User login
Can early introduction of gluten reduce risk of celiac disease?
ILLUSTRATIVE CASE
You are seeing a 2-month-old female infant for a routine well-child visit. The birth history was unremarkable. The infant is meeting appropriate developmental milestones. Growth is appropriate at the 40th percentile. The infant is exclusively breastfed. The parents report that they have heard confusing information about when to introduce solid foods, including bread, to their child’s diet. There is no known family history of CD. What anticipatory guidance can you offer regarding gluten introduction and the risk of CD?
CD is an inflammatory disease of the small intestine caused by an immune-based reaction to dietary gluten. The worldwide incidence of CD in children younger than 15 years is 21.3 per 100,000 person-years; this incidence has increased by 7.5% per year over the past several decades.2 CD has a range of both gastrointestinal and nongastrointestinal manifestations, including diarrhea, weight loss, abdominal pain, abnormal liver function test results, and iron deficiency anemia.
Diagnosis of CD in adults is based on a combination of clinical symptoms, elevated levels of immunoglobulin A anti-tissue transglutaminase antibody (tTG-IgA), and biopsy-confirmed villous atrophy of the duodenum on upper endoscopy.3 European pediatric guidelines suggest that use of certain criteria, including very high results of tTG-IgA antibody testing (> 10 times the upper limit of normal), can help to avoid endoscopic biopsies and/or human leukocyte antigens (HLA) testing for diagnosis in children.4
The mainstay of CD management is strict adherence to a gluten-free diet.3 Because this can be difficult, and yield an incomplete disease response, emphasis has been placed on primary prevention by modifying introduction of dietary gluten. Multiple prior studies examining the risk of CD have failed to demonstrate a significant association between timing of gluten introduction and development of CD among high-risk infants (eg, those with HLA-DR3 alleles or first-degree relatives with CD or type 1 diabetes).5-7 A 2016 meta-analysis concluded that there was not enough evidence to support early introduction of gluten (at 4-6 months).8 RCTs have not previously been conducted to examine the timing of gluten introduction on CD prevalence for infants at average risk, using age-appropriate doses of gluten prior to age 6 months.
Current dietary guidelines in the United States and the United Kingdom recommend introduction of nutrient-dense foods, including potentially allergenic foods, at about age 6 months to complement human milk or infant formula feedings.9,10 These guidelines do not specify the exact timing or quantity of gluten- containing food introduction for infants. A 2016 position paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition indicated that gluten could be introduced into the infant’s diet any time between 4 and 12 months. They did indicate that the amount of gluten introduced into the diet should be low to start and then increased, and that infants at high risk for CD should wait longer for gluten introduction (4 vs 6 months or 6 vs 12 months).11
STUDY SUMMARY
Gluten introduced at 4 months may be linked to lower occurrence of CD
The Enquiring About Tolerance (EAT) Study was an open-label RCT (N = 1303) with children from the general population in England and Wales. The EAT Study sought to test the prevention of food allergy by introducing allergenic foods to infants at age 4 months compared with exclusively breastfeeding until age 6 months. The median age at enrollment was 3.4 months, but allergenic food was not started until age 4 months.1,12 Most patients were White (84.%-85.4%) and lived in an urban area (77.3%-77.4%). The mean gestational age at delivery was 39.7 to 39.9 weeks.12
Infants were exclusively breastfed until age 13 weeks, at which time they were randomized into an early introduction group (EIG) or a standard introduction group (SIG). In addition to breast milk, infants in the EIG consumed 6 allergenic foods (peanut, sesame, hen’s egg, cow’s milk, cod fish, and wheat [gluten]) in a specified pattern per protocol, starting at age 4 months. Wheat (gluten) was introduced during Week 5 of the EIG protocol (median age, 20.6 weeks).12 The recommended minimum dose of gluten was 3.2 g/wk from age 16 weeks, or 4 g/wk of wheat protein (given as 2 cereal biscuits or the equivalent). Infants in the SIG avoided allergenic foods, following UK infant feeding recommendations for exclusive breastfeeding until about age 6 months. The EIG had a significantly higher rate of cesarean births than the SIG, but the study groups were otherwise balanced.13
Continue to: Families completed monthly...
Families completed monthly questionnaires on infant gluten intake and symptoms (eg, gastrointestinal, fatigue) through age 1 year, and then every 3 months through age 3 years. All children were tested for anti-transglutaminase type 2 (anti-TG2) antibodies at age 3 years as a screen for CD. Children with antibody levels > 20 IU/L were referred to independent gastroenterologists for further evaluation, which could include HLA (DQ-2/DQ-8) testing and biopsy in accordance with current European diagnostic guidelines.4
In an intention-to-treat analysis for the primary outcome, 595 children in the SIG (91.4%) and 567 in the EIG (87.0%) were included. Between ages 4 and 6 months, the mean (SD) quantity of gluten consumed in the SIG was 0.49 (1.40) g/wk; in the EIG, the mean quantity was 2.66 (1.85) g/wk (P < .001). At age 3 years, of a total of 1004 children tested for anti-TG2 antibodies, 9 had anti-TG2 levels requiring referral (7 in the SIG and 2 in the EIG). A diagnosis of CD was confirmed in 7 of 516 children in the SIG (1.4%) vs none of the 488 children in the EIG (P = .02). Using bootstrap resampling, the risk difference between the groups was 1.4% (95% CI, 0.6%-2.6%).
WHAT’S NEW
Findings have potential to change nutritional guidance
This study demonstrated that introduction of age-appropriate portions of gluten-containing products at age 4 months, in addition to breast milk, may reduce the risk of CD at 3 years in children at average risk. This finding has the potential to change anticipatory guidance given to parents regarding infant nutrition recommendations.
CAVEATS
More studies needed to confirm prevention vs delay of CD
The homogeneous study population may limit generalizability. Infants in this study were from England and Wales (84.3% were White), born at term, and were exclusively breastfed until age 13 weeks. Further studies are required to determine whether these findings can be applied to infants who are no longer breastfeeding, are more racially diverse, or are preterm in gestational age at birth. Additionally, the study followed the participants only until age 3 years. Given that the onset of CD after this age is likely, further research is needed to support that CD is truly prevented rather than delayed.
CHALLENGES TO IMPLEMENTATION
Guidance on allergen introduction may be unclear
The EAT Study protocol required parents in the EIG to sequentially introduce a minimum amount of the 6 allergenic foods specified. Only 42% of the EIG cohort reported adherence to the protocol.12 It is unclear how important this specific regimen is to the study results and whether introduction of all 6 allergenic foods simultaneously modifies the immune response to gluten. Therefore, there may be challenges to implementation if physicians do not know how to provide anticipatory guidance on the appropriate steps for allergen introduction.
1. Logan K, Perkin MR, Marrs T, et al. Early gluten introduction and celiac disease in the EAT Study: a prespecified analysis of the EAT randomized clinical trial. JAMA Pediatr. 2020;174:1041-1047. doi: 10.1001/jamapediatrics.2020.2893
2. King JA, Jeong J, Underwood FE, et al. Incidence of celiac disease is increasing over time: a systematic review and meta-analysis. Am J Gastroenterol. 2020;115:507-525. doi: 10.14309/ajg.0000000000000523
3. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656-676; quiz 677. doi: 10.1038/ajg.2013.79
4. Husby S, Koletzko S, Korponay-Szabó I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141-156. doi: 10.1097/MPG.0000000000002497
5. Vriezinga SL, Auricchio R, Bravi E, et al. Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med. 2014;371:1304-1315. doi: 10.1056/NEJMoa1404172
6. Beyerlein A, Chmiel R, Hummel S, et al. Timing of gluten introduction and islet autoimmunity in young children: updated results from the BABYDIET study. Diabetes Care. 2014;37:e194-e195. doi: 10.2337/dc14-1208
7. Lionetti E, Castellaneta S, Francavilla R, et al; SIGENP (Italian Society of Pediatric Gastroenterology, Hepatology, and Nutrition) Working Group on Weaning and CD Risk. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med. 2014;371:1295-1303. doi: 10.1056/NEJMoa1400697
8. Pinto-Sánchez MI, Verdu EF, Liu E, et al. Gluten introduction to infant feeding and risk of celiac disease: systematic review and meta-analysis. J Pediatr. 2016;168:132-143.e3. doi: 10.1016/j.jpeds.2015.09.032
9. US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025. 9th ed. December 2020. Accessed June 8, 2022. www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf
10. NHS. Food allergies in babies and young children. Last reviewed November 5, 2021. Accessed June 8, 2022. www.nhs.uk/conditions/baby/weaning-and-feeding/food-allergies-in-babies-and-young-children/
11. Szajewska H, Shamir R, Mearin L, et al. Gluten introduction and the risk of coeliac disease: a position paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2016;62:507-513. doi: 10.1097/MPG.0000000000001105
12. Perkin MR, Logan K, Marrs T, et al; EAT Study Team. Enquiring About Tolerance (EAT) study: feasibility of an early allergenic food introduction regimen. J Allergy Clin Immunol. 2016;137:1477-1486.e8. doi: 10.1016/j.jaci.2015.12.1322
13. Perkin MR, Logan K, Tseng A, et al; EAT Study Team. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743. doi: 10.1056/NEJMoa1514210
ILLUSTRATIVE CASE
You are seeing a 2-month-old female infant for a routine well-child visit. The birth history was unremarkable. The infant is meeting appropriate developmental milestones. Growth is appropriate at the 40th percentile. The infant is exclusively breastfed. The parents report that they have heard confusing information about when to introduce solid foods, including bread, to their child’s diet. There is no known family history of CD. What anticipatory guidance can you offer regarding gluten introduction and the risk of CD?
CD is an inflammatory disease of the small intestine caused by an immune-based reaction to dietary gluten. The worldwide incidence of CD in children younger than 15 years is 21.3 per 100,000 person-years; this incidence has increased by 7.5% per year over the past several decades.2 CD has a range of both gastrointestinal and nongastrointestinal manifestations, including diarrhea, weight loss, abdominal pain, abnormal liver function test results, and iron deficiency anemia.
Diagnosis of CD in adults is based on a combination of clinical symptoms, elevated levels of immunoglobulin A anti-tissue transglutaminase antibody (tTG-IgA), and biopsy-confirmed villous atrophy of the duodenum on upper endoscopy.3 European pediatric guidelines suggest that use of certain criteria, including very high results of tTG-IgA antibody testing (> 10 times the upper limit of normal), can help to avoid endoscopic biopsies and/or human leukocyte antigens (HLA) testing for diagnosis in children.4
The mainstay of CD management is strict adherence to a gluten-free diet.3 Because this can be difficult, and yield an incomplete disease response, emphasis has been placed on primary prevention by modifying introduction of dietary gluten. Multiple prior studies examining the risk of CD have failed to demonstrate a significant association between timing of gluten introduction and development of CD among high-risk infants (eg, those with HLA-DR3 alleles or first-degree relatives with CD or type 1 diabetes).5-7 A 2016 meta-analysis concluded that there was not enough evidence to support early introduction of gluten (at 4-6 months).8 RCTs have not previously been conducted to examine the timing of gluten introduction on CD prevalence for infants at average risk, using age-appropriate doses of gluten prior to age 6 months.
Current dietary guidelines in the United States and the United Kingdom recommend introduction of nutrient-dense foods, including potentially allergenic foods, at about age 6 months to complement human milk or infant formula feedings.9,10 These guidelines do not specify the exact timing or quantity of gluten- containing food introduction for infants. A 2016 position paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition indicated that gluten could be introduced into the infant’s diet any time between 4 and 12 months. They did indicate that the amount of gluten introduced into the diet should be low to start and then increased, and that infants at high risk for CD should wait longer for gluten introduction (4 vs 6 months or 6 vs 12 months).11
STUDY SUMMARY
Gluten introduced at 4 months may be linked to lower occurrence of CD
The Enquiring About Tolerance (EAT) Study was an open-label RCT (N = 1303) with children from the general population in England and Wales. The EAT Study sought to test the prevention of food allergy by introducing allergenic foods to infants at age 4 months compared with exclusively breastfeeding until age 6 months. The median age at enrollment was 3.4 months, but allergenic food was not started until age 4 months.1,12 Most patients were White (84.%-85.4%) and lived in an urban area (77.3%-77.4%). The mean gestational age at delivery was 39.7 to 39.9 weeks.12
Infants were exclusively breastfed until age 13 weeks, at which time they were randomized into an early introduction group (EIG) or a standard introduction group (SIG). In addition to breast milk, infants in the EIG consumed 6 allergenic foods (peanut, sesame, hen’s egg, cow’s milk, cod fish, and wheat [gluten]) in a specified pattern per protocol, starting at age 4 months. Wheat (gluten) was introduced during Week 5 of the EIG protocol (median age, 20.6 weeks).12 The recommended minimum dose of gluten was 3.2 g/wk from age 16 weeks, or 4 g/wk of wheat protein (given as 2 cereal biscuits or the equivalent). Infants in the SIG avoided allergenic foods, following UK infant feeding recommendations for exclusive breastfeeding until about age 6 months. The EIG had a significantly higher rate of cesarean births than the SIG, but the study groups were otherwise balanced.13
Continue to: Families completed monthly...
Families completed monthly questionnaires on infant gluten intake and symptoms (eg, gastrointestinal, fatigue) through age 1 year, and then every 3 months through age 3 years. All children were tested for anti-transglutaminase type 2 (anti-TG2) antibodies at age 3 years as a screen for CD. Children with antibody levels > 20 IU/L were referred to independent gastroenterologists for further evaluation, which could include HLA (DQ-2/DQ-8) testing and biopsy in accordance with current European diagnostic guidelines.4
In an intention-to-treat analysis for the primary outcome, 595 children in the SIG (91.4%) and 567 in the EIG (87.0%) were included. Between ages 4 and 6 months, the mean (SD) quantity of gluten consumed in the SIG was 0.49 (1.40) g/wk; in the EIG, the mean quantity was 2.66 (1.85) g/wk (P < .001). At age 3 years, of a total of 1004 children tested for anti-TG2 antibodies, 9 had anti-TG2 levels requiring referral (7 in the SIG and 2 in the EIG). A diagnosis of CD was confirmed in 7 of 516 children in the SIG (1.4%) vs none of the 488 children in the EIG (P = .02). Using bootstrap resampling, the risk difference between the groups was 1.4% (95% CI, 0.6%-2.6%).
WHAT’S NEW
Findings have potential to change nutritional guidance
This study demonstrated that introduction of age-appropriate portions of gluten-containing products at age 4 months, in addition to breast milk, may reduce the risk of CD at 3 years in children at average risk. This finding has the potential to change anticipatory guidance given to parents regarding infant nutrition recommendations.
CAVEATS
More studies needed to confirm prevention vs delay of CD
The homogeneous study population may limit generalizability. Infants in this study were from England and Wales (84.3% were White), born at term, and were exclusively breastfed until age 13 weeks. Further studies are required to determine whether these findings can be applied to infants who are no longer breastfeeding, are more racially diverse, or are preterm in gestational age at birth. Additionally, the study followed the participants only until age 3 years. Given that the onset of CD after this age is likely, further research is needed to support that CD is truly prevented rather than delayed.
CHALLENGES TO IMPLEMENTATION
Guidance on allergen introduction may be unclear
The EAT Study protocol required parents in the EIG to sequentially introduce a minimum amount of the 6 allergenic foods specified. Only 42% of the EIG cohort reported adherence to the protocol.12 It is unclear how important this specific regimen is to the study results and whether introduction of all 6 allergenic foods simultaneously modifies the immune response to gluten. Therefore, there may be challenges to implementation if physicians do not know how to provide anticipatory guidance on the appropriate steps for allergen introduction.
ILLUSTRATIVE CASE
You are seeing a 2-month-old female infant for a routine well-child visit. The birth history was unremarkable. The infant is meeting appropriate developmental milestones. Growth is appropriate at the 40th percentile. The infant is exclusively breastfed. The parents report that they have heard confusing information about when to introduce solid foods, including bread, to their child’s diet. There is no known family history of CD. What anticipatory guidance can you offer regarding gluten introduction and the risk of CD?
CD is an inflammatory disease of the small intestine caused by an immune-based reaction to dietary gluten. The worldwide incidence of CD in children younger than 15 years is 21.3 per 100,000 person-years; this incidence has increased by 7.5% per year over the past several decades.2 CD has a range of both gastrointestinal and nongastrointestinal manifestations, including diarrhea, weight loss, abdominal pain, abnormal liver function test results, and iron deficiency anemia.
Diagnosis of CD in adults is based on a combination of clinical symptoms, elevated levels of immunoglobulin A anti-tissue transglutaminase antibody (tTG-IgA), and biopsy-confirmed villous atrophy of the duodenum on upper endoscopy.3 European pediatric guidelines suggest that use of certain criteria, including very high results of tTG-IgA antibody testing (> 10 times the upper limit of normal), can help to avoid endoscopic biopsies and/or human leukocyte antigens (HLA) testing for diagnosis in children.4
The mainstay of CD management is strict adherence to a gluten-free diet.3 Because this can be difficult, and yield an incomplete disease response, emphasis has been placed on primary prevention by modifying introduction of dietary gluten. Multiple prior studies examining the risk of CD have failed to demonstrate a significant association between timing of gluten introduction and development of CD among high-risk infants (eg, those with HLA-DR3 alleles or first-degree relatives with CD or type 1 diabetes).5-7 A 2016 meta-analysis concluded that there was not enough evidence to support early introduction of gluten (at 4-6 months).8 RCTs have not previously been conducted to examine the timing of gluten introduction on CD prevalence for infants at average risk, using age-appropriate doses of gluten prior to age 6 months.
Current dietary guidelines in the United States and the United Kingdom recommend introduction of nutrient-dense foods, including potentially allergenic foods, at about age 6 months to complement human milk or infant formula feedings.9,10 These guidelines do not specify the exact timing or quantity of gluten- containing food introduction for infants. A 2016 position paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition indicated that gluten could be introduced into the infant’s diet any time between 4 and 12 months. They did indicate that the amount of gluten introduced into the diet should be low to start and then increased, and that infants at high risk for CD should wait longer for gluten introduction (4 vs 6 months or 6 vs 12 months).11
STUDY SUMMARY
Gluten introduced at 4 months may be linked to lower occurrence of CD
The Enquiring About Tolerance (EAT) Study was an open-label RCT (N = 1303) with children from the general population in England and Wales. The EAT Study sought to test the prevention of food allergy by introducing allergenic foods to infants at age 4 months compared with exclusively breastfeeding until age 6 months. The median age at enrollment was 3.4 months, but allergenic food was not started until age 4 months.1,12 Most patients were White (84.%-85.4%) and lived in an urban area (77.3%-77.4%). The mean gestational age at delivery was 39.7 to 39.9 weeks.12
Infants were exclusively breastfed until age 13 weeks, at which time they were randomized into an early introduction group (EIG) or a standard introduction group (SIG). In addition to breast milk, infants in the EIG consumed 6 allergenic foods (peanut, sesame, hen’s egg, cow’s milk, cod fish, and wheat [gluten]) in a specified pattern per protocol, starting at age 4 months. Wheat (gluten) was introduced during Week 5 of the EIG protocol (median age, 20.6 weeks).12 The recommended minimum dose of gluten was 3.2 g/wk from age 16 weeks, or 4 g/wk of wheat protein (given as 2 cereal biscuits or the equivalent). Infants in the SIG avoided allergenic foods, following UK infant feeding recommendations for exclusive breastfeeding until about age 6 months. The EIG had a significantly higher rate of cesarean births than the SIG, but the study groups were otherwise balanced.13
Continue to: Families completed monthly...
Families completed monthly questionnaires on infant gluten intake and symptoms (eg, gastrointestinal, fatigue) through age 1 year, and then every 3 months through age 3 years. All children were tested for anti-transglutaminase type 2 (anti-TG2) antibodies at age 3 years as a screen for CD. Children with antibody levels > 20 IU/L were referred to independent gastroenterologists for further evaluation, which could include HLA (DQ-2/DQ-8) testing and biopsy in accordance with current European diagnostic guidelines.4
In an intention-to-treat analysis for the primary outcome, 595 children in the SIG (91.4%) and 567 in the EIG (87.0%) were included. Between ages 4 and 6 months, the mean (SD) quantity of gluten consumed in the SIG was 0.49 (1.40) g/wk; in the EIG, the mean quantity was 2.66 (1.85) g/wk (P < .001). At age 3 years, of a total of 1004 children tested for anti-TG2 antibodies, 9 had anti-TG2 levels requiring referral (7 in the SIG and 2 in the EIG). A diagnosis of CD was confirmed in 7 of 516 children in the SIG (1.4%) vs none of the 488 children in the EIG (P = .02). Using bootstrap resampling, the risk difference between the groups was 1.4% (95% CI, 0.6%-2.6%).
WHAT’S NEW
Findings have potential to change nutritional guidance
This study demonstrated that introduction of age-appropriate portions of gluten-containing products at age 4 months, in addition to breast milk, may reduce the risk of CD at 3 years in children at average risk. This finding has the potential to change anticipatory guidance given to parents regarding infant nutrition recommendations.
CAVEATS
More studies needed to confirm prevention vs delay of CD
The homogeneous study population may limit generalizability. Infants in this study were from England and Wales (84.3% were White), born at term, and were exclusively breastfed until age 13 weeks. Further studies are required to determine whether these findings can be applied to infants who are no longer breastfeeding, are more racially diverse, or are preterm in gestational age at birth. Additionally, the study followed the participants only until age 3 years. Given that the onset of CD after this age is likely, further research is needed to support that CD is truly prevented rather than delayed.
CHALLENGES TO IMPLEMENTATION
Guidance on allergen introduction may be unclear
The EAT Study protocol required parents in the EIG to sequentially introduce a minimum amount of the 6 allergenic foods specified. Only 42% of the EIG cohort reported adherence to the protocol.12 It is unclear how important this specific regimen is to the study results and whether introduction of all 6 allergenic foods simultaneously modifies the immune response to gluten. Therefore, there may be challenges to implementation if physicians do not know how to provide anticipatory guidance on the appropriate steps for allergen introduction.
1. Logan K, Perkin MR, Marrs T, et al. Early gluten introduction and celiac disease in the EAT Study: a prespecified analysis of the EAT randomized clinical trial. JAMA Pediatr. 2020;174:1041-1047. doi: 10.1001/jamapediatrics.2020.2893
2. King JA, Jeong J, Underwood FE, et al. Incidence of celiac disease is increasing over time: a systematic review and meta-analysis. Am J Gastroenterol. 2020;115:507-525. doi: 10.14309/ajg.0000000000000523
3. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656-676; quiz 677. doi: 10.1038/ajg.2013.79
4. Husby S, Koletzko S, Korponay-Szabó I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141-156. doi: 10.1097/MPG.0000000000002497
5. Vriezinga SL, Auricchio R, Bravi E, et al. Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med. 2014;371:1304-1315. doi: 10.1056/NEJMoa1404172
6. Beyerlein A, Chmiel R, Hummel S, et al. Timing of gluten introduction and islet autoimmunity in young children: updated results from the BABYDIET study. Diabetes Care. 2014;37:e194-e195. doi: 10.2337/dc14-1208
7. Lionetti E, Castellaneta S, Francavilla R, et al; SIGENP (Italian Society of Pediatric Gastroenterology, Hepatology, and Nutrition) Working Group on Weaning and CD Risk. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med. 2014;371:1295-1303. doi: 10.1056/NEJMoa1400697
8. Pinto-Sánchez MI, Verdu EF, Liu E, et al. Gluten introduction to infant feeding and risk of celiac disease: systematic review and meta-analysis. J Pediatr. 2016;168:132-143.e3. doi: 10.1016/j.jpeds.2015.09.032
9. US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025. 9th ed. December 2020. Accessed June 8, 2022. www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf
10. NHS. Food allergies in babies and young children. Last reviewed November 5, 2021. Accessed June 8, 2022. www.nhs.uk/conditions/baby/weaning-and-feeding/food-allergies-in-babies-and-young-children/
11. Szajewska H, Shamir R, Mearin L, et al. Gluten introduction and the risk of coeliac disease: a position paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2016;62:507-513. doi: 10.1097/MPG.0000000000001105
12. Perkin MR, Logan K, Marrs T, et al; EAT Study Team. Enquiring About Tolerance (EAT) study: feasibility of an early allergenic food introduction regimen. J Allergy Clin Immunol. 2016;137:1477-1486.e8. doi: 10.1016/j.jaci.2015.12.1322
13. Perkin MR, Logan K, Tseng A, et al; EAT Study Team. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743. doi: 10.1056/NEJMoa1514210
1. Logan K, Perkin MR, Marrs T, et al. Early gluten introduction and celiac disease in the EAT Study: a prespecified analysis of the EAT randomized clinical trial. JAMA Pediatr. 2020;174:1041-1047. doi: 10.1001/jamapediatrics.2020.2893
2. King JA, Jeong J, Underwood FE, et al. Incidence of celiac disease is increasing over time: a systematic review and meta-analysis. Am J Gastroenterol. 2020;115:507-525. doi: 10.14309/ajg.0000000000000523
3. Rubio-Tapia A, Hill ID, Kelly CP, et al; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656-676; quiz 677. doi: 10.1038/ajg.2013.79
4. Husby S, Koletzko S, Korponay-Szabó I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141-156. doi: 10.1097/MPG.0000000000002497
5. Vriezinga SL, Auricchio R, Bravi E, et al. Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med. 2014;371:1304-1315. doi: 10.1056/NEJMoa1404172
6. Beyerlein A, Chmiel R, Hummel S, et al. Timing of gluten introduction and islet autoimmunity in young children: updated results from the BABYDIET study. Diabetes Care. 2014;37:e194-e195. doi: 10.2337/dc14-1208
7. Lionetti E, Castellaneta S, Francavilla R, et al; SIGENP (Italian Society of Pediatric Gastroenterology, Hepatology, and Nutrition) Working Group on Weaning and CD Risk. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med. 2014;371:1295-1303. doi: 10.1056/NEJMoa1400697
8. Pinto-Sánchez MI, Verdu EF, Liu E, et al. Gluten introduction to infant feeding and risk of celiac disease: systematic review and meta-analysis. J Pediatr. 2016;168:132-143.e3. doi: 10.1016/j.jpeds.2015.09.032
9. US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025. 9th ed. December 2020. Accessed June 8, 2022. www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf
10. NHS. Food allergies in babies and young children. Last reviewed November 5, 2021. Accessed June 8, 2022. www.nhs.uk/conditions/baby/weaning-and-feeding/food-allergies-in-babies-and-young-children/
11. Szajewska H, Shamir R, Mearin L, et al. Gluten introduction and the risk of coeliac disease: a position paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2016;62:507-513. doi: 10.1097/MPG.0000000000001105
12. Perkin MR, Logan K, Marrs T, et al; EAT Study Team. Enquiring About Tolerance (EAT) study: feasibility of an early allergenic food introduction regimen. J Allergy Clin Immunol. 2016;137:1477-1486.e8. doi: 10.1016/j.jaci.2015.12.1322
13. Perkin MR, Logan K, Tseng A, et al; EAT Study Team. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743. doi: 10.1056/NEJMoa1514210
PRACTICE CHANGER
Consider introducing gluten (wheat) in addition to breast milk or infant formula from age 4 months to potentially reduce the risk of celiac disease (CD) at age 3 years.1
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial (RCT) with a patient-oriented outcome of CD diagnosis.1
Logan K, Perkin MR, Marrs T, et al. Early gluten introduction and celiac disease in the EAT Study: a prespecified analysis of the EAT randomized clinical trial. JAMA Pediatr. 2020;174:1041-1047.
A judicious approach to ordering lab tests
CASE
A 35-year-old man arrives for an annual wellness visit with no specific complaints and no significant personal or family history. His normal exam includes a blood pressure of 110/74 mm Hg and a body mass index (BMI) of 23.6. You order “routine labs” for prevention, which include a comprehensive metabolic panel (CMP), fasting lipid profile, and thyroid-stimulating hormone (TSH) and 25(OH) vitamin D tests. Are you practicing value-based laboratory testing?
The answer to this question appears in the Case discussion at the end of the article.
Value-based care, including care provided through laboratory testing, can achieve the Institute for Healthcare Improvement’s Triple Aim of improving population health, improving the patient experience of care (including quality and satisfaction), and reducing cost: Value = (Quality x Patient experience) / Cost.1
As quality and patient experience rise and cost falls, the value of care increases. Unnecessary lab testing, however, can negatively impact this equation:
- Error introduced by unnecessary testing can adversely affect quality.
- Patients experience inconvenience and sometimes cascades of testing, in addition to financial responsibility, from unnecessary testing.
- Low-value testing also contributes to work burden and provider burnout by requiring additional review and follow-up.
Rising health care costs are approaching 18% of the US gross domestic product, driven in large part by a wasteful and inefficient care delivery system.2 One review of “waste domains” identified by the Institute of Medicine estimates that approximately one-quarter of health care costs represent waste, including overtreatment, breakdowns of care coordination, and pricing that fails to correlate to the level of care received.3 High-volume, low-cost testing contributes more to total cost than low-volume, high-cost tests.4
Provider and system factors that contribute to ongoing waste
A lack of awareness of waste and how to reduce it contribute to the problem, as does an underappreciation of the harmful effects caused by incidental abnormal results.
Provider intolerance of diagnostic uncertainty often leads to ordering even more tests.
Continue to: Also, a hope of avoiding...
Also, a hope of avoiding missed diagnoses and potential lawsuits leads to defensive practice and more testing. In addition, patients and family members can exert pressure based on a belief that more testing represents better care. Of course, financial revenues from testing may come into play, with few disincentives to forgo testing. Something that also comes into play is that evidence-based guidance on cost-effective laboratory testing may be lacking, or there may be a lack of knowledge on how to access existing evidence.
Automated systems can facilitate wasteful laboratory testing, and the heavy testing practices of hospitals and specialists may be inappropriately applied to outpatient primary care.
Factors affecting the cost of laboratory testing
Laboratory test results drive 70% of today’s medical decisions.5 Negotiated insurance payment for tests is usually much less than the direct out-of-pocket costs charged to the patient. Without insurance, lab tests can cost patients between $100 and $1000.6 If multiple tests are ordered, the costs could likely be many thousands of dollars.
Actual costs typically vary by the testing facility, the patient’s health plan, and location in the United States; hospital-based testing tends to be the most expensive. Insurers will pay for lab tests with appropriate indications that are covered in the contract with the provider.6
Choosing Wisely initiative weighs in on lab testing
Choosing Wisely, a prominent initiative of the American Board of Internal Medicine Foundation, promotes appropriate resource utilization through educational campaigns that detail how to avoid unnecessary medical tests, treatments, and procedures.7 Recommendations are based largely on specialty society consensus and disease-oriented evidence. Choosing Wisely recommendations advise against the following7:
- performing laboratory blood testing unless clinically indicated or necessary for diagnosis or management, in order to avoid iatrogenic anemia. (American Academy of Family Physicians; Society for the Advancement of Patient Blood Management)
- requesting just a serum creatinine to test adult patients with diabetes and/or hypertension for chronic kidney disease. Use the kidney profile: serum creatinine with estimated glomerular filtration rate and urinary albumin-creatinine ratio. (American Society for Clinical Pathology)
- routinely screening for prostate cancer using a prostate-specific antigen test. It should be performed only after engaging in shared decision-making with the patient. (American Academy of Family Physicians; American Urological Association)
- screening for genital herpes simplex virus infectionFrutiger LT Std in asymptomatic adults, including pregnant women. (American Academy of Family Physicians)
- performing preoperative medical tests for eye surgery unless there are specific medical indications. (American Academy of Ophthalmology)
Sequential steps to takefor value-based lab ordering
Ask the question: “How will ordering this specific test change the management of my patient?” From there, take sequential steps using sound, evidence-based pathways. Morgan and colleagues8 outline the following practical approaches to rational test ordering:
- Perform a thorough clinical assessment.
- Consider the probability and implications of a positive test result.
- Practice patient-centered communication: address the patient’s concerns and discuss the risks and benefits of tests and how they will influence management.
- Follow clinical guidelines when available.
- Avoid ordering tests to reassure the patient; unnecessary tests with insignificant results do little to reduce patient anxieties.
- Avoid letting uncertainty drive unnecessary testing. Watchful waiting can allow time for the illness to resolve or declare itself.
Let’s consider this approach in the context of 4 areas: preventive care, diagnostic evaluation, ongoing management of chronic conditions, and preoperative testing.
Continue to: Preventive guidance from the USPSTF
Preventive guidance from the USPSTF
An independent volunteer panel of 16 national experts in prevention and evidence-based medicine develop recommendations for the US Preventive Services Task Force (USPSTF).9 These guidelines are based on evidence and are updated as new evidence surfaces. Thirteen recommendations, some of which advise avoiding preventive procedures that could cause harm to patients, cover laboratory tests used in screening for conditions such as hyperlipidemia10 and prostate cancer.11 We review the ones pertinent to our patient later at the end of the Case.
While the target audience for USPSTF recommendations is clinicians who provide preventive care, the recommendations are widely followed by policymakers, managed care organizations, public and private payers, quality improvement organizations, research institutions, and patients.
Take a critical look at how you approach the diagnostic evaluation
To reduce unnecessary testing in the diagnostic evaluation of patients, first consider pretest probability, test sensitivity and specificity, narrowly out-of-range tests, habitually paired tests, and repetitive laboratory testing.
Pretest probability, and test sensitivity and specificity. Pretest probability is the estimated chance that the patient has the disease before the test result is known. In a patient with low pretest probability of a disease, the ability to conclusively arrive at the diagnosis with one positive result is limited. Similarly, for tests in patients with high pretest probability of disease, a negative test cannot be used to firmly rule out a diagnosis.12
Reliability also depends on test sensitivity (the proportion of true positive results) and specificity (the proportion of true negative results). A test with high sensitivity but low specificity will generate more false-positive results, with potential harm to patients who do not have a disease.
The pretest probability along with test sensitivity and specificity help a clinician to interpret a test result, and even decide whether to order the test at all. For example, the anti-nuclear antibody (ANA) test for systemic lupus erythematosus (SLE) has a sensitivity of 100% and a specificity of 86%13; it will always be positive in a patient with SLE. But when applied to individuals with low likelihood of SLE, false-positives are more common; the ANA is falsely positive in up to 14% of healthy individuals, depending on the population studied.13
Ordering a test may be unnecessary if the results will not change the treatment plan. For example, in a female patient with classic symptoms of an uncomplicated urinary tract infection, a urine culture and even a urinalysis may not change treatment.
Continue to: Narrowly out-of-range tests
Narrowly out-of-range tests. Test results that fall just outside the “normal” range may be of questionable significance. When an asymptomatic patient has mildly elevated liver enzymes, should additional tests be ordered to avoid missing a treatable disorder? In these scenarios, a history, including possible contributing factors such as alcohol or substance misuse, must be paired with the clinical presentation to assess pre-test probability of a particular condition.14 Repeating a narrowly out-of-range test is an option in patients when follow-up is possible. Alternatively, you could pursue watchful waiting and monitor a minor abnormality over time while being vigilant for clinical changes. This whole-patient approach will guide the decision of whether to order additional testing.
Habitually paired tests. Reflexively ordering tests together often contributes to unnecessary testing. Examples of commonly paired tests are serum lipase with amylase, C-reactive protein (CRP) with erythrocyte sedimentation rate (ESR), and TSH with free T4 to monitor patients with treated hypothyroidism. These tests add minimal value together and can be decoupled.15-17 Evidence supports ordering serum lipase alone, CRP instead of ESR, and TSH alone for monitoring thyroid status.
Some commonly paired tests may not even be necessary for diagnosis. The well-established Rotterdam Criteria for diagnosing polycystic ovary syndrome specify clinical features and ovarian ultrasound for diagnosis.18 They do not require measurement of commonly ordered follicle-stimulating hormone and luteinizing hormone for diagnosis.
Serial rather than parallel testing, a “2-step approach,” is a strategy made easier with the advent of the electronic medical record (EMR) and computerized lab systems.8 These records and lab systems allow providers to order reflex tests, and to add on additional tests, if necessary, to an existing blood specimen.
Repetitive laboratory testing. Repetitive inpatient laboratory testing in patients who are clinically stable is wasteful and potentially harmful. Interventions involving physician education alone show mixed results, but combining education with clinician audit and feedback, along with EMR-enabled restrictive ordering, have resulted in significant and sustained reductions in repetitive laboratory testing.19
Continue to: Ongoing management of chronic conditions
Ongoing management of chronic conditions
Evidence-based guidelines support choices of tests and testing intervals for ongoing management of chronic conditions such as diabetes, hyperlipidemia, and hypertension.
Diabetes. Guidelines also define quality standards that are applied to value-based contracts. For example, the American Diabetes Association recommends assessing A1C every 6 months in patients whose type 2 diabetes is under stable control.20
Hyperlipidemia. For patients diagnosed with hyperlipidemia, 2018 clinical practice guidelines published by multiple specialty societies recommend assessing adherence and response to lifestyle changes and LDL-C–lowering medications with repeat lipid measurement 4 to 12 weeks after statin initiation or dose adjustment, repeated every 3 to 12 months as needed.21
Hypertension. With a new diagnosis of hypertension, guidelines advise an initial assessment for comorbidities and end-organ damage with an electrocardiogram, urinalysis, glucose level, blood count, electrolytes, creatinine, calcium, lipids, and urinary albumin/creatinine ratio. For ongoing monitoring, guidelines recommend assessment for end-organ damage through regular measurements of creatinine, glomerular filtration rate, and urinary microalbumin/creatinine ratio. Initiation and alteration of medications should prompt appropriate additional lab follow-up—eg, a measurement of serum potassium after starting a diuretic.22
Preoperative testing
Preoperative testing is overused in low-risk, ambulatory surgery. And testing, even with abnormal results, does not affect postoperative outcomes.23
Continue to: The American Society of Anesthesiologists (ASA) Physical Status Classification System
The American Society of Anesthesiologists (ASA) Physical Status Classification System, which has been in use for more than 60 years, considers the patient’s physical status (ASA grades I-VI),24 and when paired with surgery grades of minor, intermediate, and major/complex, can help assess preoperative risk and guide preoperative testing (TABLE).24-26
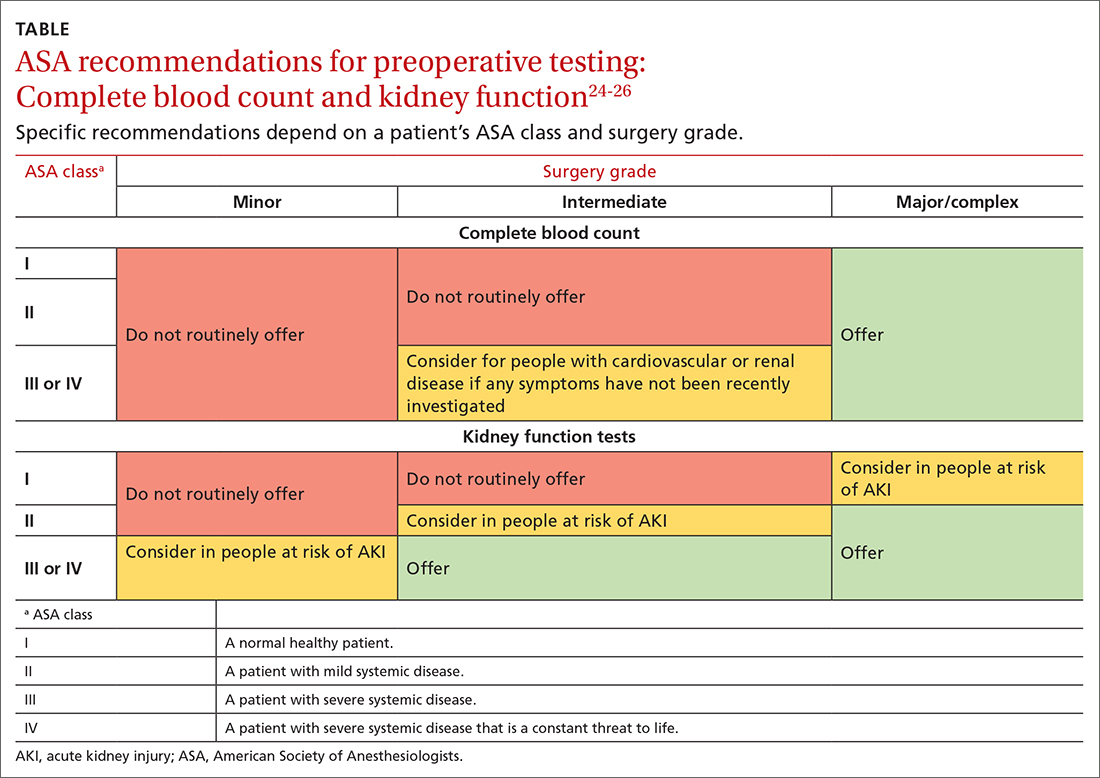
Preoperative medical testing did not reduce the risk of medical adverse events during or after cataract surgery when compared with selective or no testing.27 Unnecessary preoperative testing can lead to a nonproductive cascade of additional investigations. In a 2018 study of Medicare beneficiaries, unnecessary routine preoperative testing and testing sequelae for cataract surgery was calculated to cost Medicare up to $45.4 million annually.28
CASE
You would not be practicing value-based laboratory testing, according to the USPSTF, if you ordered a CMP, fasting lipid profile, and TSH and 25(OH) vitamin D tests for this healthy 35-year-old man whose family history, blood pressure, and BMI do not put him at elevated risk. Universal lipid screening (Grade Ba) is recommended for all adults ages 40 to 75. Thyroid screening tests and measurement of 25(OH) vitamin D level (I statementsa) are not recommended. The USPSTF has not evaluated chemistry panels for screening.
The USPSTF would recommend the following actions for this patient:
- Screen for HIV (ages 15 to 65 years; and younger or older if patient is at risk). (A recommendationa,29)
- Screen for hepatitis C virus (in those ages 18 to 79). (B recommendation30)
The following USPSTF recommendations might have come into play if this patient had certain risk factors, or if the patient had been a woman:
- Screen for diabetes if the patient is overweight or obese (B recommendation).
- Screen for hepatitis B in adults at risk (B recommendation).
- Screen for gonorrhea and chlamydia in women at risk (B recommendation). Such screening has an “I”statement for screening men at risk.
Continue to: As noted, costs of laboratory...
As noted, costs of laboratory testing vary widely, depending upon what tests are ordered, what type of insurance the patient has, and which tests the patient’s insurance covers. Who performs the testing also factors into the cost. Payers negotiate reduced fees for commercial lab testing, but potential out-of-pocket costs to patients are much higher.
For our healthy 35-year-old man, the cost of the initially proposed testing (CMP, lipid panel, TSH, and 25[OH] vitamin D level) ranges from a negotiated payer cost of $85 to potential patient out-of-pocket cost of more than $400.6
Insurance would cover the USPSTF-recommended testing (HIV and hepatitis C screening tests), which might incur only a patient co-pay, and cost the system about $65.
The USPSTF home page, found at www.uspreventiveservicestaskforce.org/uspstf/ includes recommendations that can be sorted for your patients. A web and mobile device application is also available through the website.
a USPSTF grade definitions:
A: There is high certainty that the net benefit is substantial. Offer service.
B: There is high certainty that the net benefit is moderate, or there is moderate certainty that the net benefit is moderate to substantial. Offer service.
C: There is at least moderate certainty that the net benefit is small. Offer service selectively.
D: There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. Don’t offer service.
I: Current evidence is insufficient to assess the balance of benefits and harms of the service.
CORRESPONDENCE
Mitchell Kaminski, MD, MBA, 901 Walnut Street, 10th Floor, Jefferson College of Population Health, Philadelphia, PA 19107; [email protected]
1. IHI. What is the Triple Aim? Accessed June 20, 2022. http://www.ihi.org/Topics/TripleAim/Pages/Overview.aspx#:~:text=It%20is%20IHI’s%20belief%20that,capita%20cost%20of%20health%20care
2. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. doi: 10.1001/jama.2018.1150
3. Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system estimated costs and potential for savings. JAMA. 2019;322:1501-1509. doi:10.1001/jama.2019.13978
4. Mafi JN, Russell K, Bortz BA, et al. Low-cost, high-volume health services contribute the most to unnecessary health spending. Health Aff. 2017;36:1701-1704. doi: 10.1377/hlthaff.2017.0385
5. CDC. Strengthening clinical laboratories. 2018. Accessed June 2020, 2022. www.cdc.gov/csels/dls/strengthening-clinical-labs.html
6. Vuong KT. How much do lab tests cost without insurance in 2022? Accessed May 11, 2022. www.talktomira.com/post/how-much-do-lab-test-cost-without-insurance
7. Choosing Wisely: Promoting conversations between providers and patients. Accessed June 20, 2022. www.choosingwisely.org
8. Morgan S, van Driel M, Coleman J, et al. Rational test ordering in family medicine. Can Fam Physician. 2015;61:535-537.
9. US Preventive Services Taskforce. Screening for glaucoma and impaired vision. Accessed June 20, 2022. www.uspreventiveservicestaskforce.org/uspstf
10. Arnold MJ, O’Malley PG, Downs JR. Key recommendations on managing dyslipidemia for cardiovascular risk reduction: stopping where the evidence does. Am Fam Physician. 2021;103:455-458.
11. Welch HG, Albertsen PC. Reconsidering prostate cancer mortality—the future of PSA screening. N Engl J Med. 2020;382:1557-1563. doi: 10.1056/NEJMms1914228
12. American Society for Microbiology. Why pretest and posttest probability matter in the time of COVID-19. Accessed June 20, 2022. https://asm.org/Articles/2020/June/Why-Pretest-and-Posttest-Probability-Matter-in-the
13. Slater CA, Davis RB, Shmerling RH. Antinuclear antibody testing. A study of clinical utility. Arch Intern Med. 1996;156:1421-1425.
14. Aragon G, Younossi ZM. When and how to evaluate mildly elevated liver enzymes in apparently healthy patients. Cleve Clin J Med. 2010;77:195-204. doi: 10.3949/ccjm.77a.09064
15. Ismail OZ, Bhayana V. Lipase or amylase for the diagnosis of acute pancreatitis? Clin Biochem. 2017;50:1275-1280. doi: 10.1016/j.clinbiochem.2017.07.003.
16. Gottheil S, Khemani E, Copley K, et al. Reducing inappropriate ESR testing with computerized clinical decision support. BMJ Quality Improvement Reports, 2016;5:u211376.w4582. doi: 10.1136/bmjquality.u211376.w4582
17. Schneider C, Feller M, Bauer DC, et al. Initial evaluation of thyroid dysfunction - are simultaneous TSH and fT4 tests necessary? PloS One. 2018;13:e0196631–e0196631. doi: 10.1371/journal.pone.0196631
18. Williams T, Mortada R, Porter S. Diagnosis and treatment of polycystic ovary syndrome. Am Fam Physician. 2016;94:106-113.
19. Eaton KP, Levy K, Soong C et.al. Evidence-Based Guidelines to Eliminate Repetitive Laboratory Testing. JAMA Intern Med. 2017;177:1833-1839. doi: 10.1001/jamainternmed.2017.5152
20. ADA. Glycemic targets: standards of medical care in diabetes—2021. Diabetes Care. 2021;44:S73-S84. doi: 10.2337/dc21-S006
21. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/ AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
22. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026.
23. Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256:518-528. doi: 10.1097/SLA.0b013e318265bcdb
24. ASA. ASA physical status classification system. Accessed June 22,2022. www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system
25. NLM. Preoperative tests (update): routine preoperative tests for elective surgery. Accessed June 22, 2022. www.ncbi.nlm.nih.gov/books/NBK367919/
26. ASA. American Society of Anesthesiologists releases list of commonly used tests and treatments to question-AS PART OF CHOOSING WISELY® CAMPAIGN. Accessed June 22, 2022. www.asahq.org/about-asa/newsroom/news-releases/2013/10/choosing-wisely
27. Keay L, Lindsley K, Tielsch J, et al. Routine preoperative medical testing for cataract surgery. Cochrane Database Syst Rev. 2019;1:CD007293. doi: 10.1002/14651858.CD007293.pub4
28. Chen CL, Clay TH, McLeod S, et al. A revised estimate of costs associated with routine preoperative testing in Medicare cataract patients with a procedure-specific indicator. JAMA Ophthalmol. 2018;136:231-238. doi:10.1001/jamaophthalmol.2017.6372
29. USPSTF. Human immunodeficiency virus (HIV) infection: screening. Accessed May 16, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/human-immunodeficiency-virus-hiv-infection-screening
30. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed June 20, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
CASE
A 35-year-old man arrives for an annual wellness visit with no specific complaints and no significant personal or family history. His normal exam includes a blood pressure of 110/74 mm Hg and a body mass index (BMI) of 23.6. You order “routine labs” for prevention, which include a comprehensive metabolic panel (CMP), fasting lipid profile, and thyroid-stimulating hormone (TSH) and 25(OH) vitamin D tests. Are you practicing value-based laboratory testing?
The answer to this question appears in the Case discussion at the end of the article.
Value-based care, including care provided through laboratory testing, can achieve the Institute for Healthcare Improvement’s Triple Aim of improving population health, improving the patient experience of care (including quality and satisfaction), and reducing cost: Value = (Quality x Patient experience) / Cost.1
As quality and patient experience rise and cost falls, the value of care increases. Unnecessary lab testing, however, can negatively impact this equation:
- Error introduced by unnecessary testing can adversely affect quality.
- Patients experience inconvenience and sometimes cascades of testing, in addition to financial responsibility, from unnecessary testing.
- Low-value testing also contributes to work burden and provider burnout by requiring additional review and follow-up.
Rising health care costs are approaching 18% of the US gross domestic product, driven in large part by a wasteful and inefficient care delivery system.2 One review of “waste domains” identified by the Institute of Medicine estimates that approximately one-quarter of health care costs represent waste, including overtreatment, breakdowns of care coordination, and pricing that fails to correlate to the level of care received.3 High-volume, low-cost testing contributes more to total cost than low-volume, high-cost tests.4
Provider and system factors that contribute to ongoing waste
A lack of awareness of waste and how to reduce it contribute to the problem, as does an underappreciation of the harmful effects caused by incidental abnormal results.
Provider intolerance of diagnostic uncertainty often leads to ordering even more tests.
Continue to: Also, a hope of avoiding...
Also, a hope of avoiding missed diagnoses and potential lawsuits leads to defensive practice and more testing. In addition, patients and family members can exert pressure based on a belief that more testing represents better care. Of course, financial revenues from testing may come into play, with few disincentives to forgo testing. Something that also comes into play is that evidence-based guidance on cost-effective laboratory testing may be lacking, or there may be a lack of knowledge on how to access existing evidence.
Automated systems can facilitate wasteful laboratory testing, and the heavy testing practices of hospitals and specialists may be inappropriately applied to outpatient primary care.
Factors affecting the cost of laboratory testing
Laboratory test results drive 70% of today’s medical decisions.5 Negotiated insurance payment for tests is usually much less than the direct out-of-pocket costs charged to the patient. Without insurance, lab tests can cost patients between $100 and $1000.6 If multiple tests are ordered, the costs could likely be many thousands of dollars.
Actual costs typically vary by the testing facility, the patient’s health plan, and location in the United States; hospital-based testing tends to be the most expensive. Insurers will pay for lab tests with appropriate indications that are covered in the contract with the provider.6
Choosing Wisely initiative weighs in on lab testing
Choosing Wisely, a prominent initiative of the American Board of Internal Medicine Foundation, promotes appropriate resource utilization through educational campaigns that detail how to avoid unnecessary medical tests, treatments, and procedures.7 Recommendations are based largely on specialty society consensus and disease-oriented evidence. Choosing Wisely recommendations advise against the following7:
- performing laboratory blood testing unless clinically indicated or necessary for diagnosis or management, in order to avoid iatrogenic anemia. (American Academy of Family Physicians; Society for the Advancement of Patient Blood Management)
- requesting just a serum creatinine to test adult patients with diabetes and/or hypertension for chronic kidney disease. Use the kidney profile: serum creatinine with estimated glomerular filtration rate and urinary albumin-creatinine ratio. (American Society for Clinical Pathology)
- routinely screening for prostate cancer using a prostate-specific antigen test. It should be performed only after engaging in shared decision-making with the patient. (American Academy of Family Physicians; American Urological Association)
- screening for genital herpes simplex virus infectionFrutiger LT Std in asymptomatic adults, including pregnant women. (American Academy of Family Physicians)
- performing preoperative medical tests for eye surgery unless there are specific medical indications. (American Academy of Ophthalmology)
Sequential steps to takefor value-based lab ordering
Ask the question: “How will ordering this specific test change the management of my patient?” From there, take sequential steps using sound, evidence-based pathways. Morgan and colleagues8 outline the following practical approaches to rational test ordering:
- Perform a thorough clinical assessment.
- Consider the probability and implications of a positive test result.
- Practice patient-centered communication: address the patient’s concerns and discuss the risks and benefits of tests and how they will influence management.
- Follow clinical guidelines when available.
- Avoid ordering tests to reassure the patient; unnecessary tests with insignificant results do little to reduce patient anxieties.
- Avoid letting uncertainty drive unnecessary testing. Watchful waiting can allow time for the illness to resolve or declare itself.
Let’s consider this approach in the context of 4 areas: preventive care, diagnostic evaluation, ongoing management of chronic conditions, and preoperative testing.
Continue to: Preventive guidance from the USPSTF
Preventive guidance from the USPSTF
An independent volunteer panel of 16 national experts in prevention and evidence-based medicine develop recommendations for the US Preventive Services Task Force (USPSTF).9 These guidelines are based on evidence and are updated as new evidence surfaces. Thirteen recommendations, some of which advise avoiding preventive procedures that could cause harm to patients, cover laboratory tests used in screening for conditions such as hyperlipidemia10 and prostate cancer.11 We review the ones pertinent to our patient later at the end of the Case.
While the target audience for USPSTF recommendations is clinicians who provide preventive care, the recommendations are widely followed by policymakers, managed care organizations, public and private payers, quality improvement organizations, research institutions, and patients.
Take a critical look at how you approach the diagnostic evaluation
To reduce unnecessary testing in the diagnostic evaluation of patients, first consider pretest probability, test sensitivity and specificity, narrowly out-of-range tests, habitually paired tests, and repetitive laboratory testing.
Pretest probability, and test sensitivity and specificity. Pretest probability is the estimated chance that the patient has the disease before the test result is known. In a patient with low pretest probability of a disease, the ability to conclusively arrive at the diagnosis with one positive result is limited. Similarly, for tests in patients with high pretest probability of disease, a negative test cannot be used to firmly rule out a diagnosis.12
Reliability also depends on test sensitivity (the proportion of true positive results) and specificity (the proportion of true negative results). A test with high sensitivity but low specificity will generate more false-positive results, with potential harm to patients who do not have a disease.
The pretest probability along with test sensitivity and specificity help a clinician to interpret a test result, and even decide whether to order the test at all. For example, the anti-nuclear antibody (ANA) test for systemic lupus erythematosus (SLE) has a sensitivity of 100% and a specificity of 86%13; it will always be positive in a patient with SLE. But when applied to individuals with low likelihood of SLE, false-positives are more common; the ANA is falsely positive in up to 14% of healthy individuals, depending on the population studied.13
Ordering a test may be unnecessary if the results will not change the treatment plan. For example, in a female patient with classic symptoms of an uncomplicated urinary tract infection, a urine culture and even a urinalysis may not change treatment.
Continue to: Narrowly out-of-range tests
Narrowly out-of-range tests. Test results that fall just outside the “normal” range may be of questionable significance. When an asymptomatic patient has mildly elevated liver enzymes, should additional tests be ordered to avoid missing a treatable disorder? In these scenarios, a history, including possible contributing factors such as alcohol or substance misuse, must be paired with the clinical presentation to assess pre-test probability of a particular condition.14 Repeating a narrowly out-of-range test is an option in patients when follow-up is possible. Alternatively, you could pursue watchful waiting and monitor a minor abnormality over time while being vigilant for clinical changes. This whole-patient approach will guide the decision of whether to order additional testing.
Habitually paired tests. Reflexively ordering tests together often contributes to unnecessary testing. Examples of commonly paired tests are serum lipase with amylase, C-reactive protein (CRP) with erythrocyte sedimentation rate (ESR), and TSH with free T4 to monitor patients with treated hypothyroidism. These tests add minimal value together and can be decoupled.15-17 Evidence supports ordering serum lipase alone, CRP instead of ESR, and TSH alone for monitoring thyroid status.
Some commonly paired tests may not even be necessary for diagnosis. The well-established Rotterdam Criteria for diagnosing polycystic ovary syndrome specify clinical features and ovarian ultrasound for diagnosis.18 They do not require measurement of commonly ordered follicle-stimulating hormone and luteinizing hormone for diagnosis.
Serial rather than parallel testing, a “2-step approach,” is a strategy made easier with the advent of the electronic medical record (EMR) and computerized lab systems.8 These records and lab systems allow providers to order reflex tests, and to add on additional tests, if necessary, to an existing blood specimen.
Repetitive laboratory testing. Repetitive inpatient laboratory testing in patients who are clinically stable is wasteful and potentially harmful. Interventions involving physician education alone show mixed results, but combining education with clinician audit and feedback, along with EMR-enabled restrictive ordering, have resulted in significant and sustained reductions in repetitive laboratory testing.19
Continue to: Ongoing management of chronic conditions
Ongoing management of chronic conditions
Evidence-based guidelines support choices of tests and testing intervals for ongoing management of chronic conditions such as diabetes, hyperlipidemia, and hypertension.
Diabetes. Guidelines also define quality standards that are applied to value-based contracts. For example, the American Diabetes Association recommends assessing A1C every 6 months in patients whose type 2 diabetes is under stable control.20
Hyperlipidemia. For patients diagnosed with hyperlipidemia, 2018 clinical practice guidelines published by multiple specialty societies recommend assessing adherence and response to lifestyle changes and LDL-C–lowering medications with repeat lipid measurement 4 to 12 weeks after statin initiation or dose adjustment, repeated every 3 to 12 months as needed.21
Hypertension. With a new diagnosis of hypertension, guidelines advise an initial assessment for comorbidities and end-organ damage with an electrocardiogram, urinalysis, glucose level, blood count, electrolytes, creatinine, calcium, lipids, and urinary albumin/creatinine ratio. For ongoing monitoring, guidelines recommend assessment for end-organ damage through regular measurements of creatinine, glomerular filtration rate, and urinary microalbumin/creatinine ratio. Initiation and alteration of medications should prompt appropriate additional lab follow-up—eg, a measurement of serum potassium after starting a diuretic.22
Preoperative testing
Preoperative testing is overused in low-risk, ambulatory surgery. And testing, even with abnormal results, does not affect postoperative outcomes.23
Continue to: The American Society of Anesthesiologists (ASA) Physical Status Classification System
The American Society of Anesthesiologists (ASA) Physical Status Classification System, which has been in use for more than 60 years, considers the patient’s physical status (ASA grades I-VI),24 and when paired with surgery grades of minor, intermediate, and major/complex, can help assess preoperative risk and guide preoperative testing (TABLE).24-26

Preoperative medical testing did not reduce the risk of medical adverse events during or after cataract surgery when compared with selective or no testing.27 Unnecessary preoperative testing can lead to a nonproductive cascade of additional investigations. In a 2018 study of Medicare beneficiaries, unnecessary routine preoperative testing and testing sequelae for cataract surgery was calculated to cost Medicare up to $45.4 million annually.28
CASE
You would not be practicing value-based laboratory testing, according to the USPSTF, if you ordered a CMP, fasting lipid profile, and TSH and 25(OH) vitamin D tests for this healthy 35-year-old man whose family history, blood pressure, and BMI do not put him at elevated risk. Universal lipid screening (Grade Ba) is recommended for all adults ages 40 to 75. Thyroid screening tests and measurement of 25(OH) vitamin D level (I statementsa) are not recommended. The USPSTF has not evaluated chemistry panels for screening.
The USPSTF would recommend the following actions for this patient:
- Screen for HIV (ages 15 to 65 years; and younger or older if patient is at risk). (A recommendationa,29)
- Screen for hepatitis C virus (in those ages 18 to 79). (B recommendation30)
The following USPSTF recommendations might have come into play if this patient had certain risk factors, or if the patient had been a woman:
- Screen for diabetes if the patient is overweight or obese (B recommendation).
- Screen for hepatitis B in adults at risk (B recommendation).
- Screen for gonorrhea and chlamydia in women at risk (B recommendation). Such screening has an “I”statement for screening men at risk.
Continue to: As noted, costs of laboratory...
As noted, costs of laboratory testing vary widely, depending upon what tests are ordered, what type of insurance the patient has, and which tests the patient’s insurance covers. Who performs the testing also factors into the cost. Payers negotiate reduced fees for commercial lab testing, but potential out-of-pocket costs to patients are much higher.
For our healthy 35-year-old man, the cost of the initially proposed testing (CMP, lipid panel, TSH, and 25[OH] vitamin D level) ranges from a negotiated payer cost of $85 to potential patient out-of-pocket cost of more than $400.6
Insurance would cover the USPSTF-recommended testing (HIV and hepatitis C screening tests), which might incur only a patient co-pay, and cost the system about $65.
The USPSTF home page, found at www.uspreventiveservicestaskforce.org/uspstf/ includes recommendations that can be sorted for your patients. A web and mobile device application is also available through the website.
a USPSTF grade definitions:
A: There is high certainty that the net benefit is substantial. Offer service.
B: There is high certainty that the net benefit is moderate, or there is moderate certainty that the net benefit is moderate to substantial. Offer service.
C: There is at least moderate certainty that the net benefit is small. Offer service selectively.
D: There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. Don’t offer service.
I: Current evidence is insufficient to assess the balance of benefits and harms of the service.
CORRESPONDENCE
Mitchell Kaminski, MD, MBA, 901 Walnut Street, 10th Floor, Jefferson College of Population Health, Philadelphia, PA 19107; [email protected]
CASE
A 35-year-old man arrives for an annual wellness visit with no specific complaints and no significant personal or family history. His normal exam includes a blood pressure of 110/74 mm Hg and a body mass index (BMI) of 23.6. You order “routine labs” for prevention, which include a comprehensive metabolic panel (CMP), fasting lipid profile, and thyroid-stimulating hormone (TSH) and 25(OH) vitamin D tests. Are you practicing value-based laboratory testing?
The answer to this question appears in the Case discussion at the end of the article.
Value-based care, including care provided through laboratory testing, can achieve the Institute for Healthcare Improvement’s Triple Aim of improving population health, improving the patient experience of care (including quality and satisfaction), and reducing cost: Value = (Quality x Patient experience) / Cost.1
As quality and patient experience rise and cost falls, the value of care increases. Unnecessary lab testing, however, can negatively impact this equation:
- Error introduced by unnecessary testing can adversely affect quality.
- Patients experience inconvenience and sometimes cascades of testing, in addition to financial responsibility, from unnecessary testing.
- Low-value testing also contributes to work burden and provider burnout by requiring additional review and follow-up.
Rising health care costs are approaching 18% of the US gross domestic product, driven in large part by a wasteful and inefficient care delivery system.2 One review of “waste domains” identified by the Institute of Medicine estimates that approximately one-quarter of health care costs represent waste, including overtreatment, breakdowns of care coordination, and pricing that fails to correlate to the level of care received.3 High-volume, low-cost testing contributes more to total cost than low-volume, high-cost tests.4
Provider and system factors that contribute to ongoing waste
A lack of awareness of waste and how to reduce it contribute to the problem, as does an underappreciation of the harmful effects caused by incidental abnormal results.
Provider intolerance of diagnostic uncertainty often leads to ordering even more tests.
Continue to: Also, a hope of avoiding...
Also, a hope of avoiding missed diagnoses and potential lawsuits leads to defensive practice and more testing. In addition, patients and family members can exert pressure based on a belief that more testing represents better care. Of course, financial revenues from testing may come into play, with few disincentives to forgo testing. Something that also comes into play is that evidence-based guidance on cost-effective laboratory testing may be lacking, or there may be a lack of knowledge on how to access existing evidence.
Automated systems can facilitate wasteful laboratory testing, and the heavy testing practices of hospitals and specialists may be inappropriately applied to outpatient primary care.
Factors affecting the cost of laboratory testing
Laboratory test results drive 70% of today’s medical decisions.5 Negotiated insurance payment for tests is usually much less than the direct out-of-pocket costs charged to the patient. Without insurance, lab tests can cost patients between $100 and $1000.6 If multiple tests are ordered, the costs could likely be many thousands of dollars.
Actual costs typically vary by the testing facility, the patient’s health plan, and location in the United States; hospital-based testing tends to be the most expensive. Insurers will pay for lab tests with appropriate indications that are covered in the contract with the provider.6
Choosing Wisely initiative weighs in on lab testing
Choosing Wisely, a prominent initiative of the American Board of Internal Medicine Foundation, promotes appropriate resource utilization through educational campaigns that detail how to avoid unnecessary medical tests, treatments, and procedures.7 Recommendations are based largely on specialty society consensus and disease-oriented evidence. Choosing Wisely recommendations advise against the following7:
- performing laboratory blood testing unless clinically indicated or necessary for diagnosis or management, in order to avoid iatrogenic anemia. (American Academy of Family Physicians; Society for the Advancement of Patient Blood Management)
- requesting just a serum creatinine to test adult patients with diabetes and/or hypertension for chronic kidney disease. Use the kidney profile: serum creatinine with estimated glomerular filtration rate and urinary albumin-creatinine ratio. (American Society for Clinical Pathology)
- routinely screening for prostate cancer using a prostate-specific antigen test. It should be performed only after engaging in shared decision-making with the patient. (American Academy of Family Physicians; American Urological Association)
- screening for genital herpes simplex virus infectionFrutiger LT Std in asymptomatic adults, including pregnant women. (American Academy of Family Physicians)
- performing preoperative medical tests for eye surgery unless there are specific medical indications. (American Academy of Ophthalmology)
Sequential steps to takefor value-based lab ordering
Ask the question: “How will ordering this specific test change the management of my patient?” From there, take sequential steps using sound, evidence-based pathways. Morgan and colleagues8 outline the following practical approaches to rational test ordering:
- Perform a thorough clinical assessment.
- Consider the probability and implications of a positive test result.
- Practice patient-centered communication: address the patient’s concerns and discuss the risks and benefits of tests and how they will influence management.
- Follow clinical guidelines when available.
- Avoid ordering tests to reassure the patient; unnecessary tests with insignificant results do little to reduce patient anxieties.
- Avoid letting uncertainty drive unnecessary testing. Watchful waiting can allow time for the illness to resolve or declare itself.
Let’s consider this approach in the context of 4 areas: preventive care, diagnostic evaluation, ongoing management of chronic conditions, and preoperative testing.
Continue to: Preventive guidance from the USPSTF
Preventive guidance from the USPSTF
An independent volunteer panel of 16 national experts in prevention and evidence-based medicine develop recommendations for the US Preventive Services Task Force (USPSTF).9 These guidelines are based on evidence and are updated as new evidence surfaces. Thirteen recommendations, some of which advise avoiding preventive procedures that could cause harm to patients, cover laboratory tests used in screening for conditions such as hyperlipidemia10 and prostate cancer.11 We review the ones pertinent to our patient later at the end of the Case.
While the target audience for USPSTF recommendations is clinicians who provide preventive care, the recommendations are widely followed by policymakers, managed care organizations, public and private payers, quality improvement organizations, research institutions, and patients.
Take a critical look at how you approach the diagnostic evaluation
To reduce unnecessary testing in the diagnostic evaluation of patients, first consider pretest probability, test sensitivity and specificity, narrowly out-of-range tests, habitually paired tests, and repetitive laboratory testing.
Pretest probability, and test sensitivity and specificity. Pretest probability is the estimated chance that the patient has the disease before the test result is known. In a patient with low pretest probability of a disease, the ability to conclusively arrive at the diagnosis with one positive result is limited. Similarly, for tests in patients with high pretest probability of disease, a negative test cannot be used to firmly rule out a diagnosis.12
Reliability also depends on test sensitivity (the proportion of true positive results) and specificity (the proportion of true negative results). A test with high sensitivity but low specificity will generate more false-positive results, with potential harm to patients who do not have a disease.
The pretest probability along with test sensitivity and specificity help a clinician to interpret a test result, and even decide whether to order the test at all. For example, the anti-nuclear antibody (ANA) test for systemic lupus erythematosus (SLE) has a sensitivity of 100% and a specificity of 86%13; it will always be positive in a patient with SLE. But when applied to individuals with low likelihood of SLE, false-positives are more common; the ANA is falsely positive in up to 14% of healthy individuals, depending on the population studied.13
Ordering a test may be unnecessary if the results will not change the treatment plan. For example, in a female patient with classic symptoms of an uncomplicated urinary tract infection, a urine culture and even a urinalysis may not change treatment.
Continue to: Narrowly out-of-range tests
Narrowly out-of-range tests. Test results that fall just outside the “normal” range may be of questionable significance. When an asymptomatic patient has mildly elevated liver enzymes, should additional tests be ordered to avoid missing a treatable disorder? In these scenarios, a history, including possible contributing factors such as alcohol or substance misuse, must be paired with the clinical presentation to assess pre-test probability of a particular condition.14 Repeating a narrowly out-of-range test is an option in patients when follow-up is possible. Alternatively, you could pursue watchful waiting and monitor a minor abnormality over time while being vigilant for clinical changes. This whole-patient approach will guide the decision of whether to order additional testing.
Habitually paired tests. Reflexively ordering tests together often contributes to unnecessary testing. Examples of commonly paired tests are serum lipase with amylase, C-reactive protein (CRP) with erythrocyte sedimentation rate (ESR), and TSH with free T4 to monitor patients with treated hypothyroidism. These tests add minimal value together and can be decoupled.15-17 Evidence supports ordering serum lipase alone, CRP instead of ESR, and TSH alone for monitoring thyroid status.
Some commonly paired tests may not even be necessary for diagnosis. The well-established Rotterdam Criteria for diagnosing polycystic ovary syndrome specify clinical features and ovarian ultrasound for diagnosis.18 They do not require measurement of commonly ordered follicle-stimulating hormone and luteinizing hormone for diagnosis.
Serial rather than parallel testing, a “2-step approach,” is a strategy made easier with the advent of the electronic medical record (EMR) and computerized lab systems.8 These records and lab systems allow providers to order reflex tests, and to add on additional tests, if necessary, to an existing blood specimen.
Repetitive laboratory testing. Repetitive inpatient laboratory testing in patients who are clinically stable is wasteful and potentially harmful. Interventions involving physician education alone show mixed results, but combining education with clinician audit and feedback, along with EMR-enabled restrictive ordering, have resulted in significant and sustained reductions in repetitive laboratory testing.19
Continue to: Ongoing management of chronic conditions
Ongoing management of chronic conditions
Evidence-based guidelines support choices of tests and testing intervals for ongoing management of chronic conditions such as diabetes, hyperlipidemia, and hypertension.
Diabetes. Guidelines also define quality standards that are applied to value-based contracts. For example, the American Diabetes Association recommends assessing A1C every 6 months in patients whose type 2 diabetes is under stable control.20
Hyperlipidemia. For patients diagnosed with hyperlipidemia, 2018 clinical practice guidelines published by multiple specialty societies recommend assessing adherence and response to lifestyle changes and LDL-C–lowering medications with repeat lipid measurement 4 to 12 weeks after statin initiation or dose adjustment, repeated every 3 to 12 months as needed.21
Hypertension. With a new diagnosis of hypertension, guidelines advise an initial assessment for comorbidities and end-organ damage with an electrocardiogram, urinalysis, glucose level, blood count, electrolytes, creatinine, calcium, lipids, and urinary albumin/creatinine ratio. For ongoing monitoring, guidelines recommend assessment for end-organ damage through regular measurements of creatinine, glomerular filtration rate, and urinary microalbumin/creatinine ratio. Initiation and alteration of medications should prompt appropriate additional lab follow-up—eg, a measurement of serum potassium after starting a diuretic.22
Preoperative testing
Preoperative testing is overused in low-risk, ambulatory surgery. And testing, even with abnormal results, does not affect postoperative outcomes.23
Continue to: The American Society of Anesthesiologists (ASA) Physical Status Classification System
The American Society of Anesthesiologists (ASA) Physical Status Classification System, which has been in use for more than 60 years, considers the patient’s physical status (ASA grades I-VI),24 and when paired with surgery grades of minor, intermediate, and major/complex, can help assess preoperative risk and guide preoperative testing (TABLE).24-26

Preoperative medical testing did not reduce the risk of medical adverse events during or after cataract surgery when compared with selective or no testing.27 Unnecessary preoperative testing can lead to a nonproductive cascade of additional investigations. In a 2018 study of Medicare beneficiaries, unnecessary routine preoperative testing and testing sequelae for cataract surgery was calculated to cost Medicare up to $45.4 million annually.28
CASE
You would not be practicing value-based laboratory testing, according to the USPSTF, if you ordered a CMP, fasting lipid profile, and TSH and 25(OH) vitamin D tests for this healthy 35-year-old man whose family history, blood pressure, and BMI do not put him at elevated risk. Universal lipid screening (Grade Ba) is recommended for all adults ages 40 to 75. Thyroid screening tests and measurement of 25(OH) vitamin D level (I statementsa) are not recommended. The USPSTF has not evaluated chemistry panels for screening.
The USPSTF would recommend the following actions for this patient:
- Screen for HIV (ages 15 to 65 years; and younger or older if patient is at risk). (A recommendationa,29)
- Screen for hepatitis C virus (in those ages 18 to 79). (B recommendation30)
The following USPSTF recommendations might have come into play if this patient had certain risk factors, or if the patient had been a woman:
- Screen for diabetes if the patient is overweight or obese (B recommendation).
- Screen for hepatitis B in adults at risk (B recommendation).
- Screen for gonorrhea and chlamydia in women at risk (B recommendation). Such screening has an “I”statement for screening men at risk.
Continue to: As noted, costs of laboratory...
As noted, costs of laboratory testing vary widely, depending upon what tests are ordered, what type of insurance the patient has, and which tests the patient’s insurance covers. Who performs the testing also factors into the cost. Payers negotiate reduced fees for commercial lab testing, but potential out-of-pocket costs to patients are much higher.
For our healthy 35-year-old man, the cost of the initially proposed testing (CMP, lipid panel, TSH, and 25[OH] vitamin D level) ranges from a negotiated payer cost of $85 to potential patient out-of-pocket cost of more than $400.6
Insurance would cover the USPSTF-recommended testing (HIV and hepatitis C screening tests), which might incur only a patient co-pay, and cost the system about $65.
The USPSTF home page, found at www.uspreventiveservicestaskforce.org/uspstf/ includes recommendations that can be sorted for your patients. A web and mobile device application is also available through the website.
a USPSTF grade definitions:
A: There is high certainty that the net benefit is substantial. Offer service.
B: There is high certainty that the net benefit is moderate, or there is moderate certainty that the net benefit is moderate to substantial. Offer service.
C: There is at least moderate certainty that the net benefit is small. Offer service selectively.
D: There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. Don’t offer service.
I: Current evidence is insufficient to assess the balance of benefits and harms of the service.
CORRESPONDENCE
Mitchell Kaminski, MD, MBA, 901 Walnut Street, 10th Floor, Jefferson College of Population Health, Philadelphia, PA 19107; [email protected]
1. IHI. What is the Triple Aim? Accessed June 20, 2022. http://www.ihi.org/Topics/TripleAim/Pages/Overview.aspx#:~:text=It%20is%20IHI’s%20belief%20that,capita%20cost%20of%20health%20care
2. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. doi: 10.1001/jama.2018.1150
3. Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system estimated costs and potential for savings. JAMA. 2019;322:1501-1509. doi:10.1001/jama.2019.13978
4. Mafi JN, Russell K, Bortz BA, et al. Low-cost, high-volume health services contribute the most to unnecessary health spending. Health Aff. 2017;36:1701-1704. doi: 10.1377/hlthaff.2017.0385
5. CDC. Strengthening clinical laboratories. 2018. Accessed June 2020, 2022. www.cdc.gov/csels/dls/strengthening-clinical-labs.html
6. Vuong KT. How much do lab tests cost without insurance in 2022? Accessed May 11, 2022. www.talktomira.com/post/how-much-do-lab-test-cost-without-insurance
7. Choosing Wisely: Promoting conversations between providers and patients. Accessed June 20, 2022. www.choosingwisely.org
8. Morgan S, van Driel M, Coleman J, et al. Rational test ordering in family medicine. Can Fam Physician. 2015;61:535-537.
9. US Preventive Services Taskforce. Screening for glaucoma and impaired vision. Accessed June 20, 2022. www.uspreventiveservicestaskforce.org/uspstf
10. Arnold MJ, O’Malley PG, Downs JR. Key recommendations on managing dyslipidemia for cardiovascular risk reduction: stopping where the evidence does. Am Fam Physician. 2021;103:455-458.
11. Welch HG, Albertsen PC. Reconsidering prostate cancer mortality—the future of PSA screening. N Engl J Med. 2020;382:1557-1563. doi: 10.1056/NEJMms1914228
12. American Society for Microbiology. Why pretest and posttest probability matter in the time of COVID-19. Accessed June 20, 2022. https://asm.org/Articles/2020/June/Why-Pretest-and-Posttest-Probability-Matter-in-the
13. Slater CA, Davis RB, Shmerling RH. Antinuclear antibody testing. A study of clinical utility. Arch Intern Med. 1996;156:1421-1425.
14. Aragon G, Younossi ZM. When and how to evaluate mildly elevated liver enzymes in apparently healthy patients. Cleve Clin J Med. 2010;77:195-204. doi: 10.3949/ccjm.77a.09064
15. Ismail OZ, Bhayana V. Lipase or amylase for the diagnosis of acute pancreatitis? Clin Biochem. 2017;50:1275-1280. doi: 10.1016/j.clinbiochem.2017.07.003.
16. Gottheil S, Khemani E, Copley K, et al. Reducing inappropriate ESR testing with computerized clinical decision support. BMJ Quality Improvement Reports, 2016;5:u211376.w4582. doi: 10.1136/bmjquality.u211376.w4582
17. Schneider C, Feller M, Bauer DC, et al. Initial evaluation of thyroid dysfunction - are simultaneous TSH and fT4 tests necessary? PloS One. 2018;13:e0196631–e0196631. doi: 10.1371/journal.pone.0196631
18. Williams T, Mortada R, Porter S. Diagnosis and treatment of polycystic ovary syndrome. Am Fam Physician. 2016;94:106-113.
19. Eaton KP, Levy K, Soong C et.al. Evidence-Based Guidelines to Eliminate Repetitive Laboratory Testing. JAMA Intern Med. 2017;177:1833-1839. doi: 10.1001/jamainternmed.2017.5152
20. ADA. Glycemic targets: standards of medical care in diabetes—2021. Diabetes Care. 2021;44:S73-S84. doi: 10.2337/dc21-S006
21. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/ AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
22. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026.
23. Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256:518-528. doi: 10.1097/SLA.0b013e318265bcdb
24. ASA. ASA physical status classification system. Accessed June 22,2022. www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system
25. NLM. Preoperative tests (update): routine preoperative tests for elective surgery. Accessed June 22, 2022. www.ncbi.nlm.nih.gov/books/NBK367919/
26. ASA. American Society of Anesthesiologists releases list of commonly used tests and treatments to question-AS PART OF CHOOSING WISELY® CAMPAIGN. Accessed June 22, 2022. www.asahq.org/about-asa/newsroom/news-releases/2013/10/choosing-wisely
27. Keay L, Lindsley K, Tielsch J, et al. Routine preoperative medical testing for cataract surgery. Cochrane Database Syst Rev. 2019;1:CD007293. doi: 10.1002/14651858.CD007293.pub4
28. Chen CL, Clay TH, McLeod S, et al. A revised estimate of costs associated with routine preoperative testing in Medicare cataract patients with a procedure-specific indicator. JAMA Ophthalmol. 2018;136:231-238. doi:10.1001/jamaophthalmol.2017.6372
29. USPSTF. Human immunodeficiency virus (HIV) infection: screening. Accessed May 16, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/human-immunodeficiency-virus-hiv-infection-screening
30. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed June 20, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
1. IHI. What is the Triple Aim? Accessed June 20, 2022. http://www.ihi.org/Topics/TripleAim/Pages/Overview.aspx#:~:text=It%20is%20IHI’s%20belief%20that,capita%20cost%20of%20health%20care
2. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. doi: 10.1001/jama.2018.1150
3. Shrank WH, Rogstad TL, Parekh N. Waste in the US health care system estimated costs and potential for savings. JAMA. 2019;322:1501-1509. doi:10.1001/jama.2019.13978
4. Mafi JN, Russell K, Bortz BA, et al. Low-cost, high-volume health services contribute the most to unnecessary health spending. Health Aff. 2017;36:1701-1704. doi: 10.1377/hlthaff.2017.0385
5. CDC. Strengthening clinical laboratories. 2018. Accessed June 2020, 2022. www.cdc.gov/csels/dls/strengthening-clinical-labs.html
6. Vuong KT. How much do lab tests cost without insurance in 2022? Accessed May 11, 2022. www.talktomira.com/post/how-much-do-lab-test-cost-without-insurance
7. Choosing Wisely: Promoting conversations between providers and patients. Accessed June 20, 2022. www.choosingwisely.org
8. Morgan S, van Driel M, Coleman J, et al. Rational test ordering in family medicine. Can Fam Physician. 2015;61:535-537.
9. US Preventive Services Taskforce. Screening for glaucoma and impaired vision. Accessed June 20, 2022. www.uspreventiveservicestaskforce.org/uspstf
10. Arnold MJ, O’Malley PG, Downs JR. Key recommendations on managing dyslipidemia for cardiovascular risk reduction: stopping where the evidence does. Am Fam Physician. 2021;103:455-458.
11. Welch HG, Albertsen PC. Reconsidering prostate cancer mortality—the future of PSA screening. N Engl J Med. 2020;382:1557-1563. doi: 10.1056/NEJMms1914228
12. American Society for Microbiology. Why pretest and posttest probability matter in the time of COVID-19. Accessed June 20, 2022. https://asm.org/Articles/2020/June/Why-Pretest-and-Posttest-Probability-Matter-in-the
13. Slater CA, Davis RB, Shmerling RH. Antinuclear antibody testing. A study of clinical utility. Arch Intern Med. 1996;156:1421-1425.
14. Aragon G, Younossi ZM. When and how to evaluate mildly elevated liver enzymes in apparently healthy patients. Cleve Clin J Med. 2010;77:195-204. doi: 10.3949/ccjm.77a.09064
15. Ismail OZ, Bhayana V. Lipase or amylase for the diagnosis of acute pancreatitis? Clin Biochem. 2017;50:1275-1280. doi: 10.1016/j.clinbiochem.2017.07.003.
16. Gottheil S, Khemani E, Copley K, et al. Reducing inappropriate ESR testing with computerized clinical decision support. BMJ Quality Improvement Reports, 2016;5:u211376.w4582. doi: 10.1136/bmjquality.u211376.w4582
17. Schneider C, Feller M, Bauer DC, et al. Initial evaluation of thyroid dysfunction - are simultaneous TSH and fT4 tests necessary? PloS One. 2018;13:e0196631–e0196631. doi: 10.1371/journal.pone.0196631
18. Williams T, Mortada R, Porter S. Diagnosis and treatment of polycystic ovary syndrome. Am Fam Physician. 2016;94:106-113.
19. Eaton KP, Levy K, Soong C et.al. Evidence-Based Guidelines to Eliminate Repetitive Laboratory Testing. JAMA Intern Med. 2017;177:1833-1839. doi: 10.1001/jamainternmed.2017.5152
20. ADA. Glycemic targets: standards of medical care in diabetes—2021. Diabetes Care. 2021;44:S73-S84. doi: 10.2337/dc21-S006
21. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/ AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
22. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026.
23. Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256:518-528. doi: 10.1097/SLA.0b013e318265bcdb
24. ASA. ASA physical status classification system. Accessed June 22,2022. www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system
25. NLM. Preoperative tests (update): routine preoperative tests for elective surgery. Accessed June 22, 2022. www.ncbi.nlm.nih.gov/books/NBK367919/
26. ASA. American Society of Anesthesiologists releases list of commonly used tests and treatments to question-AS PART OF CHOOSING WISELY® CAMPAIGN. Accessed June 22, 2022. www.asahq.org/about-asa/newsroom/news-releases/2013/10/choosing-wisely
27. Keay L, Lindsley K, Tielsch J, et al. Routine preoperative medical testing for cataract surgery. Cochrane Database Syst Rev. 2019;1:CD007293. doi: 10.1002/14651858.CD007293.pub4
28. Chen CL, Clay TH, McLeod S, et al. A revised estimate of costs associated with routine preoperative testing in Medicare cataract patients with a procedure-specific indicator. JAMA Ophthalmol. 2018;136:231-238. doi:10.1001/jamaophthalmol.2017.6372
29. USPSTF. Human immunodeficiency virus (HIV) infection: screening. Accessed May 16, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/human-immunodeficiency-virus-hiv-infection-screening
30. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed June 20, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
PRACTICE RECOMMENDATIONS
› Follow US Preventive Services Task Force and professional society recommendations for laboratory testing, including choice and frequency of tests. A
› Consider the pretest probability of your patient having a disease, and order the most sensitive and specific test to diagnose a new condition. Employ a 2-step approach with a second laboratory test when the first is outside the reference range. B
› Refrain from ordering routine preoperative testing for patients undergoing low-risk surgeries; these data do not improve postoperative outcomes, can lead to costly testing cascades, and may delay necessary surgical care for patients. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
‘I shall harm’
I was quite sure I had multiple sclerosis when I was a medical student. I first noticed symptoms during my neurology rotation. All the signs were there: Fatigue that was getting worse in the North Carolina heat (Uhthoff sign!). A tingle running down my neck (Lhermitte sign!). Blurry vision late at night while studying pathways in Lange Neurology. “Didn’t cousin Amy have MS?” I asked my Mom. I started researching which medical specialties didn’t require dexterity. My left eyelid began twitching and didn’t stop until I rotated to ob.gyn.
Fortunately, it was not multiple sclerosis I had, but rather nosophobia, also known as Medical Student’s Disease. The combination of intense study of symptoms, spotty knowledge of diagnoses, and grade anxiety makes nosophobia common in med students. Despite its name, it doesn’t afflict only doctors. Patients often come to us convinced they have a disease but without reason. So unshakable is their belief that multiple visits are often required to disabuse them of their self-diagnosis. I sometimes have to remind myself to appear concerned even when a “melanoma” is a freckle so small I can barely see it with a dermatoscope. Or a “genital wart” is a hair follicle that looks exactly like the hundreds on the patient’s scrotum. Tougher though, are the treatment-avoiders: patients whose imagined side effects lead them to stop or refuse treatment.
I recently saw a middle-aged man with erythroderma so severe he looked like a ghillie suit of scale. He had a lifelong history of atopic dermatitis and a 2-year history of avoiding treatments. At some point, he tried all the usual remedies: cyclosporine, methotrexate, azathioprine, light therapy, boxes of topicals. The last treatment had been dupilumab, which he tried for a few weeks. “Why did you stop that one?” I asked. The injections were making him go blind, he explained. “Not blurry? Blind?” I asked. Yes, he could not see at all after each injection. Perhaps he might have dry eyes or keratitis? Sure. But blindness? It seemed an unreasonable concern. Further discussion revealed that intolerance to medication side effects was why he had stopped all his other treatments.
Nocebo, from the Latin “I shall harm,” is the dark counterpart to the placebo. Patients experience imagined, or even real, adverse effects because they believe the treatment is causing them harm. This is true even though that treatment might not be having any unwanted physiologic effect. Statins are a good example. Studies have shown that most patient-reported side effects of statins are in fact nocebo effects rather than a result of pharmacologic causes.
Yet, many patients on statins report muscle pain or other concerns as unbearable. As a consequence, some patients who might have benefited from statins might be missing out on the protective gains. as compared with bad outcomes that occurred from not taking action. It’s frustrating when there’s a standard of care treatment, but our patient can’t get past their fear of harm to try it.
Despite my recommendations, my eczema patient insisted on continuing his nontreatment rather than take any risks with treatments for now. There are ways I might help, but I expect it will require additional visits to build trust. Today, the best I can do is to understand and respect him. At least he doesn’t think he has a genital wart – I’m not sure how I’d treat it if he did.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
I was quite sure I had multiple sclerosis when I was a medical student. I first noticed symptoms during my neurology rotation. All the signs were there: Fatigue that was getting worse in the North Carolina heat (Uhthoff sign!). A tingle running down my neck (Lhermitte sign!). Blurry vision late at night while studying pathways in Lange Neurology. “Didn’t cousin Amy have MS?” I asked my Mom. I started researching which medical specialties didn’t require dexterity. My left eyelid began twitching and didn’t stop until I rotated to ob.gyn.
Fortunately, it was not multiple sclerosis I had, but rather nosophobia, also known as Medical Student’s Disease. The combination of intense study of symptoms, spotty knowledge of diagnoses, and grade anxiety makes nosophobia common in med students. Despite its name, it doesn’t afflict only doctors. Patients often come to us convinced they have a disease but without reason. So unshakable is their belief that multiple visits are often required to disabuse them of their self-diagnosis. I sometimes have to remind myself to appear concerned even when a “melanoma” is a freckle so small I can barely see it with a dermatoscope. Or a “genital wart” is a hair follicle that looks exactly like the hundreds on the patient’s scrotum. Tougher though, are the treatment-avoiders: patients whose imagined side effects lead them to stop or refuse treatment.
I recently saw a middle-aged man with erythroderma so severe he looked like a ghillie suit of scale. He had a lifelong history of atopic dermatitis and a 2-year history of avoiding treatments. At some point, he tried all the usual remedies: cyclosporine, methotrexate, azathioprine, light therapy, boxes of topicals. The last treatment had been dupilumab, which he tried for a few weeks. “Why did you stop that one?” I asked. The injections were making him go blind, he explained. “Not blurry? Blind?” I asked. Yes, he could not see at all after each injection. Perhaps he might have dry eyes or keratitis? Sure. But blindness? It seemed an unreasonable concern. Further discussion revealed that intolerance to medication side effects was why he had stopped all his other treatments.
Nocebo, from the Latin “I shall harm,” is the dark counterpart to the placebo. Patients experience imagined, or even real, adverse effects because they believe the treatment is causing them harm. This is true even though that treatment might not be having any unwanted physiologic effect. Statins are a good example. Studies have shown that most patient-reported side effects of statins are in fact nocebo effects rather than a result of pharmacologic causes.
Yet, many patients on statins report muscle pain or other concerns as unbearable. As a consequence, some patients who might have benefited from statins might be missing out on the protective gains. as compared with bad outcomes that occurred from not taking action. It’s frustrating when there’s a standard of care treatment, but our patient can’t get past their fear of harm to try it.
Despite my recommendations, my eczema patient insisted on continuing his nontreatment rather than take any risks with treatments for now. There are ways I might help, but I expect it will require additional visits to build trust. Today, the best I can do is to understand and respect him. At least he doesn’t think he has a genital wart – I’m not sure how I’d treat it if he did.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
I was quite sure I had multiple sclerosis when I was a medical student. I first noticed symptoms during my neurology rotation. All the signs were there: Fatigue that was getting worse in the North Carolina heat (Uhthoff sign!). A tingle running down my neck (Lhermitte sign!). Blurry vision late at night while studying pathways in Lange Neurology. “Didn’t cousin Amy have MS?” I asked my Mom. I started researching which medical specialties didn’t require dexterity. My left eyelid began twitching and didn’t stop until I rotated to ob.gyn.
Fortunately, it was not multiple sclerosis I had, but rather nosophobia, also known as Medical Student’s Disease. The combination of intense study of symptoms, spotty knowledge of diagnoses, and grade anxiety makes nosophobia common in med students. Despite its name, it doesn’t afflict only doctors. Patients often come to us convinced they have a disease but without reason. So unshakable is their belief that multiple visits are often required to disabuse them of their self-diagnosis. I sometimes have to remind myself to appear concerned even when a “melanoma” is a freckle so small I can barely see it with a dermatoscope. Or a “genital wart” is a hair follicle that looks exactly like the hundreds on the patient’s scrotum. Tougher though, are the treatment-avoiders: patients whose imagined side effects lead them to stop or refuse treatment.
I recently saw a middle-aged man with erythroderma so severe he looked like a ghillie suit of scale. He had a lifelong history of atopic dermatitis and a 2-year history of avoiding treatments. At some point, he tried all the usual remedies: cyclosporine, methotrexate, azathioprine, light therapy, boxes of topicals. The last treatment had been dupilumab, which he tried for a few weeks. “Why did you stop that one?” I asked. The injections were making him go blind, he explained. “Not blurry? Blind?” I asked. Yes, he could not see at all after each injection. Perhaps he might have dry eyes or keratitis? Sure. But blindness? It seemed an unreasonable concern. Further discussion revealed that intolerance to medication side effects was why he had stopped all his other treatments.
Nocebo, from the Latin “I shall harm,” is the dark counterpart to the placebo. Patients experience imagined, or even real, adverse effects because they believe the treatment is causing them harm. This is true even though that treatment might not be having any unwanted physiologic effect. Statins are a good example. Studies have shown that most patient-reported side effects of statins are in fact nocebo effects rather than a result of pharmacologic causes.
Yet, many patients on statins report muscle pain or other concerns as unbearable. As a consequence, some patients who might have benefited from statins might be missing out on the protective gains. as compared with bad outcomes that occurred from not taking action. It’s frustrating when there’s a standard of care treatment, but our patient can’t get past their fear of harm to try it.
Despite my recommendations, my eczema patient insisted on continuing his nontreatment rather than take any risks with treatments for now. There are ways I might help, but I expect it will require additional visits to build trust. Today, the best I can do is to understand and respect him. At least he doesn’t think he has a genital wart – I’m not sure how I’d treat it if he did.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
NAFLD strongly correlated with psoriasis, PsA; risk linked to severity
NEW YORK – – and probably in those with psoriatic arthritis (PsA) as well, according to a systematic review and meta-analysis presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“Our findings imply that psoriatic patients should be screened with an ultrasonographic exam in cases where there are metabolic features that are associated with NAFLD,” reported Francesco Bellinato, MD, a researcher in the section of dermatology and venereology, University of Verona (Italy).
The data are strong. Of 76 nonduplicate publications found in the literature, the 11 observational studies included in the meta-analysis met stringent criteria, including a diagnosis of psoriasis and PsA based on objective criteria, NAFLD confirmed with liver biopsy or imaging, and odds rates calculated with 95% confidence intervals.
From these 11 studies, aggregate data were available for 249,333 psoriatic patients, of which 49% had NAFLD, and 1,491,402 were healthy controls. Among the controls, 36% had NAFLD. Four of the studies were from North America, four from Europe, and three from Asia.
In the pooled data, the risk of NAFLD among those with psoriasis relative to healthy controls fell just short of a twofold increase (odds ratio, 1.96; 95% CI, 1.70-2.26; P < .001). When stratified by studies that confirmed NAFLD by biopsy relative to ultrasonography, there was no significant heterogeneity.
Eight of the studies included an analysis of relative risk in the context of skin lesion severity defined by Psoriasis Area and Severity Index (PASI) score. Relative to those without NAFLD, psoriatic patients with NAFLD had a significant greater mean PASI score on a pooled weighted mean difference analysis (OR, 3.93; 95% CI, 2.01-5.84; P < .0001).
For PsA relative to no PsA in the five studies that compared risk between these two groups, the risk of NAFLD was again nearly twofold higher. This fell short of conventional definition of statistical significance, but it was associated with a strong trend (OR, 1.83; 95% CI, 0.98-3.43; P = .06).
The risk of NAFLD among patients with psoriasis was not found to vary significantly when assessed by univariable meta-regressions across numerous characteristics, such as sex and body mass index.
In one of the largest of the observational studies included in the meta-analysis by Alexis Ogdie, MD, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia, and colleagues, data were analyzed in more than 1.5 million patients, which included 54,251 patients with rheumatoid arthritis. While the hazard ratio of NAFLD was increased for both psoriasis (HR, 2.23) and PsA (HR, 2.11), it was not elevated in those with RA (HR, 0.96).
Risk by severity, possible mechanisms
This study also included an analysis of NAFLD risk according to psoriasis severity. While risk was still significant among those with mild disease (HR, 1.18; 95% CI, 1.07-1.30), it was almost twofold greater in those with moderate to severe psoriasis (HR, 2.23; 95% CI, 1.73-2.87).
Dr. Bellinato conceded that the mechanisms underlying the association between psoriasis and NAFLD are unknown, but he said “metaflammation” is suspected.
“The secretion of proinflammatory, prothrombotic, and oxidative stress mediators in both psoriatic skin and adipose tissue might act systemically and promote insulin resistance and other metabolic derangements that promote the development and progression of NAFLD,” Dr. Bellinato explained.
He thinks that noninvasive screening methods, such as currently used methods to calculate fibrosis score, might be useful for evaluating patients with psoriasis for NAFLD and referring them to a hepatologist when appropriate.
Given the strong association with NAFLD, Dr. Bellinato suggested that “the findings of this meta-analysis pave the way for novel, large, prospective, and histologically based studies.”
The association between psoriasis and NAFLD is clinically relevant, agreed Joel M. Gelfand, MD, vice-chair of clinical research and medical director of the clinical studies unit, department of dermatology, University of Pennsylvania, Philadelphia.
“It is not clear if psoriasis causes fatty liver disease or vice versa, but clinicians should be aware of this association,” he said in an interview. Dr. Gelfand was a coauthor of the study by Dr. Ogdie and colleagues and led another more recent population-based study that implicated methotrexate as a factor in psoriasis-related hepatotoxicity.
If NAFLD is identified in a patient with psoriasis, treatments are limited, but Dr. Gelfand suggested that patients should be made aware of the risk. “Clinicians should encourage patients with psoriasis to take measures to protect their liver, such as avoiding drinking alcohol to excess and trying to maintain a healthy body weight,” he said.
Dr. Bellinato reported no conflicts of interest. Dr. Gelfand has financial relationships with more than 10 pharmaceutical companies, including those that make therapies for psoriasis.
NEW YORK – – and probably in those with psoriatic arthritis (PsA) as well, according to a systematic review and meta-analysis presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“Our findings imply that psoriatic patients should be screened with an ultrasonographic exam in cases where there are metabolic features that are associated with NAFLD,” reported Francesco Bellinato, MD, a researcher in the section of dermatology and venereology, University of Verona (Italy).
The data are strong. Of 76 nonduplicate publications found in the literature, the 11 observational studies included in the meta-analysis met stringent criteria, including a diagnosis of psoriasis and PsA based on objective criteria, NAFLD confirmed with liver biopsy or imaging, and odds rates calculated with 95% confidence intervals.
From these 11 studies, aggregate data were available for 249,333 psoriatic patients, of which 49% had NAFLD, and 1,491,402 were healthy controls. Among the controls, 36% had NAFLD. Four of the studies were from North America, four from Europe, and three from Asia.
In the pooled data, the risk of NAFLD among those with psoriasis relative to healthy controls fell just short of a twofold increase (odds ratio, 1.96; 95% CI, 1.70-2.26; P < .001). When stratified by studies that confirmed NAFLD by biopsy relative to ultrasonography, there was no significant heterogeneity.
Eight of the studies included an analysis of relative risk in the context of skin lesion severity defined by Psoriasis Area and Severity Index (PASI) score. Relative to those without NAFLD, psoriatic patients with NAFLD had a significant greater mean PASI score on a pooled weighted mean difference analysis (OR, 3.93; 95% CI, 2.01-5.84; P < .0001).
For PsA relative to no PsA in the five studies that compared risk between these two groups, the risk of NAFLD was again nearly twofold higher. This fell short of conventional definition of statistical significance, but it was associated with a strong trend (OR, 1.83; 95% CI, 0.98-3.43; P = .06).
The risk of NAFLD among patients with psoriasis was not found to vary significantly when assessed by univariable meta-regressions across numerous characteristics, such as sex and body mass index.
In one of the largest of the observational studies included in the meta-analysis by Alexis Ogdie, MD, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia, and colleagues, data were analyzed in more than 1.5 million patients, which included 54,251 patients with rheumatoid arthritis. While the hazard ratio of NAFLD was increased for both psoriasis (HR, 2.23) and PsA (HR, 2.11), it was not elevated in those with RA (HR, 0.96).
Risk by severity, possible mechanisms
This study also included an analysis of NAFLD risk according to psoriasis severity. While risk was still significant among those with mild disease (HR, 1.18; 95% CI, 1.07-1.30), it was almost twofold greater in those with moderate to severe psoriasis (HR, 2.23; 95% CI, 1.73-2.87).
Dr. Bellinato conceded that the mechanisms underlying the association between psoriasis and NAFLD are unknown, but he said “metaflammation” is suspected.
“The secretion of proinflammatory, prothrombotic, and oxidative stress mediators in both psoriatic skin and adipose tissue might act systemically and promote insulin resistance and other metabolic derangements that promote the development and progression of NAFLD,” Dr. Bellinato explained.
He thinks that noninvasive screening methods, such as currently used methods to calculate fibrosis score, might be useful for evaluating patients with psoriasis for NAFLD and referring them to a hepatologist when appropriate.
Given the strong association with NAFLD, Dr. Bellinato suggested that “the findings of this meta-analysis pave the way for novel, large, prospective, and histologically based studies.”
The association between psoriasis and NAFLD is clinically relevant, agreed Joel M. Gelfand, MD, vice-chair of clinical research and medical director of the clinical studies unit, department of dermatology, University of Pennsylvania, Philadelphia.
“It is not clear if psoriasis causes fatty liver disease or vice versa, but clinicians should be aware of this association,” he said in an interview. Dr. Gelfand was a coauthor of the study by Dr. Ogdie and colleagues and led another more recent population-based study that implicated methotrexate as a factor in psoriasis-related hepatotoxicity.
If NAFLD is identified in a patient with psoriasis, treatments are limited, but Dr. Gelfand suggested that patients should be made aware of the risk. “Clinicians should encourage patients with psoriasis to take measures to protect their liver, such as avoiding drinking alcohol to excess and trying to maintain a healthy body weight,” he said.
Dr. Bellinato reported no conflicts of interest. Dr. Gelfand has financial relationships with more than 10 pharmaceutical companies, including those that make therapies for psoriasis.
NEW YORK – – and probably in those with psoriatic arthritis (PsA) as well, according to a systematic review and meta-analysis presented at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“Our findings imply that psoriatic patients should be screened with an ultrasonographic exam in cases where there are metabolic features that are associated with NAFLD,” reported Francesco Bellinato, MD, a researcher in the section of dermatology and venereology, University of Verona (Italy).
The data are strong. Of 76 nonduplicate publications found in the literature, the 11 observational studies included in the meta-analysis met stringent criteria, including a diagnosis of psoriasis and PsA based on objective criteria, NAFLD confirmed with liver biopsy or imaging, and odds rates calculated with 95% confidence intervals.
From these 11 studies, aggregate data were available for 249,333 psoriatic patients, of which 49% had NAFLD, and 1,491,402 were healthy controls. Among the controls, 36% had NAFLD. Four of the studies were from North America, four from Europe, and three from Asia.
In the pooled data, the risk of NAFLD among those with psoriasis relative to healthy controls fell just short of a twofold increase (odds ratio, 1.96; 95% CI, 1.70-2.26; P < .001). When stratified by studies that confirmed NAFLD by biopsy relative to ultrasonography, there was no significant heterogeneity.
Eight of the studies included an analysis of relative risk in the context of skin lesion severity defined by Psoriasis Area and Severity Index (PASI) score. Relative to those without NAFLD, psoriatic patients with NAFLD had a significant greater mean PASI score on a pooled weighted mean difference analysis (OR, 3.93; 95% CI, 2.01-5.84; P < .0001).
For PsA relative to no PsA in the five studies that compared risk between these two groups, the risk of NAFLD was again nearly twofold higher. This fell short of conventional definition of statistical significance, but it was associated with a strong trend (OR, 1.83; 95% CI, 0.98-3.43; P = .06).
The risk of NAFLD among patients with psoriasis was not found to vary significantly when assessed by univariable meta-regressions across numerous characteristics, such as sex and body mass index.
In one of the largest of the observational studies included in the meta-analysis by Alexis Ogdie, MD, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia, and colleagues, data were analyzed in more than 1.5 million patients, which included 54,251 patients with rheumatoid arthritis. While the hazard ratio of NAFLD was increased for both psoriasis (HR, 2.23) and PsA (HR, 2.11), it was not elevated in those with RA (HR, 0.96).
Risk by severity, possible mechanisms
This study also included an analysis of NAFLD risk according to psoriasis severity. While risk was still significant among those with mild disease (HR, 1.18; 95% CI, 1.07-1.30), it was almost twofold greater in those with moderate to severe psoriasis (HR, 2.23; 95% CI, 1.73-2.87).
Dr. Bellinato conceded that the mechanisms underlying the association between psoriasis and NAFLD are unknown, but he said “metaflammation” is suspected.
“The secretion of proinflammatory, prothrombotic, and oxidative stress mediators in both psoriatic skin and adipose tissue might act systemically and promote insulin resistance and other metabolic derangements that promote the development and progression of NAFLD,” Dr. Bellinato explained.
He thinks that noninvasive screening methods, such as currently used methods to calculate fibrosis score, might be useful for evaluating patients with psoriasis for NAFLD and referring them to a hepatologist when appropriate.
Given the strong association with NAFLD, Dr. Bellinato suggested that “the findings of this meta-analysis pave the way for novel, large, prospective, and histologically based studies.”
The association between psoriasis and NAFLD is clinically relevant, agreed Joel M. Gelfand, MD, vice-chair of clinical research and medical director of the clinical studies unit, department of dermatology, University of Pennsylvania, Philadelphia.
“It is not clear if psoriasis causes fatty liver disease or vice versa, but clinicians should be aware of this association,” he said in an interview. Dr. Gelfand was a coauthor of the study by Dr. Ogdie and colleagues and led another more recent population-based study that implicated methotrexate as a factor in psoriasis-related hepatotoxicity.
If NAFLD is identified in a patient with psoriasis, treatments are limited, but Dr. Gelfand suggested that patients should be made aware of the risk. “Clinicians should encourage patients with psoriasis to take measures to protect their liver, such as avoiding drinking alcohol to excess and trying to maintain a healthy body weight,” he said.
Dr. Bellinato reported no conflicts of interest. Dr. Gelfand has financial relationships with more than 10 pharmaceutical companies, including those that make therapies for psoriasis.
AT GRAPPA 2022
In some states, voters will get to decide the future of abortion rights
As states grapple with the future of abortion in the United States, Michigan, California, and Vermont could become the first states to let voters decide whether the right to abortion should be written into the state constitution.
In Michigan, a proposed constitutional amendment would override a 90-year-old state law that makes abortion a felony even in cases of rape or incest. The U.S. Supreme Court’s overturning of Roe v. Wade could revive that abortion ban – and has galvanized abortion-rights advocates to secure new protections.
Some of the momentum is coming from activists getting involved for the first time.
“I wanted to do something, but I had no political experience or really any experience in activism,” said Amanda Mazur, who lives in rural northwestern Michigan. “But I thought, ‘Maybe I can volunteer and just offer something tangible to the movement.’”
Michigan organizers like Ms. Mazur submitted more than 750,000 signatures – a record number, they said – to state election officials in hopes of having the amendment appear on the November ballot.
If just over half those signatures are validated, Michigan voters will decide whether to amend the state’s constitution to guarantee broad individual rights to “reproductive freedom” that would cover abortion, contraception, and fertility treatments. It would also prevent the state from regulating abortions later in pregnancy if the patient’s “physical or mental health” is at risk.
The ballot initiative has the backing of medical groups like the American College of Obstetricians and Gynecologists, while conservative groups have called it radical and dangerous, claiming it would “allow late-term abortions for practically any reason.”
In California, the push to expand abortion access starts from a very different vantage point: The right to abortion is protected in state statute. And voters will be asked whether they want to enshrine it in the constitution. Proposition 1, which will be on the ballot in November, would prohibit the state from interfering with Californians’ reproductive health decisions, including those related to abortion or contraception.
“I want to know for sure that that right is protected,” state Sen. Toni Atkins (D-Calif.), the Democratic leader in the Senate and lead author of the amendment, said at a legislative hearing in June. “We are protecting ourselves from future courts and future politicians.”
The amendment is one strategy that several California lawmakers are pursuing to protect abortion access in the state. Gov. Gavin Newsom, a Democrat, has signed legislation to eliminate out-of-pocket expenses for abortion for most Californians and to protect California providers that offer abortion services from lawsuits in other states. The recent state budget deal also includes $200 million for reproductive and abortion care.
Earlier in July, Vermont Gov. Phil Scott, a Republican, announced that Proposal 5 will be on the November ballot. He said in a statement: “In Vermont, we solidified the right to choose in law, and now Vermonters have the opportunity to further protect that right in our constitution.”
For Ms. Mazur, the desire to “do something” started in 2017, when she and her husband gave their daughter, then 2 years old, some happy news: She was going to be a big sister. The family was thrilled.
But then doctors told Ms. Mazur something was wrong.
“I found out halfway through the pregnancy that the baby my husband and I hoped for suffered from a rare and life-limiting genetic condition,” Ms. Mazur said. “We ultimately made the compassionate choice to end the pregnancy for my well-being, and for the well-being of our family, and the life of what we thought would be our child.”
Devastated, Ms. Mazur turned to a national online support group and met people having similar experiences. But many group members said they were having a tough time finding a way to terminate their pregnancies.
“It really broke my heart that you’re going through this already devastating experience but have to travel far away from your home across the country ... [and] advocate for yourself like crazy just to get care that you have decided with your doctor is best for you,” Ms. Mazur said.
At the time, abortion rights in Michigan seemed pretty stable, but Ms. Mazur’s political awakening found an outlet this year.
Reproductive Freedom for All, a petition group backed by the American Civil Liberties Union of Michigan and Planned Parenthood Advocates of Michigan, was gathering signatures for the constitutional amendment to enshrine abortion protections in state law. The effort took on new urgency in May after a draft of the Supreme Court’s decision in Dobbs v. Jackson Women’s Health Organization was leaked and then published.
“Folks realized that this big, scary thing that they did not think would happen might actually happen,” said Jessica Ayoub, a field organizer with the ACLU of Michigan.
Some Michiganders were registering to vote just to be eligible to sign the petition. Jaynie Hoerauf, a 62-year-old attorney in Farwell, drove 40 miles to attend a rally where she knew she could sign it.
“A bunch of us were so ticked off [about Roe being overturned], and we were talking about it. And I was like, ‘I’m just going to go on and find where I can sign the stupid petition,’” Ms. Hoerauf said.
Activists on both sides of the abortion-rights debate expect to spend millions of dollars. They predict that donations will pour in from outside Michigan and that voters in other states will be watching.
“This is just the start of our fight,” Ms. Ayoub said. “We know that it is a long road to November.”
KHN correspondent Rachel Bluth contributed to this report. This story is part of a partnership that includes Michigan Radio, NPR, and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
As states grapple with the future of abortion in the United States, Michigan, California, and Vermont could become the first states to let voters decide whether the right to abortion should be written into the state constitution.
In Michigan, a proposed constitutional amendment would override a 90-year-old state law that makes abortion a felony even in cases of rape or incest. The U.S. Supreme Court’s overturning of Roe v. Wade could revive that abortion ban – and has galvanized abortion-rights advocates to secure new protections.
Some of the momentum is coming from activists getting involved for the first time.
“I wanted to do something, but I had no political experience or really any experience in activism,” said Amanda Mazur, who lives in rural northwestern Michigan. “But I thought, ‘Maybe I can volunteer and just offer something tangible to the movement.’”
Michigan organizers like Ms. Mazur submitted more than 750,000 signatures – a record number, they said – to state election officials in hopes of having the amendment appear on the November ballot.
If just over half those signatures are validated, Michigan voters will decide whether to amend the state’s constitution to guarantee broad individual rights to “reproductive freedom” that would cover abortion, contraception, and fertility treatments. It would also prevent the state from regulating abortions later in pregnancy if the patient’s “physical or mental health” is at risk.
The ballot initiative has the backing of medical groups like the American College of Obstetricians and Gynecologists, while conservative groups have called it radical and dangerous, claiming it would “allow late-term abortions for practically any reason.”
In California, the push to expand abortion access starts from a very different vantage point: The right to abortion is protected in state statute. And voters will be asked whether they want to enshrine it in the constitution. Proposition 1, which will be on the ballot in November, would prohibit the state from interfering with Californians’ reproductive health decisions, including those related to abortion or contraception.
“I want to know for sure that that right is protected,” state Sen. Toni Atkins (D-Calif.), the Democratic leader in the Senate and lead author of the amendment, said at a legislative hearing in June. “We are protecting ourselves from future courts and future politicians.”
The amendment is one strategy that several California lawmakers are pursuing to protect abortion access in the state. Gov. Gavin Newsom, a Democrat, has signed legislation to eliminate out-of-pocket expenses for abortion for most Californians and to protect California providers that offer abortion services from lawsuits in other states. The recent state budget deal also includes $200 million for reproductive and abortion care.
Earlier in July, Vermont Gov. Phil Scott, a Republican, announced that Proposal 5 will be on the November ballot. He said in a statement: “In Vermont, we solidified the right to choose in law, and now Vermonters have the opportunity to further protect that right in our constitution.”
For Ms. Mazur, the desire to “do something” started in 2017, when she and her husband gave their daughter, then 2 years old, some happy news: She was going to be a big sister. The family was thrilled.
But then doctors told Ms. Mazur something was wrong.
“I found out halfway through the pregnancy that the baby my husband and I hoped for suffered from a rare and life-limiting genetic condition,” Ms. Mazur said. “We ultimately made the compassionate choice to end the pregnancy for my well-being, and for the well-being of our family, and the life of what we thought would be our child.”
Devastated, Ms. Mazur turned to a national online support group and met people having similar experiences. But many group members said they were having a tough time finding a way to terminate their pregnancies.
“It really broke my heart that you’re going through this already devastating experience but have to travel far away from your home across the country ... [and] advocate for yourself like crazy just to get care that you have decided with your doctor is best for you,” Ms. Mazur said.
At the time, abortion rights in Michigan seemed pretty stable, but Ms. Mazur’s political awakening found an outlet this year.
Reproductive Freedom for All, a petition group backed by the American Civil Liberties Union of Michigan and Planned Parenthood Advocates of Michigan, was gathering signatures for the constitutional amendment to enshrine abortion protections in state law. The effort took on new urgency in May after a draft of the Supreme Court’s decision in Dobbs v. Jackson Women’s Health Organization was leaked and then published.
“Folks realized that this big, scary thing that they did not think would happen might actually happen,” said Jessica Ayoub, a field organizer with the ACLU of Michigan.
Some Michiganders were registering to vote just to be eligible to sign the petition. Jaynie Hoerauf, a 62-year-old attorney in Farwell, drove 40 miles to attend a rally where she knew she could sign it.
“A bunch of us were so ticked off [about Roe being overturned], and we were talking about it. And I was like, ‘I’m just going to go on and find where I can sign the stupid petition,’” Ms. Hoerauf said.
Activists on both sides of the abortion-rights debate expect to spend millions of dollars. They predict that donations will pour in from outside Michigan and that voters in other states will be watching.
“This is just the start of our fight,” Ms. Ayoub said. “We know that it is a long road to November.”
KHN correspondent Rachel Bluth contributed to this report. This story is part of a partnership that includes Michigan Radio, NPR, and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
As states grapple with the future of abortion in the United States, Michigan, California, and Vermont could become the first states to let voters decide whether the right to abortion should be written into the state constitution.
In Michigan, a proposed constitutional amendment would override a 90-year-old state law that makes abortion a felony even in cases of rape or incest. The U.S. Supreme Court’s overturning of Roe v. Wade could revive that abortion ban – and has galvanized abortion-rights advocates to secure new protections.
Some of the momentum is coming from activists getting involved for the first time.
“I wanted to do something, but I had no political experience or really any experience in activism,” said Amanda Mazur, who lives in rural northwestern Michigan. “But I thought, ‘Maybe I can volunteer and just offer something tangible to the movement.’”
Michigan organizers like Ms. Mazur submitted more than 750,000 signatures – a record number, they said – to state election officials in hopes of having the amendment appear on the November ballot.
If just over half those signatures are validated, Michigan voters will decide whether to amend the state’s constitution to guarantee broad individual rights to “reproductive freedom” that would cover abortion, contraception, and fertility treatments. It would also prevent the state from regulating abortions later in pregnancy if the patient’s “physical or mental health” is at risk.
The ballot initiative has the backing of medical groups like the American College of Obstetricians and Gynecologists, while conservative groups have called it radical and dangerous, claiming it would “allow late-term abortions for practically any reason.”
In California, the push to expand abortion access starts from a very different vantage point: The right to abortion is protected in state statute. And voters will be asked whether they want to enshrine it in the constitution. Proposition 1, which will be on the ballot in November, would prohibit the state from interfering with Californians’ reproductive health decisions, including those related to abortion or contraception.
“I want to know for sure that that right is protected,” state Sen. Toni Atkins (D-Calif.), the Democratic leader in the Senate and lead author of the amendment, said at a legislative hearing in June. “We are protecting ourselves from future courts and future politicians.”
The amendment is one strategy that several California lawmakers are pursuing to protect abortion access in the state. Gov. Gavin Newsom, a Democrat, has signed legislation to eliminate out-of-pocket expenses for abortion for most Californians and to protect California providers that offer abortion services from lawsuits in other states. The recent state budget deal also includes $200 million for reproductive and abortion care.
Earlier in July, Vermont Gov. Phil Scott, a Republican, announced that Proposal 5 will be on the November ballot. He said in a statement: “In Vermont, we solidified the right to choose in law, and now Vermonters have the opportunity to further protect that right in our constitution.”
For Ms. Mazur, the desire to “do something” started in 2017, when she and her husband gave their daughter, then 2 years old, some happy news: She was going to be a big sister. The family was thrilled.
But then doctors told Ms. Mazur something was wrong.
“I found out halfway through the pregnancy that the baby my husband and I hoped for suffered from a rare and life-limiting genetic condition,” Ms. Mazur said. “We ultimately made the compassionate choice to end the pregnancy for my well-being, and for the well-being of our family, and the life of what we thought would be our child.”
Devastated, Ms. Mazur turned to a national online support group and met people having similar experiences. But many group members said they were having a tough time finding a way to terminate their pregnancies.
“It really broke my heart that you’re going through this already devastating experience but have to travel far away from your home across the country ... [and] advocate for yourself like crazy just to get care that you have decided with your doctor is best for you,” Ms. Mazur said.
At the time, abortion rights in Michigan seemed pretty stable, but Ms. Mazur’s political awakening found an outlet this year.
Reproductive Freedom for All, a petition group backed by the American Civil Liberties Union of Michigan and Planned Parenthood Advocates of Michigan, was gathering signatures for the constitutional amendment to enshrine abortion protections in state law. The effort took on new urgency in May after a draft of the Supreme Court’s decision in Dobbs v. Jackson Women’s Health Organization was leaked and then published.
“Folks realized that this big, scary thing that they did not think would happen might actually happen,” said Jessica Ayoub, a field organizer with the ACLU of Michigan.
Some Michiganders were registering to vote just to be eligible to sign the petition. Jaynie Hoerauf, a 62-year-old attorney in Farwell, drove 40 miles to attend a rally where she knew she could sign it.
“A bunch of us were so ticked off [about Roe being overturned], and we were talking about it. And I was like, ‘I’m just going to go on and find where I can sign the stupid petition,’” Ms. Hoerauf said.
Activists on both sides of the abortion-rights debate expect to spend millions of dollars. They predict that donations will pour in from outside Michigan and that voters in other states will be watching.
“This is just the start of our fight,” Ms. Ayoub said. “We know that it is a long road to November.”
KHN correspondent Rachel Bluth contributed to this report. This story is part of a partnership that includes Michigan Radio, NPR, and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Focal Palmoplantar Keratoderma and Gingival Keratosis Caused by a KRT16 Mutation
To the Editor:
Focal palmoplantar keratoderma and gingival keratosis (FPGK)(Online Mendelian Inheritance in Man [OMIM] 148730) is a rare autosomal-dominant syndrome featuring focal, pressure-related, painful palmoplantar keratoderma and gingival hyperkeratosis presenting as leukokeratosis. Focal palmoplantar keratoderma and gingival keratosis was first defined by Gorlin1 in 1976. Since then, only a few cases have been reported, but no causative mutations have been identified.2
Focal pressure-related palmoplantar keratoderma (PPK) and oral hyperkeratosis also are seen in pachyonychia congenita (PC)(OMIM 167200, 615726, 615728, 167210), a rare autosomal-dominant disorder of keratinization characterized by PPK and nail dystrophy. Patients with PC often present with plantar pain; more variable features include oral leukokeratosis, follicular hyperkeratosis, pilosebaceous and epidermal inclusion cysts, hoarseness, hyperhidrosis, and natal teeth. Pachyonychia congenita is caused by mutation in keratin genes KRT6A, KRT6B, KRT16, or KRT17.
Focal palmoplantar keratoderma and gingival keratosis as well as PC are distinct from other forms of PPK with gingival involvement such as
Despite the common features of FPGK and PC, they are considered distinct disorders due to absence of nail changes in FPGK and no prior evidence of a common genetic cause. We present a patient with familial FPGK found by whole exome sequencing to be caused by a mutation in KRT16.

The proband was a 57-year-old man born to unrelated parents (Figure 1). He had no skin problems at birth, and his development was normal. He had painful focal keratoderma since childhood that were most prominent at pressure points on the soles and toes (Figure 2A), in addition to gingival hyperkeratosis and oral leukokeratosis (Figure 2B). He had no associated abnormalities of the skin, hair, or teeth and no nail findings (Figure 2C). He reported that his father and 2 of his 3 sisters were affected with similar symptoms. A punch biopsy of the right fifth toe was consistent with verrucous epidermal hyperplasia with perinuclear keratinization in the spinous layer (Figure 3A). A gingival biopsy showed perinuclear eosinophilic globules and basophilic stranding in the cytoplasm (Figure 3B). His older sister had more severe and painful focal keratoderma of the soles, punctate keratoderma of the palms, gingival hyperkeratosis, and leukokeratosis of the tongue.

Whole exome sequencing of the proband revealed a heterozygous missense mutation in KRT16 (c.380G>A, p.R127H, rs57424749). Sanger sequencing confirmed this mutation and showed that it was heterozygous in both of his affected sisters and absent in his unaffected niece (Figure 1). The patient was treated with topical and systemic retinoids, keratolytics, and mechanical removal to moderate effect, with noted improvement in the appearance and associated pain of the plantar keratoderma.

Phenotypic heterogeneity is common in PC, though PC due to KRT6A mutations demonstrates more severe nail disease with oral lesions, cysts, and follicular hyperkeratosis, while PC caused by KRT16 mutations generally presents with more extensive and painful PPK.4KRT16 mutations affecting p.R127 are frequent causes of PC, and genotype-phenotype correlations have been observed. Individuals with p.R127P mutations exhibit more severe disease with earlier age of onset, more extensive nail involvement and oral leukokeratosis, and greater impact on daily quality of life than in individuals with p.R127C mutations.5 Cases of PC with KRT16 p.R127S and p.R127G mutations also have been observed. The KRT16 c.380G>A, p.R127H mutation we documented has been reported in one kindred with PC who presented with PPK, oral leukokeratosis, toenail thickening, and pilosebaceous and follicular hyperkeratosis.6
Although patients with FPGK lack the thickening of fingernails and/or toenails considered a defining feature of PC, the disorders otherwise are phenotypically similar, suggesting the possibility of common pathogenesis. One linkage study of familial FPGK excluded genetic intervals containing type I and type II keratins but was limited to a single small kindred.2 This study and our data together suggest that, similar to PC, there are multiple genes in which mutations cause FPGK.
Murine Krt16 knockouts show distinct phenotypes depending on the mouse strain in which they are propagated, ranging from perinatal lethality to differences in the severity of oral and PPK lesions.7 These observations provide evidence that additional genetic variants contribute to Krt16 phenotypes in mice and suggest the same could be true for humans.
We propose that some cases of FPGK are due to mutations in KRT16 and thus share a genetic pathogenesis with PC, underscoring the utility of whole exome sequencing in providing genetic diagnoses for disorders that are genetically and clinically heterogeneous. Further biologic investigation of phenotypes caused by KRT16 mutation may reveal respective contributions of additional genetic variation and environmental effects to the variable clinical presentations.
- Gorlin RJ. Focal palmoplantar and marginal gingival hyperkeratosis—a syndrome. Birth Defects Orig Artic Ser. 1976;12:239-242.
- Kolde G, Hennies HC, Bethke G, et al. Focal palmoplantar and gingival keratosis: a distinct palmoplantar ectodermal dysplasia with epidermolytic alterations but lack of mutations in known keratins. J Am Acad Dermatol. 2005;52(3 pt 1):403-409.
- Duchatelet S, Hovnanian A. Olmsted syndrome: clinical, molecular and therapeutic aspects. Orphanet J Rare Dis. 2015;10:33.
- Spaunhurst KM, Hogendorf AM, Smith FJ, et al. Pachyonychia congenita patients with mutations in KRT6A have more extensive disease compared with patients who have mutations in KRT16. Br J Dermatol. 2012;166:875-878.
- Fu T, Leachman SA, Wilson NJ, et al. Genotype-phenotype correlations among pachyonychia congenita patients with K16 mutations. J Invest Dermatol. 2011;131:1025-1028.
- Wilson NJ, O’Toole EA, Milstone LM, et al. The molecular genetic analysis of the expanding pachyonychia congenita case collection. Br J Dermatol. 2014;171:343-355.
- Zieman A, Coulombe PA. The keratin 16 null phenotype is modestly impacted by genetic strain background in mice. Exp Dermatol. 2018;27:672-674.
To the Editor:
Focal palmoplantar keratoderma and gingival keratosis (FPGK)(Online Mendelian Inheritance in Man [OMIM] 148730) is a rare autosomal-dominant syndrome featuring focal, pressure-related, painful palmoplantar keratoderma and gingival hyperkeratosis presenting as leukokeratosis. Focal palmoplantar keratoderma and gingival keratosis was first defined by Gorlin1 in 1976. Since then, only a few cases have been reported, but no causative mutations have been identified.2
Focal pressure-related palmoplantar keratoderma (PPK) and oral hyperkeratosis also are seen in pachyonychia congenita (PC)(OMIM 167200, 615726, 615728, 167210), a rare autosomal-dominant disorder of keratinization characterized by PPK and nail dystrophy. Patients with PC often present with plantar pain; more variable features include oral leukokeratosis, follicular hyperkeratosis, pilosebaceous and epidermal inclusion cysts, hoarseness, hyperhidrosis, and natal teeth. Pachyonychia congenita is caused by mutation in keratin genes KRT6A, KRT6B, KRT16, or KRT17.
Focal palmoplantar keratoderma and gingival keratosis as well as PC are distinct from other forms of PPK with gingival involvement such as
Despite the common features of FPGK and PC, they are considered distinct disorders due to absence of nail changes in FPGK and no prior evidence of a common genetic cause. We present a patient with familial FPGK found by whole exome sequencing to be caused by a mutation in KRT16.

The proband was a 57-year-old man born to unrelated parents (Figure 1). He had no skin problems at birth, and his development was normal. He had painful focal keratoderma since childhood that were most prominent at pressure points on the soles and toes (Figure 2A), in addition to gingival hyperkeratosis and oral leukokeratosis (Figure 2B). He had no associated abnormalities of the skin, hair, or teeth and no nail findings (Figure 2C). He reported that his father and 2 of his 3 sisters were affected with similar symptoms. A punch biopsy of the right fifth toe was consistent with verrucous epidermal hyperplasia with perinuclear keratinization in the spinous layer (Figure 3A). A gingival biopsy showed perinuclear eosinophilic globules and basophilic stranding in the cytoplasm (Figure 3B). His older sister had more severe and painful focal keratoderma of the soles, punctate keratoderma of the palms, gingival hyperkeratosis, and leukokeratosis of the tongue.

Whole exome sequencing of the proband revealed a heterozygous missense mutation in KRT16 (c.380G>A, p.R127H, rs57424749). Sanger sequencing confirmed this mutation and showed that it was heterozygous in both of his affected sisters and absent in his unaffected niece (Figure 1). The patient was treated with topical and systemic retinoids, keratolytics, and mechanical removal to moderate effect, with noted improvement in the appearance and associated pain of the plantar keratoderma.

Phenotypic heterogeneity is common in PC, though PC due to KRT6A mutations demonstrates more severe nail disease with oral lesions, cysts, and follicular hyperkeratosis, while PC caused by KRT16 mutations generally presents with more extensive and painful PPK.4KRT16 mutations affecting p.R127 are frequent causes of PC, and genotype-phenotype correlations have been observed. Individuals with p.R127P mutations exhibit more severe disease with earlier age of onset, more extensive nail involvement and oral leukokeratosis, and greater impact on daily quality of life than in individuals with p.R127C mutations.5 Cases of PC with KRT16 p.R127S and p.R127G mutations also have been observed. The KRT16 c.380G>A, p.R127H mutation we documented has been reported in one kindred with PC who presented with PPK, oral leukokeratosis, toenail thickening, and pilosebaceous and follicular hyperkeratosis.6
Although patients with FPGK lack the thickening of fingernails and/or toenails considered a defining feature of PC, the disorders otherwise are phenotypically similar, suggesting the possibility of common pathogenesis. One linkage study of familial FPGK excluded genetic intervals containing type I and type II keratins but was limited to a single small kindred.2 This study and our data together suggest that, similar to PC, there are multiple genes in which mutations cause FPGK.
Murine Krt16 knockouts show distinct phenotypes depending on the mouse strain in which they are propagated, ranging from perinatal lethality to differences in the severity of oral and PPK lesions.7 These observations provide evidence that additional genetic variants contribute to Krt16 phenotypes in mice and suggest the same could be true for humans.
We propose that some cases of FPGK are due to mutations in KRT16 and thus share a genetic pathogenesis with PC, underscoring the utility of whole exome sequencing in providing genetic diagnoses for disorders that are genetically and clinically heterogeneous. Further biologic investigation of phenotypes caused by KRT16 mutation may reveal respective contributions of additional genetic variation and environmental effects to the variable clinical presentations.
To the Editor:
Focal palmoplantar keratoderma and gingival keratosis (FPGK)(Online Mendelian Inheritance in Man [OMIM] 148730) is a rare autosomal-dominant syndrome featuring focal, pressure-related, painful palmoplantar keratoderma and gingival hyperkeratosis presenting as leukokeratosis. Focal palmoplantar keratoderma and gingival keratosis was first defined by Gorlin1 in 1976. Since then, only a few cases have been reported, but no causative mutations have been identified.2
Focal pressure-related palmoplantar keratoderma (PPK) and oral hyperkeratosis also are seen in pachyonychia congenita (PC)(OMIM 167200, 615726, 615728, 167210), a rare autosomal-dominant disorder of keratinization characterized by PPK and nail dystrophy. Patients with PC often present with plantar pain; more variable features include oral leukokeratosis, follicular hyperkeratosis, pilosebaceous and epidermal inclusion cysts, hoarseness, hyperhidrosis, and natal teeth. Pachyonychia congenita is caused by mutation in keratin genes KRT6A, KRT6B, KRT16, or KRT17.
Focal palmoplantar keratoderma and gingival keratosis as well as PC are distinct from other forms of PPK with gingival involvement such as
Despite the common features of FPGK and PC, they are considered distinct disorders due to absence of nail changes in FPGK and no prior evidence of a common genetic cause. We present a patient with familial FPGK found by whole exome sequencing to be caused by a mutation in KRT16.

The proband was a 57-year-old man born to unrelated parents (Figure 1). He had no skin problems at birth, and his development was normal. He had painful focal keratoderma since childhood that were most prominent at pressure points on the soles and toes (Figure 2A), in addition to gingival hyperkeratosis and oral leukokeratosis (Figure 2B). He had no associated abnormalities of the skin, hair, or teeth and no nail findings (Figure 2C). He reported that his father and 2 of his 3 sisters were affected with similar symptoms. A punch biopsy of the right fifth toe was consistent with verrucous epidermal hyperplasia with perinuclear keratinization in the spinous layer (Figure 3A). A gingival biopsy showed perinuclear eosinophilic globules and basophilic stranding in the cytoplasm (Figure 3B). His older sister had more severe and painful focal keratoderma of the soles, punctate keratoderma of the palms, gingival hyperkeratosis, and leukokeratosis of the tongue.

Whole exome sequencing of the proband revealed a heterozygous missense mutation in KRT16 (c.380G>A, p.R127H, rs57424749). Sanger sequencing confirmed this mutation and showed that it was heterozygous in both of his affected sisters and absent in his unaffected niece (Figure 1). The patient was treated with topical and systemic retinoids, keratolytics, and mechanical removal to moderate effect, with noted improvement in the appearance and associated pain of the plantar keratoderma.

Phenotypic heterogeneity is common in PC, though PC due to KRT6A mutations demonstrates more severe nail disease with oral lesions, cysts, and follicular hyperkeratosis, while PC caused by KRT16 mutations generally presents with more extensive and painful PPK.4KRT16 mutations affecting p.R127 are frequent causes of PC, and genotype-phenotype correlations have been observed. Individuals with p.R127P mutations exhibit more severe disease with earlier age of onset, more extensive nail involvement and oral leukokeratosis, and greater impact on daily quality of life than in individuals with p.R127C mutations.5 Cases of PC with KRT16 p.R127S and p.R127G mutations also have been observed. The KRT16 c.380G>A, p.R127H mutation we documented has been reported in one kindred with PC who presented with PPK, oral leukokeratosis, toenail thickening, and pilosebaceous and follicular hyperkeratosis.6
Although patients with FPGK lack the thickening of fingernails and/or toenails considered a defining feature of PC, the disorders otherwise are phenotypically similar, suggesting the possibility of common pathogenesis. One linkage study of familial FPGK excluded genetic intervals containing type I and type II keratins but was limited to a single small kindred.2 This study and our data together suggest that, similar to PC, there are multiple genes in which mutations cause FPGK.
Murine Krt16 knockouts show distinct phenotypes depending on the mouse strain in which they are propagated, ranging from perinatal lethality to differences in the severity of oral and PPK lesions.7 These observations provide evidence that additional genetic variants contribute to Krt16 phenotypes in mice and suggest the same could be true for humans.
We propose that some cases of FPGK are due to mutations in KRT16 and thus share a genetic pathogenesis with PC, underscoring the utility of whole exome sequencing in providing genetic diagnoses for disorders that are genetically and clinically heterogeneous. Further biologic investigation of phenotypes caused by KRT16 mutation may reveal respective contributions of additional genetic variation and environmental effects to the variable clinical presentations.
- Gorlin RJ. Focal palmoplantar and marginal gingival hyperkeratosis—a syndrome. Birth Defects Orig Artic Ser. 1976;12:239-242.
- Kolde G, Hennies HC, Bethke G, et al. Focal palmoplantar and gingival keratosis: a distinct palmoplantar ectodermal dysplasia with epidermolytic alterations but lack of mutations in known keratins. J Am Acad Dermatol. 2005;52(3 pt 1):403-409.
- Duchatelet S, Hovnanian A. Olmsted syndrome: clinical, molecular and therapeutic aspects. Orphanet J Rare Dis. 2015;10:33.
- Spaunhurst KM, Hogendorf AM, Smith FJ, et al. Pachyonychia congenita patients with mutations in KRT6A have more extensive disease compared with patients who have mutations in KRT16. Br J Dermatol. 2012;166:875-878.
- Fu T, Leachman SA, Wilson NJ, et al. Genotype-phenotype correlations among pachyonychia congenita patients with K16 mutations. J Invest Dermatol. 2011;131:1025-1028.
- Wilson NJ, O’Toole EA, Milstone LM, et al. The molecular genetic analysis of the expanding pachyonychia congenita case collection. Br J Dermatol. 2014;171:343-355.
- Zieman A, Coulombe PA. The keratin 16 null phenotype is modestly impacted by genetic strain background in mice. Exp Dermatol. 2018;27:672-674.
- Gorlin RJ. Focal palmoplantar and marginal gingival hyperkeratosis—a syndrome. Birth Defects Orig Artic Ser. 1976;12:239-242.
- Kolde G, Hennies HC, Bethke G, et al. Focal palmoplantar and gingival keratosis: a distinct palmoplantar ectodermal dysplasia with epidermolytic alterations but lack of mutations in known keratins. J Am Acad Dermatol. 2005;52(3 pt 1):403-409.
- Duchatelet S, Hovnanian A. Olmsted syndrome: clinical, molecular and therapeutic aspects. Orphanet J Rare Dis. 2015;10:33.
- Spaunhurst KM, Hogendorf AM, Smith FJ, et al. Pachyonychia congenita patients with mutations in KRT6A have more extensive disease compared with patients who have mutations in KRT16. Br J Dermatol. 2012;166:875-878.
- Fu T, Leachman SA, Wilson NJ, et al. Genotype-phenotype correlations among pachyonychia congenita patients with K16 mutations. J Invest Dermatol. 2011;131:1025-1028.
- Wilson NJ, O’Toole EA, Milstone LM, et al. The molecular genetic analysis of the expanding pachyonychia congenita case collection. Br J Dermatol. 2014;171:343-355.
- Zieman A, Coulombe PA. The keratin 16 null phenotype is modestly impacted by genetic strain background in mice. Exp Dermatol. 2018;27:672-674.
Practice Points
- Focal palmoplantar keratoderma and gingival keratosis (FPGK) is a rare autosomal-dominant syndrome featuring focal, pressure-related, painful palmoplantar keratoderma (PPK) and gingival hyperkeratosis presenting as leukokeratosis.
- Focal pressure-related PPK and oral hyperkeratosis also are seen in pachyonychia congenita (PC), which is caused by mutations in keratin genes and is distinguished from FPGK by characteristic nail changes.
- A shared causative gene suggests that FPGK should be considered part of the PC spectrum.
Statins linked to lower diabetes risk after acute pancreatitis
Use of cholesterol-lowering statins was linked to a lower risk of developing a subtype of diabetes that occurs after acute pancreatitis, according to a new report.
The benefits of statins depended on the consistency of usage, with regular users having a lower risk of developing postpancreatitis diabetes than irregular users. The results were similar with low, moderate, and high statin doses, as well as in cases of both mild and severe acute pancreatitis.
“About 15% of patients with acute pancreatitis will develop diabetes mellitus in the next 5 years, and although we can monitor for it, we can’t do anything to prevent it,” Nikhil Thiruvengadam, MD, the lead study author and a gastroenterologist at Loma Linda (Calif.) University, told this news organization.
“This could push you as a clinician to prescribe [a statin if you have a reason to] because it could provide two benefits instead of just one,” he said.
The study was published online in Clinical Gastroenterology and Hepatology.
Steady use mattered, not dose
Patients with acute pancreatitis face at least a twofold increased risk of developing postpancreatitis diabetes, the study authors write. Although previous studies have shown that statins can lower the incidence and severity of acute pancreatitis, they haven’t been studied for the prevention of postpancreatitis diabetes.
In a collaborative study with several other universities, Dr. Thiruvengadam and colleagues examined commercial insurance claims from the Optum Clinformatics database to assess the impact of statins on 118,479 patients without preexisting diabetes admitted for a first episode of acute pancreatitis between 2008 and 2020.
They compared patients who consistently used statins with irregular users and nonusers. Regular statin usage was defined as patients who had statin prescriptions filled for at least 80% of the year prior to their acute pancreatitis diagnosis. The analysis included 9,048 patients (7.6%) who used statins regularly, 27,272 (23%) who used statins irregularly, and 82,159 (69.3%) nonusers.
With a median follow-up of 3.5 years, the 5-year cumulative incidence of postpancreatitis diabetes was 7.5% among regular statin users and 12.7% among nonusers. Regular statin users had a 42% lower risk of developing postpancreatitis diabetes, compared with nonusers. Irregular statin users had a 15% lower risk of postpancreatitis diabetes.
In addition, the 5-year cumulative incidence of insulin-dependent postpancreatitis diabetes was 2.4% among regular statin users and 6.6% among nonusers. Regular statin users had a 52% lower risk of developing insulin-dependent diabetes as compared with nonusers.
Daily dosage didn’t demonstrate a linear dose-response relationship. That means high-dose statins may not be more effective in preventing diabetes as compared with lower doses, the study authors write.
Statin usage was effective across additional analyses, including sex, etiologies of pancreatitis, and in both mild and severe acute pancreatitis. According to the study authors, this suggests that a broad population of these patients may benefit from statins.
“We were pleasantly surprised by the variety of findings,” Dr. Thiruvengadam said. “We’re seeing strong signals, especially with consistency of usage.”
Ongoing studies
The results may seem paradoxical, the study authors write, given an epidemiologic association with a slight increase in new-onset diabetes with statin initiation. But, as other researchers have reported, postpancreatitis diabetes and type 2 diabetes have different clinical features and underlying pathophysiology. For example, patients with postpancreatitis diabetes have much higher rates of requiring insulin, hospitalization, and all-cause mortality, the study authors write.
In fact, postpancreatitis diabetes is thought to be driven by chronic low-grade inflammation attributable to interleukin-6 and tumor necrosis factor–alpha. Statins have been shown to reduce tumor necrosis factor–alpha secretion and the production of C-reactive protein in response to circulating interleukin-6 in hepatocytes, they write.
The results should inform long-term prospective studies of acute pancreatitis, the study authors write, as well as randomized controlled trials of statins.
In the meantime, gastroenterologists and primary care physicians who see outpatients after hospitalization for acute pancreatitis may consider using statins, particularly in those who may have another possible indication for statin therapy, such as mild hyperlipidemia.
“There appears to be a low-dose benefit, which is another reason why providers may consider using statins, though it’s not for everyone with pancreatitis,” Dr. Thiruvengadam said. “This could be an exploratory pathway and suggested for use in the right setting.”
The Type 1 Diabetes in Acute Pancreatitis Consortium, sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, is conducting an observational cohort study at more than a dozen locations across the country to investigate the incidence, etiology, and pathophysiology of diabetes after acute pancreatitis.
“Diabetes is surprisingly common after even a single attack of acute pancreatitis,” Chris Forsmark, MD, professor of medicine and chief of the division of gastroenterology, hepatology, and nutrition at the University of Florida, Gainesville, told this news organization.
Dr. Forsmark, who wasn’t involved with this study, is a member of T1DAPC and one of the principal investigators in Florida.
“The reduction of risk by 42% is quite substantial,” he said. “Like all such studies, there is risk of bias and confounding in determining the actual risk. Nonetheless, the results provide a strong reason for confirmation in other datasets and for further study.”
The study didn’t report funding support. Dr. Thiruvengadam and Dr. Forsmark report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Use of cholesterol-lowering statins was linked to a lower risk of developing a subtype of diabetes that occurs after acute pancreatitis, according to a new report.
The benefits of statins depended on the consistency of usage, with regular users having a lower risk of developing postpancreatitis diabetes than irregular users. The results were similar with low, moderate, and high statin doses, as well as in cases of both mild and severe acute pancreatitis.
“About 15% of patients with acute pancreatitis will develop diabetes mellitus in the next 5 years, and although we can monitor for it, we can’t do anything to prevent it,” Nikhil Thiruvengadam, MD, the lead study author and a gastroenterologist at Loma Linda (Calif.) University, told this news organization.
“This could push you as a clinician to prescribe [a statin if you have a reason to] because it could provide two benefits instead of just one,” he said.
The study was published online in Clinical Gastroenterology and Hepatology.
Steady use mattered, not dose
Patients with acute pancreatitis face at least a twofold increased risk of developing postpancreatitis diabetes, the study authors write. Although previous studies have shown that statins can lower the incidence and severity of acute pancreatitis, they haven’t been studied for the prevention of postpancreatitis diabetes.
In a collaborative study with several other universities, Dr. Thiruvengadam and colleagues examined commercial insurance claims from the Optum Clinformatics database to assess the impact of statins on 118,479 patients without preexisting diabetes admitted for a first episode of acute pancreatitis between 2008 and 2020.
They compared patients who consistently used statins with irregular users and nonusers. Regular statin usage was defined as patients who had statin prescriptions filled for at least 80% of the year prior to their acute pancreatitis diagnosis. The analysis included 9,048 patients (7.6%) who used statins regularly, 27,272 (23%) who used statins irregularly, and 82,159 (69.3%) nonusers.
With a median follow-up of 3.5 years, the 5-year cumulative incidence of postpancreatitis diabetes was 7.5% among regular statin users and 12.7% among nonusers. Regular statin users had a 42% lower risk of developing postpancreatitis diabetes, compared with nonusers. Irregular statin users had a 15% lower risk of postpancreatitis diabetes.
In addition, the 5-year cumulative incidence of insulin-dependent postpancreatitis diabetes was 2.4% among regular statin users and 6.6% among nonusers. Regular statin users had a 52% lower risk of developing insulin-dependent diabetes as compared with nonusers.
Daily dosage didn’t demonstrate a linear dose-response relationship. That means high-dose statins may not be more effective in preventing diabetes as compared with lower doses, the study authors write.
Statin usage was effective across additional analyses, including sex, etiologies of pancreatitis, and in both mild and severe acute pancreatitis. According to the study authors, this suggests that a broad population of these patients may benefit from statins.
“We were pleasantly surprised by the variety of findings,” Dr. Thiruvengadam said. “We’re seeing strong signals, especially with consistency of usage.”
Ongoing studies
The results may seem paradoxical, the study authors write, given an epidemiologic association with a slight increase in new-onset diabetes with statin initiation. But, as other researchers have reported, postpancreatitis diabetes and type 2 diabetes have different clinical features and underlying pathophysiology. For example, patients with postpancreatitis diabetes have much higher rates of requiring insulin, hospitalization, and all-cause mortality, the study authors write.
In fact, postpancreatitis diabetes is thought to be driven by chronic low-grade inflammation attributable to interleukin-6 and tumor necrosis factor–alpha. Statins have been shown to reduce tumor necrosis factor–alpha secretion and the production of C-reactive protein in response to circulating interleukin-6 in hepatocytes, they write.
The results should inform long-term prospective studies of acute pancreatitis, the study authors write, as well as randomized controlled trials of statins.
In the meantime, gastroenterologists and primary care physicians who see outpatients after hospitalization for acute pancreatitis may consider using statins, particularly in those who may have another possible indication for statin therapy, such as mild hyperlipidemia.
“There appears to be a low-dose benefit, which is another reason why providers may consider using statins, though it’s not for everyone with pancreatitis,” Dr. Thiruvengadam said. “This could be an exploratory pathway and suggested for use in the right setting.”
The Type 1 Diabetes in Acute Pancreatitis Consortium, sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, is conducting an observational cohort study at more than a dozen locations across the country to investigate the incidence, etiology, and pathophysiology of diabetes after acute pancreatitis.
“Diabetes is surprisingly common after even a single attack of acute pancreatitis,” Chris Forsmark, MD, professor of medicine and chief of the division of gastroenterology, hepatology, and nutrition at the University of Florida, Gainesville, told this news organization.
Dr. Forsmark, who wasn’t involved with this study, is a member of T1DAPC and one of the principal investigators in Florida.
“The reduction of risk by 42% is quite substantial,” he said. “Like all such studies, there is risk of bias and confounding in determining the actual risk. Nonetheless, the results provide a strong reason for confirmation in other datasets and for further study.”
The study didn’t report funding support. Dr. Thiruvengadam and Dr. Forsmark report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Use of cholesterol-lowering statins was linked to a lower risk of developing a subtype of diabetes that occurs after acute pancreatitis, according to a new report.
The benefits of statins depended on the consistency of usage, with regular users having a lower risk of developing postpancreatitis diabetes than irregular users. The results were similar with low, moderate, and high statin doses, as well as in cases of both mild and severe acute pancreatitis.
“About 15% of patients with acute pancreatitis will develop diabetes mellitus in the next 5 years, and although we can monitor for it, we can’t do anything to prevent it,” Nikhil Thiruvengadam, MD, the lead study author and a gastroenterologist at Loma Linda (Calif.) University, told this news organization.
“This could push you as a clinician to prescribe [a statin if you have a reason to] because it could provide two benefits instead of just one,” he said.
The study was published online in Clinical Gastroenterology and Hepatology.
Steady use mattered, not dose
Patients with acute pancreatitis face at least a twofold increased risk of developing postpancreatitis diabetes, the study authors write. Although previous studies have shown that statins can lower the incidence and severity of acute pancreatitis, they haven’t been studied for the prevention of postpancreatitis diabetes.
In a collaborative study with several other universities, Dr. Thiruvengadam and colleagues examined commercial insurance claims from the Optum Clinformatics database to assess the impact of statins on 118,479 patients without preexisting diabetes admitted for a first episode of acute pancreatitis between 2008 and 2020.
They compared patients who consistently used statins with irregular users and nonusers. Regular statin usage was defined as patients who had statin prescriptions filled for at least 80% of the year prior to their acute pancreatitis diagnosis. The analysis included 9,048 patients (7.6%) who used statins regularly, 27,272 (23%) who used statins irregularly, and 82,159 (69.3%) nonusers.
With a median follow-up of 3.5 years, the 5-year cumulative incidence of postpancreatitis diabetes was 7.5% among regular statin users and 12.7% among nonusers. Regular statin users had a 42% lower risk of developing postpancreatitis diabetes, compared with nonusers. Irregular statin users had a 15% lower risk of postpancreatitis diabetes.
In addition, the 5-year cumulative incidence of insulin-dependent postpancreatitis diabetes was 2.4% among regular statin users and 6.6% among nonusers. Regular statin users had a 52% lower risk of developing insulin-dependent diabetes as compared with nonusers.
Daily dosage didn’t demonstrate a linear dose-response relationship. That means high-dose statins may not be more effective in preventing diabetes as compared with lower doses, the study authors write.
Statin usage was effective across additional analyses, including sex, etiologies of pancreatitis, and in both mild and severe acute pancreatitis. According to the study authors, this suggests that a broad population of these patients may benefit from statins.
“We were pleasantly surprised by the variety of findings,” Dr. Thiruvengadam said. “We’re seeing strong signals, especially with consistency of usage.”
Ongoing studies
The results may seem paradoxical, the study authors write, given an epidemiologic association with a slight increase in new-onset diabetes with statin initiation. But, as other researchers have reported, postpancreatitis diabetes and type 2 diabetes have different clinical features and underlying pathophysiology. For example, patients with postpancreatitis diabetes have much higher rates of requiring insulin, hospitalization, and all-cause mortality, the study authors write.
In fact, postpancreatitis diabetes is thought to be driven by chronic low-grade inflammation attributable to interleukin-6 and tumor necrosis factor–alpha. Statins have been shown to reduce tumor necrosis factor–alpha secretion and the production of C-reactive protein in response to circulating interleukin-6 in hepatocytes, they write.
The results should inform long-term prospective studies of acute pancreatitis, the study authors write, as well as randomized controlled trials of statins.
In the meantime, gastroenterologists and primary care physicians who see outpatients after hospitalization for acute pancreatitis may consider using statins, particularly in those who may have another possible indication for statin therapy, such as mild hyperlipidemia.
“There appears to be a low-dose benefit, which is another reason why providers may consider using statins, though it’s not for everyone with pancreatitis,” Dr. Thiruvengadam said. “This could be an exploratory pathway and suggested for use in the right setting.”
The Type 1 Diabetes in Acute Pancreatitis Consortium, sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, is conducting an observational cohort study at more than a dozen locations across the country to investigate the incidence, etiology, and pathophysiology of diabetes after acute pancreatitis.
“Diabetes is surprisingly common after even a single attack of acute pancreatitis,” Chris Forsmark, MD, professor of medicine and chief of the division of gastroenterology, hepatology, and nutrition at the University of Florida, Gainesville, told this news organization.
Dr. Forsmark, who wasn’t involved with this study, is a member of T1DAPC and one of the principal investigators in Florida.
“The reduction of risk by 42% is quite substantial,” he said. “Like all such studies, there is risk of bias and confounding in determining the actual risk. Nonetheless, the results provide a strong reason for confirmation in other datasets and for further study.”
The study didn’t report funding support. Dr. Thiruvengadam and Dr. Forsmark report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
COVID-19 infection late in pregnancy linked to sevenfold risk of preterm birth
Pregnant women who get infected with SARS-CoV-2 in their third trimester are almost three times as likely to have a preterm birth, while infection after 34 weeks’ gestation raises this risk sevenfold, based on the largest matched population-based cohort study published to date.
These findings support previous studies, underscoring the need for pregnant women and their families to take preventive measures against infection, lead author Noga Fallach, MA, of the Kahn-Sagol-Maccabi Research and Innovation Center, Tel Aviv, and colleagues reported.
Past research has suggested that COVID-19 may cause low birth weights and preterm birth in pregnant women, but those studies didn’t report outcomes for each trimester, the investigators wrote in PLoS ONE, noting that “timing of viral infection during fetal development may affect birth and other health outcomes.”
To address this knowledge gap, the investigators looked back at data from 2,703 pregnant women in Israel who tested positive for SARS-CoV-2 from Feb. 21, 2020, to July 2, 2021. Pregnancy outcomes in these women were compared with outcomes in an equal number of uninfected pregnant women. Vaccination status was not reported.
Comparing the two groups showed that catching COVID-19 in the third trimester was linked with nearly triple the risk of preterm birth (odds ratio, 2.76; 95% confidence interval, 1.63-4.67), and more than quadruple the risk if COVID-19 symptoms were present (OR, 4.28; 95% CI, 1.94-9.41). Women who tested positive for SARS-CoV-2 after 34 weeks’ gestation were seven times more likely than uninfected women to deliver early (OR, 7.10; 95% CI, 2.44-20.61).
Pregnant women who caught COVID-19 in the first two trimesters were not significantly more likely to have a preterm birth. Infection was not associated with abnormally low birth rates, or pregnancy loss, in any trimester.
Tal Patalon, MD, coauthor and head of the Kahn-Sagol-Maccabi Research and Innovation Center, focused on these more optimistic findings in an interview.
“The results are encouraging, and reassuring that COVID-19 infection during pregnancy is not associated with any type of pregnancy loss,” Dr. Patalon said.
She also pointed out that the women in the study were infected with SARS-CoV-2 variants that are no longer common.
“It should be remembered that the research group tested the COVID-19 pre-Delta variants, and does not refer to the dominant variant today, which is Omicron,” Dr. Patalon said.
Still, the investigators concluded that the “results underline the importance of preventive measures taken against SARS-CoV-2 infection among pregnant women and their families.”
Sonja A. Rasmussen, MD, of the University of Florida, Gainesville, said that the issue with out-of-date variants in published research has been one of the “real challenges” in studying the ever-evolving COVID-19 pandemic; however, it’s not a good enough reason to dismiss this study.
“I think at this point, we need to assume that it applies to Omicron too,” Dr. Rasmussen said, noting that other respiratory viruses, like influenza, have also been shown to increase the risk of preterm birth when contracted in late pregnancy.
While the present findings highlight the risk of infection in the third trimester, Dr. Rasmussen advised women in all stages of pregnancy to protect themselves against COVID-19, based on the knowledge that illness in a mother can affect normal growth and development in a fetus, even if it doesn’t lead to preterm birth.
“A mom getting sick during pregnancy is not good for the baby,” Dr. Rasmussen said. “The baby’s really dependent on the mom. So you want that baby to have good nutrition throughout the pregnancy. It’s just as important earlier on as later. And you want that baby to get good oxygenation no matter what time [in the pregnancy]. I know that people want a little bit of a break [from preventive measures]. But I would emphasize that if you’re pregnant, we do all sorts of things during pregnancy to make sure that our babies are safe and healthy, and I would continue that for the whole pregnancy.”
Specifically, Dr. Rasmussen advised social distancing, use of an N95 mask, and vaccination. Getting vaccinated during pregnancy helps newborns fight off infection until 6 months of age, she added, when they become eligible for vaccination themselves. This added benefit was recently reported in a study published in the New England Journal of Medicine , for which Dr. Rasmussen cowrote an editorial .
“Vaccines have been approved for 6 months and older,” Dr. Rasmussen said. “But what do you do in those first 6 months of life? That’s a high-risk time for kids.”
Despite these risks, convincing pregnant women to get vaccinated remains a key challenge for health care providers, according to Dr. Rasmussen, even with an abundance of safety data. “Early on [in the pandemic], we said we didn’t know a lot about risks. We knew that other vaccines were safe during pregnancy, but we didn’t have a lot of information about a COVID-19 vaccine. But now we have a lot of data on safety during pregnancy, and these vaccines appear to be completely safe, based on the information we have. There have been many, many pregnant women vaccinated in the United States and in other countries.”
For reluctant expecting mothers, Dr. Rasmussen offered some words of advice: “I know that you worry about anything you do when you’re pregnant. But this is something that you can do to help your baby – now, to make a preterm birth less likely, and later, after the baby is born.
“The most important thing is for the pregnant person to hear this [vaccine recommendation] from their doctor,” she added. “If they’re going to listen to anybody, they’re going to listen to their physician. That’s what the data have shown for a long time.”
The investigators and Dr. Rasmussen disclosed no conflicts of interest.
Pregnant women who get infected with SARS-CoV-2 in their third trimester are almost three times as likely to have a preterm birth, while infection after 34 weeks’ gestation raises this risk sevenfold, based on the largest matched population-based cohort study published to date.
These findings support previous studies, underscoring the need for pregnant women and their families to take preventive measures against infection, lead author Noga Fallach, MA, of the Kahn-Sagol-Maccabi Research and Innovation Center, Tel Aviv, and colleagues reported.
Past research has suggested that COVID-19 may cause low birth weights and preterm birth in pregnant women, but those studies didn’t report outcomes for each trimester, the investigators wrote in PLoS ONE, noting that “timing of viral infection during fetal development may affect birth and other health outcomes.”
To address this knowledge gap, the investigators looked back at data from 2,703 pregnant women in Israel who tested positive for SARS-CoV-2 from Feb. 21, 2020, to July 2, 2021. Pregnancy outcomes in these women were compared with outcomes in an equal number of uninfected pregnant women. Vaccination status was not reported.
Comparing the two groups showed that catching COVID-19 in the third trimester was linked with nearly triple the risk of preterm birth (odds ratio, 2.76; 95% confidence interval, 1.63-4.67), and more than quadruple the risk if COVID-19 symptoms were present (OR, 4.28; 95% CI, 1.94-9.41). Women who tested positive for SARS-CoV-2 after 34 weeks’ gestation were seven times more likely than uninfected women to deliver early (OR, 7.10; 95% CI, 2.44-20.61).
Pregnant women who caught COVID-19 in the first two trimesters were not significantly more likely to have a preterm birth. Infection was not associated with abnormally low birth rates, or pregnancy loss, in any trimester.
Tal Patalon, MD, coauthor and head of the Kahn-Sagol-Maccabi Research and Innovation Center, focused on these more optimistic findings in an interview.
“The results are encouraging, and reassuring that COVID-19 infection during pregnancy is not associated with any type of pregnancy loss,” Dr. Patalon said.
She also pointed out that the women in the study were infected with SARS-CoV-2 variants that are no longer common.
“It should be remembered that the research group tested the COVID-19 pre-Delta variants, and does not refer to the dominant variant today, which is Omicron,” Dr. Patalon said.
Still, the investigators concluded that the “results underline the importance of preventive measures taken against SARS-CoV-2 infection among pregnant women and their families.”
Sonja A. Rasmussen, MD, of the University of Florida, Gainesville, said that the issue with out-of-date variants in published research has been one of the “real challenges” in studying the ever-evolving COVID-19 pandemic; however, it’s not a good enough reason to dismiss this study.
“I think at this point, we need to assume that it applies to Omicron too,” Dr. Rasmussen said, noting that other respiratory viruses, like influenza, have also been shown to increase the risk of preterm birth when contracted in late pregnancy.
While the present findings highlight the risk of infection in the third trimester, Dr. Rasmussen advised women in all stages of pregnancy to protect themselves against COVID-19, based on the knowledge that illness in a mother can affect normal growth and development in a fetus, even if it doesn’t lead to preterm birth.
“A mom getting sick during pregnancy is not good for the baby,” Dr. Rasmussen said. “The baby’s really dependent on the mom. So you want that baby to have good nutrition throughout the pregnancy. It’s just as important earlier on as later. And you want that baby to get good oxygenation no matter what time [in the pregnancy]. I know that people want a little bit of a break [from preventive measures]. But I would emphasize that if you’re pregnant, we do all sorts of things during pregnancy to make sure that our babies are safe and healthy, and I would continue that for the whole pregnancy.”
Specifically, Dr. Rasmussen advised social distancing, use of an N95 mask, and vaccination. Getting vaccinated during pregnancy helps newborns fight off infection until 6 months of age, she added, when they become eligible for vaccination themselves. This added benefit was recently reported in a study published in the New England Journal of Medicine , for which Dr. Rasmussen cowrote an editorial .
“Vaccines have been approved for 6 months and older,” Dr. Rasmussen said. “But what do you do in those first 6 months of life? That’s a high-risk time for kids.”
Despite these risks, convincing pregnant women to get vaccinated remains a key challenge for health care providers, according to Dr. Rasmussen, even with an abundance of safety data. “Early on [in the pandemic], we said we didn’t know a lot about risks. We knew that other vaccines were safe during pregnancy, but we didn’t have a lot of information about a COVID-19 vaccine. But now we have a lot of data on safety during pregnancy, and these vaccines appear to be completely safe, based on the information we have. There have been many, many pregnant women vaccinated in the United States and in other countries.”
For reluctant expecting mothers, Dr. Rasmussen offered some words of advice: “I know that you worry about anything you do when you’re pregnant. But this is something that you can do to help your baby – now, to make a preterm birth less likely, and later, after the baby is born.
“The most important thing is for the pregnant person to hear this [vaccine recommendation] from their doctor,” she added. “If they’re going to listen to anybody, they’re going to listen to their physician. That’s what the data have shown for a long time.”
The investigators and Dr. Rasmussen disclosed no conflicts of interest.
Pregnant women who get infected with SARS-CoV-2 in their third trimester are almost three times as likely to have a preterm birth, while infection after 34 weeks’ gestation raises this risk sevenfold, based on the largest matched population-based cohort study published to date.
These findings support previous studies, underscoring the need for pregnant women and their families to take preventive measures against infection, lead author Noga Fallach, MA, of the Kahn-Sagol-Maccabi Research and Innovation Center, Tel Aviv, and colleagues reported.
Past research has suggested that COVID-19 may cause low birth weights and preterm birth in pregnant women, but those studies didn’t report outcomes for each trimester, the investigators wrote in PLoS ONE, noting that “timing of viral infection during fetal development may affect birth and other health outcomes.”
To address this knowledge gap, the investigators looked back at data from 2,703 pregnant women in Israel who tested positive for SARS-CoV-2 from Feb. 21, 2020, to July 2, 2021. Pregnancy outcomes in these women were compared with outcomes in an equal number of uninfected pregnant women. Vaccination status was not reported.
Comparing the two groups showed that catching COVID-19 in the third trimester was linked with nearly triple the risk of preterm birth (odds ratio, 2.76; 95% confidence interval, 1.63-4.67), and more than quadruple the risk if COVID-19 symptoms were present (OR, 4.28; 95% CI, 1.94-9.41). Women who tested positive for SARS-CoV-2 after 34 weeks’ gestation were seven times more likely than uninfected women to deliver early (OR, 7.10; 95% CI, 2.44-20.61).
Pregnant women who caught COVID-19 in the first two trimesters were not significantly more likely to have a preterm birth. Infection was not associated with abnormally low birth rates, or pregnancy loss, in any trimester.
Tal Patalon, MD, coauthor and head of the Kahn-Sagol-Maccabi Research and Innovation Center, focused on these more optimistic findings in an interview.
“The results are encouraging, and reassuring that COVID-19 infection during pregnancy is not associated with any type of pregnancy loss,” Dr. Patalon said.
She also pointed out that the women in the study were infected with SARS-CoV-2 variants that are no longer common.
“It should be remembered that the research group tested the COVID-19 pre-Delta variants, and does not refer to the dominant variant today, which is Omicron,” Dr. Patalon said.
Still, the investigators concluded that the “results underline the importance of preventive measures taken against SARS-CoV-2 infection among pregnant women and their families.”
Sonja A. Rasmussen, MD, of the University of Florida, Gainesville, said that the issue with out-of-date variants in published research has been one of the “real challenges” in studying the ever-evolving COVID-19 pandemic; however, it’s not a good enough reason to dismiss this study.
“I think at this point, we need to assume that it applies to Omicron too,” Dr. Rasmussen said, noting that other respiratory viruses, like influenza, have also been shown to increase the risk of preterm birth when contracted in late pregnancy.
While the present findings highlight the risk of infection in the third trimester, Dr. Rasmussen advised women in all stages of pregnancy to protect themselves against COVID-19, based on the knowledge that illness in a mother can affect normal growth and development in a fetus, even if it doesn’t lead to preterm birth.
“A mom getting sick during pregnancy is not good for the baby,” Dr. Rasmussen said. “The baby’s really dependent on the mom. So you want that baby to have good nutrition throughout the pregnancy. It’s just as important earlier on as later. And you want that baby to get good oxygenation no matter what time [in the pregnancy]. I know that people want a little bit of a break [from preventive measures]. But I would emphasize that if you’re pregnant, we do all sorts of things during pregnancy to make sure that our babies are safe and healthy, and I would continue that for the whole pregnancy.”
Specifically, Dr. Rasmussen advised social distancing, use of an N95 mask, and vaccination. Getting vaccinated during pregnancy helps newborns fight off infection until 6 months of age, she added, when they become eligible for vaccination themselves. This added benefit was recently reported in a study published in the New England Journal of Medicine , for which Dr. Rasmussen cowrote an editorial .
“Vaccines have been approved for 6 months and older,” Dr. Rasmussen said. “But what do you do in those first 6 months of life? That’s a high-risk time for kids.”
Despite these risks, convincing pregnant women to get vaccinated remains a key challenge for health care providers, according to Dr. Rasmussen, even with an abundance of safety data. “Early on [in the pandemic], we said we didn’t know a lot about risks. We knew that other vaccines were safe during pregnancy, but we didn’t have a lot of information about a COVID-19 vaccine. But now we have a lot of data on safety during pregnancy, and these vaccines appear to be completely safe, based on the information we have. There have been many, many pregnant women vaccinated in the United States and in other countries.”
For reluctant expecting mothers, Dr. Rasmussen offered some words of advice: “I know that you worry about anything you do when you’re pregnant. But this is something that you can do to help your baby – now, to make a preterm birth less likely, and later, after the baby is born.
“The most important thing is for the pregnant person to hear this [vaccine recommendation] from their doctor,” she added. “If they’re going to listen to anybody, they’re going to listen to their physician. That’s what the data have shown for a long time.”
The investigators and Dr. Rasmussen disclosed no conflicts of interest.
FROM PLOS ONE
Safest, most effective medications for spine-related pain in older adults?
, a new comprehensive literature review suggests.
Investigators assessed the evidence for medications used for this indication in older adults by reviewing 138 double-blind, placebo-controlled trials.
Among their key findings and recommendations: Acetaminophen has a favorable safety profile for spine-related pain but nonsteroidal anti-inflammatory drugs (NSAIDs) have greater efficacy.
However, NSAIDs should be used in lower doses in the short term, with gastrointestinal precaution, the researchers note.
Corticosteroids have the least evidence for treating nonspecific back pain, they add.
“Most older people experience neck or low back pain at some point, bothersome enough to see their doctor,” coinvestigator Michael Perloff, MD, PhD, department of neurology, Boston University, said in a news release.
“Our findings provide a helpful medication guide for physicians to use for spine pain in an older population that can have a complex medical history,” Dr. Perloff added.
The results were published online in Drugs and Aging.
Recommendations, warnings
With the graying of the U.S. population, spine-related pain is increasingly common, the investigators note.
Medications play an important role in pain management, but their use has limitations in the elderly, owing to reduced liver and renal function, comorbid medical problems, and polypharmacy.
Other key findings from the literature review include that, although the nerve pain medications gabapentin and pregabalin may cause dizziness or difficulty walking, they also have some demonstrated benefit for neck and back nerve pain, such as sciatica, in older adults.
These agents should be used in lower doses with smaller dose adjustments, the researchers note.
They caution that the muscle relaxants carisoprodol, chlorzoxazone, cyclobenzaprine, metaxalone, methocarbamol, and orphenadrine should be avoided in older adults because of their association with risk for sedation and falls.
‘Rational therapeutic choices’
Three other muscle relaxants – tizanidine, baclofen, and dantrolene – may be helpful for neck and back pain. The most evidence favors tizanidine and baclofen. These should be used in reduced doses. Tizanidine should be avoided in patients with liver disease, and for patients with kidney disease, the dosing of baclofen should be reduced, the investigators write.
Other findings include the following:
- Older tricyclic antidepressants should typically be avoided in this population because of their side effects, but nortriptyline and desipramine may be better tolerated for neck and back nerve pain at lower doses.
- Newer antidepressants, particularly the selective serotonin-norepinephrine reuptake inhibitor duloxetine, have a better safety profile and good efficacy for spine-related nerve pain.
- Traditional opioids are typically avoided in the treatment of spine-related pain in older adults, owing to their associated risks.
However, low-dose opioid therapy may be helpful for severe refractory pain, with close monitoring of patients, the researchers note.
Weaker opioids, such as tramadol, may be better tolerated by older patients. They work well when combined with acetaminophen, but they carry the risk for sedation, upset stomach, and constipation.
“Medications used at the correct dose, for the correct diagnosis, adjusting for preexisting medical problems can result in better use of treatments for spine pain,” coinvestigator Jonathan Fu, MD, also with the department of neurology, Boston University, said in the release.
“Rational therapeutic choices should be targeted to spine pain diagnosis, such as NSAIDs and acetaminophen for arthritic and myofascial-based complaints, gabapentinoids or duloxetine for neuropathic and radicular symptoms, antispastic agents for myofascial-based pain, and combination therapy for mixed etiologies,” the investigators write.
They also emphasize that medications should be coupled with physical therapy and exercise programs, as well as treatment of the underlying degenerative disease process and medical illness, while keeping in mind the need for possible interventions and/or corrective surgery.
The research had no specific funding. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new comprehensive literature review suggests.
Investigators assessed the evidence for medications used for this indication in older adults by reviewing 138 double-blind, placebo-controlled trials.
Among their key findings and recommendations: Acetaminophen has a favorable safety profile for spine-related pain but nonsteroidal anti-inflammatory drugs (NSAIDs) have greater efficacy.
However, NSAIDs should be used in lower doses in the short term, with gastrointestinal precaution, the researchers note.
Corticosteroids have the least evidence for treating nonspecific back pain, they add.
“Most older people experience neck or low back pain at some point, bothersome enough to see their doctor,” coinvestigator Michael Perloff, MD, PhD, department of neurology, Boston University, said in a news release.
“Our findings provide a helpful medication guide for physicians to use for spine pain in an older population that can have a complex medical history,” Dr. Perloff added.
The results were published online in Drugs and Aging.
Recommendations, warnings
With the graying of the U.S. population, spine-related pain is increasingly common, the investigators note.
Medications play an important role in pain management, but their use has limitations in the elderly, owing to reduced liver and renal function, comorbid medical problems, and polypharmacy.
Other key findings from the literature review include that, although the nerve pain medications gabapentin and pregabalin may cause dizziness or difficulty walking, they also have some demonstrated benefit for neck and back nerve pain, such as sciatica, in older adults.
These agents should be used in lower doses with smaller dose adjustments, the researchers note.
They caution that the muscle relaxants carisoprodol, chlorzoxazone, cyclobenzaprine, metaxalone, methocarbamol, and orphenadrine should be avoided in older adults because of their association with risk for sedation and falls.
‘Rational therapeutic choices’
Three other muscle relaxants – tizanidine, baclofen, and dantrolene – may be helpful for neck and back pain. The most evidence favors tizanidine and baclofen. These should be used in reduced doses. Tizanidine should be avoided in patients with liver disease, and for patients with kidney disease, the dosing of baclofen should be reduced, the investigators write.
Other findings include the following:
- Older tricyclic antidepressants should typically be avoided in this population because of their side effects, but nortriptyline and desipramine may be better tolerated for neck and back nerve pain at lower doses.
- Newer antidepressants, particularly the selective serotonin-norepinephrine reuptake inhibitor duloxetine, have a better safety profile and good efficacy for spine-related nerve pain.
- Traditional opioids are typically avoided in the treatment of spine-related pain in older adults, owing to their associated risks.
However, low-dose opioid therapy may be helpful for severe refractory pain, with close monitoring of patients, the researchers note.
Weaker opioids, such as tramadol, may be better tolerated by older patients. They work well when combined with acetaminophen, but they carry the risk for sedation, upset stomach, and constipation.
“Medications used at the correct dose, for the correct diagnosis, adjusting for preexisting medical problems can result in better use of treatments for spine pain,” coinvestigator Jonathan Fu, MD, also with the department of neurology, Boston University, said in the release.
“Rational therapeutic choices should be targeted to spine pain diagnosis, such as NSAIDs and acetaminophen for arthritic and myofascial-based complaints, gabapentinoids or duloxetine for neuropathic and radicular symptoms, antispastic agents for myofascial-based pain, and combination therapy for mixed etiologies,” the investigators write.
They also emphasize that medications should be coupled with physical therapy and exercise programs, as well as treatment of the underlying degenerative disease process and medical illness, while keeping in mind the need for possible interventions and/or corrective surgery.
The research had no specific funding. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new comprehensive literature review suggests.
Investigators assessed the evidence for medications used for this indication in older adults by reviewing 138 double-blind, placebo-controlled trials.
Among their key findings and recommendations: Acetaminophen has a favorable safety profile for spine-related pain but nonsteroidal anti-inflammatory drugs (NSAIDs) have greater efficacy.
However, NSAIDs should be used in lower doses in the short term, with gastrointestinal precaution, the researchers note.
Corticosteroids have the least evidence for treating nonspecific back pain, they add.
“Most older people experience neck or low back pain at some point, bothersome enough to see their doctor,” coinvestigator Michael Perloff, MD, PhD, department of neurology, Boston University, said in a news release.
“Our findings provide a helpful medication guide for physicians to use for spine pain in an older population that can have a complex medical history,” Dr. Perloff added.
The results were published online in Drugs and Aging.
Recommendations, warnings
With the graying of the U.S. population, spine-related pain is increasingly common, the investigators note.
Medications play an important role in pain management, but their use has limitations in the elderly, owing to reduced liver and renal function, comorbid medical problems, and polypharmacy.
Other key findings from the literature review include that, although the nerve pain medications gabapentin and pregabalin may cause dizziness or difficulty walking, they also have some demonstrated benefit for neck and back nerve pain, such as sciatica, in older adults.
These agents should be used in lower doses with smaller dose adjustments, the researchers note.
They caution that the muscle relaxants carisoprodol, chlorzoxazone, cyclobenzaprine, metaxalone, methocarbamol, and orphenadrine should be avoided in older adults because of their association with risk for sedation and falls.
‘Rational therapeutic choices’
Three other muscle relaxants – tizanidine, baclofen, and dantrolene – may be helpful for neck and back pain. The most evidence favors tizanidine and baclofen. These should be used in reduced doses. Tizanidine should be avoided in patients with liver disease, and for patients with kidney disease, the dosing of baclofen should be reduced, the investigators write.
Other findings include the following:
- Older tricyclic antidepressants should typically be avoided in this population because of their side effects, but nortriptyline and desipramine may be better tolerated for neck and back nerve pain at lower doses.
- Newer antidepressants, particularly the selective serotonin-norepinephrine reuptake inhibitor duloxetine, have a better safety profile and good efficacy for spine-related nerve pain.
- Traditional opioids are typically avoided in the treatment of spine-related pain in older adults, owing to their associated risks.
However, low-dose opioid therapy may be helpful for severe refractory pain, with close monitoring of patients, the researchers note.
Weaker opioids, such as tramadol, may be better tolerated by older patients. They work well when combined with acetaminophen, but they carry the risk for sedation, upset stomach, and constipation.
“Medications used at the correct dose, for the correct diagnosis, adjusting for preexisting medical problems can result in better use of treatments for spine pain,” coinvestigator Jonathan Fu, MD, also with the department of neurology, Boston University, said in the release.
“Rational therapeutic choices should be targeted to spine pain diagnosis, such as NSAIDs and acetaminophen for arthritic and myofascial-based complaints, gabapentinoids or duloxetine for neuropathic and radicular symptoms, antispastic agents for myofascial-based pain, and combination therapy for mixed etiologies,” the investigators write.
They also emphasize that medications should be coupled with physical therapy and exercise programs, as well as treatment of the underlying degenerative disease process and medical illness, while keeping in mind the need for possible interventions and/or corrective surgery.
The research had no specific funding. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DRUGS AND AGING
An avocado a day doesn’t shrink belly fat, but helps with cholesterol
according to the findings of a new study.
But it did improve diet quality and led to modest lowering of total cholesterol.
More than 1,000 adults with overweight or obesity and a large waist – at least 35 inches in women and 40 inches in men – took part in this U.S. study, called the Habitual Diet and Avocado Trial (HAT).
The people in the study were divided into two groups: usual diet plus one large avocado every day and usual diet with two avocados at most per month (control group).
Those in the avocado-a-day group were given a regular supply of fresh avocados along with written instructions for how to ripen and prepare them.
They had MRI scans to measure belly fat and fat around other organs at the beginning of the study and after 6 months.
After 6 months, the people who ate an avocado a day did not have less fat around their middles – the main trial outcome – compared with people in the control group.
But at 6 months, those in the avocado-a-day group had:
- No weight gain. People’s weight remained stable in both groups.
- Improved diet quality by 8 points on a 100-point scale
- A 2.9-mg/dL decrease in total cholesterol
- A 2.5-mg/dL decrease in LDL cholesterol
The study was done by researchers at Penn State University; Tufts University; Loma Linda University; and the University of California, Los Angeles, with coordinating support from Wake Forest University.
It was published in the Journal of the American Heart Association.
“While the avocados did not affect belly fat or weight gain, the study still provides evidence that avocados can be a beneficial addition to a well-balanced diet,” Penny M. Kris-Etherton, PhD, one of the researchers and a professor of nutritional sciences at Penn State University, University Park, said in a news release.
“Incorporating an avocado per day in this study did not cause weight gain and also caused a slight decrease in LDL cholesterol, which are all important findings for better health,” she said.
Similarly, study researcher Joan Sabaté, MD, a professor at Loma Linda (Calif.) University, said: “While one avocado a day did not lead to clinically significant improvements in abdominal fat and other cardiometabolic risk factors, consuming one avocado a day did not result in body weight gain.”
“This is positive,” he said, “because eating extra calories from avocados doesn’t impact body weight or abdominal fat, and it slightly decreases total and LDL cholesterol.”
Kristina S. Petersen, PhD, another of the researchers and an assistant professor of nutritional sciences at Texas Tech University, Lubbock, pointed out that people are generally poor at adhering to the Dietary Guidelines for Americans.
This study suggests that an avocado a day can improve diet quality, she noted, which “ is important because we know a higher diet quality is associated with lower risk of several diseases, including heart disease, type 2 diabetes, and some cancers.”
But the researchers also stressed that it is important to consider the diet as a whole.
“Consistent with prior observations, a change in dietary patterns rather than a single food or nutrient may be necessary to achieve clinically significant improvements” in belly fat and other risk factors for heart attack, stroke, and diabetes, they wrote.
HAT was funded by the Hass Avocado Board, which also supplied the avocados.
A version of this article first appeared on WebMD.com.
according to the findings of a new study.
But it did improve diet quality and led to modest lowering of total cholesterol.
More than 1,000 adults with overweight or obesity and a large waist – at least 35 inches in women and 40 inches in men – took part in this U.S. study, called the Habitual Diet and Avocado Trial (HAT).
The people in the study were divided into two groups: usual diet plus one large avocado every day and usual diet with two avocados at most per month (control group).
Those in the avocado-a-day group were given a regular supply of fresh avocados along with written instructions for how to ripen and prepare them.
They had MRI scans to measure belly fat and fat around other organs at the beginning of the study and after 6 months.
After 6 months, the people who ate an avocado a day did not have less fat around their middles – the main trial outcome – compared with people in the control group.
But at 6 months, those in the avocado-a-day group had:
- No weight gain. People’s weight remained stable in both groups.
- Improved diet quality by 8 points on a 100-point scale
- A 2.9-mg/dL decrease in total cholesterol
- A 2.5-mg/dL decrease in LDL cholesterol
The study was done by researchers at Penn State University; Tufts University; Loma Linda University; and the University of California, Los Angeles, with coordinating support from Wake Forest University.
It was published in the Journal of the American Heart Association.
“While the avocados did not affect belly fat or weight gain, the study still provides evidence that avocados can be a beneficial addition to a well-balanced diet,” Penny M. Kris-Etherton, PhD, one of the researchers and a professor of nutritional sciences at Penn State University, University Park, said in a news release.
“Incorporating an avocado per day in this study did not cause weight gain and also caused a slight decrease in LDL cholesterol, which are all important findings for better health,” she said.
Similarly, study researcher Joan Sabaté, MD, a professor at Loma Linda (Calif.) University, said: “While one avocado a day did not lead to clinically significant improvements in abdominal fat and other cardiometabolic risk factors, consuming one avocado a day did not result in body weight gain.”
“This is positive,” he said, “because eating extra calories from avocados doesn’t impact body weight or abdominal fat, and it slightly decreases total and LDL cholesterol.”
Kristina S. Petersen, PhD, another of the researchers and an assistant professor of nutritional sciences at Texas Tech University, Lubbock, pointed out that people are generally poor at adhering to the Dietary Guidelines for Americans.
This study suggests that an avocado a day can improve diet quality, she noted, which “ is important because we know a higher diet quality is associated with lower risk of several diseases, including heart disease, type 2 diabetes, and some cancers.”
But the researchers also stressed that it is important to consider the diet as a whole.
“Consistent with prior observations, a change in dietary patterns rather than a single food or nutrient may be necessary to achieve clinically significant improvements” in belly fat and other risk factors for heart attack, stroke, and diabetes, they wrote.
HAT was funded by the Hass Avocado Board, which also supplied the avocados.
A version of this article first appeared on WebMD.com.
according to the findings of a new study.
But it did improve diet quality and led to modest lowering of total cholesterol.
More than 1,000 adults with overweight or obesity and a large waist – at least 35 inches in women and 40 inches in men – took part in this U.S. study, called the Habitual Diet and Avocado Trial (HAT).
The people in the study were divided into two groups: usual diet plus one large avocado every day and usual diet with two avocados at most per month (control group).
Those in the avocado-a-day group were given a regular supply of fresh avocados along with written instructions for how to ripen and prepare them.
They had MRI scans to measure belly fat and fat around other organs at the beginning of the study and after 6 months.
After 6 months, the people who ate an avocado a day did not have less fat around their middles – the main trial outcome – compared with people in the control group.
But at 6 months, those in the avocado-a-day group had:
- No weight gain. People’s weight remained stable in both groups.
- Improved diet quality by 8 points on a 100-point scale
- A 2.9-mg/dL decrease in total cholesterol
- A 2.5-mg/dL decrease in LDL cholesterol
The study was done by researchers at Penn State University; Tufts University; Loma Linda University; and the University of California, Los Angeles, with coordinating support from Wake Forest University.
It was published in the Journal of the American Heart Association.
“While the avocados did not affect belly fat or weight gain, the study still provides evidence that avocados can be a beneficial addition to a well-balanced diet,” Penny M. Kris-Etherton, PhD, one of the researchers and a professor of nutritional sciences at Penn State University, University Park, said in a news release.
“Incorporating an avocado per day in this study did not cause weight gain and also caused a slight decrease in LDL cholesterol, which are all important findings for better health,” she said.
Similarly, study researcher Joan Sabaté, MD, a professor at Loma Linda (Calif.) University, said: “While one avocado a day did not lead to clinically significant improvements in abdominal fat and other cardiometabolic risk factors, consuming one avocado a day did not result in body weight gain.”
“This is positive,” he said, “because eating extra calories from avocados doesn’t impact body weight or abdominal fat, and it slightly decreases total and LDL cholesterol.”
Kristina S. Petersen, PhD, another of the researchers and an assistant professor of nutritional sciences at Texas Tech University, Lubbock, pointed out that people are generally poor at adhering to the Dietary Guidelines for Americans.
This study suggests that an avocado a day can improve diet quality, she noted, which “ is important because we know a higher diet quality is associated with lower risk of several diseases, including heart disease, type 2 diabetes, and some cancers.”
But the researchers also stressed that it is important to consider the diet as a whole.
“Consistent with prior observations, a change in dietary patterns rather than a single food or nutrient may be necessary to achieve clinically significant improvements” in belly fat and other risk factors for heart attack, stroke, and diabetes, they wrote.
HAT was funded by the Hass Avocado Board, which also supplied the avocados.
A version of this article first appeared on WebMD.com.