User login
Misoprostol: Clinical pharmacology in obstetrics and gynecology
Oxytocin and prostaglandins are critically important regulators of uterine contraction. Obstetrician-gynecologists commonly prescribe oxytocin and prostaglandin agonists (misoprostol, dinoprostone) to stimulate uterine contraction for the induction of labor, prevention and treatment of postpartum hemorrhage, and treatment of miscarriage and fetal demise. The focus of this editorial is the clinical pharmacology of misoprostol.
Misoprostol is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of nonsteroidal anti-inflammatory drug–induced gastric ulcers and for patients at high risk for gastric ulcers, including those with a history of gastric ulcers. The approved misoprostol route and dose for this indication is oral administration of 200 µg four times daily with food.1 Recent food intake and antacid use reduces the absorption of orally administered misoprostol. There are no FDA-approved indications for the use of misoprostol as a single agent in obstetrics and gynecology. The FDA has approved the combination of mifepristone and misoprostol for medication abortion in the first trimester. In contrast to misoprostol, PGE2 (dinoprostone) is approved by the FDA as a vaginal insert containing 10 mg of dinoprostone for the initiation and/or continuation of cervical ripening in patients at or near term in whom there is a medical or obstetric indication for induction of labor (Cervidil; Ferring Pharmaceuticals Inc, Parsippany, New Jersey).2
Pharmacology of misoprostol
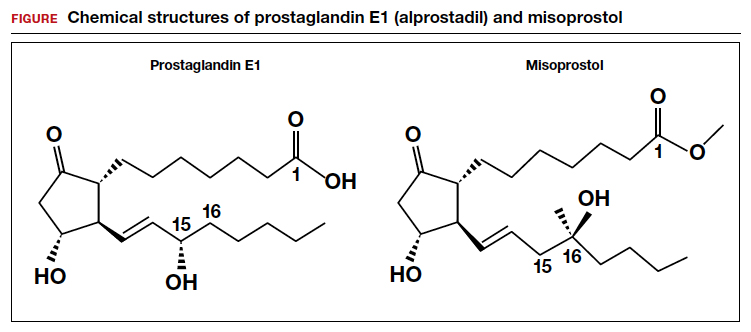
Misoprostol is a prostaglandin E1 (PGE1) agonist analogue. Prostaglandin E1 (alprostadil) is rapidly metabolized, has a half-life in the range of minutes and is not orally active, requiring administration by intravenous infusion or injection. It is indicated to maintain a patent ductus arteriosus in newborns with ductal-dependent circulation and to treat erectile dysfunction.3 In contrast to PGE1, misoprostol has a methyl ester group at carbon-1 (C-1) that increases potency and duration of action. Misoprostol also has no hydroxyl group at C-15, replacing that moiety with the addition of both a methyl- and hydroxyl- group at C-16 (FIGURE). These molecular changes improve oral activity and increase duration of action.4 Pure misoprostol is a viscous oil. It is formulated into tables by dispersing the oil on hydroxypropyl methyl cellulose before compounding into tablets. Unlike naturally occurring prostaglandins (PGE1), misoprostol tablets are stabile at room temperature for years.4
Following absorption, the methyl ester at C-1 is enzymatically cleaved, yielding misoprostol acid, the active drug.4 Misoprostol binds to the E prostanoid receptor 3 (EP-3).5 Activation of myometrial EP-3 receptor induces an increase in intracellular phosphoinositol turnover and calcium mobilization, resulting in an increase in intracellular-free calcium, triggering actin-myosin contractility.6 The increase in free calcium is propagated cell-to-cell through gap junctions that link the myometrial cells to facilitate the generation of a coordinated contraction.
Misoprostol: Various routes of administration are not equal
Misoprostol can be given by an oral, buccal, vaginal, or rectal route of administration. To study the effect of the route of administration on uterine tone and contractility, investigators randomly assigned patients at 8 to 11 weeks’ gestation to receive misoprostol 400 µg as a single dose by the oral or vaginal route. Uterine tone and contractility were measured using an intrauterine pressure transducer. Compared to vaginal administration, oral administration of misprostol was associated with rapid attainment of peak plasma level at 30 minutes, followed by a decline in concentration by 60 minutes. This rapid onset and rapid offset of plasma concentration was paralleled by the onset of uterine tone within 8 minutes, but surprisingly no sustained uterine contractions.7 By contrast, following vaginal administration of misoprostol, serum levels rose slowly and peaked in 1 to 2 hours. Uterine tone increased within 21 minutes, and sustained uterine contractions were recorded for 4 hours.7 The rapid rise and fall in plasma misoprostol following oral administration and the more sustained plasma misoprostol concentration over 4 hours has been previously reported.8 In a second study involving patients 8 to 11 weeks’ gestation, the effect of a single dose of misoprostol 400 µg by an oral or vaginal route on uterine contractility was compared using an intrauterine pressure transducer.9 Confirming previous results, the time from misoprostol administration to increased uterine tone was more rapid with oral than with vaginal administration (8 min vs 19 min). Over the course of 4 hours, uterine contraction activity was greater with vaginal than with oral administration (454 vs 166 Montevideo units).9
Both studies reported that oral administration of misoprostol resulted in more rapid onset and offset of action than vaginal administration. Oral administration of a single dose of misoprostol 400 µg did not result in sustained uterine contractions in most patients in the first trimester. Vaginal administration produced a slower onset of increased uterine tone but sustained uterine contractions over 4 hours. Compared with vaginal administration of misoprostol, the rapid onset and offset of action of oral misoprostol may reduce the rate of tachysystole and changes in fetal heart rate observed with vaginal administration.10
An important finding is that buccal and vaginal administration of misoprostol have similar effects on uterine tone in the first trimester.11 To study the effect of buccal and vaginal administration of misoprostol on uterine tone, patients 6 to 13 weeks’ gestation were randomly allocated to receive a single dose of misoprostol 400 µg by a buccal or vaginal route.11 Uterine activity over 5 hours following administration was assessed using an intrauterine pressure transducer. Uterine tone 20 to 30 minutes after buccal or vaginal administration of misoprostol (400 µg) was 27 and 28 mm Hg, respectively. Peak uterine tone, as measured by an intrauterine pressure transducer, for buccal and vaginal administration of misoprostol was 49 mm Hg and 54 mm Hg, respectively. Total Alexandria units (AU) over 5 hours following buccal or vaginal administration was 6,537 AU and 6,090 AU, respectively.11
An AU is calculated as the average amplitude of the contractions (mm Hg) multiplied by the average duration of the contractions (min) multiplied by average frequency of contraction over 10 minutes.12 By contrast, a Montevideo unit does not include an assessment of contraction duration and is calculated as average amplitude of contractions (mm Hg) multiplied by frequency of uterine contractions over 10 minutes.12
In contrast to buccal or vaginal administration, rectal administration of misoprostol resulted in much lower peak uterine tone and contractility as measured by a pressure transducer. Uterine tone 20 to 30 minutes after vaginal and rectal administration of misoprostol (400 µg) was 28 and 19 mm Hg, respectively.11 Peak uterine tone, as measured by an intrauterine pressure transducer, for vaginal and rectal administration of misoprostol was 54 and 31 mm Hg, respectively. AUs over 5 hours following vaginal and rectal administration was 6,090 AU and 2,768 AU, respectively.11 Compared with buccal and vaginal administration of misoprostol, rectal administration produced less sustained uterine contractions in the first trimester of pregnancy. To achieve maximal sustained uterine contractions, buccal and vaginal routes of administration are superior to oral and rectal administration.
Continue to: Misoprostol and cervical ripening...
Misoprostol and cervical ripening
Misoprostol is commonly used to soften and ripen the cervix. Some of the cervical ripening effects of misoprostol are likely due to increased uterine tone. In addition, misoprostol may have a direct effect on the collagen structure of the cervix. To study the effect of misoprostol on the cervix, pregnant patients in the first trimester were randomly assigned to receive misoprostol 200 µg by vaginal self-administration, isosorbide mononitrate (IMN) 40 mg by vaginal self-administration or no treatment the evening prior to pregnancy termination.13 The following day, before uterine evacuation, a cervical biopsy was obtained for electron microscopy studies and immunohistochemistry to assess the presence of enzymes involved in collagen degradation, including matrix metalloproteinase 1 (MMP-1) and matrix metalloproteinase 9 (MMP-9). Electron microscopy demonstrated that pretreatment with misoprostol resulted in a pronounced splitting and disorganization of collagen fibers.13 Compared with misoprostol treatment, IMN produced less splitting and disorganization of collagen fibers, and in the no treatment group, no marked changes in the collagen framework were observed.
Compared with no treatment, misoprostol and IMN pretreatment were associated with marked increases in MMP-1 and MMP-9 as assessed by immunohistochemistry. Misoprostol pretreatment also resulted in a significant increase in interleukin-8 concentration compared with IMN pretreatment and no treatment (8.8 vs 2.7 vs 2.4 pg/mg tissue), respectively.13 Other investigators have also reported that misoprostol increased cervical leukocyte influx and collagen disrupting enzymes MMP-8 and MMP-9.14,15
An open-label clinical trial compared the efficacy of misoprostol versus Foley catheter for labor induction at term in 1,859 patients ≥ 37 weeks’ gestation with a Bishop score <6.16 Patients were randomly allocated to misoprostol (50 µg orally every 4 hours up to 3 times in 24 hours) versus placement of a 16 F or 18 F Foley catheter introduced through the cervix, filled with 30 mL of sodium chloride or water. The investigators reported that oral misoprostol and Foley catheter cervical ripening had similar safety and effectiveness for cervical ripening as a prelude to induction of labor, including no statistically significant differences in 5-minute Apgar score <7, umbilical cord artery pH ≤ 7.05, postpartum hemorrhage, or cesarean birth rate.16
Bottom line
Misoprostol and oxytocin are commonly prescribed in obstetric practice for cervical ripening and induction of labor, respectively. The dose and route of administration of misoprostol influences the effect on the uterus. For cervical ripening, where rapid onset and offset may help to reduce the risk of uterine tachysystole and worrisome fetal heart rate changes, low-dose (50 µg) oral administration of misoprostol may be a preferred dose and route. For the treatment of miscarriage and fetal demise, to stimulate sustained uterine contractions over many hours, buccal and vaginal administration of misoprostol are preferred. Rectal administration is generally inferior to buccal and vaginal administration for stimulating sustained uterine contractions and its uses should be limited. ●
Common side effects of misoprostol are abdominal cramping, diarrhea, nausea, vomiting, headache, and fever. Elevated temperature following misoprostol administration is a concerning side effect that may require further investigation to rule out an infection, especially if the elevated temperature persists for > 4 hours. The preoptic area of the anterior hypothalamus (POAH) plays a major role in thermoregulation. When an infection causes an increase in endogenous pyrogens, including interleukin-1β, interleukin-6 and tumor necrosis factor, prostaglandins are generated in the region of the POAH, increasing the thermoregulatory set point, triggering cutaneous vasoconstriction and shivering and non-shivering thermogenesis.1 Misoprostol, especially at doses >400 µg commonly causes both patient-reported chills and temperature elevation >38° C.
In a study comparing misoprostol and oxytocin for the management of the third stage of labor, 597 patients were randomly allocated to receive oxytocin 10 units by intramuscular injection or misoprostol 400 µg or 600 µg by the oral route.2 Patient-reported shivering occurred in 13%, 19%, and 28% of patients receiving oxytocin, misoprostol 400 µg and misoprostol 800 µg, respectively. A recorded temperature >38° C occurred within 1 hour of medication administration in approximately 3%, 2%, and 7.5% of patients receiving oxytocin, misoprostol 400 µg, and misoprostol 800 µg, respectively. In another study, 453 patients scheduled for a cesarean birth were randomly allocated to receive 1 of 3 doses of rectal misoprostol 200 μg, 400 μg, or 600 μg before incision. Fever was detected in 2.6%, 9.9%, and 5.1% of the patients receiving misoprostol 200 μg, 400 μg, or 600 μg, respectively.3
References
1. Aronoff DM, Neilson EG. Antipyretics: mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111:304-315. doi: 10.1016/s0002-9343(01)00834-8.
2. Lumbiganon P, Hofmeyr J, Gumezoglu AM, et al. Misoprostol dose-related shivering and pyrexia in the third stage of labor. WHO Collaborative Trial of Misoprostol in the Management of the Third Stage of Labor. Br J Obstet Gynaecol. 1999;106:304-308. doi: 10.1111/j.1471-0528.1999.tb08266.x.
3. Sweed M, El-Said M, Abou-Gamrah AA, et al. Comparison between 200, 400 and 600 microgram rectal misoprostol before cesarean section: a randomized clinical trial. J Obstet Gynaecol Res. 2019;45:585-591. doi: 10.1111 /jog.13883.
- Cytotec [package insert]. Chicago, IL: GD Searle & Co. https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/19268slr037.pdf. Accessed June 20, 2022.
- Cervidil [package insert]. St Louis, MO: Forrest Pharmaceuticals Inc.; May 2006. Accessed June 20, 2022.
- Caverject [package insert]. New York, NY: Pfizer Inc.; March 2014. Accessed June 20, 2022.
- Collins PW. Misoprostol: discovery, development and clinical applications. Med Res Rev. 1990;10:149-172. doi: 10.1002/med.2610100202.
- Audit M, White KI, Breton B, et al. Crystal structure of misoprostol bound to the labor inducer prostaglandin E2 receptor. Nat Chem Biol. 2019;15:11-17. doi: 10.1038/s41589-018-0160-y.
- Pallliser KH, Hirst JJ, Ooi G, et al. Prostaglandin E and F receptor expression and myometrial sensitivity in labor onset in the sheep. Biol Reprod. 2005;72:937-943. doi: 10.1095/biolreprod.104.035311.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280. doi: 10.1016/s0029-7844(98)00436-0.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92. doi: 10.1016/S0029-7844(97)00111-7.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84. doi: 10.1093/humrep/deh005.
- Young DC, Delaney T, Armson BA, et al. Oral misoprostol, low dose vaginal misoprostol and vaginal dinoprostone for labor induction: randomized controlled trial. PLOS One. 2020;15:e0227245. doi: 10.1371/journal.pone.0227245.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590. doi: 10.1097/01.AOG.0000230398.32794.9d.
- el-Sahwi S, Gaafar AA, Toppozada HK. A new unit for evaluation of uterine activity. Am J Obstet Gynecol. 1967;98:900-903. doi: 10.1016/0002-9378(67)90074-9.
- Vukas N, Ekerhovd E, Abrahamsson G, et al. Cervical priming in the first trimester: morphological and biochemical effects of misoprostol and isosorbide mononitrate. Acta Obstet Gyecol. 2009;88:43-51. doi: 10.1080/00016340802585440.
- Aronsson A, Ulfgren AK, Stabi B, et al. The effect of orally and vaginally administered misoprostol on inflammatory mediators and cervical ripening during early pregnancy. Contraception. 2005;72:33-39. doi: 10.1016/j.contraception.2005.02.012.
- Denison FC, Riley SC, Elliott CL, et al. The effect of mifepristone administration on leukocyte populations, matrix metalloproteinases and inflammatory mediators in the first trimester cervix. Mol Hum Reprod. 2000;6:541-548. doi: 10.1093/molehr/6.6.541.
- ten Eikelder MLG, Rengerink KO, Jozwiak M, et al. Induction of labour at term with oral misoprostol versus a Foley catheter (PROBAAT-II): a multicentre randomised controlled non-inferiority trial. Lancet. 2016;387:1619-1628. doi: 10.1016 /S0140-6736(16)00084-2.
Oxytocin and prostaglandins are critically important regulators of uterine contraction. Obstetrician-gynecologists commonly prescribe oxytocin and prostaglandin agonists (misoprostol, dinoprostone) to stimulate uterine contraction for the induction of labor, prevention and treatment of postpartum hemorrhage, and treatment of miscarriage and fetal demise. The focus of this editorial is the clinical pharmacology of misoprostol.
Misoprostol is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of nonsteroidal anti-inflammatory drug–induced gastric ulcers and for patients at high risk for gastric ulcers, including those with a history of gastric ulcers. The approved misoprostol route and dose for this indication is oral administration of 200 µg four times daily with food.1 Recent food intake and antacid use reduces the absorption of orally administered misoprostol. There are no FDA-approved indications for the use of misoprostol as a single agent in obstetrics and gynecology. The FDA has approved the combination of mifepristone and misoprostol for medication abortion in the first trimester. In contrast to misoprostol, PGE2 (dinoprostone) is approved by the FDA as a vaginal insert containing 10 mg of dinoprostone for the initiation and/or continuation of cervical ripening in patients at or near term in whom there is a medical or obstetric indication for induction of labor (Cervidil; Ferring Pharmaceuticals Inc, Parsippany, New Jersey).2
Pharmacology of misoprostol
Misoprostol is a prostaglandin E1 (PGE1) agonist analogue. Prostaglandin E1 (alprostadil) is rapidly metabolized, has a half-life in the range of minutes and is not orally active, requiring administration by intravenous infusion or injection. It is indicated to maintain a patent ductus arteriosus in newborns with ductal-dependent circulation and to treat erectile dysfunction.3 In contrast to PGE1, misoprostol has a methyl ester group at carbon-1 (C-1) that increases potency and duration of action. Misoprostol also has no hydroxyl group at C-15, replacing that moiety with the addition of both a methyl- and hydroxyl- group at C-16 (FIGURE). These molecular changes improve oral activity and increase duration of action.4 Pure misoprostol is a viscous oil. It is formulated into tables by dispersing the oil on hydroxypropyl methyl cellulose before compounding into tablets. Unlike naturally occurring prostaglandins (PGE1), misoprostol tablets are stabile at room temperature for years.4
Following absorption, the methyl ester at C-1 is enzymatically cleaved, yielding misoprostol acid, the active drug.4 Misoprostol binds to the E prostanoid receptor 3 (EP-3).5 Activation of myometrial EP-3 receptor induces an increase in intracellular phosphoinositol turnover and calcium mobilization, resulting in an increase in intracellular-free calcium, triggering actin-myosin contractility.6 The increase in free calcium is propagated cell-to-cell through gap junctions that link the myometrial cells to facilitate the generation of a coordinated contraction.

Misoprostol: Various routes of administration are not equal
Misoprostol can be given by an oral, buccal, vaginal, or rectal route of administration. To study the effect of the route of administration on uterine tone and contractility, investigators randomly assigned patients at 8 to 11 weeks’ gestation to receive misoprostol 400 µg as a single dose by the oral or vaginal route. Uterine tone and contractility were measured using an intrauterine pressure transducer. Compared to vaginal administration, oral administration of misprostol was associated with rapid attainment of peak plasma level at 30 minutes, followed by a decline in concentration by 60 minutes. This rapid onset and rapid offset of plasma concentration was paralleled by the onset of uterine tone within 8 minutes, but surprisingly no sustained uterine contractions.7 By contrast, following vaginal administration of misoprostol, serum levels rose slowly and peaked in 1 to 2 hours. Uterine tone increased within 21 minutes, and sustained uterine contractions were recorded for 4 hours.7 The rapid rise and fall in plasma misoprostol following oral administration and the more sustained plasma misoprostol concentration over 4 hours has been previously reported.8 In a second study involving patients 8 to 11 weeks’ gestation, the effect of a single dose of misoprostol 400 µg by an oral or vaginal route on uterine contractility was compared using an intrauterine pressure transducer.9 Confirming previous results, the time from misoprostol administration to increased uterine tone was more rapid with oral than with vaginal administration (8 min vs 19 min). Over the course of 4 hours, uterine contraction activity was greater with vaginal than with oral administration (454 vs 166 Montevideo units).9
Both studies reported that oral administration of misoprostol resulted in more rapid onset and offset of action than vaginal administration. Oral administration of a single dose of misoprostol 400 µg did not result in sustained uterine contractions in most patients in the first trimester. Vaginal administration produced a slower onset of increased uterine tone but sustained uterine contractions over 4 hours. Compared with vaginal administration of misoprostol, the rapid onset and offset of action of oral misoprostol may reduce the rate of tachysystole and changes in fetal heart rate observed with vaginal administration.10
An important finding is that buccal and vaginal administration of misoprostol have similar effects on uterine tone in the first trimester.11 To study the effect of buccal and vaginal administration of misoprostol on uterine tone, patients 6 to 13 weeks’ gestation were randomly allocated to receive a single dose of misoprostol 400 µg by a buccal or vaginal route.11 Uterine activity over 5 hours following administration was assessed using an intrauterine pressure transducer. Uterine tone 20 to 30 minutes after buccal or vaginal administration of misoprostol (400 µg) was 27 and 28 mm Hg, respectively. Peak uterine tone, as measured by an intrauterine pressure transducer, for buccal and vaginal administration of misoprostol was 49 mm Hg and 54 mm Hg, respectively. Total Alexandria units (AU) over 5 hours following buccal or vaginal administration was 6,537 AU and 6,090 AU, respectively.11
An AU is calculated as the average amplitude of the contractions (mm Hg) multiplied by the average duration of the contractions (min) multiplied by average frequency of contraction over 10 minutes.12 By contrast, a Montevideo unit does not include an assessment of contraction duration and is calculated as average amplitude of contractions (mm Hg) multiplied by frequency of uterine contractions over 10 minutes.12
In contrast to buccal or vaginal administration, rectal administration of misoprostol resulted in much lower peak uterine tone and contractility as measured by a pressure transducer. Uterine tone 20 to 30 minutes after vaginal and rectal administration of misoprostol (400 µg) was 28 and 19 mm Hg, respectively.11 Peak uterine tone, as measured by an intrauterine pressure transducer, for vaginal and rectal administration of misoprostol was 54 and 31 mm Hg, respectively. AUs over 5 hours following vaginal and rectal administration was 6,090 AU and 2,768 AU, respectively.11 Compared with buccal and vaginal administration of misoprostol, rectal administration produced less sustained uterine contractions in the first trimester of pregnancy. To achieve maximal sustained uterine contractions, buccal and vaginal routes of administration are superior to oral and rectal administration.
Continue to: Misoprostol and cervical ripening...
Misoprostol and cervical ripening
Misoprostol is commonly used to soften and ripen the cervix. Some of the cervical ripening effects of misoprostol are likely due to increased uterine tone. In addition, misoprostol may have a direct effect on the collagen structure of the cervix. To study the effect of misoprostol on the cervix, pregnant patients in the first trimester were randomly assigned to receive misoprostol 200 µg by vaginal self-administration, isosorbide mononitrate (IMN) 40 mg by vaginal self-administration or no treatment the evening prior to pregnancy termination.13 The following day, before uterine evacuation, a cervical biopsy was obtained for electron microscopy studies and immunohistochemistry to assess the presence of enzymes involved in collagen degradation, including matrix metalloproteinase 1 (MMP-1) and matrix metalloproteinase 9 (MMP-9). Electron microscopy demonstrated that pretreatment with misoprostol resulted in a pronounced splitting and disorganization of collagen fibers.13 Compared with misoprostol treatment, IMN produced less splitting and disorganization of collagen fibers, and in the no treatment group, no marked changes in the collagen framework were observed.
Compared with no treatment, misoprostol and IMN pretreatment were associated with marked increases in MMP-1 and MMP-9 as assessed by immunohistochemistry. Misoprostol pretreatment also resulted in a significant increase in interleukin-8 concentration compared with IMN pretreatment and no treatment (8.8 vs 2.7 vs 2.4 pg/mg tissue), respectively.13 Other investigators have also reported that misoprostol increased cervical leukocyte influx and collagen disrupting enzymes MMP-8 and MMP-9.14,15
An open-label clinical trial compared the efficacy of misoprostol versus Foley catheter for labor induction at term in 1,859 patients ≥ 37 weeks’ gestation with a Bishop score <6.16 Patients were randomly allocated to misoprostol (50 µg orally every 4 hours up to 3 times in 24 hours) versus placement of a 16 F or 18 F Foley catheter introduced through the cervix, filled with 30 mL of sodium chloride or water. The investigators reported that oral misoprostol and Foley catheter cervical ripening had similar safety and effectiveness for cervical ripening as a prelude to induction of labor, including no statistically significant differences in 5-minute Apgar score <7, umbilical cord artery pH ≤ 7.05, postpartum hemorrhage, or cesarean birth rate.16
Bottom line
Misoprostol and oxytocin are commonly prescribed in obstetric practice for cervical ripening and induction of labor, respectively. The dose and route of administration of misoprostol influences the effect on the uterus. For cervical ripening, where rapid onset and offset may help to reduce the risk of uterine tachysystole and worrisome fetal heart rate changes, low-dose (50 µg) oral administration of misoprostol may be a preferred dose and route. For the treatment of miscarriage and fetal demise, to stimulate sustained uterine contractions over many hours, buccal and vaginal administration of misoprostol are preferred. Rectal administration is generally inferior to buccal and vaginal administration for stimulating sustained uterine contractions and its uses should be limited. ●
Common side effects of misoprostol are abdominal cramping, diarrhea, nausea, vomiting, headache, and fever. Elevated temperature following misoprostol administration is a concerning side effect that may require further investigation to rule out an infection, especially if the elevated temperature persists for > 4 hours. The preoptic area of the anterior hypothalamus (POAH) plays a major role in thermoregulation. When an infection causes an increase in endogenous pyrogens, including interleukin-1β, interleukin-6 and tumor necrosis factor, prostaglandins are generated in the region of the POAH, increasing the thermoregulatory set point, triggering cutaneous vasoconstriction and shivering and non-shivering thermogenesis.1 Misoprostol, especially at doses >400 µg commonly causes both patient-reported chills and temperature elevation >38° C.
In a study comparing misoprostol and oxytocin for the management of the third stage of labor, 597 patients were randomly allocated to receive oxytocin 10 units by intramuscular injection or misoprostol 400 µg or 600 µg by the oral route.2 Patient-reported shivering occurred in 13%, 19%, and 28% of patients receiving oxytocin, misoprostol 400 µg and misoprostol 800 µg, respectively. A recorded temperature >38° C occurred within 1 hour of medication administration in approximately 3%, 2%, and 7.5% of patients receiving oxytocin, misoprostol 400 µg, and misoprostol 800 µg, respectively. In another study, 453 patients scheduled for a cesarean birth were randomly allocated to receive 1 of 3 doses of rectal misoprostol 200 μg, 400 μg, or 600 μg before incision. Fever was detected in 2.6%, 9.9%, and 5.1% of the patients receiving misoprostol 200 μg, 400 μg, or 600 μg, respectively.3
References
1. Aronoff DM, Neilson EG. Antipyretics: mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111:304-315. doi: 10.1016/s0002-9343(01)00834-8.
2. Lumbiganon P, Hofmeyr J, Gumezoglu AM, et al. Misoprostol dose-related shivering and pyrexia in the third stage of labor. WHO Collaborative Trial of Misoprostol in the Management of the Third Stage of Labor. Br J Obstet Gynaecol. 1999;106:304-308. doi: 10.1111/j.1471-0528.1999.tb08266.x.
3. Sweed M, El-Said M, Abou-Gamrah AA, et al. Comparison between 200, 400 and 600 microgram rectal misoprostol before cesarean section: a randomized clinical trial. J Obstet Gynaecol Res. 2019;45:585-591. doi: 10.1111 /jog.13883.
Oxytocin and prostaglandins are critically important regulators of uterine contraction. Obstetrician-gynecologists commonly prescribe oxytocin and prostaglandin agonists (misoprostol, dinoprostone) to stimulate uterine contraction for the induction of labor, prevention and treatment of postpartum hemorrhage, and treatment of miscarriage and fetal demise. The focus of this editorial is the clinical pharmacology of misoprostol.
Misoprostol is approved by the US Food and Drug Administration (FDA) for the prevention and treatment of nonsteroidal anti-inflammatory drug–induced gastric ulcers and for patients at high risk for gastric ulcers, including those with a history of gastric ulcers. The approved misoprostol route and dose for this indication is oral administration of 200 µg four times daily with food.1 Recent food intake and antacid use reduces the absorption of orally administered misoprostol. There are no FDA-approved indications for the use of misoprostol as a single agent in obstetrics and gynecology. The FDA has approved the combination of mifepristone and misoprostol for medication abortion in the first trimester. In contrast to misoprostol, PGE2 (dinoprostone) is approved by the FDA as a vaginal insert containing 10 mg of dinoprostone for the initiation and/or continuation of cervical ripening in patients at or near term in whom there is a medical or obstetric indication for induction of labor (Cervidil; Ferring Pharmaceuticals Inc, Parsippany, New Jersey).2
Pharmacology of misoprostol
Misoprostol is a prostaglandin E1 (PGE1) agonist analogue. Prostaglandin E1 (alprostadil) is rapidly metabolized, has a half-life in the range of minutes and is not orally active, requiring administration by intravenous infusion or injection. It is indicated to maintain a patent ductus arteriosus in newborns with ductal-dependent circulation and to treat erectile dysfunction.3 In contrast to PGE1, misoprostol has a methyl ester group at carbon-1 (C-1) that increases potency and duration of action. Misoprostol also has no hydroxyl group at C-15, replacing that moiety with the addition of both a methyl- and hydroxyl- group at C-16 (FIGURE). These molecular changes improve oral activity and increase duration of action.4 Pure misoprostol is a viscous oil. It is formulated into tables by dispersing the oil on hydroxypropyl methyl cellulose before compounding into tablets. Unlike naturally occurring prostaglandins (PGE1), misoprostol tablets are stabile at room temperature for years.4
Following absorption, the methyl ester at C-1 is enzymatically cleaved, yielding misoprostol acid, the active drug.4 Misoprostol binds to the E prostanoid receptor 3 (EP-3).5 Activation of myometrial EP-3 receptor induces an increase in intracellular phosphoinositol turnover and calcium mobilization, resulting in an increase in intracellular-free calcium, triggering actin-myosin contractility.6 The increase in free calcium is propagated cell-to-cell through gap junctions that link the myometrial cells to facilitate the generation of a coordinated contraction.

Misoprostol: Various routes of administration are not equal
Misoprostol can be given by an oral, buccal, vaginal, or rectal route of administration. To study the effect of the route of administration on uterine tone and contractility, investigators randomly assigned patients at 8 to 11 weeks’ gestation to receive misoprostol 400 µg as a single dose by the oral or vaginal route. Uterine tone and contractility were measured using an intrauterine pressure transducer. Compared to vaginal administration, oral administration of misprostol was associated with rapid attainment of peak plasma level at 30 minutes, followed by a decline in concentration by 60 minutes. This rapid onset and rapid offset of plasma concentration was paralleled by the onset of uterine tone within 8 minutes, but surprisingly no sustained uterine contractions.7 By contrast, following vaginal administration of misoprostol, serum levels rose slowly and peaked in 1 to 2 hours. Uterine tone increased within 21 minutes, and sustained uterine contractions were recorded for 4 hours.7 The rapid rise and fall in plasma misoprostol following oral administration and the more sustained plasma misoprostol concentration over 4 hours has been previously reported.8 In a second study involving patients 8 to 11 weeks’ gestation, the effect of a single dose of misoprostol 400 µg by an oral or vaginal route on uterine contractility was compared using an intrauterine pressure transducer.9 Confirming previous results, the time from misoprostol administration to increased uterine tone was more rapid with oral than with vaginal administration (8 min vs 19 min). Over the course of 4 hours, uterine contraction activity was greater with vaginal than with oral administration (454 vs 166 Montevideo units).9
Both studies reported that oral administration of misoprostol resulted in more rapid onset and offset of action than vaginal administration. Oral administration of a single dose of misoprostol 400 µg did not result in sustained uterine contractions in most patients in the first trimester. Vaginal administration produced a slower onset of increased uterine tone but sustained uterine contractions over 4 hours. Compared with vaginal administration of misoprostol, the rapid onset and offset of action of oral misoprostol may reduce the rate of tachysystole and changes in fetal heart rate observed with vaginal administration.10
An important finding is that buccal and vaginal administration of misoprostol have similar effects on uterine tone in the first trimester.11 To study the effect of buccal and vaginal administration of misoprostol on uterine tone, patients 6 to 13 weeks’ gestation were randomly allocated to receive a single dose of misoprostol 400 µg by a buccal or vaginal route.11 Uterine activity over 5 hours following administration was assessed using an intrauterine pressure transducer. Uterine tone 20 to 30 minutes after buccal or vaginal administration of misoprostol (400 µg) was 27 and 28 mm Hg, respectively. Peak uterine tone, as measured by an intrauterine pressure transducer, for buccal and vaginal administration of misoprostol was 49 mm Hg and 54 mm Hg, respectively. Total Alexandria units (AU) over 5 hours following buccal or vaginal administration was 6,537 AU and 6,090 AU, respectively.11
An AU is calculated as the average amplitude of the contractions (mm Hg) multiplied by the average duration of the contractions (min) multiplied by average frequency of contraction over 10 minutes.12 By contrast, a Montevideo unit does not include an assessment of contraction duration and is calculated as average amplitude of contractions (mm Hg) multiplied by frequency of uterine contractions over 10 minutes.12
In contrast to buccal or vaginal administration, rectal administration of misoprostol resulted in much lower peak uterine tone and contractility as measured by a pressure transducer. Uterine tone 20 to 30 minutes after vaginal and rectal administration of misoprostol (400 µg) was 28 and 19 mm Hg, respectively.11 Peak uterine tone, as measured by an intrauterine pressure transducer, for vaginal and rectal administration of misoprostol was 54 and 31 mm Hg, respectively. AUs over 5 hours following vaginal and rectal administration was 6,090 AU and 2,768 AU, respectively.11 Compared with buccal and vaginal administration of misoprostol, rectal administration produced less sustained uterine contractions in the first trimester of pregnancy. To achieve maximal sustained uterine contractions, buccal and vaginal routes of administration are superior to oral and rectal administration.
Continue to: Misoprostol and cervical ripening...
Misoprostol and cervical ripening
Misoprostol is commonly used to soften and ripen the cervix. Some of the cervical ripening effects of misoprostol are likely due to increased uterine tone. In addition, misoprostol may have a direct effect on the collagen structure of the cervix. To study the effect of misoprostol on the cervix, pregnant patients in the first trimester were randomly assigned to receive misoprostol 200 µg by vaginal self-administration, isosorbide mononitrate (IMN) 40 mg by vaginal self-administration or no treatment the evening prior to pregnancy termination.13 The following day, before uterine evacuation, a cervical biopsy was obtained for electron microscopy studies and immunohistochemistry to assess the presence of enzymes involved in collagen degradation, including matrix metalloproteinase 1 (MMP-1) and matrix metalloproteinase 9 (MMP-9). Electron microscopy demonstrated that pretreatment with misoprostol resulted in a pronounced splitting and disorganization of collagen fibers.13 Compared with misoprostol treatment, IMN produced less splitting and disorganization of collagen fibers, and in the no treatment group, no marked changes in the collagen framework were observed.
Compared with no treatment, misoprostol and IMN pretreatment were associated with marked increases in MMP-1 and MMP-9 as assessed by immunohistochemistry. Misoprostol pretreatment also resulted in a significant increase in interleukin-8 concentration compared with IMN pretreatment and no treatment (8.8 vs 2.7 vs 2.4 pg/mg tissue), respectively.13 Other investigators have also reported that misoprostol increased cervical leukocyte influx and collagen disrupting enzymes MMP-8 and MMP-9.14,15
An open-label clinical trial compared the efficacy of misoprostol versus Foley catheter for labor induction at term in 1,859 patients ≥ 37 weeks’ gestation with a Bishop score <6.16 Patients were randomly allocated to misoprostol (50 µg orally every 4 hours up to 3 times in 24 hours) versus placement of a 16 F or 18 F Foley catheter introduced through the cervix, filled with 30 mL of sodium chloride or water. The investigators reported that oral misoprostol and Foley catheter cervical ripening had similar safety and effectiveness for cervical ripening as a prelude to induction of labor, including no statistically significant differences in 5-minute Apgar score <7, umbilical cord artery pH ≤ 7.05, postpartum hemorrhage, or cesarean birth rate.16
Bottom line
Misoprostol and oxytocin are commonly prescribed in obstetric practice for cervical ripening and induction of labor, respectively. The dose and route of administration of misoprostol influences the effect on the uterus. For cervical ripening, where rapid onset and offset may help to reduce the risk of uterine tachysystole and worrisome fetal heart rate changes, low-dose (50 µg) oral administration of misoprostol may be a preferred dose and route. For the treatment of miscarriage and fetal demise, to stimulate sustained uterine contractions over many hours, buccal and vaginal administration of misoprostol are preferred. Rectal administration is generally inferior to buccal and vaginal administration for stimulating sustained uterine contractions and its uses should be limited. ●
Common side effects of misoprostol are abdominal cramping, diarrhea, nausea, vomiting, headache, and fever. Elevated temperature following misoprostol administration is a concerning side effect that may require further investigation to rule out an infection, especially if the elevated temperature persists for > 4 hours. The preoptic area of the anterior hypothalamus (POAH) plays a major role in thermoregulation. When an infection causes an increase in endogenous pyrogens, including interleukin-1β, interleukin-6 and tumor necrosis factor, prostaglandins are generated in the region of the POAH, increasing the thermoregulatory set point, triggering cutaneous vasoconstriction and shivering and non-shivering thermogenesis.1 Misoprostol, especially at doses >400 µg commonly causes both patient-reported chills and temperature elevation >38° C.
In a study comparing misoprostol and oxytocin for the management of the third stage of labor, 597 patients were randomly allocated to receive oxytocin 10 units by intramuscular injection or misoprostol 400 µg or 600 µg by the oral route.2 Patient-reported shivering occurred in 13%, 19%, and 28% of patients receiving oxytocin, misoprostol 400 µg and misoprostol 800 µg, respectively. A recorded temperature >38° C occurred within 1 hour of medication administration in approximately 3%, 2%, and 7.5% of patients receiving oxytocin, misoprostol 400 µg, and misoprostol 800 µg, respectively. In another study, 453 patients scheduled for a cesarean birth were randomly allocated to receive 1 of 3 doses of rectal misoprostol 200 μg, 400 μg, or 600 μg before incision. Fever was detected in 2.6%, 9.9%, and 5.1% of the patients receiving misoprostol 200 μg, 400 μg, or 600 μg, respectively.3
References
1. Aronoff DM, Neilson EG. Antipyretics: mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111:304-315. doi: 10.1016/s0002-9343(01)00834-8.
2. Lumbiganon P, Hofmeyr J, Gumezoglu AM, et al. Misoprostol dose-related shivering and pyrexia in the third stage of labor. WHO Collaborative Trial of Misoprostol in the Management of the Third Stage of Labor. Br J Obstet Gynaecol. 1999;106:304-308. doi: 10.1111/j.1471-0528.1999.tb08266.x.
3. Sweed M, El-Said M, Abou-Gamrah AA, et al. Comparison between 200, 400 and 600 microgram rectal misoprostol before cesarean section: a randomized clinical trial. J Obstet Gynaecol Res. 2019;45:585-591. doi: 10.1111 /jog.13883.
- Cytotec [package insert]. Chicago, IL: GD Searle & Co. https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/19268slr037.pdf. Accessed June 20, 2022.
- Cervidil [package insert]. St Louis, MO: Forrest Pharmaceuticals Inc.; May 2006. Accessed June 20, 2022.
- Caverject [package insert]. New York, NY: Pfizer Inc.; March 2014. Accessed June 20, 2022.
- Collins PW. Misoprostol: discovery, development and clinical applications. Med Res Rev. 1990;10:149-172. doi: 10.1002/med.2610100202.
- Audit M, White KI, Breton B, et al. Crystal structure of misoprostol bound to the labor inducer prostaglandin E2 receptor. Nat Chem Biol. 2019;15:11-17. doi: 10.1038/s41589-018-0160-y.
- Pallliser KH, Hirst JJ, Ooi G, et al. Prostaglandin E and F receptor expression and myometrial sensitivity in labor onset in the sheep. Biol Reprod. 2005;72:937-943. doi: 10.1095/biolreprod.104.035311.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280. doi: 10.1016/s0029-7844(98)00436-0.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92. doi: 10.1016/S0029-7844(97)00111-7.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84. doi: 10.1093/humrep/deh005.
- Young DC, Delaney T, Armson BA, et al. Oral misoprostol, low dose vaginal misoprostol and vaginal dinoprostone for labor induction: randomized controlled trial. PLOS One. 2020;15:e0227245. doi: 10.1371/journal.pone.0227245.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590. doi: 10.1097/01.AOG.0000230398.32794.9d.
- el-Sahwi S, Gaafar AA, Toppozada HK. A new unit for evaluation of uterine activity. Am J Obstet Gynecol. 1967;98:900-903. doi: 10.1016/0002-9378(67)90074-9.
- Vukas N, Ekerhovd E, Abrahamsson G, et al. Cervical priming in the first trimester: morphological and biochemical effects of misoprostol and isosorbide mononitrate. Acta Obstet Gyecol. 2009;88:43-51. doi: 10.1080/00016340802585440.
- Aronsson A, Ulfgren AK, Stabi B, et al. The effect of orally and vaginally administered misoprostol on inflammatory mediators and cervical ripening during early pregnancy. Contraception. 2005;72:33-39. doi: 10.1016/j.contraception.2005.02.012.
- Denison FC, Riley SC, Elliott CL, et al. The effect of mifepristone administration on leukocyte populations, matrix metalloproteinases and inflammatory mediators in the first trimester cervix. Mol Hum Reprod. 2000;6:541-548. doi: 10.1093/molehr/6.6.541.
- ten Eikelder MLG, Rengerink KO, Jozwiak M, et al. Induction of labour at term with oral misoprostol versus a Foley catheter (PROBAAT-II): a multicentre randomised controlled non-inferiority trial. Lancet. 2016;387:1619-1628. doi: 10.1016 /S0140-6736(16)00084-2.
- Cytotec [package insert]. Chicago, IL: GD Searle & Co. https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/19268slr037.pdf. Accessed June 20, 2022.
- Cervidil [package insert]. St Louis, MO: Forrest Pharmaceuticals Inc.; May 2006. Accessed June 20, 2022.
- Caverject [package insert]. New York, NY: Pfizer Inc.; March 2014. Accessed June 20, 2022.
- Collins PW. Misoprostol: discovery, development and clinical applications. Med Res Rev. 1990;10:149-172. doi: 10.1002/med.2610100202.
- Audit M, White KI, Breton B, et al. Crystal structure of misoprostol bound to the labor inducer prostaglandin E2 receptor. Nat Chem Biol. 2019;15:11-17. doi: 10.1038/s41589-018-0160-y.
- Pallliser KH, Hirst JJ, Ooi G, et al. Prostaglandin E and F receptor expression and myometrial sensitivity in labor onset in the sheep. Biol Reprod. 2005;72:937-943. doi: 10.1095/biolreprod.104.035311.
- Gemzell-Danilesson K, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93:275-280. doi: 10.1016/s0029-7844(98)00436-0.
- Zieman M, Fong SK, Benowitz NL, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92. doi: 10.1016/S0029-7844(97)00111-7.
- Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81-84. doi: 10.1093/humrep/deh005.
- Young DC, Delaney T, Armson BA, et al. Oral misoprostol, low dose vaginal misoprostol and vaginal dinoprostone for labor induction: randomized controlled trial. PLOS One. 2020;15:e0227245. doi: 10.1371/journal.pone.0227245.
- Meckstroth KR, Whitaker AK, Bertisch S, et al. Misoprostol administered by epithelial routes. Drug absorption and uterine response. Obstet Gynecol. 2006;108:582-590. doi: 10.1097/01.AOG.0000230398.32794.9d.
- el-Sahwi S, Gaafar AA, Toppozada HK. A new unit for evaluation of uterine activity. Am J Obstet Gynecol. 1967;98:900-903. doi: 10.1016/0002-9378(67)90074-9.
- Vukas N, Ekerhovd E, Abrahamsson G, et al. Cervical priming in the first trimester: morphological and biochemical effects of misoprostol and isosorbide mononitrate. Acta Obstet Gyecol. 2009;88:43-51. doi: 10.1080/00016340802585440.
- Aronsson A, Ulfgren AK, Stabi B, et al. The effect of orally and vaginally administered misoprostol on inflammatory mediators and cervical ripening during early pregnancy. Contraception. 2005;72:33-39. doi: 10.1016/j.contraception.2005.02.012.
- Denison FC, Riley SC, Elliott CL, et al. The effect of mifepristone administration on leukocyte populations, matrix metalloproteinases and inflammatory mediators in the first trimester cervix. Mol Hum Reprod. 2000;6:541-548. doi: 10.1093/molehr/6.6.541.
- ten Eikelder MLG, Rengerink KO, Jozwiak M, et al. Induction of labour at term with oral misoprostol versus a Foley catheter (PROBAAT-II): a multicentre randomised controlled non-inferiority trial. Lancet. 2016;387:1619-1628. doi: 10.1016 /S0140-6736(16)00084-2.
Appropriate antibiotic selection for 12 common infections in obstetric patients
For the infections we most commonly encounter in obstetric practice, I review in this article the selection of specific antibiotics. I focus on the key pathogens that cause these infections, the most useful diagnostic tests, and the most cost-effective antibiotic therapy. Relative cost estimates (high vs low) for drugs are based on information published on the GoodRx website (https://www.goodrx.com/). Actual charges to patients, of course, may vary widely depending on contractual relationships between hospitals, insurance companies, and wholesale vendors. The infections are listed in alphabetical order, not in order of frequency or severity.
1. Bacterial vaginosis
Bacterial vaginosis (BV) is a polymicrobial infection that results from perturbation of the normal vaginal flora due to conditions such as pregnancy, hormonal therapy, and changes in the menstrual cycle. It is characterized by a decrease in the vaginal concentration of Lactobacillus crispatus, followed by an increase in Prevotella bivia, Gardnerella vaginalis, Mobiluncus species, Atopobium vaginae, and Megasphaera type 1.1,2
BV is characterized by a thin, white-gray malodorous (fishlike smell) discharge. The vaginal pH is >4.5. Clue cells are apparent on saline microscopy, and the whiff (amine) test is positive when potassium hydroxide is added to a drop of vaginal secretions. Diagnostic accuracy can be improved using one of the new vaginal panel assays such as BD MAX Vaginal Panel (Becton, Dickinson and Company).3
Antibiotic selection
Antibiotic treatment of BV is directed primarily at the anaerobic component of the infection. The preferred treatment is oral metronidazole 500 mg twice daily for 7 days. If the patient cannot tolerate metronidazole, oral clindamycin 300 mg twice daily for 7 days, can be used, although it is more expensive than metronidazole. Topical metronidazole vaginal gel (0.75%), 1 applicatorful daily for 5 days, is effective in treating the local vaginal infection, but it is not effective in preventing systemic complications such as preterm labor, chorioamnionitis, and puerperal endometritis.2 It also is significantly more expensive than the oral formulation of metronidazole. Topical clindamycin cream, 1 applicatorful daily for 5 days, is even more expensive.
Tinidazole 2 g orally daily for 2 days is an effective alternative to oral metronidazole. Single-dose therapy with oral secnidazole (2 g), a 5-nitroimidazole with a longer half-life than metronidazole, has been effective in small studies, but experience with this drug in the United States is limited. Secnidazole is also very expensive.4
2. Candidiasis
Vulvovaginal candidiasis usually is caused by Candida albicans. Other less common species include C tropicalis, C glabrata, C auris, C lusitaniae, and C krusei. The most common clinical findings are vulvovaginal pruritus in association with a curdlike white vaginal discharge. The diagnosis can be established by confirmation of a normal vaginal pH and identification of budding yeast and hyphae on a potassium hydroxide preparation. As noted above for BV, the vaginal panel assay improves the accuracy of clinical diagnosis.3 Culture usually is indicated only in patients with infections that are refractory to therapy.
Continue to: Antibiotic selection...
Antibiotic selection
In the first trimester of pregnancy, vulvovaginal candidiasis should be treated with a topical medication such as clotrimazole cream 1% (50 mg intravaginally daily for 7 days), miconazole cream 2% (100 mg intravaginally daily for 7 days), or terconazole cream 0.4% (50 g intravaginally daily for 7 days). Single-dose formulations or 3-day courses of treatment may not be quite as effective in pregnant patients, but they do offer a more convenient dosing schedule.2,5
Oral fluconazole should not be used in the first trimester of pregnancy because it has been associated with an increased risk for spontaneous abortion and with fetal cardiac septal defects. Beyond the first trimester, oral fluconazole offers an attractive option for treatment of vulvovaginal candidiasis. The appropriate dose is 150 mg initially, with a repeat dose in 3 days if symptoms persist.2,5
Ibrexafungerp (300 mg twice daily for 1 day) was recently approved by the US Food and Drug Administration (FDA) for oral treatment of vulvovaginal candidiasis. However, this drug is teratogenic and is contraindicated during pregnancy and lactation. It also is significantly more expensive than fluconazole.6
3. Cesarean delivery prophylaxis
All women having a cesarean delivery (CD) should receive antibiotic prophylaxis to reduce the risk of endometritis and wound infection.
Antibiotic selection
In my opinion, the preferred regimen is intravenous cefazolin 2 g plus azithromycin 500 mg administered preoperatively.7 Cefazolin can be administered in a rapid bolus; azithromycin should be administered over 1 hour.
In an exceptionally rigorous investigation called the C/SOAP trial (Cesarean Section Optimal Antibiotic Prophylaxis trial), Tita and colleagues showed that the combination of cefazolin plus azithromycin was superior to single-agent prophylaxis (usually with cefazolin) in preventing the composite of endometritis, wound infection, or other infection occurring within 6 weeks of surgery.8 The additive effect of azithromycin was particularly pronounced in patients having CD after labor and rupture of membranes. Harper and associates subsequently validated the cost-effectiveness of this combination regimen using a decision analytic model.9
If the patient has a serious allergy to β-lactam antibiotics, the best alternative regimen for prophylaxis is clindamycin plus gentamicin. The appropriate single intravenous dose of clindamycin is 900 mg; the single dose of gentamicin should be 5 mg/kg of ideal body weight (IBW).7
4. Chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium. In pregnant women, it typically causes urethritis, endocervicitis, and inflammatory proctitis. Along with gonorrhea, it is the cause of an unusual infection/inflammation of the liver capsule, termed Fitz-Hugh-Curtis syndrome (perihepatitis). The diagnosis of chlamydia infection is best confirmed with a nucleic acid amplification test (NAAT). The NAAT simultaneously tests for chlamydia and gonorrhea in urine or in secretions obtained from the urethra, endocervix, and rectum.2
Antibiotic selection
The drug of choice for treating chlamydia in pregnancy is azithromycin 1,000 mg orally in a single dose. Erythromycin can be used as an alternative to azithromycin, but it usually is not well tolerated because of gastrointestinal adverse effects. In my practice, the preferred alternative for a patient who cannot tolerate azithromycin is amoxicillin 500 mg orally 3 times daily for 7 days.2,10
Continue to: 5. Chorioamnionitis...
5. Chorioamnionitis
Chorioamnionitis is a polymicrobial infection caused by anaerobes, aerobic gram-negative bacilli (predominantly Escherichia coli), and aerobic gram-positive cocci (primarily group B streptococci [GBS]). The diagnosis usually is made based on clinical examination: maternal fever, maternal and fetal tachycardia, and no other localizing sign of infection. The diagnosis can be confirmed by obtaining a sample of amniotic fluid via amniocentesis or via aspiration through the intrauterine pressure catheter and demonstrating a positive Gram stain, low glucose concentration (<20 mg/dL), positive nitrites, positive leukocyte esterase, and ultimately, a positive bacteriologic culture.2
Antibiotic selection
The initial treatment of chorioamnionitis specifically targets the 2 major organisms that cause neonatal pneumonia, meningitis, and sepsis: GBS and E coli. For many years, the drugs of choice have been intravenous ampicillin (2 g every 6 hours) plus intravenous gentamicin (5 mg/kg of IBW every 24 hours). Gentamicin also can be administered intravenously at a dose of 1.5 mg/kg every 8 hours. I prefer the once-daily dosing for 3 reasons:
- Gentamicin works by a concentration-dependent mechanism; the higher the initial serum concentration, the better the killing effect.
- Once-daily dosing preserves long periods with low trough levels, an effect that minimizes ototoxicity and nephrotoxicity.
- Once-daily dosing is more convenient.
In a patient who has a contraindication to use of an aminoglycoside, aztreonam (2 g intravenously every 8 hours) may be combined with ampicillin.2
If the patient delivers vaginally, 1 dose of each drug should be administered postpartum, and then the antibiotics should be discontinued. If the patient delivers by cesarean, a single dose of a medication with strong anaerobic coverage should be administered immediately after the infant’s umbilical cord is clamped. Options include clindamycin (900 mg intravenously) or metronidazole (500 mg intravenously).11
There are 2 key exceptions to the single postpartum dose rule, however. If the patient is obese (body mass index [BMI] >30 kg/m2) or if the membranes have been ruptured for more than 24 hours, antibiotics should be continued until she has been afebrile and asymptomatic for 24 hours.12
Two single agents are excellent alternatives to the combination ampicillin-gentamicin regimen. One is ampicillin-sulbactam, 3 g intravenously every 6 hours. The other is piperacillin-tazobactam, 3.375 g intravenously every 6 hours. These extended-spectrum penicillins provide exceptionally good coverage against the major pathogens that cause chorioamnionitis. Although more expensive than the combination regimen, they avoid the potential ototoxicity and nephrotoxicity associated with gentamicin.2
6. Endometritis
Puerperal endometritis is significantly more common after CD than after vaginal delivery. The infection is polymicrobial, and the principal pathogens are anaerobic gram-positive cocci, anaerobic gram-negative bacilli, aerobic gram-negative bacilli, and aerobic gram-positive cocci. The diagnosis usually is made almost exclusively based on clinical findings: fever within 24 to 36 hours of delivery, tachycardia, mild tachypnea, and lower abdominal/pelvic pain and tenderness in the absence of any other localizing sign of infection.13
Antibiotic selection
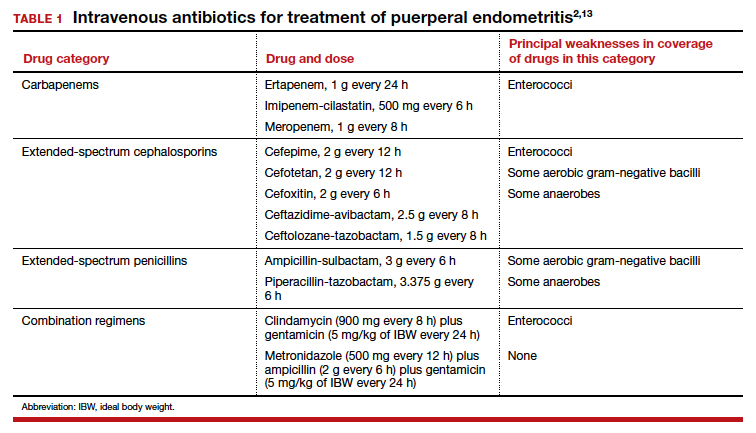
Effective treatment of endometritis requires administration of antibiotics that provide coverage against the broad range of pelvic pathogens. For many years, the gold standard of treatment has been the combination regimens of clindamycin plus gentamicin or metronidazole plus ampicillin plus gentamicin. These drugs are available in generic form and are relatively inexpensive. However, several broad-spectrum single agents are now available for treatment of endometritis. Although they are moderately more expensive than the generic combination regimens, they usually are very well tolerated, and they avoid the potential nephrotoxicity and ototoxicity associated with gentamicin. TABLE 1 summarizes the dosing regimens of these various agents and their potential weaknesses in coverage.2,13
7. Gonorrhea
Gonorrhea is caused by the gram-negative diplococcus, Neisseria gonorrhoeae. The organism has a propensity to infect columnar epithelium and uroepithelium, and, typically, it causes a localized infection of the urethra, endocervix, and rectum. The organism also can cause an oropharyngeal infection, a disseminated infection (most commonly manifested by dermatitis and arthritis), and perihepatitis.
The diagnosis is best confirmed by a NAAT that can simultaneously test for gonorrhea and chlamydia in urine or in secretions obtained from the urethra, endocervix, and rectum.2,10
Antibiotic selection
The drugs of choice for treating uncomplicated gonococcal infection in pregnancy are a single dose of ceftriaxone 500 mg intramuscularly, or cefixime 800 mg orally. If the patient is allergic to β-lactam antibiotics, the recommended treatment is gentamicin 240 mg intramuscularly in a single dose, combined with azithromycin 2,000 mg orally.14
8. Group B streptococci prophylaxis
The first-line agents for GBS prophylaxis are penicillin and ampicillin. Resistance of GBS to either of these antibiotics is extremely rare. The appropriate penicillin dose is 3 million U intravenously every 4 hours; the intravenous dose of ampicillin is 2 g initially, then 1 g every 4 hours. I prefer penicillin for prophylaxis because it has a narrower spectrum of activity and is less likely to cause antibiotic-associated diarrhea. The antibiotic should be continued until delivery of the neonate.2,15,16
If the patient has a mild allergy to penicillin, the drug of choice is cefazolin 2 g intravenously initially, then 1 g every 8 hours. If the patient’s allergy to β-lactam antibiotics is severe, the alternative agents are vancomycin (20 mg/kg intravenously every 8 hours infused over 1–2 hours; maximum single dose of 2 g) and clindamycin (900 mg intravenously every 8 hours). The latter drug should be used only if sensitivity testing has confirmed that the GBS strain is sensitive to clindamycin. Resistance to clindamycin usually ranges from 10% to 15%.2,15,16
9. Puerperal mastitis
The principal microorganisms that cause puerperal mastitis are the aerobic streptococci and staphylococci that form part of the normal skin flora. The diagnosis usually is made based on the characteristic clinical findings: erythema, tenderness, and warmth in an area of the breast accompanied by a purulent nipple discharge and fever and chills. The vast majority of cases can be treated with oral antibiotics on an outpatient basis. The key indications for hospitalization are severe illness, particularly in an immunocompromised patient, and suspicion of a breast abscess.2
Continue to: Antibiotic selection...
Antibiotic selection
The initial drug of choice for treatment of mastitis is dicloxacillin sodium 500 mg every 6 hours for 7 to 10 days. If the patient has a mild allergy to penicillin, the appropriate alternative is cephalexin 500 mg every 8 hours for 7 to 10 days. If the patient’s allergy to penicillin is severe, 2 alternatives are possible. One is clindamycin 300 mg twice daily for 7 to 10 days; the other is trimethoprim-sulfamethoxazole double strength (800 mg/160 mg), twice daily for 7 to 10 days. The latter 2 drugs are also of great value if the patient fails to respond to initial therapy and/or infection with methicillin-resistant Staphylococcus aureus (MRSA) is suspected.2 I prefer the latter agent because it is less expensive than clindamycin and is less likely to cause antibiotic-induced diarrhea.
If hospitalization is required, the drug of choice is intravenous vancomycin. The appropriate dosage is 20 mg/kg every 8 to 12 hours (maximum single dose of 2 g).2
10. Syphilis
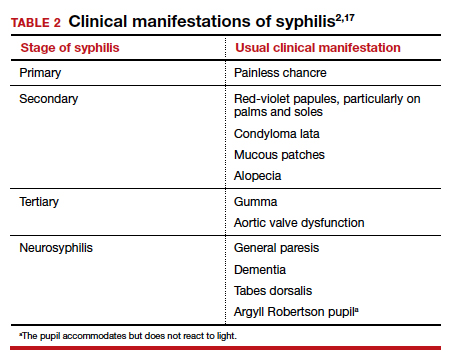
Syphilis is caused by the spirochete bacterium, Treponema pallidum. The diagnosis can be made by clinical examination if the characteristic findings listed in TABLE 2 are present.2,17 However, most patients in our practice will have latent syphilis, and the diagnosis must be established based on serologic screening.17
Antibiotic selection
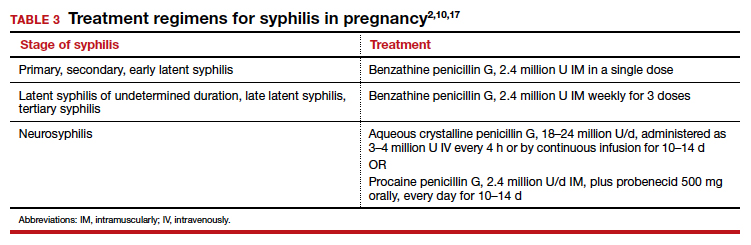
In pregnancy, the treatment of choice for syphilis is penicillin (TABLE 3).2,10,17 Only penicillin has been proven effective in treating both maternal and fetal infection. If the patient has a history of allergy to penicillin, she should undergo skin testing to determine if she is truly allergic. If hypersensitivity is confirmed, the patient should be desensitized and then treated with the appropriate regimen outlined in TABLE 3. Of interest, within a short period of time after treatment, the patient’s sensitivity to penicillin will be reestablished, and she should not be treated again with penicillin unless she undergoes another desensitization process.2,17
11. Trichomoniasis
Trichomoniasis is caused by the flagellated protozoan, Trichomonas vaginalis. The condition is characterized by a distinct yellowish-green vaginal discharge. The vaginal pH is >4.5, and motile flagellated organisms are easily visualized on saline microscopy. The vaginal panel assay also is a valuable diagnostic test.3
Antibiotic selection
The drug of choice for trichomoniasis is oral metronidazole 500 mg twice daily for 7 days. The patient’s sexual partner(s) should be treated concurrently to prevent reinfection. Most treatment failures are due to poor compliance with therapy on the part of either the patient or her partner(s); true drug resistance is uncommon. When antibiotic resistance is strongly suspected, the patient may be treated with a single 2-g oral dose of tinidazole.2
12. Urinary tract infections
Urethritis
Acute urethritis usually is caused by C trachomatis or N gonorrhoeae. The treatment of infections with these 2 organisms is discussed above.
Asymptomatic bacteriuria and acute cystitis
Bladder infections are caused primarily by E coli, Klebsiella pneumoniae, and Proteus species. Gram-positive cocci such as enterococci, Staphylococcus saprophyticus, and GBS are less common pathogens.18
The key diagnostic criterion for asymptomatic bacteriuria is a colony count greater than 100,000 organisms/mL of a single uropathogen on a clean-catch midstream urine specimen.18
The usual clinical manifestations of acute cystitis include frequency, urgency, hesitancy, suprapubic discomfort, and a low-grade fever. The diagnosis is most effectively confirmed by obtaining urine by catheterization and demonstrating a positive nitrite and positive leukocyte esterase reaction on dipstick examination. The finding of a urine pH of 8 or greater usually indicates an infection caused by Proteus species. When urine is obtained by catheterization, the criterion for defining a positive culture is greater than 100 colonies/mL.18
Antibiotic selection. In the first trimester, the preferred agents for treatment of a lower urinary tract infection are oral amoxicillin (875 mg twice daily) or cephalexin (500 mg every 8 hours). For an initial infection, a 3-day course of therapy usually is adequate. For a recurrent infection, a 7- to 10-day course is indicated.
Beyond the first trimester, nitrofurantoin monohydrate macrocrystals (100 mg orally twice daily) or trimethoprim-sulfamethoxazole double strength (800 mg/160 mg twice daily) are the preferred agents. Unless no other oral drug is likely to be effective, these 2 drugs should be avoided in the first trimester. The former has been associated with eye, heart, and cleft defects. The latter has been associated with neural tube defects, cardiac anomalies, choanal atresia, and diaphragmatic hernia.18
Acute pyelonephritis
Acute infections of the kidney usually are caused by the aerobic gram-negative bacilli: E coli, K pneumoniae, and Proteus species. Enterococci, S saprophyticus, and GBS are less likely to cause upper tract infection as opposed to bladder infection.
The typical clinical manifestations of acute pyelonephritis include high fever and chills in association with flank pain and tenderness. The diagnosis is best confirmed by obtaining urine by catheterization and documenting the presence of a positive nitrite and leukocyte esterase reaction. Again, an elevated urine pH is indicative of an infection secondary to Proteus species. The criterion for defining a positive culture from catheterized urine is greater than 100 colonies/mL.2,18
Antibiotic selection. Patients in the first half of pregnancy who are hemodynamically stable and who show no signs of preterm labor may be treated with oral antibiotics as outpatients. The 2 drugs of choice are amoxicillin-clavulanate (875 mg twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength (800 mg/160 mg twice daily for 7 to 10 days).
For unstable patients in the first half of pregnancy and for essentially all patients in the second half of pregnancy, parenteral treatment should be administered on an inpatient basis. My preference for treatment is ceftriaxone, 2 g intravenously every 24 hours. The drug provides excellent coverage against almost all the uropathogens. It has a convenient dosing schedule, and it usually is very well tolerated. Parenteral therapy should be continued until the patient has been afebrile and asymptomatic for 24 to 48 hours. At this point, the patient can be transitioned to one of the oral regimens listed above and managed as an outpatient. If the patient is allergic to β-lactam antibiotics, an excellent alternative is aztreonam, 2 g intravenously every 8 hours.2,18 ●
- Reeder CF, Duff P. A case of BV during pregnancy: best management approach. OBG Manag. 2021;33(2):38-42.
- Duff P. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies, 8th ed. Elsevier; 2021:1124-1145.
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859.
- Hiller SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Kirkpatrick K, Duff P. Candidiasis: the essentials of diagnosis and treatment. OBG Manag. 2020;32(8):27-29, 34.
- Ibrexafungerp (Brexafemme) for vulvovaginal candidiasis. Med Lett Drugs Ther. 2021;63:141-143.
- Duff P. Prevention of infection after cesarean delivery. Clin Obstet Gynecol. 2019;62:758-770.
- Tita AT, Szychowski JM, Boggess K, et al; for the C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64(RR3):1-137.
- Edwards RK, Duff P. Single additional dose postpartum therapy for women with chorioamnionitis. Obstet Gynecol. 2003;102(5 pt 1):957-961.
- Black LP, Hinson L, Duff P. Limited course of antibiotic treatment for chorioamnionitis. Obstet Gynecol. 2012;119:1102-1105.
- Duff P. Fever following cesarean delivery: what are your steps for management? OBG Manag. 2021;33(12):26-30, 35.
- St Cyr S, Barbee L, Warkowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.
- Prevention of group B streptococcal early-onset disease in newborns: ACOG committee opinion summary, number 782. Obstet Gynecol. 2019;134:1.
- Duff P. Preventing early-onset group B streptococcal disease in newborns. OBG Manag. 2019;31(12):26, 28-31.
- Finley TA, Duff P. Syphilis: cutting risk through primary prevention and prenatal screening. OBG Manag. 2020;32(11):20, 22-27.
- Duff P. UTIs in pregnancy: managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis. OBG Manag. 2022;34(1):42-46.
For the infections we most commonly encounter in obstetric practice, I review in this article the selection of specific antibiotics. I focus on the key pathogens that cause these infections, the most useful diagnostic tests, and the most cost-effective antibiotic therapy. Relative cost estimates (high vs low) for drugs are based on information published on the GoodRx website (https://www.goodrx.com/). Actual charges to patients, of course, may vary widely depending on contractual relationships between hospitals, insurance companies, and wholesale vendors. The infections are listed in alphabetical order, not in order of frequency or severity.
1. Bacterial vaginosis
Bacterial vaginosis (BV) is a polymicrobial infection that results from perturbation of the normal vaginal flora due to conditions such as pregnancy, hormonal therapy, and changes in the menstrual cycle. It is characterized by a decrease in the vaginal concentration of Lactobacillus crispatus, followed by an increase in Prevotella bivia, Gardnerella vaginalis, Mobiluncus species, Atopobium vaginae, and Megasphaera type 1.1,2
BV is characterized by a thin, white-gray malodorous (fishlike smell) discharge. The vaginal pH is >4.5. Clue cells are apparent on saline microscopy, and the whiff (amine) test is positive when potassium hydroxide is added to a drop of vaginal secretions. Diagnostic accuracy can be improved using one of the new vaginal panel assays such as BD MAX Vaginal Panel (Becton, Dickinson and Company).3
Antibiotic selection
Antibiotic treatment of BV is directed primarily at the anaerobic component of the infection. The preferred treatment is oral metronidazole 500 mg twice daily for 7 days. If the patient cannot tolerate metronidazole, oral clindamycin 300 mg twice daily for 7 days, can be used, although it is more expensive than metronidazole. Topical metronidazole vaginal gel (0.75%), 1 applicatorful daily for 5 days, is effective in treating the local vaginal infection, but it is not effective in preventing systemic complications such as preterm labor, chorioamnionitis, and puerperal endometritis.2 It also is significantly more expensive than the oral formulation of metronidazole. Topical clindamycin cream, 1 applicatorful daily for 5 days, is even more expensive.
Tinidazole 2 g orally daily for 2 days is an effective alternative to oral metronidazole. Single-dose therapy with oral secnidazole (2 g), a 5-nitroimidazole with a longer half-life than metronidazole, has been effective in small studies, but experience with this drug in the United States is limited. Secnidazole is also very expensive.4
2. Candidiasis
Vulvovaginal candidiasis usually is caused by Candida albicans. Other less common species include C tropicalis, C glabrata, C auris, C lusitaniae, and C krusei. The most common clinical findings are vulvovaginal pruritus in association with a curdlike white vaginal discharge. The diagnosis can be established by confirmation of a normal vaginal pH and identification of budding yeast and hyphae on a potassium hydroxide preparation. As noted above for BV, the vaginal panel assay improves the accuracy of clinical diagnosis.3 Culture usually is indicated only in patients with infections that are refractory to therapy.
Continue to: Antibiotic selection...
Antibiotic selection
In the first trimester of pregnancy, vulvovaginal candidiasis should be treated with a topical medication such as clotrimazole cream 1% (50 mg intravaginally daily for 7 days), miconazole cream 2% (100 mg intravaginally daily for 7 days), or terconazole cream 0.4% (50 g intravaginally daily for 7 days). Single-dose formulations or 3-day courses of treatment may not be quite as effective in pregnant patients, but they do offer a more convenient dosing schedule.2,5
Oral fluconazole should not be used in the first trimester of pregnancy because it has been associated with an increased risk for spontaneous abortion and with fetal cardiac septal defects. Beyond the first trimester, oral fluconazole offers an attractive option for treatment of vulvovaginal candidiasis. The appropriate dose is 150 mg initially, with a repeat dose in 3 days if symptoms persist.2,5
Ibrexafungerp (300 mg twice daily for 1 day) was recently approved by the US Food and Drug Administration (FDA) for oral treatment of vulvovaginal candidiasis. However, this drug is teratogenic and is contraindicated during pregnancy and lactation. It also is significantly more expensive than fluconazole.6
3. Cesarean delivery prophylaxis
All women having a cesarean delivery (CD) should receive antibiotic prophylaxis to reduce the risk of endometritis and wound infection.
Antibiotic selection
In my opinion, the preferred regimen is intravenous cefazolin 2 g plus azithromycin 500 mg administered preoperatively.7 Cefazolin can be administered in a rapid bolus; azithromycin should be administered over 1 hour.
In an exceptionally rigorous investigation called the C/SOAP trial (Cesarean Section Optimal Antibiotic Prophylaxis trial), Tita and colleagues showed that the combination of cefazolin plus azithromycin was superior to single-agent prophylaxis (usually with cefazolin) in preventing the composite of endometritis, wound infection, or other infection occurring within 6 weeks of surgery.8 The additive effect of azithromycin was particularly pronounced in patients having CD after labor and rupture of membranes. Harper and associates subsequently validated the cost-effectiveness of this combination regimen using a decision analytic model.9
If the patient has a serious allergy to β-lactam antibiotics, the best alternative regimen for prophylaxis is clindamycin plus gentamicin. The appropriate single intravenous dose of clindamycin is 900 mg; the single dose of gentamicin should be 5 mg/kg of ideal body weight (IBW).7
4. Chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium. In pregnant women, it typically causes urethritis, endocervicitis, and inflammatory proctitis. Along with gonorrhea, it is the cause of an unusual infection/inflammation of the liver capsule, termed Fitz-Hugh-Curtis syndrome (perihepatitis). The diagnosis of chlamydia infection is best confirmed with a nucleic acid amplification test (NAAT). The NAAT simultaneously tests for chlamydia and gonorrhea in urine or in secretions obtained from the urethra, endocervix, and rectum.2
Antibiotic selection
The drug of choice for treating chlamydia in pregnancy is azithromycin 1,000 mg orally in a single dose. Erythromycin can be used as an alternative to azithromycin, but it usually is not well tolerated because of gastrointestinal adverse effects. In my practice, the preferred alternative for a patient who cannot tolerate azithromycin is amoxicillin 500 mg orally 3 times daily for 7 days.2,10
Continue to: 5. Chorioamnionitis...
5. Chorioamnionitis
Chorioamnionitis is a polymicrobial infection caused by anaerobes, aerobic gram-negative bacilli (predominantly Escherichia coli), and aerobic gram-positive cocci (primarily group B streptococci [GBS]). The diagnosis usually is made based on clinical examination: maternal fever, maternal and fetal tachycardia, and no other localizing sign of infection. The diagnosis can be confirmed by obtaining a sample of amniotic fluid via amniocentesis or via aspiration through the intrauterine pressure catheter and demonstrating a positive Gram stain, low glucose concentration (<20 mg/dL), positive nitrites, positive leukocyte esterase, and ultimately, a positive bacteriologic culture.2
Antibiotic selection
The initial treatment of chorioamnionitis specifically targets the 2 major organisms that cause neonatal pneumonia, meningitis, and sepsis: GBS and E coli. For many years, the drugs of choice have been intravenous ampicillin (2 g every 6 hours) plus intravenous gentamicin (5 mg/kg of IBW every 24 hours). Gentamicin also can be administered intravenously at a dose of 1.5 mg/kg every 8 hours. I prefer the once-daily dosing for 3 reasons:
- Gentamicin works by a concentration-dependent mechanism; the higher the initial serum concentration, the better the killing effect.
- Once-daily dosing preserves long periods with low trough levels, an effect that minimizes ototoxicity and nephrotoxicity.
- Once-daily dosing is more convenient.
In a patient who has a contraindication to use of an aminoglycoside, aztreonam (2 g intravenously every 8 hours) may be combined with ampicillin.2
If the patient delivers vaginally, 1 dose of each drug should be administered postpartum, and then the antibiotics should be discontinued. If the patient delivers by cesarean, a single dose of a medication with strong anaerobic coverage should be administered immediately after the infant’s umbilical cord is clamped. Options include clindamycin (900 mg intravenously) or metronidazole (500 mg intravenously).11
There are 2 key exceptions to the single postpartum dose rule, however. If the patient is obese (body mass index [BMI] >30 kg/m2) or if the membranes have been ruptured for more than 24 hours, antibiotics should be continued until she has been afebrile and asymptomatic for 24 hours.12
Two single agents are excellent alternatives to the combination ampicillin-gentamicin regimen. One is ampicillin-sulbactam, 3 g intravenously every 6 hours. The other is piperacillin-tazobactam, 3.375 g intravenously every 6 hours. These extended-spectrum penicillins provide exceptionally good coverage against the major pathogens that cause chorioamnionitis. Although more expensive than the combination regimen, they avoid the potential ototoxicity and nephrotoxicity associated with gentamicin.2
6. Endometritis
Puerperal endometritis is significantly more common after CD than after vaginal delivery. The infection is polymicrobial, and the principal pathogens are anaerobic gram-positive cocci, anaerobic gram-negative bacilli, aerobic gram-negative bacilli, and aerobic gram-positive cocci. The diagnosis usually is made almost exclusively based on clinical findings: fever within 24 to 36 hours of delivery, tachycardia, mild tachypnea, and lower abdominal/pelvic pain and tenderness in the absence of any other localizing sign of infection.13
Antibiotic selection
Effective treatment of endometritis requires administration of antibiotics that provide coverage against the broad range of pelvic pathogens. For many years, the gold standard of treatment has been the combination regimens of clindamycin plus gentamicin or metronidazole plus ampicillin plus gentamicin. These drugs are available in generic form and are relatively inexpensive. However, several broad-spectrum single agents are now available for treatment of endometritis. Although they are moderately more expensive than the generic combination regimens, they usually are very well tolerated, and they avoid the potential nephrotoxicity and ototoxicity associated with gentamicin. TABLE 1 summarizes the dosing regimens of these various agents and their potential weaknesses in coverage.2,13

7. Gonorrhea
Gonorrhea is caused by the gram-negative diplococcus, Neisseria gonorrhoeae. The organism has a propensity to infect columnar epithelium and uroepithelium, and, typically, it causes a localized infection of the urethra, endocervix, and rectum. The organism also can cause an oropharyngeal infection, a disseminated infection (most commonly manifested by dermatitis and arthritis), and perihepatitis.
The diagnosis is best confirmed by a NAAT that can simultaneously test for gonorrhea and chlamydia in urine or in secretions obtained from the urethra, endocervix, and rectum.2,10
Antibiotic selection
The drugs of choice for treating uncomplicated gonococcal infection in pregnancy are a single dose of ceftriaxone 500 mg intramuscularly, or cefixime 800 mg orally. If the patient is allergic to β-lactam antibiotics, the recommended treatment is gentamicin 240 mg intramuscularly in a single dose, combined with azithromycin 2,000 mg orally.14
8. Group B streptococci prophylaxis
The first-line agents for GBS prophylaxis are penicillin and ampicillin. Resistance of GBS to either of these antibiotics is extremely rare. The appropriate penicillin dose is 3 million U intravenously every 4 hours; the intravenous dose of ampicillin is 2 g initially, then 1 g every 4 hours. I prefer penicillin for prophylaxis because it has a narrower spectrum of activity and is less likely to cause antibiotic-associated diarrhea. The antibiotic should be continued until delivery of the neonate.2,15,16
If the patient has a mild allergy to penicillin, the drug of choice is cefazolin 2 g intravenously initially, then 1 g every 8 hours. If the patient’s allergy to β-lactam antibiotics is severe, the alternative agents are vancomycin (20 mg/kg intravenously every 8 hours infused over 1–2 hours; maximum single dose of 2 g) and clindamycin (900 mg intravenously every 8 hours). The latter drug should be used only if sensitivity testing has confirmed that the GBS strain is sensitive to clindamycin. Resistance to clindamycin usually ranges from 10% to 15%.2,15,16
9. Puerperal mastitis
The principal microorganisms that cause puerperal mastitis are the aerobic streptococci and staphylococci that form part of the normal skin flora. The diagnosis usually is made based on the characteristic clinical findings: erythema, tenderness, and warmth in an area of the breast accompanied by a purulent nipple discharge and fever and chills. The vast majority of cases can be treated with oral antibiotics on an outpatient basis. The key indications for hospitalization are severe illness, particularly in an immunocompromised patient, and suspicion of a breast abscess.2
Continue to: Antibiotic selection...
Antibiotic selection
The initial drug of choice for treatment of mastitis is dicloxacillin sodium 500 mg every 6 hours for 7 to 10 days. If the patient has a mild allergy to penicillin, the appropriate alternative is cephalexin 500 mg every 8 hours for 7 to 10 days. If the patient’s allergy to penicillin is severe, 2 alternatives are possible. One is clindamycin 300 mg twice daily for 7 to 10 days; the other is trimethoprim-sulfamethoxazole double strength (800 mg/160 mg), twice daily for 7 to 10 days. The latter 2 drugs are also of great value if the patient fails to respond to initial therapy and/or infection with methicillin-resistant Staphylococcus aureus (MRSA) is suspected.2 I prefer the latter agent because it is less expensive than clindamycin and is less likely to cause antibiotic-induced diarrhea.
If hospitalization is required, the drug of choice is intravenous vancomycin. The appropriate dosage is 20 mg/kg every 8 to 12 hours (maximum single dose of 2 g).2
10. Syphilis
Syphilis is caused by the spirochete bacterium, Treponema pallidum. The diagnosis can be made by clinical examination if the characteristic findings listed in TABLE 2 are present.2,17 However, most patients in our practice will have latent syphilis, and the diagnosis must be established based on serologic screening.17

Antibiotic selection
In pregnancy, the treatment of choice for syphilis is penicillin (TABLE 3).2,10,17 Only penicillin has been proven effective in treating both maternal and fetal infection. If the patient has a history of allergy to penicillin, she should undergo skin testing to determine if she is truly allergic. If hypersensitivity is confirmed, the patient should be desensitized and then treated with the appropriate regimen outlined in TABLE 3. Of interest, within a short period of time after treatment, the patient’s sensitivity to penicillin will be reestablished, and she should not be treated again with penicillin unless she undergoes another desensitization process.2,17

11. Trichomoniasis
Trichomoniasis is caused by the flagellated protozoan, Trichomonas vaginalis. The condition is characterized by a distinct yellowish-green vaginal discharge. The vaginal pH is >4.5, and motile flagellated organisms are easily visualized on saline microscopy. The vaginal panel assay also is a valuable diagnostic test.3
Antibiotic selection
The drug of choice for trichomoniasis is oral metronidazole 500 mg twice daily for 7 days. The patient’s sexual partner(s) should be treated concurrently to prevent reinfection. Most treatment failures are due to poor compliance with therapy on the part of either the patient or her partner(s); true drug resistance is uncommon. When antibiotic resistance is strongly suspected, the patient may be treated with a single 2-g oral dose of tinidazole.2
12. Urinary tract infections
Urethritis
Acute urethritis usually is caused by C trachomatis or N gonorrhoeae. The treatment of infections with these 2 organisms is discussed above.
Asymptomatic bacteriuria and acute cystitis
Bladder infections are caused primarily by E coli, Klebsiella pneumoniae, and Proteus species. Gram-positive cocci such as enterococci, Staphylococcus saprophyticus, and GBS are less common pathogens.18
The key diagnostic criterion for asymptomatic bacteriuria is a colony count greater than 100,000 organisms/mL of a single uropathogen on a clean-catch midstream urine specimen.18
The usual clinical manifestations of acute cystitis include frequency, urgency, hesitancy, suprapubic discomfort, and a low-grade fever. The diagnosis is most effectively confirmed by obtaining urine by catheterization and demonstrating a positive nitrite and positive leukocyte esterase reaction on dipstick examination. The finding of a urine pH of 8 or greater usually indicates an infection caused by Proteus species. When urine is obtained by catheterization, the criterion for defining a positive culture is greater than 100 colonies/mL.18
Antibiotic selection. In the first trimester, the preferred agents for treatment of a lower urinary tract infection are oral amoxicillin (875 mg twice daily) or cephalexin (500 mg every 8 hours). For an initial infection, a 3-day course of therapy usually is adequate. For a recurrent infection, a 7- to 10-day course is indicated.
Beyond the first trimester, nitrofurantoin monohydrate macrocrystals (100 mg orally twice daily) or trimethoprim-sulfamethoxazole double strength (800 mg/160 mg twice daily) are the preferred agents. Unless no other oral drug is likely to be effective, these 2 drugs should be avoided in the first trimester. The former has been associated with eye, heart, and cleft defects. The latter has been associated with neural tube defects, cardiac anomalies, choanal atresia, and diaphragmatic hernia.18
Acute pyelonephritis
Acute infections of the kidney usually are caused by the aerobic gram-negative bacilli: E coli, K pneumoniae, and Proteus species. Enterococci, S saprophyticus, and GBS are less likely to cause upper tract infection as opposed to bladder infection.
The typical clinical manifestations of acute pyelonephritis include high fever and chills in association with flank pain and tenderness. The diagnosis is best confirmed by obtaining urine by catheterization and documenting the presence of a positive nitrite and leukocyte esterase reaction. Again, an elevated urine pH is indicative of an infection secondary to Proteus species. The criterion for defining a positive culture from catheterized urine is greater than 100 colonies/mL.2,18
Antibiotic selection. Patients in the first half of pregnancy who are hemodynamically stable and who show no signs of preterm labor may be treated with oral antibiotics as outpatients. The 2 drugs of choice are amoxicillin-clavulanate (875 mg twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength (800 mg/160 mg twice daily for 7 to 10 days).
For unstable patients in the first half of pregnancy and for essentially all patients in the second half of pregnancy, parenteral treatment should be administered on an inpatient basis. My preference for treatment is ceftriaxone, 2 g intravenously every 24 hours. The drug provides excellent coverage against almost all the uropathogens. It has a convenient dosing schedule, and it usually is very well tolerated. Parenteral therapy should be continued until the patient has been afebrile and asymptomatic for 24 to 48 hours. At this point, the patient can be transitioned to one of the oral regimens listed above and managed as an outpatient. If the patient is allergic to β-lactam antibiotics, an excellent alternative is aztreonam, 2 g intravenously every 8 hours.2,18 ●
For the infections we most commonly encounter in obstetric practice, I review in this article the selection of specific antibiotics. I focus on the key pathogens that cause these infections, the most useful diagnostic tests, and the most cost-effective antibiotic therapy. Relative cost estimates (high vs low) for drugs are based on information published on the GoodRx website (https://www.goodrx.com/). Actual charges to patients, of course, may vary widely depending on contractual relationships between hospitals, insurance companies, and wholesale vendors. The infections are listed in alphabetical order, not in order of frequency or severity.
1. Bacterial vaginosis
Bacterial vaginosis (BV) is a polymicrobial infection that results from perturbation of the normal vaginal flora due to conditions such as pregnancy, hormonal therapy, and changes in the menstrual cycle. It is characterized by a decrease in the vaginal concentration of Lactobacillus crispatus, followed by an increase in Prevotella bivia, Gardnerella vaginalis, Mobiluncus species, Atopobium vaginae, and Megasphaera type 1.1,2
BV is characterized by a thin, white-gray malodorous (fishlike smell) discharge. The vaginal pH is >4.5. Clue cells are apparent on saline microscopy, and the whiff (amine) test is positive when potassium hydroxide is added to a drop of vaginal secretions. Diagnostic accuracy can be improved using one of the new vaginal panel assays such as BD MAX Vaginal Panel (Becton, Dickinson and Company).3
Antibiotic selection
Antibiotic treatment of BV is directed primarily at the anaerobic component of the infection. The preferred treatment is oral metronidazole 500 mg twice daily for 7 days. If the patient cannot tolerate metronidazole, oral clindamycin 300 mg twice daily for 7 days, can be used, although it is more expensive than metronidazole. Topical metronidazole vaginal gel (0.75%), 1 applicatorful daily for 5 days, is effective in treating the local vaginal infection, but it is not effective in preventing systemic complications such as preterm labor, chorioamnionitis, and puerperal endometritis.2 It also is significantly more expensive than the oral formulation of metronidazole. Topical clindamycin cream, 1 applicatorful daily for 5 days, is even more expensive.
Tinidazole 2 g orally daily for 2 days is an effective alternative to oral metronidazole. Single-dose therapy with oral secnidazole (2 g), a 5-nitroimidazole with a longer half-life than metronidazole, has been effective in small studies, but experience with this drug in the United States is limited. Secnidazole is also very expensive.4
2. Candidiasis
Vulvovaginal candidiasis usually is caused by Candida albicans. Other less common species include C tropicalis, C glabrata, C auris, C lusitaniae, and C krusei. The most common clinical findings are vulvovaginal pruritus in association with a curdlike white vaginal discharge. The diagnosis can be established by confirmation of a normal vaginal pH and identification of budding yeast and hyphae on a potassium hydroxide preparation. As noted above for BV, the vaginal panel assay improves the accuracy of clinical diagnosis.3 Culture usually is indicated only in patients with infections that are refractory to therapy.
Continue to: Antibiotic selection...
Antibiotic selection
In the first trimester of pregnancy, vulvovaginal candidiasis should be treated with a topical medication such as clotrimazole cream 1% (50 mg intravaginally daily for 7 days), miconazole cream 2% (100 mg intravaginally daily for 7 days), or terconazole cream 0.4% (50 g intravaginally daily for 7 days). Single-dose formulations or 3-day courses of treatment may not be quite as effective in pregnant patients, but they do offer a more convenient dosing schedule.2,5
Oral fluconazole should not be used in the first trimester of pregnancy because it has been associated with an increased risk for spontaneous abortion and with fetal cardiac septal defects. Beyond the first trimester, oral fluconazole offers an attractive option for treatment of vulvovaginal candidiasis. The appropriate dose is 150 mg initially, with a repeat dose in 3 days if symptoms persist.2,5
Ibrexafungerp (300 mg twice daily for 1 day) was recently approved by the US Food and Drug Administration (FDA) for oral treatment of vulvovaginal candidiasis. However, this drug is teratogenic and is contraindicated during pregnancy and lactation. It also is significantly more expensive than fluconazole.6
3. Cesarean delivery prophylaxis
All women having a cesarean delivery (CD) should receive antibiotic prophylaxis to reduce the risk of endometritis and wound infection.
Antibiotic selection
In my opinion, the preferred regimen is intravenous cefazolin 2 g plus azithromycin 500 mg administered preoperatively.7 Cefazolin can be administered in a rapid bolus; azithromycin should be administered over 1 hour.
In an exceptionally rigorous investigation called the C/SOAP trial (Cesarean Section Optimal Antibiotic Prophylaxis trial), Tita and colleagues showed that the combination of cefazolin plus azithromycin was superior to single-agent prophylaxis (usually with cefazolin) in preventing the composite of endometritis, wound infection, or other infection occurring within 6 weeks of surgery.8 The additive effect of azithromycin was particularly pronounced in patients having CD after labor and rupture of membranes. Harper and associates subsequently validated the cost-effectiveness of this combination regimen using a decision analytic model.9
If the patient has a serious allergy to β-lactam antibiotics, the best alternative regimen for prophylaxis is clindamycin plus gentamicin. The appropriate single intravenous dose of clindamycin is 900 mg; the single dose of gentamicin should be 5 mg/kg of ideal body weight (IBW).7
4. Chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium. In pregnant women, it typically causes urethritis, endocervicitis, and inflammatory proctitis. Along with gonorrhea, it is the cause of an unusual infection/inflammation of the liver capsule, termed Fitz-Hugh-Curtis syndrome (perihepatitis). The diagnosis of chlamydia infection is best confirmed with a nucleic acid amplification test (NAAT). The NAAT simultaneously tests for chlamydia and gonorrhea in urine or in secretions obtained from the urethra, endocervix, and rectum.2
Antibiotic selection
The drug of choice for treating chlamydia in pregnancy is azithromycin 1,000 mg orally in a single dose. Erythromycin can be used as an alternative to azithromycin, but it usually is not well tolerated because of gastrointestinal adverse effects. In my practice, the preferred alternative for a patient who cannot tolerate azithromycin is amoxicillin 500 mg orally 3 times daily for 7 days.2,10
Continue to: 5. Chorioamnionitis...
5. Chorioamnionitis
Chorioamnionitis is a polymicrobial infection caused by anaerobes, aerobic gram-negative bacilli (predominantly Escherichia coli), and aerobic gram-positive cocci (primarily group B streptococci [GBS]). The diagnosis usually is made based on clinical examination: maternal fever, maternal and fetal tachycardia, and no other localizing sign of infection. The diagnosis can be confirmed by obtaining a sample of amniotic fluid via amniocentesis or via aspiration through the intrauterine pressure catheter and demonstrating a positive Gram stain, low glucose concentration (<20 mg/dL), positive nitrites, positive leukocyte esterase, and ultimately, a positive bacteriologic culture.2
Antibiotic selection
The initial treatment of chorioamnionitis specifically targets the 2 major organisms that cause neonatal pneumonia, meningitis, and sepsis: GBS and E coli. For many years, the drugs of choice have been intravenous ampicillin (2 g every 6 hours) plus intravenous gentamicin (5 mg/kg of IBW every 24 hours). Gentamicin also can be administered intravenously at a dose of 1.5 mg/kg every 8 hours. I prefer the once-daily dosing for 3 reasons:
- Gentamicin works by a concentration-dependent mechanism; the higher the initial serum concentration, the better the killing effect.
- Once-daily dosing preserves long periods with low trough levels, an effect that minimizes ototoxicity and nephrotoxicity.
- Once-daily dosing is more convenient.
In a patient who has a contraindication to use of an aminoglycoside, aztreonam (2 g intravenously every 8 hours) may be combined with ampicillin.2
If the patient delivers vaginally, 1 dose of each drug should be administered postpartum, and then the antibiotics should be discontinued. If the patient delivers by cesarean, a single dose of a medication with strong anaerobic coverage should be administered immediately after the infant’s umbilical cord is clamped. Options include clindamycin (900 mg intravenously) or metronidazole (500 mg intravenously).11
There are 2 key exceptions to the single postpartum dose rule, however. If the patient is obese (body mass index [BMI] >30 kg/m2) or if the membranes have been ruptured for more than 24 hours, antibiotics should be continued until she has been afebrile and asymptomatic for 24 hours.12
Two single agents are excellent alternatives to the combination ampicillin-gentamicin regimen. One is ampicillin-sulbactam, 3 g intravenously every 6 hours. The other is piperacillin-tazobactam, 3.375 g intravenously every 6 hours. These extended-spectrum penicillins provide exceptionally good coverage against the major pathogens that cause chorioamnionitis. Although more expensive than the combination regimen, they avoid the potential ototoxicity and nephrotoxicity associated with gentamicin.2
6. Endometritis
Puerperal endometritis is significantly more common after CD than after vaginal delivery. The infection is polymicrobial, and the principal pathogens are anaerobic gram-positive cocci, anaerobic gram-negative bacilli, aerobic gram-negative bacilli, and aerobic gram-positive cocci. The diagnosis usually is made almost exclusively based on clinical findings: fever within 24 to 36 hours of delivery, tachycardia, mild tachypnea, and lower abdominal/pelvic pain and tenderness in the absence of any other localizing sign of infection.13
Antibiotic selection
Effective treatment of endometritis requires administration of antibiotics that provide coverage against the broad range of pelvic pathogens. For many years, the gold standard of treatment has been the combination regimens of clindamycin plus gentamicin or metronidazole plus ampicillin plus gentamicin. These drugs are available in generic form and are relatively inexpensive. However, several broad-spectrum single agents are now available for treatment of endometritis. Although they are moderately more expensive than the generic combination regimens, they usually are very well tolerated, and they avoid the potential nephrotoxicity and ototoxicity associated with gentamicin. TABLE 1 summarizes the dosing regimens of these various agents and their potential weaknesses in coverage.2,13

7. Gonorrhea
Gonorrhea is caused by the gram-negative diplococcus, Neisseria gonorrhoeae. The organism has a propensity to infect columnar epithelium and uroepithelium, and, typically, it causes a localized infection of the urethra, endocervix, and rectum. The organism also can cause an oropharyngeal infection, a disseminated infection (most commonly manifested by dermatitis and arthritis), and perihepatitis.
The diagnosis is best confirmed by a NAAT that can simultaneously test for gonorrhea and chlamydia in urine or in secretions obtained from the urethra, endocervix, and rectum.2,10
Antibiotic selection
The drugs of choice for treating uncomplicated gonococcal infection in pregnancy are a single dose of ceftriaxone 500 mg intramuscularly, or cefixime 800 mg orally. If the patient is allergic to β-lactam antibiotics, the recommended treatment is gentamicin 240 mg intramuscularly in a single dose, combined with azithromycin 2,000 mg orally.14
8. Group B streptococci prophylaxis
The first-line agents for GBS prophylaxis are penicillin and ampicillin. Resistance of GBS to either of these antibiotics is extremely rare. The appropriate penicillin dose is 3 million U intravenously every 4 hours; the intravenous dose of ampicillin is 2 g initially, then 1 g every 4 hours. I prefer penicillin for prophylaxis because it has a narrower spectrum of activity and is less likely to cause antibiotic-associated diarrhea. The antibiotic should be continued until delivery of the neonate.2,15,16
If the patient has a mild allergy to penicillin, the drug of choice is cefazolin 2 g intravenously initially, then 1 g every 8 hours. If the patient’s allergy to β-lactam antibiotics is severe, the alternative agents are vancomycin (20 mg/kg intravenously every 8 hours infused over 1–2 hours; maximum single dose of 2 g) and clindamycin (900 mg intravenously every 8 hours). The latter drug should be used only if sensitivity testing has confirmed that the GBS strain is sensitive to clindamycin. Resistance to clindamycin usually ranges from 10% to 15%.2,15,16
9. Puerperal mastitis
The principal microorganisms that cause puerperal mastitis are the aerobic streptococci and staphylococci that form part of the normal skin flora. The diagnosis usually is made based on the characteristic clinical findings: erythema, tenderness, and warmth in an area of the breast accompanied by a purulent nipple discharge and fever and chills. The vast majority of cases can be treated with oral antibiotics on an outpatient basis. The key indications for hospitalization are severe illness, particularly in an immunocompromised patient, and suspicion of a breast abscess.2
Continue to: Antibiotic selection...
Antibiotic selection
The initial drug of choice for treatment of mastitis is dicloxacillin sodium 500 mg every 6 hours for 7 to 10 days. If the patient has a mild allergy to penicillin, the appropriate alternative is cephalexin 500 mg every 8 hours for 7 to 10 days. If the patient’s allergy to penicillin is severe, 2 alternatives are possible. One is clindamycin 300 mg twice daily for 7 to 10 days; the other is trimethoprim-sulfamethoxazole double strength (800 mg/160 mg), twice daily for 7 to 10 days. The latter 2 drugs are also of great value if the patient fails to respond to initial therapy and/or infection with methicillin-resistant Staphylococcus aureus (MRSA) is suspected.2 I prefer the latter agent because it is less expensive than clindamycin and is less likely to cause antibiotic-induced diarrhea.
If hospitalization is required, the drug of choice is intravenous vancomycin. The appropriate dosage is 20 mg/kg every 8 to 12 hours (maximum single dose of 2 g).2
10. Syphilis
Syphilis is caused by the spirochete bacterium, Treponema pallidum. The diagnosis can be made by clinical examination if the characteristic findings listed in TABLE 2 are present.2,17 However, most patients in our practice will have latent syphilis, and the diagnosis must be established based on serologic screening.17

Antibiotic selection
In pregnancy, the treatment of choice for syphilis is penicillin (TABLE 3).2,10,17 Only penicillin has been proven effective in treating both maternal and fetal infection. If the patient has a history of allergy to penicillin, she should undergo skin testing to determine if she is truly allergic. If hypersensitivity is confirmed, the patient should be desensitized and then treated with the appropriate regimen outlined in TABLE 3. Of interest, within a short period of time after treatment, the patient’s sensitivity to penicillin will be reestablished, and she should not be treated again with penicillin unless she undergoes another desensitization process.2,17

11. Trichomoniasis
Trichomoniasis is caused by the flagellated protozoan, Trichomonas vaginalis. The condition is characterized by a distinct yellowish-green vaginal discharge. The vaginal pH is >4.5, and motile flagellated organisms are easily visualized on saline microscopy. The vaginal panel assay also is a valuable diagnostic test.3
Antibiotic selection
The drug of choice for trichomoniasis is oral metronidazole 500 mg twice daily for 7 days. The patient’s sexual partner(s) should be treated concurrently to prevent reinfection. Most treatment failures are due to poor compliance with therapy on the part of either the patient or her partner(s); true drug resistance is uncommon. When antibiotic resistance is strongly suspected, the patient may be treated with a single 2-g oral dose of tinidazole.2
12. Urinary tract infections
Urethritis
Acute urethritis usually is caused by C trachomatis or N gonorrhoeae. The treatment of infections with these 2 organisms is discussed above.
Asymptomatic bacteriuria and acute cystitis
Bladder infections are caused primarily by E coli, Klebsiella pneumoniae, and Proteus species. Gram-positive cocci such as enterococci, Staphylococcus saprophyticus, and GBS are less common pathogens.18
The key diagnostic criterion for asymptomatic bacteriuria is a colony count greater than 100,000 organisms/mL of a single uropathogen on a clean-catch midstream urine specimen.18
The usual clinical manifestations of acute cystitis include frequency, urgency, hesitancy, suprapubic discomfort, and a low-grade fever. The diagnosis is most effectively confirmed by obtaining urine by catheterization and demonstrating a positive nitrite and positive leukocyte esterase reaction on dipstick examination. The finding of a urine pH of 8 or greater usually indicates an infection caused by Proteus species. When urine is obtained by catheterization, the criterion for defining a positive culture is greater than 100 colonies/mL.18
Antibiotic selection. In the first trimester, the preferred agents for treatment of a lower urinary tract infection are oral amoxicillin (875 mg twice daily) or cephalexin (500 mg every 8 hours). For an initial infection, a 3-day course of therapy usually is adequate. For a recurrent infection, a 7- to 10-day course is indicated.
Beyond the first trimester, nitrofurantoin monohydrate macrocrystals (100 mg orally twice daily) or trimethoprim-sulfamethoxazole double strength (800 mg/160 mg twice daily) are the preferred agents. Unless no other oral drug is likely to be effective, these 2 drugs should be avoided in the first trimester. The former has been associated with eye, heart, and cleft defects. The latter has been associated with neural tube defects, cardiac anomalies, choanal atresia, and diaphragmatic hernia.18
Acute pyelonephritis
Acute infections of the kidney usually are caused by the aerobic gram-negative bacilli: E coli, K pneumoniae, and Proteus species. Enterococci, S saprophyticus, and GBS are less likely to cause upper tract infection as opposed to bladder infection.
The typical clinical manifestations of acute pyelonephritis include high fever and chills in association with flank pain and tenderness. The diagnosis is best confirmed by obtaining urine by catheterization and documenting the presence of a positive nitrite and leukocyte esterase reaction. Again, an elevated urine pH is indicative of an infection secondary to Proteus species. The criterion for defining a positive culture from catheterized urine is greater than 100 colonies/mL.2,18
Antibiotic selection. Patients in the first half of pregnancy who are hemodynamically stable and who show no signs of preterm labor may be treated with oral antibiotics as outpatients. The 2 drugs of choice are amoxicillin-clavulanate (875 mg twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength (800 mg/160 mg twice daily for 7 to 10 days).
For unstable patients in the first half of pregnancy and for essentially all patients in the second half of pregnancy, parenteral treatment should be administered on an inpatient basis. My preference for treatment is ceftriaxone, 2 g intravenously every 24 hours. The drug provides excellent coverage against almost all the uropathogens. It has a convenient dosing schedule, and it usually is very well tolerated. Parenteral therapy should be continued until the patient has been afebrile and asymptomatic for 24 to 48 hours. At this point, the patient can be transitioned to one of the oral regimens listed above and managed as an outpatient. If the patient is allergic to β-lactam antibiotics, an excellent alternative is aztreonam, 2 g intravenously every 8 hours.2,18 ●
- Reeder CF, Duff P. A case of BV during pregnancy: best management approach. OBG Manag. 2021;33(2):38-42.
- Duff P. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies, 8th ed. Elsevier; 2021:1124-1145.
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859.
- Hiller SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Kirkpatrick K, Duff P. Candidiasis: the essentials of diagnosis and treatment. OBG Manag. 2020;32(8):27-29, 34.
- Ibrexafungerp (Brexafemme) for vulvovaginal candidiasis. Med Lett Drugs Ther. 2021;63:141-143.
- Duff P. Prevention of infection after cesarean delivery. Clin Obstet Gynecol. 2019;62:758-770.
- Tita AT, Szychowski JM, Boggess K, et al; for the C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64(RR3):1-137.
- Edwards RK, Duff P. Single additional dose postpartum therapy for women with chorioamnionitis. Obstet Gynecol. 2003;102(5 pt 1):957-961.
- Black LP, Hinson L, Duff P. Limited course of antibiotic treatment for chorioamnionitis. Obstet Gynecol. 2012;119:1102-1105.
- Duff P. Fever following cesarean delivery: what are your steps for management? OBG Manag. 2021;33(12):26-30, 35.
- St Cyr S, Barbee L, Warkowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.
- Prevention of group B streptococcal early-onset disease in newborns: ACOG committee opinion summary, number 782. Obstet Gynecol. 2019;134:1.
- Duff P. Preventing early-onset group B streptococcal disease in newborns. OBG Manag. 2019;31(12):26, 28-31.
- Finley TA, Duff P. Syphilis: cutting risk through primary prevention and prenatal screening. OBG Manag. 2020;32(11):20, 22-27.
- Duff P. UTIs in pregnancy: managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis. OBG Manag. 2022;34(1):42-46.
- Reeder CF, Duff P. A case of BV during pregnancy: best management approach. OBG Manag. 2021;33(2):38-42.
- Duff P. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies, 8th ed. Elsevier; 2021:1124-1145.
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859.
- Hiller SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Kirkpatrick K, Duff P. Candidiasis: the essentials of diagnosis and treatment. OBG Manag. 2020;32(8):27-29, 34.
- Ibrexafungerp (Brexafemme) for vulvovaginal candidiasis. Med Lett Drugs Ther. 2021;63:141-143.
- Duff P. Prevention of infection after cesarean delivery. Clin Obstet Gynecol. 2019;62:758-770.
- Tita AT, Szychowski JM, Boggess K, et al; for the C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64(RR3):1-137.
- Edwards RK, Duff P. Single additional dose postpartum therapy for women with chorioamnionitis. Obstet Gynecol. 2003;102(5 pt 1):957-961.
- Black LP, Hinson L, Duff P. Limited course of antibiotic treatment for chorioamnionitis. Obstet Gynecol. 2012;119:1102-1105.
- Duff P. Fever following cesarean delivery: what are your steps for management? OBG Manag. 2021;33(12):26-30, 35.
- St Cyr S, Barbee L, Warkowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.
- Prevention of group B streptococcal early-onset disease in newborns: ACOG committee opinion summary, number 782. Obstet Gynecol. 2019;134:1.
- Duff P. Preventing early-onset group B streptococcal disease in newborns. OBG Manag. 2019;31(12):26, 28-31.
- Finley TA, Duff P. Syphilis: cutting risk through primary prevention and prenatal screening. OBG Manag. 2020;32(11):20, 22-27.
- Duff P. UTIs in pregnancy: managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis. OBG Manag. 2022;34(1):42-46.
Amniotic fluid embolism: Management using a checklist
CASE Part 1: CPR initiated during induction of labor
A 32-year-old gravida 4 para 3-0-0-3 is undergoing induction of labor with intravenous (IV) oxytocin at 39 weeks of gestation. She has no significant medical or obstetric history. Fifteen minutes after reaching complete cervical dilation, she says “I don’t feel right,” then suddenly loses consciousness. The nurse finds no detectable pulse, calls a “code blue,” and initiates cardiopulmonary resuscitation (CPR). The obstetrician is notified, appears promptly, assesses the situation, and delivers a 3.6-kg baby via vacuum extraction. Apgar score is 2/10 at 1 minute and 6/10 at 5 minutes. After delivery of the placenta, there is uterine atony and brisk hemorrhage with 2 L of blood loss.
Management of AFE: A rare complication
This case demonstrates a classic presentation of amniotic fluid embolism (AFE) syndrome—a patient in labor or within 30 minutes after delivery has sudden onset of cardiorespiratory collapse followed by disseminated intravascular coagulation (DIC). AFE is rare, affecting only about 2 to 6 per 100,000 births, but classic cases have a reported maternal mortality rate that exceeds 50%.1 It is thought to reflect a complex, systemic proinflammatory response to maternal intravasation of pregnancy material, such as trophoblast, thromboplastins, fetal cells, or amniotic fluid. Because the syndrome is not necessarily directly caused by emboli or by amniotic fluid per se,2 it has been proposed that AFE be called “anaphylactoid syndrome of pregnancy,” but this terminology has not yet been widely adopted.3
Guidelines from the Society for Maternal-Fetal Medicine (SMFM) recommend several time-critical steps for the initial stabilization and management of patients with AFE.4 However, because AFE is rare, most obstetric providers may not encounter a case for many years or even decades after they have received training, so it is unrealistic to expect that they will remember these guidelines when they are needed. For this reason, when AFE occurs, it is important to have a readily accessible cognitive aid, such as a checklist that summarizes the key management steps. The SMFM provides a checklist for initial management of AFE that can be used at your institution; it is presented in the FIGURE and provides the outline for this discussion.5

Provide CPR immediately
Most AFE cases are accompanied by cardiorespiratory arrest. If the patient has no pulse, call a “code” to mobilize additional help and immediately start CPR. Use a backboard to make cardiac compressions most effective and manually displace the uterus or tilt the patient to avoid supine hypotension. Designate a timekeeper to call out 1-minute intervals and record critical data, such as medication administration and laboratory orders/results.
Expedite delivery
Immediate delivery is needed if maternal cardiac activity is not restored within 4 minutes of starting CPR, with a target to have delivery completed within 5 minutes. Operative vaginal delivery may be an option if delivery is imminent, as in the case presented, but cesarean delivery (CD) will be needed in most cases. This was previously called “perimortem cesarean” delivery, but the term “resuscitative hysterotomy” has been proposed because the primary goal is to improve the effectiveness of CPR6 and prevent both maternal and perinatal death. CPR is less effective in pregnant women because the pregnant uterus takes a substantial fraction of the maternal cardiac output, as well as compresses the vena cava. Some experts suggest that, rather than waiting 4 minutes, CD should be started as soon as an obstetrician or other surgeon is present, unless there is an immediate response to electrical cardioversion.6,7
In most cases, immediate CD should be performed wherever the patient is located rather than using precious minutes to move the patient to an operating room. Antiseptic preparation is expedited by simply pouring povidone-iodine or chlorhexidine over the lower abdomen if readily available; if not available, skip this step. Enter the abdomen and uterus as rapidly as possible using only a scalpel to make generous midline incisions.
If CPR is not required, proceed with cesarean or operative vaginal delivery as soon as the mother has been stabilized. These procedures should be performed using standard safety precautions outlined in the SMFM patient safety checklists for cesarean or operative vaginal delivery.8,9
Continue to: Anticipate hemorrhage...
Anticipate hemorrhage
Be prepared for uterine atony, coagulopathy, and catastrophic hemorrhage. Initiate IV oxytocin prophylaxis as soon as the infant is delivered. Have a low threshold for giving other uterotonic agents such as methylergonovine, carboprost, or misoprostol. If hemorrhage or DIC occurs, give tranexamic acid. Have the anesthesiologist or trauma team (if available) insert an intraosseous line for fluid resuscitation if peripheral IV access is inadequate.
Massive transfusion is often needed to treat DIC, which occurs in most AFE cases. Anticipate—do not wait—for DIC to occur. We propose activating your hospital’s massive transfusion protocol (MTP) as soon as you diagnose AFE so that blood products will be available as soon as possible. A typical MTP provides several units of red blood cells, a pheresis pack of platelets, and fresh/frozen plasma (FFP). If clinically indicated, administer cryoprecipitate instead of FFP to minimize volume overload, which may occur with FFP.
CASE Part 2: MTP initiated to treat DIC
The MTP is initiated. Laboratory results immediately pre-transfusion include hemoglobin 11.3 g/dL, platelet count 46,000 per mm3, fibrinogen 87 mg/dL, and an elevated prothrombin time international normalized ratio.
Expect heart failure
The initial hemodynamic picture in AFE is right heart failure, which should optimally be managed by a specialist from anesthesiology, cardiology, or critical care as soon as they are available. An emergency department physician may manage the hemodynamics until a specialist arrives. Avoidance of fluid overload is one important principle. If fluid challenges are needed for hypovolemic shock, boluses should be restricted to 500 mL rather than the traditional 1000 mL.
Pharmacologic treatment may include vasopressors, inotropic agents, and pulmonary vasodilators. Example medications and dosages recommended by SMFM are summarized in the checklist (FIGURE).5
After the initial phase of recovery, the hemodynamic picture often changes from right heart failure to left heart failure. Management of left heart failure is not covered in the SMFM checklist because, by the time it appears, the patient will usually be in the intensive care unit, managed by the critical care team. Management of left heart failure generally includes diuresis as needed for cardiogenic pulmonary edema, optimization of cardiac preload, and inotropic agents or vasopressors if needed to maintain cardiac output or perfusion pressure.4
Debrief, learning opportunities
Complex emergencies such as AFE are rarely handled 100% perfectly, even those with a good outcome, so they present opportunities for team learning and improvement. The team should conduct a 10- to 15-minute debrief soon after the patient is stabilized. Make an explicit statement that the main goal of the debrief is to gather suggestions as to how systems and processes could be improved for next time, not to find fault or lay blame on individuals. Encourage all personnel involved in the initial management to attend and discuss what went well and what did not. Another goal is to provide support for individuals who may feel traumatized by the dramatic, frightening events surrounding an AFE and by the poor patient outcome or guarded prognosis that frequently follows. Another goal is to discuss the plan for providing support and disclosure to the patient and family.
The vast majority of AFE cases meet criteria to be designated as “sentinel events,” because of patient transfer to the intensive care unit, multi-unit blood transfusion, other severe maternal morbidities, or maternal death. Therefore, most AFE cases will trigger a root cause analysis (RCA) or other formal sentinel event analysis conducted by the hospital’s Safety or Quality Department. As with the immediate post-event debrief, the first goal of the RCA is to identify systems issues that may have resulted in suboptimal care and that can be modified to improve future care. Specific issues regarding the checklist should also be addressed:
- Was the checklist used?
- Was the checklist available?
- Are there items on the checklist that need to be modified, added, or deleted?
The RCA concludes with the development of a performance improvement plan.
Ultimately, we encourage all AFE cases be reported to the registry maintained by the Amniotic Fluid Embolism Foundation at https://www.afesupport.org/, regardless of whether the outcome was favorable for the mother and newborn. The registry includes over 130 AFE cases since 2013 from around the world. Researchers periodically report on the registry findings.10 If providers report cases with both good and bad outcomes, the registry may provide future insights regarding which adjunctive or empiric treatments may or may not be promising.
Continue to: Empiric treatments...
Empiric treatments
From time-to-time, new regimens for empiric treatment of AFE are reported. It is important to recognize that these reports are generally uncontrolled case reports of favorable outcomes and that, without a control group, it is impossible to determine to what extent the treatment contributed to the outcome or was merely incidental. Given the rarity of AFE, it seems unlikely that there will ever be a randomized clinical trial or even a controlled prospective study comparing treatment regimens.
The “A-OK” regimen is an empiric treatment that has garnered some interest after an initial case report.11 It consists of an anticholinergic agent (atropine 0.2 mg IV), a selective 5-HT3 receptor antagonist (ondansetron 8 mg IV), and a nonsteroidal anti-inflammatory drug (ketorolac 15 mg IV). We have some reservations about this regimen, however, because atropine is relatively contraindicated if the patient has tachycardia (which is common in patients with hemorrhage) and ketorolac may suppress platelet function, which might be harmful for patients with DIC or thrombocytopenia.
Another empiric treatment is the “50-50-500” regimen, which includes an H1 antihistamine (diphenhydramine 50 mg IV), an H2 antihistamine (famotidine 50 mg IV), and a corticosteroid (hydrocortisone 500 mg IV). This regimen aims to suppress histamine-mediated and cell-mediated inflammatory responses, based on the notion that proinflammatory responses likely mediate much of the underlying pathophysiology of the AFE syndrome.
We would emphasize that these empiric regimens are not clinically validated, US Food and Drug Administration approved for treatment of AFE, or considered standard of care. Future reports of these and other regimens will be needed to evaluate their efficacy, limitations, and risks. Again, we encourage providers to report all AFE cases to the AFE Foundation registry, regardless of whether the treatments are successful.
CASE Conclusion
The hemorrhage stops after administration of oxytocin, carboprost, 6 units of cryoprecipitate, and a 6-unit platelet pheresis pack. The patient is transferred to the intensive care unit where she eventually requires a total of 10 units of red cells, 8 more units of cryoprecipitate, and another platelet pheresis pack. She is discharged to home in stable condition on postpartum day 4.
Be prepared, have the checklist ready
Because AFE is rare, most members of the health care team will have no prior experience managing a real case. It may have been years or decades since they had any education on AFE or they last read a review article such as this one. It is even possible the anesthesiologist, cardiologist, or critical care specialist has never heard of AFE. Thus if they rely on memory alone, there is substantial risk of forgetting items, getting dosages wrong, or other errors. With this in mind, what is the best way to prepare the team to expeditiously employ the management steps outlined here?
Use of a checklist that summarizes these key steps for early management, such as the SMFM checklist in the FIGURE, will help ensure that all relevant steps are performed in every AFE case. It is designed to be printed on a single sheet of letter-sized paper, and we propose that every labor and delivery (L&D) unit keep laminated copies of this checklist in several places where they will be immediately available should an AFE occur. Copies can be kept on the anesthesia carts in the L&D operating rooms, in an emergency procedures binder on the unit, and on the “crash carts” and hemorrhage supply carts in the L&D unit. Effective implementation of an AFE checklist requires all personnel know where to readily find it and have some familiarity with its contents.
An interdisciplinary team comprising representatives from nursing, obstetrics, and anesthesia should meet to discuss whether the checklist needs to be modified to fit the local hospital formulary or other unique local circumstances. The team should develop an implementation plan that includes where to keep checklist copies, a process to periodically ensure that the copies are still present and readable, a roll-out plan to inform all personnel about the checklist process, and most importantly a training plan that includes incorporating AFE cases into the schedule of multidisciplinary simulations and drills for obstetric emergencies. Other implementation strategies are outlined in the SMFM document.5
Ultimately an organized, systematic approach is recommended for management of AFE. There is no single best treatment of AFE; it is supportive and directed toward the underlying pathophysiology, which may vary from patient to patient. Therefore, although a checklist, in conjunction with regular education and simulation activities, may help optimize care and improve outcomes, there is still a high risk of maternal morbidity and mortality from AFE. ●
- Clark SL. Amniotic fluid embolism. Obstet Gynecol. 2014;123(2 Pt 1):337-348. doi:10.1097/AOG.0000000000000107.
- Funk M, Damron A, Bandi V, et al. Pulmonary vascular obstruction by squamous cells is not involved in amniotic fluid embolism. Am J Obstet Gynecol. 2018;218:460-461. doi:10.1016/j.ajog.2017.12.225.
- Gilmore DA, Wakim J, Secrest J, et al. Anaphylactoid syndrome of pregnancy: a review of the literature with latest management and outcome data. AANA J. 2003;71:120-126.
- Society for Maternal-Fetal Medicine, Pacheco LD, Saade G, et al. Amniotic fluid embolism: diagnosis and management. Am J Obstet Gynecol. 2016;215:B16-24. doi:10.1016/j.ajog.2016.03.012.
- Patient Safety and Quality Committee, Society for Maternal-Fetal Medicine; Combs CA, Montgomery DM, et al. Society for Maternal-Fetal Medicine Special Statement: checklist for initial management of amniotic fluid embolism. Am J Obstet Gynecol. 2021;224:B29-B32. doi:10.1016/j.ajog.2021.01.001.
- Rose CH, Faksh A, Traynor KD, et al. Challenging the 4- to 5-minute rule: from perimortem cesarean to resuscitative hysterotomy. Am J Obstet Gynecol. 2015;213:653-6, 653.e1. doi:10.1016/j.ajog.2015.07.019.
- Pacheco LD, Clark SL, Klassen M, et al. Amniotic fluid embolism: principles of early clinical management. Am J Obstet Gynecol. 2020;222:48-52. doi:10.1016/j.ajog.2019.07.036.
- Combs CA, Einerson BD, Toner LE, SMFM Patient Safety and Quality Committee. SMFM Special Statement: surgical safety checklists for cesarean delivery. Am J Obstet Gynecol. 2021;225:B43-B49. doi:10.1016/j.ajog.2021.07.011.
- SMFM Patient Safety and Quality Committee, Staat B, Combs CA. SMFM Special Statement: operative vaginal delivery: checklists for performance and documentation. Am J Obstet Gynecol. 2020;222:B15-B21. doi:10.1016/j.ajog.2020.02.011.
- Stafford IA, Moaddab A, Dildy GA, et al. Amniotic fluid embolism syndrome: analysis of the United States international registry. Am J Obstet Gynecol MFM. 2020;2:100083. doi:10.1016/j.ajogmf.2019.100083.
- Rezai S, Hughes AZC, Larsen TB, et al. Atypical amniotic f luid embolism managed with a novel therapeutic regimen. Case Rep Obstet Gynecol. 2017; 2017:8458375. doi:10.1155/2017/8458375.

CASE Part 1: CPR initiated during induction of labor
A 32-year-old gravida 4 para 3-0-0-3 is undergoing induction of labor with intravenous (IV) oxytocin at 39 weeks of gestation. She has no significant medical or obstetric history. Fifteen minutes after reaching complete cervical dilation, she says “I don’t feel right,” then suddenly loses consciousness. The nurse finds no detectable pulse, calls a “code blue,” and initiates cardiopulmonary resuscitation (CPR). The obstetrician is notified, appears promptly, assesses the situation, and delivers a 3.6-kg baby via vacuum extraction. Apgar score is 2/10 at 1 minute and 6/10 at 5 minutes. After delivery of the placenta, there is uterine atony and brisk hemorrhage with 2 L of blood loss.
Management of AFE: A rare complication
This case demonstrates a classic presentation of amniotic fluid embolism (AFE) syndrome—a patient in labor or within 30 minutes after delivery has sudden onset of cardiorespiratory collapse followed by disseminated intravascular coagulation (DIC). AFE is rare, affecting only about 2 to 6 per 100,000 births, but classic cases have a reported maternal mortality rate that exceeds 50%.1 It is thought to reflect a complex, systemic proinflammatory response to maternal intravasation of pregnancy material, such as trophoblast, thromboplastins, fetal cells, or amniotic fluid. Because the syndrome is not necessarily directly caused by emboli or by amniotic fluid per se,2 it has been proposed that AFE be called “anaphylactoid syndrome of pregnancy,” but this terminology has not yet been widely adopted.3
Guidelines from the Society for Maternal-Fetal Medicine (SMFM) recommend several time-critical steps for the initial stabilization and management of patients with AFE.4 However, because AFE is rare, most obstetric providers may not encounter a case for many years or even decades after they have received training, so it is unrealistic to expect that they will remember these guidelines when they are needed. For this reason, when AFE occurs, it is important to have a readily accessible cognitive aid, such as a checklist that summarizes the key management steps. The SMFM provides a checklist for initial management of AFE that can be used at your institution; it is presented in the FIGURE and provides the outline for this discussion.5

Provide CPR immediately
Most AFE cases are accompanied by cardiorespiratory arrest. If the patient has no pulse, call a “code” to mobilize additional help and immediately start CPR. Use a backboard to make cardiac compressions most effective and manually displace the uterus or tilt the patient to avoid supine hypotension. Designate a timekeeper to call out 1-minute intervals and record critical data, such as medication administration and laboratory orders/results.
Expedite delivery
Immediate delivery is needed if maternal cardiac activity is not restored within 4 minutes of starting CPR, with a target to have delivery completed within 5 minutes. Operative vaginal delivery may be an option if delivery is imminent, as in the case presented, but cesarean delivery (CD) will be needed in most cases. This was previously called “perimortem cesarean” delivery, but the term “resuscitative hysterotomy” has been proposed because the primary goal is to improve the effectiveness of CPR6 and prevent both maternal and perinatal death. CPR is less effective in pregnant women because the pregnant uterus takes a substantial fraction of the maternal cardiac output, as well as compresses the vena cava. Some experts suggest that, rather than waiting 4 minutes, CD should be started as soon as an obstetrician or other surgeon is present, unless there is an immediate response to electrical cardioversion.6,7
In most cases, immediate CD should be performed wherever the patient is located rather than using precious minutes to move the patient to an operating room. Antiseptic preparation is expedited by simply pouring povidone-iodine or chlorhexidine over the lower abdomen if readily available; if not available, skip this step. Enter the abdomen and uterus as rapidly as possible using only a scalpel to make generous midline incisions.
If CPR is not required, proceed with cesarean or operative vaginal delivery as soon as the mother has been stabilized. These procedures should be performed using standard safety precautions outlined in the SMFM patient safety checklists for cesarean or operative vaginal delivery.8,9
Continue to: Anticipate hemorrhage...
Anticipate hemorrhage
Be prepared for uterine atony, coagulopathy, and catastrophic hemorrhage. Initiate IV oxytocin prophylaxis as soon as the infant is delivered. Have a low threshold for giving other uterotonic agents such as methylergonovine, carboprost, or misoprostol. If hemorrhage or DIC occurs, give tranexamic acid. Have the anesthesiologist or trauma team (if available) insert an intraosseous line for fluid resuscitation if peripheral IV access is inadequate.
Massive transfusion is often needed to treat DIC, which occurs in most AFE cases. Anticipate—do not wait—for DIC to occur. We propose activating your hospital’s massive transfusion protocol (MTP) as soon as you diagnose AFE so that blood products will be available as soon as possible. A typical MTP provides several units of red blood cells, a pheresis pack of platelets, and fresh/frozen plasma (FFP). If clinically indicated, administer cryoprecipitate instead of FFP to minimize volume overload, which may occur with FFP.
CASE Part 2: MTP initiated to treat DIC
The MTP is initiated. Laboratory results immediately pre-transfusion include hemoglobin 11.3 g/dL, platelet count 46,000 per mm3, fibrinogen 87 mg/dL, and an elevated prothrombin time international normalized ratio.
Expect heart failure
The initial hemodynamic picture in AFE is right heart failure, which should optimally be managed by a specialist from anesthesiology, cardiology, or critical care as soon as they are available. An emergency department physician may manage the hemodynamics until a specialist arrives. Avoidance of fluid overload is one important principle. If fluid challenges are needed for hypovolemic shock, boluses should be restricted to 500 mL rather than the traditional 1000 mL.
Pharmacologic treatment may include vasopressors, inotropic agents, and pulmonary vasodilators. Example medications and dosages recommended by SMFM are summarized in the checklist (FIGURE).5
After the initial phase of recovery, the hemodynamic picture often changes from right heart failure to left heart failure. Management of left heart failure is not covered in the SMFM checklist because, by the time it appears, the patient will usually be in the intensive care unit, managed by the critical care team. Management of left heart failure generally includes diuresis as needed for cardiogenic pulmonary edema, optimization of cardiac preload, and inotropic agents or vasopressors if needed to maintain cardiac output or perfusion pressure.4
Debrief, learning opportunities
Complex emergencies such as AFE are rarely handled 100% perfectly, even those with a good outcome, so they present opportunities for team learning and improvement. The team should conduct a 10- to 15-minute debrief soon after the patient is stabilized. Make an explicit statement that the main goal of the debrief is to gather suggestions as to how systems and processes could be improved for next time, not to find fault or lay blame on individuals. Encourage all personnel involved in the initial management to attend and discuss what went well and what did not. Another goal is to provide support for individuals who may feel traumatized by the dramatic, frightening events surrounding an AFE and by the poor patient outcome or guarded prognosis that frequently follows. Another goal is to discuss the plan for providing support and disclosure to the patient and family.
The vast majority of AFE cases meet criteria to be designated as “sentinel events,” because of patient transfer to the intensive care unit, multi-unit blood transfusion, other severe maternal morbidities, or maternal death. Therefore, most AFE cases will trigger a root cause analysis (RCA) or other formal sentinel event analysis conducted by the hospital’s Safety or Quality Department. As with the immediate post-event debrief, the first goal of the RCA is to identify systems issues that may have resulted in suboptimal care and that can be modified to improve future care. Specific issues regarding the checklist should also be addressed:
- Was the checklist used?
- Was the checklist available?
- Are there items on the checklist that need to be modified, added, or deleted?
The RCA concludes with the development of a performance improvement plan.
Ultimately, we encourage all AFE cases be reported to the registry maintained by the Amniotic Fluid Embolism Foundation at https://www.afesupport.org/, regardless of whether the outcome was favorable for the mother and newborn. The registry includes over 130 AFE cases since 2013 from around the world. Researchers periodically report on the registry findings.10 If providers report cases with both good and bad outcomes, the registry may provide future insights regarding which adjunctive or empiric treatments may or may not be promising.
Continue to: Empiric treatments...
Empiric treatments
From time-to-time, new regimens for empiric treatment of AFE are reported. It is important to recognize that these reports are generally uncontrolled case reports of favorable outcomes and that, without a control group, it is impossible to determine to what extent the treatment contributed to the outcome or was merely incidental. Given the rarity of AFE, it seems unlikely that there will ever be a randomized clinical trial or even a controlled prospective study comparing treatment regimens.
The “A-OK” regimen is an empiric treatment that has garnered some interest after an initial case report.11 It consists of an anticholinergic agent (atropine 0.2 mg IV), a selective 5-HT3 receptor antagonist (ondansetron 8 mg IV), and a nonsteroidal anti-inflammatory drug (ketorolac 15 mg IV). We have some reservations about this regimen, however, because atropine is relatively contraindicated if the patient has tachycardia (which is common in patients with hemorrhage) and ketorolac may suppress platelet function, which might be harmful for patients with DIC or thrombocytopenia.
Another empiric treatment is the “50-50-500” regimen, which includes an H1 antihistamine (diphenhydramine 50 mg IV), an H2 antihistamine (famotidine 50 mg IV), and a corticosteroid (hydrocortisone 500 mg IV). This regimen aims to suppress histamine-mediated and cell-mediated inflammatory responses, based on the notion that proinflammatory responses likely mediate much of the underlying pathophysiology of the AFE syndrome.
We would emphasize that these empiric regimens are not clinically validated, US Food and Drug Administration approved for treatment of AFE, or considered standard of care. Future reports of these and other regimens will be needed to evaluate their efficacy, limitations, and risks. Again, we encourage providers to report all AFE cases to the AFE Foundation registry, regardless of whether the treatments are successful.
CASE Conclusion
The hemorrhage stops after administration of oxytocin, carboprost, 6 units of cryoprecipitate, and a 6-unit platelet pheresis pack. The patient is transferred to the intensive care unit where she eventually requires a total of 10 units of red cells, 8 more units of cryoprecipitate, and another platelet pheresis pack. She is discharged to home in stable condition on postpartum day 4.
Be prepared, have the checklist ready
Because AFE is rare, most members of the health care team will have no prior experience managing a real case. It may have been years or decades since they had any education on AFE or they last read a review article such as this one. It is even possible the anesthesiologist, cardiologist, or critical care specialist has never heard of AFE. Thus if they rely on memory alone, there is substantial risk of forgetting items, getting dosages wrong, or other errors. With this in mind, what is the best way to prepare the team to expeditiously employ the management steps outlined here?
Use of a checklist that summarizes these key steps for early management, such as the SMFM checklist in the FIGURE, will help ensure that all relevant steps are performed in every AFE case. It is designed to be printed on a single sheet of letter-sized paper, and we propose that every labor and delivery (L&D) unit keep laminated copies of this checklist in several places where they will be immediately available should an AFE occur. Copies can be kept on the anesthesia carts in the L&D operating rooms, in an emergency procedures binder on the unit, and on the “crash carts” and hemorrhage supply carts in the L&D unit. Effective implementation of an AFE checklist requires all personnel know where to readily find it and have some familiarity with its contents.
An interdisciplinary team comprising representatives from nursing, obstetrics, and anesthesia should meet to discuss whether the checklist needs to be modified to fit the local hospital formulary or other unique local circumstances. The team should develop an implementation plan that includes where to keep checklist copies, a process to periodically ensure that the copies are still present and readable, a roll-out plan to inform all personnel about the checklist process, and most importantly a training plan that includes incorporating AFE cases into the schedule of multidisciplinary simulations and drills for obstetric emergencies. Other implementation strategies are outlined in the SMFM document.5
Ultimately an organized, systematic approach is recommended for management of AFE. There is no single best treatment of AFE; it is supportive and directed toward the underlying pathophysiology, which may vary from patient to patient. Therefore, although a checklist, in conjunction with regular education and simulation activities, may help optimize care and improve outcomes, there is still a high risk of maternal morbidity and mortality from AFE. ●

CASE Part 1: CPR initiated during induction of labor
A 32-year-old gravida 4 para 3-0-0-3 is undergoing induction of labor with intravenous (IV) oxytocin at 39 weeks of gestation. She has no significant medical or obstetric history. Fifteen minutes after reaching complete cervical dilation, she says “I don’t feel right,” then suddenly loses consciousness. The nurse finds no detectable pulse, calls a “code blue,” and initiates cardiopulmonary resuscitation (CPR). The obstetrician is notified, appears promptly, assesses the situation, and delivers a 3.6-kg baby via vacuum extraction. Apgar score is 2/10 at 1 minute and 6/10 at 5 minutes. After delivery of the placenta, there is uterine atony and brisk hemorrhage with 2 L of blood loss.
Management of AFE: A rare complication
This case demonstrates a classic presentation of amniotic fluid embolism (AFE) syndrome—a patient in labor or within 30 minutes after delivery has sudden onset of cardiorespiratory collapse followed by disseminated intravascular coagulation (DIC). AFE is rare, affecting only about 2 to 6 per 100,000 births, but classic cases have a reported maternal mortality rate that exceeds 50%.1 It is thought to reflect a complex, systemic proinflammatory response to maternal intravasation of pregnancy material, such as trophoblast, thromboplastins, fetal cells, or amniotic fluid. Because the syndrome is not necessarily directly caused by emboli or by amniotic fluid per se,2 it has been proposed that AFE be called “anaphylactoid syndrome of pregnancy,” but this terminology has not yet been widely adopted.3
Guidelines from the Society for Maternal-Fetal Medicine (SMFM) recommend several time-critical steps for the initial stabilization and management of patients with AFE.4 However, because AFE is rare, most obstetric providers may not encounter a case for many years or even decades after they have received training, so it is unrealistic to expect that they will remember these guidelines when they are needed. For this reason, when AFE occurs, it is important to have a readily accessible cognitive aid, such as a checklist that summarizes the key management steps. The SMFM provides a checklist for initial management of AFE that can be used at your institution; it is presented in the FIGURE and provides the outline for this discussion.5

Provide CPR immediately
Most AFE cases are accompanied by cardiorespiratory arrest. If the patient has no pulse, call a “code” to mobilize additional help and immediately start CPR. Use a backboard to make cardiac compressions most effective and manually displace the uterus or tilt the patient to avoid supine hypotension. Designate a timekeeper to call out 1-minute intervals and record critical data, such as medication administration and laboratory orders/results.
Expedite delivery
Immediate delivery is needed if maternal cardiac activity is not restored within 4 minutes of starting CPR, with a target to have delivery completed within 5 minutes. Operative vaginal delivery may be an option if delivery is imminent, as in the case presented, but cesarean delivery (CD) will be needed in most cases. This was previously called “perimortem cesarean” delivery, but the term “resuscitative hysterotomy” has been proposed because the primary goal is to improve the effectiveness of CPR6 and prevent both maternal and perinatal death. CPR is less effective in pregnant women because the pregnant uterus takes a substantial fraction of the maternal cardiac output, as well as compresses the vena cava. Some experts suggest that, rather than waiting 4 minutes, CD should be started as soon as an obstetrician or other surgeon is present, unless there is an immediate response to electrical cardioversion.6,7
In most cases, immediate CD should be performed wherever the patient is located rather than using precious minutes to move the patient to an operating room. Antiseptic preparation is expedited by simply pouring povidone-iodine or chlorhexidine over the lower abdomen if readily available; if not available, skip this step. Enter the abdomen and uterus as rapidly as possible using only a scalpel to make generous midline incisions.
If CPR is not required, proceed with cesarean or operative vaginal delivery as soon as the mother has been stabilized. These procedures should be performed using standard safety precautions outlined in the SMFM patient safety checklists for cesarean or operative vaginal delivery.8,9
Continue to: Anticipate hemorrhage...
Anticipate hemorrhage
Be prepared for uterine atony, coagulopathy, and catastrophic hemorrhage. Initiate IV oxytocin prophylaxis as soon as the infant is delivered. Have a low threshold for giving other uterotonic agents such as methylergonovine, carboprost, or misoprostol. If hemorrhage or DIC occurs, give tranexamic acid. Have the anesthesiologist or trauma team (if available) insert an intraosseous line for fluid resuscitation if peripheral IV access is inadequate.
Massive transfusion is often needed to treat DIC, which occurs in most AFE cases. Anticipate—do not wait—for DIC to occur. We propose activating your hospital’s massive transfusion protocol (MTP) as soon as you diagnose AFE so that blood products will be available as soon as possible. A typical MTP provides several units of red blood cells, a pheresis pack of platelets, and fresh/frozen plasma (FFP). If clinically indicated, administer cryoprecipitate instead of FFP to minimize volume overload, which may occur with FFP.
CASE Part 2: MTP initiated to treat DIC
The MTP is initiated. Laboratory results immediately pre-transfusion include hemoglobin 11.3 g/dL, platelet count 46,000 per mm3, fibrinogen 87 mg/dL, and an elevated prothrombin time international normalized ratio.
Expect heart failure
The initial hemodynamic picture in AFE is right heart failure, which should optimally be managed by a specialist from anesthesiology, cardiology, or critical care as soon as they are available. An emergency department physician may manage the hemodynamics until a specialist arrives. Avoidance of fluid overload is one important principle. If fluid challenges are needed for hypovolemic shock, boluses should be restricted to 500 mL rather than the traditional 1000 mL.
Pharmacologic treatment may include vasopressors, inotropic agents, and pulmonary vasodilators. Example medications and dosages recommended by SMFM are summarized in the checklist (FIGURE).5
After the initial phase of recovery, the hemodynamic picture often changes from right heart failure to left heart failure. Management of left heart failure is not covered in the SMFM checklist because, by the time it appears, the patient will usually be in the intensive care unit, managed by the critical care team. Management of left heart failure generally includes diuresis as needed for cardiogenic pulmonary edema, optimization of cardiac preload, and inotropic agents or vasopressors if needed to maintain cardiac output or perfusion pressure.4
Debrief, learning opportunities
Complex emergencies such as AFE are rarely handled 100% perfectly, even those with a good outcome, so they present opportunities for team learning and improvement. The team should conduct a 10- to 15-minute debrief soon after the patient is stabilized. Make an explicit statement that the main goal of the debrief is to gather suggestions as to how systems and processes could be improved for next time, not to find fault or lay blame on individuals. Encourage all personnel involved in the initial management to attend and discuss what went well and what did not. Another goal is to provide support for individuals who may feel traumatized by the dramatic, frightening events surrounding an AFE and by the poor patient outcome or guarded prognosis that frequently follows. Another goal is to discuss the plan for providing support and disclosure to the patient and family.
The vast majority of AFE cases meet criteria to be designated as “sentinel events,” because of patient transfer to the intensive care unit, multi-unit blood transfusion, other severe maternal morbidities, or maternal death. Therefore, most AFE cases will trigger a root cause analysis (RCA) or other formal sentinel event analysis conducted by the hospital’s Safety or Quality Department. As with the immediate post-event debrief, the first goal of the RCA is to identify systems issues that may have resulted in suboptimal care and that can be modified to improve future care. Specific issues regarding the checklist should also be addressed:
- Was the checklist used?
- Was the checklist available?
- Are there items on the checklist that need to be modified, added, or deleted?
The RCA concludes with the development of a performance improvement plan.
Ultimately, we encourage all AFE cases be reported to the registry maintained by the Amniotic Fluid Embolism Foundation at https://www.afesupport.org/, regardless of whether the outcome was favorable for the mother and newborn. The registry includes over 130 AFE cases since 2013 from around the world. Researchers periodically report on the registry findings.10 If providers report cases with both good and bad outcomes, the registry may provide future insights regarding which adjunctive or empiric treatments may or may not be promising.
Continue to: Empiric treatments...
Empiric treatments
From time-to-time, new regimens for empiric treatment of AFE are reported. It is important to recognize that these reports are generally uncontrolled case reports of favorable outcomes and that, without a control group, it is impossible to determine to what extent the treatment contributed to the outcome or was merely incidental. Given the rarity of AFE, it seems unlikely that there will ever be a randomized clinical trial or even a controlled prospective study comparing treatment regimens.
The “A-OK” regimen is an empiric treatment that has garnered some interest after an initial case report.11 It consists of an anticholinergic agent (atropine 0.2 mg IV), a selective 5-HT3 receptor antagonist (ondansetron 8 mg IV), and a nonsteroidal anti-inflammatory drug (ketorolac 15 mg IV). We have some reservations about this regimen, however, because atropine is relatively contraindicated if the patient has tachycardia (which is common in patients with hemorrhage) and ketorolac may suppress platelet function, which might be harmful for patients with DIC or thrombocytopenia.
Another empiric treatment is the “50-50-500” regimen, which includes an H1 antihistamine (diphenhydramine 50 mg IV), an H2 antihistamine (famotidine 50 mg IV), and a corticosteroid (hydrocortisone 500 mg IV). This regimen aims to suppress histamine-mediated and cell-mediated inflammatory responses, based on the notion that proinflammatory responses likely mediate much of the underlying pathophysiology of the AFE syndrome.
We would emphasize that these empiric regimens are not clinically validated, US Food and Drug Administration approved for treatment of AFE, or considered standard of care. Future reports of these and other regimens will be needed to evaluate their efficacy, limitations, and risks. Again, we encourage providers to report all AFE cases to the AFE Foundation registry, regardless of whether the treatments are successful.
CASE Conclusion
The hemorrhage stops after administration of oxytocin, carboprost, 6 units of cryoprecipitate, and a 6-unit platelet pheresis pack. The patient is transferred to the intensive care unit where she eventually requires a total of 10 units of red cells, 8 more units of cryoprecipitate, and another platelet pheresis pack. She is discharged to home in stable condition on postpartum day 4.
Be prepared, have the checklist ready
Because AFE is rare, most members of the health care team will have no prior experience managing a real case. It may have been years or decades since they had any education on AFE or they last read a review article such as this one. It is even possible the anesthesiologist, cardiologist, or critical care specialist has never heard of AFE. Thus if they rely on memory alone, there is substantial risk of forgetting items, getting dosages wrong, or other errors. With this in mind, what is the best way to prepare the team to expeditiously employ the management steps outlined here?
Use of a checklist that summarizes these key steps for early management, such as the SMFM checklist in the FIGURE, will help ensure that all relevant steps are performed in every AFE case. It is designed to be printed on a single sheet of letter-sized paper, and we propose that every labor and delivery (L&D) unit keep laminated copies of this checklist in several places where they will be immediately available should an AFE occur. Copies can be kept on the anesthesia carts in the L&D operating rooms, in an emergency procedures binder on the unit, and on the “crash carts” and hemorrhage supply carts in the L&D unit. Effective implementation of an AFE checklist requires all personnel know where to readily find it and have some familiarity with its contents.
An interdisciplinary team comprising representatives from nursing, obstetrics, and anesthesia should meet to discuss whether the checklist needs to be modified to fit the local hospital formulary or other unique local circumstances. The team should develop an implementation plan that includes where to keep checklist copies, a process to periodically ensure that the copies are still present and readable, a roll-out plan to inform all personnel about the checklist process, and most importantly a training plan that includes incorporating AFE cases into the schedule of multidisciplinary simulations and drills for obstetric emergencies. Other implementation strategies are outlined in the SMFM document.5
Ultimately an organized, systematic approach is recommended for management of AFE. There is no single best treatment of AFE; it is supportive and directed toward the underlying pathophysiology, which may vary from patient to patient. Therefore, although a checklist, in conjunction with regular education and simulation activities, may help optimize care and improve outcomes, there is still a high risk of maternal morbidity and mortality from AFE. ●
- Clark SL. Amniotic fluid embolism. Obstet Gynecol. 2014;123(2 Pt 1):337-348. doi:10.1097/AOG.0000000000000107.
- Funk M, Damron A, Bandi V, et al. Pulmonary vascular obstruction by squamous cells is not involved in amniotic fluid embolism. Am J Obstet Gynecol. 2018;218:460-461. doi:10.1016/j.ajog.2017.12.225.
- Gilmore DA, Wakim J, Secrest J, et al. Anaphylactoid syndrome of pregnancy: a review of the literature with latest management and outcome data. AANA J. 2003;71:120-126.
- Society for Maternal-Fetal Medicine, Pacheco LD, Saade G, et al. Amniotic fluid embolism: diagnosis and management. Am J Obstet Gynecol. 2016;215:B16-24. doi:10.1016/j.ajog.2016.03.012.
- Patient Safety and Quality Committee, Society for Maternal-Fetal Medicine; Combs CA, Montgomery DM, et al. Society for Maternal-Fetal Medicine Special Statement: checklist for initial management of amniotic fluid embolism. Am J Obstet Gynecol. 2021;224:B29-B32. doi:10.1016/j.ajog.2021.01.001.
- Rose CH, Faksh A, Traynor KD, et al. Challenging the 4- to 5-minute rule: from perimortem cesarean to resuscitative hysterotomy. Am J Obstet Gynecol. 2015;213:653-6, 653.e1. doi:10.1016/j.ajog.2015.07.019.
- Pacheco LD, Clark SL, Klassen M, et al. Amniotic fluid embolism: principles of early clinical management. Am J Obstet Gynecol. 2020;222:48-52. doi:10.1016/j.ajog.2019.07.036.
- Combs CA, Einerson BD, Toner LE, SMFM Patient Safety and Quality Committee. SMFM Special Statement: surgical safety checklists for cesarean delivery. Am J Obstet Gynecol. 2021;225:B43-B49. doi:10.1016/j.ajog.2021.07.011.
- SMFM Patient Safety and Quality Committee, Staat B, Combs CA. SMFM Special Statement: operative vaginal delivery: checklists for performance and documentation. Am J Obstet Gynecol. 2020;222:B15-B21. doi:10.1016/j.ajog.2020.02.011.
- Stafford IA, Moaddab A, Dildy GA, et al. Amniotic fluid embolism syndrome: analysis of the United States international registry. Am J Obstet Gynecol MFM. 2020;2:100083. doi:10.1016/j.ajogmf.2019.100083.
- Rezai S, Hughes AZC, Larsen TB, et al. Atypical amniotic f luid embolism managed with a novel therapeutic regimen. Case Rep Obstet Gynecol. 2017; 2017:8458375. doi:10.1155/2017/8458375.
- Clark SL. Amniotic fluid embolism. Obstet Gynecol. 2014;123(2 Pt 1):337-348. doi:10.1097/AOG.0000000000000107.
- Funk M, Damron A, Bandi V, et al. Pulmonary vascular obstruction by squamous cells is not involved in amniotic fluid embolism. Am J Obstet Gynecol. 2018;218:460-461. doi:10.1016/j.ajog.2017.12.225.
- Gilmore DA, Wakim J, Secrest J, et al. Anaphylactoid syndrome of pregnancy: a review of the literature with latest management and outcome data. AANA J. 2003;71:120-126.
- Society for Maternal-Fetal Medicine, Pacheco LD, Saade G, et al. Amniotic fluid embolism: diagnosis and management. Am J Obstet Gynecol. 2016;215:B16-24. doi:10.1016/j.ajog.2016.03.012.
- Patient Safety and Quality Committee, Society for Maternal-Fetal Medicine; Combs CA, Montgomery DM, et al. Society for Maternal-Fetal Medicine Special Statement: checklist for initial management of amniotic fluid embolism. Am J Obstet Gynecol. 2021;224:B29-B32. doi:10.1016/j.ajog.2021.01.001.
- Rose CH, Faksh A, Traynor KD, et al. Challenging the 4- to 5-minute rule: from perimortem cesarean to resuscitative hysterotomy. Am J Obstet Gynecol. 2015;213:653-6, 653.e1. doi:10.1016/j.ajog.2015.07.019.
- Pacheco LD, Clark SL, Klassen M, et al. Amniotic fluid embolism: principles of early clinical management. Am J Obstet Gynecol. 2020;222:48-52. doi:10.1016/j.ajog.2019.07.036.
- Combs CA, Einerson BD, Toner LE, SMFM Patient Safety and Quality Committee. SMFM Special Statement: surgical safety checklists for cesarean delivery. Am J Obstet Gynecol. 2021;225:B43-B49. doi:10.1016/j.ajog.2021.07.011.
- SMFM Patient Safety and Quality Committee, Staat B, Combs CA. SMFM Special Statement: operative vaginal delivery: checklists for performance and documentation. Am J Obstet Gynecol. 2020;222:B15-B21. doi:10.1016/j.ajog.2020.02.011.
- Stafford IA, Moaddab A, Dildy GA, et al. Amniotic fluid embolism syndrome: analysis of the United States international registry. Am J Obstet Gynecol MFM. 2020;2:100083. doi:10.1016/j.ajogmf.2019.100083.
- Rezai S, Hughes AZC, Larsen TB, et al. Atypical amniotic f luid embolism managed with a novel therapeutic regimen. Case Rep Obstet Gynecol. 2017; 2017:8458375. doi:10.1155/2017/8458375.
Cutting dietary simple sugars may relieve GERD symptoms
Minimizing dietary consumption of simple sugars may help alleviate symptoms of gastroesophageal reflux disease (GERD), new data suggest.
People who consumed lower quantities of simple sugars experienced significant improvement in total acid exposure time, number of reflux episodes, and subjective reports of heartburn and reflux symptoms, compared with those consuming higher amounts of simple sugars, the authors report.
The authors call their study the first randomized controlled diet intervention trial to investigate both the amount and type of carbohydrate on symptomatic GERD and one of only a few to investigate any type of dietary intervention for GERD.
“There’s really almost no rigorous scientific evidence on any of the foods or ingredients or nutrients that are often recommended to avoid if you have GERD,” corresponding author Heidi J. Silver, RD, PhD, told this news organization. Dr. Silver is research professor of medicine at Vanderbilt University School of Medicine and director of the Vanderbilt Diet, Body Composition, and Human Metabolism Core in Nashville, Tenn.
Even the avoidance of fatty foods, which has been long promoted as part of GERD management, has little evidence to support it.
“With fat, there’s some belief that it may slow down gastric emptying. Therefore, if you had slower gastric emptying, you would have a longer time for the food to put pressure on the lower esophageal sphincter and create an environment for reflux. So, it’s kind of conceptually what is thought but not really tested,” she notes.
The findings were published online in the American Journal of Gastroenterology.
Greatest symptom reduction with low simple carb intake
To test the role of dietary carbohydrates, Dr. Silver and her colleagues randomly assigned 98 U.S. veterans with symptomatic GERD to intake of one of four diets with varying carbohydrate types and amounts for 9 weeks: high total/high simple (the control group), high total/low simple, low total/high simple, or low total/low simple. The total caloric intake was approximately the same for all groups.
At baseline, the average total carbohydrate consumption was 43.7% of calories, and the average simple sugar intake was 116.5 g/d. The two “low-total” groups averaged about a 10% reduction in carbohydrate calories. The “low-simple” groups reduced simple sugars by about half.
There were no changes in body weight in the control group, whereas all three of the other groups lost some weight, ranging from 1.5-2.0 kg (3.3-4.4 lb) despite calorie totals designed for weight maintenance.
There was a significant effect of diet on the two primary outcomes, total esophageal acid exposure time, and total number of reflux episodes, as measured by 24-hour ambulatory pH monitoring. The change in total acid exposure time was significantly greater for the high total/low simple group, compared with the high total/high simple group.
The participants’ ratings of symptoms assessed by the Gastroesophageal Reflux Disease Questionnaire and the GERD Symptom Assessment Scale, including heartburn frequency and severity, pain in throat/chest, and sleep disturbance, improved in all modified diet groups, compared with the control group. The mean degree of improvement in heartburn and regurgitation was twice as great for the modified diets, compared with the controls, and was greatest for the two “low-simple” carb groups.
Dr. Silver and colleagues hypothesize that the differential effects of the diets may relate to the way that dietary carbohydrates are sensed in the gastrointestinal tract after being enzymatically degraded into monosaccharides, possibly affecting lower esophageal tone via the effects of gut-derived hormones including ghrelin and glucagon-like peptide 1 that are secreted in response to macronutrient intake.
Although more data are needed about the effects of carbohydrates in GERD, Dr. Silver advised, “I do think it would be smart for clinicians, when they’re discussing diet, that they bring up the simple sugars. There’s no potential harm in reducing simple sugars. You’re only benefiting yourself in multiple ways. We know that the consumption of simple sugars is extremely excessive, not just in America but worldwide.”
Asked to comment, Philip O. Katz, MD, professor of medicine and director of the GI Function Laboratories at Weill Cornell Medicine, New York, told this news organization that “this is one of the better-designed studies with a lot of care looking at a lot of endpoints that are intriguing and useful.”
“What it says to me is there is potential for nonpharmacologic interventions for GERD that include diet change for helping patients,” he said. “This shows promise for a diet that doesn’t just concentrate on fat or acidic products and is a possible way of augmenting reflux treatment.”
However, Dr. Katz cautioned, “I don’t think anybody should do more with a 9-week study than look at it as good potential.”
“I would tell patients that this is something that they might try, but I wouldn’t make it a rigid requirement based on these data,” he added. “If I were involved in this study, the next thing I would do is transition it to real life and look at compliance to see if results were sustained at 18 weeks or 6 months.”
Diet part of an ‘overall reflux program’
Overall, Dr. Katz, who was the first author of the American College of Gastroenterology’s Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease, pointed out that the main nonpharmacologic advice for GERD management includes “Eat smaller meals, don’t eat before bed, don’t lie down after you eat, and reduce any individually known trigger foods.”
Essentially, he views dietary interventions as complementary to medication and other interventions.
“When people really have GERD – not just heartburn – dietary change is an adjunct,” Dr. Katz said. “GERD is a real disease, like diabetes. For some with GERD, maybe this is the only thing they need to do. But, in general, this will be an adjunct to augment an overall reflux program.”
And that program, both Dr. Silver and Dr. Katz said, should include referral to a dietitian or nutritionist.
“If you’re going to invest time in getting your patients to change their diet, it should be done with professional help, a qualified nutritionist who can work with the patient. This should not be a fad,” Dr. Katz said.
Dr. Silver noted, “In contrast to a physician, the dietitian looks at everything the person is eating. If they’re consuming a lot of simple sugars, I certainly would make that recommendation to reduce, along with other recommendations for GERD. It could be easily incorporated. We included examples in the paper of what we did to achieve that reduction and some things clinicians could focus on.”
One obvious approach, she said, is to advise patients to cut the amount of sugared soda they’re drinking, if not eliminate it entirely.
The study was funded by a VA Merit Award. Dr. Silver has no further disclosures. Dr. Katz is a consultant for Phathom Pharmaceuticals and Sebella Pharmaceuticals and serves on an advisory board for AstraZeneca.
A version of this article first appeared on Medscape.com.
Minimizing dietary consumption of simple sugars may help alleviate symptoms of gastroesophageal reflux disease (GERD), new data suggest.
People who consumed lower quantities of simple sugars experienced significant improvement in total acid exposure time, number of reflux episodes, and subjective reports of heartburn and reflux symptoms, compared with those consuming higher amounts of simple sugars, the authors report.
The authors call their study the first randomized controlled diet intervention trial to investigate both the amount and type of carbohydrate on symptomatic GERD and one of only a few to investigate any type of dietary intervention for GERD.
“There’s really almost no rigorous scientific evidence on any of the foods or ingredients or nutrients that are often recommended to avoid if you have GERD,” corresponding author Heidi J. Silver, RD, PhD, told this news organization. Dr. Silver is research professor of medicine at Vanderbilt University School of Medicine and director of the Vanderbilt Diet, Body Composition, and Human Metabolism Core in Nashville, Tenn.
Even the avoidance of fatty foods, which has been long promoted as part of GERD management, has little evidence to support it.
“With fat, there’s some belief that it may slow down gastric emptying. Therefore, if you had slower gastric emptying, you would have a longer time for the food to put pressure on the lower esophageal sphincter and create an environment for reflux. So, it’s kind of conceptually what is thought but not really tested,” she notes.
The findings were published online in the American Journal of Gastroenterology.
Greatest symptom reduction with low simple carb intake
To test the role of dietary carbohydrates, Dr. Silver and her colleagues randomly assigned 98 U.S. veterans with symptomatic GERD to intake of one of four diets with varying carbohydrate types and amounts for 9 weeks: high total/high simple (the control group), high total/low simple, low total/high simple, or low total/low simple. The total caloric intake was approximately the same for all groups.
At baseline, the average total carbohydrate consumption was 43.7% of calories, and the average simple sugar intake was 116.5 g/d. The two “low-total” groups averaged about a 10% reduction in carbohydrate calories. The “low-simple” groups reduced simple sugars by about half.
There were no changes in body weight in the control group, whereas all three of the other groups lost some weight, ranging from 1.5-2.0 kg (3.3-4.4 lb) despite calorie totals designed for weight maintenance.
There was a significant effect of diet on the two primary outcomes, total esophageal acid exposure time, and total number of reflux episodes, as measured by 24-hour ambulatory pH monitoring. The change in total acid exposure time was significantly greater for the high total/low simple group, compared with the high total/high simple group.
The participants’ ratings of symptoms assessed by the Gastroesophageal Reflux Disease Questionnaire and the GERD Symptom Assessment Scale, including heartburn frequency and severity, pain in throat/chest, and sleep disturbance, improved in all modified diet groups, compared with the control group. The mean degree of improvement in heartburn and regurgitation was twice as great for the modified diets, compared with the controls, and was greatest for the two “low-simple” carb groups.
Dr. Silver and colleagues hypothesize that the differential effects of the diets may relate to the way that dietary carbohydrates are sensed in the gastrointestinal tract after being enzymatically degraded into monosaccharides, possibly affecting lower esophageal tone via the effects of gut-derived hormones including ghrelin and glucagon-like peptide 1 that are secreted in response to macronutrient intake.
Although more data are needed about the effects of carbohydrates in GERD, Dr. Silver advised, “I do think it would be smart for clinicians, when they’re discussing diet, that they bring up the simple sugars. There’s no potential harm in reducing simple sugars. You’re only benefiting yourself in multiple ways. We know that the consumption of simple sugars is extremely excessive, not just in America but worldwide.”
Asked to comment, Philip O. Katz, MD, professor of medicine and director of the GI Function Laboratories at Weill Cornell Medicine, New York, told this news organization that “this is one of the better-designed studies with a lot of care looking at a lot of endpoints that are intriguing and useful.”
“What it says to me is there is potential for nonpharmacologic interventions for GERD that include diet change for helping patients,” he said. “This shows promise for a diet that doesn’t just concentrate on fat or acidic products and is a possible way of augmenting reflux treatment.”
However, Dr. Katz cautioned, “I don’t think anybody should do more with a 9-week study than look at it as good potential.”
“I would tell patients that this is something that they might try, but I wouldn’t make it a rigid requirement based on these data,” he added. “If I were involved in this study, the next thing I would do is transition it to real life and look at compliance to see if results were sustained at 18 weeks or 6 months.”
Diet part of an ‘overall reflux program’
Overall, Dr. Katz, who was the first author of the American College of Gastroenterology’s Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease, pointed out that the main nonpharmacologic advice for GERD management includes “Eat smaller meals, don’t eat before bed, don’t lie down after you eat, and reduce any individually known trigger foods.”
Essentially, he views dietary interventions as complementary to medication and other interventions.
“When people really have GERD – not just heartburn – dietary change is an adjunct,” Dr. Katz said. “GERD is a real disease, like diabetes. For some with GERD, maybe this is the only thing they need to do. But, in general, this will be an adjunct to augment an overall reflux program.”
And that program, both Dr. Silver and Dr. Katz said, should include referral to a dietitian or nutritionist.
“If you’re going to invest time in getting your patients to change their diet, it should be done with professional help, a qualified nutritionist who can work with the patient. This should not be a fad,” Dr. Katz said.
Dr. Silver noted, “In contrast to a physician, the dietitian looks at everything the person is eating. If they’re consuming a lot of simple sugars, I certainly would make that recommendation to reduce, along with other recommendations for GERD. It could be easily incorporated. We included examples in the paper of what we did to achieve that reduction and some things clinicians could focus on.”
One obvious approach, she said, is to advise patients to cut the amount of sugared soda they’re drinking, if not eliminate it entirely.
The study was funded by a VA Merit Award. Dr. Silver has no further disclosures. Dr. Katz is a consultant for Phathom Pharmaceuticals and Sebella Pharmaceuticals and serves on an advisory board for AstraZeneca.
A version of this article first appeared on Medscape.com.
Minimizing dietary consumption of simple sugars may help alleviate symptoms of gastroesophageal reflux disease (GERD), new data suggest.
People who consumed lower quantities of simple sugars experienced significant improvement in total acid exposure time, number of reflux episodes, and subjective reports of heartburn and reflux symptoms, compared with those consuming higher amounts of simple sugars, the authors report.
The authors call their study the first randomized controlled diet intervention trial to investigate both the amount and type of carbohydrate on symptomatic GERD and one of only a few to investigate any type of dietary intervention for GERD.
“There’s really almost no rigorous scientific evidence on any of the foods or ingredients or nutrients that are often recommended to avoid if you have GERD,” corresponding author Heidi J. Silver, RD, PhD, told this news organization. Dr. Silver is research professor of medicine at Vanderbilt University School of Medicine and director of the Vanderbilt Diet, Body Composition, and Human Metabolism Core in Nashville, Tenn.
Even the avoidance of fatty foods, which has been long promoted as part of GERD management, has little evidence to support it.
“With fat, there’s some belief that it may slow down gastric emptying. Therefore, if you had slower gastric emptying, you would have a longer time for the food to put pressure on the lower esophageal sphincter and create an environment for reflux. So, it’s kind of conceptually what is thought but not really tested,” she notes.
The findings were published online in the American Journal of Gastroenterology.
Greatest symptom reduction with low simple carb intake
To test the role of dietary carbohydrates, Dr. Silver and her colleagues randomly assigned 98 U.S. veterans with symptomatic GERD to intake of one of four diets with varying carbohydrate types and amounts for 9 weeks: high total/high simple (the control group), high total/low simple, low total/high simple, or low total/low simple. The total caloric intake was approximately the same for all groups.
At baseline, the average total carbohydrate consumption was 43.7% of calories, and the average simple sugar intake was 116.5 g/d. The two “low-total” groups averaged about a 10% reduction in carbohydrate calories. The “low-simple” groups reduced simple sugars by about half.
There were no changes in body weight in the control group, whereas all three of the other groups lost some weight, ranging from 1.5-2.0 kg (3.3-4.4 lb) despite calorie totals designed for weight maintenance.
There was a significant effect of diet on the two primary outcomes, total esophageal acid exposure time, and total number of reflux episodes, as measured by 24-hour ambulatory pH monitoring. The change in total acid exposure time was significantly greater for the high total/low simple group, compared with the high total/high simple group.
The participants’ ratings of symptoms assessed by the Gastroesophageal Reflux Disease Questionnaire and the GERD Symptom Assessment Scale, including heartburn frequency and severity, pain in throat/chest, and sleep disturbance, improved in all modified diet groups, compared with the control group. The mean degree of improvement in heartburn and regurgitation was twice as great for the modified diets, compared with the controls, and was greatest for the two “low-simple” carb groups.
Dr. Silver and colleagues hypothesize that the differential effects of the diets may relate to the way that dietary carbohydrates are sensed in the gastrointestinal tract after being enzymatically degraded into monosaccharides, possibly affecting lower esophageal tone via the effects of gut-derived hormones including ghrelin and glucagon-like peptide 1 that are secreted in response to macronutrient intake.
Although more data are needed about the effects of carbohydrates in GERD, Dr. Silver advised, “I do think it would be smart for clinicians, when they’re discussing diet, that they bring up the simple sugars. There’s no potential harm in reducing simple sugars. You’re only benefiting yourself in multiple ways. We know that the consumption of simple sugars is extremely excessive, not just in America but worldwide.”
Asked to comment, Philip O. Katz, MD, professor of medicine and director of the GI Function Laboratories at Weill Cornell Medicine, New York, told this news organization that “this is one of the better-designed studies with a lot of care looking at a lot of endpoints that are intriguing and useful.”
“What it says to me is there is potential for nonpharmacologic interventions for GERD that include diet change for helping patients,” he said. “This shows promise for a diet that doesn’t just concentrate on fat or acidic products and is a possible way of augmenting reflux treatment.”
However, Dr. Katz cautioned, “I don’t think anybody should do more with a 9-week study than look at it as good potential.”
“I would tell patients that this is something that they might try, but I wouldn’t make it a rigid requirement based on these data,” he added. “If I were involved in this study, the next thing I would do is transition it to real life and look at compliance to see if results were sustained at 18 weeks or 6 months.”
Diet part of an ‘overall reflux program’
Overall, Dr. Katz, who was the first author of the American College of Gastroenterology’s Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease, pointed out that the main nonpharmacologic advice for GERD management includes “Eat smaller meals, don’t eat before bed, don’t lie down after you eat, and reduce any individually known trigger foods.”
Essentially, he views dietary interventions as complementary to medication and other interventions.
“When people really have GERD – not just heartburn – dietary change is an adjunct,” Dr. Katz said. “GERD is a real disease, like diabetes. For some with GERD, maybe this is the only thing they need to do. But, in general, this will be an adjunct to augment an overall reflux program.”
And that program, both Dr. Silver and Dr. Katz said, should include referral to a dietitian or nutritionist.
“If you’re going to invest time in getting your patients to change their diet, it should be done with professional help, a qualified nutritionist who can work with the patient. This should not be a fad,” Dr. Katz said.
Dr. Silver noted, “In contrast to a physician, the dietitian looks at everything the person is eating. If they’re consuming a lot of simple sugars, I certainly would make that recommendation to reduce, along with other recommendations for GERD. It could be easily incorporated. We included examples in the paper of what we did to achieve that reduction and some things clinicians could focus on.”
One obvious approach, she said, is to advise patients to cut the amount of sugared soda they’re drinking, if not eliminate it entirely.
The study was funded by a VA Merit Award. Dr. Silver has no further disclosures. Dr. Katz is a consultant for Phathom Pharmaceuticals and Sebella Pharmaceuticals and serves on an advisory board for AstraZeneca.
A version of this article first appeared on Medscape.com.
Benzbromarone tops febuxostat for gout?
Benzbromarone is not approved in the United States because of concerns of acute liver injury but is approved in several other countries, including China, Brazil, and New Zealand.
“The results suggest that low dosing of benzbromarone may warrant stronger consideration as a safe and effective therapy to achieve serum urate target in gout without moderate chronic kidney disease,” the study team writes.
“Benzbromarone is severely hepatotoxic in some individuals and unlikely to ever gain approval in the United States,” one of the study’s investigators, Robert Terkeltaub, MD, professor of medicine, University of California, San Diego, told this news organization.
However, this study “illustrates the value and impact of uricosuric therapy in general in gout, including potentially as an initial urate-lowering monotherapy strategy, and the sheer number of subjects reaching urate target with low-dose uricosuric monotherapy was impressive,” Dr. Terkeltaub said.
The study was published online in Arthritis & Rheumatology.
“Renal uric acid underexcretion is the chief mechanism driving hyperuricemia in gout, yet the standard urate-lowering therapy recommendation is first-line xanthine oxidase inhibition irrespective of the cause of hyperuricemia,” the study team explains in their article.
Their prospective, randomized, single-center, open-labeled trial was conducted at the Gout Clinic of the Affiliated Hospital of Qingdao University, China.
A total of 196 relatively young healthy men with gout and uric acid underexcretion were randomly assigned to receive low-dose benzbromarone (25 mg/d) or low-dose febuxostat (20 mg/d) for 12 weeks.
Renal uric acid underexcretion was defined as fractional excretion of urate less than 5.5% and uric acid excretion less than or equal to 600 mg/d/1.73 m2.
A “major aspect” of this comparative effectiveness trial was its specific focus on gout-associated renal uric acid underexcretion, where the uricosuric targeted the dominant abnormality promoting the hyperuricemia, Dr. Terkeltaub told this news organization.
In addition, all participants received daily urine alkalinization with oral sodium bicarbonate. “This is not always done in clinical practice, nor in clinical trials of uricosuric agents,” Dr. Terkeltaub said.
The results showed that more participants in the benzbromarone group achieved the serum urate target of less than 6 mg/dL, compared with those in the febuxostat group (primary endpoint, 61% vs. 32%, P < .001).
Adverse events, including gout flares and urolithiasis, did not differ significantly between the two groups, with the exception of more transaminase elevation in the febuxostat group (15% vs. 4%; P = .008).
“We did not find severe hepatotoxicity with low-dose benzbromarone, but ethnic background may affect drug responses, and severe hepatotoxicity of benzbromarone has rarely been reported in Asia,” the authors write.
The incidence of urolithiasis was numerically, but not significantly, higher in the benzbromarone group (5% vs. 2%).
This study found no significant changes in participants’ triglyceride levels, though a previous study suggested febuxostat could increase serum triglycerides.
The investigators caution that the study only included patients who had baseline serum urate levels ranging from 8.0 to 10 mg/dL, who were relatively young and with few comorbidities.
The authors further noted that the “... results may not be generalizable to patients with higher serum urate levels or impaired kidney function, as well [as] patients from other geographical regions, age, and ethnicity groups. The study only included men, and the findings may not be generalizable to women with gout.”
‘Very useful’ in select cases
Weighing in on the results, Valderilio Feijó Azevedo, MD, PhD, adjunct professor of rheumatology, Federal University of Paraná, Brazil, noted that in some specific clinical circumstances, benzbromarone has been “a very useful medication, alone or combined, to treat gout patients.”
“We have great experience with the drug in Brazil. However, it is not used to treat all patients. Patients must be very well-selected in our clinical practice,” Dr. Azevedo said in an interview.
“For most patients, benzbromarone is effective for those who have failed to achieve serum uric acid goals with allopurinol treatment. We do not use it to treat patients with asymptomatic hyperuricemia. In general, we avoid patients with hepatic dysfunction due to previous hepatotoxicity reports. In every patient, we do active monitoring of enzymes,” Dr. Azevedo explained.
“We also avoid using it in patients with severe kidney disease. However, we have used it in some patients with estimated glomerular filtration rate less than 30. We also avoid dosage over 200 mg per day. On average, we use 100 mg per day combined with allopurinol or alone,” said Dr. Azevedo, who was not involved with the study.
Also weighing in, Michael Pillinger, MD, rheumatologist at NYU Langone Health, noted that while benzbromarone is not used in the United States, “in many parts of the world, it is used and is felt to be effective.” Dr. Pillinger was not associated with this current research.
This study, Dr. Pillinger said, “does underline the fact that an alternative drug that lowers urate by promoting urate excretion, if it could gain [U.S. Food and Drug Association] approval and if it were safe, could present a viable new option for therapy.”
He added, “If one conclusion to the study is that determining the basis of hyperuricemia is helpful in guiding benzbromarone use, that implies an additional layer of effort for physicians and patients in a disease that is already notoriously known for patient noncompliance – and in a case where febuxostat and allopurinol will work for both overproducers and underexcreters and would not need this additional assessment.”
The study was sponsored by Shandong Provincial Key Research and Development Plan, the National Natural Science Foundation of China, and Shandong Provincial Science Foundation for Outstanding Youth Scholarship. Dr. Terkeltaub was supported by the National Institutes of Health and the VA Research Service. Dr. Terkeltaub has received research funding from AstraZeneca, and has consulted with Horizon, Selecta, SOBI, Dyve BioSciences, Fortress, AstraZeneca, Allena, Fortress Biotech, and LG Life Sciences. Dr. Azevedo and Dr. Pillinger have no reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Benzbromarone is not approved in the United States because of concerns of acute liver injury but is approved in several other countries, including China, Brazil, and New Zealand.
“The results suggest that low dosing of benzbromarone may warrant stronger consideration as a safe and effective therapy to achieve serum urate target in gout without moderate chronic kidney disease,” the study team writes.
“Benzbromarone is severely hepatotoxic in some individuals and unlikely to ever gain approval in the United States,” one of the study’s investigators, Robert Terkeltaub, MD, professor of medicine, University of California, San Diego, told this news organization.
However, this study “illustrates the value and impact of uricosuric therapy in general in gout, including potentially as an initial urate-lowering monotherapy strategy, and the sheer number of subjects reaching urate target with low-dose uricosuric monotherapy was impressive,” Dr. Terkeltaub said.
The study was published online in Arthritis & Rheumatology.
“Renal uric acid underexcretion is the chief mechanism driving hyperuricemia in gout, yet the standard urate-lowering therapy recommendation is first-line xanthine oxidase inhibition irrespective of the cause of hyperuricemia,” the study team explains in their article.
Their prospective, randomized, single-center, open-labeled trial was conducted at the Gout Clinic of the Affiliated Hospital of Qingdao University, China.
A total of 196 relatively young healthy men with gout and uric acid underexcretion were randomly assigned to receive low-dose benzbromarone (25 mg/d) or low-dose febuxostat (20 mg/d) for 12 weeks.
Renal uric acid underexcretion was defined as fractional excretion of urate less than 5.5% and uric acid excretion less than or equal to 600 mg/d/1.73 m2.
A “major aspect” of this comparative effectiveness trial was its specific focus on gout-associated renal uric acid underexcretion, where the uricosuric targeted the dominant abnormality promoting the hyperuricemia, Dr. Terkeltaub told this news organization.
In addition, all participants received daily urine alkalinization with oral sodium bicarbonate. “This is not always done in clinical practice, nor in clinical trials of uricosuric agents,” Dr. Terkeltaub said.
The results showed that more participants in the benzbromarone group achieved the serum urate target of less than 6 mg/dL, compared with those in the febuxostat group (primary endpoint, 61% vs. 32%, P < .001).
Adverse events, including gout flares and urolithiasis, did not differ significantly between the two groups, with the exception of more transaminase elevation in the febuxostat group (15% vs. 4%; P = .008).
“We did not find severe hepatotoxicity with low-dose benzbromarone, but ethnic background may affect drug responses, and severe hepatotoxicity of benzbromarone has rarely been reported in Asia,” the authors write.
The incidence of urolithiasis was numerically, but not significantly, higher in the benzbromarone group (5% vs. 2%).
This study found no significant changes in participants’ triglyceride levels, though a previous study suggested febuxostat could increase serum triglycerides.
The investigators caution that the study only included patients who had baseline serum urate levels ranging from 8.0 to 10 mg/dL, who were relatively young and with few comorbidities.
The authors further noted that the “... results may not be generalizable to patients with higher serum urate levels or impaired kidney function, as well [as] patients from other geographical regions, age, and ethnicity groups. The study only included men, and the findings may not be generalizable to women with gout.”
‘Very useful’ in select cases
Weighing in on the results, Valderilio Feijó Azevedo, MD, PhD, adjunct professor of rheumatology, Federal University of Paraná, Brazil, noted that in some specific clinical circumstances, benzbromarone has been “a very useful medication, alone or combined, to treat gout patients.”
“We have great experience with the drug in Brazil. However, it is not used to treat all patients. Patients must be very well-selected in our clinical practice,” Dr. Azevedo said in an interview.
“For most patients, benzbromarone is effective for those who have failed to achieve serum uric acid goals with allopurinol treatment. We do not use it to treat patients with asymptomatic hyperuricemia. In general, we avoid patients with hepatic dysfunction due to previous hepatotoxicity reports. In every patient, we do active monitoring of enzymes,” Dr. Azevedo explained.
“We also avoid using it in patients with severe kidney disease. However, we have used it in some patients with estimated glomerular filtration rate less than 30. We also avoid dosage over 200 mg per day. On average, we use 100 mg per day combined with allopurinol or alone,” said Dr. Azevedo, who was not involved with the study.
Also weighing in, Michael Pillinger, MD, rheumatologist at NYU Langone Health, noted that while benzbromarone is not used in the United States, “in many parts of the world, it is used and is felt to be effective.” Dr. Pillinger was not associated with this current research.
This study, Dr. Pillinger said, “does underline the fact that an alternative drug that lowers urate by promoting urate excretion, if it could gain [U.S. Food and Drug Association] approval and if it were safe, could present a viable new option for therapy.”
He added, “If one conclusion to the study is that determining the basis of hyperuricemia is helpful in guiding benzbromarone use, that implies an additional layer of effort for physicians and patients in a disease that is already notoriously known for patient noncompliance – and in a case where febuxostat and allopurinol will work for both overproducers and underexcreters and would not need this additional assessment.”
The study was sponsored by Shandong Provincial Key Research and Development Plan, the National Natural Science Foundation of China, and Shandong Provincial Science Foundation for Outstanding Youth Scholarship. Dr. Terkeltaub was supported by the National Institutes of Health and the VA Research Service. Dr. Terkeltaub has received research funding from AstraZeneca, and has consulted with Horizon, Selecta, SOBI, Dyve BioSciences, Fortress, AstraZeneca, Allena, Fortress Biotech, and LG Life Sciences. Dr. Azevedo and Dr. Pillinger have no reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Benzbromarone is not approved in the United States because of concerns of acute liver injury but is approved in several other countries, including China, Brazil, and New Zealand.
“The results suggest that low dosing of benzbromarone may warrant stronger consideration as a safe and effective therapy to achieve serum urate target in gout without moderate chronic kidney disease,” the study team writes.
“Benzbromarone is severely hepatotoxic in some individuals and unlikely to ever gain approval in the United States,” one of the study’s investigators, Robert Terkeltaub, MD, professor of medicine, University of California, San Diego, told this news organization.
However, this study “illustrates the value and impact of uricosuric therapy in general in gout, including potentially as an initial urate-lowering monotherapy strategy, and the sheer number of subjects reaching urate target with low-dose uricosuric monotherapy was impressive,” Dr. Terkeltaub said.
The study was published online in Arthritis & Rheumatology.
“Renal uric acid underexcretion is the chief mechanism driving hyperuricemia in gout, yet the standard urate-lowering therapy recommendation is first-line xanthine oxidase inhibition irrespective of the cause of hyperuricemia,” the study team explains in their article.
Their prospective, randomized, single-center, open-labeled trial was conducted at the Gout Clinic of the Affiliated Hospital of Qingdao University, China.
A total of 196 relatively young healthy men with gout and uric acid underexcretion were randomly assigned to receive low-dose benzbromarone (25 mg/d) or low-dose febuxostat (20 mg/d) for 12 weeks.
Renal uric acid underexcretion was defined as fractional excretion of urate less than 5.5% and uric acid excretion less than or equal to 600 mg/d/1.73 m2.
A “major aspect” of this comparative effectiveness trial was its specific focus on gout-associated renal uric acid underexcretion, where the uricosuric targeted the dominant abnormality promoting the hyperuricemia, Dr. Terkeltaub told this news organization.
In addition, all participants received daily urine alkalinization with oral sodium bicarbonate. “This is not always done in clinical practice, nor in clinical trials of uricosuric agents,” Dr. Terkeltaub said.
The results showed that more participants in the benzbromarone group achieved the serum urate target of less than 6 mg/dL, compared with those in the febuxostat group (primary endpoint, 61% vs. 32%, P < .001).
Adverse events, including gout flares and urolithiasis, did not differ significantly between the two groups, with the exception of more transaminase elevation in the febuxostat group (15% vs. 4%; P = .008).
“We did not find severe hepatotoxicity with low-dose benzbromarone, but ethnic background may affect drug responses, and severe hepatotoxicity of benzbromarone has rarely been reported in Asia,” the authors write.
The incidence of urolithiasis was numerically, but not significantly, higher in the benzbromarone group (5% vs. 2%).
This study found no significant changes in participants’ triglyceride levels, though a previous study suggested febuxostat could increase serum triglycerides.
The investigators caution that the study only included patients who had baseline serum urate levels ranging from 8.0 to 10 mg/dL, who were relatively young and with few comorbidities.
The authors further noted that the “... results may not be generalizable to patients with higher serum urate levels or impaired kidney function, as well [as] patients from other geographical regions, age, and ethnicity groups. The study only included men, and the findings may not be generalizable to women with gout.”
‘Very useful’ in select cases
Weighing in on the results, Valderilio Feijó Azevedo, MD, PhD, adjunct professor of rheumatology, Federal University of Paraná, Brazil, noted that in some specific clinical circumstances, benzbromarone has been “a very useful medication, alone or combined, to treat gout patients.”
“We have great experience with the drug in Brazil. However, it is not used to treat all patients. Patients must be very well-selected in our clinical practice,” Dr. Azevedo said in an interview.
“For most patients, benzbromarone is effective for those who have failed to achieve serum uric acid goals with allopurinol treatment. We do not use it to treat patients with asymptomatic hyperuricemia. In general, we avoid patients with hepatic dysfunction due to previous hepatotoxicity reports. In every patient, we do active monitoring of enzymes,” Dr. Azevedo explained.
“We also avoid using it in patients with severe kidney disease. However, we have used it in some patients with estimated glomerular filtration rate less than 30. We also avoid dosage over 200 mg per day. On average, we use 100 mg per day combined with allopurinol or alone,” said Dr. Azevedo, who was not involved with the study.
Also weighing in, Michael Pillinger, MD, rheumatologist at NYU Langone Health, noted that while benzbromarone is not used in the United States, “in many parts of the world, it is used and is felt to be effective.” Dr. Pillinger was not associated with this current research.
This study, Dr. Pillinger said, “does underline the fact that an alternative drug that lowers urate by promoting urate excretion, if it could gain [U.S. Food and Drug Association] approval and if it were safe, could present a viable new option for therapy.”
He added, “If one conclusion to the study is that determining the basis of hyperuricemia is helpful in guiding benzbromarone use, that implies an additional layer of effort for physicians and patients in a disease that is already notoriously known for patient noncompliance – and in a case where febuxostat and allopurinol will work for both overproducers and underexcreters and would not need this additional assessment.”
The study was sponsored by Shandong Provincial Key Research and Development Plan, the National Natural Science Foundation of China, and Shandong Provincial Science Foundation for Outstanding Youth Scholarship. Dr. Terkeltaub was supported by the National Institutes of Health and the VA Research Service. Dr. Terkeltaub has received research funding from AstraZeneca, and has consulted with Horizon, Selecta, SOBI, Dyve BioSciences, Fortress, AstraZeneca, Allena, Fortress Biotech, and LG Life Sciences. Dr. Azevedo and Dr. Pillinger have no reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Coming soon: More breathable, more comfortable face masks
Sitting at his desk in Sea Girt, N.J., John Schwind is eager to demonstrate his ReadiMask 365. He holds up what looks like a white sheet of memo paper, peels off a protective liner, and sticks the mask first to his nose. He glides his fingers down his face, over his cheeks, and to his chin, sealing the mask and then demonstrating how easy it is to talk with it in place.
The mask’s medical adhesive sticks directly to the face, without causing breakouts, he said. It doesn’t let air leak and won’t fog his glasses. It’s strapless, so it won’t hurt his ears or make them stick out.
This fall, Mr. Schwind, the CEO of Global Safety First, is hoping to take home $150,000 as one of the two top winners of the federal Mask Innovation Challenge. He has made it to the top 10 but realizes he still has a ton of competition.
After the challenge launched in late 2021, nearly 1,500 submissions were received, says Kumiko Lippold, PhD, a health scientist and manager of the Mask Innovation Challenge. The challenge is run by Dr. Lippold and others at the Division of Research, Innovation, and Ventures (DRIVe), which is part of the Biomedical Advanced Research and Development Authority (BARDA) at the U.S. Department of Health & Human Services.
Like the rest of us, Dr. Lippold knows that masks desperately need a makeover. The aim is not only to get us through this pandemic but also future pandemics and other public health emergencies. “We are focused on building masks for the next pandemic, the next wildfires,” she says.
The project is a partnership among BARDA’s DRIVe, the National Institute for Occupational Safety and Health (NIOSH), and the National Institute of Standards and Technology (NIST).
While NIOSH is a partner in the challenge, giving feedback to mask developers, “the mask challenge is entirely separate from the NIOSH approval process,” Dr. Lippold says. Companies can then pursue NIOSH approval on their own, later, if they wish. The agency certifies only masks and respirators.
Preview of masks to come
“We’ve seen some really amazing things,” Dr. Lippold said of the new designs. She didn’t want to play favorites, so she gave an overview of innovations. Some designs have transparent materials, or partially see-through materials, so facial expressions can be read. “We’ve also seen really unique bio-based materials that are derived from natural products. We’ve seen sensors in some.”
One mask model has origami folds, which increase overall surface and breathing area. Some 3D-printed masks promise a custom fit and take into account whether a person’s nose bridge is low or high.
And the finalists are ...
ReadiMask 365: “I can wear this all day long,” Mr. Schwind said of his new design. It has a nano fiber filter and is flexible. Besides the one in the BARDA challenge, the company has other ReadiMasks on the market. “The most important thing is comfort,” he says. “Second is protection. If they don’t feel they have a good seal, users don’t have confidence in the mask.”
He offers various sizes of ReadiMasks, from small sizes designed for women with smaller faces to extra-large, “for NFL linemen.”
ClearMask: “We are the original clear mask,” says Aaron Hsu, CEO and co-founder of ClearMask in Baltimore. The company began in 2017, and the clear design was inspired by a company co-founder who is deaf. She was scheduled to have surgery, and her sign language interpreter did not show up, leaving her to try to communicate in the operating room with masked health care providers. There were no transparent masks available then, Mr. Hsu says.
“Being able to work with BARDA and getting their wisdom is invaluable,” he says.
The makers of ClearMask think masks are here to stay, at least for some. “I think a certain percentage of the population will continue to wear them, regardless,” said Mr. Hsu. He predicts health care settings will become stricter about wearing masks.
“Even now, when you even walk in to a hospital, you might be required to wear a mask,” he says, even as a visitor. His company’s masks are easy to adjust and are secured around the head, so your ears don’t get sore, he says.
4C Air: The BreSafe transparent mask is semi-transparent and is made of a nanomaterial that provides high levels of filtration and breathability with some transparency.
Air99: Based on origami principles, the Airgami mask is meant to improve fit, breathability, and aesthetics over existing masks. “Airgami fits better, works better and looks better,” says Min Xiao, a company spokesperson. “It won’t fall off the nose or collapse onto the mouth, and eyeglasses fog less, she says. Voices are less muffled.” It’s also reusable, rinseable and can be heat disinfected, she says. It went on the market in November 2020.
Air Flo Labs: Flo Mask Pro, like the company’s other designs, conducted over 100 3D facial scans across many ethnicities to produce a better fit, says Kevin Ngo, its creator. For the adult masks, two nose bridge sizes are offered. And users can choose a Pro Filter, with 99% filtration, or an Everyday, which is meant to be much more breathable than other masks. “Our silicone gasket is incredibly soft and gentle on the skin,” Mr. Ngo says. “In addition,we offer indents for glasses, which prevent any fogging.” The company began shipping in May; several thousand masks are in use now, Mr. Ngo said.
Georgetown University: This team’s smart mask is made of metallic foams that can be cleaned and reused.
Levi Strauss: The form of the mask can be made by any basic garment factory. It aims to activate the apparel supply chain as another source of low-cost, high-performance masks.
Matregenix: This mask, made of a transparent nanofiber, allows for easier communication while having high filtration.
SEAL Lab: The SINEW mask stands for Smart, Individualized, Near-Face, Extended Wear. The mask used technology to overcome flaws of traditional respirators, with the same degree of protection. It doesn’t make contact with the skin of the wearer’s face.
StaySafeNow: A team from Harvard University developed Crystal Guard, a reusable, cost-effective clear mask. Its developers say it’s meant to be especially useful for essential workers, teachers, and others who have to communicate to do their work.
Bye-bye N95?
“From our perspective, our goal with the mask challenge was not to replace the N95 respirator,” Dr. Lippold says. N95 masks, which NIOSH certifies, are valuable and protect people in high-risk settings. “With the mask challenge, our goal was really to provide the public with a comparable alternative that really meets their specific level of risk.” Working in a health care setting carries a different risk, she says, than going to the grocery store.
“A common complaint with the N95 is that they are very uncomfortable.” It’s a major barrier to compliance, “and we wanted to address that gap. We didn’t directly compare [the entries] to an N95,” she says, although their testing was similar to NIOSH’s. A number of finalists say they will pursue NIOSH approval, she says.
Meanwhile, some of the finalists’ masks are for sale. Air Flo Labs, for instance, has its Flo Mask Pro for sale online, noting that BARDA allowed it to release the test results from NIOSH and NIST.
Getting from 1,500 to 10
In the first phase of the challenge, Dr. Lippold says, “the goal was to engage as wide an audience as possible.” With the second phase, the bar was set a bit higher. Instead of just submitting ideas on paper, companies had to submit prototypes for lab testing. “We got about 80 submissions,” she says.
Those 80 were whittled down to 10 finalists. Teams had sent prototypes, and experts, including those from NIOSH and NIST, rated them, sometimes looking at multiple copies of the masks. Experts looked at how well the masks filtered the air, how breathable they were, and other data. Once the feedback was given to the mask companies, they entered a redesign period. “Scientists can take this data and basically make these prototypes better,” Dr. Lippold says.
The final round of testing will be in September, and the winners will be announced in the fall. The opportunity allowed companies to have their products go through testing they might not otherwise have been able to get, she says.
A version of this article first appeared on WebMD.com.
Sitting at his desk in Sea Girt, N.J., John Schwind is eager to demonstrate his ReadiMask 365. He holds up what looks like a white sheet of memo paper, peels off a protective liner, and sticks the mask first to his nose. He glides his fingers down his face, over his cheeks, and to his chin, sealing the mask and then demonstrating how easy it is to talk with it in place.
The mask’s medical adhesive sticks directly to the face, without causing breakouts, he said. It doesn’t let air leak and won’t fog his glasses. It’s strapless, so it won’t hurt his ears or make them stick out.
This fall, Mr. Schwind, the CEO of Global Safety First, is hoping to take home $150,000 as one of the two top winners of the federal Mask Innovation Challenge. He has made it to the top 10 but realizes he still has a ton of competition.
After the challenge launched in late 2021, nearly 1,500 submissions were received, says Kumiko Lippold, PhD, a health scientist and manager of the Mask Innovation Challenge. The challenge is run by Dr. Lippold and others at the Division of Research, Innovation, and Ventures (DRIVe), which is part of the Biomedical Advanced Research and Development Authority (BARDA) at the U.S. Department of Health & Human Services.
Like the rest of us, Dr. Lippold knows that masks desperately need a makeover. The aim is not only to get us through this pandemic but also future pandemics and other public health emergencies. “We are focused on building masks for the next pandemic, the next wildfires,” she says.
The project is a partnership among BARDA’s DRIVe, the National Institute for Occupational Safety and Health (NIOSH), and the National Institute of Standards and Technology (NIST).
While NIOSH is a partner in the challenge, giving feedback to mask developers, “the mask challenge is entirely separate from the NIOSH approval process,” Dr. Lippold says. Companies can then pursue NIOSH approval on their own, later, if they wish. The agency certifies only masks and respirators.
Preview of masks to come
“We’ve seen some really amazing things,” Dr. Lippold said of the new designs. She didn’t want to play favorites, so she gave an overview of innovations. Some designs have transparent materials, or partially see-through materials, so facial expressions can be read. “We’ve also seen really unique bio-based materials that are derived from natural products. We’ve seen sensors in some.”
One mask model has origami folds, which increase overall surface and breathing area. Some 3D-printed masks promise a custom fit and take into account whether a person’s nose bridge is low or high.
And the finalists are ...
ReadiMask 365: “I can wear this all day long,” Mr. Schwind said of his new design. It has a nano fiber filter and is flexible. Besides the one in the BARDA challenge, the company has other ReadiMasks on the market. “The most important thing is comfort,” he says. “Second is protection. If they don’t feel they have a good seal, users don’t have confidence in the mask.”
He offers various sizes of ReadiMasks, from small sizes designed for women with smaller faces to extra-large, “for NFL linemen.”
ClearMask: “We are the original clear mask,” says Aaron Hsu, CEO and co-founder of ClearMask in Baltimore. The company began in 2017, and the clear design was inspired by a company co-founder who is deaf. She was scheduled to have surgery, and her sign language interpreter did not show up, leaving her to try to communicate in the operating room with masked health care providers. There were no transparent masks available then, Mr. Hsu says.
“Being able to work with BARDA and getting their wisdom is invaluable,” he says.
The makers of ClearMask think masks are here to stay, at least for some. “I think a certain percentage of the population will continue to wear them, regardless,” said Mr. Hsu. He predicts health care settings will become stricter about wearing masks.
“Even now, when you even walk in to a hospital, you might be required to wear a mask,” he says, even as a visitor. His company’s masks are easy to adjust and are secured around the head, so your ears don’t get sore, he says.
4C Air: The BreSafe transparent mask is semi-transparent and is made of a nanomaterial that provides high levels of filtration and breathability with some transparency.
Air99: Based on origami principles, the Airgami mask is meant to improve fit, breathability, and aesthetics over existing masks. “Airgami fits better, works better and looks better,” says Min Xiao, a company spokesperson. “It won’t fall off the nose or collapse onto the mouth, and eyeglasses fog less, she says. Voices are less muffled.” It’s also reusable, rinseable and can be heat disinfected, she says. It went on the market in November 2020.
Air Flo Labs: Flo Mask Pro, like the company’s other designs, conducted over 100 3D facial scans across many ethnicities to produce a better fit, says Kevin Ngo, its creator. For the adult masks, two nose bridge sizes are offered. And users can choose a Pro Filter, with 99% filtration, or an Everyday, which is meant to be much more breathable than other masks. “Our silicone gasket is incredibly soft and gentle on the skin,” Mr. Ngo says. “In addition,we offer indents for glasses, which prevent any fogging.” The company began shipping in May; several thousand masks are in use now, Mr. Ngo said.
Georgetown University: This team’s smart mask is made of metallic foams that can be cleaned and reused.
Levi Strauss: The form of the mask can be made by any basic garment factory. It aims to activate the apparel supply chain as another source of low-cost, high-performance masks.
Matregenix: This mask, made of a transparent nanofiber, allows for easier communication while having high filtration.
SEAL Lab: The SINEW mask stands for Smart, Individualized, Near-Face, Extended Wear. The mask used technology to overcome flaws of traditional respirators, with the same degree of protection. It doesn’t make contact with the skin of the wearer’s face.
StaySafeNow: A team from Harvard University developed Crystal Guard, a reusable, cost-effective clear mask. Its developers say it’s meant to be especially useful for essential workers, teachers, and others who have to communicate to do their work.
Bye-bye N95?
“From our perspective, our goal with the mask challenge was not to replace the N95 respirator,” Dr. Lippold says. N95 masks, which NIOSH certifies, are valuable and protect people in high-risk settings. “With the mask challenge, our goal was really to provide the public with a comparable alternative that really meets their specific level of risk.” Working in a health care setting carries a different risk, she says, than going to the grocery store.
“A common complaint with the N95 is that they are very uncomfortable.” It’s a major barrier to compliance, “and we wanted to address that gap. We didn’t directly compare [the entries] to an N95,” she says, although their testing was similar to NIOSH’s. A number of finalists say they will pursue NIOSH approval, she says.
Meanwhile, some of the finalists’ masks are for sale. Air Flo Labs, for instance, has its Flo Mask Pro for sale online, noting that BARDA allowed it to release the test results from NIOSH and NIST.
Getting from 1,500 to 10
In the first phase of the challenge, Dr. Lippold says, “the goal was to engage as wide an audience as possible.” With the second phase, the bar was set a bit higher. Instead of just submitting ideas on paper, companies had to submit prototypes for lab testing. “We got about 80 submissions,” she says.
Those 80 were whittled down to 10 finalists. Teams had sent prototypes, and experts, including those from NIOSH and NIST, rated them, sometimes looking at multiple copies of the masks. Experts looked at how well the masks filtered the air, how breathable they were, and other data. Once the feedback was given to the mask companies, they entered a redesign period. “Scientists can take this data and basically make these prototypes better,” Dr. Lippold says.
The final round of testing will be in September, and the winners will be announced in the fall. The opportunity allowed companies to have their products go through testing they might not otherwise have been able to get, she says.
A version of this article first appeared on WebMD.com.
Sitting at his desk in Sea Girt, N.J., John Schwind is eager to demonstrate his ReadiMask 365. He holds up what looks like a white sheet of memo paper, peels off a protective liner, and sticks the mask first to his nose. He glides his fingers down his face, over his cheeks, and to his chin, sealing the mask and then demonstrating how easy it is to talk with it in place.
The mask’s medical adhesive sticks directly to the face, without causing breakouts, he said. It doesn’t let air leak and won’t fog his glasses. It’s strapless, so it won’t hurt his ears or make them stick out.
This fall, Mr. Schwind, the CEO of Global Safety First, is hoping to take home $150,000 as one of the two top winners of the federal Mask Innovation Challenge. He has made it to the top 10 but realizes he still has a ton of competition.
After the challenge launched in late 2021, nearly 1,500 submissions were received, says Kumiko Lippold, PhD, a health scientist and manager of the Mask Innovation Challenge. The challenge is run by Dr. Lippold and others at the Division of Research, Innovation, and Ventures (DRIVe), which is part of the Biomedical Advanced Research and Development Authority (BARDA) at the U.S. Department of Health & Human Services.
Like the rest of us, Dr. Lippold knows that masks desperately need a makeover. The aim is not only to get us through this pandemic but also future pandemics and other public health emergencies. “We are focused on building masks for the next pandemic, the next wildfires,” she says.
The project is a partnership among BARDA’s DRIVe, the National Institute for Occupational Safety and Health (NIOSH), and the National Institute of Standards and Technology (NIST).
While NIOSH is a partner in the challenge, giving feedback to mask developers, “the mask challenge is entirely separate from the NIOSH approval process,” Dr. Lippold says. Companies can then pursue NIOSH approval on their own, later, if they wish. The agency certifies only masks and respirators.
Preview of masks to come
“We’ve seen some really amazing things,” Dr. Lippold said of the new designs. She didn’t want to play favorites, so she gave an overview of innovations. Some designs have transparent materials, or partially see-through materials, so facial expressions can be read. “We’ve also seen really unique bio-based materials that are derived from natural products. We’ve seen sensors in some.”
One mask model has origami folds, which increase overall surface and breathing area. Some 3D-printed masks promise a custom fit and take into account whether a person’s nose bridge is low or high.
And the finalists are ...
ReadiMask 365: “I can wear this all day long,” Mr. Schwind said of his new design. It has a nano fiber filter and is flexible. Besides the one in the BARDA challenge, the company has other ReadiMasks on the market. “The most important thing is comfort,” he says. “Second is protection. If they don’t feel they have a good seal, users don’t have confidence in the mask.”
He offers various sizes of ReadiMasks, from small sizes designed for women with smaller faces to extra-large, “for NFL linemen.”
ClearMask: “We are the original clear mask,” says Aaron Hsu, CEO and co-founder of ClearMask in Baltimore. The company began in 2017, and the clear design was inspired by a company co-founder who is deaf. She was scheduled to have surgery, and her sign language interpreter did not show up, leaving her to try to communicate in the operating room with masked health care providers. There were no transparent masks available then, Mr. Hsu says.
“Being able to work with BARDA and getting their wisdom is invaluable,” he says.
The makers of ClearMask think masks are here to stay, at least for some. “I think a certain percentage of the population will continue to wear them, regardless,” said Mr. Hsu. He predicts health care settings will become stricter about wearing masks.
“Even now, when you even walk in to a hospital, you might be required to wear a mask,” he says, even as a visitor. His company’s masks are easy to adjust and are secured around the head, so your ears don’t get sore, he says.
4C Air: The BreSafe transparent mask is semi-transparent and is made of a nanomaterial that provides high levels of filtration and breathability with some transparency.
Air99: Based on origami principles, the Airgami mask is meant to improve fit, breathability, and aesthetics over existing masks. “Airgami fits better, works better and looks better,” says Min Xiao, a company spokesperson. “It won’t fall off the nose or collapse onto the mouth, and eyeglasses fog less, she says. Voices are less muffled.” It’s also reusable, rinseable and can be heat disinfected, she says. It went on the market in November 2020.
Air Flo Labs: Flo Mask Pro, like the company’s other designs, conducted over 100 3D facial scans across many ethnicities to produce a better fit, says Kevin Ngo, its creator. For the adult masks, two nose bridge sizes are offered. And users can choose a Pro Filter, with 99% filtration, or an Everyday, which is meant to be much more breathable than other masks. “Our silicone gasket is incredibly soft and gentle on the skin,” Mr. Ngo says. “In addition,we offer indents for glasses, which prevent any fogging.” The company began shipping in May; several thousand masks are in use now, Mr. Ngo said.
Georgetown University: This team’s smart mask is made of metallic foams that can be cleaned and reused.
Levi Strauss: The form of the mask can be made by any basic garment factory. It aims to activate the apparel supply chain as another source of low-cost, high-performance masks.
Matregenix: This mask, made of a transparent nanofiber, allows for easier communication while having high filtration.
SEAL Lab: The SINEW mask stands for Smart, Individualized, Near-Face, Extended Wear. The mask used technology to overcome flaws of traditional respirators, with the same degree of protection. It doesn’t make contact with the skin of the wearer’s face.
StaySafeNow: A team from Harvard University developed Crystal Guard, a reusable, cost-effective clear mask. Its developers say it’s meant to be especially useful for essential workers, teachers, and others who have to communicate to do their work.
Bye-bye N95?
“From our perspective, our goal with the mask challenge was not to replace the N95 respirator,” Dr. Lippold says. N95 masks, which NIOSH certifies, are valuable and protect people in high-risk settings. “With the mask challenge, our goal was really to provide the public with a comparable alternative that really meets their specific level of risk.” Working in a health care setting carries a different risk, she says, than going to the grocery store.
“A common complaint with the N95 is that they are very uncomfortable.” It’s a major barrier to compliance, “and we wanted to address that gap. We didn’t directly compare [the entries] to an N95,” she says, although their testing was similar to NIOSH’s. A number of finalists say they will pursue NIOSH approval, she says.
Meanwhile, some of the finalists’ masks are for sale. Air Flo Labs, for instance, has its Flo Mask Pro for sale online, noting that BARDA allowed it to release the test results from NIOSH and NIST.
Getting from 1,500 to 10
In the first phase of the challenge, Dr. Lippold says, “the goal was to engage as wide an audience as possible.” With the second phase, the bar was set a bit higher. Instead of just submitting ideas on paper, companies had to submit prototypes for lab testing. “We got about 80 submissions,” she says.
Those 80 were whittled down to 10 finalists. Teams had sent prototypes, and experts, including those from NIOSH and NIST, rated them, sometimes looking at multiple copies of the masks. Experts looked at how well the masks filtered the air, how breathable they were, and other data. Once the feedback was given to the mask companies, they entered a redesign period. “Scientists can take this data and basically make these prototypes better,” Dr. Lippold says.
The final round of testing will be in September, and the winners will be announced in the fall. The opportunity allowed companies to have their products go through testing they might not otherwise have been able to get, she says.
A version of this article first appeared on WebMD.com.
The heartache of bereavement can be fatal in heart failure
that points to the need for greater integration of psychosocial risk factors in the treatment of HF.
The adjusted relative risk of dying was nearly 30% higher among bereaved patients with HF (1.29; 95% confidence interval, 1.27-1.30) and slightly higher for those grieving the loss of more than one family member (RR, 1.35).
The highest risk was in the first week after the loss (RR, 1.78) but persisted after 5 years of follow-up (RR, 1.30).
“Heart failure is a very difficult condition and has a very poor prognosis comparable to many, many cancers,” senior author Krisztina László, PhD, Karolinska Institutet, Stockholm, said in an interview. “So it’s important for us to be aware of these increased risks and to understand them better.”
The early risk for death could be related to stress-induced cardiomyopathy, or Takotsubo syndrome, as well as activation of the hypothalamic-pituitary-adrenal axis, the renin-angiotensin-aldosterone system, and sympathetic nervous system, she explained. Higher long-term risks may reflect chronic stress, leading to poorly managed disease and an unhealthy lifestyle.
“If we understand better the underlying mechanisms maybe we can give more specific advice,” Dr. László said. “At this stage, I think having an awareness of the risk and trying to follow patients or at least not let them fall out of usual care, asking questions, trying to understand what their needs are, maybe that is what we can do well.”
A recent position paper by the European Association of Preventive Cardiology pointed out that psychosocial risk factors, like depression and social isolation, can exacerbate heart failure and calls for better integration of psychosocial factors in the treatment of patients with chronic HF.
“We don’t do a very good job of it, but I think they are very important,” observed Stuart D. Russell, MD, a professor of medicine who specializes in advanced HF at Duke University, Durham, N.C., and was not involved in the study.
“When we hear about a spouse dying, we might call and give condolences, but it’s probably a group of patients that for the next 6 months or so we need to watch more closely and see if there are things we can impact both medically as well as socially to perhaps prevent some of this increase in mortality,” he told this news organization.
Although several studies have linked bereavement with adverse health outcomes, this is just one of two studies to look specifically at its role in HF prognosis, Dr. László noted. A 2013 study of 66,000 male veterans reported that widowers had nearly a 38% higher all-cause mortality risk than did married veterans.
The present study extends those findings to 490,527 patients in the Swedish Heart Failure Registry between 2000 and 2018 and/or in the Swedish Patient Register with a primary diagnosis of HF between 1987 and 2018. During a mean follow-up of 3.7 years, 12% of participants had a family member die, and 383,674 participants died.
Results showed the HF mortality risk increased 10% after the death of a child, 20% with the death of a spouse/partner, 13% with a sibling’s death, and 5% with the death of a grandchild.
No increased risk was seen after the death of a parent, which is likely owed to a median patient age of about 75 years and “is in line with our expectations of the life cycle,” Dr. László said.
An association between bereavement and mortality risk was observed in cases of loss caused by cardiovascular disease (RR, 1.34) and other natural causes (RR, 1.27) but also in cases of unnatural deaths, such as suicide (RR, 1.13).
The overall findings were similar regardless of left ventricular ejection fraction and New York Heart Association functional class and were not affected by sex or country of birth.
Dr. Russell agreed that the death of a parent would be expected among these older patients with HF but said that “if the mechanism of this truly is kind of this increased stress hormones and Takotsubo-type mechanism, you’d think it would be worse if it was your kid that died. That shocked me a bit.”
The strong association between mortality and the loss of a spouse or partner was not surprising, given that they’re an important source of mutual social support, he added.
“If it’s a 75-year-old whose spouse dies, we need to make sure that we have the children’s phone number or other people that we can reach out to and say: ‘Can you check on them?’ ” he said. “And we need to make sure that somebody else is coming in with them because I would guess that probably at least half of what patients hear in a clinic visit goes in one ear and out the other and it’s going to make that much better. So we need to find who that new support person is for the patient.”
Asked whether there are efforts underway to incorporate psychosocial factors into current U.S. guidelines, Dr. Russell replied, “certainly within heart failure, I don’t think we’re really discussing it and, that may be the best part of this paper. It really makes us think about a different way of approaching these older patients.”
Dr. László said that future studies are needed to investigate whether less severe sources of stress may also contribute to poor HF prognosis.
“In our population, 12% of patients were affected, which is quite high, but there are patients with heart failure who experience on a daily basis other sources of stress, which are less severe but chronic and affect large numbers,” she said. “This may also have important public health implications and will be an important next step.”
The authors noted that they were unable to eliminate residual confounding by genetic factors or unmeasured socioeconomic-, lifestyle-, or health-related factors shared by family members. Other limitations are limited power to detect a modest effect in some of the subanalyses and that the findings may be generalizable only to countries with social and cultural contexts and health-related factors similar to those of Sweden.
The study was supported by grants from the Swedish Council for Working Life and Social Research, the Karolinska Institutet’s Research Foundation, and the China Scholarship Council. Dr. László is also supported by a grant from the Heart and Lung Foundation. All other authors and Dr. Russell reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
that points to the need for greater integration of psychosocial risk factors in the treatment of HF.
The adjusted relative risk of dying was nearly 30% higher among bereaved patients with HF (1.29; 95% confidence interval, 1.27-1.30) and slightly higher for those grieving the loss of more than one family member (RR, 1.35).
The highest risk was in the first week after the loss (RR, 1.78) but persisted after 5 years of follow-up (RR, 1.30).
“Heart failure is a very difficult condition and has a very poor prognosis comparable to many, many cancers,” senior author Krisztina László, PhD, Karolinska Institutet, Stockholm, said in an interview. “So it’s important for us to be aware of these increased risks and to understand them better.”
The early risk for death could be related to stress-induced cardiomyopathy, or Takotsubo syndrome, as well as activation of the hypothalamic-pituitary-adrenal axis, the renin-angiotensin-aldosterone system, and sympathetic nervous system, she explained. Higher long-term risks may reflect chronic stress, leading to poorly managed disease and an unhealthy lifestyle.
“If we understand better the underlying mechanisms maybe we can give more specific advice,” Dr. László said. “At this stage, I think having an awareness of the risk and trying to follow patients or at least not let them fall out of usual care, asking questions, trying to understand what their needs are, maybe that is what we can do well.”
A recent position paper by the European Association of Preventive Cardiology pointed out that psychosocial risk factors, like depression and social isolation, can exacerbate heart failure and calls for better integration of psychosocial factors in the treatment of patients with chronic HF.
“We don’t do a very good job of it, but I think they are very important,” observed Stuart D. Russell, MD, a professor of medicine who specializes in advanced HF at Duke University, Durham, N.C., and was not involved in the study.
“When we hear about a spouse dying, we might call and give condolences, but it’s probably a group of patients that for the next 6 months or so we need to watch more closely and see if there are things we can impact both medically as well as socially to perhaps prevent some of this increase in mortality,” he told this news organization.
Although several studies have linked bereavement with adverse health outcomes, this is just one of two studies to look specifically at its role in HF prognosis, Dr. László noted. A 2013 study of 66,000 male veterans reported that widowers had nearly a 38% higher all-cause mortality risk than did married veterans.
The present study extends those findings to 490,527 patients in the Swedish Heart Failure Registry between 2000 and 2018 and/or in the Swedish Patient Register with a primary diagnosis of HF between 1987 and 2018. During a mean follow-up of 3.7 years, 12% of participants had a family member die, and 383,674 participants died.
Results showed the HF mortality risk increased 10% after the death of a child, 20% with the death of a spouse/partner, 13% with a sibling’s death, and 5% with the death of a grandchild.
No increased risk was seen after the death of a parent, which is likely owed to a median patient age of about 75 years and “is in line with our expectations of the life cycle,” Dr. László said.
An association between bereavement and mortality risk was observed in cases of loss caused by cardiovascular disease (RR, 1.34) and other natural causes (RR, 1.27) but also in cases of unnatural deaths, such as suicide (RR, 1.13).
The overall findings were similar regardless of left ventricular ejection fraction and New York Heart Association functional class and were not affected by sex or country of birth.
Dr. Russell agreed that the death of a parent would be expected among these older patients with HF but said that “if the mechanism of this truly is kind of this increased stress hormones and Takotsubo-type mechanism, you’d think it would be worse if it was your kid that died. That shocked me a bit.”
The strong association between mortality and the loss of a spouse or partner was not surprising, given that they’re an important source of mutual social support, he added.
“If it’s a 75-year-old whose spouse dies, we need to make sure that we have the children’s phone number or other people that we can reach out to and say: ‘Can you check on them?’ ” he said. “And we need to make sure that somebody else is coming in with them because I would guess that probably at least half of what patients hear in a clinic visit goes in one ear and out the other and it’s going to make that much better. So we need to find who that new support person is for the patient.”
Asked whether there are efforts underway to incorporate psychosocial factors into current U.S. guidelines, Dr. Russell replied, “certainly within heart failure, I don’t think we’re really discussing it and, that may be the best part of this paper. It really makes us think about a different way of approaching these older patients.”
Dr. László said that future studies are needed to investigate whether less severe sources of stress may also contribute to poor HF prognosis.
“In our population, 12% of patients were affected, which is quite high, but there are patients with heart failure who experience on a daily basis other sources of stress, which are less severe but chronic and affect large numbers,” she said. “This may also have important public health implications and will be an important next step.”
The authors noted that they were unable to eliminate residual confounding by genetic factors or unmeasured socioeconomic-, lifestyle-, or health-related factors shared by family members. Other limitations are limited power to detect a modest effect in some of the subanalyses and that the findings may be generalizable only to countries with social and cultural contexts and health-related factors similar to those of Sweden.
The study was supported by grants from the Swedish Council for Working Life and Social Research, the Karolinska Institutet’s Research Foundation, and the China Scholarship Council. Dr. László is also supported by a grant from the Heart and Lung Foundation. All other authors and Dr. Russell reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
that points to the need for greater integration of psychosocial risk factors in the treatment of HF.
The adjusted relative risk of dying was nearly 30% higher among bereaved patients with HF (1.29; 95% confidence interval, 1.27-1.30) and slightly higher for those grieving the loss of more than one family member (RR, 1.35).
The highest risk was in the first week after the loss (RR, 1.78) but persisted after 5 years of follow-up (RR, 1.30).
“Heart failure is a very difficult condition and has a very poor prognosis comparable to many, many cancers,” senior author Krisztina László, PhD, Karolinska Institutet, Stockholm, said in an interview. “So it’s important for us to be aware of these increased risks and to understand them better.”
The early risk for death could be related to stress-induced cardiomyopathy, or Takotsubo syndrome, as well as activation of the hypothalamic-pituitary-adrenal axis, the renin-angiotensin-aldosterone system, and sympathetic nervous system, she explained. Higher long-term risks may reflect chronic stress, leading to poorly managed disease and an unhealthy lifestyle.
“If we understand better the underlying mechanisms maybe we can give more specific advice,” Dr. László said. “At this stage, I think having an awareness of the risk and trying to follow patients or at least not let them fall out of usual care, asking questions, trying to understand what their needs are, maybe that is what we can do well.”
A recent position paper by the European Association of Preventive Cardiology pointed out that psychosocial risk factors, like depression and social isolation, can exacerbate heart failure and calls for better integration of psychosocial factors in the treatment of patients with chronic HF.
“We don’t do a very good job of it, but I think they are very important,” observed Stuart D. Russell, MD, a professor of medicine who specializes in advanced HF at Duke University, Durham, N.C., and was not involved in the study.
“When we hear about a spouse dying, we might call and give condolences, but it’s probably a group of patients that for the next 6 months or so we need to watch more closely and see if there are things we can impact both medically as well as socially to perhaps prevent some of this increase in mortality,” he told this news organization.
Although several studies have linked bereavement with adverse health outcomes, this is just one of two studies to look specifically at its role in HF prognosis, Dr. László noted. A 2013 study of 66,000 male veterans reported that widowers had nearly a 38% higher all-cause mortality risk than did married veterans.
The present study extends those findings to 490,527 patients in the Swedish Heart Failure Registry between 2000 and 2018 and/or in the Swedish Patient Register with a primary diagnosis of HF between 1987 and 2018. During a mean follow-up of 3.7 years, 12% of participants had a family member die, and 383,674 participants died.
Results showed the HF mortality risk increased 10% after the death of a child, 20% with the death of a spouse/partner, 13% with a sibling’s death, and 5% with the death of a grandchild.
No increased risk was seen after the death of a parent, which is likely owed to a median patient age of about 75 years and “is in line with our expectations of the life cycle,” Dr. László said.
An association between bereavement and mortality risk was observed in cases of loss caused by cardiovascular disease (RR, 1.34) and other natural causes (RR, 1.27) but also in cases of unnatural deaths, such as suicide (RR, 1.13).
The overall findings were similar regardless of left ventricular ejection fraction and New York Heart Association functional class and were not affected by sex or country of birth.
Dr. Russell agreed that the death of a parent would be expected among these older patients with HF but said that “if the mechanism of this truly is kind of this increased stress hormones and Takotsubo-type mechanism, you’d think it would be worse if it was your kid that died. That shocked me a bit.”
The strong association between mortality and the loss of a spouse or partner was not surprising, given that they’re an important source of mutual social support, he added.
“If it’s a 75-year-old whose spouse dies, we need to make sure that we have the children’s phone number or other people that we can reach out to and say: ‘Can you check on them?’ ” he said. “And we need to make sure that somebody else is coming in with them because I would guess that probably at least half of what patients hear in a clinic visit goes in one ear and out the other and it’s going to make that much better. So we need to find who that new support person is for the patient.”
Asked whether there are efforts underway to incorporate psychosocial factors into current U.S. guidelines, Dr. Russell replied, “certainly within heart failure, I don’t think we’re really discussing it and, that may be the best part of this paper. It really makes us think about a different way of approaching these older patients.”
Dr. László said that future studies are needed to investigate whether less severe sources of stress may also contribute to poor HF prognosis.
“In our population, 12% of patients were affected, which is quite high, but there are patients with heart failure who experience on a daily basis other sources of stress, which are less severe but chronic and affect large numbers,” she said. “This may also have important public health implications and will be an important next step.”
The authors noted that they were unable to eliminate residual confounding by genetic factors or unmeasured socioeconomic-, lifestyle-, or health-related factors shared by family members. Other limitations are limited power to detect a modest effect in some of the subanalyses and that the findings may be generalizable only to countries with social and cultural contexts and health-related factors similar to those of Sweden.
The study was supported by grants from the Swedish Council for Working Life and Social Research, the Karolinska Institutet’s Research Foundation, and the China Scholarship Council. Dr. László is also supported by a grant from the Heart and Lung Foundation. All other authors and Dr. Russell reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JACC: HEART FAILURE
Hospital-acquired pneumonia is killing patients, yet there is a simple way to stop it
Four years ago, when Dr. Karen Giuliano went to a Boston hospital for hip replacement surgery, she was given a pale-pink bucket of toiletries issued to patients in many hospitals. Inside were tissues, bar soap, deodorant, toothpaste, and, without a doubt, the worst toothbrush she’d ever seen.
“I couldn’t believe it. I got a toothbrush with no bristles,” she said. “It must have not gone through the bristle machine. It was just a stick.”
To most patients, a useless hospital toothbrush would be a mild inconvenience. But to Dr. Giuliano, a nursing professor at the University of Massachusetts, Amherst, it was a reminder of a pervasive “blind spot” in U.S. hospitals: the stunning consequences of unbrushed teeth.
Hospital patients not getting their teeth brushed, or not brushing their teeth themselves, is believed to be a leading cause of hundreds of thousands of cases of pneumonia a year in patients who have not been put on a ventilator. Pneumonia is among the most common infections that occur in health care facilities, and a majority of cases are nonventilator hospital-acquired pneumonia, or NVHAP, which kills up to 30% of those infected, Dr. Giuliano and other experts said.
But unlike many infections that strike within hospitals, the federal government doesn’t require hospitals to report cases of NVHAP. As a result, few hospitals understand the origin of the illness, track its occurrence, or actively work to prevent it, the experts said.
, according to a growing body of peer-reviewed research papers. Instead, many hospitals often skip teeth brushing to prioritize other tasks and provide only cheap, ineffective toothbrushes, often unaware of the consequences, said Dr. Dian Baker, a Sacramento (Calif.) State nursing professor who has spent more than a decade studying NVHAP.
“I’ll tell you that today the vast majority of the tens of thousands of nurses in hospitals have no idea that pneumonia comes from germs in the mouth,” Dr. Baker said.
Pneumonia occurs when germs trigger an infection in the lungs. Although NVHAP accounts for most of the cases that occur in hospitals, it historically has not received the same attention as pneumonia tied to ventilators, which is easier to identify and study because it occurs among a narrow subset of patients.
NVHAP, a risk for virtually all hospital patients, is often caused by bacteria from the mouth that gathers in the scummy biofilm on unbrushed teeth and is aspirated into the lungs. Patients face a higher risk if they lie flat or remain immobile for long periods, so NVHAP can also be prevented by elevating their heads and getting them out of bed more often.
According to the National Organization for NV-HAP Prevention, which was founded in 2020, this pneumonia infects about 1 in every 100 hospital patients and kills 15%-30% of them. For those who survive, the illness often extends their hospital stay by up to 15 days and makes it much more likely they will be readmitted within a month or transferred to an intensive care unit.
John McCleary, 83, of Millinocket, Maine, contracted a likely case of NVHAP in 2008 after he fractured his ankle in a fall and spent 12 days in rehabilitation at a hospital, said his daughter, Kathy Day, a retired nurse and advocate with the Patient Safety Action Network.
Mr. McCleary recovered from the fracture but not from pneumonia. Two days after he returned home, the infection in his lungs caused him to be rushed back to the hospital, where he went into sepsis and spent weeks in treatment before moving to an isolation unit in a nursing home.
He died weeks later, emaciated, largely deaf, unable to eat, and often “too weak to get water through a straw,” his daughter said. After contracting pneumonia, he never walked again.
“It was an astounding assault on his body, from him being here visiting me the week before his fall, to his death just a few months later,” Ms. Day said. “And the whole thing was avoidable.”
While experts describe NVHAP as a largely ignored threat, that appears to be changing.
Last year, a group of researchers – including Dr. Giuliano and Dr. Baker, plus officials from the Centers for Disease Control and Prevention, the Veterans Health Administration, and the Joint Commission – published a “call-to-action” research paper hoping to launch “a national health care conversation about NVHAP prevention.”
The Joint Commission, a nonprofit organization whose accreditation can make or break hospitals, is considering broadening the infection control standards to include more ailments, including NVHAP, said Sylvia Garcia-Houchins, its director of infection prevention and control.
Separately, ECRI, a nonprofit focused on health care safety, this year pinpointed NVHAP as one of its top patient safety concerns.
James Davis, an ECRI infection expert, said the prevalence of NVHAP, while already alarming, is likely “underestimated” and probably worsened as hospitals swelled with patients during the coronavirus pandemic.
“We only know what’s reported,” Mr. Davis said. “Could this be the tip of the iceberg? I would say, in my opinion, probably.”
To better measure the condition, some researchers call for a standardized surveillance definition of NVHAP, which could in time open the door for the federal government to mandate reporting of cases or incentivize prevention. With increasing urgency, researchers are pushing for hospitals not to wait for the federal government to act against NVHAP.
Dr. Baker said she has spoken with hundreds of hospitals about how to prevent NVHAP, but thousands more have yet to take up the cause.
“We are not asking for some big, $300,000 piece of equipment,” Dr. Baker said. “The two things that show the best evidence of preventing this harm are things that should be happening in standard care anyway – brushing teeth and getting patients mobilized.”
That evidence comes from a smattering of studies that show those two strategies can lead to sharp reductions in infection rates.
In California, a study at 21 Kaiser Permanente hospitals used a reprioritization of oral care and getting patients out of bed to reduce rates of hospital-acquired pneumonia by around 70%. At Sutter Medical Center in Sacramento, better oral care reduced NVHAP cases by a yearly average of 35%.
At Orlando Regional Medical Center in Florida, a medical unit and a surgical unit where patients received enhanced oral care reduced NVHAP rates by 85% and 56%, respectively, when compared with similar units that received normal care. A similar study is underway at two hospitals in Illinois.
And the most compelling results come from a veterans’ hospital in Salem, Va., where a 2016 oral care pilot program reduced rates of NVHAP by 92% – saving an estimated 13 lives in just 19 months. The program, the HAPPEN Initiative, has been expanded across the Veterans Health Administration, and experts say it could serve as a model for all U.S. hospitals.
Dr. Michelle Lucatorto, a nursing official who leads HAPPEN, said the program trains nurses to most effectively brush patients’ teeth and educates patients and families on the link between oral care and preventing NVHAP. While teeth brushing may not seem to require training, Dr. Lucatorto made comparisons to how the coronavirus revealed many Americans were doing a lackluster job of another routine hygienic practice: washing their hands.
“Sometimes we are searching for the most complicated intervention,” she said. “We are always looking for that new bypass surgery, or some new technical equipment. And sometimes I think we fail to look at the simple things we can do in our practice to save people’s lives.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Four years ago, when Dr. Karen Giuliano went to a Boston hospital for hip replacement surgery, she was given a pale-pink bucket of toiletries issued to patients in many hospitals. Inside were tissues, bar soap, deodorant, toothpaste, and, without a doubt, the worst toothbrush she’d ever seen.
“I couldn’t believe it. I got a toothbrush with no bristles,” she said. “It must have not gone through the bristle machine. It was just a stick.”
To most patients, a useless hospital toothbrush would be a mild inconvenience. But to Dr. Giuliano, a nursing professor at the University of Massachusetts, Amherst, it was a reminder of a pervasive “blind spot” in U.S. hospitals: the stunning consequences of unbrushed teeth.
Hospital patients not getting their teeth brushed, or not brushing their teeth themselves, is believed to be a leading cause of hundreds of thousands of cases of pneumonia a year in patients who have not been put on a ventilator. Pneumonia is among the most common infections that occur in health care facilities, and a majority of cases are nonventilator hospital-acquired pneumonia, or NVHAP, which kills up to 30% of those infected, Dr. Giuliano and other experts said.
But unlike many infections that strike within hospitals, the federal government doesn’t require hospitals to report cases of NVHAP. As a result, few hospitals understand the origin of the illness, track its occurrence, or actively work to prevent it, the experts said.
, according to a growing body of peer-reviewed research papers. Instead, many hospitals often skip teeth brushing to prioritize other tasks and provide only cheap, ineffective toothbrushes, often unaware of the consequences, said Dr. Dian Baker, a Sacramento (Calif.) State nursing professor who has spent more than a decade studying NVHAP.
“I’ll tell you that today the vast majority of the tens of thousands of nurses in hospitals have no idea that pneumonia comes from germs in the mouth,” Dr. Baker said.
Pneumonia occurs when germs trigger an infection in the lungs. Although NVHAP accounts for most of the cases that occur in hospitals, it historically has not received the same attention as pneumonia tied to ventilators, which is easier to identify and study because it occurs among a narrow subset of patients.
NVHAP, a risk for virtually all hospital patients, is often caused by bacteria from the mouth that gathers in the scummy biofilm on unbrushed teeth and is aspirated into the lungs. Patients face a higher risk if they lie flat or remain immobile for long periods, so NVHAP can also be prevented by elevating their heads and getting them out of bed more often.
According to the National Organization for NV-HAP Prevention, which was founded in 2020, this pneumonia infects about 1 in every 100 hospital patients and kills 15%-30% of them. For those who survive, the illness often extends their hospital stay by up to 15 days and makes it much more likely they will be readmitted within a month or transferred to an intensive care unit.
John McCleary, 83, of Millinocket, Maine, contracted a likely case of NVHAP in 2008 after he fractured his ankle in a fall and spent 12 days in rehabilitation at a hospital, said his daughter, Kathy Day, a retired nurse and advocate with the Patient Safety Action Network.
Mr. McCleary recovered from the fracture but not from pneumonia. Two days after he returned home, the infection in his lungs caused him to be rushed back to the hospital, where he went into sepsis and spent weeks in treatment before moving to an isolation unit in a nursing home.
He died weeks later, emaciated, largely deaf, unable to eat, and often “too weak to get water through a straw,” his daughter said. After contracting pneumonia, he never walked again.
“It was an astounding assault on his body, from him being here visiting me the week before his fall, to his death just a few months later,” Ms. Day said. “And the whole thing was avoidable.”
While experts describe NVHAP as a largely ignored threat, that appears to be changing.
Last year, a group of researchers – including Dr. Giuliano and Dr. Baker, plus officials from the Centers for Disease Control and Prevention, the Veterans Health Administration, and the Joint Commission – published a “call-to-action” research paper hoping to launch “a national health care conversation about NVHAP prevention.”
The Joint Commission, a nonprofit organization whose accreditation can make or break hospitals, is considering broadening the infection control standards to include more ailments, including NVHAP, said Sylvia Garcia-Houchins, its director of infection prevention and control.
Separately, ECRI, a nonprofit focused on health care safety, this year pinpointed NVHAP as one of its top patient safety concerns.
James Davis, an ECRI infection expert, said the prevalence of NVHAP, while already alarming, is likely “underestimated” and probably worsened as hospitals swelled with patients during the coronavirus pandemic.
“We only know what’s reported,” Mr. Davis said. “Could this be the tip of the iceberg? I would say, in my opinion, probably.”
To better measure the condition, some researchers call for a standardized surveillance definition of NVHAP, which could in time open the door for the federal government to mandate reporting of cases or incentivize prevention. With increasing urgency, researchers are pushing for hospitals not to wait for the federal government to act against NVHAP.
Dr. Baker said she has spoken with hundreds of hospitals about how to prevent NVHAP, but thousands more have yet to take up the cause.
“We are not asking for some big, $300,000 piece of equipment,” Dr. Baker said. “The two things that show the best evidence of preventing this harm are things that should be happening in standard care anyway – brushing teeth and getting patients mobilized.”
That evidence comes from a smattering of studies that show those two strategies can lead to sharp reductions in infection rates.
In California, a study at 21 Kaiser Permanente hospitals used a reprioritization of oral care and getting patients out of bed to reduce rates of hospital-acquired pneumonia by around 70%. At Sutter Medical Center in Sacramento, better oral care reduced NVHAP cases by a yearly average of 35%.
At Orlando Regional Medical Center in Florida, a medical unit and a surgical unit where patients received enhanced oral care reduced NVHAP rates by 85% and 56%, respectively, when compared with similar units that received normal care. A similar study is underway at two hospitals in Illinois.
And the most compelling results come from a veterans’ hospital in Salem, Va., where a 2016 oral care pilot program reduced rates of NVHAP by 92% – saving an estimated 13 lives in just 19 months. The program, the HAPPEN Initiative, has been expanded across the Veterans Health Administration, and experts say it could serve as a model for all U.S. hospitals.
Dr. Michelle Lucatorto, a nursing official who leads HAPPEN, said the program trains nurses to most effectively brush patients’ teeth and educates patients and families on the link between oral care and preventing NVHAP. While teeth brushing may not seem to require training, Dr. Lucatorto made comparisons to how the coronavirus revealed many Americans were doing a lackluster job of another routine hygienic practice: washing their hands.
“Sometimes we are searching for the most complicated intervention,” she said. “We are always looking for that new bypass surgery, or some new technical equipment. And sometimes I think we fail to look at the simple things we can do in our practice to save people’s lives.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Four years ago, when Dr. Karen Giuliano went to a Boston hospital for hip replacement surgery, she was given a pale-pink bucket of toiletries issued to patients in many hospitals. Inside were tissues, bar soap, deodorant, toothpaste, and, without a doubt, the worst toothbrush she’d ever seen.
“I couldn’t believe it. I got a toothbrush with no bristles,” she said. “It must have not gone through the bristle machine. It was just a stick.”
To most patients, a useless hospital toothbrush would be a mild inconvenience. But to Dr. Giuliano, a nursing professor at the University of Massachusetts, Amherst, it was a reminder of a pervasive “blind spot” in U.S. hospitals: the stunning consequences of unbrushed teeth.
Hospital patients not getting their teeth brushed, or not brushing their teeth themselves, is believed to be a leading cause of hundreds of thousands of cases of pneumonia a year in patients who have not been put on a ventilator. Pneumonia is among the most common infections that occur in health care facilities, and a majority of cases are nonventilator hospital-acquired pneumonia, or NVHAP, which kills up to 30% of those infected, Dr. Giuliano and other experts said.
But unlike many infections that strike within hospitals, the federal government doesn’t require hospitals to report cases of NVHAP. As a result, few hospitals understand the origin of the illness, track its occurrence, or actively work to prevent it, the experts said.
, according to a growing body of peer-reviewed research papers. Instead, many hospitals often skip teeth brushing to prioritize other tasks and provide only cheap, ineffective toothbrushes, often unaware of the consequences, said Dr. Dian Baker, a Sacramento (Calif.) State nursing professor who has spent more than a decade studying NVHAP.
“I’ll tell you that today the vast majority of the tens of thousands of nurses in hospitals have no idea that pneumonia comes from germs in the mouth,” Dr. Baker said.
Pneumonia occurs when germs trigger an infection in the lungs. Although NVHAP accounts for most of the cases that occur in hospitals, it historically has not received the same attention as pneumonia tied to ventilators, which is easier to identify and study because it occurs among a narrow subset of patients.
NVHAP, a risk for virtually all hospital patients, is often caused by bacteria from the mouth that gathers in the scummy biofilm on unbrushed teeth and is aspirated into the lungs. Patients face a higher risk if they lie flat or remain immobile for long periods, so NVHAP can also be prevented by elevating their heads and getting them out of bed more often.
According to the National Organization for NV-HAP Prevention, which was founded in 2020, this pneumonia infects about 1 in every 100 hospital patients and kills 15%-30% of them. For those who survive, the illness often extends their hospital stay by up to 15 days and makes it much more likely they will be readmitted within a month or transferred to an intensive care unit.
John McCleary, 83, of Millinocket, Maine, contracted a likely case of NVHAP in 2008 after he fractured his ankle in a fall and spent 12 days in rehabilitation at a hospital, said his daughter, Kathy Day, a retired nurse and advocate with the Patient Safety Action Network.
Mr. McCleary recovered from the fracture but not from pneumonia. Two days after he returned home, the infection in his lungs caused him to be rushed back to the hospital, where he went into sepsis and spent weeks in treatment before moving to an isolation unit in a nursing home.
He died weeks later, emaciated, largely deaf, unable to eat, and often “too weak to get water through a straw,” his daughter said. After contracting pneumonia, he never walked again.
“It was an astounding assault on his body, from him being here visiting me the week before his fall, to his death just a few months later,” Ms. Day said. “And the whole thing was avoidable.”
While experts describe NVHAP as a largely ignored threat, that appears to be changing.
Last year, a group of researchers – including Dr. Giuliano and Dr. Baker, plus officials from the Centers for Disease Control and Prevention, the Veterans Health Administration, and the Joint Commission – published a “call-to-action” research paper hoping to launch “a national health care conversation about NVHAP prevention.”
The Joint Commission, a nonprofit organization whose accreditation can make or break hospitals, is considering broadening the infection control standards to include more ailments, including NVHAP, said Sylvia Garcia-Houchins, its director of infection prevention and control.
Separately, ECRI, a nonprofit focused on health care safety, this year pinpointed NVHAP as one of its top patient safety concerns.
James Davis, an ECRI infection expert, said the prevalence of NVHAP, while already alarming, is likely “underestimated” and probably worsened as hospitals swelled with patients during the coronavirus pandemic.
“We only know what’s reported,” Mr. Davis said. “Could this be the tip of the iceberg? I would say, in my opinion, probably.”
To better measure the condition, some researchers call for a standardized surveillance definition of NVHAP, which could in time open the door for the federal government to mandate reporting of cases or incentivize prevention. With increasing urgency, researchers are pushing for hospitals not to wait for the federal government to act against NVHAP.
Dr. Baker said she has spoken with hundreds of hospitals about how to prevent NVHAP, but thousands more have yet to take up the cause.
“We are not asking for some big, $300,000 piece of equipment,” Dr. Baker said. “The two things that show the best evidence of preventing this harm are things that should be happening in standard care anyway – brushing teeth and getting patients mobilized.”
That evidence comes from a smattering of studies that show those two strategies can lead to sharp reductions in infection rates.
In California, a study at 21 Kaiser Permanente hospitals used a reprioritization of oral care and getting patients out of bed to reduce rates of hospital-acquired pneumonia by around 70%. At Sutter Medical Center in Sacramento, better oral care reduced NVHAP cases by a yearly average of 35%.
At Orlando Regional Medical Center in Florida, a medical unit and a surgical unit where patients received enhanced oral care reduced NVHAP rates by 85% and 56%, respectively, when compared with similar units that received normal care. A similar study is underway at two hospitals in Illinois.
And the most compelling results come from a veterans’ hospital in Salem, Va., where a 2016 oral care pilot program reduced rates of NVHAP by 92% – saving an estimated 13 lives in just 19 months. The program, the HAPPEN Initiative, has been expanded across the Veterans Health Administration, and experts say it could serve as a model for all U.S. hospitals.
Dr. Michelle Lucatorto, a nursing official who leads HAPPEN, said the program trains nurses to most effectively brush patients’ teeth and educates patients and families on the link between oral care and preventing NVHAP. While teeth brushing may not seem to require training, Dr. Lucatorto made comparisons to how the coronavirus revealed many Americans were doing a lackluster job of another routine hygienic practice: washing their hands.
“Sometimes we are searching for the most complicated intervention,” she said. “We are always looking for that new bypass surgery, or some new technical equipment. And sometimes I think we fail to look at the simple things we can do in our practice to save people’s lives.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Uveitis in juvenile arthritis patients persists into midlife
Active uveitis remained in 43.4% of juvenile idiopathic arthritis (JIA) patients up to 40 years after a diagnosis, based on data from 30 individuals.
Uveitis occurs in approximately 10%-20% of patients with JIA, but data on the long-term activity and prevalence are limited, although previous studies suggest that uveitis can persist into adulthood, wrote Dr. Angelika Skarin of Skåne University in Lund, Sweden, and colleagues.
In a study published in Pediatric Rheumatology, the researchers reviewed ophthalmic records from 30 JIA patients at a mean of 40.7 years after uveitis onset. They compared these records to data collected from the same patient population at a mean of 7.2 and 24.0 years after onset. In the previous follow-up studies, 49% of the patients had active uveitis at 24 years, and the prevalence of cataracts and glaucoma increased between the 7-year and 24-year assessments.
In the current study, 43.4% of the population had active uveitis at the 40-year follow-up, which corresponded to 23.6% of the original study cohort. The mean age of the participants overall was 46.9 years, the mean duration of joint disease was 42.99 years, and the mean time from onset of uveitis was 40.7 years.
In addition, 66.6% of the patients in the current study had cataracts or had undergone cataract surgery in one or both eyes, and 40.0% had glaucoma.
By the time of the current study, of the original cohort of 55 individuals, 11 were deceased; rheumatic disease was declared the main cause in four patients and a contributing factor in three others.
Potential drivers of the earliest cases of glaucoma and ocular hypertension (G/OH) include increased intraocular pressure as a result of topical corticosteroid treatment, the researchers noted in their discussion. However, G/OH occurring later than the 7-year follow-up was “more likely to be the type observed in many patients with long-standing chronic uveitis, where a gradual increase in intraocular pressure is assumed to be caused by impaired aqueous outflow,” they said.
Only 4 of the 30 patients did not have regular ophthalmology visits, which suggests a study population with ocular symptoms or concerns about their eyesight, the researchers wrote. “The fact that 13% of our original cohort were reported to have severe visual impairment or worse in both eyes at any of the three follow-ups is noteworthy,” compared to reports of visual impairment of less than 0.5% in a German study in the general population for similar ages.
The findings were limited by several factors, including the retrospective design, small study population, and lack of data on 25 of the original 55-member study cohort, which may reduce the reliability of the current study, the researchers noted. However, the results reflect data from previous studies and support the need for JIA patients to continue regular ophthalmic checkups throughout life, they concluded.
The study was supported by Stiftelsen för Synskadade i f.d. Malmöhus län, Sweden, Skånes Universitetssjukhus Stiftelser och Donationer, Ögonfonden, and the Swedish Society of Medicine. The researchers had no financial conflicts to disclose.
Active uveitis remained in 43.4% of juvenile idiopathic arthritis (JIA) patients up to 40 years after a diagnosis, based on data from 30 individuals.
Uveitis occurs in approximately 10%-20% of patients with JIA, but data on the long-term activity and prevalence are limited, although previous studies suggest that uveitis can persist into adulthood, wrote Dr. Angelika Skarin of Skåne University in Lund, Sweden, and colleagues.
In a study published in Pediatric Rheumatology, the researchers reviewed ophthalmic records from 30 JIA patients at a mean of 40.7 years after uveitis onset. They compared these records to data collected from the same patient population at a mean of 7.2 and 24.0 years after onset. In the previous follow-up studies, 49% of the patients had active uveitis at 24 years, and the prevalence of cataracts and glaucoma increased between the 7-year and 24-year assessments.
In the current study, 43.4% of the population had active uveitis at the 40-year follow-up, which corresponded to 23.6% of the original study cohort. The mean age of the participants overall was 46.9 years, the mean duration of joint disease was 42.99 years, and the mean time from onset of uveitis was 40.7 years.
In addition, 66.6% of the patients in the current study had cataracts or had undergone cataract surgery in one or both eyes, and 40.0% had glaucoma.
By the time of the current study, of the original cohort of 55 individuals, 11 were deceased; rheumatic disease was declared the main cause in four patients and a contributing factor in three others.
Potential drivers of the earliest cases of glaucoma and ocular hypertension (G/OH) include increased intraocular pressure as a result of topical corticosteroid treatment, the researchers noted in their discussion. However, G/OH occurring later than the 7-year follow-up was “more likely to be the type observed in many patients with long-standing chronic uveitis, where a gradual increase in intraocular pressure is assumed to be caused by impaired aqueous outflow,” they said.
Only 4 of the 30 patients did not have regular ophthalmology visits, which suggests a study population with ocular symptoms or concerns about their eyesight, the researchers wrote. “The fact that 13% of our original cohort were reported to have severe visual impairment or worse in both eyes at any of the three follow-ups is noteworthy,” compared to reports of visual impairment of less than 0.5% in a German study in the general population for similar ages.
The findings were limited by several factors, including the retrospective design, small study population, and lack of data on 25 of the original 55-member study cohort, which may reduce the reliability of the current study, the researchers noted. However, the results reflect data from previous studies and support the need for JIA patients to continue regular ophthalmic checkups throughout life, they concluded.
The study was supported by Stiftelsen för Synskadade i f.d. Malmöhus län, Sweden, Skånes Universitetssjukhus Stiftelser och Donationer, Ögonfonden, and the Swedish Society of Medicine. The researchers had no financial conflicts to disclose.
Active uveitis remained in 43.4% of juvenile idiopathic arthritis (JIA) patients up to 40 years after a diagnosis, based on data from 30 individuals.
Uveitis occurs in approximately 10%-20% of patients with JIA, but data on the long-term activity and prevalence are limited, although previous studies suggest that uveitis can persist into adulthood, wrote Dr. Angelika Skarin of Skåne University in Lund, Sweden, and colleagues.
In a study published in Pediatric Rheumatology, the researchers reviewed ophthalmic records from 30 JIA patients at a mean of 40.7 years after uveitis onset. They compared these records to data collected from the same patient population at a mean of 7.2 and 24.0 years after onset. In the previous follow-up studies, 49% of the patients had active uveitis at 24 years, and the prevalence of cataracts and glaucoma increased between the 7-year and 24-year assessments.
In the current study, 43.4% of the population had active uveitis at the 40-year follow-up, which corresponded to 23.6% of the original study cohort. The mean age of the participants overall was 46.9 years, the mean duration of joint disease was 42.99 years, and the mean time from onset of uveitis was 40.7 years.
In addition, 66.6% of the patients in the current study had cataracts or had undergone cataract surgery in one or both eyes, and 40.0% had glaucoma.
By the time of the current study, of the original cohort of 55 individuals, 11 were deceased; rheumatic disease was declared the main cause in four patients and a contributing factor in three others.
Potential drivers of the earliest cases of glaucoma and ocular hypertension (G/OH) include increased intraocular pressure as a result of topical corticosteroid treatment, the researchers noted in their discussion. However, G/OH occurring later than the 7-year follow-up was “more likely to be the type observed in many patients with long-standing chronic uveitis, where a gradual increase in intraocular pressure is assumed to be caused by impaired aqueous outflow,” they said.
Only 4 of the 30 patients did not have regular ophthalmology visits, which suggests a study population with ocular symptoms or concerns about their eyesight, the researchers wrote. “The fact that 13% of our original cohort were reported to have severe visual impairment or worse in both eyes at any of the three follow-ups is noteworthy,” compared to reports of visual impairment of less than 0.5% in a German study in the general population for similar ages.
The findings were limited by several factors, including the retrospective design, small study population, and lack of data on 25 of the original 55-member study cohort, which may reduce the reliability of the current study, the researchers noted. However, the results reflect data from previous studies and support the need for JIA patients to continue regular ophthalmic checkups throughout life, they concluded.
The study was supported by Stiftelsen för Synskadade i f.d. Malmöhus län, Sweden, Skånes Universitetssjukhus Stiftelser och Donationer, Ögonfonden, and the Swedish Society of Medicine. The researchers had no financial conflicts to disclose.
FROM PEDIATRIC RHEUMATOLOGY
Pulse oximeters lead to less oxygen supplementation for people of color
The new research suggests that skin color–related differences in pulse oximeter readings are in fact impacting clinical decision-making, lead author Eric R. Gottlieb, MD, of Brigham and Women’s Hospital and Massachusetts Institute of Technology, both in Boston, and colleagues wrote. This suggests that technology needs to updated to improve health equity, they continued, in their paper published in JAMA Internal Medicine.
“It has been known for decades that these readings are affected by various surface pigmentations, including nail polish and skin melanin, which may affect light absorption and scattering,” the investigators wrote. “This increases the risk of hidden hypoxemia [among patients with darker skin], in which patients have falsely elevated SpO2 readings, usually defined as 92% or greater, with a blood hemoglobin oxygen saturation less than 88%.”
Although published reports on this phenomenon date back to the 1980s, clinical significance has been largely discounted, they said, citing a 2008 paper on the topic, which stated that “oximetry need not have exact accuracy” to determine if a patient needs oxygen supplementation.
‘We’re not providing equal care’
Questioning the validity of this statement, Dr. Gottlieb and colleagues conducted a retrospective cohort study involving 3,069 patients admitted to intensive care at the Beth Israel Deaconess Medical Center in Boston between 2008 and 2019, thereby excluding patients treated during the COVID-19 pandemic. The population consisted of four races/ethnicities: White (87%), Black (7%), Hispanic (4%), and Asian (3%).
Aligning with previous studies, multivariable linear regression analyses showed that Asian, Black, and Hispanic patients had significantly higher SpO2 readings than White patients in relation to hemoglobin oxygen saturation values, suggesting falsely elevated readings.
Further modeling showed that these same patient groups also received lower oxygen delivery rates, which were not explained directly by race/ethnicity, but instead were mediated by the discrepancy between SpO2 and hemoglobin oxygen saturation values. In other words, physicians were responding consistently to pulse oximetry readings, rather than exhibiting a direct racial/ethnic bias in their clinical decision-making.
“We’re not providing equal care,” Dr. Gottlieb said in an interview. “It’s not that the patients are sicker, or have other socioeconomic explanations for why this happens to them. It’s us. It’s our technology. And that’s something that really has to be fixed.”
The investigators offered a cautionary view of corrective algorithms, as these “have exacerbated disparities and are subject to ethical concerns;” for example, with glomerular filtration rate estimations in Black patients.
Dr. Gottlieb also cautioned against action by individual physicians, who may now be inclined to change how they interpret pulse oximeter readings based on a patient’s race or ethnicity.
“I don’t think that we can expect physicians, every time they see a patient, to be second guessing whether the number basically reflects the truth,” he said.
Instead, Dr. Gottlieb suggested that the burden of change rests upon the shoulders of institutions, including hospitals and device manufacturers, both of which “really need to take the responsibility” for making sure that pulse oximeters are “equitable and have similar performance across races.”
While Dr. Gottlieb said that skin color likely plays the greatest role in measurement discrepancies, he encouraged stakeholders “to think broadly about this, and not just assume that it’s entirely skin color,” noting a small amount of evidence indicating that blood chemistry may also play a role. Still, he predicted that colorimetry – the direct measurement of skin color – will probably be incorporated into pulse oximeters of the future.
Black patients 3X more likely to have hidden hypoxia than White patients
Michael Sjoding, MD, of the University of Michigan, Ann Arbor, was one of the first to raise awareness of skin color–related issues with pulse oximeters during the throes of the COVID-19 pandemic. His study, which involved more than 10,000 patients, showed that Black patients were threefold more likely to have hidden hypoxia than White patients.
The present study shows that such discrepancies are indeed clinically significant, Dr. Sjoding said in an interview. And these data are needed, he added, to bring about change.
“What is being asked is potentially a big deal,” Dr. Sjoding said. “Pulse oximeters are everywhere, and it would be a big undertaking to redesign pulse oximeters and purchase new pulse oximeters. You need a compelling body of evidence to do that. I think it’s there now, clearly. So I’m hopeful that we’re going to finally move forward, towards having devices that we are confident work accurately in everyone.”
Why it has taken so long to gather this evidence, however, is a thornier topic, considering race-related discrepancies in pulse oximeter readings were first documented more than 3 decades ago.
“We sort of rediscovered something that had been known and had been described in the past,” Dr. Sjoding said. He explained how he and many of his colleagues had completed pulmonary fellowships, yet none of them knew of these potential issues with pulse oximeters until they began to observe differences in their own patients during the pandemic.
“I’ll give previous generations of researchers the benefit of the doubt,” Dr. Sjoding said, pointing out that techniques in data gathering and analysis have advanced considerably over the years. “The types of studies that were done before were very different than what we did.”
Yet Dr. Sjoding entertained the possibility that other factors may have been at play.
“I think definitely there’s a social commentary on prioritization of research,” he said.
The study was supported by grants from the National Institutes of Health. The investigators and Dr. Sjoding reported no conflicts of interest.
The new research suggests that skin color–related differences in pulse oximeter readings are in fact impacting clinical decision-making, lead author Eric R. Gottlieb, MD, of Brigham and Women’s Hospital and Massachusetts Institute of Technology, both in Boston, and colleagues wrote. This suggests that technology needs to updated to improve health equity, they continued, in their paper published in JAMA Internal Medicine.
“It has been known for decades that these readings are affected by various surface pigmentations, including nail polish and skin melanin, which may affect light absorption and scattering,” the investigators wrote. “This increases the risk of hidden hypoxemia [among patients with darker skin], in which patients have falsely elevated SpO2 readings, usually defined as 92% or greater, with a blood hemoglobin oxygen saturation less than 88%.”
Although published reports on this phenomenon date back to the 1980s, clinical significance has been largely discounted, they said, citing a 2008 paper on the topic, which stated that “oximetry need not have exact accuracy” to determine if a patient needs oxygen supplementation.
‘We’re not providing equal care’
Questioning the validity of this statement, Dr. Gottlieb and colleagues conducted a retrospective cohort study involving 3,069 patients admitted to intensive care at the Beth Israel Deaconess Medical Center in Boston between 2008 and 2019, thereby excluding patients treated during the COVID-19 pandemic. The population consisted of four races/ethnicities: White (87%), Black (7%), Hispanic (4%), and Asian (3%).
Aligning with previous studies, multivariable linear regression analyses showed that Asian, Black, and Hispanic patients had significantly higher SpO2 readings than White patients in relation to hemoglobin oxygen saturation values, suggesting falsely elevated readings.
Further modeling showed that these same patient groups also received lower oxygen delivery rates, which were not explained directly by race/ethnicity, but instead were mediated by the discrepancy between SpO2 and hemoglobin oxygen saturation values. In other words, physicians were responding consistently to pulse oximetry readings, rather than exhibiting a direct racial/ethnic bias in their clinical decision-making.
“We’re not providing equal care,” Dr. Gottlieb said in an interview. “It’s not that the patients are sicker, or have other socioeconomic explanations for why this happens to them. It’s us. It’s our technology. And that’s something that really has to be fixed.”
The investigators offered a cautionary view of corrective algorithms, as these “have exacerbated disparities and are subject to ethical concerns;” for example, with glomerular filtration rate estimations in Black patients.
Dr. Gottlieb also cautioned against action by individual physicians, who may now be inclined to change how they interpret pulse oximeter readings based on a patient’s race or ethnicity.
“I don’t think that we can expect physicians, every time they see a patient, to be second guessing whether the number basically reflects the truth,” he said.
Instead, Dr. Gottlieb suggested that the burden of change rests upon the shoulders of institutions, including hospitals and device manufacturers, both of which “really need to take the responsibility” for making sure that pulse oximeters are “equitable and have similar performance across races.”
While Dr. Gottlieb said that skin color likely plays the greatest role in measurement discrepancies, he encouraged stakeholders “to think broadly about this, and not just assume that it’s entirely skin color,” noting a small amount of evidence indicating that blood chemistry may also play a role. Still, he predicted that colorimetry – the direct measurement of skin color – will probably be incorporated into pulse oximeters of the future.
Black patients 3X more likely to have hidden hypoxia than White patients
Michael Sjoding, MD, of the University of Michigan, Ann Arbor, was one of the first to raise awareness of skin color–related issues with pulse oximeters during the throes of the COVID-19 pandemic. His study, which involved more than 10,000 patients, showed that Black patients were threefold more likely to have hidden hypoxia than White patients.
The present study shows that such discrepancies are indeed clinically significant, Dr. Sjoding said in an interview. And these data are needed, he added, to bring about change.
“What is being asked is potentially a big deal,” Dr. Sjoding said. “Pulse oximeters are everywhere, and it would be a big undertaking to redesign pulse oximeters and purchase new pulse oximeters. You need a compelling body of evidence to do that. I think it’s there now, clearly. So I’m hopeful that we’re going to finally move forward, towards having devices that we are confident work accurately in everyone.”
Why it has taken so long to gather this evidence, however, is a thornier topic, considering race-related discrepancies in pulse oximeter readings were first documented more than 3 decades ago.
“We sort of rediscovered something that had been known and had been described in the past,” Dr. Sjoding said. He explained how he and many of his colleagues had completed pulmonary fellowships, yet none of them knew of these potential issues with pulse oximeters until they began to observe differences in their own patients during the pandemic.
“I’ll give previous generations of researchers the benefit of the doubt,” Dr. Sjoding said, pointing out that techniques in data gathering and analysis have advanced considerably over the years. “The types of studies that were done before were very different than what we did.”
Yet Dr. Sjoding entertained the possibility that other factors may have been at play.
“I think definitely there’s a social commentary on prioritization of research,” he said.
The study was supported by grants from the National Institutes of Health. The investigators and Dr. Sjoding reported no conflicts of interest.
The new research suggests that skin color–related differences in pulse oximeter readings are in fact impacting clinical decision-making, lead author Eric R. Gottlieb, MD, of Brigham and Women’s Hospital and Massachusetts Institute of Technology, both in Boston, and colleagues wrote. This suggests that technology needs to updated to improve health equity, they continued, in their paper published in JAMA Internal Medicine.
“It has been known for decades that these readings are affected by various surface pigmentations, including nail polish and skin melanin, which may affect light absorption and scattering,” the investigators wrote. “This increases the risk of hidden hypoxemia [among patients with darker skin], in which patients have falsely elevated SpO2 readings, usually defined as 92% or greater, with a blood hemoglobin oxygen saturation less than 88%.”
Although published reports on this phenomenon date back to the 1980s, clinical significance has been largely discounted, they said, citing a 2008 paper on the topic, which stated that “oximetry need not have exact accuracy” to determine if a patient needs oxygen supplementation.
‘We’re not providing equal care’
Questioning the validity of this statement, Dr. Gottlieb and colleagues conducted a retrospective cohort study involving 3,069 patients admitted to intensive care at the Beth Israel Deaconess Medical Center in Boston between 2008 and 2019, thereby excluding patients treated during the COVID-19 pandemic. The population consisted of four races/ethnicities: White (87%), Black (7%), Hispanic (4%), and Asian (3%).
Aligning with previous studies, multivariable linear regression analyses showed that Asian, Black, and Hispanic patients had significantly higher SpO2 readings than White patients in relation to hemoglobin oxygen saturation values, suggesting falsely elevated readings.
Further modeling showed that these same patient groups also received lower oxygen delivery rates, which were not explained directly by race/ethnicity, but instead were mediated by the discrepancy between SpO2 and hemoglobin oxygen saturation values. In other words, physicians were responding consistently to pulse oximetry readings, rather than exhibiting a direct racial/ethnic bias in their clinical decision-making.
“We’re not providing equal care,” Dr. Gottlieb said in an interview. “It’s not that the patients are sicker, or have other socioeconomic explanations for why this happens to them. It’s us. It’s our technology. And that’s something that really has to be fixed.”
The investigators offered a cautionary view of corrective algorithms, as these “have exacerbated disparities and are subject to ethical concerns;” for example, with glomerular filtration rate estimations in Black patients.
Dr. Gottlieb also cautioned against action by individual physicians, who may now be inclined to change how they interpret pulse oximeter readings based on a patient’s race or ethnicity.
“I don’t think that we can expect physicians, every time they see a patient, to be second guessing whether the number basically reflects the truth,” he said.
Instead, Dr. Gottlieb suggested that the burden of change rests upon the shoulders of institutions, including hospitals and device manufacturers, both of which “really need to take the responsibility” for making sure that pulse oximeters are “equitable and have similar performance across races.”
While Dr. Gottlieb said that skin color likely plays the greatest role in measurement discrepancies, he encouraged stakeholders “to think broadly about this, and not just assume that it’s entirely skin color,” noting a small amount of evidence indicating that blood chemistry may also play a role. Still, he predicted that colorimetry – the direct measurement of skin color – will probably be incorporated into pulse oximeters of the future.
Black patients 3X more likely to have hidden hypoxia than White patients
Michael Sjoding, MD, of the University of Michigan, Ann Arbor, was one of the first to raise awareness of skin color–related issues with pulse oximeters during the throes of the COVID-19 pandemic. His study, which involved more than 10,000 patients, showed that Black patients were threefold more likely to have hidden hypoxia than White patients.
The present study shows that such discrepancies are indeed clinically significant, Dr. Sjoding said in an interview. And these data are needed, he added, to bring about change.
“What is being asked is potentially a big deal,” Dr. Sjoding said. “Pulse oximeters are everywhere, and it would be a big undertaking to redesign pulse oximeters and purchase new pulse oximeters. You need a compelling body of evidence to do that. I think it’s there now, clearly. So I’m hopeful that we’re going to finally move forward, towards having devices that we are confident work accurately in everyone.”
Why it has taken so long to gather this evidence, however, is a thornier topic, considering race-related discrepancies in pulse oximeter readings were first documented more than 3 decades ago.
“We sort of rediscovered something that had been known and had been described in the past,” Dr. Sjoding said. He explained how he and many of his colleagues had completed pulmonary fellowships, yet none of them knew of these potential issues with pulse oximeters until they began to observe differences in their own patients during the pandemic.
“I’ll give previous generations of researchers the benefit of the doubt,” Dr. Sjoding said, pointing out that techniques in data gathering and analysis have advanced considerably over the years. “The types of studies that were done before were very different than what we did.”
Yet Dr. Sjoding entertained the possibility that other factors may have been at play.
“I think definitely there’s a social commentary on prioritization of research,” he said.
The study was supported by grants from the National Institutes of Health. The investigators and Dr. Sjoding reported no conflicts of interest.
FROM JAMA INTERNAL MEDICINE