User login
Patients asking about APOE gene test results? Here’s what to tell them
Advances in Alzheimer disease (AD) genes and biomarkers now allow older adults to undergo testing and learn about their risk for AD.1 Current routes for doing so include testing in cardiology, screening for enrollment in secondary prevention trials (which use these tests to determine trial eligibility),2 and direct-to-consumer (DTC) services that provide these results as part of large panels.3 Patients may also obtain apolipoprotein (APOE) genotype information as part of an assessment of the risks and benefits of treatment with aducanumab (Aduhelm) or other anti-amyloid therapies that have been developed to stop or slow the progression of AD pathologies.
Expanded access to testing, in combination with limited guidance from DTC companies, suggests more older adults may consult their primary care physicians about this testing. In this narrative review, we use a vignette-driven approach to summarize the current scientific knowledge of the topic and to offer guidance on provider-patient discussions and follow-up.
First, a look at APOE genotyping
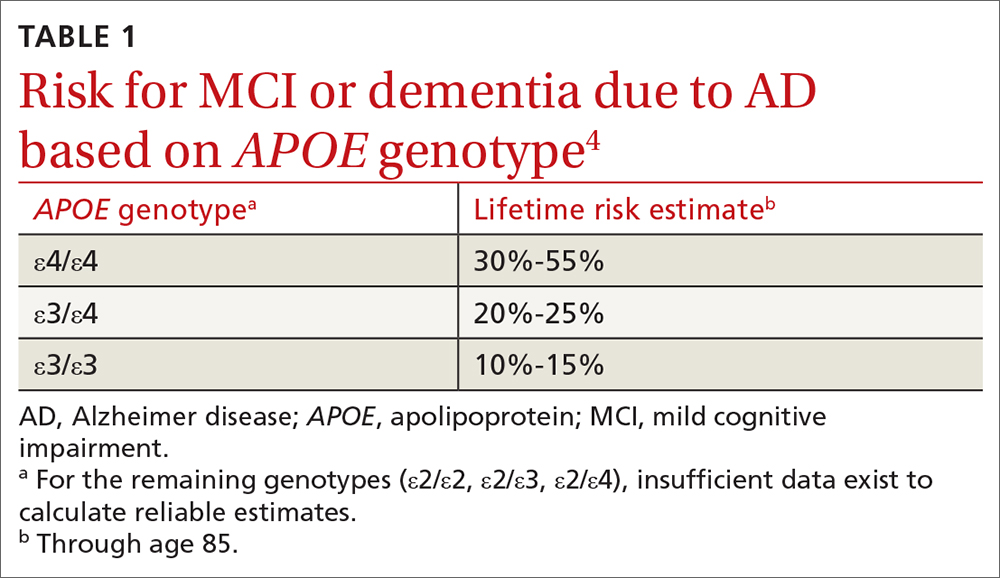
In cognitively unimpaired older adults, the APOE gene is a known risk factor for mild cognitive impairment (MCI) or AD.3 A person has 2 alleles of the APOE gene, which has 3 variants: ε2, ε3, and ε4. The combination of alleles conveys varying levels of risk for developing clinical symptoms (TABLE 14), with ε4 increasing risk and ε2 decreasing risk compared to the more common ε3; thus the ε4/ε4 genotype conveys the most risk and the ε2/ε2 the least.
The APOE gene differs from other genes that have been identified in early-onset familial AD. These other genes, which include APP, PSEN1, and PSEN2, are deterministic genes that are fully penetrant. The APOE gene is not deterministic, meaning there is no combination of APOE alleles that are necessary or sufficient to cause late-onset AD dementia.
In clinical trials of amyloid-modifying therapies, the APOE gene has been shown to convey a risk of amyloid-related imaging abnormalities (ARIA).5 That is, in addition to conveying a risk for AD, the gene also conveys a risk for adverse effects of emerging treatments that can result in serious injury or death. This includes the drug aducanumab that was recently approved by the US Food and Drug Administration (FDA).6 In this review, we focus primarily on common clinical scenarios related to APOE. However, in light of the recent controversy over aducanumab and whether the drug should be offered to patients,7-9 we also describe how a patient’s APOE genotype may factor into drug candidacy decisions.
Testing, in clinic and “at home.” To date, practice guidelines have consistently recommended against APOE genetic testing in routine clinical practice. This is primarily due to low clinical prognostic utility and the lack of actionable results. Furthermore, no lifestyle or pharmaceutical interventions based on APOE genotype currently exist (although trials are underway10).
In 2017, the FDA approved marketing of DTC testing for the APOE gene.11 While DTC companies tend to issue standardized test result reports, the content and quality can vary widely. In fact, some provide risk estimates that are too high and too definitive and may not reflect the most recent science.12
Continue to: 7 clinical scenarios and how to approach them
7 clinical scenarios and how to approach them
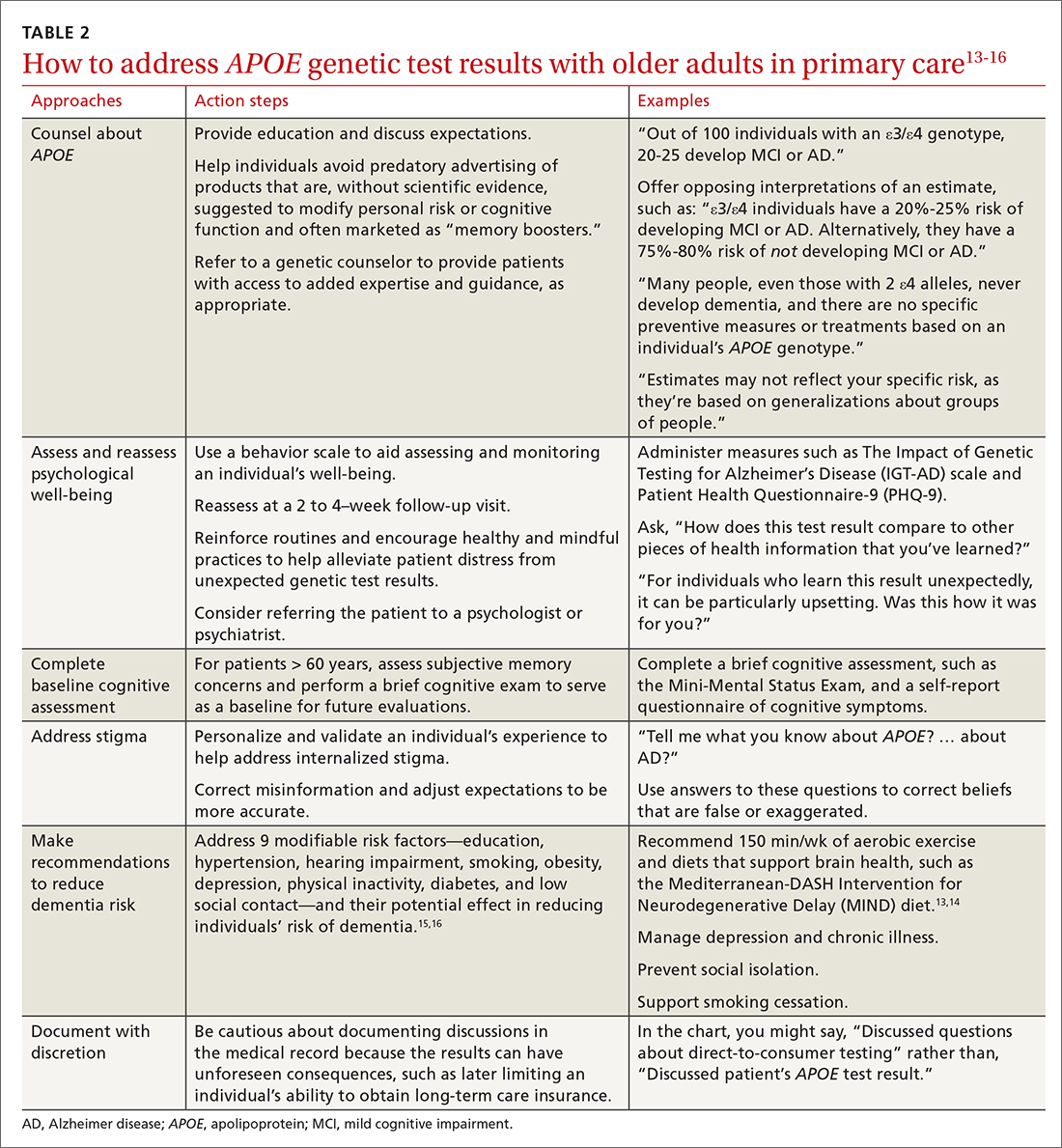
Six of the following vignettes describe common clinical scenarios in which patients seek medical advice regarding APOE test results. The seventh vignette describes a patient whose APOE genotype may play a role in possible disease-modifying treatments down the road. Each vignette is designed to guide your approach to patient discussions and follow-up. Recommendations and considerations are also summarized in TABLE 213-16.
Vignette 1
Janet W, age 65, comes to the clinic for a new patient visit. She has no concerns about her memory but recently purchased DTC genetic testing to learn about her genetic health risks. Her results showed an APOE ε4/ε4 genotype. She is now concerned about developing AD. Her mother was diagnosed with AD in her 70s.
Several important pieces of information can be conveyed by the primary care physician. First, patients such as Ms. W should be told that the APOE gene is not deterministic; many people, even those with 2 ε4 alleles, never develop dementia. Second, no specific preventive measures or treatments exist based on an individual’s APOE genotype (see Vignette 5 for additional discussion).
In this scenario, patients may ask for numeric quantification of their risk for dementia (see TABLE 14 for estimates). When conveying probabilistic risk, consider using simple percentages or pictographs (eg, out of 100 individuals with an ε4/ε4 genotype, 30 to 55 develop MCI or AD). Additionally, because people tend to exhibit confirmatory bias in thinking about probabilistic risk, providing opposing interpretations of an estimate may help them to consider alternative possibilities.17 For example
There are important caveats to the interpretation of APOE risk estimates. Because APOE risk estimates are probabilistic and averaged across a broader spectrum of people in large population cohorts,4 estimates may not accurately reflect a given individual’s risk. The ranges reflect the uncertainty in the estimates. The uncertainty arises from relatively small samples, the rareness of some genotypes (notably ε4/ε4) even in large samples, and variations in methods and sampling that can lead to differences in estimates beyond statistical variation.
Vignette 2
Eric J, age 85, presents for a new patient visit accompanied by his daughter. He lives independently, volunteers at a senior center several times a week, and exercises regularly, and neither he nor his daughter has any concerns about his memory. As a gift, he recently underwent DTC genetic testing and unexpectedly learned his APOE result, which is ε4/ε4. He wants to know about his chances of developing AD.
Risk conveyed by APOE genotype can be modified by a patient’s age. At age 85, Mr. J is healthy, highly functional, and cognitively unimpaired. Given his age, Mr. J has likely “outlived” much of the risk for dementia attributable to the ε4/ε4 genotype. His risk for dementia remains high, but this risk is likely driven more by age than by his APOE genotype. Data for individuals older than age 80 are limited, and thus risk estimates lack precision. Given Mr. J’s good health and functional status, his physician may want to perform a brief cognitive screening test to serve as a baseline for future evaluations.
Continue to: Vignette 3
Vignette 3
Audrey S is a 60-year-old African American woman who comes to the clinic for her annual visit. Because her father had AD, she recently purchased DTC genetic testing to learn about her APOE genotype and risk for AD. Her results are ε3/ε4. She is wondering what this may mean for her future.
Lack of diversity in research cohorts often limits the generalizability of estimates. For example, both the frequency and impact of APOE ε4 differ across racial groups.18 But most of the data on APOE lifetime risk estimates are from largely White patient samples. While APOE ε4 seems to confer increased risk for AD across sociocultural groups, these effects may be attenuated in African American and Hispanic populations.19,20 If Ms. S is interested in numeric risk estimates, the physician can provide the estimate for ε3/ε4 (20%-25% lifetime risk), with the important caveat that this estimate may not be reflective of her individual risk.
It may be prudent to determine whether Ms. S, at age 60, has subjective memory concerns and if she does, to perform a brief cognitive exam to serve as a baseline for future evaluations. Additionally, while the Genetic Information Nondiscrimination Act (GINA, 2008) prohibits health insurers and employers from discriminating based on genetic testing results, no legal provisions exist regarding long-term care, disability, or life insurance. Documented conversations about APOE test results in the medical record may become part of patients’ applications for these insurance products, and physicians should be cautious before documenting such discussions in the medical record.
Vignette 4
Tina L, age 60, comes to the clinic for a routine wellness visit. She recently developed an interest in genealogy and purchased a DNA testing kit to learn more about her family tree. As part of this testing, she unexpectedly learned that she has an APOE ε4/ε4 genotype. She describes feeling distraught and anxious about what the result means for her future.
Ms. L’s reaction to receiving unexpected genetic results highlights a concern of DTC APOE testing. Her experience is quite different from individuals undergoing medically recommended genetic testing or those who are participating in research studies. They receive comprehensive pre-test counseling by licensed genetic counselors. The counseling includes psychological assessment, education, and discussion of expectations.2
In Ms. L’s case, it may be helpful to explain the limits of APOE lifetime risk estimates (see Vignettes 1-3). But it’s also important to address her concerns. There are behavior scales that can aid the assessment and monitoring of an individual’s well-being. The Impact of Genetic Testing for Alzheimer’s Disease (IGT-AD) scale is a tool that assesses psychological impact. It can help physicians to identify, monitor, and address concerns.21 Other useful tools include the Patient Health Questionnaire-9 (PHQ-9) and the Geriatric Depression Scale (GDS) for depression, and a suicide or self-harm assessment.2,22,23 Finally, a follow-up visit at 2 to 4 weeks may be useful to reassess psychological well-being.
Vignette 4 (cont’d)
Ms. L returns to the clinic 2 weeks later, reporting continued anxiety about her APOE test result and feelings of hopelessness and despair.
Continue to: Some patients struggle...
Some patients struggle with knowing their APOE test result. Test result–related distress is often a combination of depression (as with Ms. L), anger, confusion, and grief.24 Cognitions often include worries about uncertainty, stereotyped threat, and internalized stigma.25,26 These issues can spill over to patient concerns about sharing an APOE test result with others.27
Intolerance of uncertainty is a transdiagnostic risk factor that can influence psychological suffering.28 Brief cognitive behavioral interventions that reinforce routines and encourage healthy and mindful practices may help alleviate patient distress from unexpected genetic test results.29 Interventions that personalize and validate an individual’s experience can help address internalized stigma.30 Referral to a psychologist or psychiatrist could be warranted. Additionally, referral to a genetic counselor may help provide patients with access to added expertise and guidance; useful web-based resources for identifying an appropriate referral include https://medlineplus.gov/genetics/understanding/consult/findingprofessional/ and https://findageneticcounselor.nsgc.org/.
Vignette 5
Bob K, age 65, comes to the clinic for his annual exam. He is a current smoker and says he’s hoping to be more physically active now that he is retired. He says that his mother and grandmother both had AD. He recently purchased DTC genetic testing to learn more about his risk for AD. His learned his APOE genotype is ε3/ε4 and is wondering what he can do to decrease his chances of developing AD.
Mr. K likely would have benefited from pre-test counseling regarding the lack of current therapies to modify one’s genetic risk for AD. A pre-test counseling session often includes education about APOE testing and a brief evaluation to assess psychological readiness to undergo testing. Posttest educational information may help Mr. K avoid predatory advertising of products claiming—without scientific evidence—to modify risk for cognitive decline or to improve cognitive function.
There are several important pieces of information that should be communicated to Mr. K. Emerging evidence from randomized controlled trials suggests that healthy lifestyle modifications may benefit cognition in individuals with APOE ε4 alleles.31 It would be prudent to address proper blood pressure control32 and counsel Mr. K on how he may be able to avoid diabetes through exercise and weight maintenance. Lifestyle recommendations for Mr. K could include: smoking cessation, regular aerobic exercise (eg, 150 min/wk), and a brain-healthy diet (eg, the Mediterranean-DASH Intervention for Neurodegenerative Delay [MIND] diet).13,14 Moreover, dementia prevention also includes appropriately managing depression and chronic illnesses and preventing social isolation and hearing loss.15,16 This information should be thoughtfully conveyed, as these interventions can improve overall (especially cardiovascular) health, as well as mitigating one’s personal risk for AD.
Vignette 6
Juan L, age 45, comes in for his annual physical exam. He has a strong family history of heart disease. His cardiologist recently ordered lipid disorder genetic testing for familial hypercholesterolemia. This panel included APOE testing and showed Mr. L’s genotype is ε2/ε4. He read that the APOE gene can be associated with an increased AD risk and asks for information about his genotype.
Mr. L received genetic testing results that were ordered by a physician for another health purpose. Current recommendations for genetic testing in cardiology advise pre-test genetic counseling.33 But this counseling may not include discussion of the relationship of APOE and risk for MCI or AD. This additional information may be unexpected for Mr. L. Moreover, its significance in the context of his present concerns about cardiovascular disease may influence his reaction.
Continue to: The ε2/ε4 genotype...
The ε2/ε4 genotype is rare. One study showed that in healthy adults, the frequency was 7 in 210 (0.02 [0.01-0.04]).34 Given the rarity of the ε2/ε4 genotype, data about it are sparse. However, since the ε4 allele increases risk but the ε2 allele decreases risk, it is likely that any increase in risk is more modest than with ε3/ε4. In addition, it would help Mr. L to know that AD occurs infrequently before age 60.35 Given his relatively young age, he is unlikely to develop AD any time in the near future. In addition, particularly if he starts early, he might be able to mitigate any increased risk through some of the advice provided to Mr. K in Vignette 5.
Vignette 7
Joe J, age 65, comes to the clinic for a new patient visit. He has no concerns about his memory but has a family history of dementia and recently purchased DTC genetic testing to learn about his genetic health risks. His results showed an APOE ε4/ε4 genotype. He is concerned about developing AD. He heard on the news that there is a drug that can treat AD and wants to know if he is a candidate for this treatment.
Mr. J would benefit from the education provided to Ms. W in Vignette 1. Patients such as Mr. J should be advised that while an APOE ε4/ε4 genotype conveys an increased risk for AD, it is not deterministic of the disease. While there are no specific preventive measures or treatments based on APOE genotype, careful medical care and lifestyle factors can offset some of the risk (see Vignette 5 for discussion).
Recently (and controversially), the FDA approved aducanumab, a drug that targets amyloid.6,36 Of note, brain amyloid is more common in individuals with the APOE ε4/ε4 genotype, such as Mr. J. However, there would be no point in testing Mr. J for brain amyloid because at present the drug is only indicated in symptomatic individuals—and, even in this setting, it is controversial. One reason for the controversy is that aducanumab has potentially severe adverse effects. Patients with the ε4/ε4 genotype should know that this genotype carries increased risk for the most serious adverse event, ARIA—which can include brain edema and microhemorrhages.
What lies ahead?
More research is needed to explore the impact that greater AD gene and biomarker testing will have on the health system and workforce development. In addition, graduate schools and training programs will need to prepare clinicians to address probabilistic risk estimates for common diseases, such as AD. Finally, health systems and medical groups that employ clinicians may want to offer simulated training—similar to the vignettes in this article—as a practice requirement or as continuing medical education. This may also allow health systems or medical groups to put in place frameworks that support clinicians in proactively answering questions for patients and families about APOE and other emerging markers of disease risk.
CORRESPONDENCE
Shana Stites, University of Pennsylvania, 3615 Chestnut Street, Philadelphia, PA 19104; [email protected]
1. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2018;14:535-562. doi: 10.1016/j.jalz.2018.02.018 PMCID:PMC5958625
2. Langlois CM, Bradbury A, Wood EM, et al. Alzheimer’s Prevention Initiative Generation Program: development of an APOE genetic counseling and disclosure process in the context of clinical trials. Alzheimers Dement Transl Res Clin Interv. 2019;5:705-716. doi: 10.1016/j.trci.2019.09.013
3. Frank L, Wesson Ashford J, Bayley PJ, et al. Genetic risk of Alzheimer’s disease: three wishes now that the genie is out of the bottle. J Alzheimers Dis. 2018;66:421-423. doi: 10.3233/JAD-180629
4. Qian J, Wolters FJ, Beiser A, et al. APOE-related risk of mild cognitive impairment and dementia for prevention trials: an analysis of four cohorts. PLOS Med. 2017;14:e1002254. doi: 10.1371/journal.pmed.1002254
5. Sperling RA, Jack CR, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367-385. doi: 10.1016/j.jalz.2011.05.2351
6. FDA. November 6, 2020: Meeting of the Peripheral and Central Nervous System Drugs Advisory Committee Meeting Announcement. Published November 12, 2020. Accessed January 14, 2021. www.fda.gov/advisory-committees/advisory-committee-calendar/november-6-2020-meeting-peripheral-and-central-nervous-system-drugs-advisory-committee-meeting
7. Cummings J. Why aducanumab is important. Nat Med. 2021;27:1498-1498. doi: 10.1038/s41591-021-01478-4
8. Alexander GC, Karlawish J. The problem of aducanumab for the treatment of Alzheimer disease. Ann Intern Med. 2021;174:1303-1304. doi: 10.7326/M21-2603
9. Mullard A. More Alzheimer’s drugs head for FDA review: what scientists are watching. Nature. 2021;599:544-545. doi: 10.1038/d41586-021-03410-9
10. Rosenberg A, Mangialasche F, Ngandu T, et al. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from finger to world-wide fingers. J Prev Alzheimers Dis. 2019:1-8. doi: 10.14283/jpad.2019.41
11. FDA. Commissioner of the FDA allows marketing of first direct-to-consumer tests that provide genetic risk information for certain conditions. Published March 24, 2020. Accessed November 7, 2020. www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-direct-consumer-tests-provide-genetic-risk-information-certain-conditions
12. Blell M, Hunter MA. Direct-to-consumer genetic testing’s red herring: “genetic ancestry” and personalized medicine. Front Med. 2019;6:48. doi: 10.3389/fmed.2019.00048
13. Ekstrand B, Scheers N, Rasmussen MK, et al. Brain foods - the role of diet in brain performance and health. Nutr Rev. 2021;79:693-708. doi: 10.1093/nutrit/nuaa091
14. Cherian L, Wang Y, Fakuda K, et al. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) diet slows cognitive decline after stroke. J Prev Alzheimers Dis. 2019;6:267-273. doi: 10.14283/jpad.2019.28
15. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396:413-446. doi: 10.1016/S0140-6736(20)30367-6
16. Livingston PG, Sommerlad A, Orgeta V, et al. The Lancet International Commission on Dementia Prevention and Care. 2017. Accessed March 30, 2022. https://discovery.ucl.ac.uk/id/eprint/1567635/1/Livingston_Dementia_prevention_intervention_care.pdf
17. Peters U. What is the function of confirmation bias? Erkenntnis. April 2020. doi: 10.1007/s10670-020-00252-1
18. Barnes LL, Bennett DA. Cognitive resilience in APOE*ε4 carriers—is race important? Nat Rev Neurol. 2015;11:190-191. doi: 10.1038/nrneurol.2015.38
19. Farrer LA. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349. doi: 10.1001/jama.1997.03550160069041
20. Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185. doi: 10.1001/archneur.60.2.185
21. Chung WW, Chen CA, Cupples LA, et al. A new scale measuring psychologic impact of genetic susceptibility testing for Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:50-56. doi: 10.1097/WAD.0b013e318188429e
22. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. doi: 10.1046/j.1525-1497.2001.016009606.x
23. Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-173. doi: 10.1300/J018v05n01_09
24. Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med. 2009;361:245-254. doi: 10.1056/NEJMoa0809578
25. Lineweaver TT, Bondi MW, Galasko D, et al. Effect of knowledge of APOE genotype on subjective and objective memory performance in healthy older adults. Am J Psychiatry. 2014;171:201-208. doi: 10.1176/appi.ajp.2013.12121590
26. Karlawish J. Understanding the impact of learning an amyloid PET scan result: preliminary findings from the SOKRATES study. Alzheimers Dement J Alzheimers Assoc. 2016;12:P325. doi: 10.1016/j.jalz.2016.06.594
27. Stites SD. Cognitively healthy individuals want to know their risk for Alzheimer’s disease: what should we do? J Alzheimers Dis. 2018;62:499-502. doi: 10.3233/JAD-171089
28. Milne S, Lomax C, Freeston MH. A review of the relationship between intolerance of uncertainty and threat appraisal in anxiety. Cogn Behav Ther. 2019;12:e38. doi: 10.1017/S1754470X19000230
29. Hebert EA, Dugas MJ. Behavioral experiments for intolerance of uncertainty: challenging the unknown in the treatment of generalized anxiety disorder. Cogn Behav Pract. 2019;26:421-436. doi: 10.1016/j.cbpra.2018.07.007
30. Stites SD, Karlawish, J. Stigma of Alzheimer’s disease dementia: considerations for practice. Pract Neurol. Published June 2018. Accessed January 31, 2019. http://practicalneurology.com/2018/06/stigma-of-alzheimers-disease-dementia/
31. Solomon A, Turunen H, Ngandu T, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75:462. doi: 10.1001/jamaneurol.2017.4365
32. Peters R, Warwick J, Anstey KJ, et al. Blood pressure and dementia: what the SPRINT-MIND trial adds and what we still need to know. Neurology. 2019;92:1017-1018. doi: 10.1212/WNL.0000000000007543
33. Musunuru K, Hershberger RE, Day SM, et al. Genetic testing for inherited cardiovascular diseases: a Scientific Statement from the American Heart Association. Circ Genom Precis Med. 2020;13: e000067. doi: 10.1161/HCG.0000000000000067
34. Margaglione M, Seripa D, Gravina C, et al. Prevalence of apolipoprotein E alleles in healthy subjects and survivors of ischemic stroke. Stroke. 1998;29:399-403. doi: 10.1161/01.STR.29.2.399
35. National Institute on Aging. Alzheimer’s disease genetics fact sheet. Reviewed December 24, 2019. Accessed April 10, 2022. www.nia.nih.gov/health/alzheimers-disease-genetics-fact-sheet
36. Belluck P, Kaplan S, Robbins R. How Aduhelm, an unproven Alzheimer’s drug, got approved. The New York Times. Published July 19, 2021. Updated Oct. 20, 2021. Accessed December 1, 2021. www.nytimes.com/2021/07/19/health/alzheimers-drug-aduhelm-fda.html
Advances in Alzheimer disease (AD) genes and biomarkers now allow older adults to undergo testing and learn about their risk for AD.1 Current routes for doing so include testing in cardiology, screening for enrollment in secondary prevention trials (which use these tests to determine trial eligibility),2 and direct-to-consumer (DTC) services that provide these results as part of large panels.3 Patients may also obtain apolipoprotein (APOE) genotype information as part of an assessment of the risks and benefits of treatment with aducanumab (Aduhelm) or other anti-amyloid therapies that have been developed to stop or slow the progression of AD pathologies.
Expanded access to testing, in combination with limited guidance from DTC companies, suggests more older adults may consult their primary care physicians about this testing. In this narrative review, we use a vignette-driven approach to summarize the current scientific knowledge of the topic and to offer guidance on provider-patient discussions and follow-up.
First, a look at APOE genotyping
In cognitively unimpaired older adults, the APOE gene is a known risk factor for mild cognitive impairment (MCI) or AD.3 A person has 2 alleles of the APOE gene, which has 3 variants: ε2, ε3, and ε4. The combination of alleles conveys varying levels of risk for developing clinical symptoms (TABLE 14), with ε4 increasing risk and ε2 decreasing risk compared to the more common ε3; thus the ε4/ε4 genotype conveys the most risk and the ε2/ε2 the least.

The APOE gene differs from other genes that have been identified in early-onset familial AD. These other genes, which include APP, PSEN1, and PSEN2, are deterministic genes that are fully penetrant. The APOE gene is not deterministic, meaning there is no combination of APOE alleles that are necessary or sufficient to cause late-onset AD dementia.
In clinical trials of amyloid-modifying therapies, the APOE gene has been shown to convey a risk of amyloid-related imaging abnormalities (ARIA).5 That is, in addition to conveying a risk for AD, the gene also conveys a risk for adverse effects of emerging treatments that can result in serious injury or death. This includes the drug aducanumab that was recently approved by the US Food and Drug Administration (FDA).6 In this review, we focus primarily on common clinical scenarios related to APOE. However, in light of the recent controversy over aducanumab and whether the drug should be offered to patients,7-9 we also describe how a patient’s APOE genotype may factor into drug candidacy decisions.
Testing, in clinic and “at home.” To date, practice guidelines have consistently recommended against APOE genetic testing in routine clinical practice. This is primarily due to low clinical prognostic utility and the lack of actionable results. Furthermore, no lifestyle or pharmaceutical interventions based on APOE genotype currently exist (although trials are underway10).
In 2017, the FDA approved marketing of DTC testing for the APOE gene.11 While DTC companies tend to issue standardized test result reports, the content and quality can vary widely. In fact, some provide risk estimates that are too high and too definitive and may not reflect the most recent science.12
Continue to: 7 clinical scenarios and how to approach them
7 clinical scenarios and how to approach them
Six of the following vignettes describe common clinical scenarios in which patients seek medical advice regarding APOE test results. The seventh vignette describes a patient whose APOE genotype may play a role in possible disease-modifying treatments down the road. Each vignette is designed to guide your approach to patient discussions and follow-up. Recommendations and considerations are also summarized in TABLE 213-16.

Vignette 1
Janet W, age 65, comes to the clinic for a new patient visit. She has no concerns about her memory but recently purchased DTC genetic testing to learn about her genetic health risks. Her results showed an APOE ε4/ε4 genotype. She is now concerned about developing AD. Her mother was diagnosed with AD in her 70s.
Several important pieces of information can be conveyed by the primary care physician. First, patients such as Ms. W should be told that the APOE gene is not deterministic; many people, even those with 2 ε4 alleles, never develop dementia. Second, no specific preventive measures or treatments exist based on an individual’s APOE genotype (see Vignette 5 for additional discussion).
In this scenario, patients may ask for numeric quantification of their risk for dementia (see TABLE 14 for estimates). When conveying probabilistic risk, consider using simple percentages or pictographs (eg, out of 100 individuals with an ε4/ε4 genotype, 30 to 55 develop MCI or AD). Additionally, because people tend to exhibit confirmatory bias in thinking about probabilistic risk, providing opposing interpretations of an estimate may help them to consider alternative possibilities.17 For example
There are important caveats to the interpretation of APOE risk estimates. Because APOE risk estimates are probabilistic and averaged across a broader spectrum of people in large population cohorts,4 estimates may not accurately reflect a given individual’s risk. The ranges reflect the uncertainty in the estimates. The uncertainty arises from relatively small samples, the rareness of some genotypes (notably ε4/ε4) even in large samples, and variations in methods and sampling that can lead to differences in estimates beyond statistical variation.
Vignette 2
Eric J, age 85, presents for a new patient visit accompanied by his daughter. He lives independently, volunteers at a senior center several times a week, and exercises regularly, and neither he nor his daughter has any concerns about his memory. As a gift, he recently underwent DTC genetic testing and unexpectedly learned his APOE result, which is ε4/ε4. He wants to know about his chances of developing AD.
Risk conveyed by APOE genotype can be modified by a patient’s age. At age 85, Mr. J is healthy, highly functional, and cognitively unimpaired. Given his age, Mr. J has likely “outlived” much of the risk for dementia attributable to the ε4/ε4 genotype. His risk for dementia remains high, but this risk is likely driven more by age than by his APOE genotype. Data for individuals older than age 80 are limited, and thus risk estimates lack precision. Given Mr. J’s good health and functional status, his physician may want to perform a brief cognitive screening test to serve as a baseline for future evaluations.
Continue to: Vignette 3
Vignette 3
Audrey S is a 60-year-old African American woman who comes to the clinic for her annual visit. Because her father had AD, she recently purchased DTC genetic testing to learn about her APOE genotype and risk for AD. Her results are ε3/ε4. She is wondering what this may mean for her future.
Lack of diversity in research cohorts often limits the generalizability of estimates. For example, both the frequency and impact of APOE ε4 differ across racial groups.18 But most of the data on APOE lifetime risk estimates are from largely White patient samples. While APOE ε4 seems to confer increased risk for AD across sociocultural groups, these effects may be attenuated in African American and Hispanic populations.19,20 If Ms. S is interested in numeric risk estimates, the physician can provide the estimate for ε3/ε4 (20%-25% lifetime risk), with the important caveat that this estimate may not be reflective of her individual risk.
It may be prudent to determine whether Ms. S, at age 60, has subjective memory concerns and if she does, to perform a brief cognitive exam to serve as a baseline for future evaluations. Additionally, while the Genetic Information Nondiscrimination Act (GINA, 2008) prohibits health insurers and employers from discriminating based on genetic testing results, no legal provisions exist regarding long-term care, disability, or life insurance. Documented conversations about APOE test results in the medical record may become part of patients’ applications for these insurance products, and physicians should be cautious before documenting such discussions in the medical record.
Vignette 4
Tina L, age 60, comes to the clinic for a routine wellness visit. She recently developed an interest in genealogy and purchased a DNA testing kit to learn more about her family tree. As part of this testing, she unexpectedly learned that she has an APOE ε4/ε4 genotype. She describes feeling distraught and anxious about what the result means for her future.
Ms. L’s reaction to receiving unexpected genetic results highlights a concern of DTC APOE testing. Her experience is quite different from individuals undergoing medically recommended genetic testing or those who are participating in research studies. They receive comprehensive pre-test counseling by licensed genetic counselors. The counseling includes psychological assessment, education, and discussion of expectations.2
In Ms. L’s case, it may be helpful to explain the limits of APOE lifetime risk estimates (see Vignettes 1-3). But it’s also important to address her concerns. There are behavior scales that can aid the assessment and monitoring of an individual’s well-being. The Impact of Genetic Testing for Alzheimer’s Disease (IGT-AD) scale is a tool that assesses psychological impact. It can help physicians to identify, monitor, and address concerns.21 Other useful tools include the Patient Health Questionnaire-9 (PHQ-9) and the Geriatric Depression Scale (GDS) for depression, and a suicide or self-harm assessment.2,22,23 Finally, a follow-up visit at 2 to 4 weeks may be useful to reassess psychological well-being.
Vignette 4 (cont’d)
Ms. L returns to the clinic 2 weeks later, reporting continued anxiety about her APOE test result and feelings of hopelessness and despair.
Continue to: Some patients struggle...
Some patients struggle with knowing their APOE test result. Test result–related distress is often a combination of depression (as with Ms. L), anger, confusion, and grief.24 Cognitions often include worries about uncertainty, stereotyped threat, and internalized stigma.25,26 These issues can spill over to patient concerns about sharing an APOE test result with others.27
Intolerance of uncertainty is a transdiagnostic risk factor that can influence psychological suffering.28 Brief cognitive behavioral interventions that reinforce routines and encourage healthy and mindful practices may help alleviate patient distress from unexpected genetic test results.29 Interventions that personalize and validate an individual’s experience can help address internalized stigma.30 Referral to a psychologist or psychiatrist could be warranted. Additionally, referral to a genetic counselor may help provide patients with access to added expertise and guidance; useful web-based resources for identifying an appropriate referral include https://medlineplus.gov/genetics/understanding/consult/findingprofessional/ and https://findageneticcounselor.nsgc.org/.
Vignette 5
Bob K, age 65, comes to the clinic for his annual exam. He is a current smoker and says he’s hoping to be more physically active now that he is retired. He says that his mother and grandmother both had AD. He recently purchased DTC genetic testing to learn more about his risk for AD. His learned his APOE genotype is ε3/ε4 and is wondering what he can do to decrease his chances of developing AD.
Mr. K likely would have benefited from pre-test counseling regarding the lack of current therapies to modify one’s genetic risk for AD. A pre-test counseling session often includes education about APOE testing and a brief evaluation to assess psychological readiness to undergo testing. Posttest educational information may help Mr. K avoid predatory advertising of products claiming—without scientific evidence—to modify risk for cognitive decline or to improve cognitive function.
There are several important pieces of information that should be communicated to Mr. K. Emerging evidence from randomized controlled trials suggests that healthy lifestyle modifications may benefit cognition in individuals with APOE ε4 alleles.31 It would be prudent to address proper blood pressure control32 and counsel Mr. K on how he may be able to avoid diabetes through exercise and weight maintenance. Lifestyle recommendations for Mr. K could include: smoking cessation, regular aerobic exercise (eg, 150 min/wk), and a brain-healthy diet (eg, the Mediterranean-DASH Intervention for Neurodegenerative Delay [MIND] diet).13,14 Moreover, dementia prevention also includes appropriately managing depression and chronic illnesses and preventing social isolation and hearing loss.15,16 This information should be thoughtfully conveyed, as these interventions can improve overall (especially cardiovascular) health, as well as mitigating one’s personal risk for AD.
Vignette 6
Juan L, age 45, comes in for his annual physical exam. He has a strong family history of heart disease. His cardiologist recently ordered lipid disorder genetic testing for familial hypercholesterolemia. This panel included APOE testing and showed Mr. L’s genotype is ε2/ε4. He read that the APOE gene can be associated with an increased AD risk and asks for information about his genotype.
Mr. L received genetic testing results that were ordered by a physician for another health purpose. Current recommendations for genetic testing in cardiology advise pre-test genetic counseling.33 But this counseling may not include discussion of the relationship of APOE and risk for MCI or AD. This additional information may be unexpected for Mr. L. Moreover, its significance in the context of his present concerns about cardiovascular disease may influence his reaction.
Continue to: The ε2/ε4 genotype...
The ε2/ε4 genotype is rare. One study showed that in healthy adults, the frequency was 7 in 210 (0.02 [0.01-0.04]).34 Given the rarity of the ε2/ε4 genotype, data about it are sparse. However, since the ε4 allele increases risk but the ε2 allele decreases risk, it is likely that any increase in risk is more modest than with ε3/ε4. In addition, it would help Mr. L to know that AD occurs infrequently before age 60.35 Given his relatively young age, he is unlikely to develop AD any time in the near future. In addition, particularly if he starts early, he might be able to mitigate any increased risk through some of the advice provided to Mr. K in Vignette 5.
Vignette 7
Joe J, age 65, comes to the clinic for a new patient visit. He has no concerns about his memory but has a family history of dementia and recently purchased DTC genetic testing to learn about his genetic health risks. His results showed an APOE ε4/ε4 genotype. He is concerned about developing AD. He heard on the news that there is a drug that can treat AD and wants to know if he is a candidate for this treatment.
Mr. J would benefit from the education provided to Ms. W in Vignette 1. Patients such as Mr. J should be advised that while an APOE ε4/ε4 genotype conveys an increased risk for AD, it is not deterministic of the disease. While there are no specific preventive measures or treatments based on APOE genotype, careful medical care and lifestyle factors can offset some of the risk (see Vignette 5 for discussion).
Recently (and controversially), the FDA approved aducanumab, a drug that targets amyloid.6,36 Of note, brain amyloid is more common in individuals with the APOE ε4/ε4 genotype, such as Mr. J. However, there would be no point in testing Mr. J for brain amyloid because at present the drug is only indicated in symptomatic individuals—and, even in this setting, it is controversial. One reason for the controversy is that aducanumab has potentially severe adverse effects. Patients with the ε4/ε4 genotype should know that this genotype carries increased risk for the most serious adverse event, ARIA—which can include brain edema and microhemorrhages.
What lies ahead?
More research is needed to explore the impact that greater AD gene and biomarker testing will have on the health system and workforce development. In addition, graduate schools and training programs will need to prepare clinicians to address probabilistic risk estimates for common diseases, such as AD. Finally, health systems and medical groups that employ clinicians may want to offer simulated training—similar to the vignettes in this article—as a practice requirement or as continuing medical education. This may also allow health systems or medical groups to put in place frameworks that support clinicians in proactively answering questions for patients and families about APOE and other emerging markers of disease risk.
CORRESPONDENCE
Shana Stites, University of Pennsylvania, 3615 Chestnut Street, Philadelphia, PA 19104; [email protected]
Advances in Alzheimer disease (AD) genes and biomarkers now allow older adults to undergo testing and learn about their risk for AD.1 Current routes for doing so include testing in cardiology, screening for enrollment in secondary prevention trials (which use these tests to determine trial eligibility),2 and direct-to-consumer (DTC) services that provide these results as part of large panels.3 Patients may also obtain apolipoprotein (APOE) genotype information as part of an assessment of the risks and benefits of treatment with aducanumab (Aduhelm) or other anti-amyloid therapies that have been developed to stop or slow the progression of AD pathologies.
Expanded access to testing, in combination with limited guidance from DTC companies, suggests more older adults may consult their primary care physicians about this testing. In this narrative review, we use a vignette-driven approach to summarize the current scientific knowledge of the topic and to offer guidance on provider-patient discussions and follow-up.
First, a look at APOE genotyping
In cognitively unimpaired older adults, the APOE gene is a known risk factor for mild cognitive impairment (MCI) or AD.3 A person has 2 alleles of the APOE gene, which has 3 variants: ε2, ε3, and ε4. The combination of alleles conveys varying levels of risk for developing clinical symptoms (TABLE 14), with ε4 increasing risk and ε2 decreasing risk compared to the more common ε3; thus the ε4/ε4 genotype conveys the most risk and the ε2/ε2 the least.

The APOE gene differs from other genes that have been identified in early-onset familial AD. These other genes, which include APP, PSEN1, and PSEN2, are deterministic genes that are fully penetrant. The APOE gene is not deterministic, meaning there is no combination of APOE alleles that are necessary or sufficient to cause late-onset AD dementia.
In clinical trials of amyloid-modifying therapies, the APOE gene has been shown to convey a risk of amyloid-related imaging abnormalities (ARIA).5 That is, in addition to conveying a risk for AD, the gene also conveys a risk for adverse effects of emerging treatments that can result in serious injury or death. This includes the drug aducanumab that was recently approved by the US Food and Drug Administration (FDA).6 In this review, we focus primarily on common clinical scenarios related to APOE. However, in light of the recent controversy over aducanumab and whether the drug should be offered to patients,7-9 we also describe how a patient’s APOE genotype may factor into drug candidacy decisions.
Testing, in clinic and “at home.” To date, practice guidelines have consistently recommended against APOE genetic testing in routine clinical practice. This is primarily due to low clinical prognostic utility and the lack of actionable results. Furthermore, no lifestyle or pharmaceutical interventions based on APOE genotype currently exist (although trials are underway10).
In 2017, the FDA approved marketing of DTC testing for the APOE gene.11 While DTC companies tend to issue standardized test result reports, the content and quality can vary widely. In fact, some provide risk estimates that are too high and too definitive and may not reflect the most recent science.12
Continue to: 7 clinical scenarios and how to approach them
7 clinical scenarios and how to approach them
Six of the following vignettes describe common clinical scenarios in which patients seek medical advice regarding APOE test results. The seventh vignette describes a patient whose APOE genotype may play a role in possible disease-modifying treatments down the road. Each vignette is designed to guide your approach to patient discussions and follow-up. Recommendations and considerations are also summarized in TABLE 213-16.

Vignette 1
Janet W, age 65, comes to the clinic for a new patient visit. She has no concerns about her memory but recently purchased DTC genetic testing to learn about her genetic health risks. Her results showed an APOE ε4/ε4 genotype. She is now concerned about developing AD. Her mother was diagnosed with AD in her 70s.
Several important pieces of information can be conveyed by the primary care physician. First, patients such as Ms. W should be told that the APOE gene is not deterministic; many people, even those with 2 ε4 alleles, never develop dementia. Second, no specific preventive measures or treatments exist based on an individual’s APOE genotype (see Vignette 5 for additional discussion).
In this scenario, patients may ask for numeric quantification of their risk for dementia (see TABLE 14 for estimates). When conveying probabilistic risk, consider using simple percentages or pictographs (eg, out of 100 individuals with an ε4/ε4 genotype, 30 to 55 develop MCI or AD). Additionally, because people tend to exhibit confirmatory bias in thinking about probabilistic risk, providing opposing interpretations of an estimate may help them to consider alternative possibilities.17 For example
There are important caveats to the interpretation of APOE risk estimates. Because APOE risk estimates are probabilistic and averaged across a broader spectrum of people in large population cohorts,4 estimates may not accurately reflect a given individual’s risk. The ranges reflect the uncertainty in the estimates. The uncertainty arises from relatively small samples, the rareness of some genotypes (notably ε4/ε4) even in large samples, and variations in methods and sampling that can lead to differences in estimates beyond statistical variation.
Vignette 2
Eric J, age 85, presents for a new patient visit accompanied by his daughter. He lives independently, volunteers at a senior center several times a week, and exercises regularly, and neither he nor his daughter has any concerns about his memory. As a gift, he recently underwent DTC genetic testing and unexpectedly learned his APOE result, which is ε4/ε4. He wants to know about his chances of developing AD.
Risk conveyed by APOE genotype can be modified by a patient’s age. At age 85, Mr. J is healthy, highly functional, and cognitively unimpaired. Given his age, Mr. J has likely “outlived” much of the risk for dementia attributable to the ε4/ε4 genotype. His risk for dementia remains high, but this risk is likely driven more by age than by his APOE genotype. Data for individuals older than age 80 are limited, and thus risk estimates lack precision. Given Mr. J’s good health and functional status, his physician may want to perform a brief cognitive screening test to serve as a baseline for future evaluations.
Continue to: Vignette 3
Vignette 3
Audrey S is a 60-year-old African American woman who comes to the clinic for her annual visit. Because her father had AD, she recently purchased DTC genetic testing to learn about her APOE genotype and risk for AD. Her results are ε3/ε4. She is wondering what this may mean for her future.
Lack of diversity in research cohorts often limits the generalizability of estimates. For example, both the frequency and impact of APOE ε4 differ across racial groups.18 But most of the data on APOE lifetime risk estimates are from largely White patient samples. While APOE ε4 seems to confer increased risk for AD across sociocultural groups, these effects may be attenuated in African American and Hispanic populations.19,20 If Ms. S is interested in numeric risk estimates, the physician can provide the estimate for ε3/ε4 (20%-25% lifetime risk), with the important caveat that this estimate may not be reflective of her individual risk.
It may be prudent to determine whether Ms. S, at age 60, has subjective memory concerns and if she does, to perform a brief cognitive exam to serve as a baseline for future evaluations. Additionally, while the Genetic Information Nondiscrimination Act (GINA, 2008) prohibits health insurers and employers from discriminating based on genetic testing results, no legal provisions exist regarding long-term care, disability, or life insurance. Documented conversations about APOE test results in the medical record may become part of patients’ applications for these insurance products, and physicians should be cautious before documenting such discussions in the medical record.
Vignette 4
Tina L, age 60, comes to the clinic for a routine wellness visit. She recently developed an interest in genealogy and purchased a DNA testing kit to learn more about her family tree. As part of this testing, she unexpectedly learned that she has an APOE ε4/ε4 genotype. She describes feeling distraught and anxious about what the result means for her future.
Ms. L’s reaction to receiving unexpected genetic results highlights a concern of DTC APOE testing. Her experience is quite different from individuals undergoing medically recommended genetic testing or those who are participating in research studies. They receive comprehensive pre-test counseling by licensed genetic counselors. The counseling includes psychological assessment, education, and discussion of expectations.2
In Ms. L’s case, it may be helpful to explain the limits of APOE lifetime risk estimates (see Vignettes 1-3). But it’s also important to address her concerns. There are behavior scales that can aid the assessment and monitoring of an individual’s well-being. The Impact of Genetic Testing for Alzheimer’s Disease (IGT-AD) scale is a tool that assesses psychological impact. It can help physicians to identify, monitor, and address concerns.21 Other useful tools include the Patient Health Questionnaire-9 (PHQ-9) and the Geriatric Depression Scale (GDS) for depression, and a suicide or self-harm assessment.2,22,23 Finally, a follow-up visit at 2 to 4 weeks may be useful to reassess psychological well-being.
Vignette 4 (cont’d)
Ms. L returns to the clinic 2 weeks later, reporting continued anxiety about her APOE test result and feelings of hopelessness and despair.
Continue to: Some patients struggle...
Some patients struggle with knowing their APOE test result. Test result–related distress is often a combination of depression (as with Ms. L), anger, confusion, and grief.24 Cognitions often include worries about uncertainty, stereotyped threat, and internalized stigma.25,26 These issues can spill over to patient concerns about sharing an APOE test result with others.27
Intolerance of uncertainty is a transdiagnostic risk factor that can influence psychological suffering.28 Brief cognitive behavioral interventions that reinforce routines and encourage healthy and mindful practices may help alleviate patient distress from unexpected genetic test results.29 Interventions that personalize and validate an individual’s experience can help address internalized stigma.30 Referral to a psychologist or psychiatrist could be warranted. Additionally, referral to a genetic counselor may help provide patients with access to added expertise and guidance; useful web-based resources for identifying an appropriate referral include https://medlineplus.gov/genetics/understanding/consult/findingprofessional/ and https://findageneticcounselor.nsgc.org/.
Vignette 5
Bob K, age 65, comes to the clinic for his annual exam. He is a current smoker and says he’s hoping to be more physically active now that he is retired. He says that his mother and grandmother both had AD. He recently purchased DTC genetic testing to learn more about his risk for AD. His learned his APOE genotype is ε3/ε4 and is wondering what he can do to decrease his chances of developing AD.
Mr. K likely would have benefited from pre-test counseling regarding the lack of current therapies to modify one’s genetic risk for AD. A pre-test counseling session often includes education about APOE testing and a brief evaluation to assess psychological readiness to undergo testing. Posttest educational information may help Mr. K avoid predatory advertising of products claiming—without scientific evidence—to modify risk for cognitive decline or to improve cognitive function.
There are several important pieces of information that should be communicated to Mr. K. Emerging evidence from randomized controlled trials suggests that healthy lifestyle modifications may benefit cognition in individuals with APOE ε4 alleles.31 It would be prudent to address proper blood pressure control32 and counsel Mr. K on how he may be able to avoid diabetes through exercise and weight maintenance. Lifestyle recommendations for Mr. K could include: smoking cessation, regular aerobic exercise (eg, 150 min/wk), and a brain-healthy diet (eg, the Mediterranean-DASH Intervention for Neurodegenerative Delay [MIND] diet).13,14 Moreover, dementia prevention also includes appropriately managing depression and chronic illnesses and preventing social isolation and hearing loss.15,16 This information should be thoughtfully conveyed, as these interventions can improve overall (especially cardiovascular) health, as well as mitigating one’s personal risk for AD.
Vignette 6
Juan L, age 45, comes in for his annual physical exam. He has a strong family history of heart disease. His cardiologist recently ordered lipid disorder genetic testing for familial hypercholesterolemia. This panel included APOE testing and showed Mr. L’s genotype is ε2/ε4. He read that the APOE gene can be associated with an increased AD risk and asks for information about his genotype.
Mr. L received genetic testing results that were ordered by a physician for another health purpose. Current recommendations for genetic testing in cardiology advise pre-test genetic counseling.33 But this counseling may not include discussion of the relationship of APOE and risk for MCI or AD. This additional information may be unexpected for Mr. L. Moreover, its significance in the context of his present concerns about cardiovascular disease may influence his reaction.
Continue to: The ε2/ε4 genotype...
The ε2/ε4 genotype is rare. One study showed that in healthy adults, the frequency was 7 in 210 (0.02 [0.01-0.04]).34 Given the rarity of the ε2/ε4 genotype, data about it are sparse. However, since the ε4 allele increases risk but the ε2 allele decreases risk, it is likely that any increase in risk is more modest than with ε3/ε4. In addition, it would help Mr. L to know that AD occurs infrequently before age 60.35 Given his relatively young age, he is unlikely to develop AD any time in the near future. In addition, particularly if he starts early, he might be able to mitigate any increased risk through some of the advice provided to Mr. K in Vignette 5.
Vignette 7
Joe J, age 65, comes to the clinic for a new patient visit. He has no concerns about his memory but has a family history of dementia and recently purchased DTC genetic testing to learn about his genetic health risks. His results showed an APOE ε4/ε4 genotype. He is concerned about developing AD. He heard on the news that there is a drug that can treat AD and wants to know if he is a candidate for this treatment.
Mr. J would benefit from the education provided to Ms. W in Vignette 1. Patients such as Mr. J should be advised that while an APOE ε4/ε4 genotype conveys an increased risk for AD, it is not deterministic of the disease. While there are no specific preventive measures or treatments based on APOE genotype, careful medical care and lifestyle factors can offset some of the risk (see Vignette 5 for discussion).
Recently (and controversially), the FDA approved aducanumab, a drug that targets amyloid.6,36 Of note, brain amyloid is more common in individuals with the APOE ε4/ε4 genotype, such as Mr. J. However, there would be no point in testing Mr. J for brain amyloid because at present the drug is only indicated in symptomatic individuals—and, even in this setting, it is controversial. One reason for the controversy is that aducanumab has potentially severe adverse effects. Patients with the ε4/ε4 genotype should know that this genotype carries increased risk for the most serious adverse event, ARIA—which can include brain edema and microhemorrhages.
What lies ahead?
More research is needed to explore the impact that greater AD gene and biomarker testing will have on the health system and workforce development. In addition, graduate schools and training programs will need to prepare clinicians to address probabilistic risk estimates for common diseases, such as AD. Finally, health systems and medical groups that employ clinicians may want to offer simulated training—similar to the vignettes in this article—as a practice requirement or as continuing medical education. This may also allow health systems or medical groups to put in place frameworks that support clinicians in proactively answering questions for patients and families about APOE and other emerging markers of disease risk.
CORRESPONDENCE
Shana Stites, University of Pennsylvania, 3615 Chestnut Street, Philadelphia, PA 19104; [email protected]
1. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2018;14:535-562. doi: 10.1016/j.jalz.2018.02.018 PMCID:PMC5958625
2. Langlois CM, Bradbury A, Wood EM, et al. Alzheimer’s Prevention Initiative Generation Program: development of an APOE genetic counseling and disclosure process in the context of clinical trials. Alzheimers Dement Transl Res Clin Interv. 2019;5:705-716. doi: 10.1016/j.trci.2019.09.013
3. Frank L, Wesson Ashford J, Bayley PJ, et al. Genetic risk of Alzheimer’s disease: three wishes now that the genie is out of the bottle. J Alzheimers Dis. 2018;66:421-423. doi: 10.3233/JAD-180629
4. Qian J, Wolters FJ, Beiser A, et al. APOE-related risk of mild cognitive impairment and dementia for prevention trials: an analysis of four cohorts. PLOS Med. 2017;14:e1002254. doi: 10.1371/journal.pmed.1002254
5. Sperling RA, Jack CR, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367-385. doi: 10.1016/j.jalz.2011.05.2351
6. FDA. November 6, 2020: Meeting of the Peripheral and Central Nervous System Drugs Advisory Committee Meeting Announcement. Published November 12, 2020. Accessed January 14, 2021. www.fda.gov/advisory-committees/advisory-committee-calendar/november-6-2020-meeting-peripheral-and-central-nervous-system-drugs-advisory-committee-meeting
7. Cummings J. Why aducanumab is important. Nat Med. 2021;27:1498-1498. doi: 10.1038/s41591-021-01478-4
8. Alexander GC, Karlawish J. The problem of aducanumab for the treatment of Alzheimer disease. Ann Intern Med. 2021;174:1303-1304. doi: 10.7326/M21-2603
9. Mullard A. More Alzheimer’s drugs head for FDA review: what scientists are watching. Nature. 2021;599:544-545. doi: 10.1038/d41586-021-03410-9
10. Rosenberg A, Mangialasche F, Ngandu T, et al. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from finger to world-wide fingers. J Prev Alzheimers Dis. 2019:1-8. doi: 10.14283/jpad.2019.41
11. FDA. Commissioner of the FDA allows marketing of first direct-to-consumer tests that provide genetic risk information for certain conditions. Published March 24, 2020. Accessed November 7, 2020. www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-direct-consumer-tests-provide-genetic-risk-information-certain-conditions
12. Blell M, Hunter MA. Direct-to-consumer genetic testing’s red herring: “genetic ancestry” and personalized medicine. Front Med. 2019;6:48. doi: 10.3389/fmed.2019.00048
13. Ekstrand B, Scheers N, Rasmussen MK, et al. Brain foods - the role of diet in brain performance and health. Nutr Rev. 2021;79:693-708. doi: 10.1093/nutrit/nuaa091
14. Cherian L, Wang Y, Fakuda K, et al. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) diet slows cognitive decline after stroke. J Prev Alzheimers Dis. 2019;6:267-273. doi: 10.14283/jpad.2019.28
15. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396:413-446. doi: 10.1016/S0140-6736(20)30367-6
16. Livingston PG, Sommerlad A, Orgeta V, et al. The Lancet International Commission on Dementia Prevention and Care. 2017. Accessed March 30, 2022. https://discovery.ucl.ac.uk/id/eprint/1567635/1/Livingston_Dementia_prevention_intervention_care.pdf
17. Peters U. What is the function of confirmation bias? Erkenntnis. April 2020. doi: 10.1007/s10670-020-00252-1
18. Barnes LL, Bennett DA. Cognitive resilience in APOE*ε4 carriers—is race important? Nat Rev Neurol. 2015;11:190-191. doi: 10.1038/nrneurol.2015.38
19. Farrer LA. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349. doi: 10.1001/jama.1997.03550160069041
20. Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185. doi: 10.1001/archneur.60.2.185
21. Chung WW, Chen CA, Cupples LA, et al. A new scale measuring psychologic impact of genetic susceptibility testing for Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:50-56. doi: 10.1097/WAD.0b013e318188429e
22. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. doi: 10.1046/j.1525-1497.2001.016009606.x
23. Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-173. doi: 10.1300/J018v05n01_09
24. Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med. 2009;361:245-254. doi: 10.1056/NEJMoa0809578
25. Lineweaver TT, Bondi MW, Galasko D, et al. Effect of knowledge of APOE genotype on subjective and objective memory performance in healthy older adults. Am J Psychiatry. 2014;171:201-208. doi: 10.1176/appi.ajp.2013.12121590
26. Karlawish J. Understanding the impact of learning an amyloid PET scan result: preliminary findings from the SOKRATES study. Alzheimers Dement J Alzheimers Assoc. 2016;12:P325. doi: 10.1016/j.jalz.2016.06.594
27. Stites SD. Cognitively healthy individuals want to know their risk for Alzheimer’s disease: what should we do? J Alzheimers Dis. 2018;62:499-502. doi: 10.3233/JAD-171089
28. Milne S, Lomax C, Freeston MH. A review of the relationship between intolerance of uncertainty and threat appraisal in anxiety. Cogn Behav Ther. 2019;12:e38. doi: 10.1017/S1754470X19000230
29. Hebert EA, Dugas MJ. Behavioral experiments for intolerance of uncertainty: challenging the unknown in the treatment of generalized anxiety disorder. Cogn Behav Pract. 2019;26:421-436. doi: 10.1016/j.cbpra.2018.07.007
30. Stites SD, Karlawish, J. Stigma of Alzheimer’s disease dementia: considerations for practice. Pract Neurol. Published June 2018. Accessed January 31, 2019. http://practicalneurology.com/2018/06/stigma-of-alzheimers-disease-dementia/
31. Solomon A, Turunen H, Ngandu T, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75:462. doi: 10.1001/jamaneurol.2017.4365
32. Peters R, Warwick J, Anstey KJ, et al. Blood pressure and dementia: what the SPRINT-MIND trial adds and what we still need to know. Neurology. 2019;92:1017-1018. doi: 10.1212/WNL.0000000000007543
33. Musunuru K, Hershberger RE, Day SM, et al. Genetic testing for inherited cardiovascular diseases: a Scientific Statement from the American Heart Association. Circ Genom Precis Med. 2020;13: e000067. doi: 10.1161/HCG.0000000000000067
34. Margaglione M, Seripa D, Gravina C, et al. Prevalence of apolipoprotein E alleles in healthy subjects and survivors of ischemic stroke. Stroke. 1998;29:399-403. doi: 10.1161/01.STR.29.2.399
35. National Institute on Aging. Alzheimer’s disease genetics fact sheet. Reviewed December 24, 2019. Accessed April 10, 2022. www.nia.nih.gov/health/alzheimers-disease-genetics-fact-sheet
36. Belluck P, Kaplan S, Robbins R. How Aduhelm, an unproven Alzheimer’s drug, got approved. The New York Times. Published July 19, 2021. Updated Oct. 20, 2021. Accessed December 1, 2021. www.nytimes.com/2021/07/19/health/alzheimers-drug-aduhelm-fda.html
1. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2018;14:535-562. doi: 10.1016/j.jalz.2018.02.018 PMCID:PMC5958625
2. Langlois CM, Bradbury A, Wood EM, et al. Alzheimer’s Prevention Initiative Generation Program: development of an APOE genetic counseling and disclosure process in the context of clinical trials. Alzheimers Dement Transl Res Clin Interv. 2019;5:705-716. doi: 10.1016/j.trci.2019.09.013
3. Frank L, Wesson Ashford J, Bayley PJ, et al. Genetic risk of Alzheimer’s disease: three wishes now that the genie is out of the bottle. J Alzheimers Dis. 2018;66:421-423. doi: 10.3233/JAD-180629
4. Qian J, Wolters FJ, Beiser A, et al. APOE-related risk of mild cognitive impairment and dementia for prevention trials: an analysis of four cohorts. PLOS Med. 2017;14:e1002254. doi: 10.1371/journal.pmed.1002254
5. Sperling RA, Jack CR, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367-385. doi: 10.1016/j.jalz.2011.05.2351
6. FDA. November 6, 2020: Meeting of the Peripheral and Central Nervous System Drugs Advisory Committee Meeting Announcement. Published November 12, 2020. Accessed January 14, 2021. www.fda.gov/advisory-committees/advisory-committee-calendar/november-6-2020-meeting-peripheral-and-central-nervous-system-drugs-advisory-committee-meeting
7. Cummings J. Why aducanumab is important. Nat Med. 2021;27:1498-1498. doi: 10.1038/s41591-021-01478-4
8. Alexander GC, Karlawish J. The problem of aducanumab for the treatment of Alzheimer disease. Ann Intern Med. 2021;174:1303-1304. doi: 10.7326/M21-2603
9. Mullard A. More Alzheimer’s drugs head for FDA review: what scientists are watching. Nature. 2021;599:544-545. doi: 10.1038/d41586-021-03410-9
10. Rosenberg A, Mangialasche F, Ngandu T, et al. Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from finger to world-wide fingers. J Prev Alzheimers Dis. 2019:1-8. doi: 10.14283/jpad.2019.41
11. FDA. Commissioner of the FDA allows marketing of first direct-to-consumer tests that provide genetic risk information for certain conditions. Published March 24, 2020. Accessed November 7, 2020. www.fda.gov/news-events/press-announcements/fda-allows-marketing-first-direct-consumer-tests-provide-genetic-risk-information-certain-conditions
12. Blell M, Hunter MA. Direct-to-consumer genetic testing’s red herring: “genetic ancestry” and personalized medicine. Front Med. 2019;6:48. doi: 10.3389/fmed.2019.00048
13. Ekstrand B, Scheers N, Rasmussen MK, et al. Brain foods - the role of diet in brain performance and health. Nutr Rev. 2021;79:693-708. doi: 10.1093/nutrit/nuaa091
14. Cherian L, Wang Y, Fakuda K, et al. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) diet slows cognitive decline after stroke. J Prev Alzheimers Dis. 2019;6:267-273. doi: 10.14283/jpad.2019.28
15. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396:413-446. doi: 10.1016/S0140-6736(20)30367-6
16. Livingston PG, Sommerlad A, Orgeta V, et al. The Lancet International Commission on Dementia Prevention and Care. 2017. Accessed March 30, 2022. https://discovery.ucl.ac.uk/id/eprint/1567635/1/Livingston_Dementia_prevention_intervention_care.pdf
17. Peters U. What is the function of confirmation bias? Erkenntnis. April 2020. doi: 10.1007/s10670-020-00252-1
18. Barnes LL, Bennett DA. Cognitive resilience in APOE*ε4 carriers—is race important? Nat Rev Neurol. 2015;11:190-191. doi: 10.1038/nrneurol.2015.38
19. Farrer LA. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349. doi: 10.1001/jama.1997.03550160069041
20. Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185. doi: 10.1001/archneur.60.2.185
21. Chung WW, Chen CA, Cupples LA, et al. A new scale measuring psychologic impact of genetic susceptibility testing for Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:50-56. doi: 10.1097/WAD.0b013e318188429e
22. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. doi: 10.1046/j.1525-1497.2001.016009606.x
23. Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-173. doi: 10.1300/J018v05n01_09
24. Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med. 2009;361:245-254. doi: 10.1056/NEJMoa0809578
25. Lineweaver TT, Bondi MW, Galasko D, et al. Effect of knowledge of APOE genotype on subjective and objective memory performance in healthy older adults. Am J Psychiatry. 2014;171:201-208. doi: 10.1176/appi.ajp.2013.12121590
26. Karlawish J. Understanding the impact of learning an amyloid PET scan result: preliminary findings from the SOKRATES study. Alzheimers Dement J Alzheimers Assoc. 2016;12:P325. doi: 10.1016/j.jalz.2016.06.594
27. Stites SD. Cognitively healthy individuals want to know their risk for Alzheimer’s disease: what should we do? J Alzheimers Dis. 2018;62:499-502. doi: 10.3233/JAD-171089
28. Milne S, Lomax C, Freeston MH. A review of the relationship between intolerance of uncertainty and threat appraisal in anxiety. Cogn Behav Ther. 2019;12:e38. doi: 10.1017/S1754470X19000230
29. Hebert EA, Dugas MJ. Behavioral experiments for intolerance of uncertainty: challenging the unknown in the treatment of generalized anxiety disorder. Cogn Behav Pract. 2019;26:421-436. doi: 10.1016/j.cbpra.2018.07.007
30. Stites SD, Karlawish, J. Stigma of Alzheimer’s disease dementia: considerations for practice. Pract Neurol. Published June 2018. Accessed January 31, 2019. http://practicalneurology.com/2018/06/stigma-of-alzheimers-disease-dementia/
31. Solomon A, Turunen H, Ngandu T, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75:462. doi: 10.1001/jamaneurol.2017.4365
32. Peters R, Warwick J, Anstey KJ, et al. Blood pressure and dementia: what the SPRINT-MIND trial adds and what we still need to know. Neurology. 2019;92:1017-1018. doi: 10.1212/WNL.0000000000007543
33. Musunuru K, Hershberger RE, Day SM, et al. Genetic testing for inherited cardiovascular diseases: a Scientific Statement from the American Heart Association. Circ Genom Precis Med. 2020;13: e000067. doi: 10.1161/HCG.0000000000000067
34. Margaglione M, Seripa D, Gravina C, et al. Prevalence of apolipoprotein E alleles in healthy subjects and survivors of ischemic stroke. Stroke. 1998;29:399-403. doi: 10.1161/01.STR.29.2.399
35. National Institute on Aging. Alzheimer’s disease genetics fact sheet. Reviewed December 24, 2019. Accessed April 10, 2022. www.nia.nih.gov/health/alzheimers-disease-genetics-fact-sheet
36. Belluck P, Kaplan S, Robbins R. How Aduhelm, an unproven Alzheimer’s drug, got approved. The New York Times. Published July 19, 2021. Updated Oct. 20, 2021. Accessed December 1, 2021. www.nytimes.com/2021/07/19/health/alzheimers-drug-aduhelm-fda.html
Ex–hospital porter a neglected giant of cancer research
We have a half-forgotten Indian immigrant to thank – a hospital night porter turned biochemist –for revolutionizing treatment of leukemia, the once deadly childhood scourge that is still the most common pediatric cancer.
Dr. Yellapragada SubbaRow has been called the “father of chemotherapy” for developing methotrexate, a powerful, inexpensive therapy for leukemia and other diseases, and he is celebrated for additional scientific achievements. Yet Dr. SubbaRow’s life was marked more by struggle than glory.
Born poor in southeastern India, he nearly succumbed to a tropical disease that killed two older brothers, and he didn’t focus on schoolwork until his father died. Later, prejudice dogged his years as an immigrant to the United States, and a blood clot took his life at the age of 53.
Scientifically, however, Dr. SubbaRow (pronounced sue-buh-rao) triumphed, despite mammoth challenges and a lack of recognition that persists to this day. National Cancer Research Month is a fitting time to look back on his extraordinary life and work and pay tribute to his accomplishments.
‘Yella,’ folic acid, and a paradigm shift
No one appreciates Dr. SubbaRow more than a cadre of Indian-born physicians who have kept his legacy alive in journal articles, presentations, and a Pulitzer Prize-winning book. Among them is author and oncologist Siddhartha Mukherjee, MD, who chronicled Dr. SubbaRow’s achievements in his New York Times No. 1 bestseller, “The Emperor of All Maladies: A Biography of Cancer.”
As Dr. Mukherjee wrote, Dr. SubbaRow was a “pioneer in many ways, a physician turned cellular physiologist, a chemist who had accidentally wandered into biology.” (Per Indian tradition, SubbaRow is the doctor’s first name, and Yellapragada is his surname, but medical literature uses SubbaRow as his cognomen, with some variations in spelling. Dr. Mukherjee wrote that his friends called him “Yella.”)
Dr. SubbaRow came to the United States in 1923, after enduring a difficult childhood and young adulthood. He’d survived bouts of religious fervor, childhood rebellion (including a bid to run away from home and become a banana trader), and a failed arranged marriage. His wife bore him a child who died in infancy. He left it all behind.
In Boston, medical officials rejected his degree. Broke, he worked for a time as a night porter at Brigham and Women’s Hospital in Boston, changing sheets and cleaning urinals. To a poor but proud high-caste Indian Brahmin, the culture shock of carrying out these tasks must have been especially jarring.
Dr. SubbaRow went on to earn a diploma from Harvard Medical School, also in Boston, and became a junior faculty member. As a foreigner, Dr. Mukherjee wrote, Dr. SubbaRow was a “reclusive, nocturnal, heavily accented vegetarian,” so different from his colleagues that advancement seemed impossible. Despite his pioneering biochemistry work, Harvard later declined to offer Dr. SubbaRow a tenured faculty position.
By the early 1940s, he took a job at an upstate New York pharmaceutical company called Lederle Labs (later purchased by Pfizer). At Lederle, Dr. SubbaRow strove to synthesize the vitamin known as folic acid. He ended up creating a kind of antivitamin, a lookalike that acted like folic acid but only succeeded in gumming up the works in receptors. But what good would it do to stop the body from absorbing folic acid? Plenty, it turned out.
Discoveries pile up, but credit and fame prove elusive
Dr. SubbaRow was no stranger to producing landmark biological work. He’d previously codiscovered phosphocreatine and ATP, which are crucial to muscular contractions. However, “in 1935, he had to disown the extent of his role in the discovery of the color test related to phosphorus, instead giving the credit to his co-author, who was being considered for promotion to a full professorship at Harvard,” wrote author Gerald Posner in his 2020 book, “Pharma: Greed, Lies and the Poisoning of America.”
Houston-area oncologist Kirtan Nautiyal, MD, who paid tribute to Dr. SubbaRow in a 2018 article, contended that “with his Indian instinct for self-effacement, he had irreparably sabotaged his own career.”
Dr. SubbaRow and his team also developed “the first effective treatment of filariasis, which causes elephantiasis of the lower limbs and genitals in millions of people, mainly in tropical countries,” Dr. Nautiyal wrote. “Later in the decade, his antibiotic program generated polymyxin, the first effective treatment against the class of bacteria called Gram negatives, and aureomycin, the first “broad-spectrum’ antibiotic.” (Aureomycin is also the first tetracycline antibiotic.)
Dr. SubbaRow’s discovery of a folic acid antagonist would again go largely unheralded. But first came the realization that folic acid made childhood leukemia worse, not better, and the prospect that this process could potentially be reversed.
Rise of methotrexate and fall of leukemia
In Boston, Sidney Farber, MD, a Boston pathologist, was desperate to help Robert Sandler, a 2-year-old leukemia patient. Dr. Farber contacted his ex-colleague Dr. SubbaRow to request a supply of aminopterin, an early version of methotrexate that Dr. SubbaRow and his team had developed. Dr. Farber injected Robert with the substance and within 3 days, the toddler’s white blood count started falling – fast. He stopped bleeding, resumed eating, and once again seemed almost identical to his twin brother, as Dr. Mukherjee wrote in his book.
Leukemia had never gone into remission before. Unfortunately, the treatment only worked temporarily. Robert, like other children treated with the drug, relapsed and died within months. But Dr. Farber “saw a door open” – a chemical, a kind of chemotherapy, that could turn back cancer. In the case of folic acid antagonists, they do so by stopping cancer cells from replicating.
Methotrexate, a related agent synthesized by Dr. SubbaRow, would become a mainstay of leukemia treatment and begin to produce long-term remission from acute lymphoblastic leukemia in 1970, when combination chemotherapy was developed.
Other cancers fell to methotrexate treatment. “Previous assumptions that cancer was nearly always fatal were revised, and the field of medical oncology (treatment of cancer with chemotherapy), which had not previously existed, was formally established in 1971,” according to the National Cancer Institute’s history of methotrexate. This account does not mention Dr. SubbaRow.
Death takes the doctor, but his legacy remains
In biographies, as well as his own words, Dr. SubbaRow comes across as a prickly, hard-driving workaholic who had little interest in intimate human connections. “It is not good to ask in every letter when I will be back,” he wrote to his wife back in India, before cutting off ties completely in the early 1930s. “I will come as early as possible. ... I do not want to write anything more.”
It seems, as his biographer S.P.K. Gupta noted, that “he was quite determined that the time allotted to him on Earth should be completely devoted to finding cures for ailments that plagued mankind.”
Still, Dr. SubbaRow’s research team was devoted to him, and he had plenty of reasons to be bitter, such as the prejudice and isolation he encountered in the United States and earlier, in British-run India. According to Mr. Posner’s book, even as a young medical student, Dr. SubbaRow heeded the call of Indian independence activist Mohandas Gandhi. He “refused the British surgical gown given him at school and instead donned a traditional and simple cotton Khadi. That act of defiance cost SubbaRow the college degree that was necessary for him to get into the State Medical College.”
During the last year of his life, Dr. SubbaRow faced yet another humiliation: In his landmark 1948 study about aminopterin as a treatment for leukemia, his colleague Dr. Farber failed to credit him, an “astonishing omission” as Yaddanapudi Ravindranath, MBBS, a pediatric hematologist/oncologist at Wayne State University, Detroit, put it. “From everything I know, Dr. Farber spent the rest of his career apologizing and trying to make amends for it,” Dr. Ravindranath said in an interview.
A career cut short, and a lasting legacy
In 1948, at the age of 53, Dr. SubbaRow suddenly died. “Many think Dr. SubbaRow would have won [the] Nobel Prize had he lived a few years longer,” said Dr. Ravindranath.
Like Dr. SubbaRow, Dr. Ravindranath was born in Andhra Pradesh state, near the city of Chennai formerly known as Madras. “Being a compatriot, in a way I continue his legacy, and I am obviously proud of him,” said Dr. Ravindranath, who has conducted his own landmark research regarding methotrexate and leukemia.
Nearly 75 years after Dr. SubbaRow’s death, Indian-born physicians like Dr. Ravindranath continue to honor him in print, trying to ensure that he’s not forgotten. Methotrexate remains a crucial treatment for leukemia, along with a long list of other ailments, including psoriasis.
Recognition for “Yella” may have come late and infrequently, but a Lederle Laboratories research library named after him offered Dr. SubbaRow a kind of immortality. A plaque there memorialized him in stone as a scientist, teacher, philosopher, and humanitarian, featuring the quote: “Science simply prolongs life. Religion deepens it.”
By all accounts, Dr. SubbaRow was a man of science and faith who had faith in science.
We have a half-forgotten Indian immigrant to thank – a hospital night porter turned biochemist –for revolutionizing treatment of leukemia, the once deadly childhood scourge that is still the most common pediatric cancer.
Dr. Yellapragada SubbaRow has been called the “father of chemotherapy” for developing methotrexate, a powerful, inexpensive therapy for leukemia and other diseases, and he is celebrated for additional scientific achievements. Yet Dr. SubbaRow’s life was marked more by struggle than glory.
Born poor in southeastern India, he nearly succumbed to a tropical disease that killed two older brothers, and he didn’t focus on schoolwork until his father died. Later, prejudice dogged his years as an immigrant to the United States, and a blood clot took his life at the age of 53.
Scientifically, however, Dr. SubbaRow (pronounced sue-buh-rao) triumphed, despite mammoth challenges and a lack of recognition that persists to this day. National Cancer Research Month is a fitting time to look back on his extraordinary life and work and pay tribute to his accomplishments.
‘Yella,’ folic acid, and a paradigm shift
No one appreciates Dr. SubbaRow more than a cadre of Indian-born physicians who have kept his legacy alive in journal articles, presentations, and a Pulitzer Prize-winning book. Among them is author and oncologist Siddhartha Mukherjee, MD, who chronicled Dr. SubbaRow’s achievements in his New York Times No. 1 bestseller, “The Emperor of All Maladies: A Biography of Cancer.”
As Dr. Mukherjee wrote, Dr. SubbaRow was a “pioneer in many ways, a physician turned cellular physiologist, a chemist who had accidentally wandered into biology.” (Per Indian tradition, SubbaRow is the doctor’s first name, and Yellapragada is his surname, but medical literature uses SubbaRow as his cognomen, with some variations in spelling. Dr. Mukherjee wrote that his friends called him “Yella.”)
Dr. SubbaRow came to the United States in 1923, after enduring a difficult childhood and young adulthood. He’d survived bouts of religious fervor, childhood rebellion (including a bid to run away from home and become a banana trader), and a failed arranged marriage. His wife bore him a child who died in infancy. He left it all behind.
In Boston, medical officials rejected his degree. Broke, he worked for a time as a night porter at Brigham and Women’s Hospital in Boston, changing sheets and cleaning urinals. To a poor but proud high-caste Indian Brahmin, the culture shock of carrying out these tasks must have been especially jarring.
Dr. SubbaRow went on to earn a diploma from Harvard Medical School, also in Boston, and became a junior faculty member. As a foreigner, Dr. Mukherjee wrote, Dr. SubbaRow was a “reclusive, nocturnal, heavily accented vegetarian,” so different from his colleagues that advancement seemed impossible. Despite his pioneering biochemistry work, Harvard later declined to offer Dr. SubbaRow a tenured faculty position.
By the early 1940s, he took a job at an upstate New York pharmaceutical company called Lederle Labs (later purchased by Pfizer). At Lederle, Dr. SubbaRow strove to synthesize the vitamin known as folic acid. He ended up creating a kind of antivitamin, a lookalike that acted like folic acid but only succeeded in gumming up the works in receptors. But what good would it do to stop the body from absorbing folic acid? Plenty, it turned out.
Discoveries pile up, but credit and fame prove elusive
Dr. SubbaRow was no stranger to producing landmark biological work. He’d previously codiscovered phosphocreatine and ATP, which are crucial to muscular contractions. However, “in 1935, he had to disown the extent of his role in the discovery of the color test related to phosphorus, instead giving the credit to his co-author, who was being considered for promotion to a full professorship at Harvard,” wrote author Gerald Posner in his 2020 book, “Pharma: Greed, Lies and the Poisoning of America.”
Houston-area oncologist Kirtan Nautiyal, MD, who paid tribute to Dr. SubbaRow in a 2018 article, contended that “with his Indian instinct for self-effacement, he had irreparably sabotaged his own career.”
Dr. SubbaRow and his team also developed “the first effective treatment of filariasis, which causes elephantiasis of the lower limbs and genitals in millions of people, mainly in tropical countries,” Dr. Nautiyal wrote. “Later in the decade, his antibiotic program generated polymyxin, the first effective treatment against the class of bacteria called Gram negatives, and aureomycin, the first “broad-spectrum’ antibiotic.” (Aureomycin is also the first tetracycline antibiotic.)
Dr. SubbaRow’s discovery of a folic acid antagonist would again go largely unheralded. But first came the realization that folic acid made childhood leukemia worse, not better, and the prospect that this process could potentially be reversed.
Rise of methotrexate and fall of leukemia
In Boston, Sidney Farber, MD, a Boston pathologist, was desperate to help Robert Sandler, a 2-year-old leukemia patient. Dr. Farber contacted his ex-colleague Dr. SubbaRow to request a supply of aminopterin, an early version of methotrexate that Dr. SubbaRow and his team had developed. Dr. Farber injected Robert with the substance and within 3 days, the toddler’s white blood count started falling – fast. He stopped bleeding, resumed eating, and once again seemed almost identical to his twin brother, as Dr. Mukherjee wrote in his book.
Leukemia had never gone into remission before. Unfortunately, the treatment only worked temporarily. Robert, like other children treated with the drug, relapsed and died within months. But Dr. Farber “saw a door open” – a chemical, a kind of chemotherapy, that could turn back cancer. In the case of folic acid antagonists, they do so by stopping cancer cells from replicating.
Methotrexate, a related agent synthesized by Dr. SubbaRow, would become a mainstay of leukemia treatment and begin to produce long-term remission from acute lymphoblastic leukemia in 1970, when combination chemotherapy was developed.
Other cancers fell to methotrexate treatment. “Previous assumptions that cancer was nearly always fatal were revised, and the field of medical oncology (treatment of cancer with chemotherapy), which had not previously existed, was formally established in 1971,” according to the National Cancer Institute’s history of methotrexate. This account does not mention Dr. SubbaRow.
Death takes the doctor, but his legacy remains
In biographies, as well as his own words, Dr. SubbaRow comes across as a prickly, hard-driving workaholic who had little interest in intimate human connections. “It is not good to ask in every letter when I will be back,” he wrote to his wife back in India, before cutting off ties completely in the early 1930s. “I will come as early as possible. ... I do not want to write anything more.”
It seems, as his biographer S.P.K. Gupta noted, that “he was quite determined that the time allotted to him on Earth should be completely devoted to finding cures for ailments that plagued mankind.”
Still, Dr. SubbaRow’s research team was devoted to him, and he had plenty of reasons to be bitter, such as the prejudice and isolation he encountered in the United States and earlier, in British-run India. According to Mr. Posner’s book, even as a young medical student, Dr. SubbaRow heeded the call of Indian independence activist Mohandas Gandhi. He “refused the British surgical gown given him at school and instead donned a traditional and simple cotton Khadi. That act of defiance cost SubbaRow the college degree that was necessary for him to get into the State Medical College.”
During the last year of his life, Dr. SubbaRow faced yet another humiliation: In his landmark 1948 study about aminopterin as a treatment for leukemia, his colleague Dr. Farber failed to credit him, an “astonishing omission” as Yaddanapudi Ravindranath, MBBS, a pediatric hematologist/oncologist at Wayne State University, Detroit, put it. “From everything I know, Dr. Farber spent the rest of his career apologizing and trying to make amends for it,” Dr. Ravindranath said in an interview.
A career cut short, and a lasting legacy
In 1948, at the age of 53, Dr. SubbaRow suddenly died. “Many think Dr. SubbaRow would have won [the] Nobel Prize had he lived a few years longer,” said Dr. Ravindranath.
Like Dr. SubbaRow, Dr. Ravindranath was born in Andhra Pradesh state, near the city of Chennai formerly known as Madras. “Being a compatriot, in a way I continue his legacy, and I am obviously proud of him,” said Dr. Ravindranath, who has conducted his own landmark research regarding methotrexate and leukemia.
Nearly 75 years after Dr. SubbaRow’s death, Indian-born physicians like Dr. Ravindranath continue to honor him in print, trying to ensure that he’s not forgotten. Methotrexate remains a crucial treatment for leukemia, along with a long list of other ailments, including psoriasis.
Recognition for “Yella” may have come late and infrequently, but a Lederle Laboratories research library named after him offered Dr. SubbaRow a kind of immortality. A plaque there memorialized him in stone as a scientist, teacher, philosopher, and humanitarian, featuring the quote: “Science simply prolongs life. Religion deepens it.”
By all accounts, Dr. SubbaRow was a man of science and faith who had faith in science.
We have a half-forgotten Indian immigrant to thank – a hospital night porter turned biochemist –for revolutionizing treatment of leukemia, the once deadly childhood scourge that is still the most common pediatric cancer.
Dr. Yellapragada SubbaRow has been called the “father of chemotherapy” for developing methotrexate, a powerful, inexpensive therapy for leukemia and other diseases, and he is celebrated for additional scientific achievements. Yet Dr. SubbaRow’s life was marked more by struggle than glory.
Born poor in southeastern India, he nearly succumbed to a tropical disease that killed two older brothers, and he didn’t focus on schoolwork until his father died. Later, prejudice dogged his years as an immigrant to the United States, and a blood clot took his life at the age of 53.
Scientifically, however, Dr. SubbaRow (pronounced sue-buh-rao) triumphed, despite mammoth challenges and a lack of recognition that persists to this day. National Cancer Research Month is a fitting time to look back on his extraordinary life and work and pay tribute to his accomplishments.
‘Yella,’ folic acid, and a paradigm shift
No one appreciates Dr. SubbaRow more than a cadre of Indian-born physicians who have kept his legacy alive in journal articles, presentations, and a Pulitzer Prize-winning book. Among them is author and oncologist Siddhartha Mukherjee, MD, who chronicled Dr. SubbaRow’s achievements in his New York Times No. 1 bestseller, “The Emperor of All Maladies: A Biography of Cancer.”
As Dr. Mukherjee wrote, Dr. SubbaRow was a “pioneer in many ways, a physician turned cellular physiologist, a chemist who had accidentally wandered into biology.” (Per Indian tradition, SubbaRow is the doctor’s first name, and Yellapragada is his surname, but medical literature uses SubbaRow as his cognomen, with some variations in spelling. Dr. Mukherjee wrote that his friends called him “Yella.”)
Dr. SubbaRow came to the United States in 1923, after enduring a difficult childhood and young adulthood. He’d survived bouts of religious fervor, childhood rebellion (including a bid to run away from home and become a banana trader), and a failed arranged marriage. His wife bore him a child who died in infancy. He left it all behind.
In Boston, medical officials rejected his degree. Broke, he worked for a time as a night porter at Brigham and Women’s Hospital in Boston, changing sheets and cleaning urinals. To a poor but proud high-caste Indian Brahmin, the culture shock of carrying out these tasks must have been especially jarring.
Dr. SubbaRow went on to earn a diploma from Harvard Medical School, also in Boston, and became a junior faculty member. As a foreigner, Dr. Mukherjee wrote, Dr. SubbaRow was a “reclusive, nocturnal, heavily accented vegetarian,” so different from his colleagues that advancement seemed impossible. Despite his pioneering biochemistry work, Harvard later declined to offer Dr. SubbaRow a tenured faculty position.
By the early 1940s, he took a job at an upstate New York pharmaceutical company called Lederle Labs (later purchased by Pfizer). At Lederle, Dr. SubbaRow strove to synthesize the vitamin known as folic acid. He ended up creating a kind of antivitamin, a lookalike that acted like folic acid but only succeeded in gumming up the works in receptors. But what good would it do to stop the body from absorbing folic acid? Plenty, it turned out.
Discoveries pile up, but credit and fame prove elusive
Dr. SubbaRow was no stranger to producing landmark biological work. He’d previously codiscovered phosphocreatine and ATP, which are crucial to muscular contractions. However, “in 1935, he had to disown the extent of his role in the discovery of the color test related to phosphorus, instead giving the credit to his co-author, who was being considered for promotion to a full professorship at Harvard,” wrote author Gerald Posner in his 2020 book, “Pharma: Greed, Lies and the Poisoning of America.”
Houston-area oncologist Kirtan Nautiyal, MD, who paid tribute to Dr. SubbaRow in a 2018 article, contended that “with his Indian instinct for self-effacement, he had irreparably sabotaged his own career.”
Dr. SubbaRow and his team also developed “the first effective treatment of filariasis, which causes elephantiasis of the lower limbs and genitals in millions of people, mainly in tropical countries,” Dr. Nautiyal wrote. “Later in the decade, his antibiotic program generated polymyxin, the first effective treatment against the class of bacteria called Gram negatives, and aureomycin, the first “broad-spectrum’ antibiotic.” (Aureomycin is also the first tetracycline antibiotic.)
Dr. SubbaRow’s discovery of a folic acid antagonist would again go largely unheralded. But first came the realization that folic acid made childhood leukemia worse, not better, and the prospect that this process could potentially be reversed.
Rise of methotrexate and fall of leukemia
In Boston, Sidney Farber, MD, a Boston pathologist, was desperate to help Robert Sandler, a 2-year-old leukemia patient. Dr. Farber contacted his ex-colleague Dr. SubbaRow to request a supply of aminopterin, an early version of methotrexate that Dr. SubbaRow and his team had developed. Dr. Farber injected Robert with the substance and within 3 days, the toddler’s white blood count started falling – fast. He stopped bleeding, resumed eating, and once again seemed almost identical to his twin brother, as Dr. Mukherjee wrote in his book.
Leukemia had never gone into remission before. Unfortunately, the treatment only worked temporarily. Robert, like other children treated with the drug, relapsed and died within months. But Dr. Farber “saw a door open” – a chemical, a kind of chemotherapy, that could turn back cancer. In the case of folic acid antagonists, they do so by stopping cancer cells from replicating.
Methotrexate, a related agent synthesized by Dr. SubbaRow, would become a mainstay of leukemia treatment and begin to produce long-term remission from acute lymphoblastic leukemia in 1970, when combination chemotherapy was developed.
Other cancers fell to methotrexate treatment. “Previous assumptions that cancer was nearly always fatal were revised, and the field of medical oncology (treatment of cancer with chemotherapy), which had not previously existed, was formally established in 1971,” according to the National Cancer Institute’s history of methotrexate. This account does not mention Dr. SubbaRow.
Death takes the doctor, but his legacy remains
In biographies, as well as his own words, Dr. SubbaRow comes across as a prickly, hard-driving workaholic who had little interest in intimate human connections. “It is not good to ask in every letter when I will be back,” he wrote to his wife back in India, before cutting off ties completely in the early 1930s. “I will come as early as possible. ... I do not want to write anything more.”
It seems, as his biographer S.P.K. Gupta noted, that “he was quite determined that the time allotted to him on Earth should be completely devoted to finding cures for ailments that plagued mankind.”
Still, Dr. SubbaRow’s research team was devoted to him, and he had plenty of reasons to be bitter, such as the prejudice and isolation he encountered in the United States and earlier, in British-run India. According to Mr. Posner’s book, even as a young medical student, Dr. SubbaRow heeded the call of Indian independence activist Mohandas Gandhi. He “refused the British surgical gown given him at school and instead donned a traditional and simple cotton Khadi. That act of defiance cost SubbaRow the college degree that was necessary for him to get into the State Medical College.”
During the last year of his life, Dr. SubbaRow faced yet another humiliation: In his landmark 1948 study about aminopterin as a treatment for leukemia, his colleague Dr. Farber failed to credit him, an “astonishing omission” as Yaddanapudi Ravindranath, MBBS, a pediatric hematologist/oncologist at Wayne State University, Detroit, put it. “From everything I know, Dr. Farber spent the rest of his career apologizing and trying to make amends for it,” Dr. Ravindranath said in an interview.
A career cut short, and a lasting legacy
In 1948, at the age of 53, Dr. SubbaRow suddenly died. “Many think Dr. SubbaRow would have won [the] Nobel Prize had he lived a few years longer,” said Dr. Ravindranath.
Like Dr. SubbaRow, Dr. Ravindranath was born in Andhra Pradesh state, near the city of Chennai formerly known as Madras. “Being a compatriot, in a way I continue his legacy, and I am obviously proud of him,” said Dr. Ravindranath, who has conducted his own landmark research regarding methotrexate and leukemia.
Nearly 75 years after Dr. SubbaRow’s death, Indian-born physicians like Dr. Ravindranath continue to honor him in print, trying to ensure that he’s not forgotten. Methotrexate remains a crucial treatment for leukemia, along with a long list of other ailments, including psoriasis.
Recognition for “Yella” may have come late and infrequently, but a Lederle Laboratories research library named after him offered Dr. SubbaRow a kind of immortality. A plaque there memorialized him in stone as a scientist, teacher, philosopher, and humanitarian, featuring the quote: “Science simply prolongs life. Religion deepens it.”
By all accounts, Dr. SubbaRow was a man of science and faith who had faith in science.
PPIs should be used ‘judiciously’ in patients with cirrhosis
In a retrospective study to evaluate the impact of proton pump inhibitors (PPIs) on all-cause mortality in patients with cirrhosis, researchers found reduced mortality only in those hospitalized for gastrointestinal bleeding. They reported increased liver-related mortality associated with PPIs in all other patients with cirrhosis.
Patients on PPIs had an 18% reduction in all-cause mortality versus other patients if they had gastrointestinal bleeding. But in those without bleeding, PPIs were associated with a 23% increase in liver-related mortality.
Further analysis suggested that the mortality increase could be related to a 21% increased risk for severe infection with PPI exposure in patients with cirrhosis, as well as a 64% increased risk for decompensation.
“My takeaway from this study is that there should be a nuanced understanding of PPIs and cirrhosis,” corresponding author Nadim Mahmud, MD, MS, University of Pennsylvania, Philadelphia, said in an interview, adding that, if they are to be used in this setting, there should be “a very compelling indication.”
Based on the new analysis, Dr. Mahmud explained, in a patient with cirrhosis hospitalized with a potentially ulcer-related upper gastrointestinal bleed, “we shouldn’t be afraid” to use PPIs “out of fear of potential infection or decompensation because our data demonstrate pretty strongly that that sort of patient may have a mortality benefit.”
In contrast, patients with cirrhosis and “vague abdominal discomfort” are often started on a PPI “just to see if that helps,” Dr. Mahmud said, and they may stay on the medication “in perpetuity, just because they’re so ubiquitously prescribed.”
“In that patient, we should recognize that there is a potential risk of increased infection and decompensation,” he said. There “should be an active effort to deprescribe the PPI or at the very least reduce it to the minimum dose needed for efficacy, if it’s treating a symptom.”
The research was published online in Gastroenterology.
Looking at the big picture of PPIs in people with cirrhosis
The authors noted that the half-life of PPIs is “prolonged in patients with cirrhosis” and that alterations in the gastrointestinal microbiota as a result of gastric acid suppression “may allow for bacterial overgrowth and translocation,” thus increasing the risk for infections.
However, studies of the impact of PPIs on adverse outcomes in patients with cirrhosis have often been hampered by numerous limitations, such as small sample sizes, a “limited ability to control for complex confounding,” or a “narrow focus” on hospitalized patients.
To overcome these problems, the team retrospectively examined data from the Veterans Outcomes and Costs Associated with Liver Diseases cohort, including all adults with incident cirrhosis between January 2008 and June 2021.
They excluded patients with Fibrosis-4 scores less than 1.45 at baseline, as well as those with prior liver transplantation, decompensated cirrhosis at baseline, a diagnosis of hepatocellular carcinoma within 6 months of the index date, and less than 6 months of follow-up.
In all, 76,251 patients with incident cirrhosis met the inclusion criteria, 21% of whom were on a PPI at baseline. The most commonly used PPIs were omeprazole (76.7%), followed by pantoprazole (22.2%) and lansoprazole (0.1%).
Those taking the drugs were more likely than other patients to be White, have metabolic and cardiovascular comorbidities, have a higher median body mass index, and were more likely to have cirrhosis because of alcohol-related liver disease or metabolic-associated fatty liver disease.
Over 49 months of follow-up, all-cause mortality was recorded for 37.5% of patients, of whom 59% experienced non–liver-related death and 41% liver-related mortality.
Multivariate analysis revealed that PPI exposure was not associated with all-cause mortality overall but was significantly associated with reduced all-cause mortality in patients with hospitalization for gastrointestinal bleeding, at a hazard ratio of 0.88.
However, PPI exposure in patients without gastrointestinal bleeding was associated with an increased risk for liver-related mortality (HR, 1.23), but a reduced risk for non–liver-related mortality (HR, 0.88).
Dr. Mahmud and colleagues found that PPI exposure was significantly associated with severe infection (HR, 1.21) and cirrhosis decompensation (HR, 1.64).
The authors suggested that these increased risks “may mediate the observed increased in liver-related mortality.”
Large study suggests limited protective PPI indication
Nancy S. Reau, MD, chair of hepatology at Rush Medical College, Chicago, said that “multiple studies” point to a link between PPI exposure and infection in cirrhosis.
“Although this is a retrospective study, it is very large so we should give credit to the associations,” she said in an interview. She was not involved with the current study.
“The most important message is that we need to be judicious with our therapy,” Dr. Reau added, qualifying that “everything is a risk-benefit ratio.”
“PPI use in cirrhosis has a role but should not overstep its boundary,” she explained. “More simply, if the PPI is indicated, you should not avoid it in a patient with cirrhosis. On the other hand, if you have a patient with advanced liver disease who is chronically taking a PPI, you should question its indication.
Paul Martin, MD, chief of the division of hepatology, University of Miami Health Systems, said in an interview that, when it comes to PPI use in patients with cirrhosis, “judicious is the right word. They should be clearly used if there’s a bona fide indication ... and probably for a finite period of time.”
In a common scenario, “a patient is put on a PPI after they’ve undergone endoscopy with obliteration of varices, and the thought is that PPIs help the ulcers induced by the banding to heal,” said Dr. Martin, who was not associated with the research. “This paper didn’t specifically tease out whether that’s beneficial or not, but it certainly suggests, in patients with a history of gastrointestinal bleeding, that PPIs are still beneficial.”
Dr. Mahmud is supported by the National Institute of Diabetes and Digestive and Kidney Diseases. One coauthor is supported by a National Institutes of Health K23 grant; another is supported by a VA Merit Grant and by a National Cancer Institute R01; a third has received unrelated support from Gilead, Glycotest, and Bayer and also is supported by VA Merit Grants. Dr. Reau and Dr. Martin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a retrospective study to evaluate the impact of proton pump inhibitors (PPIs) on all-cause mortality in patients with cirrhosis, researchers found reduced mortality only in those hospitalized for gastrointestinal bleeding. They reported increased liver-related mortality associated with PPIs in all other patients with cirrhosis.
Patients on PPIs had an 18% reduction in all-cause mortality versus other patients if they had gastrointestinal bleeding. But in those without bleeding, PPIs were associated with a 23% increase in liver-related mortality.
Further analysis suggested that the mortality increase could be related to a 21% increased risk for severe infection with PPI exposure in patients with cirrhosis, as well as a 64% increased risk for decompensation.
“My takeaway from this study is that there should be a nuanced understanding of PPIs and cirrhosis,” corresponding author Nadim Mahmud, MD, MS, University of Pennsylvania, Philadelphia, said in an interview, adding that, if they are to be used in this setting, there should be “a very compelling indication.”
Based on the new analysis, Dr. Mahmud explained, in a patient with cirrhosis hospitalized with a potentially ulcer-related upper gastrointestinal bleed, “we shouldn’t be afraid” to use PPIs “out of fear of potential infection or decompensation because our data demonstrate pretty strongly that that sort of patient may have a mortality benefit.”
In contrast, patients with cirrhosis and “vague abdominal discomfort” are often started on a PPI “just to see if that helps,” Dr. Mahmud said, and they may stay on the medication “in perpetuity, just because they’re so ubiquitously prescribed.”
“In that patient, we should recognize that there is a potential risk of increased infection and decompensation,” he said. There “should be an active effort to deprescribe the PPI or at the very least reduce it to the minimum dose needed for efficacy, if it’s treating a symptom.”
The research was published online in Gastroenterology.
Looking at the big picture of PPIs in people with cirrhosis
The authors noted that the half-life of PPIs is “prolonged in patients with cirrhosis” and that alterations in the gastrointestinal microbiota as a result of gastric acid suppression “may allow for bacterial overgrowth and translocation,” thus increasing the risk for infections.
However, studies of the impact of PPIs on adverse outcomes in patients with cirrhosis have often been hampered by numerous limitations, such as small sample sizes, a “limited ability to control for complex confounding,” or a “narrow focus” on hospitalized patients.
To overcome these problems, the team retrospectively examined data from the Veterans Outcomes and Costs Associated with Liver Diseases cohort, including all adults with incident cirrhosis between January 2008 and June 2021.
They excluded patients with Fibrosis-4 scores less than 1.45 at baseline, as well as those with prior liver transplantation, decompensated cirrhosis at baseline, a diagnosis of hepatocellular carcinoma within 6 months of the index date, and less than 6 months of follow-up.
In all, 76,251 patients with incident cirrhosis met the inclusion criteria, 21% of whom were on a PPI at baseline. The most commonly used PPIs were omeprazole (76.7%), followed by pantoprazole (22.2%) and lansoprazole (0.1%).
Those taking the drugs were more likely than other patients to be White, have metabolic and cardiovascular comorbidities, have a higher median body mass index, and were more likely to have cirrhosis because of alcohol-related liver disease or metabolic-associated fatty liver disease.
Over 49 months of follow-up, all-cause mortality was recorded for 37.5% of patients, of whom 59% experienced non–liver-related death and 41% liver-related mortality.
Multivariate analysis revealed that PPI exposure was not associated with all-cause mortality overall but was significantly associated with reduced all-cause mortality in patients with hospitalization for gastrointestinal bleeding, at a hazard ratio of 0.88.
However, PPI exposure in patients without gastrointestinal bleeding was associated with an increased risk for liver-related mortality (HR, 1.23), but a reduced risk for non–liver-related mortality (HR, 0.88).
Dr. Mahmud and colleagues found that PPI exposure was significantly associated with severe infection (HR, 1.21) and cirrhosis decompensation (HR, 1.64).
The authors suggested that these increased risks “may mediate the observed increased in liver-related mortality.”
Large study suggests limited protective PPI indication
Nancy S. Reau, MD, chair of hepatology at Rush Medical College, Chicago, said that “multiple studies” point to a link between PPI exposure and infection in cirrhosis.
“Although this is a retrospective study, it is very large so we should give credit to the associations,” she said in an interview. She was not involved with the current study.
“The most important message is that we need to be judicious with our therapy,” Dr. Reau added, qualifying that “everything is a risk-benefit ratio.”
“PPI use in cirrhosis has a role but should not overstep its boundary,” she explained. “More simply, if the PPI is indicated, you should not avoid it in a patient with cirrhosis. On the other hand, if you have a patient with advanced liver disease who is chronically taking a PPI, you should question its indication.
Paul Martin, MD, chief of the division of hepatology, University of Miami Health Systems, said in an interview that, when it comes to PPI use in patients with cirrhosis, “judicious is the right word. They should be clearly used if there’s a bona fide indication ... and probably for a finite period of time.”
In a common scenario, “a patient is put on a PPI after they’ve undergone endoscopy with obliteration of varices, and the thought is that PPIs help the ulcers induced by the banding to heal,” said Dr. Martin, who was not associated with the research. “This paper didn’t specifically tease out whether that’s beneficial or not, but it certainly suggests, in patients with a history of gastrointestinal bleeding, that PPIs are still beneficial.”
Dr. Mahmud is supported by the National Institute of Diabetes and Digestive and Kidney Diseases. One coauthor is supported by a National Institutes of Health K23 grant; another is supported by a VA Merit Grant and by a National Cancer Institute R01; a third has received unrelated support from Gilead, Glycotest, and Bayer and also is supported by VA Merit Grants. Dr. Reau and Dr. Martin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a retrospective study to evaluate the impact of proton pump inhibitors (PPIs) on all-cause mortality in patients with cirrhosis, researchers found reduced mortality only in those hospitalized for gastrointestinal bleeding. They reported increased liver-related mortality associated with PPIs in all other patients with cirrhosis.
Patients on PPIs had an 18% reduction in all-cause mortality versus other patients if they had gastrointestinal bleeding. But in those without bleeding, PPIs were associated with a 23% increase in liver-related mortality.
Further analysis suggested that the mortality increase could be related to a 21% increased risk for severe infection with PPI exposure in patients with cirrhosis, as well as a 64% increased risk for decompensation.
“My takeaway from this study is that there should be a nuanced understanding of PPIs and cirrhosis,” corresponding author Nadim Mahmud, MD, MS, University of Pennsylvania, Philadelphia, said in an interview, adding that, if they are to be used in this setting, there should be “a very compelling indication.”
Based on the new analysis, Dr. Mahmud explained, in a patient with cirrhosis hospitalized with a potentially ulcer-related upper gastrointestinal bleed, “we shouldn’t be afraid” to use PPIs “out of fear of potential infection or decompensation because our data demonstrate pretty strongly that that sort of patient may have a mortality benefit.”
In contrast, patients with cirrhosis and “vague abdominal discomfort” are often started on a PPI “just to see if that helps,” Dr. Mahmud said, and they may stay on the medication “in perpetuity, just because they’re so ubiquitously prescribed.”
“In that patient, we should recognize that there is a potential risk of increased infection and decompensation,” he said. There “should be an active effort to deprescribe the PPI or at the very least reduce it to the minimum dose needed for efficacy, if it’s treating a symptom.”
The research was published online in Gastroenterology.
Looking at the big picture of PPIs in people with cirrhosis
The authors noted that the half-life of PPIs is “prolonged in patients with cirrhosis” and that alterations in the gastrointestinal microbiota as a result of gastric acid suppression “may allow for bacterial overgrowth and translocation,” thus increasing the risk for infections.
However, studies of the impact of PPIs on adverse outcomes in patients with cirrhosis have often been hampered by numerous limitations, such as small sample sizes, a “limited ability to control for complex confounding,” or a “narrow focus” on hospitalized patients.
To overcome these problems, the team retrospectively examined data from the Veterans Outcomes and Costs Associated with Liver Diseases cohort, including all adults with incident cirrhosis between January 2008 and June 2021.
They excluded patients with Fibrosis-4 scores less than 1.45 at baseline, as well as those with prior liver transplantation, decompensated cirrhosis at baseline, a diagnosis of hepatocellular carcinoma within 6 months of the index date, and less than 6 months of follow-up.
In all, 76,251 patients with incident cirrhosis met the inclusion criteria, 21% of whom were on a PPI at baseline. The most commonly used PPIs were omeprazole (76.7%), followed by pantoprazole (22.2%) and lansoprazole (0.1%).
Those taking the drugs were more likely than other patients to be White, have metabolic and cardiovascular comorbidities, have a higher median body mass index, and were more likely to have cirrhosis because of alcohol-related liver disease or metabolic-associated fatty liver disease.
Over 49 months of follow-up, all-cause mortality was recorded for 37.5% of patients, of whom 59% experienced non–liver-related death and 41% liver-related mortality.
Multivariate analysis revealed that PPI exposure was not associated with all-cause mortality overall but was significantly associated with reduced all-cause mortality in patients with hospitalization for gastrointestinal bleeding, at a hazard ratio of 0.88.
However, PPI exposure in patients without gastrointestinal bleeding was associated with an increased risk for liver-related mortality (HR, 1.23), but a reduced risk for non–liver-related mortality (HR, 0.88).
Dr. Mahmud and colleagues found that PPI exposure was significantly associated with severe infection (HR, 1.21) and cirrhosis decompensation (HR, 1.64).
The authors suggested that these increased risks “may mediate the observed increased in liver-related mortality.”
Large study suggests limited protective PPI indication
Nancy S. Reau, MD, chair of hepatology at Rush Medical College, Chicago, said that “multiple studies” point to a link between PPI exposure and infection in cirrhosis.
“Although this is a retrospective study, it is very large so we should give credit to the associations,” she said in an interview. She was not involved with the current study.
“The most important message is that we need to be judicious with our therapy,” Dr. Reau added, qualifying that “everything is a risk-benefit ratio.”
“PPI use in cirrhosis has a role but should not overstep its boundary,” she explained. “More simply, if the PPI is indicated, you should not avoid it in a patient with cirrhosis. On the other hand, if you have a patient with advanced liver disease who is chronically taking a PPI, you should question its indication.
Paul Martin, MD, chief of the division of hepatology, University of Miami Health Systems, said in an interview that, when it comes to PPI use in patients with cirrhosis, “judicious is the right word. They should be clearly used if there’s a bona fide indication ... and probably for a finite period of time.”
In a common scenario, “a patient is put on a PPI after they’ve undergone endoscopy with obliteration of varices, and the thought is that PPIs help the ulcers induced by the banding to heal,” said Dr. Martin, who was not associated with the research. “This paper didn’t specifically tease out whether that’s beneficial or not, but it certainly suggests, in patients with a history of gastrointestinal bleeding, that PPIs are still beneficial.”
Dr. Mahmud is supported by the National Institute of Diabetes and Digestive and Kidney Diseases. One coauthor is supported by a National Institutes of Health K23 grant; another is supported by a VA Merit Grant and by a National Cancer Institute R01; a third has received unrelated support from Gilead, Glycotest, and Bayer and also is supported by VA Merit Grants. Dr. Reau and Dr. Martin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM GASTROENTEROLOGY
Decentralizing PrEP offers a road map for retention
Good solutions have great road maps.
For HIV preexposure prophylaxis (PrEP), the road map might just be that of contraceptive care. Once an onerous process, over time contraceptive care exploded into a range of options across a broad landscape in terms of approach and accessibility.
How then do organizations help vulnerable patients navigate their PrEP journeys using the contraceptive road map as a guide?
That’s what researchers at the University of Washington were intent on demonstrating, according to Julie Dombrowski, MD, MPH, an infectious disease specialist at the University of Washington, Seattle, and deputy director of the HIV/STD Program, Public Health for the city of Seattle and King County, Wash.
“The same sorts of things that happened with oral contraceptive pills – which initially required you to see a gynecologist and get a Pap smear – over time, became much more available,” said Dr. Dombrowski, coauthor of a new study published online in the Journal of Acquired Immune Deficiency Syndrome.
“The basic idea is that PrEP is not medically complicated; it can be easily protocolized,” she told this news organization.
Decentralizing HIV PrEP
In addition to her responsibilities at University of Washington, Dr. Dombrowski provides clinical services at the Public Health Sexual Health Clinic (PHSKC) at Seattle’s Harborview Medical Center – a dual-county center that provides confidential STI and HIV evaluation, screening, testing, and treatment on a walk-in basis for a sliding fee.
Sexual health clinics are ideal environments for reaching large numbers of patients, but strategies for integrating PrEP successfully into what are commonly one-time appointments have not been well-described or broadly adopted.
“Sexual health clinics in general are STD specialty clinics with walk-in access to care; often, patients come into a clinic, get seen, diagnosed, and treated, and they don’t necessarily come back,” said Dr. Dombrowski.
She said that, because most operations have been set up around same-day treatment, to offer PrEP and successfully change outcomes, there needs to be a shift in the current model toward one that promotes an ongoing relationship with the patients.
So, she and her colleagues decided to see what would happen if they implemented a decentralized PrEP model in their clinic over a 6-year period. They established a protocol that moves from an initial consultation with a clinician to review risk behaviors, ascertain HIV status, and acquire a PrEP prescription, to ongoing interactions with an STI and PrEP-trained disease intervention specialist (DIS).
As the clinic’s PrEP program coordinators, these specialists enroll patients in PrEP drug assistance programs, verify prescription fills, provide follow-up visits and adherence and adverse events assessments, and collect specimens.
“[Disease intervention specialists] are frontline public health workers who ensure that people diagnosed with HIV or an STI – or who’ve been exposed – get necessary testing and treatment,” explained Dr. Dombrowski. “They’re very similar to patient navigators.”
At the same time, clinicians remain the key providers for annual appointments, new symptoms, STI diagnoses, adverse drug reactions, and missed doses. Licensed medical providers review all labs.
Shifting responsibilities, better PrEP initiation, retention rates
After establishing the PrEP services protocol, the University of Washington team then assessed retention rates among PrEP patients who attended an initial visit (1,387) from October 2014 to December 2019. Follow-up continued through February 2020. (For study purposes, PrEP discontinuation was defined as either stopping PrEP after initiation or as lost to follow-up, i.e., either not attending a follow-up visit or not responding to more than three DIS calls or text messages).
Just over half of the participants were aged 20-29 years, and a third were aged 30-39. More than 9 out of 10 (93%) were men who sleep with men (MSM), 55% White, 26% Hispanic/Latinx, and 10% Black.
Over the course of the study, 6,887 PrEP visits were recorded. Quarterly visits increased concurrently with the program expansion, from 31 visits in 2014 to 623 in the fourth quarter (Q4) of 2019. Likewise, while 57% of visits overall were with a clinician, DIS visits increased from 3% in Q4 of 2014 to 45% in Q4 of 2019, an increase of 1,400% in 5 years.
Significant numbers of patients also initiated PrEP in the clinic, especially when prescribing practices were expanded to be part of routine, walk-in visits.
Retention rates also improved, with 43% (510/1,190) of patients still on PrEP at the end of the analysis period. Forty-one percent (490) discontinued PrEP, 21% within 3 months of initiation, and 72% within a year; another 16% moved, transferred care, or tested positive, and were considered “censored.” However, as of July 31, 2021, 54% (265) of the 490 patients who had discontinued PrEP returned to the clinic for a restart visit, 93% of whom refilled their restart prescription.
“This is really basic preventative care and is actually quite easy to do,” noted Sarah Schmalzle, MD, assistant professor of medicine and medical director of the Thrive Program at the Institute of Human Virology at the University of Maryland, Baltimore. Dr. Schmalzle was not involved in the study.
Dr. Schmalzle practices in inner-city Baltimore, so she and her colleagues have been thorough in terms of setting up PrEP (and postexposure prophylaxis, PEP) programs to ensure that patients access PrEP wherever they want to. But she also said that PrEP is only a part of the sexual wellness and prevention toolbox, and ideally, part of a whole prevention program.
“Focusing on how to get the prescription out is great but the rest is having ongoing and accurate sexual health conversations, healthy conversations about sex and prevention, to have [an] algorithm in place that says, ‘Here’s your PrEP, this is the next time that you need an appointment, the next time you need labs, I’m going to check your adherence, etc.’ ”
Both Dr. Dombrowski and Dr. Schmalzle emphasized that decentralization is not a one-size model; flexibility is key, especially when it comes to who is providing PrEP
“People overcomplicate PrEP and clinicians do this too,” said Dr. Dombrowski. “If we are going to successfully increase PrEP and improve the patient experience, we need to decrease the requirement for clinician involvement.”
Dr. Dombrowski has disclosed no relevant financial relationships. Dr. Schmalzle receives grant funding from Gilead Sciences.
A version of this article first appeared on Medscape.com.
Good solutions have great road maps.
For HIV preexposure prophylaxis (PrEP), the road map might just be that of contraceptive care. Once an onerous process, over time contraceptive care exploded into a range of options across a broad landscape in terms of approach and accessibility.
How then do organizations help vulnerable patients navigate their PrEP journeys using the contraceptive road map as a guide?
That’s what researchers at the University of Washington were intent on demonstrating, according to Julie Dombrowski, MD, MPH, an infectious disease specialist at the University of Washington, Seattle, and deputy director of the HIV/STD Program, Public Health for the city of Seattle and King County, Wash.
“The same sorts of things that happened with oral contraceptive pills – which initially required you to see a gynecologist and get a Pap smear – over time, became much more available,” said Dr. Dombrowski, coauthor of a new study published online in the Journal of Acquired Immune Deficiency Syndrome.
“The basic idea is that PrEP is not medically complicated; it can be easily protocolized,” she told this news organization.
Decentralizing HIV PrEP
In addition to her responsibilities at University of Washington, Dr. Dombrowski provides clinical services at the Public Health Sexual Health Clinic (PHSKC) at Seattle’s Harborview Medical Center – a dual-county center that provides confidential STI and HIV evaluation, screening, testing, and treatment on a walk-in basis for a sliding fee.
Sexual health clinics are ideal environments for reaching large numbers of patients, but strategies for integrating PrEP successfully into what are commonly one-time appointments have not been well-described or broadly adopted.
“Sexual health clinics in general are STD specialty clinics with walk-in access to care; often, patients come into a clinic, get seen, diagnosed, and treated, and they don’t necessarily come back,” said Dr. Dombrowski.
She said that, because most operations have been set up around same-day treatment, to offer PrEP and successfully change outcomes, there needs to be a shift in the current model toward one that promotes an ongoing relationship with the patients.
So, she and her colleagues decided to see what would happen if they implemented a decentralized PrEP model in their clinic over a 6-year period. They established a protocol that moves from an initial consultation with a clinician to review risk behaviors, ascertain HIV status, and acquire a PrEP prescription, to ongoing interactions with an STI and PrEP-trained disease intervention specialist (DIS).
As the clinic’s PrEP program coordinators, these specialists enroll patients in PrEP drug assistance programs, verify prescription fills, provide follow-up visits and adherence and adverse events assessments, and collect specimens.
“[Disease intervention specialists] are frontline public health workers who ensure that people diagnosed with HIV or an STI – or who’ve been exposed – get necessary testing and treatment,” explained Dr. Dombrowski. “They’re very similar to patient navigators.”
At the same time, clinicians remain the key providers for annual appointments, new symptoms, STI diagnoses, adverse drug reactions, and missed doses. Licensed medical providers review all labs.
Shifting responsibilities, better PrEP initiation, retention rates
After establishing the PrEP services protocol, the University of Washington team then assessed retention rates among PrEP patients who attended an initial visit (1,387) from October 2014 to December 2019. Follow-up continued through February 2020. (For study purposes, PrEP discontinuation was defined as either stopping PrEP after initiation or as lost to follow-up, i.e., either not attending a follow-up visit or not responding to more than three DIS calls or text messages).
Just over half of the participants were aged 20-29 years, and a third were aged 30-39. More than 9 out of 10 (93%) were men who sleep with men (MSM), 55% White, 26% Hispanic/Latinx, and 10% Black.
Over the course of the study, 6,887 PrEP visits were recorded. Quarterly visits increased concurrently with the program expansion, from 31 visits in 2014 to 623 in the fourth quarter (Q4) of 2019. Likewise, while 57% of visits overall were with a clinician, DIS visits increased from 3% in Q4 of 2014 to 45% in Q4 of 2019, an increase of 1,400% in 5 years.
Significant numbers of patients also initiated PrEP in the clinic, especially when prescribing practices were expanded to be part of routine, walk-in visits.
Retention rates also improved, with 43% (510/1,190) of patients still on PrEP at the end of the analysis period. Forty-one percent (490) discontinued PrEP, 21% within 3 months of initiation, and 72% within a year; another 16% moved, transferred care, or tested positive, and were considered “censored.” However, as of July 31, 2021, 54% (265) of the 490 patients who had discontinued PrEP returned to the clinic for a restart visit, 93% of whom refilled their restart prescription.
“This is really basic preventative care and is actually quite easy to do,” noted Sarah Schmalzle, MD, assistant professor of medicine and medical director of the Thrive Program at the Institute of Human Virology at the University of Maryland, Baltimore. Dr. Schmalzle was not involved in the study.
Dr. Schmalzle practices in inner-city Baltimore, so she and her colleagues have been thorough in terms of setting up PrEP (and postexposure prophylaxis, PEP) programs to ensure that patients access PrEP wherever they want to. But she also said that PrEP is only a part of the sexual wellness and prevention toolbox, and ideally, part of a whole prevention program.
“Focusing on how to get the prescription out is great but the rest is having ongoing and accurate sexual health conversations, healthy conversations about sex and prevention, to have [an] algorithm in place that says, ‘Here’s your PrEP, this is the next time that you need an appointment, the next time you need labs, I’m going to check your adherence, etc.’ ”
Both Dr. Dombrowski and Dr. Schmalzle emphasized that decentralization is not a one-size model; flexibility is key, especially when it comes to who is providing PrEP
“People overcomplicate PrEP and clinicians do this too,” said Dr. Dombrowski. “If we are going to successfully increase PrEP and improve the patient experience, we need to decrease the requirement for clinician involvement.”
Dr. Dombrowski has disclosed no relevant financial relationships. Dr. Schmalzle receives grant funding from Gilead Sciences.
A version of this article first appeared on Medscape.com.
Good solutions have great road maps.
For HIV preexposure prophylaxis (PrEP), the road map might just be that of contraceptive care. Once an onerous process, over time contraceptive care exploded into a range of options across a broad landscape in terms of approach and accessibility.
How then do organizations help vulnerable patients navigate their PrEP journeys using the contraceptive road map as a guide?
That’s what researchers at the University of Washington were intent on demonstrating, according to Julie Dombrowski, MD, MPH, an infectious disease specialist at the University of Washington, Seattle, and deputy director of the HIV/STD Program, Public Health for the city of Seattle and King County, Wash.
“The same sorts of things that happened with oral contraceptive pills – which initially required you to see a gynecologist and get a Pap smear – over time, became much more available,” said Dr. Dombrowski, coauthor of a new study published online in the Journal of Acquired Immune Deficiency Syndrome.
“The basic idea is that PrEP is not medically complicated; it can be easily protocolized,” she told this news organization.
Decentralizing HIV PrEP
In addition to her responsibilities at University of Washington, Dr. Dombrowski provides clinical services at the Public Health Sexual Health Clinic (PHSKC) at Seattle’s Harborview Medical Center – a dual-county center that provides confidential STI and HIV evaluation, screening, testing, and treatment on a walk-in basis for a sliding fee.
Sexual health clinics are ideal environments for reaching large numbers of patients, but strategies for integrating PrEP successfully into what are commonly one-time appointments have not been well-described or broadly adopted.
“Sexual health clinics in general are STD specialty clinics with walk-in access to care; often, patients come into a clinic, get seen, diagnosed, and treated, and they don’t necessarily come back,” said Dr. Dombrowski.
She said that, because most operations have been set up around same-day treatment, to offer PrEP and successfully change outcomes, there needs to be a shift in the current model toward one that promotes an ongoing relationship with the patients.
So, she and her colleagues decided to see what would happen if they implemented a decentralized PrEP model in their clinic over a 6-year period. They established a protocol that moves from an initial consultation with a clinician to review risk behaviors, ascertain HIV status, and acquire a PrEP prescription, to ongoing interactions with an STI and PrEP-trained disease intervention specialist (DIS).
As the clinic’s PrEP program coordinators, these specialists enroll patients in PrEP drug assistance programs, verify prescription fills, provide follow-up visits and adherence and adverse events assessments, and collect specimens.
“[Disease intervention specialists] are frontline public health workers who ensure that people diagnosed with HIV or an STI – or who’ve been exposed – get necessary testing and treatment,” explained Dr. Dombrowski. “They’re very similar to patient navigators.”
At the same time, clinicians remain the key providers for annual appointments, new symptoms, STI diagnoses, adverse drug reactions, and missed doses. Licensed medical providers review all labs.
Shifting responsibilities, better PrEP initiation, retention rates
After establishing the PrEP services protocol, the University of Washington team then assessed retention rates among PrEP patients who attended an initial visit (1,387) from October 2014 to December 2019. Follow-up continued through February 2020. (For study purposes, PrEP discontinuation was defined as either stopping PrEP after initiation or as lost to follow-up, i.e., either not attending a follow-up visit or not responding to more than three DIS calls or text messages).
Just over half of the participants were aged 20-29 years, and a third were aged 30-39. More than 9 out of 10 (93%) were men who sleep with men (MSM), 55% White, 26% Hispanic/Latinx, and 10% Black.
Over the course of the study, 6,887 PrEP visits were recorded. Quarterly visits increased concurrently with the program expansion, from 31 visits in 2014 to 623 in the fourth quarter (Q4) of 2019. Likewise, while 57% of visits overall were with a clinician, DIS visits increased from 3% in Q4 of 2014 to 45% in Q4 of 2019, an increase of 1,400% in 5 years.
Significant numbers of patients also initiated PrEP in the clinic, especially when prescribing practices were expanded to be part of routine, walk-in visits.
Retention rates also improved, with 43% (510/1,190) of patients still on PrEP at the end of the analysis period. Forty-one percent (490) discontinued PrEP, 21% within 3 months of initiation, and 72% within a year; another 16% moved, transferred care, or tested positive, and were considered “censored.” However, as of July 31, 2021, 54% (265) of the 490 patients who had discontinued PrEP returned to the clinic for a restart visit, 93% of whom refilled their restart prescription.
“This is really basic preventative care and is actually quite easy to do,” noted Sarah Schmalzle, MD, assistant professor of medicine and medical director of the Thrive Program at the Institute of Human Virology at the University of Maryland, Baltimore. Dr. Schmalzle was not involved in the study.
Dr. Schmalzle practices in inner-city Baltimore, so she and her colleagues have been thorough in terms of setting up PrEP (and postexposure prophylaxis, PEP) programs to ensure that patients access PrEP wherever they want to. But she also said that PrEP is only a part of the sexual wellness and prevention toolbox, and ideally, part of a whole prevention program.
“Focusing on how to get the prescription out is great but the rest is having ongoing and accurate sexual health conversations, healthy conversations about sex and prevention, to have [an] algorithm in place that says, ‘Here’s your PrEP, this is the next time that you need an appointment, the next time you need labs, I’m going to check your adherence, etc.’ ”
Both Dr. Dombrowski and Dr. Schmalzle emphasized that decentralization is not a one-size model; flexibility is key, especially when it comes to who is providing PrEP
“People overcomplicate PrEP and clinicians do this too,” said Dr. Dombrowski. “If we are going to successfully increase PrEP and improve the patient experience, we need to decrease the requirement for clinician involvement.”
Dr. Dombrowski has disclosed no relevant financial relationships. Dr. Schmalzle receives grant funding from Gilead Sciences.
A version of this article first appeared on Medscape.com.
Medical education programs tell how climate change affects health
Ms. Manivannan, copresident of Emory Medical Students for Climate Action, was in the first class of Emory’s medical students to experience the birth of a refined curriculum – lobbied for and partially created by students themselves. The new course of study addresses the myriad ways climate affects health: from air pollution and its effects on the lungs and cardiovascular system to heat-related kidney disease.
“We have known that climate has affected health for decades,” Ms. Manivannan said in a recent interview. “The narrative used to be that icebergs were melting and in 2050 polar bears would be extinct. The piece that’s different now is people are linking climate to increases in asthma and various diseases. We have a way to directly communicate that it’s not a far-off thing. It’s happening to your friends and family right now.”
Hospitals, medical schools, and public health programs are stepping up to educate the next generation of doctors as well as veteran medical workers on one of the most widespread, insidious health threats of our time – climate change – and specific ways it could affect their patients.
Although climate change may seem to many Americans like a distant threat, Marilyn Howarth, MD, a pediatrician in Philadelphia, is trying to make sure physicians are better prepared to treat a growing number of health problems associated with global warming.
“There isn’t a lot of education for pediatricians and internists on environmental health issues. It has not been a standard part of education in medical school or residency training,” Dr. Howarth, deputy director of the new Philadelphia Regional Center for Children’s Environmental Health, said. “With increasing attention on our climate, we really recognize there’s a real gap in physician knowledge, both in pediatric and adult care.”
Scientists have found that climate change can alter just about every system within the human body. Studies show that more extreme weather events, such as heat waves, thunderstorms, and floods, can worsen asthma and produce more pollen and mold, triggering debilitating respiratory problems.
According to the American Lung Association, ultrafine particles of air pollution can be inhaled and then travel throughout the bloodstream, wreaking havoc on organs and increasing risk of heart attack and stroke. Various types of air pollution also cause changes to the climate by trapping heat in the atmosphere, which leads to problems such as rising sea levels and extreme weather. Plus, in a new study published in Nature, scientists warn that warming climates are forcing animals to migrate to different areas, raising the risk that new infectious diseases will hop from animals – such as bats – to humans, a process called “zoonotic spillover” that many researchers believe is responsible for the COVID-19 pandemic.
The Philadelphia Regional Center for Children’s Environmental Health
One of the latest initiatives aimed at disseminating information about children’s health to health care providers is the Philadelphia Regional Center for Children’s Environmental Health, part of Children’s Hospital of Philadelphia and Penn Medicine. CHOP and Penn Medicine are jointly funding this center’s work, which will include educating health care providers on how to better screen for climate-caused health risks and treat related conditions, such as lead poisoning and asthma.
Outreach will focus on providers who treat patients with illnesses that researchers have linked to climate change, Dr. Howarth said. The center will offer clinicians access to seminars and webinars, along with online resources to help doctors treat environmental illnesses. For example, doctors at CHOP’s Poison Control Center are developing a toolkit for physicians to treat patients with elevated levels of lead in the blood. Scientists have linked extreme weather events related to climate change to flooding that pushes metals away from river banks where they were previously contained, allowing them to more easily contaminate homes, soils, and yards.
The initiative builds on CHOP’s Community Asthma Prevention Program (CAPP), which was launched in 1997 by Tyra Bryant-Stephens, MD, its current medical director. CAPP deploys community health workers into homes armed with supplies and tips for managing asthma. The new center will use similar tactics to provide education and resources to patients. The goal is to reach as many at-risk local children as possible.
Future generation of doctors fuel growth in climate change education
Lisa Doggett, MD, cofounder and president of the board of directors of Texas Physicians for Social Responsibility, announced in March that the University of Texas at Austin, Baylor College of Medicine, Houston, and the University of Texas Southwestern in Dallas have all decided to begin offering a course on environmental threats. Emory’s new curriculum has become more comprehensive every year since its start – thanks in part to the input of students like Ms. Manivannan. Faculty members tasked her with approving the new additions to the curriculum on how climate affects health, which in 2019 had consisted of a few slides about issues such as extreme heat exposure and air pollution and their effects on childbirth outcomes.
Material on climate change has now been woven into 13 courses. It is discussed at length in relation to pulmonology, cardiology, and gastropulmonology, for example, said Rebecca Philipsborn, MD, MPA, FAAP, faculty lead for the environmental and health curriculum at Emory.
The curriculum has only been incorporated into Emory’s program for the past 2 years. Dr. Philipsborn said the school plans to expand it to the clinical years to help trainees learn to treat conditions such as pediatric asthma.
“In the past few years, there has been so much momentum, and part of that is a testament to already seeing effects of climate change and how they affect delivery of health care,” she said.
At least one medical journal has recently ramped up its efforts to educate physicians on the links between health issues and climate change. Editors of Family Practice, from Oxford University Press, have announced that they plan to publish a special Climate Crisis and Primary Health Care issue in September.
Of course, not all climate initiatives in medicine are new. A select few have existed for decades.
But only now are physicians widely seeing the links between health and environment, according to Aaron Bernstein, MD, MPH, interim director of the Center for Climate, Health, and the Global Environment (C-CHANGE) at Harvard School of Public Health, Boston.
C-CHANGE, founded in 1996, was the first center in the world to focus on the health effects of environmental change.
“It’s taken 20 years, but what we’re seeing, I think, is the fruits of education,” Dr. Bernstein said. “There’s clearly a wave building here, and I think it really started with education and people younger than the people in charge calling them into account.”
Like the Philadelphia center, Harvard’s program conducts research on climate and health and educates people from high schoolers to health care veterans. Dr. Bernstein helps lead Climate MD, a program that aims to prepare health care workers for climate crises. The Climate MD team has published several articles in peer-reviewed journals on how to better treat patients struggling with environmental health problems. For example, an article on mapping patients in hurricane zones helped shed light on how systems can identify climate-vulnerable patients using public data.
They also developed a tool to help pediatricians provide “climate-informed primary care” – guidance on how to assess whether children are at risk of any harmful environmental exposures, a feature that is not part of standard pediatric visits.
Like the other programs, Climate MD uses community outreach to treat as many local patients as possible. Staff work with providers at more than 100 health clinics, particularly in areas where climate change disproportionately affects residents.
The next major step is to bring some of this into clinical practice, Dr. Bernstein said. In February 2020, C-CHANGE held its first symposium to address that issue.
“The key is to understand climate issues from a provider’s perspective,” he said. “Then those issues can really be brought to the bedside.”
A version of this article first appeared on Medscape.com.
Ms. Manivannan, copresident of Emory Medical Students for Climate Action, was in the first class of Emory’s medical students to experience the birth of a refined curriculum – lobbied for and partially created by students themselves. The new course of study addresses the myriad ways climate affects health: from air pollution and its effects on the lungs and cardiovascular system to heat-related kidney disease.
“We have known that climate has affected health for decades,” Ms. Manivannan said in a recent interview. “The narrative used to be that icebergs were melting and in 2050 polar bears would be extinct. The piece that’s different now is people are linking climate to increases in asthma and various diseases. We have a way to directly communicate that it’s not a far-off thing. It’s happening to your friends and family right now.”
Hospitals, medical schools, and public health programs are stepping up to educate the next generation of doctors as well as veteran medical workers on one of the most widespread, insidious health threats of our time – climate change – and specific ways it could affect their patients.
Although climate change may seem to many Americans like a distant threat, Marilyn Howarth, MD, a pediatrician in Philadelphia, is trying to make sure physicians are better prepared to treat a growing number of health problems associated with global warming.
“There isn’t a lot of education for pediatricians and internists on environmental health issues. It has not been a standard part of education in medical school or residency training,” Dr. Howarth, deputy director of the new Philadelphia Regional Center for Children’s Environmental Health, said. “With increasing attention on our climate, we really recognize there’s a real gap in physician knowledge, both in pediatric and adult care.”
Scientists have found that climate change can alter just about every system within the human body. Studies show that more extreme weather events, such as heat waves, thunderstorms, and floods, can worsen asthma and produce more pollen and mold, triggering debilitating respiratory problems.
According to the American Lung Association, ultrafine particles of air pollution can be inhaled and then travel throughout the bloodstream, wreaking havoc on organs and increasing risk of heart attack and stroke. Various types of air pollution also cause changes to the climate by trapping heat in the atmosphere, which leads to problems such as rising sea levels and extreme weather. Plus, in a new study published in Nature, scientists warn that warming climates are forcing animals to migrate to different areas, raising the risk that new infectious diseases will hop from animals – such as bats – to humans, a process called “zoonotic spillover” that many researchers believe is responsible for the COVID-19 pandemic.
The Philadelphia Regional Center for Children’s Environmental Health
One of the latest initiatives aimed at disseminating information about children’s health to health care providers is the Philadelphia Regional Center for Children’s Environmental Health, part of Children’s Hospital of Philadelphia and Penn Medicine. CHOP and Penn Medicine are jointly funding this center’s work, which will include educating health care providers on how to better screen for climate-caused health risks and treat related conditions, such as lead poisoning and asthma.
Outreach will focus on providers who treat patients with illnesses that researchers have linked to climate change, Dr. Howarth said. The center will offer clinicians access to seminars and webinars, along with online resources to help doctors treat environmental illnesses. For example, doctors at CHOP’s Poison Control Center are developing a toolkit for physicians to treat patients with elevated levels of lead in the blood. Scientists have linked extreme weather events related to climate change to flooding that pushes metals away from river banks where they were previously contained, allowing them to more easily contaminate homes, soils, and yards.
The initiative builds on CHOP’s Community Asthma Prevention Program (CAPP), which was launched in 1997 by Tyra Bryant-Stephens, MD, its current medical director. CAPP deploys community health workers into homes armed with supplies and tips for managing asthma. The new center will use similar tactics to provide education and resources to patients. The goal is to reach as many at-risk local children as possible.
Future generation of doctors fuel growth in climate change education
Lisa Doggett, MD, cofounder and president of the board of directors of Texas Physicians for Social Responsibility, announced in March that the University of Texas at Austin, Baylor College of Medicine, Houston, and the University of Texas Southwestern in Dallas have all decided to begin offering a course on environmental threats. Emory’s new curriculum has become more comprehensive every year since its start – thanks in part to the input of students like Ms. Manivannan. Faculty members tasked her with approving the new additions to the curriculum on how climate affects health, which in 2019 had consisted of a few slides about issues such as extreme heat exposure and air pollution and their effects on childbirth outcomes.
Material on climate change has now been woven into 13 courses. It is discussed at length in relation to pulmonology, cardiology, and gastropulmonology, for example, said Rebecca Philipsborn, MD, MPA, FAAP, faculty lead for the environmental and health curriculum at Emory.
The curriculum has only been incorporated into Emory’s program for the past 2 years. Dr. Philipsborn said the school plans to expand it to the clinical years to help trainees learn to treat conditions such as pediatric asthma.
“In the past few years, there has been so much momentum, and part of that is a testament to already seeing effects of climate change and how they affect delivery of health care,” she said.
At least one medical journal has recently ramped up its efforts to educate physicians on the links between health issues and climate change. Editors of Family Practice, from Oxford University Press, have announced that they plan to publish a special Climate Crisis and Primary Health Care issue in September.
Of course, not all climate initiatives in medicine are new. A select few have existed for decades.
But only now are physicians widely seeing the links between health and environment, according to Aaron Bernstein, MD, MPH, interim director of the Center for Climate, Health, and the Global Environment (C-CHANGE) at Harvard School of Public Health, Boston.
C-CHANGE, founded in 1996, was the first center in the world to focus on the health effects of environmental change.
“It’s taken 20 years, but what we’re seeing, I think, is the fruits of education,” Dr. Bernstein said. “There’s clearly a wave building here, and I think it really started with education and people younger than the people in charge calling them into account.”
Like the Philadelphia center, Harvard’s program conducts research on climate and health and educates people from high schoolers to health care veterans. Dr. Bernstein helps lead Climate MD, a program that aims to prepare health care workers for climate crises. The Climate MD team has published several articles in peer-reviewed journals on how to better treat patients struggling with environmental health problems. For example, an article on mapping patients in hurricane zones helped shed light on how systems can identify climate-vulnerable patients using public data.
They also developed a tool to help pediatricians provide “climate-informed primary care” – guidance on how to assess whether children are at risk of any harmful environmental exposures, a feature that is not part of standard pediatric visits.
Like the other programs, Climate MD uses community outreach to treat as many local patients as possible. Staff work with providers at more than 100 health clinics, particularly in areas where climate change disproportionately affects residents.
The next major step is to bring some of this into clinical practice, Dr. Bernstein said. In February 2020, C-CHANGE held its first symposium to address that issue.
“The key is to understand climate issues from a provider’s perspective,” he said. “Then those issues can really be brought to the bedside.”
A version of this article first appeared on Medscape.com.
Ms. Manivannan, copresident of Emory Medical Students for Climate Action, was in the first class of Emory’s medical students to experience the birth of a refined curriculum – lobbied for and partially created by students themselves. The new course of study addresses the myriad ways climate affects health: from air pollution and its effects on the lungs and cardiovascular system to heat-related kidney disease.
“We have known that climate has affected health for decades,” Ms. Manivannan said in a recent interview. “The narrative used to be that icebergs were melting and in 2050 polar bears would be extinct. The piece that’s different now is people are linking climate to increases in asthma and various diseases. We have a way to directly communicate that it’s not a far-off thing. It’s happening to your friends and family right now.”
Hospitals, medical schools, and public health programs are stepping up to educate the next generation of doctors as well as veteran medical workers on one of the most widespread, insidious health threats of our time – climate change – and specific ways it could affect their patients.
Although climate change may seem to many Americans like a distant threat, Marilyn Howarth, MD, a pediatrician in Philadelphia, is trying to make sure physicians are better prepared to treat a growing number of health problems associated with global warming.
“There isn’t a lot of education for pediatricians and internists on environmental health issues. It has not been a standard part of education in medical school or residency training,” Dr. Howarth, deputy director of the new Philadelphia Regional Center for Children’s Environmental Health, said. “With increasing attention on our climate, we really recognize there’s a real gap in physician knowledge, both in pediatric and adult care.”
Scientists have found that climate change can alter just about every system within the human body. Studies show that more extreme weather events, such as heat waves, thunderstorms, and floods, can worsen asthma and produce more pollen and mold, triggering debilitating respiratory problems.
According to the American Lung Association, ultrafine particles of air pollution can be inhaled and then travel throughout the bloodstream, wreaking havoc on organs and increasing risk of heart attack and stroke. Various types of air pollution also cause changes to the climate by trapping heat in the atmosphere, which leads to problems such as rising sea levels and extreme weather. Plus, in a new study published in Nature, scientists warn that warming climates are forcing animals to migrate to different areas, raising the risk that new infectious diseases will hop from animals – such as bats – to humans, a process called “zoonotic spillover” that many researchers believe is responsible for the COVID-19 pandemic.
The Philadelphia Regional Center for Children’s Environmental Health
One of the latest initiatives aimed at disseminating information about children’s health to health care providers is the Philadelphia Regional Center for Children’s Environmental Health, part of Children’s Hospital of Philadelphia and Penn Medicine. CHOP and Penn Medicine are jointly funding this center’s work, which will include educating health care providers on how to better screen for climate-caused health risks and treat related conditions, such as lead poisoning and asthma.
Outreach will focus on providers who treat patients with illnesses that researchers have linked to climate change, Dr. Howarth said. The center will offer clinicians access to seminars and webinars, along with online resources to help doctors treat environmental illnesses. For example, doctors at CHOP’s Poison Control Center are developing a toolkit for physicians to treat patients with elevated levels of lead in the blood. Scientists have linked extreme weather events related to climate change to flooding that pushes metals away from river banks where they were previously contained, allowing them to more easily contaminate homes, soils, and yards.
The initiative builds on CHOP’s Community Asthma Prevention Program (CAPP), which was launched in 1997 by Tyra Bryant-Stephens, MD, its current medical director. CAPP deploys community health workers into homes armed with supplies and tips for managing asthma. The new center will use similar tactics to provide education and resources to patients. The goal is to reach as many at-risk local children as possible.
Future generation of doctors fuel growth in climate change education
Lisa Doggett, MD, cofounder and president of the board of directors of Texas Physicians for Social Responsibility, announced in March that the University of Texas at Austin, Baylor College of Medicine, Houston, and the University of Texas Southwestern in Dallas have all decided to begin offering a course on environmental threats. Emory’s new curriculum has become more comprehensive every year since its start – thanks in part to the input of students like Ms. Manivannan. Faculty members tasked her with approving the new additions to the curriculum on how climate affects health, which in 2019 had consisted of a few slides about issues such as extreme heat exposure and air pollution and their effects on childbirth outcomes.
Material on climate change has now been woven into 13 courses. It is discussed at length in relation to pulmonology, cardiology, and gastropulmonology, for example, said Rebecca Philipsborn, MD, MPA, FAAP, faculty lead for the environmental and health curriculum at Emory.
The curriculum has only been incorporated into Emory’s program for the past 2 years. Dr. Philipsborn said the school plans to expand it to the clinical years to help trainees learn to treat conditions such as pediatric asthma.
“In the past few years, there has been so much momentum, and part of that is a testament to already seeing effects of climate change and how they affect delivery of health care,” she said.
At least one medical journal has recently ramped up its efforts to educate physicians on the links between health issues and climate change. Editors of Family Practice, from Oxford University Press, have announced that they plan to publish a special Climate Crisis and Primary Health Care issue in September.
Of course, not all climate initiatives in medicine are new. A select few have existed for decades.
But only now are physicians widely seeing the links between health and environment, according to Aaron Bernstein, MD, MPH, interim director of the Center for Climate, Health, and the Global Environment (C-CHANGE) at Harvard School of Public Health, Boston.
C-CHANGE, founded in 1996, was the first center in the world to focus on the health effects of environmental change.
“It’s taken 20 years, but what we’re seeing, I think, is the fruits of education,” Dr. Bernstein said. “There’s clearly a wave building here, and I think it really started with education and people younger than the people in charge calling them into account.”
Like the Philadelphia center, Harvard’s program conducts research on climate and health and educates people from high schoolers to health care veterans. Dr. Bernstein helps lead Climate MD, a program that aims to prepare health care workers for climate crises. The Climate MD team has published several articles in peer-reviewed journals on how to better treat patients struggling with environmental health problems. For example, an article on mapping patients in hurricane zones helped shed light on how systems can identify climate-vulnerable patients using public data.
They also developed a tool to help pediatricians provide “climate-informed primary care” – guidance on how to assess whether children are at risk of any harmful environmental exposures, a feature that is not part of standard pediatric visits.
Like the other programs, Climate MD uses community outreach to treat as many local patients as possible. Staff work with providers at more than 100 health clinics, particularly in areas where climate change disproportionately affects residents.
The next major step is to bring some of this into clinical practice, Dr. Bernstein said. In February 2020, C-CHANGE held its first symposium to address that issue.
“The key is to understand climate issues from a provider’s perspective,” he said. “Then those issues can really be brought to the bedside.”
A version of this article first appeared on Medscape.com.
Blue state alert at ACOG: Abortion seekers will head your way
SAN DIEGO – The end of the legal standards set by Roe v. Wade will likely lead to bans in as many as 26 states and send a flood of abortion seekers to the remaining states that still allow the procedure, an obstetrician-gynecologist warned colleagues at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“The blue states neighboring those states will likely see an outpouring of patients among those who can travel,” Kristyn Brandi, MD, of New Jersey Medical School, Newark, said in a presentation about legal threats to abortion rights. “These will likely flood the health care systems and delay care for everyone. Make no mistake: Virtually all ob.gyns. across the country have the potential to be impacted.”
Indeed, research suggests that thousands more Texans than usual are heading out of state for abortions each month in the wake of a new, strict antiabortion law there.
Only three sessions at the 3-day ACOG meeting directly addressed abortion. But the topic was clearly on the minds of attendees in the wake of the release of a leaked draft of a Supreme Court ruling that would eliminate federal protection for abortion rights.
The 57,000-member ACOG organization firmly supports abortion rights and declares on its website that “Abortion Is Healthcare.”
In a workshop on challenges to abortion, ACOG chief of staff Dorothea Calvano Lindquist said “we remain your steadfast partner in advocacy and guidance on all levels.”
Ivana S. Thompson, MD, an ob.gyn. at Vanderbilt University, Nashville, Tenn., explained in a presentation that Roe v. Wade established a framework for regulations around abortion; they may not be regulated during the first trimester, but states may impose rules in the second semester that are related to health. “And then in the third trimester, once the fetus reaches viability, the state may regulate abortions or even prohibit them entirely, so long as there are exceptions for medical emergencies,” she said.
The Supreme Court ruling in the 1992 case of Planned Parenthood v. Casey did away with the trimester framework, Dr. Thompson said, and declared that abortion regulations could not place an “undue burden” on women.
This change allowed laws that “are purposely designed to trap providers and clinics and to restrict their ability to provide abortions, not due to health concerns but really just to prevent pregnant people from accessing care,” she said.
In 2018, Mississippi passed a law – which never went into effect and is now challenged before the Supreme Court – that makes most abortions illegal after 15 weeks. And in September 2021, a Texas law went into effect that outlaws abortions after a fetal heartbeat is detected.
What happens if Roe is overturned and laws that ban or severely limit abortion go into effect in states across the country? In Nashville, Dr. Thompson said, patients will have to travel to Illinois – more than 300 miles away – to reach the nearest abortion clinic.
“When I think about my own clinical practice over the last year, [if the law were in place] I would not have been able to offer an abortion to a developmentally delayed, nonverbal patient who was raped by her brother,” she said. “I would not have been able to offer an abortion to the service person who was sexually assaulted by a coworker in the field. I would not have been able to offer an abortion to a person with a pregnancy complicated by a hypoplastic left heart, congenital diaphragmatic hernias with the stomach in the thorax, an unformed lumbar spine, and other anomalies.”
Bhavik Kumar, MD, a family medicine physician and medical director for primary and trans care at Planned Parenthood Gulf Coast in Houston, said the effects of the new law in Texas are already apparent. As he told ABC News last fall, he used to perform 20-30 abortions per day, but the number dwindled immediately the day the law went into effect.
At the ACOG presentation, Dr. Kumar highlighted a March 2022 research brief that reported that abortions in Texas fell by half in the month after the law was implemented compared with the previous year. And the average number of abortions performed on Texans who left the state grew by more than 10-fold from the period of September-December 2019 (514) to September-December 2021 (5,574).
Once the law went into effect, Dr. Kumar said, “we began to see longer waiting times at clinics in nearby states, wait times that started out as short as a day go to an average of 2-3 weeks to get an initial appointment. And some of these states also have mandatory delays of up to 72 hours.”
Dr. Kumar added that he’s “heard from emergency-room physicians and nurses who call and ask me what they can and cannot say when providing care for pregnant people in Texas and how they should be counseling their patients who may need emergency or urgent care after returning to Texas.”
Dr. Brandi cautioned colleagues that even ob.gyns. who don’t perform abortions will still be affected by the overturning of Roe. In some states, they’ll have to understand the rules about treating women with early ruptured membranes when cardiac motion is detected or with atopic pregnancies with cardiac activity at risk of potential tubal rupture.
The speakers urged colleagues to take action at the ballot box and their own clinics to protect patients. “While the recent leak is a truly scary moment for our country and for our practices, I’m hopeful that it will help galvanize our communities,” Dr. Brandi said. Regardless of where you live, regardless of where you practice, this ruling impacts all ob.gyns., everyone in this room. Each of us needs to go home after this conference and figure out what you are going to do to make sure that our patients can still get the care that they need.”
SAN DIEGO – The end of the legal standards set by Roe v. Wade will likely lead to bans in as many as 26 states and send a flood of abortion seekers to the remaining states that still allow the procedure, an obstetrician-gynecologist warned colleagues at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“The blue states neighboring those states will likely see an outpouring of patients among those who can travel,” Kristyn Brandi, MD, of New Jersey Medical School, Newark, said in a presentation about legal threats to abortion rights. “These will likely flood the health care systems and delay care for everyone. Make no mistake: Virtually all ob.gyns. across the country have the potential to be impacted.”
Indeed, research suggests that thousands more Texans than usual are heading out of state for abortions each month in the wake of a new, strict antiabortion law there.
Only three sessions at the 3-day ACOG meeting directly addressed abortion. But the topic was clearly on the minds of attendees in the wake of the release of a leaked draft of a Supreme Court ruling that would eliminate federal protection for abortion rights.
The 57,000-member ACOG organization firmly supports abortion rights and declares on its website that “Abortion Is Healthcare.”
In a workshop on challenges to abortion, ACOG chief of staff Dorothea Calvano Lindquist said “we remain your steadfast partner in advocacy and guidance on all levels.”
Ivana S. Thompson, MD, an ob.gyn. at Vanderbilt University, Nashville, Tenn., explained in a presentation that Roe v. Wade established a framework for regulations around abortion; they may not be regulated during the first trimester, but states may impose rules in the second semester that are related to health. “And then in the third trimester, once the fetus reaches viability, the state may regulate abortions or even prohibit them entirely, so long as there are exceptions for medical emergencies,” she said.
The Supreme Court ruling in the 1992 case of Planned Parenthood v. Casey did away with the trimester framework, Dr. Thompson said, and declared that abortion regulations could not place an “undue burden” on women.
This change allowed laws that “are purposely designed to trap providers and clinics and to restrict their ability to provide abortions, not due to health concerns but really just to prevent pregnant people from accessing care,” she said.
In 2018, Mississippi passed a law – which never went into effect and is now challenged before the Supreme Court – that makes most abortions illegal after 15 weeks. And in September 2021, a Texas law went into effect that outlaws abortions after a fetal heartbeat is detected.
What happens if Roe is overturned and laws that ban or severely limit abortion go into effect in states across the country? In Nashville, Dr. Thompson said, patients will have to travel to Illinois – more than 300 miles away – to reach the nearest abortion clinic.
“When I think about my own clinical practice over the last year, [if the law were in place] I would not have been able to offer an abortion to a developmentally delayed, nonverbal patient who was raped by her brother,” she said. “I would not have been able to offer an abortion to the service person who was sexually assaulted by a coworker in the field. I would not have been able to offer an abortion to a person with a pregnancy complicated by a hypoplastic left heart, congenital diaphragmatic hernias with the stomach in the thorax, an unformed lumbar spine, and other anomalies.”
Bhavik Kumar, MD, a family medicine physician and medical director for primary and trans care at Planned Parenthood Gulf Coast in Houston, said the effects of the new law in Texas are already apparent. As he told ABC News last fall, he used to perform 20-30 abortions per day, but the number dwindled immediately the day the law went into effect.
At the ACOG presentation, Dr. Kumar highlighted a March 2022 research brief that reported that abortions in Texas fell by half in the month after the law was implemented compared with the previous year. And the average number of abortions performed on Texans who left the state grew by more than 10-fold from the period of September-December 2019 (514) to September-December 2021 (5,574).
Once the law went into effect, Dr. Kumar said, “we began to see longer waiting times at clinics in nearby states, wait times that started out as short as a day go to an average of 2-3 weeks to get an initial appointment. And some of these states also have mandatory delays of up to 72 hours.”
Dr. Kumar added that he’s “heard from emergency-room physicians and nurses who call and ask me what they can and cannot say when providing care for pregnant people in Texas and how they should be counseling their patients who may need emergency or urgent care after returning to Texas.”
Dr. Brandi cautioned colleagues that even ob.gyns. who don’t perform abortions will still be affected by the overturning of Roe. In some states, they’ll have to understand the rules about treating women with early ruptured membranes when cardiac motion is detected or with atopic pregnancies with cardiac activity at risk of potential tubal rupture.
The speakers urged colleagues to take action at the ballot box and their own clinics to protect patients. “While the recent leak is a truly scary moment for our country and for our practices, I’m hopeful that it will help galvanize our communities,” Dr. Brandi said. Regardless of where you live, regardless of where you practice, this ruling impacts all ob.gyns., everyone in this room. Each of us needs to go home after this conference and figure out what you are going to do to make sure that our patients can still get the care that they need.”
SAN DIEGO – The end of the legal standards set by Roe v. Wade will likely lead to bans in as many as 26 states and send a flood of abortion seekers to the remaining states that still allow the procedure, an obstetrician-gynecologist warned colleagues at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
“The blue states neighboring those states will likely see an outpouring of patients among those who can travel,” Kristyn Brandi, MD, of New Jersey Medical School, Newark, said in a presentation about legal threats to abortion rights. “These will likely flood the health care systems and delay care for everyone. Make no mistake: Virtually all ob.gyns. across the country have the potential to be impacted.”
Indeed, research suggests that thousands more Texans than usual are heading out of state for abortions each month in the wake of a new, strict antiabortion law there.
Only three sessions at the 3-day ACOG meeting directly addressed abortion. But the topic was clearly on the minds of attendees in the wake of the release of a leaked draft of a Supreme Court ruling that would eliminate federal protection for abortion rights.
The 57,000-member ACOG organization firmly supports abortion rights and declares on its website that “Abortion Is Healthcare.”
In a workshop on challenges to abortion, ACOG chief of staff Dorothea Calvano Lindquist said “we remain your steadfast partner in advocacy and guidance on all levels.”
Ivana S. Thompson, MD, an ob.gyn. at Vanderbilt University, Nashville, Tenn., explained in a presentation that Roe v. Wade established a framework for regulations around abortion; they may not be regulated during the first trimester, but states may impose rules in the second semester that are related to health. “And then in the third trimester, once the fetus reaches viability, the state may regulate abortions or even prohibit them entirely, so long as there are exceptions for medical emergencies,” she said.
The Supreme Court ruling in the 1992 case of Planned Parenthood v. Casey did away with the trimester framework, Dr. Thompson said, and declared that abortion regulations could not place an “undue burden” on women.
This change allowed laws that “are purposely designed to trap providers and clinics and to restrict their ability to provide abortions, not due to health concerns but really just to prevent pregnant people from accessing care,” she said.
In 2018, Mississippi passed a law – which never went into effect and is now challenged before the Supreme Court – that makes most abortions illegal after 15 weeks. And in September 2021, a Texas law went into effect that outlaws abortions after a fetal heartbeat is detected.
What happens if Roe is overturned and laws that ban or severely limit abortion go into effect in states across the country? In Nashville, Dr. Thompson said, patients will have to travel to Illinois – more than 300 miles away – to reach the nearest abortion clinic.
“When I think about my own clinical practice over the last year, [if the law were in place] I would not have been able to offer an abortion to a developmentally delayed, nonverbal patient who was raped by her brother,” she said. “I would not have been able to offer an abortion to the service person who was sexually assaulted by a coworker in the field. I would not have been able to offer an abortion to a person with a pregnancy complicated by a hypoplastic left heart, congenital diaphragmatic hernias with the stomach in the thorax, an unformed lumbar spine, and other anomalies.”
Bhavik Kumar, MD, a family medicine physician and medical director for primary and trans care at Planned Parenthood Gulf Coast in Houston, said the effects of the new law in Texas are already apparent. As he told ABC News last fall, he used to perform 20-30 abortions per day, but the number dwindled immediately the day the law went into effect.
At the ACOG presentation, Dr. Kumar highlighted a March 2022 research brief that reported that abortions in Texas fell by half in the month after the law was implemented compared with the previous year. And the average number of abortions performed on Texans who left the state grew by more than 10-fold from the period of September-December 2019 (514) to September-December 2021 (5,574).
Once the law went into effect, Dr. Kumar said, “we began to see longer waiting times at clinics in nearby states, wait times that started out as short as a day go to an average of 2-3 weeks to get an initial appointment. And some of these states also have mandatory delays of up to 72 hours.”
Dr. Kumar added that he’s “heard from emergency-room physicians and nurses who call and ask me what they can and cannot say when providing care for pregnant people in Texas and how they should be counseling their patients who may need emergency or urgent care after returning to Texas.”
Dr. Brandi cautioned colleagues that even ob.gyns. who don’t perform abortions will still be affected by the overturning of Roe. In some states, they’ll have to understand the rules about treating women with early ruptured membranes when cardiac motion is detected or with atopic pregnancies with cardiac activity at risk of potential tubal rupture.
The speakers urged colleagues to take action at the ballot box and their own clinics to protect patients. “While the recent leak is a truly scary moment for our country and for our practices, I’m hopeful that it will help galvanize our communities,” Dr. Brandi said. Regardless of where you live, regardless of where you practice, this ruling impacts all ob.gyns., everyone in this room. Each of us needs to go home after this conference and figure out what you are going to do to make sure that our patients can still get the care that they need.”
AT ACOG 2022
Online physician reviews and ratings: The good, the bad, and the ugly
A recent article on Medscape entitled “Online Reviews Most Important Factor in Choosing a Doctor: Survey” really got me thinking about my online presence. According to the results of a new Press Ganey survey, online reviews and star ratings are the most important factor for consumers when choosing a new health care provider, even more so than the recommendation of another doctor. Almost 85% of the survey respondents said they wouldn’t make an appointment with a referred provider if they had a rating of less than 4 stars.
To be honest, I’ve rarely thought about my ratings or online reviews, and I almost never enter my own name into a web browser to see what might emerge. I don’t use most popular social media apps, I don’t have a professional website, and I was completely late in joining LinkedIn. After considering the Press Ganey survey results, though, I’m wondering how many patients (or potential patients) have found me online. And what exactly did they see?
So, I just searched online for my ratings and reviews. There weren’t many direct hits (although I’m not sure if that is a good thing or not). One of the results listed me as a “Fibromyalgia Doctor” at www.lymeforums.org. To be clear, I’m a locum tenens infectious diseases provider with a focus on inpatient consultations and teaching. I couldn’t find any reviews for myself on WebMD, but I had one rating on the Healthgrades website – I was pleased to see someone gave me 5 stars (though there weren’t any comments) until I realized the site listed my address at an ob.gyn. office; I’m not even sure if that rating was meant for me.
Use of the Internet to assess my qualities as a physician is clearly limited, and much of the online information about my specialty and practice sites is completely inaccurate. Yet, according to the Press Ganey survey, the average consumer uses three different websites and reads more than five online reviews before making a provider decision. Once they’ve seen a provider, though, patients rank practice customer service and communication as more important than “bedside manner” when considering a 5-star review. So, it appears that many physician reviews and ratings probably reflect the performance of the office staff, not the provider.
Given the few hits that I encountered when searching my own name, I’m not convinced I need to do anything differently about my online presence; essentially, I don’t really have one. However, there were certainly a lot of other physicians with the same last name as mine who did have impressive numbers of reviews and ratings. One doctor of cosmetic surgery had more than 200 reviews on multiple sites; almost all were positive, but a few patients rated him at 1 or 2 (out of 5) stars, suggesting that patients should find a new surgeon. What does one do about those outliers?
Medicine is indeed a business and patients behave as consumers when searching the web for a health care provider. Another survey has suggested that just one negative review may cause a business to risk losing 22% of its customers, and that multiple bad ratings are even worse – nearly 60% of customers will avoid a business with three or more negative online reviews.
A few years ago, The Washington Post published an article about doctors who were fighting back against bad reviews. Unfortunately, while trying to directly combat inaccuracies, some providers divulged sensitive patient information and suffered the consequences of HIPAA violations. Many legal experts suggest that, in most cases, physicians should restrain from responding publicly to negative online reviews, however tempting it may be to react.
If a negative review is attributed to a specific patient, some lawyers recommend contacting them privately by phone to address their concerns; this may clear the air enough to result in a withdrawal of the negative review or rating. Flagging and reporting fake or unsubstantiated negative reviews (or reviews that violate the Terms of Service of a specific platform) can be done. Consultation with an Internet defamation attorney might be helpful in some circumstances, though hopefully, legal action such as a lawsuit or a cease-and-desist letter can be avoided.
For physicians who do maintain an online presence, engaging with an online reputation management service can help suppress fake or negative reviews while offering strategies for building a better reputation. As for me, I think that I’m grateful my name hasn’t attracted a lot of attention at physician ranking and review sites. I guess we’ll see if it stays that way.
Dr. Devlin is president of Locum Infectious Disease Services, and an independent contractor for Weatherby Healthcare. She has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
A recent article on Medscape entitled “Online Reviews Most Important Factor in Choosing a Doctor: Survey” really got me thinking about my online presence. According to the results of a new Press Ganey survey, online reviews and star ratings are the most important factor for consumers when choosing a new health care provider, even more so than the recommendation of another doctor. Almost 85% of the survey respondents said they wouldn’t make an appointment with a referred provider if they had a rating of less than 4 stars.
To be honest, I’ve rarely thought about my ratings or online reviews, and I almost never enter my own name into a web browser to see what might emerge. I don’t use most popular social media apps, I don’t have a professional website, and I was completely late in joining LinkedIn. After considering the Press Ganey survey results, though, I’m wondering how many patients (or potential patients) have found me online. And what exactly did they see?
So, I just searched online for my ratings and reviews. There weren’t many direct hits (although I’m not sure if that is a good thing or not). One of the results listed me as a “Fibromyalgia Doctor” at www.lymeforums.org. To be clear, I’m a locum tenens infectious diseases provider with a focus on inpatient consultations and teaching. I couldn’t find any reviews for myself on WebMD, but I had one rating on the Healthgrades website – I was pleased to see someone gave me 5 stars (though there weren’t any comments) until I realized the site listed my address at an ob.gyn. office; I’m not even sure if that rating was meant for me.
Use of the Internet to assess my qualities as a physician is clearly limited, and much of the online information about my specialty and practice sites is completely inaccurate. Yet, according to the Press Ganey survey, the average consumer uses three different websites and reads more than five online reviews before making a provider decision. Once they’ve seen a provider, though, patients rank practice customer service and communication as more important than “bedside manner” when considering a 5-star review. So, it appears that many physician reviews and ratings probably reflect the performance of the office staff, not the provider.
Given the few hits that I encountered when searching my own name, I’m not convinced I need to do anything differently about my online presence; essentially, I don’t really have one. However, there were certainly a lot of other physicians with the same last name as mine who did have impressive numbers of reviews and ratings. One doctor of cosmetic surgery had more than 200 reviews on multiple sites; almost all were positive, but a few patients rated him at 1 or 2 (out of 5) stars, suggesting that patients should find a new surgeon. What does one do about those outliers?
Medicine is indeed a business and patients behave as consumers when searching the web for a health care provider. Another survey has suggested that just one negative review may cause a business to risk losing 22% of its customers, and that multiple bad ratings are even worse – nearly 60% of customers will avoid a business with three or more negative online reviews.
A few years ago, The Washington Post published an article about doctors who were fighting back against bad reviews. Unfortunately, while trying to directly combat inaccuracies, some providers divulged sensitive patient information and suffered the consequences of HIPAA violations. Many legal experts suggest that, in most cases, physicians should restrain from responding publicly to negative online reviews, however tempting it may be to react.
If a negative review is attributed to a specific patient, some lawyers recommend contacting them privately by phone to address their concerns; this may clear the air enough to result in a withdrawal of the negative review or rating. Flagging and reporting fake or unsubstantiated negative reviews (or reviews that violate the Terms of Service of a specific platform) can be done. Consultation with an Internet defamation attorney might be helpful in some circumstances, though hopefully, legal action such as a lawsuit or a cease-and-desist letter can be avoided.
For physicians who do maintain an online presence, engaging with an online reputation management service can help suppress fake or negative reviews while offering strategies for building a better reputation. As for me, I think that I’m grateful my name hasn’t attracted a lot of attention at physician ranking and review sites. I guess we’ll see if it stays that way.
Dr. Devlin is president of Locum Infectious Disease Services, and an independent contractor for Weatherby Healthcare. She has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
A recent article on Medscape entitled “Online Reviews Most Important Factor in Choosing a Doctor: Survey” really got me thinking about my online presence. According to the results of a new Press Ganey survey, online reviews and star ratings are the most important factor for consumers when choosing a new health care provider, even more so than the recommendation of another doctor. Almost 85% of the survey respondents said they wouldn’t make an appointment with a referred provider if they had a rating of less than 4 stars.
To be honest, I’ve rarely thought about my ratings or online reviews, and I almost never enter my own name into a web browser to see what might emerge. I don’t use most popular social media apps, I don’t have a professional website, and I was completely late in joining LinkedIn. After considering the Press Ganey survey results, though, I’m wondering how many patients (or potential patients) have found me online. And what exactly did they see?
So, I just searched online for my ratings and reviews. There weren’t many direct hits (although I’m not sure if that is a good thing or not). One of the results listed me as a “Fibromyalgia Doctor” at www.lymeforums.org. To be clear, I’m a locum tenens infectious diseases provider with a focus on inpatient consultations and teaching. I couldn’t find any reviews for myself on WebMD, but I had one rating on the Healthgrades website – I was pleased to see someone gave me 5 stars (though there weren’t any comments) until I realized the site listed my address at an ob.gyn. office; I’m not even sure if that rating was meant for me.
Use of the Internet to assess my qualities as a physician is clearly limited, and much of the online information about my specialty and practice sites is completely inaccurate. Yet, according to the Press Ganey survey, the average consumer uses three different websites and reads more than five online reviews before making a provider decision. Once they’ve seen a provider, though, patients rank practice customer service and communication as more important than “bedside manner” when considering a 5-star review. So, it appears that many physician reviews and ratings probably reflect the performance of the office staff, not the provider.
Given the few hits that I encountered when searching my own name, I’m not convinced I need to do anything differently about my online presence; essentially, I don’t really have one. However, there were certainly a lot of other physicians with the same last name as mine who did have impressive numbers of reviews and ratings. One doctor of cosmetic surgery had more than 200 reviews on multiple sites; almost all were positive, but a few patients rated him at 1 or 2 (out of 5) stars, suggesting that patients should find a new surgeon. What does one do about those outliers?
Medicine is indeed a business and patients behave as consumers when searching the web for a health care provider. Another survey has suggested that just one negative review may cause a business to risk losing 22% of its customers, and that multiple bad ratings are even worse – nearly 60% of customers will avoid a business with three or more negative online reviews.
A few years ago, The Washington Post published an article about doctors who were fighting back against bad reviews. Unfortunately, while trying to directly combat inaccuracies, some providers divulged sensitive patient information and suffered the consequences of HIPAA violations. Many legal experts suggest that, in most cases, physicians should restrain from responding publicly to negative online reviews, however tempting it may be to react.
If a negative review is attributed to a specific patient, some lawyers recommend contacting them privately by phone to address their concerns; this may clear the air enough to result in a withdrawal of the negative review or rating. Flagging and reporting fake or unsubstantiated negative reviews (or reviews that violate the Terms of Service of a specific platform) can be done. Consultation with an Internet defamation attorney might be helpful in some circumstances, though hopefully, legal action such as a lawsuit or a cease-and-desist letter can be avoided.
For physicians who do maintain an online presence, engaging with an online reputation management service can help suppress fake or negative reviews while offering strategies for building a better reputation. As for me, I think that I’m grateful my name hasn’t attracted a lot of attention at physician ranking and review sites. I guess we’ll see if it stays that way.
Dr. Devlin is president of Locum Infectious Disease Services, and an independent contractor for Weatherby Healthcare. She has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
SARS-CoV-2 stays in GI tract long after it clears the lungs
New data present further evidence that SARS-CoV-2 infection can settle in the gastrointestinal tract and that it can persist long after the infection has cleared the lungs.
Infection of the GI tract may figure prominently in long COVID, the study authors suggested.
Led by Aravind Natarajan, PhD, with the departments of genetics and medicine at Stanford (Calif.) University, they analyzed fecal RNA shedding up to 10 months after a COVID-19 diagnosis in 673 stool samples from 113 patients with mild to moderate disease.
They found that, in the week after diagnosis, COVID RNA remnants were present in the stool of approximately half (49.2%) of the patients. Seven months later, about 4% of them shed fecal viral RNA.
The authors noted that there was no ongoing SARS-CoV-2 RNA shedding in respiratory samples of patients at the 4-month mark.
Using self-reported symptoms regularly collected by questionnaire, they also found a correlation of long-term fecal shedding of SARS-CoV-2 RNA with abdominal pain, nausea, and vomiting.
The findings were published online in Med.
Implications of long-term viral shedding
Previous studies have found SARS-CoV-2 RNA in respiratory and fecal samples and have documented viral replication in lung and intestinal tissue. But before the current study, little had been known about long-term shedding, especially in those who have mild COVID. Most studies of viral shedding have been with severe COVID cases.
The authors noted that most studies of this kind are cross-sectional. The few other longitudinal studies have focused on early time points just after diagnosis.
Senior author Ami S. Bhatt, MD, associate professor in the departments of medicine and hematology at Stanford University, said in an interview that, though the viral genetic material in the feces lingers, on the basis of available evidence, it is highly unlikely to be contagious in most cases.
She said that understanding the dynamics of fecal shedding of SARS-CoV-2 genetic material will help interpret wastewater-based studies that are trying to determine population prevalence of the virus.
“While we don’t know the exact clinical importance of the longer-term shedding of SARS-CoV-2 in individuals with COVID-19, some have speculated that those who have long-term shedding of SARS-CoV-2 may have ongoing infections that might benefit from treatment,” she said.
“Our data support the idea that the long-term GI-related symptoms in some people might be the consequence of an ongoing infection in the GI tract, even after the respiratory infection has cleared,” Dr. Bhatt said.
“Alternatively, the presence of ongoing viral genetic material in the gut might be a trigger for the immune system to continually be active against the virus, and our immune system reaction may be the reason for long COVID–type symptoms,” she added. “This area is ripe for additional studies.”
Dr. Bhatt and colleagues will continue studying viral shedding in fecal samples as part of the nationwide RECOVER Initiative.
When reached for comment, David A. Johnson, MD, professor of medicine and chief of gastroenterology, Eastern Virginia Medical School, Norfolk, said in an interview that previous studies have indicated that the virus may be detected in the stool for a month or more and for about 2 weeks on average. Whether the virus is infectious has been in question.
But it’s not so much that the virus is infectious in the GI tract and causing symptoms, he said. Rather, there are biomic changes related to COVID, including a loss of diversity in the gut bacteria, which disrupts the balance.
“This may actually in some way predispose some patients to impaired clearance of their symptoms,” Dr. Johnson explained. “There seems to be a growing recognition that this entity called long-haul COVID may be related to specific bacterial disruptions, and the more rapidly you can resolve these disruptions, the less likely you are to continue with long-haul symptoms.”
He said that, among people who have mild COVID, the virus typically clears and gut bacteria return to normal. With severe or persistent illness, gut dysbiosis persists, he said.
“People need to be aware that the GI tract is involved in a sizable percent of patients with COVID,” Dr. Johnson said. “The GI-tract testing may reflect that the virus is there, but persistence of the detectable test positivity is very unlikely to reflect active virus.”
The authors noted that they collected only six samples from the participants over the 10-month study period.
“Follow-up studies with more frequent sampling, especially in the first 2 months after diagnosis, may help build a more nuanced model of decline of fecal viral RNA concentration over time,” they wrote.
The study was supported by a Stanford ChemH-IMA grant, fellowships from the AACR and the National Science Foundation, and the National Institutes of Health. The authors and Dr. Johnson reported no relevant financial relationships. Dr. Johnson is a regular contributor to this news organization.
A version of this article first appeared on Medscape.com.
New data present further evidence that SARS-CoV-2 infection can settle in the gastrointestinal tract and that it can persist long after the infection has cleared the lungs.
Infection of the GI tract may figure prominently in long COVID, the study authors suggested.
Led by Aravind Natarajan, PhD, with the departments of genetics and medicine at Stanford (Calif.) University, they analyzed fecal RNA shedding up to 10 months after a COVID-19 diagnosis in 673 stool samples from 113 patients with mild to moderate disease.
They found that, in the week after diagnosis, COVID RNA remnants were present in the stool of approximately half (49.2%) of the patients. Seven months later, about 4% of them shed fecal viral RNA.
The authors noted that there was no ongoing SARS-CoV-2 RNA shedding in respiratory samples of patients at the 4-month mark.
Using self-reported symptoms regularly collected by questionnaire, they also found a correlation of long-term fecal shedding of SARS-CoV-2 RNA with abdominal pain, nausea, and vomiting.
The findings were published online in Med.
Implications of long-term viral shedding
Previous studies have found SARS-CoV-2 RNA in respiratory and fecal samples and have documented viral replication in lung and intestinal tissue. But before the current study, little had been known about long-term shedding, especially in those who have mild COVID. Most studies of viral shedding have been with severe COVID cases.
The authors noted that most studies of this kind are cross-sectional. The few other longitudinal studies have focused on early time points just after diagnosis.
Senior author Ami S. Bhatt, MD, associate professor in the departments of medicine and hematology at Stanford University, said in an interview that, though the viral genetic material in the feces lingers, on the basis of available evidence, it is highly unlikely to be contagious in most cases.
She said that understanding the dynamics of fecal shedding of SARS-CoV-2 genetic material will help interpret wastewater-based studies that are trying to determine population prevalence of the virus.
“While we don’t know the exact clinical importance of the longer-term shedding of SARS-CoV-2 in individuals with COVID-19, some have speculated that those who have long-term shedding of SARS-CoV-2 may have ongoing infections that might benefit from treatment,” she said.
“Our data support the idea that the long-term GI-related symptoms in some people might be the consequence of an ongoing infection in the GI tract, even after the respiratory infection has cleared,” Dr. Bhatt said.
“Alternatively, the presence of ongoing viral genetic material in the gut might be a trigger for the immune system to continually be active against the virus, and our immune system reaction may be the reason for long COVID–type symptoms,” she added. “This area is ripe for additional studies.”
Dr. Bhatt and colleagues will continue studying viral shedding in fecal samples as part of the nationwide RECOVER Initiative.
When reached for comment, David A. Johnson, MD, professor of medicine and chief of gastroenterology, Eastern Virginia Medical School, Norfolk, said in an interview that previous studies have indicated that the virus may be detected in the stool for a month or more and for about 2 weeks on average. Whether the virus is infectious has been in question.
But it’s not so much that the virus is infectious in the GI tract and causing symptoms, he said. Rather, there are biomic changes related to COVID, including a loss of diversity in the gut bacteria, which disrupts the balance.
“This may actually in some way predispose some patients to impaired clearance of their symptoms,” Dr. Johnson explained. “There seems to be a growing recognition that this entity called long-haul COVID may be related to specific bacterial disruptions, and the more rapidly you can resolve these disruptions, the less likely you are to continue with long-haul symptoms.”
He said that, among people who have mild COVID, the virus typically clears and gut bacteria return to normal. With severe or persistent illness, gut dysbiosis persists, he said.
“People need to be aware that the GI tract is involved in a sizable percent of patients with COVID,” Dr. Johnson said. “The GI-tract testing may reflect that the virus is there, but persistence of the detectable test positivity is very unlikely to reflect active virus.”
The authors noted that they collected only six samples from the participants over the 10-month study period.
“Follow-up studies with more frequent sampling, especially in the first 2 months after diagnosis, may help build a more nuanced model of decline of fecal viral RNA concentration over time,” they wrote.
The study was supported by a Stanford ChemH-IMA grant, fellowships from the AACR and the National Science Foundation, and the National Institutes of Health. The authors and Dr. Johnson reported no relevant financial relationships. Dr. Johnson is a regular contributor to this news organization.
A version of this article first appeared on Medscape.com.
New data present further evidence that SARS-CoV-2 infection can settle in the gastrointestinal tract and that it can persist long after the infection has cleared the lungs.
Infection of the GI tract may figure prominently in long COVID, the study authors suggested.
Led by Aravind Natarajan, PhD, with the departments of genetics and medicine at Stanford (Calif.) University, they analyzed fecal RNA shedding up to 10 months after a COVID-19 diagnosis in 673 stool samples from 113 patients with mild to moderate disease.
They found that, in the week after diagnosis, COVID RNA remnants were present in the stool of approximately half (49.2%) of the patients. Seven months later, about 4% of them shed fecal viral RNA.
The authors noted that there was no ongoing SARS-CoV-2 RNA shedding in respiratory samples of patients at the 4-month mark.
Using self-reported symptoms regularly collected by questionnaire, they also found a correlation of long-term fecal shedding of SARS-CoV-2 RNA with abdominal pain, nausea, and vomiting.
The findings were published online in Med.
Implications of long-term viral shedding
Previous studies have found SARS-CoV-2 RNA in respiratory and fecal samples and have documented viral replication in lung and intestinal tissue. But before the current study, little had been known about long-term shedding, especially in those who have mild COVID. Most studies of viral shedding have been with severe COVID cases.
The authors noted that most studies of this kind are cross-sectional. The few other longitudinal studies have focused on early time points just after diagnosis.
Senior author Ami S. Bhatt, MD, associate professor in the departments of medicine and hematology at Stanford University, said in an interview that, though the viral genetic material in the feces lingers, on the basis of available evidence, it is highly unlikely to be contagious in most cases.
She said that understanding the dynamics of fecal shedding of SARS-CoV-2 genetic material will help interpret wastewater-based studies that are trying to determine population prevalence of the virus.
“While we don’t know the exact clinical importance of the longer-term shedding of SARS-CoV-2 in individuals with COVID-19, some have speculated that those who have long-term shedding of SARS-CoV-2 may have ongoing infections that might benefit from treatment,” she said.
“Our data support the idea that the long-term GI-related symptoms in some people might be the consequence of an ongoing infection in the GI tract, even after the respiratory infection has cleared,” Dr. Bhatt said.
“Alternatively, the presence of ongoing viral genetic material in the gut might be a trigger for the immune system to continually be active against the virus, and our immune system reaction may be the reason for long COVID–type symptoms,” she added. “This area is ripe for additional studies.”
Dr. Bhatt and colleagues will continue studying viral shedding in fecal samples as part of the nationwide RECOVER Initiative.
When reached for comment, David A. Johnson, MD, professor of medicine and chief of gastroenterology, Eastern Virginia Medical School, Norfolk, said in an interview that previous studies have indicated that the virus may be detected in the stool for a month or more and for about 2 weeks on average. Whether the virus is infectious has been in question.
But it’s not so much that the virus is infectious in the GI tract and causing symptoms, he said. Rather, there are biomic changes related to COVID, including a loss of diversity in the gut bacteria, which disrupts the balance.
“This may actually in some way predispose some patients to impaired clearance of their symptoms,” Dr. Johnson explained. “There seems to be a growing recognition that this entity called long-haul COVID may be related to specific bacterial disruptions, and the more rapidly you can resolve these disruptions, the less likely you are to continue with long-haul symptoms.”
He said that, among people who have mild COVID, the virus typically clears and gut bacteria return to normal. With severe or persistent illness, gut dysbiosis persists, he said.
“People need to be aware that the GI tract is involved in a sizable percent of patients with COVID,” Dr. Johnson said. “The GI-tract testing may reflect that the virus is there, but persistence of the detectable test positivity is very unlikely to reflect active virus.”
The authors noted that they collected only six samples from the participants over the 10-month study period.
“Follow-up studies with more frequent sampling, especially in the first 2 months after diagnosis, may help build a more nuanced model of decline of fecal viral RNA concentration over time,” they wrote.
The study was supported by a Stanford ChemH-IMA grant, fellowships from the AACR and the National Science Foundation, and the National Institutes of Health. The authors and Dr. Johnson reported no relevant financial relationships. Dr. Johnson is a regular contributor to this news organization.
A version of this article first appeared on Medscape.com.
FROM MED
Can sensitivity to common smells sniff out depression, anxiety?
Sensitivity to specific common odors correlates with symptoms of depression or anxiety, new research shows.
A study of more than 400 participants showed that symptoms of anxiety were associated with heightened awareness of floral scents or kitchen smells, while depression was linked to increased awareness of social odors including “good” and “bad” smells of other people.
“The assessment of meta-cognitive abilities may be a useful tool in assessing depressive, anxiety, and social anxiety symptoms,” study investigator Cinzia Cecchetto, PhD student, postdoctoral researcher, department of general psychology, University of Padua, Italy, told this news organization.
The findings were published online in the Journal of Affective Disorders.
Smell perception
Previous studies have shown a strong relationship between reduced odor detection and symptoms of depression, with less clear evidence of a link between olfactory perception and symptoms of anxiety, Dr. Cecchetto said.
However, few studies have investigated to what extent individuals with symptoms of anxiety or depression are aware of, or pay attention to, odors in their environment, she added.
The study included 429 healthy participants (76.9% women, aged 18-45 years) recruited through social media. The age cut-off was 45 because evidence shows that olfactory perceptions start to decline at that time of life, Dr. Cecchetto noted.
Participants completed psychological questionnaires, including the Beck Depression Inventory-II, the Beck Anxiety Inventory, and the Liebowitz Social Anxiety Scale.
They also completed olfactory questionnaires, including the Odor Awareness Scale for evaluating the degree to which an individual focuses on olfactory stimuli such as that from food; the Affective Impact of Odor scale for assessing how odors affect liking and memory for people, places, and things; the Vividness of Olfactory Imagery Questionnaire, which examines ability to form olfactory images such as fragrances from a garden; and the Social Odor Scale, which assesses awareness of social odors such as sweat in everyday interactions.
Results showed that general anxiety symptoms were a significant predictor of higher levels of awareness of common odors.
The investigators note that this finding is similar to that from previous research in which patients with panic disorder reported higher olfactory sensitivity, reactivity, and awareness of odors compared with a control group. It is also in line with clinical features of anxiety, in which individuals maintain heightened vigilance, hyperarousal, and action readiness to respond to sudden danger.
Individuals with social anxiety symptoms reported being less attentive toward social odors.
This finding is at odds with the tendency of individuals with social anxiety to continuously monitor the environment for signs of potential negative evaluations by others.
In addition, it contradicts findings from previous studies showing that social anxiety is associated with enhanced startle reactivity and faster processing of social odor anxiety signals, compared with healthy controls, the investigators note.
Clinical implications?
“A possible explanation for these conflicting findings could be that in our study we didn’t present real odors to participants, but asked them if they usually pay attention to these odors,” lead author Elisa Dal Bò, PhD student, Padova Neuroscience Center and department of general psychology, University of Padua, told this news organization.
“Indeed, other studies have shown individuals with social anxiety focus their attention more on themselves and avoid paying attention to other people during social interactions,” she added.
Depressive symptoms were a significant predictor of higher social odor awareness and lower affective responses to odors. Some past studies showed that “depression is characterized by an increased attention to social stimuli induced by the fear of social rejection,” Ms. Dal Bò noted.
The current findings showing that depression symptoms were associated with higher social odor awareness while social anxiety symptoms were associated with lower social odor awareness are at odds with what was hypothesized, Dr. Cecchetto said.
“Actually we were expecting the opposite pattern, and that’s why it’s important to investigate more deeply these meta-cognitive abilities,” she said.
Neither depressive nor anxiety symptoms were significant predictors of olfactory imagery.
Female respondents were more attentive to, and aware of, odors than men. This finding is “quite common in the literature,” Dr. Cecchetto noted.
Although preliminary, the results could eventually have clinical implications, the investigators note. Olfactory metacognitive abilities, obtained through questionnaires, could be used to assess potential olfactory impairment, which could signal future risk for psychiatric symptoms.
However, the relatively young age of participants in the study and the prevalence of women limits the generalization of the findings, the researchers note. In addition, clinical symptoms were self-reported and were not verified by health care professionals.
‘Not enough evidence’
Commenting for this news organization, Philip R. Muskin, MD, professor of clinical psychiatry, Columbia University Medical Center, New York, said he did not find the results surprising.
For example, that individuals with social anxiety are not particularly aware of others’ smell “makes perfect sense” because “people with social anxiety disorder are concerned with themselves,” he said.
Dr. Muskin, who has an interest in and has written about olfactory function, was not involved with the research.
He noted several study limitations. First, participants just reported on their smell awareness, but “having people actually smell stuff might have been more interesting.”
In addition, the study population was relatively young, mostly women, and women’s olfactory sensitivity changes throughout the menstrual cycle, Dr. Muskin said.
“We don’t know where these women are in their cycles when they’re reporting their awareness of odors,” he said. “It would be good to know if the women were all in the luteal phase or were premenstrual because that might correlate with their anxiety or depressive symptoms.”
Asking a patient about smell awareness may provide some insight when assessing for symptoms of depression, along with obtaining details on such things as sleep, Dr. Muskin noted.
However, he does not think the new findings are enough to include olfactory awareness in the interview process. “It’s not enough evidence to use as a clinical tool for diagnosis, and I don’t see this is clinically useful yet.”
The study was supported by the European Commission Horizon 2020 research and innovation program and the Austrian Science Fund. The investigators and Dr. Muskin have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sensitivity to specific common odors correlates with symptoms of depression or anxiety, new research shows.
A study of more than 400 participants showed that symptoms of anxiety were associated with heightened awareness of floral scents or kitchen smells, while depression was linked to increased awareness of social odors including “good” and “bad” smells of other people.
“The assessment of meta-cognitive abilities may be a useful tool in assessing depressive, anxiety, and social anxiety symptoms,” study investigator Cinzia Cecchetto, PhD student, postdoctoral researcher, department of general psychology, University of Padua, Italy, told this news organization.
The findings were published online in the Journal of Affective Disorders.
Smell perception
Previous studies have shown a strong relationship between reduced odor detection and symptoms of depression, with less clear evidence of a link between olfactory perception and symptoms of anxiety, Dr. Cecchetto said.
However, few studies have investigated to what extent individuals with symptoms of anxiety or depression are aware of, or pay attention to, odors in their environment, she added.
The study included 429 healthy participants (76.9% women, aged 18-45 years) recruited through social media. The age cut-off was 45 because evidence shows that olfactory perceptions start to decline at that time of life, Dr. Cecchetto noted.
Participants completed psychological questionnaires, including the Beck Depression Inventory-II, the Beck Anxiety Inventory, and the Liebowitz Social Anxiety Scale.
They also completed olfactory questionnaires, including the Odor Awareness Scale for evaluating the degree to which an individual focuses on olfactory stimuli such as that from food; the Affective Impact of Odor scale for assessing how odors affect liking and memory for people, places, and things; the Vividness of Olfactory Imagery Questionnaire, which examines ability to form olfactory images such as fragrances from a garden; and the Social Odor Scale, which assesses awareness of social odors such as sweat in everyday interactions.
Results showed that general anxiety symptoms were a significant predictor of higher levels of awareness of common odors.
The investigators note that this finding is similar to that from previous research in which patients with panic disorder reported higher olfactory sensitivity, reactivity, and awareness of odors compared with a control group. It is also in line with clinical features of anxiety, in which individuals maintain heightened vigilance, hyperarousal, and action readiness to respond to sudden danger.
Individuals with social anxiety symptoms reported being less attentive toward social odors.
This finding is at odds with the tendency of individuals with social anxiety to continuously monitor the environment for signs of potential negative evaluations by others.
In addition, it contradicts findings from previous studies showing that social anxiety is associated with enhanced startle reactivity and faster processing of social odor anxiety signals, compared with healthy controls, the investigators note.
Clinical implications?
“A possible explanation for these conflicting findings could be that in our study we didn’t present real odors to participants, but asked them if they usually pay attention to these odors,” lead author Elisa Dal Bò, PhD student, Padova Neuroscience Center and department of general psychology, University of Padua, told this news organization.
“Indeed, other studies have shown individuals with social anxiety focus their attention more on themselves and avoid paying attention to other people during social interactions,” she added.
Depressive symptoms were a significant predictor of higher social odor awareness and lower affective responses to odors. Some past studies showed that “depression is characterized by an increased attention to social stimuli induced by the fear of social rejection,” Ms. Dal Bò noted.
The current findings showing that depression symptoms were associated with higher social odor awareness while social anxiety symptoms were associated with lower social odor awareness are at odds with what was hypothesized, Dr. Cecchetto said.
“Actually we were expecting the opposite pattern, and that’s why it’s important to investigate more deeply these meta-cognitive abilities,” she said.
Neither depressive nor anxiety symptoms were significant predictors of olfactory imagery.
Female respondents were more attentive to, and aware of, odors than men. This finding is “quite common in the literature,” Dr. Cecchetto noted.
Although preliminary, the results could eventually have clinical implications, the investigators note. Olfactory metacognitive abilities, obtained through questionnaires, could be used to assess potential olfactory impairment, which could signal future risk for psychiatric symptoms.
However, the relatively young age of participants in the study and the prevalence of women limits the generalization of the findings, the researchers note. In addition, clinical symptoms were self-reported and were not verified by health care professionals.
‘Not enough evidence’
Commenting for this news organization, Philip R. Muskin, MD, professor of clinical psychiatry, Columbia University Medical Center, New York, said he did not find the results surprising.
For example, that individuals with social anxiety are not particularly aware of others’ smell “makes perfect sense” because “people with social anxiety disorder are concerned with themselves,” he said.
Dr. Muskin, who has an interest in and has written about olfactory function, was not involved with the research.
He noted several study limitations. First, participants just reported on their smell awareness, but “having people actually smell stuff might have been more interesting.”
In addition, the study population was relatively young, mostly women, and women’s olfactory sensitivity changes throughout the menstrual cycle, Dr. Muskin said.
“We don’t know where these women are in their cycles when they’re reporting their awareness of odors,” he said. “It would be good to know if the women were all in the luteal phase or were premenstrual because that might correlate with their anxiety or depressive symptoms.”
Asking a patient about smell awareness may provide some insight when assessing for symptoms of depression, along with obtaining details on such things as sleep, Dr. Muskin noted.
However, he does not think the new findings are enough to include olfactory awareness in the interview process. “It’s not enough evidence to use as a clinical tool for diagnosis, and I don’t see this is clinically useful yet.”
The study was supported by the European Commission Horizon 2020 research and innovation program and the Austrian Science Fund. The investigators and Dr. Muskin have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sensitivity to specific common odors correlates with symptoms of depression or anxiety, new research shows.
A study of more than 400 participants showed that symptoms of anxiety were associated with heightened awareness of floral scents or kitchen smells, while depression was linked to increased awareness of social odors including “good” and “bad” smells of other people.
“The assessment of meta-cognitive abilities may be a useful tool in assessing depressive, anxiety, and social anxiety symptoms,” study investigator Cinzia Cecchetto, PhD student, postdoctoral researcher, department of general psychology, University of Padua, Italy, told this news organization.
The findings were published online in the Journal of Affective Disorders.
Smell perception
Previous studies have shown a strong relationship between reduced odor detection and symptoms of depression, with less clear evidence of a link between olfactory perception and symptoms of anxiety, Dr. Cecchetto said.
However, few studies have investigated to what extent individuals with symptoms of anxiety or depression are aware of, or pay attention to, odors in their environment, she added.
The study included 429 healthy participants (76.9% women, aged 18-45 years) recruited through social media. The age cut-off was 45 because evidence shows that olfactory perceptions start to decline at that time of life, Dr. Cecchetto noted.
Participants completed psychological questionnaires, including the Beck Depression Inventory-II, the Beck Anxiety Inventory, and the Liebowitz Social Anxiety Scale.
They also completed olfactory questionnaires, including the Odor Awareness Scale for evaluating the degree to which an individual focuses on olfactory stimuli such as that from food; the Affective Impact of Odor scale for assessing how odors affect liking and memory for people, places, and things; the Vividness of Olfactory Imagery Questionnaire, which examines ability to form olfactory images such as fragrances from a garden; and the Social Odor Scale, which assesses awareness of social odors such as sweat in everyday interactions.
Results showed that general anxiety symptoms were a significant predictor of higher levels of awareness of common odors.
The investigators note that this finding is similar to that from previous research in which patients with panic disorder reported higher olfactory sensitivity, reactivity, and awareness of odors compared with a control group. It is also in line with clinical features of anxiety, in which individuals maintain heightened vigilance, hyperarousal, and action readiness to respond to sudden danger.
Individuals with social anxiety symptoms reported being less attentive toward social odors.
This finding is at odds with the tendency of individuals with social anxiety to continuously monitor the environment for signs of potential negative evaluations by others.
In addition, it contradicts findings from previous studies showing that social anxiety is associated with enhanced startle reactivity and faster processing of social odor anxiety signals, compared with healthy controls, the investigators note.
Clinical implications?
“A possible explanation for these conflicting findings could be that in our study we didn’t present real odors to participants, but asked them if they usually pay attention to these odors,” lead author Elisa Dal Bò, PhD student, Padova Neuroscience Center and department of general psychology, University of Padua, told this news organization.
“Indeed, other studies have shown individuals with social anxiety focus their attention more on themselves and avoid paying attention to other people during social interactions,” she added.
Depressive symptoms were a significant predictor of higher social odor awareness and lower affective responses to odors. Some past studies showed that “depression is characterized by an increased attention to social stimuli induced by the fear of social rejection,” Ms. Dal Bò noted.
The current findings showing that depression symptoms were associated with higher social odor awareness while social anxiety symptoms were associated with lower social odor awareness are at odds with what was hypothesized, Dr. Cecchetto said.
“Actually we were expecting the opposite pattern, and that’s why it’s important to investigate more deeply these meta-cognitive abilities,” she said.
Neither depressive nor anxiety symptoms were significant predictors of olfactory imagery.
Female respondents were more attentive to, and aware of, odors than men. This finding is “quite common in the literature,” Dr. Cecchetto noted.
Although preliminary, the results could eventually have clinical implications, the investigators note. Olfactory metacognitive abilities, obtained through questionnaires, could be used to assess potential olfactory impairment, which could signal future risk for psychiatric symptoms.
However, the relatively young age of participants in the study and the prevalence of women limits the generalization of the findings, the researchers note. In addition, clinical symptoms were self-reported and were not verified by health care professionals.
‘Not enough evidence’
Commenting for this news organization, Philip R. Muskin, MD, professor of clinical psychiatry, Columbia University Medical Center, New York, said he did not find the results surprising.
For example, that individuals with social anxiety are not particularly aware of others’ smell “makes perfect sense” because “people with social anxiety disorder are concerned with themselves,” he said.
Dr. Muskin, who has an interest in and has written about olfactory function, was not involved with the research.
He noted several study limitations. First, participants just reported on their smell awareness, but “having people actually smell stuff might have been more interesting.”
In addition, the study population was relatively young, mostly women, and women’s olfactory sensitivity changes throughout the menstrual cycle, Dr. Muskin said.
“We don’t know where these women are in their cycles when they’re reporting their awareness of odors,” he said. “It would be good to know if the women were all in the luteal phase or were premenstrual because that might correlate with their anxiety or depressive symptoms.”
Asking a patient about smell awareness may provide some insight when assessing for symptoms of depression, along with obtaining details on such things as sleep, Dr. Muskin noted.
However, he does not think the new findings are enough to include olfactory awareness in the interview process. “It’s not enough evidence to use as a clinical tool for diagnosis, and I don’t see this is clinically useful yet.”
The study was supported by the European Commission Horizon 2020 research and innovation program and the Austrian Science Fund. The investigators and Dr. Muskin have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Are ants the future of cancer detection?
Cancer diagnosis is frightening, invasive, time-consuming, and expensive. And more than 1.6 million people get that cancer diagnosis every year in the United States. That’s a lot of biopsies and a lot of looking at cells under highly sensitive microscopes.
But imagine if detecting cancer in those samples was as simple as taking a whiff.
We know some animals – like dogs and mice – have very sensitive noses that can sniff out disease. Inspired by those studies, French scientists decided to explore whether ants – known for their olfactory prowess – could do the same.
“Using olfaction to detect diseases is not a novel idea,” says Baptiste Piqueret, PhD, a researcher at Sorbonne Paris Nord University and lead author of the study. “Knowing how well ants can learn and how they use olfaction, we tested the abilities of ants to learn and detect diseases.”
While this is still far away from real-life clinical use, it could one day lead to a cheaper, more accessible (if not a little weird) alternative for detecting cancer. What would this new diagnostic method look like?
Pavlov’s ant
Cancer cell metabolism produces volatile organic compounds (VOCs) – organic chemicals that smell and can serve as biomarkers for diagnosis.
To train the ants to target VOCs, the researchers placed breast cancer cells and healthy cells in a petri dish – but the cancer cells included a sugary treat. “We associated a reward to the smell of cancer,” Dr. Piqueret says.
It’s a technique scientists call classical, or Pavlovian, conditioning. A neutral stimulus (cancer smell) is associated with a second stimulus (food) that elicits a behavior. After doing this a few times, the ant learns that the first stimulus predicts the second, and it will seek out the odor hoping to find that food.
Once the training was complete, the researchers presented the ant with the learned odor and a novel one – this time without a reward. Sure enough, the ants spent more time investigating the learned odor than the novel one.
“If you are hungry and you smell the odor of fresh bread, you will enter the closest bakery,” says Dr. Piqueret. “This is the same mechanism the ants are using, as you learned that fresh bread odor equals food.”
Dogs can detect VOCs via the same technique but take months and hundreds of trials to condition, the researchers note. F. fusca ants learn fast, requiring only three training trials.
Why ants?
Ants communicate primarily through olfaction or scent, and this sophisticated “language” makes them very sensitive to odors.
“Since ants are already well-attuned to detecting different chemicals, this makes them ideal for scent recognition,” says Corrie Moreau, PhD, an evolutionary biologist and entomologist at Cornell University, Ithaca, N.Y.
In their tiny ant worlds, the little creatures use chemicals, called pheromones, to convey information to other members of their nest.
“There are alarm pheromones to signal an intruder, trail pheromones so an ant knows which way to walk to a food source, and colony-level odors that signal another ant is a member of the same colony,” Dr. Moreau says.
But on closer inspection, you won’t see a nose on an ant. They “smell” with their antennas.
“These specialized structures are covered with highly sensitive receptors to be able to discern even small chemical differences,” Dr. Moreau says.
There are over 14,000 species of ants and as far as scientists like Dr. Moreau know, all of them use chemical communication, though some are better than others at detecting compounds, such as those scientists are interested in using to detect disease.
Diagnostic ants: Realistic or a curiosity?
Whether or not the new research findings could lead to a real tool for diagnosing cancer is difficult to say, says Dr. Moreau. The study only focused on pure cancer cells in a lab and not those growing inside a human body.
Anna Wanda Komorowski, MD, a medical oncologist-hematologist at Northwell Health in New York, found the study interesting and was impressed with how the researchers trained the ants. But she notes more research would be needed to parse out things like how long the ants would remember their training, and how long they could be kept in a lab for testing.
One of the attractive aspects of the research is that if it worked it might be a cheaper alternative to normal lab practices for detecting cancer cells, and possibly useful in some low-income settings where labs do not have access to cell stain technologies used to detect cancer cells.
Another glitch with the study, notes Dr. Komorowski: “The cells we’d expose them to probably would not be the same cells as those used in the study. They exposed the ants to live cell cultures. Usually, we collect material from biopsy and drop it into formaldehyde, which has such a strong odor. So the lab protocol for cancer detection would have to be different. It could be kind of tricky.”
And while ants are cheaper than stains and dyes and formaldehyde, you’d have to hire someone to train the ants – there’d still be a human factor and related costs.
“It would take much more research to figure out cost, and how applicable and reproducible it would be,” Dr. Komorowski says.
And then there’s the question of whether the ants would do their cancer-detecting work in the lab only, or if direct patient interaction might lead to a diagnosis more swiftly.
Ant expert Dr. Moreau adds, “The human body emits many other odors, so the question is whether the ants would be able to ignore all the other scents and focus only on the target scent.”
“But these results are promising,” she continues. “I guess the question is whether a patient would be willing to have trained ants crawl all over their body looking for potential cancer cells.”
A version of this article first appeared on WebMD.com.
Cancer diagnosis is frightening, invasive, time-consuming, and expensive. And more than 1.6 million people get that cancer diagnosis every year in the United States. That’s a lot of biopsies and a lot of looking at cells under highly sensitive microscopes.
But imagine if detecting cancer in those samples was as simple as taking a whiff.
We know some animals – like dogs and mice – have very sensitive noses that can sniff out disease. Inspired by those studies, French scientists decided to explore whether ants – known for their olfactory prowess – could do the same.
“Using olfaction to detect diseases is not a novel idea,” says Baptiste Piqueret, PhD, a researcher at Sorbonne Paris Nord University and lead author of the study. “Knowing how well ants can learn and how they use olfaction, we tested the abilities of ants to learn and detect diseases.”
While this is still far away from real-life clinical use, it could one day lead to a cheaper, more accessible (if not a little weird) alternative for detecting cancer. What would this new diagnostic method look like?
Pavlov’s ant
Cancer cell metabolism produces volatile organic compounds (VOCs) – organic chemicals that smell and can serve as biomarkers for diagnosis.
To train the ants to target VOCs, the researchers placed breast cancer cells and healthy cells in a petri dish – but the cancer cells included a sugary treat. “We associated a reward to the smell of cancer,” Dr. Piqueret says.
It’s a technique scientists call classical, or Pavlovian, conditioning. A neutral stimulus (cancer smell) is associated with a second stimulus (food) that elicits a behavior. After doing this a few times, the ant learns that the first stimulus predicts the second, and it will seek out the odor hoping to find that food.
Once the training was complete, the researchers presented the ant with the learned odor and a novel one – this time without a reward. Sure enough, the ants spent more time investigating the learned odor than the novel one.
“If you are hungry and you smell the odor of fresh bread, you will enter the closest bakery,” says Dr. Piqueret. “This is the same mechanism the ants are using, as you learned that fresh bread odor equals food.”
Dogs can detect VOCs via the same technique but take months and hundreds of trials to condition, the researchers note. F. fusca ants learn fast, requiring only three training trials.
Why ants?
Ants communicate primarily through olfaction or scent, and this sophisticated “language” makes them very sensitive to odors.
“Since ants are already well-attuned to detecting different chemicals, this makes them ideal for scent recognition,” says Corrie Moreau, PhD, an evolutionary biologist and entomologist at Cornell University, Ithaca, N.Y.
In their tiny ant worlds, the little creatures use chemicals, called pheromones, to convey information to other members of their nest.
“There are alarm pheromones to signal an intruder, trail pheromones so an ant knows which way to walk to a food source, and colony-level odors that signal another ant is a member of the same colony,” Dr. Moreau says.
But on closer inspection, you won’t see a nose on an ant. They “smell” with their antennas.
“These specialized structures are covered with highly sensitive receptors to be able to discern even small chemical differences,” Dr. Moreau says.
There are over 14,000 species of ants and as far as scientists like Dr. Moreau know, all of them use chemical communication, though some are better than others at detecting compounds, such as those scientists are interested in using to detect disease.
Diagnostic ants: Realistic or a curiosity?
Whether or not the new research findings could lead to a real tool for diagnosing cancer is difficult to say, says Dr. Moreau. The study only focused on pure cancer cells in a lab and not those growing inside a human body.
Anna Wanda Komorowski, MD, a medical oncologist-hematologist at Northwell Health in New York, found the study interesting and was impressed with how the researchers trained the ants. But she notes more research would be needed to parse out things like how long the ants would remember their training, and how long they could be kept in a lab for testing.
One of the attractive aspects of the research is that if it worked it might be a cheaper alternative to normal lab practices for detecting cancer cells, and possibly useful in some low-income settings where labs do not have access to cell stain technologies used to detect cancer cells.
Another glitch with the study, notes Dr. Komorowski: “The cells we’d expose them to probably would not be the same cells as those used in the study. They exposed the ants to live cell cultures. Usually, we collect material from biopsy and drop it into formaldehyde, which has such a strong odor. So the lab protocol for cancer detection would have to be different. It could be kind of tricky.”
And while ants are cheaper than stains and dyes and formaldehyde, you’d have to hire someone to train the ants – there’d still be a human factor and related costs.
“It would take much more research to figure out cost, and how applicable and reproducible it would be,” Dr. Komorowski says.
And then there’s the question of whether the ants would do their cancer-detecting work in the lab only, or if direct patient interaction might lead to a diagnosis more swiftly.
Ant expert Dr. Moreau adds, “The human body emits many other odors, so the question is whether the ants would be able to ignore all the other scents and focus only on the target scent.”
“But these results are promising,” she continues. “I guess the question is whether a patient would be willing to have trained ants crawl all over their body looking for potential cancer cells.”
A version of this article first appeared on WebMD.com.
Cancer diagnosis is frightening, invasive, time-consuming, and expensive. And more than 1.6 million people get that cancer diagnosis every year in the United States. That’s a lot of biopsies and a lot of looking at cells under highly sensitive microscopes.
But imagine if detecting cancer in those samples was as simple as taking a whiff.
We know some animals – like dogs and mice – have very sensitive noses that can sniff out disease. Inspired by those studies, French scientists decided to explore whether ants – known for their olfactory prowess – could do the same.
“Using olfaction to detect diseases is not a novel idea,” says Baptiste Piqueret, PhD, a researcher at Sorbonne Paris Nord University and lead author of the study. “Knowing how well ants can learn and how they use olfaction, we tested the abilities of ants to learn and detect diseases.”
While this is still far away from real-life clinical use, it could one day lead to a cheaper, more accessible (if not a little weird) alternative for detecting cancer. What would this new diagnostic method look like?
Pavlov’s ant
Cancer cell metabolism produces volatile organic compounds (VOCs) – organic chemicals that smell and can serve as biomarkers for diagnosis.
To train the ants to target VOCs, the researchers placed breast cancer cells and healthy cells in a petri dish – but the cancer cells included a sugary treat. “We associated a reward to the smell of cancer,” Dr. Piqueret says.
It’s a technique scientists call classical, or Pavlovian, conditioning. A neutral stimulus (cancer smell) is associated with a second stimulus (food) that elicits a behavior. After doing this a few times, the ant learns that the first stimulus predicts the second, and it will seek out the odor hoping to find that food.
Once the training was complete, the researchers presented the ant with the learned odor and a novel one – this time without a reward. Sure enough, the ants spent more time investigating the learned odor than the novel one.
“If you are hungry and you smell the odor of fresh bread, you will enter the closest bakery,” says Dr. Piqueret. “This is the same mechanism the ants are using, as you learned that fresh bread odor equals food.”
Dogs can detect VOCs via the same technique but take months and hundreds of trials to condition, the researchers note. F. fusca ants learn fast, requiring only three training trials.
Why ants?
Ants communicate primarily through olfaction or scent, and this sophisticated “language” makes them very sensitive to odors.
“Since ants are already well-attuned to detecting different chemicals, this makes them ideal for scent recognition,” says Corrie Moreau, PhD, an evolutionary biologist and entomologist at Cornell University, Ithaca, N.Y.
In their tiny ant worlds, the little creatures use chemicals, called pheromones, to convey information to other members of their nest.
“There are alarm pheromones to signal an intruder, trail pheromones so an ant knows which way to walk to a food source, and colony-level odors that signal another ant is a member of the same colony,” Dr. Moreau says.
But on closer inspection, you won’t see a nose on an ant. They “smell” with their antennas.
“These specialized structures are covered with highly sensitive receptors to be able to discern even small chemical differences,” Dr. Moreau says.
There are over 14,000 species of ants and as far as scientists like Dr. Moreau know, all of them use chemical communication, though some are better than others at detecting compounds, such as those scientists are interested in using to detect disease.
Diagnostic ants: Realistic or a curiosity?
Whether or not the new research findings could lead to a real tool for diagnosing cancer is difficult to say, says Dr. Moreau. The study only focused on pure cancer cells in a lab and not those growing inside a human body.
Anna Wanda Komorowski, MD, a medical oncologist-hematologist at Northwell Health in New York, found the study interesting and was impressed with how the researchers trained the ants. But she notes more research would be needed to parse out things like how long the ants would remember their training, and how long they could be kept in a lab for testing.
One of the attractive aspects of the research is that if it worked it might be a cheaper alternative to normal lab practices for detecting cancer cells, and possibly useful in some low-income settings where labs do not have access to cell stain technologies used to detect cancer cells.
Another glitch with the study, notes Dr. Komorowski: “The cells we’d expose them to probably would not be the same cells as those used in the study. They exposed the ants to live cell cultures. Usually, we collect material from biopsy and drop it into formaldehyde, which has such a strong odor. So the lab protocol for cancer detection would have to be different. It could be kind of tricky.”
And while ants are cheaper than stains and dyes and formaldehyde, you’d have to hire someone to train the ants – there’d still be a human factor and related costs.
“It would take much more research to figure out cost, and how applicable and reproducible it would be,” Dr. Komorowski says.
And then there’s the question of whether the ants would do their cancer-detecting work in the lab only, or if direct patient interaction might lead to a diagnosis more swiftly.
Ant expert Dr. Moreau adds, “The human body emits many other odors, so the question is whether the ants would be able to ignore all the other scents and focus only on the target scent.”
“But these results are promising,” she continues. “I guess the question is whether a patient would be willing to have trained ants crawl all over their body looking for potential cancer cells.”
A version of this article first appeared on WebMD.com.




