User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
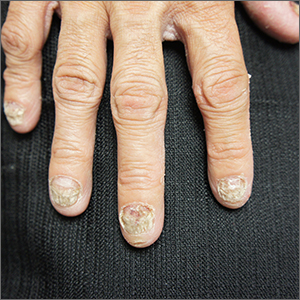
Nail dystrophy and foot pain
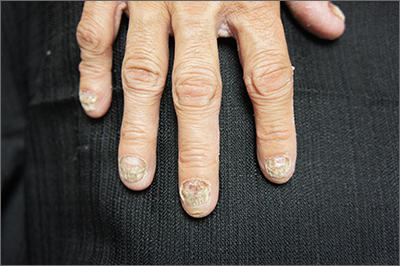
These findings are consistent with a type of heritable keratoderma called pachyonychia congenita (also called twenty-nails dystrophy). It is easy to mistake this unusual cause of thickening nails with a more common cause: onychomycosis.
Pachyonychia congenita describes a set of disorders driven by heritable defects in 1 of 5 keratin genes. The disorder is often transmitted in an autosomal dominant fashion, although a third of patients are thought to have a spontaneous mutation.1 These gene changes can cause 1 or multiple dystrophic nails, thickened nail beds, natal teeth, thick plantar or palmar nodules or plaques, and hearing difficulties. Some patients may have symptoms at birth, while other patients do not develop symptoms until later in life.1
There is currently no cure for pachyonychia congenita. Patients with suspected heritable keratoderma benefit from referral to Medical Genetics and a dermatologist who is comfortable treating keratodermas. Patients can obtain free genetic testing, educational material, and additional resources through pachyonychia.org.
This patient was prescribed topical urea 40% cream that was to be applied to the feet nightly, until the nodules became less painful. He was also evaluated for pressure-offloading orthotics. Nails may be treated with topical urea lacquer nightly until patients are satisfied with the appearance, although this patient chose to forgo the lacquer.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Smith FJD, Hansen CD, Hull PR, et al. Pachyonychia congenita. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews. Seattle (WA): University of Washington, Seattle; 2006. Updated November 30, 2017. Accessed June 27, 2022. https://www.ncbi.nlm.nih.gov/books/NBK1280/

These findings are consistent with a type of heritable keratoderma called pachyonychia congenita (also called twenty-nails dystrophy). It is easy to mistake this unusual cause of thickening nails with a more common cause: onychomycosis.
Pachyonychia congenita describes a set of disorders driven by heritable defects in 1 of 5 keratin genes. The disorder is often transmitted in an autosomal dominant fashion, although a third of patients are thought to have a spontaneous mutation.1 These gene changes can cause 1 or multiple dystrophic nails, thickened nail beds, natal teeth, thick plantar or palmar nodules or plaques, and hearing difficulties. Some patients may have symptoms at birth, while other patients do not develop symptoms until later in life.1
There is currently no cure for pachyonychia congenita. Patients with suspected heritable keratoderma benefit from referral to Medical Genetics and a dermatologist who is comfortable treating keratodermas. Patients can obtain free genetic testing, educational material, and additional resources through pachyonychia.org.
This patient was prescribed topical urea 40% cream that was to be applied to the feet nightly, until the nodules became less painful. He was also evaluated for pressure-offloading orthotics. Nails may be treated with topical urea lacquer nightly until patients are satisfied with the appearance, although this patient chose to forgo the lacquer.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

These findings are consistent with a type of heritable keratoderma called pachyonychia congenita (also called twenty-nails dystrophy). It is easy to mistake this unusual cause of thickening nails with a more common cause: onychomycosis.
Pachyonychia congenita describes a set of disorders driven by heritable defects in 1 of 5 keratin genes. The disorder is often transmitted in an autosomal dominant fashion, although a third of patients are thought to have a spontaneous mutation.1 These gene changes can cause 1 or multiple dystrophic nails, thickened nail beds, natal teeth, thick plantar or palmar nodules or plaques, and hearing difficulties. Some patients may have symptoms at birth, while other patients do not develop symptoms until later in life.1
There is currently no cure for pachyonychia congenita. Patients with suspected heritable keratoderma benefit from referral to Medical Genetics and a dermatologist who is comfortable treating keratodermas. Patients can obtain free genetic testing, educational material, and additional resources through pachyonychia.org.
This patient was prescribed topical urea 40% cream that was to be applied to the feet nightly, until the nodules became less painful. He was also evaluated for pressure-offloading orthotics. Nails may be treated with topical urea lacquer nightly until patients are satisfied with the appearance, although this patient chose to forgo the lacquer.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Smith FJD, Hansen CD, Hull PR, et al. Pachyonychia congenita. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews. Seattle (WA): University of Washington, Seattle; 2006. Updated November 30, 2017. Accessed June 27, 2022. https://www.ncbi.nlm.nih.gov/books/NBK1280/
1. Smith FJD, Hansen CD, Hull PR, et al. Pachyonychia congenita. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews. Seattle (WA): University of Washington, Seattle; 2006. Updated November 30, 2017. Accessed June 27, 2022. https://www.ncbi.nlm.nih.gov/books/NBK1280/
Skin reactions after COVID-19 vaccination have six patterns
Skin manifestations of COVID-19 were among the topics presented in several sessions at the 49th Congress of the Spanish Academy of Dermatology and Venereology. Specialists agreed that fewer skin changes associated with this virus have been seen with the latest variants of SARS-CoV-2. They highlighted the results of the most remarkable research on this topic that were presented in this forum.
In the study, which was carried out by Spanish dermatologists with the support of the AEDV, researchers analyzed skin reactions associated with the COVID-19 vaccine.
Study author Cristina Galván, MD, a dermatologist at the University Hospital of Móstoles, Madrid, said, of the dermatological manifestations caused as a reaction to these vaccines.”
The study was carried out during the first months of COVID-19 vaccination, Dr. Galván told this news organization. It was proposed as a continuation of a COVID skin study that was published in the British Journal of Dermatology. That study documented the first classification of skin lesions associated with COVID-19. Dr. Galván is the lead author of the latter study.
“The objectives of this study were to characterize and classify skin reactions after vaccination, identify their chronology, and analyze the associations with a series of antecedents: dermatological and allergic diseases, previous SARS-CoV-2 infection, and skin reactions associated with COVID-19,” said Dr. Galván. The study was a team effort, she added.
“It was conducted between Feb. 15 and May 12, 2021, and information was gathered on 405 reactions that appeared during the 21 days after any dose of the COVID-19 vaccines approved at that time in Spain: the Pfizer/BioNTech, Moderna, and University of Oxford/AstraZeneca vaccines,” she added.
Dr. Galván explained that the study shows very clear patterns and investigators reached conclusions that match those of other groups that have investigated this topic. “Six reaction patterns were described according to their frequency. The first is the ‘COVID-19 arm,’ which consists of a local reaction at the injection site and occurs almost exclusively in women and in 70% of cases after inoculation with the Moderna serum. It is a manifestation that resolves well and does not always recur in subsequent doses. More than half are of delayed onset: biopsied patients show signs of a delayed hypersensitivity reaction. In line with all the publications in this regard, it was found that this reaction is not a reason to skip or delay a dose.”
Herpes zoster reactivation
The second pattern is urticarial, which, according to the specialist, occurs with equal frequency after the administration of all vaccines and is well controlled with antihistamines. “This is a very nonspecific pattern, which does not prevent it from still being frequent. It was not associated with drug intake.
“The morbilliform pattern is more frequent after the Pfizer/BioNTech and AstraZeneca vaccines. It affects the trunk and extremities, and up to a quarter of the cases required systemic corticosteroids. The papulovesicular and pityriasis rosea–like patterns are equally frequent in all vaccines. The latter is found in a younger age group. Finally, there is the purpuric pattern, more localized in the extremities and more frequent after the Pfizer/BioNTech and AstraZeneca vaccines. On biopsy, this pattern showed small-vessel vasculitis.”
Less frequently, reactivations or de novo onset of different dermatologic diseases were found. “Varicella-zoster virus reactivations were observed with a frequency of 13.8%, being more common after the Pfizer/BioNTech vaccine,” said Dr. Galván. “Other studies have corroborated this increase in herpes zoster, although it has been seen that the absolute number is low, so the benefits of the vaccine outweigh this eventual complication. At the same time and along the same lines, vaccination against herpes zoster is recommended for those over 50 years of age.”
Another fact revealed by the study is that these reactions were not significantly more severe in people with dermatologic diseases, those with previous infection, or those with skin manifestation associated with COVID-19.
Dr. Galván highlighted that, except for the COVID-19 arm, these patterns were among those associated with the disease, “which supports [the idea] that it does not demonstrate that the host’s immune reaction to the infection was playing a role.”
Women and young people
“As for pseudoperniosis, it is poorly represented in our series: 0.7% compared to 2% in the American registry. Although neither the SARS-CoV-2–pseudoperniosis association nor its pathophysiology is clear, the idea is that if this manifestation is related to the host’s immune response during infection, pseudoperniosis after vaccination could also be linked to the immune response to the vaccine,” said Dr. Galván.
Many of these reactions are more intense in women. “Before starting to use these vaccines, we already knew that messenger RNA vaccines (a powerful activator of innate immunity) induce frequent reactions, that adjuvants and excipients (polyethylene glycol and polysorbate) also generate them, and that other factors influence reactogenicity, among those of us of the same age and sex, reactions being more frequent in younger people and in women,” said Dr. Galván. “This may be one of the reasons why the COVID-19 arm is so much more prevalent in the female population and that 80% of all reactions that were collected were in women.”
In relation to the fact that manifestations differed, depending on the type of inoculated serum, Dr. Galván said, “Some reactions are just as common after any of the vaccines. However, others are not, as is the case with the COVID-19 arm for the Moderna vaccine or reactivations of the herpes virus, more frequent after the Pfizer/BioNTech vaccine.
“Undoubtedly, behind these differences are particularities in the immune reaction caused by each of the vaccines and their composition, including the excipients,” she said.
Regarding the fact that these reactions were the same throughout the vaccine regimen or that they varied in intensity, depending on the dose, Dr. Galván said, “In our study, as in those carried out by other groups, there were no significant differences in terms of frequency after the first and second doses. One thing to keep in mind is that, due to the temporary design of our study and the time at which it was conducted, it was not possible to collect reactions after second doses of AstraZeneca.
“Manifestations have generally been mild and well controlled. Many of them did not recur after the second dose, and the vast majority did not prevent completion of the vaccination scheme, but we must not lose sight of the fact that 20% of these manifestations were assessed by the dermatologist as serious or very serious,” Dr. Galván added.
Regarding the next steps planned for this line of research, Dr. Galván commented, “We are awaiting the evolution of the reported cases and the reactions that may arise, although for now, our group does not have any open studies. The most important thing now is to be alert and report the data observed in the pharmacovigilance systems, in open registries, and in scientific literature to generate evidence.”
Dr. Galván has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Skin manifestations of COVID-19 were among the topics presented in several sessions at the 49th Congress of the Spanish Academy of Dermatology and Venereology. Specialists agreed that fewer skin changes associated with this virus have been seen with the latest variants of SARS-CoV-2. They highlighted the results of the most remarkable research on this topic that were presented in this forum.
In the study, which was carried out by Spanish dermatologists with the support of the AEDV, researchers analyzed skin reactions associated with the COVID-19 vaccine.
Study author Cristina Galván, MD, a dermatologist at the University Hospital of Móstoles, Madrid, said, of the dermatological manifestations caused as a reaction to these vaccines.”
The study was carried out during the first months of COVID-19 vaccination, Dr. Galván told this news organization. It was proposed as a continuation of a COVID skin study that was published in the British Journal of Dermatology. That study documented the first classification of skin lesions associated with COVID-19. Dr. Galván is the lead author of the latter study.
“The objectives of this study were to characterize and classify skin reactions after vaccination, identify their chronology, and analyze the associations with a series of antecedents: dermatological and allergic diseases, previous SARS-CoV-2 infection, and skin reactions associated with COVID-19,” said Dr. Galván. The study was a team effort, she added.
“It was conducted between Feb. 15 and May 12, 2021, and information was gathered on 405 reactions that appeared during the 21 days after any dose of the COVID-19 vaccines approved at that time in Spain: the Pfizer/BioNTech, Moderna, and University of Oxford/AstraZeneca vaccines,” she added.
Dr. Galván explained that the study shows very clear patterns and investigators reached conclusions that match those of other groups that have investigated this topic. “Six reaction patterns were described according to their frequency. The first is the ‘COVID-19 arm,’ which consists of a local reaction at the injection site and occurs almost exclusively in women and in 70% of cases after inoculation with the Moderna serum. It is a manifestation that resolves well and does not always recur in subsequent doses. More than half are of delayed onset: biopsied patients show signs of a delayed hypersensitivity reaction. In line with all the publications in this regard, it was found that this reaction is not a reason to skip or delay a dose.”
Herpes zoster reactivation
The second pattern is urticarial, which, according to the specialist, occurs with equal frequency after the administration of all vaccines and is well controlled with antihistamines. “This is a very nonspecific pattern, which does not prevent it from still being frequent. It was not associated with drug intake.
“The morbilliform pattern is more frequent after the Pfizer/BioNTech and AstraZeneca vaccines. It affects the trunk and extremities, and up to a quarter of the cases required systemic corticosteroids. The papulovesicular and pityriasis rosea–like patterns are equally frequent in all vaccines. The latter is found in a younger age group. Finally, there is the purpuric pattern, more localized in the extremities and more frequent after the Pfizer/BioNTech and AstraZeneca vaccines. On biopsy, this pattern showed small-vessel vasculitis.”
Less frequently, reactivations or de novo onset of different dermatologic diseases were found. “Varicella-zoster virus reactivations were observed with a frequency of 13.8%, being more common after the Pfizer/BioNTech vaccine,” said Dr. Galván. “Other studies have corroborated this increase in herpes zoster, although it has been seen that the absolute number is low, so the benefits of the vaccine outweigh this eventual complication. At the same time and along the same lines, vaccination against herpes zoster is recommended for those over 50 years of age.”
Another fact revealed by the study is that these reactions were not significantly more severe in people with dermatologic diseases, those with previous infection, or those with skin manifestation associated with COVID-19.
Dr. Galván highlighted that, except for the COVID-19 arm, these patterns were among those associated with the disease, “which supports [the idea] that it does not demonstrate that the host’s immune reaction to the infection was playing a role.”
Women and young people
“As for pseudoperniosis, it is poorly represented in our series: 0.7% compared to 2% in the American registry. Although neither the SARS-CoV-2–pseudoperniosis association nor its pathophysiology is clear, the idea is that if this manifestation is related to the host’s immune response during infection, pseudoperniosis after vaccination could also be linked to the immune response to the vaccine,” said Dr. Galván.
Many of these reactions are more intense in women. “Before starting to use these vaccines, we already knew that messenger RNA vaccines (a powerful activator of innate immunity) induce frequent reactions, that adjuvants and excipients (polyethylene glycol and polysorbate) also generate them, and that other factors influence reactogenicity, among those of us of the same age and sex, reactions being more frequent in younger people and in women,” said Dr. Galván. “This may be one of the reasons why the COVID-19 arm is so much more prevalent in the female population and that 80% of all reactions that were collected were in women.”
In relation to the fact that manifestations differed, depending on the type of inoculated serum, Dr. Galván said, “Some reactions are just as common after any of the vaccines. However, others are not, as is the case with the COVID-19 arm for the Moderna vaccine or reactivations of the herpes virus, more frequent after the Pfizer/BioNTech vaccine.
“Undoubtedly, behind these differences are particularities in the immune reaction caused by each of the vaccines and their composition, including the excipients,” she said.
Regarding the fact that these reactions were the same throughout the vaccine regimen or that they varied in intensity, depending on the dose, Dr. Galván said, “In our study, as in those carried out by other groups, there were no significant differences in terms of frequency after the first and second doses. One thing to keep in mind is that, due to the temporary design of our study and the time at which it was conducted, it was not possible to collect reactions after second doses of AstraZeneca.
“Manifestations have generally been mild and well controlled. Many of them did not recur after the second dose, and the vast majority did not prevent completion of the vaccination scheme, but we must not lose sight of the fact that 20% of these manifestations were assessed by the dermatologist as serious or very serious,” Dr. Galván added.
Regarding the next steps planned for this line of research, Dr. Galván commented, “We are awaiting the evolution of the reported cases and the reactions that may arise, although for now, our group does not have any open studies. The most important thing now is to be alert and report the data observed in the pharmacovigilance systems, in open registries, and in scientific literature to generate evidence.”
Dr. Galván has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Skin manifestations of COVID-19 were among the topics presented in several sessions at the 49th Congress of the Spanish Academy of Dermatology and Venereology. Specialists agreed that fewer skin changes associated with this virus have been seen with the latest variants of SARS-CoV-2. They highlighted the results of the most remarkable research on this topic that were presented in this forum.
In the study, which was carried out by Spanish dermatologists with the support of the AEDV, researchers analyzed skin reactions associated with the COVID-19 vaccine.
Study author Cristina Galván, MD, a dermatologist at the University Hospital of Móstoles, Madrid, said, of the dermatological manifestations caused as a reaction to these vaccines.”
The study was carried out during the first months of COVID-19 vaccination, Dr. Galván told this news organization. It was proposed as a continuation of a COVID skin study that was published in the British Journal of Dermatology. That study documented the first classification of skin lesions associated with COVID-19. Dr. Galván is the lead author of the latter study.
“The objectives of this study were to characterize and classify skin reactions after vaccination, identify their chronology, and analyze the associations with a series of antecedents: dermatological and allergic diseases, previous SARS-CoV-2 infection, and skin reactions associated with COVID-19,” said Dr. Galván. The study was a team effort, she added.
“It was conducted between Feb. 15 and May 12, 2021, and information was gathered on 405 reactions that appeared during the 21 days after any dose of the COVID-19 vaccines approved at that time in Spain: the Pfizer/BioNTech, Moderna, and University of Oxford/AstraZeneca vaccines,” she added.
Dr. Galván explained that the study shows very clear patterns and investigators reached conclusions that match those of other groups that have investigated this topic. “Six reaction patterns were described according to their frequency. The first is the ‘COVID-19 arm,’ which consists of a local reaction at the injection site and occurs almost exclusively in women and in 70% of cases after inoculation with the Moderna serum. It is a manifestation that resolves well and does not always recur in subsequent doses. More than half are of delayed onset: biopsied patients show signs of a delayed hypersensitivity reaction. In line with all the publications in this regard, it was found that this reaction is not a reason to skip or delay a dose.”
Herpes zoster reactivation
The second pattern is urticarial, which, according to the specialist, occurs with equal frequency after the administration of all vaccines and is well controlled with antihistamines. “This is a very nonspecific pattern, which does not prevent it from still being frequent. It was not associated with drug intake.
“The morbilliform pattern is more frequent after the Pfizer/BioNTech and AstraZeneca vaccines. It affects the trunk and extremities, and up to a quarter of the cases required systemic corticosteroids. The papulovesicular and pityriasis rosea–like patterns are equally frequent in all vaccines. The latter is found in a younger age group. Finally, there is the purpuric pattern, more localized in the extremities and more frequent after the Pfizer/BioNTech and AstraZeneca vaccines. On biopsy, this pattern showed small-vessel vasculitis.”
Less frequently, reactivations or de novo onset of different dermatologic diseases were found. “Varicella-zoster virus reactivations were observed with a frequency of 13.8%, being more common after the Pfizer/BioNTech vaccine,” said Dr. Galván. “Other studies have corroborated this increase in herpes zoster, although it has been seen that the absolute number is low, so the benefits of the vaccine outweigh this eventual complication. At the same time and along the same lines, vaccination against herpes zoster is recommended for those over 50 years of age.”
Another fact revealed by the study is that these reactions were not significantly more severe in people with dermatologic diseases, those with previous infection, or those with skin manifestation associated with COVID-19.
Dr. Galván highlighted that, except for the COVID-19 arm, these patterns were among those associated with the disease, “which supports [the idea] that it does not demonstrate that the host’s immune reaction to the infection was playing a role.”
Women and young people
“As for pseudoperniosis, it is poorly represented in our series: 0.7% compared to 2% in the American registry. Although neither the SARS-CoV-2–pseudoperniosis association nor its pathophysiology is clear, the idea is that if this manifestation is related to the host’s immune response during infection, pseudoperniosis after vaccination could also be linked to the immune response to the vaccine,” said Dr. Galván.
Many of these reactions are more intense in women. “Before starting to use these vaccines, we already knew that messenger RNA vaccines (a powerful activator of innate immunity) induce frequent reactions, that adjuvants and excipients (polyethylene glycol and polysorbate) also generate them, and that other factors influence reactogenicity, among those of us of the same age and sex, reactions being more frequent in younger people and in women,” said Dr. Galván. “This may be one of the reasons why the COVID-19 arm is so much more prevalent in the female population and that 80% of all reactions that were collected were in women.”
In relation to the fact that manifestations differed, depending on the type of inoculated serum, Dr. Galván said, “Some reactions are just as common after any of the vaccines. However, others are not, as is the case with the COVID-19 arm for the Moderna vaccine or reactivations of the herpes virus, more frequent after the Pfizer/BioNTech vaccine.
“Undoubtedly, behind these differences are particularities in the immune reaction caused by each of the vaccines and their composition, including the excipients,” she said.
Regarding the fact that these reactions were the same throughout the vaccine regimen or that they varied in intensity, depending on the dose, Dr. Galván said, “In our study, as in those carried out by other groups, there were no significant differences in terms of frequency after the first and second doses. One thing to keep in mind is that, due to the temporary design of our study and the time at which it was conducted, it was not possible to collect reactions after second doses of AstraZeneca.
“Manifestations have generally been mild and well controlled. Many of them did not recur after the second dose, and the vast majority did not prevent completion of the vaccination scheme, but we must not lose sight of the fact that 20% of these manifestations were assessed by the dermatologist as serious or very serious,” Dr. Galván added.
Regarding the next steps planned for this line of research, Dr. Galván commented, “We are awaiting the evolution of the reported cases and the reactions that may arise, although for now, our group does not have any open studies. The most important thing now is to be alert and report the data observed in the pharmacovigilance systems, in open registries, and in scientific literature to generate evidence.”
Dr. Galván has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monkeypox mutating faster than expected
The monkeypox virus is evolving 6-12 times faster than would be expected, according to a new study.
The virus is thought to have a single origin, the genetic data suggests, and is a likely descendant of the strain involved in the 2017-2018 monkeypox outbreak in Nigeria. It’s not clear if these mutations have aided the transmissibility of the virus among people or have any other clinical implications, João Paulo Gomes, PhD, from Portugal’s National Institute of Health, Lisbon, said in an email.
Since the monkeypox outbreak began in May, nearly 7,000 cases of monkeypox have been reported across 52 countries and territories.
Orthopoxviruses – the genus to which monkeypox belongs – are large DNA viruses that usually only gain one or two mutations every year. (For comparison, SARS-CoV-2 gains around two mutations every month.) One would expect 5 to 10 mutations in the 2022 monkeypox virus, compared with the 2017 strain, Dr. Gomes said.
In the study, Dr. Gomes and colleagues analyzed 15 monkeypox DNA sequences made available by Portugal and the National Center for Biotechnology Information, Bethesda, Md., between May 20 and May 27, 2022. The analysis revealed that this most recent strain differed by 50 single-nucleotide polymorphisms, compared with previous strains of the virus in 2017-2018.
“This is far beyond what we would expect, specifically for orthopoxvirus,” Andrew Lover, PhD, an epidemiologist at the University of Massachusetts Amherst School of Public Health & Health Sciences, told this news organization. He was not involved with the research. “That suggests [the virus] is trying to figure out the best way to deal with a new host species,” he added.
Rodents are thought to be the natural hosts of the monkeypox virus, he explained, and, in 2022, the infection transferred to humans. “Moving into a new species can ‘turbocharge’ mutations as the virus adapts to a new biological environment,” he explained, though it is not clear if the new mutations Dr. Gomes’s team detected help the 2022 virus spread more easily among people.
Researchers also found that the 2022 virus belonged in clade 3 of the virus, which is part of the less-lethal West-African clade. While the West-African clade has a fatality rate of less than 1%, the Central African clade has a fatality rate of over 10%.
The rapid changes in the viral genome could be driven by a family of proteins thought to play a role in antiviral immunity: apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3 (APOBEC3). These enzymes can make changes to a viral genome, Dr. Gomes explained, “but sometimes the system is not ‘well regulated,’ and the changes in the genome are not detrimental to the virus.” These APOBEC3-driven mutations have a signature pattern, he said, which was also detected in most of the 50 new mutations Dr. Gomes’s team identified.
However, it is not known if these mutations have clinical implications, Dr. Lover said.
The 2022 monkeypox virus does appear to behave differently than previous strains of the virus, he noted. In the current outbreak, sexual transmission appears to be very common, which is not the case for previous outbreaks, he said. Also, while monkeypox traditionally presents with a rash that can spread to all parts of the body, there have been several instances of patients presenting with just a few “very innocuous lesions,” he added.
Dr. Gomes hopes that specialized lab groups will now be able to tease out whether there is a connection between these identified mutations and changes in the behavior of the virus, including transmissibility.
While none of the findings in this analysis raises any serious concerns, the study “suggests there [are] definitely gaps in our knowledge about monkeypox,” Dr. Lover said. As for the global health response, he said, “We probably should err on the side of caution. ... There are clearly things that we absolutely don’t understand here, in terms of how quickly mutations are popping up.”
Dr. Gomes and Dr. Lover report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The monkeypox virus is evolving 6-12 times faster than would be expected, according to a new study.
The virus is thought to have a single origin, the genetic data suggests, and is a likely descendant of the strain involved in the 2017-2018 monkeypox outbreak in Nigeria. It’s not clear if these mutations have aided the transmissibility of the virus among people or have any other clinical implications, João Paulo Gomes, PhD, from Portugal’s National Institute of Health, Lisbon, said in an email.
Since the monkeypox outbreak began in May, nearly 7,000 cases of monkeypox have been reported across 52 countries and territories.
Orthopoxviruses – the genus to which monkeypox belongs – are large DNA viruses that usually only gain one or two mutations every year. (For comparison, SARS-CoV-2 gains around two mutations every month.) One would expect 5 to 10 mutations in the 2022 monkeypox virus, compared with the 2017 strain, Dr. Gomes said.
In the study, Dr. Gomes and colleagues analyzed 15 monkeypox DNA sequences made available by Portugal and the National Center for Biotechnology Information, Bethesda, Md., between May 20 and May 27, 2022. The analysis revealed that this most recent strain differed by 50 single-nucleotide polymorphisms, compared with previous strains of the virus in 2017-2018.
“This is far beyond what we would expect, specifically for orthopoxvirus,” Andrew Lover, PhD, an epidemiologist at the University of Massachusetts Amherst School of Public Health & Health Sciences, told this news organization. He was not involved with the research. “That suggests [the virus] is trying to figure out the best way to deal with a new host species,” he added.
Rodents are thought to be the natural hosts of the monkeypox virus, he explained, and, in 2022, the infection transferred to humans. “Moving into a new species can ‘turbocharge’ mutations as the virus adapts to a new biological environment,” he explained, though it is not clear if the new mutations Dr. Gomes’s team detected help the 2022 virus spread more easily among people.
Researchers also found that the 2022 virus belonged in clade 3 of the virus, which is part of the less-lethal West-African clade. While the West-African clade has a fatality rate of less than 1%, the Central African clade has a fatality rate of over 10%.
The rapid changes in the viral genome could be driven by a family of proteins thought to play a role in antiviral immunity: apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3 (APOBEC3). These enzymes can make changes to a viral genome, Dr. Gomes explained, “but sometimes the system is not ‘well regulated,’ and the changes in the genome are not detrimental to the virus.” These APOBEC3-driven mutations have a signature pattern, he said, which was also detected in most of the 50 new mutations Dr. Gomes’s team identified.
However, it is not known if these mutations have clinical implications, Dr. Lover said.
The 2022 monkeypox virus does appear to behave differently than previous strains of the virus, he noted. In the current outbreak, sexual transmission appears to be very common, which is not the case for previous outbreaks, he said. Also, while monkeypox traditionally presents with a rash that can spread to all parts of the body, there have been several instances of patients presenting with just a few “very innocuous lesions,” he added.
Dr. Gomes hopes that specialized lab groups will now be able to tease out whether there is a connection between these identified mutations and changes in the behavior of the virus, including transmissibility.
While none of the findings in this analysis raises any serious concerns, the study “suggests there [are] definitely gaps in our knowledge about monkeypox,” Dr. Lover said. As for the global health response, he said, “We probably should err on the side of caution. ... There are clearly things that we absolutely don’t understand here, in terms of how quickly mutations are popping up.”
Dr. Gomes and Dr. Lover report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The monkeypox virus is evolving 6-12 times faster than would be expected, according to a new study.
The virus is thought to have a single origin, the genetic data suggests, and is a likely descendant of the strain involved in the 2017-2018 monkeypox outbreak in Nigeria. It’s not clear if these mutations have aided the transmissibility of the virus among people or have any other clinical implications, João Paulo Gomes, PhD, from Portugal’s National Institute of Health, Lisbon, said in an email.
Since the monkeypox outbreak began in May, nearly 7,000 cases of monkeypox have been reported across 52 countries and territories.
Orthopoxviruses – the genus to which monkeypox belongs – are large DNA viruses that usually only gain one or two mutations every year. (For comparison, SARS-CoV-2 gains around two mutations every month.) One would expect 5 to 10 mutations in the 2022 monkeypox virus, compared with the 2017 strain, Dr. Gomes said.
In the study, Dr. Gomes and colleagues analyzed 15 monkeypox DNA sequences made available by Portugal and the National Center for Biotechnology Information, Bethesda, Md., between May 20 and May 27, 2022. The analysis revealed that this most recent strain differed by 50 single-nucleotide polymorphisms, compared with previous strains of the virus in 2017-2018.
“This is far beyond what we would expect, specifically for orthopoxvirus,” Andrew Lover, PhD, an epidemiologist at the University of Massachusetts Amherst School of Public Health & Health Sciences, told this news organization. He was not involved with the research. “That suggests [the virus] is trying to figure out the best way to deal with a new host species,” he added.
Rodents are thought to be the natural hosts of the monkeypox virus, he explained, and, in 2022, the infection transferred to humans. “Moving into a new species can ‘turbocharge’ mutations as the virus adapts to a new biological environment,” he explained, though it is not clear if the new mutations Dr. Gomes’s team detected help the 2022 virus spread more easily among people.
Researchers also found that the 2022 virus belonged in clade 3 of the virus, which is part of the less-lethal West-African clade. While the West-African clade has a fatality rate of less than 1%, the Central African clade has a fatality rate of over 10%.
The rapid changes in the viral genome could be driven by a family of proteins thought to play a role in antiviral immunity: apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3 (APOBEC3). These enzymes can make changes to a viral genome, Dr. Gomes explained, “but sometimes the system is not ‘well regulated,’ and the changes in the genome are not detrimental to the virus.” These APOBEC3-driven mutations have a signature pattern, he said, which was also detected in most of the 50 new mutations Dr. Gomes’s team identified.
However, it is not known if these mutations have clinical implications, Dr. Lover said.
The 2022 monkeypox virus does appear to behave differently than previous strains of the virus, he noted. In the current outbreak, sexual transmission appears to be very common, which is not the case for previous outbreaks, he said. Also, while monkeypox traditionally presents with a rash that can spread to all parts of the body, there have been several instances of patients presenting with just a few “very innocuous lesions,” he added.
Dr. Gomes hopes that specialized lab groups will now be able to tease out whether there is a connection between these identified mutations and changes in the behavior of the virus, including transmissibility.
While none of the findings in this analysis raises any serious concerns, the study “suggests there [are] definitely gaps in our knowledge about monkeypox,” Dr. Lover said. As for the global health response, he said, “We probably should err on the side of caution. ... There are clearly things that we absolutely don’t understand here, in terms of how quickly mutations are popping up.”
Dr. Gomes and Dr. Lover report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Acute Generalized Exanthematous Pustulosis Induced by the Second-Generation Antipsychotic Cariprazine
To the Editor:
A 57-year-old woman presented to an outpatient clinic with severe pruritus and burning of the skin as well as subjective fevers and chills. She had been discharged from a psychiatric hospital for attempted suicide 1 day prior. There were no recent changes in the medication regimen, which consisted of linaclotide, fluoxetine, lorazepam, and gabapentin. While admitted, the patient was started on the atypical antipsychotic cariprazine. Within 24 hours of the first dose, she developed severe facial erythema that progressed to diffuse erythema over more than 60% of the body surface area. The attending psychiatrist promptly discontinued cariprazine. During the next 24 hours, there were no reports of fever, leukocytosis, or signs of systemic organ involvement. Given the patient’s mental and medical stability, she was discharged with instructions to follow up with the outpatient dermatology clinic.
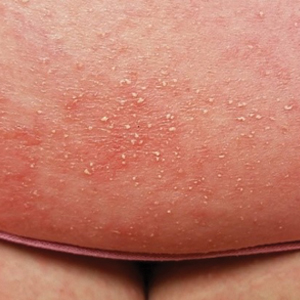
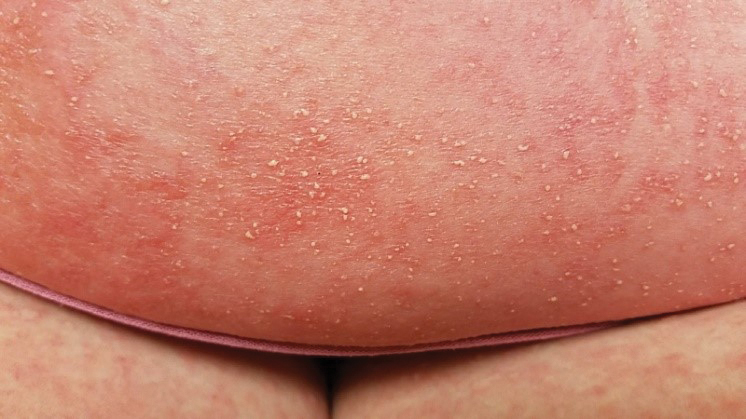
At the current presentation, physical examination revealed innumerable 1- to 4-mm pustules coalescing to lakes of pus on an erythematous base over more than 60% of the body surface area (Figure 1). The mucous membranes were clear of lesions, the Nikolsky sign was negative, and the patient’s temperature was 99.6 °F in the office. Complete blood cell count and complete metabolic panel results were within reference range.
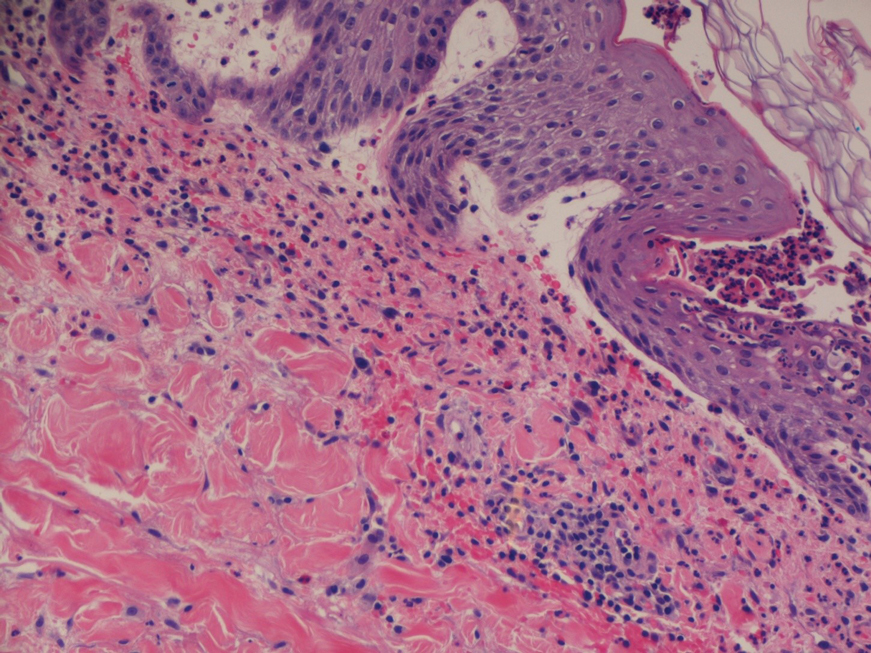
A 4-mm abdominal punch biopsy showed subcorneal neutrophilic pustules, papillary dermal edema, and superficial dermal lymphohistiocytic inflammation with numerous neutrophils, eosinophils, and extravasated red blood cells, consistent with acute generalized exanthematous pustulosis (AGEP)(Figure 2). The patient was started on wet wraps with triamcinolone cream 0.1%.
Two days later, physical examination revealed the erythema noted on initial examination had notably decreased, and the patient no longer reported burning or pruritus. One week after initial presentation to the clinic, the patient’s rash had resolved, and only a few small areas of desquamation remained.
Acute generalized exanthematous pustulosis is a severe cutaneous adverse reaction characterized by the development of numerous nonfollicular sterile pustules on an edematous and erythematous base. In almost 90% of reported cases, the cause is related to use of antibiotics, antifungals, antimalarials, or diltiazem (a calcium channel blocker). This rare cutaneous reaction occurs in 1 to 5 patients per million per year1; it carries a 1% to 2% mortality rate with proper supportive treatment.
The clinical symptoms of AGEP typically present 24 to 48 hours after drug initiation with the rapid development of dozens to thousands of 1- to 4-mm pustules, typically localized to the flexor surfaces and face. In the setting of AGEP, acute onset of fever and leukocytosis typically occur at the time of the cutaneous eruption. These features were absent in this patient. The eruption usually starts on the face and then migrates to the trunk and extremities, sparing the palms and soles. Systemic involvement most commonly presents as hepatic, renal, or pulmonary insufficiency, which has been seen in 20% of cases.2
The immunologic response associated with the reaction has been studied in vitro. Drug-specific CD8 T cells use perforin/granzyme B and Fas ligand mechanisms to induce apoptosis of the keratinocytes within the epidermis, leading to vesicle formation.3 During the very first stages of formation, vesicles mainly comprise CD8 T cells and keratinocytes. These cells then begin producing CXC-18, a potent neutrophil chemokine, leading to extensive chemotaxis of neutrophils into vesicles, which then rapidly transform to pustules.3 This rapid transformation leads to the lakes of pustules, a description often associated with AGEP.
Treatment of AGEP is mainly supportive and consists of discontinuing use of the causative agent. Topical corticosteroids can be used during the pustular phase for symptom management. There is no evidence that systemic steroids reduce the duration of the disease.2 Other supportive measures such as application of wet wraps can be used to provide comfort.
Cutaneous adverse drug reactions commonly are associated with psychiatric pharmacotherapy, but first-and second-generation antipsychotics rarely are associated with these types of reactions. In this patient, the causative agent of the AGEP was cariprazine, an atypical antipsychotic that had no reported association with AGEP or cutaneous adverse drug reactions prior to this presentation.
- Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol. 2012;53:87-92.
- Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:1214.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848.
To the Editor:
A 57-year-old woman presented to an outpatient clinic with severe pruritus and burning of the skin as well as subjective fevers and chills. She had been discharged from a psychiatric hospital for attempted suicide 1 day prior. There were no recent changes in the medication regimen, which consisted of linaclotide, fluoxetine, lorazepam, and gabapentin. While admitted, the patient was started on the atypical antipsychotic cariprazine. Within 24 hours of the first dose, she developed severe facial erythema that progressed to diffuse erythema over more than 60% of the body surface area. The attending psychiatrist promptly discontinued cariprazine. During the next 24 hours, there were no reports of fever, leukocytosis, or signs of systemic organ involvement. Given the patient’s mental and medical stability, she was discharged with instructions to follow up with the outpatient dermatology clinic.
At the current presentation, physical examination revealed innumerable 1- to 4-mm pustules coalescing to lakes of pus on an erythematous base over more than 60% of the body surface area (Figure 1). The mucous membranes were clear of lesions, the Nikolsky sign was negative, and the patient’s temperature was 99.6 °F in the office. Complete blood cell count and complete metabolic panel results were within reference range.

A 4-mm abdominal punch biopsy showed subcorneal neutrophilic pustules, papillary dermal edema, and superficial dermal lymphohistiocytic inflammation with numerous neutrophils, eosinophils, and extravasated red blood cells, consistent with acute generalized exanthematous pustulosis (AGEP)(Figure 2). The patient was started on wet wraps with triamcinolone cream 0.1%.

Two days later, physical examination revealed the erythema noted on initial examination had notably decreased, and the patient no longer reported burning or pruritus. One week after initial presentation to the clinic, the patient’s rash had resolved, and only a few small areas of desquamation remained.
Acute generalized exanthematous pustulosis is a severe cutaneous adverse reaction characterized by the development of numerous nonfollicular sterile pustules on an edematous and erythematous base. In almost 90% of reported cases, the cause is related to use of antibiotics, antifungals, antimalarials, or diltiazem (a calcium channel blocker). This rare cutaneous reaction occurs in 1 to 5 patients per million per year1; it carries a 1% to 2% mortality rate with proper supportive treatment.
The clinical symptoms of AGEP typically present 24 to 48 hours after drug initiation with the rapid development of dozens to thousands of 1- to 4-mm pustules, typically localized to the flexor surfaces and face. In the setting of AGEP, acute onset of fever and leukocytosis typically occur at the time of the cutaneous eruption. These features were absent in this patient. The eruption usually starts on the face and then migrates to the trunk and extremities, sparing the palms and soles. Systemic involvement most commonly presents as hepatic, renal, or pulmonary insufficiency, which has been seen in 20% of cases.2
The immunologic response associated with the reaction has been studied in vitro. Drug-specific CD8 T cells use perforin/granzyme B and Fas ligand mechanisms to induce apoptosis of the keratinocytes within the epidermis, leading to vesicle formation.3 During the very first stages of formation, vesicles mainly comprise CD8 T cells and keratinocytes. These cells then begin producing CXC-18, a potent neutrophil chemokine, leading to extensive chemotaxis of neutrophils into vesicles, which then rapidly transform to pustules.3 This rapid transformation leads to the lakes of pustules, a description often associated with AGEP.
Treatment of AGEP is mainly supportive and consists of discontinuing use of the causative agent. Topical corticosteroids can be used during the pustular phase for symptom management. There is no evidence that systemic steroids reduce the duration of the disease.2 Other supportive measures such as application of wet wraps can be used to provide comfort.
Cutaneous adverse drug reactions commonly are associated with psychiatric pharmacotherapy, but first-and second-generation antipsychotics rarely are associated with these types of reactions. In this patient, the causative agent of the AGEP was cariprazine, an atypical antipsychotic that had no reported association with AGEP or cutaneous adverse drug reactions prior to this presentation.
To the Editor:
A 57-year-old woman presented to an outpatient clinic with severe pruritus and burning of the skin as well as subjective fevers and chills. She had been discharged from a psychiatric hospital for attempted suicide 1 day prior. There were no recent changes in the medication regimen, which consisted of linaclotide, fluoxetine, lorazepam, and gabapentin. While admitted, the patient was started on the atypical antipsychotic cariprazine. Within 24 hours of the first dose, she developed severe facial erythema that progressed to diffuse erythema over more than 60% of the body surface area. The attending psychiatrist promptly discontinued cariprazine. During the next 24 hours, there were no reports of fever, leukocytosis, or signs of systemic organ involvement. Given the patient’s mental and medical stability, she was discharged with instructions to follow up with the outpatient dermatology clinic.
At the current presentation, physical examination revealed innumerable 1- to 4-mm pustules coalescing to lakes of pus on an erythematous base over more than 60% of the body surface area (Figure 1). The mucous membranes were clear of lesions, the Nikolsky sign was negative, and the patient’s temperature was 99.6 °F in the office. Complete blood cell count and complete metabolic panel results were within reference range.

A 4-mm abdominal punch biopsy showed subcorneal neutrophilic pustules, papillary dermal edema, and superficial dermal lymphohistiocytic inflammation with numerous neutrophils, eosinophils, and extravasated red blood cells, consistent with acute generalized exanthematous pustulosis (AGEP)(Figure 2). The patient was started on wet wraps with triamcinolone cream 0.1%.

Two days later, physical examination revealed the erythema noted on initial examination had notably decreased, and the patient no longer reported burning or pruritus. One week after initial presentation to the clinic, the patient’s rash had resolved, and only a few small areas of desquamation remained.
Acute generalized exanthematous pustulosis is a severe cutaneous adverse reaction characterized by the development of numerous nonfollicular sterile pustules on an edematous and erythematous base. In almost 90% of reported cases, the cause is related to use of antibiotics, antifungals, antimalarials, or diltiazem (a calcium channel blocker). This rare cutaneous reaction occurs in 1 to 5 patients per million per year1; it carries a 1% to 2% mortality rate with proper supportive treatment.
The clinical symptoms of AGEP typically present 24 to 48 hours after drug initiation with the rapid development of dozens to thousands of 1- to 4-mm pustules, typically localized to the flexor surfaces and face. In the setting of AGEP, acute onset of fever and leukocytosis typically occur at the time of the cutaneous eruption. These features were absent in this patient. The eruption usually starts on the face and then migrates to the trunk and extremities, sparing the palms and soles. Systemic involvement most commonly presents as hepatic, renal, or pulmonary insufficiency, which has been seen in 20% of cases.2
The immunologic response associated with the reaction has been studied in vitro. Drug-specific CD8 T cells use perforin/granzyme B and Fas ligand mechanisms to induce apoptosis of the keratinocytes within the epidermis, leading to vesicle formation.3 During the very first stages of formation, vesicles mainly comprise CD8 T cells and keratinocytes. These cells then begin producing CXC-18, a potent neutrophil chemokine, leading to extensive chemotaxis of neutrophils into vesicles, which then rapidly transform to pustules.3 This rapid transformation leads to the lakes of pustules, a description often associated with AGEP.
Treatment of AGEP is mainly supportive and consists of discontinuing use of the causative agent. Topical corticosteroids can be used during the pustular phase for symptom management. There is no evidence that systemic steroids reduce the duration of the disease.2 Other supportive measures such as application of wet wraps can be used to provide comfort.
Cutaneous adverse drug reactions commonly are associated with psychiatric pharmacotherapy, but first-and second-generation antipsychotics rarely are associated with these types of reactions. In this patient, the causative agent of the AGEP was cariprazine, an atypical antipsychotic that had no reported association with AGEP or cutaneous adverse drug reactions prior to this presentation.
- Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol. 2012;53:87-92.
- Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:1214.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848.
- Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol. 2012;53:87-92.
- Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:1214.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848.
Practice Points
- The second-generation antipsychotic cariprazine has been shown to be a potential causative agent in acute generalized exanthematous pustulosis (AGEP).
- Treatment of AGEP is mainly supportive and consists of discontinuation of the causative agent as well as symptom control using cold compresses and topical corticosteroids.
Nevus Lipomatosis Deemed Suspicious by Airport Security
To the Editor:
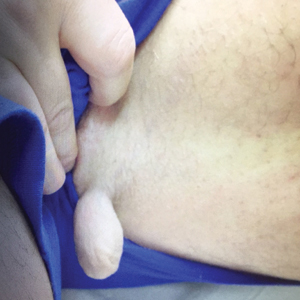
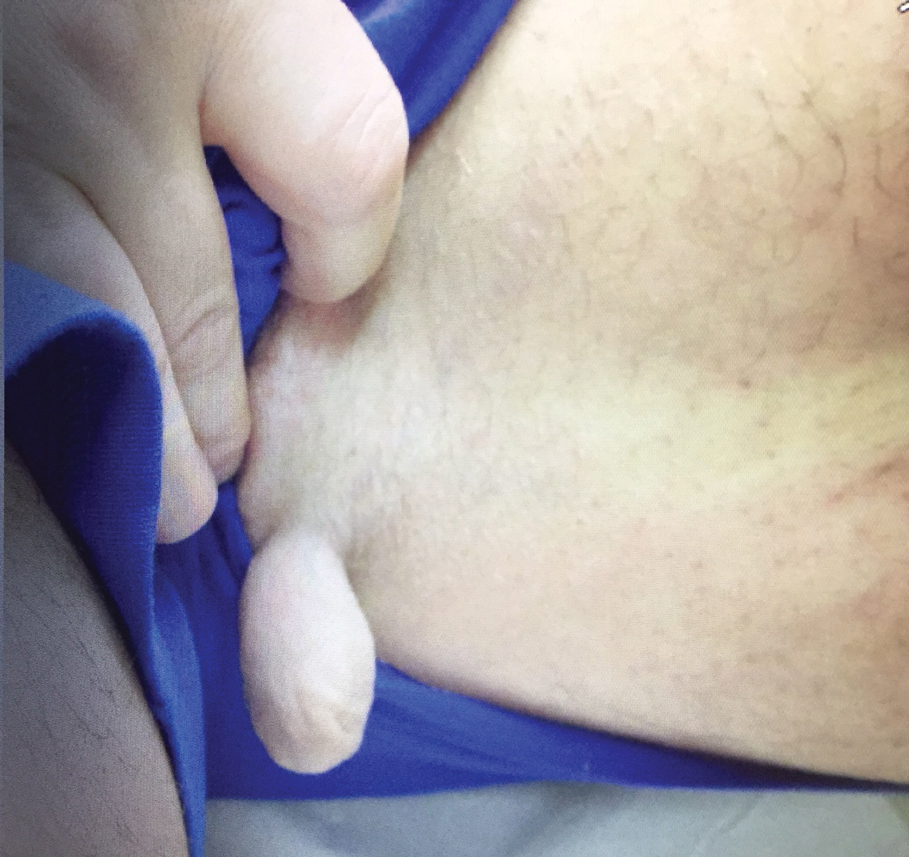
A 47-year-old man presented at the dermatology clinic with a growing lesion on the left medial thigh.
Physical examination revealed a 5-cm, pedunculated, fatty nodule on the left medial thigh that was clinically consistent with nevus lipomatosis (NL)(Figure). Although benign, trouble traveling through airport security prompted the patient to request shave removal, which subsequently was performed. Histology showed a large pedunculated nodule with prominent adipose tissue, consistent with NL. At 3-month follow-up, the patient reported getting through airport security multiple times without incident.
Nevus lipomatosis is a benign fatty lesion most commonly found on the medial thighs or trunk of adults. The lesion usually is asymptomatic but can become irritated by rubbing or catching on clothing. Our patient had symptomatic NL that caused delays getting through airport security; he experienced full resolution after simple shave removal. In rare instances, both benign and malignant skin conditions have been seen on airport scanning devices since the introduction of increased security measures following September 11, 2001. In 2016, Heymann1 reported a man with a 1.5-cm epidermal inclusion cyst detected by airport security scanners, prompting the traveler to request and carry a medically explanatory letter used to get through security. In 2015 Mayer and Adams2 described a case of nodular melanoma that was detected 20 times over a period of 2 months by airport scanners, and in 2016, Caine et al3 reported a case of desmoplastic melanoma that was detected by airport security, but after its removal was not identified by security for the next 40 flights. Noncutaneous pathology also can be detected by airport scanners. In 2013, Naraynsingh et al4 reported a man with a large left reducible inguinal hernia who was stopped by airport security and subjected to an invasive physical examination of the area. These instances demonstrate the breadth of conditions that can be cumbersome when individuals are traveling by airplane in our current security climate.
Our patient had to go through the trouble of having the benign NL lesion removed to avoid the hassle of repeatedly being stopped by airport security. The patient had the lesion removed and is doing well, but the procedure could have been avoided if systems existed to help patients with dermatologic and medical conditions at airport security. Our patient likely will never be stopped again for the suspicious lump on the left inner thigh, but many others will be stopped for similar reasons.
- Heymann WR. A cyst misinterpreted on airport scan as security threat. JAMA Dermatol. 2016;152:1388. doi:10.1001/jamadermatol.2016.3329
- Mayer JE, Adams BB. Nodular melanoma serendipitously detected by airport full body scanners. Dermatology. 2015;230:16-17. doi:10.1159/000368045
- Caine P, Javed MU, Karoo ROS. A desmoplastic melanoma detected by an airport security scanner. J Plast Reconstr Aesthet Surg. 2016;69:874-876. doi:10.1016/j.bjps.2016.02.022
- Naraynsingh V, Cawich SO, Maharaj R, et al. Inguinal hernia and airport scanners: an emerging indication for repair? 2013;2013:952835. Case Rep Med. doi:10.1155/2013/952835
To the Editor:
A 47-year-old man presented at the dermatology clinic with a growing lesion on the left medial thigh.
Physical examination revealed a 5-cm, pedunculated, fatty nodule on the left medial thigh that was clinically consistent with nevus lipomatosis (NL)(Figure). Although benign, trouble traveling through airport security prompted the patient to request shave removal, which subsequently was performed. Histology showed a large pedunculated nodule with prominent adipose tissue, consistent with NL. At 3-month follow-up, the patient reported getting through airport security multiple times without incident.

Nevus lipomatosis is a benign fatty lesion most commonly found on the medial thighs or trunk of adults. The lesion usually is asymptomatic but can become irritated by rubbing or catching on clothing. Our patient had symptomatic NL that caused delays getting through airport security; he experienced full resolution after simple shave removal. In rare instances, both benign and malignant skin conditions have been seen on airport scanning devices since the introduction of increased security measures following September 11, 2001. In 2016, Heymann1 reported a man with a 1.5-cm epidermal inclusion cyst detected by airport security scanners, prompting the traveler to request and carry a medically explanatory letter used to get through security. In 2015 Mayer and Adams2 described a case of nodular melanoma that was detected 20 times over a period of 2 months by airport scanners, and in 2016, Caine et al3 reported a case of desmoplastic melanoma that was detected by airport security, but after its removal was not identified by security for the next 40 flights. Noncutaneous pathology also can be detected by airport scanners. In 2013, Naraynsingh et al4 reported a man with a large left reducible inguinal hernia who was stopped by airport security and subjected to an invasive physical examination of the area. These instances demonstrate the breadth of conditions that can be cumbersome when individuals are traveling by airplane in our current security climate.
Our patient had to go through the trouble of having the benign NL lesion removed to avoid the hassle of repeatedly being stopped by airport security. The patient had the lesion removed and is doing well, but the procedure could have been avoided if systems existed to help patients with dermatologic and medical conditions at airport security. Our patient likely will never be stopped again for the suspicious lump on the left inner thigh, but many others will be stopped for similar reasons.
To the Editor:
A 47-year-old man presented at the dermatology clinic with a growing lesion on the left medial thigh.
Physical examination revealed a 5-cm, pedunculated, fatty nodule on the left medial thigh that was clinically consistent with nevus lipomatosis (NL)(Figure). Although benign, trouble traveling through airport security prompted the patient to request shave removal, which subsequently was performed. Histology showed a large pedunculated nodule with prominent adipose tissue, consistent with NL. At 3-month follow-up, the patient reported getting through airport security multiple times without incident.

Nevus lipomatosis is a benign fatty lesion most commonly found on the medial thighs or trunk of adults. The lesion usually is asymptomatic but can become irritated by rubbing or catching on clothing. Our patient had symptomatic NL that caused delays getting through airport security; he experienced full resolution after simple shave removal. In rare instances, both benign and malignant skin conditions have been seen on airport scanning devices since the introduction of increased security measures following September 11, 2001. In 2016, Heymann1 reported a man with a 1.5-cm epidermal inclusion cyst detected by airport security scanners, prompting the traveler to request and carry a medically explanatory letter used to get through security. In 2015 Mayer and Adams2 described a case of nodular melanoma that was detected 20 times over a period of 2 months by airport scanners, and in 2016, Caine et al3 reported a case of desmoplastic melanoma that was detected by airport security, but after its removal was not identified by security for the next 40 flights. Noncutaneous pathology also can be detected by airport scanners. In 2013, Naraynsingh et al4 reported a man with a large left reducible inguinal hernia who was stopped by airport security and subjected to an invasive physical examination of the area. These instances demonstrate the breadth of conditions that can be cumbersome when individuals are traveling by airplane in our current security climate.
Our patient had to go through the trouble of having the benign NL lesion removed to avoid the hassle of repeatedly being stopped by airport security. The patient had the lesion removed and is doing well, but the procedure could have been avoided if systems existed to help patients with dermatologic and medical conditions at airport security. Our patient likely will never be stopped again for the suspicious lump on the left inner thigh, but many others will be stopped for similar reasons.
- Heymann WR. A cyst misinterpreted on airport scan as security threat. JAMA Dermatol. 2016;152:1388. doi:10.1001/jamadermatol.2016.3329
- Mayer JE, Adams BB. Nodular melanoma serendipitously detected by airport full body scanners. Dermatology. 2015;230:16-17. doi:10.1159/000368045
- Caine P, Javed MU, Karoo ROS. A desmoplastic melanoma detected by an airport security scanner. J Plast Reconstr Aesthet Surg. 2016;69:874-876. doi:10.1016/j.bjps.2016.02.022
- Naraynsingh V, Cawich SO, Maharaj R, et al. Inguinal hernia and airport scanners: an emerging indication for repair? 2013;2013:952835. Case Rep Med. doi:10.1155/2013/952835
- Heymann WR. A cyst misinterpreted on airport scan as security threat. JAMA Dermatol. 2016;152:1388. doi:10.1001/jamadermatol.2016.3329
- Mayer JE, Adams BB. Nodular melanoma serendipitously detected by airport full body scanners. Dermatology. 2015;230:16-17. doi:10.1159/000368045
- Caine P, Javed MU, Karoo ROS. A desmoplastic melanoma detected by an airport security scanner. J Plast Reconstr Aesthet Surg. 2016;69:874-876. doi:10.1016/j.bjps.2016.02.022
- Naraynsingh V, Cawich SO, Maharaj R, et al. Inguinal hernia and airport scanners: an emerging indication for repair? 2013;2013:952835. Case Rep Med. doi:10.1155/2013/952835
Practice Points
- Nevus lipomatosis is a benign fatty lesion that most commonly is found on the medial thighs or trunk of adults.
- Both benign and malignant skin conditions have been detected on airport scanning devices.
- At times, patients must go through the hassle of having the benign lesions removed to avoid repeated problems at airport security.
Is a single dose of HPV vaccine enough?
In an April press release, the Strategic Advisory Group of Experts on Immunization (SAGE) of the World Health Organization (WHO) reported the findings of their review concerning the efficacy of various dose schedules for human papillomavirus (HPV). “A single-dose HPV vaccine delivers solid protection against HPV, the virus that causes cervical cancer, that is comparable to 2-dose schedules,” according to SAGE.
This statement comes on the heels of an article published in the November 2021 issue of Lancet Oncology about a study in India. It found that a single dose of the vaccine provides protection against persistent infection from HPV 16 and 18 similar to that provided by two or three doses.
Will this new information lead French authorities to change their recommendations? What do French specialists think? At the 45th Congress of the French Society for Colposcopy and Cervical and Vaginal Diseases (SFCPCV), Geoffroy Canlorbe, MD, PhD, of the department of gynecologic and breast surgery and oncology, Pitié-Salpêtrière Hospital, Paris, shared his thoughts.
With respect to the Indian study, Dr. Canlorbe pointed out that while its findings would need “to be confirmed by other studies,” they were, nonetheless,
India and France
During the congress press conference, he went on to say that, at this stage, the findings “cannot be extrapolated” to France. This is because the country’s situation is different. HPV vaccination coverage is low; estimates put it at 23.7%, placing the country 28th out of 31 in Europe.
“This poor coverage has nothing to do with health care–related logistical or organizational issues; instead, it has to do with people’s mistrust when it comes to vaccination. Here, people who get the first dose get the subsequent ones,” said Dr. Canlorbe. “The very fact of getting two to three doses allows the person’s body to increase the production of antibodies and get a longer-lasting response to the vaccine.”
In addition, he drew attention to several limitations of the Indian study. Initially, the team had planned to enroll 20,000 participants. In the end, there were around 17,000, and these were allocated to three cohorts: single-dose, two-dose, and three-dose. Furthermore, the primary objective, which had initially been focused on precancerous and cancerous lesions, was revised. The new aim was to compare vaccine efficacy of single dose to that of three and two doses in protecting against persistent HPV 16 and 18 infection at 10 years postvaccination. In about 90% of cases, the HPV infection went away spontaneously in 2 years without inducing lesions. Finally, the participants were women in India; therefore, the results cannot necessarily be generalized to the French population.
“This information has to be confirmed. However, as far as I know, there are no new studies going on at the moment. The Indian study, on the other hand, is still in progress,” said Dr. Canlorbe.
“In France, I think that for the time being we should stick to the studies that are currently available, which have demonstrated the efficacy and safety of two or three doses,” he concluded. In support of this approach, he cited a study on the effects of the national HPV vaccination program in England; there, the vaccination coverage is 80%.
This program was associated with a 95% risk reduction for precancerous lesions and an 87% reduction in the number of cancers, confirming the good results already achieved by Sweden and Australia.
In his comments on the WHO’s stance (which differs from that of the French experts), Jean-Luc Mergui, MD, gynecologist in the department of colposcopy and hysteroscopy at Pitié-Salpêtrière, and former president of the SFCPCV, offered an eloquent comparison: “The WHO also recommends 6 months of breastfeeding as a method of contraception, but this isn’t what’s recommended in France, for the risk of getting pregnant nevertheless remains.”
Indian study highlights
Partha Basu, MD, PhD, of the International Agency for Research on Cancer (IARC) in Lyon, France, and colleagues compared vaccine efficacy of a single dose of Gardasil (HPV 9-valent vaccine, recombinant) to that of two and three doses in protecting against persistent HPV 16 and HPV 18 infection at 10 years postvaccination.
According to the protocol, the plan was to recruit 20,000 unmarried girls, aged 10-18 years, from across India. Recruitment was initiated in September 2009. However, in response to seven unexplained deaths reported in another ongoing HPV vaccination demonstration program in the country, the Indian government issued a notification in April 2010 to stop further recruitment and HPV vaccination in all clinical trials. At this point, Dr. Basu and his team had recruited 17,729 eligible girls.
After suspension of recruitment and vaccination, their randomized trial was converted to a longitudinal, prospective, cohort study by default.
Vaccinated participants were followed up over a median duration of 9 years. In all, 4,348 participants had three doses, 4,980 had two doses (at 0 and 6 months), and 4,949 had a single dose. Cervical specimens were collected from participants 18 months after marriage or 6 months after first childbirth, whichever was earlier, to assess incident and persistent HPV infections. Participants were invited to an annual cervical cancer screening once they reached age 25 years and were married.
A single dose of HPV vaccine provides similar protection against persistent infection from HPV 16 and HPV 18, the genotypes responsible for nearly 70% of cervical cancers, compared with that provided by two or three doses. Vaccine efficacy against persistent HPV 16 and 18 infection among participants evaluable for the endpoint was 95.4% (95% confidence interval [CI], 85.0-99.9) in the single-dose default cohort (2,135 women assessed), 93.1% (95% CI, 77.3-99.8) in the two-dose cohort (1,452 women assessed), and 93.3% (95% CI, 77.5-99.7) in three-dose recipients (1,460 women assessed).
Dr. Canlorbe reported no relevant financial relationships regarding the content of this article.
This article was translated from the Medscape French edition. An English version appeared on Medscape.com.
In an April press release, the Strategic Advisory Group of Experts on Immunization (SAGE) of the World Health Organization (WHO) reported the findings of their review concerning the efficacy of various dose schedules for human papillomavirus (HPV). “A single-dose HPV vaccine delivers solid protection against HPV, the virus that causes cervical cancer, that is comparable to 2-dose schedules,” according to SAGE.
This statement comes on the heels of an article published in the November 2021 issue of Lancet Oncology about a study in India. It found that a single dose of the vaccine provides protection against persistent infection from HPV 16 and 18 similar to that provided by two or three doses.
Will this new information lead French authorities to change their recommendations? What do French specialists think? At the 45th Congress of the French Society for Colposcopy and Cervical and Vaginal Diseases (SFCPCV), Geoffroy Canlorbe, MD, PhD, of the department of gynecologic and breast surgery and oncology, Pitié-Salpêtrière Hospital, Paris, shared his thoughts.
With respect to the Indian study, Dr. Canlorbe pointed out that while its findings would need “to be confirmed by other studies,” they were, nonetheless,
India and France
During the congress press conference, he went on to say that, at this stage, the findings “cannot be extrapolated” to France. This is because the country’s situation is different. HPV vaccination coverage is low; estimates put it at 23.7%, placing the country 28th out of 31 in Europe.
“This poor coverage has nothing to do with health care–related logistical or organizational issues; instead, it has to do with people’s mistrust when it comes to vaccination. Here, people who get the first dose get the subsequent ones,” said Dr. Canlorbe. “The very fact of getting two to three doses allows the person’s body to increase the production of antibodies and get a longer-lasting response to the vaccine.”
In addition, he drew attention to several limitations of the Indian study. Initially, the team had planned to enroll 20,000 participants. In the end, there were around 17,000, and these were allocated to three cohorts: single-dose, two-dose, and three-dose. Furthermore, the primary objective, which had initially been focused on precancerous and cancerous lesions, was revised. The new aim was to compare vaccine efficacy of single dose to that of three and two doses in protecting against persistent HPV 16 and 18 infection at 10 years postvaccination. In about 90% of cases, the HPV infection went away spontaneously in 2 years without inducing lesions. Finally, the participants were women in India; therefore, the results cannot necessarily be generalized to the French population.
“This information has to be confirmed. However, as far as I know, there are no new studies going on at the moment. The Indian study, on the other hand, is still in progress,” said Dr. Canlorbe.
“In France, I think that for the time being we should stick to the studies that are currently available, which have demonstrated the efficacy and safety of two or three doses,” he concluded. In support of this approach, he cited a study on the effects of the national HPV vaccination program in England; there, the vaccination coverage is 80%.
This program was associated with a 95% risk reduction for precancerous lesions and an 87% reduction in the number of cancers, confirming the good results already achieved by Sweden and Australia.
In his comments on the WHO’s stance (which differs from that of the French experts), Jean-Luc Mergui, MD, gynecologist in the department of colposcopy and hysteroscopy at Pitié-Salpêtrière, and former president of the SFCPCV, offered an eloquent comparison: “The WHO also recommends 6 months of breastfeeding as a method of contraception, but this isn’t what’s recommended in France, for the risk of getting pregnant nevertheless remains.”
Indian study highlights
Partha Basu, MD, PhD, of the International Agency for Research on Cancer (IARC) in Lyon, France, and colleagues compared vaccine efficacy of a single dose of Gardasil (HPV 9-valent vaccine, recombinant) to that of two and three doses in protecting against persistent HPV 16 and HPV 18 infection at 10 years postvaccination.
According to the protocol, the plan was to recruit 20,000 unmarried girls, aged 10-18 years, from across India. Recruitment was initiated in September 2009. However, in response to seven unexplained deaths reported in another ongoing HPV vaccination demonstration program in the country, the Indian government issued a notification in April 2010 to stop further recruitment and HPV vaccination in all clinical trials. At this point, Dr. Basu and his team had recruited 17,729 eligible girls.
After suspension of recruitment and vaccination, their randomized trial was converted to a longitudinal, prospective, cohort study by default.
Vaccinated participants were followed up over a median duration of 9 years. In all, 4,348 participants had three doses, 4,980 had two doses (at 0 and 6 months), and 4,949 had a single dose. Cervical specimens were collected from participants 18 months after marriage or 6 months after first childbirth, whichever was earlier, to assess incident and persistent HPV infections. Participants were invited to an annual cervical cancer screening once they reached age 25 years and were married.
A single dose of HPV vaccine provides similar protection against persistent infection from HPV 16 and HPV 18, the genotypes responsible for nearly 70% of cervical cancers, compared with that provided by two or three doses. Vaccine efficacy against persistent HPV 16 and 18 infection among participants evaluable for the endpoint was 95.4% (95% confidence interval [CI], 85.0-99.9) in the single-dose default cohort (2,135 women assessed), 93.1% (95% CI, 77.3-99.8) in the two-dose cohort (1,452 women assessed), and 93.3% (95% CI, 77.5-99.7) in three-dose recipients (1,460 women assessed).
Dr. Canlorbe reported no relevant financial relationships regarding the content of this article.
This article was translated from the Medscape French edition. An English version appeared on Medscape.com.
In an April press release, the Strategic Advisory Group of Experts on Immunization (SAGE) of the World Health Organization (WHO) reported the findings of their review concerning the efficacy of various dose schedules for human papillomavirus (HPV). “A single-dose HPV vaccine delivers solid protection against HPV, the virus that causes cervical cancer, that is comparable to 2-dose schedules,” according to SAGE.
This statement comes on the heels of an article published in the November 2021 issue of Lancet Oncology about a study in India. It found that a single dose of the vaccine provides protection against persistent infection from HPV 16 and 18 similar to that provided by two or three doses.
Will this new information lead French authorities to change their recommendations? What do French specialists think? At the 45th Congress of the French Society for Colposcopy and Cervical and Vaginal Diseases (SFCPCV), Geoffroy Canlorbe, MD, PhD, of the department of gynecologic and breast surgery and oncology, Pitié-Salpêtrière Hospital, Paris, shared his thoughts.
With respect to the Indian study, Dr. Canlorbe pointed out that while its findings would need “to be confirmed by other studies,” they were, nonetheless,
India and France
During the congress press conference, he went on to say that, at this stage, the findings “cannot be extrapolated” to France. This is because the country’s situation is different. HPV vaccination coverage is low; estimates put it at 23.7%, placing the country 28th out of 31 in Europe.
“This poor coverage has nothing to do with health care–related logistical or organizational issues; instead, it has to do with people’s mistrust when it comes to vaccination. Here, people who get the first dose get the subsequent ones,” said Dr. Canlorbe. “The very fact of getting two to three doses allows the person’s body to increase the production of antibodies and get a longer-lasting response to the vaccine.”
In addition, he drew attention to several limitations of the Indian study. Initially, the team had planned to enroll 20,000 participants. In the end, there were around 17,000, and these were allocated to three cohorts: single-dose, two-dose, and three-dose. Furthermore, the primary objective, which had initially been focused on precancerous and cancerous lesions, was revised. The new aim was to compare vaccine efficacy of single dose to that of three and two doses in protecting against persistent HPV 16 and 18 infection at 10 years postvaccination. In about 90% of cases, the HPV infection went away spontaneously in 2 years without inducing lesions. Finally, the participants were women in India; therefore, the results cannot necessarily be generalized to the French population.
“This information has to be confirmed. However, as far as I know, there are no new studies going on at the moment. The Indian study, on the other hand, is still in progress,” said Dr. Canlorbe.
“In France, I think that for the time being we should stick to the studies that are currently available, which have demonstrated the efficacy and safety of two or three doses,” he concluded. In support of this approach, he cited a study on the effects of the national HPV vaccination program in England; there, the vaccination coverage is 80%.
This program was associated with a 95% risk reduction for precancerous lesions and an 87% reduction in the number of cancers, confirming the good results already achieved by Sweden and Australia.
In his comments on the WHO’s stance (which differs from that of the French experts), Jean-Luc Mergui, MD, gynecologist in the department of colposcopy and hysteroscopy at Pitié-Salpêtrière, and former president of the SFCPCV, offered an eloquent comparison: “The WHO also recommends 6 months of breastfeeding as a method of contraception, but this isn’t what’s recommended in France, for the risk of getting pregnant nevertheless remains.”
Indian study highlights
Partha Basu, MD, PhD, of the International Agency for Research on Cancer (IARC) in Lyon, France, and colleagues compared vaccine efficacy of a single dose of Gardasil (HPV 9-valent vaccine, recombinant) to that of two and three doses in protecting against persistent HPV 16 and HPV 18 infection at 10 years postvaccination.
According to the protocol, the plan was to recruit 20,000 unmarried girls, aged 10-18 years, from across India. Recruitment was initiated in September 2009. However, in response to seven unexplained deaths reported in another ongoing HPV vaccination demonstration program in the country, the Indian government issued a notification in April 2010 to stop further recruitment and HPV vaccination in all clinical trials. At this point, Dr. Basu and his team had recruited 17,729 eligible girls.
After suspension of recruitment and vaccination, their randomized trial was converted to a longitudinal, prospective, cohort study by default.
Vaccinated participants were followed up over a median duration of 9 years. In all, 4,348 participants had three doses, 4,980 had two doses (at 0 and 6 months), and 4,949 had a single dose. Cervical specimens were collected from participants 18 months after marriage or 6 months after first childbirth, whichever was earlier, to assess incident and persistent HPV infections. Participants were invited to an annual cervical cancer screening once they reached age 25 years and were married.
A single dose of HPV vaccine provides similar protection against persistent infection from HPV 16 and HPV 18, the genotypes responsible for nearly 70% of cervical cancers, compared with that provided by two or three doses. Vaccine efficacy against persistent HPV 16 and 18 infection among participants evaluable for the endpoint was 95.4% (95% confidence interval [CI], 85.0-99.9) in the single-dose default cohort (2,135 women assessed), 93.1% (95% CI, 77.3-99.8) in the two-dose cohort (1,452 women assessed), and 93.3% (95% CI, 77.5-99.7) in three-dose recipients (1,460 women assessed).
Dr. Canlorbe reported no relevant financial relationships regarding the content of this article.
This article was translated from the Medscape French edition. An English version appeared on Medscape.com.
Telemedicine and Home Pregnancy Testing for iPLEDGE: A Survey of Clinician Perspectives
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).

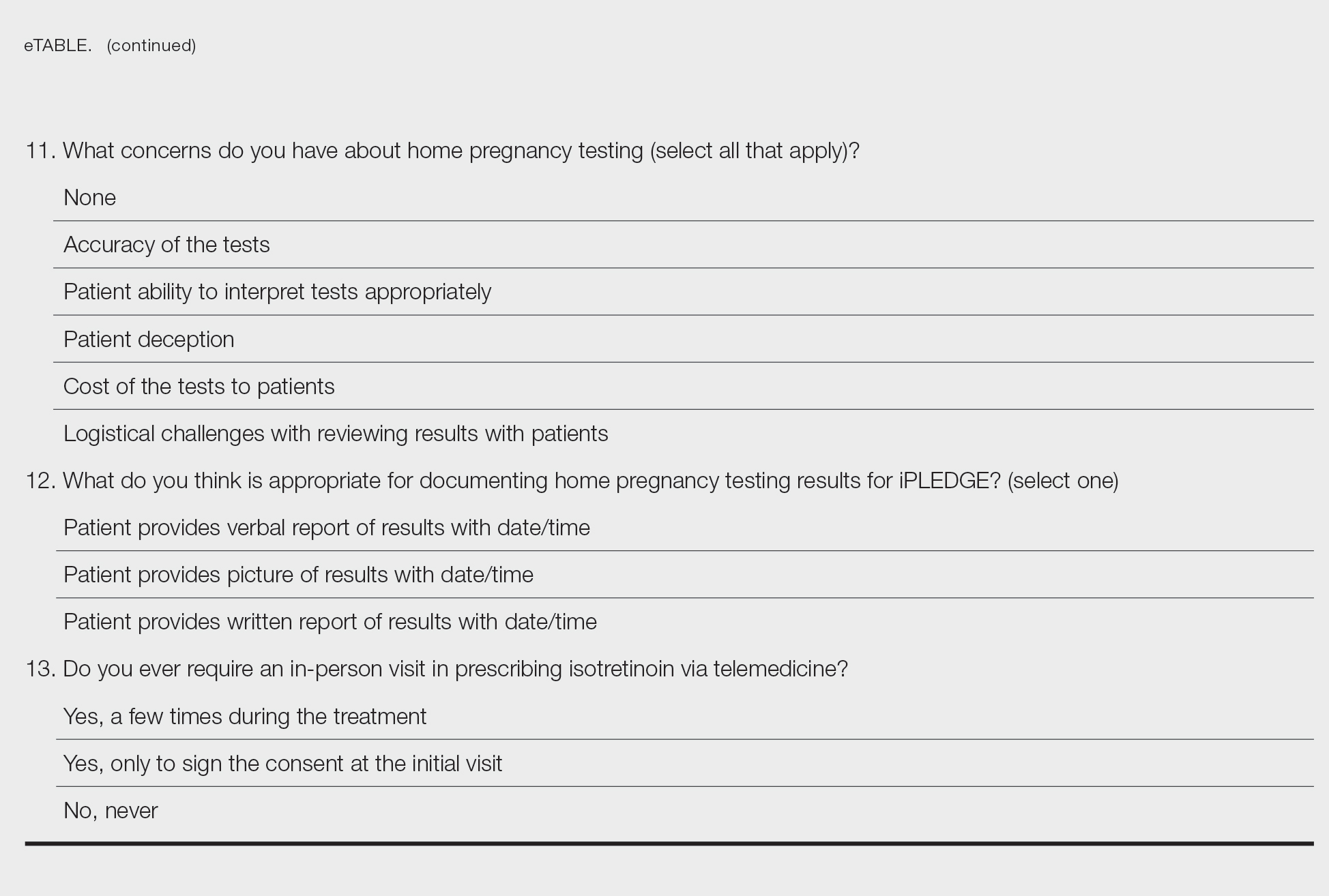
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
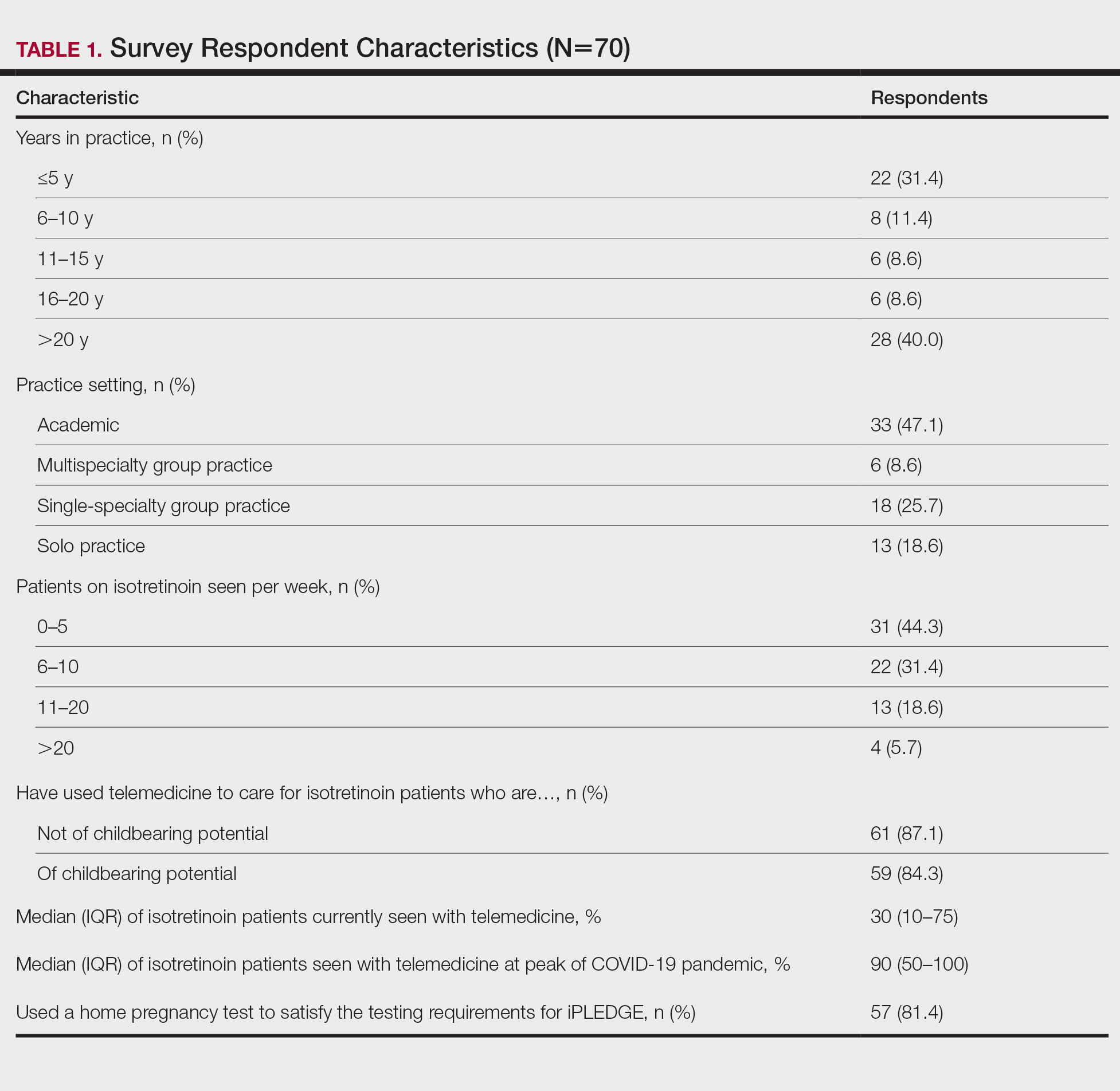
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
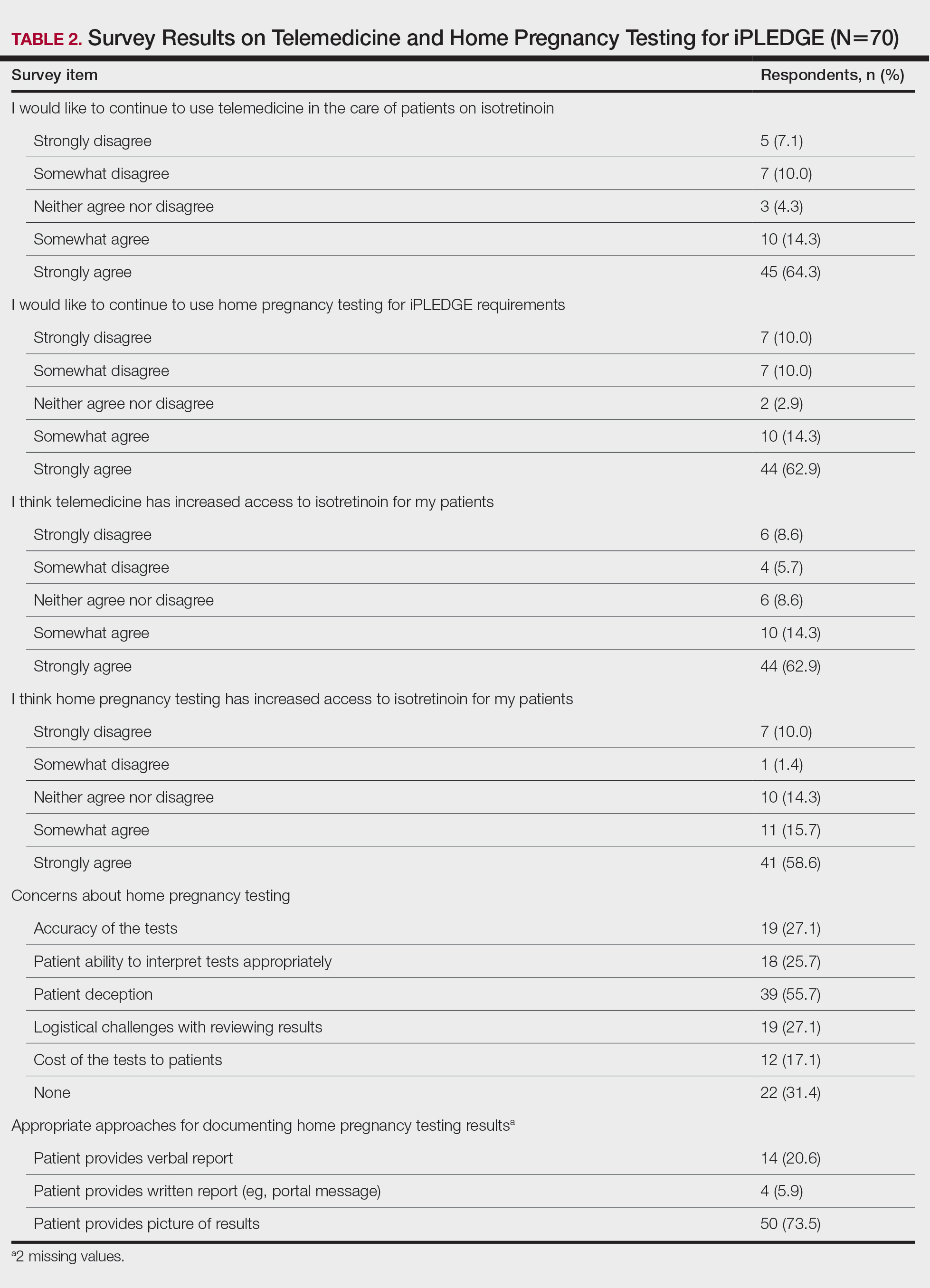
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
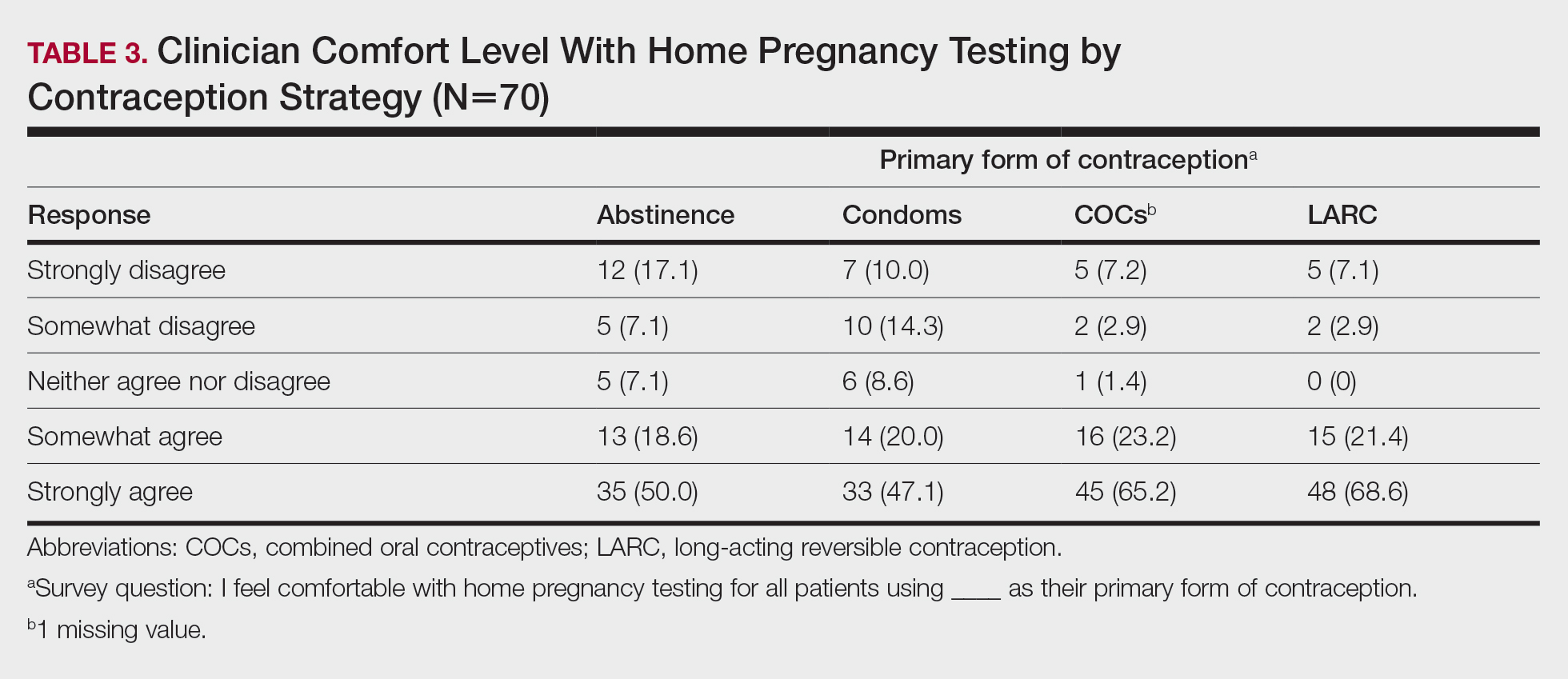
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).


Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).

The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).

In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2

Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).


Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).

The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).

In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2

Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
PRACTICE POINTS
- The majority of clinicians report that the use of telemedicine and home pregnancy testing for iPLEDGE has improved access to care and that they would like to continue these practices.
- Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care for patients treated with isotretinoin.
What’s Diet Got to Do With It? Basic and Clinical Science Behind Diet and Acne
The current understanding of the pathogenesis of acne includes altered keratinization, follicular obstruction, overproduction of sebum, and microbial colonization ( Cutibacterium acnes ) of the pilosebaceous unit resulting in perifollicular inflammation. 1 A deeper dive into the hormonal and molecular drivers of acne have implicated insulin, insulinlike growth factor 1 (IGF-1), corticotropin-releasing hormone, the phosphoinositide 3 -kinase/Akt pathway, mitogen-activated protein kinase pathway, and the nuclear factor κ B pathway. 2-4 A Western diet comprised of high glycemic index foods, carbohydrates, and dairy enhances the production of insulin and IGF-1. A downstream effect of excess insulin and IGF-1 is overactivity of the mammalian target of rapamycin complex 1 (mTORC1), a major promoter of cellular growth and proliferation that primarily is regulated through nutrient availability. 5 This article will review our understanding of the impact of the Western diet on acne pathogenesis and highlight the existing evidence behind the contributions of the mTORC1 pathway in this process. Although quality randomized controlled trials analyzing these effects are limited, dermatologists should understand the existing evidence supporting the potential impacts of diet on acne.
The Western Diet
Glycemic Index—To assess the impact of a high glycemic index diet on acne, Kwon et al6 evaluated 32 patients with mild to moderate acne and placed them on a low or high glycemic index diet for 10 weeks. The low glycemic index diet group was found to have a 70% reduction in the mean number of inflammatory acne lesions from baseline (P<.05), while the high glycemic index diet group had no significant reduction. Noninflammatory lesion counts remained statistically unchanged.6 Smith et al7 studied 43 male patients with acne on either a low glycemic index diet or a self-directed high glycemic diet that was carbohydrate dense. The low glycemic index group showed greater improvement in lesion count as well as improved insulin sensitivity at 12 weeks. Specifically, the mean lesion count (SEM) decreased by 23.5 (3.9) in the low glycemic index group and by only 12.0 (3.5) in the control group (P=.03).7 Observational studies also have supported this hypothesis. After adjustment, an analysis of 24,452 participants in the NutriNet-Santé cohort found significant associations between current acne and the consumption of sugary beverages (adjusted OR, 1.18; 95% CI, 1.01-1.38) and the consumption of fatty and sugary products (adjusted OR, 1.54; 95% CI, 1.09-2.16).8 A Cochrane review that included only 2 studies (Kwon et al6 and Smith et al7) did not find evidence to suggest a low glycemic index diet for noninflammatory lesion count reduction but did note possible benefit for a reduction in inflammatory and total lesion counts; however, Kwon et al6 had incomplete data.9
Dairy—A large retrospective study including 47,355 nurses noted the frequency of milk intake was significantly associated with increased prevalence of acne in adolescence (prevalence ratio, 1.22; 95% CI, 1.03-1.44; P=.002).10 A 2019 meta-analysis further suggested a significant relationship between acne and milk in highest vs lowest intake groups (OR, 1.48; 95% CI, 1.31-1.66) with no significant heterogeneity between the studies (I2=23.6%, P=.24 for heterogeneity), as well as a positive relationship between the highest vs lowest intake of low-fat milk (OR, 1.25; 95% CI, 1.10-1.43) and skim milk (OR, 1.82; 95% CI, 1.34-2.47). In this meta-analysis, yogurt and cheese consumption were not significantly associated with acne (OR, 0.90; 95% CI, 0.73-1.11).11 One non–evidence-based explanation for this may be that fermented dairy products have different biological actions. Pasteurized milk allows microRNAs that directly activate mTORC1 to persist, whereas the bacteria present in the fermentation process may augment this.12 A separate meta-analysis from 2018 did find that yogurt consumption was positively associated with acne (OR, 1.36; 95% CI, 1.05-1.77; P=.022), highlighting the need for larger, more rigorous studies on this topic.13
Insulin and IGF-1—As reviewed above, acne has been considered a disease of Western society, with the Western diet at the center of this association.14 A typical Western diet consists of high glycemic index foods, carbohydrates, and dairy, all of which enhance the production of insulin and IGF-1. Insulin levels increase secondary to high blood glucose and to a lesser degree by protein intake.15 Insulinlike growth factor 1 production is most influenced by age and peaks during puberty; however, high protein diets also increase liver IGF-1 production and release.16 When present in excess, insulin can function as a growth factor. Insulin exerts its anabolic effects through the IGF-1 pathway; however, insulin and IGF-1 are produced in response to different signals.17 Endocrine production of IGF-1 represents 70% of blood levels, peaks at puberty, and rapidly declines in the third decade of life.18 Insulin is produced by the pancreas, and levels correspond to lifestyle and genetically induced insulin resistance.19
Adolescents have elevated levels of IGF-1 as a major driver of puberty-associated growth.20 Despite the natural decrease in IGF-1 following puberty, acne persists in many patients and can even develop for the first time in adulthood in a subset of patients. A study of 40 acne patients and 20 controls found that patients with acne who consumed a high glycemic–load diet was significantly higher than the number of controls consuming a similar diet (P=.008). Additionally, significantly higher levels of mean (SD) serum IGF-1 on quantitative sandwich enzyme-linked immunosorbent assay in acne patients vs controls (543.2 [174.7] ng/mL vs 316.9 [95.7] ng/mL; P<.001) was identified, and these levels correlated significantly with high glycemic–load diet consumption.21 In another study, Kartal et al22 found that basal and fasting insulin levels and homeostasis model assessment scores evaluating for insulin resistance were significantly higher in 36 women compared with 24 age/sex-matched controls (P<.05). This finding remained significant even after excluding women with hyperandrogenemia (P<.05).22
Highlighting the importance of IGF-1 in the pathogenesis of acne, patients with genetic disorders characterized by IGF-1 deficiency, such as Laron syndrome, do not develop acne despite having a functional androgen receptor. Treatment with IGF-1 in these patients induces acne, further supporting the role of IGF-1 in the pathogenesis of this condition.23
The mTORC1 Pathway
Comprised of mTOR in addition to other proteins, mTORC1 is a nutrient-sensitive regulator of cellular growth, proliferation, lipid synthesis, and protein translation.5 Increased activity of mTORC1 has been described in diabetes, neurodegenerative disease, and cancer,14,24 while decreased activity may promote longevity.25 Regulation of mTORC1 occurs through several mechanisms. Growth factors such as insulin and IGF-1 promote mTORC1 activation through the PI3K/Akt pathway. Several amino acids—specifically branched chain amino acids such as alanine, arginine, asparagine, glutamine, histidine, leucine, methionine, serine, threonine, and valine—also can activate mTORC1 independently.26 Excess glucose leads to decreased adenosine monophosphate–activated protein kinase and increased activity of mTORC1, which occurs separately from insulin or IGF-1.27 Starvation blocks mTORC1 via increased adenosine monophosphate–activated protein kinase and starvation-induced hypoxia.26,28 To activate mTORC1, both the IGF-1 or insulin signal and amino acid excess must be present.29 Although not studied in acne, altering the dietary protein content in obese mice has been shown to perturb the mTORC1 pathway, leading to pathologic changes in the mTORC1-autophagy signaling axis, increased amino acid release into the blood, and an acute elevation in mTORC1 signaling.30
Another major regulator of mTORC1 is Forkhead box protein O1 (FOXO1), which is a transcription factor that regulates mTORC1 through sestrin 3.31,32 Sestrin 3 is a stress-induced protein that helps regulate blood glucose and promote insulin sensitivity.33 When FOXO1 is translocated to the cell nucleus, it upregulates the expression of sestrin 3, resulting in mTORC1 inhibition.31,32 Insulin, IGF-1, and nutrient excess lead to FOXO1 translocation to the cell cytoplasm where it can no longer mitigate mTORC1 activity, while the fasted state leads to translocation to the nucleus.34 A single study evaluated the association between FOXO1, mTORC1, a high glycemic–load diet, and acne development. Immunohistochemical detection of mTORC1 assessed by digital image analysis revealed significantly greater expression in inflamed pilosebaceous units found in acne patients (P<.001). Immunohistochemical cytoplasmic expression of FOXO1 and mTOR (used as a proxy for mTORC1) was significantly higher in patients on a high glycemic–load diet (P=.021 and P=.009, respectively) as well as in patients with more severe forms of acne (P=.005 and P=.015, respectively) and elevated IGF-1 levels (P=.004 and P=.003, respectively).21
mTORC1 contributes to the proliferation of keratinocytes and excess sebum production, both independently and through androgen-mediated processes.35-40 Insulinlike growth factor 1 binding the IGF-1 receptor leads to proliferation of keratinocytes lining the sebaceous gland and hair follicle in vivo.35 In mice with epidermis-specific deletion of mTOR, keratinocyte proliferation was decreased and hair follicles were diminished both in number and development. Genetic loss of mTOR in the epidermis led to attenuated signaling pathways of mTORC1 and mTORC2.36
Androgen function is augmented by mTORC1, FOXO1, and IGF-1 through several mechanisms, which may partially explain the hormonal relationship to acne. Androgens increase IGF-1 within the hair follicle.37 In prostate cancer cells, IGF-1 then facilitates movement of FOXO1 to the cytoplasm, preventing it from blocking mTORC1. This effective inactivation of FOXO1 thus further augments the impact of androgens by both allowing unchecked mTORC1 pathway activity and increasing translocation of the androgen receptor (AR) to the nucleus where it exerts its effects.38 Interestingly, genetic polymorphisms of the AR have been shown to cause variable affinity of FOXO1 for the AR; specifically, shorter CAG (cytosine, adenine, guanine) repeat length may lead to decreased FOXO1 binding and is associated with an increased risk for acne.41-43 In addition to its effects on the hair follicle, IGF-1 stimulates production of testosterone and dehydroepiandrosterone as well as activates 5α-reductase, leading to higher dihydrotestosterone levels, which activate the AR with higher affinity than testosterone.44 In some tissues, androgens help regulate the mTORC1 pathway through positive feedback loops.45,46 At this time, we do not know if this occurs in the pathogenesis of acne.
Isotretinoin is the treatment of choice for refractory acne. It has been hypothesized that isotretinoin induces sebocyte apoptosis via the upregulation of FOXO transcription factors and p53.47 Elevated levels of nuclear FOXO1 have been found in the sebaceous glands of patients following initiation of treatment with isotretinoin and are hypothesized to play a major role in the drug’s effectiveness. Specifically, biopsies from 14 acne patients before and after 6 weeks of isotretinoin therapy were analyzed with immunohistochemical staining and found to have a significantly improved nuclear to cytoplasmic ratio of nonphosphorylated FOXO1 (P<.001).47
Practical Recommendations
Given the available evidence, it is important for dermatologists to address dietary recommendations in acne patients. Although large randomized controlled trials on diet and acne severity are challenging to conduct in this population, the existing literature suggests that patients should avoid high glycemic index simple sugars and processed grains, and patients should focus on eating more complex carbohydrates in the form of legumes, vegetables, fruits, and tubers.6-8 With regard to dairy, milk (especially skim) has been associated with increased risks for acne.11,13 Fermented dairy products may have less impact on acne severity and include cheese, yogurt (unsweetened to keep glycemic index low), and sour cream.12
- Zaenglein AL. Acne vulgaris. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Elsevier; 2017:588-603.
- Ganceviciene R, Graziene V, Fimmel S, et al. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345-352.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Cong TX, Hao D, Wen X, et al. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311:337-349.
- Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886-1918.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Cao H, Yang G, Wang Y, et al. Complementary therapies for acne vulgaris. Cochrane Database Syst Rev. 2015;1:CD009436.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Melnik BC, Schmitz G. Pasteurized non-fermented cow’s milk but not fermented milk is a promoter of mTORC1-driven aging and increased mortality. Ageing Res Rev. 2021;67:101270.
- Juhl CR, Bergholdt HKM, Miller IM, et al. Dairy intake and acne vulgaris: a systematic review and meta-analysis of 78,529 children, adolescents, and young adults. Nutrients. 2018;10:1049. doi:10.3390/nu10081049
- Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;8:371-388.
- Smart CEM, King BR, Lopez PE. Insulin dosing for fat and protein: is it time? Diabetes Care. 2020;43:13-15.
- Wan X, Wang S, Xu J, et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation. PLoS One. 2017;12:E0173174.
- Bedinger DH, Adams SH. Metabolic, anabolic, and mitogenic insulin responses: a tissue-specific perspective for insulin receptor activators. Mol Cell Endocrinol. 2015;415:143-156.
- Gubbi S, Quipildor GF, Barzilai N, et al. 40 YEARS of IGF1: IGF1: the Jekyll and Hyde of the aging brain. J Mol Endocrinol. 2018;61:T171-T185.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Wood CL, Lane LC, Cheetham T. Puberty: normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab. 2019;33:101265.
- Agamia NF, Abdallah DM, Sorour O, et al. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br J Dermatol. 2016;174:1299-1307.
- Kartal D, Yildiz H, Ertas R, et al. Association between isolated female acne and insulin resistance: a prospective study. G Ital Dermatol Venereol. 2016;151:353-357.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191-2201.
- Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. 2018;64:127-134.
- Melick CH, Jewell JL. Regulation of mTORC1 by upstream stimuli. Genes. 2020;11:989. doi:10.3390/genes11090989
- Li M, Zhang CS, Feng JW, et al. Aldolase is a sensor for both low and high glucose, linking to AMPK and mTORC1. Cell Res. 2021;31:478-481.
- Yan T, Zhang J, Tang D, et al. Hypoxia regulates mTORC1-mediated keratinocyte motility and migration via the AMPK pathway. PLoS One. 2017;12:E0169155.
- Dennis MD, Baum JI, Kimball SR, et al. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286:8287-8296.
- Choi BSY, Daniel N, Houde VP, et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12:3377.
- Chen CC, Jeon SM, Bhaskar PT, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592-604.
- Chen Y, Huang T, Yu Z, et al. The functions and roles of sestrins in regulating human diseases. Cell Mol Biol Lett. 2022;27:2.
- Tao R, Xiong X, Liangpunsakul S, et al. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes. 2015;64:1211-1223.
- Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208-214.
- Gilhar A, Ish-Shalom S, Pillar T, et al. Effect of anti–insulin-like growth factor 1 on epidermal proliferation of human skin transplanted onto nude mice treated with growth hormone. Endocrinology. 1994;134:229-232.
- Ding X, Bloch W, Iden S, et al. mTORC1 and mTORC2 regulate skin morphogenesis and epidermal barrier formation. Nat Commun. 2016;7:13226.
- Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol. 2013;22:168-171.
- Fan W, Yanase T, Morinaga H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329-7338.
- Alestas T, Ganceviciene R, Fimmel S, et al. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med. 2006;84:75-87.
- Smith TM, Gilliland K, Clawson GA, et al. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286-1293.
- Furtado GV, Yang J, Wu D, et al. FOXO1 controls protein synthesis and transcript abundance of mutant polyglutamine proteins, preventing protein aggregation. Hum Mol Genet. 2021;30:996-1005.
- Melnik BC. Isotretinoin and FoxO1: a scientific hypothesis. Dermatoendocrinol. 2011;3:141-165.
- Heng AHS, Say YH, Sio YY, et al. Gene variants associated with acne vulgaris presentation and severity: a systematic review and meta-analysis. BMC Med Genomics. 2021;14:103.
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142-148.
- Zhao Y, Tindall DJ, Huang H. Modulation of androgen receptor by FOXA1 and FOXO1 factors in prostate cancer. Int J Biol Sci. 2014;10:614-619.
- Hamdi MM, Mutungi G. Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechanism. J Physiol. 2011;589(pt 14):3623-3640.
- Agamia NF, Hussein OM, Abdelmaksoud RE, et al. Effect of oral isotretinoin on the nucleocytoplasmic distribution of FoxO1 and FoxO3 proteins in sebaceous glands of patients with acne vulgaris. Exp Dermatol. 2018;27:1344-1351.
- Kolovou GD, Watts GF, Mikhailidis DP, et al. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profile testing: a 2019 expert panel statement, main text. Curr Vasc Pharmacol. 2019;17:498-514.
- Svoboda SA, Shields BE. Cutaneous manifestations of nutritional excess: pathophysiologic effects of hyperglycemia and hyperinsulinemia on the skin. Cutis. 2021;107:74-78.
- González-González JG, Mancillas-Adame LG, Fernández-Reyes M, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol . 2009;71:494-499.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
The current understanding of the pathogenesis of acne includes altered keratinization, follicular obstruction, overproduction of sebum, and microbial colonization ( Cutibacterium acnes ) of the pilosebaceous unit resulting in perifollicular inflammation. 1 A deeper dive into the hormonal and molecular drivers of acne have implicated insulin, insulinlike growth factor 1 (IGF-1), corticotropin-releasing hormone, the phosphoinositide 3 -kinase/Akt pathway, mitogen-activated protein kinase pathway, and the nuclear factor κ B pathway. 2-4 A Western diet comprised of high glycemic index foods, carbohydrates, and dairy enhances the production of insulin and IGF-1. A downstream effect of excess insulin and IGF-1 is overactivity of the mammalian target of rapamycin complex 1 (mTORC1), a major promoter of cellular growth and proliferation that primarily is regulated through nutrient availability. 5 This article will review our understanding of the impact of the Western diet on acne pathogenesis and highlight the existing evidence behind the contributions of the mTORC1 pathway in this process. Although quality randomized controlled trials analyzing these effects are limited, dermatologists should understand the existing evidence supporting the potential impacts of diet on acne.
The Western Diet
Glycemic Index—To assess the impact of a high glycemic index diet on acne, Kwon et al6 evaluated 32 patients with mild to moderate acne and placed them on a low or high glycemic index diet for 10 weeks. The low glycemic index diet group was found to have a 70% reduction in the mean number of inflammatory acne lesions from baseline (P<.05), while the high glycemic index diet group had no significant reduction. Noninflammatory lesion counts remained statistically unchanged.6 Smith et al7 studied 43 male patients with acne on either a low glycemic index diet or a self-directed high glycemic diet that was carbohydrate dense. The low glycemic index group showed greater improvement in lesion count as well as improved insulin sensitivity at 12 weeks. Specifically, the mean lesion count (SEM) decreased by 23.5 (3.9) in the low glycemic index group and by only 12.0 (3.5) in the control group (P=.03).7 Observational studies also have supported this hypothesis. After adjustment, an analysis of 24,452 participants in the NutriNet-Santé cohort found significant associations between current acne and the consumption of sugary beverages (adjusted OR, 1.18; 95% CI, 1.01-1.38) and the consumption of fatty and sugary products (adjusted OR, 1.54; 95% CI, 1.09-2.16).8 A Cochrane review that included only 2 studies (Kwon et al6 and Smith et al7) did not find evidence to suggest a low glycemic index diet for noninflammatory lesion count reduction but did note possible benefit for a reduction in inflammatory and total lesion counts; however, Kwon et al6 had incomplete data.9
Dairy—A large retrospective study including 47,355 nurses noted the frequency of milk intake was significantly associated with increased prevalence of acne in adolescence (prevalence ratio, 1.22; 95% CI, 1.03-1.44; P=.002).10 A 2019 meta-analysis further suggested a significant relationship between acne and milk in highest vs lowest intake groups (OR, 1.48; 95% CI, 1.31-1.66) with no significant heterogeneity between the studies (I2=23.6%, P=.24 for heterogeneity), as well as a positive relationship between the highest vs lowest intake of low-fat milk (OR, 1.25; 95% CI, 1.10-1.43) and skim milk (OR, 1.82; 95% CI, 1.34-2.47). In this meta-analysis, yogurt and cheese consumption were not significantly associated with acne (OR, 0.90; 95% CI, 0.73-1.11).11 One non–evidence-based explanation for this may be that fermented dairy products have different biological actions. Pasteurized milk allows microRNAs that directly activate mTORC1 to persist, whereas the bacteria present in the fermentation process may augment this.12 A separate meta-analysis from 2018 did find that yogurt consumption was positively associated with acne (OR, 1.36; 95% CI, 1.05-1.77; P=.022), highlighting the need for larger, more rigorous studies on this topic.13
Insulin and IGF-1—As reviewed above, acne has been considered a disease of Western society, with the Western diet at the center of this association.14 A typical Western diet consists of high glycemic index foods, carbohydrates, and dairy, all of which enhance the production of insulin and IGF-1. Insulin levels increase secondary to high blood glucose and to a lesser degree by protein intake.15 Insulinlike growth factor 1 production is most influenced by age and peaks during puberty; however, high protein diets also increase liver IGF-1 production and release.16 When present in excess, insulin can function as a growth factor. Insulin exerts its anabolic effects through the IGF-1 pathway; however, insulin and IGF-1 are produced in response to different signals.17 Endocrine production of IGF-1 represents 70% of blood levels, peaks at puberty, and rapidly declines in the third decade of life.18 Insulin is produced by the pancreas, and levels correspond to lifestyle and genetically induced insulin resistance.19
Adolescents have elevated levels of IGF-1 as a major driver of puberty-associated growth.20 Despite the natural decrease in IGF-1 following puberty, acne persists in many patients and can even develop for the first time in adulthood in a subset of patients. A study of 40 acne patients and 20 controls found that patients with acne who consumed a high glycemic–load diet was significantly higher than the number of controls consuming a similar diet (P=.008). Additionally, significantly higher levels of mean (SD) serum IGF-1 on quantitative sandwich enzyme-linked immunosorbent assay in acne patients vs controls (543.2 [174.7] ng/mL vs 316.9 [95.7] ng/mL; P<.001) was identified, and these levels correlated significantly with high glycemic–load diet consumption.21 In another study, Kartal et al22 found that basal and fasting insulin levels and homeostasis model assessment scores evaluating for insulin resistance were significantly higher in 36 women compared with 24 age/sex-matched controls (P<.05). This finding remained significant even after excluding women with hyperandrogenemia (P<.05).22
Highlighting the importance of IGF-1 in the pathogenesis of acne, patients with genetic disorders characterized by IGF-1 deficiency, such as Laron syndrome, do not develop acne despite having a functional androgen receptor. Treatment with IGF-1 in these patients induces acne, further supporting the role of IGF-1 in the pathogenesis of this condition.23
The mTORC1 Pathway
Comprised of mTOR in addition to other proteins, mTORC1 is a nutrient-sensitive regulator of cellular growth, proliferation, lipid synthesis, and protein translation.5 Increased activity of mTORC1 has been described in diabetes, neurodegenerative disease, and cancer,14,24 while decreased activity may promote longevity.25 Regulation of mTORC1 occurs through several mechanisms. Growth factors such as insulin and IGF-1 promote mTORC1 activation through the PI3K/Akt pathway. Several amino acids—specifically branched chain amino acids such as alanine, arginine, asparagine, glutamine, histidine, leucine, methionine, serine, threonine, and valine—also can activate mTORC1 independently.26 Excess glucose leads to decreased adenosine monophosphate–activated protein kinase and increased activity of mTORC1, which occurs separately from insulin or IGF-1.27 Starvation blocks mTORC1 via increased adenosine monophosphate–activated protein kinase and starvation-induced hypoxia.26,28 To activate mTORC1, both the IGF-1 or insulin signal and amino acid excess must be present.29 Although not studied in acne, altering the dietary protein content in obese mice has been shown to perturb the mTORC1 pathway, leading to pathologic changes in the mTORC1-autophagy signaling axis, increased amino acid release into the blood, and an acute elevation in mTORC1 signaling.30
Another major regulator of mTORC1 is Forkhead box protein O1 (FOXO1), which is a transcription factor that regulates mTORC1 through sestrin 3.31,32 Sestrin 3 is a stress-induced protein that helps regulate blood glucose and promote insulin sensitivity.33 When FOXO1 is translocated to the cell nucleus, it upregulates the expression of sestrin 3, resulting in mTORC1 inhibition.31,32 Insulin, IGF-1, and nutrient excess lead to FOXO1 translocation to the cell cytoplasm where it can no longer mitigate mTORC1 activity, while the fasted state leads to translocation to the nucleus.34 A single study evaluated the association between FOXO1, mTORC1, a high glycemic–load diet, and acne development. Immunohistochemical detection of mTORC1 assessed by digital image analysis revealed significantly greater expression in inflamed pilosebaceous units found in acne patients (P<.001). Immunohistochemical cytoplasmic expression of FOXO1 and mTOR (used as a proxy for mTORC1) was significantly higher in patients on a high glycemic–load diet (P=.021 and P=.009, respectively) as well as in patients with more severe forms of acne (P=.005 and P=.015, respectively) and elevated IGF-1 levels (P=.004 and P=.003, respectively).21
mTORC1 contributes to the proliferation of keratinocytes and excess sebum production, both independently and through androgen-mediated processes.35-40 Insulinlike growth factor 1 binding the IGF-1 receptor leads to proliferation of keratinocytes lining the sebaceous gland and hair follicle in vivo.35 In mice with epidermis-specific deletion of mTOR, keratinocyte proliferation was decreased and hair follicles were diminished both in number and development. Genetic loss of mTOR in the epidermis led to attenuated signaling pathways of mTORC1 and mTORC2.36
Androgen function is augmented by mTORC1, FOXO1, and IGF-1 through several mechanisms, which may partially explain the hormonal relationship to acne. Androgens increase IGF-1 within the hair follicle.37 In prostate cancer cells, IGF-1 then facilitates movement of FOXO1 to the cytoplasm, preventing it from blocking mTORC1. This effective inactivation of FOXO1 thus further augments the impact of androgens by both allowing unchecked mTORC1 pathway activity and increasing translocation of the androgen receptor (AR) to the nucleus where it exerts its effects.38 Interestingly, genetic polymorphisms of the AR have been shown to cause variable affinity of FOXO1 for the AR; specifically, shorter CAG (cytosine, adenine, guanine) repeat length may lead to decreased FOXO1 binding and is associated with an increased risk for acne.41-43 In addition to its effects on the hair follicle, IGF-1 stimulates production of testosterone and dehydroepiandrosterone as well as activates 5α-reductase, leading to higher dihydrotestosterone levels, which activate the AR with higher affinity than testosterone.44 In some tissues, androgens help regulate the mTORC1 pathway through positive feedback loops.45,46 At this time, we do not know if this occurs in the pathogenesis of acne.
Isotretinoin is the treatment of choice for refractory acne. It has been hypothesized that isotretinoin induces sebocyte apoptosis via the upregulation of FOXO transcription factors and p53.47 Elevated levels of nuclear FOXO1 have been found in the sebaceous glands of patients following initiation of treatment with isotretinoin and are hypothesized to play a major role in the drug’s effectiveness. Specifically, biopsies from 14 acne patients before and after 6 weeks of isotretinoin therapy were analyzed with immunohistochemical staining and found to have a significantly improved nuclear to cytoplasmic ratio of nonphosphorylated FOXO1 (P<.001).47
Practical Recommendations
Given the available evidence, it is important for dermatologists to address dietary recommendations in acne patients. Although large randomized controlled trials on diet and acne severity are challenging to conduct in this population, the existing literature suggests that patients should avoid high glycemic index simple sugars and processed grains, and patients should focus on eating more complex carbohydrates in the form of legumes, vegetables, fruits, and tubers.6-8 With regard to dairy, milk (especially skim) has been associated with increased risks for acne.11,13 Fermented dairy products may have less impact on acne severity and include cheese, yogurt (unsweetened to keep glycemic index low), and sour cream.12
The current understanding of the pathogenesis of acne includes altered keratinization, follicular obstruction, overproduction of sebum, and microbial colonization ( Cutibacterium acnes ) of the pilosebaceous unit resulting in perifollicular inflammation. 1 A deeper dive into the hormonal and molecular drivers of acne have implicated insulin, insulinlike growth factor 1 (IGF-1), corticotropin-releasing hormone, the phosphoinositide 3 -kinase/Akt pathway, mitogen-activated protein kinase pathway, and the nuclear factor κ B pathway. 2-4 A Western diet comprised of high glycemic index foods, carbohydrates, and dairy enhances the production of insulin and IGF-1. A downstream effect of excess insulin and IGF-1 is overactivity of the mammalian target of rapamycin complex 1 (mTORC1), a major promoter of cellular growth and proliferation that primarily is regulated through nutrient availability. 5 This article will review our understanding of the impact of the Western diet on acne pathogenesis and highlight the existing evidence behind the contributions of the mTORC1 pathway in this process. Although quality randomized controlled trials analyzing these effects are limited, dermatologists should understand the existing evidence supporting the potential impacts of diet on acne.
The Western Diet
Glycemic Index—To assess the impact of a high glycemic index diet on acne, Kwon et al6 evaluated 32 patients with mild to moderate acne and placed them on a low or high glycemic index diet for 10 weeks. The low glycemic index diet group was found to have a 70% reduction in the mean number of inflammatory acne lesions from baseline (P<.05), while the high glycemic index diet group had no significant reduction. Noninflammatory lesion counts remained statistically unchanged.6 Smith et al7 studied 43 male patients with acne on either a low glycemic index diet or a self-directed high glycemic diet that was carbohydrate dense. The low glycemic index group showed greater improvement in lesion count as well as improved insulin sensitivity at 12 weeks. Specifically, the mean lesion count (SEM) decreased by 23.5 (3.9) in the low glycemic index group and by only 12.0 (3.5) in the control group (P=.03).7 Observational studies also have supported this hypothesis. After adjustment, an analysis of 24,452 participants in the NutriNet-Santé cohort found significant associations between current acne and the consumption of sugary beverages (adjusted OR, 1.18; 95% CI, 1.01-1.38) and the consumption of fatty and sugary products (adjusted OR, 1.54; 95% CI, 1.09-2.16).8 A Cochrane review that included only 2 studies (Kwon et al6 and Smith et al7) did not find evidence to suggest a low glycemic index diet for noninflammatory lesion count reduction but did note possible benefit for a reduction in inflammatory and total lesion counts; however, Kwon et al6 had incomplete data.9
Dairy—A large retrospective study including 47,355 nurses noted the frequency of milk intake was significantly associated with increased prevalence of acne in adolescence (prevalence ratio, 1.22; 95% CI, 1.03-1.44; P=.002).10 A 2019 meta-analysis further suggested a significant relationship between acne and milk in highest vs lowest intake groups (OR, 1.48; 95% CI, 1.31-1.66) with no significant heterogeneity between the studies (I2=23.6%, P=.24 for heterogeneity), as well as a positive relationship between the highest vs lowest intake of low-fat milk (OR, 1.25; 95% CI, 1.10-1.43) and skim milk (OR, 1.82; 95% CI, 1.34-2.47). In this meta-analysis, yogurt and cheese consumption were not significantly associated with acne (OR, 0.90; 95% CI, 0.73-1.11).11 One non–evidence-based explanation for this may be that fermented dairy products have different biological actions. Pasteurized milk allows microRNAs that directly activate mTORC1 to persist, whereas the bacteria present in the fermentation process may augment this.12 A separate meta-analysis from 2018 did find that yogurt consumption was positively associated with acne (OR, 1.36; 95% CI, 1.05-1.77; P=.022), highlighting the need for larger, more rigorous studies on this topic.13
Insulin and IGF-1—As reviewed above, acne has been considered a disease of Western society, with the Western diet at the center of this association.14 A typical Western diet consists of high glycemic index foods, carbohydrates, and dairy, all of which enhance the production of insulin and IGF-1. Insulin levels increase secondary to high blood glucose and to a lesser degree by protein intake.15 Insulinlike growth factor 1 production is most influenced by age and peaks during puberty; however, high protein diets also increase liver IGF-1 production and release.16 When present in excess, insulin can function as a growth factor. Insulin exerts its anabolic effects through the IGF-1 pathway; however, insulin and IGF-1 are produced in response to different signals.17 Endocrine production of IGF-1 represents 70% of blood levels, peaks at puberty, and rapidly declines in the third decade of life.18 Insulin is produced by the pancreas, and levels correspond to lifestyle and genetically induced insulin resistance.19
Adolescents have elevated levels of IGF-1 as a major driver of puberty-associated growth.20 Despite the natural decrease in IGF-1 following puberty, acne persists in many patients and can even develop for the first time in adulthood in a subset of patients. A study of 40 acne patients and 20 controls found that patients with acne who consumed a high glycemic–load diet was significantly higher than the number of controls consuming a similar diet (P=.008). Additionally, significantly higher levels of mean (SD) serum IGF-1 on quantitative sandwich enzyme-linked immunosorbent assay in acne patients vs controls (543.2 [174.7] ng/mL vs 316.9 [95.7] ng/mL; P<.001) was identified, and these levels correlated significantly with high glycemic–load diet consumption.21 In another study, Kartal et al22 found that basal and fasting insulin levels and homeostasis model assessment scores evaluating for insulin resistance were significantly higher in 36 women compared with 24 age/sex-matched controls (P<.05). This finding remained significant even after excluding women with hyperandrogenemia (P<.05).22
Highlighting the importance of IGF-1 in the pathogenesis of acne, patients with genetic disorders characterized by IGF-1 deficiency, such as Laron syndrome, do not develop acne despite having a functional androgen receptor. Treatment with IGF-1 in these patients induces acne, further supporting the role of IGF-1 in the pathogenesis of this condition.23
The mTORC1 Pathway
Comprised of mTOR in addition to other proteins, mTORC1 is a nutrient-sensitive regulator of cellular growth, proliferation, lipid synthesis, and protein translation.5 Increased activity of mTORC1 has been described in diabetes, neurodegenerative disease, and cancer,14,24 while decreased activity may promote longevity.25 Regulation of mTORC1 occurs through several mechanisms. Growth factors such as insulin and IGF-1 promote mTORC1 activation through the PI3K/Akt pathway. Several amino acids—specifically branched chain amino acids such as alanine, arginine, asparagine, glutamine, histidine, leucine, methionine, serine, threonine, and valine—also can activate mTORC1 independently.26 Excess glucose leads to decreased adenosine monophosphate–activated protein kinase and increased activity of mTORC1, which occurs separately from insulin or IGF-1.27 Starvation blocks mTORC1 via increased adenosine monophosphate–activated protein kinase and starvation-induced hypoxia.26,28 To activate mTORC1, both the IGF-1 or insulin signal and amino acid excess must be present.29 Although not studied in acne, altering the dietary protein content in obese mice has been shown to perturb the mTORC1 pathway, leading to pathologic changes in the mTORC1-autophagy signaling axis, increased amino acid release into the blood, and an acute elevation in mTORC1 signaling.30
Another major regulator of mTORC1 is Forkhead box protein O1 (FOXO1), which is a transcription factor that regulates mTORC1 through sestrin 3.31,32 Sestrin 3 is a stress-induced protein that helps regulate blood glucose and promote insulin sensitivity.33 When FOXO1 is translocated to the cell nucleus, it upregulates the expression of sestrin 3, resulting in mTORC1 inhibition.31,32 Insulin, IGF-1, and nutrient excess lead to FOXO1 translocation to the cell cytoplasm where it can no longer mitigate mTORC1 activity, while the fasted state leads to translocation to the nucleus.34 A single study evaluated the association between FOXO1, mTORC1, a high glycemic–load diet, and acne development. Immunohistochemical detection of mTORC1 assessed by digital image analysis revealed significantly greater expression in inflamed pilosebaceous units found in acne patients (P<.001). Immunohistochemical cytoplasmic expression of FOXO1 and mTOR (used as a proxy for mTORC1) was significantly higher in patients on a high glycemic–load diet (P=.021 and P=.009, respectively) as well as in patients with more severe forms of acne (P=.005 and P=.015, respectively) and elevated IGF-1 levels (P=.004 and P=.003, respectively).21
mTORC1 contributes to the proliferation of keratinocytes and excess sebum production, both independently and through androgen-mediated processes.35-40 Insulinlike growth factor 1 binding the IGF-1 receptor leads to proliferation of keratinocytes lining the sebaceous gland and hair follicle in vivo.35 In mice with epidermis-specific deletion of mTOR, keratinocyte proliferation was decreased and hair follicles were diminished both in number and development. Genetic loss of mTOR in the epidermis led to attenuated signaling pathways of mTORC1 and mTORC2.36
Androgen function is augmented by mTORC1, FOXO1, and IGF-1 through several mechanisms, which may partially explain the hormonal relationship to acne. Androgens increase IGF-1 within the hair follicle.37 In prostate cancer cells, IGF-1 then facilitates movement of FOXO1 to the cytoplasm, preventing it from blocking mTORC1. This effective inactivation of FOXO1 thus further augments the impact of androgens by both allowing unchecked mTORC1 pathway activity and increasing translocation of the androgen receptor (AR) to the nucleus where it exerts its effects.38 Interestingly, genetic polymorphisms of the AR have been shown to cause variable affinity of FOXO1 for the AR; specifically, shorter CAG (cytosine, adenine, guanine) repeat length may lead to decreased FOXO1 binding and is associated with an increased risk for acne.41-43 In addition to its effects on the hair follicle, IGF-1 stimulates production of testosterone and dehydroepiandrosterone as well as activates 5α-reductase, leading to higher dihydrotestosterone levels, which activate the AR with higher affinity than testosterone.44 In some tissues, androgens help regulate the mTORC1 pathway through positive feedback loops.45,46 At this time, we do not know if this occurs in the pathogenesis of acne.
Isotretinoin is the treatment of choice for refractory acne. It has been hypothesized that isotretinoin induces sebocyte apoptosis via the upregulation of FOXO transcription factors and p53.47 Elevated levels of nuclear FOXO1 have been found in the sebaceous glands of patients following initiation of treatment with isotretinoin and are hypothesized to play a major role in the drug’s effectiveness. Specifically, biopsies from 14 acne patients before and after 6 weeks of isotretinoin therapy were analyzed with immunohistochemical staining and found to have a significantly improved nuclear to cytoplasmic ratio of nonphosphorylated FOXO1 (P<.001).47
Practical Recommendations
Given the available evidence, it is important for dermatologists to address dietary recommendations in acne patients. Although large randomized controlled trials on diet and acne severity are challenging to conduct in this population, the existing literature suggests that patients should avoid high glycemic index simple sugars and processed grains, and patients should focus on eating more complex carbohydrates in the form of legumes, vegetables, fruits, and tubers.6-8 With regard to dairy, milk (especially skim) has been associated with increased risks for acne.11,13 Fermented dairy products may have less impact on acne severity and include cheese, yogurt (unsweetened to keep glycemic index low), and sour cream.12
- Zaenglein AL. Acne vulgaris. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Elsevier; 2017:588-603.
- Ganceviciene R, Graziene V, Fimmel S, et al. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345-352.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Cong TX, Hao D, Wen X, et al. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311:337-349.
- Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886-1918.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Cao H, Yang G, Wang Y, et al. Complementary therapies for acne vulgaris. Cochrane Database Syst Rev. 2015;1:CD009436.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Melnik BC, Schmitz G. Pasteurized non-fermented cow’s milk but not fermented milk is a promoter of mTORC1-driven aging and increased mortality. Ageing Res Rev. 2021;67:101270.
- Juhl CR, Bergholdt HKM, Miller IM, et al. Dairy intake and acne vulgaris: a systematic review and meta-analysis of 78,529 children, adolescents, and young adults. Nutrients. 2018;10:1049. doi:10.3390/nu10081049
- Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;8:371-388.
- Smart CEM, King BR, Lopez PE. Insulin dosing for fat and protein: is it time? Diabetes Care. 2020;43:13-15.
- Wan X, Wang S, Xu J, et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation. PLoS One. 2017;12:E0173174.
- Bedinger DH, Adams SH. Metabolic, anabolic, and mitogenic insulin responses: a tissue-specific perspective for insulin receptor activators. Mol Cell Endocrinol. 2015;415:143-156.
- Gubbi S, Quipildor GF, Barzilai N, et al. 40 YEARS of IGF1: IGF1: the Jekyll and Hyde of the aging brain. J Mol Endocrinol. 2018;61:T171-T185.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Wood CL, Lane LC, Cheetham T. Puberty: normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab. 2019;33:101265.
- Agamia NF, Abdallah DM, Sorour O, et al. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br J Dermatol. 2016;174:1299-1307.
- Kartal D, Yildiz H, Ertas R, et al. Association between isolated female acne and insulin resistance: a prospective study. G Ital Dermatol Venereol. 2016;151:353-357.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191-2201.
- Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. 2018;64:127-134.
- Melick CH, Jewell JL. Regulation of mTORC1 by upstream stimuli. Genes. 2020;11:989. doi:10.3390/genes11090989
- Li M, Zhang CS, Feng JW, et al. Aldolase is a sensor for both low and high glucose, linking to AMPK and mTORC1. Cell Res. 2021;31:478-481.
- Yan T, Zhang J, Tang D, et al. Hypoxia regulates mTORC1-mediated keratinocyte motility and migration via the AMPK pathway. PLoS One. 2017;12:E0169155.
- Dennis MD, Baum JI, Kimball SR, et al. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286:8287-8296.
- Choi BSY, Daniel N, Houde VP, et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12:3377.
- Chen CC, Jeon SM, Bhaskar PT, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592-604.
- Chen Y, Huang T, Yu Z, et al. The functions and roles of sestrins in regulating human diseases. Cell Mol Biol Lett. 2022;27:2.
- Tao R, Xiong X, Liangpunsakul S, et al. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes. 2015;64:1211-1223.
- Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208-214.
- Gilhar A, Ish-Shalom S, Pillar T, et al. Effect of anti–insulin-like growth factor 1 on epidermal proliferation of human skin transplanted onto nude mice treated with growth hormone. Endocrinology. 1994;134:229-232.
- Ding X, Bloch W, Iden S, et al. mTORC1 and mTORC2 regulate skin morphogenesis and epidermal barrier formation. Nat Commun. 2016;7:13226.
- Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol. 2013;22:168-171.
- Fan W, Yanase T, Morinaga H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329-7338.
- Alestas T, Ganceviciene R, Fimmel S, et al. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med. 2006;84:75-87.
- Smith TM, Gilliland K, Clawson GA, et al. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286-1293.
- Furtado GV, Yang J, Wu D, et al. FOXO1 controls protein synthesis and transcript abundance of mutant polyglutamine proteins, preventing protein aggregation. Hum Mol Genet. 2021;30:996-1005.
- Melnik BC. Isotretinoin and FoxO1: a scientific hypothesis. Dermatoendocrinol. 2011;3:141-165.
- Heng AHS, Say YH, Sio YY, et al. Gene variants associated with acne vulgaris presentation and severity: a systematic review and meta-analysis. BMC Med Genomics. 2021;14:103.
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142-148.
- Zhao Y, Tindall DJ, Huang H. Modulation of androgen receptor by FOXA1 and FOXO1 factors in prostate cancer. Int J Biol Sci. 2014;10:614-619.
- Hamdi MM, Mutungi G. Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechanism. J Physiol. 2011;589(pt 14):3623-3640.
- Agamia NF, Hussein OM, Abdelmaksoud RE, et al. Effect of oral isotretinoin on the nucleocytoplasmic distribution of FoxO1 and FoxO3 proteins in sebaceous glands of patients with acne vulgaris. Exp Dermatol. 2018;27:1344-1351.
- Kolovou GD, Watts GF, Mikhailidis DP, et al. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profile testing: a 2019 expert panel statement, main text. Curr Vasc Pharmacol. 2019;17:498-514.
- Svoboda SA, Shields BE. Cutaneous manifestations of nutritional excess: pathophysiologic effects of hyperglycemia and hyperinsulinemia on the skin. Cutis. 2021;107:74-78.
- González-González JG, Mancillas-Adame LG, Fernández-Reyes M, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol . 2009;71:494-499.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zaenglein AL. Acne vulgaris. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Elsevier; 2017:588-603.
- Ganceviciene R, Graziene V, Fimmel S, et al. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345-352.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Cong TX, Hao D, Wen X, et al. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311:337-349.
- Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886-1918.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Cao H, Yang G, Wang Y, et al. Complementary therapies for acne vulgaris. Cochrane Database Syst Rev. 2015;1:CD009436.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Melnik BC, Schmitz G. Pasteurized non-fermented cow’s milk but not fermented milk is a promoter of mTORC1-driven aging and increased mortality. Ageing Res Rev. 2021;67:101270.
- Juhl CR, Bergholdt HKM, Miller IM, et al. Dairy intake and acne vulgaris: a systematic review and meta-analysis of 78,529 children, adolescents, and young adults. Nutrients. 2018;10:1049. doi:10.3390/nu10081049
- Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;8:371-388.
- Smart CEM, King BR, Lopez PE. Insulin dosing for fat and protein: is it time? Diabetes Care. 2020;43:13-15.
- Wan X, Wang S, Xu J, et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation. PLoS One. 2017;12:E0173174.
- Bedinger DH, Adams SH. Metabolic, anabolic, and mitogenic insulin responses: a tissue-specific perspective for insulin receptor activators. Mol Cell Endocrinol. 2015;415:143-156.
- Gubbi S, Quipildor GF, Barzilai N, et al. 40 YEARS of IGF1: IGF1: the Jekyll and Hyde of the aging brain. J Mol Endocrinol. 2018;61:T171-T185.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Wood CL, Lane LC, Cheetham T. Puberty: normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab. 2019;33:101265.
- Agamia NF, Abdallah DM, Sorour O, et al. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br J Dermatol. 2016;174:1299-1307.
- Kartal D, Yildiz H, Ertas R, et al. Association between isolated female acne and insulin resistance: a prospective study. G Ital Dermatol Venereol. 2016;151:353-357.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191-2201.
- Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. 2018;64:127-134.
- Melick CH, Jewell JL. Regulation of mTORC1 by upstream stimuli. Genes. 2020;11:989. doi:10.3390/genes11090989
- Li M, Zhang CS, Feng JW, et al. Aldolase is a sensor for both low and high glucose, linking to AMPK and mTORC1. Cell Res. 2021;31:478-481.
- Yan T, Zhang J, Tang D, et al. Hypoxia regulates mTORC1-mediated keratinocyte motility and migration via the AMPK pathway. PLoS One. 2017;12:E0169155.
- Dennis MD, Baum JI, Kimball SR, et al. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286:8287-8296.
- Choi BSY, Daniel N, Houde VP, et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12:3377.
- Chen CC, Jeon SM, Bhaskar PT, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592-604.
- Chen Y, Huang T, Yu Z, et al. The functions and roles of sestrins in regulating human diseases. Cell Mol Biol Lett. 2022;27:2.
- Tao R, Xiong X, Liangpunsakul S, et al. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes. 2015;64:1211-1223.
- Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208-214.
- Gilhar A, Ish-Shalom S, Pillar T, et al. Effect of anti–insulin-like growth factor 1 on epidermal proliferation of human skin transplanted onto nude mice treated with growth hormone. Endocrinology. 1994;134:229-232.
- Ding X, Bloch W, Iden S, et al. mTORC1 and mTORC2 regulate skin morphogenesis and epidermal barrier formation. Nat Commun. 2016;7:13226.
- Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol. 2013;22:168-171.
- Fan W, Yanase T, Morinaga H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329-7338.
- Alestas T, Ganceviciene R, Fimmel S, et al. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med. 2006;84:75-87.
- Smith TM, Gilliland K, Clawson GA, et al. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286-1293.
- Furtado GV, Yang J, Wu D, et al. FOXO1 controls protein synthesis and transcript abundance of mutant polyglutamine proteins, preventing protein aggregation. Hum Mol Genet. 2021;30:996-1005.
- Melnik BC. Isotretinoin and FoxO1: a scientific hypothesis. Dermatoendocrinol. 2011;3:141-165.
- Heng AHS, Say YH, Sio YY, et al. Gene variants associated with acne vulgaris presentation and severity: a systematic review and meta-analysis. BMC Med Genomics. 2021;14:103.
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142-148.
- Zhao Y, Tindall DJ, Huang H. Modulation of androgen receptor by FOXA1 and FOXO1 factors in prostate cancer. Int J Biol Sci. 2014;10:614-619.
- Hamdi MM, Mutungi G. Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechanism. J Physiol. 2011;589(pt 14):3623-3640.
- Agamia NF, Hussein OM, Abdelmaksoud RE, et al. Effect of oral isotretinoin on the nucleocytoplasmic distribution of FoxO1 and FoxO3 proteins in sebaceous glands of patients with acne vulgaris. Exp Dermatol. 2018;27:1344-1351.
- Kolovou GD, Watts GF, Mikhailidis DP, et al. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profile testing: a 2019 expert panel statement, main text. Curr Vasc Pharmacol. 2019;17:498-514.
- Svoboda SA, Shields BE. Cutaneous manifestations of nutritional excess: pathophysiologic effects of hyperglycemia and hyperinsulinemia on the skin. Cutis. 2021;107:74-78.
- González-González JG, Mancillas-Adame LG, Fernández-Reyes M, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol . 2009;71:494-499.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
Practice Points
- Patients are frequently interested in the role that diet plays in acne, and dermatologists should be aware of the current evidence to answer these questions effectively.
- One of the primary pathways in acne pathogenesis, mTORC1 (mammalian target of rapamycin complex 1), is partially regulated by nutrient availability, insulin, and insulinlike growth factor 1.
- Dietary recommendations for acne based on available evidence may include a low glycemic index diet and avoidance of certain dairy products.
- Insulin resistance may underlie the pathogenesis of acne in a subset of patients, and assessing insulin resistance in acne patients should be considered.
Adapting to Changes in Acne Management: Take One Step at a Time
After most dermatology residents graduate from their programs, they go out into practice and will often carry with them what they learned from their teachers, especially clinicians. Everyone else in their dermatology residency programs approaches disease management and the use of different therapies in the same way, right?
It does not take very long before these same dermatology residents realize that things are different in real-world clinical practice in many ways. Most clinicians develop a range of fairly predictable patterns in how they approach and treat common skin disorders such as acne, rosacea, psoriasis, atopic dermatitis/eczema, and seborrheic dermatitis. These patterns often include what testing is performed at baseline and at follow-up.
Recently, I have been giving thought to how clinicians—myself included—change their approaches to management of specific skin diseases over time, especially as new information and therapies emerge. Are we fast adopters, or are we slow adopters? How much evidence do we need to see before we consider adjusting our approach? Is the needle moving too fast or not fast enough?
I would like to use an example that relates to acne treatment, especially as this is one of the most common skin disorders encountered in outpatient dermatologic practice. Despite lack of US Food and Drug Administration (FDA) approval for use in acne, oral spironolactone commonly is used in females, especially adults, with acne vulgaris and has a long history as an acceptable approach in dermatology.1 Because spironolactone is a potassium-sparing diuretic, one question that commonly arises is: Do we monitor serum potassium levels at baseline and periodically during treatment with spironolactone? There has never been a definitive consensus on which approach to take. However, there has been evidence to suggest that such monitoring is not necessary in young healthy women due to a negligible risk for clinically relevant hyperkalemia.2,3
In fact, the suggestion that there is a very low risk for clinically significant hyperkalemia in healthy young women treated with spironolactone is accurate based on population-based studies. Nevertheless, the clinician is faced with confirming the patient is in fact healthy rather than assuming this is the case due to her “young” age. In addition, it is important to exclude potential drug-drug interactions that can increase the risk for hyperkalemia when coadministered with spironolactone and also to exclude an unknown underlying decrease in renal function.1 At the end of the day, I support the continued research that is being done to evaluate questions that can challenge the recycled dogma on how we manage patients, and I do not fault those who follow what they believe to be new cogent evidence. However, in the case of oral spironolactone use, I also could never fault a clinician for monitoring renal function and electrolytes including serum potassium levels in a female patient treated for acne, especially with a drug that has the known potential to cause hyperkalemia in certain clinical situations and is not FDA approved for the indication of acne (ie, the guidance that accompanies the level of investigation needed for such FDA approval is missing). The clinical judgment of the clinician who is responsible for the individual patient trumps the results from population-based studies completed thus far. Ultimately, it is the responsibility of that clinician to assure the safety of their patient in a manner that they are comfortable with.
It takes time to make changes in our approaches to patient management, and in the majority of cases, that is rightfully so. There are several potential limitations to how certain data are collected, and a reasonable verification of results over time is what tends to change behavior patterns. Ultimately, the common goal is to do what is in the best interest of our patients. No one can argue successfully against that.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Plovanich M, Weng QY, Arash Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300.
After most dermatology residents graduate from their programs, they go out into practice and will often carry with them what they learned from their teachers, especially clinicians. Everyone else in their dermatology residency programs approaches disease management and the use of different therapies in the same way, right?
It does not take very long before these same dermatology residents realize that things are different in real-world clinical practice in many ways. Most clinicians develop a range of fairly predictable patterns in how they approach and treat common skin disorders such as acne, rosacea, psoriasis, atopic dermatitis/eczema, and seborrheic dermatitis. These patterns often include what testing is performed at baseline and at follow-up.
Recently, I have been giving thought to how clinicians—myself included—change their approaches to management of specific skin diseases over time, especially as new information and therapies emerge. Are we fast adopters, or are we slow adopters? How much evidence do we need to see before we consider adjusting our approach? Is the needle moving too fast or not fast enough?
I would like to use an example that relates to acne treatment, especially as this is one of the most common skin disorders encountered in outpatient dermatologic practice. Despite lack of US Food and Drug Administration (FDA) approval for use in acne, oral spironolactone commonly is used in females, especially adults, with acne vulgaris and has a long history as an acceptable approach in dermatology.1 Because spironolactone is a potassium-sparing diuretic, one question that commonly arises is: Do we monitor serum potassium levels at baseline and periodically during treatment with spironolactone? There has never been a definitive consensus on which approach to take. However, there has been evidence to suggest that such monitoring is not necessary in young healthy women due to a negligible risk for clinically relevant hyperkalemia.2,3
In fact, the suggestion that there is a very low risk for clinically significant hyperkalemia in healthy young women treated with spironolactone is accurate based on population-based studies. Nevertheless, the clinician is faced with confirming the patient is in fact healthy rather than assuming this is the case due to her “young” age. In addition, it is important to exclude potential drug-drug interactions that can increase the risk for hyperkalemia when coadministered with spironolactone and also to exclude an unknown underlying decrease in renal function.1 At the end of the day, I support the continued research that is being done to evaluate questions that can challenge the recycled dogma on how we manage patients, and I do not fault those who follow what they believe to be new cogent evidence. However, in the case of oral spironolactone use, I also could never fault a clinician for monitoring renal function and electrolytes including serum potassium levels in a female patient treated for acne, especially with a drug that has the known potential to cause hyperkalemia in certain clinical situations and is not FDA approved for the indication of acne (ie, the guidance that accompanies the level of investigation needed for such FDA approval is missing). The clinical judgment of the clinician who is responsible for the individual patient trumps the results from population-based studies completed thus far. Ultimately, it is the responsibility of that clinician to assure the safety of their patient in a manner that they are comfortable with.
It takes time to make changes in our approaches to patient management, and in the majority of cases, that is rightfully so. There are several potential limitations to how certain data are collected, and a reasonable verification of results over time is what tends to change behavior patterns. Ultimately, the common goal is to do what is in the best interest of our patients. No one can argue successfully against that.
After most dermatology residents graduate from their programs, they go out into practice and will often carry with them what they learned from their teachers, especially clinicians. Everyone else in their dermatology residency programs approaches disease management and the use of different therapies in the same way, right?
It does not take very long before these same dermatology residents realize that things are different in real-world clinical practice in many ways. Most clinicians develop a range of fairly predictable patterns in how they approach and treat common skin disorders such as acne, rosacea, psoriasis, atopic dermatitis/eczema, and seborrheic dermatitis. These patterns often include what testing is performed at baseline and at follow-up.
Recently, I have been giving thought to how clinicians—myself included—change their approaches to management of specific skin diseases over time, especially as new information and therapies emerge. Are we fast adopters, or are we slow adopters? How much evidence do we need to see before we consider adjusting our approach? Is the needle moving too fast or not fast enough?
I would like to use an example that relates to acne treatment, especially as this is one of the most common skin disorders encountered in outpatient dermatologic practice. Despite lack of US Food and Drug Administration (FDA) approval for use in acne, oral spironolactone commonly is used in females, especially adults, with acne vulgaris and has a long history as an acceptable approach in dermatology.1 Because spironolactone is a potassium-sparing diuretic, one question that commonly arises is: Do we monitor serum potassium levels at baseline and periodically during treatment with spironolactone? There has never been a definitive consensus on which approach to take. However, there has been evidence to suggest that such monitoring is not necessary in young healthy women due to a negligible risk for clinically relevant hyperkalemia.2,3
In fact, the suggestion that there is a very low risk for clinically significant hyperkalemia in healthy young women treated with spironolactone is accurate based on population-based studies. Nevertheless, the clinician is faced with confirming the patient is in fact healthy rather than assuming this is the case due to her “young” age. In addition, it is important to exclude potential drug-drug interactions that can increase the risk for hyperkalemia when coadministered with spironolactone and also to exclude an unknown underlying decrease in renal function.1 At the end of the day, I support the continued research that is being done to evaluate questions that can challenge the recycled dogma on how we manage patients, and I do not fault those who follow what they believe to be new cogent evidence. However, in the case of oral spironolactone use, I also could never fault a clinician for monitoring renal function and electrolytes including serum potassium levels in a female patient treated for acne, especially with a drug that has the known potential to cause hyperkalemia in certain clinical situations and is not FDA approved for the indication of acne (ie, the guidance that accompanies the level of investigation needed for such FDA approval is missing). The clinical judgment of the clinician who is responsible for the individual patient trumps the results from population-based studies completed thus far. Ultimately, it is the responsibility of that clinician to assure the safety of their patient in a manner that they are comfortable with.
It takes time to make changes in our approaches to patient management, and in the majority of cases, that is rightfully so. There are several potential limitations to how certain data are collected, and a reasonable verification of results over time is what tends to change behavior patterns. Ultimately, the common goal is to do what is in the best interest of our patients. No one can argue successfully against that.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Plovanich M, Weng QY, Arash Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Plovanich M, Weng QY, Arash Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300.
Administrative Burden of iPLEDGE Deters Isotretinoin Prescriptions: Results From a Survey of Dermatologists
Isotretinoin is the most effective treatment of recalcitrant acne, but because of its teratogenicity and potential association with psychiatric adverse effects, it has been heavily regulated by the US Food and Drug Administration (FDA) through the iPLEDGE program since 2006.1,2 To manage the risk of teratogenicity associated with isotretinoin, various pregnancy prevention programs have been developed, but none of these programs have demonstrated a zero fetal exposure rate. The FDA reported 122 isotretinoin-exposed pregnancies during the first year iPLEDGE was implemented, which was a slight increase from the 120 pregnancies reported the year after the implementation of the System to Manage Accutane-Related Teratogenicity program, iPLEDGE’s predecessor.3 The iPLEDGE program requires registration of all wholesalers distributing isotretinoin, all health care providers prescribing isotretinoin, all pharmacies dispensing isotretinoin, and all female and male patients prescribed isotretinoin to create a verifiable link that only enables patients who have met all criteria to pick up their prescriptions. For patients of reproductive potential, there are additional qualification criteria and monthly requirements; before receiving their prescription every month, patients of reproductive potential must undergo a urine or serum pregnancy test with negative results, and patients must be counseled by prescribers regarding the risks of the drug and verify they are using 2 methods of contraception (or practicing abstinence) each month before completing online questions that test their understanding of the drug’s side effects and their chosen methods of contraception.4 These requirements place burdens on both patients and prescribers. Studies have shown that in the 2 years after the implementation of iPLEDGE, there was a 29% decrease in isotretinoin prescriptions.1-3
We conducted a survey study to see if clinicians chose not to prescribe isotretinoin to appropriate candidates specifically because of the administrative burden of iPLEDGE. Secondarily, we investigated the medications these clinicians would prescribe instead of isotretinoin.
Methods
In March 2020, we administered an anonymous online survey consisting of 12 multiple-choice questions to verified board-certified dermatologists in the United States using a social media group. The University of Rochester’s (Rochester, New York) institutional review board determined that our protocol met criteria for exemption (IRB STUDY00004693).
Statistical Analysis—Primary analyses used Pearson χ2 tests to identify significant differences among respondent groups, practice settings, age of respondents, and time spent registering patients for iPLEDGE.
Results
Survey results from 510 respondents are summarized in the Table.
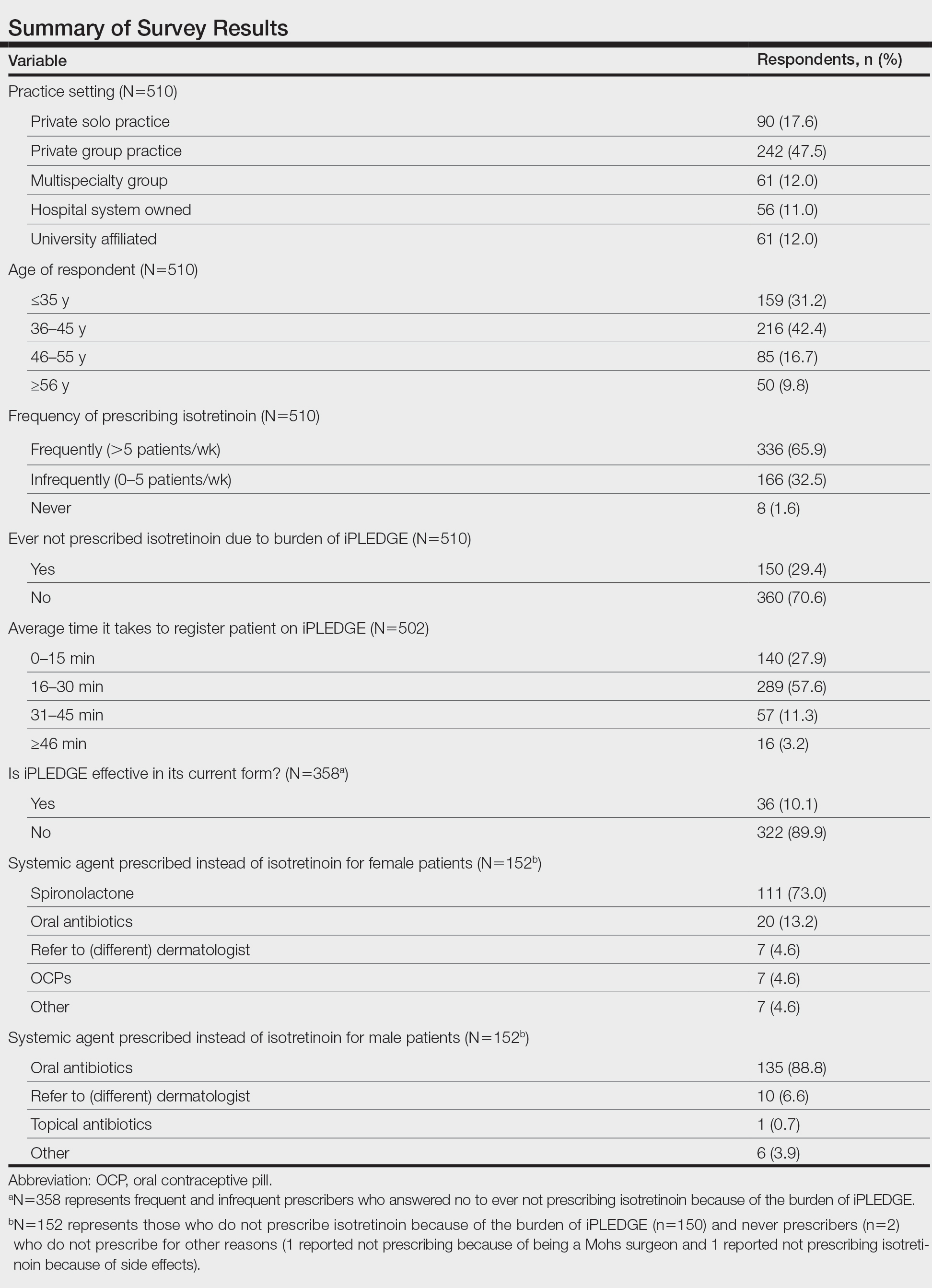
Burden of iPLEDGE—Of the respondents, 336 (65.9%) were frequent prescribers of isotretinoin, 166 (32.5%) were infrequent prescribers, and 8 (1.6%) were never prescribers. Significantly more isotretinoin prescribers estimated that their offices spend 16 to 30 minutes registering a new isotretinoin patient with the iPLEDGE program (289 [57.6%]) compared with 0 to 15 minutes (140 [27.9%]), 31 to 45 minutes (57 [11.3%]), and morethan 45 minutes (16 [3.2%])(χ23=22.09, P<.0001). Furthermore, 150 dermatologists reported sometimes not prescribing, and 2 reported never prescribing isotretinoin because of the burden of iPLEDGE.
Systemic Agents Prescribed Instead of Isotretinoin—Of the respondents, 73.0% (n=111) prescribed spironolactone to female patients and 88.8% (n=135) prescribed oral antibiotics to male patients instead of isotretinoin. Spironolactone typically is not prescribed to male patients with acne because of its feminizing side effects, such as gynecomastia.5 According to the American Academy of Dermatology guidelines on acne, systemic antibiotic usage should be limited to the shortest possible duration (ie, less than 3–4 months) because of potential bacterial resistance and reported associations with inflammatory bowel disease, Clostridium difficile infection, and candidiasis.6,7
Prescriber Demographics—The frequency of not prescribing isotretinoin did not vary by practice setting (χ 24=6.44, P=.1689) but did vary by age of the dermatologist (χ23=15.57, P=.0014). Dermatologists younger than 46 years were more likely (Figure) to report not prescribing isotretinoin because of the administrative burden of iPLEDGE. We speculate that this is because younger dermatologists are less established in their practices and therefore may have less support to complete registration without interruption of clinic workflow.
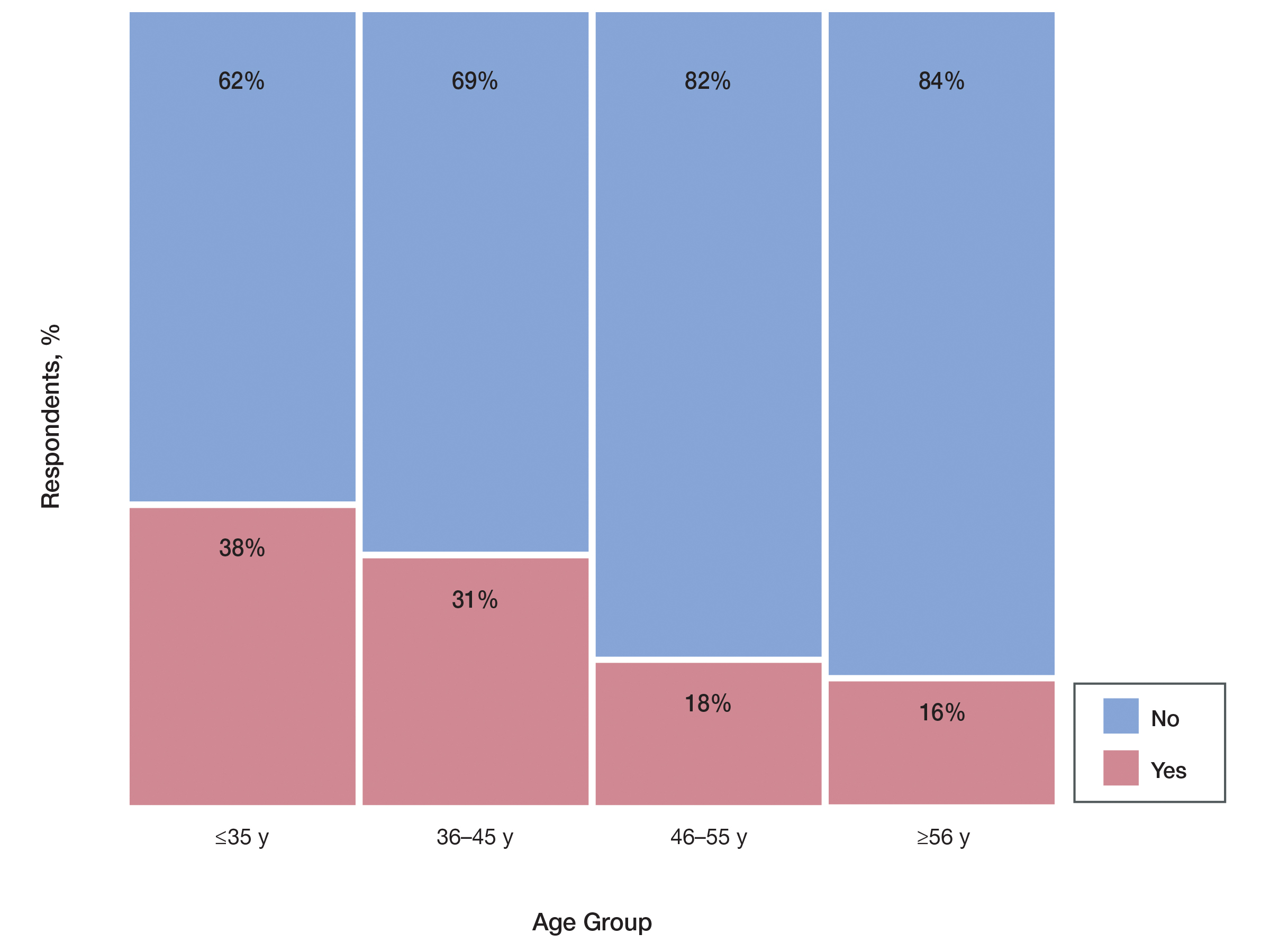
Comment
The results of our survey suggest that the administrative burden of iPLEDGE may be compelling prescribers to refrain from prescribing isotretinoin therapy to appropriate candidates when it would otherwise be the drug of choice.
Recent Changes to iPLEDGE—The FDA recently approved a modification to the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) program based on the advocacy efforts from the American Academy of Dermatology. Starting December 13, 2021, the 3 patient risk categories were consolidated into 2 gender-neutral categories: patients who can get pregnant and patients who cannot get pregnant.8 The iPLEDGE website was transitioned to a new system, and all iPLEDGE REMS users had to update their iPLEDGE accounts. After the implementation of the modified program, user access issues arose, leading to delayed treatment when patients, providers, and pharmacists were all locked out of the online system; users also experienced long hold times with the call center.8 This change highlights the ongoing critical need for a streamlined program that increases patient access to isotretinoin while maintaining safety.
Study Limitations—The main limitation of this study was the inability to calculate a true response rate to our survey. We distributed the survey via social media to maintain anonymity of the respondents. We could not track how many saw the link to compare with the number of respondents. Therefore, the only way we could calculate a response rate was with the total number of members in the group, which fluctuated around 4000 at the time we administered the survey. We calculated that we would need at least 351 responses to have a 5% margin of error at 95% confidence for our results to be generalizable and significant. We ultimately received 510 responses, which gave us a 4.05% margin of error at 95% confidence and an estimated 12.7% response rate. Since some members of the group are not active and did not see the survey link, our true response rate was likely higher. Therefore, we concluded that the survey was successful, and our significant responses were representative of US dermatologists.
Suggestions to Improve iPLEDGE Process—Our survey study should facilitate further discussions on the importance of simplifying iPLEDGE. One suggestion for improving iPLEDGE is to remove the initial registration month so care is not delayed. Currently, a patient who can get pregnant must be on 2 forms of contraception for 30 days after they register as a patient before they are eligible to fill their prescription.4 This process is unnecessarily long and arduous and could be eliminated as long as the patient has already been on an effective form of contraception and has a negative pregnancy test on the day of registration. The need to repeat contraception comprehension questions monthly is redundant and also could be removed. Another suggestion is to remove the category of patients who cannot become pregnant from the system entirely. Isotretinoin does not appear to be associated with adverse psychiatric effects as shown through the systematic review and meta-analysis of numerous studies.9 If anything, the treatment of acne with isotretinoin appears to mitigate depressive symptoms. The iPLEDGE program does not manage this largely debunked idea. Because the program’s sole goal is to manage the risk of isotretinoin’s teratogenicity, the category of those who cannot become pregnant should not be included.
Conclusion
This survey highlights the burdens of iPLEDGE for dermatologists and the need for a more streamlined risk management program. The burden was felt equally among all practice types but especially by younger dermatologists (<46 years). This time-consuming program is deterring some dermatologists from prescribing isotretinoin and ultimately limiting patient access to an effective medication.
Acknowledgment—The authors thank all of the responding clinicians who provided insight into the impact of iPLEDGE on their isotretinoin prescribing patterns.
- Prevost N, English JC. Isotretinoin: update on controversial issues. J Pediatr Adolesc Gynecol. 2013;26:290-293.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Shin J, Cheetham TC, Wong L, et al. The impact of the IPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- iPLEDGE Program. About iPLEDGE. Accessed June 13, 2022. https://ipledgeprogram.com/#Main/AboutiPledge
- Marson JW, Baldwin HE. An overview of acne therapy, part 2: hormonal therapy and isotretinoin. Dermatol Clin. 2019;37:195-203.
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
- iPLEDGE Risk Evaluation and Mitigation Strategy (REMS). Updated January 14, 2022. Accessed June 13, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/ipledge-risk-evaluation-and-mitigation-strategy-rems
- Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;76:1068-1076.e9.
Isotretinoin is the most effective treatment of recalcitrant acne, but because of its teratogenicity and potential association with psychiatric adverse effects, it has been heavily regulated by the US Food and Drug Administration (FDA) through the iPLEDGE program since 2006.1,2 To manage the risk of teratogenicity associated with isotretinoin, various pregnancy prevention programs have been developed, but none of these programs have demonstrated a zero fetal exposure rate. The FDA reported 122 isotretinoin-exposed pregnancies during the first year iPLEDGE was implemented, which was a slight increase from the 120 pregnancies reported the year after the implementation of the System to Manage Accutane-Related Teratogenicity program, iPLEDGE’s predecessor.3 The iPLEDGE program requires registration of all wholesalers distributing isotretinoin, all health care providers prescribing isotretinoin, all pharmacies dispensing isotretinoin, and all female and male patients prescribed isotretinoin to create a verifiable link that only enables patients who have met all criteria to pick up their prescriptions. For patients of reproductive potential, there are additional qualification criteria and monthly requirements; before receiving their prescription every month, patients of reproductive potential must undergo a urine or serum pregnancy test with negative results, and patients must be counseled by prescribers regarding the risks of the drug and verify they are using 2 methods of contraception (or practicing abstinence) each month before completing online questions that test their understanding of the drug’s side effects and their chosen methods of contraception.4 These requirements place burdens on both patients and prescribers. Studies have shown that in the 2 years after the implementation of iPLEDGE, there was a 29% decrease in isotretinoin prescriptions.1-3
We conducted a survey study to see if clinicians chose not to prescribe isotretinoin to appropriate candidates specifically because of the administrative burden of iPLEDGE. Secondarily, we investigated the medications these clinicians would prescribe instead of isotretinoin.
Methods
In March 2020, we administered an anonymous online survey consisting of 12 multiple-choice questions to verified board-certified dermatologists in the United States using a social media group. The University of Rochester’s (Rochester, New York) institutional review board determined that our protocol met criteria for exemption (IRB STUDY00004693).
Statistical Analysis—Primary analyses used Pearson χ2 tests to identify significant differences among respondent groups, practice settings, age of respondents, and time spent registering patients for iPLEDGE.
Results
Survey results from 510 respondents are summarized in the Table.

Burden of iPLEDGE—Of the respondents, 336 (65.9%) were frequent prescribers of isotretinoin, 166 (32.5%) were infrequent prescribers, and 8 (1.6%) were never prescribers. Significantly more isotretinoin prescribers estimated that their offices spend 16 to 30 minutes registering a new isotretinoin patient with the iPLEDGE program (289 [57.6%]) compared with 0 to 15 minutes (140 [27.9%]), 31 to 45 minutes (57 [11.3%]), and morethan 45 minutes (16 [3.2%])(χ23=22.09, P<.0001). Furthermore, 150 dermatologists reported sometimes not prescribing, and 2 reported never prescribing isotretinoin because of the burden of iPLEDGE.
Systemic Agents Prescribed Instead of Isotretinoin—Of the respondents, 73.0% (n=111) prescribed spironolactone to female patients and 88.8% (n=135) prescribed oral antibiotics to male patients instead of isotretinoin. Spironolactone typically is not prescribed to male patients with acne because of its feminizing side effects, such as gynecomastia.5 According to the American Academy of Dermatology guidelines on acne, systemic antibiotic usage should be limited to the shortest possible duration (ie, less than 3–4 months) because of potential bacterial resistance and reported associations with inflammatory bowel disease, Clostridium difficile infection, and candidiasis.6,7
Prescriber Demographics—The frequency of not prescribing isotretinoin did not vary by practice setting (χ 24=6.44, P=.1689) but did vary by age of the dermatologist (χ23=15.57, P=.0014). Dermatologists younger than 46 years were more likely (Figure) to report not prescribing isotretinoin because of the administrative burden of iPLEDGE. We speculate that this is because younger dermatologists are less established in their practices and therefore may have less support to complete registration without interruption of clinic workflow.

Comment
The results of our survey suggest that the administrative burden of iPLEDGE may be compelling prescribers to refrain from prescribing isotretinoin therapy to appropriate candidates when it would otherwise be the drug of choice.
Recent Changes to iPLEDGE—The FDA recently approved a modification to the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) program based on the advocacy efforts from the American Academy of Dermatology. Starting December 13, 2021, the 3 patient risk categories were consolidated into 2 gender-neutral categories: patients who can get pregnant and patients who cannot get pregnant.8 The iPLEDGE website was transitioned to a new system, and all iPLEDGE REMS users had to update their iPLEDGE accounts. After the implementation of the modified program, user access issues arose, leading to delayed treatment when patients, providers, and pharmacists were all locked out of the online system; users also experienced long hold times with the call center.8 This change highlights the ongoing critical need for a streamlined program that increases patient access to isotretinoin while maintaining safety.
Study Limitations—The main limitation of this study was the inability to calculate a true response rate to our survey. We distributed the survey via social media to maintain anonymity of the respondents. We could not track how many saw the link to compare with the number of respondents. Therefore, the only way we could calculate a response rate was with the total number of members in the group, which fluctuated around 4000 at the time we administered the survey. We calculated that we would need at least 351 responses to have a 5% margin of error at 95% confidence for our results to be generalizable and significant. We ultimately received 510 responses, which gave us a 4.05% margin of error at 95% confidence and an estimated 12.7% response rate. Since some members of the group are not active and did not see the survey link, our true response rate was likely higher. Therefore, we concluded that the survey was successful, and our significant responses were representative of US dermatologists.
Suggestions to Improve iPLEDGE Process—Our survey study should facilitate further discussions on the importance of simplifying iPLEDGE. One suggestion for improving iPLEDGE is to remove the initial registration month so care is not delayed. Currently, a patient who can get pregnant must be on 2 forms of contraception for 30 days after they register as a patient before they are eligible to fill their prescription.4 This process is unnecessarily long and arduous and could be eliminated as long as the patient has already been on an effective form of contraception and has a negative pregnancy test on the day of registration. The need to repeat contraception comprehension questions monthly is redundant and also could be removed. Another suggestion is to remove the category of patients who cannot become pregnant from the system entirely. Isotretinoin does not appear to be associated with adverse psychiatric effects as shown through the systematic review and meta-analysis of numerous studies.9 If anything, the treatment of acne with isotretinoin appears to mitigate depressive symptoms. The iPLEDGE program does not manage this largely debunked idea. Because the program’s sole goal is to manage the risk of isotretinoin’s teratogenicity, the category of those who cannot become pregnant should not be included.
Conclusion
This survey highlights the burdens of iPLEDGE for dermatologists and the need for a more streamlined risk management program. The burden was felt equally among all practice types but especially by younger dermatologists (<46 years). This time-consuming program is deterring some dermatologists from prescribing isotretinoin and ultimately limiting patient access to an effective medication.
Acknowledgment—The authors thank all of the responding clinicians who provided insight into the impact of iPLEDGE on their isotretinoin prescribing patterns.
Isotretinoin is the most effective treatment of recalcitrant acne, but because of its teratogenicity and potential association with psychiatric adverse effects, it has been heavily regulated by the US Food and Drug Administration (FDA) through the iPLEDGE program since 2006.1,2 To manage the risk of teratogenicity associated with isotretinoin, various pregnancy prevention programs have been developed, but none of these programs have demonstrated a zero fetal exposure rate. The FDA reported 122 isotretinoin-exposed pregnancies during the first year iPLEDGE was implemented, which was a slight increase from the 120 pregnancies reported the year after the implementation of the System to Manage Accutane-Related Teratogenicity program, iPLEDGE’s predecessor.3 The iPLEDGE program requires registration of all wholesalers distributing isotretinoin, all health care providers prescribing isotretinoin, all pharmacies dispensing isotretinoin, and all female and male patients prescribed isotretinoin to create a verifiable link that only enables patients who have met all criteria to pick up their prescriptions. For patients of reproductive potential, there are additional qualification criteria and monthly requirements; before receiving their prescription every month, patients of reproductive potential must undergo a urine or serum pregnancy test with negative results, and patients must be counseled by prescribers regarding the risks of the drug and verify they are using 2 methods of contraception (or practicing abstinence) each month before completing online questions that test their understanding of the drug’s side effects and their chosen methods of contraception.4 These requirements place burdens on both patients and prescribers. Studies have shown that in the 2 years after the implementation of iPLEDGE, there was a 29% decrease in isotretinoin prescriptions.1-3
We conducted a survey study to see if clinicians chose not to prescribe isotretinoin to appropriate candidates specifically because of the administrative burden of iPLEDGE. Secondarily, we investigated the medications these clinicians would prescribe instead of isotretinoin.
Methods
In March 2020, we administered an anonymous online survey consisting of 12 multiple-choice questions to verified board-certified dermatologists in the United States using a social media group. The University of Rochester’s (Rochester, New York) institutional review board determined that our protocol met criteria for exemption (IRB STUDY00004693).
Statistical Analysis—Primary analyses used Pearson χ2 tests to identify significant differences among respondent groups, practice settings, age of respondents, and time spent registering patients for iPLEDGE.
Results
Survey results from 510 respondents are summarized in the Table.

Burden of iPLEDGE—Of the respondents, 336 (65.9%) were frequent prescribers of isotretinoin, 166 (32.5%) were infrequent prescribers, and 8 (1.6%) were never prescribers. Significantly more isotretinoin prescribers estimated that their offices spend 16 to 30 minutes registering a new isotretinoin patient with the iPLEDGE program (289 [57.6%]) compared with 0 to 15 minutes (140 [27.9%]), 31 to 45 minutes (57 [11.3%]), and morethan 45 minutes (16 [3.2%])(χ23=22.09, P<.0001). Furthermore, 150 dermatologists reported sometimes not prescribing, and 2 reported never prescribing isotretinoin because of the burden of iPLEDGE.
Systemic Agents Prescribed Instead of Isotretinoin—Of the respondents, 73.0% (n=111) prescribed spironolactone to female patients and 88.8% (n=135) prescribed oral antibiotics to male patients instead of isotretinoin. Spironolactone typically is not prescribed to male patients with acne because of its feminizing side effects, such as gynecomastia.5 According to the American Academy of Dermatology guidelines on acne, systemic antibiotic usage should be limited to the shortest possible duration (ie, less than 3–4 months) because of potential bacterial resistance and reported associations with inflammatory bowel disease, Clostridium difficile infection, and candidiasis.6,7
Prescriber Demographics—The frequency of not prescribing isotretinoin did not vary by practice setting (χ 24=6.44, P=.1689) but did vary by age of the dermatologist (χ23=15.57, P=.0014). Dermatologists younger than 46 years were more likely (Figure) to report not prescribing isotretinoin because of the administrative burden of iPLEDGE. We speculate that this is because younger dermatologists are less established in their practices and therefore may have less support to complete registration without interruption of clinic workflow.

Comment
The results of our survey suggest that the administrative burden of iPLEDGE may be compelling prescribers to refrain from prescribing isotretinoin therapy to appropriate candidates when it would otherwise be the drug of choice.
Recent Changes to iPLEDGE—The FDA recently approved a modification to the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) program based on the advocacy efforts from the American Academy of Dermatology. Starting December 13, 2021, the 3 patient risk categories were consolidated into 2 gender-neutral categories: patients who can get pregnant and patients who cannot get pregnant.8 The iPLEDGE website was transitioned to a new system, and all iPLEDGE REMS users had to update their iPLEDGE accounts. After the implementation of the modified program, user access issues arose, leading to delayed treatment when patients, providers, and pharmacists were all locked out of the online system; users also experienced long hold times with the call center.8 This change highlights the ongoing critical need for a streamlined program that increases patient access to isotretinoin while maintaining safety.
Study Limitations—The main limitation of this study was the inability to calculate a true response rate to our survey. We distributed the survey via social media to maintain anonymity of the respondents. We could not track how many saw the link to compare with the number of respondents. Therefore, the only way we could calculate a response rate was with the total number of members in the group, which fluctuated around 4000 at the time we administered the survey. We calculated that we would need at least 351 responses to have a 5% margin of error at 95% confidence for our results to be generalizable and significant. We ultimately received 510 responses, which gave us a 4.05% margin of error at 95% confidence and an estimated 12.7% response rate. Since some members of the group are not active and did not see the survey link, our true response rate was likely higher. Therefore, we concluded that the survey was successful, and our significant responses were representative of US dermatologists.
Suggestions to Improve iPLEDGE Process—Our survey study should facilitate further discussions on the importance of simplifying iPLEDGE. One suggestion for improving iPLEDGE is to remove the initial registration month so care is not delayed. Currently, a patient who can get pregnant must be on 2 forms of contraception for 30 days after they register as a patient before they are eligible to fill their prescription.4 This process is unnecessarily long and arduous and could be eliminated as long as the patient has already been on an effective form of contraception and has a negative pregnancy test on the day of registration. The need to repeat contraception comprehension questions monthly is redundant and also could be removed. Another suggestion is to remove the category of patients who cannot become pregnant from the system entirely. Isotretinoin does not appear to be associated with adverse psychiatric effects as shown through the systematic review and meta-analysis of numerous studies.9 If anything, the treatment of acne with isotretinoin appears to mitigate depressive symptoms. The iPLEDGE program does not manage this largely debunked idea. Because the program’s sole goal is to manage the risk of isotretinoin’s teratogenicity, the category of those who cannot become pregnant should not be included.
Conclusion
This survey highlights the burdens of iPLEDGE for dermatologists and the need for a more streamlined risk management program. The burden was felt equally among all practice types but especially by younger dermatologists (<46 years). This time-consuming program is deterring some dermatologists from prescribing isotretinoin and ultimately limiting patient access to an effective medication.
Acknowledgment—The authors thank all of the responding clinicians who provided insight into the impact of iPLEDGE on their isotretinoin prescribing patterns.
- Prevost N, English JC. Isotretinoin: update on controversial issues. J Pediatr Adolesc Gynecol. 2013;26:290-293.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Shin J, Cheetham TC, Wong L, et al. The impact of the IPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- iPLEDGE Program. About iPLEDGE. Accessed June 13, 2022. https://ipledgeprogram.com/#Main/AboutiPledge
- Marson JW, Baldwin HE. An overview of acne therapy, part 2: hormonal therapy and isotretinoin. Dermatol Clin. 2019;37:195-203.
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
- iPLEDGE Risk Evaluation and Mitigation Strategy (REMS). Updated January 14, 2022. Accessed June 13, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/ipledge-risk-evaluation-and-mitigation-strategy-rems
- Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;76:1068-1076.e9.
- Prevost N, English JC. Isotretinoin: update on controversial issues. J Pediatr Adolesc Gynecol. 2013;26:290-293.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Shin J, Cheetham TC, Wong L, et al. The impact of the IPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- iPLEDGE Program. About iPLEDGE. Accessed June 13, 2022. https://ipledgeprogram.com/#Main/AboutiPledge
- Marson JW, Baldwin HE. An overview of acne therapy, part 2: hormonal therapy and isotretinoin. Dermatol Clin. 2019;37:195-203.
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
- iPLEDGE Risk Evaluation and Mitigation Strategy (REMS). Updated January 14, 2022. Accessed June 13, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/ipledge-risk-evaluation-and-mitigation-strategy-rems
- Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;76:1068-1076.e9.
Practice Points
- Of clinicians who regularly prescribe isotretinoin, approximately 30% have at times chosen not to prescribe isotretinoin to patients with severe acne because of the burden of the iPLEDGE program.
- The US Food and Drug Administration should consider further streamlining the iPLEDGE program, as it is causing physician burden and therefore suboptimal treatment plans for patients.