User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Patient-focused precautions, testing help blunt pandemic effects on heme-onc unit
Keeping hematologic oncology patients on their treatment regimens and caring for inpatients with hematologic malignancies remained “manageable” during the first 2 months of the COVID-19 pandemic at Levine Cancer Institute in Charlotte, N.C.
That level of manageability has partly been because a surge in cases so far hasn’t arrived at Levine or in most of the surrounding North Carolina and South Carolina communities it serves. As of May 15, 2020, the total number of confirmed and reported COVID-19 cases had reached about 19,000 in North Carolina, and just under 9,000 in South Carolina, out of a total population in the two states of close to 16 million. What’s happened instead at Levine Cancer Institute (LCI) has been a steady but low drumbeat of cases that, by mid-May 2020, totaled fewer than 10 patients with hematologic malignancies diagnosed with COVID-19.
“For a large system with multiple sites throughout North and South Carolina that saw 17,200 new patients in 2019 – including solid tumor, benign hematology, and malignant hematology patients – with 198,000 total patient visits, it is safe to say that we are off to a good start. However, we remain in the early throes of the pandemic and we will need to remain vigilant going forward,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in LCI’s Department of Hematologic Oncology and Blood Disorders.
The limited effects to date of COVID-19 at LCI has been thanks to a regimen of great caution for preventing infections that’s been consistently conveyed to LCI patients from before the pandemic’s onset, liberal testing that started early, a proactive plan to defer and temporarily replace infusion care when medically appropriate, a novel staffing approach designed to minimize and contain potential staff outbreaks, and an early pivot to virtual patient contact when feasible.
COVID-19 has had limited penetration into the LCI case load because patients have, in general, “been very careful,” said Dr. Voorhees.
“My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious even before the coronavirus using distancing, masking, and meticulous hand hygiene,” he said in an interview that reviewed the steps LCI took starting in March to confront and manage the effects of the then-nascent pandemic. “Since we started screening asymptomatic patients in the inpatient and outpatient settings we have identified only one patient with COVID-19 infection, which supports the low rate of infection in our patient population thus far.”
Another key step was the launch of “robust” testing for the COVID-19 virus starting on March 9, using an in-house assay from LCI’s parent health system, Atrium Health, that delivered results within 24 hours. Testing became available at LCI “earlier than at many other health systems.” At first, testing was limited to patients or staff presenting with symptoms, but in the following weeks, it expanded to more patients, including those without symptoms who were scheduled for treatment at the apheresis center, cell donors and cell recipients, patients arriving for inpatient chemotherapy or cellular therapy, patients arriving from a skilled nursing facility or similar environments, and more recently, outpatient chemotherapy patients. “We’re now doing a lot of screening,” Dr. Voorhees said. “In general, screening has been well received because patients recognize that it’s for their own safety.”
Another piece of COVID-19 preparedness was a move toward technology as an alternative to face-to-face encounters between patients and staff. “We adopted virtual technology early.” When medically appropriate, they provided either video consultations with more tech-savvy patients or telephone-based virtual visits for patients who preferred a more familiar interface. As LCI starts the process of reentry for patients whose face-to-face encounters were deferred, virtual visits will remain an important facet of maintaining care while limiting exposure for appropriate patients and facilitating adequate space for social distancing in the clinics and infusion centers.
Atrium Health also launched a “virtual hospital” geared to intensified remote management of COVID-19 patients who aren’t sick enough for hospitalization. “People who test positive automatically enter the virtual hospital and have regular interactions with their team of providers,” with LCI providing additional support for their patients who get infected. Patients receive an equipment kit that lets them monitor and transmit their vital signs. The virtual hospital program also helps expedite personal needs like delivery of prescriptions and food. “It helps patients manage at home, and has been incredibly useful,” said Dr. Voorhees.
Perhaps the most challenging step LCI clinicians took to preclude a potential COVID-19 case surge was to review all patients receiving infusional therapy or planned cellular therapy and triage those who could potentially tolerate a temporary change to either an oral, at-home regimen or to a brief hold on their treatment. Some patients on maintenance, outpatient infusion-therapy regimens “expressed concern about coming to the clinic. We looked at the patients scheduled to come for infusions and decided which visits were essential and which were deferrable without disrupting care by briefly using a noninfusional approach,” said Dr. Voorhees. The number of patients who had their regimens modified or held was “relatively small,” and with the recent recognition that a surge of infections has not occurred, “we’re now rolling out cautious reentry of those patients back to their originally prescribed chemotherapy.”
In addition to concerns of exposure at infusion clinics, there are concerns about the heightened susceptibility of immunosuppressed hematologic oncology patients to COVID-19 and their risk for more severe infection. “Our view is that, if patients tested positive, continuing immunosuppressive treatment would likely be detrimental,” so when possible treatment is temporarily suspended and then resumed when the infection has cleared. “When patients test positive for a prolonged period, a decision to resume treatment must be in the best interests of the patient and weigh the benefits of resuming therapy against the risks of incurring a more severe infection by restarting potentially immunosuppressive therapy,” Dr. Voorhees said.
The enhanced risk that cancer patients face if they develop COVID-19 was documented in a recent review of 218 cancer patients hospitalized for COVID-19 during parts of March and April in a large New York health system. The results showed an overall mortality rate of 28%, including a 37% rate among 54 patients with hematologic malignancies and a 25% rate among 164 patients with solid tumors. The mortality rate “may not be quite as high as they reported because that depends on how many patients you test, but there is no question that patients with more comorbidities are at higher risk. Patients with active cancer on chemotherapy are a particularly vulnerable population, and many have expressed concerns about their vulnerability,” he observed.
For the few LCI patients who developed COVID-19 infection, the medical staff has had several therapeutic options they could match to each patient’s needs, with help from the Atrium Health infectious disease team. LCI and Atrium Health are participating in several COVID-19 clinical treatment trials, including an investigational convalescent plasma protocol spearheaded by the Mayo Clinic. They have also opened a randomized, phase 2 trial evaluating the safety and efficacy of selinexor (Xpovio), an oral drug that’s Food and Drug Administration approved for patients with multiple myeloma, for treatment of moderate or severe COVID-19 infection. Additional studies evaluating blockade of granulocyte-macrophage colony-stimulating factor, as well as inhaled antiviral therapy, have recently launched, and several additional studies are poised to open in the coming weeks.
The LCI and Atrium Health team also has a supply of the antiviral agent remdesivir as part of the FDA’s expanded access protocol and emergency use authorization. They also have a supply of and experience administering the interleukin-6 receptor inhibitor tocilizumab (Actemra), which showed some suggestion of efficacy in limited experience treating patients with severe or critical COVID-19 infections. Clinicians at LCI have not used the investigational and unproven agents hydroxychloroquine, chloroquine, and azithromycin to either prevent or treat COVID-19.
LCI also instituted measures to try to minimize the risk that staff members could become infected and transmit the virus while asymptomatic. Following conversations held early on with COVID-19–experienced health authorities in China and Italy, the patient-facing LCI staff split into two teams starting on March 23 that alternated responsibility for direct patient interactions every 2 weeks. When one of these teams was off from direct patient contact they continued to care for patients remotely through virtual technologies. The concept was that, if a staffer became infected while remaining asymptomatic during their contact with patients, their status would either become diagnosable or resolve during their 2 weeks away from seeing any patients. Perhaps in part because of this approach infections among staff members “have not been a big issue. We’ve had an incredibly low infection rate among the LCI staff,” Dr. Voorhees noted.
By mid-May, with the imminent threat of a sudden CODIV-19 surge moderated, heme-onc operations at LCI began to cautiously revert to more normal operations. “We’re continuing patient screening for signs and symptoms of COVID-19 infection, testing for asymptomatic infections, and requiring masking and social distancing in the clinics and hospitals, but we’re starting to slowly restore the number of patients at our clinics [virtual and face to face[ and infusion centers,” and the staff’s division into two teams ended. “The idea was to get past a surge and make sure our system was not overwhelmed. We anticipated a local surge in late April, but then it kept getting pushed back. Current projections are for the infection rate among LCI patients to remain low provided that community spread remains stable or, ideally, decreases.” The LCI infectious disease staff is closely monitoring infection rates for early recognition of an outbreak, with plans to follow any new cases with contact tracing. So far, the COVID-19 pandemic at LCI “has been very manageable,” Dr. Voorhees concluded.
“We’re now better positioned to deal with a case surge if it were to happen. We could resume the two-team approach, hospital-wide plans are now in place for a future surge, and we are now up and running with robust testing and inpatient and outpatient virtual technology. The first time, we were all learning on the fly.”
The LCI biostatistics team has been prospectively collecting the Institutes’s COVID-19 patient data, with plans to report their findings.
Dr. Voorhees has had financial relationships with Bristol-Myers Squibb/Celgene, Janssen, Novartis, and Oncopeptides, none of which are relevant to this article.
Keeping hematologic oncology patients on their treatment regimens and caring for inpatients with hematologic malignancies remained “manageable” during the first 2 months of the COVID-19 pandemic at Levine Cancer Institute in Charlotte, N.C.
That level of manageability has partly been because a surge in cases so far hasn’t arrived at Levine or in most of the surrounding North Carolina and South Carolina communities it serves. As of May 15, 2020, the total number of confirmed and reported COVID-19 cases had reached about 19,000 in North Carolina, and just under 9,000 in South Carolina, out of a total population in the two states of close to 16 million. What’s happened instead at Levine Cancer Institute (LCI) has been a steady but low drumbeat of cases that, by mid-May 2020, totaled fewer than 10 patients with hematologic malignancies diagnosed with COVID-19.
“For a large system with multiple sites throughout North and South Carolina that saw 17,200 new patients in 2019 – including solid tumor, benign hematology, and malignant hematology patients – with 198,000 total patient visits, it is safe to say that we are off to a good start. However, we remain in the early throes of the pandemic and we will need to remain vigilant going forward,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in LCI’s Department of Hematologic Oncology and Blood Disorders.
The limited effects to date of COVID-19 at LCI has been thanks to a regimen of great caution for preventing infections that’s been consistently conveyed to LCI patients from before the pandemic’s onset, liberal testing that started early, a proactive plan to defer and temporarily replace infusion care when medically appropriate, a novel staffing approach designed to minimize and contain potential staff outbreaks, and an early pivot to virtual patient contact when feasible.
COVID-19 has had limited penetration into the LCI case load because patients have, in general, “been very careful,” said Dr. Voorhees.
“My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious even before the coronavirus using distancing, masking, and meticulous hand hygiene,” he said in an interview that reviewed the steps LCI took starting in March to confront and manage the effects of the then-nascent pandemic. “Since we started screening asymptomatic patients in the inpatient and outpatient settings we have identified only one patient with COVID-19 infection, which supports the low rate of infection in our patient population thus far.”
Another key step was the launch of “robust” testing for the COVID-19 virus starting on March 9, using an in-house assay from LCI’s parent health system, Atrium Health, that delivered results within 24 hours. Testing became available at LCI “earlier than at many other health systems.” At first, testing was limited to patients or staff presenting with symptoms, but in the following weeks, it expanded to more patients, including those without symptoms who were scheduled for treatment at the apheresis center, cell donors and cell recipients, patients arriving for inpatient chemotherapy or cellular therapy, patients arriving from a skilled nursing facility or similar environments, and more recently, outpatient chemotherapy patients. “We’re now doing a lot of screening,” Dr. Voorhees said. “In general, screening has been well received because patients recognize that it’s for their own safety.”
Another piece of COVID-19 preparedness was a move toward technology as an alternative to face-to-face encounters between patients and staff. “We adopted virtual technology early.” When medically appropriate, they provided either video consultations with more tech-savvy patients or telephone-based virtual visits for patients who preferred a more familiar interface. As LCI starts the process of reentry for patients whose face-to-face encounters were deferred, virtual visits will remain an important facet of maintaining care while limiting exposure for appropriate patients and facilitating adequate space for social distancing in the clinics and infusion centers.
Atrium Health also launched a “virtual hospital” geared to intensified remote management of COVID-19 patients who aren’t sick enough for hospitalization. “People who test positive automatically enter the virtual hospital and have regular interactions with their team of providers,” with LCI providing additional support for their patients who get infected. Patients receive an equipment kit that lets them monitor and transmit their vital signs. The virtual hospital program also helps expedite personal needs like delivery of prescriptions and food. “It helps patients manage at home, and has been incredibly useful,” said Dr. Voorhees.
Perhaps the most challenging step LCI clinicians took to preclude a potential COVID-19 case surge was to review all patients receiving infusional therapy or planned cellular therapy and triage those who could potentially tolerate a temporary change to either an oral, at-home regimen or to a brief hold on their treatment. Some patients on maintenance, outpatient infusion-therapy regimens “expressed concern about coming to the clinic. We looked at the patients scheduled to come for infusions and decided which visits were essential and which were deferrable without disrupting care by briefly using a noninfusional approach,” said Dr. Voorhees. The number of patients who had their regimens modified or held was “relatively small,” and with the recent recognition that a surge of infections has not occurred, “we’re now rolling out cautious reentry of those patients back to their originally prescribed chemotherapy.”
In addition to concerns of exposure at infusion clinics, there are concerns about the heightened susceptibility of immunosuppressed hematologic oncology patients to COVID-19 and their risk for more severe infection. “Our view is that, if patients tested positive, continuing immunosuppressive treatment would likely be detrimental,” so when possible treatment is temporarily suspended and then resumed when the infection has cleared. “When patients test positive for a prolonged period, a decision to resume treatment must be in the best interests of the patient and weigh the benefits of resuming therapy against the risks of incurring a more severe infection by restarting potentially immunosuppressive therapy,” Dr. Voorhees said.
The enhanced risk that cancer patients face if they develop COVID-19 was documented in a recent review of 218 cancer patients hospitalized for COVID-19 during parts of March and April in a large New York health system. The results showed an overall mortality rate of 28%, including a 37% rate among 54 patients with hematologic malignancies and a 25% rate among 164 patients with solid tumors. The mortality rate “may not be quite as high as they reported because that depends on how many patients you test, but there is no question that patients with more comorbidities are at higher risk. Patients with active cancer on chemotherapy are a particularly vulnerable population, and many have expressed concerns about their vulnerability,” he observed.
For the few LCI patients who developed COVID-19 infection, the medical staff has had several therapeutic options they could match to each patient’s needs, with help from the Atrium Health infectious disease team. LCI and Atrium Health are participating in several COVID-19 clinical treatment trials, including an investigational convalescent plasma protocol spearheaded by the Mayo Clinic. They have also opened a randomized, phase 2 trial evaluating the safety and efficacy of selinexor (Xpovio), an oral drug that’s Food and Drug Administration approved for patients with multiple myeloma, for treatment of moderate or severe COVID-19 infection. Additional studies evaluating blockade of granulocyte-macrophage colony-stimulating factor, as well as inhaled antiviral therapy, have recently launched, and several additional studies are poised to open in the coming weeks.
The LCI and Atrium Health team also has a supply of the antiviral agent remdesivir as part of the FDA’s expanded access protocol and emergency use authorization. They also have a supply of and experience administering the interleukin-6 receptor inhibitor tocilizumab (Actemra), which showed some suggestion of efficacy in limited experience treating patients with severe or critical COVID-19 infections. Clinicians at LCI have not used the investigational and unproven agents hydroxychloroquine, chloroquine, and azithromycin to either prevent or treat COVID-19.
LCI also instituted measures to try to minimize the risk that staff members could become infected and transmit the virus while asymptomatic. Following conversations held early on with COVID-19–experienced health authorities in China and Italy, the patient-facing LCI staff split into two teams starting on March 23 that alternated responsibility for direct patient interactions every 2 weeks. When one of these teams was off from direct patient contact they continued to care for patients remotely through virtual technologies. The concept was that, if a staffer became infected while remaining asymptomatic during their contact with patients, their status would either become diagnosable or resolve during their 2 weeks away from seeing any patients. Perhaps in part because of this approach infections among staff members “have not been a big issue. We’ve had an incredibly low infection rate among the LCI staff,” Dr. Voorhees noted.
By mid-May, with the imminent threat of a sudden CODIV-19 surge moderated, heme-onc operations at LCI began to cautiously revert to more normal operations. “We’re continuing patient screening for signs and symptoms of COVID-19 infection, testing for asymptomatic infections, and requiring masking and social distancing in the clinics and hospitals, but we’re starting to slowly restore the number of patients at our clinics [virtual and face to face[ and infusion centers,” and the staff’s division into two teams ended. “The idea was to get past a surge and make sure our system was not overwhelmed. We anticipated a local surge in late April, but then it kept getting pushed back. Current projections are for the infection rate among LCI patients to remain low provided that community spread remains stable or, ideally, decreases.” The LCI infectious disease staff is closely monitoring infection rates for early recognition of an outbreak, with plans to follow any new cases with contact tracing. So far, the COVID-19 pandemic at LCI “has been very manageable,” Dr. Voorhees concluded.
“We’re now better positioned to deal with a case surge if it were to happen. We could resume the two-team approach, hospital-wide plans are now in place for a future surge, and we are now up and running with robust testing and inpatient and outpatient virtual technology. The first time, we were all learning on the fly.”
The LCI biostatistics team has been prospectively collecting the Institutes’s COVID-19 patient data, with plans to report their findings.
Dr. Voorhees has had financial relationships with Bristol-Myers Squibb/Celgene, Janssen, Novartis, and Oncopeptides, none of which are relevant to this article.
Keeping hematologic oncology patients on their treatment regimens and caring for inpatients with hematologic malignancies remained “manageable” during the first 2 months of the COVID-19 pandemic at Levine Cancer Institute in Charlotte, N.C.
That level of manageability has partly been because a surge in cases so far hasn’t arrived at Levine or in most of the surrounding North Carolina and South Carolina communities it serves. As of May 15, 2020, the total number of confirmed and reported COVID-19 cases had reached about 19,000 in North Carolina, and just under 9,000 in South Carolina, out of a total population in the two states of close to 16 million. What’s happened instead at Levine Cancer Institute (LCI) has been a steady but low drumbeat of cases that, by mid-May 2020, totaled fewer than 10 patients with hematologic malignancies diagnosed with COVID-19.
“For a large system with multiple sites throughout North and South Carolina that saw 17,200 new patients in 2019 – including solid tumor, benign hematology, and malignant hematology patients – with 198,000 total patient visits, it is safe to say that we are off to a good start. However, we remain in the early throes of the pandemic and we will need to remain vigilant going forward,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in LCI’s Department of Hematologic Oncology and Blood Disorders.
The limited effects to date of COVID-19 at LCI has been thanks to a regimen of great caution for preventing infections that’s been consistently conveyed to LCI patients from before the pandemic’s onset, liberal testing that started early, a proactive plan to defer and temporarily replace infusion care when medically appropriate, a novel staffing approach designed to minimize and contain potential staff outbreaks, and an early pivot to virtual patient contact when feasible.
COVID-19 has had limited penetration into the LCI case load because patients have, in general, “been very careful,” said Dr. Voorhees.
“My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious even before the coronavirus using distancing, masking, and meticulous hand hygiene,” he said in an interview that reviewed the steps LCI took starting in March to confront and manage the effects of the then-nascent pandemic. “Since we started screening asymptomatic patients in the inpatient and outpatient settings we have identified only one patient with COVID-19 infection, which supports the low rate of infection in our patient population thus far.”
Another key step was the launch of “robust” testing for the COVID-19 virus starting on March 9, using an in-house assay from LCI’s parent health system, Atrium Health, that delivered results within 24 hours. Testing became available at LCI “earlier than at many other health systems.” At first, testing was limited to patients or staff presenting with symptoms, but in the following weeks, it expanded to more patients, including those without symptoms who were scheduled for treatment at the apheresis center, cell donors and cell recipients, patients arriving for inpatient chemotherapy or cellular therapy, patients arriving from a skilled nursing facility or similar environments, and more recently, outpatient chemotherapy patients. “We’re now doing a lot of screening,” Dr. Voorhees said. “In general, screening has been well received because patients recognize that it’s for their own safety.”
Another piece of COVID-19 preparedness was a move toward technology as an alternative to face-to-face encounters between patients and staff. “We adopted virtual technology early.” When medically appropriate, they provided either video consultations with more tech-savvy patients or telephone-based virtual visits for patients who preferred a more familiar interface. As LCI starts the process of reentry for patients whose face-to-face encounters were deferred, virtual visits will remain an important facet of maintaining care while limiting exposure for appropriate patients and facilitating adequate space for social distancing in the clinics and infusion centers.
Atrium Health also launched a “virtual hospital” geared to intensified remote management of COVID-19 patients who aren’t sick enough for hospitalization. “People who test positive automatically enter the virtual hospital and have regular interactions with their team of providers,” with LCI providing additional support for their patients who get infected. Patients receive an equipment kit that lets them monitor and transmit their vital signs. The virtual hospital program also helps expedite personal needs like delivery of prescriptions and food. “It helps patients manage at home, and has been incredibly useful,” said Dr. Voorhees.
Perhaps the most challenging step LCI clinicians took to preclude a potential COVID-19 case surge was to review all patients receiving infusional therapy or planned cellular therapy and triage those who could potentially tolerate a temporary change to either an oral, at-home regimen or to a brief hold on their treatment. Some patients on maintenance, outpatient infusion-therapy regimens “expressed concern about coming to the clinic. We looked at the patients scheduled to come for infusions and decided which visits were essential and which were deferrable without disrupting care by briefly using a noninfusional approach,” said Dr. Voorhees. The number of patients who had their regimens modified or held was “relatively small,” and with the recent recognition that a surge of infections has not occurred, “we’re now rolling out cautious reentry of those patients back to their originally prescribed chemotherapy.”
In addition to concerns of exposure at infusion clinics, there are concerns about the heightened susceptibility of immunosuppressed hematologic oncology patients to COVID-19 and their risk for more severe infection. “Our view is that, if patients tested positive, continuing immunosuppressive treatment would likely be detrimental,” so when possible treatment is temporarily suspended and then resumed when the infection has cleared. “When patients test positive for a prolonged period, a decision to resume treatment must be in the best interests of the patient and weigh the benefits of resuming therapy against the risks of incurring a more severe infection by restarting potentially immunosuppressive therapy,” Dr. Voorhees said.
The enhanced risk that cancer patients face if they develop COVID-19 was documented in a recent review of 218 cancer patients hospitalized for COVID-19 during parts of March and April in a large New York health system. The results showed an overall mortality rate of 28%, including a 37% rate among 54 patients with hematologic malignancies and a 25% rate among 164 patients with solid tumors. The mortality rate “may not be quite as high as they reported because that depends on how many patients you test, but there is no question that patients with more comorbidities are at higher risk. Patients with active cancer on chemotherapy are a particularly vulnerable population, and many have expressed concerns about their vulnerability,” he observed.
For the few LCI patients who developed COVID-19 infection, the medical staff has had several therapeutic options they could match to each patient’s needs, with help from the Atrium Health infectious disease team. LCI and Atrium Health are participating in several COVID-19 clinical treatment trials, including an investigational convalescent plasma protocol spearheaded by the Mayo Clinic. They have also opened a randomized, phase 2 trial evaluating the safety and efficacy of selinexor (Xpovio), an oral drug that’s Food and Drug Administration approved for patients with multiple myeloma, for treatment of moderate or severe COVID-19 infection. Additional studies evaluating blockade of granulocyte-macrophage colony-stimulating factor, as well as inhaled antiviral therapy, have recently launched, and several additional studies are poised to open in the coming weeks.
The LCI and Atrium Health team also has a supply of the antiviral agent remdesivir as part of the FDA’s expanded access protocol and emergency use authorization. They also have a supply of and experience administering the interleukin-6 receptor inhibitor tocilizumab (Actemra), which showed some suggestion of efficacy in limited experience treating patients with severe or critical COVID-19 infections. Clinicians at LCI have not used the investigational and unproven agents hydroxychloroquine, chloroquine, and azithromycin to either prevent or treat COVID-19.
LCI also instituted measures to try to minimize the risk that staff members could become infected and transmit the virus while asymptomatic. Following conversations held early on with COVID-19–experienced health authorities in China and Italy, the patient-facing LCI staff split into two teams starting on March 23 that alternated responsibility for direct patient interactions every 2 weeks. When one of these teams was off from direct patient contact they continued to care for patients remotely through virtual technologies. The concept was that, if a staffer became infected while remaining asymptomatic during their contact with patients, their status would either become diagnosable or resolve during their 2 weeks away from seeing any patients. Perhaps in part because of this approach infections among staff members “have not been a big issue. We’ve had an incredibly low infection rate among the LCI staff,” Dr. Voorhees noted.
By mid-May, with the imminent threat of a sudden CODIV-19 surge moderated, heme-onc operations at LCI began to cautiously revert to more normal operations. “We’re continuing patient screening for signs and symptoms of COVID-19 infection, testing for asymptomatic infections, and requiring masking and social distancing in the clinics and hospitals, but we’re starting to slowly restore the number of patients at our clinics [virtual and face to face[ and infusion centers,” and the staff’s division into two teams ended. “The idea was to get past a surge and make sure our system was not overwhelmed. We anticipated a local surge in late April, but then it kept getting pushed back. Current projections are for the infection rate among LCI patients to remain low provided that community spread remains stable or, ideally, decreases.” The LCI infectious disease staff is closely monitoring infection rates for early recognition of an outbreak, with plans to follow any new cases with contact tracing. So far, the COVID-19 pandemic at LCI “has been very manageable,” Dr. Voorhees concluded.
“We’re now better positioned to deal with a case surge if it were to happen. We could resume the two-team approach, hospital-wide plans are now in place for a future surge, and we are now up and running with robust testing and inpatient and outpatient virtual technology. The first time, we were all learning on the fly.”
The LCI biostatistics team has been prospectively collecting the Institutes’s COVID-19 patient data, with plans to report their findings.
Dr. Voorhees has had financial relationships with Bristol-Myers Squibb/Celgene, Janssen, Novartis, and Oncopeptides, none of which are relevant to this article.
Most patients with lichen sclerosus receive appropriate treatment
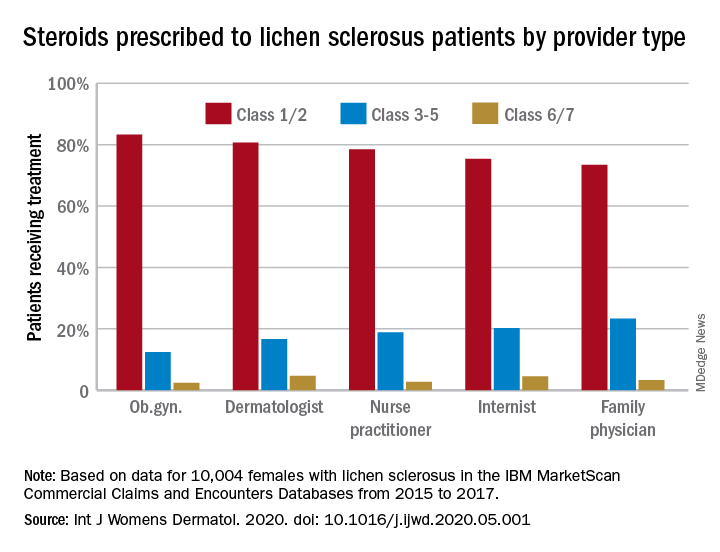
The claims-based prevalence of 0.05% found in the study is lower than previously reported, and only 16% of the diagnoses were in women aged 18-44 years, Laura E. Melnick, MD, and associates wrote after identifying 10,004 females aged 0-65 years with lichen sclerosus in the IBM MarketScan Commercial Claims and Encounters Databases from 2015 to 2017. The majority (79%) of those diagnosed were aged 45-65 years (average, 50.8 years).
In pediatric patients (up to age 17 years), the low prevalence (0.01%) “may be attributable to several factors including relative rarity, as well as variability in pediatric clinicians’ familiarity with [lichen sclerosus] and in patients’ clinical symptoms,” said Dr. Melnick and associates in the department of dermatology at New York University.
Just over half of all diagnoses (52.4%) were made by ob.gyns., with dermatologists next at 14.5%, followed by family physicians (6.5%), nurse practitioners (2.5%), and internists (0.4%), they reported in the International Journal of Women’s Dermatology.
Treatment for lichen sclerosus, in the form of high-potency topical corticosteroids, was mostly appropriate. Ob.gyns. prescribed class 1/2 steroids to 83% of their patients, tops among all clinicians. Dermatologists were just over 80%, and the other clinician categories were all over 70%, the investigators said.
“Understanding the current management of [lichen sclerosus] is important given that un- or undertreated disease can significantly impact patients’ quality of life, lead to increased lower urinary tract symptoms and irreversible architectural changes, and predispose women to squamous cell carcinoma,” they wrote.
SOURCE: Melnick LE et al. Int J Womens Dermatol. 2020. doi: 10.1016/j.ijwd.2020.05.001.

The claims-based prevalence of 0.05% found in the study is lower than previously reported, and only 16% of the diagnoses were in women aged 18-44 years, Laura E. Melnick, MD, and associates wrote after identifying 10,004 females aged 0-65 years with lichen sclerosus in the IBM MarketScan Commercial Claims and Encounters Databases from 2015 to 2017. The majority (79%) of those diagnosed were aged 45-65 years (average, 50.8 years).
In pediatric patients (up to age 17 years), the low prevalence (0.01%) “may be attributable to several factors including relative rarity, as well as variability in pediatric clinicians’ familiarity with [lichen sclerosus] and in patients’ clinical symptoms,” said Dr. Melnick and associates in the department of dermatology at New York University.
Just over half of all diagnoses (52.4%) were made by ob.gyns., with dermatologists next at 14.5%, followed by family physicians (6.5%), nurse practitioners (2.5%), and internists (0.4%), they reported in the International Journal of Women’s Dermatology.
Treatment for lichen sclerosus, in the form of high-potency topical corticosteroids, was mostly appropriate. Ob.gyns. prescribed class 1/2 steroids to 83% of their patients, tops among all clinicians. Dermatologists were just over 80%, and the other clinician categories were all over 70%, the investigators said.
“Understanding the current management of [lichen sclerosus] is important given that un- or undertreated disease can significantly impact patients’ quality of life, lead to increased lower urinary tract symptoms and irreversible architectural changes, and predispose women to squamous cell carcinoma,” they wrote.
SOURCE: Melnick LE et al. Int J Womens Dermatol. 2020. doi: 10.1016/j.ijwd.2020.05.001.

The claims-based prevalence of 0.05% found in the study is lower than previously reported, and only 16% of the diagnoses were in women aged 18-44 years, Laura E. Melnick, MD, and associates wrote after identifying 10,004 females aged 0-65 years with lichen sclerosus in the IBM MarketScan Commercial Claims and Encounters Databases from 2015 to 2017. The majority (79%) of those diagnosed were aged 45-65 years (average, 50.8 years).
In pediatric patients (up to age 17 years), the low prevalence (0.01%) “may be attributable to several factors including relative rarity, as well as variability in pediatric clinicians’ familiarity with [lichen sclerosus] and in patients’ clinical symptoms,” said Dr. Melnick and associates in the department of dermatology at New York University.
Just over half of all diagnoses (52.4%) were made by ob.gyns., with dermatologists next at 14.5%, followed by family physicians (6.5%), nurse practitioners (2.5%), and internists (0.4%), they reported in the International Journal of Women’s Dermatology.
Treatment for lichen sclerosus, in the form of high-potency topical corticosteroids, was mostly appropriate. Ob.gyns. prescribed class 1/2 steroids to 83% of their patients, tops among all clinicians. Dermatologists were just over 80%, and the other clinician categories were all over 70%, the investigators said.
“Understanding the current management of [lichen sclerosus] is important given that un- or undertreated disease can significantly impact patients’ quality of life, lead to increased lower urinary tract symptoms and irreversible architectural changes, and predispose women to squamous cell carcinoma,” they wrote.
SOURCE: Melnick LE et al. Int J Womens Dermatol. 2020. doi: 10.1016/j.ijwd.2020.05.001.
FROM THE INTERNATIONAL JOURNAL OF WOMEN’S DERMATOLOGY
COVID-19 and Mental Health Awareness Month
#howareyoureally challenge seeks to increase access to care
We are months into the COVID-19 crisis, and mental health issues are proving to be rampant. In every crisis, there is opportunity, and this one is no different. The opportunity is clear. For Mental Health Awareness Month and beyond, we must convey a powerful message that mental health is key to our well-being and must be actively addressed. Because almost everyone has felt excess anxiety these last months, we have a unique chance to engage a wider audience.
To address the urgent need, the Mental Health Coalition was formed with the understanding that the mental health crisis is fueled by a pervasive and devastating stigma, preventing millions of individuals from being able to seek the critical treatment they need. Spearheaded by social activist and fashion designer, Kenneth Cole, it is a coalition of leading mental health organizations, brands, celebrities, and advocates who have joined forces to end the stigma surrounding mental health and to change the way people talk about, and care for, mental illness. The group’s mission listed on its website states: “We must increase the conversation around mental health. We must act to end silence, reduce stigma, and engage our community to inspire hope at this essential moment.”
As most of the United States has been under stay-at-home orders, our traditional relationships have been radically disrupted. New types of relationships are forming as we are relying even more on technology to connect us. Social media seems to be on the only “social” we can now safely engage in.
The coalition’s campaign, “#howareyoureally?” is harnessing the power of social media and creating a storytelling platform to allow users to more genuinely share their feelings in these unprecedented times. Celebrities include Whoopi Goldberg, Kendall Jenner, Chris Cuomo, Deepak Chopra, Kesha, and many more have already shared their stories.
“How Are You, Really?” challenges people to answer this question using social media in an open and honest fashion while still providing hope.
The second component of the initiative is to increase access to care, and they have a long list of collaborators, including leading mental health organizations such as the American Foundation for Suicide Prevention, Anxiety and Depression Association of America, Child Mind Institute, Depression and Bipolar Support Alliance, Didi Hirsch Mental Health Services, National Alliance on Mental Illness, and many more.
We have a unique opportunity this Mental Health Awareness Month, and As a community, we must be prepared to meet the escalating needs of our population.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach, Fla. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018) and is the founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world. Dr. Ritvo also is the cofounder of the Bold Beauty Project, a nonprofit group that pairs women with disabilities with photographers who create art exhibitions to raise awareness.
#howareyoureally challenge seeks to increase access to care
#howareyoureally challenge seeks to increase access to care
We are months into the COVID-19 crisis, and mental health issues are proving to be rampant. In every crisis, there is opportunity, and this one is no different. The opportunity is clear. For Mental Health Awareness Month and beyond, we must convey a powerful message that mental health is key to our well-being and must be actively addressed. Because almost everyone has felt excess anxiety these last months, we have a unique chance to engage a wider audience.
To address the urgent need, the Mental Health Coalition was formed with the understanding that the mental health crisis is fueled by a pervasive and devastating stigma, preventing millions of individuals from being able to seek the critical treatment they need. Spearheaded by social activist and fashion designer, Kenneth Cole, it is a coalition of leading mental health organizations, brands, celebrities, and advocates who have joined forces to end the stigma surrounding mental health and to change the way people talk about, and care for, mental illness. The group’s mission listed on its website states: “We must increase the conversation around mental health. We must act to end silence, reduce stigma, and engage our community to inspire hope at this essential moment.”
As most of the United States has been under stay-at-home orders, our traditional relationships have been radically disrupted. New types of relationships are forming as we are relying even more on technology to connect us. Social media seems to be on the only “social” we can now safely engage in.
The coalition’s campaign, “#howareyoureally?” is harnessing the power of social media and creating a storytelling platform to allow users to more genuinely share their feelings in these unprecedented times. Celebrities include Whoopi Goldberg, Kendall Jenner, Chris Cuomo, Deepak Chopra, Kesha, and many more have already shared their stories.
“How Are You, Really?” challenges people to answer this question using social media in an open and honest fashion while still providing hope.
The second component of the initiative is to increase access to care, and they have a long list of collaborators, including leading mental health organizations such as the American Foundation for Suicide Prevention, Anxiety and Depression Association of America, Child Mind Institute, Depression and Bipolar Support Alliance, Didi Hirsch Mental Health Services, National Alliance on Mental Illness, and many more.
We have a unique opportunity this Mental Health Awareness Month, and As a community, we must be prepared to meet the escalating needs of our population.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach, Fla. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018) and is the founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world. Dr. Ritvo also is the cofounder of the Bold Beauty Project, a nonprofit group that pairs women with disabilities with photographers who create art exhibitions to raise awareness.
We are months into the COVID-19 crisis, and mental health issues are proving to be rampant. In every crisis, there is opportunity, and this one is no different. The opportunity is clear. For Mental Health Awareness Month and beyond, we must convey a powerful message that mental health is key to our well-being and must be actively addressed. Because almost everyone has felt excess anxiety these last months, we have a unique chance to engage a wider audience.
To address the urgent need, the Mental Health Coalition was formed with the understanding that the mental health crisis is fueled by a pervasive and devastating stigma, preventing millions of individuals from being able to seek the critical treatment they need. Spearheaded by social activist and fashion designer, Kenneth Cole, it is a coalition of leading mental health organizations, brands, celebrities, and advocates who have joined forces to end the stigma surrounding mental health and to change the way people talk about, and care for, mental illness. The group’s mission listed on its website states: “We must increase the conversation around mental health. We must act to end silence, reduce stigma, and engage our community to inspire hope at this essential moment.”
As most of the United States has been under stay-at-home orders, our traditional relationships have been radically disrupted. New types of relationships are forming as we are relying even more on technology to connect us. Social media seems to be on the only “social” we can now safely engage in.
The coalition’s campaign, “#howareyoureally?” is harnessing the power of social media and creating a storytelling platform to allow users to more genuinely share their feelings in these unprecedented times. Celebrities include Whoopi Goldberg, Kendall Jenner, Chris Cuomo, Deepak Chopra, Kesha, and many more have already shared their stories.
“How Are You, Really?” challenges people to answer this question using social media in an open and honest fashion while still providing hope.
The second component of the initiative is to increase access to care, and they have a long list of collaborators, including leading mental health organizations such as the American Foundation for Suicide Prevention, Anxiety and Depression Association of America, Child Mind Institute, Depression and Bipolar Support Alliance, Didi Hirsch Mental Health Services, National Alliance on Mental Illness, and many more.
We have a unique opportunity this Mental Health Awareness Month, and As a community, we must be prepared to meet the escalating needs of our population.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach, Fla. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018) and is the founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world. Dr. Ritvo also is the cofounder of the Bold Beauty Project, a nonprofit group that pairs women with disabilities with photographers who create art exhibitions to raise awareness.
ACE inhibitors and severe COVID-19: Protective in older patients?
.
In addition, a new meta-analysis of all the available data on the use of ACE inhibitors and angiotensin-receptor blockers (ARBs) in COVID-19–infected patients has concluded that these drugs are not associated with more severe disease and do not increase susceptibility to infection.
The observational study, which was published on the MedRxiv preprint server on May 19 and has not yet been peer reviewed, was conducted by the health insurance company United Heath Group and by Yale University, New Haven, Conn.
The investigators analyzed data from 10,000 patients from across the United States who had tested positive for COVID-19, who were enrolled in Medicare Advantage insurance plans or were commercially insured, and who had received a prescription for one or more antihypertensive medications.
Results showed that the use of ACE inhibitors was associated with an almost 40% lower risk for COVID-19 hospitalization for older people enrolled in Medicare Advantage plans. No such benefit was seen in the younger commercially insured patients or in either group with ARBs.
At a telephone media briefing on the study, senior investigator Harlan M. Krumholz, MD, said: “We don’t believe this is enough info to change practice, but we do think this is an interesting and intriguing result.
“These findings merit a clinical trial to formally test whether ACE inhibitors – which are cheap, widely available, and well-tolerated drugs – can reduce hospitalization of patients infected with COVID-19,” added Dr. Krumholz, professor of medicine at Yale and director of the Yale New Haven Hospital Center for Outcomes Research.
A pragmatic clinical trial is now being planned. In this trial, 10,000 older people who test positive for COVID-19 will be randomly assigned to receive either a low dose of an ACE inhibitor or placebo. It is hoped that recruitment for the trial will begin in June of 2020. It is open to all eligible Americans who are older than 50 years, who test negative for COVID-19, and who are not taking medications for hypertension. Prospective patients can sign up at a dedicated website.
The randomized trial, also conducted by United Health Group and Yale, is said to be “one of the first virtual COVID-19 clinical trials to be launched at scale.”
For the observational study, the researchers identified 2,263 people who were receiving medication for hypertension and who tested positive for COVID-19. Of these, approximately two-thirds were older, Medicare Advantage enrollees; one-third were younger, commercially insured individuals.
In a propensity score–matched analysis, the investigators matched 441 patients who were taking ACE inhibitors to 441 patients who were taking other antihypertensive agents; and 412 patients who were receiving an ARB to 412 patients who were receiving other antihypertensive agents.
Results showed that during a median of 30 days after testing positive, 12.7% of the cohort were hospitalized for COVID-19. In propensity score–matched analyses, neither ACE inhibitors (hazard ratio [HR], 0.77; P = .18) nor ARBs (HR, 0.88; P =.48) were significantly associated with risk for hospitalization.
However, in analyses stratified by the insurance group, ACE inhibitors (but not ARBs) were associated with a significant lower risk for hospitalization among the Medicare group (HR, 0.61; P = .02) but not among the commercially insured group (HR, 2.14; P = .12).
A second study examined outcomes of 7,933 individuals with hypertension who were hospitalized with COVID-19 (92% of these patients were Medicare Advantage enrollees). Of these, 14.2% died, 59.5% survived to discharge, and 26.3% underwent ongoing hospitalization. In propensity score–matched analyses, use of neither an ACE inhibitor (HR, 0.97; P = .74) nor an ARB (HR, 1.15; P = .15) was associated with risk of in-hospital mortality.
The researchers said their findings are consistent with prior evidence from randomized clinical trials suggesting a reduced risk for pneumonia with ACE inhibitors that is not observed with ARBs.
They also cited some preclinical evidence that they said suggests a possible protective role for ACE inhibitors in COVID-19: that ACE inhibitors, but not ARBs, are associated with the upregulation of ACE2 receptors, which modulate the local interactions of the renin-angiotensin-aldosterone system in the lung tissue.
“The presence of ACE2 receptors, therefore, exerts a protective effect against the development of acute lung injury in infections with SARS coronaviruses, which lead to dysregulation of these mechanisms and endothelial damage,” they added. “Further, our observations do not support theoretical concerns of adverse outcomes due to enhanced virulence of SARS coronaviruses due to overexpression of ACE2 receptors in cell cultures – an indirect binding site for these viruses.”
The authors also noted that their findings have “important implications” for four ongoing randomized trials of ACE inhibitors/ARBs in COVID-19, “as none of them align with the observations of our study.”
They pointed out that of the four ongoing trials, three are testing the use of ACE inhibitors or ARBs in the treatment of hospitalized COVID-19 patients, and one is testing the use of a 10-day course of ARBs after a positive SARS-CoV-2 test to prevent hospitalization.
Experts cautious
However, two cardiovascular experts who were asked to comment on this latest study were not overly optimistic about the data.
Michael A. Weber, MD, professor of medicine at the State University of New York, Brooklyn, said: “This report adds to the growing number of observational studies that show varying effects of ACE inhibitors and ARBs in increasing or decreasing hospitalizations for COVID-19 and the likelihood of in-hospital mortality. Overall, this new report differs from others in the remarkable effects of insurance coverage: In particular, for ACE inhibitors, there was a 40% reduction in fatal events in Medicare patients but a twofold increase in patients using commercial insurance – albeit the test for heterogeneity when comparing the two groups did not quite reach statistical significance.
“In essence, these authors are saying that ACE inhibitors are highly protective in patients aged 65 or older but bordering on harmful in patients aged below 65. I agree that it’s worthwhile to check this finding in a prospective trial ... but this hypothesis does seem to be a reach.”
Dr. Weber noted that both ACE inhibitors and ARBs increase the level of the ACE2 enzyme to which the COVID-19 virus binds in the lungs.
“The ACE inhibitors do so by inhibiting the enzyme’s action and thus stimulate further enzyme production; the ARBs block the effects of angiotensin II, which results in high angiotensin II levels that also upregulate ACE2 production,” he said. “Perhaps the ACE inhibitors, by binding to the ACE enzyme, can in some way interfere with the enzyme’s uptake of the COVID virus and thus provide some measure of clinical protection. This is possible, but why would this effect be apparent only in older people?”
John McMurray, MD, professor of medical cardiology at the University of Glasgow, Scotland, added: “This looks like a subgroup of a subgroup type analysis based on small numbers of events – I think there were only 77 hospitalizations among the 722 patients treated with an ACE inhibitor, and the Medicare Advantage subgroup was only 581 of those 722 patients.
“The hazard ratio had wide 95% CI [confidence interval] and a modest P value,” Dr. McMurray added. “So yes, interesting and hypothesis-generating, but not definitive.”
New meta-analysis
The new meta-analysis of all data so far available on ACE inhibitor and ARB use for patients with COVID-19 was published online in Annals of Internal Medicine on May 15.
The analysis is a living, systematic review with ongoing literature surveillance and critical appraisal, which will be updated as new data become available. It included 14 observational studies.
The authors, led by Katherine M. Mackey, MD, VA Portland Health Care System, Oregon, concluded: “High-certainty evidence suggests that ACE-inhibitor or ARB use is not associated with more severe COVID-19 disease, and moderate certainty evidence suggested no association between use of these medications and positive SARS-CoV-2 test results among symptomatic patients. Whether these medications increase the risk for mild or asymptomatic disease or are beneficial in COVID-19 treatment remains uncertain.”
In an accompanying editorial, William G. Kussmaul III, MD, Drexel University, Philadelphia, said that initial fears that these drugs may be harmful for patients with COVID-19 now seem to have been unfounded.
“We now have reasonable reassurance that drugs that alter the renin-angiotensin system do not pose substantial threats as either COVID-19 risk factors or severity multipliers,” he wrote.
A version of this article originally appeared on Medscape.com.
.
In addition, a new meta-analysis of all the available data on the use of ACE inhibitors and angiotensin-receptor blockers (ARBs) in COVID-19–infected patients has concluded that these drugs are not associated with more severe disease and do not increase susceptibility to infection.
The observational study, which was published on the MedRxiv preprint server on May 19 and has not yet been peer reviewed, was conducted by the health insurance company United Heath Group and by Yale University, New Haven, Conn.
The investigators analyzed data from 10,000 patients from across the United States who had tested positive for COVID-19, who were enrolled in Medicare Advantage insurance plans or were commercially insured, and who had received a prescription for one or more antihypertensive medications.
Results showed that the use of ACE inhibitors was associated with an almost 40% lower risk for COVID-19 hospitalization for older people enrolled in Medicare Advantage plans. No such benefit was seen in the younger commercially insured patients or in either group with ARBs.
At a telephone media briefing on the study, senior investigator Harlan M. Krumholz, MD, said: “We don’t believe this is enough info to change practice, but we do think this is an interesting and intriguing result.
“These findings merit a clinical trial to formally test whether ACE inhibitors – which are cheap, widely available, and well-tolerated drugs – can reduce hospitalization of patients infected with COVID-19,” added Dr. Krumholz, professor of medicine at Yale and director of the Yale New Haven Hospital Center for Outcomes Research.
A pragmatic clinical trial is now being planned. In this trial, 10,000 older people who test positive for COVID-19 will be randomly assigned to receive either a low dose of an ACE inhibitor or placebo. It is hoped that recruitment for the trial will begin in June of 2020. It is open to all eligible Americans who are older than 50 years, who test negative for COVID-19, and who are not taking medications for hypertension. Prospective patients can sign up at a dedicated website.
The randomized trial, also conducted by United Health Group and Yale, is said to be “one of the first virtual COVID-19 clinical trials to be launched at scale.”
For the observational study, the researchers identified 2,263 people who were receiving medication for hypertension and who tested positive for COVID-19. Of these, approximately two-thirds were older, Medicare Advantage enrollees; one-third were younger, commercially insured individuals.
In a propensity score–matched analysis, the investigators matched 441 patients who were taking ACE inhibitors to 441 patients who were taking other antihypertensive agents; and 412 patients who were receiving an ARB to 412 patients who were receiving other antihypertensive agents.
Results showed that during a median of 30 days after testing positive, 12.7% of the cohort were hospitalized for COVID-19. In propensity score–matched analyses, neither ACE inhibitors (hazard ratio [HR], 0.77; P = .18) nor ARBs (HR, 0.88; P =.48) were significantly associated with risk for hospitalization.
However, in analyses stratified by the insurance group, ACE inhibitors (but not ARBs) were associated with a significant lower risk for hospitalization among the Medicare group (HR, 0.61; P = .02) but not among the commercially insured group (HR, 2.14; P = .12).
A second study examined outcomes of 7,933 individuals with hypertension who were hospitalized with COVID-19 (92% of these patients were Medicare Advantage enrollees). Of these, 14.2% died, 59.5% survived to discharge, and 26.3% underwent ongoing hospitalization. In propensity score–matched analyses, use of neither an ACE inhibitor (HR, 0.97; P = .74) nor an ARB (HR, 1.15; P = .15) was associated with risk of in-hospital mortality.
The researchers said their findings are consistent with prior evidence from randomized clinical trials suggesting a reduced risk for pneumonia with ACE inhibitors that is not observed with ARBs.
They also cited some preclinical evidence that they said suggests a possible protective role for ACE inhibitors in COVID-19: that ACE inhibitors, but not ARBs, are associated with the upregulation of ACE2 receptors, which modulate the local interactions of the renin-angiotensin-aldosterone system in the lung tissue.
“The presence of ACE2 receptors, therefore, exerts a protective effect against the development of acute lung injury in infections with SARS coronaviruses, which lead to dysregulation of these mechanisms and endothelial damage,” they added. “Further, our observations do not support theoretical concerns of adverse outcomes due to enhanced virulence of SARS coronaviruses due to overexpression of ACE2 receptors in cell cultures – an indirect binding site for these viruses.”
The authors also noted that their findings have “important implications” for four ongoing randomized trials of ACE inhibitors/ARBs in COVID-19, “as none of them align with the observations of our study.”
They pointed out that of the four ongoing trials, three are testing the use of ACE inhibitors or ARBs in the treatment of hospitalized COVID-19 patients, and one is testing the use of a 10-day course of ARBs after a positive SARS-CoV-2 test to prevent hospitalization.
Experts cautious
However, two cardiovascular experts who were asked to comment on this latest study were not overly optimistic about the data.
Michael A. Weber, MD, professor of medicine at the State University of New York, Brooklyn, said: “This report adds to the growing number of observational studies that show varying effects of ACE inhibitors and ARBs in increasing or decreasing hospitalizations for COVID-19 and the likelihood of in-hospital mortality. Overall, this new report differs from others in the remarkable effects of insurance coverage: In particular, for ACE inhibitors, there was a 40% reduction in fatal events in Medicare patients but a twofold increase in patients using commercial insurance – albeit the test for heterogeneity when comparing the two groups did not quite reach statistical significance.
“In essence, these authors are saying that ACE inhibitors are highly protective in patients aged 65 or older but bordering on harmful in patients aged below 65. I agree that it’s worthwhile to check this finding in a prospective trial ... but this hypothesis does seem to be a reach.”
Dr. Weber noted that both ACE inhibitors and ARBs increase the level of the ACE2 enzyme to which the COVID-19 virus binds in the lungs.
“The ACE inhibitors do so by inhibiting the enzyme’s action and thus stimulate further enzyme production; the ARBs block the effects of angiotensin II, which results in high angiotensin II levels that also upregulate ACE2 production,” he said. “Perhaps the ACE inhibitors, by binding to the ACE enzyme, can in some way interfere with the enzyme’s uptake of the COVID virus and thus provide some measure of clinical protection. This is possible, but why would this effect be apparent only in older people?”
John McMurray, MD, professor of medical cardiology at the University of Glasgow, Scotland, added: “This looks like a subgroup of a subgroup type analysis based on small numbers of events – I think there were only 77 hospitalizations among the 722 patients treated with an ACE inhibitor, and the Medicare Advantage subgroup was only 581 of those 722 patients.
“The hazard ratio had wide 95% CI [confidence interval] and a modest P value,” Dr. McMurray added. “So yes, interesting and hypothesis-generating, but not definitive.”
New meta-analysis
The new meta-analysis of all data so far available on ACE inhibitor and ARB use for patients with COVID-19 was published online in Annals of Internal Medicine on May 15.
The analysis is a living, systematic review with ongoing literature surveillance and critical appraisal, which will be updated as new data become available. It included 14 observational studies.
The authors, led by Katherine M. Mackey, MD, VA Portland Health Care System, Oregon, concluded: “High-certainty evidence suggests that ACE-inhibitor or ARB use is not associated with more severe COVID-19 disease, and moderate certainty evidence suggested no association between use of these medications and positive SARS-CoV-2 test results among symptomatic patients. Whether these medications increase the risk for mild or asymptomatic disease or are beneficial in COVID-19 treatment remains uncertain.”
In an accompanying editorial, William G. Kussmaul III, MD, Drexel University, Philadelphia, said that initial fears that these drugs may be harmful for patients with COVID-19 now seem to have been unfounded.
“We now have reasonable reassurance that drugs that alter the renin-angiotensin system do not pose substantial threats as either COVID-19 risk factors or severity multipliers,” he wrote.
A version of this article originally appeared on Medscape.com.
.
In addition, a new meta-analysis of all the available data on the use of ACE inhibitors and angiotensin-receptor blockers (ARBs) in COVID-19–infected patients has concluded that these drugs are not associated with more severe disease and do not increase susceptibility to infection.
The observational study, which was published on the MedRxiv preprint server on May 19 and has not yet been peer reviewed, was conducted by the health insurance company United Heath Group and by Yale University, New Haven, Conn.
The investigators analyzed data from 10,000 patients from across the United States who had tested positive for COVID-19, who were enrolled in Medicare Advantage insurance plans or were commercially insured, and who had received a prescription for one or more antihypertensive medications.
Results showed that the use of ACE inhibitors was associated with an almost 40% lower risk for COVID-19 hospitalization for older people enrolled in Medicare Advantage plans. No such benefit was seen in the younger commercially insured patients or in either group with ARBs.
At a telephone media briefing on the study, senior investigator Harlan M. Krumholz, MD, said: “We don’t believe this is enough info to change practice, but we do think this is an interesting and intriguing result.
“These findings merit a clinical trial to formally test whether ACE inhibitors – which are cheap, widely available, and well-tolerated drugs – can reduce hospitalization of patients infected with COVID-19,” added Dr. Krumholz, professor of medicine at Yale and director of the Yale New Haven Hospital Center for Outcomes Research.
A pragmatic clinical trial is now being planned. In this trial, 10,000 older people who test positive for COVID-19 will be randomly assigned to receive either a low dose of an ACE inhibitor or placebo. It is hoped that recruitment for the trial will begin in June of 2020. It is open to all eligible Americans who are older than 50 years, who test negative for COVID-19, and who are not taking medications for hypertension. Prospective patients can sign up at a dedicated website.
The randomized trial, also conducted by United Health Group and Yale, is said to be “one of the first virtual COVID-19 clinical trials to be launched at scale.”
For the observational study, the researchers identified 2,263 people who were receiving medication for hypertension and who tested positive for COVID-19. Of these, approximately two-thirds were older, Medicare Advantage enrollees; one-third were younger, commercially insured individuals.
In a propensity score–matched analysis, the investigators matched 441 patients who were taking ACE inhibitors to 441 patients who were taking other antihypertensive agents; and 412 patients who were receiving an ARB to 412 patients who were receiving other antihypertensive agents.
Results showed that during a median of 30 days after testing positive, 12.7% of the cohort were hospitalized for COVID-19. In propensity score–matched analyses, neither ACE inhibitors (hazard ratio [HR], 0.77; P = .18) nor ARBs (HR, 0.88; P =.48) were significantly associated with risk for hospitalization.
However, in analyses stratified by the insurance group, ACE inhibitors (but not ARBs) were associated with a significant lower risk for hospitalization among the Medicare group (HR, 0.61; P = .02) but not among the commercially insured group (HR, 2.14; P = .12).
A second study examined outcomes of 7,933 individuals with hypertension who were hospitalized with COVID-19 (92% of these patients were Medicare Advantage enrollees). Of these, 14.2% died, 59.5% survived to discharge, and 26.3% underwent ongoing hospitalization. In propensity score–matched analyses, use of neither an ACE inhibitor (HR, 0.97; P = .74) nor an ARB (HR, 1.15; P = .15) was associated with risk of in-hospital mortality.
The researchers said their findings are consistent with prior evidence from randomized clinical trials suggesting a reduced risk for pneumonia with ACE inhibitors that is not observed with ARBs.
They also cited some preclinical evidence that they said suggests a possible protective role for ACE inhibitors in COVID-19: that ACE inhibitors, but not ARBs, are associated with the upregulation of ACE2 receptors, which modulate the local interactions of the renin-angiotensin-aldosterone system in the lung tissue.
“The presence of ACE2 receptors, therefore, exerts a protective effect against the development of acute lung injury in infections with SARS coronaviruses, which lead to dysregulation of these mechanisms and endothelial damage,” they added. “Further, our observations do not support theoretical concerns of adverse outcomes due to enhanced virulence of SARS coronaviruses due to overexpression of ACE2 receptors in cell cultures – an indirect binding site for these viruses.”
The authors also noted that their findings have “important implications” for four ongoing randomized trials of ACE inhibitors/ARBs in COVID-19, “as none of them align with the observations of our study.”
They pointed out that of the four ongoing trials, three are testing the use of ACE inhibitors or ARBs in the treatment of hospitalized COVID-19 patients, and one is testing the use of a 10-day course of ARBs after a positive SARS-CoV-2 test to prevent hospitalization.
Experts cautious
However, two cardiovascular experts who were asked to comment on this latest study were not overly optimistic about the data.
Michael A. Weber, MD, professor of medicine at the State University of New York, Brooklyn, said: “This report adds to the growing number of observational studies that show varying effects of ACE inhibitors and ARBs in increasing or decreasing hospitalizations for COVID-19 and the likelihood of in-hospital mortality. Overall, this new report differs from others in the remarkable effects of insurance coverage: In particular, for ACE inhibitors, there was a 40% reduction in fatal events in Medicare patients but a twofold increase in patients using commercial insurance – albeit the test for heterogeneity when comparing the two groups did not quite reach statistical significance.
“In essence, these authors are saying that ACE inhibitors are highly protective in patients aged 65 or older but bordering on harmful in patients aged below 65. I agree that it’s worthwhile to check this finding in a prospective trial ... but this hypothesis does seem to be a reach.”
Dr. Weber noted that both ACE inhibitors and ARBs increase the level of the ACE2 enzyme to which the COVID-19 virus binds in the lungs.
“The ACE inhibitors do so by inhibiting the enzyme’s action and thus stimulate further enzyme production; the ARBs block the effects of angiotensin II, which results in high angiotensin II levels that also upregulate ACE2 production,” he said. “Perhaps the ACE inhibitors, by binding to the ACE enzyme, can in some way interfere with the enzyme’s uptake of the COVID virus and thus provide some measure of clinical protection. This is possible, but why would this effect be apparent only in older people?”
John McMurray, MD, professor of medical cardiology at the University of Glasgow, Scotland, added: “This looks like a subgroup of a subgroup type analysis based on small numbers of events – I think there were only 77 hospitalizations among the 722 patients treated with an ACE inhibitor, and the Medicare Advantage subgroup was only 581 of those 722 patients.
“The hazard ratio had wide 95% CI [confidence interval] and a modest P value,” Dr. McMurray added. “So yes, interesting and hypothesis-generating, but not definitive.”
New meta-analysis
The new meta-analysis of all data so far available on ACE inhibitor and ARB use for patients with COVID-19 was published online in Annals of Internal Medicine on May 15.
The analysis is a living, systematic review with ongoing literature surveillance and critical appraisal, which will be updated as new data become available. It included 14 observational studies.
The authors, led by Katherine M. Mackey, MD, VA Portland Health Care System, Oregon, concluded: “High-certainty evidence suggests that ACE-inhibitor or ARB use is not associated with more severe COVID-19 disease, and moderate certainty evidence suggested no association between use of these medications and positive SARS-CoV-2 test results among symptomatic patients. Whether these medications increase the risk for mild or asymptomatic disease or are beneficial in COVID-19 treatment remains uncertain.”
In an accompanying editorial, William G. Kussmaul III, MD, Drexel University, Philadelphia, said that initial fears that these drugs may be harmful for patients with COVID-19 now seem to have been unfounded.
“We now have reasonable reassurance that drugs that alter the renin-angiotensin system do not pose substantial threats as either COVID-19 risk factors or severity multipliers,” he wrote.
A version of this article originally appeared on Medscape.com.
As visits for AMI drop during pandemic, deaths rise
The drastic drop in admissions for acute myocardial infarctions (AMI) during the COVID-19 pandemic in Italy has seen a parallel rise in MI fatality rates in those who do present to hospitals, according to a new report. This gives credence to suggestions that people have avoided hospitals during the pandemic despite life-threatening emergencies.
Salvatore De Rosa, MD, PhD, and colleagues reported their results in the European Heart Journal.
“These data return a frightening picture of about half of AMI patients not reaching out to the hospital at all, which will probably significantly increase mortality for AMI and bring with it a number of patients with post-MI heart failure, despite the fact that acute coronary syndrome management protocols were promptly implemented,” Dr. De Rosa, of Magna Graecia University in Catanzaro, Italy, and associates wrote.
Hospitalizations down
The study counted AMIs at 54 hospital coronary care units nationwide for the week of March 12-19, 2020, at the height of the coronavirus outbreak in northern Italy, and compared that with an equivalent week in 2019. The researchers reported 319 AMIs during the week in 2020, compared with 618 in the equivalent 2019 week, a 48% reduction (P < .001). Although the outbreak was worst in northern Italy, the decline in admissions occurred throughout the country.
An analysis of subtype determined the decline in the incidence of ST-segment elevation MI lagged significantly behind that of non-STEMI. STEMI declined from 268 in 2019 to 197 in 2020, a 27% reduction, while hospitalizations for non-STEMI went from 350 to 122, a 65% reduction.
The researchers also found substantial reductions in hospitalizations for heart failure, by 47%, and atrial fibrillation, by 53%. Incidentally, the mean age of atrial fibrillation patients was considerably younger in 2020: 64.6 vs. 70 years.
Death, complications up
AMI patients who managed to get to the hospital during the pandemic also had worse outcomes. Mortality for STEMI cases more than tripled, to 14% during the outbreak, compared with 4% in 2019 (P < .001) and complication rates increased by 80% to 19% (P = .025). Twenty-one STEMI patients were positive for COVID-19 and more than a quarter (29%) died, which was more than two and a half times the 12% death rate in non–COVID-19 STEMI patients.
Analysis of the STEMI group also found that the care gap for women with heart disease worsened significantly during the pandemic, as they comprised 20.3% of cases this year, compared with 25.4% before the pandemic. Also, the reduction in admissions for STEMI during the pandemic was statistically significant at 41% for women, but not for men at 18%.
Non-STEMI patients fared better overall than STEMI patients, but their outcomes also worsened during the pandemic. Non-STEMI patients were significantly less likely to have percutaneous coronary intervention during the pandemic than previously; the rate declined by 13%, from 77% to 66%. The non-STEMI mortality rate nearly doubled, although not statistically significantly, from 1.7% to 3.3%, whereas complication rates actually more than doubled, from 5.1% to 10.7%, a significant difference. Twelve (9.8%) of the non-STEMI patients were COVID-19 positive, but none died.
Trend extends beyond borders
Dr. De Rosa and colleagues noted that their findings are in line with studies that reported similar declines for STEMI interventions in the United States and Spain during the pandemic (J Am Coll Cardiol. 2020. doi: 10.1016/j.jacc.2020.04.011; REC Interv Cardiol. 2020. doi: 10.24875/RECIC.M20000120).
Additionally, a group at Kaiser Permanente in Northern California also reported a 50% decline in the incidence of AMI hospitalizations during the pandemic (N Engl J Med. 2020 May 19. doi: 10.1056/NEJMc2015630). Likewise, a study of aortic dissections in New York reported a sharp decline in procedures during the pandemic in the city, from 13 to 3 a month (J Am Coll Cardiol. 2020 May 15. doi: 10.1016/j.jacc.2020.05.022)
The researchers in Italy didn’t aim to determine the reasons for the decline in AMI hospitalizations, but Dr. De Rosa and colleagues speculated on the following explanations: Fear of contagion in response to media reports, concentration of resources to address COVID-19 may have engendered a sense to defer less urgent care among patients and health care systems, and a true reduction in acute cardiovascular disease because people under stay-at-home orders had low physical stress.
“The concern is fewer MIs most likely means people are dying at home or presenting later as this study suggests,” said Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix, in interpreting the results of the Italian study.
That could be a result of a mixed message from the media about accessing health care during the pandemic. “What it suggests to a lot of us is that the media has transmitted this notion that hospitals are busy taking care of COVID-19 patients, but we never said don’t come to hospital if you’re having a heart attack,” Dr. Gulati said. “I think we created some sort of fear that patients if they didn’t have COVID-19 they didn’t want to bother physicians.”
Dr. Gulati, whose practice focuses on women with CVD, said the study’s findings that interventions in women dropped more precipitously than men were concerning. “We know already that women don’t do as well after a heart attack, compared to men, and now we see it worsen it even further when women aren’t presenting,” she said. “We’re worried that this is going to increase the gap.”
Dr. DeRosa and colleagues have no relevant financial relationships to disclose.
SOURCE: De Rosa S et al. Euro Heart J. 2020 May 15. doi: 10.1093/eurheartj/ehaa409.
The drastic drop in admissions for acute myocardial infarctions (AMI) during the COVID-19 pandemic in Italy has seen a parallel rise in MI fatality rates in those who do present to hospitals, according to a new report. This gives credence to suggestions that people have avoided hospitals during the pandemic despite life-threatening emergencies.
Salvatore De Rosa, MD, PhD, and colleagues reported their results in the European Heart Journal.
“These data return a frightening picture of about half of AMI patients not reaching out to the hospital at all, which will probably significantly increase mortality for AMI and bring with it a number of patients with post-MI heart failure, despite the fact that acute coronary syndrome management protocols were promptly implemented,” Dr. De Rosa, of Magna Graecia University in Catanzaro, Italy, and associates wrote.
Hospitalizations down
The study counted AMIs at 54 hospital coronary care units nationwide for the week of March 12-19, 2020, at the height of the coronavirus outbreak in northern Italy, and compared that with an equivalent week in 2019. The researchers reported 319 AMIs during the week in 2020, compared with 618 in the equivalent 2019 week, a 48% reduction (P < .001). Although the outbreak was worst in northern Italy, the decline in admissions occurred throughout the country.
An analysis of subtype determined the decline in the incidence of ST-segment elevation MI lagged significantly behind that of non-STEMI. STEMI declined from 268 in 2019 to 197 in 2020, a 27% reduction, while hospitalizations for non-STEMI went from 350 to 122, a 65% reduction.
The researchers also found substantial reductions in hospitalizations for heart failure, by 47%, and atrial fibrillation, by 53%. Incidentally, the mean age of atrial fibrillation patients was considerably younger in 2020: 64.6 vs. 70 years.
Death, complications up
AMI patients who managed to get to the hospital during the pandemic also had worse outcomes. Mortality for STEMI cases more than tripled, to 14% during the outbreak, compared with 4% in 2019 (P < .001) and complication rates increased by 80% to 19% (P = .025). Twenty-one STEMI patients were positive for COVID-19 and more than a quarter (29%) died, which was more than two and a half times the 12% death rate in non–COVID-19 STEMI patients.
Analysis of the STEMI group also found that the care gap for women with heart disease worsened significantly during the pandemic, as they comprised 20.3% of cases this year, compared with 25.4% before the pandemic. Also, the reduction in admissions for STEMI during the pandemic was statistically significant at 41% for women, but not for men at 18%.
Non-STEMI patients fared better overall than STEMI patients, but their outcomes also worsened during the pandemic. Non-STEMI patients were significantly less likely to have percutaneous coronary intervention during the pandemic than previously; the rate declined by 13%, from 77% to 66%. The non-STEMI mortality rate nearly doubled, although not statistically significantly, from 1.7% to 3.3%, whereas complication rates actually more than doubled, from 5.1% to 10.7%, a significant difference. Twelve (9.8%) of the non-STEMI patients were COVID-19 positive, but none died.
Trend extends beyond borders
Dr. De Rosa and colleagues noted that their findings are in line with studies that reported similar declines for STEMI interventions in the United States and Spain during the pandemic (J Am Coll Cardiol. 2020. doi: 10.1016/j.jacc.2020.04.011; REC Interv Cardiol. 2020. doi: 10.24875/RECIC.M20000120).
Additionally, a group at Kaiser Permanente in Northern California also reported a 50% decline in the incidence of AMI hospitalizations during the pandemic (N Engl J Med. 2020 May 19. doi: 10.1056/NEJMc2015630). Likewise, a study of aortic dissections in New York reported a sharp decline in procedures during the pandemic in the city, from 13 to 3 a month (J Am Coll Cardiol. 2020 May 15. doi: 10.1016/j.jacc.2020.05.022)
The researchers in Italy didn’t aim to determine the reasons for the decline in AMI hospitalizations, but Dr. De Rosa and colleagues speculated on the following explanations: Fear of contagion in response to media reports, concentration of resources to address COVID-19 may have engendered a sense to defer less urgent care among patients and health care systems, and a true reduction in acute cardiovascular disease because people under stay-at-home orders had low physical stress.
“The concern is fewer MIs most likely means people are dying at home or presenting later as this study suggests,” said Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix, in interpreting the results of the Italian study.
That could be a result of a mixed message from the media about accessing health care during the pandemic. “What it suggests to a lot of us is that the media has transmitted this notion that hospitals are busy taking care of COVID-19 patients, but we never said don’t come to hospital if you’re having a heart attack,” Dr. Gulati said. “I think we created some sort of fear that patients if they didn’t have COVID-19 they didn’t want to bother physicians.”
Dr. Gulati, whose practice focuses on women with CVD, said the study’s findings that interventions in women dropped more precipitously than men were concerning. “We know already that women don’t do as well after a heart attack, compared to men, and now we see it worsen it even further when women aren’t presenting,” she said. “We’re worried that this is going to increase the gap.”
Dr. DeRosa and colleagues have no relevant financial relationships to disclose.
SOURCE: De Rosa S et al. Euro Heart J. 2020 May 15. doi: 10.1093/eurheartj/ehaa409.
The drastic drop in admissions for acute myocardial infarctions (AMI) during the COVID-19 pandemic in Italy has seen a parallel rise in MI fatality rates in those who do present to hospitals, according to a new report. This gives credence to suggestions that people have avoided hospitals during the pandemic despite life-threatening emergencies.
Salvatore De Rosa, MD, PhD, and colleagues reported their results in the European Heart Journal.
“These data return a frightening picture of about half of AMI patients not reaching out to the hospital at all, which will probably significantly increase mortality for AMI and bring with it a number of patients with post-MI heart failure, despite the fact that acute coronary syndrome management protocols were promptly implemented,” Dr. De Rosa, of Magna Graecia University in Catanzaro, Italy, and associates wrote.
Hospitalizations down
The study counted AMIs at 54 hospital coronary care units nationwide for the week of March 12-19, 2020, at the height of the coronavirus outbreak in northern Italy, and compared that with an equivalent week in 2019. The researchers reported 319 AMIs during the week in 2020, compared with 618 in the equivalent 2019 week, a 48% reduction (P < .001). Although the outbreak was worst in northern Italy, the decline in admissions occurred throughout the country.
An analysis of subtype determined the decline in the incidence of ST-segment elevation MI lagged significantly behind that of non-STEMI. STEMI declined from 268 in 2019 to 197 in 2020, a 27% reduction, while hospitalizations for non-STEMI went from 350 to 122, a 65% reduction.
The researchers also found substantial reductions in hospitalizations for heart failure, by 47%, and atrial fibrillation, by 53%. Incidentally, the mean age of atrial fibrillation patients was considerably younger in 2020: 64.6 vs. 70 years.
Death, complications up
AMI patients who managed to get to the hospital during the pandemic also had worse outcomes. Mortality for STEMI cases more than tripled, to 14% during the outbreak, compared with 4% in 2019 (P < .001) and complication rates increased by 80% to 19% (P = .025). Twenty-one STEMI patients were positive for COVID-19 and more than a quarter (29%) died, which was more than two and a half times the 12% death rate in non–COVID-19 STEMI patients.
Analysis of the STEMI group also found that the care gap for women with heart disease worsened significantly during the pandemic, as they comprised 20.3% of cases this year, compared with 25.4% before the pandemic. Also, the reduction in admissions for STEMI during the pandemic was statistically significant at 41% for women, but not for men at 18%.
Non-STEMI patients fared better overall than STEMI patients, but their outcomes also worsened during the pandemic. Non-STEMI patients were significantly less likely to have percutaneous coronary intervention during the pandemic than previously; the rate declined by 13%, from 77% to 66%. The non-STEMI mortality rate nearly doubled, although not statistically significantly, from 1.7% to 3.3%, whereas complication rates actually more than doubled, from 5.1% to 10.7%, a significant difference. Twelve (9.8%) of the non-STEMI patients were COVID-19 positive, but none died.
Trend extends beyond borders
Dr. De Rosa and colleagues noted that their findings are in line with studies that reported similar declines for STEMI interventions in the United States and Spain during the pandemic (J Am Coll Cardiol. 2020. doi: 10.1016/j.jacc.2020.04.011; REC Interv Cardiol. 2020. doi: 10.24875/RECIC.M20000120).
Additionally, a group at Kaiser Permanente in Northern California also reported a 50% decline in the incidence of AMI hospitalizations during the pandemic (N Engl J Med. 2020 May 19. doi: 10.1056/NEJMc2015630). Likewise, a study of aortic dissections in New York reported a sharp decline in procedures during the pandemic in the city, from 13 to 3 a month (J Am Coll Cardiol. 2020 May 15. doi: 10.1016/j.jacc.2020.05.022)
The researchers in Italy didn’t aim to determine the reasons for the decline in AMI hospitalizations, but Dr. De Rosa and colleagues speculated on the following explanations: Fear of contagion in response to media reports, concentration of resources to address COVID-19 may have engendered a sense to defer less urgent care among patients and health care systems, and a true reduction in acute cardiovascular disease because people under stay-at-home orders had low physical stress.
“The concern is fewer MIs most likely means people are dying at home or presenting later as this study suggests,” said Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix, in interpreting the results of the Italian study.
That could be a result of a mixed message from the media about accessing health care during the pandemic. “What it suggests to a lot of us is that the media has transmitted this notion that hospitals are busy taking care of COVID-19 patients, but we never said don’t come to hospital if you’re having a heart attack,” Dr. Gulati said. “I think we created some sort of fear that patients if they didn’t have COVID-19 they didn’t want to bother physicians.”
Dr. Gulati, whose practice focuses on women with CVD, said the study’s findings that interventions in women dropped more precipitously than men were concerning. “We know already that women don’t do as well after a heart attack, compared to men, and now we see it worsen it even further when women aren’t presenting,” she said. “We’re worried that this is going to increase the gap.”
Dr. DeRosa and colleagues have no relevant financial relationships to disclose.
SOURCE: De Rosa S et al. Euro Heart J. 2020 May 15. doi: 10.1093/eurheartj/ehaa409.
FROM THE EUROPEAN HEART JOURNAL
Today’s top news highlights: COVID-19 vaccine hurdles, new options in prostate cancer
Here are the stories our MDedge editors across specialties think you need to know about today:
COVID-19 vaccines face tough road
Vaccine-induced neutralizing antibodies may not be sufficient to reliably provide sustained protection against SARS-CoV-2 infection. Rather, a successful vaccine against coronavirus will likely need to incorporate T-cell epitopes to induce a long-term memory T-cell immune response to the virus, Mehrdad Matloubian, MD, PhD, predicted at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium. “In one study, 20 of 26 patients with SARS had lost their antibody response by 6 years post infection. And they had no B-cell immunity against the SARS antigens. The good news is they did have T-cell memory against SARS virus, and people with more severe disease tended to have more T-cell memory against SARS. All of this has really important implications for vaccine development,” observed Dr. Matloubian, a rheumatologist at the University of California, San Francisco. READ MORE
Chilblain-like lesions in children with suspected COVID-19
Reports are growing of cases of children with suspected COVID-19 and chilblain-like lesions. Most recently, there were two reports in Spain and Italy. These symptoms should be considered a sign of infection with the virus, but the symptoms themselves typically don’t require treatment, according to the authors of the two new reports, which were published in Pediatric Dermatology. READ MORE
FDA approves olaparib in metastatic prostate cancer
The Food and Drug Administration approved olaparib (Lynparza) for deleterious or suspected deleterious germline or somatic homologous recombination repair (HRR) gene-mutated metastatic castration-resistant prostate cancer (mCRPC). The drug is limited to use in men who have progressed following prior treatment with enzalutamide or abiraterone. The agency also recently approved rucaparib (Rubraca) for use in patients with mCRPC that harbor deleterious BRCA mutations (germline and/or somatic). READ MORE
Drugs, alcohol, suicide
Deaths from drugs, alcohol, and suicide are on the rise, despite recent decreases in opioid overdose deaths. A report released May 21 by the Trust for America’s Health (TFAH) and the Well Being Trust shows that 151,964 Americans died in 2018 from alcohol, drugs, and suicide. Experts warn that these deaths may increase in the wake of COVID-19. “We know what works to address deaths of despair but progress has been uneven and death rates continue to climb, with communities of color experiencing higher rates of increases in drug-induced and alcohol deaths,” said TFAH President and CEO John Auerbach. READ MORE
Guidance on managing suspected stroke during COVID-19
The American Heart Association/American Stroke Association has developed a “conceptual framework” to assist emergency medical service providers and in-hospital triage teams handle suspected cases of acute stroke during the ongoing COVID-19 crisis and future pandemics. The main factors to guide the triage decision are the likelihood of a large vessel occlusion; the magnitude of additional delays because of inter-hospital transfer and work flow efficiency at the primary stroke center or acute stroke ready hospital; the need for advanced critical care resources; and the availability of bed, staff, and PPE resources at the hospitals. READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
COVID-19 vaccines face tough road
Vaccine-induced neutralizing antibodies may not be sufficient to reliably provide sustained protection against SARS-CoV-2 infection. Rather, a successful vaccine against coronavirus will likely need to incorporate T-cell epitopes to induce a long-term memory T-cell immune response to the virus, Mehrdad Matloubian, MD, PhD, predicted at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium. “In one study, 20 of 26 patients with SARS had lost their antibody response by 6 years post infection. And they had no B-cell immunity against the SARS antigens. The good news is they did have T-cell memory against SARS virus, and people with more severe disease tended to have more T-cell memory against SARS. All of this has really important implications for vaccine development,” observed Dr. Matloubian, a rheumatologist at the University of California, San Francisco. READ MORE
Chilblain-like lesions in children with suspected COVID-19
Reports are growing of cases of children with suspected COVID-19 and chilblain-like lesions. Most recently, there were two reports in Spain and Italy. These symptoms should be considered a sign of infection with the virus, but the symptoms themselves typically don’t require treatment, according to the authors of the two new reports, which were published in Pediatric Dermatology. READ MORE
FDA approves olaparib in metastatic prostate cancer
The Food and Drug Administration approved olaparib (Lynparza) for deleterious or suspected deleterious germline or somatic homologous recombination repair (HRR) gene-mutated metastatic castration-resistant prostate cancer (mCRPC). The drug is limited to use in men who have progressed following prior treatment with enzalutamide or abiraterone. The agency also recently approved rucaparib (Rubraca) for use in patients with mCRPC that harbor deleterious BRCA mutations (germline and/or somatic). READ MORE
Drugs, alcohol, suicide
Deaths from drugs, alcohol, and suicide are on the rise, despite recent decreases in opioid overdose deaths. A report released May 21 by the Trust for America’s Health (TFAH) and the Well Being Trust shows that 151,964 Americans died in 2018 from alcohol, drugs, and suicide. Experts warn that these deaths may increase in the wake of COVID-19. “We know what works to address deaths of despair but progress has been uneven and death rates continue to climb, with communities of color experiencing higher rates of increases in drug-induced and alcohol deaths,” said TFAH President and CEO John Auerbach. READ MORE
Guidance on managing suspected stroke during COVID-19
The American Heart Association/American Stroke Association has developed a “conceptual framework” to assist emergency medical service providers and in-hospital triage teams handle suspected cases of acute stroke during the ongoing COVID-19 crisis and future pandemics. The main factors to guide the triage decision are the likelihood of a large vessel occlusion; the magnitude of additional delays because of inter-hospital transfer and work flow efficiency at the primary stroke center or acute stroke ready hospital; the need for advanced critical care resources; and the availability of bed, staff, and PPE resources at the hospitals. READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
COVID-19 vaccines face tough road
Vaccine-induced neutralizing antibodies may not be sufficient to reliably provide sustained protection against SARS-CoV-2 infection. Rather, a successful vaccine against coronavirus will likely need to incorporate T-cell epitopes to induce a long-term memory T-cell immune response to the virus, Mehrdad Matloubian, MD, PhD, predicted at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium. “In one study, 20 of 26 patients with SARS had lost their antibody response by 6 years post infection. And they had no B-cell immunity against the SARS antigens. The good news is they did have T-cell memory against SARS virus, and people with more severe disease tended to have more T-cell memory against SARS. All of this has really important implications for vaccine development,” observed Dr. Matloubian, a rheumatologist at the University of California, San Francisco. READ MORE
Chilblain-like lesions in children with suspected COVID-19
Reports are growing of cases of children with suspected COVID-19 and chilblain-like lesions. Most recently, there were two reports in Spain and Italy. These symptoms should be considered a sign of infection with the virus, but the symptoms themselves typically don’t require treatment, according to the authors of the two new reports, which were published in Pediatric Dermatology. READ MORE
FDA approves olaparib in metastatic prostate cancer
The Food and Drug Administration approved olaparib (Lynparza) for deleterious or suspected deleterious germline or somatic homologous recombination repair (HRR) gene-mutated metastatic castration-resistant prostate cancer (mCRPC). The drug is limited to use in men who have progressed following prior treatment with enzalutamide or abiraterone. The agency also recently approved rucaparib (Rubraca) for use in patients with mCRPC that harbor deleterious BRCA mutations (germline and/or somatic). READ MORE
Drugs, alcohol, suicide
Deaths from drugs, alcohol, and suicide are on the rise, despite recent decreases in opioid overdose deaths. A report released May 21 by the Trust for America’s Health (TFAH) and the Well Being Trust shows that 151,964 Americans died in 2018 from alcohol, drugs, and suicide. Experts warn that these deaths may increase in the wake of COVID-19. “We know what works to address deaths of despair but progress has been uneven and death rates continue to climb, with communities of color experiencing higher rates of increases in drug-induced and alcohol deaths,” said TFAH President and CEO John Auerbach. READ MORE
Guidance on managing suspected stroke during COVID-19
The American Heart Association/American Stroke Association has developed a “conceptual framework” to assist emergency medical service providers and in-hospital triage teams handle suspected cases of acute stroke during the ongoing COVID-19 crisis and future pandemics. The main factors to guide the triage decision are the likelihood of a large vessel occlusion; the magnitude of additional delays because of inter-hospital transfer and work flow efficiency at the primary stroke center or acute stroke ready hospital; the need for advanced critical care resources; and the availability of bed, staff, and PPE resources at the hospitals. READ MORE
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
COVID-19 may cause subacute thyroiditis
Coronavirus disease of 2019 (COVID-19) may lead to subacute thyroiditis in some patients, which is suspected to have viral or postviral origin, especially with upper respiratory tract infections, according to a case study in the Journal of Clinical Endocrinology & Metabolism.
Alessandro Brancatella, a PhD student at the University Hospital Pisa (Italy), and colleagues described the case of an 18-year-old woman who was tested Feb. 21 for SARS-CoV-2 infection after her father was hospitalized because of COVID-19. Her results were positive for the virus, and not long after, she developed mild symptoms. By March 13 and again on March 14, test swabs for SARS-CoV-2 were both negative.
On March 17, she presented with fever, fatigue, palpitations, and neck pain that radiated to her jaw. Testing and physical examination pointed to subacute thyroiditis, and she was soon diagnosed and treated with prednisone. Her neck pain and fever disappeared within 2 days, and the remaining symptoms went away within a week.
The authors noted that the woman’s thyroid had been evaluated before she tested positive for SARS-CoV-2, and at that time, thyroid disease was ruled out. They also pointed out that, although the exact etiology for subacute thyroiditis is unknown, “it is common opinion that the disease is due to a viral infection or to a post-viral inflammatory reaction in genetically predisposed subjects.” They cited examples of viruses with suspected causal associations, including mumps, Epstein-Barr virus, and HIV, and they suggested that, based on the timing of the woman’s subacute thyroiditis and the normal results of her thyroid evaluation before developing COVID-19, SARS-CoV-2 be added to that list.
“To our knowledge, this is the first case of [subacute thyroiditis] related to SARS-CoV-2,” they concluded. “We therefore believe that physicians should be alerted about the possibility of this additional clinical manifestation related to SARS-CoV-2 infection.”
One author reported funding from the University of Pisa.
SOURCE: Brancatella A et al. J Clin Endocrinol Metab. 2020 May 21. doi: 10.1210/clinem/dgaa276.
Coronavirus disease of 2019 (COVID-19) may lead to subacute thyroiditis in some patients, which is suspected to have viral or postviral origin, especially with upper respiratory tract infections, according to a case study in the Journal of Clinical Endocrinology & Metabolism.
Alessandro Brancatella, a PhD student at the University Hospital Pisa (Italy), and colleagues described the case of an 18-year-old woman who was tested Feb. 21 for SARS-CoV-2 infection after her father was hospitalized because of COVID-19. Her results were positive for the virus, and not long after, she developed mild symptoms. By March 13 and again on March 14, test swabs for SARS-CoV-2 were both negative.
On March 17, she presented with fever, fatigue, palpitations, and neck pain that radiated to her jaw. Testing and physical examination pointed to subacute thyroiditis, and she was soon diagnosed and treated with prednisone. Her neck pain and fever disappeared within 2 days, and the remaining symptoms went away within a week.
The authors noted that the woman’s thyroid had been evaluated before she tested positive for SARS-CoV-2, and at that time, thyroid disease was ruled out. They also pointed out that, although the exact etiology for subacute thyroiditis is unknown, “it is common opinion that the disease is due to a viral infection or to a post-viral inflammatory reaction in genetically predisposed subjects.” They cited examples of viruses with suspected causal associations, including mumps, Epstein-Barr virus, and HIV, and they suggested that, based on the timing of the woman’s subacute thyroiditis and the normal results of her thyroid evaluation before developing COVID-19, SARS-CoV-2 be added to that list.
“To our knowledge, this is the first case of [subacute thyroiditis] related to SARS-CoV-2,” they concluded. “We therefore believe that physicians should be alerted about the possibility of this additional clinical manifestation related to SARS-CoV-2 infection.”
One author reported funding from the University of Pisa.
SOURCE: Brancatella A et al. J Clin Endocrinol Metab. 2020 May 21. doi: 10.1210/clinem/dgaa276.
Coronavirus disease of 2019 (COVID-19) may lead to subacute thyroiditis in some patients, which is suspected to have viral or postviral origin, especially with upper respiratory tract infections, according to a case study in the Journal of Clinical Endocrinology & Metabolism.
Alessandro Brancatella, a PhD student at the University Hospital Pisa (Italy), and colleagues described the case of an 18-year-old woman who was tested Feb. 21 for SARS-CoV-2 infection after her father was hospitalized because of COVID-19. Her results were positive for the virus, and not long after, she developed mild symptoms. By March 13 and again on March 14, test swabs for SARS-CoV-2 were both negative.
On March 17, she presented with fever, fatigue, palpitations, and neck pain that radiated to her jaw. Testing and physical examination pointed to subacute thyroiditis, and she was soon diagnosed and treated with prednisone. Her neck pain and fever disappeared within 2 days, and the remaining symptoms went away within a week.
The authors noted that the woman’s thyroid had been evaluated before she tested positive for SARS-CoV-2, and at that time, thyroid disease was ruled out. They also pointed out that, although the exact etiology for subacute thyroiditis is unknown, “it is common opinion that the disease is due to a viral infection or to a post-viral inflammatory reaction in genetically predisposed subjects.” They cited examples of viruses with suspected causal associations, including mumps, Epstein-Barr virus, and HIV, and they suggested that, based on the timing of the woman’s subacute thyroiditis and the normal results of her thyroid evaluation before developing COVID-19, SARS-CoV-2 be added to that list.
“To our knowledge, this is the first case of [subacute thyroiditis] related to SARS-CoV-2,” they concluded. “We therefore believe that physicians should be alerted about the possibility of this additional clinical manifestation related to SARS-CoV-2 infection.”
One author reported funding from the University of Pisa.
SOURCE: Brancatella A et al. J Clin Endocrinol Metab. 2020 May 21. doi: 10.1210/clinem/dgaa276.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
COVID-19 vaccine won’t be a slam dunk
A successful vaccine for prevention of SARS-CoV-2 infection will probably need to incorporate T-cell epitopes to induce a long-term memory T-cell immune response to the virus, Mehrdad Matloubian, MD, PhD, predicted at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
Vaccine-induced neutralizing antibodies may not be sufficient to reliably provide sustained protection against infection. In mouse studies, T-cell immunity has protected against reinfection with the novel coronaviruses. And in some but not all studies of patients infected with the SARS virus, which shares 80% genetic overlap with the SARS-CoV-2 virus responsible for the COVID-19 pandemic, neutralizing antibodies have waned over time.
“In one study, 20 of 26 patients with SARS had lost their antibody response by 6 years post infection. And they had no B-cell immunity against the SARS antigens. The good news is they did have T-cell memory against SARS virus, and people with more severe disease tended to have more T-cell memory against SARS. All of this has really important implications for vaccine development,” observed Dr. Matloubian, a rheumatologist at the University of California, San Francisco.
Dr. Matloubian is among those who are convinced that the ongoing massive global accelerated effort to develop a safe and effective vaccine affords the best opportunity to gain the upper hand in the COVID-19 pandemic. A large array of vaccines are in development.
A key safety concern to watch for in the coming months is whether a vaccine candidate is able to sidestep the issue of antibody-dependent enhancement, whereby prior infection with a non-SARS coronavirus, such as those that cause the common cold, might result in creation of rogue subneutralizing coronavirus antibodies in response to vaccination. There is concern that these nonneutralizing antibodies could facilitate entry of the virus into monocytes and other cells lacking the ACE2 receptor, its usual portal of entry. This in turn could trigger expanded viral replication, a hyperinflammatory response, and viral spread to sites beyond the lung, such as the heart or kidneys.
Little optimism about antivirals’ impact
Dr. Matloubian predicted that antiviral medications, including the much-ballyhooed remdesivir, are unlikely to be a game changer in the COVID-19 pandemic. That’s because most patients who become symptomatic don’t do so until at least 2 days post infection. By that point, their viral load has already peaked and is waning and the B- and T-cell immune responses are starting to gear up.
“Timing seems to be everything when it comes to treatment with antivirals,” he observed. “The virus titer is usually declining by the time people present with severe COVID-19, suggesting that at this time antiviral therapy might be of little use to change the course of the disease, especially if it’s mainly immune-mediated by then. Even with influenza virus, there’s a really short window where Tamiflu [oseltamivir] is effective. It’s going to be the same case for antivirals used for treatment of COVID-19.”
He noted that in a placebo-controlled, randomized trial of remdesivir in 236 Chinese patients with severe COVID-19, intravenous remdesivir wasn’t associated with a significantly shorter time to clinical improvement, although there was a trend in that direction in the subgroup with symptom duration of 10 days or less at initiation of treatment.
A National Institutes of Health press release announcing that remdesivir had a positive impact on duration of hospitalization in a separate randomized trial drew enormous attention from a public desperate for good news. However, the full study has yet to be published, and it’s unclear when during the disease course the antiviral agent was started.
“We need a blockbuster antiviral that’s oral, highly effective, and doesn’t have any side effects to be used in prophylaxis of health care workers and for people who are exposed by family members being infected. And so far there is no such thing, even on the horizon,” according to the rheumatologist.
Fellow panelist Jinoos Yazdany, MD, concurred.
“As we talk to experts around the country, it seems like there isn’t very much optimism about such a blockbuster drug. Most people are actually putting their hope in a vaccine,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
Another research priority is identification of biomarkers in blood or bronchoalveolar lavage fluid to identify early on the subgroup of infected patients who are likely to crash and develop severe disease. That would permit a targeted approach to inhibition of the inflammatory pathways contributing to development of acute respiratory distress syndrome before this full-blown cytokine storm-like syndrome can occur. There is great interest in trying to achieve this by repurposing many biologic agents widely used by rheumatologists, including the interleukin-1 blocker anakinra (Kineret) and the IL-6 blocker tocilizumab (Actemra).
Dr. Matloubian reported having no financial conflicts of interest regarding his presentation.
A successful vaccine for prevention of SARS-CoV-2 infection will probably need to incorporate T-cell epitopes to induce a long-term memory T-cell immune response to the virus, Mehrdad Matloubian, MD, PhD, predicted at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
Vaccine-induced neutralizing antibodies may not be sufficient to reliably provide sustained protection against infection. In mouse studies, T-cell immunity has protected against reinfection with the novel coronaviruses. And in some but not all studies of patients infected with the SARS virus, which shares 80% genetic overlap with the SARS-CoV-2 virus responsible for the COVID-19 pandemic, neutralizing antibodies have waned over time.
“In one study, 20 of 26 patients with SARS had lost their antibody response by 6 years post infection. And they had no B-cell immunity against the SARS antigens. The good news is they did have T-cell memory against SARS virus, and people with more severe disease tended to have more T-cell memory against SARS. All of this has really important implications for vaccine development,” observed Dr. Matloubian, a rheumatologist at the University of California, San Francisco.
Dr. Matloubian is among those who are convinced that the ongoing massive global accelerated effort to develop a safe and effective vaccine affords the best opportunity to gain the upper hand in the COVID-19 pandemic. A large array of vaccines are in development.
A key safety concern to watch for in the coming months is whether a vaccine candidate is able to sidestep the issue of antibody-dependent enhancement, whereby prior infection with a non-SARS coronavirus, such as those that cause the common cold, might result in creation of rogue subneutralizing coronavirus antibodies in response to vaccination. There is concern that these nonneutralizing antibodies could facilitate entry of the virus into monocytes and other cells lacking the ACE2 receptor, its usual portal of entry. This in turn could trigger expanded viral replication, a hyperinflammatory response, and viral spread to sites beyond the lung, such as the heart or kidneys.
Little optimism about antivirals’ impact
Dr. Matloubian predicted that antiviral medications, including the much-ballyhooed remdesivir, are unlikely to be a game changer in the COVID-19 pandemic. That’s because most patients who become symptomatic don’t do so until at least 2 days post infection. By that point, their viral load has already peaked and is waning and the B- and T-cell immune responses are starting to gear up.
“Timing seems to be everything when it comes to treatment with antivirals,” he observed. “The virus titer is usually declining by the time people present with severe COVID-19, suggesting that at this time antiviral therapy might be of little use to change the course of the disease, especially if it’s mainly immune-mediated by then. Even with influenza virus, there’s a really short window where Tamiflu [oseltamivir] is effective. It’s going to be the same case for antivirals used for treatment of COVID-19.”
He noted that in a placebo-controlled, randomized trial of remdesivir in 236 Chinese patients with severe COVID-19, intravenous remdesivir wasn’t associated with a significantly shorter time to clinical improvement, although there was a trend in that direction in the subgroup with symptom duration of 10 days or less at initiation of treatment.
A National Institutes of Health press release announcing that remdesivir had a positive impact on duration of hospitalization in a separate randomized trial drew enormous attention from a public desperate for good news. However, the full study has yet to be published, and it’s unclear when during the disease course the antiviral agent was started.
“We need a blockbuster antiviral that’s oral, highly effective, and doesn’t have any side effects to be used in prophylaxis of health care workers and for people who are exposed by family members being infected. And so far there is no such thing, even on the horizon,” according to the rheumatologist.
Fellow panelist Jinoos Yazdany, MD, concurred.
“As we talk to experts around the country, it seems like there isn’t very much optimism about such a blockbuster drug. Most people are actually putting their hope in a vaccine,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
Another research priority is identification of biomarkers in blood or bronchoalveolar lavage fluid to identify early on the subgroup of infected patients who are likely to crash and develop severe disease. That would permit a targeted approach to inhibition of the inflammatory pathways contributing to development of acute respiratory distress syndrome before this full-blown cytokine storm-like syndrome can occur. There is great interest in trying to achieve this by repurposing many biologic agents widely used by rheumatologists, including the interleukin-1 blocker anakinra (Kineret) and the IL-6 blocker tocilizumab (Actemra).
Dr. Matloubian reported having no financial conflicts of interest regarding his presentation.
A successful vaccine for prevention of SARS-CoV-2 infection will probably need to incorporate T-cell epitopes to induce a long-term memory T-cell immune response to the virus, Mehrdad Matloubian, MD, PhD, predicted at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
Vaccine-induced neutralizing antibodies may not be sufficient to reliably provide sustained protection against infection. In mouse studies, T-cell immunity has protected against reinfection with the novel coronaviruses. And in some but not all studies of patients infected with the SARS virus, which shares 80% genetic overlap with the SARS-CoV-2 virus responsible for the COVID-19 pandemic, neutralizing antibodies have waned over time.
“In one study, 20 of 26 patients with SARS had lost their antibody response by 6 years post infection. And they had no B-cell immunity against the SARS antigens. The good news is they did have T-cell memory against SARS virus, and people with more severe disease tended to have more T-cell memory against SARS. All of this has really important implications for vaccine development,” observed Dr. Matloubian, a rheumatologist at the University of California, San Francisco.
Dr. Matloubian is among those who are convinced that the ongoing massive global accelerated effort to develop a safe and effective vaccine affords the best opportunity to gain the upper hand in the COVID-19 pandemic. A large array of vaccines are in development.
A key safety concern to watch for in the coming months is whether a vaccine candidate is able to sidestep the issue of antibody-dependent enhancement, whereby prior infection with a non-SARS coronavirus, such as those that cause the common cold, might result in creation of rogue subneutralizing coronavirus antibodies in response to vaccination. There is concern that these nonneutralizing antibodies could facilitate entry of the virus into monocytes and other cells lacking the ACE2 receptor, its usual portal of entry. This in turn could trigger expanded viral replication, a hyperinflammatory response, and viral spread to sites beyond the lung, such as the heart or kidneys.
Little optimism about antivirals’ impact
Dr. Matloubian predicted that antiviral medications, including the much-ballyhooed remdesivir, are unlikely to be a game changer in the COVID-19 pandemic. That’s because most patients who become symptomatic don’t do so until at least 2 days post infection. By that point, their viral load has already peaked and is waning and the B- and T-cell immune responses are starting to gear up.
“Timing seems to be everything when it comes to treatment with antivirals,” he observed. “The virus titer is usually declining by the time people present with severe COVID-19, suggesting that at this time antiviral therapy might be of little use to change the course of the disease, especially if it’s mainly immune-mediated by then. Even with influenza virus, there’s a really short window where Tamiflu [oseltamivir] is effective. It’s going to be the same case for antivirals used for treatment of COVID-19.”
He noted that in a placebo-controlled, randomized trial of remdesivir in 236 Chinese patients with severe COVID-19, intravenous remdesivir wasn’t associated with a significantly shorter time to clinical improvement, although there was a trend in that direction in the subgroup with symptom duration of 10 days or less at initiation of treatment.
A National Institutes of Health press release announcing that remdesivir had a positive impact on duration of hospitalization in a separate randomized trial drew enormous attention from a public desperate for good news. However, the full study has yet to be published, and it’s unclear when during the disease course the antiviral agent was started.
“We need a blockbuster antiviral that’s oral, highly effective, and doesn’t have any side effects to be used in prophylaxis of health care workers and for people who are exposed by family members being infected. And so far there is no such thing, even on the horizon,” according to the rheumatologist.
Fellow panelist Jinoos Yazdany, MD, concurred.
“As we talk to experts around the country, it seems like there isn’t very much optimism about such a blockbuster drug. Most people are actually putting their hope in a vaccine,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
Another research priority is identification of biomarkers in blood or bronchoalveolar lavage fluid to identify early on the subgroup of infected patients who are likely to crash and develop severe disease. That would permit a targeted approach to inhibition of the inflammatory pathways contributing to development of acute respiratory distress syndrome before this full-blown cytokine storm-like syndrome can occur. There is great interest in trying to achieve this by repurposing many biologic agents widely used by rheumatologists, including the interleukin-1 blocker anakinra (Kineret) and the IL-6 blocker tocilizumab (Actemra).
Dr. Matloubian reported having no financial conflicts of interest regarding his presentation.
FROM SOTA 2020
COVID-19: Delirium first, depression, anxiety, insomnia later?
Severe COVID-19 may cause delirium in the acute stage of illness, followed by the possibility of depression, anxiety, fatigue, insomnia, and posttraumatic stress disorder (PTSD) over the longer term, new research suggests.
Results from “the first systematic review and meta-analysis of the psychiatric consequences of coronavirus infection” showed that previous coronavirus epidemics were associated with a significant psychiatric burden in both the acute and post-illness stages.
“Most people with COVID-19 will not develop any mental health problems, even among those with severe cases requiring hospitalization, but given the huge numbers of people getting sick, the global impact on mental health could be considerable,” co–lead investigator Jonathan Rogers, MRCPsych, Department of Psychiatry, University College London, United Kingdom, said in a news release.
The study was published online May 18 in Lancet Psychiatry.
Need for Monitoring, Support
The researchers analyzed 65 peer-reviewed studies and seven preprint articles with data on acute and post-illness psychiatric and neuropsychiatric features of patients who had been hospitalized with COVID-19, as well as two other diseases caused by coronaviruses – severe acute respiratory syndrome (SARS), in 2002–2004, and Middle East respiratory syndrome (MERS), in 2012.
“Our main findings are that signs suggestive of delirium are common in the acute stage of SARS, MERS, and COVID-19; there is evidence of depression, anxiety, fatigue, and post-traumatic stress disorder in the post-illness stage of previous coronavirus epidemics, but there are few data yet on COVID-19,” the investigators write.
The data show that among patients acutely ill with SARS and MERS, 28% experienced confusion, 33% had depressed mood, 36% had anxiety, 34% suffered from impaired memory, and 42% had insomnia.
After recovery from SARS and MERS, sleep disorder, frequent recall of traumatic memories, emotional lability, impaired concentration, fatigue, and impaired memory were reported in more than 15% of patients during a follow-up period that ranged from 6 weeks to 39 months.
In a meta-analysis, the point prevalence in the post-illness stage was 32% for PTSD and about 15% for depression and anxiety.
In patients acutely ill with severe COVID-19, available data suggest that 65% experience delirium, 69% have agitation after withdrawal of sedation, and 21% have altered consciousness.
In one study, 33% of patients had a dysexecutive syndrome at discharge, characterized by symptoms such as inattention, disorientation, or poorly organized movements in response to command. Currently, data are very limited regarding patients who have recovered from COVID-19, the investigators caution.
“, and monitored after they recover to ensure they do not develop mental illnesses, and are able to access treatment if needed,” senior author Anthony David, FMedSci, from UCL Institute of Mental Health, said in a news release.
“While most people with COVID-19 will recover without experiencing mental illness, we need to research which factors may contribute to enduring mental health problems, and develop interventions to prevent and treat them,” he added.
Be Prepared
The coauthors of a linked commentary say it makes sense, from a biological perspective, to merge data on these three coronavirus diseases, given the degree to which they resemble each other.
They caution, however, that treatment of COVID-19 seems to be different from treatment of SARS and MERS. In addition, the social and economic situation of COVID-19 survivors’ return is completely different from that of SARS and MERS survivors.
Findings from previous coronavirus outbreaks are “useful, but might not be exact predictors of prevalences of psychiatric complications for patients with COVID-19,” write Iris Sommer, MD, PhD, from University Medical Center Groningen, the Netherlands, and P. Roberto Bakker, MD, PhD, from Maastricht University Medical Center, the Netherlands.
“The warning from [this study] that we should prepare to treat large numbers of patients with COVID-19 who go on to develop delirium, post-traumatic stress disorder, anxiety, and depression is an important message for the psychiatric community,” they add.
Sommer and Bakker also say the reported estimates of prevalence in this study should be interpreted with caution, “as true numbers of both acute and long-term psychiatric disorders for patients with COVID-19 might be considerably higher.”
Funding for the study was provided by the Wellcome Trust, the UK National Institute for Health Research (NIHR), the UK Medical Research Council, the NIHR Biomedical Research Center at the University College London Hospitals NHS Foundation Trust, and the University College London. The authors of the study and the commentary have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Severe COVID-19 may cause delirium in the acute stage of illness, followed by the possibility of depression, anxiety, fatigue, insomnia, and posttraumatic stress disorder (PTSD) over the longer term, new research suggests.
Results from “the first systematic review and meta-analysis of the psychiatric consequences of coronavirus infection” showed that previous coronavirus epidemics were associated with a significant psychiatric burden in both the acute and post-illness stages.
“Most people with COVID-19 will not develop any mental health problems, even among those with severe cases requiring hospitalization, but given the huge numbers of people getting sick, the global impact on mental health could be considerable,” co–lead investigator Jonathan Rogers, MRCPsych, Department of Psychiatry, University College London, United Kingdom, said in a news release.
The study was published online May 18 in Lancet Psychiatry.
Need for Monitoring, Support
The researchers analyzed 65 peer-reviewed studies and seven preprint articles with data on acute and post-illness psychiatric and neuropsychiatric features of patients who had been hospitalized with COVID-19, as well as two other diseases caused by coronaviruses – severe acute respiratory syndrome (SARS), in 2002–2004, and Middle East respiratory syndrome (MERS), in 2012.
“Our main findings are that signs suggestive of delirium are common in the acute stage of SARS, MERS, and COVID-19; there is evidence of depression, anxiety, fatigue, and post-traumatic stress disorder in the post-illness stage of previous coronavirus epidemics, but there are few data yet on COVID-19,” the investigators write.
The data show that among patients acutely ill with SARS and MERS, 28% experienced confusion, 33% had depressed mood, 36% had anxiety, 34% suffered from impaired memory, and 42% had insomnia.
After recovery from SARS and MERS, sleep disorder, frequent recall of traumatic memories, emotional lability, impaired concentration, fatigue, and impaired memory were reported in more than 15% of patients during a follow-up period that ranged from 6 weeks to 39 months.
In a meta-analysis, the point prevalence in the post-illness stage was 32% for PTSD and about 15% for depression and anxiety.
In patients acutely ill with severe COVID-19, available data suggest that 65% experience delirium, 69% have agitation after withdrawal of sedation, and 21% have altered consciousness.
In one study, 33% of patients had a dysexecutive syndrome at discharge, characterized by symptoms such as inattention, disorientation, or poorly organized movements in response to command. Currently, data are very limited regarding patients who have recovered from COVID-19, the investigators caution.
“, and monitored after they recover to ensure they do not develop mental illnesses, and are able to access treatment if needed,” senior author Anthony David, FMedSci, from UCL Institute of Mental Health, said in a news release.
“While most people with COVID-19 will recover without experiencing mental illness, we need to research which factors may contribute to enduring mental health problems, and develop interventions to prevent and treat them,” he added.
Be Prepared
The coauthors of a linked commentary say it makes sense, from a biological perspective, to merge data on these three coronavirus diseases, given the degree to which they resemble each other.
They caution, however, that treatment of COVID-19 seems to be different from treatment of SARS and MERS. In addition, the social and economic situation of COVID-19 survivors’ return is completely different from that of SARS and MERS survivors.
Findings from previous coronavirus outbreaks are “useful, but might not be exact predictors of prevalences of psychiatric complications for patients with COVID-19,” write Iris Sommer, MD, PhD, from University Medical Center Groningen, the Netherlands, and P. Roberto Bakker, MD, PhD, from Maastricht University Medical Center, the Netherlands.
“The warning from [this study] that we should prepare to treat large numbers of patients with COVID-19 who go on to develop delirium, post-traumatic stress disorder, anxiety, and depression is an important message for the psychiatric community,” they add.
Sommer and Bakker also say the reported estimates of prevalence in this study should be interpreted with caution, “as true numbers of both acute and long-term psychiatric disorders for patients with COVID-19 might be considerably higher.”
Funding for the study was provided by the Wellcome Trust, the UK National Institute for Health Research (NIHR), the UK Medical Research Council, the NIHR Biomedical Research Center at the University College London Hospitals NHS Foundation Trust, and the University College London. The authors of the study and the commentary have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Severe COVID-19 may cause delirium in the acute stage of illness, followed by the possibility of depression, anxiety, fatigue, insomnia, and posttraumatic stress disorder (PTSD) over the longer term, new research suggests.
Results from “the first systematic review and meta-analysis of the psychiatric consequences of coronavirus infection” showed that previous coronavirus epidemics were associated with a significant psychiatric burden in both the acute and post-illness stages.
“Most people with COVID-19 will not develop any mental health problems, even among those with severe cases requiring hospitalization, but given the huge numbers of people getting sick, the global impact on mental health could be considerable,” co–lead investigator Jonathan Rogers, MRCPsych, Department of Psychiatry, University College London, United Kingdom, said in a news release.
The study was published online May 18 in Lancet Psychiatry.
Need for Monitoring, Support
The researchers analyzed 65 peer-reviewed studies and seven preprint articles with data on acute and post-illness psychiatric and neuropsychiatric features of patients who had been hospitalized with COVID-19, as well as two other diseases caused by coronaviruses – severe acute respiratory syndrome (SARS), in 2002–2004, and Middle East respiratory syndrome (MERS), in 2012.
“Our main findings are that signs suggestive of delirium are common in the acute stage of SARS, MERS, and COVID-19; there is evidence of depression, anxiety, fatigue, and post-traumatic stress disorder in the post-illness stage of previous coronavirus epidemics, but there are few data yet on COVID-19,” the investigators write.
The data show that among patients acutely ill with SARS and MERS, 28% experienced confusion, 33% had depressed mood, 36% had anxiety, 34% suffered from impaired memory, and 42% had insomnia.
After recovery from SARS and MERS, sleep disorder, frequent recall of traumatic memories, emotional lability, impaired concentration, fatigue, and impaired memory were reported in more than 15% of patients during a follow-up period that ranged from 6 weeks to 39 months.
In a meta-analysis, the point prevalence in the post-illness stage was 32% for PTSD and about 15% for depression and anxiety.
In patients acutely ill with severe COVID-19, available data suggest that 65% experience delirium, 69% have agitation after withdrawal of sedation, and 21% have altered consciousness.
In one study, 33% of patients had a dysexecutive syndrome at discharge, characterized by symptoms such as inattention, disorientation, or poorly organized movements in response to command. Currently, data are very limited regarding patients who have recovered from COVID-19, the investigators caution.
“, and monitored after they recover to ensure they do not develop mental illnesses, and are able to access treatment if needed,” senior author Anthony David, FMedSci, from UCL Institute of Mental Health, said in a news release.
“While most people with COVID-19 will recover without experiencing mental illness, we need to research which factors may contribute to enduring mental health problems, and develop interventions to prevent and treat them,” he added.
Be Prepared
The coauthors of a linked commentary say it makes sense, from a biological perspective, to merge data on these three coronavirus diseases, given the degree to which they resemble each other.
They caution, however, that treatment of COVID-19 seems to be different from treatment of SARS and MERS. In addition, the social and economic situation of COVID-19 survivors’ return is completely different from that of SARS and MERS survivors.
Findings from previous coronavirus outbreaks are “useful, but might not be exact predictors of prevalences of psychiatric complications for patients with COVID-19,” write Iris Sommer, MD, PhD, from University Medical Center Groningen, the Netherlands, and P. Roberto Bakker, MD, PhD, from Maastricht University Medical Center, the Netherlands.
“The warning from [this study] that we should prepare to treat large numbers of patients with COVID-19 who go on to develop delirium, post-traumatic stress disorder, anxiety, and depression is an important message for the psychiatric community,” they add.
Sommer and Bakker also say the reported estimates of prevalence in this study should be interpreted with caution, “as true numbers of both acute and long-term psychiatric disorders for patients with COVID-19 might be considerably higher.”
Funding for the study was provided by the Wellcome Trust, the UK National Institute for Health Research (NIHR), the UK Medical Research Council, the NIHR Biomedical Research Center at the University College London Hospitals NHS Foundation Trust, and the University College London. The authors of the study and the commentary have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Chilblain-like lesions reported in children thought to have COVID-19
Two and elsewhere.
These symptoms should be considered a sign of infection with the virus, but the symptoms themselves typically don’t require treatment, according to the authors of the two new reports, from hospitals in Milan and Madrid, published in Pediatric Dermatology.
In the first study, Cristiana Colonna, MD, and colleagues at Hospital Maggiore Polyclinic in Milan described four cases of chilblain-like lesions in children ages 5-11 years with mild COVID-19 symptoms.
In the second, David Andina, MD, and colleagues in the ED and the departments of dermatology and pathology at the Child Jesus University Children’s Hospital in Madrid published a retrospective study of 22 cases in children and adolescents ages 6-17 years who reported to the hospital ED from April 6 to 17, the peak of the pandemic in Madrid.
In all four of the Milan cases, the skin lesions appeared several days after the onset of COVID-19 symptoms, although all four patients initially tested negative for COVID-19. However, Dr. Colonna and colleagues wrote that, “given the fact that the sensitivity and specificity of both nasopharyngeal swabs and antibody tests for COVID-19 (when available) are not 100% reliable, the question of the origin of these strange chilblain-like lesions is still elusive.” Until further studies are available, they emphasized that clinicians should be “alert to the presentation of chilblain-like findings” in children with mild symptoms “as a possible sign of COVID-19 infection.”
All the patients had lesions on their feet or toes, and a 5-year-old boy also had lesions on the right hand. One patient, an 11-year-old girl, had a biopsy that revealed dense lymphocytic perivascular cuffing and periadnexal infiltration.
“The finding of an elevated d-dimer in one of our patients, along with the clinical features suggestive of a vasoocclusive phenomenon, supports consideration of laboratory evaluation for coagulation defects in asymptomatic or mildly symptomatic children with acrovasculitis-like findings,” Dr. Colonna and colleagues wrote. None of the four cases in Milan required treatment, with three cases resolving within 5 days.
Like the Milan cases, all 22 patients in the Madrid series had foot or toe lesions and three had lesions on the fingers. This larger series also reported more detailed symptoms about the lesions: pruritus in nine patients (41%) and mild pain in seven (32%). A total of 10 patients had systemic symptoms of COVID-19, predominantly cough and rhinorrhea in 9 patients (41%), but 2 (9%) had abdominal pain and diarrhea. These symptoms, the authors said, appeared a median of 14 days (range, 1-28 days) before they developed chilblains.
A total of 19 patients were tested for COVID-19, but only 1 was positive.
This retrospective study also included contact information, with one patient having household contact with a single confirmed case of COVID-19; 12 patients recalled household contact who were considered probable cases of COVID-19, with respiratory symptoms.
Skin biopsies were obtained from the acral lesions in six patients, all showing similar results, although with varying degrees of intensity. All biopsies showed features of lymphocytic vasculopathy. Some cases showed mild dermal and perieccrine mucinosis, lymphocytic eccrine hidradenitis, vascular ectasia, red cell extravasation and focal thrombosis described as “mostly confined to scattered papillary dermal capillaries, but also in vessels of the reticular dermis.”
The only treatments Dr. Andina and colleagues reported were oral analgesics for pain and oral antihistamines for pruritus when needed. One patient was given topical corticosteroids and another a short course of oral steroids, both for erythema multiforme.
Dr. Andina and colleagues wrote that the skin lesions in these patients “were unequivocally categorized as chilblains, both clinically and histopathologically,” and, after 7-10 days, began to fade. None of the patients had complications, and had an “excellent outcome,” they noted.
Dr. Colonna and colleagues had no conflicts of interest to declare. Dr. Andina and colleagues provided no disclosure statement.
SOURCES: Colonna C et al. Ped Derm. 2020 May 6. doi: 10.1111/pde.14210; Andina D et al. Ped Derm. 2020 May 9. doi: 10.1111/pde.14215.
Two and elsewhere.
These symptoms should be considered a sign of infection with the virus, but the symptoms themselves typically don’t require treatment, according to the authors of the two new reports, from hospitals in Milan and Madrid, published in Pediatric Dermatology.
In the first study, Cristiana Colonna, MD, and colleagues at Hospital Maggiore Polyclinic in Milan described four cases of chilblain-like lesions in children ages 5-11 years with mild COVID-19 symptoms.
In the second, David Andina, MD, and colleagues in the ED and the departments of dermatology and pathology at the Child Jesus University Children’s Hospital in Madrid published a retrospective study of 22 cases in children and adolescents ages 6-17 years who reported to the hospital ED from April 6 to 17, the peak of the pandemic in Madrid.
In all four of the Milan cases, the skin lesions appeared several days after the onset of COVID-19 symptoms, although all four patients initially tested negative for COVID-19. However, Dr. Colonna and colleagues wrote that, “given the fact that the sensitivity and specificity of both nasopharyngeal swabs and antibody tests for COVID-19 (when available) are not 100% reliable, the question of the origin of these strange chilblain-like lesions is still elusive.” Until further studies are available, they emphasized that clinicians should be “alert to the presentation of chilblain-like findings” in children with mild symptoms “as a possible sign of COVID-19 infection.”
All the patients had lesions on their feet or toes, and a 5-year-old boy also had lesions on the right hand. One patient, an 11-year-old girl, had a biopsy that revealed dense lymphocytic perivascular cuffing and periadnexal infiltration.
“The finding of an elevated d-dimer in one of our patients, along with the clinical features suggestive of a vasoocclusive phenomenon, supports consideration of laboratory evaluation for coagulation defects in asymptomatic or mildly symptomatic children with acrovasculitis-like findings,” Dr. Colonna and colleagues wrote. None of the four cases in Milan required treatment, with three cases resolving within 5 days.
Like the Milan cases, all 22 patients in the Madrid series had foot or toe lesions and three had lesions on the fingers. This larger series also reported more detailed symptoms about the lesions: pruritus in nine patients (41%) and mild pain in seven (32%). A total of 10 patients had systemic symptoms of COVID-19, predominantly cough and rhinorrhea in 9 patients (41%), but 2 (9%) had abdominal pain and diarrhea. These symptoms, the authors said, appeared a median of 14 days (range, 1-28 days) before they developed chilblains.
A total of 19 patients were tested for COVID-19, but only 1 was positive.
This retrospective study also included contact information, with one patient having household contact with a single confirmed case of COVID-19; 12 patients recalled household contact who were considered probable cases of COVID-19, with respiratory symptoms.
Skin biopsies were obtained from the acral lesions in six patients, all showing similar results, although with varying degrees of intensity. All biopsies showed features of lymphocytic vasculopathy. Some cases showed mild dermal and perieccrine mucinosis, lymphocytic eccrine hidradenitis, vascular ectasia, red cell extravasation and focal thrombosis described as “mostly confined to scattered papillary dermal capillaries, but also in vessels of the reticular dermis.”
The only treatments Dr. Andina and colleagues reported were oral analgesics for pain and oral antihistamines for pruritus when needed. One patient was given topical corticosteroids and another a short course of oral steroids, both for erythema multiforme.
Dr. Andina and colleagues wrote that the skin lesions in these patients “were unequivocally categorized as chilblains, both clinically and histopathologically,” and, after 7-10 days, began to fade. None of the patients had complications, and had an “excellent outcome,” they noted.
Dr. Colonna and colleagues had no conflicts of interest to declare. Dr. Andina and colleagues provided no disclosure statement.
SOURCES: Colonna C et al. Ped Derm. 2020 May 6. doi: 10.1111/pde.14210; Andina D et al. Ped Derm. 2020 May 9. doi: 10.1111/pde.14215.
Two and elsewhere.
These symptoms should be considered a sign of infection with the virus, but the symptoms themselves typically don’t require treatment, according to the authors of the two new reports, from hospitals in Milan and Madrid, published in Pediatric Dermatology.
In the first study, Cristiana Colonna, MD, and colleagues at Hospital Maggiore Polyclinic in Milan described four cases of chilblain-like lesions in children ages 5-11 years with mild COVID-19 symptoms.
In the second, David Andina, MD, and colleagues in the ED and the departments of dermatology and pathology at the Child Jesus University Children’s Hospital in Madrid published a retrospective study of 22 cases in children and adolescents ages 6-17 years who reported to the hospital ED from April 6 to 17, the peak of the pandemic in Madrid.
In all four of the Milan cases, the skin lesions appeared several days after the onset of COVID-19 symptoms, although all four patients initially tested negative for COVID-19. However, Dr. Colonna and colleagues wrote that, “given the fact that the sensitivity and specificity of both nasopharyngeal swabs and antibody tests for COVID-19 (when available) are not 100% reliable, the question of the origin of these strange chilblain-like lesions is still elusive.” Until further studies are available, they emphasized that clinicians should be “alert to the presentation of chilblain-like findings” in children with mild symptoms “as a possible sign of COVID-19 infection.”
All the patients had lesions on their feet or toes, and a 5-year-old boy also had lesions on the right hand. One patient, an 11-year-old girl, had a biopsy that revealed dense lymphocytic perivascular cuffing and periadnexal infiltration.
“The finding of an elevated d-dimer in one of our patients, along with the clinical features suggestive of a vasoocclusive phenomenon, supports consideration of laboratory evaluation for coagulation defects in asymptomatic or mildly symptomatic children with acrovasculitis-like findings,” Dr. Colonna and colleagues wrote. None of the four cases in Milan required treatment, with three cases resolving within 5 days.
Like the Milan cases, all 22 patients in the Madrid series had foot or toe lesions and three had lesions on the fingers. This larger series also reported more detailed symptoms about the lesions: pruritus in nine patients (41%) and mild pain in seven (32%). A total of 10 patients had systemic symptoms of COVID-19, predominantly cough and rhinorrhea in 9 patients (41%), but 2 (9%) had abdominal pain and diarrhea. These symptoms, the authors said, appeared a median of 14 days (range, 1-28 days) before they developed chilblains.
A total of 19 patients were tested for COVID-19, but only 1 was positive.
This retrospective study also included contact information, with one patient having household contact with a single confirmed case of COVID-19; 12 patients recalled household contact who were considered probable cases of COVID-19, with respiratory symptoms.
Skin biopsies were obtained from the acral lesions in six patients, all showing similar results, although with varying degrees of intensity. All biopsies showed features of lymphocytic vasculopathy. Some cases showed mild dermal and perieccrine mucinosis, lymphocytic eccrine hidradenitis, vascular ectasia, red cell extravasation and focal thrombosis described as “mostly confined to scattered papillary dermal capillaries, but also in vessels of the reticular dermis.”
The only treatments Dr. Andina and colleagues reported were oral analgesics for pain and oral antihistamines for pruritus when needed. One patient was given topical corticosteroids and another a short course of oral steroids, both for erythema multiforme.
Dr. Andina and colleagues wrote that the skin lesions in these patients “were unequivocally categorized as chilblains, both clinically and histopathologically,” and, after 7-10 days, began to fade. None of the patients had complications, and had an “excellent outcome,” they noted.
Dr. Colonna and colleagues had no conflicts of interest to declare. Dr. Andina and colleagues provided no disclosure statement.
SOURCES: Colonna C et al. Ped Derm. 2020 May 6. doi: 10.1111/pde.14210; Andina D et al. Ped Derm. 2020 May 9. doi: 10.1111/pde.14215.
FROM PEDIATRIC DERMATOLOGY






