User login
Neurology Reviews covers innovative and emerging news in neurology and neuroscience every month, with a focus on practical approaches to treating Parkinson's disease, epilepsy, headache, stroke, multiple sclerosis, Alzheimer's disease, and other neurologic disorders.
PML
Progressive multifocal leukoencephalopathy
Rituxan
The leading independent newspaper covering neurology news and commentary.
High-Volume Burn Resuscitation Increases Neurologic Risk
TOPLINE:
METHODOLOGY:
- Researchers conducted a single-center review of 5176 patients with burn injuries who were admitted to a verified American Burn Association center (2003-2017); 622 of them underwent head CT within 96 hours of admission, and 83 showed intracranial abnormalities.
- Of 42 patients (mean age, 49.7 years; 80.5% men) who were admitted within 24 hours of burn, 30 patients received < 200 mL/kg and 11 received > 200 mL/kg of total resuscitation fluids, with a median total body surface area (TBSA) of 20.0.
- The primary outcome assessed was the worsening of neurologic findings on imaging related to the volume of the resuscitation fluid administered; the secondary outcomes were the incidence of new or worsening intracranial abnormalities, including hemorrhage, edema, ischemia, or infarction.
TAKEAWAY:
- Neurologic findings worsened in 47.6% patients receiving < 200 mL/kg of fluid resuscitation and 85.7% of those receiving > 200 mL/kg (P =.064).
- Repeat imaging was performed in 21 (70.0%) patients receiving < 200 mL/kg and 7 (63.6%) patients receiving > 200 mL/kg of resuscitation who underwent follow-up imaging.
- The median TBSA was 16.5 in the < 200 mL/kg group and 53.2 in the > 200 mL/kg group (P <.001).
- Intracranial abnormalities were found in 31.3% patients with hemorrhage, 18.8% with worsening edema, and 43.8% with ischemia or infarction.
IN PRACTICE:
“Patients who received over 200 mL/kg of resuscitation had an increased progression of intracranial abnormalities when compared with patients receiving less volume resuscitation,” the authors wrote. “Neurologic changes prompting imaging in burn patients may be undetectable, and our study further highlights the need for routine evaluation with neurologic imaging when undergoing large-volume resuscitations.”
SOURCE:
The study was led by Connor L. Kenney, MD, Brooke Army Medical Center, San Antonio, and was published online on November 07, 2024, in the Journal of Surgical Research.
LIMITATIONS:
Study limitations included a small patient sample and unclear guidelines for obtaining head CT scans, making it difficult to distinguish between trauma-related brain changes and disease progression. Additionally, the study lacked data on hypotensive episodes and long-term neurologic outcomes.
DISCLOSURES:
This study did not receive any specific funding. The authors declared no conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a single-center review of 5176 patients with burn injuries who were admitted to a verified American Burn Association center (2003-2017); 622 of them underwent head CT within 96 hours of admission, and 83 showed intracranial abnormalities.
- Of 42 patients (mean age, 49.7 years; 80.5% men) who were admitted within 24 hours of burn, 30 patients received < 200 mL/kg and 11 received > 200 mL/kg of total resuscitation fluids, with a median total body surface area (TBSA) of 20.0.
- The primary outcome assessed was the worsening of neurologic findings on imaging related to the volume of the resuscitation fluid administered; the secondary outcomes were the incidence of new or worsening intracranial abnormalities, including hemorrhage, edema, ischemia, or infarction.
TAKEAWAY:
- Neurologic findings worsened in 47.6% patients receiving < 200 mL/kg of fluid resuscitation and 85.7% of those receiving > 200 mL/kg (P =.064).
- Repeat imaging was performed in 21 (70.0%) patients receiving < 200 mL/kg and 7 (63.6%) patients receiving > 200 mL/kg of resuscitation who underwent follow-up imaging.
- The median TBSA was 16.5 in the < 200 mL/kg group and 53.2 in the > 200 mL/kg group (P <.001).
- Intracranial abnormalities were found in 31.3% patients with hemorrhage, 18.8% with worsening edema, and 43.8% with ischemia or infarction.
IN PRACTICE:
“Patients who received over 200 mL/kg of resuscitation had an increased progression of intracranial abnormalities when compared with patients receiving less volume resuscitation,” the authors wrote. “Neurologic changes prompting imaging in burn patients may be undetectable, and our study further highlights the need for routine evaluation with neurologic imaging when undergoing large-volume resuscitations.”
SOURCE:
The study was led by Connor L. Kenney, MD, Brooke Army Medical Center, San Antonio, and was published online on November 07, 2024, in the Journal of Surgical Research.
LIMITATIONS:
Study limitations included a small patient sample and unclear guidelines for obtaining head CT scans, making it difficult to distinguish between trauma-related brain changes and disease progression. Additionally, the study lacked data on hypotensive episodes and long-term neurologic outcomes.
DISCLOSURES:
This study did not receive any specific funding. The authors declared no conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a single-center review of 5176 patients with burn injuries who were admitted to a verified American Burn Association center (2003-2017); 622 of them underwent head CT within 96 hours of admission, and 83 showed intracranial abnormalities.
- Of 42 patients (mean age, 49.7 years; 80.5% men) who were admitted within 24 hours of burn, 30 patients received < 200 mL/kg and 11 received > 200 mL/kg of total resuscitation fluids, with a median total body surface area (TBSA) of 20.0.
- The primary outcome assessed was the worsening of neurologic findings on imaging related to the volume of the resuscitation fluid administered; the secondary outcomes were the incidence of new or worsening intracranial abnormalities, including hemorrhage, edema, ischemia, or infarction.
TAKEAWAY:
- Neurologic findings worsened in 47.6% patients receiving < 200 mL/kg of fluid resuscitation and 85.7% of those receiving > 200 mL/kg (P =.064).
- Repeat imaging was performed in 21 (70.0%) patients receiving < 200 mL/kg and 7 (63.6%) patients receiving > 200 mL/kg of resuscitation who underwent follow-up imaging.
- The median TBSA was 16.5 in the < 200 mL/kg group and 53.2 in the > 200 mL/kg group (P <.001).
- Intracranial abnormalities were found in 31.3% patients with hemorrhage, 18.8% with worsening edema, and 43.8% with ischemia or infarction.
IN PRACTICE:
“Patients who received over 200 mL/kg of resuscitation had an increased progression of intracranial abnormalities when compared with patients receiving less volume resuscitation,” the authors wrote. “Neurologic changes prompting imaging in burn patients may be undetectable, and our study further highlights the need for routine evaluation with neurologic imaging when undergoing large-volume resuscitations.”
SOURCE:
The study was led by Connor L. Kenney, MD, Brooke Army Medical Center, San Antonio, and was published online on November 07, 2024, in the Journal of Surgical Research.
LIMITATIONS:
Study limitations included a small patient sample and unclear guidelines for obtaining head CT scans, making it difficult to distinguish between trauma-related brain changes and disease progression. Additionally, the study lacked data on hypotensive episodes and long-term neurologic outcomes.
DISCLOSURES:
This study did not receive any specific funding. The authors declared no conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Smart Mattress to Reduce SUDEP?
LOS ANGELES — says one of the experts involved in its development.
When used along with a seizure detection device, Jong Woo Lee, MD, PhD, associate professor of neurology, Harvard Medical School, and Brigham and Women’s Hospital, both in Boston, Massachusetts, estimates the smart mattress could cut SUDEP by more than 50%.
In addition, early results from an observational study are backing this up, he said.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
Most SUDEP Cases Found Face Down
SUDEP is the leading cause of death in children with epilepsy and in otherwise healthy adult patients with epilepsy. When his fifth patient died of SUDEP, Lee decided it was time to try to tackle the high mortality rate associated with these unexpected deaths. “I desperately wanted to help, ” he said.
About 70% of SUDEP occurs during sleep, and victims are found face down, or in the prone position, 90% of the time, said Lee.
“Of course, the best way to prevent SUDEP is not to have a seizure, but once you have a seizure and once you’re face down, your risk for death goes up by somewhere between 30 and 100 times,” he explained.
Lee was convinced SUDEP could be prevented by simple interventions that stimulate the patient and turn them over. He noted the incidence of sudden infant death syndrome, “which has similar characteristics” to SUDEP, has been reduced by up to 75% through campaigns that simply advise placing babies on their backs.
“Most of SUDEP happens because your arousal system is knocked out and you just don’t take the breath that you’re supposed to. Just the act of turning people over and vibrating the bed will stimulate them,” he said.
However, it’s crucial that this be done quickly, said Lee. “When you look at patients who died on video and see the EEGs, everybody took their last breath within 3 minutes.”
Because the window of opportunity is so short, “we think that seizure detection devices alone are not going to really be effective because you just can’t get there or react within those 3 minutes.”
There are currently no products that detect the prone position or have the ability to reposition a patient quickly into the recovery sideways position.
Lee and his colleagues developed a smart system that can be embedded in a mattress that detects when someone is having a seizure, determines if that person is face down, and if so, safely stimulates and repositions them.
The mattress is made up of a series of programmable inflatable blocks or “cells” that have pressure, vibration, temperature, and humidity sensors embedded within. “Based on the pressure readings, we can figure out whether the patient is right side up, on their right side, on their left side, or face down,” said Lee.
If the person is face down, he or she can be repositioned within a matter of seconds. “Each of the cells can lift 1000 pounds,” he said. The mattress is “very comfortable,” said Lee, who has tried it out himself.
Eighteen normative control participants have been enrolled for development and training purposes. To date, 10 of these individuals, aged 18-53 years, weighing 100-182 lb, and with a height of 5 ft 2 in to 6 ft 1 in, underwent extensive formal testing on the prototype bed.
Researchers found the mattress responded quickly to different body positions and weights. “We were able to reposition everybody in around 20 seconds,” said Lee.
The overall accuracy of detecting the prone position was 96.8%. There were no cases of a supine or prone position being mistaken for each other.
Researchers are refining the algorithm to improve the accuracy for detecting the prone position and expect to have a completely functional prototype within a few years.
Big Step Forward
Commenting on the research, Daniel M. Goldenholz, MD, PhD, assistant professor, Division of Epilepsy, Harvard Beth Israel Deaconess Medical Center, Boston, said the study “is a big step forward in the race to provide an actionable tool to prevent SUDEP.”
The technology “appears to mostly be doing what it’s intended to do, with relatively minor technical errors being made,” he said.
However, it is not clear if this technology can truly save lives, said Goldenholz. “The data we have suggests that lying face down in bed after a seizure is correlated with SUDEP, but that does not mean that if we can simply flip people over, they for sure won’t die.”
Even if the new technology “works perfectly,” it’s still an open question, said Goldenholz. If it does save lives, “this will be a major breakthrough, and one that has been needed for a long time.”
However, even if it does not, he congratulates the team for trying to determine if reducing the prone position can help prevent SUDEP. He would like to see more “high-risk, high-reward” studies in the epilepsy field. “We are in so much need of new innovations.”
He said he was “personally very inspired” by this work. “People are dying from this terrible disease, and this team is building what they hope might save lives.”
The study was funded by the National Institutes of Health. The mattress is being developed by Soterya. Lee reported no equity in Soterya. Goldenholz reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
LOS ANGELES — says one of the experts involved in its development.
When used along with a seizure detection device, Jong Woo Lee, MD, PhD, associate professor of neurology, Harvard Medical School, and Brigham and Women’s Hospital, both in Boston, Massachusetts, estimates the smart mattress could cut SUDEP by more than 50%.
In addition, early results from an observational study are backing this up, he said.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
Most SUDEP Cases Found Face Down
SUDEP is the leading cause of death in children with epilepsy and in otherwise healthy adult patients with epilepsy. When his fifth patient died of SUDEP, Lee decided it was time to try to tackle the high mortality rate associated with these unexpected deaths. “I desperately wanted to help, ” he said.
About 70% of SUDEP occurs during sleep, and victims are found face down, or in the prone position, 90% of the time, said Lee.
“Of course, the best way to prevent SUDEP is not to have a seizure, but once you have a seizure and once you’re face down, your risk for death goes up by somewhere between 30 and 100 times,” he explained.
Lee was convinced SUDEP could be prevented by simple interventions that stimulate the patient and turn them over. He noted the incidence of sudden infant death syndrome, “which has similar characteristics” to SUDEP, has been reduced by up to 75% through campaigns that simply advise placing babies on their backs.
“Most of SUDEP happens because your arousal system is knocked out and you just don’t take the breath that you’re supposed to. Just the act of turning people over and vibrating the bed will stimulate them,” he said.
However, it’s crucial that this be done quickly, said Lee. “When you look at patients who died on video and see the EEGs, everybody took their last breath within 3 minutes.”
Because the window of opportunity is so short, “we think that seizure detection devices alone are not going to really be effective because you just can’t get there or react within those 3 minutes.”
There are currently no products that detect the prone position or have the ability to reposition a patient quickly into the recovery sideways position.
Lee and his colleagues developed a smart system that can be embedded in a mattress that detects when someone is having a seizure, determines if that person is face down, and if so, safely stimulates and repositions them.
The mattress is made up of a series of programmable inflatable blocks or “cells” that have pressure, vibration, temperature, and humidity sensors embedded within. “Based on the pressure readings, we can figure out whether the patient is right side up, on their right side, on their left side, or face down,” said Lee.
If the person is face down, he or she can be repositioned within a matter of seconds. “Each of the cells can lift 1000 pounds,” he said. The mattress is “very comfortable,” said Lee, who has tried it out himself.
Eighteen normative control participants have been enrolled for development and training purposes. To date, 10 of these individuals, aged 18-53 years, weighing 100-182 lb, and with a height of 5 ft 2 in to 6 ft 1 in, underwent extensive formal testing on the prototype bed.
Researchers found the mattress responded quickly to different body positions and weights. “We were able to reposition everybody in around 20 seconds,” said Lee.
The overall accuracy of detecting the prone position was 96.8%. There were no cases of a supine or prone position being mistaken for each other.
Researchers are refining the algorithm to improve the accuracy for detecting the prone position and expect to have a completely functional prototype within a few years.
Big Step Forward
Commenting on the research, Daniel M. Goldenholz, MD, PhD, assistant professor, Division of Epilepsy, Harvard Beth Israel Deaconess Medical Center, Boston, said the study “is a big step forward in the race to provide an actionable tool to prevent SUDEP.”
The technology “appears to mostly be doing what it’s intended to do, with relatively minor technical errors being made,” he said.
However, it is not clear if this technology can truly save lives, said Goldenholz. “The data we have suggests that lying face down in bed after a seizure is correlated with SUDEP, but that does not mean that if we can simply flip people over, they for sure won’t die.”
Even if the new technology “works perfectly,” it’s still an open question, said Goldenholz. If it does save lives, “this will be a major breakthrough, and one that has been needed for a long time.”
However, even if it does not, he congratulates the team for trying to determine if reducing the prone position can help prevent SUDEP. He would like to see more “high-risk, high-reward” studies in the epilepsy field. “We are in so much need of new innovations.”
He said he was “personally very inspired” by this work. “People are dying from this terrible disease, and this team is building what they hope might save lives.”
The study was funded by the National Institutes of Health. The mattress is being developed by Soterya. Lee reported no equity in Soterya. Goldenholz reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
LOS ANGELES — says one of the experts involved in its development.
When used along with a seizure detection device, Jong Woo Lee, MD, PhD, associate professor of neurology, Harvard Medical School, and Brigham and Women’s Hospital, both in Boston, Massachusetts, estimates the smart mattress could cut SUDEP by more than 50%.
In addition, early results from an observational study are backing this up, he said.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
Most SUDEP Cases Found Face Down
SUDEP is the leading cause of death in children with epilepsy and in otherwise healthy adult patients with epilepsy. When his fifth patient died of SUDEP, Lee decided it was time to try to tackle the high mortality rate associated with these unexpected deaths. “I desperately wanted to help, ” he said.
About 70% of SUDEP occurs during sleep, and victims are found face down, or in the prone position, 90% of the time, said Lee.
“Of course, the best way to prevent SUDEP is not to have a seizure, but once you have a seizure and once you’re face down, your risk for death goes up by somewhere between 30 and 100 times,” he explained.
Lee was convinced SUDEP could be prevented by simple interventions that stimulate the patient and turn them over. He noted the incidence of sudden infant death syndrome, “which has similar characteristics” to SUDEP, has been reduced by up to 75% through campaigns that simply advise placing babies on their backs.
“Most of SUDEP happens because your arousal system is knocked out and you just don’t take the breath that you’re supposed to. Just the act of turning people over and vibrating the bed will stimulate them,” he said.
However, it’s crucial that this be done quickly, said Lee. “When you look at patients who died on video and see the EEGs, everybody took their last breath within 3 minutes.”
Because the window of opportunity is so short, “we think that seizure detection devices alone are not going to really be effective because you just can’t get there or react within those 3 minutes.”
There are currently no products that detect the prone position or have the ability to reposition a patient quickly into the recovery sideways position.
Lee and his colleagues developed a smart system that can be embedded in a mattress that detects when someone is having a seizure, determines if that person is face down, and if so, safely stimulates and repositions them.
The mattress is made up of a series of programmable inflatable blocks or “cells” that have pressure, vibration, temperature, and humidity sensors embedded within. “Based on the pressure readings, we can figure out whether the patient is right side up, on their right side, on their left side, or face down,” said Lee.
If the person is face down, he or she can be repositioned within a matter of seconds. “Each of the cells can lift 1000 pounds,” he said. The mattress is “very comfortable,” said Lee, who has tried it out himself.
Eighteen normative control participants have been enrolled for development and training purposes. To date, 10 of these individuals, aged 18-53 years, weighing 100-182 lb, and with a height of 5 ft 2 in to 6 ft 1 in, underwent extensive formal testing on the prototype bed.
Researchers found the mattress responded quickly to different body positions and weights. “We were able to reposition everybody in around 20 seconds,” said Lee.
The overall accuracy of detecting the prone position was 96.8%. There were no cases of a supine or prone position being mistaken for each other.
Researchers are refining the algorithm to improve the accuracy for detecting the prone position and expect to have a completely functional prototype within a few years.
Big Step Forward
Commenting on the research, Daniel M. Goldenholz, MD, PhD, assistant professor, Division of Epilepsy, Harvard Beth Israel Deaconess Medical Center, Boston, said the study “is a big step forward in the race to provide an actionable tool to prevent SUDEP.”
The technology “appears to mostly be doing what it’s intended to do, with relatively minor technical errors being made,” he said.
However, it is not clear if this technology can truly save lives, said Goldenholz. “The data we have suggests that lying face down in bed after a seizure is correlated with SUDEP, but that does not mean that if we can simply flip people over, they for sure won’t die.”
Even if the new technology “works perfectly,” it’s still an open question, said Goldenholz. If it does save lives, “this will be a major breakthrough, and one that has been needed for a long time.”
However, even if it does not, he congratulates the team for trying to determine if reducing the prone position can help prevent SUDEP. He would like to see more “high-risk, high-reward” studies in the epilepsy field. “We are in so much need of new innovations.”
He said he was “personally very inspired” by this work. “People are dying from this terrible disease, and this team is building what they hope might save lives.”
The study was funded by the National Institutes of Health. The mattress is being developed by Soterya. Lee reported no equity in Soterya. Goldenholz reported no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FROM AES 2024
Sleep Apnea Linked to Heightened Mortality in Epilepsy
LOS ANGELES — according to a new analysis of over 2 million patient-years drawn from the Komodo Health Claims Database.
“A 10-year-old with uncontrolled epilepsy and central sleep apnea is about 200 times more likely to die than a general population 10-year-old. That’s comparable to a 10-year-old with {epilepsy and} congestive heart failure. Noncentral sleep apnea is comparable to being paralyzed. It’s a huge risk factor,” said poster presenter Dan Lloyd, advanced analytics lead at UCB, which sponsored the research.
The ordering of sleep apnea tests for patients with epilepsy is widely variable, according to Stefanie Dedeurwaerdere, PhD, who is the innovation and value creation lead at UCB. “Some doctors do that as a general practice, and some don’t. There’s no coherency in the way these studies are requested for epilepsy patients. We want to create some awareness around this topic,” she said, and added that treatment of sleep apnea may improve epileptic seizures.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
The study included mortality rates between January 2018 and December 2022, with a total of 2,355,410 patient-years and 968,993 patients, with an age distribution of 19.1% age 1 to less than 18 years, 23.7% age 18-35 years, and 57.2% age 36 years or older. Sleep apnea prevalences were 0.7% for central sleep apnea (CSA), 14.0% for obstructive sleep apnea (OSA), and 85.3% with no sleep apnea.
Among those aged 1-18 years, the standardized mortality ratio (SMR) for those with uncontrolled epilepsy was 27.7. For those with comorbid OSA, the SMR was 74.2, and for comorbid CSA, the SMR was 135.9. The association was less pronounced in older groups, dipping to 7.0, 11.3, and 19.5 in those aged 18-35 years, and 3.3, 3.1, and 2.8 among those aged 36 years or older.
Among the 1-18 age group, SMRs for other comorbidities included 132.3 for heart failure, 74.9 for hemiplegia/paraplegia, 55.3 for cerebrovascular disease, and 44.6 for chronic pulmonary disease.
Asked for comment, Gordon Buchanan, MD, PhD, welcomed the new work. “The results did not surprise me. I study sleep, epilepsy, and [sudden unexplained death in epilepsy (SUDEP)] in particular ... and every time I speak on these topics, someone asks me about risk of SUDEP in patients with sleep apnea. It’s great to finally have some data,” said Buchanan, a professor of neurology at the University of Iowa, Iowa City.
The authors found that patients undergoing continuous positive airway pressure (CPAP)/bi-level positive airway pressure therapy had a higher mortality risk than those not undergoing CPAP therapy but cautioned that uncontrolled confounders may be contributing to the effect.
Buchanan wondered if treatment with CPAP would be associated with a decreased mortality risk. “I think that would be interesting, but I know that, especially in children, it can be difficult to get them to remain compliant with CPAP. I think that would be interesting to know, if pushing harder to get the kids to comply with CPAP would reduce mortality,” he said.
The specific finding of heightened mortality associated with CSA is interesting, according to Buchanan. “We think of seizures propagating through the brain, maybe through direct synaptic connections or through spreading depolarization. So I think it would make sense that it would hit central regions that would then lead to sleep apnea.”
The relationship between OSA and epilepsy is likely complex. Epilepsy medications and special diets may influence body composition, which could in turn affect the risk for OSA, as could medications associated with psychiatric comorbidities, according to Buchanan.
The study is retrospective and based on claims data. It does not prove causation, and claims data do not fully capture mortality, which may lead to conservative SMR estimates. The researchers did not control for socioeconomic status, treatment status, and other comorbidities or conditions.
Lloyd and Dedeurwaerdere are employees of UCB, which sponsored the study. Buchanan had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
LOS ANGELES — according to a new analysis of over 2 million patient-years drawn from the Komodo Health Claims Database.
“A 10-year-old with uncontrolled epilepsy and central sleep apnea is about 200 times more likely to die than a general population 10-year-old. That’s comparable to a 10-year-old with {epilepsy and} congestive heart failure. Noncentral sleep apnea is comparable to being paralyzed. It’s a huge risk factor,” said poster presenter Dan Lloyd, advanced analytics lead at UCB, which sponsored the research.
The ordering of sleep apnea tests for patients with epilepsy is widely variable, according to Stefanie Dedeurwaerdere, PhD, who is the innovation and value creation lead at UCB. “Some doctors do that as a general practice, and some don’t. There’s no coherency in the way these studies are requested for epilepsy patients. We want to create some awareness around this topic,” she said, and added that treatment of sleep apnea may improve epileptic seizures.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
The study included mortality rates between January 2018 and December 2022, with a total of 2,355,410 patient-years and 968,993 patients, with an age distribution of 19.1% age 1 to less than 18 years, 23.7% age 18-35 years, and 57.2% age 36 years or older. Sleep apnea prevalences were 0.7% for central sleep apnea (CSA), 14.0% for obstructive sleep apnea (OSA), and 85.3% with no sleep apnea.
Among those aged 1-18 years, the standardized mortality ratio (SMR) for those with uncontrolled epilepsy was 27.7. For those with comorbid OSA, the SMR was 74.2, and for comorbid CSA, the SMR was 135.9. The association was less pronounced in older groups, dipping to 7.0, 11.3, and 19.5 in those aged 18-35 years, and 3.3, 3.1, and 2.8 among those aged 36 years or older.
Among the 1-18 age group, SMRs for other comorbidities included 132.3 for heart failure, 74.9 for hemiplegia/paraplegia, 55.3 for cerebrovascular disease, and 44.6 for chronic pulmonary disease.
Asked for comment, Gordon Buchanan, MD, PhD, welcomed the new work. “The results did not surprise me. I study sleep, epilepsy, and [sudden unexplained death in epilepsy (SUDEP)] in particular ... and every time I speak on these topics, someone asks me about risk of SUDEP in patients with sleep apnea. It’s great to finally have some data,” said Buchanan, a professor of neurology at the University of Iowa, Iowa City.
The authors found that patients undergoing continuous positive airway pressure (CPAP)/bi-level positive airway pressure therapy had a higher mortality risk than those not undergoing CPAP therapy but cautioned that uncontrolled confounders may be contributing to the effect.
Buchanan wondered if treatment with CPAP would be associated with a decreased mortality risk. “I think that would be interesting, but I know that, especially in children, it can be difficult to get them to remain compliant with CPAP. I think that would be interesting to know, if pushing harder to get the kids to comply with CPAP would reduce mortality,” he said.
The specific finding of heightened mortality associated with CSA is interesting, according to Buchanan. “We think of seizures propagating through the brain, maybe through direct synaptic connections or through spreading depolarization. So I think it would make sense that it would hit central regions that would then lead to sleep apnea.”
The relationship between OSA and epilepsy is likely complex. Epilepsy medications and special diets may influence body composition, which could in turn affect the risk for OSA, as could medications associated with psychiatric comorbidities, according to Buchanan.
The study is retrospective and based on claims data. It does not prove causation, and claims data do not fully capture mortality, which may lead to conservative SMR estimates. The researchers did not control for socioeconomic status, treatment status, and other comorbidities or conditions.
Lloyd and Dedeurwaerdere are employees of UCB, which sponsored the study. Buchanan had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
LOS ANGELES — according to a new analysis of over 2 million patient-years drawn from the Komodo Health Claims Database.
“A 10-year-old with uncontrolled epilepsy and central sleep apnea is about 200 times more likely to die than a general population 10-year-old. That’s comparable to a 10-year-old with {epilepsy and} congestive heart failure. Noncentral sleep apnea is comparable to being paralyzed. It’s a huge risk factor,” said poster presenter Dan Lloyd, advanced analytics lead at UCB, which sponsored the research.
The ordering of sleep apnea tests for patients with epilepsy is widely variable, according to Stefanie Dedeurwaerdere, PhD, who is the innovation and value creation lead at UCB. “Some doctors do that as a general practice, and some don’t. There’s no coherency in the way these studies are requested for epilepsy patients. We want to create some awareness around this topic,” she said, and added that treatment of sleep apnea may improve epileptic seizures.
The findings were presented at the American Epilepsy Society (AES) 78th Annual Meeting 2024.
The study included mortality rates between January 2018 and December 2022, with a total of 2,355,410 patient-years and 968,993 patients, with an age distribution of 19.1% age 1 to less than 18 years, 23.7% age 18-35 years, and 57.2% age 36 years or older. Sleep apnea prevalences were 0.7% for central sleep apnea (CSA), 14.0% for obstructive sleep apnea (OSA), and 85.3% with no sleep apnea.
Among those aged 1-18 years, the standardized mortality ratio (SMR) for those with uncontrolled epilepsy was 27.7. For those with comorbid OSA, the SMR was 74.2, and for comorbid CSA, the SMR was 135.9. The association was less pronounced in older groups, dipping to 7.0, 11.3, and 19.5 in those aged 18-35 years, and 3.3, 3.1, and 2.8 among those aged 36 years or older.
Among the 1-18 age group, SMRs for other comorbidities included 132.3 for heart failure, 74.9 for hemiplegia/paraplegia, 55.3 for cerebrovascular disease, and 44.6 for chronic pulmonary disease.
Asked for comment, Gordon Buchanan, MD, PhD, welcomed the new work. “The results did not surprise me. I study sleep, epilepsy, and [sudden unexplained death in epilepsy (SUDEP)] in particular ... and every time I speak on these topics, someone asks me about risk of SUDEP in patients with sleep apnea. It’s great to finally have some data,” said Buchanan, a professor of neurology at the University of Iowa, Iowa City.
The authors found that patients undergoing continuous positive airway pressure (CPAP)/bi-level positive airway pressure therapy had a higher mortality risk than those not undergoing CPAP therapy but cautioned that uncontrolled confounders may be contributing to the effect.
Buchanan wondered if treatment with CPAP would be associated with a decreased mortality risk. “I think that would be interesting, but I know that, especially in children, it can be difficult to get them to remain compliant with CPAP. I think that would be interesting to know, if pushing harder to get the kids to comply with CPAP would reduce mortality,” he said.
The specific finding of heightened mortality associated with CSA is interesting, according to Buchanan. “We think of seizures propagating through the brain, maybe through direct synaptic connections or through spreading depolarization. So I think it would make sense that it would hit central regions that would then lead to sleep apnea.”
The relationship between OSA and epilepsy is likely complex. Epilepsy medications and special diets may influence body composition, which could in turn affect the risk for OSA, as could medications associated with psychiatric comorbidities, according to Buchanan.
The study is retrospective and based on claims data. It does not prove causation, and claims data do not fully capture mortality, which may lead to conservative SMR estimates. The researchers did not control for socioeconomic status, treatment status, and other comorbidities or conditions.
Lloyd and Dedeurwaerdere are employees of UCB, which sponsored the study. Buchanan had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
FROM AES 2024
On the Murder of UnitedHealthcare’s CEO
On December 4, UnitedHealthcare CEO Brian Thompson was assassinated in New York City outside of a hotel. As of the time of this writing, the shooter is still at large.
I suppose I could write about how this shows that Americans are fed up with the way modern commercial healthcare companies operate. Who gets care and who doesn’t.
I could write about how industry trends of “Delay, Deny, Defend” lead to the suffering of millions of people who need healthcare that they thought they were paying for.
I could write about the callousness of the way people online are celebrating the cold-blooded murder of a married man with two children.
I might write about how insurance companies intentionally, and routinely, drag out (or deny) reimbursements for physicians (including small solo practice ones, like myself) who are legitimately caring for their patients.
I suppose I could write something about how gun violence is so pervasive in our society that it scarcely merits a second glance at the news story. If the headline just said, “Unknown Assailant Kills Man Outside Hotel,” would you have even read beyond that?
I could write about how the lack of regulations, and accelerating attempts to scrap them, can lead to insider trading.
I could write about how having insurance companies and medical facilities more beholden to shareholders than to patients is a serious conflict of interest.
I could try to make points about how the widespread availability of firearms (in this case one with a built-in silencer) in America means that anyone with a vendetta, or serious mental illness, or just a short temper, can get one — and use it.
I could talk about how “greed is good” in healthcare settings rewards a few and hurts many — no matter how much the PR spinners try to make it sound like it’s a great win-win situation all-around.
I could argue that the jubilant “good riddance” and “eat the rich” responses of many — both medical and nonmedical people — to the killing shows that, as a society, we’re losing the qualities that make us human.
I could also argue that putting financial gain for executive bonuses and stockholder dividends ahead of the health and well-being of others shows that, as a society, we’re losing the qualities that make us human.
I could make a point that , provided the target is someone they have a difference of opinion with. Which is, honestly, pretty damn scary.
I could talk about how policies of arbitrarily changing the rules about anesthesia coverage, or letting a computer decide how long a hospital stay should be, or to deny rehabilitation care, are unethical, unjust, and just plain wrong.
I could write about a lot of things based on what happened outside that New York Hilton Midtown in early December.
But as I stare at my screen, I’m well aware that no matter what I write it won’t change any opinions, solve anything, or even lead to people trying to find a solution.
Because that’s just the world we live in.
Block has a solo neurology practice in Scottsdale, Arizona.
On December 4, UnitedHealthcare CEO Brian Thompson was assassinated in New York City outside of a hotel. As of the time of this writing, the shooter is still at large.
I suppose I could write about how this shows that Americans are fed up with the way modern commercial healthcare companies operate. Who gets care and who doesn’t.
I could write about how industry trends of “Delay, Deny, Defend” lead to the suffering of millions of people who need healthcare that they thought they were paying for.
I could write about the callousness of the way people online are celebrating the cold-blooded murder of a married man with two children.
I might write about how insurance companies intentionally, and routinely, drag out (or deny) reimbursements for physicians (including small solo practice ones, like myself) who are legitimately caring for their patients.
I suppose I could write something about how gun violence is so pervasive in our society that it scarcely merits a second glance at the news story. If the headline just said, “Unknown Assailant Kills Man Outside Hotel,” would you have even read beyond that?
I could write about how the lack of regulations, and accelerating attempts to scrap them, can lead to insider trading.
I could write about how having insurance companies and medical facilities more beholden to shareholders than to patients is a serious conflict of interest.
I could try to make points about how the widespread availability of firearms (in this case one with a built-in silencer) in America means that anyone with a vendetta, or serious mental illness, or just a short temper, can get one — and use it.
I could talk about how “greed is good” in healthcare settings rewards a few and hurts many — no matter how much the PR spinners try to make it sound like it’s a great win-win situation all-around.
I could argue that the jubilant “good riddance” and “eat the rich” responses of many — both medical and nonmedical people — to the killing shows that, as a society, we’re losing the qualities that make us human.
I could also argue that putting financial gain for executive bonuses and stockholder dividends ahead of the health and well-being of others shows that, as a society, we’re losing the qualities that make us human.
I could make a point that , provided the target is someone they have a difference of opinion with. Which is, honestly, pretty damn scary.
I could talk about how policies of arbitrarily changing the rules about anesthesia coverage, or letting a computer decide how long a hospital stay should be, or to deny rehabilitation care, are unethical, unjust, and just plain wrong.
I could write about a lot of things based on what happened outside that New York Hilton Midtown in early December.
But as I stare at my screen, I’m well aware that no matter what I write it won’t change any opinions, solve anything, or even lead to people trying to find a solution.
Because that’s just the world we live in.
Block has a solo neurology practice in Scottsdale, Arizona.
On December 4, UnitedHealthcare CEO Brian Thompson was assassinated in New York City outside of a hotel. As of the time of this writing, the shooter is still at large.
I suppose I could write about how this shows that Americans are fed up with the way modern commercial healthcare companies operate. Who gets care and who doesn’t.
I could write about how industry trends of “Delay, Deny, Defend” lead to the suffering of millions of people who need healthcare that they thought they were paying for.
I could write about the callousness of the way people online are celebrating the cold-blooded murder of a married man with two children.
I might write about how insurance companies intentionally, and routinely, drag out (or deny) reimbursements for physicians (including small solo practice ones, like myself) who are legitimately caring for their patients.
I suppose I could write something about how gun violence is so pervasive in our society that it scarcely merits a second glance at the news story. If the headline just said, “Unknown Assailant Kills Man Outside Hotel,” would you have even read beyond that?
I could write about how the lack of regulations, and accelerating attempts to scrap them, can lead to insider trading.
I could write about how having insurance companies and medical facilities more beholden to shareholders than to patients is a serious conflict of interest.
I could try to make points about how the widespread availability of firearms (in this case one with a built-in silencer) in America means that anyone with a vendetta, or serious mental illness, or just a short temper, can get one — and use it.
I could talk about how “greed is good” in healthcare settings rewards a few and hurts many — no matter how much the PR spinners try to make it sound like it’s a great win-win situation all-around.
I could argue that the jubilant “good riddance” and “eat the rich” responses of many — both medical and nonmedical people — to the killing shows that, as a society, we’re losing the qualities that make us human.
I could also argue that putting financial gain for executive bonuses and stockholder dividends ahead of the health and well-being of others shows that, as a society, we’re losing the qualities that make us human.
I could make a point that , provided the target is someone they have a difference of opinion with. Which is, honestly, pretty damn scary.
I could talk about how policies of arbitrarily changing the rules about anesthesia coverage, or letting a computer decide how long a hospital stay should be, or to deny rehabilitation care, are unethical, unjust, and just plain wrong.
I could write about a lot of things based on what happened outside that New York Hilton Midtown in early December.
But as I stare at my screen, I’m well aware that no matter what I write it won’t change any opinions, solve anything, or even lead to people trying to find a solution.
Because that’s just the world we live in.
Block has a solo neurology practice in Scottsdale, Arizona.
Common Herbicide a Player in Neurodegeneration?
new research showed.
Researchers found that glyphosate exposure even at regulated levels was associated with increased neuroinflammation and accelerated Alzheimer’s disease–like pathology in mice — an effect that persisted 6 months after a recovery period when exposure was stopped.
“More research is needed to understand the consequences of glyphosate exposure to the brain in humans and to understand the appropriate dose of exposure to limit detrimental outcomes,” said co–senior author Ramon Velazquez, PhD, with Arizona State University, Tempe.
The study was published online in The Journal of Neuroinflammation.
Persistent Accumulation Within the Brain
Glyphosate is the most heavily applied herbicide in the United States, with roughly 300 million pounds used annually in agricultural communities throughout the United States. It is also used for weed control in parks, residential areas, and personal gardens.
The Environmental Protection Agency (EPA) has determined that glyphosate poses no risks to human health when used as directed. But the World Health Organization’s International Agency for Research on Cancer disagrees, classifying the herbicide as “possibly carcinogenic to humans.”
In addition to the possible cancer risk, multiple reports have also suggested potential harmful effects of glyphosate exposure on the brain.
In earlier work, Velazquez and colleagues showed that glyphosate crosses the blood-brain barrier and infiltrates the brains of mice, contributing to neuroinflammation and other detrimental effects on brain function.
In their latest study, they examined the long-term effects of glyphosate exposure on neuroinflammation and Alzheimer’s disease–like pathology using a mouse model.
They dosed 4.5-month-old mice genetically predisposed to Alzheimer’s disease and non-transgenic control mice with either 0, 50, or 500 mg/kg of glyphosate daily for 13 weeks followed by a 6-month recovery period.
The high dose is similar to levels used in earlier research, and the low dose is close to the limit used to establish the current EPA acceptable dose in humans.
Glyphosate’s metabolite, aminomethylphosphonic acid, was detectable and persisted in mouse brain tissue even 6 months after exposure ceased, the researchers reported.
Additionally, there was a significant increase in soluble and insoluble fractions of amyloid-beta (Abeta), Abeta42 plaque load and plaque size, and phosphorylated tau at Threonine 181 and Serine 396 in hippocampus and cortex brain tissue from glyphosate-exposed mice, “highlighting an exacerbation of hallmark Alzheimer’s disease–like proteinopathies,” they noted.
Glyphosate exposure was also associated with significant elevations in both pro- and anti-inflammatory cytokines and chemokines in brain tissue of transgenic and normal mice and in peripheral blood plasma of transgenic mice.
Glyphosate-exposed transgenic mice also showed heightened anxiety-like behaviors and reduced survival.
“These findings highlight that many chemicals we regularly encounter, previously considered safe, may pose potential health risks,” co–senior author Patrick Pirrotte, PhD, with the Translational Genomics Research Institute, Phoenix, Arizona, said in a statement.
“However, further research is needed to fully assess the public health impact and identify safer alternatives,” Pirrotte added.
Funding for the study was provided by the National Institutes on Aging, National Cancer Institute and the Arizona State University (ASU) Biodesign Institute. The authors have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
new research showed.
Researchers found that glyphosate exposure even at regulated levels was associated with increased neuroinflammation and accelerated Alzheimer’s disease–like pathology in mice — an effect that persisted 6 months after a recovery period when exposure was stopped.
“More research is needed to understand the consequences of glyphosate exposure to the brain in humans and to understand the appropriate dose of exposure to limit detrimental outcomes,” said co–senior author Ramon Velazquez, PhD, with Arizona State University, Tempe.
The study was published online in The Journal of Neuroinflammation.
Persistent Accumulation Within the Brain
Glyphosate is the most heavily applied herbicide in the United States, with roughly 300 million pounds used annually in agricultural communities throughout the United States. It is also used for weed control in parks, residential areas, and personal gardens.
The Environmental Protection Agency (EPA) has determined that glyphosate poses no risks to human health when used as directed. But the World Health Organization’s International Agency for Research on Cancer disagrees, classifying the herbicide as “possibly carcinogenic to humans.”
In addition to the possible cancer risk, multiple reports have also suggested potential harmful effects of glyphosate exposure on the brain.
In earlier work, Velazquez and colleagues showed that glyphosate crosses the blood-brain barrier and infiltrates the brains of mice, contributing to neuroinflammation and other detrimental effects on brain function.
In their latest study, they examined the long-term effects of glyphosate exposure on neuroinflammation and Alzheimer’s disease–like pathology using a mouse model.
They dosed 4.5-month-old mice genetically predisposed to Alzheimer’s disease and non-transgenic control mice with either 0, 50, or 500 mg/kg of glyphosate daily for 13 weeks followed by a 6-month recovery period.
The high dose is similar to levels used in earlier research, and the low dose is close to the limit used to establish the current EPA acceptable dose in humans.
Glyphosate’s metabolite, aminomethylphosphonic acid, was detectable and persisted in mouse brain tissue even 6 months after exposure ceased, the researchers reported.
Additionally, there was a significant increase in soluble and insoluble fractions of amyloid-beta (Abeta), Abeta42 plaque load and plaque size, and phosphorylated tau at Threonine 181 and Serine 396 in hippocampus and cortex brain tissue from glyphosate-exposed mice, “highlighting an exacerbation of hallmark Alzheimer’s disease–like proteinopathies,” they noted.
Glyphosate exposure was also associated with significant elevations in both pro- and anti-inflammatory cytokines and chemokines in brain tissue of transgenic and normal mice and in peripheral blood plasma of transgenic mice.
Glyphosate-exposed transgenic mice also showed heightened anxiety-like behaviors and reduced survival.
“These findings highlight that many chemicals we regularly encounter, previously considered safe, may pose potential health risks,” co–senior author Patrick Pirrotte, PhD, with the Translational Genomics Research Institute, Phoenix, Arizona, said in a statement.
“However, further research is needed to fully assess the public health impact and identify safer alternatives,” Pirrotte added.
Funding for the study was provided by the National Institutes on Aging, National Cancer Institute and the Arizona State University (ASU) Biodesign Institute. The authors have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
new research showed.
Researchers found that glyphosate exposure even at regulated levels was associated with increased neuroinflammation and accelerated Alzheimer’s disease–like pathology in mice — an effect that persisted 6 months after a recovery period when exposure was stopped.
“More research is needed to understand the consequences of glyphosate exposure to the brain in humans and to understand the appropriate dose of exposure to limit detrimental outcomes,” said co–senior author Ramon Velazquez, PhD, with Arizona State University, Tempe.
The study was published online in The Journal of Neuroinflammation.
Persistent Accumulation Within the Brain
Glyphosate is the most heavily applied herbicide in the United States, with roughly 300 million pounds used annually in agricultural communities throughout the United States. It is also used for weed control in parks, residential areas, and personal gardens.
The Environmental Protection Agency (EPA) has determined that glyphosate poses no risks to human health when used as directed. But the World Health Organization’s International Agency for Research on Cancer disagrees, classifying the herbicide as “possibly carcinogenic to humans.”
In addition to the possible cancer risk, multiple reports have also suggested potential harmful effects of glyphosate exposure on the brain.
In earlier work, Velazquez and colleagues showed that glyphosate crosses the blood-brain barrier and infiltrates the brains of mice, contributing to neuroinflammation and other detrimental effects on brain function.
In their latest study, they examined the long-term effects of glyphosate exposure on neuroinflammation and Alzheimer’s disease–like pathology using a mouse model.
They dosed 4.5-month-old mice genetically predisposed to Alzheimer’s disease and non-transgenic control mice with either 0, 50, or 500 mg/kg of glyphosate daily for 13 weeks followed by a 6-month recovery period.
The high dose is similar to levels used in earlier research, and the low dose is close to the limit used to establish the current EPA acceptable dose in humans.
Glyphosate’s metabolite, aminomethylphosphonic acid, was detectable and persisted in mouse brain tissue even 6 months after exposure ceased, the researchers reported.
Additionally, there was a significant increase in soluble and insoluble fractions of amyloid-beta (Abeta), Abeta42 plaque load and plaque size, and phosphorylated tau at Threonine 181 and Serine 396 in hippocampus and cortex brain tissue from glyphosate-exposed mice, “highlighting an exacerbation of hallmark Alzheimer’s disease–like proteinopathies,” they noted.
Glyphosate exposure was also associated with significant elevations in both pro- and anti-inflammatory cytokines and chemokines in brain tissue of transgenic and normal mice and in peripheral blood plasma of transgenic mice.
Glyphosate-exposed transgenic mice also showed heightened anxiety-like behaviors and reduced survival.
“These findings highlight that many chemicals we regularly encounter, previously considered safe, may pose potential health risks,” co–senior author Patrick Pirrotte, PhD, with the Translational Genomics Research Institute, Phoenix, Arizona, said in a statement.
“However, further research is needed to fully assess the public health impact and identify safer alternatives,” Pirrotte added.
Funding for the study was provided by the National Institutes on Aging, National Cancer Institute and the Arizona State University (ASU) Biodesign Institute. The authors have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF NEUROINFLAMMATION
New Cancer Vaccines on the Horizon: Renewed Hope or Hype?
Vaccines for treating and preventing cancer have long been considered a holy grail in oncology.
But aside from a few notable exceptions — including the human papillomavirus (HPV) vaccine, which has dramatically reduced the incidence of HPV-related cancers, and a Bacillus Calmette-Guerin vaccine, which helps prevent early-stage bladder cancer recurrence — most have failed to deliver.
Following a string of disappointments over the past decade, recent advances in the immunotherapy space are bringing renewed hope for progress.
In an American Association for Cancer Research (AACR) series earlier in 2024, Catherine J. Wu, MD, predicted big strides for cancer vaccines, especially for personalized vaccines that target patient-specific neoantigens — the proteins that form on cancer cells — as well as vaccines that can treat diverse tumor types.
said Wu, the Lavine Family Chair of Preventative Cancer Therapies at Dana-Farber Cancer Institute and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts.
A prime example is a personalized, messenger RNA (mRNA)–based vaccine designed to prevent melanoma recurrence. The mRNA-4157 vaccine encodes up to 34 different patient-specific neoantigens.
“This is one of the most exciting developments in modern cancer therapy,” said Lawrence Young, a virologist and professor of molecular oncology at the University of Warwick, Coventry, England, who commented on the investigational vaccine via the UK-based Science Media Centre.
Other promising options are on the horizon as well. In August, BioNTech announced a phase 1 global trial to study BNT116 — a vaccine to treat non–small cell lung cancer (NSCLC). BNT116, like mRNA-4157, targets specific antigens in the lung cancer cells.
“This technology is the next big phase of cancer treatment,” Siow Ming Lee, MD, a consultant medical oncologist at University College London Hospitals in England, which is leading the UK trial for the lung cancer and melanoma vaccines, told The Guardian. “We are now entering this very exciting new era of mRNA-based immunotherapy clinical trials to investigate the treatment of lung cancer.”
Still, these predictions have a familiar ring. While the prospects are exciting, delivering on them is another story. There are simply no guarantees these strategies will work as hoped.
Then: Where We Were
Cancer vaccine research began to ramp up in the 2000s, and in 2006, the first-generation HPV vaccine, Gardasil, was approved. Gardasil prevents infection from four strains of HPV that cause about 80% of cervical cancer cases.
In 2010, the Food and Drug Administration approved sipuleucel-T, the first therapeutic cancer vaccine, which improved overall survival in patients with hormone-refractory prostate cancer.
Researchers predicted this approval would “pave the way for developing innovative, next generation of vaccines with enhanced antitumor potency.”
In a 2015 AACR research forecast report, Drew Pardoll, MD, PhD, co-director of the Cancer Immunology and Hematopoiesis Program at Johns Hopkins University, Baltimore, Maryland, said that “we can expect to see encouraging results from studies using cancer vaccines.”
Despite the excitement surrounding cancer vaccines alongside a few successes, the next decade brought a longer string of late-phase disappointments.
In 2016, the phase 3 ACT IV trial of a therapeutic vaccine to treat glioblastoma multiforme (CDX-110) was terminated after it failed to demonstrate improved survival.
In 2017, a phase 3 trial of the therapeutic pancreatic cancer vaccine, GVAX, was stopped early for lack of efficacy.
That year, an attenuated Listeria monocytogenes vaccine to treat pancreatic cancer and mesothelioma also failed to come to fruition. In late 2017, concerns over listeria infections prompted Aduro Biotech to cancel its listeria-based cancer treatment program.
In 2018, a phase 3 trial of belagenpumatucel-L, a therapeutic NSCLC vaccine, failed to demonstrate a significant improvement in survival and further study was discontinued.
And in 2019, a vaccine targeting MAGE-A3, a cancer-testis antigen present in multiple tumor types, failed to meet endpoints for improved survival in a phase 3 trial, leading to discontinuation of the vaccine program.
But these disappointments and failures are normal parts of medical research and drug development and have allowed for incremental advances that helped fuel renewed interest and hope for cancer vaccines, when the timing was right, explained vaccine pioneer Larry W. Kwak, MD, PhD, deputy director of the Comprehensive Cancer Center at City of Hope, Duarte, California.
When it comes to vaccine progress, timing makes a difference. In 2011, Kwak and colleagues published promising phase 3 trial results on a personalized vaccine. The vaccine was a patient-specific tumor-derived antigen for patients with follicular lymphoma in their first remission following chemotherapy. Patients who received the vaccine demonstrated significantly longer disease-free survival.
But, at the time, personalized vaccines faced strong headwinds due, largely, to high costs, and commercial interest failed to materialize. “That’s been the major hurdle for a long time,” said Kwak.
Now, however, interest has returned alongside advances in technology and research. The big shift has been the emergence of lower-cost rapid-production mRNA and DNA platforms and a better understanding of how vaccines and potent immune stimulants, like checkpoint inhibitors, can work together to improve outcomes, he explained.
“The timing wasn’t right” back then, Kwak noted. “Now, it’s a different environment and a different time.”
A Turning Point?
Indeed, a decade later, cancer vaccine development appears to be headed in a more promising direction.
Among key cancer vaccines to watch is the mRNA-4157 vaccine, developed by Merck and Moderna, designed to prevent melanoma recurrence. In a recent phase 2 study, patients receiving the mRNA-4157 vaccine alongside pembrolizumab had nearly half the risk for melanoma recurrence or death at 3 years compared with those receiving pembrolizumab alone. Investigators are now evaluating the vaccine in a global phase 3 study in patients with high-risk, stage IIB to IV melanoma following surgery.
Another one to watch is the BNT116 NSCLC vaccine from BioNTech. This vaccine presents the immune system with NSCLC tumor markers to encourage the body to fight cancer cells expressing those markers while ignoring healthy cells. BioNTech also launched a global clinical trial for its vaccine this year.
Other notables include a pancreatic cancer mRNA vaccine, which has shown promising early results in a small trial of 16 patients. Of 16 patients who received the vaccine alongside chemotherapy and after surgery and immunotherapy, 8 responded. Of these eight, six remained recurrence free at 3 years. Investigators noted that the vaccine appeared to stimulate a durable T-cell response in patients who responded.
Kwak has also continued his work on lymphoma vaccines. In August, his team published promising first-in-human data on the use of personalized neoantigen vaccines as an early intervention in untreated patients with lymphoplasmacytic lymphoma. Among nine asymptomatic patients who received the vaccine, all achieved stable disease or better, with no dose-limiting toxicities. One patient had a minor response, and the median time to progression was greater than 72 months.
“The current setting is more for advanced disease,” Kwak explained. “It’s a tougher task, but combined with checkpoint blockade, it may be potent enough to work.”
Still, caution is important. Despite early promise, it’s too soon to tell which, if any, of these investigational vaccines will pan out in the long run. Like investigational drugs, cancer vaccines may show big promising initially but then fail in larger trials.
One key to success, according to Kwak, is to design trials so that even negative results will inform next steps.
But, he noted, failures in large clinical trials will “put a chilling effect on cancer vaccine research again.”
“That’s what keeps me up at night,” he said. “We know the science is fundamentally sound and we have seen glimpses over decades of research that cancer vaccines can work, so it’s really just a matter of tweaking things to optimize trial design.”
Companies tend to design trials to test if a vaccine works or not, without trying to understand why, he said.
“What we need to do is design those so that we can learn from negative results,” he said. That’s what he and his colleagues attempted to do in their recent trial. “We didn’t just look at clinical results; we’re interrogating the actual tumor environment to understand what worked and didn’t and how to tweak that for the next trial.”
Kwak and his colleagues found, for instance, that the vaccine had a greater effect on B cell–derived tumor cells than on cells of plasma origin, so “the most rational design for the next iteration is to combine the vaccine with agents that work directly against plasma cells,” he explained.
As for what’s next, Kwak said: “We’re just focused on trying to do good science and understand. We’ve seen glimpses of success. That’s where we are.”
A version of this article first appeared on Medscape.com.
Vaccines for treating and preventing cancer have long been considered a holy grail in oncology.
But aside from a few notable exceptions — including the human papillomavirus (HPV) vaccine, which has dramatically reduced the incidence of HPV-related cancers, and a Bacillus Calmette-Guerin vaccine, which helps prevent early-stage bladder cancer recurrence — most have failed to deliver.
Following a string of disappointments over the past decade, recent advances in the immunotherapy space are bringing renewed hope for progress.
In an American Association for Cancer Research (AACR) series earlier in 2024, Catherine J. Wu, MD, predicted big strides for cancer vaccines, especially for personalized vaccines that target patient-specific neoantigens — the proteins that form on cancer cells — as well as vaccines that can treat diverse tumor types.
said Wu, the Lavine Family Chair of Preventative Cancer Therapies at Dana-Farber Cancer Institute and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts.
A prime example is a personalized, messenger RNA (mRNA)–based vaccine designed to prevent melanoma recurrence. The mRNA-4157 vaccine encodes up to 34 different patient-specific neoantigens.
“This is one of the most exciting developments in modern cancer therapy,” said Lawrence Young, a virologist and professor of molecular oncology at the University of Warwick, Coventry, England, who commented on the investigational vaccine via the UK-based Science Media Centre.
Other promising options are on the horizon as well. In August, BioNTech announced a phase 1 global trial to study BNT116 — a vaccine to treat non–small cell lung cancer (NSCLC). BNT116, like mRNA-4157, targets specific antigens in the lung cancer cells.
“This technology is the next big phase of cancer treatment,” Siow Ming Lee, MD, a consultant medical oncologist at University College London Hospitals in England, which is leading the UK trial for the lung cancer and melanoma vaccines, told The Guardian. “We are now entering this very exciting new era of mRNA-based immunotherapy clinical trials to investigate the treatment of lung cancer.”
Still, these predictions have a familiar ring. While the prospects are exciting, delivering on them is another story. There are simply no guarantees these strategies will work as hoped.
Then: Where We Were
Cancer vaccine research began to ramp up in the 2000s, and in 2006, the first-generation HPV vaccine, Gardasil, was approved. Gardasil prevents infection from four strains of HPV that cause about 80% of cervical cancer cases.
In 2010, the Food and Drug Administration approved sipuleucel-T, the first therapeutic cancer vaccine, which improved overall survival in patients with hormone-refractory prostate cancer.
Researchers predicted this approval would “pave the way for developing innovative, next generation of vaccines with enhanced antitumor potency.”
In a 2015 AACR research forecast report, Drew Pardoll, MD, PhD, co-director of the Cancer Immunology and Hematopoiesis Program at Johns Hopkins University, Baltimore, Maryland, said that “we can expect to see encouraging results from studies using cancer vaccines.”
Despite the excitement surrounding cancer vaccines alongside a few successes, the next decade brought a longer string of late-phase disappointments.
In 2016, the phase 3 ACT IV trial of a therapeutic vaccine to treat glioblastoma multiforme (CDX-110) was terminated after it failed to demonstrate improved survival.
In 2017, a phase 3 trial of the therapeutic pancreatic cancer vaccine, GVAX, was stopped early for lack of efficacy.
That year, an attenuated Listeria monocytogenes vaccine to treat pancreatic cancer and mesothelioma also failed to come to fruition. In late 2017, concerns over listeria infections prompted Aduro Biotech to cancel its listeria-based cancer treatment program.
In 2018, a phase 3 trial of belagenpumatucel-L, a therapeutic NSCLC vaccine, failed to demonstrate a significant improvement in survival and further study was discontinued.
And in 2019, a vaccine targeting MAGE-A3, a cancer-testis antigen present in multiple tumor types, failed to meet endpoints for improved survival in a phase 3 trial, leading to discontinuation of the vaccine program.
But these disappointments and failures are normal parts of medical research and drug development and have allowed for incremental advances that helped fuel renewed interest and hope for cancer vaccines, when the timing was right, explained vaccine pioneer Larry W. Kwak, MD, PhD, deputy director of the Comprehensive Cancer Center at City of Hope, Duarte, California.
When it comes to vaccine progress, timing makes a difference. In 2011, Kwak and colleagues published promising phase 3 trial results on a personalized vaccine. The vaccine was a patient-specific tumor-derived antigen for patients with follicular lymphoma in their first remission following chemotherapy. Patients who received the vaccine demonstrated significantly longer disease-free survival.
But, at the time, personalized vaccines faced strong headwinds due, largely, to high costs, and commercial interest failed to materialize. “That’s been the major hurdle for a long time,” said Kwak.
Now, however, interest has returned alongside advances in technology and research. The big shift has been the emergence of lower-cost rapid-production mRNA and DNA platforms and a better understanding of how vaccines and potent immune stimulants, like checkpoint inhibitors, can work together to improve outcomes, he explained.
“The timing wasn’t right” back then, Kwak noted. “Now, it’s a different environment and a different time.”
A Turning Point?
Indeed, a decade later, cancer vaccine development appears to be headed in a more promising direction.
Among key cancer vaccines to watch is the mRNA-4157 vaccine, developed by Merck and Moderna, designed to prevent melanoma recurrence. In a recent phase 2 study, patients receiving the mRNA-4157 vaccine alongside pembrolizumab had nearly half the risk for melanoma recurrence or death at 3 years compared with those receiving pembrolizumab alone. Investigators are now evaluating the vaccine in a global phase 3 study in patients with high-risk, stage IIB to IV melanoma following surgery.
Another one to watch is the BNT116 NSCLC vaccine from BioNTech. This vaccine presents the immune system with NSCLC tumor markers to encourage the body to fight cancer cells expressing those markers while ignoring healthy cells. BioNTech also launched a global clinical trial for its vaccine this year.
Other notables include a pancreatic cancer mRNA vaccine, which has shown promising early results in a small trial of 16 patients. Of 16 patients who received the vaccine alongside chemotherapy and after surgery and immunotherapy, 8 responded. Of these eight, six remained recurrence free at 3 years. Investigators noted that the vaccine appeared to stimulate a durable T-cell response in patients who responded.
Kwak has also continued his work on lymphoma vaccines. In August, his team published promising first-in-human data on the use of personalized neoantigen vaccines as an early intervention in untreated patients with lymphoplasmacytic lymphoma. Among nine asymptomatic patients who received the vaccine, all achieved stable disease or better, with no dose-limiting toxicities. One patient had a minor response, and the median time to progression was greater than 72 months.
“The current setting is more for advanced disease,” Kwak explained. “It’s a tougher task, but combined with checkpoint blockade, it may be potent enough to work.”
Still, caution is important. Despite early promise, it’s too soon to tell which, if any, of these investigational vaccines will pan out in the long run. Like investigational drugs, cancer vaccines may show big promising initially but then fail in larger trials.
One key to success, according to Kwak, is to design trials so that even negative results will inform next steps.
But, he noted, failures in large clinical trials will “put a chilling effect on cancer vaccine research again.”
“That’s what keeps me up at night,” he said. “We know the science is fundamentally sound and we have seen glimpses over decades of research that cancer vaccines can work, so it’s really just a matter of tweaking things to optimize trial design.”
Companies tend to design trials to test if a vaccine works or not, without trying to understand why, he said.
“What we need to do is design those so that we can learn from negative results,” he said. That’s what he and his colleagues attempted to do in their recent trial. “We didn’t just look at clinical results; we’re interrogating the actual tumor environment to understand what worked and didn’t and how to tweak that for the next trial.”
Kwak and his colleagues found, for instance, that the vaccine had a greater effect on B cell–derived tumor cells than on cells of plasma origin, so “the most rational design for the next iteration is to combine the vaccine with agents that work directly against plasma cells,” he explained.
As for what’s next, Kwak said: “We’re just focused on trying to do good science and understand. We’ve seen glimpses of success. That’s where we are.”
A version of this article first appeared on Medscape.com.
Vaccines for treating and preventing cancer have long been considered a holy grail in oncology.
But aside from a few notable exceptions — including the human papillomavirus (HPV) vaccine, which has dramatically reduced the incidence of HPV-related cancers, and a Bacillus Calmette-Guerin vaccine, which helps prevent early-stage bladder cancer recurrence — most have failed to deliver.
Following a string of disappointments over the past decade, recent advances in the immunotherapy space are bringing renewed hope for progress.
In an American Association for Cancer Research (AACR) series earlier in 2024, Catherine J. Wu, MD, predicted big strides for cancer vaccines, especially for personalized vaccines that target patient-specific neoantigens — the proteins that form on cancer cells — as well as vaccines that can treat diverse tumor types.
said Wu, the Lavine Family Chair of Preventative Cancer Therapies at Dana-Farber Cancer Institute and a professor of medicine at Harvard Medical School, both in Boston, Massachusetts.
A prime example is a personalized, messenger RNA (mRNA)–based vaccine designed to prevent melanoma recurrence. The mRNA-4157 vaccine encodes up to 34 different patient-specific neoantigens.
“This is one of the most exciting developments in modern cancer therapy,” said Lawrence Young, a virologist and professor of molecular oncology at the University of Warwick, Coventry, England, who commented on the investigational vaccine via the UK-based Science Media Centre.
Other promising options are on the horizon as well. In August, BioNTech announced a phase 1 global trial to study BNT116 — a vaccine to treat non–small cell lung cancer (NSCLC). BNT116, like mRNA-4157, targets specific antigens in the lung cancer cells.
“This technology is the next big phase of cancer treatment,” Siow Ming Lee, MD, a consultant medical oncologist at University College London Hospitals in England, which is leading the UK trial for the lung cancer and melanoma vaccines, told The Guardian. “We are now entering this very exciting new era of mRNA-based immunotherapy clinical trials to investigate the treatment of lung cancer.”
Still, these predictions have a familiar ring. While the prospects are exciting, delivering on them is another story. There are simply no guarantees these strategies will work as hoped.
Then: Where We Were
Cancer vaccine research began to ramp up in the 2000s, and in 2006, the first-generation HPV vaccine, Gardasil, was approved. Gardasil prevents infection from four strains of HPV that cause about 80% of cervical cancer cases.
In 2010, the Food and Drug Administration approved sipuleucel-T, the first therapeutic cancer vaccine, which improved overall survival in patients with hormone-refractory prostate cancer.
Researchers predicted this approval would “pave the way for developing innovative, next generation of vaccines with enhanced antitumor potency.”
In a 2015 AACR research forecast report, Drew Pardoll, MD, PhD, co-director of the Cancer Immunology and Hematopoiesis Program at Johns Hopkins University, Baltimore, Maryland, said that “we can expect to see encouraging results from studies using cancer vaccines.”
Despite the excitement surrounding cancer vaccines alongside a few successes, the next decade brought a longer string of late-phase disappointments.
In 2016, the phase 3 ACT IV trial of a therapeutic vaccine to treat glioblastoma multiforme (CDX-110) was terminated after it failed to demonstrate improved survival.
In 2017, a phase 3 trial of the therapeutic pancreatic cancer vaccine, GVAX, was stopped early for lack of efficacy.
That year, an attenuated Listeria monocytogenes vaccine to treat pancreatic cancer and mesothelioma also failed to come to fruition. In late 2017, concerns over listeria infections prompted Aduro Biotech to cancel its listeria-based cancer treatment program.
In 2018, a phase 3 trial of belagenpumatucel-L, a therapeutic NSCLC vaccine, failed to demonstrate a significant improvement in survival and further study was discontinued.
And in 2019, a vaccine targeting MAGE-A3, a cancer-testis antigen present in multiple tumor types, failed to meet endpoints for improved survival in a phase 3 trial, leading to discontinuation of the vaccine program.
But these disappointments and failures are normal parts of medical research and drug development and have allowed for incremental advances that helped fuel renewed interest and hope for cancer vaccines, when the timing was right, explained vaccine pioneer Larry W. Kwak, MD, PhD, deputy director of the Comprehensive Cancer Center at City of Hope, Duarte, California.
When it comes to vaccine progress, timing makes a difference. In 2011, Kwak and colleagues published promising phase 3 trial results on a personalized vaccine. The vaccine was a patient-specific tumor-derived antigen for patients with follicular lymphoma in their first remission following chemotherapy. Patients who received the vaccine demonstrated significantly longer disease-free survival.
But, at the time, personalized vaccines faced strong headwinds due, largely, to high costs, and commercial interest failed to materialize. “That’s been the major hurdle for a long time,” said Kwak.
Now, however, interest has returned alongside advances in technology and research. The big shift has been the emergence of lower-cost rapid-production mRNA and DNA platforms and a better understanding of how vaccines and potent immune stimulants, like checkpoint inhibitors, can work together to improve outcomes, he explained.
“The timing wasn’t right” back then, Kwak noted. “Now, it’s a different environment and a different time.”
A Turning Point?
Indeed, a decade later, cancer vaccine development appears to be headed in a more promising direction.
Among key cancer vaccines to watch is the mRNA-4157 vaccine, developed by Merck and Moderna, designed to prevent melanoma recurrence. In a recent phase 2 study, patients receiving the mRNA-4157 vaccine alongside pembrolizumab had nearly half the risk for melanoma recurrence or death at 3 years compared with those receiving pembrolizumab alone. Investigators are now evaluating the vaccine in a global phase 3 study in patients with high-risk, stage IIB to IV melanoma following surgery.
Another one to watch is the BNT116 NSCLC vaccine from BioNTech. This vaccine presents the immune system with NSCLC tumor markers to encourage the body to fight cancer cells expressing those markers while ignoring healthy cells. BioNTech also launched a global clinical trial for its vaccine this year.
Other notables include a pancreatic cancer mRNA vaccine, which has shown promising early results in a small trial of 16 patients. Of 16 patients who received the vaccine alongside chemotherapy and after surgery and immunotherapy, 8 responded. Of these eight, six remained recurrence free at 3 years. Investigators noted that the vaccine appeared to stimulate a durable T-cell response in patients who responded.
Kwak has also continued his work on lymphoma vaccines. In August, his team published promising first-in-human data on the use of personalized neoantigen vaccines as an early intervention in untreated patients with lymphoplasmacytic lymphoma. Among nine asymptomatic patients who received the vaccine, all achieved stable disease or better, with no dose-limiting toxicities. One patient had a minor response, and the median time to progression was greater than 72 months.
“The current setting is more for advanced disease,” Kwak explained. “It’s a tougher task, but combined with checkpoint blockade, it may be potent enough to work.”
Still, caution is important. Despite early promise, it’s too soon to tell which, if any, of these investigational vaccines will pan out in the long run. Like investigational drugs, cancer vaccines may show big promising initially but then fail in larger trials.
One key to success, according to Kwak, is to design trials so that even negative results will inform next steps.
But, he noted, failures in large clinical trials will “put a chilling effect on cancer vaccine research again.”
“That’s what keeps me up at night,” he said. “We know the science is fundamentally sound and we have seen glimpses over decades of research that cancer vaccines can work, so it’s really just a matter of tweaking things to optimize trial design.”
Companies tend to design trials to test if a vaccine works or not, without trying to understand why, he said.
“What we need to do is design those so that we can learn from negative results,” he said. That’s what he and his colleagues attempted to do in their recent trial. “We didn’t just look at clinical results; we’re interrogating the actual tumor environment to understand what worked and didn’t and how to tweak that for the next trial.”
Kwak and his colleagues found, for instance, that the vaccine had a greater effect on B cell–derived tumor cells than on cells of plasma origin, so “the most rational design for the next iteration is to combine the vaccine with agents that work directly against plasma cells,” he explained.
As for what’s next, Kwak said: “We’re just focused on trying to do good science and understand. We’ve seen glimpses of success. That’s where we are.”
A version of this article first appeared on Medscape.com.
National Noncompete Ban Unlikely to Survive Under Trump, Experts Say
Even before the presidential election, the Federal Trade Commission’s (FTC) national ban on noncompete clauses faced a tough battle for survival in the courts.
Now, legal specialists forecast a grim prognosis for the ban under Donald Trump’s return to the White House.
But a federal district’s court ruling put the ban on hold, and the Trump administration isn’t expected to support lifting the ban.
“It is likely that the Trump administration will decline to defend the rule and may not even appeal the district court’s ruling, which means that the ban on noncompetes will not go into effect,” Steven Lubet, JD, a professor emeritus at Northwestern University Pritzker School of Law, Chicago, Illinois, said in an interview.
What’s in a Noncompete Clause?
Noncompete clauses in employee contracts typically restrict when and where workers can take future jobs. In medicine, supporters argue that the clauses are fair. Hospitals and practices provide a base of patients to physicians, they say, in return for their agreement not to go work for a competitor.
But those opposed to these clauses argue that the restrictions harm careers and hurt patients by unfairly preventing physicians from moving to new jobs where they’re needed.
At an April meeting, the FTC board voted 3 to 2 to ban noncompete clauses; some nonprofit organizations and senior executives were expected to be exempt. The FTC estimated that the move would save the healthcare system alone as much as $194 billion over 10 years.
“A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” declared FTC Commissioner Alvaro Bedoya.
Hospitals protested the move. In a statement, the general counsel for the American Hospital Association called it “bad law, bad policy, and a clear sign of an agency run amok” and said the FTC ignored “mountains of contrary legal precedent and evidence about its adverse impacts on the health care markets.”
Although the American Medical Association does not support a total ban, its House of Delegates adopted policies in 2023 to support the prohibition of noncompete contracts for physicians employed by for-profit and nonprofit hospitals, hospital systems, or staffing companies.
Texas Federal Judge Intervenes to Halt Ban
The ban was supposed to take effect on Sept. 4, 2024. But Texas federal judge Ada E. Brown struck down the ban in an Aug. 20 decision. She ruled that the FTC went beyond its authority.
“The district court based its ruling on a very dubious distinction between ‘unfair practices,’ which the FTC may prohibit, and ‘unfair competition,’ which, according to the court, it may not,” said Lubet.
In fact, the ban should stand, he said. “This is a classic case of the government intervening on behalf of consumers/patients by prohibiting an unfair and harmful employment practice,” Lubet said.
Amanda Hill, an attorney in Austin, Texas, who trains physicians about how to negotiate contracts, has a different take. “The Federal Trade Commission came down hard, and honestly, it really overstepped,” she said in an interview. “Congress needs to write laws, not regulatory bodies. I think all the lawyers went: ‘Good try, but you’re not going to get anywhere with that.’ ”
She noted that physicians themselves are divided over the value of noncompete clauses. “I would say 80% of my clients can’t stand noncompetes.” But another 20% own their own practices and hate the idea of losing their physicians to competitors, she said.
Trump Isn’t Seen as Likely to Support Ban
While the Biden administration firmly supported a ban on noncompete clauses, there isn’t a strict Democratic-Republican divide over whether the agreements are a good idea. Some red states have embraced bans, and Hill said this can make sense from a Republican point of view: “We don’t want to run doctors out of town and out of the state because they think they’re going to be bound by big hospitals and corporate interests.”
In fact, former Florida congressman Matt Gaetz, a Republican briefly tapped as President-elect Trump’s nominee for attorney general, supports noncompete clauses. He filed a friend-of-the-court brief with the Texas judge that supported the FTC’s ruling, saying it is a “vindication of economic freedom and free enterprise.”
But Republicans generally “believe that federal agencies are going too far and beyond the power granted to them by Congress,” Atlanta, Georgia, attorney Benjamin Fink, Esq., said in an interview.
And Trump is no fan of the FTC and its chair, Lina Khan, who may step down. Observers don’t expect that the Trump administration or a newly constituted FTC board will support an appeal of the Texas judge’s ruling.
“I don’t think anybody else — another agency or a private party — could step in place of the FTC if the FTC declines to defend the ban,” Atlanta attorney Neal F. Weinrich, Esq., said in an interview. In that case, “I think it ends.”
Attorneys Weinrich and Fink work at the same firm, which handles noncompete agreements for physicians.
Noncompete Ban Advocates Turn to States
Even if Kamala Harris had won the presidency, a national ban on noncompete clauses would have faced an uphill battle at the Supreme Court.
“The Supreme Court majority has been unsympathetic to administrative agencies, interpreting their authority very narrowly,” said Lubet.
So what happens to noncompete clauses now? While bipartisan bills in Congress have tried to ban them, legislation is unlikely to pass now that Republicans will control both the House and Senate, Fink said.
According to a recent article, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
“I definitely think states are going to continue to restrict the use of noncompetes,” Fink said.
Lubet has no disclosures. Hill, Fink, and Weinrich represent physicians in contract negotiations.
A version of this article appeared on Medscape.com.
Even before the presidential election, the Federal Trade Commission’s (FTC) national ban on noncompete clauses faced a tough battle for survival in the courts.
Now, legal specialists forecast a grim prognosis for the ban under Donald Trump’s return to the White House.
But a federal district’s court ruling put the ban on hold, and the Trump administration isn’t expected to support lifting the ban.
“It is likely that the Trump administration will decline to defend the rule and may not even appeal the district court’s ruling, which means that the ban on noncompetes will not go into effect,” Steven Lubet, JD, a professor emeritus at Northwestern University Pritzker School of Law, Chicago, Illinois, said in an interview.
What’s in a Noncompete Clause?
Noncompete clauses in employee contracts typically restrict when and where workers can take future jobs. In medicine, supporters argue that the clauses are fair. Hospitals and practices provide a base of patients to physicians, they say, in return for their agreement not to go work for a competitor.
But those opposed to these clauses argue that the restrictions harm careers and hurt patients by unfairly preventing physicians from moving to new jobs where they’re needed.
At an April meeting, the FTC board voted 3 to 2 to ban noncompete clauses; some nonprofit organizations and senior executives were expected to be exempt. The FTC estimated that the move would save the healthcare system alone as much as $194 billion over 10 years.
“A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” declared FTC Commissioner Alvaro Bedoya.
Hospitals protested the move. In a statement, the general counsel for the American Hospital Association called it “bad law, bad policy, and a clear sign of an agency run amok” and said the FTC ignored “mountains of contrary legal precedent and evidence about its adverse impacts on the health care markets.”
Although the American Medical Association does not support a total ban, its House of Delegates adopted policies in 2023 to support the prohibition of noncompete contracts for physicians employed by for-profit and nonprofit hospitals, hospital systems, or staffing companies.
Texas Federal Judge Intervenes to Halt Ban
The ban was supposed to take effect on Sept. 4, 2024. But Texas federal judge Ada E. Brown struck down the ban in an Aug. 20 decision. She ruled that the FTC went beyond its authority.
“The district court based its ruling on a very dubious distinction between ‘unfair practices,’ which the FTC may prohibit, and ‘unfair competition,’ which, according to the court, it may not,” said Lubet.
In fact, the ban should stand, he said. “This is a classic case of the government intervening on behalf of consumers/patients by prohibiting an unfair and harmful employment practice,” Lubet said.
Amanda Hill, an attorney in Austin, Texas, who trains physicians about how to negotiate contracts, has a different take. “The Federal Trade Commission came down hard, and honestly, it really overstepped,” she said in an interview. “Congress needs to write laws, not regulatory bodies. I think all the lawyers went: ‘Good try, but you’re not going to get anywhere with that.’ ”
She noted that physicians themselves are divided over the value of noncompete clauses. “I would say 80% of my clients can’t stand noncompetes.” But another 20% own their own practices and hate the idea of losing their physicians to competitors, she said.
Trump Isn’t Seen as Likely to Support Ban
While the Biden administration firmly supported a ban on noncompete clauses, there isn’t a strict Democratic-Republican divide over whether the agreements are a good idea. Some red states have embraced bans, and Hill said this can make sense from a Republican point of view: “We don’t want to run doctors out of town and out of the state because they think they’re going to be bound by big hospitals and corporate interests.”
In fact, former Florida congressman Matt Gaetz, a Republican briefly tapped as President-elect Trump’s nominee for attorney general, supports noncompete clauses. He filed a friend-of-the-court brief with the Texas judge that supported the FTC’s ruling, saying it is a “vindication of economic freedom and free enterprise.”
But Republicans generally “believe that federal agencies are going too far and beyond the power granted to them by Congress,” Atlanta, Georgia, attorney Benjamin Fink, Esq., said in an interview.
And Trump is no fan of the FTC and its chair, Lina Khan, who may step down. Observers don’t expect that the Trump administration or a newly constituted FTC board will support an appeal of the Texas judge’s ruling.
“I don’t think anybody else — another agency or a private party — could step in place of the FTC if the FTC declines to defend the ban,” Atlanta attorney Neal F. Weinrich, Esq., said in an interview. In that case, “I think it ends.”
Attorneys Weinrich and Fink work at the same firm, which handles noncompete agreements for physicians.
Noncompete Ban Advocates Turn to States
Even if Kamala Harris had won the presidency, a national ban on noncompete clauses would have faced an uphill battle at the Supreme Court.
“The Supreme Court majority has been unsympathetic to administrative agencies, interpreting their authority very narrowly,” said Lubet.
So what happens to noncompete clauses now? While bipartisan bills in Congress have tried to ban them, legislation is unlikely to pass now that Republicans will control both the House and Senate, Fink said.
According to a recent article, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
“I definitely think states are going to continue to restrict the use of noncompetes,” Fink said.
Lubet has no disclosures. Hill, Fink, and Weinrich represent physicians in contract negotiations.
A version of this article appeared on Medscape.com.
Even before the presidential election, the Federal Trade Commission’s (FTC) national ban on noncompete clauses faced a tough battle for survival in the courts.
Now, legal specialists forecast a grim prognosis for the ban under Donald Trump’s return to the White House.
But a federal district’s court ruling put the ban on hold, and the Trump administration isn’t expected to support lifting the ban.
“It is likely that the Trump administration will decline to defend the rule and may not even appeal the district court’s ruling, which means that the ban on noncompetes will not go into effect,” Steven Lubet, JD, a professor emeritus at Northwestern University Pritzker School of Law, Chicago, Illinois, said in an interview.
What’s in a Noncompete Clause?
Noncompete clauses in employee contracts typically restrict when and where workers can take future jobs. In medicine, supporters argue that the clauses are fair. Hospitals and practices provide a base of patients to physicians, they say, in return for their agreement not to go work for a competitor.
But those opposed to these clauses argue that the restrictions harm careers and hurt patients by unfairly preventing physicians from moving to new jobs where they’re needed.
At an April meeting, the FTC board voted 3 to 2 to ban noncompete clauses; some nonprofit organizations and senior executives were expected to be exempt. The FTC estimated that the move would save the healthcare system alone as much as $194 billion over 10 years.
“A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” declared FTC Commissioner Alvaro Bedoya.
Hospitals protested the move. In a statement, the general counsel for the American Hospital Association called it “bad law, bad policy, and a clear sign of an agency run amok” and said the FTC ignored “mountains of contrary legal precedent and evidence about its adverse impacts on the health care markets.”
Although the American Medical Association does not support a total ban, its House of Delegates adopted policies in 2023 to support the prohibition of noncompete contracts for physicians employed by for-profit and nonprofit hospitals, hospital systems, or staffing companies.
Texas Federal Judge Intervenes to Halt Ban
The ban was supposed to take effect on Sept. 4, 2024. But Texas federal judge Ada E. Brown struck down the ban in an Aug. 20 decision. She ruled that the FTC went beyond its authority.
“The district court based its ruling on a very dubious distinction between ‘unfair practices,’ which the FTC may prohibit, and ‘unfair competition,’ which, according to the court, it may not,” said Lubet.
In fact, the ban should stand, he said. “This is a classic case of the government intervening on behalf of consumers/patients by prohibiting an unfair and harmful employment practice,” Lubet said.
Amanda Hill, an attorney in Austin, Texas, who trains physicians about how to negotiate contracts, has a different take. “The Federal Trade Commission came down hard, and honestly, it really overstepped,” she said in an interview. “Congress needs to write laws, not regulatory bodies. I think all the lawyers went: ‘Good try, but you’re not going to get anywhere with that.’ ”
She noted that physicians themselves are divided over the value of noncompete clauses. “I would say 80% of my clients can’t stand noncompetes.” But another 20% own their own practices and hate the idea of losing their physicians to competitors, she said.
Trump Isn’t Seen as Likely to Support Ban
While the Biden administration firmly supported a ban on noncompete clauses, there isn’t a strict Democratic-Republican divide over whether the agreements are a good idea. Some red states have embraced bans, and Hill said this can make sense from a Republican point of view: “We don’t want to run doctors out of town and out of the state because they think they’re going to be bound by big hospitals and corporate interests.”
In fact, former Florida congressman Matt Gaetz, a Republican briefly tapped as President-elect Trump’s nominee for attorney general, supports noncompete clauses. He filed a friend-of-the-court brief with the Texas judge that supported the FTC’s ruling, saying it is a “vindication of economic freedom and free enterprise.”
But Republicans generally “believe that federal agencies are going too far and beyond the power granted to them by Congress,” Atlanta, Georgia, attorney Benjamin Fink, Esq., said in an interview.
And Trump is no fan of the FTC and its chair, Lina Khan, who may step down. Observers don’t expect that the Trump administration or a newly constituted FTC board will support an appeal of the Texas judge’s ruling.
“I don’t think anybody else — another agency or a private party — could step in place of the FTC if the FTC declines to defend the ban,” Atlanta attorney Neal F. Weinrich, Esq., said in an interview. In that case, “I think it ends.”
Attorneys Weinrich and Fink work at the same firm, which handles noncompete agreements for physicians.
Noncompete Ban Advocates Turn to States
Even if Kamala Harris had won the presidency, a national ban on noncompete clauses would have faced an uphill battle at the Supreme Court.
“The Supreme Court majority has been unsympathetic to administrative agencies, interpreting their authority very narrowly,” said Lubet.
So what happens to noncompete clauses now? While bipartisan bills in Congress have tried to ban them, legislation is unlikely to pass now that Republicans will control both the House and Senate, Fink said.
According to a recent article, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
“I definitely think states are going to continue to restrict the use of noncompetes,” Fink said.
Lubet has no disclosures. Hill, Fink, and Weinrich represent physicians in contract negotiations.
A version of this article appeared on Medscape.com.
Most US Adults Plan to Skip Annual COVID Vaccines
Most US adults continue to plan on skipping an annual COVID vaccine.
according to results of a new survey from the Pew Research Center.
When asked why people wouldn’t get an updated COVID vaccine, 61% said a major reason was that they don’t think they need it, and 60% said a major reason is that they are concerned about side effects. Cost was a factor for 14% of people, and 46% of people said they don’t get vaccines in general.
There were some differences in intention to get vaccinated based on a person’s age. Among people ages 65 and older, 27% said they had already gotten the vaccine, and another 27% said they probably will get the shot, leaving 45% who said they probably won’t roll up their sleeves. People ages 30-49 years old were the least likely to plan on getting a COVID shot – 66% said they probably won’t get one.
Public health officials say everyone should get an annual COVID vaccine, just as they should get a flu shot, because the vaccines are formulated each year to target virus strains predicted to be in wide circulation. Also, immunity – either from past vaccination or past infection – wanes over time.
Research shows that the vaccines reduce the likelihood of hospitalization or death caused by severe illness, particularly among people who have risk factors, like being over age 65 or having health issues that are becoming increasingly common in the United States, like diabetes, heart problems, and lung conditions.
The survey included 9,593 adults who were asked about their COVID vaccine intentions with this question: “Public health officials recently recommended an updated vaccine for COVID-19. Do you think you will probably get an updated vaccine, probably not get an updated vaccine, or have you already received an updated vaccine?” The survey was done online and by telephone from October 21 to October 27.
So far in 2024, the CDC’s ongoing immunization survey shows that 17% of adults say that, as of November 2, they have gotten vaccinated for COVID-19 this season, and 14% said they will definitely get vaccinated. The Pew Research Center survey found that 15% of people said they’ve already gotten the shot this season.
Reports of positive COVID tests, emergency room visits, and hospitalizations remain very low. About 3.6% of test results shared with the CDC were positive for COVID the week ending November 9. Less than 1% of ER visits involve a COVID diagnosis, and hospitalizations are well below the rate seen at this time last year. Last year, COVID activity in the United States began rising around Thanksgiving and continued upward, peaking in early January.
The protection from a COVID-19 vaccination usually fully kicks in about 2 weeks after you get the shot, and the vaccines are most effective for the following 3 months.
A version of this article first appeared on WebMD.com.
Most US adults continue to plan on skipping an annual COVID vaccine.
according to results of a new survey from the Pew Research Center.
When asked why people wouldn’t get an updated COVID vaccine, 61% said a major reason was that they don’t think they need it, and 60% said a major reason is that they are concerned about side effects. Cost was a factor for 14% of people, and 46% of people said they don’t get vaccines in general.
There were some differences in intention to get vaccinated based on a person’s age. Among people ages 65 and older, 27% said they had already gotten the vaccine, and another 27% said they probably will get the shot, leaving 45% who said they probably won’t roll up their sleeves. People ages 30-49 years old were the least likely to plan on getting a COVID shot – 66% said they probably won’t get one.
Public health officials say everyone should get an annual COVID vaccine, just as they should get a flu shot, because the vaccines are formulated each year to target virus strains predicted to be in wide circulation. Also, immunity – either from past vaccination or past infection – wanes over time.
Research shows that the vaccines reduce the likelihood of hospitalization or death caused by severe illness, particularly among people who have risk factors, like being over age 65 or having health issues that are becoming increasingly common in the United States, like diabetes, heart problems, and lung conditions.
The survey included 9,593 adults who were asked about their COVID vaccine intentions with this question: “Public health officials recently recommended an updated vaccine for COVID-19. Do you think you will probably get an updated vaccine, probably not get an updated vaccine, or have you already received an updated vaccine?” The survey was done online and by telephone from October 21 to October 27.
So far in 2024, the CDC’s ongoing immunization survey shows that 17% of adults say that, as of November 2, they have gotten vaccinated for COVID-19 this season, and 14% said they will definitely get vaccinated. The Pew Research Center survey found that 15% of people said they’ve already gotten the shot this season.
Reports of positive COVID tests, emergency room visits, and hospitalizations remain very low. About 3.6% of test results shared with the CDC were positive for COVID the week ending November 9. Less than 1% of ER visits involve a COVID diagnosis, and hospitalizations are well below the rate seen at this time last year. Last year, COVID activity in the United States began rising around Thanksgiving and continued upward, peaking in early January.
The protection from a COVID-19 vaccination usually fully kicks in about 2 weeks after you get the shot, and the vaccines are most effective for the following 3 months.
A version of this article first appeared on WebMD.com.
Most US adults continue to plan on skipping an annual COVID vaccine.
according to results of a new survey from the Pew Research Center.
When asked why people wouldn’t get an updated COVID vaccine, 61% said a major reason was that they don’t think they need it, and 60% said a major reason is that they are concerned about side effects. Cost was a factor for 14% of people, and 46% of people said they don’t get vaccines in general.
There were some differences in intention to get vaccinated based on a person’s age. Among people ages 65 and older, 27% said they had already gotten the vaccine, and another 27% said they probably will get the shot, leaving 45% who said they probably won’t roll up their sleeves. People ages 30-49 years old were the least likely to plan on getting a COVID shot – 66% said they probably won’t get one.
Public health officials say everyone should get an annual COVID vaccine, just as they should get a flu shot, because the vaccines are formulated each year to target virus strains predicted to be in wide circulation. Also, immunity – either from past vaccination or past infection – wanes over time.
Research shows that the vaccines reduce the likelihood of hospitalization or death caused by severe illness, particularly among people who have risk factors, like being over age 65 or having health issues that are becoming increasingly common in the United States, like diabetes, heart problems, and lung conditions.
The survey included 9,593 adults who were asked about their COVID vaccine intentions with this question: “Public health officials recently recommended an updated vaccine for COVID-19. Do you think you will probably get an updated vaccine, probably not get an updated vaccine, or have you already received an updated vaccine?” The survey was done online and by telephone from October 21 to October 27.
So far in 2024, the CDC’s ongoing immunization survey shows that 17% of adults say that, as of November 2, they have gotten vaccinated for COVID-19 this season, and 14% said they will definitely get vaccinated. The Pew Research Center survey found that 15% of people said they’ve already gotten the shot this season.
Reports of positive COVID tests, emergency room visits, and hospitalizations remain very low. About 3.6% of test results shared with the CDC were positive for COVID the week ending November 9. Less than 1% of ER visits involve a COVID diagnosis, and hospitalizations are well below the rate seen at this time last year. Last year, COVID activity in the United States began rising around Thanksgiving and continued upward, peaking in early January.
The protection from a COVID-19 vaccination usually fully kicks in about 2 weeks after you get the shot, and the vaccines are most effective for the following 3 months.
A version of this article first appeared on WebMD.com.
BCG Vaccine May Protect Against Long COVID Symptoms
TOPLINE:
METHODOLOGY:
- A phase 3 clinical trial initiated in early 2020 investigated the effect of the BCG vaccine injected during active infection on COVID-19 progression in adults with mild or moderate COVID-19. The current study summarizes the 6- and 12-month follow-up data with a focus on long-COVID symptoms.
- Patients who tested positive for severe acute respiratory syndrome coronavirus 2 were randomly assigned to receive either 0.1 mL of intradermal BCG (n = 191) or 0.9% saline placebo (n = 202) within 14 days of symptom onset and were followed up at 7, 14, 21, and 45 days and at 6 and 12 months postinjection.
- Overall, 157 BCG (median age, 40 years; 54.1% women) and 142 placebo (median age, 41 years; 65.5% women) recipients completed the 6-month follow-up, and 97 BCG (median age, 37 years; 49.5% women) and 95 placebo (median age, 40 years; 67.4% women) recipients completed the 12-month follow-up.
- The researchers primarily assessed the effect of the BCG vaccine on the development of the symptoms of long COVID at 6 and 12 months.
TAKEAWAY:
- Hearing problems were less frequent among BCG recipients at 6 months compared with those who received placebo (odds ratio [OR], 0.26; 95% CI, 0.045-1.0; P = .044).
- At 12 months, participants who received the BCG vaccine exhibited fewer issues with sleeping (P = .027), concentration (P = .009), memory (P = .009), and vision (P = .022) along with a lower long-COVID score (one-sided Wilcoxon test, P = .002) than those who received placebo.
- At 6 months, BCG demonstrated a sex-specific paradoxical effect on hair loss, decreasing it in men (P = .031), while causing a slight, though statistically nonsignificant, increase in women.
- Male sex was the strongest predictive factor for long COVID, cognitive dysfunction, and cardiopulmonary scores at both follow-up assessments.
IN PRACTICE:
“[The study] findings suggest that BCG immunotherapy for an existing ailment may be superior to prophylaxis in healthy individuals,” the authors wrote.
SOURCE:
The study was led by Mehrsa Jalalizadeh and Keini Buosi, UroScience, State University of Campinas, Unicamp, São Paulo, Brazil. It was published online on November 19, 2024, in the Journal of Internal Medicine.
LIMITATIONS:
Previous mycobacterial exposure was not tested among the study participants. A notable loss to follow-up, particularly at 12 months, may have introduced bias into the results.
DISCLOSURES:
The study was supported by the Coordination for the Improvement of Higher Education Personnel, Federal Government of Brazil, the General Coordination of the National Immunization Program, Ministry of Health (Brazil), and the National Council for Scientific and Technological Development-Research Productivity. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A phase 3 clinical trial initiated in early 2020 investigated the effect of the BCG vaccine injected during active infection on COVID-19 progression in adults with mild or moderate COVID-19. The current study summarizes the 6- and 12-month follow-up data with a focus on long-COVID symptoms.
- Patients who tested positive for severe acute respiratory syndrome coronavirus 2 were randomly assigned to receive either 0.1 mL of intradermal BCG (n = 191) or 0.9% saline placebo (n = 202) within 14 days of symptom onset and were followed up at 7, 14, 21, and 45 days and at 6 and 12 months postinjection.
- Overall, 157 BCG (median age, 40 years; 54.1% women) and 142 placebo (median age, 41 years; 65.5% women) recipients completed the 6-month follow-up, and 97 BCG (median age, 37 years; 49.5% women) and 95 placebo (median age, 40 years; 67.4% women) recipients completed the 12-month follow-up.
- The researchers primarily assessed the effect of the BCG vaccine on the development of the symptoms of long COVID at 6 and 12 months.
TAKEAWAY:
- Hearing problems were less frequent among BCG recipients at 6 months compared with those who received placebo (odds ratio [OR], 0.26; 95% CI, 0.045-1.0; P = .044).
- At 12 months, participants who received the BCG vaccine exhibited fewer issues with sleeping (P = .027), concentration (P = .009), memory (P = .009), and vision (P = .022) along with a lower long-COVID score (one-sided Wilcoxon test, P = .002) than those who received placebo.
- At 6 months, BCG demonstrated a sex-specific paradoxical effect on hair loss, decreasing it in men (P = .031), while causing a slight, though statistically nonsignificant, increase in women.
- Male sex was the strongest predictive factor for long COVID, cognitive dysfunction, and cardiopulmonary scores at both follow-up assessments.
IN PRACTICE:
“[The study] findings suggest that BCG immunotherapy for an existing ailment may be superior to prophylaxis in healthy individuals,” the authors wrote.
SOURCE:
The study was led by Mehrsa Jalalizadeh and Keini Buosi, UroScience, State University of Campinas, Unicamp, São Paulo, Brazil. It was published online on November 19, 2024, in the Journal of Internal Medicine.
LIMITATIONS:
Previous mycobacterial exposure was not tested among the study participants. A notable loss to follow-up, particularly at 12 months, may have introduced bias into the results.
DISCLOSURES:
The study was supported by the Coordination for the Improvement of Higher Education Personnel, Federal Government of Brazil, the General Coordination of the National Immunization Program, Ministry of Health (Brazil), and the National Council for Scientific and Technological Development-Research Productivity. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A phase 3 clinical trial initiated in early 2020 investigated the effect of the BCG vaccine injected during active infection on COVID-19 progression in adults with mild or moderate COVID-19. The current study summarizes the 6- and 12-month follow-up data with a focus on long-COVID symptoms.
- Patients who tested positive for severe acute respiratory syndrome coronavirus 2 were randomly assigned to receive either 0.1 mL of intradermal BCG (n = 191) or 0.9% saline placebo (n = 202) within 14 days of symptom onset and were followed up at 7, 14, 21, and 45 days and at 6 and 12 months postinjection.
- Overall, 157 BCG (median age, 40 years; 54.1% women) and 142 placebo (median age, 41 years; 65.5% women) recipients completed the 6-month follow-up, and 97 BCG (median age, 37 years; 49.5% women) and 95 placebo (median age, 40 years; 67.4% women) recipients completed the 12-month follow-up.
- The researchers primarily assessed the effect of the BCG vaccine on the development of the symptoms of long COVID at 6 and 12 months.
TAKEAWAY:
- Hearing problems were less frequent among BCG recipients at 6 months compared with those who received placebo (odds ratio [OR], 0.26; 95% CI, 0.045-1.0; P = .044).
- At 12 months, participants who received the BCG vaccine exhibited fewer issues with sleeping (P = .027), concentration (P = .009), memory (P = .009), and vision (P = .022) along with a lower long-COVID score (one-sided Wilcoxon test, P = .002) than those who received placebo.
- At 6 months, BCG demonstrated a sex-specific paradoxical effect on hair loss, decreasing it in men (P = .031), while causing a slight, though statistically nonsignificant, increase in women.
- Male sex was the strongest predictive factor for long COVID, cognitive dysfunction, and cardiopulmonary scores at both follow-up assessments.
IN PRACTICE:
“[The study] findings suggest that BCG immunotherapy for an existing ailment may be superior to prophylaxis in healthy individuals,” the authors wrote.
SOURCE:
The study was led by Mehrsa Jalalizadeh and Keini Buosi, UroScience, State University of Campinas, Unicamp, São Paulo, Brazil. It was published online on November 19, 2024, in the Journal of Internal Medicine.
LIMITATIONS:
Previous mycobacterial exposure was not tested among the study participants. A notable loss to follow-up, particularly at 12 months, may have introduced bias into the results.
DISCLOSURES:
The study was supported by the Coordination for the Improvement of Higher Education Personnel, Federal Government of Brazil, the General Coordination of the National Immunization Program, Ministry of Health (Brazil), and the National Council for Scientific and Technological Development-Research Productivity. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
How Metals Affect the Brain
This transcript has been edited for clarity.
It has always amazed me that our bodies require these tiny amounts of incredibly rare substances to function. Sure, we need oxygen. We need water. But we also need molybdenum, which makes up just 1.2 parts per million of the Earth’s crust.
Without adequate molybdenum intake, we develop seizures, developmental delays, death. Fortunately, we need so little molybdenum that true molybdenum deficiency is incredibly rare — seen only in people on total parenteral nutrition without supplementation or those with certain rare genetic conditions. But still, molybdenum is necessary for life.
Many metals are. Figure 1 colors the essential minerals on the periodic table. You can see that to stay alive, we humans need not only things like sodium, but selenium, bromine, zinc, copper, and cobalt.
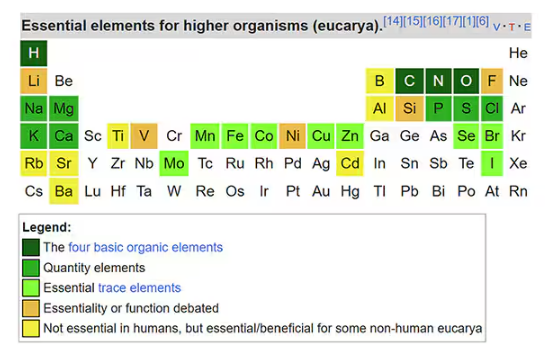
Some metals are very clearly not essential; we can all do without lead and mercury, and probably should.
But just because something is essential for life does not mean that more is better. The dose is the poison, as they say. And this week, we explore whether metals — even essential metals — might be adversely affecting our brains.
It’s not a stretch to think that metal intake could have weird effects on our nervous system. Lead exposure, primarily due to leaded gasoline, has been blamed for an average reduction of about 3 points in our national IQ, for example . But not all metals are created equal. Researchers set out to find out which might be more strongly associated with performance on cognitive tests and dementia, and reported their results in this study in JAMA Network Open.
To do this, they leveraged the MESA cohort study. This is a longitudinal study of a relatively diverse group of 6300 adults who were enrolled from 2000 to 2002 around the United States. At enrollment, they gave a urine sample and took a variety of cognitive tests. Important for this study was the digit symbol substitution test, where participants are provided a code and need to replace a list of numbers with symbols as per that code. Performance on this test worsens with age, depression, and cognitive impairment.
Participants were followed for more than a decade, and over that time, 559 (about 9%) were diagnosed with dementia.
Those baseline urine samples were assayed for a variety of metals — some essential, some very much not, as you can see in Figure 2.

Now, I have to put my kidney doctor hat on for a second and talk about urine measurement ... of anything. The problem with urine is that the concentration can change a lot — by more than 10-fold, in fact — based on how much water you drank recently. Researchers correct for this, and in the case of this study, they do what a lot of researchers do: divide the measured concentration by the urine creatinine level.
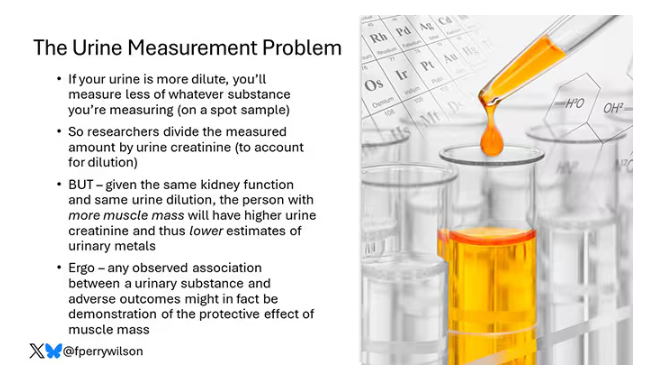
This introduces a bit of a problem. Take two people with exactly the same kidney function, who drank exactly the same water, whose urine is exactly the same concentration. The person with more muscle mass will have more creatinine in that urine sample, since creatinine is a byproduct of muscle metabolism. Because people with more muscle mass are generally healthier, when you divide your metal concentration by urine creatinine, you get a lower number, which might lead you to believe that lower levels of the metal in the urine are protective. But in fact, what you’re seeing is that higher levels of creatinine are protective. I see this issue all the time and it will always color results of studies like this.
Okay, I am doffing my kidney doctor hat now to show you the results.
The researchers first looked at the relationship between metal concentrations in the urine and performance on cognitive tests. The results were fairly equivocal, save for that digit substitution test which is shown in Figure 4.
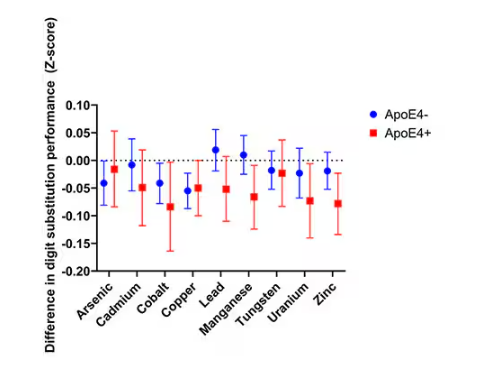
Even these results don’t ring major alarm bells for me. What you’re seeing here is the change in scores on the digit substitution test for each 25-percentile increase in urinary metal level — a pretty big change. And yet, you see really minor changes in the performance on the test. The digit substitution test is not an IQ test; but to give you a feeling for the magnitude of this change, if we looked at copper level, moving from the 25th to the 50th percentile would be associated with a loss of nine tenths of an IQ point.
You see two colors on the Figure 4 graph, by the way. That’s because the researchers stratified their findings based on whether the individual carried the ApoE4 gene allele, which is a risk factor for the development of dementia. There are reasons to believe that neurotoxic metals might be worse in this population, and I suppose you do see generally more adverse effects on scores in the red lines compared with the blue lines. But still, we’re not talking about a huge effect size here.
Let’s look at the relationship between these metals and the development of dementia itself, a clearly more important outcome than how well you can replace numeric digits with symbols. I’ll highlight a few of the results that are particularly telling.
First, the nonessential mineral cadmium, which displays the type of relationship we would expect if the metal were neurotoxic: a clear, roughly linear increase in risk for dementia as urinary concentration increases.
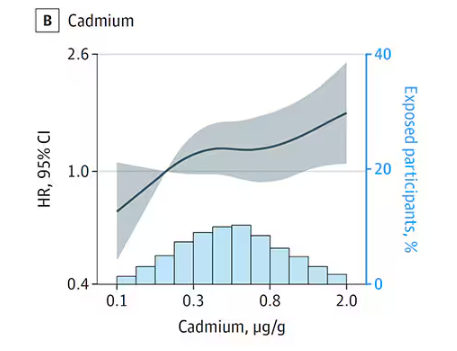
We see roughly similar patterns with the nonessential minerals tungsten and uranium, and the essential mineral zinc (beloved of respiratory-virus avoiders everywhere).
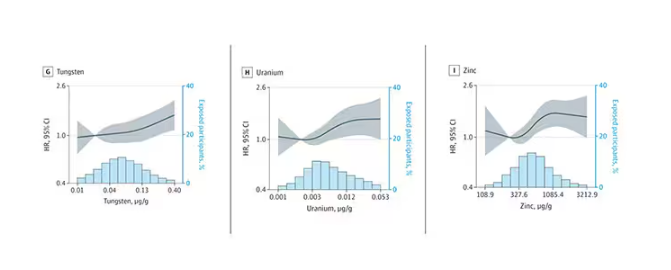
But it is very much not what we see for all metals. Strangest of all, look at lead, which shows basically no relationship with dementia.
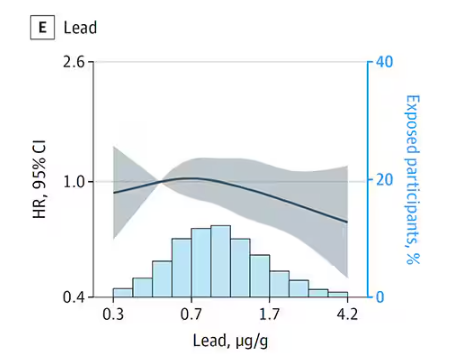
This concerns me a bit. Earlier, I discussed the issue of measuring stuff in urine and how standardizing levels to the urine creatinine level introduces a bias due to muscle mass. One way around this is to standardize urine levels to some other marker of urine dilution, like osmolality. But more fundamental than that, I like to see positive and negative controls in studies like this. For example, lead strikes me as a good positive control here. If the experimental framework were valid, I would think we’d see a relationship between lead level and dementia.
For a negative control? Well, something we are quite sure is not neurotoxic — something like sulfur, which is relatively ubiquitous, used in a variety of biological processes, and efficiently eliminated. We don’t have that in this study.
The authors close their case by creating a model that combines all the metal levels, asking the question of whether higher levels of metals in the urine in general worsen cognitive scores. And they find that the relationship exists, as you can see in Figure 8, both in carriers and noncarriers of ApoE4. But, to me, this is even more argument for the creatinine problem. If it’s not a specific metal but just the sort of general concentration of all metals, the risk for confounding by muscle mass is even higher.
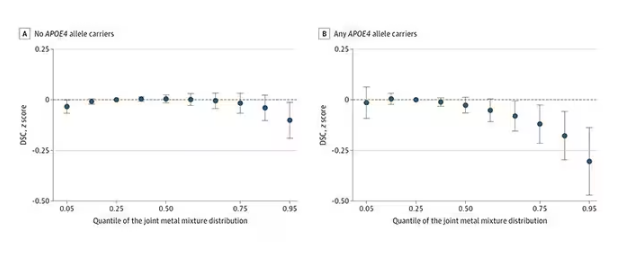
So should we worry about ingesting metals? I suppose the answer is ... kind of.
I am sure we should be avoiding lead, despite the results of this study. It’s probably best to stay away from uranium too.
As for the essential metals, I’m sure there is some toxic dose; there’s a toxic dose for everything at some point. But I don’t see evidence in this study to make me worry that a significant chunk of the population is anywhere close to that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It has always amazed me that our bodies require these tiny amounts of incredibly rare substances to function. Sure, we need oxygen. We need water. But we also need molybdenum, which makes up just 1.2 parts per million of the Earth’s crust.
Without adequate molybdenum intake, we develop seizures, developmental delays, death. Fortunately, we need so little molybdenum that true molybdenum deficiency is incredibly rare — seen only in people on total parenteral nutrition without supplementation or those with certain rare genetic conditions. But still, molybdenum is necessary for life.
Many metals are. Figure 1 colors the essential minerals on the periodic table. You can see that to stay alive, we humans need not only things like sodium, but selenium, bromine, zinc, copper, and cobalt.

Some metals are very clearly not essential; we can all do without lead and mercury, and probably should.
But just because something is essential for life does not mean that more is better. The dose is the poison, as they say. And this week, we explore whether metals — even essential metals — might be adversely affecting our brains.
It’s not a stretch to think that metal intake could have weird effects on our nervous system. Lead exposure, primarily due to leaded gasoline, has been blamed for an average reduction of about 3 points in our national IQ, for example . But not all metals are created equal. Researchers set out to find out which might be more strongly associated with performance on cognitive tests and dementia, and reported their results in this study in JAMA Network Open.
To do this, they leveraged the MESA cohort study. This is a longitudinal study of a relatively diverse group of 6300 adults who were enrolled from 2000 to 2002 around the United States. At enrollment, they gave a urine sample and took a variety of cognitive tests. Important for this study was the digit symbol substitution test, where participants are provided a code and need to replace a list of numbers with symbols as per that code. Performance on this test worsens with age, depression, and cognitive impairment.
Participants were followed for more than a decade, and over that time, 559 (about 9%) were diagnosed with dementia.
Those baseline urine samples were assayed for a variety of metals — some essential, some very much not, as you can see in Figure 2.

Now, I have to put my kidney doctor hat on for a second and talk about urine measurement ... of anything. The problem with urine is that the concentration can change a lot — by more than 10-fold, in fact — based on how much water you drank recently. Researchers correct for this, and in the case of this study, they do what a lot of researchers do: divide the measured concentration by the urine creatinine level.

This introduces a bit of a problem. Take two people with exactly the same kidney function, who drank exactly the same water, whose urine is exactly the same concentration. The person with more muscle mass will have more creatinine in that urine sample, since creatinine is a byproduct of muscle metabolism. Because people with more muscle mass are generally healthier, when you divide your metal concentration by urine creatinine, you get a lower number, which might lead you to believe that lower levels of the metal in the urine are protective. But in fact, what you’re seeing is that higher levels of creatinine are protective. I see this issue all the time and it will always color results of studies like this.
Okay, I am doffing my kidney doctor hat now to show you the results.
The researchers first looked at the relationship between metal concentrations in the urine and performance on cognitive tests. The results were fairly equivocal, save for that digit substitution test which is shown in Figure 4.

Even these results don’t ring major alarm bells for me. What you’re seeing here is the change in scores on the digit substitution test for each 25-percentile increase in urinary metal level — a pretty big change. And yet, you see really minor changes in the performance on the test. The digit substitution test is not an IQ test; but to give you a feeling for the magnitude of this change, if we looked at copper level, moving from the 25th to the 50th percentile would be associated with a loss of nine tenths of an IQ point.
You see two colors on the Figure 4 graph, by the way. That’s because the researchers stratified their findings based on whether the individual carried the ApoE4 gene allele, which is a risk factor for the development of dementia. There are reasons to believe that neurotoxic metals might be worse in this population, and I suppose you do see generally more adverse effects on scores in the red lines compared with the blue lines. But still, we’re not talking about a huge effect size here.
Let’s look at the relationship between these metals and the development of dementia itself, a clearly more important outcome than how well you can replace numeric digits with symbols. I’ll highlight a few of the results that are particularly telling.
First, the nonessential mineral cadmium, which displays the type of relationship we would expect if the metal were neurotoxic: a clear, roughly linear increase in risk for dementia as urinary concentration increases.

We see roughly similar patterns with the nonessential minerals tungsten and uranium, and the essential mineral zinc (beloved of respiratory-virus avoiders everywhere).

But it is very much not what we see for all metals. Strangest of all, look at lead, which shows basically no relationship with dementia.

This concerns me a bit. Earlier, I discussed the issue of measuring stuff in urine and how standardizing levels to the urine creatinine level introduces a bias due to muscle mass. One way around this is to standardize urine levels to some other marker of urine dilution, like osmolality. But more fundamental than that, I like to see positive and negative controls in studies like this. For example, lead strikes me as a good positive control here. If the experimental framework were valid, I would think we’d see a relationship between lead level and dementia.
For a negative control? Well, something we are quite sure is not neurotoxic — something like sulfur, which is relatively ubiquitous, used in a variety of biological processes, and efficiently eliminated. We don’t have that in this study.
The authors close their case by creating a model that combines all the metal levels, asking the question of whether higher levels of metals in the urine in general worsen cognitive scores. And they find that the relationship exists, as you can see in Figure 8, both in carriers and noncarriers of ApoE4. But, to me, this is even more argument for the creatinine problem. If it’s not a specific metal but just the sort of general concentration of all metals, the risk for confounding by muscle mass is even higher.

So should we worry about ingesting metals? I suppose the answer is ... kind of.
I am sure we should be avoiding lead, despite the results of this study. It’s probably best to stay away from uranium too.
As for the essential metals, I’m sure there is some toxic dose; there’s a toxic dose for everything at some point. But I don’t see evidence in this study to make me worry that a significant chunk of the population is anywhere close to that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It has always amazed me that our bodies require these tiny amounts of incredibly rare substances to function. Sure, we need oxygen. We need water. But we also need molybdenum, which makes up just 1.2 parts per million of the Earth’s crust.
Without adequate molybdenum intake, we develop seizures, developmental delays, death. Fortunately, we need so little molybdenum that true molybdenum deficiency is incredibly rare — seen only in people on total parenteral nutrition without supplementation or those with certain rare genetic conditions. But still, molybdenum is necessary for life.
Many metals are. Figure 1 colors the essential minerals on the periodic table. You can see that to stay alive, we humans need not only things like sodium, but selenium, bromine, zinc, copper, and cobalt.

Some metals are very clearly not essential; we can all do without lead and mercury, and probably should.
But just because something is essential for life does not mean that more is better. The dose is the poison, as they say. And this week, we explore whether metals — even essential metals — might be adversely affecting our brains.
It’s not a stretch to think that metal intake could have weird effects on our nervous system. Lead exposure, primarily due to leaded gasoline, has been blamed for an average reduction of about 3 points in our national IQ, for example . But not all metals are created equal. Researchers set out to find out which might be more strongly associated with performance on cognitive tests and dementia, and reported their results in this study in JAMA Network Open.
To do this, they leveraged the MESA cohort study. This is a longitudinal study of a relatively diverse group of 6300 adults who were enrolled from 2000 to 2002 around the United States. At enrollment, they gave a urine sample and took a variety of cognitive tests. Important for this study was the digit symbol substitution test, where participants are provided a code and need to replace a list of numbers with symbols as per that code. Performance on this test worsens with age, depression, and cognitive impairment.
Participants were followed for more than a decade, and over that time, 559 (about 9%) were diagnosed with dementia.
Those baseline urine samples were assayed for a variety of metals — some essential, some very much not, as you can see in Figure 2.

Now, I have to put my kidney doctor hat on for a second and talk about urine measurement ... of anything. The problem with urine is that the concentration can change a lot — by more than 10-fold, in fact — based on how much water you drank recently. Researchers correct for this, and in the case of this study, they do what a lot of researchers do: divide the measured concentration by the urine creatinine level.

This introduces a bit of a problem. Take two people with exactly the same kidney function, who drank exactly the same water, whose urine is exactly the same concentration. The person with more muscle mass will have more creatinine in that urine sample, since creatinine is a byproduct of muscle metabolism. Because people with more muscle mass are generally healthier, when you divide your metal concentration by urine creatinine, you get a lower number, which might lead you to believe that lower levels of the metal in the urine are protective. But in fact, what you’re seeing is that higher levels of creatinine are protective. I see this issue all the time and it will always color results of studies like this.
Okay, I am doffing my kidney doctor hat now to show you the results.
The researchers first looked at the relationship between metal concentrations in the urine and performance on cognitive tests. The results were fairly equivocal, save for that digit substitution test which is shown in Figure 4.

Even these results don’t ring major alarm bells for me. What you’re seeing here is the change in scores on the digit substitution test for each 25-percentile increase in urinary metal level — a pretty big change. And yet, you see really minor changes in the performance on the test. The digit substitution test is not an IQ test; but to give you a feeling for the magnitude of this change, if we looked at copper level, moving from the 25th to the 50th percentile would be associated with a loss of nine tenths of an IQ point.
You see two colors on the Figure 4 graph, by the way. That’s because the researchers stratified their findings based on whether the individual carried the ApoE4 gene allele, which is a risk factor for the development of dementia. There are reasons to believe that neurotoxic metals might be worse in this population, and I suppose you do see generally more adverse effects on scores in the red lines compared with the blue lines. But still, we’re not talking about a huge effect size here.
Let’s look at the relationship between these metals and the development of dementia itself, a clearly more important outcome than how well you can replace numeric digits with symbols. I’ll highlight a few of the results that are particularly telling.
First, the nonessential mineral cadmium, which displays the type of relationship we would expect if the metal were neurotoxic: a clear, roughly linear increase in risk for dementia as urinary concentration increases.

We see roughly similar patterns with the nonessential minerals tungsten and uranium, and the essential mineral zinc (beloved of respiratory-virus avoiders everywhere).

But it is very much not what we see for all metals. Strangest of all, look at lead, which shows basically no relationship with dementia.

This concerns me a bit. Earlier, I discussed the issue of measuring stuff in urine and how standardizing levels to the urine creatinine level introduces a bias due to muscle mass. One way around this is to standardize urine levels to some other marker of urine dilution, like osmolality. But more fundamental than that, I like to see positive and negative controls in studies like this. For example, lead strikes me as a good positive control here. If the experimental framework were valid, I would think we’d see a relationship between lead level and dementia.
For a negative control? Well, something we are quite sure is not neurotoxic — something like sulfur, which is relatively ubiquitous, used in a variety of biological processes, and efficiently eliminated. We don’t have that in this study.
The authors close their case by creating a model that combines all the metal levels, asking the question of whether higher levels of metals in the urine in general worsen cognitive scores. And they find that the relationship exists, as you can see in Figure 8, both in carriers and noncarriers of ApoE4. But, to me, this is even more argument for the creatinine problem. If it’s not a specific metal but just the sort of general concentration of all metals, the risk for confounding by muscle mass is even higher.

So should we worry about ingesting metals? I suppose the answer is ... kind of.
I am sure we should be avoiding lead, despite the results of this study. It’s probably best to stay away from uranium too.
As for the essential metals, I’m sure there is some toxic dose; there’s a toxic dose for everything at some point. But I don’t see evidence in this study to make me worry that a significant chunk of the population is anywhere close to that.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
