User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Simple Intraoperative Technique to Improve Wound Edge Approximation for Residents
Practice Gap
Dermatology residents can struggle with surgical closure early in their training years. Although experienced dermatologic surgeons may intuitively be able to align edges for maximal cosmesis, doing so can prove challenging in the context of learning basic surgical techniques for early residents.
Furthermore, local anesthesia can distort cutaneous anatomy and surgical landmarks, requiring the surgeon to reexamine their closure technique. Patients may require position changes or may make involuntary movements, both of which require dynamic thinking and planning on the part of the dermatologic surgeon to achieve optimal outcomes.
The Technique
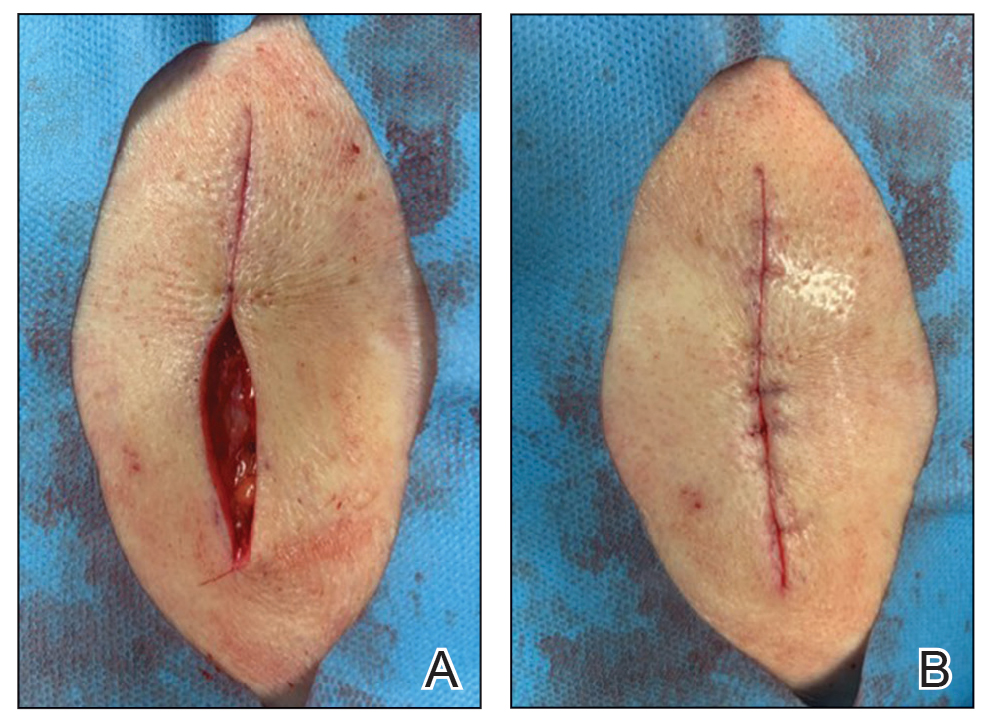
We propose the use of sutures to intraoperatively guide placement of the dermal needle. This technique can be used for various closure types; here, we demonstrate its use in a standard elliptical excision.
To begin, a standard length to width ellipse ratio of 3:1 is drawn with appropriate margins around a neoplasm.1 After excision and appropriate undermining of the ellipse, we typically use deep sutures to close the deep space. The first pass of the needle through tissue can be performed in a place of the surgeon’s preference but typically abides by the rule of halves or the zipper method (Figure 1A). To determine optimal placement of the second needle pass through tissue, we recommend applying gentle opposing traction forces to the wound apices to approximate the linear outcome of the wound edges. The surgeon can use a skin hook to guide placement of the needle to the contralateral wound edge in an unassisted method of this technique (Figure 1B). The surgeon’s assistant also can aid in applying cutaneous traction along the length of the excision if the surgeon wishes to free their hands (Figure 1C). Because the risk of needlestick injury at this step is small, it is prudent for the surgeon to advise the assistant to avoid needlestick injury by keeping their hands away from the needle path in the surgical site.
Although traction is being applied to the wound apices, the deep suture should extend across the wound with just enough pressure to leave a serosanguineous notched mark in the contralateral tissue edge (Figure 1D). After releasing traction on the wound edges, the surgeon can effortlessly visualize the target for needle placement and make a throw through the tissue accordingly.

This process can be continued until wound closure is complete (Figure 2). Top sutures or adhesive strips can be placed afterward for completing approximation of the wound edges superficially.
Practice Implications
By using this technique to align wound edges intraoperatively, the surgeon can have a functional guide for needle placement. The technique allows improvement of function and cosmesis of surgical wounds, while also accounting for topographical variations in the patient’s surgical site. Approximation of the wound edges is particularly important at the beginning of closure, as the wound edges align and approximate more with each subsequent stitch, with decreasing tension.2
In addition, when operating on a curvilinear or challenging topographical surface of the body, this technique can provide a clear template for guiding suture placement for approximating wound edges. Furthermore, local biodynamic anatomy might become distorted after excision of the tissue specimen due to release of centripetal tangential forces that were present in the pre-excised skin.1 Local change in biodynamic forces may be difficult to plan for preoperatively using other techniques.3
Although this technique can be utilized for all suture placements in closure, it is of greatest value when placing the first few sutures and when operating on nonplanar surfaces that might become distorted after excision. To ensure the best outcome, it is important to be certain that the area has been properly cleaned prior to surgery and a sterile technique is used.
- Paul SP. Biodynamic excisional skin tension lines for excisional surgery of the lower limb and the technique of using parallel relaxing incisions to further reduce wound tension. Plast Reconstr Surg Glob Open. 2017;5:E1614. doi:10.1097/GOX.0000000000001614
- Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402. doi:10.1016/j.jaad.2014.08.006
- Parikh SA, Sloan B. Clinical pearl: a simple and effective technique for improving surgical closures for the early-learning resident. Cutis. 2017;100:338-339.
Practice Gap
Dermatology residents can struggle with surgical closure early in their training years. Although experienced dermatologic surgeons may intuitively be able to align edges for maximal cosmesis, doing so can prove challenging in the context of learning basic surgical techniques for early residents.
Furthermore, local anesthesia can distort cutaneous anatomy and surgical landmarks, requiring the surgeon to reexamine their closure technique. Patients may require position changes or may make involuntary movements, both of which require dynamic thinking and planning on the part of the dermatologic surgeon to achieve optimal outcomes.
The Technique
We propose the use of sutures to intraoperatively guide placement of the dermal needle. This technique can be used for various closure types; here, we demonstrate its use in a standard elliptical excision.
To begin, a standard length to width ellipse ratio of 3:1 is drawn with appropriate margins around a neoplasm.1 After excision and appropriate undermining of the ellipse, we typically use deep sutures to close the deep space. The first pass of the needle through tissue can be performed in a place of the surgeon’s preference but typically abides by the rule of halves or the zipper method (Figure 1A). To determine optimal placement of the second needle pass through tissue, we recommend applying gentle opposing traction forces to the wound apices to approximate the linear outcome of the wound edges. The surgeon can use a skin hook to guide placement of the needle to the contralateral wound edge in an unassisted method of this technique (Figure 1B). The surgeon’s assistant also can aid in applying cutaneous traction along the length of the excision if the surgeon wishes to free their hands (Figure 1C). Because the risk of needlestick injury at this step is small, it is prudent for the surgeon to advise the assistant to avoid needlestick injury by keeping their hands away from the needle path in the surgical site.
Although traction is being applied to the wound apices, the deep suture should extend across the wound with just enough pressure to leave a serosanguineous notched mark in the contralateral tissue edge (Figure 1D). After releasing traction on the wound edges, the surgeon can effortlessly visualize the target for needle placement and make a throw through the tissue accordingly.

This process can be continued until wound closure is complete (Figure 2). Top sutures or adhesive strips can be placed afterward for completing approximation of the wound edges superficially.

Practice Implications
By using this technique to align wound edges intraoperatively, the surgeon can have a functional guide for needle placement. The technique allows improvement of function and cosmesis of surgical wounds, while also accounting for topographical variations in the patient’s surgical site. Approximation of the wound edges is particularly important at the beginning of closure, as the wound edges align and approximate more with each subsequent stitch, with decreasing tension.2
In addition, when operating on a curvilinear or challenging topographical surface of the body, this technique can provide a clear template for guiding suture placement for approximating wound edges. Furthermore, local biodynamic anatomy might become distorted after excision of the tissue specimen due to release of centripetal tangential forces that were present in the pre-excised skin.1 Local change in biodynamic forces may be difficult to plan for preoperatively using other techniques.3
Although this technique can be utilized for all suture placements in closure, it is of greatest value when placing the first few sutures and when operating on nonplanar surfaces that might become distorted after excision. To ensure the best outcome, it is important to be certain that the area has been properly cleaned prior to surgery and a sterile technique is used.
Practice Gap
Dermatology residents can struggle with surgical closure early in their training years. Although experienced dermatologic surgeons may intuitively be able to align edges for maximal cosmesis, doing so can prove challenging in the context of learning basic surgical techniques for early residents.
Furthermore, local anesthesia can distort cutaneous anatomy and surgical landmarks, requiring the surgeon to reexamine their closure technique. Patients may require position changes or may make involuntary movements, both of which require dynamic thinking and planning on the part of the dermatologic surgeon to achieve optimal outcomes.
The Technique
We propose the use of sutures to intraoperatively guide placement of the dermal needle. This technique can be used for various closure types; here, we demonstrate its use in a standard elliptical excision.
To begin, a standard length to width ellipse ratio of 3:1 is drawn with appropriate margins around a neoplasm.1 After excision and appropriate undermining of the ellipse, we typically use deep sutures to close the deep space. The first pass of the needle through tissue can be performed in a place of the surgeon’s preference but typically abides by the rule of halves or the zipper method (Figure 1A). To determine optimal placement of the second needle pass through tissue, we recommend applying gentle opposing traction forces to the wound apices to approximate the linear outcome of the wound edges. The surgeon can use a skin hook to guide placement of the needle to the contralateral wound edge in an unassisted method of this technique (Figure 1B). The surgeon’s assistant also can aid in applying cutaneous traction along the length of the excision if the surgeon wishes to free their hands (Figure 1C). Because the risk of needlestick injury at this step is small, it is prudent for the surgeon to advise the assistant to avoid needlestick injury by keeping their hands away from the needle path in the surgical site.
Although traction is being applied to the wound apices, the deep suture should extend across the wound with just enough pressure to leave a serosanguineous notched mark in the contralateral tissue edge (Figure 1D). After releasing traction on the wound edges, the surgeon can effortlessly visualize the target for needle placement and make a throw through the tissue accordingly.

This process can be continued until wound closure is complete (Figure 2). Top sutures or adhesive strips can be placed afterward for completing approximation of the wound edges superficially.

Practice Implications
By using this technique to align wound edges intraoperatively, the surgeon can have a functional guide for needle placement. The technique allows improvement of function and cosmesis of surgical wounds, while also accounting for topographical variations in the patient’s surgical site. Approximation of the wound edges is particularly important at the beginning of closure, as the wound edges align and approximate more with each subsequent stitch, with decreasing tension.2
In addition, when operating on a curvilinear or challenging topographical surface of the body, this technique can provide a clear template for guiding suture placement for approximating wound edges. Furthermore, local biodynamic anatomy might become distorted after excision of the tissue specimen due to release of centripetal tangential forces that were present in the pre-excised skin.1 Local change in biodynamic forces may be difficult to plan for preoperatively using other techniques.3
Although this technique can be utilized for all suture placements in closure, it is of greatest value when placing the first few sutures and when operating on nonplanar surfaces that might become distorted after excision. To ensure the best outcome, it is important to be certain that the area has been properly cleaned prior to surgery and a sterile technique is used.
- Paul SP. Biodynamic excisional skin tension lines for excisional surgery of the lower limb and the technique of using parallel relaxing incisions to further reduce wound tension. Plast Reconstr Surg Glob Open. 2017;5:E1614. doi:10.1097/GOX.0000000000001614
- Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402. doi:10.1016/j.jaad.2014.08.006
- Parikh SA, Sloan B. Clinical pearl: a simple and effective technique for improving surgical closures for the early-learning resident. Cutis. 2017;100:338-339.
- Paul SP. Biodynamic excisional skin tension lines for excisional surgery of the lower limb and the technique of using parallel relaxing incisions to further reduce wound tension. Plast Reconstr Surg Glob Open. 2017;5:E1614. doi:10.1097/GOX.0000000000001614
- Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402. doi:10.1016/j.jaad.2014.08.006
- Parikh SA, Sloan B. Clinical pearl: a simple and effective technique for improving surgical closures for the early-learning resident. Cutis. 2017;100:338-339.
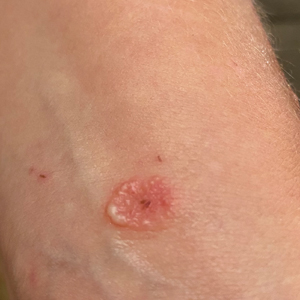
Itchy Vesicular Rash
The Diagnosis: Tinea Corporis Bullosa
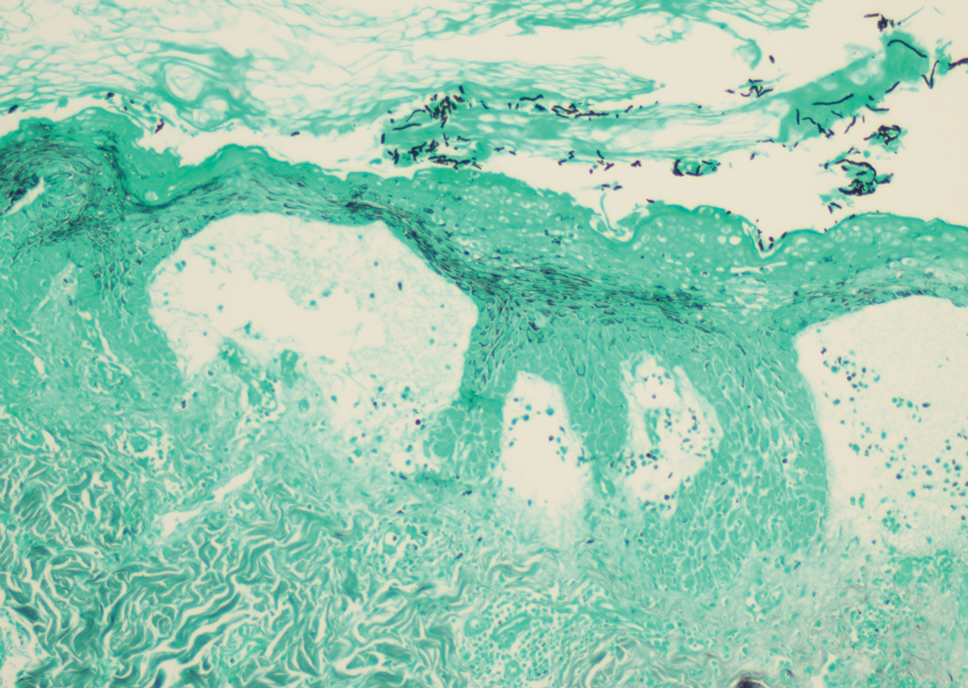
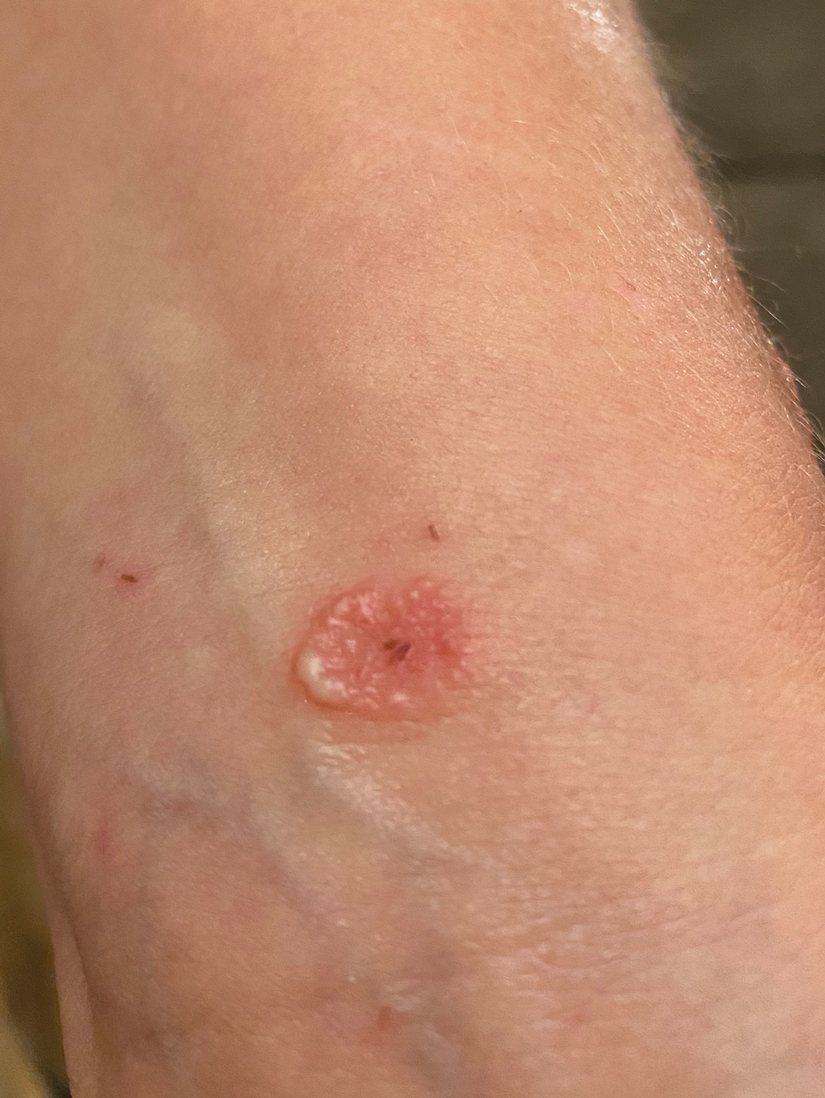
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.
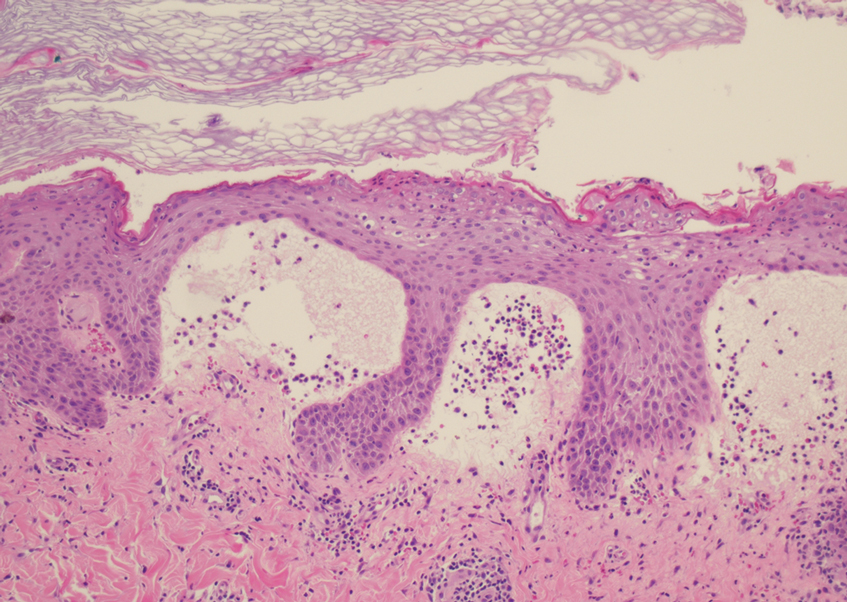
Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1
Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.

Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1

Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.

Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1

Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
A 38-year-old woman presented with a rash of 5 days’ duration that initially appeared on the wrists after playing with her kitten, with subsequent involvement of the chest, back, abdomen, and upper and lower extremities. Physical examination revealed multiple annular plaques with raised erythematous borders, rare peripheral vesicles, and superficial central scaling. Extreme pruritus accompanied the plaques, both of which developed after playing with her kitten. The patient noted that all lesions on the upper extremities evolved in areas subject to deep puncture while more superficially excoriated areas were unaffected. She denied any other prior skin conditions and had received a 5-day course of azithromycin without improvement prior to presentation; triamcinolone ointment 0.1% had provided only temporary relief. Primary care providers prescribed a short course of oral prednisone; however, she did not start it prior to presentation.
Doctors still overprescribing fluoroquinolones despite risks
When Amy Moser had a simple urinary tract infection in her late 20s, her doctor prescribed Cipro, a powerful antibiotic used to treat anthrax and some of the most fearsome bacterial infections.
Nearly 2 weeks after she finished her treatment, her left kneecap dislocated while she was trying on a swimsuit at a retail store. Shortly afterward, she had painful ligament ruptures in her wrists, then her shoulder dislocated, followed by three Achilles tendon tears.
“That’s when I fell apart,” says Ms. Moser, a Phoenix health blogger and book author. “From that moment on, for almost the next 2.5 years consistently, I had new tendon tears every few weeks.”
Ms. Moser’s doctors had no answer for what was causing her injuries, all of which required surgical fixes. A married mother of three, she was otherwise healthy and fit. So, after her third Achilles tear, she turned to the FDA’s website for answers. There, she found many warnings about side effects of Cipro, Levaquin, and other so-called fluoroquinolones, including risks for tendon and ligament injuries.
“When all the ruptures started to happen, my doctor kept asking me if I’d ever taken Levaquin, and every time I was like, ‘No.’ So I did what all doctors don’t want you to do: I Googled ‘Levaquin,’ ” she recalls.
Her search led to FDA warnings and articles about the possibility of tendon and ligament ruptures with fluroquinolones.
“That was the first time I’d ever even heard that word ‘fluroquinolones,’ and I found Cipro on that list ... and I realized that I’d just been prescribed that before everything started,” she says.
That was 12 years ago. Since then, the FDA has issued more warnings about fluoroquinolone risks. In that time, Ms. Moser, now 40, has had more than 30 surgeries to correct tendon ruptures and injuries, including a double-knee replacement this year.
“I am in chronic pain all the time,” she says. “I am chronically injured. I have a lot of tears that I’ve not fixed because they’re very complicated, and I don’t know if the rest of my body can handle the strain of recovering from those surgeries.”
Ms. Moser’s is hardly an isolated case. Since the 1980s, more than 60,000 patients have reported hundreds of thousands of serious events linked to fluoroquinolones to the FDA, including 6,575 reports of deaths.
The most common side effects were tendon rupture, as well as neurological and psychiatric symptoms. But experts estimate only 1%-10% of such events are reported to the FDA. That suggests that fluoroquinolones might have harmed hundreds of thousands of people in the United States alone, says Charles Bennett, MD, a hematologist at the University of South Carolina’s College of Pharmacy, Columbia.
Yet despite the many patient reports and FDA warnings on dangerous side effects, better treated with less risky antibiotics.
“There probably is overprescription by primary care doctors for urinary tract infections and respiratory infections, when there could be alternatives that are safer to use,” says Amesh Adalja, MD, an infectious disease specialist and senior scholar with the Johns Hopkins Center for Health Security.
“I would say that’s probably the case in the outpatient setting, not necessarily in the hospital setting or among infectious disease doctors ... but I think it’s important to say there are still some judicious uses of fluoroquinolones,” he says. “However, there probably is a lot of injudicious use of fluoroquinolones along with many other antibiotics in the primary care setting.”
FDA warnings on fluoroquinolones
Fluoroquinolones are a class of broad-spectrum antibiotics used for decades to treat certain bacterial infections.
FDA-approved fluoroquinolones include ciprofloxacin (Cipro), ciprofloxacin extended-release tablets, delafloxacin (Baxdela), gemifloxacin (Factive) levofloxacin (Levaquin), moxifloxacin (Avelox), and ofloxacin (Floxin). More than 60 generic versions of these brand-name medicines are also on the market, making them among the most prescribed antibiotics in the U.S.
Over the past 2 decades, a wide range of physical and mental health side effects have been tied to fluoroquinolones. As a result of these “adverse event reports” and research published in medical literature, the FDA has required an escalating series of warnings and safety labeling changes for doctors who prescribe these drugs.
- In 2008, the FDA first added a “black box” warning to fluoroquinolones, citing an increased risk of tendinitis and tendon rupture in patients prescribed these meds.
- In 2011, the agency required the warning label to include risks of worsening symptoms for those with myasthenia gravis, a chronic autoimmune disease that causes muscle weakness, vision problems, and speech problems.
- In 2013, regulators required updated labels noting the potential for irreversible peripheral neuropathy (serious nerve damage).
- In 2016, the FDA issued its strongest warning against the use of such antibiotics for simple bacterial infections – such as uncomplicated urinary tract infections (UTIs), acute sinusitis, and acute bronchitis – saying the “association of fluoroquinolones with disabling and potentially permanent side effects involving tendons, muscles, joints, nerves and the central nervous system ... outweighs the benefits for patients.”
- And in 2018, regulators required safety labeling changes to include warnings about the risks of aortic aneurysm – a life-threatening enlargement of the main vessel that delivers blood to the body – as well as mental health side effects and serious blood sugar disturbances.
But FDA regulators have stopped short of barring fluoroquinolone use in the treatment of bacterial infections, citing the benefits for certain conditions.
“For some patients, the benefits of fluoroquinolones may continue to outweigh the risks for treatment of serious bacterial infections, such as pneumonia or intra-abdominal infections,” said former FDA Commissioner Scott Gottlieb, MD, “but there are other serious, known risks associated with these strong antibiotics that must be carefully weighed when considering their use.”
In December 2021, a study published in the journal JAMA Network Open found the FDA’s warnings may have helped lower prescribing of the drugs in Medicare patients. But not all doctors have been responsive to those warnings, researchers found.
“An overall decline in change over time and an immediate change in fluoroquinolone prescribing was observed after the 2016 FDA warning,” the authors concluded. “Certain physicians, such as primary care physicians, were more responsive to FDA warnings than others. ... Findings of this study suggest that identifying the association of physician and organizational characteristics with fluoroquinolone prescribing practices could help in developing mechanisms for improving de-adoption.”
Some critics say the FDA should do more to spotlight the dangers of fluoroquinolones and require doctors and patients to sign checklist consent forms to show they are aware of the potential side effects of these drugs.
Rachel Brummert, a patient advocate who sits on an FDA consumer advisory board, believes the FDA needs to improve its communication to doctors on fluoroquinolone risks and get tougher with those who continue to inappropriately prescribe the drugs.
“I think there needs to be a system in place, where if something comes down from the FDA about a drug, the physician has to sign off on it, the patient has to sign off on it and mark that they understand that there are these ‘black box’ warnings,” says Ms. Brummert, 52, a representative on the FDA’s Medical Devices Advisory Committee.
As an example, she points to Australia’s medical laws requiring doctors and patients to sign a checklist before any fluoroquinolone prescription is approved.
“When a physician prescribes a fluoroquinolone antibiotic, there’s a checklist – does the patient have an infection, is it a simple infection, do they have allergies?” she notes. “And you can’t even get the prescription out – it won’t even print out, it won’t go into the system – unless you check all of the boxes. But we don’t do that here. We don’t have that type of system right now.”
Ms. Brummert says such a system might have prevented the harm from taking Levaquin her doctor prescribed for a suspected sinus infection in 2006.
Soon after she began taking the antibiotic, she ruptured her Achilles tendon, requiring surgery. By 2009, she’d had three ruptures, each needing surgical fixes. To date, she’s had more than 30 surgeries to correct tendon ruptures. She’s also had seizures, blood pressure issues, depression, chronic pain, and memory problems she attributes to taking Levaquin.
As it turns out, her doctor misdiagnosed her condition – a misstep that would have been averted with a system like Australia’s, which requires doctors to verify the presence of a bacterial infection through a simple test before prescribing a fluoroquinolone.
“When I got the Levaquin, it was for a suspected sinus infection that it turned out I didn’t even have in the first place,” she notes. “So, I took the Levaquin basically for nothing. But what I would have asked my doctor had I known is: ‘Why should I take something so strong for so simple an infection?’
“It seems common sense to me now that you don’t prescribe something that can kill anthrax for a simple sinus infection. It’s like an atom bomb killing a mosquito. I agree that there are uses for these drugs, but they are being overprescribed. And so, here I am 16 years later – I’m still rupturing, I’m still having surgery, and I’m still in pain – all for something I didn’t even need medicine for in the first place.”
Should guidelines be stronger?
So, why are so many doctors continuing to prescribe fluoroquinolones for simple infections? Dr. Adalja and other experts say several things are at work.
For one thing, Dr. Adalja notes, fluoroquinolones are broad-spectrum antibiotics that are effective against dangerous germs, including “gram-negative” bacterial infections, and are “100% bioavailable.” That means they are as effective when given in pill form as they are if put directly into a vein. So they can be used in an outpatient setting or to allow a patient to be discharged from a hospital sooner because they don’t need an IV to receive treatment.
“There are still some uses for these drugs because they are so bioavailable, and I think that drives some of the use, and those are legitimate uses, knowing that there are risks when you do it,” he says. “But no drug is without risks, and you have to weigh risks and benefits – that’s what medicine is about: deciding what the best drug is for a patient.”
But Dr. Adalja says the overprescription of fluoroquinolones is part of the larger trend of antibiotic overuse. That is driving up antibiotic resistance, which in turn is another thing leading doctors to turn to Cipro and other fluoroquinolones after other drugs have proven ineffective.
“You can’t separate this from the fact that 80% of antibiotic prescriptions in the outpatient setting are probably illegitimate or not warranted,” he notes. “And because fluoroquinolones are highly effective drugs against certain pathogens, they are the go-to [drug] for many people who are prescribing antibiotics.”
That’s why patients should be wary whenever a doctor prescribes a fluoroquinolone, or any drug to treat a suspected infection, he says.
“Any time a patient is getting prescribed an antibiotic by a physician, they should ask: ‘Do I really need this antibiotic?’ That should be the first question they ask,” he advises. “And if they’re getting a fluoroquinolone, they may want to ask: ‘Is this the best antibiotic for me?’ ”
What you can do
Ms. Brummert and Ms. Moser say they are sharing their stories to raise awareness of the dangers of fluoroquinolones.
Ms. Moser has published a book on her experiences, “The Magnificent Story of a Lame Author,” and provides a wealth of consumer resources on her blog: Mountains and Mustard Seeds.
“As much as I hate what has happened to me, it has put me in a place where I am glad that I can inform other patients,” she says.
Ms. Brummert supplements her advocacy work as an FDA adviser with useful materials she provides on her website: Drugwatch.com.
“Pain into purpose – that’s what I call it,” she says. “I can’t change what happened to me, but I can warn others.”
The upshot for patients?
- the FDA’s Drug Safety Communication on Fluoroquinolones online to learn more about the risks and benefits of these powerful antibiotics.
- If you believe you’ve been harmed by fluoroquinolones, MedWatch website to report your experiences.
Ms. Brummert also advises patients to ask 12 critical questions of any doctor who wants to prescribe a fluoroquinolone, including the following listed on her website:
- For what condition is this medication prescribed, and is there another drug specific to my condition?
- What are the risks associated with this medication, and do the benefits outweigh them?
- Will this medication interact with my other drugs and/or other health conditions?
- What are the “boxed” warnings for this medication, and where can I report adverse events?
“I would also do my own research,” she says. “I wouldn’t just take a prescription from a physician and just say, ‘OK, doctor knows best.’ ”
Ms. Moser agrees that you have to be your own patient advocate and not simply take a doctor’s advice on any medical issue without having a deeper conversation.
“I’ve had arguments with doctors who legitimately did not believe me when I told them what happened to me,” she says. “And I actually told them, ‘Go get your Physicians’ Desk Reference [for prescription drugs]’ and they opened the book in front of me and read the warnings. Obviously, they had not been keeping up with the added warnings. So, I do think that doctors do need to be better informed.”
“So, yes, it’s the FDA’s responsibility, but it is also the doctors’ responsibility to make sure that they’re watching out for the side effects and they’re reporting them when their patients come up with them and making those connections.”
A version of this article first appeared on WebMD.com.
When Amy Moser had a simple urinary tract infection in her late 20s, her doctor prescribed Cipro, a powerful antibiotic used to treat anthrax and some of the most fearsome bacterial infections.
Nearly 2 weeks after she finished her treatment, her left kneecap dislocated while she was trying on a swimsuit at a retail store. Shortly afterward, she had painful ligament ruptures in her wrists, then her shoulder dislocated, followed by three Achilles tendon tears.
“That’s when I fell apart,” says Ms. Moser, a Phoenix health blogger and book author. “From that moment on, for almost the next 2.5 years consistently, I had new tendon tears every few weeks.”
Ms. Moser’s doctors had no answer for what was causing her injuries, all of which required surgical fixes. A married mother of three, she was otherwise healthy and fit. So, after her third Achilles tear, she turned to the FDA’s website for answers. There, she found many warnings about side effects of Cipro, Levaquin, and other so-called fluoroquinolones, including risks for tendon and ligament injuries.
“When all the ruptures started to happen, my doctor kept asking me if I’d ever taken Levaquin, and every time I was like, ‘No.’ So I did what all doctors don’t want you to do: I Googled ‘Levaquin,’ ” she recalls.
Her search led to FDA warnings and articles about the possibility of tendon and ligament ruptures with fluroquinolones.
“That was the first time I’d ever even heard that word ‘fluroquinolones,’ and I found Cipro on that list ... and I realized that I’d just been prescribed that before everything started,” she says.
That was 12 years ago. Since then, the FDA has issued more warnings about fluoroquinolone risks. In that time, Ms. Moser, now 40, has had more than 30 surgeries to correct tendon ruptures and injuries, including a double-knee replacement this year.
“I am in chronic pain all the time,” she says. “I am chronically injured. I have a lot of tears that I’ve not fixed because they’re very complicated, and I don’t know if the rest of my body can handle the strain of recovering from those surgeries.”
Ms. Moser’s is hardly an isolated case. Since the 1980s, more than 60,000 patients have reported hundreds of thousands of serious events linked to fluoroquinolones to the FDA, including 6,575 reports of deaths.
The most common side effects were tendon rupture, as well as neurological and psychiatric symptoms. But experts estimate only 1%-10% of such events are reported to the FDA. That suggests that fluoroquinolones might have harmed hundreds of thousands of people in the United States alone, says Charles Bennett, MD, a hematologist at the University of South Carolina’s College of Pharmacy, Columbia.
Yet despite the many patient reports and FDA warnings on dangerous side effects, better treated with less risky antibiotics.
“There probably is overprescription by primary care doctors for urinary tract infections and respiratory infections, when there could be alternatives that are safer to use,” says Amesh Adalja, MD, an infectious disease specialist and senior scholar with the Johns Hopkins Center for Health Security.
“I would say that’s probably the case in the outpatient setting, not necessarily in the hospital setting or among infectious disease doctors ... but I think it’s important to say there are still some judicious uses of fluoroquinolones,” he says. “However, there probably is a lot of injudicious use of fluoroquinolones along with many other antibiotics in the primary care setting.”
FDA warnings on fluoroquinolones
Fluoroquinolones are a class of broad-spectrum antibiotics used for decades to treat certain bacterial infections.
FDA-approved fluoroquinolones include ciprofloxacin (Cipro), ciprofloxacin extended-release tablets, delafloxacin (Baxdela), gemifloxacin (Factive) levofloxacin (Levaquin), moxifloxacin (Avelox), and ofloxacin (Floxin). More than 60 generic versions of these brand-name medicines are also on the market, making them among the most prescribed antibiotics in the U.S.
Over the past 2 decades, a wide range of physical and mental health side effects have been tied to fluoroquinolones. As a result of these “adverse event reports” and research published in medical literature, the FDA has required an escalating series of warnings and safety labeling changes for doctors who prescribe these drugs.
- In 2008, the FDA first added a “black box” warning to fluoroquinolones, citing an increased risk of tendinitis and tendon rupture in patients prescribed these meds.
- In 2011, the agency required the warning label to include risks of worsening symptoms for those with myasthenia gravis, a chronic autoimmune disease that causes muscle weakness, vision problems, and speech problems.
- In 2013, regulators required updated labels noting the potential for irreversible peripheral neuropathy (serious nerve damage).
- In 2016, the FDA issued its strongest warning against the use of such antibiotics for simple bacterial infections – such as uncomplicated urinary tract infections (UTIs), acute sinusitis, and acute bronchitis – saying the “association of fluoroquinolones with disabling and potentially permanent side effects involving tendons, muscles, joints, nerves and the central nervous system ... outweighs the benefits for patients.”
- And in 2018, regulators required safety labeling changes to include warnings about the risks of aortic aneurysm – a life-threatening enlargement of the main vessel that delivers blood to the body – as well as mental health side effects and serious blood sugar disturbances.
But FDA regulators have stopped short of barring fluoroquinolone use in the treatment of bacterial infections, citing the benefits for certain conditions.
“For some patients, the benefits of fluoroquinolones may continue to outweigh the risks for treatment of serious bacterial infections, such as pneumonia or intra-abdominal infections,” said former FDA Commissioner Scott Gottlieb, MD, “but there are other serious, known risks associated with these strong antibiotics that must be carefully weighed when considering their use.”
In December 2021, a study published in the journal JAMA Network Open found the FDA’s warnings may have helped lower prescribing of the drugs in Medicare patients. But not all doctors have been responsive to those warnings, researchers found.
“An overall decline in change over time and an immediate change in fluoroquinolone prescribing was observed after the 2016 FDA warning,” the authors concluded. “Certain physicians, such as primary care physicians, were more responsive to FDA warnings than others. ... Findings of this study suggest that identifying the association of physician and organizational characteristics with fluoroquinolone prescribing practices could help in developing mechanisms for improving de-adoption.”
Some critics say the FDA should do more to spotlight the dangers of fluoroquinolones and require doctors and patients to sign checklist consent forms to show they are aware of the potential side effects of these drugs.
Rachel Brummert, a patient advocate who sits on an FDA consumer advisory board, believes the FDA needs to improve its communication to doctors on fluoroquinolone risks and get tougher with those who continue to inappropriately prescribe the drugs.
“I think there needs to be a system in place, where if something comes down from the FDA about a drug, the physician has to sign off on it, the patient has to sign off on it and mark that they understand that there are these ‘black box’ warnings,” says Ms. Brummert, 52, a representative on the FDA’s Medical Devices Advisory Committee.
As an example, she points to Australia’s medical laws requiring doctors and patients to sign a checklist before any fluoroquinolone prescription is approved.
“When a physician prescribes a fluoroquinolone antibiotic, there’s a checklist – does the patient have an infection, is it a simple infection, do they have allergies?” she notes. “And you can’t even get the prescription out – it won’t even print out, it won’t go into the system – unless you check all of the boxes. But we don’t do that here. We don’t have that type of system right now.”
Ms. Brummert says such a system might have prevented the harm from taking Levaquin her doctor prescribed for a suspected sinus infection in 2006.
Soon after she began taking the antibiotic, she ruptured her Achilles tendon, requiring surgery. By 2009, she’d had three ruptures, each needing surgical fixes. To date, she’s had more than 30 surgeries to correct tendon ruptures. She’s also had seizures, blood pressure issues, depression, chronic pain, and memory problems she attributes to taking Levaquin.
As it turns out, her doctor misdiagnosed her condition – a misstep that would have been averted with a system like Australia’s, which requires doctors to verify the presence of a bacterial infection through a simple test before prescribing a fluoroquinolone.
“When I got the Levaquin, it was for a suspected sinus infection that it turned out I didn’t even have in the first place,” she notes. “So, I took the Levaquin basically for nothing. But what I would have asked my doctor had I known is: ‘Why should I take something so strong for so simple an infection?’
“It seems common sense to me now that you don’t prescribe something that can kill anthrax for a simple sinus infection. It’s like an atom bomb killing a mosquito. I agree that there are uses for these drugs, but they are being overprescribed. And so, here I am 16 years later – I’m still rupturing, I’m still having surgery, and I’m still in pain – all for something I didn’t even need medicine for in the first place.”
Should guidelines be stronger?
So, why are so many doctors continuing to prescribe fluoroquinolones for simple infections? Dr. Adalja and other experts say several things are at work.
For one thing, Dr. Adalja notes, fluoroquinolones are broad-spectrum antibiotics that are effective against dangerous germs, including “gram-negative” bacterial infections, and are “100% bioavailable.” That means they are as effective when given in pill form as they are if put directly into a vein. So they can be used in an outpatient setting or to allow a patient to be discharged from a hospital sooner because they don’t need an IV to receive treatment.
“There are still some uses for these drugs because they are so bioavailable, and I think that drives some of the use, and those are legitimate uses, knowing that there are risks when you do it,” he says. “But no drug is without risks, and you have to weigh risks and benefits – that’s what medicine is about: deciding what the best drug is for a patient.”
But Dr. Adalja says the overprescription of fluoroquinolones is part of the larger trend of antibiotic overuse. That is driving up antibiotic resistance, which in turn is another thing leading doctors to turn to Cipro and other fluoroquinolones after other drugs have proven ineffective.
“You can’t separate this from the fact that 80% of antibiotic prescriptions in the outpatient setting are probably illegitimate or not warranted,” he notes. “And because fluoroquinolones are highly effective drugs against certain pathogens, they are the go-to [drug] for many people who are prescribing antibiotics.”
That’s why patients should be wary whenever a doctor prescribes a fluoroquinolone, or any drug to treat a suspected infection, he says.
“Any time a patient is getting prescribed an antibiotic by a physician, they should ask: ‘Do I really need this antibiotic?’ That should be the first question they ask,” he advises. “And if they’re getting a fluoroquinolone, they may want to ask: ‘Is this the best antibiotic for me?’ ”
What you can do
Ms. Brummert and Ms. Moser say they are sharing their stories to raise awareness of the dangers of fluoroquinolones.
Ms. Moser has published a book on her experiences, “The Magnificent Story of a Lame Author,” and provides a wealth of consumer resources on her blog: Mountains and Mustard Seeds.
“As much as I hate what has happened to me, it has put me in a place where I am glad that I can inform other patients,” she says.
Ms. Brummert supplements her advocacy work as an FDA adviser with useful materials she provides on her website: Drugwatch.com.
“Pain into purpose – that’s what I call it,” she says. “I can’t change what happened to me, but I can warn others.”
The upshot for patients?
- the FDA’s Drug Safety Communication on Fluoroquinolones online to learn more about the risks and benefits of these powerful antibiotics.
- If you believe you’ve been harmed by fluoroquinolones, MedWatch website to report your experiences.
Ms. Brummert also advises patients to ask 12 critical questions of any doctor who wants to prescribe a fluoroquinolone, including the following listed on her website:
- For what condition is this medication prescribed, and is there another drug specific to my condition?
- What are the risks associated with this medication, and do the benefits outweigh them?
- Will this medication interact with my other drugs and/or other health conditions?
- What are the “boxed” warnings for this medication, and where can I report adverse events?
“I would also do my own research,” she says. “I wouldn’t just take a prescription from a physician and just say, ‘OK, doctor knows best.’ ”
Ms. Moser agrees that you have to be your own patient advocate and not simply take a doctor’s advice on any medical issue without having a deeper conversation.
“I’ve had arguments with doctors who legitimately did not believe me when I told them what happened to me,” she says. “And I actually told them, ‘Go get your Physicians’ Desk Reference [for prescription drugs]’ and they opened the book in front of me and read the warnings. Obviously, they had not been keeping up with the added warnings. So, I do think that doctors do need to be better informed.”
“So, yes, it’s the FDA’s responsibility, but it is also the doctors’ responsibility to make sure that they’re watching out for the side effects and they’re reporting them when their patients come up with them and making those connections.”
A version of this article first appeared on WebMD.com.
When Amy Moser had a simple urinary tract infection in her late 20s, her doctor prescribed Cipro, a powerful antibiotic used to treat anthrax and some of the most fearsome bacterial infections.
Nearly 2 weeks after she finished her treatment, her left kneecap dislocated while she was trying on a swimsuit at a retail store. Shortly afterward, she had painful ligament ruptures in her wrists, then her shoulder dislocated, followed by three Achilles tendon tears.
“That’s when I fell apart,” says Ms. Moser, a Phoenix health blogger and book author. “From that moment on, for almost the next 2.5 years consistently, I had new tendon tears every few weeks.”
Ms. Moser’s doctors had no answer for what was causing her injuries, all of which required surgical fixes. A married mother of three, she was otherwise healthy and fit. So, after her third Achilles tear, she turned to the FDA’s website for answers. There, she found many warnings about side effects of Cipro, Levaquin, and other so-called fluoroquinolones, including risks for tendon and ligament injuries.
“When all the ruptures started to happen, my doctor kept asking me if I’d ever taken Levaquin, and every time I was like, ‘No.’ So I did what all doctors don’t want you to do: I Googled ‘Levaquin,’ ” she recalls.
Her search led to FDA warnings and articles about the possibility of tendon and ligament ruptures with fluroquinolones.
“That was the first time I’d ever even heard that word ‘fluroquinolones,’ and I found Cipro on that list ... and I realized that I’d just been prescribed that before everything started,” she says.
That was 12 years ago. Since then, the FDA has issued more warnings about fluoroquinolone risks. In that time, Ms. Moser, now 40, has had more than 30 surgeries to correct tendon ruptures and injuries, including a double-knee replacement this year.
“I am in chronic pain all the time,” she says. “I am chronically injured. I have a lot of tears that I’ve not fixed because they’re very complicated, and I don’t know if the rest of my body can handle the strain of recovering from those surgeries.”
Ms. Moser’s is hardly an isolated case. Since the 1980s, more than 60,000 patients have reported hundreds of thousands of serious events linked to fluoroquinolones to the FDA, including 6,575 reports of deaths.
The most common side effects were tendon rupture, as well as neurological and psychiatric symptoms. But experts estimate only 1%-10% of such events are reported to the FDA. That suggests that fluoroquinolones might have harmed hundreds of thousands of people in the United States alone, says Charles Bennett, MD, a hematologist at the University of South Carolina’s College of Pharmacy, Columbia.
Yet despite the many patient reports and FDA warnings on dangerous side effects, better treated with less risky antibiotics.
“There probably is overprescription by primary care doctors for urinary tract infections and respiratory infections, when there could be alternatives that are safer to use,” says Amesh Adalja, MD, an infectious disease specialist and senior scholar with the Johns Hopkins Center for Health Security.
“I would say that’s probably the case in the outpatient setting, not necessarily in the hospital setting or among infectious disease doctors ... but I think it’s important to say there are still some judicious uses of fluoroquinolones,” he says. “However, there probably is a lot of injudicious use of fluoroquinolones along with many other antibiotics in the primary care setting.”
FDA warnings on fluoroquinolones
Fluoroquinolones are a class of broad-spectrum antibiotics used for decades to treat certain bacterial infections.
FDA-approved fluoroquinolones include ciprofloxacin (Cipro), ciprofloxacin extended-release tablets, delafloxacin (Baxdela), gemifloxacin (Factive) levofloxacin (Levaquin), moxifloxacin (Avelox), and ofloxacin (Floxin). More than 60 generic versions of these brand-name medicines are also on the market, making them among the most prescribed antibiotics in the U.S.
Over the past 2 decades, a wide range of physical and mental health side effects have been tied to fluoroquinolones. As a result of these “adverse event reports” and research published in medical literature, the FDA has required an escalating series of warnings and safety labeling changes for doctors who prescribe these drugs.
- In 2008, the FDA first added a “black box” warning to fluoroquinolones, citing an increased risk of tendinitis and tendon rupture in patients prescribed these meds.
- In 2011, the agency required the warning label to include risks of worsening symptoms for those with myasthenia gravis, a chronic autoimmune disease that causes muscle weakness, vision problems, and speech problems.
- In 2013, regulators required updated labels noting the potential for irreversible peripheral neuropathy (serious nerve damage).
- In 2016, the FDA issued its strongest warning against the use of such antibiotics for simple bacterial infections – such as uncomplicated urinary tract infections (UTIs), acute sinusitis, and acute bronchitis – saying the “association of fluoroquinolones with disabling and potentially permanent side effects involving tendons, muscles, joints, nerves and the central nervous system ... outweighs the benefits for patients.”
- And in 2018, regulators required safety labeling changes to include warnings about the risks of aortic aneurysm – a life-threatening enlargement of the main vessel that delivers blood to the body – as well as mental health side effects and serious blood sugar disturbances.
But FDA regulators have stopped short of barring fluoroquinolone use in the treatment of bacterial infections, citing the benefits for certain conditions.
“For some patients, the benefits of fluoroquinolones may continue to outweigh the risks for treatment of serious bacterial infections, such as pneumonia or intra-abdominal infections,” said former FDA Commissioner Scott Gottlieb, MD, “but there are other serious, known risks associated with these strong antibiotics that must be carefully weighed when considering their use.”
In December 2021, a study published in the journal JAMA Network Open found the FDA’s warnings may have helped lower prescribing of the drugs in Medicare patients. But not all doctors have been responsive to those warnings, researchers found.
“An overall decline in change over time and an immediate change in fluoroquinolone prescribing was observed after the 2016 FDA warning,” the authors concluded. “Certain physicians, such as primary care physicians, were more responsive to FDA warnings than others. ... Findings of this study suggest that identifying the association of physician and organizational characteristics with fluoroquinolone prescribing practices could help in developing mechanisms for improving de-adoption.”
Some critics say the FDA should do more to spotlight the dangers of fluoroquinolones and require doctors and patients to sign checklist consent forms to show they are aware of the potential side effects of these drugs.
Rachel Brummert, a patient advocate who sits on an FDA consumer advisory board, believes the FDA needs to improve its communication to doctors on fluoroquinolone risks and get tougher with those who continue to inappropriately prescribe the drugs.
“I think there needs to be a system in place, where if something comes down from the FDA about a drug, the physician has to sign off on it, the patient has to sign off on it and mark that they understand that there are these ‘black box’ warnings,” says Ms. Brummert, 52, a representative on the FDA’s Medical Devices Advisory Committee.
As an example, she points to Australia’s medical laws requiring doctors and patients to sign a checklist before any fluoroquinolone prescription is approved.
“When a physician prescribes a fluoroquinolone antibiotic, there’s a checklist – does the patient have an infection, is it a simple infection, do they have allergies?” she notes. “And you can’t even get the prescription out – it won’t even print out, it won’t go into the system – unless you check all of the boxes. But we don’t do that here. We don’t have that type of system right now.”
Ms. Brummert says such a system might have prevented the harm from taking Levaquin her doctor prescribed for a suspected sinus infection in 2006.
Soon after she began taking the antibiotic, she ruptured her Achilles tendon, requiring surgery. By 2009, she’d had three ruptures, each needing surgical fixes. To date, she’s had more than 30 surgeries to correct tendon ruptures. She’s also had seizures, blood pressure issues, depression, chronic pain, and memory problems she attributes to taking Levaquin.
As it turns out, her doctor misdiagnosed her condition – a misstep that would have been averted with a system like Australia’s, which requires doctors to verify the presence of a bacterial infection through a simple test before prescribing a fluoroquinolone.
“When I got the Levaquin, it was for a suspected sinus infection that it turned out I didn’t even have in the first place,” she notes. “So, I took the Levaquin basically for nothing. But what I would have asked my doctor had I known is: ‘Why should I take something so strong for so simple an infection?’
“It seems common sense to me now that you don’t prescribe something that can kill anthrax for a simple sinus infection. It’s like an atom bomb killing a mosquito. I agree that there are uses for these drugs, but they are being overprescribed. And so, here I am 16 years later – I’m still rupturing, I’m still having surgery, and I’m still in pain – all for something I didn’t even need medicine for in the first place.”
Should guidelines be stronger?
So, why are so many doctors continuing to prescribe fluoroquinolones for simple infections? Dr. Adalja and other experts say several things are at work.
For one thing, Dr. Adalja notes, fluoroquinolones are broad-spectrum antibiotics that are effective against dangerous germs, including “gram-negative” bacterial infections, and are “100% bioavailable.” That means they are as effective when given in pill form as they are if put directly into a vein. So they can be used in an outpatient setting or to allow a patient to be discharged from a hospital sooner because they don’t need an IV to receive treatment.
“There are still some uses for these drugs because they are so bioavailable, and I think that drives some of the use, and those are legitimate uses, knowing that there are risks when you do it,” he says. “But no drug is without risks, and you have to weigh risks and benefits – that’s what medicine is about: deciding what the best drug is for a patient.”
But Dr. Adalja says the overprescription of fluoroquinolones is part of the larger trend of antibiotic overuse. That is driving up antibiotic resistance, which in turn is another thing leading doctors to turn to Cipro and other fluoroquinolones after other drugs have proven ineffective.
“You can’t separate this from the fact that 80% of antibiotic prescriptions in the outpatient setting are probably illegitimate or not warranted,” he notes. “And because fluoroquinolones are highly effective drugs against certain pathogens, they are the go-to [drug] for many people who are prescribing antibiotics.”
That’s why patients should be wary whenever a doctor prescribes a fluoroquinolone, or any drug to treat a suspected infection, he says.
“Any time a patient is getting prescribed an antibiotic by a physician, they should ask: ‘Do I really need this antibiotic?’ That should be the first question they ask,” he advises. “And if they’re getting a fluoroquinolone, they may want to ask: ‘Is this the best antibiotic for me?’ ”
What you can do
Ms. Brummert and Ms. Moser say they are sharing their stories to raise awareness of the dangers of fluoroquinolones.
Ms. Moser has published a book on her experiences, “The Magnificent Story of a Lame Author,” and provides a wealth of consumer resources on her blog: Mountains and Mustard Seeds.
“As much as I hate what has happened to me, it has put me in a place where I am glad that I can inform other patients,” she says.
Ms. Brummert supplements her advocacy work as an FDA adviser with useful materials she provides on her website: Drugwatch.com.
“Pain into purpose – that’s what I call it,” she says. “I can’t change what happened to me, but I can warn others.”
The upshot for patients?
- the FDA’s Drug Safety Communication on Fluoroquinolones online to learn more about the risks and benefits of these powerful antibiotics.
- If you believe you’ve been harmed by fluoroquinolones, MedWatch website to report your experiences.
Ms. Brummert also advises patients to ask 12 critical questions of any doctor who wants to prescribe a fluoroquinolone, including the following listed on her website:
- For what condition is this medication prescribed, and is there another drug specific to my condition?
- What are the risks associated with this medication, and do the benefits outweigh them?
- Will this medication interact with my other drugs and/or other health conditions?
- What are the “boxed” warnings for this medication, and where can I report adverse events?
“I would also do my own research,” she says. “I wouldn’t just take a prescription from a physician and just say, ‘OK, doctor knows best.’ ”
Ms. Moser agrees that you have to be your own patient advocate and not simply take a doctor’s advice on any medical issue without having a deeper conversation.
“I’ve had arguments with doctors who legitimately did not believe me when I told them what happened to me,” she says. “And I actually told them, ‘Go get your Physicians’ Desk Reference [for prescription drugs]’ and they opened the book in front of me and read the warnings. Obviously, they had not been keeping up with the added warnings. So, I do think that doctors do need to be better informed.”
“So, yes, it’s the FDA’s responsibility, but it is also the doctors’ responsibility to make sure that they’re watching out for the side effects and they’re reporting them when their patients come up with them and making those connections.”
A version of this article first appeared on WebMD.com.
Physicians react: Compensation isn’t worth the hassles. What’s the solution?
How satisfied are physicians that they are fairly compensated for their dedication, skills, and time? That’s a subject that seems to steer many physicians to heated emotions and to a variety of issues with today’s medical field – not all of which directly affect their pay.
Medscape’s Physician Compensation Report 2022: “Incomes Gain, Pay Gaps Remain” generally shows encouraging trends. Physician income rose from a year earlier, when it stagnated as COVID-19 restrictions led patients to stay home and medical practices to cut hours or close. And for the first time in Medscape’s 11 years of reporting on physician compensation, average income rose for every medical specialty surveyed.
Heartening findings, right? Yet the tone of comments to the report was anything but peppy. One physician even complained his plumber earns more than he does.
A family physician lamented that he has “made less in the past 3 years, with more hassles and work” and he “can’t wait to retire next year.” Meanwhile, he complained, the U.S. health system is “the costliest, yet wasteful, with worse outcomes; reactive, not preventative; and has the costliest drugs and social issues.”
Do NPs and PAs encroach on your income?
The conversation about fair compensation launched some commenters into a discussion about competition from nurse practitioners (NPs) and physician assistants (PAs). Some physicians expressed wariness at best, and anger at worst, about NPs and PAs evolving beyond traditional doctor support roles into certain direct patient support.
One-fourth of respondents in the compensation report said their income was negatively affected by competition from NPs, PAs, and other nonphysician providers. For example, with states like Arkansas expanding independent practice for certified registered nurse anesthetists (CRNAs), one commenter complained, “we are no longer needed.”
Added another physician, “primary care, especially internal medicine, is just going away for doctors. We wasted, by all accounts, 4 full years of our working lives because NPs are ‘just as good.’ ”
Greater independence for NPs and PAs strengthens the hands of health insurers and “will end up hastening the demise of primary care as we have known it,” another reader predicted. Other physicians’ takes: “For the institution, it’s much cheaper to hire NPs than to hire doctors” and “overall, physician negotiating power will decrease in the future.”
Medicare reimbursement rates grate
Although 7 in 10 respondents in the compensation report said they would continue to accept new Medicare or Medicaid patients, comments reveal resentment about the practical need to work with Medicare and its resentment rates.
“An open question to Medicare: Are you doing the dumbest thing possible by paying low wages and giving huge administrative burdens for internal medicine on purpose?” one physician wrote. “Or are you really that short-sighted?”
Another reader cited an analysis from the American College of Surgeons of Medicare’s 1998 surgical CPT codes. If Medicare had left those codes alone beyond annual inflation adjustments, the study found, reimbursement rates by 2019 would be half of what they became.
Another way of looking at the code reimbursement increases is a 50% pay cut over 20 years for doctors and medical practices, that reader insisted. “The rising cost of employee wages, particularly of the last two-and-a-half years of COVID-19, combined with the effective pay cuts over the last 20 years, equals time to quit!”
Another commenter concurred. “In the 1990s, most full-time docs were making almost double what you see [in the report], and everything cost almost half of what it does now. So, MD purchasing power is between half and one-quarter of what it was in the early 1990s.”
Are self-pay models better?
Do physicians have a better chance at consistently fair income under a self-pay practice that avoids dealing with insurance companies?
One commenter hypothesized that psychiatrists once trailed internists in income but today earn more because many “quit working for insurance and went to a cash business 15 years ago.” Many family physicians did something similar by switching to a direct primary care model, he said.
This physician said he has done the same “with great results” for patients as well: shorter office visits, faster booking of appointments, no deductibles owed. Best of all, “I love practicing medicine again, and my patients love the great health care they receive.”
Another commenter agreed. “Two words: cash practice.” But another objected, “I guess only the very rich can afford to cover your business costs.”
Regardless of the payment model, another physician argued for private practice over employed positions. “Save on the bureaucratic expenses. Go back to private practice and get rid of electronic records.”
A version of this article first appeared on Medscape.com.
How satisfied are physicians that they are fairly compensated for their dedication, skills, and time? That’s a subject that seems to steer many physicians to heated emotions and to a variety of issues with today’s medical field – not all of which directly affect their pay.
Medscape’s Physician Compensation Report 2022: “Incomes Gain, Pay Gaps Remain” generally shows encouraging trends. Physician income rose from a year earlier, when it stagnated as COVID-19 restrictions led patients to stay home and medical practices to cut hours or close. And for the first time in Medscape’s 11 years of reporting on physician compensation, average income rose for every medical specialty surveyed.
Heartening findings, right? Yet the tone of comments to the report was anything but peppy. One physician even complained his plumber earns more than he does.
A family physician lamented that he has “made less in the past 3 years, with more hassles and work” and he “can’t wait to retire next year.” Meanwhile, he complained, the U.S. health system is “the costliest, yet wasteful, with worse outcomes; reactive, not preventative; and has the costliest drugs and social issues.”
Do NPs and PAs encroach on your income?
The conversation about fair compensation launched some commenters into a discussion about competition from nurse practitioners (NPs) and physician assistants (PAs). Some physicians expressed wariness at best, and anger at worst, about NPs and PAs evolving beyond traditional doctor support roles into certain direct patient support.
One-fourth of respondents in the compensation report said their income was negatively affected by competition from NPs, PAs, and other nonphysician providers. For example, with states like Arkansas expanding independent practice for certified registered nurse anesthetists (CRNAs), one commenter complained, “we are no longer needed.”
Added another physician, “primary care, especially internal medicine, is just going away for doctors. We wasted, by all accounts, 4 full years of our working lives because NPs are ‘just as good.’ ”
Greater independence for NPs and PAs strengthens the hands of health insurers and “will end up hastening the demise of primary care as we have known it,” another reader predicted. Other physicians’ takes: “For the institution, it’s much cheaper to hire NPs than to hire doctors” and “overall, physician negotiating power will decrease in the future.”
Medicare reimbursement rates grate
Although 7 in 10 respondents in the compensation report said they would continue to accept new Medicare or Medicaid patients, comments reveal resentment about the practical need to work with Medicare and its resentment rates.
“An open question to Medicare: Are you doing the dumbest thing possible by paying low wages and giving huge administrative burdens for internal medicine on purpose?” one physician wrote. “Or are you really that short-sighted?”
Another reader cited an analysis from the American College of Surgeons of Medicare’s 1998 surgical CPT codes. If Medicare had left those codes alone beyond annual inflation adjustments, the study found, reimbursement rates by 2019 would be half of what they became.
Another way of looking at the code reimbursement increases is a 50% pay cut over 20 years for doctors and medical practices, that reader insisted. “The rising cost of employee wages, particularly of the last two-and-a-half years of COVID-19, combined with the effective pay cuts over the last 20 years, equals time to quit!”
Another commenter concurred. “In the 1990s, most full-time docs were making almost double what you see [in the report], and everything cost almost half of what it does now. So, MD purchasing power is between half and one-quarter of what it was in the early 1990s.”
Are self-pay models better?
Do physicians have a better chance at consistently fair income under a self-pay practice that avoids dealing with insurance companies?
One commenter hypothesized that psychiatrists once trailed internists in income but today earn more because many “quit working for insurance and went to a cash business 15 years ago.” Many family physicians did something similar by switching to a direct primary care model, he said.
This physician said he has done the same “with great results” for patients as well: shorter office visits, faster booking of appointments, no deductibles owed. Best of all, “I love practicing medicine again, and my patients love the great health care they receive.”
Another commenter agreed. “Two words: cash practice.” But another objected, “I guess only the very rich can afford to cover your business costs.”
Regardless of the payment model, another physician argued for private practice over employed positions. “Save on the bureaucratic expenses. Go back to private practice and get rid of electronic records.”
A version of this article first appeared on Medscape.com.
How satisfied are physicians that they are fairly compensated for their dedication, skills, and time? That’s a subject that seems to steer many physicians to heated emotions and to a variety of issues with today’s medical field – not all of which directly affect their pay.
Medscape’s Physician Compensation Report 2022: “Incomes Gain, Pay Gaps Remain” generally shows encouraging trends. Physician income rose from a year earlier, when it stagnated as COVID-19 restrictions led patients to stay home and medical practices to cut hours or close. And for the first time in Medscape’s 11 years of reporting on physician compensation, average income rose for every medical specialty surveyed.
Heartening findings, right? Yet the tone of comments to the report was anything but peppy. One physician even complained his plumber earns more than he does.
A family physician lamented that he has “made less in the past 3 years, with more hassles and work” and he “can’t wait to retire next year.” Meanwhile, he complained, the U.S. health system is “the costliest, yet wasteful, with worse outcomes; reactive, not preventative; and has the costliest drugs and social issues.”
Do NPs and PAs encroach on your income?
The conversation about fair compensation launched some commenters into a discussion about competition from nurse practitioners (NPs) and physician assistants (PAs). Some physicians expressed wariness at best, and anger at worst, about NPs and PAs evolving beyond traditional doctor support roles into certain direct patient support.
One-fourth of respondents in the compensation report said their income was negatively affected by competition from NPs, PAs, and other nonphysician providers. For example, with states like Arkansas expanding independent practice for certified registered nurse anesthetists (CRNAs), one commenter complained, “we are no longer needed.”
Added another physician, “primary care, especially internal medicine, is just going away for doctors. We wasted, by all accounts, 4 full years of our working lives because NPs are ‘just as good.’ ”
Greater independence for NPs and PAs strengthens the hands of health insurers and “will end up hastening the demise of primary care as we have known it,” another reader predicted. Other physicians’ takes: “For the institution, it’s much cheaper to hire NPs than to hire doctors” and “overall, physician negotiating power will decrease in the future.”
Medicare reimbursement rates grate
Although 7 in 10 respondents in the compensation report said they would continue to accept new Medicare or Medicaid patients, comments reveal resentment about the practical need to work with Medicare and its resentment rates.
“An open question to Medicare: Are you doing the dumbest thing possible by paying low wages and giving huge administrative burdens for internal medicine on purpose?” one physician wrote. “Or are you really that short-sighted?”
Another reader cited an analysis from the American College of Surgeons of Medicare’s 1998 surgical CPT codes. If Medicare had left those codes alone beyond annual inflation adjustments, the study found, reimbursement rates by 2019 would be half of what they became.
Another way of looking at the code reimbursement increases is a 50% pay cut over 20 years for doctors and medical practices, that reader insisted. “The rising cost of employee wages, particularly of the last two-and-a-half years of COVID-19, combined with the effective pay cuts over the last 20 years, equals time to quit!”
Another commenter concurred. “In the 1990s, most full-time docs were making almost double what you see [in the report], and everything cost almost half of what it does now. So, MD purchasing power is between half and one-quarter of what it was in the early 1990s.”
Are self-pay models better?
Do physicians have a better chance at consistently fair income under a self-pay practice that avoids dealing with insurance companies?
One commenter hypothesized that psychiatrists once trailed internists in income but today earn more because many “quit working for insurance and went to a cash business 15 years ago.” Many family physicians did something similar by switching to a direct primary care model, he said.
This physician said he has done the same “with great results” for patients as well: shorter office visits, faster booking of appointments, no deductibles owed. Best of all, “I love practicing medicine again, and my patients love the great health care they receive.”
Another commenter agreed. “Two words: cash practice.” But another objected, “I guess only the very rich can afford to cover your business costs.”
Regardless of the payment model, another physician argued for private practice over employed positions. “Save on the bureaucratic expenses. Go back to private practice and get rid of electronic records.”
A version of this article first appeared on Medscape.com.
Erythematous Pedunculated Plaque on the Dorsal Aspect of the Foot
The Diagnosis: Molluscum Contagiosum
A tangential shave removal with electrocautery was performed. Histopathology demonstrated numerous eosinophilic intracytoplasmic inclusion bodies (Figure), confirming a diagnosis of molluscum contagiosum (MC).
Molluscum contagiosum is a common poxvirus infection that is transmitted through fomites, contact, or self-inoculation.1 This infection most frequently occurs in school-aged children younger than 8 years1-3; peak incidence is 6 years of age.2,3 The worldwide estimated prevalence in children is 5.1% to 11.5%.1,3 In children cohabitating with others infected by MC, approximately 40% of households experienced a spread of infection; the risk of transmission is not associated with greater number of lesions.4 In adults, infection most commonly occurs in the setting of immunodeficiency or as a sexually transmitted infection in immunocompetent patients.3 Molluscum contagiosum infection classically presents as 1- to 3-mm, flesh- or white-colored, dome-shaped, smooth papules with central umbilication.1 Lesions often occur in clusters or lines, indicating local spread. The trunk, extremities, and face are areas that frequently are involved.2,3
Atypical presentations of MC infection can occur, as demonstrated by our case. Involvement of hair follicles by the infection can result in follicular induction.1,5 Secondary infection can mimic abscess formation.1 Inflamed MC lesions demonstrating the “beginning of the end” sign often are mistaken for primary infection, which is thought to be an inflammatory immune response to the virus.6 Lesions located on the eye or eyelid can present as unilateral conjunctivitis, conjunctival or corneal nodules, eyelid abscesses, or chalazions.1 Giant MC is a nodular variant of this infection measuring larger than 1 cm in size that can present similar to epidermoid cysts, condyloma acuminatum, or verruca vulgaris.1,7 Other reported mimicked conditions include basal cell carcinoma, trichoepithelioma, appendageal tumors, keratoacanthoma, foreign body granulomas, nevus sebaceous, or ecthyma.1,3 Molluscum contagiosum also has been reported to present as large ulcerative growths.8 In immunocompromised patients, deep fungal infection is another mimicker.1 Lesions on the plantar surfaces of the feet often are misdiagnosed as plantar verruca and present with pain during ambulation.9
The diagnosis of MC is clinical, with additional diagnostic tools reserved for more challenging situations.1 In cases with atypical presentations, dermoscopy may aid diagnosis through visualization of orifices and vascular patterns including crown, radial, and punctiform vessels.10 Biopsy or fine-needle aspiration also can be utilized as a diagnostic tool. Histopathology often reveals pathognomonic intracytoplasmic inclusions or Henderson-Paterson bodies.8,10 The appearance of MC can mimic other conditions that should be included in the differential diagnosis. Pyogenic granuloma often presents as a benign red papule that may grow rapidly and become pedunculated, sometimes with bleeding and crusting, though histology reveals groups of proliferating capillaries.11 More than half of amelanotic melanomas present in the papulonodular form as vascular or ulcerated nodules, and others may appear as erythematous macules. Diagnosis of amelanotic melanoma is made through histologic examination, which reveals atypical melanocytes in nests or cords, in conjunction with immunohistochemical stains such as S-100.12 Spitz nevi often appear as round, dome-shaped papules that most commonly are red, pink, or fleshcolored. They appear histologically similar to melanoma with nests of atypical melanocytes and nuclear atypia.13
A variety of treatment modalities can be used for MC including cantharidin, curettage, and cryotherapy.14 Imiquimod no longer is recommended due to a lack of demonstrated superiority over placebo in recent studies as well as its adverse effects.3 Topical retinoids have been recommended; however, their use frequently is limited by local irritation.3,14 Cantharidin is the most frequently utilized treatment by pediatric dermatologists. Most health care providers report subjective satisfaction with its results and efficacy, though some side effects may occur including discomfort and temporary changes in pigmentation. Treatment for MC is not required, as the condition is self-limiting.14 Therapy often is reserved for those with extensive disease, complications from lesions, cosmetic or psychological concerns, or genital involvement given the potential for sexual transmission.3 Time to resolution without treatment varies and is more prolonged in immunocompromised patients. Mean time to resolution in immunocompetent hosts has been reported as 13.3 months, but most infections are noted to clear within 2 to 4 years.1,4 Although resolution without treatment occurs, transmission to others and negative impact on quality of life (QOL) can occur and support the need for treatment. Greater impact on QOL was observed in females, those with more lesions, and patients with a longer duration of symptoms. Moderate impact on QOL was reported in 28% of patients (n=301), and severe effects were reported in 11%.4
In conclusion, MC is a common, benign, treatable cutaneous viral infection that often presents as small, flesh-colored papules in children. Its appearance can mimic a variety of other conditions. In cases with abnormal presentations, definitive diagnosis with pathology can be important to differentiate MC from more dangerous etiologies that may require further treatment.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99. doi:10.1111 /j.1365-4632.2006.02737.x
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54. doi:10.1016/j.jaad.2005.08.035
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Derm Rep. 2020;9:83-92.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195. doi:10.1016/S1473-3099(14)71053-9
- Davey J, Biswas A. Follicular induction in a case of molluscum contagiosum: possible link with secondary anetoderma-like changes? Am J Dermatopathol. 2014;36:E19-E21. doi:10.1097/DAD.0b013e31828bc7c7
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653. doi:10.1542/peds.2012-2933
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73. doi:10.5826/dpc.0603a15
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796. doi:10.1002/dc.23964
- Cohen PR, Tschen JA. Plantar molluscum contagiosum: a case report of molluscum contagiosum occurring on the sole of the foot and a review of the world literature. Cutis. 2012;90:35-41.
- Megalla M, Bronsnick T, Noor O, et al. Dermoscopic, confocal microscopic, and histologic characteristics of an atypical presentation of molluscum contagiosum. Ann Clin Pathol. 2014;2:1038.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.1525-1470.1991.tb00931.x
- Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221-230. doi:10.1097/CMR.0000000000000571
- Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 pt 1):901-913. doi:10.1016/0190-9622(92)70286-o
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
The Diagnosis: Molluscum Contagiosum
A tangential shave removal with electrocautery was performed. Histopathology demonstrated numerous eosinophilic intracytoplasmic inclusion bodies (Figure), confirming a diagnosis of molluscum contagiosum (MC).

Molluscum contagiosum is a common poxvirus infection that is transmitted through fomites, contact, or self-inoculation.1 This infection most frequently occurs in school-aged children younger than 8 years1-3; peak incidence is 6 years of age.2,3 The worldwide estimated prevalence in children is 5.1% to 11.5%.1,3 In children cohabitating with others infected by MC, approximately 40% of households experienced a spread of infection; the risk of transmission is not associated with greater number of lesions.4 In adults, infection most commonly occurs in the setting of immunodeficiency or as a sexually transmitted infection in immunocompetent patients.3 Molluscum contagiosum infection classically presents as 1- to 3-mm, flesh- or white-colored, dome-shaped, smooth papules with central umbilication.1 Lesions often occur in clusters or lines, indicating local spread. The trunk, extremities, and face are areas that frequently are involved.2,3
Atypical presentations of MC infection can occur, as demonstrated by our case. Involvement of hair follicles by the infection can result in follicular induction.1,5 Secondary infection can mimic abscess formation.1 Inflamed MC lesions demonstrating the “beginning of the end” sign often are mistaken for primary infection, which is thought to be an inflammatory immune response to the virus.6 Lesions located on the eye or eyelid can present as unilateral conjunctivitis, conjunctival or corneal nodules, eyelid abscesses, or chalazions.1 Giant MC is a nodular variant of this infection measuring larger than 1 cm in size that can present similar to epidermoid cysts, condyloma acuminatum, or verruca vulgaris.1,7 Other reported mimicked conditions include basal cell carcinoma, trichoepithelioma, appendageal tumors, keratoacanthoma, foreign body granulomas, nevus sebaceous, or ecthyma.1,3 Molluscum contagiosum also has been reported to present as large ulcerative growths.8 In immunocompromised patients, deep fungal infection is another mimicker.1 Lesions on the plantar surfaces of the feet often are misdiagnosed as plantar verruca and present with pain during ambulation.9
The diagnosis of MC is clinical, with additional diagnostic tools reserved for more challenging situations.1 In cases with atypical presentations, dermoscopy may aid diagnosis through visualization of orifices and vascular patterns including crown, radial, and punctiform vessels.10 Biopsy or fine-needle aspiration also can be utilized as a diagnostic tool. Histopathology often reveals pathognomonic intracytoplasmic inclusions or Henderson-Paterson bodies.8,10 The appearance of MC can mimic other conditions that should be included in the differential diagnosis. Pyogenic granuloma often presents as a benign red papule that may grow rapidly and become pedunculated, sometimes with bleeding and crusting, though histology reveals groups of proliferating capillaries.11 More than half of amelanotic melanomas present in the papulonodular form as vascular or ulcerated nodules, and others may appear as erythematous macules. Diagnosis of amelanotic melanoma is made through histologic examination, which reveals atypical melanocytes in nests or cords, in conjunction with immunohistochemical stains such as S-100.12 Spitz nevi often appear as round, dome-shaped papules that most commonly are red, pink, or fleshcolored. They appear histologically similar to melanoma with nests of atypical melanocytes and nuclear atypia.13
A variety of treatment modalities can be used for MC including cantharidin, curettage, and cryotherapy.14 Imiquimod no longer is recommended due to a lack of demonstrated superiority over placebo in recent studies as well as its adverse effects.3 Topical retinoids have been recommended; however, their use frequently is limited by local irritation.3,14 Cantharidin is the most frequently utilized treatment by pediatric dermatologists. Most health care providers report subjective satisfaction with its results and efficacy, though some side effects may occur including discomfort and temporary changes in pigmentation. Treatment for MC is not required, as the condition is self-limiting.14 Therapy often is reserved for those with extensive disease, complications from lesions, cosmetic or psychological concerns, or genital involvement given the potential for sexual transmission.3 Time to resolution without treatment varies and is more prolonged in immunocompromised patients. Mean time to resolution in immunocompetent hosts has been reported as 13.3 months, but most infections are noted to clear within 2 to 4 years.1,4 Although resolution without treatment occurs, transmission to others and negative impact on quality of life (QOL) can occur and support the need for treatment. Greater impact on QOL was observed in females, those with more lesions, and patients with a longer duration of symptoms. Moderate impact on QOL was reported in 28% of patients (n=301), and severe effects were reported in 11%.4
In conclusion, MC is a common, benign, treatable cutaneous viral infection that often presents as small, flesh-colored papules in children. Its appearance can mimic a variety of other conditions. In cases with abnormal presentations, definitive diagnosis with pathology can be important to differentiate MC from more dangerous etiologies that may require further treatment.
The Diagnosis: Molluscum Contagiosum
A tangential shave removal with electrocautery was performed. Histopathology demonstrated numerous eosinophilic intracytoplasmic inclusion bodies (Figure), confirming a diagnosis of molluscum contagiosum (MC).

Molluscum contagiosum is a common poxvirus infection that is transmitted through fomites, contact, or self-inoculation.1 This infection most frequently occurs in school-aged children younger than 8 years1-3; peak incidence is 6 years of age.2,3 The worldwide estimated prevalence in children is 5.1% to 11.5%.1,3 In children cohabitating with others infected by MC, approximately 40% of households experienced a spread of infection; the risk of transmission is not associated with greater number of lesions.4 In adults, infection most commonly occurs in the setting of immunodeficiency or as a sexually transmitted infection in immunocompetent patients.3 Molluscum contagiosum infection classically presents as 1- to 3-mm, flesh- or white-colored, dome-shaped, smooth papules with central umbilication.1 Lesions often occur in clusters or lines, indicating local spread. The trunk, extremities, and face are areas that frequently are involved.2,3
Atypical presentations of MC infection can occur, as demonstrated by our case. Involvement of hair follicles by the infection can result in follicular induction.1,5 Secondary infection can mimic abscess formation.1 Inflamed MC lesions demonstrating the “beginning of the end” sign often are mistaken for primary infection, which is thought to be an inflammatory immune response to the virus.6 Lesions located on the eye or eyelid can present as unilateral conjunctivitis, conjunctival or corneal nodules, eyelid abscesses, or chalazions.1 Giant MC is a nodular variant of this infection measuring larger than 1 cm in size that can present similar to epidermoid cysts, condyloma acuminatum, or verruca vulgaris.1,7 Other reported mimicked conditions include basal cell carcinoma, trichoepithelioma, appendageal tumors, keratoacanthoma, foreign body granulomas, nevus sebaceous, or ecthyma.1,3 Molluscum contagiosum also has been reported to present as large ulcerative growths.8 In immunocompromised patients, deep fungal infection is another mimicker.1 Lesions on the plantar surfaces of the feet often are misdiagnosed as plantar verruca and present with pain during ambulation.9
The diagnosis of MC is clinical, with additional diagnostic tools reserved for more challenging situations.1 In cases with atypical presentations, dermoscopy may aid diagnosis through visualization of orifices and vascular patterns including crown, radial, and punctiform vessels.10 Biopsy or fine-needle aspiration also can be utilized as a diagnostic tool. Histopathology often reveals pathognomonic intracytoplasmic inclusions or Henderson-Paterson bodies.8,10 The appearance of MC can mimic other conditions that should be included in the differential diagnosis. Pyogenic granuloma often presents as a benign red papule that may grow rapidly and become pedunculated, sometimes with bleeding and crusting, though histology reveals groups of proliferating capillaries.11 More than half of amelanotic melanomas present in the papulonodular form as vascular or ulcerated nodules, and others may appear as erythematous macules. Diagnosis of amelanotic melanoma is made through histologic examination, which reveals atypical melanocytes in nests or cords, in conjunction with immunohistochemical stains such as S-100.12 Spitz nevi often appear as round, dome-shaped papules that most commonly are red, pink, or fleshcolored. They appear histologically similar to melanoma with nests of atypical melanocytes and nuclear atypia.13
A variety of treatment modalities can be used for MC including cantharidin, curettage, and cryotherapy.14 Imiquimod no longer is recommended due to a lack of demonstrated superiority over placebo in recent studies as well as its adverse effects.3 Topical retinoids have been recommended; however, their use frequently is limited by local irritation.3,14 Cantharidin is the most frequently utilized treatment by pediatric dermatologists. Most health care providers report subjective satisfaction with its results and efficacy, though some side effects may occur including discomfort and temporary changes in pigmentation. Treatment for MC is not required, as the condition is self-limiting.14 Therapy often is reserved for those with extensive disease, complications from lesions, cosmetic or psychological concerns, or genital involvement given the potential for sexual transmission.3 Time to resolution without treatment varies and is more prolonged in immunocompromised patients. Mean time to resolution in immunocompetent hosts has been reported as 13.3 months, but most infections are noted to clear within 2 to 4 years.1,4 Although resolution without treatment occurs, transmission to others and negative impact on quality of life (QOL) can occur and support the need for treatment. Greater impact on QOL was observed in females, those with more lesions, and patients with a longer duration of symptoms. Moderate impact on QOL was reported in 28% of patients (n=301), and severe effects were reported in 11%.4
In conclusion, MC is a common, benign, treatable cutaneous viral infection that often presents as small, flesh-colored papules in children. Its appearance can mimic a variety of other conditions. In cases with abnormal presentations, definitive diagnosis with pathology can be important to differentiate MC from more dangerous etiologies that may require further treatment.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99. doi:10.1111 /j.1365-4632.2006.02737.x
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54. doi:10.1016/j.jaad.2005.08.035
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Derm Rep. 2020;9:83-92.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195. doi:10.1016/S1473-3099(14)71053-9
- Davey J, Biswas A. Follicular induction in a case of molluscum contagiosum: possible link with secondary anetoderma-like changes? Am J Dermatopathol. 2014;36:E19-E21. doi:10.1097/DAD.0b013e31828bc7c7
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653. doi:10.1542/peds.2012-2933
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73. doi:10.5826/dpc.0603a15
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796. doi:10.1002/dc.23964
- Cohen PR, Tschen JA. Plantar molluscum contagiosum: a case report of molluscum contagiosum occurring on the sole of the foot and a review of the world literature. Cutis. 2012;90:35-41.
- Megalla M, Bronsnick T, Noor O, et al. Dermoscopic, confocal microscopic, and histologic characteristics of an atypical presentation of molluscum contagiosum. Ann Clin Pathol. 2014;2:1038.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.1525-1470.1991.tb00931.x
- Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221-230. doi:10.1097/CMR.0000000000000571
- Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 pt 1):901-913. doi:10.1016/0190-9622(92)70286-o
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99. doi:10.1111 /j.1365-4632.2006.02737.x
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54. doi:10.1016/j.jaad.2005.08.035
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Derm Rep. 2020;9:83-92.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195. doi:10.1016/S1473-3099(14)71053-9
- Davey J, Biswas A. Follicular induction in a case of molluscum contagiosum: possible link with secondary anetoderma-like changes? Am J Dermatopathol. 2014;36:E19-E21. doi:10.1097/DAD.0b013e31828bc7c7
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653. doi:10.1542/peds.2012-2933
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73. doi:10.5826/dpc.0603a15
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796. doi:10.1002/dc.23964
- Cohen PR, Tschen JA. Plantar molluscum contagiosum: a case report of molluscum contagiosum occurring on the sole of the foot and a review of the world literature. Cutis. 2012;90:35-41.
- Megalla M, Bronsnick T, Noor O, et al. Dermoscopic, confocal microscopic, and histologic characteristics of an atypical presentation of molluscum contagiosum. Ann Clin Pathol. 2014;2:1038.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.1525-1470.1991.tb00931.x
- Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221-230. doi:10.1097/CMR.0000000000000571
- Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 pt 1):901-913. doi:10.1016/0190-9622(92)70286-o
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
A 13-year-old adolescent girl presented for evaluation of a lesion on the dorsal aspect of the right foot of 1 week’s duration. She had a history of acne vulgaris and seasonal allergic rhinitis. She previously had noticed a persistent, small, flesh-colored bump of unknown chronicity in the same location, which had been diagnosed as a skin tag at an outside clinic. She denied any prior treatment in this area. Approximately a week prior to presentation, the lesion became painful, larger, and darkened in color before draining yellowish fluid. Due to concern for superinfection, the patient was prescribed cephalexin by her pediatrician. Dermatologic examination revealed a 1-cm, violaceous, pedunculated plaque with hemorrhagic crust on the dorsal aspect of the right foot with surrounding erythema and tenderness.
Can dietary tweaks improve some skin diseases?
Since 1950, the terms “diet and skin” in the medical literature have markedly increased, said Vivian Shi, MD associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock, who talked about nutritional approaches for select skin diseases at MedscapeLive’s Women’s and Pediatric Dermatology Seminar.
Myths abound, but some associations of diet with skin diseases hold water, and she said.
Acne
What’s known, Dr. Shi said, is that the prevalence of acne is substantially lower in non-Westernized countries, and that diets in those countries generally have a low glycemic load, which decreases IGF-1 insulinlike growth factor 1 (IGF-1) concentrations, an accepted risk factor for acne. The Western diet also includes the hormonal effects of cow’s milk products.
Whey protein, which is popular as a supplement, isn’t good for acne, Dr. Shi said. It takes a couple of hours to digest, while casein protein digests more slowly, over 5-7 hours. If casein protein isn’t acceptable, good alternatives to whey protein are hemp seed, plant protein blends (peas, seeds, berries), egg white, brown rice isolate, and soy isolate protein.
Dairy products increase IGF-1 levels, hormonal mediators that can make acne worse. In addition, industrial cow’s milk can contain anabolic steroids and growth factor, leading to sebogenesis, Dr. Shi said. As for the type of milk, skim milk tends to be the most acnegenic and associated with the highest blood levels of IGF-1.
Supplementing with omega-3 fatty acids and gamma-linolenic acid improved mild to moderate acne in a double-blind, controlled study. Researchers randomized 45 patients with mild to moderate acne to an omega-3 fatty acid group (2,000 mg of eicosapentaenoic acid and docosahexaenoic acid), a gamma-linolenic acid group (borage oil with 400 mg gamma-linolenic acid) or a control group. After 10 weeks in both treatment groups, there was a significant reduction in inflammatory and noninflammatory lesions.
Those with acne are more likely to be deficient in Vitamin D, research suggests. Researchers also found that among those who had vitamin D deficiency, supplementing with 1,000 IU daily for 2 months reduced inflammatory lesions by 35% after 8 weeks, compared with a 6% reduction in the control group.
Other research has found that those with a low serum zinc level had more severe acne and that 30-200 mg of zinc orally for 2-4 months reduced inflammatory acne. However, Dr. Shi cautioned that those taking zinc for more than 2 months also need a copper supplement, as zinc reduces the amount of copper absorbed by the body.
Dr. Shi’s “do’s” diet list for acne patients is a follows: Paleolithic and Mediterranean diets, omega-3 fatty acids, gamma-linolenic acids, Vitamin D, zinc, tubers, legumes, vegetables, fruits, and fish.
Unknowns, she said, include chocolate, caffeine, green tea, and high salt.
Hidradenitis suppurativa
Patents with HS who follow a Mediterranean diet most closely have less severe disease, research has found. In this study, those patients with HS with the lowest adherence had a Sartorius HS score of 59.38, while those who followed it the most closely had a score of 39 (of 80).
In another study, patients with HS reported the following foods as exacerbating HS: sweets, bread/pasta/rice, dairy, and high-fat foods. Alleviating foods included vegetables, fruit, chicken, and fish.
Dr. Shi’s dietary recommendations for patients with HS: Follow a Mediterranean diet, avoid high fat foods and highly processed foods, and focus on eating more vegetables, fresh fruit, corn-based cereal, white meat, and fish.
A retrospective study of patients with Hurley stage 1 and 2 found that oral zinc gluconate, 90 mg a day, combined with 2% topical triclosan twice a day, resulted in significantly decreased HS scores and nodules and improved quality of life after 3 months. Expect vitamin D deficiency, she added.
Lastly, Dr. Shi recommended, if necessary, “weight loss to reduce the inflammatory burden.”
Rosacea
Dietary triggers for rosacea are thought to include high-fat foods, dairy foods, spicy foods, hot drinks, cinnamon, and vanilla.
A population-based case-control study in China, which evaluated 1,347 rosacea patients and 1,290 healthy controls, found that a high intake of fatty foods positively correlated with erythematotelangiectatic rosacea (ETR) and phymatous rosacea. High-frequency dairy intake negatively correlated with ETR and papulopustular rosacea, which was a surprise, she said. And in this study, no significant correlations were found between sweets, coffee, and spicy foods. That goes against the traditional thinking, she said, but this was a Chinese cohort and their diet is probably vastly different than those in the United States.
Other rosacea triggers, Dr. Shi said, are niacin-containing foods such as turkey, chicken breast, crustaceans, dried Shiitake mushrooms, peanuts, tuna, and liver, as well as cold drinks, and formalin-containing foods (fish, squid, tofu, wet noodles).
As the field of nutrigenics – how genes affect how the body responds to food – evolves, more answers about the impact of diet on these diseases will be forthcoming, Dr. Shi said.
In an interactive panel discussion, she was asked if she talks about diet with all her patients with acne, rosacea, and HS, or just those not responding to traditional therapy.
“I think it’s an important conversation to have,” Dr. Shi responded. “When I’m done with the medication [instructions], I say: ‘There is something else you can do to augment what I just told you.’ ” That’s when she explains the dietary information. She also has a handout on diet and routinely refers patients for dietary counseling.
MedscapeLive and this news organization are owned by the same parent company. Dr. Shi disclosed consulting, investigative and research funding from several sources, but not directly related to the content of her talk.
Since 1950, the terms “diet and skin” in the medical literature have markedly increased, said Vivian Shi, MD associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock, who talked about nutritional approaches for select skin diseases at MedscapeLive’s Women’s and Pediatric Dermatology Seminar.
Myths abound, but some associations of diet with skin diseases hold water, and she said.
Acne
What’s known, Dr. Shi said, is that the prevalence of acne is substantially lower in non-Westernized countries, and that diets in those countries generally have a low glycemic load, which decreases IGF-1 insulinlike growth factor 1 (IGF-1) concentrations, an accepted risk factor for acne. The Western diet also includes the hormonal effects of cow’s milk products.
Whey protein, which is popular as a supplement, isn’t good for acne, Dr. Shi said. It takes a couple of hours to digest, while casein protein digests more slowly, over 5-7 hours. If casein protein isn’t acceptable, good alternatives to whey protein are hemp seed, plant protein blends (peas, seeds, berries), egg white, brown rice isolate, and soy isolate protein.
Dairy products increase IGF-1 levels, hormonal mediators that can make acne worse. In addition, industrial cow’s milk can contain anabolic steroids and growth factor, leading to sebogenesis, Dr. Shi said. As for the type of milk, skim milk tends to be the most acnegenic and associated with the highest blood levels of IGF-1.
Supplementing with omega-3 fatty acids and gamma-linolenic acid improved mild to moderate acne in a double-blind, controlled study. Researchers randomized 45 patients with mild to moderate acne to an omega-3 fatty acid group (2,000 mg of eicosapentaenoic acid and docosahexaenoic acid), a gamma-linolenic acid group (borage oil with 400 mg gamma-linolenic acid) or a control group. After 10 weeks in both treatment groups, there was a significant reduction in inflammatory and noninflammatory lesions.
Those with acne are more likely to be deficient in Vitamin D, research suggests. Researchers also found that among those who had vitamin D deficiency, supplementing with 1,000 IU daily for 2 months reduced inflammatory lesions by 35% after 8 weeks, compared with a 6% reduction in the control group.
Other research has found that those with a low serum zinc level had more severe acne and that 30-200 mg of zinc orally for 2-4 months reduced inflammatory acne. However, Dr. Shi cautioned that those taking zinc for more than 2 months also need a copper supplement, as zinc reduces the amount of copper absorbed by the body.
Dr. Shi’s “do’s” diet list for acne patients is a follows: Paleolithic and Mediterranean diets, omega-3 fatty acids, gamma-linolenic acids, Vitamin D, zinc, tubers, legumes, vegetables, fruits, and fish.
Unknowns, she said, include chocolate, caffeine, green tea, and high salt.
Hidradenitis suppurativa
Patents with HS who follow a Mediterranean diet most closely have less severe disease, research has found. In this study, those patients with HS with the lowest adherence had a Sartorius HS score of 59.38, while those who followed it the most closely had a score of 39 (of 80).
In another study, patients with HS reported the following foods as exacerbating HS: sweets, bread/pasta/rice, dairy, and high-fat foods. Alleviating foods included vegetables, fruit, chicken, and fish.
Dr. Shi’s dietary recommendations for patients with HS: Follow a Mediterranean diet, avoid high fat foods and highly processed foods, and focus on eating more vegetables, fresh fruit, corn-based cereal, white meat, and fish.
A retrospective study of patients with Hurley stage 1 and 2 found that oral zinc gluconate, 90 mg a day, combined with 2% topical triclosan twice a day, resulted in significantly decreased HS scores and nodules and improved quality of life after 3 months. Expect vitamin D deficiency, she added.
Lastly, Dr. Shi recommended, if necessary, “weight loss to reduce the inflammatory burden.”
Rosacea
Dietary triggers for rosacea are thought to include high-fat foods, dairy foods, spicy foods, hot drinks, cinnamon, and vanilla.
A population-based case-control study in China, which evaluated 1,347 rosacea patients and 1,290 healthy controls, found that a high intake of fatty foods positively correlated with erythematotelangiectatic rosacea (ETR) and phymatous rosacea. High-frequency dairy intake negatively correlated with ETR and papulopustular rosacea, which was a surprise, she said. And in this study, no significant correlations were found between sweets, coffee, and spicy foods. That goes against the traditional thinking, she said, but this was a Chinese cohort and their diet is probably vastly different than those in the United States.
Other rosacea triggers, Dr. Shi said, are niacin-containing foods such as turkey, chicken breast, crustaceans, dried Shiitake mushrooms, peanuts, tuna, and liver, as well as cold drinks, and formalin-containing foods (fish, squid, tofu, wet noodles).
As the field of nutrigenics – how genes affect how the body responds to food – evolves, more answers about the impact of diet on these diseases will be forthcoming, Dr. Shi said.
In an interactive panel discussion, she was asked if she talks about diet with all her patients with acne, rosacea, and HS, or just those not responding to traditional therapy.
“I think it’s an important conversation to have,” Dr. Shi responded. “When I’m done with the medication [instructions], I say: ‘There is something else you can do to augment what I just told you.’ ” That’s when she explains the dietary information. She also has a handout on diet and routinely refers patients for dietary counseling.
MedscapeLive and this news organization are owned by the same parent company. Dr. Shi disclosed consulting, investigative and research funding from several sources, but not directly related to the content of her talk.
Since 1950, the terms “diet and skin” in the medical literature have markedly increased, said Vivian Shi, MD associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock, who talked about nutritional approaches for select skin diseases at MedscapeLive’s Women’s and Pediatric Dermatology Seminar.
Myths abound, but some associations of diet with skin diseases hold water, and she said.
Acne
What’s known, Dr. Shi said, is that the prevalence of acne is substantially lower in non-Westernized countries, and that diets in those countries generally have a low glycemic load, which decreases IGF-1 insulinlike growth factor 1 (IGF-1) concentrations, an accepted risk factor for acne. The Western diet also includes the hormonal effects of cow’s milk products.
Whey protein, which is popular as a supplement, isn’t good for acne, Dr. Shi said. It takes a couple of hours to digest, while casein protein digests more slowly, over 5-7 hours. If casein protein isn’t acceptable, good alternatives to whey protein are hemp seed, plant protein blends (peas, seeds, berries), egg white, brown rice isolate, and soy isolate protein.
Dairy products increase IGF-1 levels, hormonal mediators that can make acne worse. In addition, industrial cow’s milk can contain anabolic steroids and growth factor, leading to sebogenesis, Dr. Shi said. As for the type of milk, skim milk tends to be the most acnegenic and associated with the highest blood levels of IGF-1.
Supplementing with omega-3 fatty acids and gamma-linolenic acid improved mild to moderate acne in a double-blind, controlled study. Researchers randomized 45 patients with mild to moderate acne to an omega-3 fatty acid group (2,000 mg of eicosapentaenoic acid and docosahexaenoic acid), a gamma-linolenic acid group (borage oil with 400 mg gamma-linolenic acid) or a control group. After 10 weeks in both treatment groups, there was a significant reduction in inflammatory and noninflammatory lesions.
Those with acne are more likely to be deficient in Vitamin D, research suggests. Researchers also found that among those who had vitamin D deficiency, supplementing with 1,000 IU daily for 2 months reduced inflammatory lesions by 35% after 8 weeks, compared with a 6% reduction in the control group.
Other research has found that those with a low serum zinc level had more severe acne and that 30-200 mg of zinc orally for 2-4 months reduced inflammatory acne. However, Dr. Shi cautioned that those taking zinc for more than 2 months also need a copper supplement, as zinc reduces the amount of copper absorbed by the body.
Dr. Shi’s “do’s” diet list for acne patients is a follows: Paleolithic and Mediterranean diets, omega-3 fatty acids, gamma-linolenic acids, Vitamin D, zinc, tubers, legumes, vegetables, fruits, and fish.
Unknowns, she said, include chocolate, caffeine, green tea, and high salt.
Hidradenitis suppurativa
Patents with HS who follow a Mediterranean diet most closely have less severe disease, research has found. In this study, those patients with HS with the lowest adherence had a Sartorius HS score of 59.38, while those who followed it the most closely had a score of 39 (of 80).
In another study, patients with HS reported the following foods as exacerbating HS: sweets, bread/pasta/rice, dairy, and high-fat foods. Alleviating foods included vegetables, fruit, chicken, and fish.
Dr. Shi’s dietary recommendations for patients with HS: Follow a Mediterranean diet, avoid high fat foods and highly processed foods, and focus on eating more vegetables, fresh fruit, corn-based cereal, white meat, and fish.
A retrospective study of patients with Hurley stage 1 and 2 found that oral zinc gluconate, 90 mg a day, combined with 2% topical triclosan twice a day, resulted in significantly decreased HS scores and nodules and improved quality of life after 3 months. Expect vitamin D deficiency, she added.
Lastly, Dr. Shi recommended, if necessary, “weight loss to reduce the inflammatory burden.”
Rosacea
Dietary triggers for rosacea are thought to include high-fat foods, dairy foods, spicy foods, hot drinks, cinnamon, and vanilla.
A population-based case-control study in China, which evaluated 1,347 rosacea patients and 1,290 healthy controls, found that a high intake of fatty foods positively correlated with erythematotelangiectatic rosacea (ETR) and phymatous rosacea. High-frequency dairy intake negatively correlated with ETR and papulopustular rosacea, which was a surprise, she said. And in this study, no significant correlations were found between sweets, coffee, and spicy foods. That goes against the traditional thinking, she said, but this was a Chinese cohort and their diet is probably vastly different than those in the United States.
Other rosacea triggers, Dr. Shi said, are niacin-containing foods such as turkey, chicken breast, crustaceans, dried Shiitake mushrooms, peanuts, tuna, and liver, as well as cold drinks, and formalin-containing foods (fish, squid, tofu, wet noodles).
As the field of nutrigenics – how genes affect how the body responds to food – evolves, more answers about the impact of diet on these diseases will be forthcoming, Dr. Shi said.
In an interactive panel discussion, she was asked if she talks about diet with all her patients with acne, rosacea, and HS, or just those not responding to traditional therapy.
“I think it’s an important conversation to have,” Dr. Shi responded. “When I’m done with the medication [instructions], I say: ‘There is something else you can do to augment what I just told you.’ ” That’s when she explains the dietary information. She also has a handout on diet and routinely refers patients for dietary counseling.
MedscapeLive and this news organization are owned by the same parent company. Dr. Shi disclosed consulting, investigative and research funding from several sources, but not directly related to the content of her talk.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Innovations in Dermatology Spring Abstract Compendium
How much health insurers pay for almost everything is about to go public
perhaps helping answer a question that has long dogged those who buy insurance: Are we getting the best deal we can?
As of July 1, health insurers and self-insured employers must post on websites just about every price they’ve negotiated with providers for health care services, item by item. About the only thing excluded are the prices paid for prescription drugs, except those administered in hospitals or doctors’ offices.
The federally required data release could affect future prices or even how employers contract for health care. Many will see for the first time how well their insurers are doing, compared with others.
The new rules are far broader than those that went into effect in 2021 requiring hospitals to post their negotiated rates for the public to see. Now insurers must post the amounts paid for “every physician in network, every hospital, every surgery center, every nursing facility,” said Jeffrey Leibach, a partner at the consulting firm Guidehouse.
“When you start doing the math, you’re talking trillions of records,” he said. The fines the federal government could impose for noncompliance are also heftier than the penalties that hospitals face.
Federal officials learned from the hospital experience and gave insurers more direction on what was expected, said Mr. Leibach. Insurers or self-insured employers could be fined as much as $100 a day for each violation, for each affected enrollee if they fail to provide the data.
“Get your calculator out: All of a sudden you are in the millions pretty fast,” Mr. Leibach said.
Determined consumers, especially those with high-deductible health plans, may try to dig in right away and use the data to try comparing what they will have to pay at different hospitals, clinics, or doctor offices for specific services.
But each database’s enormous size may mean that most people “will find it very hard to use the data in a nuanced way,” said Katherine Baicker, dean of the University of Chicago Harris School of Public Policy.
At least at first.
Entrepreneurs are expected to quickly translate the information into more user-friendly formats so it can be incorporated into new or existing services that estimate costs for patients. And starting Jan. 1, the rules require insurers to provide online tools that will help people get up-front cost estimates for about 500 so-called “shoppable” services, meaning medical care they can schedule ahead of time.
Once those things happen, “you’ll at least have the options in front of you,” said Chris Severn, CEO of Turquoise Health, an online company that has posted price information made available under the rules for hospitals, although many hospitals have yet to comply.
With the addition of the insurers’ data, sites like his will be able to drill down further into cost variation from one place to another or among insurers.
“If you’re going to get an x-ray, you will be able to see that you can do it for $250 at this hospital, $75 at the imaging center down the road, or your specialist can do it in office for $25,” he said.
Everyone will know everyone else’s business: for example, how much insurers Aetna and Humana pay the same surgery center for a knee replacement.
The requirements stem from the Affordable Care Act and a 2019 executive order by then-President Donald Trump.
“These plans are supposed to be acting on behalf of employers in negotiating good rates, and the little insight we have on that shows it has not happened,” said Elizabeth Mitchell, president and CEO of the Purchaser Business Group on Health, an affiliation of employers who offer job-based health benefits to workers. “I do believe the dynamics are going to change.”
Other observers are more circumspect.
“Maybe at best this will reduce the wide variance of prices out there,” said Zack Cooper, director of health policy at the Yale University Institution for Social and Policy Studies, New Haven, Conn. “But it won’t be unleashing a consumer revolution.”
Still, the biggest value of the July data release may well be to shed light on how successful insurers have been at negotiating prices. It comes on the heels of research that has shown tremendous variation in what is paid for health care. A recent study by Rand, for example, shows that employers that offer job-based insurance plans paid, on average, 224% more than Medicare for the same services.
Tens of thousands of employers who buy insurance coverage for their workers will get this more-complete pricing picture – and may not like what they see.
“What we’re learning from the hospital data is that insurers are really bad at negotiating,” said Gerard Anderson, a professor in the department of health policy at the Johns Hopkins Bloomberg, Baltimore, citing research that found that negotiated rates for hospital care can be higher than what the facilities accept from patients who are not using insurance and are paying cash.
That could add to the frustration that Ms. Mitchell and others say employers have with the current health insurance system. More might try to contract with providers directly, only using insurance companies for claims processing.
Other employers may bring their insurers back to the bargaining table.
“For the first time, an employer will be able to go to an insurance company and say: ‘You have not negotiated a good-enough deal, and we know that because we can see the same provider has negotiated a better deal with another company,’ ” said James Gelfand, president of the ERISA Industry Committee, a trade group of self-insured employers.
If that happens, he added, “patients will be able to save money.”
That’s not necessarily a given, however.
Because this kind of public release of pricing data hasn’t been tried widely in health care before, how it will affect future spending remains uncertain. If insurers are pushed back to the bargaining table or providers see where they stand relative to their peers, prices could drop. However, some providers could raise their prices if they see they are charging less than their peers.
“Downward pressure may not be a given,” said Kelley Schultz, vice president of commercial policy for AHIP, the industry’s trade lobby.
Ms. Baicker said that, even after the data is out, rates will continue to be heavily influenced by local conditions, such as the size of an insurer or employer – providers often give bigger discounts, for example, to the insurers or self-insured employers that can send them the most patients. The number of hospitals in a region also matters – if an area has only one, for instance, that usually means the facility can demand higher rates.
Another unknown: Will insurers meet the deadline and provide usable data?
Ms. Schultz, at AHIP, said the industry is well on the way, partly because the original deadline was extended by 6 months. She expects insurers to do better than the hospital industry. “We saw a lot of hospitals that just decided not to post files or make them difficult to find,” she said.
So far, more than 300 noncompliant hospitals received warning letters from the government. But they could face fines of $300 a day fines for failing to comply, which is less than what insurers potentially face, although the federal government has recently upped the ante to up to $5,500 a day for the largest facilities.
Even after the pricing data is public, “I don’t think things will change overnight,” said Mr. Leibach. “Patients are still going to make care decisions based on their doctors and referrals, a lot of reasons other than price.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
perhaps helping answer a question that has long dogged those who buy insurance: Are we getting the best deal we can?
As of July 1, health insurers and self-insured employers must post on websites just about every price they’ve negotiated with providers for health care services, item by item. About the only thing excluded are the prices paid for prescription drugs, except those administered in hospitals or doctors’ offices.
The federally required data release could affect future prices or even how employers contract for health care. Many will see for the first time how well their insurers are doing, compared with others.
The new rules are far broader than those that went into effect in 2021 requiring hospitals to post their negotiated rates for the public to see. Now insurers must post the amounts paid for “every physician in network, every hospital, every surgery center, every nursing facility,” said Jeffrey Leibach, a partner at the consulting firm Guidehouse.
“When you start doing the math, you’re talking trillions of records,” he said. The fines the federal government could impose for noncompliance are also heftier than the penalties that hospitals face.
Federal officials learned from the hospital experience and gave insurers more direction on what was expected, said Mr. Leibach. Insurers or self-insured employers could be fined as much as $100 a day for each violation, for each affected enrollee if they fail to provide the data.
“Get your calculator out: All of a sudden you are in the millions pretty fast,” Mr. Leibach said.
Determined consumers, especially those with high-deductible health plans, may try to dig in right away and use the data to try comparing what they will have to pay at different hospitals, clinics, or doctor offices for specific services.
But each database’s enormous size may mean that most people “will find it very hard to use the data in a nuanced way,” said Katherine Baicker, dean of the University of Chicago Harris School of Public Policy.
At least at first.
Entrepreneurs are expected to quickly translate the information into more user-friendly formats so it can be incorporated into new or existing services that estimate costs for patients. And starting Jan. 1, the rules require insurers to provide online tools that will help people get up-front cost estimates for about 500 so-called “shoppable” services, meaning medical care they can schedule ahead of time.
Once those things happen, “you’ll at least have the options in front of you,” said Chris Severn, CEO of Turquoise Health, an online company that has posted price information made available under the rules for hospitals, although many hospitals have yet to comply.
With the addition of the insurers’ data, sites like his will be able to drill down further into cost variation from one place to another or among insurers.
“If you’re going to get an x-ray, you will be able to see that you can do it for $250 at this hospital, $75 at the imaging center down the road, or your specialist can do it in office for $25,” he said.
Everyone will know everyone else’s business: for example, how much insurers Aetna and Humana pay the same surgery center for a knee replacement.
The requirements stem from the Affordable Care Act and a 2019 executive order by then-President Donald Trump.
“These plans are supposed to be acting on behalf of employers in negotiating good rates, and the little insight we have on that shows it has not happened,” said Elizabeth Mitchell, president and CEO of the Purchaser Business Group on Health, an affiliation of employers who offer job-based health benefits to workers. “I do believe the dynamics are going to change.”
Other observers are more circumspect.
“Maybe at best this will reduce the wide variance of prices out there,” said Zack Cooper, director of health policy at the Yale University Institution for Social and Policy Studies, New Haven, Conn. “But it won’t be unleashing a consumer revolution.”
Still, the biggest value of the July data release may well be to shed light on how successful insurers have been at negotiating prices. It comes on the heels of research that has shown tremendous variation in what is paid for health care. A recent study by Rand, for example, shows that employers that offer job-based insurance plans paid, on average, 224% more than Medicare for the same services.
Tens of thousands of employers who buy insurance coverage for their workers will get this more-complete pricing picture – and may not like what they see.
“What we’re learning from the hospital data is that insurers are really bad at negotiating,” said Gerard Anderson, a professor in the department of health policy at the Johns Hopkins Bloomberg, Baltimore, citing research that found that negotiated rates for hospital care can be higher than what the facilities accept from patients who are not using insurance and are paying cash.
That could add to the frustration that Ms. Mitchell and others say employers have with the current health insurance system. More might try to contract with providers directly, only using insurance companies for claims processing.
Other employers may bring their insurers back to the bargaining table.
“For the first time, an employer will be able to go to an insurance company and say: ‘You have not negotiated a good-enough deal, and we know that because we can see the same provider has negotiated a better deal with another company,’ ” said James Gelfand, president of the ERISA Industry Committee, a trade group of self-insured employers.
If that happens, he added, “patients will be able to save money.”
That’s not necessarily a given, however.
Because this kind of public release of pricing data hasn’t been tried widely in health care before, how it will affect future spending remains uncertain. If insurers are pushed back to the bargaining table or providers see where they stand relative to their peers, prices could drop. However, some providers could raise their prices if they see they are charging less than their peers.
“Downward pressure may not be a given,” said Kelley Schultz, vice president of commercial policy for AHIP, the industry’s trade lobby.
Ms. Baicker said that, even after the data is out, rates will continue to be heavily influenced by local conditions, such as the size of an insurer or employer – providers often give bigger discounts, for example, to the insurers or self-insured employers that can send them the most patients. The number of hospitals in a region also matters – if an area has only one, for instance, that usually means the facility can demand higher rates.
Another unknown: Will insurers meet the deadline and provide usable data?
Ms. Schultz, at AHIP, said the industry is well on the way, partly because the original deadline was extended by 6 months. She expects insurers to do better than the hospital industry. “We saw a lot of hospitals that just decided not to post files or make them difficult to find,” she said.
So far, more than 300 noncompliant hospitals received warning letters from the government. But they could face fines of $300 a day fines for failing to comply, which is less than what insurers potentially face, although the federal government has recently upped the ante to up to $5,500 a day for the largest facilities.
Even after the pricing data is public, “I don’t think things will change overnight,” said Mr. Leibach. “Patients are still going to make care decisions based on their doctors and referrals, a lot of reasons other than price.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
perhaps helping answer a question that has long dogged those who buy insurance: Are we getting the best deal we can?
As of July 1, health insurers and self-insured employers must post on websites just about every price they’ve negotiated with providers for health care services, item by item. About the only thing excluded are the prices paid for prescription drugs, except those administered in hospitals or doctors’ offices.
The federally required data release could affect future prices or even how employers contract for health care. Many will see for the first time how well their insurers are doing, compared with others.
The new rules are far broader than those that went into effect in 2021 requiring hospitals to post their negotiated rates for the public to see. Now insurers must post the amounts paid for “every physician in network, every hospital, every surgery center, every nursing facility,” said Jeffrey Leibach, a partner at the consulting firm Guidehouse.
“When you start doing the math, you’re talking trillions of records,” he said. The fines the federal government could impose for noncompliance are also heftier than the penalties that hospitals face.
Federal officials learned from the hospital experience and gave insurers more direction on what was expected, said Mr. Leibach. Insurers or self-insured employers could be fined as much as $100 a day for each violation, for each affected enrollee if they fail to provide the data.
“Get your calculator out: All of a sudden you are in the millions pretty fast,” Mr. Leibach said.
Determined consumers, especially those with high-deductible health plans, may try to dig in right away and use the data to try comparing what they will have to pay at different hospitals, clinics, or doctor offices for specific services.
But each database’s enormous size may mean that most people “will find it very hard to use the data in a nuanced way,” said Katherine Baicker, dean of the University of Chicago Harris School of Public Policy.
At least at first.
Entrepreneurs are expected to quickly translate the information into more user-friendly formats so it can be incorporated into new or existing services that estimate costs for patients. And starting Jan. 1, the rules require insurers to provide online tools that will help people get up-front cost estimates for about 500 so-called “shoppable” services, meaning medical care they can schedule ahead of time.
Once those things happen, “you’ll at least have the options in front of you,” said Chris Severn, CEO of Turquoise Health, an online company that has posted price information made available under the rules for hospitals, although many hospitals have yet to comply.
With the addition of the insurers’ data, sites like his will be able to drill down further into cost variation from one place to another or among insurers.
“If you’re going to get an x-ray, you will be able to see that you can do it for $250 at this hospital, $75 at the imaging center down the road, or your specialist can do it in office for $25,” he said.
Everyone will know everyone else’s business: for example, how much insurers Aetna and Humana pay the same surgery center for a knee replacement.
The requirements stem from the Affordable Care Act and a 2019 executive order by then-President Donald Trump.
“These plans are supposed to be acting on behalf of employers in negotiating good rates, and the little insight we have on that shows it has not happened,” said Elizabeth Mitchell, president and CEO of the Purchaser Business Group on Health, an affiliation of employers who offer job-based health benefits to workers. “I do believe the dynamics are going to change.”
Other observers are more circumspect.
“Maybe at best this will reduce the wide variance of prices out there,” said Zack Cooper, director of health policy at the Yale University Institution for Social and Policy Studies, New Haven, Conn. “But it won’t be unleashing a consumer revolution.”
Still, the biggest value of the July data release may well be to shed light on how successful insurers have been at negotiating prices. It comes on the heels of research that has shown tremendous variation in what is paid for health care. A recent study by Rand, for example, shows that employers that offer job-based insurance plans paid, on average, 224% more than Medicare for the same services.
Tens of thousands of employers who buy insurance coverage for their workers will get this more-complete pricing picture – and may not like what they see.
“What we’re learning from the hospital data is that insurers are really bad at negotiating,” said Gerard Anderson, a professor in the department of health policy at the Johns Hopkins Bloomberg, Baltimore, citing research that found that negotiated rates for hospital care can be higher than what the facilities accept from patients who are not using insurance and are paying cash.
That could add to the frustration that Ms. Mitchell and others say employers have with the current health insurance system. More might try to contract with providers directly, only using insurance companies for claims processing.
Other employers may bring their insurers back to the bargaining table.
“For the first time, an employer will be able to go to an insurance company and say: ‘You have not negotiated a good-enough deal, and we know that because we can see the same provider has negotiated a better deal with another company,’ ” said James Gelfand, president of the ERISA Industry Committee, a trade group of self-insured employers.
If that happens, he added, “patients will be able to save money.”
That’s not necessarily a given, however.
Because this kind of public release of pricing data hasn’t been tried widely in health care before, how it will affect future spending remains uncertain. If insurers are pushed back to the bargaining table or providers see where they stand relative to their peers, prices could drop. However, some providers could raise their prices if they see they are charging less than their peers.
“Downward pressure may not be a given,” said Kelley Schultz, vice president of commercial policy for AHIP, the industry’s trade lobby.
Ms. Baicker said that, even after the data is out, rates will continue to be heavily influenced by local conditions, such as the size of an insurer or employer – providers often give bigger discounts, for example, to the insurers or self-insured employers that can send them the most patients. The number of hospitals in a region also matters – if an area has only one, for instance, that usually means the facility can demand higher rates.
Another unknown: Will insurers meet the deadline and provide usable data?
Ms. Schultz, at AHIP, said the industry is well on the way, partly because the original deadline was extended by 6 months. She expects insurers to do better than the hospital industry. “We saw a lot of hospitals that just decided not to post files or make them difficult to find,” she said.
So far, more than 300 noncompliant hospitals received warning letters from the government. But they could face fines of $300 a day fines for failing to comply, which is less than what insurers potentially face, although the federal government has recently upped the ante to up to $5,500 a day for the largest facilities.
Even after the pricing data is public, “I don’t think things will change overnight,” said Mr. Leibach. “Patients are still going to make care decisions based on their doctors and referrals, a lot of reasons other than price.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Diabetes devices may give children contact dermatitis
Devices that help children control their diabetes and lead fuller lives may also give them contact dermatitis, report the authors of a new study that calls for mandatory labeling of ingredients for allergy patch testing.
“A high share of patients showed positive reactions to isobornyl acrylate adhesive (IBOA) and/or their medical devices (insulin pumps or glucose devices),” the study authors write in Contact Dermatitis. “A third of patients showed positive reactions to benzoyl peroxide (BP),” used in adhesives.
“The presence of additional unidentified allergens cannot be excluded,” they add. “Overall, our experience once more highlights the importance of having access to a full description of the chemical composition of diabetes devices and related medical devices to efficiently manage patients (including children) who experience adverse skin reactions from such devices.”
Lead study author Catarina Alves da Silva, MD, of the department of dermatology and venereology of Aarhus (Denmark) University Hospital, and her colleagues conducted a retrospective study of 15 referred patients younger than 18 years who had type 1 diabetes. The children were patch tested in the university’s dermatology clinic between 2018 and 2020 in a study of skin reactions linked with diabetes devices.
Contact dermatitis from device-related allergens may be common
Many children in the study reacted to chemical compounds related to their devices.
- Of the 15 patients, seven showed positive patch test reactions to IBOA, and five showed positive reactions to BP.
- Ten children had positive patch test reactions to materials from glucose sensors and insulin pumps.
- Three showed positive reactions to adhesive remover wipes.
- Five reacted to .
Marcia Hogeling, MD, a pediatric dermatologist at UCLA Health in Santa Monica, Calif., told this news organization that she expected acrylates to cause problems but was surprised that BP caused positive patch test reactions.
BP is known to be a strong irritant but a weak allergen, the authors wrote.
“It was important to identify the allergens in these devices. Hopefully, this information will be used by manufacturers to create safer products for patients,” Dr. Hogeling, who was not involved in the study, said in an email.
Dr. Hogeling acknowledged that the small sample size is a weakness of the study, although she added that the findings may help providers select devices that do not contain their patients’ contact allergens.
Ryan J. McDonough, DO, a pediatric endocrinologist and the codirector of the Diabetes Center at Children’s Mercy Kansas City (Mo.), said in an email that, despite the small sample size, the study “highlights important device-related experiences of those living with type 1 diabetes that clinicians often encounter.
“We often spend considerable time aiding patients and their families in finding ways to mitigate the reactions,” he explained. “Having a broader understanding of these chemical compositions would help clinicians choose the right devices for their patients and prevent and treat these types of reactions.”
Dr. McDonough, who was not involved in the study, noted that the patients were in Denmark, and they were able to easily transition between insulin pumps and glucose monitoring devices.
“In the U.S., it is often more challenging to switch between devices, due to insurance-related concerns.
“The true rates of reaction in the broad type 1 diabetes population are difficult to assess,” Dr. McDonough said. “The study participants were drawn from patients referred to a dermatology clinic for evaluation of reaction. Many patients either don’t develop reactions or are treated for mild symptoms locally by their endocrinologists.
“This study should serve as a call to action for continued improvements in the transparency of the components that make up the devices and adhesives, and it can provide an opportunity to develop additional interventions to prevent these reactions,” he advised.
No information regarding funding for the study was provided. The authors, Dr. Hogeling, and Dr. McDonough reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Devices that help children control their diabetes and lead fuller lives may also give them contact dermatitis, report the authors of a new study that calls for mandatory labeling of ingredients for allergy patch testing.
“A high share of patients showed positive reactions to isobornyl acrylate adhesive (IBOA) and/or their medical devices (insulin pumps or glucose devices),” the study authors write in Contact Dermatitis. “A third of patients showed positive reactions to benzoyl peroxide (BP),” used in adhesives.
“The presence of additional unidentified allergens cannot be excluded,” they add. “Overall, our experience once more highlights the importance of having access to a full description of the chemical composition of diabetes devices and related medical devices to efficiently manage patients (including children) who experience adverse skin reactions from such devices.”
Lead study author Catarina Alves da Silva, MD, of the department of dermatology and venereology of Aarhus (Denmark) University Hospital, and her colleagues conducted a retrospective study of 15 referred patients younger than 18 years who had type 1 diabetes. The children were patch tested in the university’s dermatology clinic between 2018 and 2020 in a study of skin reactions linked with diabetes devices.
Contact dermatitis from device-related allergens may be common
Many children in the study reacted to chemical compounds related to their devices.
- Of the 15 patients, seven showed positive patch test reactions to IBOA, and five showed positive reactions to BP.
- Ten children had positive patch test reactions to materials from glucose sensors and insulin pumps.
- Three showed positive reactions to adhesive remover wipes.
- Five reacted to .
Marcia Hogeling, MD, a pediatric dermatologist at UCLA Health in Santa Monica, Calif., told this news organization that she expected acrylates to cause problems but was surprised that BP caused positive patch test reactions.
BP is known to be a strong irritant but a weak allergen, the authors wrote.
“It was important to identify the allergens in these devices. Hopefully, this information will be used by manufacturers to create safer products for patients,” Dr. Hogeling, who was not involved in the study, said in an email.
Dr. Hogeling acknowledged that the small sample size is a weakness of the study, although she added that the findings may help providers select devices that do not contain their patients’ contact allergens.
Ryan J. McDonough, DO, a pediatric endocrinologist and the codirector of the Diabetes Center at Children’s Mercy Kansas City (Mo.), said in an email that, despite the small sample size, the study “highlights important device-related experiences of those living with type 1 diabetes that clinicians often encounter.
“We often spend considerable time aiding patients and their families in finding ways to mitigate the reactions,” he explained. “Having a broader understanding of these chemical compositions would help clinicians choose the right devices for their patients and prevent and treat these types of reactions.”
Dr. McDonough, who was not involved in the study, noted that the patients were in Denmark, and they were able to easily transition between insulin pumps and glucose monitoring devices.
“In the U.S., it is often more challenging to switch between devices, due to insurance-related concerns.
“The true rates of reaction in the broad type 1 diabetes population are difficult to assess,” Dr. McDonough said. “The study participants were drawn from patients referred to a dermatology clinic for evaluation of reaction. Many patients either don’t develop reactions or are treated for mild symptoms locally by their endocrinologists.
“This study should serve as a call to action for continued improvements in the transparency of the components that make up the devices and adhesives, and it can provide an opportunity to develop additional interventions to prevent these reactions,” he advised.
No information regarding funding for the study was provided. The authors, Dr. Hogeling, and Dr. McDonough reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Devices that help children control their diabetes and lead fuller lives may also give them contact dermatitis, report the authors of a new study that calls for mandatory labeling of ingredients for allergy patch testing.
“A high share of patients showed positive reactions to isobornyl acrylate adhesive (IBOA) and/or their medical devices (insulin pumps or glucose devices),” the study authors write in Contact Dermatitis. “A third of patients showed positive reactions to benzoyl peroxide (BP),” used in adhesives.
“The presence of additional unidentified allergens cannot be excluded,” they add. “Overall, our experience once more highlights the importance of having access to a full description of the chemical composition of diabetes devices and related medical devices to efficiently manage patients (including children) who experience adverse skin reactions from such devices.”
Lead study author Catarina Alves da Silva, MD, of the department of dermatology and venereology of Aarhus (Denmark) University Hospital, and her colleagues conducted a retrospective study of 15 referred patients younger than 18 years who had type 1 diabetes. The children were patch tested in the university’s dermatology clinic between 2018 and 2020 in a study of skin reactions linked with diabetes devices.
Contact dermatitis from device-related allergens may be common
Many children in the study reacted to chemical compounds related to their devices.
- Of the 15 patients, seven showed positive patch test reactions to IBOA, and five showed positive reactions to BP.
- Ten children had positive patch test reactions to materials from glucose sensors and insulin pumps.
- Three showed positive reactions to adhesive remover wipes.
- Five reacted to .
Marcia Hogeling, MD, a pediatric dermatologist at UCLA Health in Santa Monica, Calif., told this news organization that she expected acrylates to cause problems but was surprised that BP caused positive patch test reactions.
BP is known to be a strong irritant but a weak allergen, the authors wrote.
“It was important to identify the allergens in these devices. Hopefully, this information will be used by manufacturers to create safer products for patients,” Dr. Hogeling, who was not involved in the study, said in an email.
Dr. Hogeling acknowledged that the small sample size is a weakness of the study, although she added that the findings may help providers select devices that do not contain their patients’ contact allergens.
Ryan J. McDonough, DO, a pediatric endocrinologist and the codirector of the Diabetes Center at Children’s Mercy Kansas City (Mo.), said in an email that, despite the small sample size, the study “highlights important device-related experiences of those living with type 1 diabetes that clinicians often encounter.
“We often spend considerable time aiding patients and their families in finding ways to mitigate the reactions,” he explained. “Having a broader understanding of these chemical compositions would help clinicians choose the right devices for their patients and prevent and treat these types of reactions.”
Dr. McDonough, who was not involved in the study, noted that the patients were in Denmark, and they were able to easily transition between insulin pumps and glucose monitoring devices.
“In the U.S., it is often more challenging to switch between devices, due to insurance-related concerns.
“The true rates of reaction in the broad type 1 diabetes population are difficult to assess,” Dr. McDonough said. “The study participants were drawn from patients referred to a dermatology clinic for evaluation of reaction. Many patients either don’t develop reactions or are treated for mild symptoms locally by their endocrinologists.
“This study should serve as a call to action for continued improvements in the transparency of the components that make up the devices and adhesives, and it can provide an opportunity to develop additional interventions to prevent these reactions,” he advised.
No information regarding funding for the study was provided. The authors, Dr. Hogeling, and Dr. McDonough reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Lawmakers argue for changes in prior authorization processes
Republican and Democratic members of the House called for changes in how insurer-run Medicare plans manage the prior authorization process, following testimony from a federal watchdog organization about improper denials of payment for care.
About 18% of payment denials in a sample examined by the Office of Inspector General (OIG) of the Department of Health and Human Services (HHS) either met Medicare coverage rules or the rules of the insurance plan.
As such, they should not have been denied, according to the OIG. That was the finding of an April OIG report, based on a sample of 2019 denials from large insurer-run Medicare plans.
Erin Bliss, an assistant inspector general with the OIG, appeared as a witness at a June 28 Energy and Commerce Subcommittee on Oversight and Investigations hearing to discuss this investigation and other issues with prior authorization and insurer-run Medicare, also known as the Advantage plans.
Most of these payment denials of appropriate services were due to human error during manual claims-processing reviews, Ms. Bliss told the subcommittee, such as overlooking a document, and to system processing errors, such as a Medicare insurance plan failing to program or update a system correctly.
In many cases, these denials were reversed, but patient care was still disrupted and clinicians lost time chasing clearances for services that plans already had covered, Ms. Bliss said in her testimony.
The April report was not the OIG’s first look into concerns about insurer-run plans inappropriately denying care through prior authorizations. The OIG in 2018 reported that insurer-run Medicare plans overturned 75% of their own denials during 2014-2016 when patients and clinicians appealed these decisions, overturning approximately 216,000 denials each year.
‘Numerous hoops’ unnecessary for doctors, patients
Lawmakers at the hearing supported the idea of the need for prior authorization as a screening tool to prevent unneeded care.
But they chided insurance companies for their execution of this process, with clinicians and patients often frustrated by complex steps needed. Medicare Advantage plans sometimes require prior authorization for “relatively standard medical services,” said Subcommittee on Oversight and Investigations Chair Diana DeGette (D-Colo.).
“Our seniors and their doctors should not be required to jump through numerous hoops to ensure coverage for straightforward and medically necessary procedures,” Rep. DeGette said.
Several lawmakers spoke at the hearing about the need for changes to prior authorization, including calling for action on a pending bill intended to compel insurers to streamline the review process. The Improving Seniors’ Timely Access to Care Act of 2021 already has attracted more than 300 bipartisan sponsors. A companion Senate bill has more than 30 sponsors.
The bill’s aim is to shift this process away from faxes and phone calls while also encouraging plans to adhere to evidence-based medical guidelines in consultation with physicians. The bill calls for the establishment of an electronic prior authorization program that could issue real-time decisions.
“The result will be less administrative burden for providers and more information in the hands of patients. It will allow more patients to receive care when they need it, reducing the likelihood of additional, often more severe complications,” said Rep. Larry Bucshon, MD, (R-Ind.) who is among the active sponsors of the bill.
“In the long term, I believe it would also result in cost savings for the health care system at large by identifying problems earlier and getting them treated before their patients have more complications,” Rep. Bucshon added.
Finding ‘room for improvement’ for prior authorizations
There’s strong bipartisan support in Congress for insurer-run Medicare, which has grown by 10% per year over the last several years and has doubled since 2010, according to the Medicare Payment Advisory Commission (MedPAC). About 27 million people are now enrolled in these plans.
But for that reason, insurer-run Medicare may also need more careful watching, lawmakers made clear at the hearing.
“We’ve heard quite a bit of evidence today that there is room for improvement,” said Rep. Bucshon, a strong supporter of insurer-run Medicare, which can offer patients added benefits such as dental coverage.
Rep. Ann Kuster (D-N.H.) said simplifying prior authorization would reduce stress on clinicians already dealing with burnout.
“They’re just so tired of all this paperwork and red tape,” Rep. Kuster said. “In 2022 can’t we at least consider electronic prior authorization?”
At the hearing, Rep. Michael C. Burgess, MD, (R-Tex.) noted that his home state already has taken a step toward reducing the burden of prior authorization with its “gold card” program.
In 2021, a new Texas law called on the state department of insurance to develop rules to require health plans to provide an exemption from preauthorization requirements for a particular health care service if the issuer has approved, or would have approved, at least 90% of the preauthorization requests submitted by the physician or provider for that service. The law also mandates that a physician participating in a peer-to-peer review on behalf of a health benefit plan issuer must be a Texas-licensed physician who has the same or similar specialty as the physician or clinician requesting the service, according to the state insurance department.
Separately, Rep. Suzan DelBene (D-Wash.), the sponsor of the Improving Seniors’ Timely Access to Care Act, told the American Medical Association in a recent interview that she expects the House Ways and Means Committee, on which she serves, to mark up her bill in July. (A mark-up is the process by which a House or Senate committee considers and often amends a bill and then sends it to the chamber’s leadership for a floor vote.)
In a statement issued about the hearing, America’s Health Insurance Plans (AHIP) noted that there has been work in recent years toward streamlining prior authorization. AHIP said it launched the Fast Prior Authorization Technology Highway (Fast PATH) initiative in 2020 to study electronic procedures for handling these reviews.
“The findings of this study showed that ePA delivered improvements with a strong majority of experienced providers reporting faster time to patient care, fewer phone calls and faxes, better understanding of [prior authorization] requirements, and faster time to decisions,” AHIP said.
A version of this article first appeared on Medscape.com.
Republican and Democratic members of the House called for changes in how insurer-run Medicare plans manage the prior authorization process, following testimony from a federal watchdog organization about improper denials of payment for care.
About 18% of payment denials in a sample examined by the Office of Inspector General (OIG) of the Department of Health and Human Services (HHS) either met Medicare coverage rules or the rules of the insurance plan.
As such, they should not have been denied, according to the OIG. That was the finding of an April OIG report, based on a sample of 2019 denials from large insurer-run Medicare plans.
Erin Bliss, an assistant inspector general with the OIG, appeared as a witness at a June 28 Energy and Commerce Subcommittee on Oversight and Investigations hearing to discuss this investigation and other issues with prior authorization and insurer-run Medicare, also known as the Advantage plans.
Most of these payment denials of appropriate services were due to human error during manual claims-processing reviews, Ms. Bliss told the subcommittee, such as overlooking a document, and to system processing errors, such as a Medicare insurance plan failing to program or update a system correctly.
In many cases, these denials were reversed, but patient care was still disrupted and clinicians lost time chasing clearances for services that plans already had covered, Ms. Bliss said in her testimony.
The April report was not the OIG’s first look into concerns about insurer-run plans inappropriately denying care through prior authorizations. The OIG in 2018 reported that insurer-run Medicare plans overturned 75% of their own denials during 2014-2016 when patients and clinicians appealed these decisions, overturning approximately 216,000 denials each year.
‘Numerous hoops’ unnecessary for doctors, patients
Lawmakers at the hearing supported the idea of the need for prior authorization as a screening tool to prevent unneeded care.
But they chided insurance companies for their execution of this process, with clinicians and patients often frustrated by complex steps needed. Medicare Advantage plans sometimes require prior authorization for “relatively standard medical services,” said Subcommittee on Oversight and Investigations Chair Diana DeGette (D-Colo.).
“Our seniors and their doctors should not be required to jump through numerous hoops to ensure coverage for straightforward and medically necessary procedures,” Rep. DeGette said.
Several lawmakers spoke at the hearing about the need for changes to prior authorization, including calling for action on a pending bill intended to compel insurers to streamline the review process. The Improving Seniors’ Timely Access to Care Act of 2021 already has attracted more than 300 bipartisan sponsors. A companion Senate bill has more than 30 sponsors.
The bill’s aim is to shift this process away from faxes and phone calls while also encouraging plans to adhere to evidence-based medical guidelines in consultation with physicians. The bill calls for the establishment of an electronic prior authorization program that could issue real-time decisions.
“The result will be less administrative burden for providers and more information in the hands of patients. It will allow more patients to receive care when they need it, reducing the likelihood of additional, often more severe complications,” said Rep. Larry Bucshon, MD, (R-Ind.) who is among the active sponsors of the bill.
“In the long term, I believe it would also result in cost savings for the health care system at large by identifying problems earlier and getting them treated before their patients have more complications,” Rep. Bucshon added.
Finding ‘room for improvement’ for prior authorizations
There’s strong bipartisan support in Congress for insurer-run Medicare, which has grown by 10% per year over the last several years and has doubled since 2010, according to the Medicare Payment Advisory Commission (MedPAC). About 27 million people are now enrolled in these plans.
But for that reason, insurer-run Medicare may also need more careful watching, lawmakers made clear at the hearing.
“We’ve heard quite a bit of evidence today that there is room for improvement,” said Rep. Bucshon, a strong supporter of insurer-run Medicare, which can offer patients added benefits such as dental coverage.
Rep. Ann Kuster (D-N.H.) said simplifying prior authorization would reduce stress on clinicians already dealing with burnout.
“They’re just so tired of all this paperwork and red tape,” Rep. Kuster said. “In 2022 can’t we at least consider electronic prior authorization?”
At the hearing, Rep. Michael C. Burgess, MD, (R-Tex.) noted that his home state already has taken a step toward reducing the burden of prior authorization with its “gold card” program.
In 2021, a new Texas law called on the state department of insurance to develop rules to require health plans to provide an exemption from preauthorization requirements for a particular health care service if the issuer has approved, or would have approved, at least 90% of the preauthorization requests submitted by the physician or provider for that service. The law also mandates that a physician participating in a peer-to-peer review on behalf of a health benefit plan issuer must be a Texas-licensed physician who has the same or similar specialty as the physician or clinician requesting the service, according to the state insurance department.
Separately, Rep. Suzan DelBene (D-Wash.), the sponsor of the Improving Seniors’ Timely Access to Care Act, told the American Medical Association in a recent interview that she expects the House Ways and Means Committee, on which she serves, to mark up her bill in July. (A mark-up is the process by which a House or Senate committee considers and often amends a bill and then sends it to the chamber’s leadership for a floor vote.)
In a statement issued about the hearing, America’s Health Insurance Plans (AHIP) noted that there has been work in recent years toward streamlining prior authorization. AHIP said it launched the Fast Prior Authorization Technology Highway (Fast PATH) initiative in 2020 to study electronic procedures for handling these reviews.
“The findings of this study showed that ePA delivered improvements with a strong majority of experienced providers reporting faster time to patient care, fewer phone calls and faxes, better understanding of [prior authorization] requirements, and faster time to decisions,” AHIP said.
A version of this article first appeared on Medscape.com.
Republican and Democratic members of the House called for changes in how insurer-run Medicare plans manage the prior authorization process, following testimony from a federal watchdog organization about improper denials of payment for care.
About 18% of payment denials in a sample examined by the Office of Inspector General (OIG) of the Department of Health and Human Services (HHS) either met Medicare coverage rules or the rules of the insurance plan.
As such, they should not have been denied, according to the OIG. That was the finding of an April OIG report, based on a sample of 2019 denials from large insurer-run Medicare plans.
Erin Bliss, an assistant inspector general with the OIG, appeared as a witness at a June 28 Energy and Commerce Subcommittee on Oversight and Investigations hearing to discuss this investigation and other issues with prior authorization and insurer-run Medicare, also known as the Advantage plans.
Most of these payment denials of appropriate services were due to human error during manual claims-processing reviews, Ms. Bliss told the subcommittee, such as overlooking a document, and to system processing errors, such as a Medicare insurance plan failing to program or update a system correctly.
In many cases, these denials were reversed, but patient care was still disrupted and clinicians lost time chasing clearances for services that plans already had covered, Ms. Bliss said in her testimony.
The April report was not the OIG’s first look into concerns about insurer-run plans inappropriately denying care through prior authorizations. The OIG in 2018 reported that insurer-run Medicare plans overturned 75% of their own denials during 2014-2016 when patients and clinicians appealed these decisions, overturning approximately 216,000 denials each year.
‘Numerous hoops’ unnecessary for doctors, patients
Lawmakers at the hearing supported the idea of the need for prior authorization as a screening tool to prevent unneeded care.
But they chided insurance companies for their execution of this process, with clinicians and patients often frustrated by complex steps needed. Medicare Advantage plans sometimes require prior authorization for “relatively standard medical services,” said Subcommittee on Oversight and Investigations Chair Diana DeGette (D-Colo.).
“Our seniors and their doctors should not be required to jump through numerous hoops to ensure coverage for straightforward and medically necessary procedures,” Rep. DeGette said.
Several lawmakers spoke at the hearing about the need for changes to prior authorization, including calling for action on a pending bill intended to compel insurers to streamline the review process. The Improving Seniors’ Timely Access to Care Act of 2021 already has attracted more than 300 bipartisan sponsors. A companion Senate bill has more than 30 sponsors.
The bill’s aim is to shift this process away from faxes and phone calls while also encouraging plans to adhere to evidence-based medical guidelines in consultation with physicians. The bill calls for the establishment of an electronic prior authorization program that could issue real-time decisions.
“The result will be less administrative burden for providers and more information in the hands of patients. It will allow more patients to receive care when they need it, reducing the likelihood of additional, often more severe complications,” said Rep. Larry Bucshon, MD, (R-Ind.) who is among the active sponsors of the bill.
“In the long term, I believe it would also result in cost savings for the health care system at large by identifying problems earlier and getting them treated before their patients have more complications,” Rep. Bucshon added.
Finding ‘room for improvement’ for prior authorizations
There’s strong bipartisan support in Congress for insurer-run Medicare, which has grown by 10% per year over the last several years and has doubled since 2010, according to the Medicare Payment Advisory Commission (MedPAC). About 27 million people are now enrolled in these plans.
But for that reason, insurer-run Medicare may also need more careful watching, lawmakers made clear at the hearing.
“We’ve heard quite a bit of evidence today that there is room for improvement,” said Rep. Bucshon, a strong supporter of insurer-run Medicare, which can offer patients added benefits such as dental coverage.
Rep. Ann Kuster (D-N.H.) said simplifying prior authorization would reduce stress on clinicians already dealing with burnout.
“They’re just so tired of all this paperwork and red tape,” Rep. Kuster said. “In 2022 can’t we at least consider electronic prior authorization?”
At the hearing, Rep. Michael C. Burgess, MD, (R-Tex.) noted that his home state already has taken a step toward reducing the burden of prior authorization with its “gold card” program.
In 2021, a new Texas law called on the state department of insurance to develop rules to require health plans to provide an exemption from preauthorization requirements for a particular health care service if the issuer has approved, or would have approved, at least 90% of the preauthorization requests submitted by the physician or provider for that service. The law also mandates that a physician participating in a peer-to-peer review on behalf of a health benefit plan issuer must be a Texas-licensed physician who has the same or similar specialty as the physician or clinician requesting the service, according to the state insurance department.
Separately, Rep. Suzan DelBene (D-Wash.), the sponsor of the Improving Seniors’ Timely Access to Care Act, told the American Medical Association in a recent interview that she expects the House Ways and Means Committee, on which she serves, to mark up her bill in July. (A mark-up is the process by which a House or Senate committee considers and often amends a bill and then sends it to the chamber’s leadership for a floor vote.)
In a statement issued about the hearing, America’s Health Insurance Plans (AHIP) noted that there has been work in recent years toward streamlining prior authorization. AHIP said it launched the Fast Prior Authorization Technology Highway (Fast PATH) initiative in 2020 to study electronic procedures for handling these reviews.
“The findings of this study showed that ePA delivered improvements with a strong majority of experienced providers reporting faster time to patient care, fewer phone calls and faxes, better understanding of [prior authorization] requirements, and faster time to decisions,” AHIP said.
A version of this article first appeared on Medscape.com.