then question why the patient wants to be tested when there are no medications to treat it, he added. “We really tried very hard when we did the consensus paper to say that we should perform the test and give people advice on what to do.”
Dr. Catapano believes that another major obstacle is the cost of the test, which remains high “because very few people do it,” and there is some debate over which test to use.
Taken together, these issues have meant that “payers are really struggling with the idea of funding Lp(a),” said Dr. Nicholls, adding that “there seems to be this fixation on: ‘Well, if you can’t lower Lp(a), why measure it?’ ”
Rather than blame the payers, he says there is a need to educate about the science behind testing and underline that Lp(a) is an “important risk enhancer” for cardiovascular disease.
“Because if we’re going to make people pay out of pocket, then you’re creating a massive equity issue in that only those who can afford the test have it.”
High Lp(a) Now What?
But once the test has been performed, there then comes the question as to what to do about the result.
“Before we get anywhere near an agent that effectively lowers Lp(a) and get it into the clinic, there are lots of things that we can do today,” said Dr. Nicholls.
If someone has an intermediate or high background cardiovascular risk and they have got a high Lp(a) level, they “should be treated more intensively, as we know that high Lp(a) patients do better if their LDL cholesterol and their blood pressure is lower.”
For Dr. Catapano, this means having the “same mindset as you do with [a patient with] high blood pressure, high LDL cholesterol, and so on, because it’s exactly the same thing: It’s interacting with your other risk factors to increase your overall risk.”
Dr. Gibson agreed. Through a range of measures, including weight loss and statin therapy, he was able to reduce his overall cardiovascular risk, and his LDL cholesterol level dropped to just 20 mg/dL.
A Role for Aspirin?
It gained added momentum when Pablo Corral, MD, a lipidologist and a professor in the School of Medicine, Pharmacology Department, FASTA University, Mar del Plata, Buenos Aires, Argentina, highlighted the issue on X.
He pointed to a recent study, which showed that regular aspirin use was associated with a significantly lower rate of ASCVD mortality in adults without clinical ASCVD but who had elevated Lp(a).
Dr. Nicholls said that, when you “peel away the layers” of the current evidence, there is some suggestion that Lp(a)may be prothrombotic. “So in theory, perhaps aspirin might be maybe more intuitively useful there.”
He noted that the ASPREE primary prevention study found that low-dose aspirin in older adults resulted in a significantly higher risk for major hemorrhage over placebo and did not significantly reduce the risk for cardiovascular disease.
But an analysis he and his colleagues did suggest that aspirin may indeed benefit older individuals if they have elevated Lp(a) genotypes.
An Individual Decision
For Dr. Kronenberg and Dr. Laufs, there is currently a lack of appropriate data to make a recommendation either way, particularly for primary prevention.
They warned that the risk for thrombosis in patients with mildly elevated Lp(a) cannot be discounted, and in most cases either “the existing risk of bleeding exceeds the beneficial effects [of aspirin], or it’s not indicated,” said Dr. Laufs.
“When we make a recommendation, we should have evidence-based data,” Dr. Kronenberg said, but, at the moment, people “somehow put their finger in the air and see” which way the wind is blowing.
Dr. Catapano urged patients to talk to their physician, as even low-dose aspirin is “very potent” at inhibiting platelets.
Dr. Gibson agreed, saying that he is in two minds, as the potential benefit has to be weighed against the bleeding risk.
He personally takes low-dose aspirin because “I know I have a low bleeding risk,” but it is a decision “that has to be taken individually between a patient and their physician.”
Dr. Gibson, Dr. Kronenberg, Dr. Nicholls, and Dr. Catapano all reported conflicts of interest with numerous pharmaceutical companies and organizations.
A version of this article first appeared on Medscape.com.
Should a patient with high lipoprotein (a), or Lp(a), be started on low-dose aspirin?
This is the conundrum facing many physicians and patients, but even getting to that point will require more availability and coverage of tests and a greater appreciation of the risk associated with Lp(a), said cardiologists.
Lp(a): The Silent Risk
On Lp(a) Awareness Day, C. Michael Gibson, MD, MA, CEO of the Baim Institute for Clinical Research, Boston, Massachusetts, and PERFUSE took the opportunity to talk about his experiences with testing on X.
The professor of medicine at Harvard Medical School, also in Boston, said he was surprised to find that he had a very high calcium score, despite a low-density lipoprotein (LDL) cholesterol level of just 70 mg/dL. Eventually, he found out that he had a “very, very high Lp(a),” which was particularly concerning because his grandfather died of a heart attack at 45 years of age.
But how much risk does that represent?
A 2022 consensus statement from the European Atherosclerosis Society (EAS) highlighted that epidemiologic and genetic studies “strongly support a causal and continuous association between Lp(a) concentration and cardiovascular outcomes,” even at very low LDL cholesterol levels.
This is because Lp(a) has proinflammatory and proatherosclerotic properties, and high levels are associated with both micro- and macrocalcification of the aortic valve. Findings from a US registry study also suggest the threshold related to increased cardiovascular risk may differ for primary and secondary prevention populations (J Am Coll Cardiol. 2024 Mar 5;83[9]:873-886).
Lp(a) is, however, genetically determined, and there are no drugs available that directly lower levels, although some are on the horizon. In the meantime, the experts behind the consensus statement recommend that all adults be tested at least once in their lifetime.
Testing Cost and Availability
This recommendation has been translated into guidelines in “many, many” countries, said lead author Florian Kronenberg, MD, MAE, Institute of Genetic Epidemiology, Medical University of Innsbruck, Innsbruck, Austria, but “we are far away from reaching that goal.”
“We’ve got a real problem,” added Stephen Nicholls, MD, PhD, director of the Victorian Heart Institute and a professor of cardiology at Monash University, Melbourne, Australia, as there is “not a country in the world where there’s good access to Lp(a) testing.”
Dr. Kronenberg said that the consensus statement “created a kind of momentum” toward universal testing.
Ulrich Laufs, MD, PhD, professor and chair, Department of Cardiology, University Hospital Leipzig, Leipzig, Germany, agreed, saying that, overall, Lp(a) testing has “increased dramatically,” albeit from “extremely low levels.”
Dr. Kronenberg believes that “we have to be really patient.” He cited a lack of knowledge among physicians as one of the biggest barriers to greater uptake of testing.
“There is still no appreciation of the role of Lp(a),” agreed Alberico L. Catapano, MD, PhD, director of Cardiovascular Research and of the Lipoproteins and Atherosclerosis Laboratory of IRCCS Multimedica, Milan, Italy, and past president of the EAS.
“That’s why it’s not mentioned” to patients, he said. “What is really needed is to inform physician colleagues that Lp(a) is not only a risk factor but is the cause” of atherosclerotic cardiovascular disease (ASCVD).
Dr. Kronenberg said that the pressure for testing can often come from the patient themselves.
Physicians then question why the patient wants to be tested when there are no medications to treat it, he added. “We really tried very hard when we did the consensus paper to say that we should perform the test and give people advice on what to do.”
Dr. Catapano believes that another major obstacle is the cost of the test, which remains high “because very few people do it,” and there is some debate over which test to use.
Taken together, these issues have meant that “payers are really struggling with the idea of funding Lp(a),” said Dr. Nicholls, adding that “there seems to be this fixation on: ‘Well, if you can’t lower Lp(a), why measure it?’ ”
Rather than blame the payers, he says there is a need to educate about the science behind testing and underline that Lp(a) is an “important risk enhancer” for cardiovascular disease.
“Because if we’re going to make people pay out of pocket, then you’re creating a massive equity issue in that only those who can afford the test have it.”
High Lp(a) Now What?
But once the test has been performed, there then comes the question as to what to do about the result.
“Before we get anywhere near an agent that effectively lowers Lp(a) and get it into the clinic, there are lots of things that we can do today,” said Dr. Nicholls.
If someone has an intermediate or high background cardiovascular risk and they have got a high Lp(a) level, they “should be treated more intensively, as we know that high Lp(a) patients do better if their LDL cholesterol and their blood pressure is lower.”
For Dr. Catapano, this means having the “same mindset as you do with [a patient with] high blood pressure, high LDL cholesterol, and so on, because it’s exactly the same thing: It’s interacting with your other risk factors to increase your overall risk.”
Dr. Gibson agreed. Through a range of measures, including weight loss and statin therapy, he was able to reduce his overall cardiovascular risk, and his LDL cholesterol level dropped to just 20 mg/dL.
A Role for Aspirin?
It gained added momentum when Pablo Corral, MD, a lipidologist and a professor in the School of Medicine, Pharmacology Department, FASTA University, Mar del Plata, Buenos Aires, Argentina, highlighted the issue on X.
He pointed to a recent study, which showed that regular aspirin use was associated with a significantly lower rate of ASCVD mortality in adults without clinical ASCVD but who had elevated Lp(a).
Dr. Nicholls said that, when you “peel away the layers” of the current evidence, there is some suggestion that Lp(a)may be prothrombotic. “So in theory, perhaps aspirin might be maybe more intuitively useful there.”
He noted that the ASPREE primary prevention study found that low-dose aspirin in older adults resulted in a significantly higher risk for major hemorrhage over placebo and did not significantly reduce the risk for cardiovascular disease.
But an analysis he and his colleagues did suggest that aspirin may indeed benefit older individuals if they have elevated Lp(a) genotypes.
An Individual Decision
For Dr. Kronenberg and Dr. Laufs, there is currently a lack of appropriate data to make a recommendation either way, particularly for primary prevention.
They warned that the risk for thrombosis in patients with mildly elevated Lp(a) cannot be discounted, and in most cases either “the existing risk of bleeding exceeds the beneficial effects [of aspirin], or it’s not indicated,” said Dr. Laufs.
“When we make a recommendation, we should have evidence-based data,” Dr. Kronenberg said, but, at the moment, people “somehow put their finger in the air and see” which way the wind is blowing.
Dr. Catapano urged patients to talk to their physician, as even low-dose aspirin is “very potent” at inhibiting platelets.
Dr. Gibson agreed, saying that he is in two minds, as the potential benefit has to be weighed against the bleeding risk.
He personally takes low-dose aspirin because “I know I have a low bleeding risk,” but it is a decision “that has to be taken individually between a patient and their physician.”
Dr. Gibson, Dr. Kronenberg, Dr. Nicholls, and Dr. Catapano all reported conflicts of interest with numerous pharmaceutical companies and organizations.
A version of this article first appeared on Medscape.com.
Should a patient with high lipoprotein (a), or Lp(a), be started on low-dose aspirin?
This is the conundrum facing many physicians and patients, but even getting to that point will require more availability and coverage of tests and a greater appreciation of the risk associated with Lp(a), said cardiologists.
Lp(a): The Silent Risk
On Lp(a) Awareness Day, C. Michael Gibson, MD, MA, CEO of the Baim Institute for Clinical Research, Boston, Massachusetts, and PERFUSE took the opportunity to talk about his experiences with testing on X.
The professor of medicine at Harvard Medical School, also in Boston, said he was surprised to find that he had a very high calcium score, despite a low-density lipoprotein (LDL) cholesterol level of just 70 mg/dL. Eventually, he found out that he had a “very, very high Lp(a),” which was particularly concerning because his grandfather died of a heart attack at 45 years of age.
But how much risk does that represent?
A 2022 consensus statement from the European Atherosclerosis Society (EAS) highlighted that epidemiologic and genetic studies “strongly support a causal and continuous association between Lp(a) concentration and cardiovascular outcomes,” even at very low LDL cholesterol levels.
This is because Lp(a) has proinflammatory and proatherosclerotic properties, and high levels are associated with both micro- and macrocalcification of the aortic valve. Findings from a US registry study also suggest the threshold related to increased cardiovascular risk may differ for primary and secondary prevention populations (J Am Coll Cardiol. 2024 Mar 5;83[9]:873-886).
Lp(a) is, however, genetically determined, and there are no drugs available that directly lower levels, although some are on the horizon. In the meantime, the experts behind the consensus statement recommend that all adults be tested at least once in their lifetime.
Testing Cost and Availability
This recommendation has been translated into guidelines in “many, many” countries, said lead author Florian Kronenberg, MD, MAE, Institute of Genetic Epidemiology, Medical University of Innsbruck, Innsbruck, Austria, but “we are far away from reaching that goal.”
“We’ve got a real problem,” added Stephen Nicholls, MD, PhD, director of the Victorian Heart Institute and a professor of cardiology at Monash University, Melbourne, Australia, as there is “not a country in the world where there’s good access to Lp(a) testing.”
Dr. Kronenberg said that the consensus statement “created a kind of momentum” toward universal testing.
Ulrich Laufs, MD, PhD, professor and chair, Department of Cardiology, University Hospital Leipzig, Leipzig, Germany, agreed, saying that, overall, Lp(a) testing has “increased dramatically,” albeit from “extremely low levels.”
Dr. Kronenberg believes that “we have to be really patient.” He cited a lack of knowledge among physicians as one of the biggest barriers to greater uptake of testing.
“There is still no appreciation of the role of Lp(a),” agreed Alberico L. Catapano, MD, PhD, director of Cardiovascular Research and of the Lipoproteins and Atherosclerosis Laboratory of IRCCS Multimedica, Milan, Italy, and past president of the EAS.
“That’s why it’s not mentioned” to patients, he said. “What is really needed is to inform physician colleagues that Lp(a) is not only a risk factor but is the cause” of atherosclerotic cardiovascular disease (ASCVD).
Dr. Kronenberg said that the pressure for testing can often come from the patient themselves.
Physicians then question why the patient wants to be tested when there are no medications to treat it, he added. “We really tried very hard when we did the consensus paper to say that we should perform the test and give people advice on what to do.”
Dr. Catapano believes that another major obstacle is the cost of the test, which remains high “because very few people do it,” and there is some debate over which test to use.
Taken together, these issues have meant that “payers are really struggling with the idea of funding Lp(a),” said Dr. Nicholls, adding that “there seems to be this fixation on: ‘Well, if you can’t lower Lp(a), why measure it?’ ”
Rather than blame the payers, he says there is a need to educate about the science behind testing and underline that Lp(a) is an “important risk enhancer” for cardiovascular disease.
“Because if we’re going to make people pay out of pocket, then you’re creating a massive equity issue in that only those who can afford the test have it.”
High Lp(a) Now What?
But once the test has been performed, there then comes the question as to what to do about the result.
“Before we get anywhere near an agent that effectively lowers Lp(a) and get it into the clinic, there are lots of things that we can do today,” said Dr. Nicholls.
If someone has an intermediate or high background cardiovascular risk and they have got a high Lp(a) level, they “should be treated more intensively, as we know that high Lp(a) patients do better if their LDL cholesterol and their blood pressure is lower.”
For Dr. Catapano, this means having the “same mindset as you do with [a patient with] high blood pressure, high LDL cholesterol, and so on, because it’s exactly the same thing: It’s interacting with your other risk factors to increase your overall risk.”
Dr. Gibson agreed. Through a range of measures, including weight loss and statin therapy, he was able to reduce his overall cardiovascular risk, and his LDL cholesterol level dropped to just 20 mg/dL.
A Role for Aspirin?
It gained added momentum when Pablo Corral, MD, a lipidologist and a professor in the School of Medicine, Pharmacology Department, FASTA University, Mar del Plata, Buenos Aires, Argentina, highlighted the issue on X.
He pointed to a recent study, which showed that regular aspirin use was associated with a significantly lower rate of ASCVD mortality in adults without clinical ASCVD but who had elevated Lp(a).
Dr. Nicholls said that, when you “peel away the layers” of the current evidence, there is some suggestion that Lp(a)may be prothrombotic. “So in theory, perhaps aspirin might be maybe more intuitively useful there.”
He noted that the ASPREE primary prevention study found that low-dose aspirin in older adults resulted in a significantly higher risk for major hemorrhage over placebo and did not significantly reduce the risk for cardiovascular disease.
But an analysis he and his colleagues did suggest that aspirin may indeed benefit older individuals if they have elevated Lp(a) genotypes.
An Individual Decision
For Dr. Kronenberg and Dr. Laufs, there is currently a lack of appropriate data to make a recommendation either way, particularly for primary prevention.
They warned that the risk for thrombosis in patients with mildly elevated Lp(a) cannot be discounted, and in most cases either “the existing risk of bleeding exceeds the beneficial effects [of aspirin], or it’s not indicated,” said Dr. Laufs.
“When we make a recommendation, we should have evidence-based data,” Dr. Kronenberg said, but, at the moment, people “somehow put their finger in the air and see” which way the wind is blowing.
Dr. Catapano urged patients to talk to their physician, as even low-dose aspirin is “very potent” at inhibiting platelets.
Dr. Gibson agreed, saying that he is in two minds, as the potential benefit has to be weighed against the bleeding risk.
He personally takes low-dose aspirin because “I know I have a low bleeding risk,” but it is a decision “that has to be taken individually between a patient and their physician.”
Dr. Gibson, Dr. Kronenberg, Dr. Nicholls, and Dr. Catapano all reported conflicts of interest with numerous pharmaceutical companies and organizations.
A version of this article first appeared on Medscape.com.
Facial Temperature Can Reveal Age and Disease
This transcript has been edited for clarity.
My oldest daughter is at sleepaway camp for a couple of weeks, and the camp has a photographer who goes around all day taking pictures of the kids, which get uploaded to a private Facebook group. In the past, I would go online every day (or, okay, several times a day) and scroll through all those pictures looking for one that features my kid.
I don’t have to do that anymore. This year, I simply uploaded a picture of my daughter to an app and artificial intelligence (AI) takes care of the rest, recognizing her face amidst the sea of smiling children, and flagging just those photos for me to peruse. It’s amazing, really. And a bit scary.
The fact that facial recognition has penetrated the summer camp market should tell you that the tech is truly ubiquitous. But today we’re going to think a bit more about what AI can do with a picture of your face, because the power of facial recognition is not just skin deep.
What’s got me hot and bothered about facial images is this paper, appearing in Cell Metabolism, which adds a new layer to the standard facial-analysis playbook: facial temperature.
To understand this paper, you need to understand a whole field of research that is developing various different “clocks” for age.
It turns out that age really is just a number. Our cells, our proteins, our biochemistry can be analyzed to give different numbers. These “clocks,” as distinct from the calendar we usually use to measure our age, might have more predictive power than the number itself.
There are numerous molecular clocks, such as telomere length, that not only correlate with calendar age but are superior to calendar age in predicting age-related complications. Testing telomere length typically requires a blood sample — and remains costly. But we can use other sources to estimate age; how about a photo?
I mean, we do this all the time when we meet someone new or, as a physician, when we meet a new patient. I have often written that a patient “appears younger than their stated age,” and we’ve all had the experience of hearing how old someone is and being shocked. I mean, have you seen Sharon Stone recently? She’s 66 years old. Okay — to be fair, there might be some outside help there. But you get the point.
Back to the Cell Metabolism paper. Researchers report on multiple algorithms to obtain an “age” from a picture of an individual’s face.
The first algorithm is pretty straightforward. Researchers collected 2811 images, all of Han Chinese individuals ranging in age from 20 to 90 years, and reconstructed a 3D facial map from those. 
They then trained a convolutional neural network to predict the individuals’ ages from the pictures. It was quite accurate, as you can see here.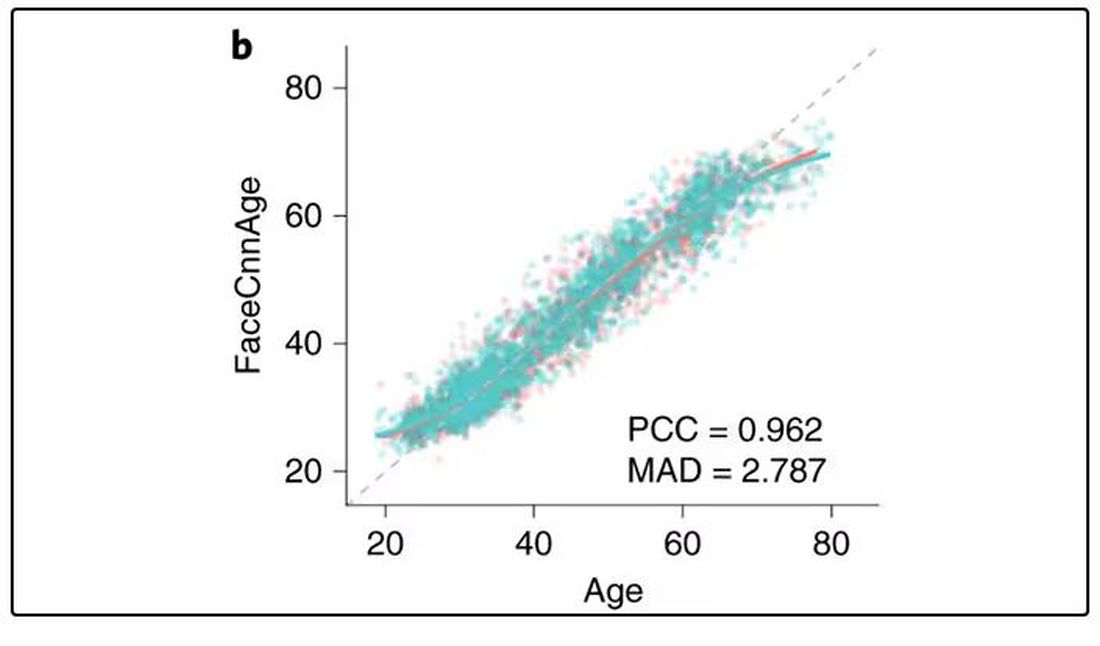
In the AI age, this may not seem that impressive. A brief search online turned up dozens of apps that promised to guess my age from a photo.
I sent this rather unflattering picture of myself to ChatGPT which, after initially demurring and saying it was not designed to guess ages, pegged me at somewhere between 35 and 45, which I am taking as a major victory.
But the Cell Metabolism paper goes deeper. Literally. 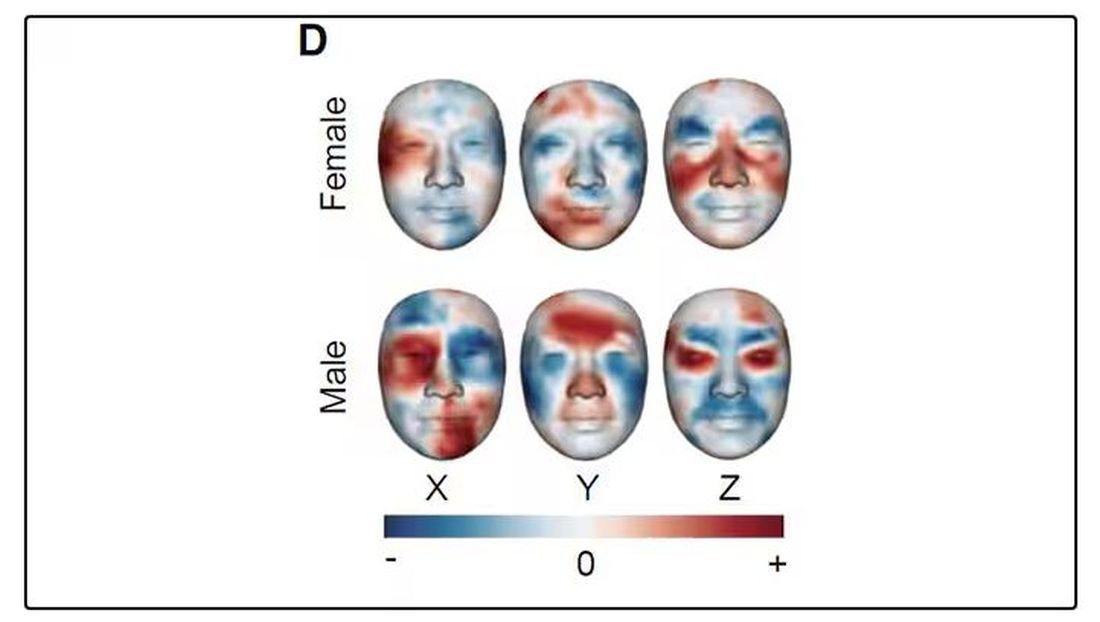
And this is where things start to get interesting. Because sure, the visible part of your face can change depending on makeup, expression, plastic surgery, and the like. But the temperature? That’s harder to fake.
It turns out that the temperature distribution in your face changes as you get older. There is a cooling of the nose and the cheeks, for example.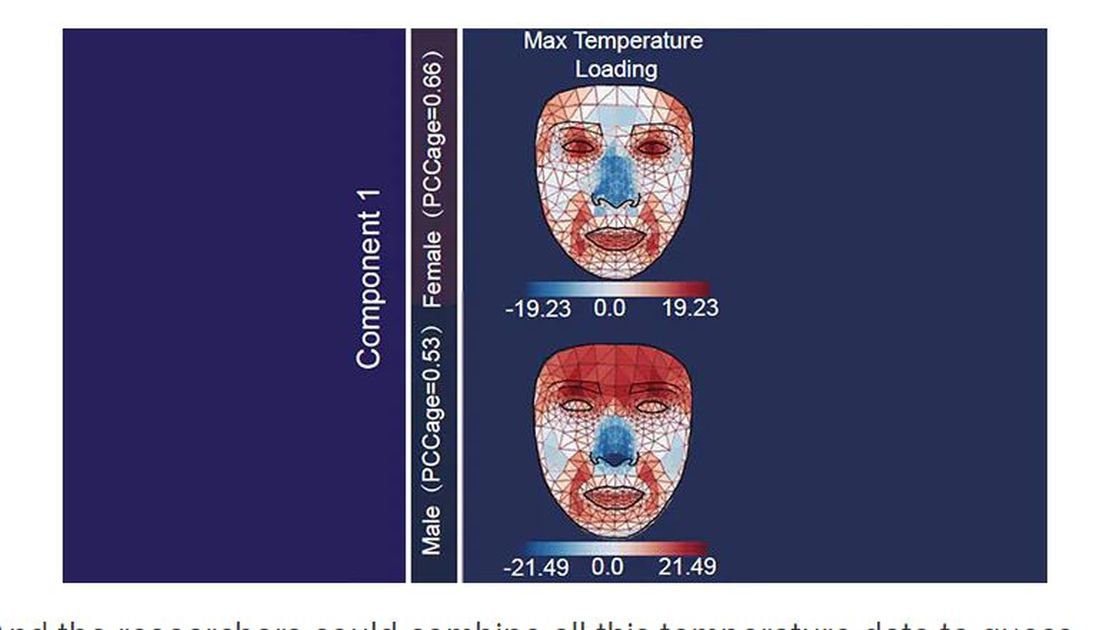
And the researchers could combine all this temperature data to guess someone’s calendar age fairly accurately, though notably not as accurately as the model that just looks at the pictures.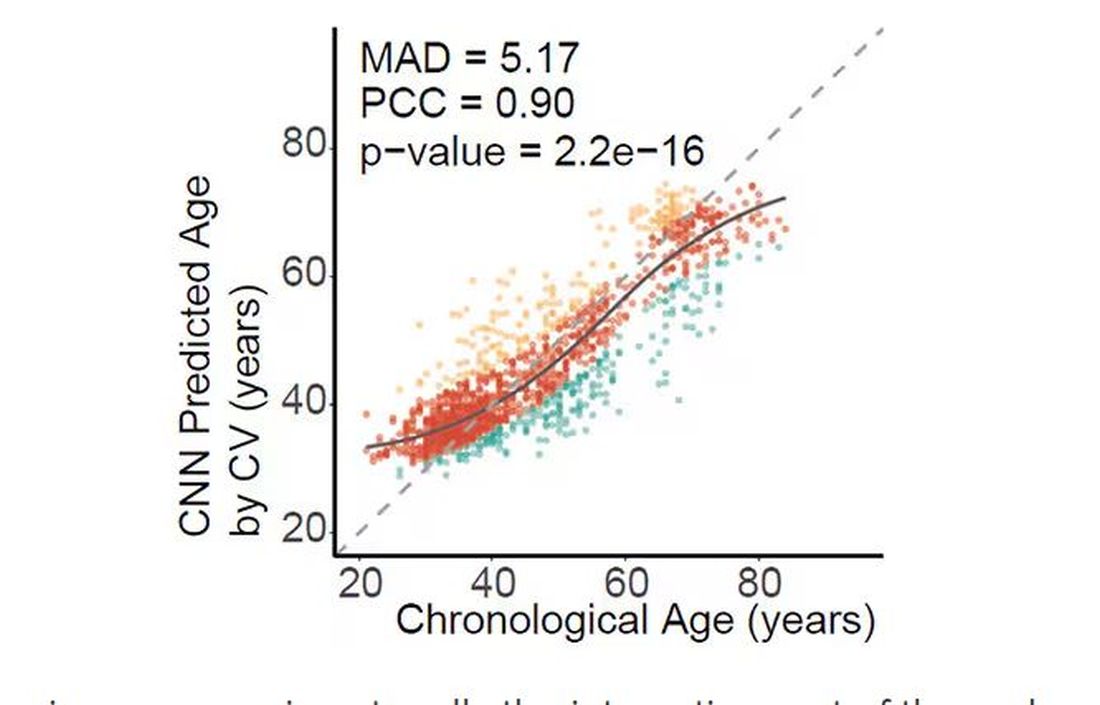
But guessing your age is not really the interesting part of thermal imaging of the face. It’s guessing — or, rather, predicting — the state of your metabolism. All these study participants had extensive metabolic testing performed, as well as detailed analysis of their lifestyle behaviors. And facial images could be used to predict those factors.
For example, the 3D reconstruction of the faces could predict who ate seafood (they tend to look younger than their actual age) compared with who ate poultry and meat (they tend to look older). The thermal imaging could predict who got more sleep (they look younger from a temperature perspective) and who ate more yogurt (also younger-appearing, temperature-wise). Facial temperature patterns could identify those with higher BMI, higher blood pressure, higher fasting glucose.
The researchers used the difference between actual and predicted age as a metric to measure illness as well. You can see here how, on average, individuals with hypertension, diabetes, and even liver cysts are “older,” at least by face temperature.
It may even be possible to use facial temperature as biofeedback. In a small study, the researchers measured the difference between facial temperature age and real age before and after 2 weeks of jump-roping. It turns out that 2 weeks of jump-roping can make you look about 5 years younger, at least as judged by a thermal camera. Or like the Predator.
Okay, this is all very cool, but I’m not saying we’ll all be doing facial temperature tests in the near future. No; what this study highlights for me is how much information about ourselves is available to those who know how to decode it. Maybe those data come from the wrinkles in our faces, or the angles of our smiles, or the speed with which we type, or the temperature of our elbows. The data have always been there, actually, but we’ve never had the tools powerful enough to analyze them until now.
When I was a kid, I was obsessed with Star Trek — I know, you’re shocked — and, of course, the famous tricorder, a scanner that could tell everything about someone’s state of health in 5 seconds from 3 feet away. That’s how I thought medicine really would be in the future. Once I got to medical school, I was disabused of that notion. But the age of data, the age of AI, may mean the tricorder age is not actually that far away.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
My oldest daughter is at sleepaway camp for a couple of weeks, and the camp has a photographer who goes around all day taking pictures of the kids, which get uploaded to a private Facebook group. In the past, I would go online every day (or, okay, several times a day) and scroll through all those pictures looking for one that features my kid.
I don’t have to do that anymore. This year, I simply uploaded a picture of my daughter to an app and artificial intelligence (AI) takes care of the rest, recognizing her face amidst the sea of smiling children, and flagging just those photos for me to peruse. It’s amazing, really. And a bit scary.
The fact that facial recognition has penetrated the summer camp market should tell you that the tech is truly ubiquitous. But today we’re going to think a bit more about what AI can do with a picture of your face, because the power of facial recognition is not just skin deep.
What’s got me hot and bothered about facial images is this paper, appearing in Cell Metabolism, which adds a new layer to the standard facial-analysis playbook: facial temperature.
To understand this paper, you need to understand a whole field of research that is developing various different “clocks” for age.
It turns out that age really is just a number. Our cells, our proteins, our biochemistry can be analyzed to give different numbers. These “clocks,” as distinct from the calendar we usually use to measure our age, might have more predictive power than the number itself.
There are numerous molecular clocks, such as telomere length, that not only correlate with calendar age but are superior to calendar age in predicting age-related complications. Testing telomere length typically requires a blood sample — and remains costly. But we can use other sources to estimate age; how about a photo?
I mean, we do this all the time when we meet someone new or, as a physician, when we meet a new patient. I have often written that a patient “appears younger than their stated age,” and we’ve all had the experience of hearing how old someone is and being shocked. I mean, have you seen Sharon Stone recently? She’s 66 years old. Okay — to be fair, there might be some outside help there. But you get the point.
Back to the Cell Metabolism paper. Researchers report on multiple algorithms to obtain an “age” from a picture of an individual’s face.
The first algorithm is pretty straightforward. Researchers collected 2811 images, all of Han Chinese individuals ranging in age from 20 to 90 years, and reconstructed a 3D facial map from those. 
They then trained a convolutional neural network to predict the individuals’ ages from the pictures. It was quite accurate, as you can see here.
In the AI age, this may not seem that impressive. A brief search online turned up dozens of apps that promised to guess my age from a photo.
I sent this rather unflattering picture of myself to ChatGPT which, after initially demurring and saying it was not designed to guess ages, pegged me at somewhere between 35 and 45, which I am taking as a major victory.
But the Cell Metabolism paper goes deeper. Literally. 
And this is where things start to get interesting. Because sure, the visible part of your face can change depending on makeup, expression, plastic surgery, and the like. But the temperature? That’s harder to fake.
It turns out that the temperature distribution in your face changes as you get older. There is a cooling of the nose and the cheeks, for example.
And the researchers could combine all this temperature data to guess someone’s calendar age fairly accurately, though notably not as accurately as the model that just looks at the pictures.
But guessing your age is not really the interesting part of thermal imaging of the face. It’s guessing — or, rather, predicting — the state of your metabolism. All these study participants had extensive metabolic testing performed, as well as detailed analysis of their lifestyle behaviors. And facial images could be used to predict those factors.
For example, the 3D reconstruction of the faces could predict who ate seafood (they tend to look younger than their actual age) compared with who ate poultry and meat (they tend to look older). The thermal imaging could predict who got more sleep (they look younger from a temperature perspective) and who ate more yogurt (also younger-appearing, temperature-wise). Facial temperature patterns could identify those with higher BMI, higher blood pressure, higher fasting glucose.
The researchers used the difference between actual and predicted age as a metric to measure illness as well. You can see here how, on average, individuals with hypertension, diabetes, and even liver cysts are “older,” at least by face temperature.
It may even be possible to use facial temperature as biofeedback. In a small study, the researchers measured the difference between facial temperature age and real age before and after 2 weeks of jump-roping. It turns out that 2 weeks of jump-roping can make you look about 5 years younger, at least as judged by a thermal camera. Or like the Predator.
Okay, this is all very cool, but I’m not saying we’ll all be doing facial temperature tests in the near future. No; what this study highlights for me is how much information about ourselves is available to those who know how to decode it. Maybe those data come from the wrinkles in our faces, or the angles of our smiles, or the speed with which we type, or the temperature of our elbows. The data have always been there, actually, but we’ve never had the tools powerful enough to analyze them until now.
When I was a kid, I was obsessed with Star Trek — I know, you’re shocked — and, of course, the famous tricorder, a scanner that could tell everything about someone’s state of health in 5 seconds from 3 feet away. That’s how I thought medicine really would be in the future. Once I got to medical school, I was disabused of that notion. But the age of data, the age of AI, may mean the tricorder age is not actually that far away.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
My oldest daughter is at sleepaway camp for a couple of weeks, and the camp has a photographer who goes around all day taking pictures of the kids, which get uploaded to a private Facebook group. In the past, I would go online every day (or, okay, several times a day) and scroll through all those pictures looking for one that features my kid.
I don’t have to do that anymore. This year, I simply uploaded a picture of my daughter to an app and artificial intelligence (AI) takes care of the rest, recognizing her face amidst the sea of smiling children, and flagging just those photos for me to peruse. It’s amazing, really. And a bit scary.
The fact that facial recognition has penetrated the summer camp market should tell you that the tech is truly ubiquitous. But today we’re going to think a bit more about what AI can do with a picture of your face, because the power of facial recognition is not just skin deep.
What’s got me hot and bothered about facial images is this paper, appearing in Cell Metabolism, which adds a new layer to the standard facial-analysis playbook: facial temperature.
To understand this paper, you need to understand a whole field of research that is developing various different “clocks” for age.
It turns out that age really is just a number. Our cells, our proteins, our biochemistry can be analyzed to give different numbers. These “clocks,” as distinct from the calendar we usually use to measure our age, might have more predictive power than the number itself.
There are numerous molecular clocks, such as telomere length, that not only correlate with calendar age but are superior to calendar age in predicting age-related complications. Testing telomere length typically requires a blood sample — and remains costly. But we can use other sources to estimate age; how about a photo?
I mean, we do this all the time when we meet someone new or, as a physician, when we meet a new patient. I have often written that a patient “appears younger than their stated age,” and we’ve all had the experience of hearing how old someone is and being shocked. I mean, have you seen Sharon Stone recently? She’s 66 years old. Okay — to be fair, there might be some outside help there. But you get the point.
Back to the Cell Metabolism paper. Researchers report on multiple algorithms to obtain an “age” from a picture of an individual’s face.
The first algorithm is pretty straightforward. Researchers collected 2811 images, all of Han Chinese individuals ranging in age from 20 to 90 years, and reconstructed a 3D facial map from those. 
They then trained a convolutional neural network to predict the individuals’ ages from the pictures. It was quite accurate, as you can see here.
In the AI age, this may not seem that impressive. A brief search online turned up dozens of apps that promised to guess my age from a photo.
I sent this rather unflattering picture of myself to ChatGPT which, after initially demurring and saying it was not designed to guess ages, pegged me at somewhere between 35 and 45, which I am taking as a major victory.
But the Cell Metabolism paper goes deeper. Literally. 
And this is where things start to get interesting. Because sure, the visible part of your face can change depending on makeup, expression, plastic surgery, and the like. But the temperature? That’s harder to fake.
It turns out that the temperature distribution in your face changes as you get older. There is a cooling of the nose and the cheeks, for example.
And the researchers could combine all this temperature data to guess someone’s calendar age fairly accurately, though notably not as accurately as the model that just looks at the pictures.
But guessing your age is not really the interesting part of thermal imaging of the face. It’s guessing — or, rather, predicting — the state of your metabolism. All these study participants had extensive metabolic testing performed, as well as detailed analysis of their lifestyle behaviors. And facial images could be used to predict those factors.
For example, the 3D reconstruction of the faces could predict who ate seafood (they tend to look younger than their actual age) compared with who ate poultry and meat (they tend to look older). The thermal imaging could predict who got more sleep (they look younger from a temperature perspective) and who ate more yogurt (also younger-appearing, temperature-wise). Facial temperature patterns could identify those with higher BMI, higher blood pressure, higher fasting glucose.
The researchers used the difference between actual and predicted age as a metric to measure illness as well. You can see here how, on average, individuals with hypertension, diabetes, and even liver cysts are “older,” at least by face temperature.
It may even be possible to use facial temperature as biofeedback. In a small study, the researchers measured the difference between facial temperature age and real age before and after 2 weeks of jump-roping. It turns out that 2 weeks of jump-roping can make you look about 5 years younger, at least as judged by a thermal camera. Or like the Predator.
Okay, this is all very cool, but I’m not saying we’ll all be doing facial temperature tests in the near future. No; what this study highlights for me is how much information about ourselves is available to those who know how to decode it. Maybe those data come from the wrinkles in our faces, or the angles of our smiles, or the speed with which we type, or the temperature of our elbows. The data have always been there, actually, but we’ve never had the tools powerful enough to analyze them until now.
When I was a kid, I was obsessed with Star Trek — I know, you’re shocked — and, of course, the famous tricorder, a scanner that could tell everything about someone’s state of health in 5 seconds from 3 feet away. That’s how I thought medicine really would be in the future. Once I got to medical school, I was disabused of that notion. But the age of data, the age of AI, may mean the tricorder age is not actually that far away.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Time Warp: Fax Machines Still Common in Oncology Practice. Why?
One minute, he’s working on sequencing a tumor genome. The next, he’s sifting through pages of disorganized data from a device that has been around for decades: the fax machine.
“If two doctors’ offices aren’t on the same electronic medical record, one of the main ways to transfer records is still by fax,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “I can go from cutting-edge innovation to relying on, at best, 1980s information technology. It just boggles my mind.”
Dr. Lewis, who has posted about his frustration with fax machines, is far from alone. Oncologists are among the many specialists across the country at the mercy of telecopiers.
According to a 2021 report by the Office of the National Coordinator for Health Information Technology, fax and mail continue to be the most common methods for hospitals and health systems to exchange care record summaries. In 2019, nearly 8 in 10 hospitals used mail or fax to send and receive health information, the report found.
Fax machines are still commonplace across the healthcare spectrum, said Robert Havasy, MS, senior director for informatics strategy at the Healthcare Information and Management Systems Society (HIMSS). Inertia, cost, and more pressing priorities for hospitals and medical institutions contribute to the technology sticking around, he explained.
“Post-COVID, my guess is we’re still at over 50% of healthcare practices using fax for some reason, on a daily basis,” Mr. Havasy said in an interview. “A lot of hospitals just don’t have the time, the money, or the staff to fix that problem because there’s always something a little higher up the priority chain they need to focus on.”
If, for instance, “you’re going to do a process redesign to reduce hospital total acquired infections, your fax machine replacement might be 10th or 12th on the list. It just never gets up to 1 or 2 because it’s ‘not that much of a problem,’ ” he added.
Or is it?
Administrators may not view fax machines as a top concern, but clinicians who deal with the machines daily see it differently.
“What worries me is we’re taking records out of an electronic storehouse [and] converting them to a paper medium,” Dr. Lewis said. “And then we are scanning into another electronic storehouse. The more steps, the more can be lost.”
And when information is lost, patient care can be compromised.
Slower Workflows, Care Concerns
Although there are no published data on fax machine use in oncology specifically, this outdated technology does come into play in a variety of ways along the cancer care continuum.
Radiation oncologist David R. Penberthy, MD, said patients often seek his cancer center’s expertise for second opinions, and that requires collecting patient records from many different practices.
“Ideally, it would come electronically, but sometimes it does come by fax,” said Dr. Penberthy, program director of radiation oncology at the University of Virginia School of Medicine in Charlottesville. “The quality of the fax is not always the best. Sometimes it’s literally a fax of a fax. You’re reading something that’s very difficult to read.”
Orders for new tests are also typically sent and received via fax temporarily while IT teams work to integrate them into the electronic health record (EHR), Dr. Penberthy said.
Insurers and third-party laboratories often send test results back by fax as well.
“Even if I haven’t actually sent my patient out of our institution, this crucial result may only be entered back into the record as a scanned document from a fax, which is not great because it can get lost in the other results that are reported electronically,” Dr. Lewis said. The risk here is that an ordering physician won’t see these results, which can lead to delayed or overlooked care for patients, he explained.
“To me, it’s like a blind spot,” Dr. Lewis said. “Every time we use a fax, I see it actually as an opportunity for oversight and missed opportunity to collect data.”
Dr. Penberthy said faxing can slow things down at his practice, particularly if he faxes a document to another office but receives no confirmation and has to track down what happened.
As for cybersecurity, data that are in transit during faxing are generally considered secure and compliant with the Health Insurance Portability and Accountability Act (HIPAA), said Mr. Havasy of HIMSS. However, the Privacy Rule also requires that data remain secure while at rest, which isn’t always possible, he added.
“That’s where faxes fall down, because generally fax machines are in public, if you will, or open areas in a hospital,” he said. “They just sit on a desk. I don’t know that the next nurse who comes up and looks through that stack was the nurse who was treating the patient.”
Important decisions or results can also be missed when sent by fax, creating headaches for physicians and care problems for patients.
Dr. Lewis recently experienced an insurance-related fax mishap over Memorial Day weekend. He believed his patient had access to the antinausea medication he had prescribed. When Dr. Lewis happened to check the fax machine over the weekend, he found a coverage denial for the medication from the insurer but, at that point, had no recourse to appeal because it was a long holiday weekend.
“Had the denial been sent by an electronic means that was quicker and more readily available, it would have been possible to appeal before the holiday weekend,” he said.
Hematologist Aaron Goodman, MD, encountered a similar problem after an insurer denied coverage of an expensive cancer drug for a patient and faxed over its reason for the denial. Dr. Goodman was not directly notified that the information arrived and didn’t learn about the denial for a week, he said.
“There’s no ‘ding’ in my inbox if something is faxed over and scanned,” said Dr. Goodman, associate professor of medicine at UC San Diego Health. “Once I realized it was denied, I was able to rectify it, but it wasted a week of a patient not getting a drug that I felt would be beneficial for them.”
Broader Health Policy Impacts
The use of outdated technology, such as fax machines, also creates ripple effects that burden the health system, health policy experts say.
Duplicate testing and unnecessary care are top impacts, said Julia Adler-Milstein, PhD, professor of medicine and chief of the division of clinical informatics and digital transformation at the University of California, San Francisco.
Studies show that 20%-30% of the $65 billion spent annually on lab tests is used on unnecessary duplicate tests, and another estimated $30 billion is spent each year on unnecessary duplicate medical imaging. These duplicate tests may be mitigated if hospitals adopt certified EHR technology, research shows.
Still, without EHR interoperability between institutions, new providers may be unaware that tests or past labs for patients exist, leading to repeat tests, said Dr. Adler-Milstein, who researches health IT policy with a focus on EHRs. Patients can sometimes fill in the gaps, but not always.
“Fax machines only help close information gaps if the clinician is aware of where to seek out the information and there is someone at the other organization to locate and transmit the information in a timely manner,” Dr. Adler-Milstein said.
Old technology and poor interoperability also greatly affect data collection for disease surveillance and monitoring, said Janet Hamilton, MPH, executive director for the Council of State and Territorial Epidemiologists. This issue was keenly demonstrated during the pandemic, Ms. Hamilton said.
“It was tragic, quite honestly,” she said. “There was such an immense amount of data that needed to be moved quickly, and that’s when computers are at their best.”
But, she said, “we didn’t have the level of systems in place to do it well.”
Specifically, the lack of electronic case reporting in place during the pandemic — where diagnoses are documented in the record and then immediately sent to the public health system — led to reports that were delayed, not made, or had missing or incomplete information, such as patients’ race and ethnicity or other health conditions, Ms. Hamilton said.
Incomplete or missing data hampered the ability of public health officials and researchers to understand how the virus might affect different patients.
“If you had a chronic condition like cancer, you were less likely to have a positive outcome with COVID,” Ms. Hamilton said. “But because electronic case reporting was not in place, we didn’t get some of those additional pieces of information. We didn’t have people’s underlying oncology status to then say, ‘Here are individuals with these types of characteristics, and these are the things that happen if they also have a cancer.’”
Slow, but Steady, Improvements
Efforts at the state and federal levels have targeted improved health information exchange, but progress takes time, Dr. Adler-Milstein said.
Most states have some form of health information exchange, such as statewide exchanges, regional health information organizations, or clinical data registries. Maryland is often held up as a notable example for its health information exchange, Dr. Adler-Milstein noted.
According to Maryland law, all hospitals under the jurisdiction of the Maryland Health Care Commission are required to electronically connect to the state-designated health information exchange. In 2012, Maryland became the first state to connect all its 46 acute care hospitals in the sharing of real-time data.
The Health Information Technology for Economic and Clinical Health (HITECH) Act provided federal-enhanced Medicaid matching funds to states through 2021 to support efforts to advance electronic exchange. Nearly all states used these funds, and most have identified other sources to sustain the efforts, according to a recent US Government Accountability Office (GAO) report. However, GAO found that small and rural providers are less likely to have the financial and technological resources to participate in or maintain electronic exchange capabilities.
Nationally, several recent initiatives have targeted health data interoperability, including for cancer care. The Centers for Disease Control and Prevention’s Data Modernization Initiative is a multiyear, multi–billion-dollar effort to improve data sharing across the federal and state public health landscape.
Meanwhile, in March 2024, the Biden-Harris administration launched United States Core Data for Interoperability Plus Cancer. The program will define a recommended minimum set of cancer-related data to be included in a patient’s EHR to enhance data exchange for research and clinical care.
EHR vendors are also key to improving the landscape, said Dr. Adler-Milstein. Vendors such as Epic have developed strong sharing capabilities for transmitting health information from site to site, but of course, that only helps if providers have Epic, she said.
“That’s where these national frameworks should help, because we don’t want it to break down by what EHR vendor you have,” she said. “It’s a patchwork. You can go to some places and hear success stories because they have Epic or a state health information exchange, but it’s very heterogeneous. In some places, they have nothing and are using a fax machine.”
Mr. Havasy believes fax machines will ultimately go extinct, particularly as a younger, more digitally savvy generation enters the healthcare workforce. He also foresees that the growing use of artificial intelligence will help eradicate the outdated technology.
But, Ms. Hamilton noted, “unless we have consistent, ongoing, sustained funding, it is very hard to move off [an older] technology that can work. That’s one of the biggest barriers.”
“Public health is about protecting the lives of every single person everywhere,” Ms. Hamilton said, “but when we don’t have the data that comes into the system, we can’t achieve our mission.”
A version of this article appeared on Medscape.com.
One minute, he’s working on sequencing a tumor genome. The next, he’s sifting through pages of disorganized data from a device that has been around for decades: the fax machine.
“If two doctors’ offices aren’t on the same electronic medical record, one of the main ways to transfer records is still by fax,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “I can go from cutting-edge innovation to relying on, at best, 1980s information technology. It just boggles my mind.”
Dr. Lewis, who has posted about his frustration with fax machines, is far from alone. Oncologists are among the many specialists across the country at the mercy of telecopiers.
According to a 2021 report by the Office of the National Coordinator for Health Information Technology, fax and mail continue to be the most common methods for hospitals and health systems to exchange care record summaries. In 2019, nearly 8 in 10 hospitals used mail or fax to send and receive health information, the report found.
Fax machines are still commonplace across the healthcare spectrum, said Robert Havasy, MS, senior director for informatics strategy at the Healthcare Information and Management Systems Society (HIMSS). Inertia, cost, and more pressing priorities for hospitals and medical institutions contribute to the technology sticking around, he explained.
“Post-COVID, my guess is we’re still at over 50% of healthcare practices using fax for some reason, on a daily basis,” Mr. Havasy said in an interview. “A lot of hospitals just don’t have the time, the money, or the staff to fix that problem because there’s always something a little higher up the priority chain they need to focus on.”
If, for instance, “you’re going to do a process redesign to reduce hospital total acquired infections, your fax machine replacement might be 10th or 12th on the list. It just never gets up to 1 or 2 because it’s ‘not that much of a problem,’ ” he added.
Or is it?
Administrators may not view fax machines as a top concern, but clinicians who deal with the machines daily see it differently.
“What worries me is we’re taking records out of an electronic storehouse [and] converting them to a paper medium,” Dr. Lewis said. “And then we are scanning into another electronic storehouse. The more steps, the more can be lost.”
And when information is lost, patient care can be compromised.
Slower Workflows, Care Concerns
Although there are no published data on fax machine use in oncology specifically, this outdated technology does come into play in a variety of ways along the cancer care continuum.
Radiation oncologist David R. Penberthy, MD, said patients often seek his cancer center’s expertise for second opinions, and that requires collecting patient records from many different practices.
“Ideally, it would come electronically, but sometimes it does come by fax,” said Dr. Penberthy, program director of radiation oncology at the University of Virginia School of Medicine in Charlottesville. “The quality of the fax is not always the best. Sometimes it’s literally a fax of a fax. You’re reading something that’s very difficult to read.”
Orders for new tests are also typically sent and received via fax temporarily while IT teams work to integrate them into the electronic health record (EHR), Dr. Penberthy said.
Insurers and third-party laboratories often send test results back by fax as well.
“Even if I haven’t actually sent my patient out of our institution, this crucial result may only be entered back into the record as a scanned document from a fax, which is not great because it can get lost in the other results that are reported electronically,” Dr. Lewis said. The risk here is that an ordering physician won’t see these results, which can lead to delayed or overlooked care for patients, he explained.
“To me, it’s like a blind spot,” Dr. Lewis said. “Every time we use a fax, I see it actually as an opportunity for oversight and missed opportunity to collect data.”
Dr. Penberthy said faxing can slow things down at his practice, particularly if he faxes a document to another office but receives no confirmation and has to track down what happened.
As for cybersecurity, data that are in transit during faxing are generally considered secure and compliant with the Health Insurance Portability and Accountability Act (HIPAA), said Mr. Havasy of HIMSS. However, the Privacy Rule also requires that data remain secure while at rest, which isn’t always possible, he added.
“That’s where faxes fall down, because generally fax machines are in public, if you will, or open areas in a hospital,” he said. “They just sit on a desk. I don’t know that the next nurse who comes up and looks through that stack was the nurse who was treating the patient.”
Important decisions or results can also be missed when sent by fax, creating headaches for physicians and care problems for patients.
Dr. Lewis recently experienced an insurance-related fax mishap over Memorial Day weekend. He believed his patient had access to the antinausea medication he had prescribed. When Dr. Lewis happened to check the fax machine over the weekend, he found a coverage denial for the medication from the insurer but, at that point, had no recourse to appeal because it was a long holiday weekend.
“Had the denial been sent by an electronic means that was quicker and more readily available, it would have been possible to appeal before the holiday weekend,” he said.
Hematologist Aaron Goodman, MD, encountered a similar problem after an insurer denied coverage of an expensive cancer drug for a patient and faxed over its reason for the denial. Dr. Goodman was not directly notified that the information arrived and didn’t learn about the denial for a week, he said.
“There’s no ‘ding’ in my inbox if something is faxed over and scanned,” said Dr. Goodman, associate professor of medicine at UC San Diego Health. “Once I realized it was denied, I was able to rectify it, but it wasted a week of a patient not getting a drug that I felt would be beneficial for them.”
Broader Health Policy Impacts
The use of outdated technology, such as fax machines, also creates ripple effects that burden the health system, health policy experts say.
Duplicate testing and unnecessary care are top impacts, said Julia Adler-Milstein, PhD, professor of medicine and chief of the division of clinical informatics and digital transformation at the University of California, San Francisco.
Studies show that 20%-30% of the $65 billion spent annually on lab tests is used on unnecessary duplicate tests, and another estimated $30 billion is spent each year on unnecessary duplicate medical imaging. These duplicate tests may be mitigated if hospitals adopt certified EHR technology, research shows.
Still, without EHR interoperability between institutions, new providers may be unaware that tests or past labs for patients exist, leading to repeat tests, said Dr. Adler-Milstein, who researches health IT policy with a focus on EHRs. Patients can sometimes fill in the gaps, but not always.
“Fax machines only help close information gaps if the clinician is aware of where to seek out the information and there is someone at the other organization to locate and transmit the information in a timely manner,” Dr. Adler-Milstein said.
Old technology and poor interoperability also greatly affect data collection for disease surveillance and monitoring, said Janet Hamilton, MPH, executive director for the Council of State and Territorial Epidemiologists. This issue was keenly demonstrated during the pandemic, Ms. Hamilton said.
“It was tragic, quite honestly,” she said. “There was such an immense amount of data that needed to be moved quickly, and that’s when computers are at their best.”
But, she said, “we didn’t have the level of systems in place to do it well.”
Specifically, the lack of electronic case reporting in place during the pandemic — where diagnoses are documented in the record and then immediately sent to the public health system — led to reports that were delayed, not made, or had missing or incomplete information, such as patients’ race and ethnicity or other health conditions, Ms. Hamilton said.
Incomplete or missing data hampered the ability of public health officials and researchers to understand how the virus might affect different patients.
“If you had a chronic condition like cancer, you were less likely to have a positive outcome with COVID,” Ms. Hamilton said. “But because electronic case reporting was not in place, we didn’t get some of those additional pieces of information. We didn’t have people’s underlying oncology status to then say, ‘Here are individuals with these types of characteristics, and these are the things that happen if they also have a cancer.’”
Slow, but Steady, Improvements
Efforts at the state and federal levels have targeted improved health information exchange, but progress takes time, Dr. Adler-Milstein said.
Most states have some form of health information exchange, such as statewide exchanges, regional health information organizations, or clinical data registries. Maryland is often held up as a notable example for its health information exchange, Dr. Adler-Milstein noted.
According to Maryland law, all hospitals under the jurisdiction of the Maryland Health Care Commission are required to electronically connect to the state-designated health information exchange. In 2012, Maryland became the first state to connect all its 46 acute care hospitals in the sharing of real-time data.
The Health Information Technology for Economic and Clinical Health (HITECH) Act provided federal-enhanced Medicaid matching funds to states through 2021 to support efforts to advance electronic exchange. Nearly all states used these funds, and most have identified other sources to sustain the efforts, according to a recent US Government Accountability Office (GAO) report. However, GAO found that small and rural providers are less likely to have the financial and technological resources to participate in or maintain electronic exchange capabilities.
Nationally, several recent initiatives have targeted health data interoperability, including for cancer care. The Centers for Disease Control and Prevention’s Data Modernization Initiative is a multiyear, multi–billion-dollar effort to improve data sharing across the federal and state public health landscape.
Meanwhile, in March 2024, the Biden-Harris administration launched United States Core Data for Interoperability Plus Cancer. The program will define a recommended minimum set of cancer-related data to be included in a patient’s EHR to enhance data exchange for research and clinical care.
EHR vendors are also key to improving the landscape, said Dr. Adler-Milstein. Vendors such as Epic have developed strong sharing capabilities for transmitting health information from site to site, but of course, that only helps if providers have Epic, she said.
“That’s where these national frameworks should help, because we don’t want it to break down by what EHR vendor you have,” she said. “It’s a patchwork. You can go to some places and hear success stories because they have Epic or a state health information exchange, but it’s very heterogeneous. In some places, they have nothing and are using a fax machine.”
Mr. Havasy believes fax machines will ultimately go extinct, particularly as a younger, more digitally savvy generation enters the healthcare workforce. He also foresees that the growing use of artificial intelligence will help eradicate the outdated technology.
But, Ms. Hamilton noted, “unless we have consistent, ongoing, sustained funding, it is very hard to move off [an older] technology that can work. That’s one of the biggest barriers.”
“Public health is about protecting the lives of every single person everywhere,” Ms. Hamilton said, “but when we don’t have the data that comes into the system, we can’t achieve our mission.”
A version of this article appeared on Medscape.com.
One minute, he’s working on sequencing a tumor genome. The next, he’s sifting through pages of disorganized data from a device that has been around for decades: the fax machine.
“If two doctors’ offices aren’t on the same electronic medical record, one of the main ways to transfer records is still by fax,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “I can go from cutting-edge innovation to relying on, at best, 1980s information technology. It just boggles my mind.”
Dr. Lewis, who has posted about his frustration with fax machines, is far from alone. Oncologists are among the many specialists across the country at the mercy of telecopiers.
According to a 2021 report by the Office of the National Coordinator for Health Information Technology, fax and mail continue to be the most common methods for hospitals and health systems to exchange care record summaries. In 2019, nearly 8 in 10 hospitals used mail or fax to send and receive health information, the report found.
Fax machines are still commonplace across the healthcare spectrum, said Robert Havasy, MS, senior director for informatics strategy at the Healthcare Information and Management Systems Society (HIMSS). Inertia, cost, and more pressing priorities for hospitals and medical institutions contribute to the technology sticking around, he explained.
“Post-COVID, my guess is we’re still at over 50% of healthcare practices using fax for some reason, on a daily basis,” Mr. Havasy said in an interview. “A lot of hospitals just don’t have the time, the money, or the staff to fix that problem because there’s always something a little higher up the priority chain they need to focus on.”
If, for instance, “you’re going to do a process redesign to reduce hospital total acquired infections, your fax machine replacement might be 10th or 12th on the list. It just never gets up to 1 or 2 because it’s ‘not that much of a problem,’ ” he added.
Or is it?
Administrators may not view fax machines as a top concern, but clinicians who deal with the machines daily see it differently.
“What worries me is we’re taking records out of an electronic storehouse [and] converting them to a paper medium,” Dr. Lewis said. “And then we are scanning into another electronic storehouse. The more steps, the more can be lost.”
And when information is lost, patient care can be compromised.
Slower Workflows, Care Concerns
Although there are no published data on fax machine use in oncology specifically, this outdated technology does come into play in a variety of ways along the cancer care continuum.
Radiation oncologist David R. Penberthy, MD, said patients often seek his cancer center’s expertise for second opinions, and that requires collecting patient records from many different practices.
“Ideally, it would come electronically, but sometimes it does come by fax,” said Dr. Penberthy, program director of radiation oncology at the University of Virginia School of Medicine in Charlottesville. “The quality of the fax is not always the best. Sometimes it’s literally a fax of a fax. You’re reading something that’s very difficult to read.”
Orders for new tests are also typically sent and received via fax temporarily while IT teams work to integrate them into the electronic health record (EHR), Dr. Penberthy said.
Insurers and third-party laboratories often send test results back by fax as well.
“Even if I haven’t actually sent my patient out of our institution, this crucial result may only be entered back into the record as a scanned document from a fax, which is not great because it can get lost in the other results that are reported electronically,” Dr. Lewis said. The risk here is that an ordering physician won’t see these results, which can lead to delayed or overlooked care for patients, he explained.
“To me, it’s like a blind spot,” Dr. Lewis said. “Every time we use a fax, I see it actually as an opportunity for oversight and missed opportunity to collect data.”
Dr. Penberthy said faxing can slow things down at his practice, particularly if he faxes a document to another office but receives no confirmation and has to track down what happened.
As for cybersecurity, data that are in transit during faxing are generally considered secure and compliant with the Health Insurance Portability and Accountability Act (HIPAA), said Mr. Havasy of HIMSS. However, the Privacy Rule also requires that data remain secure while at rest, which isn’t always possible, he added.
“That’s where faxes fall down, because generally fax machines are in public, if you will, or open areas in a hospital,” he said. “They just sit on a desk. I don’t know that the next nurse who comes up and looks through that stack was the nurse who was treating the patient.”
Important decisions or results can also be missed when sent by fax, creating headaches for physicians and care problems for patients.
Dr. Lewis recently experienced an insurance-related fax mishap over Memorial Day weekend. He believed his patient had access to the antinausea medication he had prescribed. When Dr. Lewis happened to check the fax machine over the weekend, he found a coverage denial for the medication from the insurer but, at that point, had no recourse to appeal because it was a long holiday weekend.
“Had the denial been sent by an electronic means that was quicker and more readily available, it would have been possible to appeal before the holiday weekend,” he said.
Hematologist Aaron Goodman, MD, encountered a similar problem after an insurer denied coverage of an expensive cancer drug for a patient and faxed over its reason for the denial. Dr. Goodman was not directly notified that the information arrived and didn’t learn about the denial for a week, he said.
“There’s no ‘ding’ in my inbox if something is faxed over and scanned,” said Dr. Goodman, associate professor of medicine at UC San Diego Health. “Once I realized it was denied, I was able to rectify it, but it wasted a week of a patient not getting a drug that I felt would be beneficial for them.”
Broader Health Policy Impacts
The use of outdated technology, such as fax machines, also creates ripple effects that burden the health system, health policy experts say.
Duplicate testing and unnecessary care are top impacts, said Julia Adler-Milstein, PhD, professor of medicine and chief of the division of clinical informatics and digital transformation at the University of California, San Francisco.
Studies show that 20%-30% of the $65 billion spent annually on lab tests is used on unnecessary duplicate tests, and another estimated $30 billion is spent each year on unnecessary duplicate medical imaging. These duplicate tests may be mitigated if hospitals adopt certified EHR technology, research shows.
Still, without EHR interoperability between institutions, new providers may be unaware that tests or past labs for patients exist, leading to repeat tests, said Dr. Adler-Milstein, who researches health IT policy with a focus on EHRs. Patients can sometimes fill in the gaps, but not always.
“Fax machines only help close information gaps if the clinician is aware of where to seek out the information and there is someone at the other organization to locate and transmit the information in a timely manner,” Dr. Adler-Milstein said.
Old technology and poor interoperability also greatly affect data collection for disease surveillance and monitoring, said Janet Hamilton, MPH, executive director for the Council of State and Territorial Epidemiologists. This issue was keenly demonstrated during the pandemic, Ms. Hamilton said.
“It was tragic, quite honestly,” she said. “There was such an immense amount of data that needed to be moved quickly, and that’s when computers are at their best.”
But, she said, “we didn’t have the level of systems in place to do it well.”
Specifically, the lack of electronic case reporting in place during the pandemic — where diagnoses are documented in the record and then immediately sent to the public health system — led to reports that were delayed, not made, or had missing or incomplete information, such as patients’ race and ethnicity or other health conditions, Ms. Hamilton said.
Incomplete or missing data hampered the ability of public health officials and researchers to understand how the virus might affect different patients.
“If you had a chronic condition like cancer, you were less likely to have a positive outcome with COVID,” Ms. Hamilton said. “But because electronic case reporting was not in place, we didn’t get some of those additional pieces of information. We didn’t have people’s underlying oncology status to then say, ‘Here are individuals with these types of characteristics, and these are the things that happen if they also have a cancer.’”
Slow, but Steady, Improvements
Efforts at the state and federal levels have targeted improved health information exchange, but progress takes time, Dr. Adler-Milstein said.
Most states have some form of health information exchange, such as statewide exchanges, regional health information organizations, or clinical data registries. Maryland is often held up as a notable example for its health information exchange, Dr. Adler-Milstein noted.
According to Maryland law, all hospitals under the jurisdiction of the Maryland Health Care Commission are required to electronically connect to the state-designated health information exchange. In 2012, Maryland became the first state to connect all its 46 acute care hospitals in the sharing of real-time data.
The Health Information Technology for Economic and Clinical Health (HITECH) Act provided federal-enhanced Medicaid matching funds to states through 2021 to support efforts to advance electronic exchange. Nearly all states used these funds, and most have identified other sources to sustain the efforts, according to a recent US Government Accountability Office (GAO) report. However, GAO found that small and rural providers are less likely to have the financial and technological resources to participate in or maintain electronic exchange capabilities.
Nationally, several recent initiatives have targeted health data interoperability, including for cancer care. The Centers for Disease Control and Prevention’s Data Modernization Initiative is a multiyear, multi–billion-dollar effort to improve data sharing across the federal and state public health landscape.
Meanwhile, in March 2024, the Biden-Harris administration launched United States Core Data for Interoperability Plus Cancer. The program will define a recommended minimum set of cancer-related data to be included in a patient’s EHR to enhance data exchange for research and clinical care.
EHR vendors are also key to improving the landscape, said Dr. Adler-Milstein. Vendors such as Epic have developed strong sharing capabilities for transmitting health information from site to site, but of course, that only helps if providers have Epic, she said.
“That’s where these national frameworks should help, because we don’t want it to break down by what EHR vendor you have,” she said. “It’s a patchwork. You can go to some places and hear success stories because they have Epic or a state health information exchange, but it’s very heterogeneous. In some places, they have nothing and are using a fax machine.”
Mr. Havasy believes fax machines will ultimately go extinct, particularly as a younger, more digitally savvy generation enters the healthcare workforce. He also foresees that the growing use of artificial intelligence will help eradicate the outdated technology.
But, Ms. Hamilton noted, “unless we have consistent, ongoing, sustained funding, it is very hard to move off [an older] technology that can work. That’s one of the biggest barriers.”
“Public health is about protecting the lives of every single person everywhere,” Ms. Hamilton said, “but when we don’t have the data that comes into the system, we can’t achieve our mission.”
A version of this article appeared on Medscape.com.
