User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Why scratching is so contagious
If you’ve ever felt an urge to scratch after witnessing someone else relieve their own itch, you’re certainly not alone. Itching can be contagious and the phenomenon is so common it doesn’t just affect humans. Now researchers may understand why.
Some background: In a 2007 study led by Zhou-Feng Chen, PhD, professor of anesthesiology, psychiatry, and developmental biology at the Washington University in St. Louis, researchers discovered a specific gene, the GRPR (gastrin-releasing peptide receptor), in the spinal cord and a corresponding neuropeptide, GRP (gastrin-releasing peptide). Together, the GRP system was found to transmit the “itch information” from one’s skin to the spinal cord.
This discovery was further backed by 2017 findings when Dr. Chen and his colleagues closely observed the molecular and neural basis of contagious itch behavior in mice. “We played a video that showed a mouse scratching at a very high frequency to other mice,” said Dr. Chen. “We found that, indeed, the mice who watched the video also scratched.”
To determine the inner workings at play, the researchers used molecular mapping to reveal increased neuronal activity in the suprachiasmatic nucleus (SCN), a bilateral structure found in the hypothalamus of the mouse’s brain. In other words, this part of the mouse’s brain “lit up” when a mouse displayed contagious scratching behavior.
The researchers then decided to take this one step further by manipulating the amount of GRP in the hypothalamus. “When we deleted the GRP in the SCN, the mice stopped imitating the scratch,” Dr. Chen said. “When we injected more GRP into the SCN, the mice started scratching like crazy.”
Now, after more investigating and research published in 2022 in Cell Reports, Dr. Chen and his team suspect contagious itching may have just as much to do with our eyeballs as our skin and spinal cord. Why? The phenomenon begins with a visual component: Someone seeing another person scratching.
The researchers targeted mice’s retinal ganglion cells, a type of light-capturing neuron found near the inner surface of the retina. When those cells were disabled, all scratching stopped.
This recent study argues that a previously undiscovered visual pathway may exist between the retina and the brain – bypassing the visual cortex – to provide more immediate physical reactions to potential adverse situations.
There’s more (and it could be quite relatable to some people): After the mice watched a video of another mouse scratching for half an hour, the researchers measured the mice’s stress hormone levels, finding a significant increase. This suggested that exposure to impulsive, contagious scratching behavior may have caused heightened anxiety in the mice.
said Dr. Chen. “We humans also scratch a lot, sometimes as a way to unconsciously express our internal anxiety.”
The mice may have interpreted the scratching video as a sudden negative change to their environment that they had to prepare for. “Contagious behavior is actually a very efficient way to inform other animals of what’s coming,” Dr. Chen said. “When we see other people running in a panic, there is no time to think. You just run as fast as you can. This is another example of contagious behavior that is in your own interest to survive.”
As a result, Dr. Chen believes it’s fair to infer that contagious behavior, including yawning and emotional contagion, is merely an expression of a fundamental survival mechanism that has evolved over time. “The human being is just an imitation machine. It’s often very difficult for people to act independently or as a minority because you would be working against evolution,” said Dr. Chen.
Scott Ira Krakower, DO, a child and adolescent psychiatrist at Northwell Health in Glen Oaks, N.Y., (and not party to this research), seconds this sentiment. “In regard to the physical benefits of contagion, it acts as a permanent defense and helps build collective immunity,” he said. “The social benefits when it comes to empathy or social media contagion are also important to our development. It helps us understand, adapt, and connect with others.”
Observing how empathy operates as a socially contagious behavior is something Dr. Chen and his colleagues are interested in looking into in the future.
“The definition of empathy is the sharing of emotions,” Dr. Chen said. “Shared feelings are crucial for social bonding and mental health, and for other animals, like mice, this is also the case.” Previous studies have shown that mice do, in fact, experience empathy and share feelings of pain and fear with one another.
There is still much to be explored in the study of contagious behaviors and the components of the brain that are activated during such behavior. Dr. Chen and his team intend to, ahem, scratch that particular itch.
A version of this article first appeared on Medscape.com.
If you’ve ever felt an urge to scratch after witnessing someone else relieve their own itch, you’re certainly not alone. Itching can be contagious and the phenomenon is so common it doesn’t just affect humans. Now researchers may understand why.
Some background: In a 2007 study led by Zhou-Feng Chen, PhD, professor of anesthesiology, psychiatry, and developmental biology at the Washington University in St. Louis, researchers discovered a specific gene, the GRPR (gastrin-releasing peptide receptor), in the spinal cord and a corresponding neuropeptide, GRP (gastrin-releasing peptide). Together, the GRP system was found to transmit the “itch information” from one’s skin to the spinal cord.
This discovery was further backed by 2017 findings when Dr. Chen and his colleagues closely observed the molecular and neural basis of contagious itch behavior in mice. “We played a video that showed a mouse scratching at a very high frequency to other mice,” said Dr. Chen. “We found that, indeed, the mice who watched the video also scratched.”
To determine the inner workings at play, the researchers used molecular mapping to reveal increased neuronal activity in the suprachiasmatic nucleus (SCN), a bilateral structure found in the hypothalamus of the mouse’s brain. In other words, this part of the mouse’s brain “lit up” when a mouse displayed contagious scratching behavior.
The researchers then decided to take this one step further by manipulating the amount of GRP in the hypothalamus. “When we deleted the GRP in the SCN, the mice stopped imitating the scratch,” Dr. Chen said. “When we injected more GRP into the SCN, the mice started scratching like crazy.”
Now, after more investigating and research published in 2022 in Cell Reports, Dr. Chen and his team suspect contagious itching may have just as much to do with our eyeballs as our skin and spinal cord. Why? The phenomenon begins with a visual component: Someone seeing another person scratching.
The researchers targeted mice’s retinal ganglion cells, a type of light-capturing neuron found near the inner surface of the retina. When those cells were disabled, all scratching stopped.
This recent study argues that a previously undiscovered visual pathway may exist between the retina and the brain – bypassing the visual cortex – to provide more immediate physical reactions to potential adverse situations.
There’s more (and it could be quite relatable to some people): After the mice watched a video of another mouse scratching for half an hour, the researchers measured the mice’s stress hormone levels, finding a significant increase. This suggested that exposure to impulsive, contagious scratching behavior may have caused heightened anxiety in the mice.
said Dr. Chen. “We humans also scratch a lot, sometimes as a way to unconsciously express our internal anxiety.”
The mice may have interpreted the scratching video as a sudden negative change to their environment that they had to prepare for. “Contagious behavior is actually a very efficient way to inform other animals of what’s coming,” Dr. Chen said. “When we see other people running in a panic, there is no time to think. You just run as fast as you can. This is another example of contagious behavior that is in your own interest to survive.”
As a result, Dr. Chen believes it’s fair to infer that contagious behavior, including yawning and emotional contagion, is merely an expression of a fundamental survival mechanism that has evolved over time. “The human being is just an imitation machine. It’s often very difficult for people to act independently or as a minority because you would be working against evolution,” said Dr. Chen.
Scott Ira Krakower, DO, a child and adolescent psychiatrist at Northwell Health in Glen Oaks, N.Y., (and not party to this research), seconds this sentiment. “In regard to the physical benefits of contagion, it acts as a permanent defense and helps build collective immunity,” he said. “The social benefits when it comes to empathy or social media contagion are also important to our development. It helps us understand, adapt, and connect with others.”
Observing how empathy operates as a socially contagious behavior is something Dr. Chen and his colleagues are interested in looking into in the future.
“The definition of empathy is the sharing of emotions,” Dr. Chen said. “Shared feelings are crucial for social bonding and mental health, and for other animals, like mice, this is also the case.” Previous studies have shown that mice do, in fact, experience empathy and share feelings of pain and fear with one another.
There is still much to be explored in the study of contagious behaviors and the components of the brain that are activated during such behavior. Dr. Chen and his team intend to, ahem, scratch that particular itch.
A version of this article first appeared on Medscape.com.
If you’ve ever felt an urge to scratch after witnessing someone else relieve their own itch, you’re certainly not alone. Itching can be contagious and the phenomenon is so common it doesn’t just affect humans. Now researchers may understand why.
Some background: In a 2007 study led by Zhou-Feng Chen, PhD, professor of anesthesiology, psychiatry, and developmental biology at the Washington University in St. Louis, researchers discovered a specific gene, the GRPR (gastrin-releasing peptide receptor), in the spinal cord and a corresponding neuropeptide, GRP (gastrin-releasing peptide). Together, the GRP system was found to transmit the “itch information” from one’s skin to the spinal cord.
This discovery was further backed by 2017 findings when Dr. Chen and his colleagues closely observed the molecular and neural basis of contagious itch behavior in mice. “We played a video that showed a mouse scratching at a very high frequency to other mice,” said Dr. Chen. “We found that, indeed, the mice who watched the video also scratched.”
To determine the inner workings at play, the researchers used molecular mapping to reveal increased neuronal activity in the suprachiasmatic nucleus (SCN), a bilateral structure found in the hypothalamus of the mouse’s brain. In other words, this part of the mouse’s brain “lit up” when a mouse displayed contagious scratching behavior.
The researchers then decided to take this one step further by manipulating the amount of GRP in the hypothalamus. “When we deleted the GRP in the SCN, the mice stopped imitating the scratch,” Dr. Chen said. “When we injected more GRP into the SCN, the mice started scratching like crazy.”
Now, after more investigating and research published in 2022 in Cell Reports, Dr. Chen and his team suspect contagious itching may have just as much to do with our eyeballs as our skin and spinal cord. Why? The phenomenon begins with a visual component: Someone seeing another person scratching.
The researchers targeted mice’s retinal ganglion cells, a type of light-capturing neuron found near the inner surface of the retina. When those cells were disabled, all scratching stopped.
This recent study argues that a previously undiscovered visual pathway may exist between the retina and the brain – bypassing the visual cortex – to provide more immediate physical reactions to potential adverse situations.
There’s more (and it could be quite relatable to some people): After the mice watched a video of another mouse scratching for half an hour, the researchers measured the mice’s stress hormone levels, finding a significant increase. This suggested that exposure to impulsive, contagious scratching behavior may have caused heightened anxiety in the mice.
said Dr. Chen. “We humans also scratch a lot, sometimes as a way to unconsciously express our internal anxiety.”
The mice may have interpreted the scratching video as a sudden negative change to their environment that they had to prepare for. “Contagious behavior is actually a very efficient way to inform other animals of what’s coming,” Dr. Chen said. “When we see other people running in a panic, there is no time to think. You just run as fast as you can. This is another example of contagious behavior that is in your own interest to survive.”
As a result, Dr. Chen believes it’s fair to infer that contagious behavior, including yawning and emotional contagion, is merely an expression of a fundamental survival mechanism that has evolved over time. “The human being is just an imitation machine. It’s often very difficult for people to act independently or as a minority because you would be working against evolution,” said Dr. Chen.
Scott Ira Krakower, DO, a child and adolescent psychiatrist at Northwell Health in Glen Oaks, N.Y., (and not party to this research), seconds this sentiment. “In regard to the physical benefits of contagion, it acts as a permanent defense and helps build collective immunity,” he said. “The social benefits when it comes to empathy or social media contagion are also important to our development. It helps us understand, adapt, and connect with others.”
Observing how empathy operates as a socially contagious behavior is something Dr. Chen and his colleagues are interested in looking into in the future.
“The definition of empathy is the sharing of emotions,” Dr. Chen said. “Shared feelings are crucial for social bonding and mental health, and for other animals, like mice, this is also the case.” Previous studies have shown that mice do, in fact, experience empathy and share feelings of pain and fear with one another.
There is still much to be explored in the study of contagious behaviors and the components of the brain that are activated during such behavior. Dr. Chen and his team intend to, ahem, scratch that particular itch.
A version of this article first appeared on Medscape.com.
FROM CELL REPORTS
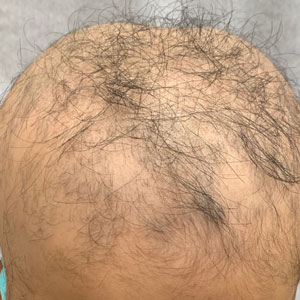
Case series supports targeted drugs in treatment of alopecia in children with AD
in children with AA and concomitant atopy.
It was only a little over a year ago that the JAK inhibitor baricitinib became the first systemic therapy approved by the Food and Drug Administration for AA in adults. In June 2023, the JAK inhibitor ritlecitinib was approved for severe AA in patients as young as 12 years of age, but there is accumulating evidence that dupilumab, which binds to the interleukin-4 receptor, might be an option for even younger children with AA.
Of those who have worked with dupilumab for controlling AA in children, Brittany Craiglow, MD, an adjunct associate professor of dermatology at Yale University, New Haven, Conn., updated a case series at the recent MedscapeLive! Annual Women’s and Pediatric Dermatology Seminar in Baltimore. A series of six children with AA treated with dupilumab was published 2 years ago in JAAD Case Reports.
Even in 2021, her case series was not the first report of benefit from dupilumab in children with AA, but instead contributed to a “growing body of literature” supporting the potential benefit in the setting of concomitant atopy, Dr. Craiglow, one of the authors of the series, said in an interview.
Of the six patients in that series, five had improvement and four had complete regrowth with dupilumab, whether as a monotherapy or in combination with other agents. The children ranged in age from 7 to 12 years. The age range at the time of AA onset was 3-11 years. All had atopic dermatitis (AD) and most had additional atopic conditions, such as food allergies or asthma.
Since publication, Dr. Craiglow has successfully treated many more patients with dupilumab, either as monotherapy or in combination with oral minoxidil, corticosteroids, and/or a topical JAK inhibitor. Dupilumab, which is approved for the treatment of AD in children as young as 6 months of age, has been well tolerated.
“Oral minoxidil is often a great adjuvant treatment in patients with AA and should be used unless there are contraindications,” based on the initial and subsequent experience treating AA with dupilumab, said Dr. Craiglow.
“Topical steroids can be used in combination with dupilumab and minoxidil, but in general dupilumab should not be combined with an oral JAK inhibitor,” she added.
Now, with the approval of ritlecitinib, Dr. Craiglow said this JAK inhibitor will become a first-line therapy in children 12 years or older with severe, persistent AA, but she considers a trial of dupilumab reasonable in younger children, given the controlled studies of safety for atopic diseases.
“I would say that dupilumab could be considered in the following clinical scenarios: children under 12 with AA and concomitant atopy, such as atopic dermatitis, asthma, allergies, and/or elevated IgE; and children over the age of 12 with concomitant atopy who either have a contraindication to a JAK inhibitor or whose families have reservations about or are unwilling to take one,” Dr. Craiglow said.
In older children, she believes that dupilumab has “a much lower chance of being effective” than an oral JAK inhibitor like ritlecitinib, but it circumvents the potential safety issues of JAK inhibitors that have been observed in adults.
With ritlecitinib providing an on-label option for AA in older children, Dr. Craiglow suggested it might be easier to obtain third-party coverage for dupilumab as an alternative to a JAK inhibitor for AA in patients younger than 12, particularly when there is an indication for a concomitant atopic condition and a rationale, such as a concern about relative safety.
Two years ago, when Dr. Craiglow and her coinvestigator published their six-patient case series, a second case series was published about the same time by investigators at the University of Pennsylvania, Philadelphia, in the Journal of the American Academy of Dermatology. This series of 16 pediatric patients with AA on dupilumab was more heterogeneous, but four of six patients with active disease and more than 4 months of follow-up had improvement in AA, including total regrowth. The improvement was concentrated in patients with moderate to severe AD at the time of treatment.
Based on this series, the authors, led by Leslie Castelo-Soccio, MD, PhD, who is now an attending physician in the Dermatology Branch of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, Md., concluded that dupilumab “may be a therapeutic option for AA” when traditional therapies have failed, “especially in patients with concurrent AD or asthma, for which the benefits of dupilumab are clear.”
When contacted about where this therapy might fit on the basis of her case series and the update on Dr. Craiglow’s experience, Dr. Castelo-Soccio, like Dr. Craiglow, stressed the importance of employing this therapy selectively.
“I do think that dupilumab is a reasonable option for AA in children with atopy and IgE levels greater than 200 IU/mL, especially if treatment is for atopic dermatitis or asthma as well,” she said.
Many clinicians, including Dr. Craiglow, have experience with oral JAK inhibitors in children younger than 12. Indeed, a recently published case study associated oral abrocitinib, a JAK inhibitor approved for moderate to severe AD in patients ages 12 and older, with hair regrowth in an 11-year-old child who had persistent AA for more than 6 years despite numerous conventional therapies.
However, the advantage of dupilumab in younger children is the greater evidence of safety, providing a level of reassurance for a treatment that is commonly used for severe atopic diseases but does not have a specific indication for AA, according to Dr. Craiglow.
Dr. Craiglow disclosed being a speaker for AbbVie and a speaker and consultant for Eli Lilly, Incyte, Pfizer, Regeneron, and Sanofi Genzyme. Dr. Castelo-Soccio had no disclosures.
in children with AA and concomitant atopy.
It was only a little over a year ago that the JAK inhibitor baricitinib became the first systemic therapy approved by the Food and Drug Administration for AA in adults. In June 2023, the JAK inhibitor ritlecitinib was approved for severe AA in patients as young as 12 years of age, but there is accumulating evidence that dupilumab, which binds to the interleukin-4 receptor, might be an option for even younger children with AA.
Of those who have worked with dupilumab for controlling AA in children, Brittany Craiglow, MD, an adjunct associate professor of dermatology at Yale University, New Haven, Conn., updated a case series at the recent MedscapeLive! Annual Women’s and Pediatric Dermatology Seminar in Baltimore. A series of six children with AA treated with dupilumab was published 2 years ago in JAAD Case Reports.
Even in 2021, her case series was not the first report of benefit from dupilumab in children with AA, but instead contributed to a “growing body of literature” supporting the potential benefit in the setting of concomitant atopy, Dr. Craiglow, one of the authors of the series, said in an interview.
Of the six patients in that series, five had improvement and four had complete regrowth with dupilumab, whether as a monotherapy or in combination with other agents. The children ranged in age from 7 to 12 years. The age range at the time of AA onset was 3-11 years. All had atopic dermatitis (AD) and most had additional atopic conditions, such as food allergies or asthma.
Since publication, Dr. Craiglow has successfully treated many more patients with dupilumab, either as monotherapy or in combination with oral minoxidil, corticosteroids, and/or a topical JAK inhibitor. Dupilumab, which is approved for the treatment of AD in children as young as 6 months of age, has been well tolerated.
“Oral minoxidil is often a great adjuvant treatment in patients with AA and should be used unless there are contraindications,” based on the initial and subsequent experience treating AA with dupilumab, said Dr. Craiglow.
“Topical steroids can be used in combination with dupilumab and minoxidil, but in general dupilumab should not be combined with an oral JAK inhibitor,” she added.
Now, with the approval of ritlecitinib, Dr. Craiglow said this JAK inhibitor will become a first-line therapy in children 12 years or older with severe, persistent AA, but she considers a trial of dupilumab reasonable in younger children, given the controlled studies of safety for atopic diseases.
“I would say that dupilumab could be considered in the following clinical scenarios: children under 12 with AA and concomitant atopy, such as atopic dermatitis, asthma, allergies, and/or elevated IgE; and children over the age of 12 with concomitant atopy who either have a contraindication to a JAK inhibitor or whose families have reservations about or are unwilling to take one,” Dr. Craiglow said.
In older children, she believes that dupilumab has “a much lower chance of being effective” than an oral JAK inhibitor like ritlecitinib, but it circumvents the potential safety issues of JAK inhibitors that have been observed in adults.
With ritlecitinib providing an on-label option for AA in older children, Dr. Craiglow suggested it might be easier to obtain third-party coverage for dupilumab as an alternative to a JAK inhibitor for AA in patients younger than 12, particularly when there is an indication for a concomitant atopic condition and a rationale, such as a concern about relative safety.
Two years ago, when Dr. Craiglow and her coinvestigator published their six-patient case series, a second case series was published about the same time by investigators at the University of Pennsylvania, Philadelphia, in the Journal of the American Academy of Dermatology. This series of 16 pediatric patients with AA on dupilumab was more heterogeneous, but four of six patients with active disease and more than 4 months of follow-up had improvement in AA, including total regrowth. The improvement was concentrated in patients with moderate to severe AD at the time of treatment.
Based on this series, the authors, led by Leslie Castelo-Soccio, MD, PhD, who is now an attending physician in the Dermatology Branch of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, Md., concluded that dupilumab “may be a therapeutic option for AA” when traditional therapies have failed, “especially in patients with concurrent AD or asthma, for which the benefits of dupilumab are clear.”
When contacted about where this therapy might fit on the basis of her case series and the update on Dr. Craiglow’s experience, Dr. Castelo-Soccio, like Dr. Craiglow, stressed the importance of employing this therapy selectively.
“I do think that dupilumab is a reasonable option for AA in children with atopy and IgE levels greater than 200 IU/mL, especially if treatment is for atopic dermatitis or asthma as well,” she said.
Many clinicians, including Dr. Craiglow, have experience with oral JAK inhibitors in children younger than 12. Indeed, a recently published case study associated oral abrocitinib, a JAK inhibitor approved for moderate to severe AD in patients ages 12 and older, with hair regrowth in an 11-year-old child who had persistent AA for more than 6 years despite numerous conventional therapies.
However, the advantage of dupilumab in younger children is the greater evidence of safety, providing a level of reassurance for a treatment that is commonly used for severe atopic diseases but does not have a specific indication for AA, according to Dr. Craiglow.
Dr. Craiglow disclosed being a speaker for AbbVie and a speaker and consultant for Eli Lilly, Incyte, Pfizer, Regeneron, and Sanofi Genzyme. Dr. Castelo-Soccio had no disclosures.
in children with AA and concomitant atopy.
It was only a little over a year ago that the JAK inhibitor baricitinib became the first systemic therapy approved by the Food and Drug Administration for AA in adults. In June 2023, the JAK inhibitor ritlecitinib was approved for severe AA in patients as young as 12 years of age, but there is accumulating evidence that dupilumab, which binds to the interleukin-4 receptor, might be an option for even younger children with AA.
Of those who have worked with dupilumab for controlling AA in children, Brittany Craiglow, MD, an adjunct associate professor of dermatology at Yale University, New Haven, Conn., updated a case series at the recent MedscapeLive! Annual Women’s and Pediatric Dermatology Seminar in Baltimore. A series of six children with AA treated with dupilumab was published 2 years ago in JAAD Case Reports.
Even in 2021, her case series was not the first report of benefit from dupilumab in children with AA, but instead contributed to a “growing body of literature” supporting the potential benefit in the setting of concomitant atopy, Dr. Craiglow, one of the authors of the series, said in an interview.
Of the six patients in that series, five had improvement and four had complete regrowth with dupilumab, whether as a monotherapy or in combination with other agents. The children ranged in age from 7 to 12 years. The age range at the time of AA onset was 3-11 years. All had atopic dermatitis (AD) and most had additional atopic conditions, such as food allergies or asthma.
Since publication, Dr. Craiglow has successfully treated many more patients with dupilumab, either as monotherapy or in combination with oral minoxidil, corticosteroids, and/or a topical JAK inhibitor. Dupilumab, which is approved for the treatment of AD in children as young as 6 months of age, has been well tolerated.
“Oral minoxidil is often a great adjuvant treatment in patients with AA and should be used unless there are contraindications,” based on the initial and subsequent experience treating AA with dupilumab, said Dr. Craiglow.
“Topical steroids can be used in combination with dupilumab and minoxidil, but in general dupilumab should not be combined with an oral JAK inhibitor,” she added.
Now, with the approval of ritlecitinib, Dr. Craiglow said this JAK inhibitor will become a first-line therapy in children 12 years or older with severe, persistent AA, but she considers a trial of dupilumab reasonable in younger children, given the controlled studies of safety for atopic diseases.
“I would say that dupilumab could be considered in the following clinical scenarios: children under 12 with AA and concomitant atopy, such as atopic dermatitis, asthma, allergies, and/or elevated IgE; and children over the age of 12 with concomitant atopy who either have a contraindication to a JAK inhibitor or whose families have reservations about or are unwilling to take one,” Dr. Craiglow said.
In older children, she believes that dupilumab has “a much lower chance of being effective” than an oral JAK inhibitor like ritlecitinib, but it circumvents the potential safety issues of JAK inhibitors that have been observed in adults.
With ritlecitinib providing an on-label option for AA in older children, Dr. Craiglow suggested it might be easier to obtain third-party coverage for dupilumab as an alternative to a JAK inhibitor for AA in patients younger than 12, particularly when there is an indication for a concomitant atopic condition and a rationale, such as a concern about relative safety.
Two years ago, when Dr. Craiglow and her coinvestigator published their six-patient case series, a second case series was published about the same time by investigators at the University of Pennsylvania, Philadelphia, in the Journal of the American Academy of Dermatology. This series of 16 pediatric patients with AA on dupilumab was more heterogeneous, but four of six patients with active disease and more than 4 months of follow-up had improvement in AA, including total regrowth. The improvement was concentrated in patients with moderate to severe AD at the time of treatment.
Based on this series, the authors, led by Leslie Castelo-Soccio, MD, PhD, who is now an attending physician in the Dermatology Branch of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, Md., concluded that dupilumab “may be a therapeutic option for AA” when traditional therapies have failed, “especially in patients with concurrent AD or asthma, for which the benefits of dupilumab are clear.”
When contacted about where this therapy might fit on the basis of her case series and the update on Dr. Craiglow’s experience, Dr. Castelo-Soccio, like Dr. Craiglow, stressed the importance of employing this therapy selectively.
“I do think that dupilumab is a reasonable option for AA in children with atopy and IgE levels greater than 200 IU/mL, especially if treatment is for atopic dermatitis or asthma as well,” she said.
Many clinicians, including Dr. Craiglow, have experience with oral JAK inhibitors in children younger than 12. Indeed, a recently published case study associated oral abrocitinib, a JAK inhibitor approved for moderate to severe AD in patients ages 12 and older, with hair regrowth in an 11-year-old child who had persistent AA for more than 6 years despite numerous conventional therapies.
However, the advantage of dupilumab in younger children is the greater evidence of safety, providing a level of reassurance for a treatment that is commonly used for severe atopic diseases but does not have a specific indication for AA, according to Dr. Craiglow.
Dr. Craiglow disclosed being a speaker for AbbVie and a speaker and consultant for Eli Lilly, Incyte, Pfizer, Regeneron, and Sanofi Genzyme. Dr. Castelo-Soccio had no disclosures.
New guidelines for laser treatment of cutaneous vascular anomalies
A new practice guideline is setting a standard for doctors who use lasers to treat cutaneous vascular anomalies.
Poor treatment has been an issue in this field because no uniform guidelines existed to inform practice, according to a press release from the American Society for Laser Medicine and Surgery.
The laser treatment settings can vary based on the type and location of the birthmark and also the patient’s skin type, which has resulted in an inconsistent approach from clinicians, according to the release.
“For decades, I have observed adverse outcomes from the improper laser treatment of vascular birthmarks,” Linda Rozell-Shannon, PhD, president and founder of the Vascular Birthmarks Foundation said in a statement from ASLMS. “As a result of these guidelines, patient outcomes will be improved.”
The guideline, published on the ASLMS website along with supporting videos, was jointly developed by ASLMS, VBF, and an international group of clinicians, marking the first consensus guideline on laser treatments for cutaneous vascular anomalies. It details 32 best practice directives for various scenarios, including advice on safety considerations, additional testing, and when to refer.
“It is important to realize that just because someone is board certified does not mean they are skilled in treating all conditions or using all lasers,” Paul Friedman, MD, a dermatologist in Houston, and former president of ASLMS, said in the ASLMS statement.
Vascular birthmarks are a common condition affecting up to 14% of children, according to VBF. Most are hemangiomas, a buildup of blood vessels that usually appears at birth or within a month after birth. Laser therapy reduces the size and color of the anomalies.
Support for this initiative was provided by Candela Medical.
A version of this article first appeared on Medscape.com.
A new practice guideline is setting a standard for doctors who use lasers to treat cutaneous vascular anomalies.
Poor treatment has been an issue in this field because no uniform guidelines existed to inform practice, according to a press release from the American Society for Laser Medicine and Surgery.
The laser treatment settings can vary based on the type and location of the birthmark and also the patient’s skin type, which has resulted in an inconsistent approach from clinicians, according to the release.
“For decades, I have observed adverse outcomes from the improper laser treatment of vascular birthmarks,” Linda Rozell-Shannon, PhD, president and founder of the Vascular Birthmarks Foundation said in a statement from ASLMS. “As a result of these guidelines, patient outcomes will be improved.”
The guideline, published on the ASLMS website along with supporting videos, was jointly developed by ASLMS, VBF, and an international group of clinicians, marking the first consensus guideline on laser treatments for cutaneous vascular anomalies. It details 32 best practice directives for various scenarios, including advice on safety considerations, additional testing, and when to refer.
“It is important to realize that just because someone is board certified does not mean they are skilled in treating all conditions or using all lasers,” Paul Friedman, MD, a dermatologist in Houston, and former president of ASLMS, said in the ASLMS statement.
Vascular birthmarks are a common condition affecting up to 14% of children, according to VBF. Most are hemangiomas, a buildup of blood vessels that usually appears at birth or within a month after birth. Laser therapy reduces the size and color of the anomalies.
Support for this initiative was provided by Candela Medical.
A version of this article first appeared on Medscape.com.
A new practice guideline is setting a standard for doctors who use lasers to treat cutaneous vascular anomalies.
Poor treatment has been an issue in this field because no uniform guidelines existed to inform practice, according to a press release from the American Society for Laser Medicine and Surgery.
The laser treatment settings can vary based on the type and location of the birthmark and also the patient’s skin type, which has resulted in an inconsistent approach from clinicians, according to the release.
“For decades, I have observed adverse outcomes from the improper laser treatment of vascular birthmarks,” Linda Rozell-Shannon, PhD, president and founder of the Vascular Birthmarks Foundation said in a statement from ASLMS. “As a result of these guidelines, patient outcomes will be improved.”
The guideline, published on the ASLMS website along with supporting videos, was jointly developed by ASLMS, VBF, and an international group of clinicians, marking the first consensus guideline on laser treatments for cutaneous vascular anomalies. It details 32 best practice directives for various scenarios, including advice on safety considerations, additional testing, and when to refer.
“It is important to realize that just because someone is board certified does not mean they are skilled in treating all conditions or using all lasers,” Paul Friedman, MD, a dermatologist in Houston, and former president of ASLMS, said in the ASLMS statement.
Vascular birthmarks are a common condition affecting up to 14% of children, according to VBF. Most are hemangiomas, a buildup of blood vessels that usually appears at birth or within a month after birth. Laser therapy reduces the size and color of the anomalies.
Support for this initiative was provided by Candela Medical.
A version of this article first appeared on Medscape.com.
Progress seen on five fronts for substantially improving treatment of epidermolysis bullosa
ASHEVILLE, N.C. – , according to a prominent EB researcher.
Not only are recent developments in EB “exciting,” the progress on multiple fronts for control of disease or its symptoms suggests “we are on the cusp of a new era,” Jemima Mellerio, BSc, MD, a consultant dermatologist, St. John’s Institute of Dermatology, London, said at the annual meeting of the Society for Pediatric Dermatology.
Published clinical studies of cell therapies and gene therapies date back at least 15 years, according to a review by Dr. Mellerio on why developments are starting to move so quickly. The difference now is that many obstacles to routine use of these options are being resolved so that viable strategies have reached or are reaching phase 3 trials.
In addition to cell therapies and gene therapies, Dr. Mellerio discussed progress in three additional areas: gene editing, protein therapy, and drug repurposing.
Summarizing progress in each, she described improvement in levels of collagen VII, an important deficit in most types of EB, that were achieved with fibroblast injections that improved levels of collagen VII and anchoring fibrils in a study published in the Journal of Investigative Dermatology. Injection of mesenchymal stromal cells (MSC) have been associated with reduced pain and itch in a series of studies, one of the earliest of which was published in the New England Journal of Medicine.
Since that time, there have been several approaches using MSC.
Of these approaches, intravenous injection of ABCB5+ MSCs might be the first to gain regulatory approval. According to Dr. Mellerio, there is an ongoing phase 3 crossover trial evaluating this approach, which followed several earlier phase studies that demonstrated adequate safety and tolerability while reducing severity scores, relieving pain and itch, and improving wound closure in patients with EB.
In 2006, correction of junctional EB (JEB) was achieved by transplantation of genetically modified epidermal cells to replace the LAMB3 gene, thereby restoring production of laminin 332, which is an essential component of the dermal-epidermal junction, according to Dr. Mellerio, citing a study in Nature Medicine.
The next attempt with this approach did not take place until 2015, resurrected to save the life of a 7-year-old Syrian boy – to generate epidermal sheets that eventually covered 80% of his body. The success is supporting further work on this approach but has also been an inspiration to other gene therapies, including a topical gene therapy recently approved in the United States.
Topically applied beremagene geperpavec (Vyjuvek, formerly known as B-VEC) was approved by the FDA in May for treating wounds in patients 6 months of age and older, with recessive or dominant dystrophic EB, on the basis of a phase 3 trial published in the New England Journal of Medicine, but others are coming. Dr. Mellerio also described a recently completed phase 3 trial with introduction of ex vivo gene-corrected keratinocytes, which has been associated with long-term improvements among patients with recessive dystrophic EB (RDEB). The responses in early phase studies included wound healing and reduction in pain and itch.
Perhaps less advanced but still promising, protein therapy, gene editing, and repurposing of existing therapies are all approaches that are moving forward. Many are supported by at least some clinical data, according to Dr. Mellerio.
As an example of protein therapy, a completed phase I/II trial associated recombinant human collagen with wound healing and pain reduction in RDEB. This study provided proof of principle for a therapy that could be applied topically or intravenously. Further development is anticipated.
Multiple platforms for gene editing have been described with the goal of simply excising pathogenic mutations or antisense oligonucleotides for sustained or permanent control of EB expression. Clinical evidence is limited, but Dr. Mellerio suggested that the theoretical potential for eliminating the source of abnormal transcription is the restoration of functional proteins essential for reversing skin fragility.
In some cases, existing drugs have the same potential. Dr. Mellerio described efforts to use an aminoglycoside to circumvent nonsense mutations that produce messenger RNA decay and impaired production of the proteins that prevent EB. In a pilot study evaluating topical gentamicin in RDEB, there were substantial improvements at 1 month and 3 months in several measures of skin fragility and encouraged studies that are now ongoing in both RDEB and JEB.
More than promising, a multinational randomized phase 3 study with birch bark extract recently published in the British Journal of Dermatology, associated treatment with this topical gel, known as Oleogel-S10, with higher rates of complete wound closure at 45 days (41.3% vs. 28.9% in the control vehicle arm) and a low risk of adverse events.
“This therapy is now approved in Europe and the United Kingdom, although, unfortunately, it is not yet available in the United States,” Dr. Mellerio noted.
Importantly, none of these therapies are necessarily effective across subtypes of EB, which often have different underlying pathogenic mechanisms, she said. However, the growing sophistication with which the pathophysiology of these subtypes is understood makes the numerous treatments in the pipeline “exciting.”
“We are at a point where we can really start to think of personalized medicine in EB,” Dr. Mellerio said. With the clinical advances already available and those expected, she suggested the recently approved treatment options are just the beginning. She expects the treatment landscape to evolve quickly over the next few years.
This does not appear to be a personal opinion. Another prominent researcher in EB, M. Peter Marinkovich, MD, director of the Stanford Bullous Disease and Psoriasis Clinics at Stanford (Calif.) University, is seeing the same real-world promise of therapies that have been in gestation for a decade or more.
“Dr. Mellerio is right. This is an exciting time for EB patients,” Dr. Marinkovich said in an interview. While the approval of B-VEC, the first gene therapy for EB, is the proof, Dr. Marinkovich, the lead author of the NEJM paper on B-VEC, noted that “many other potential EB therapies are being studied right now.” Based on promise in earlier clinical studies with many of these agents, he, like Dr. Mellerio, expects progress in real-world treatments for EB to accelerate.
Dr. Mellerio reported financial relationships with Amryt Pharma and Krystal Biotech. Dr. Marinkovich receives research support from Abeona Therapeutics, Castle Creek Pharmaceuticals, Krystal Biotech, Phoenix Tissue Repair, and WINGS Therapeutics.
A version of this article first appeared on Medscape.com.
ASHEVILLE, N.C. – , according to a prominent EB researcher.
Not only are recent developments in EB “exciting,” the progress on multiple fronts for control of disease or its symptoms suggests “we are on the cusp of a new era,” Jemima Mellerio, BSc, MD, a consultant dermatologist, St. John’s Institute of Dermatology, London, said at the annual meeting of the Society for Pediatric Dermatology.
Published clinical studies of cell therapies and gene therapies date back at least 15 years, according to a review by Dr. Mellerio on why developments are starting to move so quickly. The difference now is that many obstacles to routine use of these options are being resolved so that viable strategies have reached or are reaching phase 3 trials.
In addition to cell therapies and gene therapies, Dr. Mellerio discussed progress in three additional areas: gene editing, protein therapy, and drug repurposing.
Summarizing progress in each, she described improvement in levels of collagen VII, an important deficit in most types of EB, that were achieved with fibroblast injections that improved levels of collagen VII and anchoring fibrils in a study published in the Journal of Investigative Dermatology. Injection of mesenchymal stromal cells (MSC) have been associated with reduced pain and itch in a series of studies, one of the earliest of which was published in the New England Journal of Medicine.
Since that time, there have been several approaches using MSC.
Of these approaches, intravenous injection of ABCB5+ MSCs might be the first to gain regulatory approval. According to Dr. Mellerio, there is an ongoing phase 3 crossover trial evaluating this approach, which followed several earlier phase studies that demonstrated adequate safety and tolerability while reducing severity scores, relieving pain and itch, and improving wound closure in patients with EB.
In 2006, correction of junctional EB (JEB) was achieved by transplantation of genetically modified epidermal cells to replace the LAMB3 gene, thereby restoring production of laminin 332, which is an essential component of the dermal-epidermal junction, according to Dr. Mellerio, citing a study in Nature Medicine.
The next attempt with this approach did not take place until 2015, resurrected to save the life of a 7-year-old Syrian boy – to generate epidermal sheets that eventually covered 80% of his body. The success is supporting further work on this approach but has also been an inspiration to other gene therapies, including a topical gene therapy recently approved in the United States.
Topically applied beremagene geperpavec (Vyjuvek, formerly known as B-VEC) was approved by the FDA in May for treating wounds in patients 6 months of age and older, with recessive or dominant dystrophic EB, on the basis of a phase 3 trial published in the New England Journal of Medicine, but others are coming. Dr. Mellerio also described a recently completed phase 3 trial with introduction of ex vivo gene-corrected keratinocytes, which has been associated with long-term improvements among patients with recessive dystrophic EB (RDEB). The responses in early phase studies included wound healing and reduction in pain and itch.
Perhaps less advanced but still promising, protein therapy, gene editing, and repurposing of existing therapies are all approaches that are moving forward. Many are supported by at least some clinical data, according to Dr. Mellerio.
As an example of protein therapy, a completed phase I/II trial associated recombinant human collagen with wound healing and pain reduction in RDEB. This study provided proof of principle for a therapy that could be applied topically or intravenously. Further development is anticipated.
Multiple platforms for gene editing have been described with the goal of simply excising pathogenic mutations or antisense oligonucleotides for sustained or permanent control of EB expression. Clinical evidence is limited, but Dr. Mellerio suggested that the theoretical potential for eliminating the source of abnormal transcription is the restoration of functional proteins essential for reversing skin fragility.
In some cases, existing drugs have the same potential. Dr. Mellerio described efforts to use an aminoglycoside to circumvent nonsense mutations that produce messenger RNA decay and impaired production of the proteins that prevent EB. In a pilot study evaluating topical gentamicin in RDEB, there were substantial improvements at 1 month and 3 months in several measures of skin fragility and encouraged studies that are now ongoing in both RDEB and JEB.
More than promising, a multinational randomized phase 3 study with birch bark extract recently published in the British Journal of Dermatology, associated treatment with this topical gel, known as Oleogel-S10, with higher rates of complete wound closure at 45 days (41.3% vs. 28.9% in the control vehicle arm) and a low risk of adverse events.
“This therapy is now approved in Europe and the United Kingdom, although, unfortunately, it is not yet available in the United States,” Dr. Mellerio noted.
Importantly, none of these therapies are necessarily effective across subtypes of EB, which often have different underlying pathogenic mechanisms, she said. However, the growing sophistication with which the pathophysiology of these subtypes is understood makes the numerous treatments in the pipeline “exciting.”
“We are at a point where we can really start to think of personalized medicine in EB,” Dr. Mellerio said. With the clinical advances already available and those expected, she suggested the recently approved treatment options are just the beginning. She expects the treatment landscape to evolve quickly over the next few years.
This does not appear to be a personal opinion. Another prominent researcher in EB, M. Peter Marinkovich, MD, director of the Stanford Bullous Disease and Psoriasis Clinics at Stanford (Calif.) University, is seeing the same real-world promise of therapies that have been in gestation for a decade or more.
“Dr. Mellerio is right. This is an exciting time for EB patients,” Dr. Marinkovich said in an interview. While the approval of B-VEC, the first gene therapy for EB, is the proof, Dr. Marinkovich, the lead author of the NEJM paper on B-VEC, noted that “many other potential EB therapies are being studied right now.” Based on promise in earlier clinical studies with many of these agents, he, like Dr. Mellerio, expects progress in real-world treatments for EB to accelerate.
Dr. Mellerio reported financial relationships with Amryt Pharma and Krystal Biotech. Dr. Marinkovich receives research support from Abeona Therapeutics, Castle Creek Pharmaceuticals, Krystal Biotech, Phoenix Tissue Repair, and WINGS Therapeutics.
A version of this article first appeared on Medscape.com.
ASHEVILLE, N.C. – , according to a prominent EB researcher.
Not only are recent developments in EB “exciting,” the progress on multiple fronts for control of disease or its symptoms suggests “we are on the cusp of a new era,” Jemima Mellerio, BSc, MD, a consultant dermatologist, St. John’s Institute of Dermatology, London, said at the annual meeting of the Society for Pediatric Dermatology.
Published clinical studies of cell therapies and gene therapies date back at least 15 years, according to a review by Dr. Mellerio on why developments are starting to move so quickly. The difference now is that many obstacles to routine use of these options are being resolved so that viable strategies have reached or are reaching phase 3 trials.
In addition to cell therapies and gene therapies, Dr. Mellerio discussed progress in three additional areas: gene editing, protein therapy, and drug repurposing.
Summarizing progress in each, she described improvement in levels of collagen VII, an important deficit in most types of EB, that were achieved with fibroblast injections that improved levels of collagen VII and anchoring fibrils in a study published in the Journal of Investigative Dermatology. Injection of mesenchymal stromal cells (MSC) have been associated with reduced pain and itch in a series of studies, one of the earliest of which was published in the New England Journal of Medicine.
Since that time, there have been several approaches using MSC.
Of these approaches, intravenous injection of ABCB5+ MSCs might be the first to gain regulatory approval. According to Dr. Mellerio, there is an ongoing phase 3 crossover trial evaluating this approach, which followed several earlier phase studies that demonstrated adequate safety and tolerability while reducing severity scores, relieving pain and itch, and improving wound closure in patients with EB.
In 2006, correction of junctional EB (JEB) was achieved by transplantation of genetically modified epidermal cells to replace the LAMB3 gene, thereby restoring production of laminin 332, which is an essential component of the dermal-epidermal junction, according to Dr. Mellerio, citing a study in Nature Medicine.
The next attempt with this approach did not take place until 2015, resurrected to save the life of a 7-year-old Syrian boy – to generate epidermal sheets that eventually covered 80% of his body. The success is supporting further work on this approach but has also been an inspiration to other gene therapies, including a topical gene therapy recently approved in the United States.
Topically applied beremagene geperpavec (Vyjuvek, formerly known as B-VEC) was approved by the FDA in May for treating wounds in patients 6 months of age and older, with recessive or dominant dystrophic EB, on the basis of a phase 3 trial published in the New England Journal of Medicine, but others are coming. Dr. Mellerio also described a recently completed phase 3 trial with introduction of ex vivo gene-corrected keratinocytes, which has been associated with long-term improvements among patients with recessive dystrophic EB (RDEB). The responses in early phase studies included wound healing and reduction in pain and itch.
Perhaps less advanced but still promising, protein therapy, gene editing, and repurposing of existing therapies are all approaches that are moving forward. Many are supported by at least some clinical data, according to Dr. Mellerio.
As an example of protein therapy, a completed phase I/II trial associated recombinant human collagen with wound healing and pain reduction in RDEB. This study provided proof of principle for a therapy that could be applied topically or intravenously. Further development is anticipated.
Multiple platforms for gene editing have been described with the goal of simply excising pathogenic mutations or antisense oligonucleotides for sustained or permanent control of EB expression. Clinical evidence is limited, but Dr. Mellerio suggested that the theoretical potential for eliminating the source of abnormal transcription is the restoration of functional proteins essential for reversing skin fragility.
In some cases, existing drugs have the same potential. Dr. Mellerio described efforts to use an aminoglycoside to circumvent nonsense mutations that produce messenger RNA decay and impaired production of the proteins that prevent EB. In a pilot study evaluating topical gentamicin in RDEB, there were substantial improvements at 1 month and 3 months in several measures of skin fragility and encouraged studies that are now ongoing in both RDEB and JEB.
More than promising, a multinational randomized phase 3 study with birch bark extract recently published in the British Journal of Dermatology, associated treatment with this topical gel, known as Oleogel-S10, with higher rates of complete wound closure at 45 days (41.3% vs. 28.9% in the control vehicle arm) and a low risk of adverse events.
“This therapy is now approved in Europe and the United Kingdom, although, unfortunately, it is not yet available in the United States,” Dr. Mellerio noted.
Importantly, none of these therapies are necessarily effective across subtypes of EB, which often have different underlying pathogenic mechanisms, she said. However, the growing sophistication with which the pathophysiology of these subtypes is understood makes the numerous treatments in the pipeline “exciting.”
“We are at a point where we can really start to think of personalized medicine in EB,” Dr. Mellerio said. With the clinical advances already available and those expected, she suggested the recently approved treatment options are just the beginning. She expects the treatment landscape to evolve quickly over the next few years.
This does not appear to be a personal opinion. Another prominent researcher in EB, M. Peter Marinkovich, MD, director of the Stanford Bullous Disease and Psoriasis Clinics at Stanford (Calif.) University, is seeing the same real-world promise of therapies that have been in gestation for a decade or more.
“Dr. Mellerio is right. This is an exciting time for EB patients,” Dr. Marinkovich said in an interview. While the approval of B-VEC, the first gene therapy for EB, is the proof, Dr. Marinkovich, the lead author of the NEJM paper on B-VEC, noted that “many other potential EB therapies are being studied right now.” Based on promise in earlier clinical studies with many of these agents, he, like Dr. Mellerio, expects progress in real-world treatments for EB to accelerate.
Dr. Mellerio reported financial relationships with Amryt Pharma and Krystal Biotech. Dr. Marinkovich receives research support from Abeona Therapeutics, Castle Creek Pharmaceuticals, Krystal Biotech, Phoenix Tissue Repair, and WINGS Therapeutics.
A version of this article first appeared on Medscape.com.
AT SPD 2023
Analysis of a Pilot Curriculum for Business Education in Dermatology Residency
To the Editor:
With health care constituting one of the larger segments of the US economy, medical practice is increasingly subject to business considerations.1 Patients, providers, and organizations are all required to make decisions that reflect choices beyond clinical needs alone. Given the impact of market forces, clinicians often are asked to navigate operational and business decisions. Accordingly, education about the policy and systems that shape care delivery can improve quality and help patients.2
The ability to understand the ecosystem of health care is of utmost importance for medical providers and can be achieved through resident education. Teaching fundamental business concepts enables residents to deliver care that is responsive to the constraints and opportunities encountered by patients and organizations, which ultimately will better prepare them to serve as advocates in alignment with their principal duties as physicians.
Despite the recognizable relationship between business and medicine, training has not yet been standardized to include topics in business education, and clinicians in dermatology are remarkably positioned to benefit because of the variety of practice settings and services they can provide. In dermatology, the diversity of services provided gives rise to complex coding and use of modifiers. Proper utilization of coding and billing is critical to create accurate documentation and receive appropriate reimbursement.3 Furthermore, clinicians in dermatology have to contend with the influence of insurance at many points of care, such as with coverage of pharmaceuticals. Formularies often have wide variability in coverage and are changing as new drugs come to market in the dermatologic space.4
The landscape of practice structure also has undergone change with increasing consolidation and mergers. The acquisition of practices by private equity firms has induced changes in practice infrastructure. The impact of changing organizational and managerial influences continues to be a topic of debate, with disparate opinions on how these developments shape standards of physician satisfaction and patient care.5
The convergence of these factors points to an important question that is gaining popularity: How will young dermatologists work within the context of all these parameters to best advocate and care for their patients? These questions are garnering more attention and were recently investigated through a survey of participants in a pilot program to evaluate the importance of business education in dermatology residency.
A survey of residency program directors was created by Patrinley and Dewan,6 which found that business education during residency was important and additional training should be implemented. Despite the perceived importance of business education, only half of the programs represented by survey respondents offered any structured educational opportunities, revealing a discrepancy between believed importance and practical implementation of business training, which suggests the need to develop a standardized, dermatology-specific curriculum that could be accessed by all residents in training.6
We performed a search of the medical literature to identify models of business education in residency programs. Only a few programs were identified, in which courses were predominantly instructed to trainees in primary care–based fields. According to course graduates, the programs were beneficial.7,8 Programs that had descriptive information about curriculum structure and content were chosen for further investigation and included internal medicine programs at the University of California San Francisco (UCSF) and Columbia University Vagelos College of Physicians and Surgeons (New York, New York). UCSF implemented a Program in Residency Investigation Methods and Epidemiology (PRIME program) to deliver seven 90-minute sessions dedicated to introducing residents to medical economics. Sessions were constructed with the intent of being interactive seminars that took on a variety of forms, including reading-based discussions, case-based analysis, and simulation-based learning.7 Columbia University developed a pilot program of week-long didactic sessions that were delivered to third-year internal medicine residents. These seminars featured discussions on health policy and economics, health insurance, technology and cost assessment, legal medicine, public health, community-oriented primary care, and local health department initiatives.8 We drew on both courses to build a lecture series focused on the business of dermatology that was delivered to dermatology residents at UMass Chan Medical School (Worcester, Massachusetts). Topic selection also was informed by qualitative input collected via email from recent graduates of the UMass dermatology residency program, focusing on the following areas: the US medical economy and health care costs; billing, coding, and claims processing; quality, relative value units (RVUs), reimbursement, and the merit-based incentive payment system; coverage of pharmaceuticals and teledermatology; and management. Residents were not required to prepare for any of the sessions; they were provided with handouts and slideshow presentations for reference to review at their convenience if desired. Five seminars were virtually conducted by an MD/MBA candidate at the institution (E.H.). They were recorded over the course of an academic year at 1- to 2-month intervals. Each 45-minute session was conducted in a lecture-discussion format and included case examples to help illustrate key principles and stimulate conversation. For example, the lecture on reimbursement incorporated a fee schedule calculation for a shave biopsy, using RVU and geographic pricing cost index (GCPI) multipliers. This demonstrated the variation in Centers for Medicare & Medicaid Services reimbursement in relation to (1) constituents of the RVU calculation (ie, work, practice expense, and malpractice) and (2) practice in a particular location (ie, the GCPI). Following this example, a conversation ensued among participants regarding the factors that drive valuation, with particular interest in variation based on urban vs suburban locations across the United States. Participants also found it of interest to examine the percentage of the valuation dedicated to each constituent and how features such as lesion size informed the final assessment of the charge. Another stylistic choice in developing the model was to include prompts for further consideration prior to transitioning topics in the lectures. For example: when examining the burden of skin disease, the audience was prompted to consider: “What is driving cost escalations, and how will services of the clinical domain meet these evolving needs?” At another point in the introductory lecture, residents were asked: “How do different types of insurance plans impact the management of patients with dermatologic concerns?” These questions were intended to transition residents to the next topic of discussion and highlight take-home points of consideration for medical practice. The project was reviewed by the UMass institutional review board and met criteria for exemption.
Residents who participated in at least 1 lecture (N=10) were surveyed after attendance; there were 7 responses (70% response rate). Residents were asked to rate a series of statements on a scale of 1 (strongly disagree) to 5 (strongly agree) and to provide commentary via an online form. Respondents indicated that the course was enjoyable (average score, 4.00), provided an appropriate level of detail (average score, 4.00), would be beneficial to integrate into a dermatology residency curriculum (average score, 3.86), and informed how they would practice as a clinician (average score, 3.86)(Figure). The respondents agreed that the course met the main goals of this initiative: it helped them develop knowledge about the interface between business and dermatology (4.14) and exposed residents to topics they had not learned about previously (4.71).
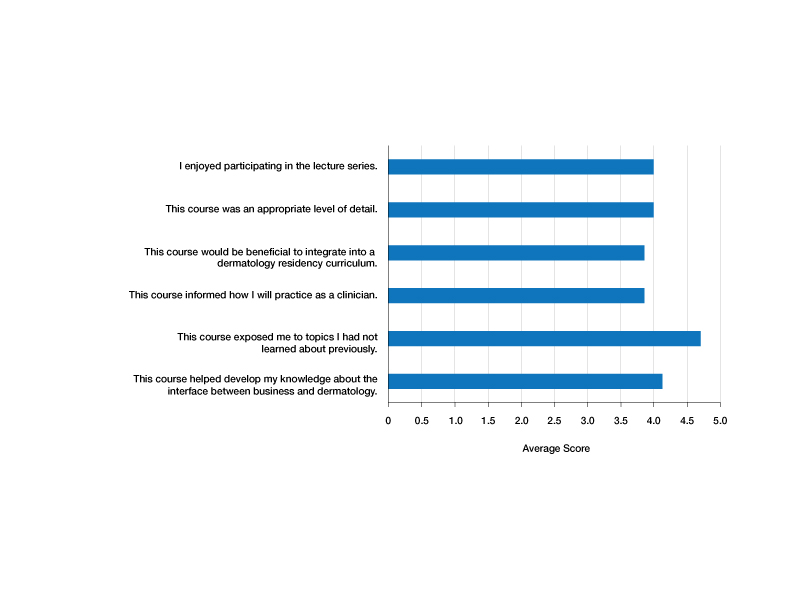
Although the course generally was well received, areas for improvement were identified from respondents’ comments, relating to audience engagement and refining the level of detail in the lectures. Recommendations included “less technical jargon and more focus on ‘big picture’ concepts, given audience’s low baseline knowledge”; “more case examples in each module”; and “more diagrams or interactive activities (polls, quizzes, break-out rooms) because the lectures were a bit dense.” This input was taken into consideration when revising the lectures for future use; they were reconstructed to have more case-based examples and prompts to encourage participation.
Resident commentary also demonstrated appreciation for education in this subject material. Statements such as “this is an important topic for future dermatologists” and “thank you so much for taking the time to implement this course” reflected the perceived value of this material during critical academic time. Another resident remarked: “This was great, thanks for putting it together.”
Given the positive experience of the residents and successful implementation of the series, this course was made available to all dermatology trainees on a network server with accompanying written documents. It is planned to be offered on a 3-year cycle in the future and will be updated to reflect inevitable changes in health care.
Although the relationship between business and medicine is increasingly important, teaching business principles has not become standardized or required in medical training. Despite the perception that this content is of value, implementation of programming has lagged behind that recognition, likely due to challenges in designing the curriculum and diffusing content into an already-saturated schedule. A model course that can be replicated in other residency programs would be valuable. We introduced a dermatology-specific lecture series to help prepare trainees for dermatology practice in a variety of clinical settings and train them with the language of business and operations that will equip them to respond to the needs of their patients, their practice, and the medical environment. Findings of this pilot study may not be generalizable to all dermatology residency programs because the sample size was small; the study was conducted at a single institution; and the content was delivered entirely online.
1. Tan S, Seiger K, Renehan P, et al. Trends in private equity acquisition of dermatology practices in the United States. JAMA Dermatol. 2019;155:1013-1021. doi:10.1001/jamadermatol.2019.1634
2. The business of health care in the United States. Harvard Online [Internet]. June 27, 2022. Accessed July 24, 2023. https://www.harvardonline.harvard.edu/blog/business-health-care-united-states
3. Ranpariya V, Cull D, Feldman SR, et al. Evaluation and management 2021 coding guidelines: key changes and implications. The Dermatologist. December 2020. Accessed July 24, 2023. https://www.hmpgloballearningnetwork.com/site/thederm/article/evaluation-and-management-2021-coding-guidelines-key-changes-and-implications?key=Ranpariya&elastic%5B0%5D=brand%3A73468
4. Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2. doi:10.1016/j.jaad.2016.12.043
5. Resneck JS Jr. Dermatology practice consolidation fueled by private equity investment: potential consequences for the specialty and patients. JAMA Dermatol. 2018;154:13-14. doi:10.1001/jamadermatol.2017.5558
6. Patrinely JR Jr, Dewan AK. Business education in dermatology residency: a survey of program directors. Cutis. 2021;108:E7-E19. doi:10.12788/cutis.0331
7. Kohlwes RJ, Chou CL. A curriculum in medical economics for residents. Acad Med. 2002;77:465-466. doi:10.1097/00001888-200205000-00040
8. Fiebach NH, Rao D, Hamm ME. A curriculum in health systems and public health for internal medicine residents. Am J Prev Med. 2011;41(4 suppl 3):S264-S269. doi:10.1016/j.amepre.2011.05.025
To the Editor:
With health care constituting one of the larger segments of the US economy, medical practice is increasingly subject to business considerations.1 Patients, providers, and organizations are all required to make decisions that reflect choices beyond clinical needs alone. Given the impact of market forces, clinicians often are asked to navigate operational and business decisions. Accordingly, education about the policy and systems that shape care delivery can improve quality and help patients.2
The ability to understand the ecosystem of health care is of utmost importance for medical providers and can be achieved through resident education. Teaching fundamental business concepts enables residents to deliver care that is responsive to the constraints and opportunities encountered by patients and organizations, which ultimately will better prepare them to serve as advocates in alignment with their principal duties as physicians.
Despite the recognizable relationship between business and medicine, training has not yet been standardized to include topics in business education, and clinicians in dermatology are remarkably positioned to benefit because of the variety of practice settings and services they can provide. In dermatology, the diversity of services provided gives rise to complex coding and use of modifiers. Proper utilization of coding and billing is critical to create accurate documentation and receive appropriate reimbursement.3 Furthermore, clinicians in dermatology have to contend with the influence of insurance at many points of care, such as with coverage of pharmaceuticals. Formularies often have wide variability in coverage and are changing as new drugs come to market in the dermatologic space.4
The landscape of practice structure also has undergone change with increasing consolidation and mergers. The acquisition of practices by private equity firms has induced changes in practice infrastructure. The impact of changing organizational and managerial influences continues to be a topic of debate, with disparate opinions on how these developments shape standards of physician satisfaction and patient care.5
The convergence of these factors points to an important question that is gaining popularity: How will young dermatologists work within the context of all these parameters to best advocate and care for their patients? These questions are garnering more attention and were recently investigated through a survey of participants in a pilot program to evaluate the importance of business education in dermatology residency.
A survey of residency program directors was created by Patrinley and Dewan,6 which found that business education during residency was important and additional training should be implemented. Despite the perceived importance of business education, only half of the programs represented by survey respondents offered any structured educational opportunities, revealing a discrepancy between believed importance and practical implementation of business training, which suggests the need to develop a standardized, dermatology-specific curriculum that could be accessed by all residents in training.6
We performed a search of the medical literature to identify models of business education in residency programs. Only a few programs were identified, in which courses were predominantly instructed to trainees in primary care–based fields. According to course graduates, the programs were beneficial.7,8 Programs that had descriptive information about curriculum structure and content were chosen for further investigation and included internal medicine programs at the University of California San Francisco (UCSF) and Columbia University Vagelos College of Physicians and Surgeons (New York, New York). UCSF implemented a Program in Residency Investigation Methods and Epidemiology (PRIME program) to deliver seven 90-minute sessions dedicated to introducing residents to medical economics. Sessions were constructed with the intent of being interactive seminars that took on a variety of forms, including reading-based discussions, case-based analysis, and simulation-based learning.7 Columbia University developed a pilot program of week-long didactic sessions that were delivered to third-year internal medicine residents. These seminars featured discussions on health policy and economics, health insurance, technology and cost assessment, legal medicine, public health, community-oriented primary care, and local health department initiatives.8 We drew on both courses to build a lecture series focused on the business of dermatology that was delivered to dermatology residents at UMass Chan Medical School (Worcester, Massachusetts). Topic selection also was informed by qualitative input collected via email from recent graduates of the UMass dermatology residency program, focusing on the following areas: the US medical economy and health care costs; billing, coding, and claims processing; quality, relative value units (RVUs), reimbursement, and the merit-based incentive payment system; coverage of pharmaceuticals and teledermatology; and management. Residents were not required to prepare for any of the sessions; they were provided with handouts and slideshow presentations for reference to review at their convenience if desired. Five seminars were virtually conducted by an MD/MBA candidate at the institution (E.H.). They were recorded over the course of an academic year at 1- to 2-month intervals. Each 45-minute session was conducted in a lecture-discussion format and included case examples to help illustrate key principles and stimulate conversation. For example, the lecture on reimbursement incorporated a fee schedule calculation for a shave biopsy, using RVU and geographic pricing cost index (GCPI) multipliers. This demonstrated the variation in Centers for Medicare & Medicaid Services reimbursement in relation to (1) constituents of the RVU calculation (ie, work, practice expense, and malpractice) and (2) practice in a particular location (ie, the GCPI). Following this example, a conversation ensued among participants regarding the factors that drive valuation, with particular interest in variation based on urban vs suburban locations across the United States. Participants also found it of interest to examine the percentage of the valuation dedicated to each constituent and how features such as lesion size informed the final assessment of the charge. Another stylistic choice in developing the model was to include prompts for further consideration prior to transitioning topics in the lectures. For example: when examining the burden of skin disease, the audience was prompted to consider: “What is driving cost escalations, and how will services of the clinical domain meet these evolving needs?” At another point in the introductory lecture, residents were asked: “How do different types of insurance plans impact the management of patients with dermatologic concerns?” These questions were intended to transition residents to the next topic of discussion and highlight take-home points of consideration for medical practice. The project was reviewed by the UMass institutional review board and met criteria for exemption.
Residents who participated in at least 1 lecture (N=10) were surveyed after attendance; there were 7 responses (70% response rate). Residents were asked to rate a series of statements on a scale of 1 (strongly disagree) to 5 (strongly agree) and to provide commentary via an online form. Respondents indicated that the course was enjoyable (average score, 4.00), provided an appropriate level of detail (average score, 4.00), would be beneficial to integrate into a dermatology residency curriculum (average score, 3.86), and informed how they would practice as a clinician (average score, 3.86)(Figure). The respondents agreed that the course met the main goals of this initiative: it helped them develop knowledge about the interface between business and dermatology (4.14) and exposed residents to topics they had not learned about previously (4.71).

Although the course generally was well received, areas for improvement were identified from respondents’ comments, relating to audience engagement and refining the level of detail in the lectures. Recommendations included “less technical jargon and more focus on ‘big picture’ concepts, given audience’s low baseline knowledge”; “more case examples in each module”; and “more diagrams or interactive activities (polls, quizzes, break-out rooms) because the lectures were a bit dense.” This input was taken into consideration when revising the lectures for future use; they were reconstructed to have more case-based examples and prompts to encourage participation.
Resident commentary also demonstrated appreciation for education in this subject material. Statements such as “this is an important topic for future dermatologists” and “thank you so much for taking the time to implement this course” reflected the perceived value of this material during critical academic time. Another resident remarked: “This was great, thanks for putting it together.”
Given the positive experience of the residents and successful implementation of the series, this course was made available to all dermatology trainees on a network server with accompanying written documents. It is planned to be offered on a 3-year cycle in the future and will be updated to reflect inevitable changes in health care.
Although the relationship between business and medicine is increasingly important, teaching business principles has not become standardized or required in medical training. Despite the perception that this content is of value, implementation of programming has lagged behind that recognition, likely due to challenges in designing the curriculum and diffusing content into an already-saturated schedule. A model course that can be replicated in other residency programs would be valuable. We introduced a dermatology-specific lecture series to help prepare trainees for dermatology practice in a variety of clinical settings and train them with the language of business and operations that will equip them to respond to the needs of their patients, their practice, and the medical environment. Findings of this pilot study may not be generalizable to all dermatology residency programs because the sample size was small; the study was conducted at a single institution; and the content was delivered entirely online.
To the Editor:
With health care constituting one of the larger segments of the US economy, medical practice is increasingly subject to business considerations.1 Patients, providers, and organizations are all required to make decisions that reflect choices beyond clinical needs alone. Given the impact of market forces, clinicians often are asked to navigate operational and business decisions. Accordingly, education about the policy and systems that shape care delivery can improve quality and help patients.2
The ability to understand the ecosystem of health care is of utmost importance for medical providers and can be achieved through resident education. Teaching fundamental business concepts enables residents to deliver care that is responsive to the constraints and opportunities encountered by patients and organizations, which ultimately will better prepare them to serve as advocates in alignment with their principal duties as physicians.
Despite the recognizable relationship between business and medicine, training has not yet been standardized to include topics in business education, and clinicians in dermatology are remarkably positioned to benefit because of the variety of practice settings and services they can provide. In dermatology, the diversity of services provided gives rise to complex coding and use of modifiers. Proper utilization of coding and billing is critical to create accurate documentation and receive appropriate reimbursement.3 Furthermore, clinicians in dermatology have to contend with the influence of insurance at many points of care, such as with coverage of pharmaceuticals. Formularies often have wide variability in coverage and are changing as new drugs come to market in the dermatologic space.4
The landscape of practice structure also has undergone change with increasing consolidation and mergers. The acquisition of practices by private equity firms has induced changes in practice infrastructure. The impact of changing organizational and managerial influences continues to be a topic of debate, with disparate opinions on how these developments shape standards of physician satisfaction and patient care.5
The convergence of these factors points to an important question that is gaining popularity: How will young dermatologists work within the context of all these parameters to best advocate and care for their patients? These questions are garnering more attention and were recently investigated through a survey of participants in a pilot program to evaluate the importance of business education in dermatology residency.
A survey of residency program directors was created by Patrinley and Dewan,6 which found that business education during residency was important and additional training should be implemented. Despite the perceived importance of business education, only half of the programs represented by survey respondents offered any structured educational opportunities, revealing a discrepancy between believed importance and practical implementation of business training, which suggests the need to develop a standardized, dermatology-specific curriculum that could be accessed by all residents in training.6
We performed a search of the medical literature to identify models of business education in residency programs. Only a few programs were identified, in which courses were predominantly instructed to trainees in primary care–based fields. According to course graduates, the programs were beneficial.7,8 Programs that had descriptive information about curriculum structure and content were chosen for further investigation and included internal medicine programs at the University of California San Francisco (UCSF) and Columbia University Vagelos College of Physicians and Surgeons (New York, New York). UCSF implemented a Program in Residency Investigation Methods and Epidemiology (PRIME program) to deliver seven 90-minute sessions dedicated to introducing residents to medical economics. Sessions were constructed with the intent of being interactive seminars that took on a variety of forms, including reading-based discussions, case-based analysis, and simulation-based learning.7 Columbia University developed a pilot program of week-long didactic sessions that were delivered to third-year internal medicine residents. These seminars featured discussions on health policy and economics, health insurance, technology and cost assessment, legal medicine, public health, community-oriented primary care, and local health department initiatives.8 We drew on both courses to build a lecture series focused on the business of dermatology that was delivered to dermatology residents at UMass Chan Medical School (Worcester, Massachusetts). Topic selection also was informed by qualitative input collected via email from recent graduates of the UMass dermatology residency program, focusing on the following areas: the US medical economy and health care costs; billing, coding, and claims processing; quality, relative value units (RVUs), reimbursement, and the merit-based incentive payment system; coverage of pharmaceuticals and teledermatology; and management. Residents were not required to prepare for any of the sessions; they were provided with handouts and slideshow presentations for reference to review at their convenience if desired. Five seminars were virtually conducted by an MD/MBA candidate at the institution (E.H.). They were recorded over the course of an academic year at 1- to 2-month intervals. Each 45-minute session was conducted in a lecture-discussion format and included case examples to help illustrate key principles and stimulate conversation. For example, the lecture on reimbursement incorporated a fee schedule calculation for a shave biopsy, using RVU and geographic pricing cost index (GCPI) multipliers. This demonstrated the variation in Centers for Medicare & Medicaid Services reimbursement in relation to (1) constituents of the RVU calculation (ie, work, practice expense, and malpractice) and (2) practice in a particular location (ie, the GCPI). Following this example, a conversation ensued among participants regarding the factors that drive valuation, with particular interest in variation based on urban vs suburban locations across the United States. Participants also found it of interest to examine the percentage of the valuation dedicated to each constituent and how features such as lesion size informed the final assessment of the charge. Another stylistic choice in developing the model was to include prompts for further consideration prior to transitioning topics in the lectures. For example: when examining the burden of skin disease, the audience was prompted to consider: “What is driving cost escalations, and how will services of the clinical domain meet these evolving needs?” At another point in the introductory lecture, residents were asked: “How do different types of insurance plans impact the management of patients with dermatologic concerns?” These questions were intended to transition residents to the next topic of discussion and highlight take-home points of consideration for medical practice. The project was reviewed by the UMass institutional review board and met criteria for exemption.
Residents who participated in at least 1 lecture (N=10) were surveyed after attendance; there were 7 responses (70% response rate). Residents were asked to rate a series of statements on a scale of 1 (strongly disagree) to 5 (strongly agree) and to provide commentary via an online form. Respondents indicated that the course was enjoyable (average score, 4.00), provided an appropriate level of detail (average score, 4.00), would be beneficial to integrate into a dermatology residency curriculum (average score, 3.86), and informed how they would practice as a clinician (average score, 3.86)(Figure). The respondents agreed that the course met the main goals of this initiative: it helped them develop knowledge about the interface between business and dermatology (4.14) and exposed residents to topics they had not learned about previously (4.71).

Although the course generally was well received, areas for improvement were identified from respondents’ comments, relating to audience engagement and refining the level of detail in the lectures. Recommendations included “less technical jargon and more focus on ‘big picture’ concepts, given audience’s low baseline knowledge”; “more case examples in each module”; and “more diagrams or interactive activities (polls, quizzes, break-out rooms) because the lectures were a bit dense.” This input was taken into consideration when revising the lectures for future use; they were reconstructed to have more case-based examples and prompts to encourage participation.
Resident commentary also demonstrated appreciation for education in this subject material. Statements such as “this is an important topic for future dermatologists” and “thank you so much for taking the time to implement this course” reflected the perceived value of this material during critical academic time. Another resident remarked: “This was great, thanks for putting it together.”
Given the positive experience of the residents and successful implementation of the series, this course was made available to all dermatology trainees on a network server with accompanying written documents. It is planned to be offered on a 3-year cycle in the future and will be updated to reflect inevitable changes in health care.
Although the relationship between business and medicine is increasingly important, teaching business principles has not become standardized or required in medical training. Despite the perception that this content is of value, implementation of programming has lagged behind that recognition, likely due to challenges in designing the curriculum and diffusing content into an already-saturated schedule. A model course that can be replicated in other residency programs would be valuable. We introduced a dermatology-specific lecture series to help prepare trainees for dermatology practice in a variety of clinical settings and train them with the language of business and operations that will equip them to respond to the needs of their patients, their practice, and the medical environment. Findings of this pilot study may not be generalizable to all dermatology residency programs because the sample size was small; the study was conducted at a single institution; and the content was delivered entirely online.
1. Tan S, Seiger K, Renehan P, et al. Trends in private equity acquisition of dermatology practices in the United States. JAMA Dermatol. 2019;155:1013-1021. doi:10.1001/jamadermatol.2019.1634
2. The business of health care in the United States. Harvard Online [Internet]. June 27, 2022. Accessed July 24, 2023. https://www.harvardonline.harvard.edu/blog/business-health-care-united-states
3. Ranpariya V, Cull D, Feldman SR, et al. Evaluation and management 2021 coding guidelines: key changes and implications. The Dermatologist. December 2020. Accessed July 24, 2023. https://www.hmpgloballearningnetwork.com/site/thederm/article/evaluation-and-management-2021-coding-guidelines-key-changes-and-implications?key=Ranpariya&elastic%5B0%5D=brand%3A73468
4. Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2. doi:10.1016/j.jaad.2016.12.043
5. Resneck JS Jr. Dermatology practice consolidation fueled by private equity investment: potential consequences for the specialty and patients. JAMA Dermatol. 2018;154:13-14. doi:10.1001/jamadermatol.2017.5558
6. Patrinely JR Jr, Dewan AK. Business education in dermatology residency: a survey of program directors. Cutis. 2021;108:E7-E19. doi:10.12788/cutis.0331
7. Kohlwes RJ, Chou CL. A curriculum in medical economics for residents. Acad Med. 2002;77:465-466. doi:10.1097/00001888-200205000-00040
8. Fiebach NH, Rao D, Hamm ME. A curriculum in health systems and public health for internal medicine residents. Am J Prev Med. 2011;41(4 suppl 3):S264-S269. doi:10.1016/j.amepre.2011.05.025
1. Tan S, Seiger K, Renehan P, et al. Trends in private equity acquisition of dermatology practices in the United States. JAMA Dermatol. 2019;155:1013-1021. doi:10.1001/jamadermatol.2019.1634
2. The business of health care in the United States. Harvard Online [Internet]. June 27, 2022. Accessed July 24, 2023. https://www.harvardonline.harvard.edu/blog/business-health-care-united-states
3. Ranpariya V, Cull D, Feldman SR, et al. Evaluation and management 2021 coding guidelines: key changes and implications. The Dermatologist. December 2020. Accessed July 24, 2023. https://www.hmpgloballearningnetwork.com/site/thederm/article/evaluation-and-management-2021-coding-guidelines-key-changes-and-implications?key=Ranpariya&elastic%5B0%5D=brand%3A73468
4. Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2. doi:10.1016/j.jaad.2016.12.043
5. Resneck JS Jr. Dermatology practice consolidation fueled by private equity investment: potential consequences for the specialty and patients. JAMA Dermatol. 2018;154:13-14. doi:10.1001/jamadermatol.2017.5558
6. Patrinely JR Jr, Dewan AK. Business education in dermatology residency: a survey of program directors. Cutis. 2021;108:E7-E19. doi:10.12788/cutis.0331
7. Kohlwes RJ, Chou CL. A curriculum in medical economics for residents. Acad Med. 2002;77:465-466. doi:10.1097/00001888-200205000-00040
8. Fiebach NH, Rao D, Hamm ME. A curriculum in health systems and public health for internal medicine residents. Am J Prev Med. 2011;41(4 suppl 3):S264-S269. doi:10.1016/j.amepre.2011.05.025
Practice Points
- Business education in dermatology residency promotes understanding of the health care ecosystem and can enable residents to more effectively deliver care that is responsive to the needs of their patients.
- Teaching fundamental business principles to residents can inform decision-making on patient, provider, and systems levels.
- A pilot curriculum supports implementation of business education teaching and will be particularly helpful in dermatology.
Adjuvant Scalp Rolling for Patients With Refractory Alopecia Areata
To the Editor:
Alopecia areata (AA) is an autoimmune nonscarring hair loss disorder that can present at any age. Patients with AA have a disproportionately high comorbidity burden and low quality of life, often grappling with anxiety, depression, and psychosocial sequelae involving identity, such as reduced self-esteem.1,2 Although conventional therapies aim to reduce hair loss, none are curative.3 Response to treatment is highly unpredictable, with current data suggesting that up to 50% of patients recover within 1 year while 14% to 25% progress to either alopecia totalis (total scalp hair loss) or alopecia universalis (total body hair loss).4 Options for therapeutic intervention remain limited and vary in safety and effectiveness, warranting further research to identify optimal modalities and minimize side effects. Interestingly, scalp rolling has been used as an adjuvant to topical triamcinolone acetonide.3,5 However, the extent of its effect in combination with other therapies remains unclear. We report 3 pediatric patients with confirmed AA refractory to conventional topical treatment who experienced remarkable scalp hair regrowth after adding biweekly scalp rolling as an adjuvant therapy.
A 7-year-old boy with AA presented with 95% scalp hair loss of 7 months’ duration (Figure 1A)(patient 1). Prior treatments included mometasone solution and clobetasol solution 0.05%. After 3 months of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth after 13 months of treatment (Figure 1B). No pain, bleeding, or other side effects were reported.

An 11-year-old girl with AA presented with 100% hair loss of 7 months’ duration (Figure 2A)(patient 2). Prior treatments included fluocinonide solution and intralesional Kenalog injections. After 4 months of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth after 13 months of treatment (Figure 2B). No pain, bleeding, or other side effects were reported.

A 16-year-old boy with AA presented with 30% hair loss of 4 years’ duration (Figure 3A)(patient 3). Prior treatments included squaric acid and intralesional Kenalog injections. After 2 years of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth at 17 months (Figure 3B). No pain, bleeding, or other side effects were reported.

Scalp rolling—also known as microneedling—provides a multifactorial approach to hair regrowth in patients with AA. The mechanism of action involves both the hair cycle and wound repair pathways by stimulation of the dermal papillae and stem cells.6 Scalp rolling has been observed to induce the expression of several hair growth pathway mediators, such as WNT3A, β-catenin, vascular endothelial growth factor, and WNT10B.7 Wnt/β-catenin pathway signaling is integral to multiple aspects of the hair regrowth process, including hair morphogenesis, follicle regeneration, and growth of the shaft itself.8,9 Scalp rolling causes microinjuries to the skin, thereby diverting blood supply to the follicles and stimulating wound regeneration, a process suggested to induce follicle regeneration. This effect is due to increased expression of vascular endothelial growth factor after cutaneous injury, a mediator of both hair growth and cycling as well as wound repair.7 Adjuvant scalp rolling creates a synergistic effect by facilitating absorption of topical and intralesional therapies. The physical breakdown of dermal capillary barriers creates microchannels that traverse the stratum corneum, improving the permeability of small-molecule substances and allowing for relatively painless and uniform delivery of combination therapies. A secondary benefit is hypertrophy, which counteracts the atrophy caused by topical steroids via collagen induction.7
Additionally, scalp rolling confers minimal risk to the patient, making it safer than conventional pharmacologic therapies such as corticosteroids or Janus kinase (JAK) inhibitors. Although intralesional steroid injections are first-line treatments for limited disease, they can cause pain and skin atrophy.10 In one cohort of 54 patients, topical steroids were inferior to both oral and intralesional treatment, and oral steroids carried a systemic side-effect profile and worsening of comorbidities including hyperglycemia and hypertension as well as negative effects on bone density.11 Baricitinib, a JAK inhibitor, was the first systemic treatment to gain US Food and Drug Administration approval for severe AA.12 However, this novel therapeutic confers adverse effects including infection, acne, and hypercholesterolemia, as reported in the BRAVE-AA trials.13 More broadly, the US Food and Drug Administration warns of serious long-term risks such as cardiovascular events and malignancy.14 Given the tremendous potential of JAK inhibitors, further research is warranted to understand both the efficacy of topical formulations as well as the possible role of scalp rolling as its adjuvant.
Finally, scalp rolling is easily accessible and affordable to patients. Scalp rolling devices are readily available and affordable online, and they can be used autonomously at home. This pragmatic option allows patients to take control of their own treatment course and offers a financially feasible alternative to navigating insurance coverage as well as the need for extra office visits for medication refills and monitoring.
We report 3 cases of the use of scalp rolling as an adjuvant to conventional therapy for refractory AA in young patients. Although prospective research is required to establish causality and characterize age-related trends in treatment response, consideration of scalp rolling as an adjuvant to conventional therapy may help to optimize treatment regimens. Given its low risk for side effects and potential benefits, we recommend scalp rolling for patients with refractory AA.
1. Senna M, Ko J, Tosti A, et al. Alopecia areata treatment patterns, healthcare resource utilization, and comorbidities in the US population using insurance claims. Adv Ther. 2021;38:4646-4658.
2. Huang CH, Fu Y, Chi CC. Health-related quality of life, depression, and self-esteem in patients with androgenetic alopecia: a systematic review and meta-analysis. JAMA Dermatol. 2021;157:963-970.
3. Deepak SH, Shwetha S. Scalp roller therapy in resistant alopecia areata. J Cutan Aesthet Surg. 2014;7:61-62.
4. Darwin E, Hirt PA, Fertig R, et al. Alopecia areata: review of epidemiology, clinical features, pathogenesis, and new treatment options.Int J Trichology. 2018;10:51-60.
5. Ito T, Yoshimasu T, Furukawa F, et al. Three-microneedle device as an effective option for intralesional corticosteroid administration for the treatment of alopecia areata. J Dermatol. 2017;44:304-305.
6. Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
7. Kim YS, Jeong KH, Kim JE, et al. Repeated microneedle stimulation induces enhanced hair growth in a murine model. Ann Dermatol. 2016;28:586-592.
8. Leirós GJ, Attorresi AI, Balañá ME. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br J Dermatol. 2012;166:1035-1042.
9. Myung PS, Takeo M, Ito M, et al. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J Invest Dermatol. 2013;133:31-41.
10. Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1-12.
11. Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276
12.
13. King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699.
14. US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. September 1, 2021.
To the Editor:
Alopecia areata (AA) is an autoimmune nonscarring hair loss disorder that can present at any age. Patients with AA have a disproportionately high comorbidity burden and low quality of life, often grappling with anxiety, depression, and psychosocial sequelae involving identity, such as reduced self-esteem.1,2 Although conventional therapies aim to reduce hair loss, none are curative.3 Response to treatment is highly unpredictable, with current data suggesting that up to 50% of patients recover within 1 year while 14% to 25% progress to either alopecia totalis (total scalp hair loss) or alopecia universalis (total body hair loss).4 Options for therapeutic intervention remain limited and vary in safety and effectiveness, warranting further research to identify optimal modalities and minimize side effects. Interestingly, scalp rolling has been used as an adjuvant to topical triamcinolone acetonide.3,5 However, the extent of its effect in combination with other therapies remains unclear. We report 3 pediatric patients with confirmed AA refractory to conventional topical treatment who experienced remarkable scalp hair regrowth after adding biweekly scalp rolling as an adjuvant therapy.
A 7-year-old boy with AA presented with 95% scalp hair loss of 7 months’ duration (Figure 1A)(patient 1). Prior treatments included mometasone solution and clobetasol solution 0.05%. After 3 months of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth after 13 months of treatment (Figure 1B). No pain, bleeding, or other side effects were reported.

An 11-year-old girl with AA presented with 100% hair loss of 7 months’ duration (Figure 2A)(patient 2). Prior treatments included fluocinonide solution and intralesional Kenalog injections. After 4 months of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth after 13 months of treatment (Figure 2B). No pain, bleeding, or other side effects were reported.

A 16-year-old boy with AA presented with 30% hair loss of 4 years’ duration (Figure 3A)(patient 3). Prior treatments included squaric acid and intralesional Kenalog injections. After 2 years of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth at 17 months (Figure 3B). No pain, bleeding, or other side effects were reported.

Scalp rolling—also known as microneedling—provides a multifactorial approach to hair regrowth in patients with AA. The mechanism of action involves both the hair cycle and wound repair pathways by stimulation of the dermal papillae and stem cells.6 Scalp rolling has been observed to induce the expression of several hair growth pathway mediators, such as WNT3A, β-catenin, vascular endothelial growth factor, and WNT10B.7 Wnt/β-catenin pathway signaling is integral to multiple aspects of the hair regrowth process, including hair morphogenesis, follicle regeneration, and growth of the shaft itself.8,9 Scalp rolling causes microinjuries to the skin, thereby diverting blood supply to the follicles and stimulating wound regeneration, a process suggested to induce follicle regeneration. This effect is due to increased expression of vascular endothelial growth factor after cutaneous injury, a mediator of both hair growth and cycling as well as wound repair.7 Adjuvant scalp rolling creates a synergistic effect by facilitating absorption of topical and intralesional therapies. The physical breakdown of dermal capillary barriers creates microchannels that traverse the stratum corneum, improving the permeability of small-molecule substances and allowing for relatively painless and uniform delivery of combination therapies. A secondary benefit is hypertrophy, which counteracts the atrophy caused by topical steroids via collagen induction.7
Additionally, scalp rolling confers minimal risk to the patient, making it safer than conventional pharmacologic therapies such as corticosteroids or Janus kinase (JAK) inhibitors. Although intralesional steroid injections are first-line treatments for limited disease, they can cause pain and skin atrophy.10 In one cohort of 54 patients, topical steroids were inferior to both oral and intralesional treatment, and oral steroids carried a systemic side-effect profile and worsening of comorbidities including hyperglycemia and hypertension as well as negative effects on bone density.11 Baricitinib, a JAK inhibitor, was the first systemic treatment to gain US Food and Drug Administration approval for severe AA.12 However, this novel therapeutic confers adverse effects including infection, acne, and hypercholesterolemia, as reported in the BRAVE-AA trials.13 More broadly, the US Food and Drug Administration warns of serious long-term risks such as cardiovascular events and malignancy.14 Given the tremendous potential of JAK inhibitors, further research is warranted to understand both the efficacy of topical formulations as well as the possible role of scalp rolling as its adjuvant.
Finally, scalp rolling is easily accessible and affordable to patients. Scalp rolling devices are readily available and affordable online, and they can be used autonomously at home. This pragmatic option allows patients to take control of their own treatment course and offers a financially feasible alternative to navigating insurance coverage as well as the need for extra office visits for medication refills and monitoring.
We report 3 cases of the use of scalp rolling as an adjuvant to conventional therapy for refractory AA in young patients. Although prospective research is required to establish causality and characterize age-related trends in treatment response, consideration of scalp rolling as an adjuvant to conventional therapy may help to optimize treatment regimens. Given its low risk for side effects and potential benefits, we recommend scalp rolling for patients with refractory AA.
To the Editor:
Alopecia areata (AA) is an autoimmune nonscarring hair loss disorder that can present at any age. Patients with AA have a disproportionately high comorbidity burden and low quality of life, often grappling with anxiety, depression, and psychosocial sequelae involving identity, such as reduced self-esteem.1,2 Although conventional therapies aim to reduce hair loss, none are curative.3 Response to treatment is highly unpredictable, with current data suggesting that up to 50% of patients recover within 1 year while 14% to 25% progress to either alopecia totalis (total scalp hair loss) or alopecia universalis (total body hair loss).4 Options for therapeutic intervention remain limited and vary in safety and effectiveness, warranting further research to identify optimal modalities and minimize side effects. Interestingly, scalp rolling has been used as an adjuvant to topical triamcinolone acetonide.3,5 However, the extent of its effect in combination with other therapies remains unclear. We report 3 pediatric patients with confirmed AA refractory to conventional topical treatment who experienced remarkable scalp hair regrowth after adding biweekly scalp rolling as an adjuvant therapy.
A 7-year-old boy with AA presented with 95% scalp hair loss of 7 months’ duration (Figure 1A)(patient 1). Prior treatments included mometasone solution and clobetasol solution 0.05%. After 3 months of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth after 13 months of treatment (Figure 1B). No pain, bleeding, or other side effects were reported.

An 11-year-old girl with AA presented with 100% hair loss of 7 months’ duration (Figure 2A)(patient 2). Prior treatments included fluocinonide solution and intralesional Kenalog injections. After 4 months of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth after 13 months of treatment (Figure 2B). No pain, bleeding, or other side effects were reported.

A 16-year-old boy with AA presented with 30% hair loss of 4 years’ duration (Figure 3A)(patient 3). Prior treatments included squaric acid and intralesional Kenalog injections. After 2 years of conventional topical therapy, twice-weekly scalp rolling with a 0.25-mm scalp roller of their choosing was added to the regimen, with clobetasol solution 0.05% and minoxidil foam 5% applied immediately after each scalp rolling session. The patient experienced 95% scalp hair regrowth at 17 months (Figure 3B). No pain, bleeding, or other side effects were reported.

Scalp rolling—also known as microneedling—provides a multifactorial approach to hair regrowth in patients with AA. The mechanism of action involves both the hair cycle and wound repair pathways by stimulation of the dermal papillae and stem cells.6 Scalp rolling has been observed to induce the expression of several hair growth pathway mediators, such as WNT3A, β-catenin, vascular endothelial growth factor, and WNT10B.7 Wnt/β-catenin pathway signaling is integral to multiple aspects of the hair regrowth process, including hair morphogenesis, follicle regeneration, and growth of the shaft itself.8,9 Scalp rolling causes microinjuries to the skin, thereby diverting blood supply to the follicles and stimulating wound regeneration, a process suggested to induce follicle regeneration. This effect is due to increased expression of vascular endothelial growth factor after cutaneous injury, a mediator of both hair growth and cycling as well as wound repair.7 Adjuvant scalp rolling creates a synergistic effect by facilitating absorption of topical and intralesional therapies. The physical breakdown of dermal capillary barriers creates microchannels that traverse the stratum corneum, improving the permeability of small-molecule substances and allowing for relatively painless and uniform delivery of combination therapies. A secondary benefit is hypertrophy, which counteracts the atrophy caused by topical steroids via collagen induction.7
Additionally, scalp rolling confers minimal risk to the patient, making it safer than conventional pharmacologic therapies such as corticosteroids or Janus kinase (JAK) inhibitors. Although intralesional steroid injections are first-line treatments for limited disease, they can cause pain and skin atrophy.10 In one cohort of 54 patients, topical steroids were inferior to both oral and intralesional treatment, and oral steroids carried a systemic side-effect profile and worsening of comorbidities including hyperglycemia and hypertension as well as negative effects on bone density.11 Baricitinib, a JAK inhibitor, was the first systemic treatment to gain US Food and Drug Administration approval for severe AA.12 However, this novel therapeutic confers adverse effects including infection, acne, and hypercholesterolemia, as reported in the BRAVE-AA trials.13 More broadly, the US Food and Drug Administration warns of serious long-term risks such as cardiovascular events and malignancy.14 Given the tremendous potential of JAK inhibitors, further research is warranted to understand both the efficacy of topical formulations as well as the possible role of scalp rolling as its adjuvant.
Finally, scalp rolling is easily accessible and affordable to patients. Scalp rolling devices are readily available and affordable online, and they can be used autonomously at home. This pragmatic option allows patients to take control of their own treatment course and offers a financially feasible alternative to navigating insurance coverage as well as the need for extra office visits for medication refills and monitoring.
We report 3 cases of the use of scalp rolling as an adjuvant to conventional therapy for refractory AA in young patients. Although prospective research is required to establish causality and characterize age-related trends in treatment response, consideration of scalp rolling as an adjuvant to conventional therapy may help to optimize treatment regimens. Given its low risk for side effects and potential benefits, we recommend scalp rolling for patients with refractory AA.
1. Senna M, Ko J, Tosti A, et al. Alopecia areata treatment patterns, healthcare resource utilization, and comorbidities in the US population using insurance claims. Adv Ther. 2021;38:4646-4658.
2. Huang CH, Fu Y, Chi CC. Health-related quality of life, depression, and self-esteem in patients with androgenetic alopecia: a systematic review and meta-analysis. JAMA Dermatol. 2021;157:963-970.
3. Deepak SH, Shwetha S. Scalp roller therapy in resistant alopecia areata. J Cutan Aesthet Surg. 2014;7:61-62.
4. Darwin E, Hirt PA, Fertig R, et al. Alopecia areata: review of epidemiology, clinical features, pathogenesis, and new treatment options.Int J Trichology. 2018;10:51-60.
5. Ito T, Yoshimasu T, Furukawa F, et al. Three-microneedle device as an effective option for intralesional corticosteroid administration for the treatment of alopecia areata. J Dermatol. 2017;44:304-305.
6. Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
7. Kim YS, Jeong KH, Kim JE, et al. Repeated microneedle stimulation induces enhanced hair growth in a murine model. Ann Dermatol. 2016;28:586-592.
8. Leirós GJ, Attorresi AI, Balañá ME. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br J Dermatol. 2012;166:1035-1042.
9. Myung PS, Takeo M, Ito M, et al. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J Invest Dermatol. 2013;133:31-41.
10. Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1-12.
11. Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276
12.
13. King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699.
14. US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. September 1, 2021.
1. Senna M, Ko J, Tosti A, et al. Alopecia areata treatment patterns, healthcare resource utilization, and comorbidities in the US population using insurance claims. Adv Ther. 2021;38:4646-4658.
2. Huang CH, Fu Y, Chi CC. Health-related quality of life, depression, and self-esteem in patients with androgenetic alopecia: a systematic review and meta-analysis. JAMA Dermatol. 2021;157:963-970.
3. Deepak SH, Shwetha S. Scalp roller therapy in resistant alopecia areata. J Cutan Aesthet Surg. 2014;7:61-62.
4. Darwin E, Hirt PA, Fertig R, et al. Alopecia areata: review of epidemiology, clinical features, pathogenesis, and new treatment options.Int J Trichology. 2018;10:51-60.
5. Ito T, Yoshimasu T, Furukawa F, et al. Three-microneedle device as an effective option for intralesional corticosteroid administration for the treatment of alopecia areata. J Dermatol. 2017;44:304-305.
6. Dhurat R, Sukesh M, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5:6-11.
7. Kim YS, Jeong KH, Kim JE, et al. Repeated microneedle stimulation induces enhanced hair growth in a murine model. Ann Dermatol. 2016;28:586-592.
8. Leirós GJ, Attorresi AI, Balañá ME. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br J Dermatol. 2012;166:1035-1042.
9. Myung PS, Takeo M, Ito M, et al. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J Invest Dermatol. 2013;133:31-41.
10. Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1-12.
11. Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276
12.
13. King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699.
14. US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. September 1, 2021.
Practice Points
- Alopecia areata (AA) is an autoimmune hair loss disorder with few effective treatments and no cure.
- Scalp rolling is a promising new treatment option that may stimulate hair regrowth by both direct collagen induction and indirect synergy with the use of topical medications.
- Dermatologists should be aware of scalp rolling as a safe, affordable, and potentially effective adjuvant to conventional therapy for AA.
Cryptococcus neoformans Panniculitis Unmasked: A Paradoxical Reaction to Therapy
To the Editor:
Cryptococcus neoformans is an opportunistic fungus with a predilection for immunocompromised hosts, including solid organ transplant recipients (SOTRs). However, the rapid emergence of diffuse panniculitis only upon the start of therapy for extracutaneous disease is a rare phenomenon. We report the case of a liver transplant recipient who developed a paradoxical inflammatory reaction after initiating liposomal amphotericin B therapy for disseminated C neoformans, which manifested as progressive indurated plaques histologically consistent with cryptococcal panniculitis.
A 44-year-old man who received an orthotopic liver transplant 12 months prior and was on prednisone (20 mg daily) and tacrolimus (7 mg total daily) was admitted for multifocal pneumonia complicated by septic shock. Blood and respiratory cultures grew C neoformans, and lumbar puncture evaluation of cerebrospinal fluid revealed the presence of Cryptococcus antigen in 1:40 titers. Liposomal amphotericin B 5 mg/kg intravenous daily and fluconazole 400 mg intravenous daily were administered starting on the fourth day of admission; maintenance tacrolimus and steroids were stopped. Within 36 hours of treatment initiation, an erythematous papular rash was noted on the extremities, which initially was deemed an infusion reaction. Over the next 6 days, the rash became progressively confluent and hyperpigmented. A dermatologist was consulted on the fifteenth day of admission.
Physical examination by dermatology revealed diffuse, hyperpigmented to erythematous macules on the torso, back, arms, and legs that coalesced into dusky indurated plaques along the thighs, right side of the flank, and right upper arm (Figure 1). Laboratory analysis revealed thrombocytopenia but was otherwise unremarkable. Histoplasma antigen and Coccidioides IgG and IgM enzyme immunoassays were negative, as were cytomegalovirus, HIV, and rapid plasma reagin test results. Blood culture testing was repeated, and the findings were negative.
The emergence of the rash after amphotericin initiation prompted concern that the cause was due to a drug reaction rather than cutaneous involvement of cryptococcal infection. Punch biopsies were obtained from the thigh plaque. Hematoxylin and eosin and Grocott-Gomori methenamine-silver stains revealed cryptococcal organisms in the dermis and subcutaneous fat (Figure 2). Bacterial, acid-fast bacillus, and fungal cultures showed no growth.
The patient was diagnosed with cryptococcal panniculitis. Induction therapy with liposomal amphotericin B 5 mg/kg daily and flucytosine 25 mg/kg twice daily was pursued. During the treatment, cutaneous involvement evolved into superficial desquamation. The patient ultimately died from shock secondary to persistent cryptococcal fungemia.
Cryptococcus neoformans is an opportunistic fungal infection that represents a notable hazard to SOTR, inflicting 1.5% to 2.8% of this population and carrying a 19% to 42% mortality rate.1,2 This infection occurs at a median of 1.6 to 2.3 years after transplantation,1,3 though liver transplant recipients and those with immune reconstitution inflammatory syndrome (IRIS)–like complications may present sooner (8.8 and 10.5 months, respectively).4 Cutaneous involvement comprises 17% to 21% of cases and is associated with extensive dissemination, including the central nervous system, lung, and bloodstream (61.5%, 23.1%, and 38.5%, respectively).1-3 When Cryptococcus infects the skin, it classically manifests as multiple nodules, umbilicated papules, ulcers, or cellulitis.3 Involvement of subcutaneous adipose tissue is uncommon and primarily is observed at initial presentation alongside disseminated disease.5-8 Our case is unique because cutaneous involvement was absent until treatment initiation.
Similar patterns of worsened or unmasked disease following treatment initiation have been observed in SOTRs with extracutaneous cryptococcus and were attributed to IRIS-like phenomena that generate a hyperactive inflammatory response to infection.4,9 Common immunosuppressive regimens, particularly tacrolimus, depress helper T cell (TH1) cytokine release and promote a TH2-dominant, anti-inflammatory state.10 In cryptococcosis, the fungus itself may stimulate a comparable cytokine milieu to promote immunologic evasion and dissemination. Cryptococcal IRIS-like responses in SOTRs are precipitated by rapid reduction or withdrawal of calcineurin inhibitors and corticosteroids, in combination with the inherent mitogenicity of the C neoformans polysaccharide capsule and antifungal agents.10 In our patient, cryptococcal yeasts may have invaded subcutaneous tissues when he became fungemic but remained subclinical due to minimal inflammatory recruitment. As treatment began and immunosuppressants diminished, fungal recognition and massive cytokine release resulted in frank panniculitis via precipitous immune dysregulation.
First-line therapy of cryptococcosis entails the use of liposomal amphotericin B and flucytosine for induction, followed by fluconazole for consolidation and maintenance. Use of corticosteroids is atypical to the antifungal regimen; however, a role for them has been suggested in severe IRIS involving individuals who are HIV positive, such as those with lesions demonstrating mass effect.11 Rare case reports have described their utility as adjunctive therapies against cryptococcus in SOTRs when treatment with antifungal agents alone failed.12 Given the paucity of prospective trials to support corticosteroid use in SOTRs as well as the worse global outcomes in cases of cryptococcal meningitis,13 therapeutic corticosteroids were not administered in our patient.
Although our case represents a rare event, cutaneous cryptococcosis and IRIS-like phenomena are clinically relevant complications in immunocompromised patients. In particular, they should be promptly considered in SOTRs receiving maintenance immunosuppressants who demonstrate symptom aggravation despite negative microbial culture results and uninterrupted antifungal therapy.
1. Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis. 2001;7:375-381.
2. Sun HY, Wagener MM, Singh N. Cryptococcosis in solid-organ, hematopoietic stem cell, and tissue transplant recipients: evidence-based evolving trends. Clin Infect Dis. 2009;48:1566-1576.
3. Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
4. Singh N, Lortholary O, Alexander BD, et al. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis. 2005;40:1756-1761.
5. Reddy BY, Shaigany S, Schulman L, et al. Resident rounds part III: case report: fatal cryptococcal panniculitis in a lung transplant recipient. J Drugs Dermatol. 2015;14:519-252.
6. Bhowmik D, Dinda AK, Xess I, et al. Fungal panniculitis in renal transplant recipients. Transpl Infect Dis. 2008;10:286-289.
7. Gloster HM, Swerlick RA, Solomon AR. Cryptococcal cellulitis in a diabetic, kidney transplant patient. J Am Acad Dermatol. 1994;30:1025-1026.
8. Carlson KC, Mehlmauer M, Evans S, et al. Cryptococcal cellulitis in renal transplant recipients. J Am Acad Dermatol. 1987;17:469-472.
9. French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis. 2009;48:101-107.
10. Singh N, Perfect JR. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect Dis. 2007;7:395-401.
11. World Health Organization. Guidelines on the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Published March 1, 2018. Accessed September 6, 2020. https://www.who.int/publications/i/item/9789241550277
12. Lanternier F, Chandesris MO, Poirée S, et al. Cellulitis revealing a cryptococcosis-related immune reconstitution inflammatory syndrome in a renal allograft recipient. Am J Transpl. 2007;7:2826-2828.
13. Beardsley J, Wolbers M, Kibengo FM, et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med. 2016;374:542-554.
To the Editor:
Cryptococcus neoformans is an opportunistic fungus with a predilection for immunocompromised hosts, including solid organ transplant recipients (SOTRs). However, the rapid emergence of diffuse panniculitis only upon the start of therapy for extracutaneous disease is a rare phenomenon. We report the case of a liver transplant recipient who developed a paradoxical inflammatory reaction after initiating liposomal amphotericin B therapy for disseminated C neoformans, which manifested as progressive indurated plaques histologically consistent with cryptococcal panniculitis.
A 44-year-old man who received an orthotopic liver transplant 12 months prior and was on prednisone (20 mg daily) and tacrolimus (7 mg total daily) was admitted for multifocal pneumonia complicated by septic shock. Blood and respiratory cultures grew C neoformans, and lumbar puncture evaluation of cerebrospinal fluid revealed the presence of Cryptococcus antigen in 1:40 titers. Liposomal amphotericin B 5 mg/kg intravenous daily and fluconazole 400 mg intravenous daily were administered starting on the fourth day of admission; maintenance tacrolimus and steroids were stopped. Within 36 hours of treatment initiation, an erythematous papular rash was noted on the extremities, which initially was deemed an infusion reaction. Over the next 6 days, the rash became progressively confluent and hyperpigmented. A dermatologist was consulted on the fifteenth day of admission.
Physical examination by dermatology revealed diffuse, hyperpigmented to erythematous macules on the torso, back, arms, and legs that coalesced into dusky indurated plaques along the thighs, right side of the flank, and right upper arm (Figure 1). Laboratory analysis revealed thrombocytopenia but was otherwise unremarkable. Histoplasma antigen and Coccidioides IgG and IgM enzyme immunoassays were negative, as were cytomegalovirus, HIV, and rapid plasma reagin test results. Blood culture testing was repeated, and the findings were negative.

The emergence of the rash after amphotericin initiation prompted concern that the cause was due to a drug reaction rather than cutaneous involvement of cryptococcal infection. Punch biopsies were obtained from the thigh plaque. Hematoxylin and eosin and Grocott-Gomori methenamine-silver stains revealed cryptococcal organisms in the dermis and subcutaneous fat (Figure 2). Bacterial, acid-fast bacillus, and fungal cultures showed no growth.

The patient was diagnosed with cryptococcal panniculitis. Induction therapy with liposomal amphotericin B 5 mg/kg daily and flucytosine 25 mg/kg twice daily was pursued. During the treatment, cutaneous involvement evolved into superficial desquamation. The patient ultimately died from shock secondary to persistent cryptococcal fungemia.
Cryptococcus neoformans is an opportunistic fungal infection that represents a notable hazard to SOTR, inflicting 1.5% to 2.8% of this population and carrying a 19% to 42% mortality rate.1,2 This infection occurs at a median of 1.6 to 2.3 years after transplantation,1,3 though liver transplant recipients and those with immune reconstitution inflammatory syndrome (IRIS)–like complications may present sooner (8.8 and 10.5 months, respectively).4 Cutaneous involvement comprises 17% to 21% of cases and is associated with extensive dissemination, including the central nervous system, lung, and bloodstream (61.5%, 23.1%, and 38.5%, respectively).1-3 When Cryptococcus infects the skin, it classically manifests as multiple nodules, umbilicated papules, ulcers, or cellulitis.3 Involvement of subcutaneous adipose tissue is uncommon and primarily is observed at initial presentation alongside disseminated disease.5-8 Our case is unique because cutaneous involvement was absent until treatment initiation.
Similar patterns of worsened or unmasked disease following treatment initiation have been observed in SOTRs with extracutaneous cryptococcus and were attributed to IRIS-like phenomena that generate a hyperactive inflammatory response to infection.4,9 Common immunosuppressive regimens, particularly tacrolimus, depress helper T cell (TH1) cytokine release and promote a TH2-dominant, anti-inflammatory state.10 In cryptococcosis, the fungus itself may stimulate a comparable cytokine milieu to promote immunologic evasion and dissemination. Cryptococcal IRIS-like responses in SOTRs are precipitated by rapid reduction or withdrawal of calcineurin inhibitors and corticosteroids, in combination with the inherent mitogenicity of the C neoformans polysaccharide capsule and antifungal agents.10 In our patient, cryptococcal yeasts may have invaded subcutaneous tissues when he became fungemic but remained subclinical due to minimal inflammatory recruitment. As treatment began and immunosuppressants diminished, fungal recognition and massive cytokine release resulted in frank panniculitis via precipitous immune dysregulation.
First-line therapy of cryptococcosis entails the use of liposomal amphotericin B and flucytosine for induction, followed by fluconazole for consolidation and maintenance. Use of corticosteroids is atypical to the antifungal regimen; however, a role for them has been suggested in severe IRIS involving individuals who are HIV positive, such as those with lesions demonstrating mass effect.11 Rare case reports have described their utility as adjunctive therapies against cryptococcus in SOTRs when treatment with antifungal agents alone failed.12 Given the paucity of prospective trials to support corticosteroid use in SOTRs as well as the worse global outcomes in cases of cryptococcal meningitis,13 therapeutic corticosteroids were not administered in our patient.
Although our case represents a rare event, cutaneous cryptococcosis and IRIS-like phenomena are clinically relevant complications in immunocompromised patients. In particular, they should be promptly considered in SOTRs receiving maintenance immunosuppressants who demonstrate symptom aggravation despite negative microbial culture results and uninterrupted antifungal therapy.
To the Editor:
Cryptococcus neoformans is an opportunistic fungus with a predilection for immunocompromised hosts, including solid organ transplant recipients (SOTRs). However, the rapid emergence of diffuse panniculitis only upon the start of therapy for extracutaneous disease is a rare phenomenon. We report the case of a liver transplant recipient who developed a paradoxical inflammatory reaction after initiating liposomal amphotericin B therapy for disseminated C neoformans, which manifested as progressive indurated plaques histologically consistent with cryptococcal panniculitis.
A 44-year-old man who received an orthotopic liver transplant 12 months prior and was on prednisone (20 mg daily) and tacrolimus (7 mg total daily) was admitted for multifocal pneumonia complicated by septic shock. Blood and respiratory cultures grew C neoformans, and lumbar puncture evaluation of cerebrospinal fluid revealed the presence of Cryptococcus antigen in 1:40 titers. Liposomal amphotericin B 5 mg/kg intravenous daily and fluconazole 400 mg intravenous daily were administered starting on the fourth day of admission; maintenance tacrolimus and steroids were stopped. Within 36 hours of treatment initiation, an erythematous papular rash was noted on the extremities, which initially was deemed an infusion reaction. Over the next 6 days, the rash became progressively confluent and hyperpigmented. A dermatologist was consulted on the fifteenth day of admission.
Physical examination by dermatology revealed diffuse, hyperpigmented to erythematous macules on the torso, back, arms, and legs that coalesced into dusky indurated plaques along the thighs, right side of the flank, and right upper arm (Figure 1). Laboratory analysis revealed thrombocytopenia but was otherwise unremarkable. Histoplasma antigen and Coccidioides IgG and IgM enzyme immunoassays were negative, as were cytomegalovirus, HIV, and rapid plasma reagin test results. Blood culture testing was repeated, and the findings were negative.

The emergence of the rash after amphotericin initiation prompted concern that the cause was due to a drug reaction rather than cutaneous involvement of cryptococcal infection. Punch biopsies were obtained from the thigh plaque. Hematoxylin and eosin and Grocott-Gomori methenamine-silver stains revealed cryptococcal organisms in the dermis and subcutaneous fat (Figure 2). Bacterial, acid-fast bacillus, and fungal cultures showed no growth.

The patient was diagnosed with cryptococcal panniculitis. Induction therapy with liposomal amphotericin B 5 mg/kg daily and flucytosine 25 mg/kg twice daily was pursued. During the treatment, cutaneous involvement evolved into superficial desquamation. The patient ultimately died from shock secondary to persistent cryptococcal fungemia.
Cryptococcus neoformans is an opportunistic fungal infection that represents a notable hazard to SOTR, inflicting 1.5% to 2.8% of this population and carrying a 19% to 42% mortality rate.1,2 This infection occurs at a median of 1.6 to 2.3 years after transplantation,1,3 though liver transplant recipients and those with immune reconstitution inflammatory syndrome (IRIS)–like complications may present sooner (8.8 and 10.5 months, respectively).4 Cutaneous involvement comprises 17% to 21% of cases and is associated with extensive dissemination, including the central nervous system, lung, and bloodstream (61.5%, 23.1%, and 38.5%, respectively).1-3 When Cryptococcus infects the skin, it classically manifests as multiple nodules, umbilicated papules, ulcers, or cellulitis.3 Involvement of subcutaneous adipose tissue is uncommon and primarily is observed at initial presentation alongside disseminated disease.5-8 Our case is unique because cutaneous involvement was absent until treatment initiation.
Similar patterns of worsened or unmasked disease following treatment initiation have been observed in SOTRs with extracutaneous cryptococcus and were attributed to IRIS-like phenomena that generate a hyperactive inflammatory response to infection.4,9 Common immunosuppressive regimens, particularly tacrolimus, depress helper T cell (TH1) cytokine release and promote a TH2-dominant, anti-inflammatory state.10 In cryptococcosis, the fungus itself may stimulate a comparable cytokine milieu to promote immunologic evasion and dissemination. Cryptococcal IRIS-like responses in SOTRs are precipitated by rapid reduction or withdrawal of calcineurin inhibitors and corticosteroids, in combination with the inherent mitogenicity of the C neoformans polysaccharide capsule and antifungal agents.10 In our patient, cryptococcal yeasts may have invaded subcutaneous tissues when he became fungemic but remained subclinical due to minimal inflammatory recruitment. As treatment began and immunosuppressants diminished, fungal recognition and massive cytokine release resulted in frank panniculitis via precipitous immune dysregulation.
First-line therapy of cryptococcosis entails the use of liposomal amphotericin B and flucytosine for induction, followed by fluconazole for consolidation and maintenance. Use of corticosteroids is atypical to the antifungal regimen; however, a role for them has been suggested in severe IRIS involving individuals who are HIV positive, such as those with lesions demonstrating mass effect.11 Rare case reports have described their utility as adjunctive therapies against cryptococcus in SOTRs when treatment with antifungal agents alone failed.12 Given the paucity of prospective trials to support corticosteroid use in SOTRs as well as the worse global outcomes in cases of cryptococcal meningitis,13 therapeutic corticosteroids were not administered in our patient.
Although our case represents a rare event, cutaneous cryptococcosis and IRIS-like phenomena are clinically relevant complications in immunocompromised patients. In particular, they should be promptly considered in SOTRs receiving maintenance immunosuppressants who demonstrate symptom aggravation despite negative microbial culture results and uninterrupted antifungal therapy.
1. Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis. 2001;7:375-381.
2. Sun HY, Wagener MM, Singh N. Cryptococcosis in solid-organ, hematopoietic stem cell, and tissue transplant recipients: evidence-based evolving trends. Clin Infect Dis. 2009;48:1566-1576.
3. Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
4. Singh N, Lortholary O, Alexander BD, et al. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis. 2005;40:1756-1761.
5. Reddy BY, Shaigany S, Schulman L, et al. Resident rounds part III: case report: fatal cryptococcal panniculitis in a lung transplant recipient. J Drugs Dermatol. 2015;14:519-252.
6. Bhowmik D, Dinda AK, Xess I, et al. Fungal panniculitis in renal transplant recipients. Transpl Infect Dis. 2008;10:286-289.
7. Gloster HM, Swerlick RA, Solomon AR. Cryptococcal cellulitis in a diabetic, kidney transplant patient. J Am Acad Dermatol. 1994;30:1025-1026.
8. Carlson KC, Mehlmauer M, Evans S, et al. Cryptococcal cellulitis in renal transplant recipients. J Am Acad Dermatol. 1987;17:469-472.
9. French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis. 2009;48:101-107.
10. Singh N, Perfect JR. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect Dis. 2007;7:395-401.
11. World Health Organization. Guidelines on the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Published March 1, 2018. Accessed September 6, 2020. https://www.who.int/publications/i/item/9789241550277
12. Lanternier F, Chandesris MO, Poirée S, et al. Cellulitis revealing a cryptococcosis-related immune reconstitution inflammatory syndrome in a renal allograft recipient. Am J Transpl. 2007;7:2826-2828.
13. Beardsley J, Wolbers M, Kibengo FM, et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med. 2016;374:542-554.
1. Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis. 2001;7:375-381.
2. Sun HY, Wagener MM, Singh N. Cryptococcosis in solid-organ, hematopoietic stem cell, and tissue transplant recipients: evidence-based evolving trends. Clin Infect Dis. 2009;48:1566-1576.
3. Sun HY, Alexander BD, Lortholary O, et al. Cutaneous cryptococcosis in solid organ transplant recipients. Med Mycol. 2010;48:785-791.
4. Singh N, Lortholary O, Alexander BD, et al. An immune reconstitution syndrome-like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin Infect Dis. 2005;40:1756-1761.
5. Reddy BY, Shaigany S, Schulman L, et al. Resident rounds part III: case report: fatal cryptococcal panniculitis in a lung transplant recipient. J Drugs Dermatol. 2015;14:519-252.
6. Bhowmik D, Dinda AK, Xess I, et al. Fungal panniculitis in renal transplant recipients. Transpl Infect Dis. 2008;10:286-289.
7. Gloster HM, Swerlick RA, Solomon AR. Cryptococcal cellulitis in a diabetic, kidney transplant patient. J Am Acad Dermatol. 1994;30:1025-1026.
8. Carlson KC, Mehlmauer M, Evans S, et al. Cryptococcal cellulitis in renal transplant recipients. J Am Acad Dermatol. 1987;17:469-472.
9. French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis. 2009;48:101-107.
10. Singh N, Perfect JR. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect Dis. 2007;7:395-401.
11. World Health Organization. Guidelines on the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Published March 1, 2018. Accessed September 6, 2020. https://www.who.int/publications/i/item/9789241550277
12. Lanternier F, Chandesris MO, Poirée S, et al. Cellulitis revealing a cryptococcosis-related immune reconstitution inflammatory syndrome in a renal allograft recipient. Am J Transpl. 2007;7:2826-2828.
13. Beardsley J, Wolbers M, Kibengo FM, et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med. 2016;374:542-554.
Practice Points
- Panniculitis caused by Cryptococcus neoformans is a rare complication in solid organ transplant recipients.
- Subclinical panniculitis from C neoformans may be unmasked during paradoxical inflammatory reactions as early as days following immunosuppressant withdrawal and treatment initiation.
Vitamin D deficiency linked to psoriasis severity
, suggesting that some people who increase their intake of the vitamin could better control this skin condition that affects up to 8 million people in the United States alone.
Brown University researchers studied almost 500 psoriasis cases taken from the National Health and Nutrition Examination Survey (NHANES), the scientists told attendees at the conference of the American Society for Nutrition. They compared the peoples’ reports on how much of their body surface was affected by psoriasis to vitamin D levels collected in blood samples.
“After adjusting for lifestyle factors such as smoking, the analysis showed that lower vitamin D levels and vitamin D deficiency were significantly associated with greater psoriasis severity,” the ASN said in a news release. “The researchers also found that patients with the least amount of body surface affected by psoriasis had the highest average vitamin D levels while those with the greatest affected area had the lowest average levels of vitamin D.”
The researchers said that people with psoriasis might improve their condition by getting more vitamin D in their diet and through supplements.
“Topical synthetic vitamin D creams are emerging as new therapies for psoriasis, but these usually require a doctor’s prescription,” said researcher Rachel K. Lim, an MD candidate at Brown University, Providence, R.I. “Our results suggest that a vitamin D–rich diet or oral vitamin D supplementation may also provide some benefit to psoriasis patients.”
The researchers said that vitamin D toxicity is rare but that people should consult with their medical caregivers before they start taking supplements.
A version of this article first appeared on WebMD.com.
, suggesting that some people who increase their intake of the vitamin could better control this skin condition that affects up to 8 million people in the United States alone.
Brown University researchers studied almost 500 psoriasis cases taken from the National Health and Nutrition Examination Survey (NHANES), the scientists told attendees at the conference of the American Society for Nutrition. They compared the peoples’ reports on how much of their body surface was affected by psoriasis to vitamin D levels collected in blood samples.
“After adjusting for lifestyle factors such as smoking, the analysis showed that lower vitamin D levels and vitamin D deficiency were significantly associated with greater psoriasis severity,” the ASN said in a news release. “The researchers also found that patients with the least amount of body surface affected by psoriasis had the highest average vitamin D levels while those with the greatest affected area had the lowest average levels of vitamin D.”
The researchers said that people with psoriasis might improve their condition by getting more vitamin D in their diet and through supplements.
“Topical synthetic vitamin D creams are emerging as new therapies for psoriasis, but these usually require a doctor’s prescription,” said researcher Rachel K. Lim, an MD candidate at Brown University, Providence, R.I. “Our results suggest that a vitamin D–rich diet or oral vitamin D supplementation may also provide some benefit to psoriasis patients.”
The researchers said that vitamin D toxicity is rare but that people should consult with their medical caregivers before they start taking supplements.
A version of this article first appeared on WebMD.com.
, suggesting that some people who increase their intake of the vitamin could better control this skin condition that affects up to 8 million people in the United States alone.
Brown University researchers studied almost 500 psoriasis cases taken from the National Health and Nutrition Examination Survey (NHANES), the scientists told attendees at the conference of the American Society for Nutrition. They compared the peoples’ reports on how much of their body surface was affected by psoriasis to vitamin D levels collected in blood samples.
“After adjusting for lifestyle factors such as smoking, the analysis showed that lower vitamin D levels and vitamin D deficiency were significantly associated with greater psoriasis severity,” the ASN said in a news release. “The researchers also found that patients with the least amount of body surface affected by psoriasis had the highest average vitamin D levels while those with the greatest affected area had the lowest average levels of vitamin D.”
The researchers said that people with psoriasis might improve their condition by getting more vitamin D in their diet and through supplements.
“Topical synthetic vitamin D creams are emerging as new therapies for psoriasis, but these usually require a doctor’s prescription,” said researcher Rachel K. Lim, an MD candidate at Brown University, Providence, R.I. “Our results suggest that a vitamin D–rich diet or oral vitamin D supplementation may also provide some benefit to psoriasis patients.”
The researchers said that vitamin D toxicity is rare but that people should consult with their medical caregivers before they start taking supplements.
A version of this article first appeared on WebMD.com.
FROM NUTRITION 2023
Multiple Nodules on the Scrotum
The Diagnosis: Scrotal Calcinosis
Scrotal calcinosis is a rare benign disease that results from the deposition of calcium, magnesium, phosphate, and carbonate within the dermis and subcutaneous layer of the skin in the absence of underlying systemic disease or serum calcium and phosphorus abnormalities.1,2 Lesions usually are asymptomatic but can be mildly painful or pruritic. They usually present in childhood or early adulthood as yellow-white firm nodules ranging in size from a few millimeters to a few centimeters that increase in size and number over time. Additionally, lesions can ulcerate and discharge a chalklike exudative material. Although benign in nature, the quality-of-life impact in patients with this condition can be substantial, specifically regarding cosmesis, which may cause patients to feel embarrassed and even avoid sexual activity. This condition rarely has been associated with infection.1
Our patient elected to undergo surgical excision under local anesthesia, and the lesions were sent for histopathologic examination. His postoperative course was unremarkable, and he was pleased with the cosmetic result of the surgery (Figure 1). Histopathology revealed calcified deposits that appeared as intradermal basophilic nodules lacking an epithelial lining (Figure 2), consistent with the diagnosis of scrotal calcinosis.2 No recurrence of the lesions was documented over the course of 18 months.
The pathogenesis of this condition is not clear. Most research supports scrotal calcinosis resulting from dystrophic calcification of epidermal inclusion cysts.3 There have been cases of scrotal calcinosis coinciding with epidermal inclusion cysts of the scrotum in varying stages of inflammation (some intact and some ruptured).2 Some research also suggests dystrophic calcification of eccrine epithelial cysts and degenerated dartos muscle as the origin of scrotal calcinosis.3
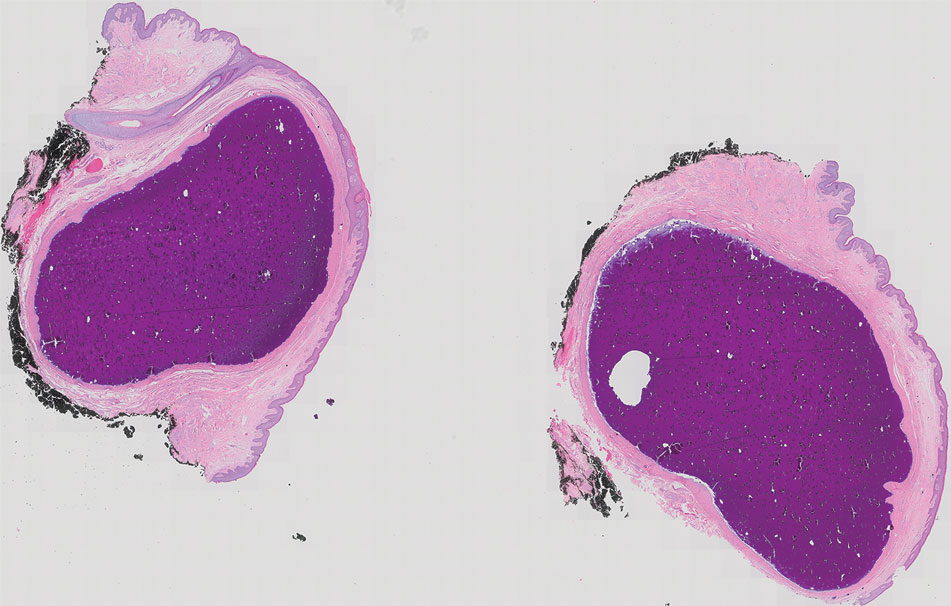
The differential diagnosis for this case included calcified steatocystoma multiplex, eruptive xanthomas, nodular scabies, and epidermal inclusion cysts. Steatocystoma multiplex can be inherited in an autosomal-dominant fashion or can develop sporadically with mutations in the KRT17 gene.4 It is characterized by multiple sebum-filled, cystic lesions of the pilosebaceous unit that may become calcified. Calcified lesions appear as yellow, firm, irregularly shaped papules or nodules ranging from a few millimeters to centimeters in size. Cysts can develop anywhere on the body with a predilection for the chest, upper extremities, axillae, trunk, groin, and scrotum.4 Histologically, our patient’s lesions were not associated with the pilosebaceous unit. Additionally, our patient denied a family history of similar skin lesions, which made calcified steatocystoma multiplex an unlikely diagnosis.
Eruptive xanthomas result from localized deposition of lipids within the dermis, typically in the setting of dyslipidemia or poorly controlled diabetes mellitus. They commonly appear on the extremities or buttocks as pruritic crops of yellow-red papules or nodules that are a few millimeters in size. Although our patient has a history of hyperlipidemia, his lesions differed substantially from eruptive xanthomas in clinical presentation.
Nodular scabies is a manifestation of classic scabies that presents with intensely pruritic erythematous papules and nodules that are a few millimeters in size and commonly occur on the axillae, groin, and genitalia. Our patient’s skin lesions were not pruritic and differed in appearance from nodular scabies.
Although research indicates scrotal calcinosis may result from dystrophic calcification of epidermal inclusion cysts,2 the latter present as dome-shaped, flesh-colored nodules with central pores representing the opening of hair follicles. Our patient lacked characteristic findings of epidermal inclusion cysts on histology.
The preferred treatment for scrotal calcinosis is surgical excision, which improves the aesthetic appearance, relieves itch, and removes ulcerative lesions.5 Additionally, surgical excision provides histological diagnostic confirmation. Recurrence with incomplete excision is possible; therefore, all lesions should be completely excised to reduce the risk for recurrence.3
- Pompeo A, Molina WR, Pohlman GD, et al. Idiopathic scrotal calcinosis: a rare entity and a review of the literature. Can Urol Assoc J. 2013;7:E439-E441. doi:10.5489/cuaj.1387
- Swinehart JM, Golitz LE. Scrotal calcinosis: dystrophic calcification of epidermoid cysts. Arch Dermatol. 1982;118:985-988. doi:10.1001 /archderm.1982.01650240029016
- Khallouk A, Yazami OE, Mellas S, et al. Idiopathic scrotal calcinosis: a nonelucidated pathogenesis and its surgical treatment. Rev Urol. 2011;13:95-97.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480. doi:10.1046/j.1365-2133.1998.02413.x
- Solanki A, Narang S, Kathpalia R, et al. Scrotal calcinosis: pathogenetic link with epidermal cyst. BMJ Case Rep. 2015;2015:bcr2015211163. doi:10.1136/bcr-2015-211163
The Diagnosis: Scrotal Calcinosis
Scrotal calcinosis is a rare benign disease that results from the deposition of calcium, magnesium, phosphate, and carbonate within the dermis and subcutaneous layer of the skin in the absence of underlying systemic disease or serum calcium and phosphorus abnormalities.1,2 Lesions usually are asymptomatic but can be mildly painful or pruritic. They usually present in childhood or early adulthood as yellow-white firm nodules ranging in size from a few millimeters to a few centimeters that increase in size and number over time. Additionally, lesions can ulcerate and discharge a chalklike exudative material. Although benign in nature, the quality-of-life impact in patients with this condition can be substantial, specifically regarding cosmesis, which may cause patients to feel embarrassed and even avoid sexual activity. This condition rarely has been associated with infection.1
Our patient elected to undergo surgical excision under local anesthesia, and the lesions were sent for histopathologic examination. His postoperative course was unremarkable, and he was pleased with the cosmetic result of the surgery (Figure 1). Histopathology revealed calcified deposits that appeared as intradermal basophilic nodules lacking an epithelial lining (Figure 2), consistent with the diagnosis of scrotal calcinosis.2 No recurrence of the lesions was documented over the course of 18 months.

The pathogenesis of this condition is not clear. Most research supports scrotal calcinosis resulting from dystrophic calcification of epidermal inclusion cysts.3 There have been cases of scrotal calcinosis coinciding with epidermal inclusion cysts of the scrotum in varying stages of inflammation (some intact and some ruptured).2 Some research also suggests dystrophic calcification of eccrine epithelial cysts and degenerated dartos muscle as the origin of scrotal calcinosis.3

The differential diagnosis for this case included calcified steatocystoma multiplex, eruptive xanthomas, nodular scabies, and epidermal inclusion cysts. Steatocystoma multiplex can be inherited in an autosomal-dominant fashion or can develop sporadically with mutations in the KRT17 gene.4 It is characterized by multiple sebum-filled, cystic lesions of the pilosebaceous unit that may become calcified. Calcified lesions appear as yellow, firm, irregularly shaped papules or nodules ranging from a few millimeters to centimeters in size. Cysts can develop anywhere on the body with a predilection for the chest, upper extremities, axillae, trunk, groin, and scrotum.4 Histologically, our patient’s lesions were not associated with the pilosebaceous unit. Additionally, our patient denied a family history of similar skin lesions, which made calcified steatocystoma multiplex an unlikely diagnosis.
Eruptive xanthomas result from localized deposition of lipids within the dermis, typically in the setting of dyslipidemia or poorly controlled diabetes mellitus. They commonly appear on the extremities or buttocks as pruritic crops of yellow-red papules or nodules that are a few millimeters in size. Although our patient has a history of hyperlipidemia, his lesions differed substantially from eruptive xanthomas in clinical presentation.
Nodular scabies is a manifestation of classic scabies that presents with intensely pruritic erythematous papules and nodules that are a few millimeters in size and commonly occur on the axillae, groin, and genitalia. Our patient’s skin lesions were not pruritic and differed in appearance from nodular scabies.
Although research indicates scrotal calcinosis may result from dystrophic calcification of epidermal inclusion cysts,2 the latter present as dome-shaped, flesh-colored nodules with central pores representing the opening of hair follicles. Our patient lacked characteristic findings of epidermal inclusion cysts on histology.
The preferred treatment for scrotal calcinosis is surgical excision, which improves the aesthetic appearance, relieves itch, and removes ulcerative lesions.5 Additionally, surgical excision provides histological diagnostic confirmation. Recurrence with incomplete excision is possible; therefore, all lesions should be completely excised to reduce the risk for recurrence.3
The Diagnosis: Scrotal Calcinosis
Scrotal calcinosis is a rare benign disease that results from the deposition of calcium, magnesium, phosphate, and carbonate within the dermis and subcutaneous layer of the skin in the absence of underlying systemic disease or serum calcium and phosphorus abnormalities.1,2 Lesions usually are asymptomatic but can be mildly painful or pruritic. They usually present in childhood or early adulthood as yellow-white firm nodules ranging in size from a few millimeters to a few centimeters that increase in size and number over time. Additionally, lesions can ulcerate and discharge a chalklike exudative material. Although benign in nature, the quality-of-life impact in patients with this condition can be substantial, specifically regarding cosmesis, which may cause patients to feel embarrassed and even avoid sexual activity. This condition rarely has been associated with infection.1
Our patient elected to undergo surgical excision under local anesthesia, and the lesions were sent for histopathologic examination. His postoperative course was unremarkable, and he was pleased with the cosmetic result of the surgery (Figure 1). Histopathology revealed calcified deposits that appeared as intradermal basophilic nodules lacking an epithelial lining (Figure 2), consistent with the diagnosis of scrotal calcinosis.2 No recurrence of the lesions was documented over the course of 18 months.

The pathogenesis of this condition is not clear. Most research supports scrotal calcinosis resulting from dystrophic calcification of epidermal inclusion cysts.3 There have been cases of scrotal calcinosis coinciding with epidermal inclusion cysts of the scrotum in varying stages of inflammation (some intact and some ruptured).2 Some research also suggests dystrophic calcification of eccrine epithelial cysts and degenerated dartos muscle as the origin of scrotal calcinosis.3

The differential diagnosis for this case included calcified steatocystoma multiplex, eruptive xanthomas, nodular scabies, and epidermal inclusion cysts. Steatocystoma multiplex can be inherited in an autosomal-dominant fashion or can develop sporadically with mutations in the KRT17 gene.4 It is characterized by multiple sebum-filled, cystic lesions of the pilosebaceous unit that may become calcified. Calcified lesions appear as yellow, firm, irregularly shaped papules or nodules ranging from a few millimeters to centimeters in size. Cysts can develop anywhere on the body with a predilection for the chest, upper extremities, axillae, trunk, groin, and scrotum.4 Histologically, our patient’s lesions were not associated with the pilosebaceous unit. Additionally, our patient denied a family history of similar skin lesions, which made calcified steatocystoma multiplex an unlikely diagnosis.
Eruptive xanthomas result from localized deposition of lipids within the dermis, typically in the setting of dyslipidemia or poorly controlled diabetes mellitus. They commonly appear on the extremities or buttocks as pruritic crops of yellow-red papules or nodules that are a few millimeters in size. Although our patient has a history of hyperlipidemia, his lesions differed substantially from eruptive xanthomas in clinical presentation.
Nodular scabies is a manifestation of classic scabies that presents with intensely pruritic erythematous papules and nodules that are a few millimeters in size and commonly occur on the axillae, groin, and genitalia. Our patient’s skin lesions were not pruritic and differed in appearance from nodular scabies.
Although research indicates scrotal calcinosis may result from dystrophic calcification of epidermal inclusion cysts,2 the latter present as dome-shaped, flesh-colored nodules with central pores representing the opening of hair follicles. Our patient lacked characteristic findings of epidermal inclusion cysts on histology.
The preferred treatment for scrotal calcinosis is surgical excision, which improves the aesthetic appearance, relieves itch, and removes ulcerative lesions.5 Additionally, surgical excision provides histological diagnostic confirmation. Recurrence with incomplete excision is possible; therefore, all lesions should be completely excised to reduce the risk for recurrence.3
- Pompeo A, Molina WR, Pohlman GD, et al. Idiopathic scrotal calcinosis: a rare entity and a review of the literature. Can Urol Assoc J. 2013;7:E439-E441. doi:10.5489/cuaj.1387
- Swinehart JM, Golitz LE. Scrotal calcinosis: dystrophic calcification of epidermoid cysts. Arch Dermatol. 1982;118:985-988. doi:10.1001 /archderm.1982.01650240029016
- Khallouk A, Yazami OE, Mellas S, et al. Idiopathic scrotal calcinosis: a nonelucidated pathogenesis and its surgical treatment. Rev Urol. 2011;13:95-97.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480. doi:10.1046/j.1365-2133.1998.02413.x
- Solanki A, Narang S, Kathpalia R, et al. Scrotal calcinosis: pathogenetic link with epidermal cyst. BMJ Case Rep. 2015;2015:bcr2015211163. doi:10.1136/bcr-2015-211163
- Pompeo A, Molina WR, Pohlman GD, et al. Idiopathic scrotal calcinosis: a rare entity and a review of the literature. Can Urol Assoc J. 2013;7:E439-E441. doi:10.5489/cuaj.1387
- Swinehart JM, Golitz LE. Scrotal calcinosis: dystrophic calcification of epidermoid cysts. Arch Dermatol. 1982;118:985-988. doi:10.1001 /archderm.1982.01650240029016
- Khallouk A, Yazami OE, Mellas S, et al. Idiopathic scrotal calcinosis: a nonelucidated pathogenesis and its surgical treatment. Rev Urol. 2011;13:95-97.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480. doi:10.1046/j.1365-2133.1998.02413.x
- Solanki A, Narang S, Kathpalia R, et al. Scrotal calcinosis: pathogenetic link with epidermal cyst. BMJ Case Rep. 2015;2015:bcr2015211163. doi:10.1136/bcr-2015-211163
A 33-year-old man presented with progressively enlarging bumps on the scrotum that were present since adolescence. He had a history of hyperlipidemia but no history of systemic or autoimmune disease. The lesions were asymptomatic without associated pruritus, pain, or discharge. No treatments had been administered, and he had no known personal or family history of similar skin conditions or skin cancer. He endorsed a monogamous relationship with his wife. Physical examination revealed 15 firm, yellow-white, subcutaneous nodules on the scrotum that varied in size.
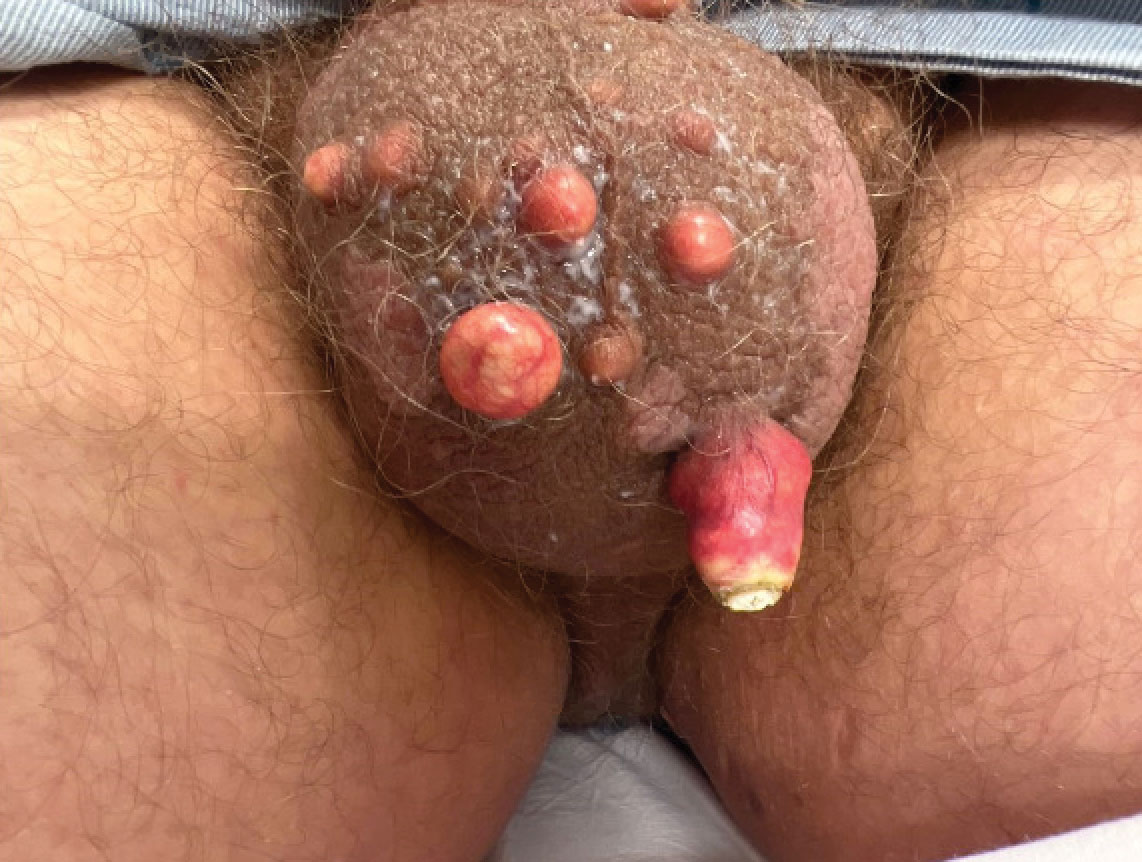
Methemoglobinemia Induced by Application of an Anesthetic Cream
To the Editor:
Methemoglobinemia (MetHb) is a condition caused by elevated levels of methemoglobin in the blood, which leads to an overall reduced ability of red blood cells to release oxygen to tissues, causing tissue hypoxia. Methemoglobinemia may be congenital or acquired. Various antibiotics and local anesthetics have been reported to induce acquired MetHb.1 We describe an adult who presented with MetHb resulting from excessive topical application of local anesthetics for painful scrotal ulcers.
A 54-year-old man presented with multiple scrotal and penile shaft ulcers of a few weeks’ duration with no systemic concerns. His medical history included chronic hepatitis C virus (HCV) and lumbar disc disease. Physical examination revealed multiple erosions and ulcers on an erythematous base involving the scrotal skin and distal penile shaft (Figure). Histopathology revealed acute leukocytoclastic vasculitis, and a laboratory workup was positive for mixed cryoglobulinemia that was thought to be HCV related. The patient was started on a systemic corticosteroid treatment in addition to sofosbuvir-velpatasvir for the treatment of HCV-related mixed cryoglobulinemic vasculitis. Concomitantly, the patient self-treated for pain with a local anesthetic cream containing lidocaine 2.5% and prilocaine 2.5%, applying it excessively every few hours daily for 2 weeks. He also intermittently used occlusive dressings.
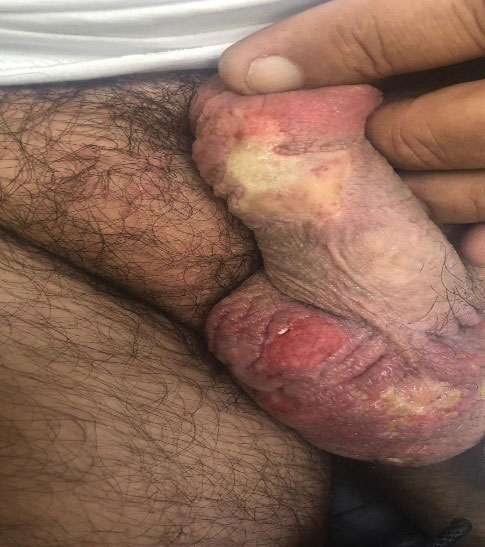
After 2 weeks of application, the patient developed lightheadedness and shortness of breath. He returned and was admitted for further evaluation. He had dyspnea and tachypnea of 22 breaths per minute. He also had mild tachycardia (109 beats per minute). He did not have a fever, and his blood pressure was normal. The oxygen saturation measured in ambient room air by pulse oximetry was 82%. A neurologic examination was normal except for mild drowsiness. The lungs were clear, and heart sounds were normal. A 12-lead electrocardiogram also was normal. A complete blood cell count showed severe macrocytic anemia with a hemoglobin level of 7 g/dL, which was a severe decline from the patient’s baseline level of 14 g/dL (reference range, 13–17 g/dL). A MetHb blood level of 11% was reported on co-oximetry. An arterial blood gas analysis revealed a pH of 7.46; partial pressure of carbon dioxide of 41 mm Hg; and partial pressure of oxygen of 63 mm Hg. The haptoglobin level was low at 2.6 mg/dL (reference range, 30–200 mg/dL). An absolute reticulocyte count was markedly elevated at 0.4×106/mL (reference range, 0.03–0.08×106/mL), lactate dehydrogenase was elevated at 430 U/L (reference range, 125–220 U/L), and indirect billirubin was high at 0.9 mg/dL (reference range, 0–0.5 mg/dL), consistent with hemolytic anemia. Electrolyte serum levels and renal function tests were within reference range. A diagnosis of MetHb induced by the lidocaine-prilocaine cream was rendered, and intravenous methylene blue 72 mg (1 mg/kg) was administered over 10 minutes. Within the next 60 minutes, the patient’s drowsiness and arterial desaturation resolved. A subsequent MetHb measurement taken several hours later was reduced to 4%. The patient remained asymptomatic and was eventually discharged.
Methemoglobinemia is an altered state of hemoglobin where the ferrous (Fe2+) ions of heme are oxidized to the ferric (Fe3+) state. These ferric ions are unable to bind oxygen, resulting in impaired oxygen delivery to tissues.1 Local anesthetics, which are strong oxidizers, have been reported to induce MetHb.2 In our patient, the extensive use of lidocaine 2.5%–prilocaine 2.5% cream resulted in severe life-threatening MetHb. The oxidizing properties of local anesthetics can be attributed to their chemical structure. Benzocaine is metabolized to potent oxidizers such as aniline, phenylhydroxylamine, and nitrobenzene.3 Prilocaine and another potent oxidizer, ortho-toluidine, which is a metabolite of prilocaine, can oxidize the iron in hemoglobin from ferrous (Fe2+) to ferric (Fe3+), leading to MetHb.2,3
Cases of anesthetic-induced MetHb primarily are associated with overuse of the product by applying it to large surface areas or using it for prolonged periods of time. In one case report, the occlusive dressing of the lidocaine-prilocaine cream applied to skin of the legs that was already abraded by laser epilation therapy resulted in MetHb.4 In our patient, applying the topical anesthetic to the eroded high-absorptive mucosal surface of the scrotal skin and the use of occlusive dressings increased the risk for toxicity. Absorption from scrotal skin is 40-times higher than the forearm.5 The face, axillae, and scalp also exhibit increased absorption compared to the forearm—10-, 4-, and 3-times higher, respectively.
In recent years, the use of topical anesthetics has greatly expanded due to the popularity of aesthetic and cosmetic procedures. These procedures often are performed in an outpatient setting.6 Dermatologists should be well aware of MetHb as a serious adverse effect and guide patients accordingly, as patients do not tend to consider a local anesthetic to be a drug. Drug interactions also may affect free lidocaine concentrations by liver cytochrome P450 metabolism; although this was not the case with our patient, special attention should be given to potential interactions that may exacerbate this serious adverse effect. Consideration should be given to patients applying the anesthetic to areas with high absorption capacity.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646-656.
- Guay J. Methemoglobinemia related to local anesthetics: a summary of 242 episodes. Anesth Analg. 2009;108:837-845.
- Jakobson B, Nilsson A. Methemoglobinemia associated with a prilocaine-lidocaine cream and trimethoprim-sulphamethoxazole. a case report. Acta Anaesthesiol Scand. 1985;29:453-455.
- Hahn I, Hoffman RS, Nelson LS. EMLA®-induced methemoglobinemia and systemic topical anesthetic toxicity. J Emerg Med. 2004;26:85-88.
- Feldmann RJ, Maibach HI. Regional variation in percutaneous penetration of 14C cortisol in man. J Invest Dermatol. 1967;48:181-183.
- Alster T. Review of lidocaine/tetracaine cream as a topical anesthetic for dermatologic laser procedures. Pain Ther. 2013;2:11-19.
To the Editor:
Methemoglobinemia (MetHb) is a condition caused by elevated levels of methemoglobin in the blood, which leads to an overall reduced ability of red blood cells to release oxygen to tissues, causing tissue hypoxia. Methemoglobinemia may be congenital or acquired. Various antibiotics and local anesthetics have been reported to induce acquired MetHb.1 We describe an adult who presented with MetHb resulting from excessive topical application of local anesthetics for painful scrotal ulcers.
A 54-year-old man presented with multiple scrotal and penile shaft ulcers of a few weeks’ duration with no systemic concerns. His medical history included chronic hepatitis C virus (HCV) and lumbar disc disease. Physical examination revealed multiple erosions and ulcers on an erythematous base involving the scrotal skin and distal penile shaft (Figure). Histopathology revealed acute leukocytoclastic vasculitis, and a laboratory workup was positive for mixed cryoglobulinemia that was thought to be HCV related. The patient was started on a systemic corticosteroid treatment in addition to sofosbuvir-velpatasvir for the treatment of HCV-related mixed cryoglobulinemic vasculitis. Concomitantly, the patient self-treated for pain with a local anesthetic cream containing lidocaine 2.5% and prilocaine 2.5%, applying it excessively every few hours daily for 2 weeks. He also intermittently used occlusive dressings.

After 2 weeks of application, the patient developed lightheadedness and shortness of breath. He returned and was admitted for further evaluation. He had dyspnea and tachypnea of 22 breaths per minute. He also had mild tachycardia (109 beats per minute). He did not have a fever, and his blood pressure was normal. The oxygen saturation measured in ambient room air by pulse oximetry was 82%. A neurologic examination was normal except for mild drowsiness. The lungs were clear, and heart sounds were normal. A 12-lead electrocardiogram also was normal. A complete blood cell count showed severe macrocytic anemia with a hemoglobin level of 7 g/dL, which was a severe decline from the patient’s baseline level of 14 g/dL (reference range, 13–17 g/dL). A MetHb blood level of 11% was reported on co-oximetry. An arterial blood gas analysis revealed a pH of 7.46; partial pressure of carbon dioxide of 41 mm Hg; and partial pressure of oxygen of 63 mm Hg. The haptoglobin level was low at 2.6 mg/dL (reference range, 30–200 mg/dL). An absolute reticulocyte count was markedly elevated at 0.4×106/mL (reference range, 0.03–0.08×106/mL), lactate dehydrogenase was elevated at 430 U/L (reference range, 125–220 U/L), and indirect billirubin was high at 0.9 mg/dL (reference range, 0–0.5 mg/dL), consistent with hemolytic anemia. Electrolyte serum levels and renal function tests were within reference range. A diagnosis of MetHb induced by the lidocaine-prilocaine cream was rendered, and intravenous methylene blue 72 mg (1 mg/kg) was administered over 10 minutes. Within the next 60 minutes, the patient’s drowsiness and arterial desaturation resolved. A subsequent MetHb measurement taken several hours later was reduced to 4%. The patient remained asymptomatic and was eventually discharged.
Methemoglobinemia is an altered state of hemoglobin where the ferrous (Fe2+) ions of heme are oxidized to the ferric (Fe3+) state. These ferric ions are unable to bind oxygen, resulting in impaired oxygen delivery to tissues.1 Local anesthetics, which are strong oxidizers, have been reported to induce MetHb.2 In our patient, the extensive use of lidocaine 2.5%–prilocaine 2.5% cream resulted in severe life-threatening MetHb. The oxidizing properties of local anesthetics can be attributed to their chemical structure. Benzocaine is metabolized to potent oxidizers such as aniline, phenylhydroxylamine, and nitrobenzene.3 Prilocaine and another potent oxidizer, ortho-toluidine, which is a metabolite of prilocaine, can oxidize the iron in hemoglobin from ferrous (Fe2+) to ferric (Fe3+), leading to MetHb.2,3
Cases of anesthetic-induced MetHb primarily are associated with overuse of the product by applying it to large surface areas or using it for prolonged periods of time. In one case report, the occlusive dressing of the lidocaine-prilocaine cream applied to skin of the legs that was already abraded by laser epilation therapy resulted in MetHb.4 In our patient, applying the topical anesthetic to the eroded high-absorptive mucosal surface of the scrotal skin and the use of occlusive dressings increased the risk for toxicity. Absorption from scrotal skin is 40-times higher than the forearm.5 The face, axillae, and scalp also exhibit increased absorption compared to the forearm—10-, 4-, and 3-times higher, respectively.
In recent years, the use of topical anesthetics has greatly expanded due to the popularity of aesthetic and cosmetic procedures. These procedures often are performed in an outpatient setting.6 Dermatologists should be well aware of MetHb as a serious adverse effect and guide patients accordingly, as patients do not tend to consider a local anesthetic to be a drug. Drug interactions also may affect free lidocaine concentrations by liver cytochrome P450 metabolism; although this was not the case with our patient, special attention should be given to potential interactions that may exacerbate this serious adverse effect. Consideration should be given to patients applying the anesthetic to areas with high absorption capacity.
To the Editor:
Methemoglobinemia (MetHb) is a condition caused by elevated levels of methemoglobin in the blood, which leads to an overall reduced ability of red blood cells to release oxygen to tissues, causing tissue hypoxia. Methemoglobinemia may be congenital or acquired. Various antibiotics and local anesthetics have been reported to induce acquired MetHb.1 We describe an adult who presented with MetHb resulting from excessive topical application of local anesthetics for painful scrotal ulcers.
A 54-year-old man presented with multiple scrotal and penile shaft ulcers of a few weeks’ duration with no systemic concerns. His medical history included chronic hepatitis C virus (HCV) and lumbar disc disease. Physical examination revealed multiple erosions and ulcers on an erythematous base involving the scrotal skin and distal penile shaft (Figure). Histopathology revealed acute leukocytoclastic vasculitis, and a laboratory workup was positive for mixed cryoglobulinemia that was thought to be HCV related. The patient was started on a systemic corticosteroid treatment in addition to sofosbuvir-velpatasvir for the treatment of HCV-related mixed cryoglobulinemic vasculitis. Concomitantly, the patient self-treated for pain with a local anesthetic cream containing lidocaine 2.5% and prilocaine 2.5%, applying it excessively every few hours daily for 2 weeks. He also intermittently used occlusive dressings.

After 2 weeks of application, the patient developed lightheadedness and shortness of breath. He returned and was admitted for further evaluation. He had dyspnea and tachypnea of 22 breaths per minute. He also had mild tachycardia (109 beats per minute). He did not have a fever, and his blood pressure was normal. The oxygen saturation measured in ambient room air by pulse oximetry was 82%. A neurologic examination was normal except for mild drowsiness. The lungs were clear, and heart sounds were normal. A 12-lead electrocardiogram also was normal. A complete blood cell count showed severe macrocytic anemia with a hemoglobin level of 7 g/dL, which was a severe decline from the patient’s baseline level of 14 g/dL (reference range, 13–17 g/dL). A MetHb blood level of 11% was reported on co-oximetry. An arterial blood gas analysis revealed a pH of 7.46; partial pressure of carbon dioxide of 41 mm Hg; and partial pressure of oxygen of 63 mm Hg. The haptoglobin level was low at 2.6 mg/dL (reference range, 30–200 mg/dL). An absolute reticulocyte count was markedly elevated at 0.4×106/mL (reference range, 0.03–0.08×106/mL), lactate dehydrogenase was elevated at 430 U/L (reference range, 125–220 U/L), and indirect billirubin was high at 0.9 mg/dL (reference range, 0–0.5 mg/dL), consistent with hemolytic anemia. Electrolyte serum levels and renal function tests were within reference range. A diagnosis of MetHb induced by the lidocaine-prilocaine cream was rendered, and intravenous methylene blue 72 mg (1 mg/kg) was administered over 10 minutes. Within the next 60 minutes, the patient’s drowsiness and arterial desaturation resolved. A subsequent MetHb measurement taken several hours later was reduced to 4%. The patient remained asymptomatic and was eventually discharged.
Methemoglobinemia is an altered state of hemoglobin where the ferrous (Fe2+) ions of heme are oxidized to the ferric (Fe3+) state. These ferric ions are unable to bind oxygen, resulting in impaired oxygen delivery to tissues.1 Local anesthetics, which are strong oxidizers, have been reported to induce MetHb.2 In our patient, the extensive use of lidocaine 2.5%–prilocaine 2.5% cream resulted in severe life-threatening MetHb. The oxidizing properties of local anesthetics can be attributed to their chemical structure. Benzocaine is metabolized to potent oxidizers such as aniline, phenylhydroxylamine, and nitrobenzene.3 Prilocaine and another potent oxidizer, ortho-toluidine, which is a metabolite of prilocaine, can oxidize the iron in hemoglobin from ferrous (Fe2+) to ferric (Fe3+), leading to MetHb.2,3
Cases of anesthetic-induced MetHb primarily are associated with overuse of the product by applying it to large surface areas or using it for prolonged periods of time. In one case report, the occlusive dressing of the lidocaine-prilocaine cream applied to skin of the legs that was already abraded by laser epilation therapy resulted in MetHb.4 In our patient, applying the topical anesthetic to the eroded high-absorptive mucosal surface of the scrotal skin and the use of occlusive dressings increased the risk for toxicity. Absorption from scrotal skin is 40-times higher than the forearm.5 The face, axillae, and scalp also exhibit increased absorption compared to the forearm—10-, 4-, and 3-times higher, respectively.
In recent years, the use of topical anesthetics has greatly expanded due to the popularity of aesthetic and cosmetic procedures. These procedures often are performed in an outpatient setting.6 Dermatologists should be well aware of MetHb as a serious adverse effect and guide patients accordingly, as patients do not tend to consider a local anesthetic to be a drug. Drug interactions also may affect free lidocaine concentrations by liver cytochrome P450 metabolism; although this was not the case with our patient, special attention should be given to potential interactions that may exacerbate this serious adverse effect. Consideration should be given to patients applying the anesthetic to areas with high absorption capacity.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646-656.
- Guay J. Methemoglobinemia related to local anesthetics: a summary of 242 episodes. Anesth Analg. 2009;108:837-845.
- Jakobson B, Nilsson A. Methemoglobinemia associated with a prilocaine-lidocaine cream and trimethoprim-sulphamethoxazole. a case report. Acta Anaesthesiol Scand. 1985;29:453-455.
- Hahn I, Hoffman RS, Nelson LS. EMLA®-induced methemoglobinemia and systemic topical anesthetic toxicity. J Emerg Med. 2004;26:85-88.
- Feldmann RJ, Maibach HI. Regional variation in percutaneous penetration of 14C cortisol in man. J Invest Dermatol. 1967;48:181-183.
- Alster T. Review of lidocaine/tetracaine cream as a topical anesthetic for dermatologic laser procedures. Pain Ther. 2013;2:11-19.
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646-656.
- Guay J. Methemoglobinemia related to local anesthetics: a summary of 242 episodes. Anesth Analg. 2009;108:837-845.
- Jakobson B, Nilsson A. Methemoglobinemia associated with a prilocaine-lidocaine cream and trimethoprim-sulphamethoxazole. a case report. Acta Anaesthesiol Scand. 1985;29:453-455.
- Hahn I, Hoffman RS, Nelson LS. EMLA®-induced methemoglobinemia and systemic topical anesthetic toxicity. J Emerg Med. 2004;26:85-88.
- Feldmann RJ, Maibach HI. Regional variation in percutaneous penetration of 14C cortisol in man. J Invest Dermatol. 1967;48:181-183.
- Alster T. Review of lidocaine/tetracaine cream as a topical anesthetic for dermatologic laser procedures. Pain Ther. 2013;2:11-19.
Practice Points
- Consideration should be given to patients applying anesthetic creams to areas with high absorption capacity.
- Dermatologists should be aware of methemoglobinemia as a serious adverse effect of local anesthetics and guide patients accordingly, as patients do not tend to consider these products to be drugs.