User login
Easing dementia caregiver burden, addressing interpersonal violence
The number of people with dementia globally is expected to reach 74.7 million by 2030 and 131.5 million by 2050.1 Because dementia is progressive, many patients will exhibit severe symptoms termed behavioral crises. Deteriorating interpersonal conduct and escalating antisocial acts result in an acquired sociopathy.2 Increasing cognitive impairment causes these patients to misunderstand intimate care and perceive it as a threat, often resulting in outbursts of violence against their caregivers.3
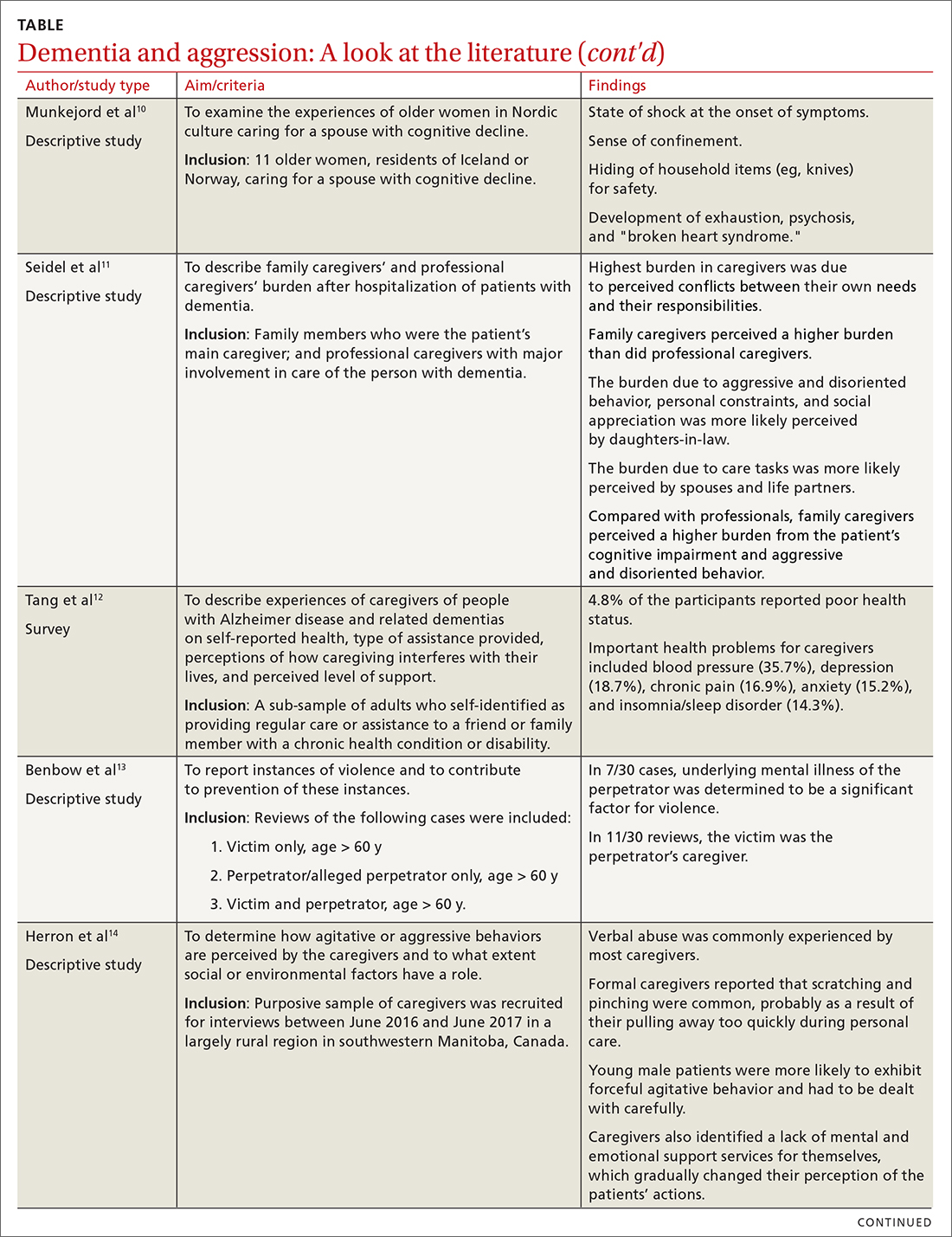
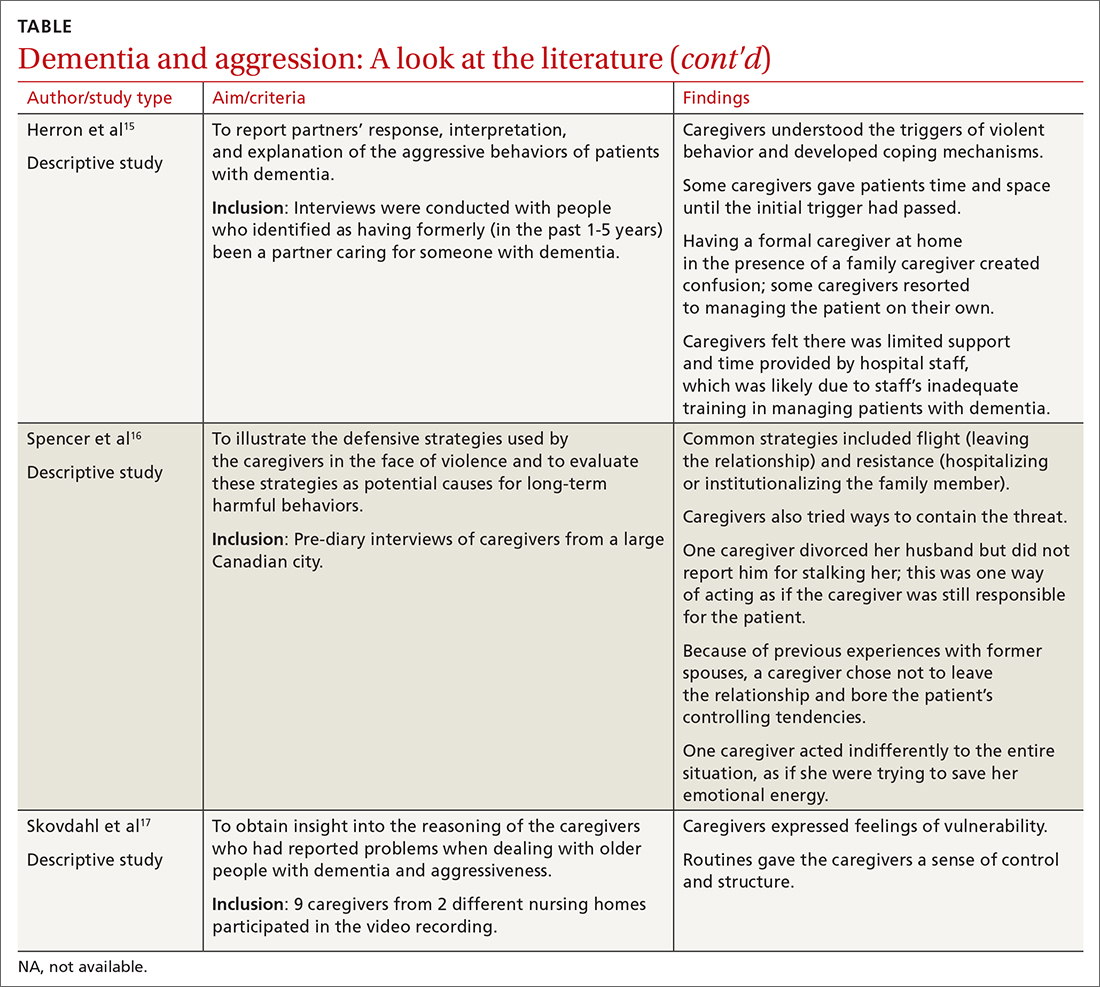
Available studies (TABLE4-17) make evident the incidence of interpersonal violence experienced by caregivers secondary to aggressive acts by patients with dementia. This violence ranges from verbal abuse, including racial slurs, to physical abuse—sometimes resulting in significant physical injury. Aggressive behavior by patients with dementia, resulting in violence towards their caregivers or partners, stems from progressive cognitive decline, which can make optimal care difficult. Such episodes may also impair the psychological and physical well-being of caregivers, increasing their risk of depression, anxiety, and even post-traumatic stress disorder (PTSD).18 The extent of the impact is also determined by the interpretation of the abuse by the caregivers themselves. One study suggested that the perception of aggressive or violent behavior as “normal” by a caregiver reduced the overall negative effect of the interactions.7Our review emphasizes the unintended burden that can fall to caregivers of patients with dementia. We also address the role of primary care providers (PCPs) in identifying these instances of violence and intervening appropriately by providing safety strategies, education, resources, and support.
CASE
A 67-year-old man with a medical history of PTSD with depression, type 2 diabetes, alcohol use disorder/dependence, hypertension, and obstructive sleep apnea was brought to his PCP by his wife. She said he had recently been unable to keep appointment times, pay bills, or take his usual medications, venlafaxine and bupropion. She also said his PTSD symptoms had worsened. He was sleeping 12 to 14 hours per day and was increasingly irritable. The patient denied any concerns or changes in his behavior.
The PCP administered a Saint Louis University Mental Status (SLUMS) examination to screen for cognitive impairment.19 The patient scored 14/30 (less than 20 is indicative of dementia). He was unable to complete a simple math problem, recall any items from a list of 5, count in reverse, draw a clock correctly, or recall a full story. Throughout the exam, the patient demonstrated minimal effort and was often only able to complete a task after further prompting by the examiner.
A computed tomography scan of the head revealed no signs of hemorrhage or damage. Thyroid-stimulating hormone levels and vitamin B12 levels were normal. A rapid plasma reagin test result was negative. The patient was given a diagnosis of Alzheimer disease. Donepezil was added to the patient’s medications, starting at 5 mg and then increased to 10 mg. His wife began to assist him with his tasks of daily living. His mood improved, and his wife noted he began to remember his appointments and take his medications with assistance.
However, the patient’s irritability continued to escalate. He grew paranoid and accused his wife of mismanaging their money. This pattern steadily worsened over the course of 6 months. The situation escalated until one day the patient’s wife called a mental health hotline reporting that her husband was holding her hostage and threatening to kill her with a gun. He told her, “I can do something to you, and they won’t even find a fingernail. It doesn’t have to be with a gun either.” She was counseled to try to stay calm to avoid aggravating the situation and to go to a safe place and stay there until help arrived.
His memory had worsened to the point that he could not recall any events from the previous 2 years. He was paranoid about anyone entering his home and would not allow his deteriorating roof to be repaired or his yard to be maintained. He did not shower for weeks at a time. He slept holding a rifle and accused his wife of embezzlement.
Continue to: The patient was evaluated...
The patient was evaluated by another specialist, who assessed his SLUMS score to be 18/30. He increased the patient’s donepezil dose, initiated a bupropion taper, and added sertraline to the regimen. The PCP spoke to the patient’s wife regarding options for her safety including leaving the home, hiding firearms, and calling the police in cases of interpersonal violence. The wife said she did not want to pursue these options. She expressed worry that he might be harmed if he was uncooperative with the police and said there was no one except her to take care of him.
Caregivers struggle to care for their loved ones
Instances of personal violence lead to shock, astonishment, heartbreak, and fear. Anticipation of a recurrence of violence causes many partners and caregivers to feel exhausted, because there is minimal hope for any chance of improvement. There are a few exceptions, however, as our case will show. In addition to emotional exhaustion, there is also a never-ending sense of self-doubt, leading many caregivers to question their ability to handle their family member.20,21 Over time, this leads to caregiver burnout, leaving them unable to understand their family member’s aggression. The sudden loss of caregiver control in dealing with the patient may also result in the family member exhibiting behavioral changes reflecting emotional trauma. For caregivers who do not live with the patient, they may choose to make fewer or shorter visits—or not visit at all—because they fear being abused.7,22
Caregivers of patients with dementia often feel helpless and powerless once abrupt and drastic changes in personality lead to some form of interpersonal violence. Additionally, caregivers with a poor health status are more likely to have lower physical function and experience greater caregiving stress overall.23 Other factors increasing stress are longer years of caregiving and the severity of a patient’s dementia and functional impairment.23
Interventions to reduce caregiver burden
Many studies have assessed the role of different interventions to reduce caregiver burden, such as teaching them problem-solving skills, increasing their knowledge of dementia, recommending social resources, providing emotional support, changing caregiver perceptions of the care situation, introducing coping strategies, relying on strengths and experiences in caregiving, help-seeking, and engaging in activity programs.24-28 For Hispanic caregivers, a structured and self-paced online telenovela format has been effective in improving care and relieving caregiver stress.29 Online positive emotion regulators helped in significantly improving quality of life and physical health in the caregivers.30 In this last intervention, caregivers had 6 online sessions with a facilitator who taught them emotional regulation skills that included: noticing positive events, capitalizing on them, and feeling gratitude; practicing mindfulness; doing a positive reappraisal; acknowledging personal strengths and setting attainable goals; and performing acts of kindness. Empowerment programs have also shown significant improvement in the well-being of caregivers.31
Caregivers may reject support.
Continue to: These practical tips can help
These practical tips can help
Based on our review of the literature, we recommend offering the following supports to caregivers:
- Counsel caregivers early on in a patient’s dementia that behavior changes are likely and may be unpredictable. Explain that dementia can involve changes to personality and behavior as well as memory difficulties.33,34
- Describe resources for support, such as day programs for senior adults, insurance coverage for caregiver respite programs, and the Alzheimer’s Association (www.alz.org/). Encourage caregivers to seek general medical and mental health care for themselves. Caregivers should have opportunities and support to discuss their experiences and to be appropriately trained for the challenge of caring for a family member with dementia.35
- Encourage disclosure about abrupt changes in the patient’s behavior. This invites families to discuss issues with you and may make them more comfortable with such conversations.
- Involve ancillary services (eg, social worker) to plan for a higher level of care well in advance of it becoming necessary.
- Discuss safety strategies for the caregiver, including when it is appropriate to alter a patient’s set routines such as bedtimes and mealtimes.33,34
- Discuss when and how to involve law enforcement, if necessary.33,34 Emphasize the importance of removing firearms from the home as a safety measure. Although federal laws do not explicitly prohibit possession of arms by patients with neurologic damage, a few states mention “organic brain syndrome” or “dementia” as conditions prohibiting use or possession of firearms.36
- Suggest, as feasible, nonpharmacologic aids for the patient such as massage therapy, animal-assisted therapy, personalized interventions, music therapy, and light therapy.37 Prescribe medications to the patient to aid in behavior modification when appropriate.
- Screen caregivers and family members for signs of interpersonal violence. Take notice of changes in caregiver behavior or irregularity in attending follow-up appointments.
CASE
Over the next month, the patient’s symptoms further deteriorated. His PCP recommended hospitalization, but the patient and his wife declined. Magnetic resonance imaging of the patient’s brain revealed severe confluent and patchy regions of white matter and T2 signal hyperintensity, consistent with chronic microvascular ischemic disease. An old, small, left parietal lobe infarct was also noted.
One month later, the patient presented to the emergency department. His symptoms were largely unchanged, but his wife indicated that she could no longer live at home due to burnout. The patient’s medications were adjusted, but he was not admitted for inpatient care. His wife said they needed help at home, but the patient opposed the idea any time that it was mentioned.
A few weeks later, the patient presented for outpatient follow-up. He was delusional, believing that the government was compelling citizens to take sertraline in order to harm their mental health. He had also begun viewing online pornography in front of his wife and attempting to remove all of his money from the bank. He was prescribed aripiprazole 15 mg, and his symptoms began to improve. Soon after, however, he threatened to kill his grandson, then took all his Lasix pills (a 7-day supply) simultaneously. The patient denied that this was a suicide attempt.
Over the course of the next month, the patient began to report hearing voices. A neuropsychological evaluation confirmed a diagnosis of dementia with psychiatric symptoms due to neurologic injury. The patient was referred to a geriatric psychiatrist and continued to be managed medically. He was assigned a multidisciplinary team comprising palliative care, social work, and care management to assist in his care and provide support to the family. His behavior improved.
Continue to: At the time of this publication...
At the time of this publication, the patient’s irritability and paranoia had subsided and he had made no further threats to his family. He has allowed a home health aide into the house and has agreed to have his roof repaired. His wife still lives with him and assists him with activities of daily living.
Interprofessional teams are key
Caregiver burnout increases the risk of patient neglect or abuse, as individuals who have been the targets of aggressive behavior are more likely to leave demented patients unattended.8,16,23 Although tools are available to screen caregivers for depression and burnout, an important step forward would be to develop an interprofessional team to aid in identifying and closely following high-risk patient–caregiver groups. This continual and varied assessment of psychosocial stressors could help prevent the development of violent interactions. These teams would allow integration with the primary health care system by frequent and effective shared communication of knowledge, development of goals, and shared decision-making.38 Setting expectations, providing support, and discussing safety strategies can improve the health and welfare of caregivers and patients with dementia alike.
CORRESPONDENCE
Abu Baker Sheikh, MD, MSC 10-5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected].
1. Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol. 2017;13:327-339.
2. Cipriani G, Borin G, Vedovello M, et al. Sociopathic behavior and dementia. Acta Neurol Belg. 2013;113:111-115.
3. Cipriani G, Lucetti C, Danti S, et al. Violent and criminal manifestations in dementia patients. Geriatr Gerontol Int. 2016;16:541-549.
4. Skovdahl K, Kihlgren AL, Kihlgren M. Different attitudes when handling aggressive behaviour in dementia—narratives from two caregiver groups. Aging Ment Health. 2003;7:277-286.
5. Kristiansen L, Hellzén O, Asplund K. Swedish assistant nurses’ experiences of job satisfaction when caring for persons suffering from dementia and behavioural disturbances. An interview study. Int J Qualitat Stud Health Well-being. 2006;1:245-256.
6. Wharton TC, Ford BK. What is known about dementia care recipient violence and aggression against caregivers? J Gerontol Soc Work. 2014;57:460-477.
7. Ostaszkiewicz J, Lakhan P, O’Connell B, et al. Ongoing challenges responding to behavioural and psychological symptoms of dementia. Int Nurs Rev. 2015;62:506-516.
8. Kim J, De Bellis AM, Xiao LD. The experience of paid family-care workers of people with dementia in South Korea. Asian Nurs Res (Korean Soc Nurs Sci). 2018;12:34-41.
9. Band-Winterstein T, Avieli H. Women coping with a partner’s dementia-related violence: a qualitative study. J Nurs Scholarsh. 2019; 51:368-379.
10. Munkejord MC, Stefansdottir OA, Sveinbjarnardottir EK. Who cares for the carer? The suffering, struggles and unmet needs of older women caring for husbands living with cognitive decline. Int Pract Devel J. 2020;10:1-11.
11. Seidel D, Thyrian JR. Burden of caring for people with dementia - comparing family caregivers and professional caregivers. A descriptive study. J Multidiscip Healthc. 2019;12:655-663.
12. Tang W, Friedman DB, Kannaley K, et al. Experiences of caregivers by care recipient’s health condition: a study of caregivers for Alzheimer’s disease and related dementias versus other chronic conditions. Geriatr Nurs. 2019;40:181-184.
13. Benbow SM, Bhattacharyya S, Kingston P. Older adults and violence: an analysis of domestic homicide reviews in England involving adults over 60 years of age. Ageing Soc. 2018;39:1097-1121.
14. Herron RV, Wrathall MA. Putting responsive behaviours in place: examining how formal and informal carers understand the actions of people with dementia. Soc Sci Med. 2018;204:9-15.
15. Herron RV, Rosenberg MW. Responding to aggression and reactive behaviours in the home. Dementia (London). 2019;18:1328-1340.
16. Spencer D, Funk LM, Herron RV, et al. Fear, defensive strategies and caring for cognitively impaired family members. J Gerontol Soc Work. 2019;62:67-85.
17. Skovdahl K, Kihlgren AL, Kihlgren M. Dementia and aggressiveness: stimulated recall interviews with caregivers after video-recorded interactions. J Clin Nurs. 2004;13:515-525.
18. Needham I, Abderhalden C, Halfens RJ, et al. Non-somatic effects of patient aggression on nurses: a systematic review. J Adv Nurs. 2005;49:283-296.
19. Tariq SH, Tumosa N, Chibnall JT, et al. The Saint Louis University Mental Status (SLUMS) Examination for detecting mild cognitive impairment and dementia is more sensitive than the Mini-Mental Status Examination (MMSE) - a pilot study. Am J Geriatr Psych. 2006;14:900-910.
20. Janzen S, Zecevic AA, Kloseck M, et al. Managing agitation using nonpharmacological interventions for seniors with dementia. Am J Alzheimers Dis Other Demen. 2013;28:524-532.
21. Zeller A, Dassen T, Kok G, et al. Nursing home caregivers’ explanations for and coping strategies with residents’ aggression: a qualitative study. J Clin Nurs. 2011;20:2469-2478.
22. Alzheimer’s Society. Fix dementia care: homecare. Accessed December 28, 2021. https://www.alzheimers.org.uk/sites/default/files/migrate/downloads/fix_dementia_care_homecare_report.pdf
23. von Känel R, Mausbach BT, Dimsdale JE, et al. Refining caregiver vulnerability for clinical practice: determinants of self-rated health in spousal dementia caregivers. BMC Geriatr. 2019;19:18.
24. Chen HM, Huang MF, Yeh YC, et al. Effectiveness of coping strategies intervention on caregiver burden among caregivers of elderly patients with dementia. Psychogeriatrics. 2015; 15:20-25.
25. Wawrziczny E, Larochette C, Papo D, et al. A customized intervention for dementia caregivers: a quasi-experimental design. J Aging Health. 2019;31:1172-1195.
26. Gitlin LN, Piersol CV, Hodgson N, et al. Reducing neuropsychiatric symptoms in persons with dementia and associated burden in family caregivers using tailored activities: Design and methods of a randomized clinical trial. Contemp Clin Trials. 2016;49:92-102.
27. de Oliveira AM, Radanovic M, Homem de Mello PC, et al. An intervention to reduce neuropsychiatric symptoms and caregiver burden in dementia: preliminary results from a randomized trial of the tailored activity program-outpatient version. Int J Geriatr Psychiatry. 2019;34:1301-1307.
28. Livingston G, Barber J, Rapaport P, et al. Clinical effectiveness of a manual based coping strategy programme (START, STrAtegies for RelaTives) in promoting the mental health of carers of family members with dementia: pragmatic randomised controlled trial. BMJ. 2013;347:f6276.
29. Kajiyama B, Fernandez G, Carter EA, et al. Helping Hispanic dementia caregivers cope with stress using technology-based resources. Clin Gerontol. 2018;41:209-216.
30. Moskowitz JT, Cheung EO, Snowberg KE, et al. Randomized controlled trial of a facilitated online positive emotion regulation intervention for dementia caregivers. Health Psychol. 2019;38:391-402.
31. Yoon HK, Kim GS. An empowerment program for family caregivers of people with dementia. Public Health Nurs. 2020;37:222-233.
32. Zwingmann I, Dreier-Wolfgramm A, Esser A, et al. Why do family dementia caregivers reject caregiver support services? Analyzing types of rejection and associated health-impairments in a cluster-randomized controlled intervention trial. BMC Health Serv Res. 2020;20:121.
33. Nybakken S, Strandås M, Bondas T. Caregivers’ perceptions of aggressive behaviour in nursing home residents living with dementia: A meta-ethnography. J Adv Nurs. 2018;74:2713-2726.
34. Nakaishi L, Moss H, Weinstein M, et al. Exploring workplace violence among home care workers in a consumer-driven home health care program. Workplace Health Saf. 2013;61:441-450.
35. Medical Advisory Secretariat. Caregiver- and patient-directed interventions for dementia: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8:1-98.
36. Betz ME, McCourt AD, Vernick JS, et al. Firearms and dementia: clinical considerations. Ann Intern Med. 2018;169:47-49.
37. Leng M, Zhao Y, Wang Z. Comparative efficacy of non-pharmacological interventions on agitation in people with dementia: a systematic review and Bayesian network meta-analysis. Int J Nurs Stud. 2020;102:103489.
38. Morgan S, Pullon S, McKinlay E. Observation of interprofessional collaborative practice in primary care teams: an integrative literature review. Int J Nurs Stud. 2015;52:1217-1230.
The number of people with dementia globally is expected to reach 74.7 million by 2030 and 131.5 million by 2050.1 Because dementia is progressive, many patients will exhibit severe symptoms termed behavioral crises. Deteriorating interpersonal conduct and escalating antisocial acts result in an acquired sociopathy.2 Increasing cognitive impairment causes these patients to misunderstand intimate care and perceive it as a threat, often resulting in outbursts of violence against their caregivers.3
Available studies (TABLE4-17) make evident the incidence of interpersonal violence experienced by caregivers secondary to aggressive acts by patients with dementia. This violence ranges from verbal abuse, including racial slurs, to physical abuse—sometimes resulting in significant physical injury. Aggressive behavior by patients with dementia, resulting in violence towards their caregivers or partners, stems from progressive cognitive decline, which can make optimal care difficult. Such episodes may also impair the psychological and physical well-being of caregivers, increasing their risk of depression, anxiety, and even post-traumatic stress disorder (PTSD).18 The extent of the impact is also determined by the interpretation of the abuse by the caregivers themselves. One study suggested that the perception of aggressive or violent behavior as “normal” by a caregiver reduced the overall negative effect of the interactions.7Our review emphasizes the unintended burden that can fall to caregivers of patients with dementia. We also address the role of primary care providers (PCPs) in identifying these instances of violence and intervening appropriately by providing safety strategies, education, resources, and support.

CASE
A 67-year-old man with a medical history of PTSD with depression, type 2 diabetes, alcohol use disorder/dependence, hypertension, and obstructive sleep apnea was brought to his PCP by his wife. She said he had recently been unable to keep appointment times, pay bills, or take his usual medications, venlafaxine and bupropion. She also said his PTSD symptoms had worsened. He was sleeping 12 to 14 hours per day and was increasingly irritable. The patient denied any concerns or changes in his behavior.
The PCP administered a Saint Louis University Mental Status (SLUMS) examination to screen for cognitive impairment.19 The patient scored 14/30 (less than 20 is indicative of dementia). He was unable to complete a simple math problem, recall any items from a list of 5, count in reverse, draw a clock correctly, or recall a full story. Throughout the exam, the patient demonstrated minimal effort and was often only able to complete a task after further prompting by the examiner.

A computed tomography scan of the head revealed no signs of hemorrhage or damage. Thyroid-stimulating hormone levels and vitamin B12 levels were normal. A rapid plasma reagin test result was negative. The patient was given a diagnosis of Alzheimer disease. Donepezil was added to the patient’s medications, starting at 5 mg and then increased to 10 mg. His wife began to assist him with his tasks of daily living. His mood improved, and his wife noted he began to remember his appointments and take his medications with assistance.

However, the patient’s irritability continued to escalate. He grew paranoid and accused his wife of mismanaging their money. This pattern steadily worsened over the course of 6 months. The situation escalated until one day the patient’s wife called a mental health hotline reporting that her husband was holding her hostage and threatening to kill her with a gun. He told her, “I can do something to you, and they won’t even find a fingernail. It doesn’t have to be with a gun either.” She was counseled to try to stay calm to avoid aggravating the situation and to go to a safe place and stay there until help arrived.
His memory had worsened to the point that he could not recall any events from the previous 2 years. He was paranoid about anyone entering his home and would not allow his deteriorating roof to be repaired or his yard to be maintained. He did not shower for weeks at a time. He slept holding a rifle and accused his wife of embezzlement.
Continue to: The patient was evaluated...
The patient was evaluated by another specialist, who assessed his SLUMS score to be 18/30. He increased the patient’s donepezil dose, initiated a bupropion taper, and added sertraline to the regimen. The PCP spoke to the patient’s wife regarding options for her safety including leaving the home, hiding firearms, and calling the police in cases of interpersonal violence. The wife said she did not want to pursue these options. She expressed worry that he might be harmed if he was uncooperative with the police and said there was no one except her to take care of him.
Caregivers struggle to care for their loved ones
Instances of personal violence lead to shock, astonishment, heartbreak, and fear. Anticipation of a recurrence of violence causes many partners and caregivers to feel exhausted, because there is minimal hope for any chance of improvement. There are a few exceptions, however, as our case will show. In addition to emotional exhaustion, there is also a never-ending sense of self-doubt, leading many caregivers to question their ability to handle their family member.20,21 Over time, this leads to caregiver burnout, leaving them unable to understand their family member’s aggression. The sudden loss of caregiver control in dealing with the patient may also result in the family member exhibiting behavioral changes reflecting emotional trauma. For caregivers who do not live with the patient, they may choose to make fewer or shorter visits—or not visit at all—because they fear being abused.7,22
Caregivers of patients with dementia often feel helpless and powerless once abrupt and drastic changes in personality lead to some form of interpersonal violence. Additionally, caregivers with a poor health status are more likely to have lower physical function and experience greater caregiving stress overall.23 Other factors increasing stress are longer years of caregiving and the severity of a patient’s dementia and functional impairment.23
Interventions to reduce caregiver burden
Many studies have assessed the role of different interventions to reduce caregiver burden, such as teaching them problem-solving skills, increasing their knowledge of dementia, recommending social resources, providing emotional support, changing caregiver perceptions of the care situation, introducing coping strategies, relying on strengths and experiences in caregiving, help-seeking, and engaging in activity programs.24-28 For Hispanic caregivers, a structured and self-paced online telenovela format has been effective in improving care and relieving caregiver stress.29 Online positive emotion regulators helped in significantly improving quality of life and physical health in the caregivers.30 In this last intervention, caregivers had 6 online sessions with a facilitator who taught them emotional regulation skills that included: noticing positive events, capitalizing on them, and feeling gratitude; practicing mindfulness; doing a positive reappraisal; acknowledging personal strengths and setting attainable goals; and performing acts of kindness. Empowerment programs have also shown significant improvement in the well-being of caregivers.31
Caregivers may reject support.
Continue to: These practical tips can help
These practical tips can help
Based on our review of the literature, we recommend offering the following supports to caregivers:
- Counsel caregivers early on in a patient’s dementia that behavior changes are likely and may be unpredictable. Explain that dementia can involve changes to personality and behavior as well as memory difficulties.33,34
- Describe resources for support, such as day programs for senior adults, insurance coverage for caregiver respite programs, and the Alzheimer’s Association (www.alz.org/). Encourage caregivers to seek general medical and mental health care for themselves. Caregivers should have opportunities and support to discuss their experiences and to be appropriately trained for the challenge of caring for a family member with dementia.35
- Encourage disclosure about abrupt changes in the patient’s behavior. This invites families to discuss issues with you and may make them more comfortable with such conversations.
- Involve ancillary services (eg, social worker) to plan for a higher level of care well in advance of it becoming necessary.
- Discuss safety strategies for the caregiver, including when it is appropriate to alter a patient’s set routines such as bedtimes and mealtimes.33,34
- Discuss when and how to involve law enforcement, if necessary.33,34 Emphasize the importance of removing firearms from the home as a safety measure. Although federal laws do not explicitly prohibit possession of arms by patients with neurologic damage, a few states mention “organic brain syndrome” or “dementia” as conditions prohibiting use or possession of firearms.36
- Suggest, as feasible, nonpharmacologic aids for the patient such as massage therapy, animal-assisted therapy, personalized interventions, music therapy, and light therapy.37 Prescribe medications to the patient to aid in behavior modification when appropriate.
- Screen caregivers and family members for signs of interpersonal violence. Take notice of changes in caregiver behavior or irregularity in attending follow-up appointments.
CASE
Over the next month, the patient’s symptoms further deteriorated. His PCP recommended hospitalization, but the patient and his wife declined. Magnetic resonance imaging of the patient’s brain revealed severe confluent and patchy regions of white matter and T2 signal hyperintensity, consistent with chronic microvascular ischemic disease. An old, small, left parietal lobe infarct was also noted.
One month later, the patient presented to the emergency department. His symptoms were largely unchanged, but his wife indicated that she could no longer live at home due to burnout. The patient’s medications were adjusted, but he was not admitted for inpatient care. His wife said they needed help at home, but the patient opposed the idea any time that it was mentioned.
A few weeks later, the patient presented for outpatient follow-up. He was delusional, believing that the government was compelling citizens to take sertraline in order to harm their mental health. He had also begun viewing online pornography in front of his wife and attempting to remove all of his money from the bank. He was prescribed aripiprazole 15 mg, and his symptoms began to improve. Soon after, however, he threatened to kill his grandson, then took all his Lasix pills (a 7-day supply) simultaneously. The patient denied that this was a suicide attempt.
Over the course of the next month, the patient began to report hearing voices. A neuropsychological evaluation confirmed a diagnosis of dementia with psychiatric symptoms due to neurologic injury. The patient was referred to a geriatric psychiatrist and continued to be managed medically. He was assigned a multidisciplinary team comprising palliative care, social work, and care management to assist in his care and provide support to the family. His behavior improved.
Continue to: At the time of this publication...
At the time of this publication, the patient’s irritability and paranoia had subsided and he had made no further threats to his family. He has allowed a home health aide into the house and has agreed to have his roof repaired. His wife still lives with him and assists him with activities of daily living.
Interprofessional teams are key
Caregiver burnout increases the risk of patient neglect or abuse, as individuals who have been the targets of aggressive behavior are more likely to leave demented patients unattended.8,16,23 Although tools are available to screen caregivers for depression and burnout, an important step forward would be to develop an interprofessional team to aid in identifying and closely following high-risk patient–caregiver groups. This continual and varied assessment of psychosocial stressors could help prevent the development of violent interactions. These teams would allow integration with the primary health care system by frequent and effective shared communication of knowledge, development of goals, and shared decision-making.38 Setting expectations, providing support, and discussing safety strategies can improve the health and welfare of caregivers and patients with dementia alike.
CORRESPONDENCE
Abu Baker Sheikh, MD, MSC 10-5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected].
The number of people with dementia globally is expected to reach 74.7 million by 2030 and 131.5 million by 2050.1 Because dementia is progressive, many patients will exhibit severe symptoms termed behavioral crises. Deteriorating interpersonal conduct and escalating antisocial acts result in an acquired sociopathy.2 Increasing cognitive impairment causes these patients to misunderstand intimate care and perceive it as a threat, often resulting in outbursts of violence against their caregivers.3
Available studies (TABLE4-17) make evident the incidence of interpersonal violence experienced by caregivers secondary to aggressive acts by patients with dementia. This violence ranges from verbal abuse, including racial slurs, to physical abuse—sometimes resulting in significant physical injury. Aggressive behavior by patients with dementia, resulting in violence towards their caregivers or partners, stems from progressive cognitive decline, which can make optimal care difficult. Such episodes may also impair the psychological and physical well-being of caregivers, increasing their risk of depression, anxiety, and even post-traumatic stress disorder (PTSD).18 The extent of the impact is also determined by the interpretation of the abuse by the caregivers themselves. One study suggested that the perception of aggressive or violent behavior as “normal” by a caregiver reduced the overall negative effect of the interactions.7Our review emphasizes the unintended burden that can fall to caregivers of patients with dementia. We also address the role of primary care providers (PCPs) in identifying these instances of violence and intervening appropriately by providing safety strategies, education, resources, and support.

CASE
A 67-year-old man with a medical history of PTSD with depression, type 2 diabetes, alcohol use disorder/dependence, hypertension, and obstructive sleep apnea was brought to his PCP by his wife. She said he had recently been unable to keep appointment times, pay bills, or take his usual medications, venlafaxine and bupropion. She also said his PTSD symptoms had worsened. He was sleeping 12 to 14 hours per day and was increasingly irritable. The patient denied any concerns or changes in his behavior.
The PCP administered a Saint Louis University Mental Status (SLUMS) examination to screen for cognitive impairment.19 The patient scored 14/30 (less than 20 is indicative of dementia). He was unable to complete a simple math problem, recall any items from a list of 5, count in reverse, draw a clock correctly, or recall a full story. Throughout the exam, the patient demonstrated minimal effort and was often only able to complete a task after further prompting by the examiner.

A computed tomography scan of the head revealed no signs of hemorrhage or damage. Thyroid-stimulating hormone levels and vitamin B12 levels were normal. A rapid plasma reagin test result was negative. The patient was given a diagnosis of Alzheimer disease. Donepezil was added to the patient’s medications, starting at 5 mg and then increased to 10 mg. His wife began to assist him with his tasks of daily living. His mood improved, and his wife noted he began to remember his appointments and take his medications with assistance.

However, the patient’s irritability continued to escalate. He grew paranoid and accused his wife of mismanaging their money. This pattern steadily worsened over the course of 6 months. The situation escalated until one day the patient’s wife called a mental health hotline reporting that her husband was holding her hostage and threatening to kill her with a gun. He told her, “I can do something to you, and they won’t even find a fingernail. It doesn’t have to be with a gun either.” She was counseled to try to stay calm to avoid aggravating the situation and to go to a safe place and stay there until help arrived.
His memory had worsened to the point that he could not recall any events from the previous 2 years. He was paranoid about anyone entering his home and would not allow his deteriorating roof to be repaired or his yard to be maintained. He did not shower for weeks at a time. He slept holding a rifle and accused his wife of embezzlement.
Continue to: The patient was evaluated...
The patient was evaluated by another specialist, who assessed his SLUMS score to be 18/30. He increased the patient’s donepezil dose, initiated a bupropion taper, and added sertraline to the regimen. The PCP spoke to the patient’s wife regarding options for her safety including leaving the home, hiding firearms, and calling the police in cases of interpersonal violence. The wife said she did not want to pursue these options. She expressed worry that he might be harmed if he was uncooperative with the police and said there was no one except her to take care of him.
Caregivers struggle to care for their loved ones
Instances of personal violence lead to shock, astonishment, heartbreak, and fear. Anticipation of a recurrence of violence causes many partners and caregivers to feel exhausted, because there is minimal hope for any chance of improvement. There are a few exceptions, however, as our case will show. In addition to emotional exhaustion, there is also a never-ending sense of self-doubt, leading many caregivers to question their ability to handle their family member.20,21 Over time, this leads to caregiver burnout, leaving them unable to understand their family member’s aggression. The sudden loss of caregiver control in dealing with the patient may also result in the family member exhibiting behavioral changes reflecting emotional trauma. For caregivers who do not live with the patient, they may choose to make fewer or shorter visits—or not visit at all—because they fear being abused.7,22
Caregivers of patients with dementia often feel helpless and powerless once abrupt and drastic changes in personality lead to some form of interpersonal violence. Additionally, caregivers with a poor health status are more likely to have lower physical function and experience greater caregiving stress overall.23 Other factors increasing stress are longer years of caregiving and the severity of a patient’s dementia and functional impairment.23
Interventions to reduce caregiver burden
Many studies have assessed the role of different interventions to reduce caregiver burden, such as teaching them problem-solving skills, increasing their knowledge of dementia, recommending social resources, providing emotional support, changing caregiver perceptions of the care situation, introducing coping strategies, relying on strengths and experiences in caregiving, help-seeking, and engaging in activity programs.24-28 For Hispanic caregivers, a structured and self-paced online telenovela format has been effective in improving care and relieving caregiver stress.29 Online positive emotion regulators helped in significantly improving quality of life and physical health in the caregivers.30 In this last intervention, caregivers had 6 online sessions with a facilitator who taught them emotional regulation skills that included: noticing positive events, capitalizing on them, and feeling gratitude; practicing mindfulness; doing a positive reappraisal; acknowledging personal strengths and setting attainable goals; and performing acts of kindness. Empowerment programs have also shown significant improvement in the well-being of caregivers.31
Caregivers may reject support.
Continue to: These practical tips can help
These practical tips can help
Based on our review of the literature, we recommend offering the following supports to caregivers:
- Counsel caregivers early on in a patient’s dementia that behavior changes are likely and may be unpredictable. Explain that dementia can involve changes to personality and behavior as well as memory difficulties.33,34
- Describe resources for support, such as day programs for senior adults, insurance coverage for caregiver respite programs, and the Alzheimer’s Association (www.alz.org/). Encourage caregivers to seek general medical and mental health care for themselves. Caregivers should have opportunities and support to discuss their experiences and to be appropriately trained for the challenge of caring for a family member with dementia.35
- Encourage disclosure about abrupt changes in the patient’s behavior. This invites families to discuss issues with you and may make them more comfortable with such conversations.
- Involve ancillary services (eg, social worker) to plan for a higher level of care well in advance of it becoming necessary.
- Discuss safety strategies for the caregiver, including when it is appropriate to alter a patient’s set routines such as bedtimes and mealtimes.33,34
- Discuss when and how to involve law enforcement, if necessary.33,34 Emphasize the importance of removing firearms from the home as a safety measure. Although federal laws do not explicitly prohibit possession of arms by patients with neurologic damage, a few states mention “organic brain syndrome” or “dementia” as conditions prohibiting use or possession of firearms.36
- Suggest, as feasible, nonpharmacologic aids for the patient such as massage therapy, animal-assisted therapy, personalized interventions, music therapy, and light therapy.37 Prescribe medications to the patient to aid in behavior modification when appropriate.
- Screen caregivers and family members for signs of interpersonal violence. Take notice of changes in caregiver behavior or irregularity in attending follow-up appointments.
CASE
Over the next month, the patient’s symptoms further deteriorated. His PCP recommended hospitalization, but the patient and his wife declined. Magnetic resonance imaging of the patient’s brain revealed severe confluent and patchy regions of white matter and T2 signal hyperintensity, consistent with chronic microvascular ischemic disease. An old, small, left parietal lobe infarct was also noted.
One month later, the patient presented to the emergency department. His symptoms were largely unchanged, but his wife indicated that she could no longer live at home due to burnout. The patient’s medications were adjusted, but he was not admitted for inpatient care. His wife said they needed help at home, but the patient opposed the idea any time that it was mentioned.
A few weeks later, the patient presented for outpatient follow-up. He was delusional, believing that the government was compelling citizens to take sertraline in order to harm their mental health. He had also begun viewing online pornography in front of his wife and attempting to remove all of his money from the bank. He was prescribed aripiprazole 15 mg, and his symptoms began to improve. Soon after, however, he threatened to kill his grandson, then took all his Lasix pills (a 7-day supply) simultaneously. The patient denied that this was a suicide attempt.
Over the course of the next month, the patient began to report hearing voices. A neuropsychological evaluation confirmed a diagnosis of dementia with psychiatric symptoms due to neurologic injury. The patient was referred to a geriatric psychiatrist and continued to be managed medically. He was assigned a multidisciplinary team comprising palliative care, social work, and care management to assist in his care and provide support to the family. His behavior improved.
Continue to: At the time of this publication...
At the time of this publication, the patient’s irritability and paranoia had subsided and he had made no further threats to his family. He has allowed a home health aide into the house and has agreed to have his roof repaired. His wife still lives with him and assists him with activities of daily living.
Interprofessional teams are key
Caregiver burnout increases the risk of patient neglect or abuse, as individuals who have been the targets of aggressive behavior are more likely to leave demented patients unattended.8,16,23 Although tools are available to screen caregivers for depression and burnout, an important step forward would be to develop an interprofessional team to aid in identifying and closely following high-risk patient–caregiver groups. This continual and varied assessment of psychosocial stressors could help prevent the development of violent interactions. These teams would allow integration with the primary health care system by frequent and effective shared communication of knowledge, development of goals, and shared decision-making.38 Setting expectations, providing support, and discussing safety strategies can improve the health and welfare of caregivers and patients with dementia alike.
CORRESPONDENCE
Abu Baker Sheikh, MD, MSC 10-5550, 1 University of New Mexico, Albuquerque, NM 87131; [email protected].
1. Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol. 2017;13:327-339.
2. Cipriani G, Borin G, Vedovello M, et al. Sociopathic behavior and dementia. Acta Neurol Belg. 2013;113:111-115.
3. Cipriani G, Lucetti C, Danti S, et al. Violent and criminal manifestations in dementia patients. Geriatr Gerontol Int. 2016;16:541-549.
4. Skovdahl K, Kihlgren AL, Kihlgren M. Different attitudes when handling aggressive behaviour in dementia—narratives from two caregiver groups. Aging Ment Health. 2003;7:277-286.
5. Kristiansen L, Hellzén O, Asplund K. Swedish assistant nurses’ experiences of job satisfaction when caring for persons suffering from dementia and behavioural disturbances. An interview study. Int J Qualitat Stud Health Well-being. 2006;1:245-256.
6. Wharton TC, Ford BK. What is known about dementia care recipient violence and aggression against caregivers? J Gerontol Soc Work. 2014;57:460-477.
7. Ostaszkiewicz J, Lakhan P, O’Connell B, et al. Ongoing challenges responding to behavioural and psychological symptoms of dementia. Int Nurs Rev. 2015;62:506-516.
8. Kim J, De Bellis AM, Xiao LD. The experience of paid family-care workers of people with dementia in South Korea. Asian Nurs Res (Korean Soc Nurs Sci). 2018;12:34-41.
9. Band-Winterstein T, Avieli H. Women coping with a partner’s dementia-related violence: a qualitative study. J Nurs Scholarsh. 2019; 51:368-379.
10. Munkejord MC, Stefansdottir OA, Sveinbjarnardottir EK. Who cares for the carer? The suffering, struggles and unmet needs of older women caring for husbands living with cognitive decline. Int Pract Devel J. 2020;10:1-11.
11. Seidel D, Thyrian JR. Burden of caring for people with dementia - comparing family caregivers and professional caregivers. A descriptive study. J Multidiscip Healthc. 2019;12:655-663.
12. Tang W, Friedman DB, Kannaley K, et al. Experiences of caregivers by care recipient’s health condition: a study of caregivers for Alzheimer’s disease and related dementias versus other chronic conditions. Geriatr Nurs. 2019;40:181-184.
13. Benbow SM, Bhattacharyya S, Kingston P. Older adults and violence: an analysis of domestic homicide reviews in England involving adults over 60 years of age. Ageing Soc. 2018;39:1097-1121.
14. Herron RV, Wrathall MA. Putting responsive behaviours in place: examining how formal and informal carers understand the actions of people with dementia. Soc Sci Med. 2018;204:9-15.
15. Herron RV, Rosenberg MW. Responding to aggression and reactive behaviours in the home. Dementia (London). 2019;18:1328-1340.
16. Spencer D, Funk LM, Herron RV, et al. Fear, defensive strategies and caring for cognitively impaired family members. J Gerontol Soc Work. 2019;62:67-85.
17. Skovdahl K, Kihlgren AL, Kihlgren M. Dementia and aggressiveness: stimulated recall interviews with caregivers after video-recorded interactions. J Clin Nurs. 2004;13:515-525.
18. Needham I, Abderhalden C, Halfens RJ, et al. Non-somatic effects of patient aggression on nurses: a systematic review. J Adv Nurs. 2005;49:283-296.
19. Tariq SH, Tumosa N, Chibnall JT, et al. The Saint Louis University Mental Status (SLUMS) Examination for detecting mild cognitive impairment and dementia is more sensitive than the Mini-Mental Status Examination (MMSE) - a pilot study. Am J Geriatr Psych. 2006;14:900-910.
20. Janzen S, Zecevic AA, Kloseck M, et al. Managing agitation using nonpharmacological interventions for seniors with dementia. Am J Alzheimers Dis Other Demen. 2013;28:524-532.
21. Zeller A, Dassen T, Kok G, et al. Nursing home caregivers’ explanations for and coping strategies with residents’ aggression: a qualitative study. J Clin Nurs. 2011;20:2469-2478.
22. Alzheimer’s Society. Fix dementia care: homecare. Accessed December 28, 2021. https://www.alzheimers.org.uk/sites/default/files/migrate/downloads/fix_dementia_care_homecare_report.pdf
23. von Känel R, Mausbach BT, Dimsdale JE, et al. Refining caregiver vulnerability for clinical practice: determinants of self-rated health in spousal dementia caregivers. BMC Geriatr. 2019;19:18.
24. Chen HM, Huang MF, Yeh YC, et al. Effectiveness of coping strategies intervention on caregiver burden among caregivers of elderly patients with dementia. Psychogeriatrics. 2015; 15:20-25.
25. Wawrziczny E, Larochette C, Papo D, et al. A customized intervention for dementia caregivers: a quasi-experimental design. J Aging Health. 2019;31:1172-1195.
26. Gitlin LN, Piersol CV, Hodgson N, et al. Reducing neuropsychiatric symptoms in persons with dementia and associated burden in family caregivers using tailored activities: Design and methods of a randomized clinical trial. Contemp Clin Trials. 2016;49:92-102.
27. de Oliveira AM, Radanovic M, Homem de Mello PC, et al. An intervention to reduce neuropsychiatric symptoms and caregiver burden in dementia: preliminary results from a randomized trial of the tailored activity program-outpatient version. Int J Geriatr Psychiatry. 2019;34:1301-1307.
28. Livingston G, Barber J, Rapaport P, et al. Clinical effectiveness of a manual based coping strategy programme (START, STrAtegies for RelaTives) in promoting the mental health of carers of family members with dementia: pragmatic randomised controlled trial. BMJ. 2013;347:f6276.
29. Kajiyama B, Fernandez G, Carter EA, et al. Helping Hispanic dementia caregivers cope with stress using technology-based resources. Clin Gerontol. 2018;41:209-216.
30. Moskowitz JT, Cheung EO, Snowberg KE, et al. Randomized controlled trial of a facilitated online positive emotion regulation intervention for dementia caregivers. Health Psychol. 2019;38:391-402.
31. Yoon HK, Kim GS. An empowerment program for family caregivers of people with dementia. Public Health Nurs. 2020;37:222-233.
32. Zwingmann I, Dreier-Wolfgramm A, Esser A, et al. Why do family dementia caregivers reject caregiver support services? Analyzing types of rejection and associated health-impairments in a cluster-randomized controlled intervention trial. BMC Health Serv Res. 2020;20:121.
33. Nybakken S, Strandås M, Bondas T. Caregivers’ perceptions of aggressive behaviour in nursing home residents living with dementia: A meta-ethnography. J Adv Nurs. 2018;74:2713-2726.
34. Nakaishi L, Moss H, Weinstein M, et al. Exploring workplace violence among home care workers in a consumer-driven home health care program. Workplace Health Saf. 2013;61:441-450.
35. Medical Advisory Secretariat. Caregiver- and patient-directed interventions for dementia: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8:1-98.
36. Betz ME, McCourt AD, Vernick JS, et al. Firearms and dementia: clinical considerations. Ann Intern Med. 2018;169:47-49.
37. Leng M, Zhao Y, Wang Z. Comparative efficacy of non-pharmacological interventions on agitation in people with dementia: a systematic review and Bayesian network meta-analysis. Int J Nurs Stud. 2020;102:103489.
38. Morgan S, Pullon S, McKinlay E. Observation of interprofessional collaborative practice in primary care teams: an integrative literature review. Int J Nurs Stud. 2015;52:1217-1230.
1. Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol. 2017;13:327-339.
2. Cipriani G, Borin G, Vedovello M, et al. Sociopathic behavior and dementia. Acta Neurol Belg. 2013;113:111-115.
3. Cipriani G, Lucetti C, Danti S, et al. Violent and criminal manifestations in dementia patients. Geriatr Gerontol Int. 2016;16:541-549.
4. Skovdahl K, Kihlgren AL, Kihlgren M. Different attitudes when handling aggressive behaviour in dementia—narratives from two caregiver groups. Aging Ment Health. 2003;7:277-286.
5. Kristiansen L, Hellzén O, Asplund K. Swedish assistant nurses’ experiences of job satisfaction when caring for persons suffering from dementia and behavioural disturbances. An interview study. Int J Qualitat Stud Health Well-being. 2006;1:245-256.
6. Wharton TC, Ford BK. What is known about dementia care recipient violence and aggression against caregivers? J Gerontol Soc Work. 2014;57:460-477.
7. Ostaszkiewicz J, Lakhan P, O’Connell B, et al. Ongoing challenges responding to behavioural and psychological symptoms of dementia. Int Nurs Rev. 2015;62:506-516.
8. Kim J, De Bellis AM, Xiao LD. The experience of paid family-care workers of people with dementia in South Korea. Asian Nurs Res (Korean Soc Nurs Sci). 2018;12:34-41.
9. Band-Winterstein T, Avieli H. Women coping with a partner’s dementia-related violence: a qualitative study. J Nurs Scholarsh. 2019; 51:368-379.
10. Munkejord MC, Stefansdottir OA, Sveinbjarnardottir EK. Who cares for the carer? The suffering, struggles and unmet needs of older women caring for husbands living with cognitive decline. Int Pract Devel J. 2020;10:1-11.
11. Seidel D, Thyrian JR. Burden of caring for people with dementia - comparing family caregivers and professional caregivers. A descriptive study. J Multidiscip Healthc. 2019;12:655-663.
12. Tang W, Friedman DB, Kannaley K, et al. Experiences of caregivers by care recipient’s health condition: a study of caregivers for Alzheimer’s disease and related dementias versus other chronic conditions. Geriatr Nurs. 2019;40:181-184.
13. Benbow SM, Bhattacharyya S, Kingston P. Older adults and violence: an analysis of domestic homicide reviews in England involving adults over 60 years of age. Ageing Soc. 2018;39:1097-1121.
14. Herron RV, Wrathall MA. Putting responsive behaviours in place: examining how formal and informal carers understand the actions of people with dementia. Soc Sci Med. 2018;204:9-15.
15. Herron RV, Rosenberg MW. Responding to aggression and reactive behaviours in the home. Dementia (London). 2019;18:1328-1340.
16. Spencer D, Funk LM, Herron RV, et al. Fear, defensive strategies and caring for cognitively impaired family members. J Gerontol Soc Work. 2019;62:67-85.
17. Skovdahl K, Kihlgren AL, Kihlgren M. Dementia and aggressiveness: stimulated recall interviews with caregivers after video-recorded interactions. J Clin Nurs. 2004;13:515-525.
18. Needham I, Abderhalden C, Halfens RJ, et al. Non-somatic effects of patient aggression on nurses: a systematic review. J Adv Nurs. 2005;49:283-296.
19. Tariq SH, Tumosa N, Chibnall JT, et al. The Saint Louis University Mental Status (SLUMS) Examination for detecting mild cognitive impairment and dementia is more sensitive than the Mini-Mental Status Examination (MMSE) - a pilot study. Am J Geriatr Psych. 2006;14:900-910.
20. Janzen S, Zecevic AA, Kloseck M, et al. Managing agitation using nonpharmacological interventions for seniors with dementia. Am J Alzheimers Dis Other Demen. 2013;28:524-532.
21. Zeller A, Dassen T, Kok G, et al. Nursing home caregivers’ explanations for and coping strategies with residents’ aggression: a qualitative study. J Clin Nurs. 2011;20:2469-2478.
22. Alzheimer’s Society. Fix dementia care: homecare. Accessed December 28, 2021. https://www.alzheimers.org.uk/sites/default/files/migrate/downloads/fix_dementia_care_homecare_report.pdf
23. von Känel R, Mausbach BT, Dimsdale JE, et al. Refining caregiver vulnerability for clinical practice: determinants of self-rated health in spousal dementia caregivers. BMC Geriatr. 2019;19:18.
24. Chen HM, Huang MF, Yeh YC, et al. Effectiveness of coping strategies intervention on caregiver burden among caregivers of elderly patients with dementia. Psychogeriatrics. 2015; 15:20-25.
25. Wawrziczny E, Larochette C, Papo D, et al. A customized intervention for dementia caregivers: a quasi-experimental design. J Aging Health. 2019;31:1172-1195.
26. Gitlin LN, Piersol CV, Hodgson N, et al. Reducing neuropsychiatric symptoms in persons with dementia and associated burden in family caregivers using tailored activities: Design and methods of a randomized clinical trial. Contemp Clin Trials. 2016;49:92-102.
27. de Oliveira AM, Radanovic M, Homem de Mello PC, et al. An intervention to reduce neuropsychiatric symptoms and caregiver burden in dementia: preliminary results from a randomized trial of the tailored activity program-outpatient version. Int J Geriatr Psychiatry. 2019;34:1301-1307.
28. Livingston G, Barber J, Rapaport P, et al. Clinical effectiveness of a manual based coping strategy programme (START, STrAtegies for RelaTives) in promoting the mental health of carers of family members with dementia: pragmatic randomised controlled trial. BMJ. 2013;347:f6276.
29. Kajiyama B, Fernandez G, Carter EA, et al. Helping Hispanic dementia caregivers cope with stress using technology-based resources. Clin Gerontol. 2018;41:209-216.
30. Moskowitz JT, Cheung EO, Snowberg KE, et al. Randomized controlled trial of a facilitated online positive emotion regulation intervention for dementia caregivers. Health Psychol. 2019;38:391-402.
31. Yoon HK, Kim GS. An empowerment program for family caregivers of people with dementia. Public Health Nurs. 2020;37:222-233.
32. Zwingmann I, Dreier-Wolfgramm A, Esser A, et al. Why do family dementia caregivers reject caregiver support services? Analyzing types of rejection and associated health-impairments in a cluster-randomized controlled intervention trial. BMC Health Serv Res. 2020;20:121.
33. Nybakken S, Strandås M, Bondas T. Caregivers’ perceptions of aggressive behaviour in nursing home residents living with dementia: A meta-ethnography. J Adv Nurs. 2018;74:2713-2726.
34. Nakaishi L, Moss H, Weinstein M, et al. Exploring workplace violence among home care workers in a consumer-driven home health care program. Workplace Health Saf. 2013;61:441-450.
35. Medical Advisory Secretariat. Caregiver- and patient-directed interventions for dementia: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8:1-98.
36. Betz ME, McCourt AD, Vernick JS, et al. Firearms and dementia: clinical considerations. Ann Intern Med. 2018;169:47-49.
37. Leng M, Zhao Y, Wang Z. Comparative efficacy of non-pharmacological interventions on agitation in people with dementia: a systematic review and Bayesian network meta-analysis. Int J Nurs Stud. 2020;102:103489.
38. Morgan S, Pullon S, McKinlay E. Observation of interprofessional collaborative practice in primary care teams: an integrative literature review. Int J Nurs Stud. 2015;52:1217-1230.
PRACTICE RECOMMENDATIONS
› Screen caregivers and family members of patients with dementia for signs of interpersonal violence. C
› Counsel caregivers early on that behavior changes in patients with dementia are likely and may be unpredictable. C
› Discuss safety strategies for the caregiver, including when it is appropriate to alter routines such as bedtimes and meals. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
An overlooked cause of dyspepsia?
Discussion of a common cause of dyspepsia was missing from your September article, “Dyspepsia: A stepwise approach to evaluation and management” (J Fam Pract. 2021;70:320-325). After more than 25 years of practice, I have found that most people with dyspepsia have hypochlorhydria1—a condition that results in the inability to produce adequate amounts of hydrochloric acid, or stomach acid. With lower amounts of stomach acid, food does not break down but ferments instead, producing gas and discomfort.
I use a simple test to diagnose patients with hypochlorhydria. The patient takes a capsule of hydrochloric acid directly after eating a meal; failure to experience epigastric burning within 30 minutes of ingesting the capsule indicates a need for additional stomach acid with a meal. If they do experience a burning sensation within 30 minutes, it indicates they do not need additional stomach acid. The burning sensation is relieved by drinking 2 teaspoons of baking soda in 4 oz of water to neutralize the excess acid.
In my experience, most people who take the test do not experience a sense of burning. I find that once these patients with hypochlorhydria start taking betaine hydrochloride with their meals, they no longer need the many over-the-counter or prescription antacids and their dyspepsia disappears. Many of my patients find that after a few months, they begin to experience burning and can discontinue the supplement, without facing a return of their dyspepsia.
Marianne Rothschild, MD
Mount Airy, MD
1. Iwai W, Abe Y, Iijima K, et al. Gastric hypochlorhydria is associated with an exacerbation of dyspeptic symptoms in female patients. J Gastoenterol. 2012;48:214-221. doi: 10.1007/s00535-012-0634-8
Editor’s note
After reading Dr. Rothschild’s letter, I decided to do a little digging to find out if there is any research evidence to support her approach to dyspepsia. I carefully searched PubMed and found only 2 observational studies showing an association between dyspepsia and hypochlorhydria. There are no randomized trials of dyspepsia treatment with hydrochloric acid to support her clinical observations. Placebo effect? Until there is a good, randomized trial, we will not know. But who would have guessed that H pylori causes peptic ulcers?
John Hickner, MD, MSc
Editor-in-Chief, The Journal of Family Practice
Discussion of a common cause of dyspepsia was missing from your September article, “Dyspepsia: A stepwise approach to evaluation and management” (J Fam Pract. 2021;70:320-325). After more than 25 years of practice, I have found that most people with dyspepsia have hypochlorhydria1—a condition that results in the inability to produce adequate amounts of hydrochloric acid, or stomach acid. With lower amounts of stomach acid, food does not break down but ferments instead, producing gas and discomfort.
I use a simple test to diagnose patients with hypochlorhydria. The patient takes a capsule of hydrochloric acid directly after eating a meal; failure to experience epigastric burning within 30 minutes of ingesting the capsule indicates a need for additional stomach acid with a meal. If they do experience a burning sensation within 30 minutes, it indicates they do not need additional stomach acid. The burning sensation is relieved by drinking 2 teaspoons of baking soda in 4 oz of water to neutralize the excess acid.
In my experience, most people who take the test do not experience a sense of burning. I find that once these patients with hypochlorhydria start taking betaine hydrochloride with their meals, they no longer need the many over-the-counter or prescription antacids and their dyspepsia disappears. Many of my patients find that after a few months, they begin to experience burning and can discontinue the supplement, without facing a return of their dyspepsia.
Marianne Rothschild, MD
Mount Airy, MD
1. Iwai W, Abe Y, Iijima K, et al. Gastric hypochlorhydria is associated with an exacerbation of dyspeptic symptoms in female patients. J Gastoenterol. 2012;48:214-221. doi: 10.1007/s00535-012-0634-8
Editor’s note
After reading Dr. Rothschild’s letter, I decided to do a little digging to find out if there is any research evidence to support her approach to dyspepsia. I carefully searched PubMed and found only 2 observational studies showing an association between dyspepsia and hypochlorhydria. There are no randomized trials of dyspepsia treatment with hydrochloric acid to support her clinical observations. Placebo effect? Until there is a good, randomized trial, we will not know. But who would have guessed that H pylori causes peptic ulcers?
John Hickner, MD, MSc
Editor-in-Chief, The Journal of Family Practice
Discussion of a common cause of dyspepsia was missing from your September article, “Dyspepsia: A stepwise approach to evaluation and management” (J Fam Pract. 2021;70:320-325). After more than 25 years of practice, I have found that most people with dyspepsia have hypochlorhydria1—a condition that results in the inability to produce adequate amounts of hydrochloric acid, or stomach acid. With lower amounts of stomach acid, food does not break down but ferments instead, producing gas and discomfort.
I use a simple test to diagnose patients with hypochlorhydria. The patient takes a capsule of hydrochloric acid directly after eating a meal; failure to experience epigastric burning within 30 minutes of ingesting the capsule indicates a need for additional stomach acid with a meal. If they do experience a burning sensation within 30 minutes, it indicates they do not need additional stomach acid. The burning sensation is relieved by drinking 2 teaspoons of baking soda in 4 oz of water to neutralize the excess acid.
In my experience, most people who take the test do not experience a sense of burning. I find that once these patients with hypochlorhydria start taking betaine hydrochloride with their meals, they no longer need the many over-the-counter or prescription antacids and their dyspepsia disappears. Many of my patients find that after a few months, they begin to experience burning and can discontinue the supplement, without facing a return of their dyspepsia.
Marianne Rothschild, MD
Mount Airy, MD
1. Iwai W, Abe Y, Iijima K, et al. Gastric hypochlorhydria is associated with an exacerbation of dyspeptic symptoms in female patients. J Gastoenterol. 2012;48:214-221. doi: 10.1007/s00535-012-0634-8
Editor’s note
After reading Dr. Rothschild’s letter, I decided to do a little digging to find out if there is any research evidence to support her approach to dyspepsia. I carefully searched PubMed and found only 2 observational studies showing an association between dyspepsia and hypochlorhydria. There are no randomized trials of dyspepsia treatment with hydrochloric acid to support her clinical observations. Placebo effect? Until there is a good, randomized trial, we will not know. But who would have guessed that H pylori causes peptic ulcers?
John Hickner, MD, MSc
Editor-in-Chief, The Journal of Family Practice
Gut Microbiota for Health World Summit 2022
Registration is now open for the Gut Microbiota for Health (GMFH) World Summit 2022, taking place March 12-13 in Washington, D.C., and virtually.
Organized by AGA and the European Society of Neurogastroenterology and Motility (ESNM), the GMFH World Summit is the preeminent international meeting on the gut microbiome for clinicians, dietitians and researchers.
Now in its 10th year, the program for this year’s conference will focus on “The Gut Microbiome in Precision Nutrition and Medicine.” Join us to gain a deeper understanding of the role of the gut microbiome in precision medicine and discover personalized approaches to modulating the gut microbiome that may promote health and improve patient outcomes for a variety of disorders and diseases.
Registration is now open for the Gut Microbiota for Health (GMFH) World Summit 2022, taking place March 12-13 in Washington, D.C., and virtually.
Organized by AGA and the European Society of Neurogastroenterology and Motility (ESNM), the GMFH World Summit is the preeminent international meeting on the gut microbiome for clinicians, dietitians and researchers.
Now in its 10th year, the program for this year’s conference will focus on “The Gut Microbiome in Precision Nutrition and Medicine.” Join us to gain a deeper understanding of the role of the gut microbiome in precision medicine and discover personalized approaches to modulating the gut microbiome that may promote health and improve patient outcomes for a variety of disorders and diseases.
Registration is now open for the Gut Microbiota for Health (GMFH) World Summit 2022, taking place March 12-13 in Washington, D.C., and virtually.
Organized by AGA and the European Society of Neurogastroenterology and Motility (ESNM), the GMFH World Summit is the preeminent international meeting on the gut microbiome for clinicians, dietitians and researchers.
Now in its 10th year, the program for this year’s conference will focus on “The Gut Microbiome in Precision Nutrition and Medicine.” Join us to gain a deeper understanding of the role of the gut microbiome in precision medicine and discover personalized approaches to modulating the gut microbiome that may promote health and improve patient outcomes for a variety of disorders and diseases.
Keeping an open mind about functional medicine
Considering the controversy surrounding functional medicine, you may be wondering why JFP published an article about it last month.1 David Gorski, MD, PhD, FACS, a vocal critic of functional medicine, commented: “Functional medicine. It sounds so … scientific and reasonable. It’s anything but. In fact, functional medicine combines the worst features of conventional medicine with a heapin’ helpin’ of quackery.”2 On its website, however, The Institute for Functional Medicine claims that “functional medicine determines how and why illness occurs and restores health by addressing the root causes of disease for each individual.”3
I suspect the truth lies somewhere in between.
Because functional medicine has gained a certain degree of popularity, I felt it was important for family physicians and other primary care clinicians to know enough about this alternative healing method to discuss it with patients who express interest.
In their review article in JFP, Orlando and colleagues tell us there are 7 defining characteristics of functional medicine.1 It is patient centered rather than disease centered, uses a “systems biology” approach, considers the dynamic balance of gene-environment interactions, is personalized based on biochemical individuality, promotes organ reserve and sustained health span, sees health as a positive vitality (not merely the absence of disease), and focuses on function rather than pathology.
Most of these statements about functional medicine apply to traditional family medicine. The clinical approach stressing lifestyle changes is mainstream, not unique. The focus on digestion and the microbiome as an important determinant of health is based on interesting basic science studies and associations noted between certain microbiome profiles and diseases.
But association is not causation. So far there is scant evidence that changing the microbiome results in better health, although some preliminary case series have generated intriguing hypotheses. And there is evidence that probiotics improve some symptoms. Ongoing research into the microbiome and health will, no doubt, be illuminating. We have much to learn.
What does seem unique, but suspect, about functional medicine is its focus on biochemical testing of unproven value and the prescribing of diets and supplements based on the test results. There are no sound scientific studies showing the benefit of this approach.
I suggest you read Orlando et al’s article. Functional medicine is an interesting, mostly unproven, approach to patient care. But I will keep an open mind until we see better research that either does—or doesn’t—support the validity of its practices.
1. Orlando FA, Chang KL, Estores IM. Functional medicine: focusing on imbalances in core metabolic processes. J Fam Pract. 2021;70:482-488,498.
2. Gorski D. Functional medicine: the ultimate misnomer in the world of integrative medicine. Science-Based Medicine. April 11, 2016. Accessed January 4, 2022. https://sciencebasedmedicine.org/functional-medicine-the-ultimate-misnomer-in-the-world-of-integrative-medicine/
3. The Institute for Functional Medicine. Accessed January 4, 2022. www.ifm.org
Considering the controversy surrounding functional medicine, you may be wondering why JFP published an article about it last month.1 David Gorski, MD, PhD, FACS, a vocal critic of functional medicine, commented: “Functional medicine. It sounds so … scientific and reasonable. It’s anything but. In fact, functional medicine combines the worst features of conventional medicine with a heapin’ helpin’ of quackery.”2 On its website, however, The Institute for Functional Medicine claims that “functional medicine determines how and why illness occurs and restores health by addressing the root causes of disease for each individual.”3
I suspect the truth lies somewhere in between.
Because functional medicine has gained a certain degree of popularity, I felt it was important for family physicians and other primary care clinicians to know enough about this alternative healing method to discuss it with patients who express interest.
In their review article in JFP, Orlando and colleagues tell us there are 7 defining characteristics of functional medicine.1 It is patient centered rather than disease centered, uses a “systems biology” approach, considers the dynamic balance of gene-environment interactions, is personalized based on biochemical individuality, promotes organ reserve and sustained health span, sees health as a positive vitality (not merely the absence of disease), and focuses on function rather than pathology.
Most of these statements about functional medicine apply to traditional family medicine. The clinical approach stressing lifestyle changes is mainstream, not unique. The focus on digestion and the microbiome as an important determinant of health is based on interesting basic science studies and associations noted between certain microbiome profiles and diseases.
But association is not causation. So far there is scant evidence that changing the microbiome results in better health, although some preliminary case series have generated intriguing hypotheses. And there is evidence that probiotics improve some symptoms. Ongoing research into the microbiome and health will, no doubt, be illuminating. We have much to learn.
What does seem unique, but suspect, about functional medicine is its focus on biochemical testing of unproven value and the prescribing of diets and supplements based on the test results. There are no sound scientific studies showing the benefit of this approach.
I suggest you read Orlando et al’s article. Functional medicine is an interesting, mostly unproven, approach to patient care. But I will keep an open mind until we see better research that either does—or doesn’t—support the validity of its practices.
Considering the controversy surrounding functional medicine, you may be wondering why JFP published an article about it last month.1 David Gorski, MD, PhD, FACS, a vocal critic of functional medicine, commented: “Functional medicine. It sounds so … scientific and reasonable. It’s anything but. In fact, functional medicine combines the worst features of conventional medicine with a heapin’ helpin’ of quackery.”2 On its website, however, The Institute for Functional Medicine claims that “functional medicine determines how and why illness occurs and restores health by addressing the root causes of disease for each individual.”3
I suspect the truth lies somewhere in between.
Because functional medicine has gained a certain degree of popularity, I felt it was important for family physicians and other primary care clinicians to know enough about this alternative healing method to discuss it with patients who express interest.
In their review article in JFP, Orlando and colleagues tell us there are 7 defining characteristics of functional medicine.1 It is patient centered rather than disease centered, uses a “systems biology” approach, considers the dynamic balance of gene-environment interactions, is personalized based on biochemical individuality, promotes organ reserve and sustained health span, sees health as a positive vitality (not merely the absence of disease), and focuses on function rather than pathology.
Most of these statements about functional medicine apply to traditional family medicine. The clinical approach stressing lifestyle changes is mainstream, not unique. The focus on digestion and the microbiome as an important determinant of health is based on interesting basic science studies and associations noted between certain microbiome profiles and diseases.
But association is not causation. So far there is scant evidence that changing the microbiome results in better health, although some preliminary case series have generated intriguing hypotheses. And there is evidence that probiotics improve some symptoms. Ongoing research into the microbiome and health will, no doubt, be illuminating. We have much to learn.
What does seem unique, but suspect, about functional medicine is its focus on biochemical testing of unproven value and the prescribing of diets and supplements based on the test results. There are no sound scientific studies showing the benefit of this approach.
I suggest you read Orlando et al’s article. Functional medicine is an interesting, mostly unproven, approach to patient care. But I will keep an open mind until we see better research that either does—or doesn’t—support the validity of its practices.
1. Orlando FA, Chang KL, Estores IM. Functional medicine: focusing on imbalances in core metabolic processes. J Fam Pract. 2021;70:482-488,498.
2. Gorski D. Functional medicine: the ultimate misnomer in the world of integrative medicine. Science-Based Medicine. April 11, 2016. Accessed January 4, 2022. https://sciencebasedmedicine.org/functional-medicine-the-ultimate-misnomer-in-the-world-of-integrative-medicine/
3. The Institute for Functional Medicine. Accessed January 4, 2022. www.ifm.org
1. Orlando FA, Chang KL, Estores IM. Functional medicine: focusing on imbalances in core metabolic processes. J Fam Pract. 2021;70:482-488,498.
2. Gorski D. Functional medicine: the ultimate misnomer in the world of integrative medicine. Science-Based Medicine. April 11, 2016. Accessed January 4, 2022. https://sciencebasedmedicine.org/functional-medicine-the-ultimate-misnomer-in-the-world-of-integrative-medicine/
3. The Institute for Functional Medicine. Accessed January 4, 2022. www.ifm.org
Nodule on the left cheek
An 85-year-old man with a history of skin cancer presented to my dermatology practice (NT) for evaluation of a “pimple” on his left cheek that failed to resolve after 2 months (FIGURE). The patient noted that the lesion had grown, but that he otherwise felt well.
On examination, the lesion was plum colored, and the area was firm and nontender to palpation. The patient was referred to a plastic surgeon for an excisional biopsy to clarify the nature of the lesion.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Merkel cell carcinoma
A biopsy performed 2 weeks after the initial visit confirmed the clinical suspicion for Merkel cell carcinoma (MCC).
MCC is a cutaneous neuroendocrine malignancy. Although its name acknowledges similarities between the tumor cells and Merkel cells, it is now considered unlikely that Merkel cells are the actual cells of origin.1
The majority of MCCs are asymptomatic despite rapid growth and are typically red or pink and occur on UV-exposed areas, as in our patient.2 A cyst or acneiform lesion is the single most common diagnosis given at the time of biopsy.2
The incidence of MCC is greatest in people of advanced age and in those who are immunosuppressed. In the United States, the estimated annual incidence rate rose from 0.5 cases per 100,000 people in 2000 to 0.7 cases per 100,000 people in 2013.3 MCC increases exponentially with advancing age, from 0.1 (per 100,000) in those ages 40 to 44 years to 9.8 in those older than 85 years.3 The growing cohort of ageing baby boomers and the increased number of immunosuppressed individuals in the community suggest that clinicians are now more likely to encounter MCC than in the past.
While UV radiation is highly associated with MCC, the major causative factor is considered to be Merkel cell polyomavirus (MCPyV).1 In fact, MCPyV has been linked to 80% of MCC cases.1,3 Most people have positive serology for MCPyV in early childhood, but the association between MCC and old age highlights the impact of immunosuppression on MCPyV activity and MCC development.1
Clinical suspicion is the first step in diagnosing MCC
The mnemonic AEIOU highlights the key clinical features of this aggressive tumor2,4:
- Asymptomatic
- Expanding rapidly (often grows in less than 3 months)
- Immune suppression (eg, chronic lymphocytic leukemia, solid organ transplant patient)
- Older than 50
- UV exposure on fair skin.
If a lesion is suspected to be MCC, the next step includes biopsy so that a definitive diagnosis can be made. A firm, nontender nodule that lacks fluctuance should raise suspicion for a neoplastic process.
Continue to: The differential is broad, ranging from cysts to melanoma
The differential is broad, ranging from cysts to melanoma
The differential diagnosis for an enlarging, plum-colored nodule on sun-exposed skin includes an abscess, a ruptured or inflamed epidermoid cyst, basal cell carcinoma, squamous cell carcinoma, and malignant melanoma.
An abscess is typically tender and expands within a matter of days rather than months.
A cyst can be ruled out by the clinical appearance and lack of an overlying pore.
Basal cell carcinoma can be characterized by a rolled border and central ulceration.
Squamous cell carcinomas often exhibit a verrucous surface with marked hyperkeratosis.
Continue to: Melanoma
Melanoma manifests with brown or irregular pigmentation and may be associated with a precursor lesion.
Tx includes excision and consistent follow-up
Complete excision is the critical first step to successful therapy. Sentinel lymph node studies are typically performed because of the high incidence of lymph node metastasis. Frequent follow-up is required because of the high risk of recurrent or persistent disease.
Local recurrence usually occurs within 1 year of diagnosis in more than 40% of patients.5 Distant metastasis can be treated with a programmed cell death ligand 1 blocking agent (avelumab) or a programmed cell death protein 1 inhibitor (nivolumab or pembrolizumab).6
Our patient was referred to a regional cancer center for sentinel lymph node evaluation, where he was found to have nodal disease. The patient was put on pembrolizumab and received radiation therapy but showed only limited response. Seven months after diagnosis, he passed away from metastatic MCC.
1. Pietropaolo V, Prezioso C, Moens U. Merkel cell polyomavirus and Merkel cell carcinoma. Cancers (Basel). 2020;12:1774. doi: 10.3390/cancers12071774
2. Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381. doi: 10.1016/j.jaad.2007.11.020
3. Paulson KG, Park SY, Vandeven NA, et al. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78:457-463.e2. doi: 10.1016/j.jaad.2017.10.028
4. Voelker R. Why Merkel cell cancer is garnering more attention. JAMA. 2018;320:18-20. doi: 10.1001/jama.2018.7042
5. Allen PJ, Browne WB, Jacques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300-2309. doi: 10.1200/JCO.2005.02.329
6. D’Angelo SP, Russell J, Lebbé C, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol. 2018;4:e180077. doi: 10.1001/jamaoncol.2018.0077
An 85-year-old man with a history of skin cancer presented to my dermatology practice (NT) for evaluation of a “pimple” on his left cheek that failed to resolve after 2 months (FIGURE). The patient noted that the lesion had grown, but that he otherwise felt well.

On examination, the lesion was plum colored, and the area was firm and nontender to palpation. The patient was referred to a plastic surgeon for an excisional biopsy to clarify the nature of the lesion.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Merkel cell carcinoma
A biopsy performed 2 weeks after the initial visit confirmed the clinical suspicion for Merkel cell carcinoma (MCC).
MCC is a cutaneous neuroendocrine malignancy. Although its name acknowledges similarities between the tumor cells and Merkel cells, it is now considered unlikely that Merkel cells are the actual cells of origin.1
The majority of MCCs are asymptomatic despite rapid growth and are typically red or pink and occur on UV-exposed areas, as in our patient.2 A cyst or acneiform lesion is the single most common diagnosis given at the time of biopsy.2
The incidence of MCC is greatest in people of advanced age and in those who are immunosuppressed. In the United States, the estimated annual incidence rate rose from 0.5 cases per 100,000 people in 2000 to 0.7 cases per 100,000 people in 2013.3 MCC increases exponentially with advancing age, from 0.1 (per 100,000) in those ages 40 to 44 years to 9.8 in those older than 85 years.3 The growing cohort of ageing baby boomers and the increased number of immunosuppressed individuals in the community suggest that clinicians are now more likely to encounter MCC than in the past.
While UV radiation is highly associated with MCC, the major causative factor is considered to be Merkel cell polyomavirus (MCPyV).1 In fact, MCPyV has been linked to 80% of MCC cases.1,3 Most people have positive serology for MCPyV in early childhood, but the association between MCC and old age highlights the impact of immunosuppression on MCPyV activity and MCC development.1
Clinical suspicion is the first step in diagnosing MCC
The mnemonic AEIOU highlights the key clinical features of this aggressive tumor2,4:
- Asymptomatic
- Expanding rapidly (often grows in less than 3 months)
- Immune suppression (eg, chronic lymphocytic leukemia, solid organ transplant patient)
- Older than 50
- UV exposure on fair skin.
If a lesion is suspected to be MCC, the next step includes biopsy so that a definitive diagnosis can be made. A firm, nontender nodule that lacks fluctuance should raise suspicion for a neoplastic process.
Continue to: The differential is broad, ranging from cysts to melanoma
The differential is broad, ranging from cysts to melanoma
The differential diagnosis for an enlarging, plum-colored nodule on sun-exposed skin includes an abscess, a ruptured or inflamed epidermoid cyst, basal cell carcinoma, squamous cell carcinoma, and malignant melanoma.
An abscess is typically tender and expands within a matter of days rather than months.
A cyst can be ruled out by the clinical appearance and lack of an overlying pore.
Basal cell carcinoma can be characterized by a rolled border and central ulceration.
Squamous cell carcinomas often exhibit a verrucous surface with marked hyperkeratosis.
Continue to: Melanoma
Melanoma manifests with brown or irregular pigmentation and may be associated with a precursor lesion.
Tx includes excision and consistent follow-up
Complete excision is the critical first step to successful therapy. Sentinel lymph node studies are typically performed because of the high incidence of lymph node metastasis. Frequent follow-up is required because of the high risk of recurrent or persistent disease.
Local recurrence usually occurs within 1 year of diagnosis in more than 40% of patients.5 Distant metastasis can be treated with a programmed cell death ligand 1 blocking agent (avelumab) or a programmed cell death protein 1 inhibitor (nivolumab or pembrolizumab).6
Our patient was referred to a regional cancer center for sentinel lymph node evaluation, where he was found to have nodal disease. The patient was put on pembrolizumab and received radiation therapy but showed only limited response. Seven months after diagnosis, he passed away from metastatic MCC.
An 85-year-old man with a history of skin cancer presented to my dermatology practice (NT) for evaluation of a “pimple” on his left cheek that failed to resolve after 2 months (FIGURE). The patient noted that the lesion had grown, but that he otherwise felt well.

On examination, the lesion was plum colored, and the area was firm and nontender to palpation. The patient was referred to a plastic surgeon for an excisional biopsy to clarify the nature of the lesion.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Merkel cell carcinoma
A biopsy performed 2 weeks after the initial visit confirmed the clinical suspicion for Merkel cell carcinoma (MCC).
MCC is a cutaneous neuroendocrine malignancy. Although its name acknowledges similarities between the tumor cells and Merkel cells, it is now considered unlikely that Merkel cells are the actual cells of origin.1
The majority of MCCs are asymptomatic despite rapid growth and are typically red or pink and occur on UV-exposed areas, as in our patient.2 A cyst or acneiform lesion is the single most common diagnosis given at the time of biopsy.2
The incidence of MCC is greatest in people of advanced age and in those who are immunosuppressed. In the United States, the estimated annual incidence rate rose from 0.5 cases per 100,000 people in 2000 to 0.7 cases per 100,000 people in 2013.3 MCC increases exponentially with advancing age, from 0.1 (per 100,000) in those ages 40 to 44 years to 9.8 in those older than 85 years.3 The growing cohort of ageing baby boomers and the increased number of immunosuppressed individuals in the community suggest that clinicians are now more likely to encounter MCC than in the past.
While UV radiation is highly associated with MCC, the major causative factor is considered to be Merkel cell polyomavirus (MCPyV).1 In fact, MCPyV has been linked to 80% of MCC cases.1,3 Most people have positive serology for MCPyV in early childhood, but the association between MCC and old age highlights the impact of immunosuppression on MCPyV activity and MCC development.1
Clinical suspicion is the first step in diagnosing MCC
The mnemonic AEIOU highlights the key clinical features of this aggressive tumor2,4:
- Asymptomatic
- Expanding rapidly (often grows in less than 3 months)
- Immune suppression (eg, chronic lymphocytic leukemia, solid organ transplant patient)
- Older than 50
- UV exposure on fair skin.
If a lesion is suspected to be MCC, the next step includes biopsy so that a definitive diagnosis can be made. A firm, nontender nodule that lacks fluctuance should raise suspicion for a neoplastic process.
Continue to: The differential is broad, ranging from cysts to melanoma
The differential is broad, ranging from cysts to melanoma
The differential diagnosis for an enlarging, plum-colored nodule on sun-exposed skin includes an abscess, a ruptured or inflamed epidermoid cyst, basal cell carcinoma, squamous cell carcinoma, and malignant melanoma.
An abscess is typically tender and expands within a matter of days rather than months.
A cyst can be ruled out by the clinical appearance and lack of an overlying pore.
Basal cell carcinoma can be characterized by a rolled border and central ulceration.
Squamous cell carcinomas often exhibit a verrucous surface with marked hyperkeratosis.
Continue to: Melanoma
Melanoma manifests with brown or irregular pigmentation and may be associated with a precursor lesion.
Tx includes excision and consistent follow-up
Complete excision is the critical first step to successful therapy. Sentinel lymph node studies are typically performed because of the high incidence of lymph node metastasis. Frequent follow-up is required because of the high risk of recurrent or persistent disease.
Local recurrence usually occurs within 1 year of diagnosis in more than 40% of patients.5 Distant metastasis can be treated with a programmed cell death ligand 1 blocking agent (avelumab) or a programmed cell death protein 1 inhibitor (nivolumab or pembrolizumab).6
Our patient was referred to a regional cancer center for sentinel lymph node evaluation, where he was found to have nodal disease. The patient was put on pembrolizumab and received radiation therapy but showed only limited response. Seven months after diagnosis, he passed away from metastatic MCC.
1. Pietropaolo V, Prezioso C, Moens U. Merkel cell polyomavirus and Merkel cell carcinoma. Cancers (Basel). 2020;12:1774. doi: 10.3390/cancers12071774
2. Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381. doi: 10.1016/j.jaad.2007.11.020
3. Paulson KG, Park SY, Vandeven NA, et al. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78:457-463.e2. doi: 10.1016/j.jaad.2017.10.028
4. Voelker R. Why Merkel cell cancer is garnering more attention. JAMA. 2018;320:18-20. doi: 10.1001/jama.2018.7042
5. Allen PJ, Browne WB, Jacques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300-2309. doi: 10.1200/JCO.2005.02.329
6. D’Angelo SP, Russell J, Lebbé C, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol. 2018;4:e180077. doi: 10.1001/jamaoncol.2018.0077
1. Pietropaolo V, Prezioso C, Moens U. Merkel cell polyomavirus and Merkel cell carcinoma. Cancers (Basel). 2020;12:1774. doi: 10.3390/cancers12071774
2. Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381. doi: 10.1016/j.jaad.2007.11.020
3. Paulson KG, Park SY, Vandeven NA, et al. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78:457-463.e2. doi: 10.1016/j.jaad.2017.10.028
4. Voelker R. Why Merkel cell cancer is garnering more attention. JAMA. 2018;320:18-20. doi: 10.1001/jama.2018.7042
5. Allen PJ, Browne WB, Jacques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300-2309. doi: 10.1200/JCO.2005.02.329
6. D’Angelo SP, Russell J, Lebbé C, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol. 2018;4:e180077. doi: 10.1001/jamaoncol.2018.0077
Can extended anticoagulation prophylaxis after discharge prevent thromboembolism?
ILLUSTRATIVE CASE
A 67-year-old man with a history of type 2 diabetes, hypertension, and chronic congestive heart failure (ejection fraction = 30%) was admitted to the intensive care unit with a diagnosis of acute hypoxic respiratory failure. He was discharged after 10 days of inpatient treatment that included daily VTE prophylaxis with low-molecular-weight heparin (LMWH). Should he go home on VTE prophylaxis?
Patients hospitalized with nonsurgical conditions such as congestive heart failure, chronic obstructive pulmonary disease, sepsis, inflammatory bowel disease, or active cancers are at increased risk for VTE due to inflammation and immobility. In a US study of 158,325 hospitalized nonsurgical patients, including those with cancer, infections, congestive heart failure, or respiratory failure, 4% of patients developed
However, use of DOACs for short-term VTE prophylaxis as an alternative to LMWH in hospitalized patients is supported by a meta-analysis showing equivalent efficacy, safety, and cost-effectiveness.1 The current study examined DOACs for extended postdischarge use.1
STUDY SUMMARY
Significant benefit of DOACs demonstrated across 4 large trials
This meta-analysis of 4 large randomized controlled trials examined the safety and efficacy of 6 weeks of postdischarge DOAC thromboprophylaxis compared with placebo in 26,408 high-risk nonsurgical hospitalized patients.1 Patients at least 40 years old were admitted with diagnoses that included New York Heart Association (NYHA) class III or IV congestive heart failure, active cancer, acute ischemic stroke, acute respiratory failure, or infectious or inflammatory disease. Study patients also had risk factors for VTE, including age 75 and older, obesity, chronic venous insufficiency, history of VTE, history of NYHA class III or IV congestive heart failure, history of cancer, thrombophilia, hormone replacement therapy, or major surgery within the 6 to 12 weeks before current medical hospitalization.
Patients were excluded if DOACs were contraindicated or if they had active or recent bleeding, renal failure, abnormal liver values, an upcoming need for surgery, or an indication for ongoing anticoagulation. Patients in 3 studies received 6 to 10 days of enoxaparin as prophylaxis during their inpatient stay. (The fourth study did not specify length of inpatient prophylaxis or drug used.) After discharge, patients were assigned to placebo or a regimen of rivaroxaban 10 mg daily, apixaban 2.5 mg twice daily, or betrixaban 80 mg daily for a range of 30 to 45 days. The primary outcome was the composite of total VTE and VTE-related death. A secondary outcome was the occurrence of nonfatal symptomatic VTE, and the primary safety outcome was the incidence of major bleeding.
The primary outcome occurred in 2.9% of the patients in the DOAC group compared with 3.6% of patients in the placebo group (odds ratio [OR] = 0.79; 95% CI, 0.69-0.91; number needed to treat [NNT] = 143). The secondary outcome occurred in 0.48% of patients in the DOAC group compared with 0.77% of patients in the placebo group (OR = 0.62; 95% CI, 0.47-0.83; NNT = 345). Major bleeding resulting in a decrease in hemoglobin concentration of more than 2 g/L, requiring transfusion of at least 2 units of packed red blood cells, reintervention at a previous surgical site, or bleeding in a critical organ or that was fatal, occurred in 0.58% of patients in the DOAC group compared with 0.3% of patients in the placebo group (OR = 1.9; 95% CI, 1.4-2.7; number needed to harm [NNH] = 357). Nonmajor bleeding was increased in the DOAC group compared with placebo (2.2% vs 1.2%; OR = 1.8; 95% CI, 1.5-2.1; NNH = 110).
The NNT to prevent a fatal VTE was 899 patients
Continue to: WHAT'S NEW
WHAT’S NEW
Mortality and morbidity benefit with small bleeding risk
Based on this study, for every 300 high-risk patients hospitalized with nonsurgical diagnoses who are given 6 weeks of DOAC prophylaxis, there will be 2 fewer cases of VTE and VTE-related death. In this same group of patients, there will be approximately 1 major bleeding event and 3 less serious bleeds.
Patients with preexisting medical conditions such as congestive heart failure, cancer, and sepsis and those admitted to an intensive care unit are at increased risk for DVT after discharge.5 Extending DOAC prophylaxis in nonsurgical patients with serious medical conditions for 6 weeks after discharge reduces the risk of VTE or VTE-related death by 0.7% compared with placebo. Treatment in this population does incur a small increased risk of major bleeding by 0.3% in the DOAC group compared with placebo.
CAVEATS
Results cannot be generalized to all patient populations
Many high-risk patients have chronic kidney disease, and because DOACs (including apixaban, rivaroxaban, and dabigatran) are renally cleared, there are limited data to establish their safety in patients with creatinine clearance ≤ 30 mL/min. Benefits seen with DOACs cannot be extrapolated to other anticoagulation agents, including warfarin or LMWH.
In accordance with new guidelines, some of the patients in this study would now receive antiplatelet therapy, eg, poststroke patients, cancer patients, and—with the ease of DOAC use—patients with atrial fibrillation. If these patients were excluded, it is not known whether the benefit would remain. Patients included in these trials were at particularly high risk for VTE, and the benefits seen in this study cannot be generalized to a patient population with fewer VTE risk factors.
CHALLENGES TO IMPLEMENTATION
High cost and lack of updated guidelines may limit DOAC thromboprophylaxis
Cost is a concern. All the new DOACs are expensive; for example, rivaroxaban costs a little less than $500 per month.6 Obtaining insurance coverage for a novel indication may be challenging. The American Society of Hematology and others have not yet endorsed extended posthospital thromboprophylaxis in nonsurgical patients, although the use of DOACs has expanded since the last guideline revisions.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Bhalla V, Lamping OF, Abdel-Latif A, et al. Contemporary meta-analysis of extended direct-acting oral anticoagulant thromboprophylaxis to prevent venous thromboembolism. Am J Med. 2020;133:1074-1081.e8. doi: 10.1016/j.amjmed.2020.01.037
2. Spyropoulos AC, Hussein M, Lin J, et al. Rates of venous thromboembolism occurrence in medical patients among the insured population. Thromb Haemost. 2009;102:951-957. doi: 10.1160/TH09-02-0073
3. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e195S-226S. doi: 10.1378/chest.11-2296
4. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198-3225. doi: 10.1182/bloodadvances.2018022954
5. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(suppl):I-4-I-8. doi: 10.1161/01.CIR.0000078468.11849.66
6. Rivaroxaban . GoodRx. Accessed August 10, 2021. www.goodrx.com/rivaroxaban
ILLUSTRATIVE CASE
A 67-year-old man with a history of type 2 diabetes, hypertension, and chronic congestive heart failure (ejection fraction = 30%) was admitted to the intensive care unit with a diagnosis of acute hypoxic respiratory failure. He was discharged after 10 days of inpatient treatment that included daily VTE prophylaxis with low-molecular-weight heparin (LMWH). Should he go home on VTE prophylaxis?
Patients hospitalized with nonsurgical conditions such as congestive heart failure, chronic obstructive pulmonary disease, sepsis, inflammatory bowel disease, or active cancers are at increased risk for VTE due to inflammation and immobility. In a US study of 158,325 hospitalized nonsurgical patients, including those with cancer, infections, congestive heart failure, or respiratory failure, 4% of patients developed
However, use of DOACs for short-term VTE prophylaxis as an alternative to LMWH in hospitalized patients is supported by a meta-analysis showing equivalent efficacy, safety, and cost-effectiveness.1 The current study examined DOACs for extended postdischarge use.1
STUDY SUMMARY
Significant benefit of DOACs demonstrated across 4 large trials
This meta-analysis of 4 large randomized controlled trials examined the safety and efficacy of 6 weeks of postdischarge DOAC thromboprophylaxis compared with placebo in 26,408 high-risk nonsurgical hospitalized patients.1 Patients at least 40 years old were admitted with diagnoses that included New York Heart Association (NYHA) class III or IV congestive heart failure, active cancer, acute ischemic stroke, acute respiratory failure, or infectious or inflammatory disease. Study patients also had risk factors for VTE, including age 75 and older, obesity, chronic venous insufficiency, history of VTE, history of NYHA class III or IV congestive heart failure, history of cancer, thrombophilia, hormone replacement therapy, or major surgery within the 6 to 12 weeks before current medical hospitalization.
Patients were excluded if DOACs were contraindicated or if they had active or recent bleeding, renal failure, abnormal liver values, an upcoming need for surgery, or an indication for ongoing anticoagulation. Patients in 3 studies received 6 to 10 days of enoxaparin as prophylaxis during their inpatient stay. (The fourth study did not specify length of inpatient prophylaxis or drug used.) After discharge, patients were assigned to placebo or a regimen of rivaroxaban 10 mg daily, apixaban 2.5 mg twice daily, or betrixaban 80 mg daily for a range of 30 to 45 days. The primary outcome was the composite of total VTE and VTE-related death. A secondary outcome was the occurrence of nonfatal symptomatic VTE, and the primary safety outcome was the incidence of major bleeding.
The primary outcome occurred in 2.9% of the patients in the DOAC group compared with 3.6% of patients in the placebo group (odds ratio [OR] = 0.79; 95% CI, 0.69-0.91; number needed to treat [NNT] = 143). The secondary outcome occurred in 0.48% of patients in the DOAC group compared with 0.77% of patients in the placebo group (OR = 0.62; 95% CI, 0.47-0.83; NNT = 345). Major bleeding resulting in a decrease in hemoglobin concentration of more than 2 g/L, requiring transfusion of at least 2 units of packed red blood cells, reintervention at a previous surgical site, or bleeding in a critical organ or that was fatal, occurred in 0.58% of patients in the DOAC group compared with 0.3% of patients in the placebo group (OR = 1.9; 95% CI, 1.4-2.7; number needed to harm [NNH] = 357). Nonmajor bleeding was increased in the DOAC group compared with placebo (2.2% vs 1.2%; OR = 1.8; 95% CI, 1.5-2.1; NNH = 110).
The NNT to prevent a fatal VTE was 899 patients
Continue to: WHAT'S NEW
WHAT’S NEW
Mortality and morbidity benefit with small bleeding risk
Based on this study, for every 300 high-risk patients hospitalized with nonsurgical diagnoses who are given 6 weeks of DOAC prophylaxis, there will be 2 fewer cases of VTE and VTE-related death. In this same group of patients, there will be approximately 1 major bleeding event and 3 less serious bleeds.
Patients with preexisting medical conditions such as congestive heart failure, cancer, and sepsis and those admitted to an intensive care unit are at increased risk for DVT after discharge.5 Extending DOAC prophylaxis in nonsurgical patients with serious medical conditions for 6 weeks after discharge reduces the risk of VTE or VTE-related death by 0.7% compared with placebo. Treatment in this population does incur a small increased risk of major bleeding by 0.3% in the DOAC group compared with placebo.
CAVEATS
Results cannot be generalized to all patient populations
Many high-risk patients have chronic kidney disease, and because DOACs (including apixaban, rivaroxaban, and dabigatran) are renally cleared, there are limited data to establish their safety in patients with creatinine clearance ≤ 30 mL/min. Benefits seen with DOACs cannot be extrapolated to other anticoagulation agents, including warfarin or LMWH.
In accordance with new guidelines, some of the patients in this study would now receive antiplatelet therapy, eg, poststroke patients, cancer patients, and—with the ease of DOAC use—patients with atrial fibrillation. If these patients were excluded, it is not known whether the benefit would remain. Patients included in these trials were at particularly high risk for VTE, and the benefits seen in this study cannot be generalized to a patient population with fewer VTE risk factors.
CHALLENGES TO IMPLEMENTATION
High cost and lack of updated guidelines may limit DOAC thromboprophylaxis
Cost is a concern. All the new DOACs are expensive; for example, rivaroxaban costs a little less than $500 per month.6 Obtaining insurance coverage for a novel indication may be challenging. The American Society of Hematology and others have not yet endorsed extended posthospital thromboprophylaxis in nonsurgical patients, although the use of DOACs has expanded since the last guideline revisions.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 67-year-old man with a history of type 2 diabetes, hypertension, and chronic congestive heart failure (ejection fraction = 30%) was admitted to the intensive care unit with a diagnosis of acute hypoxic respiratory failure. He was discharged after 10 days of inpatient treatment that included daily VTE prophylaxis with low-molecular-weight heparin (LMWH). Should he go home on VTE prophylaxis?
Patients hospitalized with nonsurgical conditions such as congestive heart failure, chronic obstructive pulmonary disease, sepsis, inflammatory bowel disease, or active cancers are at increased risk for VTE due to inflammation and immobility. In a US study of 158,325 hospitalized nonsurgical patients, including those with cancer, infections, congestive heart failure, or respiratory failure, 4% of patients developed
However, use of DOACs for short-term VTE prophylaxis as an alternative to LMWH in hospitalized patients is supported by a meta-analysis showing equivalent efficacy, safety, and cost-effectiveness.1 The current study examined DOACs for extended postdischarge use.1
STUDY SUMMARY
Significant benefit of DOACs demonstrated across 4 large trials
This meta-analysis of 4 large randomized controlled trials examined the safety and efficacy of 6 weeks of postdischarge DOAC thromboprophylaxis compared with placebo in 26,408 high-risk nonsurgical hospitalized patients.1 Patients at least 40 years old were admitted with diagnoses that included New York Heart Association (NYHA) class III or IV congestive heart failure, active cancer, acute ischemic stroke, acute respiratory failure, or infectious or inflammatory disease. Study patients also had risk factors for VTE, including age 75 and older, obesity, chronic venous insufficiency, history of VTE, history of NYHA class III or IV congestive heart failure, history of cancer, thrombophilia, hormone replacement therapy, or major surgery within the 6 to 12 weeks before current medical hospitalization.
Patients were excluded if DOACs were contraindicated or if they had active or recent bleeding, renal failure, abnormal liver values, an upcoming need for surgery, or an indication for ongoing anticoagulation. Patients in 3 studies received 6 to 10 days of enoxaparin as prophylaxis during their inpatient stay. (The fourth study did not specify length of inpatient prophylaxis or drug used.) After discharge, patients were assigned to placebo or a regimen of rivaroxaban 10 mg daily, apixaban 2.5 mg twice daily, or betrixaban 80 mg daily for a range of 30 to 45 days. The primary outcome was the composite of total VTE and VTE-related death. A secondary outcome was the occurrence of nonfatal symptomatic VTE, and the primary safety outcome was the incidence of major bleeding.
The primary outcome occurred in 2.9% of the patients in the DOAC group compared with 3.6% of patients in the placebo group (odds ratio [OR] = 0.79; 95% CI, 0.69-0.91; number needed to treat [NNT] = 143). The secondary outcome occurred in 0.48% of patients in the DOAC group compared with 0.77% of patients in the placebo group (OR = 0.62; 95% CI, 0.47-0.83; NNT = 345). Major bleeding resulting in a decrease in hemoglobin concentration of more than 2 g/L, requiring transfusion of at least 2 units of packed red blood cells, reintervention at a previous surgical site, or bleeding in a critical organ or that was fatal, occurred in 0.58% of patients in the DOAC group compared with 0.3% of patients in the placebo group (OR = 1.9; 95% CI, 1.4-2.7; number needed to harm [NNH] = 357). Nonmajor bleeding was increased in the DOAC group compared with placebo (2.2% vs 1.2%; OR = 1.8; 95% CI, 1.5-2.1; NNH = 110).
The NNT to prevent a fatal VTE was 899 patients
Continue to: WHAT'S NEW
WHAT’S NEW
Mortality and morbidity benefit with small bleeding risk
Based on this study, for every 300 high-risk patients hospitalized with nonsurgical diagnoses who are given 6 weeks of DOAC prophylaxis, there will be 2 fewer cases of VTE and VTE-related death. In this same group of patients, there will be approximately 1 major bleeding event and 3 less serious bleeds.
Patients with preexisting medical conditions such as congestive heart failure, cancer, and sepsis and those admitted to an intensive care unit are at increased risk for DVT after discharge.5 Extending DOAC prophylaxis in nonsurgical patients with serious medical conditions for 6 weeks after discharge reduces the risk of VTE or VTE-related death by 0.7% compared with placebo. Treatment in this population does incur a small increased risk of major bleeding by 0.3% in the DOAC group compared with placebo.
CAVEATS
Results cannot be generalized to all patient populations
Many high-risk patients have chronic kidney disease, and because DOACs (including apixaban, rivaroxaban, and dabigatran) are renally cleared, there are limited data to establish their safety in patients with creatinine clearance ≤ 30 mL/min. Benefits seen with DOACs cannot be extrapolated to other anticoagulation agents, including warfarin or LMWH.
In accordance with new guidelines, some of the patients in this study would now receive antiplatelet therapy, eg, poststroke patients, cancer patients, and—with the ease of DOAC use—patients with atrial fibrillation. If these patients were excluded, it is not known whether the benefit would remain. Patients included in these trials were at particularly high risk for VTE, and the benefits seen in this study cannot be generalized to a patient population with fewer VTE risk factors.
CHALLENGES TO IMPLEMENTATION
High cost and lack of updated guidelines may limit DOAC thromboprophylaxis
Cost is a concern. All the new DOACs are expensive; for example, rivaroxaban costs a little less than $500 per month.6 Obtaining insurance coverage for a novel indication may be challenging. The American Society of Hematology and others have not yet endorsed extended posthospital thromboprophylaxis in nonsurgical patients, although the use of DOACs has expanded since the last guideline revisions.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Bhalla V, Lamping OF, Abdel-Latif A, et al. Contemporary meta-analysis of extended direct-acting oral anticoagulant thromboprophylaxis to prevent venous thromboembolism. Am J Med. 2020;133:1074-1081.e8. doi: 10.1016/j.amjmed.2020.01.037
2. Spyropoulos AC, Hussein M, Lin J, et al. Rates of venous thromboembolism occurrence in medical patients among the insured population. Thromb Haemost. 2009;102:951-957. doi: 10.1160/TH09-02-0073
3. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e195S-226S. doi: 10.1378/chest.11-2296
4. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198-3225. doi: 10.1182/bloodadvances.2018022954
5. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(suppl):I-4-I-8. doi: 10.1161/01.CIR.0000078468.11849.66
6. Rivaroxaban . GoodRx. Accessed August 10, 2021. www.goodrx.com/rivaroxaban
1. Bhalla V, Lamping OF, Abdel-Latif A, et al. Contemporary meta-analysis of extended direct-acting oral anticoagulant thromboprophylaxis to prevent venous thromboembolism. Am J Med. 2020;133:1074-1081.e8. doi: 10.1016/j.amjmed.2020.01.037
2. Spyropoulos AC, Hussein M, Lin J, et al. Rates of venous thromboembolism occurrence in medical patients among the insured population. Thromb Haemost. 2009;102:951-957. doi: 10.1160/TH09-02-0073
3. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):e195S-226S. doi: 10.1378/chest.11-2296
4. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198-3225. doi: 10.1182/bloodadvances.2018022954
5. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(suppl):I-4-I-8. doi: 10.1161/01.CIR.0000078468.11849.66
6. Rivaroxaban . GoodRx. Accessed August 10, 2021. www.goodrx.com/rivaroxaban
PRACTICE CHANGER
Treat seriously ill patients with a
STRENGTH OF RECOMMENDATION
A: Meta-analysis of randomized clinical trials1
Bhalla V, Lamping OF, Abdel-Latif A, et al. Contemporary meta-analysis of extended direct-acting oral anticoagulant thromboprophylaxis to prevent venous thromboembolism. Am J Med. 2020;133:1074-1081.e8. doi: 10.1016/j.amjmed.2020.01.037
Antimicrobial resistance linked to 1.2 million global deaths in 2019
More than HIV, more than malaria.
In terms of preventable deaths, 1.27 million people could have been saved if drug-resistant infections were replaced with infections susceptible to current antibiotics. Furthermore, 4.95 million fewer people would have died if drug-resistant infections were replaced by no infections, researchers estimated.
Although the COVID-19 pandemic took some focus off the AMR burden worldwide over the past 2 years, the urgency to address risk to public health did not ebb. In fact, based on the findings, the researchers noted that AMR is now a leading cause of death worldwide.
“If left unchecked, the spread of AMR could make many bacterial pathogens much more lethal in the future than they are today,” the researchers noted in the study, published online Jan. 20, 2022, in The Lancet.
“These findings are a warning signal that antibiotic resistance is placing pressure on health care systems and leading to significant health loss,” study author Kevin Ikuta, MD, MPH, told this news organization.
“We need to continue to adhere to and support infection prevention and control programs, be thoughtful about our antibiotic use, and advocate for increased funding to vaccine discovery and the antibiotic development pipeline,” added Dr. Ikuta, health sciences assistant clinical professor of medicine at the University of California, Los Angeles.
Although many investigators have studied AMR, this study is the largest in scope, covering 204 countries and territories and incorporating data on a comprehensive range of pathogens and pathogen-drug combinations.
Dr. Ikuta, lead author Christopher J.L. Murray, DPhil, and colleagues estimated the global burden of AMR using the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019. They specifically looked at rates of death directly attributed to and separately those associated with resistance.
Regional differences
Broken down by 21 regions, Australasia had 6.5 deaths per 100,000 people attributable to AMR, the lowest rate reported. This region also had 28 deaths per 100,000 associated with AMR.
Researchers found the highest rates in western sub-Saharan Africa. Deaths attributable to AMR were 27.3 per 100,000 and associated death rate was 114.8 per 100,000.
Lower- and middle-income regions had the highest AMR death rates, although resistance remains a high-priority issue for high-income countries as well.
“It’s important to take a global perspective on resistant infections because we can learn about regions and countries that are experiencing the greatest burden, information that was previously unknown,” Dr. Ikuta said. “With these estimates policy makers can prioritize regions that are hotspots and would most benefit from additional interventions.”
Furthermore, the study emphasized the global nature of AMR. “We’ve seen over the last 2 years with COVID-19 that this sort of problem doesn’t respect country borders, and high rates of resistance in one location can spread across a region or spread globally pretty quickly,” Dr. Ikuta said.
Leading resistant infections
Lower respiratory and thorax infections, bloodstream infections, and intra-abdominal infections together accounted for almost 79% of such deaths linked to AMR.
The six leading pathogens are likely household names among infectious disease specialists. The researchers found Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, each responsible for more than 250,000 AMR-associated deaths.
The study also revealed that resistance to several first-line antibiotic agents often used empirically to treat infections accounted for more than 70% of the AMR-attributable deaths. These included fluoroquinolones and beta-lactam antibiotics such as carbapenems, cephalosporins, and penicillins.

Consistent with previous studies, MRSA stood out as a major cause of mortality. Of 88 different pathogen-drug combinations evaluated, MRSA was responsible for the most mortality: more than 100,000 deaths and 3·5 million disability-adjusted life-years.
The current study findings on MRSA “being a particularly nasty culprit” in AMR infections validates previous work that reported similar results, Vance Fowler, MD, told this news organization when asked to comment on the research. “That is reassuring.”
Potential solutions offered
Dr. Murray and colleagues outlined five strategies to address the challenge of bacterial AMR:
- Infection prevention and control remain paramount in minimizing infections in general and AMR infections in particular.
- More vaccines are needed to reduce the need for antibiotics. “Vaccines are available for only one of the six leading pathogens (S. pneumoniae), although new vaccine programs are underway for S. aureus, E. coli, and others,” the researchers wrote.
- Reduce antibiotic use unrelated to treatment of human disease.
- Avoid using antibiotics for viral infections and other unnecessary indications.
- Invest in new antibiotic development and ensure access to second-line agents in areas without widespread access.
“Identifying strategies that can work to reduce the burden of bacterial AMR – either across a wide range of settings or those that are specifically tailored to the resources available and leading pathogen-drug combinations in a particular setting – is an urgent priority,” the researchers noted.
Admirable AMR research
The results of the study are “startling, but not surprising,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C.
The authors did a “nice job” of addressing both deaths attributable and associated with AMR, Dr. Fowler added. “Those two categories unlock applications, not just in terms of how you interpret it but also what you do about it.”
The deaths attributable to AMR show that there is more work to be done regarding infection control and prevention, Dr. Fowler said, including in areas of the world like lower- and middle-income countries where infection resistance is most pronounced.
The deaths associated with AMR can be more challenging to calculate – people with infections can die for multiple reasons. However, Dr. Fowler applauded the researchers for doing “as good a job as you can” in estimating the extent of associated mortality.
‘The overlooked pandemic of antimicrobial resistance’
In an accompanying editorial in The Lancet, Ramanan Laxminarayan, PhD, MPH, wrote: “As COVID-19 rages on, the pandemic of antimicrobial resistance continues in the shadows. The toll taken by AMR on patients and their families is largely invisible but is reflected in prolonged bacterial infections that extend hospital stays and cause needless deaths.”
Dr. Laxminarayan pointed out an irony with AMR in different regions. Some of the AMR burden in sub-Saharan Africa is “probably due to inadequate access to antibiotics and high infection levels, albeit at low levels of resistance, whereas in south Asia and Latin America, it is because of high resistance even with good access to antibiotics.”
More funding to address AMR is needed, Dr. Laxminarayan noted. “Even the lower end of 911,000 deaths estimated by Murray and colleagues is higher than the number of deaths from HIV, which attracts close to U.S. $50 billion each year. However, global spending on addressing AMR is probably much lower than that.” Dr. Laxminarayan is an economist and epidemiologist affiliated with the Center for Disease Dynamics, Economics & Policy in Washington, D.C., and the Global Antibiotic Research and Development Partnership in Geneva.
An overlap with COVID-19
The Lancet report is likely “to bring more attention to AMR, especially since so many people have been distracted by COVID, and rightly so,” Dr. Fowler predicted. “The world has had its hands full with COVID.”
The two infections interact in direct ways, Dr. Fowler added. For example, some people hospitalized for COVID-19 for an extended time could develop progressively drug-resistant bacteria – leading to a superinfection.
The overlap could be illustrated by a Venn diagram, he said. A yellow circle could illustrate people with COVID-19 who are asymptomatic or who remain outpatients. Next to that would be a blue circle showing people who develop AMR infections. Where the two circles overlap would be green for those hospitalized who – because of receiving steroids, being on a ventilator, or getting a central line – develop a superinfection.
Official guidance continues
The study comes in the context of recent guidance and federal action on AMR. For example, the Infectious Diseases Society of America released new guidelines for AMR in November 2021 as part of ongoing advice on prevention and treatment of this “ongoing crisis.”
This most recent IDSA guidance addresses three pathogens in particular: AmpC beta-lactamase–producing Enterobacterales, carbapenem-resistant A. baumannii, and Stenotrophomonas maltophilia.
Also in November, the World Health Organization released an updated fact sheet on antimicrobial resistance. The WHO declared AMR one of the world’s top 10 global public health threats. The agency emphasized that misuse and overuse of antimicrobials are the main drivers in the development of drug-resistant pathogens. The WHO also pointed out that lack of clean water and sanitation in many areas of the world contribute to spread of microbes, including those resistant to current treatment options.
In September 2021, the Biden administration acknowledged the threat of AMR with allocation of more than $2 billion of the American Rescue Plan money for prevention and treatment of these infections.
Asked if there are any reasons for hope or optimism at this point, Dr. Ikuta said: “Definitely. We know what needs to be done to combat the spread of resistance. COVID-19 has demonstrated the importance of global commitment to infection control measures, such as hand washing and surveillance, and rapid investments in treatments, which can all be applied to antimicrobial resistance.”
The Bill & Melinda Gates Foundation, the Wellcome Trust, and the U.K. Department of Health and Social Care using U.K. aid funding managed by the Fleming Fund and other organizations provided funding for the study. Dr. Ikuta and Dr. Laxminarayan have disclosed no relevant financial relationships. Dr. Fowler reported receiving grants or honoraria, as well as serving as a consultant, for numerous sources. He also reported a patent pending in sepsis diagnostics and serving as chair of the V710 Scientific Advisory Committee (Merck).
A version of this article first appeared on Medscape.com.
More than HIV, more than malaria.
In terms of preventable deaths, 1.27 million people could have been saved if drug-resistant infections were replaced with infections susceptible to current antibiotics. Furthermore, 4.95 million fewer people would have died if drug-resistant infections were replaced by no infections, researchers estimated.
Although the COVID-19 pandemic took some focus off the AMR burden worldwide over the past 2 years, the urgency to address risk to public health did not ebb. In fact, based on the findings, the researchers noted that AMR is now a leading cause of death worldwide.
“If left unchecked, the spread of AMR could make many bacterial pathogens much more lethal in the future than they are today,” the researchers noted in the study, published online Jan. 20, 2022, in The Lancet.
“These findings are a warning signal that antibiotic resistance is placing pressure on health care systems and leading to significant health loss,” study author Kevin Ikuta, MD, MPH, told this news organization.
“We need to continue to adhere to and support infection prevention and control programs, be thoughtful about our antibiotic use, and advocate for increased funding to vaccine discovery and the antibiotic development pipeline,” added Dr. Ikuta, health sciences assistant clinical professor of medicine at the University of California, Los Angeles.
Although many investigators have studied AMR, this study is the largest in scope, covering 204 countries and territories and incorporating data on a comprehensive range of pathogens and pathogen-drug combinations.
Dr. Ikuta, lead author Christopher J.L. Murray, DPhil, and colleagues estimated the global burden of AMR using the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019. They specifically looked at rates of death directly attributed to and separately those associated with resistance.
Regional differences
Broken down by 21 regions, Australasia had 6.5 deaths per 100,000 people attributable to AMR, the lowest rate reported. This region also had 28 deaths per 100,000 associated with AMR.
Researchers found the highest rates in western sub-Saharan Africa. Deaths attributable to AMR were 27.3 per 100,000 and associated death rate was 114.8 per 100,000.
Lower- and middle-income regions had the highest AMR death rates, although resistance remains a high-priority issue for high-income countries as well.
“It’s important to take a global perspective on resistant infections because we can learn about regions and countries that are experiencing the greatest burden, information that was previously unknown,” Dr. Ikuta said. “With these estimates policy makers can prioritize regions that are hotspots and would most benefit from additional interventions.”
Furthermore, the study emphasized the global nature of AMR. “We’ve seen over the last 2 years with COVID-19 that this sort of problem doesn’t respect country borders, and high rates of resistance in one location can spread across a region or spread globally pretty quickly,” Dr. Ikuta said.
Leading resistant infections
Lower respiratory and thorax infections, bloodstream infections, and intra-abdominal infections together accounted for almost 79% of such deaths linked to AMR.
The six leading pathogens are likely household names among infectious disease specialists. The researchers found Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, each responsible for more than 250,000 AMR-associated deaths.
The study also revealed that resistance to several first-line antibiotic agents often used empirically to treat infections accounted for more than 70% of the AMR-attributable deaths. These included fluoroquinolones and beta-lactam antibiotics such as carbapenems, cephalosporins, and penicillins.

Consistent with previous studies, MRSA stood out as a major cause of mortality. Of 88 different pathogen-drug combinations evaluated, MRSA was responsible for the most mortality: more than 100,000 deaths and 3·5 million disability-adjusted life-years.
The current study findings on MRSA “being a particularly nasty culprit” in AMR infections validates previous work that reported similar results, Vance Fowler, MD, told this news organization when asked to comment on the research. “That is reassuring.”
Potential solutions offered
Dr. Murray and colleagues outlined five strategies to address the challenge of bacterial AMR:
- Infection prevention and control remain paramount in minimizing infections in general and AMR infections in particular.
- More vaccines are needed to reduce the need for antibiotics. “Vaccines are available for only one of the six leading pathogens (S. pneumoniae), although new vaccine programs are underway for S. aureus, E. coli, and others,” the researchers wrote.
- Reduce antibiotic use unrelated to treatment of human disease.
- Avoid using antibiotics for viral infections and other unnecessary indications.
- Invest in new antibiotic development and ensure access to second-line agents in areas without widespread access.
“Identifying strategies that can work to reduce the burden of bacterial AMR – either across a wide range of settings or those that are specifically tailored to the resources available and leading pathogen-drug combinations in a particular setting – is an urgent priority,” the researchers noted.
Admirable AMR research
The results of the study are “startling, but not surprising,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C.
The authors did a “nice job” of addressing both deaths attributable and associated with AMR, Dr. Fowler added. “Those two categories unlock applications, not just in terms of how you interpret it but also what you do about it.”
The deaths attributable to AMR show that there is more work to be done regarding infection control and prevention, Dr. Fowler said, including in areas of the world like lower- and middle-income countries where infection resistance is most pronounced.
The deaths associated with AMR can be more challenging to calculate – people with infections can die for multiple reasons. However, Dr. Fowler applauded the researchers for doing “as good a job as you can” in estimating the extent of associated mortality.
‘The overlooked pandemic of antimicrobial resistance’
In an accompanying editorial in The Lancet, Ramanan Laxminarayan, PhD, MPH, wrote: “As COVID-19 rages on, the pandemic of antimicrobial resistance continues in the shadows. The toll taken by AMR on patients and their families is largely invisible but is reflected in prolonged bacterial infections that extend hospital stays and cause needless deaths.”
Dr. Laxminarayan pointed out an irony with AMR in different regions. Some of the AMR burden in sub-Saharan Africa is “probably due to inadequate access to antibiotics and high infection levels, albeit at low levels of resistance, whereas in south Asia and Latin America, it is because of high resistance even with good access to antibiotics.”
More funding to address AMR is needed, Dr. Laxminarayan noted. “Even the lower end of 911,000 deaths estimated by Murray and colleagues is higher than the number of deaths from HIV, which attracts close to U.S. $50 billion each year. However, global spending on addressing AMR is probably much lower than that.” Dr. Laxminarayan is an economist and epidemiologist affiliated with the Center for Disease Dynamics, Economics & Policy in Washington, D.C., and the Global Antibiotic Research and Development Partnership in Geneva.
An overlap with COVID-19
The Lancet report is likely “to bring more attention to AMR, especially since so many people have been distracted by COVID, and rightly so,” Dr. Fowler predicted. “The world has had its hands full with COVID.”
The two infections interact in direct ways, Dr. Fowler added. For example, some people hospitalized for COVID-19 for an extended time could develop progressively drug-resistant bacteria – leading to a superinfection.
The overlap could be illustrated by a Venn diagram, he said. A yellow circle could illustrate people with COVID-19 who are asymptomatic or who remain outpatients. Next to that would be a blue circle showing people who develop AMR infections. Where the two circles overlap would be green for those hospitalized who – because of receiving steroids, being on a ventilator, or getting a central line – develop a superinfection.
Official guidance continues
The study comes in the context of recent guidance and federal action on AMR. For example, the Infectious Diseases Society of America released new guidelines for AMR in November 2021 as part of ongoing advice on prevention and treatment of this “ongoing crisis.”
This most recent IDSA guidance addresses three pathogens in particular: AmpC beta-lactamase–producing Enterobacterales, carbapenem-resistant A. baumannii, and Stenotrophomonas maltophilia.
Also in November, the World Health Organization released an updated fact sheet on antimicrobial resistance. The WHO declared AMR one of the world’s top 10 global public health threats. The agency emphasized that misuse and overuse of antimicrobials are the main drivers in the development of drug-resistant pathogens. The WHO also pointed out that lack of clean water and sanitation in many areas of the world contribute to spread of microbes, including those resistant to current treatment options.
In September 2021, the Biden administration acknowledged the threat of AMR with allocation of more than $2 billion of the American Rescue Plan money for prevention and treatment of these infections.
Asked if there are any reasons for hope or optimism at this point, Dr. Ikuta said: “Definitely. We know what needs to be done to combat the spread of resistance. COVID-19 has demonstrated the importance of global commitment to infection control measures, such as hand washing and surveillance, and rapid investments in treatments, which can all be applied to antimicrobial resistance.”
The Bill & Melinda Gates Foundation, the Wellcome Trust, and the U.K. Department of Health and Social Care using U.K. aid funding managed by the Fleming Fund and other organizations provided funding for the study. Dr. Ikuta and Dr. Laxminarayan have disclosed no relevant financial relationships. Dr. Fowler reported receiving grants or honoraria, as well as serving as a consultant, for numerous sources. He also reported a patent pending in sepsis diagnostics and serving as chair of the V710 Scientific Advisory Committee (Merck).
A version of this article first appeared on Medscape.com.
More than HIV, more than malaria.
In terms of preventable deaths, 1.27 million people could have been saved if drug-resistant infections were replaced with infections susceptible to current antibiotics. Furthermore, 4.95 million fewer people would have died if drug-resistant infections were replaced by no infections, researchers estimated.
Although the COVID-19 pandemic took some focus off the AMR burden worldwide over the past 2 years, the urgency to address risk to public health did not ebb. In fact, based on the findings, the researchers noted that AMR is now a leading cause of death worldwide.
“If left unchecked, the spread of AMR could make many bacterial pathogens much more lethal in the future than they are today,” the researchers noted in the study, published online Jan. 20, 2022, in The Lancet.
“These findings are a warning signal that antibiotic resistance is placing pressure on health care systems and leading to significant health loss,” study author Kevin Ikuta, MD, MPH, told this news organization.
“We need to continue to adhere to and support infection prevention and control programs, be thoughtful about our antibiotic use, and advocate for increased funding to vaccine discovery and the antibiotic development pipeline,” added Dr. Ikuta, health sciences assistant clinical professor of medicine at the University of California, Los Angeles.
Although many investigators have studied AMR, this study is the largest in scope, covering 204 countries and territories and incorporating data on a comprehensive range of pathogens and pathogen-drug combinations.
Dr. Ikuta, lead author Christopher J.L. Murray, DPhil, and colleagues estimated the global burden of AMR using the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019. They specifically looked at rates of death directly attributed to and separately those associated with resistance.
Regional differences
Broken down by 21 regions, Australasia had 6.5 deaths per 100,000 people attributable to AMR, the lowest rate reported. This region also had 28 deaths per 100,000 associated with AMR.
Researchers found the highest rates in western sub-Saharan Africa. Deaths attributable to AMR were 27.3 per 100,000 and associated death rate was 114.8 per 100,000.
Lower- and middle-income regions had the highest AMR death rates, although resistance remains a high-priority issue for high-income countries as well.
“It’s important to take a global perspective on resistant infections because we can learn about regions and countries that are experiencing the greatest burden, information that was previously unknown,” Dr. Ikuta said. “With these estimates policy makers can prioritize regions that are hotspots and would most benefit from additional interventions.”
Furthermore, the study emphasized the global nature of AMR. “We’ve seen over the last 2 years with COVID-19 that this sort of problem doesn’t respect country borders, and high rates of resistance in one location can spread across a region or spread globally pretty quickly,” Dr. Ikuta said.
Leading resistant infections
Lower respiratory and thorax infections, bloodstream infections, and intra-abdominal infections together accounted for almost 79% of such deaths linked to AMR.
The six leading pathogens are likely household names among infectious disease specialists. The researchers found Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, each responsible for more than 250,000 AMR-associated deaths.
The study also revealed that resistance to several first-line antibiotic agents often used empirically to treat infections accounted for more than 70% of the AMR-attributable deaths. These included fluoroquinolones and beta-lactam antibiotics such as carbapenems, cephalosporins, and penicillins.

Consistent with previous studies, MRSA stood out as a major cause of mortality. Of 88 different pathogen-drug combinations evaluated, MRSA was responsible for the most mortality: more than 100,000 deaths and 3·5 million disability-adjusted life-years.
The current study findings on MRSA “being a particularly nasty culprit” in AMR infections validates previous work that reported similar results, Vance Fowler, MD, told this news organization when asked to comment on the research. “That is reassuring.”
Potential solutions offered
Dr. Murray and colleagues outlined five strategies to address the challenge of bacterial AMR:
- Infection prevention and control remain paramount in minimizing infections in general and AMR infections in particular.
- More vaccines are needed to reduce the need for antibiotics. “Vaccines are available for only one of the six leading pathogens (S. pneumoniae), although new vaccine programs are underway for S. aureus, E. coli, and others,” the researchers wrote.
- Reduce antibiotic use unrelated to treatment of human disease.
- Avoid using antibiotics for viral infections and other unnecessary indications.
- Invest in new antibiotic development and ensure access to second-line agents in areas without widespread access.
“Identifying strategies that can work to reduce the burden of bacterial AMR – either across a wide range of settings or those that are specifically tailored to the resources available and leading pathogen-drug combinations in a particular setting – is an urgent priority,” the researchers noted.
Admirable AMR research
The results of the study are “startling, but not surprising,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C.
The authors did a “nice job” of addressing both deaths attributable and associated with AMR, Dr. Fowler added. “Those two categories unlock applications, not just in terms of how you interpret it but also what you do about it.”
The deaths attributable to AMR show that there is more work to be done regarding infection control and prevention, Dr. Fowler said, including in areas of the world like lower- and middle-income countries where infection resistance is most pronounced.
The deaths associated with AMR can be more challenging to calculate – people with infections can die for multiple reasons. However, Dr. Fowler applauded the researchers for doing “as good a job as you can” in estimating the extent of associated mortality.
‘The overlooked pandemic of antimicrobial resistance’
In an accompanying editorial in The Lancet, Ramanan Laxminarayan, PhD, MPH, wrote: “As COVID-19 rages on, the pandemic of antimicrobial resistance continues in the shadows. The toll taken by AMR on patients and their families is largely invisible but is reflected in prolonged bacterial infections that extend hospital stays and cause needless deaths.”
Dr. Laxminarayan pointed out an irony with AMR in different regions. Some of the AMR burden in sub-Saharan Africa is “probably due to inadequate access to antibiotics and high infection levels, albeit at low levels of resistance, whereas in south Asia and Latin America, it is because of high resistance even with good access to antibiotics.”
More funding to address AMR is needed, Dr. Laxminarayan noted. “Even the lower end of 911,000 deaths estimated by Murray and colleagues is higher than the number of deaths from HIV, which attracts close to U.S. $50 billion each year. However, global spending on addressing AMR is probably much lower than that.” Dr. Laxminarayan is an economist and epidemiologist affiliated with the Center for Disease Dynamics, Economics & Policy in Washington, D.C., and the Global Antibiotic Research and Development Partnership in Geneva.
An overlap with COVID-19
The Lancet report is likely “to bring more attention to AMR, especially since so many people have been distracted by COVID, and rightly so,” Dr. Fowler predicted. “The world has had its hands full with COVID.”
The two infections interact in direct ways, Dr. Fowler added. For example, some people hospitalized for COVID-19 for an extended time could develop progressively drug-resistant bacteria – leading to a superinfection.
The overlap could be illustrated by a Venn diagram, he said. A yellow circle could illustrate people with COVID-19 who are asymptomatic or who remain outpatients. Next to that would be a blue circle showing people who develop AMR infections. Where the two circles overlap would be green for those hospitalized who – because of receiving steroids, being on a ventilator, or getting a central line – develop a superinfection.
Official guidance continues
The study comes in the context of recent guidance and federal action on AMR. For example, the Infectious Diseases Society of America released new guidelines for AMR in November 2021 as part of ongoing advice on prevention and treatment of this “ongoing crisis.”
This most recent IDSA guidance addresses three pathogens in particular: AmpC beta-lactamase–producing Enterobacterales, carbapenem-resistant A. baumannii, and Stenotrophomonas maltophilia.
Also in November, the World Health Organization released an updated fact sheet on antimicrobial resistance. The WHO declared AMR one of the world’s top 10 global public health threats. The agency emphasized that misuse and overuse of antimicrobials are the main drivers in the development of drug-resistant pathogens. The WHO also pointed out that lack of clean water and sanitation in many areas of the world contribute to spread of microbes, including those resistant to current treatment options.
In September 2021, the Biden administration acknowledged the threat of AMR with allocation of more than $2 billion of the American Rescue Plan money for prevention and treatment of these infections.
Asked if there are any reasons for hope or optimism at this point, Dr. Ikuta said: “Definitely. We know what needs to be done to combat the spread of resistance. COVID-19 has demonstrated the importance of global commitment to infection control measures, such as hand washing and surveillance, and rapid investments in treatments, which can all be applied to antimicrobial resistance.”
The Bill & Melinda Gates Foundation, the Wellcome Trust, and the U.K. Department of Health and Social Care using U.K. aid funding managed by the Fleming Fund and other organizations provided funding for the study. Dr. Ikuta and Dr. Laxminarayan have disclosed no relevant financial relationships. Dr. Fowler reported receiving grants or honoraria, as well as serving as a consultant, for numerous sources. He also reported a patent pending in sepsis diagnostics and serving as chair of the V710 Scientific Advisory Committee (Merck).
A version of this article first appeared on Medscape.com.
‘Incomprehensible’ CABG recommendation raises concerns
BUENOS AIRES – The Latin American Association of Cardiac and Endovascular Surgery (LACES) has demanded “urgent reconsideration” of the decision to downgrade the strength of the recommendation for revascularization or coronary artery bypass graft (CABG) surgery for multivessel disease in the new guideline on coronary artery revascularization, putting it in the same class as the recommendation for percutaneous coronary intervention, which has no apparent advantage over optimal medical therapy.
With the prevalence of stable ischemic heart disease in patients with multivessel disease, the contradiction between the evidence and the new recommendation “may affect the lives and survival of millions of patients worldwide and have a major socio-economic impact,” the association warned in a public letter.
In the 2011 guideline, CABG for patients with multivessel coronary artery disease was given a class I recommendation, which means that it is considered useful and effective and should be performed in the majority of patients in most circumstances. But the new, much weaker class IIb recommendation suggests that the benefit only marginally exceeds the risk and that it should be used selectively and only after careful consideration.
“It is an incomprehensible rollercoaster drop in the recommendation level. We totally disagree. In the absence of evidence, a IIb level provides equal freedom to send a patient to surgery or not. And in patients who are not being sent to surgery, it could take years of survival before we can be sure that we are doing the right thing,” said LACES president Víctor Dayan, MD, PhD, from the cardiovascular center at the Hospital de Clínicas “Dr. Manuel Quintela”, which is part of the School of Medicine at the University of the Republic, Montevideo, Uruguay.
The change in the recommendation for this indication “reflects new evidence showing no advantage of coronary artery bypass grafting over medical therapy alone to improve survival in patients with three-vessel coronary disease with preserved left ventricular function and no left main disease,” according to the authors of the guideline, issued jointly by the American College of Cardiology (ACC), the American Heart Association, and the Society for Cardiovascular Angiography and Interventions (SCAI). In particular, they cite the 2019 ISCHEMIA clinical study that failed to show that an early invasive strategy reduces major adverse cardiovascular events, compared with optimal medical therapy and a handful of meta-analyses.
However, ISCHEMIA did not discriminate between the two types of invasive strategy – CABG and percutaneous coronary intervention (PCI) – so cannot be considered as a basis to downgrade the CABG recommendation, Dr. Dayan explained.
“Furthermore, the authors neglected previous RCTs that have shown the survival benefit of CABG in these patients and decided to put PCI in the same [class of recommendation], although no RCT has been able to show any survival advantage of PCI compared to optimal medical treatment,” the LACES letter states.
Basis should be evidence, ‘not inferences’
Three large randomized clinical trials and a 1994 meta-analysis with individual patient data from seven studies firmly established that survival is better with CABG than with medical treatment, the letter continues. However, the guideline authors did not provide any additional randomized clinical trials that refute this evidence.
“Furthermore, the committee disregarded data from the Ten-Year Follow-up Survival of the Medicine, Angioplasty, or Surgery Study (MASS II) randomized control[led] trial, which showed a lower incidence of cardiac mortality (as part of its secondary outcomes) following CABG compared to optimal medical therapy and PCI,” the letter explains.
The guideline authors might have judged current optimal medical therapy to be better than what existed 10, 15, or 30 years ago, diluting the relative benefits of surgery, but the “recommendation in a guideline must act on evidence, not inferences. And there is no evidence to support this drop in recommendation class,” Dr. Dayan said.
Other experts have drawn attention to the fact that two surgical societies – the American Association for Thoracic Surgery (AAST) and the Society of Thoracic Surgeons (STS) – did not endorse the final document, despite having participated in its review, reported this news organization.
“This is a very disappointing update that will negatively affect the lives of many people,” tweeted Marc Pelletier, MD, head of cardiac surgery at University Hospitals, Case Western Reserve University, Cleveland.
Contradictions in the text that examines the evidence and the final recommendations, are “unclear” and “open to various interpretations, when they should be a pillar for decisionmaking,” said Javier Ferrari Ayarragaray, MD, president of the Argentine College of Cardiovascular Surgeons (CACCV) and vice president of LACES.
The new guidelines “show no additional randomized controlled trial to support this downgrade in the level of evidence,” according to a recent CACCV statement. “The inclusion, approval and endorsement of this type of [recommendation,] including [other] international surgical scientific societies, such as STS, AATS, EACTS, LACES[,] is necessary to obtain a better understanding and agreement on the current evidence.”
In a Dec. 17, 2021 response to LACES, Patrick O’Gara, MD, who was chair of the ACC/AHA Joint Committee on Clinical Practice Guidelines at the time, and his successor, Joshua Beckman, MD, explained that both organizations approved the guideline for publication and support its authors “in their interpretation of the published evidence and findings.”
The pair pointed out that the drafting committee members, who have extensive clinical judgment and experience, deliberated extensively on the issue and that the change from a class I to a class IIb recommendation was “carefully considered after a review of the entire available and relevant evidence.”
“When we bring together multiple organizations to review and summarize the evidence, we work collaboratively to interpret the extensive catalog of published and peer-reviewed literature and create clinical practice recommendations,” said Thomas Getchius, director of guideline strategy and operations at the AHA.
“The final guideline reflects the latest evidence-based recommendations for coronary artery revascularization, as agreed upon by the ACC, AHA, SCAI, and the full drafting committee,” Mr. Getchius said.
Dr. Dayan and Dr. Ferrari Ayarragaray have disclosed no relevant financial relationships. Mr. Getchius is an employee of the American Heart Association.
A version of this article first appeared on Medscape.com.
BUENOS AIRES – The Latin American Association of Cardiac and Endovascular Surgery (LACES) has demanded “urgent reconsideration” of the decision to downgrade the strength of the recommendation for revascularization or coronary artery bypass graft (CABG) surgery for multivessel disease in the new guideline on coronary artery revascularization, putting it in the same class as the recommendation for percutaneous coronary intervention, which has no apparent advantage over optimal medical therapy.
With the prevalence of stable ischemic heart disease in patients with multivessel disease, the contradiction between the evidence and the new recommendation “may affect the lives and survival of millions of patients worldwide and have a major socio-economic impact,” the association warned in a public letter.
In the 2011 guideline, CABG for patients with multivessel coronary artery disease was given a class I recommendation, which means that it is considered useful and effective and should be performed in the majority of patients in most circumstances. But the new, much weaker class IIb recommendation suggests that the benefit only marginally exceeds the risk and that it should be used selectively and only after careful consideration.
“It is an incomprehensible rollercoaster drop in the recommendation level. We totally disagree. In the absence of evidence, a IIb level provides equal freedom to send a patient to surgery or not. And in patients who are not being sent to surgery, it could take years of survival before we can be sure that we are doing the right thing,” said LACES president Víctor Dayan, MD, PhD, from the cardiovascular center at the Hospital de Clínicas “Dr. Manuel Quintela”, which is part of the School of Medicine at the University of the Republic, Montevideo, Uruguay.
The change in the recommendation for this indication “reflects new evidence showing no advantage of coronary artery bypass grafting over medical therapy alone to improve survival in patients with three-vessel coronary disease with preserved left ventricular function and no left main disease,” according to the authors of the guideline, issued jointly by the American College of Cardiology (ACC), the American Heart Association, and the Society for Cardiovascular Angiography and Interventions (SCAI). In particular, they cite the 2019 ISCHEMIA clinical study that failed to show that an early invasive strategy reduces major adverse cardiovascular events, compared with optimal medical therapy and a handful of meta-analyses.
However, ISCHEMIA did not discriminate between the two types of invasive strategy – CABG and percutaneous coronary intervention (PCI) – so cannot be considered as a basis to downgrade the CABG recommendation, Dr. Dayan explained.
“Furthermore, the authors neglected previous RCTs that have shown the survival benefit of CABG in these patients and decided to put PCI in the same [class of recommendation], although no RCT has been able to show any survival advantage of PCI compared to optimal medical treatment,” the LACES letter states.
Basis should be evidence, ‘not inferences’
Three large randomized clinical trials and a 1994 meta-analysis with individual patient data from seven studies firmly established that survival is better with CABG than with medical treatment, the letter continues. However, the guideline authors did not provide any additional randomized clinical trials that refute this evidence.
“Furthermore, the committee disregarded data from the Ten-Year Follow-up Survival of the Medicine, Angioplasty, or Surgery Study (MASS II) randomized control[led] trial, which showed a lower incidence of cardiac mortality (as part of its secondary outcomes) following CABG compared to optimal medical therapy and PCI,” the letter explains.
The guideline authors might have judged current optimal medical therapy to be better than what existed 10, 15, or 30 years ago, diluting the relative benefits of surgery, but the “recommendation in a guideline must act on evidence, not inferences. And there is no evidence to support this drop in recommendation class,” Dr. Dayan said.
Other experts have drawn attention to the fact that two surgical societies – the American Association for Thoracic Surgery (AAST) and the Society of Thoracic Surgeons (STS) – did not endorse the final document, despite having participated in its review, reported this news organization.
“This is a very disappointing update that will negatively affect the lives of many people,” tweeted Marc Pelletier, MD, head of cardiac surgery at University Hospitals, Case Western Reserve University, Cleveland.
Contradictions in the text that examines the evidence and the final recommendations, are “unclear” and “open to various interpretations, when they should be a pillar for decisionmaking,” said Javier Ferrari Ayarragaray, MD, president of the Argentine College of Cardiovascular Surgeons (CACCV) and vice president of LACES.
The new guidelines “show no additional randomized controlled trial to support this downgrade in the level of evidence,” according to a recent CACCV statement. “The inclusion, approval and endorsement of this type of [recommendation,] including [other] international surgical scientific societies, such as STS, AATS, EACTS, LACES[,] is necessary to obtain a better understanding and agreement on the current evidence.”
In a Dec. 17, 2021 response to LACES, Patrick O’Gara, MD, who was chair of the ACC/AHA Joint Committee on Clinical Practice Guidelines at the time, and his successor, Joshua Beckman, MD, explained that both organizations approved the guideline for publication and support its authors “in their interpretation of the published evidence and findings.”
The pair pointed out that the drafting committee members, who have extensive clinical judgment and experience, deliberated extensively on the issue and that the change from a class I to a class IIb recommendation was “carefully considered after a review of the entire available and relevant evidence.”
“When we bring together multiple organizations to review and summarize the evidence, we work collaboratively to interpret the extensive catalog of published and peer-reviewed literature and create clinical practice recommendations,” said Thomas Getchius, director of guideline strategy and operations at the AHA.
“The final guideline reflects the latest evidence-based recommendations for coronary artery revascularization, as agreed upon by the ACC, AHA, SCAI, and the full drafting committee,” Mr. Getchius said.
Dr. Dayan and Dr. Ferrari Ayarragaray have disclosed no relevant financial relationships. Mr. Getchius is an employee of the American Heart Association.
A version of this article first appeared on Medscape.com.
BUENOS AIRES – The Latin American Association of Cardiac and Endovascular Surgery (LACES) has demanded “urgent reconsideration” of the decision to downgrade the strength of the recommendation for revascularization or coronary artery bypass graft (CABG) surgery for multivessel disease in the new guideline on coronary artery revascularization, putting it in the same class as the recommendation for percutaneous coronary intervention, which has no apparent advantage over optimal medical therapy.
With the prevalence of stable ischemic heart disease in patients with multivessel disease, the contradiction between the evidence and the new recommendation “may affect the lives and survival of millions of patients worldwide and have a major socio-economic impact,” the association warned in a public letter.
In the 2011 guideline, CABG for patients with multivessel coronary artery disease was given a class I recommendation, which means that it is considered useful and effective and should be performed in the majority of patients in most circumstances. But the new, much weaker class IIb recommendation suggests that the benefit only marginally exceeds the risk and that it should be used selectively and only after careful consideration.
“It is an incomprehensible rollercoaster drop in the recommendation level. We totally disagree. In the absence of evidence, a IIb level provides equal freedom to send a patient to surgery or not. And in patients who are not being sent to surgery, it could take years of survival before we can be sure that we are doing the right thing,” said LACES president Víctor Dayan, MD, PhD, from the cardiovascular center at the Hospital de Clínicas “Dr. Manuel Quintela”, which is part of the School of Medicine at the University of the Republic, Montevideo, Uruguay.
The change in the recommendation for this indication “reflects new evidence showing no advantage of coronary artery bypass grafting over medical therapy alone to improve survival in patients with three-vessel coronary disease with preserved left ventricular function and no left main disease,” according to the authors of the guideline, issued jointly by the American College of Cardiology (ACC), the American Heart Association, and the Society for Cardiovascular Angiography and Interventions (SCAI). In particular, they cite the 2019 ISCHEMIA clinical study that failed to show that an early invasive strategy reduces major adverse cardiovascular events, compared with optimal medical therapy and a handful of meta-analyses.
However, ISCHEMIA did not discriminate between the two types of invasive strategy – CABG and percutaneous coronary intervention (PCI) – so cannot be considered as a basis to downgrade the CABG recommendation, Dr. Dayan explained.
“Furthermore, the authors neglected previous RCTs that have shown the survival benefit of CABG in these patients and decided to put PCI in the same [class of recommendation], although no RCT has been able to show any survival advantage of PCI compared to optimal medical treatment,” the LACES letter states.
Basis should be evidence, ‘not inferences’
Three large randomized clinical trials and a 1994 meta-analysis with individual patient data from seven studies firmly established that survival is better with CABG than with medical treatment, the letter continues. However, the guideline authors did not provide any additional randomized clinical trials that refute this evidence.
“Furthermore, the committee disregarded data from the Ten-Year Follow-up Survival of the Medicine, Angioplasty, or Surgery Study (MASS II) randomized control[led] trial, which showed a lower incidence of cardiac mortality (as part of its secondary outcomes) following CABG compared to optimal medical therapy and PCI,” the letter explains.
The guideline authors might have judged current optimal medical therapy to be better than what existed 10, 15, or 30 years ago, diluting the relative benefits of surgery, but the “recommendation in a guideline must act on evidence, not inferences. And there is no evidence to support this drop in recommendation class,” Dr. Dayan said.
Other experts have drawn attention to the fact that two surgical societies – the American Association for Thoracic Surgery (AAST) and the Society of Thoracic Surgeons (STS) – did not endorse the final document, despite having participated in its review, reported this news organization.
“This is a very disappointing update that will negatively affect the lives of many people,” tweeted Marc Pelletier, MD, head of cardiac surgery at University Hospitals, Case Western Reserve University, Cleveland.
Contradictions in the text that examines the evidence and the final recommendations, are “unclear” and “open to various interpretations, when they should be a pillar for decisionmaking,” said Javier Ferrari Ayarragaray, MD, president of the Argentine College of Cardiovascular Surgeons (CACCV) and vice president of LACES.
The new guidelines “show no additional randomized controlled trial to support this downgrade in the level of evidence,” according to a recent CACCV statement. “The inclusion, approval and endorsement of this type of [recommendation,] including [other] international surgical scientific societies, such as STS, AATS, EACTS, LACES[,] is necessary to obtain a better understanding and agreement on the current evidence.”
In a Dec. 17, 2021 response to LACES, Patrick O’Gara, MD, who was chair of the ACC/AHA Joint Committee on Clinical Practice Guidelines at the time, and his successor, Joshua Beckman, MD, explained that both organizations approved the guideline for publication and support its authors “in their interpretation of the published evidence and findings.”
The pair pointed out that the drafting committee members, who have extensive clinical judgment and experience, deliberated extensively on the issue and that the change from a class I to a class IIb recommendation was “carefully considered after a review of the entire available and relevant evidence.”
“When we bring together multiple organizations to review and summarize the evidence, we work collaboratively to interpret the extensive catalog of published and peer-reviewed literature and create clinical practice recommendations,” said Thomas Getchius, director of guideline strategy and operations at the AHA.
“The final guideline reflects the latest evidence-based recommendations for coronary artery revascularization, as agreed upon by the ACC, AHA, SCAI, and the full drafting committee,” Mr. Getchius said.
Dr. Dayan and Dr. Ferrari Ayarragaray have disclosed no relevant financial relationships. Mr. Getchius is an employee of the American Heart Association.
A version of this article first appeared on Medscape.com.
52-year-old man • syncopal episode • chest pain • mild lightheadedness • Dx?
THE CASE
A 52-year-old man with a history of hypertension and gastroesophageal reflux disease (GERD) presented to the emergency department (ED) after an episode of syncope. He reported that the syncope occurred soon after he stood up to go to the kitchen to make dinner but was without prodrome or associated symptoms. He recalled little of the event, and the episode was unwitnessed. He had a few bruises on his arms but no significant injuries.
On questioning, he reported occasional palpitations but no changes in his normal exercise tolerance. His only medication was lisinopril 10 mg/d.
In the ED, his vital signs, physical exam (including orthostatic vital signs), basic labs (including troponin I), and a 12-lead EKG were normal. After a cardiology consultation, he was discharged home with a 30-day ambulatory rhythm monitor.
A few days later, while walking up and down some hills, he experienced about 15 seconds of chest pain accompanied by mild lightheadedness. Thinking it might be related to his GERD, he took some over-the-counter antacids when he returned home, since these had been effective for him in the past.
However, the rhythm monitoring company contacted the EKG lab to transmit a concerning strip (FIGURE). They also reported that the patient had been contacted and reported no further symptoms.
THE DIAGNOSIS
Most notable on the patient’s rhythm strip was a continuously varying QRS complex, which was indicative of polymorphic ventricular tachycardia and consistent with the patient’s syncope and other symptoms. Less obvious at first glance was an ST-segment elevation in the preceding beats. Comparison to a post-episode tracing (FIGURE) highlights the abnormality. Polymorphic ventricular tachycardia resolves in 1 of 2 ways: It will either stop on its own (causing syncope if it lasts more than a few seconds) or it will devolve into ventricular fibrillation, causing cardiac arrest.1
The combination of these findings and the clinical scenario prompted a recommendation that the patient report to the ED for admission (his wife drove him). He was admitted to the intensive care unit (ICU) for continuous telemetry monitoring, and a cardiac catheterization was ordered. The procedure revealed a 99% thrombotic mid-right coronary artery lesion, for which aspiration thrombectomy and uncomplicated stenting were performed.
Continue to: DISCUSSION
DISCUSSION
Guidelines from the American College of Cardiology/American Heart Association/Heart Rhythm Society recommend a detailed history and physical exam, as well as an EKG, for the initial evaluation of syncope.2 If this does not point to a diagnosis (and depending on the presentation and other factors), an ambulatory rhythm monitor can be considered. Other possible testing modalities include stress testing, resting transthoracic echocardiography, electrophysiologic testing, and cardiac magnetic resonance imaging or computed tomography.
Is the cause cardiac? The guidelines suggest that a cardiac cause of syncope is more likely if several of the following factors are present: age > 60 years; male sex; presence of known heart disease (acquired or congenital); brief prodrome (eg, palpitations) or no prodrome; exertional or supine syncope; 1 to 2 episodes; an abnormal cardiac exam; and a family history of premature sudden death.2 A noncardiac cause is suggested by other factors: younger age; no known cardiac disease; standing or a position change from supine to sitting/standing; prodrome; specific triggers (eg, dehydration, pain); and frequent and prolonged stereotypic episodes.2
While the guidelines do not specify the number of factors or endorse a specific scoring system, such tools have been developed. For example, the EGSYS (Evaluation of Guidelines in Syncope Study) Score assigns 1 point for each of 6 factors: palpitations; heart disease and/or abnormal EKG; effort syncope; supine syncope; precipitating or predisposing factors; and autonomic prodromes. A score ≥ 3 identified cardiac syncope with a sensitivity of 95%, but with a specificity of only 61%. In the derivation study, patients with a score ≥ 3 had higher mortality than those with a lower score (17 vs 3%; P < .001).3
Myocardial ischemia can trigger ventricular arrhythmias. In the GUSTO-1 trial of fibrinolytic therapy in patients with acute ST-segment elevation myocardial infarction (n = 40,895), the incidence of ventricular tachycardia or ventricular fibrillation was 10.2%.4 In a pooled analysis (4 trials; n = 26,416) of patients who were treated for non–ST-segment elevation or unstable angina-type acute coronary syndromes, the rate of these arrhythmias was markedly lower (2.1%).5 The risk of ventricular arrhythmia is one reason close monitoring (eg, continuous telemetry, ICU admission) is the standard of care for patients with acute coronary syndromes.
Our patient experienced syncope upon standing, which suggested a noncardiac cause (usually orthostatic hypotension). However, the history of palpitations increased the suspicion for a cardiac cause, and thus the rhythm monitor was ordered.
THE TAKEAWAY
This case was unusual in that ambulatory monitoring captured electrocardiographic evidence of myocardial ischemia leading directly to a ventricular arrhythmia. In the evaluation of syncope, a detailed history, physical exam, and a baseline 12-lead EKG can sometimes give clues to an arrhythmic cause of syncope (eg, Brugada syndrome, prior infarct pattern, prolonged QTc, bradycardia, heart block, arrhythmogenic right ventricular cardiomyopathy)—but prolonged rhythm monitoring is sometimes needed to identify a cause.
Michael A. Chen, MD, PhD, Harborview Medical Center, University of Washington School of Medicine, 325 9th Avenue, Box 359748 (Cardiology), Seattle, WA 98104; [email protected]
1. Viskin S, Chorin E, Viskin D, et al. Polymorphic ventricular tachycardia: terminology, mechanism, diagnosis, and emergency therapy. Circulation. 2021;144:823-839. doi: 10.1161/CIRCULATIONAHA.121.055783
2. Shen W-K, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70:620-663. doi: 10.1016/j.jacc.2017.03.002
3. Del Rosso A, Ungar A, Maggi R, et al. Clinical predictors of cardiac syncope at initial evaluation in patients referred urgently to a general hospital: the EGSYS score. Heart. 2008;94:1528-1529. doi: 10.1136/hrt.2008.143123
4. Newby KH, Thompson T, Stebbins A, et al. Sustained ventricular arrhythmias in patients receiving thrombolytic therapy: incidence and outcomes. The GUSTO Investigators. Circulation. 1998;98:2567-2573. doi: 10.1161/01.cir.98.23.2567
5. Al-Khatib SM, Granger CB, Huang Y, et al. Sustained ventricular arrhythmias among patients with acute coronary syndromes with no ST-segment elevation: incidence, predictors, and outcomes. Circulation. 2002;106:309-12. doi: 10.1161/01.cir.0000022692.49934.e3
THE CASE
A 52-year-old man with a history of hypertension and gastroesophageal reflux disease (GERD) presented to the emergency department (ED) after an episode of syncope. He reported that the syncope occurred soon after he stood up to go to the kitchen to make dinner but was without prodrome or associated symptoms. He recalled little of the event, and the episode was unwitnessed. He had a few bruises on his arms but no significant injuries.
On questioning, he reported occasional palpitations but no changes in his normal exercise tolerance. His only medication was lisinopril 10 mg/d.
In the ED, his vital signs, physical exam (including orthostatic vital signs), basic labs (including troponin I), and a 12-lead EKG were normal. After a cardiology consultation, he was discharged home with a 30-day ambulatory rhythm monitor.
A few days later, while walking up and down some hills, he experienced about 15 seconds of chest pain accompanied by mild lightheadedness. Thinking it might be related to his GERD, he took some over-the-counter antacids when he returned home, since these had been effective for him in the past.
However, the rhythm monitoring company contacted the EKG lab to transmit a concerning strip (FIGURE). They also reported that the patient had been contacted and reported no further symptoms.

THE DIAGNOSIS
Most notable on the patient’s rhythm strip was a continuously varying QRS complex, which was indicative of polymorphic ventricular tachycardia and consistent with the patient’s syncope and other symptoms. Less obvious at first glance was an ST-segment elevation in the preceding beats. Comparison to a post-episode tracing (FIGURE) highlights the abnormality. Polymorphic ventricular tachycardia resolves in 1 of 2 ways: It will either stop on its own (causing syncope if it lasts more than a few seconds) or it will devolve into ventricular fibrillation, causing cardiac arrest.1
The combination of these findings and the clinical scenario prompted a recommendation that the patient report to the ED for admission (his wife drove him). He was admitted to the intensive care unit (ICU) for continuous telemetry monitoring, and a cardiac catheterization was ordered. The procedure revealed a 99% thrombotic mid-right coronary artery lesion, for which aspiration thrombectomy and uncomplicated stenting were performed.
Continue to: DISCUSSION
DISCUSSION
Guidelines from the American College of Cardiology/American Heart Association/Heart Rhythm Society recommend a detailed history and physical exam, as well as an EKG, for the initial evaluation of syncope.2 If this does not point to a diagnosis (and depending on the presentation and other factors), an ambulatory rhythm monitor can be considered. Other possible testing modalities include stress testing, resting transthoracic echocardiography, electrophysiologic testing, and cardiac magnetic resonance imaging or computed tomography.
Is the cause cardiac? The guidelines suggest that a cardiac cause of syncope is more likely if several of the following factors are present: age > 60 years; male sex; presence of known heart disease (acquired or congenital); brief prodrome (eg, palpitations) or no prodrome; exertional or supine syncope; 1 to 2 episodes; an abnormal cardiac exam; and a family history of premature sudden death.2 A noncardiac cause is suggested by other factors: younger age; no known cardiac disease; standing or a position change from supine to sitting/standing; prodrome; specific triggers (eg, dehydration, pain); and frequent and prolonged stereotypic episodes.2
While the guidelines do not specify the number of factors or endorse a specific scoring system, such tools have been developed. For example, the EGSYS (Evaluation of Guidelines in Syncope Study) Score assigns 1 point for each of 6 factors: palpitations; heart disease and/or abnormal EKG; effort syncope; supine syncope; precipitating or predisposing factors; and autonomic prodromes. A score ≥ 3 identified cardiac syncope with a sensitivity of 95%, but with a specificity of only 61%. In the derivation study, patients with a score ≥ 3 had higher mortality than those with a lower score (17 vs 3%; P < .001).3
Myocardial ischemia can trigger ventricular arrhythmias. In the GUSTO-1 trial of fibrinolytic therapy in patients with acute ST-segment elevation myocardial infarction (n = 40,895), the incidence of ventricular tachycardia or ventricular fibrillation was 10.2%.4 In a pooled analysis (4 trials; n = 26,416) of patients who were treated for non–ST-segment elevation or unstable angina-type acute coronary syndromes, the rate of these arrhythmias was markedly lower (2.1%).5 The risk of ventricular arrhythmia is one reason close monitoring (eg, continuous telemetry, ICU admission) is the standard of care for patients with acute coronary syndromes.
Our patient experienced syncope upon standing, which suggested a noncardiac cause (usually orthostatic hypotension). However, the history of palpitations increased the suspicion for a cardiac cause, and thus the rhythm monitor was ordered.
THE TAKEAWAY
This case was unusual in that ambulatory monitoring captured electrocardiographic evidence of myocardial ischemia leading directly to a ventricular arrhythmia. In the evaluation of syncope, a detailed history, physical exam, and a baseline 12-lead EKG can sometimes give clues to an arrhythmic cause of syncope (eg, Brugada syndrome, prior infarct pattern, prolonged QTc, bradycardia, heart block, arrhythmogenic right ventricular cardiomyopathy)—but prolonged rhythm monitoring is sometimes needed to identify a cause.
Michael A. Chen, MD, PhD, Harborview Medical Center, University of Washington School of Medicine, 325 9th Avenue, Box 359748 (Cardiology), Seattle, WA 98104; [email protected]
THE CASE
A 52-year-old man with a history of hypertension and gastroesophageal reflux disease (GERD) presented to the emergency department (ED) after an episode of syncope. He reported that the syncope occurred soon after he stood up to go to the kitchen to make dinner but was without prodrome or associated symptoms. He recalled little of the event, and the episode was unwitnessed. He had a few bruises on his arms but no significant injuries.
On questioning, he reported occasional palpitations but no changes in his normal exercise tolerance. His only medication was lisinopril 10 mg/d.
In the ED, his vital signs, physical exam (including orthostatic vital signs), basic labs (including troponin I), and a 12-lead EKG were normal. After a cardiology consultation, he was discharged home with a 30-day ambulatory rhythm monitor.
A few days later, while walking up and down some hills, he experienced about 15 seconds of chest pain accompanied by mild lightheadedness. Thinking it might be related to his GERD, he took some over-the-counter antacids when he returned home, since these had been effective for him in the past.
However, the rhythm monitoring company contacted the EKG lab to transmit a concerning strip (FIGURE). They also reported that the patient had been contacted and reported no further symptoms.

THE DIAGNOSIS
Most notable on the patient’s rhythm strip was a continuously varying QRS complex, which was indicative of polymorphic ventricular tachycardia and consistent with the patient’s syncope and other symptoms. Less obvious at first glance was an ST-segment elevation in the preceding beats. Comparison to a post-episode tracing (FIGURE) highlights the abnormality. Polymorphic ventricular tachycardia resolves in 1 of 2 ways: It will either stop on its own (causing syncope if it lasts more than a few seconds) or it will devolve into ventricular fibrillation, causing cardiac arrest.1
The combination of these findings and the clinical scenario prompted a recommendation that the patient report to the ED for admission (his wife drove him). He was admitted to the intensive care unit (ICU) for continuous telemetry monitoring, and a cardiac catheterization was ordered. The procedure revealed a 99% thrombotic mid-right coronary artery lesion, for which aspiration thrombectomy and uncomplicated stenting were performed.
Continue to: DISCUSSION
DISCUSSION
Guidelines from the American College of Cardiology/American Heart Association/Heart Rhythm Society recommend a detailed history and physical exam, as well as an EKG, for the initial evaluation of syncope.2 If this does not point to a diagnosis (and depending on the presentation and other factors), an ambulatory rhythm monitor can be considered. Other possible testing modalities include stress testing, resting transthoracic echocardiography, electrophysiologic testing, and cardiac magnetic resonance imaging or computed tomography.
Is the cause cardiac? The guidelines suggest that a cardiac cause of syncope is more likely if several of the following factors are present: age > 60 years; male sex; presence of known heart disease (acquired or congenital); brief prodrome (eg, palpitations) or no prodrome; exertional or supine syncope; 1 to 2 episodes; an abnormal cardiac exam; and a family history of premature sudden death.2 A noncardiac cause is suggested by other factors: younger age; no known cardiac disease; standing or a position change from supine to sitting/standing; prodrome; specific triggers (eg, dehydration, pain); and frequent and prolonged stereotypic episodes.2
While the guidelines do not specify the number of factors or endorse a specific scoring system, such tools have been developed. For example, the EGSYS (Evaluation of Guidelines in Syncope Study) Score assigns 1 point for each of 6 factors: palpitations; heart disease and/or abnormal EKG; effort syncope; supine syncope; precipitating or predisposing factors; and autonomic prodromes. A score ≥ 3 identified cardiac syncope with a sensitivity of 95%, but with a specificity of only 61%. In the derivation study, patients with a score ≥ 3 had higher mortality than those with a lower score (17 vs 3%; P < .001).3
Myocardial ischemia can trigger ventricular arrhythmias. In the GUSTO-1 trial of fibrinolytic therapy in patients with acute ST-segment elevation myocardial infarction (n = 40,895), the incidence of ventricular tachycardia or ventricular fibrillation was 10.2%.4 In a pooled analysis (4 trials; n = 26,416) of patients who were treated for non–ST-segment elevation or unstable angina-type acute coronary syndromes, the rate of these arrhythmias was markedly lower (2.1%).5 The risk of ventricular arrhythmia is one reason close monitoring (eg, continuous telemetry, ICU admission) is the standard of care for patients with acute coronary syndromes.
Our patient experienced syncope upon standing, which suggested a noncardiac cause (usually orthostatic hypotension). However, the history of palpitations increased the suspicion for a cardiac cause, and thus the rhythm monitor was ordered.
THE TAKEAWAY
This case was unusual in that ambulatory monitoring captured electrocardiographic evidence of myocardial ischemia leading directly to a ventricular arrhythmia. In the evaluation of syncope, a detailed history, physical exam, and a baseline 12-lead EKG can sometimes give clues to an arrhythmic cause of syncope (eg, Brugada syndrome, prior infarct pattern, prolonged QTc, bradycardia, heart block, arrhythmogenic right ventricular cardiomyopathy)—but prolonged rhythm monitoring is sometimes needed to identify a cause.
Michael A. Chen, MD, PhD, Harborview Medical Center, University of Washington School of Medicine, 325 9th Avenue, Box 359748 (Cardiology), Seattle, WA 98104; [email protected]
1. Viskin S, Chorin E, Viskin D, et al. Polymorphic ventricular tachycardia: terminology, mechanism, diagnosis, and emergency therapy. Circulation. 2021;144:823-839. doi: 10.1161/CIRCULATIONAHA.121.055783
2. Shen W-K, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70:620-663. doi: 10.1016/j.jacc.2017.03.002
3. Del Rosso A, Ungar A, Maggi R, et al. Clinical predictors of cardiac syncope at initial evaluation in patients referred urgently to a general hospital: the EGSYS score. Heart. 2008;94:1528-1529. doi: 10.1136/hrt.2008.143123
4. Newby KH, Thompson T, Stebbins A, et al. Sustained ventricular arrhythmias in patients receiving thrombolytic therapy: incidence and outcomes. The GUSTO Investigators. Circulation. 1998;98:2567-2573. doi: 10.1161/01.cir.98.23.2567
5. Al-Khatib SM, Granger CB, Huang Y, et al. Sustained ventricular arrhythmias among patients with acute coronary syndromes with no ST-segment elevation: incidence, predictors, and outcomes. Circulation. 2002;106:309-12. doi: 10.1161/01.cir.0000022692.49934.e3
1. Viskin S, Chorin E, Viskin D, et al. Polymorphic ventricular tachycardia: terminology, mechanism, diagnosis, and emergency therapy. Circulation. 2021;144:823-839. doi: 10.1161/CIRCULATIONAHA.121.055783
2. Shen W-K, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70:620-663. doi: 10.1016/j.jacc.2017.03.002
3. Del Rosso A, Ungar A, Maggi R, et al. Clinical predictors of cardiac syncope at initial evaluation in patients referred urgently to a general hospital: the EGSYS score. Heart. 2008;94:1528-1529. doi: 10.1136/hrt.2008.143123
4. Newby KH, Thompson T, Stebbins A, et al. Sustained ventricular arrhythmias in patients receiving thrombolytic therapy: incidence and outcomes. The GUSTO Investigators. Circulation. 1998;98:2567-2573. doi: 10.1161/01.cir.98.23.2567
5. Al-Khatib SM, Granger CB, Huang Y, et al. Sustained ventricular arrhythmias among patients with acute coronary syndromes with no ST-segment elevation: incidence, predictors, and outcomes. Circulation. 2002;106:309-12. doi: 10.1161/01.cir.0000022692.49934.e3
How to screen for and treat teen alcohol use
THE CASE
Paul F* is a 16-year-old White boy who lives with his mother and spends some weekends with his father who has shared custody. He recently presented to the clinic for treatment due to an arrest for disorderly conduct at school. He and a friend were found drinking liquor outside the school building when they were scheduled to be in class. Paul reported that he and his friends often drink at school and at extracurricular functions. He has been using alcohol for the past 2 years, with escalating consumption (5 or more drinks per episode) in the past year. Paul has been drinking most days of the week and has even driven under the influence at times. He said, “I just feel happier when I am drinking.” An accomplished soccer player recruited by colleges, Paul recently was suspended from the team due to his poor grades. His response was, “It’s stupid anyway. What’s the point of playing?”
●
* The patient’s name and some personal details have been changed to protect his identity.
Alcohol is the number 1 substance of abuse for adolescents, used more than tobacco or drugs.1-3 In 2007 and again in 2016, the Surgeon General of the United States issued reports to highlight this important topic,1,2 noting that early and repeated exposure to alcohol during this crucial time of brain development increases the risk for future problems, including addiction.2
Adolescent alcohol use is often underestimated by parents and physicians, including misjudging how much, how often, and how young children are when they begin to drink.1 Boys and girls tend to start drinking at similar ages (13.9 and 14.4 years, respectively),3 but as girls age, they tend to drink more and binge more.4 In 2019, 1 in 4 adolescents reported drinking and more than 4 million reported at least 1 episode of binge drinking in the prior month.4 These numbers have further ramifications: early drinking is associated with alcohol dependence, relapse, use of other substances, risky sexual behaviors, injurious behaviors, suicide, motor vehicle accidents, and dating violence.4-6
Diagnosing alcohol use disorder
The range of alcohol use includes consumption, bingeing, abuse, and dependence.7,8 Consumption is defined as the drinking of alcoholic beverages. Bingeing is the consumption of more than 5 drinks for men or 4 drinks for women in 2 hours, according to the National Institute on Alcohol Abuse and Alcoholism.7 However, the criterion is slightly different for the Substance Abuse and Mental Health Services Administration, which broadens the timeframe to “on the same occasion.”9 While previously known as separate disorders, alcohol abuse (or misuse) and alcohol dependence are now diagnostically classified together as alcohol use disorders (AUDs), per the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5).8 AUD is further stratified as mild, moderate, or severe, depending on the number of criteria that are met by the patient (TABLE).8,10
Alcohol screening
Currently, the US Preventive Services Task Force (USPSTF) does not recommend screening adolescents ages 12 to 17 for AUD, and has instead issued an “I” statement (insufficient evidence).11 While the USPSTF recognizes the potential burdens of adolescent alcohol use, the potential harms of screening include “stigma, anxiety, labeling, discrimination, privacy concerns, and interference with the patient–clinician relationship.”11 The USPSTF also notes that it “did not find any evidence that specifically examined the harms of screening for alcohol use in adolescents.”11
This is at odds with recommendations from the American Academy of Pediatrics (AAP), which in 2011 released a policy statement advocating screening, brief intervention, and referral to treatment for adolescent substance use.12 In the United States, even though 83% of adolescents see a physician at least once each year,12,13 alcohol misuse screening still varies, occurring in the range of 50% to 86% of office visits.12 When screening does occur, it is often based on clinical impression only.12 Studies have shown that when a screening tool is not used, up to two-thirds of substance use disorders may be missed.12-15
Continue to: A full and complete biopsychosocial interview
A full and complete biopsychosocial interview with adolescents is a necessity, and should include queries about alcohol, drugs, and other substances. Acknowledgment of use should trigger further investigation into the substance use areas. Interviews may start with open-ended questions about alcohol use at home or at school before moving to more personalized and detailed questioning and use of screening tools.16
While various screening instruments exist, for the sake of brevity we provide as an example the Screening to Brief Intervention (S2BI) tool. It is an efficient, single-page tool that can help clinicians in their routine care of adolescents to quickly stratify the patient risk of substance use disorder as none/low, moderate, or severe.12 It can be found here: www.mcpap.com/pdf/S2Bi%20Toolkit.pdf (see page 10).
For all patients, but particularly for adolescents, confidentiality is important, and many specialty societies have created language to address this issue.12 Discuss confidentiality with both the adolescent patient and the patient’s caregiver simultaneously, with dialogue that includes: (a) the need to speak with adolescents alone during the office visit, (b) the benefits of confidentiality in the physician–patient relationship, and (c) the need to disclose selected information to keep patients safe.12 Describing the process for required disclosures is essential. Benefits of disclosure include further support for the adolescent patient as well as appropriate parental participation and support for possible referrals.12
Treating AUD
Treatment for AUD should be multifaceted. Screen for comorbid mood disorders, such as generalized anxiety,17,18 social anxiety,18 and depression,19 as well as for insomnia.18 Studies have demonstrated a strong link between insomnia and anxiety, and again between anxiety and AUD.17-19 Finally, screen for adverse childhood events such as trauma, victimization, and abuse.20 Addressing issues discovered in screening allows for more targeted and personalized treatment of AUD.
The National Institute on Drug Abuse categorizes evidence-based treatment into 3 areas: behavioral therapies, family therapies, and medications.21
Continue to: Behavioral therapies
Behavioral therapies can include group therapy, cognitive behavioral therapy (CBT), motivational enhancement therapy, 12-Step facilitation, and contingency management, in which small rewards or incentives are given for participation in treatment to reinforce positive behaviors.21
Family-based therapies, such as brief strategic family therapy, functional family therapy, and multisystem therapy recognize that adolescents exist in systems of families in communities, and that the patient’s success in treatment may be supported by these relationships.21
Some medications may achieve modest benefit for treatment of adolescents with AUD. Naltrexone, acamprosate, and disulfiram have all been used successfully to treat AUD in adults21; some physicians may choose to use these medications “off label” in adolescents. Bupropion has been used successfully in the treatment of nicotine use disorder,21 and a small study in 2005 showed some success with bupropion in treating adolescents with attention-deficit/hyperactivity disorder, comorbid depression, and substance use disorder.22 Naltrexone has also been studied in adolescents with opioid use disorder, although these were not large studies.23
Adolescents with serious, sustained issues with AUD may require more in-depth treatments such as an intensive outpatient program, a partial hospitalization program, or a residential treatment program.15 The least-restrictive environment is preferable.15 Families are generally included as part of the treatment and recovery process in those settings.21 Some patients may require detoxification prior to referral to residential treatment settings; the American Society of Addiction Medicine has published a comprehensive guideline on alcohol withdrawal.24
Paul’s family physician diagnosed his condition as AUD and referred him for CBT with a psychologist, who treated him for both the AUD and an underlying depressive disorder that was later identified. CBT focused on cognitive restructuring of depressive thoughts as well as support for continued abstinence from alcohol. The patient, with family support, declined antidepressant medication.
After 6 months of treatment, Paul and his parents were pleased with his progress. His grades improved to the point that he was permitted to play soccer again, and he was seriously looking at his future college options.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; [email protected]
1. US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2007.
2. US Department of Health and Human Services. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2016.
3. Hingson R, White A. New research findings since the 2007 Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking: A review. J Stud Alcohol Drugs Suppl. 2014; 75:158-169.
4. National Institute on Alcohol Abuse and Alcoholism. Underage drinking. National Institute of Health. Accessed December 22, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/underage-drinking.
5. Hingson R, Zha W, Iannotti R, et al. Physician advice to adolescents about drinking and other health behaviors. Pediatrics. 2013;131:249-257.
6. Schaus JF, Sole ML, McCoy TP, et al. Screening for high-risk drinking in a college student health center: characterizing students based on quantity, frequency, and harms. J Stud Alcohol Drugs Suppl. 2009;16:34-44.
7. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed December 27, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Arlington, VA; American Psychiatric Association. 2013.
9. Substance Abuse and Mental Health Services Administration. Bringing down binge drinking. Accessed December 27, 2021. www.samhsa.gov/sites/default/files/programs_campaigns/nation_prevention_week/data-binge-drinking.pdf
10. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
11. USPSTF. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:1899-1909.
12. Levy SJ, Williams JF, Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161211.
13. MacKay AP, Duran CP. Adolescent Health in the United States. National Center for Health Statistics, Centers for Disease Control and Prevention. 2007.
14. Haller DM, Meynard A, Lefebvre D, et al. Effectiveness of training family physicians to deliver a brief intervention to address excessive substance use among young patients: a cluster randomized controlled trial. CMAJ. 2014;186:E263-E272.
15. Borus J, Parhami I, Levy S. Screening, brief intervention, and referral to treatment. Child Adolesc Psychiatric Clin N Am. 2016;25:579-601.
16. Knight J, Roberts T, Gabrielli J, et al. Adolescent alcohol and substance use and abuse. Performing preventive services: A bright futures handbook. Accessed December 22, 2021. American Academy of Pediatrics. https://ocfcpacourts.us/wp-content/uploads/2020/06/Adolescent_Alcohol_and_Substance_Abuse_001005.pdf
17. Dyer ML, Heron J, Hickman M, et al. Alcohol use in late adolescence and early adulthood: the role of generalized anxiety disorder and drinking to cope motives. Drug Alcohol Depend. 2019;204:107480.
18. Blumenthal H, Taylor DJ, Cloutier RM, et al. The links between social anxiety disorder, insomnia symptoms, and alcohol use disorders: findings from a large sample of adolescents in the United States. Behav Ther. 2019;50:50-59.
19. Pedrelli P, Shapero B, Archibald A, et al. Alcohol use and depression during adolescence and young adulthood: a summary and interpretation of mixed findings. Curr Addict Rep. 2016;3:91-97.
20. Davis JP, Dworkin ER, Helton J, et al. Extending poly-victimization theory: differential effects of adolescents’ experiences of victimization on substance use disorder diagnoses upon treatment entry. Child Abuse Negl. 2019; 89:165-177.
21. NIDA. Principles of adolescent substance use disorder treatment: a research-based guide. Accessed December 22, 2021. www.drugabuse.gov/publications/principles-adolescent-substance-use-disorder-treatment-research-based-guide
22. Solhkhah R, Wilens TE, Daly J, et al. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. J Child Adolesc Psychopharmacol. 2005:15:777-786.
23. Camenga DR, Colon-Rivera HA, Muvvala SB. Medications for maintenance treatment of opioid use disorder in adolescents. J Stud Alcohol Drugs. 2019;80:393-402.
24. American Society of Addiction Medicine. The ASAM clinical practice guideline on alcohol withdrawal management. Accessed December 22, 2021. www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
THE CASE
Paul F* is a 16-year-old White boy who lives with his mother and spends some weekends with his father who has shared custody. He recently presented to the clinic for treatment due to an arrest for disorderly conduct at school. He and a friend were found drinking liquor outside the school building when they were scheduled to be in class. Paul reported that he and his friends often drink at school and at extracurricular functions. He has been using alcohol for the past 2 years, with escalating consumption (5 or more drinks per episode) in the past year. Paul has been drinking most days of the week and has even driven under the influence at times. He said, “I just feel happier when I am drinking.” An accomplished soccer player recruited by colleges, Paul recently was suspended from the team due to his poor grades. His response was, “It’s stupid anyway. What’s the point of playing?”
●
* The patient’s name and some personal details have been changed to protect his identity.
Alcohol is the number 1 substance of abuse for adolescents, used more than tobacco or drugs.1-3 In 2007 and again in 2016, the Surgeon General of the United States issued reports to highlight this important topic,1,2 noting that early and repeated exposure to alcohol during this crucial time of brain development increases the risk for future problems, including addiction.2
Adolescent alcohol use is often underestimated by parents and physicians, including misjudging how much, how often, and how young children are when they begin to drink.1 Boys and girls tend to start drinking at similar ages (13.9 and 14.4 years, respectively),3 but as girls age, they tend to drink more and binge more.4 In 2019, 1 in 4 adolescents reported drinking and more than 4 million reported at least 1 episode of binge drinking in the prior month.4 These numbers have further ramifications: early drinking is associated with alcohol dependence, relapse, use of other substances, risky sexual behaviors, injurious behaviors, suicide, motor vehicle accidents, and dating violence.4-6
Diagnosing alcohol use disorder
The range of alcohol use includes consumption, bingeing, abuse, and dependence.7,8 Consumption is defined as the drinking of alcoholic beverages. Bingeing is the consumption of more than 5 drinks for men or 4 drinks for women in 2 hours, according to the National Institute on Alcohol Abuse and Alcoholism.7 However, the criterion is slightly different for the Substance Abuse and Mental Health Services Administration, which broadens the timeframe to “on the same occasion.”9 While previously known as separate disorders, alcohol abuse (or misuse) and alcohol dependence are now diagnostically classified together as alcohol use disorders (AUDs), per the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5).8 AUD is further stratified as mild, moderate, or severe, depending on the number of criteria that are met by the patient (TABLE).8,10

Alcohol screening
Currently, the US Preventive Services Task Force (USPSTF) does not recommend screening adolescents ages 12 to 17 for AUD, and has instead issued an “I” statement (insufficient evidence).11 While the USPSTF recognizes the potential burdens of adolescent alcohol use, the potential harms of screening include “stigma, anxiety, labeling, discrimination, privacy concerns, and interference with the patient–clinician relationship.”11 The USPSTF also notes that it “did not find any evidence that specifically examined the harms of screening for alcohol use in adolescents.”11
This is at odds with recommendations from the American Academy of Pediatrics (AAP), which in 2011 released a policy statement advocating screening, brief intervention, and referral to treatment for adolescent substance use.12 In the United States, even though 83% of adolescents see a physician at least once each year,12,13 alcohol misuse screening still varies, occurring in the range of 50% to 86% of office visits.12 When screening does occur, it is often based on clinical impression only.12 Studies have shown that when a screening tool is not used, up to two-thirds of substance use disorders may be missed.12-15
Continue to: A full and complete biopsychosocial interview
A full and complete biopsychosocial interview with adolescents is a necessity, and should include queries about alcohol, drugs, and other substances. Acknowledgment of use should trigger further investigation into the substance use areas. Interviews may start with open-ended questions about alcohol use at home or at school before moving to more personalized and detailed questioning and use of screening tools.16
While various screening instruments exist, for the sake of brevity we provide as an example the Screening to Brief Intervention (S2BI) tool. It is an efficient, single-page tool that can help clinicians in their routine care of adolescents to quickly stratify the patient risk of substance use disorder as none/low, moderate, or severe.12 It can be found here: www.mcpap.com/pdf/S2Bi%20Toolkit.pdf (see page 10).
For all patients, but particularly for adolescents, confidentiality is important, and many specialty societies have created language to address this issue.12 Discuss confidentiality with both the adolescent patient and the patient’s caregiver simultaneously, with dialogue that includes: (a) the need to speak with adolescents alone during the office visit, (b) the benefits of confidentiality in the physician–patient relationship, and (c) the need to disclose selected information to keep patients safe.12 Describing the process for required disclosures is essential. Benefits of disclosure include further support for the adolescent patient as well as appropriate parental participation and support for possible referrals.12
Treating AUD
Treatment for AUD should be multifaceted. Screen for comorbid mood disorders, such as generalized anxiety,17,18 social anxiety,18 and depression,19 as well as for insomnia.18 Studies have demonstrated a strong link between insomnia and anxiety, and again between anxiety and AUD.17-19 Finally, screen for adverse childhood events such as trauma, victimization, and abuse.20 Addressing issues discovered in screening allows for more targeted and personalized treatment of AUD.
The National Institute on Drug Abuse categorizes evidence-based treatment into 3 areas: behavioral therapies, family therapies, and medications.21
Continue to: Behavioral therapies
Behavioral therapies can include group therapy, cognitive behavioral therapy (CBT), motivational enhancement therapy, 12-Step facilitation, and contingency management, in which small rewards or incentives are given for participation in treatment to reinforce positive behaviors.21
Family-based therapies, such as brief strategic family therapy, functional family therapy, and multisystem therapy recognize that adolescents exist in systems of families in communities, and that the patient’s success in treatment may be supported by these relationships.21
Some medications may achieve modest benefit for treatment of adolescents with AUD. Naltrexone, acamprosate, and disulfiram have all been used successfully to treat AUD in adults21; some physicians may choose to use these medications “off label” in adolescents. Bupropion has been used successfully in the treatment of nicotine use disorder,21 and a small study in 2005 showed some success with bupropion in treating adolescents with attention-deficit/hyperactivity disorder, comorbid depression, and substance use disorder.22 Naltrexone has also been studied in adolescents with opioid use disorder, although these were not large studies.23
Adolescents with serious, sustained issues with AUD may require more in-depth treatments such as an intensive outpatient program, a partial hospitalization program, or a residential treatment program.15 The least-restrictive environment is preferable.15 Families are generally included as part of the treatment and recovery process in those settings.21 Some patients may require detoxification prior to referral to residential treatment settings; the American Society of Addiction Medicine has published a comprehensive guideline on alcohol withdrawal.24
Paul’s family physician diagnosed his condition as AUD and referred him for CBT with a psychologist, who treated him for both the AUD and an underlying depressive disorder that was later identified. CBT focused on cognitive restructuring of depressive thoughts as well as support for continued abstinence from alcohol. The patient, with family support, declined antidepressant medication.
After 6 months of treatment, Paul and his parents were pleased with his progress. His grades improved to the point that he was permitted to play soccer again, and he was seriously looking at his future college options.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; [email protected]
THE CASE
Paul F* is a 16-year-old White boy who lives with his mother and spends some weekends with his father who has shared custody. He recently presented to the clinic for treatment due to an arrest for disorderly conduct at school. He and a friend were found drinking liquor outside the school building when they were scheduled to be in class. Paul reported that he and his friends often drink at school and at extracurricular functions. He has been using alcohol for the past 2 years, with escalating consumption (5 or more drinks per episode) in the past year. Paul has been drinking most days of the week and has even driven under the influence at times. He said, “I just feel happier when I am drinking.” An accomplished soccer player recruited by colleges, Paul recently was suspended from the team due to his poor grades. His response was, “It’s stupid anyway. What’s the point of playing?”
●
* The patient’s name and some personal details have been changed to protect his identity.
Alcohol is the number 1 substance of abuse for adolescents, used more than tobacco or drugs.1-3 In 2007 and again in 2016, the Surgeon General of the United States issued reports to highlight this important topic,1,2 noting that early and repeated exposure to alcohol during this crucial time of brain development increases the risk for future problems, including addiction.2
Adolescent alcohol use is often underestimated by parents and physicians, including misjudging how much, how often, and how young children are when they begin to drink.1 Boys and girls tend to start drinking at similar ages (13.9 and 14.4 years, respectively),3 but as girls age, they tend to drink more and binge more.4 In 2019, 1 in 4 adolescents reported drinking and more than 4 million reported at least 1 episode of binge drinking in the prior month.4 These numbers have further ramifications: early drinking is associated with alcohol dependence, relapse, use of other substances, risky sexual behaviors, injurious behaviors, suicide, motor vehicle accidents, and dating violence.4-6
Diagnosing alcohol use disorder
The range of alcohol use includes consumption, bingeing, abuse, and dependence.7,8 Consumption is defined as the drinking of alcoholic beverages. Bingeing is the consumption of more than 5 drinks for men or 4 drinks for women in 2 hours, according to the National Institute on Alcohol Abuse and Alcoholism.7 However, the criterion is slightly different for the Substance Abuse and Mental Health Services Administration, which broadens the timeframe to “on the same occasion.”9 While previously known as separate disorders, alcohol abuse (or misuse) and alcohol dependence are now diagnostically classified together as alcohol use disorders (AUDs), per the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5).8 AUD is further stratified as mild, moderate, or severe, depending on the number of criteria that are met by the patient (TABLE).8,10

Alcohol screening
Currently, the US Preventive Services Task Force (USPSTF) does not recommend screening adolescents ages 12 to 17 for AUD, and has instead issued an “I” statement (insufficient evidence).11 While the USPSTF recognizes the potential burdens of adolescent alcohol use, the potential harms of screening include “stigma, anxiety, labeling, discrimination, privacy concerns, and interference with the patient–clinician relationship.”11 The USPSTF also notes that it “did not find any evidence that specifically examined the harms of screening for alcohol use in adolescents.”11
This is at odds with recommendations from the American Academy of Pediatrics (AAP), which in 2011 released a policy statement advocating screening, brief intervention, and referral to treatment for adolescent substance use.12 In the United States, even though 83% of adolescents see a physician at least once each year,12,13 alcohol misuse screening still varies, occurring in the range of 50% to 86% of office visits.12 When screening does occur, it is often based on clinical impression only.12 Studies have shown that when a screening tool is not used, up to two-thirds of substance use disorders may be missed.12-15
Continue to: A full and complete biopsychosocial interview
A full and complete biopsychosocial interview with adolescents is a necessity, and should include queries about alcohol, drugs, and other substances. Acknowledgment of use should trigger further investigation into the substance use areas. Interviews may start with open-ended questions about alcohol use at home or at school before moving to more personalized and detailed questioning and use of screening tools.16
While various screening instruments exist, for the sake of brevity we provide as an example the Screening to Brief Intervention (S2BI) tool. It is an efficient, single-page tool that can help clinicians in their routine care of adolescents to quickly stratify the patient risk of substance use disorder as none/low, moderate, or severe.12 It can be found here: www.mcpap.com/pdf/S2Bi%20Toolkit.pdf (see page 10).
For all patients, but particularly for adolescents, confidentiality is important, and many specialty societies have created language to address this issue.12 Discuss confidentiality with both the adolescent patient and the patient’s caregiver simultaneously, with dialogue that includes: (a) the need to speak with adolescents alone during the office visit, (b) the benefits of confidentiality in the physician–patient relationship, and (c) the need to disclose selected information to keep patients safe.12 Describing the process for required disclosures is essential. Benefits of disclosure include further support for the adolescent patient as well as appropriate parental participation and support for possible referrals.12
Treating AUD
Treatment for AUD should be multifaceted. Screen for comorbid mood disorders, such as generalized anxiety,17,18 social anxiety,18 and depression,19 as well as for insomnia.18 Studies have demonstrated a strong link between insomnia and anxiety, and again between anxiety and AUD.17-19 Finally, screen for adverse childhood events such as trauma, victimization, and abuse.20 Addressing issues discovered in screening allows for more targeted and personalized treatment of AUD.
The National Institute on Drug Abuse categorizes evidence-based treatment into 3 areas: behavioral therapies, family therapies, and medications.21
Continue to: Behavioral therapies
Behavioral therapies can include group therapy, cognitive behavioral therapy (CBT), motivational enhancement therapy, 12-Step facilitation, and contingency management, in which small rewards or incentives are given for participation in treatment to reinforce positive behaviors.21
Family-based therapies, such as brief strategic family therapy, functional family therapy, and multisystem therapy recognize that adolescents exist in systems of families in communities, and that the patient’s success in treatment may be supported by these relationships.21
Some medications may achieve modest benefit for treatment of adolescents with AUD. Naltrexone, acamprosate, and disulfiram have all been used successfully to treat AUD in adults21; some physicians may choose to use these medications “off label” in adolescents. Bupropion has been used successfully in the treatment of nicotine use disorder,21 and a small study in 2005 showed some success with bupropion in treating adolescents with attention-deficit/hyperactivity disorder, comorbid depression, and substance use disorder.22 Naltrexone has also been studied in adolescents with opioid use disorder, although these were not large studies.23
Adolescents with serious, sustained issues with AUD may require more in-depth treatments such as an intensive outpatient program, a partial hospitalization program, or a residential treatment program.15 The least-restrictive environment is preferable.15 Families are generally included as part of the treatment and recovery process in those settings.21 Some patients may require detoxification prior to referral to residential treatment settings; the American Society of Addiction Medicine has published a comprehensive guideline on alcohol withdrawal.24
Paul’s family physician diagnosed his condition as AUD and referred him for CBT with a psychologist, who treated him for both the AUD and an underlying depressive disorder that was later identified. CBT focused on cognitive restructuring of depressive thoughts as well as support for continued abstinence from alcohol. The patient, with family support, declined antidepressant medication.
After 6 months of treatment, Paul and his parents were pleased with his progress. His grades improved to the point that he was permitted to play soccer again, and he was seriously looking at his future college options.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; [email protected]
1. US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2007.
2. US Department of Health and Human Services. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2016.
3. Hingson R, White A. New research findings since the 2007 Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking: A review. J Stud Alcohol Drugs Suppl. 2014; 75:158-169.
4. National Institute on Alcohol Abuse and Alcoholism. Underage drinking. National Institute of Health. Accessed December 22, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/underage-drinking.
5. Hingson R, Zha W, Iannotti R, et al. Physician advice to adolescents about drinking and other health behaviors. Pediatrics. 2013;131:249-257.
6. Schaus JF, Sole ML, McCoy TP, et al. Screening for high-risk drinking in a college student health center: characterizing students based on quantity, frequency, and harms. J Stud Alcohol Drugs Suppl. 2009;16:34-44.
7. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed December 27, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Arlington, VA; American Psychiatric Association. 2013.
9. Substance Abuse and Mental Health Services Administration. Bringing down binge drinking. Accessed December 27, 2021. www.samhsa.gov/sites/default/files/programs_campaigns/nation_prevention_week/data-binge-drinking.pdf
10. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
11. USPSTF. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:1899-1909.
12. Levy SJ, Williams JF, Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161211.
13. MacKay AP, Duran CP. Adolescent Health in the United States. National Center for Health Statistics, Centers for Disease Control and Prevention. 2007.
14. Haller DM, Meynard A, Lefebvre D, et al. Effectiveness of training family physicians to deliver a brief intervention to address excessive substance use among young patients: a cluster randomized controlled trial. CMAJ. 2014;186:E263-E272.
15. Borus J, Parhami I, Levy S. Screening, brief intervention, and referral to treatment. Child Adolesc Psychiatric Clin N Am. 2016;25:579-601.
16. Knight J, Roberts T, Gabrielli J, et al. Adolescent alcohol and substance use and abuse. Performing preventive services: A bright futures handbook. Accessed December 22, 2021. American Academy of Pediatrics. https://ocfcpacourts.us/wp-content/uploads/2020/06/Adolescent_Alcohol_and_Substance_Abuse_001005.pdf
17. Dyer ML, Heron J, Hickman M, et al. Alcohol use in late adolescence and early adulthood: the role of generalized anxiety disorder and drinking to cope motives. Drug Alcohol Depend. 2019;204:107480.
18. Blumenthal H, Taylor DJ, Cloutier RM, et al. The links between social anxiety disorder, insomnia symptoms, and alcohol use disorders: findings from a large sample of adolescents in the United States. Behav Ther. 2019;50:50-59.
19. Pedrelli P, Shapero B, Archibald A, et al. Alcohol use and depression during adolescence and young adulthood: a summary and interpretation of mixed findings. Curr Addict Rep. 2016;3:91-97.
20. Davis JP, Dworkin ER, Helton J, et al. Extending poly-victimization theory: differential effects of adolescents’ experiences of victimization on substance use disorder diagnoses upon treatment entry. Child Abuse Negl. 2019; 89:165-177.
21. NIDA. Principles of adolescent substance use disorder treatment: a research-based guide. Accessed December 22, 2021. www.drugabuse.gov/publications/principles-adolescent-substance-use-disorder-treatment-research-based-guide
22. Solhkhah R, Wilens TE, Daly J, et al. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. J Child Adolesc Psychopharmacol. 2005:15:777-786.
23. Camenga DR, Colon-Rivera HA, Muvvala SB. Medications for maintenance treatment of opioid use disorder in adolescents. J Stud Alcohol Drugs. 2019;80:393-402.
24. American Society of Addiction Medicine. The ASAM clinical practice guideline on alcohol withdrawal management. Accessed December 22, 2021. www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
1. US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2007.
2. US Department of Health and Human Services. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2016.
3. Hingson R, White A. New research findings since the 2007 Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking: A review. J Stud Alcohol Drugs Suppl. 2014; 75:158-169.
4. National Institute on Alcohol Abuse and Alcoholism. Underage drinking. National Institute of Health. Accessed December 22, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/underage-drinking.
5. Hingson R, Zha W, Iannotti R, et al. Physician advice to adolescents about drinking and other health behaviors. Pediatrics. 2013;131:249-257.
6. Schaus JF, Sole ML, McCoy TP, et al. Screening for high-risk drinking in a college student health center: characterizing students based on quantity, frequency, and harms. J Stud Alcohol Drugs Suppl. 2009;16:34-44.
7. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed December 27, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Arlington, VA; American Psychiatric Association. 2013.
9. Substance Abuse and Mental Health Services Administration. Bringing down binge drinking. Accessed December 27, 2021. www.samhsa.gov/sites/default/files/programs_campaigns/nation_prevention_week/data-binge-drinking.pdf
10. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
11. USPSTF. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:1899-1909.
12. Levy SJ, Williams JF, Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161211.
13. MacKay AP, Duran CP. Adolescent Health in the United States. National Center for Health Statistics, Centers for Disease Control and Prevention. 2007.
14. Haller DM, Meynard A, Lefebvre D, et al. Effectiveness of training family physicians to deliver a brief intervention to address excessive substance use among young patients: a cluster randomized controlled trial. CMAJ. 2014;186:E263-E272.
15. Borus J, Parhami I, Levy S. Screening, brief intervention, and referral to treatment. Child Adolesc Psychiatric Clin N Am. 2016;25:579-601.
16. Knight J, Roberts T, Gabrielli J, et al. Adolescent alcohol and substance use and abuse. Performing preventive services: A bright futures handbook. Accessed December 22, 2021. American Academy of Pediatrics. https://ocfcpacourts.us/wp-content/uploads/2020/06/Adolescent_Alcohol_and_Substance_Abuse_001005.pdf
17. Dyer ML, Heron J, Hickman M, et al. Alcohol use in late adolescence and early adulthood: the role of generalized anxiety disorder and drinking to cope motives. Drug Alcohol Depend. 2019;204:107480.
18. Blumenthal H, Taylor DJ, Cloutier RM, et al. The links between social anxiety disorder, insomnia symptoms, and alcohol use disorders: findings from a large sample of adolescents in the United States. Behav Ther. 2019;50:50-59.
19. Pedrelli P, Shapero B, Archibald A, et al. Alcohol use and depression during adolescence and young adulthood: a summary and interpretation of mixed findings. Curr Addict Rep. 2016;3:91-97.
20. Davis JP, Dworkin ER, Helton J, et al. Extending poly-victimization theory: differential effects of adolescents’ experiences of victimization on substance use disorder diagnoses upon treatment entry. Child Abuse Negl. 2019; 89:165-177.
21. NIDA. Principles of adolescent substance use disorder treatment: a research-based guide. Accessed December 22, 2021. www.drugabuse.gov/publications/principles-adolescent-substance-use-disorder-treatment-research-based-guide
22. Solhkhah R, Wilens TE, Daly J, et al. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. J Child Adolesc Psychopharmacol. 2005:15:777-786.
23. Camenga DR, Colon-Rivera HA, Muvvala SB. Medications for maintenance treatment of opioid use disorder in adolescents. J Stud Alcohol Drugs. 2019;80:393-402.
24. American Society of Addiction Medicine. The ASAM clinical practice guideline on alcohol withdrawal management. Accessed December 22, 2021. www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline