User login
VEXAS syndrome: More common, variable, and severe than expected
A recently discovered inflammatory disease known as VEXAS syndrome is more common, variable, and dangerous than previously understood, according to results of a retrospective observational study of a large health care system database. The findings, published in JAMA, found that it struck 1 in 4,269 men over the age of 50 in a largely White population and caused a wide variety of symptoms.
“The disease is quite severe,” study lead author David Beck, MD, PhD, of the department of medicine at NYU Langone Health, said in an interview. Patients with the condition “have a variety of clinical symptoms affecting different parts of the body and are being managed by different medical specialties.”
Dr. Beck and colleagues first described VEXAS (vacuoles, E1-ubiquitin-activating enzyme, X-linked, autoinflammatory, somatic) syndrome in 2020. They linked it to mutations in the UBA1 (ubiquitin-like modifier activating enzyme 1) gene. The enzyme initiates a process that identifies misfolded proteins as targets for degradation.
“VEXAS syndrome is characterized by anemia and inflammation in the skin, lungs, cartilage, and joints,” Dr. Beck said. “These symptoms are frequently mistaken for other rheumatic or hematologic diseases. However, this syndrome has a different cause, is treated differently, requires additional monitoring, and can be far more severe.”
According to him, hundreds of people have been diagnosed with the disease in the short time since it was defined. The disease is believed to be fatal in some cases. A previous report found that the median survival was 9 years among patients with a certain variant; that was significantly less than patients with two other variants.
For the new study, researchers searched for UBA1 variants in genetic data from 163,096 subjects (mean age, 52.8 years; 94% White, 61% women) who took part in the Geisinger MyCode Community Health Initiative. The 1996-2022 data comes from patients at 10 Pennsylvania hospitals.
Eleven people (9 males, 2 females) had likely UBA1 variants, and all had anemia. The cases accounted for 1 in 13,591 unrelated people (95% confidence interval, 1:7,775-1:23,758), 1 in 4,269 men older than 50 years (95% CI, 1:2,319-1:7,859), and 1 in 26,238 women older than 50 years (95% CI, 1:7,196-1:147,669).
Other common findings included macrocytosis (91%), skin problems (73%), and pulmonary disease (91%). Ten patients (91%) required transfusions.
Five of the 11 subjects didn’t meet the previously defined criteria for VEXAS syndrome. None had been diagnosed with the condition, which is not surprising considering that it hadn’t been discovered and described until recently.
Just over half of the patients – 55% – had a clinical diagnosis that was previously linked to VEXAS syndrome. “This means that slightly less than half of the patients with VEXAS syndrome had no clear associated clinical diagnosis,” Dr. Beck said. “The lack of associated clinical diagnoses may be due to the variety of nonspecific clinical characteristics that span different subspecialities in VEXAS syndrome. VEXAS syndrome represents an example of a multisystem disease where patients and their symptoms may get lost in the shuffle.”
In the future, “professionals should look out for patients with unexplained inflammation – and some combination of hematologic, rheumatologic, pulmonary, and dermatologic clinical manifestations – that either don’t carry a clinical diagnosis or don’t respond to first-line therapies,” Dr. Beck said. “These patients will also frequently be anemic, have low platelet counts, elevated markers of inflammation in the blood, and be dependent on corticosteroids.”
Diagnosis can be made via genetic testing, but the study authors note that it “is not routinely offered on standard workup for myeloid neoplasms or immune dysregulation diagnostic panels.”
As for treatment, Dr. Beck said the disease “can be partially controlled by multiple different anticytokine therapies or biologics. However, in most cases, patients still need additional steroids and/or disease-modifying antirheumatic agents [DMARDs]. In addition, bone marrow transplantation has shown signs of being a highly effective therapy.”
The study authors say more research is needed to understand the disease’s prevalence in more diverse populations.
In an interview, Matthew J. Koster, MD, a rheumatologist at Mayo Clinic in Rochester, Minn., who’s studied the disease but didn’t take part in this research project, said the findings are valid and “highly important.
“The findings of this study highlight what many academic and quaternary referral centers were wondering: Is VEXAS really more common than we think, with patients hiding in plain sight? The answer is yes,” he said. “Currently, there are less than 400 cases reported in the literature of VEXAS, but large centers are diagnosing this condition with some frequency. For example, at Mayo Clinic in Rochester, we diagnose on average one new patient with VEXAS every 7-14 days and have diagnosed 60 in the past 18 months. A national collaborative group in France has diagnosed approximately 250 patients over that same time frame when pooling patients nationwide.”
The prevalence is high enough, he said, that “clinicians should consider that some of the patients with diseases that are not responding to treatment may in fact have VEXAS rather than ‘refractory’ relapsing polychondritis or ‘recalcitrant’ rheumatoid arthritis, etc.”
The National Institute of Health funded the study. Dr. Beck, the other authors, and Dr. Koster report no disclosures.
A recently discovered inflammatory disease known as VEXAS syndrome is more common, variable, and dangerous than previously understood, according to results of a retrospective observational study of a large health care system database. The findings, published in JAMA, found that it struck 1 in 4,269 men over the age of 50 in a largely White population and caused a wide variety of symptoms.
“The disease is quite severe,” study lead author David Beck, MD, PhD, of the department of medicine at NYU Langone Health, said in an interview. Patients with the condition “have a variety of clinical symptoms affecting different parts of the body and are being managed by different medical specialties.”
Dr. Beck and colleagues first described VEXAS (vacuoles, E1-ubiquitin-activating enzyme, X-linked, autoinflammatory, somatic) syndrome in 2020. They linked it to mutations in the UBA1 (ubiquitin-like modifier activating enzyme 1) gene. The enzyme initiates a process that identifies misfolded proteins as targets for degradation.
“VEXAS syndrome is characterized by anemia and inflammation in the skin, lungs, cartilage, and joints,” Dr. Beck said. “These symptoms are frequently mistaken for other rheumatic or hematologic diseases. However, this syndrome has a different cause, is treated differently, requires additional monitoring, and can be far more severe.”
According to him, hundreds of people have been diagnosed with the disease in the short time since it was defined. The disease is believed to be fatal in some cases. A previous report found that the median survival was 9 years among patients with a certain variant; that was significantly less than patients with two other variants.
For the new study, researchers searched for UBA1 variants in genetic data from 163,096 subjects (mean age, 52.8 years; 94% White, 61% women) who took part in the Geisinger MyCode Community Health Initiative. The 1996-2022 data comes from patients at 10 Pennsylvania hospitals.
Eleven people (9 males, 2 females) had likely UBA1 variants, and all had anemia. The cases accounted for 1 in 13,591 unrelated people (95% confidence interval, 1:7,775-1:23,758), 1 in 4,269 men older than 50 years (95% CI, 1:2,319-1:7,859), and 1 in 26,238 women older than 50 years (95% CI, 1:7,196-1:147,669).
Other common findings included macrocytosis (91%), skin problems (73%), and pulmonary disease (91%). Ten patients (91%) required transfusions.
Five of the 11 subjects didn’t meet the previously defined criteria for VEXAS syndrome. None had been diagnosed with the condition, which is not surprising considering that it hadn’t been discovered and described until recently.
Just over half of the patients – 55% – had a clinical diagnosis that was previously linked to VEXAS syndrome. “This means that slightly less than half of the patients with VEXAS syndrome had no clear associated clinical diagnosis,” Dr. Beck said. “The lack of associated clinical diagnoses may be due to the variety of nonspecific clinical characteristics that span different subspecialities in VEXAS syndrome. VEXAS syndrome represents an example of a multisystem disease where patients and their symptoms may get lost in the shuffle.”
In the future, “professionals should look out for patients with unexplained inflammation – and some combination of hematologic, rheumatologic, pulmonary, and dermatologic clinical manifestations – that either don’t carry a clinical diagnosis or don’t respond to first-line therapies,” Dr. Beck said. “These patients will also frequently be anemic, have low platelet counts, elevated markers of inflammation in the blood, and be dependent on corticosteroids.”
Diagnosis can be made via genetic testing, but the study authors note that it “is not routinely offered on standard workup for myeloid neoplasms or immune dysregulation diagnostic panels.”
As for treatment, Dr. Beck said the disease “can be partially controlled by multiple different anticytokine therapies or biologics. However, in most cases, patients still need additional steroids and/or disease-modifying antirheumatic agents [DMARDs]. In addition, bone marrow transplantation has shown signs of being a highly effective therapy.”
The study authors say more research is needed to understand the disease’s prevalence in more diverse populations.
In an interview, Matthew J. Koster, MD, a rheumatologist at Mayo Clinic in Rochester, Minn., who’s studied the disease but didn’t take part in this research project, said the findings are valid and “highly important.
“The findings of this study highlight what many academic and quaternary referral centers were wondering: Is VEXAS really more common than we think, with patients hiding in plain sight? The answer is yes,” he said. “Currently, there are less than 400 cases reported in the literature of VEXAS, but large centers are diagnosing this condition with some frequency. For example, at Mayo Clinic in Rochester, we diagnose on average one new patient with VEXAS every 7-14 days and have diagnosed 60 in the past 18 months. A national collaborative group in France has diagnosed approximately 250 patients over that same time frame when pooling patients nationwide.”
The prevalence is high enough, he said, that “clinicians should consider that some of the patients with diseases that are not responding to treatment may in fact have VEXAS rather than ‘refractory’ relapsing polychondritis or ‘recalcitrant’ rheumatoid arthritis, etc.”
The National Institute of Health funded the study. Dr. Beck, the other authors, and Dr. Koster report no disclosures.
A recently discovered inflammatory disease known as VEXAS syndrome is more common, variable, and dangerous than previously understood, according to results of a retrospective observational study of a large health care system database. The findings, published in JAMA, found that it struck 1 in 4,269 men over the age of 50 in a largely White population and caused a wide variety of symptoms.
“The disease is quite severe,” study lead author David Beck, MD, PhD, of the department of medicine at NYU Langone Health, said in an interview. Patients with the condition “have a variety of clinical symptoms affecting different parts of the body and are being managed by different medical specialties.”
Dr. Beck and colleagues first described VEXAS (vacuoles, E1-ubiquitin-activating enzyme, X-linked, autoinflammatory, somatic) syndrome in 2020. They linked it to mutations in the UBA1 (ubiquitin-like modifier activating enzyme 1) gene. The enzyme initiates a process that identifies misfolded proteins as targets for degradation.
“VEXAS syndrome is characterized by anemia and inflammation in the skin, lungs, cartilage, and joints,” Dr. Beck said. “These symptoms are frequently mistaken for other rheumatic or hematologic diseases. However, this syndrome has a different cause, is treated differently, requires additional monitoring, and can be far more severe.”
According to him, hundreds of people have been diagnosed with the disease in the short time since it was defined. The disease is believed to be fatal in some cases. A previous report found that the median survival was 9 years among patients with a certain variant; that was significantly less than patients with two other variants.
For the new study, researchers searched for UBA1 variants in genetic data from 163,096 subjects (mean age, 52.8 years; 94% White, 61% women) who took part in the Geisinger MyCode Community Health Initiative. The 1996-2022 data comes from patients at 10 Pennsylvania hospitals.
Eleven people (9 males, 2 females) had likely UBA1 variants, and all had anemia. The cases accounted for 1 in 13,591 unrelated people (95% confidence interval, 1:7,775-1:23,758), 1 in 4,269 men older than 50 years (95% CI, 1:2,319-1:7,859), and 1 in 26,238 women older than 50 years (95% CI, 1:7,196-1:147,669).
Other common findings included macrocytosis (91%), skin problems (73%), and pulmonary disease (91%). Ten patients (91%) required transfusions.
Five of the 11 subjects didn’t meet the previously defined criteria for VEXAS syndrome. None had been diagnosed with the condition, which is not surprising considering that it hadn’t been discovered and described until recently.
Just over half of the patients – 55% – had a clinical diagnosis that was previously linked to VEXAS syndrome. “This means that slightly less than half of the patients with VEXAS syndrome had no clear associated clinical diagnosis,” Dr. Beck said. “The lack of associated clinical diagnoses may be due to the variety of nonspecific clinical characteristics that span different subspecialities in VEXAS syndrome. VEXAS syndrome represents an example of a multisystem disease where patients and their symptoms may get lost in the shuffle.”
In the future, “professionals should look out for patients with unexplained inflammation – and some combination of hematologic, rheumatologic, pulmonary, and dermatologic clinical manifestations – that either don’t carry a clinical diagnosis or don’t respond to first-line therapies,” Dr. Beck said. “These patients will also frequently be anemic, have low platelet counts, elevated markers of inflammation in the blood, and be dependent on corticosteroids.”
Diagnosis can be made via genetic testing, but the study authors note that it “is not routinely offered on standard workup for myeloid neoplasms or immune dysregulation diagnostic panels.”
As for treatment, Dr. Beck said the disease “can be partially controlled by multiple different anticytokine therapies or biologics. However, in most cases, patients still need additional steroids and/or disease-modifying antirheumatic agents [DMARDs]. In addition, bone marrow transplantation has shown signs of being a highly effective therapy.”
The study authors say more research is needed to understand the disease’s prevalence in more diverse populations.
In an interview, Matthew J. Koster, MD, a rheumatologist at Mayo Clinic in Rochester, Minn., who’s studied the disease but didn’t take part in this research project, said the findings are valid and “highly important.
“The findings of this study highlight what many academic and quaternary referral centers were wondering: Is VEXAS really more common than we think, with patients hiding in plain sight? The answer is yes,” he said. “Currently, there are less than 400 cases reported in the literature of VEXAS, but large centers are diagnosing this condition with some frequency. For example, at Mayo Clinic in Rochester, we diagnose on average one new patient with VEXAS every 7-14 days and have diagnosed 60 in the past 18 months. A national collaborative group in France has diagnosed approximately 250 patients over that same time frame when pooling patients nationwide.”
The prevalence is high enough, he said, that “clinicians should consider that some of the patients with diseases that are not responding to treatment may in fact have VEXAS rather than ‘refractory’ relapsing polychondritis or ‘recalcitrant’ rheumatoid arthritis, etc.”
The National Institute of Health funded the study. Dr. Beck, the other authors, and Dr. Koster report no disclosures.
FROM JAMA
Artificial intelligence applications in colonoscopy
Considerable advances in artificial intelligence (AI) and machine-learning (ML) methodologies have led to the emergence of promising tools in the field of gastrointestinal endoscopy. Computer vision is an application of AI/ML that has been successfully applied for the computer-aided detection (CADe) and computer-aided diagnosis (CADx) of colon polyps and numerous other conditions encountered during GI endoscopy. Outside of computer vision, a wide variety of other AI applications have been applied to gastroenterology, ranging from natural language processing (NLP) to optimize clinical documentation and endoscopy quality reporting to ML techniques that predict disease severity/treatment response and augment clinical decision-making.
In the United States, colonoscopy is the standard for colon cancer screening and prevention; however, precancerous polyps can be missed for various reasons, ranging from subtle surface appearance of the polyp or location behind a colonic fold to operator-dependent reasons such as inadequate mucosal inspection. Though clinical practice guidelines have set adenoma detection rate (ADR) thresholds at 20% for women and 30% for men, studies have shown a 4- to 10-fold variation in ADR among physicians in clinical practice settings,1 with an estimated adenoma miss rate (AMR) of 25% and a false-negative colonoscopy rate of 12%.2 Variability in adenoma detection affects the risk of interval colorectal cancer post colonoscopy.3,4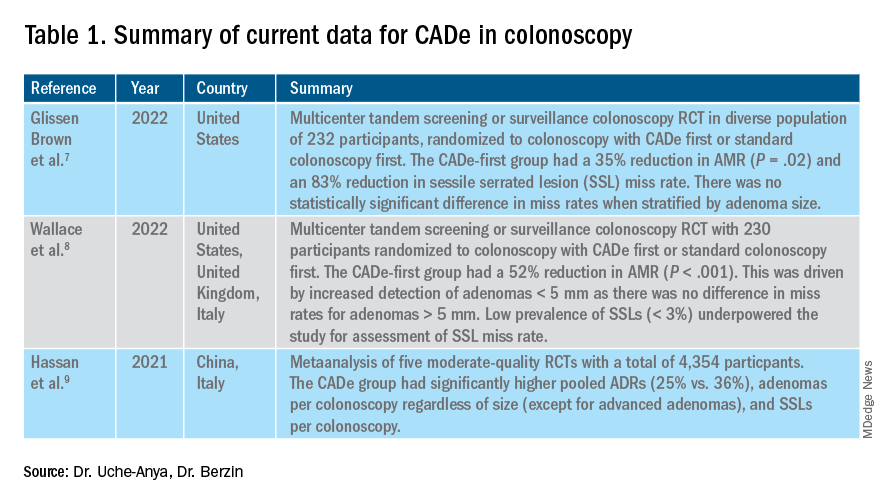
AI provides an opportunity for mitigating this risk. Advances in deep learning and computer vision have led to the development of CADe systems that automatically detect polyps in real time during colonoscopy, resulting in reduced adenoma miss rates (Table 1). In addition to polyp detection, deep-learning technologies are also being used in CADx systems for polyp diagnosis and characterization of malignancy risk. This could aid therapeutic decision-making: Unnecessary resection or histopathologic analysis could be obviated for benign hyperplastic polyps. On the other end of the polyp spectrum, an AI tool that could predict the presence or absence of submucosal invasion could be a powerful tool when evaluating early colon cancers for consideration of endoscopic submucosal dissection vs. surgery. Examples of CADe polyp detection and CADx polyp characterization are shown in Figure 1.
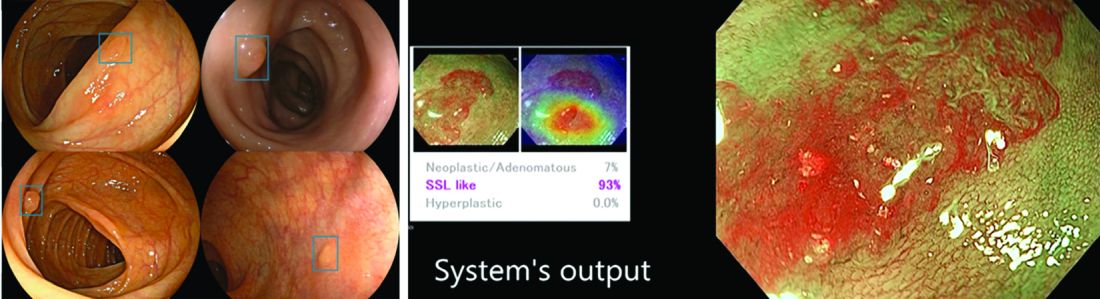
Other potential computer vision applications that may improve colonoscopy quality include tools that help measure adequacy of mucosal exposure, segmental inspection time, and a variety of other parameters associated with polyp detection performance. These are promising areas for future research. Beyond improving colonoscopy technique, natural language processing tools already are being used to optimize clinical documentation as well as extract information from colonoscopy and pathology reports that can facilitate reporting of colonoscopy quality metrics such as ADR, cecal intubation rate, withdrawal time, and bowel preparation adequacy. AI-powered analytics may help unlock large-scale reporting of colonoscopy quality metrics on a health-systems level5 or population-level,6 helping to ensure optimal performance and identifying avenues for colonoscopy quality improvement.
The majority of AI research in colonoscopy has focused on CADe for colon polyp detection and CADx for polyp diagnosis. Over the last few years, several randomized clinical trials – two in the United States – have shown that CADe significantly improves adenoma detection and reduces adenoma miss rates in comparison to standard colonoscopy. The existing data are summarized in Table 1, focusing on the two U.S. studies and an international meta-analysis.
In comparison, the data landscape for CADx is nascent and currently limited to several retrospective studies dating back to 2009 and a few prospective studies that have shown promising results.10,11 There is an expectation that integrated CADx also may support the adoption of “resect and discard” or “diagnose and leave” strategies for low-risk polyps. About two-thirds of polyps identified on average-risk screening colonoscopies are diminutive polyps (less than 5 mm in size), which rarely have advanced histologic features (about 0.5%) and are sometimes non-neoplastic (30%). Malignancy risk is even lower in the distal colon.12 As routine histopathologic assessment of such polyps is mostly of limited clinical utility and comes with added pathology costs, CADx technologies may offer a more cost-effective approach where polyps that are characterized in real-time as low-risk adenomas or non-neoplastic are “resected and discarded” or “left in” respectively. In 2011, prior to the development of current AI tools, the American Society for Gastrointestinal Endoscopy set performance thresholds for technologies supporting real-time endoscopic assessment of the histology of diminutive colorectal polyps. The ASGE recommended 90% histopathologic concordance for “resect and discard” tools and 90% negative predictive value for adenomatous histology for “diagnose and leave,” tools.13 Narrow-band imaging (NBI), for example, has been shown to meet these benchmarks14,15 with a modeling study suggesting that implementing “resect and discard” strategies with such tools could result in annual savings of $33 million without adversely affecting efficacy, although practical adoption has been limited.16 More recent work has directly explored the feasibility of leveraging CADx to support “leave-in-situ” and “resect-and-discard” strategies.17
Similarly, while CADe use in colonoscopy is associated with additional up-front costs, a modeling study suggests that its associated gains in ADR (as detailed in Table 1) make it a cost-saving strategy for colorectal cancer prevention in the long term.18 There is still uncertainty on whether the incremental CADe-associated gains in adenoma detection will necessarily translate to significant reductions in interval colorectal cancer risk, particularly for endoscopists who are already high-performing polyp detectors. A recent study suggests that, although higher ADRs were associated with lower rates of interval colorectal cancer, the gains in interval colorectal cancer risk reduction appeared to level off with ADRs above 35%-40% (this finding may be limited by statistical power).19 Further, most of the data from CADe trials suggest that gains in adenoma detection are not driven by increased detection of advanced lesions with high malignancy risk but by small polyps with long latency periods of about 5-10 years, which may not significantly alter interval cancer risk. It remains to be determined whether adoption of CADe will have an impact on hard outcomes, most importantly interval colorectal cancer risk, or merely result in increased resource utilization without moving the needle on colorectal cancer prevention. To answer this question, the OperA study – a large-scale randomized clinical trial of 200,000 patients across 18 centers from 13 countries – was launched in 2022. It will investigate the effect of colonoscopy with CADe on a number of critical measures, including long-term interval colon cancer risk.20
Despite commercial availability of regulatory-approved CADe systems and data supporting use for adenoma detection in colonoscopy, mainstream adoption in clinical practice has been sluggish. Physician survey studies have shown that, although there is considerable interest in integrating CADe into clinical practice, there are concerns about access, cost and reimbursement, integration into clinical work-flow, increased procedural times, over-reliance on AI, and algorithmic bias leading to errors.21,22 In addition, without mandatory requirements for ADR reporting or clinical practice guideline recommendations for CADe use, these systems may not be perceived as valuable or ready for prime time even though the evidence suggests otherwise.23,24 For CADe systems to see widespread adoption in clinical practice, it is important that future research studies rigorously investigate and characterize these potential barriers to better inform strategies to address AI hesitancy and implementation challenges. Such efforts can provide an integration framework for future AI applications in gastroenterology beyond colonoscopy, such as CADe of esophageal and gastric premalignant lesions in upper endoscopy, CADx for pancreatic cysts and liver lesions on imaging, NLP tools to optimizing efficient clinical documentation and reporting, and many others.
Dr. Uche-Anya is in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston. Dr. Berzin is with the Center for Advanced Endoscopy, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston. Dr. Berzin is a consultant for Wision AI, Medtronic, Magentiq Eye, RSIP Vision, and Docbot.
Corresponding Author: Eugenia Uche-Anya [email protected] Twitter: @UcheAnyaMD @tberzin
References
1. Corley DA et al. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. Sep 2011;74(3):656-65. doi: 10.1016/j.gie.2011.04.017.
2. Zhao S et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology. 05 2019;156(6):1661-74.e11. doi: 10.1053/j.gastro.2019.01.260.
3. Kaminski MF et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. May 13 2010;362(19):1795-803. doi: 10.1056/NEJMoa0907667.
4. Corley DA et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. Apr 03 2014;370(14):1298-306. doi: 10.1056/NEJMoa1309086.
5. Laique SN et al. Application of optical character recognition with natural language processing for large-scale quality metric data extraction in colonoscopy reports. Gastrointest Endosc. 03 2021;93(3):750-7. doi: 10.1016/j.gie.2020.08.038.
6. Tinmouth J et al. Validation of a natural language processing algorithm to identify adenomas and measure adenoma detection rates across a health system: a population-level study. Gastrointest Endosc. Jul 14 2022. doi: 10.1016/j.gie.2022.07.009.
7. Glissen Brown JR et al. Deep learning computer-aided polyp detection reduces adenoma miss rate: A United States multi-center randomized tandem colonoscopy study (CADeT-CS Trial). Clin Gastroenterol Hepatol. 07 2022;20(7):1499-1507.e4. doi: 10.1016/j.cgh.2021.09.009.
8. Wallace MB et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology. 07 2022;163(1):295-304.e5. doi: 10.1053/j.gastro.2022.03.007.
9. Hassan C et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 01 2021;93(1):77-85.e6. doi: 10.1016/j.gie.2020.06.059.
10. Glissen Brown JR and Berzin TM. Adoption of new technologies: Artificial intelligence. Gastrointest Endosc Clin N Am. Oct 2021;31(4):743-58. doi: 10.1016/j.giec.2021.05.010.
11. Larsen SLV and Mori Y. Artificial intelligence in colonoscopy: A review on the current status. DEN open. Apr 2022;2(1):e109. doi: 10.1002/deo2.109.
12. Gupta N et al. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc. May 2012;75(5):1022-30. doi: 10.1016/j.gie.2012.01.020.
13. Rex DK et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2011;73(3):419-22. doi: 10.1016/j.gie.2011.01.023.
14. Abu Dayyeh BK et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2015;81(3):502.e1-16. doi: 10.1016/j.gie.2014.12.022.
15. Mori Y et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy: A prospective study. Ann Intern Med. Sep 18 2018;169(6):357-66. doi: 10.7326/M18-0249.
16. Hassan C et al.. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. Oct 2010;8(10):865-9, 869.e1-3. doi: 10.1016/j.cgh.2010.05.018.
17. Hassan C et al. Artificial intelligence allows leaving-in-situ colorectal polyps. Clin Gastroenterol Hepatol. Nov 2022;20(11):2505-13.e4. doi: 10.1016/j.cgh.2022.04.045.
18. Areia M et al. Cost-effectiveness of artificial intelligence for screening colonoscopy: a modelling study. Lancet Digit Health. 06 2022;4(6):e436-44. doi: 10.1016/S2589-7500(22)00042-5.
19. Schottinger JE et al. Association of physician adenoma detection rates with postcolonoscopy colorectal cancer. JAMA. 2022 Jun 7;327(21):2114-22. doi: 10.1001/jama.2022.6644.
20. Oslo Uo. Optimising colorectal cancer prevention through personalised treatment with artificial intelligence. 2022.
21. Wadhwa V et al. Physician sentiment toward artificial intelligence (AI) in colonoscopic practice: a survey of US gastroenterologists. Endosc Int Open. Oct 2020;8(10):E1379-84. doi: 10.1055/a-1223-1926.
22. Kader R et al. Survey on the perceptions of UK gastroenterologists and endoscopists to artificial intelligence. Frontline Gastroenterol. 2022;13(5):423-9. doi: 10.1136/flgastro-2021-101994.
23. Rex DKet al. Artificial intelligence improves detection at colonoscopy: Why aren’t we all already using it? Gastroenterology. 07 2022;163(1):35-7. doi: 10.1053/j.gastro.2022.04.042.
24. Ahmad OF et al. Establishing key research questions for the implementation of artificial intelligence in colonoscopy: A modified Delphi method. Endoscopy. 09 2021;53(9):893-901. doi: 10.1055/a-1306-7590
Considerable advances in artificial intelligence (AI) and machine-learning (ML) methodologies have led to the emergence of promising tools in the field of gastrointestinal endoscopy. Computer vision is an application of AI/ML that has been successfully applied for the computer-aided detection (CADe) and computer-aided diagnosis (CADx) of colon polyps and numerous other conditions encountered during GI endoscopy. Outside of computer vision, a wide variety of other AI applications have been applied to gastroenterology, ranging from natural language processing (NLP) to optimize clinical documentation and endoscopy quality reporting to ML techniques that predict disease severity/treatment response and augment clinical decision-making.
In the United States, colonoscopy is the standard for colon cancer screening and prevention; however, precancerous polyps can be missed for various reasons, ranging from subtle surface appearance of the polyp or location behind a colonic fold to operator-dependent reasons such as inadequate mucosal inspection. Though clinical practice guidelines have set adenoma detection rate (ADR) thresholds at 20% for women and 30% for men, studies have shown a 4- to 10-fold variation in ADR among physicians in clinical practice settings,1 with an estimated adenoma miss rate (AMR) of 25% and a false-negative colonoscopy rate of 12%.2 Variability in adenoma detection affects the risk of interval colorectal cancer post colonoscopy.3,4
AI provides an opportunity for mitigating this risk. Advances in deep learning and computer vision have led to the development of CADe systems that automatically detect polyps in real time during colonoscopy, resulting in reduced adenoma miss rates (Table 1). In addition to polyp detection, deep-learning technologies are also being used in CADx systems for polyp diagnosis and characterization of malignancy risk. This could aid therapeutic decision-making: Unnecessary resection or histopathologic analysis could be obviated for benign hyperplastic polyps. On the other end of the polyp spectrum, an AI tool that could predict the presence or absence of submucosal invasion could be a powerful tool when evaluating early colon cancers for consideration of endoscopic submucosal dissection vs. surgery. Examples of CADe polyp detection and CADx polyp characterization are shown in Figure 1.

Other potential computer vision applications that may improve colonoscopy quality include tools that help measure adequacy of mucosal exposure, segmental inspection time, and a variety of other parameters associated with polyp detection performance. These are promising areas for future research. Beyond improving colonoscopy technique, natural language processing tools already are being used to optimize clinical documentation as well as extract information from colonoscopy and pathology reports that can facilitate reporting of colonoscopy quality metrics such as ADR, cecal intubation rate, withdrawal time, and bowel preparation adequacy. AI-powered analytics may help unlock large-scale reporting of colonoscopy quality metrics on a health-systems level5 or population-level,6 helping to ensure optimal performance and identifying avenues for colonoscopy quality improvement.
The majority of AI research in colonoscopy has focused on CADe for colon polyp detection and CADx for polyp diagnosis. Over the last few years, several randomized clinical trials – two in the United States – have shown that CADe significantly improves adenoma detection and reduces adenoma miss rates in comparison to standard colonoscopy. The existing data are summarized in Table 1, focusing on the two U.S. studies and an international meta-analysis.
In comparison, the data landscape for CADx is nascent and currently limited to several retrospective studies dating back to 2009 and a few prospective studies that have shown promising results.10,11 There is an expectation that integrated CADx also may support the adoption of “resect and discard” or “diagnose and leave” strategies for low-risk polyps. About two-thirds of polyps identified on average-risk screening colonoscopies are diminutive polyps (less than 5 mm in size), which rarely have advanced histologic features (about 0.5%) and are sometimes non-neoplastic (30%). Malignancy risk is even lower in the distal colon.12 As routine histopathologic assessment of such polyps is mostly of limited clinical utility and comes with added pathology costs, CADx technologies may offer a more cost-effective approach where polyps that are characterized in real-time as low-risk adenomas or non-neoplastic are “resected and discarded” or “left in” respectively. In 2011, prior to the development of current AI tools, the American Society for Gastrointestinal Endoscopy set performance thresholds for technologies supporting real-time endoscopic assessment of the histology of diminutive colorectal polyps. The ASGE recommended 90% histopathologic concordance for “resect and discard” tools and 90% negative predictive value for adenomatous histology for “diagnose and leave,” tools.13 Narrow-band imaging (NBI), for example, has been shown to meet these benchmarks14,15 with a modeling study suggesting that implementing “resect and discard” strategies with such tools could result in annual savings of $33 million without adversely affecting efficacy, although practical adoption has been limited.16 More recent work has directly explored the feasibility of leveraging CADx to support “leave-in-situ” and “resect-and-discard” strategies.17
Similarly, while CADe use in colonoscopy is associated with additional up-front costs, a modeling study suggests that its associated gains in ADR (as detailed in Table 1) make it a cost-saving strategy for colorectal cancer prevention in the long term.18 There is still uncertainty on whether the incremental CADe-associated gains in adenoma detection will necessarily translate to significant reductions in interval colorectal cancer risk, particularly for endoscopists who are already high-performing polyp detectors. A recent study suggests that, although higher ADRs were associated with lower rates of interval colorectal cancer, the gains in interval colorectal cancer risk reduction appeared to level off with ADRs above 35%-40% (this finding may be limited by statistical power).19 Further, most of the data from CADe trials suggest that gains in adenoma detection are not driven by increased detection of advanced lesions with high malignancy risk but by small polyps with long latency periods of about 5-10 years, which may not significantly alter interval cancer risk. It remains to be determined whether adoption of CADe will have an impact on hard outcomes, most importantly interval colorectal cancer risk, or merely result in increased resource utilization without moving the needle on colorectal cancer prevention. To answer this question, the OperA study – a large-scale randomized clinical trial of 200,000 patients across 18 centers from 13 countries – was launched in 2022. It will investigate the effect of colonoscopy with CADe on a number of critical measures, including long-term interval colon cancer risk.20
Despite commercial availability of regulatory-approved CADe systems and data supporting use for adenoma detection in colonoscopy, mainstream adoption in clinical practice has been sluggish. Physician survey studies have shown that, although there is considerable interest in integrating CADe into clinical practice, there are concerns about access, cost and reimbursement, integration into clinical work-flow, increased procedural times, over-reliance on AI, and algorithmic bias leading to errors.21,22 In addition, without mandatory requirements for ADR reporting or clinical practice guideline recommendations for CADe use, these systems may not be perceived as valuable or ready for prime time even though the evidence suggests otherwise.23,24 For CADe systems to see widespread adoption in clinical practice, it is important that future research studies rigorously investigate and characterize these potential barriers to better inform strategies to address AI hesitancy and implementation challenges. Such efforts can provide an integration framework for future AI applications in gastroenterology beyond colonoscopy, such as CADe of esophageal and gastric premalignant lesions in upper endoscopy, CADx for pancreatic cysts and liver lesions on imaging, NLP tools to optimizing efficient clinical documentation and reporting, and many others.
Dr. Uche-Anya is in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston. Dr. Berzin is with the Center for Advanced Endoscopy, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston. Dr. Berzin is a consultant for Wision AI, Medtronic, Magentiq Eye, RSIP Vision, and Docbot.
Corresponding Author: Eugenia Uche-Anya [email protected] Twitter: @UcheAnyaMD @tberzin
References
1. Corley DA et al. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. Sep 2011;74(3):656-65. doi: 10.1016/j.gie.2011.04.017.
2. Zhao S et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology. 05 2019;156(6):1661-74.e11. doi: 10.1053/j.gastro.2019.01.260.
3. Kaminski MF et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. May 13 2010;362(19):1795-803. doi: 10.1056/NEJMoa0907667.
4. Corley DA et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. Apr 03 2014;370(14):1298-306. doi: 10.1056/NEJMoa1309086.
5. Laique SN et al. Application of optical character recognition with natural language processing for large-scale quality metric data extraction in colonoscopy reports. Gastrointest Endosc. 03 2021;93(3):750-7. doi: 10.1016/j.gie.2020.08.038.
6. Tinmouth J et al. Validation of a natural language processing algorithm to identify adenomas and measure adenoma detection rates across a health system: a population-level study. Gastrointest Endosc. Jul 14 2022. doi: 10.1016/j.gie.2022.07.009.
7. Glissen Brown JR et al. Deep learning computer-aided polyp detection reduces adenoma miss rate: A United States multi-center randomized tandem colonoscopy study (CADeT-CS Trial). Clin Gastroenterol Hepatol. 07 2022;20(7):1499-1507.e4. doi: 10.1016/j.cgh.2021.09.009.
8. Wallace MB et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology. 07 2022;163(1):295-304.e5. doi: 10.1053/j.gastro.2022.03.007.
9. Hassan C et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 01 2021;93(1):77-85.e6. doi: 10.1016/j.gie.2020.06.059.
10. Glissen Brown JR and Berzin TM. Adoption of new technologies: Artificial intelligence. Gastrointest Endosc Clin N Am. Oct 2021;31(4):743-58. doi: 10.1016/j.giec.2021.05.010.
11. Larsen SLV and Mori Y. Artificial intelligence in colonoscopy: A review on the current status. DEN open. Apr 2022;2(1):e109. doi: 10.1002/deo2.109.
12. Gupta N et al. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc. May 2012;75(5):1022-30. doi: 10.1016/j.gie.2012.01.020.
13. Rex DK et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2011;73(3):419-22. doi: 10.1016/j.gie.2011.01.023.
14. Abu Dayyeh BK et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2015;81(3):502.e1-16. doi: 10.1016/j.gie.2014.12.022.
15. Mori Y et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy: A prospective study. Ann Intern Med. Sep 18 2018;169(6):357-66. doi: 10.7326/M18-0249.
16. Hassan C et al.. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. Oct 2010;8(10):865-9, 869.e1-3. doi: 10.1016/j.cgh.2010.05.018.
17. Hassan C et al. Artificial intelligence allows leaving-in-situ colorectal polyps. Clin Gastroenterol Hepatol. Nov 2022;20(11):2505-13.e4. doi: 10.1016/j.cgh.2022.04.045.
18. Areia M et al. Cost-effectiveness of artificial intelligence for screening colonoscopy: a modelling study. Lancet Digit Health. 06 2022;4(6):e436-44. doi: 10.1016/S2589-7500(22)00042-5.
19. Schottinger JE et al. Association of physician adenoma detection rates with postcolonoscopy colorectal cancer. JAMA. 2022 Jun 7;327(21):2114-22. doi: 10.1001/jama.2022.6644.
20. Oslo Uo. Optimising colorectal cancer prevention through personalised treatment with artificial intelligence. 2022.
21. Wadhwa V et al. Physician sentiment toward artificial intelligence (AI) in colonoscopic practice: a survey of US gastroenterologists. Endosc Int Open. Oct 2020;8(10):E1379-84. doi: 10.1055/a-1223-1926.
22. Kader R et al. Survey on the perceptions of UK gastroenterologists and endoscopists to artificial intelligence. Frontline Gastroenterol. 2022;13(5):423-9. doi: 10.1136/flgastro-2021-101994.
23. Rex DKet al. Artificial intelligence improves detection at colonoscopy: Why aren’t we all already using it? Gastroenterology. 07 2022;163(1):35-7. doi: 10.1053/j.gastro.2022.04.042.
24. Ahmad OF et al. Establishing key research questions for the implementation of artificial intelligence in colonoscopy: A modified Delphi method. Endoscopy. 09 2021;53(9):893-901. doi: 10.1055/a-1306-7590
Considerable advances in artificial intelligence (AI) and machine-learning (ML) methodologies have led to the emergence of promising tools in the field of gastrointestinal endoscopy. Computer vision is an application of AI/ML that has been successfully applied for the computer-aided detection (CADe) and computer-aided diagnosis (CADx) of colon polyps and numerous other conditions encountered during GI endoscopy. Outside of computer vision, a wide variety of other AI applications have been applied to gastroenterology, ranging from natural language processing (NLP) to optimize clinical documentation and endoscopy quality reporting to ML techniques that predict disease severity/treatment response and augment clinical decision-making.
In the United States, colonoscopy is the standard for colon cancer screening and prevention; however, precancerous polyps can be missed for various reasons, ranging from subtle surface appearance of the polyp or location behind a colonic fold to operator-dependent reasons such as inadequate mucosal inspection. Though clinical practice guidelines have set adenoma detection rate (ADR) thresholds at 20% for women and 30% for men, studies have shown a 4- to 10-fold variation in ADR among physicians in clinical practice settings,1 with an estimated adenoma miss rate (AMR) of 25% and a false-negative colonoscopy rate of 12%.2 Variability in adenoma detection affects the risk of interval colorectal cancer post colonoscopy.3,4
AI provides an opportunity for mitigating this risk. Advances in deep learning and computer vision have led to the development of CADe systems that automatically detect polyps in real time during colonoscopy, resulting in reduced adenoma miss rates (Table 1). In addition to polyp detection, deep-learning technologies are also being used in CADx systems for polyp diagnosis and characterization of malignancy risk. This could aid therapeutic decision-making: Unnecessary resection or histopathologic analysis could be obviated for benign hyperplastic polyps. On the other end of the polyp spectrum, an AI tool that could predict the presence or absence of submucosal invasion could be a powerful tool when evaluating early colon cancers for consideration of endoscopic submucosal dissection vs. surgery. Examples of CADe polyp detection and CADx polyp characterization are shown in Figure 1.

Other potential computer vision applications that may improve colonoscopy quality include tools that help measure adequacy of mucosal exposure, segmental inspection time, and a variety of other parameters associated with polyp detection performance. These are promising areas for future research. Beyond improving colonoscopy technique, natural language processing tools already are being used to optimize clinical documentation as well as extract information from colonoscopy and pathology reports that can facilitate reporting of colonoscopy quality metrics such as ADR, cecal intubation rate, withdrawal time, and bowel preparation adequacy. AI-powered analytics may help unlock large-scale reporting of colonoscopy quality metrics on a health-systems level5 or population-level,6 helping to ensure optimal performance and identifying avenues for colonoscopy quality improvement.
The majority of AI research in colonoscopy has focused on CADe for colon polyp detection and CADx for polyp diagnosis. Over the last few years, several randomized clinical trials – two in the United States – have shown that CADe significantly improves adenoma detection and reduces adenoma miss rates in comparison to standard colonoscopy. The existing data are summarized in Table 1, focusing on the two U.S. studies and an international meta-analysis.
In comparison, the data landscape for CADx is nascent and currently limited to several retrospective studies dating back to 2009 and a few prospective studies that have shown promising results.10,11 There is an expectation that integrated CADx also may support the adoption of “resect and discard” or “diagnose and leave” strategies for low-risk polyps. About two-thirds of polyps identified on average-risk screening colonoscopies are diminutive polyps (less than 5 mm in size), which rarely have advanced histologic features (about 0.5%) and are sometimes non-neoplastic (30%). Malignancy risk is even lower in the distal colon.12 As routine histopathologic assessment of such polyps is mostly of limited clinical utility and comes with added pathology costs, CADx technologies may offer a more cost-effective approach where polyps that are characterized in real-time as low-risk adenomas or non-neoplastic are “resected and discarded” or “left in” respectively. In 2011, prior to the development of current AI tools, the American Society for Gastrointestinal Endoscopy set performance thresholds for technologies supporting real-time endoscopic assessment of the histology of diminutive colorectal polyps. The ASGE recommended 90% histopathologic concordance for “resect and discard” tools and 90% negative predictive value for adenomatous histology for “diagnose and leave,” tools.13 Narrow-band imaging (NBI), for example, has been shown to meet these benchmarks14,15 with a modeling study suggesting that implementing “resect and discard” strategies with such tools could result in annual savings of $33 million without adversely affecting efficacy, although practical adoption has been limited.16 More recent work has directly explored the feasibility of leveraging CADx to support “leave-in-situ” and “resect-and-discard” strategies.17
Similarly, while CADe use in colonoscopy is associated with additional up-front costs, a modeling study suggests that its associated gains in ADR (as detailed in Table 1) make it a cost-saving strategy for colorectal cancer prevention in the long term.18 There is still uncertainty on whether the incremental CADe-associated gains in adenoma detection will necessarily translate to significant reductions in interval colorectal cancer risk, particularly for endoscopists who are already high-performing polyp detectors. A recent study suggests that, although higher ADRs were associated with lower rates of interval colorectal cancer, the gains in interval colorectal cancer risk reduction appeared to level off with ADRs above 35%-40% (this finding may be limited by statistical power).19 Further, most of the data from CADe trials suggest that gains in adenoma detection are not driven by increased detection of advanced lesions with high malignancy risk but by small polyps with long latency periods of about 5-10 years, which may not significantly alter interval cancer risk. It remains to be determined whether adoption of CADe will have an impact on hard outcomes, most importantly interval colorectal cancer risk, or merely result in increased resource utilization without moving the needle on colorectal cancer prevention. To answer this question, the OperA study – a large-scale randomized clinical trial of 200,000 patients across 18 centers from 13 countries – was launched in 2022. It will investigate the effect of colonoscopy with CADe on a number of critical measures, including long-term interval colon cancer risk.20
Despite commercial availability of regulatory-approved CADe systems and data supporting use for adenoma detection in colonoscopy, mainstream adoption in clinical practice has been sluggish. Physician survey studies have shown that, although there is considerable interest in integrating CADe into clinical practice, there are concerns about access, cost and reimbursement, integration into clinical work-flow, increased procedural times, over-reliance on AI, and algorithmic bias leading to errors.21,22 In addition, without mandatory requirements for ADR reporting or clinical practice guideline recommendations for CADe use, these systems may not be perceived as valuable or ready for prime time even though the evidence suggests otherwise.23,24 For CADe systems to see widespread adoption in clinical practice, it is important that future research studies rigorously investigate and characterize these potential barriers to better inform strategies to address AI hesitancy and implementation challenges. Such efforts can provide an integration framework for future AI applications in gastroenterology beyond colonoscopy, such as CADe of esophageal and gastric premalignant lesions in upper endoscopy, CADx for pancreatic cysts and liver lesions on imaging, NLP tools to optimizing efficient clinical documentation and reporting, and many others.
Dr. Uche-Anya is in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston. Dr. Berzin is with the Center for Advanced Endoscopy, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston. Dr. Berzin is a consultant for Wision AI, Medtronic, Magentiq Eye, RSIP Vision, and Docbot.
Corresponding Author: Eugenia Uche-Anya [email protected] Twitter: @UcheAnyaMD @tberzin
References
1. Corley DA et al. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. Sep 2011;74(3):656-65. doi: 10.1016/j.gie.2011.04.017.
2. Zhao S et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology. 05 2019;156(6):1661-74.e11. doi: 10.1053/j.gastro.2019.01.260.
3. Kaminski MF et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. May 13 2010;362(19):1795-803. doi: 10.1056/NEJMoa0907667.
4. Corley DA et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. Apr 03 2014;370(14):1298-306. doi: 10.1056/NEJMoa1309086.
5. Laique SN et al. Application of optical character recognition with natural language processing for large-scale quality metric data extraction in colonoscopy reports. Gastrointest Endosc. 03 2021;93(3):750-7. doi: 10.1016/j.gie.2020.08.038.
6. Tinmouth J et al. Validation of a natural language processing algorithm to identify adenomas and measure adenoma detection rates across a health system: a population-level study. Gastrointest Endosc. Jul 14 2022. doi: 10.1016/j.gie.2022.07.009.
7. Glissen Brown JR et al. Deep learning computer-aided polyp detection reduces adenoma miss rate: A United States multi-center randomized tandem colonoscopy study (CADeT-CS Trial). Clin Gastroenterol Hepatol. 07 2022;20(7):1499-1507.e4. doi: 10.1016/j.cgh.2021.09.009.
8. Wallace MB et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology. 07 2022;163(1):295-304.e5. doi: 10.1053/j.gastro.2022.03.007.
9. Hassan C et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 01 2021;93(1):77-85.e6. doi: 10.1016/j.gie.2020.06.059.
10. Glissen Brown JR and Berzin TM. Adoption of new technologies: Artificial intelligence. Gastrointest Endosc Clin N Am. Oct 2021;31(4):743-58. doi: 10.1016/j.giec.2021.05.010.
11. Larsen SLV and Mori Y. Artificial intelligence in colonoscopy: A review on the current status. DEN open. Apr 2022;2(1):e109. doi: 10.1002/deo2.109.
12. Gupta N et al. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc. May 2012;75(5):1022-30. doi: 10.1016/j.gie.2012.01.020.
13. Rex DK et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2011;73(3):419-22. doi: 10.1016/j.gie.2011.01.023.
14. Abu Dayyeh BK et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. Mar 2015;81(3):502.e1-16. doi: 10.1016/j.gie.2014.12.022.
15. Mori Y et al. Real-time use of artificial intelligence in identification of diminutive polyps during colonoscopy: A prospective study. Ann Intern Med. Sep 18 2018;169(6):357-66. doi: 10.7326/M18-0249.
16. Hassan C et al.. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. Oct 2010;8(10):865-9, 869.e1-3. doi: 10.1016/j.cgh.2010.05.018.
17. Hassan C et al. Artificial intelligence allows leaving-in-situ colorectal polyps. Clin Gastroenterol Hepatol. Nov 2022;20(11):2505-13.e4. doi: 10.1016/j.cgh.2022.04.045.
18. Areia M et al. Cost-effectiveness of artificial intelligence for screening colonoscopy: a modelling study. Lancet Digit Health. 06 2022;4(6):e436-44. doi: 10.1016/S2589-7500(22)00042-5.
19. Schottinger JE et al. Association of physician adenoma detection rates with postcolonoscopy colorectal cancer. JAMA. 2022 Jun 7;327(21):2114-22. doi: 10.1001/jama.2022.6644.
20. Oslo Uo. Optimising colorectal cancer prevention through personalised treatment with artificial intelligence. 2022.
21. Wadhwa V et al. Physician sentiment toward artificial intelligence (AI) in colonoscopic practice: a survey of US gastroenterologists. Endosc Int Open. Oct 2020;8(10):E1379-84. doi: 10.1055/a-1223-1926.
22. Kader R et al. Survey on the perceptions of UK gastroenterologists and endoscopists to artificial intelligence. Frontline Gastroenterol. 2022;13(5):423-9. doi: 10.1136/flgastro-2021-101994.
23. Rex DKet al. Artificial intelligence improves detection at colonoscopy: Why aren’t we all already using it? Gastroenterology. 07 2022;163(1):35-7. doi: 10.1053/j.gastro.2022.04.042.
24. Ahmad OF et al. Establishing key research questions for the implementation of artificial intelligence in colonoscopy: A modified Delphi method. Endoscopy. 09 2021;53(9):893-901. doi: 10.1055/a-1306-7590
How does SARS-CoV-2 affect other respiratory diseases?
In 2020, the rapid spread of the newly identified SARS-CoV-2 coronavirus led various global public health institutions to establish strategies to stop transmission and reduce mortality. Nonpharmacological measures – including social distancing, regular hand washing, and the use of face masks – contributed to reducing the impact of the COVID-19 pandemic on health systems in different regions of the world. However, because of the implementation of these measures, the transmission of other infectious agents also experienced a marked reduction.
Approximately 3 years after the start of the pandemic, , generating phenomena ranging from an immunity gap, which favors the increase in some diseases, to the apparent disappearance of an influenza virus lineage.
Understanding the phenomenon
In mid-2021, doctors and researchers around the world began to share their opinions about the side effect of the strict measures implemented to contain COVID-19.
In May 2021, along with some coresearchers, Emmanuel Grimprel, MD, of the Pediatric Infectious Pathology Group in Créteil, France, wrote for Infectious Disease Now, “The transmission of some pathogens is often similar to that of SARS-CoV-2, essentially large droplets, aerosols, and direct hand contact, often with lower transmissibility. The lack of immune system stimulation due to nonpharmaceutical measures induces an ‘immune debt’ that may have negative consequences when the pandemic is under control.” According to the authors, mathematical models evaluated up to that point were already suggesting that the respiratory syncytial virus (RSV) and influenza A epidemics would be more serious in subsequent years.
In July 2022, a commentary in The Lancet led by Kevin Messacar, MD, of the University of Colorado at Denver, Aurora, grew in relevance and gave prominence to the phenomenon. In the commentary, Dr. Messacar and a group of experts explained how the decrease in exposure to endemic viruses had given rise to an immunity gap.
“The immunity gap phenomenon that has been reported in articles such as The Lancet publication is mainly due to the isolation that took place to prevent SARS-CoV-2 infections. Although this distancing was a good response to combat infections, or at least delay them while coronavirus research advanced, what we are now experiencing is the increase in cases of respiratory diseases caused by other agents such as respiratory syncytial virus and influenza due to lack of exposure,” as explained to this news organization by Erandeni Martínez Jiménez, biomedicine graduate and member of the Medical Virology Laboratory of the Mexican Institute of Social Security, at the Zone No. 5 General Hospital in Metepec-Atlixco, Mexico.
“This phenomenon occurs in all age groups. However, it is more evident in children and babies, since at their age, they have been exposed to fewer pathogens and, when added to isolation, makes this immunity gap more evident. Many immunologists compare this to hygiene theory in which it is explained that a ‘sterile’ environment will cause children to avoid the everyday and common pathogens required to be able to develop an adequate immune system,” added Martínez Jimenez.
“In addition, due to the isolation, the vaccination rate in children decreased, since many parents did not risk their children going out. This causes the immunity gap to grow even further as these children are not protected against common pathogens. While a mother passes antibodies to the child through the uterus via her placenta, the mother will only pass on those antibodies to which she has been exposed and as expected due to the lockdown, exposure to other pathogens has been greatly reduced.”
On the other hand, Andreu Comas, MD, PhD, MHS, of the Center for Research in Health Sciences and Biomedicine of the Autonomous University of San Luis Potosí (Mexico), considered that there are other immunity gaps that are not limited to respiratory infections and that are related to the fall in vaccination coverage. “Children are going to experience several immunity gaps. In the middle of the previous 6-year term, we had a vaccination schedule coverage of around 70% for children. Now that vaccination coverage has fallen to 30%, today we have an immunity gap for measles, rubella, mumps, tetanus, diphtheria, whooping cough, and meningeal tuberculosis. We have a significant growth or risk for other diseases.”
Lineage extinction
Three types of influenza viruses – A, B, and C – cause infections in humans. Although influenza A virus is the main type associated with infections during seasonal periods, as of 2020, influenza B virus was considered the causative agent of about a quarter of annual influenza cases.
During the onset of the COVID-19 pandemic, cocirculation of the two distinct lineages of influenza B viruses, B/Victoria/2/1987 (B/Victoria) and B/Yamagata/16/1988 (B/Yamagata), decreased significantly. According to data from the FluNet tool, which is coordinated by the World Health Organization, since March 2020 the isolation or sequencing of viruses belonging to the Yamagata lineage was not conclusively carried out.
Specialists like John Paget, PhD, from the Netherlands Institute for Health Services Research (Nivel) in Utrecht, have indicated that determining the extinction of the B/Yamagata lineage is critical. There is the possibility of a reintroduction of the lineage, as has occurred in the past with the reemergence of influenza A (H1N1) in 1997, which could represent a risk in subsequent years.
“In the next few years, research related to viruses such as influenza B and the impact on population immunity will be important. Let’s remember that influenza changes every year due to its characteristics, so a lack of exposure will also have an impact on the development of the disease,” said Martínez Jiménez.
Vaccination is essential
According to Dr. Comas, the only way to overcome the immunity gap phenomenon is through vaccination campaigns. “There is no other way to overcome the phenomenon, and how fast it is done will depend on the effort,” he said.
“In the case of COVID-19, it is not planned to vaccinate children under 5 years of age, and if we do not vaccinate children under 5 years of age, that gap will exist. In addition, this winter season will be important to know whether we are already endemic or not. It will be the key point, and it will determine if we will have a peak or not in the summer.
“In the case of the rest of the diseases, we need to correct what has been deficient in different governments, and we are going to have the resurgence of other infectious diseases that had already been forgotten. We have the example of poliomyelitis, the increase in meningeal tuberculosis, and we will have an increase in whooping cough and pertussislike syndrome. In this sense, we are going back to the point where Mexico and the world were around the ‘60s and ‘70s, and we have to be very alert to detect, isolate, and revaccinate.”
Finally, Dr. Comas called for continuing precautionary measures before the arrival of the sixth wave. “At a national level, the sixth wave of COVID-19 has already begun, and an increase in cases is expected in January. Regarding vaccines, if you are over 18 years of age and have not had any vaccine dose, you can get Abdala, however, there are no studies on this vaccine as a booster, and it is not authorized by the Mexican government for this purpose. Therefore, it is necessary to continue with measures such as the use of face masks in crowded places or with poor ventilation, and in the event of having symptoms, avoid going out and encourage ventilation at work and schools. If we do this, at least in the case of diseases that are transmitted by the respiratory route, the impact will be minimal.”
Martínez Jiménez and Dr. Comas have disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish Edition.
A version of this article first appeared on Medscape.com.
In 2020, the rapid spread of the newly identified SARS-CoV-2 coronavirus led various global public health institutions to establish strategies to stop transmission and reduce mortality. Nonpharmacological measures – including social distancing, regular hand washing, and the use of face masks – contributed to reducing the impact of the COVID-19 pandemic on health systems in different regions of the world. However, because of the implementation of these measures, the transmission of other infectious agents also experienced a marked reduction.
Approximately 3 years after the start of the pandemic, , generating phenomena ranging from an immunity gap, which favors the increase in some diseases, to the apparent disappearance of an influenza virus lineage.
Understanding the phenomenon
In mid-2021, doctors and researchers around the world began to share their opinions about the side effect of the strict measures implemented to contain COVID-19.
In May 2021, along with some coresearchers, Emmanuel Grimprel, MD, of the Pediatric Infectious Pathology Group in Créteil, France, wrote for Infectious Disease Now, “The transmission of some pathogens is often similar to that of SARS-CoV-2, essentially large droplets, aerosols, and direct hand contact, often with lower transmissibility. The lack of immune system stimulation due to nonpharmaceutical measures induces an ‘immune debt’ that may have negative consequences when the pandemic is under control.” According to the authors, mathematical models evaluated up to that point were already suggesting that the respiratory syncytial virus (RSV) and influenza A epidemics would be more serious in subsequent years.
In July 2022, a commentary in The Lancet led by Kevin Messacar, MD, of the University of Colorado at Denver, Aurora, grew in relevance and gave prominence to the phenomenon. In the commentary, Dr. Messacar and a group of experts explained how the decrease in exposure to endemic viruses had given rise to an immunity gap.
“The immunity gap phenomenon that has been reported in articles such as The Lancet publication is mainly due to the isolation that took place to prevent SARS-CoV-2 infections. Although this distancing was a good response to combat infections, or at least delay them while coronavirus research advanced, what we are now experiencing is the increase in cases of respiratory diseases caused by other agents such as respiratory syncytial virus and influenza due to lack of exposure,” as explained to this news organization by Erandeni Martínez Jiménez, biomedicine graduate and member of the Medical Virology Laboratory of the Mexican Institute of Social Security, at the Zone No. 5 General Hospital in Metepec-Atlixco, Mexico.
“This phenomenon occurs in all age groups. However, it is more evident in children and babies, since at their age, they have been exposed to fewer pathogens and, when added to isolation, makes this immunity gap more evident. Many immunologists compare this to hygiene theory in which it is explained that a ‘sterile’ environment will cause children to avoid the everyday and common pathogens required to be able to develop an adequate immune system,” added Martínez Jimenez.
“In addition, due to the isolation, the vaccination rate in children decreased, since many parents did not risk their children going out. This causes the immunity gap to grow even further as these children are not protected against common pathogens. While a mother passes antibodies to the child through the uterus via her placenta, the mother will only pass on those antibodies to which she has been exposed and as expected due to the lockdown, exposure to other pathogens has been greatly reduced.”
On the other hand, Andreu Comas, MD, PhD, MHS, of the Center for Research in Health Sciences and Biomedicine of the Autonomous University of San Luis Potosí (Mexico), considered that there are other immunity gaps that are not limited to respiratory infections and that are related to the fall in vaccination coverage. “Children are going to experience several immunity gaps. In the middle of the previous 6-year term, we had a vaccination schedule coverage of around 70% for children. Now that vaccination coverage has fallen to 30%, today we have an immunity gap for measles, rubella, mumps, tetanus, diphtheria, whooping cough, and meningeal tuberculosis. We have a significant growth or risk for other diseases.”
Lineage extinction
Three types of influenza viruses – A, B, and C – cause infections in humans. Although influenza A virus is the main type associated with infections during seasonal periods, as of 2020, influenza B virus was considered the causative agent of about a quarter of annual influenza cases.
During the onset of the COVID-19 pandemic, cocirculation of the two distinct lineages of influenza B viruses, B/Victoria/2/1987 (B/Victoria) and B/Yamagata/16/1988 (B/Yamagata), decreased significantly. According to data from the FluNet tool, which is coordinated by the World Health Organization, since March 2020 the isolation or sequencing of viruses belonging to the Yamagata lineage was not conclusively carried out.
Specialists like John Paget, PhD, from the Netherlands Institute for Health Services Research (Nivel) in Utrecht, have indicated that determining the extinction of the B/Yamagata lineage is critical. There is the possibility of a reintroduction of the lineage, as has occurred in the past with the reemergence of influenza A (H1N1) in 1997, which could represent a risk in subsequent years.
“In the next few years, research related to viruses such as influenza B and the impact on population immunity will be important. Let’s remember that influenza changes every year due to its characteristics, so a lack of exposure will also have an impact on the development of the disease,” said Martínez Jiménez.
Vaccination is essential
According to Dr. Comas, the only way to overcome the immunity gap phenomenon is through vaccination campaigns. “There is no other way to overcome the phenomenon, and how fast it is done will depend on the effort,” he said.
“In the case of COVID-19, it is not planned to vaccinate children under 5 years of age, and if we do not vaccinate children under 5 years of age, that gap will exist. In addition, this winter season will be important to know whether we are already endemic or not. It will be the key point, and it will determine if we will have a peak or not in the summer.
“In the case of the rest of the diseases, we need to correct what has been deficient in different governments, and we are going to have the resurgence of other infectious diseases that had already been forgotten. We have the example of poliomyelitis, the increase in meningeal tuberculosis, and we will have an increase in whooping cough and pertussislike syndrome. In this sense, we are going back to the point where Mexico and the world were around the ‘60s and ‘70s, and we have to be very alert to detect, isolate, and revaccinate.”
Finally, Dr. Comas called for continuing precautionary measures before the arrival of the sixth wave. “At a national level, the sixth wave of COVID-19 has already begun, and an increase in cases is expected in January. Regarding vaccines, if you are over 18 years of age and have not had any vaccine dose, you can get Abdala, however, there are no studies on this vaccine as a booster, and it is not authorized by the Mexican government for this purpose. Therefore, it is necessary to continue with measures such as the use of face masks in crowded places or with poor ventilation, and in the event of having symptoms, avoid going out and encourage ventilation at work and schools. If we do this, at least in the case of diseases that are transmitted by the respiratory route, the impact will be minimal.”
Martínez Jiménez and Dr. Comas have disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish Edition.
A version of this article first appeared on Medscape.com.
In 2020, the rapid spread of the newly identified SARS-CoV-2 coronavirus led various global public health institutions to establish strategies to stop transmission and reduce mortality. Nonpharmacological measures – including social distancing, regular hand washing, and the use of face masks – contributed to reducing the impact of the COVID-19 pandemic on health systems in different regions of the world. However, because of the implementation of these measures, the transmission of other infectious agents also experienced a marked reduction.
Approximately 3 years after the start of the pandemic, , generating phenomena ranging from an immunity gap, which favors the increase in some diseases, to the apparent disappearance of an influenza virus lineage.
Understanding the phenomenon
In mid-2021, doctors and researchers around the world began to share their opinions about the side effect of the strict measures implemented to contain COVID-19.
In May 2021, along with some coresearchers, Emmanuel Grimprel, MD, of the Pediatric Infectious Pathology Group in Créteil, France, wrote for Infectious Disease Now, “The transmission of some pathogens is often similar to that of SARS-CoV-2, essentially large droplets, aerosols, and direct hand contact, often with lower transmissibility. The lack of immune system stimulation due to nonpharmaceutical measures induces an ‘immune debt’ that may have negative consequences when the pandemic is under control.” According to the authors, mathematical models evaluated up to that point were already suggesting that the respiratory syncytial virus (RSV) and influenza A epidemics would be more serious in subsequent years.
In July 2022, a commentary in The Lancet led by Kevin Messacar, MD, of the University of Colorado at Denver, Aurora, grew in relevance and gave prominence to the phenomenon. In the commentary, Dr. Messacar and a group of experts explained how the decrease in exposure to endemic viruses had given rise to an immunity gap.
“The immunity gap phenomenon that has been reported in articles such as The Lancet publication is mainly due to the isolation that took place to prevent SARS-CoV-2 infections. Although this distancing was a good response to combat infections, or at least delay them while coronavirus research advanced, what we are now experiencing is the increase in cases of respiratory diseases caused by other agents such as respiratory syncytial virus and influenza due to lack of exposure,” as explained to this news organization by Erandeni Martínez Jiménez, biomedicine graduate and member of the Medical Virology Laboratory of the Mexican Institute of Social Security, at the Zone No. 5 General Hospital in Metepec-Atlixco, Mexico.
“This phenomenon occurs in all age groups. However, it is more evident in children and babies, since at their age, they have been exposed to fewer pathogens and, when added to isolation, makes this immunity gap more evident. Many immunologists compare this to hygiene theory in which it is explained that a ‘sterile’ environment will cause children to avoid the everyday and common pathogens required to be able to develop an adequate immune system,” added Martínez Jimenez.
“In addition, due to the isolation, the vaccination rate in children decreased, since many parents did not risk their children going out. This causes the immunity gap to grow even further as these children are not protected against common pathogens. While a mother passes antibodies to the child through the uterus via her placenta, the mother will only pass on those antibodies to which she has been exposed and as expected due to the lockdown, exposure to other pathogens has been greatly reduced.”
On the other hand, Andreu Comas, MD, PhD, MHS, of the Center for Research in Health Sciences and Biomedicine of the Autonomous University of San Luis Potosí (Mexico), considered that there are other immunity gaps that are not limited to respiratory infections and that are related to the fall in vaccination coverage. “Children are going to experience several immunity gaps. In the middle of the previous 6-year term, we had a vaccination schedule coverage of around 70% for children. Now that vaccination coverage has fallen to 30%, today we have an immunity gap for measles, rubella, mumps, tetanus, diphtheria, whooping cough, and meningeal tuberculosis. We have a significant growth or risk for other diseases.”
Lineage extinction
Three types of influenza viruses – A, B, and C – cause infections in humans. Although influenza A virus is the main type associated with infections during seasonal periods, as of 2020, influenza B virus was considered the causative agent of about a quarter of annual influenza cases.
During the onset of the COVID-19 pandemic, cocirculation of the two distinct lineages of influenza B viruses, B/Victoria/2/1987 (B/Victoria) and B/Yamagata/16/1988 (B/Yamagata), decreased significantly. According to data from the FluNet tool, which is coordinated by the World Health Organization, since March 2020 the isolation or sequencing of viruses belonging to the Yamagata lineage was not conclusively carried out.
Specialists like John Paget, PhD, from the Netherlands Institute for Health Services Research (Nivel) in Utrecht, have indicated that determining the extinction of the B/Yamagata lineage is critical. There is the possibility of a reintroduction of the lineage, as has occurred in the past with the reemergence of influenza A (H1N1) in 1997, which could represent a risk in subsequent years.
“In the next few years, research related to viruses such as influenza B and the impact on population immunity will be important. Let’s remember that influenza changes every year due to its characteristics, so a lack of exposure will also have an impact on the development of the disease,” said Martínez Jiménez.
Vaccination is essential
According to Dr. Comas, the only way to overcome the immunity gap phenomenon is through vaccination campaigns. “There is no other way to overcome the phenomenon, and how fast it is done will depend on the effort,” he said.
“In the case of COVID-19, it is not planned to vaccinate children under 5 years of age, and if we do not vaccinate children under 5 years of age, that gap will exist. In addition, this winter season will be important to know whether we are already endemic or not. It will be the key point, and it will determine if we will have a peak or not in the summer.
“In the case of the rest of the diseases, we need to correct what has been deficient in different governments, and we are going to have the resurgence of other infectious diseases that had already been forgotten. We have the example of poliomyelitis, the increase in meningeal tuberculosis, and we will have an increase in whooping cough and pertussislike syndrome. In this sense, we are going back to the point where Mexico and the world were around the ‘60s and ‘70s, and we have to be very alert to detect, isolate, and revaccinate.”
Finally, Dr. Comas called for continuing precautionary measures before the arrival of the sixth wave. “At a national level, the sixth wave of COVID-19 has already begun, and an increase in cases is expected in January. Regarding vaccines, if you are over 18 years of age and have not had any vaccine dose, you can get Abdala, however, there are no studies on this vaccine as a booster, and it is not authorized by the Mexican government for this purpose. Therefore, it is necessary to continue with measures such as the use of face masks in crowded places or with poor ventilation, and in the event of having symptoms, avoid going out and encourage ventilation at work and schools. If we do this, at least in the case of diseases that are transmitted by the respiratory route, the impact will be minimal.”
Martínez Jiménez and Dr. Comas have disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish Edition.
A version of this article first appeared on Medscape.com.
Does appendectomy raise the risk for colorectal cancer?
In one part of a three-part analysis, researchers observed a 73% increase in CRC risk among appendectomy cases compared, with controls over a 20-year follow-up.
The study, published in Oncogene, suggests that appendectomy may promote colorectal tumorigenesis by influencing the gut microbiome and that surgeons should “more cautiously consider the necessity of appendectomy,” the authors concluded.
Charles Dinerstein, MD, who was not involved in the research, said that the findings are “intriguing,” but it’s “too soon to tell” what the potential clinical implications may be. For now, “I would not think those patients having undergone an appendectomy should have more intense screening,” said Dr. Dinerstein, medical director of the American Council on Science and Health.
A growing body of evidence suggests that microbes in the gut may play a role in CRC risk, and other research indicates that the appendix might play a role in maintaining the diversity of the gut microbiome. However, whether removing the appendix influences a person’s risk for CRC remains controversial.
In the current study, Feiyu Shi, MD, of The First Affiliated Hospital of Xi’an Jiaotong University, and colleagues sought to better understand a possible association between appendectomy and CRC risk.
The team performed a three-part study: (1) analyzed a population of 129,155 adults who had an appendectomy and those who did not to assess a possible clinical connection between appendectomy and CRC risk; (2) performed fecal metagenomics sequencing to evaluate characteristics of the gut microbiome in appendectomy cases versus matched normal controls without appendectomy; and (3) investigated a CRC mouse model with appendectomy to uncover a mechanism of appendectomy-induced colorectal tumorigenesis.
In the large epidemiological study, Dr. Shi and colleagues compared CRC risk in almost 44,000 appendectomy cases versus more than 85,000 age- and gender-matched nonappendectomy controls. The researchers found that, over the 20-year follow-up, the risk for CRC increased by 73% in appendectomy cases (adjusted hazard ratio, 1.73; P < .001). CRC risk and gut dysbiosis were more pronounced in adults older than 50 years with a history of appendectomy.
In the gut microbiome analysis, Dr. Shi’s team performed metagenomic sequencing on fecal samples from 314 participants – 157 appendectomy cases and 157 controls – and found significant alterations in the gut microbiome in appendectomy cases. The changes were characterized by enrichment of seven CRC-promoting bacteria, including Bacteroides vulgatus and Bacteroides fragilis, and depletion of five beneficial bacteria, including Collinsella aerofaciens and Enterococcus hirae.
Finally, to examine the influence of appendectomy on microbial dysbiosis and CRC tumorigenesis, Dr. Shi’s team performed an appendectomy or a sham procedure in a carcinogen-induced CRC mouse model and found that appendectomy appeared to promote CRC tumorigenesis by prompting gut dysbiosis.
Aasma Shaukat, MD, MPH, a gastroenterologist at NYU Langone Health, who was not involved in the research, urged caution in interpreting the findings, which “need confirmation in larger diverse cohorts.”
First, Dr. Shaukat explained, “the two groups are not comparable, even though [they were] matched for age and gender, and many known and unknown factors can explain the results.” For instance, information on which subjects underwent colon cancer screening is not known, which may explain differences.
Dr. Shaukat also cautioned that the researchers only profiled the microbiome in “a small group of individuals and a cross-sectional analysis is not sufficient to explain causation.”
The study had no commercial funding. Dr. Shi, Dr. Dinerstein, and Dr. Shaukat have no relevant conflicts of interest to report.
A version of this article first appeared on Medscape.com.
In one part of a three-part analysis, researchers observed a 73% increase in CRC risk among appendectomy cases compared, with controls over a 20-year follow-up.
The study, published in Oncogene, suggests that appendectomy may promote colorectal tumorigenesis by influencing the gut microbiome and that surgeons should “more cautiously consider the necessity of appendectomy,” the authors concluded.
Charles Dinerstein, MD, who was not involved in the research, said that the findings are “intriguing,” but it’s “too soon to tell” what the potential clinical implications may be. For now, “I would not think those patients having undergone an appendectomy should have more intense screening,” said Dr. Dinerstein, medical director of the American Council on Science and Health.
A growing body of evidence suggests that microbes in the gut may play a role in CRC risk, and other research indicates that the appendix might play a role in maintaining the diversity of the gut microbiome. However, whether removing the appendix influences a person’s risk for CRC remains controversial.
In the current study, Feiyu Shi, MD, of The First Affiliated Hospital of Xi’an Jiaotong University, and colleagues sought to better understand a possible association between appendectomy and CRC risk.
The team performed a three-part study: (1) analyzed a population of 129,155 adults who had an appendectomy and those who did not to assess a possible clinical connection between appendectomy and CRC risk; (2) performed fecal metagenomics sequencing to evaluate characteristics of the gut microbiome in appendectomy cases versus matched normal controls without appendectomy; and (3) investigated a CRC mouse model with appendectomy to uncover a mechanism of appendectomy-induced colorectal tumorigenesis.
In the large epidemiological study, Dr. Shi and colleagues compared CRC risk in almost 44,000 appendectomy cases versus more than 85,000 age- and gender-matched nonappendectomy controls. The researchers found that, over the 20-year follow-up, the risk for CRC increased by 73% in appendectomy cases (adjusted hazard ratio, 1.73; P < .001). CRC risk and gut dysbiosis were more pronounced in adults older than 50 years with a history of appendectomy.
In the gut microbiome analysis, Dr. Shi’s team performed metagenomic sequencing on fecal samples from 314 participants – 157 appendectomy cases and 157 controls – and found significant alterations in the gut microbiome in appendectomy cases. The changes were characterized by enrichment of seven CRC-promoting bacteria, including Bacteroides vulgatus and Bacteroides fragilis, and depletion of five beneficial bacteria, including Collinsella aerofaciens and Enterococcus hirae.
Finally, to examine the influence of appendectomy on microbial dysbiosis and CRC tumorigenesis, Dr. Shi’s team performed an appendectomy or a sham procedure in a carcinogen-induced CRC mouse model and found that appendectomy appeared to promote CRC tumorigenesis by prompting gut dysbiosis.
Aasma Shaukat, MD, MPH, a gastroenterologist at NYU Langone Health, who was not involved in the research, urged caution in interpreting the findings, which “need confirmation in larger diverse cohorts.”
First, Dr. Shaukat explained, “the two groups are not comparable, even though [they were] matched for age and gender, and many known and unknown factors can explain the results.” For instance, information on which subjects underwent colon cancer screening is not known, which may explain differences.
Dr. Shaukat also cautioned that the researchers only profiled the microbiome in “a small group of individuals and a cross-sectional analysis is not sufficient to explain causation.”
The study had no commercial funding. Dr. Shi, Dr. Dinerstein, and Dr. Shaukat have no relevant conflicts of interest to report.
A version of this article first appeared on Medscape.com.
In one part of a three-part analysis, researchers observed a 73% increase in CRC risk among appendectomy cases compared, with controls over a 20-year follow-up.
The study, published in Oncogene, suggests that appendectomy may promote colorectal tumorigenesis by influencing the gut microbiome and that surgeons should “more cautiously consider the necessity of appendectomy,” the authors concluded.
Charles Dinerstein, MD, who was not involved in the research, said that the findings are “intriguing,” but it’s “too soon to tell” what the potential clinical implications may be. For now, “I would not think those patients having undergone an appendectomy should have more intense screening,” said Dr. Dinerstein, medical director of the American Council on Science and Health.
A growing body of evidence suggests that microbes in the gut may play a role in CRC risk, and other research indicates that the appendix might play a role in maintaining the diversity of the gut microbiome. However, whether removing the appendix influences a person’s risk for CRC remains controversial.
In the current study, Feiyu Shi, MD, of The First Affiliated Hospital of Xi’an Jiaotong University, and colleagues sought to better understand a possible association between appendectomy and CRC risk.
The team performed a three-part study: (1) analyzed a population of 129,155 adults who had an appendectomy and those who did not to assess a possible clinical connection between appendectomy and CRC risk; (2) performed fecal metagenomics sequencing to evaluate characteristics of the gut microbiome in appendectomy cases versus matched normal controls without appendectomy; and (3) investigated a CRC mouse model with appendectomy to uncover a mechanism of appendectomy-induced colorectal tumorigenesis.
In the large epidemiological study, Dr. Shi and colleagues compared CRC risk in almost 44,000 appendectomy cases versus more than 85,000 age- and gender-matched nonappendectomy controls. The researchers found that, over the 20-year follow-up, the risk for CRC increased by 73% in appendectomy cases (adjusted hazard ratio, 1.73; P < .001). CRC risk and gut dysbiosis were more pronounced in adults older than 50 years with a history of appendectomy.
In the gut microbiome analysis, Dr. Shi’s team performed metagenomic sequencing on fecal samples from 314 participants – 157 appendectomy cases and 157 controls – and found significant alterations in the gut microbiome in appendectomy cases. The changes were characterized by enrichment of seven CRC-promoting bacteria, including Bacteroides vulgatus and Bacteroides fragilis, and depletion of five beneficial bacteria, including Collinsella aerofaciens and Enterococcus hirae.
Finally, to examine the influence of appendectomy on microbial dysbiosis and CRC tumorigenesis, Dr. Shi’s team performed an appendectomy or a sham procedure in a carcinogen-induced CRC mouse model and found that appendectomy appeared to promote CRC tumorigenesis by prompting gut dysbiosis.
Aasma Shaukat, MD, MPH, a gastroenterologist at NYU Langone Health, who was not involved in the research, urged caution in interpreting the findings, which “need confirmation in larger diverse cohorts.”
First, Dr. Shaukat explained, “the two groups are not comparable, even though [they were] matched for age and gender, and many known and unknown factors can explain the results.” For instance, information on which subjects underwent colon cancer screening is not known, which may explain differences.
Dr. Shaukat also cautioned that the researchers only profiled the microbiome in “a small group of individuals and a cross-sectional analysis is not sufficient to explain causation.”
The study had no commercial funding. Dr. Shi, Dr. Dinerstein, and Dr. Shaukat have no relevant conflicts of interest to report.
A version of this article first appeared on Medscape.com.
FROM ONCOGENE
Why do GI symptoms persist in some children with celiac disease?
FROM WORLD JOURNAL OF GASTROENTEROLOGY
Developing FGIDs may be linked to caloric intake and percentage of food fat, but it does not change between a GFD with processed foods or a GFD with natural products.
These are the main findings of a study run jointly by the “Federico II” University of Naples and the “Luigi Vanvitelli” University of Campania, the results of which were published in the World Journal of Gastroenterology.
Unlike in previous studies, the criteria used in this study (the Rome IV criteria) allowed investigators to diagnose FGIDs even when other organic diseases, such as celiac disease or chronic inflammatory bowel disease, were present. The evidence obtained shows that adult individuals with celiac disease are at an increased risk for functional abdominal pain, even if they adhere well to a GFD. The researchers at the University of Campania wanted to determine the prevalence of FGIDs in the pediatric age group, which has been a poorly explored area.
The study authors enrolled 104 pediatric patients (aged 1-18 years) who had been diagnosed with celiac disease. The patients were randomly divided into two groups. Group A (n = 55) received a controlled GFD with processed foods (diet 1), and group B (n = 49) received a controlled GFD with > 60% natural products (diet 2). The presence of FGIDs was assessed at diagnosis (T0) and after 12 months (T1), and any potential link to the type of diet was analyzed.
The number of symptomatic children at enrollment was 30 of 55 (54.5%) in group A and 25 of 49 (51%) in group B. After 12 months, despite negative serology for celiac disease, the prevalence of FGIDs was 10/55 (18%) in group A and 8/49 (16.3%) in group B. There was no statistically significant difference between the two groups at T1. The most common disorder was functional constipation, followed by postprandial distress syndrome. At T1, the macro- and micronutrient intake was similar between the two groups, with no significant differences in nutrient analysis. However, in both groups, the prevalence of FGIDs was lower in patients who were consuming fewer calories (odds ratio [OR], 0.99; 95% confidence interval [CI], 0.99-1.00) and fat (OR, 0.33; 95% CI, 0.65-0.95). The figure was very close to being statistically significant (P = .055).
“This is the first study to show that the presence of functional GI symptoms in children with celiac disease on a GFD are possibly related to higher caloric and fat intake,” wrote the study authors. “It remains to be determined whether the risk is due to the persistence of a chronic inflammatory process or to nutritional factors. Long-term monitoring studies will assist in determining the natural history of these functional symptoms.”
The study authors reported having no relevant financial conflicts.
This article was translated from Univadis Italy and a version appeared on Medscape.com.
FROM WORLD JOURNAL OF GASTROENTEROLOGY
Developing FGIDs may be linked to caloric intake and percentage of food fat, but it does not change between a GFD with processed foods or a GFD with natural products.
These are the main findings of a study run jointly by the “Federico II” University of Naples and the “Luigi Vanvitelli” University of Campania, the results of which were published in the World Journal of Gastroenterology.
Unlike in previous studies, the criteria used in this study (the Rome IV criteria) allowed investigators to diagnose FGIDs even when other organic diseases, such as celiac disease or chronic inflammatory bowel disease, were present. The evidence obtained shows that adult individuals with celiac disease are at an increased risk for functional abdominal pain, even if they adhere well to a GFD. The researchers at the University of Campania wanted to determine the prevalence of FGIDs in the pediatric age group, which has been a poorly explored area.
The study authors enrolled 104 pediatric patients (aged 1-18 years) who had been diagnosed with celiac disease. The patients were randomly divided into two groups. Group A (n = 55) received a controlled GFD with processed foods (diet 1), and group B (n = 49) received a controlled GFD with > 60% natural products (diet 2). The presence of FGIDs was assessed at diagnosis (T0) and after 12 months (T1), and any potential link to the type of diet was analyzed.
The number of symptomatic children at enrollment was 30 of 55 (54.5%) in group A and 25 of 49 (51%) in group B. After 12 months, despite negative serology for celiac disease, the prevalence of FGIDs was 10/55 (18%) in group A and 8/49 (16.3%) in group B. There was no statistically significant difference between the two groups at T1. The most common disorder was functional constipation, followed by postprandial distress syndrome. At T1, the macro- and micronutrient intake was similar between the two groups, with no significant differences in nutrient analysis. However, in both groups, the prevalence of FGIDs was lower in patients who were consuming fewer calories (odds ratio [OR], 0.99; 95% confidence interval [CI], 0.99-1.00) and fat (OR, 0.33; 95% CI, 0.65-0.95). The figure was very close to being statistically significant (P = .055).
“This is the first study to show that the presence of functional GI symptoms in children with celiac disease on a GFD are possibly related to higher caloric and fat intake,” wrote the study authors. “It remains to be determined whether the risk is due to the persistence of a chronic inflammatory process or to nutritional factors. Long-term monitoring studies will assist in determining the natural history of these functional symptoms.”
The study authors reported having no relevant financial conflicts.
This article was translated from Univadis Italy and a version appeared on Medscape.com.
FROM WORLD JOURNAL OF GASTROENTEROLOGY
Developing FGIDs may be linked to caloric intake and percentage of food fat, but it does not change between a GFD with processed foods or a GFD with natural products.
These are the main findings of a study run jointly by the “Federico II” University of Naples and the “Luigi Vanvitelli” University of Campania, the results of which were published in the World Journal of Gastroenterology.
Unlike in previous studies, the criteria used in this study (the Rome IV criteria) allowed investigators to diagnose FGIDs even when other organic diseases, such as celiac disease or chronic inflammatory bowel disease, were present. The evidence obtained shows that adult individuals with celiac disease are at an increased risk for functional abdominal pain, even if they adhere well to a GFD. The researchers at the University of Campania wanted to determine the prevalence of FGIDs in the pediatric age group, which has been a poorly explored area.
The study authors enrolled 104 pediatric patients (aged 1-18 years) who had been diagnosed with celiac disease. The patients were randomly divided into two groups. Group A (n = 55) received a controlled GFD with processed foods (diet 1), and group B (n = 49) received a controlled GFD with > 60% natural products (diet 2). The presence of FGIDs was assessed at diagnosis (T0) and after 12 months (T1), and any potential link to the type of diet was analyzed.
The number of symptomatic children at enrollment was 30 of 55 (54.5%) in group A and 25 of 49 (51%) in group B. After 12 months, despite negative serology for celiac disease, the prevalence of FGIDs was 10/55 (18%) in group A and 8/49 (16.3%) in group B. There was no statistically significant difference between the two groups at T1. The most common disorder was functional constipation, followed by postprandial distress syndrome. At T1, the macro- and micronutrient intake was similar between the two groups, with no significant differences in nutrient analysis. However, in both groups, the prevalence of FGIDs was lower in patients who were consuming fewer calories (odds ratio [OR], 0.99; 95% confidence interval [CI], 0.99-1.00) and fat (OR, 0.33; 95% CI, 0.65-0.95). The figure was very close to being statistically significant (P = .055).
“This is the first study to show that the presence of functional GI symptoms in children with celiac disease on a GFD are possibly related to higher caloric and fat intake,” wrote the study authors. “It remains to be determined whether the risk is due to the persistence of a chronic inflammatory process or to nutritional factors. Long-term monitoring studies will assist in determining the natural history of these functional symptoms.”
The study authors reported having no relevant financial conflicts.
This article was translated from Univadis Italy and a version appeared on Medscape.com.
‘Very doable’ low-dose workout enough to treat knee OA
Exercise helps patients with knee osteoarthritis, but more isn’t necessarily better, new research shows.
A low-dose exercise regimen helped patients with knee OA about as much as a more intense workout plan, according to trial results published online in Annals of Internal Medicine.
Both high and low doses of exercise reduced pain and improved function and quality of life.
The improvements with the lower-dose plan and its 98% adherence rate are encouraging, said Nick Trasolini, MD, assistant professor of orthopedic surgery at Atrium Health Wake Forest Baptist Medical Center, Winston-Salem, N.C.
“This is a very doable amount of medical exercise therapy for patients with knee osteoarthritis, and one that makes a big difference in patient-reported symptoms,” Dr. Trasolini, who was not involved in the study, said in an interview.
What’s the right dose?
Exercise is a go-to treatment for knee OA, but the precise dose to recommend has been unclear. To study this question, Tom Arild Torstensen, MSc, RPT, with Karolinska Institutet, Huddinge, Sweden, and Holten Institute, Stockholm, and colleagues conducted a trial at four centers in Sweden and Norway.
The study included 189 men and women with knee OA. Participants were randomly assigned to low- or high-dose exercise plans, which they performed three times per week for 12 weeks under the supervision of a physiotherapist.
Participants in the high-dose group performed 11 exercises during each session, which lasted 70-90 minutes.
The low-dose regimen consisted of five exercises – cycling, squats, step-ups, step-downs, and knee extensions – performed for 20–30 minutes.
The researchers measured outcomes using the Knee Injury and Osteoarthritis Outcome Score, which assesses pain, other symptoms, function in daily living, function in sports and recreation, and knee-related quality of life.
“Patients in both groups improved significantly over time, but high-dose exercise was not superior to low-dose exercise in most comparisons,” the study investigators reported
High-dose exercise was associated with better function in sports and recreational activity and knee-related quality of life at 6 months. Those differences did not persist at 1 year, however. The researchers reported no safety concerns with either intervention.
Adherence was “nearly perfect” in the low-dose group. It was slightly lower in the high-dose group, the researchers said.
“Interestingly, it seems that high-dose treatment could be preferable to low-dose treatment in the long run for people who lead active lives,” they wrote. “This should be the subject of future studies.”
All clinical practice guidelines for knee OA recommend exercise, but “we do not know the optimal dose,” Kim Bennell, PhD, a research physiotherapist at the University of Melbourne, said in an interview.
Dose has components, including number of times per week, number of exercises, sets and repetitions, intensity, and duration of exercise sessions, Dr. Bennell said.
“These results suggest that an exercise program that involves less time and fewer exercises can still offer benefits and may be easier for patients to undertake and stick at than one that involves greater time and effort,” she said.
The study was supported by the Swedish Rheumatic Fund. Dr. Trasolini and Dr. Bennell have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Exercise helps patients with knee osteoarthritis, but more isn’t necessarily better, new research shows.
A low-dose exercise regimen helped patients with knee OA about as much as a more intense workout plan, according to trial results published online in Annals of Internal Medicine.
Both high and low doses of exercise reduced pain and improved function and quality of life.
The improvements with the lower-dose plan and its 98% adherence rate are encouraging, said Nick Trasolini, MD, assistant professor of orthopedic surgery at Atrium Health Wake Forest Baptist Medical Center, Winston-Salem, N.C.
“This is a very doable amount of medical exercise therapy for patients with knee osteoarthritis, and one that makes a big difference in patient-reported symptoms,” Dr. Trasolini, who was not involved in the study, said in an interview.
What’s the right dose?
Exercise is a go-to treatment for knee OA, but the precise dose to recommend has been unclear. To study this question, Tom Arild Torstensen, MSc, RPT, with Karolinska Institutet, Huddinge, Sweden, and Holten Institute, Stockholm, and colleagues conducted a trial at four centers in Sweden and Norway.
The study included 189 men and women with knee OA. Participants were randomly assigned to low- or high-dose exercise plans, which they performed three times per week for 12 weeks under the supervision of a physiotherapist.
Participants in the high-dose group performed 11 exercises during each session, which lasted 70-90 minutes.
The low-dose regimen consisted of five exercises – cycling, squats, step-ups, step-downs, and knee extensions – performed for 20–30 minutes.
The researchers measured outcomes using the Knee Injury and Osteoarthritis Outcome Score, which assesses pain, other symptoms, function in daily living, function in sports and recreation, and knee-related quality of life.
“Patients in both groups improved significantly over time, but high-dose exercise was not superior to low-dose exercise in most comparisons,” the study investigators reported
High-dose exercise was associated with better function in sports and recreational activity and knee-related quality of life at 6 months. Those differences did not persist at 1 year, however. The researchers reported no safety concerns with either intervention.
Adherence was “nearly perfect” in the low-dose group. It was slightly lower in the high-dose group, the researchers said.
“Interestingly, it seems that high-dose treatment could be preferable to low-dose treatment in the long run for people who lead active lives,” they wrote. “This should be the subject of future studies.”
All clinical practice guidelines for knee OA recommend exercise, but “we do not know the optimal dose,” Kim Bennell, PhD, a research physiotherapist at the University of Melbourne, said in an interview.
Dose has components, including number of times per week, number of exercises, sets and repetitions, intensity, and duration of exercise sessions, Dr. Bennell said.
“These results suggest that an exercise program that involves less time and fewer exercises can still offer benefits and may be easier for patients to undertake and stick at than one that involves greater time and effort,” she said.
The study was supported by the Swedish Rheumatic Fund. Dr. Trasolini and Dr. Bennell have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Exercise helps patients with knee osteoarthritis, but more isn’t necessarily better, new research shows.
A low-dose exercise regimen helped patients with knee OA about as much as a more intense workout plan, according to trial results published online in Annals of Internal Medicine.
Both high and low doses of exercise reduced pain and improved function and quality of life.
The improvements with the lower-dose plan and its 98% adherence rate are encouraging, said Nick Trasolini, MD, assistant professor of orthopedic surgery at Atrium Health Wake Forest Baptist Medical Center, Winston-Salem, N.C.
“This is a very doable amount of medical exercise therapy for patients with knee osteoarthritis, and one that makes a big difference in patient-reported symptoms,” Dr. Trasolini, who was not involved in the study, said in an interview.
What’s the right dose?
Exercise is a go-to treatment for knee OA, but the precise dose to recommend has been unclear. To study this question, Tom Arild Torstensen, MSc, RPT, with Karolinska Institutet, Huddinge, Sweden, and Holten Institute, Stockholm, and colleagues conducted a trial at four centers in Sweden and Norway.
The study included 189 men and women with knee OA. Participants were randomly assigned to low- or high-dose exercise plans, which they performed three times per week for 12 weeks under the supervision of a physiotherapist.
Participants in the high-dose group performed 11 exercises during each session, which lasted 70-90 minutes.
The low-dose regimen consisted of five exercises – cycling, squats, step-ups, step-downs, and knee extensions – performed for 20–30 minutes.
The researchers measured outcomes using the Knee Injury and Osteoarthritis Outcome Score, which assesses pain, other symptoms, function in daily living, function in sports and recreation, and knee-related quality of life.
“Patients in both groups improved significantly over time, but high-dose exercise was not superior to low-dose exercise in most comparisons,” the study investigators reported
High-dose exercise was associated with better function in sports and recreational activity and knee-related quality of life at 6 months. Those differences did not persist at 1 year, however. The researchers reported no safety concerns with either intervention.
Adherence was “nearly perfect” in the low-dose group. It was slightly lower in the high-dose group, the researchers said.
“Interestingly, it seems that high-dose treatment could be preferable to low-dose treatment in the long run for people who lead active lives,” they wrote. “This should be the subject of future studies.”
All clinical practice guidelines for knee OA recommend exercise, but “we do not know the optimal dose,” Kim Bennell, PhD, a research physiotherapist at the University of Melbourne, said in an interview.
Dose has components, including number of times per week, number of exercises, sets and repetitions, intensity, and duration of exercise sessions, Dr. Bennell said.
“These results suggest that an exercise program that involves less time and fewer exercises can still offer benefits and may be easier for patients to undertake and stick at than one that involves greater time and effort,” she said.
The study was supported by the Swedish Rheumatic Fund. Dr. Trasolini and Dr. Bennell have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Update on secondary cytoreduction in recurrent ovarian cancer
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
Recurrent ovarian cancer is difficult to treat; it has high recurrence rates and poor targeted treatment options. Between 60% and 75% of patients initially diagnosed with advanced-stage ovarian cancer will relapse within 2-3 years.1 Survival for these patients is poor, with an average overall survival (OS) of 30-40 months from the time of recurrence.2 Historically, immunotherapy has shown poor efficacy for recurrent ovarian malignancy, leaving few options for patients and their providers. Given the lack of effective treatment options, secondary cytoreductive surgery (surgery at the time of recurrence) has been heavily studied as a potential therapeutic option.
The initial rationale for cytoreductive surgery (CRS) in patients with advanced ovarian cancer focused on palliation of symptoms from large, bulky disease that frequently caused obstructive symptoms and pain. Now, cytoreduction is a critical part of therapy. It decreases chemotherapy-resistant tumor cells, improves the immune response, and is thought to optimize perfusion of the residual cancer for systemic therapy. The survival benefit of surgery in the frontline setting, either with primary or interval debulking, is well established, and much of the data now demonstrate that complete resection of all macroscopic disease (also known as an R0 resection) has the greatest survival benefit.3 Given the benefits of an initial debulking surgery, secondary cytoreduction has been studied since the 1980s with mixed results. These data have demonstrated that the largest barrier to care has been appropriate patient selection for this often complex surgical procedure.
The 2020 National Comprehensive Cancer Network guidelines list secondary CRS as a treatment option; however, the procedure should only be considered in patients who have platinum sensitive disease, a performance status of 0-1, no ascites, and an isolated focus or limited focus of disease that is amenable to complete resection. Numerous retrospective studies have suggested that secondary CRS is beneficial to patients with recurrent ovarian cancer, especially if complete cytoreduction can be accomplished. Many of these studies have similarly concluded that there are benefits, such as less ascites at the time of recurrence, smaller disease burden, and a longer disease-free interval. From that foundation, multiple groups used retrospective data to investigate prognostic models to determine who would benefit most from secondary cytoreduction.
The DESKTOP Group initially published their retrospective study in 2006 and created a scoring system assessing who would benefit from secondary CRS.4 Data demonstrated that a performance status of 0, FIGO stage of I/II at the time of initial diagnosis, no residual tumor after primary surgery, and ascites less than 500 mL were associated with improved survival after secondary cytoreduction. They created the AGO score out of these data, which is positive only if three criteria are met: a performance status of 0, R0 after primary debulk, and ascites less than 500 mL at the time of recurrence.
They prospectively tested this score in DESKTOP II, which validated their findings and showed that complete secondary CRS could be achieved in 76% of those with a positive AGO score.5 Many believed that the AGO score was too restrictive, and a second retrospective study performed by a group at Memorial Sloan Kettering showed that optimal secondary cytoreduction could be achieved to prolong survival by a median of 30 months in patients with a longer disease-free interval, a single site of recurrence, and residual disease measuring less than 5 mm at time of initial/first-line surgery.6 Many individuals now use this scoring system to determine candidacy for secondary debulking: disease-free interval, number of sites of recurrence (ideally oligometastatic disease), and residual disease less than 5 mm at the time of primary debulking.
Finally, the iMODEL was developed by a group from China and found that complete R0 secondary CRS was associated with a low initial FIGO stage, no residual disease after primary surgery, longer platinum-free interval, better Eastern Cooperative Oncology Group performance status, lower CA-125 levels, as well as no ascites at the time of recurrence. Based on these criteria, individuals received either high or low iMODEL scores, and those with a low score were said to be candidates for secondary CRS. Overall, these models demonstrate that the strongest predictive factor that suggests a survival benefit from secondary CRS is the ability to achieve a complete R0 resection at the time of surgery.
Secondary debulking surgery has been tested in three large randomized controlled trials. The DESKTOP investigators and the SOC-1 trial have been the most successful groups to publish on this topic with positive results. Both groups use prognostic models for their inclusion criteria to select candidates in whom an R0 resection is believed to be most feasible. The first randomized controlled trial to publish on this topic was GOG-213,7 which did not use prognostic modeling for their inclusion criteria. Patients were randomized to secondary cytoreduction followed by platinum-based chemotherapy with or without bevacizumab versus chemotherapy alone. The median OS was 50.6 months in the surgery group and 64.7 months in the no-surgery group (P = .08), suggesting no survival benefit to secondary cytoreduction; however, an ad hoc exploratory analysis of the surgery arm showed that both overall and progression-free survival were significantly improved in the complete cytoreduction group, compared with those with residual disease at time of surgery.
The results from the GOG-213 group suggested that improved survival from secondary debulking might be achieved when prognostic modeling is used to select optimal surgical candidates. The SOC-1 trial, published in 2021, was a phase 3, randomized, controlled trial that used the iMODEL scoring system combined with PET/CT imaging for patient selection.8 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Complete cytoreduction was achieved in 73% of patients with a low iMODEL score, and these data showed improved OS in the surgery group of 58.1 months versus 53.9 months (P < .05) in the no-surgery group. Lastly, the DESKTOP group most recently published results on this topic in a large randomized, controlled trial.9 Patients were again randomized to surgery followed by platinum-based chemotherapy versus chemotherapy alone. Inclusion criteria were only met in patients with a positive AGO score. An improved OS of 7.7 months (53.7 vs. 46 months; P < .05) was demonstrated in patients that underwent surgery versus those exposed to only chemotherapy. Again, this group showed that overall survival was further improved when complete cytoreduction was achieved.
Given the results of these three trials, the Society for Gynecologic Oncology has released a statement on secondary cytoreduction in recurrent ovarian cancer (see Table).10 While it is important to use caution when comparing the three studies as study populations differed substantially, the most important takeaway the difference in survival outcomes in patients in whom complete gross resection was achieved versus no complete gross resection versus no surgery. This comparison highlights the benefit of complete cytoreduction as well as the potential harms of secondary debulking when an R0 resection cannot be achieved. Although not yet evaluated in this clinical setting, laparoscopic exploration may be useful to augment assessment of disease extent and possibility of disease resection, just as it is in frontline ovarian cancer surgery.
The importance of bevacizumab use in recurrent ovarian cancer is also highlighted in the SGO statement. In GOG-213, 84% of the total study population (in both the surgery and no surgery cohort) were treated with concurrent followed by maintenance bevacizumab with an improved survival outcome, which may suggest that this trial generalizes better than the others to contemporary management of platinum-sensitive recurrent ovarian cancer.
Overall, given the mixed data, the recommendation is for surgeons to consider all available data to guide them in treatment planning with a strong emphasis on using all available technology to assess whether complete cytoreduction can be achieved in the setting of recurrence so as to not delay the patient’s ability to receive chemotherapy.
Dr. John is a gynecologic oncology fellow at the University of North Carolina at Chapel Hill. Dr. Tucker is assistant professor of gynecologic oncology at the university.
References
1. du Bois A et al. J Natl Cancer Inst. 2003;95:1320-9.
2. Wagner U et al. Br J Cancer. 2012;107:588-91.
3. Vergote I et al. N Engl J Med. 2010;363:943-53.
4. Harter P et al. Ann Surg Oncol. 2006;13:1702-10.
5. Harter P et al. Int J Gynecol Cancer. 2011;21:289-95.
6. Chi DS et al. Cancer. 2006 106:1933-9.
7. Coleman RL et al. Lancet Oncol. 2017;18:779-1.
8. Shi T et al. Lancet Oncol. 2021;22:439-49.
9. Harter P et al. N Engl J Med 2021;385:2123-31.
10. Harrison R, et al. Gynecol Oncol. 2021;163:448-52.
High-deductible health plans detrimental for those with diabetes
Individuals with diabetes who are forced to switch to high-deductible health plans have more episodes of severe hypo- and hyperglycemia compared with those on conventional insurance plans, according to a new study.
Previous studies have shown that people with diabetes who are enrolled in high-deductible health plans (HDHPs) have an increased financial burden, lower medication adherence, and more low-severity emergency department visits, and they delay care for cardiovascular conditions.
But no study has looked at the plans’ impact on acute diabetes complications and glycemic control, wrote the authors in JAMA Network Open.
They found evidence that the high-dollar plans were associated with increased odds of severe hypoglycemic and hyperglycemic events, and that the risk increased with each successive year of enrollment. Low-income individuals, Blacks, and Hispanics were disproportionately more impacted, noted senior author Rozalina G. McCoy, MD, Mayo Clinic, Rochester, Minn., and colleagues.
Overall, “enrollees may be rationing or forgoing necessary care, which is detrimental to their health and ultimately increases the morbidity, mortality, and costs associated with diabetes,” they concluded.
A systematic review of eight studies published in Endocrine Practice in 2021 backs up this latest finding. That analysis reported enrollees in HDHPs often forgo routine care and monitoring, and that they have lower medication adherence, leading to an increase in total health care expenditures for emergency department visits, hospitalizations, and preventable complications.
Increased frequency of hypoglycemia is detrimental
The new study published in JAMA Network Open was based on data for adults enrolled in private insurance programs from 2010 to 2018. Researchers analyzed medical and pharmacy claims data contained in a large health insurance claims database, comparing adults with diabetes who had been in an HDHP for at least 1 year (and after a year of being in a conventional plan), with those who were in a conventional plan.
They identified 42,326 individuals who had been switched from a conventional plan to an HDHP. Of those, 7,375 (17.4%) were Black, 5,740 (13.6%) were Hispanic, 26,572 (62.8%) were non-Hispanic White, and 6,880 (16.3%) had a household income below $40,000 a year.
Baseline characteristics of the 202,729 people in conventional plans were similar to those in the HDHP group.
The median deductible for individuals in the HDHP group was $1,500 and for families it was $3,000, compared with $350 and $800, respectively, for those in conventional plans.
The odds of having any severe hypoglycemic event were significantly higher in the HDHP group (odds ratio [OR], 1.11; P < .001). Each year of HDHP enrollment increased the odds of a hypoglycemia-related ED or hospital visit by 2% (OR, 1.02; P = .04).
Aware that only a small number of severe hypoglycemic events, as well as an unknown number of such events, result in an emergency department visit or hospitalization, and that “the decision to seek ED or hospital care may be influenced by health plan assignment,” the authors also looked at office visits where severe, or any, hypoglycemia or hyperglycemia was coded or documented.
The proportion of HDHP enrollees where hypoglycemia was coded was 14% higher than for conventional plan enrollees (OR, 1.14; P < .001), with each year of the high-dollar plan enrollment increasing these odds by 6% (OR, 1.06; P < .001).
The tally of hypoglycemic events is an underestimate because HDHP enrollees might forgo ambulatory care for cost-related reasons, wrote the authors. Hypoglycemia might also be treated at home. But that is not necessarily a positive, they noted.
“The increased frequency of severe hypoglycemia – no matter where managed and discussed – is a sign of detrimental effects of HDHP enrollment for people living with diabetes.”
They found that individuals of racial and ethnic minorities were less likely than were White patients to have an increase in hypoglycemia-related office visits, which suggests that those patients were deferring care, wrote Dr. McCoy and colleagues.
Switching to an HDHP was associated with a significant increase in the odds of having at least one hyperglycemia-related ED or hospital visit per year (OR, 1.25; P < .001). Each successive year in the plan increased these odds by 5% (OR, 1.05; P = .02). However, the authors found no increase in hyperglycemia-related office visits.
“Because severe dysglycemic events may be prevented with optimal glycemic management, the increase in the frequency of their occurrence suggests important gaps in access to and implementation of diabetes therapy,” wrote the authors.
They noted that people with diabetes already face high out-of-pocket expenses. A high-deductible plan might make care even less affordable, they wrote.
“Individuals may be forced to ration medications, glucose-monitoring supplies, diabetes self-management education, food, and other essential cares to the detriment of their health,” they noted.
The authors added that because the study was observational, they could not delve into the root causes of the glycemic events or whether, for instance, any HDHP enrollees also had health savings accounts (HSAs) that might help defray costs.
They suggested that employers offer a wide variety of health plans, or if they are offering only a high-deductible plan that they be more transparent about potential costs. “Previous studies have shown that enrollees are not fully aware of the details within their health plans and may be focusing on reducing the cost of monthly premiums – not overall care – when choosing health plans.”
The authors said employers should find ways to fund HSAs for people with low incomes – those who appear to be most vulnerable to the effects of HDHPs.
A study published in JAMA Internal Medicine in 2017 found that low-income and HSA-eligible individuals with diabetes switched to an HDHP had major increases in emergency department visits for preventable acute diabetes complications.
The study was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the Mayo Clinic K2R Research Award, and the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. Dr. McCoy has reported receiving grants from the NIDDK, AARP, and the Patient-Centered Outcomes Research Institute, and personal fees from Emmi for the development of patient education materials about diabetes outside the submitted work.
A version of this article first appeared on Medscape.com.
Individuals with diabetes who are forced to switch to high-deductible health plans have more episodes of severe hypo- and hyperglycemia compared with those on conventional insurance plans, according to a new study.
Previous studies have shown that people with diabetes who are enrolled in high-deductible health plans (HDHPs) have an increased financial burden, lower medication adherence, and more low-severity emergency department visits, and they delay care for cardiovascular conditions.
But no study has looked at the plans’ impact on acute diabetes complications and glycemic control, wrote the authors in JAMA Network Open.
They found evidence that the high-dollar plans were associated with increased odds of severe hypoglycemic and hyperglycemic events, and that the risk increased with each successive year of enrollment. Low-income individuals, Blacks, and Hispanics were disproportionately more impacted, noted senior author Rozalina G. McCoy, MD, Mayo Clinic, Rochester, Minn., and colleagues.
Overall, “enrollees may be rationing or forgoing necessary care, which is detrimental to their health and ultimately increases the morbidity, mortality, and costs associated with diabetes,” they concluded.
A systematic review of eight studies published in Endocrine Practice in 2021 backs up this latest finding. That analysis reported enrollees in HDHPs often forgo routine care and monitoring, and that they have lower medication adherence, leading to an increase in total health care expenditures for emergency department visits, hospitalizations, and preventable complications.
Increased frequency of hypoglycemia is detrimental
The new study published in JAMA Network Open was based on data for adults enrolled in private insurance programs from 2010 to 2018. Researchers analyzed medical and pharmacy claims data contained in a large health insurance claims database, comparing adults with diabetes who had been in an HDHP for at least 1 year (and after a year of being in a conventional plan), with those who were in a conventional plan.
They identified 42,326 individuals who had been switched from a conventional plan to an HDHP. Of those, 7,375 (17.4%) were Black, 5,740 (13.6%) were Hispanic, 26,572 (62.8%) were non-Hispanic White, and 6,880 (16.3%) had a household income below $40,000 a year.
Baseline characteristics of the 202,729 people in conventional plans were similar to those in the HDHP group.
The median deductible for individuals in the HDHP group was $1,500 and for families it was $3,000, compared with $350 and $800, respectively, for those in conventional plans.
The odds of having any severe hypoglycemic event were significantly higher in the HDHP group (odds ratio [OR], 1.11; P < .001). Each year of HDHP enrollment increased the odds of a hypoglycemia-related ED or hospital visit by 2% (OR, 1.02; P = .04).
Aware that only a small number of severe hypoglycemic events, as well as an unknown number of such events, result in an emergency department visit or hospitalization, and that “the decision to seek ED or hospital care may be influenced by health plan assignment,” the authors also looked at office visits where severe, or any, hypoglycemia or hyperglycemia was coded or documented.
The proportion of HDHP enrollees where hypoglycemia was coded was 14% higher than for conventional plan enrollees (OR, 1.14; P < .001), with each year of the high-dollar plan enrollment increasing these odds by 6% (OR, 1.06; P < .001).
The tally of hypoglycemic events is an underestimate because HDHP enrollees might forgo ambulatory care for cost-related reasons, wrote the authors. Hypoglycemia might also be treated at home. But that is not necessarily a positive, they noted.
“The increased frequency of severe hypoglycemia – no matter where managed and discussed – is a sign of detrimental effects of HDHP enrollment for people living with diabetes.”
They found that individuals of racial and ethnic minorities were less likely than were White patients to have an increase in hypoglycemia-related office visits, which suggests that those patients were deferring care, wrote Dr. McCoy and colleagues.
Switching to an HDHP was associated with a significant increase in the odds of having at least one hyperglycemia-related ED or hospital visit per year (OR, 1.25; P < .001). Each successive year in the plan increased these odds by 5% (OR, 1.05; P = .02). However, the authors found no increase in hyperglycemia-related office visits.
“Because severe dysglycemic events may be prevented with optimal glycemic management, the increase in the frequency of their occurrence suggests important gaps in access to and implementation of diabetes therapy,” wrote the authors.
They noted that people with diabetes already face high out-of-pocket expenses. A high-deductible plan might make care even less affordable, they wrote.
“Individuals may be forced to ration medications, glucose-monitoring supplies, diabetes self-management education, food, and other essential cares to the detriment of their health,” they noted.
The authors added that because the study was observational, they could not delve into the root causes of the glycemic events or whether, for instance, any HDHP enrollees also had health savings accounts (HSAs) that might help defray costs.
They suggested that employers offer a wide variety of health plans, or if they are offering only a high-deductible plan that they be more transparent about potential costs. “Previous studies have shown that enrollees are not fully aware of the details within their health plans and may be focusing on reducing the cost of monthly premiums – not overall care – when choosing health plans.”
The authors said employers should find ways to fund HSAs for people with low incomes – those who appear to be most vulnerable to the effects of HDHPs.
A study published in JAMA Internal Medicine in 2017 found that low-income and HSA-eligible individuals with diabetes switched to an HDHP had major increases in emergency department visits for preventable acute diabetes complications.
The study was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the Mayo Clinic K2R Research Award, and the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. Dr. McCoy has reported receiving grants from the NIDDK, AARP, and the Patient-Centered Outcomes Research Institute, and personal fees from Emmi for the development of patient education materials about diabetes outside the submitted work.
A version of this article first appeared on Medscape.com.
Individuals with diabetes who are forced to switch to high-deductible health plans have more episodes of severe hypo- and hyperglycemia compared with those on conventional insurance plans, according to a new study.
Previous studies have shown that people with diabetes who are enrolled in high-deductible health plans (HDHPs) have an increased financial burden, lower medication adherence, and more low-severity emergency department visits, and they delay care for cardiovascular conditions.
But no study has looked at the plans’ impact on acute diabetes complications and glycemic control, wrote the authors in JAMA Network Open.
They found evidence that the high-dollar plans were associated with increased odds of severe hypoglycemic and hyperglycemic events, and that the risk increased with each successive year of enrollment. Low-income individuals, Blacks, and Hispanics were disproportionately more impacted, noted senior author Rozalina G. McCoy, MD, Mayo Clinic, Rochester, Minn., and colleagues.
Overall, “enrollees may be rationing or forgoing necessary care, which is detrimental to their health and ultimately increases the morbidity, mortality, and costs associated with diabetes,” they concluded.
A systematic review of eight studies published in Endocrine Practice in 2021 backs up this latest finding. That analysis reported enrollees in HDHPs often forgo routine care and monitoring, and that they have lower medication adherence, leading to an increase in total health care expenditures for emergency department visits, hospitalizations, and preventable complications.
Increased frequency of hypoglycemia is detrimental
The new study published in JAMA Network Open was based on data for adults enrolled in private insurance programs from 2010 to 2018. Researchers analyzed medical and pharmacy claims data contained in a large health insurance claims database, comparing adults with diabetes who had been in an HDHP for at least 1 year (and after a year of being in a conventional plan), with those who were in a conventional plan.
They identified 42,326 individuals who had been switched from a conventional plan to an HDHP. Of those, 7,375 (17.4%) were Black, 5,740 (13.6%) were Hispanic, 26,572 (62.8%) were non-Hispanic White, and 6,880 (16.3%) had a household income below $40,000 a year.
Baseline characteristics of the 202,729 people in conventional plans were similar to those in the HDHP group.
The median deductible for individuals in the HDHP group was $1,500 and for families it was $3,000, compared with $350 and $800, respectively, for those in conventional plans.
The odds of having any severe hypoglycemic event were significantly higher in the HDHP group (odds ratio [OR], 1.11; P < .001). Each year of HDHP enrollment increased the odds of a hypoglycemia-related ED or hospital visit by 2% (OR, 1.02; P = .04).
Aware that only a small number of severe hypoglycemic events, as well as an unknown number of such events, result in an emergency department visit or hospitalization, and that “the decision to seek ED or hospital care may be influenced by health plan assignment,” the authors also looked at office visits where severe, or any, hypoglycemia or hyperglycemia was coded or documented.
The proportion of HDHP enrollees where hypoglycemia was coded was 14% higher than for conventional plan enrollees (OR, 1.14; P < .001), with each year of the high-dollar plan enrollment increasing these odds by 6% (OR, 1.06; P < .001).
The tally of hypoglycemic events is an underestimate because HDHP enrollees might forgo ambulatory care for cost-related reasons, wrote the authors. Hypoglycemia might also be treated at home. But that is not necessarily a positive, they noted.
“The increased frequency of severe hypoglycemia – no matter where managed and discussed – is a sign of detrimental effects of HDHP enrollment for people living with diabetes.”
They found that individuals of racial and ethnic minorities were less likely than were White patients to have an increase in hypoglycemia-related office visits, which suggests that those patients were deferring care, wrote Dr. McCoy and colleagues.
Switching to an HDHP was associated with a significant increase in the odds of having at least one hyperglycemia-related ED or hospital visit per year (OR, 1.25; P < .001). Each successive year in the plan increased these odds by 5% (OR, 1.05; P = .02). However, the authors found no increase in hyperglycemia-related office visits.
“Because severe dysglycemic events may be prevented with optimal glycemic management, the increase in the frequency of their occurrence suggests important gaps in access to and implementation of diabetes therapy,” wrote the authors.
They noted that people with diabetes already face high out-of-pocket expenses. A high-deductible plan might make care even less affordable, they wrote.
“Individuals may be forced to ration medications, glucose-monitoring supplies, diabetes self-management education, food, and other essential cares to the detriment of their health,” they noted.
The authors added that because the study was observational, they could not delve into the root causes of the glycemic events or whether, for instance, any HDHP enrollees also had health savings accounts (HSAs) that might help defray costs.
They suggested that employers offer a wide variety of health plans, or if they are offering only a high-deductible plan that they be more transparent about potential costs. “Previous studies have shown that enrollees are not fully aware of the details within their health plans and may be focusing on reducing the cost of monthly premiums – not overall care – when choosing health plans.”
The authors said employers should find ways to fund HSAs for people with low incomes – those who appear to be most vulnerable to the effects of HDHPs.
A study published in JAMA Internal Medicine in 2017 found that low-income and HSA-eligible individuals with diabetes switched to an HDHP had major increases in emergency department visits for preventable acute diabetes complications.
The study was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the Mayo Clinic K2R Research Award, and the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. Dr. McCoy has reported receiving grants from the NIDDK, AARP, and the Patient-Centered Outcomes Research Institute, and personal fees from Emmi for the development of patient education materials about diabetes outside the submitted work.
A version of this article first appeared on Medscape.com.
Managing patients with comorbid opioid and alcohol use disorders
When left untreated, opioid use disorder (OUD) is a debilitating and potentially lethal illness. Despite the availability of safe and effective medications for OUD, the prevalence of opioid use and overdose deaths has been increasing every year.1 An additional challenge in OUD treatment is the high prevalence of comorbid alcohol use disorder (AUD).2-6 A Clinical Trials Network survey from the National Institute on Drug Abuse found 38% of persons seeking treatment for OUD also had AUD.7 Other analyses have found alcohol was involved in approximately one-fifth of opioid-related deaths.8 Research also reveals that comorbid OUD and AUD contributes to poor treatment outcomes, more medical comorbidities, and a high risk of death (including overdose death).4,9 There is no standard of care for this particular patient population.3 This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
To illustrate the various decision points, we will follow 2 hypothetical patients through various stages of treatment (Figure), from their presentation in the emergency department (ED) or outpatient clinic, through their hospital admission (if needed), and into their outpatient follow-up treatment.
CASE REPORTS
Ms. A and Ms. B present to the ED for evaluation of nausea, vomiting, sweating, anxiety, and tremor. Both patients describe their most recent use of both alcohol and opioids approximately 12 hours ago, and each has been attempting to stop using both substances at home.
Decision-making in the emergency setting
In the ED, a few important decisions need to be made regarding treatment:
- Are the presenting symptoms primarily due to alcohol withdrawal syndrome (AWS), opioid withdrawal syndrome (OWS), or both?
- Does the patient require inpatient medical withdrawal management (detoxification) based on the history and severity of the withdrawal symptoms?
- What are the patient’s treatment goals for their AUD and OUD?
- Is maintenance medication for OUD indicated? If so, which medication is most appropriate?
In the ED, the presentation of individuals affected by both OUD and AUD can be challenging because OWS shares overlapping features with AWS, including nausea, vomiting, diarrhea, sweating, anxiety, and tremor. However, although acute OWS is typically very uncomfortable, it is rarely lethal. On the other hand, severe AWS may result in delirium, seizures, and death,10 which makes it essential to recognize and treat appropriately.
Both Ms. A and Ms. B should be medically evaluated and treated by an emergency medicine physician in conjunction with psychiatric (or addiction medicine) consultation. The ED assessment of a patient presenting with both AUD and OUD should include vital signs monitoring; physical examination; blood work including comprehensive metabolic panel, serum magnesium, and phosphorus; complete blood count; pregnancy test for women of reproductive age; urine drug screen (UDS); urinalysis; and serum ethanol level. Of note, sympathetic hyperactivity is found in both alcohol and opioid withdrawal, and patients with alcohol withdrawal may also have hypokalemia, a condition associated with an increased risk of arrhythmia. Furthermore, a prolonged QTc would affect clinical decision-making about medications for OUD (ie, methadone) and withdrawal management (ie, ondansetron, trazodone, and hydroxyzine). Therefore, an electrocardiogram should be conducted, where appropriate.
Initial treatment of AWS includes vitamin supplementation (thiamine, folic acid, and multivitamins) and benzodiazepine administration (symptom-triggered and/or scheduled taper). It may also include IV fluid resuscitation, analgesics for pain, ondansetron for nausea and vomiting, and other electrolyte repletion as indicated by the laboratory results.11 Additional measures for patients in opioid withdrawal should include alpha-2 agonists such as clonidine or lofexidine for adrenergic symptoms, antiemetics, antidiarrheals, muscle relaxants, anxiolytics such as hydroxyzine, and sleep medications such as trazodone.12
Continue to: The next decision...
The next decision is whether the patient needs to be admitted for inpatient treatment. This decision is based primarily on the risk assessment and severity of AWS, including a compelling history of complicated AWS such as seizures or delirium tremens as well as consideration of the complexity and severity of any comorbid medical or psychiatric conditions. Other indications for medical withdrawal management include a history of unsuccessful ambulatory withdrawal management and pregnancy. For severe AWS, a scheduled benzodiazepine taper in addition to the symptom-triggered protocol should be considered.13-15 A psychiatric evaluation may be obtained in the ED, as long as the patient is sober enough to meaningfully participate in the psychiatric interview. Wherever possible, psychiatric interviews should be supplemented by collateral information.
CASE REPORTS CONTINUED
Ms. A admits to a 5-year history of alcohol and opioid use that meets the criteria for severe AUD and severe OUD. She has previously required inpatient treatment for seizures related to AWS. Laboratory results are notable for a serum ethanol level of 380 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Disposition of patients in alcohol and opioid withdrawal
Given Ms. A’s history of seizures while withdrawing from alcohol, she is appropriate for hospital admission for medically managed withdrawal observation. As previously mentioned, there is clinical overlap between AWS and OWS, and differentiating between the 2 syndromes is essential and may be lifesaving. Whereas anxiety, agitation, diaphoresis, tachycardia, hypertension, and insomnia can be seen in both opioid and alcohol withdrawal, OWS-specific symptoms include mydriasis, lacrimation, rhinorrhea, bone or joint aches, yawning, and piloerection. AWS may present with visual or tactile hallucinations, delirium, and grand mal seizures.15
The details of inpatient management are beyond the scope of this article; however, both patients should be started on thiamine, folic acid, and a multivitamin. For patients in alcohol withdrawal with a history of poor diet who appear malnourished or have a history of malabsorption (such as gastric bypass surgery), thiamine 100 mg/d IV should be given for 3 to 5 days to prevent Wernicke encephalopathy.16 Where there is any concern the patient may be exhibiting signs of Wernicke-Korsakoff Syndrome (impaired cognition, evident malnourishment, ataxia, or eye movement abnormalities), high-dose thiamine IV should be given presumptively as follows: 500 mg IV 3 times a day for 3 days, 250 mg/d IV for 5 days, and then oral supplementation 100 mg/d for at least 30 days.17
In summary, on presentation to the ED, both patients should be medically stabilized and started on benzodiazepines for alcohol withdrawal. The risk assessment and the severity of the AWS often determines the level of care.
CASE REPORTS CONTINUED
On hospital Day 2, Ms. A tells the consulting psychiatrist she would like to start medications to treat her substance use disorders. She has a long history of failed attempts to achieve abstinence from opioids, so she and the psychiatrist agree to initiate a trial of buprenorphine/naloxone for her OUD, 4 mg/1 mg to 8 mg/2 mg for Day 1. Although buprenorphine/naloxone seems to help her alcohol cravings somewhat, she requests additional help. She experiences migraine headaches, which is in part why she began using opioid medications. Via joint decision making with her psychiatrist, she agrees to a trial of topiramate, with a slow titration schedule starting at 25 mg/d.
Continue to: Management decisions
Management decisions: Buprenorphine for OUD
The next issue is to determine the appropriate treatment for the patient’s OUD. Although treating OWS is important in improving the patient’s health, decreasing their discomfort, and facilitating their participation in a psychosocial treatment program,18 current evidence suggests that opioid withdrawal management alone without medication for OUD rarely leads to long-term recovery.19,20 Some research suggests that the risk of accidental opioid overdose immediately following acute withdrawal management may actually be increased due to decreased tolerance in these patients.12,21,22
Three medications have the most evidence for OUD treatment: buprenorphine, methadone, and naltrexone.15 The decision to use buprenorphine, methadone, or naltrexone depends on a variety of factors, including the severity of the OUD, patient history of prior treatment successes and failures, comorbid medical and psychiatric conditions, and patient preference.4 Treatment with buprenorphine or methadone is preferred over naltrexone for patients who do not want to or cannot tolerate the physical and emotional discomfort of the opioid withdrawal process, who experience moderate to severe OUD, who have a history of failed abstinence-based treatment, or who have more severe physiological tolerance/dependence.12 Buprenorphine is a mu opioid receptor partial agonist that has been shown to reduce opioid cravings,23 provide moderate pain relief,24 and ameliorate OWS.12 It does not typically result in significant respiratory depression, which is the biggest safety concern for opioid use.12 Buprenorphine may also treat comorbid AUD at higher doses; however, the data are inconclusive.25,26 Buprenorphine should be prescribed with caution to patients with comorbid, uncontrolled AUD, due to the risk of respiratory depression when combined with alcohol. Patients who continue to drink alcohol but are able to abstain from opioids may consider starting an AUD-specific medication. Pharmacologic options are discussed in more detail in the next section.
For patients who have higher physiological dependence or more severe OUD, methadone may be a reasonable alternative to buprenorphine. Methadone, a mu-opioid receptor agonist, ameliorates OWS, reduces opioid cravings, and reduces the euphoric effects of opioid ingestion if the patient relapses. However, methadone can only be dispensed for the treatment of OUD by a federally-certified treatment program governed by restrictive and federally mandated guidelines. Compared to buprenorphine, methadone is more dangerous in overdose, has more drug interactions, and is more commonly diverted for recreational use.27 Furthermore, methadone should be prescribed with caution to patients with comorbid, uncontrolled AUD, because both alcohol and methadone can result in respiratory depression.
By contrast, the first-line treatment for individuals experiencing moderateto severe AUD is typically naltrexone.28 Naltrexone is contraindicated in Ms. A because she has a severe OUD and is unlikely to tolerate the opioid withdrawal process. Research suggests that the use of naltrexone for OUD should be limited to patients who have a mild disorder or who show low physiological dependence.29 Alternatively, acamprosate, disulfiram, topiramate, or gabapentin should be considered for Ms. A.4,28,30 Because each of these medications have specific strengths and weaknesses, medication selection should be based on individual patient factors such as comorbid psychiatric and medical conditions and/or patient preference.28
Management decisions: AUD augmentation strategies
Naltrexone is contraindicated for patients who are receiving opioids, including opioid agonist therapy for OUD. Therefore, clinicians need to consider other options for these individuals. There are several medications with good evidence, including acamprosate, disulfiram, topiramate, and gabapentin. Acamprosate and disulfiram are FDA-approved for AUD; the latter 2 have been used off-label.
Continue to: Acamprosate is a glutamate receptor modulator...
Acamprosate is a glutamate receptor modulator that reduces alcohol cravings and is recommended for patients who have achieved and wish to maintain abstinence. It can be used in patients with liver disease, because it is not hepatically metabolized.30 Topiramate is also used to reduce alcohol cravings. It antagonizes glutamate at alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) and kainite receptors, facilitates gamma-aminobutyric acid (GABA) function, and reduces the extracellular release of dopamine in the mesocorticolimbic regions of the brain.30 Topiramate is a reasonable option for patients with a seizure disorder, a history of migraine headaches,30 or who are overweight or obese and wish to lose weight.31 In a nonrandomized study, topiramate reduced alcohol intake and cravings more than naltrexone.32
Disulfiram is another second-line therapy for AUD. It is best used under close supervision because it does not reduce alcohol cravings but makes ingesting alcohol extremely aversive by preventing the breakdown of the alcohol metabolite acetaldehyde, and in doing so causes a cluster of unpleasant symptoms, including sweating, palpitations, flushing, nausea/vomiting, and increased sympathetic tone.28 Disulfiram only works if it is taken daily, and it requires a high degree of motivation and/or daily supervision at home or in the clinic.33 It is not recommended to be used as a first-line treatment based on its potential toxicity, adverse effects, and mixed findings on its efficacy. In addition, it should not be given to medically vulnerable/fragile individuals.
Lastly, gabapentin, a voltage-gated calcium channel modulator, may also be used as a second-line agent for AUD. Patients who have started alcohol withdrawal management with gabapentin may wish to continue treatment to assist with craving suppression.30 It is also a good choice for patients who have comorbid diabetic neuropathy or other neuropathic pain conditions, anxiety, or insomnia.30,34 Of note, there have been reports of gabapentin misuse.
CASE REPORTS CONTINUED
Ms. B presents to the ED with a 5-year history of moderate AUD and a 2-year history of mild OUD. She denies a history of severe or complicated AWS. Her laboratory results are significant for a serum ethanol level of 250 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Management decisions: Naltrexone for OUD
In contrast to Ms. A, Ms. B is likely able to complete the opioid withdrawal management process. It is reasonable to treat her uncomplicated, moderate alcohol withdrawal as an outpatient with gabapentin or a benzodiazepine taper. Had her AUD been as severe as Ms. A’s, or if she were unsuccessful with ambulatory withdrawal treatment attempts, Ms. B would also be a candidate for inpatient medical treatment for alcohol withdrawal regardless of the severity of her OUD. Ongoing pharmacotherapy for her AUD after withdrawal management is the same as previously outlined. After Ms. B completes the taper (typically 1 week after the ED visit), she should follow up for initiation of pharmacotherapy for AUD. Ms. B is an ideal candidate for naltrexone, which targets both AUD and OUD.
Continue to: Naltrexone is a semi-synthetic...
Naltrexone is a semi-synthetic competitive antagonist at mu-opioid receptors and a partial agonist at kappa receptors; it has little to no activity at delta receptors. Naltrexone has been shown to reduce alcohol cravings and diminish the euphoric effects of alcohol by reducing endogenous opioid release and receptor activation.35 Thus, even when patients do use alcohol while taking naltrexone, the amount of alcohol they use is typically substantially reduced.36 In fact, at a standard dose of 50 mg/d, 95% of mu-opioid receptors are occupied and are shown to yield approximately 40% alcohol abstinence rates at 1 year.36
Once Ms. B has completed withdrawal management from both alcohol and opioids, she should have a trial period of oral naltrexone to prove tolerability, and then transition to the long-acting injectable (LAI) formulation. Patients able to complete withdrawal management from opioids and transition to LAI naltrexone have been shown to have equivalent rates of successful abstinence from opioids compared to buprenorphine.37 Though Ms. B could opt to try buprenorphine to treat her mild OUD, naltrexone would be the preferred option because it has 3 advantages:
- it blocks the mu-opioid receptor, which prevents euphoria if an illicit substance is used
- it does not cause physiologic dependence or withdrawal syndrome if/when stopped
- if it is not effective, it is easy to switch to buprenorphine.
Lastly, all patients with OUD should be prescribed a rescue naloxone kit, in accordance with harm-reduction guidelines. Naloxone, a potent opioid receptor antagonist, is used to prevent or reverse respiratory depression in opioid overdose. Naloxone rescue kits include intranasal naloxone, which makes it easy for nonclinician bystanders to administer while waiting for emergency transport.38 Most states allow naloxone kits to be prescribed to individuals who have a concern for overdose among friends, family, or others in the community. The wide distribution and easy availability of naloxone rescue kits have been essential in decreasing overdose deaths among patients who misuse opioids.39
Take-home points
Patients with both OUD and AUD are relatively common and often pose significant management challenges when they present to the clinic or the ED in withdrawal. Because severe AWS can be life-threatening, hospitalization should be considered. OWS is often accompanied by intense cravings that can lead to relapse and the risk of accidental opioid overdose/death. As soon as patients are able to engage in a discussion about their treatment options, clinicians need to clarify the patient’s goals and priorities. In medications for OUD, the decision of whether to use buprenorphine, naltrexone, or methadone is guided by the severity of the OUD, the patient’s past treatment experience (illicit as well as prescribed), and patient preference. If the OUD is mild or if the patient prefers to avoid opioid agonist medications and can tolerate the opioid withdrawal process, both the AUD and OUD can be treated with naltrexone, preferably with the LAI formulation. Other AUD medications and outpatient psychotherapy may be used to augment treatment outcomes. For patients with a moderate to severe OUD, buprenorphine (preferably with immediate initiation) or methadone therapy should be offered. Patients with comorbid OUD and AUD who are treated with opioid agonists should be offered medication for AUD other than naltrexone, as outlined above. All patients with substance use disorders would benefit from psychosocial interventions, including group and individual therapy as well as community sober support groups.
Bottom Line
Patients with comorbid opioid use disorder (OUD) and alcohol use disorder (AUD) often pose significant management challenges when they present in withdrawal. This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/ cp.0305
- Eatmon CV, Trent K. Pharmacotherapy for alcohol use disorder in patients with hepatic impairment. Current Psychiatry. 2021;20(12):25-28. doi:10.12788/cp.0068
Drug Brand Names
Acamprosate • Campral
Buprenorphine/naloxone • Suboxone, Zubsolv
Clonidine • Catapres
Disulfiram • Antabuse
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Lofexidine • Lucemyra
Methadone • Methadose, Dolophine
Naloxone • Narcan
Naltrexone • ReVia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Trazodone • Desyrel, Oleptro
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207.
2. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
3. Nolan S, Klimas J, Wood E. Alcohol use in opioid agonist treatment. Addict Sci Clin Pract. 2016;11(1):17.
4. Hood LE, Leyrer-Hackson JM, Olive MF. Pharmacotherapeutic management of co-morbid alcohol and opioid use. Expert Opin Pharmacother. 2020;21(7):823-839.
5. Pikovsky M, Peacock A, Larney S, et al. Alcohol use disorder and associated physical health complications and treatment amongst individuals with and without opioid dependence: a case-control study. Drug Alcohol Depend. 2018;188:304-310.
6. Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78-82.
7. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
8. Jones CM, Paulozzi LJ, Mack KA; Centers for Disease Control and Prevention (CDC). Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(40):881-885.
9. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61.
10. Turner RC, Lichstein PR, Peden JG Jr, et al. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
11. Boba A. Management of acute alcohol intoxication. Am J Emerg Med. 1999;17(4):431.
12. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl1):1-91.
13. Shaw JM, Kolesar GS, Sellers EM, et al. Development of optimal treatment tactics for alcohol withdrawal. I. Assessment and effectiveness of supportive care. J Clin Psychopharmacol. 1981;1(6):382-389.
14. Naranjo CA, Sellers EM. Clinical assessment and pharmacotherapy of the alcohol withdrawal syndrome. Recent Dev Alcohol. 1986;4:265-281.
15. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
16. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S Suppl 1):1-72.
17. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
18. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
19. Tang Y-L, Hao W. Improving drug addiction treatment in China. Addiction. 2007;102(7):1057-1063.
20. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622.
21. Wines JD Jr, Saitz R, Horton NJ, et al. Overdose after detoxification: a prospective study. Drug Alcohol Depend. 2007;89(2-3):161-169.
22. Maughan BC, Becker EA. Drug-related mortality after discharge from treatment: a record-linkage study of substance abuse clients in Texas, 2006-2012. Drug Alcohol Depend. 2019;204:107473.
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2002;(2):CD002025.
24. Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12(5):379-384.
25. Nava F, Manzato E, Leonardi C, et al. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1867-1872.
26. Srivastava A, Kahan M, Ross S. The effect of methadone maintenance treatment on alcohol consumption: a systematic review. J Subst Abuse Treat. 2008;34(2):215-223.
27. Davids E, Gastpar M. Buprenorphine in the treatment of opioid dependence. Eur Neuropsychopharmacol. 2004;14(3):209-216.
28. American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association; 2018.
29. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol. 2014;33(6):561-567.
30. Fairbanks J, Umbreit A, Kolla BP, et al. Evidence-based pharmacotherapies for alcohol use disorder: clinical pearls. Mayo Clin Proc. 2020;95(9):1964-1977.
31. Verrotti A, Scaparrotta A, Agostinelli S, et al. Topiramate-induced weight loss: a review. Epilepsy Res. 2011;95(3):189-199.
32. Flórez G, García-Portilla P, Alvarez S, et al. Using topiramate or naltrexone for the treatment of alcohol-dependent patients. Alcohol Clin Exp Res. 2008;32(7):1251-1259.
33. Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35(10):1749-1758.
34. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
35. Sudakin D. Naltrexone: not just for opioids anymore. J Med Toxicol. 2016;12(1):71-75.
36. Rubio G, Jiménez-Arrieri MA, Ponce G, et al. Naltrexone versus acamprosate: one year follow-up of alcohol dependence treatment. Alcohol Alcohol. 2001;36(5):419-425.
37. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
38. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163.
39. Dunne RB. Prescribing naloxone for opioid overdose intervention. Pain Manag. 2018;8(3):197-208.
When left untreated, opioid use disorder (OUD) is a debilitating and potentially lethal illness. Despite the availability of safe and effective medications for OUD, the prevalence of opioid use and overdose deaths has been increasing every year.1 An additional challenge in OUD treatment is the high prevalence of comorbid alcohol use disorder (AUD).2-6 A Clinical Trials Network survey from the National Institute on Drug Abuse found 38% of persons seeking treatment for OUD also had AUD.7 Other analyses have found alcohol was involved in approximately one-fifth of opioid-related deaths.8 Research also reveals that comorbid OUD and AUD contributes to poor treatment outcomes, more medical comorbidities, and a high risk of death (including overdose death).4,9 There is no standard of care for this particular patient population.3 This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
To illustrate the various decision points, we will follow 2 hypothetical patients through various stages of treatment (Figure), from their presentation in the emergency department (ED) or outpatient clinic, through their hospital admission (if needed), and into their outpatient follow-up treatment.

CASE REPORTS
Ms. A and Ms. B present to the ED for evaluation of nausea, vomiting, sweating, anxiety, and tremor. Both patients describe their most recent use of both alcohol and opioids approximately 12 hours ago, and each has been attempting to stop using both substances at home.
Decision-making in the emergency setting
In the ED, a few important decisions need to be made regarding treatment:
- Are the presenting symptoms primarily due to alcohol withdrawal syndrome (AWS), opioid withdrawal syndrome (OWS), or both?
- Does the patient require inpatient medical withdrawal management (detoxification) based on the history and severity of the withdrawal symptoms?
- What are the patient’s treatment goals for their AUD and OUD?
- Is maintenance medication for OUD indicated? If so, which medication is most appropriate?
In the ED, the presentation of individuals affected by both OUD and AUD can be challenging because OWS shares overlapping features with AWS, including nausea, vomiting, diarrhea, sweating, anxiety, and tremor. However, although acute OWS is typically very uncomfortable, it is rarely lethal. On the other hand, severe AWS may result in delirium, seizures, and death,10 which makes it essential to recognize and treat appropriately.
Both Ms. A and Ms. B should be medically evaluated and treated by an emergency medicine physician in conjunction with psychiatric (or addiction medicine) consultation. The ED assessment of a patient presenting with both AUD and OUD should include vital signs monitoring; physical examination; blood work including comprehensive metabolic panel, serum magnesium, and phosphorus; complete blood count; pregnancy test for women of reproductive age; urine drug screen (UDS); urinalysis; and serum ethanol level. Of note, sympathetic hyperactivity is found in both alcohol and opioid withdrawal, and patients with alcohol withdrawal may also have hypokalemia, a condition associated with an increased risk of arrhythmia. Furthermore, a prolonged QTc would affect clinical decision-making about medications for OUD (ie, methadone) and withdrawal management (ie, ondansetron, trazodone, and hydroxyzine). Therefore, an electrocardiogram should be conducted, where appropriate.
Initial treatment of AWS includes vitamin supplementation (thiamine, folic acid, and multivitamins) and benzodiazepine administration (symptom-triggered and/or scheduled taper). It may also include IV fluid resuscitation, analgesics for pain, ondansetron for nausea and vomiting, and other electrolyte repletion as indicated by the laboratory results.11 Additional measures for patients in opioid withdrawal should include alpha-2 agonists such as clonidine or lofexidine for adrenergic symptoms, antiemetics, antidiarrheals, muscle relaxants, anxiolytics such as hydroxyzine, and sleep medications such as trazodone.12
Continue to: The next decision...
The next decision is whether the patient needs to be admitted for inpatient treatment. This decision is based primarily on the risk assessment and severity of AWS, including a compelling history of complicated AWS such as seizures or delirium tremens as well as consideration of the complexity and severity of any comorbid medical or psychiatric conditions. Other indications for medical withdrawal management include a history of unsuccessful ambulatory withdrawal management and pregnancy. For severe AWS, a scheduled benzodiazepine taper in addition to the symptom-triggered protocol should be considered.13-15 A psychiatric evaluation may be obtained in the ED, as long as the patient is sober enough to meaningfully participate in the psychiatric interview. Wherever possible, psychiatric interviews should be supplemented by collateral information.
CASE REPORTS CONTINUED
Ms. A admits to a 5-year history of alcohol and opioid use that meets the criteria for severe AUD and severe OUD. She has previously required inpatient treatment for seizures related to AWS. Laboratory results are notable for a serum ethanol level of 380 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Disposition of patients in alcohol and opioid withdrawal
Given Ms. A’s history of seizures while withdrawing from alcohol, she is appropriate for hospital admission for medically managed withdrawal observation. As previously mentioned, there is clinical overlap between AWS and OWS, and differentiating between the 2 syndromes is essential and may be lifesaving. Whereas anxiety, agitation, diaphoresis, tachycardia, hypertension, and insomnia can be seen in both opioid and alcohol withdrawal, OWS-specific symptoms include mydriasis, lacrimation, rhinorrhea, bone or joint aches, yawning, and piloerection. AWS may present with visual or tactile hallucinations, delirium, and grand mal seizures.15
The details of inpatient management are beyond the scope of this article; however, both patients should be started on thiamine, folic acid, and a multivitamin. For patients in alcohol withdrawal with a history of poor diet who appear malnourished or have a history of malabsorption (such as gastric bypass surgery), thiamine 100 mg/d IV should be given for 3 to 5 days to prevent Wernicke encephalopathy.16 Where there is any concern the patient may be exhibiting signs of Wernicke-Korsakoff Syndrome (impaired cognition, evident malnourishment, ataxia, or eye movement abnormalities), high-dose thiamine IV should be given presumptively as follows: 500 mg IV 3 times a day for 3 days, 250 mg/d IV for 5 days, and then oral supplementation 100 mg/d for at least 30 days.17
In summary, on presentation to the ED, both patients should be medically stabilized and started on benzodiazepines for alcohol withdrawal. The risk assessment and the severity of the AWS often determines the level of care.
CASE REPORTS CONTINUED
On hospital Day 2, Ms. A tells the consulting psychiatrist she would like to start medications to treat her substance use disorders. She has a long history of failed attempts to achieve abstinence from opioids, so she and the psychiatrist agree to initiate a trial of buprenorphine/naloxone for her OUD, 4 mg/1 mg to 8 mg/2 mg for Day 1. Although buprenorphine/naloxone seems to help her alcohol cravings somewhat, she requests additional help. She experiences migraine headaches, which is in part why she began using opioid medications. Via joint decision making with her psychiatrist, she agrees to a trial of topiramate, with a slow titration schedule starting at 25 mg/d.
Continue to: Management decisions
Management decisions: Buprenorphine for OUD
The next issue is to determine the appropriate treatment for the patient’s OUD. Although treating OWS is important in improving the patient’s health, decreasing their discomfort, and facilitating their participation in a psychosocial treatment program,18 current evidence suggests that opioid withdrawal management alone without medication for OUD rarely leads to long-term recovery.19,20 Some research suggests that the risk of accidental opioid overdose immediately following acute withdrawal management may actually be increased due to decreased tolerance in these patients.12,21,22
Three medications have the most evidence for OUD treatment: buprenorphine, methadone, and naltrexone.15 The decision to use buprenorphine, methadone, or naltrexone depends on a variety of factors, including the severity of the OUD, patient history of prior treatment successes and failures, comorbid medical and psychiatric conditions, and patient preference.4 Treatment with buprenorphine or methadone is preferred over naltrexone for patients who do not want to or cannot tolerate the physical and emotional discomfort of the opioid withdrawal process, who experience moderate to severe OUD, who have a history of failed abstinence-based treatment, or who have more severe physiological tolerance/dependence.12 Buprenorphine is a mu opioid receptor partial agonist that has been shown to reduce opioid cravings,23 provide moderate pain relief,24 and ameliorate OWS.12 It does not typically result in significant respiratory depression, which is the biggest safety concern for opioid use.12 Buprenorphine may also treat comorbid AUD at higher doses; however, the data are inconclusive.25,26 Buprenorphine should be prescribed with caution to patients with comorbid, uncontrolled AUD, due to the risk of respiratory depression when combined with alcohol. Patients who continue to drink alcohol but are able to abstain from opioids may consider starting an AUD-specific medication. Pharmacologic options are discussed in more detail in the next section.
For patients who have higher physiological dependence or more severe OUD, methadone may be a reasonable alternative to buprenorphine. Methadone, a mu-opioid receptor agonist, ameliorates OWS, reduces opioid cravings, and reduces the euphoric effects of opioid ingestion if the patient relapses. However, methadone can only be dispensed for the treatment of OUD by a federally-certified treatment program governed by restrictive and federally mandated guidelines. Compared to buprenorphine, methadone is more dangerous in overdose, has more drug interactions, and is more commonly diverted for recreational use.27 Furthermore, methadone should be prescribed with caution to patients with comorbid, uncontrolled AUD, because both alcohol and methadone can result in respiratory depression.
By contrast, the first-line treatment for individuals experiencing moderateto severe AUD is typically naltrexone.28 Naltrexone is contraindicated in Ms. A because she has a severe OUD and is unlikely to tolerate the opioid withdrawal process. Research suggests that the use of naltrexone for OUD should be limited to patients who have a mild disorder or who show low physiological dependence.29 Alternatively, acamprosate, disulfiram, topiramate, or gabapentin should be considered for Ms. A.4,28,30 Because each of these medications have specific strengths and weaknesses, medication selection should be based on individual patient factors such as comorbid psychiatric and medical conditions and/or patient preference.28
Management decisions: AUD augmentation strategies
Naltrexone is contraindicated for patients who are receiving opioids, including opioid agonist therapy for OUD. Therefore, clinicians need to consider other options for these individuals. There are several medications with good evidence, including acamprosate, disulfiram, topiramate, and gabapentin. Acamprosate and disulfiram are FDA-approved for AUD; the latter 2 have been used off-label.
Continue to: Acamprosate is a glutamate receptor modulator...
Acamprosate is a glutamate receptor modulator that reduces alcohol cravings and is recommended for patients who have achieved and wish to maintain abstinence. It can be used in patients with liver disease, because it is not hepatically metabolized.30 Topiramate is also used to reduce alcohol cravings. It antagonizes glutamate at alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) and kainite receptors, facilitates gamma-aminobutyric acid (GABA) function, and reduces the extracellular release of dopamine in the mesocorticolimbic regions of the brain.30 Topiramate is a reasonable option for patients with a seizure disorder, a history of migraine headaches,30 or who are overweight or obese and wish to lose weight.31 In a nonrandomized study, topiramate reduced alcohol intake and cravings more than naltrexone.32
Disulfiram is another second-line therapy for AUD. It is best used under close supervision because it does not reduce alcohol cravings but makes ingesting alcohol extremely aversive by preventing the breakdown of the alcohol metabolite acetaldehyde, and in doing so causes a cluster of unpleasant symptoms, including sweating, palpitations, flushing, nausea/vomiting, and increased sympathetic tone.28 Disulfiram only works if it is taken daily, and it requires a high degree of motivation and/or daily supervision at home or in the clinic.33 It is not recommended to be used as a first-line treatment based on its potential toxicity, adverse effects, and mixed findings on its efficacy. In addition, it should not be given to medically vulnerable/fragile individuals.
Lastly, gabapentin, a voltage-gated calcium channel modulator, may also be used as a second-line agent for AUD. Patients who have started alcohol withdrawal management with gabapentin may wish to continue treatment to assist with craving suppression.30 It is also a good choice for patients who have comorbid diabetic neuropathy or other neuropathic pain conditions, anxiety, or insomnia.30,34 Of note, there have been reports of gabapentin misuse.
CASE REPORTS CONTINUED
Ms. B presents to the ED with a 5-year history of moderate AUD and a 2-year history of mild OUD. She denies a history of severe or complicated AWS. Her laboratory results are significant for a serum ethanol level of 250 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Management decisions: Naltrexone for OUD
In contrast to Ms. A, Ms. B is likely able to complete the opioid withdrawal management process. It is reasonable to treat her uncomplicated, moderate alcohol withdrawal as an outpatient with gabapentin or a benzodiazepine taper. Had her AUD been as severe as Ms. A’s, or if she were unsuccessful with ambulatory withdrawal treatment attempts, Ms. B would also be a candidate for inpatient medical treatment for alcohol withdrawal regardless of the severity of her OUD. Ongoing pharmacotherapy for her AUD after withdrawal management is the same as previously outlined. After Ms. B completes the taper (typically 1 week after the ED visit), she should follow up for initiation of pharmacotherapy for AUD. Ms. B is an ideal candidate for naltrexone, which targets both AUD and OUD.
Continue to: Naltrexone is a semi-synthetic...
Naltrexone is a semi-synthetic competitive antagonist at mu-opioid receptors and a partial agonist at kappa receptors; it has little to no activity at delta receptors. Naltrexone has been shown to reduce alcohol cravings and diminish the euphoric effects of alcohol by reducing endogenous opioid release and receptor activation.35 Thus, even when patients do use alcohol while taking naltrexone, the amount of alcohol they use is typically substantially reduced.36 In fact, at a standard dose of 50 mg/d, 95% of mu-opioid receptors are occupied and are shown to yield approximately 40% alcohol abstinence rates at 1 year.36
Once Ms. B has completed withdrawal management from both alcohol and opioids, she should have a trial period of oral naltrexone to prove tolerability, and then transition to the long-acting injectable (LAI) formulation. Patients able to complete withdrawal management from opioids and transition to LAI naltrexone have been shown to have equivalent rates of successful abstinence from opioids compared to buprenorphine.37 Though Ms. B could opt to try buprenorphine to treat her mild OUD, naltrexone would be the preferred option because it has 3 advantages:
- it blocks the mu-opioid receptor, which prevents euphoria if an illicit substance is used
- it does not cause physiologic dependence or withdrawal syndrome if/when stopped
- if it is not effective, it is easy to switch to buprenorphine.
Lastly, all patients with OUD should be prescribed a rescue naloxone kit, in accordance with harm-reduction guidelines. Naloxone, a potent opioid receptor antagonist, is used to prevent or reverse respiratory depression in opioid overdose. Naloxone rescue kits include intranasal naloxone, which makes it easy for nonclinician bystanders to administer while waiting for emergency transport.38 Most states allow naloxone kits to be prescribed to individuals who have a concern for overdose among friends, family, or others in the community. The wide distribution and easy availability of naloxone rescue kits have been essential in decreasing overdose deaths among patients who misuse opioids.39
Take-home points
Patients with both OUD and AUD are relatively common and often pose significant management challenges when they present to the clinic or the ED in withdrawal. Because severe AWS can be life-threatening, hospitalization should be considered. OWS is often accompanied by intense cravings that can lead to relapse and the risk of accidental opioid overdose/death. As soon as patients are able to engage in a discussion about their treatment options, clinicians need to clarify the patient’s goals and priorities. In medications for OUD, the decision of whether to use buprenorphine, naltrexone, or methadone is guided by the severity of the OUD, the patient’s past treatment experience (illicit as well as prescribed), and patient preference. If the OUD is mild or if the patient prefers to avoid opioid agonist medications and can tolerate the opioid withdrawal process, both the AUD and OUD can be treated with naltrexone, preferably with the LAI formulation. Other AUD medications and outpatient psychotherapy may be used to augment treatment outcomes. For patients with a moderate to severe OUD, buprenorphine (preferably with immediate initiation) or methadone therapy should be offered. Patients with comorbid OUD and AUD who are treated with opioid agonists should be offered medication for AUD other than naltrexone, as outlined above. All patients with substance use disorders would benefit from psychosocial interventions, including group and individual therapy as well as community sober support groups.
Bottom Line
Patients with comorbid opioid use disorder (OUD) and alcohol use disorder (AUD) often pose significant management challenges when they present in withdrawal. This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/ cp.0305
- Eatmon CV, Trent K. Pharmacotherapy for alcohol use disorder in patients with hepatic impairment. Current Psychiatry. 2021;20(12):25-28. doi:10.12788/cp.0068
Drug Brand Names
Acamprosate • Campral
Buprenorphine/naloxone • Suboxone, Zubsolv
Clonidine • Catapres
Disulfiram • Antabuse
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Lofexidine • Lucemyra
Methadone • Methadose, Dolophine
Naloxone • Narcan
Naltrexone • ReVia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Trazodone • Desyrel, Oleptro
When left untreated, opioid use disorder (OUD) is a debilitating and potentially lethal illness. Despite the availability of safe and effective medications for OUD, the prevalence of opioid use and overdose deaths has been increasing every year.1 An additional challenge in OUD treatment is the high prevalence of comorbid alcohol use disorder (AUD).2-6 A Clinical Trials Network survey from the National Institute on Drug Abuse found 38% of persons seeking treatment for OUD also had AUD.7 Other analyses have found alcohol was involved in approximately one-fifth of opioid-related deaths.8 Research also reveals that comorbid OUD and AUD contributes to poor treatment outcomes, more medical comorbidities, and a high risk of death (including overdose death).4,9 There is no standard of care for this particular patient population.3 This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
To illustrate the various decision points, we will follow 2 hypothetical patients through various stages of treatment (Figure), from their presentation in the emergency department (ED) or outpatient clinic, through their hospital admission (if needed), and into their outpatient follow-up treatment.

CASE REPORTS
Ms. A and Ms. B present to the ED for evaluation of nausea, vomiting, sweating, anxiety, and tremor. Both patients describe their most recent use of both alcohol and opioids approximately 12 hours ago, and each has been attempting to stop using both substances at home.
Decision-making in the emergency setting
In the ED, a few important decisions need to be made regarding treatment:
- Are the presenting symptoms primarily due to alcohol withdrawal syndrome (AWS), opioid withdrawal syndrome (OWS), or both?
- Does the patient require inpatient medical withdrawal management (detoxification) based on the history and severity of the withdrawal symptoms?
- What are the patient’s treatment goals for their AUD and OUD?
- Is maintenance medication for OUD indicated? If so, which medication is most appropriate?
In the ED, the presentation of individuals affected by both OUD and AUD can be challenging because OWS shares overlapping features with AWS, including nausea, vomiting, diarrhea, sweating, anxiety, and tremor. However, although acute OWS is typically very uncomfortable, it is rarely lethal. On the other hand, severe AWS may result in delirium, seizures, and death,10 which makes it essential to recognize and treat appropriately.
Both Ms. A and Ms. B should be medically evaluated and treated by an emergency medicine physician in conjunction with psychiatric (or addiction medicine) consultation. The ED assessment of a patient presenting with both AUD and OUD should include vital signs monitoring; physical examination; blood work including comprehensive metabolic panel, serum magnesium, and phosphorus; complete blood count; pregnancy test for women of reproductive age; urine drug screen (UDS); urinalysis; and serum ethanol level. Of note, sympathetic hyperactivity is found in both alcohol and opioid withdrawal, and patients with alcohol withdrawal may also have hypokalemia, a condition associated with an increased risk of arrhythmia. Furthermore, a prolonged QTc would affect clinical decision-making about medications for OUD (ie, methadone) and withdrawal management (ie, ondansetron, trazodone, and hydroxyzine). Therefore, an electrocardiogram should be conducted, where appropriate.
Initial treatment of AWS includes vitamin supplementation (thiamine, folic acid, and multivitamins) and benzodiazepine administration (symptom-triggered and/or scheduled taper). It may also include IV fluid resuscitation, analgesics for pain, ondansetron for nausea and vomiting, and other electrolyte repletion as indicated by the laboratory results.11 Additional measures for patients in opioid withdrawal should include alpha-2 agonists such as clonidine or lofexidine for adrenergic symptoms, antiemetics, antidiarrheals, muscle relaxants, anxiolytics such as hydroxyzine, and sleep medications such as trazodone.12
Continue to: The next decision...
The next decision is whether the patient needs to be admitted for inpatient treatment. This decision is based primarily on the risk assessment and severity of AWS, including a compelling history of complicated AWS such as seizures or delirium tremens as well as consideration of the complexity and severity of any comorbid medical or psychiatric conditions. Other indications for medical withdrawal management include a history of unsuccessful ambulatory withdrawal management and pregnancy. For severe AWS, a scheduled benzodiazepine taper in addition to the symptom-triggered protocol should be considered.13-15 A psychiatric evaluation may be obtained in the ED, as long as the patient is sober enough to meaningfully participate in the psychiatric interview. Wherever possible, psychiatric interviews should be supplemented by collateral information.
CASE REPORTS CONTINUED
Ms. A admits to a 5-year history of alcohol and opioid use that meets the criteria for severe AUD and severe OUD. She has previously required inpatient treatment for seizures related to AWS. Laboratory results are notable for a serum ethanol level of 380 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Disposition of patients in alcohol and opioid withdrawal
Given Ms. A’s history of seizures while withdrawing from alcohol, she is appropriate for hospital admission for medically managed withdrawal observation. As previously mentioned, there is clinical overlap between AWS and OWS, and differentiating between the 2 syndromes is essential and may be lifesaving. Whereas anxiety, agitation, diaphoresis, tachycardia, hypertension, and insomnia can be seen in both opioid and alcohol withdrawal, OWS-specific symptoms include mydriasis, lacrimation, rhinorrhea, bone or joint aches, yawning, and piloerection. AWS may present with visual or tactile hallucinations, delirium, and grand mal seizures.15
The details of inpatient management are beyond the scope of this article; however, both patients should be started on thiamine, folic acid, and a multivitamin. For patients in alcohol withdrawal with a history of poor diet who appear malnourished or have a history of malabsorption (such as gastric bypass surgery), thiamine 100 mg/d IV should be given for 3 to 5 days to prevent Wernicke encephalopathy.16 Where there is any concern the patient may be exhibiting signs of Wernicke-Korsakoff Syndrome (impaired cognition, evident malnourishment, ataxia, or eye movement abnormalities), high-dose thiamine IV should be given presumptively as follows: 500 mg IV 3 times a day for 3 days, 250 mg/d IV for 5 days, and then oral supplementation 100 mg/d for at least 30 days.17
In summary, on presentation to the ED, both patients should be medically stabilized and started on benzodiazepines for alcohol withdrawal. The risk assessment and the severity of the AWS often determines the level of care.
CASE REPORTS CONTINUED
On hospital Day 2, Ms. A tells the consulting psychiatrist she would like to start medications to treat her substance use disorders. She has a long history of failed attempts to achieve abstinence from opioids, so she and the psychiatrist agree to initiate a trial of buprenorphine/naloxone for her OUD, 4 mg/1 mg to 8 mg/2 mg for Day 1. Although buprenorphine/naloxone seems to help her alcohol cravings somewhat, she requests additional help. She experiences migraine headaches, which is in part why she began using opioid medications. Via joint decision making with her psychiatrist, she agrees to a trial of topiramate, with a slow titration schedule starting at 25 mg/d.
Continue to: Management decisions
Management decisions: Buprenorphine for OUD
The next issue is to determine the appropriate treatment for the patient’s OUD. Although treating OWS is important in improving the patient’s health, decreasing their discomfort, and facilitating their participation in a psychosocial treatment program,18 current evidence suggests that opioid withdrawal management alone without medication for OUD rarely leads to long-term recovery.19,20 Some research suggests that the risk of accidental opioid overdose immediately following acute withdrawal management may actually be increased due to decreased tolerance in these patients.12,21,22
Three medications have the most evidence for OUD treatment: buprenorphine, methadone, and naltrexone.15 The decision to use buprenorphine, methadone, or naltrexone depends on a variety of factors, including the severity of the OUD, patient history of prior treatment successes and failures, comorbid medical and psychiatric conditions, and patient preference.4 Treatment with buprenorphine or methadone is preferred over naltrexone for patients who do not want to or cannot tolerate the physical and emotional discomfort of the opioid withdrawal process, who experience moderate to severe OUD, who have a history of failed abstinence-based treatment, or who have more severe physiological tolerance/dependence.12 Buprenorphine is a mu opioid receptor partial agonist that has been shown to reduce opioid cravings,23 provide moderate pain relief,24 and ameliorate OWS.12 It does not typically result in significant respiratory depression, which is the biggest safety concern for opioid use.12 Buprenorphine may also treat comorbid AUD at higher doses; however, the data are inconclusive.25,26 Buprenorphine should be prescribed with caution to patients with comorbid, uncontrolled AUD, due to the risk of respiratory depression when combined with alcohol. Patients who continue to drink alcohol but are able to abstain from opioids may consider starting an AUD-specific medication. Pharmacologic options are discussed in more detail in the next section.
For patients who have higher physiological dependence or more severe OUD, methadone may be a reasonable alternative to buprenorphine. Methadone, a mu-opioid receptor agonist, ameliorates OWS, reduces opioid cravings, and reduces the euphoric effects of opioid ingestion if the patient relapses. However, methadone can only be dispensed for the treatment of OUD by a federally-certified treatment program governed by restrictive and federally mandated guidelines. Compared to buprenorphine, methadone is more dangerous in overdose, has more drug interactions, and is more commonly diverted for recreational use.27 Furthermore, methadone should be prescribed with caution to patients with comorbid, uncontrolled AUD, because both alcohol and methadone can result in respiratory depression.
By contrast, the first-line treatment for individuals experiencing moderateto severe AUD is typically naltrexone.28 Naltrexone is contraindicated in Ms. A because she has a severe OUD and is unlikely to tolerate the opioid withdrawal process. Research suggests that the use of naltrexone for OUD should be limited to patients who have a mild disorder or who show low physiological dependence.29 Alternatively, acamprosate, disulfiram, topiramate, or gabapentin should be considered for Ms. A.4,28,30 Because each of these medications have specific strengths and weaknesses, medication selection should be based on individual patient factors such as comorbid psychiatric and medical conditions and/or patient preference.28
Management decisions: AUD augmentation strategies
Naltrexone is contraindicated for patients who are receiving opioids, including opioid agonist therapy for OUD. Therefore, clinicians need to consider other options for these individuals. There are several medications with good evidence, including acamprosate, disulfiram, topiramate, and gabapentin. Acamprosate and disulfiram are FDA-approved for AUD; the latter 2 have been used off-label.
Continue to: Acamprosate is a glutamate receptor modulator...
Acamprosate is a glutamate receptor modulator that reduces alcohol cravings and is recommended for patients who have achieved and wish to maintain abstinence. It can be used in patients with liver disease, because it is not hepatically metabolized.30 Topiramate is also used to reduce alcohol cravings. It antagonizes glutamate at alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) and kainite receptors, facilitates gamma-aminobutyric acid (GABA) function, and reduces the extracellular release of dopamine in the mesocorticolimbic regions of the brain.30 Topiramate is a reasonable option for patients with a seizure disorder, a history of migraine headaches,30 or who are overweight or obese and wish to lose weight.31 In a nonrandomized study, topiramate reduced alcohol intake and cravings more than naltrexone.32
Disulfiram is another second-line therapy for AUD. It is best used under close supervision because it does not reduce alcohol cravings but makes ingesting alcohol extremely aversive by preventing the breakdown of the alcohol metabolite acetaldehyde, and in doing so causes a cluster of unpleasant symptoms, including sweating, palpitations, flushing, nausea/vomiting, and increased sympathetic tone.28 Disulfiram only works if it is taken daily, and it requires a high degree of motivation and/or daily supervision at home or in the clinic.33 It is not recommended to be used as a first-line treatment based on its potential toxicity, adverse effects, and mixed findings on its efficacy. In addition, it should not be given to medically vulnerable/fragile individuals.
Lastly, gabapentin, a voltage-gated calcium channel modulator, may also be used as a second-line agent for AUD. Patients who have started alcohol withdrawal management with gabapentin may wish to continue treatment to assist with craving suppression.30 It is also a good choice for patients who have comorbid diabetic neuropathy or other neuropathic pain conditions, anxiety, or insomnia.30,34 Of note, there have been reports of gabapentin misuse.
CASE REPORTS CONTINUED
Ms. B presents to the ED with a 5-year history of moderate AUD and a 2-year history of mild OUD. She denies a history of severe or complicated AWS. Her laboratory results are significant for a serum ethanol level of 250 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Management decisions: Naltrexone for OUD
In contrast to Ms. A, Ms. B is likely able to complete the opioid withdrawal management process. It is reasonable to treat her uncomplicated, moderate alcohol withdrawal as an outpatient with gabapentin or a benzodiazepine taper. Had her AUD been as severe as Ms. A’s, or if she were unsuccessful with ambulatory withdrawal treatment attempts, Ms. B would also be a candidate for inpatient medical treatment for alcohol withdrawal regardless of the severity of her OUD. Ongoing pharmacotherapy for her AUD after withdrawal management is the same as previously outlined. After Ms. B completes the taper (typically 1 week after the ED visit), she should follow up for initiation of pharmacotherapy for AUD. Ms. B is an ideal candidate for naltrexone, which targets both AUD and OUD.
Continue to: Naltrexone is a semi-synthetic...
Naltrexone is a semi-synthetic competitive antagonist at mu-opioid receptors and a partial agonist at kappa receptors; it has little to no activity at delta receptors. Naltrexone has been shown to reduce alcohol cravings and diminish the euphoric effects of alcohol by reducing endogenous opioid release and receptor activation.35 Thus, even when patients do use alcohol while taking naltrexone, the amount of alcohol they use is typically substantially reduced.36 In fact, at a standard dose of 50 mg/d, 95% of mu-opioid receptors are occupied and are shown to yield approximately 40% alcohol abstinence rates at 1 year.36
Once Ms. B has completed withdrawal management from both alcohol and opioids, she should have a trial period of oral naltrexone to prove tolerability, and then transition to the long-acting injectable (LAI) formulation. Patients able to complete withdrawal management from opioids and transition to LAI naltrexone have been shown to have equivalent rates of successful abstinence from opioids compared to buprenorphine.37 Though Ms. B could opt to try buprenorphine to treat her mild OUD, naltrexone would be the preferred option because it has 3 advantages:
- it blocks the mu-opioid receptor, which prevents euphoria if an illicit substance is used
- it does not cause physiologic dependence or withdrawal syndrome if/when stopped
- if it is not effective, it is easy to switch to buprenorphine.
Lastly, all patients with OUD should be prescribed a rescue naloxone kit, in accordance with harm-reduction guidelines. Naloxone, a potent opioid receptor antagonist, is used to prevent or reverse respiratory depression in opioid overdose. Naloxone rescue kits include intranasal naloxone, which makes it easy for nonclinician bystanders to administer while waiting for emergency transport.38 Most states allow naloxone kits to be prescribed to individuals who have a concern for overdose among friends, family, or others in the community. The wide distribution and easy availability of naloxone rescue kits have been essential in decreasing overdose deaths among patients who misuse opioids.39
Take-home points
Patients with both OUD and AUD are relatively common and often pose significant management challenges when they present to the clinic or the ED in withdrawal. Because severe AWS can be life-threatening, hospitalization should be considered. OWS is often accompanied by intense cravings that can lead to relapse and the risk of accidental opioid overdose/death. As soon as patients are able to engage in a discussion about their treatment options, clinicians need to clarify the patient’s goals and priorities. In medications for OUD, the decision of whether to use buprenorphine, naltrexone, or methadone is guided by the severity of the OUD, the patient’s past treatment experience (illicit as well as prescribed), and patient preference. If the OUD is mild or if the patient prefers to avoid opioid agonist medications and can tolerate the opioid withdrawal process, both the AUD and OUD can be treated with naltrexone, preferably with the LAI formulation. Other AUD medications and outpatient psychotherapy may be used to augment treatment outcomes. For patients with a moderate to severe OUD, buprenorphine (preferably with immediate initiation) or methadone therapy should be offered. Patients with comorbid OUD and AUD who are treated with opioid agonists should be offered medication for AUD other than naltrexone, as outlined above. All patients with substance use disorders would benefit from psychosocial interventions, including group and individual therapy as well as community sober support groups.
Bottom Line
Patients with comorbid opioid use disorder (OUD) and alcohol use disorder (AUD) often pose significant management challenges when they present in withdrawal. This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/ cp.0305
- Eatmon CV, Trent K. Pharmacotherapy for alcohol use disorder in patients with hepatic impairment. Current Psychiatry. 2021;20(12):25-28. doi:10.12788/cp.0068
Drug Brand Names
Acamprosate • Campral
Buprenorphine/naloxone • Suboxone, Zubsolv
Clonidine • Catapres
Disulfiram • Antabuse
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Lofexidine • Lucemyra
Methadone • Methadose, Dolophine
Naloxone • Narcan
Naltrexone • ReVia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Trazodone • Desyrel, Oleptro
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207.
2. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
3. Nolan S, Klimas J, Wood E. Alcohol use in opioid agonist treatment. Addict Sci Clin Pract. 2016;11(1):17.
4. Hood LE, Leyrer-Hackson JM, Olive MF. Pharmacotherapeutic management of co-morbid alcohol and opioid use. Expert Opin Pharmacother. 2020;21(7):823-839.
5. Pikovsky M, Peacock A, Larney S, et al. Alcohol use disorder and associated physical health complications and treatment amongst individuals with and without opioid dependence: a case-control study. Drug Alcohol Depend. 2018;188:304-310.
6. Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78-82.
7. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
8. Jones CM, Paulozzi LJ, Mack KA; Centers for Disease Control and Prevention (CDC). Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(40):881-885.
9. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61.
10. Turner RC, Lichstein PR, Peden JG Jr, et al. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
11. Boba A. Management of acute alcohol intoxication. Am J Emerg Med. 1999;17(4):431.
12. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl1):1-91.
13. Shaw JM, Kolesar GS, Sellers EM, et al. Development of optimal treatment tactics for alcohol withdrawal. I. Assessment and effectiveness of supportive care. J Clin Psychopharmacol. 1981;1(6):382-389.
14. Naranjo CA, Sellers EM. Clinical assessment and pharmacotherapy of the alcohol withdrawal syndrome. Recent Dev Alcohol. 1986;4:265-281.
15. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
16. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S Suppl 1):1-72.
17. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
18. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
19. Tang Y-L, Hao W. Improving drug addiction treatment in China. Addiction. 2007;102(7):1057-1063.
20. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622.
21. Wines JD Jr, Saitz R, Horton NJ, et al. Overdose after detoxification: a prospective study. Drug Alcohol Depend. 2007;89(2-3):161-169.
22. Maughan BC, Becker EA. Drug-related mortality after discharge from treatment: a record-linkage study of substance abuse clients in Texas, 2006-2012. Drug Alcohol Depend. 2019;204:107473.
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2002;(2):CD002025.
24. Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12(5):379-384.
25. Nava F, Manzato E, Leonardi C, et al. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1867-1872.
26. Srivastava A, Kahan M, Ross S. The effect of methadone maintenance treatment on alcohol consumption: a systematic review. J Subst Abuse Treat. 2008;34(2):215-223.
27. Davids E, Gastpar M. Buprenorphine in the treatment of opioid dependence. Eur Neuropsychopharmacol. 2004;14(3):209-216.
28. American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association; 2018.
29. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol. 2014;33(6):561-567.
30. Fairbanks J, Umbreit A, Kolla BP, et al. Evidence-based pharmacotherapies for alcohol use disorder: clinical pearls. Mayo Clin Proc. 2020;95(9):1964-1977.
31. Verrotti A, Scaparrotta A, Agostinelli S, et al. Topiramate-induced weight loss: a review. Epilepsy Res. 2011;95(3):189-199.
32. Flórez G, García-Portilla P, Alvarez S, et al. Using topiramate or naltrexone for the treatment of alcohol-dependent patients. Alcohol Clin Exp Res. 2008;32(7):1251-1259.
33. Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35(10):1749-1758.
34. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
35. Sudakin D. Naltrexone: not just for opioids anymore. J Med Toxicol. 2016;12(1):71-75.
36. Rubio G, Jiménez-Arrieri MA, Ponce G, et al. Naltrexone versus acamprosate: one year follow-up of alcohol dependence treatment. Alcohol Alcohol. 2001;36(5):419-425.
37. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
38. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163.
39. Dunne RB. Prescribing naloxone for opioid overdose intervention. Pain Manag. 2018;8(3):197-208.
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207.
2. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
3. Nolan S, Klimas J, Wood E. Alcohol use in opioid agonist treatment. Addict Sci Clin Pract. 2016;11(1):17.
4. Hood LE, Leyrer-Hackson JM, Olive MF. Pharmacotherapeutic management of co-morbid alcohol and opioid use. Expert Opin Pharmacother. 2020;21(7):823-839.
5. Pikovsky M, Peacock A, Larney S, et al. Alcohol use disorder and associated physical health complications and treatment amongst individuals with and without opioid dependence: a case-control study. Drug Alcohol Depend. 2018;188:304-310.
6. Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78-82.
7. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
8. Jones CM, Paulozzi LJ, Mack KA; Centers for Disease Control and Prevention (CDC). Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(40):881-885.
9. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61.
10. Turner RC, Lichstein PR, Peden JG Jr, et al. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
11. Boba A. Management of acute alcohol intoxication. Am J Emerg Med. 1999;17(4):431.
12. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl1):1-91.
13. Shaw JM, Kolesar GS, Sellers EM, et al. Development of optimal treatment tactics for alcohol withdrawal. I. Assessment and effectiveness of supportive care. J Clin Psychopharmacol. 1981;1(6):382-389.
14. Naranjo CA, Sellers EM. Clinical assessment and pharmacotherapy of the alcohol withdrawal syndrome. Recent Dev Alcohol. 1986;4:265-281.
15. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
16. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S Suppl 1):1-72.
17. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
18. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
19. Tang Y-L, Hao W. Improving drug addiction treatment in China. Addiction. 2007;102(7):1057-1063.
20. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622.
21. Wines JD Jr, Saitz R, Horton NJ, et al. Overdose after detoxification: a prospective study. Drug Alcohol Depend. 2007;89(2-3):161-169.
22. Maughan BC, Becker EA. Drug-related mortality after discharge from treatment: a record-linkage study of substance abuse clients in Texas, 2006-2012. Drug Alcohol Depend. 2019;204:107473.
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2002;(2):CD002025.
24. Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12(5):379-384.
25. Nava F, Manzato E, Leonardi C, et al. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1867-1872.
26. Srivastava A, Kahan M, Ross S. The effect of methadone maintenance treatment on alcohol consumption: a systematic review. J Subst Abuse Treat. 2008;34(2):215-223.
27. Davids E, Gastpar M. Buprenorphine in the treatment of opioid dependence. Eur Neuropsychopharmacol. 2004;14(3):209-216.
28. American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association; 2018.
29. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol. 2014;33(6):561-567.
30. Fairbanks J, Umbreit A, Kolla BP, et al. Evidence-based pharmacotherapies for alcohol use disorder: clinical pearls. Mayo Clin Proc. 2020;95(9):1964-1977.
31. Verrotti A, Scaparrotta A, Agostinelli S, et al. Topiramate-induced weight loss: a review. Epilepsy Res. 2011;95(3):189-199.
32. Flórez G, García-Portilla P, Alvarez S, et al. Using topiramate or naltrexone for the treatment of alcohol-dependent patients. Alcohol Clin Exp Res. 2008;32(7):1251-1259.
33. Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35(10):1749-1758.
34. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
35. Sudakin D. Naltrexone: not just for opioids anymore. J Med Toxicol. 2016;12(1):71-75.
36. Rubio G, Jiménez-Arrieri MA, Ponce G, et al. Naltrexone versus acamprosate: one year follow-up of alcohol dependence treatment. Alcohol Alcohol. 2001;36(5):419-425.
37. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
38. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163.
39. Dunne RB. Prescribing naloxone for opioid overdose intervention. Pain Manag. 2018;8(3):197-208.
Evaluation after a suicide attempt: What to ask
In 2021, suicide was the 11th leading cause of death in the United States.1 Suicide resulted in 49,000 US deaths during 2021; it was the second most common cause of death in individuals age 10 to 34, and the fifth leading cause among children.1,2 Women are 3 to 4 times more likely than men to attempt suicide, but men are 4 times more likely to die by suicide.2
The evaluation of patients with suicidal ideation who have not made an attempt generally involves assessing 4 factors: the specific plan, access to lethal means, any recent social stressors, and the presence of a psychiatric disorder.3 The clinician should also assess which potential deterrents, such as religious beliefs or dependent children, might be present.
Mental health clinicians are often called upon to evaluate a patient after a suicide attempt to assess intent for continued self-harm and to determine appropriate disposition. Such an evaluation must consider multiple factors, including the method used, premeditation, consequences of the attempt, the presence of severe depression and/or psychosis, and the role of substance use. Assessment after a suicide attempt differs from the examination of individuals who harbor suicidal thoughts but have not made an attempt; the latter group may be more likely to respond to interventions such as intensive outpatient care, mobilization of family support, and religious proscriptions against suicide. However, for patients who make an attempt to end their life, whatever potential safeguards or deterrents to suicide that were in place obviously did not prevent the self-harm act. The consequences of the attempt, such as disabling injuries or medical complications, and possible involuntary commitment, need to be considered. Assessment of the patient’s feelings about having survived the attempt is important because the psychological impact of the attempt on family members may serve to intensify the patient’s depression and make a subsequent attempt more likely.
Many individuals who think of suicide have communicated self-harm thoughts or intentions, but such comments are often minimized or ignored. There is a common but erroneous belief that if patients are encouraged to discuss thoughts of self-harm, they will be more likely to act upon them. Because the opposite is true,4 clinicians should ask vulnerable patients about suicidal ideation or intent. Importantly, noncompliance with life-saving medical care, risk-taking behaviors, and substance use may also signal a desire for self-harm. Passive thoughts of death, typified by comments such as “I don’t care whether I wake up or not,” should also be elicited. Many patients who think of suicide speak of being in a “bad place” where reason and logic give way to an intense desire to end their misery.

The evaluation of a patient who has attempted suicide is an important component of providing psychiatric care. This article reflects our 45 years of evaluating such patients. As such, it reflects our clinical experience and is not evidence-based. We offer a checklist of 14 questions that we have found helpful when determining if it would be best for a patient to receive inpatient psychiatric hospitalization or a discharge referral for outpatient care (Table). Questions 1 through 6 are specific for patients who have made a suicide attempt, while questions 7 through 14 are helpful for assessing global risk factors for suicide.
1. Was the attempt premeditated?
Determining premeditation vs impulsivity is an essential element of the assessment following a suicide attempt. Many such acts may occur without forethought in response to an unexpected stressor, such as an altercation between partners or family conflicts. Impulsive attempts can occur when an individual is involved in a distressing event and/or while intoxicated. Conversely, premeditation involves forethought and planning, which may increase the risk of suicide in the near future.
Examples of premeditated behavior include:
- Contemplating the attempt days or weeks beforehand
- Researching the effects of a medication or combination of medications in terms of potential lethality
- Engaging in behavior that would decrease the likelihood of their body being discovered after the attempt
- Obtaining weapons and/or stockpiling pills
- Canvassing potential sites such as bridges or tall buildings
- Engaging in a suicide attempt “practice run”
- Leaving a suicide note or message on social media
- Making funeral arrangements, such as choosing burial clothing
- Writing a will and arranging for the custody of dependent children
- Purchasing life insurance that does not deny payment of benefits in cases of death by suicide.
Continue to: Patients with a premeditated...
Patients with a premeditated suicide attempt generally do not expect to survive and are often surprised or upset that the act was not fatal. The presence of indicators that the attempt was premeditated should direct the disposition more toward hospitalization than discharge. In assessing the impact of premeditation, it is important to gauge not just the examples listed above, but also the patient’s perception of these issues (such as potential loss of child custody). Consider how much the patient is emotionally affected by such thinking.
2. What were the consequences of the attempt?
Assessing the reason for the attempt (if any) and determining whether the inciting circumstance has changed due to the suicide attempt are an important part of the evaluation. A suicide attempt may result in reconciliation with and/or renewed support from family members or partners, who might not have been aware of the patient’s emotional distress. Such unexpected support often results in the patient exhibiting improved mood and affect, and possibly temporary resolution of suicidal thoughts. This “flight into health” may be short-lived, but it also may be enough to engage the patient in a therapeutic alliance. That may permit a discharge with safe disposition to the outpatient clinic while in the custody of a family member, partner, or close friend.
Alternatively, some people experience a troubling worsening of precipitants following a suicide attempt. Preexisting medical conditions and financial, occupational, and/or social woes may be exacerbated. Child custody determinations may be affected, assuming the patient understands the possibility of this adverse consequence. Violent methods may result in disfigurement and body image issues. Individuals from small, close-knit communities may experience stigmatization and unwanted notoriety because of their suicide attempt. Such negative consequences may render some patients more likely to make another attempt to die by suicide. It is crucial to consider how a suicide attempt may have changed the original stress that led to the attempt.
3. Which method was used?
Most fatal suicides in the US are by firearms, and many individuals who survive such attempts do so because of unfamiliarity with the weapon, gun malfunction, faulty aim, or alcohol use.5-7 Some survivors report intending to shoot themselves in the heart, but instead suffered shoulder injuries. Unfortunately, for a patient who survives self-inflicted gunshot wounds, the sequelae of chronic pain, multiple surgical procedures, disability, and disfigurement may serve as constant negative reminders of the event. Some individuals with suicidal intent eschew the idea of using firearms because they hope to avoid having a family member be the first to discover them. Witnessing the aftermath of a fatal suicide by gunshot can induce symptoms of posttraumatic stress disorder in family members and/or partners.8
For a patient with self-inflicted gunshot wounds, always determine whether the weapon has been secured or if the patient still has access to it. Asking about weapon availability is essential during the evaluation of any patient with depression, major life crises, or other factors that may yield a desire to die; this is especially true for individuals with substance use disorders (SUDs). Whenever readily available to such individuals, weapons need to be safely removed.
Continue to: Other self-harm methods...
Other self-harm methods with a high degree of lethality include jumping from bridges or buildings, poisonings, self-immolation, cutting, and hangings. Individuals who choose these approaches generally do not intend to survive. Many of these methods also entail premeditation, as in the case of individuals who canvass bridges and note time when traffic is light so they are less likely to be interrupted. Between 1937 and 2012, there were >1,600 deaths by suicide from San Francisco’s Golden Gate Bridge.9 Patients who choose highly lethal methods are often irritated during the postattempt evaluation because their plans were not fatal. Usually, patients who choose such potentially lethal methods are hospitalized initially on medical and surgical floors, and receive most of their psychiatric care from consultation psychiatrists. Following discharge, these patients may be at high risk for subsequent suicide attempts.
In the US, the most common method of attempting suicide is by overdose.4 Lethality is determined by the agent or combination of substances ingested, the amount taken, the person’s health status, and the length of time before they are discovered. Many patients mistakenly assume that readily available agents such as acetaminophen and aspirin are less likely to be fatal than prescription medications. Evaluators may want to assess for suicidality in individuals with erratic, risk-taking behaviors, who are at especially high risk for death. Learning about the method the patient used can help the clinician determine the imminent risk of another suicide attempt. The more potentially fatal the patient’s method, the more serious their suicide intent, and the higher the risk they will make another suicide attempt, possibly using an even more lethal method.
4. What was the intent?
“What did you want to happen when you made this attempt?” Many patients will respond that they wanted to die, sleep, not wake up, or did not care what happened. Others say it was a gesture to evoke a certain response from another person. If this is the case, it is important to know whether the desired outcome was achieved. These so-called gestures often involve making sure the intended person is aware of the attempt, often by writing a letter, sending a text, or posting on social media. Such behaviors may be exhibited by patients with personality disorders. While such attempts often are impulsive, if the attempt fails to generate the anticipated effect, the patient may try to gain more attention by escalating their suicide actions.
Conversely, if a spouse or partner reconciles with the patient solely because of a suicide attempt, this may set a pattern for future self-harm events in which the patient hopes to achieve the same outcome. Nevertheless, it is better to err for safety because some of these patients will make another attempt, just to prove that they should have been taken more seriously. An exploration of such intent can help the evaluation because even supposed “gestures” can have dangerous consequences. Acts that do not result in the desired outcome should precipitate hospitalization rather than discharge.
5. What facilitated the patient’s rescue?
“Why is this patient still alive?” Determine if the patient did anything to save themself, such as calling an ambulance, inducing emesis, telling someone what they did, or coming to the hospital on their own. If yes, asking them what changed their mind may provide information about what exists in their lives to potentially prevent future attempts, or about wishes to stay alive. These issues can be used to guide outpatient therapy.
Continue to: How does the patient feel about having survived?
6. How does the patient feel about having survived?
When a patient is asked how they feel about having survived a suicide attempt, some will label their act “stupid” and profess embarrassment. Others exhibit future-oriented thought, which is a very good prognostic sign. More ominous is subsequent dysphoria or lamenting that “I could not even do this right.” Patients often express anger toward anyone who rescued them, especially those whose attempts were carefully planned or were discovered by accident. Some patients might also express ambivalence about having survived.
The patient’s response to this question may be shaped by their desire to avoid hospitalization, so beyond their verbal answers, be attentive to clinical cues that may suggest the patient is not being fully transparent. Anger or ambivalence about having survived, a lack of future-oriented thought, and a restricted affect despite verbalizing joy about still being alive are features that suggest psychiatric hospitalization may be warranted.
7. Has the patient made previous suicide attempts?
Compared to individuals with no previous suicide attempts, patients with a history of suicide attempts are 30 to 40 times more likely to die by suicide.2 Many patients who present after a suicide attempt have tried to kill themselves multiple times. Exploring the number of past attempts, how recent the attempts were, and what dispositions were made can be of benefit. Reviewing the potential lethality of past attempts (eg, was hospitalization required, was the patient placed in an intensive care unit, and/or was intubation needed) is recommended. If outpatient care was suggested or medication prescribed, was the patient adherent? Consider asking about passive suicidal behavior, such as not seeking care for medical issues, discontinuing life-saving medication, or engaging in reckless behavior. While such behaviors may not have been classified as a suicide attempt, it might indicate a feeling of indifference toward staying alive. A patient with a past attempt, especially if recent, merits consideration for inpatient care. Once again, referring previously nonadherent patients to outpatient treatment is less likely to be effective.
8. Does the patient have a support network?
Before discharging a patient who has made a suicide attempt, consider the quality of their support network. Gauging the response of the family and friends to the patient’s attempt can be beneficial. Indifference or resentment on the part of loved ones is a bad sign. Some patients have access to support networks they either did not know were available or chose not to utilize. In other instances, after realizing how depressed the patient has been, the family might provide a new safety net. Strong religious affiliations can also be valuable because devout spirituality can be a deterrent to suicide behaviors.10 For an individual whose attempt was motivated by loneliness or feeling unloved or underappreciated, a newly realized support network can be an additional protective deterrent.
9. Does the patient have a family history of suicide?
There may be a familial component to suicide. Knowing about any suicide history in the family contributes to future therapeutic planning. The clinician may want to explore the patient’s family suicide history in detail because such information can have substantial impact on the patient’s motivation for attempting suicide. The evaluator may want to determine if the anniversary of a family suicide is coming. Triggers for a suicide attempt could include the anniversary of a death, birthdays, family-oriented holidays, and similar events. It is productive to understand how the patient feels about family members who have died by suicide. Some will empathize with the deceased, commenting that they did the “right thing.” Others, upon realizing how their own attempt affected others, will be remorseful and determined not to inflict more pain on their family. Such patients may need to be reminded of the misery associated with their family being left without them. These understandings are helpful at setting a safe disposition. However, a history of death by suicide in the family should always be thoroughly evaluated, regardless of the patient’s attitude about that death.
Continue to: Was the attempt the result of depression?
10. Was the attempt the result of depression?
For a patient experiencing depressive symptoms, the prognosis is less positive; they are more likely to harbor serious intent, premeditation, hopelessness, and social isolation, and less likely to express future-oriented thought. They often exhibit a temporary “flight into health.” Such progress is often transitory and may not represent recovery. Because mood disorders may still be present despite a temporary improvement, inpatient and pharmacologic treatment may be needed. If a patient’s suicide attempt occurred as a result of severe depression, it is possible they will make another suicide attempt unless their depression is addressed in a safe and secure setting, such as inpatient hospitalization, or through close family observation while the patient is receiving intensive outpatient treatment.
11. Does the patient have a psychotic disorder?
Many patients with a psychotic illness die following their first attempt without ever having contact with a mental health professional.11 Features of psychosis might include malevolent auditory hallucinations that suggest self-destruction.11 Such “voices” can be intense and self-deprecating; many patients with this type of hallucination report having made a suicide attempt “just to make the voices stop.”
Symptoms of paranoia can make it less likely for individuals with psychosis to confide in family members, friends, or medical personnel. Religious elements are often of a delusional nature and can be dangerous. Psychosis is more difficult to hide than depression and the presence of psychoses concurrent with major depressive disorder (MDD) increases the probability of suicidality.11 Psychosis secondary to substance use may diminish inhibitions and heighten impulsivity, thereby exacerbating the likelihood of self-harm. Usually, the presence of psychotic features precipitating or following a suicide attempt leads to psychiatric hospitalization.
12. Is the patient in a high-risk demographic group?
When evaluating a patient who has attempted suicide, it helps to consider not just what they did, but who they are. Specifically, does the individual belong to a demographic group that traditionally has a high rate of suicide? For example, patients who are Native American or Alaska Natives warrant extra caution.2 Older White males, especially those who are divorced, widowed, retired, and/or have chronic health problems, are also at greater risk. Compared to the general population, individuals age >80 have a massively elevated chance for self-induced death.12 Some of the reasons include:
- medical comorbidities make surviving an attempt less likely
- access to large amounts of medications
- more irreversible issues, such as chronic pain, disability, or widowhood
- living alone, which may delay discovery.
Patients who are members of any of these demographic groups may deserve serious consideration for inpatient psychiatric admission, regardless of other factors.
Continue to: Were drugs or alcohol involved?
13. Were drugs or alcohol involved?
This factor is unique in that it is both a chronic risk factor (SUDs) and a warning sign for imminent suicide, as in the case of an individual who gets intoxicated to disinhibit their fear of death so they can attempt suicide. Alcohol use disorders are associated with depression and suicide. Overdoses by fentanyl and other opiates have become more frequent.13 In many cases, fatalities are unintentional because users overestimate their tolerance or ingest contaminated substances.14 Disinhibition by alcohol and/or other drugs is a risk factor for attempting suicide and can intensify the depth of MDD. Some patients will ingest substances before an attempt just to give them the courage to act; many think of suicide only when intoxicated. Toxicology screens are indicated as part of the evaluation after a suicide attempt.
Depressive and suicidal thoughts often occur in people “coming down” from cocaine or other stimulants. These circumstances require determining whether to refer the patient for treatment for an SUD or psychiatric hospitalization.
In summary, getting intoxicated solely to diminish anxiety about suicide is a dangerous feature, whereas attempting suicide due to intoxication is less concerning. The latter patient may not consider suicide unless they become intoxicated again. When available, dual diagnosis treatment facilities can be an appropriate referral for such patients. Emergency department holding beds can allow these individuals to detoxify prior to the evaluation.
14. Does the patient have future-oriented thoughts?
When evaluating a patient who has attempted suicide, the presence of future planning and anticipation can be reassuring, but these features should be carefully assessed.14-16
After-the-fact comments may be more reliable when a patient offers them spontaneously, as opposed to in response to direct questioning.
- the specificity of the future plans
- corroboration from the family and others about the patient’s previous investment in the upcoming event
- whether the patient mentions such plans spontaneously or only in response to direct questioning
- the patient’s emotional expression or affect when discussing their future
- whether such plans are reasonable, grandiose, and/or unrealistic.
Bottom Line
When assessing a patient after a suicide attempt, both the patient’s presentation and history and the clinician’s instincts are important. Careful consideration of the method, stated intent, premeditation vs impulsivity, feelings about having survived, presence of psychiatric illness, high-risk demographic, postattempt demeanor and affect, quality of support, presence of self-rescue behaviors, future-oriented thoughts, and other factors can help in making the appropriate disposition.
Related Resources
- Kim H, Kim Y, Shin MH, et al. Early psychiatric referral after attempted suicide helps prevent suicide reattempts: a longitudinal national cohort study in South Korea. Front Psychiatry. 2022;13:607892. doi:10.3389/fpsyt.2022.607892
- Michaud L, Berva S, Ostertag L, et al. When to discharge and when to voluntary or compulsory hospitalize? Factors associated with treatment decision after self-harm. Psychiatry Res. 2022;317:114810. doi:10.1016/j.psychres.2022.114810
1. Ten Leading Causes of Death, United States 2020. Centers for Disease Control and Prevention WISQARS. Accessed March 4, 2022. https://wisqars.cdc.gov/data/lcd/home
2. Norris D, Clark MS. Evaluation and treatment of suicidal patients. Am Fam Physician. 2012;15;85(6):602-605.
3. Gliatto MF, Rai AK. Evaluation and treatment patients with suicidal ideation. Am Fam Phys. 1999;59(6):1500-1506.
4. Dazzi T, Gribble R, Wessely S, et al. Does asking about suicide and related behaviors induce suicidal ideation? What is the evidence? Psychol Med. 2014;44(16):3361-3363.
5. Lewiecki EM, Miller SA. Suicide, guns and public policy. Am J Public Health. 2013;103(1):27-31.
6. Frierson RL. Women who shoot themselves. Hosp Community Psychiatry. 1989;40(8):841-843.
7. Frierson RL, Lippmann SB. Psychiatric consultation for patients with self-inflicted gunshot wounds. Psychosomatics. 1990;31(1):67-74.
8. Mitchell AM, Terhorst L. PTSD symptoms in survivors bereaved by the suicide of a significant other. J Am Psychiatr Nurses Assoc. 2017;23(1):61-65.
9. Bateson J. The Golden Gate Bridge’s fatal flaw. Los Angeles Times. May 25, 2012. Accessed March 2, 2022. https://www.latimes.com/opinion/la-xpm-2012-may-25-la-oe-adv-bateson-golden-gate-20120525-story.html
10. Dervic K, Oquendoma MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004;161(12):2303-2308.
11. Nordentoft H, Madsen T, Fedyszyn IF. Suicidal behavior and mortality in first episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392.
12. Frierson R, Lippmann S. Suicide attempts by the old and the very old. Arch Intern Med. 1991;151(1):141-144.
13. Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opiate poisonings in the United States: 1999-2014. Am J Public Health. 2017;107(3):421-426.
14. Morin KA, Acharya S, Eibl JK, et al: Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treated patients in Ontario, Canada. Int J Drug Policy. 2021;90:103088.
15. Shobassy A, Abu-Mohammad AS. Assessing imminent suicide risk: what about future planning? Current Psychiatry. 2022;21(2):12-17.
16. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
17. Macleod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2010;44(Pt 4):495-504.
In 2021, suicide was the 11th leading cause of death in the United States.1 Suicide resulted in 49,000 US deaths during 2021; it was the second most common cause of death in individuals age 10 to 34, and the fifth leading cause among children.1,2 Women are 3 to 4 times more likely than men to attempt suicide, but men are 4 times more likely to die by suicide.2
The evaluation of patients with suicidal ideation who have not made an attempt generally involves assessing 4 factors: the specific plan, access to lethal means, any recent social stressors, and the presence of a psychiatric disorder.3 The clinician should also assess which potential deterrents, such as religious beliefs or dependent children, might be present.
Mental health clinicians are often called upon to evaluate a patient after a suicide attempt to assess intent for continued self-harm and to determine appropriate disposition. Such an evaluation must consider multiple factors, including the method used, premeditation, consequences of the attempt, the presence of severe depression and/or psychosis, and the role of substance use. Assessment after a suicide attempt differs from the examination of individuals who harbor suicidal thoughts but have not made an attempt; the latter group may be more likely to respond to interventions such as intensive outpatient care, mobilization of family support, and religious proscriptions against suicide. However, for patients who make an attempt to end their life, whatever potential safeguards or deterrents to suicide that were in place obviously did not prevent the self-harm act. The consequences of the attempt, such as disabling injuries or medical complications, and possible involuntary commitment, need to be considered. Assessment of the patient’s feelings about having survived the attempt is important because the psychological impact of the attempt on family members may serve to intensify the patient’s depression and make a subsequent attempt more likely.
Many individuals who think of suicide have communicated self-harm thoughts or intentions, but such comments are often minimized or ignored. There is a common but erroneous belief that if patients are encouraged to discuss thoughts of self-harm, they will be more likely to act upon them. Because the opposite is true,4 clinicians should ask vulnerable patients about suicidal ideation or intent. Importantly, noncompliance with life-saving medical care, risk-taking behaviors, and substance use may also signal a desire for self-harm. Passive thoughts of death, typified by comments such as “I don’t care whether I wake up or not,” should also be elicited. Many patients who think of suicide speak of being in a “bad place” where reason and logic give way to an intense desire to end their misery.

The evaluation of a patient who has attempted suicide is an important component of providing psychiatric care. This article reflects our 45 years of evaluating such patients. As such, it reflects our clinical experience and is not evidence-based. We offer a checklist of 14 questions that we have found helpful when determining if it would be best for a patient to receive inpatient psychiatric hospitalization or a discharge referral for outpatient care (Table). Questions 1 through 6 are specific for patients who have made a suicide attempt, while questions 7 through 14 are helpful for assessing global risk factors for suicide.
1. Was the attempt premeditated?
Determining premeditation vs impulsivity is an essential element of the assessment following a suicide attempt. Many such acts may occur without forethought in response to an unexpected stressor, such as an altercation between partners or family conflicts. Impulsive attempts can occur when an individual is involved in a distressing event and/or while intoxicated. Conversely, premeditation involves forethought and planning, which may increase the risk of suicide in the near future.
Examples of premeditated behavior include:
- Contemplating the attempt days or weeks beforehand
- Researching the effects of a medication or combination of medications in terms of potential lethality
- Engaging in behavior that would decrease the likelihood of their body being discovered after the attempt
- Obtaining weapons and/or stockpiling pills
- Canvassing potential sites such as bridges or tall buildings
- Engaging in a suicide attempt “practice run”
- Leaving a suicide note or message on social media
- Making funeral arrangements, such as choosing burial clothing
- Writing a will and arranging for the custody of dependent children
- Purchasing life insurance that does not deny payment of benefits in cases of death by suicide.
Continue to: Patients with a premeditated...
Patients with a premeditated suicide attempt generally do not expect to survive and are often surprised or upset that the act was not fatal. The presence of indicators that the attempt was premeditated should direct the disposition more toward hospitalization than discharge. In assessing the impact of premeditation, it is important to gauge not just the examples listed above, but also the patient’s perception of these issues (such as potential loss of child custody). Consider how much the patient is emotionally affected by such thinking.
2. What were the consequences of the attempt?
Assessing the reason for the attempt (if any) and determining whether the inciting circumstance has changed due to the suicide attempt are an important part of the evaluation. A suicide attempt may result in reconciliation with and/or renewed support from family members or partners, who might not have been aware of the patient’s emotional distress. Such unexpected support often results in the patient exhibiting improved mood and affect, and possibly temporary resolution of suicidal thoughts. This “flight into health” may be short-lived, but it also may be enough to engage the patient in a therapeutic alliance. That may permit a discharge with safe disposition to the outpatient clinic while in the custody of a family member, partner, or close friend.
Alternatively, some people experience a troubling worsening of precipitants following a suicide attempt. Preexisting medical conditions and financial, occupational, and/or social woes may be exacerbated. Child custody determinations may be affected, assuming the patient understands the possibility of this adverse consequence. Violent methods may result in disfigurement and body image issues. Individuals from small, close-knit communities may experience stigmatization and unwanted notoriety because of their suicide attempt. Such negative consequences may render some patients more likely to make another attempt to die by suicide. It is crucial to consider how a suicide attempt may have changed the original stress that led to the attempt.
3. Which method was used?
Most fatal suicides in the US are by firearms, and many individuals who survive such attempts do so because of unfamiliarity with the weapon, gun malfunction, faulty aim, or alcohol use.5-7 Some survivors report intending to shoot themselves in the heart, but instead suffered shoulder injuries. Unfortunately, for a patient who survives self-inflicted gunshot wounds, the sequelae of chronic pain, multiple surgical procedures, disability, and disfigurement may serve as constant negative reminders of the event. Some individuals with suicidal intent eschew the idea of using firearms because they hope to avoid having a family member be the first to discover them. Witnessing the aftermath of a fatal suicide by gunshot can induce symptoms of posttraumatic stress disorder in family members and/or partners.8
For a patient with self-inflicted gunshot wounds, always determine whether the weapon has been secured or if the patient still has access to it. Asking about weapon availability is essential during the evaluation of any patient with depression, major life crises, or other factors that may yield a desire to die; this is especially true for individuals with substance use disorders (SUDs). Whenever readily available to such individuals, weapons need to be safely removed.
Continue to: Other self-harm methods...
Other self-harm methods with a high degree of lethality include jumping from bridges or buildings, poisonings, self-immolation, cutting, and hangings. Individuals who choose these approaches generally do not intend to survive. Many of these methods also entail premeditation, as in the case of individuals who canvass bridges and note time when traffic is light so they are less likely to be interrupted. Between 1937 and 2012, there were >1,600 deaths by suicide from San Francisco’s Golden Gate Bridge.9 Patients who choose highly lethal methods are often irritated during the postattempt evaluation because their plans were not fatal. Usually, patients who choose such potentially lethal methods are hospitalized initially on medical and surgical floors, and receive most of their psychiatric care from consultation psychiatrists. Following discharge, these patients may be at high risk for subsequent suicide attempts.
In the US, the most common method of attempting suicide is by overdose.4 Lethality is determined by the agent or combination of substances ingested, the amount taken, the person’s health status, and the length of time before they are discovered. Many patients mistakenly assume that readily available agents such as acetaminophen and aspirin are less likely to be fatal than prescription medications. Evaluators may want to assess for suicidality in individuals with erratic, risk-taking behaviors, who are at especially high risk for death. Learning about the method the patient used can help the clinician determine the imminent risk of another suicide attempt. The more potentially fatal the patient’s method, the more serious their suicide intent, and the higher the risk they will make another suicide attempt, possibly using an even more lethal method.
4. What was the intent?
“What did you want to happen when you made this attempt?” Many patients will respond that they wanted to die, sleep, not wake up, or did not care what happened. Others say it was a gesture to evoke a certain response from another person. If this is the case, it is important to know whether the desired outcome was achieved. These so-called gestures often involve making sure the intended person is aware of the attempt, often by writing a letter, sending a text, or posting on social media. Such behaviors may be exhibited by patients with personality disorders. While such attempts often are impulsive, if the attempt fails to generate the anticipated effect, the patient may try to gain more attention by escalating their suicide actions.
Conversely, if a spouse or partner reconciles with the patient solely because of a suicide attempt, this may set a pattern for future self-harm events in which the patient hopes to achieve the same outcome. Nevertheless, it is better to err for safety because some of these patients will make another attempt, just to prove that they should have been taken more seriously. An exploration of such intent can help the evaluation because even supposed “gestures” can have dangerous consequences. Acts that do not result in the desired outcome should precipitate hospitalization rather than discharge.
5. What facilitated the patient’s rescue?
“Why is this patient still alive?” Determine if the patient did anything to save themself, such as calling an ambulance, inducing emesis, telling someone what they did, or coming to the hospital on their own. If yes, asking them what changed their mind may provide information about what exists in their lives to potentially prevent future attempts, or about wishes to stay alive. These issues can be used to guide outpatient therapy.
Continue to: How does the patient feel about having survived?
6. How does the patient feel about having survived?
When a patient is asked how they feel about having survived a suicide attempt, some will label their act “stupid” and profess embarrassment. Others exhibit future-oriented thought, which is a very good prognostic sign. More ominous is subsequent dysphoria or lamenting that “I could not even do this right.” Patients often express anger toward anyone who rescued them, especially those whose attempts were carefully planned or were discovered by accident. Some patients might also express ambivalence about having survived.
The patient’s response to this question may be shaped by their desire to avoid hospitalization, so beyond their verbal answers, be attentive to clinical cues that may suggest the patient is not being fully transparent. Anger or ambivalence about having survived, a lack of future-oriented thought, and a restricted affect despite verbalizing joy about still being alive are features that suggest psychiatric hospitalization may be warranted.
7. Has the patient made previous suicide attempts?
Compared to individuals with no previous suicide attempts, patients with a history of suicide attempts are 30 to 40 times more likely to die by suicide.2 Many patients who present after a suicide attempt have tried to kill themselves multiple times. Exploring the number of past attempts, how recent the attempts were, and what dispositions were made can be of benefit. Reviewing the potential lethality of past attempts (eg, was hospitalization required, was the patient placed in an intensive care unit, and/or was intubation needed) is recommended. If outpatient care was suggested or medication prescribed, was the patient adherent? Consider asking about passive suicidal behavior, such as not seeking care for medical issues, discontinuing life-saving medication, or engaging in reckless behavior. While such behaviors may not have been classified as a suicide attempt, it might indicate a feeling of indifference toward staying alive. A patient with a past attempt, especially if recent, merits consideration for inpatient care. Once again, referring previously nonadherent patients to outpatient treatment is less likely to be effective.
8. Does the patient have a support network?
Before discharging a patient who has made a suicide attempt, consider the quality of their support network. Gauging the response of the family and friends to the patient’s attempt can be beneficial. Indifference or resentment on the part of loved ones is a bad sign. Some patients have access to support networks they either did not know were available or chose not to utilize. In other instances, after realizing how depressed the patient has been, the family might provide a new safety net. Strong religious affiliations can also be valuable because devout spirituality can be a deterrent to suicide behaviors.10 For an individual whose attempt was motivated by loneliness or feeling unloved or underappreciated, a newly realized support network can be an additional protective deterrent.
9. Does the patient have a family history of suicide?
There may be a familial component to suicide. Knowing about any suicide history in the family contributes to future therapeutic planning. The clinician may want to explore the patient’s family suicide history in detail because such information can have substantial impact on the patient’s motivation for attempting suicide. The evaluator may want to determine if the anniversary of a family suicide is coming. Triggers for a suicide attempt could include the anniversary of a death, birthdays, family-oriented holidays, and similar events. It is productive to understand how the patient feels about family members who have died by suicide. Some will empathize with the deceased, commenting that they did the “right thing.” Others, upon realizing how their own attempt affected others, will be remorseful and determined not to inflict more pain on their family. Such patients may need to be reminded of the misery associated with their family being left without them. These understandings are helpful at setting a safe disposition. However, a history of death by suicide in the family should always be thoroughly evaluated, regardless of the patient’s attitude about that death.
Continue to: Was the attempt the result of depression?
10. Was the attempt the result of depression?
For a patient experiencing depressive symptoms, the prognosis is less positive; they are more likely to harbor serious intent, premeditation, hopelessness, and social isolation, and less likely to express future-oriented thought. They often exhibit a temporary “flight into health.” Such progress is often transitory and may not represent recovery. Because mood disorders may still be present despite a temporary improvement, inpatient and pharmacologic treatment may be needed. If a patient’s suicide attempt occurred as a result of severe depression, it is possible they will make another suicide attempt unless their depression is addressed in a safe and secure setting, such as inpatient hospitalization, or through close family observation while the patient is receiving intensive outpatient treatment.
11. Does the patient have a psychotic disorder?
Many patients with a psychotic illness die following their first attempt without ever having contact with a mental health professional.11 Features of psychosis might include malevolent auditory hallucinations that suggest self-destruction.11 Such “voices” can be intense and self-deprecating; many patients with this type of hallucination report having made a suicide attempt “just to make the voices stop.”
Symptoms of paranoia can make it less likely for individuals with psychosis to confide in family members, friends, or medical personnel. Religious elements are often of a delusional nature and can be dangerous. Psychosis is more difficult to hide than depression and the presence of psychoses concurrent with major depressive disorder (MDD) increases the probability of suicidality.11 Psychosis secondary to substance use may diminish inhibitions and heighten impulsivity, thereby exacerbating the likelihood of self-harm. Usually, the presence of psychotic features precipitating or following a suicide attempt leads to psychiatric hospitalization.
12. Is the patient in a high-risk demographic group?
When evaluating a patient who has attempted suicide, it helps to consider not just what they did, but who they are. Specifically, does the individual belong to a demographic group that traditionally has a high rate of suicide? For example, patients who are Native American or Alaska Natives warrant extra caution.2 Older White males, especially those who are divorced, widowed, retired, and/or have chronic health problems, are also at greater risk. Compared to the general population, individuals age >80 have a massively elevated chance for self-induced death.12 Some of the reasons include:
- medical comorbidities make surviving an attempt less likely
- access to large amounts of medications
- more irreversible issues, such as chronic pain, disability, or widowhood
- living alone, which may delay discovery.
Patients who are members of any of these demographic groups may deserve serious consideration for inpatient psychiatric admission, regardless of other factors.
Continue to: Were drugs or alcohol involved?
13. Were drugs or alcohol involved?
This factor is unique in that it is both a chronic risk factor (SUDs) and a warning sign for imminent suicide, as in the case of an individual who gets intoxicated to disinhibit their fear of death so they can attempt suicide. Alcohol use disorders are associated with depression and suicide. Overdoses by fentanyl and other opiates have become more frequent.13 In many cases, fatalities are unintentional because users overestimate their tolerance or ingest contaminated substances.14 Disinhibition by alcohol and/or other drugs is a risk factor for attempting suicide and can intensify the depth of MDD. Some patients will ingest substances before an attempt just to give them the courage to act; many think of suicide only when intoxicated. Toxicology screens are indicated as part of the evaluation after a suicide attempt.
Depressive and suicidal thoughts often occur in people “coming down” from cocaine or other stimulants. These circumstances require determining whether to refer the patient for treatment for an SUD or psychiatric hospitalization.
In summary, getting intoxicated solely to diminish anxiety about suicide is a dangerous feature, whereas attempting suicide due to intoxication is less concerning. The latter patient may not consider suicide unless they become intoxicated again. When available, dual diagnosis treatment facilities can be an appropriate referral for such patients. Emergency department holding beds can allow these individuals to detoxify prior to the evaluation.
14. Does the patient have future-oriented thoughts?
When evaluating a patient who has attempted suicide, the presence of future planning and anticipation can be reassuring, but these features should be carefully assessed.14-16
After-the-fact comments may be more reliable when a patient offers them spontaneously, as opposed to in response to direct questioning.
- the specificity of the future plans
- corroboration from the family and others about the patient’s previous investment in the upcoming event
- whether the patient mentions such plans spontaneously or only in response to direct questioning
- the patient’s emotional expression or affect when discussing their future
- whether such plans are reasonable, grandiose, and/or unrealistic.
Bottom Line
When assessing a patient after a suicide attempt, both the patient’s presentation and history and the clinician’s instincts are important. Careful consideration of the method, stated intent, premeditation vs impulsivity, feelings about having survived, presence of psychiatric illness, high-risk demographic, postattempt demeanor and affect, quality of support, presence of self-rescue behaviors, future-oriented thoughts, and other factors can help in making the appropriate disposition.
Related Resources
- Kim H, Kim Y, Shin MH, et al. Early psychiatric referral after attempted suicide helps prevent suicide reattempts: a longitudinal national cohort study in South Korea. Front Psychiatry. 2022;13:607892. doi:10.3389/fpsyt.2022.607892
- Michaud L, Berva S, Ostertag L, et al. When to discharge and when to voluntary or compulsory hospitalize? Factors associated with treatment decision after self-harm. Psychiatry Res. 2022;317:114810. doi:10.1016/j.psychres.2022.114810
In 2021, suicide was the 11th leading cause of death in the United States.1 Suicide resulted in 49,000 US deaths during 2021; it was the second most common cause of death in individuals age 10 to 34, and the fifth leading cause among children.1,2 Women are 3 to 4 times more likely than men to attempt suicide, but men are 4 times more likely to die by suicide.2
The evaluation of patients with suicidal ideation who have not made an attempt generally involves assessing 4 factors: the specific plan, access to lethal means, any recent social stressors, and the presence of a psychiatric disorder.3 The clinician should also assess which potential deterrents, such as religious beliefs or dependent children, might be present.
Mental health clinicians are often called upon to evaluate a patient after a suicide attempt to assess intent for continued self-harm and to determine appropriate disposition. Such an evaluation must consider multiple factors, including the method used, premeditation, consequences of the attempt, the presence of severe depression and/or psychosis, and the role of substance use. Assessment after a suicide attempt differs from the examination of individuals who harbor suicidal thoughts but have not made an attempt; the latter group may be more likely to respond to interventions such as intensive outpatient care, mobilization of family support, and religious proscriptions against suicide. However, for patients who make an attempt to end their life, whatever potential safeguards or deterrents to suicide that were in place obviously did not prevent the self-harm act. The consequences of the attempt, such as disabling injuries or medical complications, and possible involuntary commitment, need to be considered. Assessment of the patient’s feelings about having survived the attempt is important because the psychological impact of the attempt on family members may serve to intensify the patient’s depression and make a subsequent attempt more likely.
Many individuals who think of suicide have communicated self-harm thoughts or intentions, but such comments are often minimized or ignored. There is a common but erroneous belief that if patients are encouraged to discuss thoughts of self-harm, they will be more likely to act upon them. Because the opposite is true,4 clinicians should ask vulnerable patients about suicidal ideation or intent. Importantly, noncompliance with life-saving medical care, risk-taking behaviors, and substance use may also signal a desire for self-harm. Passive thoughts of death, typified by comments such as “I don’t care whether I wake up or not,” should also be elicited. Many patients who think of suicide speak of being in a “bad place” where reason and logic give way to an intense desire to end their misery.

The evaluation of a patient who has attempted suicide is an important component of providing psychiatric care. This article reflects our 45 years of evaluating such patients. As such, it reflects our clinical experience and is not evidence-based. We offer a checklist of 14 questions that we have found helpful when determining if it would be best for a patient to receive inpatient psychiatric hospitalization or a discharge referral for outpatient care (Table). Questions 1 through 6 are specific for patients who have made a suicide attempt, while questions 7 through 14 are helpful for assessing global risk factors for suicide.
1. Was the attempt premeditated?
Determining premeditation vs impulsivity is an essential element of the assessment following a suicide attempt. Many such acts may occur without forethought in response to an unexpected stressor, such as an altercation between partners or family conflicts. Impulsive attempts can occur when an individual is involved in a distressing event and/or while intoxicated. Conversely, premeditation involves forethought and planning, which may increase the risk of suicide in the near future.
Examples of premeditated behavior include:
- Contemplating the attempt days or weeks beforehand
- Researching the effects of a medication or combination of medications in terms of potential lethality
- Engaging in behavior that would decrease the likelihood of their body being discovered after the attempt
- Obtaining weapons and/or stockpiling pills
- Canvassing potential sites such as bridges or tall buildings
- Engaging in a suicide attempt “practice run”
- Leaving a suicide note or message on social media
- Making funeral arrangements, such as choosing burial clothing
- Writing a will and arranging for the custody of dependent children
- Purchasing life insurance that does not deny payment of benefits in cases of death by suicide.
Continue to: Patients with a premeditated...
Patients with a premeditated suicide attempt generally do not expect to survive and are often surprised or upset that the act was not fatal. The presence of indicators that the attempt was premeditated should direct the disposition more toward hospitalization than discharge. In assessing the impact of premeditation, it is important to gauge not just the examples listed above, but also the patient’s perception of these issues (such as potential loss of child custody). Consider how much the patient is emotionally affected by such thinking.
2. What were the consequences of the attempt?
Assessing the reason for the attempt (if any) and determining whether the inciting circumstance has changed due to the suicide attempt are an important part of the evaluation. A suicide attempt may result in reconciliation with and/or renewed support from family members or partners, who might not have been aware of the patient’s emotional distress. Such unexpected support often results in the patient exhibiting improved mood and affect, and possibly temporary resolution of suicidal thoughts. This “flight into health” may be short-lived, but it also may be enough to engage the patient in a therapeutic alliance. That may permit a discharge with safe disposition to the outpatient clinic while in the custody of a family member, partner, or close friend.
Alternatively, some people experience a troubling worsening of precipitants following a suicide attempt. Preexisting medical conditions and financial, occupational, and/or social woes may be exacerbated. Child custody determinations may be affected, assuming the patient understands the possibility of this adverse consequence. Violent methods may result in disfigurement and body image issues. Individuals from small, close-knit communities may experience stigmatization and unwanted notoriety because of their suicide attempt. Such negative consequences may render some patients more likely to make another attempt to die by suicide. It is crucial to consider how a suicide attempt may have changed the original stress that led to the attempt.
3. Which method was used?
Most fatal suicides in the US are by firearms, and many individuals who survive such attempts do so because of unfamiliarity with the weapon, gun malfunction, faulty aim, or alcohol use.5-7 Some survivors report intending to shoot themselves in the heart, but instead suffered shoulder injuries. Unfortunately, for a patient who survives self-inflicted gunshot wounds, the sequelae of chronic pain, multiple surgical procedures, disability, and disfigurement may serve as constant negative reminders of the event. Some individuals with suicidal intent eschew the idea of using firearms because they hope to avoid having a family member be the first to discover them. Witnessing the aftermath of a fatal suicide by gunshot can induce symptoms of posttraumatic stress disorder in family members and/or partners.8
For a patient with self-inflicted gunshot wounds, always determine whether the weapon has been secured or if the patient still has access to it. Asking about weapon availability is essential during the evaluation of any patient with depression, major life crises, or other factors that may yield a desire to die; this is especially true for individuals with substance use disorders (SUDs). Whenever readily available to such individuals, weapons need to be safely removed.
Continue to: Other self-harm methods...
Other self-harm methods with a high degree of lethality include jumping from bridges or buildings, poisonings, self-immolation, cutting, and hangings. Individuals who choose these approaches generally do not intend to survive. Many of these methods also entail premeditation, as in the case of individuals who canvass bridges and note time when traffic is light so they are less likely to be interrupted. Between 1937 and 2012, there were >1,600 deaths by suicide from San Francisco’s Golden Gate Bridge.9 Patients who choose highly lethal methods are often irritated during the postattempt evaluation because their plans were not fatal. Usually, patients who choose such potentially lethal methods are hospitalized initially on medical and surgical floors, and receive most of their psychiatric care from consultation psychiatrists. Following discharge, these patients may be at high risk for subsequent suicide attempts.
In the US, the most common method of attempting suicide is by overdose.4 Lethality is determined by the agent or combination of substances ingested, the amount taken, the person’s health status, and the length of time before they are discovered. Many patients mistakenly assume that readily available agents such as acetaminophen and aspirin are less likely to be fatal than prescription medications. Evaluators may want to assess for suicidality in individuals with erratic, risk-taking behaviors, who are at especially high risk for death. Learning about the method the patient used can help the clinician determine the imminent risk of another suicide attempt. The more potentially fatal the patient’s method, the more serious their suicide intent, and the higher the risk they will make another suicide attempt, possibly using an even more lethal method.
4. What was the intent?
“What did you want to happen when you made this attempt?” Many patients will respond that they wanted to die, sleep, not wake up, or did not care what happened. Others say it was a gesture to evoke a certain response from another person. If this is the case, it is important to know whether the desired outcome was achieved. These so-called gestures often involve making sure the intended person is aware of the attempt, often by writing a letter, sending a text, or posting on social media. Such behaviors may be exhibited by patients with personality disorders. While such attempts often are impulsive, if the attempt fails to generate the anticipated effect, the patient may try to gain more attention by escalating their suicide actions.
Conversely, if a spouse or partner reconciles with the patient solely because of a suicide attempt, this may set a pattern for future self-harm events in which the patient hopes to achieve the same outcome. Nevertheless, it is better to err for safety because some of these patients will make another attempt, just to prove that they should have been taken more seriously. An exploration of such intent can help the evaluation because even supposed “gestures” can have dangerous consequences. Acts that do not result in the desired outcome should precipitate hospitalization rather than discharge.
5. What facilitated the patient’s rescue?
“Why is this patient still alive?” Determine if the patient did anything to save themself, such as calling an ambulance, inducing emesis, telling someone what they did, or coming to the hospital on their own. If yes, asking them what changed their mind may provide information about what exists in their lives to potentially prevent future attempts, or about wishes to stay alive. These issues can be used to guide outpatient therapy.
Continue to: How does the patient feel about having survived?
6. How does the patient feel about having survived?
When a patient is asked how they feel about having survived a suicide attempt, some will label their act “stupid” and profess embarrassment. Others exhibit future-oriented thought, which is a very good prognostic sign. More ominous is subsequent dysphoria or lamenting that “I could not even do this right.” Patients often express anger toward anyone who rescued them, especially those whose attempts were carefully planned or were discovered by accident. Some patients might also express ambivalence about having survived.
The patient’s response to this question may be shaped by their desire to avoid hospitalization, so beyond their verbal answers, be attentive to clinical cues that may suggest the patient is not being fully transparent. Anger or ambivalence about having survived, a lack of future-oriented thought, and a restricted affect despite verbalizing joy about still being alive are features that suggest psychiatric hospitalization may be warranted.
7. Has the patient made previous suicide attempts?
Compared to individuals with no previous suicide attempts, patients with a history of suicide attempts are 30 to 40 times more likely to die by suicide.2 Many patients who present after a suicide attempt have tried to kill themselves multiple times. Exploring the number of past attempts, how recent the attempts were, and what dispositions were made can be of benefit. Reviewing the potential lethality of past attempts (eg, was hospitalization required, was the patient placed in an intensive care unit, and/or was intubation needed) is recommended. If outpatient care was suggested or medication prescribed, was the patient adherent? Consider asking about passive suicidal behavior, such as not seeking care for medical issues, discontinuing life-saving medication, or engaging in reckless behavior. While such behaviors may not have been classified as a suicide attempt, it might indicate a feeling of indifference toward staying alive. A patient with a past attempt, especially if recent, merits consideration for inpatient care. Once again, referring previously nonadherent patients to outpatient treatment is less likely to be effective.
8. Does the patient have a support network?
Before discharging a patient who has made a suicide attempt, consider the quality of their support network. Gauging the response of the family and friends to the patient’s attempt can be beneficial. Indifference or resentment on the part of loved ones is a bad sign. Some patients have access to support networks they either did not know were available or chose not to utilize. In other instances, after realizing how depressed the patient has been, the family might provide a new safety net. Strong religious affiliations can also be valuable because devout spirituality can be a deterrent to suicide behaviors.10 For an individual whose attempt was motivated by loneliness or feeling unloved or underappreciated, a newly realized support network can be an additional protective deterrent.
9. Does the patient have a family history of suicide?
There may be a familial component to suicide. Knowing about any suicide history in the family contributes to future therapeutic planning. The clinician may want to explore the patient’s family suicide history in detail because such information can have substantial impact on the patient’s motivation for attempting suicide. The evaluator may want to determine if the anniversary of a family suicide is coming. Triggers for a suicide attempt could include the anniversary of a death, birthdays, family-oriented holidays, and similar events. It is productive to understand how the patient feels about family members who have died by suicide. Some will empathize with the deceased, commenting that they did the “right thing.” Others, upon realizing how their own attempt affected others, will be remorseful and determined not to inflict more pain on their family. Such patients may need to be reminded of the misery associated with their family being left without them. These understandings are helpful at setting a safe disposition. However, a history of death by suicide in the family should always be thoroughly evaluated, regardless of the patient’s attitude about that death.
Continue to: Was the attempt the result of depression?
10. Was the attempt the result of depression?
For a patient experiencing depressive symptoms, the prognosis is less positive; they are more likely to harbor serious intent, premeditation, hopelessness, and social isolation, and less likely to express future-oriented thought. They often exhibit a temporary “flight into health.” Such progress is often transitory and may not represent recovery. Because mood disorders may still be present despite a temporary improvement, inpatient and pharmacologic treatment may be needed. If a patient’s suicide attempt occurred as a result of severe depression, it is possible they will make another suicide attempt unless their depression is addressed in a safe and secure setting, such as inpatient hospitalization, or through close family observation while the patient is receiving intensive outpatient treatment.
11. Does the patient have a psychotic disorder?
Many patients with a psychotic illness die following their first attempt without ever having contact with a mental health professional.11 Features of psychosis might include malevolent auditory hallucinations that suggest self-destruction.11 Such “voices” can be intense and self-deprecating; many patients with this type of hallucination report having made a suicide attempt “just to make the voices stop.”
Symptoms of paranoia can make it less likely for individuals with psychosis to confide in family members, friends, or medical personnel. Religious elements are often of a delusional nature and can be dangerous. Psychosis is more difficult to hide than depression and the presence of psychoses concurrent with major depressive disorder (MDD) increases the probability of suicidality.11 Psychosis secondary to substance use may diminish inhibitions and heighten impulsivity, thereby exacerbating the likelihood of self-harm. Usually, the presence of psychotic features precipitating or following a suicide attempt leads to psychiatric hospitalization.
12. Is the patient in a high-risk demographic group?
When evaluating a patient who has attempted suicide, it helps to consider not just what they did, but who they are. Specifically, does the individual belong to a demographic group that traditionally has a high rate of suicide? For example, patients who are Native American or Alaska Natives warrant extra caution.2 Older White males, especially those who are divorced, widowed, retired, and/or have chronic health problems, are also at greater risk. Compared to the general population, individuals age >80 have a massively elevated chance for self-induced death.12 Some of the reasons include:
- medical comorbidities make surviving an attempt less likely
- access to large amounts of medications
- more irreversible issues, such as chronic pain, disability, or widowhood
- living alone, which may delay discovery.
Patients who are members of any of these demographic groups may deserve serious consideration for inpatient psychiatric admission, regardless of other factors.
Continue to: Were drugs or alcohol involved?
13. Were drugs or alcohol involved?
This factor is unique in that it is both a chronic risk factor (SUDs) and a warning sign for imminent suicide, as in the case of an individual who gets intoxicated to disinhibit their fear of death so they can attempt suicide. Alcohol use disorders are associated with depression and suicide. Overdoses by fentanyl and other opiates have become more frequent.13 In many cases, fatalities are unintentional because users overestimate their tolerance or ingest contaminated substances.14 Disinhibition by alcohol and/or other drugs is a risk factor for attempting suicide and can intensify the depth of MDD. Some patients will ingest substances before an attempt just to give them the courage to act; many think of suicide only when intoxicated. Toxicology screens are indicated as part of the evaluation after a suicide attempt.
Depressive and suicidal thoughts often occur in people “coming down” from cocaine or other stimulants. These circumstances require determining whether to refer the patient for treatment for an SUD or psychiatric hospitalization.
In summary, getting intoxicated solely to diminish anxiety about suicide is a dangerous feature, whereas attempting suicide due to intoxication is less concerning. The latter patient may not consider suicide unless they become intoxicated again. When available, dual diagnosis treatment facilities can be an appropriate referral for such patients. Emergency department holding beds can allow these individuals to detoxify prior to the evaluation.
14. Does the patient have future-oriented thoughts?
When evaluating a patient who has attempted suicide, the presence of future planning and anticipation can be reassuring, but these features should be carefully assessed.14-16
After-the-fact comments may be more reliable when a patient offers them spontaneously, as opposed to in response to direct questioning.
- the specificity of the future plans
- corroboration from the family and others about the patient’s previous investment in the upcoming event
- whether the patient mentions such plans spontaneously or only in response to direct questioning
- the patient’s emotional expression or affect when discussing their future
- whether such plans are reasonable, grandiose, and/or unrealistic.
Bottom Line
When assessing a patient after a suicide attempt, both the patient’s presentation and history and the clinician’s instincts are important. Careful consideration of the method, stated intent, premeditation vs impulsivity, feelings about having survived, presence of psychiatric illness, high-risk demographic, postattempt demeanor and affect, quality of support, presence of self-rescue behaviors, future-oriented thoughts, and other factors can help in making the appropriate disposition.
Related Resources
- Kim H, Kim Y, Shin MH, et al. Early psychiatric referral after attempted suicide helps prevent suicide reattempts: a longitudinal national cohort study in South Korea. Front Psychiatry. 2022;13:607892. doi:10.3389/fpsyt.2022.607892
- Michaud L, Berva S, Ostertag L, et al. When to discharge and when to voluntary or compulsory hospitalize? Factors associated with treatment decision after self-harm. Psychiatry Res. 2022;317:114810. doi:10.1016/j.psychres.2022.114810
1. Ten Leading Causes of Death, United States 2020. Centers for Disease Control and Prevention WISQARS. Accessed March 4, 2022. https://wisqars.cdc.gov/data/lcd/home
2. Norris D, Clark MS. Evaluation and treatment of suicidal patients. Am Fam Physician. 2012;15;85(6):602-605.
3. Gliatto MF, Rai AK. Evaluation and treatment patients with suicidal ideation. Am Fam Phys. 1999;59(6):1500-1506.
4. Dazzi T, Gribble R, Wessely S, et al. Does asking about suicide and related behaviors induce suicidal ideation? What is the evidence? Psychol Med. 2014;44(16):3361-3363.
5. Lewiecki EM, Miller SA. Suicide, guns and public policy. Am J Public Health. 2013;103(1):27-31.
6. Frierson RL. Women who shoot themselves. Hosp Community Psychiatry. 1989;40(8):841-843.
7. Frierson RL, Lippmann SB. Psychiatric consultation for patients with self-inflicted gunshot wounds. Psychosomatics. 1990;31(1):67-74.
8. Mitchell AM, Terhorst L. PTSD symptoms in survivors bereaved by the suicide of a significant other. J Am Psychiatr Nurses Assoc. 2017;23(1):61-65.
9. Bateson J. The Golden Gate Bridge’s fatal flaw. Los Angeles Times. May 25, 2012. Accessed March 2, 2022. https://www.latimes.com/opinion/la-xpm-2012-may-25-la-oe-adv-bateson-golden-gate-20120525-story.html
10. Dervic K, Oquendoma MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004;161(12):2303-2308.
11. Nordentoft H, Madsen T, Fedyszyn IF. Suicidal behavior and mortality in first episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392.
12. Frierson R, Lippmann S. Suicide attempts by the old and the very old. Arch Intern Med. 1991;151(1):141-144.
13. Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opiate poisonings in the United States: 1999-2014. Am J Public Health. 2017;107(3):421-426.
14. Morin KA, Acharya S, Eibl JK, et al: Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treated patients in Ontario, Canada. Int J Drug Policy. 2021;90:103088.
15. Shobassy A, Abu-Mohammad AS. Assessing imminent suicide risk: what about future planning? Current Psychiatry. 2022;21(2):12-17.
16. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
17. Macleod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2010;44(Pt 4):495-504.
1. Ten Leading Causes of Death, United States 2020. Centers for Disease Control and Prevention WISQARS. Accessed March 4, 2022. https://wisqars.cdc.gov/data/lcd/home
2. Norris D, Clark MS. Evaluation and treatment of suicidal patients. Am Fam Physician. 2012;15;85(6):602-605.
3. Gliatto MF, Rai AK. Evaluation and treatment patients with suicidal ideation. Am Fam Phys. 1999;59(6):1500-1506.
4. Dazzi T, Gribble R, Wessely S, et al. Does asking about suicide and related behaviors induce suicidal ideation? What is the evidence? Psychol Med. 2014;44(16):3361-3363.
5. Lewiecki EM, Miller SA. Suicide, guns and public policy. Am J Public Health. 2013;103(1):27-31.
6. Frierson RL. Women who shoot themselves. Hosp Community Psychiatry. 1989;40(8):841-843.
7. Frierson RL, Lippmann SB. Psychiatric consultation for patients with self-inflicted gunshot wounds. Psychosomatics. 1990;31(1):67-74.
8. Mitchell AM, Terhorst L. PTSD symptoms in survivors bereaved by the suicide of a significant other. J Am Psychiatr Nurses Assoc. 2017;23(1):61-65.
9. Bateson J. The Golden Gate Bridge’s fatal flaw. Los Angeles Times. May 25, 2012. Accessed March 2, 2022. https://www.latimes.com/opinion/la-xpm-2012-may-25-la-oe-adv-bateson-golden-gate-20120525-story.html
10. Dervic K, Oquendoma MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004;161(12):2303-2308.
11. Nordentoft H, Madsen T, Fedyszyn IF. Suicidal behavior and mortality in first episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392.
12. Frierson R, Lippmann S. Suicide attempts by the old and the very old. Arch Intern Med. 1991;151(1):141-144.
13. Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opiate poisonings in the United States: 1999-2014. Am J Public Health. 2017;107(3):421-426.
14. Morin KA, Acharya S, Eibl JK, et al: Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treated patients in Ontario, Canada. Int J Drug Policy. 2021;90:103088.
15. Shobassy A, Abu-Mohammad AS. Assessing imminent suicide risk: what about future planning? Current Psychiatry. 2022;21(2):12-17.
16. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
17. Macleod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2010;44(Pt 4):495-504.











