User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
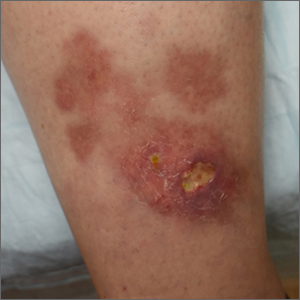
Ulcerated lower leg lesion
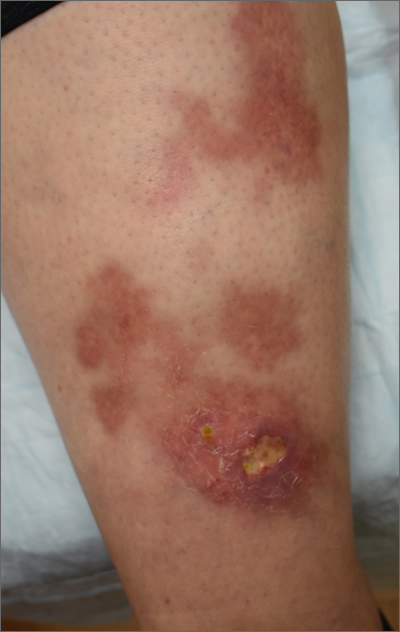
The patient’s atrophic plaques with a violaceous rim, indurated borders, and ulceration on the anterior pretibial surface were consistent with ulcerated necrobiosis lipoidica (NL).
NL typically manifests on the bilateral pretibial region as small papules or nodules that expand into yellow-brown atrophic, telangiectatic plaques with an elevated violaceous rim.1,2 Most lesions are asymptomatic due to nerve damage, but up to 35% of patients may experience pruritus and tenderness.2 Close monitoring of lesions is recommended due to risk of ulceration and potential for malignancy.2 Rare reports show development of squamous cell carcinoma within NL lesions.1
Women are 3 times more likely than men to have NL, with an average age of onset between 30 and 40 years.1 The exact pathogenesis of NL is unknown.2 Theories include vascular abnormalities (immunoglobulin deposition or microangiopathic changes leading to collagen degradation), abnormalities of collagen synthesis, neutrophil migration, and elevated tumor necrosis factor-alpha levels.1,3
While NL can be diagnosed clinically, a skin biopsy may be necessary in atypical lesions. The biopsy will reveal palisading granulomatous inflammation in the dermis, with multinucleated histiocytes palisading around degenerated collagen bundles.2
No treatment has proven to be effective for NL. Glucose control in patients with diabetes does not have a significant effect on the NL lesions.1-3 Corticosteroids (topical, intralesional, and systemic—depending on the severity) are considered first-line therapy.1-3 Lifestyle modifications, such as smoking cessation and trauma avoidance, are recommended to promote healing; proper wound care is important when there is ulceration.1,3 Other treatment options include oral pentoxifylline, topical retinoids or calcineurin inhibitors, and systemic immune system modulators (eg, tumor necrosis factor inhibitors and cyclosporine).
Since this patient did not respond to the topical betamethasone, she was started on oral pentoxifylline 400 mg tid. Unfortunately, she had to discontinue the medication because of gastrointestinal upset and was then started on doxycycline 100 mg orally bid. She was lost to follow-up.
Photo courtesy of Cyrelle F. Finan, MD. Text courtesy of Harika Echuri, MD, Tulane University School of Medicine, New Orleans, LA, Cyrelle F. Finan, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Lepe K, Riley CA, Salazar FJ. Necrobiosis lipoidica. StatPearls [Internet]. StatPearls Publishing; 2022. Updated August 26, 2021. Accessed May 31, 2022. www.ncbi.nlm.nih.gov/books/NBK459318/
2. Tong LX, Penn L, Meehan SA, Kim RH. Necrobiosis lipoidica. Dermatol Online J. 2018;24:13030/qt0qg3b3zw. doi: 10.5070/D32412042442
3. Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360. doi: 10.1016/j.det.2015.03.003

The patient’s atrophic plaques with a violaceous rim, indurated borders, and ulceration on the anterior pretibial surface were consistent with ulcerated necrobiosis lipoidica (NL).
NL typically manifests on the bilateral pretibial region as small papules or nodules that expand into yellow-brown atrophic, telangiectatic plaques with an elevated violaceous rim.1,2 Most lesions are asymptomatic due to nerve damage, but up to 35% of patients may experience pruritus and tenderness.2 Close monitoring of lesions is recommended due to risk of ulceration and potential for malignancy.2 Rare reports show development of squamous cell carcinoma within NL lesions.1
Women are 3 times more likely than men to have NL, with an average age of onset between 30 and 40 years.1 The exact pathogenesis of NL is unknown.2 Theories include vascular abnormalities (immunoglobulin deposition or microangiopathic changes leading to collagen degradation), abnormalities of collagen synthesis, neutrophil migration, and elevated tumor necrosis factor-alpha levels.1,3
While NL can be diagnosed clinically, a skin biopsy may be necessary in atypical lesions. The biopsy will reveal palisading granulomatous inflammation in the dermis, with multinucleated histiocytes palisading around degenerated collagen bundles.2
No treatment has proven to be effective for NL. Glucose control in patients with diabetes does not have a significant effect on the NL lesions.1-3 Corticosteroids (topical, intralesional, and systemic—depending on the severity) are considered first-line therapy.1-3 Lifestyle modifications, such as smoking cessation and trauma avoidance, are recommended to promote healing; proper wound care is important when there is ulceration.1,3 Other treatment options include oral pentoxifylline, topical retinoids or calcineurin inhibitors, and systemic immune system modulators (eg, tumor necrosis factor inhibitors and cyclosporine).
Since this patient did not respond to the topical betamethasone, she was started on oral pentoxifylline 400 mg tid. Unfortunately, she had to discontinue the medication because of gastrointestinal upset and was then started on doxycycline 100 mg orally bid. She was lost to follow-up.
Photo courtesy of Cyrelle F. Finan, MD. Text courtesy of Harika Echuri, MD, Tulane University School of Medicine, New Orleans, LA, Cyrelle F. Finan, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The patient’s atrophic plaques with a violaceous rim, indurated borders, and ulceration on the anterior pretibial surface were consistent with ulcerated necrobiosis lipoidica (NL).
NL typically manifests on the bilateral pretibial region as small papules or nodules that expand into yellow-brown atrophic, telangiectatic plaques with an elevated violaceous rim.1,2 Most lesions are asymptomatic due to nerve damage, but up to 35% of patients may experience pruritus and tenderness.2 Close monitoring of lesions is recommended due to risk of ulceration and potential for malignancy.2 Rare reports show development of squamous cell carcinoma within NL lesions.1
Women are 3 times more likely than men to have NL, with an average age of onset between 30 and 40 years.1 The exact pathogenesis of NL is unknown.2 Theories include vascular abnormalities (immunoglobulin deposition or microangiopathic changes leading to collagen degradation), abnormalities of collagen synthesis, neutrophil migration, and elevated tumor necrosis factor-alpha levels.1,3
While NL can be diagnosed clinically, a skin biopsy may be necessary in atypical lesions. The biopsy will reveal palisading granulomatous inflammation in the dermis, with multinucleated histiocytes palisading around degenerated collagen bundles.2
No treatment has proven to be effective for NL. Glucose control in patients with diabetes does not have a significant effect on the NL lesions.1-3 Corticosteroids (topical, intralesional, and systemic—depending on the severity) are considered first-line therapy.1-3 Lifestyle modifications, such as smoking cessation and trauma avoidance, are recommended to promote healing; proper wound care is important when there is ulceration.1,3 Other treatment options include oral pentoxifylline, topical retinoids or calcineurin inhibitors, and systemic immune system modulators (eg, tumor necrosis factor inhibitors and cyclosporine).
Since this patient did not respond to the topical betamethasone, she was started on oral pentoxifylline 400 mg tid. Unfortunately, she had to discontinue the medication because of gastrointestinal upset and was then started on doxycycline 100 mg orally bid. She was lost to follow-up.
Photo courtesy of Cyrelle F. Finan, MD. Text courtesy of Harika Echuri, MD, Tulane University School of Medicine, New Orleans, LA, Cyrelle F. Finan, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Lepe K, Riley CA, Salazar FJ. Necrobiosis lipoidica. StatPearls [Internet]. StatPearls Publishing; 2022. Updated August 26, 2021. Accessed May 31, 2022. www.ncbi.nlm.nih.gov/books/NBK459318/
2. Tong LX, Penn L, Meehan SA, Kim RH. Necrobiosis lipoidica. Dermatol Online J. 2018;24:13030/qt0qg3b3zw. doi: 10.5070/D32412042442
3. Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360. doi: 10.1016/j.det.2015.03.003
1. Lepe K, Riley CA, Salazar FJ. Necrobiosis lipoidica. StatPearls [Internet]. StatPearls Publishing; 2022. Updated August 26, 2021. Accessed May 31, 2022. www.ncbi.nlm.nih.gov/books/NBK459318/
2. Tong LX, Penn L, Meehan SA, Kim RH. Necrobiosis lipoidica. Dermatol Online J. 2018;24:13030/qt0qg3b3zw. doi: 10.5070/D32412042442
3. Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360. doi: 10.1016/j.det.2015.03.003
Omega-3 supplement sweet spot found for BP reduction
A meta-analysis of 71 randomized controlled trials has found the sweet spot for omega-3 fatty acid intake for lowering blood pressure: between 2 and 3 g/day. The investigators also reported that people at higher risk for cardiovascular disease may benefit from higher daily intake of omega-3.
The study analyzed data from randomized controlled trials involving 4,973 individuals and published from 1987 to 2020. Most of the trials used a combined supplementation of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Outcomes analysis involved the impact of combined DHA-EPA at 1, 2, 3, 4, or 5 grams daily on average changes in both systolic and diastolic BP and compared them with the placebo or control groups who had a combined intake of 0 g/day.
“We found a significant nonlinear dose-response relationship for both SBP and DBP models,” wrote senior author Xinzhi Li, MD, PhD, and colleagues. Dr. Li is program director of the school of pharmacy at Macau University of Science and Technology in Taipa, China.
Most of the trials included in the meta-analysis evaluated fish oil supplements, but a number also included EPA and DHA omega-3 fatty acids consumed in food.
When the investigators analyzed studies that used an average baseline SBP of greater than 130 mm Hg, they found that increasing omega-3 supplementation resulted in strong reductions in SBP and DBP, but not so with people with baseline SBP below 130 mm Hg.
Across the entire cohort, average SBP and DBP changes averaged –2.61 (95% confidence interval, –3.57 to –1.65) and –1.64 (95% CI, –2.29 to –0.99) mm Hg for people taking 2 g/d omega-3 supplements, and –2.61 (95% CI, –3.52 to –1.69) and –1.80 (95% CI, –2.38 to –1.23) for those on 3 g/d. The changes weren’t as robust in higher and lower intake groups overall.
However, the higher the BP, the more robust the reductions. For those with SBP greater than 130 mm Hg, 3 g/d resulted in an average change of –3.22 mm Hg (95% CI, –5.21 to –1.23). In the greater than 80 mm Hg DBP group, 3 g/d of omega-3 resulted in an average –3.81 mm Hg reduction (95% CI, –4.48 to –1.87). In patients with BP greater than 140/90 and hypertension, the reductions were even more pronounced. And in patients with BP greater than 130/80, omega-3 intake of 4-5 g/d had a greater impact than 2-3 g/d, although that benefit didn’t carry over in the greater than 140/90 group.
High cholesterol was also a factor in determining the benefits of omega-3 supplementation on BP, as Dr. Li and colleagues wrote that they found “an approximately linear relationship” between hyperlipidemia and SBP, “suggesting that increasing supplementation was associated with greater reductions in SBP.” Likewise, the study found stronger effects on BP in studies with an average patient age greater than 45 years.
In 2019, the Food and Drug Administration issued an update that consuming combined EPA and DHA may lower BP in the general population and reduce the risk of hypertension, but that “the evidence is inconsistent and inconclusive.”
“However, while our study may add a layer of credible evidence, it does not meet the threshold to make an authorized health claim for omega-3 fatty acids in compliance with FDA regulations,” Dr. Li said.
The study addresses shortcomings of previous studies of omega-3 and BP and by identifying the optimal dose, Marc George, MRCP, PhD, of the Institute of Cardiovascular Science, University College, London, and Ajay Gupta, MD, PhD, of the William Harvey Research Institute at Queen Mary University, London, wrote in an accompanying editorial. “More importantly, they have demonstrated a significantly stronger and increased BP-lowering effect in higher cardiovascular risk groups, such as those with hypertension or hyperlipidemia.”
They also noted that the 2.61–mm Hg reduction in SBP the study reported is “likely to be significant” on a population level. “A 2–mm Hg reduction in SBP is estimated to reduce stroke mortality by 10% and deaths from ischemic heart disease by 7%,” they wrote. “Expressed another way, an analysis in the U.S. population using 2010 data estimates that a population-wide reduction in SBP of 2 mm Hg in those aged 45- 64 years would translate to 30,045 fewer cardiovascular events ([coronary heart disease], stroke, and heart failure).”
The investigators and editorialists have no disclosures.
A meta-analysis of 71 randomized controlled trials has found the sweet spot for omega-3 fatty acid intake for lowering blood pressure: between 2 and 3 g/day. The investigators also reported that people at higher risk for cardiovascular disease may benefit from higher daily intake of omega-3.
The study analyzed data from randomized controlled trials involving 4,973 individuals and published from 1987 to 2020. Most of the trials used a combined supplementation of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Outcomes analysis involved the impact of combined DHA-EPA at 1, 2, 3, 4, or 5 grams daily on average changes in both systolic and diastolic BP and compared them with the placebo or control groups who had a combined intake of 0 g/day.
“We found a significant nonlinear dose-response relationship for both SBP and DBP models,” wrote senior author Xinzhi Li, MD, PhD, and colleagues. Dr. Li is program director of the school of pharmacy at Macau University of Science and Technology in Taipa, China.
Most of the trials included in the meta-analysis evaluated fish oil supplements, but a number also included EPA and DHA omega-3 fatty acids consumed in food.
When the investigators analyzed studies that used an average baseline SBP of greater than 130 mm Hg, they found that increasing omega-3 supplementation resulted in strong reductions in SBP and DBP, but not so with people with baseline SBP below 130 mm Hg.
Across the entire cohort, average SBP and DBP changes averaged –2.61 (95% confidence interval, –3.57 to –1.65) and –1.64 (95% CI, –2.29 to –0.99) mm Hg for people taking 2 g/d omega-3 supplements, and –2.61 (95% CI, –3.52 to –1.69) and –1.80 (95% CI, –2.38 to –1.23) for those on 3 g/d. The changes weren’t as robust in higher and lower intake groups overall.
However, the higher the BP, the more robust the reductions. For those with SBP greater than 130 mm Hg, 3 g/d resulted in an average change of –3.22 mm Hg (95% CI, –5.21 to –1.23). In the greater than 80 mm Hg DBP group, 3 g/d of omega-3 resulted in an average –3.81 mm Hg reduction (95% CI, –4.48 to –1.87). In patients with BP greater than 140/90 and hypertension, the reductions were even more pronounced. And in patients with BP greater than 130/80, omega-3 intake of 4-5 g/d had a greater impact than 2-3 g/d, although that benefit didn’t carry over in the greater than 140/90 group.
High cholesterol was also a factor in determining the benefits of omega-3 supplementation on BP, as Dr. Li and colleagues wrote that they found “an approximately linear relationship” between hyperlipidemia and SBP, “suggesting that increasing supplementation was associated with greater reductions in SBP.” Likewise, the study found stronger effects on BP in studies with an average patient age greater than 45 years.
In 2019, the Food and Drug Administration issued an update that consuming combined EPA and DHA may lower BP in the general population and reduce the risk of hypertension, but that “the evidence is inconsistent and inconclusive.”
“However, while our study may add a layer of credible evidence, it does not meet the threshold to make an authorized health claim for omega-3 fatty acids in compliance with FDA regulations,” Dr. Li said.
The study addresses shortcomings of previous studies of omega-3 and BP and by identifying the optimal dose, Marc George, MRCP, PhD, of the Institute of Cardiovascular Science, University College, London, and Ajay Gupta, MD, PhD, of the William Harvey Research Institute at Queen Mary University, London, wrote in an accompanying editorial. “More importantly, they have demonstrated a significantly stronger and increased BP-lowering effect in higher cardiovascular risk groups, such as those with hypertension or hyperlipidemia.”
They also noted that the 2.61–mm Hg reduction in SBP the study reported is “likely to be significant” on a population level. “A 2–mm Hg reduction in SBP is estimated to reduce stroke mortality by 10% and deaths from ischemic heart disease by 7%,” they wrote. “Expressed another way, an analysis in the U.S. population using 2010 data estimates that a population-wide reduction in SBP of 2 mm Hg in those aged 45- 64 years would translate to 30,045 fewer cardiovascular events ([coronary heart disease], stroke, and heart failure).”
The investigators and editorialists have no disclosures.
A meta-analysis of 71 randomized controlled trials has found the sweet spot for omega-3 fatty acid intake for lowering blood pressure: between 2 and 3 g/day. The investigators also reported that people at higher risk for cardiovascular disease may benefit from higher daily intake of omega-3.
The study analyzed data from randomized controlled trials involving 4,973 individuals and published from 1987 to 2020. Most of the trials used a combined supplementation of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Outcomes analysis involved the impact of combined DHA-EPA at 1, 2, 3, 4, or 5 grams daily on average changes in both systolic and diastolic BP and compared them with the placebo or control groups who had a combined intake of 0 g/day.
“We found a significant nonlinear dose-response relationship for both SBP and DBP models,” wrote senior author Xinzhi Li, MD, PhD, and colleagues. Dr. Li is program director of the school of pharmacy at Macau University of Science and Technology in Taipa, China.
Most of the trials included in the meta-analysis evaluated fish oil supplements, but a number also included EPA and DHA omega-3 fatty acids consumed in food.
When the investigators analyzed studies that used an average baseline SBP of greater than 130 mm Hg, they found that increasing omega-3 supplementation resulted in strong reductions in SBP and DBP, but not so with people with baseline SBP below 130 mm Hg.
Across the entire cohort, average SBP and DBP changes averaged –2.61 (95% confidence interval, –3.57 to –1.65) and –1.64 (95% CI, –2.29 to –0.99) mm Hg for people taking 2 g/d omega-3 supplements, and –2.61 (95% CI, –3.52 to –1.69) and –1.80 (95% CI, –2.38 to –1.23) for those on 3 g/d. The changes weren’t as robust in higher and lower intake groups overall.
However, the higher the BP, the more robust the reductions. For those with SBP greater than 130 mm Hg, 3 g/d resulted in an average change of –3.22 mm Hg (95% CI, –5.21 to –1.23). In the greater than 80 mm Hg DBP group, 3 g/d of omega-3 resulted in an average –3.81 mm Hg reduction (95% CI, –4.48 to –1.87). In patients with BP greater than 140/90 and hypertension, the reductions were even more pronounced. And in patients with BP greater than 130/80, omega-3 intake of 4-5 g/d had a greater impact than 2-3 g/d, although that benefit didn’t carry over in the greater than 140/90 group.
High cholesterol was also a factor in determining the benefits of omega-3 supplementation on BP, as Dr. Li and colleagues wrote that they found “an approximately linear relationship” between hyperlipidemia and SBP, “suggesting that increasing supplementation was associated with greater reductions in SBP.” Likewise, the study found stronger effects on BP in studies with an average patient age greater than 45 years.
In 2019, the Food and Drug Administration issued an update that consuming combined EPA and DHA may lower BP in the general population and reduce the risk of hypertension, but that “the evidence is inconsistent and inconclusive.”
“However, while our study may add a layer of credible evidence, it does not meet the threshold to make an authorized health claim for omega-3 fatty acids in compliance with FDA regulations,” Dr. Li said.
The study addresses shortcomings of previous studies of omega-3 and BP and by identifying the optimal dose, Marc George, MRCP, PhD, of the Institute of Cardiovascular Science, University College, London, and Ajay Gupta, MD, PhD, of the William Harvey Research Institute at Queen Mary University, London, wrote in an accompanying editorial. “More importantly, they have demonstrated a significantly stronger and increased BP-lowering effect in higher cardiovascular risk groups, such as those with hypertension or hyperlipidemia.”
They also noted that the 2.61–mm Hg reduction in SBP the study reported is “likely to be significant” on a population level. “A 2–mm Hg reduction in SBP is estimated to reduce stroke mortality by 10% and deaths from ischemic heart disease by 7%,” they wrote. “Expressed another way, an analysis in the U.S. population using 2010 data estimates that a population-wide reduction in SBP of 2 mm Hg in those aged 45- 64 years would translate to 30,045 fewer cardiovascular events ([coronary heart disease], stroke, and heart failure).”
The investigators and editorialists have no disclosures.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
ADA prioritizes heart failure in patients with diabetes
All U.S. patients with diabetes should undergo annual biomarker testing to allow for early diagnosis of progressive but presymptomatic heart failure, and treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class should expand among such patients to include everyone with stage B heart failure (“pre–heart failure”) or more advanced stages.
That’s a recommendation from an American Diabetes Association consensus report published June 1 in Diabetes Care.
The report notes that until now, “implementation of available strategies to detect asymptomatic heart failure [in patients with diabetes] has been suboptimal.” The remedy for this is that, “among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest heart failure stages and to implement strategies to prevent transition to symptomatic heart failure.”
Written by a 10-member panel, chaired by Rodica Pop-Busui, MD, PhD, and endorsed by the American College of Cardiology, the document also set threshold for levels of these biomarkers that are diagnostic for a more advanced stage (stage B) of heart failure in patients with diabetes but without heart failure symptoms:
- A B-type natriuretic peptide (BNP) level of ≥50 pg/mL;
- An N-terminal pro-BNP level of ≥125 pg/mL; or
- Any high sensitivity cardiac troponin value that’s above the usual upper reference limit set at >99th percentile.
‘Inexpensive’ biomarker testing
“Addition of relatively inexpensive biomarker testing as part of the standard of care may help to refine heart failure risk prediction in individuals with diabetes,” the report says.
“Substantial data indicate the ability of these biomarkers to identify those in stage A or B [heart failure] at highest risk of progressing to symptomatic heart failure or death,” and this identification is useful because “the risk in such individuals may be lowered through targeted intervention or multidisciplinary care.”
It is “impossible to understate the importance of early recognition of heart failure” in patients with heart failure, the authors declare. However, the report also cautions that, “using biomarkers to identify and in turn reduce risk for heart failure should always be done within the context of a thoughtful clinical evaluation, supported by all information available.”
The report, written during March 2021 – March 2022, cites the high prevalence and increasing incidence of heart failure in patients with diabetes as the rationale for the new recommendations.
For a person with diabetes who receives a heart failure diagnosis, the report details several management steps, starting with an evaluation for obstructive coronary artery disease, given the strong link between diabetes and atherosclerotic cardiovascular disease.
It highlights the importance of interventions that involve nutrition, smoking avoidance, minimized alcohol intake, exercise, weight loss, and relevant social determinants of health, but focuses in greater detail on a range of pharmacologic interventions. These include treatment of hypertension for people with early-stage heart failure with an ACE inhibitor or an angiotensin receptor blocker, a thiazide-type diuretic, and a mineralocorticoid receptor antagonist, such as spironolactone or the newer, nonsteroidal agent finerenone for patients with diabetic kidney disease.
Dr. Busui of the division of metabolism, endocrinology, and diabetes at the University of Michigan, Ann Arbor, and colleagues cite recent recommendations for using guidelines-directed medical therapy to treat patients with more advanced, symptomatic stages of heart failure, including heart failure with reduced or with preserved ejection fraction.
‘Prioritize’ the SGLT2-inhibitor class
The consensus report also summarizes the roles for agents in the various classes of antidiabetes drugs now available, with particular emphasis on the role for the SGLT2-inhibitor class.
SGLT2 inhibitors “are recommended for all individuals with [diabetes and] heart failure,” it says. “This consensus recommends prioritizing the use of SGLT2 inhibitors in individuals with stage B heart failure, and that SGLT2 inhibitors be an expected element of care in all individuals with diabetes and symptomatic heart failure.”
Other agents for glycemic control that receive endorsement from the report are those in the glucagonlike peptide 1 receptor agonist class. “Despite the lack of conclusive evidence of direct heart failure risk reduction” with this class, it gets a “should be considered” designation, based on its positive effects on weight loss, blood pressure, and atherothrombotic disease.
Similar acknowledgment of potential benefit in a “should be considered” role goes to metformin. But the report turned a thumb down for both the class of dipeptidyl peptidase 4 inhibitors and the thiazolidinedione class, and said that agents from the insulin and sulfonylurea classes should be used “judiciously.”
The report did not identify any commercial funding. Several of the writing committee members listed personal commercial disclosures.
All U.S. patients with diabetes should undergo annual biomarker testing to allow for early diagnosis of progressive but presymptomatic heart failure, and treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class should expand among such patients to include everyone with stage B heart failure (“pre–heart failure”) or more advanced stages.
That’s a recommendation from an American Diabetes Association consensus report published June 1 in Diabetes Care.
The report notes that until now, “implementation of available strategies to detect asymptomatic heart failure [in patients with diabetes] has been suboptimal.” The remedy for this is that, “among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest heart failure stages and to implement strategies to prevent transition to symptomatic heart failure.”
Written by a 10-member panel, chaired by Rodica Pop-Busui, MD, PhD, and endorsed by the American College of Cardiology, the document also set threshold for levels of these biomarkers that are diagnostic for a more advanced stage (stage B) of heart failure in patients with diabetes but without heart failure symptoms:
- A B-type natriuretic peptide (BNP) level of ≥50 pg/mL;
- An N-terminal pro-BNP level of ≥125 pg/mL; or
- Any high sensitivity cardiac troponin value that’s above the usual upper reference limit set at >99th percentile.
‘Inexpensive’ biomarker testing
“Addition of relatively inexpensive biomarker testing as part of the standard of care may help to refine heart failure risk prediction in individuals with diabetes,” the report says.
“Substantial data indicate the ability of these biomarkers to identify those in stage A or B [heart failure] at highest risk of progressing to symptomatic heart failure or death,” and this identification is useful because “the risk in such individuals may be lowered through targeted intervention or multidisciplinary care.”
It is “impossible to understate the importance of early recognition of heart failure” in patients with heart failure, the authors declare. However, the report also cautions that, “using biomarkers to identify and in turn reduce risk for heart failure should always be done within the context of a thoughtful clinical evaluation, supported by all information available.”
The report, written during March 2021 – March 2022, cites the high prevalence and increasing incidence of heart failure in patients with diabetes as the rationale for the new recommendations.
For a person with diabetes who receives a heart failure diagnosis, the report details several management steps, starting with an evaluation for obstructive coronary artery disease, given the strong link between diabetes and atherosclerotic cardiovascular disease.
It highlights the importance of interventions that involve nutrition, smoking avoidance, minimized alcohol intake, exercise, weight loss, and relevant social determinants of health, but focuses in greater detail on a range of pharmacologic interventions. These include treatment of hypertension for people with early-stage heart failure with an ACE inhibitor or an angiotensin receptor blocker, a thiazide-type diuretic, and a mineralocorticoid receptor antagonist, such as spironolactone or the newer, nonsteroidal agent finerenone for patients with diabetic kidney disease.
Dr. Busui of the division of metabolism, endocrinology, and diabetes at the University of Michigan, Ann Arbor, and colleagues cite recent recommendations for using guidelines-directed medical therapy to treat patients with more advanced, symptomatic stages of heart failure, including heart failure with reduced or with preserved ejection fraction.
‘Prioritize’ the SGLT2-inhibitor class
The consensus report also summarizes the roles for agents in the various classes of antidiabetes drugs now available, with particular emphasis on the role for the SGLT2-inhibitor class.
SGLT2 inhibitors “are recommended for all individuals with [diabetes and] heart failure,” it says. “This consensus recommends prioritizing the use of SGLT2 inhibitors in individuals with stage B heart failure, and that SGLT2 inhibitors be an expected element of care in all individuals with diabetes and symptomatic heart failure.”
Other agents for glycemic control that receive endorsement from the report are those in the glucagonlike peptide 1 receptor agonist class. “Despite the lack of conclusive evidence of direct heart failure risk reduction” with this class, it gets a “should be considered” designation, based on its positive effects on weight loss, blood pressure, and atherothrombotic disease.
Similar acknowledgment of potential benefit in a “should be considered” role goes to metformin. But the report turned a thumb down for both the class of dipeptidyl peptidase 4 inhibitors and the thiazolidinedione class, and said that agents from the insulin and sulfonylurea classes should be used “judiciously.”
The report did not identify any commercial funding. Several of the writing committee members listed personal commercial disclosures.
All U.S. patients with diabetes should undergo annual biomarker testing to allow for early diagnosis of progressive but presymptomatic heart failure, and treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class should expand among such patients to include everyone with stage B heart failure (“pre–heart failure”) or more advanced stages.
That’s a recommendation from an American Diabetes Association consensus report published June 1 in Diabetes Care.
The report notes that until now, “implementation of available strategies to detect asymptomatic heart failure [in patients with diabetes] has been suboptimal.” The remedy for this is that, “among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest heart failure stages and to implement strategies to prevent transition to symptomatic heart failure.”
Written by a 10-member panel, chaired by Rodica Pop-Busui, MD, PhD, and endorsed by the American College of Cardiology, the document also set threshold for levels of these biomarkers that are diagnostic for a more advanced stage (stage B) of heart failure in patients with diabetes but without heart failure symptoms:
- A B-type natriuretic peptide (BNP) level of ≥50 pg/mL;
- An N-terminal pro-BNP level of ≥125 pg/mL; or
- Any high sensitivity cardiac troponin value that’s above the usual upper reference limit set at >99th percentile.
‘Inexpensive’ biomarker testing
“Addition of relatively inexpensive biomarker testing as part of the standard of care may help to refine heart failure risk prediction in individuals with diabetes,” the report says.
“Substantial data indicate the ability of these biomarkers to identify those in stage A or B [heart failure] at highest risk of progressing to symptomatic heart failure or death,” and this identification is useful because “the risk in such individuals may be lowered through targeted intervention or multidisciplinary care.”
It is “impossible to understate the importance of early recognition of heart failure” in patients with heart failure, the authors declare. However, the report also cautions that, “using biomarkers to identify and in turn reduce risk for heart failure should always be done within the context of a thoughtful clinical evaluation, supported by all information available.”
The report, written during March 2021 – March 2022, cites the high prevalence and increasing incidence of heart failure in patients with diabetes as the rationale for the new recommendations.
For a person with diabetes who receives a heart failure diagnosis, the report details several management steps, starting with an evaluation for obstructive coronary artery disease, given the strong link between diabetes and atherosclerotic cardiovascular disease.
It highlights the importance of interventions that involve nutrition, smoking avoidance, minimized alcohol intake, exercise, weight loss, and relevant social determinants of health, but focuses in greater detail on a range of pharmacologic interventions. These include treatment of hypertension for people with early-stage heart failure with an ACE inhibitor or an angiotensin receptor blocker, a thiazide-type diuretic, and a mineralocorticoid receptor antagonist, such as spironolactone or the newer, nonsteroidal agent finerenone for patients with diabetic kidney disease.
Dr. Busui of the division of metabolism, endocrinology, and diabetes at the University of Michigan, Ann Arbor, and colleagues cite recent recommendations for using guidelines-directed medical therapy to treat patients with more advanced, symptomatic stages of heart failure, including heart failure with reduced or with preserved ejection fraction.
‘Prioritize’ the SGLT2-inhibitor class
The consensus report also summarizes the roles for agents in the various classes of antidiabetes drugs now available, with particular emphasis on the role for the SGLT2-inhibitor class.
SGLT2 inhibitors “are recommended for all individuals with [diabetes and] heart failure,” it says. “This consensus recommends prioritizing the use of SGLT2 inhibitors in individuals with stage B heart failure, and that SGLT2 inhibitors be an expected element of care in all individuals with diabetes and symptomatic heart failure.”
Other agents for glycemic control that receive endorsement from the report are those in the glucagonlike peptide 1 receptor agonist class. “Despite the lack of conclusive evidence of direct heart failure risk reduction” with this class, it gets a “should be considered” designation, based on its positive effects on weight loss, blood pressure, and atherothrombotic disease.
Similar acknowledgment of potential benefit in a “should be considered” role goes to metformin. But the report turned a thumb down for both the class of dipeptidyl peptidase 4 inhibitors and the thiazolidinedione class, and said that agents from the insulin and sulfonylurea classes should be used “judiciously.”
The report did not identify any commercial funding. Several of the writing committee members listed personal commercial disclosures.
FROM DIABETES CARE
The latest on COVID-19 and the heart in children
The 2022 Pediatric Academic Societies meeting included an excellent session on the acute and delayed effects of COVID-19 on children’s hearts. Data on the risk for cardiac injury during acute COVID-19, return-to-play guidelines after COVID-19–related heart injury, and post–vaccine-associated myocarditis were reviewed.
COVID-induced cardiac injury
The risk for COVID-induced cardiac injury is directly associated with age. Recent Centers for Disease Control and Prevention data revealed a “myocarditis or pericarditis” rate in the range of 12-17 cases per 100,000 SARS-CoV-2 infections among male children aged 5-11 years (lower rates for females); the rate jumps to 50-65 cases per 100,000 infections among male children aged 12-17 years. So cardiac injury caused by acute COVID-19 appears rare, but the risk is clearly associated with male sex and adolescent age.
Return to play after COVID-19
Clinicians may be pressed by patients and parents for advice on return to play after illness with COVID-19. In July 2020, the American College of Cardiology published an algorithm that has been adjusted over time, most recently in 2022 by the American Academy of Pediatrics. These algorithms stratify recommendations by degree of illness. One rule of thumb: Patients with severe COVID-19 (ICU care or multisystem inflammatory syndrome in children [MIS-C]) have only one box on the algorithm, and that is to rest for 3-6 months and only return to usual activity after cardiac clearance. Moderate disease (defined as ≥ 4 days of fever > 100.4 °F; ≥ 1 week of myalgia, chills, lethargy, or any non-ICU hospital stay; and no evidence of MIS-C) require undergoing an ECG to look for cardiac dysfunction, followed by at least 10 days of rest if the ECG is negative or referral for cardiac evaluation if either ECG or exam by a pediatric cardiologist is abnormal.
Clinicians can perhaps be more permissible with patients who are younger or who have had less severe disease. For example, if a patient aged younger than 12 years is asymptomatic with routine activity at the time of evaluation, an ECG is not indicated. For patients aged 12-15 years who are asymptomatic at the time of evaluation but participate in a high-intensity sport, clinicians might consider obtaining an ECG. As few as 3 days of rest might be enough for select patients who are asymptomatic at presentation. For other patients, clinicians should work with parents to introduce activity gradually and make it clear to parents that any activity intolerance requires quick reevaluation. On existing athlete registries, no deaths that are attributable to post–COVID-19 cardiac effects have been confirmed in children; however, all data presented during the session were from prior to the Omicron variant surge in early 2022, so more information may be forthcoming.
Considerations for MIS-C
Among children experiencing MIS-C, 35% had ECG changes, 40% exhibited left ventricular systolic or diastolic dysfunction, and 30% had mitral regurgitation, meaning that a large percentage of patients with MIS-C show some degree of cardiac dysfunction. Unfortunately, we are still in the data-gathering phase for long-term outcomes. Functional parameters tend to improve within a week, and most patients will return to normal cardiac function by 3-4 months.
Return to play after MIS-C is quite different from that for acute COVID-19. Patients with MIS-C should be treated much like other patients with myocarditis with an expected return to play in 3-6 months and only after cardiac follow-up. Another good-to-remember recommendation is to delay COVID-19 vaccination for at least 90 days after an episode of MIS-C.
Vaccine-related myocarditis
Once again, older age appears to be a risk factor because most patients with postvaccine myocarditis have been in their mid-teens to early 20s, with events more likely after the second vaccine dose and also more likely in male children (4:1 ratio to female children). No deaths have occurred from postvaccination myocarditis in patients younger than 30 years. Still, many individuals have exhibited residual MRI enhancement in the cardiac tissue for some time after experiencing postvaccination myocarditis; it’s currently unclear whether that has clinical implications. By comparison, CDC data demonstrates convincingly that the risk for cardiac effects is much greater after acute COVID-19 than after COVID-19 vaccination, with risk ratios often higher than 20, depending on age and condition (for example, myocarditis vs. pericarditis). Data are still insufficient to determine whether clinicians should recommend or avoid COVID-19 vaccination in children with congenital heart disease.
In summary, administering COVID-19 vaccines requires a great deal of shared decision-making with parents, and the clinician’s role is to educate parents about all potential risks related to both the vaccine and COVID-19 illness. Research has consistently shown that acute COVID-19 myocarditis and myocarditis associated with MIS-C are much more likely to occur in unvaccinated youth and more likely than postvaccination myocarditis, regardless of age.
William T. Basco, Jr., MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
The 2022 Pediatric Academic Societies meeting included an excellent session on the acute and delayed effects of COVID-19 on children’s hearts. Data on the risk for cardiac injury during acute COVID-19, return-to-play guidelines after COVID-19–related heart injury, and post–vaccine-associated myocarditis were reviewed.
COVID-induced cardiac injury
The risk for COVID-induced cardiac injury is directly associated with age. Recent Centers for Disease Control and Prevention data revealed a “myocarditis or pericarditis” rate in the range of 12-17 cases per 100,000 SARS-CoV-2 infections among male children aged 5-11 years (lower rates for females); the rate jumps to 50-65 cases per 100,000 infections among male children aged 12-17 years. So cardiac injury caused by acute COVID-19 appears rare, but the risk is clearly associated with male sex and adolescent age.
Return to play after COVID-19
Clinicians may be pressed by patients and parents for advice on return to play after illness with COVID-19. In July 2020, the American College of Cardiology published an algorithm that has been adjusted over time, most recently in 2022 by the American Academy of Pediatrics. These algorithms stratify recommendations by degree of illness. One rule of thumb: Patients with severe COVID-19 (ICU care or multisystem inflammatory syndrome in children [MIS-C]) have only one box on the algorithm, and that is to rest for 3-6 months and only return to usual activity after cardiac clearance. Moderate disease (defined as ≥ 4 days of fever > 100.4 °F; ≥ 1 week of myalgia, chills, lethargy, or any non-ICU hospital stay; and no evidence of MIS-C) require undergoing an ECG to look for cardiac dysfunction, followed by at least 10 days of rest if the ECG is negative or referral for cardiac evaluation if either ECG or exam by a pediatric cardiologist is abnormal.
Clinicians can perhaps be more permissible with patients who are younger or who have had less severe disease. For example, if a patient aged younger than 12 years is asymptomatic with routine activity at the time of evaluation, an ECG is not indicated. For patients aged 12-15 years who are asymptomatic at the time of evaluation but participate in a high-intensity sport, clinicians might consider obtaining an ECG. As few as 3 days of rest might be enough for select patients who are asymptomatic at presentation. For other patients, clinicians should work with parents to introduce activity gradually and make it clear to parents that any activity intolerance requires quick reevaluation. On existing athlete registries, no deaths that are attributable to post–COVID-19 cardiac effects have been confirmed in children; however, all data presented during the session were from prior to the Omicron variant surge in early 2022, so more information may be forthcoming.
Considerations for MIS-C
Among children experiencing MIS-C, 35% had ECG changes, 40% exhibited left ventricular systolic or diastolic dysfunction, and 30% had mitral regurgitation, meaning that a large percentage of patients with MIS-C show some degree of cardiac dysfunction. Unfortunately, we are still in the data-gathering phase for long-term outcomes. Functional parameters tend to improve within a week, and most patients will return to normal cardiac function by 3-4 months.
Return to play after MIS-C is quite different from that for acute COVID-19. Patients with MIS-C should be treated much like other patients with myocarditis with an expected return to play in 3-6 months and only after cardiac follow-up. Another good-to-remember recommendation is to delay COVID-19 vaccination for at least 90 days after an episode of MIS-C.
Vaccine-related myocarditis
Once again, older age appears to be a risk factor because most patients with postvaccine myocarditis have been in their mid-teens to early 20s, with events more likely after the second vaccine dose and also more likely in male children (4:1 ratio to female children). No deaths have occurred from postvaccination myocarditis in patients younger than 30 years. Still, many individuals have exhibited residual MRI enhancement in the cardiac tissue for some time after experiencing postvaccination myocarditis; it’s currently unclear whether that has clinical implications. By comparison, CDC data demonstrates convincingly that the risk for cardiac effects is much greater after acute COVID-19 than after COVID-19 vaccination, with risk ratios often higher than 20, depending on age and condition (for example, myocarditis vs. pericarditis). Data are still insufficient to determine whether clinicians should recommend or avoid COVID-19 vaccination in children with congenital heart disease.
In summary, administering COVID-19 vaccines requires a great deal of shared decision-making with parents, and the clinician’s role is to educate parents about all potential risks related to both the vaccine and COVID-19 illness. Research has consistently shown that acute COVID-19 myocarditis and myocarditis associated with MIS-C are much more likely to occur in unvaccinated youth and more likely than postvaccination myocarditis, regardless of age.
William T. Basco, Jr., MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
The 2022 Pediatric Academic Societies meeting included an excellent session on the acute and delayed effects of COVID-19 on children’s hearts. Data on the risk for cardiac injury during acute COVID-19, return-to-play guidelines after COVID-19–related heart injury, and post–vaccine-associated myocarditis were reviewed.
COVID-induced cardiac injury
The risk for COVID-induced cardiac injury is directly associated with age. Recent Centers for Disease Control and Prevention data revealed a “myocarditis or pericarditis” rate in the range of 12-17 cases per 100,000 SARS-CoV-2 infections among male children aged 5-11 years (lower rates for females); the rate jumps to 50-65 cases per 100,000 infections among male children aged 12-17 years. So cardiac injury caused by acute COVID-19 appears rare, but the risk is clearly associated with male sex and adolescent age.
Return to play after COVID-19
Clinicians may be pressed by patients and parents for advice on return to play after illness with COVID-19. In July 2020, the American College of Cardiology published an algorithm that has been adjusted over time, most recently in 2022 by the American Academy of Pediatrics. These algorithms stratify recommendations by degree of illness. One rule of thumb: Patients with severe COVID-19 (ICU care or multisystem inflammatory syndrome in children [MIS-C]) have only one box on the algorithm, and that is to rest for 3-6 months and only return to usual activity after cardiac clearance. Moderate disease (defined as ≥ 4 days of fever > 100.4 °F; ≥ 1 week of myalgia, chills, lethargy, or any non-ICU hospital stay; and no evidence of MIS-C) require undergoing an ECG to look for cardiac dysfunction, followed by at least 10 days of rest if the ECG is negative or referral for cardiac evaluation if either ECG or exam by a pediatric cardiologist is abnormal.
Clinicians can perhaps be more permissible with patients who are younger or who have had less severe disease. For example, if a patient aged younger than 12 years is asymptomatic with routine activity at the time of evaluation, an ECG is not indicated. For patients aged 12-15 years who are asymptomatic at the time of evaluation but participate in a high-intensity sport, clinicians might consider obtaining an ECG. As few as 3 days of rest might be enough for select patients who are asymptomatic at presentation. For other patients, clinicians should work with parents to introduce activity gradually and make it clear to parents that any activity intolerance requires quick reevaluation. On existing athlete registries, no deaths that are attributable to post–COVID-19 cardiac effects have been confirmed in children; however, all data presented during the session were from prior to the Omicron variant surge in early 2022, so more information may be forthcoming.
Considerations for MIS-C
Among children experiencing MIS-C, 35% had ECG changes, 40% exhibited left ventricular systolic or diastolic dysfunction, and 30% had mitral regurgitation, meaning that a large percentage of patients with MIS-C show some degree of cardiac dysfunction. Unfortunately, we are still in the data-gathering phase for long-term outcomes. Functional parameters tend to improve within a week, and most patients will return to normal cardiac function by 3-4 months.
Return to play after MIS-C is quite different from that for acute COVID-19. Patients with MIS-C should be treated much like other patients with myocarditis with an expected return to play in 3-6 months and only after cardiac follow-up. Another good-to-remember recommendation is to delay COVID-19 vaccination for at least 90 days after an episode of MIS-C.
Vaccine-related myocarditis
Once again, older age appears to be a risk factor because most patients with postvaccine myocarditis have been in their mid-teens to early 20s, with events more likely after the second vaccine dose and also more likely in male children (4:1 ratio to female children). No deaths have occurred from postvaccination myocarditis in patients younger than 30 years. Still, many individuals have exhibited residual MRI enhancement in the cardiac tissue for some time after experiencing postvaccination myocarditis; it’s currently unclear whether that has clinical implications. By comparison, CDC data demonstrates convincingly that the risk for cardiac effects is much greater after acute COVID-19 than after COVID-19 vaccination, with risk ratios often higher than 20, depending on age and condition (for example, myocarditis vs. pericarditis). Data are still insufficient to determine whether clinicians should recommend or avoid COVID-19 vaccination in children with congenital heart disease.
In summary, administering COVID-19 vaccines requires a great deal of shared decision-making with parents, and the clinician’s role is to educate parents about all potential risks related to both the vaccine and COVID-19 illness. Research has consistently shown that acute COVID-19 myocarditis and myocarditis associated with MIS-C are much more likely to occur in unvaccinated youth and more likely than postvaccination myocarditis, regardless of age.
William T. Basco, Jr., MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
Children & COVID: Rise in new cases slows
New cases of COVID-19 in children climbed for the seventh consecutive week, but the latest increase was the smallest of the seven, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Since the weekly total bottomed out at just under 26,000 in early April, the new-case count has risen by 28.0%, 11.8%, 43.5%, 17.4%, 50%, 14.6%, and 5.0%, based on data from the AAP/CHA weekly COVID-19 report.
The cumulative number of pediatric cases is almost 13.4 million since the pandemic began, and those infected children represent 18.9% of all cases, the AAP and CHA said based on data from 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
That 18.9% is noteworthy because it marks the first decline in that particular measure since the AAP and CHA started keeping track in April of 2020. Children’s share of the overall COVID burden had been holding at 19.0% for 14 straight weeks, the AAP/CHA data show.
Regionally, new cases were up in the South and the West, where recent rising trends continued, and down in the Midwest and Northeast, where the recent rising trends were reversed for the first time. At the state/territory level, Puerto Rico had the largest percent increase over the last 2 weeks, followed by Maryland and Delaware, the organizations noted in their joint report.
Hospital admissions in children aged 0-17 have changed little in the last week, with the Centers for Disease Control and Prevention reporting rates of 0.25 per 100,000 population on May 23 and 0.25 per 100,000 on May 29, the latest date available. There was, however, a move up to 0.26 per 100,000 from May 24 to May 28, and the CDC acknowledges a possible reporting delay over the most recent 7-day period.
Emergency department visits have dipped slightly in recent days, with children aged 0-11 years at a 7-day average of 2.0% of ED visits with diagnosed COVID on May 28, down from a 5-day stretch at 2.2% from May 19 to May 23. Children aged 12-15 years were at 1.8% on May 28, compared with 2.0% on May 23-24, and 15- to 17-year-olds were at 2.0% on May 28, down from the 2.1% reached over the previous 2 days, the CDC reported on its COVID Data Tracker.
New cases of COVID-19 in children climbed for the seventh consecutive week, but the latest increase was the smallest of the seven, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Since the weekly total bottomed out at just under 26,000 in early April, the new-case count has risen by 28.0%, 11.8%, 43.5%, 17.4%, 50%, 14.6%, and 5.0%, based on data from the AAP/CHA weekly COVID-19 report.
The cumulative number of pediatric cases is almost 13.4 million since the pandemic began, and those infected children represent 18.9% of all cases, the AAP and CHA said based on data from 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
That 18.9% is noteworthy because it marks the first decline in that particular measure since the AAP and CHA started keeping track in April of 2020. Children’s share of the overall COVID burden had been holding at 19.0% for 14 straight weeks, the AAP/CHA data show.
Regionally, new cases were up in the South and the West, where recent rising trends continued, and down in the Midwest and Northeast, where the recent rising trends were reversed for the first time. At the state/territory level, Puerto Rico had the largest percent increase over the last 2 weeks, followed by Maryland and Delaware, the organizations noted in their joint report.
Hospital admissions in children aged 0-17 have changed little in the last week, with the Centers for Disease Control and Prevention reporting rates of 0.25 per 100,000 population on May 23 and 0.25 per 100,000 on May 29, the latest date available. There was, however, a move up to 0.26 per 100,000 from May 24 to May 28, and the CDC acknowledges a possible reporting delay over the most recent 7-day period.
Emergency department visits have dipped slightly in recent days, with children aged 0-11 years at a 7-day average of 2.0% of ED visits with diagnosed COVID on May 28, down from a 5-day stretch at 2.2% from May 19 to May 23. Children aged 12-15 years were at 1.8% on May 28, compared with 2.0% on May 23-24, and 15- to 17-year-olds were at 2.0% on May 28, down from the 2.1% reached over the previous 2 days, the CDC reported on its COVID Data Tracker.
New cases of COVID-19 in children climbed for the seventh consecutive week, but the latest increase was the smallest of the seven, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Since the weekly total bottomed out at just under 26,000 in early April, the new-case count has risen by 28.0%, 11.8%, 43.5%, 17.4%, 50%, 14.6%, and 5.0%, based on data from the AAP/CHA weekly COVID-19 report.
The cumulative number of pediatric cases is almost 13.4 million since the pandemic began, and those infected children represent 18.9% of all cases, the AAP and CHA said based on data from 49 states, New York City, the District of Columbia, Puerto Rico, and Guam.
That 18.9% is noteworthy because it marks the first decline in that particular measure since the AAP and CHA started keeping track in April of 2020. Children’s share of the overall COVID burden had been holding at 19.0% for 14 straight weeks, the AAP/CHA data show.
Regionally, new cases were up in the South and the West, where recent rising trends continued, and down in the Midwest and Northeast, where the recent rising trends were reversed for the first time. At the state/territory level, Puerto Rico had the largest percent increase over the last 2 weeks, followed by Maryland and Delaware, the organizations noted in their joint report.
Hospital admissions in children aged 0-17 have changed little in the last week, with the Centers for Disease Control and Prevention reporting rates of 0.25 per 100,000 population on May 23 and 0.25 per 100,000 on May 29, the latest date available. There was, however, a move up to 0.26 per 100,000 from May 24 to May 28, and the CDC acknowledges a possible reporting delay over the most recent 7-day period.
Emergency department visits have dipped slightly in recent days, with children aged 0-11 years at a 7-day average of 2.0% of ED visits with diagnosed COVID on May 28, down from a 5-day stretch at 2.2% from May 19 to May 23. Children aged 12-15 years were at 1.8% on May 28, compared with 2.0% on May 23-24, and 15- to 17-year-olds were at 2.0% on May 28, down from the 2.1% reached over the previous 2 days, the CDC reported on its COVID Data Tracker.
Adjunctive confocal microscopy found to reduce unnecessary skin excisions
results from a large randomized clinical trial showed.
“Skin cancer management exerts a sizable burden on health systems,” researchers led by Giovanni Pellacani, MD, wrote in an article published in JAMA Dermatology. “The systematic application of RCM in the triage of high-risk patients should improve diagnostic accuracy and reduce unnecessary excisions for histopathological diagnostic confirmation, thereby reducing costs, surgical waiting lists, and delayed diagnoses.”
However, they added, “the clinical application of RCM has mainly been limited to retrospective and prospective observational studies producing hypothetical estimates of clinical applicability without intention to affect clinical and therapeutic patient pathways.”
For the current study, Dr. Pellacani, who chairs the department of dermatology at Sapienza University, Rome, and colleagues hypothesized that RCM would reduce unnecessary excisions by more than 30% and would identify all melanoma lesions 0.5 mm or thinner at baseline. They enrolled 3,165 patients with suspect lesions from three dermatology referral centers between January 2017 and December 2019, with a mean follow-up of 9.6 months in the study. Participants were randomly assigned 1:1 to standard therapeutic care, which consisted of clinical and dermoscopy evaluation with or without adjunctive RCM, a novel noninvasive technology that provides in vivo imaging of the skin, with a high diagnostic accuracy.
Histopathologic examination of all excised lesions was performed at the pathology department of the referral center. Resulting information guided prospective clinical decision-making (excision or follow-up). The mean age of patients was 49 years, 49% were women, 21% had a personal history of melanoma, and 51% had Fitzpatrick phototype 2 skin.
When compared with standard therapeutic care only, adjunctive RCM was associated with a higher positive predictive value (18.9 vs. 33.3, respectively), lower benign to malignant ratio (3.7:1.0 vs. 1.8:1.0), and a reduction in the number needed to excise of 43.4% (5.3 vs. 3.0). In addition, all 15 lesions with delayed melanoma diagnoses were thinner than 0.5 mm. Of these, eight were diagnosed as melanoma in situ.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study, said that a strength of the analysis was its follow-up and histopathologic evaluation, “which are both essentially forms of feedback. Good, relevant feedback is necessary for all of us to improve.”
She pointed out that, while RCM does appear to reduce the number of benign lesions unnecessarily removed and increase the number of skin cancers appropriately excised, the authors acknowledged that they had at least 4 years of experience with RCM. “The study also does not address the time factor (the procedure takes about 7 minutes per lesion) and the financial cost of reflectance confocal microscopy, as compared to the cost of standard follow-up alone with an increased number of excisions.”
She added that the findings “are not yet applicable to general dermatology across the world, as the authors comment, given that reflectance confocal microscopy is not yet widely available.”
The Italian Ministry of Health supported the study. Neither the researchers nor Dr. Ko reported having relevant financial conflicts.
results from a large randomized clinical trial showed.
“Skin cancer management exerts a sizable burden on health systems,” researchers led by Giovanni Pellacani, MD, wrote in an article published in JAMA Dermatology. “The systematic application of RCM in the triage of high-risk patients should improve diagnostic accuracy and reduce unnecessary excisions for histopathological diagnostic confirmation, thereby reducing costs, surgical waiting lists, and delayed diagnoses.”
However, they added, “the clinical application of RCM has mainly been limited to retrospective and prospective observational studies producing hypothetical estimates of clinical applicability without intention to affect clinical and therapeutic patient pathways.”
For the current study, Dr. Pellacani, who chairs the department of dermatology at Sapienza University, Rome, and colleagues hypothesized that RCM would reduce unnecessary excisions by more than 30% and would identify all melanoma lesions 0.5 mm or thinner at baseline. They enrolled 3,165 patients with suspect lesions from three dermatology referral centers between January 2017 and December 2019, with a mean follow-up of 9.6 months in the study. Participants were randomly assigned 1:1 to standard therapeutic care, which consisted of clinical and dermoscopy evaluation with or without adjunctive RCM, a novel noninvasive technology that provides in vivo imaging of the skin, with a high diagnostic accuracy.
Histopathologic examination of all excised lesions was performed at the pathology department of the referral center. Resulting information guided prospective clinical decision-making (excision or follow-up). The mean age of patients was 49 years, 49% were women, 21% had a personal history of melanoma, and 51% had Fitzpatrick phototype 2 skin.
When compared with standard therapeutic care only, adjunctive RCM was associated with a higher positive predictive value (18.9 vs. 33.3, respectively), lower benign to malignant ratio (3.7:1.0 vs. 1.8:1.0), and a reduction in the number needed to excise of 43.4% (5.3 vs. 3.0). In addition, all 15 lesions with delayed melanoma diagnoses were thinner than 0.5 mm. Of these, eight were diagnosed as melanoma in situ.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study, said that a strength of the analysis was its follow-up and histopathologic evaluation, “which are both essentially forms of feedback. Good, relevant feedback is necessary for all of us to improve.”
She pointed out that, while RCM does appear to reduce the number of benign lesions unnecessarily removed and increase the number of skin cancers appropriately excised, the authors acknowledged that they had at least 4 years of experience with RCM. “The study also does not address the time factor (the procedure takes about 7 minutes per lesion) and the financial cost of reflectance confocal microscopy, as compared to the cost of standard follow-up alone with an increased number of excisions.”
She added that the findings “are not yet applicable to general dermatology across the world, as the authors comment, given that reflectance confocal microscopy is not yet widely available.”
The Italian Ministry of Health supported the study. Neither the researchers nor Dr. Ko reported having relevant financial conflicts.
results from a large randomized clinical trial showed.
“Skin cancer management exerts a sizable burden on health systems,” researchers led by Giovanni Pellacani, MD, wrote in an article published in JAMA Dermatology. “The systematic application of RCM in the triage of high-risk patients should improve diagnostic accuracy and reduce unnecessary excisions for histopathological diagnostic confirmation, thereby reducing costs, surgical waiting lists, and delayed diagnoses.”
However, they added, “the clinical application of RCM has mainly been limited to retrospective and prospective observational studies producing hypothetical estimates of clinical applicability without intention to affect clinical and therapeutic patient pathways.”
For the current study, Dr. Pellacani, who chairs the department of dermatology at Sapienza University, Rome, and colleagues hypothesized that RCM would reduce unnecessary excisions by more than 30% and would identify all melanoma lesions 0.5 mm or thinner at baseline. They enrolled 3,165 patients with suspect lesions from three dermatology referral centers between January 2017 and December 2019, with a mean follow-up of 9.6 months in the study. Participants were randomly assigned 1:1 to standard therapeutic care, which consisted of clinical and dermoscopy evaluation with or without adjunctive RCM, a novel noninvasive technology that provides in vivo imaging of the skin, with a high diagnostic accuracy.
Histopathologic examination of all excised lesions was performed at the pathology department of the referral center. Resulting information guided prospective clinical decision-making (excision or follow-up). The mean age of patients was 49 years, 49% were women, 21% had a personal history of melanoma, and 51% had Fitzpatrick phototype 2 skin.
When compared with standard therapeutic care only, adjunctive RCM was associated with a higher positive predictive value (18.9 vs. 33.3, respectively), lower benign to malignant ratio (3.7:1.0 vs. 1.8:1.0), and a reduction in the number needed to excise of 43.4% (5.3 vs. 3.0). In addition, all 15 lesions with delayed melanoma diagnoses were thinner than 0.5 mm. Of these, eight were diagnosed as melanoma in situ.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study, said that a strength of the analysis was its follow-up and histopathologic evaluation, “which are both essentially forms of feedback. Good, relevant feedback is necessary for all of us to improve.”
She pointed out that, while RCM does appear to reduce the number of benign lesions unnecessarily removed and increase the number of skin cancers appropriately excised, the authors acknowledged that they had at least 4 years of experience with RCM. “The study also does not address the time factor (the procedure takes about 7 minutes per lesion) and the financial cost of reflectance confocal microscopy, as compared to the cost of standard follow-up alone with an increased number of excisions.”
She added that the findings “are not yet applicable to general dermatology across the world, as the authors comment, given that reflectance confocal microscopy is not yet widely available.”
The Italian Ministry of Health supported the study. Neither the researchers nor Dr. Ko reported having relevant financial conflicts.
FROM JAMA DERMATOLOGY
Tin in permanent contraception implants causes toxicity
Essure implants arrived on the market in 2002 as permanent contraception for women older than age 45 years with children. They were recalled in 2017. Presented as an alternative to laparoscopic tubal ligation, this medical device resulted in rare side effects affecting thousands of women, most notably the nervous system, cardiovascular system, endocrine system, and musculoskeletal system.
Implant analysis protocol
“My research focuses on a variety of medical devices, mostly joint replacements, and more specifically, hip replacements. I look at how these materials behave in humans and how the wear debris affects the body,” explained Ana Maria Trunfio-Sfarghiu, bioengineering expert and research associate with the French National Center for Scientific Research at the Lyon National Institute of Applied Sciences’ Contact and Structure Mechanics Laboratory.
“The problems with Essure implants started with a woman who had been using one for about 10 years and was experiencing side effects such as trouble concentrating and focusing, significant vaginal bleeding, extreme tiredness, hair loss, etc. She had the implant removed, and we retrieved it from her gynecologist and analyzed it alongside other implants,” said Ms. Trunfio-Sfarghiu.
“Together with the hospital, we set up an implant analysis protocol. We visited hospital teams to demonstrate how to prepare the biopsies, embedded in paraffin blocks, before sending them to us for analysis. We gave the same specimen preparation instructions for all subjects,” Ms. Trunfio-Sfarghiu explained.
After a year of clinical analysis, the Journal of Trace Elements in Medicine and Biology published an article about 18 cases.
Implant weld corrosion
The Essure implant measures a few centimeters long and resembles a small spring. Once it is released inside the fallopian tube, its goal is to create inflammation and block the tube. It triggers fibrosis, which prevents the sperm from reaching the egg. Premarketing tests had shown that the fibrosis surrounding the implant would keep it from moving. However, the pharmaceutical company hadn’t assessed the mechanical integrity of the spring weld, which was made of silver-tin.
During their analysis in collaboration with the Minapath laboratory, Ms. Trunfio-Sfarghiu’s team found that the weld had corroded and that tin particles had been released into the subjects’ bodies. “The study included about 40 women, and we found tin in all of them,” said Ms. Trunfio-Sfarghiu.
This weld corrosion has several possible consequences. “When the implant degrades, it can travel anywhere in the pelvis, like a needle moving through the body with no apparent destination. The surgeons who operate to remove it describe similar surgeries in military medicine when the patient has been hit by a bullet!”
Organotin toxicity
Although tin is not especially toxic for the body when ingested, it can bind to organic compounds if it passes through to the blood. “When tin binds to a carbon atom, it becomes organotin, a neurotoxin,” said Ms. Trunfio-Sfarghiu.
She said that this organotin can travel to the brain and trigger symptoms like those found in patients with Essure implants. “For the time being, there is insufficient data to assert that we found organotin in all subjects. Another more in-depth study would be needed to assess migration to the brain. For the past 2 years, we have tried to obtain academic funding to continue our research, so far without success. Academic and political authorities seem to be a bit scared of what we’ve found,” said Ms. Trunfio-Sfarghiu.
For her, “it’s how the implant was marketed that is problematic. The implant was designed to create local inflammation, inflammation in itself being difficult to control. Some women need to have their entire uterus and ovaries removed to resolve problems caused by the implant.”
Harm in the United States
Ms. Trunfio-Sfarghiu’s research has helped American victims obtain acknowledgment of their suffering in the United States. “But the harm caused to women by defective implants has yet to be acknowledged in France,” she added.
She explained that Essure was recalled in 2017 because sales were poor, not because it was deemed dangerous. Her conclusion? “No implant that creates inflammation should be authorized, especially if there is a surgical alternative, which there is here: tubal ligation.”
A version of this article appeared on Medscape.com. This article was translated from the Medscape French edition.
Essure implants arrived on the market in 2002 as permanent contraception for women older than age 45 years with children. They were recalled in 2017. Presented as an alternative to laparoscopic tubal ligation, this medical device resulted in rare side effects affecting thousands of women, most notably the nervous system, cardiovascular system, endocrine system, and musculoskeletal system.
Implant analysis protocol
“My research focuses on a variety of medical devices, mostly joint replacements, and more specifically, hip replacements. I look at how these materials behave in humans and how the wear debris affects the body,” explained Ana Maria Trunfio-Sfarghiu, bioengineering expert and research associate with the French National Center for Scientific Research at the Lyon National Institute of Applied Sciences’ Contact and Structure Mechanics Laboratory.
“The problems with Essure implants started with a woman who had been using one for about 10 years and was experiencing side effects such as trouble concentrating and focusing, significant vaginal bleeding, extreme tiredness, hair loss, etc. She had the implant removed, and we retrieved it from her gynecologist and analyzed it alongside other implants,” said Ms. Trunfio-Sfarghiu.
“Together with the hospital, we set up an implant analysis protocol. We visited hospital teams to demonstrate how to prepare the biopsies, embedded in paraffin blocks, before sending them to us for analysis. We gave the same specimen preparation instructions for all subjects,” Ms. Trunfio-Sfarghiu explained.
After a year of clinical analysis, the Journal of Trace Elements in Medicine and Biology published an article about 18 cases.
Implant weld corrosion
The Essure implant measures a few centimeters long and resembles a small spring. Once it is released inside the fallopian tube, its goal is to create inflammation and block the tube. It triggers fibrosis, which prevents the sperm from reaching the egg. Premarketing tests had shown that the fibrosis surrounding the implant would keep it from moving. However, the pharmaceutical company hadn’t assessed the mechanical integrity of the spring weld, which was made of silver-tin.
During their analysis in collaboration with the Minapath laboratory, Ms. Trunfio-Sfarghiu’s team found that the weld had corroded and that tin particles had been released into the subjects’ bodies. “The study included about 40 women, and we found tin in all of them,” said Ms. Trunfio-Sfarghiu.
This weld corrosion has several possible consequences. “When the implant degrades, it can travel anywhere in the pelvis, like a needle moving through the body with no apparent destination. The surgeons who operate to remove it describe similar surgeries in military medicine when the patient has been hit by a bullet!”
Organotin toxicity
Although tin is not especially toxic for the body when ingested, it can bind to organic compounds if it passes through to the blood. “When tin binds to a carbon atom, it becomes organotin, a neurotoxin,” said Ms. Trunfio-Sfarghiu.
She said that this organotin can travel to the brain and trigger symptoms like those found in patients with Essure implants. “For the time being, there is insufficient data to assert that we found organotin in all subjects. Another more in-depth study would be needed to assess migration to the brain. For the past 2 years, we have tried to obtain academic funding to continue our research, so far without success. Academic and political authorities seem to be a bit scared of what we’ve found,” said Ms. Trunfio-Sfarghiu.
For her, “it’s how the implant was marketed that is problematic. The implant was designed to create local inflammation, inflammation in itself being difficult to control. Some women need to have their entire uterus and ovaries removed to resolve problems caused by the implant.”
Harm in the United States
Ms. Trunfio-Sfarghiu’s research has helped American victims obtain acknowledgment of their suffering in the United States. “But the harm caused to women by defective implants has yet to be acknowledged in France,” she added.
She explained that Essure was recalled in 2017 because sales were poor, not because it was deemed dangerous. Her conclusion? “No implant that creates inflammation should be authorized, especially if there is a surgical alternative, which there is here: tubal ligation.”
A version of this article appeared on Medscape.com. This article was translated from the Medscape French edition.
Essure implants arrived on the market in 2002 as permanent contraception for women older than age 45 years with children. They were recalled in 2017. Presented as an alternative to laparoscopic tubal ligation, this medical device resulted in rare side effects affecting thousands of women, most notably the nervous system, cardiovascular system, endocrine system, and musculoskeletal system.
Implant analysis protocol
“My research focuses on a variety of medical devices, mostly joint replacements, and more specifically, hip replacements. I look at how these materials behave in humans and how the wear debris affects the body,” explained Ana Maria Trunfio-Sfarghiu, bioengineering expert and research associate with the French National Center for Scientific Research at the Lyon National Institute of Applied Sciences’ Contact and Structure Mechanics Laboratory.
“The problems with Essure implants started with a woman who had been using one for about 10 years and was experiencing side effects such as trouble concentrating and focusing, significant vaginal bleeding, extreme tiredness, hair loss, etc. She had the implant removed, and we retrieved it from her gynecologist and analyzed it alongside other implants,” said Ms. Trunfio-Sfarghiu.
“Together with the hospital, we set up an implant analysis protocol. We visited hospital teams to demonstrate how to prepare the biopsies, embedded in paraffin blocks, before sending them to us for analysis. We gave the same specimen preparation instructions for all subjects,” Ms. Trunfio-Sfarghiu explained.
After a year of clinical analysis, the Journal of Trace Elements in Medicine and Biology published an article about 18 cases.
Implant weld corrosion
The Essure implant measures a few centimeters long and resembles a small spring. Once it is released inside the fallopian tube, its goal is to create inflammation and block the tube. It triggers fibrosis, which prevents the sperm from reaching the egg. Premarketing tests had shown that the fibrosis surrounding the implant would keep it from moving. However, the pharmaceutical company hadn’t assessed the mechanical integrity of the spring weld, which was made of silver-tin.
During their analysis in collaboration with the Minapath laboratory, Ms. Trunfio-Sfarghiu’s team found that the weld had corroded and that tin particles had been released into the subjects’ bodies. “The study included about 40 women, and we found tin in all of them,” said Ms. Trunfio-Sfarghiu.
This weld corrosion has several possible consequences. “When the implant degrades, it can travel anywhere in the pelvis, like a needle moving through the body with no apparent destination. The surgeons who operate to remove it describe similar surgeries in military medicine when the patient has been hit by a bullet!”
Organotin toxicity
Although tin is not especially toxic for the body when ingested, it can bind to organic compounds if it passes through to the blood. “When tin binds to a carbon atom, it becomes organotin, a neurotoxin,” said Ms. Trunfio-Sfarghiu.
She said that this organotin can travel to the brain and trigger symptoms like those found in patients with Essure implants. “For the time being, there is insufficient data to assert that we found organotin in all subjects. Another more in-depth study would be needed to assess migration to the brain. For the past 2 years, we have tried to obtain academic funding to continue our research, so far without success. Academic and political authorities seem to be a bit scared of what we’ve found,” said Ms. Trunfio-Sfarghiu.
For her, “it’s how the implant was marketed that is problematic. The implant was designed to create local inflammation, inflammation in itself being difficult to control. Some women need to have their entire uterus and ovaries removed to resolve problems caused by the implant.”
Harm in the United States
Ms. Trunfio-Sfarghiu’s research has helped American victims obtain acknowledgment of their suffering in the United States. “But the harm caused to women by defective implants has yet to be acknowledged in France,” she added.
She explained that Essure was recalled in 2017 because sales were poor, not because it was deemed dangerous. Her conclusion? “No implant that creates inflammation should be authorized, especially if there is a surgical alternative, which there is here: tubal ligation.”
A version of this article appeared on Medscape.com. This article was translated from the Medscape French edition.
FDA clears Abbott Freestyle Libre 3 glucose sensor
The Food and Drug Administration has cleared Abbot’s Freestyle Libre 3 system for use by people aged 4 years and older with diabetes.
The new system was cleared for use for both iOS- and Android-compatible mobile apps, enabling real-time glucose readings in contrast to the “intermittently scanned” capability of prior Libre versions. The Libre 3 allows for optional alarms and notifications of urgent low or high glucose levels, as well as remote monitoring by health care professionals or the patient’s family members and/or friends.
The FreeStyle Libre 3 was granted a CE Mark in Europe in October 2020.
Smaller, thinner, and better integration
According to Abbott, the Libre 3 is the first continuous glucose monitoring (CGM) system to show a mean absolute relative difference (MARD) of less than 8% compared with a gold-standard glucose measure. The average Libre 3 MARD is 7.9%, compared with 9.3% for the Libre 2. The Libre 3 is also the “smallest and thinnest” CGM, roughly the size of two stacked U.S. pennies, worn on the upper arm.
And, the company said, the Libre 3 has a Bluetooth integration of up to 33 feet, a range 50% further than other CGMs.
This version follows the FreeStyle Libre 2, approved in June 2020, and its compatible iPhone app, approved in August 2021.
The Libre 3 will be priced the same as the Libre 2, at about one-third the cost of other CGM systems. However, it is not currently eligible for Medicare reimbursement. Medicaid eligibility may vary by state.
“I applaud Abbott for making their CGM system the most affordable and addressing disparities in care so patients living with diabetes can avoid complications and optimize their quality of life,” Eugene E. Wright Jr., MD, of Duke University, Durham, N.C., said in an Abbott statement.
“I have seen real-world evidence that diabetes technologies like CGMs have helped my patients safely achieve improved glycemic control,” he said.
The FreeStyle Libre 3 sensor will be available at participating pharmacies later this year.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared Abbot’s Freestyle Libre 3 system for use by people aged 4 years and older with diabetes.
The new system was cleared for use for both iOS- and Android-compatible mobile apps, enabling real-time glucose readings in contrast to the “intermittently scanned” capability of prior Libre versions. The Libre 3 allows for optional alarms and notifications of urgent low or high glucose levels, as well as remote monitoring by health care professionals or the patient’s family members and/or friends.
The FreeStyle Libre 3 was granted a CE Mark in Europe in October 2020.
Smaller, thinner, and better integration
According to Abbott, the Libre 3 is the first continuous glucose monitoring (CGM) system to show a mean absolute relative difference (MARD) of less than 8% compared with a gold-standard glucose measure. The average Libre 3 MARD is 7.9%, compared with 9.3% for the Libre 2. The Libre 3 is also the “smallest and thinnest” CGM, roughly the size of two stacked U.S. pennies, worn on the upper arm.
And, the company said, the Libre 3 has a Bluetooth integration of up to 33 feet, a range 50% further than other CGMs.
This version follows the FreeStyle Libre 2, approved in June 2020, and its compatible iPhone app, approved in August 2021.
The Libre 3 will be priced the same as the Libre 2, at about one-third the cost of other CGM systems. However, it is not currently eligible for Medicare reimbursement. Medicaid eligibility may vary by state.
“I applaud Abbott for making their CGM system the most affordable and addressing disparities in care so patients living with diabetes can avoid complications and optimize their quality of life,” Eugene E. Wright Jr., MD, of Duke University, Durham, N.C., said in an Abbott statement.
“I have seen real-world evidence that diabetes technologies like CGMs have helped my patients safely achieve improved glycemic control,” he said.
The FreeStyle Libre 3 sensor will be available at participating pharmacies later this year.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared Abbot’s Freestyle Libre 3 system for use by people aged 4 years and older with diabetes.
The new system was cleared for use for both iOS- and Android-compatible mobile apps, enabling real-time glucose readings in contrast to the “intermittently scanned” capability of prior Libre versions. The Libre 3 allows for optional alarms and notifications of urgent low or high glucose levels, as well as remote monitoring by health care professionals or the patient’s family members and/or friends.
The FreeStyle Libre 3 was granted a CE Mark in Europe in October 2020.
Smaller, thinner, and better integration
According to Abbott, the Libre 3 is the first continuous glucose monitoring (CGM) system to show a mean absolute relative difference (MARD) of less than 8% compared with a gold-standard glucose measure. The average Libre 3 MARD is 7.9%, compared with 9.3% for the Libre 2. The Libre 3 is also the “smallest and thinnest” CGM, roughly the size of two stacked U.S. pennies, worn on the upper arm.
And, the company said, the Libre 3 has a Bluetooth integration of up to 33 feet, a range 50% further than other CGMs.
This version follows the FreeStyle Libre 2, approved in June 2020, and its compatible iPhone app, approved in August 2021.
The Libre 3 will be priced the same as the Libre 2, at about one-third the cost of other CGM systems. However, it is not currently eligible for Medicare reimbursement. Medicaid eligibility may vary by state.
“I applaud Abbott for making their CGM system the most affordable and addressing disparities in care so patients living with diabetes can avoid complications and optimize their quality of life,” Eugene E. Wright Jr., MD, of Duke University, Durham, N.C., said in an Abbott statement.
“I have seen real-world evidence that diabetes technologies like CGMs have helped my patients safely achieve improved glycemic control,” he said.
The FreeStyle Libre 3 sensor will be available at participating pharmacies later this year.
A version of this article first appeared on Medscape.com.
Long COVID neuropsychiatric deficits greater than expected
NEW ORLEANS – , adding to mounting evidence of the significant toll the chronic condition can have on mental health.
“Many clinicians have observed the symptoms we describe in this study, however this report is among the first which identify the specific deficits using neuropsychological testing to better characterize the syndrome,” Sean T. Lynch, MD, first author of a study on the issue presented at the annual meeting of the American Psychiatric Association, said in an interview.
Dr. Lynch, of the department of psychiatry, Westchester Medical Center Health System, Valhalla, N.Y., and his colleagues enrolled 60 participants who had experienced acute COVID-19 disease 6-8 months earlier and had undergone neuropsychological, psychiatric, medical, functional, and quality-of-life assessments. Results from the study were published online in the Journal of the Academy of Consultation–Liaison Psychiatry (2022 Jan 25. doi: 10.1016/j.jaclp.2022.01.003).
Among the study participants, 32 were seeking treatment for brain fog in a clinical program for survivors of COVID-19, while the remaining 28 were part of an ongoing longitudinal investigation of neuropsychological, medical, and psychiatric sequelae of COVID-19, but were not seeking care for the persistent symptoms.
Assessments for neurocognitive impairment included a battery of tests used in infectious and other diseases, including the Test of Premorbid Function, the Patient Assessment of Own Function, the Trail Making Test parts A and B, the Stroop Color and Word Test, and others.
Overall, the battery of assessments showed that 37 (62%) of participants had neuropsychological test impairment, with results below the 16th percentile in two tests, while 16 (27%) showed scores indicative of severe impairment (below the second percentile in at least one test and below the 16th percentile in one test).
Those reporting brain fog had scores that were even lower than expected on tests of attention, processing speed, memory, and executive function. And among those reporting brain fog, significantly more had scores reflecting severe impairment compared with the controls (38% vs. 14%; P < .04).
“Based on what we’ve observed in our patients and what others have previously reported, we did expect to find some impairment in this study sample,” Dr. Lynch noted.
“However, we were surprised to find that 27% of the study sample had extremely low neuropsychological test scores, meaning that they scored at least two standard deviations below the expected score on at least one neuropsychological test based on their age and level of education.”
The brain fog group also reported significantly higher levels of depression, fatigue, PTSD, and functional difficulties, and lower quality of life.
Severe impairment on the neuropsychological tests correlated with the extent of acute COVID-19 symptoms, as well as depression scores, number of medical comorbidities, and subjective cognitive complaints.
An analysis of serum levels of the inflammatory markers among 50 of the 60 participants showed that 45% of the patients had an elevated IL-6, 20% had elevated TNF-alpha, and 41% had elevated CRP, compared with reference ranges.
IL-6 levels were found to correlate with acute COVID-19 symptoms, the number of medical comorbidities, fatigue, and measures of executive function, while C-reactive protein (CRP) correlated with current COVID-19 symptoms and depression scores.
In terms of clinical factors that might predict low neuropsychological test scores, Dr. Lynch noted that the “markers that we found to be significant included severity of acute COVID-19 illness, current post-COVID-19 symptoms, measures of depression and anxiety, level of fatigue, and number of medical comorbidities.”
Dr. Lynch noted that the ongoing study will include up to 18-month follow-ups that are currently underway. “The [follow-ups] will examine if symptoms improve over time and evaluate if any intervention that took place was successful,” he said.
Survey supports findings
The detrimental effects of mental health symptoms in long COVID were further supported in another study at the APA meeting, an online survey of 787 survivors of acute COVID-19.
In the community survey, presented by Michael Van Ameringen, MD, a professor in the department of psychiatry and behavioral neurosciences at McMaster University, in Hamilton, Ont., all respondents (100%) reported having persistent symptoms of the virus, and as many as 68% indicated that they had not returned to normal functioning, despite only 15% of the respondents having been hospitalized with COVID-19.
A large proportion showed significant depression, anxiety, and posttraumatic stress disorder (PTSD), and the most commonly reported persistent symptoms were fatigue in 75.9% of respondents, brain fog in 67.9%, concentration difficulties in 61.1%, and weakness in 51.2%.
As many as 88.2% of patients said they experienced persistent neurocognitive symptoms, with poor memory and concentration; 56% reported problems with word finding; and 54.1% had slowed thinking.
The respondents showed high rates of anxiety (41.7%) as well as depression (61.4%) as determined by scores above 9 on the Generalized Anxiety Disorder–7 (GAD-7) and Patient Health Questionnaires (PHQ-9).
As many as 40.5% of respondents showed probable PTSD, with scores above 30 on the PTSD checklist (PCL-5). Their mean resilience score on the Brief Resilient Coping Scale was 13.5, suggesting low resilience.
Among the respondents, 43.3% said they had received past treatment for mental health, while 33.5% were currently receiving mental health treatment.
Dr. Van Ameringen noted the important limitation of the study being an online survey with no control group, but said the responses nevertheless raise the question of the role of prior psychiatric disorders in long COVID.
“In our sample, 40% of respondents had a past psychiatric history, so you wonder if that also makes you vulnerable to long COVID,” he said in an interview.
“About a third were getting psychiatric help, but I think the more impaired you are, the more likely you are to seek help.”
Those who were hospitalized with COVID-19 were at a higher risk of PTSD compared with those not hospitalized (P < .001), as were those under the age of 30 (P < .05) or between 31 and 50 vs. over 50 (P < .01).
Dr. Van Ameringen noted that the survey’s high rate of subjects who had not returned to normal functioning was especially striking.
“This is not a minor issue – these are people who are no longer functioning in society,” he said.
In pandemics, the brain tends to be ‘overlooked’
Further addressing the neurological effects of COVID-19 at the APA meeting, Avindra Nath, MD, clinical director of the National Institutes of Neurologic Disorders and Stroke in Bethesda, Md., noted that the persisting cognitive and psychiatric symptoms after illness, such as brain fog and depression and anxiety, are not necessarily unique to COVID-19.
“We have seen this before,” he said. “There have been at least seven or eight human coronaviruses, and the interesting thing is each one affects the brain and causes neurological complications.”
The effects are classified differently and have slightly different receptors, “but the consequences are the same.”
Of note, however, research published in The Lancet Psychiatry (2021 May. doi: 10.1016/S2215-0366[21]00084-5) revealed that symptoms such as dementia, mood, and anxiety are significantly higher after COVID-19 compared with other respiratory infections, with the differences increasing at 180 days since the index event.
Dr. Nath noted that, over the decades, he has observed that in pandemics “the brain tends to get overlooked.” He explained that “what can be most important in the end is what happened in the brain, because those are the things that really cause the long-term consequences.”
“These patients are depressed; they have dementia, they have brain fog, and even now that we recognize these issues, we haven’t done a very good job of studying them,” he said. “There’s so much we still don’t know, and a lot of patients are left with these symptoms and nowhere to go.”
Dr. Lynch, Dr. Van Ameringen, and Dr. Nath had no disclosures to report.
NEW ORLEANS – , adding to mounting evidence of the significant toll the chronic condition can have on mental health.
“Many clinicians have observed the symptoms we describe in this study, however this report is among the first which identify the specific deficits using neuropsychological testing to better characterize the syndrome,” Sean T. Lynch, MD, first author of a study on the issue presented at the annual meeting of the American Psychiatric Association, said in an interview.
Dr. Lynch, of the department of psychiatry, Westchester Medical Center Health System, Valhalla, N.Y., and his colleagues enrolled 60 participants who had experienced acute COVID-19 disease 6-8 months earlier and had undergone neuropsychological, psychiatric, medical, functional, and quality-of-life assessments. Results from the study were published online in the Journal of the Academy of Consultation–Liaison Psychiatry (2022 Jan 25. doi: 10.1016/j.jaclp.2022.01.003).
Among the study participants, 32 were seeking treatment for brain fog in a clinical program for survivors of COVID-19, while the remaining 28 were part of an ongoing longitudinal investigation of neuropsychological, medical, and psychiatric sequelae of COVID-19, but were not seeking care for the persistent symptoms.
Assessments for neurocognitive impairment included a battery of tests used in infectious and other diseases, including the Test of Premorbid Function, the Patient Assessment of Own Function, the Trail Making Test parts A and B, the Stroop Color and Word Test, and others.
Overall, the battery of assessments showed that 37 (62%) of participants had neuropsychological test impairment, with results below the 16th percentile in two tests, while 16 (27%) showed scores indicative of severe impairment (below the second percentile in at least one test and below the 16th percentile in one test).
Those reporting brain fog had scores that were even lower than expected on tests of attention, processing speed, memory, and executive function. And among those reporting brain fog, significantly more had scores reflecting severe impairment compared with the controls (38% vs. 14%; P < .04).
“Based on what we’ve observed in our patients and what others have previously reported, we did expect to find some impairment in this study sample,” Dr. Lynch noted.
“However, we were surprised to find that 27% of the study sample had extremely low neuropsychological test scores, meaning that they scored at least two standard deviations below the expected score on at least one neuropsychological test based on their age and level of education.”
The brain fog group also reported significantly higher levels of depression, fatigue, PTSD, and functional difficulties, and lower quality of life.
Severe impairment on the neuropsychological tests correlated with the extent of acute COVID-19 symptoms, as well as depression scores, number of medical comorbidities, and subjective cognitive complaints.
An analysis of serum levels of the inflammatory markers among 50 of the 60 participants showed that 45% of the patients had an elevated IL-6, 20% had elevated TNF-alpha, and 41% had elevated CRP, compared with reference ranges.
IL-6 levels were found to correlate with acute COVID-19 symptoms, the number of medical comorbidities, fatigue, and measures of executive function, while C-reactive protein (CRP) correlated with current COVID-19 symptoms and depression scores.
In terms of clinical factors that might predict low neuropsychological test scores, Dr. Lynch noted that the “markers that we found to be significant included severity of acute COVID-19 illness, current post-COVID-19 symptoms, measures of depression and anxiety, level of fatigue, and number of medical comorbidities.”
Dr. Lynch noted that the ongoing study will include up to 18-month follow-ups that are currently underway. “The [follow-ups] will examine if symptoms improve over time and evaluate if any intervention that took place was successful,” he said.
Survey supports findings
The detrimental effects of mental health symptoms in long COVID were further supported in another study at the APA meeting, an online survey of 787 survivors of acute COVID-19.
In the community survey, presented by Michael Van Ameringen, MD, a professor in the department of psychiatry and behavioral neurosciences at McMaster University, in Hamilton, Ont., all respondents (100%) reported having persistent symptoms of the virus, and as many as 68% indicated that they had not returned to normal functioning, despite only 15% of the respondents having been hospitalized with COVID-19.
A large proportion showed significant depression, anxiety, and posttraumatic stress disorder (PTSD), and the most commonly reported persistent symptoms were fatigue in 75.9% of respondents, brain fog in 67.9%, concentration difficulties in 61.1%, and weakness in 51.2%.
As many as 88.2% of patients said they experienced persistent neurocognitive symptoms, with poor memory and concentration; 56% reported problems with word finding; and 54.1% had slowed thinking.
The respondents showed high rates of anxiety (41.7%) as well as depression (61.4%) as determined by scores above 9 on the Generalized Anxiety Disorder–7 (GAD-7) and Patient Health Questionnaires (PHQ-9).
As many as 40.5% of respondents showed probable PTSD, with scores above 30 on the PTSD checklist (PCL-5). Their mean resilience score on the Brief Resilient Coping Scale was 13.5, suggesting low resilience.
Among the respondents, 43.3% said they had received past treatment for mental health, while 33.5% were currently receiving mental health treatment.
Dr. Van Ameringen noted the important limitation of the study being an online survey with no control group, but said the responses nevertheless raise the question of the role of prior psychiatric disorders in long COVID.
“In our sample, 40% of respondents had a past psychiatric history, so you wonder if that also makes you vulnerable to long COVID,” he said in an interview.
“About a third were getting psychiatric help, but I think the more impaired you are, the more likely you are to seek help.”
Those who were hospitalized with COVID-19 were at a higher risk of PTSD compared with those not hospitalized (P < .001), as were those under the age of 30 (P < .05) or between 31 and 50 vs. over 50 (P < .01).
Dr. Van Ameringen noted that the survey’s high rate of subjects who had not returned to normal functioning was especially striking.
“This is not a minor issue – these are people who are no longer functioning in society,” he said.
In pandemics, the brain tends to be ‘overlooked’
Further addressing the neurological effects of COVID-19 at the APA meeting, Avindra Nath, MD, clinical director of the National Institutes of Neurologic Disorders and Stroke in Bethesda, Md., noted that the persisting cognitive and psychiatric symptoms after illness, such as brain fog and depression and anxiety, are not necessarily unique to COVID-19.
“We have seen this before,” he said. “There have been at least seven or eight human coronaviruses, and the interesting thing is each one affects the brain and causes neurological complications.”
The effects are classified differently and have slightly different receptors, “but the consequences are the same.”
Of note, however, research published in The Lancet Psychiatry (2021 May. doi: 10.1016/S2215-0366[21]00084-5) revealed that symptoms such as dementia, mood, and anxiety are significantly higher after COVID-19 compared with other respiratory infections, with the differences increasing at 180 days since the index event.
Dr. Nath noted that, over the decades, he has observed that in pandemics “the brain tends to get overlooked.” He explained that “what can be most important in the end is what happened in the brain, because those are the things that really cause the long-term consequences.”
“These patients are depressed; they have dementia, they have brain fog, and even now that we recognize these issues, we haven’t done a very good job of studying them,” he said. “There’s so much we still don’t know, and a lot of patients are left with these symptoms and nowhere to go.”
Dr. Lynch, Dr. Van Ameringen, and Dr. Nath had no disclosures to report.
NEW ORLEANS – , adding to mounting evidence of the significant toll the chronic condition can have on mental health.
“Many clinicians have observed the symptoms we describe in this study, however this report is among the first which identify the specific deficits using neuropsychological testing to better characterize the syndrome,” Sean T. Lynch, MD, first author of a study on the issue presented at the annual meeting of the American Psychiatric Association, said in an interview.
Dr. Lynch, of the department of psychiatry, Westchester Medical Center Health System, Valhalla, N.Y., and his colleagues enrolled 60 participants who had experienced acute COVID-19 disease 6-8 months earlier and had undergone neuropsychological, psychiatric, medical, functional, and quality-of-life assessments. Results from the study were published online in the Journal of the Academy of Consultation–Liaison Psychiatry (2022 Jan 25. doi: 10.1016/j.jaclp.2022.01.003).
Among the study participants, 32 were seeking treatment for brain fog in a clinical program for survivors of COVID-19, while the remaining 28 were part of an ongoing longitudinal investigation of neuropsychological, medical, and psychiatric sequelae of COVID-19, but were not seeking care for the persistent symptoms.
Assessments for neurocognitive impairment included a battery of tests used in infectious and other diseases, including the Test of Premorbid Function, the Patient Assessment of Own Function, the Trail Making Test parts A and B, the Stroop Color and Word Test, and others.
Overall, the battery of assessments showed that 37 (62%) of participants had neuropsychological test impairment, with results below the 16th percentile in two tests, while 16 (27%) showed scores indicative of severe impairment (below the second percentile in at least one test and below the 16th percentile in one test).
Those reporting brain fog had scores that were even lower than expected on tests of attention, processing speed, memory, and executive function. And among those reporting brain fog, significantly more had scores reflecting severe impairment compared with the controls (38% vs. 14%; P < .04).
“Based on what we’ve observed in our patients and what others have previously reported, we did expect to find some impairment in this study sample,” Dr. Lynch noted.
“However, we were surprised to find that 27% of the study sample had extremely low neuropsychological test scores, meaning that they scored at least two standard deviations below the expected score on at least one neuropsychological test based on their age and level of education.”
The brain fog group also reported significantly higher levels of depression, fatigue, PTSD, and functional difficulties, and lower quality of life.
Severe impairment on the neuropsychological tests correlated with the extent of acute COVID-19 symptoms, as well as depression scores, number of medical comorbidities, and subjective cognitive complaints.
An analysis of serum levels of the inflammatory markers among 50 of the 60 participants showed that 45% of the patients had an elevated IL-6, 20% had elevated TNF-alpha, and 41% had elevated CRP, compared with reference ranges.
IL-6 levels were found to correlate with acute COVID-19 symptoms, the number of medical comorbidities, fatigue, and measures of executive function, while C-reactive protein (CRP) correlated with current COVID-19 symptoms and depression scores.
In terms of clinical factors that might predict low neuropsychological test scores, Dr. Lynch noted that the “markers that we found to be significant included severity of acute COVID-19 illness, current post-COVID-19 symptoms, measures of depression and anxiety, level of fatigue, and number of medical comorbidities.”
Dr. Lynch noted that the ongoing study will include up to 18-month follow-ups that are currently underway. “The [follow-ups] will examine if symptoms improve over time and evaluate if any intervention that took place was successful,” he said.
Survey supports findings
The detrimental effects of mental health symptoms in long COVID were further supported in another study at the APA meeting, an online survey of 787 survivors of acute COVID-19.
In the community survey, presented by Michael Van Ameringen, MD, a professor in the department of psychiatry and behavioral neurosciences at McMaster University, in Hamilton, Ont., all respondents (100%) reported having persistent symptoms of the virus, and as many as 68% indicated that they had not returned to normal functioning, despite only 15% of the respondents having been hospitalized with COVID-19.
A large proportion showed significant depression, anxiety, and posttraumatic stress disorder (PTSD), and the most commonly reported persistent symptoms were fatigue in 75.9% of respondents, brain fog in 67.9%, concentration difficulties in 61.1%, and weakness in 51.2%.
As many as 88.2% of patients said they experienced persistent neurocognitive symptoms, with poor memory and concentration; 56% reported problems with word finding; and 54.1% had slowed thinking.
The respondents showed high rates of anxiety (41.7%) as well as depression (61.4%) as determined by scores above 9 on the Generalized Anxiety Disorder–7 (GAD-7) and Patient Health Questionnaires (PHQ-9).
As many as 40.5% of respondents showed probable PTSD, with scores above 30 on the PTSD checklist (PCL-5). Their mean resilience score on the Brief Resilient Coping Scale was 13.5, suggesting low resilience.
Among the respondents, 43.3% said they had received past treatment for mental health, while 33.5% were currently receiving mental health treatment.
Dr. Van Ameringen noted the important limitation of the study being an online survey with no control group, but said the responses nevertheless raise the question of the role of prior psychiatric disorders in long COVID.
“In our sample, 40% of respondents had a past psychiatric history, so you wonder if that also makes you vulnerable to long COVID,” he said in an interview.
“About a third were getting psychiatric help, but I think the more impaired you are, the more likely you are to seek help.”
Those who were hospitalized with COVID-19 were at a higher risk of PTSD compared with those not hospitalized (P < .001), as were those under the age of 30 (P < .05) or between 31 and 50 vs. over 50 (P < .01).
Dr. Van Ameringen noted that the survey’s high rate of subjects who had not returned to normal functioning was especially striking.
“This is not a minor issue – these are people who are no longer functioning in society,” he said.
In pandemics, the brain tends to be ‘overlooked’
Further addressing the neurological effects of COVID-19 at the APA meeting, Avindra Nath, MD, clinical director of the National Institutes of Neurologic Disorders and Stroke in Bethesda, Md., noted that the persisting cognitive and psychiatric symptoms after illness, such as brain fog and depression and anxiety, are not necessarily unique to COVID-19.
“We have seen this before,” he said. “There have been at least seven or eight human coronaviruses, and the interesting thing is each one affects the brain and causes neurological complications.”
The effects are classified differently and have slightly different receptors, “but the consequences are the same.”
Of note, however, research published in The Lancet Psychiatry (2021 May. doi: 10.1016/S2215-0366[21]00084-5) revealed that symptoms such as dementia, mood, and anxiety are significantly higher after COVID-19 compared with other respiratory infections, with the differences increasing at 180 days since the index event.
Dr. Nath noted that, over the decades, he has observed that in pandemics “the brain tends to get overlooked.” He explained that “what can be most important in the end is what happened in the brain, because those are the things that really cause the long-term consequences.”
“These patients are depressed; they have dementia, they have brain fog, and even now that we recognize these issues, we haven’t done a very good job of studying them,” he said. “There’s so much we still don’t know, and a lot of patients are left with these symptoms and nowhere to go.”
Dr. Lynch, Dr. Van Ameringen, and Dr. Nath had no disclosures to report.
AT APA 2022
Coffee drinkers – even those with a sweet tooth – live longer
Among more than 170,000 people in the United Kingdom, those who drank about two to four cups of coffee a day, with or without sugar, had a lower rate of death than those who didn’t drink coffee, reported lead author Dan Liu, MD, of the department of epidemiology at Southern Medical University, Guangzhou, China.
“Previous observational studies have suggested an association between coffee intake and reduced risk for death, but they did not distinguish between coffee consumed with sugar or artificial sweeteners and coffee consumed without,” Dr. Liu, who is also of the department of public health and preventive medicine, Jinan University, Guangzhou, China, and colleagues wrote in Annals of Internal Medicine.
To learn more, the investigators turned to the UK Biobank, which recruited approximately half a million participants in the United Kingdom between 2006 and 2010 to undergo a variety of questionnaires, interviews, physical measurements, and medical tests. Out of this group, 171,616 participants completed at least one dietary questionnaire and met the criteria for the present study, including lack of cancer or cardiovascular disease upon enrollment.
Results from these questionnaires showed that 55.4% of participants drank coffee without any sweetener, 14.3% drank coffee with sugar, 6.1% drank coffee with artificial sweetener, and 24.2% did not drink coffee at all. Coffee drinkers were further sorted into groups based on how many cups of coffee they drank per day.
Coffee drinkers were significantly less likely to die from any cause
Over the course of about 7 years, 3,177 of the participants died, including 1,725 who died from cancer and 628 who died from cardiovascular disease.
After accounting for other factors that might impact risk of death, like lifestyle choices, the investigators found that coffee drinkers were significantly less likely to die from any cause, cardiovascular disease, or cancer, than those who didn’t drink coffee at all. This benefit was observed across types of coffee, including ground, instant, and decaffeinated varieties. The protective effects of coffee were most apparent in people who drank about two to four cups a day, among whom death was about 30% less likely, regardless of whether they added sugar to their coffee or not. Individuals who drank coffee with artificial sweetener did not live significantly longer than those who drank no coffee at all; however, the investigators suggested that this result may have been skewed by higher rates of negative health factors, such as obesity and hypertension, in the artificial sweetener group.
Dr. Liu and colleagues noted that their findings align with previous studies linking coffee consumption with survival. Like those other studies, the present data revealed a “U-shaped” benefit curve, in which moderate coffee consumption was associated with longer life, whereas low or no consumption and high consumption were not.
Experts caution against drinking sweetened beverages despite new findings
Although the present findings suggested that adding sugar did not eliminate the health benefits of coffee, Dr. Liu and colleagues still cautioned against sweetened beverages, citing widely known associations between sugar consumption and poor health.
In an accompanying editorial, Christina C. Wee, MD, MPH, deputy editor of Annals of Internal Medicine, pointed out a key detail from the data: the amount of sugar added to coffee in the U.K. study may be dwarfed by the amount consumed by some coffee drinkers across the pond.
“The average dose of added sugar per cup of sweetened coffee [in the study] was only a little over a teaspoon, or about 4 grams,” Dr. Wee wrote. “This is a far cry from the 15 grams of sugar in an 8-ounce cup of caramel macchiato at a popular U.S. coffee chain.”
Still, Dr. Wee, an associate professor of medicine at Harvard Medical School, Boston, and director of the obesity research program in the division of general medicine at Beth Israel Deaconess Medical Center, Boston, suggested that your typical coffee drinker can feel safe in their daily habit.
“The evidence does not suggest a need for most coffee drinkers – particularly those who drink it with no or modest amounts of sugar – to eliminate coffee,” she wrote. “So drink up – but it would be prudent to avoid too many caramel macchiatos while more evidence brews.”
Estefanía Toledo, MD, MPH, PhD, of the department of preventive medicine and public health at the University of Navarra, Pamplona, Spain, offered a similar takeaway.
“For those who enjoy drinking coffee, are not pregnant or lactating, and do not have special health conditions, coffee consumption could be considered part of a healthy lifestyle,” Dr. Toledo said in a written comment. “I would recommend adding as little sugar as possible to coffee until more evidence has been accrued.”
Dr. Toledo, who previously published a study showing a link between coffee and extended survival, noted that moderate coffee consumption has “repeatedly” been associated with lower rates of “several chronic diseases” and death, but there still isn’t enough evidence to recommend coffee for those who don’t already drink it.
More long-term research is needed, Dr. Toledo said, ideally with studies comparing changes in coffee consumption and health outcomes over time. These may not be forthcoming, however, as such trials are “not easy and feasible to conduct.”
David Kao, MD, assistant professor of medicine-cardiology and medical director of the school of medicine at the University of Colorado at Denver, Aurora, said that the study conducted by Dr. Liu and colleagues is a “very well-executed analysis” that strengthens our confidence in the safety of long-term coffee consumption, even for patients with heart disease.
Dr. Kao, who recently published an analysis showing that higher coffee intake is associated with a lower risk of heart failure, refrained from advising anyone to up their coffee quota.
“I remain cautious about stating too strongly that people should increase coffee intake purely to improve survival,” Dr. Kao said in a written comment. “That said, it does not appear harmful to increase it some, until you drink consistently more than six to seven cups per day.”
The study was supported by the National Natural Science Foundation of China, the Young Elite Scientist Sponsorship Program by CAST, the Guangdong Basic and Applied Basic Research Foundation, and others. Dr. Toledo and Dr. Kao disclosed no relevant conflicts of interest.
Among more than 170,000 people in the United Kingdom, those who drank about two to four cups of coffee a day, with or without sugar, had a lower rate of death than those who didn’t drink coffee, reported lead author Dan Liu, MD, of the department of epidemiology at Southern Medical University, Guangzhou, China.
“Previous observational studies have suggested an association between coffee intake and reduced risk for death, but they did not distinguish between coffee consumed with sugar or artificial sweeteners and coffee consumed without,” Dr. Liu, who is also of the department of public health and preventive medicine, Jinan University, Guangzhou, China, and colleagues wrote in Annals of Internal Medicine.
To learn more, the investigators turned to the UK Biobank, which recruited approximately half a million participants in the United Kingdom between 2006 and 2010 to undergo a variety of questionnaires, interviews, physical measurements, and medical tests. Out of this group, 171,616 participants completed at least one dietary questionnaire and met the criteria for the present study, including lack of cancer or cardiovascular disease upon enrollment.
Results from these questionnaires showed that 55.4% of participants drank coffee without any sweetener, 14.3% drank coffee with sugar, 6.1% drank coffee with artificial sweetener, and 24.2% did not drink coffee at all. Coffee drinkers were further sorted into groups based on how many cups of coffee they drank per day.
Coffee drinkers were significantly less likely to die from any cause
Over the course of about 7 years, 3,177 of the participants died, including 1,725 who died from cancer and 628 who died from cardiovascular disease.
After accounting for other factors that might impact risk of death, like lifestyle choices, the investigators found that coffee drinkers were significantly less likely to die from any cause, cardiovascular disease, or cancer, than those who didn’t drink coffee at all. This benefit was observed across types of coffee, including ground, instant, and decaffeinated varieties. The protective effects of coffee were most apparent in people who drank about two to four cups a day, among whom death was about 30% less likely, regardless of whether they added sugar to their coffee or not. Individuals who drank coffee with artificial sweetener did not live significantly longer than those who drank no coffee at all; however, the investigators suggested that this result may have been skewed by higher rates of negative health factors, such as obesity and hypertension, in the artificial sweetener group.
Dr. Liu and colleagues noted that their findings align with previous studies linking coffee consumption with survival. Like those other studies, the present data revealed a “U-shaped” benefit curve, in which moderate coffee consumption was associated with longer life, whereas low or no consumption and high consumption were not.
Experts caution against drinking sweetened beverages despite new findings
Although the present findings suggested that adding sugar did not eliminate the health benefits of coffee, Dr. Liu and colleagues still cautioned against sweetened beverages, citing widely known associations between sugar consumption and poor health.
In an accompanying editorial, Christina C. Wee, MD, MPH, deputy editor of Annals of Internal Medicine, pointed out a key detail from the data: the amount of sugar added to coffee in the U.K. study may be dwarfed by the amount consumed by some coffee drinkers across the pond.
“The average dose of added sugar per cup of sweetened coffee [in the study] was only a little over a teaspoon, or about 4 grams,” Dr. Wee wrote. “This is a far cry from the 15 grams of sugar in an 8-ounce cup of caramel macchiato at a popular U.S. coffee chain.”
Still, Dr. Wee, an associate professor of medicine at Harvard Medical School, Boston, and director of the obesity research program in the division of general medicine at Beth Israel Deaconess Medical Center, Boston, suggested that your typical coffee drinker can feel safe in their daily habit.
“The evidence does not suggest a need for most coffee drinkers – particularly those who drink it with no or modest amounts of sugar – to eliminate coffee,” she wrote. “So drink up – but it would be prudent to avoid too many caramel macchiatos while more evidence brews.”
Estefanía Toledo, MD, MPH, PhD, of the department of preventive medicine and public health at the University of Navarra, Pamplona, Spain, offered a similar takeaway.
“For those who enjoy drinking coffee, are not pregnant or lactating, and do not have special health conditions, coffee consumption could be considered part of a healthy lifestyle,” Dr. Toledo said in a written comment. “I would recommend adding as little sugar as possible to coffee until more evidence has been accrued.”
Dr. Toledo, who previously published a study showing a link between coffee and extended survival, noted that moderate coffee consumption has “repeatedly” been associated with lower rates of “several chronic diseases” and death, but there still isn’t enough evidence to recommend coffee for those who don’t already drink it.
More long-term research is needed, Dr. Toledo said, ideally with studies comparing changes in coffee consumption and health outcomes over time. These may not be forthcoming, however, as such trials are “not easy and feasible to conduct.”
David Kao, MD, assistant professor of medicine-cardiology and medical director of the school of medicine at the University of Colorado at Denver, Aurora, said that the study conducted by Dr. Liu and colleagues is a “very well-executed analysis” that strengthens our confidence in the safety of long-term coffee consumption, even for patients with heart disease.
Dr. Kao, who recently published an analysis showing that higher coffee intake is associated with a lower risk of heart failure, refrained from advising anyone to up their coffee quota.
“I remain cautious about stating too strongly that people should increase coffee intake purely to improve survival,” Dr. Kao said in a written comment. “That said, it does not appear harmful to increase it some, until you drink consistently more than six to seven cups per day.”
The study was supported by the National Natural Science Foundation of China, the Young Elite Scientist Sponsorship Program by CAST, the Guangdong Basic and Applied Basic Research Foundation, and others. Dr. Toledo and Dr. Kao disclosed no relevant conflicts of interest.
Among more than 170,000 people in the United Kingdom, those who drank about two to four cups of coffee a day, with or without sugar, had a lower rate of death than those who didn’t drink coffee, reported lead author Dan Liu, MD, of the department of epidemiology at Southern Medical University, Guangzhou, China.
“Previous observational studies have suggested an association between coffee intake and reduced risk for death, but they did not distinguish between coffee consumed with sugar or artificial sweeteners and coffee consumed without,” Dr. Liu, who is also of the department of public health and preventive medicine, Jinan University, Guangzhou, China, and colleagues wrote in Annals of Internal Medicine.
To learn more, the investigators turned to the UK Biobank, which recruited approximately half a million participants in the United Kingdom between 2006 and 2010 to undergo a variety of questionnaires, interviews, physical measurements, and medical tests. Out of this group, 171,616 participants completed at least one dietary questionnaire and met the criteria for the present study, including lack of cancer or cardiovascular disease upon enrollment.
Results from these questionnaires showed that 55.4% of participants drank coffee without any sweetener, 14.3% drank coffee with sugar, 6.1% drank coffee with artificial sweetener, and 24.2% did not drink coffee at all. Coffee drinkers were further sorted into groups based on how many cups of coffee they drank per day.
Coffee drinkers were significantly less likely to die from any cause
Over the course of about 7 years, 3,177 of the participants died, including 1,725 who died from cancer and 628 who died from cardiovascular disease.
After accounting for other factors that might impact risk of death, like lifestyle choices, the investigators found that coffee drinkers were significantly less likely to die from any cause, cardiovascular disease, or cancer, than those who didn’t drink coffee at all. This benefit was observed across types of coffee, including ground, instant, and decaffeinated varieties. The protective effects of coffee were most apparent in people who drank about two to four cups a day, among whom death was about 30% less likely, regardless of whether they added sugar to their coffee or not. Individuals who drank coffee with artificial sweetener did not live significantly longer than those who drank no coffee at all; however, the investigators suggested that this result may have been skewed by higher rates of negative health factors, such as obesity and hypertension, in the artificial sweetener group.
Dr. Liu and colleagues noted that their findings align with previous studies linking coffee consumption with survival. Like those other studies, the present data revealed a “U-shaped” benefit curve, in which moderate coffee consumption was associated with longer life, whereas low or no consumption and high consumption were not.
Experts caution against drinking sweetened beverages despite new findings
Although the present findings suggested that adding sugar did not eliminate the health benefits of coffee, Dr. Liu and colleagues still cautioned against sweetened beverages, citing widely known associations between sugar consumption and poor health.
In an accompanying editorial, Christina C. Wee, MD, MPH, deputy editor of Annals of Internal Medicine, pointed out a key detail from the data: the amount of sugar added to coffee in the U.K. study may be dwarfed by the amount consumed by some coffee drinkers across the pond.
“The average dose of added sugar per cup of sweetened coffee [in the study] was only a little over a teaspoon, or about 4 grams,” Dr. Wee wrote. “This is a far cry from the 15 grams of sugar in an 8-ounce cup of caramel macchiato at a popular U.S. coffee chain.”
Still, Dr. Wee, an associate professor of medicine at Harvard Medical School, Boston, and director of the obesity research program in the division of general medicine at Beth Israel Deaconess Medical Center, Boston, suggested that your typical coffee drinker can feel safe in their daily habit.
“The evidence does not suggest a need for most coffee drinkers – particularly those who drink it with no or modest amounts of sugar – to eliminate coffee,” she wrote. “So drink up – but it would be prudent to avoid too many caramel macchiatos while more evidence brews.”
Estefanía Toledo, MD, MPH, PhD, of the department of preventive medicine and public health at the University of Navarra, Pamplona, Spain, offered a similar takeaway.
“For those who enjoy drinking coffee, are not pregnant or lactating, and do not have special health conditions, coffee consumption could be considered part of a healthy lifestyle,” Dr. Toledo said in a written comment. “I would recommend adding as little sugar as possible to coffee until more evidence has been accrued.”
Dr. Toledo, who previously published a study showing a link between coffee and extended survival, noted that moderate coffee consumption has “repeatedly” been associated with lower rates of “several chronic diseases” and death, but there still isn’t enough evidence to recommend coffee for those who don’t already drink it.
More long-term research is needed, Dr. Toledo said, ideally with studies comparing changes in coffee consumption and health outcomes over time. These may not be forthcoming, however, as such trials are “not easy and feasible to conduct.”
David Kao, MD, assistant professor of medicine-cardiology and medical director of the school of medicine at the University of Colorado at Denver, Aurora, said that the study conducted by Dr. Liu and colleagues is a “very well-executed analysis” that strengthens our confidence in the safety of long-term coffee consumption, even for patients with heart disease.
Dr. Kao, who recently published an analysis showing that higher coffee intake is associated with a lower risk of heart failure, refrained from advising anyone to up their coffee quota.
“I remain cautious about stating too strongly that people should increase coffee intake purely to improve survival,” Dr. Kao said in a written comment. “That said, it does not appear harmful to increase it some, until you drink consistently more than six to seven cups per day.”
The study was supported by the National Natural Science Foundation of China, the Young Elite Scientist Sponsorship Program by CAST, the Guangdong Basic and Applied Basic Research Foundation, and others. Dr. Toledo and Dr. Kao disclosed no relevant conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE