User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Hydrogen peroxide reduces C. acnes cultures following shoulder surgery
Prior to shoulder surgery, application of 3% hydrogen peroxide is a simple and inexpensive strategy to reduce the risk of postoperative cultures of Cutibacterium acnes, according to findings from a prospective randomized trial. The results were reported in an abstract scheduled for release at the annual meeting of the American Academy of Orthopaedic Surgeons. The meeting was canceled because of COVID-19.
“This approach is simple, cheap, and does not rely on patient compliance,” explained Surena Namdari, MD, associate professor of orthopedic surgery at Thomas Jefferson University, Philadelphia.
C. acnes, formerly known as Propionibacterium acnes, is increasingly seen as an important target for prevention of postoperative shoulder infections because of published reports that it is the most commonly isolated bacterium from such infections, Dr. Namdari said in an interview.
In the prospective, randomized trial, male patients scheduled for shoulder arthroscopy were recruited if they did not have active acne, history of psoriatic or eczematous lesions, or recent antibiotic use. Most of the preoperative preparation of the surgical site was the same in the experimental and control arms. This included hair clipping, application of 2% chlorhexidine, and cleansing with saturated 7.5% povidone-iodine solution surgical scrub brushes.
The difference was that 3% hydrogen peroxide–soaked gauzes were applied to perioperative skin of those randomized to the experimental group but not to controls. All patients received routine preoperative oral antibiotics as well as perioperative applications of a formulation containing 2% chlorhexidine gluconate and 70% isopropyl alcohol.
Following surgery, 11 (18.6%) of the 59 patients in the experimental arm versus 23 (34.8%) of the 66 patients randomized to the control group had positive cultures for C. acnes (P = .047), according to the trial results, which have now been published (J Shoulder Elbow Surg. 2020;29:212-6).
There were no cases of skin reactions in either the experimental or control groups.
Topical skin cleansers that contain peroxide, such as benzoyl peroxide, have been shown to have a C. acnes decolonizing effect if applied repeatedly in the days prior to surgery, but Dr. Namdari suggested the problem with this approach is that it depends on patient compliance. A prophylaxis included in the preoperative routine eliminates this potential problem.
C. acnes is an anaerobic bacterium that is part of the resident flora of the skin around several joints, including the knee and the hip, but it is particularly common in the posterior shoulder. Colonization has been found substantially more common in men than in women, according to Dr. Namdari.
The specific threat posed by C. acnes to risk of postoperative infections “is still being defined,” and this trial was not large enough to associate the reduction in postoperative C. acnes cultures with a reduced risk of an adverse clinical outcome, but Dr. Namdari says that the data do show that the nearly 50% reduction in positive cultures was achieved efficiently and inexpensively with no apparent risk.
Several previous studies have also evaluated strategies for reducing C. acnes skin burden on the basis of expected protection against postoperative infection. In one, which associated a 3-day preoperative course of benzoyl peroxide with a reduction in the skin burden of C. acnes, the authors also concluded that this approach deserves consideration in routine skin preparation for shoulder arthroplasty (J Shoulder Elbow Surg. 2018;27:1539-44).
“We believe that a preoperative skin prep protocol that reduces C. acnes load on the skin would likely lead to reduced postoperative infections,” reported the senior author, Mohit N. Gilotra, MD, assistant professor, University of Maryland, Baltimore. Contacted about the rationale for reducing C. acnes skin burden without objective evidence of an impact on postoperative infection risk, Dr. Gilotra indicated these strategies make sense.
“It seems to be true for staph infections and is a reasonable assumption to make here,” he added. “Future work will help determine how much benzoyl peroxide, hydrogen peroxide, or other skin prep can reduce surgical site infection.”
Dr. Namdari reports financial relationships with multiple device and pharmaceutical companies but none relevant to this study.
SOURCE: Namdari S et al. AAOS 2020. Abstract P0808.
Prior to shoulder surgery, application of 3% hydrogen peroxide is a simple and inexpensive strategy to reduce the risk of postoperative cultures of Cutibacterium acnes, according to findings from a prospective randomized trial. The results were reported in an abstract scheduled for release at the annual meeting of the American Academy of Orthopaedic Surgeons. The meeting was canceled because of COVID-19.
“This approach is simple, cheap, and does not rely on patient compliance,” explained Surena Namdari, MD, associate professor of orthopedic surgery at Thomas Jefferson University, Philadelphia.
C. acnes, formerly known as Propionibacterium acnes, is increasingly seen as an important target for prevention of postoperative shoulder infections because of published reports that it is the most commonly isolated bacterium from such infections, Dr. Namdari said in an interview.
In the prospective, randomized trial, male patients scheduled for shoulder arthroscopy were recruited if they did not have active acne, history of psoriatic or eczematous lesions, or recent antibiotic use. Most of the preoperative preparation of the surgical site was the same in the experimental and control arms. This included hair clipping, application of 2% chlorhexidine, and cleansing with saturated 7.5% povidone-iodine solution surgical scrub brushes.
The difference was that 3% hydrogen peroxide–soaked gauzes were applied to perioperative skin of those randomized to the experimental group but not to controls. All patients received routine preoperative oral antibiotics as well as perioperative applications of a formulation containing 2% chlorhexidine gluconate and 70% isopropyl alcohol.
Following surgery, 11 (18.6%) of the 59 patients in the experimental arm versus 23 (34.8%) of the 66 patients randomized to the control group had positive cultures for C. acnes (P = .047), according to the trial results, which have now been published (J Shoulder Elbow Surg. 2020;29:212-6).
There were no cases of skin reactions in either the experimental or control groups.
Topical skin cleansers that contain peroxide, such as benzoyl peroxide, have been shown to have a C. acnes decolonizing effect if applied repeatedly in the days prior to surgery, but Dr. Namdari suggested the problem with this approach is that it depends on patient compliance. A prophylaxis included in the preoperative routine eliminates this potential problem.
C. acnes is an anaerobic bacterium that is part of the resident flora of the skin around several joints, including the knee and the hip, but it is particularly common in the posterior shoulder. Colonization has been found substantially more common in men than in women, according to Dr. Namdari.
The specific threat posed by C. acnes to risk of postoperative infections “is still being defined,” and this trial was not large enough to associate the reduction in postoperative C. acnes cultures with a reduced risk of an adverse clinical outcome, but Dr. Namdari says that the data do show that the nearly 50% reduction in positive cultures was achieved efficiently and inexpensively with no apparent risk.
Several previous studies have also evaluated strategies for reducing C. acnes skin burden on the basis of expected protection against postoperative infection. In one, which associated a 3-day preoperative course of benzoyl peroxide with a reduction in the skin burden of C. acnes, the authors also concluded that this approach deserves consideration in routine skin preparation for shoulder arthroplasty (J Shoulder Elbow Surg. 2018;27:1539-44).
“We believe that a preoperative skin prep protocol that reduces C. acnes load on the skin would likely lead to reduced postoperative infections,” reported the senior author, Mohit N. Gilotra, MD, assistant professor, University of Maryland, Baltimore. Contacted about the rationale for reducing C. acnes skin burden without objective evidence of an impact on postoperative infection risk, Dr. Gilotra indicated these strategies make sense.
“It seems to be true for staph infections and is a reasonable assumption to make here,” he added. “Future work will help determine how much benzoyl peroxide, hydrogen peroxide, or other skin prep can reduce surgical site infection.”
Dr. Namdari reports financial relationships with multiple device and pharmaceutical companies but none relevant to this study.
SOURCE: Namdari S et al. AAOS 2020. Abstract P0808.
Prior to shoulder surgery, application of 3% hydrogen peroxide is a simple and inexpensive strategy to reduce the risk of postoperative cultures of Cutibacterium acnes, according to findings from a prospective randomized trial. The results were reported in an abstract scheduled for release at the annual meeting of the American Academy of Orthopaedic Surgeons. The meeting was canceled because of COVID-19.
“This approach is simple, cheap, and does not rely on patient compliance,” explained Surena Namdari, MD, associate professor of orthopedic surgery at Thomas Jefferson University, Philadelphia.
C. acnes, formerly known as Propionibacterium acnes, is increasingly seen as an important target for prevention of postoperative shoulder infections because of published reports that it is the most commonly isolated bacterium from such infections, Dr. Namdari said in an interview.
In the prospective, randomized trial, male patients scheduled for shoulder arthroscopy were recruited if they did not have active acne, history of psoriatic or eczematous lesions, or recent antibiotic use. Most of the preoperative preparation of the surgical site was the same in the experimental and control arms. This included hair clipping, application of 2% chlorhexidine, and cleansing with saturated 7.5% povidone-iodine solution surgical scrub brushes.
The difference was that 3% hydrogen peroxide–soaked gauzes were applied to perioperative skin of those randomized to the experimental group but not to controls. All patients received routine preoperative oral antibiotics as well as perioperative applications of a formulation containing 2% chlorhexidine gluconate and 70% isopropyl alcohol.
Following surgery, 11 (18.6%) of the 59 patients in the experimental arm versus 23 (34.8%) of the 66 patients randomized to the control group had positive cultures for C. acnes (P = .047), according to the trial results, which have now been published (J Shoulder Elbow Surg. 2020;29:212-6).
There were no cases of skin reactions in either the experimental or control groups.
Topical skin cleansers that contain peroxide, such as benzoyl peroxide, have been shown to have a C. acnes decolonizing effect if applied repeatedly in the days prior to surgery, but Dr. Namdari suggested the problem with this approach is that it depends on patient compliance. A prophylaxis included in the preoperative routine eliminates this potential problem.
C. acnes is an anaerobic bacterium that is part of the resident flora of the skin around several joints, including the knee and the hip, but it is particularly common in the posterior shoulder. Colonization has been found substantially more common in men than in women, according to Dr. Namdari.
The specific threat posed by C. acnes to risk of postoperative infections “is still being defined,” and this trial was not large enough to associate the reduction in postoperative C. acnes cultures with a reduced risk of an adverse clinical outcome, but Dr. Namdari says that the data do show that the nearly 50% reduction in positive cultures was achieved efficiently and inexpensively with no apparent risk.
Several previous studies have also evaluated strategies for reducing C. acnes skin burden on the basis of expected protection against postoperative infection. In one, which associated a 3-day preoperative course of benzoyl peroxide with a reduction in the skin burden of C. acnes, the authors also concluded that this approach deserves consideration in routine skin preparation for shoulder arthroplasty (J Shoulder Elbow Surg. 2018;27:1539-44).
“We believe that a preoperative skin prep protocol that reduces C. acnes load on the skin would likely lead to reduced postoperative infections,” reported the senior author, Mohit N. Gilotra, MD, assistant professor, University of Maryland, Baltimore. Contacted about the rationale for reducing C. acnes skin burden without objective evidence of an impact on postoperative infection risk, Dr. Gilotra indicated these strategies make sense.
“It seems to be true for staph infections and is a reasonable assumption to make here,” he added. “Future work will help determine how much benzoyl peroxide, hydrogen peroxide, or other skin prep can reduce surgical site infection.”
Dr. Namdari reports financial relationships with multiple device and pharmaceutical companies but none relevant to this study.
SOURCE: Namdari S et al. AAOS 2020. Abstract P0808.
FROM AAOS 2020
Pandemic effect: All other health care visits can wait
according to survey conducted at the end of April.
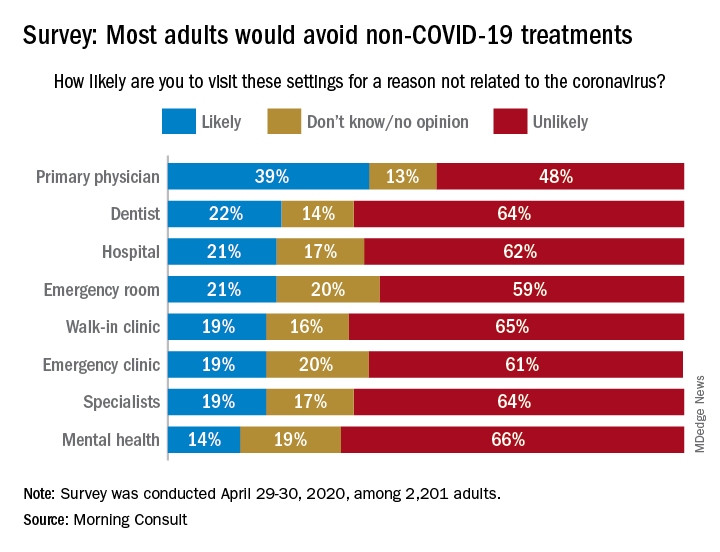
When asked how likely they were to visit a variety of health care settings for treatment not related to the coronavirus, 62% of respondents said it was unlikely that they would go to a hospital, 64% wouldn’t go to a specialist, and 65% would avoid walk-in clinics, digital media company Morning Consult reported May 4.
The only setting with less than a majority on the unlikely-to-visit side was primary physicians, who managed to combine a 39% likely vote with a 13% undecided/no-opinion tally, Morning Consult said after surveying 2,201 adults on April 29-30 (margin of error, ±2 percentage points).
As to when they might feel comfortable making such an in-person visit with their primary physician, 24% of respondents said they would willing to go in the next month, 14% said 2 months, 18% said 3 months, 13% said 6 months, and 10% said more than 6 months, the Morning Consult data show.
“Hospitals, despite being overburdened in recent weeks in coronavirus hot spots such as New York City, have reported dips in revenue as a result of potential patients opting against receiving elective surgeries out of fear of contracting COVID-19,” Morning Consult wrote, and these poll results suggest that “health care companies could continue to feel the pinch as long as the coronavirus lingers.”
according to survey conducted at the end of April.

When asked how likely they were to visit a variety of health care settings for treatment not related to the coronavirus, 62% of respondents said it was unlikely that they would go to a hospital, 64% wouldn’t go to a specialist, and 65% would avoid walk-in clinics, digital media company Morning Consult reported May 4.
The only setting with less than a majority on the unlikely-to-visit side was primary physicians, who managed to combine a 39% likely vote with a 13% undecided/no-opinion tally, Morning Consult said after surveying 2,201 adults on April 29-30 (margin of error, ±2 percentage points).
As to when they might feel comfortable making such an in-person visit with their primary physician, 24% of respondents said they would willing to go in the next month, 14% said 2 months, 18% said 3 months, 13% said 6 months, and 10% said more than 6 months, the Morning Consult data show.
“Hospitals, despite being overburdened in recent weeks in coronavirus hot spots such as New York City, have reported dips in revenue as a result of potential patients opting against receiving elective surgeries out of fear of contracting COVID-19,” Morning Consult wrote, and these poll results suggest that “health care companies could continue to feel the pinch as long as the coronavirus lingers.”
according to survey conducted at the end of April.

When asked how likely they were to visit a variety of health care settings for treatment not related to the coronavirus, 62% of respondents said it was unlikely that they would go to a hospital, 64% wouldn’t go to a specialist, and 65% would avoid walk-in clinics, digital media company Morning Consult reported May 4.
The only setting with less than a majority on the unlikely-to-visit side was primary physicians, who managed to combine a 39% likely vote with a 13% undecided/no-opinion tally, Morning Consult said after surveying 2,201 adults on April 29-30 (margin of error, ±2 percentage points).
As to when they might feel comfortable making such an in-person visit with their primary physician, 24% of respondents said they would willing to go in the next month, 14% said 2 months, 18% said 3 months, 13% said 6 months, and 10% said more than 6 months, the Morning Consult data show.
“Hospitals, despite being overburdened in recent weeks in coronavirus hot spots such as New York City, have reported dips in revenue as a result of potential patients opting against receiving elective surgeries out of fear of contracting COVID-19,” Morning Consult wrote, and these poll results suggest that “health care companies could continue to feel the pinch as long as the coronavirus lingers.”
FDA grants EUA to muscle stimulator to reduce mechanical ventilator usage
The Food and Drug Administration has issued an Emergency Use Authorization (EUA) for the VentFree Respiratory Muscle Stimulator in order to potentially reduce the number of days adult patients, including those with COVID-19, require mechanical ventilation, according to a press release from Liberate Medical.
In comparison with mechanical ventilation, which is invasive and commonly weakens the breathing muscles, the VentFree system uses noninvasive neuromuscular electrical stimulation to contract the abdominal wall muscles in synchrony with exhalation during mechanical ventilation, according to the press release. This allows patients to begin treatment during the early stages of ventilation while they are sedated and to continue until they are weaned off of ventilation.
A pair of pilot randomized, controlled studies, completed in Europe and Australia, showed that VentFree helped to reduce ventilation duration and ICU length of stay, compared with placebo stimulation. The FDA granted VentFree Breakthrough Device status in 2019.
“We are grateful to the FDA for recognizing the potential of VentFree and feel privileged to have the opportunity to help patients on mechanical ventilation during the COVID-19 pandemic,” Angus McLachlan PhD, cofounder and CEO of Liberate Medical, said in the press release.
VentFree has been authorized for use only for the duration of the current COVID-19 emergency, as it has not yet been approved or cleared for usage by primary care providers.
The Food and Drug Administration has issued an Emergency Use Authorization (EUA) for the VentFree Respiratory Muscle Stimulator in order to potentially reduce the number of days adult patients, including those with COVID-19, require mechanical ventilation, according to a press release from Liberate Medical.
In comparison with mechanical ventilation, which is invasive and commonly weakens the breathing muscles, the VentFree system uses noninvasive neuromuscular electrical stimulation to contract the abdominal wall muscles in synchrony with exhalation during mechanical ventilation, according to the press release. This allows patients to begin treatment during the early stages of ventilation while they are sedated and to continue until they are weaned off of ventilation.
A pair of pilot randomized, controlled studies, completed in Europe and Australia, showed that VentFree helped to reduce ventilation duration and ICU length of stay, compared with placebo stimulation. The FDA granted VentFree Breakthrough Device status in 2019.
“We are grateful to the FDA for recognizing the potential of VentFree and feel privileged to have the opportunity to help patients on mechanical ventilation during the COVID-19 pandemic,” Angus McLachlan PhD, cofounder and CEO of Liberate Medical, said in the press release.
VentFree has been authorized for use only for the duration of the current COVID-19 emergency, as it has not yet been approved or cleared for usage by primary care providers.
The Food and Drug Administration has issued an Emergency Use Authorization (EUA) for the VentFree Respiratory Muscle Stimulator in order to potentially reduce the number of days adult patients, including those with COVID-19, require mechanical ventilation, according to a press release from Liberate Medical.
In comparison with mechanical ventilation, which is invasive and commonly weakens the breathing muscles, the VentFree system uses noninvasive neuromuscular electrical stimulation to contract the abdominal wall muscles in synchrony with exhalation during mechanical ventilation, according to the press release. This allows patients to begin treatment during the early stages of ventilation while they are sedated and to continue until they are weaned off of ventilation.
A pair of pilot randomized, controlled studies, completed in Europe and Australia, showed that VentFree helped to reduce ventilation duration and ICU length of stay, compared with placebo stimulation. The FDA granted VentFree Breakthrough Device status in 2019.
“We are grateful to the FDA for recognizing the potential of VentFree and feel privileged to have the opportunity to help patients on mechanical ventilation during the COVID-19 pandemic,” Angus McLachlan PhD, cofounder and CEO of Liberate Medical, said in the press release.
VentFree has been authorized for use only for the duration of the current COVID-19 emergency, as it has not yet been approved or cleared for usage by primary care providers.
Cell and gene research raise hopes for recessive dystrophic EB treatments
LONDON – .
“I think there is a palpable sense that we are close to some breakthroughs for EB,” which may include “a cure for this intractable disease,” said Jouni Uitto, MD, PhD, in welcoming delegates to the meeting, held in January 2020.
Dr. Uitto, professor of dermatology and cutaneous biology, and biochemistry and molecular biology, at Sidney Kimmel Medical College, Philadelphia, said that the “breadth of academia-based basic science has been tremendous over the past 3 decades. We can now identify 21 different genes harboring mutations associated with different EB phenotypes, and we have a pretty good understanding how those mutations actually explain the phenotypic spectrum of different forms of EB.”
Importantly, “there are now perhaps as many as a dozen different clinical trials that are in the early stages of trying to find a permanent cure for this disease,” Dr. Uitto said, with some that are looking at fixing the underlying defect once and for all, or at the very least, counteracting subsequent complications. “The spectrum varies from attempting to enhance wound healing to gene repair, gene replacement, protein replacement therapies, cell-based therapies. There is a whole spectrum of often complementary approaches that we believe will lead to a cure and treatment for this disease. We look forward to developing therapies which will be helpful to the benefit of all the patients with EB,” said Dr. Uitto, who is also chair of the department of dermatology and cutaneous biology at Sidney Kimmel Medical College.
EB research is gathering ‘momentum’
John McGrath, MD, professor of molecular dermatology, King’s College, London, chaired a session on the latest in cell manipulation research and made the following comment: “A few years ago, we were making progress, but we were chatting about a lot of the same things; but now, suddenly there seems to be momentum, re-energy, rediscovery, real progress.”
Dr. McGrath noted that gene and cell research, and preclinical development, were culminating in clinical trials and potentially products that could change the way clinicians thought about managing patients with EB. “That prospect of getting closer and closer to real treatments, and maybe even a cure” is becoming more of a reality, he said.
Dr. McGrath is also head of the genetic skin disease group at King’s College London, and an honorary consultant dermatologist at St. John’s Institute of Dermatology, part of the Guy’s and St. Thomas’ NHS Foundation Trust in London. He has been a principal investigator for clinical trials of fibroblast cell therapy and allogeneic intravenous mesenchymal stromal cells (MSCs) therapy.
“It has been a joy for me to see the benefits of those clinical trials. There is nothing like it as an investigator when you see an intervention make a difference to a patient,” Dr. McGrath said. “For me, it was just a real eye opener when I saw the skin changes in a child that received intravenous allogeneic MSCs. The skin changed dramatically, it went from red and inflamed to calm and pink, [giving a] first glimpse into something that might be reversible, treatable, not just papering over the cracks.”
Correcting the genetic defect
The most severe form of RDEB is caused by mutations in COL7A1, the gene for collagen type 7 (COL7), the major connective component of the skin, anchoring the epidermis to the dermis. Its absence results in skin that can be so fragile it has been likened to the wings of a butterfly and results in severe blistering after very little trauma.
There is a lot of research on how to correct the underlying genetic defect, either by replacing COL7A1 entirely, repairing the gene, or editing the gene so that COL 7 can be produced in situ and prevent the formation of wounds and heal those that might already be present.
“The excitement is obvious,” said Jakub Tolar, MD, PhD, professor in the department of pediatrics, blood and marrow transplantation, and dean of the University of Minnesota Medical School, Minneapolis, who chaired a session on gene and gene manipulation therapies. “If one can go and correct that information, it follows that everything else is going to be okay,” he said. “Only it’s not. I think that it’s pretty clear that more than gene correction is needed.”
Some of the approaches to replace the faulty gene discussed at the meeting involved taking skin biopsy samples from a healthy area of skin from a patient with RDEB, isolating specific skin cells (fibroblasts, keratinocytes, or both), transferring a healthy copy of the COL7A1 gene into those cells – then expanding the population to form sheets of cells that can be grafted onto the wounds of the same patient.
Clinical trials of gene therapy for RDEB
Clinical trials with these novel gene-corrected, tissue-engineered grafts have already started, including EBGraft, a phase 1/2 open, nonrandomized, proof-of-concept trial using genetically corrected sheets of fibroblasts and keratinocytes, conducted by Alain Hovnanian, MD, PhD, Necker-Enfants Malades Hospital in Paris, and associates.
Then there is the phase 3 VIITAL trial being conducted at Stanford (Calif.) University by Jean Tang, MD, PhD, and colleagues. Recruitment in this open trial, which will enroll 10-15 patients with RDEB, has just started. The aim of the study is to investigate the efficacy and safety of EB-101, an autologous cell therapy that corrects COL7A1 in keratinocytes.
Positive findings from a phase 1/2 study with EB-101 were presented in a poster at the meeting by Emily Gorell, DO, a postdoctoral medical fellow in dermatology, at Stanford University and her associates. The trial included seven patients with RDEB who were treated and followed for 3 to 6 years. Data from that study showed that there were no serious adverse events and 95% of patients’ wounds that were treated (36/38) were healed by at least 50%, based on an Investigator Global Assessment at 6 months. In comparison, none of the untreated wounds had healed by that time point. “There was evidence of C7 [collagen 7] restoration at 2 years in two participants,” and wound healing was associated with both reduced pain and itch, the investigators wrote in the poster.
Another approach to this so-called ‘ex-vivo’ gene therapy is to take the patient’s cells via a small skin biopsy, genetically modify them, expand the population of these modified cells, and then inject them back into the patient. This approach was described by Peter Marinkovich, MD, of the department of dermatology at Stanford University, during an oral presentation and in a poster at the meeting.
Dr. Marinkovich discussed the results of an ongoing phase 1/2 study in which six subjects with RDEB – five adults and one child – were treated intradermally with genetically modified fibroblasts in a preparation currently known as FCX-007.
“Before we had to graft the cells, take the patients into the OR [operating room], with the risks of general anesthesia, but here we don’t have to take the patients to the OR, we just take them into the hospital for a day, inject their wounds and then send them on their way,” Dr. Marinkovich said. Interim findings show that the patients have tolerated the therapy very well up to 52 weeks, he noted.
A greater percentage of wounds were healed by more than 50% following treatment with FCX-007 than those left untreated at weeks 4 (80% versus 20%), 12 (90% versus 44%), 25 (75% versus 50%), and 52 (83% versus 33%).
These results have been used to inform the design of the upcoming phase 3 study, DEFI-RDEB. The multicenter intrapatient randomized, controlled, open-label study is evaluating FCX-007 in the treatment of persistent nonhealing wounds in about 20 people with RDEB.
The promise of ‘off-the-shelf’ topical gene therapy
Another study Dr. Marinkovich is involved with is a phase 1/2 study of beremagene geperpavec (B-VEC), an “in-vivo” gene therapy. B-VEC is a topically administered therapy containing a replication-deficient, nonintegrating viral vector that contains two functional COL7A1 genes. The concept is that, when applied directly onto the skin, the virus gets into the skin and carries with it the healthy gene copies; these get taken up by the skin cells, which then produce COL7.
Initially, two patients with generalized severe RDEB were studied. B-VEC was applied to one of two wounds and a placebo to the other wound in each patient. Another four patients were then enrolled and studied for 3 months. Nine of 10 wounds closed completely after initial administration of B-VEC, with an average time to 100% wound closure of 17.4 days. The average duration of wound closure has been 113 days so far.
“One chronic wound that was originally open for over 4 years closed completely following B-VEC readministration. The wound has remained closed for 100 days,” Dr. Marinkovich and associates reported in a poster at the meeting. A postimaging study showed that COL7 was being produced from 48 hours to up to 90 days later.
“I’m really excited about this type of therapy,” Dr. Marinkovich said during an oral presentation. Unlike the ex-vivo gene therapy approach, where each patient’s cells have to be taken by a biopsy, altered, engineered, and expanded, which takes specialized facilities that can vary by country and location, this in-vivo gene therapy can be considered an “off-the shelf” treatment that can be shipped all over the world and could reach many patients. “It’s another weapon in our armamentarium against this deadly disease that we are all fighting against together,” Dr. Marinkovich added.
EBGRAFT is supported by Cure EB. The VIITAL trial is sponsored by Abeona Therapeutics. The phase 1/2 trials of EB-101 were funded by grants from the National Institutes of Health, EB Research Partnership, EM Medical Research Foundation, and Abeona Therapeutics. The FCX-007 phase 1/2 study was supported by Fibrocell Technologies. The upcoming phase 3 will be funded by Fibrocell Technologies in collaboration with Castle Creek Pharmaceuticals. The B-VEC study is supported by Krystal Biotech.
Dr. Uitto and Dr. McGrath had no potential conflicts of interest to report. Dr. Tolar has received funding from the National Institutes of Health, various EB charities and the Richard M. Schulze Family Foundation (RMSFF). He disclosed receiving honoraria or consultation fees from Ticeba/RHEACELL GmbH and Taiga Biosciences. Dr. Marinkovich disclosed being an investigator working on RDEB-related research projects in collaboration with Krystal Biotech, Fibrocell Technologies, Abeona Therapeutics, and Wings (formerly ProQR).
SOURCES: Gorell E et al. EB 2020, Poster 124; Marinkovich MP et al. EB 2020, Poster 123; Marinkovich MP et al. EB 2020, Poster 52.
LONDON – .
“I think there is a palpable sense that we are close to some breakthroughs for EB,” which may include “a cure for this intractable disease,” said Jouni Uitto, MD, PhD, in welcoming delegates to the meeting, held in January 2020.
Dr. Uitto, professor of dermatology and cutaneous biology, and biochemistry and molecular biology, at Sidney Kimmel Medical College, Philadelphia, said that the “breadth of academia-based basic science has been tremendous over the past 3 decades. We can now identify 21 different genes harboring mutations associated with different EB phenotypes, and we have a pretty good understanding how those mutations actually explain the phenotypic spectrum of different forms of EB.”
Importantly, “there are now perhaps as many as a dozen different clinical trials that are in the early stages of trying to find a permanent cure for this disease,” Dr. Uitto said, with some that are looking at fixing the underlying defect once and for all, or at the very least, counteracting subsequent complications. “The spectrum varies from attempting to enhance wound healing to gene repair, gene replacement, protein replacement therapies, cell-based therapies. There is a whole spectrum of often complementary approaches that we believe will lead to a cure and treatment for this disease. We look forward to developing therapies which will be helpful to the benefit of all the patients with EB,” said Dr. Uitto, who is also chair of the department of dermatology and cutaneous biology at Sidney Kimmel Medical College.
EB research is gathering ‘momentum’
John McGrath, MD, professor of molecular dermatology, King’s College, London, chaired a session on the latest in cell manipulation research and made the following comment: “A few years ago, we were making progress, but we were chatting about a lot of the same things; but now, suddenly there seems to be momentum, re-energy, rediscovery, real progress.”
Dr. McGrath noted that gene and cell research, and preclinical development, were culminating in clinical trials and potentially products that could change the way clinicians thought about managing patients with EB. “That prospect of getting closer and closer to real treatments, and maybe even a cure” is becoming more of a reality, he said.
Dr. McGrath is also head of the genetic skin disease group at King’s College London, and an honorary consultant dermatologist at St. John’s Institute of Dermatology, part of the Guy’s and St. Thomas’ NHS Foundation Trust in London. He has been a principal investigator for clinical trials of fibroblast cell therapy and allogeneic intravenous mesenchymal stromal cells (MSCs) therapy.
“It has been a joy for me to see the benefits of those clinical trials. There is nothing like it as an investigator when you see an intervention make a difference to a patient,” Dr. McGrath said. “For me, it was just a real eye opener when I saw the skin changes in a child that received intravenous allogeneic MSCs. The skin changed dramatically, it went from red and inflamed to calm and pink, [giving a] first glimpse into something that might be reversible, treatable, not just papering over the cracks.”
Correcting the genetic defect
The most severe form of RDEB is caused by mutations in COL7A1, the gene for collagen type 7 (COL7), the major connective component of the skin, anchoring the epidermis to the dermis. Its absence results in skin that can be so fragile it has been likened to the wings of a butterfly and results in severe blistering after very little trauma.
There is a lot of research on how to correct the underlying genetic defect, either by replacing COL7A1 entirely, repairing the gene, or editing the gene so that COL 7 can be produced in situ and prevent the formation of wounds and heal those that might already be present.
“The excitement is obvious,” said Jakub Tolar, MD, PhD, professor in the department of pediatrics, blood and marrow transplantation, and dean of the University of Minnesota Medical School, Minneapolis, who chaired a session on gene and gene manipulation therapies. “If one can go and correct that information, it follows that everything else is going to be okay,” he said. “Only it’s not. I think that it’s pretty clear that more than gene correction is needed.”
Some of the approaches to replace the faulty gene discussed at the meeting involved taking skin biopsy samples from a healthy area of skin from a patient with RDEB, isolating specific skin cells (fibroblasts, keratinocytes, or both), transferring a healthy copy of the COL7A1 gene into those cells – then expanding the population to form sheets of cells that can be grafted onto the wounds of the same patient.
Clinical trials of gene therapy for RDEB
Clinical trials with these novel gene-corrected, tissue-engineered grafts have already started, including EBGraft, a phase 1/2 open, nonrandomized, proof-of-concept trial using genetically corrected sheets of fibroblasts and keratinocytes, conducted by Alain Hovnanian, MD, PhD, Necker-Enfants Malades Hospital in Paris, and associates.
Then there is the phase 3 VIITAL trial being conducted at Stanford (Calif.) University by Jean Tang, MD, PhD, and colleagues. Recruitment in this open trial, which will enroll 10-15 patients with RDEB, has just started. The aim of the study is to investigate the efficacy and safety of EB-101, an autologous cell therapy that corrects COL7A1 in keratinocytes.
Positive findings from a phase 1/2 study with EB-101 were presented in a poster at the meeting by Emily Gorell, DO, a postdoctoral medical fellow in dermatology, at Stanford University and her associates. The trial included seven patients with RDEB who were treated and followed for 3 to 6 years. Data from that study showed that there were no serious adverse events and 95% of patients’ wounds that were treated (36/38) were healed by at least 50%, based on an Investigator Global Assessment at 6 months. In comparison, none of the untreated wounds had healed by that time point. “There was evidence of C7 [collagen 7] restoration at 2 years in two participants,” and wound healing was associated with both reduced pain and itch, the investigators wrote in the poster.
Another approach to this so-called ‘ex-vivo’ gene therapy is to take the patient’s cells via a small skin biopsy, genetically modify them, expand the population of these modified cells, and then inject them back into the patient. This approach was described by Peter Marinkovich, MD, of the department of dermatology at Stanford University, during an oral presentation and in a poster at the meeting.
Dr. Marinkovich discussed the results of an ongoing phase 1/2 study in which six subjects with RDEB – five adults and one child – were treated intradermally with genetically modified fibroblasts in a preparation currently known as FCX-007.
“Before we had to graft the cells, take the patients into the OR [operating room], with the risks of general anesthesia, but here we don’t have to take the patients to the OR, we just take them into the hospital for a day, inject their wounds and then send them on their way,” Dr. Marinkovich said. Interim findings show that the patients have tolerated the therapy very well up to 52 weeks, he noted.
A greater percentage of wounds were healed by more than 50% following treatment with FCX-007 than those left untreated at weeks 4 (80% versus 20%), 12 (90% versus 44%), 25 (75% versus 50%), and 52 (83% versus 33%).
These results have been used to inform the design of the upcoming phase 3 study, DEFI-RDEB. The multicenter intrapatient randomized, controlled, open-label study is evaluating FCX-007 in the treatment of persistent nonhealing wounds in about 20 people with RDEB.
The promise of ‘off-the-shelf’ topical gene therapy
Another study Dr. Marinkovich is involved with is a phase 1/2 study of beremagene geperpavec (B-VEC), an “in-vivo” gene therapy. B-VEC is a topically administered therapy containing a replication-deficient, nonintegrating viral vector that contains two functional COL7A1 genes. The concept is that, when applied directly onto the skin, the virus gets into the skin and carries with it the healthy gene copies; these get taken up by the skin cells, which then produce COL7.
Initially, two patients with generalized severe RDEB were studied. B-VEC was applied to one of two wounds and a placebo to the other wound in each patient. Another four patients were then enrolled and studied for 3 months. Nine of 10 wounds closed completely after initial administration of B-VEC, with an average time to 100% wound closure of 17.4 days. The average duration of wound closure has been 113 days so far.
“One chronic wound that was originally open for over 4 years closed completely following B-VEC readministration. The wound has remained closed for 100 days,” Dr. Marinkovich and associates reported in a poster at the meeting. A postimaging study showed that COL7 was being produced from 48 hours to up to 90 days later.
“I’m really excited about this type of therapy,” Dr. Marinkovich said during an oral presentation. Unlike the ex-vivo gene therapy approach, where each patient’s cells have to be taken by a biopsy, altered, engineered, and expanded, which takes specialized facilities that can vary by country and location, this in-vivo gene therapy can be considered an “off-the shelf” treatment that can be shipped all over the world and could reach many patients. “It’s another weapon in our armamentarium against this deadly disease that we are all fighting against together,” Dr. Marinkovich added.
EBGRAFT is supported by Cure EB. The VIITAL trial is sponsored by Abeona Therapeutics. The phase 1/2 trials of EB-101 were funded by grants from the National Institutes of Health, EB Research Partnership, EM Medical Research Foundation, and Abeona Therapeutics. The FCX-007 phase 1/2 study was supported by Fibrocell Technologies. The upcoming phase 3 will be funded by Fibrocell Technologies in collaboration with Castle Creek Pharmaceuticals. The B-VEC study is supported by Krystal Biotech.
Dr. Uitto and Dr. McGrath had no potential conflicts of interest to report. Dr. Tolar has received funding from the National Institutes of Health, various EB charities and the Richard M. Schulze Family Foundation (RMSFF). He disclosed receiving honoraria or consultation fees from Ticeba/RHEACELL GmbH and Taiga Biosciences. Dr. Marinkovich disclosed being an investigator working on RDEB-related research projects in collaboration with Krystal Biotech, Fibrocell Technologies, Abeona Therapeutics, and Wings (formerly ProQR).
SOURCES: Gorell E et al. EB 2020, Poster 124; Marinkovich MP et al. EB 2020, Poster 123; Marinkovich MP et al. EB 2020, Poster 52.
LONDON – .
“I think there is a palpable sense that we are close to some breakthroughs for EB,” which may include “a cure for this intractable disease,” said Jouni Uitto, MD, PhD, in welcoming delegates to the meeting, held in January 2020.
Dr. Uitto, professor of dermatology and cutaneous biology, and biochemistry and molecular biology, at Sidney Kimmel Medical College, Philadelphia, said that the “breadth of academia-based basic science has been tremendous over the past 3 decades. We can now identify 21 different genes harboring mutations associated with different EB phenotypes, and we have a pretty good understanding how those mutations actually explain the phenotypic spectrum of different forms of EB.”
Importantly, “there are now perhaps as many as a dozen different clinical trials that are in the early stages of trying to find a permanent cure for this disease,” Dr. Uitto said, with some that are looking at fixing the underlying defect once and for all, or at the very least, counteracting subsequent complications. “The spectrum varies from attempting to enhance wound healing to gene repair, gene replacement, protein replacement therapies, cell-based therapies. There is a whole spectrum of often complementary approaches that we believe will lead to a cure and treatment for this disease. We look forward to developing therapies which will be helpful to the benefit of all the patients with EB,” said Dr. Uitto, who is also chair of the department of dermatology and cutaneous biology at Sidney Kimmel Medical College.
EB research is gathering ‘momentum’
John McGrath, MD, professor of molecular dermatology, King’s College, London, chaired a session on the latest in cell manipulation research and made the following comment: “A few years ago, we were making progress, but we were chatting about a lot of the same things; but now, suddenly there seems to be momentum, re-energy, rediscovery, real progress.”
Dr. McGrath noted that gene and cell research, and preclinical development, were culminating in clinical trials and potentially products that could change the way clinicians thought about managing patients with EB. “That prospect of getting closer and closer to real treatments, and maybe even a cure” is becoming more of a reality, he said.
Dr. McGrath is also head of the genetic skin disease group at King’s College London, and an honorary consultant dermatologist at St. John’s Institute of Dermatology, part of the Guy’s and St. Thomas’ NHS Foundation Trust in London. He has been a principal investigator for clinical trials of fibroblast cell therapy and allogeneic intravenous mesenchymal stromal cells (MSCs) therapy.
“It has been a joy for me to see the benefits of those clinical trials. There is nothing like it as an investigator when you see an intervention make a difference to a patient,” Dr. McGrath said. “For me, it was just a real eye opener when I saw the skin changes in a child that received intravenous allogeneic MSCs. The skin changed dramatically, it went from red and inflamed to calm and pink, [giving a] first glimpse into something that might be reversible, treatable, not just papering over the cracks.”
Correcting the genetic defect
The most severe form of RDEB is caused by mutations in COL7A1, the gene for collagen type 7 (COL7), the major connective component of the skin, anchoring the epidermis to the dermis. Its absence results in skin that can be so fragile it has been likened to the wings of a butterfly and results in severe blistering after very little trauma.
There is a lot of research on how to correct the underlying genetic defect, either by replacing COL7A1 entirely, repairing the gene, or editing the gene so that COL 7 can be produced in situ and prevent the formation of wounds and heal those that might already be present.
“The excitement is obvious,” said Jakub Tolar, MD, PhD, professor in the department of pediatrics, blood and marrow transplantation, and dean of the University of Minnesota Medical School, Minneapolis, who chaired a session on gene and gene manipulation therapies. “If one can go and correct that information, it follows that everything else is going to be okay,” he said. “Only it’s not. I think that it’s pretty clear that more than gene correction is needed.”
Some of the approaches to replace the faulty gene discussed at the meeting involved taking skin biopsy samples from a healthy area of skin from a patient with RDEB, isolating specific skin cells (fibroblasts, keratinocytes, or both), transferring a healthy copy of the COL7A1 gene into those cells – then expanding the population to form sheets of cells that can be grafted onto the wounds of the same patient.
Clinical trials of gene therapy for RDEB
Clinical trials with these novel gene-corrected, tissue-engineered grafts have already started, including EBGraft, a phase 1/2 open, nonrandomized, proof-of-concept trial using genetically corrected sheets of fibroblasts and keratinocytes, conducted by Alain Hovnanian, MD, PhD, Necker-Enfants Malades Hospital in Paris, and associates.
Then there is the phase 3 VIITAL trial being conducted at Stanford (Calif.) University by Jean Tang, MD, PhD, and colleagues. Recruitment in this open trial, which will enroll 10-15 patients with RDEB, has just started. The aim of the study is to investigate the efficacy and safety of EB-101, an autologous cell therapy that corrects COL7A1 in keratinocytes.
Positive findings from a phase 1/2 study with EB-101 were presented in a poster at the meeting by Emily Gorell, DO, a postdoctoral medical fellow in dermatology, at Stanford University and her associates. The trial included seven patients with RDEB who were treated and followed for 3 to 6 years. Data from that study showed that there were no serious adverse events and 95% of patients’ wounds that were treated (36/38) were healed by at least 50%, based on an Investigator Global Assessment at 6 months. In comparison, none of the untreated wounds had healed by that time point. “There was evidence of C7 [collagen 7] restoration at 2 years in two participants,” and wound healing was associated with both reduced pain and itch, the investigators wrote in the poster.
Another approach to this so-called ‘ex-vivo’ gene therapy is to take the patient’s cells via a small skin biopsy, genetically modify them, expand the population of these modified cells, and then inject them back into the patient. This approach was described by Peter Marinkovich, MD, of the department of dermatology at Stanford University, during an oral presentation and in a poster at the meeting.
Dr. Marinkovich discussed the results of an ongoing phase 1/2 study in which six subjects with RDEB – five adults and one child – were treated intradermally with genetically modified fibroblasts in a preparation currently known as FCX-007.
“Before we had to graft the cells, take the patients into the OR [operating room], with the risks of general anesthesia, but here we don’t have to take the patients to the OR, we just take them into the hospital for a day, inject their wounds and then send them on their way,” Dr. Marinkovich said. Interim findings show that the patients have tolerated the therapy very well up to 52 weeks, he noted.
A greater percentage of wounds were healed by more than 50% following treatment with FCX-007 than those left untreated at weeks 4 (80% versus 20%), 12 (90% versus 44%), 25 (75% versus 50%), and 52 (83% versus 33%).
These results have been used to inform the design of the upcoming phase 3 study, DEFI-RDEB. The multicenter intrapatient randomized, controlled, open-label study is evaluating FCX-007 in the treatment of persistent nonhealing wounds in about 20 people with RDEB.
The promise of ‘off-the-shelf’ topical gene therapy
Another study Dr. Marinkovich is involved with is a phase 1/2 study of beremagene geperpavec (B-VEC), an “in-vivo” gene therapy. B-VEC is a topically administered therapy containing a replication-deficient, nonintegrating viral vector that contains two functional COL7A1 genes. The concept is that, when applied directly onto the skin, the virus gets into the skin and carries with it the healthy gene copies; these get taken up by the skin cells, which then produce COL7.
Initially, two patients with generalized severe RDEB were studied. B-VEC was applied to one of two wounds and a placebo to the other wound in each patient. Another four patients were then enrolled and studied for 3 months. Nine of 10 wounds closed completely after initial administration of B-VEC, with an average time to 100% wound closure of 17.4 days. The average duration of wound closure has been 113 days so far.
“One chronic wound that was originally open for over 4 years closed completely following B-VEC readministration. The wound has remained closed for 100 days,” Dr. Marinkovich and associates reported in a poster at the meeting. A postimaging study showed that COL7 was being produced from 48 hours to up to 90 days later.
“I’m really excited about this type of therapy,” Dr. Marinkovich said during an oral presentation. Unlike the ex-vivo gene therapy approach, where each patient’s cells have to be taken by a biopsy, altered, engineered, and expanded, which takes specialized facilities that can vary by country and location, this in-vivo gene therapy can be considered an “off-the shelf” treatment that can be shipped all over the world and could reach many patients. “It’s another weapon in our armamentarium against this deadly disease that we are all fighting against together,” Dr. Marinkovich added.
EBGRAFT is supported by Cure EB. The VIITAL trial is sponsored by Abeona Therapeutics. The phase 1/2 trials of EB-101 were funded by grants from the National Institutes of Health, EB Research Partnership, EM Medical Research Foundation, and Abeona Therapeutics. The FCX-007 phase 1/2 study was supported by Fibrocell Technologies. The upcoming phase 3 will be funded by Fibrocell Technologies in collaboration with Castle Creek Pharmaceuticals. The B-VEC study is supported by Krystal Biotech.
Dr. Uitto and Dr. McGrath had no potential conflicts of interest to report. Dr. Tolar has received funding from the National Institutes of Health, various EB charities and the Richard M. Schulze Family Foundation (RMSFF). He disclosed receiving honoraria or consultation fees from Ticeba/RHEACELL GmbH and Taiga Biosciences. Dr. Marinkovich disclosed being an investigator working on RDEB-related research projects in collaboration with Krystal Biotech, Fibrocell Technologies, Abeona Therapeutics, and Wings (formerly ProQR).
SOURCES: Gorell E et al. EB 2020, Poster 124; Marinkovich MP et al. EB 2020, Poster 123; Marinkovich MP et al. EB 2020, Poster 52.
REPORTING FROM EB 2020
COVID-19 death rate was twice as high in cancer patients in NYC study
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
FROM CANCER DISCOVERY
Sensitizer prevalent in many hypoallergenic products for children
(AD) and allergic contact dermatitis, according to a research letter in the Journal of the American Academy of Dermatology.
In the letter, the authors, Reid W. Collis, of Washington University in St. Louis, and David M. Sheinbein, MD, of the division of dermatology at the university, referred to a previous study showing an association between contact sensitivity with CAPB and people with a history of AD. This was supported by the results of their own recent study in pediatric patients, they wrote, which found that reactions to CAPB were “exclusively” in patients with AD.
In the survey, they looked at children’s shampoo and soap products available on online databases of six of the biggest retailers, and analyzed the top 20 best-selling products for each retailer in 2018. Of the unique products, CAPB was found to be an ingredient in 52% (39 of 75) of the shampoos and 44% (29 of 66) of the soap products. But each of these products “contained the term ‘hypoallergenic; on the product itself or in the product’s description,” they noted.
“CAPB is a prevalent sensitizer in pediatric patients and should be avoided in patients with AD,” the investigators wrote. That said, it’s not included among the 35 prevalent allergens in the T.R.U.E. test, and they recommended that pediatricians and dermatologists “be aware of common products containing CAPB when counseling patients about their product choices,” considering that CAPB sensitivity is more likely in patients with AD.
The study had no funding source, and the authors had no disclosures.
[email protected]
SOURCE: Cho SI et al. J Am Acad Dermatol. 2020 May. doi: 10.1016/j.jaad.2019.12.036.
(AD) and allergic contact dermatitis, according to a research letter in the Journal of the American Academy of Dermatology.
In the letter, the authors, Reid W. Collis, of Washington University in St. Louis, and David M. Sheinbein, MD, of the division of dermatology at the university, referred to a previous study showing an association between contact sensitivity with CAPB and people with a history of AD. This was supported by the results of their own recent study in pediatric patients, they wrote, which found that reactions to CAPB were “exclusively” in patients with AD.
In the survey, they looked at children’s shampoo and soap products available on online databases of six of the biggest retailers, and analyzed the top 20 best-selling products for each retailer in 2018. Of the unique products, CAPB was found to be an ingredient in 52% (39 of 75) of the shampoos and 44% (29 of 66) of the soap products. But each of these products “contained the term ‘hypoallergenic; on the product itself or in the product’s description,” they noted.
“CAPB is a prevalent sensitizer in pediatric patients and should be avoided in patients with AD,” the investigators wrote. That said, it’s not included among the 35 prevalent allergens in the T.R.U.E. test, and they recommended that pediatricians and dermatologists “be aware of common products containing CAPB when counseling patients about their product choices,” considering that CAPB sensitivity is more likely in patients with AD.
The study had no funding source, and the authors had no disclosures.
[email protected]
SOURCE: Cho SI et al. J Am Acad Dermatol. 2020 May. doi: 10.1016/j.jaad.2019.12.036.
(AD) and allergic contact dermatitis, according to a research letter in the Journal of the American Academy of Dermatology.
In the letter, the authors, Reid W. Collis, of Washington University in St. Louis, and David M. Sheinbein, MD, of the division of dermatology at the university, referred to a previous study showing an association between contact sensitivity with CAPB and people with a history of AD. This was supported by the results of their own recent study in pediatric patients, they wrote, which found that reactions to CAPB were “exclusively” in patients with AD.
In the survey, they looked at children’s shampoo and soap products available on online databases of six of the biggest retailers, and analyzed the top 20 best-selling products for each retailer in 2018. Of the unique products, CAPB was found to be an ingredient in 52% (39 of 75) of the shampoos and 44% (29 of 66) of the soap products. But each of these products “contained the term ‘hypoallergenic; on the product itself or in the product’s description,” they noted.
“CAPB is a prevalent sensitizer in pediatric patients and should be avoided in patients with AD,” the investigators wrote. That said, it’s not included among the 35 prevalent allergens in the T.R.U.E. test, and they recommended that pediatricians and dermatologists “be aware of common products containing CAPB when counseling patients about their product choices,” considering that CAPB sensitivity is more likely in patients with AD.
The study had no funding source, and the authors had no disclosures.
[email protected]
SOURCE: Cho SI et al. J Am Acad Dermatol. 2020 May. doi: 10.1016/j.jaad.2019.12.036.
Fountains of Wayne, and a hospitalist’s first day, remembered
Like many in the health care field, I have found it hard to watch the news over these past couple of months when it seems that almost every story is about COVID-19 or its repercussions. Luckily, I have two young daughters who “encourage” me to listen to the Frozen 2 soundtrack instead of putting on the evening news when I get home from work. Still, news manages to seep through my defenses. As I scrolled through some headlines recently, I learned of the death of musician Adam Schlesinger from COVID-19. He wasn’t a household name, but his death still hit me in unexpected ways.
I started internship in late June 2005, in a city (Portland, Ore.) about as different from my previous home (Dallas) as any two places can possibly be. I think the day before internship started still ranks as the most nervous of my life. I’m not sure how I slept at all that night, but somehow I did and arrived at the Portland Veterans Affairs Hospital the following morning to start my new career.
And then … nothing happened. Early on that first day, the electronic medical records crashed, and no patients were admitted during our time on “short call.” My upper level resident took care of the one or two established patients on the team (both discharged), so I ended the day with records that would not be broken during the remainder of my residency: 0 notes written, 0 patients seen. Perhaps the most successful first day that any intern, anywhere has ever had, although it prepared me quite poorly for all the subsequent days.
Since I had some time on my hands, I made the 20-minute walk to one of my new hometown’s record stores where Fountains of Wayne (FOW) was playing an acoustic in-store set. Their album from a few years prior, “Welcome Interstate Managers,” was in heavy rotation when I made the drive from Dallas to Portland. It was (and is) a great album for long drives – melodic, catchy, and (mostly) up-tempo. Adam and the band’s singer, Chris Collingwood, played several songs that night on the store’s stage. Then they headed out to the next city, and I headed back home and on to many far-busier days of residency.
We would cross paths again a decade later. I moved back to Texas and became a hospitalist. It turns out that, if you have enough hospitalists of a certain age and if enough of those hospitalists have unearned confidence in their musical ability, then a covers band will undoubtedly be formed. And so, it happened here in San Antonio. We were not selective in our song choices – we played songs from every decade of the last 50 years, bands as popular as the Beatles and as indie as the Rentals. And we played some FOW.
Our band (which will go nameless here so that our YouTube recordings are more difficult to find) played a grand total of one gig during our years of intermittent practicing. That one gig was my wedding rehearsal dinner and the penultimate song we played was “Stacy’s Mom,” which is notable for being both FOW’s biggest hit and a completely inappropriate song to play at a wedding rehearsal dinner. The crowd was probably around the same size as the one that had seen Adam and Chris play in Portland 10 years prior. I don’t think the applause we received was quite as genuine or deserved, though.
After Adam and Chris played their gig, there was an autograph session and I took home a signed poster. Last year, I decided to take it out of storage and hang it in my office. The date of the show and the first day of my physician career, a date now nearly 15 years ago, is written in psychedelic typography at the bottom. The store that I went to that day is no longer there, a victim of progress like so many other record stores across the country. Another location of the same store is still open in Portland. I hope that it and all the other small book and music stores across the country can survive this current crisis, but I know that many will not.
So, here’s to you Adam, and to all the others who have lost their lives to this terrible illness. As a small token of remembrance, I’ll be playing some Fountains of Wayne on the drive home tonight. It’s not quite the same as playing it on a cross-country drive, but hopefully, we will all be able to do that again soon.
Dr. Sehgal is a clinical associate professor of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System and UT-Health San Antonio. He is a member of the editorial advisory board for The Hospitalist.
Like many in the health care field, I have found it hard to watch the news over these past couple of months when it seems that almost every story is about COVID-19 or its repercussions. Luckily, I have two young daughters who “encourage” me to listen to the Frozen 2 soundtrack instead of putting on the evening news when I get home from work. Still, news manages to seep through my defenses. As I scrolled through some headlines recently, I learned of the death of musician Adam Schlesinger from COVID-19. He wasn’t a household name, but his death still hit me in unexpected ways.
I started internship in late June 2005, in a city (Portland, Ore.) about as different from my previous home (Dallas) as any two places can possibly be. I think the day before internship started still ranks as the most nervous of my life. I’m not sure how I slept at all that night, but somehow I did and arrived at the Portland Veterans Affairs Hospital the following morning to start my new career.
And then … nothing happened. Early on that first day, the electronic medical records crashed, and no patients were admitted during our time on “short call.” My upper level resident took care of the one or two established patients on the team (both discharged), so I ended the day with records that would not be broken during the remainder of my residency: 0 notes written, 0 patients seen. Perhaps the most successful first day that any intern, anywhere has ever had, although it prepared me quite poorly for all the subsequent days.
Since I had some time on my hands, I made the 20-minute walk to one of my new hometown’s record stores where Fountains of Wayne (FOW) was playing an acoustic in-store set. Their album from a few years prior, “Welcome Interstate Managers,” was in heavy rotation when I made the drive from Dallas to Portland. It was (and is) a great album for long drives – melodic, catchy, and (mostly) up-tempo. Adam and the band’s singer, Chris Collingwood, played several songs that night on the store’s stage. Then they headed out to the next city, and I headed back home and on to many far-busier days of residency.
We would cross paths again a decade later. I moved back to Texas and became a hospitalist. It turns out that, if you have enough hospitalists of a certain age and if enough of those hospitalists have unearned confidence in their musical ability, then a covers band will undoubtedly be formed. And so, it happened here in San Antonio. We were not selective in our song choices – we played songs from every decade of the last 50 years, bands as popular as the Beatles and as indie as the Rentals. And we played some FOW.
Our band (which will go nameless here so that our YouTube recordings are more difficult to find) played a grand total of one gig during our years of intermittent practicing. That one gig was my wedding rehearsal dinner and the penultimate song we played was “Stacy’s Mom,” which is notable for being both FOW’s biggest hit and a completely inappropriate song to play at a wedding rehearsal dinner. The crowd was probably around the same size as the one that had seen Adam and Chris play in Portland 10 years prior. I don’t think the applause we received was quite as genuine or deserved, though.
After Adam and Chris played their gig, there was an autograph session and I took home a signed poster. Last year, I decided to take it out of storage and hang it in my office. The date of the show and the first day of my physician career, a date now nearly 15 years ago, is written in psychedelic typography at the bottom. The store that I went to that day is no longer there, a victim of progress like so many other record stores across the country. Another location of the same store is still open in Portland. I hope that it and all the other small book and music stores across the country can survive this current crisis, but I know that many will not.
So, here’s to you Adam, and to all the others who have lost their lives to this terrible illness. As a small token of remembrance, I’ll be playing some Fountains of Wayne on the drive home tonight. It’s not quite the same as playing it on a cross-country drive, but hopefully, we will all be able to do that again soon.
Dr. Sehgal is a clinical associate professor of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System and UT-Health San Antonio. He is a member of the editorial advisory board for The Hospitalist.
Like many in the health care field, I have found it hard to watch the news over these past couple of months when it seems that almost every story is about COVID-19 or its repercussions. Luckily, I have two young daughters who “encourage” me to listen to the Frozen 2 soundtrack instead of putting on the evening news when I get home from work. Still, news manages to seep through my defenses. As I scrolled through some headlines recently, I learned of the death of musician Adam Schlesinger from COVID-19. He wasn’t a household name, but his death still hit me in unexpected ways.
I started internship in late June 2005, in a city (Portland, Ore.) about as different from my previous home (Dallas) as any two places can possibly be. I think the day before internship started still ranks as the most nervous of my life. I’m not sure how I slept at all that night, but somehow I did and arrived at the Portland Veterans Affairs Hospital the following morning to start my new career.
And then … nothing happened. Early on that first day, the electronic medical records crashed, and no patients were admitted during our time on “short call.” My upper level resident took care of the one or two established patients on the team (both discharged), so I ended the day with records that would not be broken during the remainder of my residency: 0 notes written, 0 patients seen. Perhaps the most successful first day that any intern, anywhere has ever had, although it prepared me quite poorly for all the subsequent days.
Since I had some time on my hands, I made the 20-minute walk to one of my new hometown’s record stores where Fountains of Wayne (FOW) was playing an acoustic in-store set. Their album from a few years prior, “Welcome Interstate Managers,” was in heavy rotation when I made the drive from Dallas to Portland. It was (and is) a great album for long drives – melodic, catchy, and (mostly) up-tempo. Adam and the band’s singer, Chris Collingwood, played several songs that night on the store’s stage. Then they headed out to the next city, and I headed back home and on to many far-busier days of residency.
We would cross paths again a decade later. I moved back to Texas and became a hospitalist. It turns out that, if you have enough hospitalists of a certain age and if enough of those hospitalists have unearned confidence in their musical ability, then a covers band will undoubtedly be formed. And so, it happened here in San Antonio. We were not selective in our song choices – we played songs from every decade of the last 50 years, bands as popular as the Beatles and as indie as the Rentals. And we played some FOW.
Our band (which will go nameless here so that our YouTube recordings are more difficult to find) played a grand total of one gig during our years of intermittent practicing. That one gig was my wedding rehearsal dinner and the penultimate song we played was “Stacy’s Mom,” which is notable for being both FOW’s biggest hit and a completely inappropriate song to play at a wedding rehearsal dinner. The crowd was probably around the same size as the one that had seen Adam and Chris play in Portland 10 years prior. I don’t think the applause we received was quite as genuine or deserved, though.
After Adam and Chris played their gig, there was an autograph session and I took home a signed poster. Last year, I decided to take it out of storage and hang it in my office. The date of the show and the first day of my physician career, a date now nearly 15 years ago, is written in psychedelic typography at the bottom. The store that I went to that day is no longer there, a victim of progress like so many other record stores across the country. Another location of the same store is still open in Portland. I hope that it and all the other small book and music stores across the country can survive this current crisis, but I know that many will not.
So, here’s to you Adam, and to all the others who have lost their lives to this terrible illness. As a small token of remembrance, I’ll be playing some Fountains of Wayne on the drive home tonight. It’s not quite the same as playing it on a cross-country drive, but hopefully, we will all be able to do that again soon.
Dr. Sehgal is a clinical associate professor of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System and UT-Health San Antonio. He is a member of the editorial advisory board for The Hospitalist.
Doctor with a mask: Enhancing communication and empathy
Delivering a goodbye monologue to an elderly patient, I said: “Tomorrow, my colleague Dr. XYZ, who is an excellent physician, will be here in my place, and I will leave a detailed sign out for them.” I was on the last day of a 7-day-long block on hospital medicine service. Typically, when I say goodbye, some patients respond “thank you, enjoy your time,” some don’t care, and some show disappointment at the transition. This patient became uneasy, choking back tears, and said: “But, I don’t want a new doctor. You know me well. ... They don’t even allow my family in the hospital.”
That expression of anxiety, of having to build rapport with a new provider, concerns about continuity of care, and missing support of family members were not alien to me. As I instinctively took a step toward him to offer a comforting hug, an unsolicited voice in my head said, “social distancing.” I steered back, handing him a box of tissues. I continued: “You have come a long way, and things are looking good from here,” providing more details before I left the room. There was a change in my practice that week. I didn’t shake hands with my patients; I didn’t sit on any unassigned chair; I had no family members in the room asking me questions or supporting my patients. I was trying to show empathy or a smile behind a mask and protective eyewear. The business card with photograph had become more critical than ever for patients to “see” their doctor.
Moving from room to room and examining patients, it felt like the coronavirus was changing the practice of medicine beyond concerns of virus transmission, losing a patient, or putting in extra hours. I realized I was missing so-called “nonverbal communication” amid social distancing: facial expressions, social touch, and the support of family or friends to motivate or destress patients. With no visitors and curbed health care staff entries into patient’s rooms, social distancing was amounting to social isolation. My protective gear and social distancing seemed to be reducing my perceived empathy with patients, and the ability to build a good patient-physician relationship.
Amid alarms, beeps, and buzzes, patients were not only missing their families but also the familiar faces of their physicians. I needed to raise my game while embracing the “new normal” of health care. Cut to the next 13 patients: I paid more attention to voice, tone, and posture. I called patient families from the bedside instead of the office. I translated my emotions with words, loud and clear, replacing “your renal function looks better” (said without a smile) with “I am happy to see your renal function better.”
Through years of practice, I felt prepared to deal with feelings of denial, grief, anxiety, and much more, but the emotions arising as a result of this pandemic were unique. “I knew my mother was old, and this day would come,” said one of the inconsolable family members of a critically ill patient. “However, I wished to be at her side that day, not like this.” I spend my days listening to patient and family concerns about unemployment with quarantine, fears of spreading the disease to loved ones, and the possibility of medications not working.
After a long day, I went back to that first elderly patient to see if he was comfortable with the transition of care. I did a video conference with his daughter, and repeated my goodbyes. The patient smiled and said: “Doc, you deserve a break.” That day I learned about the challenges of good clinical rounding in coronavirus times, and how to overcome them. For “millennial” physicians, it is our first pandemic, and we are learning from it every day.
Driving home through empty streets, I concluded that my answers to the clinical questions asked by patients and families lean heavily on ever-changing data, and the treatments offered have yet to prove their mettle. As a result, I will continue to focus as much on the time-tested fundamentals of clinical practice: communication and empathy. I cannot allow the social distancing and the mask to hide my compassion, or take away from patient satisfaction. Shifting gears, I turned on my car radio, using music to reset my mind before attending to my now-homeschooling kids.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Wong CK et al. Effect of facemasks on empathy and relational continuity: A randomised controlled trial in primary care. BMC Fam Pract. 2013;14:200.
2. Little P et al. Randomised controlled trial of a brief intervention targeting predominantly nonverbal communication in general practice consultations. Br J Gen Pract. 2015;65(635):e351-6.
3. Varghese A. A doctor’s touch. TEDGlobal 2011. 2011 Jul. https://www.ted.com/talks/abraham_verghese_a_doctor_s_touch?language=en
Delivering a goodbye monologue to an elderly patient, I said: “Tomorrow, my colleague Dr. XYZ, who is an excellent physician, will be here in my place, and I will leave a detailed sign out for them.” I was on the last day of a 7-day-long block on hospital medicine service. Typically, when I say goodbye, some patients respond “thank you, enjoy your time,” some don’t care, and some show disappointment at the transition. This patient became uneasy, choking back tears, and said: “But, I don’t want a new doctor. You know me well. ... They don’t even allow my family in the hospital.”
That expression of anxiety, of having to build rapport with a new provider, concerns about continuity of care, and missing support of family members were not alien to me. As I instinctively took a step toward him to offer a comforting hug, an unsolicited voice in my head said, “social distancing.” I steered back, handing him a box of tissues. I continued: “You have come a long way, and things are looking good from here,” providing more details before I left the room. There was a change in my practice that week. I didn’t shake hands with my patients; I didn’t sit on any unassigned chair; I had no family members in the room asking me questions or supporting my patients. I was trying to show empathy or a smile behind a mask and protective eyewear. The business card with photograph had become more critical than ever for patients to “see” their doctor.
Moving from room to room and examining patients, it felt like the coronavirus was changing the practice of medicine beyond concerns of virus transmission, losing a patient, or putting in extra hours. I realized I was missing so-called “nonverbal communication” amid social distancing: facial expressions, social touch, and the support of family or friends to motivate or destress patients. With no visitors and curbed health care staff entries into patient’s rooms, social distancing was amounting to social isolation. My protective gear and social distancing seemed to be reducing my perceived empathy with patients, and the ability to build a good patient-physician relationship.
Amid alarms, beeps, and buzzes, patients were not only missing their families but also the familiar faces of their physicians. I needed to raise my game while embracing the “new normal” of health care. Cut to the next 13 patients: I paid more attention to voice, tone, and posture. I called patient families from the bedside instead of the office. I translated my emotions with words, loud and clear, replacing “your renal function looks better” (said without a smile) with “I am happy to see your renal function better.”
Through years of practice, I felt prepared to deal with feelings of denial, grief, anxiety, and much more, but the emotions arising as a result of this pandemic were unique. “I knew my mother was old, and this day would come,” said one of the inconsolable family members of a critically ill patient. “However, I wished to be at her side that day, not like this.” I spend my days listening to patient and family concerns about unemployment with quarantine, fears of spreading the disease to loved ones, and the possibility of medications not working.
After a long day, I went back to that first elderly patient to see if he was comfortable with the transition of care. I did a video conference with his daughter, and repeated my goodbyes. The patient smiled and said: “Doc, you deserve a break.” That day I learned about the challenges of good clinical rounding in coronavirus times, and how to overcome them. For “millennial” physicians, it is our first pandemic, and we are learning from it every day.
Driving home through empty streets, I concluded that my answers to the clinical questions asked by patients and families lean heavily on ever-changing data, and the treatments offered have yet to prove their mettle. As a result, I will continue to focus as much on the time-tested fundamentals of clinical practice: communication and empathy. I cannot allow the social distancing and the mask to hide my compassion, or take away from patient satisfaction. Shifting gears, I turned on my car radio, using music to reset my mind before attending to my now-homeschooling kids.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Wong CK et al. Effect of facemasks on empathy and relational continuity: A randomised controlled trial in primary care. BMC Fam Pract. 2013;14:200.
2. Little P et al. Randomised controlled trial of a brief intervention targeting predominantly nonverbal communication in general practice consultations. Br J Gen Pract. 2015;65(635):e351-6.
3. Varghese A. A doctor’s touch. TEDGlobal 2011. 2011 Jul. https://www.ted.com/talks/abraham_verghese_a_doctor_s_touch?language=en
Delivering a goodbye monologue to an elderly patient, I said: “Tomorrow, my colleague Dr. XYZ, who is an excellent physician, will be here in my place, and I will leave a detailed sign out for them.” I was on the last day of a 7-day-long block on hospital medicine service. Typically, when I say goodbye, some patients respond “thank you, enjoy your time,” some don’t care, and some show disappointment at the transition. This patient became uneasy, choking back tears, and said: “But, I don’t want a new doctor. You know me well. ... They don’t even allow my family in the hospital.”
That expression of anxiety, of having to build rapport with a new provider, concerns about continuity of care, and missing support of family members were not alien to me. As I instinctively took a step toward him to offer a comforting hug, an unsolicited voice in my head said, “social distancing.” I steered back, handing him a box of tissues. I continued: “You have come a long way, and things are looking good from here,” providing more details before I left the room. There was a change in my practice that week. I didn’t shake hands with my patients; I didn’t sit on any unassigned chair; I had no family members in the room asking me questions or supporting my patients. I was trying to show empathy or a smile behind a mask and protective eyewear. The business card with photograph had become more critical than ever for patients to “see” their doctor.
Moving from room to room and examining patients, it felt like the coronavirus was changing the practice of medicine beyond concerns of virus transmission, losing a patient, or putting in extra hours. I realized I was missing so-called “nonverbal communication” amid social distancing: facial expressions, social touch, and the support of family or friends to motivate or destress patients. With no visitors and curbed health care staff entries into patient’s rooms, social distancing was amounting to social isolation. My protective gear and social distancing seemed to be reducing my perceived empathy with patients, and the ability to build a good patient-physician relationship.
Amid alarms, beeps, and buzzes, patients were not only missing their families but also the familiar faces of their physicians. I needed to raise my game while embracing the “new normal” of health care. Cut to the next 13 patients: I paid more attention to voice, tone, and posture. I called patient families from the bedside instead of the office. I translated my emotions with words, loud and clear, replacing “your renal function looks better” (said without a smile) with “I am happy to see your renal function better.”
Through years of practice, I felt prepared to deal with feelings of denial, grief, anxiety, and much more, but the emotions arising as a result of this pandemic were unique. “I knew my mother was old, and this day would come,” said one of the inconsolable family members of a critically ill patient. “However, I wished to be at her side that day, not like this.” I spend my days listening to patient and family concerns about unemployment with quarantine, fears of spreading the disease to loved ones, and the possibility of medications not working.
After a long day, I went back to that first elderly patient to see if he was comfortable with the transition of care. I did a video conference with his daughter, and repeated my goodbyes. The patient smiled and said: “Doc, you deserve a break.” That day I learned about the challenges of good clinical rounding in coronavirus times, and how to overcome them. For “millennial” physicians, it is our first pandemic, and we are learning from it every day.
Driving home through empty streets, I concluded that my answers to the clinical questions asked by patients and families lean heavily on ever-changing data, and the treatments offered have yet to prove their mettle. As a result, I will continue to focus as much on the time-tested fundamentals of clinical practice: communication and empathy. I cannot allow the social distancing and the mask to hide my compassion, or take away from patient satisfaction. Shifting gears, I turned on my car radio, using music to reset my mind before attending to my now-homeschooling kids.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Wong CK et al. Effect of facemasks on empathy and relational continuity: A randomised controlled trial in primary care. BMC Fam Pract. 2013;14:200.
2. Little P et al. Randomised controlled trial of a brief intervention targeting predominantly nonverbal communication in general practice consultations. Br J Gen Pract. 2015;65(635):e351-6.
3. Varghese A. A doctor’s touch. TEDGlobal 2011. 2011 Jul. https://www.ted.com/talks/abraham_verghese_a_doctor_s_touch?language=en
Case reports illustrate heterogeneity of skin manifestations in COVID patients
Two infection.
It is not yet clear from these or other case reports which, if any, skin eruptions accompanying COVID-19 infections are caused by the virus, but the authors of the editorial, led by Lauren M. Madigan, MD, of the department of dermatology at the University of Utah, Salt Lake City, urged dermatologists to lead efforts to find out.
“To fully characterize skin manifestations, it may be necessary for dermatologists to evaluate these patients directly; comprehensive evaluation could reveal important morphologic clues, such as the subtle purpuric nature of skin lesions or the characteristic mucosal or ophthalmologic features of COVID-19,” the authors of the editorial stated.
So far, the patterns of skin symptoms, which have been identified in up to 20% of COVID-19–infected patients in some series, have been heterogeneous as demonstrated in the two published case reports.
In one case, a papulosquamous and erythematous periumbilical patch that appeared on the trunk in an elderly patient 1 day after hospital admission for acute respiratory distress rapidly evolved into a digitate papulosquamous eruption involving the upper arms, shoulder, and back. It was described as “clinically reminiscent” of pityriasis rosea by the authors, from the divisions of dermatology and venereology, pathology, intensive care, and the virology laboratory, of the Hôpital Cochin, Paris.
In the other, pruritic erythematous macules, papules, and petechiae affecting the buttocks, popliteal fossae, anterior thighs, and lower abdomen appeared 3 days after the onset of fever in a 48-year-old man hospitalized in Madrid. A biopsy demonstrated a superficial perivascular lymphocytic infiltrate with red cell extravasation and focal papillary edema, “along with focal parakeratosis and isolated dyskeratotic cells,” according to the authors of this report, from the department of dermatology at Ramon y Cajal University, Madrid.
It was unclear whether COVID-19 directly caused either skin eruption. In the patient with the digitate papulosquamous eruption, no virus could be isolated from the skin. Based on high levels of proinflammatory cytokines, it was hypothesized that the rash might have been secondary to an immune response. The rash resolved within a week, but the patient subsequently died of the infection.
In the second case, the petechial lesions, which developed before any treatment was initiated, were said to resemble those associated with other viruses, such as parvovirus B19. This led the investigators to speculate that SARS-CoV-2 “could affect the skin in a similar way,” even though other potential etiologies could not be excluded. Treated with a topical steroid and an oral antihistamine, the skin lesions resolved after 5 days. This patient was discharged after recovering from the respiratory illness after 12 days.
Like previously reported cutaneous eruptions associated with COVID-19 infection, these cases “raise more questions than they provide answers,” wrote the authors of the editorial, but the limited information currently available was the basis for encouraging dermatologists to get involved.
To participate, dermatologists need not necessarily be affiliated with an academic center, according to one of the editorial coauthors, Kanade Shinkai, MD, PhD, professor of dermatology at the University of California, San Francisco. She noted that any health professional is invited to submit cases of COVID-19–associated dermatoses to a registry set up by the American Academy of Dermatology.
It is hoped that cases captured in this registry will create sufficient data to allow clinically relevant patterns and etiologies to be characterized.
The need for data is clear to those on the front lines. Kirsten Lo Sicco, MD, associate director of the skin and cancer unit at New York University, reported that her center is already set up to collect data systematically. “At NYU, we are currently working on standardizing laboratory and histopathology work up for COVID-19 patients who present with various skin eruptions.”
The goal, she added, is “to better determine COVID-19 pathophysiology, systemic associations, patient outcomes, and potential therapeutics.”
“Presumably, many of the eruptions seen in the setting of COVID-19 infection are related,” Dr. Lo Sicco explained in an interview. However, skin complications of infection “may overlap with or be a result of other etiologies as well.”
While better testing for COVID-19 and more lesion biopsies will play a critical role in differentiating etiologies, “we must not overcall COVID-19–related skin eruptions and potentially overlook other diagnoses,” Dr. Lo Sicco said.
In recounting some challenges from the NYU experience so far, Dr. Lo Sicco described the difficulty of differentiating COVID-19–related skin eruptions from skin eruptions caused by treatments, such as antibiotics and antivirals, when the presentation is delayed.
“This is where collaboration with our dermatopathologists becomes important. Drug eruptions, viral exanthems, urticarial eruptions, vasculopathy, and vasculitis can all be differentiated on dermpath,” she said.
One early obstacle to the skin biopsies essential for these types of studies was the limited supply of personal protective equipment at many centers, including hospitals in New York. Biopsies could not be safely performed if supplies of masks and gowns were limited.
Recent evidence suggests that some of the more common morphologies, such as purpuric eruptions, livedo reticularis, and retiform purpura, are linked to the vasculopathy associated with COVID-19 infection, according to Dr. Lo Sicco, but this invites a new set of questions.
One is whether vasculopathies can be prevented with prophylactic anticoagulation. Many hospitalized COVID-19 patients are already receiving therapeutic anticoagulation, but Dr. Lo Sicco questioned whether prophylactic anticoagulation might improve prognosis for outpatients, such as those discharged or those never hospitalized. This is a strategy now being investigated.
Ultimately, she agreed with the thrust of the JAMA Dermatology editorial.
“Dermatologists are vital to determine if various morphologies, such as urticarial, vesicular, purpuric, or papulosquamous lesions, have any specific systemic implications or relate to differences in patient outcomes,” she said.
These are exactly the types of issues being actively investigated at her center.
Neither the authors of the case reports nor of the editorial reported any conflicts of interest.
SOURCEs: Madigan LM et al. JAMA Dermatol. 2020 Apr 30. doi:10.1001/jamadermatol.2020.1438; Diaz-Guimaraens B et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1741; Sanchez A et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1704.
Two infection.
It is not yet clear from these or other case reports which, if any, skin eruptions accompanying COVID-19 infections are caused by the virus, but the authors of the editorial, led by Lauren M. Madigan, MD, of the department of dermatology at the University of Utah, Salt Lake City, urged dermatologists to lead efforts to find out.
“To fully characterize skin manifestations, it may be necessary for dermatologists to evaluate these patients directly; comprehensive evaluation could reveal important morphologic clues, such as the subtle purpuric nature of skin lesions or the characteristic mucosal or ophthalmologic features of COVID-19,” the authors of the editorial stated.
So far, the patterns of skin symptoms, which have been identified in up to 20% of COVID-19–infected patients in some series, have been heterogeneous as demonstrated in the two published case reports.
In one case, a papulosquamous and erythematous periumbilical patch that appeared on the trunk in an elderly patient 1 day after hospital admission for acute respiratory distress rapidly evolved into a digitate papulosquamous eruption involving the upper arms, shoulder, and back. It was described as “clinically reminiscent” of pityriasis rosea by the authors, from the divisions of dermatology and venereology, pathology, intensive care, and the virology laboratory, of the Hôpital Cochin, Paris.
In the other, pruritic erythematous macules, papules, and petechiae affecting the buttocks, popliteal fossae, anterior thighs, and lower abdomen appeared 3 days after the onset of fever in a 48-year-old man hospitalized in Madrid. A biopsy demonstrated a superficial perivascular lymphocytic infiltrate with red cell extravasation and focal papillary edema, “along with focal parakeratosis and isolated dyskeratotic cells,” according to the authors of this report, from the department of dermatology at Ramon y Cajal University, Madrid.
It was unclear whether COVID-19 directly caused either skin eruption. In the patient with the digitate papulosquamous eruption, no virus could be isolated from the skin. Based on high levels of proinflammatory cytokines, it was hypothesized that the rash might have been secondary to an immune response. The rash resolved within a week, but the patient subsequently died of the infection.
In the second case, the petechial lesions, which developed before any treatment was initiated, were said to resemble those associated with other viruses, such as parvovirus B19. This led the investigators to speculate that SARS-CoV-2 “could affect the skin in a similar way,” even though other potential etiologies could not be excluded. Treated with a topical steroid and an oral antihistamine, the skin lesions resolved after 5 days. This patient was discharged after recovering from the respiratory illness after 12 days.
Like previously reported cutaneous eruptions associated with COVID-19 infection, these cases “raise more questions than they provide answers,” wrote the authors of the editorial, but the limited information currently available was the basis for encouraging dermatologists to get involved.
To participate, dermatologists need not necessarily be affiliated with an academic center, according to one of the editorial coauthors, Kanade Shinkai, MD, PhD, professor of dermatology at the University of California, San Francisco. She noted that any health professional is invited to submit cases of COVID-19–associated dermatoses to a registry set up by the American Academy of Dermatology.
It is hoped that cases captured in this registry will create sufficient data to allow clinically relevant patterns and etiologies to be characterized.
The need for data is clear to those on the front lines. Kirsten Lo Sicco, MD, associate director of the skin and cancer unit at New York University, reported that her center is already set up to collect data systematically. “At NYU, we are currently working on standardizing laboratory and histopathology work up for COVID-19 patients who present with various skin eruptions.”
The goal, she added, is “to better determine COVID-19 pathophysiology, systemic associations, patient outcomes, and potential therapeutics.”
“Presumably, many of the eruptions seen in the setting of COVID-19 infection are related,” Dr. Lo Sicco explained in an interview. However, skin complications of infection “may overlap with or be a result of other etiologies as well.”
While better testing for COVID-19 and more lesion biopsies will play a critical role in differentiating etiologies, “we must not overcall COVID-19–related skin eruptions and potentially overlook other diagnoses,” Dr. Lo Sicco said.
In recounting some challenges from the NYU experience so far, Dr. Lo Sicco described the difficulty of differentiating COVID-19–related skin eruptions from skin eruptions caused by treatments, such as antibiotics and antivirals, when the presentation is delayed.
“This is where collaboration with our dermatopathologists becomes important. Drug eruptions, viral exanthems, urticarial eruptions, vasculopathy, and vasculitis can all be differentiated on dermpath,” she said.
One early obstacle to the skin biopsies essential for these types of studies was the limited supply of personal protective equipment at many centers, including hospitals in New York. Biopsies could not be safely performed if supplies of masks and gowns were limited.
Recent evidence suggests that some of the more common morphologies, such as purpuric eruptions, livedo reticularis, and retiform purpura, are linked to the vasculopathy associated with COVID-19 infection, according to Dr. Lo Sicco, but this invites a new set of questions.
One is whether vasculopathies can be prevented with prophylactic anticoagulation. Many hospitalized COVID-19 patients are already receiving therapeutic anticoagulation, but Dr. Lo Sicco questioned whether prophylactic anticoagulation might improve prognosis for outpatients, such as those discharged or those never hospitalized. This is a strategy now being investigated.
Ultimately, she agreed with the thrust of the JAMA Dermatology editorial.
“Dermatologists are vital to determine if various morphologies, such as urticarial, vesicular, purpuric, or papulosquamous lesions, have any specific systemic implications or relate to differences in patient outcomes,” she said.
These are exactly the types of issues being actively investigated at her center.
Neither the authors of the case reports nor of the editorial reported any conflicts of interest.
SOURCEs: Madigan LM et al. JAMA Dermatol. 2020 Apr 30. doi:10.1001/jamadermatol.2020.1438; Diaz-Guimaraens B et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1741; Sanchez A et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1704.
Two infection.
It is not yet clear from these or other case reports which, if any, skin eruptions accompanying COVID-19 infections are caused by the virus, but the authors of the editorial, led by Lauren M. Madigan, MD, of the department of dermatology at the University of Utah, Salt Lake City, urged dermatologists to lead efforts to find out.
“To fully characterize skin manifestations, it may be necessary for dermatologists to evaluate these patients directly; comprehensive evaluation could reveal important morphologic clues, such as the subtle purpuric nature of skin lesions or the characteristic mucosal or ophthalmologic features of COVID-19,” the authors of the editorial stated.
So far, the patterns of skin symptoms, which have been identified in up to 20% of COVID-19–infected patients in some series, have been heterogeneous as demonstrated in the two published case reports.
In one case, a papulosquamous and erythematous periumbilical patch that appeared on the trunk in an elderly patient 1 day after hospital admission for acute respiratory distress rapidly evolved into a digitate papulosquamous eruption involving the upper arms, shoulder, and back. It was described as “clinically reminiscent” of pityriasis rosea by the authors, from the divisions of dermatology and venereology, pathology, intensive care, and the virology laboratory, of the Hôpital Cochin, Paris.
In the other, pruritic erythematous macules, papules, and petechiae affecting the buttocks, popliteal fossae, anterior thighs, and lower abdomen appeared 3 days after the onset of fever in a 48-year-old man hospitalized in Madrid. A biopsy demonstrated a superficial perivascular lymphocytic infiltrate with red cell extravasation and focal papillary edema, “along with focal parakeratosis and isolated dyskeratotic cells,” according to the authors of this report, from the department of dermatology at Ramon y Cajal University, Madrid.
It was unclear whether COVID-19 directly caused either skin eruption. In the patient with the digitate papulosquamous eruption, no virus could be isolated from the skin. Based on high levels of proinflammatory cytokines, it was hypothesized that the rash might have been secondary to an immune response. The rash resolved within a week, but the patient subsequently died of the infection.
In the second case, the petechial lesions, which developed before any treatment was initiated, were said to resemble those associated with other viruses, such as parvovirus B19. This led the investigators to speculate that SARS-CoV-2 “could affect the skin in a similar way,” even though other potential etiologies could not be excluded. Treated with a topical steroid and an oral antihistamine, the skin lesions resolved after 5 days. This patient was discharged after recovering from the respiratory illness after 12 days.
Like previously reported cutaneous eruptions associated with COVID-19 infection, these cases “raise more questions than they provide answers,” wrote the authors of the editorial, but the limited information currently available was the basis for encouraging dermatologists to get involved.
To participate, dermatologists need not necessarily be affiliated with an academic center, according to one of the editorial coauthors, Kanade Shinkai, MD, PhD, professor of dermatology at the University of California, San Francisco. She noted that any health professional is invited to submit cases of COVID-19–associated dermatoses to a registry set up by the American Academy of Dermatology.
It is hoped that cases captured in this registry will create sufficient data to allow clinically relevant patterns and etiologies to be characterized.
The need for data is clear to those on the front lines. Kirsten Lo Sicco, MD, associate director of the skin and cancer unit at New York University, reported that her center is already set up to collect data systematically. “At NYU, we are currently working on standardizing laboratory and histopathology work up for COVID-19 patients who present with various skin eruptions.”
The goal, she added, is “to better determine COVID-19 pathophysiology, systemic associations, patient outcomes, and potential therapeutics.”
“Presumably, many of the eruptions seen in the setting of COVID-19 infection are related,” Dr. Lo Sicco explained in an interview. However, skin complications of infection “may overlap with or be a result of other etiologies as well.”
While better testing for COVID-19 and more lesion biopsies will play a critical role in differentiating etiologies, “we must not overcall COVID-19–related skin eruptions and potentially overlook other diagnoses,” Dr. Lo Sicco said.
In recounting some challenges from the NYU experience so far, Dr. Lo Sicco described the difficulty of differentiating COVID-19–related skin eruptions from skin eruptions caused by treatments, such as antibiotics and antivirals, when the presentation is delayed.
“This is where collaboration with our dermatopathologists becomes important. Drug eruptions, viral exanthems, urticarial eruptions, vasculopathy, and vasculitis can all be differentiated on dermpath,” she said.
One early obstacle to the skin biopsies essential for these types of studies was the limited supply of personal protective equipment at many centers, including hospitals in New York. Biopsies could not be safely performed if supplies of masks and gowns were limited.
Recent evidence suggests that some of the more common morphologies, such as purpuric eruptions, livedo reticularis, and retiform purpura, are linked to the vasculopathy associated with COVID-19 infection, according to Dr. Lo Sicco, but this invites a new set of questions.
One is whether vasculopathies can be prevented with prophylactic anticoagulation. Many hospitalized COVID-19 patients are already receiving therapeutic anticoagulation, but Dr. Lo Sicco questioned whether prophylactic anticoagulation might improve prognosis for outpatients, such as those discharged or those never hospitalized. This is a strategy now being investigated.
Ultimately, she agreed with the thrust of the JAMA Dermatology editorial.
“Dermatologists are vital to determine if various morphologies, such as urticarial, vesicular, purpuric, or papulosquamous lesions, have any specific systemic implications or relate to differences in patient outcomes,” she said.
These are exactly the types of issues being actively investigated at her center.
Neither the authors of the case reports nor of the editorial reported any conflicts of interest.
SOURCEs: Madigan LM et al. JAMA Dermatol. 2020 Apr 30. doi:10.1001/jamadermatol.2020.1438; Diaz-Guimaraens B et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1741; Sanchez A et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1704.
Cancer screening, monitoring down during pandemic
according to a report by the IQVIA Institute for Human Data Science.
There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
according to a report by the IQVIA Institute for Human Data Science.

There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
according to a report by the IQVIA Institute for Human Data Science.

There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.










