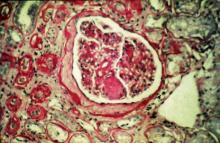
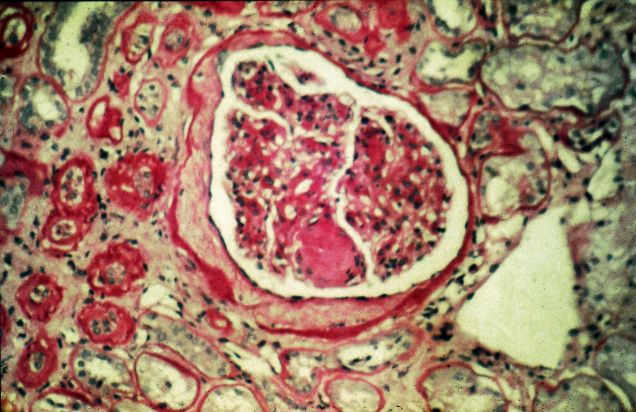
User login
AGA issues position statements on reducing CRC burden
The American Gastroenterological Association has published eight position statements aimed at reducing the burden of colorectal cancer (CRC).
The evidence-based statements, published in Gastroenterology, call for a national approach to CRC screening, outline the elements of a high-quality screening program, and make clear that payers should cover all costs, from bowel prep through pathology, plus follow-up for high-risk patients.
“There is strong evidence that CRC screening is effective [at reducing CRC incidence and mortality] ... but less than 70% of eligible individuals have been screened,” wrote authors led by David Lieberman, MD, who is on the AGA Executive Committee on the Screening Continuum and affiliated with Oregon Health and Science University, Portland, noting the recent expansion of eligibility to include individuals in the 45- to 49-year age group.
“CRC screening saves lives, but only if people get screened,” Dr. Lieberman said in a press release from the AGA. “Cost sharing is an important barrier to screening, which contributes to racial, ethnic and socioeconomic inequities in colorectal cancer outcomes. The full cost of screening – including noninvasive tests and follow-up colonoscopies – should be covered without cost to patients.”
He added: “AGA wishes to collaborate with stakeholders to eliminate obstacles to screening, which disproportionately impact those with low income and lack of insurance.”
Eliminating disparities in screening
Among the position statements, Dr. Lieberman and colleagues first called for “development of a national approach to CRC screening” to patch gaps in access across the United States.
“Systematic outreach occurs infrequently,” they noted. “CRC screening prevalence is much lower among individuals who do not have access to health care due to lack of insurance, do not have a primary care provider, or are part of a medically underserved community.”
According to Dr. Lieberman and colleagues, the AGA is also “working with a broad coalition of stakeholders,” such as the American Cancer Society, payers, patient advocacy groups, and others, to create a “national resource ... focused on ensuring high-quality CRC screening and eliminating barriers to CRC screening.”
Specifically, the coalition will work to collectively tackle “disparities created by social determinants of health, which includes lack of access to screening, transportation, and even work hours and child care.
“The AGA recognizes that moving the needle to achieve a CRC screening participation goal of 80% will take a village,” they wrote.
Elements of high-quality CRC screening
The investigators went on to describe the key features of a high-quality CRC screening program, including “colonoscopy and noninvasive screening options, patient education, outreach, and navigation support.”
Dr. Lieberman and colleagues pointed out that offering more than one type of screening test “acknowledges patient preferences and improves participation.”
Certain noninvasive methods, such as fecal immunochemical testing (FIT), eliminate “important barriers” to screening, they noted, such as the need for special preparation, time off work, and transportation to a medical facility.
For individuals who have high-risk adenomas (HRAs) or advanced sessile serrated lesions (SSLs), screening should be expanded to include follow-up, the investigators added.
“Evidence from a systematic review demonstrates that individuals with HRAs at baseline have a 3- to 4-fold higher risk of incident CRC during follow-up compared with individuals with no adenoma or low-risk adenomas,” they wrote. “There is also evidence that individuals with advanced SSLs have a three= to fourfold higher risk of CRC, compared with individuals with nonadvanced SSLs.”
Payers should cover costs
To further improve access to care, payers should cover the full costs of CRC screening because “copays and deductibles are barriers to screening and contribute to socioeconomic disparities,” that “disproportionately impact those with low income and lack of insurance,” according to Dr. Lieberman and colleagues.
They noted that the Affordable Care Act “eliminated copayments for preventive services,” yet a recent study showed that almost half of patients with commercial insurance and more than three-quarters of patients with Medicare still share some cost of CRC screening.
The investigators made clear that payers need to cover costs from start to finish, including “bowel preparation, facility and professional fees, anesthesia, and pathology,” as well as follow-up screening for high-risk patients identified by noninvasive methods.
“Noninvasive colorectal screening should be considered as programs with multiple steps, each of which, including follow-up colonoscopy if the test is positive, should be covered by payers without cost sharing as part of the screening continuum,” Dr. Lieberman and colleagues wrote.
Changes underway
According to Steven Itzkowitz, MD, professor of medicine and oncological sciences and director of the gastroenterology fellowship training program at the Icahn School of Medicine at Mount Sinai, New York, the AGA publication is important because it “consolidates many of the critical issues related to decreasing the burden of colorectal cancer in the United States.”
Dr. Itzkowitz noted that changes are already underway to eliminate cost as a barrier to screening.
“The good news is that, in the past year, the Departments of Health & Human Services, Labor, and Treasury declared that cost sharing should not be imposed, and plans are required to cover screening colonoscopy with polyp removal and colonoscopy that is performed to follow-up after an abnormal noninvasive CRC screening test,” Dr. Itzkowitz said in an interview. “Many plans are following suit, but it will take time for this coverage to take effect across all plans.”
For individual gastroenterologists who would like to do their part in reducing screening inequity, Dr. Itzkowitz suggested leveraging noninvasive testing, as the AGA recommends.
“This publication is the latest to call for using noninvasive, stool-based testing in addition to colonoscopy,” Dr. Itzkowitz said. “FIT and multitarget stool DNA tests all have proven efficacy in this regard, so gastroenterologists should have those conversations with their patients. GIs can also make it easier for patients to complete colonoscopy by developing patient navigation programs, direct access referrals, and systems for communicating with primary care providers for easier referrals and communicating colonoscopy results.”
Many practices are already instituting such improvements in response to the restrictions imposed by the COVID-19 pandemic, according to Dr. Itzkowitz.“These changes, plus better coverage by payers, will make a huge impact on health equity when it comes to colorectal cancer screening.”
The publication was supported by the AGA. The investigators disclosed relationships with Geneoscopy, ColoWrap, UniversalDx, and others. Dr. Itzkowitz disclosed no relevant conflicts of interest.
Groups interested in collaborating with AGA should contact Kathleen Teixeira, AGA Vice President, Public Policy and Advocacy, at [email protected].
The American Gastroenterological Association has published eight position statements aimed at reducing the burden of colorectal cancer (CRC).
The evidence-based statements, published in Gastroenterology, call for a national approach to CRC screening, outline the elements of a high-quality screening program, and make clear that payers should cover all costs, from bowel prep through pathology, plus follow-up for high-risk patients.
“There is strong evidence that CRC screening is effective [at reducing CRC incidence and mortality] ... but less than 70% of eligible individuals have been screened,” wrote authors led by David Lieberman, MD, who is on the AGA Executive Committee on the Screening Continuum and affiliated with Oregon Health and Science University, Portland, noting the recent expansion of eligibility to include individuals in the 45- to 49-year age group.
“CRC screening saves lives, but only if people get screened,” Dr. Lieberman said in a press release from the AGA. “Cost sharing is an important barrier to screening, which contributes to racial, ethnic and socioeconomic inequities in colorectal cancer outcomes. The full cost of screening – including noninvasive tests and follow-up colonoscopies – should be covered without cost to patients.”
He added: “AGA wishes to collaborate with stakeholders to eliminate obstacles to screening, which disproportionately impact those with low income and lack of insurance.”
Eliminating disparities in screening
Among the position statements, Dr. Lieberman and colleagues first called for “development of a national approach to CRC screening” to patch gaps in access across the United States.
“Systematic outreach occurs infrequently,” they noted. “CRC screening prevalence is much lower among individuals who do not have access to health care due to lack of insurance, do not have a primary care provider, or are part of a medically underserved community.”
According to Dr. Lieberman and colleagues, the AGA is also “working with a broad coalition of stakeholders,” such as the American Cancer Society, payers, patient advocacy groups, and others, to create a “national resource ... focused on ensuring high-quality CRC screening and eliminating barriers to CRC screening.”
Specifically, the coalition will work to collectively tackle “disparities created by social determinants of health, which includes lack of access to screening, transportation, and even work hours and child care.
“The AGA recognizes that moving the needle to achieve a CRC screening participation goal of 80% will take a village,” they wrote.
Elements of high-quality CRC screening
The investigators went on to describe the key features of a high-quality CRC screening program, including “colonoscopy and noninvasive screening options, patient education, outreach, and navigation support.”
Dr. Lieberman and colleagues pointed out that offering more than one type of screening test “acknowledges patient preferences and improves participation.”
Certain noninvasive methods, such as fecal immunochemical testing (FIT), eliminate “important barriers” to screening, they noted, such as the need for special preparation, time off work, and transportation to a medical facility.
For individuals who have high-risk adenomas (HRAs) or advanced sessile serrated lesions (SSLs), screening should be expanded to include follow-up, the investigators added.
“Evidence from a systematic review demonstrates that individuals with HRAs at baseline have a 3- to 4-fold higher risk of incident CRC during follow-up compared with individuals with no adenoma or low-risk adenomas,” they wrote. “There is also evidence that individuals with advanced SSLs have a three= to fourfold higher risk of CRC, compared with individuals with nonadvanced SSLs.”
Payers should cover costs
To further improve access to care, payers should cover the full costs of CRC screening because “copays and deductibles are barriers to screening and contribute to socioeconomic disparities,” that “disproportionately impact those with low income and lack of insurance,” according to Dr. Lieberman and colleagues.
They noted that the Affordable Care Act “eliminated copayments for preventive services,” yet a recent study showed that almost half of patients with commercial insurance and more than three-quarters of patients with Medicare still share some cost of CRC screening.
The investigators made clear that payers need to cover costs from start to finish, including “bowel preparation, facility and professional fees, anesthesia, and pathology,” as well as follow-up screening for high-risk patients identified by noninvasive methods.
“Noninvasive colorectal screening should be considered as programs with multiple steps, each of which, including follow-up colonoscopy if the test is positive, should be covered by payers without cost sharing as part of the screening continuum,” Dr. Lieberman and colleagues wrote.
Changes underway
According to Steven Itzkowitz, MD, professor of medicine and oncological sciences and director of the gastroenterology fellowship training program at the Icahn School of Medicine at Mount Sinai, New York, the AGA publication is important because it “consolidates many of the critical issues related to decreasing the burden of colorectal cancer in the United States.”
Dr. Itzkowitz noted that changes are already underway to eliminate cost as a barrier to screening.
“The good news is that, in the past year, the Departments of Health & Human Services, Labor, and Treasury declared that cost sharing should not be imposed, and plans are required to cover screening colonoscopy with polyp removal and colonoscopy that is performed to follow-up after an abnormal noninvasive CRC screening test,” Dr. Itzkowitz said in an interview. “Many plans are following suit, but it will take time for this coverage to take effect across all plans.”
For individual gastroenterologists who would like to do their part in reducing screening inequity, Dr. Itzkowitz suggested leveraging noninvasive testing, as the AGA recommends.
“This publication is the latest to call for using noninvasive, stool-based testing in addition to colonoscopy,” Dr. Itzkowitz said. “FIT and multitarget stool DNA tests all have proven efficacy in this regard, so gastroenterologists should have those conversations with their patients. GIs can also make it easier for patients to complete colonoscopy by developing patient navigation programs, direct access referrals, and systems for communicating with primary care providers for easier referrals and communicating colonoscopy results.”
Many practices are already instituting such improvements in response to the restrictions imposed by the COVID-19 pandemic, according to Dr. Itzkowitz.“These changes, plus better coverage by payers, will make a huge impact on health equity when it comes to colorectal cancer screening.”
The publication was supported by the AGA. The investigators disclosed relationships with Geneoscopy, ColoWrap, UniversalDx, and others. Dr. Itzkowitz disclosed no relevant conflicts of interest.
Groups interested in collaborating with AGA should contact Kathleen Teixeira, AGA Vice President, Public Policy and Advocacy, at [email protected].
The American Gastroenterological Association has published eight position statements aimed at reducing the burden of colorectal cancer (CRC).
The evidence-based statements, published in Gastroenterology, call for a national approach to CRC screening, outline the elements of a high-quality screening program, and make clear that payers should cover all costs, from bowel prep through pathology, plus follow-up for high-risk patients.
“There is strong evidence that CRC screening is effective [at reducing CRC incidence and mortality] ... but less than 70% of eligible individuals have been screened,” wrote authors led by David Lieberman, MD, who is on the AGA Executive Committee on the Screening Continuum and affiliated with Oregon Health and Science University, Portland, noting the recent expansion of eligibility to include individuals in the 45- to 49-year age group.
“CRC screening saves lives, but only if people get screened,” Dr. Lieberman said in a press release from the AGA. “Cost sharing is an important barrier to screening, which contributes to racial, ethnic and socioeconomic inequities in colorectal cancer outcomes. The full cost of screening – including noninvasive tests and follow-up colonoscopies – should be covered without cost to patients.”
He added: “AGA wishes to collaborate with stakeholders to eliminate obstacles to screening, which disproportionately impact those with low income and lack of insurance.”
Eliminating disparities in screening
Among the position statements, Dr. Lieberman and colleagues first called for “development of a national approach to CRC screening” to patch gaps in access across the United States.
“Systematic outreach occurs infrequently,” they noted. “CRC screening prevalence is much lower among individuals who do not have access to health care due to lack of insurance, do not have a primary care provider, or are part of a medically underserved community.”
According to Dr. Lieberman and colleagues, the AGA is also “working with a broad coalition of stakeholders,” such as the American Cancer Society, payers, patient advocacy groups, and others, to create a “national resource ... focused on ensuring high-quality CRC screening and eliminating barriers to CRC screening.”
Specifically, the coalition will work to collectively tackle “disparities created by social determinants of health, which includes lack of access to screening, transportation, and even work hours and child care.
“The AGA recognizes that moving the needle to achieve a CRC screening participation goal of 80% will take a village,” they wrote.
Elements of high-quality CRC screening
The investigators went on to describe the key features of a high-quality CRC screening program, including “colonoscopy and noninvasive screening options, patient education, outreach, and navigation support.”
Dr. Lieberman and colleagues pointed out that offering more than one type of screening test “acknowledges patient preferences and improves participation.”
Certain noninvasive methods, such as fecal immunochemical testing (FIT), eliminate “important barriers” to screening, they noted, such as the need for special preparation, time off work, and transportation to a medical facility.
For individuals who have high-risk adenomas (HRAs) or advanced sessile serrated lesions (SSLs), screening should be expanded to include follow-up, the investigators added.
“Evidence from a systematic review demonstrates that individuals with HRAs at baseline have a 3- to 4-fold higher risk of incident CRC during follow-up compared with individuals with no adenoma or low-risk adenomas,” they wrote. “There is also evidence that individuals with advanced SSLs have a three= to fourfold higher risk of CRC, compared with individuals with nonadvanced SSLs.”
Payers should cover costs
To further improve access to care, payers should cover the full costs of CRC screening because “copays and deductibles are barriers to screening and contribute to socioeconomic disparities,” that “disproportionately impact those with low income and lack of insurance,” according to Dr. Lieberman and colleagues.
They noted that the Affordable Care Act “eliminated copayments for preventive services,” yet a recent study showed that almost half of patients with commercial insurance and more than three-quarters of patients with Medicare still share some cost of CRC screening.
The investigators made clear that payers need to cover costs from start to finish, including “bowel preparation, facility and professional fees, anesthesia, and pathology,” as well as follow-up screening for high-risk patients identified by noninvasive methods.
“Noninvasive colorectal screening should be considered as programs with multiple steps, each of which, including follow-up colonoscopy if the test is positive, should be covered by payers without cost sharing as part of the screening continuum,” Dr. Lieberman and colleagues wrote.
Changes underway
According to Steven Itzkowitz, MD, professor of medicine and oncological sciences and director of the gastroenterology fellowship training program at the Icahn School of Medicine at Mount Sinai, New York, the AGA publication is important because it “consolidates many of the critical issues related to decreasing the burden of colorectal cancer in the United States.”
Dr. Itzkowitz noted that changes are already underway to eliminate cost as a barrier to screening.
“The good news is that, in the past year, the Departments of Health & Human Services, Labor, and Treasury declared that cost sharing should not be imposed, and plans are required to cover screening colonoscopy with polyp removal and colonoscopy that is performed to follow-up after an abnormal noninvasive CRC screening test,” Dr. Itzkowitz said in an interview. “Many plans are following suit, but it will take time for this coverage to take effect across all plans.”
For individual gastroenterologists who would like to do their part in reducing screening inequity, Dr. Itzkowitz suggested leveraging noninvasive testing, as the AGA recommends.
“This publication is the latest to call for using noninvasive, stool-based testing in addition to colonoscopy,” Dr. Itzkowitz said. “FIT and multitarget stool DNA tests all have proven efficacy in this regard, so gastroenterologists should have those conversations with their patients. GIs can also make it easier for patients to complete colonoscopy by developing patient navigation programs, direct access referrals, and systems for communicating with primary care providers for easier referrals and communicating colonoscopy results.”
Many practices are already instituting such improvements in response to the restrictions imposed by the COVID-19 pandemic, according to Dr. Itzkowitz.“These changes, plus better coverage by payers, will make a huge impact on health equity when it comes to colorectal cancer screening.”
The publication was supported by the AGA. The investigators disclosed relationships with Geneoscopy, ColoWrap, UniversalDx, and others. Dr. Itzkowitz disclosed no relevant conflicts of interest.
Groups interested in collaborating with AGA should contact Kathleen Teixeira, AGA Vice President, Public Policy and Advocacy, at [email protected].
FROM GASTROENTEROLOGY
AGA issues position statements on reducing CRC burden
The American Gastroenterological Association has published eight position statements aimed at reducing the burden of colorectal cancer (CRC).
The evidence-based statements, published in Gastroenterology, call for a national approach to CRC screening, outline the elements of a high-quality screening program, and make clear that payers should cover all costs, from bowel prep through pathology, plus follow-up for high-risk patients.
“There is strong evidence that CRC screening is effective [at reducing CRC incidence and mortality] ... but less than 70% of eligible individuals have been screened,” wrote authors led by David Lieberman, MD, who is on the AGA Executive Committee on the Screening Continuum and affiliated with Oregon Health and Science University, Portland, noting the recent expansion of eligibility to include individuals in the 45- to 49-year age group.
“CRC screening saves lives, but only if people get screened,” Dr. Lieberman said in a press release from the AGA. “Cost sharing is an important barrier to screening, which contributes to racial, ethnic and socioeconomic inequities in colorectal cancer outcomes. The full cost of screening – including noninvasive tests and follow-up colonoscopies – should be covered without cost to patients.”

He added: “AGA wishes to collaborate with stakeholders to eliminate obstacles to screening, which disproportionately impact those with low income and lack of insurance.”
Eliminating disparities in screening
Among the position statements, Dr. Lieberman and colleagues first called for “development of a national approach to CRC screening” to patch gaps in access across the United States.
“Systematic outreach occurs infrequently,” they noted. “CRC screening prevalence is much lower among individuals who do not have access to health care due to lack of insurance, do not have a primary care provider, or are part of a medically underserved community.”
According to Dr. Lieberman and colleagues, the AGA is also “working with a broad coalition of stakeholders,” such as the American Cancer Society, payers, patient advocacy groups, and others, to create a “national resource ... focused on ensuring high-quality CRC screening and eliminating barriers to CRC screening.”
Specifically, the coalition will work to collectively tackle “disparities created by social determinants of health, which includes lack of access to screening, transportation, and even work hours and child care.
“The AGA recognizes that moving the needle to achieve a CRC screening participation goal of 80% will take a village,” they wrote.
Elements of high-quality CRC screening
The investigators went on to describe the key features of a high-quality CRC screening program, including “colonoscopy and noninvasive screening options, patient education, outreach, and navigation support.”
Dr. Lieberman and colleagues pointed out that offering more than one type of screening test “acknowledges patient preferences and improves participation.”
Certain noninvasive methods, such as fecal immunochemical testing (FIT), eliminate “important barriers” to screening, they noted, such as the need for special preparation, time off work, and transportation to a medical facility.
For individuals who have high-risk adenomas (HRAs) or advanced sessile serrated lesions (SSLs), screening should be expanded to include follow-up, the investigators added.
“Evidence from a systematic review demonstrates that individuals with HRAs at baseline have a 3- to 4-fold higher risk of incident CRC during follow-up compared with individuals with no adenoma or low-risk adenomas,” they wrote. “There is also evidence that individuals with advanced SSLs have a three= to fourfold higher risk of CRC, compared with individuals with nonadvanced SSLs.”
Payers should cover costs
To further improve access to care, payers should cover the full costs of CRC screening because “copays and deductibles are barriers to screening and contribute to socioeconomic disparities,” that “disproportionately impact those with low income and lack of insurance,” according to Dr. Lieberman and colleagues.
They noted that the Affordable Care Act “eliminated copayments for preventive services,” yet a recent study showed that almost half of patients with commercial insurance and more than three-quarters of patients with Medicare still share some cost of CRC screening.
The investigators made clear that payers need to cover costs from start to finish, including “bowel preparation, facility and professional fees, anesthesia, and pathology,” as well as follow-up screening for high-risk patients identified by noninvasive methods.
“Noninvasive colorectal screening should be considered as programs with multiple steps, each of which, including follow-up colonoscopy if the test is positive, should be covered by payers without cost sharing as part of the screening continuum,” Dr. Lieberman and colleagues wrote.
Changes underway
According to Steven Itzkowitz, MD, professor of medicine and oncological sciences and director of the gastroenterology fellowship training program at the Icahn School of Medicine at Mount Sinai, New York, the AGA publication is important because it “consolidates many of the critical issues related to decreasing the burden of colorectal cancer in the United States.”
Dr. Itzkowitz noted that changes are already underway to eliminate cost as a barrier to screening.
“The good news is that, in the past year, the Departments of Health & Human Services, Labor, and Treasury declared that cost sharing should not be imposed, and plans are required to cover screening colonoscopy with polyp removal and colonoscopy that is performed to follow-up after an abnormal noninvasive CRC screening test,” Dr. Itzkowitz said in an interview. “Many plans are following suit, but it will take time for this coverage to take effect across all plans.”
For individual gastroenterologists who would like to do their part in reducing screening inequity, Dr. Itzkowitz suggested leveraging noninvasive testing, as the AGA recommends.
“This publication is the latest to call for using noninvasive, stool-based testing in addition to colonoscopy,” Dr. Itzkowitz said. “FIT and multitarget stool DNA tests all have proven efficacy in this regard, so gastroenterologists should have those conversations with their patients. GIs can also make it easier for patients to complete colonoscopy by developing patient navigation programs, direct access referrals, and systems for communicating with primary care providers for easier referrals and communicating colonoscopy results.”
Many practices are already instituting such improvements in response to the restrictions imposed by the COVID-19 pandemic, according to Dr. Itzkowitz.“These changes, plus better coverage by payers, will make a huge impact on health equity when it comes to colorectal cancer screening.”
The publication was supported by the AGA. The investigators disclosed relationships with Geneoscopy, ColoWrap, UniversalDx, and others. Dr. Itzkowitz disclosed no relevant conflicts of interest.
The American Gastroenterological Association has published eight position statements aimed at reducing the burden of colorectal cancer (CRC).
The evidence-based statements, published in Gastroenterology, call for a national approach to CRC screening, outline the elements of a high-quality screening program, and make clear that payers should cover all costs, from bowel prep through pathology, plus follow-up for high-risk patients.
“There is strong evidence that CRC screening is effective [at reducing CRC incidence and mortality] ... but less than 70% of eligible individuals have been screened,” wrote authors led by David Lieberman, MD, who is on the AGA Executive Committee on the Screening Continuum and affiliated with Oregon Health and Science University, Portland, noting the recent expansion of eligibility to include individuals in the 45- to 49-year age group.
“CRC screening saves lives, but only if people get screened,” Dr. Lieberman said in a press release from the AGA. “Cost sharing is an important barrier to screening, which contributes to racial, ethnic and socioeconomic inequities in colorectal cancer outcomes. The full cost of screening – including noninvasive tests and follow-up colonoscopies – should be covered without cost to patients.”

He added: “AGA wishes to collaborate with stakeholders to eliminate obstacles to screening, which disproportionately impact those with low income and lack of insurance.”
Eliminating disparities in screening
Among the position statements, Dr. Lieberman and colleagues first called for “development of a national approach to CRC screening” to patch gaps in access across the United States.
“Systematic outreach occurs infrequently,” they noted. “CRC screening prevalence is much lower among individuals who do not have access to health care due to lack of insurance, do not have a primary care provider, or are part of a medically underserved community.”
According to Dr. Lieberman and colleagues, the AGA is also “working with a broad coalition of stakeholders,” such as the American Cancer Society, payers, patient advocacy groups, and others, to create a “national resource ... focused on ensuring high-quality CRC screening and eliminating barriers to CRC screening.”
Specifically, the coalition will work to collectively tackle “disparities created by social determinants of health, which includes lack of access to screening, transportation, and even work hours and child care.
“The AGA recognizes that moving the needle to achieve a CRC screening participation goal of 80% will take a village,” they wrote.
Elements of high-quality CRC screening
The investigators went on to describe the key features of a high-quality CRC screening program, including “colonoscopy and noninvasive screening options, patient education, outreach, and navigation support.”
Dr. Lieberman and colleagues pointed out that offering more than one type of screening test “acknowledges patient preferences and improves participation.”
Certain noninvasive methods, such as fecal immunochemical testing (FIT), eliminate “important barriers” to screening, they noted, such as the need for special preparation, time off work, and transportation to a medical facility.
For individuals who have high-risk adenomas (HRAs) or advanced sessile serrated lesions (SSLs), screening should be expanded to include follow-up, the investigators added.
“Evidence from a systematic review demonstrates that individuals with HRAs at baseline have a 3- to 4-fold higher risk of incident CRC during follow-up compared with individuals with no adenoma or low-risk adenomas,” they wrote. “There is also evidence that individuals with advanced SSLs have a three= to fourfold higher risk of CRC, compared with individuals with nonadvanced SSLs.”
Payers should cover costs
To further improve access to care, payers should cover the full costs of CRC screening because “copays and deductibles are barriers to screening and contribute to socioeconomic disparities,” that “disproportionately impact those with low income and lack of insurance,” according to Dr. Lieberman and colleagues.
They noted that the Affordable Care Act “eliminated copayments for preventive services,” yet a recent study showed that almost half of patients with commercial insurance and more than three-quarters of patients with Medicare still share some cost of CRC screening.
The investigators made clear that payers need to cover costs from start to finish, including “bowel preparation, facility and professional fees, anesthesia, and pathology,” as well as follow-up screening for high-risk patients identified by noninvasive methods.
“Noninvasive colorectal screening should be considered as programs with multiple steps, each of which, including follow-up colonoscopy if the test is positive, should be covered by payers without cost sharing as part of the screening continuum,” Dr. Lieberman and colleagues wrote.
Changes underway
According to Steven Itzkowitz, MD, professor of medicine and oncological sciences and director of the gastroenterology fellowship training program at the Icahn School of Medicine at Mount Sinai, New York, the AGA publication is important because it “consolidates many of the critical issues related to decreasing the burden of colorectal cancer in the United States.”
Dr. Itzkowitz noted that changes are already underway to eliminate cost as a barrier to screening.
“The good news is that, in the past year, the Departments of Health & Human Services, Labor, and Treasury declared that cost sharing should not be imposed, and plans are required to cover screening colonoscopy with polyp removal and colonoscopy that is performed to follow-up after an abnormal noninvasive CRC screening test,” Dr. Itzkowitz said in an interview. “Many plans are following suit, but it will take time for this coverage to take effect across all plans.”
For individual gastroenterologists who would like to do their part in reducing screening inequity, Dr. Itzkowitz suggested leveraging noninvasive testing, as the AGA recommends.
“This publication is the latest to call for using noninvasive, stool-based testing in addition to colonoscopy,” Dr. Itzkowitz said. “FIT and multitarget stool DNA tests all have proven efficacy in this regard, so gastroenterologists should have those conversations with their patients. GIs can also make it easier for patients to complete colonoscopy by developing patient navigation programs, direct access referrals, and systems for communicating with primary care providers for easier referrals and communicating colonoscopy results.”
Many practices are already instituting such improvements in response to the restrictions imposed by the COVID-19 pandemic, according to Dr. Itzkowitz.“These changes, plus better coverage by payers, will make a huge impact on health equity when it comes to colorectal cancer screening.”
The publication was supported by the AGA. The investigators disclosed relationships with Geneoscopy, ColoWrap, UniversalDx, and others. Dr. Itzkowitz disclosed no relevant conflicts of interest.
The American Gastroenterological Association has published eight position statements aimed at reducing the burden of colorectal cancer (CRC).
The evidence-based statements, published in Gastroenterology, call for a national approach to CRC screening, outline the elements of a high-quality screening program, and make clear that payers should cover all costs, from bowel prep through pathology, plus follow-up for high-risk patients.
“There is strong evidence that CRC screening is effective [at reducing CRC incidence and mortality] ... but less than 70% of eligible individuals have been screened,” wrote authors led by David Lieberman, MD, who is on the AGA Executive Committee on the Screening Continuum and affiliated with Oregon Health and Science University, Portland, noting the recent expansion of eligibility to include individuals in the 45- to 49-year age group.
“CRC screening saves lives, but only if people get screened,” Dr. Lieberman said in a press release from the AGA. “Cost sharing is an important barrier to screening, which contributes to racial, ethnic and socioeconomic inequities in colorectal cancer outcomes. The full cost of screening – including noninvasive tests and follow-up colonoscopies – should be covered without cost to patients.”

He added: “AGA wishes to collaborate with stakeholders to eliminate obstacles to screening, which disproportionately impact those with low income and lack of insurance.”
Eliminating disparities in screening
Among the position statements, Dr. Lieberman and colleagues first called for “development of a national approach to CRC screening” to patch gaps in access across the United States.
“Systematic outreach occurs infrequently,” they noted. “CRC screening prevalence is much lower among individuals who do not have access to health care due to lack of insurance, do not have a primary care provider, or are part of a medically underserved community.”
According to Dr. Lieberman and colleagues, the AGA is also “working with a broad coalition of stakeholders,” such as the American Cancer Society, payers, patient advocacy groups, and others, to create a “national resource ... focused on ensuring high-quality CRC screening and eliminating barriers to CRC screening.”
Specifically, the coalition will work to collectively tackle “disparities created by social determinants of health, which includes lack of access to screening, transportation, and even work hours and child care.
“The AGA recognizes that moving the needle to achieve a CRC screening participation goal of 80% will take a village,” they wrote.
Elements of high-quality CRC screening
The investigators went on to describe the key features of a high-quality CRC screening program, including “colonoscopy and noninvasive screening options, patient education, outreach, and navigation support.”
Dr. Lieberman and colleagues pointed out that offering more than one type of screening test “acknowledges patient preferences and improves participation.”
Certain noninvasive methods, such as fecal immunochemical testing (FIT), eliminate “important barriers” to screening, they noted, such as the need for special preparation, time off work, and transportation to a medical facility.
For individuals who have high-risk adenomas (HRAs) or advanced sessile serrated lesions (SSLs), screening should be expanded to include follow-up, the investigators added.
“Evidence from a systematic review demonstrates that individuals with HRAs at baseline have a 3- to 4-fold higher risk of incident CRC during follow-up compared with individuals with no adenoma or low-risk adenomas,” they wrote. “There is also evidence that individuals with advanced SSLs have a three= to fourfold higher risk of CRC, compared with individuals with nonadvanced SSLs.”
Payers should cover costs
To further improve access to care, payers should cover the full costs of CRC screening because “copays and deductibles are barriers to screening and contribute to socioeconomic disparities,” that “disproportionately impact those with low income and lack of insurance,” according to Dr. Lieberman and colleagues.
They noted that the Affordable Care Act “eliminated copayments for preventive services,” yet a recent study showed that almost half of patients with commercial insurance and more than three-quarters of patients with Medicare still share some cost of CRC screening.
The investigators made clear that payers need to cover costs from start to finish, including “bowel preparation, facility and professional fees, anesthesia, and pathology,” as well as follow-up screening for high-risk patients identified by noninvasive methods.
“Noninvasive colorectal screening should be considered as programs with multiple steps, each of which, including follow-up colonoscopy if the test is positive, should be covered by payers without cost sharing as part of the screening continuum,” Dr. Lieberman and colleagues wrote.
Changes underway
According to Steven Itzkowitz, MD, professor of medicine and oncological sciences and director of the gastroenterology fellowship training program at the Icahn School of Medicine at Mount Sinai, New York, the AGA publication is important because it “consolidates many of the critical issues related to decreasing the burden of colorectal cancer in the United States.”
Dr. Itzkowitz noted that changes are already underway to eliminate cost as a barrier to screening.
“The good news is that, in the past year, the Departments of Health & Human Services, Labor, and Treasury declared that cost sharing should not be imposed, and plans are required to cover screening colonoscopy with polyp removal and colonoscopy that is performed to follow-up after an abnormal noninvasive CRC screening test,” Dr. Itzkowitz said in an interview. “Many plans are following suit, but it will take time for this coverage to take effect across all plans.”
For individual gastroenterologists who would like to do their part in reducing screening inequity, Dr. Itzkowitz suggested leveraging noninvasive testing, as the AGA recommends.
“This publication is the latest to call for using noninvasive, stool-based testing in addition to colonoscopy,” Dr. Itzkowitz said. “FIT and multitarget stool DNA tests all have proven efficacy in this regard, so gastroenterologists should have those conversations with their patients. GIs can also make it easier for patients to complete colonoscopy by developing patient navigation programs, direct access referrals, and systems for communicating with primary care providers for easier referrals and communicating colonoscopy results.”
Many practices are already instituting such improvements in response to the restrictions imposed by the COVID-19 pandemic, according to Dr. Itzkowitz.“These changes, plus better coverage by payers, will make a huge impact on health equity when it comes to colorectal cancer screening.”
The publication was supported by the AGA. The investigators disclosed relationships with Geneoscopy, ColoWrap, UniversalDx, and others. Dr. Itzkowitz disclosed no relevant conflicts of interest.
FROM GASTROENTEROLOGY
Sex Differences in MS
The X vs Y chromosome
The impact of sex on MS is not surprising. The normal human CNS and immune system show fundamental sex-based differences in regional gray matter volumes1 and brain aerobic glycolysis, which is higher in females.2 Females across virtually all species are known to have stronger innate and adaptive immune system responses, both cellular and humoral.3 Genetically, the X chromosome contains immune regulatory genes, such as TLR7 and Foxp3, while sex hormones are known to have an immune modulatory impact.4 Environmental MS risk factors appear to be influenced by sex as well.3
MS is more common in women by a 3:1 ratio. About 80% of all autoimmune/immune-mediated diseases show such a female predominance4; exceptions include male predominance in ankylosing spondylitis and equal sex ratio in inflammatory bowel disease. The MS female-to-male sex ratio has increased over time, but only for the relapsing clinical phenotype. This is not true for primary progressive MS (PPMS), which is essentially 1:1.5 The explanation for this is unknown.
Prognosis
Sex impacts MS outcomes, with males showing a worse prognosis. This is not simply due to their increased risk for PPMS. Men are less likely to recover from relapses, they have more cognitive deficits and greater disability development, they have higher rates of transitioning from relapsing to secondary progressive MS (SPMS), and they have higher rates of brain volume loss.5-7 In a large global database study of 15,826 MS subjects, men with relapse-onset MS showed greater annual expanded disability status scale (EDSS) increase (0.133 vs 0.112, P <.01) than women, while women showed a decreased risk of SPMS (P =.001). In contrast, patients with PPMS did not show sex-based EDSS worsening8.
In a recent observational and retrospective study of a national Argentinean MS registry of 3099 patients with MS, 34.7% (n=1074) were men.9 Presentation with PPMS occurred in 11% of men vs 5% of women. Exclusively infratentorial lesions were found more frequently in men with relapse-onset than in women (P=.00006). Worse EDSS scores were confirmed only in men with relapse-onset MS (P=.02), but this study confirmed no difference based on sex for PPMS.9
Lesion volumes
Sex-based differences in brain magnetic resonance imaging (MRI) have been reported in those with MS. In an ongoing prospective study of 106 MS subjects, men and women showed similar average lesion volumes on MRI.10 However, men showed higher whole brain lesion numbers (P=.033) and volume (P=.043). While brain volumes were higher in men in this study (P<.001), age- and sex-appropriate normative whole brain volume percentiles were smaller in men (P=.05). The greatest percentile difference involved normative hippocampal volume percentiles (mean 62 ± 32 in women vs 40 ± 31 in men, (P<.001). Men showed more spinal cord lesions (P=.018), and it was observed that their age-associated cervical spine volume loss started a decade earlier.
A review of data in a large, real-world MRI database (N=2199), a greater proportion of men were diagnosed with progressive MS. Compared with women with progressive MS, they had lower normalized whole brain volume (P<.001) and gray matter volume (P<.001) and greater lateral ventricular volume (P<.001).11 Both sex and age affected lateral ventricular gray matter volumes. Men over the age of 60 years did not show significant sex-based differences.
MS and hormones
Hormonal states seem to have a strong impact on MS onset. MS is rare before puberty (<1%). It begins to present in young adulthood, with an average age at onset of about 30 years. Progressive MS is even more age related and presents closer to mid-life, around 40 to 45 years of age. This is approaching female menopause and well into andropause.
Pregnancy is the best studied hormonal state. MS has no negative impact on fertility or pregnancy, at least for relapsing MS.5 However, pregnancy has a strong impact on MS. Disease activity decreases during pregnancy, particularly in the last trimester. In the immediate postpartum period, there is an approximately 3-month risk for increased disease activity.5 In a recent study, postpartum relapses occurred in about 14% of untreated individuals. The protective factors are believed to involve sex hormones, which peak in the last trimester and then rapidly fall postpartum. These observations have led to estriol treatment studies in women with relapsing MS and indirectly to testosterone studies in men with MS.5 Regarding the safe use of disease-modifying therapies (DMTs) while pregnant, only glatiramer acetate and the interferon betas have had thousands of human exposures.
No teratogenicity is documented; our study12 showed that branded glatiramer acetate did not expose a pregnancy to a higher risk for congenital anomalies than a pregnancy13 in the general population. No pregnancy washout14 is needed, and it can be used during pregnancy and breastfeeding.
It is increasingly accepted not to use a pregnancy washout with the fumarates (their half-life is ≤1 hour) and with natalizumab. Due to its rebound risk, natalizumab is often continued into the first and even second trimester. Both natalizumab and fingolimod (sphingosine-1-phosphate receptor modulators) are recognized to carry risk of rebound relapses during pregnancy, which can be severe.15,16
Breastfeeding (particularly exclusive, <1 bottle daily) appears to decrease postpartum risk for breakthrough activity. It is considered safe with the needle injectables (interferon betas and glatiramer acetate). Monoclonal antibodies are also considered acceptable, based on poor excretion into milk and negligible infant absorption. For example, a recent study of natalizumab showed the relative infant dose was 0.04% of maternal exposure.17 The MS oral DMTs carry unknown risk and, in general, are not used while breastfeeding.18
Assisted reproductive technology has been associated with an increased annualized relapse rate in the 3 months after the procedure fails (P≤.01).19 A recent review found that continuing DMTs during the assisted reproductive technology procedure lowered this risk.20
MS and menstruation
Formal MS studies on the menstrual cycle are limited.21 Occasional subjects note menstrual-related relapses or pseudo relapses.19 Some women report worsening of symptoms prior to their cycle. This could reflect increased body temperature or hormonal fluctuations. In 1 study, cognitive and physical performance worsened in the premenstrual vs ovulation phase.22 Another small study reported that the number and volume of contrast lesions correlated with the progesterone-to-estradiol ratio in the luteal phase.19 This is clearly an understudied area.
Hormone therapy was examined in 333 women in the Danish MS registry. There was no association with hormone therapy and 6-month confirmed or sustained disability, particularly when it was used for <5 years.23 In a small study of women with MS, 19 of whom had relapsing MS and were on continuous oral contraception and 27 who were taking cyclic contraception, no difference was noted in time to relapse.24 However, continuous users had a longer time to contrast lesion activity (P =.05) and a trend toward a longer time to T2 lesion formation (P =.09). In those observed for at least 1 year, the longer time to T2 lesion (P=.03) and contrast lesion (P =.02) development was more significant for continuous users. The authors suggested that this finding associated with continuous contraception use indicated less inflammatory MRI activity. Clearly, further studies are needed.
MS and menopause
Menopause is another hormonal state that has been studied in MS. MS does not affect age at menopause. Anti-Mullerian hormone (AMH) is a biomarker of ovarian aging (reflecting follicular reserve) that can be measured in blood. Levels peak around age 25, tapering to undetectable levels at menopause.25 Studies have been inconsistent about whether AMH levels are lower in women with MS. Most studies suggest menopause is associated with a transient worsening of MS symptoms.25 A recent review concluded that hormone replacement therapy for menopausal women did not show consistent benefits.26 In another study that looked at the association between menopause and MS disease progression, 20 postmenopausal women were compared with 35 premenopausal women and 30 men with MS for 24 months.27 The postmenopausal group had higher age and disease duration (P<.0001), with higher initial and final EDSS scores. Similar proportions progressed. There was a significant association between final EDSS score and age, number of comorbidities, and menopause. All 3 may be cofactors in progression.
Studies suggest menopause is associated with greater disability but with a lower relapse rate. This is expected based on the time course of falling relapses and increasing disability progression with age. In women with clinically isolated syndrome enrolled in the Barcelona prospective cohort, menopause was not associated with increased disability risk for women with MS.28 A Mayo Clinic population-based cohort study evaluated 1376 subjects and 396 female control subjects. Premature or early menopause or nulliparity was associated with earlier onset of progressive MS; pregnancies appeared to have a “dose effect” on delaying progressive disease.29 The authors’ interpretation of this finding was that estrogen had a possible beneficial impact on delaying MS progression.
In summary, sex-based differences in MS continue to be a hot topic, with ongoing studies providing new data that require verification and larger-scale studies. Studying women and men with MS should ultimately give us important new insights into this major neurologic disorder of young adults.
- Liu S, Seidlitz J, Blumethal JD, et al. Integrative, structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proc Natl Acad Sci. 2020;117(31):18788-18798.
- Lee JW, Profant M, Wang C. Metabolic sex dimorphism of the brain at the gene, cell, and tissue level. J Immunol. 2022;208(2):212-220.
- Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626-638.
- Leffler J, Trend S, Gorman S, Hart PH. Sex-specific environmental impacts on initiation and progression of multiple sclerosis. Front Neurol. 2022;13:835162.
- Coyle PK. What can we learn from sex differences in MS? J Pers Med. 2021;11(10):1006.
- Safi NV, Krieger S. Men with multiple sclerosis. Pract Neurol. 2021;37-40.
- Golden LC, Voskuhl R. The importance of studying sex differences in disease: the example of multiple sclerosis. J Neurosci Res. 2017;95(1-2):633-643.
- Ribbons KA, McElduff P, Boz C, et al. Male sex is independently associated with faster disability accumulation in relapse-onset MS but not in primary progressive MS. PLoS One. 2015;10(6):e0122686.
- Luetic GG, Menichini ML, Vrech C, et al. Clinical and demographic characteristics of male MS patients included in the national registry—RelevarEM. Does sex or phenotype make the difference in the association with poor prognosis? Mult Scler Relat Disord. 2022;58:103401.
- Zeydan B, Neyal N, Son J, et al. Sex and age differences in MS imaging biomarkers. Paper presented at: ACTRIMS 2022 Forum; February 24-26, 2022; West Palm Beach, FL; P203.
- Jakimovski D, Zivadinov R, Bersland N, et al. Sex-specific differences in life span brain volumes in multiple sclerosis. J Neuroimaging. 2020;30(3):342-350.
- Sandberg-Wollheim M, Neudorfer O, Grinspan A, et al. Pregnancy outcomes from the Branded Glatiramer Acetate Pregnancy Database. Int J MS Care. 2018;20(1):9-14.
- Langer-Gould AM. Pregnancy and family planning in multiple sclerosis. Continuum (Minneap Minn). 2019;25(3):773-792.
- Ciplea AI, Langer-Gould A, Stahl A, et al. Safety of potential breast milk exposure to IFN-β or glatiramer acetate: one-year infant outcomes. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e757.
- Bianco A, Lucchini M, Totaro R, et al. Disease reactivation after fingolimod discontinuation in pregnant multiple sclerosis patients. Neurotherapeutics. 2021;18(4):2598-2607.
- Hellwig K, Tokic M, Thiel S, et al. Multiple sclerosis disease activity and disability following discontinuation of natalizumab for pregnancy. JAMA Netw Open. 2022;5(1):e2144750.
- Proschmann U, Haase R, Inojosa H, et al. Drug and neurofilament levels in serum and breastmilk of women with multiple sclerosis exposed to natalizumab during pregnancy and lactation. Front Immunol. 2021;12:715195.
- Bove RM, Houtchens MK. Pregnancy management in multiple sclerosis and other demyelinating diseases. Continuum (Minneap Minn). 2022;28(1):12-33.
- Bove R, Rankin K, Lin C, et al. Effect of assisted reproductive technology on multiple sclerosis relapses: case series and meta-analysis. Mult Scler. 2020;26(11):1410-1419.
- Graham E, Bakkensen J, Anderson A, et al. Impact of continuing disease modifying therapy during assisted reproductive technologies in women with MS: a multicenter analysis of inflammatory activity. Paper presented at: ACTRIMS 2022 Forum; February 24-26, 2022; West Palm Beach, FL; P411.
- Roeder HJ, Leira EC. Effects of the menstrual cycle on neurological disorders. Curr Neurol Neurosci Rep. 2021;21(7):34.
- Yorgun YG, Ozakbas S. Effect of hormonal changes on the neurological status in the menstrual cycle of patient with multiple sclerosis. Clin Neurol Neurosurg. 2019;186:105499.
- Kopp TI, Lidegaard Ø, Magyari M. Hormone therapy and disease activity in Danish women with multiple sclerosis: a population-based cohort study. Eur J Neurol. 2022;29(6):1753-1762.
- Chen CS, Krishnakumar T, Rowles W, et al. Comparison of MS inflammatory activity in women using continuous versus cyclic combined oral contraceptives. Mult Scler Relat Disord. 2020;41:101970.
- Bove R, Okai A, Houtchens M, et al. Effects of menopause in women with multiple sclerosis: an evidence-based review. Front Neurol. 2021;12:554375.
- Midaglia L, Otero S, Baró F, et al. Menopause and multiple sclerosis: influence on prognosis and role of disease-modifying drugs and hormonal replacement therapy. Mult Scler. 2022;28(2):173-182.
- De Caneda MA, Silva CB, de Vecino MC. The association between menopause and the multiple sclerosis progression. Paper presented at: ACTRIMS 2022 Forum; February 24-26, 2022; West Palm Beach, FL; P205.
- Otero-Romero S, Midaglia L, Carbonell-Mirabent P, et al. Menopause does not modify disability trajectories in a longitudinal cohort of women with clinically isolated syndrome and multiple sclerosis followed from disease onset. Eur J Neurol. 2022;29(4):1075-1081.
- Zeydan B, Atkinson EJ, Weis DM, et al. Reproductive history and progressive multiple sclerosis risk in women. Brain Commun. 2020;2(2):fcaa185.
The X vs Y chromosome
The impact of sex on MS is not surprising. The normal human CNS and immune system show fundamental sex-based differences in regional gray matter volumes1 and brain aerobic glycolysis, which is higher in females.2 Females across virtually all species are known to have stronger innate and adaptive immune system responses, both cellular and humoral.3 Genetically, the X chromosome contains immune regulatory genes, such as TLR7 and Foxp3, while sex hormones are known to have an immune modulatory impact.4 Environmental MS risk factors appear to be influenced by sex as well.3
MS is more common in women by a 3:1 ratio. About 80% of all autoimmune/immune-mediated diseases show such a female predominance4; exceptions include male predominance in ankylosing spondylitis and equal sex ratio in inflammatory bowel disease. The MS female-to-male sex ratio has increased over time, but only for the relapsing clinical phenotype. This is not true for primary progressive MS (PPMS), which is essentially 1:1.5 The explanation for this is unknown.
Prognosis
Sex impacts MS outcomes, with males showing a worse prognosis. This is not simply due to their increased risk for PPMS. Men are less likely to recover from relapses, they have more cognitive deficits and greater disability development, they have higher rates of transitioning from relapsing to secondary progressive MS (SPMS), and they have higher rates of brain volume loss.5-7 In a large global database study of 15,826 MS subjects, men with relapse-onset MS showed greater annual expanded disability status scale (EDSS) increase (0.133 vs 0.112, P <.01) than women, while women showed a decreased risk of SPMS (P =.001). In contrast, patients with PPMS did not show sex-based EDSS worsening8.
In a recent observational and retrospective study of a national Argentinean MS registry of 3099 patients with MS, 34.7% (n=1074) were men.9 Presentation with PPMS occurred in 11% of men vs 5% of women. Exclusively infratentorial lesions were found more frequently in men with relapse-onset than in women (P=.00006). Worse EDSS scores were confirmed only in men with relapse-onset MS (P=.02), but this study confirmed no difference based on sex for PPMS.9
Lesion volumes
Sex-based differences in brain magnetic resonance imaging (MRI) have been reported in those with MS. In an ongoing prospective study of 106 MS subjects, men and women showed similar average lesion volumes on MRI.10 However, men showed higher whole brain lesion numbers (P=.033) and volume (P=.043). While brain volumes were higher in men in this study (P<.001), age- and sex-appropriate normative whole brain volume percentiles were smaller in men (P=.05). The greatest percentile difference involved normative hippocampal volume percentiles (mean 62 ± 32 in women vs 40 ± 31 in men, (P<.001). Men showed more spinal cord lesions (P=.018), and it was observed that their age-associated cervical spine volume loss started a decade earlier.
A review of data in a large, real-world MRI database (N=2199), a greater proportion of men were diagnosed with progressive MS. Compared with women with progressive MS, they had lower normalized whole brain volume (P<.001) and gray matter volume (P<.001) and greater lateral ventricular volume (P<.001).11 Both sex and age affected lateral ventricular gray matter volumes. Men over the age of 60 years did not show significant sex-based differences.
MS and hormones
Hormonal states seem to have a strong impact on MS onset. MS is rare before puberty (<1%). It begins to present in young adulthood, with an average age at onset of about 30 years. Progressive MS is even more age related and presents closer to mid-life, around 40 to 45 years of age. This is approaching female menopause and well into andropause.
Pregnancy is the best studied hormonal state. MS has no negative impact on fertility or pregnancy, at least for relapsing MS.5 However, pregnancy has a strong impact on MS. Disease activity decreases during pregnancy, particularly in the last trimester. In the immediate postpartum period, there is an approximately 3-month risk for increased disease activity.5 In a recent study, postpartum relapses occurred in about 14% of untreated individuals. The protective factors are believed to involve sex hormones, which peak in the last trimester and then rapidly fall postpartum. These observations have led to estriol treatment studies in women with relapsing MS and indirectly to testosterone studies in men with MS.5 Regarding the safe use of disease-modifying therapies (DMTs) while pregnant, only glatiramer acetate and the interferon betas have had thousands of human exposures.
No teratogenicity is documented; our study12 showed that branded glatiramer acetate did not expose a pregnancy to a higher risk for congenital anomalies than a pregnancy13 in the general population. No pregnancy washout14 is needed, and it can be used during pregnancy and breastfeeding.
It is increasingly accepted not to use a pregnancy washout with the fumarates (their half-life is ≤1 hour) and with natalizumab. Due to its rebound risk, natalizumab is often continued into the first and even second trimester. Both natalizumab and fingolimod (sphingosine-1-phosphate receptor modulators) are recognized to carry risk of rebound relapses during pregnancy, which can be severe.15,16
Breastfeeding (particularly exclusive, <1 bottle daily) appears to decrease postpartum risk for breakthrough activity. It is considered safe with the needle injectables (interferon betas and glatiramer acetate). Monoclonal antibodies are also considered acceptable, based on poor excretion into milk and negligible infant absorption. For example, a recent study of natalizumab showed the relative infant dose was 0.04% of maternal exposure.17 The MS oral DMTs carry unknown risk and, in general, are not used while breastfeeding.18
Assisted reproductive technology has been associated with an increased annualized relapse rate in the 3 months after the procedure fails (P≤.01).19 A recent review found that continuing DMTs during the assisted reproductive technology procedure lowered this risk.20
MS and menstruation
Formal MS studies on the menstrual cycle are limited.21 Occasional subjects note menstrual-related relapses or pseudo relapses.19 Some women report worsening of symptoms prior to their cycle. This could reflect increased body temperature or hormonal fluctuations. In 1 study, cognitive and physical performance worsened in the premenstrual vs ovulation phase.22 Another small study reported that the number and volume of contrast lesions correlated with the progesterone-to-estradiol ratio in the luteal phase.19 This is clearly an understudied area.
Hormone therapy was examined in 333 women in the Danish MS registry. There was no association with hormone therapy and 6-month confirmed or sustained disability, particularly when it was used for <5 years.23 In a small study of women with MS, 19 of whom had relapsing MS and were on continuous oral contraception and 27 who were taking cyclic contraception, no difference was noted in time to relapse.24 However, continuous users had a longer time to contrast lesion activity (P =.05) and a trend toward a longer time to T2 lesion formation (P =.09). In those observed for at least 1 year, the longer time to T2 lesion (P=.03) and contrast lesion (P =.02) development was more significant for continuous users. The authors suggested that this finding associated with continuous contraception use indicated less inflammatory MRI activity. Clearly, further studies are needed.
MS and menopause
Menopause is another hormonal state that has been studied in MS. MS does not affect age at menopause. Anti-Mullerian hormone (AMH) is a biomarker of ovarian aging (reflecting follicular reserve) that can be measured in blood. Levels peak around age 25, tapering to undetectable levels at menopause.25 Studies have been inconsistent about whether AMH levels are lower in women with MS. Most studies suggest menopause is associated with a transient worsening of MS symptoms.25 A recent review concluded that hormone replacement therapy for menopausal women did not show consistent benefits.26 In another study that looked at the association between menopause and MS disease progression, 20 postmenopausal women were compared with 35 premenopausal women and 30 men with MS for 24 months.27 The postmenopausal group had higher age and disease duration (P<.0001), with higher initial and final EDSS scores. Similar proportions progressed. There was a significant association between final EDSS score and age, number of comorbidities, and menopause. All 3 may be cofactors in progression.
Studies suggest menopause is associated with greater disability but with a lower relapse rate. This is expected based on the time course of falling relapses and increasing disability progression with age. In women with clinically isolated syndrome enrolled in the Barcelona prospective cohort, menopause was not associated with increased disability risk for women with MS.28 A Mayo Clinic population-based cohort study evaluated 1376 subjects and 396 female control subjects. Premature or early menopause or nulliparity was associated with earlier onset of progressive MS; pregnancies appeared to have a “dose effect” on delaying progressive disease.29 The authors’ interpretation of this finding was that estrogen had a possible beneficial impact on delaying MS progression.
In summary, sex-based differences in MS continue to be a hot topic, with ongoing studies providing new data that require verification and larger-scale studies. Studying women and men with MS should ultimately give us important new insights into this major neurologic disorder of young adults.
The X vs Y chromosome
The impact of sex on MS is not surprising. The normal human CNS and immune system show fundamental sex-based differences in regional gray matter volumes1 and brain aerobic glycolysis, which is higher in females.2 Females across virtually all species are known to have stronger innate and adaptive immune system responses, both cellular and humoral.3 Genetically, the X chromosome contains immune regulatory genes, such as TLR7 and Foxp3, while sex hormones are known to have an immune modulatory impact.4 Environmental MS risk factors appear to be influenced by sex as well.3
MS is more common in women by a 3:1 ratio. About 80% of all autoimmune/immune-mediated diseases show such a female predominance4; exceptions include male predominance in ankylosing spondylitis and equal sex ratio in inflammatory bowel disease. The MS female-to-male sex ratio has increased over time, but only for the relapsing clinical phenotype. This is not true for primary progressive MS (PPMS), which is essentially 1:1.5 The explanation for this is unknown.
Prognosis
Sex impacts MS outcomes, with males showing a worse prognosis. This is not simply due to their increased risk for PPMS. Men are less likely to recover from relapses, they have more cognitive deficits and greater disability development, they have higher rates of transitioning from relapsing to secondary progressive MS (SPMS), and they have higher rates of brain volume loss.5-7 In a large global database study of 15,826 MS subjects, men with relapse-onset MS showed greater annual expanded disability status scale (EDSS) increase (0.133 vs 0.112, P <.01) than women, while women showed a decreased risk of SPMS (P =.001). In contrast, patients with PPMS did not show sex-based EDSS worsening8.
In a recent observational and retrospective study of a national Argentinean MS registry of 3099 patients with MS, 34.7% (n=1074) were men.9 Presentation with PPMS occurred in 11% of men vs 5% of women. Exclusively infratentorial lesions were found more frequently in men with relapse-onset than in women (P=.00006). Worse EDSS scores were confirmed only in men with relapse-onset MS (P=.02), but this study confirmed no difference based on sex for PPMS.9
Lesion volumes
Sex-based differences in brain magnetic resonance imaging (MRI) have been reported in those with MS. In an ongoing prospective study of 106 MS subjects, men and women showed similar average lesion volumes on MRI.10 However, men showed higher whole brain lesion numbers (P=.033) and volume (P=.043). While brain volumes were higher in men in this study (P<.001), age- and sex-appropriate normative whole brain volume percentiles were smaller in men (P=.05). The greatest percentile difference involved normative hippocampal volume percentiles (mean 62 ± 32 in women vs 40 ± 31 in men, (P<.001). Men showed more spinal cord lesions (P=.018), and it was observed that their age-associated cervical spine volume loss started a decade earlier.
A review of data in a large, real-world MRI database (N=2199), a greater proportion of men were diagnosed with progressive MS. Compared with women with progressive MS, they had lower normalized whole brain volume (P<.001) and gray matter volume (P<.001) and greater lateral ventricular volume (P<.001).11 Both sex and age affected lateral ventricular gray matter volumes. Men over the age of 60 years did not show significant sex-based differences.
MS and hormones
Hormonal states seem to have a strong impact on MS onset. MS is rare before puberty (<1%). It begins to present in young adulthood, with an average age at onset of about 30 years. Progressive MS is even more age related and presents closer to mid-life, around 40 to 45 years of age. This is approaching female menopause and well into andropause.
Pregnancy is the best studied hormonal state. MS has no negative impact on fertility or pregnancy, at least for relapsing MS.5 However, pregnancy has a strong impact on MS. Disease activity decreases during pregnancy, particularly in the last trimester. In the immediate postpartum period, there is an approximately 3-month risk for increased disease activity.5 In a recent study, postpartum relapses occurred in about 14% of untreated individuals. The protective factors are believed to involve sex hormones, which peak in the last trimester and then rapidly fall postpartum. These observations have led to estriol treatment studies in women with relapsing MS and indirectly to testosterone studies in men with MS.5 Regarding the safe use of disease-modifying therapies (DMTs) while pregnant, only glatiramer acetate and the interferon betas have had thousands of human exposures.
No teratogenicity is documented; our study12 showed that branded glatiramer acetate did not expose a pregnancy to a higher risk for congenital anomalies than a pregnancy13 in the general population. No pregnancy washout14 is needed, and it can be used during pregnancy and breastfeeding.
It is increasingly accepted not to use a pregnancy washout with the fumarates (their half-life is ≤1 hour) and with natalizumab. Due to its rebound risk, natalizumab is often continued into the first and even second trimester. Both natalizumab and fingolimod (sphingosine-1-phosphate receptor modulators) are recognized to carry risk of rebound relapses during pregnancy, which can be severe.15,16
Breastfeeding (particularly exclusive, <1 bottle daily) appears to decrease postpartum risk for breakthrough activity. It is considered safe with the needle injectables (interferon betas and glatiramer acetate). Monoclonal antibodies are also considered acceptable, based on poor excretion into milk and negligible infant absorption. For example, a recent study of natalizumab showed the relative infant dose was 0.04% of maternal exposure.17 The MS oral DMTs carry unknown risk and, in general, are not used while breastfeeding.18
Assisted reproductive technology has been associated with an increased annualized relapse rate in the 3 months after the procedure fails (P≤.01).19 A recent review found that continuing DMTs during the assisted reproductive technology procedure lowered this risk.20
MS and menstruation
Formal MS studies on the menstrual cycle are limited.21 Occasional subjects note menstrual-related relapses or pseudo relapses.19 Some women report worsening of symptoms prior to their cycle. This could reflect increased body temperature or hormonal fluctuations. In 1 study, cognitive and physical performance worsened in the premenstrual vs ovulation phase.22 Another small study reported that the number and volume of contrast lesions correlated with the progesterone-to-estradiol ratio in the luteal phase.19 This is clearly an understudied area.
Hormone therapy was examined in 333 women in the Danish MS registry. There was no association with hormone therapy and 6-month confirmed or sustained disability, particularly when it was used for <5 years.23 In a small study of women with MS, 19 of whom had relapsing MS and were on continuous oral contraception and 27 who were taking cyclic contraception, no difference was noted in time to relapse.24 However, continuous users had a longer time to contrast lesion activity (P =.05) and a trend toward a longer time to T2 lesion formation (P =.09). In those observed for at least 1 year, the longer time to T2 lesion (P=.03) and contrast lesion (P =.02) development was more significant for continuous users. The authors suggested that this finding associated with continuous contraception use indicated less inflammatory MRI activity. Clearly, further studies are needed.
MS and menopause
Menopause is another hormonal state that has been studied in MS. MS does not affect age at menopause. Anti-Mullerian hormone (AMH) is a biomarker of ovarian aging (reflecting follicular reserve) that can be measured in blood. Levels peak around age 25, tapering to undetectable levels at menopause.25 Studies have been inconsistent about whether AMH levels are lower in women with MS. Most studies suggest menopause is associated with a transient worsening of MS symptoms.25 A recent review concluded that hormone replacement therapy for menopausal women did not show consistent benefits.26 In another study that looked at the association between menopause and MS disease progression, 20 postmenopausal women were compared with 35 premenopausal women and 30 men with MS for 24 months.27 The postmenopausal group had higher age and disease duration (P<.0001), with higher initial and final EDSS scores. Similar proportions progressed. There was a significant association between final EDSS score and age, number of comorbidities, and menopause. All 3 may be cofactors in progression.
Studies suggest menopause is associated with greater disability but with a lower relapse rate. This is expected based on the time course of falling relapses and increasing disability progression with age. In women with clinically isolated syndrome enrolled in the Barcelona prospective cohort, menopause was not associated with increased disability risk for women with MS.28 A Mayo Clinic population-based cohort study evaluated 1376 subjects and 396 female control subjects. Premature or early menopause or nulliparity was associated with earlier onset of progressive MS; pregnancies appeared to have a “dose effect” on delaying progressive disease.29 The authors’ interpretation of this finding was that estrogen had a possible beneficial impact on delaying MS progression.
In summary, sex-based differences in MS continue to be a hot topic, with ongoing studies providing new data that require verification and larger-scale studies. Studying women and men with MS should ultimately give us important new insights into this major neurologic disorder of young adults.
- Liu S, Seidlitz J, Blumethal JD, et al. Integrative, structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proc Natl Acad Sci. 2020;117(31):18788-18798.
- Lee JW, Profant M, Wang C. Metabolic sex dimorphism of the brain at the gene, cell, and tissue level. J Immunol. 2022;208(2):212-220.
- Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626-638.
- Leffler J, Trend S, Gorman S, Hart PH. Sex-specific environmental impacts on initiation and progression of multiple sclerosis. Front Neurol. 2022;13:835162.
- Coyle PK. What can we learn from sex differences in MS? J Pers Med. 2021;11(10):1006.
- Safi NV, Krieger S. Men with multiple sclerosis. Pract Neurol. 2021;37-40.
- Golden LC, Voskuhl R. The importance of studying sex differences in disease: the example of multiple sclerosis. J Neurosci Res. 2017;95(1-2):633-643.
- Ribbons KA, McElduff P, Boz C, et al. Male sex is independently associated with faster disability accumulation in relapse-onset MS but not in primary progressive MS. PLoS One. 2015;10(6):e0122686.
- Luetic GG, Menichini ML, Vrech C, et al. Clinical and demographic characteristics of male MS patients included in the national registry—RelevarEM. Does sex or phenotype make the difference in the association with poor prognosis? Mult Scler Relat Disord. 2022;58:103401.
- Zeydan B, Neyal N, Son J, et al. Sex and age differences in MS imaging biomarkers. Paper presented at: ACTRIMS 2022 Forum; February 24-26, 2022; West Palm Beach, FL; P203.
- Jakimovski D, Zivadinov R, Bersland N, et al. Sex-specific differences in life span brain volumes in multiple sclerosis. J Neuroimaging. 2020;30(3):342-350.
- Sandberg-Wollheim M, Neudorfer O, Grinspan A, et al. Pregnancy outcomes from the Branded Glatiramer Acetate Pregnancy Database. Int J MS Care. 2018;20(1):9-14.
- Langer-Gould AM. Pregnancy and family planning in multiple sclerosis. Continuum (Minneap Minn). 2019;25(3):773-792.
- Ciplea AI, Langer-Gould A, Stahl A, et al. Safety of potential breast milk exposure to IFN-β or glatiramer acetate: one-year infant outcomes. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e757.
- Bianco A, Lucchini M, Totaro R, et al. Disease reactivation after fingolimod discontinuation in pregnant multiple sclerosis patients. Neurotherapeutics. 2021;18(4):2598-2607.
- Hellwig K, Tokic M, Thiel S, et al. Multiple sclerosis disease activity and disability following discontinuation of natalizumab for pregnancy. JAMA Netw Open. 2022;5(1):e2144750.
- Proschmann U, Haase R, Inojosa H, et al. Drug and neurofilament levels in serum and breastmilk of women with multiple sclerosis exposed to natalizumab during pregnancy and lactation. Front Immunol. 2021;12:715195.
- Bove RM, Houtchens MK. Pregnancy management in multiple sclerosis and other demyelinating diseases. Continuum (Minneap Minn). 2022;28(1):12-33.
- Bove R, Rankin K, Lin C, et al. Effect of assisted reproductive technology on multiple sclerosis relapses: case series and meta-analysis. Mult Scler. 2020;26(11):1410-1419.
- Graham E, Bakkensen J, Anderson A, et al. Impact of continuing disease modifying therapy during assisted reproductive technologies in women with MS: a multicenter analysis of inflammatory activity. Paper presented at: ACTRIMS 2022 Forum; February 24-26, 2022; West Palm Beach, FL; P411.
- Roeder HJ, Leira EC. Effects of the menstrual cycle on neurological disorders. Curr Neurol Neurosci Rep. 2021;21(7):34.
- Yorgun YG, Ozakbas S. Effect of hormonal changes on the neurological status in the menstrual cycle of patient with multiple sclerosis. Clin Neurol Neurosurg. 2019;186:105499.
- Kopp TI, Lidegaard Ø, Magyari M. Hormone therapy and disease activity in Danish women with multiple sclerosis: a population-based cohort study. Eur J Neurol. 2022;29(6):1753-1762.
- Chen CS, Krishnakumar T, Rowles W, et al. Comparison of MS inflammatory activity in women using continuous versus cyclic combined oral contraceptives. Mult Scler Relat Disord. 2020;41:101970.
- Bove R, Okai A, Houtchens M, et al. Effects of menopause in women with multiple sclerosis: an evidence-based review. Front Neurol. 2021;12:554375.
- Midaglia L, Otero S, Baró F, et al. Menopause and multiple sclerosis: influence on prognosis and role of disease-modifying drugs and hormonal replacement therapy. Mult Scler. 2022;28(2):173-182.
- De Caneda MA, Silva CB, de Vecino MC. The association between menopause and the multiple sclerosis progression. Paper presented at: ACTRIMS 2022 Forum; February 24-26, 2022; West Palm Beach, FL; P205.
- Otero-Romero S, Midaglia L, Carbonell-Mirabent P, et al. Menopause does not modify disability trajectories in a longitudinal cohort of women with clinically isolated syndrome and multiple sclerosis followed from disease onset. Eur J Neurol. 2022;29(4):1075-1081.
- Zeydan B, Atkinson EJ, Weis DM, et al. Reproductive history and progressive multiple sclerosis risk in women. Brain Commun. 2020;2(2):fcaa185.
- Liu S, Seidlitz J, Blumethal JD, et al. Integrative, structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proc Natl Acad Sci. 2020;117(31):18788-18798.
- Lee JW, Profant M, Wang C. Metabolic sex dimorphism of the brain at the gene, cell, and tissue level. J Immunol. 2022;208(2):212-220.
- Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626-638.
- Leffler J, Trend S, Gorman S, Hart PH. Sex-specific environmental impacts on initiation and progression of multiple sclerosis. Front Neurol. 2022;13:835162.
- Coyle PK. What can we learn from sex differences in MS? J Pers Med. 2021;11(10):1006.
- Safi NV, Krieger S. Men with multiple sclerosis. Pract Neurol. 2021;37-40.
- Golden LC, Voskuhl R. The importance of studying sex differences in disease: the example of multiple sclerosis. J Neurosci Res. 2017;95(1-2):633-643.
- Ribbons KA, McElduff P, Boz C, et al. Male sex is independently associated with faster disability accumulation in relapse-onset MS but not in primary progressive MS. PLoS One. 2015;10(6):e0122686.
- Luetic GG, Menichini ML, Vrech C, et al. Clinical and demographic characteristics of male MS patients included in the national registry—RelevarEM. Does sex or phenotype make the difference in the association with poor prognosis? Mult Scler Relat Disord. 2022;58:103401.
- Zeydan B, Neyal N, Son J, et al. Sex and age differences in MS imaging biomarkers. Paper presented at: ACTRIMS 2022 Forum; February 24-26, 2022; West Palm Beach, FL; P203.
- Jakimovski D, Zivadinov R, Bersland N, et al. Sex-specific differences in life span brain volumes in multiple sclerosis. J Neuroimaging. 2020;30(3):342-350.
- Sandberg-Wollheim M, Neudorfer O, Grinspan A, et al. Pregnancy outcomes from the Branded Glatiramer Acetate Pregnancy Database. Int J MS Care. 2018;20(1):9-14.
- Langer-Gould AM. Pregnancy and family planning in multiple sclerosis. Continuum (Minneap Minn). 2019;25(3):773-792.
- Ciplea AI, Langer-Gould A, Stahl A, et al. Safety of potential breast milk exposure to IFN-β or glatiramer acetate: one-year infant outcomes. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e757.
- Bianco A, Lucchini M, Totaro R, et al. Disease reactivation after fingolimod discontinuation in pregnant multiple sclerosis patients. Neurotherapeutics. 2021;18(4):2598-2607.
- Hellwig K, Tokic M, Thiel S, et al. Multiple sclerosis disease activity and disability following discontinuation of natalizumab for pregnancy. JAMA Netw Open. 2022;5(1):e2144750.
- Proschmann U, Haase R, Inojosa H, et al. Drug and neurofilament levels in serum and breastmilk of women with multiple sclerosis exposed to natalizumab during pregnancy and lactation. Front Immunol. 2021;12:715195.
- Bove RM, Houtchens MK. Pregnancy management in multiple sclerosis and other demyelinating diseases. Continuum (Minneap Minn). 2022;28(1):12-33.
- Bove R, Rankin K, Lin C, et al. Effect of assisted reproductive technology on multiple sclerosis relapses: case series and meta-analysis. Mult Scler. 2020;26(11):1410-1419.
- Graham E, Bakkensen J, Anderson A, et al. Impact of continuing disease modifying therapy during assisted reproductive technologies in women with MS: a multicenter analysis of inflammatory activity. Paper presented at: ACTRIMS 2022 Forum; February 24-26, 2022; West Palm Beach, FL; P411.
- Roeder HJ, Leira EC. Effects of the menstrual cycle on neurological disorders. Curr Neurol Neurosci Rep. 2021;21(7):34.
- Yorgun YG, Ozakbas S. Effect of hormonal changes on the neurological status in the menstrual cycle of patient with multiple sclerosis. Clin Neurol Neurosurg. 2019;186:105499.
- Kopp TI, Lidegaard Ø, Magyari M. Hormone therapy and disease activity in Danish women with multiple sclerosis: a population-based cohort study. Eur J Neurol. 2022;29(6):1753-1762.
- Chen CS, Krishnakumar T, Rowles W, et al. Comparison of MS inflammatory activity in women using continuous versus cyclic combined oral contraceptives. Mult Scler Relat Disord. 2020;41:101970.
- Bove R, Okai A, Houtchens M, et al. Effects of menopause in women with multiple sclerosis: an evidence-based review. Front Neurol. 2021;12:554375.
- Midaglia L, Otero S, Baró F, et al. Menopause and multiple sclerosis: influence on prognosis and role of disease-modifying drugs and hormonal replacement therapy. Mult Scler. 2022;28(2):173-182.
- De Caneda MA, Silva CB, de Vecino MC. The association between menopause and the multiple sclerosis progression. Paper presented at: ACTRIMS 2022 Forum; February 24-26, 2022; West Palm Beach, FL; P205.
- Otero-Romero S, Midaglia L, Carbonell-Mirabent P, et al. Menopause does not modify disability trajectories in a longitudinal cohort of women with clinically isolated syndrome and multiple sclerosis followed from disease onset. Eur J Neurol. 2022;29(4):1075-1081.
- Zeydan B, Atkinson EJ, Weis DM, et al. Reproductive history and progressive multiple sclerosis risk in women. Brain Commun. 2020;2(2):fcaa185.
Stimulants may not improve academic learning in children with ADHD
Extended-release methylphenidate (Concerta) had no effect on learning academic material taught in a small group of children with attention-deficit/hyperactivity disorder (ADHD), a controlled crossover study found.
As in previous studies, however, the stimulant did improve seat work productivity and classroom behavior, but these benefits did not translate into better learning of individual academic learning units, according to William E. Pelham Jr., PhD, of the department of psychology at Florida International University in Miami, and colleagues.
The results were published online in the Journal of Consulting and Clinical Psychology.
The authors said the finding raises questions about how stimulant medication leads to improved academic achievement over time. “This is important given that many parents and pediatricians believe that medication will improve academic achievement; parents are more likely to pursue medication (vs. other treatment options) when they identify academic achievement as a primary goal for treatment. The current findings suggest this emphasis may be misguided,” they wrote.
In their view, efforts to improve learning in children with ADHD should focus on delivering effective academic instruction and support such as individualized educational plans rather than stimulant therapy.
The study
The study cohort consisted of 173 children aged 7-12 (77% male, 86% Hispanic) who met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria for ADHD and were participating in a therapeutic summer camp classroom.
The experimental design was a triple-masked, within-subject, AB/BA crossover trial. Children completed two consecutive phases of daily, 25-minute instruction in both subject-area content (science and social studies) and vocabulary. Each phase was a standard instructional unit lasting for 3 weeks and lessons were given by credentialed teachers via small-group, evidence-based instruction.
Each child was randomized to receive daily osmotic-release oral system methylphenidate (OROS-MPH) during either the first or second instructional phase and to receive placebo during the other.
Seat work referred to the amount of work a pupil completed in a fixed duration of independent work time, and classroom behavior referred to the frequency of violating classroom rules. Learning was measured by tests, and multilevel models were fit separately to the subject and vocabulary test scores, with four observations per child: pretest and posttest in the two academic subject areas.
The results showed that medication had large, salutary, statistically significant effects on children’s academic seat work productivity and classroom behavior on every single day of the instructional period.
Pupils completed 37% more arithmetic problems per minute when taking OROS-MPH and committed 53% fewer rule violations per hour. In terms of learning the material taught during instruction, however, tests showed that children learned the same amount of subject-area and vocabulary content whether they were taking OROS-MPH or placebo during the instructional period.
Consistent with previous studies, medication slightly helped to improve test scores when taken on the day of a test, but not enough to boost most children’s grades. For example, medication helped children increase on average 1.7 percentage points out of 100 on science and social studies tests.
“This finding has relevance for parents deciding whether to medicate their child for occasions such as a psychoeducational evaluation or high-stakes academic testing – while the effect size was small, findings suggest being medicated would improve scores,” the investigators wrote.
Sharing his perspective on the study but not involved in it, Herschel R. Lessin, MD, a pediatrician at The Children’s Medical Group in Poughkeepsie, N.Y., and coauthor of the American Academy of Pediatrics (AAP) guidelines on ADHD, said, “If you ignore the sensationalized headlines, this study is an interesting but preliminary first step, and justifies further research on the topic. It also has several potential defects, which the authors in fact address in the supplements.” The cohort size was small, for example, the doses of medication were very low, and the study took place in a controlled therapeutic setting – not the everyday classroom.
Furthermore, Dr. Lessin added, the study’s conclusions “are contrary to my 40 years of experience in treating ADHD. If they had used standard measures of assessment, as in previous studies, they would have found medication did impact learning. More research is clearly needed.”
In other comments, Holly K. Harris, MD, assistant professor of pediatrics-development at Baylor College of Medicine and Texas Children’s Hospital in Houston, said the core symptoms of ADHD are primarily behavioral in nature, not academic learning related.
“Stimulant medications are targeting these core behavioral symptoms of ADHD ... but the goal of treatment is more than just the reduction of symptoms; it is to improve a child’s overall functioning so that they succeed at what is expected of them and avoid developing even more impairments,” Dr. Harris said, adding that symptom improvement can sometimes allow a child to learn better in the classroom and achieve more academically.
Children with ADHD may have diagnosed or undiagnosed comorbid learning disabilities, with one 2013 study suggesting a rate of 31%-45%.
With such learning disabilities being distinct from core behavioral symptoms, stimulant medications would not be expected to address a child’s learning disability. “In fact, best practice is for a child with ADHD who is not responding to stimulant medication (doctors might refer to this as complex ADHD) to undergo full individual evaluations either through the school system or an outside psychological assessment to assess for potential learning disabilities or other comorbid developmental/learning or psychiatric diagnosis,” Dr. Harris said.
Rather than changing prescribing patterns, she continued, pediatricians could consider advising parents to request learning evaluations through the school system if the child continues to struggle academically with no change in learning outcomes despite improvement in some behavioral outcomes.
As a reference, Dr. Harris recommended the Society for Developmental and Behavioral Pediatrics guidelines for complex ADHD.
This study was funded by the National Institute on Mental Health with additional support from the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the Institute of Education Sciences. Coauthor James Waxmonsky, MD, has received research funding from the National Institutes of Health, Supernus, and Pfizer and served on the advisory board for Iron Shore, NLS Pharma, and Purdue Pharma.
Extended-release methylphenidate (Concerta) had no effect on learning academic material taught in a small group of children with attention-deficit/hyperactivity disorder (ADHD), a controlled crossover study found.
As in previous studies, however, the stimulant did improve seat work productivity and classroom behavior, but these benefits did not translate into better learning of individual academic learning units, according to William E. Pelham Jr., PhD, of the department of psychology at Florida International University in Miami, and colleagues.
The results were published online in the Journal of Consulting and Clinical Psychology.
The authors said the finding raises questions about how stimulant medication leads to improved academic achievement over time. “This is important given that many parents and pediatricians believe that medication will improve academic achievement; parents are more likely to pursue medication (vs. other treatment options) when they identify academic achievement as a primary goal for treatment. The current findings suggest this emphasis may be misguided,” they wrote.
In their view, efforts to improve learning in children with ADHD should focus on delivering effective academic instruction and support such as individualized educational plans rather than stimulant therapy.
The study
The study cohort consisted of 173 children aged 7-12 (77% male, 86% Hispanic) who met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria for ADHD and were participating in a therapeutic summer camp classroom.
The experimental design was a triple-masked, within-subject, AB/BA crossover trial. Children completed two consecutive phases of daily, 25-minute instruction in both subject-area content (science and social studies) and vocabulary. Each phase was a standard instructional unit lasting for 3 weeks and lessons were given by credentialed teachers via small-group, evidence-based instruction.
Each child was randomized to receive daily osmotic-release oral system methylphenidate (OROS-MPH) during either the first or second instructional phase and to receive placebo during the other.
Seat work referred to the amount of work a pupil completed in a fixed duration of independent work time, and classroom behavior referred to the frequency of violating classroom rules. Learning was measured by tests, and multilevel models were fit separately to the subject and vocabulary test scores, with four observations per child: pretest and posttest in the two academic subject areas.
The results showed that medication had large, salutary, statistically significant effects on children’s academic seat work productivity and classroom behavior on every single day of the instructional period.
Pupils completed 37% more arithmetic problems per minute when taking OROS-MPH and committed 53% fewer rule violations per hour. In terms of learning the material taught during instruction, however, tests showed that children learned the same amount of subject-area and vocabulary content whether they were taking OROS-MPH or placebo during the instructional period.
Consistent with previous studies, medication slightly helped to improve test scores when taken on the day of a test, but not enough to boost most children’s grades. For example, medication helped children increase on average 1.7 percentage points out of 100 on science and social studies tests.
“This finding has relevance for parents deciding whether to medicate their child for occasions such as a psychoeducational evaluation or high-stakes academic testing – while the effect size was small, findings suggest being medicated would improve scores,” the investigators wrote.
Sharing his perspective on the study but not involved in it, Herschel R. Lessin, MD, a pediatrician at The Children’s Medical Group in Poughkeepsie, N.Y., and coauthor of the American Academy of Pediatrics (AAP) guidelines on ADHD, said, “If you ignore the sensationalized headlines, this study is an interesting but preliminary first step, and justifies further research on the topic. It also has several potential defects, which the authors in fact address in the supplements.” The cohort size was small, for example, the doses of medication were very low, and the study took place in a controlled therapeutic setting – not the everyday classroom.
Furthermore, Dr. Lessin added, the study’s conclusions “are contrary to my 40 years of experience in treating ADHD. If they had used standard measures of assessment, as in previous studies, they would have found medication did impact learning. More research is clearly needed.”
In other comments, Holly K. Harris, MD, assistant professor of pediatrics-development at Baylor College of Medicine and Texas Children’s Hospital in Houston, said the core symptoms of ADHD are primarily behavioral in nature, not academic learning related.
“Stimulant medications are targeting these core behavioral symptoms of ADHD ... but the goal of treatment is more than just the reduction of symptoms; it is to improve a child’s overall functioning so that they succeed at what is expected of them and avoid developing even more impairments,” Dr. Harris said, adding that symptom improvement can sometimes allow a child to learn better in the classroom and achieve more academically.
Children with ADHD may have diagnosed or undiagnosed comorbid learning disabilities, with one 2013 study suggesting a rate of 31%-45%.
With such learning disabilities being distinct from core behavioral symptoms, stimulant medications would not be expected to address a child’s learning disability. “In fact, best practice is for a child with ADHD who is not responding to stimulant medication (doctors might refer to this as complex ADHD) to undergo full individual evaluations either through the school system or an outside psychological assessment to assess for potential learning disabilities or other comorbid developmental/learning or psychiatric diagnosis,” Dr. Harris said.
Rather than changing prescribing patterns, she continued, pediatricians could consider advising parents to request learning evaluations through the school system if the child continues to struggle academically with no change in learning outcomes despite improvement in some behavioral outcomes.
As a reference, Dr. Harris recommended the Society for Developmental and Behavioral Pediatrics guidelines for complex ADHD.
This study was funded by the National Institute on Mental Health with additional support from the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the Institute of Education Sciences. Coauthor James Waxmonsky, MD, has received research funding from the National Institutes of Health, Supernus, and Pfizer and served on the advisory board for Iron Shore, NLS Pharma, and Purdue Pharma.
Extended-release methylphenidate (Concerta) had no effect on learning academic material taught in a small group of children with attention-deficit/hyperactivity disorder (ADHD), a controlled crossover study found.
As in previous studies, however, the stimulant did improve seat work productivity and classroom behavior, but these benefits did not translate into better learning of individual academic learning units, according to William E. Pelham Jr., PhD, of the department of psychology at Florida International University in Miami, and colleagues.
The results were published online in the Journal of Consulting and Clinical Psychology.
The authors said the finding raises questions about how stimulant medication leads to improved academic achievement over time. “This is important given that many parents and pediatricians believe that medication will improve academic achievement; parents are more likely to pursue medication (vs. other treatment options) when they identify academic achievement as a primary goal for treatment. The current findings suggest this emphasis may be misguided,” they wrote.
In their view, efforts to improve learning in children with ADHD should focus on delivering effective academic instruction and support such as individualized educational plans rather than stimulant therapy.
The study
The study cohort consisted of 173 children aged 7-12 (77% male, 86% Hispanic) who met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria for ADHD and were participating in a therapeutic summer camp classroom.
The experimental design was a triple-masked, within-subject, AB/BA crossover trial. Children completed two consecutive phases of daily, 25-minute instruction in both subject-area content (science and social studies) and vocabulary. Each phase was a standard instructional unit lasting for 3 weeks and lessons were given by credentialed teachers via small-group, evidence-based instruction.
Each child was randomized to receive daily osmotic-release oral system methylphenidate (OROS-MPH) during either the first or second instructional phase and to receive placebo during the other.
Seat work referred to the amount of work a pupil completed in a fixed duration of independent work time, and classroom behavior referred to the frequency of violating classroom rules. Learning was measured by tests, and multilevel models were fit separately to the subject and vocabulary test scores, with four observations per child: pretest and posttest in the two academic subject areas.
The results showed that medication had large, salutary, statistically significant effects on children’s academic seat work productivity and classroom behavior on every single day of the instructional period.
Pupils completed 37% more arithmetic problems per minute when taking OROS-MPH and committed 53% fewer rule violations per hour. In terms of learning the material taught during instruction, however, tests showed that children learned the same amount of subject-area and vocabulary content whether they were taking OROS-MPH or placebo during the instructional period.
Consistent with previous studies, medication slightly helped to improve test scores when taken on the day of a test, but not enough to boost most children’s grades. For example, medication helped children increase on average 1.7 percentage points out of 100 on science and social studies tests.
“This finding has relevance for parents deciding whether to medicate their child for occasions such as a psychoeducational evaluation or high-stakes academic testing – while the effect size was small, findings suggest being medicated would improve scores,” the investigators wrote.
Sharing his perspective on the study but not involved in it, Herschel R. Lessin, MD, a pediatrician at The Children’s Medical Group in Poughkeepsie, N.Y., and coauthor of the American Academy of Pediatrics (AAP) guidelines on ADHD, said, “If you ignore the sensationalized headlines, this study is an interesting but preliminary first step, and justifies further research on the topic. It also has several potential defects, which the authors in fact address in the supplements.” The cohort size was small, for example, the doses of medication were very low, and the study took place in a controlled therapeutic setting – not the everyday classroom.
Furthermore, Dr. Lessin added, the study’s conclusions “are contrary to my 40 years of experience in treating ADHD. If they had used standard measures of assessment, as in previous studies, they would have found medication did impact learning. More research is clearly needed.”
In other comments, Holly K. Harris, MD, assistant professor of pediatrics-development at Baylor College of Medicine and Texas Children’s Hospital in Houston, said the core symptoms of ADHD are primarily behavioral in nature, not academic learning related.
“Stimulant medications are targeting these core behavioral symptoms of ADHD ... but the goal of treatment is more than just the reduction of symptoms; it is to improve a child’s overall functioning so that they succeed at what is expected of them and avoid developing even more impairments,” Dr. Harris said, adding that symptom improvement can sometimes allow a child to learn better in the classroom and achieve more academically.
Children with ADHD may have diagnosed or undiagnosed comorbid learning disabilities, with one 2013 study suggesting a rate of 31%-45%.
With such learning disabilities being distinct from core behavioral symptoms, stimulant medications would not be expected to address a child’s learning disability. “In fact, best practice is for a child with ADHD who is not responding to stimulant medication (doctors might refer to this as complex ADHD) to undergo full individual evaluations either through the school system or an outside psychological assessment to assess for potential learning disabilities or other comorbid developmental/learning or psychiatric diagnosis,” Dr. Harris said.
Rather than changing prescribing patterns, she continued, pediatricians could consider advising parents to request learning evaluations through the school system if the child continues to struggle academically with no change in learning outcomes despite improvement in some behavioral outcomes.
As a reference, Dr. Harris recommended the Society for Developmental and Behavioral Pediatrics guidelines for complex ADHD.
This study was funded by the National Institute on Mental Health with additional support from the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the Institute of Education Sciences. Coauthor James Waxmonsky, MD, has received research funding from the National Institutes of Health, Supernus, and Pfizer and served on the advisory board for Iron Shore, NLS Pharma, and Purdue Pharma.
FROM JOURNAL OF CONSULTING AND CLINICAL PSYCHOLOGY
Momelotinib hits the mark for deadly bone marrow cancer
“The current state for the treatment of myelofibrosis relies on JAK2,” said Ruben Mesa, MD, of the Mays Cancer Center at the UT Health San Antonio MD Anderson Cancer Center.
“Momelotinib is a JAK1 and JAK2 inhibitor.” However, in the early days of studying momelotinib,“it became clear that there was also potentially an improvement in anemia,” which may be related to the additional inhibition of ACVR1, he explained.
Data suggest that the ability to curb anemia in anemic myelofibrosis patients prolongs their lives for up to 8 years, Dr. Mesa added.
Previous studies, notably the phase 3 SIMPLIFY study, showed that momelotinib was associated with comparable effects on spleen volume, transfusion, and total symptom scores from baseline that were similar to ruxolitinib.
In the current study, known as MOMENTUM, a daily dose of momelotinib was compared to danazol for treatment of symptomatic and anemic myelofibrosis (MF) patients who had previously received standard JAK-inhibitor therapy.
In the study, the researchers randomized 130 patients to momelotinib and 65 to danazol. After 24 weeks, those in the danazol group were allowed to cross over to momelotinib. The primary endpoint of the study was total symptom score (TSS) response after 24 weeks. Secondary endpoints included transfusion independence and splenic response at 24 weeks. The median age of the patients in the momelotinib group was 71 years, 60.8% were male, and 82% were white. The baseline demographics were not significantly different in the danazol group.
Overall, 24.6% of momelotinib patients responded with improved total symptom scores at 24 weeks vs. 9.2% of the danazol group. Spleen response also was significantly higher in the momelotinib group; 40% of patients showed a 25% reduction and 23% showed a 35% reduction, compared with 6.2% and 3.1%, respectively, of patients in the danazol group. Transfusion independence at week 24 also was higher for momelotinib patients, compared with danazol patients (31% vs. 20%, respectively, P = 0064).
Adverse events of grade 3 or higher occurred in 53.8% of momelotinib patients and 64.6% of danazol patients, and serious adverse events occurred in 34.6% and 40.0%, respectively. Nearly all patients had anemia, but only 27.7% and 26.2% of the momelotinib and danazol groups, respectively, had thrombocytopenia of grade 3 or higher. The most common nonhematologic adverse events were diarrhea, nausea, and increased blood creatinine. A total of 27.7% of the patients in the momelotinib group discontinued treatment; 16 of whom did so because of an adverse event.
Also, at 24 weeks, patients in the momelotinib group showed a trend towards increased overall survival, compared with danazol (HR, 0.506, P = 0.719).
With momelotinib, there is a consistent thrombocytopenic profile across subgroups, the data on which were presented separately at ASCO (poster 7061), Dr. Mesa added.
“We feel that these findings support the future use of momelotinib as an effective treatment in MF patients, especially those with anemia,” he concluded.
Cytopenia data are exciting
The key finding in the current study is that “momelotinib leads to important endpoints including significant improvement in symptoms and spleen reduction,” said Dr. Gabriela Hobbs of Harvard Medical School, Boston, who served as the discussant for the study.
“I think a novel finding of momelotinib that is definitely exciting from the treatment perspective is that momelotinib can also lead to improvement in cytopenias,” she said. “We often have to decide between treating the symptoms of the spleen at the expense of blood counts,” in MF patients, she noted.
The study was sponsored by Sierra Oncology. Dr. Mesa disclosed relationships with companies including Constellation Pharmaceutical, La Jolla Pharma, and study sponsor Sierra Oncology, as well as funding from AbbVie, Celgene, Constellation Pharmaceuticals, CTI, Genentech, Incyte, Mays Cancer Center, NCI, Promedior, and Samus. Dr. Hobbs had no financial conflicts to disclose.
This article was updated 06/14/2022.
“The current state for the treatment of myelofibrosis relies on JAK2,” said Ruben Mesa, MD, of the Mays Cancer Center at the UT Health San Antonio MD Anderson Cancer Center.
“Momelotinib is a JAK1 and JAK2 inhibitor.” However, in the early days of studying momelotinib,“it became clear that there was also potentially an improvement in anemia,” which may be related to the additional inhibition of ACVR1, he explained.
Data suggest that the ability to curb anemia in anemic myelofibrosis patients prolongs their lives for up to 8 years, Dr. Mesa added.
Previous studies, notably the phase 3 SIMPLIFY study, showed that momelotinib was associated with comparable effects on spleen volume, transfusion, and total symptom scores from baseline that were similar to ruxolitinib.
In the current study, known as MOMENTUM, a daily dose of momelotinib was compared to danazol for treatment of symptomatic and anemic myelofibrosis (MF) patients who had previously received standard JAK-inhibitor therapy.
In the study, the researchers randomized 130 patients to momelotinib and 65 to danazol. After 24 weeks, those in the danazol group were allowed to cross over to momelotinib. The primary endpoint of the study was total symptom score (TSS) response after 24 weeks. Secondary endpoints included transfusion independence and splenic response at 24 weeks. The median age of the patients in the momelotinib group was 71 years, 60.8% were male, and 82% were white. The baseline demographics were not significantly different in the danazol group.
Overall, 24.6% of momelotinib patients responded with improved total symptom scores at 24 weeks vs. 9.2% of the danazol group. Spleen response also was significantly higher in the momelotinib group; 40% of patients showed a 25% reduction and 23% showed a 35% reduction, compared with 6.2% and 3.1%, respectively, of patients in the danazol group. Transfusion independence at week 24 also was higher for momelotinib patients, compared with danazol patients (31% vs. 20%, respectively, P = 0064).
Adverse events of grade 3 or higher occurred in 53.8% of momelotinib patients and 64.6% of danazol patients, and serious adverse events occurred in 34.6% and 40.0%, respectively. Nearly all patients had anemia, but only 27.7% and 26.2% of the momelotinib and danazol groups, respectively, had thrombocytopenia of grade 3 or higher. The most common nonhematologic adverse events were diarrhea, nausea, and increased blood creatinine. A total of 27.7% of the patients in the momelotinib group discontinued treatment; 16 of whom did so because of an adverse event.
Also, at 24 weeks, patients in the momelotinib group showed a trend towards increased overall survival, compared with danazol (HR, 0.506, P = 0.719).
With momelotinib, there is a consistent thrombocytopenic profile across subgroups, the data on which were presented separately at ASCO (poster 7061), Dr. Mesa added.
“We feel that these findings support the future use of momelotinib as an effective treatment in MF patients, especially those with anemia,” he concluded.
Cytopenia data are exciting
The key finding in the current study is that “momelotinib leads to important endpoints including significant improvement in symptoms and spleen reduction,” said Dr. Gabriela Hobbs of Harvard Medical School, Boston, who served as the discussant for the study.
“I think a novel finding of momelotinib that is definitely exciting from the treatment perspective is that momelotinib can also lead to improvement in cytopenias,” she said. “We often have to decide between treating the symptoms of the spleen at the expense of blood counts,” in MF patients, she noted.
The study was sponsored by Sierra Oncology. Dr. Mesa disclosed relationships with companies including Constellation Pharmaceutical, La Jolla Pharma, and study sponsor Sierra Oncology, as well as funding from AbbVie, Celgene, Constellation Pharmaceuticals, CTI, Genentech, Incyte, Mays Cancer Center, NCI, Promedior, and Samus. Dr. Hobbs had no financial conflicts to disclose.
This article was updated 06/14/2022.
“The current state for the treatment of myelofibrosis relies on JAK2,” said Ruben Mesa, MD, of the Mays Cancer Center at the UT Health San Antonio MD Anderson Cancer Center.
“Momelotinib is a JAK1 and JAK2 inhibitor.” However, in the early days of studying momelotinib,“it became clear that there was also potentially an improvement in anemia,” which may be related to the additional inhibition of ACVR1, he explained.
Data suggest that the ability to curb anemia in anemic myelofibrosis patients prolongs their lives for up to 8 years, Dr. Mesa added.
Previous studies, notably the phase 3 SIMPLIFY study, showed that momelotinib was associated with comparable effects on spleen volume, transfusion, and total symptom scores from baseline that were similar to ruxolitinib.
In the current study, known as MOMENTUM, a daily dose of momelotinib was compared to danazol for treatment of symptomatic and anemic myelofibrosis (MF) patients who had previously received standard JAK-inhibitor therapy.
In the study, the researchers randomized 130 patients to momelotinib and 65 to danazol. After 24 weeks, those in the danazol group were allowed to cross over to momelotinib. The primary endpoint of the study was total symptom score (TSS) response after 24 weeks. Secondary endpoints included transfusion independence and splenic response at 24 weeks. The median age of the patients in the momelotinib group was 71 years, 60.8% were male, and 82% were white. The baseline demographics were not significantly different in the danazol group.
Overall, 24.6% of momelotinib patients responded with improved total symptom scores at 24 weeks vs. 9.2% of the danazol group. Spleen response also was significantly higher in the momelotinib group; 40% of patients showed a 25% reduction and 23% showed a 35% reduction, compared with 6.2% and 3.1%, respectively, of patients in the danazol group. Transfusion independence at week 24 also was higher for momelotinib patients, compared with danazol patients (31% vs. 20%, respectively, P = 0064).
Adverse events of grade 3 or higher occurred in 53.8% of momelotinib patients and 64.6% of danazol patients, and serious adverse events occurred in 34.6% and 40.0%, respectively. Nearly all patients had anemia, but only 27.7% and 26.2% of the momelotinib and danazol groups, respectively, had thrombocytopenia of grade 3 or higher. The most common nonhematologic adverse events were diarrhea, nausea, and increased blood creatinine. A total of 27.7% of the patients in the momelotinib group discontinued treatment; 16 of whom did so because of an adverse event.
Also, at 24 weeks, patients in the momelotinib group showed a trend towards increased overall survival, compared with danazol (HR, 0.506, P = 0.719).
With momelotinib, there is a consistent thrombocytopenic profile across subgroups, the data on which were presented separately at ASCO (poster 7061), Dr. Mesa added.
“We feel that these findings support the future use of momelotinib as an effective treatment in MF patients, especially those with anemia,” he concluded.
Cytopenia data are exciting
The key finding in the current study is that “momelotinib leads to important endpoints including significant improvement in symptoms and spleen reduction,” said Dr. Gabriela Hobbs of Harvard Medical School, Boston, who served as the discussant for the study.
“I think a novel finding of momelotinib that is definitely exciting from the treatment perspective is that momelotinib can also lead to improvement in cytopenias,” she said. “We often have to decide between treating the symptoms of the spleen at the expense of blood counts,” in MF patients, she noted.
The study was sponsored by Sierra Oncology. Dr. Mesa disclosed relationships with companies including Constellation Pharmaceutical, La Jolla Pharma, and study sponsor Sierra Oncology, as well as funding from AbbVie, Celgene, Constellation Pharmaceuticals, CTI, Genentech, Incyte, Mays Cancer Center, NCI, Promedior, and Samus. Dr. Hobbs had no financial conflicts to disclose.
This article was updated 06/14/2022.
FROM ASCO 2022
A prescription for de-diagnosing
In 2016, Gupta and Cahill challenged the field of psychiatry to reexamine prescribing patterns.1 They warned against the use of polypharmacy when not attached to improved patient functioning. They were concerned with the limited evidence for polypharmacy as well as DSM diagnostic criteria. In their inspiring article, they described a process of deprescribing.
In an effort to study and practice their recommendations, we have noticed a lack of literature examining the elimination of diagnostic labels. While there have been some studies looking at comorbidity, especially with substance use disorders,2 there is a paucity of scientific evidence on patients with numerous diagnoses. Yet our practices are filled with patients who have been labeled with multiple conflicting or redundant diagnoses throughout their lives depending on the setting or the orientation of the practitioner.
The DSM-5 warns against diagnosing disorders when “the occurrence … is not better explained by” another disorder.3 A mix of diagnoses creates confusion for patients as well as clinicians trying to sort through their reported psychiatric histories.
A routine example would include a patient presenting for an initial evaluation and stating “I’ve been diagnosed as manic-depressive, high anxiety, split personality, posttraumatic stress, insomnia, ADD, and depression.” A review of the medical record will reveal a list of diagnoses, including bipolar II, generalized anxiety disorder, borderline personality disorder, posttraumatic stress disorder, unspecified insomnia, attention-deficit/hyperactivity disorder, and major depressive disorder. The medication list includes lamotrigine, valproic acid, citalopram, bupropion, buspirone, prazosin, methylphenidate, clonazepam, hydroxyzine, and low-dose quetiapine at night as needed.
This is an example of polypharmacy treating multiple, and at times conflicting, diagnoses. While an extreme case, in our experience, cases like this are not uncommon. It was actually in our efforts to examine deprescribing that we noticed this quandary. When inquiring about patients on many psychotropic medications, we often receive this retort: the patient is only prescribed one medication per disorder. Some providers have the belief that multiple disorders justify multiple medications, and that this tautological thinking legitimizes polypharmacy.
A patient who has varying moods, some fears, a fluctuating temperament, past traumas, occasional difficulty sleeping, intermittent inattention, and some sadness may be given all the diagnoses listed above and the resulting medication list. The multiplication of diagnoses, “polydiagnosing,” is a convenient justification for future polypharmacy. A lack of careful assessment and thinking in the application of new diagnoses permits the use of increasing numbers of pharmacological agents. A constellation of symptoms of anxiety, concentration deficits, affective dysregulation, and psychosis may justify the combination of benzodiazepines, stimulants, mood stabilizers, and antipsychotics, while a patient with “just” schizophrenia who is sometimes sad, scared, or distracted is more likely to be kept on just one medication, likely an antipsychotic.
Contrary to most medical disorders (for example, tuberculosis) but similar to others (for example, chronic pain), psychiatric disorders are based on the opinion of a “modest number of ‘expert’ classifications.”4 While the broad categories of disorders are justifiable, individual diagnoses are burdened with high rates of comorbidity; lack of treatment specificity; and evidence that distinct syndromes share a genetic basis. Those concerns were exemplified in the study examining the inter-rater reliability of DSM-5 diagnoses, where many disorders were found to have questionable validity.5
A psychiatric diagnosis should be based on biological, psychological, and social factors, which align with our understanding of the natural course of an illness. A patient presenting with transient symptoms of sadness in the context of significant social factors like homelessness and/or significant biological factors associated with schizophrenia should not reflexively receive an additional diagnosis of a depressive disorder. A patient reporting poor concentration in the context of a manic episode should not receive an additional diagnosis of attention-deficit disorder. An older patient with depression on multiple antipsychotics for adjunctive treatment should not necessarily receive a diagnosis of cognitive disorder at the first sign of memory problems.
The cavalier and inconsistent use of diagnoses renders the patients with no clear narrative of who they are. They end up integrating the varying providers’ opinions as a cacophony of labels of unclear significance. Many patients have contradictory diagnoses like major depressive disorder and bipolar disorder, or schizophrenia and schizoaffective disorder. Those inaccurate diagnoses could not only lead to treatment mistakes, but also psychological harm.6
A clearer diagnostic picture is not only more scientifically sound but also more coherent to the patient. This in turn can lead to an improved treatment alliance and buy-in from the patient.
How should a provider practice de-diagnosing? Based on the work of Reeve, et al.,7 on the principles crucial to deprescribing, and subsequent research by Gupta and Cahill,8 we compiled a list of considerations for practitioners wishing to engage in this type of work with their patients.
Choose the right time. While insurance companies require diagnostic findings from the first visit, abrupt de-diagnosing for the sake of simplifying the record from that first visit could be detrimental. Patients can become attached to and find meaning in their diagnostic labels. This was exemplified with the removal of Asperger’s syndrome from the DSM-5.9 Acute symptomatology may be an opportune time to revisit the core pathology of a patient, or a poor time for a patient to have this discussion.
Compile a list of all the patient’s diagnoses. Our initial visits are often illuminated when patients enumerate the vast number of diagnoses they have been given by different providers. Patients will often list half a dozen diagnoses. The patterns often follow life courses with ADHD, conduct disorder, and learning disability in childhood; with anxiety, depression, and/or bipolar disorder in early adulthood; to complicated grief, depression with pseudodementia, and neurocognitive disorders in older adults. Yet patients rarely appreciate the temporary or episodic nature of mental disorders and instead accumulate diagnoses at each change of provider.
Initiate discussion with the patient. It is meaningful to see if patients resonate with the question, “Do you ever feel like every psychiatrist you have seen has given you a different diagnosis?” In our experience, patients’ reactions to this question usually exemplify the problematic nature of the vast array of diagnoses our patients are given. The majority of them are unable to confidently explain the meaning of those diagnoses, the context in which they were given, or their significance. This simple exercise has a powerful effect on raising awareness to patients of the problematic nature of polydiagnosing.
Introduce de-diagnosing. The engagement of patients in the diagnostic process has a significant effect. Reviewing not only diagnostic criteria but also nosology and debates in our understanding of diagnoses can provide patients with further engagement in their care. A simple review of the debate of the bereavement exclusion may permit a patient to not only understand the complexity, but also the changing nature of diagnoses. Suddenly, they are no longer bystanders, but informed participants in their care.
Identify diagnoses most appropriate for removal. Contradictory diagnoses are common in the clinical settings we work in. We routinely see patients carrying multiple mood diagnoses, despite our diagnostic systems not permitting one to have both unipolar and bipolar depression. Superfluous diagnoses are also frequent, with patients receiving depressive, or anxious labels when in an acute state of psychosis or mania. This is exemplified by patients suffering from thought blocking and receiving cognitive or attention-related diagnoses. Concurrent yet different diagnoses are also common in patients with a different list of diagnoses by their primary care provider, their therapist, and their psychiatrist. This is particularly problematic as it forces the patient to alternate their thinking or choose between their providers.
Create a new narrative for the patient. Once diagnoses are explained, clarified, and understood, patients with the help of their providers can reexamine their life story under a new and simplified construct. This process often leads to a less confusing sense of self, an increased dedication to the treatment process, whether behavioral, social, psychological, or pharmacologic.
Consider deprescribing. With a more straightforward and more grounded list of diagnoses (or simply one diagnosis), we find the process of deprescribing to be simpler and more engaging for patients. For example, patients can clearly understand the lack of necessity of an antipsychotic prescription for a resolved substance-induced psychosis. Patients are more engaged in their care, leading to improved medication compliance and less attachment to discontinued medications.
Monitor and adapt. One should of course reevaluate diagnoses as the course of illness provides us with additional information. However, we suggest waiting for a manic episode to emerge prior to diagnosing bipolar rather than suggesting the diagnosis because a patient was wearing red shoes, spoke multiple languages, had multiple degrees and was creative.10 The contextual basis and progression of the symptoms should lead to continual reassessment of diagnoses.
Physicians are aware of the balance between Occam’s razor, which promotes the simplest single explanation for a problem, versus Hickam’s dictum that reminds us that patients can have as many diseases as they please. However, similarly to polypharmacy, “polydiagnosing” has negative effects. While the field of psychiatry’s advancing knowledge may encourage providers to diagnose their patients with the growing number of diagnoses, patients still need and benefit from a coherent and clear medical narrative. Psychiatry would be wise to recognize this concerning trend, in its attempt at rectifying polypharmacy.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest. Dr. Lehman is a professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He has no conflicts of interest.
References
1. Gupta S & Cahill JD. A prescription for “deprescribing” in psychiatry. Psychiatr Serv. 2016 Aug 1;67(8):904-7. doi: 10.1176/appi.ps.201500359.
2. Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction. 2006 Sep;101 Suppl 1:76-88. doi: 10.1111/j.1360-0443.2006.01592.x.
3. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). American Psychiatric Association, 2022. https://psychiatry.org/psychiatrists/practice/dsm.
4. Kendler KS. An historical framework for psychiatric nosology. Psychol Med. 2009 Dec;39(12):1935-41. doi: 10.1017/S0033291709005753.
5. Regier DA et al. DSM-5 field trials in the United States and Canada. Am J Psychiatry. 2013 Jan;170(1):59-70. doi: 10.1176/appi.ajp.2012.12070999.
6. Bhattacharya R et al. When good news is bad news: psychological impact of false-positive diagnosis of HIV. AIDS Care. 2008 May;20(5):560-4. doi: 10.1080/09540120701867206.
7. Reeve E et al. Review of deprescribing processes and development of an evidence‐based, patient‐centred deprescribing process. Br J Clin Pharmacol. 2014 Oct;78(4):738-47. doi: 10.1111/bcp.12386.
8. Gupta S and Cahill JD. A prescription for “deprescribing” in psychiatry.
9. Solomon M. “On the appearance and disappearance of Asperger’s syndrome” in Kendler and Parnas (eds.) Philosophical Issues in Psychiatry IV: Classification of Psychiatric Illness. Oxford University Press, 2017. doi: 10.1093/med/9780198796022.003.0023.
10. Akiskal HS. Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: The “red sign,” the “rule of three,” and other biographic signs of temperamental extravagance, activation, and hypomania. J Affect Disord. 2005 Feb;84(2-3):279-90. doi: 10.1016/j.jad.2004.06.002.
In 2016, Gupta and Cahill challenged the field of psychiatry to reexamine prescribing patterns.1 They warned against the use of polypharmacy when not attached to improved patient functioning. They were concerned with the limited evidence for polypharmacy as well as DSM diagnostic criteria. In their inspiring article, they described a process of deprescribing.
In an effort to study and practice their recommendations, we have noticed a lack of literature examining the elimination of diagnostic labels. While there have been some studies looking at comorbidity, especially with substance use disorders,2 there is a paucity of scientific evidence on patients with numerous diagnoses. Yet our practices are filled with patients who have been labeled with multiple conflicting or redundant diagnoses throughout their lives depending on the setting or the orientation of the practitioner.
The DSM-5 warns against diagnosing disorders when “the occurrence … is not better explained by” another disorder.3 A mix of diagnoses creates confusion for patients as well as clinicians trying to sort through their reported psychiatric histories.
A routine example would include a patient presenting for an initial evaluation and stating “I’ve been diagnosed as manic-depressive, high anxiety, split personality, posttraumatic stress, insomnia, ADD, and depression.” A review of the medical record will reveal a list of diagnoses, including bipolar II, generalized anxiety disorder, borderline personality disorder, posttraumatic stress disorder, unspecified insomnia, attention-deficit/hyperactivity disorder, and major depressive disorder. The medication list includes lamotrigine, valproic acid, citalopram, bupropion, buspirone, prazosin, methylphenidate, clonazepam, hydroxyzine, and low-dose quetiapine at night as needed.
This is an example of polypharmacy treating multiple, and at times conflicting, diagnoses. While an extreme case, in our experience, cases like this are not uncommon. It was actually in our efforts to examine deprescribing that we noticed this quandary. When inquiring about patients on many psychotropic medications, we often receive this retort: the patient is only prescribed one medication per disorder. Some providers have the belief that multiple disorders justify multiple medications, and that this tautological thinking legitimizes polypharmacy.
A patient who has varying moods, some fears, a fluctuating temperament, past traumas, occasional difficulty sleeping, intermittent inattention, and some sadness may be given all the diagnoses listed above and the resulting medication list. The multiplication of diagnoses, “polydiagnosing,” is a convenient justification for future polypharmacy. A lack of careful assessment and thinking in the application of new diagnoses permits the use of increasing numbers of pharmacological agents. A constellation of symptoms of anxiety, concentration deficits, affective dysregulation, and psychosis may justify the combination of benzodiazepines, stimulants, mood stabilizers, and antipsychotics, while a patient with “just” schizophrenia who is sometimes sad, scared, or distracted is more likely to be kept on just one medication, likely an antipsychotic.
Contrary to most medical disorders (for example, tuberculosis) but similar to others (for example, chronic pain), psychiatric disorders are based on the opinion of a “modest number of ‘expert’ classifications.”4 While the broad categories of disorders are justifiable, individual diagnoses are burdened with high rates of comorbidity; lack of treatment specificity; and evidence that distinct syndromes share a genetic basis. Those concerns were exemplified in the study examining the inter-rater reliability of DSM-5 diagnoses, where many disorders were found to have questionable validity.5
A psychiatric diagnosis should be based on biological, psychological, and social factors, which align with our understanding of the natural course of an illness. A patient presenting with transient symptoms of sadness in the context of significant social factors like homelessness and/or significant biological factors associated with schizophrenia should not reflexively receive an additional diagnosis of a depressive disorder. A patient reporting poor concentration in the context of a manic episode should not receive an additional diagnosis of attention-deficit disorder. An older patient with depression on multiple antipsychotics for adjunctive treatment should not necessarily receive a diagnosis of cognitive disorder at the first sign of memory problems.
The cavalier and inconsistent use of diagnoses renders the patients with no clear narrative of who they are. They end up integrating the varying providers’ opinions as a cacophony of labels of unclear significance. Many patients have contradictory diagnoses like major depressive disorder and bipolar disorder, or schizophrenia and schizoaffective disorder. Those inaccurate diagnoses could not only lead to treatment mistakes, but also psychological harm.6
A clearer diagnostic picture is not only more scientifically sound but also more coherent to the patient. This in turn can lead to an improved treatment alliance and buy-in from the patient.
How should a provider practice de-diagnosing? Based on the work of Reeve, et al.,7 on the principles crucial to deprescribing, and subsequent research by Gupta and Cahill,8 we compiled a list of considerations for practitioners wishing to engage in this type of work with their patients.
Choose the right time. While insurance companies require diagnostic findings from the first visit, abrupt de-diagnosing for the sake of simplifying the record from that first visit could be detrimental. Patients can become attached to and find meaning in their diagnostic labels. This was exemplified with the removal of Asperger’s syndrome from the DSM-5.9 Acute symptomatology may be an opportune time to revisit the core pathology of a patient, or a poor time for a patient to have this discussion.
Compile a list of all the patient’s diagnoses. Our initial visits are often illuminated when patients enumerate the vast number of diagnoses they have been given by different providers. Patients will often list half a dozen diagnoses. The patterns often follow life courses with ADHD, conduct disorder, and learning disability in childhood; with anxiety, depression, and/or bipolar disorder in early adulthood; to complicated grief, depression with pseudodementia, and neurocognitive disorders in older adults. Yet patients rarely appreciate the temporary or episodic nature of mental disorders and instead accumulate diagnoses at each change of provider.
Initiate discussion with the patient. It is meaningful to see if patients resonate with the question, “Do you ever feel like every psychiatrist you have seen has given you a different diagnosis?” In our experience, patients’ reactions to this question usually exemplify the problematic nature of the vast array of diagnoses our patients are given. The majority of them are unable to confidently explain the meaning of those diagnoses, the context in which they were given, or their significance. This simple exercise has a powerful effect on raising awareness to patients of the problematic nature of polydiagnosing.
Introduce de-diagnosing. The engagement of patients in the diagnostic process has a significant effect. Reviewing not only diagnostic criteria but also nosology and debates in our understanding of diagnoses can provide patients with further engagement in their care. A simple review of the debate of the bereavement exclusion may permit a patient to not only understand the complexity, but also the changing nature of diagnoses. Suddenly, they are no longer bystanders, but informed participants in their care.
Identify diagnoses most appropriate for removal. Contradictory diagnoses are common in the clinical settings we work in. We routinely see patients carrying multiple mood diagnoses, despite our diagnostic systems not permitting one to have both unipolar and bipolar depression. Superfluous diagnoses are also frequent, with patients receiving depressive, or anxious labels when in an acute state of psychosis or mania. This is exemplified by patients suffering from thought blocking and receiving cognitive or attention-related diagnoses. Concurrent yet different diagnoses are also common in patients with a different list of diagnoses by their primary care provider, their therapist, and their psychiatrist. This is particularly problematic as it forces the patient to alternate their thinking or choose between their providers.
Create a new narrative for the patient. Once diagnoses are explained, clarified, and understood, patients with the help of their providers can reexamine their life story under a new and simplified construct. This process often leads to a less confusing sense of self, an increased dedication to the treatment process, whether behavioral, social, psychological, or pharmacologic.
Consider deprescribing. With a more straightforward and more grounded list of diagnoses (or simply one diagnosis), we find the process of deprescribing to be simpler and more engaging for patients. For example, patients can clearly understand the lack of necessity of an antipsychotic prescription for a resolved substance-induced psychosis. Patients are more engaged in their care, leading to improved medication compliance and less attachment to discontinued medications.
Monitor and adapt. One should of course reevaluate diagnoses as the course of illness provides us with additional information. However, we suggest waiting for a manic episode to emerge prior to diagnosing bipolar rather than suggesting the diagnosis because a patient was wearing red shoes, spoke multiple languages, had multiple degrees and was creative.10 The contextual basis and progression of the symptoms should lead to continual reassessment of diagnoses.
Physicians are aware of the balance between Occam’s razor, which promotes the simplest single explanation for a problem, versus Hickam’s dictum that reminds us that patients can have as many diseases as they please. However, similarly to polypharmacy, “polydiagnosing” has negative effects. While the field of psychiatry’s advancing knowledge may encourage providers to diagnose their patients with the growing number of diagnoses, patients still need and benefit from a coherent and clear medical narrative. Psychiatry would be wise to recognize this concerning trend, in its attempt at rectifying polypharmacy.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest. Dr. Lehman is a professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He has no conflicts of interest.
References
1. Gupta S & Cahill JD. A prescription for “deprescribing” in psychiatry. Psychiatr Serv. 2016 Aug 1;67(8):904-7. doi: 10.1176/appi.ps.201500359.
2. Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction. 2006 Sep;101 Suppl 1:76-88. doi: 10.1111/j.1360-0443.2006.01592.x.
3. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). American Psychiatric Association, 2022. https://psychiatry.org/psychiatrists/practice/dsm.
4. Kendler KS. An historical framework for psychiatric nosology. Psychol Med. 2009 Dec;39(12):1935-41. doi: 10.1017/S0033291709005753.
5. Regier DA et al. DSM-5 field trials in the United States and Canada. Am J Psychiatry. 2013 Jan;170(1):59-70. doi: 10.1176/appi.ajp.2012.12070999.
6. Bhattacharya R et al. When good news is bad news: psychological impact of false-positive diagnosis of HIV. AIDS Care. 2008 May;20(5):560-4. doi: 10.1080/09540120701867206.
7. Reeve E et al. Review of deprescribing processes and development of an evidence‐based, patient‐centred deprescribing process. Br J Clin Pharmacol. 2014 Oct;78(4):738-47. doi: 10.1111/bcp.12386.
8. Gupta S and Cahill JD. A prescription for “deprescribing” in psychiatry.
9. Solomon M. “On the appearance and disappearance of Asperger’s syndrome” in Kendler and Parnas (eds.) Philosophical Issues in Psychiatry IV: Classification of Psychiatric Illness. Oxford University Press, 2017. doi: 10.1093/med/9780198796022.003.0023.
10. Akiskal HS. Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: The “red sign,” the “rule of three,” and other biographic signs of temperamental extravagance, activation, and hypomania. J Affect Disord. 2005 Feb;84(2-3):279-90. doi: 10.1016/j.jad.2004.06.002.
In 2016, Gupta and Cahill challenged the field of psychiatry to reexamine prescribing patterns.1 They warned against the use of polypharmacy when not attached to improved patient functioning. They were concerned with the limited evidence for polypharmacy as well as DSM diagnostic criteria. In their inspiring article, they described a process of deprescribing.
In an effort to study and practice their recommendations, we have noticed a lack of literature examining the elimination of diagnostic labels. While there have been some studies looking at comorbidity, especially with substance use disorders,2 there is a paucity of scientific evidence on patients with numerous diagnoses. Yet our practices are filled with patients who have been labeled with multiple conflicting or redundant diagnoses throughout their lives depending on the setting or the orientation of the practitioner.
The DSM-5 warns against diagnosing disorders when “the occurrence … is not better explained by” another disorder.3 A mix of diagnoses creates confusion for patients as well as clinicians trying to sort through their reported psychiatric histories.
A routine example would include a patient presenting for an initial evaluation and stating “I’ve been diagnosed as manic-depressive, high anxiety, split personality, posttraumatic stress, insomnia, ADD, and depression.” A review of the medical record will reveal a list of diagnoses, including bipolar II, generalized anxiety disorder, borderline personality disorder, posttraumatic stress disorder, unspecified insomnia, attention-deficit/hyperactivity disorder, and major depressive disorder. The medication list includes lamotrigine, valproic acid, citalopram, bupropion, buspirone, prazosin, methylphenidate, clonazepam, hydroxyzine, and low-dose quetiapine at night as needed.
This is an example of polypharmacy treating multiple, and at times conflicting, diagnoses. While an extreme case, in our experience, cases like this are not uncommon. It was actually in our efforts to examine deprescribing that we noticed this quandary. When inquiring about patients on many psychotropic medications, we often receive this retort: the patient is only prescribed one medication per disorder. Some providers have the belief that multiple disorders justify multiple medications, and that this tautological thinking legitimizes polypharmacy.
A patient who has varying moods, some fears, a fluctuating temperament, past traumas, occasional difficulty sleeping, intermittent inattention, and some sadness may be given all the diagnoses listed above and the resulting medication list. The multiplication of diagnoses, “polydiagnosing,” is a convenient justification for future polypharmacy. A lack of careful assessment and thinking in the application of new diagnoses permits the use of increasing numbers of pharmacological agents. A constellation of symptoms of anxiety, concentration deficits, affective dysregulation, and psychosis may justify the combination of benzodiazepines, stimulants, mood stabilizers, and antipsychotics, while a patient with “just” schizophrenia who is sometimes sad, scared, or distracted is more likely to be kept on just one medication, likely an antipsychotic.
Contrary to most medical disorders (for example, tuberculosis) but similar to others (for example, chronic pain), psychiatric disorders are based on the opinion of a “modest number of ‘expert’ classifications.”4 While the broad categories of disorders are justifiable, individual diagnoses are burdened with high rates of comorbidity; lack of treatment specificity; and evidence that distinct syndromes share a genetic basis. Those concerns were exemplified in the study examining the inter-rater reliability of DSM-5 diagnoses, where many disorders were found to have questionable validity.5
A psychiatric diagnosis should be based on biological, psychological, and social factors, which align with our understanding of the natural course of an illness. A patient presenting with transient symptoms of sadness in the context of significant social factors like homelessness and/or significant biological factors associated with schizophrenia should not reflexively receive an additional diagnosis of a depressive disorder. A patient reporting poor concentration in the context of a manic episode should not receive an additional diagnosis of attention-deficit disorder. An older patient with depression on multiple antipsychotics for adjunctive treatment should not necessarily receive a diagnosis of cognitive disorder at the first sign of memory problems.
The cavalier and inconsistent use of diagnoses renders the patients with no clear narrative of who they are. They end up integrating the varying providers’ opinions as a cacophony of labels of unclear significance. Many patients have contradictory diagnoses like major depressive disorder and bipolar disorder, or schizophrenia and schizoaffective disorder. Those inaccurate diagnoses could not only lead to treatment mistakes, but also psychological harm.6
A clearer diagnostic picture is not only more scientifically sound but also more coherent to the patient. This in turn can lead to an improved treatment alliance and buy-in from the patient.
How should a provider practice de-diagnosing? Based on the work of Reeve, et al.,7 on the principles crucial to deprescribing, and subsequent research by Gupta and Cahill,8 we compiled a list of considerations for practitioners wishing to engage in this type of work with their patients.
Choose the right time. While insurance companies require diagnostic findings from the first visit, abrupt de-diagnosing for the sake of simplifying the record from that first visit could be detrimental. Patients can become attached to and find meaning in their diagnostic labels. This was exemplified with the removal of Asperger’s syndrome from the DSM-5.9 Acute symptomatology may be an opportune time to revisit the core pathology of a patient, or a poor time for a patient to have this discussion.
Compile a list of all the patient’s diagnoses. Our initial visits are often illuminated when patients enumerate the vast number of diagnoses they have been given by different providers. Patients will often list half a dozen diagnoses. The patterns often follow life courses with ADHD, conduct disorder, and learning disability in childhood; with anxiety, depression, and/or bipolar disorder in early adulthood; to complicated grief, depression with pseudodementia, and neurocognitive disorders in older adults. Yet patients rarely appreciate the temporary or episodic nature of mental disorders and instead accumulate diagnoses at each change of provider.
Initiate discussion with the patient. It is meaningful to see if patients resonate with the question, “Do you ever feel like every psychiatrist you have seen has given you a different diagnosis?” In our experience, patients’ reactions to this question usually exemplify the problematic nature of the vast array of diagnoses our patients are given. The majority of them are unable to confidently explain the meaning of those diagnoses, the context in which they were given, or their significance. This simple exercise has a powerful effect on raising awareness to patients of the problematic nature of polydiagnosing.
Introduce de-diagnosing. The engagement of patients in the diagnostic process has a significant effect. Reviewing not only diagnostic criteria but also nosology and debates in our understanding of diagnoses can provide patients with further engagement in their care. A simple review of the debate of the bereavement exclusion may permit a patient to not only understand the complexity, but also the changing nature of diagnoses. Suddenly, they are no longer bystanders, but informed participants in their care.
Identify diagnoses most appropriate for removal. Contradictory diagnoses are common in the clinical settings we work in. We routinely see patients carrying multiple mood diagnoses, despite our diagnostic systems not permitting one to have both unipolar and bipolar depression. Superfluous diagnoses are also frequent, with patients receiving depressive, or anxious labels when in an acute state of psychosis or mania. This is exemplified by patients suffering from thought blocking and receiving cognitive or attention-related diagnoses. Concurrent yet different diagnoses are also common in patients with a different list of diagnoses by their primary care provider, their therapist, and their psychiatrist. This is particularly problematic as it forces the patient to alternate their thinking or choose between their providers.
Create a new narrative for the patient. Once diagnoses are explained, clarified, and understood, patients with the help of their providers can reexamine their life story under a new and simplified construct. This process often leads to a less confusing sense of self, an increased dedication to the treatment process, whether behavioral, social, psychological, or pharmacologic.
Consider deprescribing. With a more straightforward and more grounded list of diagnoses (or simply one diagnosis), we find the process of deprescribing to be simpler and more engaging for patients. For example, patients can clearly understand the lack of necessity of an antipsychotic prescription for a resolved substance-induced psychosis. Patients are more engaged in their care, leading to improved medication compliance and less attachment to discontinued medications.
Monitor and adapt. One should of course reevaluate diagnoses as the course of illness provides us with additional information. However, we suggest waiting for a manic episode to emerge prior to diagnosing bipolar rather than suggesting the diagnosis because a patient was wearing red shoes, spoke multiple languages, had multiple degrees and was creative.10 The contextual basis and progression of the symptoms should lead to continual reassessment of diagnoses.
Physicians are aware of the balance between Occam’s razor, which promotes the simplest single explanation for a problem, versus Hickam’s dictum that reminds us that patients can have as many diseases as they please. However, similarly to polypharmacy, “polydiagnosing” has negative effects. While the field of psychiatry’s advancing knowledge may encourage providers to diagnose their patients with the growing number of diagnoses, patients still need and benefit from a coherent and clear medical narrative. Psychiatry would be wise to recognize this concerning trend, in its attempt at rectifying polypharmacy.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. He has no conflicts of interest. Dr. Lehman is a professor of psychiatry at the University of California, San Diego. He is codirector of all acute and intensive psychiatric treatment at the Veterans Affairs Medical Center in San Diego, where he practices clinical psychiatry. He has no conflicts of interest.
References
1. Gupta S & Cahill JD. A prescription for “deprescribing” in psychiatry. Psychiatr Serv. 2016 Aug 1;67(8):904-7. doi: 10.1176/appi.ps.201500359.
2. Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction. 2006 Sep;101 Suppl 1:76-88. doi: 10.1111/j.1360-0443.2006.01592.x.
3. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). American Psychiatric Association, 2022. https://psychiatry.org/psychiatrists/practice/dsm.
4. Kendler KS. An historical framework for psychiatric nosology. Psychol Med. 2009 Dec;39(12):1935-41. doi: 10.1017/S0033291709005753.
5. Regier DA et al. DSM-5 field trials in the United States and Canada. Am J Psychiatry. 2013 Jan;170(1):59-70. doi: 10.1176/appi.ajp.2012.12070999.
6. Bhattacharya R et al. When good news is bad news: psychological impact of false-positive diagnosis of HIV. AIDS Care. 2008 May;20(5):560-4. doi: 10.1080/09540120701867206.
7. Reeve E et al. Review of deprescribing processes and development of an evidence‐based, patient‐centred deprescribing process. Br J Clin Pharmacol. 2014 Oct;78(4):738-47. doi: 10.1111/bcp.12386.
8. Gupta S and Cahill JD. A prescription for “deprescribing” in psychiatry.
9. Solomon M. “On the appearance and disappearance of Asperger’s syndrome” in Kendler and Parnas (eds.) Philosophical Issues in Psychiatry IV: Classification of Psychiatric Illness. Oxford University Press, 2017. doi: 10.1093/med/9780198796022.003.0023.
10. Akiskal HS. Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: The “red sign,” the “rule of three,” and other biographic signs of temperamental extravagance, activation, and hypomania. J Affect Disord. 2005 Feb;84(2-3):279-90. doi: 10.1016/j.jad.2004.06.002.
Pruritus and pitting edema
The 2020 Kidney Disease Improving Global Outcomes (KDIGO) diabetes management in CKD guideline states that most patients with diabetic nephropathy and an eGFR ≥ 30 mL/min/1.73 m2 benefit from treatment with both metformin and a sodium-glucose cotransporter 2 (SGLT2) inhibitor, which have been demonstrated to offer substantial benefits in reducing the risks for diabetic nephropathy and cardiovascular disease.
In patients who do not reach individualized targets with metformin and an SGLT2 inhibitor, or who are unable to use these medications, a long-acting glucagon-like peptide 1 (GLP-1) receptor antagonist may be used.
Metformin should be administered with caution to patients with CKD because it may increase the risk for lactic acidosis. It is contraindicated in patients with an eGFR < 30, but this patient's eGFR is adequate. Many clinicians might use a lower metformin dosage (1500 mg) as a precaution. Given how high his A1c is, adding a GLP-1 receptor antagonist is probably going to be needed because an SGLT2 inhibitor is only intermediate in terms of glucose reduction.
For control of his hypertension, the American Diabetes Association recommends either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) as first-line treatment. However, one agent alone is unlikely to control this patient's hypertension. At his level of eGFR, a thiazide diuretic is unlikely to be very effective. Therefore, a loop diuretic should be initiated with the ACE inhibitor or ARB, especially because he has edema.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The 2020 Kidney Disease Improving Global Outcomes (KDIGO) diabetes management in CKD guideline states that most patients with diabetic nephropathy and an eGFR ≥ 30 mL/min/1.73 m2 benefit from treatment with both metformin and a sodium-glucose cotransporter 2 (SGLT2) inhibitor, which have been demonstrated to offer substantial benefits in reducing the risks for diabetic nephropathy and cardiovascular disease.
In patients who do not reach individualized targets with metformin and an SGLT2 inhibitor, or who are unable to use these medications, a long-acting glucagon-like peptide 1 (GLP-1) receptor antagonist may be used.
Metformin should be administered with caution to patients with CKD because it may increase the risk for lactic acidosis. It is contraindicated in patients with an eGFR < 30, but this patient's eGFR is adequate. Many clinicians might use a lower metformin dosage (1500 mg) as a precaution. Given how high his A1c is, adding a GLP-1 receptor antagonist is probably going to be needed because an SGLT2 inhibitor is only intermediate in terms of glucose reduction.
For control of his hypertension, the American Diabetes Association recommends either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) as first-line treatment. However, one agent alone is unlikely to control this patient's hypertension. At his level of eGFR, a thiazide diuretic is unlikely to be very effective. Therefore, a loop diuretic should be initiated with the ACE inhibitor or ARB, especially because he has edema.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The 2020 Kidney Disease Improving Global Outcomes (KDIGO) diabetes management in CKD guideline states that most patients with diabetic nephropathy and an eGFR ≥ 30 mL/min/1.73 m2 benefit from treatment with both metformin and a sodium-glucose cotransporter 2 (SGLT2) inhibitor, which have been demonstrated to offer substantial benefits in reducing the risks for diabetic nephropathy and cardiovascular disease.
In patients who do not reach individualized targets with metformin and an SGLT2 inhibitor, or who are unable to use these medications, a long-acting glucagon-like peptide 1 (GLP-1) receptor antagonist may be used.
Metformin should be administered with caution to patients with CKD because it may increase the risk for lactic acidosis. It is contraindicated in patients with an eGFR < 30, but this patient's eGFR is adequate. Many clinicians might use a lower metformin dosage (1500 mg) as a precaution. Given how high his A1c is, adding a GLP-1 receptor antagonist is probably going to be needed because an SGLT2 inhibitor is only intermediate in terms of glucose reduction.
For control of his hypertension, the American Diabetes Association recommends either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) as first-line treatment. However, one agent alone is unlikely to control this patient's hypertension. At his level of eGFR, a thiazide diuretic is unlikely to be very effective. Therefore, a loop diuretic should be initiated with the ACE inhibitor or ARB, especially because he has edema.
Romesh K. Khardori, MD, PhD, Professor, Department of Internal Medicine, Division of Diabetes, Endocrine, and Metabolic Disorders, Eastern Virginia Medical School; EVMS Medical Group, Norfolk, Virginia
Romesh K. Khardori, MD, PhD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 47-year-old Black man presents with shortness of breath, pruritus, and pitting edema of the bilateral extremities, which have been present for 6 weeks. He has a 7-year history of type 2 diabetes, hypertension, and hyperlipidemia, as well as a 30–pack-year history of smoking. His blood pressure is 160/95 mm Hg, heart rate is 97 beats/min (regular rate and rhythm), and respiration is 26 breaths/min. He also has proliferative retinopathy. He is 5 ft 10 in and weighs 220 lb (BMI 31.6). He is taking metformin 2550 mg/d. Other medications include simvastatin 20 mg, amlodipine 10 mg, and hydrochlorothiazide 25 mg. He admits to being nonadherent to his medication regimen. A year ago, his estimated glomerular filtration rate (eGFR) was 66 mL/min/1.73 m2 and he had 1+ proteinuria.
Laboratory tests reveal hemoglobin of 8.7 g/dL, creatinine of 3.4 g/dL, eGFR of 32 mL/min/1.73 m2, serum albumin of 3.3 g/dL, A1c of 8.8%, low-density lipoprotein of 143 mg/dL, high-density lipoprotein of 43 mg/dL, random glucose of 186 mg/dL, albumin-creatinine ratio of 3250 mg/g, calcium of 8.7 mg/dL, phosphorus of 4.2 mg/dL, plasma parathyroid hormone of 77 pg/mL, and C-reactive protein of 12.
In summary, this patient has normal albumin levels and increased proteinuria with decreased eGFR. His glucose level and A1c are not controlled. In addition, he has anemia, a low serum albumin level, and edema.
This patient has diabetic nephropathy and is at risk for a cardiovascular event because of his eGFR and long history of diabetes, hypertension, tobacco use, and hyperlipidemia. Intervention to control these risk factors should start immediately to prevent progression to chronic kidney disease (CKD).
Just 20 minutes of vigorous activity daily benefits teens
Vigorous physical activity for 20 minutes a day was enough to maximize cardiorespiratory benefits in adolescents, based on data from more than 300 individuals.
Current recommendations for physical activity in children and adolescents from the World Health Organization call for moderate to vigorous physical activity (MVPA) for an average of 60 minutes a day for physical and mental health; however, guidance on how much physical activity teens need to maximize cardiorespiratory fitness (CRF) has not been determined, Samuel Joseph Burden, BMedSci, of John Radcliffe Hospital Oxford (England), and colleagues wrote.
“Although data in young people are limited, adult studies have shown that regular, brief vigorous physical activity is highly effective at improving health markers, including CRF, which is also an important marker of health in youth,” the researchers wrote.
In a study published in Pediatrics, the researchers examined the associations between physical activity intensity and maximal CRF. The study population included 339 adolescents aged 13-14 years who were evaluated during the 2018-2019 and 2019-2020 school years. Participants wore wrist accelerometers to measure the intensity of physical activity and participated in 20-meter shuttle runs to demonstrate CRF. The researchers used partial multivariable linear regression to assess variables at different intensities including moderate physical activity (MPA), light physical activity (LPA), and sedentary time, as well as vigorous physical activity (VPA).
The wrist monitors measured the intensities of physical activity based on the bandpass-filtered followed by Euclidean norm metric (BFEN), a validated metric. “Previously validated thresholds for BFEN were used to determine the average duration of daily physical activity at each intensity: 0.1 g for LPA, 0.314 g for MPA, and 0.998 g for VPA,” the researchers wrote. Physical activity below the threshold for LPA was categorized as sedentary time.
Participants wore the accelerometers for 1 week; value recording included at least 3 weekdays and 1 weekend day, and each valid day required more than 6 hours of awake time.
Overall, VPA for up to 20 minutes was significantly associated with improved CRF. However, the benefits on CRF plateaued after that time, and longer duration of VPA was not associated with significantly greater improvements in CRF. Neither MPA nor LPA were associated with any improvements in CRF.
Participants who engaged in an average of 14 minutes (range, 12-17 minutes) of VPA per day met the median CRF.
The researchers also conducted independent t tests to assess differences in VPA at different CRF thresholds.
Those in the highest quartile of VPA had CRF z scores 1.03 higher, compared with those in the lower quartiles.
Given that current PA guidelines involve a combination of moderate and vigorous PA that could be met by MPA with no VPA, the current findings have public health implications for improving CRF in adolescents, the researchers wrote.
Even with MPA as an option, most adolescents fail to meet the recommendations of at least 60 minutes of MVPA, they said. “One possible reason is that this duration is quite long, requiring a daily time commitment that some may find difficult to maintain. A shorter target of 20 minutes might be easier to schedule daily and a focus on VPA would simplify messages about the intensity of activity that is likely to improve CRF.”
The study findings were limited by several factors including the use of data from only two schools in the United Kingdom, which may limit generalizability, and future research would ideally include a more direct assessment of VO2 max, the researchers wrote. However, the results were strengthened by the large and diverse study population, including teens with a wide range of body mass index as well as CRF.
Future research is needed to test whether interventions based on a target of 20 minutes of VPA creates significant improvements in adolescent cardiometabolic health, the researchers concluded.
Any activity has value for sedentary teens
The current study suggests that counseling teens about physical activity may be less challenging for clinicians if optimal cardiorespiratory benefits can be reached with shorter bouts of activity, Michele LaBotz, MD, of Intermed Sports Medicine, South Portland, Maine, and Sarah Hoffman, DO, of Tufts University, Boston, wrote in an accompanying editorial.
The results have two key implications for pediatricians, the authors said. First, “optimal CRF can be achieved with much shorter periods of activity than previously recommended.” Second, “current ‘moderate to vigorous’ PA recommendations may not be sufficient to improve CRF in adolescents, if achieved through moderate activity only.”
However, the editorialists emphasized that, although shorter periods of higher-intensity exercise reduce the time burden for teens and families, specific education is needed to explain the extra effort involved in exercising vigorously enough for cardiorespiratory benefits.
Patients can be counseled that activity is vigorous when they start to sweat, their face gets red, and they feel short of breath and unable to talk during activity,” they explained. These sensations may be new and uncomfortable for children and teens who have been quite sedentary or used to low-intensity activity. Dr. LaBotz and Dr. Hoffman advised pediatricians to counsel patients to build intensity gradually, with “exercise snacks,” that involve several minutes of activity that become more challenging over time.
“Exercise snacks can include anything that elevates the heart rate for a minute or more, such as running up and down the stairs a few times; chasing the dog around the backyard; or just putting on some music and dancing hard,” the editorialists wrote.
“Some exercise is better than none, and extrapolating from adult data, the biggest benefit likely occurs when we can help our most sedentary and least fit patients become a bit more active, even if it falls short of currently recommended levels,” they concluded.
The study was supported by grants to various researchers from the British Heart Foundation, the Elizabeth Casson Trust, the U.K. National Institute of Health Research, the Professor Nigel Groome Studentship scheme (Oxford Brookes University), and the U.K. Department of Health. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose.
Vigorous physical activity for 20 minutes a day was enough to maximize cardiorespiratory benefits in adolescents, based on data from more than 300 individuals.
Current recommendations for physical activity in children and adolescents from the World Health Organization call for moderate to vigorous physical activity (MVPA) for an average of 60 minutes a day for physical and mental health; however, guidance on how much physical activity teens need to maximize cardiorespiratory fitness (CRF) has not been determined, Samuel Joseph Burden, BMedSci, of John Radcliffe Hospital Oxford (England), and colleagues wrote.
“Although data in young people are limited, adult studies have shown that regular, brief vigorous physical activity is highly effective at improving health markers, including CRF, which is also an important marker of health in youth,” the researchers wrote.
In a study published in Pediatrics, the researchers examined the associations between physical activity intensity and maximal CRF. The study population included 339 adolescents aged 13-14 years who were evaluated during the 2018-2019 and 2019-2020 school years. Participants wore wrist accelerometers to measure the intensity of physical activity and participated in 20-meter shuttle runs to demonstrate CRF. The researchers used partial multivariable linear regression to assess variables at different intensities including moderate physical activity (MPA), light physical activity (LPA), and sedentary time, as well as vigorous physical activity (VPA).
The wrist monitors measured the intensities of physical activity based on the bandpass-filtered followed by Euclidean norm metric (BFEN), a validated metric. “Previously validated thresholds for BFEN were used to determine the average duration of daily physical activity at each intensity: 0.1 g for LPA, 0.314 g for MPA, and 0.998 g for VPA,” the researchers wrote. Physical activity below the threshold for LPA was categorized as sedentary time.
Participants wore the accelerometers for 1 week; value recording included at least 3 weekdays and 1 weekend day, and each valid day required more than 6 hours of awake time.
Overall, VPA for up to 20 minutes was significantly associated with improved CRF. However, the benefits on CRF plateaued after that time, and longer duration of VPA was not associated with significantly greater improvements in CRF. Neither MPA nor LPA were associated with any improvements in CRF.
Participants who engaged in an average of 14 minutes (range, 12-17 minutes) of VPA per day met the median CRF.
The researchers also conducted independent t tests to assess differences in VPA at different CRF thresholds.
Those in the highest quartile of VPA had CRF z scores 1.03 higher, compared with those in the lower quartiles.
Given that current PA guidelines involve a combination of moderate and vigorous PA that could be met by MPA with no VPA, the current findings have public health implications for improving CRF in adolescents, the researchers wrote.
Even with MPA as an option, most adolescents fail to meet the recommendations of at least 60 minutes of MVPA, they said. “One possible reason is that this duration is quite long, requiring a daily time commitment that some may find difficult to maintain. A shorter target of 20 minutes might be easier to schedule daily and a focus on VPA would simplify messages about the intensity of activity that is likely to improve CRF.”
The study findings were limited by several factors including the use of data from only two schools in the United Kingdom, which may limit generalizability, and future research would ideally include a more direct assessment of VO2 max, the researchers wrote. However, the results were strengthened by the large and diverse study population, including teens with a wide range of body mass index as well as CRF.
Future research is needed to test whether interventions based on a target of 20 minutes of VPA creates significant improvements in adolescent cardiometabolic health, the researchers concluded.
Any activity has value for sedentary teens
The current study suggests that counseling teens about physical activity may be less challenging for clinicians if optimal cardiorespiratory benefits can be reached with shorter bouts of activity, Michele LaBotz, MD, of Intermed Sports Medicine, South Portland, Maine, and Sarah Hoffman, DO, of Tufts University, Boston, wrote in an accompanying editorial.
The results have two key implications for pediatricians, the authors said. First, “optimal CRF can be achieved with much shorter periods of activity than previously recommended.” Second, “current ‘moderate to vigorous’ PA recommendations may not be sufficient to improve CRF in adolescents, if achieved through moderate activity only.”
However, the editorialists emphasized that, although shorter periods of higher-intensity exercise reduce the time burden for teens and families, specific education is needed to explain the extra effort involved in exercising vigorously enough for cardiorespiratory benefits.
Patients can be counseled that activity is vigorous when they start to sweat, their face gets red, and they feel short of breath and unable to talk during activity,” they explained. These sensations may be new and uncomfortable for children and teens who have been quite sedentary or used to low-intensity activity. Dr. LaBotz and Dr. Hoffman advised pediatricians to counsel patients to build intensity gradually, with “exercise snacks,” that involve several minutes of activity that become more challenging over time.
“Exercise snacks can include anything that elevates the heart rate for a minute or more, such as running up and down the stairs a few times; chasing the dog around the backyard; or just putting on some music and dancing hard,” the editorialists wrote.
“Some exercise is better than none, and extrapolating from adult data, the biggest benefit likely occurs when we can help our most sedentary and least fit patients become a bit more active, even if it falls short of currently recommended levels,” they concluded.
The study was supported by grants to various researchers from the British Heart Foundation, the Elizabeth Casson Trust, the U.K. National Institute of Health Research, the Professor Nigel Groome Studentship scheme (Oxford Brookes University), and the U.K. Department of Health. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose.
Vigorous physical activity for 20 minutes a day was enough to maximize cardiorespiratory benefits in adolescents, based on data from more than 300 individuals.
Current recommendations for physical activity in children and adolescents from the World Health Organization call for moderate to vigorous physical activity (MVPA) for an average of 60 minutes a day for physical and mental health; however, guidance on how much physical activity teens need to maximize cardiorespiratory fitness (CRF) has not been determined, Samuel Joseph Burden, BMedSci, of John Radcliffe Hospital Oxford (England), and colleagues wrote.
“Although data in young people are limited, adult studies have shown that regular, brief vigorous physical activity is highly effective at improving health markers, including CRF, which is also an important marker of health in youth,” the researchers wrote.
In a study published in Pediatrics, the researchers examined the associations between physical activity intensity and maximal CRF. The study population included 339 adolescents aged 13-14 years who were evaluated during the 2018-2019 and 2019-2020 school years. Participants wore wrist accelerometers to measure the intensity of physical activity and participated in 20-meter shuttle runs to demonstrate CRF. The researchers used partial multivariable linear regression to assess variables at different intensities including moderate physical activity (MPA), light physical activity (LPA), and sedentary time, as well as vigorous physical activity (VPA).
The wrist monitors measured the intensities of physical activity based on the bandpass-filtered followed by Euclidean norm metric (BFEN), a validated metric. “Previously validated thresholds for BFEN were used to determine the average duration of daily physical activity at each intensity: 0.1 g for LPA, 0.314 g for MPA, and 0.998 g for VPA,” the researchers wrote. Physical activity below the threshold for LPA was categorized as sedentary time.
Participants wore the accelerometers for 1 week; value recording included at least 3 weekdays and 1 weekend day, and each valid day required more than 6 hours of awake time.
Overall, VPA for up to 20 minutes was significantly associated with improved CRF. However, the benefits on CRF plateaued after that time, and longer duration of VPA was not associated with significantly greater improvements in CRF. Neither MPA nor LPA were associated with any improvements in CRF.
Participants who engaged in an average of 14 minutes (range, 12-17 minutes) of VPA per day met the median CRF.
The researchers also conducted independent t tests to assess differences in VPA at different CRF thresholds.
Those in the highest quartile of VPA had CRF z scores 1.03 higher, compared with those in the lower quartiles.
Given that current PA guidelines involve a combination of moderate and vigorous PA that could be met by MPA with no VPA, the current findings have public health implications for improving CRF in adolescents, the researchers wrote.
Even with MPA as an option, most adolescents fail to meet the recommendations of at least 60 minutes of MVPA, they said. “One possible reason is that this duration is quite long, requiring a daily time commitment that some may find difficult to maintain. A shorter target of 20 minutes might be easier to schedule daily and a focus on VPA would simplify messages about the intensity of activity that is likely to improve CRF.”
The study findings were limited by several factors including the use of data from only two schools in the United Kingdom, which may limit generalizability, and future research would ideally include a more direct assessment of VO2 max, the researchers wrote. However, the results were strengthened by the large and diverse study population, including teens with a wide range of body mass index as well as CRF.
Future research is needed to test whether interventions based on a target of 20 minutes of VPA creates significant improvements in adolescent cardiometabolic health, the researchers concluded.
Any activity has value for sedentary teens
The current study suggests that counseling teens about physical activity may be less challenging for clinicians if optimal cardiorespiratory benefits can be reached with shorter bouts of activity, Michele LaBotz, MD, of Intermed Sports Medicine, South Portland, Maine, and Sarah Hoffman, DO, of Tufts University, Boston, wrote in an accompanying editorial.
The results have two key implications for pediatricians, the authors said. First, “optimal CRF can be achieved with much shorter periods of activity than previously recommended.” Second, “current ‘moderate to vigorous’ PA recommendations may not be sufficient to improve CRF in adolescents, if achieved through moderate activity only.”
However, the editorialists emphasized that, although shorter periods of higher-intensity exercise reduce the time burden for teens and families, specific education is needed to explain the extra effort involved in exercising vigorously enough for cardiorespiratory benefits.
Patients can be counseled that activity is vigorous when they start to sweat, their face gets red, and they feel short of breath and unable to talk during activity,” they explained. These sensations may be new and uncomfortable for children and teens who have been quite sedentary or used to low-intensity activity. Dr. LaBotz and Dr. Hoffman advised pediatricians to counsel patients to build intensity gradually, with “exercise snacks,” that involve several minutes of activity that become more challenging over time.
“Exercise snacks can include anything that elevates the heart rate for a minute or more, such as running up and down the stairs a few times; chasing the dog around the backyard; or just putting on some music and dancing hard,” the editorialists wrote.
“Some exercise is better than none, and extrapolating from adult data, the biggest benefit likely occurs when we can help our most sedentary and least fit patients become a bit more active, even if it falls short of currently recommended levels,” they concluded.
The study was supported by grants to various researchers from the British Heart Foundation, the Elizabeth Casson Trust, the U.K. National Institute of Health Research, the Professor Nigel Groome Studentship scheme (Oxford Brookes University), and the U.K. Department of Health. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose.
FROM PEDIATRICS
Time to toss the tomes
This past weekend, because of a series of unfortunate events, I had to move a lot of furniture. This included the bookshelves in my home office. I began by taking books off the shelves to make the bookcase easier to move.
After blowing away a few pounds of dust, I found myself staring at tomes that were once the center of my life: Robbin’s “Pathological Basis of Disease,” Cecil’s “Essentials of Medicine,” Stryer’s “Biochemistry, Grant’s Method of Anatomy,” Stedman’s “Medical Dictionary,” and a few others. All of them more than 30 years old.
I piled the books up on a table as I moved the bookcase, thinking about them. I hadn’t opened any of them in at least 20 years, probably more.
When it was time to put them back, I stared at the pile. They’re big and heavy, qualities that we assume are good things in textbooks. Especially in medical school.
Books have heft. Their knowledge and supposed wisdom are measured by weight and size as you slowly turn the pages under a desk lamp. Not like today, where all the libraries of the world are accessible from a single lightweight iPad.
I remember carrying those books around, stuffed in a backpack draped over my left shoulder. In retrospect it’s amazing I didn’t develop a long thoracic nerve palsy during those years.
They were expensive. I mean, in 1989 dollars, they were all between $50 and $100. I long ago shredded my credit card statements from that era, but my spending for books was pretty high. Fortunately my dad stood behind me for a big chunk of this, and told me to get whatever I needed. Believe me, I know how lucky I am.
I looked at the books. We’d been through a lot together. Long nights at my apartment across the street from Creighton, reading and rereading them. The pages still marked with the yellow highlighter pen that never left my side back then. A younger version of myself traced these pages, committing things to memory that I now have no recollection of. (If you can still draw the Krebs cycle from memory you’re way ahead of me.)
Realistically, though, there was no reason to hold onto them anymore. I’m about two-thirds of the way through my career.
Plus, they’re out of date. Basic anatomy knowledge hasn’t changed much, but most everything else has. I started med school in 1989, and if I’d been looking things up in 1959 textbooks then, I probably wouldn’t have gotten very far. When I need to look things up these days I go to UpToDate, or Epocrates, or other online sources or apps.
I carried the majority of the books out to the recycling can. (It took a few trips.)
Facing some now-empty space on my bookshelf, I put my next challenge there: A pile of 33-RPM records that I still can’t bring myself to get rid of.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This past weekend, because of a series of unfortunate events, I had to move a lot of furniture. This included the bookshelves in my home office. I began by taking books off the shelves to make the bookcase easier to move.
After blowing away a few pounds of dust, I found myself staring at tomes that were once the center of my life: Robbin’s “Pathological Basis of Disease,” Cecil’s “Essentials of Medicine,” Stryer’s “Biochemistry, Grant’s Method of Anatomy,” Stedman’s “Medical Dictionary,” and a few others. All of them more than 30 years old.
I piled the books up on a table as I moved the bookcase, thinking about them. I hadn’t opened any of them in at least 20 years, probably more.
When it was time to put them back, I stared at the pile. They’re big and heavy, qualities that we assume are good things in textbooks. Especially in medical school.
Books have heft. Their knowledge and supposed wisdom are measured by weight and size as you slowly turn the pages under a desk lamp. Not like today, where all the libraries of the world are accessible from a single lightweight iPad.
I remember carrying those books around, stuffed in a backpack draped over my left shoulder. In retrospect it’s amazing I didn’t develop a long thoracic nerve palsy during those years.
They were expensive. I mean, in 1989 dollars, they were all between $50 and $100. I long ago shredded my credit card statements from that era, but my spending for books was pretty high. Fortunately my dad stood behind me for a big chunk of this, and told me to get whatever I needed. Believe me, I know how lucky I am.
I looked at the books. We’d been through a lot together. Long nights at my apartment across the street from Creighton, reading and rereading them. The pages still marked with the yellow highlighter pen that never left my side back then. A younger version of myself traced these pages, committing things to memory that I now have no recollection of. (If you can still draw the Krebs cycle from memory you’re way ahead of me.)
Realistically, though, there was no reason to hold onto them anymore. I’m about two-thirds of the way through my career.
Plus, they’re out of date. Basic anatomy knowledge hasn’t changed much, but most everything else has. I started med school in 1989, and if I’d been looking things up in 1959 textbooks then, I probably wouldn’t have gotten very far. When I need to look things up these days I go to UpToDate, or Epocrates, or other online sources or apps.
I carried the majority of the books out to the recycling can. (It took a few trips.)
Facing some now-empty space on my bookshelf, I put my next challenge there: A pile of 33-RPM records that I still can’t bring myself to get rid of.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This past weekend, because of a series of unfortunate events, I had to move a lot of furniture. This included the bookshelves in my home office. I began by taking books off the shelves to make the bookcase easier to move.
After blowing away a few pounds of dust, I found myself staring at tomes that were once the center of my life: Robbin’s “Pathological Basis of Disease,” Cecil’s “Essentials of Medicine,” Stryer’s “Biochemistry, Grant’s Method of Anatomy,” Stedman’s “Medical Dictionary,” and a few others. All of them more than 30 years old.
I piled the books up on a table as I moved the bookcase, thinking about them. I hadn’t opened any of them in at least 20 years, probably more.
When it was time to put them back, I stared at the pile. They’re big and heavy, qualities that we assume are good things in textbooks. Especially in medical school.
Books have heft. Their knowledge and supposed wisdom are measured by weight and size as you slowly turn the pages under a desk lamp. Not like today, where all the libraries of the world are accessible from a single lightweight iPad.
I remember carrying those books around, stuffed in a backpack draped over my left shoulder. In retrospect it’s amazing I didn’t develop a long thoracic nerve palsy during those years.
They were expensive. I mean, in 1989 dollars, they were all between $50 and $100. I long ago shredded my credit card statements from that era, but my spending for books was pretty high. Fortunately my dad stood behind me for a big chunk of this, and told me to get whatever I needed. Believe me, I know how lucky I am.
I looked at the books. We’d been through a lot together. Long nights at my apartment across the street from Creighton, reading and rereading them. The pages still marked with the yellow highlighter pen that never left my side back then. A younger version of myself traced these pages, committing things to memory that I now have no recollection of. (If you can still draw the Krebs cycle from memory you’re way ahead of me.)
Realistically, though, there was no reason to hold onto them anymore. I’m about two-thirds of the way through my career.
Plus, they’re out of date. Basic anatomy knowledge hasn’t changed much, but most everything else has. I started med school in 1989, and if I’d been looking things up in 1959 textbooks then, I probably wouldn’t have gotten very far. When I need to look things up these days I go to UpToDate, or Epocrates, or other online sources or apps.
I carried the majority of the books out to the recycling can. (It took a few trips.)
Facing some now-empty space on my bookshelf, I put my next challenge there: A pile of 33-RPM records that I still can’t bring myself to get rid of.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Risk of drug interactions is on the rise as MS drugs evolve
NATIONAL HARBOR, MD – How often do patients with multiple sclerosis (MS) end up taking drugs that could dangerously interact with other medications they’re taking? A new German study provides a disturbing hint, a pharmacist who spoke at the annual meeting of the Consortium of Multiple Sclerosis Centers told colleagues: Out of 627 patients who took an average of 5.3 drugs each, about 1 in 25 faced a potentially severe interaction, and nearly two-thirds had at least one potentially risky interaction.
It’s crucial to “work on identifying those interactions,” said Jenelle H. Montgomery, PharmD, of Duke University Hospital, Durham, N.C., and to understand the risks. As she noted, interactions don’t just put patients at risk of adverse effects and hospitalization. They can also lead to secondary comorbidities and therapeutic failures.
Newer versus older drugs
Drug interactions in MS have become more common as disease-modifying therapies have evolved, she said. Some older drugs – such as glatiramer acetate, beta-interferons, and fumarates – have low interaction profiles. But newer drugs have more drug interactions caused in part by their side-effect profiles, oral routes of administration, and immunosuppressive instead of immunomodulatory effects, she said. Teriflunomide, for example, interacts with rosuvastatin and warfarin.
S1P modulators are especially complex on the interaction front, Dr. Montgomery said. Cardiology consults are recommended for patients taking siponimod, ozanimod, and ponesimod, and there are a number of potential interactions between these drugs and other medications.
In regard to other MS drugs, other medications can disrupt the metabolism of cladribine, she said, and the manufacturer recommends separating any other oral drug doses by 3 hours. Even MS-related drugs can interact: carbamazepine, used to treat MS-related neuropathic pain, interacts with drugs such as siponimod.
Who is most at risk?
One strategy could be to focus on patients who may be more susceptible. Dr. Montgomery highlighted the kinds of patients who were most at risk of polypharmacy, per the 2022 German study: older people, those with lower education levels, and those with more disability. And she pointed out that 77% of all drug interactions were between prescription drugs. Another 19% were between prescription drugs and over-the-counter medications, and 4% were between OTC drugs.
She also emphasized the importance of asking about everything that a patient is taking, including herbal supplements, as nearly 60% of people aged 20 and over take them, and about 75% of those over 60. A quarter of people over age 60 take at least four supplements.
Information about interactions with supplements isn’t always available, she said, but she did mention concerns about St. John’s wort interactions with siponimod and cladribine.
Dr. Montgomery also offered several tips: Periodically ask patients to bring in medication bottles or pillboxes; encourage annual checkups with primary physicians; and use drug resources such as Facts and Comparisons, Lexicomp, Clinical Pharmacology, Micromedex, and Natural Medicines.
Disclosures for Dr. Montgomery were not available.
NATIONAL HARBOR, MD – How often do patients with multiple sclerosis (MS) end up taking drugs that could dangerously interact with other medications they’re taking? A new German study provides a disturbing hint, a pharmacist who spoke at the annual meeting of the Consortium of Multiple Sclerosis Centers told colleagues: Out of 627 patients who took an average of 5.3 drugs each, about 1 in 25 faced a potentially severe interaction, and nearly two-thirds had at least one potentially risky interaction.
It’s crucial to “work on identifying those interactions,” said Jenelle H. Montgomery, PharmD, of Duke University Hospital, Durham, N.C., and to understand the risks. As she noted, interactions don’t just put patients at risk of adverse effects and hospitalization. They can also lead to secondary comorbidities and therapeutic failures.
Newer versus older drugs
Drug interactions in MS have become more common as disease-modifying therapies have evolved, she said. Some older drugs – such as glatiramer acetate, beta-interferons, and fumarates – have low interaction profiles. But newer drugs have more drug interactions caused in part by their side-effect profiles, oral routes of administration, and immunosuppressive instead of immunomodulatory effects, she said. Teriflunomide, for example, interacts with rosuvastatin and warfarin.
S1P modulators are especially complex on the interaction front, Dr. Montgomery said. Cardiology consults are recommended for patients taking siponimod, ozanimod, and ponesimod, and there are a number of potential interactions between these drugs and other medications.
In regard to other MS drugs, other medications can disrupt the metabolism of cladribine, she said, and the manufacturer recommends separating any other oral drug doses by 3 hours. Even MS-related drugs can interact: carbamazepine, used to treat MS-related neuropathic pain, interacts with drugs such as siponimod.
Who is most at risk?
One strategy could be to focus on patients who may be more susceptible. Dr. Montgomery highlighted the kinds of patients who were most at risk of polypharmacy, per the 2022 German study: older people, those with lower education levels, and those with more disability. And she pointed out that 77% of all drug interactions were between prescription drugs. Another 19% were between prescription drugs and over-the-counter medications, and 4% were between OTC drugs.
She also emphasized the importance of asking about everything that a patient is taking, including herbal supplements, as nearly 60% of people aged 20 and over take them, and about 75% of those over 60. A quarter of people over age 60 take at least four supplements.
Information about interactions with supplements isn’t always available, she said, but she did mention concerns about St. John’s wort interactions with siponimod and cladribine.
Dr. Montgomery also offered several tips: Periodically ask patients to bring in medication bottles or pillboxes; encourage annual checkups with primary physicians; and use drug resources such as Facts and Comparisons, Lexicomp, Clinical Pharmacology, Micromedex, and Natural Medicines.
Disclosures for Dr. Montgomery were not available.
NATIONAL HARBOR, MD – How often do patients with multiple sclerosis (MS) end up taking drugs that could dangerously interact with other medications they’re taking? A new German study provides a disturbing hint, a pharmacist who spoke at the annual meeting of the Consortium of Multiple Sclerosis Centers told colleagues: Out of 627 patients who took an average of 5.3 drugs each, about 1 in 25 faced a potentially severe interaction, and nearly two-thirds had at least one potentially risky interaction.
It’s crucial to “work on identifying those interactions,” said Jenelle H. Montgomery, PharmD, of Duke University Hospital, Durham, N.C., and to understand the risks. As she noted, interactions don’t just put patients at risk of adverse effects and hospitalization. They can also lead to secondary comorbidities and therapeutic failures.
Newer versus older drugs
Drug interactions in MS have become more common as disease-modifying therapies have evolved, she said. Some older drugs – such as glatiramer acetate, beta-interferons, and fumarates – have low interaction profiles. But newer drugs have more drug interactions caused in part by their side-effect profiles, oral routes of administration, and immunosuppressive instead of immunomodulatory effects, she said. Teriflunomide, for example, interacts with rosuvastatin and warfarin.
S1P modulators are especially complex on the interaction front, Dr. Montgomery said. Cardiology consults are recommended for patients taking siponimod, ozanimod, and ponesimod, and there are a number of potential interactions between these drugs and other medications.
In regard to other MS drugs, other medications can disrupt the metabolism of cladribine, she said, and the manufacturer recommends separating any other oral drug doses by 3 hours. Even MS-related drugs can interact: carbamazepine, used to treat MS-related neuropathic pain, interacts with drugs such as siponimod.
Who is most at risk?
One strategy could be to focus on patients who may be more susceptible. Dr. Montgomery highlighted the kinds of patients who were most at risk of polypharmacy, per the 2022 German study: older people, those with lower education levels, and those with more disability. And she pointed out that 77% of all drug interactions were between prescription drugs. Another 19% were between prescription drugs and over-the-counter medications, and 4% were between OTC drugs.
She also emphasized the importance of asking about everything that a patient is taking, including herbal supplements, as nearly 60% of people aged 20 and over take them, and about 75% of those over 60. A quarter of people over age 60 take at least four supplements.
Information about interactions with supplements isn’t always available, she said, but she did mention concerns about St. John’s wort interactions with siponimod and cladribine.
Dr. Montgomery also offered several tips: Periodically ask patients to bring in medication bottles or pillboxes; encourage annual checkups with primary physicians; and use drug resources such as Facts and Comparisons, Lexicomp, Clinical Pharmacology, Micromedex, and Natural Medicines.
Disclosures for Dr. Montgomery were not available.
AT CMSC 2022