User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Concurrent Atopic Dermatitis and Psoriasis Successfully Treated With Dual Biologic Therapy
Atopic dermatitis (AD) and psoriasis are common skin diseases in which dysfunction of the epidermal barrier leads to skin inflammation and altered expression of proinflammatory cytokines.1 There often is overlap in the clinical and histopathologic features of AD and psoriasis, which can make diagnosis a challenge. Persistent late-stage AD can present with psoriasiform lichenified changes, and psoriasis lesions in the acute stage can have an eczematous appearance.2 Histologically, chronic psoriasis lesions share many overlapping features with AD, and some subsets of AD with IL-17 predominance (ie, intrinsic, pediatric, presentation in Asian patients) exhibit a psoriasiform appearance.3,4
Atopic dermatitis and psoriasis are considered 2 distinct conditions because AD is a helper T cell (TH2)–driven disease with subsequent overproduction of IL-4 and IL-13 and psoriasis is a TH17 cell–driven disease with overproduction of IL-173; however, the shared features of AD and psoriasis represent an underlying immunopathological spectrum2,5,6 in which one condition can develop following treatment of the other condition (immunological shift in pathways), both conditions can occur at different times in a patient’s life with alternating cycles of disease flares, or both conditions can coexist as an overlapping syndrome.1,2 A retrospective study from 2012 to 2019 estimated the prevalence of concomitant AD and psoriasis in the United States at 1.3%, with AD following the diagnosis of psoriasis in 67% of cases.1 Concurrent AD and psoriasis—when both diseases flaresimultaneously—is the rarest scenario.2,5
Treatment modalities for AD include topical corticosteroids, which act on immune cells to suppress the release of proinflammatory cytokines, as well as dupilumab, which offers targeted blockade of involved cytokines IL-4 and IL-13. Psoriasis can be treated with multiple immune modulators, including topical corticosteroids and vitamin D analogs, as well as systemic medications that reduce T-cell activation and inflammatory cytokines through targeting of IFN-γ, IL-2, tumor necrosis factor α, IL-17, and IL-23.7,8
We present the case of a patient with long-standing concurrent, treatment-resistant AD and psoriasis who was successfully treated with dual biologic therapy with guselkumab and dupilumab.
Case Report
A 62-year-old woman presented to our dermatology clinic with red itchy scales and painful fissures on the palms, hands, and soles of more than 12 years’ duration. Her medical history included an allergy to amoxicillin-clavulanate as well as an allergy to both dog and cat dander on prick testing. Her family history included dyshidrotic eczema in her mother. A complete blood cell count with differential was within reference range. A shave biopsy of the right dorsal hand performed at the onset of symptoms at an outside facility revealed hyperkeratotic acanthotic epidermis with a mild perivascular lymphocytic infiltrate.
Results of patch testing indicated contact hypersensitivity to the botanical rosin colophonium (or colophony); carba mix (1, 3-diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethydithiocarbamate); thiuram mix (tetramethylthiuram disulfide, tetramethylthiuram monosulfide, and tetraethylthiuram disulfide); n,n-diphenylguanidine; and tixocortol-21-pivalate. Our patient was given guidance on avoiding these agents, as it was suspected that exposure may be exacerbating the psoriasis. The psoriasis was treated with topical corticosteroids, keratolytics, and calcineurin inhibitors, all of which offered minimal or no relief. Trials of systemic agents, including methotrexate (discontinued because transaminitis developed), etanercept, adalimumab, and apremilast for 6 to 10 months did not provide improvement.
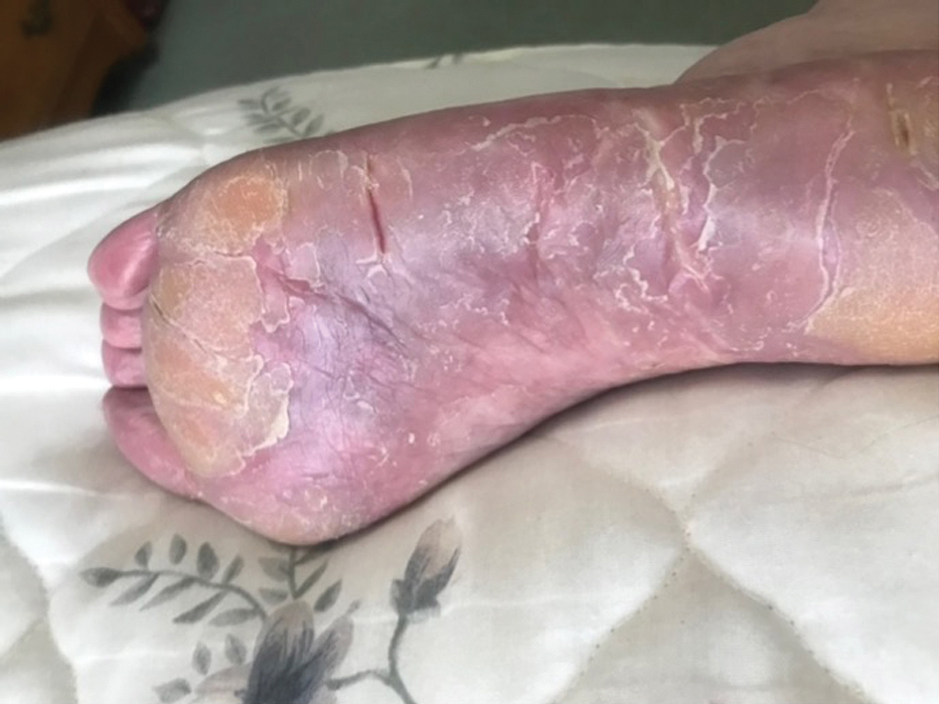
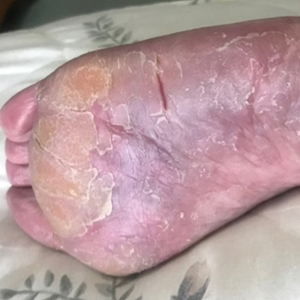
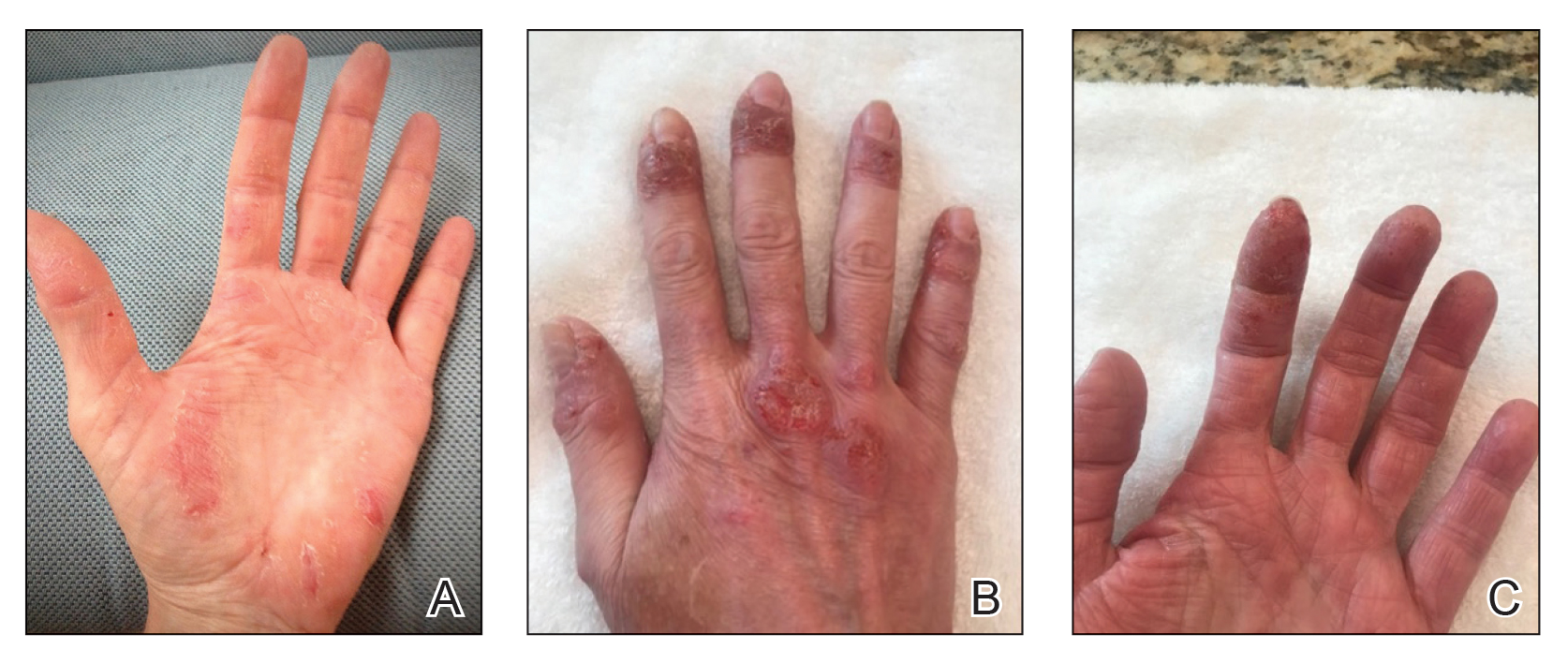
Two years prior to the current presentation, our patient had been treated with the IL-23 inhibitor guselkumab, which provided moderate improvement. When she presented to our clinic, physical examination while she was taking guselkumab demonstrated prurigo with excoriations of the extremities, hyperkeratosis with scaling and fissures of the soles, erythematous scaly plaques on the palms and dorsal surface of the hands, and mild onycholysis of the nails (Figures 1 and 2). Because we were concerned about concomitant intrinsic AD, dupilumab was initiated in conjunction with guselkumab. A second biopsy was considered but deferred in favor of clinical monitoring.
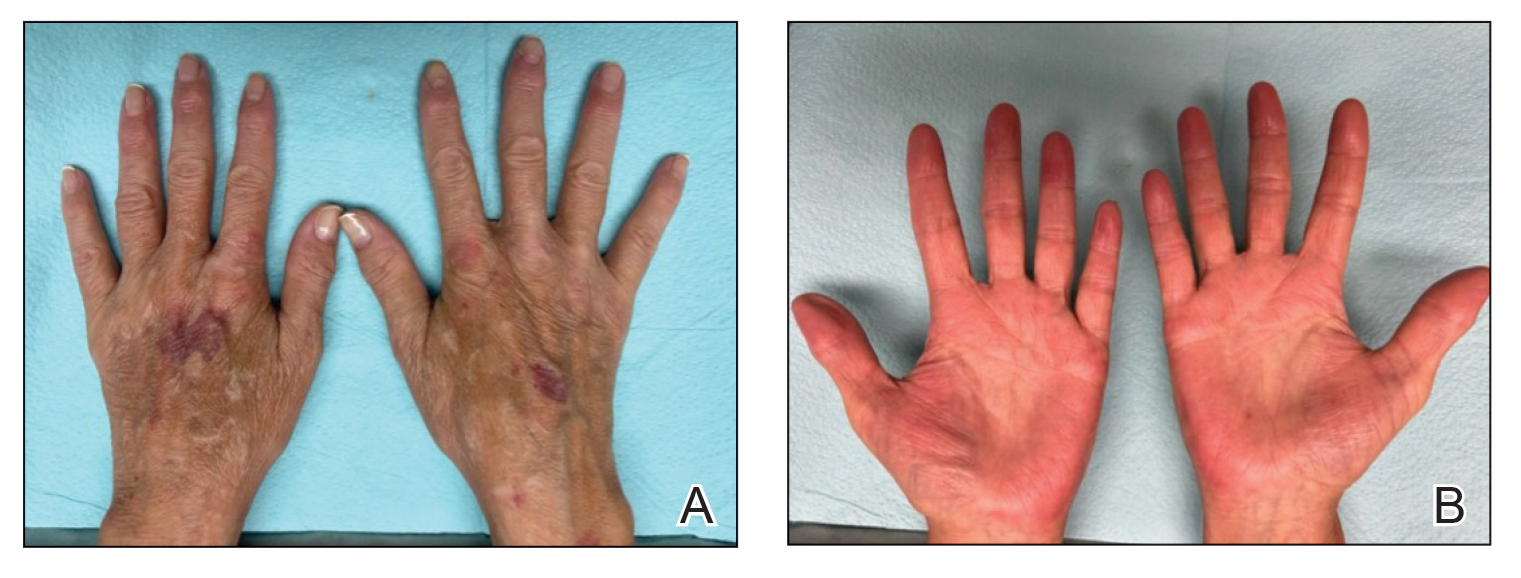
After 1 year of dual biologic therapy, the patient experienced near-complete resolution of symptoms. The psoriasis completely resolved from an initial body surface area of 5%, and the AD body surface area decreased from 30% to 2% (Figure 3). The patient reported no adverse effects from treatment.
Comment
Atopic dermatitis and psoriasis involve complex immunopathology and a spectrum of cytokines that might explain the overlap in their clinical and histopathologic presentations.
Atopic dermatitis—Atopic dermatitis involves TH1, TH2, TH9, TH17, and TH22 cells; TH2 cells release IL-4, IL-5, and IL-13, all of which are key cytokines in the inflammatory pathway of AD.9,10 Activation of the helper T-cell subset and the release of cytokines differ slightly based on the subcategory of AD and the stage of exacerbation. In addition to TH2-cell activation, TH1 cells and TH22 cells—which release IL-12 and IL-22, respectively—are active in both intrinsic and extrinsic AD. TH17 cells and TH9 cells—which release IL-17 and IL-9, respectively—are more prominent in the intrinsic pathway than in the extrinsic pathway.9 Intrinsic AD is recognized by a lack of eosinophilia, female predominance, and delayed onset compared to extrinsic AD; there also is a lack of history of atopy.1 Extrinsic AD is characterized by eosinophilia as well as a personal and family history of atopy.11 Our patient—a female with onset in older adulthood, lack of eosinophilia, and a family history of atopy—displayed features of both intrinsic and extrinsic AD.
Psoriasis—The immunopathology of psoriasis involves stimulation of dendritic cells, which activate TH17 cells through IL-23. TH17 cells then release IL-17 and IL-22. Therefore, both AD and psoriasis involve activation of TH22 and TH1 cells, with increased IL-17 and IL-22 production.3,10,12 IL-17 and IL-22 induce epidermal hyperplasia; IL-22 also contributes to skin barrier dysfunction.12 Therefore, it might be reasonable to consider psoriasis and AD as diseases that exist across a T-cell axis spectrum, thereby accounting for some overlap in disease characteristics.3
Dual Biologic Therapy—Dupilumab blocks the IL-4 receptor α subunit, a receptor for IL-4 and IL-13, which are key cytokines in the pathogenesis of AD.10 Guselkumab inhibits IL-23, thus blocking the inflammatory cascade of TH17 cell activation and release of IL-17 and IL-22 in the psoriasis pathway.13 Although an immunopathological spectrum exists between the 2 diseases, the continued presence of AD symptoms after blocking the IL-23 cascade suggests that additional blockade of TH2 cells is required to control AD in patients with true concurrent disease.
Accurate diagnosis of AD and/or psoriasis is important when considering targeted treatment of these conditions with biologics. The use of dual biologics is limited by a paucity of data regarding the safety of these agents when given in combination. A recent meta-analysis of dual biologic therapy in patients with inflammatory bowel disease demonstrated acceptable safety results with a pooled adverse reaction rate of 31%.14
Anchoring Bias—Anchoring bias can occur when a clinician’s decisions are influenced by a particular event or reference point, which might cause them to disregard subsequent evidence. Our case illustrates the importance of critically assessing the response to treatment and being mindful of the potential influence of anchoring bias on the differential diagnosis. Although overcoming biases in conditions with clinical overlap can be challenging, it is important to consider coexisting AD and psoriasis in patients with extensive hand involvement when multiple treatments have failed and only a partial response to targeted pathways has been achieved. In our case, the patient also had contact hypersensitivity to tixocortol-21-pivalate, which indicates hypersensitivity to many prescription topical corticosteroids, oral prednisone, and over-the-counter hydrocortisone; however, topical corticosteroids continued to be prescribed for her, which might have contributed to the lack of improvement and even exacerbated the rash.
Future Considerations—A consideration for the future in this case is discontinuing guselkumab to observe whether symptoms recur. We discussed this option with the patient, but she opted to continue treatment with dupilumab and guselkumab because of the symptom resolution.
Conclusion
Concomitant disease can present as an overlapping pattern in the same area, whereas other regions might have geographically isolated disease. Our patient’s overlap of symptoms, the failure of multiple treatments, and the partial improvement she experienced on guselkumab made diagnosis and management challenging; however, dual biologic therapy was successful.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis—a retrospective review. J Dermatolog Treat. 2021;32:716-720. doi:10.1080/09546634.2019.1702147
- Bozek A, Zajac M, Krupka M. Atopic dermatitis and psoriasis as overlapping syndromes. Mediators Inflamm. 2020;2020:7527859. doi:10.1155/2020/7527859
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73. doi:10.1016/j.coi.2017.08.008
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Docampo A, MJ, I, et al. Response to letter to the editor: ‘psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children.’ J Eur Acad Dermatol Venereol. 2019;33:E410-E412. doi:10.1111/jdv.15716
- Johnson MC, Bowers NL, Strowd LC. Concurrent atopic dermatitis and psoriasis vulgaris: implications for targeted biologic therapy. Cutis. 2022;109:110-112. doi:10.12788/cutis.0453
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Klonowska J, Glen J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci. 2018;19:3086. doi:10.3390/ijms19103086
- Ratchataswan T, Banzon TM, Thyssen JP, et al. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-1065. doi:10.1016/j.jaip.2020.11.034
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:7488. doi:10.3390/ijms21207488
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139:2437-2446.e1. doi:10.1016/j.jid.2019.05.016
- Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17:406-414.
Atopic dermatitis (AD) and psoriasis are common skin diseases in which dysfunction of the epidermal barrier leads to skin inflammation and altered expression of proinflammatory cytokines.1 There often is overlap in the clinical and histopathologic features of AD and psoriasis, which can make diagnosis a challenge. Persistent late-stage AD can present with psoriasiform lichenified changes, and psoriasis lesions in the acute stage can have an eczematous appearance.2 Histologically, chronic psoriasis lesions share many overlapping features with AD, and some subsets of AD with IL-17 predominance (ie, intrinsic, pediatric, presentation in Asian patients) exhibit a psoriasiform appearance.3,4
Atopic dermatitis and psoriasis are considered 2 distinct conditions because AD is a helper T cell (TH2)–driven disease with subsequent overproduction of IL-4 and IL-13 and psoriasis is a TH17 cell–driven disease with overproduction of IL-173; however, the shared features of AD and psoriasis represent an underlying immunopathological spectrum2,5,6 in which one condition can develop following treatment of the other condition (immunological shift in pathways), both conditions can occur at different times in a patient’s life with alternating cycles of disease flares, or both conditions can coexist as an overlapping syndrome.1,2 A retrospective study from 2012 to 2019 estimated the prevalence of concomitant AD and psoriasis in the United States at 1.3%, with AD following the diagnosis of psoriasis in 67% of cases.1 Concurrent AD and psoriasis—when both diseases flaresimultaneously—is the rarest scenario.2,5
Treatment modalities for AD include topical corticosteroids, which act on immune cells to suppress the release of proinflammatory cytokines, as well as dupilumab, which offers targeted blockade of involved cytokines IL-4 and IL-13. Psoriasis can be treated with multiple immune modulators, including topical corticosteroids and vitamin D analogs, as well as systemic medications that reduce T-cell activation and inflammatory cytokines through targeting of IFN-γ, IL-2, tumor necrosis factor α, IL-17, and IL-23.7,8
We present the case of a patient with long-standing concurrent, treatment-resistant AD and psoriasis who was successfully treated with dual biologic therapy with guselkumab and dupilumab.
Case Report
A 62-year-old woman presented to our dermatology clinic with red itchy scales and painful fissures on the palms, hands, and soles of more than 12 years’ duration. Her medical history included an allergy to amoxicillin-clavulanate as well as an allergy to both dog and cat dander on prick testing. Her family history included dyshidrotic eczema in her mother. A complete blood cell count with differential was within reference range. A shave biopsy of the right dorsal hand performed at the onset of symptoms at an outside facility revealed hyperkeratotic acanthotic epidermis with a mild perivascular lymphocytic infiltrate.
Results of patch testing indicated contact hypersensitivity to the botanical rosin colophonium (or colophony); carba mix (1, 3-diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethydithiocarbamate); thiuram mix (tetramethylthiuram disulfide, tetramethylthiuram monosulfide, and tetraethylthiuram disulfide); n,n-diphenylguanidine; and tixocortol-21-pivalate. Our patient was given guidance on avoiding these agents, as it was suspected that exposure may be exacerbating the psoriasis. The psoriasis was treated with topical corticosteroids, keratolytics, and calcineurin inhibitors, all of which offered minimal or no relief. Trials of systemic agents, including methotrexate (discontinued because transaminitis developed), etanercept, adalimumab, and apremilast for 6 to 10 months did not provide improvement.

Two years prior to the current presentation, our patient had been treated with the IL-23 inhibitor guselkumab, which provided moderate improvement. When she presented to our clinic, physical examination while she was taking guselkumab demonstrated prurigo with excoriations of the extremities, hyperkeratosis with scaling and fissures of the soles, erythematous scaly plaques on the palms and dorsal surface of the hands, and mild onycholysis of the nails (Figures 1 and 2). Because we were concerned about concomitant intrinsic AD, dupilumab was initiated in conjunction with guselkumab. A second biopsy was considered but deferred in favor of clinical monitoring.

After 1 year of dual biologic therapy, the patient experienced near-complete resolution of symptoms. The psoriasis completely resolved from an initial body surface area of 5%, and the AD body surface area decreased from 30% to 2% (Figure 3). The patient reported no adverse effects from treatment.

Comment
Atopic dermatitis and psoriasis involve complex immunopathology and a spectrum of cytokines that might explain the overlap in their clinical and histopathologic presentations.
Atopic dermatitis—Atopic dermatitis involves TH1, TH2, TH9, TH17, and TH22 cells; TH2 cells release IL-4, IL-5, and IL-13, all of which are key cytokines in the inflammatory pathway of AD.9,10 Activation of the helper T-cell subset and the release of cytokines differ slightly based on the subcategory of AD and the stage of exacerbation. In addition to TH2-cell activation, TH1 cells and TH22 cells—which release IL-12 and IL-22, respectively—are active in both intrinsic and extrinsic AD. TH17 cells and TH9 cells—which release IL-17 and IL-9, respectively—are more prominent in the intrinsic pathway than in the extrinsic pathway.9 Intrinsic AD is recognized by a lack of eosinophilia, female predominance, and delayed onset compared to extrinsic AD; there also is a lack of history of atopy.1 Extrinsic AD is characterized by eosinophilia as well as a personal and family history of atopy.11 Our patient—a female with onset in older adulthood, lack of eosinophilia, and a family history of atopy—displayed features of both intrinsic and extrinsic AD.
Psoriasis—The immunopathology of psoriasis involves stimulation of dendritic cells, which activate TH17 cells through IL-23. TH17 cells then release IL-17 and IL-22. Therefore, both AD and psoriasis involve activation of TH22 and TH1 cells, with increased IL-17 and IL-22 production.3,10,12 IL-17 and IL-22 induce epidermal hyperplasia; IL-22 also contributes to skin barrier dysfunction.12 Therefore, it might be reasonable to consider psoriasis and AD as diseases that exist across a T-cell axis spectrum, thereby accounting for some overlap in disease characteristics.3
Dual Biologic Therapy—Dupilumab blocks the IL-4 receptor α subunit, a receptor for IL-4 and IL-13, which are key cytokines in the pathogenesis of AD.10 Guselkumab inhibits IL-23, thus blocking the inflammatory cascade of TH17 cell activation and release of IL-17 and IL-22 in the psoriasis pathway.13 Although an immunopathological spectrum exists between the 2 diseases, the continued presence of AD symptoms after blocking the IL-23 cascade suggests that additional blockade of TH2 cells is required to control AD in patients with true concurrent disease.
Accurate diagnosis of AD and/or psoriasis is important when considering targeted treatment of these conditions with biologics. The use of dual biologics is limited by a paucity of data regarding the safety of these agents when given in combination. A recent meta-analysis of dual biologic therapy in patients with inflammatory bowel disease demonstrated acceptable safety results with a pooled adverse reaction rate of 31%.14
Anchoring Bias—Anchoring bias can occur when a clinician’s decisions are influenced by a particular event or reference point, which might cause them to disregard subsequent evidence. Our case illustrates the importance of critically assessing the response to treatment and being mindful of the potential influence of anchoring bias on the differential diagnosis. Although overcoming biases in conditions with clinical overlap can be challenging, it is important to consider coexisting AD and psoriasis in patients with extensive hand involvement when multiple treatments have failed and only a partial response to targeted pathways has been achieved. In our case, the patient also had contact hypersensitivity to tixocortol-21-pivalate, which indicates hypersensitivity to many prescription topical corticosteroids, oral prednisone, and over-the-counter hydrocortisone; however, topical corticosteroids continued to be prescribed for her, which might have contributed to the lack of improvement and even exacerbated the rash.
Future Considerations—A consideration for the future in this case is discontinuing guselkumab to observe whether symptoms recur. We discussed this option with the patient, but she opted to continue treatment with dupilumab and guselkumab because of the symptom resolution.
Conclusion
Concomitant disease can present as an overlapping pattern in the same area, whereas other regions might have geographically isolated disease. Our patient’s overlap of symptoms, the failure of multiple treatments, and the partial improvement she experienced on guselkumab made diagnosis and management challenging; however, dual biologic therapy was successful.
Atopic dermatitis (AD) and psoriasis are common skin diseases in which dysfunction of the epidermal barrier leads to skin inflammation and altered expression of proinflammatory cytokines.1 There often is overlap in the clinical and histopathologic features of AD and psoriasis, which can make diagnosis a challenge. Persistent late-stage AD can present with psoriasiform lichenified changes, and psoriasis lesions in the acute stage can have an eczematous appearance.2 Histologically, chronic psoriasis lesions share many overlapping features with AD, and some subsets of AD with IL-17 predominance (ie, intrinsic, pediatric, presentation in Asian patients) exhibit a psoriasiform appearance.3,4
Atopic dermatitis and psoriasis are considered 2 distinct conditions because AD is a helper T cell (TH2)–driven disease with subsequent overproduction of IL-4 and IL-13 and psoriasis is a TH17 cell–driven disease with overproduction of IL-173; however, the shared features of AD and psoriasis represent an underlying immunopathological spectrum2,5,6 in which one condition can develop following treatment of the other condition (immunological shift in pathways), both conditions can occur at different times in a patient’s life with alternating cycles of disease flares, or both conditions can coexist as an overlapping syndrome.1,2 A retrospective study from 2012 to 2019 estimated the prevalence of concomitant AD and psoriasis in the United States at 1.3%, with AD following the diagnosis of psoriasis in 67% of cases.1 Concurrent AD and psoriasis—when both diseases flaresimultaneously—is the rarest scenario.2,5
Treatment modalities for AD include topical corticosteroids, which act on immune cells to suppress the release of proinflammatory cytokines, as well as dupilumab, which offers targeted blockade of involved cytokines IL-4 and IL-13. Psoriasis can be treated with multiple immune modulators, including topical corticosteroids and vitamin D analogs, as well as systemic medications that reduce T-cell activation and inflammatory cytokines through targeting of IFN-γ, IL-2, tumor necrosis factor α, IL-17, and IL-23.7,8
We present the case of a patient with long-standing concurrent, treatment-resistant AD and psoriasis who was successfully treated with dual biologic therapy with guselkumab and dupilumab.
Case Report
A 62-year-old woman presented to our dermatology clinic with red itchy scales and painful fissures on the palms, hands, and soles of more than 12 years’ duration. Her medical history included an allergy to amoxicillin-clavulanate as well as an allergy to both dog and cat dander on prick testing. Her family history included dyshidrotic eczema in her mother. A complete blood cell count with differential was within reference range. A shave biopsy of the right dorsal hand performed at the onset of symptoms at an outside facility revealed hyperkeratotic acanthotic epidermis with a mild perivascular lymphocytic infiltrate.
Results of patch testing indicated contact hypersensitivity to the botanical rosin colophonium (or colophony); carba mix (1, 3-diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethydithiocarbamate); thiuram mix (tetramethylthiuram disulfide, tetramethylthiuram monosulfide, and tetraethylthiuram disulfide); n,n-diphenylguanidine; and tixocortol-21-pivalate. Our patient was given guidance on avoiding these agents, as it was suspected that exposure may be exacerbating the psoriasis. The psoriasis was treated with topical corticosteroids, keratolytics, and calcineurin inhibitors, all of which offered minimal or no relief. Trials of systemic agents, including methotrexate (discontinued because transaminitis developed), etanercept, adalimumab, and apremilast for 6 to 10 months did not provide improvement.

Two years prior to the current presentation, our patient had been treated with the IL-23 inhibitor guselkumab, which provided moderate improvement. When she presented to our clinic, physical examination while she was taking guselkumab demonstrated prurigo with excoriations of the extremities, hyperkeratosis with scaling and fissures of the soles, erythematous scaly plaques on the palms and dorsal surface of the hands, and mild onycholysis of the nails (Figures 1 and 2). Because we were concerned about concomitant intrinsic AD, dupilumab was initiated in conjunction with guselkumab. A second biopsy was considered but deferred in favor of clinical monitoring.

After 1 year of dual biologic therapy, the patient experienced near-complete resolution of symptoms. The psoriasis completely resolved from an initial body surface area of 5%, and the AD body surface area decreased from 30% to 2% (Figure 3). The patient reported no adverse effects from treatment.

Comment
Atopic dermatitis and psoriasis involve complex immunopathology and a spectrum of cytokines that might explain the overlap in their clinical and histopathologic presentations.
Atopic dermatitis—Atopic dermatitis involves TH1, TH2, TH9, TH17, and TH22 cells; TH2 cells release IL-4, IL-5, and IL-13, all of which are key cytokines in the inflammatory pathway of AD.9,10 Activation of the helper T-cell subset and the release of cytokines differ slightly based on the subcategory of AD and the stage of exacerbation. In addition to TH2-cell activation, TH1 cells and TH22 cells—which release IL-12 and IL-22, respectively—are active in both intrinsic and extrinsic AD. TH17 cells and TH9 cells—which release IL-17 and IL-9, respectively—are more prominent in the intrinsic pathway than in the extrinsic pathway.9 Intrinsic AD is recognized by a lack of eosinophilia, female predominance, and delayed onset compared to extrinsic AD; there also is a lack of history of atopy.1 Extrinsic AD is characterized by eosinophilia as well as a personal and family history of atopy.11 Our patient—a female with onset in older adulthood, lack of eosinophilia, and a family history of atopy—displayed features of both intrinsic and extrinsic AD.
Psoriasis—The immunopathology of psoriasis involves stimulation of dendritic cells, which activate TH17 cells through IL-23. TH17 cells then release IL-17 and IL-22. Therefore, both AD and psoriasis involve activation of TH22 and TH1 cells, with increased IL-17 and IL-22 production.3,10,12 IL-17 and IL-22 induce epidermal hyperplasia; IL-22 also contributes to skin barrier dysfunction.12 Therefore, it might be reasonable to consider psoriasis and AD as diseases that exist across a T-cell axis spectrum, thereby accounting for some overlap in disease characteristics.3
Dual Biologic Therapy—Dupilumab blocks the IL-4 receptor α subunit, a receptor for IL-4 and IL-13, which are key cytokines in the pathogenesis of AD.10 Guselkumab inhibits IL-23, thus blocking the inflammatory cascade of TH17 cell activation and release of IL-17 and IL-22 in the psoriasis pathway.13 Although an immunopathological spectrum exists between the 2 diseases, the continued presence of AD symptoms after blocking the IL-23 cascade suggests that additional blockade of TH2 cells is required to control AD in patients with true concurrent disease.
Accurate diagnosis of AD and/or psoriasis is important when considering targeted treatment of these conditions with biologics. The use of dual biologics is limited by a paucity of data regarding the safety of these agents when given in combination. A recent meta-analysis of dual biologic therapy in patients with inflammatory bowel disease demonstrated acceptable safety results with a pooled adverse reaction rate of 31%.14
Anchoring Bias—Anchoring bias can occur when a clinician’s decisions are influenced by a particular event or reference point, which might cause them to disregard subsequent evidence. Our case illustrates the importance of critically assessing the response to treatment and being mindful of the potential influence of anchoring bias on the differential diagnosis. Although overcoming biases in conditions with clinical overlap can be challenging, it is important to consider coexisting AD and psoriasis in patients with extensive hand involvement when multiple treatments have failed and only a partial response to targeted pathways has been achieved. In our case, the patient also had contact hypersensitivity to tixocortol-21-pivalate, which indicates hypersensitivity to many prescription topical corticosteroids, oral prednisone, and over-the-counter hydrocortisone; however, topical corticosteroids continued to be prescribed for her, which might have contributed to the lack of improvement and even exacerbated the rash.
Future Considerations—A consideration for the future in this case is discontinuing guselkumab to observe whether symptoms recur. We discussed this option with the patient, but she opted to continue treatment with dupilumab and guselkumab because of the symptom resolution.
Conclusion
Concomitant disease can present as an overlapping pattern in the same area, whereas other regions might have geographically isolated disease. Our patient’s overlap of symptoms, the failure of multiple treatments, and the partial improvement she experienced on guselkumab made diagnosis and management challenging; however, dual biologic therapy was successful.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis—a retrospective review. J Dermatolog Treat. 2021;32:716-720. doi:10.1080/09546634.2019.1702147
- Bozek A, Zajac M, Krupka M. Atopic dermatitis and psoriasis as overlapping syndromes. Mediators Inflamm. 2020;2020:7527859. doi:10.1155/2020/7527859
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73. doi:10.1016/j.coi.2017.08.008
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Docampo A, MJ, I, et al. Response to letter to the editor: ‘psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children.’ J Eur Acad Dermatol Venereol. 2019;33:E410-E412. doi:10.1111/jdv.15716
- Johnson MC, Bowers NL, Strowd LC. Concurrent atopic dermatitis and psoriasis vulgaris: implications for targeted biologic therapy. Cutis. 2022;109:110-112. doi:10.12788/cutis.0453
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Klonowska J, Glen J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci. 2018;19:3086. doi:10.3390/ijms19103086
- Ratchataswan T, Banzon TM, Thyssen JP, et al. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-1065. doi:10.1016/j.jaip.2020.11.034
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:7488. doi:10.3390/ijms21207488
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139:2437-2446.e1. doi:10.1016/j.jid.2019.05.016
- Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17:406-414.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis—a retrospective review. J Dermatolog Treat. 2021;32:716-720. doi:10.1080/09546634.2019.1702147
- Bozek A, Zajac M, Krupka M. Atopic dermatitis and psoriasis as overlapping syndromes. Mediators Inflamm. 2020;2020:7527859. doi:10.1155/2020/7527859
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73. doi:10.1016/j.coi.2017.08.008
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Docampo A, MJ, I, et al. Response to letter to the editor: ‘psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children.’ J Eur Acad Dermatol Venereol. 2019;33:E410-E412. doi:10.1111/jdv.15716
- Johnson MC, Bowers NL, Strowd LC. Concurrent atopic dermatitis and psoriasis vulgaris: implications for targeted biologic therapy. Cutis. 2022;109:110-112. doi:10.12788/cutis.0453
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Klonowska J, Glen J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci. 2018;19:3086. doi:10.3390/ijms19103086
- Ratchataswan T, Banzon TM, Thyssen JP, et al. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-1065. doi:10.1016/j.jaip.2020.11.034
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:7488. doi:10.3390/ijms21207488
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139:2437-2446.e1. doi:10.1016/j.jid.2019.05.016
- Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17:406-414.
Practice Points
- Atopic dermatitis and psoriasis can share clinical and histopathologic features, which represents their underlying immunopathologic spectrum.
- Atopic dermatitis and psoriasis can coexist in a single patient, which may be suspected from a clinical picture of treatment-resistant disease, a partial response to targeted therapies, or extensive hand involvement.
Endocrine Mucin-Producing Sweat Gland Carcinoma and Primary Cutaneous Mucinous Carcinoma: A Case Series
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) and
Methods
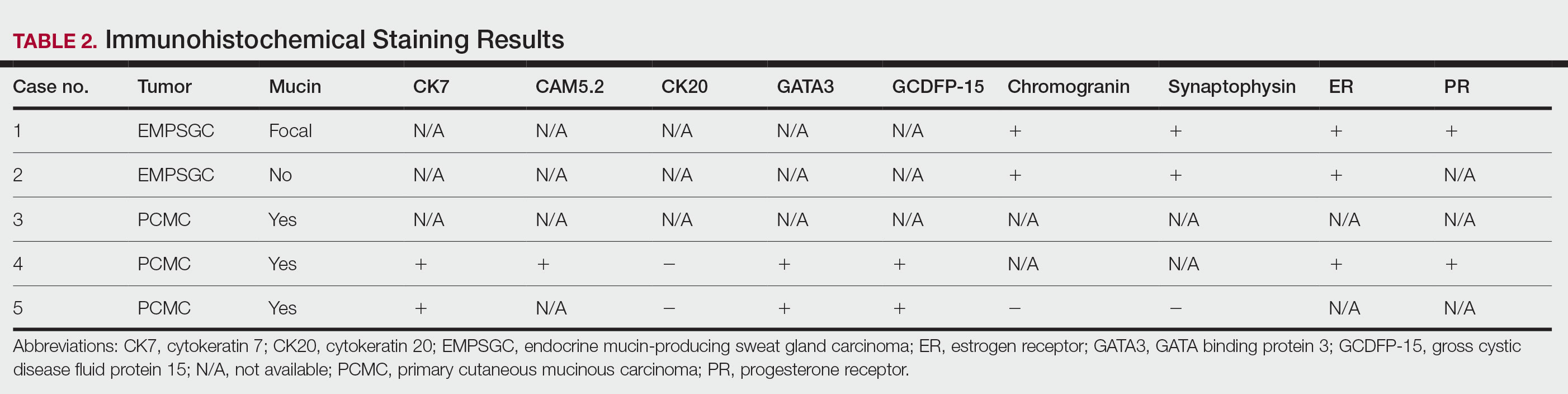
Following institutional review board approval, we conducted a retrospective, single-institution case series. We searched electronic medical records dating from 2000 to 2019 for tumors diagnosed as PCMC or extramammary Paget disease treated with MMS. We gathered demographic, clinical, pathologic, and follow-up information from the electronic medical records for each case (Tables 1 and 2). Two dermatopathologists (B.P. and B.F.K.) reviewed the hematoxylin and eosin–stained slides of each tumor as well as all available immunohistochemical stains. One of the reviewers (B.F.K.) is a board-certified dermatologist, dermatopathologist, and fellowship-trained Mohs surgeon.
Results
Demographic and Clinical Information—We identified 2 cases of EMPSGC and 3 cases of PCMC diagnosed and treated at our institution; 4 of these cases had been treated within the last 2 years. One had been treated 18 years prior; case information was limited due to planned institutional record destruction. Three of the patients were female and 2 were male. The mean age at presentation was 71 years (range, 62–87 years). None had experienced recurrence or metastases after a mean follow-up of 30 months.
Case 1—A 68-year-old woman noted a slow-growing, flesh-colored papule measuring 12×10 mm on the right lower eyelid. An excisional biopsy was completed with 2-mm clinical margins, and the defect was closed in a linear fashion. Histologic sections demonstrated EMPSGC with uninvolved margins. The patient desired no further intervention and was clinically followed. Magnetic resonance imaging (MRI) of the head and neck found no evidence of metastasis. She has had no recurrence after 15 months.
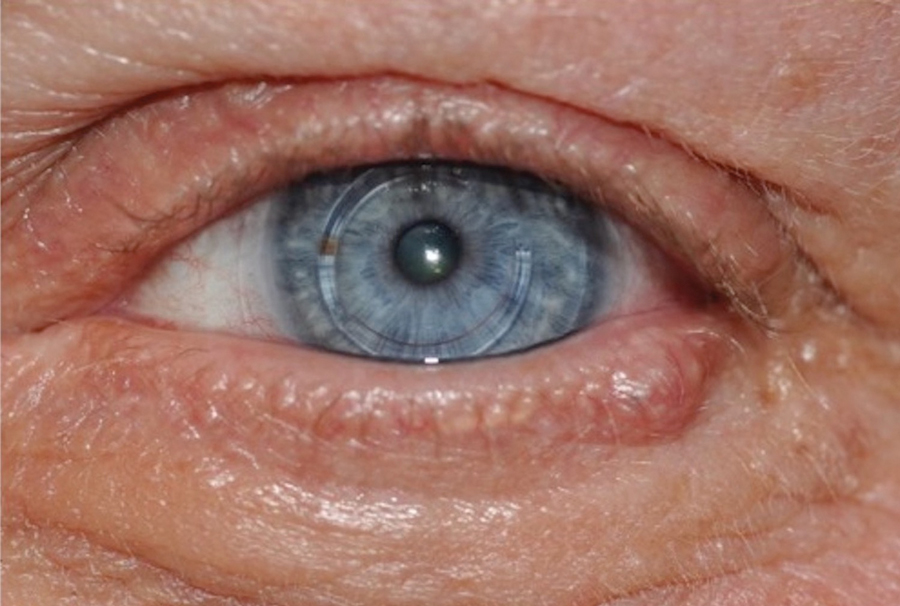
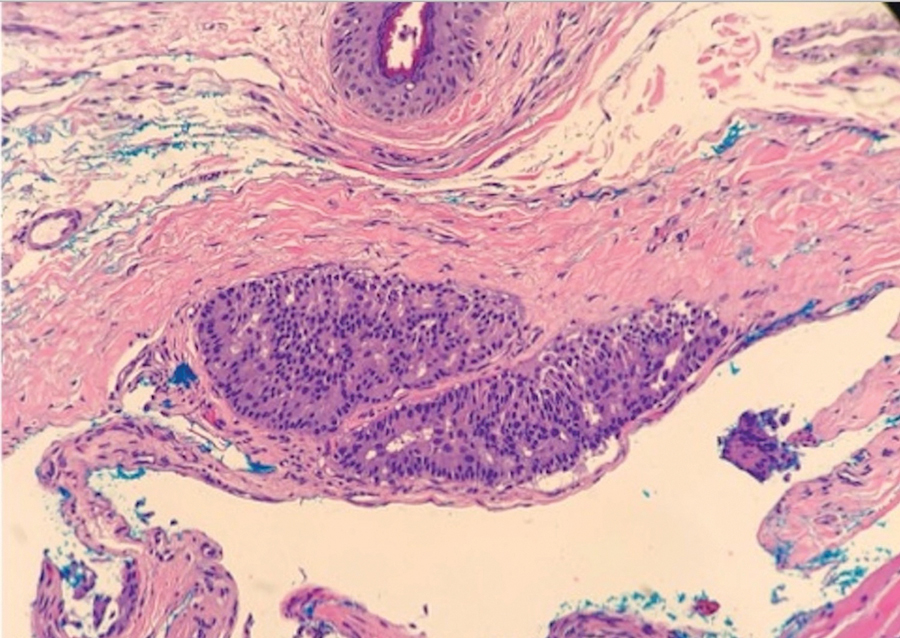
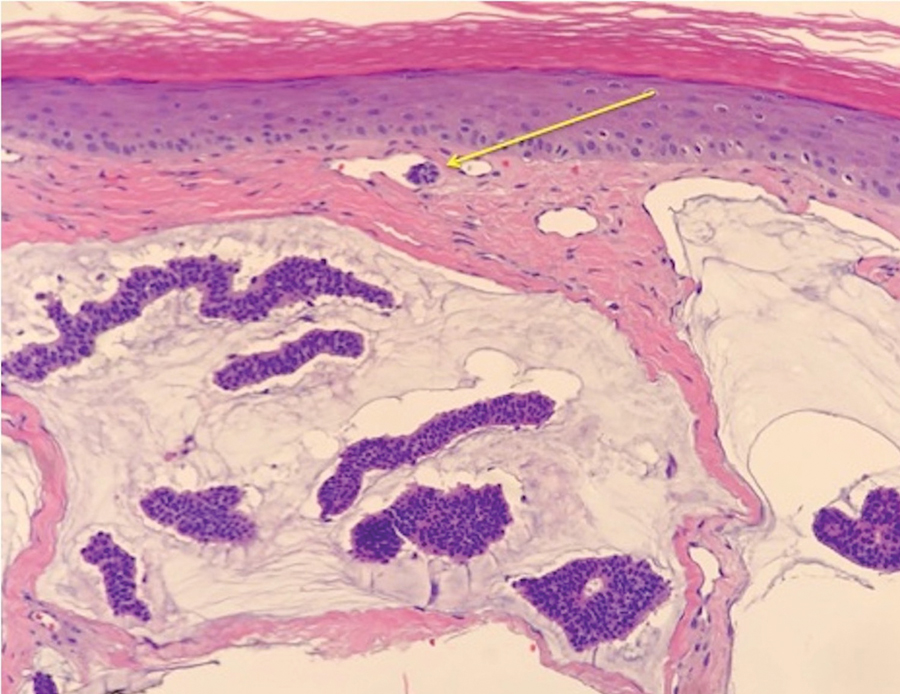
Case 2—A 62-year-old man presented with a 7×5-mm, flesh-colored papule on the left lower eyelid margin (Figure 1). It was previously treated conservatively as a hordeolum but was biopsied after it failed to resolve with 3-mm margins. Histopathology demonstrated an EMPSGC (Figure 2). The lesion was treated with modified MMS with permanent en face section processing and cleared after 1 stage. Computed tomography of the head and neck showed no abnormalities. He has had no recurrence after 9 months.
Case 3—A 72-year-old man presented with a nontender papule near the right lateral canthus. A punch biopsy demonstrated PCMC. He was treated via modified MMS with permanent en face section processing. The tumor was cleared in 1 stage. He showed no evidence of recurrence after 112 months and died of unrelated causes. The rest of his clinical information was limited because of planned institutional destruction of records.
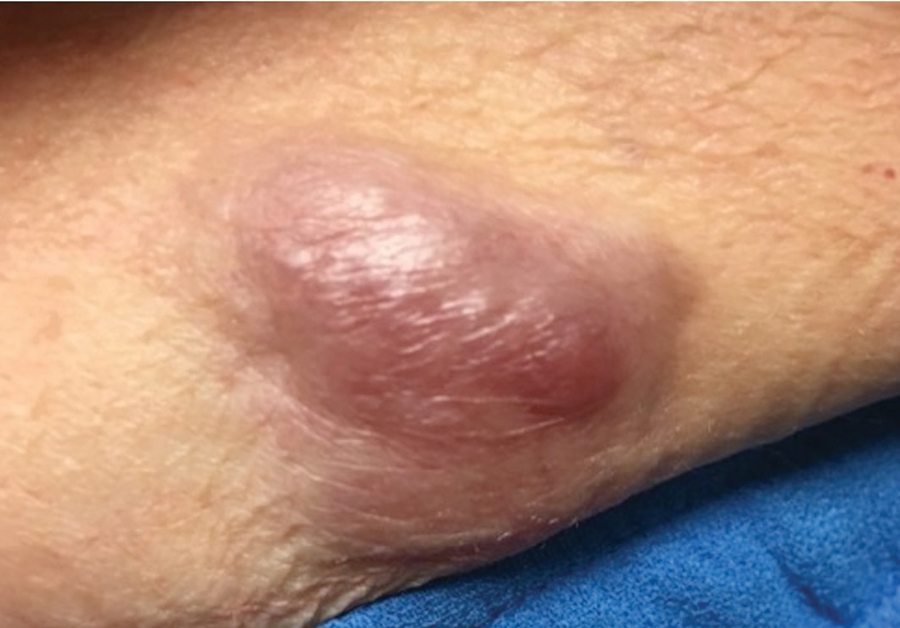
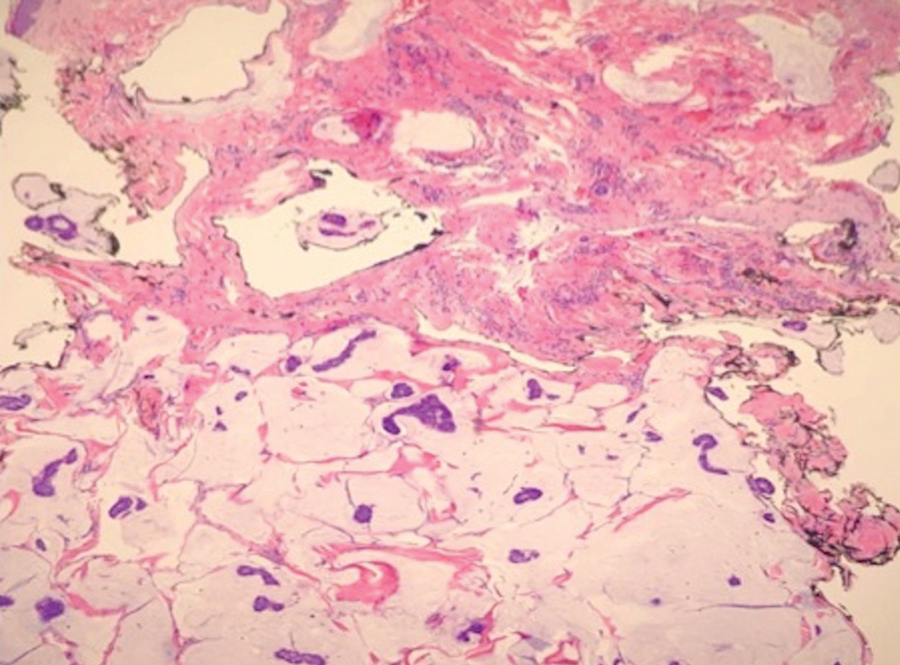
Case 4—An 87-year-old woman presented with a 25×25-mm, slow-growing mass of 12 months’ duration on the left lower abdomen (Figure 3). A biopsy demonstrated PCMC (Figure 4). Because of the size of the lesion, she underwent WLE with 20- to 30-mm margins by a general surgeon under general anesthesia. Positron emission tomography/computed tomography was unremarkable. She has remained disease free for 11 months.
Case 5—A 66-year-old woman presented for evaluation of a posterior scalp mass measuring 23×18 mm that had grown over the last 24 months. Biopsy showed mucinous carcinoma with lymphovascular invasion consistent with PCMC (Figure 5) confirmed on multiple tissue levels and with the aid of immunohistochemistry. She was sent for an MRI of the head, neck, chest, abdomen, and pelvis, which demonstrated 2 enlarged postauricular lymph nodes and raised suspicion for metastatic disease vs reactive lymphadenopathy. Mohs micrographic surgery with frozen sections was performed with 1- to 3-mm margins; the final layer was sent for permanent processing and confirmed negative margins. Sentinel lymph node biopsy and lymphadenectomy of the 2 nodes present on imaging showed no evidence of metastasis. The patient had no recurrence in 1 month.
Comment
Endocrine mucin-producing sweat gland carcinoma and PCMC are sweat gland malignancies that carry low metastatic potential but are locally aggressive. Endocrine mucin-producing sweat gland carcinoma has a strong predilection for the periorbital region, especially the lower eyelids of older women.3 Primary cutaneous mucinous carcinoma may arise on the eyelids, scalp, axillae, and trunk and has been reported more often in older men. These slow-growing tumors appear as nonspecific nodules.3 Lesions frequently are asymptomatic but rarely may cause pruritus and bleeding. Histologically, EMPSGC appears as solid or cystic nodules of cells with a papillary, cribriform, or pseudopapillary appearance. Intracellular or extracellular mucin as well as malignant spread of tumor cells along pre-existing ductlike structures make it difficult to histologically distinguish EMPSGC from ductal carcinoma in situ.3
A key histopathologic feature of PCMC is basophilic epithelioid cell nests in mucinous lakes.4 Rosettelike structures are seen within solid areas of the tumor. Fibrous septae separate individual collections of mucin, creating a lobulated appearance. The histopathologic differential diagnosis of EMPSGC and PCMC is broad, including basal cell carcinoma, hidradenoma, hidradenocarcinoma, apocrine adenoma, and dermal duct tumor. Positive expression of at least 1 neuroendocrine marker (ie, synaptophysin, neuron-specific enolase, chromogranin) and low-molecular cytokeratin (cytokeratin 7, CAM5.2, Ber-EP4) can aid in the diagnosis of both EMPSGC and PCMC.4 The use of p63 immunostaining is beneficial in delineating adnexal neoplasms. Adnexal tumors that stain positively with p63 are more likely to be of primary cutaneous origin, whereas lack of p63 staining usually denotes a secondary metastatic process. However, p63 staining is less reliable when distinguishing primary and metastatic mucinous neoplasms. Metastatic mucinous carcinomas often stain positive with p63, while PCMC usually stains negative despite its primary cutaneous origin, decreasing the clinical utility of p63. The tumor may be identical to metastatic mucinous adenocarcinoma of the breast, gastrointestinal tract, lung, ovary, and pancreas. Tumor islands floating in mucin are identified in both primary cutaneous and metastatic disease to the skin.3,6 Areas of tumor necrosis, notable atypia, and perineural or lymphovascular invasion are infrequently reported in EMPSGC or PCMC, though lymphatic invasion was identified in case 5 presented herein.
A metastatic workup is warranted in all cases of PCMC, including a thorough history, review of systems, breast examination, and imaging. A workup may be considered in cases of EMPSGC depending on histologic features or clinical history.
There is uncertainty regarding the optimal management of these slow-growing yet locally destructive tumors.5 The incidence of local recurrence of PCMC after WLE with narrow margins of at least 1 cm can be as high as 30% to 40%, especially on the eyelid.4 There is no consensus on surgical care for either of these tumors.5 Because of the high recurrence rate and the predilection for the eyelid and face, MMS provides an excellent alternative to WLE for tissue preservation and meticulous margin control. We advocate for the use of the Mohs technique with permanent sectioning, which may delay the repair, but reviewing tissue with permanent fixation improves the quality and accuracy of the margin evaluation because these tumors often are infiltrative and difficult to delineate under frozen section processing. Permanent en face sectioning allows the laboratory to utilize the full array of immunohistochemical stains for these tumors, providing accurate and timely results.
Limitations to our retrospective uncontrolled study include missing or incomplete data points and short follow-up time. Additionally, there was no standardization to the margins removed with MMS or WLE because of the limited available data that comment on appropriate margins.
- Held L, Ruetten A, Kutzner H, et al. Endocrine mucin‐producing sweat gland carcinoma: clinicopathologic, immunohistochemical and molecular analysis of 11 cases with emphasis on MYB immunoexpression. J Cutan Pathol. 2018;45:674-680.
- Navrazhina K, Petukhova T, Wildman HF, et al. Endocrine mucin-producing sweat gland carcinoma of the scalp treated with Mohs micrographic surgery. JAAD Case Rep. 2018;4:887-889.
- Scott BL, Anyanwu CO, Vandergriff T, et al. Endocrine mucin–producing sweat gland carcinoma treated with Mohs micrographic surgery. Dermatol Surg. 2017;43:1498-1500.
- Chang S, Shim SH, Joo M, et al. A case of endocrine mucin-producing sweat gland carcinoma co-existing with mucinous carcinoma: a case report. Korean J Pathol. 2010;44:97-100.
- Kamalpour L, Brindise RT, Nodzenski M, et al. Primary cutaneous mucinous carcinoma: a systematic review and meta-analysis of outcomes after surgery. JAMA Dermatol. 2014;150:380-384.
- Bulliard C, Murali R, Maloof A, et al. Endocrine mucin‐producing sweat gland carcinoma: report of a case and review of the literature. J Cutan Pathol. 2006;33:812-816.
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) and

Methods
Following institutional review board approval, we conducted a retrospective, single-institution case series. We searched electronic medical records dating from 2000 to 2019 for tumors diagnosed as PCMC or extramammary Paget disease treated with MMS. We gathered demographic, clinical, pathologic, and follow-up information from the electronic medical records for each case (Tables 1 and 2). Two dermatopathologists (B.P. and B.F.K.) reviewed the hematoxylin and eosin–stained slides of each tumor as well as all available immunohistochemical stains. One of the reviewers (B.F.K.) is a board-certified dermatologist, dermatopathologist, and fellowship-trained Mohs surgeon.

Results
Demographic and Clinical Information—We identified 2 cases of EMPSGC and 3 cases of PCMC diagnosed and treated at our institution; 4 of these cases had been treated within the last 2 years. One had been treated 18 years prior; case information was limited due to planned institutional record destruction. Three of the patients were female and 2 were male. The mean age at presentation was 71 years (range, 62–87 years). None had experienced recurrence or metastases after a mean follow-up of 30 months.
Case 1—A 68-year-old woman noted a slow-growing, flesh-colored papule measuring 12×10 mm on the right lower eyelid. An excisional biopsy was completed with 2-mm clinical margins, and the defect was closed in a linear fashion. Histologic sections demonstrated EMPSGC with uninvolved margins. The patient desired no further intervention and was clinically followed. Magnetic resonance imaging (MRI) of the head and neck found no evidence of metastasis. She has had no recurrence after 15 months.

Case 2—A 62-year-old man presented with a 7×5-mm, flesh-colored papule on the left lower eyelid margin (Figure 1). It was previously treated conservatively as a hordeolum but was biopsied after it failed to resolve with 3-mm margins. Histopathology demonstrated an EMPSGC (Figure 2). The lesion was treated with modified MMS with permanent en face section processing and cleared after 1 stage. Computed tomography of the head and neck showed no abnormalities. He has had no recurrence after 9 months.

Case 3—A 72-year-old man presented with a nontender papule near the right lateral canthus. A punch biopsy demonstrated PCMC. He was treated via modified MMS with permanent en face section processing. The tumor was cleared in 1 stage. He showed no evidence of recurrence after 112 months and died of unrelated causes. The rest of his clinical information was limited because of planned institutional destruction of records.

Case 4—An 87-year-old woman presented with a 25×25-mm, slow-growing mass of 12 months’ duration on the left lower abdomen (Figure 3). A biopsy demonstrated PCMC (Figure 4). Because of the size of the lesion, she underwent WLE with 20- to 30-mm margins by a general surgeon under general anesthesia. Positron emission tomography/computed tomography was unremarkable. She has remained disease free for 11 months.

Case 5—A 66-year-old woman presented for evaluation of a posterior scalp mass measuring 23×18 mm that had grown over the last 24 months. Biopsy showed mucinous carcinoma with lymphovascular invasion consistent with PCMC (Figure 5) confirmed on multiple tissue levels and with the aid of immunohistochemistry. She was sent for an MRI of the head, neck, chest, abdomen, and pelvis, which demonstrated 2 enlarged postauricular lymph nodes and raised suspicion for metastatic disease vs reactive lymphadenopathy. Mohs micrographic surgery with frozen sections was performed with 1- to 3-mm margins; the final layer was sent for permanent processing and confirmed negative margins. Sentinel lymph node biopsy and lymphadenectomy of the 2 nodes present on imaging showed no evidence of metastasis. The patient had no recurrence in 1 month.

Comment
Endocrine mucin-producing sweat gland carcinoma and PCMC are sweat gland malignancies that carry low metastatic potential but are locally aggressive. Endocrine mucin-producing sweat gland carcinoma has a strong predilection for the periorbital region, especially the lower eyelids of older women.3 Primary cutaneous mucinous carcinoma may arise on the eyelids, scalp, axillae, and trunk and has been reported more often in older men. These slow-growing tumors appear as nonspecific nodules.3 Lesions frequently are asymptomatic but rarely may cause pruritus and bleeding. Histologically, EMPSGC appears as solid or cystic nodules of cells with a papillary, cribriform, or pseudopapillary appearance. Intracellular or extracellular mucin as well as malignant spread of tumor cells along pre-existing ductlike structures make it difficult to histologically distinguish EMPSGC from ductal carcinoma in situ.3
A key histopathologic feature of PCMC is basophilic epithelioid cell nests in mucinous lakes.4 Rosettelike structures are seen within solid areas of the tumor. Fibrous septae separate individual collections of mucin, creating a lobulated appearance. The histopathologic differential diagnosis of EMPSGC and PCMC is broad, including basal cell carcinoma, hidradenoma, hidradenocarcinoma, apocrine adenoma, and dermal duct tumor. Positive expression of at least 1 neuroendocrine marker (ie, synaptophysin, neuron-specific enolase, chromogranin) and low-molecular cytokeratin (cytokeratin 7, CAM5.2, Ber-EP4) can aid in the diagnosis of both EMPSGC and PCMC.4 The use of p63 immunostaining is beneficial in delineating adnexal neoplasms. Adnexal tumors that stain positively with p63 are more likely to be of primary cutaneous origin, whereas lack of p63 staining usually denotes a secondary metastatic process. However, p63 staining is less reliable when distinguishing primary and metastatic mucinous neoplasms. Metastatic mucinous carcinomas often stain positive with p63, while PCMC usually stains negative despite its primary cutaneous origin, decreasing the clinical utility of p63. The tumor may be identical to metastatic mucinous adenocarcinoma of the breast, gastrointestinal tract, lung, ovary, and pancreas. Tumor islands floating in mucin are identified in both primary cutaneous and metastatic disease to the skin.3,6 Areas of tumor necrosis, notable atypia, and perineural or lymphovascular invasion are infrequently reported in EMPSGC or PCMC, though lymphatic invasion was identified in case 5 presented herein.
A metastatic workup is warranted in all cases of PCMC, including a thorough history, review of systems, breast examination, and imaging. A workup may be considered in cases of EMPSGC depending on histologic features or clinical history.
There is uncertainty regarding the optimal management of these slow-growing yet locally destructive tumors.5 The incidence of local recurrence of PCMC after WLE with narrow margins of at least 1 cm can be as high as 30% to 40%, especially on the eyelid.4 There is no consensus on surgical care for either of these tumors.5 Because of the high recurrence rate and the predilection for the eyelid and face, MMS provides an excellent alternative to WLE for tissue preservation and meticulous margin control. We advocate for the use of the Mohs technique with permanent sectioning, which may delay the repair, but reviewing tissue with permanent fixation improves the quality and accuracy of the margin evaluation because these tumors often are infiltrative and difficult to delineate under frozen section processing. Permanent en face sectioning allows the laboratory to utilize the full array of immunohistochemical stains for these tumors, providing accurate and timely results.
Limitations to our retrospective uncontrolled study include missing or incomplete data points and short follow-up time. Additionally, there was no standardization to the margins removed with MMS or WLE because of the limited available data that comment on appropriate margins.
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) and

Methods
Following institutional review board approval, we conducted a retrospective, single-institution case series. We searched electronic medical records dating from 2000 to 2019 for tumors diagnosed as PCMC or extramammary Paget disease treated with MMS. We gathered demographic, clinical, pathologic, and follow-up information from the electronic medical records for each case (Tables 1 and 2). Two dermatopathologists (B.P. and B.F.K.) reviewed the hematoxylin and eosin–stained slides of each tumor as well as all available immunohistochemical stains. One of the reviewers (B.F.K.) is a board-certified dermatologist, dermatopathologist, and fellowship-trained Mohs surgeon.

Results
Demographic and Clinical Information—We identified 2 cases of EMPSGC and 3 cases of PCMC diagnosed and treated at our institution; 4 of these cases had been treated within the last 2 years. One had been treated 18 years prior; case information was limited due to planned institutional record destruction. Three of the patients were female and 2 were male. The mean age at presentation was 71 years (range, 62–87 years). None had experienced recurrence or metastases after a mean follow-up of 30 months.
Case 1—A 68-year-old woman noted a slow-growing, flesh-colored papule measuring 12×10 mm on the right lower eyelid. An excisional biopsy was completed with 2-mm clinical margins, and the defect was closed in a linear fashion. Histologic sections demonstrated EMPSGC with uninvolved margins. The patient desired no further intervention and was clinically followed. Magnetic resonance imaging (MRI) of the head and neck found no evidence of metastasis. She has had no recurrence after 15 months.

Case 2—A 62-year-old man presented with a 7×5-mm, flesh-colored papule on the left lower eyelid margin (Figure 1). It was previously treated conservatively as a hordeolum but was biopsied after it failed to resolve with 3-mm margins. Histopathology demonstrated an EMPSGC (Figure 2). The lesion was treated with modified MMS with permanent en face section processing and cleared after 1 stage. Computed tomography of the head and neck showed no abnormalities. He has had no recurrence after 9 months.

Case 3—A 72-year-old man presented with a nontender papule near the right lateral canthus. A punch biopsy demonstrated PCMC. He was treated via modified MMS with permanent en face section processing. The tumor was cleared in 1 stage. He showed no evidence of recurrence after 112 months and died of unrelated causes. The rest of his clinical information was limited because of planned institutional destruction of records.

Case 4—An 87-year-old woman presented with a 25×25-mm, slow-growing mass of 12 months’ duration on the left lower abdomen (Figure 3). A biopsy demonstrated PCMC (Figure 4). Because of the size of the lesion, she underwent WLE with 20- to 30-mm margins by a general surgeon under general anesthesia. Positron emission tomography/computed tomography was unremarkable. She has remained disease free for 11 months.

Case 5—A 66-year-old woman presented for evaluation of a posterior scalp mass measuring 23×18 mm that had grown over the last 24 months. Biopsy showed mucinous carcinoma with lymphovascular invasion consistent with PCMC (Figure 5) confirmed on multiple tissue levels and with the aid of immunohistochemistry. She was sent for an MRI of the head, neck, chest, abdomen, and pelvis, which demonstrated 2 enlarged postauricular lymph nodes and raised suspicion for metastatic disease vs reactive lymphadenopathy. Mohs micrographic surgery with frozen sections was performed with 1- to 3-mm margins; the final layer was sent for permanent processing and confirmed negative margins. Sentinel lymph node biopsy and lymphadenectomy of the 2 nodes present on imaging showed no evidence of metastasis. The patient had no recurrence in 1 month.

Comment
Endocrine mucin-producing sweat gland carcinoma and PCMC are sweat gland malignancies that carry low metastatic potential but are locally aggressive. Endocrine mucin-producing sweat gland carcinoma has a strong predilection for the periorbital region, especially the lower eyelids of older women.3 Primary cutaneous mucinous carcinoma may arise on the eyelids, scalp, axillae, and trunk and has been reported more often in older men. These slow-growing tumors appear as nonspecific nodules.3 Lesions frequently are asymptomatic but rarely may cause pruritus and bleeding. Histologically, EMPSGC appears as solid or cystic nodules of cells with a papillary, cribriform, or pseudopapillary appearance. Intracellular or extracellular mucin as well as malignant spread of tumor cells along pre-existing ductlike structures make it difficult to histologically distinguish EMPSGC from ductal carcinoma in situ.3
A key histopathologic feature of PCMC is basophilic epithelioid cell nests in mucinous lakes.4 Rosettelike structures are seen within solid areas of the tumor. Fibrous septae separate individual collections of mucin, creating a lobulated appearance. The histopathologic differential diagnosis of EMPSGC and PCMC is broad, including basal cell carcinoma, hidradenoma, hidradenocarcinoma, apocrine adenoma, and dermal duct tumor. Positive expression of at least 1 neuroendocrine marker (ie, synaptophysin, neuron-specific enolase, chromogranin) and low-molecular cytokeratin (cytokeratin 7, CAM5.2, Ber-EP4) can aid in the diagnosis of both EMPSGC and PCMC.4 The use of p63 immunostaining is beneficial in delineating adnexal neoplasms. Adnexal tumors that stain positively with p63 are more likely to be of primary cutaneous origin, whereas lack of p63 staining usually denotes a secondary metastatic process. However, p63 staining is less reliable when distinguishing primary and metastatic mucinous neoplasms. Metastatic mucinous carcinomas often stain positive with p63, while PCMC usually stains negative despite its primary cutaneous origin, decreasing the clinical utility of p63. The tumor may be identical to metastatic mucinous adenocarcinoma of the breast, gastrointestinal tract, lung, ovary, and pancreas. Tumor islands floating in mucin are identified in both primary cutaneous and metastatic disease to the skin.3,6 Areas of tumor necrosis, notable atypia, and perineural or lymphovascular invasion are infrequently reported in EMPSGC or PCMC, though lymphatic invasion was identified in case 5 presented herein.
A metastatic workup is warranted in all cases of PCMC, including a thorough history, review of systems, breast examination, and imaging. A workup may be considered in cases of EMPSGC depending on histologic features or clinical history.
There is uncertainty regarding the optimal management of these slow-growing yet locally destructive tumors.5 The incidence of local recurrence of PCMC after WLE with narrow margins of at least 1 cm can be as high as 30% to 40%, especially on the eyelid.4 There is no consensus on surgical care for either of these tumors.5 Because of the high recurrence rate and the predilection for the eyelid and face, MMS provides an excellent alternative to WLE for tissue preservation and meticulous margin control. We advocate for the use of the Mohs technique with permanent sectioning, which may delay the repair, but reviewing tissue with permanent fixation improves the quality and accuracy of the margin evaluation because these tumors often are infiltrative and difficult to delineate under frozen section processing. Permanent en face sectioning allows the laboratory to utilize the full array of immunohistochemical stains for these tumors, providing accurate and timely results.
Limitations to our retrospective uncontrolled study include missing or incomplete data points and short follow-up time. Additionally, there was no standardization to the margins removed with MMS or WLE because of the limited available data that comment on appropriate margins.
- Held L, Ruetten A, Kutzner H, et al. Endocrine mucin‐producing sweat gland carcinoma: clinicopathologic, immunohistochemical and molecular analysis of 11 cases with emphasis on MYB immunoexpression. J Cutan Pathol. 2018;45:674-680.
- Navrazhina K, Petukhova T, Wildman HF, et al. Endocrine mucin-producing sweat gland carcinoma of the scalp treated with Mohs micrographic surgery. JAAD Case Rep. 2018;4:887-889.
- Scott BL, Anyanwu CO, Vandergriff T, et al. Endocrine mucin–producing sweat gland carcinoma treated with Mohs micrographic surgery. Dermatol Surg. 2017;43:1498-1500.
- Chang S, Shim SH, Joo M, et al. A case of endocrine mucin-producing sweat gland carcinoma co-existing with mucinous carcinoma: a case report. Korean J Pathol. 2010;44:97-100.
- Kamalpour L, Brindise RT, Nodzenski M, et al. Primary cutaneous mucinous carcinoma: a systematic review and meta-analysis of outcomes after surgery. JAMA Dermatol. 2014;150:380-384.
- Bulliard C, Murali R, Maloof A, et al. Endocrine mucin‐producing sweat gland carcinoma: report of a case and review of the literature. J Cutan Pathol. 2006;33:812-816.
- Held L, Ruetten A, Kutzner H, et al. Endocrine mucin‐producing sweat gland carcinoma: clinicopathologic, immunohistochemical and molecular analysis of 11 cases with emphasis on MYB immunoexpression. J Cutan Pathol. 2018;45:674-680.
- Navrazhina K, Petukhova T, Wildman HF, et al. Endocrine mucin-producing sweat gland carcinoma of the scalp treated with Mohs micrographic surgery. JAAD Case Rep. 2018;4:887-889.
- Scott BL, Anyanwu CO, Vandergriff T, et al. Endocrine mucin–producing sweat gland carcinoma treated with Mohs micrographic surgery. Dermatol Surg. 2017;43:1498-1500.
- Chang S, Shim SH, Joo M, et al. A case of endocrine mucin-producing sweat gland carcinoma co-existing with mucinous carcinoma: a case report. Korean J Pathol. 2010;44:97-100.
- Kamalpour L, Brindise RT, Nodzenski M, et al. Primary cutaneous mucinous carcinoma: a systematic review and meta-analysis of outcomes after surgery. JAMA Dermatol. 2014;150:380-384.
- Bulliard C, Murali R, Maloof A, et al. Endocrine mucin‐producing sweat gland carcinoma: report of a case and review of the literature. J Cutan Pathol. 2006;33:812-816.
Practice Points
- Endocrine mucin-producing sweat gland carcinoma and primary cutaneous mucinous carcinoma are rare low-grade neoplasms thought to arise from apocrine glands that are morphologically and immunohistochemically analogous to ductal carcinoma in situ and mucinous carcinoma of the breast, respectively.
- Management involves a metastatic workup and either wide local excision with margins greater than 5 mm or Mohs micrographic surgery in anatomically sensitive areas.
Cat Scratch Disease Presenting With Concurrent Pityriasis Rosea in a 10-Year-Old Girl
To the Editor:
Cat scratch disease (CSD) is caused by Bartonella henselae and Bartonella clarridgeiae bacteria transferred from cats to humans that results in an inflamed inoculation site and tender lymphadenopathy. Pityriasis rosea (PR) and PR-like eruptions are self-limited, acute exanthems that have been associated with infections, vaccinations, and medications. We report a case of PR occurring in a 10-year-old girl with CSD, which may suggest an association between the 2 diseases.

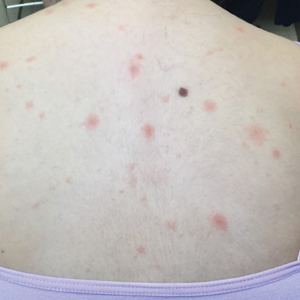
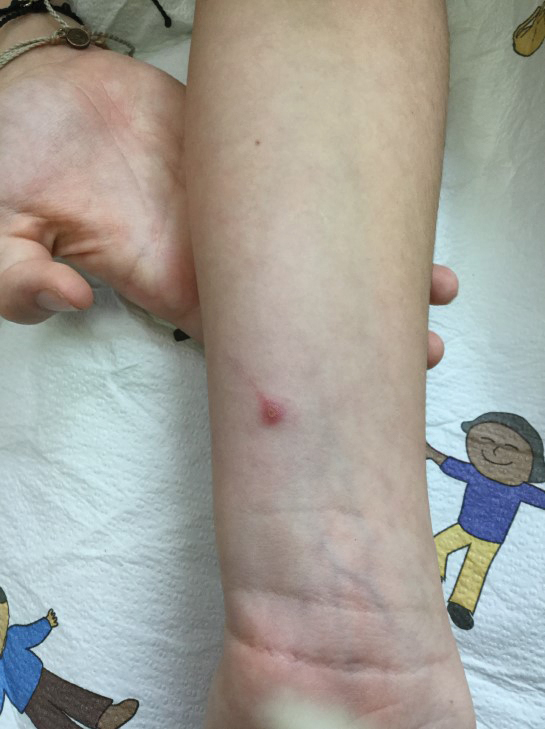
A 10-year-old girl who was otherwise healthy presented in the winter with a rash of 5 days’ duration. Fourteen days prior to the rash, the patient reported being scratched by a new kitten and noted a pinpoint “puncture” on the left forearm that developed into a red papule over the following week. Seven days after the cat scratch, the patient experienced pain and swelling in the left axilla. Approximately 1 week after the onset of lymphadenopathy, the patient developed an asymptomatic rash that started with a large spot on the left chest, followed by smaller spots appearing over the next 2 days and spreading to the rest of the trunk. Four days after the rash onset, the patient experienced a mild headache, low-grade subjective fever, and chills. She denied any recent travel, bug bites, sore throat, and diarrhea. She was up-to-date on all vaccinations and had not received any vaccines preceding the symptoms. Physical examination revealed a 2-cm pink, scaly, thin plaque with a collarette of scale on the left upper chest (herald patch), along with multiple thin pink papules and small plaques with central scale on the trunk (Figure 1). A pustule with adjacent linear erosion was present on the left ventral forearm (Figure 2). The patient had a tender subcutaneous nodule in the left axilla as well as bilateral anterior and posterior cervical-chain subcutaneous tender nodules. There was no involvement of the palms, soles, or mucosae.
The patient was empirically treated for CSD with azithromycin (200 mg/5 mL), 404 mg on day 1 followed by 202 mg daily for 4 days. The rash was treated with hydrocortisone cream 2.5% twice daily for 2 weeks. A wound culture of the pustule on the left forearm was negative for neutrophils and organisms. Antibody serologies obtained 4 weeks after presentation were notable for an elevated B henselae IgG titer of 1:640, confirming the diagnosis of CSD. Following treatment with azithromycin and hydrocortisone, all of the patient’s symptoms resolved after 1 to 2 weeks.
Cat scratch disease is a zoonotic infection caused by the bacteria B henselae and the more recently described pathogen B clarridgeiae. Cat fleas spread these bacteria among cats, which subsequently inoculate the bacteria into humans through bites and scratches. The incidence of CSD in the United States is estimated to be 4.5 to 9.3 per 100,000 individuals in the outpatient setting and 0.19 to 0.86 per 100,000 individuals in the inpatient setting.1 Geographic variance can occur based on flea populations, resulting in higher incidence in warm humid climates and lower incidence in mountainous arid climates. The incidence of CSD in the pediatric population is highest in children aged 5 to 9 years. A national representative survey (N=3011) from 2017 revealed that 37.2% of primary care providers had diagnosed CSD in the prior year.1
Classic CSD presents as an erythematous papule at the inoculation site lasting days to weeks, with progression to tender lymphadenopathy lasting weeks to months. Fever, malaise, and chills also can be seen. Atypical CSD occurs in up to 24% of cases in immunocompetent patients.1 Atypical and systemic presentations are varied and can include fever of unknown origin, neuroretinitis, uveitis, retinal vessel occlusion, encephalitis, hepatosplenic lesions, Parinaud oculoglandular syndrome, osteomyelitis, and endocarditis.1,2 Atypical dermatologic presentations of CSD include maculopapular rash in 7% of cases and erythema nodosum in 2.5% of cases, as well as rare reports of cutaneous vasculitis, urticaria, immune thrombocytopenic purpura, and papuloedematous eruption.3 Treatment guidelines for CSD vary widely depending on the clinical presentation as well as the immunocompetence of the infected individual. Our patient had limited regional lymphadenopathy with no signs of dissemination or neurologic involvement and was successfully treated with a 5-day course of oral azithromycin (weight based, 10 mg/kg). More extensive disease such as hepatosplenic or neurologic CSD may require multiple antibiotics for up to 6 weeks. Alternative or additional antibiotics used for CSD include rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, doxycycline, gentamicin, and clarithromycin. Opinions vary as to whether all patients or just those with complicated infections warrant antibiotic therapy.4-6
Pityriasis rosea is a self-limited acute exanthematous disease that is classically associated with a systemic reactivation of human herpesvirus (HHV) 6 and/or HHV-7. The incidence of PR is estimated to be 480 per 100,000 dermatologic patients. It is slightly more common in females and occurs most often in patients aged 10 to 35 years.7 Clinically, PR appears with the abrupt onset of a single erythematous scaly patch (termed the herald patch), followed by a secondary eruption of smaller erythematous scaly macules and patches along the trunk’s cleavage lines. The secondary eruption on the back is sometimes termed a Christmas or fir tree pattern.7,8
In addition to the classic presentation of PR, there have been reports of numerous atypical clinical presentations. The herald patch, which classically presents on the trunk, also has been reported to present on the extremities; PR of the extremities is defined by lesions that appear as large scaly plaques on the extremities only. Inverse PR presents with lesions occurring in flexural areas and acral surfaces but not on the trunk. There also is an acral PR variant in which lesions appear only on the palms, wrists, and soles. Purpuric or hemorrhagic PR has been described and presents with purpura and petechiae with or without collarettes of scale in diffuse locations, including the palate. Oral PR presents more commonly in patients of color as erosions, ulcers, hemorrhagic lesions, bullae, or geographic tongue. Erythema multiforme–like PR appears with targetoid lesions on the trunk, face, neck, and arms without a history of herpes simplex virus infection. A large pear-shaped herald patch has been reported and characterizes the gigantea PR of Darier variant. Irritated PR occurs with typical PR findings, but afflicted patients report severe pain and burning with diaphoresis. Relapsing PR can occur within 1 year of a prior episode of PR and presents without a herald patch. Persistent PR is defined by PR lasting more than 3 months, and most reported cases have included oral lesions. Finally, other PR variants that have been described include urticarial, papular, follicular, vesicular, and hypopigmented types.7-9
Furthermore, there have been reports of multiple atypical presentations occurring simultaneously in the same patient.10 Although PR classically has been associated with HHV-6 and/or HHV-7 reactivation, it has been reported with a few other clinical situations and conditions. Pityriasislike eruption specifically refers to an exanthem secondary to drugs or vaccination that resembles PR but shows clinical differences, including diffuse and confluent dusky-red macules and/or plaques with or without desquamation on the trunk, extremities, and face. Drugs that have been implicated as triggers include ACE inhibitors, gold, isotretinoin, nonsteroidal anti-inflammatory agents, omeprazole, terbinafine, and tyrosine kinase inhibitors. Smallpox, tuberculosis, poliomyelitis, influenza, diphtheria, tetanus, hepatitis B virus, pneumococcus, papillomavirus, yellow fever, and pertussis vaccinations also have been associated with PR.7,11,12 Additionally, PR has been reported to occur with active systemic infections, specifically H1N1 influenza, though it is rare.13 Because of its self-limited course, treatment of PR most often involves only reassurance. Topical corticosteroids may be appropriate for pruritus.7,8
Pediatric health care providers including dermatologists should be familiar with both CSD and PR because they are common diseases that more often are encountered in the pediatric population. We present a unique case of CSD presenting with concurrent PR, which highlights a potential new etiology for PR and a rare cutaneous manifestation of CSD. Further investigation into a possible relationship between CSD and PR may be warranted. Patients with any signs and symptoms of fever, tender lymphadenopathy, worsening rash, or exposure to cats warrant a thorough history and physical examination to ensure that neither entity is overlooked.
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge [published online July 14, 2017]. Zoonoses Public Health. 2018;65:67-73. doi:10.1111/zph.12368
- Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96:E524-E532. doi:10.1111/aos.13684
- Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online February 16, 2018]. BMJ Case Rep. doi:10.1136/bcr-2017-222511
- Shorbatli L, Koranyi K, Nahata M. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. doi: 10.1007/s11096-018-0746-1
- Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. doi:10.1097/00006454-199806000-00002
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. Updated December 9, 2021. Accessed September 12, 2023. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease
- Drago F, Ciccarese G, Rebora A, et al. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232:431-437. doi:10.1159/000445375
- Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. 2017;5:203-211. doi:10.12998/wjcc.v5.i6.203
- Alzahrani NA, Al Jasser MI. Geographic tonguelike presentation in a child with pityriasis rosea: case report and review of oral manifestations of pityriasis rosea. Pediatr Dermatol. 2018;35:E124-E127. doi:10.1111/pde.13417
- Sinha S, Sardana K, Garg V. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29:538-540. doi:10.1111/j.1525-1470.2011.01549.x
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4:800-801. doi:10.1016/j.jdcr.2018.04.002
- Drago F, Ciccarese G, Javor S, et al. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30:544-545. doi:10.1111/jdv.12942
- Mubki TF, Bin Dayel SA, Kadry R. A case of pityriasis rosea concurrent with the novel influenza A (H1N1) infection. Pediatr Dermatol. 2011;28:341-342. doi:10.1111/j.1525-1470.2010.01090.x
To the Editor:
Cat scratch disease (CSD) is caused by Bartonella henselae and Bartonella clarridgeiae bacteria transferred from cats to humans that results in an inflamed inoculation site and tender lymphadenopathy. Pityriasis rosea (PR) and PR-like eruptions are self-limited, acute exanthems that have been associated with infections, vaccinations, and medications. We report a case of PR occurring in a 10-year-old girl with CSD, which may suggest an association between the 2 diseases.

A 10-year-old girl who was otherwise healthy presented in the winter with a rash of 5 days’ duration. Fourteen days prior to the rash, the patient reported being scratched by a new kitten and noted a pinpoint “puncture” on the left forearm that developed into a red papule over the following week. Seven days after the cat scratch, the patient experienced pain and swelling in the left axilla. Approximately 1 week after the onset of lymphadenopathy, the patient developed an asymptomatic rash that started with a large spot on the left chest, followed by smaller spots appearing over the next 2 days and spreading to the rest of the trunk. Four days after the rash onset, the patient experienced a mild headache, low-grade subjective fever, and chills. She denied any recent travel, bug bites, sore throat, and diarrhea. She was up-to-date on all vaccinations and had not received any vaccines preceding the symptoms. Physical examination revealed a 2-cm pink, scaly, thin plaque with a collarette of scale on the left upper chest (herald patch), along with multiple thin pink papules and small plaques with central scale on the trunk (Figure 1). A pustule with adjacent linear erosion was present on the left ventral forearm (Figure 2). The patient had a tender subcutaneous nodule in the left axilla as well as bilateral anterior and posterior cervical-chain subcutaneous tender nodules. There was no involvement of the palms, soles, or mucosae.

The patient was empirically treated for CSD with azithromycin (200 mg/5 mL), 404 mg on day 1 followed by 202 mg daily for 4 days. The rash was treated with hydrocortisone cream 2.5% twice daily for 2 weeks. A wound culture of the pustule on the left forearm was negative for neutrophils and organisms. Antibody serologies obtained 4 weeks after presentation were notable for an elevated B henselae IgG titer of 1:640, confirming the diagnosis of CSD. Following treatment with azithromycin and hydrocortisone, all of the patient’s symptoms resolved after 1 to 2 weeks.
Cat scratch disease is a zoonotic infection caused by the bacteria B henselae and the more recently described pathogen B clarridgeiae. Cat fleas spread these bacteria among cats, which subsequently inoculate the bacteria into humans through bites and scratches. The incidence of CSD in the United States is estimated to be 4.5 to 9.3 per 100,000 individuals in the outpatient setting and 0.19 to 0.86 per 100,000 individuals in the inpatient setting.1 Geographic variance can occur based on flea populations, resulting in higher incidence in warm humid climates and lower incidence in mountainous arid climates. The incidence of CSD in the pediatric population is highest in children aged 5 to 9 years. A national representative survey (N=3011) from 2017 revealed that 37.2% of primary care providers had diagnosed CSD in the prior year.1
Classic CSD presents as an erythematous papule at the inoculation site lasting days to weeks, with progression to tender lymphadenopathy lasting weeks to months. Fever, malaise, and chills also can be seen. Atypical CSD occurs in up to 24% of cases in immunocompetent patients.1 Atypical and systemic presentations are varied and can include fever of unknown origin, neuroretinitis, uveitis, retinal vessel occlusion, encephalitis, hepatosplenic lesions, Parinaud oculoglandular syndrome, osteomyelitis, and endocarditis.1,2 Atypical dermatologic presentations of CSD include maculopapular rash in 7% of cases and erythema nodosum in 2.5% of cases, as well as rare reports of cutaneous vasculitis, urticaria, immune thrombocytopenic purpura, and papuloedematous eruption.3 Treatment guidelines for CSD vary widely depending on the clinical presentation as well as the immunocompetence of the infected individual. Our patient had limited regional lymphadenopathy with no signs of dissemination or neurologic involvement and was successfully treated with a 5-day course of oral azithromycin (weight based, 10 mg/kg). More extensive disease such as hepatosplenic or neurologic CSD may require multiple antibiotics for up to 6 weeks. Alternative or additional antibiotics used for CSD include rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, doxycycline, gentamicin, and clarithromycin. Opinions vary as to whether all patients or just those with complicated infections warrant antibiotic therapy.4-6
Pityriasis rosea is a self-limited acute exanthematous disease that is classically associated with a systemic reactivation of human herpesvirus (HHV) 6 and/or HHV-7. The incidence of PR is estimated to be 480 per 100,000 dermatologic patients. It is slightly more common in females and occurs most often in patients aged 10 to 35 years.7 Clinically, PR appears with the abrupt onset of a single erythematous scaly patch (termed the herald patch), followed by a secondary eruption of smaller erythematous scaly macules and patches along the trunk’s cleavage lines. The secondary eruption on the back is sometimes termed a Christmas or fir tree pattern.7,8
In addition to the classic presentation of PR, there have been reports of numerous atypical clinical presentations. The herald patch, which classically presents on the trunk, also has been reported to present on the extremities; PR of the extremities is defined by lesions that appear as large scaly plaques on the extremities only. Inverse PR presents with lesions occurring in flexural areas and acral surfaces but not on the trunk. There also is an acral PR variant in which lesions appear only on the palms, wrists, and soles. Purpuric or hemorrhagic PR has been described and presents with purpura and petechiae with or without collarettes of scale in diffuse locations, including the palate. Oral PR presents more commonly in patients of color as erosions, ulcers, hemorrhagic lesions, bullae, or geographic tongue. Erythema multiforme–like PR appears with targetoid lesions on the trunk, face, neck, and arms without a history of herpes simplex virus infection. A large pear-shaped herald patch has been reported and characterizes the gigantea PR of Darier variant. Irritated PR occurs with typical PR findings, but afflicted patients report severe pain and burning with diaphoresis. Relapsing PR can occur within 1 year of a prior episode of PR and presents without a herald patch. Persistent PR is defined by PR lasting more than 3 months, and most reported cases have included oral lesions. Finally, other PR variants that have been described include urticarial, papular, follicular, vesicular, and hypopigmented types.7-9
Furthermore, there have been reports of multiple atypical presentations occurring simultaneously in the same patient.10 Although PR classically has been associated with HHV-6 and/or HHV-7 reactivation, it has been reported with a few other clinical situations and conditions. Pityriasislike eruption specifically refers to an exanthem secondary to drugs or vaccination that resembles PR but shows clinical differences, including diffuse and confluent dusky-red macules and/or plaques with or without desquamation on the trunk, extremities, and face. Drugs that have been implicated as triggers include ACE inhibitors, gold, isotretinoin, nonsteroidal anti-inflammatory agents, omeprazole, terbinafine, and tyrosine kinase inhibitors. Smallpox, tuberculosis, poliomyelitis, influenza, diphtheria, tetanus, hepatitis B virus, pneumococcus, papillomavirus, yellow fever, and pertussis vaccinations also have been associated with PR.7,11,12 Additionally, PR has been reported to occur with active systemic infections, specifically H1N1 influenza, though it is rare.13 Because of its self-limited course, treatment of PR most often involves only reassurance. Topical corticosteroids may be appropriate for pruritus.7,8
Pediatric health care providers including dermatologists should be familiar with both CSD and PR because they are common diseases that more often are encountered in the pediatric population. We present a unique case of CSD presenting with concurrent PR, which highlights a potential new etiology for PR and a rare cutaneous manifestation of CSD. Further investigation into a possible relationship between CSD and PR may be warranted. Patients with any signs and symptoms of fever, tender lymphadenopathy, worsening rash, or exposure to cats warrant a thorough history and physical examination to ensure that neither entity is overlooked.
To the Editor:
Cat scratch disease (CSD) is caused by Bartonella henselae and Bartonella clarridgeiae bacteria transferred from cats to humans that results in an inflamed inoculation site and tender lymphadenopathy. Pityriasis rosea (PR) and PR-like eruptions are self-limited, acute exanthems that have been associated with infections, vaccinations, and medications. We report a case of PR occurring in a 10-year-old girl with CSD, which may suggest an association between the 2 diseases.

A 10-year-old girl who was otherwise healthy presented in the winter with a rash of 5 days’ duration. Fourteen days prior to the rash, the patient reported being scratched by a new kitten and noted a pinpoint “puncture” on the left forearm that developed into a red papule over the following week. Seven days after the cat scratch, the patient experienced pain and swelling in the left axilla. Approximately 1 week after the onset of lymphadenopathy, the patient developed an asymptomatic rash that started with a large spot on the left chest, followed by smaller spots appearing over the next 2 days and spreading to the rest of the trunk. Four days after the rash onset, the patient experienced a mild headache, low-grade subjective fever, and chills. She denied any recent travel, bug bites, sore throat, and diarrhea. She was up-to-date on all vaccinations and had not received any vaccines preceding the symptoms. Physical examination revealed a 2-cm pink, scaly, thin plaque with a collarette of scale on the left upper chest (herald patch), along with multiple thin pink papules and small plaques with central scale on the trunk (Figure 1). A pustule with adjacent linear erosion was present on the left ventral forearm (Figure 2). The patient had a tender subcutaneous nodule in the left axilla as well as bilateral anterior and posterior cervical-chain subcutaneous tender nodules. There was no involvement of the palms, soles, or mucosae.

The patient was empirically treated for CSD with azithromycin (200 mg/5 mL), 404 mg on day 1 followed by 202 mg daily for 4 days. The rash was treated with hydrocortisone cream 2.5% twice daily for 2 weeks. A wound culture of the pustule on the left forearm was negative for neutrophils and organisms. Antibody serologies obtained 4 weeks after presentation were notable for an elevated B henselae IgG titer of 1:640, confirming the diagnosis of CSD. Following treatment with azithromycin and hydrocortisone, all of the patient’s symptoms resolved after 1 to 2 weeks.
Cat scratch disease is a zoonotic infection caused by the bacteria B henselae and the more recently described pathogen B clarridgeiae. Cat fleas spread these bacteria among cats, which subsequently inoculate the bacteria into humans through bites and scratches. The incidence of CSD in the United States is estimated to be 4.5 to 9.3 per 100,000 individuals in the outpatient setting and 0.19 to 0.86 per 100,000 individuals in the inpatient setting.1 Geographic variance can occur based on flea populations, resulting in higher incidence in warm humid climates and lower incidence in mountainous arid climates. The incidence of CSD in the pediatric population is highest in children aged 5 to 9 years. A national representative survey (N=3011) from 2017 revealed that 37.2% of primary care providers had diagnosed CSD in the prior year.1
Classic CSD presents as an erythematous papule at the inoculation site lasting days to weeks, with progression to tender lymphadenopathy lasting weeks to months. Fever, malaise, and chills also can be seen. Atypical CSD occurs in up to 24% of cases in immunocompetent patients.1 Atypical and systemic presentations are varied and can include fever of unknown origin, neuroretinitis, uveitis, retinal vessel occlusion, encephalitis, hepatosplenic lesions, Parinaud oculoglandular syndrome, osteomyelitis, and endocarditis.1,2 Atypical dermatologic presentations of CSD include maculopapular rash in 7% of cases and erythema nodosum in 2.5% of cases, as well as rare reports of cutaneous vasculitis, urticaria, immune thrombocytopenic purpura, and papuloedematous eruption.3 Treatment guidelines for CSD vary widely depending on the clinical presentation as well as the immunocompetence of the infected individual. Our patient had limited regional lymphadenopathy with no signs of dissemination or neurologic involvement and was successfully treated with a 5-day course of oral azithromycin (weight based, 10 mg/kg). More extensive disease such as hepatosplenic or neurologic CSD may require multiple antibiotics for up to 6 weeks. Alternative or additional antibiotics used for CSD include rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, doxycycline, gentamicin, and clarithromycin. Opinions vary as to whether all patients or just those with complicated infections warrant antibiotic therapy.4-6
Pityriasis rosea is a self-limited acute exanthematous disease that is classically associated with a systemic reactivation of human herpesvirus (HHV) 6 and/or HHV-7. The incidence of PR is estimated to be 480 per 100,000 dermatologic patients. It is slightly more common in females and occurs most often in patients aged 10 to 35 years.7 Clinically, PR appears with the abrupt onset of a single erythematous scaly patch (termed the herald patch), followed by a secondary eruption of smaller erythematous scaly macules and patches along the trunk’s cleavage lines. The secondary eruption on the back is sometimes termed a Christmas or fir tree pattern.7,8
In addition to the classic presentation of PR, there have been reports of numerous atypical clinical presentations. The herald patch, which classically presents on the trunk, also has been reported to present on the extremities; PR of the extremities is defined by lesions that appear as large scaly plaques on the extremities only. Inverse PR presents with lesions occurring in flexural areas and acral surfaces but not on the trunk. There also is an acral PR variant in which lesions appear only on the palms, wrists, and soles. Purpuric or hemorrhagic PR has been described and presents with purpura and petechiae with or without collarettes of scale in diffuse locations, including the palate. Oral PR presents more commonly in patients of color as erosions, ulcers, hemorrhagic lesions, bullae, or geographic tongue. Erythema multiforme–like PR appears with targetoid lesions on the trunk, face, neck, and arms without a history of herpes simplex virus infection. A large pear-shaped herald patch has been reported and characterizes the gigantea PR of Darier variant. Irritated PR occurs with typical PR findings, but afflicted patients report severe pain and burning with diaphoresis. Relapsing PR can occur within 1 year of a prior episode of PR and presents without a herald patch. Persistent PR is defined by PR lasting more than 3 months, and most reported cases have included oral lesions. Finally, other PR variants that have been described include urticarial, papular, follicular, vesicular, and hypopigmented types.7-9
Furthermore, there have been reports of multiple atypical presentations occurring simultaneously in the same patient.10 Although PR classically has been associated with HHV-6 and/or HHV-7 reactivation, it has been reported with a few other clinical situations and conditions. Pityriasislike eruption specifically refers to an exanthem secondary to drugs or vaccination that resembles PR but shows clinical differences, including diffuse and confluent dusky-red macules and/or plaques with or without desquamation on the trunk, extremities, and face. Drugs that have been implicated as triggers include ACE inhibitors, gold, isotretinoin, nonsteroidal anti-inflammatory agents, omeprazole, terbinafine, and tyrosine kinase inhibitors. Smallpox, tuberculosis, poliomyelitis, influenza, diphtheria, tetanus, hepatitis B virus, pneumococcus, papillomavirus, yellow fever, and pertussis vaccinations also have been associated with PR.7,11,12 Additionally, PR has been reported to occur with active systemic infections, specifically H1N1 influenza, though it is rare.13 Because of its self-limited course, treatment of PR most often involves only reassurance. Topical corticosteroids may be appropriate for pruritus.7,8
Pediatric health care providers including dermatologists should be familiar with both CSD and PR because they are common diseases that more often are encountered in the pediatric population. We present a unique case of CSD presenting with concurrent PR, which highlights a potential new etiology for PR and a rare cutaneous manifestation of CSD. Further investigation into a possible relationship between CSD and PR may be warranted. Patients with any signs and symptoms of fever, tender lymphadenopathy, worsening rash, or exposure to cats warrant a thorough history and physical examination to ensure that neither entity is overlooked.
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge [published online July 14, 2017]. Zoonoses Public Health. 2018;65:67-73. doi:10.1111/zph.12368
- Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96:E524-E532. doi:10.1111/aos.13684
- Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online February 16, 2018]. BMJ Case Rep. doi:10.1136/bcr-2017-222511
- Shorbatli L, Koranyi K, Nahata M. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. doi: 10.1007/s11096-018-0746-1
- Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. doi:10.1097/00006454-199806000-00002
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. Updated December 9, 2021. Accessed September 12, 2023. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease
- Drago F, Ciccarese G, Rebora A, et al. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232:431-437. doi:10.1159/000445375
- Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. 2017;5:203-211. doi:10.12998/wjcc.v5.i6.203
- Alzahrani NA, Al Jasser MI. Geographic tonguelike presentation in a child with pityriasis rosea: case report and review of oral manifestations of pityriasis rosea. Pediatr Dermatol. 2018;35:E124-E127. doi:10.1111/pde.13417
- Sinha S, Sardana K, Garg V. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29:538-540. doi:10.1111/j.1525-1470.2011.01549.x
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4:800-801. doi:10.1016/j.jdcr.2018.04.002
- Drago F, Ciccarese G, Javor S, et al. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30:544-545. doi:10.1111/jdv.12942
- Mubki TF, Bin Dayel SA, Kadry R. A case of pityriasis rosea concurrent with the novel influenza A (H1N1) infection. Pediatr Dermatol. 2011;28:341-342. doi:10.1111/j.1525-1470.2010.01090.x
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge [published online July 14, 2017]. Zoonoses Public Health. 2018;65:67-73. doi:10.1111/zph.12368
- Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96:E524-E532. doi:10.1111/aos.13684
- Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online February 16, 2018]. BMJ Case Rep. doi:10.1136/bcr-2017-222511
- Shorbatli L, Koranyi K, Nahata M. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. doi: 10.1007/s11096-018-0746-1
- Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. doi:10.1097/00006454-199806000-00002
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. Updated December 9, 2021. Accessed September 12, 2023. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease
- Drago F, Ciccarese G, Rebora A, et al. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232:431-437. doi:10.1159/000445375
- Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. 2017;5:203-211. doi:10.12998/wjcc.v5.i6.203
- Alzahrani NA, Al Jasser MI. Geographic tonguelike presentation in a child with pityriasis rosea: case report and review of oral manifestations of pityriasis rosea. Pediatr Dermatol. 2018;35:E124-E127. doi:10.1111/pde.13417
- Sinha S, Sardana K, Garg V. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29:538-540. doi:10.1111/j.1525-1470.2011.01549.x
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4:800-801. doi:10.1016/j.jdcr.2018.04.002
- Drago F, Ciccarese G, Javor S, et al. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30:544-545. doi:10.1111/jdv.12942
- Mubki TF, Bin Dayel SA, Kadry R. A case of pityriasis rosea concurrent with the novel influenza A (H1N1) infection. Pediatr Dermatol. 2011;28:341-342. doi:10.1111/j.1525-1470.2010.01090.x
Practice Points
- Dermatologists should familiarize themselves with the physical examination findings of cat scratch disease.
- There are numerous clinical variants and triggers of pityriasis rosea (PR).
- There may be a new infectious trigger for PR, and exposure to cats prior to a classic PR eruption should raise one’s suspicion as a possible cause.
Severe psoriasis linked to a higher risk for heart disease, study confirms
TOPLINE:
METHODOLOGY:
- Prior studies with small sample sizes have shown that CMD predicts poor cardiovascular outcomes in patients with severe psoriasis.
- In a prospective multicenter study, researchers enrolled 448 patients with moderate to severe psoriasis with no documented clinical cardiovascular disease who underwent transthoracic Doppler echocardiography to evaluate coronary microcirculation.
- The outcome variable of interest was CMD, defined as a coronary flow rate of 2.5 mL or less.
- The researchers used multivariable linear regression to model the associations of the characteristics of patients with psoriasis with CMD.
TAKEAWAY:
- Of the 448 patients, 141 (31.5%) showed CMD.
- Multivariable regression revealed four variables independently associated with CMD: higher Psoriasis Area Severity Index (PASI) score (per unit, odds ratio, 1.058; P < .001), duration of psoriasis (per year; OR, 1.046; P < .001), the presence of psoriatic arthritis (OR, 1.938; P = .015), and hypertension (OR, 2.169; P = .010).
- An increase of 1 point in the PASI score and 1 year of psoriasis duration were associated with a 5.8% and a 4.6% increased risk for CMD, respectively.
IN PRACTICE:
“We should diagnose and actively search for microvascular dysfunction in patients with psoriasis, as this population is at particularly high risk,” the researchers wrote.
SOURCE:
Stefano Piaserico, MD, PhD, of the University of Padova (Italy), led the research. The study was published in the Journal of Investigative Dermatology.
LIMITATIONS:
A small proportion of patients in the study were being treated for psoriasis, and other tools for assessing CMD were not used, such as PET-CT and cardiovascular MRI.
DISCLOSURES:
The authors reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Prior studies with small sample sizes have shown that CMD predicts poor cardiovascular outcomes in patients with severe psoriasis.
- In a prospective multicenter study, researchers enrolled 448 patients with moderate to severe psoriasis with no documented clinical cardiovascular disease who underwent transthoracic Doppler echocardiography to evaluate coronary microcirculation.
- The outcome variable of interest was CMD, defined as a coronary flow rate of 2.5 mL or less.
- The researchers used multivariable linear regression to model the associations of the characteristics of patients with psoriasis with CMD.
TAKEAWAY:
- Of the 448 patients, 141 (31.5%) showed CMD.
- Multivariable regression revealed four variables independently associated with CMD: higher Psoriasis Area Severity Index (PASI) score (per unit, odds ratio, 1.058; P < .001), duration of psoriasis (per year; OR, 1.046; P < .001), the presence of psoriatic arthritis (OR, 1.938; P = .015), and hypertension (OR, 2.169; P = .010).
- An increase of 1 point in the PASI score and 1 year of psoriasis duration were associated with a 5.8% and a 4.6% increased risk for CMD, respectively.
IN PRACTICE:
“We should diagnose and actively search for microvascular dysfunction in patients with psoriasis, as this population is at particularly high risk,” the researchers wrote.
SOURCE:
Stefano Piaserico, MD, PhD, of the University of Padova (Italy), led the research. The study was published in the Journal of Investigative Dermatology.
LIMITATIONS:
A small proportion of patients in the study were being treated for psoriasis, and other tools for assessing CMD were not used, such as PET-CT and cardiovascular MRI.
DISCLOSURES:
The authors reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Prior studies with small sample sizes have shown that CMD predicts poor cardiovascular outcomes in patients with severe psoriasis.
- In a prospective multicenter study, researchers enrolled 448 patients with moderate to severe psoriasis with no documented clinical cardiovascular disease who underwent transthoracic Doppler echocardiography to evaluate coronary microcirculation.
- The outcome variable of interest was CMD, defined as a coronary flow rate of 2.5 mL or less.
- The researchers used multivariable linear regression to model the associations of the characteristics of patients with psoriasis with CMD.
TAKEAWAY:
- Of the 448 patients, 141 (31.5%) showed CMD.
- Multivariable regression revealed four variables independently associated with CMD: higher Psoriasis Area Severity Index (PASI) score (per unit, odds ratio, 1.058; P < .001), duration of psoriasis (per year; OR, 1.046; P < .001), the presence of psoriatic arthritis (OR, 1.938; P = .015), and hypertension (OR, 2.169; P = .010).
- An increase of 1 point in the PASI score and 1 year of psoriasis duration were associated with a 5.8% and a 4.6% increased risk for CMD, respectively.
IN PRACTICE:
“We should diagnose and actively search for microvascular dysfunction in patients with psoriasis, as this population is at particularly high risk,” the researchers wrote.
SOURCE:
Stefano Piaserico, MD, PhD, of the University of Padova (Italy), led the research. The study was published in the Journal of Investigative Dermatology.
LIMITATIONS:
A small proportion of patients in the study were being treated for psoriasis, and other tools for assessing CMD were not used, such as PET-CT and cardiovascular MRI.
DISCLOSURES:
The authors reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Commentary: Are "significant" results necessarily clinically meaningful? October 2023
I was excited to see that the study included the use of objective electronic monitoring of sleep quality. This was done using wrist actigraphy, devices on the wrist that measure acceleration movements. What a great tool this could be for measuring how much scratching our patients are doing! With devices like these measuring movements objectively, we wouldn't have to rely on patients' self-report of itch or sleep quality. Sadly, these monitors did not show any meaningful differences between the dupilumab and placebo groups. This technology holds great promise but it isn't yet ready for prime-time assessment of scratching or sleep.
The title of Chiesa Fuxench and colleagues' article, "Risk of Inflammatory Bowel Disease in Patients With Atopic Dermatitis," might be scary to our patients. The authors reported that "children and adults with AD had an increased risk of IBD [inflammatory bowel disease]." The authors concluded, "Clinicians should be aware of these risks, particularly when selecting systemic treatments for AD in patients who may have coincident gastrointestinal symptoms." Bah, humbug, I say!
Be careful when someone tells you there is increased risk. This study was done exceptionally well by an exceptionally good research team. They were working with a huge database and included many controls to ensure that their findings weren't due to chance. And while they did find an "increased risk," they proved — rather conclusively, I believe — that the increased risk is tiny and not something we need to worry about.
The results of this study suggest that there is a scientific link between AD and IBD, probably some genetic inflammatory signaling contributing to both conditions. But even in the highest-risk group, it would take seeing well over 1000 patients for a year to see one more case of IBD due to AD. This article is a good foundation for researchers who want to explore the underlying connection between AD and IBD. The study is an even better foundation for physicians who want to reassure patients that there is little to no meaningful increased risk for IBD in patients with AD.
Am I allowed to just say "Ditto!"? Wan and colleagues' article "Incidence of Cardiovascular Disease and Venous Thromboembolism in Patients with Atopic Dermatitis" does show a statistically significant increased risk for cardiovascular (CV) disease in patients with AD. Is that increase clinically significant? This study was also exceptionally well done by an exceptionally good research team. They concluded, "Atopic dermatitis, particularly when severe, is associated with increased risks of venous thromboembolism and CV disease, which may influence the monitoring of patients and selection of treatments for AD." I look at their findings and conclude that AD, even when severe, is associated with little if any clinically meaningful increased risks for venous thromboembolism or CV disease, and we don't need to add any special CV monitoring of AD patients.
The key data are presented in Table 2 of their manuscript. In children, the risk for deep vein thrombosis (DVT) in those with severe AD was about 3 times (0.16) that of those with no AD (0.05). But those numbers are per 1000 patient-years. Therefore, the increased risk is 0.16 - 0.05 = 0.11/1000 patient-years. Thus, you'd expect to see one more case of DVT per year in every 9000 children with severe AD. Does that mean we need to monitor all 9000 for DVT? Would that be cost-effective? Might the monitoring cause more problems than it would solve?
CV disease is much more common in adults than in children, but still, with a difference in risk of about 0.5-1 per 1000 patient-years, you'd only expect one more event due to AD in every 1000-2000 patients, and even that is assuming that the entire risk difference was due to AD and not to some other variable that wasn't measured.
With so much drug development for AD, I think we are going to be inundated with companies wanting us to hear their message over and over again. One way to do that is to mine clinical trial data for more papers. In Merola and colleagues' article "Safety and Efficacy of Tralokinumab in Older Adults With Moderate-to-Severe Atopic Dermatitis" we see just that. We already know that tralokinumab is effective for moderate to severe AD from past publications of clinical trial data. Here, the investigators report on a subset of the clinical trial data — the data on older adults — and, not surprisingly, the drug worked. The efficacy rate, 17% getting clear or almost clear, doesn't sound particularly exciting compared with the higher rates we've seen for other products, but perhaps that lower rate is due in part to differences in studies. Instead of more cuts of data from the same trials, it would be nice to see how tralokinumab compares with other AD treatments on a head-to-head basis.
I was excited to see that the study included the use of objective electronic monitoring of sleep quality. This was done using wrist actigraphy, devices on the wrist that measure acceleration movements. What a great tool this could be for measuring how much scratching our patients are doing! With devices like these measuring movements objectively, we wouldn't have to rely on patients' self-report of itch or sleep quality. Sadly, these monitors did not show any meaningful differences between the dupilumab and placebo groups. This technology holds great promise but it isn't yet ready for prime-time assessment of scratching or sleep.
The title of Chiesa Fuxench and colleagues' article, "Risk of Inflammatory Bowel Disease in Patients With Atopic Dermatitis," might be scary to our patients. The authors reported that "children and adults with AD had an increased risk of IBD [inflammatory bowel disease]." The authors concluded, "Clinicians should be aware of these risks, particularly when selecting systemic treatments for AD in patients who may have coincident gastrointestinal symptoms." Bah, humbug, I say!
Be careful when someone tells you there is increased risk. This study was done exceptionally well by an exceptionally good research team. They were working with a huge database and included many controls to ensure that their findings weren't due to chance. And while they did find an "increased risk," they proved — rather conclusively, I believe — that the increased risk is tiny and not something we need to worry about.
The results of this study suggest that there is a scientific link between AD and IBD, probably some genetic inflammatory signaling contributing to both conditions. But even in the highest-risk group, it would take seeing well over 1000 patients for a year to see one more case of IBD due to AD. This article is a good foundation for researchers who want to explore the underlying connection between AD and IBD. The study is an even better foundation for physicians who want to reassure patients that there is little to no meaningful increased risk for IBD in patients with AD.
Am I allowed to just say "Ditto!"? Wan and colleagues' article "Incidence of Cardiovascular Disease and Venous Thromboembolism in Patients with Atopic Dermatitis" does show a statistically significant increased risk for cardiovascular (CV) disease in patients with AD. Is that increase clinically significant? This study was also exceptionally well done by an exceptionally good research team. They concluded, "Atopic dermatitis, particularly when severe, is associated with increased risks of venous thromboembolism and CV disease, which may influence the monitoring of patients and selection of treatments for AD." I look at their findings and conclude that AD, even when severe, is associated with little if any clinically meaningful increased risks for venous thromboembolism or CV disease, and we don't need to add any special CV monitoring of AD patients.
The key data are presented in Table 2 of their manuscript. In children, the risk for deep vein thrombosis (DVT) in those with severe AD was about 3 times (0.16) that of those with no AD (0.05). But those numbers are per 1000 patient-years. Therefore, the increased risk is 0.16 - 0.05 = 0.11/1000 patient-years. Thus, you'd expect to see one more case of DVT per year in every 9000 children with severe AD. Does that mean we need to monitor all 9000 for DVT? Would that be cost-effective? Might the monitoring cause more problems than it would solve?
CV disease is much more common in adults than in children, but still, with a difference in risk of about 0.5-1 per 1000 patient-years, you'd only expect one more event due to AD in every 1000-2000 patients, and even that is assuming that the entire risk difference was due to AD and not to some other variable that wasn't measured.
With so much drug development for AD, I think we are going to be inundated with companies wanting us to hear their message over and over again. One way to do that is to mine clinical trial data for more papers. In Merola and colleagues' article "Safety and Efficacy of Tralokinumab in Older Adults With Moderate-to-Severe Atopic Dermatitis" we see just that. We already know that tralokinumab is effective for moderate to severe AD from past publications of clinical trial data. Here, the investigators report on a subset of the clinical trial data — the data on older adults — and, not surprisingly, the drug worked. The efficacy rate, 17% getting clear or almost clear, doesn't sound particularly exciting compared with the higher rates we've seen for other products, but perhaps that lower rate is due in part to differences in studies. Instead of more cuts of data from the same trials, it would be nice to see how tralokinumab compares with other AD treatments on a head-to-head basis.
I was excited to see that the study included the use of objective electronic monitoring of sleep quality. This was done using wrist actigraphy, devices on the wrist that measure acceleration movements. What a great tool this could be for measuring how much scratching our patients are doing! With devices like these measuring movements objectively, we wouldn't have to rely on patients' self-report of itch or sleep quality. Sadly, these monitors did not show any meaningful differences between the dupilumab and placebo groups. This technology holds great promise but it isn't yet ready for prime-time assessment of scratching or sleep.
The title of Chiesa Fuxench and colleagues' article, "Risk of Inflammatory Bowel Disease in Patients With Atopic Dermatitis," might be scary to our patients. The authors reported that "children and adults with AD had an increased risk of IBD [inflammatory bowel disease]." The authors concluded, "Clinicians should be aware of these risks, particularly when selecting systemic treatments for AD in patients who may have coincident gastrointestinal symptoms." Bah, humbug, I say!
Be careful when someone tells you there is increased risk. This study was done exceptionally well by an exceptionally good research team. They were working with a huge database and included many controls to ensure that their findings weren't due to chance. And while they did find an "increased risk," they proved — rather conclusively, I believe — that the increased risk is tiny and not something we need to worry about.
The results of this study suggest that there is a scientific link between AD and IBD, probably some genetic inflammatory signaling contributing to both conditions. But even in the highest-risk group, it would take seeing well over 1000 patients for a year to see one more case of IBD due to AD. This article is a good foundation for researchers who want to explore the underlying connection between AD and IBD. The study is an even better foundation for physicians who want to reassure patients that there is little to no meaningful increased risk for IBD in patients with AD.
Am I allowed to just say "Ditto!"? Wan and colleagues' article "Incidence of Cardiovascular Disease and Venous Thromboembolism in Patients with Atopic Dermatitis" does show a statistically significant increased risk for cardiovascular (CV) disease in patients with AD. Is that increase clinically significant? This study was also exceptionally well done by an exceptionally good research team. They concluded, "Atopic dermatitis, particularly when severe, is associated with increased risks of venous thromboembolism and CV disease, which may influence the monitoring of patients and selection of treatments for AD." I look at their findings and conclude that AD, even when severe, is associated with little if any clinically meaningful increased risks for venous thromboembolism or CV disease, and we don't need to add any special CV monitoring of AD patients.
The key data are presented in Table 2 of their manuscript. In children, the risk for deep vein thrombosis (DVT) in those with severe AD was about 3 times (0.16) that of those with no AD (0.05). But those numbers are per 1000 patient-years. Therefore, the increased risk is 0.16 - 0.05 = 0.11/1000 patient-years. Thus, you'd expect to see one more case of DVT per year in every 9000 children with severe AD. Does that mean we need to monitor all 9000 for DVT? Would that be cost-effective? Might the monitoring cause more problems than it would solve?
CV disease is much more common in adults than in children, but still, with a difference in risk of about 0.5-1 per 1000 patient-years, you'd only expect one more event due to AD in every 1000-2000 patients, and even that is assuming that the entire risk difference was due to AD and not to some other variable that wasn't measured.
With so much drug development for AD, I think we are going to be inundated with companies wanting us to hear their message over and over again. One way to do that is to mine clinical trial data for more papers. In Merola and colleagues' article "Safety and Efficacy of Tralokinumab in Older Adults With Moderate-to-Severe Atopic Dermatitis" we see just that. We already know that tralokinumab is effective for moderate to severe AD from past publications of clinical trial data. Here, the investigators report on a subset of the clinical trial data — the data on older adults — and, not surprisingly, the drug worked. The efficacy rate, 17% getting clear or almost clear, doesn't sound particularly exciting compared with the higher rates we've seen for other products, but perhaps that lower rate is due in part to differences in studies. Instead of more cuts of data from the same trials, it would be nice to see how tralokinumab compares with other AD treatments on a head-to-head basis.
Diffuse Pruritic Eruption in an Immunocompromised Patient
The Diagnosis: Scabies Infestation
Direct microscopy revealed the presence of a live scabies mite and numerous eggs (Figure), confirming the diagnosis of a scabies infestation. Scabies, caused by the Sarcoptes scabiei var hominis mite, characteristically presents in adults as pruritic hyperkeratotic plaques of the interdigital web spaces of the hands, flexor surfaces of the wrists and elbows, axillae, male genitalia, and breasts; however, an atypical presentation is common in immunocompromised or immunosuppressed individuals, such as our patient. In children, the palms, soles, and head (ie, face, scalp, neck) are common sites of involvement. Although dermatologists generally are familiar with severe atypical presentations such as Norwegian crusted scabies or bullous scabies, it is important that they are aware of other atypical presentations, such as the diffuse papulonodular variant observed in our patient.1 As such, a low threshold of suspicion for scabies infestations should be employed in immunocompromised patients with new-onset pruritic eruptions.
Direct microscopy is widely accepted as the gold standard for the diagnosis of scabies infestations; it is a fast and low-cost diagnostic tool. However, this technique displays variable sensitivity in clinical practice, requiring experience and a skilled hand.1,2 Other more sensitive diagnostic options for suspected scabies infestations include histopathology, serology, and molecular-based techniques such as DNA isolation and polymerase chain reaction. Although these tests do demonstrate greater sensitivity, they also are more invasive, time intensive, and costly.2 Therefore, they typically are not the first choice for a suspected scabies infestation. Dermoscopy has emerged as another tool to aid in the diagnosis of a suspected scabies infestation, enabling visualization of scaly burrows, eggs, and live mites. Classically, findings resembling a delta wing with contrail are seen on dermoscopic examination. The delta wing represents the brown triangular structure of the pigmented scabies mite head and anterior legs; the contrail is the lighter linear structures streaming behind the scabies mite (similar to visible vapor streams occurring behind flying jets), representing the burrow of the mite.
Although treatment of scabies infestations typically can be accomplished with permethrin cream 5%, the diffuse nature of our patient’s lesions in combination with his immunocompromised state made oral therapy a more appropriate choice. Based on Centers for Disease Control and Prevention recommendations, the patient received 2 doses of oral weight-based ivermectin (200 μg/kg per dose) administered 1 week apart.1,3 The initial dose at day 1 serves to eliminate any scabies mites that are present, while the second dose 1 week later eliminates any residual eggs. Our patient experienced complete resolution of the symptoms following this treatment regimen.
It was important to differentiate our patient’s scabies infestation from other intensely pruritic conditions and morphologic mimics including papular urticaria, lichenoid drug eruptions, tinea corporis, and prurigo nodularis. Papular urticaria is an intensely pruritic hypersensitivity reaction to insect bites that commonly affects the extremities or other exposed areas. Visible puncta may be present.4 Our patient’s lesion distribution involved areas covered by clothing, no puncta were present, and he had no history of a recent arthropod assault, making the diagnosis of papular urticaria less likely.
Lichenoid drug eruptions classically present with symmetric, diffuse, pruritic, violaceous, scaling papules and plaques that present 2 to 3 months after exposure to an offending agent.5 Our patient’s eruption was papulonodular with no violaceous plaques, and he did not report changes to his medications, making a lichenoid drug eruption less likely.
Tinea corporis is another intensely pruritic condition that should be considered, especially in immunocompromised patients. It is caused by dermatophytes and classically presents as erythematous pruritic plaques with an annular, advancing, scaling border.6 Although immunocompromised patients may display extensive involvement, our patient’s lesions were papulonodular with no annular morphology or scale, rendering tinea corporis less likely.
Prurigo nodularis is a chronic condition characterized by pruritic, violaceous, dome-shaped, smooth or crusted nodules secondary to repeated scratching or pressure. Although prurigo nodules can develop as a secondary change due to chronic excoriations in scabies infestations, prurigo nodules usually do not develop in areas such as the midline of the back that are not easily reached by the fingernails,7 which made prurigo nodularis less likely in our patient.
This case describes a unique papulonodular variant of scabies presenting in an immunocompromised cancer patient. Timely recognition and diagnosis of atypical scabies infestations can decrease morbidity and improve the quality of life of these patients.
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Siddig EE, Hay R. Laboratory-based diagnosis of scabies: a review of the current status. Trans R Soc Trop Med Hyg. 2022;116:4-9. doi:10.1093/trstmh/trab049
- Centers for Disease Control and Prevention. Parasites—scabies. medications. Accessed September 19, 2023. https://www.cdc.gov/parasites/ scabies/health_professionals/meds.html
- Örnek S, Zuberbier T, Kocatürk E. Annular urticarial lesions. Clin Dermatol. 2022;40:480-504. doi:10.1016/j.clindermatol .2021.12.010
- Cheraghlou S, Levy LL. Fixed drug eruptions, bullous drug eruptions, and lichenoid drug eruptions. Clin Dermatol. 2020;38:679-692. doi:10.1016/j.clindermatol.2020.06.010
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6. doi:10.7573/dic.2020-5-6
- Kwon CD, Khanna R, Williams KA, et al. Diagnostic workup and evaluation of patients with prurigo nodularis. Medicines (Basel). 2019;6:97. doi:10.3390/medicines6040097
The Diagnosis: Scabies Infestation
Direct microscopy revealed the presence of a live scabies mite and numerous eggs (Figure), confirming the diagnosis of a scabies infestation. Scabies, caused by the Sarcoptes scabiei var hominis mite, characteristically presents in adults as pruritic hyperkeratotic plaques of the interdigital web spaces of the hands, flexor surfaces of the wrists and elbows, axillae, male genitalia, and breasts; however, an atypical presentation is common in immunocompromised or immunosuppressed individuals, such as our patient. In children, the palms, soles, and head (ie, face, scalp, neck) are common sites of involvement. Although dermatologists generally are familiar with severe atypical presentations such as Norwegian crusted scabies or bullous scabies, it is important that they are aware of other atypical presentations, such as the diffuse papulonodular variant observed in our patient.1 As such, a low threshold of suspicion for scabies infestations should be employed in immunocompromised patients with new-onset pruritic eruptions.

Direct microscopy is widely accepted as the gold standard for the diagnosis of scabies infestations; it is a fast and low-cost diagnostic tool. However, this technique displays variable sensitivity in clinical practice, requiring experience and a skilled hand.1,2 Other more sensitive diagnostic options for suspected scabies infestations include histopathology, serology, and molecular-based techniques such as DNA isolation and polymerase chain reaction. Although these tests do demonstrate greater sensitivity, they also are more invasive, time intensive, and costly.2 Therefore, they typically are not the first choice for a suspected scabies infestation. Dermoscopy has emerged as another tool to aid in the diagnosis of a suspected scabies infestation, enabling visualization of scaly burrows, eggs, and live mites. Classically, findings resembling a delta wing with contrail are seen on dermoscopic examination. The delta wing represents the brown triangular structure of the pigmented scabies mite head and anterior legs; the contrail is the lighter linear structures streaming behind the scabies mite (similar to visible vapor streams occurring behind flying jets), representing the burrow of the mite.
Although treatment of scabies infestations typically can be accomplished with permethrin cream 5%, the diffuse nature of our patient’s lesions in combination with his immunocompromised state made oral therapy a more appropriate choice. Based on Centers for Disease Control and Prevention recommendations, the patient received 2 doses of oral weight-based ivermectin (200 μg/kg per dose) administered 1 week apart.1,3 The initial dose at day 1 serves to eliminate any scabies mites that are present, while the second dose 1 week later eliminates any residual eggs. Our patient experienced complete resolution of the symptoms following this treatment regimen.
It was important to differentiate our patient’s scabies infestation from other intensely pruritic conditions and morphologic mimics including papular urticaria, lichenoid drug eruptions, tinea corporis, and prurigo nodularis. Papular urticaria is an intensely pruritic hypersensitivity reaction to insect bites that commonly affects the extremities or other exposed areas. Visible puncta may be present.4 Our patient’s lesion distribution involved areas covered by clothing, no puncta were present, and he had no history of a recent arthropod assault, making the diagnosis of papular urticaria less likely.
Lichenoid drug eruptions classically present with symmetric, diffuse, pruritic, violaceous, scaling papules and plaques that present 2 to 3 months after exposure to an offending agent.5 Our patient’s eruption was papulonodular with no violaceous plaques, and he did not report changes to his medications, making a lichenoid drug eruption less likely.
Tinea corporis is another intensely pruritic condition that should be considered, especially in immunocompromised patients. It is caused by dermatophytes and classically presents as erythematous pruritic plaques with an annular, advancing, scaling border.6 Although immunocompromised patients may display extensive involvement, our patient’s lesions were papulonodular with no annular morphology or scale, rendering tinea corporis less likely.
Prurigo nodularis is a chronic condition characterized by pruritic, violaceous, dome-shaped, smooth or crusted nodules secondary to repeated scratching or pressure. Although prurigo nodules can develop as a secondary change due to chronic excoriations in scabies infestations, prurigo nodules usually do not develop in areas such as the midline of the back that are not easily reached by the fingernails,7 which made prurigo nodularis less likely in our patient.
This case describes a unique papulonodular variant of scabies presenting in an immunocompromised cancer patient. Timely recognition and diagnosis of atypical scabies infestations can decrease morbidity and improve the quality of life of these patients.
The Diagnosis: Scabies Infestation
Direct microscopy revealed the presence of a live scabies mite and numerous eggs (Figure), confirming the diagnosis of a scabies infestation. Scabies, caused by the Sarcoptes scabiei var hominis mite, characteristically presents in adults as pruritic hyperkeratotic plaques of the interdigital web spaces of the hands, flexor surfaces of the wrists and elbows, axillae, male genitalia, and breasts; however, an atypical presentation is common in immunocompromised or immunosuppressed individuals, such as our patient. In children, the palms, soles, and head (ie, face, scalp, neck) are common sites of involvement. Although dermatologists generally are familiar with severe atypical presentations such as Norwegian crusted scabies or bullous scabies, it is important that they are aware of other atypical presentations, such as the diffuse papulonodular variant observed in our patient.1 As such, a low threshold of suspicion for scabies infestations should be employed in immunocompromised patients with new-onset pruritic eruptions.

Direct microscopy is widely accepted as the gold standard for the diagnosis of scabies infestations; it is a fast and low-cost diagnostic tool. However, this technique displays variable sensitivity in clinical practice, requiring experience and a skilled hand.1,2 Other more sensitive diagnostic options for suspected scabies infestations include histopathology, serology, and molecular-based techniques such as DNA isolation and polymerase chain reaction. Although these tests do demonstrate greater sensitivity, they also are more invasive, time intensive, and costly.2 Therefore, they typically are not the first choice for a suspected scabies infestation. Dermoscopy has emerged as another tool to aid in the diagnosis of a suspected scabies infestation, enabling visualization of scaly burrows, eggs, and live mites. Classically, findings resembling a delta wing with contrail are seen on dermoscopic examination. The delta wing represents the brown triangular structure of the pigmented scabies mite head and anterior legs; the contrail is the lighter linear structures streaming behind the scabies mite (similar to visible vapor streams occurring behind flying jets), representing the burrow of the mite.
Although treatment of scabies infestations typically can be accomplished with permethrin cream 5%, the diffuse nature of our patient’s lesions in combination with his immunocompromised state made oral therapy a more appropriate choice. Based on Centers for Disease Control and Prevention recommendations, the patient received 2 doses of oral weight-based ivermectin (200 μg/kg per dose) administered 1 week apart.1,3 The initial dose at day 1 serves to eliminate any scabies mites that are present, while the second dose 1 week later eliminates any residual eggs. Our patient experienced complete resolution of the symptoms following this treatment regimen.
It was important to differentiate our patient’s scabies infestation from other intensely pruritic conditions and morphologic mimics including papular urticaria, lichenoid drug eruptions, tinea corporis, and prurigo nodularis. Papular urticaria is an intensely pruritic hypersensitivity reaction to insect bites that commonly affects the extremities or other exposed areas. Visible puncta may be present.4 Our patient’s lesion distribution involved areas covered by clothing, no puncta were present, and he had no history of a recent arthropod assault, making the diagnosis of papular urticaria less likely.
Lichenoid drug eruptions classically present with symmetric, diffuse, pruritic, violaceous, scaling papules and plaques that present 2 to 3 months after exposure to an offending agent.5 Our patient’s eruption was papulonodular with no violaceous plaques, and he did not report changes to his medications, making a lichenoid drug eruption less likely.
Tinea corporis is another intensely pruritic condition that should be considered, especially in immunocompromised patients. It is caused by dermatophytes and classically presents as erythematous pruritic plaques with an annular, advancing, scaling border.6 Although immunocompromised patients may display extensive involvement, our patient’s lesions were papulonodular with no annular morphology or scale, rendering tinea corporis less likely.
Prurigo nodularis is a chronic condition characterized by pruritic, violaceous, dome-shaped, smooth or crusted nodules secondary to repeated scratching or pressure. Although prurigo nodules can develop as a secondary change due to chronic excoriations in scabies infestations, prurigo nodules usually do not develop in areas such as the midline of the back that are not easily reached by the fingernails,7 which made prurigo nodularis less likely in our patient.
This case describes a unique papulonodular variant of scabies presenting in an immunocompromised cancer patient. Timely recognition and diagnosis of atypical scabies infestations can decrease morbidity and improve the quality of life of these patients.
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Siddig EE, Hay R. Laboratory-based diagnosis of scabies: a review of the current status. Trans R Soc Trop Med Hyg. 2022;116:4-9. doi:10.1093/trstmh/trab049
- Centers for Disease Control and Prevention. Parasites—scabies. medications. Accessed September 19, 2023. https://www.cdc.gov/parasites/ scabies/health_professionals/meds.html
- Örnek S, Zuberbier T, Kocatürk E. Annular urticarial lesions. Clin Dermatol. 2022;40:480-504. doi:10.1016/j.clindermatol .2021.12.010
- Cheraghlou S, Levy LL. Fixed drug eruptions, bullous drug eruptions, and lichenoid drug eruptions. Clin Dermatol. 2020;38:679-692. doi:10.1016/j.clindermatol.2020.06.010
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6. doi:10.7573/dic.2020-5-6
- Kwon CD, Khanna R, Williams KA, et al. Diagnostic workup and evaluation of patients with prurigo nodularis. Medicines (Basel). 2019;6:97. doi:10.3390/medicines6040097
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Siddig EE, Hay R. Laboratory-based diagnosis of scabies: a review of the current status. Trans R Soc Trop Med Hyg. 2022;116:4-9. doi:10.1093/trstmh/trab049
- Centers for Disease Control and Prevention. Parasites—scabies. medications. Accessed September 19, 2023. https://www.cdc.gov/parasites/ scabies/health_professionals/meds.html
- Örnek S, Zuberbier T, Kocatürk E. Annular urticarial lesions. Clin Dermatol. 2022;40:480-504. doi:10.1016/j.clindermatol .2021.12.010
- Cheraghlou S, Levy LL. Fixed drug eruptions, bullous drug eruptions, and lichenoid drug eruptions. Clin Dermatol. 2020;38:679-692. doi:10.1016/j.clindermatol.2020.06.010
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6. doi:10.7573/dic.2020-5-6
- Kwon CD, Khanna R, Williams KA, et al. Diagnostic workup and evaluation of patients with prurigo nodularis. Medicines (Basel). 2019;6:97. doi:10.3390/medicines6040097
A 54-year-old man presented to our dermatology clinic for evaluation of a widespread intensely pruritic rash of 4 weeks’ duration. Calamine lotion and oral hydroxyzine provided minimal relief. He was being treated for a myeloproliferative disorder with immunosuppressive therapy consisting of a combination of cladribine, low-dose cytarabine, and fedratinib. Physical examination revealed multiple excoriated papules and indurated nodules on the extensor and flexor surfaces of the arms and legs (top), chest, midline of the back (bottom), and groin. No lesions were noted on the volar aspect of the patient’s wrists or interdigital spaces, and no central puncta or scales were present. He denied any preceding arthropod bites, trauma, new environmental exposures, or changes to his medications. Scrapings from several representative lesions were obtained for mineral oil preparation and microscopic evaluation.
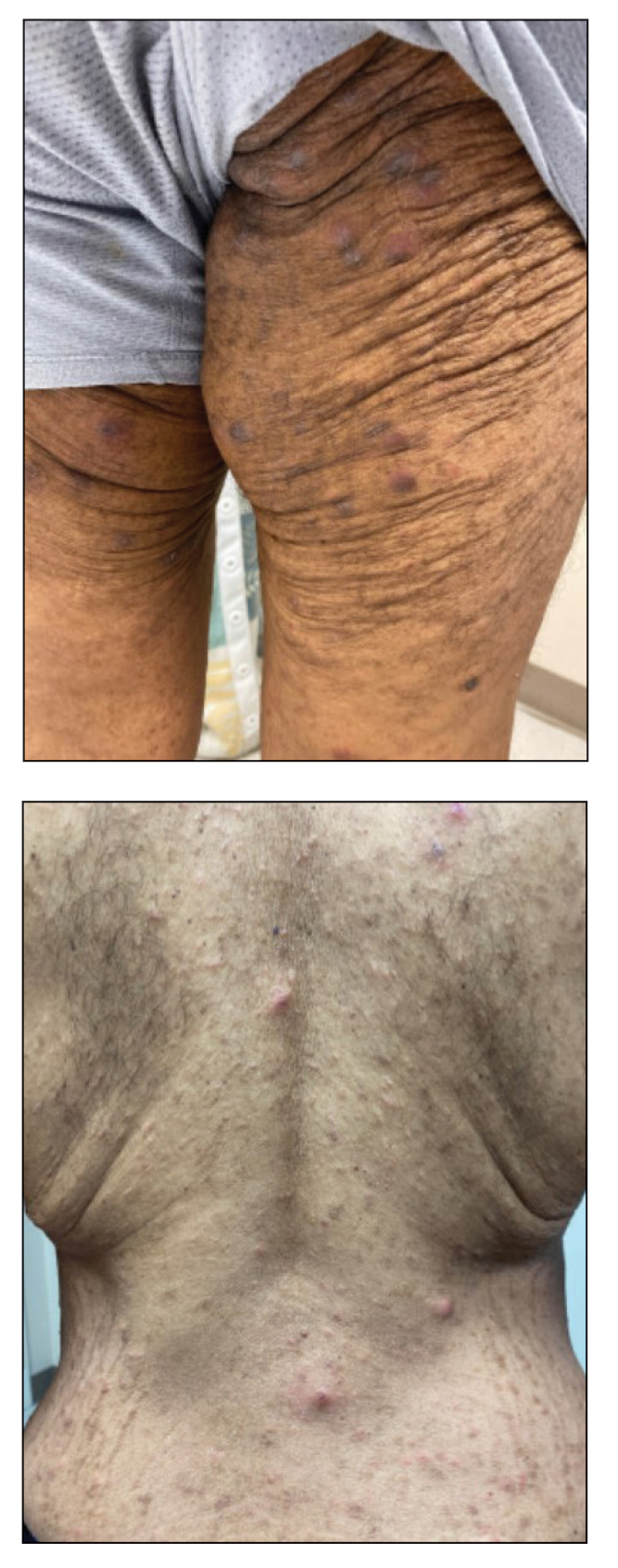
European Commission grants approval of ritlecitinib for severe alopecia areata
This makes ritlecitinib the first medicine authorized by the EC to treat individuals with severe alopecia areata as young as 12 years of age.
Taken as a once-daily pill, ritlecitinib is a dual inhibitor of the TEC family of tyrosine kinases and of Janus kinase 3. In June of 2023, the drug received FDA approval for the treatment of severe alopecia areata in people ages 12 and older in the United States.
According to a press release from Pfizer, which developed the drug, EC approval was based on the pivotal ALLEGRO clinical trial program, which included the ALLEGRO phase 2b/3 study that evaluated ritlecitinib in patients aged 12 years and older with alopecia areata with 50% or more scalp hair loss, including patients with alopecia totalis (total scalp hair loss) and alopecia universalis (total body hair loss). Results from this study showed that 13.4% of adults and adolescents achieved 90% or more scalp hair coverage (Severity of Alopecia Tool score of 10 or less) after 24 weeks of treatment with ritlecitinib 50 mg, compared with 1.5% of those on placebo.
The study also measured Patient Global Impression of Change (PGI-C). At week 24, 49.2% of participants treated with ritlecitinib reported a PGI-C response of “moderate” to “great” improvement in their alopecia areata, compared with 9.2% with placebo.
According to results from an ongoing, long-term phase 3 study of ritlecitinib known as ALLEGRO-LT, the most common adverse reactions reported from use of the drug included diarrhea (9.2%), acne (6.2%), upper respiratory tract infections (6.2%), urticaria (4.6%), rash (3.8%), folliculitis (3.1%), and dizziness (2.3%), the company press release said.
This makes ritlecitinib the first medicine authorized by the EC to treat individuals with severe alopecia areata as young as 12 years of age.
Taken as a once-daily pill, ritlecitinib is a dual inhibitor of the TEC family of tyrosine kinases and of Janus kinase 3. In June of 2023, the drug received FDA approval for the treatment of severe alopecia areata in people ages 12 and older in the United States.
According to a press release from Pfizer, which developed the drug, EC approval was based on the pivotal ALLEGRO clinical trial program, which included the ALLEGRO phase 2b/3 study that evaluated ritlecitinib in patients aged 12 years and older with alopecia areata with 50% or more scalp hair loss, including patients with alopecia totalis (total scalp hair loss) and alopecia universalis (total body hair loss). Results from this study showed that 13.4% of adults and adolescents achieved 90% or more scalp hair coverage (Severity of Alopecia Tool score of 10 or less) after 24 weeks of treatment with ritlecitinib 50 mg, compared with 1.5% of those on placebo.
The study also measured Patient Global Impression of Change (PGI-C). At week 24, 49.2% of participants treated with ritlecitinib reported a PGI-C response of “moderate” to “great” improvement in their alopecia areata, compared with 9.2% with placebo.
According to results from an ongoing, long-term phase 3 study of ritlecitinib known as ALLEGRO-LT, the most common adverse reactions reported from use of the drug included diarrhea (9.2%), acne (6.2%), upper respiratory tract infections (6.2%), urticaria (4.6%), rash (3.8%), folliculitis (3.1%), and dizziness (2.3%), the company press release said.
This makes ritlecitinib the first medicine authorized by the EC to treat individuals with severe alopecia areata as young as 12 years of age.
Taken as a once-daily pill, ritlecitinib is a dual inhibitor of the TEC family of tyrosine kinases and of Janus kinase 3. In June of 2023, the drug received FDA approval for the treatment of severe alopecia areata in people ages 12 and older in the United States.
According to a press release from Pfizer, which developed the drug, EC approval was based on the pivotal ALLEGRO clinical trial program, which included the ALLEGRO phase 2b/3 study that evaluated ritlecitinib in patients aged 12 years and older with alopecia areata with 50% or more scalp hair loss, including patients with alopecia totalis (total scalp hair loss) and alopecia universalis (total body hair loss). Results from this study showed that 13.4% of adults and adolescents achieved 90% or more scalp hair coverage (Severity of Alopecia Tool score of 10 or less) after 24 weeks of treatment with ritlecitinib 50 mg, compared with 1.5% of those on placebo.
The study also measured Patient Global Impression of Change (PGI-C). At week 24, 49.2% of participants treated with ritlecitinib reported a PGI-C response of “moderate” to “great” improvement in their alopecia areata, compared with 9.2% with placebo.
According to results from an ongoing, long-term phase 3 study of ritlecitinib known as ALLEGRO-LT, the most common adverse reactions reported from use of the drug included diarrhea (9.2%), acne (6.2%), upper respiratory tract infections (6.2%), urticaria (4.6%), rash (3.8%), folliculitis (3.1%), and dizziness (2.3%), the company press release said.
Do doctors have a legal right to work from home because of health issues or disability?
A radiologist who claims he was forced to resign after requesting to work from home has settled his discrimination lawsuit with a New York hospital.
Although the case was resolved without a definitive win, legal analysts say the complaint raises important questions about whether some physicians have the right to work from home.
Since the pandemic, employers across the country have become more accepting of professionals working remotely.
Richard Heiden, MD, sued New York City Health and Hospitals in 2020, claiming discrimination and retaliation violations under the American with Disabilities Act (ADA) and the New York State Human Rights Law. Dr. Heiden, who has ulcerative colitis, had asked to work off-site during the start of the pandemic, but the hospital denied his accommodation request. Shortly later, administrators accused Dr. Heiden of poor performance and requested he resign or administrators would terminate him, according to his lawsuit.
Attorneys for New York City Health and Hospitals contended that Dr. Heiden was a poorly performing radiologist who was undergoing a performance review at the time of his accommodation request. The radiologist’s departure was related to the results of the review and had nothing to do with his disability or accommodation request, according to the hospital.
The undisclosed settlement ends a 3-year court battle between Dr. Heiden and the hospital corporation.
In an email, Laura Williams, an attorney for the hospital corporation, said that “the settlement was in the best interest of all parties.”
Dr. Heiden and his attorneys also did not respond to requests for comment.
A critical piece to the puzzle is understanding who is protected under the ADA and is therefore entitled to reasonable accommodations, said Doron Dorfman, JSD, an associate professor at Seton Hall University Law School in Newark, N.J., who focuses on disability law.
A common misconception is that only physicians with a physical disability are “disabled,” he said. However, under the law, a disabled individual is anyone with a physical or mental impairment – including mental illness – that limits major life activities; a person with a history of such impairment; or a person who is perceived by others as having an impairment.
“The law is much broader than many people think,” he said. “I think a lot of people don’t think about those with invisible disabilities, such as people with allergies, those who are immunocompromised, those with chronic illnesses. A lot of people don’t see themselves as disabled, and a lot of employers don’t see them as disabled.”
Working from home has not historically been considered a “reasonable accommodation” under the ADA, Mr. Dorfman said. However, that appears to be changing.
“There has been a sea change,” Mr. Dorfman said. “The question is coming before the courts more frequently, and recent legal decisions show judges may be altering their views on the subject.”
What led to the doctor’s lawsuit?
Dr. Heiden, a longtime radiologist, had practiced at Lincoln Medical and Mental Health Center for about a year when he requested to work remotely. (Lincoln is operated by New York City Health and Hospitals.) At the time, the governor of New York had ordered a statewide lockdown because of COVID-19, and Dr. Heiden expressed concern that his ulcerative colitis made him a high-risk individual for the virus, according to court documents.
In his March 22, 2020, request, Dr. Heiden said that, except for fluoroscopy, his job could be done entirely from his home, according to a district court summary of the case. He also offered to pay for any costs associated with the remote work setup.
Around the same time, New York City Health and Hospitals permitted its facilities to issue a limited number of workstations to radiologists to facilitate remote work in the event of COVID-related staffing shortages. Administrators were in the process of acquiring remote radiology workstations and determining which radiologists at Lincoln would receive them, according to the case summary.
On March 24, the chair of radiology at Lincoln met with Dr. Heiden to review the results of a recent focused professional practice evaluation (FPPE). An FPPE refers to an intensive review of an expansive selection of patient cases handled by the subject physician. During the meeting, the chair that claimed Dr. Heiden was a poor performer and was accurate in his assessments 93.8% of the time, which was below the hospital’s 97% threshold, according to Dr. Heiden’s lawsuit. Dr. Heiden disagreed with the results, and the two engaged in several more meetings.
Meanwhile, Dr. Heiden’s accommodation request was forwarded to other administrators. In an email introduced into court evidence, the chair indicated he did not support the accommodation, writing that Dr. Heiden’s “skill set does not meet the criteria for the initial installations” of the workstations.
On March 26, 2020, the chair allegedly asked Dr. Heiden to either resign or he would be terminated and reported to the New York State Office of Professional Medical Conduct. Four days later, Dr. Heiden learned that his accommodation request had been denied. He resigned on April 2, 2020.
In his lawsuit, Dr. Heiden claimed that the hospital discriminated against him on the basis of his disability in violation of ADA by denying him equal terms and conditions of employment and failing to provide a reasonable accommodation.
The defendants, who included the radiology chair, did not dispute that Dr. Heiden was asked to resign or that administrators warned termination, but they argued the impetus was his FPPE results and a history of inaccurate interpretations. Other clinicians and physicians had expressed concerns about Dr. Heiden’s “lack of clarity [and] interpretive errors,” according to deposition testimony. The hospital emphasized the FPPE had concluded before Dr. Heiden’s accommodation request was made.
New York City Health and Hospitals requested a federal judge dismiss the lawsuit for lack of valid claims. In January 2023, U.S. District Judge Lewis Liman allowed the case to proceed, ruling that some of Dr. Heiden’s claims had merit.
“Plaintiff has satisfied his obligation to proffer sufficient evidence to create an inference of retaliatory or discriminatory intent,” Judge Liman wrote in his decision. “[The chair] had not always planned to ask for plaintiff’s resignation based on the results of the FPPE completed on March 10, 2020. The decision to ask for that resignation arose shortly after the request for the accommodation. And there is evidence from which the jury could find that [the chair] was not receptive to making the accommodation.”
A jury trial was scheduled for July 2023, but the parties reached a settlement on May 31, 2023.
Is working from home reasonable for physicians?
The widespread swing to remote work in recent years has paved a smoother road for physicians who request the accommodation, said Peter Poullos, MD, clinical associate professor of radiology, gastroenterology, and hepatology at Stanford (Calif.) University and founder and cochair of the Stanford Medicine Alliance for Disability Inclusion and Equity.
“There is now a precedent and examples all over that working from home for some is a viable alternative to working in the hospital or a clinic,” Dr. Poullos said. “If a lawyer can point to instances of other people having received the same accommodation, even if the accommodation was given to someone without a disability, it’s much harder for an employer to say: ‘It’s not possible.’ Because clearly, it is.”
A key factor is the employee’s job duties and whether the employee can complete them remotely, said Mr. Dorfman. With physicians, the reasonableness would heavily depend on their specialty.
A radiologist, for example, would probably have a stronger case for performing their duties remotely compared with a surgeon, Dr. Poullos said.
In general, whether an accommodation is reasonable is decided on a case-by-case basis and usually includes reviewing supporting documentation from a medical provider, said Emily Harvey, a Denver-based disability law attorney. Employers are allowed to deny accommodations if they would cause an undue burden to the employer or fundamentally alter the nature or operation of the job or business.
“When it comes to the ADA, and disability rights in general, the analysis is based on the need of the individual,” she said. “Two people with identical diagnoses could need vastly different accommodations to be successful in the same job.”
Mr. Dorfman added that employers are only required to provide an accommodation that is reasonable under the circumstances, whether or not that accommodation meets the preferred request of the employee. For instance, if an immunocompromised physician asked to work from home, but the employer could ensure that all those working around the physician will mask, that could be reasonable enough.
A recent case analysis by Bloomberg Law shows that more courts are siding with employees who request remote work, compared with in past years. Employees who made disability-related remote work requests prevailed in 40% of federal court rulings from 2021 to 2023 versusa success rate of 30% from 2017 to 2019, according to the July 2023 analysis.
The analysis shows that employers still win the majority of the time, but that the gap is closing, Mr. Dorfman said.
In a September 2020 decision, for example, a Massachusetts District Court ruled in favor of an employee with asthma who was precluding from working at home by a behavioral and mental health agency. U.S. Magistrate Judge Katherine Robertson said that the manager was entitled to telework as a reasonable accommodation under the ADA for 60 days or until further notice. The lawsuit was settled in 2021.
“I think judges are much more used to working from home themselves,” Mr. Dorfman said. “That may affect their sense of accepting remote work as a reasonable accommodation. Their personal experience with it [may] actually inform their view of the topic.”
Your accommodation request was denied: Now what?
If you are unsure about your rights under the ADA, a first step is understanding the law’s protections and learning the obligations of your employer.
Keep in mind that not everyone at your workplace may understand the law and what is required, said Dr. Poullos. When making a request to work from home, ensure that you’re using the right words and asking the right people, he advised. Some physicians, for instance, may only discuss the request with their direct supervisor and give up when the request is denied. “The employee might say, ‘I’ve been dealing with some medical issues and I’m really tired and need to adjust my schedule.’ They don’t mention the word ‘disability,’ they don’t mention the ADA, they don’t mention the word ‘accommodation,’ and so that might not trigger the appropriate response.”
Lisa Meeks, PhD, an expert and researcher in disabilities in medical education, encourages physicians and others to follow the appeals process at their institution if they feel their accommodation request has been unjustly denied.
Research shows that physicians who make accommodation requests rarely escalate denials to an appeal, grievance, or complaint, said Dr. Meeks, cohost of the Docs With Disabilities podcast and director of the Docs With Disabilities Initiative. The initiative aims to use research, education, and stories to drive change in perceptions, disability policy, and procedures in health professions and in biomedical and science education.
If an accommodation cannot be agreed on, doctors can reach out the Equal Employment Opportunity Commission and file a discrimination charge. The agency will review the case and provide an opinion on whether the charge has merit. The EEOC’s decision is not binding in court, and even if the agency believes the charge has no merit, employees still have the right to sue, he said.
Ms. Harvey added that the EEOC has many resources on its website, and that most states also have civil rights agencies that have additional resources. Every state and U.S. territory also has a protection and advocacy organization that may be able to help. Physicians can also review their state bar to locate and consult with disability rights attorneys.
Although it may seem like an uphill battle to push for an accommodation, it can be worth it in the end, said Michael Argenyi, MD, an addiction medicine specialist and assistant professor at the University of Massachusetts, Worcester. Dr. Argenyi, who has hearing loss, was featured on the Docs With Disabilities podcast.
“It’s difficult to ‘rock the boat’ and ask for support from the C-suite for employees with disabilities, or to rearrange a small medical office budget to establish a byline just for accommodations,” Dr. Argenyi said. “Yet, the payoff is worthwhile – patients and fellow colleagues notice commitments to diversity building and inclusion.”
A version of this article appeared on Medscape.com.
A radiologist who claims he was forced to resign after requesting to work from home has settled his discrimination lawsuit with a New York hospital.
Although the case was resolved without a definitive win, legal analysts say the complaint raises important questions about whether some physicians have the right to work from home.
Since the pandemic, employers across the country have become more accepting of professionals working remotely.
Richard Heiden, MD, sued New York City Health and Hospitals in 2020, claiming discrimination and retaliation violations under the American with Disabilities Act (ADA) and the New York State Human Rights Law. Dr. Heiden, who has ulcerative colitis, had asked to work off-site during the start of the pandemic, but the hospital denied his accommodation request. Shortly later, administrators accused Dr. Heiden of poor performance and requested he resign or administrators would terminate him, according to his lawsuit.
Attorneys for New York City Health and Hospitals contended that Dr. Heiden was a poorly performing radiologist who was undergoing a performance review at the time of his accommodation request. The radiologist’s departure was related to the results of the review and had nothing to do with his disability or accommodation request, according to the hospital.
The undisclosed settlement ends a 3-year court battle between Dr. Heiden and the hospital corporation.
In an email, Laura Williams, an attorney for the hospital corporation, said that “the settlement was in the best interest of all parties.”
Dr. Heiden and his attorneys also did not respond to requests for comment.
A critical piece to the puzzle is understanding who is protected under the ADA and is therefore entitled to reasonable accommodations, said Doron Dorfman, JSD, an associate professor at Seton Hall University Law School in Newark, N.J., who focuses on disability law.
A common misconception is that only physicians with a physical disability are “disabled,” he said. However, under the law, a disabled individual is anyone with a physical or mental impairment – including mental illness – that limits major life activities; a person with a history of such impairment; or a person who is perceived by others as having an impairment.
“The law is much broader than many people think,” he said. “I think a lot of people don’t think about those with invisible disabilities, such as people with allergies, those who are immunocompromised, those with chronic illnesses. A lot of people don’t see themselves as disabled, and a lot of employers don’t see them as disabled.”
Working from home has not historically been considered a “reasonable accommodation” under the ADA, Mr. Dorfman said. However, that appears to be changing.
“There has been a sea change,” Mr. Dorfman said. “The question is coming before the courts more frequently, and recent legal decisions show judges may be altering their views on the subject.”
What led to the doctor’s lawsuit?
Dr. Heiden, a longtime radiologist, had practiced at Lincoln Medical and Mental Health Center for about a year when he requested to work remotely. (Lincoln is operated by New York City Health and Hospitals.) At the time, the governor of New York had ordered a statewide lockdown because of COVID-19, and Dr. Heiden expressed concern that his ulcerative colitis made him a high-risk individual for the virus, according to court documents.
In his March 22, 2020, request, Dr. Heiden said that, except for fluoroscopy, his job could be done entirely from his home, according to a district court summary of the case. He also offered to pay for any costs associated with the remote work setup.
Around the same time, New York City Health and Hospitals permitted its facilities to issue a limited number of workstations to radiologists to facilitate remote work in the event of COVID-related staffing shortages. Administrators were in the process of acquiring remote radiology workstations and determining which radiologists at Lincoln would receive them, according to the case summary.
On March 24, the chair of radiology at Lincoln met with Dr. Heiden to review the results of a recent focused professional practice evaluation (FPPE). An FPPE refers to an intensive review of an expansive selection of patient cases handled by the subject physician. During the meeting, the chair that claimed Dr. Heiden was a poor performer and was accurate in his assessments 93.8% of the time, which was below the hospital’s 97% threshold, according to Dr. Heiden’s lawsuit. Dr. Heiden disagreed with the results, and the two engaged in several more meetings.
Meanwhile, Dr. Heiden’s accommodation request was forwarded to other administrators. In an email introduced into court evidence, the chair indicated he did not support the accommodation, writing that Dr. Heiden’s “skill set does not meet the criteria for the initial installations” of the workstations.
On March 26, 2020, the chair allegedly asked Dr. Heiden to either resign or he would be terminated and reported to the New York State Office of Professional Medical Conduct. Four days later, Dr. Heiden learned that his accommodation request had been denied. He resigned on April 2, 2020.
In his lawsuit, Dr. Heiden claimed that the hospital discriminated against him on the basis of his disability in violation of ADA by denying him equal terms and conditions of employment and failing to provide a reasonable accommodation.
The defendants, who included the radiology chair, did not dispute that Dr. Heiden was asked to resign or that administrators warned termination, but they argued the impetus was his FPPE results and a history of inaccurate interpretations. Other clinicians and physicians had expressed concerns about Dr. Heiden’s “lack of clarity [and] interpretive errors,” according to deposition testimony. The hospital emphasized the FPPE had concluded before Dr. Heiden’s accommodation request was made.
New York City Health and Hospitals requested a federal judge dismiss the lawsuit for lack of valid claims. In January 2023, U.S. District Judge Lewis Liman allowed the case to proceed, ruling that some of Dr. Heiden’s claims had merit.
“Plaintiff has satisfied his obligation to proffer sufficient evidence to create an inference of retaliatory or discriminatory intent,” Judge Liman wrote in his decision. “[The chair] had not always planned to ask for plaintiff’s resignation based on the results of the FPPE completed on March 10, 2020. The decision to ask for that resignation arose shortly after the request for the accommodation. And there is evidence from which the jury could find that [the chair] was not receptive to making the accommodation.”
A jury trial was scheduled for July 2023, but the parties reached a settlement on May 31, 2023.
Is working from home reasonable for physicians?
The widespread swing to remote work in recent years has paved a smoother road for physicians who request the accommodation, said Peter Poullos, MD, clinical associate professor of radiology, gastroenterology, and hepatology at Stanford (Calif.) University and founder and cochair of the Stanford Medicine Alliance for Disability Inclusion and Equity.
“There is now a precedent and examples all over that working from home for some is a viable alternative to working in the hospital or a clinic,” Dr. Poullos said. “If a lawyer can point to instances of other people having received the same accommodation, even if the accommodation was given to someone without a disability, it’s much harder for an employer to say: ‘It’s not possible.’ Because clearly, it is.”
A key factor is the employee’s job duties and whether the employee can complete them remotely, said Mr. Dorfman. With physicians, the reasonableness would heavily depend on their specialty.
A radiologist, for example, would probably have a stronger case for performing their duties remotely compared with a surgeon, Dr. Poullos said.
In general, whether an accommodation is reasonable is decided on a case-by-case basis and usually includes reviewing supporting documentation from a medical provider, said Emily Harvey, a Denver-based disability law attorney. Employers are allowed to deny accommodations if they would cause an undue burden to the employer or fundamentally alter the nature or operation of the job or business.
“When it comes to the ADA, and disability rights in general, the analysis is based on the need of the individual,” she said. “Two people with identical diagnoses could need vastly different accommodations to be successful in the same job.”
Mr. Dorfman added that employers are only required to provide an accommodation that is reasonable under the circumstances, whether or not that accommodation meets the preferred request of the employee. For instance, if an immunocompromised physician asked to work from home, but the employer could ensure that all those working around the physician will mask, that could be reasonable enough.
A recent case analysis by Bloomberg Law shows that more courts are siding with employees who request remote work, compared with in past years. Employees who made disability-related remote work requests prevailed in 40% of federal court rulings from 2021 to 2023 versusa success rate of 30% from 2017 to 2019, according to the July 2023 analysis.
The analysis shows that employers still win the majority of the time, but that the gap is closing, Mr. Dorfman said.
In a September 2020 decision, for example, a Massachusetts District Court ruled in favor of an employee with asthma who was precluding from working at home by a behavioral and mental health agency. U.S. Magistrate Judge Katherine Robertson said that the manager was entitled to telework as a reasonable accommodation under the ADA for 60 days or until further notice. The lawsuit was settled in 2021.
“I think judges are much more used to working from home themselves,” Mr. Dorfman said. “That may affect their sense of accepting remote work as a reasonable accommodation. Their personal experience with it [may] actually inform their view of the topic.”
Your accommodation request was denied: Now what?
If you are unsure about your rights under the ADA, a first step is understanding the law’s protections and learning the obligations of your employer.
Keep in mind that not everyone at your workplace may understand the law and what is required, said Dr. Poullos. When making a request to work from home, ensure that you’re using the right words and asking the right people, he advised. Some physicians, for instance, may only discuss the request with their direct supervisor and give up when the request is denied. “The employee might say, ‘I’ve been dealing with some medical issues and I’m really tired and need to adjust my schedule.’ They don’t mention the word ‘disability,’ they don’t mention the ADA, they don’t mention the word ‘accommodation,’ and so that might not trigger the appropriate response.”
Lisa Meeks, PhD, an expert and researcher in disabilities in medical education, encourages physicians and others to follow the appeals process at their institution if they feel their accommodation request has been unjustly denied.
Research shows that physicians who make accommodation requests rarely escalate denials to an appeal, grievance, or complaint, said Dr. Meeks, cohost of the Docs With Disabilities podcast and director of the Docs With Disabilities Initiative. The initiative aims to use research, education, and stories to drive change in perceptions, disability policy, and procedures in health professions and in biomedical and science education.
If an accommodation cannot be agreed on, doctors can reach out the Equal Employment Opportunity Commission and file a discrimination charge. The agency will review the case and provide an opinion on whether the charge has merit. The EEOC’s decision is not binding in court, and even if the agency believes the charge has no merit, employees still have the right to sue, he said.
Ms. Harvey added that the EEOC has many resources on its website, and that most states also have civil rights agencies that have additional resources. Every state and U.S. territory also has a protection and advocacy organization that may be able to help. Physicians can also review their state bar to locate and consult with disability rights attorneys.
Although it may seem like an uphill battle to push for an accommodation, it can be worth it in the end, said Michael Argenyi, MD, an addiction medicine specialist and assistant professor at the University of Massachusetts, Worcester. Dr. Argenyi, who has hearing loss, was featured on the Docs With Disabilities podcast.
“It’s difficult to ‘rock the boat’ and ask for support from the C-suite for employees with disabilities, or to rearrange a small medical office budget to establish a byline just for accommodations,” Dr. Argenyi said. “Yet, the payoff is worthwhile – patients and fellow colleagues notice commitments to diversity building and inclusion.”
A version of this article appeared on Medscape.com.
A radiologist who claims he was forced to resign after requesting to work from home has settled his discrimination lawsuit with a New York hospital.
Although the case was resolved without a definitive win, legal analysts say the complaint raises important questions about whether some physicians have the right to work from home.
Since the pandemic, employers across the country have become more accepting of professionals working remotely.
Richard Heiden, MD, sued New York City Health and Hospitals in 2020, claiming discrimination and retaliation violations under the American with Disabilities Act (ADA) and the New York State Human Rights Law. Dr. Heiden, who has ulcerative colitis, had asked to work off-site during the start of the pandemic, but the hospital denied his accommodation request. Shortly later, administrators accused Dr. Heiden of poor performance and requested he resign or administrators would terminate him, according to his lawsuit.
Attorneys for New York City Health and Hospitals contended that Dr. Heiden was a poorly performing radiologist who was undergoing a performance review at the time of his accommodation request. The radiologist’s departure was related to the results of the review and had nothing to do with his disability or accommodation request, according to the hospital.
The undisclosed settlement ends a 3-year court battle between Dr. Heiden and the hospital corporation.
In an email, Laura Williams, an attorney for the hospital corporation, said that “the settlement was in the best interest of all parties.”
Dr. Heiden and his attorneys also did not respond to requests for comment.
A critical piece to the puzzle is understanding who is protected under the ADA and is therefore entitled to reasonable accommodations, said Doron Dorfman, JSD, an associate professor at Seton Hall University Law School in Newark, N.J., who focuses on disability law.
A common misconception is that only physicians with a physical disability are “disabled,” he said. However, under the law, a disabled individual is anyone with a physical or mental impairment – including mental illness – that limits major life activities; a person with a history of such impairment; or a person who is perceived by others as having an impairment.
“The law is much broader than many people think,” he said. “I think a lot of people don’t think about those with invisible disabilities, such as people with allergies, those who are immunocompromised, those with chronic illnesses. A lot of people don’t see themselves as disabled, and a lot of employers don’t see them as disabled.”
Working from home has not historically been considered a “reasonable accommodation” under the ADA, Mr. Dorfman said. However, that appears to be changing.
“There has been a sea change,” Mr. Dorfman said. “The question is coming before the courts more frequently, and recent legal decisions show judges may be altering their views on the subject.”
What led to the doctor’s lawsuit?
Dr. Heiden, a longtime radiologist, had practiced at Lincoln Medical and Mental Health Center for about a year when he requested to work remotely. (Lincoln is operated by New York City Health and Hospitals.) At the time, the governor of New York had ordered a statewide lockdown because of COVID-19, and Dr. Heiden expressed concern that his ulcerative colitis made him a high-risk individual for the virus, according to court documents.
In his March 22, 2020, request, Dr. Heiden said that, except for fluoroscopy, his job could be done entirely from his home, according to a district court summary of the case. He also offered to pay for any costs associated with the remote work setup.
Around the same time, New York City Health and Hospitals permitted its facilities to issue a limited number of workstations to radiologists to facilitate remote work in the event of COVID-related staffing shortages. Administrators were in the process of acquiring remote radiology workstations and determining which radiologists at Lincoln would receive them, according to the case summary.
On March 24, the chair of radiology at Lincoln met with Dr. Heiden to review the results of a recent focused professional practice evaluation (FPPE). An FPPE refers to an intensive review of an expansive selection of patient cases handled by the subject physician. During the meeting, the chair that claimed Dr. Heiden was a poor performer and was accurate in his assessments 93.8% of the time, which was below the hospital’s 97% threshold, according to Dr. Heiden’s lawsuit. Dr. Heiden disagreed with the results, and the two engaged in several more meetings.
Meanwhile, Dr. Heiden’s accommodation request was forwarded to other administrators. In an email introduced into court evidence, the chair indicated he did not support the accommodation, writing that Dr. Heiden’s “skill set does not meet the criteria for the initial installations” of the workstations.
On March 26, 2020, the chair allegedly asked Dr. Heiden to either resign or he would be terminated and reported to the New York State Office of Professional Medical Conduct. Four days later, Dr. Heiden learned that his accommodation request had been denied. He resigned on April 2, 2020.
In his lawsuit, Dr. Heiden claimed that the hospital discriminated against him on the basis of his disability in violation of ADA by denying him equal terms and conditions of employment and failing to provide a reasonable accommodation.
The defendants, who included the radiology chair, did not dispute that Dr. Heiden was asked to resign or that administrators warned termination, but they argued the impetus was his FPPE results and a history of inaccurate interpretations. Other clinicians and physicians had expressed concerns about Dr. Heiden’s “lack of clarity [and] interpretive errors,” according to deposition testimony. The hospital emphasized the FPPE had concluded before Dr. Heiden’s accommodation request was made.
New York City Health and Hospitals requested a federal judge dismiss the lawsuit for lack of valid claims. In January 2023, U.S. District Judge Lewis Liman allowed the case to proceed, ruling that some of Dr. Heiden’s claims had merit.
“Plaintiff has satisfied his obligation to proffer sufficient evidence to create an inference of retaliatory or discriminatory intent,” Judge Liman wrote in his decision. “[The chair] had not always planned to ask for plaintiff’s resignation based on the results of the FPPE completed on March 10, 2020. The decision to ask for that resignation arose shortly after the request for the accommodation. And there is evidence from which the jury could find that [the chair] was not receptive to making the accommodation.”
A jury trial was scheduled for July 2023, but the parties reached a settlement on May 31, 2023.
Is working from home reasonable for physicians?
The widespread swing to remote work in recent years has paved a smoother road for physicians who request the accommodation, said Peter Poullos, MD, clinical associate professor of radiology, gastroenterology, and hepatology at Stanford (Calif.) University and founder and cochair of the Stanford Medicine Alliance for Disability Inclusion and Equity.
“There is now a precedent and examples all over that working from home for some is a viable alternative to working in the hospital or a clinic,” Dr. Poullos said. “If a lawyer can point to instances of other people having received the same accommodation, even if the accommodation was given to someone without a disability, it’s much harder for an employer to say: ‘It’s not possible.’ Because clearly, it is.”
A key factor is the employee’s job duties and whether the employee can complete them remotely, said Mr. Dorfman. With physicians, the reasonableness would heavily depend on their specialty.
A radiologist, for example, would probably have a stronger case for performing their duties remotely compared with a surgeon, Dr. Poullos said.
In general, whether an accommodation is reasonable is decided on a case-by-case basis and usually includes reviewing supporting documentation from a medical provider, said Emily Harvey, a Denver-based disability law attorney. Employers are allowed to deny accommodations if they would cause an undue burden to the employer or fundamentally alter the nature or operation of the job or business.
“When it comes to the ADA, and disability rights in general, the analysis is based on the need of the individual,” she said. “Two people with identical diagnoses could need vastly different accommodations to be successful in the same job.”
Mr. Dorfman added that employers are only required to provide an accommodation that is reasonable under the circumstances, whether or not that accommodation meets the preferred request of the employee. For instance, if an immunocompromised physician asked to work from home, but the employer could ensure that all those working around the physician will mask, that could be reasonable enough.
A recent case analysis by Bloomberg Law shows that more courts are siding with employees who request remote work, compared with in past years. Employees who made disability-related remote work requests prevailed in 40% of federal court rulings from 2021 to 2023 versusa success rate of 30% from 2017 to 2019, according to the July 2023 analysis.
The analysis shows that employers still win the majority of the time, but that the gap is closing, Mr. Dorfman said.
In a September 2020 decision, for example, a Massachusetts District Court ruled in favor of an employee with asthma who was precluding from working at home by a behavioral and mental health agency. U.S. Magistrate Judge Katherine Robertson said that the manager was entitled to telework as a reasonable accommodation under the ADA for 60 days or until further notice. The lawsuit was settled in 2021.
“I think judges are much more used to working from home themselves,” Mr. Dorfman said. “That may affect their sense of accepting remote work as a reasonable accommodation. Their personal experience with it [may] actually inform their view of the topic.”
Your accommodation request was denied: Now what?
If you are unsure about your rights under the ADA, a first step is understanding the law’s protections and learning the obligations of your employer.
Keep in mind that not everyone at your workplace may understand the law and what is required, said Dr. Poullos. When making a request to work from home, ensure that you’re using the right words and asking the right people, he advised. Some physicians, for instance, may only discuss the request with their direct supervisor and give up when the request is denied. “The employee might say, ‘I’ve been dealing with some medical issues and I’m really tired and need to adjust my schedule.’ They don’t mention the word ‘disability,’ they don’t mention the ADA, they don’t mention the word ‘accommodation,’ and so that might not trigger the appropriate response.”
Lisa Meeks, PhD, an expert and researcher in disabilities in medical education, encourages physicians and others to follow the appeals process at their institution if they feel their accommodation request has been unjustly denied.
Research shows that physicians who make accommodation requests rarely escalate denials to an appeal, grievance, or complaint, said Dr. Meeks, cohost of the Docs With Disabilities podcast and director of the Docs With Disabilities Initiative. The initiative aims to use research, education, and stories to drive change in perceptions, disability policy, and procedures in health professions and in biomedical and science education.
If an accommodation cannot be agreed on, doctors can reach out the Equal Employment Opportunity Commission and file a discrimination charge. The agency will review the case and provide an opinion on whether the charge has merit. The EEOC’s decision is not binding in court, and even if the agency believes the charge has no merit, employees still have the right to sue, he said.
Ms. Harvey added that the EEOC has many resources on its website, and that most states also have civil rights agencies that have additional resources. Every state and U.S. territory also has a protection and advocacy organization that may be able to help. Physicians can also review their state bar to locate and consult with disability rights attorneys.
Although it may seem like an uphill battle to push for an accommodation, it can be worth it in the end, said Michael Argenyi, MD, an addiction medicine specialist and assistant professor at the University of Massachusetts, Worcester. Dr. Argenyi, who has hearing loss, was featured on the Docs With Disabilities podcast.
“It’s difficult to ‘rock the boat’ and ask for support from the C-suite for employees with disabilities, or to rearrange a small medical office budget to establish a byline just for accommodations,” Dr. Argenyi said. “Yet, the payoff is worthwhile – patients and fellow colleagues notice commitments to diversity building and inclusion.”
A version of this article appeared on Medscape.com.
Combining lasers: A recipe for maximizing results and patient satisfaction
SAN DIEGO –
“Using a fractional laser as a solo treatment is missing an opportunity to achieve more dramatic improvement for your patients,” Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said at the annual Masters of Aesthetics Symposium. Among the laser treatments he performs, “combination fractional treatments, typically using the 1927-nm laser” are associated with the highest patient satisfaction, he said.
The order of device use matters, he noted. First, he recommended, use a pulsed dye laser, KTP, or intense pulsed light (IPL) for erythema and telangiectasias, and/or a Q-switched or picosecond laser for pigment. Second, use an ablative or nonablative fractional laser for resurfacing. “A lot of seborrheic keratoses don’t respond to selective photothermolysis well, so I’ll use liquid nitrogen at the time of treatment and before or after treat with a picosecond laser,” added Dr. Avram. “This combined treatment approach is less painful than ablative fractional treatment. You’re going to have downtime anyway, so why not maximize the results at that one treatment session?”
The fractional 1927 laser delivers hundreds of thousands of microscopic pulses and fosters high water absorption, so it targets superficial skin conditions such as actinic keratoses, lentigines, and ephelides at depths of 200-250 microns. It thermally coagulates 30%-40% of skin, which heals without affecting surrounding skin and leaves no perceptible scar, he said.
Clinicians can also combine devices to treat scars. “For red scars, it’s often best to treat both erythema and scar texture with two lasers at the same session,” Dr. Avram said. Again, the order matters. First, he recommended using the pulse dye laser, IPL, or KTP at low fluence and short pulse duration. Second, treat with an ablative or nonablative fractional laser at a low density. “In my experience the ablative fractional lasers are far more efficacious,” he said. “Then we typically add a little Kenalog and 5-FU via laser-assisted drug delivery.”
Dr. Avram disclosed that he has received consulting fees from Allergan. He also reported holding shareholder interest and intellectual property rights with Cytrellis Biosystems.
SAN DIEGO –
“Using a fractional laser as a solo treatment is missing an opportunity to achieve more dramatic improvement for your patients,” Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said at the annual Masters of Aesthetics Symposium. Among the laser treatments he performs, “combination fractional treatments, typically using the 1927-nm laser” are associated with the highest patient satisfaction, he said.
The order of device use matters, he noted. First, he recommended, use a pulsed dye laser, KTP, or intense pulsed light (IPL) for erythema and telangiectasias, and/or a Q-switched or picosecond laser for pigment. Second, use an ablative or nonablative fractional laser for resurfacing. “A lot of seborrheic keratoses don’t respond to selective photothermolysis well, so I’ll use liquid nitrogen at the time of treatment and before or after treat with a picosecond laser,” added Dr. Avram. “This combined treatment approach is less painful than ablative fractional treatment. You’re going to have downtime anyway, so why not maximize the results at that one treatment session?”
The fractional 1927 laser delivers hundreds of thousands of microscopic pulses and fosters high water absorption, so it targets superficial skin conditions such as actinic keratoses, lentigines, and ephelides at depths of 200-250 microns. It thermally coagulates 30%-40% of skin, which heals without affecting surrounding skin and leaves no perceptible scar, he said.
Clinicians can also combine devices to treat scars. “For red scars, it’s often best to treat both erythema and scar texture with two lasers at the same session,” Dr. Avram said. Again, the order matters. First, he recommended using the pulse dye laser, IPL, or KTP at low fluence and short pulse duration. Second, treat with an ablative or nonablative fractional laser at a low density. “In my experience the ablative fractional lasers are far more efficacious,” he said. “Then we typically add a little Kenalog and 5-FU via laser-assisted drug delivery.”
Dr. Avram disclosed that he has received consulting fees from Allergan. He also reported holding shareholder interest and intellectual property rights with Cytrellis Biosystems.
SAN DIEGO –
“Using a fractional laser as a solo treatment is missing an opportunity to achieve more dramatic improvement for your patients,” Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said at the annual Masters of Aesthetics Symposium. Among the laser treatments he performs, “combination fractional treatments, typically using the 1927-nm laser” are associated with the highest patient satisfaction, he said.
The order of device use matters, he noted. First, he recommended, use a pulsed dye laser, KTP, or intense pulsed light (IPL) for erythema and telangiectasias, and/or a Q-switched or picosecond laser for pigment. Second, use an ablative or nonablative fractional laser for resurfacing. “A lot of seborrheic keratoses don’t respond to selective photothermolysis well, so I’ll use liquid nitrogen at the time of treatment and before or after treat with a picosecond laser,” added Dr. Avram. “This combined treatment approach is less painful than ablative fractional treatment. You’re going to have downtime anyway, so why not maximize the results at that one treatment session?”
The fractional 1927 laser delivers hundreds of thousands of microscopic pulses and fosters high water absorption, so it targets superficial skin conditions such as actinic keratoses, lentigines, and ephelides at depths of 200-250 microns. It thermally coagulates 30%-40% of skin, which heals without affecting surrounding skin and leaves no perceptible scar, he said.
Clinicians can also combine devices to treat scars. “For red scars, it’s often best to treat both erythema and scar texture with two lasers at the same session,” Dr. Avram said. Again, the order matters. First, he recommended using the pulse dye laser, IPL, or KTP at low fluence and short pulse duration. Second, treat with an ablative or nonablative fractional laser at a low density. “In my experience the ablative fractional lasers are far more efficacious,” he said. “Then we typically add a little Kenalog and 5-FU via laser-assisted drug delivery.”
Dr. Avram disclosed that he has received consulting fees from Allergan. He also reported holding shareholder interest and intellectual property rights with Cytrellis Biosystems.
AT MOAS 2023
The differential diagnosis you’re missing
I’m not the smartest dermatologist in our department. We’re fortunate to have a few super-smarties, you know, the ones who can still recite all the genes in Jean Bolognia’s dermatology textbook and have “Dermpath Bowl Champion” plaques covering their walls. Yet as our chief, I often get requests for a second or third opinion, hoping somehow I’ll discover a diagnosis that others missed. Sometimes they are real diagnostic dilemmas. Oftentimes they’re just itchy.
Recently an itchy 73-year-old woman came to see me. She had seen several competent dermatologists, had comprehensive workups, and had reasonable, even aggressive, attempts at treating. Not much interesting in her history. Nothing on exam. Cancer workup was negative as was pretty much any autoimmune or allergic cause. Biopsy? Maybe a touch of “dermal hypersensitivity.” She was still upset at being told previously she might have scabies. “Scabies!” she said indignantly. “How could I have scabies? No one has touched this body in nearly 4 years!” That’s interesting, I thought.
The electronic medical record holds a lot of useful information. We spend hours combing through histories, labs, pathology, scans, drugs to search for clues that might help with diagnoses. One tab we hardly visit is demographics. Why should that matter, of course? Age, phone number, and address are typically not contributory. But for this woman there was a bit of data that mattered; I checked right after her remark. Marital status: Widowed. She couldn’t have had scabies because no one touches her. Anymore. As our comprehensive workup did not find a cause nor did treatments mitigate her symptoms, I wondered if loneliness might be a contributing factor. I asked if anyone else was itching, any family, any friends? “No, I live alone. I don’t have anyone.”
, and dementia for example. According to the U.S. Surgeon General, it increases the risk for premature death comparable to smoking 15 cigarettes a day. Yet, we rarely (ever?) ask people if they’re lonely. In part because we don’t have good treatments. Remedies for loneliness are mostly societal – reaching out to the widowed, creating spaces that encourage connection, organizing events that bring people together. I cannot type any of these into the EMR orders. However, merely mentioning that a patient could be lonely can be therapeutic. They might not recognize its impact or that they have agency to make it better. They also might not see how their lives still have meaning, an important comorbidity of loneliness.
Not long after her appointment was a 63-year-old man who complained of a burning scrotum. He worked as a knife sharpener, setting up a folding table at local groceries and farmers markets. COVID killed most of his gigs. Like the woman who didn’t have scabies, comprehensive workups turned up nothing. And seemingly nothing, including antibiotics, gabapentin, indomethacin, lidocaine, helped. At his last visit, we talked about his condition. We had also talked about the proper way to sharpen a knife. I came in prepared to offer something dramatic this visit, methotrexate, dupilumab? But before I could speak, he opened a recycled plastic grocery bag and dumped out knives of various sizes. Also a small ax. He then proceeded to show me how each knife has to be sharpened in its own way. Before leaving he handed me a well-worn Arkansas sharpening stone. “For you,” he said. I gave him no additional recommendations or treatments. He hasn’t been back to dermatology since.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
I’m not the smartest dermatologist in our department. We’re fortunate to have a few super-smarties, you know, the ones who can still recite all the genes in Jean Bolognia’s dermatology textbook and have “Dermpath Bowl Champion” plaques covering their walls. Yet as our chief, I often get requests for a second or third opinion, hoping somehow I’ll discover a diagnosis that others missed. Sometimes they are real diagnostic dilemmas. Oftentimes they’re just itchy.
Recently an itchy 73-year-old woman came to see me. She had seen several competent dermatologists, had comprehensive workups, and had reasonable, even aggressive, attempts at treating. Not much interesting in her history. Nothing on exam. Cancer workup was negative as was pretty much any autoimmune or allergic cause. Biopsy? Maybe a touch of “dermal hypersensitivity.” She was still upset at being told previously she might have scabies. “Scabies!” she said indignantly. “How could I have scabies? No one has touched this body in nearly 4 years!” That’s interesting, I thought.
The electronic medical record holds a lot of useful information. We spend hours combing through histories, labs, pathology, scans, drugs to search for clues that might help with diagnoses. One tab we hardly visit is demographics. Why should that matter, of course? Age, phone number, and address are typically not contributory. But for this woman there was a bit of data that mattered; I checked right after her remark. Marital status: Widowed. She couldn’t have had scabies because no one touches her. Anymore. As our comprehensive workup did not find a cause nor did treatments mitigate her symptoms, I wondered if loneliness might be a contributing factor. I asked if anyone else was itching, any family, any friends? “No, I live alone. I don’t have anyone.”
, and dementia for example. According to the U.S. Surgeon General, it increases the risk for premature death comparable to smoking 15 cigarettes a day. Yet, we rarely (ever?) ask people if they’re lonely. In part because we don’t have good treatments. Remedies for loneliness are mostly societal – reaching out to the widowed, creating spaces that encourage connection, organizing events that bring people together. I cannot type any of these into the EMR orders. However, merely mentioning that a patient could be lonely can be therapeutic. They might not recognize its impact or that they have agency to make it better. They also might not see how their lives still have meaning, an important comorbidity of loneliness.
Not long after her appointment was a 63-year-old man who complained of a burning scrotum. He worked as a knife sharpener, setting up a folding table at local groceries and farmers markets. COVID killed most of his gigs. Like the woman who didn’t have scabies, comprehensive workups turned up nothing. And seemingly nothing, including antibiotics, gabapentin, indomethacin, lidocaine, helped. At his last visit, we talked about his condition. We had also talked about the proper way to sharpen a knife. I came in prepared to offer something dramatic this visit, methotrexate, dupilumab? But before I could speak, he opened a recycled plastic grocery bag and dumped out knives of various sizes. Also a small ax. He then proceeded to show me how each knife has to be sharpened in its own way. Before leaving he handed me a well-worn Arkansas sharpening stone. “For you,” he said. I gave him no additional recommendations or treatments. He hasn’t been back to dermatology since.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
I’m not the smartest dermatologist in our department. We’re fortunate to have a few super-smarties, you know, the ones who can still recite all the genes in Jean Bolognia’s dermatology textbook and have “Dermpath Bowl Champion” plaques covering their walls. Yet as our chief, I often get requests for a second or third opinion, hoping somehow I’ll discover a diagnosis that others missed. Sometimes they are real diagnostic dilemmas. Oftentimes they’re just itchy.
Recently an itchy 73-year-old woman came to see me. She had seen several competent dermatologists, had comprehensive workups, and had reasonable, even aggressive, attempts at treating. Not much interesting in her history. Nothing on exam. Cancer workup was negative as was pretty much any autoimmune or allergic cause. Biopsy? Maybe a touch of “dermal hypersensitivity.” She was still upset at being told previously she might have scabies. “Scabies!” she said indignantly. “How could I have scabies? No one has touched this body in nearly 4 years!” That’s interesting, I thought.
The electronic medical record holds a lot of useful information. We spend hours combing through histories, labs, pathology, scans, drugs to search for clues that might help with diagnoses. One tab we hardly visit is demographics. Why should that matter, of course? Age, phone number, and address are typically not contributory. But for this woman there was a bit of data that mattered; I checked right after her remark. Marital status: Widowed. She couldn’t have had scabies because no one touches her. Anymore. As our comprehensive workup did not find a cause nor did treatments mitigate her symptoms, I wondered if loneliness might be a contributing factor. I asked if anyone else was itching, any family, any friends? “No, I live alone. I don’t have anyone.”
, and dementia for example. According to the U.S. Surgeon General, it increases the risk for premature death comparable to smoking 15 cigarettes a day. Yet, we rarely (ever?) ask people if they’re lonely. In part because we don’t have good treatments. Remedies for loneliness are mostly societal – reaching out to the widowed, creating spaces that encourage connection, organizing events that bring people together. I cannot type any of these into the EMR orders. However, merely mentioning that a patient could be lonely can be therapeutic. They might not recognize its impact or that they have agency to make it better. They also might not see how their lives still have meaning, an important comorbidity of loneliness.
Not long after her appointment was a 63-year-old man who complained of a burning scrotum. He worked as a knife sharpener, setting up a folding table at local groceries and farmers markets. COVID killed most of his gigs. Like the woman who didn’t have scabies, comprehensive workups turned up nothing. And seemingly nothing, including antibiotics, gabapentin, indomethacin, lidocaine, helped. At his last visit, we talked about his condition. We had also talked about the proper way to sharpen a knife. I came in prepared to offer something dramatic this visit, methotrexate, dupilumab? But before I could speak, he opened a recycled plastic grocery bag and dumped out knives of various sizes. Also a small ax. He then proceeded to show me how each knife has to be sharpened in its own way. Before leaving he handed me a well-worn Arkansas sharpening stone. “For you,” he said. I gave him no additional recommendations or treatments. He hasn’t been back to dermatology since.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].