User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
CDC: 20% of people in the U.S. are infected with an STD
Among the more than 320 million people in the United States, there was a prevalence estimate of 67.6 million sexually transmitted infections at the time of assessment in 2018, according to the results of an epidemiologic study using multiple data sources, including the National Health and Nutrition Examination Survey (NHANES).
In addition, almost half of the incident STIs occurred in the 15- to 24-year age bracket, according to a report published online in Sexually Transmitted Diseases. Researchers estimated the combined number of prevalent and incident infections of eight STIs in the United States in 2018: chlamydia, gonorrhea, trichomoniasis, syphilis, genital herpes (caused by herpes simplex virus type 2 [HSV-2]), human papillomavirus (HPV), sexually transmitted hepatitis B virus (HBV), and sexually transmitted HIV.
The estimated incidences of these STIs in this update, the first since 2008, were made using more recent data and improved estimation methods to provide updated STI prevalence and incidence estimates for 2018, both overall and by disease. “Having a combined estimate is crucial for policy purposes to illustrate the importance of STIs in the United States,” according to Kristen M. Kreisel, PhD, an epidemiologist at the Centers for Disease Control and Prevention, division of STD prevention, and colleagues.
The number of prevalent and incident infections were obtained by multiplying each STI’s updated per capita estimates by the 2018 full resident population estimates from the American Community Survey.
Detailed results
Chlamydia. The prevalence of chlamydia was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. There were an estimated 2.4 million prevalent urogenital chlamydial infections among persons aged 15-39 years in 2018; 1.1 and 1.3 million infections among men and women, respectively. Individuals aged 15-24 years comprised 56.7% and 75.8% of all infections in men and women respectively.
Gonorrhea. The prevalence of gonorrhea was estimated using ordinary differential equation based modeling. The number of prevalent urogenital gonococcal infections in 2018 among 15- to 39-year-olds was 209,000 overall; 50,000 in men and 155,000 in women. Of these, 113,000 (54.1%) occurred in 15- to 24-year-olds.
Trichomoniasis. The prevalence of trichomoniasis was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. The number of prevalent Trichomonas infections among 15- to 59-year-olds was 2.6 million, with 470,000 in men and 2.1 million in women. Persons aged 15-24 years comprised 15.6% of all prevalent infections, according to the authors.
Syphilis. The number of estimated prevalent syphilitic infections (all stages) among 14- to 49-year-old persons in 2018 was 156,000, with infections in men comprising 71.8% of all infections. Infections in both men and women aged 14-24 years accounted for about 25% of all infections, with 36,000 total prevalent syphilitic infections among 14- to 24-year-olds in 2018.
Genital herpes. The prevalence of genital herpes (caused by HSV-2) was estimated using 2015-2018 NHANES data, according to the authors. In persons aged 15-49 years in 2018, there were 18.6 million prevalent HSV-2 infections; 6.4 million among men and 12.2 million among women. Infections in 15- to 24-year-olds comprised 7.1% of all prevalent HSV-2 infections.
HPV. The prevalence of HPV was estimated using 2013-2016 NHANES data, which was assumed to reflect stable prevalence in 2018, according to the authors. Among 15- to 59-year-olds, the estimated number of persons, men, and women infected with one or more disease-associated HPV types in 2018 was 42.5, 23.4, and 19.2 million, respectively, with an estimated 9.0 million (21%) 15- to 24-year-olds infected,
HBV. NHANES 2013-2018 data were used to estimate the prevalence of sexually transmitted chronic HBV infections in 2018, according to the authors. The estimated number of infections among persons aged 15 years and older in 2018 was 103,000 (51,000 men and 52,000 women). There small sample size of individuals aged 15-24 years in the NHANES database made it impossible to obtain an accurate estimate for this group, according to the authors.
HIV. Data from the National HIV Surveillance System were used to estimate the prevalence and incidence of sexually transmitted HIV infections for persons aged 13 years and older in 2018. A total of 984,000 individuals aged 13 years and older were estimated to be living with sexually transmitted HIV at the end of 2018, according to the authors. Nearly 80% were men. In the 13- to 24-year-old age bracket, there were an estimated 45,400 living with sexually transmitted HIV.
Billions in costs
Commenting on the study by the CDC researchers, Raul Romaguera, acting director for CDC’s division of STD prevention, stated in a press release: “There are significant human and financial costs associated with these infections, and we know from other studies that cuts in STI prevention efforts result in higher costs down the road. Preventing STIs could save billions in medical costs, but more importantly, prevention would improve the health and lives of millions of people.”
“About 20% of the total U.S. population had an STI at a given point in 2018, while nearly half of all incident infections occurred in people aged 15-24 years. Focusing STI prevention efforts on the 15- to 24-year-old population may be key to lowering the STI burden in the U.S.,” the researchers concluded.
The authors reported that they had no disclosures.
Among the more than 320 million people in the United States, there was a prevalence estimate of 67.6 million sexually transmitted infections at the time of assessment in 2018, according to the results of an epidemiologic study using multiple data sources, including the National Health and Nutrition Examination Survey (NHANES).
In addition, almost half of the incident STIs occurred in the 15- to 24-year age bracket, according to a report published online in Sexually Transmitted Diseases. Researchers estimated the combined number of prevalent and incident infections of eight STIs in the United States in 2018: chlamydia, gonorrhea, trichomoniasis, syphilis, genital herpes (caused by herpes simplex virus type 2 [HSV-2]), human papillomavirus (HPV), sexually transmitted hepatitis B virus (HBV), and sexually transmitted HIV.
The estimated incidences of these STIs in this update, the first since 2008, were made using more recent data and improved estimation methods to provide updated STI prevalence and incidence estimates for 2018, both overall and by disease. “Having a combined estimate is crucial for policy purposes to illustrate the importance of STIs in the United States,” according to Kristen M. Kreisel, PhD, an epidemiologist at the Centers for Disease Control and Prevention, division of STD prevention, and colleagues.
The number of prevalent and incident infections were obtained by multiplying each STI’s updated per capita estimates by the 2018 full resident population estimates from the American Community Survey.
Detailed results
Chlamydia. The prevalence of chlamydia was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. There were an estimated 2.4 million prevalent urogenital chlamydial infections among persons aged 15-39 years in 2018; 1.1 and 1.3 million infections among men and women, respectively. Individuals aged 15-24 years comprised 56.7% and 75.8% of all infections in men and women respectively.
Gonorrhea. The prevalence of gonorrhea was estimated using ordinary differential equation based modeling. The number of prevalent urogenital gonococcal infections in 2018 among 15- to 39-year-olds was 209,000 overall; 50,000 in men and 155,000 in women. Of these, 113,000 (54.1%) occurred in 15- to 24-year-olds.
Trichomoniasis. The prevalence of trichomoniasis was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. The number of prevalent Trichomonas infections among 15- to 59-year-olds was 2.6 million, with 470,000 in men and 2.1 million in women. Persons aged 15-24 years comprised 15.6% of all prevalent infections, according to the authors.
Syphilis. The number of estimated prevalent syphilitic infections (all stages) among 14- to 49-year-old persons in 2018 was 156,000, with infections in men comprising 71.8% of all infections. Infections in both men and women aged 14-24 years accounted for about 25% of all infections, with 36,000 total prevalent syphilitic infections among 14- to 24-year-olds in 2018.
Genital herpes. The prevalence of genital herpes (caused by HSV-2) was estimated using 2015-2018 NHANES data, according to the authors. In persons aged 15-49 years in 2018, there were 18.6 million prevalent HSV-2 infections; 6.4 million among men and 12.2 million among women. Infections in 15- to 24-year-olds comprised 7.1% of all prevalent HSV-2 infections.
HPV. The prevalence of HPV was estimated using 2013-2016 NHANES data, which was assumed to reflect stable prevalence in 2018, according to the authors. Among 15- to 59-year-olds, the estimated number of persons, men, and women infected with one or more disease-associated HPV types in 2018 was 42.5, 23.4, and 19.2 million, respectively, with an estimated 9.0 million (21%) 15- to 24-year-olds infected,
HBV. NHANES 2013-2018 data were used to estimate the prevalence of sexually transmitted chronic HBV infections in 2018, according to the authors. The estimated number of infections among persons aged 15 years and older in 2018 was 103,000 (51,000 men and 52,000 women). There small sample size of individuals aged 15-24 years in the NHANES database made it impossible to obtain an accurate estimate for this group, according to the authors.
HIV. Data from the National HIV Surveillance System were used to estimate the prevalence and incidence of sexually transmitted HIV infections for persons aged 13 years and older in 2018. A total of 984,000 individuals aged 13 years and older were estimated to be living with sexually transmitted HIV at the end of 2018, according to the authors. Nearly 80% were men. In the 13- to 24-year-old age bracket, there were an estimated 45,400 living with sexually transmitted HIV.
Billions in costs
Commenting on the study by the CDC researchers, Raul Romaguera, acting director for CDC’s division of STD prevention, stated in a press release: “There are significant human and financial costs associated with these infections, and we know from other studies that cuts in STI prevention efforts result in higher costs down the road. Preventing STIs could save billions in medical costs, but more importantly, prevention would improve the health and lives of millions of people.”
“About 20% of the total U.S. population had an STI at a given point in 2018, while nearly half of all incident infections occurred in people aged 15-24 years. Focusing STI prevention efforts on the 15- to 24-year-old population may be key to lowering the STI burden in the U.S.,” the researchers concluded.
The authors reported that they had no disclosures.
Among the more than 320 million people in the United States, there was a prevalence estimate of 67.6 million sexually transmitted infections at the time of assessment in 2018, according to the results of an epidemiologic study using multiple data sources, including the National Health and Nutrition Examination Survey (NHANES).
In addition, almost half of the incident STIs occurred in the 15- to 24-year age bracket, according to a report published online in Sexually Transmitted Diseases. Researchers estimated the combined number of prevalent and incident infections of eight STIs in the United States in 2018: chlamydia, gonorrhea, trichomoniasis, syphilis, genital herpes (caused by herpes simplex virus type 2 [HSV-2]), human papillomavirus (HPV), sexually transmitted hepatitis B virus (HBV), and sexually transmitted HIV.
The estimated incidences of these STIs in this update, the first since 2008, were made using more recent data and improved estimation methods to provide updated STI prevalence and incidence estimates for 2018, both overall and by disease. “Having a combined estimate is crucial for policy purposes to illustrate the importance of STIs in the United States,” according to Kristen M. Kreisel, PhD, an epidemiologist at the Centers for Disease Control and Prevention, division of STD prevention, and colleagues.
The number of prevalent and incident infections were obtained by multiplying each STI’s updated per capita estimates by the 2018 full resident population estimates from the American Community Survey.
Detailed results
Chlamydia. The prevalence of chlamydia was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. There were an estimated 2.4 million prevalent urogenital chlamydial infections among persons aged 15-39 years in 2018; 1.1 and 1.3 million infections among men and women, respectively. Individuals aged 15-24 years comprised 56.7% and 75.8% of all infections in men and women respectively.
Gonorrhea. The prevalence of gonorrhea was estimated using ordinary differential equation based modeling. The number of prevalent urogenital gonococcal infections in 2018 among 15- to 39-year-olds was 209,000 overall; 50,000 in men and 155,000 in women. Of these, 113,000 (54.1%) occurred in 15- to 24-year-olds.
Trichomoniasis. The prevalence of trichomoniasis was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. The number of prevalent Trichomonas infections among 15- to 59-year-olds was 2.6 million, with 470,000 in men and 2.1 million in women. Persons aged 15-24 years comprised 15.6% of all prevalent infections, according to the authors.
Syphilis. The number of estimated prevalent syphilitic infections (all stages) among 14- to 49-year-old persons in 2018 was 156,000, with infections in men comprising 71.8% of all infections. Infections in both men and women aged 14-24 years accounted for about 25% of all infections, with 36,000 total prevalent syphilitic infections among 14- to 24-year-olds in 2018.
Genital herpes. The prevalence of genital herpes (caused by HSV-2) was estimated using 2015-2018 NHANES data, according to the authors. In persons aged 15-49 years in 2018, there were 18.6 million prevalent HSV-2 infections; 6.4 million among men and 12.2 million among women. Infections in 15- to 24-year-olds comprised 7.1% of all prevalent HSV-2 infections.
HPV. The prevalence of HPV was estimated using 2013-2016 NHANES data, which was assumed to reflect stable prevalence in 2018, according to the authors. Among 15- to 59-year-olds, the estimated number of persons, men, and women infected with one or more disease-associated HPV types in 2018 was 42.5, 23.4, and 19.2 million, respectively, with an estimated 9.0 million (21%) 15- to 24-year-olds infected,
HBV. NHANES 2013-2018 data were used to estimate the prevalence of sexually transmitted chronic HBV infections in 2018, according to the authors. The estimated number of infections among persons aged 15 years and older in 2018 was 103,000 (51,000 men and 52,000 women). There small sample size of individuals aged 15-24 years in the NHANES database made it impossible to obtain an accurate estimate for this group, according to the authors.
HIV. Data from the National HIV Surveillance System were used to estimate the prevalence and incidence of sexually transmitted HIV infections for persons aged 13 years and older in 2018. A total of 984,000 individuals aged 13 years and older were estimated to be living with sexually transmitted HIV at the end of 2018, according to the authors. Nearly 80% were men. In the 13- to 24-year-old age bracket, there were an estimated 45,400 living with sexually transmitted HIV.
Billions in costs
Commenting on the study by the CDC researchers, Raul Romaguera, acting director for CDC’s division of STD prevention, stated in a press release: “There are significant human and financial costs associated with these infections, and we know from other studies that cuts in STI prevention efforts result in higher costs down the road. Preventing STIs could save billions in medical costs, but more importantly, prevention would improve the health and lives of millions of people.”
“About 20% of the total U.S. population had an STI at a given point in 2018, while nearly half of all incident infections occurred in people aged 15-24 years. Focusing STI prevention efforts on the 15- to 24-year-old population may be key to lowering the STI burden in the U.S.,” the researchers concluded.
The authors reported that they had no disclosures.
FROM SEXUALLY TRANSMITTED DISEASES
Monoclonal antibody drops fat, ups muscle in obesity, diabetes
In a phase 2 randomized clinical trial of adults with type 2 diabetes and obesity, investigational drug bimagrumab (BYM338, Novartis) – a monoclonal antibody that blocks activin type II receptors and stimulates skeletal muscle growth – led to big reductions in total body fat mass and A1c and significant increases in lean mass compared with placebo.
The efficacy and safety findings “suggest that blockade of the activin receptor with bimagrumab could provide a novel pharmacologic approach for managing patients with type 2 diabetes with excess adiposity,” Steven B. Heymsfield, MD, Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, and colleagues reported in their study, published online Jan. 13 in JAMA Network Open.
Preliminary findings from the study of 75 patients treated for 48 weeks – in which neither group ate less despite intensive nutrition advice – were presented at Obesity Week in 2019.
As reported then, Lee M. Kaplan, MD, PhD, noted that the 6.5% weight loss in the bimagrumab group was similar to that seen with antiobesity medications that suppress appetite.
“What it suggests,” he said in an interview, “is that there may be a completely new mechanism at play here,” because patients receiving bimagrumab weren’t eating less but were losing the same amount of weight as reported for weight-loss drugs that work by decreasing appetite.
“Is this going to be the kind of complementary drug with a different mechanism that’s going to augment the effects of other drugs?” wondered Dr. Kaplan, director of the Obesity, Metabolism & Nutrition Institute at Massachusetts General Hospital, Boston, who has previously served as a scientific consultant to Novartis.
Asked about future plans for bimagrumab, a Novartis spokesperson said in an interview, “We are currently reviewing the program strategy and considering next steps.”
Four FDA-approved weight-loss drugs now approved
The Food and Drug Administration approval for lorcaserin (Belviq, Belviq XR, Eisai) for weight loss was rescinded on Feb. 13, 2020, when a postmarketing trial revealed an increased occurrence of cancer, leaving four drugs approved for weight loss in the United States, plus several drugs in development, Dr. Heymsfield and colleagues wrote.
The current phase 2 trial was designed to determine the safety and efficacy of bimagrumab – which had originally been studied to see if it would increase lean muscle mass in people with sarcopenia – on total body fat mass and glycemic control in patients with type 2 diabetes and overweight or obesity.
Researchers enrolled 75 adults at eight sites in the United States and one in Wales, United Kingdom, from 2017 to 2019.
On average, patients were 60 years old with an A1c of 7.8% and a body mass index of 32.9 kg/m2; they weighed 93.6 kg and had a fat mass of 35 kg.
Patients received an intravenous infusion of bimagrumab (10 mg/kg up to 1,200 mg in 5% dextrose solution) or placebo (5% dextrose solution) every 4 weeks for 48 weeks. They met with a registered dietitian at each monthly study visit and had a virtual check-in between visits.
Participants were advised to follow a diet that would cut 500 calories a day and encouraged to follow the American Diabetes Association walking program.
Body fat mass was measured by dual-energy x-ray absorptiometry (DEXA).
There were more women in the bimagrumab group than in the placebo group (62% vs. 32%), but baseline BMI, total body fat mass, and A1c were similar in both groups.
Same caloric intake, less fat tissue, more muscle, smaller waist
At 48 weeks in the bimagrumab vs. placebo group, there was on average (all P < .001):
- A loss of 20.5% vs. 0.5% (−7.5 vs. −0.2 kg) of total body fat mass.
- A loss of 6.5% vs. 0.8% (−5.9 vs. −0.8 kg) of body weight.
- A gain of 3.6% vs. a loss of 0.8% (1.7 vs. −0.4 kg) of lean mass.
Similarly, the relatively large between-group differences in total body fat mass and body weight at 48 weeks with bimagrumab were accompanied by favorable differences in BMI (−2.19 vs. −0.28 kg/m2; P < .001) and waist circumference (−9.0 vs. 0.5 cm; P < .001), the investigators pointed out.
Moreover, the reduction of abdominal visceral adipose tissue and waist circumference with bimagrumab “was nearly twice that observed in a recently published study of patients with type 2 diabetes treated with an intensive lifestyle program and the glucagon-like peptide 1 (GLP-1) agonist liraglutide,” they noted.
This highlights “the importance of moving away from body weight as a primary efficacy marker of drugs to more metabolically relevant endpoints.”
Also, A1c decreased by 0.76% in the bimagrumab group and increased by 0.04% in the placebo group (P = .005).
Serious adverse events occurred in three patients (8%) in the bimagrumab group (elevated lipase, epigastric pain, pancreatitis, pneumonia) and three patients (8%) in the placebo group (cellulitis, acute coronary syndrome, acute myocardial infarction, worsening gastroparesis, thermal burn).
Adverse events were reported by 31 of 37 patients in the bimagrumab group, most often mild diarrhea (41%) and muscle spasms (41%), and 31 of 38 patients in the placebo group, most often headache (13%) and upper respiratory tract infection (13%).
The study was funded by Novartis. Dr. Heymsfield has reported receiving personal fees from Tanita and Medifast outside the submitted work. Disclosures for the other authors are listed in the article. Dr. Kaplan has reported previously serving as a scientific consultant to Novartis.
A version of this article first appeared on Medscape.com.
In a phase 2 randomized clinical trial of adults with type 2 diabetes and obesity, investigational drug bimagrumab (BYM338, Novartis) – a monoclonal antibody that blocks activin type II receptors and stimulates skeletal muscle growth – led to big reductions in total body fat mass and A1c and significant increases in lean mass compared with placebo.
The efficacy and safety findings “suggest that blockade of the activin receptor with bimagrumab could provide a novel pharmacologic approach for managing patients with type 2 diabetes with excess adiposity,” Steven B. Heymsfield, MD, Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, and colleagues reported in their study, published online Jan. 13 in JAMA Network Open.
Preliminary findings from the study of 75 patients treated for 48 weeks – in which neither group ate less despite intensive nutrition advice – were presented at Obesity Week in 2019.
As reported then, Lee M. Kaplan, MD, PhD, noted that the 6.5% weight loss in the bimagrumab group was similar to that seen with antiobesity medications that suppress appetite.
“What it suggests,” he said in an interview, “is that there may be a completely new mechanism at play here,” because patients receiving bimagrumab weren’t eating less but were losing the same amount of weight as reported for weight-loss drugs that work by decreasing appetite.
“Is this going to be the kind of complementary drug with a different mechanism that’s going to augment the effects of other drugs?” wondered Dr. Kaplan, director of the Obesity, Metabolism & Nutrition Institute at Massachusetts General Hospital, Boston, who has previously served as a scientific consultant to Novartis.
Asked about future plans for bimagrumab, a Novartis spokesperson said in an interview, “We are currently reviewing the program strategy and considering next steps.”
Four FDA-approved weight-loss drugs now approved
The Food and Drug Administration approval for lorcaserin (Belviq, Belviq XR, Eisai) for weight loss was rescinded on Feb. 13, 2020, when a postmarketing trial revealed an increased occurrence of cancer, leaving four drugs approved for weight loss in the United States, plus several drugs in development, Dr. Heymsfield and colleagues wrote.
The current phase 2 trial was designed to determine the safety and efficacy of bimagrumab – which had originally been studied to see if it would increase lean muscle mass in people with sarcopenia – on total body fat mass and glycemic control in patients with type 2 diabetes and overweight or obesity.
Researchers enrolled 75 adults at eight sites in the United States and one in Wales, United Kingdom, from 2017 to 2019.
On average, patients were 60 years old with an A1c of 7.8% and a body mass index of 32.9 kg/m2; they weighed 93.6 kg and had a fat mass of 35 kg.
Patients received an intravenous infusion of bimagrumab (10 mg/kg up to 1,200 mg in 5% dextrose solution) or placebo (5% dextrose solution) every 4 weeks for 48 weeks. They met with a registered dietitian at each monthly study visit and had a virtual check-in between visits.
Participants were advised to follow a diet that would cut 500 calories a day and encouraged to follow the American Diabetes Association walking program.
Body fat mass was measured by dual-energy x-ray absorptiometry (DEXA).
There were more women in the bimagrumab group than in the placebo group (62% vs. 32%), but baseline BMI, total body fat mass, and A1c were similar in both groups.
Same caloric intake, less fat tissue, more muscle, smaller waist
At 48 weeks in the bimagrumab vs. placebo group, there was on average (all P < .001):
- A loss of 20.5% vs. 0.5% (−7.5 vs. −0.2 kg) of total body fat mass.
- A loss of 6.5% vs. 0.8% (−5.9 vs. −0.8 kg) of body weight.
- A gain of 3.6% vs. a loss of 0.8% (1.7 vs. −0.4 kg) of lean mass.
Similarly, the relatively large between-group differences in total body fat mass and body weight at 48 weeks with bimagrumab were accompanied by favorable differences in BMI (−2.19 vs. −0.28 kg/m2; P < .001) and waist circumference (−9.0 vs. 0.5 cm; P < .001), the investigators pointed out.
Moreover, the reduction of abdominal visceral adipose tissue and waist circumference with bimagrumab “was nearly twice that observed in a recently published study of patients with type 2 diabetes treated with an intensive lifestyle program and the glucagon-like peptide 1 (GLP-1) agonist liraglutide,” they noted.
This highlights “the importance of moving away from body weight as a primary efficacy marker of drugs to more metabolically relevant endpoints.”
Also, A1c decreased by 0.76% in the bimagrumab group and increased by 0.04% in the placebo group (P = .005).
Serious adverse events occurred in three patients (8%) in the bimagrumab group (elevated lipase, epigastric pain, pancreatitis, pneumonia) and three patients (8%) in the placebo group (cellulitis, acute coronary syndrome, acute myocardial infarction, worsening gastroparesis, thermal burn).
Adverse events were reported by 31 of 37 patients in the bimagrumab group, most often mild diarrhea (41%) and muscle spasms (41%), and 31 of 38 patients in the placebo group, most often headache (13%) and upper respiratory tract infection (13%).
The study was funded by Novartis. Dr. Heymsfield has reported receiving personal fees from Tanita and Medifast outside the submitted work. Disclosures for the other authors are listed in the article. Dr. Kaplan has reported previously serving as a scientific consultant to Novartis.
A version of this article first appeared on Medscape.com.
In a phase 2 randomized clinical trial of adults with type 2 diabetes and obesity, investigational drug bimagrumab (BYM338, Novartis) – a monoclonal antibody that blocks activin type II receptors and stimulates skeletal muscle growth – led to big reductions in total body fat mass and A1c and significant increases in lean mass compared with placebo.
The efficacy and safety findings “suggest that blockade of the activin receptor with bimagrumab could provide a novel pharmacologic approach for managing patients with type 2 diabetes with excess adiposity,” Steven B. Heymsfield, MD, Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, and colleagues reported in their study, published online Jan. 13 in JAMA Network Open.
Preliminary findings from the study of 75 patients treated for 48 weeks – in which neither group ate less despite intensive nutrition advice – were presented at Obesity Week in 2019.
As reported then, Lee M. Kaplan, MD, PhD, noted that the 6.5% weight loss in the bimagrumab group was similar to that seen with antiobesity medications that suppress appetite.
“What it suggests,” he said in an interview, “is that there may be a completely new mechanism at play here,” because patients receiving bimagrumab weren’t eating less but were losing the same amount of weight as reported for weight-loss drugs that work by decreasing appetite.
“Is this going to be the kind of complementary drug with a different mechanism that’s going to augment the effects of other drugs?” wondered Dr. Kaplan, director of the Obesity, Metabolism & Nutrition Institute at Massachusetts General Hospital, Boston, who has previously served as a scientific consultant to Novartis.
Asked about future plans for bimagrumab, a Novartis spokesperson said in an interview, “We are currently reviewing the program strategy and considering next steps.”
Four FDA-approved weight-loss drugs now approved
The Food and Drug Administration approval for lorcaserin (Belviq, Belviq XR, Eisai) for weight loss was rescinded on Feb. 13, 2020, when a postmarketing trial revealed an increased occurrence of cancer, leaving four drugs approved for weight loss in the United States, plus several drugs in development, Dr. Heymsfield and colleagues wrote.
The current phase 2 trial was designed to determine the safety and efficacy of bimagrumab – which had originally been studied to see if it would increase lean muscle mass in people with sarcopenia – on total body fat mass and glycemic control in patients with type 2 diabetes and overweight or obesity.
Researchers enrolled 75 adults at eight sites in the United States and one in Wales, United Kingdom, from 2017 to 2019.
On average, patients were 60 years old with an A1c of 7.8% and a body mass index of 32.9 kg/m2; they weighed 93.6 kg and had a fat mass of 35 kg.
Patients received an intravenous infusion of bimagrumab (10 mg/kg up to 1,200 mg in 5% dextrose solution) or placebo (5% dextrose solution) every 4 weeks for 48 weeks. They met with a registered dietitian at each monthly study visit and had a virtual check-in between visits.
Participants were advised to follow a diet that would cut 500 calories a day and encouraged to follow the American Diabetes Association walking program.
Body fat mass was measured by dual-energy x-ray absorptiometry (DEXA).
There were more women in the bimagrumab group than in the placebo group (62% vs. 32%), but baseline BMI, total body fat mass, and A1c were similar in both groups.
Same caloric intake, less fat tissue, more muscle, smaller waist
At 48 weeks in the bimagrumab vs. placebo group, there was on average (all P < .001):
- A loss of 20.5% vs. 0.5% (−7.5 vs. −0.2 kg) of total body fat mass.
- A loss of 6.5% vs. 0.8% (−5.9 vs. −0.8 kg) of body weight.
- A gain of 3.6% vs. a loss of 0.8% (1.7 vs. −0.4 kg) of lean mass.
Similarly, the relatively large between-group differences in total body fat mass and body weight at 48 weeks with bimagrumab were accompanied by favorable differences in BMI (−2.19 vs. −0.28 kg/m2; P < .001) and waist circumference (−9.0 vs. 0.5 cm; P < .001), the investigators pointed out.
Moreover, the reduction of abdominal visceral adipose tissue and waist circumference with bimagrumab “was nearly twice that observed in a recently published study of patients with type 2 diabetes treated with an intensive lifestyle program and the glucagon-like peptide 1 (GLP-1) agonist liraglutide,” they noted.
This highlights “the importance of moving away from body weight as a primary efficacy marker of drugs to more metabolically relevant endpoints.”
Also, A1c decreased by 0.76% in the bimagrumab group and increased by 0.04% in the placebo group (P = .005).
Serious adverse events occurred in three patients (8%) in the bimagrumab group (elevated lipase, epigastric pain, pancreatitis, pneumonia) and three patients (8%) in the placebo group (cellulitis, acute coronary syndrome, acute myocardial infarction, worsening gastroparesis, thermal burn).
Adverse events were reported by 31 of 37 patients in the bimagrumab group, most often mild diarrhea (41%) and muscle spasms (41%), and 31 of 38 patients in the placebo group, most often headache (13%) and upper respiratory tract infection (13%).
The study was funded by Novartis. Dr. Heymsfield has reported receiving personal fees from Tanita and Medifast outside the submitted work. Disclosures for the other authors are listed in the article. Dr. Kaplan has reported previously serving as a scientific consultant to Novartis.
A version of this article first appeared on Medscape.com.
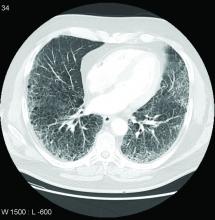
Lung disease raises mortality risk in older RA patients
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
FROM RHEUMATOLOGY
Racial, social inequities persist in IBD
Although inflammatory bowel disease (IBD) affects primarily White patients, about one-quarter of cases are found in non-White racial and ethnic groups. Various factors have combined to lead to disparities in treatment and outcomes for non-Whites with IBD.
Ethnic and racial disparities, along with socioeconomic factors, were the subject of a presentation by Ruby Greywoode, MD, at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“Historical and present-day realities of racial inequity and factors that contribute to socioeconomic status [include] educational and housing policies, employment practices, and generational wealth. Addressing health disparities requires acknowledging these systemic factors,” said Dr. Greywoode, who is with Montefiore Medical Center in New York.
An important concept in discussing health disparity is social determinants of health, which refers to nonbiological factors that affect health and health outcomes. These are “the conditions in which people live, work, learn, and play that affect their health and their quality of life,” said Dr. Greywoode.
Dr. Greywoode shared examples of social determinants that affect economic stability and financial worry. One study found that one in six IBD patients reported not taking their medications because of cost considerations. A survey of about 900 adults showed that 1 in 4 delayed medical care – half of those because of cost; patients who delayed care were 2.5 times more likely to report an IBD flare in the previous year.
Another important issue is food insecurity. Other presenters at the session emphasized the importance of high-quality nutrition in IBD, and Dr. Greywoode presented one survey showing that only 9% of patients who had both food security and social support reported cost-related medication nonadherence. Among those that had either food insecurity or poor social support, 12% reported cost-related medication nonadherence, but the proportion jumped to 57% among patients who had both food insecurity and lack of social support.
Session comoderator Kelly Issokson noted that socioeconomic factors often interfere with adoption of healthy diets. Whole foods and plant-based foods are expensive, and the financial pressures of the COVID-19 epidemic have made that worse. “Millions of people are slipping into poverty and food insecurity. This is one of the things she highlighted as factors in medication nonadherence,” said Ms. Issokson, who is the clinical nutritional coordinator at the digestive disease clinic at Cedars-Sinai Medical Center in Los Angeles.
Dr. Greywoode also described studies that looked at race, socioeconomic status, and IBD outcomes. A review from 2013 showed disparities among Whites, African Americans, and Hispanics with respect to undergoing ulcerative colitis–related colectomy and Crohn’s disease–related bowel resection. Ulcerative colitis patients on Medicaid had 230% greater in-patient mortality, compared with patients with private insurance, even after adjustment for multiple confounders.
But inequities are not static. “Since this publication, we have numerous other studies drawing conclusions that sometimes agree with and sometimes conflict with it. My belief is that health disparities in IBD will continue to be an active area of research. We know that it takes vigilance to identify, track, and address any disparities when they do arise,” said Dr. Greywoode.
Dr. Greywoode also noted that phenotypic differences based on race and ethnicity influence disparities. She showed results from a meta-analysis that found a difference in the frequency of perianal Crohn’s disease by race and ethnicity; the highest frequency occurred in Black patients (31%), followed by Asians (22%), Whites (14%), and Hispanics (13%). Another study showed that African American patients with Crohn’s disease were more likely to develop a new abscess (adjusted odds ratio, 2.27; 95% confidence interval, 1.31-3.93) or anal fissure (aOR, 1.76; 95% CI, 1.01-3.07), and were also more likely to be initiated on an anti–tumor necrosis factor drug (aOR, 1.85; 95% CI, 1.09-3.14).
Those differences underscore the need to recognize that IBD is not just a disease for White patients. “As we move forward in IBD research, we recognize that individuals of European ancestry are not the only ones who have IBD. There is a growing diverse racial and ethnic population with IBD,” said Dr. Greywoode.
She noted that, in the United States, it is estimated that about one in four adult patients are non-Hispanic African American, Hispanic, Asians, or other ethnicities. Nevertheless, Whites are overrepresented among participants in IBD clinical trials. Some trials are composed of as much as 95% White patients, and sometimes race isn’t even listed. “It’s unclear if [race/ethnicity data are] not collected or not deemed important, but we know that what is not collected is not measured, and what is not measured can’t be evaluated, either to praise or constructively criticize,” said Dr. Greywoode.
Fortunately, there are efforts in place to improve representation in clinical trials. There has been a mandate for almost 3 decades that federally funded research must include racial and ethnic minorities who have been traditionally underrepresented. The Food and Drug Administration has also provided guidance to industry to improve diversity in clinical trial participation, and industry groups have developed strategies, including improved representation among investigators and related early-career development programs. At the community and independent health care practice levels, clinical trial networks encourage patient participation with regulatory and data management support to bolster practices with insufficient resources.
Underrepresentation in clinical trials resonated with comoderator Tina Aswani Omprakash, who is a patient advocate and a master’s in public health student at the Icahn School of Medicine at Mount Sinai, New York. She called for greater awareness among physicians that IBD can occur among people of all backgrounds. “[Providers] would look at me and [say]: ‘There’s no way that, as a South Asian woman, you have that kind of disease.’ There’s that lack of believability,” said Ms. Aswani Omprakash.
Greater recognition of the diversity of patients, as well as the phenotypic differences found among ethnicities, could also inform clinical trial participation and, ultimately, more personalized medicine. “We have to look at these things, observe how they’re affecting populations differently, so that we can have proper medication solutions,” said Ms. Aswani Omprakash.
Dr. Greywoode and Ms. Issokson have no relevant financial disclosures. Ms. Aswani Omprakash has consulted for Genentech, AbbVie, Janssen, and Arena.
The AGA applauds researchers who are working to raise our awareness of health disparities in digestive diseases. The AGA is committed to addressing this important societal issue head on. Learn more about AGA’s commitment through the AGA Equity Project.
Updated Feb. 17, 2021.
Although inflammatory bowel disease (IBD) affects primarily White patients, about one-quarter of cases are found in non-White racial and ethnic groups. Various factors have combined to lead to disparities in treatment and outcomes for non-Whites with IBD.
Ethnic and racial disparities, along with socioeconomic factors, were the subject of a presentation by Ruby Greywoode, MD, at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“Historical and present-day realities of racial inequity and factors that contribute to socioeconomic status [include] educational and housing policies, employment practices, and generational wealth. Addressing health disparities requires acknowledging these systemic factors,” said Dr. Greywoode, who is with Montefiore Medical Center in New York.
An important concept in discussing health disparity is social determinants of health, which refers to nonbiological factors that affect health and health outcomes. These are “the conditions in which people live, work, learn, and play that affect their health and their quality of life,” said Dr. Greywoode.
Dr. Greywoode shared examples of social determinants that affect economic stability and financial worry. One study found that one in six IBD patients reported not taking their medications because of cost considerations. A survey of about 900 adults showed that 1 in 4 delayed medical care – half of those because of cost; patients who delayed care were 2.5 times more likely to report an IBD flare in the previous year.
Another important issue is food insecurity. Other presenters at the session emphasized the importance of high-quality nutrition in IBD, and Dr. Greywoode presented one survey showing that only 9% of patients who had both food security and social support reported cost-related medication nonadherence. Among those that had either food insecurity or poor social support, 12% reported cost-related medication nonadherence, but the proportion jumped to 57% among patients who had both food insecurity and lack of social support.
Session comoderator Kelly Issokson noted that socioeconomic factors often interfere with adoption of healthy diets. Whole foods and plant-based foods are expensive, and the financial pressures of the COVID-19 epidemic have made that worse. “Millions of people are slipping into poverty and food insecurity. This is one of the things she highlighted as factors in medication nonadherence,” said Ms. Issokson, who is the clinical nutritional coordinator at the digestive disease clinic at Cedars-Sinai Medical Center in Los Angeles.
Dr. Greywoode also described studies that looked at race, socioeconomic status, and IBD outcomes. A review from 2013 showed disparities among Whites, African Americans, and Hispanics with respect to undergoing ulcerative colitis–related colectomy and Crohn’s disease–related bowel resection. Ulcerative colitis patients on Medicaid had 230% greater in-patient mortality, compared with patients with private insurance, even after adjustment for multiple confounders.
But inequities are not static. “Since this publication, we have numerous other studies drawing conclusions that sometimes agree with and sometimes conflict with it. My belief is that health disparities in IBD will continue to be an active area of research. We know that it takes vigilance to identify, track, and address any disparities when they do arise,” said Dr. Greywoode.
Dr. Greywoode also noted that phenotypic differences based on race and ethnicity influence disparities. She showed results from a meta-analysis that found a difference in the frequency of perianal Crohn’s disease by race and ethnicity; the highest frequency occurred in Black patients (31%), followed by Asians (22%), Whites (14%), and Hispanics (13%). Another study showed that African American patients with Crohn’s disease were more likely to develop a new abscess (adjusted odds ratio, 2.27; 95% confidence interval, 1.31-3.93) or anal fissure (aOR, 1.76; 95% CI, 1.01-3.07), and were also more likely to be initiated on an anti–tumor necrosis factor drug (aOR, 1.85; 95% CI, 1.09-3.14).
Those differences underscore the need to recognize that IBD is not just a disease for White patients. “As we move forward in IBD research, we recognize that individuals of European ancestry are not the only ones who have IBD. There is a growing diverse racial and ethnic population with IBD,” said Dr. Greywoode.
She noted that, in the United States, it is estimated that about one in four adult patients are non-Hispanic African American, Hispanic, Asians, or other ethnicities. Nevertheless, Whites are overrepresented among participants in IBD clinical trials. Some trials are composed of as much as 95% White patients, and sometimes race isn’t even listed. “It’s unclear if [race/ethnicity data are] not collected or not deemed important, but we know that what is not collected is not measured, and what is not measured can’t be evaluated, either to praise or constructively criticize,” said Dr. Greywoode.
Fortunately, there are efforts in place to improve representation in clinical trials. There has been a mandate for almost 3 decades that federally funded research must include racial and ethnic minorities who have been traditionally underrepresented. The Food and Drug Administration has also provided guidance to industry to improve diversity in clinical trial participation, and industry groups have developed strategies, including improved representation among investigators and related early-career development programs. At the community and independent health care practice levels, clinical trial networks encourage patient participation with regulatory and data management support to bolster practices with insufficient resources.
Underrepresentation in clinical trials resonated with comoderator Tina Aswani Omprakash, who is a patient advocate and a master’s in public health student at the Icahn School of Medicine at Mount Sinai, New York. She called for greater awareness among physicians that IBD can occur among people of all backgrounds. “[Providers] would look at me and [say]: ‘There’s no way that, as a South Asian woman, you have that kind of disease.’ There’s that lack of believability,” said Ms. Aswani Omprakash.
Greater recognition of the diversity of patients, as well as the phenotypic differences found among ethnicities, could also inform clinical trial participation and, ultimately, more personalized medicine. “We have to look at these things, observe how they’re affecting populations differently, so that we can have proper medication solutions,” said Ms. Aswani Omprakash.
Dr. Greywoode and Ms. Issokson have no relevant financial disclosures. Ms. Aswani Omprakash has consulted for Genentech, AbbVie, Janssen, and Arena.
The AGA applauds researchers who are working to raise our awareness of health disparities in digestive diseases. The AGA is committed to addressing this important societal issue head on. Learn more about AGA’s commitment through the AGA Equity Project.
Updated Feb. 17, 2021.
Although inflammatory bowel disease (IBD) affects primarily White patients, about one-quarter of cases are found in non-White racial and ethnic groups. Various factors have combined to lead to disparities in treatment and outcomes for non-Whites with IBD.
Ethnic and racial disparities, along with socioeconomic factors, were the subject of a presentation by Ruby Greywoode, MD, at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“Historical and present-day realities of racial inequity and factors that contribute to socioeconomic status [include] educational and housing policies, employment practices, and generational wealth. Addressing health disparities requires acknowledging these systemic factors,” said Dr. Greywoode, who is with Montefiore Medical Center in New York.
An important concept in discussing health disparity is social determinants of health, which refers to nonbiological factors that affect health and health outcomes. These are “the conditions in which people live, work, learn, and play that affect their health and their quality of life,” said Dr. Greywoode.
Dr. Greywoode shared examples of social determinants that affect economic stability and financial worry. One study found that one in six IBD patients reported not taking their medications because of cost considerations. A survey of about 900 adults showed that 1 in 4 delayed medical care – half of those because of cost; patients who delayed care were 2.5 times more likely to report an IBD flare in the previous year.
Another important issue is food insecurity. Other presenters at the session emphasized the importance of high-quality nutrition in IBD, and Dr. Greywoode presented one survey showing that only 9% of patients who had both food security and social support reported cost-related medication nonadherence. Among those that had either food insecurity or poor social support, 12% reported cost-related medication nonadherence, but the proportion jumped to 57% among patients who had both food insecurity and lack of social support.
Session comoderator Kelly Issokson noted that socioeconomic factors often interfere with adoption of healthy diets. Whole foods and plant-based foods are expensive, and the financial pressures of the COVID-19 epidemic have made that worse. “Millions of people are slipping into poverty and food insecurity. This is one of the things she highlighted as factors in medication nonadherence,” said Ms. Issokson, who is the clinical nutritional coordinator at the digestive disease clinic at Cedars-Sinai Medical Center in Los Angeles.
Dr. Greywoode also described studies that looked at race, socioeconomic status, and IBD outcomes. A review from 2013 showed disparities among Whites, African Americans, and Hispanics with respect to undergoing ulcerative colitis–related colectomy and Crohn’s disease–related bowel resection. Ulcerative colitis patients on Medicaid had 230% greater in-patient mortality, compared with patients with private insurance, even after adjustment for multiple confounders.
But inequities are not static. “Since this publication, we have numerous other studies drawing conclusions that sometimes agree with and sometimes conflict with it. My belief is that health disparities in IBD will continue to be an active area of research. We know that it takes vigilance to identify, track, and address any disparities when they do arise,” said Dr. Greywoode.
Dr. Greywoode also noted that phenotypic differences based on race and ethnicity influence disparities. She showed results from a meta-analysis that found a difference in the frequency of perianal Crohn’s disease by race and ethnicity; the highest frequency occurred in Black patients (31%), followed by Asians (22%), Whites (14%), and Hispanics (13%). Another study showed that African American patients with Crohn’s disease were more likely to develop a new abscess (adjusted odds ratio, 2.27; 95% confidence interval, 1.31-3.93) or anal fissure (aOR, 1.76; 95% CI, 1.01-3.07), and were also more likely to be initiated on an anti–tumor necrosis factor drug (aOR, 1.85; 95% CI, 1.09-3.14).
Those differences underscore the need to recognize that IBD is not just a disease for White patients. “As we move forward in IBD research, we recognize that individuals of European ancestry are not the only ones who have IBD. There is a growing diverse racial and ethnic population with IBD,” said Dr. Greywoode.
She noted that, in the United States, it is estimated that about one in four adult patients are non-Hispanic African American, Hispanic, Asians, or other ethnicities. Nevertheless, Whites are overrepresented among participants in IBD clinical trials. Some trials are composed of as much as 95% White patients, and sometimes race isn’t even listed. “It’s unclear if [race/ethnicity data are] not collected or not deemed important, but we know that what is not collected is not measured, and what is not measured can’t be evaluated, either to praise or constructively criticize,” said Dr. Greywoode.
Fortunately, there are efforts in place to improve representation in clinical trials. There has been a mandate for almost 3 decades that federally funded research must include racial and ethnic minorities who have been traditionally underrepresented. The Food and Drug Administration has also provided guidance to industry to improve diversity in clinical trial participation, and industry groups have developed strategies, including improved representation among investigators and related early-career development programs. At the community and independent health care practice levels, clinical trial networks encourage patient participation with regulatory and data management support to bolster practices with insufficient resources.
Underrepresentation in clinical trials resonated with comoderator Tina Aswani Omprakash, who is a patient advocate and a master’s in public health student at the Icahn School of Medicine at Mount Sinai, New York. She called for greater awareness among physicians that IBD can occur among people of all backgrounds. “[Providers] would look at me and [say]: ‘There’s no way that, as a South Asian woman, you have that kind of disease.’ There’s that lack of believability,” said Ms. Aswani Omprakash.
Greater recognition of the diversity of patients, as well as the phenotypic differences found among ethnicities, could also inform clinical trial participation and, ultimately, more personalized medicine. “We have to look at these things, observe how they’re affecting populations differently, so that we can have proper medication solutions,” said Ms. Aswani Omprakash.
Dr. Greywoode and Ms. Issokson have no relevant financial disclosures. Ms. Aswani Omprakash has consulted for Genentech, AbbVie, Janssen, and Arena.
The AGA applauds researchers who are working to raise our awareness of health disparities in digestive diseases. The AGA is committed to addressing this important societal issue head on. Learn more about AGA’s commitment through the AGA Equity Project.
Updated Feb. 17, 2021.
FROM THE CROHN’S & COLITIS CONGRESS
Weekly COVID-19 cases in children dropped 22%
according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
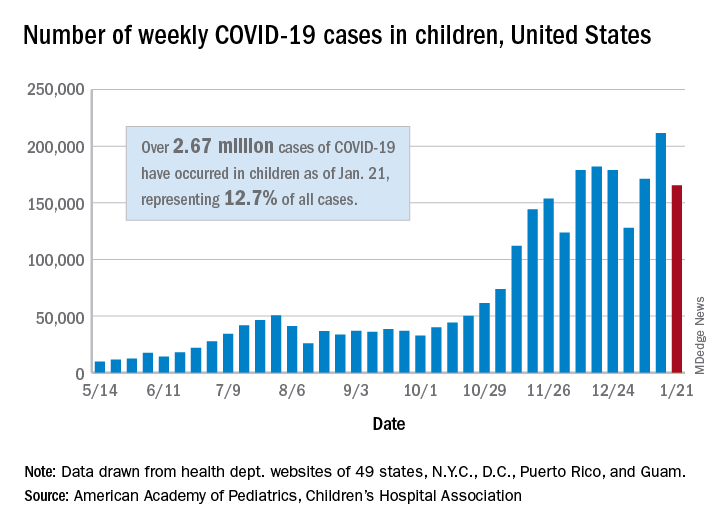
The 165,000 new cases reported during the week of Jan. 15-21 were down by almost 22% from the previous week’s 211,000, when the new-case count reached its highest point in the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Cumulative cases in children now stand at just over 2.67 million, and children represent 12.7% of all COVID-19 cases reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. For the week of Jan. 15-21, children made up 14.8% of all new cases, the highest proportion since late September, the AAP/CHA data show.
The cumulative rate of infection among children is up to 3,556 per 100,000 nationally, with states ranging from 943 per 100,000 in Hawaii to 8,195 in North Dakota. California has the most reported cases at 383,000, while Vermont has the fewest at 1,820, the two organizations reported.
There were 14 more deaths among children in the last week, bringing the total to 205 in the 43 states (plus New York City and Guam) reporting such data. Children represent just 0.06% of all coronavirus-related deaths, and only 0.01% of all cases in children have resulted in death, the AAP and CHA said. There are still 10 states where no children have died from COVID-19.
Although severe illness appears to be rare in children, the AAP and CHA noted, “there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The 165,000 new cases reported during the week of Jan. 15-21 were down by almost 22% from the previous week’s 211,000, when the new-case count reached its highest point in the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Cumulative cases in children now stand at just over 2.67 million, and children represent 12.7% of all COVID-19 cases reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. For the week of Jan. 15-21, children made up 14.8% of all new cases, the highest proportion since late September, the AAP/CHA data show.
The cumulative rate of infection among children is up to 3,556 per 100,000 nationally, with states ranging from 943 per 100,000 in Hawaii to 8,195 in North Dakota. California has the most reported cases at 383,000, while Vermont has the fewest at 1,820, the two organizations reported.
There were 14 more deaths among children in the last week, bringing the total to 205 in the 43 states (plus New York City and Guam) reporting such data. Children represent just 0.06% of all coronavirus-related deaths, and only 0.01% of all cases in children have resulted in death, the AAP and CHA said. There are still 10 states where no children have died from COVID-19.
Although severe illness appears to be rare in children, the AAP and CHA noted, “there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The 165,000 new cases reported during the week of Jan. 15-21 were down by almost 22% from the previous week’s 211,000, when the new-case count reached its highest point in the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Cumulative cases in children now stand at just over 2.67 million, and children represent 12.7% of all COVID-19 cases reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. For the week of Jan. 15-21, children made up 14.8% of all new cases, the highest proportion since late September, the AAP/CHA data show.
The cumulative rate of infection among children is up to 3,556 per 100,000 nationally, with states ranging from 943 per 100,000 in Hawaii to 8,195 in North Dakota. California has the most reported cases at 383,000, while Vermont has the fewest at 1,820, the two organizations reported.
There were 14 more deaths among children in the last week, bringing the total to 205 in the 43 states (plus New York City and Guam) reporting such data. Children represent just 0.06% of all coronavirus-related deaths, and only 0.01% of all cases in children have resulted in death, the AAP and CHA said. There are still 10 states where no children have died from COVID-19.
Although severe illness appears to be rare in children, the AAP and CHA noted, “there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
COVID-19 risks linked to medications in IBD
Multicenter and population cohort studies suggest that patients with inflammatory bowel disease (IBD) are not at unique risk of contracting COVID-19 or experiencing worse outcomes, with the exception of a few risk factors such as corticosteroid use and combination therapy that appear tied to greater risk of hospitalization and mortality. The findings line up well with previous experience with infectious disease and are reassuring, but they also underscore the need to taper steroids and de-escalate from combination therapy, when possible.
“There is not a clear increased risk of getting COVID-19 among IBD patients compared to the general population, and that seems to hold even if you look at certain medication types, [even] if patients are on immunosuppressives like thiopurines or anti-TNF [anti–tumor necrosis factor] drugs,” Ryan C. Ungaro, MD, said in an interview. Dr. Ungaro, who is with the Icahn School of Medicine at Mount Sinai, New York, discussed IBD and COVID-19 risks at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
A systematic review showed that 0.3% of IBD patients contracted COVID-19 during study periods, compared with 0.2%-4.0% of the general population, and a matched-cohort analysis of a national Veterans Affairs database showed an infection prevalence of 0.23% among patients with IBD versus 0.20% among those without (P = .29). The analysis also showed use of anti-TNF therapies or thiopurines was not associated with an increased risk.
Studies show that patients with IBD in general do not appear to be at greater risk of severe disease outcomes such as hospitalization or 30-day mortality. For example, a U.S. national database study of more than 40 million patients compared 232 patients with IBD who were diagnosed with COVID-19 with 19,776 non-IBD patients and found that, after propensity matching, there were no significant association between IBD and worse outcomes (risk ratio, 0.93; 95% confidence interval, 0.68-1.27; P = .86) or hospitalizations (RR, 1.10; 95% CI, 0.74-1.40; P = .91)).
However, some risk factors could be red flags. Data from the international SECURE-IBD registry showed an association between combined endpoint of ICU, requiring a ventilator, or death and advanced age (adjusted odds ratio, 1.04; 95% CI, 1.01-1.06; P < .01) and two or more comorbidities (aOR, 2.87; 95% CI, 1.05-7.85; P < .04). More specifically to IBD, severe COVID-19 was associated with use of corticosteroids (aOR, 6.87; 95% CI, 2.30-20.51; P < .001). In terms of other therapies, another study found a similar effect with thiopurines (compared with TNF monotherapy; aOR, 4.08; 95% CI, 1.65-9.78; Bonferroni adjusted P = .008), and combined use of anti-TNF drugs and a thiopurine (compared with TNF monotherapy; aOR, 4.01; 95% CI, 1.73-9.61; Bonferroni adjusted P = .013), but anti-TNF therapies alone trended toward a protective effect (compared with no anti-TNF therapy; aOR, 0.69; Bonferroni adjusted P = .52). That study found no significant association between severe outcomes and anti-IL 12/23 (compared with anti-TNF monotherapy; aOR, 0.98; 95% CI, 0.12-8.06; P = .98) or anti-integrin biologics (compared with anti-TNF monotherapy; aOR, 2.42; 95% CI, 0.59-9.96; P = .22).
Overall, the data are “generally consistent with prior data on infections and IBD: That steroids and combination therapy increase the risk of infection and bad outcomes and that interestingly biologic monotherapy may actually confer a little bit of protection against emergent outcomes and at a minimum appears to be neutral,” said Dr. Ungaro.
He noted that the recommendations from the IOIBD COVID-19 Task Force were based on expert opinion, but the new data have largely supported them overall. He did suggest some potential modifications, including reducing thiopurine use among patients on combination therapy. According to Dr. Ungaro, the recommendations do call for withholding all IBD therapy for 10 days after positive SARS-CoV-2 tests, whether the patient is symptomatic or not. “I think the recent data is reassuring that potentially in asymptomatic and maybe even mild cases, the monotherapy biologics – we can consider not delaying administering those. I think we need more data about that, but it’s reassuring that patients on those had no worse outcomes and [in fact did] slightly better,” Dr. Ungaro said during the presentation.
The data reinforced the need to consider tapering patients off corticosteroids or combination therapies, if possible. “It’s something we were doing in regular IBD care beforehand, but the COVID-19 pandemic offers another reason to limit the use of steroids and evaluate if patients are able to de-escalate from combination therapies,” said Dr. Ungaro.
On the other hand, there was concern among some patients early in the pandemic that their immunotherapy drugs may put them at risk of contracting COVID-19, which led some to discontinue medications. Ongoing studies are illustrating the problem with this, according to David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the congress’s organizing committee. “The data do not in general suggest you should do that to protect yourself. In fact, being on the therapies may have a better outcome. Patients always want to come off their therapies, [but] during the pandemic that is a risk not worth taking. Getting sick from your Crohn’s disease or colitis, when there are limited health care resources and, in some places, limited hospital beds and where the rescue therapy might include steroids, is a risky proposition. It’s not the time to do this,” said Dr. Rubin.
With respect to vaccines, it appears so far that there is no increased risk of adverse events associated with IBD. Patients who are on immunosuppressive drugs may experience a lower response to immunization, which has been seen with other vaccines. “The benefits likely outweigh the risks based on our prior experience with other vaccinations. It’s an area of ongoing study, but I do think we should recommend that our IBD patients get the COVID-19 vaccine, especially if they have risk factors for severe disease,” said Dr. Ungaro.
Dr. Ungaro is on the advisory board for Bristol-Myers Squibb, Janssen, Pfizer, and Takeda. He has received funding from AbbVie, Boehringer Ingelheim, Eli Lilly, and Pfizer. He has been a speaker or received consulting fees from AbbVie and Eli Lilly. Dr. Rubin is a consultant for Janssen, Pfizer, Takeda, and AbbVie.
This article was updated Jan. 27, 2021.
Multicenter and population cohort studies suggest that patients with inflammatory bowel disease (IBD) are not at unique risk of contracting COVID-19 or experiencing worse outcomes, with the exception of a few risk factors such as corticosteroid use and combination therapy that appear tied to greater risk of hospitalization and mortality. The findings line up well with previous experience with infectious disease and are reassuring, but they also underscore the need to taper steroids and de-escalate from combination therapy, when possible.
“There is not a clear increased risk of getting COVID-19 among IBD patients compared to the general population, and that seems to hold even if you look at certain medication types, [even] if patients are on immunosuppressives like thiopurines or anti-TNF [anti–tumor necrosis factor] drugs,” Ryan C. Ungaro, MD, said in an interview. Dr. Ungaro, who is with the Icahn School of Medicine at Mount Sinai, New York, discussed IBD and COVID-19 risks at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
A systematic review showed that 0.3% of IBD patients contracted COVID-19 during study periods, compared with 0.2%-4.0% of the general population, and a matched-cohort analysis of a national Veterans Affairs database showed an infection prevalence of 0.23% among patients with IBD versus 0.20% among those without (P = .29). The analysis also showed use of anti-TNF therapies or thiopurines was not associated with an increased risk.
Studies show that patients with IBD in general do not appear to be at greater risk of severe disease outcomes such as hospitalization or 30-day mortality. For example, a U.S. national database study of more than 40 million patients compared 232 patients with IBD who were diagnosed with COVID-19 with 19,776 non-IBD patients and found that, after propensity matching, there were no significant association between IBD and worse outcomes (risk ratio, 0.93; 95% confidence interval, 0.68-1.27; P = .86) or hospitalizations (RR, 1.10; 95% CI, 0.74-1.40; P = .91)).
However, some risk factors could be red flags. Data from the international SECURE-IBD registry showed an association between combined endpoint of ICU, requiring a ventilator, or death and advanced age (adjusted odds ratio, 1.04; 95% CI, 1.01-1.06; P < .01) and two or more comorbidities (aOR, 2.87; 95% CI, 1.05-7.85; P < .04). More specifically to IBD, severe COVID-19 was associated with use of corticosteroids (aOR, 6.87; 95% CI, 2.30-20.51; P < .001). In terms of other therapies, another study found a similar effect with thiopurines (compared with TNF monotherapy; aOR, 4.08; 95% CI, 1.65-9.78; Bonferroni adjusted P = .008), and combined use of anti-TNF drugs and a thiopurine (compared with TNF monotherapy; aOR, 4.01; 95% CI, 1.73-9.61; Bonferroni adjusted P = .013), but anti-TNF therapies alone trended toward a protective effect (compared with no anti-TNF therapy; aOR, 0.69; Bonferroni adjusted P = .52). That study found no significant association between severe outcomes and anti-IL 12/23 (compared with anti-TNF monotherapy; aOR, 0.98; 95% CI, 0.12-8.06; P = .98) or anti-integrin biologics (compared with anti-TNF monotherapy; aOR, 2.42; 95% CI, 0.59-9.96; P = .22).
Overall, the data are “generally consistent with prior data on infections and IBD: That steroids and combination therapy increase the risk of infection and bad outcomes and that interestingly biologic monotherapy may actually confer a little bit of protection against emergent outcomes and at a minimum appears to be neutral,” said Dr. Ungaro.
He noted that the recommendations from the IOIBD COVID-19 Task Force were based on expert opinion, but the new data have largely supported them overall. He did suggest some potential modifications, including reducing thiopurine use among patients on combination therapy. According to Dr. Ungaro, the recommendations do call for withholding all IBD therapy for 10 days after positive SARS-CoV-2 tests, whether the patient is symptomatic or not. “I think the recent data is reassuring that potentially in asymptomatic and maybe even mild cases, the monotherapy biologics – we can consider not delaying administering those. I think we need more data about that, but it’s reassuring that patients on those had no worse outcomes and [in fact did] slightly better,” Dr. Ungaro said during the presentation.
The data reinforced the need to consider tapering patients off corticosteroids or combination therapies, if possible. “It’s something we were doing in regular IBD care beforehand, but the COVID-19 pandemic offers another reason to limit the use of steroids and evaluate if patients are able to de-escalate from combination therapies,” said Dr. Ungaro.
On the other hand, there was concern among some patients early in the pandemic that their immunotherapy drugs may put them at risk of contracting COVID-19, which led some to discontinue medications. Ongoing studies are illustrating the problem with this, according to David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the congress’s organizing committee. “The data do not in general suggest you should do that to protect yourself. In fact, being on the therapies may have a better outcome. Patients always want to come off their therapies, [but] during the pandemic that is a risk not worth taking. Getting sick from your Crohn’s disease or colitis, when there are limited health care resources and, in some places, limited hospital beds and where the rescue therapy might include steroids, is a risky proposition. It’s not the time to do this,” said Dr. Rubin.
With respect to vaccines, it appears so far that there is no increased risk of adverse events associated with IBD. Patients who are on immunosuppressive drugs may experience a lower response to immunization, which has been seen with other vaccines. “The benefits likely outweigh the risks based on our prior experience with other vaccinations. It’s an area of ongoing study, but I do think we should recommend that our IBD patients get the COVID-19 vaccine, especially if they have risk factors for severe disease,” said Dr. Ungaro.
Dr. Ungaro is on the advisory board for Bristol-Myers Squibb, Janssen, Pfizer, and Takeda. He has received funding from AbbVie, Boehringer Ingelheim, Eli Lilly, and Pfizer. He has been a speaker or received consulting fees from AbbVie and Eli Lilly. Dr. Rubin is a consultant for Janssen, Pfizer, Takeda, and AbbVie.
This article was updated Jan. 27, 2021.
Multicenter and population cohort studies suggest that patients with inflammatory bowel disease (IBD) are not at unique risk of contracting COVID-19 or experiencing worse outcomes, with the exception of a few risk factors such as corticosteroid use and combination therapy that appear tied to greater risk of hospitalization and mortality. The findings line up well with previous experience with infectious disease and are reassuring, but they also underscore the need to taper steroids and de-escalate from combination therapy, when possible.
“There is not a clear increased risk of getting COVID-19 among IBD patients compared to the general population, and that seems to hold even if you look at certain medication types, [even] if patients are on immunosuppressives like thiopurines or anti-TNF [anti–tumor necrosis factor] drugs,” Ryan C. Ungaro, MD, said in an interview. Dr. Ungaro, who is with the Icahn School of Medicine at Mount Sinai, New York, discussed IBD and COVID-19 risks at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
A systematic review showed that 0.3% of IBD patients contracted COVID-19 during study periods, compared with 0.2%-4.0% of the general population, and a matched-cohort analysis of a national Veterans Affairs database showed an infection prevalence of 0.23% among patients with IBD versus 0.20% among those without (P = .29). The analysis also showed use of anti-TNF therapies or thiopurines was not associated with an increased risk.
Studies show that patients with IBD in general do not appear to be at greater risk of severe disease outcomes such as hospitalization or 30-day mortality. For example, a U.S. national database study of more than 40 million patients compared 232 patients with IBD who were diagnosed with COVID-19 with 19,776 non-IBD patients and found that, after propensity matching, there were no significant association between IBD and worse outcomes (risk ratio, 0.93; 95% confidence interval, 0.68-1.27; P = .86) or hospitalizations (RR, 1.10; 95% CI, 0.74-1.40; P = .91)).
However, some risk factors could be red flags. Data from the international SECURE-IBD registry showed an association between combined endpoint of ICU, requiring a ventilator, or death and advanced age (adjusted odds ratio, 1.04; 95% CI, 1.01-1.06; P < .01) and two or more comorbidities (aOR, 2.87; 95% CI, 1.05-7.85; P < .04). More specifically to IBD, severe COVID-19 was associated with use of corticosteroids (aOR, 6.87; 95% CI, 2.30-20.51; P < .001). In terms of other therapies, another study found a similar effect with thiopurines (compared with TNF monotherapy; aOR, 4.08; 95% CI, 1.65-9.78; Bonferroni adjusted P = .008), and combined use of anti-TNF drugs and a thiopurine (compared with TNF monotherapy; aOR, 4.01; 95% CI, 1.73-9.61; Bonferroni adjusted P = .013), but anti-TNF therapies alone trended toward a protective effect (compared with no anti-TNF therapy; aOR, 0.69; Bonferroni adjusted P = .52). That study found no significant association between severe outcomes and anti-IL 12/23 (compared with anti-TNF monotherapy; aOR, 0.98; 95% CI, 0.12-8.06; P = .98) or anti-integrin biologics (compared with anti-TNF monotherapy; aOR, 2.42; 95% CI, 0.59-9.96; P = .22).
Overall, the data are “generally consistent with prior data on infections and IBD: That steroids and combination therapy increase the risk of infection and bad outcomes and that interestingly biologic monotherapy may actually confer a little bit of protection against emergent outcomes and at a minimum appears to be neutral,” said Dr. Ungaro.
He noted that the recommendations from the IOIBD COVID-19 Task Force were based on expert opinion, but the new data have largely supported them overall. He did suggest some potential modifications, including reducing thiopurine use among patients on combination therapy. According to Dr. Ungaro, the recommendations do call for withholding all IBD therapy for 10 days after positive SARS-CoV-2 tests, whether the patient is symptomatic or not. “I think the recent data is reassuring that potentially in asymptomatic and maybe even mild cases, the monotherapy biologics – we can consider not delaying administering those. I think we need more data about that, but it’s reassuring that patients on those had no worse outcomes and [in fact did] slightly better,” Dr. Ungaro said during the presentation.
The data reinforced the need to consider tapering patients off corticosteroids or combination therapies, if possible. “It’s something we were doing in regular IBD care beforehand, but the COVID-19 pandemic offers another reason to limit the use of steroids and evaluate if patients are able to de-escalate from combination therapies,” said Dr. Ungaro.
On the other hand, there was concern among some patients early in the pandemic that their immunotherapy drugs may put them at risk of contracting COVID-19, which led some to discontinue medications. Ongoing studies are illustrating the problem with this, according to David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the congress’s organizing committee. “The data do not in general suggest you should do that to protect yourself. In fact, being on the therapies may have a better outcome. Patients always want to come off their therapies, [but] during the pandemic that is a risk not worth taking. Getting sick from your Crohn’s disease or colitis, when there are limited health care resources and, in some places, limited hospital beds and where the rescue therapy might include steroids, is a risky proposition. It’s not the time to do this,” said Dr. Rubin.
With respect to vaccines, it appears so far that there is no increased risk of adverse events associated with IBD. Patients who are on immunosuppressive drugs may experience a lower response to immunization, which has been seen with other vaccines. “The benefits likely outweigh the risks based on our prior experience with other vaccinations. It’s an area of ongoing study, but I do think we should recommend that our IBD patients get the COVID-19 vaccine, especially if they have risk factors for severe disease,” said Dr. Ungaro.
Dr. Ungaro is on the advisory board for Bristol-Myers Squibb, Janssen, Pfizer, and Takeda. He has received funding from AbbVie, Boehringer Ingelheim, Eli Lilly, and Pfizer. He has been a speaker or received consulting fees from AbbVie and Eli Lilly. Dr. Rubin is a consultant for Janssen, Pfizer, Takeda, and AbbVie.
This article was updated Jan. 27, 2021.
FROM THE CROHN’S & COLITIS CONGRESS
Bathing now more widely accepted as an eczema treatment strategy
According to Noreen Heer Nicol, PhD, RN, FNP, frustration still exists for patients, families, and health care providers regarding the lack of consensus that routine bathing is good for patients with atopic dermatitis.
During the Revolutionizing Atopic Dermatitis symposium, she said that conflicting and vague guidelines currently exist on the topic.
“This stems from the fact that we just don’t have good studies,” said Dr. Nicol, associate dean and associate professor of nursing at the University of Colorado at Denver, Aurora. “Particularly, we don’t have randomized, controlled trials on the effects of water and bathing. It’s not just parents that are frustrated, but health care providers are as well.”
In an observational analysis, researchers evaluated results from three online surveys of dermatologists, allergists, and immunologists, and primary care physicians regarding routine bathing frequency recommendations for children with AD. It found that PCPs recommended daily bathing less than 50% of the time, while specialists recommended daily bathing more than 50% of the time.
“It seems like the PCPs have embraced that old dermatology notion when bathing was avoided in patients with AD,” Dr. Nicol said. “This lack of consensus on the basic daily care steps in AD management causes a great deal of confusion amongst patients, families, and young health care providers, in particular,” she added.
She believes that this goes back to a century-long debate about the pros and cons of bathing in AD. “We used to say that bathing will dry the skin out if you take a bath or a shower without immediately applying something like a good moisturizer. That’s where the 3-minute rule came along from the National Eczema Association, meaning that bathing hydrates the stratum corneum if you take a bath or a shower and you immediately apply that good moisturizer within 3 minutes to retain that hydration and keep the barrier intact and flexible.”
Dr. Nicol presented a stepwise management model that she has published many times over the years (see Pediatr Nursing 2020;46[2]:92-8 and J Allergy Clin Immunol Pract 2019;7[1]:1-16).
Step 1 consists of basic care, including skin hydration/bathing, application of a daily moisturizer, avoiding irritants, and identifying and addressing specific triggers. “This is the foundation for every step as you go forward,” she explained. Soak and seal has been a mainstay of treatment at National Jewish Health, she noted. “By that, I mean taking a soaking 10-15 minute bath in warm water daily. Gently pat away excess water. Immediately apply skin medications or moisturizer within 3 minutes. Using a gentle fragrance-free, dye-free cleanser to clean skin is also important. Avoid scrubbing.”
A review article on bathing and associated treatments in AD was published in 2017 and includes 144 references to bathing studies. A separate recommendation known as the “AD Yardstick” published by Dr. Nicol’s colleague at National Jewish Health, Mark Boguniewicz, MD, and coauthors, elaborated on the definition of basic skin care for nonlesional AD. Besides recommending the liberal and frequent application of moisturizers, it suggests management with warm baths or showers using nonsoap cleansers, usually once per day, followed by application of a moisturizer, even on clear areas.
“This is now what people are thinking as the basis of skin care in patients with AD,” Dr. Nicol said. “Warm baths and showers don’t look so controversial anymore. This model nicely lays out what we want people to remember. In the past, many times we just skipped that important step of telling people about bathing.”
In a small 2009 study, researchers conducted a quantitative assessment of combination bathing and moisturizing regimens on skin hydration in AD. They found that bathing followed by application of a moisturizer provides modest hydration benefits, though less than that of simply applying moisturizer alone. “That has not been the case for most of us who are bathing advocates,” Dr. Nicol said. “We believe that there is an additional hydration that’s gained from bathing and moisturizers done properly.”
In an earlier retrospective study of 28 patients referred to a tertiary care center for refractory chronic pruritic eruptions, researchers found that a plain-water 20-minute soak followed by smearing of midstrength corticosteroid ointment led to clearing or dramatic improvement of the lesions (Arch Dermatol 2005;14:1556-9). The authors recommended prospective studies to confirm the findings.
In a separate review of medical literature, researchers explored the role of frequent bathing in the treatment of pediatric AD (Ann Allergy Asthma Immunol 2016;117[1]:9-13). They found that the weight of evidence suggests that the frequent soak and smear bathing is preferred to infrequent bathing in the management of AD. Frequent bathing was defined as bathing at least once a day, while infrequent bathing was defined as bathing less than once a day.
“Bleach baths have received much attention in recent years, and have been endorsed by multiple AD guidelines, though not to the same degree as regular bathing,” Dr. Nicol said. “Right now, you can find almost as much literature for this practice as against it. The populations that seem to value from beach baths the most, however, are those with frequent infections, particularly those who are methicillin resistant. Most people recommend a maximum of two to three times per week but only with an active infection. Care must be taken to avoid additional drying or irritation of the skin from bleach.”
Many bleach bath recipes call for adding one-eighth to one-half of a cup of bleach to a tub full or water.
Dr. Nicol disclosed that she has served as an advisory board member for Eli Lilly.
According to Noreen Heer Nicol, PhD, RN, FNP, frustration still exists for patients, families, and health care providers regarding the lack of consensus that routine bathing is good for patients with atopic dermatitis.
During the Revolutionizing Atopic Dermatitis symposium, she said that conflicting and vague guidelines currently exist on the topic.
“This stems from the fact that we just don’t have good studies,” said Dr. Nicol, associate dean and associate professor of nursing at the University of Colorado at Denver, Aurora. “Particularly, we don’t have randomized, controlled trials on the effects of water and bathing. It’s not just parents that are frustrated, but health care providers are as well.”
In an observational analysis, researchers evaluated results from three online surveys of dermatologists, allergists, and immunologists, and primary care physicians regarding routine bathing frequency recommendations for children with AD. It found that PCPs recommended daily bathing less than 50% of the time, while specialists recommended daily bathing more than 50% of the time.
“It seems like the PCPs have embraced that old dermatology notion when bathing was avoided in patients with AD,” Dr. Nicol said. “This lack of consensus on the basic daily care steps in AD management causes a great deal of confusion amongst patients, families, and young health care providers, in particular,” she added.
She believes that this goes back to a century-long debate about the pros and cons of bathing in AD. “We used to say that bathing will dry the skin out if you take a bath or a shower without immediately applying something like a good moisturizer. That’s where the 3-minute rule came along from the National Eczema Association, meaning that bathing hydrates the stratum corneum if you take a bath or a shower and you immediately apply that good moisturizer within 3 minutes to retain that hydration and keep the barrier intact and flexible.”
Dr. Nicol presented a stepwise management model that she has published many times over the years (see Pediatr Nursing 2020;46[2]:92-8 and J Allergy Clin Immunol Pract 2019;7[1]:1-16).
Step 1 consists of basic care, including skin hydration/bathing, application of a daily moisturizer, avoiding irritants, and identifying and addressing specific triggers. “This is the foundation for every step as you go forward,” she explained. Soak and seal has been a mainstay of treatment at National Jewish Health, she noted. “By that, I mean taking a soaking 10-15 minute bath in warm water daily. Gently pat away excess water. Immediately apply skin medications or moisturizer within 3 minutes. Using a gentle fragrance-free, dye-free cleanser to clean skin is also important. Avoid scrubbing.”
A review article on bathing and associated treatments in AD was published in 2017 and includes 144 references to bathing studies. A separate recommendation known as the “AD Yardstick” published by Dr. Nicol’s colleague at National Jewish Health, Mark Boguniewicz, MD, and coauthors, elaborated on the definition of basic skin care for nonlesional AD. Besides recommending the liberal and frequent application of moisturizers, it suggests management with warm baths or showers using nonsoap cleansers, usually once per day, followed by application of a moisturizer, even on clear areas.
“This is now what people are thinking as the basis of skin care in patients with AD,” Dr. Nicol said. “Warm baths and showers don’t look so controversial anymore. This model nicely lays out what we want people to remember. In the past, many times we just skipped that important step of telling people about bathing.”
In a small 2009 study, researchers conducted a quantitative assessment of combination bathing and moisturizing regimens on skin hydration in AD. They found that bathing followed by application of a moisturizer provides modest hydration benefits, though less than that of simply applying moisturizer alone. “That has not been the case for most of us who are bathing advocates,” Dr. Nicol said. “We believe that there is an additional hydration that’s gained from bathing and moisturizers done properly.”
In an earlier retrospective study of 28 patients referred to a tertiary care center for refractory chronic pruritic eruptions, researchers found that a plain-water 20-minute soak followed by smearing of midstrength corticosteroid ointment led to clearing or dramatic improvement of the lesions (Arch Dermatol 2005;14:1556-9). The authors recommended prospective studies to confirm the findings.
In a separate review of medical literature, researchers explored the role of frequent bathing in the treatment of pediatric AD (Ann Allergy Asthma Immunol 2016;117[1]:9-13). They found that the weight of evidence suggests that the frequent soak and smear bathing is preferred to infrequent bathing in the management of AD. Frequent bathing was defined as bathing at least once a day, while infrequent bathing was defined as bathing less than once a day.
“Bleach baths have received much attention in recent years, and have been endorsed by multiple AD guidelines, though not to the same degree as regular bathing,” Dr. Nicol said. “Right now, you can find almost as much literature for this practice as against it. The populations that seem to value from beach baths the most, however, are those with frequent infections, particularly those who are methicillin resistant. Most people recommend a maximum of two to three times per week but only with an active infection. Care must be taken to avoid additional drying or irritation of the skin from bleach.”
Many bleach bath recipes call for adding one-eighth to one-half of a cup of bleach to a tub full or water.
Dr. Nicol disclosed that she has served as an advisory board member for Eli Lilly.
According to Noreen Heer Nicol, PhD, RN, FNP, frustration still exists for patients, families, and health care providers regarding the lack of consensus that routine bathing is good for patients with atopic dermatitis.
During the Revolutionizing Atopic Dermatitis symposium, she said that conflicting and vague guidelines currently exist on the topic.
“This stems from the fact that we just don’t have good studies,” said Dr. Nicol, associate dean and associate professor of nursing at the University of Colorado at Denver, Aurora. “Particularly, we don’t have randomized, controlled trials on the effects of water and bathing. It’s not just parents that are frustrated, but health care providers are as well.”
In an observational analysis, researchers evaluated results from three online surveys of dermatologists, allergists, and immunologists, and primary care physicians regarding routine bathing frequency recommendations for children with AD. It found that PCPs recommended daily bathing less than 50% of the time, while specialists recommended daily bathing more than 50% of the time.
“It seems like the PCPs have embraced that old dermatology notion when bathing was avoided in patients with AD,” Dr. Nicol said. “This lack of consensus on the basic daily care steps in AD management causes a great deal of confusion amongst patients, families, and young health care providers, in particular,” she added.
She believes that this goes back to a century-long debate about the pros and cons of bathing in AD. “We used to say that bathing will dry the skin out if you take a bath or a shower without immediately applying something like a good moisturizer. That’s where the 3-minute rule came along from the National Eczema Association, meaning that bathing hydrates the stratum corneum if you take a bath or a shower and you immediately apply that good moisturizer within 3 minutes to retain that hydration and keep the barrier intact and flexible.”
Dr. Nicol presented a stepwise management model that she has published many times over the years (see Pediatr Nursing 2020;46[2]:92-8 and J Allergy Clin Immunol Pract 2019;7[1]:1-16).
Step 1 consists of basic care, including skin hydration/bathing, application of a daily moisturizer, avoiding irritants, and identifying and addressing specific triggers. “This is the foundation for every step as you go forward,” she explained. Soak and seal has been a mainstay of treatment at National Jewish Health, she noted. “By that, I mean taking a soaking 10-15 minute bath in warm water daily. Gently pat away excess water. Immediately apply skin medications or moisturizer within 3 minutes. Using a gentle fragrance-free, dye-free cleanser to clean skin is also important. Avoid scrubbing.”
A review article on bathing and associated treatments in AD was published in 2017 and includes 144 references to bathing studies. A separate recommendation known as the “AD Yardstick” published by Dr. Nicol’s colleague at National Jewish Health, Mark Boguniewicz, MD, and coauthors, elaborated on the definition of basic skin care for nonlesional AD. Besides recommending the liberal and frequent application of moisturizers, it suggests management with warm baths or showers using nonsoap cleansers, usually once per day, followed by application of a moisturizer, even on clear areas.
“This is now what people are thinking as the basis of skin care in patients with AD,” Dr. Nicol said. “Warm baths and showers don’t look so controversial anymore. This model nicely lays out what we want people to remember. In the past, many times we just skipped that important step of telling people about bathing.”
In a small 2009 study, researchers conducted a quantitative assessment of combination bathing and moisturizing regimens on skin hydration in AD. They found that bathing followed by application of a moisturizer provides modest hydration benefits, though less than that of simply applying moisturizer alone. “That has not been the case for most of us who are bathing advocates,” Dr. Nicol said. “We believe that there is an additional hydration that’s gained from bathing and moisturizers done properly.”
In an earlier retrospective study of 28 patients referred to a tertiary care center for refractory chronic pruritic eruptions, researchers found that a plain-water 20-minute soak followed by smearing of midstrength corticosteroid ointment led to clearing or dramatic improvement of the lesions (Arch Dermatol 2005;14:1556-9). The authors recommended prospective studies to confirm the findings.
In a separate review of medical literature, researchers explored the role of frequent bathing in the treatment of pediatric AD (Ann Allergy Asthma Immunol 2016;117[1]:9-13). They found that the weight of evidence suggests that the frequent soak and smear bathing is preferred to infrequent bathing in the management of AD. Frequent bathing was defined as bathing at least once a day, while infrequent bathing was defined as bathing less than once a day.
“Bleach baths have received much attention in recent years, and have been endorsed by multiple AD guidelines, though not to the same degree as regular bathing,” Dr. Nicol said. “Right now, you can find almost as much literature for this practice as against it. The populations that seem to value from beach baths the most, however, are those with frequent infections, particularly those who are methicillin resistant. Most people recommend a maximum of two to three times per week but only with an active infection. Care must be taken to avoid additional drying or irritation of the skin from bleach.”
Many bleach bath recipes call for adding one-eighth to one-half of a cup of bleach to a tub full or water.
Dr. Nicol disclosed that she has served as an advisory board member for Eli Lilly.
FROM REVOLUTIONIZING AD 2020
Erythema, Blisters, and Scars on the Elbows, Knees, and Legs
The Diagnosis: Epidermolysis Bullosa Acquisita
The diagnosis of epidermolysis bullosa acquisita (EBA) was made based on the clinical and pathologic findings. A blistering disorder that resolves with milia is characteristic of EBA. Hematoxylin and eosin staining demonstrated a pauci-inflammatory separation between the epidermis and dermis (Figure 1). Direct immunofluorescence studies showed linear IgG deposition along the basement membrane zone while C3 was negative (Figure 2). Salt-split skin was essential, as it revealed IgG deposition to the floor of the split (Figure 3), a pattern seen in EBA and not bullous pemphigoid (BP).1
Epidermolysis bullosa acquisita is an acquired autoimmune bullous disorder that results from antibodies to type VII collagen, an anchoring fibril that attaches the lamina densa to the dermis. The epidemiology and etiology of the trigger that leads to antibody production are not well known, but an association between EBA and inflammatory bowel disease has been described.2 Although this disease may present in childhood, EBA most commonly is a disorder seen in adults and the elderly. A classic noninflammatory mechanobullous form as well as an inflammatory BP-like form are the most commonly encountered presentations. Light microscopy demonstrates subepidermal cleavage without acantholysis. In the inflammatory BP-like subtype, an inflammatory infiltrate may be present. Direct immunofluorescence is remarkable for a linear band of IgG deposits along the basement membrane zone, with or without C3 deposition in a similar pattern.1
Bullous pemphigoid is within the differential of EBA. It can be difficult to differentiate clinically, especially when a patient has the BP-like variant of EBA because, as the name implies, it mimics BP. Patients with BP often will report a pruritic patch that will then develop into an urticarial plaque. Scarring and milia rarely are seen in BP but can be observed in the multiple presentations of EBA. Hematoxylin and eosin staining and direct immunofluorescence may be almost identical, and differentiating between the 2 disorders can be a challenge. Immunodeposition in EBA occurs in a U-shaped, serrated pattern, while the pattern in BP is N-shaped and serrated.3 Although the U-shaped, serrated pattern is relatively specific, it is not always easy to interpret and requires a high-quality biopsy specimen, which can be difficult to discern with certainty in suboptimal preparations. Another way to differentiate between the 2 entities is to utilize the salt-split skin technique, as performed in our patient. With salt-split skin, the biopsy is placed into a solution of 1 mol/L sodium chloride and incubated at 4 °C (39 °F) for 18 to 24 hours. A blister is then produced at the level of the lamina lucida, which allows for the staining of immunoreactants to occur either above or below that split (commonly referred to as staining on the roof or floor of the blister cavity). With EBA, there is immunoreactant deposition on the floor of the blister, while the opposite occurs in BP.4
Epidermolysis bullosa simplex is the most common type of epidermolysis bullosa, with keratin genes KRT5 and KRT14 as frequent mutations. Patients develop blisters, vesicles, bullae, and milia on traumatized areas of the body such as the hands, elbows, knees, and feet. This disease presents early in childhood. Histology exhibits a cell-poor subepidermal blister.5 With porphyria cutanea tarda, reduced activity of uroporphyrinogen decarboxylase, a major enzyme in the heme synthesis pathway, leads to blisters with erosions and milia on sun-exposed areas of the body. Histologic evaluation reveals a subepidermal pauci-inflammatory vesicle with festooning of the dermal papillae and amphophilic basement membrane within the epidermis. Direct immunofluorescence of porphyria cutanea tarda demonstrates IgM and C3 in the vessels.6 Sweet syndrome is a neutrophilic dermatosis that presents as erythematous, edematous, hot, and tender plaques along with fever and leukocytosis. It is associated with myeloproliferative disorders. Biopsy demonstrates papillary dermal edema along with diffuse neutrophilic infiltrate.7
Numerous medications have been recommended for the treatment of EBA, ranging from steroids to steroid-sparing drugs such as colchicine and dapsone.8,9 Our patient was educated on physical precautions and was started on dapsone alone due to comorbid diabetes mellitus and renal disease. Within a few weeks of initiating dapsone, he observed a reduction in erythema, and within months he experienced a decrease in blister eruption frequency.
- Vorobyev A, Ludwig RJ, Schmidt E. Clinical features and diagnosis of epidermolysis bullosa acquisita. Expert Rev Clin Immunol. 2017;13:157-169.
- Reddy H, Shipman AR, Wojnarowska F. Epidermolysis bullosa acquisita and inflammatory bowel disease: a review of the literature. Clin Exp Dermatol. 2013;38:225-230.
- Vodegel RM, Jonkman MF, Pas HH, et al. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol. 2004;151:112-118.
- Gardner KM, Crawford RI. Distinguishing epidermolysis bullosa acquisita from bullous pemphigoid without direct immunofluorescence. J Cutan Med Surg. 2018;22:22-24.
- Sprecher E. Epidermolysis bullosa simplex. Dermatol Clin. 2010;28:23-32.
- Maynard B, Peters MS. Histologic and immunofluorescence study of cutaneous porphyrias. J Cutan Pathol. 1992;19:40-47.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018:79:987-1006.
- Kirtschig G, Murrell D, Wojnarowska F, et al. Interventions for mucous membrane pemphigoid and epidermolysis bullosa acquisita. Cochrane Database Syst Rev. 2003;1:CD004056
- Gürcan HM, Ahmed AR. Current concepts in the treatment of epidermolysis bullosa acquisita. Expert Opin Pharmacother. 2011;12:1259-1268.
The Diagnosis: Epidermolysis Bullosa Acquisita
The diagnosis of epidermolysis bullosa acquisita (EBA) was made based on the clinical and pathologic findings. A blistering disorder that resolves with milia is characteristic of EBA. Hematoxylin and eosin staining demonstrated a pauci-inflammatory separation between the epidermis and dermis (Figure 1). Direct immunofluorescence studies showed linear IgG deposition along the basement membrane zone while C3 was negative (Figure 2). Salt-split skin was essential, as it revealed IgG deposition to the floor of the split (Figure 3), a pattern seen in EBA and not bullous pemphigoid (BP).1



Epidermolysis bullosa acquisita is an acquired autoimmune bullous disorder that results from antibodies to type VII collagen, an anchoring fibril that attaches the lamina densa to the dermis. The epidemiology and etiology of the trigger that leads to antibody production are not well known, but an association between EBA and inflammatory bowel disease has been described.2 Although this disease may present in childhood, EBA most commonly is a disorder seen in adults and the elderly. A classic noninflammatory mechanobullous form as well as an inflammatory BP-like form are the most commonly encountered presentations. Light microscopy demonstrates subepidermal cleavage without acantholysis. In the inflammatory BP-like subtype, an inflammatory infiltrate may be present. Direct immunofluorescence is remarkable for a linear band of IgG deposits along the basement membrane zone, with or without C3 deposition in a similar pattern.1
Bullous pemphigoid is within the differential of EBA. It can be difficult to differentiate clinically, especially when a patient has the BP-like variant of EBA because, as the name implies, it mimics BP. Patients with BP often will report a pruritic patch that will then develop into an urticarial plaque. Scarring and milia rarely are seen in BP but can be observed in the multiple presentations of EBA. Hematoxylin and eosin staining and direct immunofluorescence may be almost identical, and differentiating between the 2 disorders can be a challenge. Immunodeposition in EBA occurs in a U-shaped, serrated pattern, while the pattern in BP is N-shaped and serrated.3 Although the U-shaped, serrated pattern is relatively specific, it is not always easy to interpret and requires a high-quality biopsy specimen, which can be difficult to discern with certainty in suboptimal preparations. Another way to differentiate between the 2 entities is to utilize the salt-split skin technique, as performed in our patient. With salt-split skin, the biopsy is placed into a solution of 1 mol/L sodium chloride and incubated at 4 °C (39 °F) for 18 to 24 hours. A blister is then produced at the level of the lamina lucida, which allows for the staining of immunoreactants to occur either above or below that split (commonly referred to as staining on the roof or floor of the blister cavity). With EBA, there is immunoreactant deposition on the floor of the blister, while the opposite occurs in BP.4
Epidermolysis bullosa simplex is the most common type of epidermolysis bullosa, with keratin genes KRT5 and KRT14 as frequent mutations. Patients develop blisters, vesicles, bullae, and milia on traumatized areas of the body such as the hands, elbows, knees, and feet. This disease presents early in childhood. Histology exhibits a cell-poor subepidermal blister.5 With porphyria cutanea tarda, reduced activity of uroporphyrinogen decarboxylase, a major enzyme in the heme synthesis pathway, leads to blisters with erosions and milia on sun-exposed areas of the body. Histologic evaluation reveals a subepidermal pauci-inflammatory vesicle with festooning of the dermal papillae and amphophilic basement membrane within the epidermis. Direct immunofluorescence of porphyria cutanea tarda demonstrates IgM and C3 in the vessels.6 Sweet syndrome is a neutrophilic dermatosis that presents as erythematous, edematous, hot, and tender plaques along with fever and leukocytosis. It is associated with myeloproliferative disorders. Biopsy demonstrates papillary dermal edema along with diffuse neutrophilic infiltrate.7
Numerous medications have been recommended for the treatment of EBA, ranging from steroids to steroid-sparing drugs such as colchicine and dapsone.8,9 Our patient was educated on physical precautions and was started on dapsone alone due to comorbid diabetes mellitus and renal disease. Within a few weeks of initiating dapsone, he observed a reduction in erythema, and within months he experienced a decrease in blister eruption frequency.
The Diagnosis: Epidermolysis Bullosa Acquisita
The diagnosis of epidermolysis bullosa acquisita (EBA) was made based on the clinical and pathologic findings. A blistering disorder that resolves with milia is characteristic of EBA. Hematoxylin and eosin staining demonstrated a pauci-inflammatory separation between the epidermis and dermis (Figure 1). Direct immunofluorescence studies showed linear IgG deposition along the basement membrane zone while C3 was negative (Figure 2). Salt-split skin was essential, as it revealed IgG deposition to the floor of the split (Figure 3), a pattern seen in EBA and not bullous pemphigoid (BP).1



Epidermolysis bullosa acquisita is an acquired autoimmune bullous disorder that results from antibodies to type VII collagen, an anchoring fibril that attaches the lamina densa to the dermis. The epidemiology and etiology of the trigger that leads to antibody production are not well known, but an association between EBA and inflammatory bowel disease has been described.2 Although this disease may present in childhood, EBA most commonly is a disorder seen in adults and the elderly. A classic noninflammatory mechanobullous form as well as an inflammatory BP-like form are the most commonly encountered presentations. Light microscopy demonstrates subepidermal cleavage without acantholysis. In the inflammatory BP-like subtype, an inflammatory infiltrate may be present. Direct immunofluorescence is remarkable for a linear band of IgG deposits along the basement membrane zone, with or without C3 deposition in a similar pattern.1
Bullous pemphigoid is within the differential of EBA. It can be difficult to differentiate clinically, especially when a patient has the BP-like variant of EBA because, as the name implies, it mimics BP. Patients with BP often will report a pruritic patch that will then develop into an urticarial plaque. Scarring and milia rarely are seen in BP but can be observed in the multiple presentations of EBA. Hematoxylin and eosin staining and direct immunofluorescence may be almost identical, and differentiating between the 2 disorders can be a challenge. Immunodeposition in EBA occurs in a U-shaped, serrated pattern, while the pattern in BP is N-shaped and serrated.3 Although the U-shaped, serrated pattern is relatively specific, it is not always easy to interpret and requires a high-quality biopsy specimen, which can be difficult to discern with certainty in suboptimal preparations. Another way to differentiate between the 2 entities is to utilize the salt-split skin technique, as performed in our patient. With salt-split skin, the biopsy is placed into a solution of 1 mol/L sodium chloride and incubated at 4 °C (39 °F) for 18 to 24 hours. A blister is then produced at the level of the lamina lucida, which allows for the staining of immunoreactants to occur either above or below that split (commonly referred to as staining on the roof or floor of the blister cavity). With EBA, there is immunoreactant deposition on the floor of the blister, while the opposite occurs in BP.4
Epidermolysis bullosa simplex is the most common type of epidermolysis bullosa, with keratin genes KRT5 and KRT14 as frequent mutations. Patients develop blisters, vesicles, bullae, and milia on traumatized areas of the body such as the hands, elbows, knees, and feet. This disease presents early in childhood. Histology exhibits a cell-poor subepidermal blister.5 With porphyria cutanea tarda, reduced activity of uroporphyrinogen decarboxylase, a major enzyme in the heme synthesis pathway, leads to blisters with erosions and milia on sun-exposed areas of the body. Histologic evaluation reveals a subepidermal pauci-inflammatory vesicle with festooning of the dermal papillae and amphophilic basement membrane within the epidermis. Direct immunofluorescence of porphyria cutanea tarda demonstrates IgM and C3 in the vessels.6 Sweet syndrome is a neutrophilic dermatosis that presents as erythematous, edematous, hot, and tender plaques along with fever and leukocytosis. It is associated with myeloproliferative disorders. Biopsy demonstrates papillary dermal edema along with diffuse neutrophilic infiltrate.7
Numerous medications have been recommended for the treatment of EBA, ranging from steroids to steroid-sparing drugs such as colchicine and dapsone.8,9 Our patient was educated on physical precautions and was started on dapsone alone due to comorbid diabetes mellitus and renal disease. Within a few weeks of initiating dapsone, he observed a reduction in erythema, and within months he experienced a decrease in blister eruption frequency.
- Vorobyev A, Ludwig RJ, Schmidt E. Clinical features and diagnosis of epidermolysis bullosa acquisita. Expert Rev Clin Immunol. 2017;13:157-169.
- Reddy H, Shipman AR, Wojnarowska F. Epidermolysis bullosa acquisita and inflammatory bowel disease: a review of the literature. Clin Exp Dermatol. 2013;38:225-230.
- Vodegel RM, Jonkman MF, Pas HH, et al. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol. 2004;151:112-118.
- Gardner KM, Crawford RI. Distinguishing epidermolysis bullosa acquisita from bullous pemphigoid without direct immunofluorescence. J Cutan Med Surg. 2018;22:22-24.
- Sprecher E. Epidermolysis bullosa simplex. Dermatol Clin. 2010;28:23-32.
- Maynard B, Peters MS. Histologic and immunofluorescence study of cutaneous porphyrias. J Cutan Pathol. 1992;19:40-47.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018:79:987-1006.
- Kirtschig G, Murrell D, Wojnarowska F, et al. Interventions for mucous membrane pemphigoid and epidermolysis bullosa acquisita. Cochrane Database Syst Rev. 2003;1:CD004056
- Gürcan HM, Ahmed AR. Current concepts in the treatment of epidermolysis bullosa acquisita. Expert Opin Pharmacother. 2011;12:1259-1268.
- Vorobyev A, Ludwig RJ, Schmidt E. Clinical features and diagnosis of epidermolysis bullosa acquisita. Expert Rev Clin Immunol. 2017;13:157-169.
- Reddy H, Shipman AR, Wojnarowska F. Epidermolysis bullosa acquisita and inflammatory bowel disease: a review of the literature. Clin Exp Dermatol. 2013;38:225-230.
- Vodegel RM, Jonkman MF, Pas HH, et al. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol. 2004;151:112-118.
- Gardner KM, Crawford RI. Distinguishing epidermolysis bullosa acquisita from bullous pemphigoid without direct immunofluorescence. J Cutan Med Surg. 2018;22:22-24.
- Sprecher E. Epidermolysis bullosa simplex. Dermatol Clin. 2010;28:23-32.
- Maynard B, Peters MS. Histologic and immunofluorescence study of cutaneous porphyrias. J Cutan Pathol. 1992;19:40-47.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018:79:987-1006.
- Kirtschig G, Murrell D, Wojnarowska F, et al. Interventions for mucous membrane pemphigoid and epidermolysis bullosa acquisita. Cochrane Database Syst Rev. 2003;1:CD004056
- Gürcan HM, Ahmed AR. Current concepts in the treatment of epidermolysis bullosa acquisita. Expert Opin Pharmacother. 2011;12:1259-1268.
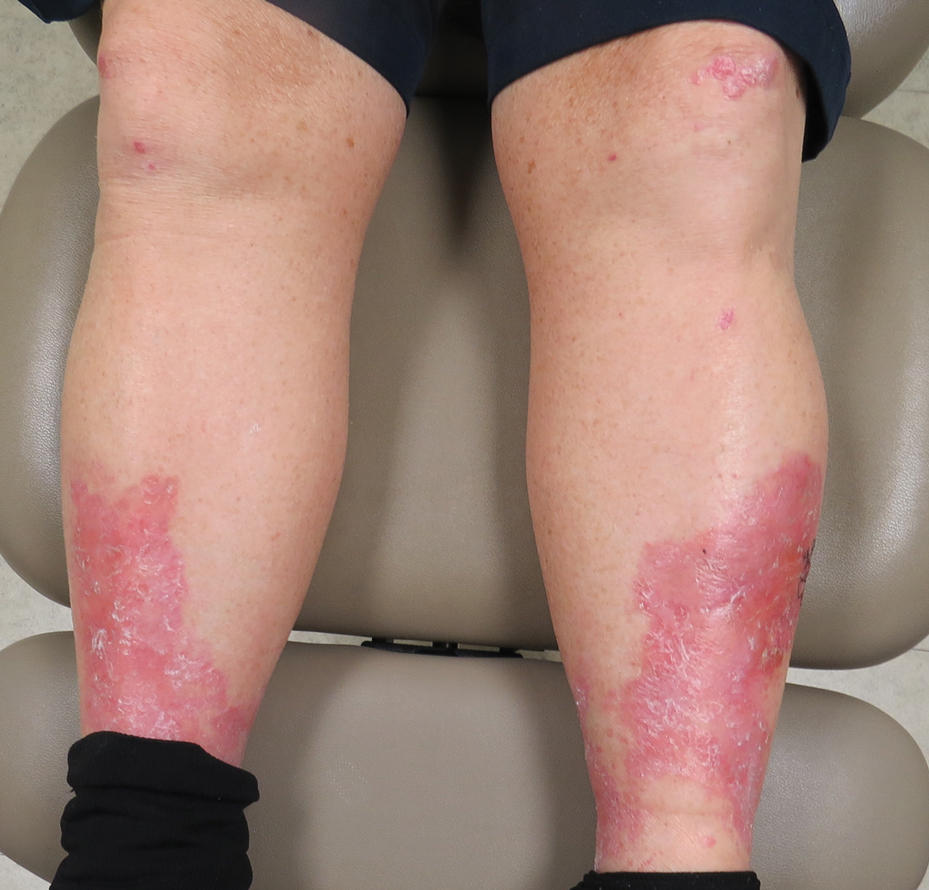
A 69-year-old man presented with an asymptomatic rash on the extensor surfaces of 2 years' duration. He reported recurrent blisters that would then scar over. The lesions did not occur in relation to any known trauma. The patient's medical history revealed dialysis-dependent end-stage renal disease secondary to type 2 diabetes mellitus. His medications were noncontributory, and there was no family history of blistering disorders. He had tried triamcinolone cream without any improvement. Physical examination was remarkable for erythematous blisters and bullae with scales and milia on the elbows, knees, and lower legs. The oral mucosa was unremarkable. Shave biopsies of the skin for direct immunofluorescence and salt-split skin studies were obtained.
Guselkumab maintains psoriasis efficacy long after discontinuation
Fully half of patients with moderate to severe psoriasis who achieve complete clearance after their first four doses of guselkumab (Tremfya) continue to maintain a PASI 90 response nearly 6 months after withdrawal of the biologic, according to a post hoc analysis of the pivotal phase 3 VOYAGE 2 trial.
“That’s impressive maintenance of efficacy,” said Curdin Conrad, MD, who presented the data at the virtual annual congress of the European Academy of Dermatology and Venereology.
“These findings are reassuring when you have to interrupt guselkumab therapy: For example, due to acute infection, pregnancy, or surgery. But it might also help when considering in the future a flexible dosing interval, particularly for patients who had complete clearance,” added Dr. Conrad, professor of dermatology and head of the polyclinic and the Center of Excellence for Psoriasis at Lausanne (Switzerland) University Hospital.
The intriguing implication from VOYAGE 2 that guselkumab might lend itself to flexible dosing featuring lengthy drug-free intervals is being prospectively examined in the ongoing phase 3b GUIDE trial. This is a double-blind, placebo-controlled trial including 888 French and German patients with moderate to severe psoriasis and a study hypothesis that those who have a Psoriasis Area and Severity Index score of 0 at weeks 20 and 28 in response to on-label dosing – the so-called ‘super responders’ – will maintain disease control until week 68 if their dosing is reduced to 100 mg of guselkumab every 16 weeks instead of the standard 8-week intervals.
Dr. Conrad reported that in VOYAGE 2, 106 patients on standard-dose guselkumab who had a PASI score of 0 at weeks 20 and 28 were randomized to discontinue the interleukin-23 inhibitor after receiving their fourth dose at week 20. It took 25 weeks for 50% of them to lose their PASI 90 response as defined by regression to a PASI score of 1 or greater. Using a less stringent definition of maintenance of efficacy, the super responders’ median time off guselkumab until reaching a PASI score of 3 or more was 30.7 weeks, with a median of 35.4 weeks to a PASI score of 5 or more.
In addition, 34 other VOYAGE 2 participants who were almost clear on guselkumab at weeks 20 and 28, with a PASI score of more than 0 but less than 1, were randomized to guselkumab withdrawal after their week-20 dose. Median time to loss of their PASI 90 response was shorter than that of the super responders – not surprising since their mean PASI score when the biologic was halted was 0.5, rather than 0 as for the super responders. But Dr. Conrad said the maintenance of response was still impressive: A median of 16.2 weeks to reach a PASI score of 1 or more, 27.2 weeks for a PASI 3, and 33.7 weeks for a PASI score of 5.
He reported receiving research funding from and serving as a scientific adviser to Janssen, the study sponsor, as well as to more than a dozen other pharmaceutical companies.
Fully half of patients with moderate to severe psoriasis who achieve complete clearance after their first four doses of guselkumab (Tremfya) continue to maintain a PASI 90 response nearly 6 months after withdrawal of the biologic, according to a post hoc analysis of the pivotal phase 3 VOYAGE 2 trial.
“That’s impressive maintenance of efficacy,” said Curdin Conrad, MD, who presented the data at the virtual annual congress of the European Academy of Dermatology and Venereology.
“These findings are reassuring when you have to interrupt guselkumab therapy: For example, due to acute infection, pregnancy, or surgery. But it might also help when considering in the future a flexible dosing interval, particularly for patients who had complete clearance,” added Dr. Conrad, professor of dermatology and head of the polyclinic and the Center of Excellence for Psoriasis at Lausanne (Switzerland) University Hospital.
The intriguing implication from VOYAGE 2 that guselkumab might lend itself to flexible dosing featuring lengthy drug-free intervals is being prospectively examined in the ongoing phase 3b GUIDE trial. This is a double-blind, placebo-controlled trial including 888 French and German patients with moderate to severe psoriasis and a study hypothesis that those who have a Psoriasis Area and Severity Index score of 0 at weeks 20 and 28 in response to on-label dosing – the so-called ‘super responders’ – will maintain disease control until week 68 if their dosing is reduced to 100 mg of guselkumab every 16 weeks instead of the standard 8-week intervals.
Dr. Conrad reported that in VOYAGE 2, 106 patients on standard-dose guselkumab who had a PASI score of 0 at weeks 20 and 28 were randomized to discontinue the interleukin-23 inhibitor after receiving their fourth dose at week 20. It took 25 weeks for 50% of them to lose their PASI 90 response as defined by regression to a PASI score of 1 or greater. Using a less stringent definition of maintenance of efficacy, the super responders’ median time off guselkumab until reaching a PASI score of 3 or more was 30.7 weeks, with a median of 35.4 weeks to a PASI score of 5 or more.
In addition, 34 other VOYAGE 2 participants who were almost clear on guselkumab at weeks 20 and 28, with a PASI score of more than 0 but less than 1, were randomized to guselkumab withdrawal after their week-20 dose. Median time to loss of their PASI 90 response was shorter than that of the super responders – not surprising since their mean PASI score when the biologic was halted was 0.5, rather than 0 as for the super responders. But Dr. Conrad said the maintenance of response was still impressive: A median of 16.2 weeks to reach a PASI score of 1 or more, 27.2 weeks for a PASI 3, and 33.7 weeks for a PASI score of 5.
He reported receiving research funding from and serving as a scientific adviser to Janssen, the study sponsor, as well as to more than a dozen other pharmaceutical companies.
Fully half of patients with moderate to severe psoriasis who achieve complete clearance after their first four doses of guselkumab (Tremfya) continue to maintain a PASI 90 response nearly 6 months after withdrawal of the biologic, according to a post hoc analysis of the pivotal phase 3 VOYAGE 2 trial.
“That’s impressive maintenance of efficacy,” said Curdin Conrad, MD, who presented the data at the virtual annual congress of the European Academy of Dermatology and Venereology.
“These findings are reassuring when you have to interrupt guselkumab therapy: For example, due to acute infection, pregnancy, or surgery. But it might also help when considering in the future a flexible dosing interval, particularly for patients who had complete clearance,” added Dr. Conrad, professor of dermatology and head of the polyclinic and the Center of Excellence for Psoriasis at Lausanne (Switzerland) University Hospital.
The intriguing implication from VOYAGE 2 that guselkumab might lend itself to flexible dosing featuring lengthy drug-free intervals is being prospectively examined in the ongoing phase 3b GUIDE trial. This is a double-blind, placebo-controlled trial including 888 French and German patients with moderate to severe psoriasis and a study hypothesis that those who have a Psoriasis Area and Severity Index score of 0 at weeks 20 and 28 in response to on-label dosing – the so-called ‘super responders’ – will maintain disease control until week 68 if their dosing is reduced to 100 mg of guselkumab every 16 weeks instead of the standard 8-week intervals.
Dr. Conrad reported that in VOYAGE 2, 106 patients on standard-dose guselkumab who had a PASI score of 0 at weeks 20 and 28 were randomized to discontinue the interleukin-23 inhibitor after receiving their fourth dose at week 20. It took 25 weeks for 50% of them to lose their PASI 90 response as defined by regression to a PASI score of 1 or greater. Using a less stringent definition of maintenance of efficacy, the super responders’ median time off guselkumab until reaching a PASI score of 3 or more was 30.7 weeks, with a median of 35.4 weeks to a PASI score of 5 or more.
In addition, 34 other VOYAGE 2 participants who were almost clear on guselkumab at weeks 20 and 28, with a PASI score of more than 0 but less than 1, were randomized to guselkumab withdrawal after their week-20 dose. Median time to loss of their PASI 90 response was shorter than that of the super responders – not surprising since their mean PASI score when the biologic was halted was 0.5, rather than 0 as for the super responders. But Dr. Conrad said the maintenance of response was still impressive: A median of 16.2 weeks to reach a PASI score of 1 or more, 27.2 weeks for a PASI 3, and 33.7 weeks for a PASI score of 5.
He reported receiving research funding from and serving as a scientific adviser to Janssen, the study sponsor, as well as to more than a dozen other pharmaceutical companies.
FROM THE EADV CONGRESS
Daily moisturizers a bedrock of atopic dermatitis management
Mounting .
In an updated review of clinical evidence on the topic, Adelaide A. Hebert, MD, Noreen Heer Nicol, PhD, RN, FNP, and colleagues evaluated 13 trials that assessed daily moisturization for the treatment of AD published between 2006 and 2019. “The bottom line is, daily moisturization increased skin hydration and it decreased transepidermal water loss in all children and adults in the 13 studies we looked at,” Dr. Nicol, associate dean and associate professor of nursing at the University of Colorado, Denver, said at the Revolutionizing Atopic Dermatitis symposium.
Based on published evidence in the review, she and her coauthors assembled six points regarding the importance of essential skin repair in AD:
1. It strengthens the barrier that protects against environmental triggers such as skin irritants aeroallergens, dust mites, and pet dander.
2. It decreases moisture loss that perpetuates damage and can provoke inflammatory processes.
3. It promotes a healthy microbiome via induction of antimicrobial peptides.
4. It maintains stratum corneum acidification, which protects against pathogens.
5. It reduces recurrence of flares when used daily.
6. It prevents the onset of AD when applied early in life to at-risk children.
A separate review of optimal AD care authored by Dr. Nicol underscores the importance of foundational management, “meaning that we want you to use hydration and daily moisturizers as part of your everyday management,” she said. “Without good barrier repair, infections and allergens can break through. The intention is to have that barrier repair a key point of moisturizer use.”
In a 2014 published study, researchers investigated the role of proactive emollient therapy in preventing AD in 124 neonates in the United States and the United Kingdom with a first-degree relative with a history of allergic rhinitis, asthma, or AD. The treatment group received daily total body application of Aquaphor Healing Ointment, Cetaphil Cream, or sunflower seed oil, starting at 3 weeks of age, while the control group received no moisturizers. They found that daily emollient therapy significantly reduced the cumulative incidence of AD at 6 months (22% vs. 42% among controls). A follow-up study confirmed a protective but nonsignificant effect of daily moisturizer use at 12 months (AD was diagnosed in 13.2% of those in the treatment group vs. 25% in the control group), most likely due to the study being underpowered.
“The message here is simple,” Dr. Nicol said. “Wouldn’t it be wonderful if we could reduce the burden of AD by doing something as straightforward as moisturizer use in our neonates?”
With so many moisturizers on the market today, considerations include active ingredients, side effects, absorption, and amount required for efficacy. “On the average adult, head to toe, front to back, one time it takes about 30 grams or one ounce of something to cover them completely, so you want to make sure people are using enough,” Dr. Nicol said. “You don’t want to be prescribing people 15- and 30-gram tubes of product and hoping they have enough to cover their bodies multiple times.”
Ten years ago, a randomized, controlled trial found that Aquaphor Healing Ointment was 47 times more cost-effective than prescription barrier creams Atopiclair nonsteroidal cream and EpiCeram controlled release skin barrier emulsion. “The most expensive things do not have to be the best things to be used,” Dr. Nicol said. “Recognize what the properties of these products are and what the benefit is.”
A basic principle of skin care for AD patients recommended by Dr. Nicol and colleagues at National Jewish Health, Denver, includes applying a fragrance-free moisturizer within 3 minutes of finishing a bath or a shower. They recommend products sold in 1-pound jars or large tubes, such as Aquaphor Healing Ointment, Vaniply Ointment, Eucerin Creme (various formulations), Vanicream, CeraVe Cream, or Cetaphil cream. “Vaseline is a good occlusive preparation to seal in but is most effective after bath or shower,” the recommendations continue. “Topical maintenance medications may be used in place of moisturizers or sealer when prescribed.”
She recommends including a list of preferred moisturizers for patients to use into written action plans for skin care. “This adds a lot of benefit to patients,” she said. “Always put the patient at the center of your decision-making. Spend time listening to them, give them options of things that they are willing to use so that they can trust you.”
Dr. Nicol disclosed that she has served as an advisory board member for Eli Lilly & Co.
Mounting .
In an updated review of clinical evidence on the topic, Adelaide A. Hebert, MD, Noreen Heer Nicol, PhD, RN, FNP, and colleagues evaluated 13 trials that assessed daily moisturization for the treatment of AD published between 2006 and 2019. “The bottom line is, daily moisturization increased skin hydration and it decreased transepidermal water loss in all children and adults in the 13 studies we looked at,” Dr. Nicol, associate dean and associate professor of nursing at the University of Colorado, Denver, said at the Revolutionizing Atopic Dermatitis symposium.
Based on published evidence in the review, she and her coauthors assembled six points regarding the importance of essential skin repair in AD:
1. It strengthens the barrier that protects against environmental triggers such as skin irritants aeroallergens, dust mites, and pet dander.
2. It decreases moisture loss that perpetuates damage and can provoke inflammatory processes.
3. It promotes a healthy microbiome via induction of antimicrobial peptides.
4. It maintains stratum corneum acidification, which protects against pathogens.
5. It reduces recurrence of flares when used daily.
6. It prevents the onset of AD when applied early in life to at-risk children.
A separate review of optimal AD care authored by Dr. Nicol underscores the importance of foundational management, “meaning that we want you to use hydration and daily moisturizers as part of your everyday management,” she said. “Without good barrier repair, infections and allergens can break through. The intention is to have that barrier repair a key point of moisturizer use.”
In a 2014 published study, researchers investigated the role of proactive emollient therapy in preventing AD in 124 neonates in the United States and the United Kingdom with a first-degree relative with a history of allergic rhinitis, asthma, or AD. The treatment group received daily total body application of Aquaphor Healing Ointment, Cetaphil Cream, or sunflower seed oil, starting at 3 weeks of age, while the control group received no moisturizers. They found that daily emollient therapy significantly reduced the cumulative incidence of AD at 6 months (22% vs. 42% among controls). A follow-up study confirmed a protective but nonsignificant effect of daily moisturizer use at 12 months (AD was diagnosed in 13.2% of those in the treatment group vs. 25% in the control group), most likely due to the study being underpowered.
“The message here is simple,” Dr. Nicol said. “Wouldn’t it be wonderful if we could reduce the burden of AD by doing something as straightforward as moisturizer use in our neonates?”
With so many moisturizers on the market today, considerations include active ingredients, side effects, absorption, and amount required for efficacy. “On the average adult, head to toe, front to back, one time it takes about 30 grams or one ounce of something to cover them completely, so you want to make sure people are using enough,” Dr. Nicol said. “You don’t want to be prescribing people 15- and 30-gram tubes of product and hoping they have enough to cover their bodies multiple times.”
Ten years ago, a randomized, controlled trial found that Aquaphor Healing Ointment was 47 times more cost-effective than prescription barrier creams Atopiclair nonsteroidal cream and EpiCeram controlled release skin barrier emulsion. “The most expensive things do not have to be the best things to be used,” Dr. Nicol said. “Recognize what the properties of these products are and what the benefit is.”
A basic principle of skin care for AD patients recommended by Dr. Nicol and colleagues at National Jewish Health, Denver, includes applying a fragrance-free moisturizer within 3 minutes of finishing a bath or a shower. They recommend products sold in 1-pound jars or large tubes, such as Aquaphor Healing Ointment, Vaniply Ointment, Eucerin Creme (various formulations), Vanicream, CeraVe Cream, or Cetaphil cream. “Vaseline is a good occlusive preparation to seal in but is most effective after bath or shower,” the recommendations continue. “Topical maintenance medications may be used in place of moisturizers or sealer when prescribed.”
She recommends including a list of preferred moisturizers for patients to use into written action plans for skin care. “This adds a lot of benefit to patients,” she said. “Always put the patient at the center of your decision-making. Spend time listening to them, give them options of things that they are willing to use so that they can trust you.”
Dr. Nicol disclosed that she has served as an advisory board member for Eli Lilly & Co.
Mounting .
In an updated review of clinical evidence on the topic, Adelaide A. Hebert, MD, Noreen Heer Nicol, PhD, RN, FNP, and colleagues evaluated 13 trials that assessed daily moisturization for the treatment of AD published between 2006 and 2019. “The bottom line is, daily moisturization increased skin hydration and it decreased transepidermal water loss in all children and adults in the 13 studies we looked at,” Dr. Nicol, associate dean and associate professor of nursing at the University of Colorado, Denver, said at the Revolutionizing Atopic Dermatitis symposium.
Based on published evidence in the review, she and her coauthors assembled six points regarding the importance of essential skin repair in AD:
1. It strengthens the barrier that protects against environmental triggers such as skin irritants aeroallergens, dust mites, and pet dander.
2. It decreases moisture loss that perpetuates damage and can provoke inflammatory processes.
3. It promotes a healthy microbiome via induction of antimicrobial peptides.
4. It maintains stratum corneum acidification, which protects against pathogens.
5. It reduces recurrence of flares when used daily.
6. It prevents the onset of AD when applied early in life to at-risk children.
A separate review of optimal AD care authored by Dr. Nicol underscores the importance of foundational management, “meaning that we want you to use hydration and daily moisturizers as part of your everyday management,” she said. “Without good barrier repair, infections and allergens can break through. The intention is to have that barrier repair a key point of moisturizer use.”
In a 2014 published study, researchers investigated the role of proactive emollient therapy in preventing AD in 124 neonates in the United States and the United Kingdom with a first-degree relative with a history of allergic rhinitis, asthma, or AD. The treatment group received daily total body application of Aquaphor Healing Ointment, Cetaphil Cream, or sunflower seed oil, starting at 3 weeks of age, while the control group received no moisturizers. They found that daily emollient therapy significantly reduced the cumulative incidence of AD at 6 months (22% vs. 42% among controls). A follow-up study confirmed a protective but nonsignificant effect of daily moisturizer use at 12 months (AD was diagnosed in 13.2% of those in the treatment group vs. 25% in the control group), most likely due to the study being underpowered.
“The message here is simple,” Dr. Nicol said. “Wouldn’t it be wonderful if we could reduce the burden of AD by doing something as straightforward as moisturizer use in our neonates?”
With so many moisturizers on the market today, considerations include active ingredients, side effects, absorption, and amount required for efficacy. “On the average adult, head to toe, front to back, one time it takes about 30 grams or one ounce of something to cover them completely, so you want to make sure people are using enough,” Dr. Nicol said. “You don’t want to be prescribing people 15- and 30-gram tubes of product and hoping they have enough to cover their bodies multiple times.”
Ten years ago, a randomized, controlled trial found that Aquaphor Healing Ointment was 47 times more cost-effective than prescription barrier creams Atopiclair nonsteroidal cream and EpiCeram controlled release skin barrier emulsion. “The most expensive things do not have to be the best things to be used,” Dr. Nicol said. “Recognize what the properties of these products are and what the benefit is.”
A basic principle of skin care for AD patients recommended by Dr. Nicol and colleagues at National Jewish Health, Denver, includes applying a fragrance-free moisturizer within 3 minutes of finishing a bath or a shower. They recommend products sold in 1-pound jars or large tubes, such as Aquaphor Healing Ointment, Vaniply Ointment, Eucerin Creme (various formulations), Vanicream, CeraVe Cream, or Cetaphil cream. “Vaseline is a good occlusive preparation to seal in but is most effective after bath or shower,” the recommendations continue. “Topical maintenance medications may be used in place of moisturizers or sealer when prescribed.”
She recommends including a list of preferred moisturizers for patients to use into written action plans for skin care. “This adds a lot of benefit to patients,” she said. “Always put the patient at the center of your decision-making. Spend time listening to them, give them options of things that they are willing to use so that they can trust you.”
Dr. Nicol disclosed that she has served as an advisory board member for Eli Lilly & Co.
FROM REVOLUTIONIZING AD 2020