User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


‘Brutal’ plan to restrict palliative radiation during pandemic
A major comprehensive cancer center at the epicenter of the New York City COVID-19 storm is preparing to scale back palliative radiation therapy (RT), anticipating a focus on only oncologic emergencies.
“We’re not there yet, but we’re anticipating when the time comes in the next few weeks that we will have a system in place so we are able to handle it,” Jonathan Yang, MD, PhD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, told Medscape Medical News.
Yang and an expert panel of colleagues reviewed high-impact evidence, prior systematic reviews, and national guidelines to compile a set of recommendations for triage and shortened palliative rRT at their center, should the need arise.
The recommendations on palliative radiotherapy for oncologic emergencies in the setting of COVID-19 appear in a preprint version in Advances in Radiation Oncology, released by the American Society of Radiation Oncology.
Yang says the recommendations are a careful balance between the risk of COVID-19 exposure of staff and patients with the potential morbidity of delaying treatment.
“Everyone is conscious of decisions about whether patients need treatment now or can wait,” he told Medscape Medical News. “It’s a juggling act every single day, but by having this guideline in place, when we face the situation where we do have to make decisions, is helpful.”
The document aims to enable swift decisions based on best practice, including a three-tiered system prioritizing only “clinically urgent cases, in which delaying treatment would result in compromised outcomes or serious morbidity.”
“It’s brutal, that’s the only word for it. Not that I disagree with it,” commented Padraig Warde, MB BCh, professor, Department of Radiation Oncology, University of Toronto, and radiation oncologist, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
Like many places, Toronto is not yet experiencing the COVID-19 burden of New York City, but Warde says the MSKCC guideline is useful for everyone. “Other centers should review it and see how they could deal with resource limitations,” he said. “It’s sobering and sad, but if you don’t have the staff to treat all patients, which particular patients do you choose to treat?”
In a nutshell, the MSKCC recommendations defines Tier 1 patients as having oncologic emergencies that require palliative RT, including “cord compression, symptomatic brain metastases requiring whole-brain radiotherapy, life-threatening tumor bleeding, and malignant airway obstruction.”
According to the decision-making guideline, patients in Tiers 2 and 3 would have their palliative RT delayed. This would include Tier 2 patients whose needs are not classified as emergencies, but who have either symptomatic disease for which RT is usually the standard of care or asymptomatic disease for which RT is recommended “to prevent imminent functional deficits.” Tier 3 would be symptomatic or asymptomatic patients for whom RT is “one of the effective treatment options.”
“Rationing is always very difficult because as physicians you always want to do everything you can for your patients but we really have to strike the balance on when to do what, said Yang. The plan that he authored anticipates both reduced availability of radiation therapists as well as aggressive attempts to limit patients’ infection exposure.
“If a patient’s radiation is being considered for delay due to COVID-19, other means are utilized to achieve the goal of palliation in the interim, and in addition to the tier system, this decision is also made on a case-by-case basis with departmental discussion on the risks and benefits,” he explained.
“There are layers of checks and balances for these decisions...Obviously for oncologic emergencies, radiation will be implemented. However for less urgent situations, bringing them into the hospital when there are other ways to achieve the same goal, potential risk of exposure to COVID-19 is higher than the benefit we would be able to provide.”
The document also recommends shorter courses of RT when radiation is deemed appropriate.
“We have good evidence showing shorter courses of radiation can effectively treat the goal of palliation compared to longer courses of radiation,” he explained. “Going through this pandemic actually forces radiation oncologists in the United States to put that evidence into practice. It’s not suboptimal care in the sense that we are achieving the same goal — palliation. This paper is to remind people there are equally effective courses of palliation we can be using.”
“[There’s] nothing like a crisis to get people to do the right thing,” commented Louis Potters, MD, professor and chair of radiation medicine at the Feinstein Institutes, the research arm of Northwell Health, New York’s largest healthcare provider.
Northwell Health has been at the epicenter of the New York outbreak of COVID-19. Potters writes on an ASTRO blog that, as of March 26, Northwell Health “has diagnosed 4399 positive COVID-19 patients, which is about 20% of New York state and 1.2% of all cases in the world. All cancer surgery was discontinued as of March 20 and all of our 23 hospitals are seeing COVID-19 admissions, and ICU care became the primary focus of the entire system. As of today, we have reserved one floor in two hospitals for non-COVID care such as trauma. That’s it.”
Before the crisis, radiation medicine at Northwell consisted of eight separate locations treating on average 280 EBRT cases a day, not including SBRT/SRS and brachytherapy cases. “That of course was 3 weeks ago,” he notes.
Commenting on the recommendations from the MSKCC group, Potters told Medscape Medical News that the primary goal “was to document what are acceptable alternatives for accelerated care.”
“Ironically, these guidelines represent best practices with evidence that — in a non–COVID-19 world — make sense for the majority of patients requiring palliative radiotherapy,” he said.
Potters said there has been hesitance to transition to shorter radiation treatments for several reasons.
“Historically, palliative radiotherapy has been delivered over 2 to 4 weeks with good results. And, as is typical in medicine, the transition to shorter course care is slowed by financial incentives to protract care,” he explained.
“In a value-based future where payment is based on outcomes, this transition to shorter care will evolve very quickly. But given the current COVID-19 crisis, and the risk to patients and staff, the incentive for shorter treatment courses has been thrust upon us and the MSKCC outline helps to define how to do this safely and with evidence-based expected efficacy.”
This article first appeared on Medscape.com.
A major comprehensive cancer center at the epicenter of the New York City COVID-19 storm is preparing to scale back palliative radiation therapy (RT), anticipating a focus on only oncologic emergencies.
“We’re not there yet, but we’re anticipating when the time comes in the next few weeks that we will have a system in place so we are able to handle it,” Jonathan Yang, MD, PhD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, told Medscape Medical News.
Yang and an expert panel of colleagues reviewed high-impact evidence, prior systematic reviews, and national guidelines to compile a set of recommendations for triage and shortened palliative rRT at their center, should the need arise.
The recommendations on palliative radiotherapy for oncologic emergencies in the setting of COVID-19 appear in a preprint version in Advances in Radiation Oncology, released by the American Society of Radiation Oncology.
Yang says the recommendations are a careful balance between the risk of COVID-19 exposure of staff and patients with the potential morbidity of delaying treatment.
“Everyone is conscious of decisions about whether patients need treatment now or can wait,” he told Medscape Medical News. “It’s a juggling act every single day, but by having this guideline in place, when we face the situation where we do have to make decisions, is helpful.”
The document aims to enable swift decisions based on best practice, including a three-tiered system prioritizing only “clinically urgent cases, in which delaying treatment would result in compromised outcomes or serious morbidity.”
“It’s brutal, that’s the only word for it. Not that I disagree with it,” commented Padraig Warde, MB BCh, professor, Department of Radiation Oncology, University of Toronto, and radiation oncologist, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
Like many places, Toronto is not yet experiencing the COVID-19 burden of New York City, but Warde says the MSKCC guideline is useful for everyone. “Other centers should review it and see how they could deal with resource limitations,” he said. “It’s sobering and sad, but if you don’t have the staff to treat all patients, which particular patients do you choose to treat?”
In a nutshell, the MSKCC recommendations defines Tier 1 patients as having oncologic emergencies that require palliative RT, including “cord compression, symptomatic brain metastases requiring whole-brain radiotherapy, life-threatening tumor bleeding, and malignant airway obstruction.”
According to the decision-making guideline, patients in Tiers 2 and 3 would have their palliative RT delayed. This would include Tier 2 patients whose needs are not classified as emergencies, but who have either symptomatic disease for which RT is usually the standard of care or asymptomatic disease for which RT is recommended “to prevent imminent functional deficits.” Tier 3 would be symptomatic or asymptomatic patients for whom RT is “one of the effective treatment options.”
“Rationing is always very difficult because as physicians you always want to do everything you can for your patients but we really have to strike the balance on when to do what, said Yang. The plan that he authored anticipates both reduced availability of radiation therapists as well as aggressive attempts to limit patients’ infection exposure.
“If a patient’s radiation is being considered for delay due to COVID-19, other means are utilized to achieve the goal of palliation in the interim, and in addition to the tier system, this decision is also made on a case-by-case basis with departmental discussion on the risks and benefits,” he explained.
“There are layers of checks and balances for these decisions...Obviously for oncologic emergencies, radiation will be implemented. However for less urgent situations, bringing them into the hospital when there are other ways to achieve the same goal, potential risk of exposure to COVID-19 is higher than the benefit we would be able to provide.”
The document also recommends shorter courses of RT when radiation is deemed appropriate.
“We have good evidence showing shorter courses of radiation can effectively treat the goal of palliation compared to longer courses of radiation,” he explained. “Going through this pandemic actually forces radiation oncologists in the United States to put that evidence into practice. It’s not suboptimal care in the sense that we are achieving the same goal — palliation. This paper is to remind people there are equally effective courses of palliation we can be using.”
“[There’s] nothing like a crisis to get people to do the right thing,” commented Louis Potters, MD, professor and chair of radiation medicine at the Feinstein Institutes, the research arm of Northwell Health, New York’s largest healthcare provider.
Northwell Health has been at the epicenter of the New York outbreak of COVID-19. Potters writes on an ASTRO blog that, as of March 26, Northwell Health “has diagnosed 4399 positive COVID-19 patients, which is about 20% of New York state and 1.2% of all cases in the world. All cancer surgery was discontinued as of March 20 and all of our 23 hospitals are seeing COVID-19 admissions, and ICU care became the primary focus of the entire system. As of today, we have reserved one floor in two hospitals for non-COVID care such as trauma. That’s it.”
Before the crisis, radiation medicine at Northwell consisted of eight separate locations treating on average 280 EBRT cases a day, not including SBRT/SRS and brachytherapy cases. “That of course was 3 weeks ago,” he notes.
Commenting on the recommendations from the MSKCC group, Potters told Medscape Medical News that the primary goal “was to document what are acceptable alternatives for accelerated care.”
“Ironically, these guidelines represent best practices with evidence that — in a non–COVID-19 world — make sense for the majority of patients requiring palliative radiotherapy,” he said.
Potters said there has been hesitance to transition to shorter radiation treatments for several reasons.
“Historically, palliative radiotherapy has been delivered over 2 to 4 weeks with good results. And, as is typical in medicine, the transition to shorter course care is slowed by financial incentives to protract care,” he explained.
“In a value-based future where payment is based on outcomes, this transition to shorter care will evolve very quickly. But given the current COVID-19 crisis, and the risk to patients and staff, the incentive for shorter treatment courses has been thrust upon us and the MSKCC outline helps to define how to do this safely and with evidence-based expected efficacy.”
This article first appeared on Medscape.com.
A major comprehensive cancer center at the epicenter of the New York City COVID-19 storm is preparing to scale back palliative radiation therapy (RT), anticipating a focus on only oncologic emergencies.
“We’re not there yet, but we’re anticipating when the time comes in the next few weeks that we will have a system in place so we are able to handle it,” Jonathan Yang, MD, PhD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, told Medscape Medical News.
Yang and an expert panel of colleagues reviewed high-impact evidence, prior systematic reviews, and national guidelines to compile a set of recommendations for triage and shortened palliative rRT at their center, should the need arise.
The recommendations on palliative radiotherapy for oncologic emergencies in the setting of COVID-19 appear in a preprint version in Advances in Radiation Oncology, released by the American Society of Radiation Oncology.
Yang says the recommendations are a careful balance between the risk of COVID-19 exposure of staff and patients with the potential morbidity of delaying treatment.
“Everyone is conscious of decisions about whether patients need treatment now or can wait,” he told Medscape Medical News. “It’s a juggling act every single day, but by having this guideline in place, when we face the situation where we do have to make decisions, is helpful.”
The document aims to enable swift decisions based on best practice, including a three-tiered system prioritizing only “clinically urgent cases, in which delaying treatment would result in compromised outcomes or serious morbidity.”
“It’s brutal, that’s the only word for it. Not that I disagree with it,” commented Padraig Warde, MB BCh, professor, Department of Radiation Oncology, University of Toronto, and radiation oncologist, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
Like many places, Toronto is not yet experiencing the COVID-19 burden of New York City, but Warde says the MSKCC guideline is useful for everyone. “Other centers should review it and see how they could deal with resource limitations,” he said. “It’s sobering and sad, but if you don’t have the staff to treat all patients, which particular patients do you choose to treat?”
In a nutshell, the MSKCC recommendations defines Tier 1 patients as having oncologic emergencies that require palliative RT, including “cord compression, symptomatic brain metastases requiring whole-brain radiotherapy, life-threatening tumor bleeding, and malignant airway obstruction.”
According to the decision-making guideline, patients in Tiers 2 and 3 would have their palliative RT delayed. This would include Tier 2 patients whose needs are not classified as emergencies, but who have either symptomatic disease for which RT is usually the standard of care or asymptomatic disease for which RT is recommended “to prevent imminent functional deficits.” Tier 3 would be symptomatic or asymptomatic patients for whom RT is “one of the effective treatment options.”
“Rationing is always very difficult because as physicians you always want to do everything you can for your patients but we really have to strike the balance on when to do what, said Yang. The plan that he authored anticipates both reduced availability of radiation therapists as well as aggressive attempts to limit patients’ infection exposure.
“If a patient’s radiation is being considered for delay due to COVID-19, other means are utilized to achieve the goal of palliation in the interim, and in addition to the tier system, this decision is also made on a case-by-case basis with departmental discussion on the risks and benefits,” he explained.
“There are layers of checks and balances for these decisions...Obviously for oncologic emergencies, radiation will be implemented. However for less urgent situations, bringing them into the hospital when there are other ways to achieve the same goal, potential risk of exposure to COVID-19 is higher than the benefit we would be able to provide.”
The document also recommends shorter courses of RT when radiation is deemed appropriate.
“We have good evidence showing shorter courses of radiation can effectively treat the goal of palliation compared to longer courses of radiation,” he explained. “Going through this pandemic actually forces radiation oncologists in the United States to put that evidence into practice. It’s not suboptimal care in the sense that we are achieving the same goal — palliation. This paper is to remind people there are equally effective courses of palliation we can be using.”
“[There’s] nothing like a crisis to get people to do the right thing,” commented Louis Potters, MD, professor and chair of radiation medicine at the Feinstein Institutes, the research arm of Northwell Health, New York’s largest healthcare provider.
Northwell Health has been at the epicenter of the New York outbreak of COVID-19. Potters writes on an ASTRO blog that, as of March 26, Northwell Health “has diagnosed 4399 positive COVID-19 patients, which is about 20% of New York state and 1.2% of all cases in the world. All cancer surgery was discontinued as of March 20 and all of our 23 hospitals are seeing COVID-19 admissions, and ICU care became the primary focus of the entire system. As of today, we have reserved one floor in two hospitals for non-COVID care such as trauma. That’s it.”
Before the crisis, radiation medicine at Northwell consisted of eight separate locations treating on average 280 EBRT cases a day, not including SBRT/SRS and brachytherapy cases. “That of course was 3 weeks ago,” he notes.
Commenting on the recommendations from the MSKCC group, Potters told Medscape Medical News that the primary goal “was to document what are acceptable alternatives for accelerated care.”
“Ironically, these guidelines represent best practices with evidence that — in a non–COVID-19 world — make sense for the majority of patients requiring palliative radiotherapy,” he said.
Potters said there has been hesitance to transition to shorter radiation treatments for several reasons.
“Historically, palliative radiotherapy has been delivered over 2 to 4 weeks with good results. And, as is typical in medicine, the transition to shorter course care is slowed by financial incentives to protract care,” he explained.
“In a value-based future where payment is based on outcomes, this transition to shorter care will evolve very quickly. But given the current COVID-19 crisis, and the risk to patients and staff, the incentive for shorter treatment courses has been thrust upon us and the MSKCC outline helps to define how to do this safely and with evidence-based expected efficacy.”
This article first appeared on Medscape.com.
Flu activity down from its third peak of the season, COVID-19 still a factor
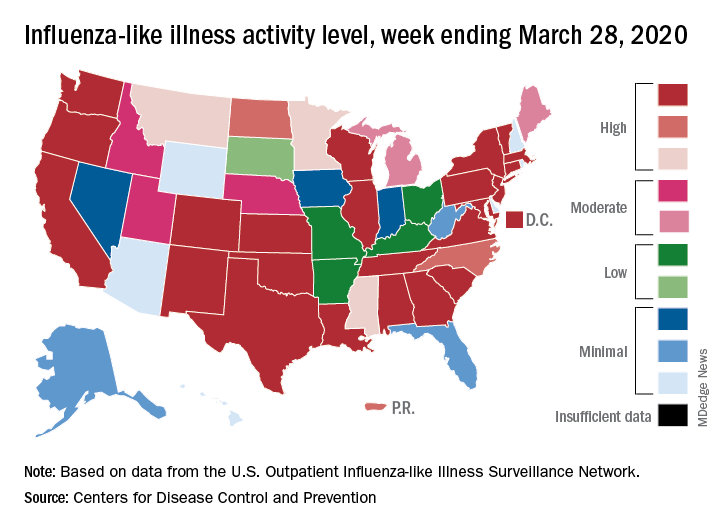
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
First report of MM patient successfully treated for COVID-19 with tocilizumab
Recent research has shown that severe cases of COVID-19 show an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor (GSF) and interleukin-6 (IL-6). Following up on that research, investigators from China reported the first case of COVID-19 in a patient with multiple myeloma (MM) who was successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab (an off-label use in the United States). The exceptional case report was published online in Blood Advances, an American Society of Hematology journal.
A 60-year-old man working in Wuhan, China, developed chest tightness without fever and cough on Feb. 1, 2020, and was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days while swab specimens were collected and tested by real-time reverse transcriptase–polymerase chain reaction. A positive result for SARS-CoV-2 infection was received 3 days later. The patient was subsequently given 200-mg umifenovir (Arbidol) tablets orally, three times daily, for antiviral treatment.
The patient had a history of symptomatic MM, which was diagnosed in 2015. The patient received two cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he received thalidomide for maintenance.
Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained, and laboratory investigations revealed a high level of serum IL-6. On hospital day 9, the patient was given a single, one-time dose of 8 mg/kg tocilizumab, administered by IV. On hospital day 12, his chest tightness disappeared. “After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: Instead, this might be attributed to the recovery of the normal T cells,” the authors wrote.
On hospital day 19, the patient’s chest CT scan showed that the range of ground-glass opacities had obviously decreased, and he was declared cured and discharged from the hospital. The patient had no symptoms of MM, and related laboratory findings were all in normal ranges, according to the researchers.
“This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab,” the researchers concluded.
The authors declared that they had no conflicts of interest.
SOURCE: Zhang X et al. Blood Adv. 2020;4(7):1307-10.
Recent research has shown that severe cases of COVID-19 show an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor (GSF) and interleukin-6 (IL-6). Following up on that research, investigators from China reported the first case of COVID-19 in a patient with multiple myeloma (MM) who was successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab (an off-label use in the United States). The exceptional case report was published online in Blood Advances, an American Society of Hematology journal.
A 60-year-old man working in Wuhan, China, developed chest tightness without fever and cough on Feb. 1, 2020, and was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days while swab specimens were collected and tested by real-time reverse transcriptase–polymerase chain reaction. A positive result for SARS-CoV-2 infection was received 3 days later. The patient was subsequently given 200-mg umifenovir (Arbidol) tablets orally, three times daily, for antiviral treatment.
The patient had a history of symptomatic MM, which was diagnosed in 2015. The patient received two cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he received thalidomide for maintenance.
Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained, and laboratory investigations revealed a high level of serum IL-6. On hospital day 9, the patient was given a single, one-time dose of 8 mg/kg tocilizumab, administered by IV. On hospital day 12, his chest tightness disappeared. “After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: Instead, this might be attributed to the recovery of the normal T cells,” the authors wrote.
On hospital day 19, the patient’s chest CT scan showed that the range of ground-glass opacities had obviously decreased, and he was declared cured and discharged from the hospital. The patient had no symptoms of MM, and related laboratory findings were all in normal ranges, according to the researchers.
“This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab,” the researchers concluded.
The authors declared that they had no conflicts of interest.
SOURCE: Zhang X et al. Blood Adv. 2020;4(7):1307-10.
Recent research has shown that severe cases of COVID-19 show an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor (GSF) and interleukin-6 (IL-6). Following up on that research, investigators from China reported the first case of COVID-19 in a patient with multiple myeloma (MM) who was successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab (an off-label use in the United States). The exceptional case report was published online in Blood Advances, an American Society of Hematology journal.
A 60-year-old man working in Wuhan, China, developed chest tightness without fever and cough on Feb. 1, 2020, and was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days while swab specimens were collected and tested by real-time reverse transcriptase–polymerase chain reaction. A positive result for SARS-CoV-2 infection was received 3 days later. The patient was subsequently given 200-mg umifenovir (Arbidol) tablets orally, three times daily, for antiviral treatment.
The patient had a history of symptomatic MM, which was diagnosed in 2015. The patient received two cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he received thalidomide for maintenance.
Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained, and laboratory investigations revealed a high level of serum IL-6. On hospital day 9, the patient was given a single, one-time dose of 8 mg/kg tocilizumab, administered by IV. On hospital day 12, his chest tightness disappeared. “After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: Instead, this might be attributed to the recovery of the normal T cells,” the authors wrote.
On hospital day 19, the patient’s chest CT scan showed that the range of ground-glass opacities had obviously decreased, and he was declared cured and discharged from the hospital. The patient had no symptoms of MM, and related laboratory findings were all in normal ranges, according to the researchers.
“This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab,” the researchers concluded.
The authors declared that they had no conflicts of interest.
SOURCE: Zhang X et al. Blood Adv. 2020;4(7):1307-10.
FROM BLOOD ADVANCES
Meet the new SHM president: Dr. Danielle Scheurer
Danielle Scheurer, MD, MSRC, SFHM, is the chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is the outgoing medical editor of The Hospitalist, and the new president of the Society of Hospital Medicine. She assumes the role from immediate past-president Christopher Frost, MD, SFHM.
As a hospitalist for 17 years, Dr. Scheurer has practiced in both academic tertiary care, as well as community hospital settings. As a chief quality officer, she has worked to improve quality and safety in all health care settings, including ambulatory care, nursing homes, home health, and surgical centers. She brings a broad experience in the medical industry to the SHM presidency.
At what point in your education/training did you decide to practice hospital medicine?
I always loved inpatient medicine throughout my entire meds-peds residency training at Duke University in Durham, N.C. I honestly never had a doubt that hospital medicine was going to be my career. What appeals to me is that each hour and each day is different, which is invigorating.
What are your favorite aspects of clinical practice and of your administrative duties?
I like doing both administrative work and clinical work because I believe having a view of both worlds helps me to be a better physician and a better administrator. It greatly helps me bring realistic solutions to the front lines since I have a good understanding of what needs to be done, but also what is likely to actually work.
As president of SHM over the next year, what are your primary goals?
My primary goal is to deeply connect with the SHM membership and understand what their needs are. There is enormous change happening in the medical industry, and SHM should be a conduit for information sharing, resources, and most importantly, answers to all our difficult problems. Hospitalists are critical to success for our hospitals and our communities during the COVID-19 pandemic. We must be able to give and receive information quickly and seamlessly to effectively help each other across the country and the world. SHM must be seen as a critical convener, especially in times of crisis.
Additionally, SHM has always fostered a “big tent” philosophy, so we will continue to explore ways to expand membership beyond “the core” of internal medicine, family medicine, and pediatrics and reach a better understanding of what our constituents need and how we can add value to their work lives and careers. In addition to expanding membership within our borders, other expansions already include working with international chapters and members with an “all teach, all learn” attitude to better understand mutually beneficial partnerships with international members. Through all these expansions, we will come closer to truly realizing our mission at SHM, which is to “promote exceptional care for hospitalized patients.”
You mention COVID-19. What resources is SHM offering to members?
We have opened up the SHM Learning Portal to help members and non-members address upcoming challenges, such as expanding ICU coverage or cross-training providers for hospital medicine. Several modules in SHM’s “Critical Care for the Hospitalist” series may be especially relevant during the COVID-19 crisis:
- Fluid Resuscitation in the Critically Ill
- Mechanical Ventilation Part I – The Basics
- Mechanical Ventilation Part II – Beyond the Basics
- Mechanical Ventilation Part III – ARDS
Finally, in this time when so many hospitalists are busy dealing with COVID-19, SHM is committed to offering valuable resources and is in the process of offering new material, including Twitter chats, webinars, blogs, and podcasts to help hospitalists share best practices. Please bookmark SHM’s compilation of COVID-19 resources at hospitalmedicine.org/coronavirus.
We also continue to forge ahead with our publications, The Hospitalist and the Journal of Hospital Medicine, by adding online content as it becomes available. Visit the COVID-19 news feed on The Hospitalist website at www.the-hospitalist.org/hospitalist/coronavirus-updates.
In this trying time, we can still connect as a community and continue to learn from each other. We encourage you to use SHM’s online community, HMX, to share resources and crowd-source solutions. Ideas for SHM resources can be submitted via email at [email protected].
What are some of the current challenges for hospital medicine?
The demands placed on hospitalists are greater than ever. With shortening length of stay, rising acuity and complexity, increasing administrative burdens, and high emphasis on care transitions, our skills (and our patience) need to rise to these increasing demands. As a member-based society, SHM (and the board of directors) seeks to ensure we are helping hospitalists be the very best they can be, regardless of hospitalist type or practice setting.
The good news is that we are still in high demand. Within the medical industry, there has been an explosive growth in the need for hospitalists, and we can now be found in almost every hospital setting in the United States. But as a current commodity, it is imperative that we continue to prove the value we are adding to our patients and their families, the systems in which we work, and the industry as a whole.
How will hospital medicine change in the next decade?
I believe one of the biggest changes we will see is the shift to ambulatory settings and the use of telehealth, and we all need to gain significant comfort with both to be effective.
Do you have any advice for students and residents interested in hospital medicine?
It is an incredibly dynamic and invigorating career; I can’t imagine doing anything else.
Danielle Scheurer, MD, MSRC, SFHM, is the chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is the outgoing medical editor of The Hospitalist, and the new president of the Society of Hospital Medicine. She assumes the role from immediate past-president Christopher Frost, MD, SFHM.
As a hospitalist for 17 years, Dr. Scheurer has practiced in both academic tertiary care, as well as community hospital settings. As a chief quality officer, she has worked to improve quality and safety in all health care settings, including ambulatory care, nursing homes, home health, and surgical centers. She brings a broad experience in the medical industry to the SHM presidency.
At what point in your education/training did you decide to practice hospital medicine?
I always loved inpatient medicine throughout my entire meds-peds residency training at Duke University in Durham, N.C. I honestly never had a doubt that hospital medicine was going to be my career. What appeals to me is that each hour and each day is different, which is invigorating.
What are your favorite aspects of clinical practice and of your administrative duties?
I like doing both administrative work and clinical work because I believe having a view of both worlds helps me to be a better physician and a better administrator. It greatly helps me bring realistic solutions to the front lines since I have a good understanding of what needs to be done, but also what is likely to actually work.
As president of SHM over the next year, what are your primary goals?
My primary goal is to deeply connect with the SHM membership and understand what their needs are. There is enormous change happening in the medical industry, and SHM should be a conduit for information sharing, resources, and most importantly, answers to all our difficult problems. Hospitalists are critical to success for our hospitals and our communities during the COVID-19 pandemic. We must be able to give and receive information quickly and seamlessly to effectively help each other across the country and the world. SHM must be seen as a critical convener, especially in times of crisis.
Additionally, SHM has always fostered a “big tent” philosophy, so we will continue to explore ways to expand membership beyond “the core” of internal medicine, family medicine, and pediatrics and reach a better understanding of what our constituents need and how we can add value to their work lives and careers. In addition to expanding membership within our borders, other expansions already include working with international chapters and members with an “all teach, all learn” attitude to better understand mutually beneficial partnerships with international members. Through all these expansions, we will come closer to truly realizing our mission at SHM, which is to “promote exceptional care for hospitalized patients.”
You mention COVID-19. What resources is SHM offering to members?
We have opened up the SHM Learning Portal to help members and non-members address upcoming challenges, such as expanding ICU coverage or cross-training providers for hospital medicine. Several modules in SHM’s “Critical Care for the Hospitalist” series may be especially relevant during the COVID-19 crisis:
- Fluid Resuscitation in the Critically Ill
- Mechanical Ventilation Part I – The Basics
- Mechanical Ventilation Part II – Beyond the Basics
- Mechanical Ventilation Part III – ARDS
Finally, in this time when so many hospitalists are busy dealing with COVID-19, SHM is committed to offering valuable resources and is in the process of offering new material, including Twitter chats, webinars, blogs, and podcasts to help hospitalists share best practices. Please bookmark SHM’s compilation of COVID-19 resources at hospitalmedicine.org/coronavirus.
We also continue to forge ahead with our publications, The Hospitalist and the Journal of Hospital Medicine, by adding online content as it becomes available. Visit the COVID-19 news feed on The Hospitalist website at www.the-hospitalist.org/hospitalist/coronavirus-updates.
In this trying time, we can still connect as a community and continue to learn from each other. We encourage you to use SHM’s online community, HMX, to share resources and crowd-source solutions. Ideas for SHM resources can be submitted via email at [email protected].
What are some of the current challenges for hospital medicine?
The demands placed on hospitalists are greater than ever. With shortening length of stay, rising acuity and complexity, increasing administrative burdens, and high emphasis on care transitions, our skills (and our patience) need to rise to these increasing demands. As a member-based society, SHM (and the board of directors) seeks to ensure we are helping hospitalists be the very best they can be, regardless of hospitalist type or practice setting.
The good news is that we are still in high demand. Within the medical industry, there has been an explosive growth in the need for hospitalists, and we can now be found in almost every hospital setting in the United States. But as a current commodity, it is imperative that we continue to prove the value we are adding to our patients and their families, the systems in which we work, and the industry as a whole.
How will hospital medicine change in the next decade?
I believe one of the biggest changes we will see is the shift to ambulatory settings and the use of telehealth, and we all need to gain significant comfort with both to be effective.
Do you have any advice for students and residents interested in hospital medicine?
It is an incredibly dynamic and invigorating career; I can’t imagine doing anything else.
Danielle Scheurer, MD, MSRC, SFHM, is the chief quality officer and professor of medicine at the Medical University of South Carolina, Charleston. She is the outgoing medical editor of The Hospitalist, and the new president of the Society of Hospital Medicine. She assumes the role from immediate past-president Christopher Frost, MD, SFHM.
As a hospitalist for 17 years, Dr. Scheurer has practiced in both academic tertiary care, as well as community hospital settings. As a chief quality officer, she has worked to improve quality and safety in all health care settings, including ambulatory care, nursing homes, home health, and surgical centers. She brings a broad experience in the medical industry to the SHM presidency.
At what point in your education/training did you decide to practice hospital medicine?
I always loved inpatient medicine throughout my entire meds-peds residency training at Duke University in Durham, N.C. I honestly never had a doubt that hospital medicine was going to be my career. What appeals to me is that each hour and each day is different, which is invigorating.
What are your favorite aspects of clinical practice and of your administrative duties?
I like doing both administrative work and clinical work because I believe having a view of both worlds helps me to be a better physician and a better administrator. It greatly helps me bring realistic solutions to the front lines since I have a good understanding of what needs to be done, but also what is likely to actually work.
As president of SHM over the next year, what are your primary goals?
My primary goal is to deeply connect with the SHM membership and understand what their needs are. There is enormous change happening in the medical industry, and SHM should be a conduit for information sharing, resources, and most importantly, answers to all our difficult problems. Hospitalists are critical to success for our hospitals and our communities during the COVID-19 pandemic. We must be able to give and receive information quickly and seamlessly to effectively help each other across the country and the world. SHM must be seen as a critical convener, especially in times of crisis.
Additionally, SHM has always fostered a “big tent” philosophy, so we will continue to explore ways to expand membership beyond “the core” of internal medicine, family medicine, and pediatrics and reach a better understanding of what our constituents need and how we can add value to their work lives and careers. In addition to expanding membership within our borders, other expansions already include working with international chapters and members with an “all teach, all learn” attitude to better understand mutually beneficial partnerships with international members. Through all these expansions, we will come closer to truly realizing our mission at SHM, which is to “promote exceptional care for hospitalized patients.”
You mention COVID-19. What resources is SHM offering to members?
We have opened up the SHM Learning Portal to help members and non-members address upcoming challenges, such as expanding ICU coverage or cross-training providers for hospital medicine. Several modules in SHM’s “Critical Care for the Hospitalist” series may be especially relevant during the COVID-19 crisis:
- Fluid Resuscitation in the Critically Ill
- Mechanical Ventilation Part I – The Basics
- Mechanical Ventilation Part II – Beyond the Basics
- Mechanical Ventilation Part III – ARDS
Finally, in this time when so many hospitalists are busy dealing with COVID-19, SHM is committed to offering valuable resources and is in the process of offering new material, including Twitter chats, webinars, blogs, and podcasts to help hospitalists share best practices. Please bookmark SHM’s compilation of COVID-19 resources at hospitalmedicine.org/coronavirus.
We also continue to forge ahead with our publications, The Hospitalist and the Journal of Hospital Medicine, by adding online content as it becomes available. Visit the COVID-19 news feed on The Hospitalist website at www.the-hospitalist.org/hospitalist/coronavirus-updates.
In this trying time, we can still connect as a community and continue to learn from each other. We encourage you to use SHM’s online community, HMX, to share resources and crowd-source solutions. Ideas for SHM resources can be submitted via email at [email protected].
What are some of the current challenges for hospital medicine?
The demands placed on hospitalists are greater than ever. With shortening length of stay, rising acuity and complexity, increasing administrative burdens, and high emphasis on care transitions, our skills (and our patience) need to rise to these increasing demands. As a member-based society, SHM (and the board of directors) seeks to ensure we are helping hospitalists be the very best they can be, regardless of hospitalist type or practice setting.
The good news is that we are still in high demand. Within the medical industry, there has been an explosive growth in the need for hospitalists, and we can now be found in almost every hospital setting in the United States. But as a current commodity, it is imperative that we continue to prove the value we are adding to our patients and their families, the systems in which we work, and the industry as a whole.
How will hospital medicine change in the next decade?
I believe one of the biggest changes we will see is the shift to ambulatory settings and the use of telehealth, and we all need to gain significant comfort with both to be effective.
Do you have any advice for students and residents interested in hospital medicine?
It is an incredibly dynamic and invigorating career; I can’t imagine doing anything else.
HM20 canceled: SHM explains why
COVID-19 made holding meeting impossible
In mid-March, the Society of Hospital Medicine board of directors concluded that it was impossible for SHM to move forward with Hospital Medicine 2020 because of the continued spread of virus that causes Coronavirus Disease 2019 (COVID-19).
Given the most recent information available from the Centers for Disease Control & Prevention and the World Health Organization about the evolving global pandemic and the number of institutions that had travel bans in place, SHM leadership concluded that canceling the Annual Conference was the only path forward.
“Canceling the conference during this unprecedented time is the right thing to do,” said Benji K. Mathews, MD, SFHM, CLHM, course director for HM20. “With the evolving circumstances out of our control, there were risks to our community as it would have gathered, communities we connect with on our travels, and our home communities and hospitals – canceling was the best way to mitigate these risks. Through it all, I couldn’t have asked for a better leadership team and the larger SHM community for their support.”
Because hospitalists are on the front lines of patient care at their institutions, they will be needed more than ever as the pandemic continues to grow in order to manage care of hospitalized patients with COVID-19 and other illnesses. As the only medical society dedicated to hospital medicine, SHM will continue to support hospitalists with resources and research specific to COVID-19 and its impact on the practice of hospital medicine.
SHM is aware that this necessary cancellation impacts many from both a financial and logistical perspective. As such, SHM will refund all conference registration fees for HM20 in full. SHM is also providing the opportunity to defer your HM20 registration to HM21, taking place May 4-7, 2021 in Las Vegas, or Pediatric Hospital Medicine 2020, taking place July 23-26, 2020 in Lake Buena Vista, Fla.
For accommodation or travel cancellations, SHM requests that individuals please refer to their respective hotel or carrier’s customer service team and related cancellation policies.
To provide the world-class education that conference attendees have come to expect from SHM over the years, the SHM team is exploring virtual options to offer select content originally anticipated at HM20. SHM also offers online education via the SHM Learning Portal and the new SHM Education app.
Visit shmannualconference.org/faqs for a full list of FAQs. For additional questions, please contact [email protected].
SHM will continue to monitor the COVID-19 pandemic and provide hospitalists with useful resources in this time of need at hospitalmedicine.org/coronavirus. For news coverage of COVID-19, visit https://www.the-hospitalist.org/hospitalist/coronavirus-updates.
COVID-19 made holding meeting impossible
COVID-19 made holding meeting impossible
In mid-March, the Society of Hospital Medicine board of directors concluded that it was impossible for SHM to move forward with Hospital Medicine 2020 because of the continued spread of virus that causes Coronavirus Disease 2019 (COVID-19).
Given the most recent information available from the Centers for Disease Control & Prevention and the World Health Organization about the evolving global pandemic and the number of institutions that had travel bans in place, SHM leadership concluded that canceling the Annual Conference was the only path forward.
“Canceling the conference during this unprecedented time is the right thing to do,” said Benji K. Mathews, MD, SFHM, CLHM, course director for HM20. “With the evolving circumstances out of our control, there were risks to our community as it would have gathered, communities we connect with on our travels, and our home communities and hospitals – canceling was the best way to mitigate these risks. Through it all, I couldn’t have asked for a better leadership team and the larger SHM community for their support.”
Because hospitalists are on the front lines of patient care at their institutions, they will be needed more than ever as the pandemic continues to grow in order to manage care of hospitalized patients with COVID-19 and other illnesses. As the only medical society dedicated to hospital medicine, SHM will continue to support hospitalists with resources and research specific to COVID-19 and its impact on the practice of hospital medicine.
SHM is aware that this necessary cancellation impacts many from both a financial and logistical perspective. As such, SHM will refund all conference registration fees for HM20 in full. SHM is also providing the opportunity to defer your HM20 registration to HM21, taking place May 4-7, 2021 in Las Vegas, or Pediatric Hospital Medicine 2020, taking place July 23-26, 2020 in Lake Buena Vista, Fla.
For accommodation or travel cancellations, SHM requests that individuals please refer to their respective hotel or carrier’s customer service team and related cancellation policies.
To provide the world-class education that conference attendees have come to expect from SHM over the years, the SHM team is exploring virtual options to offer select content originally anticipated at HM20. SHM also offers online education via the SHM Learning Portal and the new SHM Education app.
Visit shmannualconference.org/faqs for a full list of FAQs. For additional questions, please contact [email protected].
SHM will continue to monitor the COVID-19 pandemic and provide hospitalists with useful resources in this time of need at hospitalmedicine.org/coronavirus. For news coverage of COVID-19, visit https://www.the-hospitalist.org/hospitalist/coronavirus-updates.
In mid-March, the Society of Hospital Medicine board of directors concluded that it was impossible for SHM to move forward with Hospital Medicine 2020 because of the continued spread of virus that causes Coronavirus Disease 2019 (COVID-19).
Given the most recent information available from the Centers for Disease Control & Prevention and the World Health Organization about the evolving global pandemic and the number of institutions that had travel bans in place, SHM leadership concluded that canceling the Annual Conference was the only path forward.
“Canceling the conference during this unprecedented time is the right thing to do,” said Benji K. Mathews, MD, SFHM, CLHM, course director for HM20. “With the evolving circumstances out of our control, there were risks to our community as it would have gathered, communities we connect with on our travels, and our home communities and hospitals – canceling was the best way to mitigate these risks. Through it all, I couldn’t have asked for a better leadership team and the larger SHM community for their support.”
Because hospitalists are on the front lines of patient care at their institutions, they will be needed more than ever as the pandemic continues to grow in order to manage care of hospitalized patients with COVID-19 and other illnesses. As the only medical society dedicated to hospital medicine, SHM will continue to support hospitalists with resources and research specific to COVID-19 and its impact on the practice of hospital medicine.
SHM is aware that this necessary cancellation impacts many from both a financial and logistical perspective. As such, SHM will refund all conference registration fees for HM20 in full. SHM is also providing the opportunity to defer your HM20 registration to HM21, taking place May 4-7, 2021 in Las Vegas, or Pediatric Hospital Medicine 2020, taking place July 23-26, 2020 in Lake Buena Vista, Fla.
For accommodation or travel cancellations, SHM requests that individuals please refer to their respective hotel or carrier’s customer service team and related cancellation policies.
To provide the world-class education that conference attendees have come to expect from SHM over the years, the SHM team is exploring virtual options to offer select content originally anticipated at HM20. SHM also offers online education via the SHM Learning Portal and the new SHM Education app.
Visit shmannualconference.org/faqs for a full list of FAQs. For additional questions, please contact [email protected].
SHM will continue to monitor the COVID-19 pandemic and provide hospitalists with useful resources in this time of need at hospitalmedicine.org/coronavirus. For news coverage of COVID-19, visit https://www.the-hospitalist.org/hospitalist/coronavirus-updates.
FDA grants emergency authorization for first rapid antibody test for COVID-19
The U.S. Food and Drug Administration has granted Cellex an emergency use authorization to market a rapid antibody test for COVID-19, the first antibody test released amidst the pandemic.
“It is reasonable to believe that your product may be effective in diagnosing COVID-19,” and “there is no adequate, approved, and available alternative,” the agency said in a letter to Cellex.
A drop of serum, plasma, or whole blood is placed into a well on a small cartridge, and the results are read 15-20 minutes later; lines indicate the presence of IgM, IgG, or both antibodies against the SARS-CoV-2 virus.
Of 128 samples confirmed positive by reverse transcription polymerase chain reaction in premarket testing, 120 tested positive by IgG, IgM, or both. Of 250 confirmed negative, 239 were negative by the rapid test.
The numbers translated to a positive percent agreement with RT-PCR of 93.8% (95% CI: 88.06-97.26%) and a negative percent agreement of 96.4% (95% CI: 92.26-97.78%), according to labeling.
“Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection,” the labeling states.
Negative results do not rule out infection; antibodies might not have had enough time to form or the virus could have had a minor amino acid mutation in the epitope recognized by the antibodies screened for in the test. False positives can occur due to cross-reactivity with antibodies from previous infections, such as from other coronaviruses.
Labeling suggests that people who test negative should be checked again in a few days, and positive results should be confirmed by other methods. Also, the intensity of the test lines do not necessarily correlate with SARS-CoV-2 antibody titers.
As part of its authorization, the FDA waived good manufacturing practice requirements, but stipulated that advertising must state that the test has not been formally approved by the agency.
Testing is limited to Clinical Laboratory Improvement Amendments-certified labs. Positive results are required to be reported to public health authorities. The test can be ordered through Cellex distributors or directly from the company.
IgM antibodies are generally detectable several days after the initial infection, while IgG antibodies take longer. It’s not known how long COVID-19 antibodies persist after the infection has cleared, the agency said.
The U.S. Food and Drug Administration has granted Cellex an emergency use authorization to market a rapid antibody test for COVID-19, the first antibody test released amidst the pandemic.
“It is reasonable to believe that your product may be effective in diagnosing COVID-19,” and “there is no adequate, approved, and available alternative,” the agency said in a letter to Cellex.
A drop of serum, plasma, or whole blood is placed into a well on a small cartridge, and the results are read 15-20 minutes later; lines indicate the presence of IgM, IgG, or both antibodies against the SARS-CoV-2 virus.
Of 128 samples confirmed positive by reverse transcription polymerase chain reaction in premarket testing, 120 tested positive by IgG, IgM, or both. Of 250 confirmed negative, 239 were negative by the rapid test.
The numbers translated to a positive percent agreement with RT-PCR of 93.8% (95% CI: 88.06-97.26%) and a negative percent agreement of 96.4% (95% CI: 92.26-97.78%), according to labeling.
“Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection,” the labeling states.
Negative results do not rule out infection; antibodies might not have had enough time to form or the virus could have had a minor amino acid mutation in the epitope recognized by the antibodies screened for in the test. False positives can occur due to cross-reactivity with antibodies from previous infections, such as from other coronaviruses.
Labeling suggests that people who test negative should be checked again in a few days, and positive results should be confirmed by other methods. Also, the intensity of the test lines do not necessarily correlate with SARS-CoV-2 antibody titers.
As part of its authorization, the FDA waived good manufacturing practice requirements, but stipulated that advertising must state that the test has not been formally approved by the agency.
Testing is limited to Clinical Laboratory Improvement Amendments-certified labs. Positive results are required to be reported to public health authorities. The test can be ordered through Cellex distributors or directly from the company.
IgM antibodies are generally detectable several days after the initial infection, while IgG antibodies take longer. It’s not known how long COVID-19 antibodies persist after the infection has cleared, the agency said.
The U.S. Food and Drug Administration has granted Cellex an emergency use authorization to market a rapid antibody test for COVID-19, the first antibody test released amidst the pandemic.
“It is reasonable to believe that your product may be effective in diagnosing COVID-19,” and “there is no adequate, approved, and available alternative,” the agency said in a letter to Cellex.
A drop of serum, plasma, or whole blood is placed into a well on a small cartridge, and the results are read 15-20 minutes later; lines indicate the presence of IgM, IgG, or both antibodies against the SARS-CoV-2 virus.
Of 128 samples confirmed positive by reverse transcription polymerase chain reaction in premarket testing, 120 tested positive by IgG, IgM, or both. Of 250 confirmed negative, 239 were negative by the rapid test.
The numbers translated to a positive percent agreement with RT-PCR of 93.8% (95% CI: 88.06-97.26%) and a negative percent agreement of 96.4% (95% CI: 92.26-97.78%), according to labeling.
“Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection,” the labeling states.
Negative results do not rule out infection; antibodies might not have had enough time to form or the virus could have had a minor amino acid mutation in the epitope recognized by the antibodies screened for in the test. False positives can occur due to cross-reactivity with antibodies from previous infections, such as from other coronaviruses.
Labeling suggests that people who test negative should be checked again in a few days, and positive results should be confirmed by other methods. Also, the intensity of the test lines do not necessarily correlate with SARS-CoV-2 antibody titers.
As part of its authorization, the FDA waived good manufacturing practice requirements, but stipulated that advertising must state that the test has not been formally approved by the agency.
Testing is limited to Clinical Laboratory Improvement Amendments-certified labs. Positive results are required to be reported to public health authorities. The test can be ordered through Cellex distributors or directly from the company.
IgM antibodies are generally detectable several days after the initial infection, while IgG antibodies take longer. It’s not known how long COVID-19 antibodies persist after the infection has cleared, the agency said.
Survey: COVID-19 is getting in our heads
As the COVID-19 pandemic sweeps across the United States, it is increasingly affecting those who are not infected. Social bonds are being broken, businesses are closing, jobs are being lost, and the stress is mounting.

In a poll conducted March 25-30, 45% of Americans said that stress resulting from the pandemic is having a negative impact on their mental health, compared with 32% expressing that view just 2 weeks earlier, the Kaiser Family Foundation reported April 2.
In the later survey, the effect looked like this: 19% of all respondents said that the pandemic has had a major negative impact and 26% said it has been minor so far. Women were more likely than men (24% vs. 15%) to report a major impact, as were blacks and Hispanic adults (both at 24%) compared with whites (17%), the KFF investigators said.
More Hispanic (44%) and black (42%) respondents also said that they had already lost their job, lost income, or had their hours reduced without pay as a result of the pandemic, compared with whites (36%). Among all respondents, 26% had lost income from a job or business and 28% had lost their job, been laid off, or had their hours reduced without pay, according to KFF.
A majority of respondents (57%) reported “being worried they will put themselves at risk of exposure to coronavirus because they can’t afford to stay home and miss work,” the researchers said. That figure is up from 35% in the earlier survey.
Anxiety about work-related exposure was even higher among hourly workers or those who get paid by the job (61%) and among employed adults who earn less than $40,000 annually (72%), they reported.
Overall, 72% of respondents said that their lives have been disrupted “a lot” or “some” by the coronavirus outbreak, and that is a jump of 32 percentage points over the previous poll, the investigators noted.
The disruption is expected to continue, it seems, as 74% believe that the worst is yet to come “in spite of the health, social and economic upheaval that Americans are already experiencing,” they wrote.
As the COVID-19 pandemic sweeps across the United States, it is increasingly affecting those who are not infected. Social bonds are being broken, businesses are closing, jobs are being lost, and the stress is mounting.

In a poll conducted March 25-30, 45% of Americans said that stress resulting from the pandemic is having a negative impact on their mental health, compared with 32% expressing that view just 2 weeks earlier, the Kaiser Family Foundation reported April 2.
In the later survey, the effect looked like this: 19% of all respondents said that the pandemic has had a major negative impact and 26% said it has been minor so far. Women were more likely than men (24% vs. 15%) to report a major impact, as were blacks and Hispanic adults (both at 24%) compared with whites (17%), the KFF investigators said.
More Hispanic (44%) and black (42%) respondents also said that they had already lost their job, lost income, or had their hours reduced without pay as a result of the pandemic, compared with whites (36%). Among all respondents, 26% had lost income from a job or business and 28% had lost their job, been laid off, or had their hours reduced without pay, according to KFF.
A majority of respondents (57%) reported “being worried they will put themselves at risk of exposure to coronavirus because they can’t afford to stay home and miss work,” the researchers said. That figure is up from 35% in the earlier survey.
Anxiety about work-related exposure was even higher among hourly workers or those who get paid by the job (61%) and among employed adults who earn less than $40,000 annually (72%), they reported.
Overall, 72% of respondents said that their lives have been disrupted “a lot” or “some” by the coronavirus outbreak, and that is a jump of 32 percentage points over the previous poll, the investigators noted.
The disruption is expected to continue, it seems, as 74% believe that the worst is yet to come “in spite of the health, social and economic upheaval that Americans are already experiencing,” they wrote.
As the COVID-19 pandemic sweeps across the United States, it is increasingly affecting those who are not infected. Social bonds are being broken, businesses are closing, jobs are being lost, and the stress is mounting.

In a poll conducted March 25-30, 45% of Americans said that stress resulting from the pandemic is having a negative impact on their mental health, compared with 32% expressing that view just 2 weeks earlier, the Kaiser Family Foundation reported April 2.
In the later survey, the effect looked like this: 19% of all respondents said that the pandemic has had a major negative impact and 26% said it has been minor so far. Women were more likely than men (24% vs. 15%) to report a major impact, as were blacks and Hispanic adults (both at 24%) compared with whites (17%), the KFF investigators said.
More Hispanic (44%) and black (42%) respondents also said that they had already lost their job, lost income, or had their hours reduced without pay as a result of the pandemic, compared with whites (36%). Among all respondents, 26% had lost income from a job or business and 28% had lost their job, been laid off, or had their hours reduced without pay, according to KFF.
A majority of respondents (57%) reported “being worried they will put themselves at risk of exposure to coronavirus because they can’t afford to stay home and miss work,” the researchers said. That figure is up from 35% in the earlier survey.
Anxiety about work-related exposure was even higher among hourly workers or those who get paid by the job (61%) and among employed adults who earn less than $40,000 annually (72%), they reported.
Overall, 72% of respondents said that their lives have been disrupted “a lot” or “some” by the coronavirus outbreak, and that is a jump of 32 percentage points over the previous poll, the investigators noted.
The disruption is expected to continue, it seems, as 74% believe that the worst is yet to come “in spite of the health, social and economic upheaval that Americans are already experiencing,” they wrote.
First presumptive case of encephalitis linked to COVID-19 reported
“As the number of patients with COVID-19 increases worldwide, clinicians and radiologists should be watching for this presentation among patients presenting with COVID-19 and altered mental status,” the clinicians advise in a report published online March 31 in Radiology.
“This is significant for all providers to be aware of and looking out for in [COVID-19] patients who present with an altered level of consciousness. This complication is as devastating as severe lung disease,” Elissa Fory, MD, a neurologist with Henry Ford who was part of the team of medical experts that made the diagnosis, said in a statement.
“We need to be thinking of how we’re going to incorporate patients with severe neurological disease into our treatment paradigm,” Fory added.
Brent Griffith, MD, radiologist with Henry Ford and senior author of the case report, said the case shows “the important role that imaging can play in COVID-19 cases.”
Diagnosed via neuroimaging
The 58-year-old woman presented with a 3-day history of fever, cough, and muscle aches ― symptoms consistent with COVID-19. She was transported by ambulance to the emergency department and showed signs of confusion, lethargy, and disorientation.
The woman tested negative for influenza, but a rapid COVID-19 test confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. She was later diagnosed with acute hemorrhagic necrotizing encephalopathy.
“The team had suspected encephalitis at the outset, but then back-to-back CT and MRI scans made the diagnosis,” Fory said in the statement.
Noncontrast head CT revealed “symmetric hypoattenuation within the bilateral medial thalami with a normal CT angiogram and CT venogram,” the team reports in their article. Brain MRI showed “hemorrhagic rim enhancing lesions within the bilateral thalami, medial temporal lobes, and subinsular regions.”
The patient was started on intravenous immunoglobulin but not high-dose steroids, because of concern for respiratory compromise. As of April 1, the patient was hospitalized in serious condition. Henry Ford Hospital has not provided an update.
Acute necrotizing encephalopathy (ANE) is a rare complication of viral infections, but until now, it has not been known to have occurred as a result of COVID-19 infection. ANE has been associated with intracranial “cytokine storms,” and a recent report in the Lancet suggested that a subgroup of patients with severe COVID-19 might develop a cytokine storm syndrome.
Commenting for Medscape Medical News, Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University in St. Louis, Missouri, said, “Since this is just one report of one patient, the findings are the most preliminary we can conceive, and more research is needed to determine the extent to which COVID-19 may affect the central nervous system.”
Fory, Griffith, and Raji have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
“As the number of patients with COVID-19 increases worldwide, clinicians and radiologists should be watching for this presentation among patients presenting with COVID-19 and altered mental status,” the clinicians advise in a report published online March 31 in Radiology.
“This is significant for all providers to be aware of and looking out for in [COVID-19] patients who present with an altered level of consciousness. This complication is as devastating as severe lung disease,” Elissa Fory, MD, a neurologist with Henry Ford who was part of the team of medical experts that made the diagnosis, said in a statement.
“We need to be thinking of how we’re going to incorporate patients with severe neurological disease into our treatment paradigm,” Fory added.
Brent Griffith, MD, radiologist with Henry Ford and senior author of the case report, said the case shows “the important role that imaging can play in COVID-19 cases.”
Diagnosed via neuroimaging
The 58-year-old woman presented with a 3-day history of fever, cough, and muscle aches ― symptoms consistent with COVID-19. She was transported by ambulance to the emergency department and showed signs of confusion, lethargy, and disorientation.
The woman tested negative for influenza, but a rapid COVID-19 test confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. She was later diagnosed with acute hemorrhagic necrotizing encephalopathy.
“The team had suspected encephalitis at the outset, but then back-to-back CT and MRI scans made the diagnosis,” Fory said in the statement.
Noncontrast head CT revealed “symmetric hypoattenuation within the bilateral medial thalami with a normal CT angiogram and CT venogram,” the team reports in their article. Brain MRI showed “hemorrhagic rim enhancing lesions within the bilateral thalami, medial temporal lobes, and subinsular regions.”
The patient was started on intravenous immunoglobulin but not high-dose steroids, because of concern for respiratory compromise. As of April 1, the patient was hospitalized in serious condition. Henry Ford Hospital has not provided an update.
Acute necrotizing encephalopathy (ANE) is a rare complication of viral infections, but until now, it has not been known to have occurred as a result of COVID-19 infection. ANE has been associated with intracranial “cytokine storms,” and a recent report in the Lancet suggested that a subgroup of patients with severe COVID-19 might develop a cytokine storm syndrome.
Commenting for Medscape Medical News, Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University in St. Louis, Missouri, said, “Since this is just one report of one patient, the findings are the most preliminary we can conceive, and more research is needed to determine the extent to which COVID-19 may affect the central nervous system.”
Fory, Griffith, and Raji have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
“As the number of patients with COVID-19 increases worldwide, clinicians and radiologists should be watching for this presentation among patients presenting with COVID-19 and altered mental status,” the clinicians advise in a report published online March 31 in Radiology.
“This is significant for all providers to be aware of and looking out for in [COVID-19] patients who present with an altered level of consciousness. This complication is as devastating as severe lung disease,” Elissa Fory, MD, a neurologist with Henry Ford who was part of the team of medical experts that made the diagnosis, said in a statement.
“We need to be thinking of how we’re going to incorporate patients with severe neurological disease into our treatment paradigm,” Fory added.
Brent Griffith, MD, radiologist with Henry Ford and senior author of the case report, said the case shows “the important role that imaging can play in COVID-19 cases.”
Diagnosed via neuroimaging
The 58-year-old woman presented with a 3-day history of fever, cough, and muscle aches ― symptoms consistent with COVID-19. She was transported by ambulance to the emergency department and showed signs of confusion, lethargy, and disorientation.
The woman tested negative for influenza, but a rapid COVID-19 test confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. She was later diagnosed with acute hemorrhagic necrotizing encephalopathy.
“The team had suspected encephalitis at the outset, but then back-to-back CT and MRI scans made the diagnosis,” Fory said in the statement.
Noncontrast head CT revealed “symmetric hypoattenuation within the bilateral medial thalami with a normal CT angiogram and CT venogram,” the team reports in their article. Brain MRI showed “hemorrhagic rim enhancing lesions within the bilateral thalami, medial temporal lobes, and subinsular regions.”
The patient was started on intravenous immunoglobulin but not high-dose steroids, because of concern for respiratory compromise. As of April 1, the patient was hospitalized in serious condition. Henry Ford Hospital has not provided an update.
Acute necrotizing encephalopathy (ANE) is a rare complication of viral infections, but until now, it has not been known to have occurred as a result of COVID-19 infection. ANE has been associated with intracranial “cytokine storms,” and a recent report in the Lancet suggested that a subgroup of patients with severe COVID-19 might develop a cytokine storm syndrome.
Commenting for Medscape Medical News, Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University in St. Louis, Missouri, said, “Since this is just one report of one patient, the findings are the most preliminary we can conceive, and more research is needed to determine the extent to which COVID-19 may affect the central nervous system.”
Fory, Griffith, and Raji have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Survey shows just how dire PPE shortages are at many hospitals
As the COVID-19 pandemic spreads over the country, nearly half (48%) of US healthcare facilities — of various types and sizes — are already or almost out of respirators for treating patients, according to the results of a national online survey of infection prevention professionals.
Conducted during March 23-25 by the Association for Professionals in Infection Control and Epidemiology (APIC), the survey asked APIC’s 11,922 US-based infection preventionist members to rank their facilities’ supply of personal protective equipment (PPE) and key items, such as hand sanitizer and cleaning products, on a 5-point scale from having “plenty” to “none.”
Overall, 1,140 (9.6%) infection preventionists responded. Almost 70% of respondents represented a healthcare system rather than a single facility, and facilities ranged from hospitals (42.7%) to ambulatory care (17.4%) and dialysis (2.7%). The centers, from all 50 states and Washington, D.C., ranged in size from those with 1 to 50 beds to those with more than 300 beds.
and 317 (27.8%) said they were almost out of the devices, which are needed to protect healthcare workers managing patients with COVID-19 and different infectious diseases.
The survey was posted Friday on the APIC website.
Other findings from the survey include:
- Nearly half of respondents (49.2%) said their centers lack sufficient enough face shields, with 36.5% reporting being almost out and 12.6% reporting being completely out.
- Approximately one third (31.7%) of respondents reported being completely or nearly out of face masks.
- Even simple hand sanitizer is in short supply at more than 1 in 4 facilities surveyed; 25.6% of respondents said they are almost out and 2.6% are completely out.
- Nearly 30% of respondents reported accessing supplemental PPE through state or local resources, while 24.6% said they accepted private donations of supplies.
- Fewer than one-third (31.5%) said they had sufficient gowns.
- About 28% said they were almost out of protective respirators, while 20.5% said they have none.
- Only 12.3% said they have received supplies from federal resources, including the Strategic National Stockpile, which is controlled by the Department of Health and Human Services.
- 17.2% of respondents reported resorting to DIY measures such as sewing their own masks.
In terms of staffing resources, 67% of respondents said their center has only one (or fewer) full-time–equivalent infection preventionist on staff to develop protocols for managing COVID-19. That is not surprising given the general underresourcing of infection control programs, the survey compilers said.
“Hospitals and health facilities with fewer than one full-time person on staff to direct infection prevention activities may have been disadvantaged even before the COVID-19 pandemic,” said APIC president Connie Steed, MSN, RN, in a related news release.
On a more positive note, about two thirds of facilities said they have sufficient supplies of gloves (63.4%) and hand washing soap (67.1%).
“I am concerned that many facilities will not be able to protect healthcare workers and patients from not only COVID-19, but also MRSA, C diff., and other antibiotic-resistant infections,” Steed said.
At some centers, however, the situation is not so grim — yet. The large Harris Health System in Houston has enough PPE on hand to support all infection prevention protocols in place, according to Bryan McLeod, director of corporate communications. “The PPE inventory varies from a few weeks to well over a month depending on the specific item,” McLeod told Medscape Medical News. “But everything is dependent on the utilization rate, which can vary with patient volume. Our concern is long-term resupply while demand is peaking around the world, and we continue to pursue all avenues to secure resupply.”
Above all, Steed emphasizes healthcare workers’ need for clarity. “They need to know when exactly they can expect desperately needed supplies to arrive so they don’t have to turn to unproven crisis methods for PPE,” she said. “There have been grim reports from health officials about the supply shortage for weeks and we’re not getting any answers. This is unacceptable.”
APIC is urging the federal government for immediate activation of the Cold War–era Defense Production Act and any other available means to quickly manufacture vital supplies to protect healthcare workers treating the escalating numbers of COVID-19 patients.
In the meantime, frontline healthcare workers are scouring the Internet for suppliers and begging online for donations of masks.
APIC notes that the COVID-19 pandemic is compounded by this year’s particularly severe influenza season, which had already led overcrowded healthcare facilities.
This article first appeared on Medscape.com.
As the COVID-19 pandemic spreads over the country, nearly half (48%) of US healthcare facilities — of various types and sizes — are already or almost out of respirators for treating patients, according to the results of a national online survey of infection prevention professionals.
Conducted during March 23-25 by the Association for Professionals in Infection Control and Epidemiology (APIC), the survey asked APIC’s 11,922 US-based infection preventionist members to rank their facilities’ supply of personal protective equipment (PPE) and key items, such as hand sanitizer and cleaning products, on a 5-point scale from having “plenty” to “none.”
Overall, 1,140 (9.6%) infection preventionists responded. Almost 70% of respondents represented a healthcare system rather than a single facility, and facilities ranged from hospitals (42.7%) to ambulatory care (17.4%) and dialysis (2.7%). The centers, from all 50 states and Washington, D.C., ranged in size from those with 1 to 50 beds to those with more than 300 beds.
and 317 (27.8%) said they were almost out of the devices, which are needed to protect healthcare workers managing patients with COVID-19 and different infectious diseases.
The survey was posted Friday on the APIC website.
Other findings from the survey include:
- Nearly half of respondents (49.2%) said their centers lack sufficient enough face shields, with 36.5% reporting being almost out and 12.6% reporting being completely out.
- Approximately one third (31.7%) of respondents reported being completely or nearly out of face masks.
- Even simple hand sanitizer is in short supply at more than 1 in 4 facilities surveyed; 25.6% of respondents said they are almost out and 2.6% are completely out.
- Nearly 30% of respondents reported accessing supplemental PPE through state or local resources, while 24.6% said they accepted private donations of supplies.
- Fewer than one-third (31.5%) said they had sufficient gowns.
- About 28% said they were almost out of protective respirators, while 20.5% said they have none.
- Only 12.3% said they have received supplies from federal resources, including the Strategic National Stockpile, which is controlled by the Department of Health and Human Services.
- 17.2% of respondents reported resorting to DIY measures such as sewing their own masks.
In terms of staffing resources, 67% of respondents said their center has only one (or fewer) full-time–equivalent infection preventionist on staff to develop protocols for managing COVID-19. That is not surprising given the general underresourcing of infection control programs, the survey compilers said.
“Hospitals and health facilities with fewer than one full-time person on staff to direct infection prevention activities may have been disadvantaged even before the COVID-19 pandemic,” said APIC president Connie Steed, MSN, RN, in a related news release.
On a more positive note, about two thirds of facilities said they have sufficient supplies of gloves (63.4%) and hand washing soap (67.1%).
“I am concerned that many facilities will not be able to protect healthcare workers and patients from not only COVID-19, but also MRSA, C diff., and other antibiotic-resistant infections,” Steed said.
At some centers, however, the situation is not so grim — yet. The large Harris Health System in Houston has enough PPE on hand to support all infection prevention protocols in place, according to Bryan McLeod, director of corporate communications. “The PPE inventory varies from a few weeks to well over a month depending on the specific item,” McLeod told Medscape Medical News. “But everything is dependent on the utilization rate, which can vary with patient volume. Our concern is long-term resupply while demand is peaking around the world, and we continue to pursue all avenues to secure resupply.”
Above all, Steed emphasizes healthcare workers’ need for clarity. “They need to know when exactly they can expect desperately needed supplies to arrive so they don’t have to turn to unproven crisis methods for PPE,” she said. “There have been grim reports from health officials about the supply shortage for weeks and we’re not getting any answers. This is unacceptable.”
APIC is urging the federal government for immediate activation of the Cold War–era Defense Production Act and any other available means to quickly manufacture vital supplies to protect healthcare workers treating the escalating numbers of COVID-19 patients.
In the meantime, frontline healthcare workers are scouring the Internet for suppliers and begging online for donations of masks.
APIC notes that the COVID-19 pandemic is compounded by this year’s particularly severe influenza season, which had already led overcrowded healthcare facilities.
This article first appeared on Medscape.com.
As the COVID-19 pandemic spreads over the country, nearly half (48%) of US healthcare facilities — of various types and sizes — are already or almost out of respirators for treating patients, according to the results of a national online survey of infection prevention professionals.
Conducted during March 23-25 by the Association for Professionals in Infection Control and Epidemiology (APIC), the survey asked APIC’s 11,922 US-based infection preventionist members to rank their facilities’ supply of personal protective equipment (PPE) and key items, such as hand sanitizer and cleaning products, on a 5-point scale from having “plenty” to “none.”
Overall, 1,140 (9.6%) infection preventionists responded. Almost 70% of respondents represented a healthcare system rather than a single facility, and facilities ranged from hospitals (42.7%) to ambulatory care (17.4%) and dialysis (2.7%). The centers, from all 50 states and Washington, D.C., ranged in size from those with 1 to 50 beds to those with more than 300 beds.
and 317 (27.8%) said they were almost out of the devices, which are needed to protect healthcare workers managing patients with COVID-19 and different infectious diseases.
The survey was posted Friday on the APIC website.
Other findings from the survey include:
- Nearly half of respondents (49.2%) said their centers lack sufficient enough face shields, with 36.5% reporting being almost out and 12.6% reporting being completely out.
- Approximately one third (31.7%) of respondents reported being completely or nearly out of face masks.
- Even simple hand sanitizer is in short supply at more than 1 in 4 facilities surveyed; 25.6% of respondents said they are almost out and 2.6% are completely out.
- Nearly 30% of respondents reported accessing supplemental PPE through state or local resources, while 24.6% said they accepted private donations of supplies.
- Fewer than one-third (31.5%) said they had sufficient gowns.
- About 28% said they were almost out of protective respirators, while 20.5% said they have none.
- Only 12.3% said they have received supplies from federal resources, including the Strategic National Stockpile, which is controlled by the Department of Health and Human Services.
- 17.2% of respondents reported resorting to DIY measures such as sewing their own masks.
In terms of staffing resources, 67% of respondents said their center has only one (or fewer) full-time–equivalent infection preventionist on staff to develop protocols for managing COVID-19. That is not surprising given the general underresourcing of infection control programs, the survey compilers said.
“Hospitals and health facilities with fewer than one full-time person on staff to direct infection prevention activities may have been disadvantaged even before the COVID-19 pandemic,” said APIC president Connie Steed, MSN, RN, in a related news release.
On a more positive note, about two thirds of facilities said they have sufficient supplies of gloves (63.4%) and hand washing soap (67.1%).
“I am concerned that many facilities will not be able to protect healthcare workers and patients from not only COVID-19, but also MRSA, C diff., and other antibiotic-resistant infections,” Steed said.
At some centers, however, the situation is not so grim — yet. The large Harris Health System in Houston has enough PPE on hand to support all infection prevention protocols in place, according to Bryan McLeod, director of corporate communications. “The PPE inventory varies from a few weeks to well over a month depending on the specific item,” McLeod told Medscape Medical News. “But everything is dependent on the utilization rate, which can vary with patient volume. Our concern is long-term resupply while demand is peaking around the world, and we continue to pursue all avenues to secure resupply.”
Above all, Steed emphasizes healthcare workers’ need for clarity. “They need to know when exactly they can expect desperately needed supplies to arrive so they don’t have to turn to unproven crisis methods for PPE,” she said. “There have been grim reports from health officials about the supply shortage for weeks and we’re not getting any answers. This is unacceptable.”
APIC is urging the federal government for immediate activation of the Cold War–era Defense Production Act and any other available means to quickly manufacture vital supplies to protect healthcare workers treating the escalating numbers of COVID-19 patients.
In the meantime, frontline healthcare workers are scouring the Internet for suppliers and begging online for donations of masks.
APIC notes that the COVID-19 pandemic is compounded by this year’s particularly severe influenza season, which had already led overcrowded healthcare facilities.
This article first appeared on Medscape.com.
iPLEDGE allows at-home pregnancy tests during pandemic
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.