User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Meet our newest genetically engineered frenemy, herpes
Herpes to the rescue
Let’s face it: When people hear the word “herpes,” their first thoughts are not positive. But what if herpes could be a hero?
Scientists have found a way to make a strain of herpes that kills cancer because, hey, it’s 2022, and anything is possible. Trials have been going well and this seems like a safe and effective way to fight cancer.
Viruses may be one of our oldest enemies, but it’s also been said that the enemy of my enemy is my friend. So why not make herpes the enemy of cancer, thereby turning it into our friend? The genetically modified herpes virus is injected directly into tumors, where it destroys cancer cells from within. But wait, there’s more! The patient’s immune system also senses the virus and springs into action against it and the cancer in which it is residing.
During the phase 1 trial, three of the nine patients saw tumor reduction and the therapy proved safe as well. Future trials will be able to more specifically target various cancer types and make the treatment better. For once, we are rooting for you, herpes.
A breath of not-so-fresh air
There’s nothing quite like that first real warm day of spring. You can finally open the windows and clear out the old stuffy air that’s been hanging around all winter long. It’s a ritual that’s now backed up with some science in the form of a new study. Turns out that there’s actually a fair amount of smog in the average home. That’s right, smog’s not just for the big city anymore.
As part of the HOMEChem project, a whole host of scientists gathered together under one roof in a typical suburban house and immediately started doing chores. Cooking, cleaning, the works. No, it wasn’t because they had trashed the place the night before. They had set up instrumentation all around the house to measure the chemical makeup of the air inside. A scientist’s idea of a wild party.
The results are perhaps not all that surprising, but interesting nonetheless. Your homemade smog certainly won’t kill you, but there’s both an increased amount and higher concentration of airborne toxins in indoor air, compared with outdoors. Benzene and formaldehyde were common, as were acrolein (a pulmonary toxicant emitted by lumber and burning fats) and isocyanic acid (which can react with proteins in the human body). The researchers noted that most of these chemicals can be removed with proper ventilation.
Although cleaning is certainly responsible for a fair share of the chemicals, cooking generally produced more toxic compounds, similar to what’s found in wildfire smoke. One of the researchers said this makes sense, since a wildfire can be considered an “extreme form of cooking.” Scientists may not know how to party, but their idea of a barbecue sounds … interesting. We’re looking forward to an upcoming study out of California: Can a 1-million acre wildfire adequately cook a ribeye steak?
We’re dying to try composting ... with humans, that is
We here at LOTME are not really fans of politicians, except as objects of ridicule. That is kind of fun. Whether we’re watching Fox News, listening to NPR, or reading Vladimir Putin’s fashion blog, one thing remains clear: If you want actual information, don’t ask a politician.
There are, of course, always exceptions, and we just found one: California state representative Cristina Garcia. Rep. Garcia sponsored a bill just signed into law by Gov. Gavin Newsom that legalizes the practice of human composting, the reduction of remains by “placing bodies in individual vessels and fostering gentle transformation into a nutrient-dense soil.”
Since we’ve written about this sort of thing before – Washington was the first state to legalize the process back in 2019 – we’re more interested now in what Rep. Garcia told NBC News while describing her motivation: “I’ve always wanted to be a tree. The idea of having my family sitting under my shade one day – that brings a lot of joy.” How great is that? Tree-hugging is just not enough. Be the tree.
California is the fifth state to provide its residents with the human composting option, the other three being Colorado, Oregon, and Vermont. The process “typically involves putting a body into a steel vessel, then covering it with organic materials like straw, wood chips and alfalfa. Microbes break down the corpse and the plant matter, transforming the various components into nutrient-rich soil in roughly 30 days,” Smithsonian Magazine explained.
We just happen to have some good news for Rep. Garcia about that wanting-to-be-a-tree business. She’s already pretty close. For more on that, we go to our correspondent from beyond the grave, Carl Sagan, who shares a thought about trees. And no, we couldn’t just write out his quote here. You have to hear it in Dr. Sagan’s own voice.
That’ll be one pandemic with extra distress. Hold the goals
When the COVID-19 pandemic first hit it put a lot of stuff on hold for everyone. Couldn’t eat inside at your favorite restaurant, attend that long-awaited concert, or travel out of the country. Those were all pretty bad, but it was the disruption of pursuing long-term goals that seemed to have the most effect on people’s mental health.
Investigators from the University of Waterloo (Ont.) looked at how putting such goals on hold affected people’s mental well-being. The study’s 226 participants were asked about their “COVID-frozen” goals and the degree to which they were able to actively pursue each goal and how committed they were to achieving it.
What they found was that the participants’ COVID-frozen goals were associated with feelings of psychological distress, such as anxiety, depressive symptoms, stress, and lowered life satisfaction. It was only when participants were able to disengage from goal rumination that well-being was impacted positively.
“Goal rumination is compulsive and can aggravate worries and frustrations while also taking away mental resources from other goals,” Candice Hubley, lead author and a PhD candidate in psychology, said in a written statement. So in short, you’re only stressing yourself out more about something that is far off in the distance when you could be focusing more on short-term, tangible goals instead.
Now, no one is saying to give up on your goals. Just take them one at a time. You’ll have better life satisfaction and your COVID-frozen goals will thaw out before you know it.
Herpes to the rescue
Let’s face it: When people hear the word “herpes,” their first thoughts are not positive. But what if herpes could be a hero?
Scientists have found a way to make a strain of herpes that kills cancer because, hey, it’s 2022, and anything is possible. Trials have been going well and this seems like a safe and effective way to fight cancer.
Viruses may be one of our oldest enemies, but it’s also been said that the enemy of my enemy is my friend. So why not make herpes the enemy of cancer, thereby turning it into our friend? The genetically modified herpes virus is injected directly into tumors, where it destroys cancer cells from within. But wait, there’s more! The patient’s immune system also senses the virus and springs into action against it and the cancer in which it is residing.
During the phase 1 trial, three of the nine patients saw tumor reduction and the therapy proved safe as well. Future trials will be able to more specifically target various cancer types and make the treatment better. For once, we are rooting for you, herpes.
A breath of not-so-fresh air
There’s nothing quite like that first real warm day of spring. You can finally open the windows and clear out the old stuffy air that’s been hanging around all winter long. It’s a ritual that’s now backed up with some science in the form of a new study. Turns out that there’s actually a fair amount of smog in the average home. That’s right, smog’s not just for the big city anymore.
As part of the HOMEChem project, a whole host of scientists gathered together under one roof in a typical suburban house and immediately started doing chores. Cooking, cleaning, the works. No, it wasn’t because they had trashed the place the night before. They had set up instrumentation all around the house to measure the chemical makeup of the air inside. A scientist’s idea of a wild party.
The results are perhaps not all that surprising, but interesting nonetheless. Your homemade smog certainly won’t kill you, but there’s both an increased amount and higher concentration of airborne toxins in indoor air, compared with outdoors. Benzene and formaldehyde were common, as were acrolein (a pulmonary toxicant emitted by lumber and burning fats) and isocyanic acid (which can react with proteins in the human body). The researchers noted that most of these chemicals can be removed with proper ventilation.
Although cleaning is certainly responsible for a fair share of the chemicals, cooking generally produced more toxic compounds, similar to what’s found in wildfire smoke. One of the researchers said this makes sense, since a wildfire can be considered an “extreme form of cooking.” Scientists may not know how to party, but their idea of a barbecue sounds … interesting. We’re looking forward to an upcoming study out of California: Can a 1-million acre wildfire adequately cook a ribeye steak?
We’re dying to try composting ... with humans, that is
We here at LOTME are not really fans of politicians, except as objects of ridicule. That is kind of fun. Whether we’re watching Fox News, listening to NPR, or reading Vladimir Putin’s fashion blog, one thing remains clear: If you want actual information, don’t ask a politician.
There are, of course, always exceptions, and we just found one: California state representative Cristina Garcia. Rep. Garcia sponsored a bill just signed into law by Gov. Gavin Newsom that legalizes the practice of human composting, the reduction of remains by “placing bodies in individual vessels and fostering gentle transformation into a nutrient-dense soil.”
Since we’ve written about this sort of thing before – Washington was the first state to legalize the process back in 2019 – we’re more interested now in what Rep. Garcia told NBC News while describing her motivation: “I’ve always wanted to be a tree. The idea of having my family sitting under my shade one day – that brings a lot of joy.” How great is that? Tree-hugging is just not enough. Be the tree.
California is the fifth state to provide its residents with the human composting option, the other three being Colorado, Oregon, and Vermont. The process “typically involves putting a body into a steel vessel, then covering it with organic materials like straw, wood chips and alfalfa. Microbes break down the corpse and the plant matter, transforming the various components into nutrient-rich soil in roughly 30 days,” Smithsonian Magazine explained.
We just happen to have some good news for Rep. Garcia about that wanting-to-be-a-tree business. She’s already pretty close. For more on that, we go to our correspondent from beyond the grave, Carl Sagan, who shares a thought about trees. And no, we couldn’t just write out his quote here. You have to hear it in Dr. Sagan’s own voice.
That’ll be one pandemic with extra distress. Hold the goals
When the COVID-19 pandemic first hit it put a lot of stuff on hold for everyone. Couldn’t eat inside at your favorite restaurant, attend that long-awaited concert, or travel out of the country. Those were all pretty bad, but it was the disruption of pursuing long-term goals that seemed to have the most effect on people’s mental health.
Investigators from the University of Waterloo (Ont.) looked at how putting such goals on hold affected people’s mental well-being. The study’s 226 participants were asked about their “COVID-frozen” goals and the degree to which they were able to actively pursue each goal and how committed they were to achieving it.
What they found was that the participants’ COVID-frozen goals were associated with feelings of psychological distress, such as anxiety, depressive symptoms, stress, and lowered life satisfaction. It was only when participants were able to disengage from goal rumination that well-being was impacted positively.
“Goal rumination is compulsive and can aggravate worries and frustrations while also taking away mental resources from other goals,” Candice Hubley, lead author and a PhD candidate in psychology, said in a written statement. So in short, you’re only stressing yourself out more about something that is far off in the distance when you could be focusing more on short-term, tangible goals instead.
Now, no one is saying to give up on your goals. Just take them one at a time. You’ll have better life satisfaction and your COVID-frozen goals will thaw out before you know it.
Herpes to the rescue
Let’s face it: When people hear the word “herpes,” their first thoughts are not positive. But what if herpes could be a hero?
Scientists have found a way to make a strain of herpes that kills cancer because, hey, it’s 2022, and anything is possible. Trials have been going well and this seems like a safe and effective way to fight cancer.
Viruses may be one of our oldest enemies, but it’s also been said that the enemy of my enemy is my friend. So why not make herpes the enemy of cancer, thereby turning it into our friend? The genetically modified herpes virus is injected directly into tumors, where it destroys cancer cells from within. But wait, there’s more! The patient’s immune system also senses the virus and springs into action against it and the cancer in which it is residing.
During the phase 1 trial, three of the nine patients saw tumor reduction and the therapy proved safe as well. Future trials will be able to more specifically target various cancer types and make the treatment better. For once, we are rooting for you, herpes.
A breath of not-so-fresh air
There’s nothing quite like that first real warm day of spring. You can finally open the windows and clear out the old stuffy air that’s been hanging around all winter long. It’s a ritual that’s now backed up with some science in the form of a new study. Turns out that there’s actually a fair amount of smog in the average home. That’s right, smog’s not just for the big city anymore.
As part of the HOMEChem project, a whole host of scientists gathered together under one roof in a typical suburban house and immediately started doing chores. Cooking, cleaning, the works. No, it wasn’t because they had trashed the place the night before. They had set up instrumentation all around the house to measure the chemical makeup of the air inside. A scientist’s idea of a wild party.
The results are perhaps not all that surprising, but interesting nonetheless. Your homemade smog certainly won’t kill you, but there’s both an increased amount and higher concentration of airborne toxins in indoor air, compared with outdoors. Benzene and formaldehyde were common, as were acrolein (a pulmonary toxicant emitted by lumber and burning fats) and isocyanic acid (which can react with proteins in the human body). The researchers noted that most of these chemicals can be removed with proper ventilation.
Although cleaning is certainly responsible for a fair share of the chemicals, cooking generally produced more toxic compounds, similar to what’s found in wildfire smoke. One of the researchers said this makes sense, since a wildfire can be considered an “extreme form of cooking.” Scientists may not know how to party, but their idea of a barbecue sounds … interesting. We’re looking forward to an upcoming study out of California: Can a 1-million acre wildfire adequately cook a ribeye steak?
We’re dying to try composting ... with humans, that is
We here at LOTME are not really fans of politicians, except as objects of ridicule. That is kind of fun. Whether we’re watching Fox News, listening to NPR, or reading Vladimir Putin’s fashion blog, one thing remains clear: If you want actual information, don’t ask a politician.
There are, of course, always exceptions, and we just found one: California state representative Cristina Garcia. Rep. Garcia sponsored a bill just signed into law by Gov. Gavin Newsom that legalizes the practice of human composting, the reduction of remains by “placing bodies in individual vessels and fostering gentle transformation into a nutrient-dense soil.”
Since we’ve written about this sort of thing before – Washington was the first state to legalize the process back in 2019 – we’re more interested now in what Rep. Garcia told NBC News while describing her motivation: “I’ve always wanted to be a tree. The idea of having my family sitting under my shade one day – that brings a lot of joy.” How great is that? Tree-hugging is just not enough. Be the tree.
California is the fifth state to provide its residents with the human composting option, the other three being Colorado, Oregon, and Vermont. The process “typically involves putting a body into a steel vessel, then covering it with organic materials like straw, wood chips and alfalfa. Microbes break down the corpse and the plant matter, transforming the various components into nutrient-rich soil in roughly 30 days,” Smithsonian Magazine explained.
We just happen to have some good news for Rep. Garcia about that wanting-to-be-a-tree business. She’s already pretty close. For more on that, we go to our correspondent from beyond the grave, Carl Sagan, who shares a thought about trees. And no, we couldn’t just write out his quote here. You have to hear it in Dr. Sagan’s own voice.
That’ll be one pandemic with extra distress. Hold the goals
When the COVID-19 pandemic first hit it put a lot of stuff on hold for everyone. Couldn’t eat inside at your favorite restaurant, attend that long-awaited concert, or travel out of the country. Those were all pretty bad, but it was the disruption of pursuing long-term goals that seemed to have the most effect on people’s mental health.
Investigators from the University of Waterloo (Ont.) looked at how putting such goals on hold affected people’s mental well-being. The study’s 226 participants were asked about their “COVID-frozen” goals and the degree to which they were able to actively pursue each goal and how committed they were to achieving it.
What they found was that the participants’ COVID-frozen goals were associated with feelings of psychological distress, such as anxiety, depressive symptoms, stress, and lowered life satisfaction. It was only when participants were able to disengage from goal rumination that well-being was impacted positively.
“Goal rumination is compulsive and can aggravate worries and frustrations while also taking away mental resources from other goals,” Candice Hubley, lead author and a PhD candidate in psychology, said in a written statement. So in short, you’re only stressing yourself out more about something that is far off in the distance when you could be focusing more on short-term, tangible goals instead.
Now, no one is saying to give up on your goals. Just take them one at a time. You’ll have better life satisfaction and your COVID-frozen goals will thaw out before you know it.
Continued monkeypox spread can lead to viral mutations
Monkeypox cases are declining in the United States and the United Kingdom, but experts are urging the public to continue efforts to stanch the spread of the virus. Continued transmission of monkeypox provides more opportunities for the virus to mutate, according to Philip Johnson, PhD, assistant professor of biology at the University of Maryland, College Park, and colleagues.
the authors wrote in a correspondence published in The Lancet.
When case numbers are lower – and therefore less of a public health concern – viral transmission chains can be longer without causing alarm, Dr. Johnson explained. “The more generations of transmission, the more opportunities there are for mutations to occur,” he told this news organization. While it is difficult to anticipate how mutations can affect a virus, these changes in genetic code could be advantageous to the virus, making it more transmissible from human to human and therefore much more difficult to control.
This applies to any virus. The large Ebola outbreak from 2013 to 2016 is an example; a retrospective analysis found that specific amino acid changes in the Ebola virus increased growth in human cells and may have made the virus more infectious. More recently, the Delta and Omicron variants of SARS-CoV-2 each contained mutations that were associated with higher transmissibility. A recent study suggested that monkeypox appears to be mutating faster than expected, though it is not clear if these genetic mutations have changed the virus’ behavior.
Zoonotic infections, or viruses that originate from nonhuman animals, at first are expected to be less adapted to people, but that can change over time. When a virus continues to jump from animals to humans – as monkeypox has done since it was first identified in humans in 1970 – chances are it will gain a mutation that allows it to spread more effectively between people, said Rachel Roper, PhD, a professor of microbiology and immunology at East Carolina University, Greenville, N.C. She was not involved with The Lancet article.
“We discounted monkeypox; we didn’t pay much attention to it because it had not been that big of a problem,” she said in an interview. “We think this virus has been circulating now since 2017 and we really just realized it in May.”
Although monkeypox received global attention this past summer, the outbreak is now receiving less news coverage, and the public’s attention may be waning. Furthermore, the U.S. Congress just dropped billions of dollars from a short-term spending bill that would have provided additional COVID-19 and monkeypox funding.
Although new cases are trending downward, now is not the time to take our foot off the gas, Dr. Johnson and colleagues warned. “The epidemic is far from over, and continued drive toward elimination is essential,” the authors wrote. Because the virus exists in rodent populations in areas of central and west Africa, it is not possible to eradicate monkeypox as we did smallpox; however, “we could, through vaccination, eliminate any significant human to human transmission,” Dr. Johnson said.
Dr. Johnson also urges a more proactive approach to combating emerging infectious diseases in the future. “We wrote this article to raise awareness about the importance of dedicating resources to controlling these diseases all the way down to ideally elimination in the countries where they develop, and not just waiting until [these diseases] reach wealthier countries,” he said.
Dr. Roper agrees that a more global perspective is needed in monitoring and controlling zoonotic disease, but resources are limited. “The problem is there are a whole bunch of virus groups and a whole bunch of viruses jumping into humans all the time,” she said. “We can’t predict which virus group is going to be the next one with a big hit. I worked on SARS-CoV-1 back in 2003 to 2009, and I would have predicted that a virus from some other group would have jumped into humans next, before COVID hit,” she added.
Dr. Johnson acknowledged that it is hard to know where to focus public health resources, considering the hundreds of thousands of zoonotic viruses that may exist. He thought the best approach was to target emerging diseases that already appear to have extended transmission chains, “not just things that are hopping from animals to humans and sputtering out and disappearing, but diseases that appear to have any sustained human to human transmission.”
Dr. Johnson and Dr. Roper report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monkeypox cases are declining in the United States and the United Kingdom, but experts are urging the public to continue efforts to stanch the spread of the virus. Continued transmission of monkeypox provides more opportunities for the virus to mutate, according to Philip Johnson, PhD, assistant professor of biology at the University of Maryland, College Park, and colleagues.
the authors wrote in a correspondence published in The Lancet.
When case numbers are lower – and therefore less of a public health concern – viral transmission chains can be longer without causing alarm, Dr. Johnson explained. “The more generations of transmission, the more opportunities there are for mutations to occur,” he told this news organization. While it is difficult to anticipate how mutations can affect a virus, these changes in genetic code could be advantageous to the virus, making it more transmissible from human to human and therefore much more difficult to control.
This applies to any virus. The large Ebola outbreak from 2013 to 2016 is an example; a retrospective analysis found that specific amino acid changes in the Ebola virus increased growth in human cells and may have made the virus more infectious. More recently, the Delta and Omicron variants of SARS-CoV-2 each contained mutations that were associated with higher transmissibility. A recent study suggested that monkeypox appears to be mutating faster than expected, though it is not clear if these genetic mutations have changed the virus’ behavior.
Zoonotic infections, or viruses that originate from nonhuman animals, at first are expected to be less adapted to people, but that can change over time. When a virus continues to jump from animals to humans – as monkeypox has done since it was first identified in humans in 1970 – chances are it will gain a mutation that allows it to spread more effectively between people, said Rachel Roper, PhD, a professor of microbiology and immunology at East Carolina University, Greenville, N.C. She was not involved with The Lancet article.
“We discounted monkeypox; we didn’t pay much attention to it because it had not been that big of a problem,” she said in an interview. “We think this virus has been circulating now since 2017 and we really just realized it in May.”
Although monkeypox received global attention this past summer, the outbreak is now receiving less news coverage, and the public’s attention may be waning. Furthermore, the U.S. Congress just dropped billions of dollars from a short-term spending bill that would have provided additional COVID-19 and monkeypox funding.
Although new cases are trending downward, now is not the time to take our foot off the gas, Dr. Johnson and colleagues warned. “The epidemic is far from over, and continued drive toward elimination is essential,” the authors wrote. Because the virus exists in rodent populations in areas of central and west Africa, it is not possible to eradicate monkeypox as we did smallpox; however, “we could, through vaccination, eliminate any significant human to human transmission,” Dr. Johnson said.
Dr. Johnson also urges a more proactive approach to combating emerging infectious diseases in the future. “We wrote this article to raise awareness about the importance of dedicating resources to controlling these diseases all the way down to ideally elimination in the countries where they develop, and not just waiting until [these diseases] reach wealthier countries,” he said.
Dr. Roper agrees that a more global perspective is needed in monitoring and controlling zoonotic disease, but resources are limited. “The problem is there are a whole bunch of virus groups and a whole bunch of viruses jumping into humans all the time,” she said. “We can’t predict which virus group is going to be the next one with a big hit. I worked on SARS-CoV-1 back in 2003 to 2009, and I would have predicted that a virus from some other group would have jumped into humans next, before COVID hit,” she added.
Dr. Johnson acknowledged that it is hard to know where to focus public health resources, considering the hundreds of thousands of zoonotic viruses that may exist. He thought the best approach was to target emerging diseases that already appear to have extended transmission chains, “not just things that are hopping from animals to humans and sputtering out and disappearing, but diseases that appear to have any sustained human to human transmission.”
Dr. Johnson and Dr. Roper report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monkeypox cases are declining in the United States and the United Kingdom, but experts are urging the public to continue efforts to stanch the spread of the virus. Continued transmission of monkeypox provides more opportunities for the virus to mutate, according to Philip Johnson, PhD, assistant professor of biology at the University of Maryland, College Park, and colleagues.
the authors wrote in a correspondence published in The Lancet.
When case numbers are lower – and therefore less of a public health concern – viral transmission chains can be longer without causing alarm, Dr. Johnson explained. “The more generations of transmission, the more opportunities there are for mutations to occur,” he told this news organization. While it is difficult to anticipate how mutations can affect a virus, these changes in genetic code could be advantageous to the virus, making it more transmissible from human to human and therefore much more difficult to control.
This applies to any virus. The large Ebola outbreak from 2013 to 2016 is an example; a retrospective analysis found that specific amino acid changes in the Ebola virus increased growth in human cells and may have made the virus more infectious. More recently, the Delta and Omicron variants of SARS-CoV-2 each contained mutations that were associated with higher transmissibility. A recent study suggested that monkeypox appears to be mutating faster than expected, though it is not clear if these genetic mutations have changed the virus’ behavior.
Zoonotic infections, or viruses that originate from nonhuman animals, at first are expected to be less adapted to people, but that can change over time. When a virus continues to jump from animals to humans – as monkeypox has done since it was first identified in humans in 1970 – chances are it will gain a mutation that allows it to spread more effectively between people, said Rachel Roper, PhD, a professor of microbiology and immunology at East Carolina University, Greenville, N.C. She was not involved with The Lancet article.
“We discounted monkeypox; we didn’t pay much attention to it because it had not been that big of a problem,” she said in an interview. “We think this virus has been circulating now since 2017 and we really just realized it in May.”
Although monkeypox received global attention this past summer, the outbreak is now receiving less news coverage, and the public’s attention may be waning. Furthermore, the U.S. Congress just dropped billions of dollars from a short-term spending bill that would have provided additional COVID-19 and monkeypox funding.
Although new cases are trending downward, now is not the time to take our foot off the gas, Dr. Johnson and colleagues warned. “The epidemic is far from over, and continued drive toward elimination is essential,” the authors wrote. Because the virus exists in rodent populations in areas of central and west Africa, it is not possible to eradicate monkeypox as we did smallpox; however, “we could, through vaccination, eliminate any significant human to human transmission,” Dr. Johnson said.
Dr. Johnson also urges a more proactive approach to combating emerging infectious diseases in the future. “We wrote this article to raise awareness about the importance of dedicating resources to controlling these diseases all the way down to ideally elimination in the countries where they develop, and not just waiting until [these diseases] reach wealthier countries,” he said.
Dr. Roper agrees that a more global perspective is needed in monitoring and controlling zoonotic disease, but resources are limited. “The problem is there are a whole bunch of virus groups and a whole bunch of viruses jumping into humans all the time,” she said. “We can’t predict which virus group is going to be the next one with a big hit. I worked on SARS-CoV-1 back in 2003 to 2009, and I would have predicted that a virus from some other group would have jumped into humans next, before COVID hit,” she added.
Dr. Johnson acknowledged that it is hard to know where to focus public health resources, considering the hundreds of thousands of zoonotic viruses that may exist. He thought the best approach was to target emerging diseases that already appear to have extended transmission chains, “not just things that are hopping from animals to humans and sputtering out and disappearing, but diseases that appear to have any sustained human to human transmission.”
Dr. Johnson and Dr. Roper report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dual-Physician Marriages: Understanding the Challenges and Rewards
Dual-physician marriages are becoming increasingly common. The estimated median age of first marriage has been increasing; the US Census Bureau reported a median age of 30.4 years for men and 28.6 years for women in early 2021.1 According to the Association of American Medical Colleges 2020 Matriculating Student Questionnaire, the median age at matriculation for medical students was 23 years (N=16,956), and 92.4% (N=15,932) reported their marital status as single and never legally married.2 Thus, it is likely that the majority of physicians get married at some point during medical school or residency training. A survey of over 10,000 physicians in more than 29 specialties showed that 24% of female physicians and 15% of male physicians are married to other physicians.3
Challenges
There are common challenges to all dual-career households, including coordinating demanding career schedules that compete with each other, balancing childrearing with career advancement, and harmonizing economic and personal goals. However, there are challenges that can be amplified in and unique to dual-physician marriages.
The Couples Match—Medical students, trainees, and even physicians in later stages of their careers may have less autonomy over their schedules compared to professionals in other fields. An early obstacle that many dual-physician marriages must overcome is navigating the National Resident Matching Program as a couple. The number of individuals participating as a couple in the 2022 Main Residency Match was 2444, and the postgraduate year 1 (PGY-1) match rate for individuals participating as a couple was 93.7%. The overall PGY-1 match rate for MD seniors in the United States was 92.9%.4 Thus, entering the match as a couple does not necessarily pose a disadvantage to successfully matching, but these statistics may be misleading. When applicants participate in the Match as a couple, their rank order lists form pairs of program choices that are processed by the matching algorithm to match the couple to the most preferred pair of programs on their rank order lists where each partner has been offered a position. Although many couples coordinate their rank order lists geographically, there is no guarantee that the couple will actually match together in the same city, let alone in the same time zone. Also, the statistics do not take into account if an individual in the couple is only partially matched (eg, if one applicant matches to a preliminary year position but not to an advanced dermatology position). The couples’ Match is only available to partners in the same application cycle, and couples that are not in sync may be more restricted when applying for residency positions.
Lack of Synchronization—Dual-physician couples are challenged to achieve synchronization not only in their day-to-day lives but also over the course of their careers. After matching to residency, the dual-physician couple faces additional scheduling stressors during training. Varied demanding patient schedules and competing call schedules may take a toll on the ability to spend time together. Coordination between both training programs to ensure weekend schedules and vacations are aligned can be helpful to try to maximize time together. If the couple’s education is staggered, their training schedules may not align when proceeding to fellowship or starting off with a new job as an attending. It is not uncommon for couples in medicine to be long-distance for a period of time, and partners may find themselves sacrificing ideal positions or self-restricting application to certain programs or jobs to secure a position near a partner who is already in training in a certain geographic location.
Domestic Work-Life Balance—Juxtaposing 2 highly demanding careers in the same household can be associated with certain tensions, as the weight of household and childrearing responsibilities as well as professional productivity and advancement is divided by the couple. In a 2008 survey of the American College of Surgeons on burnout, work-home conflict, and career satisfaction, surgeons in dual-physician relationships experienced a recent career conflict with their domestic partner and a work-home conflict more often than surgeons whose partners were working nonphysicians.5 The hours worked between men and women in dual-physician families differed according to a national sample of 9868 physicians in dual-physician relationships. The study showed that weekly hours worked by women with children were lower than among those without children, whereas similar differences were not observed among men.6 It is not understood if this suggests that women in dual-physician families work fewer hours due to the pressures of historical gender norms and increased household responsibilities. A 1988 survey of female physicians (N=382) in which 247 respondents indicated that they had domestic partners showed that women physicians whose partners also were physicians (n=91) were more than twice as likely to interrupt their own careers for their partners’ careers compared to female physicians whose partners were not physicians (n=156)(25% vs 11%, respectively). In contrast, the male partners who were not physicians were significantly more likely to interrupt their careers than male partners who were physicians (41% vs 15%, respectively, P<.05).7
Divorce—There have been mixed reports on the incidence of divorce in physicians compared to the general population, but studies suggest that physicians’ marriages tend to be more stable than those of other societal groups.8 Of 203 respondents of a survey of female physician members of the Minnesota Medical Association who were or had been married to another physician, 11.3% (22/203) were divorced, and medicine was reported to play a role in 69.6% of those separations.9 A retrospective analysis of nationally representative surveys by the US Census showed that divorce among physicians is less common than among non–health care workers and several other health professions.10
Rewards
The benefits of medical marriages are multifold and include increased job satisfaction, stability, financial security, shared passions, and mutual understanding. Common passions and interests form the foundation for many relationships, which is true for the dual-physician marriage. In a 2009 study, Perlman et al11 performed qualitative interviews with 25 physicians and their partners—10 of which were in dual-physician relationships—about the challenges and strengths of their relationships. A key theme that emerged during the interviews was the acknowledgment of the benefits of being a physician to the relationship. Participants discussed both the financial security in a physician marriage and the security that medical knowledge adds to a relationship when caring for ill or injured family members. Other key themes identified were relying on mutual support in the relationship, recognizing the important role of each family member, and having shared values.11
Financial Security—The financial security attributed to being in a medical marriage was highlighted in a series of interviews with physicians and their spouses.11 A cross-sectional survey of a random sample of physicians showed that both men and women in dual-physician families had lower personal incomes than physicians married to nonphysicians. However, men and women in dual-physician families had spouses with higher incomes compared to spouses of physicians married to nonphysicians. Thus, the total family incomes were substantially higher in dual-physician households than the family incomes of physicians married to nonphysicians.12
Satisfaction—Dual-physician marriages benefit from a shared camaraderie and understanding of the joys and sacrifices that accompany pursuing a career in medicine. Medical spouses can communicate in mutually understood medical jargon. Compared to physicians married to nonphysicians, a statistically significant difference (P<.001) was found in physicians in dual-physicians families who more frequently reported enjoyment in discussing work with their spouses and more frequently reported satisfaction from shared work interests with their spouses.12
Final Thoughts
From the start of medical training, physicians and physicians-in-training experience unique benefits and challenges that are compounded in distinctive ways when 2 physicians get married. In an era where dual-physician marriage is becoming more common, it is important to acknowledge how this can both enrich and challenge the relationship.
Acknowledgment—The author thanks her husband Joshua L. Weinstock, MD (Camden, New Jersey), for his contribution to this article and their marriage.
- Census Bureau releases new estimates on America’s families and living arrangements. News release. US Census Bureau; November 29, 2021. Accessed September 23, 2022. https://www.census.gov/newsroom/press-releases/2021/families-and-living-arrangements.html
- Association of American Medical Colleges. Matriculating Student Questionnaire: 2020 All Schools Summary Report. Published December 2020. Accessed September 12, 2022. https://www.aamc.org/media/50081/download
- Baggett SM, Martin KL. Medscape physician lifestyle & happiness report 2022. Medscape. January 14, 2022. Accessed September 19, 2022. https://www.medscape.com/slideshow/2022-lifestyle-happiness-6014665
- National Resident Matching Program. Results and Data 2022 Main Residency Match. Published May 2022. Accessed September 12, 2022. https://www.nrmp.org/wp-content/uploads/2022/05/2022-Main-Match-Results-and-Data_Final.pdf
- Dyrbye LN, Shanafelt TD, Balch CM, et al. Physicians married or partnered to physicians: a comparative study in the American College of Surgeons. J Am Coll Surg. 2010;211:663-671. doi:10.1016/j.jamcollsurg.2010.03.032
- Ly DP, Seabury SA, Jena AB. Hours worked among US dual physician couples with children, 2000 to 2015. JAMA Intern Med. 2017;177:1524-1525. doi:10.1001/jamainternmed.2017.3437
- Tesch BJ, Osborne J, Simpson DE, et al. Women physicians in dual-physician relationships compared with those in other dual-career relationships. Acad Med. 1992;67:542-544. doi:10.1097/00001888-199208000-00014
- Doherty WJ, Burge SK. Divorce among physicians. comparisons with other occupational groups. JAMA. 1989;261:2374-2377.
- Smith C, Boulger J, Beattie K. Exploring the dual-physician marriage. Minn Med. 2002;85:39-43.
- Ly DP, Seabury SA, Jena AB. Divorce among physicians and other healthcare professionals in the United States: analysis of census survey data. BMJ. 2015;350:h706. doi:10.1136/bmj.h706
- Perlman RL, Ross PT, Lypson ML. Understanding the medical marriage: physicians and their partners share strategies for success. Acad Med. 2015;90:63-68. doi:10.1097/ACM.0000000000000449
- Sobecks NW, Justice AC, Hinze S, et al. When doctors marry doctors: a survey exploring the professional and family lives of young physicians. Ann Intern Med. 1999;130(4 pt 1):312-319. doi:10.7326/0003-4819-130-4-199902160-00017
Dual-physician marriages are becoming increasingly common. The estimated median age of first marriage has been increasing; the US Census Bureau reported a median age of 30.4 years for men and 28.6 years for women in early 2021.1 According to the Association of American Medical Colleges 2020 Matriculating Student Questionnaire, the median age at matriculation for medical students was 23 years (N=16,956), and 92.4% (N=15,932) reported their marital status as single and never legally married.2 Thus, it is likely that the majority of physicians get married at some point during medical school or residency training. A survey of over 10,000 physicians in more than 29 specialties showed that 24% of female physicians and 15% of male physicians are married to other physicians.3
Challenges
There are common challenges to all dual-career households, including coordinating demanding career schedules that compete with each other, balancing childrearing with career advancement, and harmonizing economic and personal goals. However, there are challenges that can be amplified in and unique to dual-physician marriages.
The Couples Match—Medical students, trainees, and even physicians in later stages of their careers may have less autonomy over their schedules compared to professionals in other fields. An early obstacle that many dual-physician marriages must overcome is navigating the National Resident Matching Program as a couple. The number of individuals participating as a couple in the 2022 Main Residency Match was 2444, and the postgraduate year 1 (PGY-1) match rate for individuals participating as a couple was 93.7%. The overall PGY-1 match rate for MD seniors in the United States was 92.9%.4 Thus, entering the match as a couple does not necessarily pose a disadvantage to successfully matching, but these statistics may be misleading. When applicants participate in the Match as a couple, their rank order lists form pairs of program choices that are processed by the matching algorithm to match the couple to the most preferred pair of programs on their rank order lists where each partner has been offered a position. Although many couples coordinate their rank order lists geographically, there is no guarantee that the couple will actually match together in the same city, let alone in the same time zone. Also, the statistics do not take into account if an individual in the couple is only partially matched (eg, if one applicant matches to a preliminary year position but not to an advanced dermatology position). The couples’ Match is only available to partners in the same application cycle, and couples that are not in sync may be more restricted when applying for residency positions.
Lack of Synchronization—Dual-physician couples are challenged to achieve synchronization not only in their day-to-day lives but also over the course of their careers. After matching to residency, the dual-physician couple faces additional scheduling stressors during training. Varied demanding patient schedules and competing call schedules may take a toll on the ability to spend time together. Coordination between both training programs to ensure weekend schedules and vacations are aligned can be helpful to try to maximize time together. If the couple’s education is staggered, their training schedules may not align when proceeding to fellowship or starting off with a new job as an attending. It is not uncommon for couples in medicine to be long-distance for a period of time, and partners may find themselves sacrificing ideal positions or self-restricting application to certain programs or jobs to secure a position near a partner who is already in training in a certain geographic location.
Domestic Work-Life Balance—Juxtaposing 2 highly demanding careers in the same household can be associated with certain tensions, as the weight of household and childrearing responsibilities as well as professional productivity and advancement is divided by the couple. In a 2008 survey of the American College of Surgeons on burnout, work-home conflict, and career satisfaction, surgeons in dual-physician relationships experienced a recent career conflict with their domestic partner and a work-home conflict more often than surgeons whose partners were working nonphysicians.5 The hours worked between men and women in dual-physician families differed according to a national sample of 9868 physicians in dual-physician relationships. The study showed that weekly hours worked by women with children were lower than among those without children, whereas similar differences were not observed among men.6 It is not understood if this suggests that women in dual-physician families work fewer hours due to the pressures of historical gender norms and increased household responsibilities. A 1988 survey of female physicians (N=382) in which 247 respondents indicated that they had domestic partners showed that women physicians whose partners also were physicians (n=91) were more than twice as likely to interrupt their own careers for their partners’ careers compared to female physicians whose partners were not physicians (n=156)(25% vs 11%, respectively). In contrast, the male partners who were not physicians were significantly more likely to interrupt their careers than male partners who were physicians (41% vs 15%, respectively, P<.05).7
Divorce—There have been mixed reports on the incidence of divorce in physicians compared to the general population, but studies suggest that physicians’ marriages tend to be more stable than those of other societal groups.8 Of 203 respondents of a survey of female physician members of the Minnesota Medical Association who were or had been married to another physician, 11.3% (22/203) were divorced, and medicine was reported to play a role in 69.6% of those separations.9 A retrospective analysis of nationally representative surveys by the US Census showed that divorce among physicians is less common than among non–health care workers and several other health professions.10
Rewards
The benefits of medical marriages are multifold and include increased job satisfaction, stability, financial security, shared passions, and mutual understanding. Common passions and interests form the foundation for many relationships, which is true for the dual-physician marriage. In a 2009 study, Perlman et al11 performed qualitative interviews with 25 physicians and their partners—10 of which were in dual-physician relationships—about the challenges and strengths of their relationships. A key theme that emerged during the interviews was the acknowledgment of the benefits of being a physician to the relationship. Participants discussed both the financial security in a physician marriage and the security that medical knowledge adds to a relationship when caring for ill or injured family members. Other key themes identified were relying on mutual support in the relationship, recognizing the important role of each family member, and having shared values.11
Financial Security—The financial security attributed to being in a medical marriage was highlighted in a series of interviews with physicians and their spouses.11 A cross-sectional survey of a random sample of physicians showed that both men and women in dual-physician families had lower personal incomes than physicians married to nonphysicians. However, men and women in dual-physician families had spouses with higher incomes compared to spouses of physicians married to nonphysicians. Thus, the total family incomes were substantially higher in dual-physician households than the family incomes of physicians married to nonphysicians.12
Satisfaction—Dual-physician marriages benefit from a shared camaraderie and understanding of the joys and sacrifices that accompany pursuing a career in medicine. Medical spouses can communicate in mutually understood medical jargon. Compared to physicians married to nonphysicians, a statistically significant difference (P<.001) was found in physicians in dual-physicians families who more frequently reported enjoyment in discussing work with their spouses and more frequently reported satisfaction from shared work interests with their spouses.12
Final Thoughts
From the start of medical training, physicians and physicians-in-training experience unique benefits and challenges that are compounded in distinctive ways when 2 physicians get married. In an era where dual-physician marriage is becoming more common, it is important to acknowledge how this can both enrich and challenge the relationship.
Acknowledgment—The author thanks her husband Joshua L. Weinstock, MD (Camden, New Jersey), for his contribution to this article and their marriage.
Dual-physician marriages are becoming increasingly common. The estimated median age of first marriage has been increasing; the US Census Bureau reported a median age of 30.4 years for men and 28.6 years for women in early 2021.1 According to the Association of American Medical Colleges 2020 Matriculating Student Questionnaire, the median age at matriculation for medical students was 23 years (N=16,956), and 92.4% (N=15,932) reported their marital status as single and never legally married.2 Thus, it is likely that the majority of physicians get married at some point during medical school or residency training. A survey of over 10,000 physicians in more than 29 specialties showed that 24% of female physicians and 15% of male physicians are married to other physicians.3
Challenges
There are common challenges to all dual-career households, including coordinating demanding career schedules that compete with each other, balancing childrearing with career advancement, and harmonizing economic and personal goals. However, there are challenges that can be amplified in and unique to dual-physician marriages.
The Couples Match—Medical students, trainees, and even physicians in later stages of their careers may have less autonomy over their schedules compared to professionals in other fields. An early obstacle that many dual-physician marriages must overcome is navigating the National Resident Matching Program as a couple. The number of individuals participating as a couple in the 2022 Main Residency Match was 2444, and the postgraduate year 1 (PGY-1) match rate for individuals participating as a couple was 93.7%. The overall PGY-1 match rate for MD seniors in the United States was 92.9%.4 Thus, entering the match as a couple does not necessarily pose a disadvantage to successfully matching, but these statistics may be misleading. When applicants participate in the Match as a couple, their rank order lists form pairs of program choices that are processed by the matching algorithm to match the couple to the most preferred pair of programs on their rank order lists where each partner has been offered a position. Although many couples coordinate their rank order lists geographically, there is no guarantee that the couple will actually match together in the same city, let alone in the same time zone. Also, the statistics do not take into account if an individual in the couple is only partially matched (eg, if one applicant matches to a preliminary year position but not to an advanced dermatology position). The couples’ Match is only available to partners in the same application cycle, and couples that are not in sync may be more restricted when applying for residency positions.
Lack of Synchronization—Dual-physician couples are challenged to achieve synchronization not only in their day-to-day lives but also over the course of their careers. After matching to residency, the dual-physician couple faces additional scheduling stressors during training. Varied demanding patient schedules and competing call schedules may take a toll on the ability to spend time together. Coordination between both training programs to ensure weekend schedules and vacations are aligned can be helpful to try to maximize time together. If the couple’s education is staggered, their training schedules may not align when proceeding to fellowship or starting off with a new job as an attending. It is not uncommon for couples in medicine to be long-distance for a period of time, and partners may find themselves sacrificing ideal positions or self-restricting application to certain programs or jobs to secure a position near a partner who is already in training in a certain geographic location.
Domestic Work-Life Balance—Juxtaposing 2 highly demanding careers in the same household can be associated with certain tensions, as the weight of household and childrearing responsibilities as well as professional productivity and advancement is divided by the couple. In a 2008 survey of the American College of Surgeons on burnout, work-home conflict, and career satisfaction, surgeons in dual-physician relationships experienced a recent career conflict with their domestic partner and a work-home conflict more often than surgeons whose partners were working nonphysicians.5 The hours worked between men and women in dual-physician families differed according to a national sample of 9868 physicians in dual-physician relationships. The study showed that weekly hours worked by women with children were lower than among those without children, whereas similar differences were not observed among men.6 It is not understood if this suggests that women in dual-physician families work fewer hours due to the pressures of historical gender norms and increased household responsibilities. A 1988 survey of female physicians (N=382) in which 247 respondents indicated that they had domestic partners showed that women physicians whose partners also were physicians (n=91) were more than twice as likely to interrupt their own careers for their partners’ careers compared to female physicians whose partners were not physicians (n=156)(25% vs 11%, respectively). In contrast, the male partners who were not physicians were significantly more likely to interrupt their careers than male partners who were physicians (41% vs 15%, respectively, P<.05).7
Divorce—There have been mixed reports on the incidence of divorce in physicians compared to the general population, but studies suggest that physicians’ marriages tend to be more stable than those of other societal groups.8 Of 203 respondents of a survey of female physician members of the Minnesota Medical Association who were or had been married to another physician, 11.3% (22/203) were divorced, and medicine was reported to play a role in 69.6% of those separations.9 A retrospective analysis of nationally representative surveys by the US Census showed that divorce among physicians is less common than among non–health care workers and several other health professions.10
Rewards
The benefits of medical marriages are multifold and include increased job satisfaction, stability, financial security, shared passions, and mutual understanding. Common passions and interests form the foundation for many relationships, which is true for the dual-physician marriage. In a 2009 study, Perlman et al11 performed qualitative interviews with 25 physicians and their partners—10 of which were in dual-physician relationships—about the challenges and strengths of their relationships. A key theme that emerged during the interviews was the acknowledgment of the benefits of being a physician to the relationship. Participants discussed both the financial security in a physician marriage and the security that medical knowledge adds to a relationship when caring for ill or injured family members. Other key themes identified were relying on mutual support in the relationship, recognizing the important role of each family member, and having shared values.11
Financial Security—The financial security attributed to being in a medical marriage was highlighted in a series of interviews with physicians and their spouses.11 A cross-sectional survey of a random sample of physicians showed that both men and women in dual-physician families had lower personal incomes than physicians married to nonphysicians. However, men and women in dual-physician families had spouses with higher incomes compared to spouses of physicians married to nonphysicians. Thus, the total family incomes were substantially higher in dual-physician households than the family incomes of physicians married to nonphysicians.12
Satisfaction—Dual-physician marriages benefit from a shared camaraderie and understanding of the joys and sacrifices that accompany pursuing a career in medicine. Medical spouses can communicate in mutually understood medical jargon. Compared to physicians married to nonphysicians, a statistically significant difference (P<.001) was found in physicians in dual-physicians families who more frequently reported enjoyment in discussing work with their spouses and more frequently reported satisfaction from shared work interests with their spouses.12
Final Thoughts
From the start of medical training, physicians and physicians-in-training experience unique benefits and challenges that are compounded in distinctive ways when 2 physicians get married. In an era where dual-physician marriage is becoming more common, it is important to acknowledge how this can both enrich and challenge the relationship.
Acknowledgment—The author thanks her husband Joshua L. Weinstock, MD (Camden, New Jersey), for his contribution to this article and their marriage.
- Census Bureau releases new estimates on America’s families and living arrangements. News release. US Census Bureau; November 29, 2021. Accessed September 23, 2022. https://www.census.gov/newsroom/press-releases/2021/families-and-living-arrangements.html
- Association of American Medical Colleges. Matriculating Student Questionnaire: 2020 All Schools Summary Report. Published December 2020. Accessed September 12, 2022. https://www.aamc.org/media/50081/download
- Baggett SM, Martin KL. Medscape physician lifestyle & happiness report 2022. Medscape. January 14, 2022. Accessed September 19, 2022. https://www.medscape.com/slideshow/2022-lifestyle-happiness-6014665
- National Resident Matching Program. Results and Data 2022 Main Residency Match. Published May 2022. Accessed September 12, 2022. https://www.nrmp.org/wp-content/uploads/2022/05/2022-Main-Match-Results-and-Data_Final.pdf
- Dyrbye LN, Shanafelt TD, Balch CM, et al. Physicians married or partnered to physicians: a comparative study in the American College of Surgeons. J Am Coll Surg. 2010;211:663-671. doi:10.1016/j.jamcollsurg.2010.03.032
- Ly DP, Seabury SA, Jena AB. Hours worked among US dual physician couples with children, 2000 to 2015. JAMA Intern Med. 2017;177:1524-1525. doi:10.1001/jamainternmed.2017.3437
- Tesch BJ, Osborne J, Simpson DE, et al. Women physicians in dual-physician relationships compared with those in other dual-career relationships. Acad Med. 1992;67:542-544. doi:10.1097/00001888-199208000-00014
- Doherty WJ, Burge SK. Divorce among physicians. comparisons with other occupational groups. JAMA. 1989;261:2374-2377.
- Smith C, Boulger J, Beattie K. Exploring the dual-physician marriage. Minn Med. 2002;85:39-43.
- Ly DP, Seabury SA, Jena AB. Divorce among physicians and other healthcare professionals in the United States: analysis of census survey data. BMJ. 2015;350:h706. doi:10.1136/bmj.h706
- Perlman RL, Ross PT, Lypson ML. Understanding the medical marriage: physicians and their partners share strategies for success. Acad Med. 2015;90:63-68. doi:10.1097/ACM.0000000000000449
- Sobecks NW, Justice AC, Hinze S, et al. When doctors marry doctors: a survey exploring the professional and family lives of young physicians. Ann Intern Med. 1999;130(4 pt 1):312-319. doi:10.7326/0003-4819-130-4-199902160-00017
- Census Bureau releases new estimates on America’s families and living arrangements. News release. US Census Bureau; November 29, 2021. Accessed September 23, 2022. https://www.census.gov/newsroom/press-releases/2021/families-and-living-arrangements.html
- Association of American Medical Colleges. Matriculating Student Questionnaire: 2020 All Schools Summary Report. Published December 2020. Accessed September 12, 2022. https://www.aamc.org/media/50081/download
- Baggett SM, Martin KL. Medscape physician lifestyle & happiness report 2022. Medscape. January 14, 2022. Accessed September 19, 2022. https://www.medscape.com/slideshow/2022-lifestyle-happiness-6014665
- National Resident Matching Program. Results and Data 2022 Main Residency Match. Published May 2022. Accessed September 12, 2022. https://www.nrmp.org/wp-content/uploads/2022/05/2022-Main-Match-Results-and-Data_Final.pdf
- Dyrbye LN, Shanafelt TD, Balch CM, et al. Physicians married or partnered to physicians: a comparative study in the American College of Surgeons. J Am Coll Surg. 2010;211:663-671. doi:10.1016/j.jamcollsurg.2010.03.032
- Ly DP, Seabury SA, Jena AB. Hours worked among US dual physician couples with children, 2000 to 2015. JAMA Intern Med. 2017;177:1524-1525. doi:10.1001/jamainternmed.2017.3437
- Tesch BJ, Osborne J, Simpson DE, et al. Women physicians in dual-physician relationships compared with those in other dual-career relationships. Acad Med. 1992;67:542-544. doi:10.1097/00001888-199208000-00014
- Doherty WJ, Burge SK. Divorce among physicians. comparisons with other occupational groups. JAMA. 1989;261:2374-2377.
- Smith C, Boulger J, Beattie K. Exploring the dual-physician marriage. Minn Med. 2002;85:39-43.
- Ly DP, Seabury SA, Jena AB. Divorce among physicians and other healthcare professionals in the United States: analysis of census survey data. BMJ. 2015;350:h706. doi:10.1136/bmj.h706
- Perlman RL, Ross PT, Lypson ML. Understanding the medical marriage: physicians and their partners share strategies for success. Acad Med. 2015;90:63-68. doi:10.1097/ACM.0000000000000449
- Sobecks NW, Justice AC, Hinze S, et al. When doctors marry doctors: a survey exploring the professional and family lives of young physicians. Ann Intern Med. 1999;130(4 pt 1):312-319. doi:10.7326/0003-4819-130-4-199902160-00017
Resident Pearl
- As more physicians marry other physicians, there is an increasing need to understand the challenges and rewards of these relationships.
HIV Pre-exposure Prophylaxis (PrEP): A Survey of Dermatologists’ Knowledge and Practice Patterns
To the Editor:
In a 2010 landmark paper, researchers reported that the Preexposure Prophylaxis Initiative (iPrEx) trial demonstrated that once-daily pre-exposure prophylaxis (PrEP) with emtricitabine plus tenofovir disoproxil fumarate, which was approved by the US Food and Drug Administration (FDA) and packaged together as Truvada (Gilead Sciences, Inc), achieved a 44% reduction in the incidence of HIV infection compared to the placebo arm of the study (64/1248 HIV infections in the placebo group vs 36/1251 in the intervention group).1 Subsequently, the US Department of Health and Human Services proposed an initiative to reduce new HIV infections by 90% by 2030.2 The Centers for Disease Control and Prevention estimates that 1.1 million Americans have an indication for PrEP, yet only approximately 400,000 individuals currently take PrEP.3,4
Increasing awareness of PrEP and its indications is essential because PrEP exerts its greatest benefit when used broadly. Awareness among primary care and infectious disease physicians was reported at 76%5; awareness among other medical specialists remains unknown. Awareness of PrEP among dermatologists is important because dermatologists play an important role in the diagnosis and treatment of many sexually transmitted infections (STIs), which are a risk factor for transmission of HIV. As providers who treat STIs, dermatologists are in a prime position to educate patients about PrEP, refer them for treatment, and prescribe the regimen. We conducted a survey to assess dermatologists’ knowledge about and attitudes toward PrEP. We also provide a brief summary of prescribing information about common PrEP regimens to fill in the knowledge gap among dermatologists as a way to promote its utilization.
An electronic survey was distributed to 486 members of the Association of Professors of Dermatology based in the United States using the web-based survey application REDCap. The study was approved by the New York University Grossman School of Medicine (New York, New York) institutional review board. Eighty-one anonymous survey responses were completed and returned (response rate, 16.6%). Data were analyzed using descriptive statistics.
The mean age (SD) of respondents was 39.1 (9.7) years; 49.4% (40/81) were male; and 74.1% (60/81) were attending physicians, with a mean (SD) of 9.4 (8.6) years of practice. Clinical practices were predominantly from the northeast (46.9% [38/81]) and mostly in an academic setting (74.1% [60/81]). As shown in Table 1, most surveyed dermatologists reported being aware of PrEP (93.8% [76/81]), but a minority (42.0% [34/81]) were familiar with indications for its use; even fewer (4.9% [4/81]) were current prescribers. Referral to other physicians for PrEP was reported by 58.0% (47/81) of respondents.
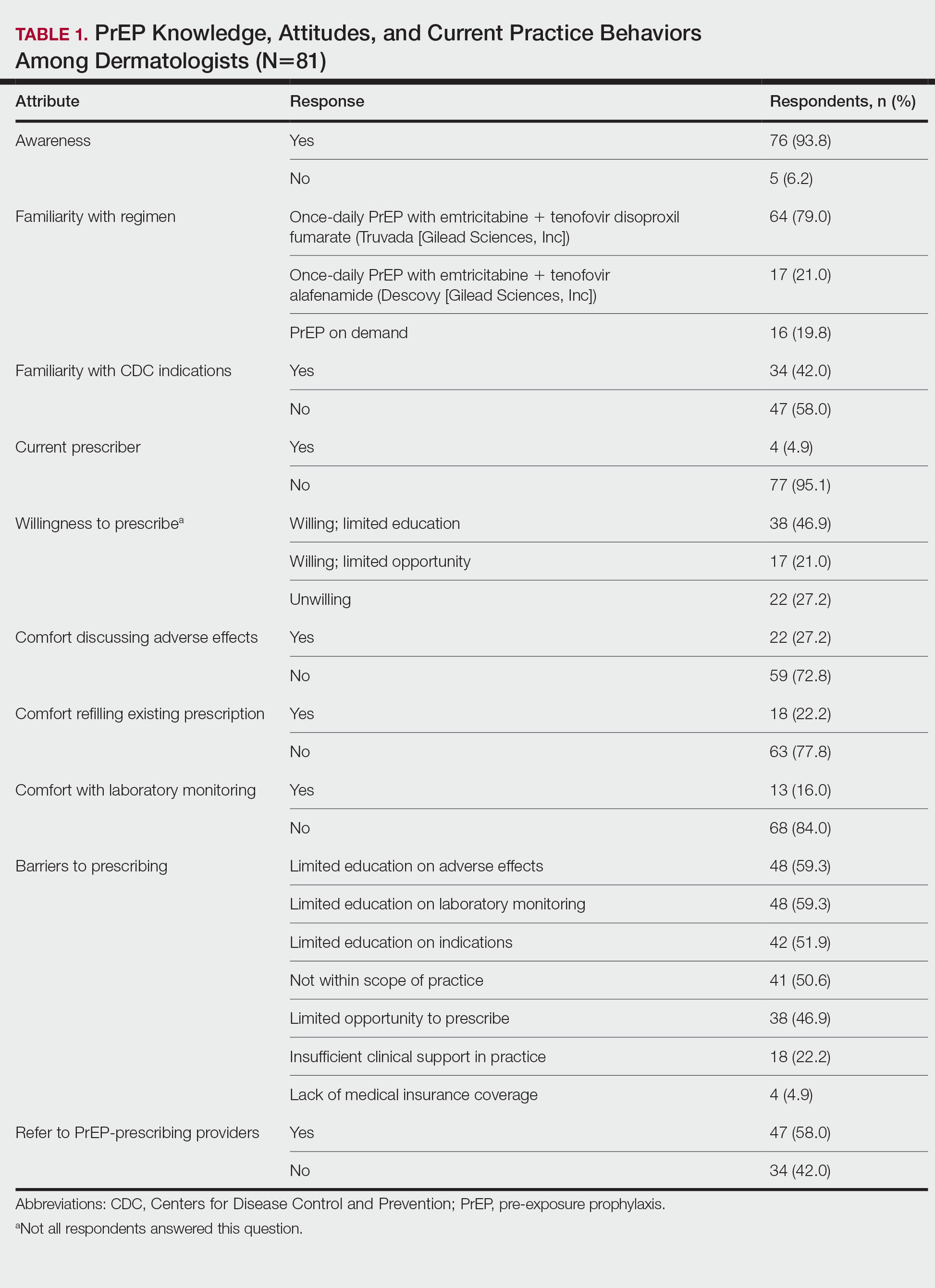
Despite respondents’ awareness of PrEP as a preventive measure (93.8% [76/81]) and their willingness to prescribe it (67.9% [55/81]), many reported being largely unfamiliar with its indications (58.0% [47/81]) and uncomfortable discussing its adverse effects (72.8% [59/81]), conducting appropriate laboratory monitoring (84.0% [68/81]), and refilling existing prescriptions (77.8% [63/81]). Respondents’ lack of education about PrEP was a barrier to prescribing (51.9% [42/81] to 59.3% [48/81]) and explains why a small minority (4.9% [4/81]) currently prescribe the regimen.
Our study sought to characterize current clinical knowledge about and practice patterns of PrEP among dermatologists. Dermatologists often encounter patients who present with an STI, which is a risk factor for HIV infection, but our survey respondents reported several barriers to utilizing PrEP. The difference in the degree of respondents’ willingness to prescribe PrEP (67.9%) and those who self-identified as prescribers (4.9%) suggests a role for dermatologists in prescribing or discussing PrEP with their patients—albeit a currently undefined role.
The results of our study suggested that half (41/81) of dermatologists believe that PrEP prescription is out of their scope of practice, likely due to a combination of scheduling, laboratory monitoring, and medicolegal concerns. For dermatologists who are interested in being PrEP prescribers, our results suggested that closing the knowledge gap around PrEP among dermatologists through training and education could improve comfort with this medication and lead to changes in practice to prevent the spread of HIV infection.
PrEP is indicated for HIV-negative patients who have HIV-positive sexual partners, utilize barrier protection methods inconsistently, or had a diagnosis of an STI in the last 6 months.6 In 2012, the FDA approved once-daily use of emtricitabine plus tenofovir for primary prevention of HIV infection. Post hoc analysis of iPrEx trial data revealed that once-daily PrEP taken regularly had a 92% to 100% protective effect against HIV.7
Regrettably, real-world uptake of PrEP has been slower than desired. The most recent data (2021) show that nearly 1 million individuals worldwide take PrEP; however, this represents only approximately one-third of those eligible.8 Utilization is notably lower among Black and Latino populations who stand to gain the most from PrEP given their higher risk of contracting HIV compared to their White counterparts.9 As such, improving access to PrEP through expanded provider awareness is essential to decrease the risk for HIV infection and transmission.
Emtricitabine plus tenofovir is safe and well tolerated; more common adverse effects are headache, nausea, vomiting, rash, and loss of appetite. Tenofovir likely decreases bone mineral density, even in HIV-negative patients10; mineralization seems to recover after the medication is discontinued.11 Rarely, tenofovir can increase the level of creatinine and hepatic transaminases; a recent report on its long-term side effects has shown small nonprogressive decreases in glomerular filtration rate.12 Monitoring kidney function is a component of prescribing PrEP (Table 2).
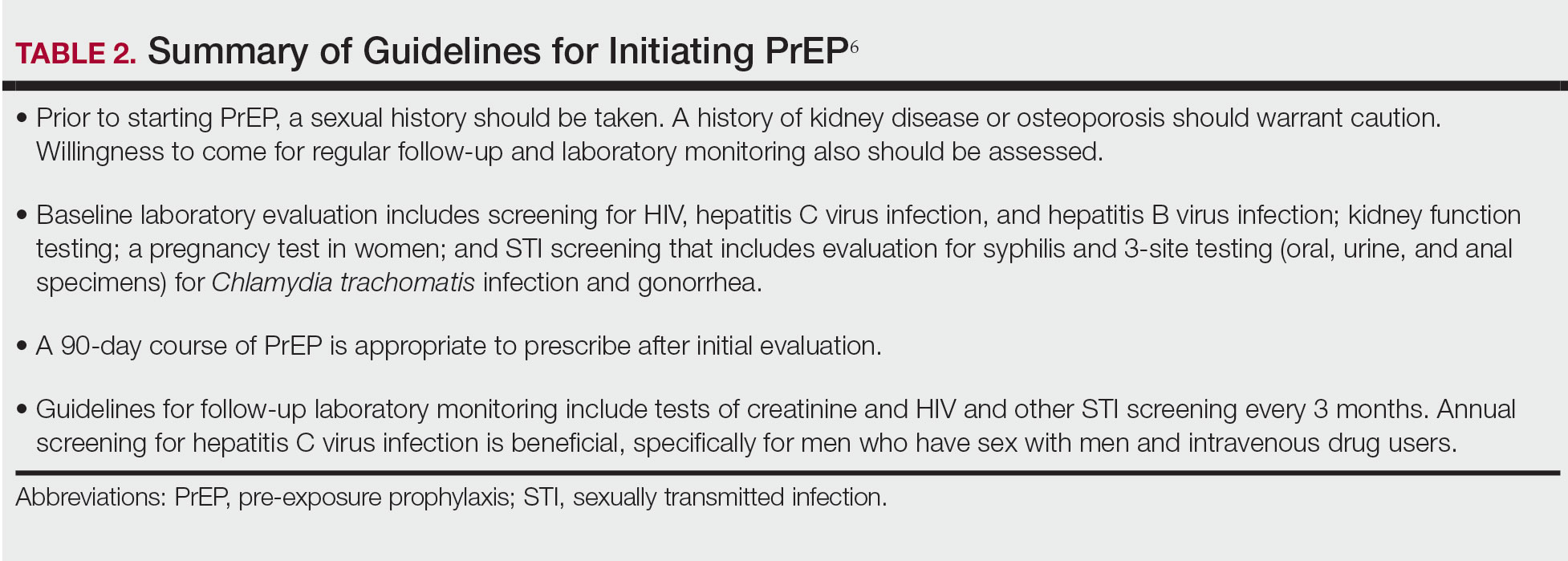
In 2019, emtricitabine plus tenofovir was reformulated with tenofovir alafenamide; the new combination regimen received FDA approval for once-daily PrEP under the brand name Descovy (Gilead Sciences, Inc). The new formulation results in a lower blood concentration of tenofovir and has been reported to present less of a risk for bone and kidney toxicity.13,14
Notably, emtricitabine plus tenofovir alafenamide might accumulate faster in peripheral lymphatic tissue than emtricitabine plus tenofovir disoproxil fumarate. This property has led to a new regimen known as “on-demand PrEP,” which follows a 2-1-1 dosing regimen: Patients take a double dose 2 to 24 hours before sexual activity, 1 dose on the day of sexual activity, and 1 dose the day after sexual activity.15 Because some patients at risk for HIV infection might not be consistently sexually active, on-demand PrEP allows them to cycle on and off the medication. Barriers to implementing on-demand PrEP include requiring that sexual activity be planned and an adverse effect profile similar to daily-use PrEP.16
The FDA recently approved a long-acting, once-monthly combination injectable PrEP of cabotegravir and rilpivirine.17 The long duration of action of this PrEP will benefit patients who report problems with medication adherence.
Our study demonstrates low frequency in prescribing patterns of PrEP among dermatologists and suggests that an addressable barrier to such prescribing is the lack of knowledge on how to prescribe it safely, which warrants further clinical investigation. We summarize an approach to prescribing PrEP in Table 2. Our study was limited by a small sample of mostly academic dermatologists and selection bias, which may diminish the generalizability of findings. A study of a larger, more representative group of dermatologists likely would show different prescribing patterns and degrees of knowledge about PrEP. Research is needed to study the impact of educational interventions that aim to increase both knowledge and prescribing of PrEP among dermatologists.
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599. doi:10.1056/NEJMoa1011205
- Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845. doi:10.1001/jama.2019.1343
- Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28:850-857.e9. doi:10.1016/j.annepidem.2018.05.003
- Song HJ, Squires P, Wilson D, et al. Trends in HIV preexposure prophylaxis prescribing in the United States, 2012-2018. JAMA. 2020;324:395-397. doi:10.1001/jama.2020.7312
- Petroll AE, Walsh JL, Owczarzak JL, et al. PrEP awareness, familiarity, comfort, and prescribing experience among US primary care providers and HIV specialists. AIDS Behav. 2017;21:1256-1267. doi:10.1007/s10461-016-1625-1
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. a clinical practice guideline. Centers for Disease Control and Prevention. Accessed September 15, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- Riddell J 4th, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA. 2018;319:1261-1268. doi:10.1001/JAMA.2018.1917
- Segal K, Fitch L, Riaz F, et al. The evolution of oral PrEP access: tracking trends in global oral PrEP use over time. J Int AIDS Soc. 2021;24:27-28.
- Elion RA, Kabiri M, Mayer KH, et al. Estimated impact of targeted pre-exposure prophylaxis: strategies for men who have sex with men in the United States. Int J Environ Res Public Health. 2019;16:1592. doi:10.3390/ijerph16091592
- Kasonde M, Niska RW, Rose C, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One. 2014;9:e90111. doi:10.1371/journal.pone.0090111
- Glidden DV, Mulligan K, McMahan V, et al. Brief report: recovery of bone mineral density after discontinuation of tenofovir-based HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2017;76:177-182. doi:10.1097/QAI.0000000000001475
- Tang EC, Vittinghoff E, Anderson PL, et al. Changes in kidney function associated with daily tenofovir disoproxil fumarate/emtricitabine for HIV preexposure prophylaxis use in the United States Demonstration Project. J Acquir Immune Defic Syndr. 2018;77:193-198. doi:10.1097/QAI.0000000000001566
- Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33:1455-1465. doi:10.1097/QAD.0000000000002223
- Agarwal K, Brunetto M, Seto WK, et al; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection [published online January 17, 2018]. J Hepatol. 2018;68:672-681. doi:10.1016/j.jhep.2017.11.039
- Molina JM, Capitant C, Spire B, et al; ANRS IPERGAY Study Group. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection [published online December 1, 2015]. N Engl J Med. 2015;3;2237-2246. doi:10.1056/NEJMoa1506273
- Saberi P, Scott HM. On-demand oral pre-exposure prophylaxis with tenofovir/emtricitabine: what every clinician needs to know. J Gen Intern Med. 2020;35:1285-1288. doi:10.1007/s11606-020-05651-2
- Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15:e1002690. doi:10.1371/journal.pmed.1002690
To the Editor:
In a 2010 landmark paper, researchers reported that the Preexposure Prophylaxis Initiative (iPrEx) trial demonstrated that once-daily pre-exposure prophylaxis (PrEP) with emtricitabine plus tenofovir disoproxil fumarate, which was approved by the US Food and Drug Administration (FDA) and packaged together as Truvada (Gilead Sciences, Inc), achieved a 44% reduction in the incidence of HIV infection compared to the placebo arm of the study (64/1248 HIV infections in the placebo group vs 36/1251 in the intervention group).1 Subsequently, the US Department of Health and Human Services proposed an initiative to reduce new HIV infections by 90% by 2030.2 The Centers for Disease Control and Prevention estimates that 1.1 million Americans have an indication for PrEP, yet only approximately 400,000 individuals currently take PrEP.3,4
Increasing awareness of PrEP and its indications is essential because PrEP exerts its greatest benefit when used broadly. Awareness among primary care and infectious disease physicians was reported at 76%5; awareness among other medical specialists remains unknown. Awareness of PrEP among dermatologists is important because dermatologists play an important role in the diagnosis and treatment of many sexually transmitted infections (STIs), which are a risk factor for transmission of HIV. As providers who treat STIs, dermatologists are in a prime position to educate patients about PrEP, refer them for treatment, and prescribe the regimen. We conducted a survey to assess dermatologists’ knowledge about and attitudes toward PrEP. We also provide a brief summary of prescribing information about common PrEP regimens to fill in the knowledge gap among dermatologists as a way to promote its utilization.
An electronic survey was distributed to 486 members of the Association of Professors of Dermatology based in the United States using the web-based survey application REDCap. The study was approved by the New York University Grossman School of Medicine (New York, New York) institutional review board. Eighty-one anonymous survey responses were completed and returned (response rate, 16.6%). Data were analyzed using descriptive statistics.
The mean age (SD) of respondents was 39.1 (9.7) years; 49.4% (40/81) were male; and 74.1% (60/81) were attending physicians, with a mean (SD) of 9.4 (8.6) years of practice. Clinical practices were predominantly from the northeast (46.9% [38/81]) and mostly in an academic setting (74.1% [60/81]). As shown in Table 1, most surveyed dermatologists reported being aware of PrEP (93.8% [76/81]), but a minority (42.0% [34/81]) were familiar with indications for its use; even fewer (4.9% [4/81]) were current prescribers. Referral to other physicians for PrEP was reported by 58.0% (47/81) of respondents.

Despite respondents’ awareness of PrEP as a preventive measure (93.8% [76/81]) and their willingness to prescribe it (67.9% [55/81]), many reported being largely unfamiliar with its indications (58.0% [47/81]) and uncomfortable discussing its adverse effects (72.8% [59/81]), conducting appropriate laboratory monitoring (84.0% [68/81]), and refilling existing prescriptions (77.8% [63/81]). Respondents’ lack of education about PrEP was a barrier to prescribing (51.9% [42/81] to 59.3% [48/81]) and explains why a small minority (4.9% [4/81]) currently prescribe the regimen.
Our study sought to characterize current clinical knowledge about and practice patterns of PrEP among dermatologists. Dermatologists often encounter patients who present with an STI, which is a risk factor for HIV infection, but our survey respondents reported several barriers to utilizing PrEP. The difference in the degree of respondents’ willingness to prescribe PrEP (67.9%) and those who self-identified as prescribers (4.9%) suggests a role for dermatologists in prescribing or discussing PrEP with their patients—albeit a currently undefined role.
The results of our study suggested that half (41/81) of dermatologists believe that PrEP prescription is out of their scope of practice, likely due to a combination of scheduling, laboratory monitoring, and medicolegal concerns. For dermatologists who are interested in being PrEP prescribers, our results suggested that closing the knowledge gap around PrEP among dermatologists through training and education could improve comfort with this medication and lead to changes in practice to prevent the spread of HIV infection.
PrEP is indicated for HIV-negative patients who have HIV-positive sexual partners, utilize barrier protection methods inconsistently, or had a diagnosis of an STI in the last 6 months.6 In 2012, the FDA approved once-daily use of emtricitabine plus tenofovir for primary prevention of HIV infection. Post hoc analysis of iPrEx trial data revealed that once-daily PrEP taken regularly had a 92% to 100% protective effect against HIV.7
Regrettably, real-world uptake of PrEP has been slower than desired. The most recent data (2021) show that nearly 1 million individuals worldwide take PrEP; however, this represents only approximately one-third of those eligible.8 Utilization is notably lower among Black and Latino populations who stand to gain the most from PrEP given their higher risk of contracting HIV compared to their White counterparts.9 As such, improving access to PrEP through expanded provider awareness is essential to decrease the risk for HIV infection and transmission.
Emtricitabine plus tenofovir is safe and well tolerated; more common adverse effects are headache, nausea, vomiting, rash, and loss of appetite. Tenofovir likely decreases bone mineral density, even in HIV-negative patients10; mineralization seems to recover after the medication is discontinued.11 Rarely, tenofovir can increase the level of creatinine and hepatic transaminases; a recent report on its long-term side effects has shown small nonprogressive decreases in glomerular filtration rate.12 Monitoring kidney function is a component of prescribing PrEP (Table 2).

In 2019, emtricitabine plus tenofovir was reformulated with tenofovir alafenamide; the new combination regimen received FDA approval for once-daily PrEP under the brand name Descovy (Gilead Sciences, Inc). The new formulation results in a lower blood concentration of tenofovir and has been reported to present less of a risk for bone and kidney toxicity.13,14
Notably, emtricitabine plus tenofovir alafenamide might accumulate faster in peripheral lymphatic tissue than emtricitabine plus tenofovir disoproxil fumarate. This property has led to a new regimen known as “on-demand PrEP,” which follows a 2-1-1 dosing regimen: Patients take a double dose 2 to 24 hours before sexual activity, 1 dose on the day of sexual activity, and 1 dose the day after sexual activity.15 Because some patients at risk for HIV infection might not be consistently sexually active, on-demand PrEP allows them to cycle on and off the medication. Barriers to implementing on-demand PrEP include requiring that sexual activity be planned and an adverse effect profile similar to daily-use PrEP.16
The FDA recently approved a long-acting, once-monthly combination injectable PrEP of cabotegravir and rilpivirine.17 The long duration of action of this PrEP will benefit patients who report problems with medication adherence.
Our study demonstrates low frequency in prescribing patterns of PrEP among dermatologists and suggests that an addressable barrier to such prescribing is the lack of knowledge on how to prescribe it safely, which warrants further clinical investigation. We summarize an approach to prescribing PrEP in Table 2. Our study was limited by a small sample of mostly academic dermatologists and selection bias, which may diminish the generalizability of findings. A study of a larger, more representative group of dermatologists likely would show different prescribing patterns and degrees of knowledge about PrEP. Research is needed to study the impact of educational interventions that aim to increase both knowledge and prescribing of PrEP among dermatologists.
To the Editor:
In a 2010 landmark paper, researchers reported that the Preexposure Prophylaxis Initiative (iPrEx) trial demonstrated that once-daily pre-exposure prophylaxis (PrEP) with emtricitabine plus tenofovir disoproxil fumarate, which was approved by the US Food and Drug Administration (FDA) and packaged together as Truvada (Gilead Sciences, Inc), achieved a 44% reduction in the incidence of HIV infection compared to the placebo arm of the study (64/1248 HIV infections in the placebo group vs 36/1251 in the intervention group).1 Subsequently, the US Department of Health and Human Services proposed an initiative to reduce new HIV infections by 90% by 2030.2 The Centers for Disease Control and Prevention estimates that 1.1 million Americans have an indication for PrEP, yet only approximately 400,000 individuals currently take PrEP.3,4
Increasing awareness of PrEP and its indications is essential because PrEP exerts its greatest benefit when used broadly. Awareness among primary care and infectious disease physicians was reported at 76%5; awareness among other medical specialists remains unknown. Awareness of PrEP among dermatologists is important because dermatologists play an important role in the diagnosis and treatment of many sexually transmitted infections (STIs), which are a risk factor for transmission of HIV. As providers who treat STIs, dermatologists are in a prime position to educate patients about PrEP, refer them for treatment, and prescribe the regimen. We conducted a survey to assess dermatologists’ knowledge about and attitudes toward PrEP. We also provide a brief summary of prescribing information about common PrEP regimens to fill in the knowledge gap among dermatologists as a way to promote its utilization.
An electronic survey was distributed to 486 members of the Association of Professors of Dermatology based in the United States using the web-based survey application REDCap. The study was approved by the New York University Grossman School of Medicine (New York, New York) institutional review board. Eighty-one anonymous survey responses were completed and returned (response rate, 16.6%). Data were analyzed using descriptive statistics.
The mean age (SD) of respondents was 39.1 (9.7) years; 49.4% (40/81) were male; and 74.1% (60/81) were attending physicians, with a mean (SD) of 9.4 (8.6) years of practice. Clinical practices were predominantly from the northeast (46.9% [38/81]) and mostly in an academic setting (74.1% [60/81]). As shown in Table 1, most surveyed dermatologists reported being aware of PrEP (93.8% [76/81]), but a minority (42.0% [34/81]) were familiar with indications for its use; even fewer (4.9% [4/81]) were current prescribers. Referral to other physicians for PrEP was reported by 58.0% (47/81) of respondents.

Despite respondents’ awareness of PrEP as a preventive measure (93.8% [76/81]) and their willingness to prescribe it (67.9% [55/81]), many reported being largely unfamiliar with its indications (58.0% [47/81]) and uncomfortable discussing its adverse effects (72.8% [59/81]), conducting appropriate laboratory monitoring (84.0% [68/81]), and refilling existing prescriptions (77.8% [63/81]). Respondents’ lack of education about PrEP was a barrier to prescribing (51.9% [42/81] to 59.3% [48/81]) and explains why a small minority (4.9% [4/81]) currently prescribe the regimen.
Our study sought to characterize current clinical knowledge about and practice patterns of PrEP among dermatologists. Dermatologists often encounter patients who present with an STI, which is a risk factor for HIV infection, but our survey respondents reported several barriers to utilizing PrEP. The difference in the degree of respondents’ willingness to prescribe PrEP (67.9%) and those who self-identified as prescribers (4.9%) suggests a role for dermatologists in prescribing or discussing PrEP with their patients—albeit a currently undefined role.
The results of our study suggested that half (41/81) of dermatologists believe that PrEP prescription is out of their scope of practice, likely due to a combination of scheduling, laboratory monitoring, and medicolegal concerns. For dermatologists who are interested in being PrEP prescribers, our results suggested that closing the knowledge gap around PrEP among dermatologists through training and education could improve comfort with this medication and lead to changes in practice to prevent the spread of HIV infection.
PrEP is indicated for HIV-negative patients who have HIV-positive sexual partners, utilize barrier protection methods inconsistently, or had a diagnosis of an STI in the last 6 months.6 In 2012, the FDA approved once-daily use of emtricitabine plus tenofovir for primary prevention of HIV infection. Post hoc analysis of iPrEx trial data revealed that once-daily PrEP taken regularly had a 92% to 100% protective effect against HIV.7
Regrettably, real-world uptake of PrEP has been slower than desired. The most recent data (2021) show that nearly 1 million individuals worldwide take PrEP; however, this represents only approximately one-third of those eligible.8 Utilization is notably lower among Black and Latino populations who stand to gain the most from PrEP given their higher risk of contracting HIV compared to their White counterparts.9 As such, improving access to PrEP through expanded provider awareness is essential to decrease the risk for HIV infection and transmission.
Emtricitabine plus tenofovir is safe and well tolerated; more common adverse effects are headache, nausea, vomiting, rash, and loss of appetite. Tenofovir likely decreases bone mineral density, even in HIV-negative patients10; mineralization seems to recover after the medication is discontinued.11 Rarely, tenofovir can increase the level of creatinine and hepatic transaminases; a recent report on its long-term side effects has shown small nonprogressive decreases in glomerular filtration rate.12 Monitoring kidney function is a component of prescribing PrEP (Table 2).

In 2019, emtricitabine plus tenofovir was reformulated with tenofovir alafenamide; the new combination regimen received FDA approval for once-daily PrEP under the brand name Descovy (Gilead Sciences, Inc). The new formulation results in a lower blood concentration of tenofovir and has been reported to present less of a risk for bone and kidney toxicity.13,14
Notably, emtricitabine plus tenofovir alafenamide might accumulate faster in peripheral lymphatic tissue than emtricitabine plus tenofovir disoproxil fumarate. This property has led to a new regimen known as “on-demand PrEP,” which follows a 2-1-1 dosing regimen: Patients take a double dose 2 to 24 hours before sexual activity, 1 dose on the day of sexual activity, and 1 dose the day after sexual activity.15 Because some patients at risk for HIV infection might not be consistently sexually active, on-demand PrEP allows them to cycle on and off the medication. Barriers to implementing on-demand PrEP include requiring that sexual activity be planned and an adverse effect profile similar to daily-use PrEP.16
The FDA recently approved a long-acting, once-monthly combination injectable PrEP of cabotegravir and rilpivirine.17 The long duration of action of this PrEP will benefit patients who report problems with medication adherence.
Our study demonstrates low frequency in prescribing patterns of PrEP among dermatologists and suggests that an addressable barrier to such prescribing is the lack of knowledge on how to prescribe it safely, which warrants further clinical investigation. We summarize an approach to prescribing PrEP in Table 2. Our study was limited by a small sample of mostly academic dermatologists and selection bias, which may diminish the generalizability of findings. A study of a larger, more representative group of dermatologists likely would show different prescribing patterns and degrees of knowledge about PrEP. Research is needed to study the impact of educational interventions that aim to increase both knowledge and prescribing of PrEP among dermatologists.
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599. doi:10.1056/NEJMoa1011205
- Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845. doi:10.1001/jama.2019.1343
- Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28:850-857.e9. doi:10.1016/j.annepidem.2018.05.003
- Song HJ, Squires P, Wilson D, et al. Trends in HIV preexposure prophylaxis prescribing in the United States, 2012-2018. JAMA. 2020;324:395-397. doi:10.1001/jama.2020.7312
- Petroll AE, Walsh JL, Owczarzak JL, et al. PrEP awareness, familiarity, comfort, and prescribing experience among US primary care providers and HIV specialists. AIDS Behav. 2017;21:1256-1267. doi:10.1007/s10461-016-1625-1
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. a clinical practice guideline. Centers for Disease Control and Prevention. Accessed September 15, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- Riddell J 4th, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA. 2018;319:1261-1268. doi:10.1001/JAMA.2018.1917
- Segal K, Fitch L, Riaz F, et al. The evolution of oral PrEP access: tracking trends in global oral PrEP use over time. J Int AIDS Soc. 2021;24:27-28.
- Elion RA, Kabiri M, Mayer KH, et al. Estimated impact of targeted pre-exposure prophylaxis: strategies for men who have sex with men in the United States. Int J Environ Res Public Health. 2019;16:1592. doi:10.3390/ijerph16091592
- Kasonde M, Niska RW, Rose C, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One. 2014;9:e90111. doi:10.1371/journal.pone.0090111
- Glidden DV, Mulligan K, McMahan V, et al. Brief report: recovery of bone mineral density after discontinuation of tenofovir-based HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2017;76:177-182. doi:10.1097/QAI.0000000000001475
- Tang EC, Vittinghoff E, Anderson PL, et al. Changes in kidney function associated with daily tenofovir disoproxil fumarate/emtricitabine for HIV preexposure prophylaxis use in the United States Demonstration Project. J Acquir Immune Defic Syndr. 2018;77:193-198. doi:10.1097/QAI.0000000000001566
- Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33:1455-1465. doi:10.1097/QAD.0000000000002223
- Agarwal K, Brunetto M, Seto WK, et al; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection [published online January 17, 2018]. J Hepatol. 2018;68:672-681. doi:10.1016/j.jhep.2017.11.039
- Molina JM, Capitant C, Spire B, et al; ANRS IPERGAY Study Group. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection [published online December 1, 2015]. N Engl J Med. 2015;3;2237-2246. doi:10.1056/NEJMoa1506273
- Saberi P, Scott HM. On-demand oral pre-exposure prophylaxis with tenofovir/emtricitabine: what every clinician needs to know. J Gen Intern Med. 2020;35:1285-1288. doi:10.1007/s11606-020-05651-2
- Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15:e1002690. doi:10.1371/journal.pmed.1002690
- Grant RM, Lama JR, Anderson PL, et al; iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587-2599. doi:10.1056/NEJMoa1011205
- Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: a plan for the United States. JAMA. 2019;321:844-845. doi:10.1001/jama.2019.1343
- Smith DK, Van Handel M, Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018;28:850-857.e9. doi:10.1016/j.annepidem.2018.05.003
- Song HJ, Squires P, Wilson D, et al. Trends in HIV preexposure prophylaxis prescribing in the United States, 2012-2018. JAMA. 2020;324:395-397. doi:10.1001/jama.2020.7312
- Petroll AE, Walsh JL, Owczarzak JL, et al. PrEP awareness, familiarity, comfort, and prescribing experience among US primary care providers and HIV specialists. AIDS Behav. 2017;21:1256-1267. doi:10.1007/s10461-016-1625-1
- US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. a clinical practice guideline. Centers for Disease Control and Prevention. Accessed September 15, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- Riddell J 4th, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA. 2018;319:1261-1268. doi:10.1001/JAMA.2018.1917
- Segal K, Fitch L, Riaz F, et al. The evolution of oral PrEP access: tracking trends in global oral PrEP use over time. J Int AIDS Soc. 2021;24:27-28.
- Elion RA, Kabiri M, Mayer KH, et al. Estimated impact of targeted pre-exposure prophylaxis: strategies for men who have sex with men in the United States. Int J Environ Res Public Health. 2019;16:1592. doi:10.3390/ijerph16091592
- Kasonde M, Niska RW, Rose C, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS One. 2014;9:e90111. doi:10.1371/journal.pone.0090111
- Glidden DV, Mulligan K, McMahan V, et al. Brief report: recovery of bone mineral density after discontinuation of tenofovir-based HIV pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2017;76:177-182. doi:10.1097/QAI.0000000000001475
- Tang EC, Vittinghoff E, Anderson PL, et al. Changes in kidney function associated with daily tenofovir disoproxil fumarate/emtricitabine for HIV preexposure prophylaxis use in the United States Demonstration Project. J Acquir Immune Defic Syndr. 2018;77:193-198. doi:10.1097/QAI.0000000000001566
- Gupta SK, Post FA, Arribas JR, et al. Renal safety of tenofovir alafenamide vs. tenofovir disoproxil fumarate: a pooled analysis of 26 clinical trials. AIDS. 2019;33:1455-1465. doi:10.1097/QAD.0000000000002223
- Agarwal K, Brunetto M, Seto WK, et al; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection [published online January 17, 2018]. J Hepatol. 2018;68:672-681. doi:10.1016/j.jhep.2017.11.039
- Molina JM, Capitant C, Spire B, et al; ANRS IPERGAY Study Group. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection [published online December 1, 2015]. N Engl J Med. 2015;3;2237-2246. doi:10.1056/NEJMoa1506273
- Saberi P, Scott HM. On-demand oral pre-exposure prophylaxis with tenofovir/emtricitabine: what every clinician needs to know. J Gen Intern Med. 2020;35:1285-1288. doi:10.1007/s11606-020-05651-2
- Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med. 2018;15:e1002690. doi:10.1371/journal.pmed.1002690
Practice Points
- Sexually transmitted infections (STIs) often have skin manifestations, with patients presenting to dermatologists.
- Pre-exposure prophylaxis (PrEP) uses antiretrovirals taken prophylactically to prevent transmission of and infection with HIV. Dermatologists are aware of PrEP, but several barriers prevent them from being prescribers.
- Patients with a history of an STI should be considered for PrEP.
Out-of-state telehealth visits could help more patients if restrictions eased: Study
About 5% of traditional Medicare patients who had telehealth visits were seen virtually by out-of-state clinicians in the first half of 2021, according to a new study in JAMA Health Forum.
Since then, however, many states have restored restrictions that prevent physicians who are licensed in one state from having telehealth visits with patients unless they’re licensed in the state where the patients live.
This is not fair to many people who live in areas near state borders, the authors argued. For those patients, it is much more convenient to see their primary care physician in a virtual visit from home than to travel to the doctor’s office in another state. This convenience is enjoyed by most patients who reside elsewhere in their state because they’re seeing physicians who are licensed there.
Moreover, the paper said, patients who live in rural areas and in counties with relatively few physicians per capita would also benefit from relaxed telemedicine restrictions.
Using Medicare claims data, the researchers examined the characteristics of out-of-state (OOS) telemedicine visits for the 6 months from January to June 2021. They chose that period for two reasons: by then, health care had stabilized after the chaotic early phase of the pandemic, and in most states, the relaxation of licensing rules for OOS telehealth had not yet lapsed. Earlier periods of time were also used for certain types of comparisons.
Among fee-for-service Medicare beneficiaries, the number of OOS telemedicine visits peaked at 451,086 in April 2020 and slowly fell to 175,545 in June 2021, according to the study. The fraction of OOS telehealth visits among all virtual visits was 4.5% in April 2020 and increased to 5.6% by June 2021.
Staying close to home
Of all beneficiaries with a telemedicine visit in the study period, 33% lived within 15 miles of a state border. That cohort accounted for 57.2% of all OOS telemedicine visits.
The highest rates of OOS telehealth visits were seen in the District of Columbia (38.5%), Wyoming (25.6%), and North Dakota (21.1%). California (1%), Texas (2%), and Massachusetts (2.1%) had the lowest rates.
Though intuitive in retrospect, the correlation of OOS telemedicine use with proximity to state borders was one of the study’s most important findings, lead author Ateev Mehrotra, MD, a professor at Harvard Medical School, Boston, said in an interview. “It makes sense,” he said. “If you’re in D.C. and you need a cardiologist, you don’t think: ‘I’ll stay in D.C.’ No, Maryland is right there, so you might use a Maryland cardiologist. Now you’re out of state, even though that office might be only half a mile away from you.”
Similar dynamics, he noted, are seen in many metropolitan areas that border on other states, such as Cincinnati; Philadelphia; and Portland, Ore.
This finding lines up with another result of the study: The majority of patients who had OOS telemedicine visits had previously seen in person the doctor who conducted the virtual visit.
Across all OOS telemedicine visits in the first half of 2021, the researchers observed a prior in-person visit between March 2019 and the date of the virtual visit with the same patient and the same clinician in 62.8% of those visits. Across all in-state telehealth visits, 75.8% of them were made by patients who had seen the same clinician in person since March 2019. This preponderance of virtual visits to clinicians whom the patients had already seen in person reflects the fact that, during the pandemic, most physicians began conducting telehealth visits with their own patients, Dr. Mehrotra said.
It also lays to rest the concern that some states have had about allowing OOS telemedicine visits to physicians not licensed in those states, he added. “They think that all these docs from far away are going to start taking care of patients they don’t even know. But our study shows that isn’t the case. Most of the time, doctors are seeing a patient who’s switching over from in-person visits to out-of-state telemedicine.”
More specialty care sought
The dominant conditions that patients presented with were the same in OOS telemedicine and within-state virtual visits. However, the use of OOS telemedicine was higher for some types of specialized care.
For example, the rate of OOS telemedicine use, compared with all telemedicine use, was highest for cancer care (9.8%). Drilling down to more specific conditions, the top three in OOS telemedicine visits were assessment of organ transplant (13%); male reproductive cancers, such as prostate cancer (11.3%); and graft-related issues (10.2%).
The specialty trend was also evident in the types of OOS clinicians from whom Medicare patients sought virtual care. The rates of OOS telemedicine use as a percentage of all telemedicine use in particular specialties were highest for uncommon specialties, such as hematology/oncology, rheumatology, urology, medical oncology, and orthopedic surgery (8.5%). There was less use of OOS telemedicine as a percentage of all telemedicine among more common medical specialties (6.4%), mental health specialties (4.4%), and primary care (4.4%).
Despite its relatively low showing in this category, however, behavioral health was the leading condition treated in both within-state and OOS telemedicine visits, accounting for 30.7% and 25.8%, respectively, of those encounters.
States backslide on OOS telehealth
Since the end of the study period, over half of the states have restored some or all of the restrictions on OOS telemedicine that they had lifted during the pandemic.
According to Dr. Mehrotra, 22 states have some kind of regulation in place to allow an OOS clinician to conduct telehealth visits without being licensed in the state. This varies all the way from complete reciprocity with other states’ licenses to “emergency” telemedicine licenses. The other 28 states and Washington, D.C., require an OOS telemedicine practitioner to get a state license.
Various proposals have been floated to ameliorate this situation, the JAMA paper noted. These proposals include an expansion of the Interstate Medical Licensure Compact that the Federation of State Medical Boards organized in 2014. Since the pact became effective in 2014, at least 35 states and the District of Columbia have joined it. Those states have made it simpler for physicians to gain licensure in states other than their original state of licensure. However, Mehrotra said, it’s still not easy, and not many physicians have taken advantage of it.
One new wrinkle has emerged in this policy debate as a result of the Supreme Court decision overturning Roe v. Wade, he noted. Because people are using OOS telemedicine visits to get prescriptions to abort their fetuses, “that has changed the enthusiasm level for it among many states,” he said.
Dr. Mehrotra reported personal fees from the Pew Charitable Trust, Sanofi Pasteur, and Black Opal Ventures outside the submitted work. One coauthor reported receiving grants from Patient-Centered Outcomes Research, National Institute on Aging, Roundtrip, Independence Blue Cross; personal fees or salary from RAND Corporation from Verily Life Sciences; and that the American Telemedicine Association covered a conference fee. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
About 5% of traditional Medicare patients who had telehealth visits were seen virtually by out-of-state clinicians in the first half of 2021, according to a new study in JAMA Health Forum.
Since then, however, many states have restored restrictions that prevent physicians who are licensed in one state from having telehealth visits with patients unless they’re licensed in the state where the patients live.
This is not fair to many people who live in areas near state borders, the authors argued. For those patients, it is much more convenient to see their primary care physician in a virtual visit from home than to travel to the doctor’s office in another state. This convenience is enjoyed by most patients who reside elsewhere in their state because they’re seeing physicians who are licensed there.
Moreover, the paper said, patients who live in rural areas and in counties with relatively few physicians per capita would also benefit from relaxed telemedicine restrictions.
Using Medicare claims data, the researchers examined the characteristics of out-of-state (OOS) telemedicine visits for the 6 months from January to June 2021. They chose that period for two reasons: by then, health care had stabilized after the chaotic early phase of the pandemic, and in most states, the relaxation of licensing rules for OOS telehealth had not yet lapsed. Earlier periods of time were also used for certain types of comparisons.
Among fee-for-service Medicare beneficiaries, the number of OOS telemedicine visits peaked at 451,086 in April 2020 and slowly fell to 175,545 in June 2021, according to the study. The fraction of OOS telehealth visits among all virtual visits was 4.5% in April 2020 and increased to 5.6% by June 2021.
Staying close to home
Of all beneficiaries with a telemedicine visit in the study period, 33% lived within 15 miles of a state border. That cohort accounted for 57.2% of all OOS telemedicine visits.
The highest rates of OOS telehealth visits were seen in the District of Columbia (38.5%), Wyoming (25.6%), and North Dakota (21.1%). California (1%), Texas (2%), and Massachusetts (2.1%) had the lowest rates.
Though intuitive in retrospect, the correlation of OOS telemedicine use with proximity to state borders was one of the study’s most important findings, lead author Ateev Mehrotra, MD, a professor at Harvard Medical School, Boston, said in an interview. “It makes sense,” he said. “If you’re in D.C. and you need a cardiologist, you don’t think: ‘I’ll stay in D.C.’ No, Maryland is right there, so you might use a Maryland cardiologist. Now you’re out of state, even though that office might be only half a mile away from you.”
Similar dynamics, he noted, are seen in many metropolitan areas that border on other states, such as Cincinnati; Philadelphia; and Portland, Ore.
This finding lines up with another result of the study: The majority of patients who had OOS telemedicine visits had previously seen in person the doctor who conducted the virtual visit.
Across all OOS telemedicine visits in the first half of 2021, the researchers observed a prior in-person visit between March 2019 and the date of the virtual visit with the same patient and the same clinician in 62.8% of those visits. Across all in-state telehealth visits, 75.8% of them were made by patients who had seen the same clinician in person since March 2019. This preponderance of virtual visits to clinicians whom the patients had already seen in person reflects the fact that, during the pandemic, most physicians began conducting telehealth visits with their own patients, Dr. Mehrotra said.
It also lays to rest the concern that some states have had about allowing OOS telemedicine visits to physicians not licensed in those states, he added. “They think that all these docs from far away are going to start taking care of patients they don’t even know. But our study shows that isn’t the case. Most of the time, doctors are seeing a patient who’s switching over from in-person visits to out-of-state telemedicine.”
More specialty care sought
The dominant conditions that patients presented with were the same in OOS telemedicine and within-state virtual visits. However, the use of OOS telemedicine was higher for some types of specialized care.
For example, the rate of OOS telemedicine use, compared with all telemedicine use, was highest for cancer care (9.8%). Drilling down to more specific conditions, the top three in OOS telemedicine visits were assessment of organ transplant (13%); male reproductive cancers, such as prostate cancer (11.3%); and graft-related issues (10.2%).
The specialty trend was also evident in the types of OOS clinicians from whom Medicare patients sought virtual care. The rates of OOS telemedicine use as a percentage of all telemedicine use in particular specialties were highest for uncommon specialties, such as hematology/oncology, rheumatology, urology, medical oncology, and orthopedic surgery (8.5%). There was less use of OOS telemedicine as a percentage of all telemedicine among more common medical specialties (6.4%), mental health specialties (4.4%), and primary care (4.4%).
Despite its relatively low showing in this category, however, behavioral health was the leading condition treated in both within-state and OOS telemedicine visits, accounting for 30.7% and 25.8%, respectively, of those encounters.
States backslide on OOS telehealth
Since the end of the study period, over half of the states have restored some or all of the restrictions on OOS telemedicine that they had lifted during the pandemic.
According to Dr. Mehrotra, 22 states have some kind of regulation in place to allow an OOS clinician to conduct telehealth visits without being licensed in the state. This varies all the way from complete reciprocity with other states’ licenses to “emergency” telemedicine licenses. The other 28 states and Washington, D.C., require an OOS telemedicine practitioner to get a state license.
Various proposals have been floated to ameliorate this situation, the JAMA paper noted. These proposals include an expansion of the Interstate Medical Licensure Compact that the Federation of State Medical Boards organized in 2014. Since the pact became effective in 2014, at least 35 states and the District of Columbia have joined it. Those states have made it simpler for physicians to gain licensure in states other than their original state of licensure. However, Mehrotra said, it’s still not easy, and not many physicians have taken advantage of it.
One new wrinkle has emerged in this policy debate as a result of the Supreme Court decision overturning Roe v. Wade, he noted. Because people are using OOS telemedicine visits to get prescriptions to abort their fetuses, “that has changed the enthusiasm level for it among many states,” he said.
Dr. Mehrotra reported personal fees from the Pew Charitable Trust, Sanofi Pasteur, and Black Opal Ventures outside the submitted work. One coauthor reported receiving grants from Patient-Centered Outcomes Research, National Institute on Aging, Roundtrip, Independence Blue Cross; personal fees or salary from RAND Corporation from Verily Life Sciences; and that the American Telemedicine Association covered a conference fee. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
About 5% of traditional Medicare patients who had telehealth visits were seen virtually by out-of-state clinicians in the first half of 2021, according to a new study in JAMA Health Forum.
Since then, however, many states have restored restrictions that prevent physicians who are licensed in one state from having telehealth visits with patients unless they’re licensed in the state where the patients live.
This is not fair to many people who live in areas near state borders, the authors argued. For those patients, it is much more convenient to see their primary care physician in a virtual visit from home than to travel to the doctor’s office in another state. This convenience is enjoyed by most patients who reside elsewhere in their state because they’re seeing physicians who are licensed there.
Moreover, the paper said, patients who live in rural areas and in counties with relatively few physicians per capita would also benefit from relaxed telemedicine restrictions.
Using Medicare claims data, the researchers examined the characteristics of out-of-state (OOS) telemedicine visits for the 6 months from January to June 2021. They chose that period for two reasons: by then, health care had stabilized after the chaotic early phase of the pandemic, and in most states, the relaxation of licensing rules for OOS telehealth had not yet lapsed. Earlier periods of time were also used for certain types of comparisons.
Among fee-for-service Medicare beneficiaries, the number of OOS telemedicine visits peaked at 451,086 in April 2020 and slowly fell to 175,545 in June 2021, according to the study. The fraction of OOS telehealth visits among all virtual visits was 4.5% in April 2020 and increased to 5.6% by June 2021.
Staying close to home
Of all beneficiaries with a telemedicine visit in the study period, 33% lived within 15 miles of a state border. That cohort accounted for 57.2% of all OOS telemedicine visits.
The highest rates of OOS telehealth visits were seen in the District of Columbia (38.5%), Wyoming (25.6%), and North Dakota (21.1%). California (1%), Texas (2%), and Massachusetts (2.1%) had the lowest rates.
Though intuitive in retrospect, the correlation of OOS telemedicine use with proximity to state borders was one of the study’s most important findings, lead author Ateev Mehrotra, MD, a professor at Harvard Medical School, Boston, said in an interview. “It makes sense,” he said. “If you’re in D.C. and you need a cardiologist, you don’t think: ‘I’ll stay in D.C.’ No, Maryland is right there, so you might use a Maryland cardiologist. Now you’re out of state, even though that office might be only half a mile away from you.”
Similar dynamics, he noted, are seen in many metropolitan areas that border on other states, such as Cincinnati; Philadelphia; and Portland, Ore.
This finding lines up with another result of the study: The majority of patients who had OOS telemedicine visits had previously seen in person the doctor who conducted the virtual visit.
Across all OOS telemedicine visits in the first half of 2021, the researchers observed a prior in-person visit between March 2019 and the date of the virtual visit with the same patient and the same clinician in 62.8% of those visits. Across all in-state telehealth visits, 75.8% of them were made by patients who had seen the same clinician in person since March 2019. This preponderance of virtual visits to clinicians whom the patients had already seen in person reflects the fact that, during the pandemic, most physicians began conducting telehealth visits with their own patients, Dr. Mehrotra said.
It also lays to rest the concern that some states have had about allowing OOS telemedicine visits to physicians not licensed in those states, he added. “They think that all these docs from far away are going to start taking care of patients they don’t even know. But our study shows that isn’t the case. Most of the time, doctors are seeing a patient who’s switching over from in-person visits to out-of-state telemedicine.”
More specialty care sought
The dominant conditions that patients presented with were the same in OOS telemedicine and within-state virtual visits. However, the use of OOS telemedicine was higher for some types of specialized care.
For example, the rate of OOS telemedicine use, compared with all telemedicine use, was highest for cancer care (9.8%). Drilling down to more specific conditions, the top three in OOS telemedicine visits were assessment of organ transplant (13%); male reproductive cancers, such as prostate cancer (11.3%); and graft-related issues (10.2%).
The specialty trend was also evident in the types of OOS clinicians from whom Medicare patients sought virtual care. The rates of OOS telemedicine use as a percentage of all telemedicine use in particular specialties were highest for uncommon specialties, such as hematology/oncology, rheumatology, urology, medical oncology, and orthopedic surgery (8.5%). There was less use of OOS telemedicine as a percentage of all telemedicine among more common medical specialties (6.4%), mental health specialties (4.4%), and primary care (4.4%).
Despite its relatively low showing in this category, however, behavioral health was the leading condition treated in both within-state and OOS telemedicine visits, accounting for 30.7% and 25.8%, respectively, of those encounters.
States backslide on OOS telehealth
Since the end of the study period, over half of the states have restored some or all of the restrictions on OOS telemedicine that they had lifted during the pandemic.
According to Dr. Mehrotra, 22 states have some kind of regulation in place to allow an OOS clinician to conduct telehealth visits without being licensed in the state. This varies all the way from complete reciprocity with other states’ licenses to “emergency” telemedicine licenses. The other 28 states and Washington, D.C., require an OOS telemedicine practitioner to get a state license.
Various proposals have been floated to ameliorate this situation, the JAMA paper noted. These proposals include an expansion of the Interstate Medical Licensure Compact that the Federation of State Medical Boards organized in 2014. Since the pact became effective in 2014, at least 35 states and the District of Columbia have joined it. Those states have made it simpler for physicians to gain licensure in states other than their original state of licensure. However, Mehrotra said, it’s still not easy, and not many physicians have taken advantage of it.
One new wrinkle has emerged in this policy debate as a result of the Supreme Court decision overturning Roe v. Wade, he noted. Because people are using OOS telemedicine visits to get prescriptions to abort their fetuses, “that has changed the enthusiasm level for it among many states,” he said.
Dr. Mehrotra reported personal fees from the Pew Charitable Trust, Sanofi Pasteur, and Black Opal Ventures outside the submitted work. One coauthor reported receiving grants from Patient-Centered Outcomes Research, National Institute on Aging, Roundtrip, Independence Blue Cross; personal fees or salary from RAND Corporation from Verily Life Sciences; and that the American Telemedicine Association covered a conference fee. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA HEALTH FORUM
Tender Nonhealing Lesion on the Leg
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
A 50-year-old woman presented to our dermatology clinic with an exquisitely tender, nonhealing lesion on the left leg of 2 weeks’ duration that began as a small red-purplish spot. She applied a triple antibiotic ointment and wrapped the area with gauze daily but reported that it continued to enlarge and darken in color before forming a “scab.” She noted occasional seropurulent discharge and denied any trauma or new exposures to the area. She was seen at a local emergency department 3 days prior to presentation and was prescribed oral clindamycin for suspected cellulitis, but she denied any improvement with the initiation of antibiotics. Her medical history was notable for obesity, depression, hypothyroidism, and liver disease secondary to alcohol use disorder. She reported that she drank a pint of vodka daily. Her medications included pantoprazole, spironolactone, bumetanide, citalopram, levothyroxine, naltrexone, tramadol, and a multivitamin. Physical examination revealed violaceous mottling with areas of superficial erythema and ulceration with necrotic eschars on the proximal left thigh that were extremely painful. A biopsy was obtained for confirmation of diagnosis, but the patient died before the results were returned.
Consider the mnemonic ‘CLEAR’ when counseling acne patients
to use when treating this group of patients.
During a presentation at Medscape Live’s annual Coastal Dermatology Symposium, Dr. Harper, who practices at Dermatology and Skin Care of Birmingham, Ala., elaborated on the mnemonic, as follows:
C: Communicate expectations. “I look right at the acne patient and say, ‘I know you don’t just want to be better; I know you want to be clear,’ ” she said at the meeting. “ ‘That’s my goal for you, too. That may take us more than one visit and more than one treatment, but I am on your team, and that’s what we’re shooting for.’ If you don’t communicate that, they’re going to think that their acne is not that important to you.”
L: Listen for clues to customize the patient’s treatment. “We’re quick to say, ‘my patients don’t do what I recommend,’ or ‘they didn’t do what the last doctor recommended,’ ” Dr. Harper said. “Sometimes that is true, but there may be a reason why. Maybe the medication was too expensive. Maybe it was bleaching their fabrics. Maybe the regimen was too complex. Listen for opportunities to make adjustments to get their acne closer to clear.”
E: Treat early to improve quality of life and to decrease the risk of scarring. “I have a laser in my practice that is good at treating acne scarring,” she said. “Do I ever look at my patient and say, ‘don’t worry about those scars; I can make them go away?’ No. I look at them and say, ‘we can maybe make this 40% better,’ something like that. We have to prevent acne scars, because we’re not good at treating them.”
A: Treat aggressively with more combination therapies, more hormonal therapies, more isotretinoin, and perhaps more prior authorizations. She characterized the effort to obtain a prior authorization as “our megaphone back to insurance companies that says, ‘we think it is worth taking the time to do this prior authorization because the acne patient will benefit.’ ”
R: Don’t resist isotretinoin. Dr. Harper, who began practicing dermatology more than 20 years ago, said that over time, she has gradually prescribed more isotretinoin for her patients with acne. “It’s not a first-line [treatment], but I’m not afraid of it. If I can’t get somebody clear on other oral or topical treatments, we are going to try isotretinoin.”
The goal of acne treatment, she added, is to affect four key aspects of pathogenesis: follicular epithelial hyperproliferation, inflammation, Cutibacterium acnes (C. acnes), and sebum. “That’s what we’re always shooting for,” she said.
Dr. Harper is a past president of the American Acne & Rosacea Society. She disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speaker’s bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
to use when treating this group of patients.
During a presentation at Medscape Live’s annual Coastal Dermatology Symposium, Dr. Harper, who practices at Dermatology and Skin Care of Birmingham, Ala., elaborated on the mnemonic, as follows:
C: Communicate expectations. “I look right at the acne patient and say, ‘I know you don’t just want to be better; I know you want to be clear,’ ” she said at the meeting. “ ‘That’s my goal for you, too. That may take us more than one visit and more than one treatment, but I am on your team, and that’s what we’re shooting for.’ If you don’t communicate that, they’re going to think that their acne is not that important to you.”
L: Listen for clues to customize the patient’s treatment. “We’re quick to say, ‘my patients don’t do what I recommend,’ or ‘they didn’t do what the last doctor recommended,’ ” Dr. Harper said. “Sometimes that is true, but there may be a reason why. Maybe the medication was too expensive. Maybe it was bleaching their fabrics. Maybe the regimen was too complex. Listen for opportunities to make adjustments to get their acne closer to clear.”
E: Treat early to improve quality of life and to decrease the risk of scarring. “I have a laser in my practice that is good at treating acne scarring,” she said. “Do I ever look at my patient and say, ‘don’t worry about those scars; I can make them go away?’ No. I look at them and say, ‘we can maybe make this 40% better,’ something like that. We have to prevent acne scars, because we’re not good at treating them.”
A: Treat aggressively with more combination therapies, more hormonal therapies, more isotretinoin, and perhaps more prior authorizations. She characterized the effort to obtain a prior authorization as “our megaphone back to insurance companies that says, ‘we think it is worth taking the time to do this prior authorization because the acne patient will benefit.’ ”
R: Don’t resist isotretinoin. Dr. Harper, who began practicing dermatology more than 20 years ago, said that over time, she has gradually prescribed more isotretinoin for her patients with acne. “It’s not a first-line [treatment], but I’m not afraid of it. If I can’t get somebody clear on other oral or topical treatments, we are going to try isotretinoin.”
The goal of acne treatment, she added, is to affect four key aspects of pathogenesis: follicular epithelial hyperproliferation, inflammation, Cutibacterium acnes (C. acnes), and sebum. “That’s what we’re always shooting for,” she said.
Dr. Harper is a past president of the American Acne & Rosacea Society. She disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speaker’s bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
to use when treating this group of patients.
During a presentation at Medscape Live’s annual Coastal Dermatology Symposium, Dr. Harper, who practices at Dermatology and Skin Care of Birmingham, Ala., elaborated on the mnemonic, as follows:
C: Communicate expectations. “I look right at the acne patient and say, ‘I know you don’t just want to be better; I know you want to be clear,’ ” she said at the meeting. “ ‘That’s my goal for you, too. That may take us more than one visit and more than one treatment, but I am on your team, and that’s what we’re shooting for.’ If you don’t communicate that, they’re going to think that their acne is not that important to you.”
L: Listen for clues to customize the patient’s treatment. “We’re quick to say, ‘my patients don’t do what I recommend,’ or ‘they didn’t do what the last doctor recommended,’ ” Dr. Harper said. “Sometimes that is true, but there may be a reason why. Maybe the medication was too expensive. Maybe it was bleaching their fabrics. Maybe the regimen was too complex. Listen for opportunities to make adjustments to get their acne closer to clear.”
E: Treat early to improve quality of life and to decrease the risk of scarring. “I have a laser in my practice that is good at treating acne scarring,” she said. “Do I ever look at my patient and say, ‘don’t worry about those scars; I can make them go away?’ No. I look at them and say, ‘we can maybe make this 40% better,’ something like that. We have to prevent acne scars, because we’re not good at treating them.”
A: Treat aggressively with more combination therapies, more hormonal therapies, more isotretinoin, and perhaps more prior authorizations. She characterized the effort to obtain a prior authorization as “our megaphone back to insurance companies that says, ‘we think it is worth taking the time to do this prior authorization because the acne patient will benefit.’ ”
R: Don’t resist isotretinoin. Dr. Harper, who began practicing dermatology more than 20 years ago, said that over time, she has gradually prescribed more isotretinoin for her patients with acne. “It’s not a first-line [treatment], but I’m not afraid of it. If I can’t get somebody clear on other oral or topical treatments, we are going to try isotretinoin.”
The goal of acne treatment, she added, is to affect four key aspects of pathogenesis: follicular epithelial hyperproliferation, inflammation, Cutibacterium acnes (C. acnes), and sebum. “That’s what we’re always shooting for,” she said.
Dr. Harper is a past president of the American Acne & Rosacea Society. She disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speaker’s bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
FROM MEDSCAPE LIVE COASTAL DERM
Many factors linked with higher, lower risk for hand eczema
“In this population-based study, all atopic diseases, not only atopic dermatitis, were found as individual risk factors for HE. In addition, female gender, obesity and mold exposure increased the risk of HE,” wrote Marjut Koskelo, MD, and her colleagues at the University of Oulu in Finland. Their report was published in Contact Dermatitis.
“Parental allergy was also a risk factor of offspring’s HE. Moderate or high physical activity as well as owning a dog appeared as protective factors of HE. No association was found between other lifestyle factors and HE,” they added.
Hand eczema is one of the most common skin disorders and is the most common occupational skin disease, the authors wrote. Many risk factors, including atopic dermatitis, are known to be linked with HE, but whether various other factors might also be linked has not been well studied.
The research team investigated the link between HE and atopic diseases, parental factors, environmental factors (exposure to mold, keeping animals), and lifestyle factors (physical activity, obesity, tobacco and alcohol use).
They analyzed data of people who took part in the Northern Finland Birth Cohort 1966 Study. The data, collected since 1965, includes details about 12,055 mothers in northern Finland who were expected to deliver babies in 1966, and their 12,058 live-born children. The children have been followed over the years with questionnaires and clinical examinations, and their parents have been followed by national registers and medical reports.
For the 46-year follow-up, 6,830 respondents aged 45-46 years, roughly half of them women, completed a 132-question form covering physical health, lifestyle, environmental factors, socioeconomic status, and history of hand eczema and other atopic diseases.
In the statistical analysis, the researchers adjusted for atopic dermatitis, asthma, allergic rhinoconjunctivitis, education level, body mass index, maternal BMI, parental allergy, physical activity, living on a farm, and mold exposure and symptoms.
Of the 900 respondents who reported having had HE, 592 (65.8%) were women and 308 (34.2%) were men (odds ratio [OR], 1.73; 95% confidence interval [CI], 1.49-2.0).
Various factors linked with hand eczema risk
The authors found the following:
- Atopic diseases and HE were linked: atopic dermatitis (adjusted odds ratio [aOR], 9.66; 95% CI, 8.03-11.66), asthma (aOR, 1.38; 95% CI, 1.12-1.71), and allergic rhinoconjunctivitis (aOR, 1.28; 95% CI, 1.04-1.56). Sex did not affect the link between atopic diseases and HE.
- Respondents who reported visible mold or mold odor in their apartments had higher risk for HE than did those without a history of mold exposure (OR, 1.32; 95% CI, 1.07-1.61).
- Obesity was linked with HE (OR, 3.44; 95% CI, 1.05-22.8), but smoking status, alcohol intake, and education level were not statistically significant risk factors for HE.
- Participants who reported moderate or high physical activity had lower risk for HE (OR, 0.78; 95% CI, 0.64-0.94; and OR, 0.56; 95% CI, 0.33-0.91, respectively) than those who were less active.
- Parental allergy increased risk for HE (OR, 1.98; 95% CI, 1.70-2.30); as maternal age, BMI, and menarche age increased, so did the risk for the child’s HE, but the increases were not statistically significant; and no significant links were found between maternal tobacco smoking, parental asthma, birth weight, parity, gestational age, and HE.
- Dog owners had less risk for HE than did people without a dog (OR, 0.83; 95% CI, 0.71-0.97); links between cat or farm animal owners and HE were not significant.
“There is a strong association between hand eczema and atopic diseases,” Maya Jonas, MD, clinical assistant professor of dermatology at The Ohio State University Wexner Medical Center in Columbus, told this news organization.
“When evaluating patients with hand eczema, it is important to ask if they have a history of atopic dermatitis, asthma, or allergic rhinitis,” said Dr. Jonas, who was not involved in the study.
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University, Cleveland, was surprised by the inverse link between physical activity and HE.
“What struck me as interesting is the inverse association between hand eczema and physical activity, that greater physical activity will decrease the risk for hand eczema,” she said in an interview. “It’s an interesting finding that’s worth exploring.
“Dermatologists have also speculated about the association with the female gender, because women are more likely to be in situations that involve frequent hand washing or in occupations, such as hairdressing, that involve known irritants and allergens,” added Dr. Baron, who was not involved in the study.
The main weakness, she noted, is the reliance on self-reported diagnosis. “Hand eczema is a common condition, but the etiologies of reported hand eczema may vary.
“Being cognizant of these associations can help us prescribe appropriate medications and advise patients about how they can avoid exposures that will aggravate their condition,” Dr. Baron advised.
The authors recommend further related studies.
The authors, Dr. Jonas, and Dr. Baron report no relevant financial relationships. The study was not funded.
A version of this article first appeared on Medscape.com.
“In this population-based study, all atopic diseases, not only atopic dermatitis, were found as individual risk factors for HE. In addition, female gender, obesity and mold exposure increased the risk of HE,” wrote Marjut Koskelo, MD, and her colleagues at the University of Oulu in Finland. Their report was published in Contact Dermatitis.
“Parental allergy was also a risk factor of offspring’s HE. Moderate or high physical activity as well as owning a dog appeared as protective factors of HE. No association was found between other lifestyle factors and HE,” they added.
Hand eczema is one of the most common skin disorders and is the most common occupational skin disease, the authors wrote. Many risk factors, including atopic dermatitis, are known to be linked with HE, but whether various other factors might also be linked has not been well studied.
The research team investigated the link between HE and atopic diseases, parental factors, environmental factors (exposure to mold, keeping animals), and lifestyle factors (physical activity, obesity, tobacco and alcohol use).
They analyzed data of people who took part in the Northern Finland Birth Cohort 1966 Study. The data, collected since 1965, includes details about 12,055 mothers in northern Finland who were expected to deliver babies in 1966, and their 12,058 live-born children. The children have been followed over the years with questionnaires and clinical examinations, and their parents have been followed by national registers and medical reports.
For the 46-year follow-up, 6,830 respondents aged 45-46 years, roughly half of them women, completed a 132-question form covering physical health, lifestyle, environmental factors, socioeconomic status, and history of hand eczema and other atopic diseases.
In the statistical analysis, the researchers adjusted for atopic dermatitis, asthma, allergic rhinoconjunctivitis, education level, body mass index, maternal BMI, parental allergy, physical activity, living on a farm, and mold exposure and symptoms.
Of the 900 respondents who reported having had HE, 592 (65.8%) were women and 308 (34.2%) were men (odds ratio [OR], 1.73; 95% confidence interval [CI], 1.49-2.0).
Various factors linked with hand eczema risk
The authors found the following:
- Atopic diseases and HE were linked: atopic dermatitis (adjusted odds ratio [aOR], 9.66; 95% CI, 8.03-11.66), asthma (aOR, 1.38; 95% CI, 1.12-1.71), and allergic rhinoconjunctivitis (aOR, 1.28; 95% CI, 1.04-1.56). Sex did not affect the link between atopic diseases and HE.
- Respondents who reported visible mold or mold odor in their apartments had higher risk for HE than did those without a history of mold exposure (OR, 1.32; 95% CI, 1.07-1.61).
- Obesity was linked with HE (OR, 3.44; 95% CI, 1.05-22.8), but smoking status, alcohol intake, and education level were not statistically significant risk factors for HE.
- Participants who reported moderate or high physical activity had lower risk for HE (OR, 0.78; 95% CI, 0.64-0.94; and OR, 0.56; 95% CI, 0.33-0.91, respectively) than those who were less active.
- Parental allergy increased risk for HE (OR, 1.98; 95% CI, 1.70-2.30); as maternal age, BMI, and menarche age increased, so did the risk for the child’s HE, but the increases were not statistically significant; and no significant links were found between maternal tobacco smoking, parental asthma, birth weight, parity, gestational age, and HE.
- Dog owners had less risk for HE than did people without a dog (OR, 0.83; 95% CI, 0.71-0.97); links between cat or farm animal owners and HE were not significant.
“There is a strong association between hand eczema and atopic diseases,” Maya Jonas, MD, clinical assistant professor of dermatology at The Ohio State University Wexner Medical Center in Columbus, told this news organization.
“When evaluating patients with hand eczema, it is important to ask if they have a history of atopic dermatitis, asthma, or allergic rhinitis,” said Dr. Jonas, who was not involved in the study.
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University, Cleveland, was surprised by the inverse link between physical activity and HE.
“What struck me as interesting is the inverse association between hand eczema and physical activity, that greater physical activity will decrease the risk for hand eczema,” she said in an interview. “It’s an interesting finding that’s worth exploring.
“Dermatologists have also speculated about the association with the female gender, because women are more likely to be in situations that involve frequent hand washing or in occupations, such as hairdressing, that involve known irritants and allergens,” added Dr. Baron, who was not involved in the study.
The main weakness, she noted, is the reliance on self-reported diagnosis. “Hand eczema is a common condition, but the etiologies of reported hand eczema may vary.
“Being cognizant of these associations can help us prescribe appropriate medications and advise patients about how they can avoid exposures that will aggravate their condition,” Dr. Baron advised.
The authors recommend further related studies.
The authors, Dr. Jonas, and Dr. Baron report no relevant financial relationships. The study was not funded.
A version of this article first appeared on Medscape.com.
“In this population-based study, all atopic diseases, not only atopic dermatitis, were found as individual risk factors for HE. In addition, female gender, obesity and mold exposure increased the risk of HE,” wrote Marjut Koskelo, MD, and her colleagues at the University of Oulu in Finland. Their report was published in Contact Dermatitis.
“Parental allergy was also a risk factor of offspring’s HE. Moderate or high physical activity as well as owning a dog appeared as protective factors of HE. No association was found between other lifestyle factors and HE,” they added.
Hand eczema is one of the most common skin disorders and is the most common occupational skin disease, the authors wrote. Many risk factors, including atopic dermatitis, are known to be linked with HE, but whether various other factors might also be linked has not been well studied.
The research team investigated the link between HE and atopic diseases, parental factors, environmental factors (exposure to mold, keeping animals), and lifestyle factors (physical activity, obesity, tobacco and alcohol use).
They analyzed data of people who took part in the Northern Finland Birth Cohort 1966 Study. The data, collected since 1965, includes details about 12,055 mothers in northern Finland who were expected to deliver babies in 1966, and their 12,058 live-born children. The children have been followed over the years with questionnaires and clinical examinations, and their parents have been followed by national registers and medical reports.
For the 46-year follow-up, 6,830 respondents aged 45-46 years, roughly half of them women, completed a 132-question form covering physical health, lifestyle, environmental factors, socioeconomic status, and history of hand eczema and other atopic diseases.
In the statistical analysis, the researchers adjusted for atopic dermatitis, asthma, allergic rhinoconjunctivitis, education level, body mass index, maternal BMI, parental allergy, physical activity, living on a farm, and mold exposure and symptoms.
Of the 900 respondents who reported having had HE, 592 (65.8%) were women and 308 (34.2%) were men (odds ratio [OR], 1.73; 95% confidence interval [CI], 1.49-2.0).
Various factors linked with hand eczema risk
The authors found the following:
- Atopic diseases and HE were linked: atopic dermatitis (adjusted odds ratio [aOR], 9.66; 95% CI, 8.03-11.66), asthma (aOR, 1.38; 95% CI, 1.12-1.71), and allergic rhinoconjunctivitis (aOR, 1.28; 95% CI, 1.04-1.56). Sex did not affect the link between atopic diseases and HE.
- Respondents who reported visible mold or mold odor in their apartments had higher risk for HE than did those without a history of mold exposure (OR, 1.32; 95% CI, 1.07-1.61).
- Obesity was linked with HE (OR, 3.44; 95% CI, 1.05-22.8), but smoking status, alcohol intake, and education level were not statistically significant risk factors for HE.
- Participants who reported moderate or high physical activity had lower risk for HE (OR, 0.78; 95% CI, 0.64-0.94; and OR, 0.56; 95% CI, 0.33-0.91, respectively) than those who were less active.
- Parental allergy increased risk for HE (OR, 1.98; 95% CI, 1.70-2.30); as maternal age, BMI, and menarche age increased, so did the risk for the child’s HE, but the increases were not statistically significant; and no significant links were found between maternal tobacco smoking, parental asthma, birth weight, parity, gestational age, and HE.
- Dog owners had less risk for HE than did people without a dog (OR, 0.83; 95% CI, 0.71-0.97); links between cat or farm animal owners and HE were not significant.
“There is a strong association between hand eczema and atopic diseases,” Maya Jonas, MD, clinical assistant professor of dermatology at The Ohio State University Wexner Medical Center in Columbus, told this news organization.
“When evaluating patients with hand eczema, it is important to ask if they have a history of atopic dermatitis, asthma, or allergic rhinitis,” said Dr. Jonas, who was not involved in the study.
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University, Cleveland, was surprised by the inverse link between physical activity and HE.
“What struck me as interesting is the inverse association between hand eczema and physical activity, that greater physical activity will decrease the risk for hand eczema,” she said in an interview. “It’s an interesting finding that’s worth exploring.
“Dermatologists have also speculated about the association with the female gender, because women are more likely to be in situations that involve frequent hand washing or in occupations, such as hairdressing, that involve known irritants and allergens,” added Dr. Baron, who was not involved in the study.
The main weakness, she noted, is the reliance on self-reported diagnosis. “Hand eczema is a common condition, but the etiologies of reported hand eczema may vary.
“Being cognizant of these associations can help us prescribe appropriate medications and advise patients about how they can avoid exposures that will aggravate their condition,” Dr. Baron advised.
The authors recommend further related studies.
The authors, Dr. Jonas, and Dr. Baron report no relevant financial relationships. The study was not funded.
A version of this article first appeared on Medscape.com.
FROM CONTACT DERMATITIS
Apremilast may have some cardiometabolic benefits in patients with psoriasis
The trial, led by Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology and vice chair of clinical research in dermatology at the University of Pennsylvania, Philadelphia, found that apremilast (Otezla) has a neutral effect on aortic vascular inflammation in patients with moderate to severe psoriasis.
It also had variable, but generally favorable, associations with 68 cardiometabolic biomarkers tested and associations with reductions in both visceral and subcutaneous fat. Findings of the study were published online in JAMA Dermatology.
Fat reductions maintained at 1-year mark
The researchers found a 5%-6% reduction in subcutaneous and visceral fat at week 16 of the study that was maintained at the 1-year mark. “The fact that it was rock stable a year later is pretty encouraging,” Dr. Gelfand told this news organization.
As for effects on vascular inflammation, Dr. Gelfand said, “The good news is we didn’t find any adverse effects on aortic vascular inflammation, but we didn’t find any beneficial effects either. That was a little disappointing.
“The most surprising thing was really the effects on visceral adiposity,” he added. “I’m not aware of any other drug having demonstrated that effect.”
Michael S. Garshick, MD, a cardiologist with NYU Langone Health in New York, who was not involved with the trial, told this news organization that despite seemingly good epidemiologic evidence in observational studies that by treating psoriasis surrogates of cardiovascular risk can be reduced, this trial, like others before it, failed to reduce aortic vascular inflammation.
The trial does help answer the question of whether apremilast can induce weight loss, he said, something that earlier trials suggested. “This trial confirms that, which is exciting,” he said. The reduction in both visceral and subcutaneous fat “deserves a lot further study.”
Several questions remain, Dr. Garshick said. Both he and Dr. Gelfand pointed to the need for large, placebo-controlled trials. “We still don’t know which medications may be preferrable in psoriasis to reduce [cardiovascular] risk if any at all,” Dr. Garshick said.
Seventy patients enrolled
In total, 70 patients with moderate to severe psoriasis were enrolled, 60 completed week 16, and 39 completed week 52 of the single-arm, open-label trial conducted between April 2017 and August 2021 at seven dermatology sites in the United States.
Participants took 30 mg of apremilast, an oral phosphodiesterase-4 (PDE-4) inhibitor approved for treating psoriasis and psoriatic arthritis, twice daily. Participants’ average age was 47.5 years; most were male (77.1%) and White (82.9%); almost 6% were Black. Average body mass index was 30 kg/m2. Patients could not have received biologics within 90 days of study baseline (or 180 days for ustekinumab [Stelara]).
There was no change in aortic vascular inflammation at week 16 (target to background ratio, −0.02; 95% confidence interval [CI], −0.08 to 0.05; P = .61) or week 52 (target to background ratio, −0.07; 95% CI, −0.15 to 0.01; P = .09) compared with baseline.
“At week 16, there were reductions in levels of interleukin-1b, fetuin A, valine, leucine, and isoleucine,” the authors wrote, adding that at week 52, compared with baseline, “there were reductions in levels of ferritin, cholesterol efflux capacity, beta-hydroxybutyrate, acetone, and ketone bodies, and an increase in levels of apolipoprotein A-1.”
This study highlights the importance of screening, Dr. Garshick said.
He and Dr. Gelfand said people with psoriatic disease tend to be vastly underscreened for cardiovascular risk factors.
Dr. Gelfand said, “If we did what we knew worked – meaning we screened them for diabetes, we screen their cholesterol, we check their blood pressure, and we adequately treated those traditional cardiovascular risk factors, we probably could narrow the gap quite a bit” in terms of the lower life expectancy people face when they have more significant psoriasis.
Celgene was the initial funding sponsor; sponsorship was then transferred to Amgen. The authors designed, executed, analyzed, and reported the study. Celgene provided nonbinding input into study design, and Amgen provided nonbinding input into the reporting of results. Dr. Gelfand reported numerous disclosures with various pharmaceutical companies and organizations. Dr. Garshick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The trial, led by Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology and vice chair of clinical research in dermatology at the University of Pennsylvania, Philadelphia, found that apremilast (Otezla) has a neutral effect on aortic vascular inflammation in patients with moderate to severe psoriasis.
It also had variable, but generally favorable, associations with 68 cardiometabolic biomarkers tested and associations with reductions in both visceral and subcutaneous fat. Findings of the study were published online in JAMA Dermatology.
Fat reductions maintained at 1-year mark
The researchers found a 5%-6% reduction in subcutaneous and visceral fat at week 16 of the study that was maintained at the 1-year mark. “The fact that it was rock stable a year later is pretty encouraging,” Dr. Gelfand told this news organization.
As for effects on vascular inflammation, Dr. Gelfand said, “The good news is we didn’t find any adverse effects on aortic vascular inflammation, but we didn’t find any beneficial effects either. That was a little disappointing.
“The most surprising thing was really the effects on visceral adiposity,” he added. “I’m not aware of any other drug having demonstrated that effect.”
Michael S. Garshick, MD, a cardiologist with NYU Langone Health in New York, who was not involved with the trial, told this news organization that despite seemingly good epidemiologic evidence in observational studies that by treating psoriasis surrogates of cardiovascular risk can be reduced, this trial, like others before it, failed to reduce aortic vascular inflammation.
The trial does help answer the question of whether apremilast can induce weight loss, he said, something that earlier trials suggested. “This trial confirms that, which is exciting,” he said. The reduction in both visceral and subcutaneous fat “deserves a lot further study.”
Several questions remain, Dr. Garshick said. Both he and Dr. Gelfand pointed to the need for large, placebo-controlled trials. “We still don’t know which medications may be preferrable in psoriasis to reduce [cardiovascular] risk if any at all,” Dr. Garshick said.
Seventy patients enrolled
In total, 70 patients with moderate to severe psoriasis were enrolled, 60 completed week 16, and 39 completed week 52 of the single-arm, open-label trial conducted between April 2017 and August 2021 at seven dermatology sites in the United States.
Participants took 30 mg of apremilast, an oral phosphodiesterase-4 (PDE-4) inhibitor approved for treating psoriasis and psoriatic arthritis, twice daily. Participants’ average age was 47.5 years; most were male (77.1%) and White (82.9%); almost 6% were Black. Average body mass index was 30 kg/m2. Patients could not have received biologics within 90 days of study baseline (or 180 days for ustekinumab [Stelara]).
There was no change in aortic vascular inflammation at week 16 (target to background ratio, −0.02; 95% confidence interval [CI], −0.08 to 0.05; P = .61) or week 52 (target to background ratio, −0.07; 95% CI, −0.15 to 0.01; P = .09) compared with baseline.
“At week 16, there were reductions in levels of interleukin-1b, fetuin A, valine, leucine, and isoleucine,” the authors wrote, adding that at week 52, compared with baseline, “there were reductions in levels of ferritin, cholesterol efflux capacity, beta-hydroxybutyrate, acetone, and ketone bodies, and an increase in levels of apolipoprotein A-1.”
This study highlights the importance of screening, Dr. Garshick said.
He and Dr. Gelfand said people with psoriatic disease tend to be vastly underscreened for cardiovascular risk factors.
Dr. Gelfand said, “If we did what we knew worked – meaning we screened them for diabetes, we screen their cholesterol, we check their blood pressure, and we adequately treated those traditional cardiovascular risk factors, we probably could narrow the gap quite a bit” in terms of the lower life expectancy people face when they have more significant psoriasis.
Celgene was the initial funding sponsor; sponsorship was then transferred to Amgen. The authors designed, executed, analyzed, and reported the study. Celgene provided nonbinding input into study design, and Amgen provided nonbinding input into the reporting of results. Dr. Gelfand reported numerous disclosures with various pharmaceutical companies and organizations. Dr. Garshick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The trial, led by Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology and vice chair of clinical research in dermatology at the University of Pennsylvania, Philadelphia, found that apremilast (Otezla) has a neutral effect on aortic vascular inflammation in patients with moderate to severe psoriasis.
It also had variable, but generally favorable, associations with 68 cardiometabolic biomarkers tested and associations with reductions in both visceral and subcutaneous fat. Findings of the study were published online in JAMA Dermatology.
Fat reductions maintained at 1-year mark
The researchers found a 5%-6% reduction in subcutaneous and visceral fat at week 16 of the study that was maintained at the 1-year mark. “The fact that it was rock stable a year later is pretty encouraging,” Dr. Gelfand told this news organization.
As for effects on vascular inflammation, Dr. Gelfand said, “The good news is we didn’t find any adverse effects on aortic vascular inflammation, but we didn’t find any beneficial effects either. That was a little disappointing.
“The most surprising thing was really the effects on visceral adiposity,” he added. “I’m not aware of any other drug having demonstrated that effect.”
Michael S. Garshick, MD, a cardiologist with NYU Langone Health in New York, who was not involved with the trial, told this news organization that despite seemingly good epidemiologic evidence in observational studies that by treating psoriasis surrogates of cardiovascular risk can be reduced, this trial, like others before it, failed to reduce aortic vascular inflammation.
The trial does help answer the question of whether apremilast can induce weight loss, he said, something that earlier trials suggested. “This trial confirms that, which is exciting,” he said. The reduction in both visceral and subcutaneous fat “deserves a lot further study.”
Several questions remain, Dr. Garshick said. Both he and Dr. Gelfand pointed to the need for large, placebo-controlled trials. “We still don’t know which medications may be preferrable in psoriasis to reduce [cardiovascular] risk if any at all,” Dr. Garshick said.
Seventy patients enrolled
In total, 70 patients with moderate to severe psoriasis were enrolled, 60 completed week 16, and 39 completed week 52 of the single-arm, open-label trial conducted between April 2017 and August 2021 at seven dermatology sites in the United States.
Participants took 30 mg of apremilast, an oral phosphodiesterase-4 (PDE-4) inhibitor approved for treating psoriasis and psoriatic arthritis, twice daily. Participants’ average age was 47.5 years; most were male (77.1%) and White (82.9%); almost 6% were Black. Average body mass index was 30 kg/m2. Patients could not have received biologics within 90 days of study baseline (or 180 days for ustekinumab [Stelara]).
There was no change in aortic vascular inflammation at week 16 (target to background ratio, −0.02; 95% confidence interval [CI], −0.08 to 0.05; P = .61) or week 52 (target to background ratio, −0.07; 95% CI, −0.15 to 0.01; P = .09) compared with baseline.
“At week 16, there were reductions in levels of interleukin-1b, fetuin A, valine, leucine, and isoleucine,” the authors wrote, adding that at week 52, compared with baseline, “there were reductions in levels of ferritin, cholesterol efflux capacity, beta-hydroxybutyrate, acetone, and ketone bodies, and an increase in levels of apolipoprotein A-1.”
This study highlights the importance of screening, Dr. Garshick said.
He and Dr. Gelfand said people with psoriatic disease tend to be vastly underscreened for cardiovascular risk factors.
Dr. Gelfand said, “If we did what we knew worked – meaning we screened them for diabetes, we screen their cholesterol, we check their blood pressure, and we adequately treated those traditional cardiovascular risk factors, we probably could narrow the gap quite a bit” in terms of the lower life expectancy people face when they have more significant psoriasis.
Celgene was the initial funding sponsor; sponsorship was then transferred to Amgen. The authors designed, executed, analyzed, and reported the study. Celgene provided nonbinding input into study design, and Amgen provided nonbinding input into the reporting of results. Dr. Gelfand reported numerous disclosures with various pharmaceutical companies and organizations. Dr. Garshick reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Early emollient use reduces dermatitis in at-risk infants
Recent study findings published in Allergy (2022 Aug 23. doi: 10.1111/all.15491) suggest that
The single-center STOP-AD clinical trial recruited term infants within 4 days of birth who were at high risk for AD, as determined on the basis of a parent-reported history of the disease or asthma or allergic rhinitis. Infants were randomly assigned to undergo either a standard skin care routine (control group; n = 160) or twice-daily emollient application for the first 8 weeks of life (intervention group; n = 161).
In the intervention group, infants received an emollient that was specifically formulated for AD-prone skin. The control group received standard skin care advice, which did not include specific advice on bathing frequency or regular emollient use.
The mean age of the infants at randomization was 1.9 days. A total of 41 infants in the intervention group and 20 infants in the control group were withdrawn from the study. Most withdrawals (80%) occurred prior to the 2-week visit.
At 12 months, the cumulative incidence of AD was 32.8% in the intervention group and 46.4% in the control group (P = .036). The investigators note that daily emollient use was associated with a 29% lower risk of cumulative AD at 1 year in comparison with the control intervention.
No significant difference was observed between the groups regarding the incidence of parent-reported skin infections during the treatment period (5.0% vs. 5.7%; P > .05).
Study investigator Jonathan O’Brien Hourihane, MBBS, of the Royal College of Surgeons in Dublin, said in an interview that previously published findings from the BASELINE study supported the rationale for the early use of emollients in infancy to prevent AD.
The investigators of the BASELINE study found that skin barrier function, as measured by transepidermal water loss, increased from birth to 8 weeks but then became stable at 6 months. These observations suggest that the period during early infancy “could be a critical window in which to protect the skin barrier” of infants at risk for AD, Dr. Hourihane added.
Dr. Hourihane, who serves as the head of department of pediatrics at the Royal College of Surgeons, explained that the long-term clinical burden of AD is often more significant if the condition begins earlier in life, underscoring the importance of early prevention and control.
“The casual role [of AD] in other allergic conditions remains suspected but not proven, but its association is clear,” he said. He noted that infants with eczema “also have poorer sleep, and the condition causes increased family disruption,” highlighting the far-reaching burden of AD.
Commenting on the study, Adelaide Hebert, MD, professor of pediatric dermatology at the University of Texas, Houston, said in an interview that the barrier defect observed in AD is one of the prime areas to address as a means of controlling the chronic, relapsing disorder. She noted that the use of emollients can repair this defective barrier.
“Early initiation of emollients has the potential to reduce dryness, itching, transgression of allergens, and infectious agents,” explained Dr. Hebert, who wasn’t involved in the study. “Emollient application also allows the parent to inspect the skin surface and address any challenges in a timely manner.”
In the STOP-AD trial, Dr. Hourihane and colleagues also found that, among patients with loss-of-function (LoF) mutations in the filaggrin gene (FLG), the prevalence of AD at 6 and 12 months seemed to be a higher than among patients with the wild-type gene, but the difference did not reach statistical significance.
Commenting on this finding, Dr. Hebert noted that LoF FLG mutation carriers may benefit especially from emollient use, given that LoF mutations in FLG is associated with reduced production of natural moisturizing factors in the skin.
Regarding future research directions, Dr. Hourihane stated that there is a need for replication and validation of the findings in studies that include infants from different ethnic backgrounds as well as those from various social settings. These studies should also include variable treatment windows to determine both short- and longer-term effects of emollient use in this population, Dr. Hourihane explained.
Dr. Hourihane added that he and the investigators do not yet understand which aspect of the study’s program was key for reducing the incidence of AD in the first year of life. “The timing of emollient initiation, the duration of treatment, the products, or maybe just a combination of these” could be possible explanations.
The study was independently supported. Dr. Hourihand reported receiving grant funding from Aimmune Therapeutics and DBV Technologies. Dr. Hebert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Recent study findings published in Allergy (2022 Aug 23. doi: 10.1111/all.15491) suggest that
The single-center STOP-AD clinical trial recruited term infants within 4 days of birth who were at high risk for AD, as determined on the basis of a parent-reported history of the disease or asthma or allergic rhinitis. Infants were randomly assigned to undergo either a standard skin care routine (control group; n = 160) or twice-daily emollient application for the first 8 weeks of life (intervention group; n = 161).
In the intervention group, infants received an emollient that was specifically formulated for AD-prone skin. The control group received standard skin care advice, which did not include specific advice on bathing frequency or regular emollient use.
The mean age of the infants at randomization was 1.9 days. A total of 41 infants in the intervention group and 20 infants in the control group were withdrawn from the study. Most withdrawals (80%) occurred prior to the 2-week visit.
At 12 months, the cumulative incidence of AD was 32.8% in the intervention group and 46.4% in the control group (P = .036). The investigators note that daily emollient use was associated with a 29% lower risk of cumulative AD at 1 year in comparison with the control intervention.
No significant difference was observed between the groups regarding the incidence of parent-reported skin infections during the treatment period (5.0% vs. 5.7%; P > .05).
Study investigator Jonathan O’Brien Hourihane, MBBS, of the Royal College of Surgeons in Dublin, said in an interview that previously published findings from the BASELINE study supported the rationale for the early use of emollients in infancy to prevent AD.
The investigators of the BASELINE study found that skin barrier function, as measured by transepidermal water loss, increased from birth to 8 weeks but then became stable at 6 months. These observations suggest that the period during early infancy “could be a critical window in which to protect the skin barrier” of infants at risk for AD, Dr. Hourihane added.
Dr. Hourihane, who serves as the head of department of pediatrics at the Royal College of Surgeons, explained that the long-term clinical burden of AD is often more significant if the condition begins earlier in life, underscoring the importance of early prevention and control.
“The casual role [of AD] in other allergic conditions remains suspected but not proven, but its association is clear,” he said. He noted that infants with eczema “also have poorer sleep, and the condition causes increased family disruption,” highlighting the far-reaching burden of AD.
Commenting on the study, Adelaide Hebert, MD, professor of pediatric dermatology at the University of Texas, Houston, said in an interview that the barrier defect observed in AD is one of the prime areas to address as a means of controlling the chronic, relapsing disorder. She noted that the use of emollients can repair this defective barrier.
“Early initiation of emollients has the potential to reduce dryness, itching, transgression of allergens, and infectious agents,” explained Dr. Hebert, who wasn’t involved in the study. “Emollient application also allows the parent to inspect the skin surface and address any challenges in a timely manner.”
In the STOP-AD trial, Dr. Hourihane and colleagues also found that, among patients with loss-of-function (LoF) mutations in the filaggrin gene (FLG), the prevalence of AD at 6 and 12 months seemed to be a higher than among patients with the wild-type gene, but the difference did not reach statistical significance.
Commenting on this finding, Dr. Hebert noted that LoF FLG mutation carriers may benefit especially from emollient use, given that LoF mutations in FLG is associated with reduced production of natural moisturizing factors in the skin.
Regarding future research directions, Dr. Hourihane stated that there is a need for replication and validation of the findings in studies that include infants from different ethnic backgrounds as well as those from various social settings. These studies should also include variable treatment windows to determine both short- and longer-term effects of emollient use in this population, Dr. Hourihane explained.
Dr. Hourihane added that he and the investigators do not yet understand which aspect of the study’s program was key for reducing the incidence of AD in the first year of life. “The timing of emollient initiation, the duration of treatment, the products, or maybe just a combination of these” could be possible explanations.
The study was independently supported. Dr. Hourihand reported receiving grant funding from Aimmune Therapeutics and DBV Technologies. Dr. Hebert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Recent study findings published in Allergy (2022 Aug 23. doi: 10.1111/all.15491) suggest that
The single-center STOP-AD clinical trial recruited term infants within 4 days of birth who were at high risk for AD, as determined on the basis of a parent-reported history of the disease or asthma or allergic rhinitis. Infants were randomly assigned to undergo either a standard skin care routine (control group; n = 160) or twice-daily emollient application for the first 8 weeks of life (intervention group; n = 161).
In the intervention group, infants received an emollient that was specifically formulated for AD-prone skin. The control group received standard skin care advice, which did not include specific advice on bathing frequency or regular emollient use.
The mean age of the infants at randomization was 1.9 days. A total of 41 infants in the intervention group and 20 infants in the control group were withdrawn from the study. Most withdrawals (80%) occurred prior to the 2-week visit.
At 12 months, the cumulative incidence of AD was 32.8% in the intervention group and 46.4% in the control group (P = .036). The investigators note that daily emollient use was associated with a 29% lower risk of cumulative AD at 1 year in comparison with the control intervention.
No significant difference was observed between the groups regarding the incidence of parent-reported skin infections during the treatment period (5.0% vs. 5.7%; P > .05).
Study investigator Jonathan O’Brien Hourihane, MBBS, of the Royal College of Surgeons in Dublin, said in an interview that previously published findings from the BASELINE study supported the rationale for the early use of emollients in infancy to prevent AD.
The investigators of the BASELINE study found that skin barrier function, as measured by transepidermal water loss, increased from birth to 8 weeks but then became stable at 6 months. These observations suggest that the period during early infancy “could be a critical window in which to protect the skin barrier” of infants at risk for AD, Dr. Hourihane added.
Dr. Hourihane, who serves as the head of department of pediatrics at the Royal College of Surgeons, explained that the long-term clinical burden of AD is often more significant if the condition begins earlier in life, underscoring the importance of early prevention and control.
“The casual role [of AD] in other allergic conditions remains suspected but not proven, but its association is clear,” he said. He noted that infants with eczema “also have poorer sleep, and the condition causes increased family disruption,” highlighting the far-reaching burden of AD.
Commenting on the study, Adelaide Hebert, MD, professor of pediatric dermatology at the University of Texas, Houston, said in an interview that the barrier defect observed in AD is one of the prime areas to address as a means of controlling the chronic, relapsing disorder. She noted that the use of emollients can repair this defective barrier.
“Early initiation of emollients has the potential to reduce dryness, itching, transgression of allergens, and infectious agents,” explained Dr. Hebert, who wasn’t involved in the study. “Emollient application also allows the parent to inspect the skin surface and address any challenges in a timely manner.”
In the STOP-AD trial, Dr. Hourihane and colleagues also found that, among patients with loss-of-function (LoF) mutations in the filaggrin gene (FLG), the prevalence of AD at 6 and 12 months seemed to be a higher than among patients with the wild-type gene, but the difference did not reach statistical significance.
Commenting on this finding, Dr. Hebert noted that LoF FLG mutation carriers may benefit especially from emollient use, given that LoF mutations in FLG is associated with reduced production of natural moisturizing factors in the skin.
Regarding future research directions, Dr. Hourihane stated that there is a need for replication and validation of the findings in studies that include infants from different ethnic backgrounds as well as those from various social settings. These studies should also include variable treatment windows to determine both short- and longer-term effects of emollient use in this population, Dr. Hourihane explained.
Dr. Hourihane added that he and the investigators do not yet understand which aspect of the study’s program was key for reducing the incidence of AD in the first year of life. “The timing of emollient initiation, the duration of treatment, the products, or maybe just a combination of these” could be possible explanations.
The study was independently supported. Dr. Hourihand reported receiving grant funding from Aimmune Therapeutics and DBV Technologies. Dr. Hebert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALLERGY