User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Advice from the front lines: How cancer centers can cope with COVID-19
according to the medical director of a cancer care alliance in the first U.S. epicenter of the coronavirus outbreak.
Jennie R. Crews, MD, the medical director of the Seattle Cancer Care Alliance (SCCA), discussed the SCCA experience and offered advice for other cancer centers in a webinar hosted by the Association of Community Cancer Centers.
Dr. Crews highlighted the SCCA’s use of algorithms to predict which patients can be managed via telehealth and which require face-to-face visits, human resource issues that arose at SCCA, screening and testing procedures, and the importance of communication with patients, caregivers, and staff.
Communication
Dr. Crews stressed the value of clear, regular, and internally consistent staff communication in a variety of formats. SCCA sends daily email blasts to their personnel regarding policies and procedures, which are archived on the SCCA intranet site.
SCCA also holds weekly town hall meetings at which leaders respond to staff questions regarding practical matters they have encountered and future plans. Providers’ up-to-the-minute familiarity with policies and procedures enables all team members to uniformly and clearly communicate to patients and caregivers.
Dr. Crews emphasized the value of consistency and “over-communication” in projecting confidence and preparedness to patients and caregivers during an unsettling time. SCCA has developed fact sheets, posted current information on the SCCA website, and provided education during doorway screenings.
Screening and testing
All SCCA staff members are screened daily at the practice entrance so they have personal experience with the process utilized for patients. Because symptoms associated with coronavirus infection may overlap with cancer treatment–related complaints, SCCA clinicians have expanded the typical coronavirus screening questionnaire for patients on cancer treatment.
Patients with ambiguous symptoms are masked, taken to a physically separate area of the SCCA clinics, and screened further by an advanced practice provider. The patients are then triaged to either the clinic for treatment or to the emergency department for further triage and care.
Although testing processes and procedures have been modified, Dr. Crews advised codifying those policies and procedures, including notification of results and follow-up for both patients and staff. Dr. Crews also stressed the importance of clearly articulated return-to-work policies for staff who have potential exposure and/or positive test results.
At the University of Washington’s virology laboratory, they have a test turnaround time of less than 12 hours.
Planning ahead
Dr. Crews highlighted the importance of community-based surge planning, utilizing predictive models to assess inpatient capacity requirements and potential repurposing of providers.
The SCCA is prepared to close selected community sites and shift personnel to other locations if personnel needs cannot be met because of illness or quarantine. Contingency plans include specialized pharmacy services for patients requiring chemotherapy.
The SCCA has not yet experienced shortages of personal protective equipment (PPE). However, Dr. Crews said staff require detailed education regarding the use of PPE in order to safeguard the supply while providing maximal staff protection.
Helping the helpers
During the pandemic, SCCA has dealt with a variety of challenging human resource issues, including:
- Extending sick time beyond what was previously “stored” in staff members’ earned time off.
- Childcare during an extended hiatus in school and daycare schedules.
- Programs to maintain and/or restore employee wellness (including staff-centered support services, spiritual care, mindfulness exercises, and town halls).
Dr. Crews also discussed recruitment of community resources to provide meals for staff from local restaurants with restricted hours and transportation resources for staff and patients, as visitors are restricted (currently one per patient).
Managing care
Dr. Crews noted that the University of Washington had a foundational structure for a telehealth program prior to the pandemic. Their telehealth committee enabled SCCA to scale up the service quickly with their academic partners, including training modules for and certification of providers, outfitting off-site personnel with dedicated lines and hardware, and provision of personal Zoom accounts.
SCCA also devised algorithms for determining when face-to-face visits, remote management, or deferred visits are appropriate in various scenarios. The algorithms were developed by disease-specialized teams.
As a general rule, routine chemotherapy and radiation are administered on schedule. On-treatment and follow-up office visits are conducted via telehealth if possible. In some cases, initiation of chemotherapy and radiation has been delayed, and screening services have been suspended.
In response to questions about palliative care during the pandemic, Dr. Crews said SCCA has encouraged their patients to complete, review, or update their advance directives. The SCCA has not had the need to resuscitate a coronavirus-infected outpatient but has instituted policies for utilizing full PPE on any patient requiring resuscitation.
In her closing remarks, Dr. Crews stressed that the response to COVID-19 in Washington state has required an intense collaboration among colleagues, the community, and government leaders, as the actions required extended far beyond medical decision makers alone.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
according to the medical director of a cancer care alliance in the first U.S. epicenter of the coronavirus outbreak.
Jennie R. Crews, MD, the medical director of the Seattle Cancer Care Alliance (SCCA), discussed the SCCA experience and offered advice for other cancer centers in a webinar hosted by the Association of Community Cancer Centers.
Dr. Crews highlighted the SCCA’s use of algorithms to predict which patients can be managed via telehealth and which require face-to-face visits, human resource issues that arose at SCCA, screening and testing procedures, and the importance of communication with patients, caregivers, and staff.
Communication
Dr. Crews stressed the value of clear, regular, and internally consistent staff communication in a variety of formats. SCCA sends daily email blasts to their personnel regarding policies and procedures, which are archived on the SCCA intranet site.
SCCA also holds weekly town hall meetings at which leaders respond to staff questions regarding practical matters they have encountered and future plans. Providers’ up-to-the-minute familiarity with policies and procedures enables all team members to uniformly and clearly communicate to patients and caregivers.
Dr. Crews emphasized the value of consistency and “over-communication” in projecting confidence and preparedness to patients and caregivers during an unsettling time. SCCA has developed fact sheets, posted current information on the SCCA website, and provided education during doorway screenings.
Screening and testing
All SCCA staff members are screened daily at the practice entrance so they have personal experience with the process utilized for patients. Because symptoms associated with coronavirus infection may overlap with cancer treatment–related complaints, SCCA clinicians have expanded the typical coronavirus screening questionnaire for patients on cancer treatment.
Patients with ambiguous symptoms are masked, taken to a physically separate area of the SCCA clinics, and screened further by an advanced practice provider. The patients are then triaged to either the clinic for treatment or to the emergency department for further triage and care.
Although testing processes and procedures have been modified, Dr. Crews advised codifying those policies and procedures, including notification of results and follow-up for both patients and staff. Dr. Crews also stressed the importance of clearly articulated return-to-work policies for staff who have potential exposure and/or positive test results.
At the University of Washington’s virology laboratory, they have a test turnaround time of less than 12 hours.
Planning ahead
Dr. Crews highlighted the importance of community-based surge planning, utilizing predictive models to assess inpatient capacity requirements and potential repurposing of providers.
The SCCA is prepared to close selected community sites and shift personnel to other locations if personnel needs cannot be met because of illness or quarantine. Contingency plans include specialized pharmacy services for patients requiring chemotherapy.
The SCCA has not yet experienced shortages of personal protective equipment (PPE). However, Dr. Crews said staff require detailed education regarding the use of PPE in order to safeguard the supply while providing maximal staff protection.
Helping the helpers
During the pandemic, SCCA has dealt with a variety of challenging human resource issues, including:
- Extending sick time beyond what was previously “stored” in staff members’ earned time off.
- Childcare during an extended hiatus in school and daycare schedules.
- Programs to maintain and/or restore employee wellness (including staff-centered support services, spiritual care, mindfulness exercises, and town halls).
Dr. Crews also discussed recruitment of community resources to provide meals for staff from local restaurants with restricted hours and transportation resources for staff and patients, as visitors are restricted (currently one per patient).
Managing care
Dr. Crews noted that the University of Washington had a foundational structure for a telehealth program prior to the pandemic. Their telehealth committee enabled SCCA to scale up the service quickly with their academic partners, including training modules for and certification of providers, outfitting off-site personnel with dedicated lines and hardware, and provision of personal Zoom accounts.
SCCA also devised algorithms for determining when face-to-face visits, remote management, or deferred visits are appropriate in various scenarios. The algorithms were developed by disease-specialized teams.
As a general rule, routine chemotherapy and radiation are administered on schedule. On-treatment and follow-up office visits are conducted via telehealth if possible. In some cases, initiation of chemotherapy and radiation has been delayed, and screening services have been suspended.
In response to questions about palliative care during the pandemic, Dr. Crews said SCCA has encouraged their patients to complete, review, or update their advance directives. The SCCA has not had the need to resuscitate a coronavirus-infected outpatient but has instituted policies for utilizing full PPE on any patient requiring resuscitation.
In her closing remarks, Dr. Crews stressed that the response to COVID-19 in Washington state has required an intense collaboration among colleagues, the community, and government leaders, as the actions required extended far beyond medical decision makers alone.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
according to the medical director of a cancer care alliance in the first U.S. epicenter of the coronavirus outbreak.
Jennie R. Crews, MD, the medical director of the Seattle Cancer Care Alliance (SCCA), discussed the SCCA experience and offered advice for other cancer centers in a webinar hosted by the Association of Community Cancer Centers.
Dr. Crews highlighted the SCCA’s use of algorithms to predict which patients can be managed via telehealth and which require face-to-face visits, human resource issues that arose at SCCA, screening and testing procedures, and the importance of communication with patients, caregivers, and staff.
Communication
Dr. Crews stressed the value of clear, regular, and internally consistent staff communication in a variety of formats. SCCA sends daily email blasts to their personnel regarding policies and procedures, which are archived on the SCCA intranet site.
SCCA also holds weekly town hall meetings at which leaders respond to staff questions regarding practical matters they have encountered and future plans. Providers’ up-to-the-minute familiarity with policies and procedures enables all team members to uniformly and clearly communicate to patients and caregivers.
Dr. Crews emphasized the value of consistency and “over-communication” in projecting confidence and preparedness to patients and caregivers during an unsettling time. SCCA has developed fact sheets, posted current information on the SCCA website, and provided education during doorway screenings.
Screening and testing
All SCCA staff members are screened daily at the practice entrance so they have personal experience with the process utilized for patients. Because symptoms associated with coronavirus infection may overlap with cancer treatment–related complaints, SCCA clinicians have expanded the typical coronavirus screening questionnaire for patients on cancer treatment.
Patients with ambiguous symptoms are masked, taken to a physically separate area of the SCCA clinics, and screened further by an advanced practice provider. The patients are then triaged to either the clinic for treatment or to the emergency department for further triage and care.
Although testing processes and procedures have been modified, Dr. Crews advised codifying those policies and procedures, including notification of results and follow-up for both patients and staff. Dr. Crews also stressed the importance of clearly articulated return-to-work policies for staff who have potential exposure and/or positive test results.
At the University of Washington’s virology laboratory, they have a test turnaround time of less than 12 hours.
Planning ahead
Dr. Crews highlighted the importance of community-based surge planning, utilizing predictive models to assess inpatient capacity requirements and potential repurposing of providers.
The SCCA is prepared to close selected community sites and shift personnel to other locations if personnel needs cannot be met because of illness or quarantine. Contingency plans include specialized pharmacy services for patients requiring chemotherapy.
The SCCA has not yet experienced shortages of personal protective equipment (PPE). However, Dr. Crews said staff require detailed education regarding the use of PPE in order to safeguard the supply while providing maximal staff protection.
Helping the helpers
During the pandemic, SCCA has dealt with a variety of challenging human resource issues, including:
- Extending sick time beyond what was previously “stored” in staff members’ earned time off.
- Childcare during an extended hiatus in school and daycare schedules.
- Programs to maintain and/or restore employee wellness (including staff-centered support services, spiritual care, mindfulness exercises, and town halls).
Dr. Crews also discussed recruitment of community resources to provide meals for staff from local restaurants with restricted hours and transportation resources for staff and patients, as visitors are restricted (currently one per patient).
Managing care
Dr. Crews noted that the University of Washington had a foundational structure for a telehealth program prior to the pandemic. Their telehealth committee enabled SCCA to scale up the service quickly with their academic partners, including training modules for and certification of providers, outfitting off-site personnel with dedicated lines and hardware, and provision of personal Zoom accounts.
SCCA also devised algorithms for determining when face-to-face visits, remote management, or deferred visits are appropriate in various scenarios. The algorithms were developed by disease-specialized teams.
As a general rule, routine chemotherapy and radiation are administered on schedule. On-treatment and follow-up office visits are conducted via telehealth if possible. In some cases, initiation of chemotherapy and radiation has been delayed, and screening services have been suspended.
In response to questions about palliative care during the pandemic, Dr. Crews said SCCA has encouraged their patients to complete, review, or update their advance directives. The SCCA has not had the need to resuscitate a coronavirus-infected outpatient but has instituted policies for utilizing full PPE on any patient requiring resuscitation.
In her closing remarks, Dr. Crews stressed that the response to COVID-19 in Washington state has required an intense collaboration among colleagues, the community, and government leaders, as the actions required extended far beyond medical decision makers alone.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
NYU med student joins COVID fight: ‘Time to step up’
On the evening of March 24, I got the email. When the bolded letters “We ask for your help” flashed across my screen, I knew exactly what was being asked of me: to graduate early and join the fight against COVID-19.
For the 120 fourth-year medical students in my class at NYU Grossman School of Medicine, the arrival of that email was always more a question of when than if. Similar moves had already been made in Italy as well as the United Kingdom, where the surge in patients with COVID-19 has devastated hospitals and left healthcare workers dead or drained. The New York hospitals where I’ve trained, places I have grown to love over the past 4 years, are now experiencing similar horrors. Residents and attending doctors – mentors and teachers – are burned out and exhausted. They need help.
Like most medical students, I chose to pursue medicine out of a desire to help. On both my medical school and residency applications, I spoke about my resolve to bear witness to and provide support to those suffering. Yet, being recruited to the front lines of a global pandemic felt deeply unsettling. Is this how I want to finally enter the world of medicine? The scope of what is actually being asked of me was immense.
Given the onslaught of bad news coming in on every device I had cozied up to during my social distancing, how could I want to do this? I’ve seen the death toll climb in Italy, with dozens of doctors dead. I’ve seen the photos of faces marred by masks worn for 12-16 hours at a time. I’ve been repeatedly reminded that we are just behind Italy. Things are certainly going to get worse.
It sounds selfish and petty, but I feel like COVID-19 has already robbed me of so much. Yet that was my first thought when I received the email. The end of fourth year in medical school is supposed to be a joyous, celebratory time. We have worked years for this moment. So many of us have fought burnout to reach this time, a brief moment of rest between being a medical student and becoming a full-fledged physician.
I matched into residency just 4 days before being asked to join the front lines of the pandemic. I found out my match results without the usual fanfare, sitting on a bench in Madison Square Park, FaceTiming my dad and safely social-distanced from my mom. They both cried tears of joy. Like so many people around the world right now, I couldn’t even embrace my parents. Would they want me to volunteer?
I reached out to my classmates. I thought that some of them would certainly share my worries. I thought they also had to be carrying this uncomfortable kind of grief, a heavy and acidic feeling of dreams collapsing into a moral duty. I received a unanimous reply: “We are needed. It’s our time to step up.” No matter how many “what ifs” I voiced, they wouldn’t crack or waver. Still, even if they never admitted it to me, I wondered whether they privately shared some of my concerns and fears.
Everyone knows information is shared instantly in our Twitter-centric world, but I was still shocked and unprepared for how quickly I was at the center of a major news story. Within an hour of that email, I was contacted by an old acquaintance from elementary school, now a journalist. He had found me through Facebook and asked, “Will you be one of the NYU students graduating early? Would love to get a comment.” Another friend texted me a photo of the leaked email, quipping, “Are you going to save us from the pandemic, Dr. Gabe?!” “It’s not a small decision!” I snapped back.
I went through something like the seven stages of grief in rapid succession. I found that with each excuse I made why I shouldn’t volunteer, I somehow became increasingly more anxious. To my surprise, when I decided I would join 50 of my peers at NYU, graduate early, and volunteer, my mind settled. The more I thought about it, the more I was overtaken by the selfless beauty of the profession I’m entering. This is what it means to be a doctor. I recalled a key part of the Hippocratic Oath: “I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.”
I am going to fulfill my special obligations.
The fear is still there. I’m scared of COVID. I’m scared to infect others. I’m scared of winding up paralyzed and intubated. But I have also realized that all we have is each other. Healthcare workers supporting healthcare workers. New Yorkers supporting New Yorkers. Citizens of the world supporting citizens of the world. This is my time to be there for others, unwaveringly.
Logistical details continue to roll in, although they feel trivial in relation to the decision I have already made. The paperwork tells me that I will be onboarded to NYU’s internal medicine residency program. I will be compensated and protected under a similar contract to what current NYU residents sign. I have been promised that I will remain insured until I start my official residency program in July. My student loans won’t begin accruing interest until my normally planned graduation date. I am told that I will have personal protective equipment in line with the Centers for Disease Control and Prevention recommendations.
Questions still linger. Is it safe for me and my newly minted physician peers to continue living with our spouses, children, and friends? How long will I need to quarantine after my contract ends? Will there be a virtual graduation ceremony for my parents and loved ones to enjoy? In these challenging times, each day gives me a little more clarity about what exactly I am signing up for, but there are still so many uncertainties.
Am I naive to say that I do feel prepared? Or at least as prepared as anyone can be. With respect to my training, I have completed the requirements to graduate, which is why I am being permitted to graduate early in the first place. Our faculty points to our professionalism as the most promising indicator of our preparedness. They are heartened that we have embraced this truest test: our duty to others.
There is an eerie calm to New York City that contradicts what is shown on the news. With stores closed and streets quiet, it almost feels like Christmas morning here. Yet, inside the hospital, a fire rages. All the metaphors being used right now speak about violence, devastation, and immeasurable human suffering. “A war is being fought.” Or so I have heard. I guess I am about to find out.
Gabriel Redel-Traub is a fourth-year medical student at NYU Grossman School of Medicine. He will be starting residency in internal medicine at Columbia Presbyterian this summer. He is the former editor-in-chief of Dartmouth College’s Mouth Magazine, an editor of NYU’s LitMed Database, and has published most recently in the Hasting’s Center Magazine. Gabriel Redel-Traub has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
On the evening of March 24, I got the email. When the bolded letters “We ask for your help” flashed across my screen, I knew exactly what was being asked of me: to graduate early and join the fight against COVID-19.
For the 120 fourth-year medical students in my class at NYU Grossman School of Medicine, the arrival of that email was always more a question of when than if. Similar moves had already been made in Italy as well as the United Kingdom, where the surge in patients with COVID-19 has devastated hospitals and left healthcare workers dead or drained. The New York hospitals where I’ve trained, places I have grown to love over the past 4 years, are now experiencing similar horrors. Residents and attending doctors – mentors and teachers – are burned out and exhausted. They need help.
Like most medical students, I chose to pursue medicine out of a desire to help. On both my medical school and residency applications, I spoke about my resolve to bear witness to and provide support to those suffering. Yet, being recruited to the front lines of a global pandemic felt deeply unsettling. Is this how I want to finally enter the world of medicine? The scope of what is actually being asked of me was immense.
Given the onslaught of bad news coming in on every device I had cozied up to during my social distancing, how could I want to do this? I’ve seen the death toll climb in Italy, with dozens of doctors dead. I’ve seen the photos of faces marred by masks worn for 12-16 hours at a time. I’ve been repeatedly reminded that we are just behind Italy. Things are certainly going to get worse.
It sounds selfish and petty, but I feel like COVID-19 has already robbed me of so much. Yet that was my first thought when I received the email. The end of fourth year in medical school is supposed to be a joyous, celebratory time. We have worked years for this moment. So many of us have fought burnout to reach this time, a brief moment of rest between being a medical student and becoming a full-fledged physician.
I matched into residency just 4 days before being asked to join the front lines of the pandemic. I found out my match results without the usual fanfare, sitting on a bench in Madison Square Park, FaceTiming my dad and safely social-distanced from my mom. They both cried tears of joy. Like so many people around the world right now, I couldn’t even embrace my parents. Would they want me to volunteer?
I reached out to my classmates. I thought that some of them would certainly share my worries. I thought they also had to be carrying this uncomfortable kind of grief, a heavy and acidic feeling of dreams collapsing into a moral duty. I received a unanimous reply: “We are needed. It’s our time to step up.” No matter how many “what ifs” I voiced, they wouldn’t crack or waver. Still, even if they never admitted it to me, I wondered whether they privately shared some of my concerns and fears.
Everyone knows information is shared instantly in our Twitter-centric world, but I was still shocked and unprepared for how quickly I was at the center of a major news story. Within an hour of that email, I was contacted by an old acquaintance from elementary school, now a journalist. He had found me through Facebook and asked, “Will you be one of the NYU students graduating early? Would love to get a comment.” Another friend texted me a photo of the leaked email, quipping, “Are you going to save us from the pandemic, Dr. Gabe?!” “It’s not a small decision!” I snapped back.
I went through something like the seven stages of grief in rapid succession. I found that with each excuse I made why I shouldn’t volunteer, I somehow became increasingly more anxious. To my surprise, when I decided I would join 50 of my peers at NYU, graduate early, and volunteer, my mind settled. The more I thought about it, the more I was overtaken by the selfless beauty of the profession I’m entering. This is what it means to be a doctor. I recalled a key part of the Hippocratic Oath: “I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.”
I am going to fulfill my special obligations.
The fear is still there. I’m scared of COVID. I’m scared to infect others. I’m scared of winding up paralyzed and intubated. But I have also realized that all we have is each other. Healthcare workers supporting healthcare workers. New Yorkers supporting New Yorkers. Citizens of the world supporting citizens of the world. This is my time to be there for others, unwaveringly.
Logistical details continue to roll in, although they feel trivial in relation to the decision I have already made. The paperwork tells me that I will be onboarded to NYU’s internal medicine residency program. I will be compensated and protected under a similar contract to what current NYU residents sign. I have been promised that I will remain insured until I start my official residency program in July. My student loans won’t begin accruing interest until my normally planned graduation date. I am told that I will have personal protective equipment in line with the Centers for Disease Control and Prevention recommendations.
Questions still linger. Is it safe for me and my newly minted physician peers to continue living with our spouses, children, and friends? How long will I need to quarantine after my contract ends? Will there be a virtual graduation ceremony for my parents and loved ones to enjoy? In these challenging times, each day gives me a little more clarity about what exactly I am signing up for, but there are still so many uncertainties.
Am I naive to say that I do feel prepared? Or at least as prepared as anyone can be. With respect to my training, I have completed the requirements to graduate, which is why I am being permitted to graduate early in the first place. Our faculty points to our professionalism as the most promising indicator of our preparedness. They are heartened that we have embraced this truest test: our duty to others.
There is an eerie calm to New York City that contradicts what is shown on the news. With stores closed and streets quiet, it almost feels like Christmas morning here. Yet, inside the hospital, a fire rages. All the metaphors being used right now speak about violence, devastation, and immeasurable human suffering. “A war is being fought.” Or so I have heard. I guess I am about to find out.
Gabriel Redel-Traub is a fourth-year medical student at NYU Grossman School of Medicine. He will be starting residency in internal medicine at Columbia Presbyterian this summer. He is the former editor-in-chief of Dartmouth College’s Mouth Magazine, an editor of NYU’s LitMed Database, and has published most recently in the Hasting’s Center Magazine. Gabriel Redel-Traub has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
On the evening of March 24, I got the email. When the bolded letters “We ask for your help” flashed across my screen, I knew exactly what was being asked of me: to graduate early and join the fight against COVID-19.
For the 120 fourth-year medical students in my class at NYU Grossman School of Medicine, the arrival of that email was always more a question of when than if. Similar moves had already been made in Italy as well as the United Kingdom, where the surge in patients with COVID-19 has devastated hospitals and left healthcare workers dead or drained. The New York hospitals where I’ve trained, places I have grown to love over the past 4 years, are now experiencing similar horrors. Residents and attending doctors – mentors and teachers – are burned out and exhausted. They need help.
Like most medical students, I chose to pursue medicine out of a desire to help. On both my medical school and residency applications, I spoke about my resolve to bear witness to and provide support to those suffering. Yet, being recruited to the front lines of a global pandemic felt deeply unsettling. Is this how I want to finally enter the world of medicine? The scope of what is actually being asked of me was immense.
Given the onslaught of bad news coming in on every device I had cozied up to during my social distancing, how could I want to do this? I’ve seen the death toll climb in Italy, with dozens of doctors dead. I’ve seen the photos of faces marred by masks worn for 12-16 hours at a time. I’ve been repeatedly reminded that we are just behind Italy. Things are certainly going to get worse.
It sounds selfish and petty, but I feel like COVID-19 has already robbed me of so much. Yet that was my first thought when I received the email. The end of fourth year in medical school is supposed to be a joyous, celebratory time. We have worked years for this moment. So many of us have fought burnout to reach this time, a brief moment of rest between being a medical student and becoming a full-fledged physician.
I matched into residency just 4 days before being asked to join the front lines of the pandemic. I found out my match results without the usual fanfare, sitting on a bench in Madison Square Park, FaceTiming my dad and safely social-distanced from my mom. They both cried tears of joy. Like so many people around the world right now, I couldn’t even embrace my parents. Would they want me to volunteer?
I reached out to my classmates. I thought that some of them would certainly share my worries. I thought they also had to be carrying this uncomfortable kind of grief, a heavy and acidic feeling of dreams collapsing into a moral duty. I received a unanimous reply: “We are needed. It’s our time to step up.” No matter how many “what ifs” I voiced, they wouldn’t crack or waver. Still, even if they never admitted it to me, I wondered whether they privately shared some of my concerns and fears.
Everyone knows information is shared instantly in our Twitter-centric world, but I was still shocked and unprepared for how quickly I was at the center of a major news story. Within an hour of that email, I was contacted by an old acquaintance from elementary school, now a journalist. He had found me through Facebook and asked, “Will you be one of the NYU students graduating early? Would love to get a comment.” Another friend texted me a photo of the leaked email, quipping, “Are you going to save us from the pandemic, Dr. Gabe?!” “It’s not a small decision!” I snapped back.
I went through something like the seven stages of grief in rapid succession. I found that with each excuse I made why I shouldn’t volunteer, I somehow became increasingly more anxious. To my surprise, when I decided I would join 50 of my peers at NYU, graduate early, and volunteer, my mind settled. The more I thought about it, the more I was overtaken by the selfless beauty of the profession I’m entering. This is what it means to be a doctor. I recalled a key part of the Hippocratic Oath: “I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.”
I am going to fulfill my special obligations.
The fear is still there. I’m scared of COVID. I’m scared to infect others. I’m scared of winding up paralyzed and intubated. But I have also realized that all we have is each other. Healthcare workers supporting healthcare workers. New Yorkers supporting New Yorkers. Citizens of the world supporting citizens of the world. This is my time to be there for others, unwaveringly.
Logistical details continue to roll in, although they feel trivial in relation to the decision I have already made. The paperwork tells me that I will be onboarded to NYU’s internal medicine residency program. I will be compensated and protected under a similar contract to what current NYU residents sign. I have been promised that I will remain insured until I start my official residency program in July. My student loans won’t begin accruing interest until my normally planned graduation date. I am told that I will have personal protective equipment in line with the Centers for Disease Control and Prevention recommendations.
Questions still linger. Is it safe for me and my newly minted physician peers to continue living with our spouses, children, and friends? How long will I need to quarantine after my contract ends? Will there be a virtual graduation ceremony for my parents and loved ones to enjoy? In these challenging times, each day gives me a little more clarity about what exactly I am signing up for, but there are still so many uncertainties.
Am I naive to say that I do feel prepared? Or at least as prepared as anyone can be. With respect to my training, I have completed the requirements to graduate, which is why I am being permitted to graduate early in the first place. Our faculty points to our professionalism as the most promising indicator of our preparedness. They are heartened that we have embraced this truest test: our duty to others.
There is an eerie calm to New York City that contradicts what is shown on the news. With stores closed and streets quiet, it almost feels like Christmas morning here. Yet, inside the hospital, a fire rages. All the metaphors being used right now speak about violence, devastation, and immeasurable human suffering. “A war is being fought.” Or so I have heard. I guess I am about to find out.
Gabriel Redel-Traub is a fourth-year medical student at NYU Grossman School of Medicine. He will be starting residency in internal medicine at Columbia Presbyterian this summer. He is the former editor-in-chief of Dartmouth College’s Mouth Magazine, an editor of NYU’s LitMed Database, and has published most recently in the Hasting’s Center Magazine. Gabriel Redel-Traub has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
FDA removes pregnancy category C warning from certain MS medications
The FDA based the decision on data from more than 1,000 real-world pregnancies, including pregnancies from a large epidemiologic study and published studies over several decades, which found no connection between use of interferon-beta products during early pregnancy and an increased risk of major birth defects, according to the FDA.
As a result, the labels for both medications will no longer have the pregnancy category C designation; however, patients should continue to notify their health care provider if they are pregnant or plan to become pregnant.
The FDA decision to remove the warning follows a similar decision by the European Medicines Agency last year.
“Many women with MS are diagnosed during their childbearing years. With this important update for Plegridy and Avonex, healthcare providers have more data to inform appropriate treatment paths for patients who may be pregnant or planning for pregnancy,” said Bernd Kieseier, MD, MHBA, executive director and head of global MS at Worldwide Medical, Biogen, in a press release.
The FDA based the decision on data from more than 1,000 real-world pregnancies, including pregnancies from a large epidemiologic study and published studies over several decades, which found no connection between use of interferon-beta products during early pregnancy and an increased risk of major birth defects, according to the FDA.
As a result, the labels for both medications will no longer have the pregnancy category C designation; however, patients should continue to notify their health care provider if they are pregnant or plan to become pregnant.
The FDA decision to remove the warning follows a similar decision by the European Medicines Agency last year.
“Many women with MS are diagnosed during their childbearing years. With this important update for Plegridy and Avonex, healthcare providers have more data to inform appropriate treatment paths for patients who may be pregnant or planning for pregnancy,” said Bernd Kieseier, MD, MHBA, executive director and head of global MS at Worldwide Medical, Biogen, in a press release.
The FDA based the decision on data from more than 1,000 real-world pregnancies, including pregnancies from a large epidemiologic study and published studies over several decades, which found no connection between use of interferon-beta products during early pregnancy and an increased risk of major birth defects, according to the FDA.
As a result, the labels for both medications will no longer have the pregnancy category C designation; however, patients should continue to notify their health care provider if they are pregnant or plan to become pregnant.
The FDA decision to remove the warning follows a similar decision by the European Medicines Agency last year.
“Many women with MS are diagnosed during their childbearing years. With this important update for Plegridy and Avonex, healthcare providers have more data to inform appropriate treatment paths for patients who may be pregnant or planning for pregnancy,” said Bernd Kieseier, MD, MHBA, executive director and head of global MS at Worldwide Medical, Biogen, in a press release.
Neurologists navigate unknown territory during COVID-19 pandemic
Neurologic disorders are among the “underlying medical conditions that may increase the risk of serious COVID-19 for individuals of any age,” according to the Centers for Disease Control and Prevention.
Potentially relevant drug interactions, how immunosuppressive medications may influence the risk of COVID-19, and neurologic diseases that may be associated with greater risk are among the questions that experts and groups have addressed.
According to the CDC, neurologic conditions that may heighten the risk of severe COVID-19 include “disorders of the brain, spinal cord, peripheral nerve, and muscle such as cerebral palsy, epilepsy (seizure disorders), stroke, intellectual disability, moderate to severe developmental delay, muscular dystrophy, or spinal cord injury.” Many patients, however, may not have substantially increased risks, neurologists suggest.
“Patients with conditions that do not affect their swallowing or breathing muscles and in whom the immune system is working normally are not considered to be at increased risk from COVID-19,” according to March 26 guidance from the Association of British Neurologists (ABN). “Milder or moderate forms of many of the commoner neurological disorders, such as Parkinson’s disease, multiple sclerosis, epilepsy, are not currently considered to confer increased risk, so long as the breathing and swallowing muscles are functioning well.”
Neurologists should tailor treatment decisions to individual patients, according to the ABN. “Although some neurological conditions or treatments increase the risk of complicated COVID-19, most patients in these groups will overcome the infection,” the association noted.
Interactions with potential COVID-19 treatments
Standard drugs in neurology may interact with potential COVID-19 treatments. For example, “preliminary experience suggests that there is a possible benefit from hydroxychloroquine and azithromycin treatment in COVID-19 infection,” but either of those drugs “may lead to a deterioration in myasthenia gravis,” the ABN notes. “Doctors will have to balance the risks from myasthenia and COVID-19 on a case-by-case basis.” The Liverpool Drug Interactions Group has published tables that describe interactions between potential COVID-19 treatments and anticonvulsants, analgesics, immunosuppressants, and other medication classes.
Many muscle diseases and neuromuscular junction diseases may entail higher risks of complicated COVID-19, the ABN suggested. For patients on immunotherapy, the medication may be a more important consideration for COVID-19 than the underlying disease. Other comorbidities such as hypertension, renal impairment, neutropenia, lymphopenia, liver disease, diabetes mellitus, ischemic heart disease, and lung disease may be important factors, according to the association.
Seizures may not worsen
After the CDC added epilepsy to its list of conditions that entail higher risk of severe COVID-19, M. Scott Perry, MD, medical director of neurology at Cook Children’s Medical Center in Fort Worth, Tex., commented on Twitter that “most healthy people with controlled epilepsy [are] probably at no more risk than others.”
“Those treated with steroids or other immunosuppressive drugs are likely higher risk,” Dr. Perry said. “Likewise, patients with other medical comorbidities such as muscle weakness, swallowing or breathing problems, and other complex cases of epilepsy are likely higher risk. Regardless: be responsible, avoid crowds, wash your hands, avoid sick contacts.”
Doctors in Italy, based on small numbers of cases, have found that seizures are not worse in patients with epilepsy and COVID-19, said Dr. Perry. A few children, including several patients with Dravet syndrome, “had uncomplicated illness and seizures were no worse,” he said. “That is reassuring.”
“Until now, there is no evidence of a direct effect of COVID-19 on seizures or epilepsy,” according to the International League Against Epilepsy (ILAE). “However, patients may experience worsening of seizures due to systemic illnesses, drug interactions, decreased access to antiseizure medications, and increased stress.”
“In younger children, the fever that accompanies COVID-19 may exacerbate seizures, as might any febrile illness,” according to an American Epilepsy Society (AES) resource for epilepsy clinicians. “The main known elevated risk factors related to COVID-19 are age, respiratory disease, and other chronic medical conditions not related to epilepsy. As for all, people with epilepsy should adhere to the CDC recommendations for reducing risk of infection.” Neurologists should review with patients the importance of treatment adherence, update plans for managing breakthrough seizures, and ensure necessary medications are on hand, according to the AES.
The Epilepsy Foundation created a page with information about COVID-19 for patients with epilepsy and recorded a discussion with epilepsy specialists. DEE-P (Developmental Epileptic Encephalopathy–Project) Connections recorded a webinar about protecting medically complex or immune-suppressed children with epilepsy from COVID-19.
MS DMTs and the coronavirus
The National Multiple Sclerosis (MS) Society has provided guidance on the use of disease-modifying therapies (DMTs) during the COVID-19 pandemic. “There are numerous recommendations circulating that attempt to provide clarity and guidance, however, differences among the recommendations have created confusion,” the society says. “DMT decision making varies significantly from country to country, ranging from highly provider-directed to a collaborative decision-making model. ... DMT decisions should be individualized and made collaboratively between the person with MS and his/her healthcare provider.”
Patients with MS and their physicians should weigh risks and benefits before starting cell-depleting DMTs such as alemtuzumab, cladribine, ocrelizumab, or rituximab, according the National MS Society. They also should consider the risks and benefits of DMTs that carry warnings of a potentially severe increase in disability after stopping therapy, such as fingolimod and natalizumab. “We endorse the global advice provided by the MS International Federation (MSIF) – but emphasize that DMT decision making must be individualized and based upon multiple factors,” the National MS Society said.
Neurologists currently lack evidence about how COVID-19 affects patients with MS, according to the MSIF, which based its DMT guidance on advice from MS neurologists and research experts from member organizations. Many DMTs suppress or modify the immune system, and “some MS medications might increase the likelihood of developing complications from a COVID-19 infection but this risk needs to be balanced with the risks of stopping treatment,” according to the federation.
Patients currently taking DMTs should continue treatment, and those who develop symptoms of COVID-19 or test positive for the infection should discuss their DMT with a health care professional familiar with their care, the MSIF recommends. Decisions about starting a DMT should take into account a patient’s disease course, disease activity, and regional COVID-19 risks, according to the federation. For patients due to start DMT, treatments that do not reduce lymphocytes, such as interferons, glatiramer acetate, or natalizumab, should be considered.
Fingolimod, dimethyl fumarate, teriflunomide, and siponimod “may reduce the ability of the immune system to respond to an infection,” and “people should carefully consider the risks and benefits of initiating these treatments during the COVID-19 pandemic,” according to the federation. “People with MS who are currently taking alemtuzumab, cladribine, ocrelizumab, rituximab, fingolimod, dimethyl fumarate, teriflunomide or siponimod and are living in a community with a COVID-19 outbreak should isolate as much as possible to reduce their risk of infection.”
Extended isolation during the COVID-19 outbreak may be warranted for patients with MS who have recently undergone autologous hematopoietic stem cell treatment, which entails intensive chemotherapy, the guidance says. In addition, postponement of this procedure should be considered.
Child neurology, migraine, movement disorders, and stroke
The Child Neurology Foundation (CNF) and Child Neurology Society (CNS) published a joint statement about COVID-19. “Most children who contract COVID-19 appear to exhibit only mild symptoms,” said Scott Pomeroy, MD, president of CNF’s board of directors and chair of the department of neurology at Boston Children’s Hospital, in the statement. “However, if your child is taking a medication such as steroids that can lower their immune system response, there could be an increased risk for more significant symptoms. In addition, children with lung disease, such as asthma, may also be at higher risk. Therefore, it is important to practice preventative precautions. We hope that this information will help to reduce some of the fears that families in our community may be experiencing.”
The American Migraine Foundation shared COVID-19 considerations for patients with migraine from Mia Minen, MD, associate professor of neurology and population health at NYU Langone in New York. Patients with migraine who are otherwise in good health are not expected to be at increased risk of severe COVID-19, according to Dr. Minen. Best practices include having an adequate supply of medicine, considering alternatives to in-person doctor visits, and being “mindful of routine and diet to reduce migraine triggers,” the foundation suggests. In addition, patients should try to limit stress and seek out “alternative methods of social interaction.”
“The relationship between COVID-19 and Parkinson’s disease or other movement disorders remains unknown,” the International Parkinson and Movement Disorder Society said. “In general, we recommend that our movement disorder patients do not assume they are at extreme risks, which for the time being are uncertain. Nevertheless, we strongly recommend following the standard measures strictly to avoid exposures to the virus.”
The American Heart Association (AHA) cautions that older patients with coronary heart disease or hypertension “may be more likely than others to be infected by the coronavirus that causes COVID-19 and to develop more severe symptoms.” In addition, people with a history of stroke “may face a higher risk of complications,” according to the AHA. “As a result, people who have heart disease or another underlying condition should stay home to limit their risk of contracting the virus.”
Several groups emphasized the importance of telemedicine as an option for patients with neurologic conditions during the pandemic. The American Headache Society has hosted discussions on conducting neurologic exams via telemedicine. The American Academy of Neurology also conducted a webinar on telemedicine and COVID-19 and created a page with COVID-19 resources. The journal Neurology is publishing invited commentaries about neurologic aspects of the COVID-19 pandemic.
Neurologic disorders are among the “underlying medical conditions that may increase the risk of serious COVID-19 for individuals of any age,” according to the Centers for Disease Control and Prevention.
Potentially relevant drug interactions, how immunosuppressive medications may influence the risk of COVID-19, and neurologic diseases that may be associated with greater risk are among the questions that experts and groups have addressed.
According to the CDC, neurologic conditions that may heighten the risk of severe COVID-19 include “disorders of the brain, spinal cord, peripheral nerve, and muscle such as cerebral palsy, epilepsy (seizure disorders), stroke, intellectual disability, moderate to severe developmental delay, muscular dystrophy, or spinal cord injury.” Many patients, however, may not have substantially increased risks, neurologists suggest.
“Patients with conditions that do not affect their swallowing or breathing muscles and in whom the immune system is working normally are not considered to be at increased risk from COVID-19,” according to March 26 guidance from the Association of British Neurologists (ABN). “Milder or moderate forms of many of the commoner neurological disorders, such as Parkinson’s disease, multiple sclerosis, epilepsy, are not currently considered to confer increased risk, so long as the breathing and swallowing muscles are functioning well.”
Neurologists should tailor treatment decisions to individual patients, according to the ABN. “Although some neurological conditions or treatments increase the risk of complicated COVID-19, most patients in these groups will overcome the infection,” the association noted.
Interactions with potential COVID-19 treatments
Standard drugs in neurology may interact with potential COVID-19 treatments. For example, “preliminary experience suggests that there is a possible benefit from hydroxychloroquine and azithromycin treatment in COVID-19 infection,” but either of those drugs “may lead to a deterioration in myasthenia gravis,” the ABN notes. “Doctors will have to balance the risks from myasthenia and COVID-19 on a case-by-case basis.” The Liverpool Drug Interactions Group has published tables that describe interactions between potential COVID-19 treatments and anticonvulsants, analgesics, immunosuppressants, and other medication classes.
Many muscle diseases and neuromuscular junction diseases may entail higher risks of complicated COVID-19, the ABN suggested. For patients on immunotherapy, the medication may be a more important consideration for COVID-19 than the underlying disease. Other comorbidities such as hypertension, renal impairment, neutropenia, lymphopenia, liver disease, diabetes mellitus, ischemic heart disease, and lung disease may be important factors, according to the association.
Seizures may not worsen
After the CDC added epilepsy to its list of conditions that entail higher risk of severe COVID-19, M. Scott Perry, MD, medical director of neurology at Cook Children’s Medical Center in Fort Worth, Tex., commented on Twitter that “most healthy people with controlled epilepsy [are] probably at no more risk than others.”
“Those treated with steroids or other immunosuppressive drugs are likely higher risk,” Dr. Perry said. “Likewise, patients with other medical comorbidities such as muscle weakness, swallowing or breathing problems, and other complex cases of epilepsy are likely higher risk. Regardless: be responsible, avoid crowds, wash your hands, avoid sick contacts.”
Doctors in Italy, based on small numbers of cases, have found that seizures are not worse in patients with epilepsy and COVID-19, said Dr. Perry. A few children, including several patients with Dravet syndrome, “had uncomplicated illness and seizures were no worse,” he said. “That is reassuring.”
“Until now, there is no evidence of a direct effect of COVID-19 on seizures or epilepsy,” according to the International League Against Epilepsy (ILAE). “However, patients may experience worsening of seizures due to systemic illnesses, drug interactions, decreased access to antiseizure medications, and increased stress.”
“In younger children, the fever that accompanies COVID-19 may exacerbate seizures, as might any febrile illness,” according to an American Epilepsy Society (AES) resource for epilepsy clinicians. “The main known elevated risk factors related to COVID-19 are age, respiratory disease, and other chronic medical conditions not related to epilepsy. As for all, people with epilepsy should adhere to the CDC recommendations for reducing risk of infection.” Neurologists should review with patients the importance of treatment adherence, update plans for managing breakthrough seizures, and ensure necessary medications are on hand, according to the AES.
The Epilepsy Foundation created a page with information about COVID-19 for patients with epilepsy and recorded a discussion with epilepsy specialists. DEE-P (Developmental Epileptic Encephalopathy–Project) Connections recorded a webinar about protecting medically complex or immune-suppressed children with epilepsy from COVID-19.
MS DMTs and the coronavirus
The National Multiple Sclerosis (MS) Society has provided guidance on the use of disease-modifying therapies (DMTs) during the COVID-19 pandemic. “There are numerous recommendations circulating that attempt to provide clarity and guidance, however, differences among the recommendations have created confusion,” the society says. “DMT decision making varies significantly from country to country, ranging from highly provider-directed to a collaborative decision-making model. ... DMT decisions should be individualized and made collaboratively between the person with MS and his/her healthcare provider.”
Patients with MS and their physicians should weigh risks and benefits before starting cell-depleting DMTs such as alemtuzumab, cladribine, ocrelizumab, or rituximab, according the National MS Society. They also should consider the risks and benefits of DMTs that carry warnings of a potentially severe increase in disability after stopping therapy, such as fingolimod and natalizumab. “We endorse the global advice provided by the MS International Federation (MSIF) – but emphasize that DMT decision making must be individualized and based upon multiple factors,” the National MS Society said.
Neurologists currently lack evidence about how COVID-19 affects patients with MS, according to the MSIF, which based its DMT guidance on advice from MS neurologists and research experts from member organizations. Many DMTs suppress or modify the immune system, and “some MS medications might increase the likelihood of developing complications from a COVID-19 infection but this risk needs to be balanced with the risks of stopping treatment,” according to the federation.
Patients currently taking DMTs should continue treatment, and those who develop symptoms of COVID-19 or test positive for the infection should discuss their DMT with a health care professional familiar with their care, the MSIF recommends. Decisions about starting a DMT should take into account a patient’s disease course, disease activity, and regional COVID-19 risks, according to the federation. For patients due to start DMT, treatments that do not reduce lymphocytes, such as interferons, glatiramer acetate, or natalizumab, should be considered.
Fingolimod, dimethyl fumarate, teriflunomide, and siponimod “may reduce the ability of the immune system to respond to an infection,” and “people should carefully consider the risks and benefits of initiating these treatments during the COVID-19 pandemic,” according to the federation. “People with MS who are currently taking alemtuzumab, cladribine, ocrelizumab, rituximab, fingolimod, dimethyl fumarate, teriflunomide or siponimod and are living in a community with a COVID-19 outbreak should isolate as much as possible to reduce their risk of infection.”
Extended isolation during the COVID-19 outbreak may be warranted for patients with MS who have recently undergone autologous hematopoietic stem cell treatment, which entails intensive chemotherapy, the guidance says. In addition, postponement of this procedure should be considered.
Child neurology, migraine, movement disorders, and stroke
The Child Neurology Foundation (CNF) and Child Neurology Society (CNS) published a joint statement about COVID-19. “Most children who contract COVID-19 appear to exhibit only mild symptoms,” said Scott Pomeroy, MD, president of CNF’s board of directors and chair of the department of neurology at Boston Children’s Hospital, in the statement. “However, if your child is taking a medication such as steroids that can lower their immune system response, there could be an increased risk for more significant symptoms. In addition, children with lung disease, such as asthma, may also be at higher risk. Therefore, it is important to practice preventative precautions. We hope that this information will help to reduce some of the fears that families in our community may be experiencing.”
The American Migraine Foundation shared COVID-19 considerations for patients with migraine from Mia Minen, MD, associate professor of neurology and population health at NYU Langone in New York. Patients with migraine who are otherwise in good health are not expected to be at increased risk of severe COVID-19, according to Dr. Minen. Best practices include having an adequate supply of medicine, considering alternatives to in-person doctor visits, and being “mindful of routine and diet to reduce migraine triggers,” the foundation suggests. In addition, patients should try to limit stress and seek out “alternative methods of social interaction.”
“The relationship between COVID-19 and Parkinson’s disease or other movement disorders remains unknown,” the International Parkinson and Movement Disorder Society said. “In general, we recommend that our movement disorder patients do not assume they are at extreme risks, which for the time being are uncertain. Nevertheless, we strongly recommend following the standard measures strictly to avoid exposures to the virus.”
The American Heart Association (AHA) cautions that older patients with coronary heart disease or hypertension “may be more likely than others to be infected by the coronavirus that causes COVID-19 and to develop more severe symptoms.” In addition, people with a history of stroke “may face a higher risk of complications,” according to the AHA. “As a result, people who have heart disease or another underlying condition should stay home to limit their risk of contracting the virus.”
Several groups emphasized the importance of telemedicine as an option for patients with neurologic conditions during the pandemic. The American Headache Society has hosted discussions on conducting neurologic exams via telemedicine. The American Academy of Neurology also conducted a webinar on telemedicine and COVID-19 and created a page with COVID-19 resources. The journal Neurology is publishing invited commentaries about neurologic aspects of the COVID-19 pandemic.
Neurologic disorders are among the “underlying medical conditions that may increase the risk of serious COVID-19 for individuals of any age,” according to the Centers for Disease Control and Prevention.
Potentially relevant drug interactions, how immunosuppressive medications may influence the risk of COVID-19, and neurologic diseases that may be associated with greater risk are among the questions that experts and groups have addressed.
According to the CDC, neurologic conditions that may heighten the risk of severe COVID-19 include “disorders of the brain, spinal cord, peripheral nerve, and muscle such as cerebral palsy, epilepsy (seizure disorders), stroke, intellectual disability, moderate to severe developmental delay, muscular dystrophy, or spinal cord injury.” Many patients, however, may not have substantially increased risks, neurologists suggest.
“Patients with conditions that do not affect their swallowing or breathing muscles and in whom the immune system is working normally are not considered to be at increased risk from COVID-19,” according to March 26 guidance from the Association of British Neurologists (ABN). “Milder or moderate forms of many of the commoner neurological disorders, such as Parkinson’s disease, multiple sclerosis, epilepsy, are not currently considered to confer increased risk, so long as the breathing and swallowing muscles are functioning well.”
Neurologists should tailor treatment decisions to individual patients, according to the ABN. “Although some neurological conditions or treatments increase the risk of complicated COVID-19, most patients in these groups will overcome the infection,” the association noted.
Interactions with potential COVID-19 treatments
Standard drugs in neurology may interact with potential COVID-19 treatments. For example, “preliminary experience suggests that there is a possible benefit from hydroxychloroquine and azithromycin treatment in COVID-19 infection,” but either of those drugs “may lead to a deterioration in myasthenia gravis,” the ABN notes. “Doctors will have to balance the risks from myasthenia and COVID-19 on a case-by-case basis.” The Liverpool Drug Interactions Group has published tables that describe interactions between potential COVID-19 treatments and anticonvulsants, analgesics, immunosuppressants, and other medication classes.
Many muscle diseases and neuromuscular junction diseases may entail higher risks of complicated COVID-19, the ABN suggested. For patients on immunotherapy, the medication may be a more important consideration for COVID-19 than the underlying disease. Other comorbidities such as hypertension, renal impairment, neutropenia, lymphopenia, liver disease, diabetes mellitus, ischemic heart disease, and lung disease may be important factors, according to the association.
Seizures may not worsen
After the CDC added epilepsy to its list of conditions that entail higher risk of severe COVID-19, M. Scott Perry, MD, medical director of neurology at Cook Children’s Medical Center in Fort Worth, Tex., commented on Twitter that “most healthy people with controlled epilepsy [are] probably at no more risk than others.”
“Those treated with steroids or other immunosuppressive drugs are likely higher risk,” Dr. Perry said. “Likewise, patients with other medical comorbidities such as muscle weakness, swallowing or breathing problems, and other complex cases of epilepsy are likely higher risk. Regardless: be responsible, avoid crowds, wash your hands, avoid sick contacts.”
Doctors in Italy, based on small numbers of cases, have found that seizures are not worse in patients with epilepsy and COVID-19, said Dr. Perry. A few children, including several patients with Dravet syndrome, “had uncomplicated illness and seizures were no worse,” he said. “That is reassuring.”
“Until now, there is no evidence of a direct effect of COVID-19 on seizures or epilepsy,” according to the International League Against Epilepsy (ILAE). “However, patients may experience worsening of seizures due to systemic illnesses, drug interactions, decreased access to antiseizure medications, and increased stress.”
“In younger children, the fever that accompanies COVID-19 may exacerbate seizures, as might any febrile illness,” according to an American Epilepsy Society (AES) resource for epilepsy clinicians. “The main known elevated risk factors related to COVID-19 are age, respiratory disease, and other chronic medical conditions not related to epilepsy. As for all, people with epilepsy should adhere to the CDC recommendations for reducing risk of infection.” Neurologists should review with patients the importance of treatment adherence, update plans for managing breakthrough seizures, and ensure necessary medications are on hand, according to the AES.
The Epilepsy Foundation created a page with information about COVID-19 for patients with epilepsy and recorded a discussion with epilepsy specialists. DEE-P (Developmental Epileptic Encephalopathy–Project) Connections recorded a webinar about protecting medically complex or immune-suppressed children with epilepsy from COVID-19.
MS DMTs and the coronavirus
The National Multiple Sclerosis (MS) Society has provided guidance on the use of disease-modifying therapies (DMTs) during the COVID-19 pandemic. “There are numerous recommendations circulating that attempt to provide clarity and guidance, however, differences among the recommendations have created confusion,” the society says. “DMT decision making varies significantly from country to country, ranging from highly provider-directed to a collaborative decision-making model. ... DMT decisions should be individualized and made collaboratively between the person with MS and his/her healthcare provider.”
Patients with MS and their physicians should weigh risks and benefits before starting cell-depleting DMTs such as alemtuzumab, cladribine, ocrelizumab, or rituximab, according the National MS Society. They also should consider the risks and benefits of DMTs that carry warnings of a potentially severe increase in disability after stopping therapy, such as fingolimod and natalizumab. “We endorse the global advice provided by the MS International Federation (MSIF) – but emphasize that DMT decision making must be individualized and based upon multiple factors,” the National MS Society said.
Neurologists currently lack evidence about how COVID-19 affects patients with MS, according to the MSIF, which based its DMT guidance on advice from MS neurologists and research experts from member organizations. Many DMTs suppress or modify the immune system, and “some MS medications might increase the likelihood of developing complications from a COVID-19 infection but this risk needs to be balanced with the risks of stopping treatment,” according to the federation.
Patients currently taking DMTs should continue treatment, and those who develop symptoms of COVID-19 or test positive for the infection should discuss their DMT with a health care professional familiar with their care, the MSIF recommends. Decisions about starting a DMT should take into account a patient’s disease course, disease activity, and regional COVID-19 risks, according to the federation. For patients due to start DMT, treatments that do not reduce lymphocytes, such as interferons, glatiramer acetate, or natalizumab, should be considered.
Fingolimod, dimethyl fumarate, teriflunomide, and siponimod “may reduce the ability of the immune system to respond to an infection,” and “people should carefully consider the risks and benefits of initiating these treatments during the COVID-19 pandemic,” according to the federation. “People with MS who are currently taking alemtuzumab, cladribine, ocrelizumab, rituximab, fingolimod, dimethyl fumarate, teriflunomide or siponimod and are living in a community with a COVID-19 outbreak should isolate as much as possible to reduce their risk of infection.”
Extended isolation during the COVID-19 outbreak may be warranted for patients with MS who have recently undergone autologous hematopoietic stem cell treatment, which entails intensive chemotherapy, the guidance says. In addition, postponement of this procedure should be considered.
Child neurology, migraine, movement disorders, and stroke
The Child Neurology Foundation (CNF) and Child Neurology Society (CNS) published a joint statement about COVID-19. “Most children who contract COVID-19 appear to exhibit only mild symptoms,” said Scott Pomeroy, MD, president of CNF’s board of directors and chair of the department of neurology at Boston Children’s Hospital, in the statement. “However, if your child is taking a medication such as steroids that can lower their immune system response, there could be an increased risk for more significant symptoms. In addition, children with lung disease, such as asthma, may also be at higher risk. Therefore, it is important to practice preventative precautions. We hope that this information will help to reduce some of the fears that families in our community may be experiencing.”
The American Migraine Foundation shared COVID-19 considerations for patients with migraine from Mia Minen, MD, associate professor of neurology and population health at NYU Langone in New York. Patients with migraine who are otherwise in good health are not expected to be at increased risk of severe COVID-19, according to Dr. Minen. Best practices include having an adequate supply of medicine, considering alternatives to in-person doctor visits, and being “mindful of routine and diet to reduce migraine triggers,” the foundation suggests. In addition, patients should try to limit stress and seek out “alternative methods of social interaction.”
“The relationship between COVID-19 and Parkinson’s disease or other movement disorders remains unknown,” the International Parkinson and Movement Disorder Society said. “In general, we recommend that our movement disorder patients do not assume they are at extreme risks, which for the time being are uncertain. Nevertheless, we strongly recommend following the standard measures strictly to avoid exposures to the virus.”
The American Heart Association (AHA) cautions that older patients with coronary heart disease or hypertension “may be more likely than others to be infected by the coronavirus that causes COVID-19 and to develop more severe symptoms.” In addition, people with a history of stroke “may face a higher risk of complications,” according to the AHA. “As a result, people who have heart disease or another underlying condition should stay home to limit their risk of contracting the virus.”
Several groups emphasized the importance of telemedicine as an option for patients with neurologic conditions during the pandemic. The American Headache Society has hosted discussions on conducting neurologic exams via telemedicine. The American Academy of Neurology also conducted a webinar on telemedicine and COVID-19 and created a page with COVID-19 resources. The journal Neurology is publishing invited commentaries about neurologic aspects of the COVID-19 pandemic.
Surge in firearm sales tied to COVID-19 fears, uncertainty presents risks
Use gentle assumptions and focus on home access to elicit positive answers.
In the wake of the 2012 shooting at Sandy Hook Elementary, in Newtown, Conn., after 20 children and seven adults were murdered, American gun sales surged on fears of new restrictions.
In the ensuing months, 20 more children and 40 more adults died from unintentional shootings believed to be tied to that surge in gun purchases.1 More recently, American gun sales surged in response to the COVID-19 pandemic with heated legal battles brewing over whether gun sales are essential.2,3 The results of this surge in sales are yet to fully manifest, but I would like to discuss several risks.
The public health risks of firearm access are well established: Nearly every measure of harm, from suicide to negligent injury and death to homicide to shootings of police, increase along with access to firearms.4 That firearms in the home are associated with greater likelihoods of suicide, negligent injury and death, and intrafamilial homicide has been recognized for decades as has the substantially heightened risk in the immediate period after a firearm is brought into the home.5,6 Defensive gun use is rare despite this being the nominal reason for firearm ownership among many.7 Even prior to recent events, there had been concerns of increased unsafe carrying and handling of firearms.8 It seems reasonable to expect such trends not to be diminished by recent events.
Added to this are several stressors, which one can reasonably expect to be associated with increased risks for unsafe use. There are new, broad social stressors from fear and uncertainty about COVID-19. Unemployment rates have skyrocketed, clinical care has been disrupted, and basic necessities have become scant. Children are home from school, unable to play with friends and unable to access mental health services as easily as before; risks of negligent and suicidal injuries and death may ensue. Couples and families are isolated in homes together for longer periods and with fewer avenues for relief; previously peaceful homes may see conflicts increase and homes with abuse have now trapped victims with their assailants. Social isolation is difficult for any person and may be even more traumatic for people with underlying vulnerabilities, including mental illness. The risks of being isolated in a home – struggling with worsening symptoms – with ready access to a firearm are self-evident.
- Consider reassessing for firearm access. Patients may be in new homes, or there may be new firearms in their homes. Use gentle assumptions and focus on home access over personal access to elicit the most true, positive answers, for example: “I understand there have been a lot of changes recently; how many guns are in the home now?”
- Reinforce safer storage practices. Simple measures, such as storing ammunition separately and using trigger locks or safes, can make a substantial difference in injury risks.
- Do not forget aging clients; suicide risk increases with age, and there may be substantial risks among the geriatric population for suicide and murder-suicide. If using telepsychiatry, realize that the abuser might be in the home or within earshot of any clinical encounter, and this might put the client at heightened risk, during and after telesessions.
- Highlight access to local and national resources, including the Disaster Distress Hotline (800-985-5990) and the National Suicide Prevention Lifeline (800-273-TALK). Promote both numbers, and note that some people may be more comfortable reaching out for help for “distress” than for “suicide.”
References
1. Levine PB and McKnight R. Science. 2017 Dec 8;358(6368):1324-8.
2. Levin D. “Coronavirus and firearms: Are gun shops essential businesses?” The New York Times. 2020 Mar 25.
3. Robertson L. “Neither hurricanes nor 9/11 caused as big a surge in gun sales as coronavirus.” Miami Herald. 2020 Mar 25.
4. Moyer MW. Scientific American. 2017 Oct;317(4):54-63.
5. Kellermann AL et al. J Trauma. 1998 Aug;45(2):263-7.
6. Wintemute GJ et al. New Engl J Med. 1999 Nov 18;341(21):1583-9.
7. Firearm Justifiable Homicides and Non-Fatal Self-Defense Gun Use: An Analysis of Federal Bureau of Investigation and National Crime Victimization Survey Data. Washington: Violence Policy Center; 2019 Jul.
8. Towers S et al. bioRxiv. 2019 Apr 18;613687.
Dr. Rozel is the medical director of resolve Crisis Services at UPMC Western Psychiatric Hospital and president of the American Association for Emergency Psychiatry. He also is associate professor of psychiatry and an adjunct professor of law at the University of Pittsburgh. He has no conflicts of interest but has worked for a gun dealer to teach sales staff how to recognize people in crisis (rather than sell a gun).
Use gentle assumptions and focus on home access to elicit positive answers.
Use gentle assumptions and focus on home access to elicit positive answers.
In the wake of the 2012 shooting at Sandy Hook Elementary, in Newtown, Conn., after 20 children and seven adults were murdered, American gun sales surged on fears of new restrictions.
In the ensuing months, 20 more children and 40 more adults died from unintentional shootings believed to be tied to that surge in gun purchases.1 More recently, American gun sales surged in response to the COVID-19 pandemic with heated legal battles brewing over whether gun sales are essential.2,3 The results of this surge in sales are yet to fully manifest, but I would like to discuss several risks.
The public health risks of firearm access are well established: Nearly every measure of harm, from suicide to negligent injury and death to homicide to shootings of police, increase along with access to firearms.4 That firearms in the home are associated with greater likelihoods of suicide, negligent injury and death, and intrafamilial homicide has been recognized for decades as has the substantially heightened risk in the immediate period after a firearm is brought into the home.5,6 Defensive gun use is rare despite this being the nominal reason for firearm ownership among many.7 Even prior to recent events, there had been concerns of increased unsafe carrying and handling of firearms.8 It seems reasonable to expect such trends not to be diminished by recent events.
Added to this are several stressors, which one can reasonably expect to be associated with increased risks for unsafe use. There are new, broad social stressors from fear and uncertainty about COVID-19. Unemployment rates have skyrocketed, clinical care has been disrupted, and basic necessities have become scant. Children are home from school, unable to play with friends and unable to access mental health services as easily as before; risks of negligent and suicidal injuries and death may ensue. Couples and families are isolated in homes together for longer periods and with fewer avenues for relief; previously peaceful homes may see conflicts increase and homes with abuse have now trapped victims with their assailants. Social isolation is difficult for any person and may be even more traumatic for people with underlying vulnerabilities, including mental illness. The risks of being isolated in a home – struggling with worsening symptoms – with ready access to a firearm are self-evident.
- Consider reassessing for firearm access. Patients may be in new homes, or there may be new firearms in their homes. Use gentle assumptions and focus on home access over personal access to elicit the most true, positive answers, for example: “I understand there have been a lot of changes recently; how many guns are in the home now?”
- Reinforce safer storage practices. Simple measures, such as storing ammunition separately and using trigger locks or safes, can make a substantial difference in injury risks.
- Do not forget aging clients; suicide risk increases with age, and there may be substantial risks among the geriatric population for suicide and murder-suicide. If using telepsychiatry, realize that the abuser might be in the home or within earshot of any clinical encounter, and this might put the client at heightened risk, during and after telesessions.
- Highlight access to local and national resources, including the Disaster Distress Hotline (800-985-5990) and the National Suicide Prevention Lifeline (800-273-TALK). Promote both numbers, and note that some people may be more comfortable reaching out for help for “distress” than for “suicide.”
References
1. Levine PB and McKnight R. Science. 2017 Dec 8;358(6368):1324-8.
2. Levin D. “Coronavirus and firearms: Are gun shops essential businesses?” The New York Times. 2020 Mar 25.
3. Robertson L. “Neither hurricanes nor 9/11 caused as big a surge in gun sales as coronavirus.” Miami Herald. 2020 Mar 25.
4. Moyer MW. Scientific American. 2017 Oct;317(4):54-63.
5. Kellermann AL et al. J Trauma. 1998 Aug;45(2):263-7.
6. Wintemute GJ et al. New Engl J Med. 1999 Nov 18;341(21):1583-9.
7. Firearm Justifiable Homicides and Non-Fatal Self-Defense Gun Use: An Analysis of Federal Bureau of Investigation and National Crime Victimization Survey Data. Washington: Violence Policy Center; 2019 Jul.
8. Towers S et al. bioRxiv. 2019 Apr 18;613687.
Dr. Rozel is the medical director of resolve Crisis Services at UPMC Western Psychiatric Hospital and president of the American Association for Emergency Psychiatry. He also is associate professor of psychiatry and an adjunct professor of law at the University of Pittsburgh. He has no conflicts of interest but has worked for a gun dealer to teach sales staff how to recognize people in crisis (rather than sell a gun).
In the wake of the 2012 shooting at Sandy Hook Elementary, in Newtown, Conn., after 20 children and seven adults were murdered, American gun sales surged on fears of new restrictions.
In the ensuing months, 20 more children and 40 more adults died from unintentional shootings believed to be tied to that surge in gun purchases.1 More recently, American gun sales surged in response to the COVID-19 pandemic with heated legal battles brewing over whether gun sales are essential.2,3 The results of this surge in sales are yet to fully manifest, but I would like to discuss several risks.
The public health risks of firearm access are well established: Nearly every measure of harm, from suicide to negligent injury and death to homicide to shootings of police, increase along with access to firearms.4 That firearms in the home are associated with greater likelihoods of suicide, negligent injury and death, and intrafamilial homicide has been recognized for decades as has the substantially heightened risk in the immediate period after a firearm is brought into the home.5,6 Defensive gun use is rare despite this being the nominal reason for firearm ownership among many.7 Even prior to recent events, there had been concerns of increased unsafe carrying and handling of firearms.8 It seems reasonable to expect such trends not to be diminished by recent events.
Added to this are several stressors, which one can reasonably expect to be associated with increased risks for unsafe use. There are new, broad social stressors from fear and uncertainty about COVID-19. Unemployment rates have skyrocketed, clinical care has been disrupted, and basic necessities have become scant. Children are home from school, unable to play with friends and unable to access mental health services as easily as before; risks of negligent and suicidal injuries and death may ensue. Couples and families are isolated in homes together for longer periods and with fewer avenues for relief; previously peaceful homes may see conflicts increase and homes with abuse have now trapped victims with their assailants. Social isolation is difficult for any person and may be even more traumatic for people with underlying vulnerabilities, including mental illness. The risks of being isolated in a home – struggling with worsening symptoms – with ready access to a firearm are self-evident.
- Consider reassessing for firearm access. Patients may be in new homes, or there may be new firearms in their homes. Use gentle assumptions and focus on home access over personal access to elicit the most true, positive answers, for example: “I understand there have been a lot of changes recently; how many guns are in the home now?”
- Reinforce safer storage practices. Simple measures, such as storing ammunition separately and using trigger locks or safes, can make a substantial difference in injury risks.
- Do not forget aging clients; suicide risk increases with age, and there may be substantial risks among the geriatric population for suicide and murder-suicide. If using telepsychiatry, realize that the abuser might be in the home or within earshot of any clinical encounter, and this might put the client at heightened risk, during and after telesessions.
- Highlight access to local and national resources, including the Disaster Distress Hotline (800-985-5990) and the National Suicide Prevention Lifeline (800-273-TALK). Promote both numbers, and note that some people may be more comfortable reaching out for help for “distress” than for “suicide.”
References
1. Levine PB and McKnight R. Science. 2017 Dec 8;358(6368):1324-8.
2. Levin D. “Coronavirus and firearms: Are gun shops essential businesses?” The New York Times. 2020 Mar 25.
3. Robertson L. “Neither hurricanes nor 9/11 caused as big a surge in gun sales as coronavirus.” Miami Herald. 2020 Mar 25.
4. Moyer MW. Scientific American. 2017 Oct;317(4):54-63.
5. Kellermann AL et al. J Trauma. 1998 Aug;45(2):263-7.
6. Wintemute GJ et al. New Engl J Med. 1999 Nov 18;341(21):1583-9.
7. Firearm Justifiable Homicides and Non-Fatal Self-Defense Gun Use: An Analysis of Federal Bureau of Investigation and National Crime Victimization Survey Data. Washington: Violence Policy Center; 2019 Jul.
8. Towers S et al. bioRxiv. 2019 Apr 18;613687.
Dr. Rozel is the medical director of resolve Crisis Services at UPMC Western Psychiatric Hospital and president of the American Association for Emergency Psychiatry. He also is associate professor of psychiatry and an adjunct professor of law at the University of Pittsburgh. He has no conflicts of interest but has worked for a gun dealer to teach sales staff how to recognize people in crisis (rather than sell a gun).
No staff COVID-19 diagnoses after plan at Chinese cancer center
Short-term results
No staff members or patients were diagnosed with COVID-19 after “strict protective measures” for screening and managing patients were implemented at the National Cancer Center/Cancer Hospital, Chinese Academy of Sciences, in Beijing, according to a report published online April 1 in JAMA Oncology.
However, the time period for the analysis, which included nearly 3000 patients, was short — only about 3 weeks (February 12 to March 3). Also, Beijing is more than 1100 kilometers from Wuhan, the center of the Chinese outbreak of COVID-19.
The Beijing cancer hospital implemented a multipronged safety plan in February in order to “avoid COVID-19 related nosocomial cross-infection between patients and medical staff,” explain the authors, led by medical oncologist Zhijie Wang, MD.
Notably, “all of the measures taken in China are actively being implemented and used in major oncology centers in the United States,” Robert Carlson, MD, chief executive officer, National Comprehensive Cancer Network (NCCN), told Medscape Medical News.
John Greene, MD, section chief, Infectious Disease and Tropical Medicine, Moffitt Cancer Center, Tampa, Florida, pointed out that the Chinese safety plan, which is full of “good measures,” is being largely used at his center. However, he observed that one tool — doing a temperature check at the hospital front door — is not well supported by most of the literature. “It gives good optics and looks like you are doing the most you possibly can, but scientifically it may not be as effective [as other screening measures],” he said.
The Chinese plan consists of four broad elements
First, the above-mentioned on-site temperature tests are performed at the entrances of the hospital, outpatient clinic, and wards. Contact and travel histories related to the Wuhan epidemic area are also established and recorded.
Second, an outpatient appointment scheduling system allows both online scheduling and on-site registration. Online consultation channels are open daily, featuring instruction on medication taking and cancer-related symptom management. These “substantially reduced the flow of people in the hospital,” write the authors. On-site patients must wear a mask and have their own disinfectant.
Third, for patients with cancer preparing to be admitted to hospital, symptoms associated with COVID-19, such as fever and cough, are recorded. Mandatory blood tests and CT scans of the lungs are performed. COVID-19 virus nucleic acid tests are performed for patients with suspected pneumonia on imaging.
Fourth, some anticancer drugs conventionally administered by infusion have been changed to oral administration, such as etoposide and vinorelbine. For adjuvant or maintenance chemotherapy, the infusion intervals were appropriately prolonged depending on patients’ conditions.
Eight out of 2,900 patients had imaging suspicious for infection
The Chinese authors report that a total of 2,944 patients with cancer were seen for clinic consultation and treatment in the wards (2795 outpatients and 149 inpatients).
Patients with cancer are believed to have a higher probability of severe illness and increased mortality compared with the healthy population once infected with COVID-19, point out the authors.
Under the new “strict screening strategy,” 27 patients showed radiologic manifestations of inflammatory changes or multiple-site exudative pneumonia in the lungs, including eight suspected of having COVID-19 infection. “Fortunately, negative results from nucleic acid testing ultimately excluded COVID-19 infection in all these patients,” the authors report.
However, two of these patients “presented with recovered pneumonia after symptomatic treatment.” Commenting on this finding, Moffitt’s Greene said that may mean these two patients were tested and found to be positive but were early in the infection and not yet shedding the virus, or they were infected after the initial negative result.
Greene said his center has implemented some measures not mentioned in the Chinese plan. For example, the Florida center no longer allows inpatient visitation. Also, one third of staff now work from home, resulting in less social interaction. Social distancing in meetings, the cafeteria, and hallways is being observed “aggressively,” and most meetings are now on Zoom, he said.
Moffitt has not been hard hit with COVID-19 and is at level one preparedness, the lowest rung. The center has performed 60 tests to date, with only one positive for the virus (< 2%), Greene told Medscape Medical News.
Currently, in the larger Tampa Bay community setting, about 12% of tests are positive.
The low percentage found among the Moffitt patients “tells you that a lot of cancer patients have fever and respiratory symptoms due to other viruses and, more importantly, other reasons, whether it’s their immunotherapy or chemotherapy or their cancer,” said Greene.
NCCN’s Carlson said the publication of the Chinese data was a good sign in terms of international science.
“This is a strong example of how the global oncology community rapidly shares information and experience whenever it makes a difference in patient care,” he commented.
The authors, as well as Carlson and Greene, have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Short-term results
Short-term results
No staff members or patients were diagnosed with COVID-19 after “strict protective measures” for screening and managing patients were implemented at the National Cancer Center/Cancer Hospital, Chinese Academy of Sciences, in Beijing, according to a report published online April 1 in JAMA Oncology.
However, the time period for the analysis, which included nearly 3000 patients, was short — only about 3 weeks (February 12 to March 3). Also, Beijing is more than 1100 kilometers from Wuhan, the center of the Chinese outbreak of COVID-19.
The Beijing cancer hospital implemented a multipronged safety plan in February in order to “avoid COVID-19 related nosocomial cross-infection between patients and medical staff,” explain the authors, led by medical oncologist Zhijie Wang, MD.
Notably, “all of the measures taken in China are actively being implemented and used in major oncology centers in the United States,” Robert Carlson, MD, chief executive officer, National Comprehensive Cancer Network (NCCN), told Medscape Medical News.
John Greene, MD, section chief, Infectious Disease and Tropical Medicine, Moffitt Cancer Center, Tampa, Florida, pointed out that the Chinese safety plan, which is full of “good measures,” is being largely used at his center. However, he observed that one tool — doing a temperature check at the hospital front door — is not well supported by most of the literature. “It gives good optics and looks like you are doing the most you possibly can, but scientifically it may not be as effective [as other screening measures],” he said.
The Chinese plan consists of four broad elements
First, the above-mentioned on-site temperature tests are performed at the entrances of the hospital, outpatient clinic, and wards. Contact and travel histories related to the Wuhan epidemic area are also established and recorded.
Second, an outpatient appointment scheduling system allows both online scheduling and on-site registration. Online consultation channels are open daily, featuring instruction on medication taking and cancer-related symptom management. These “substantially reduced the flow of people in the hospital,” write the authors. On-site patients must wear a mask and have their own disinfectant.
Third, for patients with cancer preparing to be admitted to hospital, symptoms associated with COVID-19, such as fever and cough, are recorded. Mandatory blood tests and CT scans of the lungs are performed. COVID-19 virus nucleic acid tests are performed for patients with suspected pneumonia on imaging.
Fourth, some anticancer drugs conventionally administered by infusion have been changed to oral administration, such as etoposide and vinorelbine. For adjuvant or maintenance chemotherapy, the infusion intervals were appropriately prolonged depending on patients’ conditions.
Eight out of 2,900 patients had imaging suspicious for infection
The Chinese authors report that a total of 2,944 patients with cancer were seen for clinic consultation and treatment in the wards (2795 outpatients and 149 inpatients).
Patients with cancer are believed to have a higher probability of severe illness and increased mortality compared with the healthy population once infected with COVID-19, point out the authors.
Under the new “strict screening strategy,” 27 patients showed radiologic manifestations of inflammatory changes or multiple-site exudative pneumonia in the lungs, including eight suspected of having COVID-19 infection. “Fortunately, negative results from nucleic acid testing ultimately excluded COVID-19 infection in all these patients,” the authors report.
However, two of these patients “presented with recovered pneumonia after symptomatic treatment.” Commenting on this finding, Moffitt’s Greene said that may mean these two patients were tested and found to be positive but were early in the infection and not yet shedding the virus, or they were infected after the initial negative result.
Greene said his center has implemented some measures not mentioned in the Chinese plan. For example, the Florida center no longer allows inpatient visitation. Also, one third of staff now work from home, resulting in less social interaction. Social distancing in meetings, the cafeteria, and hallways is being observed “aggressively,” and most meetings are now on Zoom, he said.
Moffitt has not been hard hit with COVID-19 and is at level one preparedness, the lowest rung. The center has performed 60 tests to date, with only one positive for the virus (< 2%), Greene told Medscape Medical News.
Currently, in the larger Tampa Bay community setting, about 12% of tests are positive.
The low percentage found among the Moffitt patients “tells you that a lot of cancer patients have fever and respiratory symptoms due to other viruses and, more importantly, other reasons, whether it’s their immunotherapy or chemotherapy or their cancer,” said Greene.
NCCN’s Carlson said the publication of the Chinese data was a good sign in terms of international science.
“This is a strong example of how the global oncology community rapidly shares information and experience whenever it makes a difference in patient care,” he commented.
The authors, as well as Carlson and Greene, have reported no relevant financial relationships.
This article first appeared on Medscape.com.
No staff members or patients were diagnosed with COVID-19 after “strict protective measures” for screening and managing patients were implemented at the National Cancer Center/Cancer Hospital, Chinese Academy of Sciences, in Beijing, according to a report published online April 1 in JAMA Oncology.
However, the time period for the analysis, which included nearly 3000 patients, was short — only about 3 weeks (February 12 to March 3). Also, Beijing is more than 1100 kilometers from Wuhan, the center of the Chinese outbreak of COVID-19.
The Beijing cancer hospital implemented a multipronged safety plan in February in order to “avoid COVID-19 related nosocomial cross-infection between patients and medical staff,” explain the authors, led by medical oncologist Zhijie Wang, MD.
Notably, “all of the measures taken in China are actively being implemented and used in major oncology centers in the United States,” Robert Carlson, MD, chief executive officer, National Comprehensive Cancer Network (NCCN), told Medscape Medical News.
John Greene, MD, section chief, Infectious Disease and Tropical Medicine, Moffitt Cancer Center, Tampa, Florida, pointed out that the Chinese safety plan, which is full of “good measures,” is being largely used at his center. However, he observed that one tool — doing a temperature check at the hospital front door — is not well supported by most of the literature. “It gives good optics and looks like you are doing the most you possibly can, but scientifically it may not be as effective [as other screening measures],” he said.
The Chinese plan consists of four broad elements
First, the above-mentioned on-site temperature tests are performed at the entrances of the hospital, outpatient clinic, and wards. Contact and travel histories related to the Wuhan epidemic area are also established and recorded.
Second, an outpatient appointment scheduling system allows both online scheduling and on-site registration. Online consultation channels are open daily, featuring instruction on medication taking and cancer-related symptom management. These “substantially reduced the flow of people in the hospital,” write the authors. On-site patients must wear a mask and have their own disinfectant.
Third, for patients with cancer preparing to be admitted to hospital, symptoms associated with COVID-19, such as fever and cough, are recorded. Mandatory blood tests and CT scans of the lungs are performed. COVID-19 virus nucleic acid tests are performed for patients with suspected pneumonia on imaging.
Fourth, some anticancer drugs conventionally administered by infusion have been changed to oral administration, such as etoposide and vinorelbine. For adjuvant or maintenance chemotherapy, the infusion intervals were appropriately prolonged depending on patients’ conditions.
Eight out of 2,900 patients had imaging suspicious for infection
The Chinese authors report that a total of 2,944 patients with cancer were seen for clinic consultation and treatment in the wards (2795 outpatients and 149 inpatients).
Patients with cancer are believed to have a higher probability of severe illness and increased mortality compared with the healthy population once infected with COVID-19, point out the authors.
Under the new “strict screening strategy,” 27 patients showed radiologic manifestations of inflammatory changes or multiple-site exudative pneumonia in the lungs, including eight suspected of having COVID-19 infection. “Fortunately, negative results from nucleic acid testing ultimately excluded COVID-19 infection in all these patients,” the authors report.
However, two of these patients “presented with recovered pneumonia after symptomatic treatment.” Commenting on this finding, Moffitt’s Greene said that may mean these two patients were tested and found to be positive but were early in the infection and not yet shedding the virus, or they were infected after the initial negative result.
Greene said his center has implemented some measures not mentioned in the Chinese plan. For example, the Florida center no longer allows inpatient visitation. Also, one third of staff now work from home, resulting in less social interaction. Social distancing in meetings, the cafeteria, and hallways is being observed “aggressively,” and most meetings are now on Zoom, he said.
Moffitt has not been hard hit with COVID-19 and is at level one preparedness, the lowest rung. The center has performed 60 tests to date, with only one positive for the virus (< 2%), Greene told Medscape Medical News.
Currently, in the larger Tampa Bay community setting, about 12% of tests are positive.
The low percentage found among the Moffitt patients “tells you that a lot of cancer patients have fever and respiratory symptoms due to other viruses and, more importantly, other reasons, whether it’s their immunotherapy or chemotherapy or their cancer,” said Greene.
NCCN’s Carlson said the publication of the Chinese data was a good sign in terms of international science.
“This is a strong example of how the global oncology community rapidly shares information and experience whenever it makes a difference in patient care,” he commented.
The authors, as well as Carlson and Greene, have reported no relevant financial relationships.
This article first appeared on Medscape.com.
What Happens When COVID-19 Breaks Out on a Nuclear Aircraft Carrier?
Updated April 2, 2020.
The commander of a US Navy aircraft carrier in the midst of a COVID-19 outbreak was swiftly fired by Acting Secretary of the Navy Thomas Modly following media coverage of the plight of more than 200 COVID-19 positive sailors on the USS Theodore Roosevelt.
In a statement released April 2, Modly announced the removal of Capt. Brett Crozier for writing a memo that was later leaked to the San Francisco Chronicle newspaper. According to Acting Secretary Modly, the memo was sent “outside the chain of command” and his action “made his Sailors, their families, and many in the public believe that his letter was the only reason help from our larger Navy family was forthcoming, which was hardly the case.”
On Monday, March 30, Capt. Crozier, commanding officer of the nuclear aircraft carrier USS Theodore Roosevelt, sent an urgent request for assistance to senior Navy officials: “[I]n combat we are willing to take certain risks that are not acceptable in peacetime. However, we are not at war, and therefore cannot allow a single Sailor to perish as a result of this pandemic unnecessarily. Decisive action is required now in order to comply with CDC and NAVADMIN 083/20 guidance and prevent tragic outcomes.”
Even as a number of cruise ships with ill and dying passengers were—are—waiting to be allowed to dock in Florida and elsewhere, the USS Theodore Roosevelt was also dealing with a COVID-19 outbreak onboard—and awaiting permission to let the crew of more than 4,000 on shore so they could quarantine safely.
Crozier pointed to “lessons learned” from the Diamond Princess—the only comparable situation at the time. He quoted from the abstract to an epidemiological research study: An index case on board the cruise ship was reported in late January; a month later, 619 of 3,700 passengers and crew had tested positive. Without any interventions, the abstract noted, between January 21st and February 19th an estimated 2,920 of the passengers would have been infected. Isolation and quarantine, it concluded, prevented 2,307 cases. Further, an early evacuation would have been associated with 76 infected persons.
The Diamond Princess, Crozier wrote, was able to more effectively isolate people due to a higher percentage of individual and compartmentalized accommodations. However, due to a warship’s “inherent limitations of space,” his crew could not comply with orders to practice social distancing. “With the exceptions of a handful of senior officer staterooms,” he wrote, “none of the berthing onboard a warship is appropriate for quarantine or isolation.” He also pointed to other obstacles: shared bathrooms, shared sleeping quarters, group mealtimes, and ladders and other surfaces touched and possibly contaminated as crew move around the ship.
Moreover, Crozier wrote, “The spread of the disease is ongoing and accelerating.” By Tuesday March 31st, nearly 1,300 sailors had been tested, and hundreds were testing negative, but 243 sailors had tested positive and 87 more were showing symptoms, according to the latest reports. So far, none are showing serious symptoms.
“If we do not act now, we are failing to take care of our most trusted asset—our sailors,” Capt Crozier wrote. At first, no one seemed to be listening, but after the Chronicle broke the story and it began circulating in the media—things changed. “I heard about the letter from Capt. Crozier [Tuesday] morning,” said Acting Secretary Modly in an interview with the Chronicle. “I know that our command organization has been aware of this for about 24 hours and we have been working actually the last 7 days to move those sailors off the ship and get them into accommodations in Guam. The problem is that Guam doesn’t have enough beds right now and we’re having to talk to the government there to see if we can get some hotel space, create tent-type facilities.”
He noted that the situation for the USS Theodore Roosevelt is “a little bit different and unique” in that it has aircraft and armaments on it, fire hazards, and “we have to run a nuclear power plant.” Crozier had proposed that approximately 10% of the crew remain on board to take care of the duties such as tending to the nuclear reactor.
As of April 1, the Navy plans to remove some 2,700 sailors to the hotel rooms government officials on Guam have secured for them. Secretary Modly made no mention of the care or treatment of infected sailors in his April 2nd statement, but offered this reassurance: "You can offer comfort to your fellow citizens who are struggling and fearful here at home by standing the watch, and working your way through this pandemic with courage and optimism and set the example for the nation. We have an obligation to ensure you have everything you need as fast as we can get it there, and you have my commitment that we will not let you down."
Updated April 2, 2020.
The commander of a US Navy aircraft carrier in the midst of a COVID-19 outbreak was swiftly fired by Acting Secretary of the Navy Thomas Modly following media coverage of the plight of more than 200 COVID-19 positive sailors on the USS Theodore Roosevelt.
In a statement released April 2, Modly announced the removal of Capt. Brett Crozier for writing a memo that was later leaked to the San Francisco Chronicle newspaper. According to Acting Secretary Modly, the memo was sent “outside the chain of command” and his action “made his Sailors, their families, and many in the public believe that his letter was the only reason help from our larger Navy family was forthcoming, which was hardly the case.”
On Monday, March 30, Capt. Crozier, commanding officer of the nuclear aircraft carrier USS Theodore Roosevelt, sent an urgent request for assistance to senior Navy officials: “[I]n combat we are willing to take certain risks that are not acceptable in peacetime. However, we are not at war, and therefore cannot allow a single Sailor to perish as a result of this pandemic unnecessarily. Decisive action is required now in order to comply with CDC and NAVADMIN 083/20 guidance and prevent tragic outcomes.”
Even as a number of cruise ships with ill and dying passengers were—are—waiting to be allowed to dock in Florida and elsewhere, the USS Theodore Roosevelt was also dealing with a COVID-19 outbreak onboard—and awaiting permission to let the crew of more than 4,000 on shore so they could quarantine safely.
Crozier pointed to “lessons learned” from the Diamond Princess—the only comparable situation at the time. He quoted from the abstract to an epidemiological research study: An index case on board the cruise ship was reported in late January; a month later, 619 of 3,700 passengers and crew had tested positive. Without any interventions, the abstract noted, between January 21st and February 19th an estimated 2,920 of the passengers would have been infected. Isolation and quarantine, it concluded, prevented 2,307 cases. Further, an early evacuation would have been associated with 76 infected persons.
The Diamond Princess, Crozier wrote, was able to more effectively isolate people due to a higher percentage of individual and compartmentalized accommodations. However, due to a warship’s “inherent limitations of space,” his crew could not comply with orders to practice social distancing. “With the exceptions of a handful of senior officer staterooms,” he wrote, “none of the berthing onboard a warship is appropriate for quarantine or isolation.” He also pointed to other obstacles: shared bathrooms, shared sleeping quarters, group mealtimes, and ladders and other surfaces touched and possibly contaminated as crew move around the ship.
Moreover, Crozier wrote, “The spread of the disease is ongoing and accelerating.” By Tuesday March 31st, nearly 1,300 sailors had been tested, and hundreds were testing negative, but 243 sailors had tested positive and 87 more were showing symptoms, according to the latest reports. So far, none are showing serious symptoms.
“If we do not act now, we are failing to take care of our most trusted asset—our sailors,” Capt Crozier wrote. At first, no one seemed to be listening, but after the Chronicle broke the story and it began circulating in the media—things changed. “I heard about the letter from Capt. Crozier [Tuesday] morning,” said Acting Secretary Modly in an interview with the Chronicle. “I know that our command organization has been aware of this for about 24 hours and we have been working actually the last 7 days to move those sailors off the ship and get them into accommodations in Guam. The problem is that Guam doesn’t have enough beds right now and we’re having to talk to the government there to see if we can get some hotel space, create tent-type facilities.”
He noted that the situation for the USS Theodore Roosevelt is “a little bit different and unique” in that it has aircraft and armaments on it, fire hazards, and “we have to run a nuclear power plant.” Crozier had proposed that approximately 10% of the crew remain on board to take care of the duties such as tending to the nuclear reactor.
As of April 1, the Navy plans to remove some 2,700 sailors to the hotel rooms government officials on Guam have secured for them. Secretary Modly made no mention of the care or treatment of infected sailors in his April 2nd statement, but offered this reassurance: "You can offer comfort to your fellow citizens who are struggling and fearful here at home by standing the watch, and working your way through this pandemic with courage and optimism and set the example for the nation. We have an obligation to ensure you have everything you need as fast as we can get it there, and you have my commitment that we will not let you down."
Updated April 2, 2020.
The commander of a US Navy aircraft carrier in the midst of a COVID-19 outbreak was swiftly fired by Acting Secretary of the Navy Thomas Modly following media coverage of the plight of more than 200 COVID-19 positive sailors on the USS Theodore Roosevelt.
In a statement released April 2, Modly announced the removal of Capt. Brett Crozier for writing a memo that was later leaked to the San Francisco Chronicle newspaper. According to Acting Secretary Modly, the memo was sent “outside the chain of command” and his action “made his Sailors, their families, and many in the public believe that his letter was the only reason help from our larger Navy family was forthcoming, which was hardly the case.”
On Monday, March 30, Capt. Crozier, commanding officer of the nuclear aircraft carrier USS Theodore Roosevelt, sent an urgent request for assistance to senior Navy officials: “[I]n combat we are willing to take certain risks that are not acceptable in peacetime. However, we are not at war, and therefore cannot allow a single Sailor to perish as a result of this pandemic unnecessarily. Decisive action is required now in order to comply with CDC and NAVADMIN 083/20 guidance and prevent tragic outcomes.”
Even as a number of cruise ships with ill and dying passengers were—are—waiting to be allowed to dock in Florida and elsewhere, the USS Theodore Roosevelt was also dealing with a COVID-19 outbreak onboard—and awaiting permission to let the crew of more than 4,000 on shore so they could quarantine safely.
Crozier pointed to “lessons learned” from the Diamond Princess—the only comparable situation at the time. He quoted from the abstract to an epidemiological research study: An index case on board the cruise ship was reported in late January; a month later, 619 of 3,700 passengers and crew had tested positive. Without any interventions, the abstract noted, between January 21st and February 19th an estimated 2,920 of the passengers would have been infected. Isolation and quarantine, it concluded, prevented 2,307 cases. Further, an early evacuation would have been associated with 76 infected persons.
The Diamond Princess, Crozier wrote, was able to more effectively isolate people due to a higher percentage of individual and compartmentalized accommodations. However, due to a warship’s “inherent limitations of space,” his crew could not comply with orders to practice social distancing. “With the exceptions of a handful of senior officer staterooms,” he wrote, “none of the berthing onboard a warship is appropriate for quarantine or isolation.” He also pointed to other obstacles: shared bathrooms, shared sleeping quarters, group mealtimes, and ladders and other surfaces touched and possibly contaminated as crew move around the ship.
Moreover, Crozier wrote, “The spread of the disease is ongoing and accelerating.” By Tuesday March 31st, nearly 1,300 sailors had been tested, and hundreds were testing negative, but 243 sailors had tested positive and 87 more were showing symptoms, according to the latest reports. So far, none are showing serious symptoms.
“If we do not act now, we are failing to take care of our most trusted asset—our sailors,” Capt Crozier wrote. At first, no one seemed to be listening, but after the Chronicle broke the story and it began circulating in the media—things changed. “I heard about the letter from Capt. Crozier [Tuesday] morning,” said Acting Secretary Modly in an interview with the Chronicle. “I know that our command organization has been aware of this for about 24 hours and we have been working actually the last 7 days to move those sailors off the ship and get them into accommodations in Guam. The problem is that Guam doesn’t have enough beds right now and we’re having to talk to the government there to see if we can get some hotel space, create tent-type facilities.”
He noted that the situation for the USS Theodore Roosevelt is “a little bit different and unique” in that it has aircraft and armaments on it, fire hazards, and “we have to run a nuclear power plant.” Crozier had proposed that approximately 10% of the crew remain on board to take care of the duties such as tending to the nuclear reactor.
As of April 1, the Navy plans to remove some 2,700 sailors to the hotel rooms government officials on Guam have secured for them. Secretary Modly made no mention of the care or treatment of infected sailors in his April 2nd statement, but offered this reassurance: "You can offer comfort to your fellow citizens who are struggling and fearful here at home by standing the watch, and working your way through this pandemic with courage and optimism and set the example for the nation. We have an obligation to ensure you have everything you need as fast as we can get it there, and you have my commitment that we will not let you down."
Comorbidities more common in hospitalized COVID-19 patients
Greater prevalence of underlying health conditions such as diabetes and chronic lung disease was seen among nearly 7,200 Americans hospitalized with coronavirus disease 2019 (COVID-19), according to the Centers for Disease Control and Prevention.
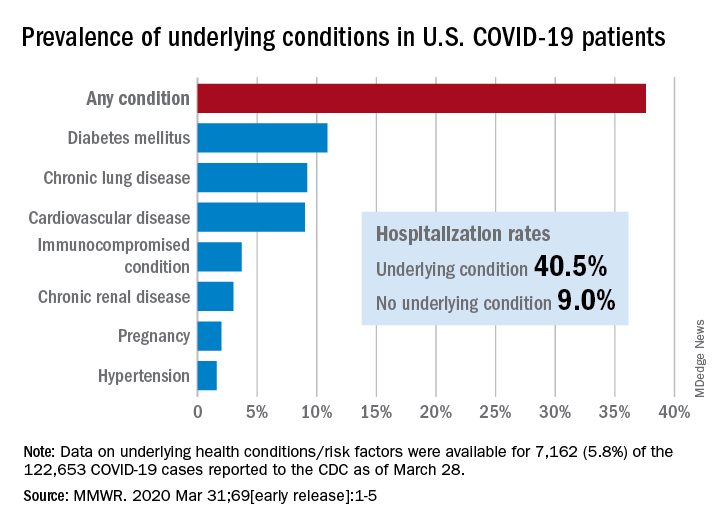
Of the 122,653 laboratory-confirmed COVID-19 cases reported to the CDC as of March 28, the COVID-19 Response Team had access to data on the presence or absence of underlying health conditions and other recognized risk factors for severe outcomes from respiratory infections for 7,162 (5.8%) patients.
“Among these patients, higher percentages of patients with underlying conditions were admitted to the hospital and to an ICU than patients without reported underlying conditions. These results are consistent with findings from China and Italy,” Katherine Fleming-Dutra, MD, and associates said in the MMWR.
Individuals with underlying health conditions/risk factors made up 37.6% of all COVID-19 patients in the study but represented a majority of ICU (78%) and non-ICU (71%) hospital admissions. In contrast, 73% of COVID-19 patients who were not hospitalized had no underlying conditions, Dr. Fleming-Dutra and the CDC COVID-19 Response Team reported.
With a prevalence of 10.9%, diabetes mellitus was the most common condition reported among all COVID-19 patients, followed by chronic lung disease (9.2%) and cardiovascular disease (9.0%), the investigators said.
Another look at the data shows that 40.5% of those with underlying conditions were hospitalized, compared with 9.0% of the 4,470 COVID-19 patients without any risk factors.
“Strategies to protect all persons and especially those with underlying health conditions, including social distancing and handwashing, should be implemented by all communities and all persons to help slow the spread of COVID-19,” the response team wrote.
SOURCE: Fleming-Dutra K et al. MMWR. 2020 Mar 31;69 (early release):1-5.
Greater prevalence of underlying health conditions such as diabetes and chronic lung disease was seen among nearly 7,200 Americans hospitalized with coronavirus disease 2019 (COVID-19), according to the Centers for Disease Control and Prevention.

Of the 122,653 laboratory-confirmed COVID-19 cases reported to the CDC as of March 28, the COVID-19 Response Team had access to data on the presence or absence of underlying health conditions and other recognized risk factors for severe outcomes from respiratory infections for 7,162 (5.8%) patients.
“Among these patients, higher percentages of patients with underlying conditions were admitted to the hospital and to an ICU than patients without reported underlying conditions. These results are consistent with findings from China and Italy,” Katherine Fleming-Dutra, MD, and associates said in the MMWR.
Individuals with underlying health conditions/risk factors made up 37.6% of all COVID-19 patients in the study but represented a majority of ICU (78%) and non-ICU (71%) hospital admissions. In contrast, 73% of COVID-19 patients who were not hospitalized had no underlying conditions, Dr. Fleming-Dutra and the CDC COVID-19 Response Team reported.
With a prevalence of 10.9%, diabetes mellitus was the most common condition reported among all COVID-19 patients, followed by chronic lung disease (9.2%) and cardiovascular disease (9.0%), the investigators said.
Another look at the data shows that 40.5% of those with underlying conditions were hospitalized, compared with 9.0% of the 4,470 COVID-19 patients without any risk factors.
“Strategies to protect all persons and especially those with underlying health conditions, including social distancing and handwashing, should be implemented by all communities and all persons to help slow the spread of COVID-19,” the response team wrote.
SOURCE: Fleming-Dutra K et al. MMWR. 2020 Mar 31;69 (early release):1-5.
Greater prevalence of underlying health conditions such as diabetes and chronic lung disease was seen among nearly 7,200 Americans hospitalized with coronavirus disease 2019 (COVID-19), according to the Centers for Disease Control and Prevention.

Of the 122,653 laboratory-confirmed COVID-19 cases reported to the CDC as of March 28, the COVID-19 Response Team had access to data on the presence or absence of underlying health conditions and other recognized risk factors for severe outcomes from respiratory infections for 7,162 (5.8%) patients.
“Among these patients, higher percentages of patients with underlying conditions were admitted to the hospital and to an ICU than patients without reported underlying conditions. These results are consistent with findings from China and Italy,” Katherine Fleming-Dutra, MD, and associates said in the MMWR.
Individuals with underlying health conditions/risk factors made up 37.6% of all COVID-19 patients in the study but represented a majority of ICU (78%) and non-ICU (71%) hospital admissions. In contrast, 73% of COVID-19 patients who were not hospitalized had no underlying conditions, Dr. Fleming-Dutra and the CDC COVID-19 Response Team reported.
With a prevalence of 10.9%, diabetes mellitus was the most common condition reported among all COVID-19 patients, followed by chronic lung disease (9.2%) and cardiovascular disease (9.0%), the investigators said.
Another look at the data shows that 40.5% of those with underlying conditions were hospitalized, compared with 9.0% of the 4,470 COVID-19 patients without any risk factors.
“Strategies to protect all persons and especially those with underlying health conditions, including social distancing and handwashing, should be implemented by all communities and all persons to help slow the spread of COVID-19,” the response team wrote.
SOURCE: Fleming-Dutra K et al. MMWR. 2020 Mar 31;69 (early release):1-5.
FROM MMWR
SARS serum neutralizing antibodies may inform the treatment of COVID-19
The immune responses of specific antibodies were maintained in more than 90% of recovered SARS-CoV patients for 2 years, raising the likelihood that the similarly behaving SARS-CoV-2 might provoke the same response, according to an online communication published in the Journal of Microbiology, Immunology and Infection.
The authors cited a cohort study of convalescent SARS-CoV patients (56 cases, from the Beijing hospital of the Armed Forces Police, China) that showed that specific IgG antibodies and neutralizing antibodies were highly correlated, peaking at month 4 after the onset of disease and decreasing gradually thereafter.
This and other studies suggest that the immune responses of specific antibodies were maintained in more than 90% of recovered SARS-CoV patients for 2 years, according to the authors.
However, of particular concern is the fact that only 11.8% of patients acquire specific SARS-CoV Abs in the early period after recovery at day 7, not reaching 100% until day 90, which highlights the importance of the detection of antibody titers for convalescent COVID-19 patients, according to the authors. “Otherwise, these patients with low titers of antibodies may not be efficient for the clearance of SARS-CoV-2.”
The authors also cited a recent study that showed how neutralizing antibody from a convalescent SARS patient could block the SARS-CoV-2 from entering into target cells in vitro, and suggested that previous experimental SARS-CoV vaccines and neutralizing antibodies could provide novel preventive and therapeutic options for COVID-19.
“These experiences from SARS-CoV are expected to have some implications for the treatment, management and surveillance of SARS-CoV-2 patients,” the authors concluded.
SOURCE: Lin Q et al. J Microbiol Immunol Infect. 2020 Mar 25. https://doi.org/10.1016/j.jmii.2020.03.015.
The immune responses of specific antibodies were maintained in more than 90% of recovered SARS-CoV patients for 2 years, raising the likelihood that the similarly behaving SARS-CoV-2 might provoke the same response, according to an online communication published in the Journal of Microbiology, Immunology and Infection.
The authors cited a cohort study of convalescent SARS-CoV patients (56 cases, from the Beijing hospital of the Armed Forces Police, China) that showed that specific IgG antibodies and neutralizing antibodies were highly correlated, peaking at month 4 after the onset of disease and decreasing gradually thereafter.
This and other studies suggest that the immune responses of specific antibodies were maintained in more than 90% of recovered SARS-CoV patients for 2 years, according to the authors.
However, of particular concern is the fact that only 11.8% of patients acquire specific SARS-CoV Abs in the early period after recovery at day 7, not reaching 100% until day 90, which highlights the importance of the detection of antibody titers for convalescent COVID-19 patients, according to the authors. “Otherwise, these patients with low titers of antibodies may not be efficient for the clearance of SARS-CoV-2.”
The authors also cited a recent study that showed how neutralizing antibody from a convalescent SARS patient could block the SARS-CoV-2 from entering into target cells in vitro, and suggested that previous experimental SARS-CoV vaccines and neutralizing antibodies could provide novel preventive and therapeutic options for COVID-19.
“These experiences from SARS-CoV are expected to have some implications for the treatment, management and surveillance of SARS-CoV-2 patients,” the authors concluded.
SOURCE: Lin Q et al. J Microbiol Immunol Infect. 2020 Mar 25. https://doi.org/10.1016/j.jmii.2020.03.015.
The immune responses of specific antibodies were maintained in more than 90% of recovered SARS-CoV patients for 2 years, raising the likelihood that the similarly behaving SARS-CoV-2 might provoke the same response, according to an online communication published in the Journal of Microbiology, Immunology and Infection.
The authors cited a cohort study of convalescent SARS-CoV patients (56 cases, from the Beijing hospital of the Armed Forces Police, China) that showed that specific IgG antibodies and neutralizing antibodies were highly correlated, peaking at month 4 after the onset of disease and decreasing gradually thereafter.
This and other studies suggest that the immune responses of specific antibodies were maintained in more than 90% of recovered SARS-CoV patients for 2 years, according to the authors.
However, of particular concern is the fact that only 11.8% of patients acquire specific SARS-CoV Abs in the early period after recovery at day 7, not reaching 100% until day 90, which highlights the importance of the detection of antibody titers for convalescent COVID-19 patients, according to the authors. “Otherwise, these patients with low titers of antibodies may not be efficient for the clearance of SARS-CoV-2.”
The authors also cited a recent study that showed how neutralizing antibody from a convalescent SARS patient could block the SARS-CoV-2 from entering into target cells in vitro, and suggested that previous experimental SARS-CoV vaccines and neutralizing antibodies could provide novel preventive and therapeutic options for COVID-19.
“These experiences from SARS-CoV are expected to have some implications for the treatment, management and surveillance of SARS-CoV-2 patients,” the authors concluded.
SOURCE: Lin Q et al. J Microbiol Immunol Infect. 2020 Mar 25. https://doi.org/10.1016/j.jmii.2020.03.015.
FROM THE JOURNAL OF MICROBIOLOGY, IMMUNOLOGY AND INFECTION
COVID-19: More hydroxychloroquine data from France, more questions
A controversial study led by Didier Raoult, MD, PhD, on the combination of hydroxychloroquine and azithromycin in patients with COVID-19 was published March 20. The latest results from the same Marseille team, which involve 80 patients, were reported on March 27.
The investigators report a significant reduction in the viral load (83% patients had negative results on quantitative polymerase chain reaction testing at day 7, and 93% had negative results on day 8). There was a “clinical improvement compared to the natural progression.” One death occurred, and three patients were transferred to intensive care units.
If the data seem encouraging, the lack of a control arm in the study leaves clinicians perplexed, however.
Benjamin Davido, MD, an infectious disease specialist at Raymond-Poincaré Hospital in Garches, Paris, spoke in an interview about the implications of these new results.
What do you think about the new results presented by Prof. Raoult’s team? Do they confirm the effectiveness of hydroxychloroquine?
These results are complementary [to the original results] but don’t offer any new information or new statistical evidence. They are absolutely superimposable and say overall that, between 5 and 7 days [of treatment], very few patients shed the virus. But that is not the question that everyone is asking.
Even if we don’t necessarily have to conduct a randomized study, we should at least compare the treatment, either against another therapy – which could be hydroxychloroquine monotherapy, or just standard of care. It needed an authentic control arm.
To recruit 80 patients so quickly, the researchers probably took people with essentially ambulatory forms of the disease (there was a call for screening in the south of France) – therefore, by definition, less severe cases.
But to describe such a population of patients as going home and saying, “There were very few hospitalizations and it is going well,” does not in any way prove that the treatment reduces hospitalizations.
The argument for not having a control arm in this study was that it would be unethical. What do you think?
I agree with this argument when it comes to patients presenting with risk factors or who are starting to develop pneumonia.
But I don’t think this is the case at the beginning of the illness. Of course, you don’t want to wait to have severe disease or for the patient to be in intensive care to start treatment. In these cases, it is indeed very difficult to find a control arm.
In the ongoing Discovery trial, which involves more than 3,000 patients in Europe, including 800 in France, the patients have severe disease, and there are five treatment arms. Moreover, hydroxychloroquine is given without azithromycin. What do you think of this?
I think it’s a mistake. It will not answer the question of the effectiveness of hydroxychloroquine in COVID-19, especially as they’re not studying azithromycin in a situation where the compound seems necessary for the effectiveness of the treatment.
In addition, Discovery reinforces the notion of studying Kaletra [lopinavir/ritonavir, AbbVie] again, while Chinese researchers have shown that it does not work, the argument being that Kaletra was given too late (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282). Therefore, if we make the same mistakes from a methodological point of view, we will end up with negative results.
What should have been done in the Marseille study?
The question is: Are there more or fewer hospitalizations when we treat a homogeneous population straight away?
The answer could be very clear, as a control already exists! They are the patients that flow into our hospitals every day – ironically, these 80 patients [in the latest results, presented March 27] could be among the 80% who had a form similar to nasopharyngitis and resolved.
In this illness, we know that there are 80% spontaneous recoveries and 20% so-called severe forms. Therefore, with 80 patients, we are very underpowered. The cohort is too small for a disease in which 80% of the evolution is benign.
It would take 1,000 patients, and then, even without a control arm, we would have an answer.
On March 26, Didier Raoult’s team also announced having already treated 700 patients with hydroxychloroquine, with only one death. Therefore, if this cohort increases significantly in Marseille and we see that, on the map, there are fewer issues with patient flow and saturation in Marseille and that there are fewer patients in intensive care, you will have to wonder about the effect of hydroxychloroquine.
We will find out very quickly. If it really works, and they treat all the patients presenting at Timone Hospital, we will soon have the answer. It will be a real-life study.
What are the other studies on hydroxychloroquine that could give us answers?
There was a Chinese study that did not show a difference in effectiveness between hydroxychloroquine and placebo, but that was, again, conducted in only around 20 patients (J Zhejiang Univ (Med Sci). 2020. doi: 10.3785/j.issn.1008-9292.2020.03.03). This cohort is too small and tells us nothing; it cannot show anything. We must wait for the results of larger trials being conducted in China.
It surprises me that, today, we still do not have Italian data on the use of chloroquine-type drugs ... perhaps because they have a care pathway that means there is no outpatient treatment and that they arrive already with severe disease. The Italian recommendations nevertheless indicate the use of hydroxychloroquine.
I also wonder about the lack of studies of cohorts where, in retrospect, we could have followed people previously treated with hydroxychloroquine for chronic diseases (e.g., rheumatoid arthritis, lupus, etc.). Or we could identify all those patients on the health insurance system who had prescriptions.
That is how we discovered the AIDS epidemic in San Francisco: There was an increase in the number of prescriptions for trimethoprim/sulfamethoxazole (Bactrim) that corresponded to a population subtype (homosexual), and we realized that it was for a disease that resembled pneumocystosis. We discovered that via the drug!
If hydroxychloroquine is effective, it is enough to look at people who took it before the epidemic and see how they fared. And there, we do not need a control arm. This could give us some direction. The March 26 decree of the new Véran Law states that community pharmacies can dispense to patients with a previous prescription, so we can find these individuals.
Do you think that the lack of, or difficulty in setting up, studies on hydroxychloroquine in France is linked to decisions that are more political than scientific?
Perhaps the contaminated blood scandal still casts a shadow in France, and there is a great deal of anxiety over the fact that we are already in a crisis, and we do not want a second one. I can understand that.
However, just a week ago, access to this drug (and others with market approval that have been on the market for several years) was blocked in hospital central pharmacies, while we are the medical specialists with the authorization! It was unacceptable.
It was sorted out 48 hours ago: hydroxychloroquine is now available in the hospital, and to my knowledge, we no longer have a problem obtaining it.
It took time to alleviate doubts over the major health risks with this drug. [Officials] seemed almost like amateurs in their hesitation; I think they lacked foresight. We have forgotten that the treatment advocated by Prof. Didier Raoult is not chloroquine but rather hydroxychloroquine, and we know that the adverse effects are less [with hydroxychloroquine] than with chloroquine.
You yourself have treated patients with chloroquine, despite the risk for toxicity highlighted by some.
Initially, when we first started treating patients, we thought of chloroquine because we did not have data on hydroxychloroquine, only Chinese data with chloroquine. We therefore prescribed chloroquine several days before prescribing hydroxychloroquine.
The question of the toxicity of chloroquine was not unjustified, but I think we took far too much time to decide on the toxicity of hydroxychloroquine. Is [the latter] political? I don’t know. It was widely publicized, which amazes me for a drug that is already available.
On the other hand, everyone was talking at the same time about the toxicity of NSAIDs. ... One has the impression it was to create a diversion. I think there were double standards at play and a scapegoat was needed to gain some time and ask questions.
What is sure is that it is probably not for financial reasons, as hydroxychloroquine costs nothing. That’s to say there were probably pharmaceutical issues at stake for possible competitors of hydroxychloroquine; I do not want to get into this debate, and it doesn’t matter, as long as we have an answer.
Today, the only thing we have advanced on is the “safety” of hydroxychloroquine, the low risk to the general population. ... On the other hand, we have still not made any progress on the evidence of efficacy, compared with other treatments.
Personally, I really believe in hydroxychloroquine. It would nevertheless be a shame to think we had found the fountain of youth and realize, in 4 weeks, that we have the same number of deaths. That is the problem. I hope that we will soon have solid data so we do not waste time focusing solely on hydroxychloroquine.
What are the other avenues of research that grab your attention?
The Discovery trial will probably give an answer on remdesivir [GS-5734, Gilead], which is a direct antiviral and could be interesting. But there are other studies being conducted currently in China.
There is also favipiravir [T-705, Avigan, Toyama Chemical], which is an anti-influenza drug used in Japan, which could explain, in part, the control of the epidemic in that country. There are effects in vitro on coronavirus. But it is not at all studied in France at the moment. Therefore, we should not focus exclusively on hydroxychloroquine; we must keep a close eye on other molecules, in particular the “old” drugs, like this antiviral.
The study was supported by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, the National Research Agency, under the Investissements d’avenir program, Région Provence Alpes Côte d’Azur, and European funding FEDER PRIMI. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A controversial study led by Didier Raoult, MD, PhD, on the combination of hydroxychloroquine and azithromycin in patients with COVID-19 was published March 20. The latest results from the same Marseille team, which involve 80 patients, were reported on March 27.
The investigators report a significant reduction in the viral load (83% patients had negative results on quantitative polymerase chain reaction testing at day 7, and 93% had negative results on day 8). There was a “clinical improvement compared to the natural progression.” One death occurred, and three patients were transferred to intensive care units.
If the data seem encouraging, the lack of a control arm in the study leaves clinicians perplexed, however.
Benjamin Davido, MD, an infectious disease specialist at Raymond-Poincaré Hospital in Garches, Paris, spoke in an interview about the implications of these new results.
What do you think about the new results presented by Prof. Raoult’s team? Do they confirm the effectiveness of hydroxychloroquine?
These results are complementary [to the original results] but don’t offer any new information or new statistical evidence. They are absolutely superimposable and say overall that, between 5 and 7 days [of treatment], very few patients shed the virus. But that is not the question that everyone is asking.
Even if we don’t necessarily have to conduct a randomized study, we should at least compare the treatment, either against another therapy – which could be hydroxychloroquine monotherapy, or just standard of care. It needed an authentic control arm.
To recruit 80 patients so quickly, the researchers probably took people with essentially ambulatory forms of the disease (there was a call for screening in the south of France) – therefore, by definition, less severe cases.
But to describe such a population of patients as going home and saying, “There were very few hospitalizations and it is going well,” does not in any way prove that the treatment reduces hospitalizations.
The argument for not having a control arm in this study was that it would be unethical. What do you think?
I agree with this argument when it comes to patients presenting with risk factors or who are starting to develop pneumonia.
But I don’t think this is the case at the beginning of the illness. Of course, you don’t want to wait to have severe disease or for the patient to be in intensive care to start treatment. In these cases, it is indeed very difficult to find a control arm.
In the ongoing Discovery trial, which involves more than 3,000 patients in Europe, including 800 in France, the patients have severe disease, and there are five treatment arms. Moreover, hydroxychloroquine is given without azithromycin. What do you think of this?
I think it’s a mistake. It will not answer the question of the effectiveness of hydroxychloroquine in COVID-19, especially as they’re not studying azithromycin in a situation where the compound seems necessary for the effectiveness of the treatment.
In addition, Discovery reinforces the notion of studying Kaletra [lopinavir/ritonavir, AbbVie] again, while Chinese researchers have shown that it does not work, the argument being that Kaletra was given too late (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282). Therefore, if we make the same mistakes from a methodological point of view, we will end up with negative results.
What should have been done in the Marseille study?
The question is: Are there more or fewer hospitalizations when we treat a homogeneous population straight away?
The answer could be very clear, as a control already exists! They are the patients that flow into our hospitals every day – ironically, these 80 patients [in the latest results, presented March 27] could be among the 80% who had a form similar to nasopharyngitis and resolved.
In this illness, we know that there are 80% spontaneous recoveries and 20% so-called severe forms. Therefore, with 80 patients, we are very underpowered. The cohort is too small for a disease in which 80% of the evolution is benign.
It would take 1,000 patients, and then, even without a control arm, we would have an answer.
On March 26, Didier Raoult’s team also announced having already treated 700 patients with hydroxychloroquine, with only one death. Therefore, if this cohort increases significantly in Marseille and we see that, on the map, there are fewer issues with patient flow and saturation in Marseille and that there are fewer patients in intensive care, you will have to wonder about the effect of hydroxychloroquine.
We will find out very quickly. If it really works, and they treat all the patients presenting at Timone Hospital, we will soon have the answer. It will be a real-life study.
What are the other studies on hydroxychloroquine that could give us answers?
There was a Chinese study that did not show a difference in effectiveness between hydroxychloroquine and placebo, but that was, again, conducted in only around 20 patients (J Zhejiang Univ (Med Sci). 2020. doi: 10.3785/j.issn.1008-9292.2020.03.03). This cohort is too small and tells us nothing; it cannot show anything. We must wait for the results of larger trials being conducted in China.
It surprises me that, today, we still do not have Italian data on the use of chloroquine-type drugs ... perhaps because they have a care pathway that means there is no outpatient treatment and that they arrive already with severe disease. The Italian recommendations nevertheless indicate the use of hydroxychloroquine.
I also wonder about the lack of studies of cohorts where, in retrospect, we could have followed people previously treated with hydroxychloroquine for chronic diseases (e.g., rheumatoid arthritis, lupus, etc.). Or we could identify all those patients on the health insurance system who had prescriptions.
That is how we discovered the AIDS epidemic in San Francisco: There was an increase in the number of prescriptions for trimethoprim/sulfamethoxazole (Bactrim) that corresponded to a population subtype (homosexual), and we realized that it was for a disease that resembled pneumocystosis. We discovered that via the drug!
If hydroxychloroquine is effective, it is enough to look at people who took it before the epidemic and see how they fared. And there, we do not need a control arm. This could give us some direction. The March 26 decree of the new Véran Law states that community pharmacies can dispense to patients with a previous prescription, so we can find these individuals.
Do you think that the lack of, or difficulty in setting up, studies on hydroxychloroquine in France is linked to decisions that are more political than scientific?
Perhaps the contaminated blood scandal still casts a shadow in France, and there is a great deal of anxiety over the fact that we are already in a crisis, and we do not want a second one. I can understand that.
However, just a week ago, access to this drug (and others with market approval that have been on the market for several years) was blocked in hospital central pharmacies, while we are the medical specialists with the authorization! It was unacceptable.
It was sorted out 48 hours ago: hydroxychloroquine is now available in the hospital, and to my knowledge, we no longer have a problem obtaining it.
It took time to alleviate doubts over the major health risks with this drug. [Officials] seemed almost like amateurs in their hesitation; I think they lacked foresight. We have forgotten that the treatment advocated by Prof. Didier Raoult is not chloroquine but rather hydroxychloroquine, and we know that the adverse effects are less [with hydroxychloroquine] than with chloroquine.
You yourself have treated patients with chloroquine, despite the risk for toxicity highlighted by some.
Initially, when we first started treating patients, we thought of chloroquine because we did not have data on hydroxychloroquine, only Chinese data with chloroquine. We therefore prescribed chloroquine several days before prescribing hydroxychloroquine.
The question of the toxicity of chloroquine was not unjustified, but I think we took far too much time to decide on the toxicity of hydroxychloroquine. Is [the latter] political? I don’t know. It was widely publicized, which amazes me for a drug that is already available.
On the other hand, everyone was talking at the same time about the toxicity of NSAIDs. ... One has the impression it was to create a diversion. I think there were double standards at play and a scapegoat was needed to gain some time and ask questions.
What is sure is that it is probably not for financial reasons, as hydroxychloroquine costs nothing. That’s to say there were probably pharmaceutical issues at stake for possible competitors of hydroxychloroquine; I do not want to get into this debate, and it doesn’t matter, as long as we have an answer.
Today, the only thing we have advanced on is the “safety” of hydroxychloroquine, the low risk to the general population. ... On the other hand, we have still not made any progress on the evidence of efficacy, compared with other treatments.
Personally, I really believe in hydroxychloroquine. It would nevertheless be a shame to think we had found the fountain of youth and realize, in 4 weeks, that we have the same number of deaths. That is the problem. I hope that we will soon have solid data so we do not waste time focusing solely on hydroxychloroquine.
What are the other avenues of research that grab your attention?
The Discovery trial will probably give an answer on remdesivir [GS-5734, Gilead], which is a direct antiviral and could be interesting. But there are other studies being conducted currently in China.
There is also favipiravir [T-705, Avigan, Toyama Chemical], which is an anti-influenza drug used in Japan, which could explain, in part, the control of the epidemic in that country. There are effects in vitro on coronavirus. But it is not at all studied in France at the moment. Therefore, we should not focus exclusively on hydroxychloroquine; we must keep a close eye on other molecules, in particular the “old” drugs, like this antiviral.
The study was supported by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, the National Research Agency, under the Investissements d’avenir program, Région Provence Alpes Côte d’Azur, and European funding FEDER PRIMI. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A controversial study led by Didier Raoult, MD, PhD, on the combination of hydroxychloroquine and azithromycin in patients with COVID-19 was published March 20. The latest results from the same Marseille team, which involve 80 patients, were reported on March 27.
The investigators report a significant reduction in the viral load (83% patients had negative results on quantitative polymerase chain reaction testing at day 7, and 93% had negative results on day 8). There was a “clinical improvement compared to the natural progression.” One death occurred, and three patients were transferred to intensive care units.
If the data seem encouraging, the lack of a control arm in the study leaves clinicians perplexed, however.
Benjamin Davido, MD, an infectious disease specialist at Raymond-Poincaré Hospital in Garches, Paris, spoke in an interview about the implications of these new results.
What do you think about the new results presented by Prof. Raoult’s team? Do they confirm the effectiveness of hydroxychloroquine?
These results are complementary [to the original results] but don’t offer any new information or new statistical evidence. They are absolutely superimposable and say overall that, between 5 and 7 days [of treatment], very few patients shed the virus. But that is not the question that everyone is asking.
Even if we don’t necessarily have to conduct a randomized study, we should at least compare the treatment, either against another therapy – which could be hydroxychloroquine monotherapy, or just standard of care. It needed an authentic control arm.
To recruit 80 patients so quickly, the researchers probably took people with essentially ambulatory forms of the disease (there was a call for screening in the south of France) – therefore, by definition, less severe cases.
But to describe such a population of patients as going home and saying, “There were very few hospitalizations and it is going well,” does not in any way prove that the treatment reduces hospitalizations.
The argument for not having a control arm in this study was that it would be unethical. What do you think?
I agree with this argument when it comes to patients presenting with risk factors or who are starting to develop pneumonia.
But I don’t think this is the case at the beginning of the illness. Of course, you don’t want to wait to have severe disease or for the patient to be in intensive care to start treatment. In these cases, it is indeed very difficult to find a control arm.
In the ongoing Discovery trial, which involves more than 3,000 patients in Europe, including 800 in France, the patients have severe disease, and there are five treatment arms. Moreover, hydroxychloroquine is given without azithromycin. What do you think of this?
I think it’s a mistake. It will not answer the question of the effectiveness of hydroxychloroquine in COVID-19, especially as they’re not studying azithromycin in a situation where the compound seems necessary for the effectiveness of the treatment.
In addition, Discovery reinforces the notion of studying Kaletra [lopinavir/ritonavir, AbbVie] again, while Chinese researchers have shown that it does not work, the argument being that Kaletra was given too late (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282). Therefore, if we make the same mistakes from a methodological point of view, we will end up with negative results.
What should have been done in the Marseille study?
The question is: Are there more or fewer hospitalizations when we treat a homogeneous population straight away?
The answer could be very clear, as a control already exists! They are the patients that flow into our hospitals every day – ironically, these 80 patients [in the latest results, presented March 27] could be among the 80% who had a form similar to nasopharyngitis and resolved.
In this illness, we know that there are 80% spontaneous recoveries and 20% so-called severe forms. Therefore, with 80 patients, we are very underpowered. The cohort is too small for a disease in which 80% of the evolution is benign.
It would take 1,000 patients, and then, even without a control arm, we would have an answer.
On March 26, Didier Raoult’s team also announced having already treated 700 patients with hydroxychloroquine, with only one death. Therefore, if this cohort increases significantly in Marseille and we see that, on the map, there are fewer issues with patient flow and saturation in Marseille and that there are fewer patients in intensive care, you will have to wonder about the effect of hydroxychloroquine.
We will find out very quickly. If it really works, and they treat all the patients presenting at Timone Hospital, we will soon have the answer. It will be a real-life study.
What are the other studies on hydroxychloroquine that could give us answers?
There was a Chinese study that did not show a difference in effectiveness between hydroxychloroquine and placebo, but that was, again, conducted in only around 20 patients (J Zhejiang Univ (Med Sci). 2020. doi: 10.3785/j.issn.1008-9292.2020.03.03). This cohort is too small and tells us nothing; it cannot show anything. We must wait for the results of larger trials being conducted in China.
It surprises me that, today, we still do not have Italian data on the use of chloroquine-type drugs ... perhaps because they have a care pathway that means there is no outpatient treatment and that they arrive already with severe disease. The Italian recommendations nevertheless indicate the use of hydroxychloroquine.
I also wonder about the lack of studies of cohorts where, in retrospect, we could have followed people previously treated with hydroxychloroquine for chronic diseases (e.g., rheumatoid arthritis, lupus, etc.). Or we could identify all those patients on the health insurance system who had prescriptions.
That is how we discovered the AIDS epidemic in San Francisco: There was an increase in the number of prescriptions for trimethoprim/sulfamethoxazole (Bactrim) that corresponded to a population subtype (homosexual), and we realized that it was for a disease that resembled pneumocystosis. We discovered that via the drug!
If hydroxychloroquine is effective, it is enough to look at people who took it before the epidemic and see how they fared. And there, we do not need a control arm. This could give us some direction. The March 26 decree of the new Véran Law states that community pharmacies can dispense to patients with a previous prescription, so we can find these individuals.
Do you think that the lack of, or difficulty in setting up, studies on hydroxychloroquine in France is linked to decisions that are more political than scientific?
Perhaps the contaminated blood scandal still casts a shadow in France, and there is a great deal of anxiety over the fact that we are already in a crisis, and we do not want a second one. I can understand that.
However, just a week ago, access to this drug (and others with market approval that have been on the market for several years) was blocked in hospital central pharmacies, while we are the medical specialists with the authorization! It was unacceptable.
It was sorted out 48 hours ago: hydroxychloroquine is now available in the hospital, and to my knowledge, we no longer have a problem obtaining it.
It took time to alleviate doubts over the major health risks with this drug. [Officials] seemed almost like amateurs in their hesitation; I think they lacked foresight. We have forgotten that the treatment advocated by Prof. Didier Raoult is not chloroquine but rather hydroxychloroquine, and we know that the adverse effects are less [with hydroxychloroquine] than with chloroquine.
You yourself have treated patients with chloroquine, despite the risk for toxicity highlighted by some.
Initially, when we first started treating patients, we thought of chloroquine because we did not have data on hydroxychloroquine, only Chinese data with chloroquine. We therefore prescribed chloroquine several days before prescribing hydroxychloroquine.
The question of the toxicity of chloroquine was not unjustified, but I think we took far too much time to decide on the toxicity of hydroxychloroquine. Is [the latter] political? I don’t know. It was widely publicized, which amazes me for a drug that is already available.
On the other hand, everyone was talking at the same time about the toxicity of NSAIDs. ... One has the impression it was to create a diversion. I think there were double standards at play and a scapegoat was needed to gain some time and ask questions.
What is sure is that it is probably not for financial reasons, as hydroxychloroquine costs nothing. That’s to say there were probably pharmaceutical issues at stake for possible competitors of hydroxychloroquine; I do not want to get into this debate, and it doesn’t matter, as long as we have an answer.
Today, the only thing we have advanced on is the “safety” of hydroxychloroquine, the low risk to the general population. ... On the other hand, we have still not made any progress on the evidence of efficacy, compared with other treatments.
Personally, I really believe in hydroxychloroquine. It would nevertheless be a shame to think we had found the fountain of youth and realize, in 4 weeks, that we have the same number of deaths. That is the problem. I hope that we will soon have solid data so we do not waste time focusing solely on hydroxychloroquine.
What are the other avenues of research that grab your attention?
The Discovery trial will probably give an answer on remdesivir [GS-5734, Gilead], which is a direct antiviral and could be interesting. But there are other studies being conducted currently in China.
There is also favipiravir [T-705, Avigan, Toyama Chemical], which is an anti-influenza drug used in Japan, which could explain, in part, the control of the epidemic in that country. There are effects in vitro on coronavirus. But it is not at all studied in France at the moment. Therefore, we should not focus exclusively on hydroxychloroquine; we must keep a close eye on other molecules, in particular the “old” drugs, like this antiviral.
The study was supported by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, the National Research Agency, under the Investissements d’avenir program, Région Provence Alpes Côte d’Azur, and European funding FEDER PRIMI. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.






