User login
AGA Clinical Practice Update: Expert review of dietary options for IBS
The American Gastroenterological Association has published a clinical practice update on dietary interventions for patients with irritable bowel syndrome (IBS). The topics range from identification of suitable candidates for dietary interventions, to levels of evidence for specific diets, which are becoming increasingly recognized for their key role in managing patients with IBS, according to lead author William D. Chey, MD, of the University of Michigan, Ann Arbor, and colleagues.
“Most medical therapies for IBS improve global symptoms in fewer than one-half of patients, with a therapeutic gain of 7%-15% over placebo,” the researchers wrote in Gastroenterology. “Most patients with IBS associate their GI symptoms with eating food.”
According to Dr. Chey and colleagues, clinicians who are considering dietary modifications for treating IBS should first recognize the inherent challenges presented by this process and be aware that new diets won’t work for everyone.
“Specialty diets require planning and preparation, which may be impractical for some patients,” they wrote, noting that individuals with “decreased cognitive abilities and significant psychiatric disease” may be unable to follow diets or interpret their own responses to specific foods. Special diets may also be inappropriate for patients with financial constraints, and “should be avoided in patients with an eating disorder.”
Because of the challenges involved in dietary interventions, Dr. Chey and colleagues advised clinical support from a registered dietitian nutritionist or other resource.
Patients who are suitable candidates for intervention and willing to try a new diet should first provide information about their current eating habits. A food trial can then be personalized and implemented for a predetermined amount of time. If the patient does not respond, then the diet should be stopped and changed to a new diet or another intervention.
Dr. Chey and colleagues discussed three specific dietary interventions and their corresponding levels of evidence: soluble fiber; the low-fermentable oligo-, di-, and monosaccharides and polyols (FODMAP) diet; and a gluten-free diet.
“Soluble fiber is efficacious in treating global symptoms of IBS,” they wrote, citing 15 randomized controlled trials. Soluble fiber is most suitable for patients with constipation-predominant IBS, and different soluble fibers may yield different outcomes based on characteristics such as rate of fermentation and viscosity. In contrast, insoluble fiber is unlikely to help with IBS, and may worsen abdominal pain and bloating.
The low-FODMAP diet is “currently the most evidence-based diet intervention for IBS,” especially for patients with diarrhea-predominant IBS. Dr. Chey and colleagues offered a clear roadmap for employing the diet. First, patients should eat only low-FODMAP foods for 4-6 weeks. If symptoms don’t improve, the diet should be stopped. If symptoms do improve, foods containing a single FODMAP should be reintroduced one at a time, each in increasing quantities over 3 days, alongside documentation of symptom responses. Finally, the diet should be personalized based on these responses. The majority of patients (close to 80%) “can liberalize” a low-FODMAP diet based on their responses.
In contrast with the low-FODMAP diet, which has a relatively solid body of supporting evidence, efficacy data are still limited for treating IBS with a gluten-free diet. “Although observational studies found that most patients with IBS improve with a gluten-free diet, randomized controlled trials have yielded mixed results,” Dr. Chey and colleagues explained.
Their report cited a recent monograph on the topic that concluded that gluten-free eating offered no significant benefit over placebo (relative risk, 0.46; 95% confidence interval, 0.16-1.28). While some studies have documented positive results with a gluten-free diet, Dr. Chey and colleagues suggested that confounding variables such as the nocebo effect and the impact of other dietary factors have yet to be ruled out. “At present, it remains unclear whether a gluten-free diet is of benefit to patients with IBS.”
Dr. Chey and colleagues also explored IBS biomarkers. While some early data have shown that biomarkers may predict dietary responses, “there is insufficient evidence to support their routine use in clinical practice. ... Further efforts to identify and validate biomarkers that predict response to dietary interventions are needed to deliver ‘personalized nutrition.’ ”
The clinical practice update was commissioned and approved by the AGA CPU Committee and the AGA Governing Board. The researchers disclosed relationships with Biomerica, Salix, Mauna Kea Technologies, and others.
This article was updated May 19, 2022.
The American Gastroenterological Association has published a clinical practice update on dietary interventions for patients with irritable bowel syndrome (IBS). The topics range from identification of suitable candidates for dietary interventions, to levels of evidence for specific diets, which are becoming increasingly recognized for their key role in managing patients with IBS, according to lead author William D. Chey, MD, of the University of Michigan, Ann Arbor, and colleagues.
“Most medical therapies for IBS improve global symptoms in fewer than one-half of patients, with a therapeutic gain of 7%-15% over placebo,” the researchers wrote in Gastroenterology. “Most patients with IBS associate their GI symptoms with eating food.”
According to Dr. Chey and colleagues, clinicians who are considering dietary modifications for treating IBS should first recognize the inherent challenges presented by this process and be aware that new diets won’t work for everyone.
“Specialty diets require planning and preparation, which may be impractical for some patients,” they wrote, noting that individuals with “decreased cognitive abilities and significant psychiatric disease” may be unable to follow diets or interpret their own responses to specific foods. Special diets may also be inappropriate for patients with financial constraints, and “should be avoided in patients with an eating disorder.”
Because of the challenges involved in dietary interventions, Dr. Chey and colleagues advised clinical support from a registered dietitian nutritionist or other resource.
Patients who are suitable candidates for intervention and willing to try a new diet should first provide information about their current eating habits. A food trial can then be personalized and implemented for a predetermined amount of time. If the patient does not respond, then the diet should be stopped and changed to a new diet or another intervention.
Dr. Chey and colleagues discussed three specific dietary interventions and their corresponding levels of evidence: soluble fiber; the low-fermentable oligo-, di-, and monosaccharides and polyols (FODMAP) diet; and a gluten-free diet.
“Soluble fiber is efficacious in treating global symptoms of IBS,” they wrote, citing 15 randomized controlled trials. Soluble fiber is most suitable for patients with constipation-predominant IBS, and different soluble fibers may yield different outcomes based on characteristics such as rate of fermentation and viscosity. In contrast, insoluble fiber is unlikely to help with IBS, and may worsen abdominal pain and bloating.
The low-FODMAP diet is “currently the most evidence-based diet intervention for IBS,” especially for patients with diarrhea-predominant IBS. Dr. Chey and colleagues offered a clear roadmap for employing the diet. First, patients should eat only low-FODMAP foods for 4-6 weeks. If symptoms don’t improve, the diet should be stopped. If symptoms do improve, foods containing a single FODMAP should be reintroduced one at a time, each in increasing quantities over 3 days, alongside documentation of symptom responses. Finally, the diet should be personalized based on these responses. The majority of patients (close to 80%) “can liberalize” a low-FODMAP diet based on their responses.
In contrast with the low-FODMAP diet, which has a relatively solid body of supporting evidence, efficacy data are still limited for treating IBS with a gluten-free diet. “Although observational studies found that most patients with IBS improve with a gluten-free diet, randomized controlled trials have yielded mixed results,” Dr. Chey and colleagues explained.
Their report cited a recent monograph on the topic that concluded that gluten-free eating offered no significant benefit over placebo (relative risk, 0.46; 95% confidence interval, 0.16-1.28). While some studies have documented positive results with a gluten-free diet, Dr. Chey and colleagues suggested that confounding variables such as the nocebo effect and the impact of other dietary factors have yet to be ruled out. “At present, it remains unclear whether a gluten-free diet is of benefit to patients with IBS.”
Dr. Chey and colleagues also explored IBS biomarkers. While some early data have shown that biomarkers may predict dietary responses, “there is insufficient evidence to support their routine use in clinical practice. ... Further efforts to identify and validate biomarkers that predict response to dietary interventions are needed to deliver ‘personalized nutrition.’ ”
The clinical practice update was commissioned and approved by the AGA CPU Committee and the AGA Governing Board. The researchers disclosed relationships with Biomerica, Salix, Mauna Kea Technologies, and others.
This article was updated May 19, 2022.
The American Gastroenterological Association has published a clinical practice update on dietary interventions for patients with irritable bowel syndrome (IBS). The topics range from identification of suitable candidates for dietary interventions, to levels of evidence for specific diets, which are becoming increasingly recognized for their key role in managing patients with IBS, according to lead author William D. Chey, MD, of the University of Michigan, Ann Arbor, and colleagues.
“Most medical therapies for IBS improve global symptoms in fewer than one-half of patients, with a therapeutic gain of 7%-15% over placebo,” the researchers wrote in Gastroenterology. “Most patients with IBS associate their GI symptoms with eating food.”
According to Dr. Chey and colleagues, clinicians who are considering dietary modifications for treating IBS should first recognize the inherent challenges presented by this process and be aware that new diets won’t work for everyone.
“Specialty diets require planning and preparation, which may be impractical for some patients,” they wrote, noting that individuals with “decreased cognitive abilities and significant psychiatric disease” may be unable to follow diets or interpret their own responses to specific foods. Special diets may also be inappropriate for patients with financial constraints, and “should be avoided in patients with an eating disorder.”
Because of the challenges involved in dietary interventions, Dr. Chey and colleagues advised clinical support from a registered dietitian nutritionist or other resource.
Patients who are suitable candidates for intervention and willing to try a new diet should first provide information about their current eating habits. A food trial can then be personalized and implemented for a predetermined amount of time. If the patient does not respond, then the diet should be stopped and changed to a new diet or another intervention.
Dr. Chey and colleagues discussed three specific dietary interventions and their corresponding levels of evidence: soluble fiber; the low-fermentable oligo-, di-, and monosaccharides and polyols (FODMAP) diet; and a gluten-free diet.
“Soluble fiber is efficacious in treating global symptoms of IBS,” they wrote, citing 15 randomized controlled trials. Soluble fiber is most suitable for patients with constipation-predominant IBS, and different soluble fibers may yield different outcomes based on characteristics such as rate of fermentation and viscosity. In contrast, insoluble fiber is unlikely to help with IBS, and may worsen abdominal pain and bloating.
The low-FODMAP diet is “currently the most evidence-based diet intervention for IBS,” especially for patients with diarrhea-predominant IBS. Dr. Chey and colleagues offered a clear roadmap for employing the diet. First, patients should eat only low-FODMAP foods for 4-6 weeks. If symptoms don’t improve, the diet should be stopped. If symptoms do improve, foods containing a single FODMAP should be reintroduced one at a time, each in increasing quantities over 3 days, alongside documentation of symptom responses. Finally, the diet should be personalized based on these responses. The majority of patients (close to 80%) “can liberalize” a low-FODMAP diet based on their responses.
In contrast with the low-FODMAP diet, which has a relatively solid body of supporting evidence, efficacy data are still limited for treating IBS with a gluten-free diet. “Although observational studies found that most patients with IBS improve with a gluten-free diet, randomized controlled trials have yielded mixed results,” Dr. Chey and colleagues explained.
Their report cited a recent monograph on the topic that concluded that gluten-free eating offered no significant benefit over placebo (relative risk, 0.46; 95% confidence interval, 0.16-1.28). While some studies have documented positive results with a gluten-free diet, Dr. Chey and colleagues suggested that confounding variables such as the nocebo effect and the impact of other dietary factors have yet to be ruled out. “At present, it remains unclear whether a gluten-free diet is of benefit to patients with IBS.”
Dr. Chey and colleagues also explored IBS biomarkers. While some early data have shown that biomarkers may predict dietary responses, “there is insufficient evidence to support their routine use in clinical practice. ... Further efforts to identify and validate biomarkers that predict response to dietary interventions are needed to deliver ‘personalized nutrition.’ ”
The clinical practice update was commissioned and approved by the AGA CPU Committee and the AGA Governing Board. The researchers disclosed relationships with Biomerica, Salix, Mauna Kea Technologies, and others.
This article was updated May 19, 2022.
FROM GASTROENTEROLOGY
Taking cardiac pacing from boring to super cool
For the past 2 decades, catheter ablation stole most of the excitement in electrophysiology. Cardiac pacing was seen as necessary but boring. His-bundle pacing earned only modest attention.
But at the annual scientific sessions of the Heart Rhythm Society, cardiac pacing consolidated its comeback and entered the super-cool category.
Not one but three late-breaking clinical trials considered the role of pacing the heart’s conduction system for both preventive and therapeutic purposes. Conduction system pacing, or CSP as we call it, includes pacing the His bundle or the left bundle branch. Left bundle–branch pacing has now largely replaced His-bundle pacing.
Before I tell you about the studies, let’s review why CSP disrupts the status quo.
The core idea goes back to basic physiology: After the impulse leaves the atrioventricular node, the heart’s specialized conduction system allows rapid and synchronous conduction to both the right and left ventricles.
Standard cardiac pacing means fixing a pacing lead into the muscle of the right ventricle. From that spot, conduction spreads via slower muscle-to-muscle conduction, which leads to a wide QRS complex and the right ventricle contracts before the left ventricle.
While such dyssynchronous contraction is better than no contraction, this approach leads to a pacing-induced cardiomyopathy in a substantial number of cases. (The incidence reported in many studies varies widely.)
The most disruptive effect of conduction system pacing is that it is a form of cardiac resynchronization therapy (CRT). And that is nifty because, until recently, resynchronizing the ventricles required placing two ventricular leads: one in the right ventricle and the other in the coronary sinus to pace the left ventricle.
Left bundle-branch pacing vs. biventricular pacing
The first of the three HRS studies is the LBBP-RESYNC randomized controlled trial led by Jiangang Zou, MD, PhD, and performed in multiple centers in China. It compared the efficacy of left bundle–branch pacing (LBBP) with that of conventional biventricular pacing in 40 patients with heart failure who were eligible for CRT. The primary endpoint was the change in left ventricular ejection fraction (LVEF) from baseline to 6-month follow-up.
The results favored LBBP. Although both pacing techniques improved LVEF from baseline, the between-group difference in LVEF was greater in the LBBP arm than the biventricular pacing arm by a statistically significant 5.6% (95% confidence interval, 0.3%-10.9%). Secondary endpoints, such as reductions in left ventricular end-systolic volume, N-terminal of the prohormone brain natriuretic peptide, and QRS duration, also favored LBBP.
Conduction system pacing vs. biventricular pacing
A second late-breaking study, from the Geisinger group, led by Pugazhendhi Vijayaraman, MD, was simultaneously published in Heart Rhythm.
This nonrandomized observational study compared nearly 500 patients eligible for CRT treated at two health systems. One group favors conduction system pacing and the other does traditional biventricular pacing, which set up a two-armed comparison.
CSP was accomplished by LBBP (65%) and His-bundle pacing (35%).
The primary endpoint of death or first hospitalization for heart failure occurred in 28.3% of patients in the CSP arm versus 38.4% of the biventricular arm (hazard ratio, 1.52; 95% CI, 1.08-2.09). QRS duration and LVEF also improved from baseline in both groups.
LBB area pacing as a bailout for failed CRT
The Geisinger group also presented and published an international multicenter study that assessed the feasibility of LBBP as a bailout when standard biventricular pacing did not work – because of inadequate coronary sinus anatomy or CRT nonresponse, defined as lack of clinical or echocardiographic improvement.
This series included 212 patients in whom CRT failed and who underwent attempted LBBP pacing. The bailout was successful in 200 patients (91%). The primary endpoint was defined as an increase in LVEF above 5% on echocardiography.
During 12-month follow-up, 61% of patients had an improvement in LVEF above 5% and nearly 30% had a “super-response,” defined as a 20% or greater increase or normalization of LVEF. Similar to the previous studies, LBBP resulted in shorter QRS duration and improved echocardiography parameters.
Am I persuaded?
I was an early adopter of His-bundle pacing. When successful, it delivered both aesthetically pleasing QRS complexes and clinical efficacy. But there were many challenges: it is technically difficult, and capture thresholds are often high at implant and get higher over time, which leads to shorter battery life.
Pacing the left bundle branch mitigates these challenges. Here, the operator approaches from the right side and screws the lead a few millimeters into the septum, so the tip of the lead can capture the left bundle or one of its branches. This allows activation of the heart’s specialized conduction system and thus synchronizes right and left ventricle contraction.
Although there is a learning curve, LBBP is technically easier than His-bundle pacing and ultimately results in far better pacing and sensing parameters. What’s more, the preferred lead for LBBP has a stellar efficacy record – over years.
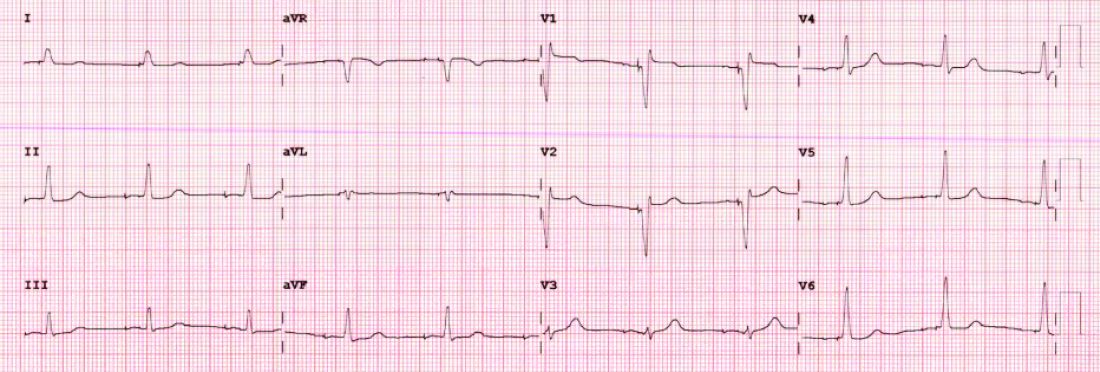
I have become enthralled by the gorgeous QRS complexes from LBBP. The ability to pace the heart without creating dyssynchrony infuses me with joy. I chose cardiology largely because of the beauty of the ECG.
But as a medical conservative who is cautious about unproven therapies, I have questions. How is LBBP defined? Is left septal pacing good enough, or do you need actual left bundle capture? What about long-term performance of a lead in the septum?
Biventricular pacing has set a high bar because it has been proven effective for reducing hard clinical outcomes in large randomized controlled trials.
The studies at HRS begin to answer these questions. The randomized controlled trial from China supports the notion that effective LBBP (the investigators rigorously defined left bundle capture) leads to favorable effects on cardiac contraction. The two observational studies reported similarly encouraging findings on cardiac function.
The three studies therefore tentatively support the notion that LBBP actually produces favorable cardiac performance.
Whether LBBP leads to better clinical outcomes remains uncertain. The nonrandomized comparison study, which found better hard outcomes in the CSP arm, cannot be used to infer causality. There is too much risk for selection bias.
But the LBBP bailout study does suggest that this strategy is reasonable when coronary sinus leads fail – especially since the alternative is surgical placement of an epicardial lead on the left ventricle.
At minimum, the HRS studies persuade me that LBBP will likely prevent pacing-induced cardiomyopathy. If I or a family member required a pacemaker, I’d surely want the operator to be skilled at placing a left bundle lead.
While I am confident that conduction system pacing will become a transformative advance in cardiac pacing, aesthetically pleasing ECG patterns are not enough. There remains much to learn with this nascent approach.
The barriers to getting more CSP trials
The challenge going forward will be funding new trials. CSP stands to prevent pacing-induced cardiomyopathy and offer less costly alternatives to standard biventricular pacing for CRT. This is great for patients, but it would mean that fewer higher-cost CRT devices will be sold.
Heart rhythm research is largely industry-funded because in most cases better therapies for patients mean more profits for industry. In the case of CSP, there is no such confluence of interests.
Conduction system pacing has come about because of the efforts of a few tireless champions who not only published extensively but were also skilled at using social media to spread the excitement. Trials have been small and often self-funded.
The data presented at HRS provides enough equipoise to support a large outcomes-based randomized controlled trial. Imagine if our CSP champions were able to find public-funding sources for such future trials.
Now that would be super cool.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky., and is a writer and podcaster for Medscape. He participates in clinical research and writes often about the state of medical evidence. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
For the past 2 decades, catheter ablation stole most of the excitement in electrophysiology. Cardiac pacing was seen as necessary but boring. His-bundle pacing earned only modest attention.
But at the annual scientific sessions of the Heart Rhythm Society, cardiac pacing consolidated its comeback and entered the super-cool category.
Not one but three late-breaking clinical trials considered the role of pacing the heart’s conduction system for both preventive and therapeutic purposes. Conduction system pacing, or CSP as we call it, includes pacing the His bundle or the left bundle branch. Left bundle–branch pacing has now largely replaced His-bundle pacing.
Before I tell you about the studies, let’s review why CSP disrupts the status quo.
The core idea goes back to basic physiology: After the impulse leaves the atrioventricular node, the heart’s specialized conduction system allows rapid and synchronous conduction to both the right and left ventricles.
Standard cardiac pacing means fixing a pacing lead into the muscle of the right ventricle. From that spot, conduction spreads via slower muscle-to-muscle conduction, which leads to a wide QRS complex and the right ventricle contracts before the left ventricle.
While such dyssynchronous contraction is better than no contraction, this approach leads to a pacing-induced cardiomyopathy in a substantial number of cases. (The incidence reported in many studies varies widely.)
The most disruptive effect of conduction system pacing is that it is a form of cardiac resynchronization therapy (CRT). And that is nifty because, until recently, resynchronizing the ventricles required placing two ventricular leads: one in the right ventricle and the other in the coronary sinus to pace the left ventricle.
Left bundle-branch pacing vs. biventricular pacing
The first of the three HRS studies is the LBBP-RESYNC randomized controlled trial led by Jiangang Zou, MD, PhD, and performed in multiple centers in China. It compared the efficacy of left bundle–branch pacing (LBBP) with that of conventional biventricular pacing in 40 patients with heart failure who were eligible for CRT. The primary endpoint was the change in left ventricular ejection fraction (LVEF) from baseline to 6-month follow-up.
The results favored LBBP. Although both pacing techniques improved LVEF from baseline, the between-group difference in LVEF was greater in the LBBP arm than the biventricular pacing arm by a statistically significant 5.6% (95% confidence interval, 0.3%-10.9%). Secondary endpoints, such as reductions in left ventricular end-systolic volume, N-terminal of the prohormone brain natriuretic peptide, and QRS duration, also favored LBBP.
Conduction system pacing vs. biventricular pacing
A second late-breaking study, from the Geisinger group, led by Pugazhendhi Vijayaraman, MD, was simultaneously published in Heart Rhythm.
This nonrandomized observational study compared nearly 500 patients eligible for CRT treated at two health systems. One group favors conduction system pacing and the other does traditional biventricular pacing, which set up a two-armed comparison.
CSP was accomplished by LBBP (65%) and His-bundle pacing (35%).
The primary endpoint of death or first hospitalization for heart failure occurred in 28.3% of patients in the CSP arm versus 38.4% of the biventricular arm (hazard ratio, 1.52; 95% CI, 1.08-2.09). QRS duration and LVEF also improved from baseline in both groups.
LBB area pacing as a bailout for failed CRT
The Geisinger group also presented and published an international multicenter study that assessed the feasibility of LBBP as a bailout when standard biventricular pacing did not work – because of inadequate coronary sinus anatomy or CRT nonresponse, defined as lack of clinical or echocardiographic improvement.
This series included 212 patients in whom CRT failed and who underwent attempted LBBP pacing. The bailout was successful in 200 patients (91%). The primary endpoint was defined as an increase in LVEF above 5% on echocardiography.
During 12-month follow-up, 61% of patients had an improvement in LVEF above 5% and nearly 30% had a “super-response,” defined as a 20% or greater increase or normalization of LVEF. Similar to the previous studies, LBBP resulted in shorter QRS duration and improved echocardiography parameters.
Am I persuaded?
I was an early adopter of His-bundle pacing. When successful, it delivered both aesthetically pleasing QRS complexes and clinical efficacy. But there were many challenges: it is technically difficult, and capture thresholds are often high at implant and get higher over time, which leads to shorter battery life.
Pacing the left bundle branch mitigates these challenges. Here, the operator approaches from the right side and screws the lead a few millimeters into the septum, so the tip of the lead can capture the left bundle or one of its branches. This allows activation of the heart’s specialized conduction system and thus synchronizes right and left ventricle contraction.
Although there is a learning curve, LBBP is technically easier than His-bundle pacing and ultimately results in far better pacing and sensing parameters. What’s more, the preferred lead for LBBP has a stellar efficacy record – over years.

I have become enthralled by the gorgeous QRS complexes from LBBP. The ability to pace the heart without creating dyssynchrony infuses me with joy. I chose cardiology largely because of the beauty of the ECG.
But as a medical conservative who is cautious about unproven therapies, I have questions. How is LBBP defined? Is left septal pacing good enough, or do you need actual left bundle capture? What about long-term performance of a lead in the septum?
Biventricular pacing has set a high bar because it has been proven effective for reducing hard clinical outcomes in large randomized controlled trials.
The studies at HRS begin to answer these questions. The randomized controlled trial from China supports the notion that effective LBBP (the investigators rigorously defined left bundle capture) leads to favorable effects on cardiac contraction. The two observational studies reported similarly encouraging findings on cardiac function.
The three studies therefore tentatively support the notion that LBBP actually produces favorable cardiac performance.
Whether LBBP leads to better clinical outcomes remains uncertain. The nonrandomized comparison study, which found better hard outcomes in the CSP arm, cannot be used to infer causality. There is too much risk for selection bias.
But the LBBP bailout study does suggest that this strategy is reasonable when coronary sinus leads fail – especially since the alternative is surgical placement of an epicardial lead on the left ventricle.
At minimum, the HRS studies persuade me that LBBP will likely prevent pacing-induced cardiomyopathy. If I or a family member required a pacemaker, I’d surely want the operator to be skilled at placing a left bundle lead.
While I am confident that conduction system pacing will become a transformative advance in cardiac pacing, aesthetically pleasing ECG patterns are not enough. There remains much to learn with this nascent approach.
The barriers to getting more CSP trials
The challenge going forward will be funding new trials. CSP stands to prevent pacing-induced cardiomyopathy and offer less costly alternatives to standard biventricular pacing for CRT. This is great for patients, but it would mean that fewer higher-cost CRT devices will be sold.
Heart rhythm research is largely industry-funded because in most cases better therapies for patients mean more profits for industry. In the case of CSP, there is no such confluence of interests.
Conduction system pacing has come about because of the efforts of a few tireless champions who not only published extensively but were also skilled at using social media to spread the excitement. Trials have been small and often self-funded.
The data presented at HRS provides enough equipoise to support a large outcomes-based randomized controlled trial. Imagine if our CSP champions were able to find public-funding sources for such future trials.
Now that would be super cool.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky., and is a writer and podcaster for Medscape. He participates in clinical research and writes often about the state of medical evidence. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
For the past 2 decades, catheter ablation stole most of the excitement in electrophysiology. Cardiac pacing was seen as necessary but boring. His-bundle pacing earned only modest attention.
But at the annual scientific sessions of the Heart Rhythm Society, cardiac pacing consolidated its comeback and entered the super-cool category.
Not one but three late-breaking clinical trials considered the role of pacing the heart’s conduction system for both preventive and therapeutic purposes. Conduction system pacing, or CSP as we call it, includes pacing the His bundle or the left bundle branch. Left bundle–branch pacing has now largely replaced His-bundle pacing.
Before I tell you about the studies, let’s review why CSP disrupts the status quo.
The core idea goes back to basic physiology: After the impulse leaves the atrioventricular node, the heart’s specialized conduction system allows rapid and synchronous conduction to both the right and left ventricles.
Standard cardiac pacing means fixing a pacing lead into the muscle of the right ventricle. From that spot, conduction spreads via slower muscle-to-muscle conduction, which leads to a wide QRS complex and the right ventricle contracts before the left ventricle.
While such dyssynchronous contraction is better than no contraction, this approach leads to a pacing-induced cardiomyopathy in a substantial number of cases. (The incidence reported in many studies varies widely.)
The most disruptive effect of conduction system pacing is that it is a form of cardiac resynchronization therapy (CRT). And that is nifty because, until recently, resynchronizing the ventricles required placing two ventricular leads: one in the right ventricle and the other in the coronary sinus to pace the left ventricle.
Left bundle-branch pacing vs. biventricular pacing
The first of the three HRS studies is the LBBP-RESYNC randomized controlled trial led by Jiangang Zou, MD, PhD, and performed in multiple centers in China. It compared the efficacy of left bundle–branch pacing (LBBP) with that of conventional biventricular pacing in 40 patients with heart failure who were eligible for CRT. The primary endpoint was the change in left ventricular ejection fraction (LVEF) from baseline to 6-month follow-up.
The results favored LBBP. Although both pacing techniques improved LVEF from baseline, the between-group difference in LVEF was greater in the LBBP arm than the biventricular pacing arm by a statistically significant 5.6% (95% confidence interval, 0.3%-10.9%). Secondary endpoints, such as reductions in left ventricular end-systolic volume, N-terminal of the prohormone brain natriuretic peptide, and QRS duration, also favored LBBP.
Conduction system pacing vs. biventricular pacing
A second late-breaking study, from the Geisinger group, led by Pugazhendhi Vijayaraman, MD, was simultaneously published in Heart Rhythm.
This nonrandomized observational study compared nearly 500 patients eligible for CRT treated at two health systems. One group favors conduction system pacing and the other does traditional biventricular pacing, which set up a two-armed comparison.
CSP was accomplished by LBBP (65%) and His-bundle pacing (35%).
The primary endpoint of death or first hospitalization for heart failure occurred in 28.3% of patients in the CSP arm versus 38.4% of the biventricular arm (hazard ratio, 1.52; 95% CI, 1.08-2.09). QRS duration and LVEF also improved from baseline in both groups.
LBB area pacing as a bailout for failed CRT
The Geisinger group also presented and published an international multicenter study that assessed the feasibility of LBBP as a bailout when standard biventricular pacing did not work – because of inadequate coronary sinus anatomy or CRT nonresponse, defined as lack of clinical or echocardiographic improvement.
This series included 212 patients in whom CRT failed and who underwent attempted LBBP pacing. The bailout was successful in 200 patients (91%). The primary endpoint was defined as an increase in LVEF above 5% on echocardiography.
During 12-month follow-up, 61% of patients had an improvement in LVEF above 5% and nearly 30% had a “super-response,” defined as a 20% or greater increase or normalization of LVEF. Similar to the previous studies, LBBP resulted in shorter QRS duration and improved echocardiography parameters.
Am I persuaded?
I was an early adopter of His-bundle pacing. When successful, it delivered both aesthetically pleasing QRS complexes and clinical efficacy. But there were many challenges: it is technically difficult, and capture thresholds are often high at implant and get higher over time, which leads to shorter battery life.
Pacing the left bundle branch mitigates these challenges. Here, the operator approaches from the right side and screws the lead a few millimeters into the septum, so the tip of the lead can capture the left bundle or one of its branches. This allows activation of the heart’s specialized conduction system and thus synchronizes right and left ventricle contraction.
Although there is a learning curve, LBBP is technically easier than His-bundle pacing and ultimately results in far better pacing and sensing parameters. What’s more, the preferred lead for LBBP has a stellar efficacy record – over years.

I have become enthralled by the gorgeous QRS complexes from LBBP. The ability to pace the heart without creating dyssynchrony infuses me with joy. I chose cardiology largely because of the beauty of the ECG.
But as a medical conservative who is cautious about unproven therapies, I have questions. How is LBBP defined? Is left septal pacing good enough, or do you need actual left bundle capture? What about long-term performance of a lead in the septum?
Biventricular pacing has set a high bar because it has been proven effective for reducing hard clinical outcomes in large randomized controlled trials.
The studies at HRS begin to answer these questions. The randomized controlled trial from China supports the notion that effective LBBP (the investigators rigorously defined left bundle capture) leads to favorable effects on cardiac contraction. The two observational studies reported similarly encouraging findings on cardiac function.
The three studies therefore tentatively support the notion that LBBP actually produces favorable cardiac performance.
Whether LBBP leads to better clinical outcomes remains uncertain. The nonrandomized comparison study, which found better hard outcomes in the CSP arm, cannot be used to infer causality. There is too much risk for selection bias.
But the LBBP bailout study does suggest that this strategy is reasonable when coronary sinus leads fail – especially since the alternative is surgical placement of an epicardial lead on the left ventricle.
At minimum, the HRS studies persuade me that LBBP will likely prevent pacing-induced cardiomyopathy. If I or a family member required a pacemaker, I’d surely want the operator to be skilled at placing a left bundle lead.
While I am confident that conduction system pacing will become a transformative advance in cardiac pacing, aesthetically pleasing ECG patterns are not enough. There remains much to learn with this nascent approach.
The barriers to getting more CSP trials
The challenge going forward will be funding new trials. CSP stands to prevent pacing-induced cardiomyopathy and offer less costly alternatives to standard biventricular pacing for CRT. This is great for patients, but it would mean that fewer higher-cost CRT devices will be sold.
Heart rhythm research is largely industry-funded because in most cases better therapies for patients mean more profits for industry. In the case of CSP, there is no such confluence of interests.
Conduction system pacing has come about because of the efforts of a few tireless champions who not only published extensively but were also skilled at using social media to spread the excitement. Trials have been small and often self-funded.
The data presented at HRS provides enough equipoise to support a large outcomes-based randomized controlled trial. Imagine if our CSP champions were able to find public-funding sources for such future trials.
Now that would be super cool.
Dr. Mandrola practices cardiac electrophysiology in Louisville, Ky., and is a writer and podcaster for Medscape. He participates in clinical research and writes often about the state of medical evidence. He has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Colorado law would lift veil of secrecy on sperm donations
Legislation nearing passage in Colorado would lift a veil of secrecy around sperm donation and grant other protections to people conceived with donated gametes.
The bipartisan bill, which was passed by the state’s house of representatives May 10 after previous approval by the senate, would enable offspring to learn the identity of a sperm or egg donor when they turn 18 and receive a donor’s medical information prior to that. Fertility clinics would be required to update donors’ contact information and medical records every 3 years.
In addition, clinics would have to make “good-faith efforts” to track births to ensure that no more than 25 families conceive babies from a single donor’s sperm. Egg donors could donate up to six times, based on medical risk.
The bill would establish a minimum donor age of 21 years and require dissemination of educational materials to donors and prospective parents about the psychological needs of donor-conceived children.
The provisions would take effect with donations collected on or after Jan. 1, 2025. Violators would be subject to fines of up to $20,000 per day.
Advocates point out that in addition to the benefits of knowing one’s genetic identity, the anonymity of sperm donors has been scuttled by the availability of commercial genetic testing. (Egg donation has tended to be more open.)
Some sperm banks already have adopted systems in which adult offspring can learn the identity of donors if both parties agree. However, a survey by the United States Donor Conceived Council, an advocacy group, found “significant problems” with some of those policies, such as requirements that donor-conceived offspring sign nondisclosure agreements or sperm banks refusing to release information if a donor-conceived person’s parents never registered the child’s birth with the bank.
Some measures in the bill reflect the guidelines of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology, although not all companies follow them, according to the council’s survey. For example, no sperm bank adheres to a recommendation that donors be at least 21 years old.
“The industry is shifting very fast, but there are definitely banks that I think need an extra push to protect the rights of the people that they’re producing,” Tiffany Gardner, a spokesperson for the council, told this news organization.
At a senate hearing, fertility care providers voiced concerns that the legislation would impose undue burdens on the industry and discourage men from donating sperm. In response, sponsors made several amendments, including capping a licensing fee for clinics and banks at $500 and increasing the family limit for each donor, which was originally set at 10.
Still, some in the industry said the bill, introduced April 22, was too rushed to receive adequate scrutiny. While everyone agreed that limiting the number of a person’s half siblings is a good thing, for example, the best way to go about it is unclear, they said.
“There wasn’t enough time to really get experts together to provide more formal, thoughtful, evidence-based feedback on what should be on this bill,” said Cassandra Roeca, MD, of Shady Grove Fertility, which has clinics in Denver and Colorado Springs. Dr. Roeca testified on behalf of Colorado Fertility Advocates, a nonprofit that promotes access to fertility care.
Gov. Jared Polis (D) is expected to sign the bill, an aide to one of the co-sponsors, Rep. Kerry Tipper (D-Lakewood), said in an interview.
Colorado is not the only state considering transparency for donor-conceived offspring. A New York bill would require fertility clinics to verify the medical, educational, and criminal histories of donors and allow donor-conceived people access to the information.
The New York measure is championed by the family of Steven Gunner, a 27-year-old man who died in May 2020 of an opioid overdose. The Wall Street Journal reported that Mr. Gunner’s family had been unaware of his biological father’s history of psychiatric problems.
A version of this article first appeared on Medscape.com.
Legislation nearing passage in Colorado would lift a veil of secrecy around sperm donation and grant other protections to people conceived with donated gametes.
The bipartisan bill, which was passed by the state’s house of representatives May 10 after previous approval by the senate, would enable offspring to learn the identity of a sperm or egg donor when they turn 18 and receive a donor’s medical information prior to that. Fertility clinics would be required to update donors’ contact information and medical records every 3 years.
In addition, clinics would have to make “good-faith efforts” to track births to ensure that no more than 25 families conceive babies from a single donor’s sperm. Egg donors could donate up to six times, based on medical risk.
The bill would establish a minimum donor age of 21 years and require dissemination of educational materials to donors and prospective parents about the psychological needs of donor-conceived children.
The provisions would take effect with donations collected on or after Jan. 1, 2025. Violators would be subject to fines of up to $20,000 per day.
Advocates point out that in addition to the benefits of knowing one’s genetic identity, the anonymity of sperm donors has been scuttled by the availability of commercial genetic testing. (Egg donation has tended to be more open.)
Some sperm banks already have adopted systems in which adult offspring can learn the identity of donors if both parties agree. However, a survey by the United States Donor Conceived Council, an advocacy group, found “significant problems” with some of those policies, such as requirements that donor-conceived offspring sign nondisclosure agreements or sperm banks refusing to release information if a donor-conceived person’s parents never registered the child’s birth with the bank.
Some measures in the bill reflect the guidelines of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology, although not all companies follow them, according to the council’s survey. For example, no sperm bank adheres to a recommendation that donors be at least 21 years old.
“The industry is shifting very fast, but there are definitely banks that I think need an extra push to protect the rights of the people that they’re producing,” Tiffany Gardner, a spokesperson for the council, told this news organization.
At a senate hearing, fertility care providers voiced concerns that the legislation would impose undue burdens on the industry and discourage men from donating sperm. In response, sponsors made several amendments, including capping a licensing fee for clinics and banks at $500 and increasing the family limit for each donor, which was originally set at 10.
Still, some in the industry said the bill, introduced April 22, was too rushed to receive adequate scrutiny. While everyone agreed that limiting the number of a person’s half siblings is a good thing, for example, the best way to go about it is unclear, they said.
“There wasn’t enough time to really get experts together to provide more formal, thoughtful, evidence-based feedback on what should be on this bill,” said Cassandra Roeca, MD, of Shady Grove Fertility, which has clinics in Denver and Colorado Springs. Dr. Roeca testified on behalf of Colorado Fertility Advocates, a nonprofit that promotes access to fertility care.
Gov. Jared Polis (D) is expected to sign the bill, an aide to one of the co-sponsors, Rep. Kerry Tipper (D-Lakewood), said in an interview.
Colorado is not the only state considering transparency for donor-conceived offspring. A New York bill would require fertility clinics to verify the medical, educational, and criminal histories of donors and allow donor-conceived people access to the information.
The New York measure is championed by the family of Steven Gunner, a 27-year-old man who died in May 2020 of an opioid overdose. The Wall Street Journal reported that Mr. Gunner’s family had been unaware of his biological father’s history of psychiatric problems.
A version of this article first appeared on Medscape.com.
Legislation nearing passage in Colorado would lift a veil of secrecy around sperm donation and grant other protections to people conceived with donated gametes.
The bipartisan bill, which was passed by the state’s house of representatives May 10 after previous approval by the senate, would enable offspring to learn the identity of a sperm or egg donor when they turn 18 and receive a donor’s medical information prior to that. Fertility clinics would be required to update donors’ contact information and medical records every 3 years.
In addition, clinics would have to make “good-faith efforts” to track births to ensure that no more than 25 families conceive babies from a single donor’s sperm. Egg donors could donate up to six times, based on medical risk.
The bill would establish a minimum donor age of 21 years and require dissemination of educational materials to donors and prospective parents about the psychological needs of donor-conceived children.
The provisions would take effect with donations collected on or after Jan. 1, 2025. Violators would be subject to fines of up to $20,000 per day.
Advocates point out that in addition to the benefits of knowing one’s genetic identity, the anonymity of sperm donors has been scuttled by the availability of commercial genetic testing. (Egg donation has tended to be more open.)
Some sperm banks already have adopted systems in which adult offspring can learn the identity of donors if both parties agree. However, a survey by the United States Donor Conceived Council, an advocacy group, found “significant problems” with some of those policies, such as requirements that donor-conceived offspring sign nondisclosure agreements or sperm banks refusing to release information if a donor-conceived person’s parents never registered the child’s birth with the bank.
Some measures in the bill reflect the guidelines of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology, although not all companies follow them, according to the council’s survey. For example, no sperm bank adheres to a recommendation that donors be at least 21 years old.
“The industry is shifting very fast, but there are definitely banks that I think need an extra push to protect the rights of the people that they’re producing,” Tiffany Gardner, a spokesperson for the council, told this news organization.
At a senate hearing, fertility care providers voiced concerns that the legislation would impose undue burdens on the industry and discourage men from donating sperm. In response, sponsors made several amendments, including capping a licensing fee for clinics and banks at $500 and increasing the family limit for each donor, which was originally set at 10.
Still, some in the industry said the bill, introduced April 22, was too rushed to receive adequate scrutiny. While everyone agreed that limiting the number of a person’s half siblings is a good thing, for example, the best way to go about it is unclear, they said.
“There wasn’t enough time to really get experts together to provide more formal, thoughtful, evidence-based feedback on what should be on this bill,” said Cassandra Roeca, MD, of Shady Grove Fertility, which has clinics in Denver and Colorado Springs. Dr. Roeca testified on behalf of Colorado Fertility Advocates, a nonprofit that promotes access to fertility care.
Gov. Jared Polis (D) is expected to sign the bill, an aide to one of the co-sponsors, Rep. Kerry Tipper (D-Lakewood), said in an interview.
Colorado is not the only state considering transparency for donor-conceived offspring. A New York bill would require fertility clinics to verify the medical, educational, and criminal histories of donors and allow donor-conceived people access to the information.
The New York measure is championed by the family of Steven Gunner, a 27-year-old man who died in May 2020 of an opioid overdose. The Wall Street Journal reported that Mr. Gunner’s family had been unaware of his biological father’s history of psychiatric problems.
A version of this article first appeared on Medscape.com.
Senate GOP Puts Up Roadblocks to Bipartisan House Bill for Veterans’ Burn Pit Care
Thousands of military veterans who are sick after being exposed to toxic smoke and dust while on duty are facing a Senate roadblock to ambitious legislation designed to provide them care.
The Senate could start work as soon as this week on a bipartisan bill, called the Honoring Our PACT Act, that passed the House of Representatives in March. It would make it much easier for veterans to get health care and benefits from the Veterans Health Administration if they get sick because of the air they breathed around massive, open-air incineration pits. The military used those pits in war zones around the globe — sometimes the size of football fields — to burn anything from human and medical waste to plastics and munitions, setting it alight with jet fuel.
As it stands now, more than three-quarters of all veterans who submit claims for cancer, breathing disorders, and other illnesses that they believe are caused by inhaling poisonous burn pit smoke have their claims denied, according to estimates from the Department of Veterans Affairs and service organizations.
The reason so few are approved is that the military and VA require injured war fighters to prove an illness is directly connected to their service — something that is extremely difficult when it comes to toxic exposures. The House’s PACT Act would make that easier by declaring that any of the 3.5 million veterans who served in the global war on terror — including operations in Afghanistan, Iraq, and the Persian Gulf — would be presumed eligible for benefits if they come down with any of 23 ailments linked to the burn pits.
Although 34 Republicans voted with Democrats to pass the bill in the House, only one Republican, Sen. Marco Rubio of Florida, has signaled support for the measure. At least 10 GOP members would have to join all Democrats to avoid the threat of a filibuster in the Senate and allow the bill to advance to President Joe Biden’s desk. Biden called on Congress to pass such legislation in his State of the Union address, citing the death of his son Beau Biden, who served in Iraq in 2008 and died in 2015 of glioblastoma, a brain cancer included on the bill’s list of qualifying conditions.
Senate Republicans are raising concerns about the measure, however, suggesting it won’t be paid for, that it is too big, too ambitious, and could end up promising more than the government can deliver.
The Congressional Budget Office estimates the bill would cost more than $300 billion over 10 years, and the VA already has struggled for years to meet surging demand from troops serving deployments since the 2001 terror attacks on America, with a backlog of delayed claims running into the hundreds of thousands. Besides addressing burn pits, the bill would expand benefits for veterans who served at certain nuclear sites, and cover more conditions related to Agent Orange exposure in Vietnam, among several other issues.
While the bill phases in coverage for new groups of beneficiaries over 10 years, some Republicans involved in writing legislation about burn pits fear it is all too much.
Sen. Mike Rounds (R-S.D.), a member of the Veterans’ Affairs Committee, summed up the concern as stemming from promising lots of assistance “that might look really good,” but the bottom line is that those “who really need the care would never get into a VA facility.”
Sen. Thom Tillis (R-N.C.), another member of the panel, agreed. “What we’re concerned with is that you’ve got a backlog of 222,000 cases now, and if you implement, by legislative fiat, the 23 presumptions, we’re gonna go to a million and a half to two and a half million backlog,” he said. Tillis has advanced his own burn pits bill that would leave it to the military and VA to determine which illnesses automatically were presumed to be service-connected. That tally is likely to cover fewer people. “So the question we have is, while making a new promise, are we going to be breaking a promise for all those veterans that need care today?”
Republicans have insisted they want to do something to help veterans who are increasingly getting sick with illnesses that appear related to toxic exposure. About 300,000 veterans have signed up with the VA’s burn pits registry.
Sen. Jerry Moran from Kansas, the top Republican on the Veterans’ Affairs Committee, held a press conference in February with Sen. Jon Tester (D-Mont.), the committee chairman, advocating a more gradual process to expand access to benefits and define the illnesses that would qualify.
The event was designed to show what would easily gain bipartisan support in the Senate while the House was still working on its bill.
Veterans’ service organizations, which try to avoid taking partisan positions, have praised such efforts. But they’ve also made clear they like the House bill. More than 40 of the groups endorsed the PACT Act before it passed the lower chamber.
Aleks Morosky, a governmental affairs specialist for the Wounded Warrior Project, plans to meet with senators this month in hope of advancing the PACT Act.
“This is an urgent issue. I mean, people are dying,” Morosky said.
He added that he believes some minor changes and input from the VA would eliminate the sorts of problems senators are raising.
“This bill was meticulously put together, and these are the provisions that veterans need,” Morosky said. “The VA is telling us that they can implement it the way they’ve implemented large numbers of people coming into the system in the past.”
He pointed to the recent expansion of Agent Orange benefits to Navy veterans and to VA Secretary Denis McDonough’s testimony to the Senate Veterans’ Affairs committee in March. McDonough largely supported the legislation but said the VA would need new leasing authority to ensure it had adequate facilities, as well as more say over adding illnesses to be covered.
Senate Republicans are not so sure about the VA’s ability to absorb such a large group of new patients. Tillis and Rounds suggested one solution would be to greatly expand the access to care veterans can seek outside the VA. They pointed to the Mission Act, a law passed in 2018 that was meant to grant veterans access to private health care. Some critics say it has not lived up to its promise. It’s also been expensive, requiring emergency appropriations from Congress.
“You better think about having community care — because there’s no way you’re going to be able to ramp up the medical infrastructure to provide that purely through the VA,” Tillis said.
Tester said in a statement that the committee was working on McDonough’s requests — and could have a modified bill for a vote before Memorial Day.
“In addition to delivering historic reform for all generations of toxic-exposed veterans, I’m working to ensure this legislation provides VA with additional resources and authorities to hire more staff, establish new facilities, and make critical investments to better ensure it can meet the current and future needs of our nation’s veterans,” Tester said.
Whether or not those changes satisfy enough Republicans remains to be seen.
Sen. Kirsten Gillibrand (D-N.Y.), who chairs the Armed Services subcommittee on personnel and earlier wrote a burn pits bill, said neither cost nor fears about problems on implementation should get in the way of passing the bill. Her proposal was incorporated into the House’s PACT Act.
“To deny service because of a lack of resources or a lack of personnel is an outrageous statement,” Gillibrand said. “We promised these men and women when they went to war that when they came back, we would protect them. And that is our solemn obligation. And if it needs more resources, we will get them more resources.”
She predicted Republicans would come along to help pass a bill.
“I’m optimistic, actually. I think we just need a little more time to talk to more Republicans to get everybody on board,” she said.
Thousands of military veterans who are sick after being exposed to toxic smoke and dust while on duty are facing a Senate roadblock to ambitious legislation designed to provide them care.
The Senate could start work as soon as this week on a bipartisan bill, called the Honoring Our PACT Act, that passed the House of Representatives in March. It would make it much easier for veterans to get health care and benefits from the Veterans Health Administration if they get sick because of the air they breathed around massive, open-air incineration pits. The military used those pits in war zones around the globe — sometimes the size of football fields — to burn anything from human and medical waste to plastics and munitions, setting it alight with jet fuel.
As it stands now, more than three-quarters of all veterans who submit claims for cancer, breathing disorders, and other illnesses that they believe are caused by inhaling poisonous burn pit smoke have their claims denied, according to estimates from the Department of Veterans Affairs and service organizations.
The reason so few are approved is that the military and VA require injured war fighters to prove an illness is directly connected to their service — something that is extremely difficult when it comes to toxic exposures. The House’s PACT Act would make that easier by declaring that any of the 3.5 million veterans who served in the global war on terror — including operations in Afghanistan, Iraq, and the Persian Gulf — would be presumed eligible for benefits if they come down with any of 23 ailments linked to the burn pits.
Although 34 Republicans voted with Democrats to pass the bill in the House, only one Republican, Sen. Marco Rubio of Florida, has signaled support for the measure. At least 10 GOP members would have to join all Democrats to avoid the threat of a filibuster in the Senate and allow the bill to advance to President Joe Biden’s desk. Biden called on Congress to pass such legislation in his State of the Union address, citing the death of his son Beau Biden, who served in Iraq in 2008 and died in 2015 of glioblastoma, a brain cancer included on the bill’s list of qualifying conditions.
Senate Republicans are raising concerns about the measure, however, suggesting it won’t be paid for, that it is too big, too ambitious, and could end up promising more than the government can deliver.
The Congressional Budget Office estimates the bill would cost more than $300 billion over 10 years, and the VA already has struggled for years to meet surging demand from troops serving deployments since the 2001 terror attacks on America, with a backlog of delayed claims running into the hundreds of thousands. Besides addressing burn pits, the bill would expand benefits for veterans who served at certain nuclear sites, and cover more conditions related to Agent Orange exposure in Vietnam, among several other issues.
While the bill phases in coverage for new groups of beneficiaries over 10 years, some Republicans involved in writing legislation about burn pits fear it is all too much.
Sen. Mike Rounds (R-S.D.), a member of the Veterans’ Affairs Committee, summed up the concern as stemming from promising lots of assistance “that might look really good,” but the bottom line is that those “who really need the care would never get into a VA facility.”
Sen. Thom Tillis (R-N.C.), another member of the panel, agreed. “What we’re concerned with is that you’ve got a backlog of 222,000 cases now, and if you implement, by legislative fiat, the 23 presumptions, we’re gonna go to a million and a half to two and a half million backlog,” he said. Tillis has advanced his own burn pits bill that would leave it to the military and VA to determine which illnesses automatically were presumed to be service-connected. That tally is likely to cover fewer people. “So the question we have is, while making a new promise, are we going to be breaking a promise for all those veterans that need care today?”
Republicans have insisted they want to do something to help veterans who are increasingly getting sick with illnesses that appear related to toxic exposure. About 300,000 veterans have signed up with the VA’s burn pits registry.
Sen. Jerry Moran from Kansas, the top Republican on the Veterans’ Affairs Committee, held a press conference in February with Sen. Jon Tester (D-Mont.), the committee chairman, advocating a more gradual process to expand access to benefits and define the illnesses that would qualify.
The event was designed to show what would easily gain bipartisan support in the Senate while the House was still working on its bill.
Veterans’ service organizations, which try to avoid taking partisan positions, have praised such efforts. But they’ve also made clear they like the House bill. More than 40 of the groups endorsed the PACT Act before it passed the lower chamber.
Aleks Morosky, a governmental affairs specialist for the Wounded Warrior Project, plans to meet with senators this month in hope of advancing the PACT Act.
“This is an urgent issue. I mean, people are dying,” Morosky said.
He added that he believes some minor changes and input from the VA would eliminate the sorts of problems senators are raising.
“This bill was meticulously put together, and these are the provisions that veterans need,” Morosky said. “The VA is telling us that they can implement it the way they’ve implemented large numbers of people coming into the system in the past.”
He pointed to the recent expansion of Agent Orange benefits to Navy veterans and to VA Secretary Denis McDonough’s testimony to the Senate Veterans’ Affairs committee in March. McDonough largely supported the legislation but said the VA would need new leasing authority to ensure it had adequate facilities, as well as more say over adding illnesses to be covered.
Senate Republicans are not so sure about the VA’s ability to absorb such a large group of new patients. Tillis and Rounds suggested one solution would be to greatly expand the access to care veterans can seek outside the VA. They pointed to the Mission Act, a law passed in 2018 that was meant to grant veterans access to private health care. Some critics say it has not lived up to its promise. It’s also been expensive, requiring emergency appropriations from Congress.
“You better think about having community care — because there’s no way you’re going to be able to ramp up the medical infrastructure to provide that purely through the VA,” Tillis said.
Tester said in a statement that the committee was working on McDonough’s requests — and could have a modified bill for a vote before Memorial Day.
“In addition to delivering historic reform for all generations of toxic-exposed veterans, I’m working to ensure this legislation provides VA with additional resources and authorities to hire more staff, establish new facilities, and make critical investments to better ensure it can meet the current and future needs of our nation’s veterans,” Tester said.
Whether or not those changes satisfy enough Republicans remains to be seen.
Sen. Kirsten Gillibrand (D-N.Y.), who chairs the Armed Services subcommittee on personnel and earlier wrote a burn pits bill, said neither cost nor fears about problems on implementation should get in the way of passing the bill. Her proposal was incorporated into the House’s PACT Act.
“To deny service because of a lack of resources or a lack of personnel is an outrageous statement,” Gillibrand said. “We promised these men and women when they went to war that when they came back, we would protect them. And that is our solemn obligation. And if it needs more resources, we will get them more resources.”
She predicted Republicans would come along to help pass a bill.
“I’m optimistic, actually. I think we just need a little more time to talk to more Republicans to get everybody on board,” she said.
Thousands of military veterans who are sick after being exposed to toxic smoke and dust while on duty are facing a Senate roadblock to ambitious legislation designed to provide them care.
The Senate could start work as soon as this week on a bipartisan bill, called the Honoring Our PACT Act, that passed the House of Representatives in March. It would make it much easier for veterans to get health care and benefits from the Veterans Health Administration if they get sick because of the air they breathed around massive, open-air incineration pits. The military used those pits in war zones around the globe — sometimes the size of football fields — to burn anything from human and medical waste to plastics and munitions, setting it alight with jet fuel.
As it stands now, more than three-quarters of all veterans who submit claims for cancer, breathing disorders, and other illnesses that they believe are caused by inhaling poisonous burn pit smoke have their claims denied, according to estimates from the Department of Veterans Affairs and service organizations.
The reason so few are approved is that the military and VA require injured war fighters to prove an illness is directly connected to their service — something that is extremely difficult when it comes to toxic exposures. The House’s PACT Act would make that easier by declaring that any of the 3.5 million veterans who served in the global war on terror — including operations in Afghanistan, Iraq, and the Persian Gulf — would be presumed eligible for benefits if they come down with any of 23 ailments linked to the burn pits.
Although 34 Republicans voted with Democrats to pass the bill in the House, only one Republican, Sen. Marco Rubio of Florida, has signaled support for the measure. At least 10 GOP members would have to join all Democrats to avoid the threat of a filibuster in the Senate and allow the bill to advance to President Joe Biden’s desk. Biden called on Congress to pass such legislation in his State of the Union address, citing the death of his son Beau Biden, who served in Iraq in 2008 and died in 2015 of glioblastoma, a brain cancer included on the bill’s list of qualifying conditions.
Senate Republicans are raising concerns about the measure, however, suggesting it won’t be paid for, that it is too big, too ambitious, and could end up promising more than the government can deliver.
The Congressional Budget Office estimates the bill would cost more than $300 billion over 10 years, and the VA already has struggled for years to meet surging demand from troops serving deployments since the 2001 terror attacks on America, with a backlog of delayed claims running into the hundreds of thousands. Besides addressing burn pits, the bill would expand benefits for veterans who served at certain nuclear sites, and cover more conditions related to Agent Orange exposure in Vietnam, among several other issues.
While the bill phases in coverage for new groups of beneficiaries over 10 years, some Republicans involved in writing legislation about burn pits fear it is all too much.
Sen. Mike Rounds (R-S.D.), a member of the Veterans’ Affairs Committee, summed up the concern as stemming from promising lots of assistance “that might look really good,” but the bottom line is that those “who really need the care would never get into a VA facility.”
Sen. Thom Tillis (R-N.C.), another member of the panel, agreed. “What we’re concerned with is that you’ve got a backlog of 222,000 cases now, and if you implement, by legislative fiat, the 23 presumptions, we’re gonna go to a million and a half to two and a half million backlog,” he said. Tillis has advanced his own burn pits bill that would leave it to the military and VA to determine which illnesses automatically were presumed to be service-connected. That tally is likely to cover fewer people. “So the question we have is, while making a new promise, are we going to be breaking a promise for all those veterans that need care today?”
Republicans have insisted they want to do something to help veterans who are increasingly getting sick with illnesses that appear related to toxic exposure. About 300,000 veterans have signed up with the VA’s burn pits registry.
Sen. Jerry Moran from Kansas, the top Republican on the Veterans’ Affairs Committee, held a press conference in February with Sen. Jon Tester (D-Mont.), the committee chairman, advocating a more gradual process to expand access to benefits and define the illnesses that would qualify.
The event was designed to show what would easily gain bipartisan support in the Senate while the House was still working on its bill.
Veterans’ service organizations, which try to avoid taking partisan positions, have praised such efforts. But they’ve also made clear they like the House bill. More than 40 of the groups endorsed the PACT Act before it passed the lower chamber.
Aleks Morosky, a governmental affairs specialist for the Wounded Warrior Project, plans to meet with senators this month in hope of advancing the PACT Act.
“This is an urgent issue. I mean, people are dying,” Morosky said.
He added that he believes some minor changes and input from the VA would eliminate the sorts of problems senators are raising.
“This bill was meticulously put together, and these are the provisions that veterans need,” Morosky said. “The VA is telling us that they can implement it the way they’ve implemented large numbers of people coming into the system in the past.”
He pointed to the recent expansion of Agent Orange benefits to Navy veterans and to VA Secretary Denis McDonough’s testimony to the Senate Veterans’ Affairs committee in March. McDonough largely supported the legislation but said the VA would need new leasing authority to ensure it had adequate facilities, as well as more say over adding illnesses to be covered.
Senate Republicans are not so sure about the VA’s ability to absorb such a large group of new patients. Tillis and Rounds suggested one solution would be to greatly expand the access to care veterans can seek outside the VA. They pointed to the Mission Act, a law passed in 2018 that was meant to grant veterans access to private health care. Some critics say it has not lived up to its promise. It’s also been expensive, requiring emergency appropriations from Congress.
“You better think about having community care — because there’s no way you’re going to be able to ramp up the medical infrastructure to provide that purely through the VA,” Tillis said.
Tester said in a statement that the committee was working on McDonough’s requests — and could have a modified bill for a vote before Memorial Day.
“In addition to delivering historic reform for all generations of toxic-exposed veterans, I’m working to ensure this legislation provides VA with additional resources and authorities to hire more staff, establish new facilities, and make critical investments to better ensure it can meet the current and future needs of our nation’s veterans,” Tester said.
Whether or not those changes satisfy enough Republicans remains to be seen.
Sen. Kirsten Gillibrand (D-N.Y.), who chairs the Armed Services subcommittee on personnel and earlier wrote a burn pits bill, said neither cost nor fears about problems on implementation should get in the way of passing the bill. Her proposal was incorporated into the House’s PACT Act.
“To deny service because of a lack of resources or a lack of personnel is an outrageous statement,” Gillibrand said. “We promised these men and women when they went to war that when they came back, we would protect them. And that is our solemn obligation. And if it needs more resources, we will get them more resources.”
She predicted Republicans would come along to help pass a bill.
“I’m optimistic, actually. I think we just need a little more time to talk to more Republicans to get everybody on board,” she said.
Does noninvasive brain stimulation augment CBT for depression?
Results of a multicenter, placebo-controlled randomized clinical trials showed adjunctive transcranial direct current stimulation (tDCS) was not superior to sham-tDCS plus CBT or CBT alone.
“Combining these interventions does not lead to added value. This is an example where negative findings guide the way of future studies. What we learned is that we might change things in a few dimensions,” study investigator Malek Bajbouj, MD, Charité University Hospital, Berlin, told this news organization.
The study was published online in JAMA Psychiatry.
Urgent need for better treatment
MDD affects 10% of the global population. However, up to 30% of patients have an inadequate response to standard treatment of CBT, pharmacotherapy, or a combination of the two, highlighting the need to develop more effective therapeutic strategies, the investigators note.
A noninvasive approach, tDCS, in healthy populations, has been shown to enhance cognitive function in brain regions that are also relevant for CBT. Specifically, the investigators point out that tDCS can “positively modulate neuronal activity in prefrontal structures central for affective and cognitive processes,” including emotion regulation, cognitive control working memory, and learning.
Based on this early data, the investigators conducted a randomized, placebo-controlled trial to determine whether tDCS combined with CBT might have clinically relevant synergistic effects.
The multicenter study included adults aged 20-65 years with a single or recurrent depressive episode who were either not receiving medication or receiving a stable regimen of selective serotonin reuptake inhibitors (SSRIs) or mirtazapine (Remeron).
A total of 148 participants (89 women, 59 men) with a mean age of 41 years were randomly assigned to receive CBT alone (n = 53), CBT+ tDCS (n = 48) or CBT + sham tDCS (n = 47).
Participants attended a 6-week group intervention of 12 sessions of CBT. If assigned, tDCS was applied simultaneously. Active tDCS included stimulation with an intensity of 2 milliamps for 30 minutes.
The study’s primary outcome was the change in Montgomery-Åsberg Depression Rating Scale (MADRS) from baseline to post treatment in the intention-to-treat sample. A total of 126 patients completed the study.
At baseline, the average MADRS score was 23.0. In each of the study groups, MADRS scores were reduced by a mean of 6.5 points (95% confidence interval, 3.82-9.14 points). The Cohen d value was -0.90 (95% CI, -1.43 to -0.50), indicating a significant effect over time, the researchers report. However, they add that “there was not significant effect of group and no significant interaction of group x time, indicating the estimated additive effects were not statistically significant.”
Results suggest that more research is needed to optimize treatment synchronization to achieve synergies between noninvasive brain stimulation and psychotherapeutic interventions.
Beauty and promise
Commenting on the findings, Mark George, MD, director of the Medical University of South Carolina Center for Advanced Imaging Research and the Brain Stimulation Laboratory, Charleston, described the study as “a really good effort by a great group of researchers.”
It’s unclear, he added, why tDCS failed to augment CBT. “It may be about the nongeneralizability of tDCS to complex functions, it may be that they didn’t get the dose right, or it might be due to a placebo response,” he speculated.
Furthermore, “tDCS is the most simple form of brain stimulation. The beauty and promise of tDCS is that it is so inexpensive and safe,” Dr. George added.
If proven effective, tDCS could potentially be used at home and rolled out as a frontline therapy for depression, he added. “Everybody wants the technology to work as an antidepressant, since it could have a very big positive public health impact,” said Dr. George.
Referring to previous research showing tDCS’ ability to improve specific brain functions in healthy controls, Dr. George noted that the potential of tDCS may be limited to augmenting specific brain functions such as memory but not more complex behaviors like depression.
However, Dr. George believes a more plausible explanation is that the optimal dose for tDCS has not yet been determined.
With other types of neuromodulation, such as electroconvulsive therapy, “we know that we’re in the brain with the right dose. But for tDCS, we don’t know that, and we’ve got to figure that out before it’s ever really going to make it [as a treatment],” he said.
“There have been great advances through the years in the field of brain stimulation and the treatment of depression. But rates of depression and suicide are continuing to grow, and we have not yet made a significant dent in treatment, in part because these technologies require equipment, [and] they’re expensive. So when we figure out tDCS, it will be a very important piece of our toolkit – a real game changer,” Dr. George added.
A version of this article first appeared on Medscape.com.
Results of a multicenter, placebo-controlled randomized clinical trials showed adjunctive transcranial direct current stimulation (tDCS) was not superior to sham-tDCS plus CBT or CBT alone.
“Combining these interventions does not lead to added value. This is an example where negative findings guide the way of future studies. What we learned is that we might change things in a few dimensions,” study investigator Malek Bajbouj, MD, Charité University Hospital, Berlin, told this news organization.
The study was published online in JAMA Psychiatry.
Urgent need for better treatment
MDD affects 10% of the global population. However, up to 30% of patients have an inadequate response to standard treatment of CBT, pharmacotherapy, or a combination of the two, highlighting the need to develop more effective therapeutic strategies, the investigators note.
A noninvasive approach, tDCS, in healthy populations, has been shown to enhance cognitive function in brain regions that are also relevant for CBT. Specifically, the investigators point out that tDCS can “positively modulate neuronal activity in prefrontal structures central for affective and cognitive processes,” including emotion regulation, cognitive control working memory, and learning.
Based on this early data, the investigators conducted a randomized, placebo-controlled trial to determine whether tDCS combined with CBT might have clinically relevant synergistic effects.
The multicenter study included adults aged 20-65 years with a single or recurrent depressive episode who were either not receiving medication or receiving a stable regimen of selective serotonin reuptake inhibitors (SSRIs) or mirtazapine (Remeron).
A total of 148 participants (89 women, 59 men) with a mean age of 41 years were randomly assigned to receive CBT alone (n = 53), CBT+ tDCS (n = 48) or CBT + sham tDCS (n = 47).
Participants attended a 6-week group intervention of 12 sessions of CBT. If assigned, tDCS was applied simultaneously. Active tDCS included stimulation with an intensity of 2 milliamps for 30 minutes.
The study’s primary outcome was the change in Montgomery-Åsberg Depression Rating Scale (MADRS) from baseline to post treatment in the intention-to-treat sample. A total of 126 patients completed the study.
At baseline, the average MADRS score was 23.0. In each of the study groups, MADRS scores were reduced by a mean of 6.5 points (95% confidence interval, 3.82-9.14 points). The Cohen d value was -0.90 (95% CI, -1.43 to -0.50), indicating a significant effect over time, the researchers report. However, they add that “there was not significant effect of group and no significant interaction of group x time, indicating the estimated additive effects were not statistically significant.”
Results suggest that more research is needed to optimize treatment synchronization to achieve synergies between noninvasive brain stimulation and psychotherapeutic interventions.
Beauty and promise
Commenting on the findings, Mark George, MD, director of the Medical University of South Carolina Center for Advanced Imaging Research and the Brain Stimulation Laboratory, Charleston, described the study as “a really good effort by a great group of researchers.”
It’s unclear, he added, why tDCS failed to augment CBT. “It may be about the nongeneralizability of tDCS to complex functions, it may be that they didn’t get the dose right, or it might be due to a placebo response,” he speculated.
Furthermore, “tDCS is the most simple form of brain stimulation. The beauty and promise of tDCS is that it is so inexpensive and safe,” Dr. George added.
If proven effective, tDCS could potentially be used at home and rolled out as a frontline therapy for depression, he added. “Everybody wants the technology to work as an antidepressant, since it could have a very big positive public health impact,” said Dr. George.
Referring to previous research showing tDCS’ ability to improve specific brain functions in healthy controls, Dr. George noted that the potential of tDCS may be limited to augmenting specific brain functions such as memory but not more complex behaviors like depression.
However, Dr. George believes a more plausible explanation is that the optimal dose for tDCS has not yet been determined.
With other types of neuromodulation, such as electroconvulsive therapy, “we know that we’re in the brain with the right dose. But for tDCS, we don’t know that, and we’ve got to figure that out before it’s ever really going to make it [as a treatment],” he said.
“There have been great advances through the years in the field of brain stimulation and the treatment of depression. But rates of depression and suicide are continuing to grow, and we have not yet made a significant dent in treatment, in part because these technologies require equipment, [and] they’re expensive. So when we figure out tDCS, it will be a very important piece of our toolkit – a real game changer,” Dr. George added.
A version of this article first appeared on Medscape.com.
Results of a multicenter, placebo-controlled randomized clinical trials showed adjunctive transcranial direct current stimulation (tDCS) was not superior to sham-tDCS plus CBT or CBT alone.
“Combining these interventions does not lead to added value. This is an example where negative findings guide the way of future studies. What we learned is that we might change things in a few dimensions,” study investigator Malek Bajbouj, MD, Charité University Hospital, Berlin, told this news organization.
The study was published online in JAMA Psychiatry.
Urgent need for better treatment
MDD affects 10% of the global population. However, up to 30% of patients have an inadequate response to standard treatment of CBT, pharmacotherapy, or a combination of the two, highlighting the need to develop more effective therapeutic strategies, the investigators note.
A noninvasive approach, tDCS, in healthy populations, has been shown to enhance cognitive function in brain regions that are also relevant for CBT. Specifically, the investigators point out that tDCS can “positively modulate neuronal activity in prefrontal structures central for affective and cognitive processes,” including emotion regulation, cognitive control working memory, and learning.
Based on this early data, the investigators conducted a randomized, placebo-controlled trial to determine whether tDCS combined with CBT might have clinically relevant synergistic effects.
The multicenter study included adults aged 20-65 years with a single or recurrent depressive episode who were either not receiving medication or receiving a stable regimen of selective serotonin reuptake inhibitors (SSRIs) or mirtazapine (Remeron).
A total of 148 participants (89 women, 59 men) with a mean age of 41 years were randomly assigned to receive CBT alone (n = 53), CBT+ tDCS (n = 48) or CBT + sham tDCS (n = 47).
Participants attended a 6-week group intervention of 12 sessions of CBT. If assigned, tDCS was applied simultaneously. Active tDCS included stimulation with an intensity of 2 milliamps for 30 minutes.
The study’s primary outcome was the change in Montgomery-Åsberg Depression Rating Scale (MADRS) from baseline to post treatment in the intention-to-treat sample. A total of 126 patients completed the study.
At baseline, the average MADRS score was 23.0. In each of the study groups, MADRS scores were reduced by a mean of 6.5 points (95% confidence interval, 3.82-9.14 points). The Cohen d value was -0.90 (95% CI, -1.43 to -0.50), indicating a significant effect over time, the researchers report. However, they add that “there was not significant effect of group and no significant interaction of group x time, indicating the estimated additive effects were not statistically significant.”
Results suggest that more research is needed to optimize treatment synchronization to achieve synergies between noninvasive brain stimulation and psychotherapeutic interventions.
Beauty and promise
Commenting on the findings, Mark George, MD, director of the Medical University of South Carolina Center for Advanced Imaging Research and the Brain Stimulation Laboratory, Charleston, described the study as “a really good effort by a great group of researchers.”
It’s unclear, he added, why tDCS failed to augment CBT. “It may be about the nongeneralizability of tDCS to complex functions, it may be that they didn’t get the dose right, or it might be due to a placebo response,” he speculated.
Furthermore, “tDCS is the most simple form of brain stimulation. The beauty and promise of tDCS is that it is so inexpensive and safe,” Dr. George added.
If proven effective, tDCS could potentially be used at home and rolled out as a frontline therapy for depression, he added. “Everybody wants the technology to work as an antidepressant, since it could have a very big positive public health impact,” said Dr. George.
Referring to previous research showing tDCS’ ability to improve specific brain functions in healthy controls, Dr. George noted that the potential of tDCS may be limited to augmenting specific brain functions such as memory but not more complex behaviors like depression.
However, Dr. George believes a more plausible explanation is that the optimal dose for tDCS has not yet been determined.
With other types of neuromodulation, such as electroconvulsive therapy, “we know that we’re in the brain with the right dose. But for tDCS, we don’t know that, and we’ve got to figure that out before it’s ever really going to make it [as a treatment],” he said.
“There have been great advances through the years in the field of brain stimulation and the treatment of depression. But rates of depression and suicide are continuing to grow, and we have not yet made a significant dent in treatment, in part because these technologies require equipment, [and] they’re expensive. So when we figure out tDCS, it will be a very important piece of our toolkit – a real game changer,” Dr. George added.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Neurology, psychiatry studies overlook sex as a variable
A large percentage of studies in neurology and psychiatry over the past decade have failed to account for differences between the sexes, according to a team of Canadian researchers.
“Despite the fact there are papers that are using males and females in the studies, they’re not using the males and females in the way that would optimally find the possibility of sex differences,” lead author Liisa A.M. Galea, PhD, told this news organization. Dr. Galea is a professor and distinguished scholar at the Djavad Mowafaghian Center for Brain Health at the University of British Columbia in Vancouver.
The study was published online in Nature Communications.
Optimal design uncommon
Differences in how neurologic and psychiatric diseases affect men and women have been well documented. Women, for example, are more susceptible to severe stroke, and men are more prone to cognitive decline with schizophrenia. With Alzheimer’s disease, women typically have more severe cognitive defects.
The researchers surveyed 3,193 papers that included a multitude of studies. Although most of the papers reported studies that included both sexes, only 19% of surveyed studies used what Dr. Galea called an optimal design for the discovery of sex differences. “What I mean by ‘optimally’ is the design of the experiments and the analysis of sex as a variable,” she said. And in 2019, only 5% of the studies used sex as a variable for determining differences between the sexes, the study found.
In the current research, two authors read the methods and results of each study described in each paper, Dr. Galea said. The readers noted whether the paper reported the study sample size and whether the studies used a balanced design. The surveyed journals include Nature Neuroscience, Neuron, Journal of Neuroscience, Molecular Psychiatry, Biological Psychiatry, and Neuropsychopharmacology.
‘Not much is changing’
“I had a suspicion that this was happening,” Dr. Galea said. “I didn’t know that it’s so bad, to be fair.” The “good news story,” she said, is that more papers considered sex as a factor in the later years surveyed. In 2019, more than 95% of papers across both disciplines reported participants’ sex, compared with about 70% in 2009. However, less than 20% of the papers in all study years reported studies that used sex optimally to determine differences between the sexes.
“The other thing that shocked me,” Dr. Galea said, “was that even despite the fact that we saw this increase in the number of papers that were using males and females, we didn’t see the sort of corresponding increase in those that were using ‘optimal design’ or ‘optimal analysis,’ ” Dr. Galea said. In 2009, 14% of papers used optimal design and 2% used optimal analysis for determining sex differences. By 2019, those percentages were 19% and 5%, respectively.
But even the papers that used both sexes had shortcomings, the study found. Just over one-third of these papers (34.5%) didn’t use a balanced design. Just over one-quarter (25.9%) didn’t identify the sample size, a shortcoming that marked 18% of these studies in 2009 and 33% in 2019. Fifteen percent of papers examined included studies that used both sexes inconsistently.
“That matters, because other studies have found that about 20% of papers are doing some kind of analysis with sex, but we had a suspicion that a lot of studies would include sex as a covariate,” Dr. Galea said. “Essentially what that does is, you remove that variable from the data. So, any statistical variation due to sex is then gone.
“The problem with that,” she added, “is you’re not actually looking to see if there’s an influence of sex; you’re removing it.”
Dr. Galea noted that this study points to a need for funding agencies to demand that researchers meet their mandates on sex- and gender-based analysis. “Despite the mandates, not much is really changing as far as the analysis or design of experiments, and we need to figure out how to change that,” she said. “We need to figure out how to get researchers more interested to use the power of studying sex differences.”
‘Not surprising, but disappointing’
Vladimir Hachinski, MD, professor of neurology and epidemiology at Western University in London, Ont., and former editor in chief of Stroke, told this news organization that women have almost twice the life risk of developing dementia, are at higher risk of stroke below age 35 years, and have more severe strokes and higher rates of disability at any age.
Commenting on the current study, Dr. Hachinski said, “It’s not surprising, but it’s disappointing, because we’ve known the difference for a long time.” He added, “The paper is very important because we were not aware that it was that bad.”
Dr. Hachinski also stated, “This paper needs a lot of reading. It’s a great resource, and it should be highlighted as one of those things that needs to be addressed, because it matters.”
The study was funded by a Natural Sciences and Engineering Research Council of Canada grant and by the British Columbia Women’s Foundation. Dr. Galea and Hachinski had no relevant disclosures.
A version of this article first appeared on Medscape.com.
A large percentage of studies in neurology and psychiatry over the past decade have failed to account for differences between the sexes, according to a team of Canadian researchers.
“Despite the fact there are papers that are using males and females in the studies, they’re not using the males and females in the way that would optimally find the possibility of sex differences,” lead author Liisa A.M. Galea, PhD, told this news organization. Dr. Galea is a professor and distinguished scholar at the Djavad Mowafaghian Center for Brain Health at the University of British Columbia in Vancouver.
The study was published online in Nature Communications.
Optimal design uncommon
Differences in how neurologic and psychiatric diseases affect men and women have been well documented. Women, for example, are more susceptible to severe stroke, and men are more prone to cognitive decline with schizophrenia. With Alzheimer’s disease, women typically have more severe cognitive defects.
The researchers surveyed 3,193 papers that included a multitude of studies. Although most of the papers reported studies that included both sexes, only 19% of surveyed studies used what Dr. Galea called an optimal design for the discovery of sex differences. “What I mean by ‘optimally’ is the design of the experiments and the analysis of sex as a variable,” she said. And in 2019, only 5% of the studies used sex as a variable for determining differences between the sexes, the study found.
In the current research, two authors read the methods and results of each study described in each paper, Dr. Galea said. The readers noted whether the paper reported the study sample size and whether the studies used a balanced design. The surveyed journals include Nature Neuroscience, Neuron, Journal of Neuroscience, Molecular Psychiatry, Biological Psychiatry, and Neuropsychopharmacology.
‘Not much is changing’
“I had a suspicion that this was happening,” Dr. Galea said. “I didn’t know that it’s so bad, to be fair.” The “good news story,” she said, is that more papers considered sex as a factor in the later years surveyed. In 2019, more than 95% of papers across both disciplines reported participants’ sex, compared with about 70% in 2009. However, less than 20% of the papers in all study years reported studies that used sex optimally to determine differences between the sexes.
“The other thing that shocked me,” Dr. Galea said, “was that even despite the fact that we saw this increase in the number of papers that were using males and females, we didn’t see the sort of corresponding increase in those that were using ‘optimal design’ or ‘optimal analysis,’ ” Dr. Galea said. In 2009, 14% of papers used optimal design and 2% used optimal analysis for determining sex differences. By 2019, those percentages were 19% and 5%, respectively.
But even the papers that used both sexes had shortcomings, the study found. Just over one-third of these papers (34.5%) didn’t use a balanced design. Just over one-quarter (25.9%) didn’t identify the sample size, a shortcoming that marked 18% of these studies in 2009 and 33% in 2019. Fifteen percent of papers examined included studies that used both sexes inconsistently.
“That matters, because other studies have found that about 20% of papers are doing some kind of analysis with sex, but we had a suspicion that a lot of studies would include sex as a covariate,” Dr. Galea said. “Essentially what that does is, you remove that variable from the data. So, any statistical variation due to sex is then gone.
“The problem with that,” she added, “is you’re not actually looking to see if there’s an influence of sex; you’re removing it.”
Dr. Galea noted that this study points to a need for funding agencies to demand that researchers meet their mandates on sex- and gender-based analysis. “Despite the mandates, not much is really changing as far as the analysis or design of experiments, and we need to figure out how to change that,” she said. “We need to figure out how to get researchers more interested to use the power of studying sex differences.”
‘Not surprising, but disappointing’
Vladimir Hachinski, MD, professor of neurology and epidemiology at Western University in London, Ont., and former editor in chief of Stroke, told this news organization that women have almost twice the life risk of developing dementia, are at higher risk of stroke below age 35 years, and have more severe strokes and higher rates of disability at any age.
Commenting on the current study, Dr. Hachinski said, “It’s not surprising, but it’s disappointing, because we’ve known the difference for a long time.” He added, “The paper is very important because we were not aware that it was that bad.”
Dr. Hachinski also stated, “This paper needs a lot of reading. It’s a great resource, and it should be highlighted as one of those things that needs to be addressed, because it matters.”
The study was funded by a Natural Sciences and Engineering Research Council of Canada grant and by the British Columbia Women’s Foundation. Dr. Galea and Hachinski had no relevant disclosures.
A version of this article first appeared on Medscape.com.
A large percentage of studies in neurology and psychiatry over the past decade have failed to account for differences between the sexes, according to a team of Canadian researchers.
“Despite the fact there are papers that are using males and females in the studies, they’re not using the males and females in the way that would optimally find the possibility of sex differences,” lead author Liisa A.M. Galea, PhD, told this news organization. Dr. Galea is a professor and distinguished scholar at the Djavad Mowafaghian Center for Brain Health at the University of British Columbia in Vancouver.
The study was published online in Nature Communications.
Optimal design uncommon
Differences in how neurologic and psychiatric diseases affect men and women have been well documented. Women, for example, are more susceptible to severe stroke, and men are more prone to cognitive decline with schizophrenia. With Alzheimer’s disease, women typically have more severe cognitive defects.
The researchers surveyed 3,193 papers that included a multitude of studies. Although most of the papers reported studies that included both sexes, only 19% of surveyed studies used what Dr. Galea called an optimal design for the discovery of sex differences. “What I mean by ‘optimally’ is the design of the experiments and the analysis of sex as a variable,” she said. And in 2019, only 5% of the studies used sex as a variable for determining differences between the sexes, the study found.
In the current research, two authors read the methods and results of each study described in each paper, Dr. Galea said. The readers noted whether the paper reported the study sample size and whether the studies used a balanced design. The surveyed journals include Nature Neuroscience, Neuron, Journal of Neuroscience, Molecular Psychiatry, Biological Psychiatry, and Neuropsychopharmacology.
‘Not much is changing’
“I had a suspicion that this was happening,” Dr. Galea said. “I didn’t know that it’s so bad, to be fair.” The “good news story,” she said, is that more papers considered sex as a factor in the later years surveyed. In 2019, more than 95% of papers across both disciplines reported participants’ sex, compared with about 70% in 2009. However, less than 20% of the papers in all study years reported studies that used sex optimally to determine differences between the sexes.
“The other thing that shocked me,” Dr. Galea said, “was that even despite the fact that we saw this increase in the number of papers that were using males and females, we didn’t see the sort of corresponding increase in those that were using ‘optimal design’ or ‘optimal analysis,’ ” Dr. Galea said. In 2009, 14% of papers used optimal design and 2% used optimal analysis for determining sex differences. By 2019, those percentages were 19% and 5%, respectively.
But even the papers that used both sexes had shortcomings, the study found. Just over one-third of these papers (34.5%) didn’t use a balanced design. Just over one-quarter (25.9%) didn’t identify the sample size, a shortcoming that marked 18% of these studies in 2009 and 33% in 2019. Fifteen percent of papers examined included studies that used both sexes inconsistently.
“That matters, because other studies have found that about 20% of papers are doing some kind of analysis with sex, but we had a suspicion that a lot of studies would include sex as a covariate,” Dr. Galea said. “Essentially what that does is, you remove that variable from the data. So, any statistical variation due to sex is then gone.
“The problem with that,” she added, “is you’re not actually looking to see if there’s an influence of sex; you’re removing it.”
Dr. Galea noted that this study points to a need for funding agencies to demand that researchers meet their mandates on sex- and gender-based analysis. “Despite the mandates, not much is really changing as far as the analysis or design of experiments, and we need to figure out how to change that,” she said. “We need to figure out how to get researchers more interested to use the power of studying sex differences.”
‘Not surprising, but disappointing’
Vladimir Hachinski, MD, professor of neurology and epidemiology at Western University in London, Ont., and former editor in chief of Stroke, told this news organization that women have almost twice the life risk of developing dementia, are at higher risk of stroke below age 35 years, and have more severe strokes and higher rates of disability at any age.
Commenting on the current study, Dr. Hachinski said, “It’s not surprising, but it’s disappointing, because we’ve known the difference for a long time.” He added, “The paper is very important because we were not aware that it was that bad.”
Dr. Hachinski also stated, “This paper needs a lot of reading. It’s a great resource, and it should be highlighted as one of those things that needs to be addressed, because it matters.”
The study was funded by a Natural Sciences and Engineering Research Council of Canada grant and by the British Columbia Women’s Foundation. Dr. Galea and Hachinski had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Do psychotropic meds raise or lower COVID risk in psych patients?
Investigators found that second-generation antipsychotics were associated with a 48% lower risk of COVID-19, while valproic acid was associated with a 39% increased risk of the disease.
“Exposures to several psychotropic medications were associated with risk of COVID-19 infection among inpatients with serious mental illness; decreased risk was observed with the use of second generation antipsychotics, with paliperidone use associated with the largest effect size. Valproic acid use was associated with an increased risk of infection,” the investigators, led by Katlyn Nemani, MD, at NYU Langone Medical Center, New York, write.
The study was published online in JAMA Network Open.
Vulnerable population
Patients with serious mental illness are particularly vulnerable to COVID-19. Several psychotropic medications have been identified as potential therapeutic agents to prevent or treat COVID-19, but they have not been systematically studied in this patient population.
The researchers analyzed data from 1,958 adults who were continuously hospitalized with serious mental illness from March 8 to July 1, 2020. The mean age was 51.4 years, and 1,442 (74%) were men.
A total of 969 patients (49.5%) had laboratory-confirmed COVID-19 while hospitalized, and 38 (3.9%) died – a mortality rate four times higher than estimates from the general population in New York during the same time frame, the researchers note.
“This finding is consistent with prior studies that have found increased rates of infection in congregate settings and increased mortality after infection among patients with serious mental illness,” the investigators write.
The use of second-generation antipsychotic medications, as a class, was associated with a lower likelihood of COVID-19 (odds ratio, 0.62; 95% confidence interval, 0.45-0.86), while the use of mood stabilizers was associated with increased likelihood of infection (OR, 1.23; 95% CI, 1.03-1.47).
In a multivariable model of individual medications, use of the long-acting atypical antipsychotic paliperidone was associated with a lower odds of infection (OR, 0.59; 95% CI, 0.41-0.84), and use of valproic acid was associated with increased odds of infection (OR, 1.39; 95% CI, 1.10-1.76).
Valproic acid downregulates angiotensin-converting enzyme 2 in endothelial cells, which may impair immune function and contribute to poor outcomes for patients with COVID-19, the researchers say.
The use of clozapine was associated with reduced odds of COVID-related death (unadjusted OR, 0.25; 95% CI, 0.10-0.62; fully adjusted OR, 0.43; 95% CI, 0.17-1.12).
“Although there have been concerns about clozapine use during the pandemic as a risk factor for pneumonia and potential toxic effects during acute infection, clozapine use was not associated with an increased risk of COVID-19 infection or death in the present study. In fact, unadjusted estimates suggested a significant protective association,” the investigators write.
However, they note, data on clozapine and COVID-19 have been mixed.
Two prior studies of health record data showed an increased risk of COVID-19 associated with clozapine treatment, while a study that was limited to inpatients found a lower risk of infection and a lower risk of symptomatic disease in association with clozapine use.
The researchers also found a lower mortality risk in patients taking antidepressants; there were no COVID-related deaths among patients taking escitalopram, venlafaxine, bupropion, or fluvoxamine.
Although the association was not statistically significant, this observation is in line with larger studies that showed reduced risk of adverse outcomes associated with antidepressant use, the researchers note.
A matter of debate
In an accompanying commentary, Benedetta Vai, PhD, and Mario Gennaro Mazza, MD, with IRCCS San Raffaele Scientific Institute, Milan, point out that the link between psychopharmacologic compounds, in particular antipsychotics, and severe COVID-19 outcomes remains “a matter of debate, with inconsistent findings between studies.”
They note further research is needed to determine whether the protective role of second-generation antipsychotics on risk of COVID-19 is mediated by an immune effect or by the direct antiviral properties of these molecules.
The study had no specific funding. Dr. Nemani, Dr. Vai, and Dr. Mazza have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators found that second-generation antipsychotics were associated with a 48% lower risk of COVID-19, while valproic acid was associated with a 39% increased risk of the disease.
“Exposures to several psychotropic medications were associated with risk of COVID-19 infection among inpatients with serious mental illness; decreased risk was observed with the use of second generation antipsychotics, with paliperidone use associated with the largest effect size. Valproic acid use was associated with an increased risk of infection,” the investigators, led by Katlyn Nemani, MD, at NYU Langone Medical Center, New York, write.
The study was published online in JAMA Network Open.
Vulnerable population
Patients with serious mental illness are particularly vulnerable to COVID-19. Several psychotropic medications have been identified as potential therapeutic agents to prevent or treat COVID-19, but they have not been systematically studied in this patient population.
The researchers analyzed data from 1,958 adults who were continuously hospitalized with serious mental illness from March 8 to July 1, 2020. The mean age was 51.4 years, and 1,442 (74%) were men.
A total of 969 patients (49.5%) had laboratory-confirmed COVID-19 while hospitalized, and 38 (3.9%) died – a mortality rate four times higher than estimates from the general population in New York during the same time frame, the researchers note.
“This finding is consistent with prior studies that have found increased rates of infection in congregate settings and increased mortality after infection among patients with serious mental illness,” the investigators write.
The use of second-generation antipsychotic medications, as a class, was associated with a lower likelihood of COVID-19 (odds ratio, 0.62; 95% confidence interval, 0.45-0.86), while the use of mood stabilizers was associated with increased likelihood of infection (OR, 1.23; 95% CI, 1.03-1.47).
In a multivariable model of individual medications, use of the long-acting atypical antipsychotic paliperidone was associated with a lower odds of infection (OR, 0.59; 95% CI, 0.41-0.84), and use of valproic acid was associated with increased odds of infection (OR, 1.39; 95% CI, 1.10-1.76).
Valproic acid downregulates angiotensin-converting enzyme 2 in endothelial cells, which may impair immune function and contribute to poor outcomes for patients with COVID-19, the researchers say.
The use of clozapine was associated with reduced odds of COVID-related death (unadjusted OR, 0.25; 95% CI, 0.10-0.62; fully adjusted OR, 0.43; 95% CI, 0.17-1.12).
“Although there have been concerns about clozapine use during the pandemic as a risk factor for pneumonia and potential toxic effects during acute infection, clozapine use was not associated with an increased risk of COVID-19 infection or death in the present study. In fact, unadjusted estimates suggested a significant protective association,” the investigators write.
However, they note, data on clozapine and COVID-19 have been mixed.
Two prior studies of health record data showed an increased risk of COVID-19 associated with clozapine treatment, while a study that was limited to inpatients found a lower risk of infection and a lower risk of symptomatic disease in association with clozapine use.
The researchers also found a lower mortality risk in patients taking antidepressants; there were no COVID-related deaths among patients taking escitalopram, venlafaxine, bupropion, or fluvoxamine.
Although the association was not statistically significant, this observation is in line with larger studies that showed reduced risk of adverse outcomes associated with antidepressant use, the researchers note.
A matter of debate
In an accompanying commentary, Benedetta Vai, PhD, and Mario Gennaro Mazza, MD, with IRCCS San Raffaele Scientific Institute, Milan, point out that the link between psychopharmacologic compounds, in particular antipsychotics, and severe COVID-19 outcomes remains “a matter of debate, with inconsistent findings between studies.”
They note further research is needed to determine whether the protective role of second-generation antipsychotics on risk of COVID-19 is mediated by an immune effect or by the direct antiviral properties of these molecules.
The study had no specific funding. Dr. Nemani, Dr. Vai, and Dr. Mazza have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators found that second-generation antipsychotics were associated with a 48% lower risk of COVID-19, while valproic acid was associated with a 39% increased risk of the disease.
“Exposures to several psychotropic medications were associated with risk of COVID-19 infection among inpatients with serious mental illness; decreased risk was observed with the use of second generation antipsychotics, with paliperidone use associated with the largest effect size. Valproic acid use was associated with an increased risk of infection,” the investigators, led by Katlyn Nemani, MD, at NYU Langone Medical Center, New York, write.
The study was published online in JAMA Network Open.
Vulnerable population
Patients with serious mental illness are particularly vulnerable to COVID-19. Several psychotropic medications have been identified as potential therapeutic agents to prevent or treat COVID-19, but they have not been systematically studied in this patient population.
The researchers analyzed data from 1,958 adults who were continuously hospitalized with serious mental illness from March 8 to July 1, 2020. The mean age was 51.4 years, and 1,442 (74%) were men.
A total of 969 patients (49.5%) had laboratory-confirmed COVID-19 while hospitalized, and 38 (3.9%) died – a mortality rate four times higher than estimates from the general population in New York during the same time frame, the researchers note.
“This finding is consistent with prior studies that have found increased rates of infection in congregate settings and increased mortality after infection among patients with serious mental illness,” the investigators write.
The use of second-generation antipsychotic medications, as a class, was associated with a lower likelihood of COVID-19 (odds ratio, 0.62; 95% confidence interval, 0.45-0.86), while the use of mood stabilizers was associated with increased likelihood of infection (OR, 1.23; 95% CI, 1.03-1.47).
In a multivariable model of individual medications, use of the long-acting atypical antipsychotic paliperidone was associated with a lower odds of infection (OR, 0.59; 95% CI, 0.41-0.84), and use of valproic acid was associated with increased odds of infection (OR, 1.39; 95% CI, 1.10-1.76).
Valproic acid downregulates angiotensin-converting enzyme 2 in endothelial cells, which may impair immune function and contribute to poor outcomes for patients with COVID-19, the researchers say.
The use of clozapine was associated with reduced odds of COVID-related death (unadjusted OR, 0.25; 95% CI, 0.10-0.62; fully adjusted OR, 0.43; 95% CI, 0.17-1.12).
“Although there have been concerns about clozapine use during the pandemic as a risk factor for pneumonia and potential toxic effects during acute infection, clozapine use was not associated with an increased risk of COVID-19 infection or death in the present study. In fact, unadjusted estimates suggested a significant protective association,” the investigators write.
However, they note, data on clozapine and COVID-19 have been mixed.
Two prior studies of health record data showed an increased risk of COVID-19 associated with clozapine treatment, while a study that was limited to inpatients found a lower risk of infection and a lower risk of symptomatic disease in association with clozapine use.
The researchers also found a lower mortality risk in patients taking antidepressants; there were no COVID-related deaths among patients taking escitalopram, venlafaxine, bupropion, or fluvoxamine.
Although the association was not statistically significant, this observation is in line with larger studies that showed reduced risk of adverse outcomes associated with antidepressant use, the researchers note.
A matter of debate
In an accompanying commentary, Benedetta Vai, PhD, and Mario Gennaro Mazza, MD, with IRCCS San Raffaele Scientific Institute, Milan, point out that the link between psychopharmacologic compounds, in particular antipsychotics, and severe COVID-19 outcomes remains “a matter of debate, with inconsistent findings between studies.”
They note further research is needed to determine whether the protective role of second-generation antipsychotics on risk of COVID-19 is mediated by an immune effect or by the direct antiviral properties of these molecules.
The study had no specific funding. Dr. Nemani, Dr. Vai, and Dr. Mazza have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
TikTok challenge hits Taco Bell right in its ‘Stuft Nacho’
Losing weight for TikTok: Taco Bell edition
There are many reasons why a person would want to lose weight. Too numerous to list. Losing weight to improve your health, however, doesn’t bring in a few hundred thousand TikTok subscribers. Losing weight to convince Taco Bell to bring back an obscure menu item, on the other hand ...
Chris Sandberg, a 37-year-old man from San Francisco, has struggled with his weight for years, losing and gaining hundreds of pounds in an endless cycle of feast and famine. In an unrelated development, at the start of the pandemic he also started making videos on TikTok. As the pandemic wore on, he realized that his excess weight put him at increased risk for severe COVID, as well as other chronic diseases, and he resolved to lose weight. He decided to turn his weight-loss journey into a TikTok challenge but, as we said, losing weight for its own sake isn’t enough for the almighty algorithm. He needed a different goal, preferably something offbeat and a little silly.
Back in 2013, Taco Bell introduced the Grilled Stuft Nacho, “a flour tortilla, shaped like a nacho, stuffed with beef, cheesy jalapeño sauce, sour cream and crunchy red strips,” according to its website. Mr. Sandberg discovered the item in 2015 and instantly fell in love, purchasing one every day for a week. After that first week, however, he discovered, to his horror, that the Grilled Stuft Nacho had been discontinued.
That loss haunted him for years, until inspiration struck in 2021. He pledged to work out every day on TikTok until Taco Bell brought back the Grilled Stuft Nacho. A bit incongruous, exercising for notoriously unhealthy fast food, but that’s kind of the point. He began the challenge on Jan. 4, 2021, and has continued it every day since, nearly 500 days. Over that time, he’s lost 87 pounds (from 275 at the start to under 190) and currently has 450,000 TikTok subscribers.
A year into the challenge, a local Taco Bell made Mr. Sandberg his beloved Grilled Stuft Nacho, but since the challenge was to exercise until Taco Bell brings the item back to all its restaurants, not just for him, the great journey continues. And we admire him for it. In fact, he’s inspired us: We will write a LOTME every week until it receives a Pulitzer Prize. This is important journalism we do here. Don’t deny it!
Episode XIX: COVID strikes back
So what’s next for COVID? Is Disney going to turn it into a series? Can it support a spin-off? Did James Cameron really buy the movie rights? Can it compete against the NFL in the all-important 18-34 demographic? When are Star Wars characters going to get involved?
COVID’s motivations and negotiations are pretty much a mystery to us, but we can answer that last question. They already are involved. Well, one of them anyway.
The Chinese government has been enforcing a COVID lockdown in Shanghai for over a month now, but authorities had started letting people out of their homes for short periods of time. A recent push to bring down transmission, however, has made residents increasingly frustrated and argumentative, according to Reuters.
A now-unavailable video, which Reuters could not verify, surfaced on Chinese social media showing police in hazmat suits arguing with people who were being told that they were going to be quarantined because a neighbor had tested positive.
That’s when the Force kicks in, and this next bit comes directly from the Reuters report: “This is so that we can thoroughly remove any positive cases,” one of the officers is heard saying. “Stop asking me why, there is no why.”
There is no why? Does that remind you of someone? Someone short and green, with an odd syntax? That’s right. Clearly, Yoda it is. Yoda is alive and working for the Chinese government in Shanghai. You read it here first.
Your coffee may be guilty of sexual discrimination
How do you take your coffee? Espresso, drip, instant, or brewed from a regular old coffee machine? Well, a recent study published in Open Heart suggests that gender and brewing method can alter your coffee’s effect on cholesterol levels.
Besides caffeine, coffee beans have naturally occurring chemicals such as diterpenes, cafestol, and kahweol that raise cholesterol levels in the blood. And then there are the various brewing methods, which are going to release different amounts of chemicals from the beans. According to Consumer Reports, an ounce of espresso has 63 mg of caffeine and an ounce of regular coffee has 12-16 mg. That’s a bit deceiving, though, since no one ever drinks an ounce of regular coffee, so figure 96-128 mg of caffeine for an 8-ounce cup. That’s enough to make anyone’s heart race.
Data from 21,083 participants in the seventh survey of the Tromsø Study who were aged 40 and older showed that women drank a mean of 3.8 cups per day while men drank 4.9 cups. Drinking six or more cups of plunger-brewed coffee was associated with increased cholesterol in both genders, but drinking three to five cups of espresso was significantly associated with high cholesterol in men only. Having six or more cups of filtered coffee daily raised cholesterol in women, but instant coffee increased cholesterol levels in both genders, regardless of how many cups they drank.
People all over the planet drink coffee, some of us like our lives depend on it. Since “coffee is the most frequently consumed central stimulant worldwide,” the investigators said, “even small health effects can have considerable health consequences.”
We’ll drink to that.
Have you ever dreamed of having a clone?
When will science grace us with the ability to clone ourselves? It sounds like a dream come true. Our clones can do the stuff that we don’t want to do, like sit in on that 3-hour meeting or do our grocery shopping – really just all the boring stuff we don’t want to do.
In 1996, when a sheep named Dolly became the first mammal cloned successfully, people thought it was the start of an amazing cloning era, but, alas, we haven’t made it to cloning humans yet, as LiveScience discovered when it took a look at the subject.
The idea of cloning was quite exciting for science, as people looked forward to eradicating genetic diseases and birth defects. Research done in 1999, however, countered those hopes by suggesting that cloning might increase birth defects.
So why do you think we haven’t advanced to truly cloning humans? Ethics? Time and effort? Technological barriers? “Human cloning is a particularly dramatic action, and was one of the topics that helped launch American bioethics,” Hank Greely, professor of law and genetics at Stanford (Calif.) University, told LiveScience.
What if the clones turned evil and were bent on destroying the world?
We might imagine a clone of ourselves being completely identical to us in our thoughts, actions, and physical looks. However, that’s not necessarily true; a clone would be its own person even if it looks exactly like you.
So what do the professionals think? Is it worth giving human cloning a shot? Are there benefits? Mr. Greely said that “there are none that we should be willing to consider.”
The dream of having a clone to help your son with his math homework may have gone down the drain, but maybe it’s best not to open doors that could lead to drastic changes in our world.
Losing weight for TikTok: Taco Bell edition
There are many reasons why a person would want to lose weight. Too numerous to list. Losing weight to improve your health, however, doesn’t bring in a few hundred thousand TikTok subscribers. Losing weight to convince Taco Bell to bring back an obscure menu item, on the other hand ...
Chris Sandberg, a 37-year-old man from San Francisco, has struggled with his weight for years, losing and gaining hundreds of pounds in an endless cycle of feast and famine. In an unrelated development, at the start of the pandemic he also started making videos on TikTok. As the pandemic wore on, he realized that his excess weight put him at increased risk for severe COVID, as well as other chronic diseases, and he resolved to lose weight. He decided to turn his weight-loss journey into a TikTok challenge but, as we said, losing weight for its own sake isn’t enough for the almighty algorithm. He needed a different goal, preferably something offbeat and a little silly.
Back in 2013, Taco Bell introduced the Grilled Stuft Nacho, “a flour tortilla, shaped like a nacho, stuffed with beef, cheesy jalapeño sauce, sour cream and crunchy red strips,” according to its website. Mr. Sandberg discovered the item in 2015 and instantly fell in love, purchasing one every day for a week. After that first week, however, he discovered, to his horror, that the Grilled Stuft Nacho had been discontinued.
That loss haunted him for years, until inspiration struck in 2021. He pledged to work out every day on TikTok until Taco Bell brought back the Grilled Stuft Nacho. A bit incongruous, exercising for notoriously unhealthy fast food, but that’s kind of the point. He began the challenge on Jan. 4, 2021, and has continued it every day since, nearly 500 days. Over that time, he’s lost 87 pounds (from 275 at the start to under 190) and currently has 450,000 TikTok subscribers.
A year into the challenge, a local Taco Bell made Mr. Sandberg his beloved Grilled Stuft Nacho, but since the challenge was to exercise until Taco Bell brings the item back to all its restaurants, not just for him, the great journey continues. And we admire him for it. In fact, he’s inspired us: We will write a LOTME every week until it receives a Pulitzer Prize. This is important journalism we do here. Don’t deny it!
Episode XIX: COVID strikes back
So what’s next for COVID? Is Disney going to turn it into a series? Can it support a spin-off? Did James Cameron really buy the movie rights? Can it compete against the NFL in the all-important 18-34 demographic? When are Star Wars characters going to get involved?
COVID’s motivations and negotiations are pretty much a mystery to us, but we can answer that last question. They already are involved. Well, one of them anyway.
The Chinese government has been enforcing a COVID lockdown in Shanghai for over a month now, but authorities had started letting people out of their homes for short periods of time. A recent push to bring down transmission, however, has made residents increasingly frustrated and argumentative, according to Reuters.
A now-unavailable video, which Reuters could not verify, surfaced on Chinese social media showing police in hazmat suits arguing with people who were being told that they were going to be quarantined because a neighbor had tested positive.
That’s when the Force kicks in, and this next bit comes directly from the Reuters report: “This is so that we can thoroughly remove any positive cases,” one of the officers is heard saying. “Stop asking me why, there is no why.”
There is no why? Does that remind you of someone? Someone short and green, with an odd syntax? That’s right. Clearly, Yoda it is. Yoda is alive and working for the Chinese government in Shanghai. You read it here first.
Your coffee may be guilty of sexual discrimination
How do you take your coffee? Espresso, drip, instant, or brewed from a regular old coffee machine? Well, a recent study published in Open Heart suggests that gender and brewing method can alter your coffee’s effect on cholesterol levels.
Besides caffeine, coffee beans have naturally occurring chemicals such as diterpenes, cafestol, and kahweol that raise cholesterol levels in the blood. And then there are the various brewing methods, which are going to release different amounts of chemicals from the beans. According to Consumer Reports, an ounce of espresso has 63 mg of caffeine and an ounce of regular coffee has 12-16 mg. That’s a bit deceiving, though, since no one ever drinks an ounce of regular coffee, so figure 96-128 mg of caffeine for an 8-ounce cup. That’s enough to make anyone’s heart race.
Data from 21,083 participants in the seventh survey of the Tromsø Study who were aged 40 and older showed that women drank a mean of 3.8 cups per day while men drank 4.9 cups. Drinking six or more cups of plunger-brewed coffee was associated with increased cholesterol in both genders, but drinking three to five cups of espresso was significantly associated with high cholesterol in men only. Having six or more cups of filtered coffee daily raised cholesterol in women, but instant coffee increased cholesterol levels in both genders, regardless of how many cups they drank.
People all over the planet drink coffee, some of us like our lives depend on it. Since “coffee is the most frequently consumed central stimulant worldwide,” the investigators said, “even small health effects can have considerable health consequences.”
We’ll drink to that.
Have you ever dreamed of having a clone?
When will science grace us with the ability to clone ourselves? It sounds like a dream come true. Our clones can do the stuff that we don’t want to do, like sit in on that 3-hour meeting or do our grocery shopping – really just all the boring stuff we don’t want to do.
In 1996, when a sheep named Dolly became the first mammal cloned successfully, people thought it was the start of an amazing cloning era, but, alas, we haven’t made it to cloning humans yet, as LiveScience discovered when it took a look at the subject.
The idea of cloning was quite exciting for science, as people looked forward to eradicating genetic diseases and birth defects. Research done in 1999, however, countered those hopes by suggesting that cloning might increase birth defects.
So why do you think we haven’t advanced to truly cloning humans? Ethics? Time and effort? Technological barriers? “Human cloning is a particularly dramatic action, and was one of the topics that helped launch American bioethics,” Hank Greely, professor of law and genetics at Stanford (Calif.) University, told LiveScience.
What if the clones turned evil and were bent on destroying the world?
We might imagine a clone of ourselves being completely identical to us in our thoughts, actions, and physical looks. However, that’s not necessarily true; a clone would be its own person even if it looks exactly like you.
So what do the professionals think? Is it worth giving human cloning a shot? Are there benefits? Mr. Greely said that “there are none that we should be willing to consider.”
The dream of having a clone to help your son with his math homework may have gone down the drain, but maybe it’s best not to open doors that could lead to drastic changes in our world.
Losing weight for TikTok: Taco Bell edition
There are many reasons why a person would want to lose weight. Too numerous to list. Losing weight to improve your health, however, doesn’t bring in a few hundred thousand TikTok subscribers. Losing weight to convince Taco Bell to bring back an obscure menu item, on the other hand ...
Chris Sandberg, a 37-year-old man from San Francisco, has struggled with his weight for years, losing and gaining hundreds of pounds in an endless cycle of feast and famine. In an unrelated development, at the start of the pandemic he also started making videos on TikTok. As the pandemic wore on, he realized that his excess weight put him at increased risk for severe COVID, as well as other chronic diseases, and he resolved to lose weight. He decided to turn his weight-loss journey into a TikTok challenge but, as we said, losing weight for its own sake isn’t enough for the almighty algorithm. He needed a different goal, preferably something offbeat and a little silly.
Back in 2013, Taco Bell introduced the Grilled Stuft Nacho, “a flour tortilla, shaped like a nacho, stuffed with beef, cheesy jalapeño sauce, sour cream and crunchy red strips,” according to its website. Mr. Sandberg discovered the item in 2015 and instantly fell in love, purchasing one every day for a week. After that first week, however, he discovered, to his horror, that the Grilled Stuft Nacho had been discontinued.
That loss haunted him for years, until inspiration struck in 2021. He pledged to work out every day on TikTok until Taco Bell brought back the Grilled Stuft Nacho. A bit incongruous, exercising for notoriously unhealthy fast food, but that’s kind of the point. He began the challenge on Jan. 4, 2021, and has continued it every day since, nearly 500 days. Over that time, he’s lost 87 pounds (from 275 at the start to under 190) and currently has 450,000 TikTok subscribers.
A year into the challenge, a local Taco Bell made Mr. Sandberg his beloved Grilled Stuft Nacho, but since the challenge was to exercise until Taco Bell brings the item back to all its restaurants, not just for him, the great journey continues. And we admire him for it. In fact, he’s inspired us: We will write a LOTME every week until it receives a Pulitzer Prize. This is important journalism we do here. Don’t deny it!
Episode XIX: COVID strikes back
So what’s next for COVID? Is Disney going to turn it into a series? Can it support a spin-off? Did James Cameron really buy the movie rights? Can it compete against the NFL in the all-important 18-34 demographic? When are Star Wars characters going to get involved?
COVID’s motivations and negotiations are pretty much a mystery to us, but we can answer that last question. They already are involved. Well, one of them anyway.
The Chinese government has been enforcing a COVID lockdown in Shanghai for over a month now, but authorities had started letting people out of their homes for short periods of time. A recent push to bring down transmission, however, has made residents increasingly frustrated and argumentative, according to Reuters.
A now-unavailable video, which Reuters could not verify, surfaced on Chinese social media showing police in hazmat suits arguing with people who were being told that they were going to be quarantined because a neighbor had tested positive.
That’s when the Force kicks in, and this next bit comes directly from the Reuters report: “This is so that we can thoroughly remove any positive cases,” one of the officers is heard saying. “Stop asking me why, there is no why.”
There is no why? Does that remind you of someone? Someone short and green, with an odd syntax? That’s right. Clearly, Yoda it is. Yoda is alive and working for the Chinese government in Shanghai. You read it here first.
Your coffee may be guilty of sexual discrimination
How do you take your coffee? Espresso, drip, instant, or brewed from a regular old coffee machine? Well, a recent study published in Open Heart suggests that gender and brewing method can alter your coffee’s effect on cholesterol levels.
Besides caffeine, coffee beans have naturally occurring chemicals such as diterpenes, cafestol, and kahweol that raise cholesterol levels in the blood. And then there are the various brewing methods, which are going to release different amounts of chemicals from the beans. According to Consumer Reports, an ounce of espresso has 63 mg of caffeine and an ounce of regular coffee has 12-16 mg. That’s a bit deceiving, though, since no one ever drinks an ounce of regular coffee, so figure 96-128 mg of caffeine for an 8-ounce cup. That’s enough to make anyone’s heart race.
Data from 21,083 participants in the seventh survey of the Tromsø Study who were aged 40 and older showed that women drank a mean of 3.8 cups per day while men drank 4.9 cups. Drinking six or more cups of plunger-brewed coffee was associated with increased cholesterol in both genders, but drinking three to five cups of espresso was significantly associated with high cholesterol in men only. Having six or more cups of filtered coffee daily raised cholesterol in women, but instant coffee increased cholesterol levels in both genders, regardless of how many cups they drank.
People all over the planet drink coffee, some of us like our lives depend on it. Since “coffee is the most frequently consumed central stimulant worldwide,” the investigators said, “even small health effects can have considerable health consequences.”
We’ll drink to that.
Have you ever dreamed of having a clone?
When will science grace us with the ability to clone ourselves? It sounds like a dream come true. Our clones can do the stuff that we don’t want to do, like sit in on that 3-hour meeting or do our grocery shopping – really just all the boring stuff we don’t want to do.
In 1996, when a sheep named Dolly became the first mammal cloned successfully, people thought it was the start of an amazing cloning era, but, alas, we haven’t made it to cloning humans yet, as LiveScience discovered when it took a look at the subject.
The idea of cloning was quite exciting for science, as people looked forward to eradicating genetic diseases and birth defects. Research done in 1999, however, countered those hopes by suggesting that cloning might increase birth defects.
So why do you think we haven’t advanced to truly cloning humans? Ethics? Time and effort? Technological barriers? “Human cloning is a particularly dramatic action, and was one of the topics that helped launch American bioethics,” Hank Greely, professor of law and genetics at Stanford (Calif.) University, told LiveScience.
What if the clones turned evil and were bent on destroying the world?
We might imagine a clone of ourselves being completely identical to us in our thoughts, actions, and physical looks. However, that’s not necessarily true; a clone would be its own person even if it looks exactly like you.
So what do the professionals think? Is it worth giving human cloning a shot? Are there benefits? Mr. Greely said that “there are none that we should be willing to consider.”
The dream of having a clone to help your son with his math homework may have gone down the drain, but maybe it’s best not to open doors that could lead to drastic changes in our world.
Recommendations for improving federal diabetes programs: How primary care clinicians can help with implementation
Recently the National Clinical Care Commission provided recommendations to Congress for improving federal diabetes programs in a report. This commission was put together after Congress passed the National Clinical Care Commission Act in 2017.
The report provides a wide range of recommendations that look to combat and prevent diabetes at many levels. An exciting aspect of the recommendations is that they consider how all agencies, including those that are not specifically health care, can fight diabetes.
The report acknowledges that many recent advances in diabetes treatments have made huge differences for clinicians and patients alike. Unfortunately, they have not been translated quickly into practice and when they have been, there have been disparities in the rollouts.
The document also states that many other factors, including housing, health care access, and food access, greatly affect the prevention and control of diabetes, according to a paper published in Annals of Internal Medicine. These factors have led to significant disparities in the population impacted by diabetes.
The topic areas of the recommendations include federal programs and policies; population-level programs to prevent diabetes, facilitate treatments, and promote health equity; type 2 diabetes prevention; insurance coverage; diabetes care delivery; and diabetes research.
Supporting recommendations in clinics
Family physicians, internists, and pediatricians can directly support many of the recommendations in their clinics. For those recommendations that are not directed at primary care clinics specifically, physicians should provide advocacy for their implementation.
If implemented, some of these recommendations will allow primary care physicians to improve at providing treatments to their patients for diabetes prevention and treatment of the disease. For example, the recommendations call for requirements of insurance companies to cover screening for prediabetes with the use of hemoglobin A1c and the participation in Centers for Disease Control and Prevention–recognized diabetes prevention programs.
The recommendations also call for the requirement of high-value diabetes services and treatment to be covered predeductible by insurers. If more consistently covered by insurers, it would be easier for us to implement these opportunities including educational groups in our practices. Additionally, if they were available predeductible, we could recommend these to our patients with less worry about cost.
Within care delivery recommendations, they also highlight the importance of an adequate and sustainable team to enhance care for patients with diabetes. Many of us know that it takes more than just the medications, but also significant counseling on diet, exercise and other lifestyle aspects – which need to be tailored to each patient for both prevention and treatment of diabetes.
The recommendations also call for the education and treatment modalities to be able to be provided and covered via virtual methods, while potentially increasing physicians’ ability to provide and patients’ ability to access. Ensuring both the workforce is available and that insurance provides coverage would make these programs accessible to so many more physician offices and ultimately patients.
Importance of social factors
As stated earlier, one of the great aspects of this report is its acknowledgment of the importance of social factors on the prevention and treatment of diabetes.
The report recommends expanding housing opportunities for low-income individuals as individuals cannot focus on their health when worried about housing. It also recommends increasing assistance with programs focused on food security. Primary care physicians should advocate for the adoption of these and other recommendations, because of the potentially meaningful impact these changes could have.
Ensuring adequate housing and access to healthy food would go a long way in the prevention and treatment of diabetes. If there are increases in these resources, team members within primary care physician offices would be wonderful allies to help direct patients to these resources. As these concerns may be top of mind for some patients, linking patients to these resources in the physician’s office may reinforce for patients that physicians understand our patients’ biggest concerns.
Ultimately, if the sweeping recommendations in this report are adopted and enforced, it could mean significant improvements for many patients at risk for and living with diabetes. They would provide payment for these resources making them more accessible for patients and physicians alike.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
Recently the National Clinical Care Commission provided recommendations to Congress for improving federal diabetes programs in a report. This commission was put together after Congress passed the National Clinical Care Commission Act in 2017.
The report provides a wide range of recommendations that look to combat and prevent diabetes at many levels. An exciting aspect of the recommendations is that they consider how all agencies, including those that are not specifically health care, can fight diabetes.
The report acknowledges that many recent advances in diabetes treatments have made huge differences for clinicians and patients alike. Unfortunately, they have not been translated quickly into practice and when they have been, there have been disparities in the rollouts.
The document also states that many other factors, including housing, health care access, and food access, greatly affect the prevention and control of diabetes, according to a paper published in Annals of Internal Medicine. These factors have led to significant disparities in the population impacted by diabetes.
The topic areas of the recommendations include federal programs and policies; population-level programs to prevent diabetes, facilitate treatments, and promote health equity; type 2 diabetes prevention; insurance coverage; diabetes care delivery; and diabetes research.
Supporting recommendations in clinics
Family physicians, internists, and pediatricians can directly support many of the recommendations in their clinics. For those recommendations that are not directed at primary care clinics specifically, physicians should provide advocacy for their implementation.
If implemented, some of these recommendations will allow primary care physicians to improve at providing treatments to their patients for diabetes prevention and treatment of the disease. For example, the recommendations call for requirements of insurance companies to cover screening for prediabetes with the use of hemoglobin A1c and the participation in Centers for Disease Control and Prevention–recognized diabetes prevention programs.
The recommendations also call for the requirement of high-value diabetes services and treatment to be covered predeductible by insurers. If more consistently covered by insurers, it would be easier for us to implement these opportunities including educational groups in our practices. Additionally, if they were available predeductible, we could recommend these to our patients with less worry about cost.
Within care delivery recommendations, they also highlight the importance of an adequate and sustainable team to enhance care for patients with diabetes. Many of us know that it takes more than just the medications, but also significant counseling on diet, exercise and other lifestyle aspects – which need to be tailored to each patient for both prevention and treatment of diabetes.
The recommendations also call for the education and treatment modalities to be able to be provided and covered via virtual methods, while potentially increasing physicians’ ability to provide and patients’ ability to access. Ensuring both the workforce is available and that insurance provides coverage would make these programs accessible to so many more physician offices and ultimately patients.
Importance of social factors
As stated earlier, one of the great aspects of this report is its acknowledgment of the importance of social factors on the prevention and treatment of diabetes.
The report recommends expanding housing opportunities for low-income individuals as individuals cannot focus on their health when worried about housing. It also recommends increasing assistance with programs focused on food security. Primary care physicians should advocate for the adoption of these and other recommendations, because of the potentially meaningful impact these changes could have.
Ensuring adequate housing and access to healthy food would go a long way in the prevention and treatment of diabetes. If there are increases in these resources, team members within primary care physician offices would be wonderful allies to help direct patients to these resources. As these concerns may be top of mind for some patients, linking patients to these resources in the physician’s office may reinforce for patients that physicians understand our patients’ biggest concerns.
Ultimately, if the sweeping recommendations in this report are adopted and enforced, it could mean significant improvements for many patients at risk for and living with diabetes. They would provide payment for these resources making them more accessible for patients and physicians alike.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
Recently the National Clinical Care Commission provided recommendations to Congress for improving federal diabetes programs in a report. This commission was put together after Congress passed the National Clinical Care Commission Act in 2017.
The report provides a wide range of recommendations that look to combat and prevent diabetes at many levels. An exciting aspect of the recommendations is that they consider how all agencies, including those that are not specifically health care, can fight diabetes.
The report acknowledges that many recent advances in diabetes treatments have made huge differences for clinicians and patients alike. Unfortunately, they have not been translated quickly into practice and when they have been, there have been disparities in the rollouts.
The document also states that many other factors, including housing, health care access, and food access, greatly affect the prevention and control of diabetes, according to a paper published in Annals of Internal Medicine. These factors have led to significant disparities in the population impacted by diabetes.
The topic areas of the recommendations include federal programs and policies; population-level programs to prevent diabetes, facilitate treatments, and promote health equity; type 2 diabetes prevention; insurance coverage; diabetes care delivery; and diabetes research.
Supporting recommendations in clinics
Family physicians, internists, and pediatricians can directly support many of the recommendations in their clinics. For those recommendations that are not directed at primary care clinics specifically, physicians should provide advocacy for their implementation.
If implemented, some of these recommendations will allow primary care physicians to improve at providing treatments to their patients for diabetes prevention and treatment of the disease. For example, the recommendations call for requirements of insurance companies to cover screening for prediabetes with the use of hemoglobin A1c and the participation in Centers for Disease Control and Prevention–recognized diabetes prevention programs.
The recommendations also call for the requirement of high-value diabetes services and treatment to be covered predeductible by insurers. If more consistently covered by insurers, it would be easier for us to implement these opportunities including educational groups in our practices. Additionally, if they were available predeductible, we could recommend these to our patients with less worry about cost.
Within care delivery recommendations, they also highlight the importance of an adequate and sustainable team to enhance care for patients with diabetes. Many of us know that it takes more than just the medications, but also significant counseling on diet, exercise and other lifestyle aspects – which need to be tailored to each patient for both prevention and treatment of diabetes.
The recommendations also call for the education and treatment modalities to be able to be provided and covered via virtual methods, while potentially increasing physicians’ ability to provide and patients’ ability to access. Ensuring both the workforce is available and that insurance provides coverage would make these programs accessible to so many more physician offices and ultimately patients.
Importance of social factors
As stated earlier, one of the great aspects of this report is its acknowledgment of the importance of social factors on the prevention and treatment of diabetes.
The report recommends expanding housing opportunities for low-income individuals as individuals cannot focus on their health when worried about housing. It also recommends increasing assistance with programs focused on food security. Primary care physicians should advocate for the adoption of these and other recommendations, because of the potentially meaningful impact these changes could have.
Ensuring adequate housing and access to healthy food would go a long way in the prevention and treatment of diabetes. If there are increases in these resources, team members within primary care physician offices would be wonderful allies to help direct patients to these resources. As these concerns may be top of mind for some patients, linking patients to these resources in the physician’s office may reinforce for patients that physicians understand our patients’ biggest concerns.
Ultimately, if the sweeping recommendations in this report are adopted and enforced, it could mean significant improvements for many patients at risk for and living with diabetes. They would provide payment for these resources making them more accessible for patients and physicians alike.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
Smooth plaque on ankle
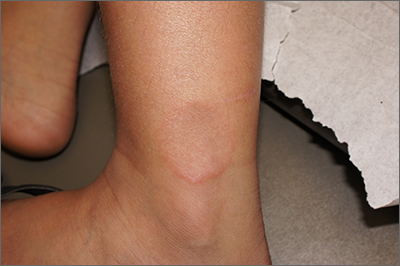
A 4-mm punch biopsy of the annular border confirmed a diagnosis of localized granuloma annulare (GA).
There is a long list of differential diagnoses for annular patches and plaques; it includes tinea corporis and important systemic diseases such as sarcoidosis and Lyme disease. Clinical features of GA include annular, minimally scaly patches to plaques with central clearing on extensor surfaces in children and adults. Sometimes GA is much more widespread. Often, the diagnosis can be made clinically, but a punch biopsy of the deep dermis will confirm the diagnosis by showing palisading or interstitial granulomatous inflammation, necrobiotic collagen, and often mucin.
GA is a common inflammatory disorder with an uncertain etiology. Localized GA affects children and adults and is often self limiting. It may, however, last for months or years before resolving. Disseminated disease is much more recalcitrant with few good treatment options if topical steroids or phototherapy fails. Treatment for localized disease is much more successful with topical or intralesional steroids.
Trauma can cause a localized plaque to resolve; a lesion may resolve soon after a biopsy is performed. Possible related conditions include diabetes, thyroid disease, hepatitis C, and hyperlipidemia; but there is no consensus on focused screening. Similarly, associations or nonassociations with malignancy in adults have been cited, but evidence is lacking.1
In this case, the patient and his family were reassured that the diagnosis wasn’t serious. In a single visit, he received a series of 6 to 7 injections of 10 mg/mL triamcinolone which led to resolution of the lesion in 4 weeks.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi: 10.1016/j.jaad.2015.03.055

A 4-mm punch biopsy of the annular border confirmed a diagnosis of localized granuloma annulare (GA).
There is a long list of differential diagnoses for annular patches and plaques; it includes tinea corporis and important systemic diseases such as sarcoidosis and Lyme disease. Clinical features of GA include annular, minimally scaly patches to plaques with central clearing on extensor surfaces in children and adults. Sometimes GA is much more widespread. Often, the diagnosis can be made clinically, but a punch biopsy of the deep dermis will confirm the diagnosis by showing palisading or interstitial granulomatous inflammation, necrobiotic collagen, and often mucin.
GA is a common inflammatory disorder with an uncertain etiology. Localized GA affects children and adults and is often self limiting. It may, however, last for months or years before resolving. Disseminated disease is much more recalcitrant with few good treatment options if topical steroids or phototherapy fails. Treatment for localized disease is much more successful with topical or intralesional steroids.
Trauma can cause a localized plaque to resolve; a lesion may resolve soon after a biopsy is performed. Possible related conditions include diabetes, thyroid disease, hepatitis C, and hyperlipidemia; but there is no consensus on focused screening. Similarly, associations or nonassociations with malignancy in adults have been cited, but evidence is lacking.1
In this case, the patient and his family were reassured that the diagnosis wasn’t serious. In a single visit, he received a series of 6 to 7 injections of 10 mg/mL triamcinolone which led to resolution of the lesion in 4 weeks.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

A 4-mm punch biopsy of the annular border confirmed a diagnosis of localized granuloma annulare (GA).
There is a long list of differential diagnoses for annular patches and plaques; it includes tinea corporis and important systemic diseases such as sarcoidosis and Lyme disease. Clinical features of GA include annular, minimally scaly patches to plaques with central clearing on extensor surfaces in children and adults. Sometimes GA is much more widespread. Often, the diagnosis can be made clinically, but a punch biopsy of the deep dermis will confirm the diagnosis by showing palisading or interstitial granulomatous inflammation, necrobiotic collagen, and often mucin.
GA is a common inflammatory disorder with an uncertain etiology. Localized GA affects children and adults and is often self limiting. It may, however, last for months or years before resolving. Disseminated disease is much more recalcitrant with few good treatment options if topical steroids or phototherapy fails. Treatment for localized disease is much more successful with topical or intralesional steroids.
Trauma can cause a localized plaque to resolve; a lesion may resolve soon after a biopsy is performed. Possible related conditions include diabetes, thyroid disease, hepatitis C, and hyperlipidemia; but there is no consensus on focused screening. Similarly, associations or nonassociations with malignancy in adults have been cited, but evidence is lacking.1
In this case, the patient and his family were reassured that the diagnosis wasn’t serious. In a single visit, he received a series of 6 to 7 injections of 10 mg/mL triamcinolone which led to resolution of the lesion in 4 weeks.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi: 10.1016/j.jaad.2015.03.055
1. Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi: 10.1016/j.jaad.2015.03.055