User login
Steroids no cure for obstructive sleep apnea in children
Children with obstructive sleep apnea syndrome (OSAS) who undergo treatment with intranasal corticosteroids (INCS) did not experience significant improvement in polysomnographic, neurobehavioral, and other symptoms at 3 and 12 months of treatment. At 12 months of INCS treatment, there was a statistically significant but not clinically relevant reduction in the obstructive apnea hypopnea index (OHAI).
“Previous studies were done in children with OSA with an obstructive apnea hypopnea index of less than 5, so they had very mild OSA,” Ignacio Tapia, MD, associate professor of pediatrics, University of Pennsylvania, Philadelphia, said in an interview.
“But then people started using the INCS for a whole range of OSA, so this is why we wanted to do the trial, to make sure that these drugs were being used correctly,” he added.
“The main message from this paper is that I think INCS may still have a role to play in OSA to treat some of the symptoms, like snoring, and in our study, quality of life indices also improved, ,” Dr. Tapia emphasized.
The study was published online in the journal Chest.
3 months of INCS
A total of 134 children between 5 and 12 years of age were randomly assigned to receive INCS for 3 months or placebo. Children in the original INCS arm were then reassigned to receive 9 more months of the same treatment or placebo. Symptoms as well as polysomnographic and neurobehavioral findings were measured at baseline, at 3 months, and again at 12 months.
“The primary outcome was OAHI change at 3 months, available for 122 children,” the authors explained. The OSAS was defined as an OAHI of between two and three events per hour. The median age of the children at baseline was 7.9 years, and the median OAHI baseline score was 5.8/hr (95% confidence interval, 3.6-9.7/hr). The total daily dose of the INCS used was 110 mcg.
At 3 months, the mean change in the OAHI from baseline was –1.73/hr (95% CI, –3.91 to 1.92/hr), while at 12 months, the mean change in the same index was –1.21 (–4.22 to 1.71/hr). These changes were not significantly different from OAHI changes observed among control participants. “OSAS symptoms and neurobehavioral results were not different [either] between the INCS and placebo groups at 3 and12 months,” the authors added.
However, among those children who received INCS treatment for the entire 12 months, the OAHI decreased significantly from 7.2/hr (95% CI, 3.62-9.88/hr) at baseline to 3.71/hr (95% CI, 1.56-6.4/hr; P = .039), although the OAHI did not normalize, the authors noted. Asked to clarify whether this change was not significant, Dr. Tapia said that it did meet statistical significance, but clinically, it meant that the children still needed some form of treatment, because they still had OSA in the range needing treatment.
The placebo group had more asthma exacerbations, upper respiratory tract infections, and exacerbations of OSAS symptoms, compared with children in the INCS group. It is possible that INCS provided a certain degree of protection from asthma exacerbation, the authors suggested.
However, recent guidelines from the American Academy of Pediatrics suggest that clinicians may prescribe these agents for children with mild OSAS in whom adenotonsillectomy is contraindicated; for those with mild postoperative OSAS, adenotonsillectomy remains the treatment of choice for childhood OSA. “The low level of enthusiasm for INCS in these guidelines is based on results from studies of INCS treatment of OSAS that had been limited by small sample size, lack of placebo control, limited duration, and variability in baseline data,” the authors wrote.
“The results of the current larger and more rigorous study of children with a wider range of OSAS also do not support the currently liberal use of INCS for the treatment of OSAS,” they wrote.
Complex issue
In a comment, Rakesh Bhattacharjee, MD, associate professor of pediatrics, University of California, San Diego, noted that he does prescribe INCS for children with mild OSA but not for all children. “We based our decisions on polysomnography, which we use to categorize OSA as mild, moderate, or severe.
“But we certainly do offer this treatment for children with mild sleep apnea as a way to avoid surgical treatment,” Dr. Bhattacharjee added. He also uses INCS for residual sleep apnea that some children experience following adenotonsillectomy. As the current study suggests, many people are treating sleep apnea empirically without confirming the severity of the disorder by a sleep study.
“If a sleep study is not done, we don’t know how severe it is, so this would advocate for the utility of a sleep study so that you can quantify the severity of symptoms and target your therapy to children who might be appropriate for INCS therapy,” Dr. Bhattacharjee said.
On the other hand, surgery is not always relevant even if a child has enlarged adenoids and tonsils, as, for example, a child with obesity. In these children, physicians need to think about other treatments, such as continuous positive airways pressure. “CPAP is not perfect,” Dr. Bhattacharjee observed. “And as pediatricians, we need to do a lot of work to improve the use of CPAP, but, that said, there are children for whom INCS and surgery might be a waste of time, and this is where CPAP might be an alternative.”
Dr. Bhattacharjee previously was the lead author of a large study of children who underwent treatment with CPAP. While findings suggested that adherence to treatment is lower in children than it is for adults, the authors also showed that numerous actionable factors could used to improve adherence to CPAP among children who might otherwise benefit from it.
The authors disclosed no relevant financial relationships. Dr. Bhattacharjee has served as a scientific adviser for Jazz Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Children with obstructive sleep apnea syndrome (OSAS) who undergo treatment with intranasal corticosteroids (INCS) did not experience significant improvement in polysomnographic, neurobehavioral, and other symptoms at 3 and 12 months of treatment. At 12 months of INCS treatment, there was a statistically significant but not clinically relevant reduction in the obstructive apnea hypopnea index (OHAI).
“Previous studies were done in children with OSA with an obstructive apnea hypopnea index of less than 5, so they had very mild OSA,” Ignacio Tapia, MD, associate professor of pediatrics, University of Pennsylvania, Philadelphia, said in an interview.
“But then people started using the INCS for a whole range of OSA, so this is why we wanted to do the trial, to make sure that these drugs were being used correctly,” he added.
“The main message from this paper is that I think INCS may still have a role to play in OSA to treat some of the symptoms, like snoring, and in our study, quality of life indices also improved, ,” Dr. Tapia emphasized.
The study was published online in the journal Chest.
3 months of INCS
A total of 134 children between 5 and 12 years of age were randomly assigned to receive INCS for 3 months or placebo. Children in the original INCS arm were then reassigned to receive 9 more months of the same treatment or placebo. Symptoms as well as polysomnographic and neurobehavioral findings were measured at baseline, at 3 months, and again at 12 months.
“The primary outcome was OAHI change at 3 months, available for 122 children,” the authors explained. The OSAS was defined as an OAHI of between two and three events per hour. The median age of the children at baseline was 7.9 years, and the median OAHI baseline score was 5.8/hr (95% confidence interval, 3.6-9.7/hr). The total daily dose of the INCS used was 110 mcg.
At 3 months, the mean change in the OAHI from baseline was –1.73/hr (95% CI, –3.91 to 1.92/hr), while at 12 months, the mean change in the same index was –1.21 (–4.22 to 1.71/hr). These changes were not significantly different from OAHI changes observed among control participants. “OSAS symptoms and neurobehavioral results were not different [either] between the INCS and placebo groups at 3 and12 months,” the authors added.
However, among those children who received INCS treatment for the entire 12 months, the OAHI decreased significantly from 7.2/hr (95% CI, 3.62-9.88/hr) at baseline to 3.71/hr (95% CI, 1.56-6.4/hr; P = .039), although the OAHI did not normalize, the authors noted. Asked to clarify whether this change was not significant, Dr. Tapia said that it did meet statistical significance, but clinically, it meant that the children still needed some form of treatment, because they still had OSA in the range needing treatment.
The placebo group had more asthma exacerbations, upper respiratory tract infections, and exacerbations of OSAS symptoms, compared with children in the INCS group. It is possible that INCS provided a certain degree of protection from asthma exacerbation, the authors suggested.
However, recent guidelines from the American Academy of Pediatrics suggest that clinicians may prescribe these agents for children with mild OSAS in whom adenotonsillectomy is contraindicated; for those with mild postoperative OSAS, adenotonsillectomy remains the treatment of choice for childhood OSA. “The low level of enthusiasm for INCS in these guidelines is based on results from studies of INCS treatment of OSAS that had been limited by small sample size, lack of placebo control, limited duration, and variability in baseline data,” the authors wrote.
“The results of the current larger and more rigorous study of children with a wider range of OSAS also do not support the currently liberal use of INCS for the treatment of OSAS,” they wrote.
Complex issue
In a comment, Rakesh Bhattacharjee, MD, associate professor of pediatrics, University of California, San Diego, noted that he does prescribe INCS for children with mild OSA but not for all children. “We based our decisions on polysomnography, which we use to categorize OSA as mild, moderate, or severe.
“But we certainly do offer this treatment for children with mild sleep apnea as a way to avoid surgical treatment,” Dr. Bhattacharjee added. He also uses INCS for residual sleep apnea that some children experience following adenotonsillectomy. As the current study suggests, many people are treating sleep apnea empirically without confirming the severity of the disorder by a sleep study.
“If a sleep study is not done, we don’t know how severe it is, so this would advocate for the utility of a sleep study so that you can quantify the severity of symptoms and target your therapy to children who might be appropriate for INCS therapy,” Dr. Bhattacharjee said.
On the other hand, surgery is not always relevant even if a child has enlarged adenoids and tonsils, as, for example, a child with obesity. In these children, physicians need to think about other treatments, such as continuous positive airways pressure. “CPAP is not perfect,” Dr. Bhattacharjee observed. “And as pediatricians, we need to do a lot of work to improve the use of CPAP, but, that said, there are children for whom INCS and surgery might be a waste of time, and this is where CPAP might be an alternative.”
Dr. Bhattacharjee previously was the lead author of a large study of children who underwent treatment with CPAP. While findings suggested that adherence to treatment is lower in children than it is for adults, the authors also showed that numerous actionable factors could used to improve adherence to CPAP among children who might otherwise benefit from it.
The authors disclosed no relevant financial relationships. Dr. Bhattacharjee has served as a scientific adviser for Jazz Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Children with obstructive sleep apnea syndrome (OSAS) who undergo treatment with intranasal corticosteroids (INCS) did not experience significant improvement in polysomnographic, neurobehavioral, and other symptoms at 3 and 12 months of treatment. At 12 months of INCS treatment, there was a statistically significant but not clinically relevant reduction in the obstructive apnea hypopnea index (OHAI).
“Previous studies were done in children with OSA with an obstructive apnea hypopnea index of less than 5, so they had very mild OSA,” Ignacio Tapia, MD, associate professor of pediatrics, University of Pennsylvania, Philadelphia, said in an interview.
“But then people started using the INCS for a whole range of OSA, so this is why we wanted to do the trial, to make sure that these drugs were being used correctly,” he added.
“The main message from this paper is that I think INCS may still have a role to play in OSA to treat some of the symptoms, like snoring, and in our study, quality of life indices also improved, ,” Dr. Tapia emphasized.
The study was published online in the journal Chest.
3 months of INCS
A total of 134 children between 5 and 12 years of age were randomly assigned to receive INCS for 3 months or placebo. Children in the original INCS arm were then reassigned to receive 9 more months of the same treatment or placebo. Symptoms as well as polysomnographic and neurobehavioral findings were measured at baseline, at 3 months, and again at 12 months.
“The primary outcome was OAHI change at 3 months, available for 122 children,” the authors explained. The OSAS was defined as an OAHI of between two and three events per hour. The median age of the children at baseline was 7.9 years, and the median OAHI baseline score was 5.8/hr (95% confidence interval, 3.6-9.7/hr). The total daily dose of the INCS used was 110 mcg.
At 3 months, the mean change in the OAHI from baseline was –1.73/hr (95% CI, –3.91 to 1.92/hr), while at 12 months, the mean change in the same index was –1.21 (–4.22 to 1.71/hr). These changes were not significantly different from OAHI changes observed among control participants. “OSAS symptoms and neurobehavioral results were not different [either] between the INCS and placebo groups at 3 and12 months,” the authors added.
However, among those children who received INCS treatment for the entire 12 months, the OAHI decreased significantly from 7.2/hr (95% CI, 3.62-9.88/hr) at baseline to 3.71/hr (95% CI, 1.56-6.4/hr; P = .039), although the OAHI did not normalize, the authors noted. Asked to clarify whether this change was not significant, Dr. Tapia said that it did meet statistical significance, but clinically, it meant that the children still needed some form of treatment, because they still had OSA in the range needing treatment.
The placebo group had more asthma exacerbations, upper respiratory tract infections, and exacerbations of OSAS symptoms, compared with children in the INCS group. It is possible that INCS provided a certain degree of protection from asthma exacerbation, the authors suggested.
However, recent guidelines from the American Academy of Pediatrics suggest that clinicians may prescribe these agents for children with mild OSAS in whom adenotonsillectomy is contraindicated; for those with mild postoperative OSAS, adenotonsillectomy remains the treatment of choice for childhood OSA. “The low level of enthusiasm for INCS in these guidelines is based on results from studies of INCS treatment of OSAS that had been limited by small sample size, lack of placebo control, limited duration, and variability in baseline data,” the authors wrote.
“The results of the current larger and more rigorous study of children with a wider range of OSAS also do not support the currently liberal use of INCS for the treatment of OSAS,” they wrote.
Complex issue
In a comment, Rakesh Bhattacharjee, MD, associate professor of pediatrics, University of California, San Diego, noted that he does prescribe INCS for children with mild OSA but not for all children. “We based our decisions on polysomnography, which we use to categorize OSA as mild, moderate, or severe.
“But we certainly do offer this treatment for children with mild sleep apnea as a way to avoid surgical treatment,” Dr. Bhattacharjee added. He also uses INCS for residual sleep apnea that some children experience following adenotonsillectomy. As the current study suggests, many people are treating sleep apnea empirically without confirming the severity of the disorder by a sleep study.
“If a sleep study is not done, we don’t know how severe it is, so this would advocate for the utility of a sleep study so that you can quantify the severity of symptoms and target your therapy to children who might be appropriate for INCS therapy,” Dr. Bhattacharjee said.
On the other hand, surgery is not always relevant even if a child has enlarged adenoids and tonsils, as, for example, a child with obesity. In these children, physicians need to think about other treatments, such as continuous positive airways pressure. “CPAP is not perfect,” Dr. Bhattacharjee observed. “And as pediatricians, we need to do a lot of work to improve the use of CPAP, but, that said, there are children for whom INCS and surgery might be a waste of time, and this is where CPAP might be an alternative.”
Dr. Bhattacharjee previously was the lead author of a large study of children who underwent treatment with CPAP. While findings suggested that adherence to treatment is lower in children than it is for adults, the authors also showed that numerous actionable factors could used to improve adherence to CPAP among children who might otherwise benefit from it.
The authors disclosed no relevant financial relationships. Dr. Bhattacharjee has served as a scientific adviser for Jazz Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM CHEST
Global data provide new insight into problem gambling
Across the globe few individuals at risk for gambling problems seek help for the issue, new research shows.
In the first study to examine global prevalence of help-seeking for problem gambling, the systematic review showed that
“Our findings suggest a considerable need for help among those experiencing problems related to their gambling,” Rimke Bijker, PhD, of the University of Auckland (New Zealand) and colleagues wrote.
The findings were published online in Addiction.
A public health concern
An increase in online gambling and stress and isolation during the COVID-19 pandemic led to experts sounding the alarm about gambling disorders. But despite its emergence as a public health concern, systematic investigation of help-seeking for problem gambling has been lacking, the investigators noted.
In their review, they included 24 studies conducted between 2010 and 2020 and involving a total of 188,234 individuals. More than 70% of the studies were conducted in Australia and New Zealand and 25% were conducted in the United States and Canada.
The overall prevalence of help-seeking for problem gambling among adults worldwide was 0.23% (95% confidence interval, 0.16-0.33).
Prevalence estimates were significantly higher in studies assessing lifetime help-seeking (0.50%; 95% CI, 0.35-0.71), compared with studies that examined current help-seeking (0.14%; 95% CI, 0.1-0.2, P < .001).
There were no significant differences in prevalence by gambling participation, region, type of help-seeking (professional only or mixed options), or year of data collection.
Gambling severity was measured by the Problem Gambling Severity Index as low risk, as moderate risk, or as problem gambling. Help-seeking was highest in the problem gambling and the moderate-risk groups, compared with the low-risk group (20.63%, 3.73%, and 0.27%, respectively; P < .001).
“A public health approach to gambling problems should be grounded in robust evidence on what people currently do to minimize and reduce their gambling harm and this should be inclusive of professional and nonprofessional support and self-help,” the investigators wrote.
Around 40% of individuals with problem gambling recover with or without professional oversight, they added.
Historically, gambling interventions have focused on those with more severe gambling problems. To truly address the issue, gambling reduction efforts should consider individuals with problems across the full continuum of risk, including those experiencing less severe problem gambling, the researchers wrote.
They added that those with more severe gambling issues “are likely to have comorbidities and may require more intensive interventions, guided by professionals,” such as general practitioners, psychiatrists, or psychologists.
Those with a less severe form “may prefer non-professional options and self-help strategies, which highlights the importance of information on such sources of help being promoted and easily accessible,” the investigators wrote.
No funding source for the study was reported. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Across the globe few individuals at risk for gambling problems seek help for the issue, new research shows.
In the first study to examine global prevalence of help-seeking for problem gambling, the systematic review showed that
“Our findings suggest a considerable need for help among those experiencing problems related to their gambling,” Rimke Bijker, PhD, of the University of Auckland (New Zealand) and colleagues wrote.
The findings were published online in Addiction.
A public health concern
An increase in online gambling and stress and isolation during the COVID-19 pandemic led to experts sounding the alarm about gambling disorders. But despite its emergence as a public health concern, systematic investigation of help-seeking for problem gambling has been lacking, the investigators noted.
In their review, they included 24 studies conducted between 2010 and 2020 and involving a total of 188,234 individuals. More than 70% of the studies were conducted in Australia and New Zealand and 25% were conducted in the United States and Canada.
The overall prevalence of help-seeking for problem gambling among adults worldwide was 0.23% (95% confidence interval, 0.16-0.33).
Prevalence estimates were significantly higher in studies assessing lifetime help-seeking (0.50%; 95% CI, 0.35-0.71), compared with studies that examined current help-seeking (0.14%; 95% CI, 0.1-0.2, P < .001).
There were no significant differences in prevalence by gambling participation, region, type of help-seeking (professional only or mixed options), or year of data collection.
Gambling severity was measured by the Problem Gambling Severity Index as low risk, as moderate risk, or as problem gambling. Help-seeking was highest in the problem gambling and the moderate-risk groups, compared with the low-risk group (20.63%, 3.73%, and 0.27%, respectively; P < .001).
“A public health approach to gambling problems should be grounded in robust evidence on what people currently do to minimize and reduce their gambling harm and this should be inclusive of professional and nonprofessional support and self-help,” the investigators wrote.
Around 40% of individuals with problem gambling recover with or without professional oversight, they added.
Historically, gambling interventions have focused on those with more severe gambling problems. To truly address the issue, gambling reduction efforts should consider individuals with problems across the full continuum of risk, including those experiencing less severe problem gambling, the researchers wrote.
They added that those with more severe gambling issues “are likely to have comorbidities and may require more intensive interventions, guided by professionals,” such as general practitioners, psychiatrists, or psychologists.
Those with a less severe form “may prefer non-professional options and self-help strategies, which highlights the importance of information on such sources of help being promoted and easily accessible,” the investigators wrote.
No funding source for the study was reported. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Across the globe few individuals at risk for gambling problems seek help for the issue, new research shows.
In the first study to examine global prevalence of help-seeking for problem gambling, the systematic review showed that
“Our findings suggest a considerable need for help among those experiencing problems related to their gambling,” Rimke Bijker, PhD, of the University of Auckland (New Zealand) and colleagues wrote.
The findings were published online in Addiction.
A public health concern
An increase in online gambling and stress and isolation during the COVID-19 pandemic led to experts sounding the alarm about gambling disorders. But despite its emergence as a public health concern, systematic investigation of help-seeking for problem gambling has been lacking, the investigators noted.
In their review, they included 24 studies conducted between 2010 and 2020 and involving a total of 188,234 individuals. More than 70% of the studies were conducted in Australia and New Zealand and 25% were conducted in the United States and Canada.
The overall prevalence of help-seeking for problem gambling among adults worldwide was 0.23% (95% confidence interval, 0.16-0.33).
Prevalence estimates were significantly higher in studies assessing lifetime help-seeking (0.50%; 95% CI, 0.35-0.71), compared with studies that examined current help-seeking (0.14%; 95% CI, 0.1-0.2, P < .001).
There were no significant differences in prevalence by gambling participation, region, type of help-seeking (professional only or mixed options), or year of data collection.
Gambling severity was measured by the Problem Gambling Severity Index as low risk, as moderate risk, or as problem gambling. Help-seeking was highest in the problem gambling and the moderate-risk groups, compared with the low-risk group (20.63%, 3.73%, and 0.27%, respectively; P < .001).
“A public health approach to gambling problems should be grounded in robust evidence on what people currently do to minimize and reduce their gambling harm and this should be inclusive of professional and nonprofessional support and self-help,” the investigators wrote.
Around 40% of individuals with problem gambling recover with or without professional oversight, they added.
Historically, gambling interventions have focused on those with more severe gambling problems. To truly address the issue, gambling reduction efforts should consider individuals with problems across the full continuum of risk, including those experiencing less severe problem gambling, the researchers wrote.
They added that those with more severe gambling issues “are likely to have comorbidities and may require more intensive interventions, guided by professionals,” such as general practitioners, psychiatrists, or psychologists.
Those with a less severe form “may prefer non-professional options and self-help strategies, which highlights the importance of information on such sources of help being promoted and easily accessible,” the investigators wrote.
No funding source for the study was reported. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ADDICTION
Electroacupuncture promising for depression-related insomnia
In a study of more than 200 adults with depression and comorbid insomnia, change from baseline to week 8 on the Pittsburgh Sleep Quality Index (PSQI) was 3 points greater in the group receiving EA versus a group receiving sham acupuncture (SA) plus standard care, and 5 points greater vs a control group receiving standard care only. The improvements were sustained during a 24-week postintervention follow-up.
The EA group also showed significant improvement in depression, insomnia, self-rated anxiety, and total sleep time – all of which were not found in the SA or control groups.
“Based on the results of our trial, we recommend patients with depression and insomnia seek the treatment of EA as an alternative and complementary therapy for better results,” study investigator Shifen Xu, PhD, Shanghai (China) Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, told this news organization.
The findings were published online in JAMA Network Open.
Bidirectional relationship
“Sleep disturbance is the prominent symptom in patients with depression,” the investigators noted.
Depression and sleep issues have a bidirectional relationship, in that “poor sleep quality contributes to the development of depression, and having depression makes a person more likely to develop sleep issues,” they wrote.
Patients with co-occurring depression and sleep disorders are more difficult to treat and have a greater risk for relapse and recurrence of depression, they added.
Acupuncture may be an “effective drug-free approach to help treat mental illness and sleep disorders,” the researchers noted. A previous study suggested acupuncture may improve sleep efficacy and prolong total sleep in patients with primary insomnia.
“EA is the combination of traditional Chinese acupuncture with electric-impulse stimulation, and it can enhance the therapeutic effect of the acupoints throughout the needle retention time,” Dr. Xu said.
A previous pilot study of EA for depression-related insomnia showed significant improvements in sleep quality after EA treatment, but the sample size was small.
The current researchers, therefore, undertook the present study – with a larger sample size and comparison with SA and standard care. They divided 270 adults (mean age, 50.3 years; 71.9% women) at three tertiary hospitals in Shanghai into three groups, each consisting of 90 participants.
The EA plus standard care group and the SA plus standard care group received 30-minute treatments three times per week for 8 weeks. The control group received standard care only.
All participants had DSM-5–diagnosed depression; baseline PSQI scores greater than 7, with higher scores indicating worse sleep quality and a greater number or sleep disorders; and Hamilton Depression Rating Scales (HDRS-17) scores of 20-35, with higher scores indicating higher depression levels.
Patients with secondary depressive disorders caused by other conditions, medication, or psychotic disorders were excluded, as were patients with a history of alcohol abuse or drug dependence or those who had received acupuncture within the previous year.
Of the patients who completed the 8-week intervention, 83 were in the EA group, 81 in the SA group, and 83 in the control group. Almost all participants (91.5%) completed all outcome measurements by the end of the 24-week follow-up period (also known as week 32).
Calm mind, balanced mood
At the 8-week posttreatment assessment, which was the primary endpoint, the EA group had a mean reduction from baseline of 6.2 points (95% confidence interval, −6.9 to −5.6) in PSQI score.
There was a significant difference in PSQI score between the EA versus the SA group (−3.6 points; 95% CI, −4.4 to −2.8; P < .001) and vs the control group (−5.1 points; 95% CI, −6.0 to −4.2; P < .001).
The efficacy of EA in treating insomnia was sustained during the postintervention follow-up period when the EA group had a significantly greater reduction in PSQI score, compared with the SA group (−4.7; 95% CI, −5.4 to −3.9; P < .001) and the control group (−5.0; 95% CI, −5.8 to −4.1; P < .001).
Patients receiving EA also experienced significant (all P values < .001) improvement from baseline on secondary outcomes, including:
- Scores on the HDRS (−10.7; 95% CI, −11.8 to −9.7)
- Scores on the Insomnia Severity Index, (−7.6; 95% CI,−8.5 to −6.7)
- Scores on the Self-rated Anxiety Scale (−2.9; 95% CI, −4.1 to −1.7)
- Total sleep time, as recorded by sleep actigraphy (29.1 minutes; 95% CI, 21.5-36.7)
In addition, the EA group showed significant improvement in depression scores compared with the SA and control groups at both 8 and 32 weeks (all P values < .001).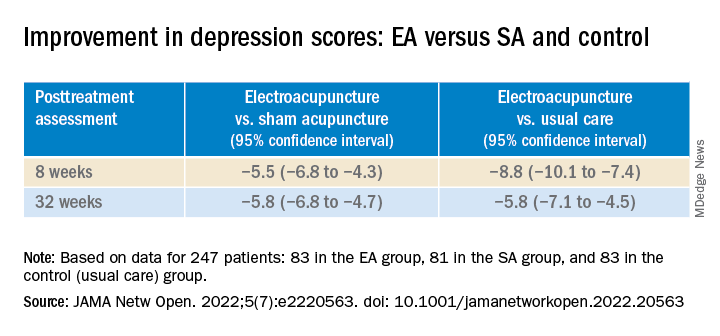
Participants in the EA group also had a 4.2% (95% CI, 2.6% - 5.8%) higher sleep efficiency score at week 8, compared with those in the SA group (P < .001).
In addition, they had lower scores on the Insomnia Severity Index and the Self-rated Anxiety Scale, and longer total sleep time, compared with the control group at week 8.
None of the participants reported any serious adverse events.
“Our findings constitute subjective and objective evidence of the efficacy and safety of EA with standard care in treating comorbid depression and insomnia compared with SA with standard care or standard care alone,” the investigators wrote.
“The acupoints we used in this trial mainly act on calming mind, relieving negative mood, and balancing the yin-yang,” Dr. Xu added.
Viable adjunctive treatment
Commenting on the study, Albert Yeung, MD, ScD, associate director of the Mass General Depression and Clinical Research Program and associate professor of psychiatry, Harvard Medical School, Boston, said that, with the evidence from this study, “acupuncture and/or electroacupuncture could be a viable adjunctive treatment for depressed patients who suffer from insomnia.”
Dr. Yeung, who was not involved with the study, is the coauthor of an accompanying editorial.
“More well-designed studies are warranted to provide evidence for integrating holistic treatment in medicine,” he said.
The study was funded by grants from the National Natural Science Foundation of China, and Shanghai Municipal Health. The investigators and Dr. Yeung reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a study of more than 200 adults with depression and comorbid insomnia, change from baseline to week 8 on the Pittsburgh Sleep Quality Index (PSQI) was 3 points greater in the group receiving EA versus a group receiving sham acupuncture (SA) plus standard care, and 5 points greater vs a control group receiving standard care only. The improvements were sustained during a 24-week postintervention follow-up.
The EA group also showed significant improvement in depression, insomnia, self-rated anxiety, and total sleep time – all of which were not found in the SA or control groups.
“Based on the results of our trial, we recommend patients with depression and insomnia seek the treatment of EA as an alternative and complementary therapy for better results,” study investigator Shifen Xu, PhD, Shanghai (China) Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, told this news organization.
The findings were published online in JAMA Network Open.
Bidirectional relationship
“Sleep disturbance is the prominent symptom in patients with depression,” the investigators noted.
Depression and sleep issues have a bidirectional relationship, in that “poor sleep quality contributes to the development of depression, and having depression makes a person more likely to develop sleep issues,” they wrote.
Patients with co-occurring depression and sleep disorders are more difficult to treat and have a greater risk for relapse and recurrence of depression, they added.
Acupuncture may be an “effective drug-free approach to help treat mental illness and sleep disorders,” the researchers noted. A previous study suggested acupuncture may improve sleep efficacy and prolong total sleep in patients with primary insomnia.
“EA is the combination of traditional Chinese acupuncture with electric-impulse stimulation, and it can enhance the therapeutic effect of the acupoints throughout the needle retention time,” Dr. Xu said.
A previous pilot study of EA for depression-related insomnia showed significant improvements in sleep quality after EA treatment, but the sample size was small.
The current researchers, therefore, undertook the present study – with a larger sample size and comparison with SA and standard care. They divided 270 adults (mean age, 50.3 years; 71.9% women) at three tertiary hospitals in Shanghai into three groups, each consisting of 90 participants.
The EA plus standard care group and the SA plus standard care group received 30-minute treatments three times per week for 8 weeks. The control group received standard care only.
All participants had DSM-5–diagnosed depression; baseline PSQI scores greater than 7, with higher scores indicating worse sleep quality and a greater number or sleep disorders; and Hamilton Depression Rating Scales (HDRS-17) scores of 20-35, with higher scores indicating higher depression levels.
Patients with secondary depressive disorders caused by other conditions, medication, or psychotic disorders were excluded, as were patients with a history of alcohol abuse or drug dependence or those who had received acupuncture within the previous year.
Of the patients who completed the 8-week intervention, 83 were in the EA group, 81 in the SA group, and 83 in the control group. Almost all participants (91.5%) completed all outcome measurements by the end of the 24-week follow-up period (also known as week 32).
Calm mind, balanced mood
At the 8-week posttreatment assessment, which was the primary endpoint, the EA group had a mean reduction from baseline of 6.2 points (95% confidence interval, −6.9 to −5.6) in PSQI score.
There was a significant difference in PSQI score between the EA versus the SA group (−3.6 points; 95% CI, −4.4 to −2.8; P < .001) and vs the control group (−5.1 points; 95% CI, −6.0 to −4.2; P < .001).
The efficacy of EA in treating insomnia was sustained during the postintervention follow-up period when the EA group had a significantly greater reduction in PSQI score, compared with the SA group (−4.7; 95% CI, −5.4 to −3.9; P < .001) and the control group (−5.0; 95% CI, −5.8 to −4.1; P < .001).
Patients receiving EA also experienced significant (all P values < .001) improvement from baseline on secondary outcomes, including:
- Scores on the HDRS (−10.7; 95% CI, −11.8 to −9.7)
- Scores on the Insomnia Severity Index, (−7.6; 95% CI,−8.5 to −6.7)
- Scores on the Self-rated Anxiety Scale (−2.9; 95% CI, −4.1 to −1.7)
- Total sleep time, as recorded by sleep actigraphy (29.1 minutes; 95% CI, 21.5-36.7)
In addition, the EA group showed significant improvement in depression scores compared with the SA and control groups at both 8 and 32 weeks (all P values < .001).
Participants in the EA group also had a 4.2% (95% CI, 2.6% - 5.8%) higher sleep efficiency score at week 8, compared with those in the SA group (P < .001).
In addition, they had lower scores on the Insomnia Severity Index and the Self-rated Anxiety Scale, and longer total sleep time, compared with the control group at week 8.
None of the participants reported any serious adverse events.
“Our findings constitute subjective and objective evidence of the efficacy and safety of EA with standard care in treating comorbid depression and insomnia compared with SA with standard care or standard care alone,” the investigators wrote.
“The acupoints we used in this trial mainly act on calming mind, relieving negative mood, and balancing the yin-yang,” Dr. Xu added.
Viable adjunctive treatment
Commenting on the study, Albert Yeung, MD, ScD, associate director of the Mass General Depression and Clinical Research Program and associate professor of psychiatry, Harvard Medical School, Boston, said that, with the evidence from this study, “acupuncture and/or electroacupuncture could be a viable adjunctive treatment for depressed patients who suffer from insomnia.”
Dr. Yeung, who was not involved with the study, is the coauthor of an accompanying editorial.
“More well-designed studies are warranted to provide evidence for integrating holistic treatment in medicine,” he said.
The study was funded by grants from the National Natural Science Foundation of China, and Shanghai Municipal Health. The investigators and Dr. Yeung reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a study of more than 200 adults with depression and comorbid insomnia, change from baseline to week 8 on the Pittsburgh Sleep Quality Index (PSQI) was 3 points greater in the group receiving EA versus a group receiving sham acupuncture (SA) plus standard care, and 5 points greater vs a control group receiving standard care only. The improvements were sustained during a 24-week postintervention follow-up.
The EA group also showed significant improvement in depression, insomnia, self-rated anxiety, and total sleep time – all of which were not found in the SA or control groups.
“Based on the results of our trial, we recommend patients with depression and insomnia seek the treatment of EA as an alternative and complementary therapy for better results,” study investigator Shifen Xu, PhD, Shanghai (China) Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, told this news organization.
The findings were published online in JAMA Network Open.
Bidirectional relationship
“Sleep disturbance is the prominent symptom in patients with depression,” the investigators noted.
Depression and sleep issues have a bidirectional relationship, in that “poor sleep quality contributes to the development of depression, and having depression makes a person more likely to develop sleep issues,” they wrote.
Patients with co-occurring depression and sleep disorders are more difficult to treat and have a greater risk for relapse and recurrence of depression, they added.
Acupuncture may be an “effective drug-free approach to help treat mental illness and sleep disorders,” the researchers noted. A previous study suggested acupuncture may improve sleep efficacy and prolong total sleep in patients with primary insomnia.
“EA is the combination of traditional Chinese acupuncture with electric-impulse stimulation, and it can enhance the therapeutic effect of the acupoints throughout the needle retention time,” Dr. Xu said.
A previous pilot study of EA for depression-related insomnia showed significant improvements in sleep quality after EA treatment, but the sample size was small.
The current researchers, therefore, undertook the present study – with a larger sample size and comparison with SA and standard care. They divided 270 adults (mean age, 50.3 years; 71.9% women) at three tertiary hospitals in Shanghai into three groups, each consisting of 90 participants.
The EA plus standard care group and the SA plus standard care group received 30-minute treatments three times per week for 8 weeks. The control group received standard care only.
All participants had DSM-5–diagnosed depression; baseline PSQI scores greater than 7, with higher scores indicating worse sleep quality and a greater number or sleep disorders; and Hamilton Depression Rating Scales (HDRS-17) scores of 20-35, with higher scores indicating higher depression levels.
Patients with secondary depressive disorders caused by other conditions, medication, or psychotic disorders were excluded, as were patients with a history of alcohol abuse or drug dependence or those who had received acupuncture within the previous year.
Of the patients who completed the 8-week intervention, 83 were in the EA group, 81 in the SA group, and 83 in the control group. Almost all participants (91.5%) completed all outcome measurements by the end of the 24-week follow-up period (also known as week 32).
Calm mind, balanced mood
At the 8-week posttreatment assessment, which was the primary endpoint, the EA group had a mean reduction from baseline of 6.2 points (95% confidence interval, −6.9 to −5.6) in PSQI score.
There was a significant difference in PSQI score between the EA versus the SA group (−3.6 points; 95% CI, −4.4 to −2.8; P < .001) and vs the control group (−5.1 points; 95% CI, −6.0 to −4.2; P < .001).
The efficacy of EA in treating insomnia was sustained during the postintervention follow-up period when the EA group had a significantly greater reduction in PSQI score, compared with the SA group (−4.7; 95% CI, −5.4 to −3.9; P < .001) and the control group (−5.0; 95% CI, −5.8 to −4.1; P < .001).
Patients receiving EA also experienced significant (all P values < .001) improvement from baseline on secondary outcomes, including:
- Scores on the HDRS (−10.7; 95% CI, −11.8 to −9.7)
- Scores on the Insomnia Severity Index, (−7.6; 95% CI,−8.5 to −6.7)
- Scores on the Self-rated Anxiety Scale (−2.9; 95% CI, −4.1 to −1.7)
- Total sleep time, as recorded by sleep actigraphy (29.1 minutes; 95% CI, 21.5-36.7)
In addition, the EA group showed significant improvement in depression scores compared with the SA and control groups at both 8 and 32 weeks (all P values < .001).
Participants in the EA group also had a 4.2% (95% CI, 2.6% - 5.8%) higher sleep efficiency score at week 8, compared with those in the SA group (P < .001).
In addition, they had lower scores on the Insomnia Severity Index and the Self-rated Anxiety Scale, and longer total sleep time, compared with the control group at week 8.
None of the participants reported any serious adverse events.
“Our findings constitute subjective and objective evidence of the efficacy and safety of EA with standard care in treating comorbid depression and insomnia compared with SA with standard care or standard care alone,” the investigators wrote.
“The acupoints we used in this trial mainly act on calming mind, relieving negative mood, and balancing the yin-yang,” Dr. Xu added.
Viable adjunctive treatment
Commenting on the study, Albert Yeung, MD, ScD, associate director of the Mass General Depression and Clinical Research Program and associate professor of psychiatry, Harvard Medical School, Boston, said that, with the evidence from this study, “acupuncture and/or electroacupuncture could be a viable adjunctive treatment for depressed patients who suffer from insomnia.”
Dr. Yeung, who was not involved with the study, is the coauthor of an accompanying editorial.
“More well-designed studies are warranted to provide evidence for integrating holistic treatment in medicine,” he said.
The study was funded by grants from the National Natural Science Foundation of China, and Shanghai Municipal Health. The investigators and Dr. Yeung reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Children and COVID: Does latest rise in new cases point toward stabilization?
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.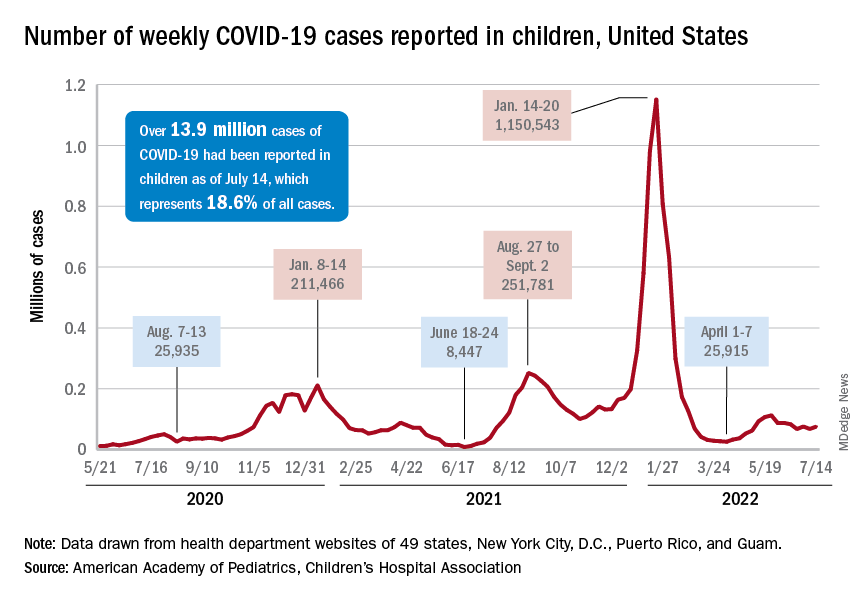
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.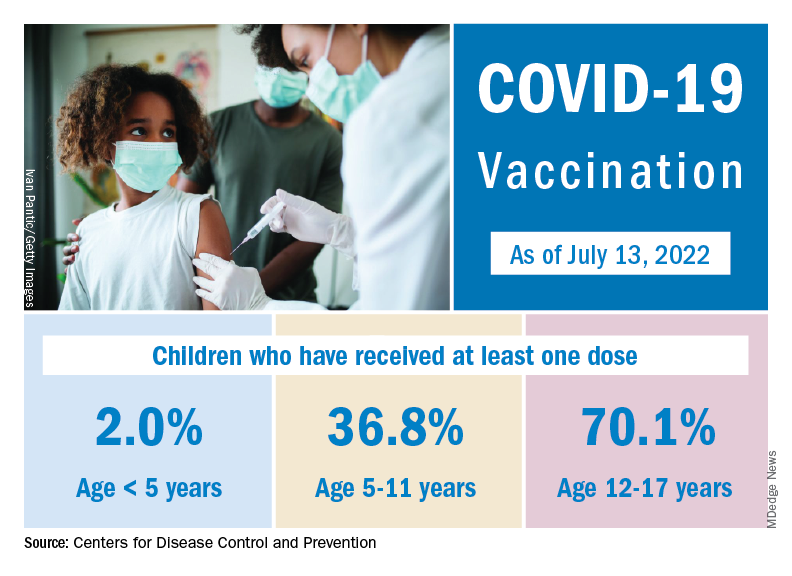
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
New COVID-19 cases rose for the second time in 3 weeks, as the effort to vaccinate the youngest children continued to slow after just 3 full weeks.
Nationally, over 75,000 children under age 5 years received their first dose of COVID-19 vaccine during the week of July 7-13. That number is down from the previous week – 118,000 from June 30 to July 6 – which, in turn, was lower than the 206,000 doses administered through the first 10 days after approval, based on data from the Centers for Disease Control and Prevention. That all adds up to just under 400,000 vaccinated children, or 2% of the eligible population under age 5, as of July 13.
State-level data, meanwhile, show considerable variation, the American Academy of Pediatrics noted in its weekly analysis of the CDC vaccine data. Vermont has already vaccinated 10.0% of children under age 5 years, and Massachusetts is at 9.5%, while Mississippi (0.3%), Alabama (0.5%), and Louisiana (0.8%) are still below 1%, the AAP said.
New cases show signs of steadying
The national count was up by 11.1% for the week of July 8-14, rising to 75,000 new cases, compared with 68,000 the previous week, but the recent trend seems to be leaning toward steadiness. The overall number has been between 67,000 and 76,000 over the past 4 weeks, alternating between rising and falling in that time span, according to data gathered by the AAP and the Children’s Hospital Association from state and territorial health departments.
the two groups said, also noting that several states have stopped updating their online dashboards over the past year, making the current total artificially low in comparison.
Taken with that grain of salt, the cumulative number of child cases since the start of the pandemic is just over 13.9 million, which represents 18.6% of all cases in the United States. That proportion has been declining in recent weeks and was as high as 19.0% as late as mid-May. “While COVID-19 cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases,” the AAP and CHA said.
RV dysfunction slams survival in acute COVID, flu, pneumonia
The study covered in this summary was published in medRxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- Right ventricular (RV) dilation or dysfunction in patients hospitalized with acute COVID-19 is associated with an elevated risk for in-hospital death.
- The impact of RV dilation or dysfunction on in-hospital mortality is similar for patients with acute COVID-19 and those with influenza, pneumonia, or acute respiratory distress syndrome (ARDS), but COVID-19 patients have greater absolute in-hospital mortality.
- RV dilatation or dysfunction in patients with acute COVID-19 is associated with a diagnosis of venous thromboembolism and subsequent intubation and mechanical ventilation.
Why this matters
- Right ventricular dysfunction increases mortality risk in acute COVID-19, and this study shows that
- The findings suggest that abnormal RV findings should be considered a mortality risk marker in patients with acute respiratory illness, especially COVID-19.
Study design
- The retrospective study involved 225 consecutive patients admitted for acute COVID-19 from March 2020 to February 2021 at four major hospitals in the same metropolitan region and a control group of 6,150 adults admitted to the hospital for influenza, pneumonia, or ARDS; mean age in the study cohort was 63 years.
- All participants underwent echocardiography during their hospitalization, including evaluation of any RV dilation or dysfunction.
- Associations between RV measurements and in-hospital mortality, the primary outcome, were adjusted for potential confounders.
Key results
- Patients in the COVID-19 group were more likely than were those in the control group to be male (66% vs. 54%; P < .001), to identify as Hispanic (38% vs. 15%; P < .001), and to have a higher mean body mass index (29.4 vs. 27.9 kg/m2; P = .008).
- Compared with the control group, patients in the COVID-19 group more often required admission to the intensive care unit (75% vs. 54%; P < .001), mechanical ventilation (P < .001), and initiation of renal replacement therapy (P = .002), and more often were diagnosed with deep-vein thrombosis or pulmonary embolism (25% vs. 14%; P < .001). The median length of hospital stay was 20 days in the COVID-19 group, compared with 10 days in the control group (P < .001).
- In-hospital mortality was 21.3% in the COVID-19 group and 11.8% in the control group (P = .001). Those hospitalized with COVID-19 had an adjusted relative risk (RR) of 1.54 (95% confidence interval [CI], 1.06-2.24; P = .02) for in-hospital mortality, compared with those hospitalized for other respiratory illnesses.
- Mild RV dilation was associated with an adjusted RR of 1.4 (95% CI, 1.17-1.69; P = .0003) for in-hospital death, and moderate to severe RV dilation was associated with an adjusted RR of 2.0 (95% CI, 1.62-2.47; P < .0001).
- The corresponding adjusted risks for mild RV dysfunction and greater-than-mild RV dysfunction were, respectively, 1.39 (95% CI, 1.10-1.77; P = .007) and 1.68 (95% CI, 1.17-2.42; P = .005).
- The RR for in-hospital mortality associated with RV dilation and dysfunction was similar in those with COVID-19 and those with other respiratory illness, but the former had a higher baseline risk that yielded a greater absolute risk in the COVID-19 group.
Limitations
- The study was based primarily on a retrospective review of electronic health records, which poses a risk for misclassification.
- Echocardiography was performed without blinding operators to patient clinical status, and echocardiograms were interpreted in a single university hospital system, so were not externally validated.
- Because echocardiograms obtained during hospitalization could not be compared with previous echocardiograms, it could not be determined whether any of the patients had preexisting RV dilation or dysfunction.
- Strain imaging was not feasible in many cases.
Disclosures
- The study received no commercial funding.
- The authors disclosed no financial relationships.
This is a summary of a preprint research study, Association of Right Ventricular Dilation and Dysfunction on Echocardiogram With In-Hospital Mortality Among Patients Hospitalized with COVID-19 Compared With Other Acute Respiratory Illness, written by researchers at the University of California, San Francisco, department of medicine, and Zuckerberg San Francisco General Hospital, division of cardiology. A version of this article first appeared on Medscape.com.
The study covered in this summary was published in medRxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- Right ventricular (RV) dilation or dysfunction in patients hospitalized with acute COVID-19 is associated with an elevated risk for in-hospital death.
- The impact of RV dilation or dysfunction on in-hospital mortality is similar for patients with acute COVID-19 and those with influenza, pneumonia, or acute respiratory distress syndrome (ARDS), but COVID-19 patients have greater absolute in-hospital mortality.
- RV dilatation or dysfunction in patients with acute COVID-19 is associated with a diagnosis of venous thromboembolism and subsequent intubation and mechanical ventilation.
Why this matters
- Right ventricular dysfunction increases mortality risk in acute COVID-19, and this study shows that
- The findings suggest that abnormal RV findings should be considered a mortality risk marker in patients with acute respiratory illness, especially COVID-19.
Study design
- The retrospective study involved 225 consecutive patients admitted for acute COVID-19 from March 2020 to February 2021 at four major hospitals in the same metropolitan region and a control group of 6,150 adults admitted to the hospital for influenza, pneumonia, or ARDS; mean age in the study cohort was 63 years.
- All participants underwent echocardiography during their hospitalization, including evaluation of any RV dilation or dysfunction.
- Associations between RV measurements and in-hospital mortality, the primary outcome, were adjusted for potential confounders.
Key results
- Patients in the COVID-19 group were more likely than were those in the control group to be male (66% vs. 54%; P < .001), to identify as Hispanic (38% vs. 15%; P < .001), and to have a higher mean body mass index (29.4 vs. 27.9 kg/m2; P = .008).
- Compared with the control group, patients in the COVID-19 group more often required admission to the intensive care unit (75% vs. 54%; P < .001), mechanical ventilation (P < .001), and initiation of renal replacement therapy (P = .002), and more often were diagnosed with deep-vein thrombosis or pulmonary embolism (25% vs. 14%; P < .001). The median length of hospital stay was 20 days in the COVID-19 group, compared with 10 days in the control group (P < .001).
- In-hospital mortality was 21.3% in the COVID-19 group and 11.8% in the control group (P = .001). Those hospitalized with COVID-19 had an adjusted relative risk (RR) of 1.54 (95% confidence interval [CI], 1.06-2.24; P = .02) for in-hospital mortality, compared with those hospitalized for other respiratory illnesses.
- Mild RV dilation was associated with an adjusted RR of 1.4 (95% CI, 1.17-1.69; P = .0003) for in-hospital death, and moderate to severe RV dilation was associated with an adjusted RR of 2.0 (95% CI, 1.62-2.47; P < .0001).
- The corresponding adjusted risks for mild RV dysfunction and greater-than-mild RV dysfunction were, respectively, 1.39 (95% CI, 1.10-1.77; P = .007) and 1.68 (95% CI, 1.17-2.42; P = .005).
- The RR for in-hospital mortality associated with RV dilation and dysfunction was similar in those with COVID-19 and those with other respiratory illness, but the former had a higher baseline risk that yielded a greater absolute risk in the COVID-19 group.
Limitations
- The study was based primarily on a retrospective review of electronic health records, which poses a risk for misclassification.
- Echocardiography was performed without blinding operators to patient clinical status, and echocardiograms were interpreted in a single university hospital system, so were not externally validated.
- Because echocardiograms obtained during hospitalization could not be compared with previous echocardiograms, it could not be determined whether any of the patients had preexisting RV dilation or dysfunction.
- Strain imaging was not feasible in many cases.
Disclosures
- The study received no commercial funding.
- The authors disclosed no financial relationships.
This is a summary of a preprint research study, Association of Right Ventricular Dilation and Dysfunction on Echocardiogram With In-Hospital Mortality Among Patients Hospitalized with COVID-19 Compared With Other Acute Respiratory Illness, written by researchers at the University of California, San Francisco, department of medicine, and Zuckerberg San Francisco General Hospital, division of cardiology. A version of this article first appeared on Medscape.com.
The study covered in this summary was published in medRxiv.org as a preprint and has not yet been peer reviewed.
Key takeaways
- Right ventricular (RV) dilation or dysfunction in patients hospitalized with acute COVID-19 is associated with an elevated risk for in-hospital death.
- The impact of RV dilation or dysfunction on in-hospital mortality is similar for patients with acute COVID-19 and those with influenza, pneumonia, or acute respiratory distress syndrome (ARDS), but COVID-19 patients have greater absolute in-hospital mortality.
- RV dilatation or dysfunction in patients with acute COVID-19 is associated with a diagnosis of venous thromboembolism and subsequent intubation and mechanical ventilation.
Why this matters
- Right ventricular dysfunction increases mortality risk in acute COVID-19, and this study shows that
- The findings suggest that abnormal RV findings should be considered a mortality risk marker in patients with acute respiratory illness, especially COVID-19.
Study design
- The retrospective study involved 225 consecutive patients admitted for acute COVID-19 from March 2020 to February 2021 at four major hospitals in the same metropolitan region and a control group of 6,150 adults admitted to the hospital for influenza, pneumonia, or ARDS; mean age in the study cohort was 63 years.
- All participants underwent echocardiography during their hospitalization, including evaluation of any RV dilation or dysfunction.
- Associations between RV measurements and in-hospital mortality, the primary outcome, were adjusted for potential confounders.
Key results
- Patients in the COVID-19 group were more likely than were those in the control group to be male (66% vs. 54%; P < .001), to identify as Hispanic (38% vs. 15%; P < .001), and to have a higher mean body mass index (29.4 vs. 27.9 kg/m2; P = .008).
- Compared with the control group, patients in the COVID-19 group more often required admission to the intensive care unit (75% vs. 54%; P < .001), mechanical ventilation (P < .001), and initiation of renal replacement therapy (P = .002), and more often were diagnosed with deep-vein thrombosis or pulmonary embolism (25% vs. 14%; P < .001). The median length of hospital stay was 20 days in the COVID-19 group, compared with 10 days in the control group (P < .001).
- In-hospital mortality was 21.3% in the COVID-19 group and 11.8% in the control group (P = .001). Those hospitalized with COVID-19 had an adjusted relative risk (RR) of 1.54 (95% confidence interval [CI], 1.06-2.24; P = .02) for in-hospital mortality, compared with those hospitalized for other respiratory illnesses.
- Mild RV dilation was associated with an adjusted RR of 1.4 (95% CI, 1.17-1.69; P = .0003) for in-hospital death, and moderate to severe RV dilation was associated with an adjusted RR of 2.0 (95% CI, 1.62-2.47; P < .0001).
- The corresponding adjusted risks for mild RV dysfunction and greater-than-mild RV dysfunction were, respectively, 1.39 (95% CI, 1.10-1.77; P = .007) and 1.68 (95% CI, 1.17-2.42; P = .005).
- The RR for in-hospital mortality associated with RV dilation and dysfunction was similar in those with COVID-19 and those with other respiratory illness, but the former had a higher baseline risk that yielded a greater absolute risk in the COVID-19 group.
Limitations
- The study was based primarily on a retrospective review of electronic health records, which poses a risk for misclassification.
- Echocardiography was performed without blinding operators to patient clinical status, and echocardiograms were interpreted in a single university hospital system, so were not externally validated.
- Because echocardiograms obtained during hospitalization could not be compared with previous echocardiograms, it could not be determined whether any of the patients had preexisting RV dilation or dysfunction.
- Strain imaging was not feasible in many cases.
Disclosures
- The study received no commercial funding.
- The authors disclosed no financial relationships.
This is a summary of a preprint research study, Association of Right Ventricular Dilation and Dysfunction on Echocardiogram With In-Hospital Mortality Among Patients Hospitalized with COVID-19 Compared With Other Acute Respiratory Illness, written by researchers at the University of California, San Francisco, department of medicine, and Zuckerberg San Francisco General Hospital, division of cardiology. A version of this article first appeared on Medscape.com.
Erenumab effective and well-tolerated in chronic migraine
Key clinical point: Erenumab is effective and well tolerated in patients with chronic migraine who did not respond to previous migraine treatments.
Major finding: Overall, 71.4% of patients treated with erenumab achieved ≥30% reduction in monthly migraine days from baseline to 9-12 weeks and 34.0% of patients at all assessment periods through 52 weeks. Constipation was the most common adverse event and 13.7% of patients discontinued treatment because of a lack of tolerability.
Study details: The data come from a 52-week, prospective, observational study including 300 patients with chronic migraine who received ≥1 dose of erenumab, of which 273 and 119 patients completed 12 and 52 weeks of treatment, respectively.
Disclosures: This study was funded by and conducted in collaboration with Novartis Pharma AG, Basel, Switzerland. Some authors reported being consultants, speakers, or scientific advisors for or receiving personal fees from various sources, including Novartis. Two authors declared being employees of and holding stocks in Novartis.
Source: Cullum CK et al. Real-world long-term efficacy and safety of erenumab in adults with chronic migraine: A 52-week, single-center, prospective, observational study. J Headache Pain. 2022;23(1):61 Jun 2). Doi:10.1186/s10194-022-01433-9
Key clinical point: Erenumab is effective and well tolerated in patients with chronic migraine who did not respond to previous migraine treatments.
Major finding: Overall, 71.4% of patients treated with erenumab achieved ≥30% reduction in monthly migraine days from baseline to 9-12 weeks and 34.0% of patients at all assessment periods through 52 weeks. Constipation was the most common adverse event and 13.7% of patients discontinued treatment because of a lack of tolerability.
Study details: The data come from a 52-week, prospective, observational study including 300 patients with chronic migraine who received ≥1 dose of erenumab, of which 273 and 119 patients completed 12 and 52 weeks of treatment, respectively.
Disclosures: This study was funded by and conducted in collaboration with Novartis Pharma AG, Basel, Switzerland. Some authors reported being consultants, speakers, or scientific advisors for or receiving personal fees from various sources, including Novartis. Two authors declared being employees of and holding stocks in Novartis.
Source: Cullum CK et al. Real-world long-term efficacy and safety of erenumab in adults with chronic migraine: A 52-week, single-center, prospective, observational study. J Headache Pain. 2022;23(1):61 Jun 2). Doi:10.1186/s10194-022-01433-9
Key clinical point: Erenumab is effective and well tolerated in patients with chronic migraine who did not respond to previous migraine treatments.
Major finding: Overall, 71.4% of patients treated with erenumab achieved ≥30% reduction in monthly migraine days from baseline to 9-12 weeks and 34.0% of patients at all assessment periods through 52 weeks. Constipation was the most common adverse event and 13.7% of patients discontinued treatment because of a lack of tolerability.
Study details: The data come from a 52-week, prospective, observational study including 300 patients with chronic migraine who received ≥1 dose of erenumab, of which 273 and 119 patients completed 12 and 52 weeks of treatment, respectively.
Disclosures: This study was funded by and conducted in collaboration with Novartis Pharma AG, Basel, Switzerland. Some authors reported being consultants, speakers, or scientific advisors for or receiving personal fees from various sources, including Novartis. Two authors declared being employees of and holding stocks in Novartis.
Source: Cullum CK et al. Real-world long-term efficacy and safety of erenumab in adults with chronic migraine: A 52-week, single-center, prospective, observational study. J Headache Pain. 2022;23(1):61 Jun 2). Doi:10.1186/s10194-022-01433-9
Women with severe migraine with aura have a higher risk for atrial fibrillation
Key clinical point: Severe migraine without aura increased the long-term risk for atrial fibrillation (AF) by 16%-21% in both men and women. The risk for future AF was highest in women with severe migraine with aura but not significant in male counterparts.
Major finding: Men (adjusted hazard ratio [aHR] 1.21; 95% CI 1.12-1.31) and women (aHR 1.16; 95% CI 1.09-1.22) with severe migraine without aura had a modest but significantly higher risk for AF. The risk was most prominent in women with severe migraine with aura (aHR 1.48; 95% CI 1.18-1.85), but was not significant in men.
Study details: Findings are from a large-scale population-based study including 4,020,488 participants without AF, of which 4986 and 105,029 had migraine with and without aura, respectively.
Disclosures: This study was supported by a Korea Medical Device Development Fund grant funded by the Korea Government. E-K Choi and GYH Lip reported receiving research grants or speaking fees or serving as consultants or speakers for various sources.
Source: Rhee T-M et al. Type and severity of migraine determines risk of atrial fibrillation in women.Front Cardiovasc Med. 2022;9:910225(May 31). Doi:10.3389/fcvm.2022.910225
Key clinical point: Severe migraine without aura increased the long-term risk for atrial fibrillation (AF) by 16%-21% in both men and women. The risk for future AF was highest in women with severe migraine with aura but not significant in male counterparts.
Major finding: Men (adjusted hazard ratio [aHR] 1.21; 95% CI 1.12-1.31) and women (aHR 1.16; 95% CI 1.09-1.22) with severe migraine without aura had a modest but significantly higher risk for AF. The risk was most prominent in women with severe migraine with aura (aHR 1.48; 95% CI 1.18-1.85), but was not significant in men.
Study details: Findings are from a large-scale population-based study including 4,020,488 participants without AF, of which 4986 and 105,029 had migraine with and without aura, respectively.
Disclosures: This study was supported by a Korea Medical Device Development Fund grant funded by the Korea Government. E-K Choi and GYH Lip reported receiving research grants or speaking fees or serving as consultants or speakers for various sources.
Source: Rhee T-M et al. Type and severity of migraine determines risk of atrial fibrillation in women.Front Cardiovasc Med. 2022;9:910225(May 31). Doi:10.3389/fcvm.2022.910225
Key clinical point: Severe migraine without aura increased the long-term risk for atrial fibrillation (AF) by 16%-21% in both men and women. The risk for future AF was highest in women with severe migraine with aura but not significant in male counterparts.
Major finding: Men (adjusted hazard ratio [aHR] 1.21; 95% CI 1.12-1.31) and women (aHR 1.16; 95% CI 1.09-1.22) with severe migraine without aura had a modest but significantly higher risk for AF. The risk was most prominent in women with severe migraine with aura (aHR 1.48; 95% CI 1.18-1.85), but was not significant in men.
Study details: Findings are from a large-scale population-based study including 4,020,488 participants without AF, of which 4986 and 105,029 had migraine with and without aura, respectively.
Disclosures: This study was supported by a Korea Medical Device Development Fund grant funded by the Korea Government. E-K Choi and GYH Lip reported receiving research grants or speaking fees or serving as consultants or speakers for various sources.
Source: Rhee T-M et al. Type and severity of migraine determines risk of atrial fibrillation in women.Front Cardiovasc Med. 2022;9:910225(May 31). Doi:10.3389/fcvm.2022.910225
Migraine: Atogepant effective and well tolerated as preventive treatment
Key clinical point: A higher proportion of patients with migraine treated with atogepant vs. placebo showed a significant reduction in the monthly migraine days (MMD) during the 12 weeks of treatment.
Major finding: At 12 weeks, ≥50% reduction in the mean MMD was achieved by a significantly higher proportion of patients receiving 10 mg (55.6%), 30 mg (58.7%), or 60 mg (60.8%) atogepantcompared with placebo (29.0%; all P < .001), with findings being similar for ≥25%, ≥75%, and 100% reduction in mean MMD. The incidence of treatment-emergent adverse events was similar among the treatment groups.
Study details: This was a secondary analysis of the ADVANCE trial including 873 patients with a ≥1-year history of migraine with or without aura who were randomly assigned to receive atogepant (10, 30, or 60 mg; n=659) or placebo (n=214).
Disclosures: This study was sponsored by Allergan. Some authors declared receiving speaking fees, consulting fees, personal fees, research grants, or royalties or owing stocks or stock options in various sources, including Allergan/AbbVie.
Source: Lipton RB et al.Rates of response to atogepant for migraine prophylaxis among adults:
A secondary analysis of a randomized clinical trial.JAMA Netw Open. 2022;5(6):e2215499 Jun 8). Doi: 10.1001/jamanetworkopen.2022.15499
Key clinical point: A higher proportion of patients with migraine treated with atogepant vs. placebo showed a significant reduction in the monthly migraine days (MMD) during the 12 weeks of treatment.
Major finding: At 12 weeks, ≥50% reduction in the mean MMD was achieved by a significantly higher proportion of patients receiving 10 mg (55.6%), 30 mg (58.7%), or 60 mg (60.8%) atogepantcompared with placebo (29.0%; all P < .001), with findings being similar for ≥25%, ≥75%, and 100% reduction in mean MMD. The incidence of treatment-emergent adverse events was similar among the treatment groups.
Study details: This was a secondary analysis of the ADVANCE trial including 873 patients with a ≥1-year history of migraine with or without aura who were randomly assigned to receive atogepant (10, 30, or 60 mg; n=659) or placebo (n=214).
Disclosures: This study was sponsored by Allergan. Some authors declared receiving speaking fees, consulting fees, personal fees, research grants, or royalties or owing stocks or stock options in various sources, including Allergan/AbbVie.
Source: Lipton RB et al.Rates of response to atogepant for migraine prophylaxis among adults:
A secondary analysis of a randomized clinical trial.JAMA Netw Open. 2022;5(6):e2215499 Jun 8). Doi: 10.1001/jamanetworkopen.2022.15499
Key clinical point: A higher proportion of patients with migraine treated with atogepant vs. placebo showed a significant reduction in the monthly migraine days (MMD) during the 12 weeks of treatment.
Major finding: At 12 weeks, ≥50% reduction in the mean MMD was achieved by a significantly higher proportion of patients receiving 10 mg (55.6%), 30 mg (58.7%), or 60 mg (60.8%) atogepantcompared with placebo (29.0%; all P < .001), with findings being similar for ≥25%, ≥75%, and 100% reduction in mean MMD. The incidence of treatment-emergent adverse events was similar among the treatment groups.
Study details: This was a secondary analysis of the ADVANCE trial including 873 patients with a ≥1-year history of migraine with or without aura who were randomly assigned to receive atogepant (10, 30, or 60 mg; n=659) or placebo (n=214).
Disclosures: This study was sponsored by Allergan. Some authors declared receiving speaking fees, consulting fees, personal fees, research grants, or royalties or owing stocks or stock options in various sources, including Allergan/AbbVie.
Source: Lipton RB et al.Rates of response to atogepant for migraine prophylaxis among adults:
A secondary analysis of a randomized clinical trial.JAMA Netw Open. 2022;5(6):e2215499 Jun 8). Doi: 10.1001/jamanetworkopen.2022.15499
Anxiety spreads from mother to daughter, father to son
The new findings suggest that children learn anxious behavior from their parents, study investigator Barbara Pavlova, PhD, clinical psychologist with Nova Scotia Health Authority, told this news organization.
“This means that transmission of anxiety from parents to children may be preventable,” said Dr. Pavlova, assistant professor, department of psychiatry, Dalhousie University, Halifax, Canada.
“Treating parents’ anxiety is not just important for their own health but also for the health of their children. This may be especially true if the child and the parent are the same sex,” Dr. Pavlova added.
The study was published online in JAMA Network Open.
Parental anxiety a disruptor
Anxiety disorders run in families. Both genes and environment are thought to be at play, but there are few data on sex-specific transmission from parent to child.
To investigate, the researchers conducted a cross-sectional study of 203 girls and 195 boys and their parents. The average age of the children was 11 years, and they had a familial risk for mood disorders.
Anxiety disorder in a same-sex parent was significantly associated with anxiety disorder in offspring (odds ratio, 2.85; 95% confidence interval, 1.52-5.34; P = .001) but not in an opposite-sex parent (OR, 1.51; 95% CI, 0.81-2.81; P = .20).
Living with a same-sex parent without anxiety was associated with lower rates of offspring anxiety (OR, 0.38; 95% CI, 0.22-0.67; P = .001).
Among all 398 children, 108 (27%) had been diagnosed with one or more anxiety disorders, including generalized anxiety disorder (7.8%), social anxiety disorder (6.3%), separation anxiety disorder (8.6%), specific phobia (8%), and anxiety disorder not otherwise specified (5%).
Rates of anxiety disorders in children increased with age, from 14% in those younger than 9 years to 52% in those older than 15 years. Anxiety disorders were similarly common among boys (24%) and girls (30%).
Rates of anxiety disorders were lowest (24%) in children of two parents without anxiety disorders and highest (41%) in cases in which both parents had anxiety disorders.
The findings point to the possible role of environmental factors, “such as modeling and vicarious learning,” in the transmission of anxiety from parents to their children, the researchers note.
“A child receives [a] similar amount of genetic information from each biological parent. A strong same-sex parent effect suggests children learn resilience by modeling the behavior of their same-sex parent. A parent’s anxiety disorder may disrupt this protective learning,” said Dr. Pavlova.
Early diagnosis, treatment essential
Reached for comment, Jill Emanuele, PhD, vice president of clinical training for the Child MIND Institute, New York, said that when it comes to anxiety, it’s important to assess and treat both the parent and the child.
“We know that both environment and genetics play a role in anxiety disorders. From a clinical perspective, if we see a parent with an anxiety disorder, we know that there is a chance that that is also going to affect the child – whether or not the child has an anxiety disorder,” Dr. Emanuele said in an interview.
“Anxiety disorders are the most common psychiatric disorders diagnosed. We also know that anxiety disorders emerge earlier than mood disorders and certainly can emerge in childhood. It’s important to address anxiety early because those same problems can continue into adulthood if left untreated,” Dr. Emanuele added.
The study was supported by the Canada Research Chairs Program, the Canadian Institutes of Health Research, the Brain & Behavior Research Foundation, the Nova Scotia Health Research Foundation, and the Dalhousie Medical Research Foundation. The authors have disclosed no relevant financial relationships. Dr. Emanuele is a board member with the Anxiety and Depression Association of America.
A version of this article first appeared on Medscape.com.
The new findings suggest that children learn anxious behavior from their parents, study investigator Barbara Pavlova, PhD, clinical psychologist with Nova Scotia Health Authority, told this news organization.
“This means that transmission of anxiety from parents to children may be preventable,” said Dr. Pavlova, assistant professor, department of psychiatry, Dalhousie University, Halifax, Canada.
“Treating parents’ anxiety is not just important for their own health but also for the health of their children. This may be especially true if the child and the parent are the same sex,” Dr. Pavlova added.
The study was published online in JAMA Network Open.
Parental anxiety a disruptor
Anxiety disorders run in families. Both genes and environment are thought to be at play, but there are few data on sex-specific transmission from parent to child.
To investigate, the researchers conducted a cross-sectional study of 203 girls and 195 boys and their parents. The average age of the children was 11 years, and they had a familial risk for mood disorders.
Anxiety disorder in a same-sex parent was significantly associated with anxiety disorder in offspring (odds ratio, 2.85; 95% confidence interval, 1.52-5.34; P = .001) but not in an opposite-sex parent (OR, 1.51; 95% CI, 0.81-2.81; P = .20).
Living with a same-sex parent without anxiety was associated with lower rates of offspring anxiety (OR, 0.38; 95% CI, 0.22-0.67; P = .001).
Among all 398 children, 108 (27%) had been diagnosed with one or more anxiety disorders, including generalized anxiety disorder (7.8%), social anxiety disorder (6.3%), separation anxiety disorder (8.6%), specific phobia (8%), and anxiety disorder not otherwise specified (5%).
Rates of anxiety disorders in children increased with age, from 14% in those younger than 9 years to 52% in those older than 15 years. Anxiety disorders were similarly common among boys (24%) and girls (30%).
Rates of anxiety disorders were lowest (24%) in children of two parents without anxiety disorders and highest (41%) in cases in which both parents had anxiety disorders.
The findings point to the possible role of environmental factors, “such as modeling and vicarious learning,” in the transmission of anxiety from parents to their children, the researchers note.
“A child receives [a] similar amount of genetic information from each biological parent. A strong same-sex parent effect suggests children learn resilience by modeling the behavior of their same-sex parent. A parent’s anxiety disorder may disrupt this protective learning,” said Dr. Pavlova.
Early diagnosis, treatment essential
Reached for comment, Jill Emanuele, PhD, vice president of clinical training for the Child MIND Institute, New York, said that when it comes to anxiety, it’s important to assess and treat both the parent and the child.
“We know that both environment and genetics play a role in anxiety disorders. From a clinical perspective, if we see a parent with an anxiety disorder, we know that there is a chance that that is also going to affect the child – whether or not the child has an anxiety disorder,” Dr. Emanuele said in an interview.
“Anxiety disorders are the most common psychiatric disorders diagnosed. We also know that anxiety disorders emerge earlier than mood disorders and certainly can emerge in childhood. It’s important to address anxiety early because those same problems can continue into adulthood if left untreated,” Dr. Emanuele added.
The study was supported by the Canada Research Chairs Program, the Canadian Institutes of Health Research, the Brain & Behavior Research Foundation, the Nova Scotia Health Research Foundation, and the Dalhousie Medical Research Foundation. The authors have disclosed no relevant financial relationships. Dr. Emanuele is a board member with the Anxiety and Depression Association of America.
A version of this article first appeared on Medscape.com.
The new findings suggest that children learn anxious behavior from their parents, study investigator Barbara Pavlova, PhD, clinical psychologist with Nova Scotia Health Authority, told this news organization.
“This means that transmission of anxiety from parents to children may be preventable,” said Dr. Pavlova, assistant professor, department of psychiatry, Dalhousie University, Halifax, Canada.
“Treating parents’ anxiety is not just important for their own health but also for the health of their children. This may be especially true if the child and the parent are the same sex,” Dr. Pavlova added.
The study was published online in JAMA Network Open.
Parental anxiety a disruptor
Anxiety disorders run in families. Both genes and environment are thought to be at play, but there are few data on sex-specific transmission from parent to child.
To investigate, the researchers conducted a cross-sectional study of 203 girls and 195 boys and their parents. The average age of the children was 11 years, and they had a familial risk for mood disorders.
Anxiety disorder in a same-sex parent was significantly associated with anxiety disorder in offspring (odds ratio, 2.85; 95% confidence interval, 1.52-5.34; P = .001) but not in an opposite-sex parent (OR, 1.51; 95% CI, 0.81-2.81; P = .20).
Living with a same-sex parent without anxiety was associated with lower rates of offspring anxiety (OR, 0.38; 95% CI, 0.22-0.67; P = .001).
Among all 398 children, 108 (27%) had been diagnosed with one or more anxiety disorders, including generalized anxiety disorder (7.8%), social anxiety disorder (6.3%), separation anxiety disorder (8.6%), specific phobia (8%), and anxiety disorder not otherwise specified (5%).
Rates of anxiety disorders in children increased with age, from 14% in those younger than 9 years to 52% in those older than 15 years. Anxiety disorders were similarly common among boys (24%) and girls (30%).
Rates of anxiety disorders were lowest (24%) in children of two parents without anxiety disorders and highest (41%) in cases in which both parents had anxiety disorders.
The findings point to the possible role of environmental factors, “such as modeling and vicarious learning,” in the transmission of anxiety from parents to their children, the researchers note.
“A child receives [a] similar amount of genetic information from each biological parent. A strong same-sex parent effect suggests children learn resilience by modeling the behavior of their same-sex parent. A parent’s anxiety disorder may disrupt this protective learning,” said Dr. Pavlova.
Early diagnosis, treatment essential
Reached for comment, Jill Emanuele, PhD, vice president of clinical training for the Child MIND Institute, New York, said that when it comes to anxiety, it’s important to assess and treat both the parent and the child.
“We know that both environment and genetics play a role in anxiety disorders. From a clinical perspective, if we see a parent with an anxiety disorder, we know that there is a chance that that is also going to affect the child – whether or not the child has an anxiety disorder,” Dr. Emanuele said in an interview.
“Anxiety disorders are the most common psychiatric disorders diagnosed. We also know that anxiety disorders emerge earlier than mood disorders and certainly can emerge in childhood. It’s important to address anxiety early because those same problems can continue into adulthood if left untreated,” Dr. Emanuele added.
The study was supported by the Canada Research Chairs Program, the Canadian Institutes of Health Research, the Brain & Behavior Research Foundation, the Nova Scotia Health Research Foundation, and the Dalhousie Medical Research Foundation. The authors have disclosed no relevant financial relationships. Dr. Emanuele is a board member with the Anxiety and Depression Association of America.
A version of this article first appeared on Medscape.com.
Migraine significantly correlates with fetal-type posterior cerebral artery in ischemic stroke
Key clinical point: Migraine was significantly associated with fetal-type posterior cerebral artery (PCA) status in patients with ischemic stroke.
Major finding: Fetal-type PCA status was independently associated with migraine (adjusted odds ratio [aOR] 2.06; P = .005). The other factors independently associated with migraine were hypertension (aOR 1.97; P = .011), female gender (aOR 2.03; P = .017) and National Institutes of Health Stroke Scale score at admission (aOR 1.08; P = .018).
Study details: This cross-sectional study included 750 patients aged ≥18 yearswith ischemic stroke, of which 11.3% had migraine history.
Disclosures: This study received no specific funding. The authors declared no conflicts of interest.
Source: Zhang Y et al. The association between migraine and fetal-type posterior cerebral artery in patients with ischemic stroke. Cerebrovasc Dis. 2022 (May 13). Doi:10.1159/000524616
Key clinical point: Migraine was significantly associated with fetal-type posterior cerebral artery (PCA) status in patients with ischemic stroke.
Major finding: Fetal-type PCA status was independently associated with migraine (adjusted odds ratio [aOR] 2.06; P = .005). The other factors independently associated with migraine were hypertension (aOR 1.97; P = .011), female gender (aOR 2.03; P = .017) and National Institutes of Health Stroke Scale score at admission (aOR 1.08; P = .018).
Study details: This cross-sectional study included 750 patients aged ≥18 yearswith ischemic stroke, of which 11.3% had migraine history.
Disclosures: This study received no specific funding. The authors declared no conflicts of interest.
Source: Zhang Y et al. The association between migraine and fetal-type posterior cerebral artery in patients with ischemic stroke. Cerebrovasc Dis. 2022 (May 13). Doi:10.1159/000524616
Key clinical point: Migraine was significantly associated with fetal-type posterior cerebral artery (PCA) status in patients with ischemic stroke.
Major finding: Fetal-type PCA status was independently associated with migraine (adjusted odds ratio [aOR] 2.06; P = .005). The other factors independently associated with migraine were hypertension (aOR 1.97; P = .011), female gender (aOR 2.03; P = .017) and National Institutes of Health Stroke Scale score at admission (aOR 1.08; P = .018).
Study details: This cross-sectional study included 750 patients aged ≥18 yearswith ischemic stroke, of which 11.3% had migraine history.
Disclosures: This study received no specific funding. The authors declared no conflicts of interest.
Source: Zhang Y et al. The association between migraine and fetal-type posterior cerebral artery in patients with ischemic stroke. Cerebrovasc Dis. 2022 (May 13). Doi:10.1159/000524616




