User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Improving statewide reporting of melanoma cases
For years, . I have audited my melanoma cases (biopsies and excisions sent to me) and discovered that of the 240 cases confirmed over the past 5 years, only 41 were reported to the Ohio state health department and are in that database. That amounts to 199 unreported cases – nearly 83% of the total.
This raises the question as to who is responsible for reporting these cases. Dermatology is unique in that our pathology specimens are not routinely passed through a hospital pathology laboratory. The big difference in reporting is that hospital labs have trained data registrars to report all reportable cancers to state health departments. Therefore, in my case, only patients sent to a hospital-based surgeon for sentinel node biopsies or exceptionally large excisions get reported. When I have spoken about this to my dermatology lab and biopsying physicians, the discussion rapidly turns into a finger pointing game of who is responsible. No one, except perhaps the dermatologist who did the biopsy, has all the data.
Unfortunately, these cases are tedious and time consuming to report. Despite state laws requiring reporting, even with penalties for nonreporters, many small dermatology practices do not report these cases and expect their dermatopathology labs to do so, but the labs expect the biopsying dermatologist to report the cases. This is a classic case of an unfunded mandate since small dermatology practices do not have the time or resources for reporting.
I have worked with the Ohio Department of Health to remove any unnecessary data fields and they have managed to reduce the reporting fields (to 59!). This is the minimum amount required to be included in the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) database. Many of these fields are not applicable to thin melanomas and after reviewing the 1-hour online training course, each patient can be entered (once the necessary data are collected) in about 15 minutes. This is still a formidable task for small offices, which cannot be blamed for ducking and hoping someone else reports.
While there is controversy regarding the relevance of thin melanomas to overall survival, more accurate reporting can only bolster either argument.
A solution to underreporting
I believe we have developed a unique solution to this conundrum. Our office is partnering with the local melanoma support group (Melanoma Know More) to train volunteers to help with the data collection and reporting of these thin melanomas. We have also discovered that the local community college has students who are majoring in pathology data registry reporting and are happy to gain a little experience before graduating.
We eventually hope to become a clearinghouse for the entire state of Ohio. The state health department has agreed not to apply punitive measures to physicians who are new reporters. It is our plan to obtain melanoma pathology reports, run these past the state database, identify unreported cases, and obtain further data as needed from the biopsying physicians, and then complete the reporting.
I think dermatologic oncologists in every state should view this as an opportunity for a significant quality improvement project, and as a terrific service to the general dermatology community.
The ramifications of more comprehensive reporting of melanomas are great. I would expect more attention to the disease by researchers, and much more clout with state and national legislators. Think about increased funding for melanoma research, allowing sunscreen use for school children, sunshades for playgrounds, and more responsible tanning bed restrictions.
Now, I must inform you that this is my last column, but I plan to continue writing. Over the past 6 years, I have been able to cover a wide range of topics ranging from human trafficking and the American Medical Association, to the many problems faced by small practices. I have enjoyed myself hugely. To quote Douglas Adams, from The Hitchhiker’s Guide to the Galaxy, “So long and thanks for all the fish!” Keep in touch at [email protected].
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
For years, . I have audited my melanoma cases (biopsies and excisions sent to me) and discovered that of the 240 cases confirmed over the past 5 years, only 41 were reported to the Ohio state health department and are in that database. That amounts to 199 unreported cases – nearly 83% of the total.
This raises the question as to who is responsible for reporting these cases. Dermatology is unique in that our pathology specimens are not routinely passed through a hospital pathology laboratory. The big difference in reporting is that hospital labs have trained data registrars to report all reportable cancers to state health departments. Therefore, in my case, only patients sent to a hospital-based surgeon for sentinel node biopsies or exceptionally large excisions get reported. When I have spoken about this to my dermatology lab and biopsying physicians, the discussion rapidly turns into a finger pointing game of who is responsible. No one, except perhaps the dermatologist who did the biopsy, has all the data.
Unfortunately, these cases are tedious and time consuming to report. Despite state laws requiring reporting, even with penalties for nonreporters, many small dermatology practices do not report these cases and expect their dermatopathology labs to do so, but the labs expect the biopsying dermatologist to report the cases. This is a classic case of an unfunded mandate since small dermatology practices do not have the time or resources for reporting.
I have worked with the Ohio Department of Health to remove any unnecessary data fields and they have managed to reduce the reporting fields (to 59!). This is the minimum amount required to be included in the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) database. Many of these fields are not applicable to thin melanomas and after reviewing the 1-hour online training course, each patient can be entered (once the necessary data are collected) in about 15 minutes. This is still a formidable task for small offices, which cannot be blamed for ducking and hoping someone else reports.
While there is controversy regarding the relevance of thin melanomas to overall survival, more accurate reporting can only bolster either argument.
A solution to underreporting
I believe we have developed a unique solution to this conundrum. Our office is partnering with the local melanoma support group (Melanoma Know More) to train volunteers to help with the data collection and reporting of these thin melanomas. We have also discovered that the local community college has students who are majoring in pathology data registry reporting and are happy to gain a little experience before graduating.
We eventually hope to become a clearinghouse for the entire state of Ohio. The state health department has agreed not to apply punitive measures to physicians who are new reporters. It is our plan to obtain melanoma pathology reports, run these past the state database, identify unreported cases, and obtain further data as needed from the biopsying physicians, and then complete the reporting.
I think dermatologic oncologists in every state should view this as an opportunity for a significant quality improvement project, and as a terrific service to the general dermatology community.
The ramifications of more comprehensive reporting of melanomas are great. I would expect more attention to the disease by researchers, and much more clout with state and national legislators. Think about increased funding for melanoma research, allowing sunscreen use for school children, sunshades for playgrounds, and more responsible tanning bed restrictions.
Now, I must inform you that this is my last column, but I plan to continue writing. Over the past 6 years, I have been able to cover a wide range of topics ranging from human trafficking and the American Medical Association, to the many problems faced by small practices. I have enjoyed myself hugely. To quote Douglas Adams, from The Hitchhiker’s Guide to the Galaxy, “So long and thanks for all the fish!” Keep in touch at [email protected].
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
For years, . I have audited my melanoma cases (biopsies and excisions sent to me) and discovered that of the 240 cases confirmed over the past 5 years, only 41 were reported to the Ohio state health department and are in that database. That amounts to 199 unreported cases – nearly 83% of the total.
This raises the question as to who is responsible for reporting these cases. Dermatology is unique in that our pathology specimens are not routinely passed through a hospital pathology laboratory. The big difference in reporting is that hospital labs have trained data registrars to report all reportable cancers to state health departments. Therefore, in my case, only patients sent to a hospital-based surgeon for sentinel node biopsies or exceptionally large excisions get reported. When I have spoken about this to my dermatology lab and biopsying physicians, the discussion rapidly turns into a finger pointing game of who is responsible. No one, except perhaps the dermatologist who did the biopsy, has all the data.
Unfortunately, these cases are tedious and time consuming to report. Despite state laws requiring reporting, even with penalties for nonreporters, many small dermatology practices do not report these cases and expect their dermatopathology labs to do so, but the labs expect the biopsying dermatologist to report the cases. This is a classic case of an unfunded mandate since small dermatology practices do not have the time or resources for reporting.
I have worked with the Ohio Department of Health to remove any unnecessary data fields and they have managed to reduce the reporting fields (to 59!). This is the minimum amount required to be included in the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) database. Many of these fields are not applicable to thin melanomas and after reviewing the 1-hour online training course, each patient can be entered (once the necessary data are collected) in about 15 minutes. This is still a formidable task for small offices, which cannot be blamed for ducking and hoping someone else reports.
While there is controversy regarding the relevance of thin melanomas to overall survival, more accurate reporting can only bolster either argument.
A solution to underreporting
I believe we have developed a unique solution to this conundrum. Our office is partnering with the local melanoma support group (Melanoma Know More) to train volunteers to help with the data collection and reporting of these thin melanomas. We have also discovered that the local community college has students who are majoring in pathology data registry reporting and are happy to gain a little experience before graduating.
We eventually hope to become a clearinghouse for the entire state of Ohio. The state health department has agreed not to apply punitive measures to physicians who are new reporters. It is our plan to obtain melanoma pathology reports, run these past the state database, identify unreported cases, and obtain further data as needed from the biopsying physicians, and then complete the reporting.
I think dermatologic oncologists in every state should view this as an opportunity for a significant quality improvement project, and as a terrific service to the general dermatology community.
The ramifications of more comprehensive reporting of melanomas are great. I would expect more attention to the disease by researchers, and much more clout with state and national legislators. Think about increased funding for melanoma research, allowing sunscreen use for school children, sunshades for playgrounds, and more responsible tanning bed restrictions.
Now, I must inform you that this is my last column, but I plan to continue writing. Over the past 6 years, I have been able to cover a wide range of topics ranging from human trafficking and the American Medical Association, to the many problems faced by small practices. I have enjoyed myself hugely. To quote Douglas Adams, from The Hitchhiker’s Guide to the Galaxy, “So long and thanks for all the fish!” Keep in touch at [email protected].
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
The neurological super powers of grandma are real
Deer, COVID, how?
Usually humans cannot get close enough to a deer to really be face-to-face, so it’s easy to question how on Earth deer are contracting COVID-19. Well, stranger things have happened, and honestly, we’ve just stopped questioning most of them.
Exhibit A comes to us from a Penn State University study: Eighty percent of deer sampled in Iowa in December 2020 and January 2021 – as part of the state’s chronic wasting disease surveillance program – were found to be positive for COVID-19.
A statement from the university said that “white-tailed deer may be a reservoir for the virus to continually circulate and raise concerns about the emergence of new strains that may prove a threat to wildlife and, possibly, to humans.” The investigators also suggested that deer probably caught the virus from humans and then transmitted it to other deer.
If you or someone you know is a hunter or a white-tailed deer, it’s best to proceed with caution. There’s no evidence that COVID-19 has jumped from deer to humans, but hunters should wear masks and gloves while working with deer, worrying not just about the deer’s face, but also … you know, the gastrointestinal parts, Robert Salata, MD, of University Hospitals Cleveland Medical Center, told Syracuse.com. It also shouldn’t be too risky to eat venison, he said, just make sure the meat is cooked thoroughly.
The more you know!
The neurological super powers of grandma are real
What is it about grandmothers that makes them seem almost magical at times? They somehow always know how you feel. And they can almost always tell when something is wrong. They also seem to be the biggest ally a child will have against his or her parents.
So what makes these super matriarchs? The answer is in the brain.
Apparently there’s a function in the brains of grandmothers geared toward “emotional empathy.” James Rilling, PhD, of Emory University, lead author of a recent study focused on looking at the brain function of grandmothers, suggested that they’re neurologically tapped into feeling how their grandchildren feel: “If their grandchild is smiling, they’re feeling the child’s joy. And if their grandchild is crying, they’re feeling the child’s pain and distress.”
And then there’s the cute factor. Never underestimate a child’s ability to manipulate his or her grandmother’s brain.
So how do the researchers know this? Functional MRI showed more brain activity in the parts of the brain that deal with emotional empathy and movement in the participating grandmas when shown pictures of their grandchildren. Images of their own adult children lit up areas more associated with cognitive empathy. So less emotional and more mental/logical understanding.
Kids, don’t tell Mom about the secret midnight snacks with grandma. She wouldn’t get it.
Then there’s the grandmother hypothesis, which suggests that women tend to live longer to provide some kind of evolutionary benefit to their children and grandchildren. Evidence also exists that children with positive engagement from their grandmothers tend to have better social and academic outcomes, behavior, and physical health.
A lot of credit on how children turn out, of course, goes to parents, but more can be said about grandmas. Don’t let the age and freshly baked cookies fool you. They have neurologic superpowers within.
Brain cleanup on aisle 5
You’ve got your local grocery store down. You know the ins and outs; you know where everything is. Last week you did your trip in record time. This week, however, you have to stop at a different store. Same chain, but a different location. You stroll in, confidently walk toward the first aisle for your fruits and veggies, and ... it’s all ice cream. Oops.
There’s a lot we don’t understand about the brain, including how it remembers familiar environments to avoid confusion. Or why it fails to do so, as with our grocery store example. However, thanks to a study from the University of Arizona, we may have an answer.
For the experiment, a group of participants watched a video tour of three virtual cities. Those cities were very similar, being laid out in basically identical fashion. Stores could be found in the same places, but the identity of those stores varied. Some stores were in all three cities, some were in two, and some were unique. Participants were asked to memorize the layouts, and those who got things more than 80% correct ran through the test again, only this time their brain activity was monitored through MRI.
In general, brain activity was similar for the participants; after all, they were recalling similar environments. However, when asked about stores that appeared in multiple cities, brain activity varied dramatically. This indicated to the researchers that the brain was recalling shared stores as if they were more dissimilar than two completely disparate and unique stores, a concept often known to brain scientists as “repulsion.” It also indicates that the memories regarding shared environments are stored in the prefrontal cortex, not the hippocampus, which typically handles memory.
The researchers plan to apply this information to questions about diseases such as Alzheimer’s, so the next time you get turned around in a weirdly unfamiliar grocery store, just think: “It’s okay, I’m helping to solve a terrible brain disease.”
The real endgame: Friction is the winner
Spoiler alert! If you haven’t seen “Avengers: Infinity War” yet, we’re about to ruin it for you.
For those still with us, here’s the spoiler: Thanos would not have been able to snap his fingers while wearing the Infinity Gauntlet.
Saad Bhamla, PhD, of Georgia Tech University’s school of chemical and biomolecular engineering, had been studying powerful and ultrafast motions in living organisms along with several colleagues before the movie came out in 2018, and when they saw the finger-snapping scene it got them wondering.
Being scientists of course, they had no choice. They got out their high-speed imaging equipment, automated image processing software, and dynamic force sensors and analyzed finger snaps, paying close attention to friction by covering fingers with “different materials, including metallic thimbles to simulate the effects of trying to snap while wearing a metallic gauntlet, much like Thanos,” according to a statement on Eurekalert.
With finger snaps, it’s all about the rotational velocity. The angular acceleration involved is the fastest ever measured in a human, with a professional baseball pitcher’s throwing arm a distant second.
Dr. Bhamla’s reaction to their work explains why scientists are the ones doing science. “When I first saw the data, I jumped out of my chair,” he said in the written statement.
Rotational velocities dropped dramatically when the friction-reducing thimbles were used, so there was no snap. Which means that billions and billions of fictional lives could have been saved if the filmmakers had just talked to the right scientist.
That scientist, clearly, is Dr. Bhamla, who said that “this is the only scientific project in my lab in which we could snap our fingers and get data.”
Deer, COVID, how?
Usually humans cannot get close enough to a deer to really be face-to-face, so it’s easy to question how on Earth deer are contracting COVID-19. Well, stranger things have happened, and honestly, we’ve just stopped questioning most of them.
Exhibit A comes to us from a Penn State University study: Eighty percent of deer sampled in Iowa in December 2020 and January 2021 – as part of the state’s chronic wasting disease surveillance program – were found to be positive for COVID-19.
A statement from the university said that “white-tailed deer may be a reservoir for the virus to continually circulate and raise concerns about the emergence of new strains that may prove a threat to wildlife and, possibly, to humans.” The investigators also suggested that deer probably caught the virus from humans and then transmitted it to other deer.
If you or someone you know is a hunter or a white-tailed deer, it’s best to proceed with caution. There’s no evidence that COVID-19 has jumped from deer to humans, but hunters should wear masks and gloves while working with deer, worrying not just about the deer’s face, but also … you know, the gastrointestinal parts, Robert Salata, MD, of University Hospitals Cleveland Medical Center, told Syracuse.com. It also shouldn’t be too risky to eat venison, he said, just make sure the meat is cooked thoroughly.
The more you know!
The neurological super powers of grandma are real
What is it about grandmothers that makes them seem almost magical at times? They somehow always know how you feel. And they can almost always tell when something is wrong. They also seem to be the biggest ally a child will have against his or her parents.
So what makes these super matriarchs? The answer is in the brain.
Apparently there’s a function in the brains of grandmothers geared toward “emotional empathy.” James Rilling, PhD, of Emory University, lead author of a recent study focused on looking at the brain function of grandmothers, suggested that they’re neurologically tapped into feeling how their grandchildren feel: “If their grandchild is smiling, they’re feeling the child’s joy. And if their grandchild is crying, they’re feeling the child’s pain and distress.”
And then there’s the cute factor. Never underestimate a child’s ability to manipulate his or her grandmother’s brain.
So how do the researchers know this? Functional MRI showed more brain activity in the parts of the brain that deal with emotional empathy and movement in the participating grandmas when shown pictures of their grandchildren. Images of their own adult children lit up areas more associated with cognitive empathy. So less emotional and more mental/logical understanding.
Kids, don’t tell Mom about the secret midnight snacks with grandma. She wouldn’t get it.
Then there’s the grandmother hypothesis, which suggests that women tend to live longer to provide some kind of evolutionary benefit to their children and grandchildren. Evidence also exists that children with positive engagement from their grandmothers tend to have better social and academic outcomes, behavior, and physical health.
A lot of credit on how children turn out, of course, goes to parents, but more can be said about grandmas. Don’t let the age and freshly baked cookies fool you. They have neurologic superpowers within.
Brain cleanup on aisle 5
You’ve got your local grocery store down. You know the ins and outs; you know where everything is. Last week you did your trip in record time. This week, however, you have to stop at a different store. Same chain, but a different location. You stroll in, confidently walk toward the first aisle for your fruits and veggies, and ... it’s all ice cream. Oops.
There’s a lot we don’t understand about the brain, including how it remembers familiar environments to avoid confusion. Or why it fails to do so, as with our grocery store example. However, thanks to a study from the University of Arizona, we may have an answer.
For the experiment, a group of participants watched a video tour of three virtual cities. Those cities were very similar, being laid out in basically identical fashion. Stores could be found in the same places, but the identity of those stores varied. Some stores were in all three cities, some were in two, and some were unique. Participants were asked to memorize the layouts, and those who got things more than 80% correct ran through the test again, only this time their brain activity was monitored through MRI.
In general, brain activity was similar for the participants; after all, they were recalling similar environments. However, when asked about stores that appeared in multiple cities, brain activity varied dramatically. This indicated to the researchers that the brain was recalling shared stores as if they were more dissimilar than two completely disparate and unique stores, a concept often known to brain scientists as “repulsion.” It also indicates that the memories regarding shared environments are stored in the prefrontal cortex, not the hippocampus, which typically handles memory.
The researchers plan to apply this information to questions about diseases such as Alzheimer’s, so the next time you get turned around in a weirdly unfamiliar grocery store, just think: “It’s okay, I’m helping to solve a terrible brain disease.”
The real endgame: Friction is the winner
Spoiler alert! If you haven’t seen “Avengers: Infinity War” yet, we’re about to ruin it for you.
For those still with us, here’s the spoiler: Thanos would not have been able to snap his fingers while wearing the Infinity Gauntlet.
Saad Bhamla, PhD, of Georgia Tech University’s school of chemical and biomolecular engineering, had been studying powerful and ultrafast motions in living organisms along with several colleagues before the movie came out in 2018, and when they saw the finger-snapping scene it got them wondering.
Being scientists of course, they had no choice. They got out their high-speed imaging equipment, automated image processing software, and dynamic force sensors and analyzed finger snaps, paying close attention to friction by covering fingers with “different materials, including metallic thimbles to simulate the effects of trying to snap while wearing a metallic gauntlet, much like Thanos,” according to a statement on Eurekalert.
With finger snaps, it’s all about the rotational velocity. The angular acceleration involved is the fastest ever measured in a human, with a professional baseball pitcher’s throwing arm a distant second.
Dr. Bhamla’s reaction to their work explains why scientists are the ones doing science. “When I first saw the data, I jumped out of my chair,” he said in the written statement.
Rotational velocities dropped dramatically when the friction-reducing thimbles were used, so there was no snap. Which means that billions and billions of fictional lives could have been saved if the filmmakers had just talked to the right scientist.
That scientist, clearly, is Dr. Bhamla, who said that “this is the only scientific project in my lab in which we could snap our fingers and get data.”
Deer, COVID, how?
Usually humans cannot get close enough to a deer to really be face-to-face, so it’s easy to question how on Earth deer are contracting COVID-19. Well, stranger things have happened, and honestly, we’ve just stopped questioning most of them.
Exhibit A comes to us from a Penn State University study: Eighty percent of deer sampled in Iowa in December 2020 and January 2021 – as part of the state’s chronic wasting disease surveillance program – were found to be positive for COVID-19.
A statement from the university said that “white-tailed deer may be a reservoir for the virus to continually circulate and raise concerns about the emergence of new strains that may prove a threat to wildlife and, possibly, to humans.” The investigators also suggested that deer probably caught the virus from humans and then transmitted it to other deer.
If you or someone you know is a hunter or a white-tailed deer, it’s best to proceed with caution. There’s no evidence that COVID-19 has jumped from deer to humans, but hunters should wear masks and gloves while working with deer, worrying not just about the deer’s face, but also … you know, the gastrointestinal parts, Robert Salata, MD, of University Hospitals Cleveland Medical Center, told Syracuse.com. It also shouldn’t be too risky to eat venison, he said, just make sure the meat is cooked thoroughly.
The more you know!
The neurological super powers of grandma are real
What is it about grandmothers that makes them seem almost magical at times? They somehow always know how you feel. And they can almost always tell when something is wrong. They also seem to be the biggest ally a child will have against his or her parents.
So what makes these super matriarchs? The answer is in the brain.
Apparently there’s a function in the brains of grandmothers geared toward “emotional empathy.” James Rilling, PhD, of Emory University, lead author of a recent study focused on looking at the brain function of grandmothers, suggested that they’re neurologically tapped into feeling how their grandchildren feel: “If their grandchild is smiling, they’re feeling the child’s joy. And if their grandchild is crying, they’re feeling the child’s pain and distress.”
And then there’s the cute factor. Never underestimate a child’s ability to manipulate his or her grandmother’s brain.
So how do the researchers know this? Functional MRI showed more brain activity in the parts of the brain that deal with emotional empathy and movement in the participating grandmas when shown pictures of their grandchildren. Images of their own adult children lit up areas more associated with cognitive empathy. So less emotional and more mental/logical understanding.
Kids, don’t tell Mom about the secret midnight snacks with grandma. She wouldn’t get it.
Then there’s the grandmother hypothesis, which suggests that women tend to live longer to provide some kind of evolutionary benefit to their children and grandchildren. Evidence also exists that children with positive engagement from their grandmothers tend to have better social and academic outcomes, behavior, and physical health.
A lot of credit on how children turn out, of course, goes to parents, but more can be said about grandmas. Don’t let the age and freshly baked cookies fool you. They have neurologic superpowers within.
Brain cleanup on aisle 5
You’ve got your local grocery store down. You know the ins and outs; you know where everything is. Last week you did your trip in record time. This week, however, you have to stop at a different store. Same chain, but a different location. You stroll in, confidently walk toward the first aisle for your fruits and veggies, and ... it’s all ice cream. Oops.
There’s a lot we don’t understand about the brain, including how it remembers familiar environments to avoid confusion. Or why it fails to do so, as with our grocery store example. However, thanks to a study from the University of Arizona, we may have an answer.
For the experiment, a group of participants watched a video tour of three virtual cities. Those cities were very similar, being laid out in basically identical fashion. Stores could be found in the same places, but the identity of those stores varied. Some stores were in all three cities, some were in two, and some were unique. Participants were asked to memorize the layouts, and those who got things more than 80% correct ran through the test again, only this time their brain activity was monitored through MRI.
In general, brain activity was similar for the participants; after all, they were recalling similar environments. However, when asked about stores that appeared in multiple cities, brain activity varied dramatically. This indicated to the researchers that the brain was recalling shared stores as if they were more dissimilar than two completely disparate and unique stores, a concept often known to brain scientists as “repulsion.” It also indicates that the memories regarding shared environments are stored in the prefrontal cortex, not the hippocampus, which typically handles memory.
The researchers plan to apply this information to questions about diseases such as Alzheimer’s, so the next time you get turned around in a weirdly unfamiliar grocery store, just think: “It’s okay, I’m helping to solve a terrible brain disease.”
The real endgame: Friction is the winner
Spoiler alert! If you haven’t seen “Avengers: Infinity War” yet, we’re about to ruin it for you.
For those still with us, here’s the spoiler: Thanos would not have been able to snap his fingers while wearing the Infinity Gauntlet.
Saad Bhamla, PhD, of Georgia Tech University’s school of chemical and biomolecular engineering, had been studying powerful and ultrafast motions in living organisms along with several colleagues before the movie came out in 2018, and when they saw the finger-snapping scene it got them wondering.
Being scientists of course, they had no choice. They got out their high-speed imaging equipment, automated image processing software, and dynamic force sensors and analyzed finger snaps, paying close attention to friction by covering fingers with “different materials, including metallic thimbles to simulate the effects of trying to snap while wearing a metallic gauntlet, much like Thanos,” according to a statement on Eurekalert.
With finger snaps, it’s all about the rotational velocity. The angular acceleration involved is the fastest ever measured in a human, with a professional baseball pitcher’s throwing arm a distant second.
Dr. Bhamla’s reaction to their work explains why scientists are the ones doing science. “When I first saw the data, I jumped out of my chair,” he said in the written statement.
Rotational velocities dropped dramatically when the friction-reducing thimbles were used, so there was no snap. Which means that billions and billions of fictional lives could have been saved if the filmmakers had just talked to the right scientist.
That scientist, clearly, is Dr. Bhamla, who said that “this is the only scientific project in my lab in which we could snap our fingers and get data.”
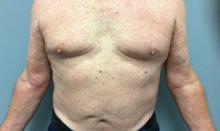
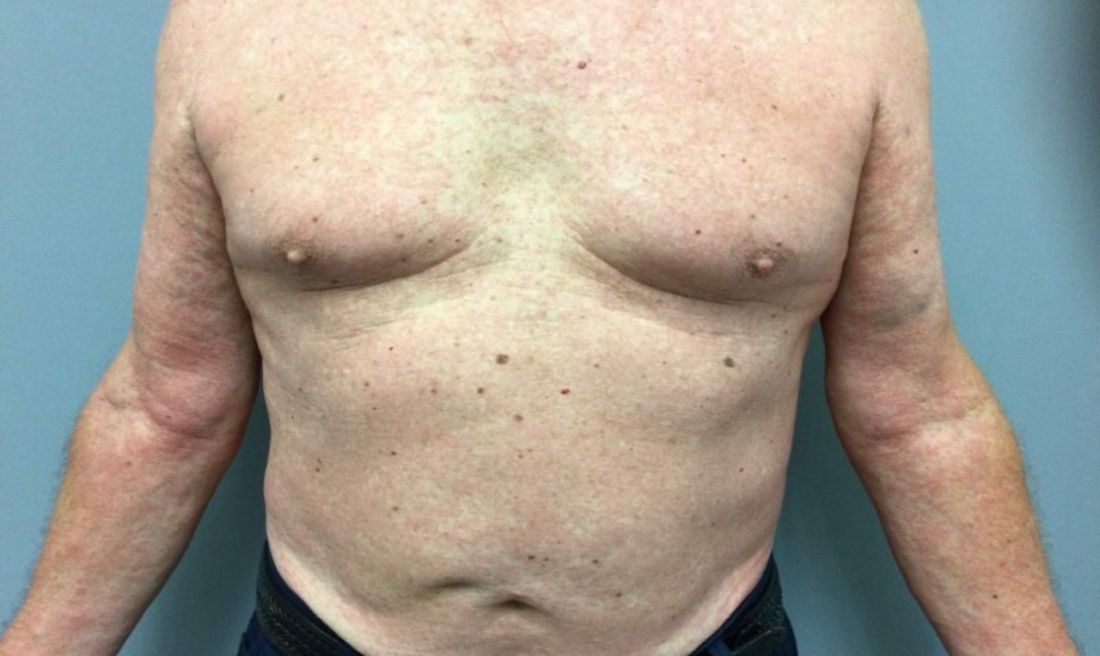
A 73-year-old White male presented with 2 days of a very pruritic rash
Reactions can occur anytime from within the first 2 weeks of treatment up to 10 days after the treatment has been discontinued. If a drug is rechallenged, eruptions may occur sooner. Pruritus is commonly seen. Clinically, erythematous papules and macules present symmetrically on the trunk and upper extremities and then become more generalized. A low-grade fever may be present.
Antibiotics are the most common causes of exanthematous drug eruptions. Penicillins and trimethoprim-sulfamethoxazole are common offenders. Cephalosporins, anticonvulsants, and allopurinol may also induce a reaction. As this condition is diagnosed clinically, skin biopsy is often not necessary. Histology is nonspecific and shows a mild perivascular lymphocytic infiltrate and few epidermal necrotic keratinocytes.
In drug reaction with eosinophilia and systemic symptoms (DRESS), symptoms present 2-6 weeks after the offending medication has been started. The cutaneous rash appears similar to an exanthematous drug eruption; however, lesions will also present on the face, and facial edema may occur. Fever is often present. Laboratory findings include a marked peripheral blood hypereosinophilia. Elevated liver function tests may be seen. Viruses such as Epstein-Barr virus, enteroviruses, adenovirus, early HIV, human herpesvirus 6, and parvovirus B19 have a similar clinical appearance to an exanthematous drug eruption. A mild eosinophilia, as seen in a drug eruption, helps to distinguish between a drug eruption and viral exanthem. In Stevens-Johnson Syndrome, mucosal membranes are involved and skin is often painful or appears dusky.
Treatment of exanthematous drug eruptions is largely supportive. Discontinuing the drug will help speed resolution and topical steroids may alleviate pruritus.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Bolognia J et al. “Dermatology” (St. Louis: Mosby/Elsevier, 2008).
2. James W et al. “Andrews’ Diseases of the Skin,” 13th ed. (Philadelphia: Saunders Elsevier, 2006).
Reactions can occur anytime from within the first 2 weeks of treatment up to 10 days after the treatment has been discontinued. If a drug is rechallenged, eruptions may occur sooner. Pruritus is commonly seen. Clinically, erythematous papules and macules present symmetrically on the trunk and upper extremities and then become more generalized. A low-grade fever may be present.
Antibiotics are the most common causes of exanthematous drug eruptions. Penicillins and trimethoprim-sulfamethoxazole are common offenders. Cephalosporins, anticonvulsants, and allopurinol may also induce a reaction. As this condition is diagnosed clinically, skin biopsy is often not necessary. Histology is nonspecific and shows a mild perivascular lymphocytic infiltrate and few epidermal necrotic keratinocytes.
In drug reaction with eosinophilia and systemic symptoms (DRESS), symptoms present 2-6 weeks after the offending medication has been started. The cutaneous rash appears similar to an exanthematous drug eruption; however, lesions will also present on the face, and facial edema may occur. Fever is often present. Laboratory findings include a marked peripheral blood hypereosinophilia. Elevated liver function tests may be seen. Viruses such as Epstein-Barr virus, enteroviruses, adenovirus, early HIV, human herpesvirus 6, and parvovirus B19 have a similar clinical appearance to an exanthematous drug eruption. A mild eosinophilia, as seen in a drug eruption, helps to distinguish between a drug eruption and viral exanthem. In Stevens-Johnson Syndrome, mucosal membranes are involved and skin is often painful or appears dusky.
Treatment of exanthematous drug eruptions is largely supportive. Discontinuing the drug will help speed resolution and topical steroids may alleviate pruritus.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Bolognia J et al. “Dermatology” (St. Louis: Mosby/Elsevier, 2008).
2. James W et al. “Andrews’ Diseases of the Skin,” 13th ed. (Philadelphia: Saunders Elsevier, 2006).
Reactions can occur anytime from within the first 2 weeks of treatment up to 10 days after the treatment has been discontinued. If a drug is rechallenged, eruptions may occur sooner. Pruritus is commonly seen. Clinically, erythematous papules and macules present symmetrically on the trunk and upper extremities and then become more generalized. A low-grade fever may be present.
Antibiotics are the most common causes of exanthematous drug eruptions. Penicillins and trimethoprim-sulfamethoxazole are common offenders. Cephalosporins, anticonvulsants, and allopurinol may also induce a reaction. As this condition is diagnosed clinically, skin biopsy is often not necessary. Histology is nonspecific and shows a mild perivascular lymphocytic infiltrate and few epidermal necrotic keratinocytes.
In drug reaction with eosinophilia and systemic symptoms (DRESS), symptoms present 2-6 weeks after the offending medication has been started. The cutaneous rash appears similar to an exanthematous drug eruption; however, lesions will also present on the face, and facial edema may occur. Fever is often present. Laboratory findings include a marked peripheral blood hypereosinophilia. Elevated liver function tests may be seen. Viruses such as Epstein-Barr virus, enteroviruses, adenovirus, early HIV, human herpesvirus 6, and parvovirus B19 have a similar clinical appearance to an exanthematous drug eruption. A mild eosinophilia, as seen in a drug eruption, helps to distinguish between a drug eruption and viral exanthem. In Stevens-Johnson Syndrome, mucosal membranes are involved and skin is often painful or appears dusky.
Treatment of exanthematous drug eruptions is largely supportive. Discontinuing the drug will help speed resolution and topical steroids may alleviate pruritus.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Bolognia J et al. “Dermatology” (St. Louis: Mosby/Elsevier, 2008).
2. James W et al. “Andrews’ Diseases of the Skin,” 13th ed. (Philadelphia: Saunders Elsevier, 2006).
Innovations in Dermatology Fall Abstract Compendium
Words from the wise
“When 900-years-old you reach, look as good you will not.” –Yoda
I’ve been on a roll lately: 100, 94, 90, 97, 94. These aren’t grades or even what I scratched on my scorecard for 18 holes (that’s more like 112), but rather patients I’ve seen.
Our oldest-old have been in COVID-19 protection for the last couple of years and only now feel safe to come out again. Many have skin cancers. Some of them have many. I’m grateful that for all their health problems, basal cell carcinomas at least I can cure. And
From a 94-year-old woman who was just discharged from the hospital for sepsis: First, sepsis can sneak up from behind and jump you when you’re 94. She was sitting in a waiting room for a routine exam when she passed out and woke up in the ICU. She made it home and is back on her feet, literally. When I asked her how she made it though, she was very matter of fact. Trust that the doctors know what’s right. Trust that someone will tell you what to do next. Trust that you know your own body and what you can and cannot do. Ask for help, then simply trust it will all work out. It usually does.
From a 97-year-old fighter pilot who fought in the Korean War: Let regrets drop away and live to fight another day. He’s had multiple marriages, built and lost companies, been fired and fired at, and made some doozy mistakes, some that caused considerable pain and collateral damage. But each day is new and requires your best. He has lived long enough to love dozens of grandkids and give away more than what most people ever make. His bottom line, if you worry and fret and regret, you’ll make even more mistakes ahead. Look ahead, the ground never comes up from behind you.
From a 94-year-old whose son was killed in a car accident nearly 60 years ago: You can be both happy and sad. When she retold the story of how the police knocked on her door with the news that her son was dead, she started to cry. Even 60 years isn’t long enough to blunt such pain. She still thinks of him often and to this day sometimes finds it difficult to believe he’s gone. Such pain never leaves you. But she is still a happy person with countless joys and is still having such fun. If you live long enough, both will likely be true.
From a 90-year old who still played tennis: “Just one and one.” That is, one beer and one shot, every day. No more. No less. I daren’t say I recommend this one; however, it might also be the social aspect of drinking that matters. He also advised to be free with friendships. You’ll have many people come in and out of your life; be open to new ones all the time. Also sometimes let your friends win.
From a 100-year-old, I asked how he managed to get through the Great Depression, WWII, civil unrest of the 1950s, and the Vietnam War. His reply? “To be honest, I’ve never seen anything quite like this before.”
When there’s time, consider asking for advice from those elders who happen to have an appointment with you. Bring you wisdom, they will.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“When 900-years-old you reach, look as good you will not.” –Yoda
I’ve been on a roll lately: 100, 94, 90, 97, 94. These aren’t grades or even what I scratched on my scorecard for 18 holes (that’s more like 112), but rather patients I’ve seen.
Our oldest-old have been in COVID-19 protection for the last couple of years and only now feel safe to come out again. Many have skin cancers. Some of them have many. I’m grateful that for all their health problems, basal cell carcinomas at least I can cure. And
From a 94-year-old woman who was just discharged from the hospital for sepsis: First, sepsis can sneak up from behind and jump you when you’re 94. She was sitting in a waiting room for a routine exam when she passed out and woke up in the ICU. She made it home and is back on her feet, literally. When I asked her how she made it though, she was very matter of fact. Trust that the doctors know what’s right. Trust that someone will tell you what to do next. Trust that you know your own body and what you can and cannot do. Ask for help, then simply trust it will all work out. It usually does.
From a 97-year-old fighter pilot who fought in the Korean War: Let regrets drop away and live to fight another day. He’s had multiple marriages, built and lost companies, been fired and fired at, and made some doozy mistakes, some that caused considerable pain and collateral damage. But each day is new and requires your best. He has lived long enough to love dozens of grandkids and give away more than what most people ever make. His bottom line, if you worry and fret and regret, you’ll make even more mistakes ahead. Look ahead, the ground never comes up from behind you.
From a 94-year-old whose son was killed in a car accident nearly 60 years ago: You can be both happy and sad. When she retold the story of how the police knocked on her door with the news that her son was dead, she started to cry. Even 60 years isn’t long enough to blunt such pain. She still thinks of him often and to this day sometimes finds it difficult to believe he’s gone. Such pain never leaves you. But she is still a happy person with countless joys and is still having such fun. If you live long enough, both will likely be true.
From a 90-year old who still played tennis: “Just one and one.” That is, one beer and one shot, every day. No more. No less. I daren’t say I recommend this one; however, it might also be the social aspect of drinking that matters. He also advised to be free with friendships. You’ll have many people come in and out of your life; be open to new ones all the time. Also sometimes let your friends win.
From a 100-year-old, I asked how he managed to get through the Great Depression, WWII, civil unrest of the 1950s, and the Vietnam War. His reply? “To be honest, I’ve never seen anything quite like this before.”
When there’s time, consider asking for advice from those elders who happen to have an appointment with you. Bring you wisdom, they will.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“When 900-years-old you reach, look as good you will not.” –Yoda
I’ve been on a roll lately: 100, 94, 90, 97, 94. These aren’t grades or even what I scratched on my scorecard for 18 holes (that’s more like 112), but rather patients I’ve seen.
Our oldest-old have been in COVID-19 protection for the last couple of years and only now feel safe to come out again. Many have skin cancers. Some of them have many. I’m grateful that for all their health problems, basal cell carcinomas at least I can cure. And
From a 94-year-old woman who was just discharged from the hospital for sepsis: First, sepsis can sneak up from behind and jump you when you’re 94. She was sitting in a waiting room for a routine exam when she passed out and woke up in the ICU. She made it home and is back on her feet, literally. When I asked her how she made it though, she was very matter of fact. Trust that the doctors know what’s right. Trust that someone will tell you what to do next. Trust that you know your own body and what you can and cannot do. Ask for help, then simply trust it will all work out. It usually does.
From a 97-year-old fighter pilot who fought in the Korean War: Let regrets drop away and live to fight another day. He’s had multiple marriages, built and lost companies, been fired and fired at, and made some doozy mistakes, some that caused considerable pain and collateral damage. But each day is new and requires your best. He has lived long enough to love dozens of grandkids and give away more than what most people ever make. His bottom line, if you worry and fret and regret, you’ll make even more mistakes ahead. Look ahead, the ground never comes up from behind you.
From a 94-year-old whose son was killed in a car accident nearly 60 years ago: You can be both happy and sad. When she retold the story of how the police knocked on her door with the news that her son was dead, she started to cry. Even 60 years isn’t long enough to blunt such pain. She still thinks of him often and to this day sometimes finds it difficult to believe he’s gone. Such pain never leaves you. But she is still a happy person with countless joys and is still having such fun. If you live long enough, both will likely be true.
From a 90-year old who still played tennis: “Just one and one.” That is, one beer and one shot, every day. No more. No less. I daren’t say I recommend this one; however, it might also be the social aspect of drinking that matters. He also advised to be free with friendships. You’ll have many people come in and out of your life; be open to new ones all the time. Also sometimes let your friends win.
From a 100-year-old, I asked how he managed to get through the Great Depression, WWII, civil unrest of the 1950s, and the Vietnam War. His reply? “To be honest, I’ve never seen anything quite like this before.”
When there’s time, consider asking for advice from those elders who happen to have an appointment with you. Bring you wisdom, they will.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Adding rituximab to belimumab offers no help for lupus
Adding a single cycle of rituximab to belimumab (Benlysta) did not improve disease control for patients with systemic lupus erythematosus (SLE) in comparison with belimumab alone in a phase 3, randomized, controlled trial.
Among patients with SLE who were randomly assigned to receive belimumab with either rituximab, placebo, or standard care, there were no statistically significant differences between the rituximab and placebo arms for the primary endpoint of the proportion of patients with disease control at week 52 or in the secondary endpoints of clinical remission at week 64 or disease control at week 104, Cynthia Aranow, MD, reported in a late-breaking poster session presented during the virtual annual meeting of the American College of Rheumatology.
“Using a new, clinically meaningful endpoint underscores the efficacy of belimumab for disease control, with some patients maintaining disease control with considerable reductions in steroids, and no immunosuppressants,” said Dr. Aranow, a rheumatologist specializing in SLE and RA in New York and director of the Clinical Autoimmunity Center of Excellence at Feinstein Institutes for Medical Research, Manhasset, N.Y.
Use of the combination of belimumab and rituximab was, however, associated with significant improvement over belimumab and placebo in several secondary efficacy endpoints.
Investigators in the randomized, controlled trial, dubbed BLISS-BELIEVE, had previously published a rationale for sequential therapy with belimumab, a human monoclonal antibody that binds to soluble B-lymphocyte stimulator, and rituximab, a B-cell–depleting anti-CD20 monoclonal antibody.
“These biologics, which operate through complementary mechanisms, might result in an enhanced depletion of circulating and tissue-resident autoreactive B lymphocytes when administered together. Thus, belimumab and rituximab combination may be a highly effective treatment of SLE,” they wrote in an article published in 2019 in BMJ Open.
Three-arm trial
The investigators screened 396 patients, of whom 292 were randomly assigned in a 1:2:1 ratio to receive either subcutaneous belimumab 200 mg/wk plus intravenous placebo at weeks 4 and 6 (BEL/PBO, 72 patients), belimumab plus IV rituximab 1,000 mg at weeks 4 and 6 (BEL/RTX, 144 patients), or open-label belimumab plus standard therapy. Patients were allowed to continue taking antimalarial and nonsteroidal anti-inflammatory drugs throughout the study.
The primary disease-control endpoint was defined as a Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) score of 2 or less, achieved without other immunosuppression, equivalent to that achieved with prednisone 5 mg/day or less.
As noted before, there were no significant differences between the BEL/RTX and BEL/PBO arms in either disease control at week 52 or in the secondary endpoints of clinical remission at week 64 (SLEDAI-2K score, 0) or in the proportion of patients with disease control at week 104.
However, use of BEL/RTX was associated with a significantly longer duration of disease control through 52 weeks than was BEL/PBO (mean, 105.4 days vs. 60.1 days; P = .0188) and with a large SLEDAI-2K mean change from baseline at week 104 (–7.2 vs 5.1; P = .0033).
In addition, there was a trend toward a shift in proteinuria from baseline high (>0.5 g/24 h) to normal in the BEL/RTX group at week 52 and a significantly greater shift at week 104 (P = .0085).
The overall adverse event profiles were generally consistent with those of the individual agents, although serious infections and infestations occurred more frequently with BEL/RTX than BEL/PBO.
Further analyses planned to look for subgroups that benefit
In a poster discussion session, Akshat Khanna, PhD, of Newtown, Pa., a consultant with Effimed Life Sciences Research, asked Dr. Aranow about the rationale for giving rituximab and belimumab concurrently and noted that, in the BEAT-LUPUS and CALIBRATE trials, anti-CD20 agents were given first, followed by belimumab, to prevent activation of humoral immunity.
“The two B-cell agents were given sequentially. Belimumab was given first to maximize the effect of peripheral B-cell depletion and [was] then continued after rituximab to suppress the elevation [of B-lymphocyte stimulator] that occurs after rituximab monotherapy. We used this approach (instead of that used in CALIBRATE and BEAT LUPUS), as we thought this might be more efficacious,” she explained.
When asked whether there were subgroups of patients who might still benefit from the combination, compared with belimumab alone, Dr. Aranow replied: “There may be individual patients in which it might be considered. Further analyses of the data are ongoing/planned.”
The study was supported by GlaxoSmithKline. Dr. Aranow has received grant/research support from GlaxoSmithKline and has consulted for Bristol-Myers Squibb. Dr. Khanna has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adding a single cycle of rituximab to belimumab (Benlysta) did not improve disease control for patients with systemic lupus erythematosus (SLE) in comparison with belimumab alone in a phase 3, randomized, controlled trial.
Among patients with SLE who were randomly assigned to receive belimumab with either rituximab, placebo, or standard care, there were no statistically significant differences between the rituximab and placebo arms for the primary endpoint of the proportion of patients with disease control at week 52 or in the secondary endpoints of clinical remission at week 64 or disease control at week 104, Cynthia Aranow, MD, reported in a late-breaking poster session presented during the virtual annual meeting of the American College of Rheumatology.
“Using a new, clinically meaningful endpoint underscores the efficacy of belimumab for disease control, with some patients maintaining disease control with considerable reductions in steroids, and no immunosuppressants,” said Dr. Aranow, a rheumatologist specializing in SLE and RA in New York and director of the Clinical Autoimmunity Center of Excellence at Feinstein Institutes for Medical Research, Manhasset, N.Y.
Use of the combination of belimumab and rituximab was, however, associated with significant improvement over belimumab and placebo in several secondary efficacy endpoints.
Investigators in the randomized, controlled trial, dubbed BLISS-BELIEVE, had previously published a rationale for sequential therapy with belimumab, a human monoclonal antibody that binds to soluble B-lymphocyte stimulator, and rituximab, a B-cell–depleting anti-CD20 monoclonal antibody.
“These biologics, which operate through complementary mechanisms, might result in an enhanced depletion of circulating and tissue-resident autoreactive B lymphocytes when administered together. Thus, belimumab and rituximab combination may be a highly effective treatment of SLE,” they wrote in an article published in 2019 in BMJ Open.
Three-arm trial
The investigators screened 396 patients, of whom 292 were randomly assigned in a 1:2:1 ratio to receive either subcutaneous belimumab 200 mg/wk plus intravenous placebo at weeks 4 and 6 (BEL/PBO, 72 patients), belimumab plus IV rituximab 1,000 mg at weeks 4 and 6 (BEL/RTX, 144 patients), or open-label belimumab plus standard therapy. Patients were allowed to continue taking antimalarial and nonsteroidal anti-inflammatory drugs throughout the study.
The primary disease-control endpoint was defined as a Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) score of 2 or less, achieved without other immunosuppression, equivalent to that achieved with prednisone 5 mg/day or less.
As noted before, there were no significant differences between the BEL/RTX and BEL/PBO arms in either disease control at week 52 or in the secondary endpoints of clinical remission at week 64 (SLEDAI-2K score, 0) or in the proportion of patients with disease control at week 104.
However, use of BEL/RTX was associated with a significantly longer duration of disease control through 52 weeks than was BEL/PBO (mean, 105.4 days vs. 60.1 days; P = .0188) and with a large SLEDAI-2K mean change from baseline at week 104 (–7.2 vs 5.1; P = .0033).
In addition, there was a trend toward a shift in proteinuria from baseline high (>0.5 g/24 h) to normal in the BEL/RTX group at week 52 and a significantly greater shift at week 104 (P = .0085).
The overall adverse event profiles were generally consistent with those of the individual agents, although serious infections and infestations occurred more frequently with BEL/RTX than BEL/PBO.
Further analyses planned to look for subgroups that benefit
In a poster discussion session, Akshat Khanna, PhD, of Newtown, Pa., a consultant with Effimed Life Sciences Research, asked Dr. Aranow about the rationale for giving rituximab and belimumab concurrently and noted that, in the BEAT-LUPUS and CALIBRATE trials, anti-CD20 agents were given first, followed by belimumab, to prevent activation of humoral immunity.
“The two B-cell agents were given sequentially. Belimumab was given first to maximize the effect of peripheral B-cell depletion and [was] then continued after rituximab to suppress the elevation [of B-lymphocyte stimulator] that occurs after rituximab monotherapy. We used this approach (instead of that used in CALIBRATE and BEAT LUPUS), as we thought this might be more efficacious,” she explained.
When asked whether there were subgroups of patients who might still benefit from the combination, compared with belimumab alone, Dr. Aranow replied: “There may be individual patients in which it might be considered. Further analyses of the data are ongoing/planned.”
The study was supported by GlaxoSmithKline. Dr. Aranow has received grant/research support from GlaxoSmithKline and has consulted for Bristol-Myers Squibb. Dr. Khanna has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adding a single cycle of rituximab to belimumab (Benlysta) did not improve disease control for patients with systemic lupus erythematosus (SLE) in comparison with belimumab alone in a phase 3, randomized, controlled trial.
Among patients with SLE who were randomly assigned to receive belimumab with either rituximab, placebo, or standard care, there were no statistically significant differences between the rituximab and placebo arms for the primary endpoint of the proportion of patients with disease control at week 52 or in the secondary endpoints of clinical remission at week 64 or disease control at week 104, Cynthia Aranow, MD, reported in a late-breaking poster session presented during the virtual annual meeting of the American College of Rheumatology.
“Using a new, clinically meaningful endpoint underscores the efficacy of belimumab for disease control, with some patients maintaining disease control with considerable reductions in steroids, and no immunosuppressants,” said Dr. Aranow, a rheumatologist specializing in SLE and RA in New York and director of the Clinical Autoimmunity Center of Excellence at Feinstein Institutes for Medical Research, Manhasset, N.Y.
Use of the combination of belimumab and rituximab was, however, associated with significant improvement over belimumab and placebo in several secondary efficacy endpoints.
Investigators in the randomized, controlled trial, dubbed BLISS-BELIEVE, had previously published a rationale for sequential therapy with belimumab, a human monoclonal antibody that binds to soluble B-lymphocyte stimulator, and rituximab, a B-cell–depleting anti-CD20 monoclonal antibody.
“These biologics, which operate through complementary mechanisms, might result in an enhanced depletion of circulating and tissue-resident autoreactive B lymphocytes when administered together. Thus, belimumab and rituximab combination may be a highly effective treatment of SLE,” they wrote in an article published in 2019 in BMJ Open.
Three-arm trial
The investigators screened 396 patients, of whom 292 were randomly assigned in a 1:2:1 ratio to receive either subcutaneous belimumab 200 mg/wk plus intravenous placebo at weeks 4 and 6 (BEL/PBO, 72 patients), belimumab plus IV rituximab 1,000 mg at weeks 4 and 6 (BEL/RTX, 144 patients), or open-label belimumab plus standard therapy. Patients were allowed to continue taking antimalarial and nonsteroidal anti-inflammatory drugs throughout the study.
The primary disease-control endpoint was defined as a Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) score of 2 or less, achieved without other immunosuppression, equivalent to that achieved with prednisone 5 mg/day or less.
As noted before, there were no significant differences between the BEL/RTX and BEL/PBO arms in either disease control at week 52 or in the secondary endpoints of clinical remission at week 64 (SLEDAI-2K score, 0) or in the proportion of patients with disease control at week 104.
However, use of BEL/RTX was associated with a significantly longer duration of disease control through 52 weeks than was BEL/PBO (mean, 105.4 days vs. 60.1 days; P = .0188) and with a large SLEDAI-2K mean change from baseline at week 104 (–7.2 vs 5.1; P = .0033).
In addition, there was a trend toward a shift in proteinuria from baseline high (>0.5 g/24 h) to normal in the BEL/RTX group at week 52 and a significantly greater shift at week 104 (P = .0085).
The overall adverse event profiles were generally consistent with those of the individual agents, although serious infections and infestations occurred more frequently with BEL/RTX than BEL/PBO.
Further analyses planned to look for subgroups that benefit
In a poster discussion session, Akshat Khanna, PhD, of Newtown, Pa., a consultant with Effimed Life Sciences Research, asked Dr. Aranow about the rationale for giving rituximab and belimumab concurrently and noted that, in the BEAT-LUPUS and CALIBRATE trials, anti-CD20 agents were given first, followed by belimumab, to prevent activation of humoral immunity.
“The two B-cell agents were given sequentially. Belimumab was given first to maximize the effect of peripheral B-cell depletion and [was] then continued after rituximab to suppress the elevation [of B-lymphocyte stimulator] that occurs after rituximab monotherapy. We used this approach (instead of that used in CALIBRATE and BEAT LUPUS), as we thought this might be more efficacious,” she explained.
When asked whether there were subgroups of patients who might still benefit from the combination, compared with belimumab alone, Dr. Aranow replied: “There may be individual patients in which it might be considered. Further analyses of the data are ongoing/planned.”
The study was supported by GlaxoSmithKline. Dr. Aranow has received grant/research support from GlaxoSmithKline and has consulted for Bristol-Myers Squibb. Dr. Khanna has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACR 2021
Tofacitinib postmarketing trial data shed light on JAK inhibitor risks
Additional analyses of a postmarketing trial that was required after the Food and Drug Administration’s approval of the Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR) has identified characteristics of older patients with rheumatoid arthritis with at least one cardiovascular risk factor who may be at higher risk for major adverse cardiovascular events (MACE) when taking the drug.
Results from the phase 3b/4 ORAL Surveillance trial presented at the virtual annual meeting of the American College of Rheumatology show that people taking tofacitinib for RA with at least one cardiovascular (CV) risk factor had a nonsignificant higher risk for MACE than did people taking tumor necrosis factor inhibitors (TNFi), with the risk from tofacitinib more pronounced in current smokers, aspirin users, people older than 65 years, and men, compared with women.
“It is the first large, randomized safety study of active RA patients with increased CV risk comparing tofacitinib to TNF inhibition,” study author Christina Charles-Schoeman, MD, said in an interview. “These data emphasize the importance of assessing baseline CV risk when treating patients with RA.” Dr. Charles-Schoeman is chief of rheumatology at the University of California, Los Angeles.
The results shed further light on the trial’s findings, which the FDA used in September 2021 to mandate boxed warnings about the risk of MI or stroke, cancer, venous thromboembolism, and death, as well as updated indications, for tofacitinib and other JAK inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq). The FDA limited all approved uses of these three medications to patients who have not responded well to TNFi to ensure their benefits outweigh their risks.
Tofacitinib is indicated for RA, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis. Baricitinib and upadacitinib are approved only for RA.
While the overall results of the trial results show nonsignificant increased incidence rates for MACE in tofacitinib users versus TNFI users, Katherine Liao, MD, a rheumatologist at Brigham and Women’s Hospital in Boston, noted that more information is needed to determine who is at greatest risk. “Another thing to keep in mind is, while there was evidence of an elevated relative risk for MACE, compared to TNFi, the absolute risk, based on the numbers what we know so far, is small,” she said.
The trial compared two different doses of tofacitinib – 5 mg (1,455 patients) and 10 mg (n = 1,456) twice daily – and TNFi (n = 1,451) in people with moderate to severe RA over age 50. Patient characteristics were similar across all three treatment arms, Dr. Charles-Schoeman said. All patients had inadequate response to methotrexate, and about 57% in all three treatment groups were taking corticosteroids. The 10-mg tofacitinib patients switched to the 5-mg dose in February 2019 but represent the 10-mg group in the study analysis.
ORAL Surveillance demonstrated a 24% greater risk of MACE in the 5-mg tofacitinib patients and a 43% heightened risk the 10-mg group, compared with patients who received a TNFi.
The differentiating factor for MACE incidence was MI. The higher- and lower-dose tofacitinib groups had 69% and 80% greater risk for MI. While the risk for fatal MI were similar across all three treatment groups, the risk for nonfatal MI were more than doubled in the respective tofacitinib groups: hazard ratios of 2.32 and 2.08. The incidence of stroke was similar across all three arms, Dr. Charles-Schoeman said.
The study identified a number of baseline characteristics as independent overall risk factors for MACE across all treatment groups. Current smoking and aspirin use more than doubled the risk (HR, 2.18; P < .0001 and HR, 2.11; P = .004, respectively), while age greater than 65 years and male sex approached that level (HR, 1.81; P = .0011 and HR, 1.81; P = .0015) approached that level. Other factors that elevated the risk of MACE to a lesser extent were a history of diabetes, hypertension or coronary artery procedures, and a total cholesterol to HDL ratio greater than4.
Other ORAL Surveillance subanalyses and tofacitinib real-world data reported
This was one of several analyses presented at ACR 2021 that compared adverse event risks for tofacitinib versus TNFi drugs. A separate analysis of claims data from patients with RA in two U.S. insurance databases plus Medicare found a statistically nonsignificant increased risk of adverse CV outcomes (MI or stroke) with tofacitinib, compared with TNFi users, among patients who met the same inclusion and exclusion criteria of the ORAL Surveillance trial but not in a “real-world evidence” cohort of more than 102,000 patients with RA in routine care from the databases.
Two additional ORAL Surveillance analyses presented at ACR 2021 gave details about risk factors for higher rates of malignancies and venous thromboembolic events found in patients taking tofacitinib with at least one CV risk factor. As would be expected, older age (≥65 vs. 50-64 years) and current or past smoking (vs. never smoking) were independent risk factors for higher malignancy rates across all treatment arms. Pulmonary embolism events across treatment groups were independently associated with a history of venous thromboembolism, baseline use of oral contraceptives or hormone replacement therapy, baseline body mass index of at least 30 kg/m2, age 65 or older, and history of hypertension.
The ORAL Surveillance findings are worth considering when determining treatments for RA patients with CV risk factors, Dr. Charles-Schoeman said. “Tofacitinib remains an effective RA treatment,” she said. “The choice of specific RA treatment for any patient remains an individual decision between the patient and physician, which is decided based on a number of different factors. This new study provides additional information regarding both tofacitinib as well as traditional CV risk factors for discussion with the patient.”
The ORAL Surveillance results may give rheumatologists reason to rethink use of tofacitinib in some patients with CV risk, said Dr. Liao of Brigham and Women’s Hospital in Boston. “Currently, we have limited data and are still awaiting a report of the full trial results,” she said in an interview. “Based on the data available, I can think of a few patients in my clinic where I would reconsider use of these drugs, i.e., history of heart attack with stable angina, especially if there are other options.” However, she noted that many patients on tofacitinib have already failed on older treatments.
These data emphasize the importance of addressing CV risk with patients, said Brittany N. Weber, MD, PhD, a cardio-rheumatologist at Brigham and Women’s Hospital who works with Dr. Liao. “It is also an opportunity to discuss modification of risk factors and to discuss primary prevention therapies, such as statin therapy, where appropriate,” she added. “Based on the individual’s cardiovascular risk, there may be a role for further risk stratification to further understand an individual’s risk, which can also inform primary prevention cardiovascular therapies and help guide these discussions.” Risk stratification could include cardiac CT for calcium scoring or cardiac coronary CT angiography for determining atherosclerotic burden.
The study was sponsored by Pfizer. Dr. Charles-Schoeman disclosed relationships with AbbVie, Bristol-Myers Squibb, Gilead Sciences, Pfizer, and Regeneron-Sanofi. Dr. Liao and Dr. Weber have no relevant disclosures.
Additional analyses of a postmarketing trial that was required after the Food and Drug Administration’s approval of the Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR) has identified characteristics of older patients with rheumatoid arthritis with at least one cardiovascular risk factor who may be at higher risk for major adverse cardiovascular events (MACE) when taking the drug.
Results from the phase 3b/4 ORAL Surveillance trial presented at the virtual annual meeting of the American College of Rheumatology show that people taking tofacitinib for RA with at least one cardiovascular (CV) risk factor had a nonsignificant higher risk for MACE than did people taking tumor necrosis factor inhibitors (TNFi), with the risk from tofacitinib more pronounced in current smokers, aspirin users, people older than 65 years, and men, compared with women.
“It is the first large, randomized safety study of active RA patients with increased CV risk comparing tofacitinib to TNF inhibition,” study author Christina Charles-Schoeman, MD, said in an interview. “These data emphasize the importance of assessing baseline CV risk when treating patients with RA.” Dr. Charles-Schoeman is chief of rheumatology at the University of California, Los Angeles.
The results shed further light on the trial’s findings, which the FDA used in September 2021 to mandate boxed warnings about the risk of MI or stroke, cancer, venous thromboembolism, and death, as well as updated indications, for tofacitinib and other JAK inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq). The FDA limited all approved uses of these three medications to patients who have not responded well to TNFi to ensure their benefits outweigh their risks.
Tofacitinib is indicated for RA, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis. Baricitinib and upadacitinib are approved only for RA.
While the overall results of the trial results show nonsignificant increased incidence rates for MACE in tofacitinib users versus TNFI users, Katherine Liao, MD, a rheumatologist at Brigham and Women’s Hospital in Boston, noted that more information is needed to determine who is at greatest risk. “Another thing to keep in mind is, while there was evidence of an elevated relative risk for MACE, compared to TNFi, the absolute risk, based on the numbers what we know so far, is small,” she said.
The trial compared two different doses of tofacitinib – 5 mg (1,455 patients) and 10 mg (n = 1,456) twice daily – and TNFi (n = 1,451) in people with moderate to severe RA over age 50. Patient characteristics were similar across all three treatment arms, Dr. Charles-Schoeman said. All patients had inadequate response to methotrexate, and about 57% in all three treatment groups were taking corticosteroids. The 10-mg tofacitinib patients switched to the 5-mg dose in February 2019 but represent the 10-mg group in the study analysis.
ORAL Surveillance demonstrated a 24% greater risk of MACE in the 5-mg tofacitinib patients and a 43% heightened risk the 10-mg group, compared with patients who received a TNFi.
The differentiating factor for MACE incidence was MI. The higher- and lower-dose tofacitinib groups had 69% and 80% greater risk for MI. While the risk for fatal MI were similar across all three treatment groups, the risk for nonfatal MI were more than doubled in the respective tofacitinib groups: hazard ratios of 2.32 and 2.08. The incidence of stroke was similar across all three arms, Dr. Charles-Schoeman said.
The study identified a number of baseline characteristics as independent overall risk factors for MACE across all treatment groups. Current smoking and aspirin use more than doubled the risk (HR, 2.18; P < .0001 and HR, 2.11; P = .004, respectively), while age greater than 65 years and male sex approached that level (HR, 1.81; P = .0011 and HR, 1.81; P = .0015) approached that level. Other factors that elevated the risk of MACE to a lesser extent were a history of diabetes, hypertension or coronary artery procedures, and a total cholesterol to HDL ratio greater than4.
Other ORAL Surveillance subanalyses and tofacitinib real-world data reported
This was one of several analyses presented at ACR 2021 that compared adverse event risks for tofacitinib versus TNFi drugs. A separate analysis of claims data from patients with RA in two U.S. insurance databases plus Medicare found a statistically nonsignificant increased risk of adverse CV outcomes (MI or stroke) with tofacitinib, compared with TNFi users, among patients who met the same inclusion and exclusion criteria of the ORAL Surveillance trial but not in a “real-world evidence” cohort of more than 102,000 patients with RA in routine care from the databases.
Two additional ORAL Surveillance analyses presented at ACR 2021 gave details about risk factors for higher rates of malignancies and venous thromboembolic events found in patients taking tofacitinib with at least one CV risk factor. As would be expected, older age (≥65 vs. 50-64 years) and current or past smoking (vs. never smoking) were independent risk factors for higher malignancy rates across all treatment arms. Pulmonary embolism events across treatment groups were independently associated with a history of venous thromboembolism, baseline use of oral contraceptives or hormone replacement therapy, baseline body mass index of at least 30 kg/m2, age 65 or older, and history of hypertension.
The ORAL Surveillance findings are worth considering when determining treatments for RA patients with CV risk factors, Dr. Charles-Schoeman said. “Tofacitinib remains an effective RA treatment,” she said. “The choice of specific RA treatment for any patient remains an individual decision between the patient and physician, which is decided based on a number of different factors. This new study provides additional information regarding both tofacitinib as well as traditional CV risk factors for discussion with the patient.”
The ORAL Surveillance results may give rheumatologists reason to rethink use of tofacitinib in some patients with CV risk, said Dr. Liao of Brigham and Women’s Hospital in Boston. “Currently, we have limited data and are still awaiting a report of the full trial results,” she said in an interview. “Based on the data available, I can think of a few patients in my clinic where I would reconsider use of these drugs, i.e., history of heart attack with stable angina, especially if there are other options.” However, she noted that many patients on tofacitinib have already failed on older treatments.
These data emphasize the importance of addressing CV risk with patients, said Brittany N. Weber, MD, PhD, a cardio-rheumatologist at Brigham and Women’s Hospital who works with Dr. Liao. “It is also an opportunity to discuss modification of risk factors and to discuss primary prevention therapies, such as statin therapy, where appropriate,” she added. “Based on the individual’s cardiovascular risk, there may be a role for further risk stratification to further understand an individual’s risk, which can also inform primary prevention cardiovascular therapies and help guide these discussions.” Risk stratification could include cardiac CT for calcium scoring or cardiac coronary CT angiography for determining atherosclerotic burden.
The study was sponsored by Pfizer. Dr. Charles-Schoeman disclosed relationships with AbbVie, Bristol-Myers Squibb, Gilead Sciences, Pfizer, and Regeneron-Sanofi. Dr. Liao and Dr. Weber have no relevant disclosures.
Additional analyses of a postmarketing trial that was required after the Food and Drug Administration’s approval of the Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR) has identified characteristics of older patients with rheumatoid arthritis with at least one cardiovascular risk factor who may be at higher risk for major adverse cardiovascular events (MACE) when taking the drug.
Results from the phase 3b/4 ORAL Surveillance trial presented at the virtual annual meeting of the American College of Rheumatology show that people taking tofacitinib for RA with at least one cardiovascular (CV) risk factor had a nonsignificant higher risk for MACE than did people taking tumor necrosis factor inhibitors (TNFi), with the risk from tofacitinib more pronounced in current smokers, aspirin users, people older than 65 years, and men, compared with women.
“It is the first large, randomized safety study of active RA patients with increased CV risk comparing tofacitinib to TNF inhibition,” study author Christina Charles-Schoeman, MD, said in an interview. “These data emphasize the importance of assessing baseline CV risk when treating patients with RA.” Dr. Charles-Schoeman is chief of rheumatology at the University of California, Los Angeles.
The results shed further light on the trial’s findings, which the FDA used in September 2021 to mandate boxed warnings about the risk of MI or stroke, cancer, venous thromboembolism, and death, as well as updated indications, for tofacitinib and other JAK inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq). The FDA limited all approved uses of these three medications to patients who have not responded well to TNFi to ensure their benefits outweigh their risks.
Tofacitinib is indicated for RA, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis. Baricitinib and upadacitinib are approved only for RA.
While the overall results of the trial results show nonsignificant increased incidence rates for MACE in tofacitinib users versus TNFI users, Katherine Liao, MD, a rheumatologist at Brigham and Women’s Hospital in Boston, noted that more information is needed to determine who is at greatest risk. “Another thing to keep in mind is, while there was evidence of an elevated relative risk for MACE, compared to TNFi, the absolute risk, based on the numbers what we know so far, is small,” she said.
The trial compared two different doses of tofacitinib – 5 mg (1,455 patients) and 10 mg (n = 1,456) twice daily – and TNFi (n = 1,451) in people with moderate to severe RA over age 50. Patient characteristics were similar across all three treatment arms, Dr. Charles-Schoeman said. All patients had inadequate response to methotrexate, and about 57% in all three treatment groups were taking corticosteroids. The 10-mg tofacitinib patients switched to the 5-mg dose in February 2019 but represent the 10-mg group in the study analysis.
ORAL Surveillance demonstrated a 24% greater risk of MACE in the 5-mg tofacitinib patients and a 43% heightened risk the 10-mg group, compared with patients who received a TNFi.
The differentiating factor for MACE incidence was MI. The higher- and lower-dose tofacitinib groups had 69% and 80% greater risk for MI. While the risk for fatal MI were similar across all three treatment groups, the risk for nonfatal MI were more than doubled in the respective tofacitinib groups: hazard ratios of 2.32 and 2.08. The incidence of stroke was similar across all three arms, Dr. Charles-Schoeman said.
The study identified a number of baseline characteristics as independent overall risk factors for MACE across all treatment groups. Current smoking and aspirin use more than doubled the risk (HR, 2.18; P < .0001 and HR, 2.11; P = .004, respectively), while age greater than 65 years and male sex approached that level (HR, 1.81; P = .0011 and HR, 1.81; P = .0015) approached that level. Other factors that elevated the risk of MACE to a lesser extent were a history of diabetes, hypertension or coronary artery procedures, and a total cholesterol to HDL ratio greater than4.
Other ORAL Surveillance subanalyses and tofacitinib real-world data reported
This was one of several analyses presented at ACR 2021 that compared adverse event risks for tofacitinib versus TNFi drugs. A separate analysis of claims data from patients with RA in two U.S. insurance databases plus Medicare found a statistically nonsignificant increased risk of adverse CV outcomes (MI or stroke) with tofacitinib, compared with TNFi users, among patients who met the same inclusion and exclusion criteria of the ORAL Surveillance trial but not in a “real-world evidence” cohort of more than 102,000 patients with RA in routine care from the databases.
Two additional ORAL Surveillance analyses presented at ACR 2021 gave details about risk factors for higher rates of malignancies and venous thromboembolic events found in patients taking tofacitinib with at least one CV risk factor. As would be expected, older age (≥65 vs. 50-64 years) and current or past smoking (vs. never smoking) were independent risk factors for higher malignancy rates across all treatment arms. Pulmonary embolism events across treatment groups were independently associated with a history of venous thromboembolism, baseline use of oral contraceptives or hormone replacement therapy, baseline body mass index of at least 30 kg/m2, age 65 or older, and history of hypertension.
The ORAL Surveillance findings are worth considering when determining treatments for RA patients with CV risk factors, Dr. Charles-Schoeman said. “Tofacitinib remains an effective RA treatment,” she said. “The choice of specific RA treatment for any patient remains an individual decision between the patient and physician, which is decided based on a number of different factors. This new study provides additional information regarding both tofacitinib as well as traditional CV risk factors for discussion with the patient.”
The ORAL Surveillance results may give rheumatologists reason to rethink use of tofacitinib in some patients with CV risk, said Dr. Liao of Brigham and Women’s Hospital in Boston. “Currently, we have limited data and are still awaiting a report of the full trial results,” she said in an interview. “Based on the data available, I can think of a few patients in my clinic where I would reconsider use of these drugs, i.e., history of heart attack with stable angina, especially if there are other options.” However, she noted that many patients on tofacitinib have already failed on older treatments.
These data emphasize the importance of addressing CV risk with patients, said Brittany N. Weber, MD, PhD, a cardio-rheumatologist at Brigham and Women’s Hospital who works with Dr. Liao. “It is also an opportunity to discuss modification of risk factors and to discuss primary prevention therapies, such as statin therapy, where appropriate,” she added. “Based on the individual’s cardiovascular risk, there may be a role for further risk stratification to further understand an individual’s risk, which can also inform primary prevention cardiovascular therapies and help guide these discussions.” Risk stratification could include cardiac CT for calcium scoring or cardiac coronary CT angiography for determining atherosclerotic burden.
The study was sponsored by Pfizer. Dr. Charles-Schoeman disclosed relationships with AbbVie, Bristol-Myers Squibb, Gilead Sciences, Pfizer, and Regeneron-Sanofi. Dr. Liao and Dr. Weber have no relevant disclosures.
FROM ACR 2021
Embezzlement: It can happen to you
In November, the office manager of a San Antonio dermatology practice was sentenced to 46 months in prison for defrauding the practice of nearly $350,000 from patient billings and employee profit sharing accounts.
Per the indictment, the practice conducted a nonprofit educational symposium in 2012. A bank account was established to collect contributions for that event, which was supposed to be closed at its conclusion; but the office manager kept it open, and deposited practice receipts into it. She then used the account as her slush fund for travel, property payments, meal purchases, and other personal expenses on credit cards she fraudulently opened in the practice’s name. This continued for several years.
Because this case has received national attention, I am republishing my column on embezzlement, which includes recommendations that could prevent such unfortunate situations from occurring.
Few crimes are more easily overlooked than theft from within. who think everything is fine. Most embezzlers are not skillful or discreet; but their transgressions may go undetected for years, simply because no one suspects it is happening.
Detecting fraud is an inexact science. There is no textbook approach that one can follow, but a few simple measures will prevent or expose the most common forms:
Make it more difficult. Theft and embezzlement are usually products of opportunity, so minimize those opportunities. No one person should be in charge of the entire bookkeeping process: the person who enters charges should be different from the one who enters payments. The one who writes checks or makes electronic fund transfers should not balance the books, and so on. Internal audits should be done on a regular basis, and all employees should know that. Your accountant can help.
Reconcile cash receipts daily. Embezzlement does not require sophisticated technology; the most common form is simply taking cash out of the till. In a typical scenario, a patient pays a copay of $15 in cash; the receptionist records the payment as $5, and pockets the rest. Make sure a receipt is generated for every cash transaction, and that someone other than the person accepting cash reconciles the charges, receipts, and cash totals daily.
Inventory your stock. Cash isn’t the only susceptible commodity. If you sell cosmetics or other products, inventory your stock frequently. And office personnel are not the only potential thieves: Over a year ago, a locum tenens physician down the street conspired with a receptionist to take cash transactions for cosmetic neurotoxins and fillers “off the books” and split the spoils. That office was being ripped off twice; first for the neurotoxin and filler materials themselves, and then for the cash proceeds.
Separate all accounting duties. Another popular ploy is false invoicing for imaginary supplies. A friend’s experience provides a good example (retold with his permission): His bookkeeper wrote sizable checks to herself, disguising them in the ledger as payments to vendors commonly used by his practice. Since the same employee also balanced the checkbook, she got away with it for years. “It wasn’t at all clever,” he told me, “and I’m embarrassed to admit that it happened to me.”
Once again, separation of duties is the key to prevention. One employee should enter invoices into the data system, another should issue the check or make the electronic transfer, and a third should match invoices to goods and services received.
Verify expense reports. False expense reporting is a subset of the fake invoice scam. When an employee asks for reimbursement of expenses, make sure those expenses are real.
Ask about computer safeguards. Computers facilitate a lot of financial chores, but they also consolidate financial data in one place, where it is potentially accessible to anybody, anywhere. Your computer vendor should be aware of this, and should have safeguards built into your system. Ask about them. If they aren’t there, ask why.
Hire honest employees. All applicants look great on paper, so check their references; and with their permission, you can run background checks for a few dollars on any of several public information websites. (See my previous columns on hiring at http://www.mdedge.com/dermatology/managing-your-practice.)
Look for “red flags.” Examples are employees who refuse to take vacations, because someone else will have do their work; or who insist on posting expenses that are a coworker’s responsibility, “just to be nice.” Anyone obviously living beyond his or her means merits suspicion as well.
Consider bonding your employees. Dishonesty bonds are relatively inexpensive, and you will be assured of some measure of recovery should your safeguards fail. In addition, the mere knowledge that your staff is bonded will frighten off most dishonest applicants. One effective screen is a question on your employment application: “Would you object to being bonded?”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
In November, the office manager of a San Antonio dermatology practice was sentenced to 46 months in prison for defrauding the practice of nearly $350,000 from patient billings and employee profit sharing accounts.
Per the indictment, the practice conducted a nonprofit educational symposium in 2012. A bank account was established to collect contributions for that event, which was supposed to be closed at its conclusion; but the office manager kept it open, and deposited practice receipts into it. She then used the account as her slush fund for travel, property payments, meal purchases, and other personal expenses on credit cards she fraudulently opened in the practice’s name. This continued for several years.
Because this case has received national attention, I am republishing my column on embezzlement, which includes recommendations that could prevent such unfortunate situations from occurring.
Few crimes are more easily overlooked than theft from within. who think everything is fine. Most embezzlers are not skillful or discreet; but their transgressions may go undetected for years, simply because no one suspects it is happening.
Detecting fraud is an inexact science. There is no textbook approach that one can follow, but a few simple measures will prevent or expose the most common forms:
Make it more difficult. Theft and embezzlement are usually products of opportunity, so minimize those opportunities. No one person should be in charge of the entire bookkeeping process: the person who enters charges should be different from the one who enters payments. The one who writes checks or makes electronic fund transfers should not balance the books, and so on. Internal audits should be done on a regular basis, and all employees should know that. Your accountant can help.
Reconcile cash receipts daily. Embezzlement does not require sophisticated technology; the most common form is simply taking cash out of the till. In a typical scenario, a patient pays a copay of $15 in cash; the receptionist records the payment as $5, and pockets the rest. Make sure a receipt is generated for every cash transaction, and that someone other than the person accepting cash reconciles the charges, receipts, and cash totals daily.
Inventory your stock. Cash isn’t the only susceptible commodity. If you sell cosmetics or other products, inventory your stock frequently. And office personnel are not the only potential thieves: Over a year ago, a locum tenens physician down the street conspired with a receptionist to take cash transactions for cosmetic neurotoxins and fillers “off the books” and split the spoils. That office was being ripped off twice; first for the neurotoxin and filler materials themselves, and then for the cash proceeds.
Separate all accounting duties. Another popular ploy is false invoicing for imaginary supplies. A friend’s experience provides a good example (retold with his permission): His bookkeeper wrote sizable checks to herself, disguising them in the ledger as payments to vendors commonly used by his practice. Since the same employee also balanced the checkbook, she got away with it for years. “It wasn’t at all clever,” he told me, “and I’m embarrassed to admit that it happened to me.”
Once again, separation of duties is the key to prevention. One employee should enter invoices into the data system, another should issue the check or make the electronic transfer, and a third should match invoices to goods and services received.
Verify expense reports. False expense reporting is a subset of the fake invoice scam. When an employee asks for reimbursement of expenses, make sure those expenses are real.
Ask about computer safeguards. Computers facilitate a lot of financial chores, but they also consolidate financial data in one place, where it is potentially accessible to anybody, anywhere. Your computer vendor should be aware of this, and should have safeguards built into your system. Ask about them. If they aren’t there, ask why.
Hire honest employees. All applicants look great on paper, so check their references; and with their permission, you can run background checks for a few dollars on any of several public information websites. (See my previous columns on hiring at http://www.mdedge.com/dermatology/managing-your-practice.)
Look for “red flags.” Examples are employees who refuse to take vacations, because someone else will have do their work; or who insist on posting expenses that are a coworker’s responsibility, “just to be nice.” Anyone obviously living beyond his or her means merits suspicion as well.
Consider bonding your employees. Dishonesty bonds are relatively inexpensive, and you will be assured of some measure of recovery should your safeguards fail. In addition, the mere knowledge that your staff is bonded will frighten off most dishonest applicants. One effective screen is a question on your employment application: “Would you object to being bonded?”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
In November, the office manager of a San Antonio dermatology practice was sentenced to 46 months in prison for defrauding the practice of nearly $350,000 from patient billings and employee profit sharing accounts.
Per the indictment, the practice conducted a nonprofit educational symposium in 2012. A bank account was established to collect contributions for that event, which was supposed to be closed at its conclusion; but the office manager kept it open, and deposited practice receipts into it. She then used the account as her slush fund for travel, property payments, meal purchases, and other personal expenses on credit cards she fraudulently opened in the practice’s name. This continued for several years.
Because this case has received national attention, I am republishing my column on embezzlement, which includes recommendations that could prevent such unfortunate situations from occurring.
Few crimes are more easily overlooked than theft from within. who think everything is fine. Most embezzlers are not skillful or discreet; but their transgressions may go undetected for years, simply because no one suspects it is happening.
Detecting fraud is an inexact science. There is no textbook approach that one can follow, but a few simple measures will prevent or expose the most common forms:
Make it more difficult. Theft and embezzlement are usually products of opportunity, so minimize those opportunities. No one person should be in charge of the entire bookkeeping process: the person who enters charges should be different from the one who enters payments. The one who writes checks or makes electronic fund transfers should not balance the books, and so on. Internal audits should be done on a regular basis, and all employees should know that. Your accountant can help.
Reconcile cash receipts daily. Embezzlement does not require sophisticated technology; the most common form is simply taking cash out of the till. In a typical scenario, a patient pays a copay of $15 in cash; the receptionist records the payment as $5, and pockets the rest. Make sure a receipt is generated for every cash transaction, and that someone other than the person accepting cash reconciles the charges, receipts, and cash totals daily.
Inventory your stock. Cash isn’t the only susceptible commodity. If you sell cosmetics or other products, inventory your stock frequently. And office personnel are not the only potential thieves: Over a year ago, a locum tenens physician down the street conspired with a receptionist to take cash transactions for cosmetic neurotoxins and fillers “off the books” and split the spoils. That office was being ripped off twice; first for the neurotoxin and filler materials themselves, and then for the cash proceeds.
Separate all accounting duties. Another popular ploy is false invoicing for imaginary supplies. A friend’s experience provides a good example (retold with his permission): His bookkeeper wrote sizable checks to herself, disguising them in the ledger as payments to vendors commonly used by his practice. Since the same employee also balanced the checkbook, she got away with it for years. “It wasn’t at all clever,” he told me, “and I’m embarrassed to admit that it happened to me.”
Once again, separation of duties is the key to prevention. One employee should enter invoices into the data system, another should issue the check or make the electronic transfer, and a third should match invoices to goods and services received.
Verify expense reports. False expense reporting is a subset of the fake invoice scam. When an employee asks for reimbursement of expenses, make sure those expenses are real.
Ask about computer safeguards. Computers facilitate a lot of financial chores, but they also consolidate financial data in one place, where it is potentially accessible to anybody, anywhere. Your computer vendor should be aware of this, and should have safeguards built into your system. Ask about them. If they aren’t there, ask why.
Hire honest employees. All applicants look great on paper, so check their references; and with their permission, you can run background checks for a few dollars on any of several public information websites. (See my previous columns on hiring at http://www.mdedge.com/dermatology/managing-your-practice.)
Look for “red flags.” Examples are employees who refuse to take vacations, because someone else will have do their work; or who insist on posting expenses that are a coworker’s responsibility, “just to be nice.” Anyone obviously living beyond his or her means merits suspicion as well.
Consider bonding your employees. Dishonesty bonds are relatively inexpensive, and you will be assured of some measure of recovery should your safeguards fail. In addition, the mere knowledge that your staff is bonded will frighten off most dishonest applicants. One effective screen is a question on your employment application: “Would you object to being bonded?”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Erythematous Nodule With Central Erosions on the Calf
The Diagnosis: Osteoma Cutis
Osteoma cutis is the heterotopic development of cutaneous ossifications in the dermis or subcutaneous fat and presents as plaquelike, stony, hard nodules. It can manifest as either a primary or secondary condition based on the presence or absence of a prior skin insult at the lesion site. Primary osteoma cutis occurs in 15% of patients and arises either de novo or in association with any of several inflammatory, neoplastic, and metabolic diseases that provide a favorable environment for abnormal mesenchymal stem cell commitment to osteoid,1 including Albright hereditary osteodystrophy, myositis ossificans progressiva, and progressive osseous heteroplasia, which are all associated with mutations in the heterotrimeric G-protein alpha subunit encoding gene, GNAS. 1,2 It is suggested that an insufficiency of Gsα leads to uncontrolled negative regulation of nonosseous connective tissue differentiation, forming osteoid.3 Additionally, diseases involving gain-of-function mutations in the activin A receptor type 1 encoding gene, ACVR1, such as fibrodysplasia ossificans progressiva, have been associated with osteoma cutis.4 These mutations lead to decreased receptor affinity to molecular safeguards of bone morphogenetic protein signaling, ultimately contributing to progressive ectopic bone formation.5 Secondary osteoma cutis occurs in 85% of patients and develops at the site of prior skin damage due to inflammation, neoplasm, or trauma.6 It is believed that tissue damage and degeneration lead to mesenchymal stem cell proliferation and skeletogenicinducing factor recruitment forming cartilaginous tissue, later replaced by bone through endochondral ossification.7
Although osteoma cutis previously was believed to be rare, more recent radiologic studies suggest otherwise, detecting cutaneous osteomas in up to 42.1% of patients.8 Consequently, it is likely that osteoma cutis is underdiagnosed due to its subclinical nature. Our patient, however, presented with a solitary osteoma cutis with perforation of the epidermis, a rare phenomenon.9-12
A shave biopsy in our patient revealed moderate to focally marked, irregular epidermal hyperplasia with a large focus of moderate, compact, parakeratotic crust overlying the epidermis in the center of the specimen. The papillary dermis in the center of the specimen revealed large foci of dark pink to purple bone fragments surrounded by moderate lymphocytic infiltrate with few foci perforating through the overlying epidermis (Figure, A). These findings were characteristic of osteoma cutis with perforation through the overlying epidermis.
The diagnosis of osteoma cutis at the age of 62 years suggested that the lesion was not primary in association with previously described diseases. Furthermore, the lack of phenotypic features of these diseases including obesity, developmental disability, and high parathyroid hormone levels essentially excluded this possibility. The presence of the lesion on the lower extremities initially may have suggested osteoma cutis secondary to chronic venous insufficiency13; however, the absence of visible varicose veins or obvious signs of stasis disease made this unlikely. No further cutaneous disorders at or around the lesion site clinically and histologically suggested that our patient’s lesion was primary and of idiopathic nature. Dermatofibroma can present similarly in appearance but would characteristically dimple centrally when pinched. Keratoacanthoma presents with central ulceration and keratin plugging. Pilomatricoma more commonly presents on the head and neck and less frequently as a firm nodule. Lastly, prurigo nodularis more commonly presents as a symmetrically diffuse rash compared to an isolated nodule.
Osteoma cutis is a cutaneous ossification that may be primary or secondary in nature and less rare than originally thought. Workup for potentially associated inflammatory, neoplastic, and metabolic diseases should be considered in patients with this condition. Perforating osteoma cutis is a rare variant that presents as solitary or multiple nodules with central erosion and crust. The mechanism of transepidermal elimination leading to skin perforation is hypothesized to involve epidermal hyperproliferation leading to upward movement.14 Shave biopsy establishes a definitive histopathologic diagnosis and often is curative. Given that lesions of osteoma cutis themselves are benign, removal may not be necessary.
- Falsey RR, Ackerman L. Eruptive, hard cutaneous nodules in a 61-yearold woman. osteoma cutis in a patient with Albright hereditary osteodystrophy (AHO). JAMA Dermatol. 2013;149:975-976.
- Martin J, Tucker M, Browning JC. Infantile osteoma cutis as a presentation of a GNAS mutation. Pediatr Dermatol. 2012;29:483-484.
- Shore EM, Ahn J, de Beur SJ, et al. Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N Engl J Med. 2002;346:99-106.
- Kaplan FS, Le Merrer M, Glaser DL, et al. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol. 2008;22:191-205.
- Song GA, Kim HJ, Woo KM, et al. Molecular consequences of the ACVR1(R206H) mutation of fibrodysplasia ossificans progressiva. J Biol Chem. 2010;285:22542-22553.
- Roth SI, Stowell RE, Helwig EB, et al. Cutaneous ossification. report of 120 cases and review of the literature. Arch Pathol. 1963;76:44-54.
- Shimono K, Uchibe K, Kuboki T, et al. The pathophysiology of heterotopic ossification: current treatment considerations in dentistry. Japanese Dental Science Review. 2014;50:1-8.
- Kim D, Franco GA, Shigehara H, et al. Benign miliary osteoma cutis of the face: a common incidental CT finding. AJNR Am J Neuroradiol. 2017;38:789-794.
- Basu P, Erickson CP, Calame A, et al. Osteoma cutis: an adverse event following tattoo placement. Cureus. 2019;11:E4323.
- Cohen PR. Perforating osteoma cutis: case report and literature review of patients with a solitary perforating osteoma cutis lesion. Dermatol Online J. 2018;24:13030/qt6kt5n92w.
- Hong SH, Kang HY. A case of perforating osteoma cutis. Ann Dermatol. 2003;15:153-155.
- Kim BK, Ahn SK. Acquired perforating osteoma cutis. Ann Dermatol. 2015;27:452-453.
- Lippmann HI, Goldin RR. Subcutaneous ossification of the legs in chronic venous insufficiency. Radiology. 1960;74:279-288.
- Haro R, Revelles JM, Angulo J, et al. Plaque-like osteoma cutis with transepidermal elimination. J Cutan Pathol. 2009;36:591-593.
The Diagnosis: Osteoma Cutis
Osteoma cutis is the heterotopic development of cutaneous ossifications in the dermis or subcutaneous fat and presents as plaquelike, stony, hard nodules. It can manifest as either a primary or secondary condition based on the presence or absence of a prior skin insult at the lesion site. Primary osteoma cutis occurs in 15% of patients and arises either de novo or in association with any of several inflammatory, neoplastic, and metabolic diseases that provide a favorable environment for abnormal mesenchymal stem cell commitment to osteoid,1 including Albright hereditary osteodystrophy, myositis ossificans progressiva, and progressive osseous heteroplasia, which are all associated with mutations in the heterotrimeric G-protein alpha subunit encoding gene, GNAS. 1,2 It is suggested that an insufficiency of Gsα leads to uncontrolled negative regulation of nonosseous connective tissue differentiation, forming osteoid.3 Additionally, diseases involving gain-of-function mutations in the activin A receptor type 1 encoding gene, ACVR1, such as fibrodysplasia ossificans progressiva, have been associated with osteoma cutis.4 These mutations lead to decreased receptor affinity to molecular safeguards of bone morphogenetic protein signaling, ultimately contributing to progressive ectopic bone formation.5 Secondary osteoma cutis occurs in 85% of patients and develops at the site of prior skin damage due to inflammation, neoplasm, or trauma.6 It is believed that tissue damage and degeneration lead to mesenchymal stem cell proliferation and skeletogenicinducing factor recruitment forming cartilaginous tissue, later replaced by bone through endochondral ossification.7
Although osteoma cutis previously was believed to be rare, more recent radiologic studies suggest otherwise, detecting cutaneous osteomas in up to 42.1% of patients.8 Consequently, it is likely that osteoma cutis is underdiagnosed due to its subclinical nature. Our patient, however, presented with a solitary osteoma cutis with perforation of the epidermis, a rare phenomenon.9-12
A shave biopsy in our patient revealed moderate to focally marked, irregular epidermal hyperplasia with a large focus of moderate, compact, parakeratotic crust overlying the epidermis in the center of the specimen. The papillary dermis in the center of the specimen revealed large foci of dark pink to purple bone fragments surrounded by moderate lymphocytic infiltrate with few foci perforating through the overlying epidermis (Figure, A). These findings were characteristic of osteoma cutis with perforation through the overlying epidermis.

The diagnosis of osteoma cutis at the age of 62 years suggested that the lesion was not primary in association with previously described diseases. Furthermore, the lack of phenotypic features of these diseases including obesity, developmental disability, and high parathyroid hormone levels essentially excluded this possibility. The presence of the lesion on the lower extremities initially may have suggested osteoma cutis secondary to chronic venous insufficiency13; however, the absence of visible varicose veins or obvious signs of stasis disease made this unlikely. No further cutaneous disorders at or around the lesion site clinically and histologically suggested that our patient’s lesion was primary and of idiopathic nature. Dermatofibroma can present similarly in appearance but would characteristically dimple centrally when pinched. Keratoacanthoma presents with central ulceration and keratin plugging. Pilomatricoma more commonly presents on the head and neck and less frequently as a firm nodule. Lastly, prurigo nodularis more commonly presents as a symmetrically diffuse rash compared to an isolated nodule.
Osteoma cutis is a cutaneous ossification that may be primary or secondary in nature and less rare than originally thought. Workup for potentially associated inflammatory, neoplastic, and metabolic diseases should be considered in patients with this condition. Perforating osteoma cutis is a rare variant that presents as solitary or multiple nodules with central erosion and crust. The mechanism of transepidermal elimination leading to skin perforation is hypothesized to involve epidermal hyperproliferation leading to upward movement.14 Shave biopsy establishes a definitive histopathologic diagnosis and often is curative. Given that lesions of osteoma cutis themselves are benign, removal may not be necessary.
The Diagnosis: Osteoma Cutis
Osteoma cutis is the heterotopic development of cutaneous ossifications in the dermis or subcutaneous fat and presents as plaquelike, stony, hard nodules. It can manifest as either a primary or secondary condition based on the presence or absence of a prior skin insult at the lesion site. Primary osteoma cutis occurs in 15% of patients and arises either de novo or in association with any of several inflammatory, neoplastic, and metabolic diseases that provide a favorable environment for abnormal mesenchymal stem cell commitment to osteoid,1 including Albright hereditary osteodystrophy, myositis ossificans progressiva, and progressive osseous heteroplasia, which are all associated with mutations in the heterotrimeric G-protein alpha subunit encoding gene, GNAS. 1,2 It is suggested that an insufficiency of Gsα leads to uncontrolled negative regulation of nonosseous connective tissue differentiation, forming osteoid.3 Additionally, diseases involving gain-of-function mutations in the activin A receptor type 1 encoding gene, ACVR1, such as fibrodysplasia ossificans progressiva, have been associated with osteoma cutis.4 These mutations lead to decreased receptor affinity to molecular safeguards of bone morphogenetic protein signaling, ultimately contributing to progressive ectopic bone formation.5 Secondary osteoma cutis occurs in 85% of patients and develops at the site of prior skin damage due to inflammation, neoplasm, or trauma.6 It is believed that tissue damage and degeneration lead to mesenchymal stem cell proliferation and skeletogenicinducing factor recruitment forming cartilaginous tissue, later replaced by bone through endochondral ossification.7
Although osteoma cutis previously was believed to be rare, more recent radiologic studies suggest otherwise, detecting cutaneous osteomas in up to 42.1% of patients.8 Consequently, it is likely that osteoma cutis is underdiagnosed due to its subclinical nature. Our patient, however, presented with a solitary osteoma cutis with perforation of the epidermis, a rare phenomenon.9-12
A shave biopsy in our patient revealed moderate to focally marked, irregular epidermal hyperplasia with a large focus of moderate, compact, parakeratotic crust overlying the epidermis in the center of the specimen. The papillary dermis in the center of the specimen revealed large foci of dark pink to purple bone fragments surrounded by moderate lymphocytic infiltrate with few foci perforating through the overlying epidermis (Figure, A). These findings were characteristic of osteoma cutis with perforation through the overlying epidermis.

The diagnosis of osteoma cutis at the age of 62 years suggested that the lesion was not primary in association with previously described diseases. Furthermore, the lack of phenotypic features of these diseases including obesity, developmental disability, and high parathyroid hormone levels essentially excluded this possibility. The presence of the lesion on the lower extremities initially may have suggested osteoma cutis secondary to chronic venous insufficiency13; however, the absence of visible varicose veins or obvious signs of stasis disease made this unlikely. No further cutaneous disorders at or around the lesion site clinically and histologically suggested that our patient’s lesion was primary and of idiopathic nature. Dermatofibroma can present similarly in appearance but would characteristically dimple centrally when pinched. Keratoacanthoma presents with central ulceration and keratin plugging. Pilomatricoma more commonly presents on the head and neck and less frequently as a firm nodule. Lastly, prurigo nodularis more commonly presents as a symmetrically diffuse rash compared to an isolated nodule.
Osteoma cutis is a cutaneous ossification that may be primary or secondary in nature and less rare than originally thought. Workup for potentially associated inflammatory, neoplastic, and metabolic diseases should be considered in patients with this condition. Perforating osteoma cutis is a rare variant that presents as solitary or multiple nodules with central erosion and crust. The mechanism of transepidermal elimination leading to skin perforation is hypothesized to involve epidermal hyperproliferation leading to upward movement.14 Shave biopsy establishes a definitive histopathologic diagnosis and often is curative. Given that lesions of osteoma cutis themselves are benign, removal may not be necessary.
- Falsey RR, Ackerman L. Eruptive, hard cutaneous nodules in a 61-yearold woman. osteoma cutis in a patient with Albright hereditary osteodystrophy (AHO). JAMA Dermatol. 2013;149:975-976.
- Martin J, Tucker M, Browning JC. Infantile osteoma cutis as a presentation of a GNAS mutation. Pediatr Dermatol. 2012;29:483-484.
- Shore EM, Ahn J, de Beur SJ, et al. Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N Engl J Med. 2002;346:99-106.
- Kaplan FS, Le Merrer M, Glaser DL, et al. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol. 2008;22:191-205.
- Song GA, Kim HJ, Woo KM, et al. Molecular consequences of the ACVR1(R206H) mutation of fibrodysplasia ossificans progressiva. J Biol Chem. 2010;285:22542-22553.
- Roth SI, Stowell RE, Helwig EB, et al. Cutaneous ossification. report of 120 cases and review of the literature. Arch Pathol. 1963;76:44-54.
- Shimono K, Uchibe K, Kuboki T, et al. The pathophysiology of heterotopic ossification: current treatment considerations in dentistry. Japanese Dental Science Review. 2014;50:1-8.
- Kim D, Franco GA, Shigehara H, et al. Benign miliary osteoma cutis of the face: a common incidental CT finding. AJNR Am J Neuroradiol. 2017;38:789-794.
- Basu P, Erickson CP, Calame A, et al. Osteoma cutis: an adverse event following tattoo placement. Cureus. 2019;11:E4323.
- Cohen PR. Perforating osteoma cutis: case report and literature review of patients with a solitary perforating osteoma cutis lesion. Dermatol Online J. 2018;24:13030/qt6kt5n92w.
- Hong SH, Kang HY. A case of perforating osteoma cutis. Ann Dermatol. 2003;15:153-155.
- Kim BK, Ahn SK. Acquired perforating osteoma cutis. Ann Dermatol. 2015;27:452-453.
- Lippmann HI, Goldin RR. Subcutaneous ossification of the legs in chronic venous insufficiency. Radiology. 1960;74:279-288.
- Haro R, Revelles JM, Angulo J, et al. Plaque-like osteoma cutis with transepidermal elimination. J Cutan Pathol. 2009;36:591-593.
- Falsey RR, Ackerman L. Eruptive, hard cutaneous nodules in a 61-yearold woman. osteoma cutis in a patient with Albright hereditary osteodystrophy (AHO). JAMA Dermatol. 2013;149:975-976.
- Martin J, Tucker M, Browning JC. Infantile osteoma cutis as a presentation of a GNAS mutation. Pediatr Dermatol. 2012;29:483-484.
- Shore EM, Ahn J, de Beur SJ, et al. Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N Engl J Med. 2002;346:99-106.
- Kaplan FS, Le Merrer M, Glaser DL, et al. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol. 2008;22:191-205.
- Song GA, Kim HJ, Woo KM, et al. Molecular consequences of the ACVR1(R206H) mutation of fibrodysplasia ossificans progressiva. J Biol Chem. 2010;285:22542-22553.
- Roth SI, Stowell RE, Helwig EB, et al. Cutaneous ossification. report of 120 cases and review of the literature. Arch Pathol. 1963;76:44-54.
- Shimono K, Uchibe K, Kuboki T, et al. The pathophysiology of heterotopic ossification: current treatment considerations in dentistry. Japanese Dental Science Review. 2014;50:1-8.
- Kim D, Franco GA, Shigehara H, et al. Benign miliary osteoma cutis of the face: a common incidental CT finding. AJNR Am J Neuroradiol. 2017;38:789-794.
- Basu P, Erickson CP, Calame A, et al. Osteoma cutis: an adverse event following tattoo placement. Cureus. 2019;11:E4323.
- Cohen PR. Perforating osteoma cutis: case report and literature review of patients with a solitary perforating osteoma cutis lesion. Dermatol Online J. 2018;24:13030/qt6kt5n92w.
- Hong SH, Kang HY. A case of perforating osteoma cutis. Ann Dermatol. 2003;15:153-155.
- Kim BK, Ahn SK. Acquired perforating osteoma cutis. Ann Dermatol. 2015;27:452-453.
- Lippmann HI, Goldin RR. Subcutaneous ossification of the legs in chronic venous insufficiency. Radiology. 1960;74:279-288.
- Haro R, Revelles JM, Angulo J, et al. Plaque-like osteoma cutis with transepidermal elimination. J Cutan Pathol. 2009;36:591-593.
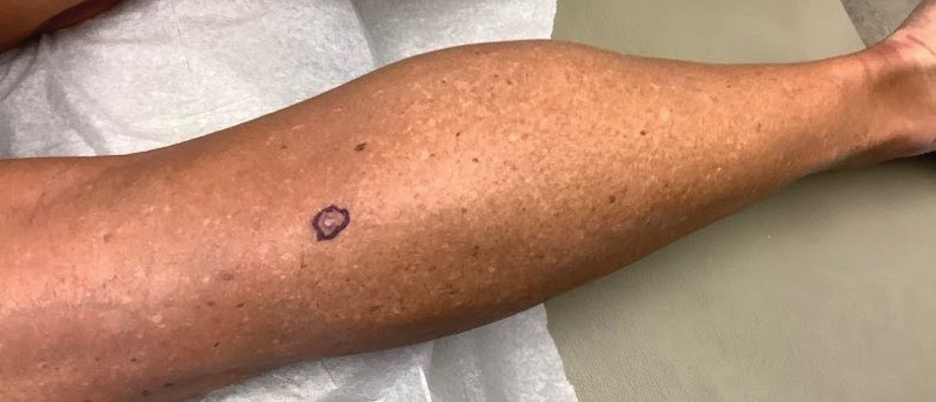
A 62-year-old woman presented with an irregular, erythematous, 4-mm nodule with central erosions on the left proximal calf of 2 months’ duration. The patient had a history of actinic keratoses and dysplastic nevi. She had no other notable medical history. She was not taking any medications and reported no history of trauma to the area. A shave biopsy of the lesion (encircled by black ink) was performed.
AI: Skin of color underrepresented in datasets used to identify skin cancer
An in the databases, researchers in the United Kingdom report.
Out of 106,950 skin lesions documented in 21 open-access databases and 17 open-access atlases identified by David Wen, BMBCh, from the University of Oxford (England), and colleagues, 2,436 images contained information on Fitzpatrick skin type. Of these, “only 10 images were from individuals with Fitzpatrick skin type V, and only a single image was from an individual with Fitzpatrick skin type VI,” the researchers said. “The ethnicity of these individuals was either Brazilian or unknown.”
In two datasets containing 1,585 images with ethnicity data, “no images were from individuals with an African, Afro-Caribbean, or South Asian background,” Dr. Wen and colleagues noted. “Coupled with the geographical origins of datasets, there was massive under-representation of skin lesion images from darker-skinned populations.”
The results of their systematic review were presented at the National Cancer Research Institute Festival and published on Nov. 9, 2021, in The Lancet Digital Health. To the best of their knowledge, they wrote, this is “the first systematic review of publicly available skin lesion images comprising predominantly dermoscopic and macroscopic images available through open access datasets and atlases.”
Overall, 11 of 14 datasets (79%) were from North America, Europe, or Oceania among datasets with information on country of origin, the researchers said. Either dermoscopic images or macroscopic photographs were the only types of images available in 19 of 21 (91%) datasets. There was some variation in the clinical information available, with 81,662 images (76.4%) containing information on age, 82,848 images (77.5%) having information on gender, and 79,561 images having information about body site (74.4%).
The researchers explained that these datasets might be of limited use in a real-world setting where the images aren’t representative of the population. Artificial intelligence (AI) programs that train using images of patients with one skin type, for example, can potentially misdiagnose patients of another skin type, they said.
“AI programs hold a lot of potential for diagnosing skin cancer because it can look at pictures and quickly and cost-effectively evaluate any worrying spots on the skin,” Dr. Wen said in a press release from the NCRI Festival. “However, it’s important to know about the images and patients used to develop programs, as these influence which groups of people the programs will be most effective for in real-life settings. Research has shown that programs trained on images taken from people with lighter skin types only might not be as accurate for people with darker skin, and vice versa.”
There was also “limited information on who, how and why the images were taken,” Dr. Wen said in the release. “This has implications for the programs developed from these images, due to uncertainty around how they may perform in different groups of people, especially in those who aren’t well represented in datasets, such as those with darker skin. This can potentially lead to the exclusion or even harm of these groups from AI technologies.”
While there are no current guidelines for developing skin image datasets, quality standards are needed, according to the researchers.
“Ensuring equitable digital health includes building unbiased, representative datasets to ensure that the algorithms that are created benefit people of all backgrounds and skin types,” they concluded in the study.
Neil Steven, MBBS, MA, PhD, FRCP, an NCRI Skin Group member who was not involved with the research, stated in the press release that the results from the study by Dr. Wen and colleagues “raise concerns about the ability of AI to assist in skin cancer diagnosis, especially in a global context.”
“I hope this work will continue and help ensure that the progress we make in using AI in medicine will benefit all patients, recognizing that human skin color is highly diverse,” said Dr. Steven, honorary consultant in medical oncology at University Hospitals Birmingham (England) NHS Foundation Trust.
‘We need more images of everybody’
Dermatologist Adewole Adamson, MD, MPP, assistant professor in the department of internal medicine (division of dermatology) at the University of Texas at Austin, said in an interview that a “major potential downside” of algorithms not trained on diverse datasets is the potential for incorrect diagnoses.
“The harms of algorithms used for diagnostic purposes in the skin can be particularly significant because of the scalability of this technology. A lot of thought needs to be put into how these algorithms are developed and tested,” said Dr. Adamson, who reviewed the manuscript of The Lancet Digital Health study but was not involved with the research.
He referred to the results of a recently published study in JAMA Dermatology, which found that only 10% of studies used to develop or test deep-learning algorithms contained metadata on skin tone. “Furthermore, most datasets are from countries where darker skin types are not represented. [These] algorithms therefore likely underperform on people of darker skin types and thus, users should be wary,” Dr. Adamson said.
A consensus guideline should be developed for public AI algorithms, he said, which should have metadata containing information on sex, race/ethnicity, geographic location, skin type, and part of the body. “This distribution should also be reported in any publication of an algorithm so that users can see if the distribution of the population in the training data mirrors that of the population in which it is intended to be used,” he added.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was not involved with the research, said that, while this issue of underrepresentation has been known in dermatology for some time, the strength of the Lancet study is that it is a large study, with a message of “we need more images of everybody.”
“This is probably the broadest study looking at every possible accessible resource and taking an organized approach,” Dr. Friedman said in an interview. “But I think it also raises some important points about how we think about skin tones and how we refer to them as well with respect to misusing classification schemes that we currently have.”
While using ethnicity data and certain Fitzpatrick skin types as a proxy for darker skin is a limitation of the metadata the study authors had available, it also highlights “a broader problem with respect to lexicon regarding skin tone,” he explained.
“Skin does not have a race, it doesn’t have an ethnicity,” Dr. Friedman said.
A dataset that contains not only different skin tones but how different dermatologic conditions look across skin tones is important. “If you just look at one photo of one skin tone, you missed the fact that clinical presentations can be so polymorphic, especially because of different skin tones,” Dr. Friedman said.
“We need to keep pushing this message to ensure that images keep getting collected. We [need to] ensure that there’s quality control with these images and that we’re disseminating them in a way that everyone has access, both from self-learning, but also to teach others,” said Dr. Friedman, coeditor of a recently introduced dermatology atlas showing skin conditions in different skin tones.
Adamson reports no relevant financial relationships. Dr. Friedman is a coeditor of a dermatology atlas supported by Allergan Aesthetics and SkinBetter Science. This study was funded by NHSX and the Health Foundation. Three authors reported being paid employees of Databiology at the time of the study. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An in the databases, researchers in the United Kingdom report.
Out of 106,950 skin lesions documented in 21 open-access databases and 17 open-access atlases identified by David Wen, BMBCh, from the University of Oxford (England), and colleagues, 2,436 images contained information on Fitzpatrick skin type. Of these, “only 10 images were from individuals with Fitzpatrick skin type V, and only a single image was from an individual with Fitzpatrick skin type VI,” the researchers said. “The ethnicity of these individuals was either Brazilian or unknown.”
In two datasets containing 1,585 images with ethnicity data, “no images were from individuals with an African, Afro-Caribbean, or South Asian background,” Dr. Wen and colleagues noted. “Coupled with the geographical origins of datasets, there was massive under-representation of skin lesion images from darker-skinned populations.”
The results of their systematic review were presented at the National Cancer Research Institute Festival and published on Nov. 9, 2021, in The Lancet Digital Health. To the best of their knowledge, they wrote, this is “the first systematic review of publicly available skin lesion images comprising predominantly dermoscopic and macroscopic images available through open access datasets and atlases.”
Overall, 11 of 14 datasets (79%) were from North America, Europe, or Oceania among datasets with information on country of origin, the researchers said. Either dermoscopic images or macroscopic photographs were the only types of images available in 19 of 21 (91%) datasets. There was some variation in the clinical information available, with 81,662 images (76.4%) containing information on age, 82,848 images (77.5%) having information on gender, and 79,561 images having information about body site (74.4%).
The researchers explained that these datasets might be of limited use in a real-world setting where the images aren’t representative of the population. Artificial intelligence (AI) programs that train using images of patients with one skin type, for example, can potentially misdiagnose patients of another skin type, they said.
“AI programs hold a lot of potential for diagnosing skin cancer because it can look at pictures and quickly and cost-effectively evaluate any worrying spots on the skin,” Dr. Wen said in a press release from the NCRI Festival. “However, it’s important to know about the images and patients used to develop programs, as these influence which groups of people the programs will be most effective for in real-life settings. Research has shown that programs trained on images taken from people with lighter skin types only might not be as accurate for people with darker skin, and vice versa.”
There was also “limited information on who, how and why the images were taken,” Dr. Wen said in the release. “This has implications for the programs developed from these images, due to uncertainty around how they may perform in different groups of people, especially in those who aren’t well represented in datasets, such as those with darker skin. This can potentially lead to the exclusion or even harm of these groups from AI technologies.”
While there are no current guidelines for developing skin image datasets, quality standards are needed, according to the researchers.
“Ensuring equitable digital health includes building unbiased, representative datasets to ensure that the algorithms that are created benefit people of all backgrounds and skin types,” they concluded in the study.
Neil Steven, MBBS, MA, PhD, FRCP, an NCRI Skin Group member who was not involved with the research, stated in the press release that the results from the study by Dr. Wen and colleagues “raise concerns about the ability of AI to assist in skin cancer diagnosis, especially in a global context.”
“I hope this work will continue and help ensure that the progress we make in using AI in medicine will benefit all patients, recognizing that human skin color is highly diverse,” said Dr. Steven, honorary consultant in medical oncology at University Hospitals Birmingham (England) NHS Foundation Trust.
‘We need more images of everybody’
Dermatologist Adewole Adamson, MD, MPP, assistant professor in the department of internal medicine (division of dermatology) at the University of Texas at Austin, said in an interview that a “major potential downside” of algorithms not trained on diverse datasets is the potential for incorrect diagnoses.
“The harms of algorithms used for diagnostic purposes in the skin can be particularly significant because of the scalability of this technology. A lot of thought needs to be put into how these algorithms are developed and tested,” said Dr. Adamson, who reviewed the manuscript of The Lancet Digital Health study but was not involved with the research.
He referred to the results of a recently published study in JAMA Dermatology, which found that only 10% of studies used to develop or test deep-learning algorithms contained metadata on skin tone. “Furthermore, most datasets are from countries where darker skin types are not represented. [These] algorithms therefore likely underperform on people of darker skin types and thus, users should be wary,” Dr. Adamson said.
A consensus guideline should be developed for public AI algorithms, he said, which should have metadata containing information on sex, race/ethnicity, geographic location, skin type, and part of the body. “This distribution should also be reported in any publication of an algorithm so that users can see if the distribution of the population in the training data mirrors that of the population in which it is intended to be used,” he added.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was not involved with the research, said that, while this issue of underrepresentation has been known in dermatology for some time, the strength of the Lancet study is that it is a large study, with a message of “we need more images of everybody.”
“This is probably the broadest study looking at every possible accessible resource and taking an organized approach,” Dr. Friedman said in an interview. “But I think it also raises some important points about how we think about skin tones and how we refer to them as well with respect to misusing classification schemes that we currently have.”
While using ethnicity data and certain Fitzpatrick skin types as a proxy for darker skin is a limitation of the metadata the study authors had available, it also highlights “a broader problem with respect to lexicon regarding skin tone,” he explained.
“Skin does not have a race, it doesn’t have an ethnicity,” Dr. Friedman said.
A dataset that contains not only different skin tones but how different dermatologic conditions look across skin tones is important. “If you just look at one photo of one skin tone, you missed the fact that clinical presentations can be so polymorphic, especially because of different skin tones,” Dr. Friedman said.
“We need to keep pushing this message to ensure that images keep getting collected. We [need to] ensure that there’s quality control with these images and that we’re disseminating them in a way that everyone has access, both from self-learning, but also to teach others,” said Dr. Friedman, coeditor of a recently introduced dermatology atlas showing skin conditions in different skin tones.
Adamson reports no relevant financial relationships. Dr. Friedman is a coeditor of a dermatology atlas supported by Allergan Aesthetics and SkinBetter Science. This study was funded by NHSX and the Health Foundation. Three authors reported being paid employees of Databiology at the time of the study. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An in the databases, researchers in the United Kingdom report.
Out of 106,950 skin lesions documented in 21 open-access databases and 17 open-access atlases identified by David Wen, BMBCh, from the University of Oxford (England), and colleagues, 2,436 images contained information on Fitzpatrick skin type. Of these, “only 10 images were from individuals with Fitzpatrick skin type V, and only a single image was from an individual with Fitzpatrick skin type VI,” the researchers said. “The ethnicity of these individuals was either Brazilian or unknown.”
In two datasets containing 1,585 images with ethnicity data, “no images were from individuals with an African, Afro-Caribbean, or South Asian background,” Dr. Wen and colleagues noted. “Coupled with the geographical origins of datasets, there was massive under-representation of skin lesion images from darker-skinned populations.”
The results of their systematic review were presented at the National Cancer Research Institute Festival and published on Nov. 9, 2021, in The Lancet Digital Health. To the best of their knowledge, they wrote, this is “the first systematic review of publicly available skin lesion images comprising predominantly dermoscopic and macroscopic images available through open access datasets and atlases.”
Overall, 11 of 14 datasets (79%) were from North America, Europe, or Oceania among datasets with information on country of origin, the researchers said. Either dermoscopic images or macroscopic photographs were the only types of images available in 19 of 21 (91%) datasets. There was some variation in the clinical information available, with 81,662 images (76.4%) containing information on age, 82,848 images (77.5%) having information on gender, and 79,561 images having information about body site (74.4%).
The researchers explained that these datasets might be of limited use in a real-world setting where the images aren’t representative of the population. Artificial intelligence (AI) programs that train using images of patients with one skin type, for example, can potentially misdiagnose patients of another skin type, they said.
“AI programs hold a lot of potential for diagnosing skin cancer because it can look at pictures and quickly and cost-effectively evaluate any worrying spots on the skin,” Dr. Wen said in a press release from the NCRI Festival. “However, it’s important to know about the images and patients used to develop programs, as these influence which groups of people the programs will be most effective for in real-life settings. Research has shown that programs trained on images taken from people with lighter skin types only might not be as accurate for people with darker skin, and vice versa.”
There was also “limited information on who, how and why the images were taken,” Dr. Wen said in the release. “This has implications for the programs developed from these images, due to uncertainty around how they may perform in different groups of people, especially in those who aren’t well represented in datasets, such as those with darker skin. This can potentially lead to the exclusion or even harm of these groups from AI technologies.”
While there are no current guidelines for developing skin image datasets, quality standards are needed, according to the researchers.
“Ensuring equitable digital health includes building unbiased, representative datasets to ensure that the algorithms that are created benefit people of all backgrounds and skin types,” they concluded in the study.
Neil Steven, MBBS, MA, PhD, FRCP, an NCRI Skin Group member who was not involved with the research, stated in the press release that the results from the study by Dr. Wen and colleagues “raise concerns about the ability of AI to assist in skin cancer diagnosis, especially in a global context.”
“I hope this work will continue and help ensure that the progress we make in using AI in medicine will benefit all patients, recognizing that human skin color is highly diverse,” said Dr. Steven, honorary consultant in medical oncology at University Hospitals Birmingham (England) NHS Foundation Trust.
‘We need more images of everybody’
Dermatologist Adewole Adamson, MD, MPP, assistant professor in the department of internal medicine (division of dermatology) at the University of Texas at Austin, said in an interview that a “major potential downside” of algorithms not trained on diverse datasets is the potential for incorrect diagnoses.
“The harms of algorithms used for diagnostic purposes in the skin can be particularly significant because of the scalability of this technology. A lot of thought needs to be put into how these algorithms are developed and tested,” said Dr. Adamson, who reviewed the manuscript of The Lancet Digital Health study but was not involved with the research.
He referred to the results of a recently published study in JAMA Dermatology, which found that only 10% of studies used to develop or test deep-learning algorithms contained metadata on skin tone. “Furthermore, most datasets are from countries where darker skin types are not represented. [These] algorithms therefore likely underperform on people of darker skin types and thus, users should be wary,” Dr. Adamson said.
A consensus guideline should be developed for public AI algorithms, he said, which should have metadata containing information on sex, race/ethnicity, geographic location, skin type, and part of the body. “This distribution should also be reported in any publication of an algorithm so that users can see if the distribution of the population in the training data mirrors that of the population in which it is intended to be used,” he added.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was not involved with the research, said that, while this issue of underrepresentation has been known in dermatology for some time, the strength of the Lancet study is that it is a large study, with a message of “we need more images of everybody.”
“This is probably the broadest study looking at every possible accessible resource and taking an organized approach,” Dr. Friedman said in an interview. “But I think it also raises some important points about how we think about skin tones and how we refer to them as well with respect to misusing classification schemes that we currently have.”
While using ethnicity data and certain Fitzpatrick skin types as a proxy for darker skin is a limitation of the metadata the study authors had available, it also highlights “a broader problem with respect to lexicon regarding skin tone,” he explained.
“Skin does not have a race, it doesn’t have an ethnicity,” Dr. Friedman said.
A dataset that contains not only different skin tones but how different dermatologic conditions look across skin tones is important. “If you just look at one photo of one skin tone, you missed the fact that clinical presentations can be so polymorphic, especially because of different skin tones,” Dr. Friedman said.
“We need to keep pushing this message to ensure that images keep getting collected. We [need to] ensure that there’s quality control with these images and that we’re disseminating them in a way that everyone has access, both from self-learning, but also to teach others,” said Dr. Friedman, coeditor of a recently introduced dermatology atlas showing skin conditions in different skin tones.
Adamson reports no relevant financial relationships. Dr. Friedman is a coeditor of a dermatology atlas supported by Allergan Aesthetics and SkinBetter Science. This study was funded by NHSX and the Health Foundation. Three authors reported being paid employees of Databiology at the time of the study. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.