User login
Cold forceps on par with cold snare polypectomy for tiny polyps
For nonpedunculated polyps measuring 3 mm or less, cold forceps polypectomy is noninferior to cold snare polypectomy and takes significantly less time, according to the results of the TINYPOLYP trial.
“In our trial, which is the largest to date evaluating complete resection of polyps ≤ 3 mm using cold forceps versus cold snare, we demonstrate that it is acceptable to remove ≤ 3 mm polyps with either cold snare or cold forceps,” lead author Mike Wei, MD, a gastroenterology and hepatology fellow at Stanford University, California, told this news organization.
“Cold forceps can oftentimes be the more efficient way to remove polyps compared to cold snare, and, as such, it was important to provide validation for this practice,” Dr. Wei said.
The study was published online in The American Journal of Gastroenterology.
Evaluating two techniques
Both the U.S. Multi-Society Task Force on Colorectal Cancer and the European Society of Gastrointestinal Endoscopy recommend that diminutive (< 5 mm) and small (6-9 mm) polyps be removed by cold snare polypectomy (CSP).
But whether CSP has a significant advantage over cold forceps polypectomy (CFP) for polyps ≤ 3 mm was unclear.
The TINYPOLYP trial enrolled 179 adults aged 18 years and older who underwent colonoscopy for any indication; colonoscopy was performed by four board-certified endoscopists who each had at least 4 years of experience after completing their fellowship.
A total of 279 nonpedunculated polyps ≤ 3 mm were identified; 138 were removed by CSP, and 141 were removed by CFP. Patient and procedure characteristics were similar in the two groups.
The polyps were similar in size in the CSP and CFP groups (2.5 and 2.6 mm, respectively), as was the distribution of polyps (33.3% and 26.2% in the ascending colon; 26.8% and 24.8% in the transverse colon). A higher proportion of tubular adenomas were removed by CSP than by CFP (79.7% vs. 66.0%).
CSP took significantly longer to perform than CFP (42.3 sec vs. 23.2 sec, P < .001). But with CFP, it was significantly more likely that polyps would need to be removed in more than one piece, compared with CSP (15.6% vs. 3.6%, P < .001).
Hemostatic clip was deployed for one polyp in the CFP group (0.7%); none were used in the CSP group, which was a nonsignificant difference.
There was also no significant difference in positive margins on biopsy (two cases in each group; 1.7%) or in the rate of complete resection (98.3% in both groups), demonstrating noninferiority of CFP, compared with CSP, the study team says.
There were no 30-day complications in either group, including perforation, postpolypectomy bleeding, and postpolypectomy syndrome, and no patient required management of postpolypectomy bleeding. No patient died within 30 days of colonoscopy.
On the basis of their results, Dr. Wei and colleagues say, “When an endoscopist encounters a diminutive polyp ≤ 3 mm, either a cold forceps or cold snare can be utilized during the procedure.”
Guidance for endoscopists
Reached for comment, Emre Gorgun, MD, in the department of colorectal surgery at the Cleveland Clinic, Ohio, said this is an “interesting” study that attempts to pinpoint the “best endoscopic management of tiny polyps.”
“From previously published, well-designed studies, we know that the cold snare technique works very well for polyps up to 10 mm. There have been more recent studies showing that the cold snare technique can be used even in larger polyps, 10-15 mm,” Dr. Gorgun said in an interview.
On the other hand, for polyps < 5 mm, “cold snare technique may take longer and may not provide any added benefits,” he noted. “It may be associated with higher cost due to utilizing more tools, as well as more procedure time and provider services.”
Dr. Gorgun said that the results of the TINYPOLYP study “can help endoscopists in decisionmaking when they come across polyps smaller than 5 mm.”
The study demonstrates that these tiny polyps can “certainly be destroyed/removed by the cold forceps approach,” he added.
The trial had no specific funding. Dr. Wei reports no relevant financial relationships. Dr. Gorgun is a consultant for Boston Scientific, Olympus, and Dilumen.
A version of this article first appeared on Medscape.com.
For nonpedunculated polyps measuring 3 mm or less, cold forceps polypectomy is noninferior to cold snare polypectomy and takes significantly less time, according to the results of the TINYPOLYP trial.
“In our trial, which is the largest to date evaluating complete resection of polyps ≤ 3 mm using cold forceps versus cold snare, we demonstrate that it is acceptable to remove ≤ 3 mm polyps with either cold snare or cold forceps,” lead author Mike Wei, MD, a gastroenterology and hepatology fellow at Stanford University, California, told this news organization.
“Cold forceps can oftentimes be the more efficient way to remove polyps compared to cold snare, and, as such, it was important to provide validation for this practice,” Dr. Wei said.
The study was published online in The American Journal of Gastroenterology.
Evaluating two techniques
Both the U.S. Multi-Society Task Force on Colorectal Cancer and the European Society of Gastrointestinal Endoscopy recommend that diminutive (< 5 mm) and small (6-9 mm) polyps be removed by cold snare polypectomy (CSP).
But whether CSP has a significant advantage over cold forceps polypectomy (CFP) for polyps ≤ 3 mm was unclear.
The TINYPOLYP trial enrolled 179 adults aged 18 years and older who underwent colonoscopy for any indication; colonoscopy was performed by four board-certified endoscopists who each had at least 4 years of experience after completing their fellowship.
A total of 279 nonpedunculated polyps ≤ 3 mm were identified; 138 were removed by CSP, and 141 were removed by CFP. Patient and procedure characteristics were similar in the two groups.
The polyps were similar in size in the CSP and CFP groups (2.5 and 2.6 mm, respectively), as was the distribution of polyps (33.3% and 26.2% in the ascending colon; 26.8% and 24.8% in the transverse colon). A higher proportion of tubular adenomas were removed by CSP than by CFP (79.7% vs. 66.0%).
CSP took significantly longer to perform than CFP (42.3 sec vs. 23.2 sec, P < .001). But with CFP, it was significantly more likely that polyps would need to be removed in more than one piece, compared with CSP (15.6% vs. 3.6%, P < .001).
Hemostatic clip was deployed for one polyp in the CFP group (0.7%); none were used in the CSP group, which was a nonsignificant difference.
There was also no significant difference in positive margins on biopsy (two cases in each group; 1.7%) or in the rate of complete resection (98.3% in both groups), demonstrating noninferiority of CFP, compared with CSP, the study team says.
There were no 30-day complications in either group, including perforation, postpolypectomy bleeding, and postpolypectomy syndrome, and no patient required management of postpolypectomy bleeding. No patient died within 30 days of colonoscopy.
On the basis of their results, Dr. Wei and colleagues say, “When an endoscopist encounters a diminutive polyp ≤ 3 mm, either a cold forceps or cold snare can be utilized during the procedure.”
Guidance for endoscopists
Reached for comment, Emre Gorgun, MD, in the department of colorectal surgery at the Cleveland Clinic, Ohio, said this is an “interesting” study that attempts to pinpoint the “best endoscopic management of tiny polyps.”
“From previously published, well-designed studies, we know that the cold snare technique works very well for polyps up to 10 mm. There have been more recent studies showing that the cold snare technique can be used even in larger polyps, 10-15 mm,” Dr. Gorgun said in an interview.
On the other hand, for polyps < 5 mm, “cold snare technique may take longer and may not provide any added benefits,” he noted. “It may be associated with higher cost due to utilizing more tools, as well as more procedure time and provider services.”
Dr. Gorgun said that the results of the TINYPOLYP study “can help endoscopists in decisionmaking when they come across polyps smaller than 5 mm.”
The study demonstrates that these tiny polyps can “certainly be destroyed/removed by the cold forceps approach,” he added.
The trial had no specific funding. Dr. Wei reports no relevant financial relationships. Dr. Gorgun is a consultant for Boston Scientific, Olympus, and Dilumen.
A version of this article first appeared on Medscape.com.
For nonpedunculated polyps measuring 3 mm or less, cold forceps polypectomy is noninferior to cold snare polypectomy and takes significantly less time, according to the results of the TINYPOLYP trial.
“In our trial, which is the largest to date evaluating complete resection of polyps ≤ 3 mm using cold forceps versus cold snare, we demonstrate that it is acceptable to remove ≤ 3 mm polyps with either cold snare or cold forceps,” lead author Mike Wei, MD, a gastroenterology and hepatology fellow at Stanford University, California, told this news organization.
“Cold forceps can oftentimes be the more efficient way to remove polyps compared to cold snare, and, as such, it was important to provide validation for this practice,” Dr. Wei said.
The study was published online in The American Journal of Gastroenterology.
Evaluating two techniques
Both the U.S. Multi-Society Task Force on Colorectal Cancer and the European Society of Gastrointestinal Endoscopy recommend that diminutive (< 5 mm) and small (6-9 mm) polyps be removed by cold snare polypectomy (CSP).
But whether CSP has a significant advantage over cold forceps polypectomy (CFP) for polyps ≤ 3 mm was unclear.
The TINYPOLYP trial enrolled 179 adults aged 18 years and older who underwent colonoscopy for any indication; colonoscopy was performed by four board-certified endoscopists who each had at least 4 years of experience after completing their fellowship.
A total of 279 nonpedunculated polyps ≤ 3 mm were identified; 138 were removed by CSP, and 141 were removed by CFP. Patient and procedure characteristics were similar in the two groups.
The polyps were similar in size in the CSP and CFP groups (2.5 and 2.6 mm, respectively), as was the distribution of polyps (33.3% and 26.2% in the ascending colon; 26.8% and 24.8% in the transverse colon). A higher proportion of tubular adenomas were removed by CSP than by CFP (79.7% vs. 66.0%).
CSP took significantly longer to perform than CFP (42.3 sec vs. 23.2 sec, P < .001). But with CFP, it was significantly more likely that polyps would need to be removed in more than one piece, compared with CSP (15.6% vs. 3.6%, P < .001).
Hemostatic clip was deployed for one polyp in the CFP group (0.7%); none were used in the CSP group, which was a nonsignificant difference.
There was also no significant difference in positive margins on biopsy (two cases in each group; 1.7%) or in the rate of complete resection (98.3% in both groups), demonstrating noninferiority of CFP, compared with CSP, the study team says.
There were no 30-day complications in either group, including perforation, postpolypectomy bleeding, and postpolypectomy syndrome, and no patient required management of postpolypectomy bleeding. No patient died within 30 days of colonoscopy.
On the basis of their results, Dr. Wei and colleagues say, “When an endoscopist encounters a diminutive polyp ≤ 3 mm, either a cold forceps or cold snare can be utilized during the procedure.”
Guidance for endoscopists
Reached for comment, Emre Gorgun, MD, in the department of colorectal surgery at the Cleveland Clinic, Ohio, said this is an “interesting” study that attempts to pinpoint the “best endoscopic management of tiny polyps.”
“From previously published, well-designed studies, we know that the cold snare technique works very well for polyps up to 10 mm. There have been more recent studies showing that the cold snare technique can be used even in larger polyps, 10-15 mm,” Dr. Gorgun said in an interview.
On the other hand, for polyps < 5 mm, “cold snare technique may take longer and may not provide any added benefits,” he noted. “It may be associated with higher cost due to utilizing more tools, as well as more procedure time and provider services.”
Dr. Gorgun said that the results of the TINYPOLYP study “can help endoscopists in decisionmaking when they come across polyps smaller than 5 mm.”
The study demonstrates that these tiny polyps can “certainly be destroyed/removed by the cold forceps approach,” he added.
The trial had no specific funding. Dr. Wei reports no relevant financial relationships. Dr. Gorgun is a consultant for Boston Scientific, Olympus, and Dilumen.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Anorexia nervosa in adolescent patients: What pediatricians need to know
Eating disorders are among the most prevalent, disabling, and potentially fatal psychiatric illnesses, and the COVID-19 pandemic has exacerbated their burden, with a 15.3% increase in incidence in 2020 compared with previous years.1 This increase was almost solely among adolescent girls with anorexia nervosa (AN), which is often insidious in onset and more difficult to treat as it advances. Adolescents with AN are most likely to present to their pediatricians, so awareness and early recognition of the symptoms is critical. Pediatricians are also an integral part of the treatment team in AN and can offer monitoring for serious complications, alongside valuable guidance to parents, who are central to treatment and the reestablishment of healthy eating habits in their children. Here we will review the epidemiology, diagnosis, and treatment of anorexia, with an emphasis on what pediatricians need to know to screen and to facilitate treatment.
Epidemiology
AN is marked by a fear of gaining weight or behaviors that interfere with weight gain and a self-evaluation unduly influenced by weight and body shape. Youth with AN often deny the seriousness of their malnutrition, although that is not required for diagnosis. AN can be of a restrictive or binge-purge subtype, and amenorrhea is no longer a requirement for diagnosis. There is not a specific weight or body mass index cutoff for the diagnosis, but the severity of AN is determined by the BMI percentile normed to age and sex. The average age of onset is 18, and the prepandemic prevalence of AN was about 1% of the population. It affects about 10 times as many females as males. It is quite rare prior to puberty, affecting about 0.01% of that age group. There is a heritable component, with a fivefold relative risk in youth with a parent with AN, and twin studies suggest heritability rates as high as 75%. Youth with rigid cognitive styles appear more vulnerable, as do those who participate in activities such as ballet, gymnastics, modeling, and wrestling because of the role of appearance and weight in performance. More than half of patients with AN will have another psychiatric illness, most commonly anxiety disorders, depression, or obsessive-compulsive disorder. AN becomes chronic in up to 15% of sufferers and the mortality rate is close to 10%, with approximately half dying from medical complications and half dying by suicide.
Screening
Parents and pediatricians are usually the first to notice that a child has started to lose weight or is falling off the growth curve. But weight changes usually emerge after feelings of preoccupation with weight, body shape, and body satisfaction. If parents report escalating pickiness around food, increased or compulsive exercise, persistent self-consciousness and self-criticism around weight and body shape, it is worth starting with screening questions.
If you notice preoccupation or anxiety around being weighed, even if the weight or growth curve are still normal, it is worthwhile to screen. Screening questions, such as the SCOFF questionnaire with five simple questions, can be very sensitive for both AN and bulimia nervosa.2 There are also many validated screening instruments, such as the Eating Disorder Inventory or Eating Attitudes Test (for adolescents) and the Kids Eating Disorder Survey and the Child Eating Attitudes Test (for younger children), that are short self-reports that you can have your patients fill out when you have a higher index of suspicion. Weight loss or growth failure without a preoccupation around weight or appearance needs a thorough a medical workup, and could be a function of other psychiatric problems, such as depression.
If a child screens positive for an eating disorder, your full physical examination, growth curves, and longitudinal growth charts are critical for diagnosis. Percentile BMIs must be used, given the inaccuracy of standard BMI calculations in this age group. (Centers for Disease Control and Prevention age and sex growth charts include methods for this calculation). Laboratory assessment, including metabolic, kidney, pancreatic, and thyroid function, and an EKG can illuminate if there are consequences of restricting or purging. Of course, you want to evaluate for significant medical symptoms, including bradycardia, orthostasis, and hypokalemia. These medical symptoms are not limited to the severely underweight and merit referral to an emergency department and possible medical admission.
Then, a referral to a clinician who is expert in the assessment and treatment of eating disorders is needed. This may be a child psychiatrist, psychologist, or a colleague pediatrician with this specialization. It is also very important to begin the conversation with the family to introduce your concerns, describe what you have noticed, and discuss the need for further assessment and possibly treatment.
Be mindful that discussing this in front of your patient may heighten the patient’s anxiety or distress. Be prepared to offer support and understanding for your patient’s anxiety, while steadfastly providing absolute clarity for the parents about the necessity of further evaluation and treatment. Many parents will be concerned and ready to do whatever is needed to get their child’s eating and growth back on track. But some parents may have more difficulty. They may have their own history with an eating disorder. They may be avoiding a sense of shame or alarm. They may be eager to avoid adding to their child’s stress. They may be tired of engaging in power struggles with the child. They may be proud of their ambitious, accomplished young athlete. Their trust in you makes you uniquely positioned to complicate their thinking. And treatment will hinge on them, so this is a critical bridge to care.
Beyond telling parents that they will need to bring more structure and supervision to mealtimes to begin addressing their child’s nutrition, you might offer guidance on other strategies. Empower parents to limit their child’s use of social media sites such as Instagram, YouTube, and TikTok, where they may be immersed in comparing themselves to idealized (and airbrushed) influencers. Empower them to make their child’s participation in beloved sports contingent on eating meals together and completely or on a stabilized weight (as will be common in treatment). Remind them that there are no bad foods, that the goal is health, and that they are not in a power struggle with their child, but instead allied with their child to treat AN. Remind them to also look for chances to have fun with their child, to help everyone remember what matters.
Treatment
Family-based therapy (FBT) is the first-line treatment of shorter-duration AN in children and adolescents. It focuses on the parents, helping them to calmly and effectively manage their child’s eating behaviors until their weight and behaviors have normalized. As a patient’s nutritional status improves, so does cognitive function, emotional flexibility, and mood. Individual therapy and psychopharmacologic treatment can be very effective for comorbid anxiety, mood, attentional, and thought disorders. Family-based work does include the child and is often done in group-based settings with clinicians from multiple disciplines. Dietitians provide education and guidance about healthy nutrition to the child and parents. Therapists may work with the child, parents, or full family to focus on behavior modification and managing distress. Most academic medical centers provide access to FBT, but there are many regions with no providers of this evidence-based treatment. One of the silver linings of the COVID-19 pandemic is that several online services have emerged offering FBT, working with families to manage mealtimes and treatment entirely at home.3 Pediatricians provide regular medical checks to measure progress and help with decisions about when it is safe to permit exercise or advance privileges and independence around eating. Some pediatricians have discovered a deep interest in this area of pediatrics and built their practices on it. Given the surge in prevalence of AN and the needs for adolescent mental health services, we hope more will do so.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Taquet M et al. Br J Psychiatry. 2022;220:262-4.
2. Morgan JF et al. West J Med. 2000 Mar;172(3):164-5.
3. Matheson BE et al. Int J Eat Disord. 2020 Jul;53(7):1142-54.
Eating disorders are among the most prevalent, disabling, and potentially fatal psychiatric illnesses, and the COVID-19 pandemic has exacerbated their burden, with a 15.3% increase in incidence in 2020 compared with previous years.1 This increase was almost solely among adolescent girls with anorexia nervosa (AN), which is often insidious in onset and more difficult to treat as it advances. Adolescents with AN are most likely to present to their pediatricians, so awareness and early recognition of the symptoms is critical. Pediatricians are also an integral part of the treatment team in AN and can offer monitoring for serious complications, alongside valuable guidance to parents, who are central to treatment and the reestablishment of healthy eating habits in their children. Here we will review the epidemiology, diagnosis, and treatment of anorexia, with an emphasis on what pediatricians need to know to screen and to facilitate treatment.
Epidemiology
AN is marked by a fear of gaining weight or behaviors that interfere with weight gain and a self-evaluation unduly influenced by weight and body shape. Youth with AN often deny the seriousness of their malnutrition, although that is not required for diagnosis. AN can be of a restrictive or binge-purge subtype, and amenorrhea is no longer a requirement for diagnosis. There is not a specific weight or body mass index cutoff for the diagnosis, but the severity of AN is determined by the BMI percentile normed to age and sex. The average age of onset is 18, and the prepandemic prevalence of AN was about 1% of the population. It affects about 10 times as many females as males. It is quite rare prior to puberty, affecting about 0.01% of that age group. There is a heritable component, with a fivefold relative risk in youth with a parent with AN, and twin studies suggest heritability rates as high as 75%. Youth with rigid cognitive styles appear more vulnerable, as do those who participate in activities such as ballet, gymnastics, modeling, and wrestling because of the role of appearance and weight in performance. More than half of patients with AN will have another psychiatric illness, most commonly anxiety disorders, depression, or obsessive-compulsive disorder. AN becomes chronic in up to 15% of sufferers and the mortality rate is close to 10%, with approximately half dying from medical complications and half dying by suicide.
Screening
Parents and pediatricians are usually the first to notice that a child has started to lose weight or is falling off the growth curve. But weight changes usually emerge after feelings of preoccupation with weight, body shape, and body satisfaction. If parents report escalating pickiness around food, increased or compulsive exercise, persistent self-consciousness and self-criticism around weight and body shape, it is worth starting with screening questions.
If you notice preoccupation or anxiety around being weighed, even if the weight or growth curve are still normal, it is worthwhile to screen. Screening questions, such as the SCOFF questionnaire with five simple questions, can be very sensitive for both AN and bulimia nervosa.2 There are also many validated screening instruments, such as the Eating Disorder Inventory or Eating Attitudes Test (for adolescents) and the Kids Eating Disorder Survey and the Child Eating Attitudes Test (for younger children), that are short self-reports that you can have your patients fill out when you have a higher index of suspicion. Weight loss or growth failure without a preoccupation around weight or appearance needs a thorough a medical workup, and could be a function of other psychiatric problems, such as depression.
If a child screens positive for an eating disorder, your full physical examination, growth curves, and longitudinal growth charts are critical for diagnosis. Percentile BMIs must be used, given the inaccuracy of standard BMI calculations in this age group. (Centers for Disease Control and Prevention age and sex growth charts include methods for this calculation). Laboratory assessment, including metabolic, kidney, pancreatic, and thyroid function, and an EKG can illuminate if there are consequences of restricting or purging. Of course, you want to evaluate for significant medical symptoms, including bradycardia, orthostasis, and hypokalemia. These medical symptoms are not limited to the severely underweight and merit referral to an emergency department and possible medical admission.
Then, a referral to a clinician who is expert in the assessment and treatment of eating disorders is needed. This may be a child psychiatrist, psychologist, or a colleague pediatrician with this specialization. It is also very important to begin the conversation with the family to introduce your concerns, describe what you have noticed, and discuss the need for further assessment and possibly treatment.
Be mindful that discussing this in front of your patient may heighten the patient’s anxiety or distress. Be prepared to offer support and understanding for your patient’s anxiety, while steadfastly providing absolute clarity for the parents about the necessity of further evaluation and treatment. Many parents will be concerned and ready to do whatever is needed to get their child’s eating and growth back on track. But some parents may have more difficulty. They may have their own history with an eating disorder. They may be avoiding a sense of shame or alarm. They may be eager to avoid adding to their child’s stress. They may be tired of engaging in power struggles with the child. They may be proud of their ambitious, accomplished young athlete. Their trust in you makes you uniquely positioned to complicate their thinking. And treatment will hinge on them, so this is a critical bridge to care.
Beyond telling parents that they will need to bring more structure and supervision to mealtimes to begin addressing their child’s nutrition, you might offer guidance on other strategies. Empower parents to limit their child’s use of social media sites such as Instagram, YouTube, and TikTok, where they may be immersed in comparing themselves to idealized (and airbrushed) influencers. Empower them to make their child’s participation in beloved sports contingent on eating meals together and completely or on a stabilized weight (as will be common in treatment). Remind them that there are no bad foods, that the goal is health, and that they are not in a power struggle with their child, but instead allied with their child to treat AN. Remind them to also look for chances to have fun with their child, to help everyone remember what matters.
Treatment
Family-based therapy (FBT) is the first-line treatment of shorter-duration AN in children and adolescents. It focuses on the parents, helping them to calmly and effectively manage their child’s eating behaviors until their weight and behaviors have normalized. As a patient’s nutritional status improves, so does cognitive function, emotional flexibility, and mood. Individual therapy and psychopharmacologic treatment can be very effective for comorbid anxiety, mood, attentional, and thought disorders. Family-based work does include the child and is often done in group-based settings with clinicians from multiple disciplines. Dietitians provide education and guidance about healthy nutrition to the child and parents. Therapists may work with the child, parents, or full family to focus on behavior modification and managing distress. Most academic medical centers provide access to FBT, but there are many regions with no providers of this evidence-based treatment. One of the silver linings of the COVID-19 pandemic is that several online services have emerged offering FBT, working with families to manage mealtimes and treatment entirely at home.3 Pediatricians provide regular medical checks to measure progress and help with decisions about when it is safe to permit exercise or advance privileges and independence around eating. Some pediatricians have discovered a deep interest in this area of pediatrics and built their practices on it. Given the surge in prevalence of AN and the needs for adolescent mental health services, we hope more will do so.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Taquet M et al. Br J Psychiatry. 2022;220:262-4.
2. Morgan JF et al. West J Med. 2000 Mar;172(3):164-5.
3. Matheson BE et al. Int J Eat Disord. 2020 Jul;53(7):1142-54.
Eating disorders are among the most prevalent, disabling, and potentially fatal psychiatric illnesses, and the COVID-19 pandemic has exacerbated their burden, with a 15.3% increase in incidence in 2020 compared with previous years.1 This increase was almost solely among adolescent girls with anorexia nervosa (AN), which is often insidious in onset and more difficult to treat as it advances. Adolescents with AN are most likely to present to their pediatricians, so awareness and early recognition of the symptoms is critical. Pediatricians are also an integral part of the treatment team in AN and can offer monitoring for serious complications, alongside valuable guidance to parents, who are central to treatment and the reestablishment of healthy eating habits in their children. Here we will review the epidemiology, diagnosis, and treatment of anorexia, with an emphasis on what pediatricians need to know to screen and to facilitate treatment.
Epidemiology
AN is marked by a fear of gaining weight or behaviors that interfere with weight gain and a self-evaluation unduly influenced by weight and body shape. Youth with AN often deny the seriousness of their malnutrition, although that is not required for diagnosis. AN can be of a restrictive or binge-purge subtype, and amenorrhea is no longer a requirement for diagnosis. There is not a specific weight or body mass index cutoff for the diagnosis, but the severity of AN is determined by the BMI percentile normed to age and sex. The average age of onset is 18, and the prepandemic prevalence of AN was about 1% of the population. It affects about 10 times as many females as males. It is quite rare prior to puberty, affecting about 0.01% of that age group. There is a heritable component, with a fivefold relative risk in youth with a parent with AN, and twin studies suggest heritability rates as high as 75%. Youth with rigid cognitive styles appear more vulnerable, as do those who participate in activities such as ballet, gymnastics, modeling, and wrestling because of the role of appearance and weight in performance. More than half of patients with AN will have another psychiatric illness, most commonly anxiety disorders, depression, or obsessive-compulsive disorder. AN becomes chronic in up to 15% of sufferers and the mortality rate is close to 10%, with approximately half dying from medical complications and half dying by suicide.
Screening
Parents and pediatricians are usually the first to notice that a child has started to lose weight or is falling off the growth curve. But weight changes usually emerge after feelings of preoccupation with weight, body shape, and body satisfaction. If parents report escalating pickiness around food, increased or compulsive exercise, persistent self-consciousness and self-criticism around weight and body shape, it is worth starting with screening questions.
If you notice preoccupation or anxiety around being weighed, even if the weight or growth curve are still normal, it is worthwhile to screen. Screening questions, such as the SCOFF questionnaire with five simple questions, can be very sensitive for both AN and bulimia nervosa.2 There are also many validated screening instruments, such as the Eating Disorder Inventory or Eating Attitudes Test (for adolescents) and the Kids Eating Disorder Survey and the Child Eating Attitudes Test (for younger children), that are short self-reports that you can have your patients fill out when you have a higher index of suspicion. Weight loss or growth failure without a preoccupation around weight or appearance needs a thorough a medical workup, and could be a function of other psychiatric problems, such as depression.
If a child screens positive for an eating disorder, your full physical examination, growth curves, and longitudinal growth charts are critical for diagnosis. Percentile BMIs must be used, given the inaccuracy of standard BMI calculations in this age group. (Centers for Disease Control and Prevention age and sex growth charts include methods for this calculation). Laboratory assessment, including metabolic, kidney, pancreatic, and thyroid function, and an EKG can illuminate if there are consequences of restricting or purging. Of course, you want to evaluate for significant medical symptoms, including bradycardia, orthostasis, and hypokalemia. These medical symptoms are not limited to the severely underweight and merit referral to an emergency department and possible medical admission.
Then, a referral to a clinician who is expert in the assessment and treatment of eating disorders is needed. This may be a child psychiatrist, psychologist, or a colleague pediatrician with this specialization. It is also very important to begin the conversation with the family to introduce your concerns, describe what you have noticed, and discuss the need for further assessment and possibly treatment.
Be mindful that discussing this in front of your patient may heighten the patient’s anxiety or distress. Be prepared to offer support and understanding for your patient’s anxiety, while steadfastly providing absolute clarity for the parents about the necessity of further evaluation and treatment. Many parents will be concerned and ready to do whatever is needed to get their child’s eating and growth back on track. But some parents may have more difficulty. They may have their own history with an eating disorder. They may be avoiding a sense of shame or alarm. They may be eager to avoid adding to their child’s stress. They may be tired of engaging in power struggles with the child. They may be proud of their ambitious, accomplished young athlete. Their trust in you makes you uniquely positioned to complicate their thinking. And treatment will hinge on them, so this is a critical bridge to care.
Beyond telling parents that they will need to bring more structure and supervision to mealtimes to begin addressing their child’s nutrition, you might offer guidance on other strategies. Empower parents to limit their child’s use of social media sites such as Instagram, YouTube, and TikTok, where they may be immersed in comparing themselves to idealized (and airbrushed) influencers. Empower them to make their child’s participation in beloved sports contingent on eating meals together and completely or on a stabilized weight (as will be common in treatment). Remind them that there are no bad foods, that the goal is health, and that they are not in a power struggle with their child, but instead allied with their child to treat AN. Remind them to also look for chances to have fun with their child, to help everyone remember what matters.
Treatment
Family-based therapy (FBT) is the first-line treatment of shorter-duration AN in children and adolescents. It focuses on the parents, helping them to calmly and effectively manage their child’s eating behaviors until their weight and behaviors have normalized. As a patient’s nutritional status improves, so does cognitive function, emotional flexibility, and mood. Individual therapy and psychopharmacologic treatment can be very effective for comorbid anxiety, mood, attentional, and thought disorders. Family-based work does include the child and is often done in group-based settings with clinicians from multiple disciplines. Dietitians provide education and guidance about healthy nutrition to the child and parents. Therapists may work with the child, parents, or full family to focus on behavior modification and managing distress. Most academic medical centers provide access to FBT, but there are many regions with no providers of this evidence-based treatment. One of the silver linings of the COVID-19 pandemic is that several online services have emerged offering FBT, working with families to manage mealtimes and treatment entirely at home.3 Pediatricians provide regular medical checks to measure progress and help with decisions about when it is safe to permit exercise or advance privileges and independence around eating. Some pediatricians have discovered a deep interest in this area of pediatrics and built their practices on it. Given the surge in prevalence of AN and the needs for adolescent mental health services, we hope more will do so.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Taquet M et al. Br J Psychiatry. 2022;220:262-4.
2. Morgan JF et al. West J Med. 2000 Mar;172(3):164-5.
3. Matheson BE et al. Int J Eat Disord. 2020 Jul;53(7):1142-54.
Skull Base Regeneration During Treatment With Chemoradiation for Nasopharyngeal Carcinoma: A Case Report
Nasopharyngeal carcinoma (NPC) differs from other head and neck (H&N) cancers in its epidemiology and treatment. Unlike other H&N cancers, NPC has a distinct geographical distribution with a much higher incidence in endemic areas, such as southern China, than in areas where it is relatively uncommon, such as the United States.1 The etiology of NPC varies based on the geographical distribution, with Epstein-Barr virus (EBV) thought to be the primary etiologic agent in endemic areas. On the other hand, in North America 2 additional subsets of NPC have been identified: human papillomavirus (HPV)–positive/EBV-negative and HPV-negative/EBV-negative.2,3 NPC arises from the epithelial lining of the nasopharynx, often in the fossa of Rosenmuller, and is the most seen tumor in the nasopharynx.4 NPC is less surgically accessible than other H&N cancers, and surgery to the nasopharynx poses more risks given the proximity of critical surrounding structures. NPC is radiosensitive, and therefore radiotherapy (RT), in combination with chemotherapy for locally advanced tumors, has become the mainstay of treatment for nonmetastatic NPC.4
NPC often presents with an asymptomatic neck mass or with symptoms of epistaxis, nasal obstruction, and otitis media.5 Advanced cases of NPC can present with direct extension into the skull base, paranasal sinuses, and orbit, as well as involvement of cranial nerves. Radiation planning for tumors of the nasopharynx is complicated by the need to deliver an adequate dose to the tumor while limiting dose and toxicity to nearby critical structures such as the brainstem, optic chiasm, eyes, spinal cord (SC), temporal lobes, and cochleae. Achieving an adequate dose to nasopharyngeal primary tumors is especially complicated for T4 tumors invading the skull base with intracranial extension, in direct contact with these critical structures (Table 1).
Skull base invasion is a poor prognostic factor, predicting for an increased risk of locoregional recurrence and worse overall survival. Furthermore, the extent of skull base invasion in NPC affects overall prognosis, with cranial nerve involvement and intracranial extension predictive for worse outcomes.5 Depending on the extent of destruction, a bony defect along the skull base could develop with tumor shrinkage during RT, resulting in complications such as cerebrospinal fluid leaks, herniation, and atlantoaxial instability.6
There is a paucity of literature on the ability of bone to regenerate during or after RT for cases of NPC with skull base destruction. To our knowledge, nothing has been published detailing the extent of bony regeneration that can occur during treatment itself, as the tumor regresses and poses a threat of a skull base defect. Here we present a case of T4 HPV-positive/EBV-negative NPC with intracranial extension and describe the RT planning methods leading to prolonged local control, limited toxicities, and bony regeneration of the skull base during treatment.
Case Presentation
A 34-year-old male patient with no previous medical history presented to the emergency department with worsening diplopia, nasal obstruction, facial pain, and neck stiffness. The patient reported a 3 pack-year smoking history with recent smoking cessation. His physical examination was notable for a right abducens nerve palsy and an ulcerated nasopharyngeal mass on endoscopy.
Computed tomography (CT) scan revealed a 7-cm mass in the nasopharynx, eroding through the skull base with destruction and replacement of the clivus by tumor. Also noted was erosion of the petrous apices, carotid canals, sella turcica, dens, and the bilateral occipital condyles. There was intracranial extension with replacement of portions of the cavernous sinuses as well as mass effect on the prepontine cistern. Additional brain imaging studies, including magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, were obtained for completion of the staging workup. The MRI correlated with the findings noted on CT and demonstrated involvement of Meckel cave, foramen ovale, foramen rotundum, Dorello canal, and the hypoglossal canals. No cervical lymphadenopathy or distant metastases were noted on imaging. Pathology from biopsy revealed poorly differentiated squamous cell carcinoma, EBV-negative, strongly p16-positive, HPV-16 positive, and P53-negative.
The H&N multidisciplinary tumor board recommended concurrent chemoradiation for this stage IVA (T4N0M0) EBV-negative, HPV-positive, Word Health Organization type I NPC (Table 2). The patient underwent CT simulation for RT planning, and both tumor volumes and critical normal structures were contoured. The goal was to deliver 70 Gy to the gross tumor. However, given the inability to deliver this dose while meeting the SC dose tolerance of < 45 Gy, a 2-Gy fraction was removed. Therefore, 34 fractions of 2 Gy were delivered to the tumor volume for a total dose of 68 Gy. Weekly cisplatin, at a dose of 40 mg/m2, was administered concurrently with RT.
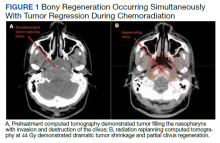
RT planning was complicated by the tumor’s contact with the brainstem and upper cervical SC, as well as proximity of the tumor to the optic apparatus. The patient underwent 2 replanning CT scans at 26 Gy and 44 Gy to evaluate for tumor shrinkage. These CT scans demonstrated shrinkage of the tumor away from critical neural structures, allowing the treatment volume to be reduced away from these structures in order to achieve required dose tolerances (brainstem < 54 Gy, optic nerves and chiasm < 50 Gy, SC < 45 Gy for this case). The replanning CT scan at 44 Gy, 5 weeks after treatment initiation, demonstrated that dramatic tumor shrinkage had occurred early in treatment, with separation of the remaining tumor from the area of the SC and brainstem with which it was initially in contact (Figure 1). This improvement allowed for shrinkage of the high-dose radiation field away from these critical neural structures.
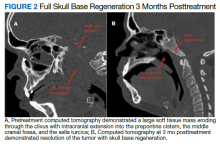
Baseline destruction of the skull base by tumor raised concern for craniospinal instability with tumor response. The patient was evaluated by neurosurgery before the start of RT, and the recommendation was for reimaging during treatment and close follow-up of the patient’s symptoms to determine whether surgical fixation would be indicated during or after treatment. The patient underwent a replanning CT scan at 44 Gy, 5 weeks after treatment initiation, that demonstrated impressive bony regeneration occurring during chemoradiation. New bone formation was noted in the region of the clivus and bilateral occipital condyles, which had been absent on CT prior to treatment initiation. Another CT at 54 Gy demonstrated further ossification of the clivus and bilateral occipital condyles, and bony regeneration occurring rapidly during chemoradiation. The posttreatment CT 3 months after completion of chemoradiation demonstrated complete skull base regeneration, maintaining stability of this area and precluding the need for neurosurgical intervention (Figure 2).
During RT,
The patient had no evidence of disease at 5 years posttreatment. After completing treatment, the patient experienced ongoing intermittent nasal congestion and occasional aural fullness. He experienced an early decay of several teeth starting 1 year after completion of RT, and he continues to visit his dentist for management. He experienced no other treatment-related toxicities. In particular, he has exhibited no signs of neurologic toxicity to date.
Discussion
RT for NPC is complicated by the proximity of these tumors to critical surrounding neural structures. It is challenging to achieve the required dose constraints to surrounding neural tissues while delivering the usual 70-Gy dose to the gross tumor, especially when the tumor comes into direct contact with these structures.
This case provides an example of response-adapted RT using imaging during treatment to shrink the high-dose target as the tumor shrinks away from critical surrounding structures.7 This strategy permits delivery of the maximum dose to the tumor while minimizing radiation dose, and therefore risk of toxicity, to normal surrounding structures. While it is typical to deliver 70 Gy to the full extent of tumor involvement for H&N tumors, this was not possible in this case as the tumor was in contact with the brainstem and upper cervical SC. Delivering the full 70 Gy to these areas of tumor would have placed this patient at substantial risk of brainstem and/or SC toxicity. This report demonstrates that response-adapted RT with shrinking fields can allow for tumor control while avoiding toxicity to critical neural structures for cases of locally advanced NPC in which tumor is abutting these structures.
Bony regeneration of the skull base following RT has been reported in the literature, but in limited reviews. Early reports used plain radiography to follow changes. Unger and colleagues demonstrated the regeneration of bone using skull radiographs 4 to 6 months after completion of RT for NPC.8 More recent literature details the ability of bone to regenerate after RT based on CT findings. Fang and colleagues reported on 90 cases of NPC with skull base destruction, with 63% having bony regeneration on posttreatment CT.9 Most of the patients in Fang’s report had bony regeneration within 1 year of treatment, and in general, bony regeneration became more evident on imaging with longer follow-up. Of note, local control was significantly greater in patients with regeneration vs persistent destruction (77% vs 21%, P < .001). On multivariate analysis, complete tumor response was significantly associated with bony regeneration; other factors such as age, sex, radiation dose, and chemotherapy were not significantly associated with the likelihood of bony regeneration.
Our report details a nasopharyngeal tumor that destroyed the skull base with no intact bony barrier. In such cases, concern arises regarding craniospinal instability with tumor regression if there is not simultaneous bone regeneration. Tumor invasion of the skull base and C1-2 vertebral bodies and complications from treatment of such tumor extent can lead to symptoms of craniospinal instability, including pain, difficulty with neck range of motion, and loss of strength and sensation in the upper and lower extremities.10 A case report of a woman treated with chemoradiation for a plasmacytoma of the skull base detailed her posttreatment presentation with quadriparesis resulting from craniospinal instability after tumor regression.11 Such instability is generally treated surgically, and during this woman’s surgery, there was an injury to the right vertebral artery, although this did not cause any additional neurologic deficits.
RT leads to hypocellularity, hypovascularity, and hypoxia of treated tissues, resulting in a reduced ability for growth and healing. Studies demonstrate that irradiated bone contains fewer osteoblast cells and osteocytes than unirradiated bone, resulting in reduced regenerative capacity.12,13 Furthermore, the reconstruction of bony defects resulting after cancer treatment has been shown to be difficult and associated with a high risk of complications.14 Given the impaired ability of irradiated bone to regenerate, studies have evaluated the use of growth factors and gene therapy to promote bone formation after treatment.15 Bone marrow stem cells have been shown to reverse radiation-induced cellular depletion and to increase osteocyte counts in animal studies.12 Further, overexpression of miR-34a, a tumor suppressor involved in tissue development, has been shown to improve osteoblastic differentiation of irradiated bone marrow stem cells and promote bone regeneration in vitro and in animal studies.13 While several techniques are being studied in vitro and in animal studies to promote bony regeneration after RT, there is a lack of data on use of these techniques in humans with cancer.
With our case, there was great uncertainty related to the ability of bone to regenerate during treatment and concern regarding consequences of formation of a skull base defect during treatment. CT imaging revealed bony regeneration of the central skull base and clivus, as well as occipital condyles, that occurred throughout the RT course. There was clear evidence of bone regeneration on the replanning CT obtained 5 weeks after treatment initiation. To our knowledge, this is the first report to demonstrate rapid bony regeneration during RT, thereby maintaining the integrity of the skull base and precluding the need for neurosurgical intervention. Moving forward, imaging should be considered during treatment for patients with tumor-related destruction of the skull base and upper cervical spine to evaluate the extent of bony regeneration during treatment and estimate the potential risk of craniocervical instability. Further studies with imaging during treatment are needed for more information on the likelihood of bony regeneration and factors that correlate with bony regeneration during treatment. As in other reports, our case demonstrates that bony regeneration may predict complete response to RT.9
Our patient’s tumor was HPV-positive and EBV-negative. In the US, the rate of HPV-positive NPC is 35%.16 However, HPV-positive NPC is much less common in endemic areas. A recent study from China of 1,328 patients with NPC revealed a 6.4% rate of HPV-positive/EBV-negative cases.17 In that study, patients with HPV-positive/EBV-negative tumors had improved survival compared to patients whose tumors were HPV-negative/EBV-positive. Another study suggests that the impact of HPV in NPC varies according to race, with HPV-positivity predicting for improved outcomes in East Asian patients and worse outcomes in White patients.17 A study from the University of Michigan suggests that both HPV-positive/EBV-negative and HPV-negative/EBV-negative NPC are associated with worse overall survival and locoregional control than EBV-positive NPC.2 Overall, the prognostic role of HPV in NPC remains unclear given conflicting information in the literature and the lack of large population studies.18
Conclusions
There is a paucity of literature on bony regeneration in patients with skull base destruction from advanced NPC, and in particular, the ability of skull base regeneration to occur during treatment simultaneous with tumor regression. Our patient had HPV-positive/EBV-negative NPC, but it is unclear how this subtype affected his prognosis. Factors such as tumor histology, radiosensitivity with rapid tumor regression, and young age may have all contributed to the rapidity of bone regeneration in our patient. This case report demonstrates that an impressive tumor response to chemoradiation with simultaneous bony regeneration is possible among patients presenting with tumor destruction of the skull base, precluding the need for neurosurgical intervention.
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777. doi:10.1158/1055-9965.EPI-06-0353
2. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi:10.1016/j.ijrobp.2013.11.246
3. Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562-567. doi:10.1002/hed.21216
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-0
5. Roh JL, Sung MW, Kim KH, et al.. Nasopharyngeal carcinoma with skull base invasion: a necessity of staging subdivision. Am J Otolaryngol. 2004;25(1):26-32. doi:10.1016/j.amjoto.2003.09.011
6. Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine. 1998;23(11):1280-1282. doi:10.1097/00007632-199806010-00021
7. Morgan HE, Sher DJ. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck. 2020;5:1. doi:10.1186/s41199-019-0046-z
8. Unger JD, Chiang LC, Unger GF. Apparent reformation of the base of the skull following radiotherapy for nasopharyngeal carcinoma. Radiology. 1978;126(3):779-782. doi:10.1148/126.3.779
9. Fang FM, Leung SW, Wang CJ, et al. Computed tomography findings of bony regeneration after radiotherapy for nasopharyngeal carcinoma with skull base destruction: implications for local control. Int J Radiat Oncol Biol Phys. 1999;44(2):305-309. doi:10.1016/s0360-3016(99)00004-8
10. Tiruchelvarayan R, Lee KA, Ng I. Surgery for atlanto-axial (C1-2) involvement or instability in nasopharyngeal carcinoma patients. Singapore Med J. 2012;53(6):416-421.
11. Samprón N, Arrazola M, Urculo E. Skull-base plasmacytoma with craniocervical instability [in Spanish]. Neurocirugia (Astur). 2009;20(5):478-483.
12. Zheutlin AR, Deshpande SS, Nelson NS, et al. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: a histomorphometric evaluation. Cytotherapy. 2016;18(5):664-672. doi:10.1016/j.jcyt.2016.01.013
13. Liu H, Dong Y, Feng X, et al. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblast differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi:10.1186/s13287-019-1285-y
14. Holzapfel BM, Wagner F, Martine LC, et al. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35(3):475-487. doi:10.1007/s10555-016-9635-z
15. Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77-81. doi:10.1177/0022034509352151
16. Wotman M, Oh EJ, Ahn S, Kraus D, Constantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi:10.1016/j.amjoto.2019.06.00717. Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530-536. doi:10.1002/cncr.31031.
18. Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi:10.1002/hed.24978
19. Lee AWM, Lydiatt WM, Colevas AD, et al. Nasopharynx. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:103.
20. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and genetics of head and neck tumors. In: World Health Organization Classification of Tumours. IARC Press; 2005.
Nasopharyngeal carcinoma (NPC) differs from other head and neck (H&N) cancers in its epidemiology and treatment. Unlike other H&N cancers, NPC has a distinct geographical distribution with a much higher incidence in endemic areas, such as southern China, than in areas where it is relatively uncommon, such as the United States.1 The etiology of NPC varies based on the geographical distribution, with Epstein-Barr virus (EBV) thought to be the primary etiologic agent in endemic areas. On the other hand, in North America 2 additional subsets of NPC have been identified: human papillomavirus (HPV)–positive/EBV-negative and HPV-negative/EBV-negative.2,3 NPC arises from the epithelial lining of the nasopharynx, often in the fossa of Rosenmuller, and is the most seen tumor in the nasopharynx.4 NPC is less surgically accessible than other H&N cancers, and surgery to the nasopharynx poses more risks given the proximity of critical surrounding structures. NPC is radiosensitive, and therefore radiotherapy (RT), in combination with chemotherapy for locally advanced tumors, has become the mainstay of treatment for nonmetastatic NPC.4
NPC often presents with an asymptomatic neck mass or with symptoms of epistaxis, nasal obstruction, and otitis media.5 Advanced cases of NPC can present with direct extension into the skull base, paranasal sinuses, and orbit, as well as involvement of cranial nerves. Radiation planning for tumors of the nasopharynx is complicated by the need to deliver an adequate dose to the tumor while limiting dose and toxicity to nearby critical structures such as the brainstem, optic chiasm, eyes, spinal cord (SC), temporal lobes, and cochleae. Achieving an adequate dose to nasopharyngeal primary tumors is especially complicated for T4 tumors invading the skull base with intracranial extension, in direct contact with these critical structures (Table 1).
Skull base invasion is a poor prognostic factor, predicting for an increased risk of locoregional recurrence and worse overall survival. Furthermore, the extent of skull base invasion in NPC affects overall prognosis, with cranial nerve involvement and intracranial extension predictive for worse outcomes.5 Depending on the extent of destruction, a bony defect along the skull base could develop with tumor shrinkage during RT, resulting in complications such as cerebrospinal fluid leaks, herniation, and atlantoaxial instability.6
There is a paucity of literature on the ability of bone to regenerate during or after RT for cases of NPC with skull base destruction. To our knowledge, nothing has been published detailing the extent of bony regeneration that can occur during treatment itself, as the tumor regresses and poses a threat of a skull base defect. Here we present a case of T4 HPV-positive/EBV-negative NPC with intracranial extension and describe the RT planning methods leading to prolonged local control, limited toxicities, and bony regeneration of the skull base during treatment.
Case Presentation
A 34-year-old male patient with no previous medical history presented to the emergency department with worsening diplopia, nasal obstruction, facial pain, and neck stiffness. The patient reported a 3 pack-year smoking history with recent smoking cessation. His physical examination was notable for a right abducens nerve palsy and an ulcerated nasopharyngeal mass on endoscopy.
Computed tomography (CT) scan revealed a 7-cm mass in the nasopharynx, eroding through the skull base with destruction and replacement of the clivus by tumor. Also noted was erosion of the petrous apices, carotid canals, sella turcica, dens, and the bilateral occipital condyles. There was intracranial extension with replacement of portions of the cavernous sinuses as well as mass effect on the prepontine cistern. Additional brain imaging studies, including magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, were obtained for completion of the staging workup. The MRI correlated with the findings noted on CT and demonstrated involvement of Meckel cave, foramen ovale, foramen rotundum, Dorello canal, and the hypoglossal canals. No cervical lymphadenopathy or distant metastases were noted on imaging. Pathology from biopsy revealed poorly differentiated squamous cell carcinoma, EBV-negative, strongly p16-positive, HPV-16 positive, and P53-negative.
The H&N multidisciplinary tumor board recommended concurrent chemoradiation for this stage IVA (T4N0M0) EBV-negative, HPV-positive, Word Health Organization type I NPC (Table 2). The patient underwent CT simulation for RT planning, and both tumor volumes and critical normal structures were contoured. The goal was to deliver 70 Gy to the gross tumor. However, given the inability to deliver this dose while meeting the SC dose tolerance of < 45 Gy, a 2-Gy fraction was removed. Therefore, 34 fractions of 2 Gy were delivered to the tumor volume for a total dose of 68 Gy. Weekly cisplatin, at a dose of 40 mg/m2, was administered concurrently with RT.
RT planning was complicated by the tumor’s contact with the brainstem and upper cervical SC, as well as proximity of the tumor to the optic apparatus. The patient underwent 2 replanning CT scans at 26 Gy and 44 Gy to evaluate for tumor shrinkage. These CT scans demonstrated shrinkage of the tumor away from critical neural structures, allowing the treatment volume to be reduced away from these structures in order to achieve required dose tolerances (brainstem < 54 Gy, optic nerves and chiasm < 50 Gy, SC < 45 Gy for this case). The replanning CT scan at 44 Gy, 5 weeks after treatment initiation, demonstrated that dramatic tumor shrinkage had occurred early in treatment, with separation of the remaining tumor from the area of the SC and brainstem with which it was initially in contact (Figure 1). This improvement allowed for shrinkage of the high-dose radiation field away from these critical neural structures.
Baseline destruction of the skull base by tumor raised concern for craniospinal instability with tumor response. The patient was evaluated by neurosurgery before the start of RT, and the recommendation was for reimaging during treatment and close follow-up of the patient’s symptoms to determine whether surgical fixation would be indicated during or after treatment. The patient underwent a replanning CT scan at 44 Gy, 5 weeks after treatment initiation, that demonstrated impressive bony regeneration occurring during chemoradiation. New bone formation was noted in the region of the clivus and bilateral occipital condyles, which had been absent on CT prior to treatment initiation. Another CT at 54 Gy demonstrated further ossification of the clivus and bilateral occipital condyles, and bony regeneration occurring rapidly during chemoradiation. The posttreatment CT 3 months after completion of chemoradiation demonstrated complete skull base regeneration, maintaining stability of this area and precluding the need for neurosurgical intervention (Figure 2).
During RT,
The patient had no evidence of disease at 5 years posttreatment. After completing treatment, the patient experienced ongoing intermittent nasal congestion and occasional aural fullness. He experienced an early decay of several teeth starting 1 year after completion of RT, and he continues to visit his dentist for management. He experienced no other treatment-related toxicities. In particular, he has exhibited no signs of neurologic toxicity to date.
Discussion
RT for NPC is complicated by the proximity of these tumors to critical surrounding neural structures. It is challenging to achieve the required dose constraints to surrounding neural tissues while delivering the usual 70-Gy dose to the gross tumor, especially when the tumor comes into direct contact with these structures.
This case provides an example of response-adapted RT using imaging during treatment to shrink the high-dose target as the tumor shrinks away from critical surrounding structures.7 This strategy permits delivery of the maximum dose to the tumor while minimizing radiation dose, and therefore risk of toxicity, to normal surrounding structures. While it is typical to deliver 70 Gy to the full extent of tumor involvement for H&N tumors, this was not possible in this case as the tumor was in contact with the brainstem and upper cervical SC. Delivering the full 70 Gy to these areas of tumor would have placed this patient at substantial risk of brainstem and/or SC toxicity. This report demonstrates that response-adapted RT with shrinking fields can allow for tumor control while avoiding toxicity to critical neural structures for cases of locally advanced NPC in which tumor is abutting these structures.
Bony regeneration of the skull base following RT has been reported in the literature, but in limited reviews. Early reports used plain radiography to follow changes. Unger and colleagues demonstrated the regeneration of bone using skull radiographs 4 to 6 months after completion of RT for NPC.8 More recent literature details the ability of bone to regenerate after RT based on CT findings. Fang and colleagues reported on 90 cases of NPC with skull base destruction, with 63% having bony regeneration on posttreatment CT.9 Most of the patients in Fang’s report had bony regeneration within 1 year of treatment, and in general, bony regeneration became more evident on imaging with longer follow-up. Of note, local control was significantly greater in patients with regeneration vs persistent destruction (77% vs 21%, P < .001). On multivariate analysis, complete tumor response was significantly associated with bony regeneration; other factors such as age, sex, radiation dose, and chemotherapy were not significantly associated with the likelihood of bony regeneration.
Our report details a nasopharyngeal tumor that destroyed the skull base with no intact bony barrier. In such cases, concern arises regarding craniospinal instability with tumor regression if there is not simultaneous bone regeneration. Tumor invasion of the skull base and C1-2 vertebral bodies and complications from treatment of such tumor extent can lead to symptoms of craniospinal instability, including pain, difficulty with neck range of motion, and loss of strength and sensation in the upper and lower extremities.10 A case report of a woman treated with chemoradiation for a plasmacytoma of the skull base detailed her posttreatment presentation with quadriparesis resulting from craniospinal instability after tumor regression.11 Such instability is generally treated surgically, and during this woman’s surgery, there was an injury to the right vertebral artery, although this did not cause any additional neurologic deficits.
RT leads to hypocellularity, hypovascularity, and hypoxia of treated tissues, resulting in a reduced ability for growth and healing. Studies demonstrate that irradiated bone contains fewer osteoblast cells and osteocytes than unirradiated bone, resulting in reduced regenerative capacity.12,13 Furthermore, the reconstruction of bony defects resulting after cancer treatment has been shown to be difficult and associated with a high risk of complications.14 Given the impaired ability of irradiated bone to regenerate, studies have evaluated the use of growth factors and gene therapy to promote bone formation after treatment.15 Bone marrow stem cells have been shown to reverse radiation-induced cellular depletion and to increase osteocyte counts in animal studies.12 Further, overexpression of miR-34a, a tumor suppressor involved in tissue development, has been shown to improve osteoblastic differentiation of irradiated bone marrow stem cells and promote bone regeneration in vitro and in animal studies.13 While several techniques are being studied in vitro and in animal studies to promote bony regeneration after RT, there is a lack of data on use of these techniques in humans with cancer.
With our case, there was great uncertainty related to the ability of bone to regenerate during treatment and concern regarding consequences of formation of a skull base defect during treatment. CT imaging revealed bony regeneration of the central skull base and clivus, as well as occipital condyles, that occurred throughout the RT course. There was clear evidence of bone regeneration on the replanning CT obtained 5 weeks after treatment initiation. To our knowledge, this is the first report to demonstrate rapid bony regeneration during RT, thereby maintaining the integrity of the skull base and precluding the need for neurosurgical intervention. Moving forward, imaging should be considered during treatment for patients with tumor-related destruction of the skull base and upper cervical spine to evaluate the extent of bony regeneration during treatment and estimate the potential risk of craniocervical instability. Further studies with imaging during treatment are needed for more information on the likelihood of bony regeneration and factors that correlate with bony regeneration during treatment. As in other reports, our case demonstrates that bony regeneration may predict complete response to RT.9
Our patient’s tumor was HPV-positive and EBV-negative. In the US, the rate of HPV-positive NPC is 35%.16 However, HPV-positive NPC is much less common in endemic areas. A recent study from China of 1,328 patients with NPC revealed a 6.4% rate of HPV-positive/EBV-negative cases.17 In that study, patients with HPV-positive/EBV-negative tumors had improved survival compared to patients whose tumors were HPV-negative/EBV-positive. Another study suggests that the impact of HPV in NPC varies according to race, with HPV-positivity predicting for improved outcomes in East Asian patients and worse outcomes in White patients.17 A study from the University of Michigan suggests that both HPV-positive/EBV-negative and HPV-negative/EBV-negative NPC are associated with worse overall survival and locoregional control than EBV-positive NPC.2 Overall, the prognostic role of HPV in NPC remains unclear given conflicting information in the literature and the lack of large population studies.18
Conclusions
There is a paucity of literature on bony regeneration in patients with skull base destruction from advanced NPC, and in particular, the ability of skull base regeneration to occur during treatment simultaneous with tumor regression. Our patient had HPV-positive/EBV-negative NPC, but it is unclear how this subtype affected his prognosis. Factors such as tumor histology, radiosensitivity with rapid tumor regression, and young age may have all contributed to the rapidity of bone regeneration in our patient. This case report demonstrates that an impressive tumor response to chemoradiation with simultaneous bony regeneration is possible among patients presenting with tumor destruction of the skull base, precluding the need for neurosurgical intervention.
Nasopharyngeal carcinoma (NPC) differs from other head and neck (H&N) cancers in its epidemiology and treatment. Unlike other H&N cancers, NPC has a distinct geographical distribution with a much higher incidence in endemic areas, such as southern China, than in areas where it is relatively uncommon, such as the United States.1 The etiology of NPC varies based on the geographical distribution, with Epstein-Barr virus (EBV) thought to be the primary etiologic agent in endemic areas. On the other hand, in North America 2 additional subsets of NPC have been identified: human papillomavirus (HPV)–positive/EBV-negative and HPV-negative/EBV-negative.2,3 NPC arises from the epithelial lining of the nasopharynx, often in the fossa of Rosenmuller, and is the most seen tumor in the nasopharynx.4 NPC is less surgically accessible than other H&N cancers, and surgery to the nasopharynx poses more risks given the proximity of critical surrounding structures. NPC is radiosensitive, and therefore radiotherapy (RT), in combination with chemotherapy for locally advanced tumors, has become the mainstay of treatment for nonmetastatic NPC.4
NPC often presents with an asymptomatic neck mass or with symptoms of epistaxis, nasal obstruction, and otitis media.5 Advanced cases of NPC can present with direct extension into the skull base, paranasal sinuses, and orbit, as well as involvement of cranial nerves. Radiation planning for tumors of the nasopharynx is complicated by the need to deliver an adequate dose to the tumor while limiting dose and toxicity to nearby critical structures such as the brainstem, optic chiasm, eyes, spinal cord (SC), temporal lobes, and cochleae. Achieving an adequate dose to nasopharyngeal primary tumors is especially complicated for T4 tumors invading the skull base with intracranial extension, in direct contact with these critical structures (Table 1).
Skull base invasion is a poor prognostic factor, predicting for an increased risk of locoregional recurrence and worse overall survival. Furthermore, the extent of skull base invasion in NPC affects overall prognosis, with cranial nerve involvement and intracranial extension predictive for worse outcomes.5 Depending on the extent of destruction, a bony defect along the skull base could develop with tumor shrinkage during RT, resulting in complications such as cerebrospinal fluid leaks, herniation, and atlantoaxial instability.6
There is a paucity of literature on the ability of bone to regenerate during or after RT for cases of NPC with skull base destruction. To our knowledge, nothing has been published detailing the extent of bony regeneration that can occur during treatment itself, as the tumor regresses and poses a threat of a skull base defect. Here we present a case of T4 HPV-positive/EBV-negative NPC with intracranial extension and describe the RT planning methods leading to prolonged local control, limited toxicities, and bony regeneration of the skull base during treatment.
Case Presentation
A 34-year-old male patient with no previous medical history presented to the emergency department with worsening diplopia, nasal obstruction, facial pain, and neck stiffness. The patient reported a 3 pack-year smoking history with recent smoking cessation. His physical examination was notable for a right abducens nerve palsy and an ulcerated nasopharyngeal mass on endoscopy.
Computed tomography (CT) scan revealed a 7-cm mass in the nasopharynx, eroding through the skull base with destruction and replacement of the clivus by tumor. Also noted was erosion of the petrous apices, carotid canals, sella turcica, dens, and the bilateral occipital condyles. There was intracranial extension with replacement of portions of the cavernous sinuses as well as mass effect on the prepontine cistern. Additional brain imaging studies, including magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, were obtained for completion of the staging workup. The MRI correlated with the findings noted on CT and demonstrated involvement of Meckel cave, foramen ovale, foramen rotundum, Dorello canal, and the hypoglossal canals. No cervical lymphadenopathy or distant metastases were noted on imaging. Pathology from biopsy revealed poorly differentiated squamous cell carcinoma, EBV-negative, strongly p16-positive, HPV-16 positive, and P53-negative.
The H&N multidisciplinary tumor board recommended concurrent chemoradiation for this stage IVA (T4N0M0) EBV-negative, HPV-positive, Word Health Organization type I NPC (Table 2). The patient underwent CT simulation for RT planning, and both tumor volumes and critical normal structures were contoured. The goal was to deliver 70 Gy to the gross tumor. However, given the inability to deliver this dose while meeting the SC dose tolerance of < 45 Gy, a 2-Gy fraction was removed. Therefore, 34 fractions of 2 Gy were delivered to the tumor volume for a total dose of 68 Gy. Weekly cisplatin, at a dose of 40 mg/m2, was administered concurrently with RT.
RT planning was complicated by the tumor’s contact with the brainstem and upper cervical SC, as well as proximity of the tumor to the optic apparatus. The patient underwent 2 replanning CT scans at 26 Gy and 44 Gy to evaluate for tumor shrinkage. These CT scans demonstrated shrinkage of the tumor away from critical neural structures, allowing the treatment volume to be reduced away from these structures in order to achieve required dose tolerances (brainstem < 54 Gy, optic nerves and chiasm < 50 Gy, SC < 45 Gy for this case). The replanning CT scan at 44 Gy, 5 weeks after treatment initiation, demonstrated that dramatic tumor shrinkage had occurred early in treatment, with separation of the remaining tumor from the area of the SC and brainstem with which it was initially in contact (Figure 1). This improvement allowed for shrinkage of the high-dose radiation field away from these critical neural structures.
Baseline destruction of the skull base by tumor raised concern for craniospinal instability with tumor response. The patient was evaluated by neurosurgery before the start of RT, and the recommendation was for reimaging during treatment and close follow-up of the patient’s symptoms to determine whether surgical fixation would be indicated during or after treatment. The patient underwent a replanning CT scan at 44 Gy, 5 weeks after treatment initiation, that demonstrated impressive bony regeneration occurring during chemoradiation. New bone formation was noted in the region of the clivus and bilateral occipital condyles, which had been absent on CT prior to treatment initiation. Another CT at 54 Gy demonstrated further ossification of the clivus and bilateral occipital condyles, and bony regeneration occurring rapidly during chemoradiation. The posttreatment CT 3 months after completion of chemoradiation demonstrated complete skull base regeneration, maintaining stability of this area and precluding the need for neurosurgical intervention (Figure 2).
During RT,
The patient had no evidence of disease at 5 years posttreatment. After completing treatment, the patient experienced ongoing intermittent nasal congestion and occasional aural fullness. He experienced an early decay of several teeth starting 1 year after completion of RT, and he continues to visit his dentist for management. He experienced no other treatment-related toxicities. In particular, he has exhibited no signs of neurologic toxicity to date.
Discussion
RT for NPC is complicated by the proximity of these tumors to critical surrounding neural structures. It is challenging to achieve the required dose constraints to surrounding neural tissues while delivering the usual 70-Gy dose to the gross tumor, especially when the tumor comes into direct contact with these structures.
This case provides an example of response-adapted RT using imaging during treatment to shrink the high-dose target as the tumor shrinks away from critical surrounding structures.7 This strategy permits delivery of the maximum dose to the tumor while minimizing radiation dose, and therefore risk of toxicity, to normal surrounding structures. While it is typical to deliver 70 Gy to the full extent of tumor involvement for H&N tumors, this was not possible in this case as the tumor was in contact with the brainstem and upper cervical SC. Delivering the full 70 Gy to these areas of tumor would have placed this patient at substantial risk of brainstem and/or SC toxicity. This report demonstrates that response-adapted RT with shrinking fields can allow for tumor control while avoiding toxicity to critical neural structures for cases of locally advanced NPC in which tumor is abutting these structures.
Bony regeneration of the skull base following RT has been reported in the literature, but in limited reviews. Early reports used plain radiography to follow changes. Unger and colleagues demonstrated the regeneration of bone using skull radiographs 4 to 6 months after completion of RT for NPC.8 More recent literature details the ability of bone to regenerate after RT based on CT findings. Fang and colleagues reported on 90 cases of NPC with skull base destruction, with 63% having bony regeneration on posttreatment CT.9 Most of the patients in Fang’s report had bony regeneration within 1 year of treatment, and in general, bony regeneration became more evident on imaging with longer follow-up. Of note, local control was significantly greater in patients with regeneration vs persistent destruction (77% vs 21%, P < .001). On multivariate analysis, complete tumor response was significantly associated with bony regeneration; other factors such as age, sex, radiation dose, and chemotherapy were not significantly associated with the likelihood of bony regeneration.
Our report details a nasopharyngeal tumor that destroyed the skull base with no intact bony barrier. In such cases, concern arises regarding craniospinal instability with tumor regression if there is not simultaneous bone regeneration. Tumor invasion of the skull base and C1-2 vertebral bodies and complications from treatment of such tumor extent can lead to symptoms of craniospinal instability, including pain, difficulty with neck range of motion, and loss of strength and sensation in the upper and lower extremities.10 A case report of a woman treated with chemoradiation for a plasmacytoma of the skull base detailed her posttreatment presentation with quadriparesis resulting from craniospinal instability after tumor regression.11 Such instability is generally treated surgically, and during this woman’s surgery, there was an injury to the right vertebral artery, although this did not cause any additional neurologic deficits.
RT leads to hypocellularity, hypovascularity, and hypoxia of treated tissues, resulting in a reduced ability for growth and healing. Studies demonstrate that irradiated bone contains fewer osteoblast cells and osteocytes than unirradiated bone, resulting in reduced regenerative capacity.12,13 Furthermore, the reconstruction of bony defects resulting after cancer treatment has been shown to be difficult and associated with a high risk of complications.14 Given the impaired ability of irradiated bone to regenerate, studies have evaluated the use of growth factors and gene therapy to promote bone formation after treatment.15 Bone marrow stem cells have been shown to reverse radiation-induced cellular depletion and to increase osteocyte counts in animal studies.12 Further, overexpression of miR-34a, a tumor suppressor involved in tissue development, has been shown to improve osteoblastic differentiation of irradiated bone marrow stem cells and promote bone regeneration in vitro and in animal studies.13 While several techniques are being studied in vitro and in animal studies to promote bony regeneration after RT, there is a lack of data on use of these techniques in humans with cancer.
With our case, there was great uncertainty related to the ability of bone to regenerate during treatment and concern regarding consequences of formation of a skull base defect during treatment. CT imaging revealed bony regeneration of the central skull base and clivus, as well as occipital condyles, that occurred throughout the RT course. There was clear evidence of bone regeneration on the replanning CT obtained 5 weeks after treatment initiation. To our knowledge, this is the first report to demonstrate rapid bony regeneration during RT, thereby maintaining the integrity of the skull base and precluding the need for neurosurgical intervention. Moving forward, imaging should be considered during treatment for patients with tumor-related destruction of the skull base and upper cervical spine to evaluate the extent of bony regeneration during treatment and estimate the potential risk of craniocervical instability. Further studies with imaging during treatment are needed for more information on the likelihood of bony regeneration and factors that correlate with bony regeneration during treatment. As in other reports, our case demonstrates that bony regeneration may predict complete response to RT.9
Our patient’s tumor was HPV-positive and EBV-negative. In the US, the rate of HPV-positive NPC is 35%.16 However, HPV-positive NPC is much less common in endemic areas. A recent study from China of 1,328 patients with NPC revealed a 6.4% rate of HPV-positive/EBV-negative cases.17 In that study, patients with HPV-positive/EBV-negative tumors had improved survival compared to patients whose tumors were HPV-negative/EBV-positive. Another study suggests that the impact of HPV in NPC varies according to race, with HPV-positivity predicting for improved outcomes in East Asian patients and worse outcomes in White patients.17 A study from the University of Michigan suggests that both HPV-positive/EBV-negative and HPV-negative/EBV-negative NPC are associated with worse overall survival and locoregional control than EBV-positive NPC.2 Overall, the prognostic role of HPV in NPC remains unclear given conflicting information in the literature and the lack of large population studies.18
Conclusions
There is a paucity of literature on bony regeneration in patients with skull base destruction from advanced NPC, and in particular, the ability of skull base regeneration to occur during treatment simultaneous with tumor regression. Our patient had HPV-positive/EBV-negative NPC, but it is unclear how this subtype affected his prognosis. Factors such as tumor histology, radiosensitivity with rapid tumor regression, and young age may have all contributed to the rapidity of bone regeneration in our patient. This case report demonstrates that an impressive tumor response to chemoradiation with simultaneous bony regeneration is possible among patients presenting with tumor destruction of the skull base, precluding the need for neurosurgical intervention.
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777. doi:10.1158/1055-9965.EPI-06-0353
2. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi:10.1016/j.ijrobp.2013.11.246
3. Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562-567. doi:10.1002/hed.21216
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-0
5. Roh JL, Sung MW, Kim KH, et al.. Nasopharyngeal carcinoma with skull base invasion: a necessity of staging subdivision. Am J Otolaryngol. 2004;25(1):26-32. doi:10.1016/j.amjoto.2003.09.011
6. Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine. 1998;23(11):1280-1282. doi:10.1097/00007632-199806010-00021
7. Morgan HE, Sher DJ. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck. 2020;5:1. doi:10.1186/s41199-019-0046-z
8. Unger JD, Chiang LC, Unger GF. Apparent reformation of the base of the skull following radiotherapy for nasopharyngeal carcinoma. Radiology. 1978;126(3):779-782. doi:10.1148/126.3.779
9. Fang FM, Leung SW, Wang CJ, et al. Computed tomography findings of bony regeneration after radiotherapy for nasopharyngeal carcinoma with skull base destruction: implications for local control. Int J Radiat Oncol Biol Phys. 1999;44(2):305-309. doi:10.1016/s0360-3016(99)00004-8
10. Tiruchelvarayan R, Lee KA, Ng I. Surgery for atlanto-axial (C1-2) involvement or instability in nasopharyngeal carcinoma patients. Singapore Med J. 2012;53(6):416-421.
11. Samprón N, Arrazola M, Urculo E. Skull-base plasmacytoma with craniocervical instability [in Spanish]. Neurocirugia (Astur). 2009;20(5):478-483.
12. Zheutlin AR, Deshpande SS, Nelson NS, et al. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: a histomorphometric evaluation. Cytotherapy. 2016;18(5):664-672. doi:10.1016/j.jcyt.2016.01.013
13. Liu H, Dong Y, Feng X, et al. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblast differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi:10.1186/s13287-019-1285-y
14. Holzapfel BM, Wagner F, Martine LC, et al. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35(3):475-487. doi:10.1007/s10555-016-9635-z
15. Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77-81. doi:10.1177/0022034509352151
16. Wotman M, Oh EJ, Ahn S, Kraus D, Constantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi:10.1016/j.amjoto.2019.06.00717. Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530-536. doi:10.1002/cncr.31031.
18. Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi:10.1002/hed.24978
19. Lee AWM, Lydiatt WM, Colevas AD, et al. Nasopharynx. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:103.
20. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and genetics of head and neck tumors. In: World Health Organization Classification of Tumours. IARC Press; 2005.
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777. doi:10.1158/1055-9965.EPI-06-0353
2. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi:10.1016/j.ijrobp.2013.11.246
3. Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562-567. doi:10.1002/hed.21216
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-0
5. Roh JL, Sung MW, Kim KH, et al.. Nasopharyngeal carcinoma with skull base invasion: a necessity of staging subdivision. Am J Otolaryngol. 2004;25(1):26-32. doi:10.1016/j.amjoto.2003.09.011
6. Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine. 1998;23(11):1280-1282. doi:10.1097/00007632-199806010-00021
7. Morgan HE, Sher DJ. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck. 2020;5:1. doi:10.1186/s41199-019-0046-z
8. Unger JD, Chiang LC, Unger GF. Apparent reformation of the base of the skull following radiotherapy for nasopharyngeal carcinoma. Radiology. 1978;126(3):779-782. doi:10.1148/126.3.779
9. Fang FM, Leung SW, Wang CJ, et al. Computed tomography findings of bony regeneration after radiotherapy for nasopharyngeal carcinoma with skull base destruction: implications for local control. Int J Radiat Oncol Biol Phys. 1999;44(2):305-309. doi:10.1016/s0360-3016(99)00004-8
10. Tiruchelvarayan R, Lee KA, Ng I. Surgery for atlanto-axial (C1-2) involvement or instability in nasopharyngeal carcinoma patients. Singapore Med J. 2012;53(6):416-421.
11. Samprón N, Arrazola M, Urculo E. Skull-base plasmacytoma with craniocervical instability [in Spanish]. Neurocirugia (Astur). 2009;20(5):478-483.
12. Zheutlin AR, Deshpande SS, Nelson NS, et al. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: a histomorphometric evaluation. Cytotherapy. 2016;18(5):664-672. doi:10.1016/j.jcyt.2016.01.013
13. Liu H, Dong Y, Feng X, et al. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblast differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi:10.1186/s13287-019-1285-y
14. Holzapfel BM, Wagner F, Martine LC, et al. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35(3):475-487. doi:10.1007/s10555-016-9635-z
15. Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77-81. doi:10.1177/0022034509352151
16. Wotman M, Oh EJ, Ahn S, Kraus D, Constantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi:10.1016/j.amjoto.2019.06.00717. Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530-536. doi:10.1002/cncr.31031.
18. Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi:10.1002/hed.24978
19. Lee AWM, Lydiatt WM, Colevas AD, et al. Nasopharynx. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:103.
20. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and genetics of head and neck tumors. In: World Health Organization Classification of Tumours. IARC Press; 2005.
My choice? Unvaccinated pose outsize risk to vaccinated
according to a mathematical modeling study.
The study, which simulated patterns of infection among vaccinated and unvaccinated populations, showed that, as the populations mixed less, attack rates decreased among vaccinated people (from 15% to 10%) and increased among unvaccinated people (from 62% to 79%). The unvaccinated increasingly became the source of infection, however.
“When the vaccinated and unvaccinated mix, indirect protection is conferred upon the unvaccinated by the buffering effect of vaccinated individuals, and by contrast, risk in the vaccinated goes up,” lead author David Fisman, MD, professor of epidemiology at the University of Toronto, told this news organization.
As the groups mix less and less, the size of the epidemic increases among the unvaccinated and decreases among the vaccinated. “But the impact of the unvaccinated on risk in the vaccinated is disproportionate to the numbers of contacts between the two groups,” said Dr. Fisman.
The study was published online in the Canadian Medical Association Journal.
Relative contributions to risk
The researchers used a model of a respiratory viral disease “similar to SARS-CoV-2 infection with Delta variant.” They included reproduction values to capture the dynamics of the Omicron variant, which was emerging at the time. In the study, vaccines ranged in effectiveness from 40% to 80%. The study incorporated various levels of mixing between a partially vaccinated and an unvaccinated population. The mixing ranged from random mixing to like-with-like mixing (“assortativity”). There were three possible “compartments” of people in the model: those considered susceptible to infection, those considered infected and infectious, and those considered immune because of recovery.
The model showed that, as mixing between the vaccinated and the unvaccinated populations increased, case numbers rose, “with cases in the unvaccinated subpopulation accounting for a substantial proportion of infections.” However, as mixing between the populations decreased, the final attack rate decreased among vaccinated people, but the relative “contribution of risk to vaccinated people caused by infection acquired from contact with unvaccinated people ... increased.”
When the vaccination rate was increased in the model, case numbers among the vaccinated declined “as expected, owing to indirect protective effects,” the researchers noted. But this also “further increased the relative contribution to risk in vaccinated people by those who were unvaccinated.”
Self-regarding risk?
The findings show that “choices made by people who forgo vaccination contribute disproportionately to risk among those who do get vaccinated,” the researchers wrote. “Although risk associated with avoiding vaccination during a virulent pandemic accrues chiefly to those who are unvaccinated, the choice of some individuals to refuse vaccination is likely to affect the health and safety of vaccinated people in a manner disproportionate to the fraction of unvaccinated people in the population.”
The fact that like-with-like mixing cannot mitigate the risk to vaccinated people “undermines the assertion that vaccine choice is best left to the individual and supports strong public actions aimed at enhancing vaccine uptake and limiting access to public spaces for unvaccinated people,” they wrote.
Mandates and passports
“Our model provides support for vaccine mandates and passports during epidemics, such that vaccination is required for people to take part in nonessential activities,” said Dr. Fisman. The choice to not be vaccinated against COVID-19 should not be considered “self-regarding,” he added. “Risk is self-regarding when it only impacts the person engaging in the activity. Something like smoking cigarettes (alone, without others around) creates a lot of risk over time, but if nobody is breathing your secondhand smoke, you’re only creating risk for yourself. By contrast, we regulate, in Ontario, your right to smoke in public indoor spaces such as restaurants, because once other people are around, the risk isn’t self-regarding anymore. You’re creating risk for others.”
The authors also noted that the risks created by the unvaccinated extend beyond those of infection by “creating a risk that those around them may not be able to obtain the care they need.” They recommended that considerations of equity and justice for people who do choose to be vaccinated, as well as those who choose not to be, need to be included in formulating vaccination policy.
Illuminating the discussion
Asked to comment on the study, Matthew Oughton, MD, assistant professor of medicine at McGill University, Montreal, said: “It is easy to dismiss a mathematical model as a series of assumptions that leads to an implausible conclusion. ... However, they can serve to illustrate and, to an extent, quantify the results of complex interactions, and this study does just that.” Dr. Oughton was not involved in the research.
During the past 2 years, the scientific press and the general press have often discussed the individual and collective effects of disease-prevention methods, including nonpharmaceutical interventions. “Models like this can help illuminate those discussions by highlighting important consequences of preventive measures,” said Dr. Oughton, who also works in the division of infectious diseases at the Jewish General Hospital, Montreal.
It’s worth noting that the authors modeled vaccine effectiveness against all infection, “rather than the generally greater and more durable effects we have seen for vaccines in prevention of severe infection,” said Dr. Oughton. He added that the authors did not include the effect of vaccination in reducing forward transmission. “Inclusion of this effect would presumably have reduced overall infectious burden in mixed populations and increased the difference between groups at lower levels of mixing between populations.”
The research was supported by a grant from the Canadian Institutes of Health Research. Dr. Fisman has served on advisory boards related to influenza and SARS-CoV-2 vaccines for Seqirus, Pfizer, AstraZeneca, and Sanofi-Pasteur Vaccines and has served as a legal expert on issues related to COVID-19 epidemiology for the Elementary Teachers Federation of Ontario and the Registered Nurses Association of Ontario. Dr. Oughton disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a mathematical modeling study.
The study, which simulated patterns of infection among vaccinated and unvaccinated populations, showed that, as the populations mixed less, attack rates decreased among vaccinated people (from 15% to 10%) and increased among unvaccinated people (from 62% to 79%). The unvaccinated increasingly became the source of infection, however.
“When the vaccinated and unvaccinated mix, indirect protection is conferred upon the unvaccinated by the buffering effect of vaccinated individuals, and by contrast, risk in the vaccinated goes up,” lead author David Fisman, MD, professor of epidemiology at the University of Toronto, told this news organization.
As the groups mix less and less, the size of the epidemic increases among the unvaccinated and decreases among the vaccinated. “But the impact of the unvaccinated on risk in the vaccinated is disproportionate to the numbers of contacts between the two groups,” said Dr. Fisman.
The study was published online in the Canadian Medical Association Journal.
Relative contributions to risk
The researchers used a model of a respiratory viral disease “similar to SARS-CoV-2 infection with Delta variant.” They included reproduction values to capture the dynamics of the Omicron variant, which was emerging at the time. In the study, vaccines ranged in effectiveness from 40% to 80%. The study incorporated various levels of mixing between a partially vaccinated and an unvaccinated population. The mixing ranged from random mixing to like-with-like mixing (“assortativity”). There were three possible “compartments” of people in the model: those considered susceptible to infection, those considered infected and infectious, and those considered immune because of recovery.
The model showed that, as mixing between the vaccinated and the unvaccinated populations increased, case numbers rose, “with cases in the unvaccinated subpopulation accounting for a substantial proportion of infections.” However, as mixing between the populations decreased, the final attack rate decreased among vaccinated people, but the relative “contribution of risk to vaccinated people caused by infection acquired from contact with unvaccinated people ... increased.”
When the vaccination rate was increased in the model, case numbers among the vaccinated declined “as expected, owing to indirect protective effects,” the researchers noted. But this also “further increased the relative contribution to risk in vaccinated people by those who were unvaccinated.”
Self-regarding risk?
The findings show that “choices made by people who forgo vaccination contribute disproportionately to risk among those who do get vaccinated,” the researchers wrote. “Although risk associated with avoiding vaccination during a virulent pandemic accrues chiefly to those who are unvaccinated, the choice of some individuals to refuse vaccination is likely to affect the health and safety of vaccinated people in a manner disproportionate to the fraction of unvaccinated people in the population.”
The fact that like-with-like mixing cannot mitigate the risk to vaccinated people “undermines the assertion that vaccine choice is best left to the individual and supports strong public actions aimed at enhancing vaccine uptake and limiting access to public spaces for unvaccinated people,” they wrote.
Mandates and passports
“Our model provides support for vaccine mandates and passports during epidemics, such that vaccination is required for people to take part in nonessential activities,” said Dr. Fisman. The choice to not be vaccinated against COVID-19 should not be considered “self-regarding,” he added. “Risk is self-regarding when it only impacts the person engaging in the activity. Something like smoking cigarettes (alone, without others around) creates a lot of risk over time, but if nobody is breathing your secondhand smoke, you’re only creating risk for yourself. By contrast, we regulate, in Ontario, your right to smoke in public indoor spaces such as restaurants, because once other people are around, the risk isn’t self-regarding anymore. You’re creating risk for others.”
The authors also noted that the risks created by the unvaccinated extend beyond those of infection by “creating a risk that those around them may not be able to obtain the care they need.” They recommended that considerations of equity and justice for people who do choose to be vaccinated, as well as those who choose not to be, need to be included in formulating vaccination policy.
Illuminating the discussion
Asked to comment on the study, Matthew Oughton, MD, assistant professor of medicine at McGill University, Montreal, said: “It is easy to dismiss a mathematical model as a series of assumptions that leads to an implausible conclusion. ... However, they can serve to illustrate and, to an extent, quantify the results of complex interactions, and this study does just that.” Dr. Oughton was not involved in the research.
During the past 2 years, the scientific press and the general press have often discussed the individual and collective effects of disease-prevention methods, including nonpharmaceutical interventions. “Models like this can help illuminate those discussions by highlighting important consequences of preventive measures,” said Dr. Oughton, who also works in the division of infectious diseases at the Jewish General Hospital, Montreal.
It’s worth noting that the authors modeled vaccine effectiveness against all infection, “rather than the generally greater and more durable effects we have seen for vaccines in prevention of severe infection,” said Dr. Oughton. He added that the authors did not include the effect of vaccination in reducing forward transmission. “Inclusion of this effect would presumably have reduced overall infectious burden in mixed populations and increased the difference between groups at lower levels of mixing between populations.”
The research was supported by a grant from the Canadian Institutes of Health Research. Dr. Fisman has served on advisory boards related to influenza and SARS-CoV-2 vaccines for Seqirus, Pfizer, AstraZeneca, and Sanofi-Pasteur Vaccines and has served as a legal expert on issues related to COVID-19 epidemiology for the Elementary Teachers Federation of Ontario and the Registered Nurses Association of Ontario. Dr. Oughton disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a mathematical modeling study.
The study, which simulated patterns of infection among vaccinated and unvaccinated populations, showed that, as the populations mixed less, attack rates decreased among vaccinated people (from 15% to 10%) and increased among unvaccinated people (from 62% to 79%). The unvaccinated increasingly became the source of infection, however.
“When the vaccinated and unvaccinated mix, indirect protection is conferred upon the unvaccinated by the buffering effect of vaccinated individuals, and by contrast, risk in the vaccinated goes up,” lead author David Fisman, MD, professor of epidemiology at the University of Toronto, told this news organization.
As the groups mix less and less, the size of the epidemic increases among the unvaccinated and decreases among the vaccinated. “But the impact of the unvaccinated on risk in the vaccinated is disproportionate to the numbers of contacts between the two groups,” said Dr. Fisman.
The study was published online in the Canadian Medical Association Journal.
Relative contributions to risk
The researchers used a model of a respiratory viral disease “similar to SARS-CoV-2 infection with Delta variant.” They included reproduction values to capture the dynamics of the Omicron variant, which was emerging at the time. In the study, vaccines ranged in effectiveness from 40% to 80%. The study incorporated various levels of mixing between a partially vaccinated and an unvaccinated population. The mixing ranged from random mixing to like-with-like mixing (“assortativity”). There were three possible “compartments” of people in the model: those considered susceptible to infection, those considered infected and infectious, and those considered immune because of recovery.
The model showed that, as mixing between the vaccinated and the unvaccinated populations increased, case numbers rose, “with cases in the unvaccinated subpopulation accounting for a substantial proportion of infections.” However, as mixing between the populations decreased, the final attack rate decreased among vaccinated people, but the relative “contribution of risk to vaccinated people caused by infection acquired from contact with unvaccinated people ... increased.”
When the vaccination rate was increased in the model, case numbers among the vaccinated declined “as expected, owing to indirect protective effects,” the researchers noted. But this also “further increased the relative contribution to risk in vaccinated people by those who were unvaccinated.”
Self-regarding risk?
The findings show that “choices made by people who forgo vaccination contribute disproportionately to risk among those who do get vaccinated,” the researchers wrote. “Although risk associated with avoiding vaccination during a virulent pandemic accrues chiefly to those who are unvaccinated, the choice of some individuals to refuse vaccination is likely to affect the health and safety of vaccinated people in a manner disproportionate to the fraction of unvaccinated people in the population.”
The fact that like-with-like mixing cannot mitigate the risk to vaccinated people “undermines the assertion that vaccine choice is best left to the individual and supports strong public actions aimed at enhancing vaccine uptake and limiting access to public spaces for unvaccinated people,” they wrote.
Mandates and passports
“Our model provides support for vaccine mandates and passports during epidemics, such that vaccination is required for people to take part in nonessential activities,” said Dr. Fisman. The choice to not be vaccinated against COVID-19 should not be considered “self-regarding,” he added. “Risk is self-regarding when it only impacts the person engaging in the activity. Something like smoking cigarettes (alone, without others around) creates a lot of risk over time, but if nobody is breathing your secondhand smoke, you’re only creating risk for yourself. By contrast, we regulate, in Ontario, your right to smoke in public indoor spaces such as restaurants, because once other people are around, the risk isn’t self-regarding anymore. You’re creating risk for others.”
The authors also noted that the risks created by the unvaccinated extend beyond those of infection by “creating a risk that those around them may not be able to obtain the care they need.” They recommended that considerations of equity and justice for people who do choose to be vaccinated, as well as those who choose not to be, need to be included in formulating vaccination policy.
Illuminating the discussion
Asked to comment on the study, Matthew Oughton, MD, assistant professor of medicine at McGill University, Montreal, said: “It is easy to dismiss a mathematical model as a series of assumptions that leads to an implausible conclusion. ... However, they can serve to illustrate and, to an extent, quantify the results of complex interactions, and this study does just that.” Dr. Oughton was not involved in the research.
During the past 2 years, the scientific press and the general press have often discussed the individual and collective effects of disease-prevention methods, including nonpharmaceutical interventions. “Models like this can help illuminate those discussions by highlighting important consequences of preventive measures,” said Dr. Oughton, who also works in the division of infectious diseases at the Jewish General Hospital, Montreal.
It’s worth noting that the authors modeled vaccine effectiveness against all infection, “rather than the generally greater and more durable effects we have seen for vaccines in prevention of severe infection,” said Dr. Oughton. He added that the authors did not include the effect of vaccination in reducing forward transmission. “Inclusion of this effect would presumably have reduced overall infectious burden in mixed populations and increased the difference between groups at lower levels of mixing between populations.”
The research was supported by a grant from the Canadian Institutes of Health Research. Dr. Fisman has served on advisory boards related to influenza and SARS-CoV-2 vaccines for Seqirus, Pfizer, AstraZeneca, and Sanofi-Pasteur Vaccines and has served as a legal expert on issues related to COVID-19 epidemiology for the Elementary Teachers Federation of Ontario and the Registered Nurses Association of Ontario. Dr. Oughton disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
How social determinants of health impact disparities in IBD care, outcomes
The incidence of inflammatory bowel disease (IBD) is on the rise among racial and ethnic minority groups in the United States, and social determinants of health (SDOH) contribute to disparities in IBD care and outcome, say the authors of a new paper on the topic.
It’s an “overdue priority to acknowledge the weight and influence of the SDOH on health disparities in IBD care,” write Adjoa Anyane-Yeboa, MD, PhD, with Massachusetts General Hospital, Boston, and co-authors.
“Only after this acknowledgement can we begin to develop alternative systems that work to rectify the deleterious effects of our current policies in a more longitudinal and effective manner,” they say.
Their paper was published online in Clinical Gastroenterology and Hepatology.
Upstream factors propagate downstream outcomes
The authors found multiple examples in the literature of how upstream SDOH (for example, racism, poverty, neighborhood violence, and under-insurance) lead to midstream SDOH (for example, lack of social support, lack of access to specialized IBD care, poor housing conditions, and food insecurity) that result in poor downstream outcomes in IBD (for example, delayed diagnosis, increased disease activity, IBD flares, and suboptimal medical management).
The IBD literature shows that Black/African American adults with IBD often have worse outcomes across the IBD care continuum than White peers, with higher hospitalization rates, longer stays, increased hospitalization costs, higher readmission rates, and more complications after IBD surgery.
Unequal access to specialized IBD care is a factor, with Black/African American patients less likely to undergo annual visits to a gastroenterologist or IBD specialist, twice as likely than White patients to visit the emergency department over a 12-month period, and less likely to receive treatment with infliximab.
As has been shown for other chronic digestive diseases and cancers, disparities in outcomes related to IBD exist across race, ethnicity, differential insurance status and coverage, and socioeconomic status, the authors note.
Yet, they point out that, interestingly, a 2021 study of patients with Medicaid insurance from four states revealed no disparities in the use of IBD-specific medications between Black/African American and White patients, suggesting that when access to care is equal, disparities diminish.
Target multiple stakeholders to achieve IBD health equity
Achieving health equity in IBD will require strategies targeting medical trainees, providers, practices, and health systems, as well as community and industry leaders and policymakers, Dr. Anyane-Yeboa and colleagues say.
At the medical trainee level, racism and bias should be addressed early in medical student, resident, and fellow training and education. Curricula should move away from race-based training, where race is considered an independent risk factor for disease and often used to guide differential diagnoses and treatment, they suggest.
At the provider level, they say self-reflection around one’s own beliefs, biases, perceptions, and interactions with diverse and vulnerable patient groups is “paramount.” Individual self-reflection should be coupled with mandatory and effective implicit bias and anti-racism training.
At the practice or hospital system level, screening for SDOH at the point of care, addressing barriers to needed treatment, and connecting patients to appropriate resources are all important, they write.
The researchers also call for policy-level changes to increase funding for health equity research, which is historically undervalued and underfunded.
“Focusing on SDOH as the root cause of health inequity in IBD is essential to improve outcomes for marginalized patients,” they write.
Given that research describing specific interventions to address SDOH in IBD is currently nonexistent, “our paper serves as a call to action for more work to be done in this area,” they say.
“As medical providers and health care organizations, we all have a responsibility to address the SDOH when caring for our patients in order to provide each patient with IBD the opportunity to achieve the best health possible,” they conclude.
This research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The incidence of inflammatory bowel disease (IBD) is on the rise among racial and ethnic minority groups in the United States, and social determinants of health (SDOH) contribute to disparities in IBD care and outcome, say the authors of a new paper on the topic.
It’s an “overdue priority to acknowledge the weight and influence of the SDOH on health disparities in IBD care,” write Adjoa Anyane-Yeboa, MD, PhD, with Massachusetts General Hospital, Boston, and co-authors.
“Only after this acknowledgement can we begin to develop alternative systems that work to rectify the deleterious effects of our current policies in a more longitudinal and effective manner,” they say.
Their paper was published online in Clinical Gastroenterology and Hepatology.
Upstream factors propagate downstream outcomes
The authors found multiple examples in the literature of how upstream SDOH (for example, racism, poverty, neighborhood violence, and under-insurance) lead to midstream SDOH (for example, lack of social support, lack of access to specialized IBD care, poor housing conditions, and food insecurity) that result in poor downstream outcomes in IBD (for example, delayed diagnosis, increased disease activity, IBD flares, and suboptimal medical management).
The IBD literature shows that Black/African American adults with IBD often have worse outcomes across the IBD care continuum than White peers, with higher hospitalization rates, longer stays, increased hospitalization costs, higher readmission rates, and more complications after IBD surgery.
Unequal access to specialized IBD care is a factor, with Black/African American patients less likely to undergo annual visits to a gastroenterologist or IBD specialist, twice as likely than White patients to visit the emergency department over a 12-month period, and less likely to receive treatment with infliximab.
As has been shown for other chronic digestive diseases and cancers, disparities in outcomes related to IBD exist across race, ethnicity, differential insurance status and coverage, and socioeconomic status, the authors note.
Yet, they point out that, interestingly, a 2021 study of patients with Medicaid insurance from four states revealed no disparities in the use of IBD-specific medications between Black/African American and White patients, suggesting that when access to care is equal, disparities diminish.
Target multiple stakeholders to achieve IBD health equity
Achieving health equity in IBD will require strategies targeting medical trainees, providers, practices, and health systems, as well as community and industry leaders and policymakers, Dr. Anyane-Yeboa and colleagues say.
At the medical trainee level, racism and bias should be addressed early in medical student, resident, and fellow training and education. Curricula should move away from race-based training, where race is considered an independent risk factor for disease and often used to guide differential diagnoses and treatment, they suggest.
At the provider level, they say self-reflection around one’s own beliefs, biases, perceptions, and interactions with diverse and vulnerable patient groups is “paramount.” Individual self-reflection should be coupled with mandatory and effective implicit bias and anti-racism training.
At the practice or hospital system level, screening for SDOH at the point of care, addressing barriers to needed treatment, and connecting patients to appropriate resources are all important, they write.
The researchers also call for policy-level changes to increase funding for health equity research, which is historically undervalued and underfunded.
“Focusing on SDOH as the root cause of health inequity in IBD is essential to improve outcomes for marginalized patients,” they write.
Given that research describing specific interventions to address SDOH in IBD is currently nonexistent, “our paper serves as a call to action for more work to be done in this area,” they say.
“As medical providers and health care organizations, we all have a responsibility to address the SDOH when caring for our patients in order to provide each patient with IBD the opportunity to achieve the best health possible,” they conclude.
This research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The incidence of inflammatory bowel disease (IBD) is on the rise among racial and ethnic minority groups in the United States, and social determinants of health (SDOH) contribute to disparities in IBD care and outcome, say the authors of a new paper on the topic.
It’s an “overdue priority to acknowledge the weight and influence of the SDOH on health disparities in IBD care,” write Adjoa Anyane-Yeboa, MD, PhD, with Massachusetts General Hospital, Boston, and co-authors.
“Only after this acknowledgement can we begin to develop alternative systems that work to rectify the deleterious effects of our current policies in a more longitudinal and effective manner,” they say.
Their paper was published online in Clinical Gastroenterology and Hepatology.
Upstream factors propagate downstream outcomes
The authors found multiple examples in the literature of how upstream SDOH (for example, racism, poverty, neighborhood violence, and under-insurance) lead to midstream SDOH (for example, lack of social support, lack of access to specialized IBD care, poor housing conditions, and food insecurity) that result in poor downstream outcomes in IBD (for example, delayed diagnosis, increased disease activity, IBD flares, and suboptimal medical management).
The IBD literature shows that Black/African American adults with IBD often have worse outcomes across the IBD care continuum than White peers, with higher hospitalization rates, longer stays, increased hospitalization costs, higher readmission rates, and more complications after IBD surgery.
Unequal access to specialized IBD care is a factor, with Black/African American patients less likely to undergo annual visits to a gastroenterologist or IBD specialist, twice as likely than White patients to visit the emergency department over a 12-month period, and less likely to receive treatment with infliximab.
As has been shown for other chronic digestive diseases and cancers, disparities in outcomes related to IBD exist across race, ethnicity, differential insurance status and coverage, and socioeconomic status, the authors note.
Yet, they point out that, interestingly, a 2021 study of patients with Medicaid insurance from four states revealed no disparities in the use of IBD-specific medications between Black/African American and White patients, suggesting that when access to care is equal, disparities diminish.
Target multiple stakeholders to achieve IBD health equity
Achieving health equity in IBD will require strategies targeting medical trainees, providers, practices, and health systems, as well as community and industry leaders and policymakers, Dr. Anyane-Yeboa and colleagues say.
At the medical trainee level, racism and bias should be addressed early in medical student, resident, and fellow training and education. Curricula should move away from race-based training, where race is considered an independent risk factor for disease and often used to guide differential diagnoses and treatment, they suggest.
At the provider level, they say self-reflection around one’s own beliefs, biases, perceptions, and interactions with diverse and vulnerable patient groups is “paramount.” Individual self-reflection should be coupled with mandatory and effective implicit bias and anti-racism training.
At the practice or hospital system level, screening for SDOH at the point of care, addressing barriers to needed treatment, and connecting patients to appropriate resources are all important, they write.
The researchers also call for policy-level changes to increase funding for health equity research, which is historically undervalued and underfunded.
“Focusing on SDOH as the root cause of health inequity in IBD is essential to improve outcomes for marginalized patients,” they write.
Given that research describing specific interventions to address SDOH in IBD is currently nonexistent, “our paper serves as a call to action for more work to be done in this area,” they say.
“As medical providers and health care organizations, we all have a responsibility to address the SDOH when caring for our patients in order to provide each patient with IBD the opportunity to achieve the best health possible,” they conclude.
This research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
When coping skills and parenting behavioral interventions ‘don’t work’
You have an appointment with a 14-year-old youth you last saw for an annual camp physical. He had screened positive for depression, and you had referred him to a local therapist. He did not have an appointment until after camp, and you have only met a few times, but since you had spoken with him about his depression, he set up an appointment with you to ask about medications. When you meet him you ask about what he had been doing in therapy and he says, “I’m learning ‘coping skills,’ but they don’t work.”
From breathing exercises and sticker charts to mindfulness and grounding exercise, coping skills can be crucial for learning how to manage distress, regulate emotions, become more effective interpersonally, and function better. Similarly, parenting interventions, which change the way parents and youth interact, are a central family intervention for behavioral problems in youth.
It is very common, however, to hear that they “don’t work” or have a parent say, “We tried that, it doesn’t work.”
When kids and parents reject coping skills and behavioral interventions by saying they do not work, the consequences can be substantial. It can mean the rejection of coping skills and strategies that actually would have helped, given time and support; that kids and families bounce between services with increasing frustration; that they search for a magic bullet (which also won’t work); and, particularly concerning for physicians, a belief that the youth have not received the right medication, resulting in potentially unhelpful concoctions of medication.
One of the biggest challenges in helping youth and parents overcome their difficulties – whether these difficulties are depression and anxiety or being better parents to struggling kids – is helping them understand that despite the fact that coping skills and behavioral interventions do not seem to work, they work.
We just have to do a better job explaining what that “work” is.
There are five points you can make.
- First, the coping skill or behavioral intervention is not supposed to work if that means solving the underlying problem. Coping skills and behavioral interventions do not immediately cure anxiety, mend broken hearts, correct disruptive behaviors, disentangle power struggles, or alleviate depression. That is not what their job is. Coping skills and behavioral interventions are there to help us get better at handling complex situations and feelings. In particular, they are good at helping us manage our thoughts (“I can’t do it,” “He should behave better”) and our affect (anger, frustration, rage, anxiety, sadness), so that over time we get better at solving the problems, and break out of the patterns that perpetuate these problems.
- Second, kids and parents do not give skills credit for when they do work. That time you were spiraling out of control and told your mom you needed a break and watched some YouTube videos and then joined the family for dinner? Your coping skills worked, but nobody noticed because they worked. We need to help our young patients and families identify those times that coping skills and behavioral interventions worked.
- Third, let’s face it: Nothing works all the time. It is no wonder kids and families are disappointed by coping skills and behavioral interventions if they think they magically work once and forever. We need to manage expectations.
- Fourth, we know they are supposed to fail, and we should discuss this openly up front. This may sound surprising, but challenging behaviors often get worse when we begin to work on them. “Extinction bursts” is probably the easiest explanation, but for psychodynamically oriented youth and families we could talk about “resistance.” No matter what, things tend to get worse before they get better. We should let people know this ahead of time.
- Fifth, and this is the one that forces youth and parents to ask how hard they actually tried, these skills need to be practiced. You can’t be in the middle of a panic attack and for the first time start trying to pace your breathing with a technique a therapist told you about 3 weeks ago. This makes about as much sense as not training for a marathon. You need to practice and build up the skills, recognizing that as you become more familiar with them, they will help you manage during stressful situations. Every skill should be practiced, preferably several times or more in sessions, maybe every session, and definitely outside of sessions when not in distress.
We cannot blame children and parents for thinking that coping skills and behavioral interventions do not work. They are struggling, suffering, fighting, frightened, angry, anxious, frustrated, and often desperate for something to make everything better. Helping them recognize this desire for things to be better while managing expectations is an essential complement to supporting the use of coping skills and behavioral interventions, and a fairly easy conversation to have with youth.
So when you are talking about coping skills and parental behavioral interventions, it is important to be prepared for the “it didn’t work” conversation, and even to address these issues up front. After all, these strategies may not solve all the problems in the world, but can be lifelong ways of coping with life’s challenges.
Dr. Henderson is associate professor of clinical psychiatry at New York University and deputy director of child and adolescent psychiatry at Bellevue Hospital, New York.
You have an appointment with a 14-year-old youth you last saw for an annual camp physical. He had screened positive for depression, and you had referred him to a local therapist. He did not have an appointment until after camp, and you have only met a few times, but since you had spoken with him about his depression, he set up an appointment with you to ask about medications. When you meet him you ask about what he had been doing in therapy and he says, “I’m learning ‘coping skills,’ but they don’t work.”
From breathing exercises and sticker charts to mindfulness and grounding exercise, coping skills can be crucial for learning how to manage distress, regulate emotions, become more effective interpersonally, and function better. Similarly, parenting interventions, which change the way parents and youth interact, are a central family intervention for behavioral problems in youth.
It is very common, however, to hear that they “don’t work” or have a parent say, “We tried that, it doesn’t work.”
When kids and parents reject coping skills and behavioral interventions by saying they do not work, the consequences can be substantial. It can mean the rejection of coping skills and strategies that actually would have helped, given time and support; that kids and families bounce between services with increasing frustration; that they search for a magic bullet (which also won’t work); and, particularly concerning for physicians, a belief that the youth have not received the right medication, resulting in potentially unhelpful concoctions of medication.
One of the biggest challenges in helping youth and parents overcome their difficulties – whether these difficulties are depression and anxiety or being better parents to struggling kids – is helping them understand that despite the fact that coping skills and behavioral interventions do not seem to work, they work.
We just have to do a better job explaining what that “work” is.
There are five points you can make.
- First, the coping skill or behavioral intervention is not supposed to work if that means solving the underlying problem. Coping skills and behavioral interventions do not immediately cure anxiety, mend broken hearts, correct disruptive behaviors, disentangle power struggles, or alleviate depression. That is not what their job is. Coping skills and behavioral interventions are there to help us get better at handling complex situations and feelings. In particular, they are good at helping us manage our thoughts (“I can’t do it,” “He should behave better”) and our affect (anger, frustration, rage, anxiety, sadness), so that over time we get better at solving the problems, and break out of the patterns that perpetuate these problems.
- Second, kids and parents do not give skills credit for when they do work. That time you were spiraling out of control and told your mom you needed a break and watched some YouTube videos and then joined the family for dinner? Your coping skills worked, but nobody noticed because they worked. We need to help our young patients and families identify those times that coping skills and behavioral interventions worked.
- Third, let’s face it: Nothing works all the time. It is no wonder kids and families are disappointed by coping skills and behavioral interventions if they think they magically work once and forever. We need to manage expectations.
- Fourth, we know they are supposed to fail, and we should discuss this openly up front. This may sound surprising, but challenging behaviors often get worse when we begin to work on them. “Extinction bursts” is probably the easiest explanation, but for psychodynamically oriented youth and families we could talk about “resistance.” No matter what, things tend to get worse before they get better. We should let people know this ahead of time.
- Fifth, and this is the one that forces youth and parents to ask how hard they actually tried, these skills need to be practiced. You can’t be in the middle of a panic attack and for the first time start trying to pace your breathing with a technique a therapist told you about 3 weeks ago. This makes about as much sense as not training for a marathon. You need to practice and build up the skills, recognizing that as you become more familiar with them, they will help you manage during stressful situations. Every skill should be practiced, preferably several times or more in sessions, maybe every session, and definitely outside of sessions when not in distress.
We cannot blame children and parents for thinking that coping skills and behavioral interventions do not work. They are struggling, suffering, fighting, frightened, angry, anxious, frustrated, and often desperate for something to make everything better. Helping them recognize this desire for things to be better while managing expectations is an essential complement to supporting the use of coping skills and behavioral interventions, and a fairly easy conversation to have with youth.
So when you are talking about coping skills and parental behavioral interventions, it is important to be prepared for the “it didn’t work” conversation, and even to address these issues up front. After all, these strategies may not solve all the problems in the world, but can be lifelong ways of coping with life’s challenges.
Dr. Henderson is associate professor of clinical psychiatry at New York University and deputy director of child and adolescent psychiatry at Bellevue Hospital, New York.
You have an appointment with a 14-year-old youth you last saw for an annual camp physical. He had screened positive for depression, and you had referred him to a local therapist. He did not have an appointment until after camp, and you have only met a few times, but since you had spoken with him about his depression, he set up an appointment with you to ask about medications. When you meet him you ask about what he had been doing in therapy and he says, “I’m learning ‘coping skills,’ but they don’t work.”
From breathing exercises and sticker charts to mindfulness and grounding exercise, coping skills can be crucial for learning how to manage distress, regulate emotions, become more effective interpersonally, and function better. Similarly, parenting interventions, which change the way parents and youth interact, are a central family intervention for behavioral problems in youth.
It is very common, however, to hear that they “don’t work” or have a parent say, “We tried that, it doesn’t work.”
When kids and parents reject coping skills and behavioral interventions by saying they do not work, the consequences can be substantial. It can mean the rejection of coping skills and strategies that actually would have helped, given time and support; that kids and families bounce between services with increasing frustration; that they search for a magic bullet (which also won’t work); and, particularly concerning for physicians, a belief that the youth have not received the right medication, resulting in potentially unhelpful concoctions of medication.
One of the biggest challenges in helping youth and parents overcome their difficulties – whether these difficulties are depression and anxiety or being better parents to struggling kids – is helping them understand that despite the fact that coping skills and behavioral interventions do not seem to work, they work.
We just have to do a better job explaining what that “work” is.
There are five points you can make.
- First, the coping skill or behavioral intervention is not supposed to work if that means solving the underlying problem. Coping skills and behavioral interventions do not immediately cure anxiety, mend broken hearts, correct disruptive behaviors, disentangle power struggles, or alleviate depression. That is not what their job is. Coping skills and behavioral interventions are there to help us get better at handling complex situations and feelings. In particular, they are good at helping us manage our thoughts (“I can’t do it,” “He should behave better”) and our affect (anger, frustration, rage, anxiety, sadness), so that over time we get better at solving the problems, and break out of the patterns that perpetuate these problems.
- Second, kids and parents do not give skills credit for when they do work. That time you were spiraling out of control and told your mom you needed a break and watched some YouTube videos and then joined the family for dinner? Your coping skills worked, but nobody noticed because they worked. We need to help our young patients and families identify those times that coping skills and behavioral interventions worked.
- Third, let’s face it: Nothing works all the time. It is no wonder kids and families are disappointed by coping skills and behavioral interventions if they think they magically work once and forever. We need to manage expectations.
- Fourth, we know they are supposed to fail, and we should discuss this openly up front. This may sound surprising, but challenging behaviors often get worse when we begin to work on them. “Extinction bursts” is probably the easiest explanation, but for psychodynamically oriented youth and families we could talk about “resistance.” No matter what, things tend to get worse before they get better. We should let people know this ahead of time.
- Fifth, and this is the one that forces youth and parents to ask how hard they actually tried, these skills need to be practiced. You can’t be in the middle of a panic attack and for the first time start trying to pace your breathing with a technique a therapist told you about 3 weeks ago. This makes about as much sense as not training for a marathon. You need to practice and build up the skills, recognizing that as you become more familiar with them, they will help you manage during stressful situations. Every skill should be practiced, preferably several times or more in sessions, maybe every session, and definitely outside of sessions when not in distress.
We cannot blame children and parents for thinking that coping skills and behavioral interventions do not work. They are struggling, suffering, fighting, frightened, angry, anxious, frustrated, and often desperate for something to make everything better. Helping them recognize this desire for things to be better while managing expectations is an essential complement to supporting the use of coping skills and behavioral interventions, and a fairly easy conversation to have with youth.
So when you are talking about coping skills and parental behavioral interventions, it is important to be prepared for the “it didn’t work” conversation, and even to address these issues up front. After all, these strategies may not solve all the problems in the world, but can be lifelong ways of coping with life’s challenges.
Dr. Henderson is associate professor of clinical psychiatry at New York University and deputy director of child and adolescent psychiatry at Bellevue Hospital, New York.
Hospital factors tied to lower maternal morbidity
A new study of hospitals in New York City suggests ways to reduce severe maternal morbidity (SMM). The researchers interviewed health care professionals in four institutions with low performance and four with high performance, and identified various themes associated with good performance.
“Our results raise the hypothesis that hospital learning collaboratives focused on optimizing organizational practices and policies, increasing clinician and staff awareness and education on maternal health disparities, and addressing structural racism may be important tools for improving equity in maternal outcomes,” the authors wrote in the study, published in Obstetrics & Gynecology.
The researchers conducted 50 semistructured interviews with health care professionals at lower-performing and higher-performing New York City hospitals, which were selected based on risk-adjusted morbidity metrics. The interviews explored various topics, including structural characteristics like staffing, organizational characteristics like culture and communication, labor and delivery practices such as teamwork and use of evidence-based practices, and racial and ethnic disparities.
The analysis revealed six broad areas that were stronger in high-performing hospitals: day-to-day involvement of leadership in quality activities, an emphasis on standards and standardized care, good communication and teamwork between nurses and physicians, good staffing and supervision among physicians and nurses, sharing of performance data with health care workers, and acknowledgment of the existence of racial and ethnic disparities and that bias can cause treatment differences.
“I think this qualitative approach is an important lens to pair with the quantitative approach. With such variability in severe maternal morbidity between hospitals in New York, it is not enough to just look at the quantitative data. To understand how to improve you must examine structures and processes. The structures, which are the physical and organizational characteristics in health care, and the process, which is how health care is delivered,” Veronica Gillispie-Bell, MD, wrote in a comment. Dr. Gillispie-Bell is medical director at Louisiana Perinatal Quality Collaborative and the Pregnancy-Associated Mortality Review for the Louisiana Department of Health.
“We know that high reliability organizations are those who are preoccupied with quality and safety. That means accountability from leadership (structure) and stability in standardization of care (processes). However, none of this matters if you do not have a culture that promotes safety. Based on the key findings of the high-performing hospitals, there was a culture that promoted safety and quality evidenced in the nurse-physician communication and the transparency around data through a lens of equity,” wrote Dr. Gillispie-Bell.
She noted that the study should encourage low-performing hospitals, since it illustrates avenues for improvement. Her personal experience reflects that, though she said that hospitals need help. The Louisiana Perinatal Quality Collaborative addressed severe maternal morbidity at birthing centers by implementing evidence-based best practices for management of hypertension and hemorrhage along with health equity measures. The team conducted coaching calls, in-person learning sessions, and in-person visits through a “Listening Tour.”
The result was a 35% reduction in hemorrhage overall and a reduction of 49% in hemorrhage in Black women, as well as hypertension by 12% overall between August 2018 and May 2020. Not all the news was good, as Black women still had an increase in severe maternal morbidity, possibly because of the COVID epidemic, since it is a risk factor for hypertension during pregnancy and infection rates are higher among Black individuals. “We need support for state based perinatal quality collaboratives to do this work and we need accountability as we are now seeing from metrics being implemented by [the Centers for Medicare & Medicaid Services]. Hospitals need to stratify their data by race and ethnicity to see where there are disparities in their outcomes,” said Dr. Gillispie-Bell.
The improvements are needed, given that the United States has the highest rates of maternal mortality and morbidity among developed countries, “most of which is preventable, and we have significant inequities by race and ethnicity,” said Laurie Zephyrin, MD, vice president for advancing health equity at the Commonwealth Fund. The question becomes how to effect change, and “there’s a lot happening in the policy space. Some of this policy change is directed at expanding insurance coverage, including more opportunities, including funding for community health workers and doulas, and thinking about how to incorporate midwives. There’s also work around how do we actually improve the care delivered by our health system.” Dr. Zephyrin added that the Department of Health & Human Services has contracted with the health improvement company Premier to use data and best-practices to improve maternal health.
The new work has the potential to be complementary to such approaches. “It provides some structure around how to approach some of the solutions, none of which I think is rocket science. It’s just something that needs to be focused on more intentionally,” said Dr. Zephyrin.
For example, the report found that high-performing hospitals had leaders who collaborated with frontline clinicians to share performance data, and this occurred in person, at departmental quality meetings, and during grand rounds. In contrast, staff in low-performing hospitals did not mention data feedback and some said that their institution made little effort to communicate performance metrics to frontline staff.
“One of the key lessons from the pandemic is that we need to have better data, and we need to have data around race and ethnicity to be able to understand the impact on marginalized communities. This study highlights that there’s more to be done around data to ensure that we can truly move the needle on advancing health equity,” said Dr. Zephyrin.
The researchers also found that clinicians in low-performing institutions did not acknowledge the presence of structural racism or differences in care associated with race or ethnicity. When they acknowledge differences in care, they attributed them to factors outside of the hospital’s control, such as patients not seeking out health care or not maintaining a healthy weight. Clinicians at high-performing hospitals were more likely to explicitly mention racism and bias and acknowledged that these factors could contribute to differences in care.
Dr. Gillispie-Bell and Dr. Zephyrin have no relevant financial disclosures.
A new study of hospitals in New York City suggests ways to reduce severe maternal morbidity (SMM). The researchers interviewed health care professionals in four institutions with low performance and four with high performance, and identified various themes associated with good performance.
“Our results raise the hypothesis that hospital learning collaboratives focused on optimizing organizational practices and policies, increasing clinician and staff awareness and education on maternal health disparities, and addressing structural racism may be important tools for improving equity in maternal outcomes,” the authors wrote in the study, published in Obstetrics & Gynecology.
The researchers conducted 50 semistructured interviews with health care professionals at lower-performing and higher-performing New York City hospitals, which were selected based on risk-adjusted morbidity metrics. The interviews explored various topics, including structural characteristics like staffing, organizational characteristics like culture and communication, labor and delivery practices such as teamwork and use of evidence-based practices, and racial and ethnic disparities.
The analysis revealed six broad areas that were stronger in high-performing hospitals: day-to-day involvement of leadership in quality activities, an emphasis on standards and standardized care, good communication and teamwork between nurses and physicians, good staffing and supervision among physicians and nurses, sharing of performance data with health care workers, and acknowledgment of the existence of racial and ethnic disparities and that bias can cause treatment differences.
“I think this qualitative approach is an important lens to pair with the quantitative approach. With such variability in severe maternal morbidity between hospitals in New York, it is not enough to just look at the quantitative data. To understand how to improve you must examine structures and processes. The structures, which are the physical and organizational characteristics in health care, and the process, which is how health care is delivered,” Veronica Gillispie-Bell, MD, wrote in a comment. Dr. Gillispie-Bell is medical director at Louisiana Perinatal Quality Collaborative and the Pregnancy-Associated Mortality Review for the Louisiana Department of Health.
“We know that high reliability organizations are those who are preoccupied with quality and safety. That means accountability from leadership (structure) and stability in standardization of care (processes). However, none of this matters if you do not have a culture that promotes safety. Based on the key findings of the high-performing hospitals, there was a culture that promoted safety and quality evidenced in the nurse-physician communication and the transparency around data through a lens of equity,” wrote Dr. Gillispie-Bell.
She noted that the study should encourage low-performing hospitals, since it illustrates avenues for improvement. Her personal experience reflects that, though she said that hospitals need help. The Louisiana Perinatal Quality Collaborative addressed severe maternal morbidity at birthing centers by implementing evidence-based best practices for management of hypertension and hemorrhage along with health equity measures. The team conducted coaching calls, in-person learning sessions, and in-person visits through a “Listening Tour.”
The result was a 35% reduction in hemorrhage overall and a reduction of 49% in hemorrhage in Black women, as well as hypertension by 12% overall between August 2018 and May 2020. Not all the news was good, as Black women still had an increase in severe maternal morbidity, possibly because of the COVID epidemic, since it is a risk factor for hypertension during pregnancy and infection rates are higher among Black individuals. “We need support for state based perinatal quality collaboratives to do this work and we need accountability as we are now seeing from metrics being implemented by [the Centers for Medicare & Medicaid Services]. Hospitals need to stratify their data by race and ethnicity to see where there are disparities in their outcomes,” said Dr. Gillispie-Bell.
The improvements are needed, given that the United States has the highest rates of maternal mortality and morbidity among developed countries, “most of which is preventable, and we have significant inequities by race and ethnicity,” said Laurie Zephyrin, MD, vice president for advancing health equity at the Commonwealth Fund. The question becomes how to effect change, and “there’s a lot happening in the policy space. Some of this policy change is directed at expanding insurance coverage, including more opportunities, including funding for community health workers and doulas, and thinking about how to incorporate midwives. There’s also work around how do we actually improve the care delivered by our health system.” Dr. Zephyrin added that the Department of Health & Human Services has contracted with the health improvement company Premier to use data and best-practices to improve maternal health.
The new work has the potential to be complementary to such approaches. “It provides some structure around how to approach some of the solutions, none of which I think is rocket science. It’s just something that needs to be focused on more intentionally,” said Dr. Zephyrin.
For example, the report found that high-performing hospitals had leaders who collaborated with frontline clinicians to share performance data, and this occurred in person, at departmental quality meetings, and during grand rounds. In contrast, staff in low-performing hospitals did not mention data feedback and some said that their institution made little effort to communicate performance metrics to frontline staff.
“One of the key lessons from the pandemic is that we need to have better data, and we need to have data around race and ethnicity to be able to understand the impact on marginalized communities. This study highlights that there’s more to be done around data to ensure that we can truly move the needle on advancing health equity,” said Dr. Zephyrin.
The researchers also found that clinicians in low-performing institutions did not acknowledge the presence of structural racism or differences in care associated with race or ethnicity. When they acknowledge differences in care, they attributed them to factors outside of the hospital’s control, such as patients not seeking out health care or not maintaining a healthy weight. Clinicians at high-performing hospitals were more likely to explicitly mention racism and bias and acknowledged that these factors could contribute to differences in care.
Dr. Gillispie-Bell and Dr. Zephyrin have no relevant financial disclosures.
A new study of hospitals in New York City suggests ways to reduce severe maternal morbidity (SMM). The researchers interviewed health care professionals in four institutions with low performance and four with high performance, and identified various themes associated with good performance.
“Our results raise the hypothesis that hospital learning collaboratives focused on optimizing organizational practices and policies, increasing clinician and staff awareness and education on maternal health disparities, and addressing structural racism may be important tools for improving equity in maternal outcomes,” the authors wrote in the study, published in Obstetrics & Gynecology.
The researchers conducted 50 semistructured interviews with health care professionals at lower-performing and higher-performing New York City hospitals, which were selected based on risk-adjusted morbidity metrics. The interviews explored various topics, including structural characteristics like staffing, organizational characteristics like culture and communication, labor and delivery practices such as teamwork and use of evidence-based practices, and racial and ethnic disparities.
The analysis revealed six broad areas that were stronger in high-performing hospitals: day-to-day involvement of leadership in quality activities, an emphasis on standards and standardized care, good communication and teamwork between nurses and physicians, good staffing and supervision among physicians and nurses, sharing of performance data with health care workers, and acknowledgment of the existence of racial and ethnic disparities and that bias can cause treatment differences.
“I think this qualitative approach is an important lens to pair with the quantitative approach. With such variability in severe maternal morbidity between hospitals in New York, it is not enough to just look at the quantitative data. To understand how to improve you must examine structures and processes. The structures, which are the physical and organizational characteristics in health care, and the process, which is how health care is delivered,” Veronica Gillispie-Bell, MD, wrote in a comment. Dr. Gillispie-Bell is medical director at Louisiana Perinatal Quality Collaborative and the Pregnancy-Associated Mortality Review for the Louisiana Department of Health.
“We know that high reliability organizations are those who are preoccupied with quality and safety. That means accountability from leadership (structure) and stability in standardization of care (processes). However, none of this matters if you do not have a culture that promotes safety. Based on the key findings of the high-performing hospitals, there was a culture that promoted safety and quality evidenced in the nurse-physician communication and the transparency around data through a lens of equity,” wrote Dr. Gillispie-Bell.
She noted that the study should encourage low-performing hospitals, since it illustrates avenues for improvement. Her personal experience reflects that, though she said that hospitals need help. The Louisiana Perinatal Quality Collaborative addressed severe maternal morbidity at birthing centers by implementing evidence-based best practices for management of hypertension and hemorrhage along with health equity measures. The team conducted coaching calls, in-person learning sessions, and in-person visits through a “Listening Tour.”
The result was a 35% reduction in hemorrhage overall and a reduction of 49% in hemorrhage in Black women, as well as hypertension by 12% overall between August 2018 and May 2020. Not all the news was good, as Black women still had an increase in severe maternal morbidity, possibly because of the COVID epidemic, since it is a risk factor for hypertension during pregnancy and infection rates are higher among Black individuals. “We need support for state based perinatal quality collaboratives to do this work and we need accountability as we are now seeing from metrics being implemented by [the Centers for Medicare & Medicaid Services]. Hospitals need to stratify their data by race and ethnicity to see where there are disparities in their outcomes,” said Dr. Gillispie-Bell.
The improvements are needed, given that the United States has the highest rates of maternal mortality and morbidity among developed countries, “most of which is preventable, and we have significant inequities by race and ethnicity,” said Laurie Zephyrin, MD, vice president for advancing health equity at the Commonwealth Fund. The question becomes how to effect change, and “there’s a lot happening in the policy space. Some of this policy change is directed at expanding insurance coverage, including more opportunities, including funding for community health workers and doulas, and thinking about how to incorporate midwives. There’s also work around how do we actually improve the care delivered by our health system.” Dr. Zephyrin added that the Department of Health & Human Services has contracted with the health improvement company Premier to use data and best-practices to improve maternal health.
The new work has the potential to be complementary to such approaches. “It provides some structure around how to approach some of the solutions, none of which I think is rocket science. It’s just something that needs to be focused on more intentionally,” said Dr. Zephyrin.
For example, the report found that high-performing hospitals had leaders who collaborated with frontline clinicians to share performance data, and this occurred in person, at departmental quality meetings, and during grand rounds. In contrast, staff in low-performing hospitals did not mention data feedback and some said that their institution made little effort to communicate performance metrics to frontline staff.
“One of the key lessons from the pandemic is that we need to have better data, and we need to have data around race and ethnicity to be able to understand the impact on marginalized communities. This study highlights that there’s more to be done around data to ensure that we can truly move the needle on advancing health equity,” said Dr. Zephyrin.
The researchers also found that clinicians in low-performing institutions did not acknowledge the presence of structural racism or differences in care associated with race or ethnicity. When they acknowledge differences in care, they attributed them to factors outside of the hospital’s control, such as patients not seeking out health care or not maintaining a healthy weight. Clinicians at high-performing hospitals were more likely to explicitly mention racism and bias and acknowledged that these factors could contribute to differences in care.
Dr. Gillispie-Bell and Dr. Zephyrin have no relevant financial disclosures.
FROM OBSTETRICS & GYNECOLOGY
COVID fallout: ‘Alarming’ dip in routine vax for pregnant women
The percentage of low-income pregnant mothers who received influenza and Tdap vaccinations fell sharply during the COVID-19 pandemic, especially in Black and Hispanic patients, a new study finds.
The percentage of patients who received the influenza vaccines at two Medicaid clinics in Houston dropped from 78% before the pandemic to 61% during it (adjusted odds ratio, 0.38; 95% CI, 0.26-0.53; P < .01), researchers reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. The percentage receiving the Tdap vaccine dipped from 85% to 76% (aOR, 0.56; 95% CI, 0.40-0.79; P < .01).
New York–Presbyterian/Weill Cornell Medical Center pediatrician Sallie Permar, MD, PhD, who’s familiar with the study findings, called them “alarming” and said in an interview that they should be “a call to action for providers.”
“Continuing the status quo in our routine preventative health care and clinic operations means that we are losing ground in reduction and elimination of vaccine-preventable diseases,” Dr. Permar said in an interview.
According to corresponding author Bani Ratan, MD, an ob.gyn. with the Baylor College of Medicine, Houston, there’s been little if any previous research into routine, non-COVID vaccination in pregnant women during the pandemic.
For the study, researchers retrospectively analyzed the records of 939 pregnant women who entered prenatal care before 20 weeks (462 from May–November 2019, and 477 from May–November 2020) and delivered at full term.
Among ethnic groups, non-Hispanic Blacks saw the largest decline in influenza vaccines. Among them, the percentage who got them fell from 64% (73/114) to 35% (35/101; aOR, 0.30; 95% CI, 0.17-0.52; P < .01). Only Hispanics had a statistically significant decline in Tdap vaccination (OR, 0.52, 95% CI, 0.34-0.80; P < .01, percentages not provided).
Another study presented at ACOG examined vaccination rates during the pandemic and found that Tdap vaccination rates dipped among pregnant women in a Philadelphia-area health care system.
Possible causes for the decline in routine vaccination include hesitancy linked to the COVID-19 vaccines and fewer office visits because of telemedicine, said Dr. Batan in an interview.
Dr. Permar blamed the role of vaccine misinformation during the pandemic and the mistrust caused by the exclusion of pregnant women from early vaccine trials. She added that “challenges in health care staffing and issues of health care provider burnout that worsened during the pandemic likely contributed to a fraying of the focus on preventive health maintenance simply due to bandwidth of health professionals.”
In a separate study presented at ACOG, researchers at the State University of New York, Syracuse, reported on a survey of 157 pregnant women of whom just 38.2% were vaccinated against COVID-19. Among the unvaccinated, who were more likely to have less education, 66% reported that lack of data about vaccination was their primary concern.
No funding or disclosures are reported by study authors. Dr. Permar reported consulting for Merck, Moderna, GlaxoSmithKline, Pfizer, Dynavax, and Hookipa on cytomegalovirus vaccine programs.
*This story was updated on 5/11/2022.
The percentage of low-income pregnant mothers who received influenza and Tdap vaccinations fell sharply during the COVID-19 pandemic, especially in Black and Hispanic patients, a new study finds.
The percentage of patients who received the influenza vaccines at two Medicaid clinics in Houston dropped from 78% before the pandemic to 61% during it (adjusted odds ratio, 0.38; 95% CI, 0.26-0.53; P < .01), researchers reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. The percentage receiving the Tdap vaccine dipped from 85% to 76% (aOR, 0.56; 95% CI, 0.40-0.79; P < .01).
New York–Presbyterian/Weill Cornell Medical Center pediatrician Sallie Permar, MD, PhD, who’s familiar with the study findings, called them “alarming” and said in an interview that they should be “a call to action for providers.”
“Continuing the status quo in our routine preventative health care and clinic operations means that we are losing ground in reduction and elimination of vaccine-preventable diseases,” Dr. Permar said in an interview.
According to corresponding author Bani Ratan, MD, an ob.gyn. with the Baylor College of Medicine, Houston, there’s been little if any previous research into routine, non-COVID vaccination in pregnant women during the pandemic.
For the study, researchers retrospectively analyzed the records of 939 pregnant women who entered prenatal care before 20 weeks (462 from May–November 2019, and 477 from May–November 2020) and delivered at full term.
Among ethnic groups, non-Hispanic Blacks saw the largest decline in influenza vaccines. Among them, the percentage who got them fell from 64% (73/114) to 35% (35/101; aOR, 0.30; 95% CI, 0.17-0.52; P < .01). Only Hispanics had a statistically significant decline in Tdap vaccination (OR, 0.52, 95% CI, 0.34-0.80; P < .01, percentages not provided).
Another study presented at ACOG examined vaccination rates during the pandemic and found that Tdap vaccination rates dipped among pregnant women in a Philadelphia-area health care system.
Possible causes for the decline in routine vaccination include hesitancy linked to the COVID-19 vaccines and fewer office visits because of telemedicine, said Dr. Batan in an interview.
Dr. Permar blamed the role of vaccine misinformation during the pandemic and the mistrust caused by the exclusion of pregnant women from early vaccine trials. She added that “challenges in health care staffing and issues of health care provider burnout that worsened during the pandemic likely contributed to a fraying of the focus on preventive health maintenance simply due to bandwidth of health professionals.”
In a separate study presented at ACOG, researchers at the State University of New York, Syracuse, reported on a survey of 157 pregnant women of whom just 38.2% were vaccinated against COVID-19. Among the unvaccinated, who were more likely to have less education, 66% reported that lack of data about vaccination was their primary concern.
No funding or disclosures are reported by study authors. Dr. Permar reported consulting for Merck, Moderna, GlaxoSmithKline, Pfizer, Dynavax, and Hookipa on cytomegalovirus vaccine programs.
*This story was updated on 5/11/2022.
The percentage of low-income pregnant mothers who received influenza and Tdap vaccinations fell sharply during the COVID-19 pandemic, especially in Black and Hispanic patients, a new study finds.
The percentage of patients who received the influenza vaccines at two Medicaid clinics in Houston dropped from 78% before the pandemic to 61% during it (adjusted odds ratio, 0.38; 95% CI, 0.26-0.53; P < .01), researchers reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. The percentage receiving the Tdap vaccine dipped from 85% to 76% (aOR, 0.56; 95% CI, 0.40-0.79; P < .01).
New York–Presbyterian/Weill Cornell Medical Center pediatrician Sallie Permar, MD, PhD, who’s familiar with the study findings, called them “alarming” and said in an interview that they should be “a call to action for providers.”
“Continuing the status quo in our routine preventative health care and clinic operations means that we are losing ground in reduction and elimination of vaccine-preventable diseases,” Dr. Permar said in an interview.
According to corresponding author Bani Ratan, MD, an ob.gyn. with the Baylor College of Medicine, Houston, there’s been little if any previous research into routine, non-COVID vaccination in pregnant women during the pandemic.
For the study, researchers retrospectively analyzed the records of 939 pregnant women who entered prenatal care before 20 weeks (462 from May–November 2019, and 477 from May–November 2020) and delivered at full term.
Among ethnic groups, non-Hispanic Blacks saw the largest decline in influenza vaccines. Among them, the percentage who got them fell from 64% (73/114) to 35% (35/101; aOR, 0.30; 95% CI, 0.17-0.52; P < .01). Only Hispanics had a statistically significant decline in Tdap vaccination (OR, 0.52, 95% CI, 0.34-0.80; P < .01, percentages not provided).
Another study presented at ACOG examined vaccination rates during the pandemic and found that Tdap vaccination rates dipped among pregnant women in a Philadelphia-area health care system.
Possible causes for the decline in routine vaccination include hesitancy linked to the COVID-19 vaccines and fewer office visits because of telemedicine, said Dr. Batan in an interview.
Dr. Permar blamed the role of vaccine misinformation during the pandemic and the mistrust caused by the exclusion of pregnant women from early vaccine trials. She added that “challenges in health care staffing and issues of health care provider burnout that worsened during the pandemic likely contributed to a fraying of the focus on preventive health maintenance simply due to bandwidth of health professionals.”
In a separate study presented at ACOG, researchers at the State University of New York, Syracuse, reported on a survey of 157 pregnant women of whom just 38.2% were vaccinated against COVID-19. Among the unvaccinated, who were more likely to have less education, 66% reported that lack of data about vaccination was their primary concern.
No funding or disclosures are reported by study authors. Dr. Permar reported consulting for Merck, Moderna, GlaxoSmithKline, Pfizer, Dynavax, and Hookipa on cytomegalovirus vaccine programs.
*This story was updated on 5/11/2022.
FROM ACOG 2022
CDC predicts a rise in COVID-19 hospitalizations and deaths in coming weeks
, according to a national forecast used by the Centers for Disease Control and Prevention.
The national model also predicts that about 5,000 deaths will occur over the next two weeks, with Ohio, New Jersey, and New York projected to see the largest totals of daily deaths in upcoming weeks.
The numbers follow several weeks of steady increases in infections across the country. More than 67,000 new cases are being reported daily, according to the data tracker from The New York Times, marking a 59% increase in the past two weeks.
In the Northeast, infection rates have risen by nearly 65%. In the New York and New Jersey region, infection rates are up about 55% in the past two weeks.
Hospitalizations have already begun to climb as well, with about 19,000 COVID-19 patients hospitalized nationwide and 1,725 in intensive care, according to the latest data from the Department of Health and Human Services. In the last week, hospital admissions have jumped by 20%, and emergency department visits are up by 18%.
The CDC forecast shows that 42 states and territories will see increases in hospital admissions during the next two weeks. Florida, Minnesota, New York, and Wisconsin will see some of the largest increases.
On average, more than 2,200 COVID-19 patients are entering the hospital each day, which has increased about 20% in the last week, according to ABC News. This also marks the highest number of COVID-19 patients needing hospital care since mid-March.
Public health officials have cited several factors for the increase in cases, such as states lifting mask mandates and other safety restrictions, ABC News reported. Highly contagious Omicron subvariants, such as BA.2 and BA.2.12.1, continue to spread in the United States and escape immunity from previous infections.
The BA.2 subvariant accounts for 62% of new national cases, according to the latest CDC data. The BA.2.12.1 subvariant makes up about 36% of new cases across the United States but 62% in the New York area.
A version of this article first appeared on WebMD.com.
, according to a national forecast used by the Centers for Disease Control and Prevention.
The national model also predicts that about 5,000 deaths will occur over the next two weeks, with Ohio, New Jersey, and New York projected to see the largest totals of daily deaths in upcoming weeks.
The numbers follow several weeks of steady increases in infections across the country. More than 67,000 new cases are being reported daily, according to the data tracker from The New York Times, marking a 59% increase in the past two weeks.
In the Northeast, infection rates have risen by nearly 65%. In the New York and New Jersey region, infection rates are up about 55% in the past two weeks.
Hospitalizations have already begun to climb as well, with about 19,000 COVID-19 patients hospitalized nationwide and 1,725 in intensive care, according to the latest data from the Department of Health and Human Services. In the last week, hospital admissions have jumped by 20%, and emergency department visits are up by 18%.
The CDC forecast shows that 42 states and territories will see increases in hospital admissions during the next two weeks. Florida, Minnesota, New York, and Wisconsin will see some of the largest increases.
On average, more than 2,200 COVID-19 patients are entering the hospital each day, which has increased about 20% in the last week, according to ABC News. This also marks the highest number of COVID-19 patients needing hospital care since mid-March.
Public health officials have cited several factors for the increase in cases, such as states lifting mask mandates and other safety restrictions, ABC News reported. Highly contagious Omicron subvariants, such as BA.2 and BA.2.12.1, continue to spread in the United States and escape immunity from previous infections.
The BA.2 subvariant accounts for 62% of new national cases, according to the latest CDC data. The BA.2.12.1 subvariant makes up about 36% of new cases across the United States but 62% in the New York area.
A version of this article first appeared on WebMD.com.
, according to a national forecast used by the Centers for Disease Control and Prevention.
The national model also predicts that about 5,000 deaths will occur over the next two weeks, with Ohio, New Jersey, and New York projected to see the largest totals of daily deaths in upcoming weeks.
The numbers follow several weeks of steady increases in infections across the country. More than 67,000 new cases are being reported daily, according to the data tracker from The New York Times, marking a 59% increase in the past two weeks.
In the Northeast, infection rates have risen by nearly 65%. In the New York and New Jersey region, infection rates are up about 55% in the past two weeks.
Hospitalizations have already begun to climb as well, with about 19,000 COVID-19 patients hospitalized nationwide and 1,725 in intensive care, according to the latest data from the Department of Health and Human Services. In the last week, hospital admissions have jumped by 20%, and emergency department visits are up by 18%.
The CDC forecast shows that 42 states and territories will see increases in hospital admissions during the next two weeks. Florida, Minnesota, New York, and Wisconsin will see some of the largest increases.
On average, more than 2,200 COVID-19 patients are entering the hospital each day, which has increased about 20% in the last week, according to ABC News. This also marks the highest number of COVID-19 patients needing hospital care since mid-March.
Public health officials have cited several factors for the increase in cases, such as states lifting mask mandates and other safety restrictions, ABC News reported. Highly contagious Omicron subvariants, such as BA.2 and BA.2.12.1, continue to spread in the United States and escape immunity from previous infections.
The BA.2 subvariant accounts for 62% of new national cases, according to the latest CDC data. The BA.2.12.1 subvariant makes up about 36% of new cases across the United States but 62% in the New York area.
A version of this article first appeared on WebMD.com.
‘Bane of my existence:’ The burden of Medicare Advantage denials
, a recent analysis suggests.
The report from the Office of Inspector General (OIG) of the U.S. Department of Health & Human Services found that 13% of prior authorization denials were for service requests, which included cancer care, that met Medicare coverage rules and 18% of payment denials were for claims that met Medicare coverage and MAO billing rules, delaying or halting payments for services that clinicians had provided.
MAO denials are the “bane of my existence,” said Michael Buckstein, MD, PhD, a radiation oncologist at Mount Sinai Hospital in New York.
“Working at a large hospital in a metropolitan city, we spend enormous and increasing amounts of time on prior approvals and we get denials quite frequently, which certainly can lead to delays in treatment,” said Dr. Buckstein, who reviewed the OIG report for this news organization.
According to Dr. Buckstein, once a claim is denied, staff must spend time filing and scheduling an appeal, and if the appeal is denied in a physician peer-to-peer review, staff could face secondary and tertiary appeals. “We have been living with this frustration for a long time,” Dr. Buckstein said.
Widespread and persistent problems
Medicare Advantage plans, which are approved by the Centers for Medicare & Medicaid Services but run by private companies, continue to grow in popularity.
In 2021, 26.4 million Medicare beneficiaries (42%) were enrolled in a Medicare Advantage plan, and by 2030, about 51% of all Medicare beneficiaries will be enrolled, according to estimates from the Kaiser Family Foundation.
“Although MAOs approve the vast majority of prior authorization requests and provider payment requests, MAOs also deny millions of requests each year,” the OIG wrote. “CMS’s annual audits of MAOs have highlighted widespread and persistent problems related to inappropriate denials of services and payment.”
In the current report, the OIG reviewed case files for 247 denials of prior authorization requests and 183 payment denials issued by 15 of the largest MAOs during 1 week in June of 2019.
The authors found that 13% of prior authorization denials occurred for service requests that met Medicare coverage rules, meaning these services would likely have been approved had the patient been enrolled in traditional Medicare.
The most prominent service types among these denials included imaging services, stays in postacute facilities, and injections.
In one case, for example, the MAO stated that a beneficiary would need to wait at least 1 year for a follow-up MRI because the size of the patient’s adrenal lesion (< 2 cm) was too small to warrant follow-up before 1 year. However, this restriction is not included in Medicare coverage rules. And an OIG physician panel found that the documentation in the original request demonstrated that the MRI was medically necessary to determine whether the lesion seen on an earlier CT scan was malignant.
Upon appeal, the MAO reversed its original denial.
Among the payment requests that MAOs denied, almost one in five were for claims that met Medicare coverage and billing rules, which delayed or prevented payments for services already delivered. Most payment denials were caused by human error during manual claims-processing reviews and system processing errors, the OIG report found.
In one case, for example, a MAO denied payment for radiation treatment for a patient with a tumor on the pancreas, incorrectly claiming that no prior authorization had been submitted for the service. However, the physician subsequently provided a screenshot demonstrating that the MAO had granted prior authorization for the billed claim, and the MAO reversed the denial.
Most of these prior authorization denial reversals occurred because of an appeal filed by the beneficiary or the provider, which can take weeks.
In one case, an MAO denied a request for a CT scan of the chest and pelvis for a beneficiary with endometrial cancer. It took 5 weeks for the provider to get the denial reversed. The OIG panel determined that the original request included sufficient documentation to demonstrate the CT was needed to assess the stage of the cancer and determine the appropriate course of treatment.
These denials and reversals not only waste time but may also cause harm. In a 2021 American Medical Association survey, 34% of physicians reported that prior authorization led to a serious adverse event for a patient in their care, including hospitalization, medical intervention to prevent permanent impairment, and even disability or death.
Almost 90% of the physicians surveyed described the burden associated with prior authorizations as ‘high’ or ‘extremely high.’ More specifically, physicians and their staff spend nearly 2 days a week on prior authorizations and 40% of physicians have staff who work exclusively on prior authorizations.
“It’s just not the way medicine should be practiced, especially for cancer patients who are very vulnerable and want rapid care,” Dr. Buckstein said.
Time for action
Weighing in on the OIG report, Robert E. Wailes, MD, president of the California Medical Association, noted that “it has become common practice for health insurance companies to create obstacles for patients in hopes of not having to pay for essential healthcare.”
The reason for these obstacles is simple, he said: “Fewer procedures performed translates to larger insurance company profits.”
America’s Health Insurance Plans (AHIP) defended prior authorization, saying it is “an important patient safety, cost-saving, and waste-prevention tool.”
The group also called out the OIG review for its “extraordinarily small” sample of 247 prior authorization requests over 1 week.
“Drawing far-reaching conclusions based on a very small sample of data and misleading headlines is not a productive way to improve our healthcare system for patients,” the AHIP statement reads.
But, according to Anna Schwamlein Howard, who works on policy development at the American Cancer Society Cancer Action Network, the recent OIG report is in line with previous OIG reports.
And, Ms. Howard emphasized, the current report and others like it “highlight the need for CMS to utilize its audit authority and ensure that beneficiaries have access to medically necessary treatments, particularly cancer treatments.”
Along those lines, the OIG report recommends that the CMS should issue new guidance on the appropriate use of MAO clinical criteria in medical necessity reviews, update its audit protocols to address issues identified in the report, and direct MAOs to take additional steps to identify and address vulnerabilities that can lead to manual review and system errors.
In a statement, the CMS said it is committed to oversight and enforcement of the requirements of the Medicare Advantage program and agreed with the OIG recommendations.
“Lawmakers must act now to place patient needs before corporate profits and simplify by streamlining prior authorization processes,” Dr. Wailes said.
The ACS recently released a paper on this topic entitled, “The Medicare Appeals Process: Reforms Needed to Ensure Beneficiary Access.”
A version of this article first appeared on Medscape.com.
, a recent analysis suggests.
The report from the Office of Inspector General (OIG) of the U.S. Department of Health & Human Services found that 13% of prior authorization denials were for service requests, which included cancer care, that met Medicare coverage rules and 18% of payment denials were for claims that met Medicare coverage and MAO billing rules, delaying or halting payments for services that clinicians had provided.
MAO denials are the “bane of my existence,” said Michael Buckstein, MD, PhD, a radiation oncologist at Mount Sinai Hospital in New York.
“Working at a large hospital in a metropolitan city, we spend enormous and increasing amounts of time on prior approvals and we get denials quite frequently, which certainly can lead to delays in treatment,” said Dr. Buckstein, who reviewed the OIG report for this news organization.
According to Dr. Buckstein, once a claim is denied, staff must spend time filing and scheduling an appeal, and if the appeal is denied in a physician peer-to-peer review, staff could face secondary and tertiary appeals. “We have been living with this frustration for a long time,” Dr. Buckstein said.
Widespread and persistent problems
Medicare Advantage plans, which are approved by the Centers for Medicare & Medicaid Services but run by private companies, continue to grow in popularity.
In 2021, 26.4 million Medicare beneficiaries (42%) were enrolled in a Medicare Advantage plan, and by 2030, about 51% of all Medicare beneficiaries will be enrolled, according to estimates from the Kaiser Family Foundation.
“Although MAOs approve the vast majority of prior authorization requests and provider payment requests, MAOs also deny millions of requests each year,” the OIG wrote. “CMS’s annual audits of MAOs have highlighted widespread and persistent problems related to inappropriate denials of services and payment.”
In the current report, the OIG reviewed case files for 247 denials of prior authorization requests and 183 payment denials issued by 15 of the largest MAOs during 1 week in June of 2019.
The authors found that 13% of prior authorization denials occurred for service requests that met Medicare coverage rules, meaning these services would likely have been approved had the patient been enrolled in traditional Medicare.
The most prominent service types among these denials included imaging services, stays in postacute facilities, and injections.
In one case, for example, the MAO stated that a beneficiary would need to wait at least 1 year for a follow-up MRI because the size of the patient’s adrenal lesion (< 2 cm) was too small to warrant follow-up before 1 year. However, this restriction is not included in Medicare coverage rules. And an OIG physician panel found that the documentation in the original request demonstrated that the MRI was medically necessary to determine whether the lesion seen on an earlier CT scan was malignant.
Upon appeal, the MAO reversed its original denial.
Among the payment requests that MAOs denied, almost one in five were for claims that met Medicare coverage and billing rules, which delayed or prevented payments for services already delivered. Most payment denials were caused by human error during manual claims-processing reviews and system processing errors, the OIG report found.
In one case, for example, a MAO denied payment for radiation treatment for a patient with a tumor on the pancreas, incorrectly claiming that no prior authorization had been submitted for the service. However, the physician subsequently provided a screenshot demonstrating that the MAO had granted prior authorization for the billed claim, and the MAO reversed the denial.
Most of these prior authorization denial reversals occurred because of an appeal filed by the beneficiary or the provider, which can take weeks.
In one case, an MAO denied a request for a CT scan of the chest and pelvis for a beneficiary with endometrial cancer. It took 5 weeks for the provider to get the denial reversed. The OIG panel determined that the original request included sufficient documentation to demonstrate the CT was needed to assess the stage of the cancer and determine the appropriate course of treatment.
These denials and reversals not only waste time but may also cause harm. In a 2021 American Medical Association survey, 34% of physicians reported that prior authorization led to a serious adverse event for a patient in their care, including hospitalization, medical intervention to prevent permanent impairment, and even disability or death.
Almost 90% of the physicians surveyed described the burden associated with prior authorizations as ‘high’ or ‘extremely high.’ More specifically, physicians and their staff spend nearly 2 days a week on prior authorizations and 40% of physicians have staff who work exclusively on prior authorizations.
“It’s just not the way medicine should be practiced, especially for cancer patients who are very vulnerable and want rapid care,” Dr. Buckstein said.
Time for action
Weighing in on the OIG report, Robert E. Wailes, MD, president of the California Medical Association, noted that “it has become common practice for health insurance companies to create obstacles for patients in hopes of not having to pay for essential healthcare.”
The reason for these obstacles is simple, he said: “Fewer procedures performed translates to larger insurance company profits.”
America’s Health Insurance Plans (AHIP) defended prior authorization, saying it is “an important patient safety, cost-saving, and waste-prevention tool.”
The group also called out the OIG review for its “extraordinarily small” sample of 247 prior authorization requests over 1 week.
“Drawing far-reaching conclusions based on a very small sample of data and misleading headlines is not a productive way to improve our healthcare system for patients,” the AHIP statement reads.
But, according to Anna Schwamlein Howard, who works on policy development at the American Cancer Society Cancer Action Network, the recent OIG report is in line with previous OIG reports.
And, Ms. Howard emphasized, the current report and others like it “highlight the need for CMS to utilize its audit authority and ensure that beneficiaries have access to medically necessary treatments, particularly cancer treatments.”
Along those lines, the OIG report recommends that the CMS should issue new guidance on the appropriate use of MAO clinical criteria in medical necessity reviews, update its audit protocols to address issues identified in the report, and direct MAOs to take additional steps to identify and address vulnerabilities that can lead to manual review and system errors.
In a statement, the CMS said it is committed to oversight and enforcement of the requirements of the Medicare Advantage program and agreed with the OIG recommendations.
“Lawmakers must act now to place patient needs before corporate profits and simplify by streamlining prior authorization processes,” Dr. Wailes said.
The ACS recently released a paper on this topic entitled, “The Medicare Appeals Process: Reforms Needed to Ensure Beneficiary Access.”
A version of this article first appeared on Medscape.com.
, a recent analysis suggests.
The report from the Office of Inspector General (OIG) of the U.S. Department of Health & Human Services found that 13% of prior authorization denials were for service requests, which included cancer care, that met Medicare coverage rules and 18% of payment denials were for claims that met Medicare coverage and MAO billing rules, delaying or halting payments for services that clinicians had provided.
MAO denials are the “bane of my existence,” said Michael Buckstein, MD, PhD, a radiation oncologist at Mount Sinai Hospital in New York.
“Working at a large hospital in a metropolitan city, we spend enormous and increasing amounts of time on prior approvals and we get denials quite frequently, which certainly can lead to delays in treatment,” said Dr. Buckstein, who reviewed the OIG report for this news organization.
According to Dr. Buckstein, once a claim is denied, staff must spend time filing and scheduling an appeal, and if the appeal is denied in a physician peer-to-peer review, staff could face secondary and tertiary appeals. “We have been living with this frustration for a long time,” Dr. Buckstein said.
Widespread and persistent problems
Medicare Advantage plans, which are approved by the Centers for Medicare & Medicaid Services but run by private companies, continue to grow in popularity.
In 2021, 26.4 million Medicare beneficiaries (42%) were enrolled in a Medicare Advantage plan, and by 2030, about 51% of all Medicare beneficiaries will be enrolled, according to estimates from the Kaiser Family Foundation.
“Although MAOs approve the vast majority of prior authorization requests and provider payment requests, MAOs also deny millions of requests each year,” the OIG wrote. “CMS’s annual audits of MAOs have highlighted widespread and persistent problems related to inappropriate denials of services and payment.”
In the current report, the OIG reviewed case files for 247 denials of prior authorization requests and 183 payment denials issued by 15 of the largest MAOs during 1 week in June of 2019.
The authors found that 13% of prior authorization denials occurred for service requests that met Medicare coverage rules, meaning these services would likely have been approved had the patient been enrolled in traditional Medicare.
The most prominent service types among these denials included imaging services, stays in postacute facilities, and injections.
In one case, for example, the MAO stated that a beneficiary would need to wait at least 1 year for a follow-up MRI because the size of the patient’s adrenal lesion (< 2 cm) was too small to warrant follow-up before 1 year. However, this restriction is not included in Medicare coverage rules. And an OIG physician panel found that the documentation in the original request demonstrated that the MRI was medically necessary to determine whether the lesion seen on an earlier CT scan was malignant.
Upon appeal, the MAO reversed its original denial.
Among the payment requests that MAOs denied, almost one in five were for claims that met Medicare coverage and billing rules, which delayed or prevented payments for services already delivered. Most payment denials were caused by human error during manual claims-processing reviews and system processing errors, the OIG report found.
In one case, for example, a MAO denied payment for radiation treatment for a patient with a tumor on the pancreas, incorrectly claiming that no prior authorization had been submitted for the service. However, the physician subsequently provided a screenshot demonstrating that the MAO had granted prior authorization for the billed claim, and the MAO reversed the denial.
Most of these prior authorization denial reversals occurred because of an appeal filed by the beneficiary or the provider, which can take weeks.
In one case, an MAO denied a request for a CT scan of the chest and pelvis for a beneficiary with endometrial cancer. It took 5 weeks for the provider to get the denial reversed. The OIG panel determined that the original request included sufficient documentation to demonstrate the CT was needed to assess the stage of the cancer and determine the appropriate course of treatment.
These denials and reversals not only waste time but may also cause harm. In a 2021 American Medical Association survey, 34% of physicians reported that prior authorization led to a serious adverse event for a patient in their care, including hospitalization, medical intervention to prevent permanent impairment, and even disability or death.
Almost 90% of the physicians surveyed described the burden associated with prior authorizations as ‘high’ or ‘extremely high.’ More specifically, physicians and their staff spend nearly 2 days a week on prior authorizations and 40% of physicians have staff who work exclusively on prior authorizations.
“It’s just not the way medicine should be practiced, especially for cancer patients who are very vulnerable and want rapid care,” Dr. Buckstein said.
Time for action
Weighing in on the OIG report, Robert E. Wailes, MD, president of the California Medical Association, noted that “it has become common practice for health insurance companies to create obstacles for patients in hopes of not having to pay for essential healthcare.”
The reason for these obstacles is simple, he said: “Fewer procedures performed translates to larger insurance company profits.”
America’s Health Insurance Plans (AHIP) defended prior authorization, saying it is “an important patient safety, cost-saving, and waste-prevention tool.”
The group also called out the OIG review for its “extraordinarily small” sample of 247 prior authorization requests over 1 week.
“Drawing far-reaching conclusions based on a very small sample of data and misleading headlines is not a productive way to improve our healthcare system for patients,” the AHIP statement reads.
But, according to Anna Schwamlein Howard, who works on policy development at the American Cancer Society Cancer Action Network, the recent OIG report is in line with previous OIG reports.
And, Ms. Howard emphasized, the current report and others like it “highlight the need for CMS to utilize its audit authority and ensure that beneficiaries have access to medically necessary treatments, particularly cancer treatments.”
Along those lines, the OIG report recommends that the CMS should issue new guidance on the appropriate use of MAO clinical criteria in medical necessity reviews, update its audit protocols to address issues identified in the report, and direct MAOs to take additional steps to identify and address vulnerabilities that can lead to manual review and system errors.
In a statement, the CMS said it is committed to oversight and enforcement of the requirements of the Medicare Advantage program and agreed with the OIG recommendations.
“Lawmakers must act now to place patient needs before corporate profits and simplify by streamlining prior authorization processes,” Dr. Wailes said.
The ACS recently released a paper on this topic entitled, “The Medicare Appeals Process: Reforms Needed to Ensure Beneficiary Access.”
A version of this article first appeared on Medscape.com.