User login
2022 billing and coding updates
Telehealth and Teaching Physician Services and ICD-10 codes updates
In my previous article in June, 2022, we plowed through the billing and coding updates regarding critical care services, and, I hope that it helped our readers get more acquainted with the nuances of billing and coding in the ICU. In this piece, I would like to briefly elucidate three other areas of practice, which will be relevant to all physicians across various specialties.
Telehealth services
(PHE). Initially, the plan was to remove these from the list of covered services by the latter end of the COVID-19 PHE, which, created some uncertainty, or by December 31, 2021. Fortunately, CMS finalized that they will extend it through the end of the calendar year (CY) 2023. So, now all the telehealth services will remain on the CMS list until December 31, 2023. The general principle behind this ruling is to allow for more time for CMS and stakeholders to gather data and to submit support for requesting these services to be permanently added to the Medicare telehealth services list.
Not only has CMS extended the deadline for telehealth services but also they have gone far and beyond to extend some of the codes for cardiac and intensive cardiac rehabilitation until December 31, 2023, as well.
There has been a lot of debate regarding the geographic restrictions when it comes to telehealth visits for diagnosis, evaluation, or treatment of a mental health disorder. As per the latest Consolidated Appropriations Act of 2021 (Section 123), the home of the patient is a permissible site. But, the caveat is that there must be an in-person service with the practitioner/physician within 6 months prior to the initial telehealth visit. Additionally, there has to be a set frequency for subsequent in-person visits. And, usually the subsequent visits will need to be provided at least every 12 months. These requirements are not set in stone and can be changed on a case-by-case basis provided there is appropriate documentation in the chart.
Lastly, it is important to understand and use the appropriate telecommunication systems for the telehealth visits and the modifiers that are associated with them. By definition, it has to be audio and video equipment that allows two-way, real-time interactive communication between the patient and the provider when used for telehealth services for the diagnosis, evaluation, or treatment of mental health disorders. But, CMS is in the process of amending it to include audio-only communications technology. At this time, the use of audio-only interactive telecommunications system is limited to practitioners who have the capability to provide two-way audio/video communications but, where the patient is not capable, or does not consent to, the use of two-way audio/video technology. Modifier FQ should be attached to all the mental health services that were furnished using audio-only communications. And, mental health services can include services for treatment of substance use disorders (SUD). Please do not confuse modifier FQ with modifier 93 as FQ is only for behavioral health services. And, remember that the totality of the communication of information exchanged between the provider and the patient during the course of the synchronous telemedicine service (rendered via telephone or other real-time interactive audio only telecommunication system) must be of an amount and nature that is sufficient to meet the key components and/or requirements of the same service when rendered via a face-to-face interaction.
Teaching physician services
As a general rule, a teaching physician can bill for the resident services only if they are present for the critical (key) portion of the service. But, there is one exception called the “primary care exception” under which in certain teaching hospital primary care centers, the teaching physician can bill for certain services as furnished independently by the resident without the teaching physician being physically present, but with the teaching physician’s review.
The current model to bill for office/outpatient E/M visit level is either based on either total time spent (personally) or medical-decision-making (MDM). When time is used to select the visit level only the time spent by the teaching physician in qualifying activities can be included for the purposes of the visit level selection. And, this includes the time the teaching physician was present with the resident performing those qualifying activities. Also, under the primary care exception, time cannot be used to select the visit level. This is to guard against the possibility of inappropriate coding that reflects residents’ inefficiencies rather than a measure of the total medically necessary time required to furnish the E/M services.
ICD-10 updates
Usually, the ICD-10 codes are updated annually and take effect every October 1. Some of the most relevant updates are as follows:
1. U09.9 Post COVID-19 condition, unspecified: This should be used to document sequelae of COVID-19 or “long COVID” conditions, after the acute illness has resolved. But, remember to code the conditions related to COVID-19 first and do not use this code with an active or current COVID-19 infection.
2. U07.0 Vaping-related disorder: This should be used for all vaping-related illnesses. However, additional codes for other diagnoses such as acute respiratory failure, acute respiratory distress syndrome, or pneumonitis can also be used with this code. Other respiratory signs and symptoms such as cough and shortness of breath should not be coded separately.
3. Cough is one of the most common reasons for referral to a pulmonologist. The CDC has expanded these codes so please remember to code the most specific diagnosis as deemed appropriate.
R05.1 Acute cough
R05.2 Subacute cough
R05.3 Chronic cough
R05.4 Cough, syncope
R05.8 Other specified cough
R05.9 Cough, unspecified
We will be back with some more exciting and intriguing billing and coding updates in our next article and hope to see everyone at CHEST 2022 in Nashville., TN.
Telehealth and Teaching Physician Services and ICD-10 codes updates
Telehealth and Teaching Physician Services and ICD-10 codes updates
In my previous article in June, 2022, we plowed through the billing and coding updates regarding critical care services, and, I hope that it helped our readers get more acquainted with the nuances of billing and coding in the ICU. In this piece, I would like to briefly elucidate three other areas of practice, which will be relevant to all physicians across various specialties.
Telehealth services
(PHE). Initially, the plan was to remove these from the list of covered services by the latter end of the COVID-19 PHE, which, created some uncertainty, or by December 31, 2021. Fortunately, CMS finalized that they will extend it through the end of the calendar year (CY) 2023. So, now all the telehealth services will remain on the CMS list until December 31, 2023. The general principle behind this ruling is to allow for more time for CMS and stakeholders to gather data and to submit support for requesting these services to be permanently added to the Medicare telehealth services list.
Not only has CMS extended the deadline for telehealth services but also they have gone far and beyond to extend some of the codes for cardiac and intensive cardiac rehabilitation until December 31, 2023, as well.
There has been a lot of debate regarding the geographic restrictions when it comes to telehealth visits for diagnosis, evaluation, or treatment of a mental health disorder. As per the latest Consolidated Appropriations Act of 2021 (Section 123), the home of the patient is a permissible site. But, the caveat is that there must be an in-person service with the practitioner/physician within 6 months prior to the initial telehealth visit. Additionally, there has to be a set frequency for subsequent in-person visits. And, usually the subsequent visits will need to be provided at least every 12 months. These requirements are not set in stone and can be changed on a case-by-case basis provided there is appropriate documentation in the chart.
Lastly, it is important to understand and use the appropriate telecommunication systems for the telehealth visits and the modifiers that are associated with them. By definition, it has to be audio and video equipment that allows two-way, real-time interactive communication between the patient and the provider when used for telehealth services for the diagnosis, evaluation, or treatment of mental health disorders. But, CMS is in the process of amending it to include audio-only communications technology. At this time, the use of audio-only interactive telecommunications system is limited to practitioners who have the capability to provide two-way audio/video communications but, where the patient is not capable, or does not consent to, the use of two-way audio/video technology. Modifier FQ should be attached to all the mental health services that were furnished using audio-only communications. And, mental health services can include services for treatment of substance use disorders (SUD). Please do not confuse modifier FQ with modifier 93 as FQ is only for behavioral health services. And, remember that the totality of the communication of information exchanged between the provider and the patient during the course of the synchronous telemedicine service (rendered via telephone or other real-time interactive audio only telecommunication system) must be of an amount and nature that is sufficient to meet the key components and/or requirements of the same service when rendered via a face-to-face interaction.
Teaching physician services
As a general rule, a teaching physician can bill for the resident services only if they are present for the critical (key) portion of the service. But, there is one exception called the “primary care exception” under which in certain teaching hospital primary care centers, the teaching physician can bill for certain services as furnished independently by the resident without the teaching physician being physically present, but with the teaching physician’s review.
The current model to bill for office/outpatient E/M visit level is either based on either total time spent (personally) or medical-decision-making (MDM). When time is used to select the visit level only the time spent by the teaching physician in qualifying activities can be included for the purposes of the visit level selection. And, this includes the time the teaching physician was present with the resident performing those qualifying activities. Also, under the primary care exception, time cannot be used to select the visit level. This is to guard against the possibility of inappropriate coding that reflects residents’ inefficiencies rather than a measure of the total medically necessary time required to furnish the E/M services.
ICD-10 updates
Usually, the ICD-10 codes are updated annually and take effect every October 1. Some of the most relevant updates are as follows:
1. U09.9 Post COVID-19 condition, unspecified: This should be used to document sequelae of COVID-19 or “long COVID” conditions, after the acute illness has resolved. But, remember to code the conditions related to COVID-19 first and do not use this code with an active or current COVID-19 infection.
2. U07.0 Vaping-related disorder: This should be used for all vaping-related illnesses. However, additional codes for other diagnoses such as acute respiratory failure, acute respiratory distress syndrome, or pneumonitis can also be used with this code. Other respiratory signs and symptoms such as cough and shortness of breath should not be coded separately.
3. Cough is one of the most common reasons for referral to a pulmonologist. The CDC has expanded these codes so please remember to code the most specific diagnosis as deemed appropriate.
R05.1 Acute cough
R05.2 Subacute cough
R05.3 Chronic cough
R05.4 Cough, syncope
R05.8 Other specified cough
R05.9 Cough, unspecified
We will be back with some more exciting and intriguing billing and coding updates in our next article and hope to see everyone at CHEST 2022 in Nashville., TN.
In my previous article in June, 2022, we plowed through the billing and coding updates regarding critical care services, and, I hope that it helped our readers get more acquainted with the nuances of billing and coding in the ICU. In this piece, I would like to briefly elucidate three other areas of practice, which will be relevant to all physicians across various specialties.
Telehealth services
(PHE). Initially, the plan was to remove these from the list of covered services by the latter end of the COVID-19 PHE, which, created some uncertainty, or by December 31, 2021. Fortunately, CMS finalized that they will extend it through the end of the calendar year (CY) 2023. So, now all the telehealth services will remain on the CMS list until December 31, 2023. The general principle behind this ruling is to allow for more time for CMS and stakeholders to gather data and to submit support for requesting these services to be permanently added to the Medicare telehealth services list.
Not only has CMS extended the deadline for telehealth services but also they have gone far and beyond to extend some of the codes for cardiac and intensive cardiac rehabilitation until December 31, 2023, as well.
There has been a lot of debate regarding the geographic restrictions when it comes to telehealth visits for diagnosis, evaluation, or treatment of a mental health disorder. As per the latest Consolidated Appropriations Act of 2021 (Section 123), the home of the patient is a permissible site. But, the caveat is that there must be an in-person service with the practitioner/physician within 6 months prior to the initial telehealth visit. Additionally, there has to be a set frequency for subsequent in-person visits. And, usually the subsequent visits will need to be provided at least every 12 months. These requirements are not set in stone and can be changed on a case-by-case basis provided there is appropriate documentation in the chart.
Lastly, it is important to understand and use the appropriate telecommunication systems for the telehealth visits and the modifiers that are associated with them. By definition, it has to be audio and video equipment that allows two-way, real-time interactive communication between the patient and the provider when used for telehealth services for the diagnosis, evaluation, or treatment of mental health disorders. But, CMS is in the process of amending it to include audio-only communications technology. At this time, the use of audio-only interactive telecommunications system is limited to practitioners who have the capability to provide two-way audio/video communications but, where the patient is not capable, or does not consent to, the use of two-way audio/video technology. Modifier FQ should be attached to all the mental health services that were furnished using audio-only communications. And, mental health services can include services for treatment of substance use disorders (SUD). Please do not confuse modifier FQ with modifier 93 as FQ is only for behavioral health services. And, remember that the totality of the communication of information exchanged between the provider and the patient during the course of the synchronous telemedicine service (rendered via telephone or other real-time interactive audio only telecommunication system) must be of an amount and nature that is sufficient to meet the key components and/or requirements of the same service when rendered via a face-to-face interaction.
Teaching physician services
As a general rule, a teaching physician can bill for the resident services only if they are present for the critical (key) portion of the service. But, there is one exception called the “primary care exception” under which in certain teaching hospital primary care centers, the teaching physician can bill for certain services as furnished independently by the resident without the teaching physician being physically present, but with the teaching physician’s review.
The current model to bill for office/outpatient E/M visit level is either based on either total time spent (personally) or medical-decision-making (MDM). When time is used to select the visit level only the time spent by the teaching physician in qualifying activities can be included for the purposes of the visit level selection. And, this includes the time the teaching physician was present with the resident performing those qualifying activities. Also, under the primary care exception, time cannot be used to select the visit level. This is to guard against the possibility of inappropriate coding that reflects residents’ inefficiencies rather than a measure of the total medically necessary time required to furnish the E/M services.
ICD-10 updates
Usually, the ICD-10 codes are updated annually and take effect every October 1. Some of the most relevant updates are as follows:
1. U09.9 Post COVID-19 condition, unspecified: This should be used to document sequelae of COVID-19 or “long COVID” conditions, after the acute illness has resolved. But, remember to code the conditions related to COVID-19 first and do not use this code with an active or current COVID-19 infection.
2. U07.0 Vaping-related disorder: This should be used for all vaping-related illnesses. However, additional codes for other diagnoses such as acute respiratory failure, acute respiratory distress syndrome, or pneumonitis can also be used with this code. Other respiratory signs and symptoms such as cough and shortness of breath should not be coded separately.
3. Cough is one of the most common reasons for referral to a pulmonologist. The CDC has expanded these codes so please remember to code the most specific diagnosis as deemed appropriate.
R05.1 Acute cough
R05.2 Subacute cough
R05.3 Chronic cough
R05.4 Cough, syncope
R05.8 Other specified cough
R05.9 Cough, unspecified
We will be back with some more exciting and intriguing billing and coding updates in our next article and hope to see everyone at CHEST 2022 in Nashville., TN.
Access unmatched asthma education from anywhere
CHEST is proud to announce the launch of the newest addition to our e-learning options: the CHEST Asthma Curriculum Pathway.
This unique offering combines a variety of bite-sized educational resources from among CHEST’s most popular and effective products, including case-based CHEST SEEK™ questions, podcasts and videos from asthma experts, the latest research from the journal CHEST®, and more.
The pathway comprises several different “paths,” or tracks, that enable clinicians to target their education based on their knowledge gaps and career level. Users can opt to follow the curriculum from start to finish to gain a comprehensive overview of asthma management. Or, they can select individual paths to focus their learning on topics including asthma pathophysiology, diagnosis and classification, exacerbations, phenotypes, and more.
According to early learners of the pathway: It helped a lot with the knowledge check-in.” Another commented: “It is very comprehensive on all aspects of asthma. I enjoyed the higher-level learning on the choice of biologics and asthma mimickers.” The education modalities were highlighted, as well, with this feedback: “I really enjoyed the variety of media (lectures, discussions, papers, games).”
Exploring the education
The Asthma Curriculum Pathway offers targeted education options to fit the career level and clinical interest of clinicians, ranging from trainees and early career physicians to experienced asthma specialists and advanced practice providers.
Paths include:
• Path 1: Pathophysiology
• Path 2: Diagnosis & Classification
• Path 3: Management
• Path 4: Mimickers
• Path 5: Comorbidities
• Path 6: Phenotypes
• Path 7: Exacerbations
• Path 8: Special Situations
Plus, each path offers claiming credit, including CME, for completion—all while driving clinicians to consistently advance best outcomes for their patients with asthma.
Visit (https://bit.ly/asthma-pathway) to access the best of CHEST’s asthma education with the new Asthma Curriculum Pathway, accessible via web or mobile device.
CHEST is proud to announce the launch of the newest addition to our e-learning options: the CHEST Asthma Curriculum Pathway.
This unique offering combines a variety of bite-sized educational resources from among CHEST’s most popular and effective products, including case-based CHEST SEEK™ questions, podcasts and videos from asthma experts, the latest research from the journal CHEST®, and more.
The pathway comprises several different “paths,” or tracks, that enable clinicians to target their education based on their knowledge gaps and career level. Users can opt to follow the curriculum from start to finish to gain a comprehensive overview of asthma management. Or, they can select individual paths to focus their learning on topics including asthma pathophysiology, diagnosis and classification, exacerbations, phenotypes, and more.
According to early learners of the pathway: It helped a lot with the knowledge check-in.” Another commented: “It is very comprehensive on all aspects of asthma. I enjoyed the higher-level learning on the choice of biologics and asthma mimickers.” The education modalities were highlighted, as well, with this feedback: “I really enjoyed the variety of media (lectures, discussions, papers, games).”
Exploring the education
The Asthma Curriculum Pathway offers targeted education options to fit the career level and clinical interest of clinicians, ranging from trainees and early career physicians to experienced asthma specialists and advanced practice providers.
Paths include:
• Path 1: Pathophysiology
• Path 2: Diagnosis & Classification
• Path 3: Management
• Path 4: Mimickers
• Path 5: Comorbidities
• Path 6: Phenotypes
• Path 7: Exacerbations
• Path 8: Special Situations
Plus, each path offers claiming credit, including CME, for completion—all while driving clinicians to consistently advance best outcomes for their patients with asthma.
Visit (https://bit.ly/asthma-pathway) to access the best of CHEST’s asthma education with the new Asthma Curriculum Pathway, accessible via web or mobile device.
CHEST is proud to announce the launch of the newest addition to our e-learning options: the CHEST Asthma Curriculum Pathway.
This unique offering combines a variety of bite-sized educational resources from among CHEST’s most popular and effective products, including case-based CHEST SEEK™ questions, podcasts and videos from asthma experts, the latest research from the journal CHEST®, and more.
The pathway comprises several different “paths,” or tracks, that enable clinicians to target their education based on their knowledge gaps and career level. Users can opt to follow the curriculum from start to finish to gain a comprehensive overview of asthma management. Or, they can select individual paths to focus their learning on topics including asthma pathophysiology, diagnosis and classification, exacerbations, phenotypes, and more.
According to early learners of the pathway: It helped a lot with the knowledge check-in.” Another commented: “It is very comprehensive on all aspects of asthma. I enjoyed the higher-level learning on the choice of biologics and asthma mimickers.” The education modalities were highlighted, as well, with this feedback: “I really enjoyed the variety of media (lectures, discussions, papers, games).”
Exploring the education
The Asthma Curriculum Pathway offers targeted education options to fit the career level and clinical interest of clinicians, ranging from trainees and early career physicians to experienced asthma specialists and advanced practice providers.
Paths include:
• Path 1: Pathophysiology
• Path 2: Diagnosis & Classification
• Path 3: Management
• Path 4: Mimickers
• Path 5: Comorbidities
• Path 6: Phenotypes
• Path 7: Exacerbations
• Path 8: Special Situations
Plus, each path offers claiming credit, including CME, for completion—all while driving clinicians to consistently advance best outcomes for their patients with asthma.
Visit (https://bit.ly/asthma-pathway) to access the best of CHEST’s asthma education with the new Asthma Curriculum Pathway, accessible via web or mobile device.
Advanced POCUS for us all?
Point-of-care ultrasound (POCUS) is a useful, practice-changing bedside tool that spans all medical and surgical specialties. While the definition of POCUS varies, most would agree it is an abbreviated exam that helps to answer a specific clinical question. With the expansion of POCUS training, the clinical questions being asked and answered have increased in scope and volume. The types of exams being utilized in “point of care ultrasound” have also increased and include transthoracic echocardiography; trans-esophageal echocardiography; and lung, gastric, abdominal, and ocular ultrasound. POCUS is used across multiple specialties, including critical care, anesthesiology, emergency medicine, and primary care.
Not only has POCUS become increasingly important clinically, but specialties now test these skills on their respective board examinations. Anesthesia is one of many such examples. The content outline for the American Board of Anesthesiology includes POCUS as a tested item on both the written and applied components of the exam. POCUS training must be directed toward both optimizing patient management and preparing learners for their board examination. A method for teaching this has yet to be defined (Naji A, et al. Cureus. 2021;13[5]:e15217).
One question – how should different specialties approach this educational challenge and should specialties train together? The answer is complicated. Many POCUS courses and certifications exist, and all vary in their content, didactics, and length. No true gold standard exists for POCUS certification for radiology or noncardiology providers. Additionally, there are no defined expectations or testing processes that certify a provider is “certified” to perform POCUS. While waiting for medical society guidelines to address these issues, many in graduate medical education (GME) are coming up with their own ways to incorporate POCUS into their respective training programs (Atkinson P, et al. CJEM. 2015 Mar;17[2]:161).
Who’s training whom?
Over the past decade, several expert committees, including those in critical care, have developed recommendations and consensus statements urging training facilities to independently create POCUS curriculums. The threshold for many programs to enter this realm of expertise is high and oftentimes unobtainable. We’ve seen emergency medicine and anesthesia raise the bar for ultrasound education in their residencies, but it’s unclear whether all fellowship-trained physicians can and should be tasked with obtaining official POCUS certification.
While specific specialties may require tailored certifications, there’s a considerable overlap in POCUS exam content across specialties. One approach to POCUS training could be developing and implementing a multidisciplinary curriculum. This would allow for pooling of resources (equipment, staff) and harnessing knowledge from providers familiar with different phases of patient care (ICU, perioperative, ED, outpatient clinics). By approaching POCUS from a multidisciplinary perspective, the quality of education may be enhanced (Mayo PH, et al. Intensive Care Med. 2014;40[5]:654). Is it then prudent for providers and trainees alike to share in didactics across all areas of the hospital and clinic? Would this close the knowledge gap between specialties who are facile with ultrasound and those not?
Determining the role of transesophageal echocardiography in a POCUS curriculum
This modality of imaging has been, until recently, reserved for cardiologists and anesthesiologists. More recently transesophageal echocardiography (TEE) has been utilized by emergency and critical care medicine physicians. TEE is part of recommended training for these specialties as a tool for diagnostic and rescue measures, including ventilator management, emergency procedures, and medication titration. Rescue TEE can also be utilized perioperatively where the transthoracic exam is limited by poor windows or the operative procedure precludes access to the chest. While transthoracic echocardiography (TTE) is often used in a point of care fashion, TEE is utilized less often. This may stem from the invasive nature of the procedure but likely also results from lack of equipment and training. Like POCUS overall, TEE POCUS will require incorporation into training programs to achieve widespread use and acceptance.
A deluge of research on TEE for the noncardiologist shows this modality is minimally invasive, safe, and effective. As it becomes more readily available and technology improves, there is no reason why an esophageal probe can’t be used in a patient with a secured airway (Wray TC, et al. J Intensive Care Med. 2021;36[1]:123).
Ultrasound for hemodynamic monitoring
There are many methods employed for hemodynamic monitoring in the ICU. Although echocardiographic and vascular parameters have been validated in the cardiac and perioperative fields, their application in the ICU setting for resuscitation and volume management remain somewhat controversial. The use of TEE and more advanced understanding of spectral doppler and pulmonary ultrasonography using TEE has revolutionized the way providers are managing critically ill patients. (Garcia YA, et al. Chest. 2017;152[4]:736).
In our opinion, physiology and imaging training for residents and fellows should be required for critical care medicine trainees. Delving into the nuances of frank-starling curves, stroke work, and diastolic function will enrich their understanding and highlight the applicability of ultrasonography. Furthermore, all clinicians caring for patients with critical illness should be privy to the nuances of physiologic derangement, and to that end, advanced echocardiographic principles and image acquisition. The heart-lung interactions are demonstrated in real time using POCUS and can clearly delineate treatment goals (Vieillard-Baron A, et al. Intensive Care Med. 2019;45[6]:770).
Documentation and billing
If clinicians are making medical decisions based off imaging gathered at the bedside and interpreted in real-time, documentation should reflect that. That documentation will invariably lead to billing and possibly audit or quality review by colleagues or other healthcare staff. Radiology and cardiology have perfected the billing process for image interpretation, but their form of documentation and interpretation may not easily be implemented in the perioperative or critical care settings. An abbreviated document with focused information should take the place of the formal study. With that, the credentialing and board certification process will allow providers to feel empowered to make clinical decisions based off these focused examinations.
Dr. Goertzen is Chief Fellow, Pulmonary/Critical Care; Dr. Knuf is Program Director, Department of Anesthesia; and Dr. Villalobos is Director of Medical ICU, Department of Internal Medicine, San Antonio Military Medical Center, San Antonio, Texas.
Point-of-care ultrasound (POCUS) is a useful, practice-changing bedside tool that spans all medical and surgical specialties. While the definition of POCUS varies, most would agree it is an abbreviated exam that helps to answer a specific clinical question. With the expansion of POCUS training, the clinical questions being asked and answered have increased in scope and volume. The types of exams being utilized in “point of care ultrasound” have also increased and include transthoracic echocardiography; trans-esophageal echocardiography; and lung, gastric, abdominal, and ocular ultrasound. POCUS is used across multiple specialties, including critical care, anesthesiology, emergency medicine, and primary care.
Not only has POCUS become increasingly important clinically, but specialties now test these skills on their respective board examinations. Anesthesia is one of many such examples. The content outline for the American Board of Anesthesiology includes POCUS as a tested item on both the written and applied components of the exam. POCUS training must be directed toward both optimizing patient management and preparing learners for their board examination. A method for teaching this has yet to be defined (Naji A, et al. Cureus. 2021;13[5]:e15217).
One question – how should different specialties approach this educational challenge and should specialties train together? The answer is complicated. Many POCUS courses and certifications exist, and all vary in their content, didactics, and length. No true gold standard exists for POCUS certification for radiology or noncardiology providers. Additionally, there are no defined expectations or testing processes that certify a provider is “certified” to perform POCUS. While waiting for medical society guidelines to address these issues, many in graduate medical education (GME) are coming up with their own ways to incorporate POCUS into their respective training programs (Atkinson P, et al. CJEM. 2015 Mar;17[2]:161).
Who’s training whom?
Over the past decade, several expert committees, including those in critical care, have developed recommendations and consensus statements urging training facilities to independently create POCUS curriculums. The threshold for many programs to enter this realm of expertise is high and oftentimes unobtainable. We’ve seen emergency medicine and anesthesia raise the bar for ultrasound education in their residencies, but it’s unclear whether all fellowship-trained physicians can and should be tasked with obtaining official POCUS certification.
While specific specialties may require tailored certifications, there’s a considerable overlap in POCUS exam content across specialties. One approach to POCUS training could be developing and implementing a multidisciplinary curriculum. This would allow for pooling of resources (equipment, staff) and harnessing knowledge from providers familiar with different phases of patient care (ICU, perioperative, ED, outpatient clinics). By approaching POCUS from a multidisciplinary perspective, the quality of education may be enhanced (Mayo PH, et al. Intensive Care Med. 2014;40[5]:654). Is it then prudent for providers and trainees alike to share in didactics across all areas of the hospital and clinic? Would this close the knowledge gap between specialties who are facile with ultrasound and those not?
Determining the role of transesophageal echocardiography in a POCUS curriculum
This modality of imaging has been, until recently, reserved for cardiologists and anesthesiologists. More recently transesophageal echocardiography (TEE) has been utilized by emergency and critical care medicine physicians. TEE is part of recommended training for these specialties as a tool for diagnostic and rescue measures, including ventilator management, emergency procedures, and medication titration. Rescue TEE can also be utilized perioperatively where the transthoracic exam is limited by poor windows or the operative procedure precludes access to the chest. While transthoracic echocardiography (TTE) is often used in a point of care fashion, TEE is utilized less often. This may stem from the invasive nature of the procedure but likely also results from lack of equipment and training. Like POCUS overall, TEE POCUS will require incorporation into training programs to achieve widespread use and acceptance.
A deluge of research on TEE for the noncardiologist shows this modality is minimally invasive, safe, and effective. As it becomes more readily available and technology improves, there is no reason why an esophageal probe can’t be used in a patient with a secured airway (Wray TC, et al. J Intensive Care Med. 2021;36[1]:123).
Ultrasound for hemodynamic monitoring
There are many methods employed for hemodynamic monitoring in the ICU. Although echocardiographic and vascular parameters have been validated in the cardiac and perioperative fields, their application in the ICU setting for resuscitation and volume management remain somewhat controversial. The use of TEE and more advanced understanding of spectral doppler and pulmonary ultrasonography using TEE has revolutionized the way providers are managing critically ill patients. (Garcia YA, et al. Chest. 2017;152[4]:736).
In our opinion, physiology and imaging training for residents and fellows should be required for critical care medicine trainees. Delving into the nuances of frank-starling curves, stroke work, and diastolic function will enrich their understanding and highlight the applicability of ultrasonography. Furthermore, all clinicians caring for patients with critical illness should be privy to the nuances of physiologic derangement, and to that end, advanced echocardiographic principles and image acquisition. The heart-lung interactions are demonstrated in real time using POCUS and can clearly delineate treatment goals (Vieillard-Baron A, et al. Intensive Care Med. 2019;45[6]:770).
Documentation and billing
If clinicians are making medical decisions based off imaging gathered at the bedside and interpreted in real-time, documentation should reflect that. That documentation will invariably lead to billing and possibly audit or quality review by colleagues or other healthcare staff. Radiology and cardiology have perfected the billing process for image interpretation, but their form of documentation and interpretation may not easily be implemented in the perioperative or critical care settings. An abbreviated document with focused information should take the place of the formal study. With that, the credentialing and board certification process will allow providers to feel empowered to make clinical decisions based off these focused examinations.
Dr. Goertzen is Chief Fellow, Pulmonary/Critical Care; Dr. Knuf is Program Director, Department of Anesthesia; and Dr. Villalobos is Director of Medical ICU, Department of Internal Medicine, San Antonio Military Medical Center, San Antonio, Texas.
Point-of-care ultrasound (POCUS) is a useful, practice-changing bedside tool that spans all medical and surgical specialties. While the definition of POCUS varies, most would agree it is an abbreviated exam that helps to answer a specific clinical question. With the expansion of POCUS training, the clinical questions being asked and answered have increased in scope and volume. The types of exams being utilized in “point of care ultrasound” have also increased and include transthoracic echocardiography; trans-esophageal echocardiography; and lung, gastric, abdominal, and ocular ultrasound. POCUS is used across multiple specialties, including critical care, anesthesiology, emergency medicine, and primary care.
Not only has POCUS become increasingly important clinically, but specialties now test these skills on their respective board examinations. Anesthesia is one of many such examples. The content outline for the American Board of Anesthesiology includes POCUS as a tested item on both the written and applied components of the exam. POCUS training must be directed toward both optimizing patient management and preparing learners for their board examination. A method for teaching this has yet to be defined (Naji A, et al. Cureus. 2021;13[5]:e15217).
One question – how should different specialties approach this educational challenge and should specialties train together? The answer is complicated. Many POCUS courses and certifications exist, and all vary in their content, didactics, and length. No true gold standard exists for POCUS certification for radiology or noncardiology providers. Additionally, there are no defined expectations or testing processes that certify a provider is “certified” to perform POCUS. While waiting for medical society guidelines to address these issues, many in graduate medical education (GME) are coming up with their own ways to incorporate POCUS into their respective training programs (Atkinson P, et al. CJEM. 2015 Mar;17[2]:161).
Who’s training whom?
Over the past decade, several expert committees, including those in critical care, have developed recommendations and consensus statements urging training facilities to independently create POCUS curriculums. The threshold for many programs to enter this realm of expertise is high and oftentimes unobtainable. We’ve seen emergency medicine and anesthesia raise the bar for ultrasound education in their residencies, but it’s unclear whether all fellowship-trained physicians can and should be tasked with obtaining official POCUS certification.
While specific specialties may require tailored certifications, there’s a considerable overlap in POCUS exam content across specialties. One approach to POCUS training could be developing and implementing a multidisciplinary curriculum. This would allow for pooling of resources (equipment, staff) and harnessing knowledge from providers familiar with different phases of patient care (ICU, perioperative, ED, outpatient clinics). By approaching POCUS from a multidisciplinary perspective, the quality of education may be enhanced (Mayo PH, et al. Intensive Care Med. 2014;40[5]:654). Is it then prudent for providers and trainees alike to share in didactics across all areas of the hospital and clinic? Would this close the knowledge gap between specialties who are facile with ultrasound and those not?
Determining the role of transesophageal echocardiography in a POCUS curriculum
This modality of imaging has been, until recently, reserved for cardiologists and anesthesiologists. More recently transesophageal echocardiography (TEE) has been utilized by emergency and critical care medicine physicians. TEE is part of recommended training for these specialties as a tool for diagnostic and rescue measures, including ventilator management, emergency procedures, and medication titration. Rescue TEE can also be utilized perioperatively where the transthoracic exam is limited by poor windows or the operative procedure precludes access to the chest. While transthoracic echocardiography (TTE) is often used in a point of care fashion, TEE is utilized less often. This may stem from the invasive nature of the procedure but likely also results from lack of equipment and training. Like POCUS overall, TEE POCUS will require incorporation into training programs to achieve widespread use and acceptance.
A deluge of research on TEE for the noncardiologist shows this modality is minimally invasive, safe, and effective. As it becomes more readily available and technology improves, there is no reason why an esophageal probe can’t be used in a patient with a secured airway (Wray TC, et al. J Intensive Care Med. 2021;36[1]:123).
Ultrasound for hemodynamic monitoring
There are many methods employed for hemodynamic monitoring in the ICU. Although echocardiographic and vascular parameters have been validated in the cardiac and perioperative fields, their application in the ICU setting for resuscitation and volume management remain somewhat controversial. The use of TEE and more advanced understanding of spectral doppler and pulmonary ultrasonography using TEE has revolutionized the way providers are managing critically ill patients. (Garcia YA, et al. Chest. 2017;152[4]:736).
In our opinion, physiology and imaging training for residents and fellows should be required for critical care medicine trainees. Delving into the nuances of frank-starling curves, stroke work, and diastolic function will enrich their understanding and highlight the applicability of ultrasonography. Furthermore, all clinicians caring for patients with critical illness should be privy to the nuances of physiologic derangement, and to that end, advanced echocardiographic principles and image acquisition. The heart-lung interactions are demonstrated in real time using POCUS and can clearly delineate treatment goals (Vieillard-Baron A, et al. Intensive Care Med. 2019;45[6]:770).
Documentation and billing
If clinicians are making medical decisions based off imaging gathered at the bedside and interpreted in real-time, documentation should reflect that. That documentation will invariably lead to billing and possibly audit or quality review by colleagues or other healthcare staff. Radiology and cardiology have perfected the billing process for image interpretation, but their form of documentation and interpretation may not easily be implemented in the perioperative or critical care settings. An abbreviated document with focused information should take the place of the formal study. With that, the credentialing and board certification process will allow providers to feel empowered to make clinical decisions based off these focused examinations.
Dr. Goertzen is Chief Fellow, Pulmonary/Critical Care; Dr. Knuf is Program Director, Department of Anesthesia; and Dr. Villalobos is Director of Medical ICU, Department of Internal Medicine, San Antonio Military Medical Center, San Antonio, Texas.
The possibilities are endless: A chat with the incoming CHEST Foundation President, Robert De Marco, MD, FCCP
As the presidency of the American College of Chest Physicians changes hands in January 2023, so will the role of President of the CHEST Foundation. To get to know the incoming President of the CHEST Foundation, we spoke with Robert (Bob) De Marco, MD, FCCP, about his philanthropy work and his goals for the philanthropic arm of CHEST.
Tell me about your history with philanthropy work.
My philanthropy work started long before the CHEST Foundation. While I’ve been a member of CHEST since my second year of fellowship, it wasn’t until much later that I became involved with the philanthropic side of the organization. Earlier in my career, I was involved more so with the American Cancer Society. I had gotten involved with them by chance – participating in an event of theirs – and was encouraged to get more involved by one of their board members. Being involved with them made a lot of sense seeing as a strong percentage of my patients at the time were being treated for lung cancer. My most notable accomplishments with the American Cancer Society were in serving as the Chairmen of my local Relay for Life program for 10 years, as a board member, and then as a president of my local chapter.
When did you get involved with the CHEST Foundation?
I had served in a handful of positions within CHEST, including Chair of the (since reinvented) Practice Management Committee, so I was deeply involved in the association, and I thought to myself, “I have experience in fundraising through my work with the American Cancer Society, why don’t I use it to help our association?” When I moved to Florida, I no longer had the local connection to the American Cancer Society, so it was an opportune time to transition over to the CHEST Foundation.
How has the Foundation changed in the time that you’ve been involved?
The Foundation has changed drastically since I first joined the Board of Trustees 9 years ago. When I first got involved, the primary goal of the Foundation was staying “out of the red.” At that time, we were an organization that gave away more than we made.
After years of building a corpus to fund our own projects, we’re in a really good place now with some phenomenal goals and some excellent initiatives to fundraise around, including a CHEST diversity initiative, First 5 Minutes™, and Bridging Specialties™: Timely Diagnosis for ILD Patients, which seeks to break down silos within medicine to improve patient care.
What will be a focus of your Foundation presidency?
You know, one thing I always appreciated about the American Cancer Society was that there were always notable accomplishments to point back to when supporting fundraising efforts. You could say, “Did you know that bone marrow transplantation was initially funded by the American Cancer Society?” and other examples that would truly inspire someone to want to get involved in supporting those efforts.
The CHEST Foundation may not have funded bone marrow transplantation, but in 25 years of awarding grants, there are equally good stories to share. The impact of the Foundation is tremendous, and we’ve only just begun to share examples of where grant recipients went with their research or community service projects.
A recent grant story that was shared with me was that of Panagis Galiatsatos, MD, MHS, who received a community service grant to start a program educating children in the Baltimore community about lung health. This program was so moving that it inspired one of the Baltimore teachers to pursue a career in medicine and that individual is now a practicing MD.
This is just one example of the Foundation’s impact and it’s through these stories that we share the “why” behind every dollar that is raised, and my first goal is to tell these stories.
Another key focus of not only my presidency, but Dr. Ian Nathanson’s, as well, as we collaborated a lot on our roles, will be on member involvement and awareness. Even I wasn’t involved in the CHEST Foundation until years into my CHEST membership, so I understand that there are competing demands. But I also know that there is a lot to be gained from the work with the Foundation. I want the CHEST members to be excited about the Foundation and to want to support its efforts.
These two goals go hand in hand, and I look forward to sharing the Foundation’s impact with a new audience and reinvigorating the support of our existing donors.
Is there anything else you’d like to say to the reader?
We cannot accomplish anything without the support of our donors, and I want to sincerely thank everyone who has donated to the CHEST Foundation. I also encourage those who have never donated or have yet to donate this year to visit the Foundation’s website (foundation.chestnet.org) and explore some of the inspiring initiatives you can support to strengthen the impact of the CHEST Foundation because the possibilities are truly endless.
As the presidency of the American College of Chest Physicians changes hands in January 2023, so will the role of President of the CHEST Foundation. To get to know the incoming President of the CHEST Foundation, we spoke with Robert (Bob) De Marco, MD, FCCP, about his philanthropy work and his goals for the philanthropic arm of CHEST.
Tell me about your history with philanthropy work.
My philanthropy work started long before the CHEST Foundation. While I’ve been a member of CHEST since my second year of fellowship, it wasn’t until much later that I became involved with the philanthropic side of the organization. Earlier in my career, I was involved more so with the American Cancer Society. I had gotten involved with them by chance – participating in an event of theirs – and was encouraged to get more involved by one of their board members. Being involved with them made a lot of sense seeing as a strong percentage of my patients at the time were being treated for lung cancer. My most notable accomplishments with the American Cancer Society were in serving as the Chairmen of my local Relay for Life program for 10 years, as a board member, and then as a president of my local chapter.
When did you get involved with the CHEST Foundation?
I had served in a handful of positions within CHEST, including Chair of the (since reinvented) Practice Management Committee, so I was deeply involved in the association, and I thought to myself, “I have experience in fundraising through my work with the American Cancer Society, why don’t I use it to help our association?” When I moved to Florida, I no longer had the local connection to the American Cancer Society, so it was an opportune time to transition over to the CHEST Foundation.
How has the Foundation changed in the time that you’ve been involved?
The Foundation has changed drastically since I first joined the Board of Trustees 9 years ago. When I first got involved, the primary goal of the Foundation was staying “out of the red.” At that time, we were an organization that gave away more than we made.
After years of building a corpus to fund our own projects, we’re in a really good place now with some phenomenal goals and some excellent initiatives to fundraise around, including a CHEST diversity initiative, First 5 Minutes™, and Bridging Specialties™: Timely Diagnosis for ILD Patients, which seeks to break down silos within medicine to improve patient care.
What will be a focus of your Foundation presidency?
You know, one thing I always appreciated about the American Cancer Society was that there were always notable accomplishments to point back to when supporting fundraising efforts. You could say, “Did you know that bone marrow transplantation was initially funded by the American Cancer Society?” and other examples that would truly inspire someone to want to get involved in supporting those efforts.
The CHEST Foundation may not have funded bone marrow transplantation, but in 25 years of awarding grants, there are equally good stories to share. The impact of the Foundation is tremendous, and we’ve only just begun to share examples of where grant recipients went with their research or community service projects.
A recent grant story that was shared with me was that of Panagis Galiatsatos, MD, MHS, who received a community service grant to start a program educating children in the Baltimore community about lung health. This program was so moving that it inspired one of the Baltimore teachers to pursue a career in medicine and that individual is now a practicing MD.
This is just one example of the Foundation’s impact and it’s through these stories that we share the “why” behind every dollar that is raised, and my first goal is to tell these stories.
Another key focus of not only my presidency, but Dr. Ian Nathanson’s, as well, as we collaborated a lot on our roles, will be on member involvement and awareness. Even I wasn’t involved in the CHEST Foundation until years into my CHEST membership, so I understand that there are competing demands. But I also know that there is a lot to be gained from the work with the Foundation. I want the CHEST members to be excited about the Foundation and to want to support its efforts.
These two goals go hand in hand, and I look forward to sharing the Foundation’s impact with a new audience and reinvigorating the support of our existing donors.
Is there anything else you’d like to say to the reader?
We cannot accomplish anything without the support of our donors, and I want to sincerely thank everyone who has donated to the CHEST Foundation. I also encourage those who have never donated or have yet to donate this year to visit the Foundation’s website (foundation.chestnet.org) and explore some of the inspiring initiatives you can support to strengthen the impact of the CHEST Foundation because the possibilities are truly endless.
As the presidency of the American College of Chest Physicians changes hands in January 2023, so will the role of President of the CHEST Foundation. To get to know the incoming President of the CHEST Foundation, we spoke with Robert (Bob) De Marco, MD, FCCP, about his philanthropy work and his goals for the philanthropic arm of CHEST.
Tell me about your history with philanthropy work.
My philanthropy work started long before the CHEST Foundation. While I’ve been a member of CHEST since my second year of fellowship, it wasn’t until much later that I became involved with the philanthropic side of the organization. Earlier in my career, I was involved more so with the American Cancer Society. I had gotten involved with them by chance – participating in an event of theirs – and was encouraged to get more involved by one of their board members. Being involved with them made a lot of sense seeing as a strong percentage of my patients at the time were being treated for lung cancer. My most notable accomplishments with the American Cancer Society were in serving as the Chairmen of my local Relay for Life program for 10 years, as a board member, and then as a president of my local chapter.
When did you get involved with the CHEST Foundation?
I had served in a handful of positions within CHEST, including Chair of the (since reinvented) Practice Management Committee, so I was deeply involved in the association, and I thought to myself, “I have experience in fundraising through my work with the American Cancer Society, why don’t I use it to help our association?” When I moved to Florida, I no longer had the local connection to the American Cancer Society, so it was an opportune time to transition over to the CHEST Foundation.
How has the Foundation changed in the time that you’ve been involved?
The Foundation has changed drastically since I first joined the Board of Trustees 9 years ago. When I first got involved, the primary goal of the Foundation was staying “out of the red.” At that time, we were an organization that gave away more than we made.
After years of building a corpus to fund our own projects, we’re in a really good place now with some phenomenal goals and some excellent initiatives to fundraise around, including a CHEST diversity initiative, First 5 Minutes™, and Bridging Specialties™: Timely Diagnosis for ILD Patients, which seeks to break down silos within medicine to improve patient care.
What will be a focus of your Foundation presidency?
You know, one thing I always appreciated about the American Cancer Society was that there were always notable accomplishments to point back to when supporting fundraising efforts. You could say, “Did you know that bone marrow transplantation was initially funded by the American Cancer Society?” and other examples that would truly inspire someone to want to get involved in supporting those efforts.
The CHEST Foundation may not have funded bone marrow transplantation, but in 25 years of awarding grants, there are equally good stories to share. The impact of the Foundation is tremendous, and we’ve only just begun to share examples of where grant recipients went with their research or community service projects.
A recent grant story that was shared with me was that of Panagis Galiatsatos, MD, MHS, who received a community service grant to start a program educating children in the Baltimore community about lung health. This program was so moving that it inspired one of the Baltimore teachers to pursue a career in medicine and that individual is now a practicing MD.
This is just one example of the Foundation’s impact and it’s through these stories that we share the “why” behind every dollar that is raised, and my first goal is to tell these stories.
Another key focus of not only my presidency, but Dr. Ian Nathanson’s, as well, as we collaborated a lot on our roles, will be on member involvement and awareness. Even I wasn’t involved in the CHEST Foundation until years into my CHEST membership, so I understand that there are competing demands. But I also know that there is a lot to be gained from the work with the Foundation. I want the CHEST members to be excited about the Foundation and to want to support its efforts.
These two goals go hand in hand, and I look forward to sharing the Foundation’s impact with a new audience and reinvigorating the support of our existing donors.
Is there anything else you’d like to say to the reader?
We cannot accomplish anything without the support of our donors, and I want to sincerely thank everyone who has donated to the CHEST Foundation. I also encourage those who have never donated or have yet to donate this year to visit the Foundation’s website (foundation.chestnet.org) and explore some of the inspiring initiatives you can support to strengthen the impact of the CHEST Foundation because the possibilities are truly endless.
Reverse-Grip Technique of Scissors in Dermatologic Surgery: Tips to Improve Undermining Efficiency
Practice Gap
One of the most important elements of successful reconstruction is effective undermining prior to placement of buried sutures. The main benefit of an evenly undermined plane is that tension is reduced, thus permitting seamless tissue mobilization and wound edge approximation.1
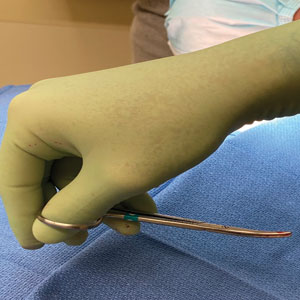
However, achieving a consistent and appropriate plane can present challenges in certain blind spots within one’s field of work. A right hand–dominant surgeon might find it difficult to undermine tissue between the 3-o’clock and 6-o’clock positions (Figure 1) and often must resort to unnatural positioning to obtain adequate reach.
We propose a technique of reversing the grip on undermining scissors that improves efficiency without sacrificing technique.
Technique
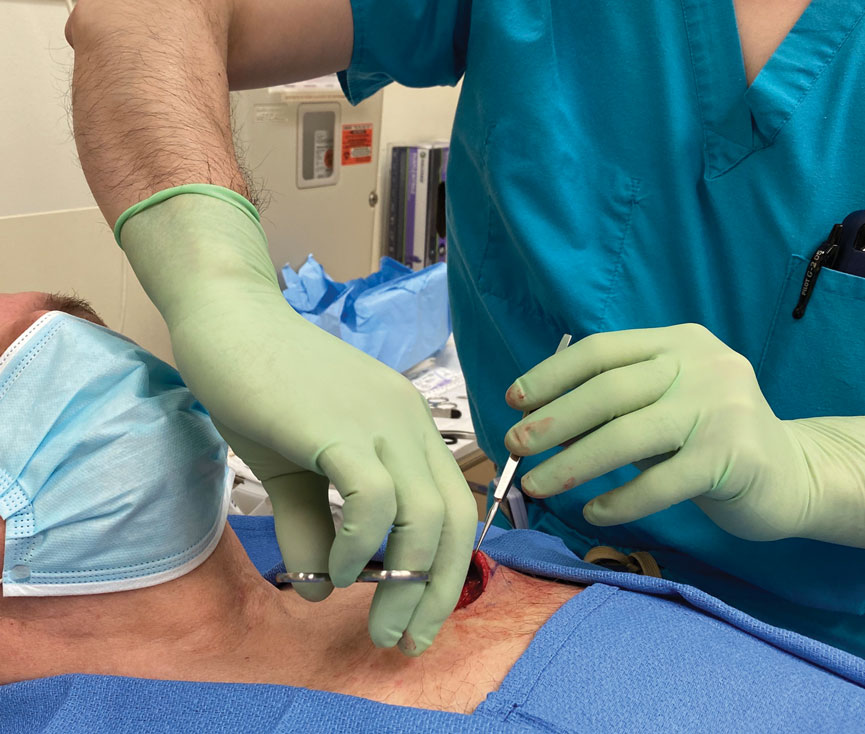
The surgeon simply grasps the ring handles with the ring finger and thumb with the tip pointing to the wrist (Figure 2). Most of the control comes from rotating the wrist while spreading with the thumb (Figure 3).
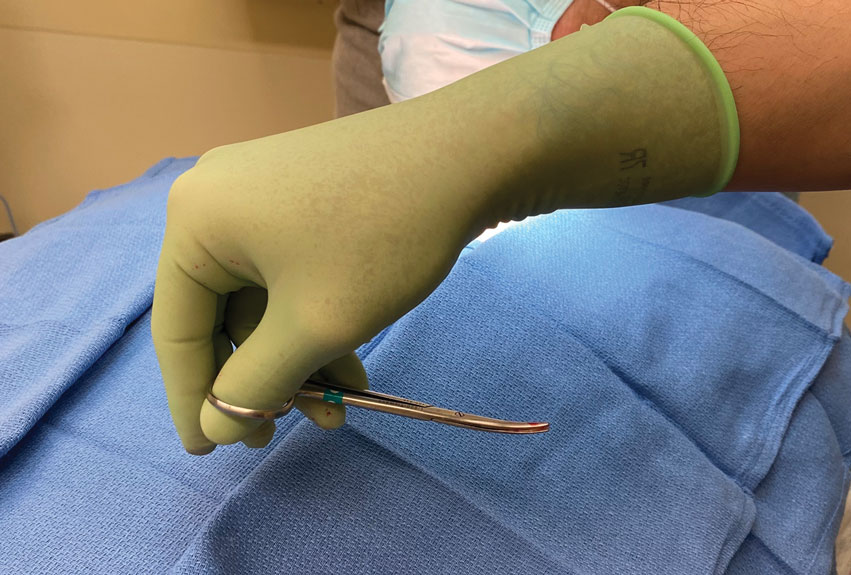
The main advantage of the reverse-grip technique is that it prevents abduction of the arm at the shoulder joint, which reduces shoulder fatigue and keeps the elbow close to the trunk and away from the sterile surgical field. Achieving optimal ergonomics during surgery has been shown to reduce pain and likely prolong the surgeon’s career.2
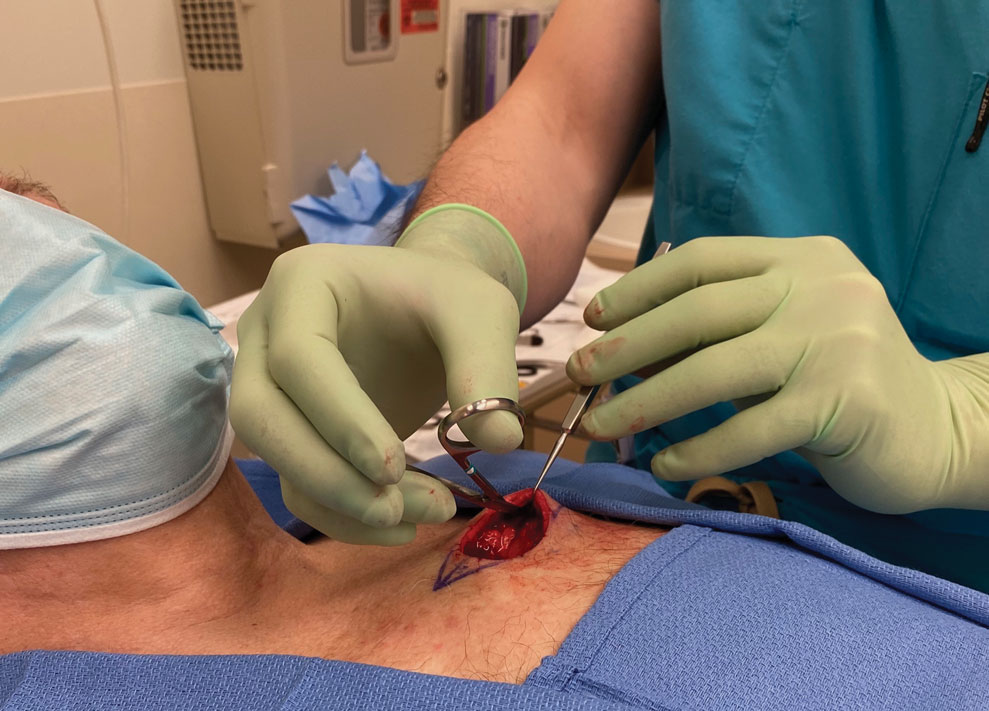
A limitation of the reverse-grip technique is that direct visualization of the undermining plane is not achieved; however, direct visualization also is not obtained when undermining in the standard fashion unless the instruments are passed to the surgical assistant or the surgeon moves to the other side of the table.
Undermining can be performed safely without direct visualization as long as several rules are followed:
• The undermining plane is first established under direct visualization on the far side of the wound—at the 6-o’clock to 12-o’clock positions—and then followed to the area where direct visualization is not obtained.
• A blunt-tipped scissor is used to prevent penetrating trauma to neurovascular bundles. Blunt-tipped instruments allow more “feel” through tactile feedback to the surgeon and prevent accidental injury to these critical structures.
• A curved scissor is used with “tips up,” such that the surgeon does not unintentionally make the undermining plane deeper than anticipated.
Practice Implications
With practice, one can perform circumferential undermining independently with few alterations in stance and while maintaining a natural position throughout. Use of skin hooks to elevate the skin can further aid in visualizing the correct depth of undermining. If executed correctly, the reverse-grip technique can expand the surgeon’s work field, thus providing ease of dissection in difficult-to-reach areas.
- Chen DL, Carlson EO, Fathi R, et al. Undermining and hemostasis. Dermatol Surg. 2015;41(suppl 10):S201-S215. doi:10.1097/DSS.0000000000000489
- Chan J, Kim DJ, Kassira-Carley S, et al. Ergonomics in dermatologic surgery: lessons learned across related specialties and opportunities for improvement. Dermatol Surg. 2020;46:763-772. doi:10.1097/DSS.0000000000002295
Practice Gap
One of the most important elements of successful reconstruction is effective undermining prior to placement of buried sutures. The main benefit of an evenly undermined plane is that tension is reduced, thus permitting seamless tissue mobilization and wound edge approximation.1
However, achieving a consistent and appropriate plane can present challenges in certain blind spots within one’s field of work. A right hand–dominant surgeon might find it difficult to undermine tissue between the 3-o’clock and 6-o’clock positions (Figure 1) and often must resort to unnatural positioning to obtain adequate reach.

We propose a technique of reversing the grip on undermining scissors that improves efficiency without sacrificing technique.
Technique
The surgeon simply grasps the ring handles with the ring finger and thumb with the tip pointing to the wrist (Figure 2). Most of the control comes from rotating the wrist while spreading with the thumb (Figure 3).

The main advantage of the reverse-grip technique is that it prevents abduction of the arm at the shoulder joint, which reduces shoulder fatigue and keeps the elbow close to the trunk and away from the sterile surgical field. Achieving optimal ergonomics during surgery has been shown to reduce pain and likely prolong the surgeon’s career.2

A limitation of the reverse-grip technique is that direct visualization of the undermining plane is not achieved; however, direct visualization also is not obtained when undermining in the standard fashion unless the instruments are passed to the surgical assistant or the surgeon moves to the other side of the table.
Undermining can be performed safely without direct visualization as long as several rules are followed:
• The undermining plane is first established under direct visualization on the far side of the wound—at the 6-o’clock to 12-o’clock positions—and then followed to the area where direct visualization is not obtained.
• A blunt-tipped scissor is used to prevent penetrating trauma to neurovascular bundles. Blunt-tipped instruments allow more “feel” through tactile feedback to the surgeon and prevent accidental injury to these critical structures.
• A curved scissor is used with “tips up,” such that the surgeon does not unintentionally make the undermining plane deeper than anticipated.
Practice Implications
With practice, one can perform circumferential undermining independently with few alterations in stance and while maintaining a natural position throughout. Use of skin hooks to elevate the skin can further aid in visualizing the correct depth of undermining. If executed correctly, the reverse-grip technique can expand the surgeon’s work field, thus providing ease of dissection in difficult-to-reach areas.
Practice Gap
One of the most important elements of successful reconstruction is effective undermining prior to placement of buried sutures. The main benefit of an evenly undermined plane is that tension is reduced, thus permitting seamless tissue mobilization and wound edge approximation.1
However, achieving a consistent and appropriate plane can present challenges in certain blind spots within one’s field of work. A right hand–dominant surgeon might find it difficult to undermine tissue between the 3-o’clock and 6-o’clock positions (Figure 1) and often must resort to unnatural positioning to obtain adequate reach.

We propose a technique of reversing the grip on undermining scissors that improves efficiency without sacrificing technique.
Technique
The surgeon simply grasps the ring handles with the ring finger and thumb with the tip pointing to the wrist (Figure 2). Most of the control comes from rotating the wrist while spreading with the thumb (Figure 3).

The main advantage of the reverse-grip technique is that it prevents abduction of the arm at the shoulder joint, which reduces shoulder fatigue and keeps the elbow close to the trunk and away from the sterile surgical field. Achieving optimal ergonomics during surgery has been shown to reduce pain and likely prolong the surgeon’s career.2

A limitation of the reverse-grip technique is that direct visualization of the undermining plane is not achieved; however, direct visualization also is not obtained when undermining in the standard fashion unless the instruments are passed to the surgical assistant or the surgeon moves to the other side of the table.
Undermining can be performed safely without direct visualization as long as several rules are followed:
• The undermining plane is first established under direct visualization on the far side of the wound—at the 6-o’clock to 12-o’clock positions—and then followed to the area where direct visualization is not obtained.
• A blunt-tipped scissor is used to prevent penetrating trauma to neurovascular bundles. Blunt-tipped instruments allow more “feel” through tactile feedback to the surgeon and prevent accidental injury to these critical structures.
• A curved scissor is used with “tips up,” such that the surgeon does not unintentionally make the undermining plane deeper than anticipated.
Practice Implications
With practice, one can perform circumferential undermining independently with few alterations in stance and while maintaining a natural position throughout. Use of skin hooks to elevate the skin can further aid in visualizing the correct depth of undermining. If executed correctly, the reverse-grip technique can expand the surgeon’s work field, thus providing ease of dissection in difficult-to-reach areas.
- Chen DL, Carlson EO, Fathi R, et al. Undermining and hemostasis. Dermatol Surg. 2015;41(suppl 10):S201-S215. doi:10.1097/DSS.0000000000000489
- Chan J, Kim DJ, Kassira-Carley S, et al. Ergonomics in dermatologic surgery: lessons learned across related specialties and opportunities for improvement. Dermatol Surg. 2020;46:763-772. doi:10.1097/DSS.0000000000002295
- Chen DL, Carlson EO, Fathi R, et al. Undermining and hemostasis. Dermatol Surg. 2015;41(suppl 10):S201-S215. doi:10.1097/DSS.0000000000000489
- Chan J, Kim DJ, Kassira-Carley S, et al. Ergonomics in dermatologic surgery: lessons learned across related specialties and opportunities for improvement. Dermatol Surg. 2020;46:763-772. doi:10.1097/DSS.0000000000002295
Medicare Part D Prescription Claims for Brodalumab: Analysis of Annual Trends for 2017-2019
To the Editor:
Brodalumab, a monoclonal antibody targeting IL-17RA, was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe chronic plaque psoriasis. The drug is the only biologic agent available for the treatment of psoriasis for which a psoriasis area severity index score of 100 is a primary end point.1,2 Brodalumab is associated with an FDA boxed warning due to an increased risk for suicidal ideation and behavior (SIB), including completed suicides, during clinical trials.
We sought to characterize national utilization of this effective yet underutilized drug among Medicare beneficiaries by surveying the Medicare Part D Prescriber dataset.3 We tabulated brodalumab utilization statistics and characteristics of high-volume prescribers who had 11 or more annual claims for brodalumab.
Despite its associated boxed warning, the number of Medicare D claims for brodalumab increased by 1756 from 2017 to 2019, surpassing $7 million in costs by 2019. The number of beneficiaries also increased from 11 to 292—a 415.2% annual increase in beneficiaries for whom brodalumab was prescribed (Table 1).
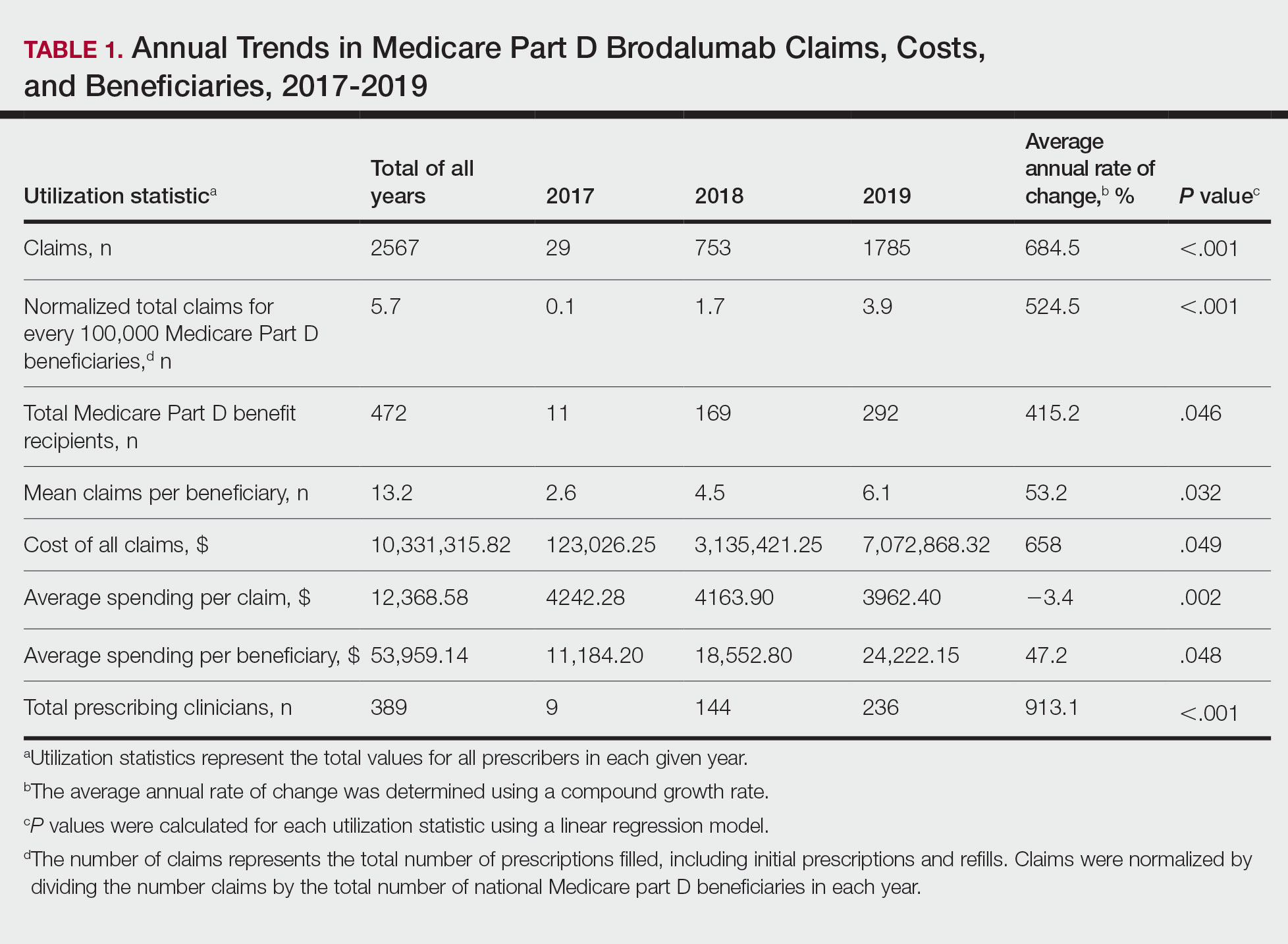
In addition, states in the West and South had the highest utilization rates of brodalumab in 2019. There also was an increasing trend toward high-volume prescribers of brodalumab, with private practice clinicians constituting the majority (Table 2).
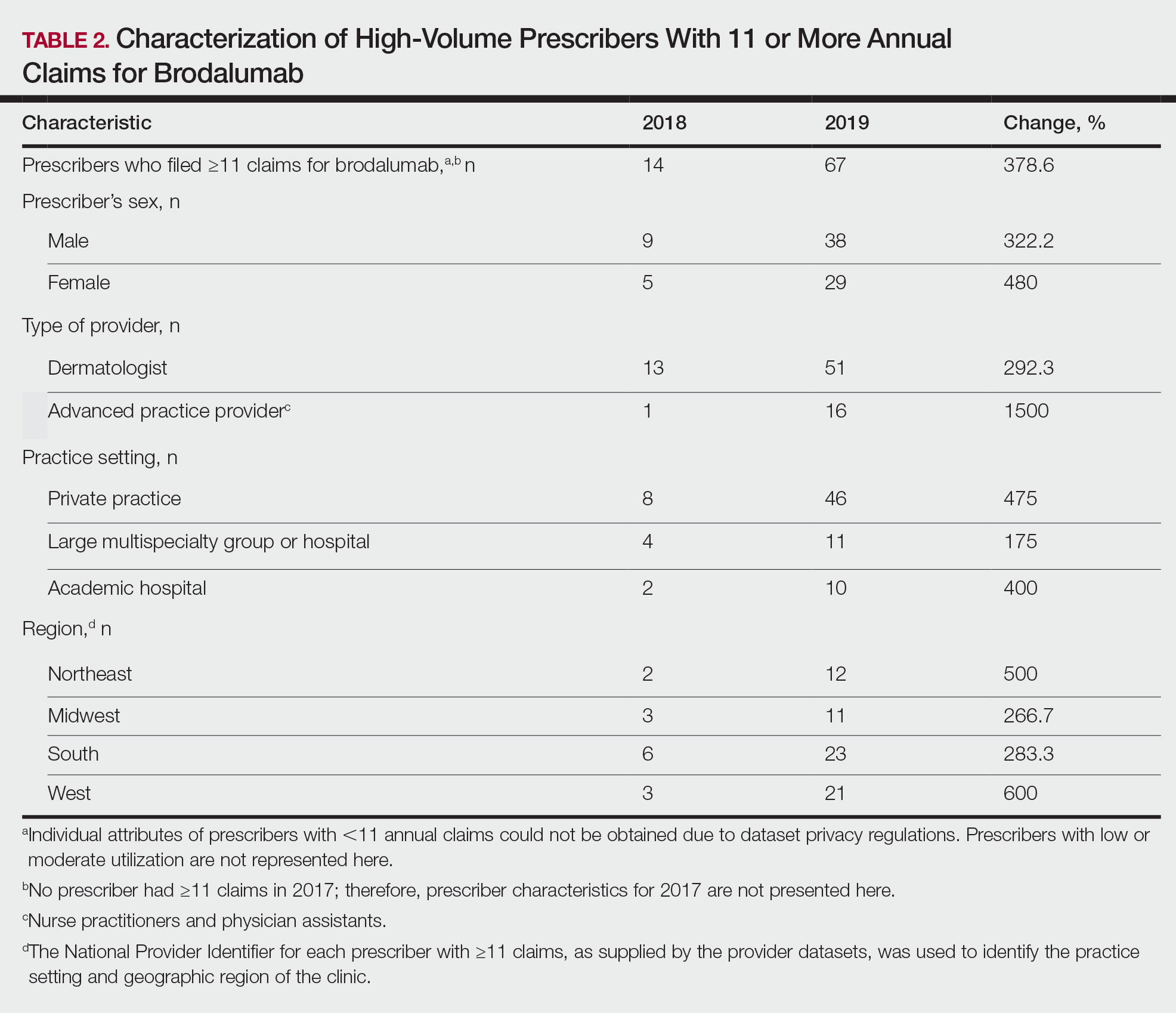
There was a substantial increase in advanced practice providers including nurse practitioners and physician assistants who were brodalumab prescribers. Although this trend might promote greater access to brodalumab, it is vital to ensure that advanced practice providers receive targeted training to properly understand the complexities of treatment with brodalumab.
Although the utilization of brodalumab has increased since 2017 (P<.001), it is still underutilized compared to the other IL-17 inhibitors secukinumab and ixekizumab. Secukinumab was FDA approved for the treatment of moderate to severe plaque psoriasis in 2015, followed by ixekizumab in 2016.4
According to the Medicare Part D database, both secukinumab and ixekizumab had a higher number of total claims and prescribers compared to brodalumab in the years of their debut.3 In 2015, there were 3593 claims for and 862 prescribers of secukinumab; in 2016, there were 1731 claims for and 681 prescribers of ixekizumab. In contrast, there were only 29 claims for and 11 prescribers of brodalumab in 2017, the year that the drug was approved by the FDA. During the same 3-year period, secukinumab and ixekizumab had a substantially greater number of claims—totals of 176,823 and 55,289, respectively—than brodalumab. The higher number of claims for secukinumab and ixekizumab compared to brodalumab may reflect clinicians’ increasing confidence in prescribing those drugs, given their long-term safety and efficacy. In addition, secukinumab and ixekizumab do not require completion of a Risk Evaluation and Mitigation Strategy (REMS) program, which makes them more readily prescribable.3
Overall, most experts agree that there is no increase in the risk for suicide associated with brodalumab compared to the general population. A 2-year pharmacovigilance report on brodalumab supports the safety of this drug.5 All participants who completed suicide during the clinical trials harbored an underlying psychiatric disorder or stressor(s).6
Although causation between brodalumab and SIB has not been demonstrated, it remains imperative that prescribers diligently assess patients’ risk of SIB and subsequently their access to appropriate psychiatric services as a precaution, if necessary. This is particularly important for private practice prescribers, who constitute the majority of Medicare D brodalumab claims, because they must ensure collaboration with a multidisciplinary team involving mental health providers. Lastly, considering that the highest number of brodalumab Medicare D claims were in western and southern states, it is critical to note that those 2 regions also harbor comparatively fewer mental health facilities that accept Medicare than other regions of the country.7 Prescribers in western and southern states must be mindful of mental health coverage limitations when treating psoriasis patients with brodalumab.
The increase in the number of claims, beneficiaries, and prescribers of brodalumab during its first 3 years of availability might be attributed to its efficacy and safety. On the other hand, the boxed warning and REMS associated with brodalumab might have led to underutilization of this drug compared to other IL-17 inhibitors.
Our analysis is limited by its representative restriction to Medicare patients. There also are limited data on brodalumab given its novelty. Individual attributes of prescribers with fewer than 11 annual claims for brodalumab could not be obtained because of dataset regulations; however, aggregated utilization statistics provide an indication of brodalumab prescribing patterns among all providers. Furthermore, during this analysis, data on the Medicare D database were limited to 2013 through 2020. Studies are needed to determine prescribing patterns of brodalumab since this study period.
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. doi:10.7573/dic.212570
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292. doi:10.1080/14712598.2019.1579794
- Centers for Medicare & Medicaid Services. Medicare Part D Prescribers. Updated July 27, 2022. Accessed September 23, 2022. https://data.cms.gov/provider-summary-by-type-of-service/medicare-part-d-prescribers/medicare-part-d-prescribers-by-provider
- Drugs. US Food and Drug Administration website. Accessed September 23, 2022. https://www.fda.gov/drugs
- Lebwohl M, Leonardi C, Wu JJ, et al. Two-year US pharmacovigilance report on brodalumab. Dermatol Ther (Heidelb). 2021;11:173-180. doi:10.1007/s13555-020-00472-x
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81-89.e5. doi:10.1016/j.jaad.2017.08.024
- Substance Abuse and Mental Health Services Administration. National Mental Health Services Survey (N-MHSS): 2019, Data On Mental Health Treatment Facilities. Rockville, MD: Substance Abuse and Mental Health Services Administration; August 13, 2020. Accessed September 21, 2022. https://www.samhsa.gov/data/report/national-mental-health-services-survey-n-mhss-2019-data-mental-health-treatment-facilities
To the Editor:
Brodalumab, a monoclonal antibody targeting IL-17RA, was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe chronic plaque psoriasis. The drug is the only biologic agent available for the treatment of psoriasis for which a psoriasis area severity index score of 100 is a primary end point.1,2 Brodalumab is associated with an FDA boxed warning due to an increased risk for suicidal ideation and behavior (SIB), including completed suicides, during clinical trials.
We sought to characterize national utilization of this effective yet underutilized drug among Medicare beneficiaries by surveying the Medicare Part D Prescriber dataset.3 We tabulated brodalumab utilization statistics and characteristics of high-volume prescribers who had 11 or more annual claims for brodalumab.
Despite its associated boxed warning, the number of Medicare D claims for brodalumab increased by 1756 from 2017 to 2019, surpassing $7 million in costs by 2019. The number of beneficiaries also increased from 11 to 292—a 415.2% annual increase in beneficiaries for whom brodalumab was prescribed (Table 1).

In addition, states in the West and South had the highest utilization rates of brodalumab in 2019. There also was an increasing trend toward high-volume prescribers of brodalumab, with private practice clinicians constituting the majority (Table 2).

There was a substantial increase in advanced practice providers including nurse practitioners and physician assistants who were brodalumab prescribers. Although this trend might promote greater access to brodalumab, it is vital to ensure that advanced practice providers receive targeted training to properly understand the complexities of treatment with brodalumab.
Although the utilization of brodalumab has increased since 2017 (P<.001), it is still underutilized compared to the other IL-17 inhibitors secukinumab and ixekizumab. Secukinumab was FDA approved for the treatment of moderate to severe plaque psoriasis in 2015, followed by ixekizumab in 2016.4
According to the Medicare Part D database, both secukinumab and ixekizumab had a higher number of total claims and prescribers compared to brodalumab in the years of their debut.3 In 2015, there were 3593 claims for and 862 prescribers of secukinumab; in 2016, there were 1731 claims for and 681 prescribers of ixekizumab. In contrast, there were only 29 claims for and 11 prescribers of brodalumab in 2017, the year that the drug was approved by the FDA. During the same 3-year period, secukinumab and ixekizumab had a substantially greater number of claims—totals of 176,823 and 55,289, respectively—than brodalumab. The higher number of claims for secukinumab and ixekizumab compared to brodalumab may reflect clinicians’ increasing confidence in prescribing those drugs, given their long-term safety and efficacy. In addition, secukinumab and ixekizumab do not require completion of a Risk Evaluation and Mitigation Strategy (REMS) program, which makes them more readily prescribable.3
Overall, most experts agree that there is no increase in the risk for suicide associated with brodalumab compared to the general population. A 2-year pharmacovigilance report on brodalumab supports the safety of this drug.5 All participants who completed suicide during the clinical trials harbored an underlying psychiatric disorder or stressor(s).6
Although causation between brodalumab and SIB has not been demonstrated, it remains imperative that prescribers diligently assess patients’ risk of SIB and subsequently their access to appropriate psychiatric services as a precaution, if necessary. This is particularly important for private practice prescribers, who constitute the majority of Medicare D brodalumab claims, because they must ensure collaboration with a multidisciplinary team involving mental health providers. Lastly, considering that the highest number of brodalumab Medicare D claims were in western and southern states, it is critical to note that those 2 regions also harbor comparatively fewer mental health facilities that accept Medicare than other regions of the country.7 Prescribers in western and southern states must be mindful of mental health coverage limitations when treating psoriasis patients with brodalumab.
The increase in the number of claims, beneficiaries, and prescribers of brodalumab during its first 3 years of availability might be attributed to its efficacy and safety. On the other hand, the boxed warning and REMS associated with brodalumab might have led to underutilization of this drug compared to other IL-17 inhibitors.
Our analysis is limited by its representative restriction to Medicare patients. There also are limited data on brodalumab given its novelty. Individual attributes of prescribers with fewer than 11 annual claims for brodalumab could not be obtained because of dataset regulations; however, aggregated utilization statistics provide an indication of brodalumab prescribing patterns among all providers. Furthermore, during this analysis, data on the Medicare D database were limited to 2013 through 2020. Studies are needed to determine prescribing patterns of brodalumab since this study period.
To the Editor:
Brodalumab, a monoclonal antibody targeting IL-17RA, was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe chronic plaque psoriasis. The drug is the only biologic agent available for the treatment of psoriasis for which a psoriasis area severity index score of 100 is a primary end point.1,2 Brodalumab is associated with an FDA boxed warning due to an increased risk for suicidal ideation and behavior (SIB), including completed suicides, during clinical trials.
We sought to characterize national utilization of this effective yet underutilized drug among Medicare beneficiaries by surveying the Medicare Part D Prescriber dataset.3 We tabulated brodalumab utilization statistics and characteristics of high-volume prescribers who had 11 or more annual claims for brodalumab.
Despite its associated boxed warning, the number of Medicare D claims for brodalumab increased by 1756 from 2017 to 2019, surpassing $7 million in costs by 2019. The number of beneficiaries also increased from 11 to 292—a 415.2% annual increase in beneficiaries for whom brodalumab was prescribed (Table 1).

In addition, states in the West and South had the highest utilization rates of brodalumab in 2019. There also was an increasing trend toward high-volume prescribers of brodalumab, with private practice clinicians constituting the majority (Table 2).

There was a substantial increase in advanced practice providers including nurse practitioners and physician assistants who were brodalumab prescribers. Although this trend might promote greater access to brodalumab, it is vital to ensure that advanced practice providers receive targeted training to properly understand the complexities of treatment with brodalumab.
Although the utilization of brodalumab has increased since 2017 (P<.001), it is still underutilized compared to the other IL-17 inhibitors secukinumab and ixekizumab. Secukinumab was FDA approved for the treatment of moderate to severe plaque psoriasis in 2015, followed by ixekizumab in 2016.4
According to the Medicare Part D database, both secukinumab and ixekizumab had a higher number of total claims and prescribers compared to brodalumab in the years of their debut.3 In 2015, there were 3593 claims for and 862 prescribers of secukinumab; in 2016, there were 1731 claims for and 681 prescribers of ixekizumab. In contrast, there were only 29 claims for and 11 prescribers of brodalumab in 2017, the year that the drug was approved by the FDA. During the same 3-year period, secukinumab and ixekizumab had a substantially greater number of claims—totals of 176,823 and 55,289, respectively—than brodalumab. The higher number of claims for secukinumab and ixekizumab compared to brodalumab may reflect clinicians’ increasing confidence in prescribing those drugs, given their long-term safety and efficacy. In addition, secukinumab and ixekizumab do not require completion of a Risk Evaluation and Mitigation Strategy (REMS) program, which makes them more readily prescribable.3
Overall, most experts agree that there is no increase in the risk for suicide associated with brodalumab compared to the general population. A 2-year pharmacovigilance report on brodalumab supports the safety of this drug.5 All participants who completed suicide during the clinical trials harbored an underlying psychiatric disorder or stressor(s).6
Although causation between brodalumab and SIB has not been demonstrated, it remains imperative that prescribers diligently assess patients’ risk of SIB and subsequently their access to appropriate psychiatric services as a precaution, if necessary. This is particularly important for private practice prescribers, who constitute the majority of Medicare D brodalumab claims, because they must ensure collaboration with a multidisciplinary team involving mental health providers. Lastly, considering that the highest number of brodalumab Medicare D claims were in western and southern states, it is critical to note that those 2 regions also harbor comparatively fewer mental health facilities that accept Medicare than other regions of the country.7 Prescribers in western and southern states must be mindful of mental health coverage limitations when treating psoriasis patients with brodalumab.
The increase in the number of claims, beneficiaries, and prescribers of brodalumab during its first 3 years of availability might be attributed to its efficacy and safety. On the other hand, the boxed warning and REMS associated with brodalumab might have led to underutilization of this drug compared to other IL-17 inhibitors.
Our analysis is limited by its representative restriction to Medicare patients. There also are limited data on brodalumab given its novelty. Individual attributes of prescribers with fewer than 11 annual claims for brodalumab could not be obtained because of dataset regulations; however, aggregated utilization statistics provide an indication of brodalumab prescribing patterns among all providers. Furthermore, during this analysis, data on the Medicare D database were limited to 2013 through 2020. Studies are needed to determine prescribing patterns of brodalumab since this study period.
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. doi:10.7573/dic.212570
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292. doi:10.1080/14712598.2019.1579794
- Centers for Medicare & Medicaid Services. Medicare Part D Prescribers. Updated July 27, 2022. Accessed September 23, 2022. https://data.cms.gov/provider-summary-by-type-of-service/medicare-part-d-prescribers/medicare-part-d-prescribers-by-provider
- Drugs. US Food and Drug Administration website. Accessed September 23, 2022. https://www.fda.gov/drugs
- Lebwohl M, Leonardi C, Wu JJ, et al. Two-year US pharmacovigilance report on brodalumab. Dermatol Ther (Heidelb). 2021;11:173-180. doi:10.1007/s13555-020-00472-x
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81-89.e5. doi:10.1016/j.jaad.2017.08.024
- Substance Abuse and Mental Health Services Administration. National Mental Health Services Survey (N-MHSS): 2019, Data On Mental Health Treatment Facilities. Rockville, MD: Substance Abuse and Mental Health Services Administration; August 13, 2020. Accessed September 21, 2022. https://www.samhsa.gov/data/report/national-mental-health-services-survey-n-mhss-2019-data-mental-health-treatment-facilities
- Foulkes AC, Warren RB. Brodalumab in psoriasis: evidence to date and clinical potential. Drugs Context. 2019;8:212570. doi:10.7573/dic.212570
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292. doi:10.1080/14712598.2019.1579794
- Centers for Medicare & Medicaid Services. Medicare Part D Prescribers. Updated July 27, 2022. Accessed September 23, 2022. https://data.cms.gov/provider-summary-by-type-of-service/medicare-part-d-prescribers/medicare-part-d-prescribers-by-provider
- Drugs. US Food and Drug Administration website. Accessed September 23, 2022. https://www.fda.gov/drugs
- Lebwohl M, Leonardi C, Wu JJ, et al. Two-year US pharmacovigilance report on brodalumab. Dermatol Ther (Heidelb). 2021;11:173-180. doi:10.1007/s13555-020-00472-x
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78:81-89.e5. doi:10.1016/j.jaad.2017.08.024
- Substance Abuse and Mental Health Services Administration. National Mental Health Services Survey (N-MHSS): 2019, Data On Mental Health Treatment Facilities. Rockville, MD: Substance Abuse and Mental Health Services Administration; August 13, 2020. Accessed September 21, 2022. https://www.samhsa.gov/data/report/national-mental-health-services-survey-n-mhss-2019-data-mental-health-treatment-facilities
Practice Points
- Brodalumab is associated with a boxed warning due to increased suicidal ideation and behavior (SIB), including completed suicides, during clinical trials.
- Brodalumab is underutilized compared to the other US Food and Drug Administration–approved IL-17 inhibitors used to treat psoriasis.
- Most experts agree that there is no increased risk for suicide associated with brodalumab. However, it remains imperative that prescribers assess patients’ risk of SIB and subsequently their access to appropriate psychiatric services prior to initiating and during treatment with brodalumab.
Glucocorticoid-Induced Bone Loss: Dietary Supplementation Recommendations to Reduce the Risk for Osteoporosis and Osteoporotic Fractures
Glucocorticoids (GCs) are among the most widely prescribed medications in dermatologic practice. Although GCs are highly effective anti-inflammatory agents, long-term systemic therapy can result in dangerous adverse effects, including GC-induced osteoporosis (GIO), a bone disease associated with a heightened risk for fragility fractures.1,2 In the United States, an estimated 10.2 million adults have osteoporosis—defined as a T-score lower than −2.5 measured via a bone densitometry scan—and 43.4 million adults have low bone mineral density (BMD).3,4 The prevalence of osteoporosis is increasing, and the diagnosis is more common in females and adults 55 years and older.2 More than 2 million individuals have osteoporosis-related fractures annually, and the mortality risk is increased at 5 and 10 years following low-energy osteoporosis-related fractures.3-5
Glucocorticoid therapy is the leading iatrogenic cause of secondary osteoporosis. As many as 30% of all patients treated with systemic GCs for more than 6 months develop GIO.1,6,7 Glucocorticoid-induced BMD loss occurs at a rate of 6% to 12% of total BMD during the first year, slowing to approximately 3% per year during subsequent therapy.1 The risk for insufficiency fractures increases by as much as 75% from baseline in adults with rheumatic, pulmonary, and skin disorders within the first 3 months of therapy and peaks at approximately 12 months.1,2
Despite the risks, many long-term GC users never receive therapy to prevent bone loss; others are only started on therapy once they have sustained an insufficiency fracture. A 5-year international observational study including more than 40,000 postmenopausal women found that only 51% of patients who were on continuous GC therapy were undergoing BMD testing and appropriate medical management.8 This review highlights the existing evidence on the risks of osteoporosis and osteoporotic (OP) fractures in the setting of topical, intralesional, intramuscular, and systemic GC treatment, as well as recommendations for nutritional supplementation to reduce these risks.
Pathophysiology
The pathophysiology of GIO is multifactorial and occurs in both early and late phases.9,10 The early phase is characterized by rapid BMD reduction due to excessive bone resorption. The late phase is characterized by slower and more progressive BMD reduction due to impaired bone formation.9 At the osteocyte level, GCs decrease cell viability and induce apoptosis.11 At the osteoblast level, GCs impair cell replication and differentiation and have proapoptotic effects, resulting in decreased cell numbers and subsequent bone formation.10 At the osteoclast level, GCs increase expression of pro-osteoclastic cytokines and decrease mature osteoclast apoptosis, resulting in an expanded osteoclastic life span and prolonged bone resorption.12,13 Indirectly, GCs alter calcium metabolism by decreasing gastrointestinal calcium absorption and impairing renal absorption.14,15
GCs and Osteoporosis
Oral GCs—Glucocorticoid-induced osteoporosis and fracture risk are dose and duration dependent.6 A study of 244,235 patients taking GCs and 244,235 controls found the relative risk of vertebral fracture was 1.55 (range, 1.20–2.01) for daily prednisone use at less than 2.5 mg, 2.59 (range, 2.16–3.10) for daily prednisone use from 2.5 to 7.4 mg, and 5.18 (range, 4.25–6.31) for daily doses of 7.5 mg or higher; the relative risk for hip fractures was 0.99 (range, 0.82–1.20), 1.77 (range, 1.55–2.02), and 2.27 (range, 1.94–2.66), respectively.16 Another large retrospective cohort study found that continuous treatment with prednisone 10 mg/d for more than 90 days compared to no GC exposure increased the risk for hip fractures 7-fold and 17-fold for vertebral fractures.17 Although the minimum cumulative dose of GCs known to cause osteoporosis is not clearly established, the American College of Rheumatology has proposed an algorithm as a basic approach to anticipate, prevent, and treat GIO (Figure).18,19 Fracture risk should be assessed in all patients who are prescribed prednisone 2.5 mg/d for 3 months or longer or an anticipated cumulative dose of more than 1 g per year. Patients 40 years and older with anticipated GC use of 3 months or longer should have both a bone densitometry scan and a Fracture Risk Assessment (FRAX) score. The FRAX tool estimates the 10-year probability of fracture in patients aged 40 to 80 years, and those patients can be further risk stratified as low (FRAX <10%), moderate (FRAX 10%–19%), or high (FRAX ≥20%) risk. In patients with moderate to high risk of fracture (FRAX >10%), initiation of pharmacologic treatment or referral to a metabolic bone specialist should be considered.18,19 First-line therapy is an oral bisphosphonate, and second-line therapies include intravenous bisphosphonates, teriparatide, denosumab, or raloxifene for patients at high risk for GIO.19 Adults younger than 40 years with a history of OP fracture or considerable risk factors for OP fractures should have a bone densitometry scan, and, if results are abnormal, the patient should be referred to a metabolic bone specialist. Those with low fracture risk based on bone densitometry and FRAX and those with no risk factors should be assessed annually for bone health (additional risk factors, GC dose and duration, bone densitometry/FRAX if indicated).18 In addition to GC dose and duration, additional risk factors for GIO, which are factored into the FRAX tool, include advanced age, low body mass index, history of bone fracture, smoking, excessive alcohol use (≥3 drinks/d), history of falls, low BMD, family history of bone fracture, and hypovitaminosis D.6
Topical GCs—Although there is strong evidence and clear guidelines regarding oral GIO, there is a dearth of data surrounding OP risk due to treatment with topical GCs. A recent retrospective nationwide Danish study evaluating the risk of osteoporosis and major OP fracture in 723,251 adults treated with potent or very potent topical steroids sought to evaluate these risks.20 Patients were included if they had filled prescriptions of at least 500 g of topical mometasone or an equivalent alternative. The investigators reported a 3% increase in relative risk of osteoporosis and major OP fracture with doubling of the cumulative topical GC dose (hazard ratio [HR], 1.03 [95% CI, 1.02-1.04] for both). The overall population-attributable risk was 4.3% (95% CI, 2.7%-5.8%) for osteoporosis and 2.7% (95% CI, 1.7%-3.8%) for major OP fracture. Notably, at least 10,000 g of mometasone was required for 1 additional patient to have a major OP fracture.20 In a commentary based on this study, Jackson21 noted that the number of patient-years of topical GC use needed for 1 fracture was 4-fold higher than that for high-dose oral GCs (40 mg/d prednisolone for ≥30 days). Another study assessed the effects of topical GCs on BMD in adults with moderate to severe atopic dermatitis over a 2-year period.22 No significant difference in BMD assessed via bone densitometry of either the lumbar spine or total hip at baseline or at 2-year follow-up was reported for either group treated with corticosteroids (<75 g per month or ≥75 g per month). Of note, the authors did not account for steroid potency, which ranged from class 1 through class 4.22 Although limited data exist, these studies suggest topical GCs used at conventional doses with appropriate breaks in therapy will not substantially increase risk for GIO or OP fracture; however, in the small subset of patients requiring chronic use of superpotent topical corticosteroids with other OP risk factors, transitioning to non–GC-based therapy or initiating bone health therapy may be advised to improve patient outcomes. Risk assessment, as in cases of chronic topical GC use, may be beneficial.
Intralesional GCs—Intralesional GCs are indicated for numerous inflammatory conditions including alopecia areata, discoid lupus erythematosus, keloids, and granuloma annulare. It generally is accepted that doses of triamcinolone acetonide should not exceed 20 mg per session spaced at least 3 weeks apart or up to 40 mg per month.18 One study demonstrated that doses of triamcinolone diacetate of 25 mg or less were unlikely to produce systemic effects and were determined to be a safe dose for intralesional injections.23 A retrospective cross-sectional case series including 18 patients with alopecia areata reported decreased BMD in 9 patients receiving intralesional triamcinolone acetonide 10 mg/mL at 4- to 8-week intervals for at least 20 months, with cumulative doses greater than 500 mg. This was particularly notable in postmenopausal women and men older than 50 years; participants with a body mass index less than 18.5 kg/m2, history of a stress fracture, family history of osteopenia or osteoporosis, and history of smoking; and those who did not regularly engage in weight-bearing exercises.24 Patients receiving long-term (ie, >1 year) intralesional steroids should be evaluated for osteoporosis risk and preventative strategies should be considered (ie, regular weight-bearing exercises, calcium and vitamin D supplementation, bisphosphate therapy). As with topical GCs, there are no clear guidelines for risk assessment or treatment recommendations for GIO.
Intramuscular GCs—The data regarding intramuscular (IM) GCs and dermatologic disease is severely limited, and to the best of our knowledge, no studies specifically assess the risk for GIO or fracture secondary to intramuscular GCs; however, a retrospective study of 27 patients (4 female, 23 male; mean age, 33 years [range, 12–61 years]) with refractory alopecia areata receiving IM triamcinolone acetonide (40 mg every 4 weeks for 3–6 months) reported 1 patient (a 56-year-old woman) with notably decreased bone densitometry from baseline requiring treatment discontinuation.25 No other patients at risk for osteoporosis had decreased BMD from treatment with IM triamcinolone; however, it was noted that 1 month following treatment, 10 of 11 assessed patients demonstrated decreased levels of morning serum cortisol and plasma adrenocorticotropic hormone—despite baseline levels within reference range—that resolved 3 months after treatment completion,25 which suggests a prolonged release of IM triamcinolone and sustained systemic effect. One systematic review of 342 patients with dermatologic diseases treated with IM corticosteroids found the primary side effects included dysmenorrhea, injection-site lipoatrophy, and adrenocortical suppression, with only a single reported case of low BMD.26 Given the paucity of evidence, additional studies are required to assess the effect of IM triamcinolone on BMD and risk for major OP fractures with regard to dosing and frequency. As there are no clear guidelines for osteoporosis evaluation in the setting of intramuscular GCs, it may be prudent to follow the algorithmic model recommended for oral steroids when anticipating at least 3 months of intramuscular GCs.
Diet and Prevention of Bone Loss
Given the profound impact that systemic GCs have on osteoporosis and fracture risk and the sparse data regarding risk from topical, intralesional, or intramuscular GCs, diet and nutrition represent a simple, safe, and potentially preventative method of slowing BMD loss and minimizing fracture risk. In higher-risk patients, nutritional assessment in combination with medical therapy also is likely warranted.
Calcium and Vitamin D3—Patients treated with any GC dose longer than 3 months should undergo calcium and vitamin D optimization.19 Exceptions for supplementation include certain patients with sarcoidosis, which can be associated with high vitamin D levels; patients with a history of hypercalcemia or hypercalciuria; and patients with chronic kidney disease.6 In a meta-analysis including 30,970 patients in 8 randomized controlled trials, calcium (500–1200 mg/d) and vitamin D (400–800 IU/d) supplementation reduced the risk of total fractures by 15% (summary relative risk estimate, 0.85 [95% CI, 0.73-0.98]) and hip fractures by 30% (summary relative risk estimate, 0.70 [95% CI, 0.56-0.87]).4 One double-blind, placebo-controlled clinical trial conducted by the Women’s Health Initiative that included 36,282 postmenopausal women who were taking 1000 mg of calcium and 400 IU of vitamin D3 daily for more than 5 years reported an HR of 0.62 (95% CI, 0.38-1.00) for hip fracture for supplementation vs placebo.27 Lastly, a 2016 Cochrane Review including 12 randomized trials and 1343 participants reported a 43% lower risk of new vertebral fractures following supplementation with calcium, vitamin D, or both compared with controls.28
Specific recommendations for calcium and vitamin D3 supplementation vary based on age and sex. The US Preventive Services Task Force concluded that insufficient evidence exists to support calcium and vitamin D3 supplementation in asymptomatic men and premenopausal women.29 The National Osteoporosis Foundation (NOF) supports the use of calcium supplementation for fracture risk reduction in middle-aged and older adults.4 Furthermore, the NOF supports the Institute of Medicine recommendations31 that men aged 50 to 70 years consume 1000 mg/d of calcium and that women 51 years and older as well as men 71 years and older consume 1200 mg/d of calcium.30 The NOF recommends 800 to 1000 IU/d of vitamin D in adults 50 years and older, while the Institute of Medicine recommends 600 IU/d in adults 70 years and younger and 800 IU/d in adults 71 years and older.31 These recommendations are similar to both the Endocrine Society and the American Geriatric Society.32,33 Total calcium should not exceed 2000 mg/d due to risk of adverse effects.
Dietary sources of vitamin D include fatty fish, mushrooms, and fortified dairy products, though recommended doses rarely can be achieved through diet alone.34 Dairy products are the primary source of dietary calcium. Other high-calcium foods include green leafy vegetables, nuts and seeds, soft-boned fish, and fortified beverages and cereals.35
Probiotics—A growing body of evidence suggests that probiotics may be beneficial in promoting bone health by improving calcium homeostasis, reducing risk for hyperparathyroidism secondary to GC therapy, and decreasing age-related bone resorption.36 An animal study demonstrated that probiotics can regulate bone resorption and formation as well as reduce bone loss secondary to GC therapy.37 A randomized, double-blind, placebo-controlled, multicenter trial randomly assigned 249 healthy, early postmenopausal women to receive probiotic treatment containing 3 lactobacillus strains (Lactobacillus paracasei DSM 13434, Lactobacillus plantarum DSM 15312, and L plantarum DSM 15313) or placebo once daily for 12 months.38 Bone mineral density was measured at baseline and at 12 months. Of the 234 participants who completed the study, lactobacillus treatment reduced lumbosacral BMD loss compared to the placebo group (mean difference, 0.71% [95% CI, 0.06-1.35]). They also reported significant lumbosacral BMD loss in the placebo group (−0.72% [95% CI, −1.22 to −0.22]) compared to no BMD loss in the group treated with lactobacillus (−0.01% [95% CI, −0.50 to 0.48]).38 Although the data may be encouraging, more studies are needed to determine if probiotics should be regarded as an adjuvant treatment to calcium, vitamin D, and pharmacologic therapy for long-term prevention of bone loss in the setting of GIO.39 Because existing studies on probiotics include varying compositions and doses, larger studies with consistent supplementation are required. Encouraging probiotic intake through fermented dairy products may represent a simple low-risk intervention to support bone health.
Anti-inflammatory Diet—The traditional Mediterranean diet is rich in fruits, vegetables, fish, nuts, whole grains, legumes, and monounsaturated fats and low in meat and dairy products. The Mediterranean diet has been shown to be modestly protective against osteoporosis and fracture risk. A large US observational study including 93,676 women showed that those with the highest quintile of the alternate Mediterranean diet score had a lower risk for hip fracture (HR, 0.80 [95% CI, 0.66-0.97]), with an absolute risk reduction of 0.29% and number needed to treat at 342.40 A multicenter study involving adults from 8 European countries found that increased adherence to the Mediterranean diet was associated with a 7% reduction in hip fracture incidence (HR per 1 unit increase in Mediterranean diet, 0.93 [95% CI, 0.89-0.98]). High vegetable and fruit intake was associated with decreased hip fracture incidence (HR, 0.86 and 0.89 [95% CI, 0.79-0.94 and 0.82-0.97, respectively]), and high meat and excessive ethanol consumption were associated with increased fracture incidence (HR, 1.18 and 1.74 [95% CI, 1.06-1.31 and 1.32-2.31, respectively]).41 Similarly, a large observational study in Sweden that included 37,903 men and 33,403 women reported similar findings, noting a 6% lower hip fracture rate per one unit increase in alternate Mediterranean diet score (adjusted HR, 0.94 [95% CI, 0.92-0.96]).42 This is thought to be due in part to higher levels of dietary vitamin D present in many foods traditionally included in the Mediterranean diet.43 Additionally, olive oil, a staple in the Mediterranean diet, appears to reduce bone loss by promoting osteoblast proliferation and maturation, inhibiting bone resorption, suppressing oxidative stress and inflammation, and increasing calcium deposition in the extracellular matrix.44,45 Fruits, vegetables, legumes, and nuts also are rich in minerals including potassium and magnesium, which are important in bone health to promote osteoblast proliferation and vitamin D activation.36,46-48
Final Thoughts
Osteoporosis-related fractures are common and are associated with high morbidity and health care costs. Dermatologists using and prescribing corticosteroids must be aware of the risk for GIO, particularly in patients with a pre-existing diagnosis of osteopenia or osteoporosis. There likely is no oral corticosteroid dose that does not increase a patient’s risk for osteoporosis; therefore, oral GCs should be used at the lowest effective daily dose for the shortest duration possible. Patients with an anticipated duration of at least 3 months—regardless of dose—should be assessed for their risk for GIO. Patients using topical and intralesional corticosteroids are unlikely to develop GIO; however, those with risk factors and a considerable cumulative dose may warrant further evaluation. In all cases, we advocate for supplementing with calcium and vitamin D as well as promoting probiotic intake and the Mediterranean diet. Those at moderate to high risk for fracture may require additional medical therapy. Dermatologists are uniquely positioned to identify this at-risk population, and because osteoporosis is a chronic illness, primary care providers should be notified of prolonged GC therapy to help with risk assessment, initiation of vitamin and mineral supplementation, and follow-up with metabolic bone health specialists. Through a multidisciplinary approach and patient education, GIO and the potential risk for fracture can be successfully mitigated in most patients.
- Weinstein RS. Clinical practice. glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62-70.
- Buckley L, Humphrey MB. Glucocorticoid-induced osteoporosis. N Engl J Med. 2018;379:2547-2556.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526.
- Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27:367-376.
- Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
- Caplan A, Fett N, Rosenbach M, et al. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76:1-9.
- Gudbjornsson B, Juliusson UI, Gudjonsson FV. Prevalence of long term steroid treatment and the frequency of decision making to prevent steroid induced osteoporosis in daily clinical practice. Ann Rheum Dis. 2002;61:32-36.
- Silverman S, Curtis J, Saag K, et al. International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteoporos Int. 2015;26:419-420.
- Canalis E, Bilezikian JP, Angeli A, et al. Perspectives on glucocorticoid-induced osteoporosis. Bone. 2004;34:593-598.
- Canalis E, Mazziotti G, Giustina A, et al. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319-1328.
- Lane NE, Yao W, Balooch M, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21:466-476.
- Hofbauer LC, Gori F, Riggs BL, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382-4389.
- Jia D, O’Brien CA, Stewart SA, et al. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592-5599.
- Mazziotti G, Angeli A, Bilezikian JP, et al. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab. 2006;17:144-149.
- Huybers S, Naber TH, Bindels RJ, et al. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am J Physiol Gastrointest Liver Physiol. 2007;292:G92-G97.
- Van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993-1000.
- Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15:323-328.
- Lupsa BC, Insogna KL, Micheletti RG, et al. Corticosteroid use in chronic dermatologic disorders and osteoporosis. Int J Womens Dermatol. 2021;7:545-551.
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2017;69:1095-1110.
- Egeberg A, Schwarz P, Harsløf T, et al. Association of potent and very potent topical corticosteroids and the risk of osteoporosis and major osteoporotic fractures. JAMA Dermatol. 2021;157:275-282.
- Jackson RD. Topical corticosteroids and glucocorticoid-induced osteoporosis-cumulative dose and duration matter. JAMA Dermatol. 2021;157:269-270.
- van Velsen SG, Haeck IM, Knol MJ, et al. Two-year assessment of effect of topical corticosteroids on bone mineral density in adults with moderate to severe atopic dermatitis. J Am Acad Dermatol. 2012;66:691-693.
- McGugan AD, Shuster S, Bottoms E. Adrenal suppression from intradermal triamcinolone. J Invest Dermatol. 1963;40:271-272.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Seo J, Lee YI, Hwang S, et al. Intramuscular triamcinolone acetonide: an undervalued option for refractory alopecia areata. J Dermatol. 2017;44:173-179.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Prentice RL, Pettinger MB, Jackson RD, et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567-580.
- Allen CS, Yeung JH, Vandermeer B, et al. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev. 2016;10:CD001347. doi:10.1002/14651858.CD001347.pub2
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1592-1599.
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381.
- Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Vitamin D fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated August 12, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Calcium fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated June 2, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/
- Muñoz-Garach A, García-Fontana B, Muñoz-Torres M. Nutrients and dietary patterns related to osteoporosis. Nutrients. 2020;12:1986.
- Schepper JD, Collins F, Rios-Arce ND, et al. Involvement of the gut microbiota and barrier function in glucocorticoid-induced osteoporosis. J Bone Miner Res. 2020;35:801-820.
- Jansson PA, Curiac D, Ahrén IL, et al. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019;1:E154-E162.
- Rizzoli R, Biver E. Are probiotics the new calcium and vitamin D for bone health? Curr Osteoporos Rep. 2020;18:273-284.
- Haring B, Crandall CJ, Wu C, et al. Dietary patterns and fractures in postmenopausal women: results from the Women’s Health Initiative. JAMA Intern Med. 2016;176:645-652.
- Benetou V, Orfanos P, Pettersson-Kymmer U, et al. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporos Int. 2013;24:1587-1598.
- Byberg L, Bellavia A, Larsson SC, et al. Mediterranean diet and hip fracture in Swedish men and women. J Bone Miner Res. 2016;31:2098-2105.
- Zupo R, Lampignano L, Lattanzio A, et al. Association between adherence to the Mediterranean diet and circulating vitamin D levels. Int J Food Sci Nutr. 2020;71:884-890.
- Chin KY, Ima-Nirwana S. Olives and bone: a green osteoporosis prevention option. Int J Environ Res Public Health. 2016;13:755.
- García-Martínez O, Rivas A, Ramos-Torrecillas J, et al. The effect of olive oil on osteoporosis prevention. Int J Food Sci Nutr. 2014;65:834-840.
- Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118:181-189.
- Veronese N, Stubbs B, Solmi M, et al. Dietary magnesium intake and fracture risk: data from a large prospective study. Br J Nutr. 2017;117:1570-1576.
- Kong SH, Kim JH, Hong AR, et al. Dietary potassium intake is beneficial to bone health in a low calcium intake population: the Korean National Health and Nutrition Examination Survey (KNHANES)(2008-2011). Osteoporos Int. 2017;28:1577-1585.
Glucocorticoids (GCs) are among the most widely prescribed medications in dermatologic practice. Although GCs are highly effective anti-inflammatory agents, long-term systemic therapy can result in dangerous adverse effects, including GC-induced osteoporosis (GIO), a bone disease associated with a heightened risk for fragility fractures.1,2 In the United States, an estimated 10.2 million adults have osteoporosis—defined as a T-score lower than −2.5 measured via a bone densitometry scan—and 43.4 million adults have low bone mineral density (BMD).3,4 The prevalence of osteoporosis is increasing, and the diagnosis is more common in females and adults 55 years and older.2 More than 2 million individuals have osteoporosis-related fractures annually, and the mortality risk is increased at 5 and 10 years following low-energy osteoporosis-related fractures.3-5
Glucocorticoid therapy is the leading iatrogenic cause of secondary osteoporosis. As many as 30% of all patients treated with systemic GCs for more than 6 months develop GIO.1,6,7 Glucocorticoid-induced BMD loss occurs at a rate of 6% to 12% of total BMD during the first year, slowing to approximately 3% per year during subsequent therapy.1 The risk for insufficiency fractures increases by as much as 75% from baseline in adults with rheumatic, pulmonary, and skin disorders within the first 3 months of therapy and peaks at approximately 12 months.1,2
Despite the risks, many long-term GC users never receive therapy to prevent bone loss; others are only started on therapy once they have sustained an insufficiency fracture. A 5-year international observational study including more than 40,000 postmenopausal women found that only 51% of patients who were on continuous GC therapy were undergoing BMD testing and appropriate medical management.8 This review highlights the existing evidence on the risks of osteoporosis and osteoporotic (OP) fractures in the setting of topical, intralesional, intramuscular, and systemic GC treatment, as well as recommendations for nutritional supplementation to reduce these risks.
Pathophysiology
The pathophysiology of GIO is multifactorial and occurs in both early and late phases.9,10 The early phase is characterized by rapid BMD reduction due to excessive bone resorption. The late phase is characterized by slower and more progressive BMD reduction due to impaired bone formation.9 At the osteocyte level, GCs decrease cell viability and induce apoptosis.11 At the osteoblast level, GCs impair cell replication and differentiation and have proapoptotic effects, resulting in decreased cell numbers and subsequent bone formation.10 At the osteoclast level, GCs increase expression of pro-osteoclastic cytokines and decrease mature osteoclast apoptosis, resulting in an expanded osteoclastic life span and prolonged bone resorption.12,13 Indirectly, GCs alter calcium metabolism by decreasing gastrointestinal calcium absorption and impairing renal absorption.14,15
GCs and Osteoporosis
Oral GCs—Glucocorticoid-induced osteoporosis and fracture risk are dose and duration dependent.6 A study of 244,235 patients taking GCs and 244,235 controls found the relative risk of vertebral fracture was 1.55 (range, 1.20–2.01) for daily prednisone use at less than 2.5 mg, 2.59 (range, 2.16–3.10) for daily prednisone use from 2.5 to 7.4 mg, and 5.18 (range, 4.25–6.31) for daily doses of 7.5 mg or higher; the relative risk for hip fractures was 0.99 (range, 0.82–1.20), 1.77 (range, 1.55–2.02), and 2.27 (range, 1.94–2.66), respectively.16 Another large retrospective cohort study found that continuous treatment with prednisone 10 mg/d for more than 90 days compared to no GC exposure increased the risk for hip fractures 7-fold and 17-fold for vertebral fractures.17 Although the minimum cumulative dose of GCs known to cause osteoporosis is not clearly established, the American College of Rheumatology has proposed an algorithm as a basic approach to anticipate, prevent, and treat GIO (Figure).18,19 Fracture risk should be assessed in all patients who are prescribed prednisone 2.5 mg/d for 3 months or longer or an anticipated cumulative dose of more than 1 g per year. Patients 40 years and older with anticipated GC use of 3 months or longer should have both a bone densitometry scan and a Fracture Risk Assessment (FRAX) score. The FRAX tool estimates the 10-year probability of fracture in patients aged 40 to 80 years, and those patients can be further risk stratified as low (FRAX <10%), moderate (FRAX 10%–19%), or high (FRAX ≥20%) risk. In patients with moderate to high risk of fracture (FRAX >10%), initiation of pharmacologic treatment or referral to a metabolic bone specialist should be considered.18,19 First-line therapy is an oral bisphosphonate, and second-line therapies include intravenous bisphosphonates, teriparatide, denosumab, or raloxifene for patients at high risk for GIO.19 Adults younger than 40 years with a history of OP fracture or considerable risk factors for OP fractures should have a bone densitometry scan, and, if results are abnormal, the patient should be referred to a metabolic bone specialist. Those with low fracture risk based on bone densitometry and FRAX and those with no risk factors should be assessed annually for bone health (additional risk factors, GC dose and duration, bone densitometry/FRAX if indicated).18 In addition to GC dose and duration, additional risk factors for GIO, which are factored into the FRAX tool, include advanced age, low body mass index, history of bone fracture, smoking, excessive alcohol use (≥3 drinks/d), history of falls, low BMD, family history of bone fracture, and hypovitaminosis D.6

Topical GCs—Although there is strong evidence and clear guidelines regarding oral GIO, there is a dearth of data surrounding OP risk due to treatment with topical GCs. A recent retrospective nationwide Danish study evaluating the risk of osteoporosis and major OP fracture in 723,251 adults treated with potent or very potent topical steroids sought to evaluate these risks.20 Patients were included if they had filled prescriptions of at least 500 g of topical mometasone or an equivalent alternative. The investigators reported a 3% increase in relative risk of osteoporosis and major OP fracture with doubling of the cumulative topical GC dose (hazard ratio [HR], 1.03 [95% CI, 1.02-1.04] for both). The overall population-attributable risk was 4.3% (95% CI, 2.7%-5.8%) for osteoporosis and 2.7% (95% CI, 1.7%-3.8%) for major OP fracture. Notably, at least 10,000 g of mometasone was required for 1 additional patient to have a major OP fracture.20 In a commentary based on this study, Jackson21 noted that the number of patient-years of topical GC use needed for 1 fracture was 4-fold higher than that for high-dose oral GCs (40 mg/d prednisolone for ≥30 days). Another study assessed the effects of topical GCs on BMD in adults with moderate to severe atopic dermatitis over a 2-year period.22 No significant difference in BMD assessed via bone densitometry of either the lumbar spine or total hip at baseline or at 2-year follow-up was reported for either group treated with corticosteroids (<75 g per month or ≥75 g per month). Of note, the authors did not account for steroid potency, which ranged from class 1 through class 4.22 Although limited data exist, these studies suggest topical GCs used at conventional doses with appropriate breaks in therapy will not substantially increase risk for GIO or OP fracture; however, in the small subset of patients requiring chronic use of superpotent topical corticosteroids with other OP risk factors, transitioning to non–GC-based therapy or initiating bone health therapy may be advised to improve patient outcomes. Risk assessment, as in cases of chronic topical GC use, may be beneficial.
Intralesional GCs—Intralesional GCs are indicated for numerous inflammatory conditions including alopecia areata, discoid lupus erythematosus, keloids, and granuloma annulare. It generally is accepted that doses of triamcinolone acetonide should not exceed 20 mg per session spaced at least 3 weeks apart or up to 40 mg per month.18 One study demonstrated that doses of triamcinolone diacetate of 25 mg or less were unlikely to produce systemic effects and were determined to be a safe dose for intralesional injections.23 A retrospective cross-sectional case series including 18 patients with alopecia areata reported decreased BMD in 9 patients receiving intralesional triamcinolone acetonide 10 mg/mL at 4- to 8-week intervals for at least 20 months, with cumulative doses greater than 500 mg. This was particularly notable in postmenopausal women and men older than 50 years; participants with a body mass index less than 18.5 kg/m2, history of a stress fracture, family history of osteopenia or osteoporosis, and history of smoking; and those who did not regularly engage in weight-bearing exercises.24 Patients receiving long-term (ie, >1 year) intralesional steroids should be evaluated for osteoporosis risk and preventative strategies should be considered (ie, regular weight-bearing exercises, calcium and vitamin D supplementation, bisphosphate therapy). As with topical GCs, there are no clear guidelines for risk assessment or treatment recommendations for GIO.
Intramuscular GCs—The data regarding intramuscular (IM) GCs and dermatologic disease is severely limited, and to the best of our knowledge, no studies specifically assess the risk for GIO or fracture secondary to intramuscular GCs; however, a retrospective study of 27 patients (4 female, 23 male; mean age, 33 years [range, 12–61 years]) with refractory alopecia areata receiving IM triamcinolone acetonide (40 mg every 4 weeks for 3–6 months) reported 1 patient (a 56-year-old woman) with notably decreased bone densitometry from baseline requiring treatment discontinuation.25 No other patients at risk for osteoporosis had decreased BMD from treatment with IM triamcinolone; however, it was noted that 1 month following treatment, 10 of 11 assessed patients demonstrated decreased levels of morning serum cortisol and plasma adrenocorticotropic hormone—despite baseline levels within reference range—that resolved 3 months after treatment completion,25 which suggests a prolonged release of IM triamcinolone and sustained systemic effect. One systematic review of 342 patients with dermatologic diseases treated with IM corticosteroids found the primary side effects included dysmenorrhea, injection-site lipoatrophy, and adrenocortical suppression, with only a single reported case of low BMD.26 Given the paucity of evidence, additional studies are required to assess the effect of IM triamcinolone on BMD and risk for major OP fractures with regard to dosing and frequency. As there are no clear guidelines for osteoporosis evaluation in the setting of intramuscular GCs, it may be prudent to follow the algorithmic model recommended for oral steroids when anticipating at least 3 months of intramuscular GCs.
Diet and Prevention of Bone Loss
Given the profound impact that systemic GCs have on osteoporosis and fracture risk and the sparse data regarding risk from topical, intralesional, or intramuscular GCs, diet and nutrition represent a simple, safe, and potentially preventative method of slowing BMD loss and minimizing fracture risk. In higher-risk patients, nutritional assessment in combination with medical therapy also is likely warranted.
Calcium and Vitamin D3—Patients treated with any GC dose longer than 3 months should undergo calcium and vitamin D optimization.19 Exceptions for supplementation include certain patients with sarcoidosis, which can be associated with high vitamin D levels; patients with a history of hypercalcemia or hypercalciuria; and patients with chronic kidney disease.6 In a meta-analysis including 30,970 patients in 8 randomized controlled trials, calcium (500–1200 mg/d) and vitamin D (400–800 IU/d) supplementation reduced the risk of total fractures by 15% (summary relative risk estimate, 0.85 [95% CI, 0.73-0.98]) and hip fractures by 30% (summary relative risk estimate, 0.70 [95% CI, 0.56-0.87]).4 One double-blind, placebo-controlled clinical trial conducted by the Women’s Health Initiative that included 36,282 postmenopausal women who were taking 1000 mg of calcium and 400 IU of vitamin D3 daily for more than 5 years reported an HR of 0.62 (95% CI, 0.38-1.00) for hip fracture for supplementation vs placebo.27 Lastly, a 2016 Cochrane Review including 12 randomized trials and 1343 participants reported a 43% lower risk of new vertebral fractures following supplementation with calcium, vitamin D, or both compared with controls.28
Specific recommendations for calcium and vitamin D3 supplementation vary based on age and sex. The US Preventive Services Task Force concluded that insufficient evidence exists to support calcium and vitamin D3 supplementation in asymptomatic men and premenopausal women.29 The National Osteoporosis Foundation (NOF) supports the use of calcium supplementation for fracture risk reduction in middle-aged and older adults.4 Furthermore, the NOF supports the Institute of Medicine recommendations31 that men aged 50 to 70 years consume 1000 mg/d of calcium and that women 51 years and older as well as men 71 years and older consume 1200 mg/d of calcium.30 The NOF recommends 800 to 1000 IU/d of vitamin D in adults 50 years and older, while the Institute of Medicine recommends 600 IU/d in adults 70 years and younger and 800 IU/d in adults 71 years and older.31 These recommendations are similar to both the Endocrine Society and the American Geriatric Society.32,33 Total calcium should not exceed 2000 mg/d due to risk of adverse effects.
Dietary sources of vitamin D include fatty fish, mushrooms, and fortified dairy products, though recommended doses rarely can be achieved through diet alone.34 Dairy products are the primary source of dietary calcium. Other high-calcium foods include green leafy vegetables, nuts and seeds, soft-boned fish, and fortified beverages and cereals.35
Probiotics—A growing body of evidence suggests that probiotics may be beneficial in promoting bone health by improving calcium homeostasis, reducing risk for hyperparathyroidism secondary to GC therapy, and decreasing age-related bone resorption.36 An animal study demonstrated that probiotics can regulate bone resorption and formation as well as reduce bone loss secondary to GC therapy.37 A randomized, double-blind, placebo-controlled, multicenter trial randomly assigned 249 healthy, early postmenopausal women to receive probiotic treatment containing 3 lactobacillus strains (Lactobacillus paracasei DSM 13434, Lactobacillus plantarum DSM 15312, and L plantarum DSM 15313) or placebo once daily for 12 months.38 Bone mineral density was measured at baseline and at 12 months. Of the 234 participants who completed the study, lactobacillus treatment reduced lumbosacral BMD loss compared to the placebo group (mean difference, 0.71% [95% CI, 0.06-1.35]). They also reported significant lumbosacral BMD loss in the placebo group (−0.72% [95% CI, −1.22 to −0.22]) compared to no BMD loss in the group treated with lactobacillus (−0.01% [95% CI, −0.50 to 0.48]).38 Although the data may be encouraging, more studies are needed to determine if probiotics should be regarded as an adjuvant treatment to calcium, vitamin D, and pharmacologic therapy for long-term prevention of bone loss in the setting of GIO.39 Because existing studies on probiotics include varying compositions and doses, larger studies with consistent supplementation are required. Encouraging probiotic intake through fermented dairy products may represent a simple low-risk intervention to support bone health.
Anti-inflammatory Diet—The traditional Mediterranean diet is rich in fruits, vegetables, fish, nuts, whole grains, legumes, and monounsaturated fats and low in meat and dairy products. The Mediterranean diet has been shown to be modestly protective against osteoporosis and fracture risk. A large US observational study including 93,676 women showed that those with the highest quintile of the alternate Mediterranean diet score had a lower risk for hip fracture (HR, 0.80 [95% CI, 0.66-0.97]), with an absolute risk reduction of 0.29% and number needed to treat at 342.40 A multicenter study involving adults from 8 European countries found that increased adherence to the Mediterranean diet was associated with a 7% reduction in hip fracture incidence (HR per 1 unit increase in Mediterranean diet, 0.93 [95% CI, 0.89-0.98]). High vegetable and fruit intake was associated with decreased hip fracture incidence (HR, 0.86 and 0.89 [95% CI, 0.79-0.94 and 0.82-0.97, respectively]), and high meat and excessive ethanol consumption were associated with increased fracture incidence (HR, 1.18 and 1.74 [95% CI, 1.06-1.31 and 1.32-2.31, respectively]).41 Similarly, a large observational study in Sweden that included 37,903 men and 33,403 women reported similar findings, noting a 6% lower hip fracture rate per one unit increase in alternate Mediterranean diet score (adjusted HR, 0.94 [95% CI, 0.92-0.96]).42 This is thought to be due in part to higher levels of dietary vitamin D present in many foods traditionally included in the Mediterranean diet.43 Additionally, olive oil, a staple in the Mediterranean diet, appears to reduce bone loss by promoting osteoblast proliferation and maturation, inhibiting bone resorption, suppressing oxidative stress and inflammation, and increasing calcium deposition in the extracellular matrix.44,45 Fruits, vegetables, legumes, and nuts also are rich in minerals including potassium and magnesium, which are important in bone health to promote osteoblast proliferation and vitamin D activation.36,46-48
Final Thoughts
Osteoporosis-related fractures are common and are associated with high morbidity and health care costs. Dermatologists using and prescribing corticosteroids must be aware of the risk for GIO, particularly in patients with a pre-existing diagnosis of osteopenia or osteoporosis. There likely is no oral corticosteroid dose that does not increase a patient’s risk for osteoporosis; therefore, oral GCs should be used at the lowest effective daily dose for the shortest duration possible. Patients with an anticipated duration of at least 3 months—regardless of dose—should be assessed for their risk for GIO. Patients using topical and intralesional corticosteroids are unlikely to develop GIO; however, those with risk factors and a considerable cumulative dose may warrant further evaluation. In all cases, we advocate for supplementing with calcium and vitamin D as well as promoting probiotic intake and the Mediterranean diet. Those at moderate to high risk for fracture may require additional medical therapy. Dermatologists are uniquely positioned to identify this at-risk population, and because osteoporosis is a chronic illness, primary care providers should be notified of prolonged GC therapy to help with risk assessment, initiation of vitamin and mineral supplementation, and follow-up with metabolic bone health specialists. Through a multidisciplinary approach and patient education, GIO and the potential risk for fracture can be successfully mitigated in most patients.
Glucocorticoids (GCs) are among the most widely prescribed medications in dermatologic practice. Although GCs are highly effective anti-inflammatory agents, long-term systemic therapy can result in dangerous adverse effects, including GC-induced osteoporosis (GIO), a bone disease associated with a heightened risk for fragility fractures.1,2 In the United States, an estimated 10.2 million adults have osteoporosis—defined as a T-score lower than −2.5 measured via a bone densitometry scan—and 43.4 million adults have low bone mineral density (BMD).3,4 The prevalence of osteoporosis is increasing, and the diagnosis is more common in females and adults 55 years and older.2 More than 2 million individuals have osteoporosis-related fractures annually, and the mortality risk is increased at 5 and 10 years following low-energy osteoporosis-related fractures.3-5
Glucocorticoid therapy is the leading iatrogenic cause of secondary osteoporosis. As many as 30% of all patients treated with systemic GCs for more than 6 months develop GIO.1,6,7 Glucocorticoid-induced BMD loss occurs at a rate of 6% to 12% of total BMD during the first year, slowing to approximately 3% per year during subsequent therapy.1 The risk for insufficiency fractures increases by as much as 75% from baseline in adults with rheumatic, pulmonary, and skin disorders within the first 3 months of therapy and peaks at approximately 12 months.1,2
Despite the risks, many long-term GC users never receive therapy to prevent bone loss; others are only started on therapy once they have sustained an insufficiency fracture. A 5-year international observational study including more than 40,000 postmenopausal women found that only 51% of patients who were on continuous GC therapy were undergoing BMD testing and appropriate medical management.8 This review highlights the existing evidence on the risks of osteoporosis and osteoporotic (OP) fractures in the setting of topical, intralesional, intramuscular, and systemic GC treatment, as well as recommendations for nutritional supplementation to reduce these risks.
Pathophysiology
The pathophysiology of GIO is multifactorial and occurs in both early and late phases.9,10 The early phase is characterized by rapid BMD reduction due to excessive bone resorption. The late phase is characterized by slower and more progressive BMD reduction due to impaired bone formation.9 At the osteocyte level, GCs decrease cell viability and induce apoptosis.11 At the osteoblast level, GCs impair cell replication and differentiation and have proapoptotic effects, resulting in decreased cell numbers and subsequent bone formation.10 At the osteoclast level, GCs increase expression of pro-osteoclastic cytokines and decrease mature osteoclast apoptosis, resulting in an expanded osteoclastic life span and prolonged bone resorption.12,13 Indirectly, GCs alter calcium metabolism by decreasing gastrointestinal calcium absorption and impairing renal absorption.14,15
GCs and Osteoporosis
Oral GCs—Glucocorticoid-induced osteoporosis and fracture risk are dose and duration dependent.6 A study of 244,235 patients taking GCs and 244,235 controls found the relative risk of vertebral fracture was 1.55 (range, 1.20–2.01) for daily prednisone use at less than 2.5 mg, 2.59 (range, 2.16–3.10) for daily prednisone use from 2.5 to 7.4 mg, and 5.18 (range, 4.25–6.31) for daily doses of 7.5 mg or higher; the relative risk for hip fractures was 0.99 (range, 0.82–1.20), 1.77 (range, 1.55–2.02), and 2.27 (range, 1.94–2.66), respectively.16 Another large retrospective cohort study found that continuous treatment with prednisone 10 mg/d for more than 90 days compared to no GC exposure increased the risk for hip fractures 7-fold and 17-fold for vertebral fractures.17 Although the minimum cumulative dose of GCs known to cause osteoporosis is not clearly established, the American College of Rheumatology has proposed an algorithm as a basic approach to anticipate, prevent, and treat GIO (Figure).18,19 Fracture risk should be assessed in all patients who are prescribed prednisone 2.5 mg/d for 3 months or longer or an anticipated cumulative dose of more than 1 g per year. Patients 40 years and older with anticipated GC use of 3 months or longer should have both a bone densitometry scan and a Fracture Risk Assessment (FRAX) score. The FRAX tool estimates the 10-year probability of fracture in patients aged 40 to 80 years, and those patients can be further risk stratified as low (FRAX <10%), moderate (FRAX 10%–19%), or high (FRAX ≥20%) risk. In patients with moderate to high risk of fracture (FRAX >10%), initiation of pharmacologic treatment or referral to a metabolic bone specialist should be considered.18,19 First-line therapy is an oral bisphosphonate, and second-line therapies include intravenous bisphosphonates, teriparatide, denosumab, or raloxifene for patients at high risk for GIO.19 Adults younger than 40 years with a history of OP fracture or considerable risk factors for OP fractures should have a bone densitometry scan, and, if results are abnormal, the patient should be referred to a metabolic bone specialist. Those with low fracture risk based on bone densitometry and FRAX and those with no risk factors should be assessed annually for bone health (additional risk factors, GC dose and duration, bone densitometry/FRAX if indicated).18 In addition to GC dose and duration, additional risk factors for GIO, which are factored into the FRAX tool, include advanced age, low body mass index, history of bone fracture, smoking, excessive alcohol use (≥3 drinks/d), history of falls, low BMD, family history of bone fracture, and hypovitaminosis D.6

Topical GCs—Although there is strong evidence and clear guidelines regarding oral GIO, there is a dearth of data surrounding OP risk due to treatment with topical GCs. A recent retrospective nationwide Danish study evaluating the risk of osteoporosis and major OP fracture in 723,251 adults treated with potent or very potent topical steroids sought to evaluate these risks.20 Patients were included if they had filled prescriptions of at least 500 g of topical mometasone or an equivalent alternative. The investigators reported a 3% increase in relative risk of osteoporosis and major OP fracture with doubling of the cumulative topical GC dose (hazard ratio [HR], 1.03 [95% CI, 1.02-1.04] for both). The overall population-attributable risk was 4.3% (95% CI, 2.7%-5.8%) for osteoporosis and 2.7% (95% CI, 1.7%-3.8%) for major OP fracture. Notably, at least 10,000 g of mometasone was required for 1 additional patient to have a major OP fracture.20 In a commentary based on this study, Jackson21 noted that the number of patient-years of topical GC use needed for 1 fracture was 4-fold higher than that for high-dose oral GCs (40 mg/d prednisolone for ≥30 days). Another study assessed the effects of topical GCs on BMD in adults with moderate to severe atopic dermatitis over a 2-year period.22 No significant difference in BMD assessed via bone densitometry of either the lumbar spine or total hip at baseline or at 2-year follow-up was reported for either group treated with corticosteroids (<75 g per month or ≥75 g per month). Of note, the authors did not account for steroid potency, which ranged from class 1 through class 4.22 Although limited data exist, these studies suggest topical GCs used at conventional doses with appropriate breaks in therapy will not substantially increase risk for GIO or OP fracture; however, in the small subset of patients requiring chronic use of superpotent topical corticosteroids with other OP risk factors, transitioning to non–GC-based therapy or initiating bone health therapy may be advised to improve patient outcomes. Risk assessment, as in cases of chronic topical GC use, may be beneficial.
Intralesional GCs—Intralesional GCs are indicated for numerous inflammatory conditions including alopecia areata, discoid lupus erythematosus, keloids, and granuloma annulare. It generally is accepted that doses of triamcinolone acetonide should not exceed 20 mg per session spaced at least 3 weeks apart or up to 40 mg per month.18 One study demonstrated that doses of triamcinolone diacetate of 25 mg or less were unlikely to produce systemic effects and were determined to be a safe dose for intralesional injections.23 A retrospective cross-sectional case series including 18 patients with alopecia areata reported decreased BMD in 9 patients receiving intralesional triamcinolone acetonide 10 mg/mL at 4- to 8-week intervals for at least 20 months, with cumulative doses greater than 500 mg. This was particularly notable in postmenopausal women and men older than 50 years; participants with a body mass index less than 18.5 kg/m2, history of a stress fracture, family history of osteopenia or osteoporosis, and history of smoking; and those who did not regularly engage in weight-bearing exercises.24 Patients receiving long-term (ie, >1 year) intralesional steroids should be evaluated for osteoporosis risk and preventative strategies should be considered (ie, regular weight-bearing exercises, calcium and vitamin D supplementation, bisphosphate therapy). As with topical GCs, there are no clear guidelines for risk assessment or treatment recommendations for GIO.
Intramuscular GCs—The data regarding intramuscular (IM) GCs and dermatologic disease is severely limited, and to the best of our knowledge, no studies specifically assess the risk for GIO or fracture secondary to intramuscular GCs; however, a retrospective study of 27 patients (4 female, 23 male; mean age, 33 years [range, 12–61 years]) with refractory alopecia areata receiving IM triamcinolone acetonide (40 mg every 4 weeks for 3–6 months) reported 1 patient (a 56-year-old woman) with notably decreased bone densitometry from baseline requiring treatment discontinuation.25 No other patients at risk for osteoporosis had decreased BMD from treatment with IM triamcinolone; however, it was noted that 1 month following treatment, 10 of 11 assessed patients demonstrated decreased levels of morning serum cortisol and plasma adrenocorticotropic hormone—despite baseline levels within reference range—that resolved 3 months after treatment completion,25 which suggests a prolonged release of IM triamcinolone and sustained systemic effect. One systematic review of 342 patients with dermatologic diseases treated with IM corticosteroids found the primary side effects included dysmenorrhea, injection-site lipoatrophy, and adrenocortical suppression, with only a single reported case of low BMD.26 Given the paucity of evidence, additional studies are required to assess the effect of IM triamcinolone on BMD and risk for major OP fractures with regard to dosing and frequency. As there are no clear guidelines for osteoporosis evaluation in the setting of intramuscular GCs, it may be prudent to follow the algorithmic model recommended for oral steroids when anticipating at least 3 months of intramuscular GCs.
Diet and Prevention of Bone Loss
Given the profound impact that systemic GCs have on osteoporosis and fracture risk and the sparse data regarding risk from topical, intralesional, or intramuscular GCs, diet and nutrition represent a simple, safe, and potentially preventative method of slowing BMD loss and minimizing fracture risk. In higher-risk patients, nutritional assessment in combination with medical therapy also is likely warranted.
Calcium and Vitamin D3—Patients treated with any GC dose longer than 3 months should undergo calcium and vitamin D optimization.19 Exceptions for supplementation include certain patients with sarcoidosis, which can be associated with high vitamin D levels; patients with a history of hypercalcemia or hypercalciuria; and patients with chronic kidney disease.6 In a meta-analysis including 30,970 patients in 8 randomized controlled trials, calcium (500–1200 mg/d) and vitamin D (400–800 IU/d) supplementation reduced the risk of total fractures by 15% (summary relative risk estimate, 0.85 [95% CI, 0.73-0.98]) and hip fractures by 30% (summary relative risk estimate, 0.70 [95% CI, 0.56-0.87]).4 One double-blind, placebo-controlled clinical trial conducted by the Women’s Health Initiative that included 36,282 postmenopausal women who were taking 1000 mg of calcium and 400 IU of vitamin D3 daily for more than 5 years reported an HR of 0.62 (95% CI, 0.38-1.00) for hip fracture for supplementation vs placebo.27 Lastly, a 2016 Cochrane Review including 12 randomized trials and 1343 participants reported a 43% lower risk of new vertebral fractures following supplementation with calcium, vitamin D, or both compared with controls.28
Specific recommendations for calcium and vitamin D3 supplementation vary based on age and sex. The US Preventive Services Task Force concluded that insufficient evidence exists to support calcium and vitamin D3 supplementation in asymptomatic men and premenopausal women.29 The National Osteoporosis Foundation (NOF) supports the use of calcium supplementation for fracture risk reduction in middle-aged and older adults.4 Furthermore, the NOF supports the Institute of Medicine recommendations31 that men aged 50 to 70 years consume 1000 mg/d of calcium and that women 51 years and older as well as men 71 years and older consume 1200 mg/d of calcium.30 The NOF recommends 800 to 1000 IU/d of vitamin D in adults 50 years and older, while the Institute of Medicine recommends 600 IU/d in adults 70 years and younger and 800 IU/d in adults 71 years and older.31 These recommendations are similar to both the Endocrine Society and the American Geriatric Society.32,33 Total calcium should not exceed 2000 mg/d due to risk of adverse effects.
Dietary sources of vitamin D include fatty fish, mushrooms, and fortified dairy products, though recommended doses rarely can be achieved through diet alone.34 Dairy products are the primary source of dietary calcium. Other high-calcium foods include green leafy vegetables, nuts and seeds, soft-boned fish, and fortified beverages and cereals.35
Probiotics—A growing body of evidence suggests that probiotics may be beneficial in promoting bone health by improving calcium homeostasis, reducing risk for hyperparathyroidism secondary to GC therapy, and decreasing age-related bone resorption.36 An animal study demonstrated that probiotics can regulate bone resorption and formation as well as reduce bone loss secondary to GC therapy.37 A randomized, double-blind, placebo-controlled, multicenter trial randomly assigned 249 healthy, early postmenopausal women to receive probiotic treatment containing 3 lactobacillus strains (Lactobacillus paracasei DSM 13434, Lactobacillus plantarum DSM 15312, and L plantarum DSM 15313) or placebo once daily for 12 months.38 Bone mineral density was measured at baseline and at 12 months. Of the 234 participants who completed the study, lactobacillus treatment reduced lumbosacral BMD loss compared to the placebo group (mean difference, 0.71% [95% CI, 0.06-1.35]). They also reported significant lumbosacral BMD loss in the placebo group (−0.72% [95% CI, −1.22 to −0.22]) compared to no BMD loss in the group treated with lactobacillus (−0.01% [95% CI, −0.50 to 0.48]).38 Although the data may be encouraging, more studies are needed to determine if probiotics should be regarded as an adjuvant treatment to calcium, vitamin D, and pharmacologic therapy for long-term prevention of bone loss in the setting of GIO.39 Because existing studies on probiotics include varying compositions and doses, larger studies with consistent supplementation are required. Encouraging probiotic intake through fermented dairy products may represent a simple low-risk intervention to support bone health.
Anti-inflammatory Diet—The traditional Mediterranean diet is rich in fruits, vegetables, fish, nuts, whole grains, legumes, and monounsaturated fats and low in meat and dairy products. The Mediterranean diet has been shown to be modestly protective against osteoporosis and fracture risk. A large US observational study including 93,676 women showed that those with the highest quintile of the alternate Mediterranean diet score had a lower risk for hip fracture (HR, 0.80 [95% CI, 0.66-0.97]), with an absolute risk reduction of 0.29% and number needed to treat at 342.40 A multicenter study involving adults from 8 European countries found that increased adherence to the Mediterranean diet was associated with a 7% reduction in hip fracture incidence (HR per 1 unit increase in Mediterranean diet, 0.93 [95% CI, 0.89-0.98]). High vegetable and fruit intake was associated with decreased hip fracture incidence (HR, 0.86 and 0.89 [95% CI, 0.79-0.94 and 0.82-0.97, respectively]), and high meat and excessive ethanol consumption were associated with increased fracture incidence (HR, 1.18 and 1.74 [95% CI, 1.06-1.31 and 1.32-2.31, respectively]).41 Similarly, a large observational study in Sweden that included 37,903 men and 33,403 women reported similar findings, noting a 6% lower hip fracture rate per one unit increase in alternate Mediterranean diet score (adjusted HR, 0.94 [95% CI, 0.92-0.96]).42 This is thought to be due in part to higher levels of dietary vitamin D present in many foods traditionally included in the Mediterranean diet.43 Additionally, olive oil, a staple in the Mediterranean diet, appears to reduce bone loss by promoting osteoblast proliferation and maturation, inhibiting bone resorption, suppressing oxidative stress and inflammation, and increasing calcium deposition in the extracellular matrix.44,45 Fruits, vegetables, legumes, and nuts also are rich in minerals including potassium and magnesium, which are important in bone health to promote osteoblast proliferation and vitamin D activation.36,46-48
Final Thoughts
Osteoporosis-related fractures are common and are associated with high morbidity and health care costs. Dermatologists using and prescribing corticosteroids must be aware of the risk for GIO, particularly in patients with a pre-existing diagnosis of osteopenia or osteoporosis. There likely is no oral corticosteroid dose that does not increase a patient’s risk for osteoporosis; therefore, oral GCs should be used at the lowest effective daily dose for the shortest duration possible. Patients with an anticipated duration of at least 3 months—regardless of dose—should be assessed for their risk for GIO. Patients using topical and intralesional corticosteroids are unlikely to develop GIO; however, those with risk factors and a considerable cumulative dose may warrant further evaluation. In all cases, we advocate for supplementing with calcium and vitamin D as well as promoting probiotic intake and the Mediterranean diet. Those at moderate to high risk for fracture may require additional medical therapy. Dermatologists are uniquely positioned to identify this at-risk population, and because osteoporosis is a chronic illness, primary care providers should be notified of prolonged GC therapy to help with risk assessment, initiation of vitamin and mineral supplementation, and follow-up with metabolic bone health specialists. Through a multidisciplinary approach and patient education, GIO and the potential risk for fracture can be successfully mitigated in most patients.
- Weinstein RS. Clinical practice. glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62-70.
- Buckley L, Humphrey MB. Glucocorticoid-induced osteoporosis. N Engl J Med. 2018;379:2547-2556.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526.
- Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27:367-376.
- Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
- Caplan A, Fett N, Rosenbach M, et al. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76:1-9.
- Gudbjornsson B, Juliusson UI, Gudjonsson FV. Prevalence of long term steroid treatment and the frequency of decision making to prevent steroid induced osteoporosis in daily clinical practice. Ann Rheum Dis. 2002;61:32-36.
- Silverman S, Curtis J, Saag K, et al. International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteoporos Int. 2015;26:419-420.
- Canalis E, Bilezikian JP, Angeli A, et al. Perspectives on glucocorticoid-induced osteoporosis. Bone. 2004;34:593-598.
- Canalis E, Mazziotti G, Giustina A, et al. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319-1328.
- Lane NE, Yao W, Balooch M, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21:466-476.
- Hofbauer LC, Gori F, Riggs BL, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382-4389.
- Jia D, O’Brien CA, Stewart SA, et al. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592-5599.
- Mazziotti G, Angeli A, Bilezikian JP, et al. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab. 2006;17:144-149.
- Huybers S, Naber TH, Bindels RJ, et al. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am J Physiol Gastrointest Liver Physiol. 2007;292:G92-G97.
- Van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993-1000.
- Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15:323-328.
- Lupsa BC, Insogna KL, Micheletti RG, et al. Corticosteroid use in chronic dermatologic disorders and osteoporosis. Int J Womens Dermatol. 2021;7:545-551.
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2017;69:1095-1110.
- Egeberg A, Schwarz P, Harsløf T, et al. Association of potent and very potent topical corticosteroids and the risk of osteoporosis and major osteoporotic fractures. JAMA Dermatol. 2021;157:275-282.
- Jackson RD. Topical corticosteroids and glucocorticoid-induced osteoporosis-cumulative dose and duration matter. JAMA Dermatol. 2021;157:269-270.
- van Velsen SG, Haeck IM, Knol MJ, et al. Two-year assessment of effect of topical corticosteroids on bone mineral density in adults with moderate to severe atopic dermatitis. J Am Acad Dermatol. 2012;66:691-693.
- McGugan AD, Shuster S, Bottoms E. Adrenal suppression from intradermal triamcinolone. J Invest Dermatol. 1963;40:271-272.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Seo J, Lee YI, Hwang S, et al. Intramuscular triamcinolone acetonide: an undervalued option for refractory alopecia areata. J Dermatol. 2017;44:173-179.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Prentice RL, Pettinger MB, Jackson RD, et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567-580.
- Allen CS, Yeung JH, Vandermeer B, et al. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev. 2016;10:CD001347. doi:10.1002/14651858.CD001347.pub2
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1592-1599.
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381.
- Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Vitamin D fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated August 12, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Calcium fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated June 2, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/
- Muñoz-Garach A, García-Fontana B, Muñoz-Torres M. Nutrients and dietary patterns related to osteoporosis. Nutrients. 2020;12:1986.
- Schepper JD, Collins F, Rios-Arce ND, et al. Involvement of the gut microbiota and barrier function in glucocorticoid-induced osteoporosis. J Bone Miner Res. 2020;35:801-820.
- Jansson PA, Curiac D, Ahrén IL, et al. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019;1:E154-E162.
- Rizzoli R, Biver E. Are probiotics the new calcium and vitamin D for bone health? Curr Osteoporos Rep. 2020;18:273-284.
- Haring B, Crandall CJ, Wu C, et al. Dietary patterns and fractures in postmenopausal women: results from the Women’s Health Initiative. JAMA Intern Med. 2016;176:645-652.
- Benetou V, Orfanos P, Pettersson-Kymmer U, et al. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporos Int. 2013;24:1587-1598.
- Byberg L, Bellavia A, Larsson SC, et al. Mediterranean diet and hip fracture in Swedish men and women. J Bone Miner Res. 2016;31:2098-2105.
- Zupo R, Lampignano L, Lattanzio A, et al. Association between adherence to the Mediterranean diet and circulating vitamin D levels. Int J Food Sci Nutr. 2020;71:884-890.
- Chin KY, Ima-Nirwana S. Olives and bone: a green osteoporosis prevention option. Int J Environ Res Public Health. 2016;13:755.
- García-Martínez O, Rivas A, Ramos-Torrecillas J, et al. The effect of olive oil on osteoporosis prevention. Int J Food Sci Nutr. 2014;65:834-840.
- Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118:181-189.
- Veronese N, Stubbs B, Solmi M, et al. Dietary magnesium intake and fracture risk: data from a large prospective study. Br J Nutr. 2017;117:1570-1576.
- Kong SH, Kim JH, Hong AR, et al. Dietary potassium intake is beneficial to bone health in a low calcium intake population: the Korean National Health and Nutrition Examination Survey (KNHANES)(2008-2011). Osteoporos Int. 2017;28:1577-1585.
- Weinstein RS. Clinical practice. glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62-70.
- Buckley L, Humphrey MB. Glucocorticoid-induced osteoporosis. N Engl J Med. 2018;379:2547-2556.
- Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526.
- Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27:367-376.
- Bliuc D, Nguyen ND, Milch VE, et al. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513-521.
- Caplan A, Fett N, Rosenbach M, et al. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76:1-9.
- Gudbjornsson B, Juliusson UI, Gudjonsson FV. Prevalence of long term steroid treatment and the frequency of decision making to prevent steroid induced osteoporosis in daily clinical practice. Ann Rheum Dis. 2002;61:32-36.
- Silverman S, Curtis J, Saag K, et al. International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteoporos Int. 2015;26:419-420.
- Canalis E, Bilezikian JP, Angeli A, et al. Perspectives on glucocorticoid-induced osteoporosis. Bone. 2004;34:593-598.
- Canalis E, Mazziotti G, Giustina A, et al. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319-1328.
- Lane NE, Yao W, Balooch M, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21:466-476.
- Hofbauer LC, Gori F, Riggs BL, et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382-4389.
- Jia D, O’Brien CA, Stewart SA, et al. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592-5599.
- Mazziotti G, Angeli A, Bilezikian JP, et al. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab. 2006;17:144-149.
- Huybers S, Naber TH, Bindels RJ, et al. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am J Physiol Gastrointest Liver Physiol. 2007;292:G92-G97.
- Van Staa TP, Leufkens HG, Abenhaim L, et al. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993-1000.
- Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15:323-328.
- Lupsa BC, Insogna KL, Micheletti RG, et al. Corticosteroid use in chronic dermatologic disorders and osteoporosis. Int J Womens Dermatol. 2021;7:545-551.
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2017;69:1095-1110.
- Egeberg A, Schwarz P, Harsløf T, et al. Association of potent and very potent topical corticosteroids and the risk of osteoporosis and major osteoporotic fractures. JAMA Dermatol. 2021;157:275-282.
- Jackson RD. Topical corticosteroids and glucocorticoid-induced osteoporosis-cumulative dose and duration matter. JAMA Dermatol. 2021;157:269-270.
- van Velsen SG, Haeck IM, Knol MJ, et al. Two-year assessment of effect of topical corticosteroids on bone mineral density in adults with moderate to severe atopic dermatitis. J Am Acad Dermatol. 2012;66:691-693.
- McGugan AD, Shuster S, Bottoms E. Adrenal suppression from intradermal triamcinolone. J Invest Dermatol. 1963;40:271-272.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Seo J, Lee YI, Hwang S, et al. Intramuscular triamcinolone acetonide: an undervalued option for refractory alopecia areata. J Dermatol. 2017;44:173-179.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Prentice RL, Pettinger MB, Jackson RD, et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567-580.
- Allen CS, Yeung JH, Vandermeer B, et al. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev. 2016;10:CD001347. doi:10.1002/14651858.CD001347.pub2
- US Preventive Services Task Force; Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1592-1599.
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359-2381.
- Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147-152.
- Vitamin D fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated August 12, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Calcium fact sheet for health professionals. National Institutes of Health Office of Dietary Supplements website. Updated June 2, 2022. Accessed September 16, 2022. https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/
- Muñoz-Garach A, García-Fontana B, Muñoz-Torres M. Nutrients and dietary patterns related to osteoporosis. Nutrients. 2020;12:1986.
- Schepper JD, Collins F, Rios-Arce ND, et al. Involvement of the gut microbiota and barrier function in glucocorticoid-induced osteoporosis. J Bone Miner Res. 2020;35:801-820.
- Jansson PA, Curiac D, Ahrén IL, et al. Probiotic treatment using a mix of three Lactobacillus strains for lumbar spine bone loss in postmenopausal women: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. 2019;1:E154-E162.
- Rizzoli R, Biver E. Are probiotics the new calcium and vitamin D for bone health? Curr Osteoporos Rep. 2020;18:273-284.
- Haring B, Crandall CJ, Wu C, et al. Dietary patterns and fractures in postmenopausal women: results from the Women’s Health Initiative. JAMA Intern Med. 2016;176:645-652.
- Benetou V, Orfanos P, Pettersson-Kymmer U, et al. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporos Int. 2013;24:1587-1598.
- Byberg L, Bellavia A, Larsson SC, et al. Mediterranean diet and hip fracture in Swedish men and women. J Bone Miner Res. 2016;31:2098-2105.
- Zupo R, Lampignano L, Lattanzio A, et al. Association between adherence to the Mediterranean diet and circulating vitamin D levels. Int J Food Sci Nutr. 2020;71:884-890.
- Chin KY, Ima-Nirwana S. Olives and bone: a green osteoporosis prevention option. Int J Environ Res Public Health. 2016;13:755.
- García-Martínez O, Rivas A, Ramos-Torrecillas J, et al. The effect of olive oil on osteoporosis prevention. Int J Food Sci Nutr. 2014;65:834-840.
- Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118:181-189.
- Veronese N, Stubbs B, Solmi M, et al. Dietary magnesium intake and fracture risk: data from a large prospective study. Br J Nutr. 2017;117:1570-1576.
- Kong SH, Kim JH, Hong AR, et al. Dietary potassium intake is beneficial to bone health in a low calcium intake population: the Korean National Health and Nutrition Examination Survey (KNHANES)(2008-2011). Osteoporos Int. 2017;28:1577-1585.
Practice Points
- Many long-term glucocorticoid (GC) users never receive therapy to prevent bone loss, and others are only started on therapy once they have sustained an insufficiency fracture.
- Oral GCs should be used at the lowest effective daily dose for the shortest duration possible.
- Patients using topical and intralesional corticosteroids are unlikely to develop GC-induced osteoporosis.
The CROWNing Event on Hair Loss in Women of Color: A Framework for Advocacy and Community Engagement (FACE) Survey Analysis
Hair loss is a primary reason why women with skin of color seek dermatologic care.1-3 In addition to physical disfigurement, patients with hair loss are more likely to report feelings of depression, anxiety, and low self-esteem compared to the general population.4 There is a critical gap in advocacy efforts and educational information intended for women with skin of color. The American Academy of Dermatology (AAD) has 6 main public health programs (https://www.aad.org/public/public-health) and 8 stated advocacy priorities (https://www.aad.org/member/advocacy/priorities) but none of them focus on outreach to minority communities.
Historically, hair in patients with skin of color also has been a systemic tangible target for race-based discrimination. The Create a Respectful and Open World for Natural Hair (CROWN) Act was passed to protect against discrimination based on race-based hairstyles in schools and workplaces.5 Health care providers play an important role in advocating for their patients, but studies have shown that barriers to effective advocacy include a lack of knowledge, resources, or time.6-8 Virtual advocacy events improve participants’ understanding and interest in community engagement and advocacy.6,7 With the mission to engage, educate, and empower women with skin of color and the dermatologists who treat them, the Virginia Dermatology Society hosted the virtual CROWNing Event on Hair Loss in Women of Color in July 2021. We believe that this event, as well as this column, can serve as a template to improve advocacy and educational efforts for additional topics and diseases that affect marginalized or underserved populations. Survey data were collected and analyzed to establish a baseline of awareness and understanding of hair loss in women with skin of color and to evaluate the impact of a virtual event on participants’ empowerment and familiarity with resources for this population.
Methods
The Virginia Dermatology Society organized a virtual event focused on hair loss and practical political advocacy for women with skin of color. As members of the Virginia Dermatology Society and as part of the planning and execution of this event, the authors engaged relevant stakeholder organizations and collaborated with faculty at a local historically Black university to create a targeted, culturally sensitive communication strategy known as the Framework for Advocacy and Community Engagement (FACE) model (Figure). The agenda included presentations by 2 patients of color living with a hair loss disorder, a dermatologist with experience in advocacy, a Virginia state legislator, and a dermatologic hair loss expert, followed by a final question-and-answer session.
We created pre- and postevent Likert scale surveys assessing participant attitudes, knowledge, and awareness surrounding hair loss that were distributed electronically to all 399 registrants before and after the event, respectively. The responses were analyzed using a Mann-Whitney U test.
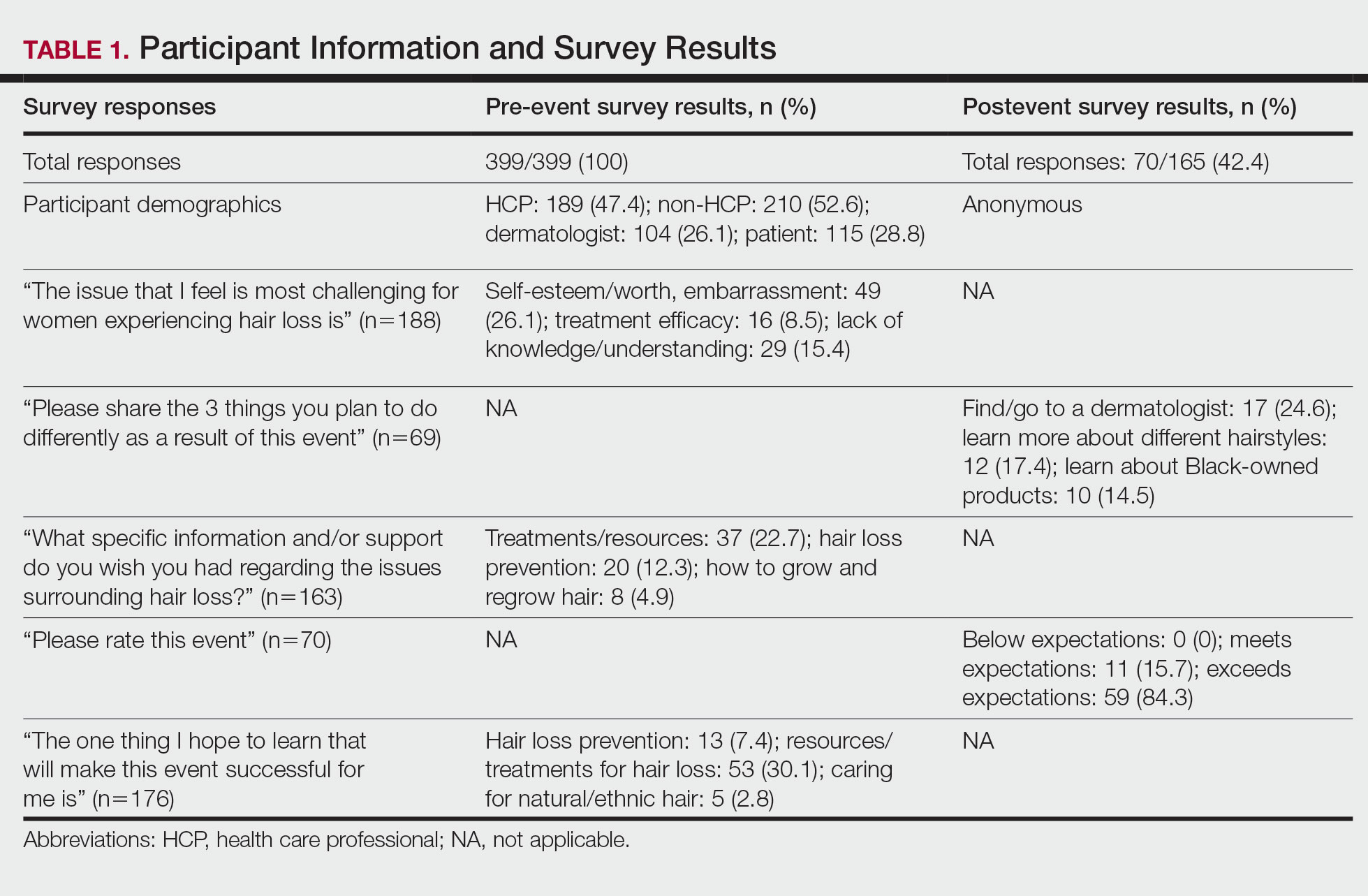
Based on preliminary pre-event survey data, we created a resource toolkit (https://bit.ly/vadermhairlosstoolkit) for distribution to both patients and physicians. The toolkit included articles about evaluating, diagnosing, and treating different types of hair loss that would be beneficial for dermatologists, as well as informational articles, online resources, and videos that would be helpful to patients.
Of the 399 registrants, 165 (41.4%) attended the live virtual event. The postevent survey was completed by 70 (42.4%) participants and showed that familiarity with resources and treatments (z=−3.34, P=.0008) and feelings of empowerment (z=−3.55, P=.0004) significantly increased from before the event (Table 2). Participants indicated that the event exceeded (84.3%) or met (15.7%) their expectations.
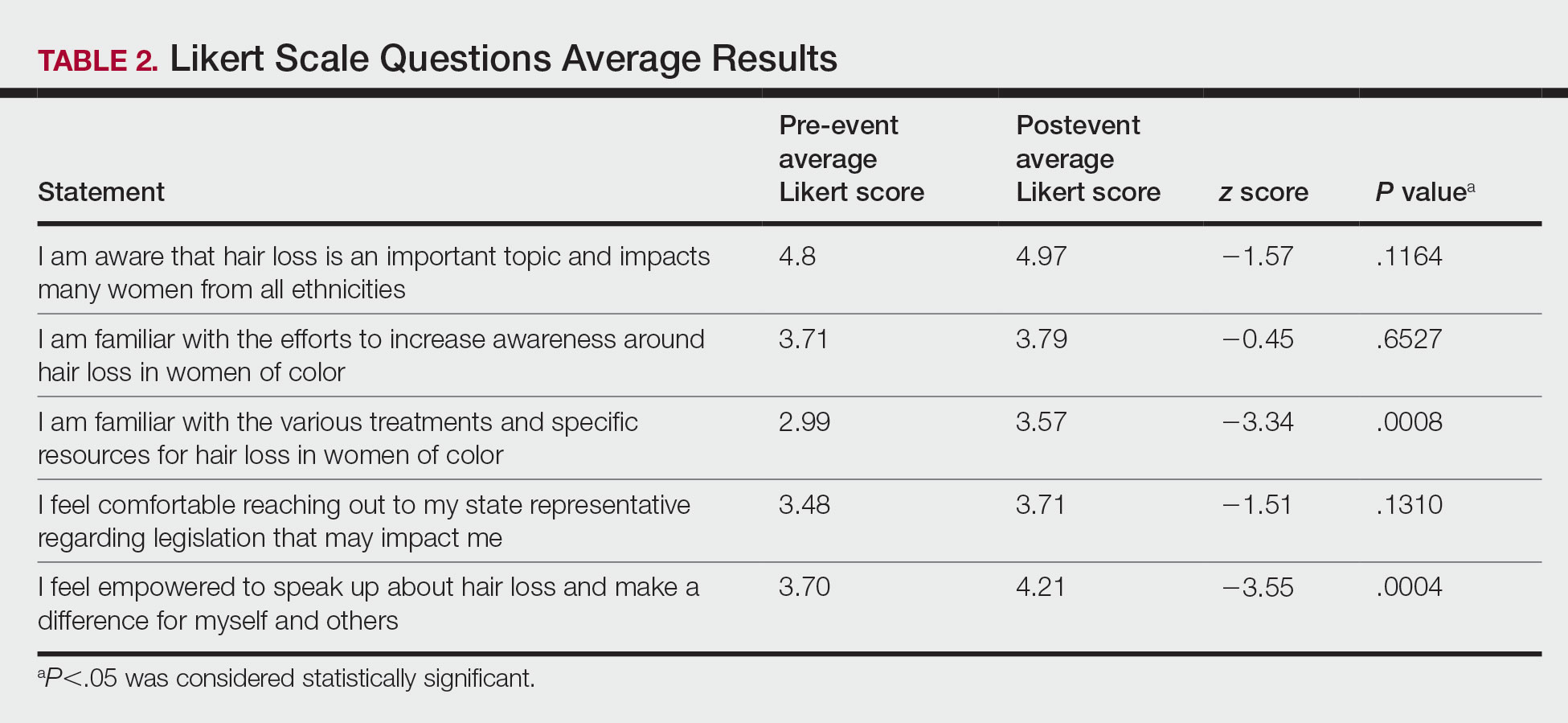
Comment
Hair Loss Is Prevalent in Skin of Color Patients—Alopecia is the fourth most common reason women with skin of color seek care from a dermatologist, accounting for 8.3% of all visits in a study of 1412 patient visits; however, it was not among the leading 10 diagnoses made during visits for White patients.3 Traction alopecia, discoid lupus erythematosus, and central centrifugal cicatricial alopecia occur more commonly in Black women,9 many of whom do not feel their dermatologists understand hair in this population.10,11 Lack of skin of color education in medical school and dermatology residency programs has been reported and must be improved to eliminate the knowledge gaps, acquire cultural competence, and improve all aspects of care for patients with skin of color.11-14 Our survey results similarly demonstrated that only 66% of board-certified dermatologists reported being familiar with the various and specific resources and treatments for hair loss in women of color. Improved understanding of hair in patients of color is a first step in diagnosing and treating hair loss.15 Expertise of dermatologists in skin of color improves the dermatology experience of patients of color.11
Hair loss is more than a cosmetic issue, and it is essential that it is regarded as such. Patients with hair loss have an increased prevalence of depression and anxiety compared to the general population and report lower self-esteem, heightened self-consciousness, and loss of confidence.4,9 Historically, the lives of patients of color have been drastically affected by society’s perceptions of their skin color and hairstyle.16
Hair-Based Discrimination in the Workplace—To compound the problem, hair also is a common target of race-based discrimination behind the illusion of “professionalism.” Hair-based discrimination keeps people of color out of professional workplaces; for instance, women of color are more likely to be sent home due to hair appearance than White women.5 The CROWN Act, created in 2019, extends statutory protection to hair texture and protective hairstyles such as braids, locs, twists, and knots in the workplace and public schools to protect against discrimination due to race-based hairstyles. The CROWN Act provides an opportunity for dermatologists to support legislation that protects patients of color and the fundamental human right to nondiscrimination. As societal pressure for damaging hair practices such as hot combing or chemical relaxants decreases, patient outcomes will improve.5
How to Support the CROWN Act—There are various meaningful ways for dermatologists to support the CROWN act, including but not limited to signing petitions, sending letters of support to elected representatives, joining the CROWN Coalition, raising awareness and educating the public through social media, vocalizing against hair discrimination in our own workplaces and communities, and asking patients about their experiences with hair discrimination.5 In addition to advocacy, other antiracist actions suggested to improve health equity include creating curricula on racial inequity and increasing diversity in dermatology.16
There are many advocacy and public health campaigns promoted on the AAD website; however, despite the AAD’s formation of the Access to Dermatologic Care Task Force (ATDCTF) with the goal to raise awareness among dermatologists of health disparities affecting marginalized and underserved populations and to develop policies that increase access to care for these groups, there are still critical gaps in advocacy and information.13 This gap in both advocacy and understanding of hair loss conditions in women of color is one reason the CROWNing Event in July 2021 was held, and we believe this event along with this column can serve as a template for addressing additional topics and diseases that affect marginalized or underserved populations.
Dermatologists can play a vital role in advocating for skin and hair needs in all patient populations from the personal or clinical encounter level to population-level policy legislation.5,8 As experts in skin and hair, dermatologists are best prepared to assume leadership in addressing racial health inequities, educating the public, and improving awareness.5,16 Dermatologists must be able to diagnose and manage skin conditions in people of color.12 However, health advocacy should extend beyond changes to health behavior or health interventions and instead address the root causes of systemic issues that drive disparate health outcomes.6 Every dermatologist has a contribution to make; it is time for us to acknowledge that patients’ ailments neither begin nor end at the clinic door.8,16 As dermatologists, we must speak out against the racial inequities and discriminatory policies affecting the lives of patients of color.16
Although the CROWNing event should be considered successful, reflection in hindsight has allowed us to find ways to improve the impact of future events, including incorporating more lay members of the respective community in the planning process, allocating more time during the event programming for questions, and streamlining the distribution of pre-event and postevent surveys to better gauge knowledge retention among participants and gain crucial feedback for future event planning.
How to Use the FACE Model—We believe that the FACE model (Figure) can help providers engage lay members of the community with additional topics and diseases that affect marginalized and underserved populations. We recommend that future organizers engage stakeholders early during the design, planning, and implementation phases to ensure that the community’s most pressing needs are addressed. Dermatologists possess the knowledge and influence to serve as powerful advocates and champions for health equity. As physicians on the front lines of dermatologic health, we are uniquely positioned to engage and partner with patients through educational and advocacy events such as ours. Similarly, informed and empowered patients can advocate for policies and be proponents for greater research funding.5 We call on the AAD and other dermatologic organizations to expand community outreach and advocacy efforts to include underserved and underrepresented populations.
Acknowledgments—The authors would like to thank and acknowledge the faculty at Hampton University (Hampton, Virginia)—specifically Ms. B. DáVida Plummer, MA—for assistance with communication strategies, including organizing the radio and television announcements and proofreading the public service announcements. We also would like to thank other CROWNing Event Planning Committee members, including Natalia Mendoza, MD (Newport News, Virginia); Farhaad Riyaz, MD (Gainesville, Virginia); Deborah Elder, MD (Charlottesville, Virginia); and David Rowe, MD (Charlottesville, Virginia), as well as Sandra Ring, MS, CCLS, CNP (Chicago, Illinois), from the AAD and the various speakers at the event, including the 2 patients; Victoria Barbosa, MD, MPH, MBA (Chicago, Illinois); Avery LaChance, MD, MPH (Boston, Massachusetts); and Senator Lionell Spruill Sr (Chesapeake, Virginia). We acknowledge Marieke K. Jones, PhD, at the Claude Moore Health Sciences Library at the University of Virginia (Charlottesville, Virginia), for her statistical expertise.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(suppl 1):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105:599-610. doi:10.1016/j.mcna.2021.04.002
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Tran A, Gohara M. Community engagement matters: a call for greater advocacy in dermatology. Int J Womens Dermatol. 2021;7:189-190. doi:10.1016/j.ijwd.2021.01.008
- Yu Z, Moustafa D, Kwak R, et al. Engaging in advocacy during medical training: assessing the impact of a virtual COVID-19-focused state advocacy day [published online January 13, 2021]. Postgrad Med J. doi:10.1136/postgradmedj-2020-139362
- Earnest MA, Wong SL, Federico SG. Perspective: physician advocacy: what is it and how do we do it? Acad Med J Assoc Am Med Coll. 2010;85:63-67. doi:10.1097/ACM.0b013e3181c40d40
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319. doi:10.1016/j.ijwd.2019.08.005
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Taylor SC. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155:1109-1110. doi:10.1001/jamadermatol.2019.1963
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Smith RJ, Oliver BU. Advocating for Black lives—a call to dermatologists to dismantle institutionalized racism and address racial health inequities. JAMA Dermatol. 2021;157:155-156. doi:10.1001/jamadermatol.2020.4392
Hair loss is a primary reason why women with skin of color seek dermatologic care.1-3 In addition to physical disfigurement, patients with hair loss are more likely to report feelings of depression, anxiety, and low self-esteem compared to the general population.4 There is a critical gap in advocacy efforts and educational information intended for women with skin of color. The American Academy of Dermatology (AAD) has 6 main public health programs (https://www.aad.org/public/public-health) and 8 stated advocacy priorities (https://www.aad.org/member/advocacy/priorities) but none of them focus on outreach to minority communities.
Historically, hair in patients with skin of color also has been a systemic tangible target for race-based discrimination. The Create a Respectful and Open World for Natural Hair (CROWN) Act was passed to protect against discrimination based on race-based hairstyles in schools and workplaces.5 Health care providers play an important role in advocating for their patients, but studies have shown that barriers to effective advocacy include a lack of knowledge, resources, or time.6-8 Virtual advocacy events improve participants’ understanding and interest in community engagement and advocacy.6,7 With the mission to engage, educate, and empower women with skin of color and the dermatologists who treat them, the Virginia Dermatology Society hosted the virtual CROWNing Event on Hair Loss in Women of Color in July 2021. We believe that this event, as well as this column, can serve as a template to improve advocacy and educational efforts for additional topics and diseases that affect marginalized or underserved populations. Survey data were collected and analyzed to establish a baseline of awareness and understanding of hair loss in women with skin of color and to evaluate the impact of a virtual event on participants’ empowerment and familiarity with resources for this population.
Methods
The Virginia Dermatology Society organized a virtual event focused on hair loss and practical political advocacy for women with skin of color. As members of the Virginia Dermatology Society and as part of the planning and execution of this event, the authors engaged relevant stakeholder organizations and collaborated with faculty at a local historically Black university to create a targeted, culturally sensitive communication strategy known as the Framework for Advocacy and Community Engagement (FACE) model (Figure). The agenda included presentations by 2 patients of color living with a hair loss disorder, a dermatologist with experience in advocacy, a Virginia state legislator, and a dermatologic hair loss expert, followed by a final question-and-answer session.

We created pre- and postevent Likert scale surveys assessing participant attitudes, knowledge, and awareness surrounding hair loss that were distributed electronically to all 399 registrants before and after the event, respectively. The responses were analyzed using a Mann-Whitney U test.

Based on preliminary pre-event survey data, we created a resource toolkit (https://bit.ly/vadermhairlosstoolkit) for distribution to both patients and physicians. The toolkit included articles about evaluating, diagnosing, and treating different types of hair loss that would be beneficial for dermatologists, as well as informational articles, online resources, and videos that would be helpful to patients.
Of the 399 registrants, 165 (41.4%) attended the live virtual event. The postevent survey was completed by 70 (42.4%) participants and showed that familiarity with resources and treatments (z=−3.34, P=.0008) and feelings of empowerment (z=−3.55, P=.0004) significantly increased from before the event (Table 2). Participants indicated that the event exceeded (84.3%) or met (15.7%) their expectations.

Comment
Hair Loss Is Prevalent in Skin of Color Patients—Alopecia is the fourth most common reason women with skin of color seek care from a dermatologist, accounting for 8.3% of all visits in a study of 1412 patient visits; however, it was not among the leading 10 diagnoses made during visits for White patients.3 Traction alopecia, discoid lupus erythematosus, and central centrifugal cicatricial alopecia occur more commonly in Black women,9 many of whom do not feel their dermatologists understand hair in this population.10,11 Lack of skin of color education in medical school and dermatology residency programs has been reported and must be improved to eliminate the knowledge gaps, acquire cultural competence, and improve all aspects of care for patients with skin of color.11-14 Our survey results similarly demonstrated that only 66% of board-certified dermatologists reported being familiar with the various and specific resources and treatments for hair loss in women of color. Improved understanding of hair in patients of color is a first step in diagnosing and treating hair loss.15 Expertise of dermatologists in skin of color improves the dermatology experience of patients of color.11
Hair loss is more than a cosmetic issue, and it is essential that it is regarded as such. Patients with hair loss have an increased prevalence of depression and anxiety compared to the general population and report lower self-esteem, heightened self-consciousness, and loss of confidence.4,9 Historically, the lives of patients of color have been drastically affected by society’s perceptions of their skin color and hairstyle.16
Hair-Based Discrimination in the Workplace—To compound the problem, hair also is a common target of race-based discrimination behind the illusion of “professionalism.” Hair-based discrimination keeps people of color out of professional workplaces; for instance, women of color are more likely to be sent home due to hair appearance than White women.5 The CROWN Act, created in 2019, extends statutory protection to hair texture and protective hairstyles such as braids, locs, twists, and knots in the workplace and public schools to protect against discrimination due to race-based hairstyles. The CROWN Act provides an opportunity for dermatologists to support legislation that protects patients of color and the fundamental human right to nondiscrimination. As societal pressure for damaging hair practices such as hot combing or chemical relaxants decreases, patient outcomes will improve.5
How to Support the CROWN Act—There are various meaningful ways for dermatologists to support the CROWN act, including but not limited to signing petitions, sending letters of support to elected representatives, joining the CROWN Coalition, raising awareness and educating the public through social media, vocalizing against hair discrimination in our own workplaces and communities, and asking patients about their experiences with hair discrimination.5 In addition to advocacy, other antiracist actions suggested to improve health equity include creating curricula on racial inequity and increasing diversity in dermatology.16
There are many advocacy and public health campaigns promoted on the AAD website; however, despite the AAD’s formation of the Access to Dermatologic Care Task Force (ATDCTF) with the goal to raise awareness among dermatologists of health disparities affecting marginalized and underserved populations and to develop policies that increase access to care for these groups, there are still critical gaps in advocacy and information.13 This gap in both advocacy and understanding of hair loss conditions in women of color is one reason the CROWNing Event in July 2021 was held, and we believe this event along with this column can serve as a template for addressing additional topics and diseases that affect marginalized or underserved populations.
Dermatologists can play a vital role in advocating for skin and hair needs in all patient populations from the personal or clinical encounter level to population-level policy legislation.5,8 As experts in skin and hair, dermatologists are best prepared to assume leadership in addressing racial health inequities, educating the public, and improving awareness.5,16 Dermatologists must be able to diagnose and manage skin conditions in people of color.12 However, health advocacy should extend beyond changes to health behavior or health interventions and instead address the root causes of systemic issues that drive disparate health outcomes.6 Every dermatologist has a contribution to make; it is time for us to acknowledge that patients’ ailments neither begin nor end at the clinic door.8,16 As dermatologists, we must speak out against the racial inequities and discriminatory policies affecting the lives of patients of color.16
Although the CROWNing event should be considered successful, reflection in hindsight has allowed us to find ways to improve the impact of future events, including incorporating more lay members of the respective community in the planning process, allocating more time during the event programming for questions, and streamlining the distribution of pre-event and postevent surveys to better gauge knowledge retention among participants and gain crucial feedback for future event planning.
How to Use the FACE Model—We believe that the FACE model (Figure) can help providers engage lay members of the community with additional topics and diseases that affect marginalized and underserved populations. We recommend that future organizers engage stakeholders early during the design, planning, and implementation phases to ensure that the community’s most pressing needs are addressed. Dermatologists possess the knowledge and influence to serve as powerful advocates and champions for health equity. As physicians on the front lines of dermatologic health, we are uniquely positioned to engage and partner with patients through educational and advocacy events such as ours. Similarly, informed and empowered patients can advocate for policies and be proponents for greater research funding.5 We call on the AAD and other dermatologic organizations to expand community outreach and advocacy efforts to include underserved and underrepresented populations.
Acknowledgments—The authors would like to thank and acknowledge the faculty at Hampton University (Hampton, Virginia)—specifically Ms. B. DáVida Plummer, MA—for assistance with communication strategies, including organizing the radio and television announcements and proofreading the public service announcements. We also would like to thank other CROWNing Event Planning Committee members, including Natalia Mendoza, MD (Newport News, Virginia); Farhaad Riyaz, MD (Gainesville, Virginia); Deborah Elder, MD (Charlottesville, Virginia); and David Rowe, MD (Charlottesville, Virginia), as well as Sandra Ring, MS, CCLS, CNP (Chicago, Illinois), from the AAD and the various speakers at the event, including the 2 patients; Victoria Barbosa, MD, MPH, MBA (Chicago, Illinois); Avery LaChance, MD, MPH (Boston, Massachusetts); and Senator Lionell Spruill Sr (Chesapeake, Virginia). We acknowledge Marieke K. Jones, PhD, at the Claude Moore Health Sciences Library at the University of Virginia (Charlottesville, Virginia), for her statistical expertise.
Hair loss is a primary reason why women with skin of color seek dermatologic care.1-3 In addition to physical disfigurement, patients with hair loss are more likely to report feelings of depression, anxiety, and low self-esteem compared to the general population.4 There is a critical gap in advocacy efforts and educational information intended for women with skin of color. The American Academy of Dermatology (AAD) has 6 main public health programs (https://www.aad.org/public/public-health) and 8 stated advocacy priorities (https://www.aad.org/member/advocacy/priorities) but none of them focus on outreach to minority communities.
Historically, hair in patients with skin of color also has been a systemic tangible target for race-based discrimination. The Create a Respectful and Open World for Natural Hair (CROWN) Act was passed to protect against discrimination based on race-based hairstyles in schools and workplaces.5 Health care providers play an important role in advocating for their patients, but studies have shown that barriers to effective advocacy include a lack of knowledge, resources, or time.6-8 Virtual advocacy events improve participants’ understanding and interest in community engagement and advocacy.6,7 With the mission to engage, educate, and empower women with skin of color and the dermatologists who treat them, the Virginia Dermatology Society hosted the virtual CROWNing Event on Hair Loss in Women of Color in July 2021. We believe that this event, as well as this column, can serve as a template to improve advocacy and educational efforts for additional topics and diseases that affect marginalized or underserved populations. Survey data were collected and analyzed to establish a baseline of awareness and understanding of hair loss in women with skin of color and to evaluate the impact of a virtual event on participants’ empowerment and familiarity with resources for this population.
Methods
The Virginia Dermatology Society organized a virtual event focused on hair loss and practical political advocacy for women with skin of color. As members of the Virginia Dermatology Society and as part of the planning and execution of this event, the authors engaged relevant stakeholder organizations and collaborated with faculty at a local historically Black university to create a targeted, culturally sensitive communication strategy known as the Framework for Advocacy and Community Engagement (FACE) model (Figure). The agenda included presentations by 2 patients of color living with a hair loss disorder, a dermatologist with experience in advocacy, a Virginia state legislator, and a dermatologic hair loss expert, followed by a final question-and-answer session.

We created pre- and postevent Likert scale surveys assessing participant attitudes, knowledge, and awareness surrounding hair loss that were distributed electronically to all 399 registrants before and after the event, respectively. The responses were analyzed using a Mann-Whitney U test.

Based on preliminary pre-event survey data, we created a resource toolkit (https://bit.ly/vadermhairlosstoolkit) for distribution to both patients and physicians. The toolkit included articles about evaluating, diagnosing, and treating different types of hair loss that would be beneficial for dermatologists, as well as informational articles, online resources, and videos that would be helpful to patients.
Of the 399 registrants, 165 (41.4%) attended the live virtual event. The postevent survey was completed by 70 (42.4%) participants and showed that familiarity with resources and treatments (z=−3.34, P=.0008) and feelings of empowerment (z=−3.55, P=.0004) significantly increased from before the event (Table 2). Participants indicated that the event exceeded (84.3%) or met (15.7%) their expectations.

Comment
Hair Loss Is Prevalent in Skin of Color Patients—Alopecia is the fourth most common reason women with skin of color seek care from a dermatologist, accounting for 8.3% of all visits in a study of 1412 patient visits; however, it was not among the leading 10 diagnoses made during visits for White patients.3 Traction alopecia, discoid lupus erythematosus, and central centrifugal cicatricial alopecia occur more commonly in Black women,9 many of whom do not feel their dermatologists understand hair in this population.10,11 Lack of skin of color education in medical school and dermatology residency programs has been reported and must be improved to eliminate the knowledge gaps, acquire cultural competence, and improve all aspects of care for patients with skin of color.11-14 Our survey results similarly demonstrated that only 66% of board-certified dermatologists reported being familiar with the various and specific resources and treatments for hair loss in women of color. Improved understanding of hair in patients of color is a first step in diagnosing and treating hair loss.15 Expertise of dermatologists in skin of color improves the dermatology experience of patients of color.11
Hair loss is more than a cosmetic issue, and it is essential that it is regarded as such. Patients with hair loss have an increased prevalence of depression and anxiety compared to the general population and report lower self-esteem, heightened self-consciousness, and loss of confidence.4,9 Historically, the lives of patients of color have been drastically affected by society’s perceptions of their skin color and hairstyle.16
Hair-Based Discrimination in the Workplace—To compound the problem, hair also is a common target of race-based discrimination behind the illusion of “professionalism.” Hair-based discrimination keeps people of color out of professional workplaces; for instance, women of color are more likely to be sent home due to hair appearance than White women.5 The CROWN Act, created in 2019, extends statutory protection to hair texture and protective hairstyles such as braids, locs, twists, and knots in the workplace and public schools to protect against discrimination due to race-based hairstyles. The CROWN Act provides an opportunity for dermatologists to support legislation that protects patients of color and the fundamental human right to nondiscrimination. As societal pressure for damaging hair practices such as hot combing or chemical relaxants decreases, patient outcomes will improve.5
How to Support the CROWN Act—There are various meaningful ways for dermatologists to support the CROWN act, including but not limited to signing petitions, sending letters of support to elected representatives, joining the CROWN Coalition, raising awareness and educating the public through social media, vocalizing against hair discrimination in our own workplaces and communities, and asking patients about their experiences with hair discrimination.5 In addition to advocacy, other antiracist actions suggested to improve health equity include creating curricula on racial inequity and increasing diversity in dermatology.16
There are many advocacy and public health campaigns promoted on the AAD website; however, despite the AAD’s formation of the Access to Dermatologic Care Task Force (ATDCTF) with the goal to raise awareness among dermatologists of health disparities affecting marginalized and underserved populations and to develop policies that increase access to care for these groups, there are still critical gaps in advocacy and information.13 This gap in both advocacy and understanding of hair loss conditions in women of color is one reason the CROWNing Event in July 2021 was held, and we believe this event along with this column can serve as a template for addressing additional topics and diseases that affect marginalized or underserved populations.
Dermatologists can play a vital role in advocating for skin and hair needs in all patient populations from the personal or clinical encounter level to population-level policy legislation.5,8 As experts in skin and hair, dermatologists are best prepared to assume leadership in addressing racial health inequities, educating the public, and improving awareness.5,16 Dermatologists must be able to diagnose and manage skin conditions in people of color.12 However, health advocacy should extend beyond changes to health behavior or health interventions and instead address the root causes of systemic issues that drive disparate health outcomes.6 Every dermatologist has a contribution to make; it is time for us to acknowledge that patients’ ailments neither begin nor end at the clinic door.8,16 As dermatologists, we must speak out against the racial inequities and discriminatory policies affecting the lives of patients of color.16
Although the CROWNing event should be considered successful, reflection in hindsight has allowed us to find ways to improve the impact of future events, including incorporating more lay members of the respective community in the planning process, allocating more time during the event programming for questions, and streamlining the distribution of pre-event and postevent surveys to better gauge knowledge retention among participants and gain crucial feedback for future event planning.
How to Use the FACE Model—We believe that the FACE model (Figure) can help providers engage lay members of the community with additional topics and diseases that affect marginalized and underserved populations. We recommend that future organizers engage stakeholders early during the design, planning, and implementation phases to ensure that the community’s most pressing needs are addressed. Dermatologists possess the knowledge and influence to serve as powerful advocates and champions for health equity. As physicians on the front lines of dermatologic health, we are uniquely positioned to engage and partner with patients through educational and advocacy events such as ours. Similarly, informed and empowered patients can advocate for policies and be proponents for greater research funding.5 We call on the AAD and other dermatologic organizations to expand community outreach and advocacy efforts to include underserved and underrepresented populations.
Acknowledgments—The authors would like to thank and acknowledge the faculty at Hampton University (Hampton, Virginia)—specifically Ms. B. DáVida Plummer, MA—for assistance with communication strategies, including organizing the radio and television announcements and proofreading the public service announcements. We also would like to thank other CROWNing Event Planning Committee members, including Natalia Mendoza, MD (Newport News, Virginia); Farhaad Riyaz, MD (Gainesville, Virginia); Deborah Elder, MD (Charlottesville, Virginia); and David Rowe, MD (Charlottesville, Virginia), as well as Sandra Ring, MS, CCLS, CNP (Chicago, Illinois), from the AAD and the various speakers at the event, including the 2 patients; Victoria Barbosa, MD, MPH, MBA (Chicago, Illinois); Avery LaChance, MD, MPH (Boston, Massachusetts); and Senator Lionell Spruill Sr (Chesapeake, Virginia). We acknowledge Marieke K. Jones, PhD, at the Claude Moore Health Sciences Library at the University of Virginia (Charlottesville, Virginia), for her statistical expertise.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(suppl 1):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105:599-610. doi:10.1016/j.mcna.2021.04.002
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Tran A, Gohara M. Community engagement matters: a call for greater advocacy in dermatology. Int J Womens Dermatol. 2021;7:189-190. doi:10.1016/j.ijwd.2021.01.008
- Yu Z, Moustafa D, Kwak R, et al. Engaging in advocacy during medical training: assessing the impact of a virtual COVID-19-focused state advocacy day [published online January 13, 2021]. Postgrad Med J. doi:10.1136/postgradmedj-2020-139362
- Earnest MA, Wong SL, Federico SG. Perspective: physician advocacy: what is it and how do we do it? Acad Med J Assoc Am Med Coll. 2010;85:63-67. doi:10.1097/ACM.0b013e3181c40d40
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319. doi:10.1016/j.ijwd.2019.08.005
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Taylor SC. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155:1109-1110. doi:10.1001/jamadermatol.2019.1963
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Smith RJ, Oliver BU. Advocating for Black lives—a call to dermatologists to dismantle institutionalized racism and address racial health inequities. JAMA Dermatol. 2021;157:155-156. doi:10.1001/jamadermatol.2020.4392
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3(suppl 1):S21-S37. doi:10.1016/j.ijwd.2017.02.006
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Jamerson TA, Aguh C. An approach to patients with alopecia. Med Clin North Am. 2021;105:599-610. doi:10.1016/j.mcna.2021.04.002
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Tran A, Gohara M. Community engagement matters: a call for greater advocacy in dermatology. Int J Womens Dermatol. 2021;7:189-190. doi:10.1016/j.ijwd.2021.01.008
- Yu Z, Moustafa D, Kwak R, et al. Engaging in advocacy during medical training: assessing the impact of a virtual COVID-19-focused state advocacy day [published online January 13, 2021]. Postgrad Med J. doi:10.1136/postgradmedj-2020-139362
- Earnest MA, Wong SL, Federico SG. Perspective: physician advocacy: what is it and how do we do it? Acad Med J Assoc Am Med Coll. 2010;85:63-67. doi:10.1097/ACM.0b013e3181c40d40
- Raffi J, Suresh R, Agbai O. Clinical recognition and management of alopecia in women of color. Int J Womens Dermatol. 2019;5:314-319. doi:10.1016/j.ijwd.2019.08.005
- Gathers RC, Mahan MG. African American women, hair care, and health barriers. J Clin Aesthet Dermatol. 2014;7:26-29.
- Gorbatenko-Roth K, Prose N, Kundu RV, et al. Assessment of Black patients’ perception of their dermatology care. JAMA Dermatol. 2019;155:1129-1134. doi:10.1001/jamadermatol.2019.2063
- Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol. 2006;55:687-690. doi:10.1016/j.jaad.2005.10.068
- Buster KJ, Stevens EI, Elmets CA. Dermatologic health disparities. Dermatol Clin. 2012;30:53-59, viii. doi:10.1016/j.det.2011.08.002
- Taylor SC. Meeting the unique dermatologic needs of black patients. JAMA Dermatol. 2019;155:1109-1110. doi:10.1001/jamadermatol.2019.1963
- Dlova NC, Salkey KS, Callender VD, et al. Central centrifugal cicatricial alopecia: new insights and a call for action. J Investig Dermatol Symp Proc. 2017;18:S54-S56. doi:10.1016/j.jisp.2017.01.004
- Smith RJ, Oliver BU. Advocating for Black lives—a call to dermatologists to dismantle institutionalized racism and address racial health inequities. JAMA Dermatol. 2021;157:155-156. doi:10.1001/jamadermatol.2020.4392
Practice Points
- Hair loss is associated with low self-esteem in women with skin of color; therefore, it is important to both acknowledge the social and psychological impacts of hair loss in this population and provide educational resources and community events that address patient concerns.
- There is a deficit of dermatology advocacy efforts that address conditions affecting patients with skin of color. Highlighting this disparity is the first step to catalyzing change.
- Dermatologists are responsible for advocating for women with skin of color and for addressing the social issues that impact their quality of life.
- The Framework for Advocacy and Community Efforts (FACE) model is a template for others to use when planning community engagement and advocacy efforts.
Petrolatum Is Effective as a Moisturizer, But There Are More Uses for It
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
Petrolatum recently has received substantial social media attention. In the last year, the number of TikTok and Instagram videos mentioning petrolatum increased by 46% and 93%, respectively. According to Unilever, the company that manufactures Vaseline, mentions of the product have gone up by 327% on social media compared to last year largely due to a trend known as “slugging,” or the practice of slathering on petrolatum overnight to improve skin hydration.1 However, petrolatum has a variety of other uses. Given its increase in popularity, we review the many uses of petrolatum within dermatology.
The main reason for petrolatum’s presence on social media is its effectiveness as a moisturizer, which is due to its occlusive property. Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL). Among available oil-based moisturizers, petrolatum is the most effective in reducing TEWL by 98%, while others only provide reductions of 20% to 30%,2 which makes it ideal for soothing itch and irritation in several skin conditions, including dry skin, cheilitis, chafing, and diaper rash. Petrolatum is particularly helpful in sensitive areas where the skin is thinner, such as the eyelids or lips, as it is less irritating than lotions.
Petrolatum also may be used to treat dry skin and mild atopic dermatitis with the soak-and-smear technique,3 which entails soaking the affected skin—or the entire body, if needed—in a plain water bath for 20 minutes and then immediately smearing the skin with petrolatum. Soaking hydrates the damaged stratum corneum and enhances desquamation. The moist stratum corneum absorbs topical treatments more effectively, and desquamation leaves a thinner stratum corneum for the product to traverse. Smearing with petrolatum then traps the moisture in the skin and thus has a dual function by both delivering the petrolatum to the skin and trapping the moisture from the soak. The result is decreased TEWL, improved hydration, and increased penetration, thereby enhancing skin barrier repair.3,4
Smearing solely with petrolatum is effective in cases not accompanied by considerable inflammation. In cases involving notable inflammation or severe xerosis, a steroidal ointment may be required.3 This generally is done for several nights to 2 weeks before conversion to maintenance therapy. In these cases, petrolatum may then be used as maintenance therapy or bridge therapy for maintenance with simple moisturizers, which decreases recurrence and flares of dermatitis and also prevents continuous exposure to steroidal agents that can result in atrophy and purpura at application sites. The soak-and-smear technique has been found to be effective, with 90% of patients having 90% to 100% clearance.3
Petrolatum also is particularly useful for wound healing. A study on the molecular responses induced by petrolatum found that it significantly upregulated innate immune genes (P<.01), increased antimicrobial peptides (P<.001), and improved epidermal differentiation.5 Additionally, it keeps wound edges moist, which enhances angiogenesis, improves collagen synthesis, and increases the breakdown of dead tissue and fibrin.6 It also prevents scab formation, which can prolong healing time.7
Petrolatum is superior to antibiotic use after clean cutaneous surgery given its excellent safety profile. In one randomized controlled trial comparing petrolatum to bacitracin, petrolatum was found to be just as effective for wound healing with a similar infection rate. Although 4 patients developed allergic contact dermatitis (ACD) with bacitracin use, no patients who used petrolatum developed ACD.8 There are numerous other reports of bacitracin causing ACD,9,10 with a prevalence as high as 22% in chronic leg ulcer patients.10 There are even multiple reports of bacitracin causing contact urticaria and life-threatening anaphylaxis.11 In the most recent report from the North American Contact Dermatitis Group’s list of top allergens, bacitracin placed 11th with an ACD prevalence of 5.5%. Neomycin, another common postwound emollient, has similar adverse effects and ranked 12th with an ACD prevalence of 5.4%.12 Despite the risk for ACD with antibiotics, one study on wound care handouts from dermatologists (N=169) found that nearly half (43%) still advocated for the use of antibiotics.13 Likewise, another study among nondermatologists found that 40% (10/25) recommended the use of antibiotics for wound care14 despite strong evidence that topical antibiotics in clean dermatologic procedures offer no additional benefit compared with petrolatum. Additionally, topical antibiotics carry a risk of antibiotic resistance, adverse reactions such as ACD and anaphylaxis, and higher health care costs.9 Thus, petrolatum should be used as standard care after clean cutaneous procedures, and the application of antibiotics should be abandoned.
Petrolatum also is an effective treatment for pruritus scroti.15 It is particularly helpful for recalcitrant disease when several topical medications have failed or ACD or irritant contact dermatitis to medications or cleansing products is suspected. Although topical corticosteroids are the mainstay of treatment, severe burning or redness may occur with prolonged use of these medications, thus it often is useful to discontinue topical medications and treat with plain water sitz baths at night followed by petrolatum immediately applied over wet skin. This approach has several benefits, including soothing the area, providing an occlusive barrier, retaining moisture, and eliminating contact with steroids and potential allergens and irritants. This may be followed with patch testing to determine if ACD from cleansing products or medications is the culprit. This treatment also may be used in pruritus ani or pruritus vulvae.15
Finally, petrolatum may even be used to treat parasitic skin infections such as cutaneous furuncular myiasis,16 a condition most commonly caused by the human botfly (Dermatobia hominis) or the African tumbu fly (Cordylobia anthropophaga). The larvae infest the skin by penetrating the dermis and burrowing into the subdermal layer. It is characterized by furuncular nodules with a central black punctum formed by larvae burrowed underneath the skin. An inflammatory reaction occurs in the sites surrounding the larvae with erythematous, edematous, and tender skin. Symptoms range from mild pruritus and a prickly heat sensation to intense cutaneous pain, agitation, and insomnia. Occluding the punctum, or breathing hole, of the infectious organism with petrolatum will asphyxiate the larvae, causing it to emerge within and leading to definitive diagnosis and treatment. This permits rapid removal and avoids extensive incision and extraction.16
The increased social media attention of petrolatum has raised the awareness of its utility as a moisturizer; however, it has many other uses, including soothing itch and irritation, improving wound healing, alleviating scrotal itch, and treating parasitic skin infections. It not only is an effective product but also is a particularly safe one. Petrolatum is well deserving of its positive reputation in dermatology and its current popularity among the general public
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
- Cramer M. A staple of grandma’s medicine cabinet gets hot on TikTok. New York Times. Published February 11, 2022. Accessed September 15, 2022. https://www.nytimes.com/2022/02/11/business/vaseline-slugging-tiktok.html
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Gutman AB, Kligman AM, Sciacca J, et al. Soak and smear: a standard technique revisited. 2005;141:1556-1559. doi:10.1001/archderm.141.12.1556
- Ghadially R, Halkier-Sorensen L, Elias PM. Effects of petrolatum on stratum corneum structure and function. J Am Acad Dermatol. 1992;26:387-396. doi:10.1016/0190-9622(92)70060-S
- Czarnowicki T, Malajian D, Khattri S, et al. Petrolatum: barrier repair and antimicrobial responses underlying this “inert” moisturizer. J Allergy Clin Immunol. 2016;137:1091-1102.e7. doi:10.1016/j.jaci.2015.08.013
- Field CK, Kerstein MD. Overview of wound healing in a moist environment. Am J Surg. 1994;167:2S-6S.
- Winter GD. Some factors affecting skin and wound healing. J Tissue Viability. 2006;16:20-23. doi:10.1016/S0965-206X(06)62006-8
- Smack DP, Harrington AC, Dunn C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. a randomized controlled trial. JAMA. 1996;276:972-977.
- Jacob SE, James WD. From road rash to top allergen in a flash: bacitracin. 2004;30(4 pt 1):521-524. doi:10.1111/j.1524-4725.2004.30168.x..
- Zaki I, Shall L, Dalziel KL. Bacitracin: a significant sensitizer in leg ulcer patients? Contact Dermatitis. 1994;31:92-94. doi:10.1111/j.1600-0536.1994.tb01924.x
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermatitis. 1995;6:28-31. doi:10.1016/1046-199X(95)90066-7
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Nguyen JK, Huang A, Siegel DM, et al. Variability in wound care recommendations following dermatologic procedures. Dermatol Surg. 2020;46:186-191. doi:10.1097/DSS.0000000000001952
- Fathy R, Chu B, Singh P, et al. Variation in topical antibiotics recommendations in wound care instructions by non-dermatologists. J Gen Intern Med. 2021;36:238-239. doi:10.1007/s11606-020-05689-2
- James WD, Elston DM, Treat JR, et al. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
Learning Experiences in LGBT Health During Dermatology Residency
Approximately 4.5% of adults within the United States identify as members of the lesbian, gay, bisexual, transgender (LGBT) community.1 This is an umbrella term inclusive of all individuals identifying as nonheterosexual or noncisgender. Although the LGBT community has increasingly become more recognized and accepted by society over time, health care disparities persist and have been well documented in the literature.2-4 Dermatologists have the potential to greatly impact LGBT health, as many health concerns in this population are cutaneous, such as sun-protection behaviors, side effects of gender-affirming hormone therapy and gender-affirming procedures, and cutaneous manifestations of sexually transmitted infections.5-7
An education gap has been demonstrated in both medical students and resident physicians regarding LGBT health and cultural competency. In a large-scale, multi-institutional survey study published in 2015, approximately two-thirds of medical students rated their schools’ LGBT curriculum as fair, poor, or very poor.8 Additional studies have echoed these results and have demonstrated not only the need but the desire for additional training on LGBT issues in medical school.9-11 The Association of American Medical Colleges has begun implementing curricular and institutional changes to fulfill this need.12,13
The LGBT education gap has been shown to extend into residency training. Multiple studies performed within a variety of medical specialties have demonstrated that resident physicians receive insufficient training in LGBT health issues, lack comfort in caring for LGBT patients, and would benefit from dedicated curricula on these topics.14-18 Currently, the 2022 Accreditation Council for Graduate Medical Education (ACGME) guidelines related to LGBT health are minimal and nonspecific.19
Ensuring that dermatology trainees are well equipped to manage these issues while providing culturally competent care to LGBT patients is paramount. However, research suggests that dedicated training on these topics likely is insufficient. A survey study of dermatology residency program directors (N=90) revealed that although 81% (72/89) viewed training in LGBT health as either very important or somewhat important, 46% (41/90) of programs did not dedicate any time to this content and 37% (33/90) only dedicated 1 to 2 hours per year.20
To further explore this potential education gap, we surveyed dermatology residents directly to better understand LGBT education within residency training, resident preparedness to care for LGBT patients, and outness/discrimination of LGBT-identifying residents. We believe this study should drive future research on the development and implementation of LGBT-specific curricula in dermatology training programs.
Methods
A cross-sectional survey study of dermatology residents in the United States was conducted. The study was deemed exempt from review by The Ohio State University (Columbus, Ohio) institutional review board. Survey responses were collected from October 7, 2020, to November 13, 2020. Qualtrics software was used to create the 20-question survey, which included a combination of categorical, dichotomous, and optional free-text questions related to patient demographics, LGBT training experiences, perceived areas of curriculum improvement, comfort level managing LGBT health issues, and personal experiences. Some questions were adapted from prior surveys.15,21 Validated survey tools used included the 2020 US Census to collect information regarding race and ethnicity, the Mohr and Fassinger Outness Inventory to measure outness regarding sexual orientation, and select questions from the 2020 Association of American Medical Colleges Medical School Graduation Questionnaire regarding discrimination.22-24
The survey was distributed to current allopathic and osteopathic dermatology residents by a variety of methods, including emails to program director and program coordinator listserves. The survey also was posted in the American Academy of Dermatology Expert Resource Group on LGBTQ Health October 2020 newsletter, as well as dermatology social media groups, including a messaging forum limited to dermatology residents, a Facebook group open to dermatologists and dermatology residents, and the Facebook group of the Gay and Lesbian Dermatology Association. Current dermatology residents, including those in combined dermatology and internal medicine programs, were included. Individuals who had been accepted to dermatology training programs but had not yet started were excluded. A follow-up email was sent to the program director listserve approximately 3 weeks after the initial distribution.
Statistical Analysis—The data were analyzed in Qualtrics and Microsoft Excel using descriptive statistics. Stata software (Stata 15.1, StataCorp) was used to perform a Kruskal-Wallis equality-of-populations rank test to compare the means of education level and feelings of preparedness.
Results
Demographics of Respondents—A total of 126 responses were recorded, 12 of which were blank and were removed from the database. A total of 114 dermatology residents’ responses were collected in Qualtrics and analyzed; 91 completed the entire survey (an 80% completion rate). Based on the 2020-2021 ACGME data listing, there were 1612 dermatology residents in the United States, which is an estimated response rate of 7% (114/1612).25 The eTable outlines the demographics of the survey respondents. Most were cisgender females (60%), followed by cisgender males (35%); the remainder preferred not to answer. Regarding sexual orientation, 77% identified as straight or heterosexual; 17% as gay, lesbian, or homosexual; 1% as queer; and 1% as bisexual. The training programs were in 26 states, the majority of which were in the Midwest (34%) and in urban settings (69%). A wide range of postgraduate levels and residency sizes were represented in the survey.
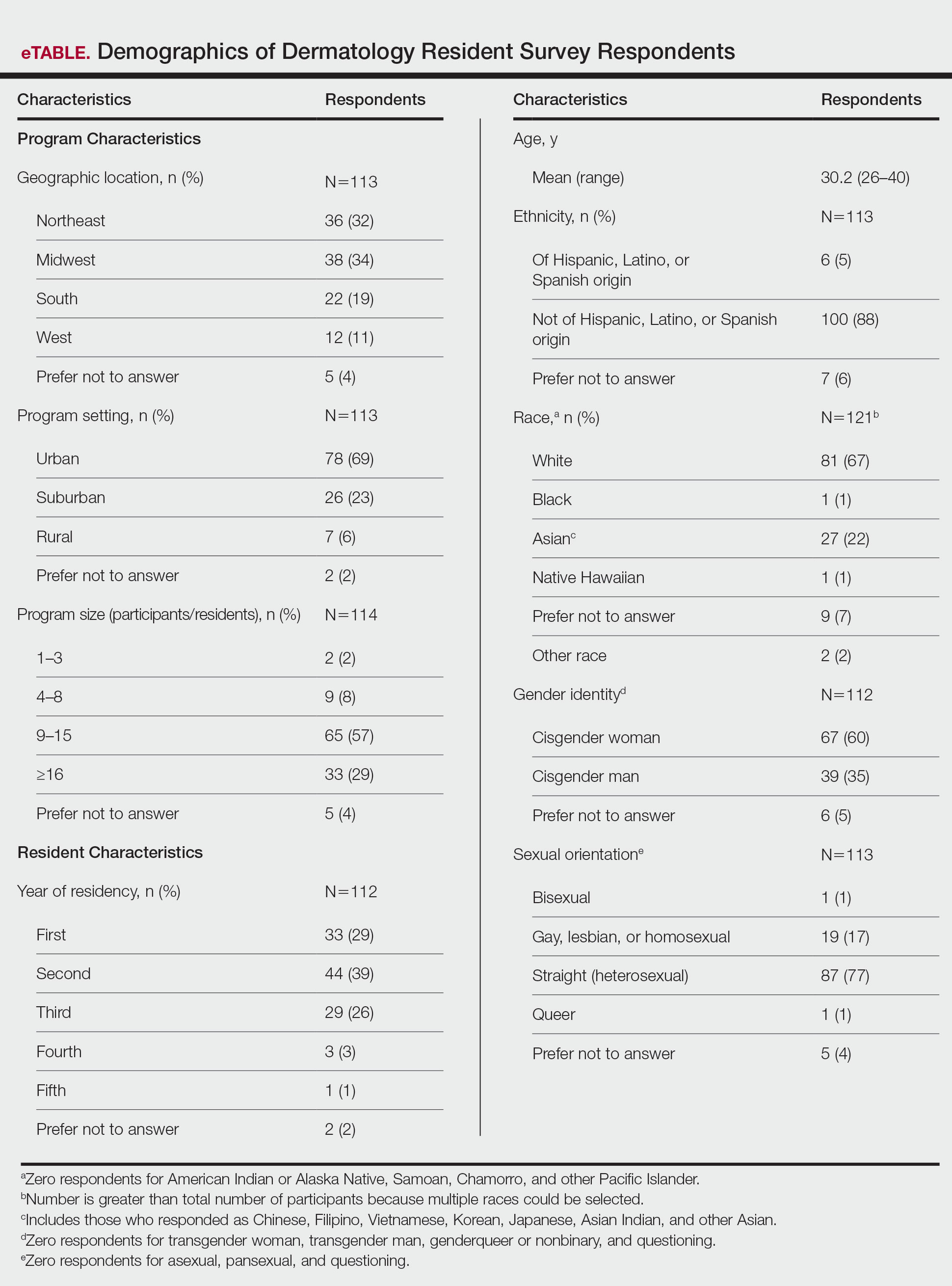
LGBT Education—Fifty-one percent of respondents reported that their programs offer 1 hour or less of LGBT-related curricula per year; 34% reported no time dedicated to this topic. A small portion of residents (5%) reported 10 or more hours of LGBT education per year. Residents also were asked the average number of hours of LGBT education they thought they should receive. The discrepancy between these measures can be visualized in Figure 1. The median hours of education received was 1 hour (IQR, 0–4 hours), whereas the median hours of education desired was 4 hours (IQR, 2–5 hours). The most common and most helpful methods of education reported were clinical experiences with faculty or patients and live lectures.
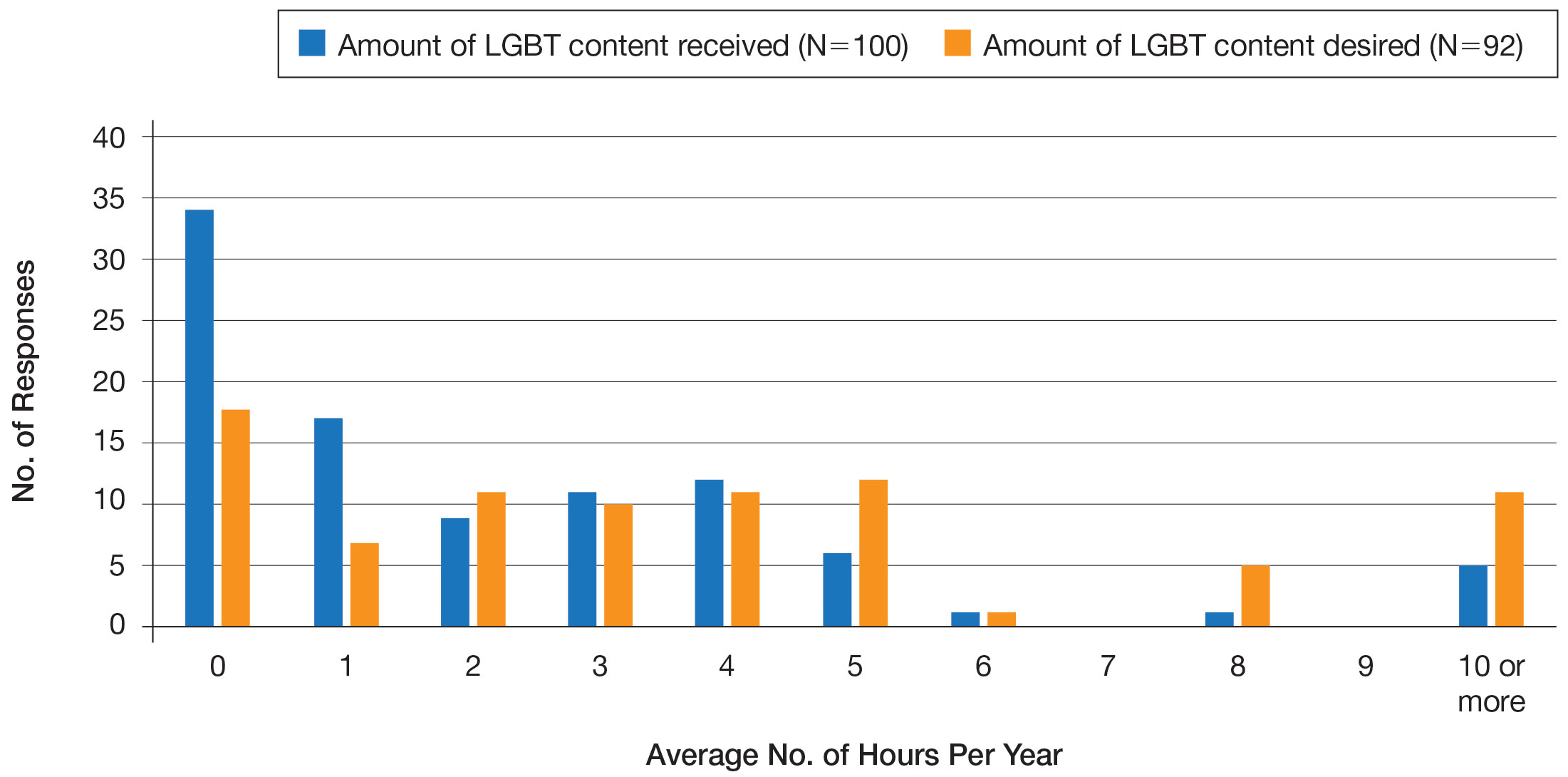
Overall, 45% of survey respondents felt that LGBT topics were covered poorly or not at all in dermatology residency, whereas 26% thought the coverage was good or excellent. The topics that residents were most likely to report receiving good or excellent coverage were dermatologic manifestations of HIV/AIDS (70%) and sexually transmitted diseases in LGBT patients (48%). The topics that were most likely to be reported as not taught or poorly taught included dermatologic concerns associated with puberty blockers (71%), body image (58%), dermatologic concerns associated with gender-affirming surgery (55%), skin cancer risk (53%), taking an LGBT-oriented history and physical examination (52%), and effects of gender-affirming hormone therapy on the skin (50%). A detailed breakdown of coverage level by topic can be found in Figure 2.
Preparedness to Care for LGBT Patients—Only 68% of survey respondents agreed or strongly agreed that they feel comfortable treating LGBT patients. Furthermore, 49% of dermatology residents reported that they feel not at all prepared or insufficiently prepared to provide care to LGBT individuals (Figure 2), and 60% believed that LGBT training needed to be improved at their residency programs.
There was a significant association between reported level of education and feelings of preparedness. A high ranking of provided education was associated with higher levels of feeling prepared to care for LGBT patients (Kruskal-Wallis rank test, P<.001).
Discrimination/Outness—Approximately one-fourth (24%; 4/17) of nonheterosexual dermatology residents reported that they had been subjected to offensive remarks about their sexual orientation in the workplace. One respondent commented that they were less “out” at their residency program due to fear of discrimination. Nearly one-third of the overall group of dermatology residents surveyed (29%; 27/92) reported that they had witnessed inappropriate or discriminatory comments about LGBT persons made by employees or staff at their programs. Most residents surveyed (96%; 88/92) agreed or strongly agreed that they feel comfortable working alongside LGBT physicians.
There were 18 nonheterosexual dermatologyresidents who completed the Mohr and Fassinger Outness Inventory.23 In general, respondents reported that they were more “out” with friends and family than work peers and were least “out” with work supervisors and strangers.
Comment
Dermatology Residents Desire More Time on LGBT Health—This cross-sectional survey study explored dermatology residents’ educational experiences with LGBT health during residency training. Similar studies have been performed in other specialties, including a study from 2019 surveying emergency medicine residents that demonstrated residents find caring for LGBT patients more challenging.15 Another 2019 study surveying psychiatry residents found that 42.4% (N=99) reported no coverage of LGBT topics.18 Our study is unique in that it surveyed dermatology residents directly regarding this topic. Although most dermatology program directors view LGBT dermatologic health as an important topic, a prior study revealed that many programs are lacking dedicated LGBT educational experiences. The most common barriers reported were insufficient time in the didactic schedule and lack of experienced faculty.20
Our study revealed that dermatology residents overall tend to agree with residents from other specialties and dermatology program directors. Most of the dermatology residents surveyed reported desiring more time per year spent on LGBT health education than they receive, and 60% expressed that LGBT educational experiences need to be improved at their residency programs. Education on and subsequent comfort level with LGBT health issues varied by subtopic, with most residents feeling comfortable dealing with dermatologic manifestations of HIV/AIDS and other sexually transmitted diseases and less comfortable with topics such as puberty blockers, gender-affirming surgery and hormone therapy, body image, and skin cancer risk.
Overall, LGBT health training is viewed as important and in need of improvement by both program directors and residents, yet implementation lags at many programs. A small proportion of the represented programs are excelling in this area—just over 5% of respondents reported receiving 10 or more hours of LGBT-relevant education per year, and approximately 26% of residents felt that LGBT coverage was good or excellent at their programs. Our study showed a clear relationship between feelings of preparedness and education level. The lack of LGBT education at some dermatology residency programs translated into a large portion of dermatology residents feeling ill equipped to care for LGBT patients after graduation—nearly 50% of those surveyed reported feeling insufficiently prepared to care for the LGBT community.
Discrimination in Residency Programs—Dermatology residency programs also are not free from sexual orientation–related and gender identity–related workplace discrimination. Although 96% of dermatology residents reported that they feel comfortable working alongside LGBT physicians, 24% of nonheterosexual respondents stated they had been subjected to offensive remarks about their sexual orientation, and 29% of the overall group of dermatology residents had witnessed discriminatory comments to LGBT individuals at their programs. In addition, some nonheterosexual dermatology residents reported being less “out” with their workplace supervisors and strangers, such as patients, than with their family and friends, and 50% of this group reported that their sexual identity was not openly discussed with their workplace supervisors. It has been demonstrated that individuals are more likely to “come out” in perceived LGBT-friendly workplace environments and that being “out” positively impacts psychological health because of the effects of perceived social support and self-coherence.26,27
Study Strengths and Limitations—Strengths of this study include the modest sample size of dermatology residents that participated, high completion rate, and the anonymity of the survey. Limitations include the risk of sampling bias by posting the survey on LGBT-specific groups. The survey also took place in the fall, so the results may not accurately reflect programs that cover this material later in the academic year. Lastly, not all survey questions were validated.
Implementing Change in Residency Programs—Although the results of this study exposed the need for increasing LGBT education in dermatology residency, they do not provide guidelines for the best strategy to begin implementing change. A study from 2020 provides some guidance for incorporating LGBT health training into dermatology residency programs through a combination of curricular modifications and climate optimization.28 Additional future research should focus on the best methods for preparing dermatology residents to care for this population. In this study, residents reported that the most effective teaching methods were real encounters with LGBT patients or faculty educated on LGBT health as well as live lectures from experts. There also appeared to be a correlation between hours spent on LGBT health, including various subtopics, and residents’ perceived preparedness in these areas. Potential actionable items include clarifying the ACGME guidelines on LGBT health topics; increasing the sexual and gender diversity of the faculty, staff, residents, and patients; and dedicating additional didactic and clinical time to LGBT topics and experiences.
Conclusion
This survey study of dermatology residents regarding LGBT learning experiences in residency training provided evidence that dermatology residents as a whole are not adequately taught LGBT health topics and therefore feel unprepared to take care of this patient population. Additionally, most residents desire improvement of LGBT health education and training. Further studies focusing on the best methods for implementing LGBT-specific curricula are needed.
- Newport F. In U.S., estimate of LGBT population rises to 4.5%. Gallup. May 22, 2018. Accessed September 19, 2022. https://news.gallup.com/poll/234863/estimate-lgbt-population-rises.aspx
- Hafeez H, Zeshan M, Tahir MA, et al. Health care disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Gonzales G, Henning-Smith C. Barriers to care among transgender and gender nonconforming adults. Millbank Q. 2017;95:726-748.
- Quinn GP, Sanchez JA, Sutton SK, et al. Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA Cancer J Clin. 2015;65:384-400.
- Sullivan P, Trinidad J, Hamann D. Issues in transgender dermatology: a systematic review of the literature. J Am Acad Dermatol. 2019;81:438-447.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
- White W, Brenman S, Paradis E, et al. Lesbian, gay, bisexual, and transgender patient care: medical students’ preparedness and comfort. Teach Learn Med. 2015;27:254-263.
- Nama N, MacPherson P, Sampson M, et al. Medical students’ perception of lesbian, gay, bisexual, and transgender (LGBT) discrimination in their learning environment and their self-reported comfort level for caring for LGBT patients: a survey study. Med Educ Online. 2017;22:1-8.
- Phelan SM, Burke SE, Hardeman RR, et al. Medical school factors associated with changes in implicit and explicit bias against gay and lesbian people among 3492 graduating medical students. J Gen Intern Med. 2017;32:1193-1201.
- Cherabie J, Nilsen K, Houssayni S. Transgender health medical education intervention and its effects on beliefs, attitudes, comfort, and knowledge. Kans J Med. 2018;11:106-109.
- Integrating LGBT and DSD content into medical school curricula. Association of American Medical Colleges website. Published November 2015. Accessed September 23, 2022. https://www.aamc.org/what-we-do/equity-diversity-inclusion/lgbt-health-resources/videos/curricula-integration
- Cooper MB, Chacko M, Christner J. Incorporating LGBT health in an undergraduate medical education curriculum through the construct of social determinants of health. MedEdPORTAL. 2018;14:10781.
- Moll J, Krieger P, Moreno-Walton L, et al. The prevalence of lesbian, gay, bisexual, and transgender health education and training in emergency medicine residency programs: what do we know? Acad Emerg Med. 2014;21:608-611.
- Moll J, Krieger P, Heron SL, et al. Attitudes, behavior, and comfort of emergency medicine residents in caring for LGBT patients: what do we know? AEM Educ Train. 2019;3:129-135.
- Hirschtritt ME, Noy G, Haller E, et al. LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:41-45.
- Ufomata E, Eckstrand KL, Spagnoletti C, et al. Comprehensive curriculum for internal medicine residents on primary care of patients identifying as lesbian, gay, bisexual, or transgender. MedEdPORTAL. 2020;16:10875.
- Zonana J, Batchelder S, Pula J, et al. Comment on: LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:547-548.
- Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Dermatology. Revised June 12, 2022. Accessed September 23, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/080_dermatology_2022.pdf
- Jia JL, Nord KM, Sarin KY, et al. Sexual and gender minority curricula within US dermatology residency programs. JAMA Dermatol. 2020;156:593-594.
- Mansh M, White W, Gee-Tong L, et al. Sexual and gender minority identity disclosure during undergraduate medical education: “in the closet” in medical school. Acad Med. 2015;90:634-644.
- US Census Bureau. 2020 Census Informational Questionnaire. Accessed September 19, 2022. https://www2.census.gov/programs-surveys/decennial/2020/technical-documentation/questionnaires-and-instructions/questionnaires/2020-informational-questionnaire-english_DI-Q1.pdf
- Mohr JJ, Fassinger RE. Measuring dimensions of lesbian and gay male experience. Meas Eval Couns Dev. 2000;33:66-90.
- Association of American Medical Colleges. Medical School Graduation Questionnaire: 2020 All Schools Summary Report. Published July 2020. Accessed September 19, 2022. https://www.aamc.org/media/46851/download
- Accreditation Council for Graduate Medical Education. Data Resource Book: Academic Year 2019-2020. Accessed September 19, 2022. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2019-2020_acgme_databook_document.pdf
- Mohr JJ, Jackson SD, Sheets RL. Sexual orientation self-presentation among bisexual-identified women and men: patterns and predictors. Arch Sex Behav. 2017;46:1465-1479.
- Tatum AK. Workplace climate and job satisfaction: a test of social cognitive career theory (SCCT)’s workplace self-management model with sexual minority employees. Semantic Scholar. 2018. Accessed September 19, 2022. https://www.semanticscholar.org/paper/Workplace-Climate-and-Job-Satisfaction%3A-A-Test-of-Tatum/5af75ab70acfb73c54e34b95597576d30e07df12
- Fakhoury JW, Daveluy S. Incorporating lesbian, gay, bisexual, and transgender training into a residency program. Dermatol Clin. 2020;38:285-292.
Approximately 4.5% of adults within the United States identify as members of the lesbian, gay, bisexual, transgender (LGBT) community.1 This is an umbrella term inclusive of all individuals identifying as nonheterosexual or noncisgender. Although the LGBT community has increasingly become more recognized and accepted by society over time, health care disparities persist and have been well documented in the literature.2-4 Dermatologists have the potential to greatly impact LGBT health, as many health concerns in this population are cutaneous, such as sun-protection behaviors, side effects of gender-affirming hormone therapy and gender-affirming procedures, and cutaneous manifestations of sexually transmitted infections.5-7
An education gap has been demonstrated in both medical students and resident physicians regarding LGBT health and cultural competency. In a large-scale, multi-institutional survey study published in 2015, approximately two-thirds of medical students rated their schools’ LGBT curriculum as fair, poor, or very poor.8 Additional studies have echoed these results and have demonstrated not only the need but the desire for additional training on LGBT issues in medical school.9-11 The Association of American Medical Colleges has begun implementing curricular and institutional changes to fulfill this need.12,13
The LGBT education gap has been shown to extend into residency training. Multiple studies performed within a variety of medical specialties have demonstrated that resident physicians receive insufficient training in LGBT health issues, lack comfort in caring for LGBT patients, and would benefit from dedicated curricula on these topics.14-18 Currently, the 2022 Accreditation Council for Graduate Medical Education (ACGME) guidelines related to LGBT health are minimal and nonspecific.19
Ensuring that dermatology trainees are well equipped to manage these issues while providing culturally competent care to LGBT patients is paramount. However, research suggests that dedicated training on these topics likely is insufficient. A survey study of dermatology residency program directors (N=90) revealed that although 81% (72/89) viewed training in LGBT health as either very important or somewhat important, 46% (41/90) of programs did not dedicate any time to this content and 37% (33/90) only dedicated 1 to 2 hours per year.20
To further explore this potential education gap, we surveyed dermatology residents directly to better understand LGBT education within residency training, resident preparedness to care for LGBT patients, and outness/discrimination of LGBT-identifying residents. We believe this study should drive future research on the development and implementation of LGBT-specific curricula in dermatology training programs.
Methods
A cross-sectional survey study of dermatology residents in the United States was conducted. The study was deemed exempt from review by The Ohio State University (Columbus, Ohio) institutional review board. Survey responses were collected from October 7, 2020, to November 13, 2020. Qualtrics software was used to create the 20-question survey, which included a combination of categorical, dichotomous, and optional free-text questions related to patient demographics, LGBT training experiences, perceived areas of curriculum improvement, comfort level managing LGBT health issues, and personal experiences. Some questions were adapted from prior surveys.15,21 Validated survey tools used included the 2020 US Census to collect information regarding race and ethnicity, the Mohr and Fassinger Outness Inventory to measure outness regarding sexual orientation, and select questions from the 2020 Association of American Medical Colleges Medical School Graduation Questionnaire regarding discrimination.22-24
The survey was distributed to current allopathic and osteopathic dermatology residents by a variety of methods, including emails to program director and program coordinator listserves. The survey also was posted in the American Academy of Dermatology Expert Resource Group on LGBTQ Health October 2020 newsletter, as well as dermatology social media groups, including a messaging forum limited to dermatology residents, a Facebook group open to dermatologists and dermatology residents, and the Facebook group of the Gay and Lesbian Dermatology Association. Current dermatology residents, including those in combined dermatology and internal medicine programs, were included. Individuals who had been accepted to dermatology training programs but had not yet started were excluded. A follow-up email was sent to the program director listserve approximately 3 weeks after the initial distribution.
Statistical Analysis—The data were analyzed in Qualtrics and Microsoft Excel using descriptive statistics. Stata software (Stata 15.1, StataCorp) was used to perform a Kruskal-Wallis equality-of-populations rank test to compare the means of education level and feelings of preparedness.
Results
Demographics of Respondents—A total of 126 responses were recorded, 12 of which were blank and were removed from the database. A total of 114 dermatology residents’ responses were collected in Qualtrics and analyzed; 91 completed the entire survey (an 80% completion rate). Based on the 2020-2021 ACGME data listing, there were 1612 dermatology residents in the United States, which is an estimated response rate of 7% (114/1612).25 The eTable outlines the demographics of the survey respondents. Most were cisgender females (60%), followed by cisgender males (35%); the remainder preferred not to answer. Regarding sexual orientation, 77% identified as straight or heterosexual; 17% as gay, lesbian, or homosexual; 1% as queer; and 1% as bisexual. The training programs were in 26 states, the majority of which were in the Midwest (34%) and in urban settings (69%). A wide range of postgraduate levels and residency sizes were represented in the survey.

LGBT Education—Fifty-one percent of respondents reported that their programs offer 1 hour or less of LGBT-related curricula per year; 34% reported no time dedicated to this topic. A small portion of residents (5%) reported 10 or more hours of LGBT education per year. Residents also were asked the average number of hours of LGBT education they thought they should receive. The discrepancy between these measures can be visualized in Figure 1. The median hours of education received was 1 hour (IQR, 0–4 hours), whereas the median hours of education desired was 4 hours (IQR, 2–5 hours). The most common and most helpful methods of education reported were clinical experiences with faculty or patients and live lectures.

Overall, 45% of survey respondents felt that LGBT topics were covered poorly or not at all in dermatology residency, whereas 26% thought the coverage was good or excellent. The topics that residents were most likely to report receiving good or excellent coverage were dermatologic manifestations of HIV/AIDS (70%) and sexually transmitted diseases in LGBT patients (48%). The topics that were most likely to be reported as not taught or poorly taught included dermatologic concerns associated with puberty blockers (71%), body image (58%), dermatologic concerns associated with gender-affirming surgery (55%), skin cancer risk (53%), taking an LGBT-oriented history and physical examination (52%), and effects of gender-affirming hormone therapy on the skin (50%). A detailed breakdown of coverage level by topic can be found in Figure 2.

Preparedness to Care for LGBT Patients—Only 68% of survey respondents agreed or strongly agreed that they feel comfortable treating LGBT patients. Furthermore, 49% of dermatology residents reported that they feel not at all prepared or insufficiently prepared to provide care to LGBT individuals (Figure 2), and 60% believed that LGBT training needed to be improved at their residency programs.
There was a significant association between reported level of education and feelings of preparedness. A high ranking of provided education was associated with higher levels of feeling prepared to care for LGBT patients (Kruskal-Wallis rank test, P<.001).
Discrimination/Outness—Approximately one-fourth (24%; 4/17) of nonheterosexual dermatology residents reported that they had been subjected to offensive remarks about their sexual orientation in the workplace. One respondent commented that they were less “out” at their residency program due to fear of discrimination. Nearly one-third of the overall group of dermatology residents surveyed (29%; 27/92) reported that they had witnessed inappropriate or discriminatory comments about LGBT persons made by employees or staff at their programs. Most residents surveyed (96%; 88/92) agreed or strongly agreed that they feel comfortable working alongside LGBT physicians.
There were 18 nonheterosexual dermatologyresidents who completed the Mohr and Fassinger Outness Inventory.23 In general, respondents reported that they were more “out” with friends and family than work peers and were least “out” with work supervisors and strangers.
Comment
Dermatology Residents Desire More Time on LGBT Health—This cross-sectional survey study explored dermatology residents’ educational experiences with LGBT health during residency training. Similar studies have been performed in other specialties, including a study from 2019 surveying emergency medicine residents that demonstrated residents find caring for LGBT patients more challenging.15 Another 2019 study surveying psychiatry residents found that 42.4% (N=99) reported no coverage of LGBT topics.18 Our study is unique in that it surveyed dermatology residents directly regarding this topic. Although most dermatology program directors view LGBT dermatologic health as an important topic, a prior study revealed that many programs are lacking dedicated LGBT educational experiences. The most common barriers reported were insufficient time in the didactic schedule and lack of experienced faculty.20
Our study revealed that dermatology residents overall tend to agree with residents from other specialties and dermatology program directors. Most of the dermatology residents surveyed reported desiring more time per year spent on LGBT health education than they receive, and 60% expressed that LGBT educational experiences need to be improved at their residency programs. Education on and subsequent comfort level with LGBT health issues varied by subtopic, with most residents feeling comfortable dealing with dermatologic manifestations of HIV/AIDS and other sexually transmitted diseases and less comfortable with topics such as puberty blockers, gender-affirming surgery and hormone therapy, body image, and skin cancer risk.
Overall, LGBT health training is viewed as important and in need of improvement by both program directors and residents, yet implementation lags at many programs. A small proportion of the represented programs are excelling in this area—just over 5% of respondents reported receiving 10 or more hours of LGBT-relevant education per year, and approximately 26% of residents felt that LGBT coverage was good or excellent at their programs. Our study showed a clear relationship between feelings of preparedness and education level. The lack of LGBT education at some dermatology residency programs translated into a large portion of dermatology residents feeling ill equipped to care for LGBT patients after graduation—nearly 50% of those surveyed reported feeling insufficiently prepared to care for the LGBT community.
Discrimination in Residency Programs—Dermatology residency programs also are not free from sexual orientation–related and gender identity–related workplace discrimination. Although 96% of dermatology residents reported that they feel comfortable working alongside LGBT physicians, 24% of nonheterosexual respondents stated they had been subjected to offensive remarks about their sexual orientation, and 29% of the overall group of dermatology residents had witnessed discriminatory comments to LGBT individuals at their programs. In addition, some nonheterosexual dermatology residents reported being less “out” with their workplace supervisors and strangers, such as patients, than with their family and friends, and 50% of this group reported that their sexual identity was not openly discussed with their workplace supervisors. It has been demonstrated that individuals are more likely to “come out” in perceived LGBT-friendly workplace environments and that being “out” positively impacts psychological health because of the effects of perceived social support and self-coherence.26,27
Study Strengths and Limitations—Strengths of this study include the modest sample size of dermatology residents that participated, high completion rate, and the anonymity of the survey. Limitations include the risk of sampling bias by posting the survey on LGBT-specific groups. The survey also took place in the fall, so the results may not accurately reflect programs that cover this material later in the academic year. Lastly, not all survey questions were validated.
Implementing Change in Residency Programs—Although the results of this study exposed the need for increasing LGBT education in dermatology residency, they do not provide guidelines for the best strategy to begin implementing change. A study from 2020 provides some guidance for incorporating LGBT health training into dermatology residency programs through a combination of curricular modifications and climate optimization.28 Additional future research should focus on the best methods for preparing dermatology residents to care for this population. In this study, residents reported that the most effective teaching methods were real encounters with LGBT patients or faculty educated on LGBT health as well as live lectures from experts. There also appeared to be a correlation between hours spent on LGBT health, including various subtopics, and residents’ perceived preparedness in these areas. Potential actionable items include clarifying the ACGME guidelines on LGBT health topics; increasing the sexual and gender diversity of the faculty, staff, residents, and patients; and dedicating additional didactic and clinical time to LGBT topics and experiences.
Conclusion
This survey study of dermatology residents regarding LGBT learning experiences in residency training provided evidence that dermatology residents as a whole are not adequately taught LGBT health topics and therefore feel unprepared to take care of this patient population. Additionally, most residents desire improvement of LGBT health education and training. Further studies focusing on the best methods for implementing LGBT-specific curricula are needed.
Approximately 4.5% of adults within the United States identify as members of the lesbian, gay, bisexual, transgender (LGBT) community.1 This is an umbrella term inclusive of all individuals identifying as nonheterosexual or noncisgender. Although the LGBT community has increasingly become more recognized and accepted by society over time, health care disparities persist and have been well documented in the literature.2-4 Dermatologists have the potential to greatly impact LGBT health, as many health concerns in this population are cutaneous, such as sun-protection behaviors, side effects of gender-affirming hormone therapy and gender-affirming procedures, and cutaneous manifestations of sexually transmitted infections.5-7
An education gap has been demonstrated in both medical students and resident physicians regarding LGBT health and cultural competency. In a large-scale, multi-institutional survey study published in 2015, approximately two-thirds of medical students rated their schools’ LGBT curriculum as fair, poor, or very poor.8 Additional studies have echoed these results and have demonstrated not only the need but the desire for additional training on LGBT issues in medical school.9-11 The Association of American Medical Colleges has begun implementing curricular and institutional changes to fulfill this need.12,13
The LGBT education gap has been shown to extend into residency training. Multiple studies performed within a variety of medical specialties have demonstrated that resident physicians receive insufficient training in LGBT health issues, lack comfort in caring for LGBT patients, and would benefit from dedicated curricula on these topics.14-18 Currently, the 2022 Accreditation Council for Graduate Medical Education (ACGME) guidelines related to LGBT health are minimal and nonspecific.19
Ensuring that dermatology trainees are well equipped to manage these issues while providing culturally competent care to LGBT patients is paramount. However, research suggests that dedicated training on these topics likely is insufficient. A survey study of dermatology residency program directors (N=90) revealed that although 81% (72/89) viewed training in LGBT health as either very important or somewhat important, 46% (41/90) of programs did not dedicate any time to this content and 37% (33/90) only dedicated 1 to 2 hours per year.20
To further explore this potential education gap, we surveyed dermatology residents directly to better understand LGBT education within residency training, resident preparedness to care for LGBT patients, and outness/discrimination of LGBT-identifying residents. We believe this study should drive future research on the development and implementation of LGBT-specific curricula in dermatology training programs.
Methods
A cross-sectional survey study of dermatology residents in the United States was conducted. The study was deemed exempt from review by The Ohio State University (Columbus, Ohio) institutional review board. Survey responses were collected from October 7, 2020, to November 13, 2020. Qualtrics software was used to create the 20-question survey, which included a combination of categorical, dichotomous, and optional free-text questions related to patient demographics, LGBT training experiences, perceived areas of curriculum improvement, comfort level managing LGBT health issues, and personal experiences. Some questions were adapted from prior surveys.15,21 Validated survey tools used included the 2020 US Census to collect information regarding race and ethnicity, the Mohr and Fassinger Outness Inventory to measure outness regarding sexual orientation, and select questions from the 2020 Association of American Medical Colleges Medical School Graduation Questionnaire regarding discrimination.22-24
The survey was distributed to current allopathic and osteopathic dermatology residents by a variety of methods, including emails to program director and program coordinator listserves. The survey also was posted in the American Academy of Dermatology Expert Resource Group on LGBTQ Health October 2020 newsletter, as well as dermatology social media groups, including a messaging forum limited to dermatology residents, a Facebook group open to dermatologists and dermatology residents, and the Facebook group of the Gay and Lesbian Dermatology Association. Current dermatology residents, including those in combined dermatology and internal medicine programs, were included. Individuals who had been accepted to dermatology training programs but had not yet started were excluded. A follow-up email was sent to the program director listserve approximately 3 weeks after the initial distribution.
Statistical Analysis—The data were analyzed in Qualtrics and Microsoft Excel using descriptive statistics. Stata software (Stata 15.1, StataCorp) was used to perform a Kruskal-Wallis equality-of-populations rank test to compare the means of education level and feelings of preparedness.
Results
Demographics of Respondents—A total of 126 responses were recorded, 12 of which were blank and were removed from the database. A total of 114 dermatology residents’ responses were collected in Qualtrics and analyzed; 91 completed the entire survey (an 80% completion rate). Based on the 2020-2021 ACGME data listing, there were 1612 dermatology residents in the United States, which is an estimated response rate of 7% (114/1612).25 The eTable outlines the demographics of the survey respondents. Most were cisgender females (60%), followed by cisgender males (35%); the remainder preferred not to answer. Regarding sexual orientation, 77% identified as straight or heterosexual; 17% as gay, lesbian, or homosexual; 1% as queer; and 1% as bisexual. The training programs were in 26 states, the majority of which were in the Midwest (34%) and in urban settings (69%). A wide range of postgraduate levels and residency sizes were represented in the survey.

LGBT Education—Fifty-one percent of respondents reported that their programs offer 1 hour or less of LGBT-related curricula per year; 34% reported no time dedicated to this topic. A small portion of residents (5%) reported 10 or more hours of LGBT education per year. Residents also were asked the average number of hours of LGBT education they thought they should receive. The discrepancy between these measures can be visualized in Figure 1. The median hours of education received was 1 hour (IQR, 0–4 hours), whereas the median hours of education desired was 4 hours (IQR, 2–5 hours). The most common and most helpful methods of education reported were clinical experiences with faculty or patients and live lectures.

Overall, 45% of survey respondents felt that LGBT topics were covered poorly or not at all in dermatology residency, whereas 26% thought the coverage was good or excellent. The topics that residents were most likely to report receiving good or excellent coverage were dermatologic manifestations of HIV/AIDS (70%) and sexually transmitted diseases in LGBT patients (48%). The topics that were most likely to be reported as not taught or poorly taught included dermatologic concerns associated with puberty blockers (71%), body image (58%), dermatologic concerns associated with gender-affirming surgery (55%), skin cancer risk (53%), taking an LGBT-oriented history and physical examination (52%), and effects of gender-affirming hormone therapy on the skin (50%). A detailed breakdown of coverage level by topic can be found in Figure 2.

Preparedness to Care for LGBT Patients—Only 68% of survey respondents agreed or strongly agreed that they feel comfortable treating LGBT patients. Furthermore, 49% of dermatology residents reported that they feel not at all prepared or insufficiently prepared to provide care to LGBT individuals (Figure 2), and 60% believed that LGBT training needed to be improved at their residency programs.
There was a significant association between reported level of education and feelings of preparedness. A high ranking of provided education was associated with higher levels of feeling prepared to care for LGBT patients (Kruskal-Wallis rank test, P<.001).
Discrimination/Outness—Approximately one-fourth (24%; 4/17) of nonheterosexual dermatology residents reported that they had been subjected to offensive remarks about their sexual orientation in the workplace. One respondent commented that they were less “out” at their residency program due to fear of discrimination. Nearly one-third of the overall group of dermatology residents surveyed (29%; 27/92) reported that they had witnessed inappropriate or discriminatory comments about LGBT persons made by employees or staff at their programs. Most residents surveyed (96%; 88/92) agreed or strongly agreed that they feel comfortable working alongside LGBT physicians.
There were 18 nonheterosexual dermatologyresidents who completed the Mohr and Fassinger Outness Inventory.23 In general, respondents reported that they were more “out” with friends and family than work peers and were least “out” with work supervisors and strangers.
Comment
Dermatology Residents Desire More Time on LGBT Health—This cross-sectional survey study explored dermatology residents’ educational experiences with LGBT health during residency training. Similar studies have been performed in other specialties, including a study from 2019 surveying emergency medicine residents that demonstrated residents find caring for LGBT patients more challenging.15 Another 2019 study surveying psychiatry residents found that 42.4% (N=99) reported no coverage of LGBT topics.18 Our study is unique in that it surveyed dermatology residents directly regarding this topic. Although most dermatology program directors view LGBT dermatologic health as an important topic, a prior study revealed that many programs are lacking dedicated LGBT educational experiences. The most common barriers reported were insufficient time in the didactic schedule and lack of experienced faculty.20
Our study revealed that dermatology residents overall tend to agree with residents from other specialties and dermatology program directors. Most of the dermatology residents surveyed reported desiring more time per year spent on LGBT health education than they receive, and 60% expressed that LGBT educational experiences need to be improved at their residency programs. Education on and subsequent comfort level with LGBT health issues varied by subtopic, with most residents feeling comfortable dealing with dermatologic manifestations of HIV/AIDS and other sexually transmitted diseases and less comfortable with topics such as puberty blockers, gender-affirming surgery and hormone therapy, body image, and skin cancer risk.
Overall, LGBT health training is viewed as important and in need of improvement by both program directors and residents, yet implementation lags at many programs. A small proportion of the represented programs are excelling in this area—just over 5% of respondents reported receiving 10 or more hours of LGBT-relevant education per year, and approximately 26% of residents felt that LGBT coverage was good or excellent at their programs. Our study showed a clear relationship between feelings of preparedness and education level. The lack of LGBT education at some dermatology residency programs translated into a large portion of dermatology residents feeling ill equipped to care for LGBT patients after graduation—nearly 50% of those surveyed reported feeling insufficiently prepared to care for the LGBT community.
Discrimination in Residency Programs—Dermatology residency programs also are not free from sexual orientation–related and gender identity–related workplace discrimination. Although 96% of dermatology residents reported that they feel comfortable working alongside LGBT physicians, 24% of nonheterosexual respondents stated they had been subjected to offensive remarks about their sexual orientation, and 29% of the overall group of dermatology residents had witnessed discriminatory comments to LGBT individuals at their programs. In addition, some nonheterosexual dermatology residents reported being less “out” with their workplace supervisors and strangers, such as patients, than with their family and friends, and 50% of this group reported that their sexual identity was not openly discussed with their workplace supervisors. It has been demonstrated that individuals are more likely to “come out” in perceived LGBT-friendly workplace environments and that being “out” positively impacts psychological health because of the effects of perceived social support and self-coherence.26,27
Study Strengths and Limitations—Strengths of this study include the modest sample size of dermatology residents that participated, high completion rate, and the anonymity of the survey. Limitations include the risk of sampling bias by posting the survey on LGBT-specific groups. The survey also took place in the fall, so the results may not accurately reflect programs that cover this material later in the academic year. Lastly, not all survey questions were validated.
Implementing Change in Residency Programs—Although the results of this study exposed the need for increasing LGBT education in dermatology residency, they do not provide guidelines for the best strategy to begin implementing change. A study from 2020 provides some guidance for incorporating LGBT health training into dermatology residency programs through a combination of curricular modifications and climate optimization.28 Additional future research should focus on the best methods for preparing dermatology residents to care for this population. In this study, residents reported that the most effective teaching methods were real encounters with LGBT patients or faculty educated on LGBT health as well as live lectures from experts. There also appeared to be a correlation between hours spent on LGBT health, including various subtopics, and residents’ perceived preparedness in these areas. Potential actionable items include clarifying the ACGME guidelines on LGBT health topics; increasing the sexual and gender diversity of the faculty, staff, residents, and patients; and dedicating additional didactic and clinical time to LGBT topics and experiences.
Conclusion
This survey study of dermatology residents regarding LGBT learning experiences in residency training provided evidence that dermatology residents as a whole are not adequately taught LGBT health topics and therefore feel unprepared to take care of this patient population. Additionally, most residents desire improvement of LGBT health education and training. Further studies focusing on the best methods for implementing LGBT-specific curricula are needed.
- Newport F. In U.S., estimate of LGBT population rises to 4.5%. Gallup. May 22, 2018. Accessed September 19, 2022. https://news.gallup.com/poll/234863/estimate-lgbt-population-rises.aspx
- Hafeez H, Zeshan M, Tahir MA, et al. Health care disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Gonzales G, Henning-Smith C. Barriers to care among transgender and gender nonconforming adults. Millbank Q. 2017;95:726-748.
- Quinn GP, Sanchez JA, Sutton SK, et al. Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA Cancer J Clin. 2015;65:384-400.
- Sullivan P, Trinidad J, Hamann D. Issues in transgender dermatology: a systematic review of the literature. J Am Acad Dermatol. 2019;81:438-447.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
- White W, Brenman S, Paradis E, et al. Lesbian, gay, bisexual, and transgender patient care: medical students’ preparedness and comfort. Teach Learn Med. 2015;27:254-263.
- Nama N, MacPherson P, Sampson M, et al. Medical students’ perception of lesbian, gay, bisexual, and transgender (LGBT) discrimination in their learning environment and their self-reported comfort level for caring for LGBT patients: a survey study. Med Educ Online. 2017;22:1-8.
- Phelan SM, Burke SE, Hardeman RR, et al. Medical school factors associated with changes in implicit and explicit bias against gay and lesbian people among 3492 graduating medical students. J Gen Intern Med. 2017;32:1193-1201.
- Cherabie J, Nilsen K, Houssayni S. Transgender health medical education intervention and its effects on beliefs, attitudes, comfort, and knowledge. Kans J Med. 2018;11:106-109.
- Integrating LGBT and DSD content into medical school curricula. Association of American Medical Colleges website. Published November 2015. Accessed September 23, 2022. https://www.aamc.org/what-we-do/equity-diversity-inclusion/lgbt-health-resources/videos/curricula-integration
- Cooper MB, Chacko M, Christner J. Incorporating LGBT health in an undergraduate medical education curriculum through the construct of social determinants of health. MedEdPORTAL. 2018;14:10781.
- Moll J, Krieger P, Moreno-Walton L, et al. The prevalence of lesbian, gay, bisexual, and transgender health education and training in emergency medicine residency programs: what do we know? Acad Emerg Med. 2014;21:608-611.
- Moll J, Krieger P, Heron SL, et al. Attitudes, behavior, and comfort of emergency medicine residents in caring for LGBT patients: what do we know? AEM Educ Train. 2019;3:129-135.
- Hirschtritt ME, Noy G, Haller E, et al. LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:41-45.
- Ufomata E, Eckstrand KL, Spagnoletti C, et al. Comprehensive curriculum for internal medicine residents on primary care of patients identifying as lesbian, gay, bisexual, or transgender. MedEdPORTAL. 2020;16:10875.
- Zonana J, Batchelder S, Pula J, et al. Comment on: LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:547-548.
- Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Dermatology. Revised June 12, 2022. Accessed September 23, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/080_dermatology_2022.pdf
- Jia JL, Nord KM, Sarin KY, et al. Sexual and gender minority curricula within US dermatology residency programs. JAMA Dermatol. 2020;156:593-594.
- Mansh M, White W, Gee-Tong L, et al. Sexual and gender minority identity disclosure during undergraduate medical education: “in the closet” in medical school. Acad Med. 2015;90:634-644.
- US Census Bureau. 2020 Census Informational Questionnaire. Accessed September 19, 2022. https://www2.census.gov/programs-surveys/decennial/2020/technical-documentation/questionnaires-and-instructions/questionnaires/2020-informational-questionnaire-english_DI-Q1.pdf
- Mohr JJ, Fassinger RE. Measuring dimensions of lesbian and gay male experience. Meas Eval Couns Dev. 2000;33:66-90.
- Association of American Medical Colleges. Medical School Graduation Questionnaire: 2020 All Schools Summary Report. Published July 2020. Accessed September 19, 2022. https://www.aamc.org/media/46851/download
- Accreditation Council for Graduate Medical Education. Data Resource Book: Academic Year 2019-2020. Accessed September 19, 2022. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2019-2020_acgme_databook_document.pdf
- Mohr JJ, Jackson SD, Sheets RL. Sexual orientation self-presentation among bisexual-identified women and men: patterns and predictors. Arch Sex Behav. 2017;46:1465-1479.
- Tatum AK. Workplace climate and job satisfaction: a test of social cognitive career theory (SCCT)’s workplace self-management model with sexual minority employees. Semantic Scholar. 2018. Accessed September 19, 2022. https://www.semanticscholar.org/paper/Workplace-Climate-and-Job-Satisfaction%3A-A-Test-of-Tatum/5af75ab70acfb73c54e34b95597576d30e07df12
- Fakhoury JW, Daveluy S. Incorporating lesbian, gay, bisexual, and transgender training into a residency program. Dermatol Clin. 2020;38:285-292.
- Newport F. In U.S., estimate of LGBT population rises to 4.5%. Gallup. May 22, 2018. Accessed September 19, 2022. https://news.gallup.com/poll/234863/estimate-lgbt-population-rises.aspx
- Hafeez H, Zeshan M, Tahir MA, et al. Health care disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Gonzales G, Henning-Smith C. Barriers to care among transgender and gender nonconforming adults. Millbank Q. 2017;95:726-748.
- Quinn GP, Sanchez JA, Sutton SK, et al. Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA Cancer J Clin. 2015;65:384-400.
- Sullivan P, Trinidad J, Hamann D. Issues in transgender dermatology: a systematic review of the literature. J Am Acad Dermatol. 2019;81:438-447.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
- White W, Brenman S, Paradis E, et al. Lesbian, gay, bisexual, and transgender patient care: medical students’ preparedness and comfort. Teach Learn Med. 2015;27:254-263.
- Nama N, MacPherson P, Sampson M, et al. Medical students’ perception of lesbian, gay, bisexual, and transgender (LGBT) discrimination in their learning environment and their self-reported comfort level for caring for LGBT patients: a survey study. Med Educ Online. 2017;22:1-8.
- Phelan SM, Burke SE, Hardeman RR, et al. Medical school factors associated with changes in implicit and explicit bias against gay and lesbian people among 3492 graduating medical students. J Gen Intern Med. 2017;32:1193-1201.
- Cherabie J, Nilsen K, Houssayni S. Transgender health medical education intervention and its effects on beliefs, attitudes, comfort, and knowledge. Kans J Med. 2018;11:106-109.
- Integrating LGBT and DSD content into medical school curricula. Association of American Medical Colleges website. Published November 2015. Accessed September 23, 2022. https://www.aamc.org/what-we-do/equity-diversity-inclusion/lgbt-health-resources/videos/curricula-integration
- Cooper MB, Chacko M, Christner J. Incorporating LGBT health in an undergraduate medical education curriculum through the construct of social determinants of health. MedEdPORTAL. 2018;14:10781.
- Moll J, Krieger P, Moreno-Walton L, et al. The prevalence of lesbian, gay, bisexual, and transgender health education and training in emergency medicine residency programs: what do we know? Acad Emerg Med. 2014;21:608-611.
- Moll J, Krieger P, Heron SL, et al. Attitudes, behavior, and comfort of emergency medicine residents in caring for LGBT patients: what do we know? AEM Educ Train. 2019;3:129-135.
- Hirschtritt ME, Noy G, Haller E, et al. LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:41-45.
- Ufomata E, Eckstrand KL, Spagnoletti C, et al. Comprehensive curriculum for internal medicine residents on primary care of patients identifying as lesbian, gay, bisexual, or transgender. MedEdPORTAL. 2020;16:10875.
- Zonana J, Batchelder S, Pula J, et al. Comment on: LGBT-specific education in general psychiatry residency programs: a survey of program directors. Acad Psychiatry. 2019;43:547-548.
- Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Dermatology. Revised June 12, 2022. Accessed September 23, 2022. https://www.acgme.org/globalassets/pfassets/programrequirements/080_dermatology_2022.pdf
- Jia JL, Nord KM, Sarin KY, et al. Sexual and gender minority curricula within US dermatology residency programs. JAMA Dermatol. 2020;156:593-594.
- Mansh M, White W, Gee-Tong L, et al. Sexual and gender minority identity disclosure during undergraduate medical education: “in the closet” in medical school. Acad Med. 2015;90:634-644.
- US Census Bureau. 2020 Census Informational Questionnaire. Accessed September 19, 2022. https://www2.census.gov/programs-surveys/decennial/2020/technical-documentation/questionnaires-and-instructions/questionnaires/2020-informational-questionnaire-english_DI-Q1.pdf
- Mohr JJ, Fassinger RE. Measuring dimensions of lesbian and gay male experience. Meas Eval Couns Dev. 2000;33:66-90.
- Association of American Medical Colleges. Medical School Graduation Questionnaire: 2020 All Schools Summary Report. Published July 2020. Accessed September 19, 2022. https://www.aamc.org/media/46851/download
- Accreditation Council for Graduate Medical Education. Data Resource Book: Academic Year 2019-2020. Accessed September 19, 2022. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2019-2020_acgme_databook_document.pdf
- Mohr JJ, Jackson SD, Sheets RL. Sexual orientation self-presentation among bisexual-identified women and men: patterns and predictors. Arch Sex Behav. 2017;46:1465-1479.
- Tatum AK. Workplace climate and job satisfaction: a test of social cognitive career theory (SCCT)’s workplace self-management model with sexual minority employees. Semantic Scholar. 2018. Accessed September 19, 2022. https://www.semanticscholar.org/paper/Workplace-Climate-and-Job-Satisfaction%3A-A-Test-of-Tatum/5af75ab70acfb73c54e34b95597576d30e07df12
- Fakhoury JW, Daveluy S. Incorporating lesbian, gay, bisexual, and transgender training into a residency program. Dermatol Clin. 2020;38:285-292.
Practice Points
- Dermatologists have the potential to greatly impact lesbian, gay, bisexual, transgender (LGBT) health since many health concerns in this population are cutaneous.
- Improving LGBT health education and training in dermatology residency likely will increase dermatology residents' comfort level in treating this population.