User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
CDC unveils mental health protection plan for health care workers
Federal health officials have outlined a five-part plan to improve and protect the mental health and well-being of America’s health care workers (HCWs) and create sustainable change for the next generation of HCWs.
“It’s long past time for us to care for the people who care for all of us and address burnout in our health care workers,” U.S. Surgeon General Vivek H. Murthy, MD, MBA, said during a webinar hosted by the National Institute for Occupational Safety and Health, part of the U.S. Centers for Disease Control and Prevention.
“My hope is that, going forward, we will be able to embark on this journey together to create a health care system, a health care environment, a country where we can not only provide extraordinary care to all those who need it, but where we can take good care of those who have sacrificed so much and make sure that they are well,” Dr. Murthy said.
Burnout is not selective
There are 20 million HCWs in the United States, and no one is immune from burnout, said NIOSH Director John Howard, MD.
He noted that from June through Sept. of 2020 – the height of the COVID-19 pandemic – 93% of HCWs experienced some degree of stress, with 22% reporting moderate depression and post-traumatic stress disorder.
Looking at subsets of HCWs, a recent survey showed that one in five nurses contemplated leaving the profession because of insufficient staffing, intensity of workload, emotional and physical toll of the job, and lack of support, Dr. Howard noted.
Physician burnout was a significant issue even before the pandemic, with about 79% of physicians reporting burnout. , Dr. Howard said.
Women in health care jobs are especially vulnerable to burnout; 76% of health care jobs are held by women and 64% of physicians that feel burned-out are women, according to federal data.
“We have significant work to do in shoring up the safety and health of women in health care,” Dr. Howard said.
Mental health is also suffering among local and state public health workers. In a recent CDC survey of 26,000 of these workers, 53% reported symptoms of at least one mental health condition in the past 2 weeks.
“That is really an alarming proportion of public health workers who are as vital and essential as nurses and doctors are in our health care system,” Dr. Howard said.
Primary prevention approach
To tackle the burnout crisis, NIOSH plans to:
- Take a deep dive into understanding the personal, social, and economic burdens HCWs face on a daily basis.
- Assimilate the evidence and create a repository of best practices, resources, and interventions.
- Partner with key stakeholders, including the American Hospital Association, the American Nurses Association, National Nurses United, the Joint Commission.
- Identify and adapt tools for the health care workplace that emphasize stress reduction.
NIOSH also plans to “generate awareness through a national, multidimensional social marketing campaign to get the word out about stress so health care workers don’t feel so alone,” Dr. Howard said.
This five-part plan takes a primary prevention approach to identifying and eliminating risk factors for burnout and stress, he added.
Secondary prevention, “when damage has already been done and you’re trying to save a health care worker who is suffering from a mental health issue, that’s a lot harder than taking a good look at what you can do to organizational practices that lead to health care workers’ stress and burnout,” Dr. Howard said.
A version of this article first appeared on Medscape.com.
Federal health officials have outlined a five-part plan to improve and protect the mental health and well-being of America’s health care workers (HCWs) and create sustainable change for the next generation of HCWs.
“It’s long past time for us to care for the people who care for all of us and address burnout in our health care workers,” U.S. Surgeon General Vivek H. Murthy, MD, MBA, said during a webinar hosted by the National Institute for Occupational Safety and Health, part of the U.S. Centers for Disease Control and Prevention.
“My hope is that, going forward, we will be able to embark on this journey together to create a health care system, a health care environment, a country where we can not only provide extraordinary care to all those who need it, but where we can take good care of those who have sacrificed so much and make sure that they are well,” Dr. Murthy said.
Burnout is not selective
There are 20 million HCWs in the United States, and no one is immune from burnout, said NIOSH Director John Howard, MD.
He noted that from June through Sept. of 2020 – the height of the COVID-19 pandemic – 93% of HCWs experienced some degree of stress, with 22% reporting moderate depression and post-traumatic stress disorder.
Looking at subsets of HCWs, a recent survey showed that one in five nurses contemplated leaving the profession because of insufficient staffing, intensity of workload, emotional and physical toll of the job, and lack of support, Dr. Howard noted.
Physician burnout was a significant issue even before the pandemic, with about 79% of physicians reporting burnout. , Dr. Howard said.
Women in health care jobs are especially vulnerable to burnout; 76% of health care jobs are held by women and 64% of physicians that feel burned-out are women, according to federal data.
“We have significant work to do in shoring up the safety and health of women in health care,” Dr. Howard said.
Mental health is also suffering among local and state public health workers. In a recent CDC survey of 26,000 of these workers, 53% reported symptoms of at least one mental health condition in the past 2 weeks.
“That is really an alarming proportion of public health workers who are as vital and essential as nurses and doctors are in our health care system,” Dr. Howard said.
Primary prevention approach
To tackle the burnout crisis, NIOSH plans to:
- Take a deep dive into understanding the personal, social, and economic burdens HCWs face on a daily basis.
- Assimilate the evidence and create a repository of best practices, resources, and interventions.
- Partner with key stakeholders, including the American Hospital Association, the American Nurses Association, National Nurses United, the Joint Commission.
- Identify and adapt tools for the health care workplace that emphasize stress reduction.
NIOSH also plans to “generate awareness through a national, multidimensional social marketing campaign to get the word out about stress so health care workers don’t feel so alone,” Dr. Howard said.
This five-part plan takes a primary prevention approach to identifying and eliminating risk factors for burnout and stress, he added.
Secondary prevention, “when damage has already been done and you’re trying to save a health care worker who is suffering from a mental health issue, that’s a lot harder than taking a good look at what you can do to organizational practices that lead to health care workers’ stress and burnout,” Dr. Howard said.
A version of this article first appeared on Medscape.com.
Federal health officials have outlined a five-part plan to improve and protect the mental health and well-being of America’s health care workers (HCWs) and create sustainable change for the next generation of HCWs.
“It’s long past time for us to care for the people who care for all of us and address burnout in our health care workers,” U.S. Surgeon General Vivek H. Murthy, MD, MBA, said during a webinar hosted by the National Institute for Occupational Safety and Health, part of the U.S. Centers for Disease Control and Prevention.
“My hope is that, going forward, we will be able to embark on this journey together to create a health care system, a health care environment, a country where we can not only provide extraordinary care to all those who need it, but where we can take good care of those who have sacrificed so much and make sure that they are well,” Dr. Murthy said.
Burnout is not selective
There are 20 million HCWs in the United States, and no one is immune from burnout, said NIOSH Director John Howard, MD.
He noted that from June through Sept. of 2020 – the height of the COVID-19 pandemic – 93% of HCWs experienced some degree of stress, with 22% reporting moderate depression and post-traumatic stress disorder.
Looking at subsets of HCWs, a recent survey showed that one in five nurses contemplated leaving the profession because of insufficient staffing, intensity of workload, emotional and physical toll of the job, and lack of support, Dr. Howard noted.
Physician burnout was a significant issue even before the pandemic, with about 79% of physicians reporting burnout. , Dr. Howard said.
Women in health care jobs are especially vulnerable to burnout; 76% of health care jobs are held by women and 64% of physicians that feel burned-out are women, according to federal data.
“We have significant work to do in shoring up the safety and health of women in health care,” Dr. Howard said.
Mental health is also suffering among local and state public health workers. In a recent CDC survey of 26,000 of these workers, 53% reported symptoms of at least one mental health condition in the past 2 weeks.
“That is really an alarming proportion of public health workers who are as vital and essential as nurses and doctors are in our health care system,” Dr. Howard said.
Primary prevention approach
To tackle the burnout crisis, NIOSH plans to:
- Take a deep dive into understanding the personal, social, and economic burdens HCWs face on a daily basis.
- Assimilate the evidence and create a repository of best practices, resources, and interventions.
- Partner with key stakeholders, including the American Hospital Association, the American Nurses Association, National Nurses United, the Joint Commission.
- Identify and adapt tools for the health care workplace that emphasize stress reduction.
NIOSH also plans to “generate awareness through a national, multidimensional social marketing campaign to get the word out about stress so health care workers don’t feel so alone,” Dr. Howard said.
This five-part plan takes a primary prevention approach to identifying and eliminating risk factors for burnout and stress, he added.
Secondary prevention, “when damage has already been done and you’re trying to save a health care worker who is suffering from a mental health issue, that’s a lot harder than taking a good look at what you can do to organizational practices that lead to health care workers’ stress and burnout,” Dr. Howard said.
A version of this article first appeared on Medscape.com.
CDC: All adults should be eligible for Pfizer, Moderna boosters
on its vaccine recommendations.
The Advisory Committee on Immunization Practices, or ACIP, recommended that all adults be eligible for a third dose of a Pfizer or Moderna mRNA vaccine, at least 6 months after their second dose.
They also strengthened a recommendation that everyone over the age of 50 should get a third dose, whether or not they have an underlying health condition that may increase their risk from a COVID-19 infection.
The committee voted 11 to 0 in favor of both policies.
CDC Director Rochelle Walensky, MD, must now sign off on both policies, which she is expected to do.
More than 70 million adults are now eligible for booster shots in the United States, but only about 31 million people have received one. About half of those who have been boosted are over the age of 65.
In a recent survey, the Kaiser Family Foundation found that about 4 in 10 younger adults said they were unsure if they qualified for a booster.
Under the current policy, boosters are recommended for everyone age 65 and older. But people who are younger than age 65 are eligible for boosters if they have an underlying health condition or live or work in a high-risk situation—something individuals have to determine on their own. Experts said that shading of the policy had created confusion that was holding people back.
Nirav Shah, MD, JD, president of the Association of State and Territorial Health Officials, noted that public health officials have been swamped with calls from people who are trying to figure out if they are eligible to get a booster dose.
He said that in a call the evening of Nov. 18 with state health departments, “There was not a single state that voiced opposition to this move,” he told the ACIP.
Dr. Shah said that the current guidelines were well intentioned, but “in pursuit of precision, they create confusion.”
“Our concern is that eligible individuals are not receiving boosters right now as a result of this confusion,” he said.
The committee based its decision on the results of a new study of boosters in Pfizer vaccine recipients, as well as reassuring safety information that’s being collected through the CDC and FDA’s monitoring systems.
Pfizer presented the early results from a study of 10,000 people who had all received two doses of its vaccine. Half of the study participants received a third shot, or booster. The other half got a placebo.
The study is ongoing, but so far, six of the people in the booster group have gotten a COVID-19 infection with symptoms compared to 123 people who got COVID-19 in the placebo group, making boosters 95% effective at keeping people from getting sick. Most people in the study had gotten their original doses about 10 months earlier. They’ve been followed for about 10 weeks since their booster. Importantly, there were no study participants hospitalized for COVID-19 infections in either the placebo or booster group, indicating that the first two doses were still very effective at preventing severe outcomes from infection.
The majority of side effects after a third Pfizer dose were mild and temporary. Side effects like sore arms, swelling, fever, headache, and fatigue were more common in the booster group — affecting about 1 in 4 people who got a third shot. Vaccination side effects were less common after boosters than have been seen after the second dose of the vaccine.
Some cases of myocarditis and pericarditis have been reported after people received vaccine boosters, but the risk for this heart inflammation appears to be extremely low, about two cases for every million doses given. There were 54 cases of myocarditis reported so far to the Vaccine Adverse Event Reporting System, or VAERS. So far, only 12 have met the case definition and are considered related to vaccination. Most of the reported cases are still being studied.
on its vaccine recommendations.
The Advisory Committee on Immunization Practices, or ACIP, recommended that all adults be eligible for a third dose of a Pfizer or Moderna mRNA vaccine, at least 6 months after their second dose.
They also strengthened a recommendation that everyone over the age of 50 should get a third dose, whether or not they have an underlying health condition that may increase their risk from a COVID-19 infection.
The committee voted 11 to 0 in favor of both policies.
CDC Director Rochelle Walensky, MD, must now sign off on both policies, which she is expected to do.
More than 70 million adults are now eligible for booster shots in the United States, but only about 31 million people have received one. About half of those who have been boosted are over the age of 65.
In a recent survey, the Kaiser Family Foundation found that about 4 in 10 younger adults said they were unsure if they qualified for a booster.
Under the current policy, boosters are recommended for everyone age 65 and older. But people who are younger than age 65 are eligible for boosters if they have an underlying health condition or live or work in a high-risk situation—something individuals have to determine on their own. Experts said that shading of the policy had created confusion that was holding people back.
Nirav Shah, MD, JD, president of the Association of State and Territorial Health Officials, noted that public health officials have been swamped with calls from people who are trying to figure out if they are eligible to get a booster dose.
He said that in a call the evening of Nov. 18 with state health departments, “There was not a single state that voiced opposition to this move,” he told the ACIP.
Dr. Shah said that the current guidelines were well intentioned, but “in pursuit of precision, they create confusion.”
“Our concern is that eligible individuals are not receiving boosters right now as a result of this confusion,” he said.
The committee based its decision on the results of a new study of boosters in Pfizer vaccine recipients, as well as reassuring safety information that’s being collected through the CDC and FDA’s monitoring systems.
Pfizer presented the early results from a study of 10,000 people who had all received two doses of its vaccine. Half of the study participants received a third shot, or booster. The other half got a placebo.
The study is ongoing, but so far, six of the people in the booster group have gotten a COVID-19 infection with symptoms compared to 123 people who got COVID-19 in the placebo group, making boosters 95% effective at keeping people from getting sick. Most people in the study had gotten their original doses about 10 months earlier. They’ve been followed for about 10 weeks since their booster. Importantly, there were no study participants hospitalized for COVID-19 infections in either the placebo or booster group, indicating that the first two doses were still very effective at preventing severe outcomes from infection.
The majority of side effects after a third Pfizer dose were mild and temporary. Side effects like sore arms, swelling, fever, headache, and fatigue were more common in the booster group — affecting about 1 in 4 people who got a third shot. Vaccination side effects were less common after boosters than have been seen after the second dose of the vaccine.
Some cases of myocarditis and pericarditis have been reported after people received vaccine boosters, but the risk for this heart inflammation appears to be extremely low, about two cases for every million doses given. There were 54 cases of myocarditis reported so far to the Vaccine Adverse Event Reporting System, or VAERS. So far, only 12 have met the case definition and are considered related to vaccination. Most of the reported cases are still being studied.
on its vaccine recommendations.
The Advisory Committee on Immunization Practices, or ACIP, recommended that all adults be eligible for a third dose of a Pfizer or Moderna mRNA vaccine, at least 6 months after their second dose.
They also strengthened a recommendation that everyone over the age of 50 should get a third dose, whether or not they have an underlying health condition that may increase their risk from a COVID-19 infection.
The committee voted 11 to 0 in favor of both policies.
CDC Director Rochelle Walensky, MD, must now sign off on both policies, which she is expected to do.
More than 70 million adults are now eligible for booster shots in the United States, but only about 31 million people have received one. About half of those who have been boosted are over the age of 65.
In a recent survey, the Kaiser Family Foundation found that about 4 in 10 younger adults said they were unsure if they qualified for a booster.
Under the current policy, boosters are recommended for everyone age 65 and older. But people who are younger than age 65 are eligible for boosters if they have an underlying health condition or live or work in a high-risk situation—something individuals have to determine on their own. Experts said that shading of the policy had created confusion that was holding people back.
Nirav Shah, MD, JD, president of the Association of State and Territorial Health Officials, noted that public health officials have been swamped with calls from people who are trying to figure out if they are eligible to get a booster dose.
He said that in a call the evening of Nov. 18 with state health departments, “There was not a single state that voiced opposition to this move,” he told the ACIP.
Dr. Shah said that the current guidelines were well intentioned, but “in pursuit of precision, they create confusion.”
“Our concern is that eligible individuals are not receiving boosters right now as a result of this confusion,” he said.
The committee based its decision on the results of a new study of boosters in Pfizer vaccine recipients, as well as reassuring safety information that’s being collected through the CDC and FDA’s monitoring systems.
Pfizer presented the early results from a study of 10,000 people who had all received two doses of its vaccine. Half of the study participants received a third shot, or booster. The other half got a placebo.
The study is ongoing, but so far, six of the people in the booster group have gotten a COVID-19 infection with symptoms compared to 123 people who got COVID-19 in the placebo group, making boosters 95% effective at keeping people from getting sick. Most people in the study had gotten their original doses about 10 months earlier. They’ve been followed for about 10 weeks since their booster. Importantly, there were no study participants hospitalized for COVID-19 infections in either the placebo or booster group, indicating that the first two doses were still very effective at preventing severe outcomes from infection.
The majority of side effects after a third Pfizer dose were mild and temporary. Side effects like sore arms, swelling, fever, headache, and fatigue were more common in the booster group — affecting about 1 in 4 people who got a third shot. Vaccination side effects were less common after boosters than have been seen after the second dose of the vaccine.
Some cases of myocarditis and pericarditis have been reported after people received vaccine boosters, but the risk for this heart inflammation appears to be extremely low, about two cases for every million doses given. There were 54 cases of myocarditis reported so far to the Vaccine Adverse Event Reporting System, or VAERS. So far, only 12 have met the case definition and are considered related to vaccination. Most of the reported cases are still being studied.
Black young adults: Remember this when facing discrimination
Joel Bervell recalls leaving his hometown of Seattle for the East Coast after being accepted into Yale University.
Still getting accustomed to the big move, Mr. Bervell, who had breezed through high school with straight As, went to see his chemistry professor for advice after getting a low grade on a test.
“He took one look at me and said, ‘Oh, if you’re on the football team, you don’t need to worry about it. So many people from the football team come into the class and end up dropping out, so if you need to drop this class, you can,’ ” Mr. Bervell says.
Mr. Bervell, who is Black, was not on the football team, nor did he receive a sports scholarship of any kind.
“For that professor to make an assumption of me, which to me felt like it was based on my race, made me less likely to want to go into a science field, where I felt like I was being judged before I even had a chance to prove myself,” Mr. Bervell says.
.
Researchers studied health data on 1,834 Americans ages 18-28 over a 10-year span. Findings show that the more instances of discrimination they experienced – including ageism, sexism, and racism – the more likely they were to face mental and behavioral struggles, like mental illness, drug use, severe psychological distress, and poor overall health.
Mr. Bervell, now 26, says he feels lucky that growing up, he was taught healthy ways to process his feelings and emotions.
“Instead of taking that and internalizing it, I said, ‘how can I use this to prove him wrong?’” he says. “Does that mean I need to work harder or does that mean I need to find a different mentor? Surround myself with different people?”
Mr. Bervell is currently a 3rd-year medical student at Washington State University.
When he’s not at the hospital seeing patients, you can find him educating his nearly 340,000 TikTok followers on topics like racial bias in medicine.
Acknowledge the impact
Most Black people don’t tie psychological distress to acts of racism, according to Rheeda Walker, PhD, psychology professor at the University of Houston and author of “The Unapologetic Guide to Black Mental Health” (Oakland, Calif.: New Harbinger Publications, 2020).
Many Black people even normalize it.
“Individuals deal with it [racism] as just another thing, like paying bills, going to work, and studying for class and not as the overwhelming psychological burden that it is,” says Dr. Walker.
And despite what some may say, racial discrimination is not merely “a thing of the past,” Dr. Walker says.
“Instead, discrimination has shifted form from more overt forms of discrimination to less obvious microaggression,” she says.
It’s also critical that young adults are taught how to deal with racism to avoid the risk of “internalizing that they deserve to be mistreated, and/or that they have to work twice as hard to overcome racism,” says Dr. Walker.
“Both scenarios can escalate hopelessness and worry, psychological features of depression and anxiety, respectively,” Dr. Walker says.
Embrace your emotions
Known around the office as “a big teddy bear,” Frederick Herman, a mortgage loan originator based in Charlotte, Va., was coaching a newer employee on how to make sales calls, a common practice in his line of work.
He says a day or 2 days later, his manager let him know that he had made an employee “very uncomfortable” by intimidating them while they were on the phone. Mr. Herman, 29, was told to watch his “aggressive” behavior.
“I’m a bigger Black man. I’m like 6’2, 300 lbs., somewhat muscular. So, if me talking or trying to coach her came off as intimidating, then there’s nothing that I could do or say differently than I was already doing to make her not feel intimidated,” Mr. Herman says.
“If a big teddy bear is now intimidating to you, that just tells me everything I need to know.”
This wasn’t the first time Mr. Herman had been reprimanded for being “too aggressive” or “showing off” when trying to help colleagues at work.
“I’ve had other experiences at work where I may not share my ideas, or I may get super anxious,” says Mr. Herman, a Black man of Haitian descent.
It’s important to allow yourself to feel your emotions after facing acts of discrimination, says Ebony Butler, PhD, a licensed psychologist and creator of My Therapy Cards, a card deck tailored for men, women, and teens of color, with self-care and reflection prompts.
This is a practice called “self-validation” and can reduce the tendency to blame oneself for the mistreatment, says Dr. Butler.
Mr. Herman, 29, says that he recently signed up for therapy to work through his struggles with anxiety.
Relaxation techniques, like grounding and mindfulness, can also be helpful, says Dr. Butler.
“Some example ways to practice grounding are immersing oneself in nature, walking bare feet on the ground, lying on the floor, practicing slow, deep breathing, or engaging the senses,” she says.
“When we are grounded and present, we can better manage our responses and plan our action steps.”
Utilize unique
If you find yourself in a racially charged school or workplace setting, don’t be intimidated, says Wendy Osefo, PhD, education professor at Johns Hopkins University, Baltimore, political commentator, and television personality.
Dr. Osefo made history in 2016 as the first Black woman to earn a PhD in public affairs/community development from Rutgers University.
“Your attitude should be that, no matter how different you might be, you belong, and you earned the right to occupy this space. You’re not less qualified than others who surround you,” she says.
Dr. Ofeso is also CEO of The 1954 Equity Project, an organization that gives minority students tools to succeed in higher education – like mentorships, peer support groups, and other resources and services – all while remaining their authentic selves.
No matter how uncomfortable it might be, staying true to who you are vs. conforming to the masses pays off, says Dr. Osefo.
“Being different is unique and allows you to bring a new and fresh perspective into an environment,” she says.
“Leaning into this uniqueness builds a level of confidence that will aid in your ability to be successful.”
A version of this article first appeared on WebMD.com.
Joel Bervell recalls leaving his hometown of Seattle for the East Coast after being accepted into Yale University.
Still getting accustomed to the big move, Mr. Bervell, who had breezed through high school with straight As, went to see his chemistry professor for advice after getting a low grade on a test.
“He took one look at me and said, ‘Oh, if you’re on the football team, you don’t need to worry about it. So many people from the football team come into the class and end up dropping out, so if you need to drop this class, you can,’ ” Mr. Bervell says.
Mr. Bervell, who is Black, was not on the football team, nor did he receive a sports scholarship of any kind.
“For that professor to make an assumption of me, which to me felt like it was based on my race, made me less likely to want to go into a science field, where I felt like I was being judged before I even had a chance to prove myself,” Mr. Bervell says.
.
Researchers studied health data on 1,834 Americans ages 18-28 over a 10-year span. Findings show that the more instances of discrimination they experienced – including ageism, sexism, and racism – the more likely they were to face mental and behavioral struggles, like mental illness, drug use, severe psychological distress, and poor overall health.
Mr. Bervell, now 26, says he feels lucky that growing up, he was taught healthy ways to process his feelings and emotions.
“Instead of taking that and internalizing it, I said, ‘how can I use this to prove him wrong?’” he says. “Does that mean I need to work harder or does that mean I need to find a different mentor? Surround myself with different people?”
Mr. Bervell is currently a 3rd-year medical student at Washington State University.
When he’s not at the hospital seeing patients, you can find him educating his nearly 340,000 TikTok followers on topics like racial bias in medicine.
Acknowledge the impact
Most Black people don’t tie psychological distress to acts of racism, according to Rheeda Walker, PhD, psychology professor at the University of Houston and author of “The Unapologetic Guide to Black Mental Health” (Oakland, Calif.: New Harbinger Publications, 2020).
Many Black people even normalize it.
“Individuals deal with it [racism] as just another thing, like paying bills, going to work, and studying for class and not as the overwhelming psychological burden that it is,” says Dr. Walker.
And despite what some may say, racial discrimination is not merely “a thing of the past,” Dr. Walker says.
“Instead, discrimination has shifted form from more overt forms of discrimination to less obvious microaggression,” she says.
It’s also critical that young adults are taught how to deal with racism to avoid the risk of “internalizing that they deserve to be mistreated, and/or that they have to work twice as hard to overcome racism,” says Dr. Walker.
“Both scenarios can escalate hopelessness and worry, psychological features of depression and anxiety, respectively,” Dr. Walker says.
Embrace your emotions
Known around the office as “a big teddy bear,” Frederick Herman, a mortgage loan originator based in Charlotte, Va., was coaching a newer employee on how to make sales calls, a common practice in his line of work.
He says a day or 2 days later, his manager let him know that he had made an employee “very uncomfortable” by intimidating them while they were on the phone. Mr. Herman, 29, was told to watch his “aggressive” behavior.
“I’m a bigger Black man. I’m like 6’2, 300 lbs., somewhat muscular. So, if me talking or trying to coach her came off as intimidating, then there’s nothing that I could do or say differently than I was already doing to make her not feel intimidated,” Mr. Herman says.
“If a big teddy bear is now intimidating to you, that just tells me everything I need to know.”
This wasn’t the first time Mr. Herman had been reprimanded for being “too aggressive” or “showing off” when trying to help colleagues at work.
“I’ve had other experiences at work where I may not share my ideas, or I may get super anxious,” says Mr. Herman, a Black man of Haitian descent.
It’s important to allow yourself to feel your emotions after facing acts of discrimination, says Ebony Butler, PhD, a licensed psychologist and creator of My Therapy Cards, a card deck tailored for men, women, and teens of color, with self-care and reflection prompts.
This is a practice called “self-validation” and can reduce the tendency to blame oneself for the mistreatment, says Dr. Butler.
Mr. Herman, 29, says that he recently signed up for therapy to work through his struggles with anxiety.
Relaxation techniques, like grounding and mindfulness, can also be helpful, says Dr. Butler.
“Some example ways to practice grounding are immersing oneself in nature, walking bare feet on the ground, lying on the floor, practicing slow, deep breathing, or engaging the senses,” she says.
“When we are grounded and present, we can better manage our responses and plan our action steps.”
Utilize unique
If you find yourself in a racially charged school or workplace setting, don’t be intimidated, says Wendy Osefo, PhD, education professor at Johns Hopkins University, Baltimore, political commentator, and television personality.
Dr. Osefo made history in 2016 as the first Black woman to earn a PhD in public affairs/community development from Rutgers University.
“Your attitude should be that, no matter how different you might be, you belong, and you earned the right to occupy this space. You’re not less qualified than others who surround you,” she says.
Dr. Ofeso is also CEO of The 1954 Equity Project, an organization that gives minority students tools to succeed in higher education – like mentorships, peer support groups, and other resources and services – all while remaining their authentic selves.
No matter how uncomfortable it might be, staying true to who you are vs. conforming to the masses pays off, says Dr. Osefo.
“Being different is unique and allows you to bring a new and fresh perspective into an environment,” she says.
“Leaning into this uniqueness builds a level of confidence that will aid in your ability to be successful.”
A version of this article first appeared on WebMD.com.
Joel Bervell recalls leaving his hometown of Seattle for the East Coast after being accepted into Yale University.
Still getting accustomed to the big move, Mr. Bervell, who had breezed through high school with straight As, went to see his chemistry professor for advice after getting a low grade on a test.
“He took one look at me and said, ‘Oh, if you’re on the football team, you don’t need to worry about it. So many people from the football team come into the class and end up dropping out, so if you need to drop this class, you can,’ ” Mr. Bervell says.
Mr. Bervell, who is Black, was not on the football team, nor did he receive a sports scholarship of any kind.
“For that professor to make an assumption of me, which to me felt like it was based on my race, made me less likely to want to go into a science field, where I felt like I was being judged before I even had a chance to prove myself,” Mr. Bervell says.
.
Researchers studied health data on 1,834 Americans ages 18-28 over a 10-year span. Findings show that the more instances of discrimination they experienced – including ageism, sexism, and racism – the more likely they were to face mental and behavioral struggles, like mental illness, drug use, severe psychological distress, and poor overall health.
Mr. Bervell, now 26, says he feels lucky that growing up, he was taught healthy ways to process his feelings and emotions.
“Instead of taking that and internalizing it, I said, ‘how can I use this to prove him wrong?’” he says. “Does that mean I need to work harder or does that mean I need to find a different mentor? Surround myself with different people?”
Mr. Bervell is currently a 3rd-year medical student at Washington State University.
When he’s not at the hospital seeing patients, you can find him educating his nearly 340,000 TikTok followers on topics like racial bias in medicine.
Acknowledge the impact
Most Black people don’t tie psychological distress to acts of racism, according to Rheeda Walker, PhD, psychology professor at the University of Houston and author of “The Unapologetic Guide to Black Mental Health” (Oakland, Calif.: New Harbinger Publications, 2020).
Many Black people even normalize it.
“Individuals deal with it [racism] as just another thing, like paying bills, going to work, and studying for class and not as the overwhelming psychological burden that it is,” says Dr. Walker.
And despite what some may say, racial discrimination is not merely “a thing of the past,” Dr. Walker says.
“Instead, discrimination has shifted form from more overt forms of discrimination to less obvious microaggression,” she says.
It’s also critical that young adults are taught how to deal with racism to avoid the risk of “internalizing that they deserve to be mistreated, and/or that they have to work twice as hard to overcome racism,” says Dr. Walker.
“Both scenarios can escalate hopelessness and worry, psychological features of depression and anxiety, respectively,” Dr. Walker says.
Embrace your emotions
Known around the office as “a big teddy bear,” Frederick Herman, a mortgage loan originator based in Charlotte, Va., was coaching a newer employee on how to make sales calls, a common practice in his line of work.
He says a day or 2 days later, his manager let him know that he had made an employee “very uncomfortable” by intimidating them while they were on the phone. Mr. Herman, 29, was told to watch his “aggressive” behavior.
“I’m a bigger Black man. I’m like 6’2, 300 lbs., somewhat muscular. So, if me talking or trying to coach her came off as intimidating, then there’s nothing that I could do or say differently than I was already doing to make her not feel intimidated,” Mr. Herman says.
“If a big teddy bear is now intimidating to you, that just tells me everything I need to know.”
This wasn’t the first time Mr. Herman had been reprimanded for being “too aggressive” or “showing off” when trying to help colleagues at work.
“I’ve had other experiences at work where I may not share my ideas, or I may get super anxious,” says Mr. Herman, a Black man of Haitian descent.
It’s important to allow yourself to feel your emotions after facing acts of discrimination, says Ebony Butler, PhD, a licensed psychologist and creator of My Therapy Cards, a card deck tailored for men, women, and teens of color, with self-care and reflection prompts.
This is a practice called “self-validation” and can reduce the tendency to blame oneself for the mistreatment, says Dr. Butler.
Mr. Herman, 29, says that he recently signed up for therapy to work through his struggles with anxiety.
Relaxation techniques, like grounding and mindfulness, can also be helpful, says Dr. Butler.
“Some example ways to practice grounding are immersing oneself in nature, walking bare feet on the ground, lying on the floor, practicing slow, deep breathing, or engaging the senses,” she says.
“When we are grounded and present, we can better manage our responses and plan our action steps.”
Utilize unique
If you find yourself in a racially charged school or workplace setting, don’t be intimidated, says Wendy Osefo, PhD, education professor at Johns Hopkins University, Baltimore, political commentator, and television personality.
Dr. Osefo made history in 2016 as the first Black woman to earn a PhD in public affairs/community development from Rutgers University.
“Your attitude should be that, no matter how different you might be, you belong, and you earned the right to occupy this space. You’re not less qualified than others who surround you,” she says.
Dr. Ofeso is also CEO of The 1954 Equity Project, an organization that gives minority students tools to succeed in higher education – like mentorships, peer support groups, and other resources and services – all while remaining their authentic selves.
No matter how uncomfortable it might be, staying true to who you are vs. conforming to the masses pays off, says Dr. Osefo.
“Being different is unique and allows you to bring a new and fresh perspective into an environment,” she says.
“Leaning into this uniqueness builds a level of confidence that will aid in your ability to be successful.”
A version of this article first appeared on WebMD.com.
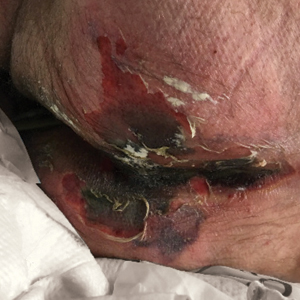
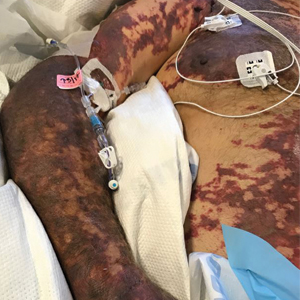
Serious infection hospitalizations have declined in patients with PsA
The rate of U.S. hospitalizations for three types of serious infections in patients with psoriatic arthritis (PsA) appears to have declined from 2012 to 2017, according to research presented at the virtual annual meeting of the American College of Rheumatology.
Several of the standard treatments for PsA have an increased risk of infections, but the rates vary amongst conventional disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, biologics, and other therapies.
“Given the uptake of biological therapies has increased over recent years, we sought to investigate the national trends in serious infections in patients with psoriatic arthritis from the years 2012 to 2017,” Vagishwari Murugesan, MBBS, a psoriatic arthritis clinical fellow at the University of Toronto, told attendees in a prerecorded poster presentation. Dr. Murugesan was a fellow at Boston University when she conducted the research.
The researchers analyzed data from 2012 to 2017 in the U.S. National Inpatient Sample (NIS), which includes approximately 20% of all discharges from U.S. community hospitals except rehabilitation and long-term acute care institutions. Using ICD-9 and ICD-10 codes, the researchers identified all discharge records containing a diagnosis of PsA as well as pneumonia, sepsis, urinary tract infection (UTI), and skin and soft-tissue infections. After making adjustments to match U.S. population age distributions over the years, they examined trends in serious infections among patients with PsA for that 6-year period.
Demographics over those years changed little: The average age of discharged patients was 59.5 in 2012 and 60.8 in 2017. Similarly, the patient population was 56% women and 88.5% Whites in 2012 and 57.7% women and 88.4% Whites in 2017. The average length of stay was also similar: 4.7 days in 2012, compared with 4.9 days in 2017.
Among 50,700 discharges of patients with PsA in 2012, the researchers identified 125 with pneumonia, 230 with sepsis, 312 with skin and soft-tissue infections, and 174 with a UTI. Among the 179,400 discharges in 2017 of patients with PsA, 344 had pneumonia, 374 had sepsis, 681 had skin and soft-tissue infections, and 348 had a UTI. After statistical analysis, the researchers found no significant differences in pneumonia diagnoses during the years studied, but they did find a statistically significant decline in sepsis, skin and soft tissue infections, and UTI discharges (P < .001).
A notable limitation of the study is the NIS database’s lack of data on treatments or outpatient data, making it impossible to determine if more infections were occurring but simply being treated in outpatient settings, although it’s not clear why such a substantial shift would occur in just 5 years. It’s also possible that coding practices differ across hospital, but, presumably, the ways they might differ in 2012 would be similar to any differences in 2017.
Arthur Kavanaugh, MD, a professor of medicine and director of the Center for Innovative Therapy at the University of California, San Diego, found the results interesting for what he considers an important topic.
“What makes these data interesting is the same thing that limits their reliability: The authors note that infections decreased ‘despite the increase in use of biologics over this time,’ ” Dr. Kavanaugh said in an interview. “These are claims data, so there is no way to support any association between those serious infections and biologic use. Indeed, multiple factors could have also impacted these data. It is not possible to tell from claims data.”
Dr. Kavanaugh said the question is worth investigating further with data from other sources.
The research was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One study coauthor reported ties to UCB; Dr. Murugesan and her other coauthors reported no disclosures. Dr. Kavanaugh had no disclosures.
The rate of U.S. hospitalizations for three types of serious infections in patients with psoriatic arthritis (PsA) appears to have declined from 2012 to 2017, according to research presented at the virtual annual meeting of the American College of Rheumatology.
Several of the standard treatments for PsA have an increased risk of infections, but the rates vary amongst conventional disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, biologics, and other therapies.
“Given the uptake of biological therapies has increased over recent years, we sought to investigate the national trends in serious infections in patients with psoriatic arthritis from the years 2012 to 2017,” Vagishwari Murugesan, MBBS, a psoriatic arthritis clinical fellow at the University of Toronto, told attendees in a prerecorded poster presentation. Dr. Murugesan was a fellow at Boston University when she conducted the research.
The researchers analyzed data from 2012 to 2017 in the U.S. National Inpatient Sample (NIS), which includes approximately 20% of all discharges from U.S. community hospitals except rehabilitation and long-term acute care institutions. Using ICD-9 and ICD-10 codes, the researchers identified all discharge records containing a diagnosis of PsA as well as pneumonia, sepsis, urinary tract infection (UTI), and skin and soft-tissue infections. After making adjustments to match U.S. population age distributions over the years, they examined trends in serious infections among patients with PsA for that 6-year period.
Demographics over those years changed little: The average age of discharged patients was 59.5 in 2012 and 60.8 in 2017. Similarly, the patient population was 56% women and 88.5% Whites in 2012 and 57.7% women and 88.4% Whites in 2017. The average length of stay was also similar: 4.7 days in 2012, compared with 4.9 days in 2017.
Among 50,700 discharges of patients with PsA in 2012, the researchers identified 125 with pneumonia, 230 with sepsis, 312 with skin and soft-tissue infections, and 174 with a UTI. Among the 179,400 discharges in 2017 of patients with PsA, 344 had pneumonia, 374 had sepsis, 681 had skin and soft-tissue infections, and 348 had a UTI. After statistical analysis, the researchers found no significant differences in pneumonia diagnoses during the years studied, but they did find a statistically significant decline in sepsis, skin and soft tissue infections, and UTI discharges (P < .001).
A notable limitation of the study is the NIS database’s lack of data on treatments or outpatient data, making it impossible to determine if more infections were occurring but simply being treated in outpatient settings, although it’s not clear why such a substantial shift would occur in just 5 years. It’s also possible that coding practices differ across hospital, but, presumably, the ways they might differ in 2012 would be similar to any differences in 2017.
Arthur Kavanaugh, MD, a professor of medicine and director of the Center for Innovative Therapy at the University of California, San Diego, found the results interesting for what he considers an important topic.
“What makes these data interesting is the same thing that limits their reliability: The authors note that infections decreased ‘despite the increase in use of biologics over this time,’ ” Dr. Kavanaugh said in an interview. “These are claims data, so there is no way to support any association between those serious infections and biologic use. Indeed, multiple factors could have also impacted these data. It is not possible to tell from claims data.”
Dr. Kavanaugh said the question is worth investigating further with data from other sources.
The research was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One study coauthor reported ties to UCB; Dr. Murugesan and her other coauthors reported no disclosures. Dr. Kavanaugh had no disclosures.
The rate of U.S. hospitalizations for three types of serious infections in patients with psoriatic arthritis (PsA) appears to have declined from 2012 to 2017, according to research presented at the virtual annual meeting of the American College of Rheumatology.
Several of the standard treatments for PsA have an increased risk of infections, but the rates vary amongst conventional disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, biologics, and other therapies.
“Given the uptake of biological therapies has increased over recent years, we sought to investigate the national trends in serious infections in patients with psoriatic arthritis from the years 2012 to 2017,” Vagishwari Murugesan, MBBS, a psoriatic arthritis clinical fellow at the University of Toronto, told attendees in a prerecorded poster presentation. Dr. Murugesan was a fellow at Boston University when she conducted the research.
The researchers analyzed data from 2012 to 2017 in the U.S. National Inpatient Sample (NIS), which includes approximately 20% of all discharges from U.S. community hospitals except rehabilitation and long-term acute care institutions. Using ICD-9 and ICD-10 codes, the researchers identified all discharge records containing a diagnosis of PsA as well as pneumonia, sepsis, urinary tract infection (UTI), and skin and soft-tissue infections. After making adjustments to match U.S. population age distributions over the years, they examined trends in serious infections among patients with PsA for that 6-year period.
Demographics over those years changed little: The average age of discharged patients was 59.5 in 2012 and 60.8 in 2017. Similarly, the patient population was 56% women and 88.5% Whites in 2012 and 57.7% women and 88.4% Whites in 2017. The average length of stay was also similar: 4.7 days in 2012, compared with 4.9 days in 2017.
Among 50,700 discharges of patients with PsA in 2012, the researchers identified 125 with pneumonia, 230 with sepsis, 312 with skin and soft-tissue infections, and 174 with a UTI. Among the 179,400 discharges in 2017 of patients with PsA, 344 had pneumonia, 374 had sepsis, 681 had skin and soft-tissue infections, and 348 had a UTI. After statistical analysis, the researchers found no significant differences in pneumonia diagnoses during the years studied, but they did find a statistically significant decline in sepsis, skin and soft tissue infections, and UTI discharges (P < .001).
A notable limitation of the study is the NIS database’s lack of data on treatments or outpatient data, making it impossible to determine if more infections were occurring but simply being treated in outpatient settings, although it’s not clear why such a substantial shift would occur in just 5 years. It’s also possible that coding practices differ across hospital, but, presumably, the ways they might differ in 2012 would be similar to any differences in 2017.
Arthur Kavanaugh, MD, a professor of medicine and director of the Center for Innovative Therapy at the University of California, San Diego, found the results interesting for what he considers an important topic.
“What makes these data interesting is the same thing that limits their reliability: The authors note that infections decreased ‘despite the increase in use of biologics over this time,’ ” Dr. Kavanaugh said in an interview. “These are claims data, so there is no way to support any association between those serious infections and biologic use. Indeed, multiple factors could have also impacted these data. It is not possible to tell from claims data.”
Dr. Kavanaugh said the question is worth investigating further with data from other sources.
The research was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One study coauthor reported ties to UCB; Dr. Murugesan and her other coauthors reported no disclosures. Dr. Kavanaugh had no disclosures.
FROM ACR 2021
CDC: Thirty percent of hospital workers in U.S. still unvaccinated
, according to a new survey by the Centers for Disease Control and Prevention.
The snapshot in time – Jan. 20, 2021 to Sept. 15, 2021 – is based on voluntary weekly reports from hospitals. Only about 48% of the 5,085 hospitals in the U.S. Health and Human Services department’s Unified Hospital Data Surveillance System reported data on vaccination coverage during the period, and, after validation checks, the study included reports from 2,086 facilities, or just 41% of all hospitals, covering 3.35 million workers.
Overall, the number who were fully vaccinated rose from 36.1% in Jan. 2021 to 60.2% in April 2021, and then crept slowly up to 70% by Sept. 15, the CDC researchers reported in the American Journal of Infection Control.
The slowdown among hospital workers seems to mirror the same decline as in the general population.
Arjun Srinivasan, MD, associate director for health care–associated infection prevention programs at the CDC, said the decline in part may be the result of misinformation.
Health care personnel “are not fully immune from vaccine misinformation,” he said, adding that such misinformation “is contributing to decreased vaccine uptake among non–health care personnel.”
“The take-home message is that there is a lot of work to do in health care settings in order to get all of our health care personnel vaccinated,” Dr. Srinivasan told this news organization. “We need them to be vaccinated to protect themselves. It is also really important that we as health care personnel get vaccinated to protect our patients.”
Vaccine mandates
The analysis shows that workers were more likely to be vaccinated if they worked at a children’s hospital (77%), lived in metropolitan counties (71%), or worked in a hospital with lower cumulative admissions of COVID-19 patients, or lower cumulative COVID-19 cases.
The odds of being fully vaccinated were lower if the surrounding community had lower vaccination coverage. Workers in non-metropolitan counties (63.3%) and in rural counties (65.1%) were also less likely to be fully vaccinated, as well as those who were in critical access hospitals (64%) or long-term acute care hospitals (68.8%).
Surveys have shown that health care personnel who are vaccine-hesitant cited concerns they had about vaccine efficacy, adverse effects, the speed of vaccine development, and lack of full Food and Drug Administration approval, the study authors noted. In addition, many reported low trust in the government.
A Medscape survey this past April found that 25% of health care workers said they did not plan to be fully vaccinated. Some 40% of the 9,349 workers who responded said that employers should never require a COVID-19 vaccine for clinicians.
But the Centers for Medicare & Medicaid Services is attempting to require all health care facilities that receive Medicare or Medicaid payment to vaccinate workers. All eligible staff must receive the first dose of a two-dose COVID-19 vaccine or a one-dose vaccine by Dec. 6, and a second dose by Jan. 4, 2022. The policy allows exemptions based on recognized medical conditions or religious beliefs.
Some hospitals and health systems and various states and cities have already begun implementing vaccine mandates. Northwell Health in New York, for instance, lost 1,400 workers (evenly split between clinical and nonclinical staff), or 2% of its 77,000 employees, as a result of the state’s mandate.
Northwell’s workforce is now considered 100% vaccinated, a hospital spokesman said in an interview. In addition, “we have allowed for team members who changed their minds and presented proof of vaccination to return,” said the spokesman, adding that “a couple of hundred employees have done just that.”
Ten states sued the Biden administration recently, aiming to stop the health care worker vaccine mandate. Other challenges to vaccine mandates have generally been unsuccessful. The U.S. Supreme Court, for example, in October declined to hear a challenge to Maine’s mandate for health care workers, even though it did not allow religious exemptions, according to the Washington Post.
“The courts seem to agree that health care personnel are different, and could be subject to these mandates,” said Dr. Srinivasan.
A version of this article first appeared on Medscape.com.
, according to a new survey by the Centers for Disease Control and Prevention.
The snapshot in time – Jan. 20, 2021 to Sept. 15, 2021 – is based on voluntary weekly reports from hospitals. Only about 48% of the 5,085 hospitals in the U.S. Health and Human Services department’s Unified Hospital Data Surveillance System reported data on vaccination coverage during the period, and, after validation checks, the study included reports from 2,086 facilities, or just 41% of all hospitals, covering 3.35 million workers.
Overall, the number who were fully vaccinated rose from 36.1% in Jan. 2021 to 60.2% in April 2021, and then crept slowly up to 70% by Sept. 15, the CDC researchers reported in the American Journal of Infection Control.
The slowdown among hospital workers seems to mirror the same decline as in the general population.
Arjun Srinivasan, MD, associate director for health care–associated infection prevention programs at the CDC, said the decline in part may be the result of misinformation.
Health care personnel “are not fully immune from vaccine misinformation,” he said, adding that such misinformation “is contributing to decreased vaccine uptake among non–health care personnel.”
“The take-home message is that there is a lot of work to do in health care settings in order to get all of our health care personnel vaccinated,” Dr. Srinivasan told this news organization. “We need them to be vaccinated to protect themselves. It is also really important that we as health care personnel get vaccinated to protect our patients.”
Vaccine mandates
The analysis shows that workers were more likely to be vaccinated if they worked at a children’s hospital (77%), lived in metropolitan counties (71%), or worked in a hospital with lower cumulative admissions of COVID-19 patients, or lower cumulative COVID-19 cases.
The odds of being fully vaccinated were lower if the surrounding community had lower vaccination coverage. Workers in non-metropolitan counties (63.3%) and in rural counties (65.1%) were also less likely to be fully vaccinated, as well as those who were in critical access hospitals (64%) or long-term acute care hospitals (68.8%).
Surveys have shown that health care personnel who are vaccine-hesitant cited concerns they had about vaccine efficacy, adverse effects, the speed of vaccine development, and lack of full Food and Drug Administration approval, the study authors noted. In addition, many reported low trust in the government.
A Medscape survey this past April found that 25% of health care workers said they did not plan to be fully vaccinated. Some 40% of the 9,349 workers who responded said that employers should never require a COVID-19 vaccine for clinicians.
But the Centers for Medicare & Medicaid Services is attempting to require all health care facilities that receive Medicare or Medicaid payment to vaccinate workers. All eligible staff must receive the first dose of a two-dose COVID-19 vaccine or a one-dose vaccine by Dec. 6, and a second dose by Jan. 4, 2022. The policy allows exemptions based on recognized medical conditions or religious beliefs.
Some hospitals and health systems and various states and cities have already begun implementing vaccine mandates. Northwell Health in New York, for instance, lost 1,400 workers (evenly split between clinical and nonclinical staff), or 2% of its 77,000 employees, as a result of the state’s mandate.
Northwell’s workforce is now considered 100% vaccinated, a hospital spokesman said in an interview. In addition, “we have allowed for team members who changed their minds and presented proof of vaccination to return,” said the spokesman, adding that “a couple of hundred employees have done just that.”
Ten states sued the Biden administration recently, aiming to stop the health care worker vaccine mandate. Other challenges to vaccine mandates have generally been unsuccessful. The U.S. Supreme Court, for example, in October declined to hear a challenge to Maine’s mandate for health care workers, even though it did not allow religious exemptions, according to the Washington Post.
“The courts seem to agree that health care personnel are different, and could be subject to these mandates,” said Dr. Srinivasan.
A version of this article first appeared on Medscape.com.
, according to a new survey by the Centers for Disease Control and Prevention.
The snapshot in time – Jan. 20, 2021 to Sept. 15, 2021 – is based on voluntary weekly reports from hospitals. Only about 48% of the 5,085 hospitals in the U.S. Health and Human Services department’s Unified Hospital Data Surveillance System reported data on vaccination coverage during the period, and, after validation checks, the study included reports from 2,086 facilities, or just 41% of all hospitals, covering 3.35 million workers.
Overall, the number who were fully vaccinated rose from 36.1% in Jan. 2021 to 60.2% in April 2021, and then crept slowly up to 70% by Sept. 15, the CDC researchers reported in the American Journal of Infection Control.
The slowdown among hospital workers seems to mirror the same decline as in the general population.
Arjun Srinivasan, MD, associate director for health care–associated infection prevention programs at the CDC, said the decline in part may be the result of misinformation.
Health care personnel “are not fully immune from vaccine misinformation,” he said, adding that such misinformation “is contributing to decreased vaccine uptake among non–health care personnel.”
“The take-home message is that there is a lot of work to do in health care settings in order to get all of our health care personnel vaccinated,” Dr. Srinivasan told this news organization. “We need them to be vaccinated to protect themselves. It is also really important that we as health care personnel get vaccinated to protect our patients.”
Vaccine mandates
The analysis shows that workers were more likely to be vaccinated if they worked at a children’s hospital (77%), lived in metropolitan counties (71%), or worked in a hospital with lower cumulative admissions of COVID-19 patients, or lower cumulative COVID-19 cases.
The odds of being fully vaccinated were lower if the surrounding community had lower vaccination coverage. Workers in non-metropolitan counties (63.3%) and in rural counties (65.1%) were also less likely to be fully vaccinated, as well as those who were in critical access hospitals (64%) or long-term acute care hospitals (68.8%).
Surveys have shown that health care personnel who are vaccine-hesitant cited concerns they had about vaccine efficacy, adverse effects, the speed of vaccine development, and lack of full Food and Drug Administration approval, the study authors noted. In addition, many reported low trust in the government.
A Medscape survey this past April found that 25% of health care workers said they did not plan to be fully vaccinated. Some 40% of the 9,349 workers who responded said that employers should never require a COVID-19 vaccine for clinicians.
But the Centers for Medicare & Medicaid Services is attempting to require all health care facilities that receive Medicare or Medicaid payment to vaccinate workers. All eligible staff must receive the first dose of a two-dose COVID-19 vaccine or a one-dose vaccine by Dec. 6, and a second dose by Jan. 4, 2022. The policy allows exemptions based on recognized medical conditions or religious beliefs.
Some hospitals and health systems and various states and cities have already begun implementing vaccine mandates. Northwell Health in New York, for instance, lost 1,400 workers (evenly split between clinical and nonclinical staff), or 2% of its 77,000 employees, as a result of the state’s mandate.
Northwell’s workforce is now considered 100% vaccinated, a hospital spokesman said in an interview. In addition, “we have allowed for team members who changed their minds and presented proof of vaccination to return,” said the spokesman, adding that “a couple of hundred employees have done just that.”
Ten states sued the Biden administration recently, aiming to stop the health care worker vaccine mandate. Other challenges to vaccine mandates have generally been unsuccessful. The U.S. Supreme Court, for example, in October declined to hear a challenge to Maine’s mandate for health care workers, even though it did not allow religious exemptions, according to the Washington Post.
“The courts seem to agree that health care personnel are different, and could be subject to these mandates,” said Dr. Srinivasan.
A version of this article first appeared on Medscape.com.
How to deal with offensive or impaired doctors
Knowing what to say and do can lead to a positive outcome for the physician involved and the organization.
Misbehaving and impaired physicians put their organizations at risk, which can lead to malpractice/patient injury lawsuits, labor law and harassment claims, and a damaged reputation through negative social media reviews, said Debra Phairas, MBA, president of Practice and Liability Consultants LLC, at the annual meeting of the Medical Group Management Association (MGMA) .
“Verbal harassment or bullying claims can result in large dollar awards against the organizations that knew about the behavior and did nothing to stop it. Organizations can be sued for that,” says Ms. Phairas.
She recalls a doctor who called a female doctor “an entitled bitch” and the administrator “incompetent” in front of other staff. “He would pick on one department manager at every meeting and humiliate them in front of the others,” says Ms. Phairas.
After working with a human resources (HR) attorney and conducting independent reviews, they used a strategy Ms. Phairas calls her “3 C’s” for dealing with disruptive doctors.
Confront, correct, and/or counsel
The three C’s can work individually or together, depending on the doctor’s situation. Confronting a physician can start with an informal discussion; correcting can involve seeking a written apology that directly addresses the problem or sending a letter of admonition; and coaching or counseling can be offered. If the doctor resists those efforts, practice administrators can issue a final letter of warning and then suspend or terminate the physician, says Ms. Phairas.
Sometimes having a conversation with a disruptive doctor about the risks and consequences is enough to change the offending behavior, says Ms. Phairas.
She recalled being asked by a medical group to meet with a physician who she says was “snapping the bra straps of medical assistants in the hall — everyone there was horrified. I told him that’s not appropriate, that he was placing everyone at risk and they will terminate him if he didn’t stop. I asked for his commitment to stop, and he agreed,” says Ms. Phairas.
She also recommends implementing these strategies to prevent and deal with disruptive physicians:
- Implement a code of conduct and share it during interviews;
- Have zero tolerance policies and procedures for documenting behavior;
- Get advice from a good employment attorney;
- Implement written performance improvement plans;
- Provide resources to change the behavior;
- Follow through with suspension and termination; and
- Add to shareholder agreements a clause stating that partners/shareholders can gently ask or insist that the physician obtain counseling or help.
Getting impaired doctors help
Doctors can be impaired through substance abuse, a serious medical illness, mental illness, or age-related deterioration.
Life events such as divorce or the death of a spouse, child, or a physician partner can affect a doctor’s mental health. “In those cases, you need to have the courage to say you’re really depressed and we all agree you need to get help,” says Ms. Phairas.
She recalls one occasion in which a practice administration staff member could not locate a doctor whose patients were waiting to be seen. “He was so devastated from his divorce that he had crawled into a ball beneath his desk. She had to coax him out and tell him that they were worried about him and he needed to get help.”
Another reason doctors may not be performing well may be because of an undiagnosed medical illness. Doctors in an orthopedic group were mad at another partner who had slowed down and couldn’t help pay the expenses. “They were ready to terminate him when he went to the doctor and learned he had colon cancer,” says Ms. Phairas.
Ms. Phairas recommends that practices update their partner shareholder agreements regularly with the following:
- Include “fit for duty” examinations, especially after age 65.
- Insist that a physician be evaluated by a doctor outside the practice. The doctor may be one that they agree upon or one chosen by the local medical society president.
- Include in the agreement the clause, “Partners and employees will be subject to review for impairment due to matters including but not limited to age-related, physical, or mental conditions.”
- Establish a voting mechanism for terminating a physician.
Aging doctors who won’t retire
Some doctors have retired early because of COVID, whereas others are staying on because they are feeling financial pressures — they lost a lot of money last year and need to make up for it, says Ms. Phairas.
She warned that administrators have to be careful in dealing with older doctors because of age discrimination laws.
Doctors may not notice they are declining mentally until it becomes a problem. Ms. Phairas recalls an internist senior partner who started behaving erratically when he was 78 years old. “He wrote himself a $25,000 check from the organization’s funds without telling his partners, left a patient he should have been watching and she fell over and sued the practice, and the staff started noticing that he was forgetting or not doing things,” says Ms. Phairas.
She sought guidance from a good HR attorney and involved a malpractice attorney. She then met with the senior partner. “I reminded him of his Hippocratic Oath that he took when he became a doctor and told him that his actions were harming patients. I pleaded with him that it was time to retire. He didn’t.”
Because this physician wouldn’t retire, the practice referred to their updated shareholder agreement, which stated that they could insist that the physician undergo a neuropsychiatric assessment from a certified specialist. He didn’t pass the evaluation, which then provided evidence of his declining cognitive skills.
“All the doctors, myself, and the HR attorney talked to him about this and laid out all the facts. It was hard to say these things, but he listened and left. We went through the termination process to protect the practice and avoid litigation. The malpractice insurer also refused to renew his policy,” says Ms. Phairas.
A version of this article first appeared on Medscape.com.
Knowing what to say and do can lead to a positive outcome for the physician involved and the organization.
Misbehaving and impaired physicians put their organizations at risk, which can lead to malpractice/patient injury lawsuits, labor law and harassment claims, and a damaged reputation through negative social media reviews, said Debra Phairas, MBA, president of Practice and Liability Consultants LLC, at the annual meeting of the Medical Group Management Association (MGMA) .
“Verbal harassment or bullying claims can result in large dollar awards against the organizations that knew about the behavior and did nothing to stop it. Organizations can be sued for that,” says Ms. Phairas.
She recalls a doctor who called a female doctor “an entitled bitch” and the administrator “incompetent” in front of other staff. “He would pick on one department manager at every meeting and humiliate them in front of the others,” says Ms. Phairas.
After working with a human resources (HR) attorney and conducting independent reviews, they used a strategy Ms. Phairas calls her “3 C’s” for dealing with disruptive doctors.
Confront, correct, and/or counsel
The three C’s can work individually or together, depending on the doctor’s situation. Confronting a physician can start with an informal discussion; correcting can involve seeking a written apology that directly addresses the problem or sending a letter of admonition; and coaching or counseling can be offered. If the doctor resists those efforts, practice administrators can issue a final letter of warning and then suspend or terminate the physician, says Ms. Phairas.
Sometimes having a conversation with a disruptive doctor about the risks and consequences is enough to change the offending behavior, says Ms. Phairas.
She recalled being asked by a medical group to meet with a physician who she says was “snapping the bra straps of medical assistants in the hall — everyone there was horrified. I told him that’s not appropriate, that he was placing everyone at risk and they will terminate him if he didn’t stop. I asked for his commitment to stop, and he agreed,” says Ms. Phairas.
She also recommends implementing these strategies to prevent and deal with disruptive physicians:
- Implement a code of conduct and share it during interviews;
- Have zero tolerance policies and procedures for documenting behavior;
- Get advice from a good employment attorney;
- Implement written performance improvement plans;
- Provide resources to change the behavior;
- Follow through with suspension and termination; and
- Add to shareholder agreements a clause stating that partners/shareholders can gently ask or insist that the physician obtain counseling or help.
Getting impaired doctors help
Doctors can be impaired through substance abuse, a serious medical illness, mental illness, or age-related deterioration.
Life events such as divorce or the death of a spouse, child, or a physician partner can affect a doctor’s mental health. “In those cases, you need to have the courage to say you’re really depressed and we all agree you need to get help,” says Ms. Phairas.
She recalls one occasion in which a practice administration staff member could not locate a doctor whose patients were waiting to be seen. “He was so devastated from his divorce that he had crawled into a ball beneath his desk. She had to coax him out and tell him that they were worried about him and he needed to get help.”
Another reason doctors may not be performing well may be because of an undiagnosed medical illness. Doctors in an orthopedic group were mad at another partner who had slowed down and couldn’t help pay the expenses. “They were ready to terminate him when he went to the doctor and learned he had colon cancer,” says Ms. Phairas.
Ms. Phairas recommends that practices update their partner shareholder agreements regularly with the following:
- Include “fit for duty” examinations, especially after age 65.
- Insist that a physician be evaluated by a doctor outside the practice. The doctor may be one that they agree upon or one chosen by the local medical society president.
- Include in the agreement the clause, “Partners and employees will be subject to review for impairment due to matters including but not limited to age-related, physical, or mental conditions.”
- Establish a voting mechanism for terminating a physician.
Aging doctors who won’t retire
Some doctors have retired early because of COVID, whereas others are staying on because they are feeling financial pressures — they lost a lot of money last year and need to make up for it, says Ms. Phairas.
She warned that administrators have to be careful in dealing with older doctors because of age discrimination laws.
Doctors may not notice they are declining mentally until it becomes a problem. Ms. Phairas recalls an internist senior partner who started behaving erratically when he was 78 years old. “He wrote himself a $25,000 check from the organization’s funds without telling his partners, left a patient he should have been watching and she fell over and sued the practice, and the staff started noticing that he was forgetting or not doing things,” says Ms. Phairas.
She sought guidance from a good HR attorney and involved a malpractice attorney. She then met with the senior partner. “I reminded him of his Hippocratic Oath that he took when he became a doctor and told him that his actions were harming patients. I pleaded with him that it was time to retire. He didn’t.”
Because this physician wouldn’t retire, the practice referred to their updated shareholder agreement, which stated that they could insist that the physician undergo a neuropsychiatric assessment from a certified specialist. He didn’t pass the evaluation, which then provided evidence of his declining cognitive skills.
“All the doctors, myself, and the HR attorney talked to him about this and laid out all the facts. It was hard to say these things, but he listened and left. We went through the termination process to protect the practice and avoid litigation. The malpractice insurer also refused to renew his policy,” says Ms. Phairas.
A version of this article first appeared on Medscape.com.
Knowing what to say and do can lead to a positive outcome for the physician involved and the organization.
Misbehaving and impaired physicians put their organizations at risk, which can lead to malpractice/patient injury lawsuits, labor law and harassment claims, and a damaged reputation through negative social media reviews, said Debra Phairas, MBA, president of Practice and Liability Consultants LLC, at the annual meeting of the Medical Group Management Association (MGMA) .
“Verbal harassment or bullying claims can result in large dollar awards against the organizations that knew about the behavior and did nothing to stop it. Organizations can be sued for that,” says Ms. Phairas.
She recalls a doctor who called a female doctor “an entitled bitch” and the administrator “incompetent” in front of other staff. “He would pick on one department manager at every meeting and humiliate them in front of the others,” says Ms. Phairas.
After working with a human resources (HR) attorney and conducting independent reviews, they used a strategy Ms. Phairas calls her “3 C’s” for dealing with disruptive doctors.
Confront, correct, and/or counsel
The three C’s can work individually or together, depending on the doctor’s situation. Confronting a physician can start with an informal discussion; correcting can involve seeking a written apology that directly addresses the problem or sending a letter of admonition; and coaching or counseling can be offered. If the doctor resists those efforts, practice administrators can issue a final letter of warning and then suspend or terminate the physician, says Ms. Phairas.
Sometimes having a conversation with a disruptive doctor about the risks and consequences is enough to change the offending behavior, says Ms. Phairas.
She recalled being asked by a medical group to meet with a physician who she says was “snapping the bra straps of medical assistants in the hall — everyone there was horrified. I told him that’s not appropriate, that he was placing everyone at risk and they will terminate him if he didn’t stop. I asked for his commitment to stop, and he agreed,” says Ms. Phairas.
She also recommends implementing these strategies to prevent and deal with disruptive physicians:
- Implement a code of conduct and share it during interviews;
- Have zero tolerance policies and procedures for documenting behavior;
- Get advice from a good employment attorney;
- Implement written performance improvement plans;
- Provide resources to change the behavior;
- Follow through with suspension and termination; and
- Add to shareholder agreements a clause stating that partners/shareholders can gently ask or insist that the physician obtain counseling or help.
Getting impaired doctors help
Doctors can be impaired through substance abuse, a serious medical illness, mental illness, or age-related deterioration.
Life events such as divorce or the death of a spouse, child, or a physician partner can affect a doctor’s mental health. “In those cases, you need to have the courage to say you’re really depressed and we all agree you need to get help,” says Ms. Phairas.
She recalls one occasion in which a practice administration staff member could not locate a doctor whose patients were waiting to be seen. “He was so devastated from his divorce that he had crawled into a ball beneath his desk. She had to coax him out and tell him that they were worried about him and he needed to get help.”
Another reason doctors may not be performing well may be because of an undiagnosed medical illness. Doctors in an orthopedic group were mad at another partner who had slowed down and couldn’t help pay the expenses. “They were ready to terminate him when he went to the doctor and learned he had colon cancer,” says Ms. Phairas.
Ms. Phairas recommends that practices update their partner shareholder agreements regularly with the following:
- Include “fit for duty” examinations, especially after age 65.
- Insist that a physician be evaluated by a doctor outside the practice. The doctor may be one that they agree upon or one chosen by the local medical society president.
- Include in the agreement the clause, “Partners and employees will be subject to review for impairment due to matters including but not limited to age-related, physical, or mental conditions.”
- Establish a voting mechanism for terminating a physician.
Aging doctors who won’t retire
Some doctors have retired early because of COVID, whereas others are staying on because they are feeling financial pressures — they lost a lot of money last year and need to make up for it, says Ms. Phairas.
She warned that administrators have to be careful in dealing with older doctors because of age discrimination laws.
Doctors may not notice they are declining mentally until it becomes a problem. Ms. Phairas recalls an internist senior partner who started behaving erratically when he was 78 years old. “He wrote himself a $25,000 check from the organization’s funds without telling his partners, left a patient he should have been watching and she fell over and sued the practice, and the staff started noticing that he was forgetting or not doing things,” says Ms. Phairas.
She sought guidance from a good HR attorney and involved a malpractice attorney. She then met with the senior partner. “I reminded him of his Hippocratic Oath that he took when he became a doctor and told him that his actions were harming patients. I pleaded with him that it was time to retire. He didn’t.”
Because this physician wouldn’t retire, the practice referred to their updated shareholder agreement, which stated that they could insist that the physician undergo a neuropsychiatric assessment from a certified specialist. He didn’t pass the evaluation, which then provided evidence of his declining cognitive skills.
“All the doctors, myself, and the HR attorney talked to him about this and laid out all the facts. It was hard to say these things, but he listened and left. We went through the termination process to protect the practice and avoid litigation. The malpractice insurer also refused to renew his policy,” says Ms. Phairas.
A version of this article first appeared on Medscape.com.
Mask-wearing cuts new COVID-19 cases by 53%, study says
Social distancing and handwashing were also effective at lowering the number of cases, but wearing masks was the most effective tool against the coronavirus.
“Personal and social measures, including handwashing, mask wearing, and physical distancing are effective at reducing the incidence of COVID-19,” the study authors wrote.
The research team, which included public health and infectious disease specialists in Australia, China, and the U.K., evaluated 72 studies of COVID-19 precautions during the pandemic. They later looked at eight studies that focused on handwashing, mask wearing, and physical distancing.
Among six studies that looked at mask wearing, the researchers found a 53% reduction in COVID-19 cases. In the broader analysis with additional studies, wearing a mask reduced coronavirus transmission, cases, and deaths.
In one study across 200 countries, mandatory mask wearing resulted in nearly 46% fewer negative outcomes from COVID-19. In another study in the U.S., coronavirus transmission was reduced 29% in states where masks were mandatory.
But the research team couldn’t analyze the impact of the type of face mask used, the frequency of mask wearing, or the overall compliance with wearing face masks.
Among five studies that looked at physical distancing, the researchers found a 25% reduction in the rate of COVID-19. A study in the U.S. showed a 12% decrease in coronavirus transmission, while another study in Iran reported a reduction in COVID-19 mortality.
Handwashing interventions also suggested a substantial reduction of COVID-19 cases up to 53%, the researchers wrote. But in adjusted models, the results weren’t statistically significant due to the small number of studies included.
Other studies found significant decreases related to other public health measures, such as quarantines, broad lockdowns, border closures, school closures, business closures, and travel restrictions. Still, the research team couldn’t analyze the overall effectiveness of these measures due to the different ways the studies were conducted.
The study lines up with other research conducted so far during the pandemic, the research team wrote, which indicates that wearing masks and physical distancing can reduce transmission, cases, and deaths.
That said, more studies are needed, particularly now that vaccinations are available and contagious coronavirus variants have become prevalent.
“Further research is needed to assess the effectiveness of public health measures after adequate vaccination coverage has been achieved,” they wrote.
“It is likely that further control of the COVID-19 pandemic depends not only on high vaccination coverage and its effectiveness but also on ongoing adherence to effective and sustainable public health measures,” they concluded.
A version of this article first appeared on WebMD.com.
Social distancing and handwashing were also effective at lowering the number of cases, but wearing masks was the most effective tool against the coronavirus.
“Personal and social measures, including handwashing, mask wearing, and physical distancing are effective at reducing the incidence of COVID-19,” the study authors wrote.
The research team, which included public health and infectious disease specialists in Australia, China, and the U.K., evaluated 72 studies of COVID-19 precautions during the pandemic. They later looked at eight studies that focused on handwashing, mask wearing, and physical distancing.
Among six studies that looked at mask wearing, the researchers found a 53% reduction in COVID-19 cases. In the broader analysis with additional studies, wearing a mask reduced coronavirus transmission, cases, and deaths.
In one study across 200 countries, mandatory mask wearing resulted in nearly 46% fewer negative outcomes from COVID-19. In another study in the U.S., coronavirus transmission was reduced 29% in states where masks were mandatory.
But the research team couldn’t analyze the impact of the type of face mask used, the frequency of mask wearing, or the overall compliance with wearing face masks.
Among five studies that looked at physical distancing, the researchers found a 25% reduction in the rate of COVID-19. A study in the U.S. showed a 12% decrease in coronavirus transmission, while another study in Iran reported a reduction in COVID-19 mortality.
Handwashing interventions also suggested a substantial reduction of COVID-19 cases up to 53%, the researchers wrote. But in adjusted models, the results weren’t statistically significant due to the small number of studies included.
Other studies found significant decreases related to other public health measures, such as quarantines, broad lockdowns, border closures, school closures, business closures, and travel restrictions. Still, the research team couldn’t analyze the overall effectiveness of these measures due to the different ways the studies were conducted.
The study lines up with other research conducted so far during the pandemic, the research team wrote, which indicates that wearing masks and physical distancing can reduce transmission, cases, and deaths.
That said, more studies are needed, particularly now that vaccinations are available and contagious coronavirus variants have become prevalent.
“Further research is needed to assess the effectiveness of public health measures after adequate vaccination coverage has been achieved,” they wrote.
“It is likely that further control of the COVID-19 pandemic depends not only on high vaccination coverage and its effectiveness but also on ongoing adherence to effective and sustainable public health measures,” they concluded.
A version of this article first appeared on WebMD.com.
Social distancing and handwashing were also effective at lowering the number of cases, but wearing masks was the most effective tool against the coronavirus.
“Personal and social measures, including handwashing, mask wearing, and physical distancing are effective at reducing the incidence of COVID-19,” the study authors wrote.
The research team, which included public health and infectious disease specialists in Australia, China, and the U.K., evaluated 72 studies of COVID-19 precautions during the pandemic. They later looked at eight studies that focused on handwashing, mask wearing, and physical distancing.
Among six studies that looked at mask wearing, the researchers found a 53% reduction in COVID-19 cases. In the broader analysis with additional studies, wearing a mask reduced coronavirus transmission, cases, and deaths.
In one study across 200 countries, mandatory mask wearing resulted in nearly 46% fewer negative outcomes from COVID-19. In another study in the U.S., coronavirus transmission was reduced 29% in states where masks were mandatory.
But the research team couldn’t analyze the impact of the type of face mask used, the frequency of mask wearing, or the overall compliance with wearing face masks.
Among five studies that looked at physical distancing, the researchers found a 25% reduction in the rate of COVID-19. A study in the U.S. showed a 12% decrease in coronavirus transmission, while another study in Iran reported a reduction in COVID-19 mortality.
Handwashing interventions also suggested a substantial reduction of COVID-19 cases up to 53%, the researchers wrote. But in adjusted models, the results weren’t statistically significant due to the small number of studies included.
Other studies found significant decreases related to other public health measures, such as quarantines, broad lockdowns, border closures, school closures, business closures, and travel restrictions. Still, the research team couldn’t analyze the overall effectiveness of these measures due to the different ways the studies were conducted.
The study lines up with other research conducted so far during the pandemic, the research team wrote, which indicates that wearing masks and physical distancing can reduce transmission, cases, and deaths.
That said, more studies are needed, particularly now that vaccinations are available and contagious coronavirus variants have become prevalent.
“Further research is needed to assess the effectiveness of public health measures after adequate vaccination coverage has been achieved,” they wrote.
“It is likely that further control of the COVID-19 pandemic depends not only on high vaccination coverage and its effectiveness but also on ongoing adherence to effective and sustainable public health measures,” they concluded.
A version of this article first appeared on WebMD.com.
FROM THE BMJ
Medical technology should keep patient in mind
Indeed, science and technology provide opportunities to improve outcomes in ways not even imagined 100 years ago, yet we must acknowledge that technology also threatens to erect barriers between us and our patients. We can be easily tempted to confuse new care delivery tools with the actual care itself.
Threats to the physician-patient relationship
Medical history provides many examples of how our zeal to innovate can have untoward consequences to the physician-patient relationship.
In the late 1800s, for example, to convey a sense of science, purity of intent, and trust, the medical community began wearing white coats. Those white coats have been discussed as creating emotional distance between physicians and their patients.1
Even when we in the medical community are slow and reluctant to change, the external forces propelling us forward often seem unstoppable; kinetic aspirations to innovate electronic information systems and new applications seem suddenly to revolutionize care delivery when we least expect it. The rapidity of change in technology can sometimes be dizzying but can at the same time can occur so swiftly we don’t even notice it.
After René Laennec invented the stethoscope in the early 1800s, clinicians no longer needed to physically lean in and place an ear directly onto patients to hear their hearts beating. This created a distance from patients that was still lamented 50 years later, when a professor of medicine is reported to have said, “he that hath ears to hear, let him use his ears and not a stethoscope.” Still, while the stethoscope has literally distanced us from patients, it is such an important tool that we no longer think about this distancing. We have adapted over time to remain close to our patients, to sincerely listen to their thoughts and reassure them that we hear them without the need to feel our ears on their chests.
Francis Peabody, the eminent Harvard physician, wrote an essay in 1927 titled, “The Care of the Patient.” At the end of the first paragraph, he states: “The most common criticism made at present by older practitioners is that young graduates ... are too “scientific” and do not know how to take care of patients.” He goes on to say that “one of the essential qualities of the clinician is interest in humanity, for the secret of the care of the patient is in caring for the patient.”2
We agree with Dr. Peabody. As we embrace science and technology that can change health outcomes, our patients’ needs to feel understood and cared for will not diminish. Instead, that need will continue to be an important aspect of our struggle and joy in providing holistic, humane, competent care into the future.
Twenty-first century physicians have access to an ever-growing trove of data, yet our ability to truly know our patients seems somehow less accessible. Home health devices have begun to provide a flow of information about parameters, ranging from continuous glucose readings to home blood pressures, weights, and inspiratory flow readings. These data can provide much more accurate insight into patients than what we can glean from one point in time during an office visit. Yet we need to remember that behind the data are people with dreams and desires, not just table entries in an electronic health record.
In 1923, the German philosopher Martin Buber published the book for which he is best known, “I and Thou.” In that book, Mr. Buber says that there are two ways we can approach relationships: “I-Thou” or “I-It.” In I-It relationships, we view the other person as an “it” to be used to accomplish a purpose, or to be experienced without his or her full involvement. In an I-Thou relationship, we appreciate the other people for all their complexity, in their full humanness. We must consciously remind ourselves amid the rush of technology that there are real people behind those data. We must acknowledge and approach each person as a unique individual who has dreams, goals, fears, and wishes that may be different from ours but to which we can still relate.
‘From the Beating End of the Stethoscope’
John Ciardi, an American poet, said the following in a poem titled, “Lines From the Beating End of the Stethoscope”:
I speak, as I say, the patient’s point of view.
But, given time, doctors are patients, too.
And there’s our bond: beyond anatomy,
Or in it, through it, to the mystery
Medicine takes the pulse of and lets go
Forever unexplained. It’s art, we know,
Not science at the heart. Doctor be whole,
I won’t insist the patient is a soul,
But he’s a something, possibly laughable,
Or possibly sublime, but not quite graphable.
Not quite containable on a bed chart.
Where science touches man it turns to art.3
This poem is a reminder of the subtle needs of patients during their encounters with doctors, especially around many of the most important decisions and events in their lives. Patients’ needs are varied, complex, difficult to discern, and not able to be fully explained or understood through math and science.
Einstein warned us that the modern age would be characterized by a perfection of means and a confusion of goals.4 As clinicians, we should strive to clarify and align our goals with those of our patients, providing care that is real, compassionate, and personal, not just an optimized means to achieve standardized metrics. While technology can assist us in this pursuit, we’ll need be careful that our enchantment with innovation does not cloud our actual goal: truly caring for our patients.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
References
1. Jones VA. The white coat: Why not follow suit? JAMA. 1999;281(5):478. doi: 10.1001/jama.281.5.478-JMS0203-5-1
2. Peabody, Francis (1927). “The care of the patient.” JAMA. 88(12):877-82. doi: 10.1001/jama.1927.02680380001001.
3. Ciardi, John. Lines from the Beating End of the Stethoscope. Saturday Review, Nov. 18, 1968.
4. Albert Einstein, Out of My Later Years, 1950.
Indeed, science and technology provide opportunities to improve outcomes in ways not even imagined 100 years ago, yet we must acknowledge that technology also threatens to erect barriers between us and our patients. We can be easily tempted to confuse new care delivery tools with the actual care itself.
Threats to the physician-patient relationship
Medical history provides many examples of how our zeal to innovate can have untoward consequences to the physician-patient relationship.
In the late 1800s, for example, to convey a sense of science, purity of intent, and trust, the medical community began wearing white coats. Those white coats have been discussed as creating emotional distance between physicians and their patients.1
Even when we in the medical community are slow and reluctant to change, the external forces propelling us forward often seem unstoppable; kinetic aspirations to innovate electronic information systems and new applications seem suddenly to revolutionize care delivery when we least expect it. The rapidity of change in technology can sometimes be dizzying but can at the same time can occur so swiftly we don’t even notice it.
After René Laennec invented the stethoscope in the early 1800s, clinicians no longer needed to physically lean in and place an ear directly onto patients to hear their hearts beating. This created a distance from patients that was still lamented 50 years later, when a professor of medicine is reported to have said, “he that hath ears to hear, let him use his ears and not a stethoscope.” Still, while the stethoscope has literally distanced us from patients, it is such an important tool that we no longer think about this distancing. We have adapted over time to remain close to our patients, to sincerely listen to their thoughts and reassure them that we hear them without the need to feel our ears on their chests.
Francis Peabody, the eminent Harvard physician, wrote an essay in 1927 titled, “The Care of the Patient.” At the end of the first paragraph, he states: “The most common criticism made at present by older practitioners is that young graduates ... are too “scientific” and do not know how to take care of patients.” He goes on to say that “one of the essential qualities of the clinician is interest in humanity, for the secret of the care of the patient is in caring for the patient.”2
We agree with Dr. Peabody. As we embrace science and technology that can change health outcomes, our patients’ needs to feel understood and cared for will not diminish. Instead, that need will continue to be an important aspect of our struggle and joy in providing holistic, humane, competent care into the future.
Twenty-first century physicians have access to an ever-growing trove of data, yet our ability to truly know our patients seems somehow less accessible. Home health devices have begun to provide a flow of information about parameters, ranging from continuous glucose readings to home blood pressures, weights, and inspiratory flow readings. These data can provide much more accurate insight into patients than what we can glean from one point in time during an office visit. Yet we need to remember that behind the data are people with dreams and desires, not just table entries in an electronic health record.
In 1923, the German philosopher Martin Buber published the book for which he is best known, “I and Thou.” In that book, Mr. Buber says that there are two ways we can approach relationships: “I-Thou” or “I-It.” In I-It relationships, we view the other person as an “it” to be used to accomplish a purpose, or to be experienced without his or her full involvement. In an I-Thou relationship, we appreciate the other people for all their complexity, in their full humanness. We must consciously remind ourselves amid the rush of technology that there are real people behind those data. We must acknowledge and approach each person as a unique individual who has dreams, goals, fears, and wishes that may be different from ours but to which we can still relate.
‘From the Beating End of the Stethoscope’
John Ciardi, an American poet, said the following in a poem titled, “Lines From the Beating End of the Stethoscope”:
I speak, as I say, the patient’s point of view.
But, given time, doctors are patients, too.
And there’s our bond: beyond anatomy,
Or in it, through it, to the mystery
Medicine takes the pulse of and lets go
Forever unexplained. It’s art, we know,
Not science at the heart. Doctor be whole,
I won’t insist the patient is a soul,
But he’s a something, possibly laughable,
Or possibly sublime, but not quite graphable.
Not quite containable on a bed chart.
Where science touches man it turns to art.3
This poem is a reminder of the subtle needs of patients during their encounters with doctors, especially around many of the most important decisions and events in their lives. Patients’ needs are varied, complex, difficult to discern, and not able to be fully explained or understood through math and science.
Einstein warned us that the modern age would be characterized by a perfection of means and a confusion of goals.4 As clinicians, we should strive to clarify and align our goals with those of our patients, providing care that is real, compassionate, and personal, not just an optimized means to achieve standardized metrics. While technology can assist us in this pursuit, we’ll need be careful that our enchantment with innovation does not cloud our actual goal: truly caring for our patients.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
References
1. Jones VA. The white coat: Why not follow suit? JAMA. 1999;281(5):478. doi: 10.1001/jama.281.5.478-JMS0203-5-1
2. Peabody, Francis (1927). “The care of the patient.” JAMA. 88(12):877-82. doi: 10.1001/jama.1927.02680380001001.
3. Ciardi, John. Lines from the Beating End of the Stethoscope. Saturday Review, Nov. 18, 1968.
4. Albert Einstein, Out of My Later Years, 1950.
Indeed, science and technology provide opportunities to improve outcomes in ways not even imagined 100 years ago, yet we must acknowledge that technology also threatens to erect barriers between us and our patients. We can be easily tempted to confuse new care delivery tools with the actual care itself.
Threats to the physician-patient relationship
Medical history provides many examples of how our zeal to innovate can have untoward consequences to the physician-patient relationship.
In the late 1800s, for example, to convey a sense of science, purity of intent, and trust, the medical community began wearing white coats. Those white coats have been discussed as creating emotional distance between physicians and their patients.1
Even when we in the medical community are slow and reluctant to change, the external forces propelling us forward often seem unstoppable; kinetic aspirations to innovate electronic information systems and new applications seem suddenly to revolutionize care delivery when we least expect it. The rapidity of change in technology can sometimes be dizzying but can at the same time can occur so swiftly we don’t even notice it.
After René Laennec invented the stethoscope in the early 1800s, clinicians no longer needed to physically lean in and place an ear directly onto patients to hear their hearts beating. This created a distance from patients that was still lamented 50 years later, when a professor of medicine is reported to have said, “he that hath ears to hear, let him use his ears and not a stethoscope.” Still, while the stethoscope has literally distanced us from patients, it is such an important tool that we no longer think about this distancing. We have adapted over time to remain close to our patients, to sincerely listen to their thoughts and reassure them that we hear them without the need to feel our ears on their chests.
Francis Peabody, the eminent Harvard physician, wrote an essay in 1927 titled, “The Care of the Patient.” At the end of the first paragraph, he states: “The most common criticism made at present by older practitioners is that young graduates ... are too “scientific” and do not know how to take care of patients.” He goes on to say that “one of the essential qualities of the clinician is interest in humanity, for the secret of the care of the patient is in caring for the patient.”2
We agree with Dr. Peabody. As we embrace science and technology that can change health outcomes, our patients’ needs to feel understood and cared for will not diminish. Instead, that need will continue to be an important aspect of our struggle and joy in providing holistic, humane, competent care into the future.
Twenty-first century physicians have access to an ever-growing trove of data, yet our ability to truly know our patients seems somehow less accessible. Home health devices have begun to provide a flow of information about parameters, ranging from continuous glucose readings to home blood pressures, weights, and inspiratory flow readings. These data can provide much more accurate insight into patients than what we can glean from one point in time during an office visit. Yet we need to remember that behind the data are people with dreams and desires, not just table entries in an electronic health record.
In 1923, the German philosopher Martin Buber published the book for which he is best known, “I and Thou.” In that book, Mr. Buber says that there are two ways we can approach relationships: “I-Thou” or “I-It.” In I-It relationships, we view the other person as an “it” to be used to accomplish a purpose, or to be experienced without his or her full involvement. In an I-Thou relationship, we appreciate the other people for all their complexity, in their full humanness. We must consciously remind ourselves amid the rush of technology that there are real people behind those data. We must acknowledge and approach each person as a unique individual who has dreams, goals, fears, and wishes that may be different from ours but to which we can still relate.
‘From the Beating End of the Stethoscope’
John Ciardi, an American poet, said the following in a poem titled, “Lines From the Beating End of the Stethoscope”:
I speak, as I say, the patient’s point of view.
But, given time, doctors are patients, too.
And there’s our bond: beyond anatomy,
Or in it, through it, to the mystery
Medicine takes the pulse of and lets go
Forever unexplained. It’s art, we know,
Not science at the heart. Doctor be whole,
I won’t insist the patient is a soul,
But he’s a something, possibly laughable,
Or possibly sublime, but not quite graphable.
Not quite containable on a bed chart.
Where science touches man it turns to art.3
This poem is a reminder of the subtle needs of patients during their encounters with doctors, especially around many of the most important decisions and events in their lives. Patients’ needs are varied, complex, difficult to discern, and not able to be fully explained or understood through math and science.
Einstein warned us that the modern age would be characterized by a perfection of means and a confusion of goals.4 As clinicians, we should strive to clarify and align our goals with those of our patients, providing care that is real, compassionate, and personal, not just an optimized means to achieve standardized metrics. While technology can assist us in this pursuit, we’ll need be careful that our enchantment with innovation does not cloud our actual goal: truly caring for our patients.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
References
1. Jones VA. The white coat: Why not follow suit? JAMA. 1999;281(5):478. doi: 10.1001/jama.281.5.478-JMS0203-5-1
2. Peabody, Francis (1927). “The care of the patient.” JAMA. 88(12):877-82. doi: 10.1001/jama.1927.02680380001001.
3. Ciardi, John. Lines from the Beating End of the Stethoscope. Saturday Review, Nov. 18, 1968.
4. Albert Einstein, Out of My Later Years, 1950.
Retiform Purpura on the Buttocks in 6 Critically Ill COVID-19 Patients
To the Editor:
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.
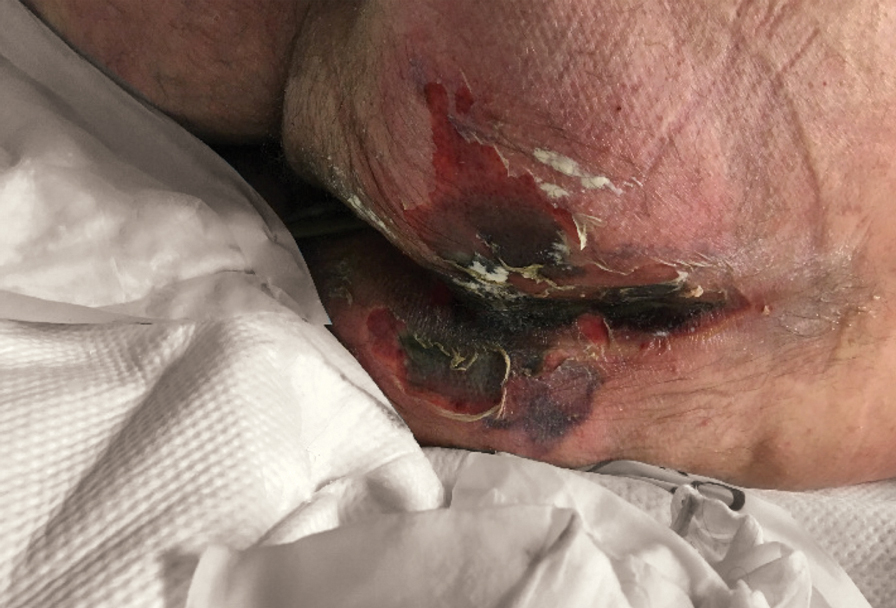
Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.
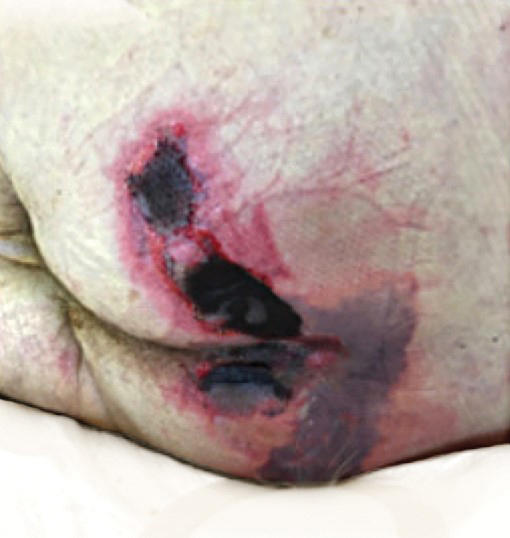
Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
To the Editor:
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.

Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.

Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
To the Editor:
There is emerging evidence of skin findings in patients with COVID-19, including perniolike changes of the toes as well as urticarial and vesicular eruptions.1 Magro et al2 reported 3 cases of livedoid and purpuric skin eruptions in critically ill COVID-19 patients with evidence of thrombotic vasculopathy on skin biopsy, including a 32-year-old man with striking buttocks retiform purpura. Histopathologic analysis revealed thrombotic vasculopathy and pressure-induced ischemic necrosis. Since that patient was first evaluated (March 2020), we identified 6 more cases of critically ill COVID-19 patients from a single academic hospital in New York City with essentially identical clinical findings. Herein, we report those 6 cases of critically ill and intubated patients with COVID-19 who developed retiform purpura on the buttocks only, approximately 11 to 21 days after onset of COVID-19 symptoms.
We provided consultation for 5 men and 1 woman (age range, 42–78 years) who were critically ill with COVID-19 and developed retiform purpura on the buttocks (Figures 1 and 2). All had an elevated D-dimer concentration: 2 patients, >700 ng/mL; 2 patients, >2000 ng/mL; 2 patients, >6000 ng/mL (reference, 229 ng/mL). Three patients experienced a peak D-dimer concentration on the day retiform purpura was reported.

Further evidence of coagulopathy in these patients included 1 patient with a newly diagnosed left popliteal deep vein thrombosis and 1 patient with a known history of protein C deficiency and deep vein thromboses. Five patients were receiving anticoagulation on the day the skin changes were documented; anticoagulation was contraindicated in the sixth patient because of oropharyngeal bleeding. Anticoagulation was continued at the treatment dosage (enoxaparin 80 mg twice daily) in 3 patients, and in 2 patients receiving a prophylactic dose (enoxaparin 40 mg daily), anticoagulation was escalated to treatment dose due to rising D-dimer levels and newly diagnosed retiform purpura. Skin biopsy was deferred for all patients due to positional and ventilatory restrictions. At that point in their care, 3 patients remained admitted on medicine floors, 2 were in the intensive care unit, and 1 had died.

Although the differential diagnosis for retiform purpura is broad and should be fully considered in any patient with this finding, based on the elevated D-dimer concentration, critical illness secondary to COVID-19, and striking similarity to earlier reported case of buttocks retiform purpura with thrombotic vasculopathy and pressure injury noted histopathologically,2 we suspect the buttocks retiform purpura in our 6 cases also represent a combination of cutaneous thrombosis and pressure injury. In addition to acral livedoid eruptions (also reported by Magro and colleagues2), we suspect that this cutaneous manifestation might be associated with a hypercoagulable state in some patients, especially in the setting of a rising D-dimer concentration. One study found that 31% of 184 patients with severe COVID-19 had thrombotic complications,3 a clinical picture that portends a poor prognosis.4
COVID-19 patients presenting with retiform purpura should be fully evaluated based on the broad differential for this morphology. We present 6 cases of buttocks retiform purpura in critically ill COVID-19 patients—all with strikingly similar morphologic findings, an elevated D-dimer concentration, and critical illness due to COVID-19—to alert clinicians to this constellation of findings and propose that this cutaneous manifestation could indicate an associated hypercoaguable state and should prompt a hematology consultation. Additionally, biopsy of this skin finding should be considered, especially if biopsy results might serve to guide management; however, obtaining a biopsy specimen can be technically difficult because of ventilatory requirements.
Given the magnitude of the COVID-19 pandemic and the propensity of these patients to experience thrombotic events, recognition of this skin finding in COVID-19 is important and might allow timely intervention.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212-e213. doi:10.1111/jdv.16387
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. doi:10.1016/j.trsl.2020.04.007
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
- Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. doi:10.1111/jth.14768
Practice Points
- Retiform purpura in a severely ill patient with COVID-19 and a markedly elevated D-dimer concentration might be a cutaneous sign of systemic coagulopathy.
- This constellation of findings should prompt consideration of skin biopsy and hematology consultation.
Purpura Fulminans in an Asplenic Intravenous Drug User
To the Editor:
A 56-year-old man with a history of opioid abuse and splenectomy decades prior due to a motor vehicle accident was brought to an outside emergency department with confusion, slurred speech, and difficulty breathing. Over the next few days, he became febrile and hypotensive, requiring vasopressors. Clinical laboratory testing revealed a urine drug screen positive for opioids and a low platelet count in the setting of a rapidly evolving retiform purpuric rash.
The patient was transferred to our institution 6 days after initial presentation with primary diagnoses of septic shock with multiorgan failure and disseminated intravascular coagulation (DIC). Blood cultures were positive for gram-negative rods. After several days of broad-spectrum antibiotics and supportive care, cultures were reported as positive for Capnocytophaga canimorsus. Upon further questioning, the patient’s wife reported that the couple had a new puppy and that the patient often allowed the dog to bite him playfully and lick abrasions on his hands and legs. He had not received medical treatment for any of the dog’s bites.
On initial examination at the time of transfer, the patient’s skin was remarkable for diffuse areas of stellate and retiform purpura with dusky centers and necrosis of the nasal tip and earlobes. Both hands were purpuric, with necrosis of the fingertips (Figure 1A). The flank was marked by large areas of full-thickness sloughing of the skin (Figure 1B). The lower extremities were edematous, with some areas of stellate purpura and numerous large bullae that drained straw-colored fluid (Figure 1C). Lower extremity pulses were found with Doppler ultrasonography.
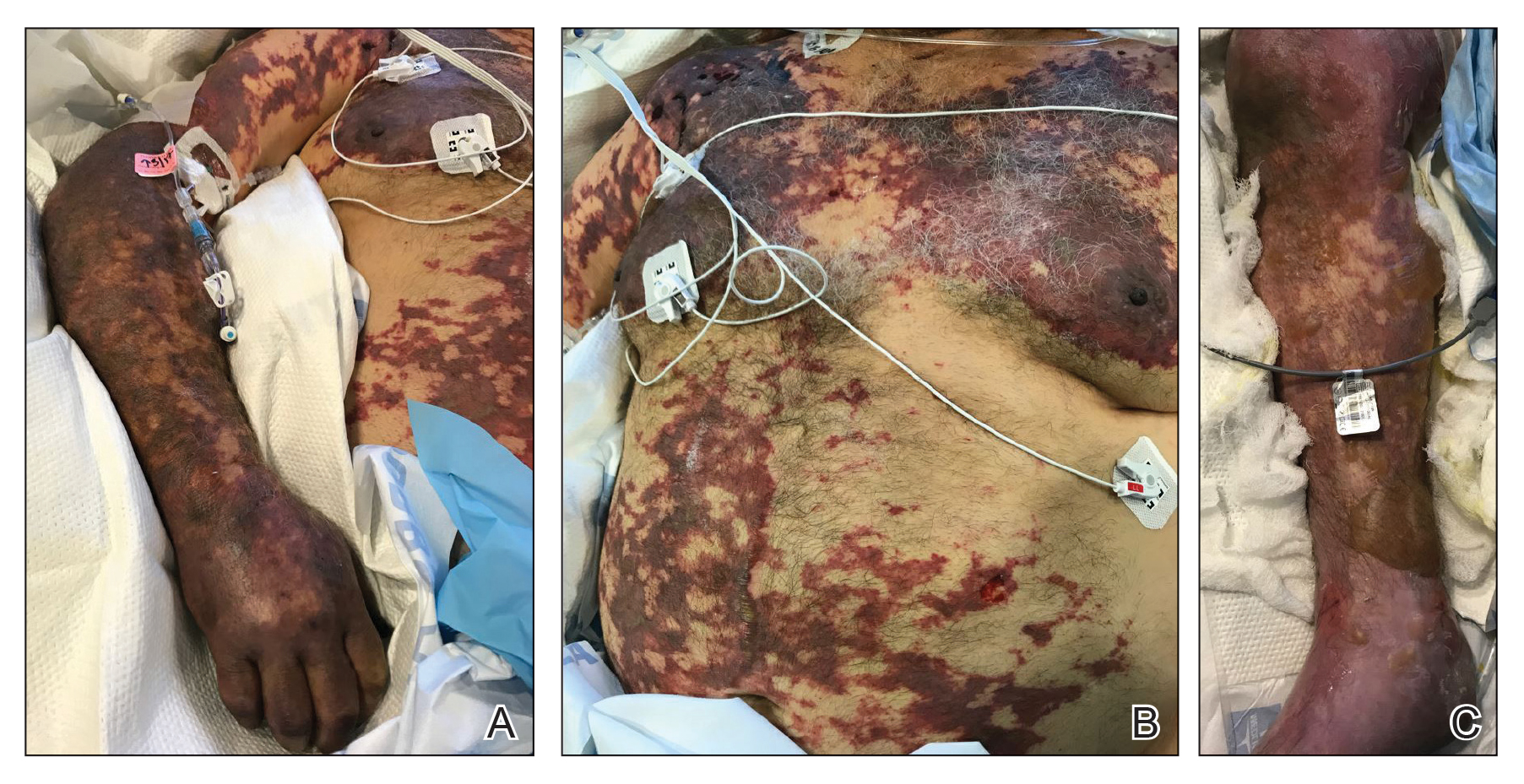
Given the presence of rapidly developing retiform purpura in the clinical context of severe sepsis, purpura fulminans (PF) was the primary consideration in the differential diagnosis. Levamisole-induced necrosis syndrome also was considered because of necrosis of the ears and nose as well as the history of substance use; however, the patient was not known to have a history of cocaine abuse, and a test of antineutrophil cytoplasmic antibody was negative.
A punch biopsy of the abdomen revealed intravascular thrombi with epidermal and sweat gland necrosis, consistent with PF (Figure 2). Gram, Giemsa, and Gomori methenamine-silver stains were negative for organisms. Tissue culture remained negative. Repeat blood cultures demonstrated Candida parapsilosis fungemia. Respiratory culture was positive for budding yeast.
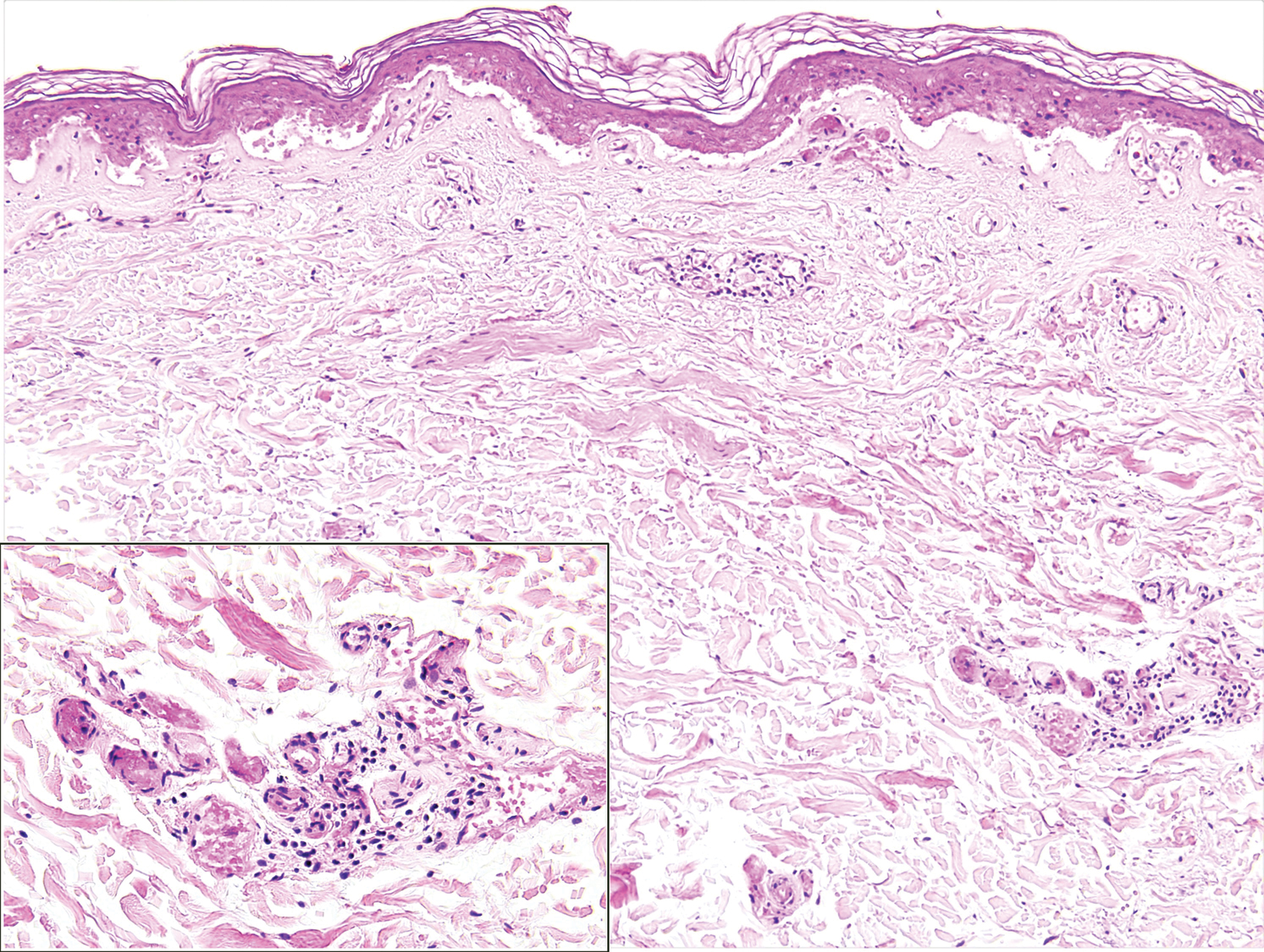
The patient was treated with antimicrobials, intravenous argatroban, and subcutaneous heparin. Purpura and bullae on the trunk slowly resolved with systemic therapy and wound care with petrolatum and nonadherent dressings. However, lesions on the nasal tip, all fingers of both hands, and several toes evolved into dry gangrene. The hospital course was complicated by renal failure requiring continuous renal replacement therapy; respiratory failure requiring ventilator support; and elevated levels of liver enzymes, consistent with involvement of the hepatic microvasculature.
The patient was in the medical intensive care unit at our institution for 2 weeks and was transferred to a burn center for specialized wound care. At transfer, he was still on a ventilator and receiving continuous renal replacement therapy. Subsequently, the patient required a left above-the-knee amputation, right below-the-knee amputation, and amputation of several digits of the upper extremities. In the months after the amputations, he required multiple stump revisions and experienced surgical site infections that complicated healing.
Purpura fulminans is an uncommon syndrome characterized by intravascular thrombosis and hemorrhagic infarction of the skin. The condition commonly is associated with septic shock, causing vascular collapse and DIC. It often develops rapidly.
Because of associated high mortality, it is important to differentiate PF from other causes of cutaneous retiform purpura, including other causes of thrombosis and large vessel vasculitis. Leading causes of PF include infection and hereditary or acquired deficiency of protein C, protein S, or antithrombin III. Regardless of cause, biopsy results demonstrate vascular thrombosis out of proportion to vasculitis. The mortality rate is 42% to 50%. The incidence of postinfectious sepsis sequelae in PF is higher than in survivors of sepsis only, especially amputation.1-3 Most patients do not die from complications of sepsis but from sequelae of the hypercoagulable and prothrombotic state associated with PF.4 Hemorrhagic infarction can affect the kidneys, brain, lungs, heart, eyes, and adrenal glands (ie, necrosis, namely Waterhouse-Friderichsen syndrome).5
The most common infectious cause of PF is sepsis secondary to Neisseria meningitidis, with as many as 25% of infected patients developing PF.6Streptococcus pneumoniae is another common cause. Other important causative organisms include Streptococcus pyogenes; Staphylococcus aureus (in the setting of intravenous substance use); Klebsiella oxytoca; Klebsiella aerogenes; rickettsial organisms; and viruses, including cytomegalovirus and varicella-zoster virus.2,7-13 Two earlier cases associated with Capnocytophaga were characterized by concomitant renal failure, metabolic acidosis, hemolytic anemia, and DIC.14
It is estimated that Capnocytophaga causes 11% to 46% of all cases of sepsis15; sepsis resulting from Capnocytophaga has extremely poor outcomes, with mortality reaching as high as 60%. The organism is part of the normal oral flora of cats and dogs, and a bite (less often, a scratch) is the cause of most Capnocytophaga infections. The clinical spectrum of C canimorsus infection associated with dog saliva exposure more commonly includes cellulitis at or around the site of inoculation, meningitis, and endocarditis.16
Although patients affected by PF can be young and healthy, several risk factors for PF have been identified2,6,16: asplenia, an immunocompromised state, systemic corticosteroid use, cirrhosis, and alcoholism. Asplenic patients have been shown to be particularly susceptible to systemic Capnocytophaga infection; when bitten by a dog, they should be treated with prophylactic antibiotics to cover Capnocytophaga.17 Immunocompetent patients rarely develop severe infection with Capnocytophaga.16,18,19 The complement system in particular is critically important in defending against C canimorsus.20
The underlying pathophysiology of acute infectious PF is multifactorial, encompassing increased expression of procoagulant tissue factor by monocytes and endothelial cells in the presence of bacterial pathogens. Dysfunction of protein C, an anticoagulant component of the coagulation cascade, often is cited as a crucial derangement leading to the development of a prothrombotic state in acute infectious PF.21 Serum protein S and antithrombin deficiency also can play a role.22 Specific in vitro examination of C canimorsus has revealed a protease that catalyzes N-terminal cleavage of procoagulant factor X, resulting in loss of function.15
Retiform purpura is a hallmark feature of PF, often beginning as nonblanching erythema with localized edema and petechiae before evolving into the characteristic stellate lesions with hemorrhagic bullae and subsequent necrosis.23 Pathologic examination reveals microthrombi involving arterioles and smaller vessels.24 There typically is laboratory evidence of DIC in PF, including elevated prothrombin time and partial thromboplastin time, thrombocytopenia, elevated D-dimer, and a decreased fibrinogen level.6,23
Capnocytophaga bacteria are challenging to grow on standard culture media. Optimal media for growth include 5% sheep’s blood and chocolate agar.16 Polymerase chain reaction can identify Capnocytophaga; in cases in which blood culture does not produce growth, 16S ribosomal RNA gene sequencing of tissue from skin biopsy has identified the pathogen.25
Some Capnocytophaga isolates have been shown to produce beta-lactamase; individual strains can be resistant to penicillins, cephalosporins, and imipenem.26 Factors associated with an increased risk for death include decreased leukocyte and platelet counts and an increased level of arterial lactate.27
Empiric antibiotic therapy for Capnocytophaga sepsis should include a beta-lactam and beta-lactamase inhibitor, such as piperacillin-tazobactam. Management of DIC can include therapeutic heparin or low-molecular-weight heparin and prophylactic platelet transfusion to maintain a pre-established value.28-30 Debridement should be conservative; it is important to wait for definite delineation between viable and necrotic tissue,31 which might take several months.32 Human skin allografts, in addition to artificial skin, are utilized as supplemental therapy for more rapid wound closure after removal of necrotic tissue.33,34 Hyperoxygenated fatty acids have been noted to aid in more rapid wound healing in infants with PF.35
Fresh frozen plasma is one method to replace missing factors, but it contains little protein C.36 Outcomes with recombinant human activated protein C (drotrecogin alfa) are mixed, and studies have shown no benefit in reducing the risk for death.37,38 Protein C concentrate has shown therapeutic benefit in some case reports and small retrospective studies.4 In one case report, protein C concentrate and heparin were utilized in combination with antithrombin III.21
Hyperbaric O2 might be of benefit when initiated within 5 days after onset of PF. However, hyperbaric O2 does carry risk; O2 toxicity, barotrauma, and barriers to timely resuscitation when the patient is inside the pressurized chamber can occur.2
There is a single report of successful use of the vasodilator iloprost for meningococcal PF without need for surgical intervention; the team also utilized topical nitroglycerin patches on the fingers to avoid digital amputation.39 Epoprostenol, tissue plasminogen activator, and antithrombin have been utilized in cases of extensive PF. Fibrinolytic therapy might have some utility, but only in a setting of malignancy-associated DIC.40
Treatment of acute infectious PF lacks a high level of evidence. Options include replacement of anticoagulant factors, anticoagulant therapy, hyperbaric O2, topical and systemic vasodilators, and, in the setting of underlying cancer, fibrinolytics. Even with therapy, prognosis is guarded.
- Ghosh SK, Bandyopadhyay D, Dutta A. Purpura fulminans: a cutaneous marker of disseminated intravascular coagulation. West J Emerg Med. 2009;10:41.
- Ursin Rein P, Jacobsen D, Ormaasen V, et al. Pneumococcal sepsis requiring mechanical ventilation: cohort study in 38 patients with rapid progression to septic shock. Acta Anaesthesiol Scand. 2018;62:1428-1435. doi:10.1111/aas
- Contou D, Canoui-Poitrine F, Coudroy R, et al; Hopeful Study Group. Long-term quality of life in adult patients surviving purpura fulminans: an exposed-unexposed multicenter cohort study. Clin Infect Dis. 2019;69:332-340. doi:10.1093/cid/ciy901
- Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071. doi:10.1136/adc.2010.199919
- Karimi K, Odhav A, Kollipara R, et al. Acute cutaneous necrosis: a guide to early diagnosis and treatment. J Cutan Med Surg. 2017;21:425-437. doi:10.1177/1203475417708164
- Colling ME, Bendapudi PK. Purpura fulminans: mechanism and management of dysregulated hemostasis. Transfus Med Rev. 2018;32:69-76. doi:10.1016/j.tmrv.2017.10.001
- Kankeu Fonkoua L, Zhang S, Canty E, et al. Purpura fulminans from reduced protein S following cytomegalovirus and varicella infection. Am J Hematol. 2019;94:491-495. doi:10.1002/ajh.25386
- Okuzono S, Ishimura M, Kanno S, et al. Streptococcus pyogenes-purpura fulminans as an invasive form of group A streptococcal infection. Ann Clin Microbiol Antimicrob. 2018;17:31. doi:10.1186/s12941-018-0282-9
- Gupta D, Chandrashekar L, Srinivas BH, et al. Acute infectious purpura fulminans caused by group A β-hemolytic Streptococcus: an uncommon organism. Indian Dermatol Online J. 2016;7:132-133. doi:10.4103/2229-5178.178093
- Saini S, Duncan RA. Sloughing skin in intravenous drug user. IDCases. 2018;12:74-75. doi:10.1016/j.idcr.2018.03.007
- Tsubouchi N, Tsurukiri J, Numata J, et al. Acute infectious purpura fulminans caused by Klebsiella oxytoca. Intern Med. 2019;58:1801-1802. doi:10.2169/internalmedicine.2350-18
- Yamamoto S, Ito R. Acute infectious purpura fulminans with Enterobacter aerogenes post-neurosurgery. IDCases. 2019;15:e00514. doi:10.1016/j.idcr.2019.e00514
- Dalugama C, Gawarammana IB. Rare presentation of rickettsial infection as purpura fulminans: a case report. J Med Case Rep. 2018;12:145. doi:10.1186/s13256-018-1672-5
- Kazandjieva J, Antonov D, Kamarashev J, et al. Acrally distributed dermatoses: vascular dermatoses (purpura and vasculitis). Clin Dermatol. 2017;35:68-80. doi:10.1016/j.clindermatol.2016.09.013
- Hack K, Renzi F, Hess E, et al. Inactivation of human coagulation factor X by a protease of the pathogen Capnocytophaga canimorsus. J Thromb Haemost. 2017;15:487-499. doi:10.1111/jth.13605
- Zajkowska J, M, Falkowski D, et al. Capnocytophaga canimorsus—an underestimated danger after dog or cat bite - review of literature. Przegl Epidemiol. 2016;70:289-295.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86-97. doi:10.1016/S0140-6736(10)61493-6
- Behrend Christiansen C, Berg RMG, Plovsing RR, et al. Two cases of infectious purpura fulminans and septic shock caused by Capnocytophaga canimorsus transmitted from dogs. Scand J Infect Dis. 2012;44:635-639. doi:10.3109/00365548.2012.672765
- Ruddock TL, Rindler JM, Bergfeld WF. Capnocytophaga canimorsus septicemia in an asplenic patient. Cutis. 1997;60:95-97.
- Mantovani E, Busani S, Biagioni E, et al. Purpura fulminans and septic shock due to Capnocytophaga canimorsus after dog bite: a case report and review of the literature. Case Rep Crit Care. 2018;2018:7090268. doi:10.1155/2018/7090268
- Bendapudi PK, Robbins A, LeBoeuf N, et al. Persistence of endothelial thrombomodulin in a patient with infectious purpura fulminans treated with protein C concentrate. Blood Adv. 2018;2:2917-2921. doi:10.1182/bloodadvances.2018024430
- Lerolle N, Carlotti A, Melican K, et al. Assessment of the interplay between blood and skin vascular abnormalities in adult purpura fulminans. Am J Respir Crit Care Med. 2013;188:684-692. doi:10.1164/rccm.201302-0228OC.
- Thornsberry LA, LoSicco KI, English JC III. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462. doi:10.1016/j.jaad.2013.01.043
- Adcock DM, Hicks MJ. Dermatopathology of skin necrosis associated with purpura fulminans. Semin Thromb Hemost. 1990;16:283-292. doi:10.1055/s-2007-1002681
- Dautzenberg KHW, Polderman FN, van Suylen RJ, et al. Purpura fulminans mimicking toxic epidermal necrolysis—additional value of 16S rRNA sequencing and skin biopsy. Neth J Med. 2017;75:165-168.
- Zangenah S, Andersson AF, V, et al. Genomic analysis reveals the presence of a class D beta-lactamase with broad substrate specificity in animal bite associated Capnocytophaga species. Eur J Clin Microbiol Infect Dis. 2017;36:657-662. doi:10.1007/s10096-016-2842-2
- Contou D, Sonneville R, Canoui-Poitrine F, et al; Hopeful Study Group. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: a French multicenter retrospective cohort study. Intensive Care Med. 2018;44:1502-1511. doi:10.1007/s00134-018-5341-3
- Zenz W, Zoehrer B, Levin M, et al; . Use of recombinant tissue plasminogen activator in children with meningococcal purpura fulminans: a retrospective study. Crit Care Med. 2004;32:1777-1780. doi:10.1097/01.ccm.0000133667.86429.5d
- Wallace JS, Hall JC. Use of drug therapy to manage acute cutaneous necrosis of the skin. J Drugs Dermatol. 2010;9:341-349.
- Squizzato A, Hunt BJ, Kinasewitz GT, et al. Supportive management strategies for disseminated intravascular coagulation. an international consensus. Thromb Haemost. 2016;115:896-904. doi:10.1160/TH15-09-0740
- Herrera R, Hobar PC, Ginsburg CM. Surgical intervention for the complications of meningococcal-induced purpura fulminans. Pediatr Infect Dis J. 1994;13:734-737. doi:10.1097/00006454-199408000-00011
- Pino PA, JA, F. Delayed surgical debridement and use of semiocclusive dressings for salvage of fingers after purpura fulminans. Hand (N Y). 2016;11:NP34-NP37. doi:10.1177/1558944716661996
- Gaucher S, J, Jarraya M. Human skin allografts as a useful adjunct in the treatment of purpura fulminans. J Wound Care. 2010;19:355-358. doi:10.12968/jowc.2010.19.8.77714
- Mazzone L, Schiestl C. Management of septic skin necroses. Eur J Pediatr Surg. 2013;23:349-358. doi:10.1055/s-0033-1352530
- G, Torra-Bou JE, Manzano-Canillas ML, et al. Management of purpura fulminans skin lesions in a premature neonate with sepsis: a case study. J Wound Care. 2019;28:198-203. doi:10.12968/jowc.2019.28.4.198
- Kizilocak H, Ozdemir N, Dikme G, et al. Homozygous protein C deficiency presenting as neonatal purpura fulminans: management with fresh frozen plasma, low molecular weight heparin and protein C concentrate. J Thromb Thrombolysis. 2018;45:315-318. doi:10.1007/s11239-017-1606-x
- Ranieri VM, Thompson BT, Barie PS, et al; . Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064. doi:10.1056/NEJMoa1202290
- Bernard GR, Vincent J-L, Laterre P-F, et al; . Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. doi:10.1056/NEJM200103083441001
- Hage-Sleiman M, Derre N, Verdet C, et al. Meningococcal purpura fulminans and severe myocarditis with clinical meningitis but no meningeal inflammation: a case report. BMC Infect Dis. 2019;19:252. doi:10.1186/s12879-019-3866-x
- Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. doi:10.1111/j.1365-2141.2009.07600.x
To the Editor:
A 56-year-old man with a history of opioid abuse and splenectomy decades prior due to a motor vehicle accident was brought to an outside emergency department with confusion, slurred speech, and difficulty breathing. Over the next few days, he became febrile and hypotensive, requiring vasopressors. Clinical laboratory testing revealed a urine drug screen positive for opioids and a low platelet count in the setting of a rapidly evolving retiform purpuric rash.
The patient was transferred to our institution 6 days after initial presentation with primary diagnoses of septic shock with multiorgan failure and disseminated intravascular coagulation (DIC). Blood cultures were positive for gram-negative rods. After several days of broad-spectrum antibiotics and supportive care, cultures were reported as positive for Capnocytophaga canimorsus. Upon further questioning, the patient’s wife reported that the couple had a new puppy and that the patient often allowed the dog to bite him playfully and lick abrasions on his hands and legs. He had not received medical treatment for any of the dog’s bites.
On initial examination at the time of transfer, the patient’s skin was remarkable for diffuse areas of stellate and retiform purpura with dusky centers and necrosis of the nasal tip and earlobes. Both hands were purpuric, with necrosis of the fingertips (Figure 1A). The flank was marked by large areas of full-thickness sloughing of the skin (Figure 1B). The lower extremities were edematous, with some areas of stellate purpura and numerous large bullae that drained straw-colored fluid (Figure 1C). Lower extremity pulses were found with Doppler ultrasonography.

Given the presence of rapidly developing retiform purpura in the clinical context of severe sepsis, purpura fulminans (PF) was the primary consideration in the differential diagnosis. Levamisole-induced necrosis syndrome also was considered because of necrosis of the ears and nose as well as the history of substance use; however, the patient was not known to have a history of cocaine abuse, and a test of antineutrophil cytoplasmic antibody was negative.
A punch biopsy of the abdomen revealed intravascular thrombi with epidermal and sweat gland necrosis, consistent with PF (Figure 2). Gram, Giemsa, and Gomori methenamine-silver stains were negative for organisms. Tissue culture remained negative. Repeat blood cultures demonstrated Candida parapsilosis fungemia. Respiratory culture was positive for budding yeast.

The patient was treated with antimicrobials, intravenous argatroban, and subcutaneous heparin. Purpura and bullae on the trunk slowly resolved with systemic therapy and wound care with petrolatum and nonadherent dressings. However, lesions on the nasal tip, all fingers of both hands, and several toes evolved into dry gangrene. The hospital course was complicated by renal failure requiring continuous renal replacement therapy; respiratory failure requiring ventilator support; and elevated levels of liver enzymes, consistent with involvement of the hepatic microvasculature.
The patient was in the medical intensive care unit at our institution for 2 weeks and was transferred to a burn center for specialized wound care. At transfer, he was still on a ventilator and receiving continuous renal replacement therapy. Subsequently, the patient required a left above-the-knee amputation, right below-the-knee amputation, and amputation of several digits of the upper extremities. In the months after the amputations, he required multiple stump revisions and experienced surgical site infections that complicated healing.
Purpura fulminans is an uncommon syndrome characterized by intravascular thrombosis and hemorrhagic infarction of the skin. The condition commonly is associated with septic shock, causing vascular collapse and DIC. It often develops rapidly.
Because of associated high mortality, it is important to differentiate PF from other causes of cutaneous retiform purpura, including other causes of thrombosis and large vessel vasculitis. Leading causes of PF include infection and hereditary or acquired deficiency of protein C, protein S, or antithrombin III. Regardless of cause, biopsy results demonstrate vascular thrombosis out of proportion to vasculitis. The mortality rate is 42% to 50%. The incidence of postinfectious sepsis sequelae in PF is higher than in survivors of sepsis only, especially amputation.1-3 Most patients do not die from complications of sepsis but from sequelae of the hypercoagulable and prothrombotic state associated with PF.4 Hemorrhagic infarction can affect the kidneys, brain, lungs, heart, eyes, and adrenal glands (ie, necrosis, namely Waterhouse-Friderichsen syndrome).5
The most common infectious cause of PF is sepsis secondary to Neisseria meningitidis, with as many as 25% of infected patients developing PF.6Streptococcus pneumoniae is another common cause. Other important causative organisms include Streptococcus pyogenes; Staphylococcus aureus (in the setting of intravenous substance use); Klebsiella oxytoca; Klebsiella aerogenes; rickettsial organisms; and viruses, including cytomegalovirus and varicella-zoster virus.2,7-13 Two earlier cases associated with Capnocytophaga were characterized by concomitant renal failure, metabolic acidosis, hemolytic anemia, and DIC.14
It is estimated that Capnocytophaga causes 11% to 46% of all cases of sepsis15; sepsis resulting from Capnocytophaga has extremely poor outcomes, with mortality reaching as high as 60%. The organism is part of the normal oral flora of cats and dogs, and a bite (less often, a scratch) is the cause of most Capnocytophaga infections. The clinical spectrum of C canimorsus infection associated with dog saliva exposure more commonly includes cellulitis at or around the site of inoculation, meningitis, and endocarditis.16
Although patients affected by PF can be young and healthy, several risk factors for PF have been identified2,6,16: asplenia, an immunocompromised state, systemic corticosteroid use, cirrhosis, and alcoholism. Asplenic patients have been shown to be particularly susceptible to systemic Capnocytophaga infection; when bitten by a dog, they should be treated with prophylactic antibiotics to cover Capnocytophaga.17 Immunocompetent patients rarely develop severe infection with Capnocytophaga.16,18,19 The complement system in particular is critically important in defending against C canimorsus.20
The underlying pathophysiology of acute infectious PF is multifactorial, encompassing increased expression of procoagulant tissue factor by monocytes and endothelial cells in the presence of bacterial pathogens. Dysfunction of protein C, an anticoagulant component of the coagulation cascade, often is cited as a crucial derangement leading to the development of a prothrombotic state in acute infectious PF.21 Serum protein S and antithrombin deficiency also can play a role.22 Specific in vitro examination of C canimorsus has revealed a protease that catalyzes N-terminal cleavage of procoagulant factor X, resulting in loss of function.15
Retiform purpura is a hallmark feature of PF, often beginning as nonblanching erythema with localized edema and petechiae before evolving into the characteristic stellate lesions with hemorrhagic bullae and subsequent necrosis.23 Pathologic examination reveals microthrombi involving arterioles and smaller vessels.24 There typically is laboratory evidence of DIC in PF, including elevated prothrombin time and partial thromboplastin time, thrombocytopenia, elevated D-dimer, and a decreased fibrinogen level.6,23
Capnocytophaga bacteria are challenging to grow on standard culture media. Optimal media for growth include 5% sheep’s blood and chocolate agar.16 Polymerase chain reaction can identify Capnocytophaga; in cases in which blood culture does not produce growth, 16S ribosomal RNA gene sequencing of tissue from skin biopsy has identified the pathogen.25
Some Capnocytophaga isolates have been shown to produce beta-lactamase; individual strains can be resistant to penicillins, cephalosporins, and imipenem.26 Factors associated with an increased risk for death include decreased leukocyte and platelet counts and an increased level of arterial lactate.27
Empiric antibiotic therapy for Capnocytophaga sepsis should include a beta-lactam and beta-lactamase inhibitor, such as piperacillin-tazobactam. Management of DIC can include therapeutic heparin or low-molecular-weight heparin and prophylactic platelet transfusion to maintain a pre-established value.28-30 Debridement should be conservative; it is important to wait for definite delineation between viable and necrotic tissue,31 which might take several months.32 Human skin allografts, in addition to artificial skin, are utilized as supplemental therapy for more rapid wound closure after removal of necrotic tissue.33,34 Hyperoxygenated fatty acids have been noted to aid in more rapid wound healing in infants with PF.35
Fresh frozen plasma is one method to replace missing factors, but it contains little protein C.36 Outcomes with recombinant human activated protein C (drotrecogin alfa) are mixed, and studies have shown no benefit in reducing the risk for death.37,38 Protein C concentrate has shown therapeutic benefit in some case reports and small retrospective studies.4 In one case report, protein C concentrate and heparin were utilized in combination with antithrombin III.21
Hyperbaric O2 might be of benefit when initiated within 5 days after onset of PF. However, hyperbaric O2 does carry risk; O2 toxicity, barotrauma, and barriers to timely resuscitation when the patient is inside the pressurized chamber can occur.2
There is a single report of successful use of the vasodilator iloprost for meningococcal PF without need for surgical intervention; the team also utilized topical nitroglycerin patches on the fingers to avoid digital amputation.39 Epoprostenol, tissue plasminogen activator, and antithrombin have been utilized in cases of extensive PF. Fibrinolytic therapy might have some utility, but only in a setting of malignancy-associated DIC.40
Treatment of acute infectious PF lacks a high level of evidence. Options include replacement of anticoagulant factors, anticoagulant therapy, hyperbaric O2, topical and systemic vasodilators, and, in the setting of underlying cancer, fibrinolytics. Even with therapy, prognosis is guarded.
To the Editor:
A 56-year-old man with a history of opioid abuse and splenectomy decades prior due to a motor vehicle accident was brought to an outside emergency department with confusion, slurred speech, and difficulty breathing. Over the next few days, he became febrile and hypotensive, requiring vasopressors. Clinical laboratory testing revealed a urine drug screen positive for opioids and a low platelet count in the setting of a rapidly evolving retiform purpuric rash.
The patient was transferred to our institution 6 days after initial presentation with primary diagnoses of septic shock with multiorgan failure and disseminated intravascular coagulation (DIC). Blood cultures were positive for gram-negative rods. After several days of broad-spectrum antibiotics and supportive care, cultures were reported as positive for Capnocytophaga canimorsus. Upon further questioning, the patient’s wife reported that the couple had a new puppy and that the patient often allowed the dog to bite him playfully and lick abrasions on his hands and legs. He had not received medical treatment for any of the dog’s bites.
On initial examination at the time of transfer, the patient’s skin was remarkable for diffuse areas of stellate and retiform purpura with dusky centers and necrosis of the nasal tip and earlobes. Both hands were purpuric, with necrosis of the fingertips (Figure 1A). The flank was marked by large areas of full-thickness sloughing of the skin (Figure 1B). The lower extremities were edematous, with some areas of stellate purpura and numerous large bullae that drained straw-colored fluid (Figure 1C). Lower extremity pulses were found with Doppler ultrasonography.

Given the presence of rapidly developing retiform purpura in the clinical context of severe sepsis, purpura fulminans (PF) was the primary consideration in the differential diagnosis. Levamisole-induced necrosis syndrome also was considered because of necrosis of the ears and nose as well as the history of substance use; however, the patient was not known to have a history of cocaine abuse, and a test of antineutrophil cytoplasmic antibody was negative.
A punch biopsy of the abdomen revealed intravascular thrombi with epidermal and sweat gland necrosis, consistent with PF (Figure 2). Gram, Giemsa, and Gomori methenamine-silver stains were negative for organisms. Tissue culture remained negative. Repeat blood cultures demonstrated Candida parapsilosis fungemia. Respiratory culture was positive for budding yeast.

The patient was treated with antimicrobials, intravenous argatroban, and subcutaneous heparin. Purpura and bullae on the trunk slowly resolved with systemic therapy and wound care with petrolatum and nonadherent dressings. However, lesions on the nasal tip, all fingers of both hands, and several toes evolved into dry gangrene. The hospital course was complicated by renal failure requiring continuous renal replacement therapy; respiratory failure requiring ventilator support; and elevated levels of liver enzymes, consistent with involvement of the hepatic microvasculature.
The patient was in the medical intensive care unit at our institution for 2 weeks and was transferred to a burn center for specialized wound care. At transfer, he was still on a ventilator and receiving continuous renal replacement therapy. Subsequently, the patient required a left above-the-knee amputation, right below-the-knee amputation, and amputation of several digits of the upper extremities. In the months after the amputations, he required multiple stump revisions and experienced surgical site infections that complicated healing.
Purpura fulminans is an uncommon syndrome characterized by intravascular thrombosis and hemorrhagic infarction of the skin. The condition commonly is associated with septic shock, causing vascular collapse and DIC. It often develops rapidly.
Because of associated high mortality, it is important to differentiate PF from other causes of cutaneous retiform purpura, including other causes of thrombosis and large vessel vasculitis. Leading causes of PF include infection and hereditary or acquired deficiency of protein C, protein S, or antithrombin III. Regardless of cause, biopsy results demonstrate vascular thrombosis out of proportion to vasculitis. The mortality rate is 42% to 50%. The incidence of postinfectious sepsis sequelae in PF is higher than in survivors of sepsis only, especially amputation.1-3 Most patients do not die from complications of sepsis but from sequelae of the hypercoagulable and prothrombotic state associated with PF.4 Hemorrhagic infarction can affect the kidneys, brain, lungs, heart, eyes, and adrenal glands (ie, necrosis, namely Waterhouse-Friderichsen syndrome).5
The most common infectious cause of PF is sepsis secondary to Neisseria meningitidis, with as many as 25% of infected patients developing PF.6Streptococcus pneumoniae is another common cause. Other important causative organisms include Streptococcus pyogenes; Staphylococcus aureus (in the setting of intravenous substance use); Klebsiella oxytoca; Klebsiella aerogenes; rickettsial organisms; and viruses, including cytomegalovirus and varicella-zoster virus.2,7-13 Two earlier cases associated with Capnocytophaga were characterized by concomitant renal failure, metabolic acidosis, hemolytic anemia, and DIC.14
It is estimated that Capnocytophaga causes 11% to 46% of all cases of sepsis15; sepsis resulting from Capnocytophaga has extremely poor outcomes, with mortality reaching as high as 60%. The organism is part of the normal oral flora of cats and dogs, and a bite (less often, a scratch) is the cause of most Capnocytophaga infections. The clinical spectrum of C canimorsus infection associated with dog saliva exposure more commonly includes cellulitis at or around the site of inoculation, meningitis, and endocarditis.16
Although patients affected by PF can be young and healthy, several risk factors for PF have been identified2,6,16: asplenia, an immunocompromised state, systemic corticosteroid use, cirrhosis, and alcoholism. Asplenic patients have been shown to be particularly susceptible to systemic Capnocytophaga infection; when bitten by a dog, they should be treated with prophylactic antibiotics to cover Capnocytophaga.17 Immunocompetent patients rarely develop severe infection with Capnocytophaga.16,18,19 The complement system in particular is critically important in defending against C canimorsus.20
The underlying pathophysiology of acute infectious PF is multifactorial, encompassing increased expression of procoagulant tissue factor by monocytes and endothelial cells in the presence of bacterial pathogens. Dysfunction of protein C, an anticoagulant component of the coagulation cascade, often is cited as a crucial derangement leading to the development of a prothrombotic state in acute infectious PF.21 Serum protein S and antithrombin deficiency also can play a role.22 Specific in vitro examination of C canimorsus has revealed a protease that catalyzes N-terminal cleavage of procoagulant factor X, resulting in loss of function.15
Retiform purpura is a hallmark feature of PF, often beginning as nonblanching erythema with localized edema and petechiae before evolving into the characteristic stellate lesions with hemorrhagic bullae and subsequent necrosis.23 Pathologic examination reveals microthrombi involving arterioles and smaller vessels.24 There typically is laboratory evidence of DIC in PF, including elevated prothrombin time and partial thromboplastin time, thrombocytopenia, elevated D-dimer, and a decreased fibrinogen level.6,23
Capnocytophaga bacteria are challenging to grow on standard culture media. Optimal media for growth include 5% sheep’s blood and chocolate agar.16 Polymerase chain reaction can identify Capnocytophaga; in cases in which blood culture does not produce growth, 16S ribosomal RNA gene sequencing of tissue from skin biopsy has identified the pathogen.25
Some Capnocytophaga isolates have been shown to produce beta-lactamase; individual strains can be resistant to penicillins, cephalosporins, and imipenem.26 Factors associated with an increased risk for death include decreased leukocyte and platelet counts and an increased level of arterial lactate.27
Empiric antibiotic therapy for Capnocytophaga sepsis should include a beta-lactam and beta-lactamase inhibitor, such as piperacillin-tazobactam. Management of DIC can include therapeutic heparin or low-molecular-weight heparin and prophylactic platelet transfusion to maintain a pre-established value.28-30 Debridement should be conservative; it is important to wait for definite delineation between viable and necrotic tissue,31 which might take several months.32 Human skin allografts, in addition to artificial skin, are utilized as supplemental therapy for more rapid wound closure after removal of necrotic tissue.33,34 Hyperoxygenated fatty acids have been noted to aid in more rapid wound healing in infants with PF.35
Fresh frozen plasma is one method to replace missing factors, but it contains little protein C.36 Outcomes with recombinant human activated protein C (drotrecogin alfa) are mixed, and studies have shown no benefit in reducing the risk for death.37,38 Protein C concentrate has shown therapeutic benefit in some case reports and small retrospective studies.4 In one case report, protein C concentrate and heparin were utilized in combination with antithrombin III.21
Hyperbaric O2 might be of benefit when initiated within 5 days after onset of PF. However, hyperbaric O2 does carry risk; O2 toxicity, barotrauma, and barriers to timely resuscitation when the patient is inside the pressurized chamber can occur.2
There is a single report of successful use of the vasodilator iloprost for meningococcal PF without need for surgical intervention; the team also utilized topical nitroglycerin patches on the fingers to avoid digital amputation.39 Epoprostenol, tissue plasminogen activator, and antithrombin have been utilized in cases of extensive PF. Fibrinolytic therapy might have some utility, but only in a setting of malignancy-associated DIC.40
Treatment of acute infectious PF lacks a high level of evidence. Options include replacement of anticoagulant factors, anticoagulant therapy, hyperbaric O2, topical and systemic vasodilators, and, in the setting of underlying cancer, fibrinolytics. Even with therapy, prognosis is guarded.
- Ghosh SK, Bandyopadhyay D, Dutta A. Purpura fulminans: a cutaneous marker of disseminated intravascular coagulation. West J Emerg Med. 2009;10:41.
- Ursin Rein P, Jacobsen D, Ormaasen V, et al. Pneumococcal sepsis requiring mechanical ventilation: cohort study in 38 patients with rapid progression to septic shock. Acta Anaesthesiol Scand. 2018;62:1428-1435. doi:10.1111/aas
- Contou D, Canoui-Poitrine F, Coudroy R, et al; Hopeful Study Group. Long-term quality of life in adult patients surviving purpura fulminans: an exposed-unexposed multicenter cohort study. Clin Infect Dis. 2019;69:332-340. doi:10.1093/cid/ciy901
- Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071. doi:10.1136/adc.2010.199919
- Karimi K, Odhav A, Kollipara R, et al. Acute cutaneous necrosis: a guide to early diagnosis and treatment. J Cutan Med Surg. 2017;21:425-437. doi:10.1177/1203475417708164
- Colling ME, Bendapudi PK. Purpura fulminans: mechanism and management of dysregulated hemostasis. Transfus Med Rev. 2018;32:69-76. doi:10.1016/j.tmrv.2017.10.001
- Kankeu Fonkoua L, Zhang S, Canty E, et al. Purpura fulminans from reduced protein S following cytomegalovirus and varicella infection. Am J Hematol. 2019;94:491-495. doi:10.1002/ajh.25386
- Okuzono S, Ishimura M, Kanno S, et al. Streptococcus pyogenes-purpura fulminans as an invasive form of group A streptococcal infection. Ann Clin Microbiol Antimicrob. 2018;17:31. doi:10.1186/s12941-018-0282-9
- Gupta D, Chandrashekar L, Srinivas BH, et al. Acute infectious purpura fulminans caused by group A β-hemolytic Streptococcus: an uncommon organism. Indian Dermatol Online J. 2016;7:132-133. doi:10.4103/2229-5178.178093
- Saini S, Duncan RA. Sloughing skin in intravenous drug user. IDCases. 2018;12:74-75. doi:10.1016/j.idcr.2018.03.007
- Tsubouchi N, Tsurukiri J, Numata J, et al. Acute infectious purpura fulminans caused by Klebsiella oxytoca. Intern Med. 2019;58:1801-1802. doi:10.2169/internalmedicine.2350-18
- Yamamoto S, Ito R. Acute infectious purpura fulminans with Enterobacter aerogenes post-neurosurgery. IDCases. 2019;15:e00514. doi:10.1016/j.idcr.2019.e00514
- Dalugama C, Gawarammana IB. Rare presentation of rickettsial infection as purpura fulminans: a case report. J Med Case Rep. 2018;12:145. doi:10.1186/s13256-018-1672-5
- Kazandjieva J, Antonov D, Kamarashev J, et al. Acrally distributed dermatoses: vascular dermatoses (purpura and vasculitis). Clin Dermatol. 2017;35:68-80. doi:10.1016/j.clindermatol.2016.09.013
- Hack K, Renzi F, Hess E, et al. Inactivation of human coagulation factor X by a protease of the pathogen Capnocytophaga canimorsus. J Thromb Haemost. 2017;15:487-499. doi:10.1111/jth.13605
- Zajkowska J, M, Falkowski D, et al. Capnocytophaga canimorsus—an underestimated danger after dog or cat bite - review of literature. Przegl Epidemiol. 2016;70:289-295.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86-97. doi:10.1016/S0140-6736(10)61493-6
- Behrend Christiansen C, Berg RMG, Plovsing RR, et al. Two cases of infectious purpura fulminans and septic shock caused by Capnocytophaga canimorsus transmitted from dogs. Scand J Infect Dis. 2012;44:635-639. doi:10.3109/00365548.2012.672765
- Ruddock TL, Rindler JM, Bergfeld WF. Capnocytophaga canimorsus septicemia in an asplenic patient. Cutis. 1997;60:95-97.
- Mantovani E, Busani S, Biagioni E, et al. Purpura fulminans and septic shock due to Capnocytophaga canimorsus after dog bite: a case report and review of the literature. Case Rep Crit Care. 2018;2018:7090268. doi:10.1155/2018/7090268
- Bendapudi PK, Robbins A, LeBoeuf N, et al. Persistence of endothelial thrombomodulin in a patient with infectious purpura fulminans treated with protein C concentrate. Blood Adv. 2018;2:2917-2921. doi:10.1182/bloodadvances.2018024430
- Lerolle N, Carlotti A, Melican K, et al. Assessment of the interplay between blood and skin vascular abnormalities in adult purpura fulminans. Am J Respir Crit Care Med. 2013;188:684-692. doi:10.1164/rccm.201302-0228OC.
- Thornsberry LA, LoSicco KI, English JC III. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462. doi:10.1016/j.jaad.2013.01.043
- Adcock DM, Hicks MJ. Dermatopathology of skin necrosis associated with purpura fulminans. Semin Thromb Hemost. 1990;16:283-292. doi:10.1055/s-2007-1002681
- Dautzenberg KHW, Polderman FN, van Suylen RJ, et al. Purpura fulminans mimicking toxic epidermal necrolysis—additional value of 16S rRNA sequencing and skin biopsy. Neth J Med. 2017;75:165-168.
- Zangenah S, Andersson AF, V, et al. Genomic analysis reveals the presence of a class D beta-lactamase with broad substrate specificity in animal bite associated Capnocytophaga species. Eur J Clin Microbiol Infect Dis. 2017;36:657-662. doi:10.1007/s10096-016-2842-2
- Contou D, Sonneville R, Canoui-Poitrine F, et al; Hopeful Study Group. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: a French multicenter retrospective cohort study. Intensive Care Med. 2018;44:1502-1511. doi:10.1007/s00134-018-5341-3
- Zenz W, Zoehrer B, Levin M, et al; . Use of recombinant tissue plasminogen activator in children with meningococcal purpura fulminans: a retrospective study. Crit Care Med. 2004;32:1777-1780. doi:10.1097/01.ccm.0000133667.86429.5d
- Wallace JS, Hall JC. Use of drug therapy to manage acute cutaneous necrosis of the skin. J Drugs Dermatol. 2010;9:341-349.
- Squizzato A, Hunt BJ, Kinasewitz GT, et al. Supportive management strategies for disseminated intravascular coagulation. an international consensus. Thromb Haemost. 2016;115:896-904. doi:10.1160/TH15-09-0740
- Herrera R, Hobar PC, Ginsburg CM. Surgical intervention for the complications of meningococcal-induced purpura fulminans. Pediatr Infect Dis J. 1994;13:734-737. doi:10.1097/00006454-199408000-00011
- Pino PA, JA, F. Delayed surgical debridement and use of semiocclusive dressings for salvage of fingers after purpura fulminans. Hand (N Y). 2016;11:NP34-NP37. doi:10.1177/1558944716661996
- Gaucher S, J, Jarraya M. Human skin allografts as a useful adjunct in the treatment of purpura fulminans. J Wound Care. 2010;19:355-358. doi:10.12968/jowc.2010.19.8.77714
- Mazzone L, Schiestl C. Management of septic skin necroses. Eur J Pediatr Surg. 2013;23:349-358. doi:10.1055/s-0033-1352530
- G, Torra-Bou JE, Manzano-Canillas ML, et al. Management of purpura fulminans skin lesions in a premature neonate with sepsis: a case study. J Wound Care. 2019;28:198-203. doi:10.12968/jowc.2019.28.4.198
- Kizilocak H, Ozdemir N, Dikme G, et al. Homozygous protein C deficiency presenting as neonatal purpura fulminans: management with fresh frozen plasma, low molecular weight heparin and protein C concentrate. J Thromb Thrombolysis. 2018;45:315-318. doi:10.1007/s11239-017-1606-x
- Ranieri VM, Thompson BT, Barie PS, et al; . Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064. doi:10.1056/NEJMoa1202290
- Bernard GR, Vincent J-L, Laterre P-F, et al; . Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. doi:10.1056/NEJM200103083441001
- Hage-Sleiman M, Derre N, Verdet C, et al. Meningococcal purpura fulminans and severe myocarditis with clinical meningitis but no meningeal inflammation: a case report. BMC Infect Dis. 2019;19:252. doi:10.1186/s12879-019-3866-x
- Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. doi:10.1111/j.1365-2141.2009.07600.x
- Ghosh SK, Bandyopadhyay D, Dutta A. Purpura fulminans: a cutaneous marker of disseminated intravascular coagulation. West J Emerg Med. 2009;10:41.
- Ursin Rein P, Jacobsen D, Ormaasen V, et al. Pneumococcal sepsis requiring mechanical ventilation: cohort study in 38 patients with rapid progression to septic shock. Acta Anaesthesiol Scand. 2018;62:1428-1435. doi:10.1111/aas
- Contou D, Canoui-Poitrine F, Coudroy R, et al; Hopeful Study Group. Long-term quality of life in adult patients surviving purpura fulminans: an exposed-unexposed multicenter cohort study. Clin Infect Dis. 2019;69:332-340. doi:10.1093/cid/ciy901
- Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: recognition, diagnosis and management. Arch Dis Child. 2011;96:1066-1071. doi:10.1136/adc.2010.199919
- Karimi K, Odhav A, Kollipara R, et al. Acute cutaneous necrosis: a guide to early diagnosis and treatment. J Cutan Med Surg. 2017;21:425-437. doi:10.1177/1203475417708164
- Colling ME, Bendapudi PK. Purpura fulminans: mechanism and management of dysregulated hemostasis. Transfus Med Rev. 2018;32:69-76. doi:10.1016/j.tmrv.2017.10.001
- Kankeu Fonkoua L, Zhang S, Canty E, et al. Purpura fulminans from reduced protein S following cytomegalovirus and varicella infection. Am J Hematol. 2019;94:491-495. doi:10.1002/ajh.25386
- Okuzono S, Ishimura M, Kanno S, et al. Streptococcus pyogenes-purpura fulminans as an invasive form of group A streptococcal infection. Ann Clin Microbiol Antimicrob. 2018;17:31. doi:10.1186/s12941-018-0282-9
- Gupta D, Chandrashekar L, Srinivas BH, et al. Acute infectious purpura fulminans caused by group A β-hemolytic Streptococcus: an uncommon organism. Indian Dermatol Online J. 2016;7:132-133. doi:10.4103/2229-5178.178093
- Saini S, Duncan RA. Sloughing skin in intravenous drug user. IDCases. 2018;12:74-75. doi:10.1016/j.idcr.2018.03.007
- Tsubouchi N, Tsurukiri J, Numata J, et al. Acute infectious purpura fulminans caused by Klebsiella oxytoca. Intern Med. 2019;58:1801-1802. doi:10.2169/internalmedicine.2350-18
- Yamamoto S, Ito R. Acute infectious purpura fulminans with Enterobacter aerogenes post-neurosurgery. IDCases. 2019;15:e00514. doi:10.1016/j.idcr.2019.e00514
- Dalugama C, Gawarammana IB. Rare presentation of rickettsial infection as purpura fulminans: a case report. J Med Case Rep. 2018;12:145. doi:10.1186/s13256-018-1672-5
- Kazandjieva J, Antonov D, Kamarashev J, et al. Acrally distributed dermatoses: vascular dermatoses (purpura and vasculitis). Clin Dermatol. 2017;35:68-80. doi:10.1016/j.clindermatol.2016.09.013
- Hack K, Renzi F, Hess E, et al. Inactivation of human coagulation factor X by a protease of the pathogen Capnocytophaga canimorsus. J Thromb Haemost. 2017;15:487-499. doi:10.1111/jth.13605
- Zajkowska J, M, Falkowski D, et al. Capnocytophaga canimorsus—an underestimated danger after dog or cat bite - review of literature. Przegl Epidemiol. 2016;70:289-295.
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86-97. doi:10.1016/S0140-6736(10)61493-6
- Behrend Christiansen C, Berg RMG, Plovsing RR, et al. Two cases of infectious purpura fulminans and septic shock caused by Capnocytophaga canimorsus transmitted from dogs. Scand J Infect Dis. 2012;44:635-639. doi:10.3109/00365548.2012.672765
- Ruddock TL, Rindler JM, Bergfeld WF. Capnocytophaga canimorsus septicemia in an asplenic patient. Cutis. 1997;60:95-97.
- Mantovani E, Busani S, Biagioni E, et al. Purpura fulminans and septic shock due to Capnocytophaga canimorsus after dog bite: a case report and review of the literature. Case Rep Crit Care. 2018;2018:7090268. doi:10.1155/2018/7090268
- Bendapudi PK, Robbins A, LeBoeuf N, et al. Persistence of endothelial thrombomodulin in a patient with infectious purpura fulminans treated with protein C concentrate. Blood Adv. 2018;2:2917-2921. doi:10.1182/bloodadvances.2018024430
- Lerolle N, Carlotti A, Melican K, et al. Assessment of the interplay between blood and skin vascular abnormalities in adult purpura fulminans. Am J Respir Crit Care Med. 2013;188:684-692. doi:10.1164/rccm.201302-0228OC.
- Thornsberry LA, LoSicco KI, English JC III. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462. doi:10.1016/j.jaad.2013.01.043
- Adcock DM, Hicks MJ. Dermatopathology of skin necrosis associated with purpura fulminans. Semin Thromb Hemost. 1990;16:283-292. doi:10.1055/s-2007-1002681
- Dautzenberg KHW, Polderman FN, van Suylen RJ, et al. Purpura fulminans mimicking toxic epidermal necrolysis—additional value of 16S rRNA sequencing and skin biopsy. Neth J Med. 2017;75:165-168.
- Zangenah S, Andersson AF, V, et al. Genomic analysis reveals the presence of a class D beta-lactamase with broad substrate specificity in animal bite associated Capnocytophaga species. Eur J Clin Microbiol Infect Dis. 2017;36:657-662. doi:10.1007/s10096-016-2842-2
- Contou D, Sonneville R, Canoui-Poitrine F, et al; Hopeful Study Group. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: a French multicenter retrospective cohort study. Intensive Care Med. 2018;44:1502-1511. doi:10.1007/s00134-018-5341-3
- Zenz W, Zoehrer B, Levin M, et al; . Use of recombinant tissue plasminogen activator in children with meningococcal purpura fulminans: a retrospective study. Crit Care Med. 2004;32:1777-1780. doi:10.1097/01.ccm.0000133667.86429.5d
- Wallace JS, Hall JC. Use of drug therapy to manage acute cutaneous necrosis of the skin. J Drugs Dermatol. 2010;9:341-349.
- Squizzato A, Hunt BJ, Kinasewitz GT, et al. Supportive management strategies for disseminated intravascular coagulation. an international consensus. Thromb Haemost. 2016;115:896-904. doi:10.1160/TH15-09-0740
- Herrera R, Hobar PC, Ginsburg CM. Surgical intervention for the complications of meningococcal-induced purpura fulminans. Pediatr Infect Dis J. 1994;13:734-737. doi:10.1097/00006454-199408000-00011
- Pino PA, JA, F. Delayed surgical debridement and use of semiocclusive dressings for salvage of fingers after purpura fulminans. Hand (N Y). 2016;11:NP34-NP37. doi:10.1177/1558944716661996
- Gaucher S, J, Jarraya M. Human skin allografts as a useful adjunct in the treatment of purpura fulminans. J Wound Care. 2010;19:355-358. doi:10.12968/jowc.2010.19.8.77714
- Mazzone L, Schiestl C. Management of septic skin necroses. Eur J Pediatr Surg. 2013;23:349-358. doi:10.1055/s-0033-1352530
- G, Torra-Bou JE, Manzano-Canillas ML, et al. Management of purpura fulminans skin lesions in a premature neonate with sepsis: a case study. J Wound Care. 2019;28:198-203. doi:10.12968/jowc.2019.28.4.198
- Kizilocak H, Ozdemir N, Dikme G, et al. Homozygous protein C deficiency presenting as neonatal purpura fulminans: management with fresh frozen plasma, low molecular weight heparin and protein C concentrate. J Thromb Thrombolysis. 2018;45:315-318. doi:10.1007/s11239-017-1606-x
- Ranieri VM, Thompson BT, Barie PS, et al; . Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064. doi:10.1056/NEJMoa1202290
- Bernard GR, Vincent J-L, Laterre P-F, et al; . Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709. doi:10.1056/NEJM200103083441001
- Hage-Sleiman M, Derre N, Verdet C, et al. Meningococcal purpura fulminans and severe myocarditis with clinical meningitis but no meningeal inflammation: a case report. BMC Infect Dis. 2019;19:252. doi:10.1186/s12879-019-3866-x
- Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24-33. doi:10.1111/j.1365-2141.2009.07600.x
Practice Points
- Capnocytophaga species are fastidious, slow-growing microorganisms. It is important, therefore, to maintain a high degree of suspicion and alertthe microbiology laboratory to increase the likelihood of isolation.
- Patients should be cautioned regarding the need for prophylactic antibiotics in the event of an animal bite; asplenic patients are at particular risk for infection.
- In patients with severe purpura fulminans and a gangrenous limb, it is important to allow adequate time for demarcation of gangrene and not rush to amputation.