User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
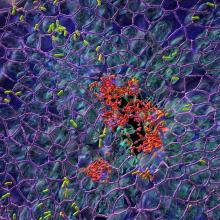
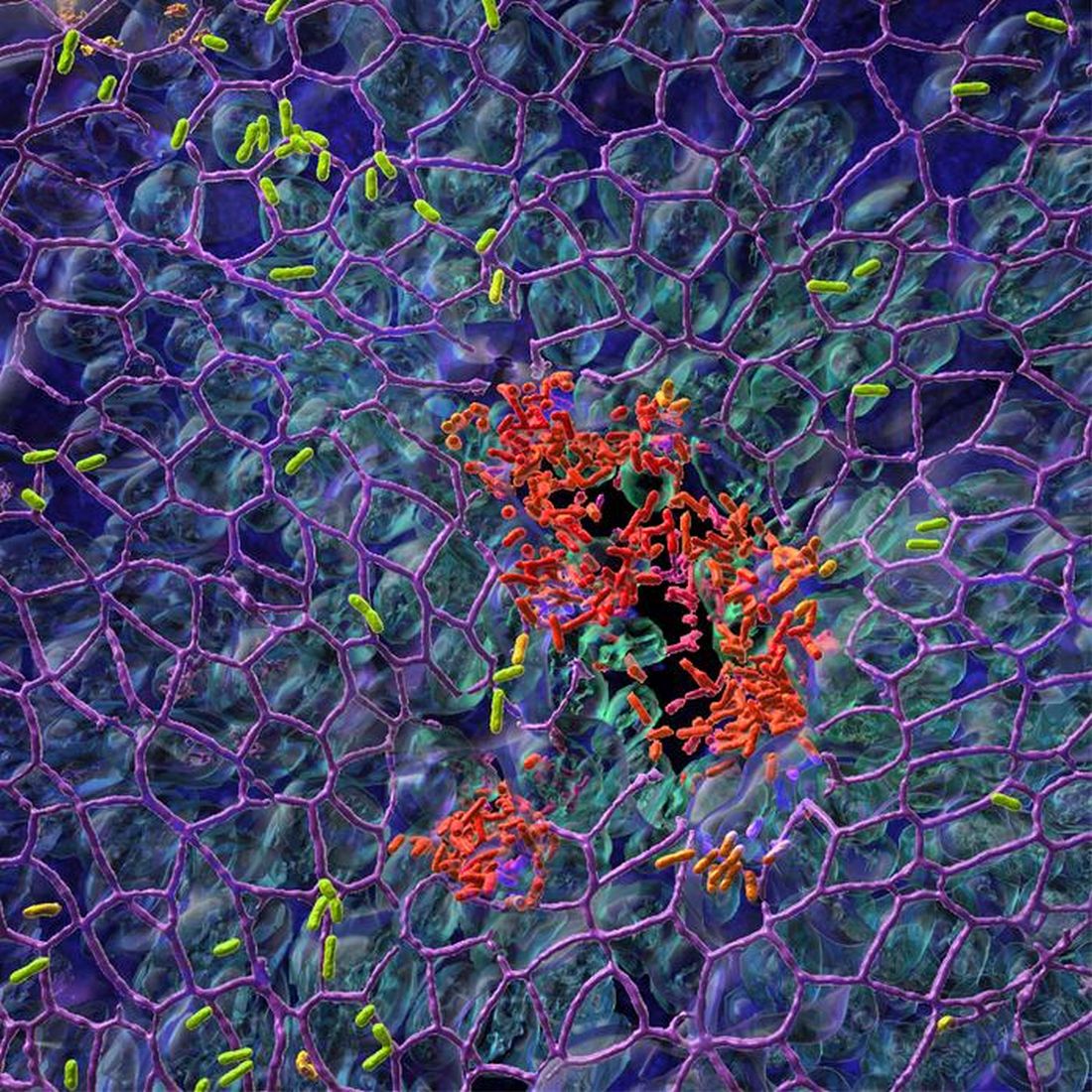
FDA passes on olorofim despite critical need for antifungals
The U.S. Food and Drug Administration is declining to approve the investigational antifungal olorofim and is asking for more data, according to a news release from the manufacturer, F2G.
Olorofim, (formerly known as F901318) is the first in the orotomide class of antifungals to be evaluated clinically for the treatment of invasive mold infections. Its maker, F2G, is a biotech company based in Manchester, England, that focuses on developing drugs for rare fungal diseases.
The company says it remains optimistic and will address the FDA’s requirements and continue to seek approval.
The FDA’s denial comes as fungal infections are becoming increasingly common and resistant to treatment. There are only four antifungal classes currently available, and there are few new candidates in the pipeline. No new classes of antifungals have been developed in 2 decades.
David Andes, MD, chief of the division of infectious diseases at the University of Wisconsin–Madison, told this news organization he shares the hope that the company can meet the requirements to gain approval.
“Some of the early results were really exciting,” he said. “People are enthusiastic about the compound because it has a novel mechanism of action, and it is active against a group of fungi that we have limited to no options for.”
Early results ‘exciting’
Dr. Andes said several physicians have been able to prescribe olorofim under the compassionate use program “and have witnessed success.”
Olorofim is the first antifungal agent to be granted breakthrough therapy designation, which the FDA granted in November 2019 for the treatment of invasive mold infections for patients with limited or no treatment options, including patients with refractory aspergillosis or those who are intolerant of currently available therapy. It is also indicated for infections due to Lomentospora prolificans, Scedosporium, and Scopulariopsis species.
Olorofim received a second breakthrough therapy designation in October 2020. The second designation was granted for treatment of central nervous system coccidioidomycosis that is refractory or for cases that cannot be treated with standard-of-care therapy.
It is very difficult for patients to be approved to receive compassionate use medicines, Dr. Andes pointed out. “I’d like to have access sooner rather than later,” he added.
Dr. Andes says the drugs are expensive and are time consuming to produce. And with antifungals, it is difficult to demonstrate safety in comparison with other antimicrobial agents because “it’s hard to hurt a fungus without having toxicity with human cells.”
Complete response letter issued
F2G received a complete response letter from the FDA regarding its new drug application for olorofim, according to the news release issued by the company. “While F2G is disappointed with this outcome, we remain optimistic about olorofim’s potential to address an unmet need for patients with invasive fungal infections who have exhausted their treatment alternatives,” Francesco Maria Lavino, chief executive officer, said in the release. “We are assessing the details of the Complete Response Letter, and we plan to meet with the FDA to discuss it further.”
Dr. Andes says few other antifungals have made it as far as olorofim in clinical trials.
Lance B. Price, PhD, codirector of the Antibiotic Resistance Action Center at George Washington University in Washington, told this news organization that despite the lack of antifungals in the pipeline, “We can’t allow our desperation to override the checkpoints that ensure that antifungals are safe to use in people.”
In the meantime, he said, it is important to preserve the utility of current antifungals by avoiding overusing them in medicine and agriculture.
“Sadly,” he said, “a drug called ipflufenoquin, which works by a similar mode of action as olorofim, has already been approved by the U.S. Environmental Protection Agency for use in plant agriculture. This could weaken the effectiveness of olorofim for treating things like Aspergillus infections even before the drug has been approved for use in humans.”
Plant drug undermining olorofim efficacy in humans
“While I’m sure this makes financial sense for the makers of ipflufenoquin, it borders on insanity from a public health perspective,” Dr. Price said.
Meanwhile, the global threat of fungal infections grows. The World Health Organization has launched its first-ever list of health-threatening fungi. Authors of a WHO report that contains the list write, “The invasive forms of these fungal infections often affect severely ill patients and those with significant underlying immune system–related conditions.”
F2G will continue to expand olorofim’s clinical trial program, according to the company’s statement. Along with its partner, Shionogi, it is enrolling patients with proven or probable invasive aspergillosis in a global phase 3 trial (OASIS), which will compare outcomes after treatment with olorofim in comparison with amphotericin B liposome (AmBisome) followed by standard of care.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration is declining to approve the investigational antifungal olorofim and is asking for more data, according to a news release from the manufacturer, F2G.
Olorofim, (formerly known as F901318) is the first in the orotomide class of antifungals to be evaluated clinically for the treatment of invasive mold infections. Its maker, F2G, is a biotech company based in Manchester, England, that focuses on developing drugs for rare fungal diseases.
The company says it remains optimistic and will address the FDA’s requirements and continue to seek approval.
The FDA’s denial comes as fungal infections are becoming increasingly common and resistant to treatment. There are only four antifungal classes currently available, and there are few new candidates in the pipeline. No new classes of antifungals have been developed in 2 decades.
David Andes, MD, chief of the division of infectious diseases at the University of Wisconsin–Madison, told this news organization he shares the hope that the company can meet the requirements to gain approval.
“Some of the early results were really exciting,” he said. “People are enthusiastic about the compound because it has a novel mechanism of action, and it is active against a group of fungi that we have limited to no options for.”
Early results ‘exciting’
Dr. Andes said several physicians have been able to prescribe olorofim under the compassionate use program “and have witnessed success.”
Olorofim is the first antifungal agent to be granted breakthrough therapy designation, which the FDA granted in November 2019 for the treatment of invasive mold infections for patients with limited or no treatment options, including patients with refractory aspergillosis or those who are intolerant of currently available therapy. It is also indicated for infections due to Lomentospora prolificans, Scedosporium, and Scopulariopsis species.
Olorofim received a second breakthrough therapy designation in October 2020. The second designation was granted for treatment of central nervous system coccidioidomycosis that is refractory or for cases that cannot be treated with standard-of-care therapy.
It is very difficult for patients to be approved to receive compassionate use medicines, Dr. Andes pointed out. “I’d like to have access sooner rather than later,” he added.
Dr. Andes says the drugs are expensive and are time consuming to produce. And with antifungals, it is difficult to demonstrate safety in comparison with other antimicrobial agents because “it’s hard to hurt a fungus without having toxicity with human cells.”
Complete response letter issued
F2G received a complete response letter from the FDA regarding its new drug application for olorofim, according to the news release issued by the company. “While F2G is disappointed with this outcome, we remain optimistic about olorofim’s potential to address an unmet need for patients with invasive fungal infections who have exhausted their treatment alternatives,” Francesco Maria Lavino, chief executive officer, said in the release. “We are assessing the details of the Complete Response Letter, and we plan to meet with the FDA to discuss it further.”
Dr. Andes says few other antifungals have made it as far as olorofim in clinical trials.
Lance B. Price, PhD, codirector of the Antibiotic Resistance Action Center at George Washington University in Washington, told this news organization that despite the lack of antifungals in the pipeline, “We can’t allow our desperation to override the checkpoints that ensure that antifungals are safe to use in people.”
In the meantime, he said, it is important to preserve the utility of current antifungals by avoiding overusing them in medicine and agriculture.
“Sadly,” he said, “a drug called ipflufenoquin, which works by a similar mode of action as olorofim, has already been approved by the U.S. Environmental Protection Agency for use in plant agriculture. This could weaken the effectiveness of olorofim for treating things like Aspergillus infections even before the drug has been approved for use in humans.”
Plant drug undermining olorofim efficacy in humans
“While I’m sure this makes financial sense for the makers of ipflufenoquin, it borders on insanity from a public health perspective,” Dr. Price said.
Meanwhile, the global threat of fungal infections grows. The World Health Organization has launched its first-ever list of health-threatening fungi. Authors of a WHO report that contains the list write, “The invasive forms of these fungal infections often affect severely ill patients and those with significant underlying immune system–related conditions.”
F2G will continue to expand olorofim’s clinical trial program, according to the company’s statement. Along with its partner, Shionogi, it is enrolling patients with proven or probable invasive aspergillosis in a global phase 3 trial (OASIS), which will compare outcomes after treatment with olorofim in comparison with amphotericin B liposome (AmBisome) followed by standard of care.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration is declining to approve the investigational antifungal olorofim and is asking for more data, according to a news release from the manufacturer, F2G.
Olorofim, (formerly known as F901318) is the first in the orotomide class of antifungals to be evaluated clinically for the treatment of invasive mold infections. Its maker, F2G, is a biotech company based in Manchester, England, that focuses on developing drugs for rare fungal diseases.
The company says it remains optimistic and will address the FDA’s requirements and continue to seek approval.
The FDA’s denial comes as fungal infections are becoming increasingly common and resistant to treatment. There are only four antifungal classes currently available, and there are few new candidates in the pipeline. No new classes of antifungals have been developed in 2 decades.
David Andes, MD, chief of the division of infectious diseases at the University of Wisconsin–Madison, told this news organization he shares the hope that the company can meet the requirements to gain approval.
“Some of the early results were really exciting,” he said. “People are enthusiastic about the compound because it has a novel mechanism of action, and it is active against a group of fungi that we have limited to no options for.”
Early results ‘exciting’
Dr. Andes said several physicians have been able to prescribe olorofim under the compassionate use program “and have witnessed success.”
Olorofim is the first antifungal agent to be granted breakthrough therapy designation, which the FDA granted in November 2019 for the treatment of invasive mold infections for patients with limited or no treatment options, including patients with refractory aspergillosis or those who are intolerant of currently available therapy. It is also indicated for infections due to Lomentospora prolificans, Scedosporium, and Scopulariopsis species.
Olorofim received a second breakthrough therapy designation in October 2020. The second designation was granted for treatment of central nervous system coccidioidomycosis that is refractory or for cases that cannot be treated with standard-of-care therapy.
It is very difficult for patients to be approved to receive compassionate use medicines, Dr. Andes pointed out. “I’d like to have access sooner rather than later,” he added.
Dr. Andes says the drugs are expensive and are time consuming to produce. And with antifungals, it is difficult to demonstrate safety in comparison with other antimicrobial agents because “it’s hard to hurt a fungus without having toxicity with human cells.”
Complete response letter issued
F2G received a complete response letter from the FDA regarding its new drug application for olorofim, according to the news release issued by the company. “While F2G is disappointed with this outcome, we remain optimistic about olorofim’s potential to address an unmet need for patients with invasive fungal infections who have exhausted their treatment alternatives,” Francesco Maria Lavino, chief executive officer, said in the release. “We are assessing the details of the Complete Response Letter, and we plan to meet with the FDA to discuss it further.”
Dr. Andes says few other antifungals have made it as far as olorofim in clinical trials.
Lance B. Price, PhD, codirector of the Antibiotic Resistance Action Center at George Washington University in Washington, told this news organization that despite the lack of antifungals in the pipeline, “We can’t allow our desperation to override the checkpoints that ensure that antifungals are safe to use in people.”
In the meantime, he said, it is important to preserve the utility of current antifungals by avoiding overusing them in medicine and agriculture.
“Sadly,” he said, “a drug called ipflufenoquin, which works by a similar mode of action as olorofim, has already been approved by the U.S. Environmental Protection Agency for use in plant agriculture. This could weaken the effectiveness of olorofim for treating things like Aspergillus infections even before the drug has been approved for use in humans.”
Plant drug undermining olorofim efficacy in humans
“While I’m sure this makes financial sense for the makers of ipflufenoquin, it borders on insanity from a public health perspective,” Dr. Price said.
Meanwhile, the global threat of fungal infections grows. The World Health Organization has launched its first-ever list of health-threatening fungi. Authors of a WHO report that contains the list write, “The invasive forms of these fungal infections often affect severely ill patients and those with significant underlying immune system–related conditions.”
F2G will continue to expand olorofim’s clinical trial program, according to the company’s statement. Along with its partner, Shionogi, it is enrolling patients with proven or probable invasive aspergillosis in a global phase 3 trial (OASIS), which will compare outcomes after treatment with olorofim in comparison with amphotericin B liposome (AmBisome) followed by standard of care.
A version of this article first appeared on Medscape.com.
Low-calorie tastes sweeter with a little salt
Low-calorie tastes sweeter with a little salt
Diet and sugar-free foods and drinks seem like a good idea, but it’s hard to get past that strange aftertaste, right? It’s the calling card for the noncaloric aspartame- and stevia-containing sweeteners that we consume to make us feel like we can have the best of both worlds.
That weird lingering taste can be a total turn-off for some (raises hand), but researchers have found an almost facepalm solution to the not-so-sweet problem, and it’s salt.
Now, the concept of sweet and salty is not a far-fetched partnership when it comes to snack consumption (try M&Ms in your popcorn). The researchers at Almendra, a manufacturer of stevia sweeteners, put that iconic flavor pair to the test by adding mineral salts that have some nutritional value to lessen the effect of a stevia compound, rebaudioside A, found in noncaloric sweeteners.
The researchers added in magnesium chloride, calcium chloride, and potassium chloride separately to lessen rebaudioside A’s intensity, but they needed so much salt that it killed the sweet taste completely. A blend of the three mineral salts, however, reduced the lingering taste by 79% and improved the real sugar-like taste. The researchers tried this blend in reduced-calorie orange juice and a citrus-flavored soft drink, improving the taste in both.
The salty and sweet match comes in for the win once again. This time helping against the fight of obesity instead of making it worse.
Pseudomonas’ Achilles’ heel is more of an Achilles’ genetic switch
Today, on the long-awaited return of “Bacteria vs. the World,” we meet one of the rock stars of infectious disease.
LOTME: Through the use of imaginary technology, we’re talking to Pseudomonas aeruginosa. Thanks for joining us on such short notice, after Neisseria gonorrhoeae canceled at the last minute.
P. aeruginosa: No problem. I think we can all guess what that little devil is up to.
LOTME: Bacterial resistance to antibiotics is a huge problem for our species. What makes you so hard to fight?
P. aeruginosa: We’ve been trying to keep that a secret, actually, but now that researchers in Switzerland and Denmark seem to have figured it out, I guess it’s okay for me to spill the beans.
LOTME: Beans? What do beans have to do with it?
P. aeruginosa: Nothing, it’s just a colloquial expression that means I’m sharing previously private information.
LOTME: Sure, we knew that. Please, continue your spilling.
P. aeruginosa: The secret is … Well, let’s just say we were a little worried when the Clash released “Should I Stay or Should I Go” back in the 1980s.
LOTME: The Clash? Now we’re really confused.
P. aeruginosa: The answer to their question, “Should I stay or should I go? is yes. Successful invasion of a human is all about division of labor. “While one fraction of the bacterial population adheres to the mucosal surface and forms a biofilm, the other subpopulation spreads to distant tissue sites,” is how the investigators described it. We can increase surface colonization by using a “job-sharing” process, they said, and even resist antibiotics because most of us remain in the protective biofilm.
LOTME: And they say you guys don’t have brains.
P. aeruginosa: But wait, there’s more. We don’t just divide the labor randomly. After the initial colonization we form two functionally distinct subpopulations. One has high levels of the bacterial signaling molecule c-di-GMP and stays put to work on the biofilm. The other group, with low levels of c-di-GMP, heads out to the surrounding tissue to continue the colonization. As project leader Urs Jenal put it, “By identifying the genetic switch, we have tracked down the Achilles heel of the pathogen.”
LOTME: Pretty clever stuff, for humans, anyway.
P. aeruginosa: We agree, but now that you know our secret, we can’t let you share it.
LOTME: Wait! The journal article’s already been published. Your secret is out. You can’t stop that by infecting me.
P. aeruginosa: True enough, but are you familiar with the fable of the scorpion and the frog? It’s our nature.
LOTME: Nooooo! N. gonorrhoeae wouldn’t have done this!
What a pain in the Butt
Businesses rise and businesses fall. We all know that one cursed location, that spot in town where we see businesses move in and close up in a matter of months. At the same time, though, there are also businesses that have been around as long as anyone can remember, pillars of the community.
Corydon, IN., likely has a few such long-lived shops, but it is officially down one 70-year-old family business as of late April, with the unfortunate passing of beloved local pharmacy Butt Drugs. Prescription pick-up in rear.
The business dates back to 1952, when it was founded as William H. Butt Drugs. We’re sure William Butt was never teased about his last name. Nope. No one would ever do that. After he passed the store to his children, it underwent a stint as Butt Rexall Drugs. When the shop was passed down to its third-generation and ultimately final owner, Katie Butt Beckort, she decided to simplify the name. Get right down to the bottom of things, as it were.
Butt Drugs was a popular spot, featuring an old-school soda fountain and themed souvenirs. According to Ms. Butt Beckort, people would come from miles away to buy “I love Butt Drugs” T-shirts, magnets, and so on. Yes, they knew perfectly well what they were sitting on.
So, if was such a hit, why did it close? Butt Drugs may have a hilarious name and merchandise to match, but the pharmacy portion of the pharmacy had been losing money for years. You know, the actual point of the business. As with so many things, we can blame it on the insurance companies. More than half the drugs that passed through Butt Drugs’ doors were sold at a loss, because the insurance companies refused to reimburse the store more than the wholesale price of the drug. Not even a good butt drug could clear up that financial diarrhea.
And so, we’ve lost Butt Drugs forever. Spicy food enthusiasts, coffee drinkers, and all patrons of Taco Bell, take a moment to reflect and mourn on what you’ve lost. No more Butt Drugs to relieve your suffering. A true kick in the butt indeed.
Low-calorie tastes sweeter with a little salt
Diet and sugar-free foods and drinks seem like a good idea, but it’s hard to get past that strange aftertaste, right? It’s the calling card for the noncaloric aspartame- and stevia-containing sweeteners that we consume to make us feel like we can have the best of both worlds.
That weird lingering taste can be a total turn-off for some (raises hand), but researchers have found an almost facepalm solution to the not-so-sweet problem, and it’s salt.
Now, the concept of sweet and salty is not a far-fetched partnership when it comes to snack consumption (try M&Ms in your popcorn). The researchers at Almendra, a manufacturer of stevia sweeteners, put that iconic flavor pair to the test by adding mineral salts that have some nutritional value to lessen the effect of a stevia compound, rebaudioside A, found in noncaloric sweeteners.
The researchers added in magnesium chloride, calcium chloride, and potassium chloride separately to lessen rebaudioside A’s intensity, but they needed so much salt that it killed the sweet taste completely. A blend of the three mineral salts, however, reduced the lingering taste by 79% and improved the real sugar-like taste. The researchers tried this blend in reduced-calorie orange juice and a citrus-flavored soft drink, improving the taste in both.
The salty and sweet match comes in for the win once again. This time helping against the fight of obesity instead of making it worse.
Pseudomonas’ Achilles’ heel is more of an Achilles’ genetic switch
Today, on the long-awaited return of “Bacteria vs. the World,” we meet one of the rock stars of infectious disease.
LOTME: Through the use of imaginary technology, we’re talking to Pseudomonas aeruginosa. Thanks for joining us on such short notice, after Neisseria gonorrhoeae canceled at the last minute.
P. aeruginosa: No problem. I think we can all guess what that little devil is up to.
LOTME: Bacterial resistance to antibiotics is a huge problem for our species. What makes you so hard to fight?
P. aeruginosa: We’ve been trying to keep that a secret, actually, but now that researchers in Switzerland and Denmark seem to have figured it out, I guess it’s okay for me to spill the beans.
LOTME: Beans? What do beans have to do with it?
P. aeruginosa: Nothing, it’s just a colloquial expression that means I’m sharing previously private information.
LOTME: Sure, we knew that. Please, continue your spilling.
P. aeruginosa: The secret is … Well, let’s just say we were a little worried when the Clash released “Should I Stay or Should I Go” back in the 1980s.
LOTME: The Clash? Now we’re really confused.
P. aeruginosa: The answer to their question, “Should I stay or should I go? is yes. Successful invasion of a human is all about division of labor. “While one fraction of the bacterial population adheres to the mucosal surface and forms a biofilm, the other subpopulation spreads to distant tissue sites,” is how the investigators described it. We can increase surface colonization by using a “job-sharing” process, they said, and even resist antibiotics because most of us remain in the protective biofilm.
LOTME: And they say you guys don’t have brains.
P. aeruginosa: But wait, there’s more. We don’t just divide the labor randomly. After the initial colonization we form two functionally distinct subpopulations. One has high levels of the bacterial signaling molecule c-di-GMP and stays put to work on the biofilm. The other group, with low levels of c-di-GMP, heads out to the surrounding tissue to continue the colonization. As project leader Urs Jenal put it, “By identifying the genetic switch, we have tracked down the Achilles heel of the pathogen.”
LOTME: Pretty clever stuff, for humans, anyway.
P. aeruginosa: We agree, but now that you know our secret, we can’t let you share it.
LOTME: Wait! The journal article’s already been published. Your secret is out. You can’t stop that by infecting me.
P. aeruginosa: True enough, but are you familiar with the fable of the scorpion and the frog? It’s our nature.
LOTME: Nooooo! N. gonorrhoeae wouldn’t have done this!
What a pain in the Butt
Businesses rise and businesses fall. We all know that one cursed location, that spot in town where we see businesses move in and close up in a matter of months. At the same time, though, there are also businesses that have been around as long as anyone can remember, pillars of the community.
Corydon, IN., likely has a few such long-lived shops, but it is officially down one 70-year-old family business as of late April, with the unfortunate passing of beloved local pharmacy Butt Drugs. Prescription pick-up in rear.
The business dates back to 1952, when it was founded as William H. Butt Drugs. We’re sure William Butt was never teased about his last name. Nope. No one would ever do that. After he passed the store to his children, it underwent a stint as Butt Rexall Drugs. When the shop was passed down to its third-generation and ultimately final owner, Katie Butt Beckort, she decided to simplify the name. Get right down to the bottom of things, as it were.
Butt Drugs was a popular spot, featuring an old-school soda fountain and themed souvenirs. According to Ms. Butt Beckort, people would come from miles away to buy “I love Butt Drugs” T-shirts, magnets, and so on. Yes, they knew perfectly well what they were sitting on.
So, if was such a hit, why did it close? Butt Drugs may have a hilarious name and merchandise to match, but the pharmacy portion of the pharmacy had been losing money for years. You know, the actual point of the business. As with so many things, we can blame it on the insurance companies. More than half the drugs that passed through Butt Drugs’ doors were sold at a loss, because the insurance companies refused to reimburse the store more than the wholesale price of the drug. Not even a good butt drug could clear up that financial diarrhea.
And so, we’ve lost Butt Drugs forever. Spicy food enthusiasts, coffee drinkers, and all patrons of Taco Bell, take a moment to reflect and mourn on what you’ve lost. No more Butt Drugs to relieve your suffering. A true kick in the butt indeed.
Low-calorie tastes sweeter with a little salt
Diet and sugar-free foods and drinks seem like a good idea, but it’s hard to get past that strange aftertaste, right? It’s the calling card for the noncaloric aspartame- and stevia-containing sweeteners that we consume to make us feel like we can have the best of both worlds.
That weird lingering taste can be a total turn-off for some (raises hand), but researchers have found an almost facepalm solution to the not-so-sweet problem, and it’s salt.
Now, the concept of sweet and salty is not a far-fetched partnership when it comes to snack consumption (try M&Ms in your popcorn). The researchers at Almendra, a manufacturer of stevia sweeteners, put that iconic flavor pair to the test by adding mineral salts that have some nutritional value to lessen the effect of a stevia compound, rebaudioside A, found in noncaloric sweeteners.
The researchers added in magnesium chloride, calcium chloride, and potassium chloride separately to lessen rebaudioside A’s intensity, but they needed so much salt that it killed the sweet taste completely. A blend of the three mineral salts, however, reduced the lingering taste by 79% and improved the real sugar-like taste. The researchers tried this blend in reduced-calorie orange juice and a citrus-flavored soft drink, improving the taste in both.
The salty and sweet match comes in for the win once again. This time helping against the fight of obesity instead of making it worse.
Pseudomonas’ Achilles’ heel is more of an Achilles’ genetic switch
Today, on the long-awaited return of “Bacteria vs. the World,” we meet one of the rock stars of infectious disease.
LOTME: Through the use of imaginary technology, we’re talking to Pseudomonas aeruginosa. Thanks for joining us on such short notice, after Neisseria gonorrhoeae canceled at the last minute.
P. aeruginosa: No problem. I think we can all guess what that little devil is up to.
LOTME: Bacterial resistance to antibiotics is a huge problem for our species. What makes you so hard to fight?
P. aeruginosa: We’ve been trying to keep that a secret, actually, but now that researchers in Switzerland and Denmark seem to have figured it out, I guess it’s okay for me to spill the beans.
LOTME: Beans? What do beans have to do with it?
P. aeruginosa: Nothing, it’s just a colloquial expression that means I’m sharing previously private information.
LOTME: Sure, we knew that. Please, continue your spilling.
P. aeruginosa: The secret is … Well, let’s just say we were a little worried when the Clash released “Should I Stay or Should I Go” back in the 1980s.
LOTME: The Clash? Now we’re really confused.
P. aeruginosa: The answer to their question, “Should I stay or should I go? is yes. Successful invasion of a human is all about division of labor. “While one fraction of the bacterial population adheres to the mucosal surface and forms a biofilm, the other subpopulation spreads to distant tissue sites,” is how the investigators described it. We can increase surface colonization by using a “job-sharing” process, they said, and even resist antibiotics because most of us remain in the protective biofilm.
LOTME: And they say you guys don’t have brains.
P. aeruginosa: But wait, there’s more. We don’t just divide the labor randomly. After the initial colonization we form two functionally distinct subpopulations. One has high levels of the bacterial signaling molecule c-di-GMP and stays put to work on the biofilm. The other group, with low levels of c-di-GMP, heads out to the surrounding tissue to continue the colonization. As project leader Urs Jenal put it, “By identifying the genetic switch, we have tracked down the Achilles heel of the pathogen.”
LOTME: Pretty clever stuff, for humans, anyway.
P. aeruginosa: We agree, but now that you know our secret, we can’t let you share it.
LOTME: Wait! The journal article’s already been published. Your secret is out. You can’t stop that by infecting me.
P. aeruginosa: True enough, but are you familiar with the fable of the scorpion and the frog? It’s our nature.
LOTME: Nooooo! N. gonorrhoeae wouldn’t have done this!
What a pain in the Butt
Businesses rise and businesses fall. We all know that one cursed location, that spot in town where we see businesses move in and close up in a matter of months. At the same time, though, there are also businesses that have been around as long as anyone can remember, pillars of the community.
Corydon, IN., likely has a few such long-lived shops, but it is officially down one 70-year-old family business as of late April, with the unfortunate passing of beloved local pharmacy Butt Drugs. Prescription pick-up in rear.
The business dates back to 1952, when it was founded as William H. Butt Drugs. We’re sure William Butt was never teased about his last name. Nope. No one would ever do that. After he passed the store to his children, it underwent a stint as Butt Rexall Drugs. When the shop was passed down to its third-generation and ultimately final owner, Katie Butt Beckort, she decided to simplify the name. Get right down to the bottom of things, as it were.
Butt Drugs was a popular spot, featuring an old-school soda fountain and themed souvenirs. According to Ms. Butt Beckort, people would come from miles away to buy “I love Butt Drugs” T-shirts, magnets, and so on. Yes, they knew perfectly well what they were sitting on.
So, if was such a hit, why did it close? Butt Drugs may have a hilarious name and merchandise to match, but the pharmacy portion of the pharmacy had been losing money for years. You know, the actual point of the business. As with so many things, we can blame it on the insurance companies. More than half the drugs that passed through Butt Drugs’ doors were sold at a loss, because the insurance companies refused to reimburse the store more than the wholesale price of the drug. Not even a good butt drug could clear up that financial diarrhea.
And so, we’ve lost Butt Drugs forever. Spicy food enthusiasts, coffee drinkers, and all patrons of Taco Bell, take a moment to reflect and mourn on what you’ve lost. No more Butt Drugs to relieve your suffering. A true kick in the butt indeed.
Proposal to cap Part B pay on some drugs draws opposition
An influential panel proposed capping Medicare Part B pay for some drugs, arguing this would remove financial incentives to use more costly medicines when there are less expensive equivalents.
Medical groups have objected to both this recommendation from the Medicare Payment Advisory Commission (MedPAC) and the panel’s underlying premise. MedPAC said financial as well as clinical factors can come into play in clinicians’ choices of drugs for patients.
In an interview, Christina Downey, MD, chair of the Government Affairs Committee of the American College of Rheumatology, said physicians in her field cannot switch patients’ medicines to try to make a profit.
“Patients only respond to the drugs that they respond to,” Dr. Downey said. “It’s frankly very insulting to say that physicians just force patients to go on medicines that are going to make them a bunch of money.”
In a June report to Congress, MedPAC recommended reducing the add-on payment for many drugs given in hospitals and clinics, which are thus covered by Part B, as part of a package of suggestions for addressing rising costs. Part B drug spending grew about 9% annually between 2009 and 2021, rising from $15.4 billion to $42.9 billion, MedPAC said.
Medicare’s current Part B drug pricing model starts with the reported average sales price (ASP) and then adds about 4.3% or 6%, depending on current budget-sequester law, to the cost of medicines.
MedPAC members voted 17-0 in April in favor of a general recommendation to revise the Part B payment approach. In the June report, MedPAC fleshes out this idea. It mentions a model in which the add-on Part B payment would be the lesser of either 6% of the ASP, 3% plus $24, or $220.
The majority of Part B drug administrations are for very low-priced drugs, MedPAC said. But for some of the more costly ones, annual prices can be more than $400,000 per patient, and future launch prices may be even higher for certain types of products, such as gene therapies, MedPAC said.
“There is no evidence that the costs of a drug’s administration are proportionate to the price of the drug,” MedPAC said.
Concerns about how well Medicare covers the cost of drug administration should be addressed through other pathways, such as the American Medical
Association’s Specialty Society Relative Value Scale Update Committee (RUC), MedPAC said. AMA’s RUC advises the Centers for Medicare & Medicaid Services on the physician fee schedule.
Congress is not obliged to act on or to even consider MedPAC’s work. In general, lawmakers and CMS often pay heed to the panel’s recommendations, sometimes incorporating them into new policy.
But this new MedPAC Part B recommendation has drawn strong opposition, similar to the response to a 2016 CMS plan to cut the Part B add-on payment. That plan, which CMS later abandoned, would have cut the markup on Part B drugs to 2.5% and added a flat fee to cover administration costs.
Why not focus on PBMs instead?
The timing of the MedPAC recommendation is poor, given that CMS already is trying to implement the Inflation Reduction Act and create a new system of direct Medicare drug price negotiations, as ordered by Congress, said Madelaine A. Feldman, MD, a rheumatologist based in New Orleans.
A better approach for lowering drug prices would be to focus more on the operations of pharmacy benefit managers (PBMs), said Dr. Feldman, who also is vice president for advocacy and government affairs for the Coalition of State Rheumatology Organizations. A pending bipartisan Senate bill, for example, would prohibit PBM compensation based on the price of a drug as a condition of entering into a contract with a Medicare Part D plan.
Congress needs to take steps to unlink the profits of PBMs from higher drug prices, Dr. Feldman said.
“Until that happens, we can put all the lipstick we want on this big pig, but it’s not going to really fix the problem,” she said.
Reduced pay for drugs acquired through 340B program?
In an interview about the new MedPAC proposal, Ted Okon, executive director of the Community Oncology Alliance, urged renewed attention to what he sees as unintended consequences of the 340B discount drug program.
Under this program, certain hospitals can acquire drugs at steeply reduced prices, but they are not obliged to share those discounts with patients. Hospitals that participate in the 340B program can gain funds when patients and their insurers, including Medicare, pay more for the medicines hospitals and other organizations acquired with the 340B discount. Hospitals say they use the money from the 340B program to expand resources in their communities.
But rapid growth of the program in recent years has led to questions, especially about the role of contract pharmacies that manage the program. Congress created the 340B program in 1992 as a workaround to then new rules on Medicaid drug coverage.
In 2021, participating hospitals and clinics and organizations purchased about $44 billion worth of medicines through the 340B drug program. This was an increase of 16% from the previous year, according to a report from the nonprofit Commonwealth Fund. The number of sites, including hospitals and pharmacies, enrolled in the 340B program rose from 8,100 in 2000 to 50,000 by 2020, the report said.
MedPAC in 2016 urged CMS to reduce the amount Medicare pays for drugs acquired through the 340B program. CMS did so during the Trump administration, a policy later defended by the Biden administration.
But the U.S. Supreme Court last year said Medicare erred in its approach to making this cut, as earlier reported. Federal law required that the Department of Health and Human Services conduct a survey to support such a step, and HHS did not do this, the court said. CMS thus was ordered to return Medicare to the ASP+6% payment model for drugs purchased through the 340B discount program.
In the June report, though, MedPAC stuck by its 2016 recommendation that Medicare reduce its payments for drugs purchased through the 340B discount program despite this setback.
“We continue to believe that this approach is appropriate, and the specific level of payment reduction could be considered further as newer data become available,” MedPAC said.
Hospital, PhRMA split
Hospitals would certainly contest any renewed bid by CMS to drop Medicare’s pay for drugs purchased through the 340B program. The American Hospital Association objected to the MedPAC proposal regarding the add-on payment in Part B drug pricing.
MedPAC commissioners discussed this idea at a January meeting, prompting a February letter from the AHA to the panel. Like Dr. Feldman, AHA said it would be “premature” to launch into a revision of Part B drug pricing while the impact of the IRA on drug prices was still unclear.
AHA also noted that a reduction in Part B drug reimbursement would “shift the responsibility for the rapid increase in drug prices away from drug manufacturers, and instead places the burden on hospitals and patients.”
But the AHA gave a much warmer reception to another proposal MedPAC considered this year and that it included in its June report, which is a plan to address the high cost of certain drugs of as yet unconfirmed clinical benefit.
In April, the AHA said it supports a move toward a “value-based approach” in certain cases in which first-in-class medicines are sold under U.S. Food and Drug Administration’s accelerated approvals. Medicare could then cap payment for such drugs that have excessively high launch prices and uncertain clinical benefit, AHA said.
In the June report, MedPAC recommended that Medicare be able to place such a limit on Part B payments in certain cases, including ones in which companies do not meet FDA deadlines for postmarketing confirmatory trials.
The Pharmaceutical Research and Manufacturers of America (PhRMA) objected to this proposed change. The trade group for drugmakers said the FDA often revises and extends enrollment milestones for pending confirmatory trials when companies hit snags, such as challenges in enrolling patients, PhRMA said.
Reducing Part B payment for drugs for which confirmatory trials have been delayed would have a “disproportionate impact” on smaller and rural communities, where independent practices struggle to keep their doors open as it is, PhRMA spokeswoman Nicole Longo wrote in a blog post.
“If physicians can’t afford to administer a medicine, then they won’t and that means their patients won’t have access to them either,” Ms. Longo wrote.
A version of this article first appeared on Medscape.com.
An influential panel proposed capping Medicare Part B pay for some drugs, arguing this would remove financial incentives to use more costly medicines when there are less expensive equivalents.
Medical groups have objected to both this recommendation from the Medicare Payment Advisory Commission (MedPAC) and the panel’s underlying premise. MedPAC said financial as well as clinical factors can come into play in clinicians’ choices of drugs for patients.
In an interview, Christina Downey, MD, chair of the Government Affairs Committee of the American College of Rheumatology, said physicians in her field cannot switch patients’ medicines to try to make a profit.
“Patients only respond to the drugs that they respond to,” Dr. Downey said. “It’s frankly very insulting to say that physicians just force patients to go on medicines that are going to make them a bunch of money.”
In a June report to Congress, MedPAC recommended reducing the add-on payment for many drugs given in hospitals and clinics, which are thus covered by Part B, as part of a package of suggestions for addressing rising costs. Part B drug spending grew about 9% annually between 2009 and 2021, rising from $15.4 billion to $42.9 billion, MedPAC said.
Medicare’s current Part B drug pricing model starts with the reported average sales price (ASP) and then adds about 4.3% or 6%, depending on current budget-sequester law, to the cost of medicines.
MedPAC members voted 17-0 in April in favor of a general recommendation to revise the Part B payment approach. In the June report, MedPAC fleshes out this idea. It mentions a model in which the add-on Part B payment would be the lesser of either 6% of the ASP, 3% plus $24, or $220.
The majority of Part B drug administrations are for very low-priced drugs, MedPAC said. But for some of the more costly ones, annual prices can be more than $400,000 per patient, and future launch prices may be even higher for certain types of products, such as gene therapies, MedPAC said.
“There is no evidence that the costs of a drug’s administration are proportionate to the price of the drug,” MedPAC said.
Concerns about how well Medicare covers the cost of drug administration should be addressed through other pathways, such as the American Medical
Association’s Specialty Society Relative Value Scale Update Committee (RUC), MedPAC said. AMA’s RUC advises the Centers for Medicare & Medicaid Services on the physician fee schedule.
Congress is not obliged to act on or to even consider MedPAC’s work. In general, lawmakers and CMS often pay heed to the panel’s recommendations, sometimes incorporating them into new policy.
But this new MedPAC Part B recommendation has drawn strong opposition, similar to the response to a 2016 CMS plan to cut the Part B add-on payment. That plan, which CMS later abandoned, would have cut the markup on Part B drugs to 2.5% and added a flat fee to cover administration costs.
Why not focus on PBMs instead?
The timing of the MedPAC recommendation is poor, given that CMS already is trying to implement the Inflation Reduction Act and create a new system of direct Medicare drug price negotiations, as ordered by Congress, said Madelaine A. Feldman, MD, a rheumatologist based in New Orleans.
A better approach for lowering drug prices would be to focus more on the operations of pharmacy benefit managers (PBMs), said Dr. Feldman, who also is vice president for advocacy and government affairs for the Coalition of State Rheumatology Organizations. A pending bipartisan Senate bill, for example, would prohibit PBM compensation based on the price of a drug as a condition of entering into a contract with a Medicare Part D plan.
Congress needs to take steps to unlink the profits of PBMs from higher drug prices, Dr. Feldman said.
“Until that happens, we can put all the lipstick we want on this big pig, but it’s not going to really fix the problem,” she said.
Reduced pay for drugs acquired through 340B program?
In an interview about the new MedPAC proposal, Ted Okon, executive director of the Community Oncology Alliance, urged renewed attention to what he sees as unintended consequences of the 340B discount drug program.
Under this program, certain hospitals can acquire drugs at steeply reduced prices, but they are not obliged to share those discounts with patients. Hospitals that participate in the 340B program can gain funds when patients and their insurers, including Medicare, pay more for the medicines hospitals and other organizations acquired with the 340B discount. Hospitals say they use the money from the 340B program to expand resources in their communities.
But rapid growth of the program in recent years has led to questions, especially about the role of contract pharmacies that manage the program. Congress created the 340B program in 1992 as a workaround to then new rules on Medicaid drug coverage.
In 2021, participating hospitals and clinics and organizations purchased about $44 billion worth of medicines through the 340B drug program. This was an increase of 16% from the previous year, according to a report from the nonprofit Commonwealth Fund. The number of sites, including hospitals and pharmacies, enrolled in the 340B program rose from 8,100 in 2000 to 50,000 by 2020, the report said.
MedPAC in 2016 urged CMS to reduce the amount Medicare pays for drugs acquired through the 340B program. CMS did so during the Trump administration, a policy later defended by the Biden administration.
But the U.S. Supreme Court last year said Medicare erred in its approach to making this cut, as earlier reported. Federal law required that the Department of Health and Human Services conduct a survey to support such a step, and HHS did not do this, the court said. CMS thus was ordered to return Medicare to the ASP+6% payment model for drugs purchased through the 340B discount program.
In the June report, though, MedPAC stuck by its 2016 recommendation that Medicare reduce its payments for drugs purchased through the 340B discount program despite this setback.
“We continue to believe that this approach is appropriate, and the specific level of payment reduction could be considered further as newer data become available,” MedPAC said.
Hospital, PhRMA split
Hospitals would certainly contest any renewed bid by CMS to drop Medicare’s pay for drugs purchased through the 340B program. The American Hospital Association objected to the MedPAC proposal regarding the add-on payment in Part B drug pricing.
MedPAC commissioners discussed this idea at a January meeting, prompting a February letter from the AHA to the panel. Like Dr. Feldman, AHA said it would be “premature” to launch into a revision of Part B drug pricing while the impact of the IRA on drug prices was still unclear.
AHA also noted that a reduction in Part B drug reimbursement would “shift the responsibility for the rapid increase in drug prices away from drug manufacturers, and instead places the burden on hospitals and patients.”
But the AHA gave a much warmer reception to another proposal MedPAC considered this year and that it included in its June report, which is a plan to address the high cost of certain drugs of as yet unconfirmed clinical benefit.
In April, the AHA said it supports a move toward a “value-based approach” in certain cases in which first-in-class medicines are sold under U.S. Food and Drug Administration’s accelerated approvals. Medicare could then cap payment for such drugs that have excessively high launch prices and uncertain clinical benefit, AHA said.
In the June report, MedPAC recommended that Medicare be able to place such a limit on Part B payments in certain cases, including ones in which companies do not meet FDA deadlines for postmarketing confirmatory trials.
The Pharmaceutical Research and Manufacturers of America (PhRMA) objected to this proposed change. The trade group for drugmakers said the FDA often revises and extends enrollment milestones for pending confirmatory trials when companies hit snags, such as challenges in enrolling patients, PhRMA said.
Reducing Part B payment for drugs for which confirmatory trials have been delayed would have a “disproportionate impact” on smaller and rural communities, where independent practices struggle to keep their doors open as it is, PhRMA spokeswoman Nicole Longo wrote in a blog post.
“If physicians can’t afford to administer a medicine, then they won’t and that means their patients won’t have access to them either,” Ms. Longo wrote.
A version of this article first appeared on Medscape.com.
An influential panel proposed capping Medicare Part B pay for some drugs, arguing this would remove financial incentives to use more costly medicines when there are less expensive equivalents.
Medical groups have objected to both this recommendation from the Medicare Payment Advisory Commission (MedPAC) and the panel’s underlying premise. MedPAC said financial as well as clinical factors can come into play in clinicians’ choices of drugs for patients.
In an interview, Christina Downey, MD, chair of the Government Affairs Committee of the American College of Rheumatology, said physicians in her field cannot switch patients’ medicines to try to make a profit.
“Patients only respond to the drugs that they respond to,” Dr. Downey said. “It’s frankly very insulting to say that physicians just force patients to go on medicines that are going to make them a bunch of money.”
In a June report to Congress, MedPAC recommended reducing the add-on payment for many drugs given in hospitals and clinics, which are thus covered by Part B, as part of a package of suggestions for addressing rising costs. Part B drug spending grew about 9% annually between 2009 and 2021, rising from $15.4 billion to $42.9 billion, MedPAC said.
Medicare’s current Part B drug pricing model starts with the reported average sales price (ASP) and then adds about 4.3% or 6%, depending on current budget-sequester law, to the cost of medicines.
MedPAC members voted 17-0 in April in favor of a general recommendation to revise the Part B payment approach. In the June report, MedPAC fleshes out this idea. It mentions a model in which the add-on Part B payment would be the lesser of either 6% of the ASP, 3% plus $24, or $220.
The majority of Part B drug administrations are for very low-priced drugs, MedPAC said. But for some of the more costly ones, annual prices can be more than $400,000 per patient, and future launch prices may be even higher for certain types of products, such as gene therapies, MedPAC said.
“There is no evidence that the costs of a drug’s administration are proportionate to the price of the drug,” MedPAC said.
Concerns about how well Medicare covers the cost of drug administration should be addressed through other pathways, such as the American Medical
Association’s Specialty Society Relative Value Scale Update Committee (RUC), MedPAC said. AMA’s RUC advises the Centers for Medicare & Medicaid Services on the physician fee schedule.
Congress is not obliged to act on or to even consider MedPAC’s work. In general, lawmakers and CMS often pay heed to the panel’s recommendations, sometimes incorporating them into new policy.
But this new MedPAC Part B recommendation has drawn strong opposition, similar to the response to a 2016 CMS plan to cut the Part B add-on payment. That plan, which CMS later abandoned, would have cut the markup on Part B drugs to 2.5% and added a flat fee to cover administration costs.
Why not focus on PBMs instead?
The timing of the MedPAC recommendation is poor, given that CMS already is trying to implement the Inflation Reduction Act and create a new system of direct Medicare drug price negotiations, as ordered by Congress, said Madelaine A. Feldman, MD, a rheumatologist based in New Orleans.
A better approach for lowering drug prices would be to focus more on the operations of pharmacy benefit managers (PBMs), said Dr. Feldman, who also is vice president for advocacy and government affairs for the Coalition of State Rheumatology Organizations. A pending bipartisan Senate bill, for example, would prohibit PBM compensation based on the price of a drug as a condition of entering into a contract with a Medicare Part D plan.
Congress needs to take steps to unlink the profits of PBMs from higher drug prices, Dr. Feldman said.
“Until that happens, we can put all the lipstick we want on this big pig, but it’s not going to really fix the problem,” she said.
Reduced pay for drugs acquired through 340B program?
In an interview about the new MedPAC proposal, Ted Okon, executive director of the Community Oncology Alliance, urged renewed attention to what he sees as unintended consequences of the 340B discount drug program.
Under this program, certain hospitals can acquire drugs at steeply reduced prices, but they are not obliged to share those discounts with patients. Hospitals that participate in the 340B program can gain funds when patients and their insurers, including Medicare, pay more for the medicines hospitals and other organizations acquired with the 340B discount. Hospitals say they use the money from the 340B program to expand resources in their communities.
But rapid growth of the program in recent years has led to questions, especially about the role of contract pharmacies that manage the program. Congress created the 340B program in 1992 as a workaround to then new rules on Medicaid drug coverage.
In 2021, participating hospitals and clinics and organizations purchased about $44 billion worth of medicines through the 340B drug program. This was an increase of 16% from the previous year, according to a report from the nonprofit Commonwealth Fund. The number of sites, including hospitals and pharmacies, enrolled in the 340B program rose from 8,100 in 2000 to 50,000 by 2020, the report said.
MedPAC in 2016 urged CMS to reduce the amount Medicare pays for drugs acquired through the 340B program. CMS did so during the Trump administration, a policy later defended by the Biden administration.
But the U.S. Supreme Court last year said Medicare erred in its approach to making this cut, as earlier reported. Federal law required that the Department of Health and Human Services conduct a survey to support such a step, and HHS did not do this, the court said. CMS thus was ordered to return Medicare to the ASP+6% payment model for drugs purchased through the 340B discount program.
In the June report, though, MedPAC stuck by its 2016 recommendation that Medicare reduce its payments for drugs purchased through the 340B discount program despite this setback.
“We continue to believe that this approach is appropriate, and the specific level of payment reduction could be considered further as newer data become available,” MedPAC said.
Hospital, PhRMA split
Hospitals would certainly contest any renewed bid by CMS to drop Medicare’s pay for drugs purchased through the 340B program. The American Hospital Association objected to the MedPAC proposal regarding the add-on payment in Part B drug pricing.
MedPAC commissioners discussed this idea at a January meeting, prompting a February letter from the AHA to the panel. Like Dr. Feldman, AHA said it would be “premature” to launch into a revision of Part B drug pricing while the impact of the IRA on drug prices was still unclear.
AHA also noted that a reduction in Part B drug reimbursement would “shift the responsibility for the rapid increase in drug prices away from drug manufacturers, and instead places the burden on hospitals and patients.”
But the AHA gave a much warmer reception to another proposal MedPAC considered this year and that it included in its June report, which is a plan to address the high cost of certain drugs of as yet unconfirmed clinical benefit.
In April, the AHA said it supports a move toward a “value-based approach” in certain cases in which first-in-class medicines are sold under U.S. Food and Drug Administration’s accelerated approvals. Medicare could then cap payment for such drugs that have excessively high launch prices and uncertain clinical benefit, AHA said.
In the June report, MedPAC recommended that Medicare be able to place such a limit on Part B payments in certain cases, including ones in which companies do not meet FDA deadlines for postmarketing confirmatory trials.
The Pharmaceutical Research and Manufacturers of America (PhRMA) objected to this proposed change. The trade group for drugmakers said the FDA often revises and extends enrollment milestones for pending confirmatory trials when companies hit snags, such as challenges in enrolling patients, PhRMA said.
Reducing Part B payment for drugs for which confirmatory trials have been delayed would have a “disproportionate impact” on smaller and rural communities, where independent practices struggle to keep their doors open as it is, PhRMA spokeswoman Nicole Longo wrote in a blog post.
“If physicians can’t afford to administer a medicine, then they won’t and that means their patients won’t have access to them either,” Ms. Longo wrote.
A version of this article first appeared on Medscape.com.
How not to establish rapport with your patient
1. Stride confidently into the room to greet your 84-year-old female patient.
2. Introduce yourself saying, “Hi, I’m Dr. Jeff Benabio.”
3. Extend your clenched fist toward her chest and wait for her to reciprocate.
4. Smile awkwardly behind your mask while you wait.
5. Advise that you are doing a fist bump instead of a handshake to prevent the spread of viruses.
6. Wait.
7. Explain that she can bump, also known as “dap,” you back by extending her clenched fist and bumping into yours.
8. Wait a bit more.
9. Lower your fist and pat her on the shoulder with your left hand. Do so gently so it doesn’t seem like you just did a quick right jab followed by a left hook.
10. Sit down diffidently and pray that you can help her so this office visit is not an utter disaster.
It seemed a good idea for 2020: Let’s stop shaking hands while we wait out this viral apocalypse. Sensible, but entering a patient room and just sitting down didn’t work. It felt cold, impolite – this isn’t the DMV. In medicine, a complete stranger has to trust us to get naked, tell intimate secrets, even be stuck by needles all within minutes of meeting. We needed a trust-building substitute greeting.
There was the Muslim hand-on-my-heart greeting. Or the Hindu “namaste” or Buddhist “amituofo” folded hands. Or perhaps the paternalistic shoulder pat? I went with the fist bump. With some of my partner docs, my old MBA squad, my neighbor, the fist bump felt natural, reciprocated without hesitation. But it fails with many patients. To understand why, it’s helpful to know the history of the fist bump, also known as the dap.
Dap is an acronym for Dignity And Pride. It’s a variation of a handshake that originated among Black soldiers in the Vietnam war as a means of showing fraternity and establishing connectedness. In Vietnam, 30% of the combat battalions were Black. Marginalized in the military and at home, they created a greeting that was meaningful and unique. The dap was a series of shakes, bumps, slaps, and hugs that was symbolic. It was a means of showing respect and humility, that no one is above others, that I’ve got your back and you’ve got mine. It was a powerful recognition of humanity and effective means of personal connection. It spread from the Black community to the general population and it exists still today. The choreographed pregame handshake you see so many NBA players engage in is a descendant of the dap. Like many rituals, it reinforces bonds with those who are your people, your team, those you trust.
The more generalized version is the simple fist bump. It is widely used, notably by President Obama, and in the appropriate circumstance, will almost always be reciprocated. But it doesn’t work well to create trust with a stranger. With a patient for example, you are not showing them respect for some accomplishment. Nor are we connecting with them as a member of your team. Unless this is a patient whom you’ve seen many times before, a fist bump attempt might be met with “are you serious?” In fact, a survey done in 2016 asking infectious disease professionals what they thought of fist bumps as a greeting, very few replied it was a good idea. Most felt it was unprofessional. Not to mention that a fist bump does not symbolize an agreement in the way that a handshake does (and has done since at least the 9th century BC).
With COVID waning and masks doffed, I’ve found myself back to handshaking. Yes, I sanitize before and after, another ritual that has symbolic as well as practical significance. I get fewer sideways glances from my geriatric patients for sure. But I do still offer a little dap for my liquid nitrogen–survivor kids and for the occasional fellow Gen Xer. “Wonder Twin powers, activate!”
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
1. Stride confidently into the room to greet your 84-year-old female patient.
2. Introduce yourself saying, “Hi, I’m Dr. Jeff Benabio.”
3. Extend your clenched fist toward her chest and wait for her to reciprocate.
4. Smile awkwardly behind your mask while you wait.
5. Advise that you are doing a fist bump instead of a handshake to prevent the spread of viruses.
6. Wait.
7. Explain that she can bump, also known as “dap,” you back by extending her clenched fist and bumping into yours.
8. Wait a bit more.
9. Lower your fist and pat her on the shoulder with your left hand. Do so gently so it doesn’t seem like you just did a quick right jab followed by a left hook.
10. Sit down diffidently and pray that you can help her so this office visit is not an utter disaster.
It seemed a good idea for 2020: Let’s stop shaking hands while we wait out this viral apocalypse. Sensible, but entering a patient room and just sitting down didn’t work. It felt cold, impolite – this isn’t the DMV. In medicine, a complete stranger has to trust us to get naked, tell intimate secrets, even be stuck by needles all within minutes of meeting. We needed a trust-building substitute greeting.
There was the Muslim hand-on-my-heart greeting. Or the Hindu “namaste” or Buddhist “amituofo” folded hands. Or perhaps the paternalistic shoulder pat? I went with the fist bump. With some of my partner docs, my old MBA squad, my neighbor, the fist bump felt natural, reciprocated without hesitation. But it fails with many patients. To understand why, it’s helpful to know the history of the fist bump, also known as the dap.
Dap is an acronym for Dignity And Pride. It’s a variation of a handshake that originated among Black soldiers in the Vietnam war as a means of showing fraternity and establishing connectedness. In Vietnam, 30% of the combat battalions were Black. Marginalized in the military and at home, they created a greeting that was meaningful and unique. The dap was a series of shakes, bumps, slaps, and hugs that was symbolic. It was a means of showing respect and humility, that no one is above others, that I’ve got your back and you’ve got mine. It was a powerful recognition of humanity and effective means of personal connection. It spread from the Black community to the general population and it exists still today. The choreographed pregame handshake you see so many NBA players engage in is a descendant of the dap. Like many rituals, it reinforces bonds with those who are your people, your team, those you trust.
The more generalized version is the simple fist bump. It is widely used, notably by President Obama, and in the appropriate circumstance, will almost always be reciprocated. But it doesn’t work well to create trust with a stranger. With a patient for example, you are not showing them respect for some accomplishment. Nor are we connecting with them as a member of your team. Unless this is a patient whom you’ve seen many times before, a fist bump attempt might be met with “are you serious?” In fact, a survey done in 2016 asking infectious disease professionals what they thought of fist bumps as a greeting, very few replied it was a good idea. Most felt it was unprofessional. Not to mention that a fist bump does not symbolize an agreement in the way that a handshake does (and has done since at least the 9th century BC).
With COVID waning and masks doffed, I’ve found myself back to handshaking. Yes, I sanitize before and after, another ritual that has symbolic as well as practical significance. I get fewer sideways glances from my geriatric patients for sure. But I do still offer a little dap for my liquid nitrogen–survivor kids and for the occasional fellow Gen Xer. “Wonder Twin powers, activate!”
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
1. Stride confidently into the room to greet your 84-year-old female patient.
2. Introduce yourself saying, “Hi, I’m Dr. Jeff Benabio.”
3. Extend your clenched fist toward her chest and wait for her to reciprocate.
4. Smile awkwardly behind your mask while you wait.
5. Advise that you are doing a fist bump instead of a handshake to prevent the spread of viruses.
6. Wait.
7. Explain that she can bump, also known as “dap,” you back by extending her clenched fist and bumping into yours.
8. Wait a bit more.
9. Lower your fist and pat her on the shoulder with your left hand. Do so gently so it doesn’t seem like you just did a quick right jab followed by a left hook.
10. Sit down diffidently and pray that you can help her so this office visit is not an utter disaster.
It seemed a good idea for 2020: Let’s stop shaking hands while we wait out this viral apocalypse. Sensible, but entering a patient room and just sitting down didn’t work. It felt cold, impolite – this isn’t the DMV. In medicine, a complete stranger has to trust us to get naked, tell intimate secrets, even be stuck by needles all within minutes of meeting. We needed a trust-building substitute greeting.
There was the Muslim hand-on-my-heart greeting. Or the Hindu “namaste” or Buddhist “amituofo” folded hands. Or perhaps the paternalistic shoulder pat? I went with the fist bump. With some of my partner docs, my old MBA squad, my neighbor, the fist bump felt natural, reciprocated without hesitation. But it fails with many patients. To understand why, it’s helpful to know the history of the fist bump, also known as the dap.
Dap is an acronym for Dignity And Pride. It’s a variation of a handshake that originated among Black soldiers in the Vietnam war as a means of showing fraternity and establishing connectedness. In Vietnam, 30% of the combat battalions were Black. Marginalized in the military and at home, they created a greeting that was meaningful and unique. The dap was a series of shakes, bumps, slaps, and hugs that was symbolic. It was a means of showing respect and humility, that no one is above others, that I’ve got your back and you’ve got mine. It was a powerful recognition of humanity and effective means of personal connection. It spread from the Black community to the general population and it exists still today. The choreographed pregame handshake you see so many NBA players engage in is a descendant of the dap. Like many rituals, it reinforces bonds with those who are your people, your team, those you trust.
The more generalized version is the simple fist bump. It is widely used, notably by President Obama, and in the appropriate circumstance, will almost always be reciprocated. But it doesn’t work well to create trust with a stranger. With a patient for example, you are not showing them respect for some accomplishment. Nor are we connecting with them as a member of your team. Unless this is a patient whom you’ve seen many times before, a fist bump attempt might be met with “are you serious?” In fact, a survey done in 2016 asking infectious disease professionals what they thought of fist bumps as a greeting, very few replied it was a good idea. Most felt it was unprofessional. Not to mention that a fist bump does not symbolize an agreement in the way that a handshake does (and has done since at least the 9th century BC).
With COVID waning and masks doffed, I’ve found myself back to handshaking. Yes, I sanitize before and after, another ritual that has symbolic as well as practical significance. I get fewer sideways glances from my geriatric patients for sure. But I do still offer a little dap for my liquid nitrogen–survivor kids and for the occasional fellow Gen Xer. “Wonder Twin powers, activate!”
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Methotrexate does not impair sperm quality, small study finds
TOPLINE:
Methotrexate (MTX) is not associated with testicular toxicity, so therapy can be safety started in men pursuing parenthood, a small study finds.
METHODOLOGY:
- Lack of evidence regarding MTX’s effect on sperm quality has resulted in inconsistent recommendations for men actively pursuing parenthood.
- Researchers enrolled 20 men aged 18 years or older with an immune-mediated inflammatory disease (IMID) who were about to begin MTX therapy and 25 healthy men as controls.
- Participants provided semen samples prior to beginning MTX therapy and 13 weeks after beginning therapy.
- Researchers tested samples in both groups for markers of testicular toxicity.
- Also evaluated whether MTX polyglutamates could be detected in sperm of seminal fluid, as a secondary outcome.
TAKEAWAY:
- Found no significant differences in conventional semen parameters, sperm DNA damage, or male reproductive endocrine axis between the MTX group and controls.
- The concentration of MTX polyglutamates is low in both sperm and seminal fluid and is particularly low in sperm.
IN PRACTICE:
“Therapy with MTX can be safely started or continued in men diagnosed with an IMID and with an active wish to become a father,” the authors write.
STUDY DETAILS:
Luis Fernando Perez-Garcia, MD, Erasmus Medical Center, Rotterdam, the Netherlands, led the research. The study was published online in Annals of the Rheumatic Diseases on June 1, 2023.
LIMITATIONS:
The small number of participants and that the study included only MTX starters and not those who have taken MTX longer term.
DISCLOSURES:
Grants from the Dutch Arthritis Foundation, The Netherlands Organization for Health Research and Development, and Consejo Nacional de Ciencia y Tecnologia funded the project. Researchers disclosed financial relationships with Galapagos NV and UCB.
A version of this article first appeared on Medscape.com.
TOPLINE:
Methotrexate (MTX) is not associated with testicular toxicity, so therapy can be safety started in men pursuing parenthood, a small study finds.
METHODOLOGY:
- Lack of evidence regarding MTX’s effect on sperm quality has resulted in inconsistent recommendations for men actively pursuing parenthood.
- Researchers enrolled 20 men aged 18 years or older with an immune-mediated inflammatory disease (IMID) who were about to begin MTX therapy and 25 healthy men as controls.
- Participants provided semen samples prior to beginning MTX therapy and 13 weeks after beginning therapy.
- Researchers tested samples in both groups for markers of testicular toxicity.
- Also evaluated whether MTX polyglutamates could be detected in sperm of seminal fluid, as a secondary outcome.
TAKEAWAY:
- Found no significant differences in conventional semen parameters, sperm DNA damage, or male reproductive endocrine axis between the MTX group and controls.
- The concentration of MTX polyglutamates is low in both sperm and seminal fluid and is particularly low in sperm.
IN PRACTICE:
“Therapy with MTX can be safely started or continued in men diagnosed with an IMID and with an active wish to become a father,” the authors write.
STUDY DETAILS:
Luis Fernando Perez-Garcia, MD, Erasmus Medical Center, Rotterdam, the Netherlands, led the research. The study was published online in Annals of the Rheumatic Diseases on June 1, 2023.
LIMITATIONS:
The small number of participants and that the study included only MTX starters and not those who have taken MTX longer term.
DISCLOSURES:
Grants from the Dutch Arthritis Foundation, The Netherlands Organization for Health Research and Development, and Consejo Nacional de Ciencia y Tecnologia funded the project. Researchers disclosed financial relationships with Galapagos NV and UCB.
A version of this article first appeared on Medscape.com.
TOPLINE:
Methotrexate (MTX) is not associated with testicular toxicity, so therapy can be safety started in men pursuing parenthood, a small study finds.
METHODOLOGY:
- Lack of evidence regarding MTX’s effect on sperm quality has resulted in inconsistent recommendations for men actively pursuing parenthood.
- Researchers enrolled 20 men aged 18 years or older with an immune-mediated inflammatory disease (IMID) who were about to begin MTX therapy and 25 healthy men as controls.
- Participants provided semen samples prior to beginning MTX therapy and 13 weeks after beginning therapy.
- Researchers tested samples in both groups for markers of testicular toxicity.
- Also evaluated whether MTX polyglutamates could be detected in sperm of seminal fluid, as a secondary outcome.
TAKEAWAY:
- Found no significant differences in conventional semen parameters, sperm DNA damage, or male reproductive endocrine axis between the MTX group and controls.
- The concentration of MTX polyglutamates is low in both sperm and seminal fluid and is particularly low in sperm.
IN PRACTICE:
“Therapy with MTX can be safely started or continued in men diagnosed with an IMID and with an active wish to become a father,” the authors write.
STUDY DETAILS:
Luis Fernando Perez-Garcia, MD, Erasmus Medical Center, Rotterdam, the Netherlands, led the research. The study was published online in Annals of the Rheumatic Diseases on June 1, 2023.
LIMITATIONS:
The small number of participants and that the study included only MTX starters and not those who have taken MTX longer term.
DISCLOSURES:
Grants from the Dutch Arthritis Foundation, The Netherlands Organization for Health Research and Development, and Consejo Nacional de Ciencia y Tecnologia funded the project. Researchers disclosed financial relationships with Galapagos NV and UCB.
A version of this article first appeared on Medscape.com.
Warts difficult to eradicate in immunocompromised children
.
Only a quarter of patients (24%) who were undergoing active cancer treatment experienced complete resolution of their warts, compared with 63.3% of patients who were not on active treatment.
In addition, warts persisted or worsened in 56.0% of patients receiving active treatment compared with 13.4% of those who were not receiving it.
“These data enable providers treating warts in children with cancer to have an educated discussion regarding the expected clinical progression of warts and the likelihood of response to wart therapy while on and off anti-cancer treatment,” the authors wrote in the study, published in Pediatric Dermatology.
In immunocompromised children, warts are more common than in the general pediatric population, and more resistant to treatment. But as the authors noted, data on the course and prognosis of warts in pediatric patients who are actively receiving anti-cancer therapy compared with patients who have completed treatment are limited.
Tina Ho, MD, PhD, of the department of dermatology, and colleagues from Boston Children’s Hospital, sought to analyze the clinical course of warts treated in this patient population at their institution over a 10-year period. They conducted a retrospective study of 72 children who were treated for cancer between 2011 and 2021, and who had also been treated for warts.
The median age of the cohort was 12 years, and they were followed for a median of 2 years following their diagnosis of warts. Within this group, more than half (55%) had hematologic malignancies, while 27% had a history of bone marrow transplantation.
Of note, the authors pointed out, 54% of the patients had plantar warts, and 60% of patients (38 of 63) with a documented number of warts had more than five at the time of presentation.
The treatment regimens that the children had received varied, with 81% of patients receiving cytotoxic chemotherapy and 23% of patients on targeted therapies that included immunotherapy.
The warts were most commonly treated with cryotherapy and topical salicylic acid; this was the case for those actively receiving oncology treatment or those who had completed their treatment regimens.
Outcomes of wart treatments were available in 25 of the patients undergoing active cancer treatment and in 30 of those who had completed treatment. For children on active oncology treatment, 5 (20%) achieved partial resolution, 6 (24%) achieved complete resolution, and 14 (56%) experienced persistence or worsening of their warts following therapy. Those who had completed treatment had better outcomes: Seven (23.3%) had a partial response, 19 (63.3%) had complete resolution, and 4 (13.4%) had persistence or worsening of warts after treatment of warts.
The authors also pointed out the treatment of warts can be painful, expensive, and time-consuming. “It is thus imperative that the risks and benefits of these treatments are carefully considered before proceeding with treatment,” wrote Dr. Ho and colleagues. “This is especially true in medically complex children with cancer who may be fearful of procedures and spend significant portions of their young lives within the medical system.”
Limitations to the study include its retrospective design and small sample size. Clinical data were not uniformly complete, and follow-up intervals varied among the participants. Also, it was conducted at a single-institution and at a large tertiary center, so the results may not be fully generalizable.
The authors declared no conflict of interest. No outside funding source was listed.
.
Only a quarter of patients (24%) who were undergoing active cancer treatment experienced complete resolution of their warts, compared with 63.3% of patients who were not on active treatment.
In addition, warts persisted or worsened in 56.0% of patients receiving active treatment compared with 13.4% of those who were not receiving it.
“These data enable providers treating warts in children with cancer to have an educated discussion regarding the expected clinical progression of warts and the likelihood of response to wart therapy while on and off anti-cancer treatment,” the authors wrote in the study, published in Pediatric Dermatology.
In immunocompromised children, warts are more common than in the general pediatric population, and more resistant to treatment. But as the authors noted, data on the course and prognosis of warts in pediatric patients who are actively receiving anti-cancer therapy compared with patients who have completed treatment are limited.
Tina Ho, MD, PhD, of the department of dermatology, and colleagues from Boston Children’s Hospital, sought to analyze the clinical course of warts treated in this patient population at their institution over a 10-year period. They conducted a retrospective study of 72 children who were treated for cancer between 2011 and 2021, and who had also been treated for warts.
The median age of the cohort was 12 years, and they were followed for a median of 2 years following their diagnosis of warts. Within this group, more than half (55%) had hematologic malignancies, while 27% had a history of bone marrow transplantation.
Of note, the authors pointed out, 54% of the patients had plantar warts, and 60% of patients (38 of 63) with a documented number of warts had more than five at the time of presentation.
The treatment regimens that the children had received varied, with 81% of patients receiving cytotoxic chemotherapy and 23% of patients on targeted therapies that included immunotherapy.
The warts were most commonly treated with cryotherapy and topical salicylic acid; this was the case for those actively receiving oncology treatment or those who had completed their treatment regimens.
Outcomes of wart treatments were available in 25 of the patients undergoing active cancer treatment and in 30 of those who had completed treatment. For children on active oncology treatment, 5 (20%) achieved partial resolution, 6 (24%) achieved complete resolution, and 14 (56%) experienced persistence or worsening of their warts following therapy. Those who had completed treatment had better outcomes: Seven (23.3%) had a partial response, 19 (63.3%) had complete resolution, and 4 (13.4%) had persistence or worsening of warts after treatment of warts.
The authors also pointed out the treatment of warts can be painful, expensive, and time-consuming. “It is thus imperative that the risks and benefits of these treatments are carefully considered before proceeding with treatment,” wrote Dr. Ho and colleagues. “This is especially true in medically complex children with cancer who may be fearful of procedures and spend significant portions of their young lives within the medical system.”
Limitations to the study include its retrospective design and small sample size. Clinical data were not uniformly complete, and follow-up intervals varied among the participants. Also, it was conducted at a single-institution and at a large tertiary center, so the results may not be fully generalizable.
The authors declared no conflict of interest. No outside funding source was listed.
.
Only a quarter of patients (24%) who were undergoing active cancer treatment experienced complete resolution of their warts, compared with 63.3% of patients who were not on active treatment.
In addition, warts persisted or worsened in 56.0% of patients receiving active treatment compared with 13.4% of those who were not receiving it.
“These data enable providers treating warts in children with cancer to have an educated discussion regarding the expected clinical progression of warts and the likelihood of response to wart therapy while on and off anti-cancer treatment,” the authors wrote in the study, published in Pediatric Dermatology.
In immunocompromised children, warts are more common than in the general pediatric population, and more resistant to treatment. But as the authors noted, data on the course and prognosis of warts in pediatric patients who are actively receiving anti-cancer therapy compared with patients who have completed treatment are limited.
Tina Ho, MD, PhD, of the department of dermatology, and colleagues from Boston Children’s Hospital, sought to analyze the clinical course of warts treated in this patient population at their institution over a 10-year period. They conducted a retrospective study of 72 children who were treated for cancer between 2011 and 2021, and who had also been treated for warts.
The median age of the cohort was 12 years, and they were followed for a median of 2 years following their diagnosis of warts. Within this group, more than half (55%) had hematologic malignancies, while 27% had a history of bone marrow transplantation.
Of note, the authors pointed out, 54% of the patients had plantar warts, and 60% of patients (38 of 63) with a documented number of warts had more than five at the time of presentation.
The treatment regimens that the children had received varied, with 81% of patients receiving cytotoxic chemotherapy and 23% of patients on targeted therapies that included immunotherapy.
The warts were most commonly treated with cryotherapy and topical salicylic acid; this was the case for those actively receiving oncology treatment or those who had completed their treatment regimens.
Outcomes of wart treatments were available in 25 of the patients undergoing active cancer treatment and in 30 of those who had completed treatment. For children on active oncology treatment, 5 (20%) achieved partial resolution, 6 (24%) achieved complete resolution, and 14 (56%) experienced persistence or worsening of their warts following therapy. Those who had completed treatment had better outcomes: Seven (23.3%) had a partial response, 19 (63.3%) had complete resolution, and 4 (13.4%) had persistence or worsening of warts after treatment of warts.
The authors also pointed out the treatment of warts can be painful, expensive, and time-consuming. “It is thus imperative that the risks and benefits of these treatments are carefully considered before proceeding with treatment,” wrote Dr. Ho and colleagues. “This is especially true in medically complex children with cancer who may be fearful of procedures and spend significant portions of their young lives within the medical system.”
Limitations to the study include its retrospective design and small sample size. Clinical data were not uniformly complete, and follow-up intervals varied among the participants. Also, it was conducted at a single-institution and at a large tertiary center, so the results may not be fully generalizable.
The authors declared no conflict of interest. No outside funding source was listed.
FROM PEDIATRIC DERMATOLOGY
Prognostic factors of SCCs in organ transplant recipients worse compared with general population
, results from a dual cohort study demonstrated.
The findings build on previous research and underscore the need for early diagnosis and aggressive surveillance in this patient population, corresponding author Adele C. Green, MBBS, PhD, professor and senior scientist at the QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published online in JAMA Dermatology. “Squamous cell carcinomas (SCCs) of the skin develop up to 77 times more frequently in immunosuppressed organ transplant recipients (OTRs) than the general population,” they wrote. “Because SCCs cause substantially more morbidity and death in the former, they are postulated to be innately more aggressive than in immunocompetent patients, but OTRs’ higher SCC mortality may simply reflect greater SCC tumor burdens per patient.”
In what is believed to be the first study of its kind, Dr. Green and colleagues drew data from two cohort studies to evaluate five key clinicopathologic indicators of poor SCC outcomes in organ transplant recipients, and in those from the general population in Queensland, Australia: cephalic location, perineural invasion, invasion to/beyond subcutaneous fat, poor differentiation, and tumor size greater than 20 mm. The study population included organ transplant recipients at high risk of skin cancer, who were enrolled in the Skin Tumours in Allograft Recipients (STAR) study, and those from a population-based cohort, the QSkin Sun and Health Study. STAR consisted of lung transplant recipients and kidney and liver transplant recipients at high risk of skin cancer who were recruited from tertiary centers and diagnosed with histopathologically confirmed SCC from 2012 to 2015. QSkin consisted of individuals from Queensland’s general adult population diagnosed with SCCs from 2012 to 2015.
SCC cases in QSkin were ascertained through Australia’s universal health insurance agency and linked with histopathology records. Next, the researchers performed data analysis from both cohort studies to determine the prevalence ratio (PR) of head/neck location, perineural invasion, tumor invasion to/beyond subcutaneous fat, poor cellular differentiation, and tumor diameter greater than 20 mm among SCCs among organ transplant recipients compared with the general population.
After combining the two studies, the researchers compared 741 SCCs excised from 191 organ transplant recipients and 2,558 SCCs excised from 1,507 individuals in the general population. Their median ages were similar (62.7 and 63.7 years, respectively) and most were male (78% and 63.4%, respectively).
As for site of involvement, SCCs developed most often on the head and neck in the transplant recipients (38.6%) and on the arms and hands in the general population (35.2%). After adjustment for age and sex, perineural invasion of SCCs was more than twice as common in transplant recipients than among cases in the general population, as was invasion to/beyond subcutaneous fat (PR of 2.37 for both associations).
In other findings, compared with SCCs in the general population, poorly vs. well-differentiated SCCs were more than threefold more common in transplant recipients (PR, 3.45), while the prevalence of tumors greater than 20 mm vs. 20 mm or smaller was moderately higher in transplant recipients (PR, 1.52).
“These findings are considered generalizable, confirming that OTRs’ poorer SCC outcomes are associated with not only their sheer numbers of SCC tumors, but also with a strong shift toward more invasive, less differentiated, and larger SCC tumors, in agreement with previous findings,” the researchers wrote. “This shift is likely associated with decreased immunosurveillance resulting from immunosuppressive therapy (since carcinogenesis decelerates with therapy cessation) interacting with effects of high UV radiation exposure.”
They acknowledged certain limitations of their analysis, chiefly the lack of central review of SCCs to ensure standard assessment of histopathologic features “including caliber of nerves with perineural invasion and cell differentiation; such a review would not have been feasible logistically.”
The study was supported by grants from the National Health and Medical Research Council of Australia. The researchers reported having no disclosures related to the submitted work.
, results from a dual cohort study demonstrated.
The findings build on previous research and underscore the need for early diagnosis and aggressive surveillance in this patient population, corresponding author Adele C. Green, MBBS, PhD, professor and senior scientist at the QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published online in JAMA Dermatology. “Squamous cell carcinomas (SCCs) of the skin develop up to 77 times more frequently in immunosuppressed organ transplant recipients (OTRs) than the general population,” they wrote. “Because SCCs cause substantially more morbidity and death in the former, they are postulated to be innately more aggressive than in immunocompetent patients, but OTRs’ higher SCC mortality may simply reflect greater SCC tumor burdens per patient.”
In what is believed to be the first study of its kind, Dr. Green and colleagues drew data from two cohort studies to evaluate five key clinicopathologic indicators of poor SCC outcomes in organ transplant recipients, and in those from the general population in Queensland, Australia: cephalic location, perineural invasion, invasion to/beyond subcutaneous fat, poor differentiation, and tumor size greater than 20 mm. The study population included organ transplant recipients at high risk of skin cancer, who were enrolled in the Skin Tumours in Allograft Recipients (STAR) study, and those from a population-based cohort, the QSkin Sun and Health Study. STAR consisted of lung transplant recipients and kidney and liver transplant recipients at high risk of skin cancer who were recruited from tertiary centers and diagnosed with histopathologically confirmed SCC from 2012 to 2015. QSkin consisted of individuals from Queensland’s general adult population diagnosed with SCCs from 2012 to 2015.
SCC cases in QSkin were ascertained through Australia’s universal health insurance agency and linked with histopathology records. Next, the researchers performed data analysis from both cohort studies to determine the prevalence ratio (PR) of head/neck location, perineural invasion, tumor invasion to/beyond subcutaneous fat, poor cellular differentiation, and tumor diameter greater than 20 mm among SCCs among organ transplant recipients compared with the general population.
After combining the two studies, the researchers compared 741 SCCs excised from 191 organ transplant recipients and 2,558 SCCs excised from 1,507 individuals in the general population. Their median ages were similar (62.7 and 63.7 years, respectively) and most were male (78% and 63.4%, respectively).
As for site of involvement, SCCs developed most often on the head and neck in the transplant recipients (38.6%) and on the arms and hands in the general population (35.2%). After adjustment for age and sex, perineural invasion of SCCs was more than twice as common in transplant recipients than among cases in the general population, as was invasion to/beyond subcutaneous fat (PR of 2.37 for both associations).
In other findings, compared with SCCs in the general population, poorly vs. well-differentiated SCCs were more than threefold more common in transplant recipients (PR, 3.45), while the prevalence of tumors greater than 20 mm vs. 20 mm or smaller was moderately higher in transplant recipients (PR, 1.52).
“These findings are considered generalizable, confirming that OTRs’ poorer SCC outcomes are associated with not only their sheer numbers of SCC tumors, but also with a strong shift toward more invasive, less differentiated, and larger SCC tumors, in agreement with previous findings,” the researchers wrote. “This shift is likely associated with decreased immunosurveillance resulting from immunosuppressive therapy (since carcinogenesis decelerates with therapy cessation) interacting with effects of high UV radiation exposure.”
They acknowledged certain limitations of their analysis, chiefly the lack of central review of SCCs to ensure standard assessment of histopathologic features “including caliber of nerves with perineural invasion and cell differentiation; such a review would not have been feasible logistically.”
The study was supported by grants from the National Health and Medical Research Council of Australia. The researchers reported having no disclosures related to the submitted work.
, results from a dual cohort study demonstrated.
The findings build on previous research and underscore the need for early diagnosis and aggressive surveillance in this patient population, corresponding author Adele C. Green, MBBS, PhD, professor and senior scientist at the QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published online in JAMA Dermatology. “Squamous cell carcinomas (SCCs) of the skin develop up to 77 times more frequently in immunosuppressed organ transplant recipients (OTRs) than the general population,” they wrote. “Because SCCs cause substantially more morbidity and death in the former, they are postulated to be innately more aggressive than in immunocompetent patients, but OTRs’ higher SCC mortality may simply reflect greater SCC tumor burdens per patient.”
In what is believed to be the first study of its kind, Dr. Green and colleagues drew data from two cohort studies to evaluate five key clinicopathologic indicators of poor SCC outcomes in organ transplant recipients, and in those from the general population in Queensland, Australia: cephalic location, perineural invasion, invasion to/beyond subcutaneous fat, poor differentiation, and tumor size greater than 20 mm. The study population included organ transplant recipients at high risk of skin cancer, who were enrolled in the Skin Tumours in Allograft Recipients (STAR) study, and those from a population-based cohort, the QSkin Sun and Health Study. STAR consisted of lung transplant recipients and kidney and liver transplant recipients at high risk of skin cancer who were recruited from tertiary centers and diagnosed with histopathologically confirmed SCC from 2012 to 2015. QSkin consisted of individuals from Queensland’s general adult population diagnosed with SCCs from 2012 to 2015.
SCC cases in QSkin were ascertained through Australia’s universal health insurance agency and linked with histopathology records. Next, the researchers performed data analysis from both cohort studies to determine the prevalence ratio (PR) of head/neck location, perineural invasion, tumor invasion to/beyond subcutaneous fat, poor cellular differentiation, and tumor diameter greater than 20 mm among SCCs among organ transplant recipients compared with the general population.
After combining the two studies, the researchers compared 741 SCCs excised from 191 organ transplant recipients and 2,558 SCCs excised from 1,507 individuals in the general population. Their median ages were similar (62.7 and 63.7 years, respectively) and most were male (78% and 63.4%, respectively).
As for site of involvement, SCCs developed most often on the head and neck in the transplant recipients (38.6%) and on the arms and hands in the general population (35.2%). After adjustment for age and sex, perineural invasion of SCCs was more than twice as common in transplant recipients than among cases in the general population, as was invasion to/beyond subcutaneous fat (PR of 2.37 for both associations).
In other findings, compared with SCCs in the general population, poorly vs. well-differentiated SCCs were more than threefold more common in transplant recipients (PR, 3.45), while the prevalence of tumors greater than 20 mm vs. 20 mm or smaller was moderately higher in transplant recipients (PR, 1.52).
“These findings are considered generalizable, confirming that OTRs’ poorer SCC outcomes are associated with not only their sheer numbers of SCC tumors, but also with a strong shift toward more invasive, less differentiated, and larger SCC tumors, in agreement with previous findings,” the researchers wrote. “This shift is likely associated with decreased immunosurveillance resulting from immunosuppressive therapy (since carcinogenesis decelerates with therapy cessation) interacting with effects of high UV radiation exposure.”
They acknowledged certain limitations of their analysis, chiefly the lack of central review of SCCs to ensure standard assessment of histopathologic features “including caliber of nerves with perineural invasion and cell differentiation; such a review would not have been feasible logistically.”
The study was supported by grants from the National Health and Medical Research Council of Australia. The researchers reported having no disclosures related to the submitted work.
FROM JAMA DERMATOLOGY
Erythematous Dermal Facial Plaques in a Neutropenic Patient
THE DIAGNOSIS: Neutrophilic Eccrine Hidradenitis
A biopsy from the left preauricular cheek demonstrated dermal neutrophilic inflammation around eccrine coils with focal necrosis (Figure). No notable diffuse dermal neutrophilic infiltrate was present, ruling out Sweet syndrome, and no notable interstitial neutrophilic infiltrate was present, making cellulitis and erysipelas less likely; panculture of tissue also was negative.1,2 Atypical cells in the deep dermis were positive for CD163 and negative for CD117, CD34, CD123, and myeloperoxidase, consistent with a diagnosis of neutrophilic eccrine hidradenitis (NEH) and reactive histiocytes.3 Treatment with oral prednisone resulted in rapid improvement of symptoms.
Neutrophilic eccrine hidradenitis is a rare reactive neutrophilic dermatosis characterized by eccrine gland involvement. This benign and self-limited condition presents as asymmetric erythematous papules and plaques.2 Among 8 granulocytopenic patients with neutrophilic dermatoses, 5 were diagnosed with NEH.4 Although first identified in 1982, NEH remains poorly understood.2 Initial theories suggested that NEH developed due to cytotoxic substances secreted in sweat glands causing necrosis and neutrophil chemotaxis; however, chemotherapy exposure cannot be linked to every case of NEH. Neutrophilic eccrine hidradenitis can be extremely difficult to differentiate clinically from conditions such as cellulitis and Sweet syndrome.
A patient history can be helpful in identifying triggering factors. Neutrophilic eccrine hidradenitis most commonly is associated with malignant, drug-induced, or infectious triggers, while Sweet syndrome has a broad range of associations including infections, vaccines, inflammatory bowel disease, pregnancy, malignancy, and drug-induced etiologies (Table).1 On average, NEH presents 10 days after chemotherapy induction, with 70% of cases presenting after the first chemotherapy cycle.5 Bacterial cellulitis or erysipelas have an infectious etiology, and patients may report symptoms such as fever, chills, or malaise. Immunosuppressed patients are at greater risk for infection; therefore, clinical signs of infection in a granulocytopenic patient should be addressed urgently.
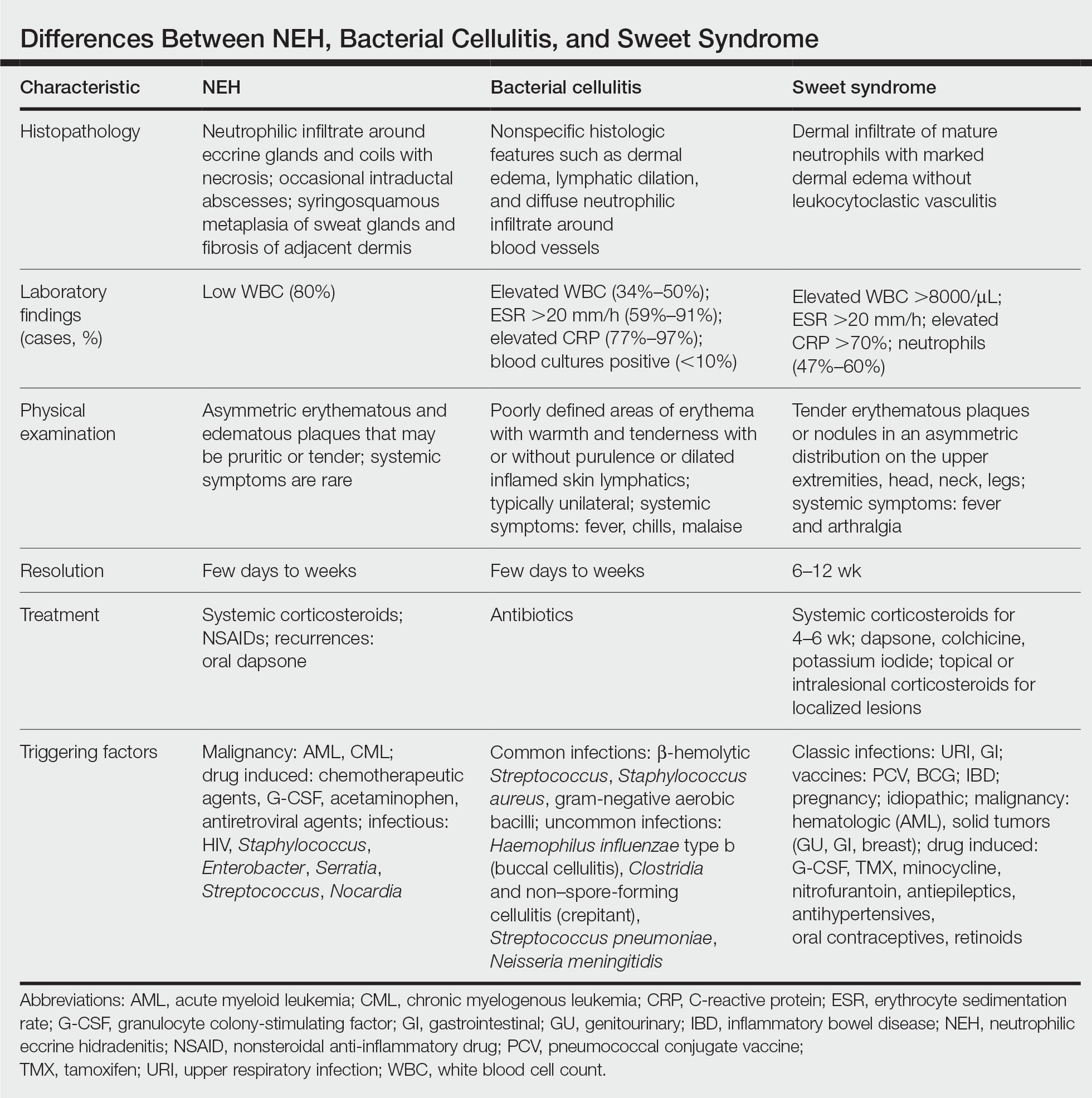
Physical examination may have limited value in differentiating between these diagnoses, as neutrophilic dermatoses notoriously mimic infection. Cutaneous lesions can appear as pruritic or tender erythematous plaques, papules, or nodules in these conditions, though cellulitis and erysipelas tend to be unilateral and may have associated purulence or inflamed skin lymphatics. Given the potential for misdiagnosis, approaching patients with a broad differential can be helpful. In our patient, the differential diagnosis included Sweet syndrome, NEH, bacterial cellulitis, erysipelas, leukemia cutis, sarcoid, and eosinophilic cellulitis.
Leukemia cutis refers to the infiltration of neoplastic leukocytes in the skin and often occurs in patients with peripheral leukemia, most often acute myeloid leukemia or chronic lymphocytic leukemia. Patients with leukemia cutis often have a worse prognosis, as this finding signifies extramedullary spread of disease.6 Clinically, lesions can appear similar to those seen in our patient, though they typically are not symptomatic, can be nodular, tend to exhibit a violaceous hue, and occasionally may be hemorrhagic. Wells syndrome (also known as eosinophilic cellulitis) is an inflammatory dermatosis that presents as painful or pruritic, edematous and erythematous plaques.7,8 A green hue on resolution is present in some cases and may help clinicians differentiate this disease from mimickers.7 Often, eosinophilic cellulitis is misdiagnosed as bacterial cellulitis and treated with antibiotics. The presence of systemic symptoms such as fever or arthralgia is more typical of bacterial cellulitis, erysipelas, eosinophilic cellulitis, or Sweet syndrome than of NEH.1 Additionally, inflammatory markers (ie, C-reactive protein) and white blood cell counts tend to be elevated in bacterial cellulitis and Sweet syndrome, while leukopenia often is seen in NEH.
Histopathology is crucial in distinguishing these disease etiologies. Neutrophilic eccrine hidradenitis is diagnosed by the characteristic neutrophilic infiltrate and necrosis surrounding eccrine glands and coils. There also may be occasional intraductal abscesses and syringosquamous metaplasia of the sweat glands along with fibrosis of the adjacent dermis. In contrast, histologic sections of Sweet syndrome show numerous mature neutrophils infiltrating the dermis with marked papillary dermal edema. The histopathology of bacterial cellulitis and erysipelas is less specific, but common features include dermal edema, lymphatic dilation, and a diffuse neutrophilic infiltrate surrounding blood vessels. Pathogenic organisms may be seen on histopathology but are not required for the diagnosis of bacterial cellulitis or erysipelas.2 Additionally, blood and tissue culture can assist in identification of both the source of infection and the causative organism, but cultures may not always be positive.
Comparatively, the histopathologic features of eosinophilic cellulitis include dermal edema, eosinophilic infiltration, and flame figures that form when eosinophils degranulate and coat collagen fibers with major basic protein. Flame figures are characteristic but not pathognomonic for eosinophilic cellulitis.7 The histopathology of leukemia cutis varies based on the leukemia classification; generally, in acute myeloid leukemia the infiltrate is composed of neoplastic cells in the early stages of development that are positive for myeloid markers such as myeloperoxidase. Atypical and immature granulocytes within the leukocytic infiltrate differentiate this condition from the other diagnoses. Treatment may entail chemotherapy or radiotherapy, and this diagnosis generally carries the worst prognosis of all the conditions in the differential.6
Differentiating between these conditions is important in guiding treatment, especially in patients with febrile neutropenia. Unnecessary steroids in immunosuppressed patients can be dangerous, especially if the patient has an infection such as bacterial cellulitis. Furthermore, unwarranted antibiotic use for noninfectious conditions may expose patients to substantial side effects and not improve the condition. Neutrophilic eccrine hidradenitis typically is self-limited and treated symptomatically with systemic corticosteroids and nonsteroidal anti-inflammatory drugs.3 Sweet syndrome often requires a longer course of oral steroids. Leukemia cutis worsens as the leukemia advances, and treatment of the underlying malignancy is the most effective treatment.9
Early and accurate recognition of the diagnosis can prevent harmful diagnostic delay, unnecessary antibiotic use, or extended steroid taper in neutropenic patients. Appreciating the differences between these diagnoses can assist clinicians in investigating and tailoring a broad differential to specific patient presentations, which is especially critical when considering common mimickers for life-threatening conditions.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.0642
- Srivastava M, Scharf S, Meehan SA, et al. Neutrophilic eccrine hidradenitis masquerading as facial cellulitis. J Am Acad Dermatol. 2007;56:693-696. doi:10.1016/j.jaad.2006.07.032
- Copaescu AM, Castilloux JF, Chababi-Atallah M, et al. A classic clinical case: neutrophilic eccrine hidradenitis. Case Rep Dermatol. 2013; 5:340-346. doi:10.1159/000356229
- Aractingi S, Mallet V, Pinquier L, et al. Neutrophilic dermatoses during granulocytopenia. Arch Dermatol. 1995;131:1141-1145.
- Cohen PR. Neutrophilic dermatoses occurring in oncology patients. Int J Dermatol. 2007;46:106-111. doi:10.1111/j.1365-4632.2006.02605.x
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Chung CL, Cusack CA. Wells syndrome: an enigmatic and therapeutically challenging disease. J Drugs Dermatol. 2006;5:908-911.
- Räßler F, Lukács J, Elsner P. Treatment of eosinophilic cellulitis (Wells syndrome): a systematic review. J Eur Acad Dermatol Venereol. 2016;30:1465-1479. doi:10.1111/jdv.13706
- Hobbs LK, Carr PC, Gru AA, et al. Case and review: cutaneous involvement by chronic neutrophilic leukemia vs Sweet syndrome: a diagnostic dilemma. J Cutan Pathol. 2021;48:644-649. doi:10.1111 /cup.13925
THE DIAGNOSIS: Neutrophilic Eccrine Hidradenitis
A biopsy from the left preauricular cheek demonstrated dermal neutrophilic inflammation around eccrine coils with focal necrosis (Figure). No notable diffuse dermal neutrophilic infiltrate was present, ruling out Sweet syndrome, and no notable interstitial neutrophilic infiltrate was present, making cellulitis and erysipelas less likely; panculture of tissue also was negative.1,2 Atypical cells in the deep dermis were positive for CD163 and negative for CD117, CD34, CD123, and myeloperoxidase, consistent with a diagnosis of neutrophilic eccrine hidradenitis (NEH) and reactive histiocytes.3 Treatment with oral prednisone resulted in rapid improvement of symptoms.

Neutrophilic eccrine hidradenitis is a rare reactive neutrophilic dermatosis characterized by eccrine gland involvement. This benign and self-limited condition presents as asymmetric erythematous papules and plaques.2 Among 8 granulocytopenic patients with neutrophilic dermatoses, 5 were diagnosed with NEH.4 Although first identified in 1982, NEH remains poorly understood.2 Initial theories suggested that NEH developed due to cytotoxic substances secreted in sweat glands causing necrosis and neutrophil chemotaxis; however, chemotherapy exposure cannot be linked to every case of NEH. Neutrophilic eccrine hidradenitis can be extremely difficult to differentiate clinically from conditions such as cellulitis and Sweet syndrome.
A patient history can be helpful in identifying triggering factors. Neutrophilic eccrine hidradenitis most commonly is associated with malignant, drug-induced, or infectious triggers, while Sweet syndrome has a broad range of associations including infections, vaccines, inflammatory bowel disease, pregnancy, malignancy, and drug-induced etiologies (Table).1 On average, NEH presents 10 days after chemotherapy induction, with 70% of cases presenting after the first chemotherapy cycle.5 Bacterial cellulitis or erysipelas have an infectious etiology, and patients may report symptoms such as fever, chills, or malaise. Immunosuppressed patients are at greater risk for infection; therefore, clinical signs of infection in a granulocytopenic patient should be addressed urgently.

Physical examination may have limited value in differentiating between these diagnoses, as neutrophilic dermatoses notoriously mimic infection. Cutaneous lesions can appear as pruritic or tender erythematous plaques, papules, or nodules in these conditions, though cellulitis and erysipelas tend to be unilateral and may have associated purulence or inflamed skin lymphatics. Given the potential for misdiagnosis, approaching patients with a broad differential can be helpful. In our patient, the differential diagnosis included Sweet syndrome, NEH, bacterial cellulitis, erysipelas, leukemia cutis, sarcoid, and eosinophilic cellulitis.
Leukemia cutis refers to the infiltration of neoplastic leukocytes in the skin and often occurs in patients with peripheral leukemia, most often acute myeloid leukemia or chronic lymphocytic leukemia. Patients with leukemia cutis often have a worse prognosis, as this finding signifies extramedullary spread of disease.6 Clinically, lesions can appear similar to those seen in our patient, though they typically are not symptomatic, can be nodular, tend to exhibit a violaceous hue, and occasionally may be hemorrhagic. Wells syndrome (also known as eosinophilic cellulitis) is an inflammatory dermatosis that presents as painful or pruritic, edematous and erythematous plaques.7,8 A green hue on resolution is present in some cases and may help clinicians differentiate this disease from mimickers.7 Often, eosinophilic cellulitis is misdiagnosed as bacterial cellulitis and treated with antibiotics. The presence of systemic symptoms such as fever or arthralgia is more typical of bacterial cellulitis, erysipelas, eosinophilic cellulitis, or Sweet syndrome than of NEH.1 Additionally, inflammatory markers (ie, C-reactive protein) and white blood cell counts tend to be elevated in bacterial cellulitis and Sweet syndrome, while leukopenia often is seen in NEH.
Histopathology is crucial in distinguishing these disease etiologies. Neutrophilic eccrine hidradenitis is diagnosed by the characteristic neutrophilic infiltrate and necrosis surrounding eccrine glands and coils. There also may be occasional intraductal abscesses and syringosquamous metaplasia of the sweat glands along with fibrosis of the adjacent dermis. In contrast, histologic sections of Sweet syndrome show numerous mature neutrophils infiltrating the dermis with marked papillary dermal edema. The histopathology of bacterial cellulitis and erysipelas is less specific, but common features include dermal edema, lymphatic dilation, and a diffuse neutrophilic infiltrate surrounding blood vessels. Pathogenic organisms may be seen on histopathology but are not required for the diagnosis of bacterial cellulitis or erysipelas.2 Additionally, blood and tissue culture can assist in identification of both the source of infection and the causative organism, but cultures may not always be positive.
Comparatively, the histopathologic features of eosinophilic cellulitis include dermal edema, eosinophilic infiltration, and flame figures that form when eosinophils degranulate and coat collagen fibers with major basic protein. Flame figures are characteristic but not pathognomonic for eosinophilic cellulitis.7 The histopathology of leukemia cutis varies based on the leukemia classification; generally, in acute myeloid leukemia the infiltrate is composed of neoplastic cells in the early stages of development that are positive for myeloid markers such as myeloperoxidase. Atypical and immature granulocytes within the leukocytic infiltrate differentiate this condition from the other diagnoses. Treatment may entail chemotherapy or radiotherapy, and this diagnosis generally carries the worst prognosis of all the conditions in the differential.6
Differentiating between these conditions is important in guiding treatment, especially in patients with febrile neutropenia. Unnecessary steroids in immunosuppressed patients can be dangerous, especially if the patient has an infection such as bacterial cellulitis. Furthermore, unwarranted antibiotic use for noninfectious conditions may expose patients to substantial side effects and not improve the condition. Neutrophilic eccrine hidradenitis typically is self-limited and treated symptomatically with systemic corticosteroids and nonsteroidal anti-inflammatory drugs.3 Sweet syndrome often requires a longer course of oral steroids. Leukemia cutis worsens as the leukemia advances, and treatment of the underlying malignancy is the most effective treatment.9
Early and accurate recognition of the diagnosis can prevent harmful diagnostic delay, unnecessary antibiotic use, or extended steroid taper in neutropenic patients. Appreciating the differences between these diagnoses can assist clinicians in investigating and tailoring a broad differential to specific patient presentations, which is especially critical when considering common mimickers for life-threatening conditions.
THE DIAGNOSIS: Neutrophilic Eccrine Hidradenitis
A biopsy from the left preauricular cheek demonstrated dermal neutrophilic inflammation around eccrine coils with focal necrosis (Figure). No notable diffuse dermal neutrophilic infiltrate was present, ruling out Sweet syndrome, and no notable interstitial neutrophilic infiltrate was present, making cellulitis and erysipelas less likely; panculture of tissue also was negative.1,2 Atypical cells in the deep dermis were positive for CD163 and negative for CD117, CD34, CD123, and myeloperoxidase, consistent with a diagnosis of neutrophilic eccrine hidradenitis (NEH) and reactive histiocytes.3 Treatment with oral prednisone resulted in rapid improvement of symptoms.

Neutrophilic eccrine hidradenitis is a rare reactive neutrophilic dermatosis characterized by eccrine gland involvement. This benign and self-limited condition presents as asymmetric erythematous papules and plaques.2 Among 8 granulocytopenic patients with neutrophilic dermatoses, 5 were diagnosed with NEH.4 Although first identified in 1982, NEH remains poorly understood.2 Initial theories suggested that NEH developed due to cytotoxic substances secreted in sweat glands causing necrosis and neutrophil chemotaxis; however, chemotherapy exposure cannot be linked to every case of NEH. Neutrophilic eccrine hidradenitis can be extremely difficult to differentiate clinically from conditions such as cellulitis and Sweet syndrome.
A patient history can be helpful in identifying triggering factors. Neutrophilic eccrine hidradenitis most commonly is associated with malignant, drug-induced, or infectious triggers, while Sweet syndrome has a broad range of associations including infections, vaccines, inflammatory bowel disease, pregnancy, malignancy, and drug-induced etiologies (Table).1 On average, NEH presents 10 days after chemotherapy induction, with 70% of cases presenting after the first chemotherapy cycle.5 Bacterial cellulitis or erysipelas have an infectious etiology, and patients may report symptoms such as fever, chills, or malaise. Immunosuppressed patients are at greater risk for infection; therefore, clinical signs of infection in a granulocytopenic patient should be addressed urgently.

Physical examination may have limited value in differentiating between these diagnoses, as neutrophilic dermatoses notoriously mimic infection. Cutaneous lesions can appear as pruritic or tender erythematous plaques, papules, or nodules in these conditions, though cellulitis and erysipelas tend to be unilateral and may have associated purulence or inflamed skin lymphatics. Given the potential for misdiagnosis, approaching patients with a broad differential can be helpful. In our patient, the differential diagnosis included Sweet syndrome, NEH, bacterial cellulitis, erysipelas, leukemia cutis, sarcoid, and eosinophilic cellulitis.
Leukemia cutis refers to the infiltration of neoplastic leukocytes in the skin and often occurs in patients with peripheral leukemia, most often acute myeloid leukemia or chronic lymphocytic leukemia. Patients with leukemia cutis often have a worse prognosis, as this finding signifies extramedullary spread of disease.6 Clinically, lesions can appear similar to those seen in our patient, though they typically are not symptomatic, can be nodular, tend to exhibit a violaceous hue, and occasionally may be hemorrhagic. Wells syndrome (also known as eosinophilic cellulitis) is an inflammatory dermatosis that presents as painful or pruritic, edematous and erythematous plaques.7,8 A green hue on resolution is present in some cases and may help clinicians differentiate this disease from mimickers.7 Often, eosinophilic cellulitis is misdiagnosed as bacterial cellulitis and treated with antibiotics. The presence of systemic symptoms such as fever or arthralgia is more typical of bacterial cellulitis, erysipelas, eosinophilic cellulitis, or Sweet syndrome than of NEH.1 Additionally, inflammatory markers (ie, C-reactive protein) and white blood cell counts tend to be elevated in bacterial cellulitis and Sweet syndrome, while leukopenia often is seen in NEH.
Histopathology is crucial in distinguishing these disease etiologies. Neutrophilic eccrine hidradenitis is diagnosed by the characteristic neutrophilic infiltrate and necrosis surrounding eccrine glands and coils. There also may be occasional intraductal abscesses and syringosquamous metaplasia of the sweat glands along with fibrosis of the adjacent dermis. In contrast, histologic sections of Sweet syndrome show numerous mature neutrophils infiltrating the dermis with marked papillary dermal edema. The histopathology of bacterial cellulitis and erysipelas is less specific, but common features include dermal edema, lymphatic dilation, and a diffuse neutrophilic infiltrate surrounding blood vessels. Pathogenic organisms may be seen on histopathology but are not required for the diagnosis of bacterial cellulitis or erysipelas.2 Additionally, blood and tissue culture can assist in identification of both the source of infection and the causative organism, but cultures may not always be positive.
Comparatively, the histopathologic features of eosinophilic cellulitis include dermal edema, eosinophilic infiltration, and flame figures that form when eosinophils degranulate and coat collagen fibers with major basic protein. Flame figures are characteristic but not pathognomonic for eosinophilic cellulitis.7 The histopathology of leukemia cutis varies based on the leukemia classification; generally, in acute myeloid leukemia the infiltrate is composed of neoplastic cells in the early stages of development that are positive for myeloid markers such as myeloperoxidase. Atypical and immature granulocytes within the leukocytic infiltrate differentiate this condition from the other diagnoses. Treatment may entail chemotherapy or radiotherapy, and this diagnosis generally carries the worst prognosis of all the conditions in the differential.6
Differentiating between these conditions is important in guiding treatment, especially in patients with febrile neutropenia. Unnecessary steroids in immunosuppressed patients can be dangerous, especially if the patient has an infection such as bacterial cellulitis. Furthermore, unwarranted antibiotic use for noninfectious conditions may expose patients to substantial side effects and not improve the condition. Neutrophilic eccrine hidradenitis typically is self-limited and treated symptomatically with systemic corticosteroids and nonsteroidal anti-inflammatory drugs.3 Sweet syndrome often requires a longer course of oral steroids. Leukemia cutis worsens as the leukemia advances, and treatment of the underlying malignancy is the most effective treatment.9
Early and accurate recognition of the diagnosis can prevent harmful diagnostic delay, unnecessary antibiotic use, or extended steroid taper in neutropenic patients. Appreciating the differences between these diagnoses can assist clinicians in investigating and tailoring a broad differential to specific patient presentations, which is especially critical when considering common mimickers for life-threatening conditions.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.0642
- Srivastava M, Scharf S, Meehan SA, et al. Neutrophilic eccrine hidradenitis masquerading as facial cellulitis. J Am Acad Dermatol. 2007;56:693-696. doi:10.1016/j.jaad.2006.07.032
- Copaescu AM, Castilloux JF, Chababi-Atallah M, et al. A classic clinical case: neutrophilic eccrine hidradenitis. Case Rep Dermatol. 2013; 5:340-346. doi:10.1159/000356229
- Aractingi S, Mallet V, Pinquier L, et al. Neutrophilic dermatoses during granulocytopenia. Arch Dermatol. 1995;131:1141-1145.
- Cohen PR. Neutrophilic dermatoses occurring in oncology patients. Int J Dermatol. 2007;46:106-111. doi:10.1111/j.1365-4632.2006.02605.x
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Chung CL, Cusack CA. Wells syndrome: an enigmatic and therapeutically challenging disease. J Drugs Dermatol. 2006;5:908-911.
- Räßler F, Lukács J, Elsner P. Treatment of eosinophilic cellulitis (Wells syndrome): a systematic review. J Eur Acad Dermatol Venereol. 2016;30:1465-1479. doi:10.1111/jdv.13706
- Hobbs LK, Carr PC, Gru AA, et al. Case and review: cutaneous involvement by chronic neutrophilic leukemia vs Sweet syndrome: a diagnostic dilemma. J Cutan Pathol. 2021;48:644-649. doi:10.1111 /cup.13925
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.0642
- Srivastava M, Scharf S, Meehan SA, et al. Neutrophilic eccrine hidradenitis masquerading as facial cellulitis. J Am Acad Dermatol. 2007;56:693-696. doi:10.1016/j.jaad.2006.07.032
- Copaescu AM, Castilloux JF, Chababi-Atallah M, et al. A classic clinical case: neutrophilic eccrine hidradenitis. Case Rep Dermatol. 2013; 5:340-346. doi:10.1159/000356229
- Aractingi S, Mallet V, Pinquier L, et al. Neutrophilic dermatoses during granulocytopenia. Arch Dermatol. 1995;131:1141-1145.
- Cohen PR. Neutrophilic dermatoses occurring in oncology patients. Int J Dermatol. 2007;46:106-111. doi:10.1111/j.1365-4632.2006.02605.x
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Chung CL, Cusack CA. Wells syndrome: an enigmatic and therapeutically challenging disease. J Drugs Dermatol. 2006;5:908-911.
- Räßler F, Lukács J, Elsner P. Treatment of eosinophilic cellulitis (Wells syndrome): a systematic review. J Eur Acad Dermatol Venereol. 2016;30:1465-1479. doi:10.1111/jdv.13706
- Hobbs LK, Carr PC, Gru AA, et al. Case and review: cutaneous involvement by chronic neutrophilic leukemia vs Sweet syndrome: a diagnostic dilemma. J Cutan Pathol. 2021;48:644-649. doi:10.1111 /cup.13925
A 50-year-old woman undergoing cytarabine induction therapy for acute myeloid leukemia developed tender, erythematous, dermal plaques on the nasal dorsum, left medial eyebrow, left preauricular cheek, and right cheek. The rash erupted 7 days after receiving the cytarabine induction regimen. She had a fever (temperature, 39.9 °C [103.8 °F]) and also was neutropenic.
A 63-year-old male presented for evaluation of worsening genital lesions and associated swelling
.1 Clinically, ENV presents as verrucous, hyperkeratotic, cobblestone-like patches, plaques, and nodules with associated nonpitting edema of the affected body area.1 Secondary bacterial infections are common and often worsen the clinical course. The etiology of ENV involves chronic lymphatic obstruction and venous insufficiency, with additional risk factors including obesity, chronic lymphedema, bacterial infection, surgery or trauma, neoplasia, radiation, congestive heart failure, or scleroderma.2,3 While most commonly presenting on the lower extremities, cases have been reported involving the abdomen, sacrum, ears, buttocks, and penoscrotal area.1,2
Regardless of location, the pathogenesis of ENV remains the same. Chronic lymphatic obstruction results in accumulation and lymphostasis of protein-rich dermal fluid, which subsequently precipitates fibroblast proliferation and activation, suppression of the local immune response and development of recurrent lymphangitis, chronic inflammation, and potential secondary bacterial infection.2,4
There is no standard of care for the treatment and management of ENV and recurrence is common. Interventions often involve those used for chronic lymphedema – including leg elevation, compression stockings or devices, skin hygiene, and lymphatic pumping.2,3 Medical management with topical and oral retinoids has been reported, as well as emphasis on weight loss and infection control.1,4 Surgical intervention is often reserved for refractory cases that fail to respond to more conservative management, or severe presentations resulting in extensive functional and aesthetic impairment. Less commonly reported treatment modalities include lymphaticovenular anastomosis and ablative carbon dioxide laser use, although this latter intervention demonstrated minimal improvement in this patient.5,6
Penoscrotal ENV is a rare form of ENV affecting the genital region of males, often resulting in significant disfigurement, functional impairment, and psychosocial distress. Penoscrotal elephantiasis can be idiopathic, due to filarial infections, scleroinflammatory stricture of the urethra, Chlamydia trachomatis infection, and lymphostasis secondary to chronic inflammatory conditions such as streptococcal infections, radiotherapy, surgery, chronic venous stasis, or Kaposi sarcoma.7
In addition, hidradenitis suppurativa (HS) has been documented multiple times in the literature in association with the development of ENV, detailing lymphatic scarring secondary to chronic inguinal HS as the main pathogenic factor.8,9
Surgery is the mainstay of treatment for penoscrotal ENV, which not only improves functionality and cosmesis, but also aids in prevention of rare malignant sequelae, such as lymphangiosarcoma.10 Such interventions can involve lymphangioplasty to aid in lymphatic drainage or excision of the mass and subcutaneous tissue with full-thickness skin grafting for reconstruction.7 Collaboration between urology, plastic surgery, and dermatology is often essential to obtain adequate care with satisfactory outcomes and minimal recurrence for patients with this uncommon condition.
This case and photo were submitted by Marlee Hill, a medical student at the University of Oklahoma, Oklahoma City; and Michael Franzetti, MD, and Jeffrey McBride, MD, department of dermatology, University of Oklahoma Health Sciences Center. The column was edited by Donna Bilu Martin, MD.
Dr. Donna Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to d[email protected].
References
1. Hadian Y et al. Dermatol Online J. 2019 Dec 15;25(12):13030/qt6rn1s8ff.
2. Judge N and Kilic A. J Dermatol Case Rep. 2016 Nov 13;10(2):32-4.
3. Dean SM et al. J Am Acad Dermatol. 2011 Jun;64(6):1104-10.
4. Sisto K and Khachemoune A. Am J Clin Dermatol. 2008;9(3):141-6.
5. Motegi S et al. Dermatology. 2007;215(2):147-51.
6. Robinson CG et al. J Cutan Med Surg. 2018;22(6):611-3.
7. Koualla S et al. Ann Chir Plast Esthet. 2023 Apr 10;S0294-1260(23)00035-3.
8. Lelonek E et al. Acta Derm Venereol. 2021 Feb 11;101(2):adv00389.
9. Good LM et al. J Am Acad Dermatol. 2011 May;64(5):993-4.
10. Cerri A et al. Eur J Dermatol. 1998 Oct-Nov;8(7):511-4.
.1 Clinically, ENV presents as verrucous, hyperkeratotic, cobblestone-like patches, plaques, and nodules with associated nonpitting edema of the affected body area.1 Secondary bacterial infections are common and often worsen the clinical course. The etiology of ENV involves chronic lymphatic obstruction and venous insufficiency, with additional risk factors including obesity, chronic lymphedema, bacterial infection, surgery or trauma, neoplasia, radiation, congestive heart failure, or scleroderma.2,3 While most commonly presenting on the lower extremities, cases have been reported involving the abdomen, sacrum, ears, buttocks, and penoscrotal area.1,2
Regardless of location, the pathogenesis of ENV remains the same. Chronic lymphatic obstruction results in accumulation and lymphostasis of protein-rich dermal fluid, which subsequently precipitates fibroblast proliferation and activation, suppression of the local immune response and development of recurrent lymphangitis, chronic inflammation, and potential secondary bacterial infection.2,4
There is no standard of care for the treatment and management of ENV and recurrence is common. Interventions often involve those used for chronic lymphedema – including leg elevation, compression stockings or devices, skin hygiene, and lymphatic pumping.2,3 Medical management with topical and oral retinoids has been reported, as well as emphasis on weight loss and infection control.1,4 Surgical intervention is often reserved for refractory cases that fail to respond to more conservative management, or severe presentations resulting in extensive functional and aesthetic impairment. Less commonly reported treatment modalities include lymphaticovenular anastomosis and ablative carbon dioxide laser use, although this latter intervention demonstrated minimal improvement in this patient.5,6
Penoscrotal ENV is a rare form of ENV affecting the genital region of males, often resulting in significant disfigurement, functional impairment, and psychosocial distress. Penoscrotal elephantiasis can be idiopathic, due to filarial infections, scleroinflammatory stricture of the urethra, Chlamydia trachomatis infection, and lymphostasis secondary to chronic inflammatory conditions such as streptococcal infections, radiotherapy, surgery, chronic venous stasis, or Kaposi sarcoma.7
In addition, hidradenitis suppurativa (HS) has been documented multiple times in the literature in association with the development of ENV, detailing lymphatic scarring secondary to chronic inguinal HS as the main pathogenic factor.8,9
Surgery is the mainstay of treatment for penoscrotal ENV, which not only improves functionality and cosmesis, but also aids in prevention of rare malignant sequelae, such as lymphangiosarcoma.10 Such interventions can involve lymphangioplasty to aid in lymphatic drainage or excision of the mass and subcutaneous tissue with full-thickness skin grafting for reconstruction.7 Collaboration between urology, plastic surgery, and dermatology is often essential to obtain adequate care with satisfactory outcomes and minimal recurrence for patients with this uncommon condition.
This case and photo were submitted by Marlee Hill, a medical student at the University of Oklahoma, Oklahoma City; and Michael Franzetti, MD, and Jeffrey McBride, MD, department of dermatology, University of Oklahoma Health Sciences Center. The column was edited by Donna Bilu Martin, MD.
Dr. Donna Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to d[email protected].
References
1. Hadian Y et al. Dermatol Online J. 2019 Dec 15;25(12):13030/qt6rn1s8ff.
2. Judge N and Kilic A. J Dermatol Case Rep. 2016 Nov 13;10(2):32-4.
3. Dean SM et al. J Am Acad Dermatol. 2011 Jun;64(6):1104-10.
4. Sisto K and Khachemoune A. Am J Clin Dermatol. 2008;9(3):141-6.
5. Motegi S et al. Dermatology. 2007;215(2):147-51.
6. Robinson CG et al. J Cutan Med Surg. 2018;22(6):611-3.
7. Koualla S et al. Ann Chir Plast Esthet. 2023 Apr 10;S0294-1260(23)00035-3.
8. Lelonek E et al. Acta Derm Venereol. 2021 Feb 11;101(2):adv00389.
9. Good LM et al. J Am Acad Dermatol. 2011 May;64(5):993-4.
10. Cerri A et al. Eur J Dermatol. 1998 Oct-Nov;8(7):511-4.
.1 Clinically, ENV presents as verrucous, hyperkeratotic, cobblestone-like patches, plaques, and nodules with associated nonpitting edema of the affected body area.1 Secondary bacterial infections are common and often worsen the clinical course. The etiology of ENV involves chronic lymphatic obstruction and venous insufficiency, with additional risk factors including obesity, chronic lymphedema, bacterial infection, surgery or trauma, neoplasia, radiation, congestive heart failure, or scleroderma.2,3 While most commonly presenting on the lower extremities, cases have been reported involving the abdomen, sacrum, ears, buttocks, and penoscrotal area.1,2
Regardless of location, the pathogenesis of ENV remains the same. Chronic lymphatic obstruction results in accumulation and lymphostasis of protein-rich dermal fluid, which subsequently precipitates fibroblast proliferation and activation, suppression of the local immune response and development of recurrent lymphangitis, chronic inflammation, and potential secondary bacterial infection.2,4
There is no standard of care for the treatment and management of ENV and recurrence is common. Interventions often involve those used for chronic lymphedema – including leg elevation, compression stockings or devices, skin hygiene, and lymphatic pumping.2,3 Medical management with topical and oral retinoids has been reported, as well as emphasis on weight loss and infection control.1,4 Surgical intervention is often reserved for refractory cases that fail to respond to more conservative management, or severe presentations resulting in extensive functional and aesthetic impairment. Less commonly reported treatment modalities include lymphaticovenular anastomosis and ablative carbon dioxide laser use, although this latter intervention demonstrated minimal improvement in this patient.5,6
Penoscrotal ENV is a rare form of ENV affecting the genital region of males, often resulting in significant disfigurement, functional impairment, and psychosocial distress. Penoscrotal elephantiasis can be idiopathic, due to filarial infections, scleroinflammatory stricture of the urethra, Chlamydia trachomatis infection, and lymphostasis secondary to chronic inflammatory conditions such as streptococcal infections, radiotherapy, surgery, chronic venous stasis, or Kaposi sarcoma.7
In addition, hidradenitis suppurativa (HS) has been documented multiple times in the literature in association with the development of ENV, detailing lymphatic scarring secondary to chronic inguinal HS as the main pathogenic factor.8,9
Surgery is the mainstay of treatment for penoscrotal ENV, which not only improves functionality and cosmesis, but also aids in prevention of rare malignant sequelae, such as lymphangiosarcoma.10 Such interventions can involve lymphangioplasty to aid in lymphatic drainage or excision of the mass and subcutaneous tissue with full-thickness skin grafting for reconstruction.7 Collaboration between urology, plastic surgery, and dermatology is often essential to obtain adequate care with satisfactory outcomes and minimal recurrence for patients with this uncommon condition.
This case and photo were submitted by Marlee Hill, a medical student at the University of Oklahoma, Oklahoma City; and Michael Franzetti, MD, and Jeffrey McBride, MD, department of dermatology, University of Oklahoma Health Sciences Center. The column was edited by Donna Bilu Martin, MD.
Dr. Donna Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to d[email protected].
References
1. Hadian Y et al. Dermatol Online J. 2019 Dec 15;25(12):13030/qt6rn1s8ff.
2. Judge N and Kilic A. J Dermatol Case Rep. 2016 Nov 13;10(2):32-4.
3. Dean SM et al. J Am Acad Dermatol. 2011 Jun;64(6):1104-10.
4. Sisto K and Khachemoune A. Am J Clin Dermatol. 2008;9(3):141-6.
5. Motegi S et al. Dermatology. 2007;215(2):147-51.
6. Robinson CG et al. J Cutan Med Surg. 2018;22(6):611-3.
7. Koualla S et al. Ann Chir Plast Esthet. 2023 Apr 10;S0294-1260(23)00035-3.
8. Lelonek E et al. Acta Derm Venereol. 2021 Feb 11;101(2):adv00389.
9. Good LM et al. J Am Acad Dermatol. 2011 May;64(5):993-4.
10. Cerri A et al. Eur J Dermatol. 1998 Oct-Nov;8(7):511-4.
FDA warns of tattoo ink tied to dangerous infections
The Food and Drug Administration draft guidance released recently on possible contamination of tattoo ink was not concerning Whitney Donohue, 34, owner of Forget Me Not Tattoo in Billings, Mont.
“I get our ink directly through the manufacturer – not at a store or through Amazon or eBay,” she said. “You never know if it’s going to be repackaged.”
Tattoo artists themselves, she said, regulate the quality of ink they use.
Still, the threat is real, said Bruce Brod, MD, a clinical professor of dermatology at the University of Pennsylvania Health System. “I’ve seen several different infections from tattooing, and they are from organisms that tend to contaminate things in damp, liquid-type environments.”
, dermatologists said.
“Tattooing involves puncturing the epidermis about 100 times per second with needles and depositing ink 1.5 to 2 millimeters below the surface of the skin, deep into the dermis,” the guidance states. “Contaminated tattoo ink can cause infections and serious injuries. Because these inks are injected, pathogens or other harmful substances in these inks can travel from the injection site through the blood and lymphatic systems to other parts of the body.”
The guidance comes as body art continues to get more popular. According to a 2019 poll, 30% of Americans had at least one tattoo – up from 21% in 2012. Forty percent of people 18-34 and 36% of those ages 35-54 had at least one tattoo. And though they are commonplace, tattoos come with medical risks that should be known beforehand, doctors said.
Commonly reported symptoms of tattoo ink–associated infections include rashes, blisters, painful nodules, and severe abscesses. One of the most common bacteria found in contaminated tattoo ink is nontuberculous mycobacteria, which is related to the bacteria that causes tuberculosis and can be found in soil and water.
The guidance lists several unsanitary manufacturing conditions that may lead to ink contamination, including:
- Preparing or packing of tattoo inks in facilities that are hard to sanitize, such as carpeted areas
- Ink or ink components left uncovered, especially near open air ducts
- Unsanitary mixing of tattoo inks, including with unclean utensils or containers
- Lack of appropriate attire by staff, failure to use hairnets, lab coats, aprons, gowns, masks, or gloves
“Infections will often spread along the drainage channels in the skin and create squiggly, uneven lines of big red, lumpy nodules,” Dr. Brod said.
Between 2003 and 2023, there were 18 recalls of tattoo inks that were contaminated with various microorganisms, according to the FDA. In May 2019, the FDA issued a safety alert advising consumers, tattoo artists, and retailers to avoid using or selling certain tattoo inks contaminated with microorganisms.
Reputable ink manufacturers use a process called gamma radiation, which refers to electromagnetic radiation of high frequencies to kill microorganisms in the ink and its packaging.
Most of the trustworthy, high-quality ink manufacturers are well-known among tattoo artists, Ms. Donohue said.
While she has seen customers with sensitive skin have allergic reactions, she has not seen someone come back with an infection in her 9 years working in the tattoo industry.
Because tattoo ink is considered a cosmetic product, there is not much regulatory oversight involved, which means the sterility and quality of ingredients vary, said Teo Soleymani, MD, an assistant clinical professor of dermatology and dermatological surgery at the UCLA David Geffen School of Medicine.
“Cosmeceuticals aren’t regulated by the FDA like prescription medication,” he said. “What we’ve seen many times is inadvertent contamination during the application process or contamination while the inks are being made.”
In years past, unclean needles spreading hepatitis and HIV were more of a concern, but those rates have dropped significantly, Dr. Soleymani said.
The infections that have increased are from rare bacteria that exist in stagnant water. And they are injected into a part of the body that allows them to evade the immune system, he said: shallow enough that there aren’t many associated blood vessels, but not still below the layer of skin that gets sloughed off every 28 days.
Sometimes, antibiotics alone won’t cut it, and the tattoo will require surgical removal.
“The aesthetic you were going for has to be not only removed, but you’re left with a surgical scar,” Dr. Soleymani said. “Tattoos can be beautiful, but they can come with unwanted visitors that can cause months of misery.”
A version of this article first appeared on WebMD.com.
The Food and Drug Administration draft guidance released recently on possible contamination of tattoo ink was not concerning Whitney Donohue, 34, owner of Forget Me Not Tattoo in Billings, Mont.
“I get our ink directly through the manufacturer – not at a store or through Amazon or eBay,” she said. “You never know if it’s going to be repackaged.”
Tattoo artists themselves, she said, regulate the quality of ink they use.
Still, the threat is real, said Bruce Brod, MD, a clinical professor of dermatology at the University of Pennsylvania Health System. “I’ve seen several different infections from tattooing, and they are from organisms that tend to contaminate things in damp, liquid-type environments.”
, dermatologists said.
“Tattooing involves puncturing the epidermis about 100 times per second with needles and depositing ink 1.5 to 2 millimeters below the surface of the skin, deep into the dermis,” the guidance states. “Contaminated tattoo ink can cause infections and serious injuries. Because these inks are injected, pathogens or other harmful substances in these inks can travel from the injection site through the blood and lymphatic systems to other parts of the body.”
The guidance comes as body art continues to get more popular. According to a 2019 poll, 30% of Americans had at least one tattoo – up from 21% in 2012. Forty percent of people 18-34 and 36% of those ages 35-54 had at least one tattoo. And though they are commonplace, tattoos come with medical risks that should be known beforehand, doctors said.
Commonly reported symptoms of tattoo ink–associated infections include rashes, blisters, painful nodules, and severe abscesses. One of the most common bacteria found in contaminated tattoo ink is nontuberculous mycobacteria, which is related to the bacteria that causes tuberculosis and can be found in soil and water.
The guidance lists several unsanitary manufacturing conditions that may lead to ink contamination, including:
- Preparing or packing of tattoo inks in facilities that are hard to sanitize, such as carpeted areas
- Ink or ink components left uncovered, especially near open air ducts
- Unsanitary mixing of tattoo inks, including with unclean utensils or containers
- Lack of appropriate attire by staff, failure to use hairnets, lab coats, aprons, gowns, masks, or gloves
“Infections will often spread along the drainage channels in the skin and create squiggly, uneven lines of big red, lumpy nodules,” Dr. Brod said.
Between 2003 and 2023, there were 18 recalls of tattoo inks that were contaminated with various microorganisms, according to the FDA. In May 2019, the FDA issued a safety alert advising consumers, tattoo artists, and retailers to avoid using or selling certain tattoo inks contaminated with microorganisms.
Reputable ink manufacturers use a process called gamma radiation, which refers to electromagnetic radiation of high frequencies to kill microorganisms in the ink and its packaging.
Most of the trustworthy, high-quality ink manufacturers are well-known among tattoo artists, Ms. Donohue said.
While she has seen customers with sensitive skin have allergic reactions, she has not seen someone come back with an infection in her 9 years working in the tattoo industry.
Because tattoo ink is considered a cosmetic product, there is not much regulatory oversight involved, which means the sterility and quality of ingredients vary, said Teo Soleymani, MD, an assistant clinical professor of dermatology and dermatological surgery at the UCLA David Geffen School of Medicine.
“Cosmeceuticals aren’t regulated by the FDA like prescription medication,” he said. “What we’ve seen many times is inadvertent contamination during the application process or contamination while the inks are being made.”
In years past, unclean needles spreading hepatitis and HIV were more of a concern, but those rates have dropped significantly, Dr. Soleymani said.
The infections that have increased are from rare bacteria that exist in stagnant water. And they are injected into a part of the body that allows them to evade the immune system, he said: shallow enough that there aren’t many associated blood vessels, but not still below the layer of skin that gets sloughed off every 28 days.
Sometimes, antibiotics alone won’t cut it, and the tattoo will require surgical removal.
“The aesthetic you were going for has to be not only removed, but you’re left with a surgical scar,” Dr. Soleymani said. “Tattoos can be beautiful, but they can come with unwanted visitors that can cause months of misery.”
A version of this article first appeared on WebMD.com.
The Food and Drug Administration draft guidance released recently on possible contamination of tattoo ink was not concerning Whitney Donohue, 34, owner of Forget Me Not Tattoo in Billings, Mont.
“I get our ink directly through the manufacturer – not at a store or through Amazon or eBay,” she said. “You never know if it’s going to be repackaged.”
Tattoo artists themselves, she said, regulate the quality of ink they use.
Still, the threat is real, said Bruce Brod, MD, a clinical professor of dermatology at the University of Pennsylvania Health System. “I’ve seen several different infections from tattooing, and they are from organisms that tend to contaminate things in damp, liquid-type environments.”
, dermatologists said.
“Tattooing involves puncturing the epidermis about 100 times per second with needles and depositing ink 1.5 to 2 millimeters below the surface of the skin, deep into the dermis,” the guidance states. “Contaminated tattoo ink can cause infections and serious injuries. Because these inks are injected, pathogens or other harmful substances in these inks can travel from the injection site through the blood and lymphatic systems to other parts of the body.”
The guidance comes as body art continues to get more popular. According to a 2019 poll, 30% of Americans had at least one tattoo – up from 21% in 2012. Forty percent of people 18-34 and 36% of those ages 35-54 had at least one tattoo. And though they are commonplace, tattoos come with medical risks that should be known beforehand, doctors said.
Commonly reported symptoms of tattoo ink–associated infections include rashes, blisters, painful nodules, and severe abscesses. One of the most common bacteria found in contaminated tattoo ink is nontuberculous mycobacteria, which is related to the bacteria that causes tuberculosis and can be found in soil and water.
The guidance lists several unsanitary manufacturing conditions that may lead to ink contamination, including:
- Preparing or packing of tattoo inks in facilities that are hard to sanitize, such as carpeted areas
- Ink or ink components left uncovered, especially near open air ducts
- Unsanitary mixing of tattoo inks, including with unclean utensils or containers
- Lack of appropriate attire by staff, failure to use hairnets, lab coats, aprons, gowns, masks, or gloves
“Infections will often spread along the drainage channels in the skin and create squiggly, uneven lines of big red, lumpy nodules,” Dr. Brod said.
Between 2003 and 2023, there were 18 recalls of tattoo inks that were contaminated with various microorganisms, according to the FDA. In May 2019, the FDA issued a safety alert advising consumers, tattoo artists, and retailers to avoid using or selling certain tattoo inks contaminated with microorganisms.
Reputable ink manufacturers use a process called gamma radiation, which refers to electromagnetic radiation of high frequencies to kill microorganisms in the ink and its packaging.
Most of the trustworthy, high-quality ink manufacturers are well-known among tattoo artists, Ms. Donohue said.
While she has seen customers with sensitive skin have allergic reactions, she has not seen someone come back with an infection in her 9 years working in the tattoo industry.
Because tattoo ink is considered a cosmetic product, there is not much regulatory oversight involved, which means the sterility and quality of ingredients vary, said Teo Soleymani, MD, an assistant clinical professor of dermatology and dermatological surgery at the UCLA David Geffen School of Medicine.
“Cosmeceuticals aren’t regulated by the FDA like prescription medication,” he said. “What we’ve seen many times is inadvertent contamination during the application process or contamination while the inks are being made.”
In years past, unclean needles spreading hepatitis and HIV were more of a concern, but those rates have dropped significantly, Dr. Soleymani said.
The infections that have increased are from rare bacteria that exist in stagnant water. And they are injected into a part of the body that allows them to evade the immune system, he said: shallow enough that there aren’t many associated blood vessels, but not still below the layer of skin that gets sloughed off every 28 days.
Sometimes, antibiotics alone won’t cut it, and the tattoo will require surgical removal.
“The aesthetic you were going for has to be not only removed, but you’re left with a surgical scar,” Dr. Soleymani said. “Tattoos can be beautiful, but they can come with unwanted visitors that can cause months of misery.”
A version of this article first appeared on WebMD.com.