User login
COVID at 2 years: Preparing for a different ‘normal’
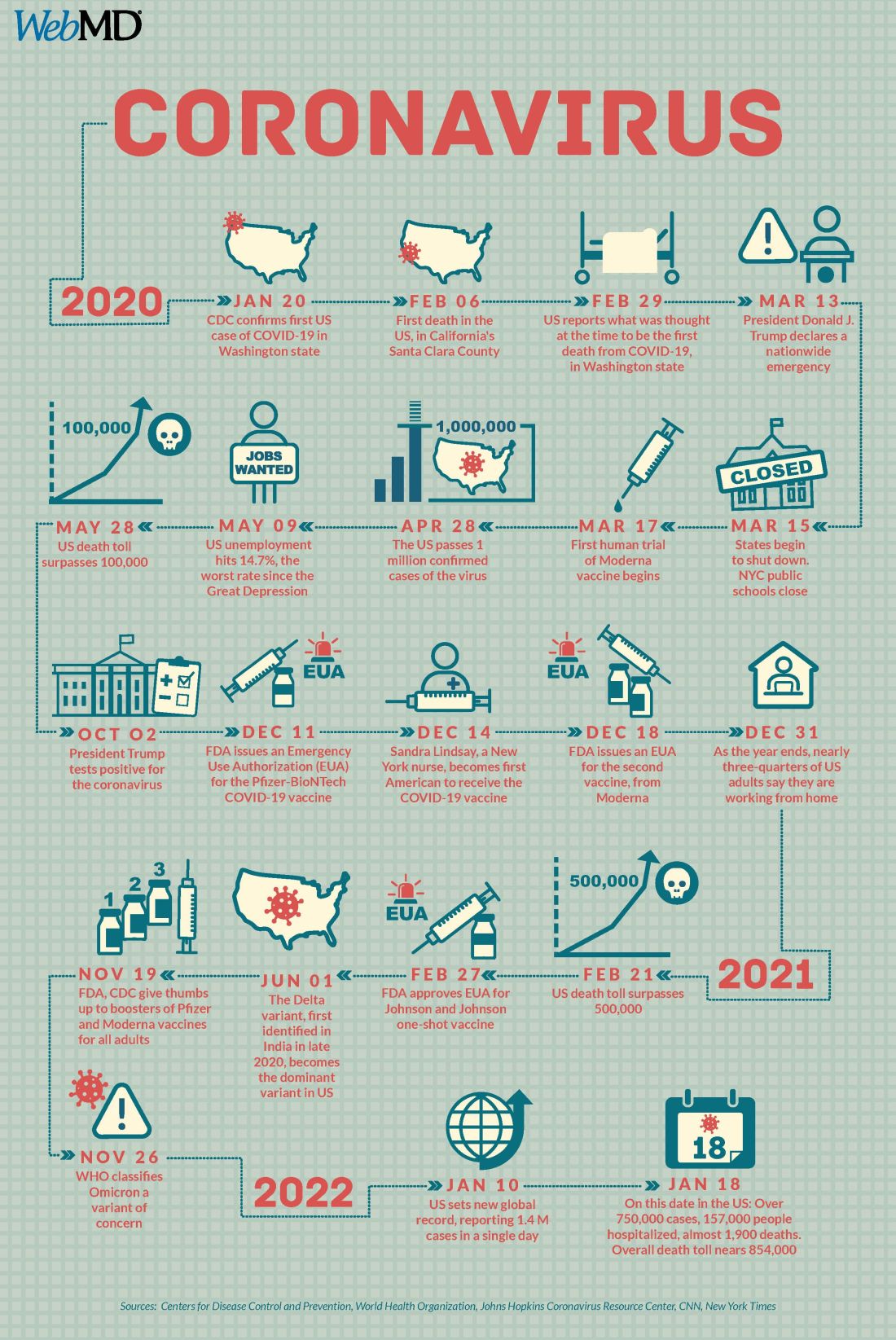
Two years into the COVID-19 pandemic, the United States is still breaking records in hospital overcrowding and new cases.
The United States is logging nearly 800,000 cases a day, hospitals are starting to fray, and deaths have topped 850,000. Schools oscillate from remote to in-person learning, polarizing communities.
The vaccines are lifesaving for many, yet frustration mounts as the numbers of unvaccinated people in this country stays relatively stagnant (63% in the United States are fully vaccinated) and other parts of the world have seen hardly a single dose. Africa has the slowest vaccination rate among continents, with only 14% of the population receiving one shot, according to the New York Times tracker.
Yet
Effective vaccines and treatments that can keep people out of the hospital were developed at an astounding pace, and advances in tracking and testing – in both access and effectiveness – are starting to pay off.
Some experts say it’s possible that the raging Omicron surge will slow by late spring, providing some relief and maybe shifting the pandemic to a slower-burning endemic.
But other experts caution to keep our guard up, saying it’s time to settle into a “new normal” and upend the strategy for fighting COVID-19.
Time to change COVID thinking
Three former members of the Biden-Harris Transition COVID-19 Advisory Board wrote recently in JAMA that COVID-19 has now become one of the many viral respiratory diseases that health care providers and patients will manage each year.
The group of experts from the University of Pennsylvania, University of Minnesota, and New York University write that “many of the measures to reduce transmission of SARS-CoV-2 (for example, ventilation) will also reduce transmission of other respiratory viruses. Thus, policy makers should retire previous public health categorizations, including deaths from pneumonia and influenza or pneumonia, influenza, and COVID-19, and focus on a new category: the aggregate risk of all respiratory virus infections.”
Other experts, including Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore, have said it’s been clear since the early days of SARS-CoV-2 that we must learn to live with the virus because it “will be ever present for the remaining history of our species.”
But that doesn’t mean the virus will always have the upper hand. Although the United States has been reaching record numbers of hospitalizations in January, these hospitalizations differ from those of last year – marked by fewer extreme lifesaving measures, fewer deaths, and shorter hospital stays – caused in part by medical and therapeutic advances and in part to the nature of the Omicron variant itself.
One sign of progress, Dr. Adalja said, will be the widespread decoupling of cases from hospitalizations, something that has already happened in countries such as the United Kingdom.
“That’s a reflection of how well they have vaccinated their high-risk population and how poorly we have vaccinated our high-risk population,” he said.
Omicron will bump up natural immunity
Dr. Adalja said though the numbers of unvaccinated in the United States appear to be stuck, Omicron’s sweep will make the difference, leaving behind more natural immunity in the population.
Currently, hospitals are struggling with staffing concerns as a “direct result” of too many unvaccinated people, he said.
Andrew Badley, MD, an infectious diseases specialist at Mayo Clinic in Rochester, Minn., and director of the clinic’s COVID-19 Task Force, said the good news with Omicron is that nearly all people it infects will recover.
Over time, when the body sees foreign antigens repeatedly, the quantity and quality of the antibodies the immune system produces increase and the body becomes better at fighting disease.
So “a large amount of the population will have recovered and have a degree of immunity,” Dr. Badley said.
His optimism is tempered by his belief that “it’s going to get worse before it gets better.”
But Dr. Badley still predicts a turnaround. “We’ll see a downturn in COVID in late spring or early summer,” and well into the second quarter of 2022, “we’ll see a reemergence of control.”
Right now, with Omicron, one infected person is infecting three to five others, he said. The hope is that it will eventually reach one-to-one endemic levels.
As for the threat of new variants, Badley said, “it’s not predictable whether they will be stronger or weaker.”
Masks may be around for years
Many experts predict that masks will continue to be part of the national wardrobe for the foreseeable future.
“We will continue to see new cases for years and years to come. Some will respond to that with masks in public places for a very long time. I personally will do so,” Dr. Badley said.
Two mindsets: Inside/outside the hospital
Emily Landon, MD, an infectious disease doctor and the executive medical director of infection prevention and control at University of Chicago Medicine, told this news organization she views the pandemic from two different vantage points.
As a health care provider, she sees her hospital, like others worldwide, overwhelmed. Supplies of a major weapon to help prevent hospitalization, the monoclonal antibody sotrovimab, are running out. Dr. Landon said she has been calling other hospitals to see if they have supplies and, if so, whether Omicron patients can transfer there.
Bottom line: The things they relied on a month ago to keep people out of the hospital are no longer there, she said.
Meanwhile, “We have more COVID patients than we have ever had,” Dr. Landon said.
Last year, UChicago hit a high of 170 people hospitalized with COVID. This year, so far, the peak was 270.
Dr. Landon said she is frustrated when she leaves that overburdened world inside the hospital for the outside world, where people wear no masks or ineffective face coverings and gather unsafely. Although some of that behavior reflects an intention to flout the advice of medical experts, some is caused in part, she said, by the lack of a clear national health strategy and garbled communication from those in charge of public safety.
Americans are deciding for themselves, on an a la carte basis, whether to wear a mask or get tested or travel, and school districts decide individually when it’s time to go virtual.
“People are exhausted from having to do a risk-benefit analysis for every single activity they, their friends, their kids want to participate in,” she said.
U.S. behind in several areas
Despite our self-image as the global leader in science and medicine, the United States stumbled badly in its response to the pandemic, with grave consequences both at home and abroad, experts say.
In a recent commentary in JAMA, Lawrence Gostin, JD, from Georgetown University, Washington, and Jennifer Nuzzo, DrPH, at Johns Hopkins University, Baltimore, pointed to several critical shortfalls in the nation’s efforts to control the disease.
One such shortfall is public trust.
This news organization reported in June 2021 that a poll of its readers found that 44% said their trust in the CDC had waned during the pandemic, and 33% said their trust in the FDA had eroded as well.
Health care providers who responded to the poll lost trust as well. About half of the doctors and nurses who responded said they disagreed with the FDA’s decision-making during the pandemic. Nearly 60% of doctors and 65% of nurses said they disagreed with the CDC’s overall pandemic guidance.
Lack of trust can make people resist vaccines and efforts to fight the virus, the authors wrote.
“This will become really relevant when we have ample supply of Pfizer’s antiviral medication,” Mr. Gostin, who directs the O’Neill Institute for National and Global Health Law at Georgetown, told this news organization. “The next phase of the pandemic is not to link testing to contact tracing, because we’re way past that, but to link testing to treatment.”
Lack of regional manufacturing of products is also thwarting global progress.
“It is extraordinarily important that our pharmaceutical industry transfer technology in a pandemic,” Mr. Gostin said. “The most glaring failure to do that is the mRNA vaccines. We’ve got this enormously effective vaccine and the two manufacturers – Pfizer and Moderna – are refusing to share the technology with producers in other countries. That keeps coming back to haunt us.”
Another problem: When the vaccines are shared with other countries, they are being delivered close to the date they expire or arriving at a shipyards without warning, so even some of the doses that get delivered are going to waste, Mr. Gostin said.
“It’s one of the greatest moral failures of my lifetime,” he said.
Also a failure is the “jaw-dropping” state of testing 2 years into the pandemic, he said, as people continue to pay high prices for tests or endure long lines.
The U.S. government updated its calculations and ordered 1 billion tests for the general public. The COVIDtests.gov website to order the free tests is now live.
It’s a step in the right direction. Mr. Gostin and Dr. Nuzzo wrote that there is every reason to expect future epidemics that are as serious or more serious than COVID.
“Failure to address clearly observed weaknesses in the COVID-19 response will have preventable adverse health, social, and economic consequences when the next novel outbreak occurs,” they wrote.
A version of this article first appeared on WebMD.com.
Two years into the COVID-19 pandemic, the United States is still breaking records in hospital overcrowding and new cases.
The United States is logging nearly 800,000 cases a day, hospitals are starting to fray, and deaths have topped 850,000. Schools oscillate from remote to in-person learning, polarizing communities.
The vaccines are lifesaving for many, yet frustration mounts as the numbers of unvaccinated people in this country stays relatively stagnant (63% in the United States are fully vaccinated) and other parts of the world have seen hardly a single dose. Africa has the slowest vaccination rate among continents, with only 14% of the population receiving one shot, according to the New York Times tracker.
Yet
Effective vaccines and treatments that can keep people out of the hospital were developed at an astounding pace, and advances in tracking and testing – in both access and effectiveness – are starting to pay off.
Some experts say it’s possible that the raging Omicron surge will slow by late spring, providing some relief and maybe shifting the pandemic to a slower-burning endemic.
But other experts caution to keep our guard up, saying it’s time to settle into a “new normal” and upend the strategy for fighting COVID-19.
Time to change COVID thinking
Three former members of the Biden-Harris Transition COVID-19 Advisory Board wrote recently in JAMA that COVID-19 has now become one of the many viral respiratory diseases that health care providers and patients will manage each year.
The group of experts from the University of Pennsylvania, University of Minnesota, and New York University write that “many of the measures to reduce transmission of SARS-CoV-2 (for example, ventilation) will also reduce transmission of other respiratory viruses. Thus, policy makers should retire previous public health categorizations, including deaths from pneumonia and influenza or pneumonia, influenza, and COVID-19, and focus on a new category: the aggregate risk of all respiratory virus infections.”
Other experts, including Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore, have said it’s been clear since the early days of SARS-CoV-2 that we must learn to live with the virus because it “will be ever present for the remaining history of our species.”
But that doesn’t mean the virus will always have the upper hand. Although the United States has been reaching record numbers of hospitalizations in January, these hospitalizations differ from those of last year – marked by fewer extreme lifesaving measures, fewer deaths, and shorter hospital stays – caused in part by medical and therapeutic advances and in part to the nature of the Omicron variant itself.
One sign of progress, Dr. Adalja said, will be the widespread decoupling of cases from hospitalizations, something that has already happened in countries such as the United Kingdom.
“That’s a reflection of how well they have vaccinated their high-risk population and how poorly we have vaccinated our high-risk population,” he said.
Omicron will bump up natural immunity
Dr. Adalja said though the numbers of unvaccinated in the United States appear to be stuck, Omicron’s sweep will make the difference, leaving behind more natural immunity in the population.
Currently, hospitals are struggling with staffing concerns as a “direct result” of too many unvaccinated people, he said.
Andrew Badley, MD, an infectious diseases specialist at Mayo Clinic in Rochester, Minn., and director of the clinic’s COVID-19 Task Force, said the good news with Omicron is that nearly all people it infects will recover.
Over time, when the body sees foreign antigens repeatedly, the quantity and quality of the antibodies the immune system produces increase and the body becomes better at fighting disease.
So “a large amount of the population will have recovered and have a degree of immunity,” Dr. Badley said.
His optimism is tempered by his belief that “it’s going to get worse before it gets better.”
But Dr. Badley still predicts a turnaround. “We’ll see a downturn in COVID in late spring or early summer,” and well into the second quarter of 2022, “we’ll see a reemergence of control.”
Right now, with Omicron, one infected person is infecting three to five others, he said. The hope is that it will eventually reach one-to-one endemic levels.
As for the threat of new variants, Badley said, “it’s not predictable whether they will be stronger or weaker.”
Masks may be around for years
Many experts predict that masks will continue to be part of the national wardrobe for the foreseeable future.
“We will continue to see new cases for years and years to come. Some will respond to that with masks in public places for a very long time. I personally will do so,” Dr. Badley said.
Two mindsets: Inside/outside the hospital
Emily Landon, MD, an infectious disease doctor and the executive medical director of infection prevention and control at University of Chicago Medicine, told this news organization she views the pandemic from two different vantage points.
As a health care provider, she sees her hospital, like others worldwide, overwhelmed. Supplies of a major weapon to help prevent hospitalization, the monoclonal antibody sotrovimab, are running out. Dr. Landon said she has been calling other hospitals to see if they have supplies and, if so, whether Omicron patients can transfer there.
Bottom line: The things they relied on a month ago to keep people out of the hospital are no longer there, she said.
Meanwhile, “We have more COVID patients than we have ever had,” Dr. Landon said.
Last year, UChicago hit a high of 170 people hospitalized with COVID. This year, so far, the peak was 270.
Dr. Landon said she is frustrated when she leaves that overburdened world inside the hospital for the outside world, where people wear no masks or ineffective face coverings and gather unsafely. Although some of that behavior reflects an intention to flout the advice of medical experts, some is caused in part, she said, by the lack of a clear national health strategy and garbled communication from those in charge of public safety.
Americans are deciding for themselves, on an a la carte basis, whether to wear a mask or get tested or travel, and school districts decide individually when it’s time to go virtual.
“People are exhausted from having to do a risk-benefit analysis for every single activity they, their friends, their kids want to participate in,” she said.
U.S. behind in several areas
Despite our self-image as the global leader in science and medicine, the United States stumbled badly in its response to the pandemic, with grave consequences both at home and abroad, experts say.
In a recent commentary in JAMA, Lawrence Gostin, JD, from Georgetown University, Washington, and Jennifer Nuzzo, DrPH, at Johns Hopkins University, Baltimore, pointed to several critical shortfalls in the nation’s efforts to control the disease.
One such shortfall is public trust.
This news organization reported in June 2021 that a poll of its readers found that 44% said their trust in the CDC had waned during the pandemic, and 33% said their trust in the FDA had eroded as well.
Health care providers who responded to the poll lost trust as well. About half of the doctors and nurses who responded said they disagreed with the FDA’s decision-making during the pandemic. Nearly 60% of doctors and 65% of nurses said they disagreed with the CDC’s overall pandemic guidance.
Lack of trust can make people resist vaccines and efforts to fight the virus, the authors wrote.
“This will become really relevant when we have ample supply of Pfizer’s antiviral medication,” Mr. Gostin, who directs the O’Neill Institute for National and Global Health Law at Georgetown, told this news organization. “The next phase of the pandemic is not to link testing to contact tracing, because we’re way past that, but to link testing to treatment.”
Lack of regional manufacturing of products is also thwarting global progress.
“It is extraordinarily important that our pharmaceutical industry transfer technology in a pandemic,” Mr. Gostin said. “The most glaring failure to do that is the mRNA vaccines. We’ve got this enormously effective vaccine and the two manufacturers – Pfizer and Moderna – are refusing to share the technology with producers in other countries. That keeps coming back to haunt us.”
Another problem: When the vaccines are shared with other countries, they are being delivered close to the date they expire or arriving at a shipyards without warning, so even some of the doses that get delivered are going to waste, Mr. Gostin said.
“It’s one of the greatest moral failures of my lifetime,” he said.
Also a failure is the “jaw-dropping” state of testing 2 years into the pandemic, he said, as people continue to pay high prices for tests or endure long lines.
The U.S. government updated its calculations and ordered 1 billion tests for the general public. The COVIDtests.gov website to order the free tests is now live.
It’s a step in the right direction. Mr. Gostin and Dr. Nuzzo wrote that there is every reason to expect future epidemics that are as serious or more serious than COVID.
“Failure to address clearly observed weaknesses in the COVID-19 response will have preventable adverse health, social, and economic consequences when the next novel outbreak occurs,” they wrote.

A version of this article first appeared on WebMD.com.
Two years into the COVID-19 pandemic, the United States is still breaking records in hospital overcrowding and new cases.
The United States is logging nearly 800,000 cases a day, hospitals are starting to fray, and deaths have topped 850,000. Schools oscillate from remote to in-person learning, polarizing communities.
The vaccines are lifesaving for many, yet frustration mounts as the numbers of unvaccinated people in this country stays relatively stagnant (63% in the United States are fully vaccinated) and other parts of the world have seen hardly a single dose. Africa has the slowest vaccination rate among continents, with only 14% of the population receiving one shot, according to the New York Times tracker.
Yet
Effective vaccines and treatments that can keep people out of the hospital were developed at an astounding pace, and advances in tracking and testing – in both access and effectiveness – are starting to pay off.
Some experts say it’s possible that the raging Omicron surge will slow by late spring, providing some relief and maybe shifting the pandemic to a slower-burning endemic.
But other experts caution to keep our guard up, saying it’s time to settle into a “new normal” and upend the strategy for fighting COVID-19.
Time to change COVID thinking
Three former members of the Biden-Harris Transition COVID-19 Advisory Board wrote recently in JAMA that COVID-19 has now become one of the many viral respiratory diseases that health care providers and patients will manage each year.
The group of experts from the University of Pennsylvania, University of Minnesota, and New York University write that “many of the measures to reduce transmission of SARS-CoV-2 (for example, ventilation) will also reduce transmission of other respiratory viruses. Thus, policy makers should retire previous public health categorizations, including deaths from pneumonia and influenza or pneumonia, influenza, and COVID-19, and focus on a new category: the aggregate risk of all respiratory virus infections.”
Other experts, including Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore, have said it’s been clear since the early days of SARS-CoV-2 that we must learn to live with the virus because it “will be ever present for the remaining history of our species.”
But that doesn’t mean the virus will always have the upper hand. Although the United States has been reaching record numbers of hospitalizations in January, these hospitalizations differ from those of last year – marked by fewer extreme lifesaving measures, fewer deaths, and shorter hospital stays – caused in part by medical and therapeutic advances and in part to the nature of the Omicron variant itself.
One sign of progress, Dr. Adalja said, will be the widespread decoupling of cases from hospitalizations, something that has already happened in countries such as the United Kingdom.
“That’s a reflection of how well they have vaccinated their high-risk population and how poorly we have vaccinated our high-risk population,” he said.
Omicron will bump up natural immunity
Dr. Adalja said though the numbers of unvaccinated in the United States appear to be stuck, Omicron’s sweep will make the difference, leaving behind more natural immunity in the population.
Currently, hospitals are struggling with staffing concerns as a “direct result” of too many unvaccinated people, he said.
Andrew Badley, MD, an infectious diseases specialist at Mayo Clinic in Rochester, Minn., and director of the clinic’s COVID-19 Task Force, said the good news with Omicron is that nearly all people it infects will recover.
Over time, when the body sees foreign antigens repeatedly, the quantity and quality of the antibodies the immune system produces increase and the body becomes better at fighting disease.
So “a large amount of the population will have recovered and have a degree of immunity,” Dr. Badley said.
His optimism is tempered by his belief that “it’s going to get worse before it gets better.”
But Dr. Badley still predicts a turnaround. “We’ll see a downturn in COVID in late spring or early summer,” and well into the second quarter of 2022, “we’ll see a reemergence of control.”
Right now, with Omicron, one infected person is infecting three to five others, he said. The hope is that it will eventually reach one-to-one endemic levels.
As for the threat of new variants, Badley said, “it’s not predictable whether they will be stronger or weaker.”
Masks may be around for years
Many experts predict that masks will continue to be part of the national wardrobe for the foreseeable future.
“We will continue to see new cases for years and years to come. Some will respond to that with masks in public places for a very long time. I personally will do so,” Dr. Badley said.
Two mindsets: Inside/outside the hospital
Emily Landon, MD, an infectious disease doctor and the executive medical director of infection prevention and control at University of Chicago Medicine, told this news organization she views the pandemic from two different vantage points.
As a health care provider, she sees her hospital, like others worldwide, overwhelmed. Supplies of a major weapon to help prevent hospitalization, the monoclonal antibody sotrovimab, are running out. Dr. Landon said she has been calling other hospitals to see if they have supplies and, if so, whether Omicron patients can transfer there.
Bottom line: The things they relied on a month ago to keep people out of the hospital are no longer there, she said.
Meanwhile, “We have more COVID patients than we have ever had,” Dr. Landon said.
Last year, UChicago hit a high of 170 people hospitalized with COVID. This year, so far, the peak was 270.
Dr. Landon said she is frustrated when she leaves that overburdened world inside the hospital for the outside world, where people wear no masks or ineffective face coverings and gather unsafely. Although some of that behavior reflects an intention to flout the advice of medical experts, some is caused in part, she said, by the lack of a clear national health strategy and garbled communication from those in charge of public safety.
Americans are deciding for themselves, on an a la carte basis, whether to wear a mask or get tested or travel, and school districts decide individually when it’s time to go virtual.
“People are exhausted from having to do a risk-benefit analysis for every single activity they, their friends, their kids want to participate in,” she said.
U.S. behind in several areas
Despite our self-image as the global leader in science and medicine, the United States stumbled badly in its response to the pandemic, with grave consequences both at home and abroad, experts say.
In a recent commentary in JAMA, Lawrence Gostin, JD, from Georgetown University, Washington, and Jennifer Nuzzo, DrPH, at Johns Hopkins University, Baltimore, pointed to several critical shortfalls in the nation’s efforts to control the disease.
One such shortfall is public trust.
This news organization reported in June 2021 that a poll of its readers found that 44% said their trust in the CDC had waned during the pandemic, and 33% said their trust in the FDA had eroded as well.
Health care providers who responded to the poll lost trust as well. About half of the doctors and nurses who responded said they disagreed with the FDA’s decision-making during the pandemic. Nearly 60% of doctors and 65% of nurses said they disagreed with the CDC’s overall pandemic guidance.
Lack of trust can make people resist vaccines and efforts to fight the virus, the authors wrote.
“This will become really relevant when we have ample supply of Pfizer’s antiviral medication,” Mr. Gostin, who directs the O’Neill Institute for National and Global Health Law at Georgetown, told this news organization. “The next phase of the pandemic is not to link testing to contact tracing, because we’re way past that, but to link testing to treatment.”
Lack of regional manufacturing of products is also thwarting global progress.
“It is extraordinarily important that our pharmaceutical industry transfer technology in a pandemic,” Mr. Gostin said. “The most glaring failure to do that is the mRNA vaccines. We’ve got this enormously effective vaccine and the two manufacturers – Pfizer and Moderna – are refusing to share the technology with producers in other countries. That keeps coming back to haunt us.”
Another problem: When the vaccines are shared with other countries, they are being delivered close to the date they expire or arriving at a shipyards without warning, so even some of the doses that get delivered are going to waste, Mr. Gostin said.
“It’s one of the greatest moral failures of my lifetime,” he said.
Also a failure is the “jaw-dropping” state of testing 2 years into the pandemic, he said, as people continue to pay high prices for tests or endure long lines.
The U.S. government updated its calculations and ordered 1 billion tests for the general public. The COVIDtests.gov website to order the free tests is now live.
It’s a step in the right direction. Mr. Gostin and Dr. Nuzzo wrote that there is every reason to expect future epidemics that are as serious or more serious than COVID.
“Failure to address clearly observed weaknesses in the COVID-19 response will have preventable adverse health, social, and economic consequences when the next novel outbreak occurs,” they wrote.

A version of this article first appeared on WebMD.com.
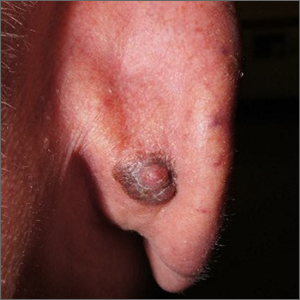
Pioneering test predicts return of malignant melanoma
Their research, published in the British Journal of Dermatology, describes how early-stage melanomas at risk of spreading secrete transforming growth factor beta2 (TGF-beta2), which causes the reduction, or down-regulation, of the proteins AMBRA1 and loricrin, both of which are found in the skin overlaying the tumor. TGF-beta2 also causes the loss of claudin-1, which in turn leads to loss of skin integrity, facilitating ulceration.
Senior author Penny Lovat, PhD, professor of cellular dermatology and oncology at Newcastle University, and chief scientific officer at AMLo Biosciences, explained: “AMBRA1, loricrin, and claudin-1 are all proteins key to maintaining the integrity of the upper layer of the skin,” and that the loss of these proteins causes gaps to develop, allowing the tumor to spread and ulcerate – a process associated with high-risk tumors. Dr. Lovat likened the process to that of “mortar and bricks holding together a wall”, with the loss of these proteins being “like the mortar crumbling away in the wall.”
According to Cancer Research UK, there are over 16,000 new cases of melanoma skin cancer each year in the United Kingdom, with over 2,000 deaths annually. After being surgically removed, primary tumors are histologically staged, with even low-risk cases being followed up for a number of years, a process that can be time-consuming for patients and costly for the NHS.
Some reassurance for those with melanoma
The creators of the new test say that it is these low-risk patients that the test is able to identify, offering a degree of reassurance to those diagnosed with the disease, and potentially reducing the number of hospital clinic visits they require.
Dr. Lovat commented: “Our test offers a personalized prognosis as it more accurately predicts if your skin cancer is unlikely to spread.”
She added that the test will aid clinicians to identify genuinely low-risk patients diagnosed with an early-stage melanoma, reducing the number of follow-up appointments for those identified as low risk. It, therefore, offers the opportunity to save the NHS time and money.
Excellent news for those with skin cancer
Phil Brady, chief operating officer of the British Skin Foundation, echoed Dr. Lovat’s comments, saying: “The test can alleviate stress and anxiety for patients caused by this potentially deadly skin cancer, whilst increasing efficiency and reducing costs to the NHS.”
Nick Levell, MD, consultant dermatologist & British Skin Foundation spokesperson, who has not been involved in the research, commented how the arrival of the test was “excellent news,” adding that “people at low risk can be reassured and will not have to attend hospital so often for check-ups”.
The development of the new test AMBLor has been led by Dr. Lovat, in association with the university spin-out company AMLo Biosciences, and is accredited by the National Accreditation Body for the United Kingdom. The test involves tissue sections from the standard biopsy being sent in the post to the lab for analysis and costs £293 plus VAT. Currently available through a private referral service, the Newcastle team have applied for the test to be made available on the NHS.
A version of this article first appeared on Medscape UK.
Their research, published in the British Journal of Dermatology, describes how early-stage melanomas at risk of spreading secrete transforming growth factor beta2 (TGF-beta2), which causes the reduction, or down-regulation, of the proteins AMBRA1 and loricrin, both of which are found in the skin overlaying the tumor. TGF-beta2 also causes the loss of claudin-1, which in turn leads to loss of skin integrity, facilitating ulceration.
Senior author Penny Lovat, PhD, professor of cellular dermatology and oncology at Newcastle University, and chief scientific officer at AMLo Biosciences, explained: “AMBRA1, loricrin, and claudin-1 are all proteins key to maintaining the integrity of the upper layer of the skin,” and that the loss of these proteins causes gaps to develop, allowing the tumor to spread and ulcerate – a process associated with high-risk tumors. Dr. Lovat likened the process to that of “mortar and bricks holding together a wall”, with the loss of these proteins being “like the mortar crumbling away in the wall.”
According to Cancer Research UK, there are over 16,000 new cases of melanoma skin cancer each year in the United Kingdom, with over 2,000 deaths annually. After being surgically removed, primary tumors are histologically staged, with even low-risk cases being followed up for a number of years, a process that can be time-consuming for patients and costly for the NHS.
Some reassurance for those with melanoma
The creators of the new test say that it is these low-risk patients that the test is able to identify, offering a degree of reassurance to those diagnosed with the disease, and potentially reducing the number of hospital clinic visits they require.
Dr. Lovat commented: “Our test offers a personalized prognosis as it more accurately predicts if your skin cancer is unlikely to spread.”
She added that the test will aid clinicians to identify genuinely low-risk patients diagnosed with an early-stage melanoma, reducing the number of follow-up appointments for those identified as low risk. It, therefore, offers the opportunity to save the NHS time and money.
Excellent news for those with skin cancer
Phil Brady, chief operating officer of the British Skin Foundation, echoed Dr. Lovat’s comments, saying: “The test can alleviate stress and anxiety for patients caused by this potentially deadly skin cancer, whilst increasing efficiency and reducing costs to the NHS.”
Nick Levell, MD, consultant dermatologist & British Skin Foundation spokesperson, who has not been involved in the research, commented how the arrival of the test was “excellent news,” adding that “people at low risk can be reassured and will not have to attend hospital so often for check-ups”.
The development of the new test AMBLor has been led by Dr. Lovat, in association with the university spin-out company AMLo Biosciences, and is accredited by the National Accreditation Body for the United Kingdom. The test involves tissue sections from the standard biopsy being sent in the post to the lab for analysis and costs £293 plus VAT. Currently available through a private referral service, the Newcastle team have applied for the test to be made available on the NHS.
A version of this article first appeared on Medscape UK.
Their research, published in the British Journal of Dermatology, describes how early-stage melanomas at risk of spreading secrete transforming growth factor beta2 (TGF-beta2), which causes the reduction, or down-regulation, of the proteins AMBRA1 and loricrin, both of which are found in the skin overlaying the tumor. TGF-beta2 also causes the loss of claudin-1, which in turn leads to loss of skin integrity, facilitating ulceration.
Senior author Penny Lovat, PhD, professor of cellular dermatology and oncology at Newcastle University, and chief scientific officer at AMLo Biosciences, explained: “AMBRA1, loricrin, and claudin-1 are all proteins key to maintaining the integrity of the upper layer of the skin,” and that the loss of these proteins causes gaps to develop, allowing the tumor to spread and ulcerate – a process associated with high-risk tumors. Dr. Lovat likened the process to that of “mortar and bricks holding together a wall”, with the loss of these proteins being “like the mortar crumbling away in the wall.”
According to Cancer Research UK, there are over 16,000 new cases of melanoma skin cancer each year in the United Kingdom, with over 2,000 deaths annually. After being surgically removed, primary tumors are histologically staged, with even low-risk cases being followed up for a number of years, a process that can be time-consuming for patients and costly for the NHS.
Some reassurance for those with melanoma
The creators of the new test say that it is these low-risk patients that the test is able to identify, offering a degree of reassurance to those diagnosed with the disease, and potentially reducing the number of hospital clinic visits they require.
Dr. Lovat commented: “Our test offers a personalized prognosis as it more accurately predicts if your skin cancer is unlikely to spread.”
She added that the test will aid clinicians to identify genuinely low-risk patients diagnosed with an early-stage melanoma, reducing the number of follow-up appointments for those identified as low risk. It, therefore, offers the opportunity to save the NHS time and money.
Excellent news for those with skin cancer
Phil Brady, chief operating officer of the British Skin Foundation, echoed Dr. Lovat’s comments, saying: “The test can alleviate stress and anxiety for patients caused by this potentially deadly skin cancer, whilst increasing efficiency and reducing costs to the NHS.”
Nick Levell, MD, consultant dermatologist & British Skin Foundation spokesperson, who has not been involved in the research, commented how the arrival of the test was “excellent news,” adding that “people at low risk can be reassured and will not have to attend hospital so often for check-ups”.
The development of the new test AMBLor has been led by Dr. Lovat, in association with the university spin-out company AMLo Biosciences, and is accredited by the National Accreditation Body for the United Kingdom. The test involves tissue sections from the standard biopsy being sent in the post to the lab for analysis and costs £293 plus VAT. Currently available through a private referral service, the Newcastle team have applied for the test to be made available on the NHS.
A version of this article first appeared on Medscape UK.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Detransition, baby: Examining factors leading to ‘detransitioning’ and regret in the transgender community
Over the holiday season I had the pleasure of finally reading the national bestseller, Detransition, Baby. On the surface, the story depicts the complex relationships between Reese, a transgender woman who strongly desires a family, her ex-wife, Ames – a transgender woman who detransitioned to live as a cisgender man – and Ames’ cisgender female partner, who is unexpectedly pregnant with his child. The story delves much deeper than the relationships between these characters, as it exceptionally articulates many of the emotional intricacies of the transgender experience and addresses one of the most taboo topics in the transgender community – detransitioning and regret.
The terms “transition” and “detransition” have fallen out of favor in the vernacular of the transgender population as they incorrectly imply that gender identity is contingent upon gender-affirmation processes.1,2 More importantly, the terms “detransition” and regret are not synonymous. Conflating these terms has undermined the intrinsic nature of gender identity, which has resulted in political and legal consequences seeking to limit or outright ban care for transgender patients.
As a gender-affirming surgeon, one of the most common questions I get asked is the rate of regret patients have after their surgeries. While I have no issue answering the question when it is presented, I do not hesitate to point out some of the problematic subtext inherent in such inquiries. Within the line of questioning, many often comment, “It’s so permanent,” “I can’t believe people can do this to their bodies,” or “How sure are patients before undergoing these surgeries?” While these comments and queries can be downright offensive, they seem to stem from the difficulty people have comprehending gender dysphoria and the painstaking steps people take to affirm their identity. The implication of these comments also reveals a more deep-seated issue – general distrust of individual bodily autonomy, personal identity, and choice.
For the obstetrician-gynecologist, understanding the concept of autonomous, patient-centered decision-making should be second nature, as we face a similar line of interrogation when discussing abortion, contraception, and pregnancy. No other field faces this level of scrutiny when it comes to defending a patient’s bodily autonomy. For example, given the history of reproductive injustice with tubal ligation procedures, the American College of Obstetricians and Gynecologists has issued clear guidelines regarding counseling of women while acknowledging the tenuous history of these procedures with minority subgroups. According to their committee opinion, ethical counseling for such a permanent procedure involves understanding the content of information presented to the patient, how that information is conveyed, and self-reflection on the part of the provider.3 The approach to counseling and understanding gender-affirming care is no different.
I want to be clear that regret after gender-affirming surgery is rare, occurring in 0%-3.8% of patients.4-6 In a separate study, 91% of patients expressed significant improvement in quality of life after surgery.7 However, what is disheartening about patients who experience surgical regret is that it originates from continued difficulty from the transition process itself and ongoing discrimination – even though the patient’s physical characteristics match their gender identity.4-6 Similarly, in another survey which examined 17,151 participants who had pursued gender affirmation (broadly defined), approximately 2,242 (13.1%) reported a history of detransition.2 Among these adults, the vast majority (82.5%), cited external factors such as school harassment, sexual violence, family pressure, and social stigma as reasons for detransitioning.2 Other associated factors included male sex assigned at birth, nonbinary gender identity, bisexual orientation, and having an unsupportive family.2
When Ames is explaining his “detransition” to his cisfemale partner, he states: “I got sick of living as trans …[sic]… I am trans, but I don’t need to do trans.”8 While there is still more research needed to further understand detransitioning and surgical regret, these few studies demonstrate a heart-breaking reality – in many aspects of our society it is still extremely difficult to live as a transgender person.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa. She did not report any disclosures.
References
1. National LGBTQIA+ Health Education Center, A program of the Fenway Institute: LGBTQIA+ glossary of terms for health care teams. 2020. Available at www.lgbtqiahealtheducation.org/wp-content/uploads/2020/10/Glossary-2020.08.30.pdf. Accessed Dec. 30, 2021.
2. Turban JL et al. LGBT Health 2021;8(4):273-80.
3. Sterilization of women: Ethical issues and considerations. Committee Opinion No. 695. American College of Obstetricians and Gynecologists. Obstet Gynecol 2017;129:e109-16.
4. Ruppin U, Pfafflin F. Arch Sex Behav. 2015;44:1321-9.
5. Lawrence AA. Arch Sex Behav. 2003;32:299-315.
6. Landen M et al. Acta Psychiatr Scand. 1998;97:284-9.
7. Papdopulos NA et al. J Sex Med. 2017;14(5):721-30.
8. Peters T. Detransition, Baby. New York: Penguin Random House, 2021.
Over the holiday season I had the pleasure of finally reading the national bestseller, Detransition, Baby. On the surface, the story depicts the complex relationships between Reese, a transgender woman who strongly desires a family, her ex-wife, Ames – a transgender woman who detransitioned to live as a cisgender man – and Ames’ cisgender female partner, who is unexpectedly pregnant with his child. The story delves much deeper than the relationships between these characters, as it exceptionally articulates many of the emotional intricacies of the transgender experience and addresses one of the most taboo topics in the transgender community – detransitioning and regret.
The terms “transition” and “detransition” have fallen out of favor in the vernacular of the transgender population as they incorrectly imply that gender identity is contingent upon gender-affirmation processes.1,2 More importantly, the terms “detransition” and regret are not synonymous. Conflating these terms has undermined the intrinsic nature of gender identity, which has resulted in political and legal consequences seeking to limit or outright ban care for transgender patients.
As a gender-affirming surgeon, one of the most common questions I get asked is the rate of regret patients have after their surgeries. While I have no issue answering the question when it is presented, I do not hesitate to point out some of the problematic subtext inherent in such inquiries. Within the line of questioning, many often comment, “It’s so permanent,” “I can’t believe people can do this to their bodies,” or “How sure are patients before undergoing these surgeries?” While these comments and queries can be downright offensive, they seem to stem from the difficulty people have comprehending gender dysphoria and the painstaking steps people take to affirm their identity. The implication of these comments also reveals a more deep-seated issue – general distrust of individual bodily autonomy, personal identity, and choice.
For the obstetrician-gynecologist, understanding the concept of autonomous, patient-centered decision-making should be second nature, as we face a similar line of interrogation when discussing abortion, contraception, and pregnancy. No other field faces this level of scrutiny when it comes to defending a patient’s bodily autonomy. For example, given the history of reproductive injustice with tubal ligation procedures, the American College of Obstetricians and Gynecologists has issued clear guidelines regarding counseling of women while acknowledging the tenuous history of these procedures with minority subgroups. According to their committee opinion, ethical counseling for such a permanent procedure involves understanding the content of information presented to the patient, how that information is conveyed, and self-reflection on the part of the provider.3 The approach to counseling and understanding gender-affirming care is no different.
I want to be clear that regret after gender-affirming surgery is rare, occurring in 0%-3.8% of patients.4-6 In a separate study, 91% of patients expressed significant improvement in quality of life after surgery.7 However, what is disheartening about patients who experience surgical regret is that it originates from continued difficulty from the transition process itself and ongoing discrimination – even though the patient’s physical characteristics match their gender identity.4-6 Similarly, in another survey which examined 17,151 participants who had pursued gender affirmation (broadly defined), approximately 2,242 (13.1%) reported a history of detransition.2 Among these adults, the vast majority (82.5%), cited external factors such as school harassment, sexual violence, family pressure, and social stigma as reasons for detransitioning.2 Other associated factors included male sex assigned at birth, nonbinary gender identity, bisexual orientation, and having an unsupportive family.2
When Ames is explaining his “detransition” to his cisfemale partner, he states: “I got sick of living as trans …[sic]… I am trans, but I don’t need to do trans.”8 While there is still more research needed to further understand detransitioning and surgical regret, these few studies demonstrate a heart-breaking reality – in many aspects of our society it is still extremely difficult to live as a transgender person.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa. She did not report any disclosures.
References
1. National LGBTQIA+ Health Education Center, A program of the Fenway Institute: LGBTQIA+ glossary of terms for health care teams. 2020. Available at www.lgbtqiahealtheducation.org/wp-content/uploads/2020/10/Glossary-2020.08.30.pdf. Accessed Dec. 30, 2021.
2. Turban JL et al. LGBT Health 2021;8(4):273-80.
3. Sterilization of women: Ethical issues and considerations. Committee Opinion No. 695. American College of Obstetricians and Gynecologists. Obstet Gynecol 2017;129:e109-16.
4. Ruppin U, Pfafflin F. Arch Sex Behav. 2015;44:1321-9.
5. Lawrence AA. Arch Sex Behav. 2003;32:299-315.
6. Landen M et al. Acta Psychiatr Scand. 1998;97:284-9.
7. Papdopulos NA et al. J Sex Med. 2017;14(5):721-30.
8. Peters T. Detransition, Baby. New York: Penguin Random House, 2021.
Over the holiday season I had the pleasure of finally reading the national bestseller, Detransition, Baby. On the surface, the story depicts the complex relationships between Reese, a transgender woman who strongly desires a family, her ex-wife, Ames – a transgender woman who detransitioned to live as a cisgender man – and Ames’ cisgender female partner, who is unexpectedly pregnant with his child. The story delves much deeper than the relationships between these characters, as it exceptionally articulates many of the emotional intricacies of the transgender experience and addresses one of the most taboo topics in the transgender community – detransitioning and regret.
The terms “transition” and “detransition” have fallen out of favor in the vernacular of the transgender population as they incorrectly imply that gender identity is contingent upon gender-affirmation processes.1,2 More importantly, the terms “detransition” and regret are not synonymous. Conflating these terms has undermined the intrinsic nature of gender identity, which has resulted in political and legal consequences seeking to limit or outright ban care for transgender patients.
As a gender-affirming surgeon, one of the most common questions I get asked is the rate of regret patients have after their surgeries. While I have no issue answering the question when it is presented, I do not hesitate to point out some of the problematic subtext inherent in such inquiries. Within the line of questioning, many often comment, “It’s so permanent,” “I can’t believe people can do this to their bodies,” or “How sure are patients before undergoing these surgeries?” While these comments and queries can be downright offensive, they seem to stem from the difficulty people have comprehending gender dysphoria and the painstaking steps people take to affirm their identity. The implication of these comments also reveals a more deep-seated issue – general distrust of individual bodily autonomy, personal identity, and choice.
For the obstetrician-gynecologist, understanding the concept of autonomous, patient-centered decision-making should be second nature, as we face a similar line of interrogation when discussing abortion, contraception, and pregnancy. No other field faces this level of scrutiny when it comes to defending a patient’s bodily autonomy. For example, given the history of reproductive injustice with tubal ligation procedures, the American College of Obstetricians and Gynecologists has issued clear guidelines regarding counseling of women while acknowledging the tenuous history of these procedures with minority subgroups. According to their committee opinion, ethical counseling for such a permanent procedure involves understanding the content of information presented to the patient, how that information is conveyed, and self-reflection on the part of the provider.3 The approach to counseling and understanding gender-affirming care is no different.
I want to be clear that regret after gender-affirming surgery is rare, occurring in 0%-3.8% of patients.4-6 In a separate study, 91% of patients expressed significant improvement in quality of life after surgery.7 However, what is disheartening about patients who experience surgical regret is that it originates from continued difficulty from the transition process itself and ongoing discrimination – even though the patient’s physical characteristics match their gender identity.4-6 Similarly, in another survey which examined 17,151 participants who had pursued gender affirmation (broadly defined), approximately 2,242 (13.1%) reported a history of detransition.2 Among these adults, the vast majority (82.5%), cited external factors such as school harassment, sexual violence, family pressure, and social stigma as reasons for detransitioning.2 Other associated factors included male sex assigned at birth, nonbinary gender identity, bisexual orientation, and having an unsupportive family.2
When Ames is explaining his “detransition” to his cisfemale partner, he states: “I got sick of living as trans …[sic]… I am trans, but I don’t need to do trans.”8 While there is still more research needed to further understand detransitioning and surgical regret, these few studies demonstrate a heart-breaking reality – in many aspects of our society it is still extremely difficult to live as a transgender person.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa. She did not report any disclosures.
References
1. National LGBTQIA+ Health Education Center, A program of the Fenway Institute: LGBTQIA+ glossary of terms for health care teams. 2020. Available at www.lgbtqiahealtheducation.org/wp-content/uploads/2020/10/Glossary-2020.08.30.pdf. Accessed Dec. 30, 2021.
2. Turban JL et al. LGBT Health 2021;8(4):273-80.
3. Sterilization of women: Ethical issues and considerations. Committee Opinion No. 695. American College of Obstetricians and Gynecologists. Obstet Gynecol 2017;129:e109-16.
4. Ruppin U, Pfafflin F. Arch Sex Behav. 2015;44:1321-9.
5. Lawrence AA. Arch Sex Behav. 2003;32:299-315.
6. Landen M et al. Acta Psychiatr Scand. 1998;97:284-9.
7. Papdopulos NA et al. J Sex Med. 2017;14(5):721-30.
8. Peters T. Detransition, Baby. New York: Penguin Random House, 2021.
Antibiotic choices for inpatients with SSTIs vary by race
– in a national cross-sectional study involving over 1,000 patients in 91 hospitals.
The potential racial disparity in management of SSTI was detected after data were adjusted for penicillin allergy history and for MRSA colonization/infection. The data were also adjusted for hospital day (since admission) in order to control for the administration of more empiric therapy early on.
Clindamycin, a beta-lactam alternative, is not recommended as an SSTI treatment given its frequent dosing requirements and high potential for adverse events including Clostridioides difficile infection (DCI). “Clindamycin is an option but it’s considered inferior. ... It covers MRSA but it shouldn’t be a go-to for skin and soft-tissue infections,” said senior author Kimberly Blumenthal, MD, MSc, assistant professor of medicine at Harvard University, and an allergist, immunologist, and drug allergy and epidemiology researcher at Massachusetts General Hospital, both in Boston.
Cefazolin, on the other hand, does not cover MRSA but is “a guideline-recommended first-line antibiotic for cellulitis SSTI in the hospital,” she said in an interview.
The findings, recently published in JAMA Network Open, offer a valuable portrait of the antibiotics being prescribed in the inpatient setting for SSTIs. Vancomycin, which typically is reserved for MRSA, was the most commonly prescribed antibiotic, regardless of race. Piperacillin-tazobactam, a beta-lactam, was the second most commonly prescribed antibiotic, again regardless of race.
Intravenously administered cefazolin was used in 13% of White inpatients versus 5% of Black inpatients. After controlling for kidney disease, diabetes, and ICU location (in addition to hospital day, penicillin allergy history, and MRSA), White inpatients had an increased likelihood of being prescribed cefazolin (adjusted odds ratio, 2.82; 95% confidence interval, 1.41-5.63) and a decreased likelihood of clindamycin use (aOR, 0.54; 95% CI, 0.30-0.96), compared with Black inpatients.
The investigators utilized the Acute Care Hospital Groups network within Vizient, a member-driven health care performance improvement company, to collect data for the study. Most of the hospitals (91%) that submitted data on adult inpatients with cellulitis or SSTIs (without other infections) were in urban settings and 9% were in rural settings; 60% were community hospitals and 40% were academic medical centers. The researchers accounted for “clustering by hospital” – such as the use of internal guidelines – in their methodology.
Differential management and prescribing practices associated with race and ethnicity have been demonstrated for cardiovascular disease and other chronic problems, but “to see such racial differences play out in acute care is striking,” Utibe R. Essien, MD, MPH, assistant professor of medicine at the University of Pittsburgh and a core investigator with the Center for Health Equity Research and Promotion at the Veterans Affairs Pittsburgh Healthcare System, said in an interview.
“In acute care, we tend to practice pretty similarly across the board ... so the findings give me pause,” said Dr. Essien, an internist and a coauthor of the study, who also works with the University of Pittsburgh’s Center for Pharmaceutical Policy and Prescribing.
Also notable was the prevalence of historical penicillin allergy documented in the dataset: 23% in Black inpatients and 18% in White inpatients with SSTI. It’s a surprisingly high prevalence overall, Dr. Blumenthal said, and the racial difference was surprising because penicillin allergy has been commonly described in the literature as being more common in the White population.
Even though penicillin allergy was controlled for in the study, “given that historical penicillin allergies are associated with increased clindamycin use and risk of CDI, but are often disproved with formal testing, racial disparities in penicillin allergy documentation and assessment require additional study,” she and her coauthors wrote.
Ideally, Dr. Blumenthal said, all inpatients would have access to allergy consultations or testing or some sort of infrastructure for assessing a history of penicillin allergy. At Mass General, allergy consults and challenge doses of beta-lactams (also called graded challenges) are frequently employed.
The study did not collect data on income, educational level, and other structural vulnerability factors. More research is needed to better understand “what’s going on in acute care settings and what the potential drivers of disparities may be,” said Dr. Essien, who co-authored a recent JAMA editorial on “achieving pharmacoequity” to reduce health disparities.
“If guidelines suggest that medication A is the ideal and optimal treatment, we really have to do our best to ensure that every patient, regardless of race or ethnicity, can get that treatment,” he said.
In the study, race was extracted from the medical record and may not have been correctly assigned, the authors noted. “Other race” was not specified in the dataset, and Hispanic ethnicity was not captured. The number of individuals identified as Asian and other races was small, prompting the researchers to focus on antibiotic use in Black and White patients (224 and 854 patients, respectively).
Dr. Blumenthal and Dr. Essien both reported that they had no relevant disclosures. The study was supported with National Institutes of Health grants and the Massachusetts General Hospital department of medicine transformative scholar program.
– in a national cross-sectional study involving over 1,000 patients in 91 hospitals.
The potential racial disparity in management of SSTI was detected after data were adjusted for penicillin allergy history and for MRSA colonization/infection. The data were also adjusted for hospital day (since admission) in order to control for the administration of more empiric therapy early on.
Clindamycin, a beta-lactam alternative, is not recommended as an SSTI treatment given its frequent dosing requirements and high potential for adverse events including Clostridioides difficile infection (DCI). “Clindamycin is an option but it’s considered inferior. ... It covers MRSA but it shouldn’t be a go-to for skin and soft-tissue infections,” said senior author Kimberly Blumenthal, MD, MSc, assistant professor of medicine at Harvard University, and an allergist, immunologist, and drug allergy and epidemiology researcher at Massachusetts General Hospital, both in Boston.
Cefazolin, on the other hand, does not cover MRSA but is “a guideline-recommended first-line antibiotic for cellulitis SSTI in the hospital,” she said in an interview.
The findings, recently published in JAMA Network Open, offer a valuable portrait of the antibiotics being prescribed in the inpatient setting for SSTIs. Vancomycin, which typically is reserved for MRSA, was the most commonly prescribed antibiotic, regardless of race. Piperacillin-tazobactam, a beta-lactam, was the second most commonly prescribed antibiotic, again regardless of race.
Intravenously administered cefazolin was used in 13% of White inpatients versus 5% of Black inpatients. After controlling for kidney disease, diabetes, and ICU location (in addition to hospital day, penicillin allergy history, and MRSA), White inpatients had an increased likelihood of being prescribed cefazolin (adjusted odds ratio, 2.82; 95% confidence interval, 1.41-5.63) and a decreased likelihood of clindamycin use (aOR, 0.54; 95% CI, 0.30-0.96), compared with Black inpatients.
The investigators utilized the Acute Care Hospital Groups network within Vizient, a member-driven health care performance improvement company, to collect data for the study. Most of the hospitals (91%) that submitted data on adult inpatients with cellulitis or SSTIs (without other infections) were in urban settings and 9% were in rural settings; 60% were community hospitals and 40% were academic medical centers. The researchers accounted for “clustering by hospital” – such as the use of internal guidelines – in their methodology.
Differential management and prescribing practices associated with race and ethnicity have been demonstrated for cardiovascular disease and other chronic problems, but “to see such racial differences play out in acute care is striking,” Utibe R. Essien, MD, MPH, assistant professor of medicine at the University of Pittsburgh and a core investigator with the Center for Health Equity Research and Promotion at the Veterans Affairs Pittsburgh Healthcare System, said in an interview.
“In acute care, we tend to practice pretty similarly across the board ... so the findings give me pause,” said Dr. Essien, an internist and a coauthor of the study, who also works with the University of Pittsburgh’s Center for Pharmaceutical Policy and Prescribing.
Also notable was the prevalence of historical penicillin allergy documented in the dataset: 23% in Black inpatients and 18% in White inpatients with SSTI. It’s a surprisingly high prevalence overall, Dr. Blumenthal said, and the racial difference was surprising because penicillin allergy has been commonly described in the literature as being more common in the White population.
Even though penicillin allergy was controlled for in the study, “given that historical penicillin allergies are associated with increased clindamycin use and risk of CDI, but are often disproved with formal testing, racial disparities in penicillin allergy documentation and assessment require additional study,” she and her coauthors wrote.
Ideally, Dr. Blumenthal said, all inpatients would have access to allergy consultations or testing or some sort of infrastructure for assessing a history of penicillin allergy. At Mass General, allergy consults and challenge doses of beta-lactams (also called graded challenges) are frequently employed.
The study did not collect data on income, educational level, and other structural vulnerability factors. More research is needed to better understand “what’s going on in acute care settings and what the potential drivers of disparities may be,” said Dr. Essien, who co-authored a recent JAMA editorial on “achieving pharmacoequity” to reduce health disparities.
“If guidelines suggest that medication A is the ideal and optimal treatment, we really have to do our best to ensure that every patient, regardless of race or ethnicity, can get that treatment,” he said.
In the study, race was extracted from the medical record and may not have been correctly assigned, the authors noted. “Other race” was not specified in the dataset, and Hispanic ethnicity was not captured. The number of individuals identified as Asian and other races was small, prompting the researchers to focus on antibiotic use in Black and White patients (224 and 854 patients, respectively).
Dr. Blumenthal and Dr. Essien both reported that they had no relevant disclosures. The study was supported with National Institutes of Health grants and the Massachusetts General Hospital department of medicine transformative scholar program.
– in a national cross-sectional study involving over 1,000 patients in 91 hospitals.
The potential racial disparity in management of SSTI was detected after data were adjusted for penicillin allergy history and for MRSA colonization/infection. The data were also adjusted for hospital day (since admission) in order to control for the administration of more empiric therapy early on.
Clindamycin, a beta-lactam alternative, is not recommended as an SSTI treatment given its frequent dosing requirements and high potential for adverse events including Clostridioides difficile infection (DCI). “Clindamycin is an option but it’s considered inferior. ... It covers MRSA but it shouldn’t be a go-to for skin and soft-tissue infections,” said senior author Kimberly Blumenthal, MD, MSc, assistant professor of medicine at Harvard University, and an allergist, immunologist, and drug allergy and epidemiology researcher at Massachusetts General Hospital, both in Boston.
Cefazolin, on the other hand, does not cover MRSA but is “a guideline-recommended first-line antibiotic for cellulitis SSTI in the hospital,” she said in an interview.
The findings, recently published in JAMA Network Open, offer a valuable portrait of the antibiotics being prescribed in the inpatient setting for SSTIs. Vancomycin, which typically is reserved for MRSA, was the most commonly prescribed antibiotic, regardless of race. Piperacillin-tazobactam, a beta-lactam, was the second most commonly prescribed antibiotic, again regardless of race.
Intravenously administered cefazolin was used in 13% of White inpatients versus 5% of Black inpatients. After controlling for kidney disease, diabetes, and ICU location (in addition to hospital day, penicillin allergy history, and MRSA), White inpatients had an increased likelihood of being prescribed cefazolin (adjusted odds ratio, 2.82; 95% confidence interval, 1.41-5.63) and a decreased likelihood of clindamycin use (aOR, 0.54; 95% CI, 0.30-0.96), compared with Black inpatients.
The investigators utilized the Acute Care Hospital Groups network within Vizient, a member-driven health care performance improvement company, to collect data for the study. Most of the hospitals (91%) that submitted data on adult inpatients with cellulitis or SSTIs (without other infections) were in urban settings and 9% were in rural settings; 60% were community hospitals and 40% were academic medical centers. The researchers accounted for “clustering by hospital” – such as the use of internal guidelines – in their methodology.
Differential management and prescribing practices associated with race and ethnicity have been demonstrated for cardiovascular disease and other chronic problems, but “to see such racial differences play out in acute care is striking,” Utibe R. Essien, MD, MPH, assistant professor of medicine at the University of Pittsburgh and a core investigator with the Center for Health Equity Research and Promotion at the Veterans Affairs Pittsburgh Healthcare System, said in an interview.
“In acute care, we tend to practice pretty similarly across the board ... so the findings give me pause,” said Dr. Essien, an internist and a coauthor of the study, who also works with the University of Pittsburgh’s Center for Pharmaceutical Policy and Prescribing.
Also notable was the prevalence of historical penicillin allergy documented in the dataset: 23% in Black inpatients and 18% in White inpatients with SSTI. It’s a surprisingly high prevalence overall, Dr. Blumenthal said, and the racial difference was surprising because penicillin allergy has been commonly described in the literature as being more common in the White population.
Even though penicillin allergy was controlled for in the study, “given that historical penicillin allergies are associated with increased clindamycin use and risk of CDI, but are often disproved with formal testing, racial disparities in penicillin allergy documentation and assessment require additional study,” she and her coauthors wrote.
Ideally, Dr. Blumenthal said, all inpatients would have access to allergy consultations or testing or some sort of infrastructure for assessing a history of penicillin allergy. At Mass General, allergy consults and challenge doses of beta-lactams (also called graded challenges) are frequently employed.
The study did not collect data on income, educational level, and other structural vulnerability factors. More research is needed to better understand “what’s going on in acute care settings and what the potential drivers of disparities may be,” said Dr. Essien, who co-authored a recent JAMA editorial on “achieving pharmacoequity” to reduce health disparities.
“If guidelines suggest that medication A is the ideal and optimal treatment, we really have to do our best to ensure that every patient, regardless of race or ethnicity, can get that treatment,” he said.
In the study, race was extracted from the medical record and may not have been correctly assigned, the authors noted. “Other race” was not specified in the dataset, and Hispanic ethnicity was not captured. The number of individuals identified as Asian and other races was small, prompting the researchers to focus on antibiotic use in Black and White patients (224 and 854 patients, respectively).
Dr. Blumenthal and Dr. Essien both reported that they had no relevant disclosures. The study was supported with National Institutes of Health grants and the Massachusetts General Hospital department of medicine transformative scholar program.
FROM JAMA NETWORK OPEN
Make America beautiful: Support mask mandates
In space, no one can hear your red blood cells scream
There are many reasons why space is the final frontier, not least of which are the major health issues space travel places on the human body. So until a shady billionaire finds an alien protomolecule on a Saturnian moon and starts splicing it with human DNA so we can hang out in space all day without a spacesuit, we’re stuck with things like space anemia, a condition many astronauts develop after extended time in space.
Space anemia has been known for many years, but it was assumed that it developed as a reaction to microgravity and was a short-term phenomenon only – a temporary compensation as fluids and blood volume adjusted themselves. But as new research shows, that assumption seems to be wrong.
For the study, published in Nature Medicine, 13 astronauts who were in space for at least 120 days – long enough for all their red blood cells to have been produced in space – had their blood tested consistently. Before their flights, the astronauts created and destroyed 2 million red blood cells per second, but while they were in space, they destroyed 3 million cells per second. Notably, this process continued for the entire duration of the space flight. So, not a temporary reaction.
Consequently, 5 of the 13 astronauts developed anemia when they returned to Earth. (Interesting space fact: Having fewer blood cells isn’t a problem while you’re in space; the effects of anemia only manifest when the body returns to full gravity.) The anemia disappeared after a few months, but the astronauts were still destroying 30% more red blood cells a year after their spaceflight than they were before leaving Earth.
You may be thinking: Well, if they were destroying 50% more red blood cells while in space, how come they didn’t all develop severe anemia? The researchers theorized that production was boosted as well, which sounds like a good thing. The body is compensating, as it should. Unfortunately, that increased production stresses bone marrow function and the demand for energy spikes. That’s not such a good thing. And of course, many of the astronauts got anemia anyway.
To tackle the issue, the researchers emphasized the importance of feeding astronauts a proper diet, plus potential supplements before spaceflight. So don’t worry, Captain Kirk will be able to arm wrestle Klingons and romance suspiciously human-looking aliens without fear of keeling over from anemia-induced fatigue. Earth will stay safe.
Tell me with your eyes
Communication can be hard, even under the best of circumstances, but for many nonverbal patients in the intensive care unit who can’t move, getting a point across to the health care team can be a huge struggle in itself.
Health care professionals have been making do with eye-blinking or head-nodding, but what if that’s just not enough? New research shows that it’s not, and there’s a more effective way for patients to say what they mean just by looking.
In a study published in the Journal of Trauma and Acute Care Surgery, researchers looked into using eye-tracking systems for nonverbal ICU patients to communicate. Eye-tracking isn’t anything new, but using it as a form of communication among nonverbal patients with critical illness hasn’t been looked at before.
How does it work? The eye-tracking system is set up in the patient’s line of sight and its various algorithms and software collect data to calculate where exactly the patient is looking. Established scores and scales assess the patient’s mood, quality of life, pain, and self-esteem.
The researchers found that participating patients were actually experiencing more negative moods, pain, and feelings of frustration than was once believed. Making this tool even more valuable for treatment adjustment and meeting patients’ needs.
In this case, it means that health care providers are getting an eyeful … of communication.
Make America grave again
Here we go again. Somebody just found something else that the United States is not the best at. To go along with math and science education, infrastructure investment, quality of life …
That’s going to go on for a while, so let’s get to the new stuff. An international group of researchers surveyed end-of-life care in 81 countries and ranked them based on the assessment of 181 experts in those countries. They looked at 13 different factors, including proper management of pain and comfort, having a clean and safe space, being treated kindly, lack of cost barriers to appropriate care, and treatments that address quality of life and don’t just extend life.
… press freedom, industrial production, racial equality, Internet connectivity …
Their report card, published in the Journal of Pain and Symptom Management, gave six countries an A, with Great Britain at the top. The other five were Ireland, Taiwan, Australia, South Korea, and Costa Rica. The lowest grade went to Paraguay in 81st place, with Lebanon, Brazil, Senegal, and Haiti just ahead.
… environmental stewardship, body-mass index, social mobility, COVID safeness …
The United States, getting a firm grasp on mediocrity, ranked 43rd. Here are some countries that did better: North Macedonia (7th), Sri Lanka (16th), Uganda (31st), and Uruguay 33rd). In the United States, “we spend so much money trying to get people to live longer, but we don’t spend enough money in helping people die better,” lead author Eric A. Finkelstein, PhD, said in a written statement.
… economic stability, and soccer; we’re also not the best at dying. Wait, did we already say that?
The face mask that launched a thousand ships
Face masks, clearly, have been a source of social strife during the pandemic. People may not agree on mandates, but a mask can be a pretty-low-maintenance face shield if you don’t feel like putting on make-up or want to cover up some blemishes.
Before the pandemic, people thought that those wearing face masks were less attractive because the masks represented illness or disease, according to Dr. Michael Lewis of Cardiff (Wales) University. Back then, no one really wore masks besides doctors and nurses, so if you saw someone wearing one on the street, you probably wondered what they were trying to hide.
Now, though, the subject of face mask attractiveness has been revisited by Dr. Lewis and his associate, Oliver Hies, who found that face masks now make people more attractive.
“Our study suggests faces are considered most attractive when covered by medical face masks. … At a time when we feel vulnerable, we may find the wearing of medical masks reassuring and so feel more positive towards the wearer,” Dr. Lewis told the Guardian.
He suggested that we’re no longer looking at people wearing a mask as disease riddled, but rather doing their part to protect society. Or maybe we focus more on someone’s eyes when that’s all there is to look at. Or, maybe we wind up making up what the rest of someone’s face looks like to meet our attractiveness criteria.
However you feel about masks, they’re cheaper than plastic surgery. And you can go out wearing a new face every day.
In space, no one can hear your red blood cells scream
There are many reasons why space is the final frontier, not least of which are the major health issues space travel places on the human body. So until a shady billionaire finds an alien protomolecule on a Saturnian moon and starts splicing it with human DNA so we can hang out in space all day without a spacesuit, we’re stuck with things like space anemia, a condition many astronauts develop after extended time in space.
Space anemia has been known for many years, but it was assumed that it developed as a reaction to microgravity and was a short-term phenomenon only – a temporary compensation as fluids and blood volume adjusted themselves. But as new research shows, that assumption seems to be wrong.
For the study, published in Nature Medicine, 13 astronauts who were in space for at least 120 days – long enough for all their red blood cells to have been produced in space – had their blood tested consistently. Before their flights, the astronauts created and destroyed 2 million red blood cells per second, but while they were in space, they destroyed 3 million cells per second. Notably, this process continued for the entire duration of the space flight. So, not a temporary reaction.
Consequently, 5 of the 13 astronauts developed anemia when they returned to Earth. (Interesting space fact: Having fewer blood cells isn’t a problem while you’re in space; the effects of anemia only manifest when the body returns to full gravity.) The anemia disappeared after a few months, but the astronauts were still destroying 30% more red blood cells a year after their spaceflight than they were before leaving Earth.
You may be thinking: Well, if they were destroying 50% more red blood cells while in space, how come they didn’t all develop severe anemia? The researchers theorized that production was boosted as well, which sounds like a good thing. The body is compensating, as it should. Unfortunately, that increased production stresses bone marrow function and the demand for energy spikes. That’s not such a good thing. And of course, many of the astronauts got anemia anyway.
To tackle the issue, the researchers emphasized the importance of feeding astronauts a proper diet, plus potential supplements before spaceflight. So don’t worry, Captain Kirk will be able to arm wrestle Klingons and romance suspiciously human-looking aliens without fear of keeling over from anemia-induced fatigue. Earth will stay safe.
Tell me with your eyes
Communication can be hard, even under the best of circumstances, but for many nonverbal patients in the intensive care unit who can’t move, getting a point across to the health care team can be a huge struggle in itself.
Health care professionals have been making do with eye-blinking or head-nodding, but what if that’s just not enough? New research shows that it’s not, and there’s a more effective way for patients to say what they mean just by looking.
In a study published in the Journal of Trauma and Acute Care Surgery, researchers looked into using eye-tracking systems for nonverbal ICU patients to communicate. Eye-tracking isn’t anything new, but using it as a form of communication among nonverbal patients with critical illness hasn’t been looked at before.
How does it work? The eye-tracking system is set up in the patient’s line of sight and its various algorithms and software collect data to calculate where exactly the patient is looking. Established scores and scales assess the patient’s mood, quality of life, pain, and self-esteem.
The researchers found that participating patients were actually experiencing more negative moods, pain, and feelings of frustration than was once believed. Making this tool even more valuable for treatment adjustment and meeting patients’ needs.
In this case, it means that health care providers are getting an eyeful … of communication.
Make America grave again
Here we go again. Somebody just found something else that the United States is not the best at. To go along with math and science education, infrastructure investment, quality of life …
That’s going to go on for a while, so let’s get to the new stuff. An international group of researchers surveyed end-of-life care in 81 countries and ranked them based on the assessment of 181 experts in those countries. They looked at 13 different factors, including proper management of pain and comfort, having a clean and safe space, being treated kindly, lack of cost barriers to appropriate care, and treatments that address quality of life and don’t just extend life.
… press freedom, industrial production, racial equality, Internet connectivity …
Their report card, published in the Journal of Pain and Symptom Management, gave six countries an A, with Great Britain at the top. The other five were Ireland, Taiwan, Australia, South Korea, and Costa Rica. The lowest grade went to Paraguay in 81st place, with Lebanon, Brazil, Senegal, and Haiti just ahead.
… environmental stewardship, body-mass index, social mobility, COVID safeness …
The United States, getting a firm grasp on mediocrity, ranked 43rd. Here are some countries that did better: North Macedonia (7th), Sri Lanka (16th), Uganda (31st), and Uruguay 33rd). In the United States, “we spend so much money trying to get people to live longer, but we don’t spend enough money in helping people die better,” lead author Eric A. Finkelstein, PhD, said in a written statement.
… economic stability, and soccer; we’re also not the best at dying. Wait, did we already say that?
The face mask that launched a thousand ships
Face masks, clearly, have been a source of social strife during the pandemic. People may not agree on mandates, but a mask can be a pretty-low-maintenance face shield if you don’t feel like putting on make-up or want to cover up some blemishes.
Before the pandemic, people thought that those wearing face masks were less attractive because the masks represented illness or disease, according to Dr. Michael Lewis of Cardiff (Wales) University. Back then, no one really wore masks besides doctors and nurses, so if you saw someone wearing one on the street, you probably wondered what they were trying to hide.
Now, though, the subject of face mask attractiveness has been revisited by Dr. Lewis and his associate, Oliver Hies, who found that face masks now make people more attractive.
“Our study suggests faces are considered most attractive when covered by medical face masks. … At a time when we feel vulnerable, we may find the wearing of medical masks reassuring and so feel more positive towards the wearer,” Dr. Lewis told the Guardian.
He suggested that we’re no longer looking at people wearing a mask as disease riddled, but rather doing their part to protect society. Or maybe we focus more on someone’s eyes when that’s all there is to look at. Or, maybe we wind up making up what the rest of someone’s face looks like to meet our attractiveness criteria.
However you feel about masks, they’re cheaper than plastic surgery. And you can go out wearing a new face every day.
In space, no one can hear your red blood cells scream
There are many reasons why space is the final frontier, not least of which are the major health issues space travel places on the human body. So until a shady billionaire finds an alien protomolecule on a Saturnian moon and starts splicing it with human DNA so we can hang out in space all day without a spacesuit, we’re stuck with things like space anemia, a condition many astronauts develop after extended time in space.
Space anemia has been known for many years, but it was assumed that it developed as a reaction to microgravity and was a short-term phenomenon only – a temporary compensation as fluids and blood volume adjusted themselves. But as new research shows, that assumption seems to be wrong.
For the study, published in Nature Medicine, 13 astronauts who were in space for at least 120 days – long enough for all their red blood cells to have been produced in space – had their blood tested consistently. Before their flights, the astronauts created and destroyed 2 million red blood cells per second, but while they were in space, they destroyed 3 million cells per second. Notably, this process continued for the entire duration of the space flight. So, not a temporary reaction.
Consequently, 5 of the 13 astronauts developed anemia when they returned to Earth. (Interesting space fact: Having fewer blood cells isn’t a problem while you’re in space; the effects of anemia only manifest when the body returns to full gravity.) The anemia disappeared after a few months, but the astronauts were still destroying 30% more red blood cells a year after their spaceflight than they were before leaving Earth.
You may be thinking: Well, if they were destroying 50% more red blood cells while in space, how come they didn’t all develop severe anemia? The researchers theorized that production was boosted as well, which sounds like a good thing. The body is compensating, as it should. Unfortunately, that increased production stresses bone marrow function and the demand for energy spikes. That’s not such a good thing. And of course, many of the astronauts got anemia anyway.
To tackle the issue, the researchers emphasized the importance of feeding astronauts a proper diet, plus potential supplements before spaceflight. So don’t worry, Captain Kirk will be able to arm wrestle Klingons and romance suspiciously human-looking aliens without fear of keeling over from anemia-induced fatigue. Earth will stay safe.
Tell me with your eyes
Communication can be hard, even under the best of circumstances, but for many nonverbal patients in the intensive care unit who can’t move, getting a point across to the health care team can be a huge struggle in itself.
Health care professionals have been making do with eye-blinking or head-nodding, but what if that’s just not enough? New research shows that it’s not, and there’s a more effective way for patients to say what they mean just by looking.
In a study published in the Journal of Trauma and Acute Care Surgery, researchers looked into using eye-tracking systems for nonverbal ICU patients to communicate. Eye-tracking isn’t anything new, but using it as a form of communication among nonverbal patients with critical illness hasn’t been looked at before.
How does it work? The eye-tracking system is set up in the patient’s line of sight and its various algorithms and software collect data to calculate where exactly the patient is looking. Established scores and scales assess the patient’s mood, quality of life, pain, and self-esteem.
The researchers found that participating patients were actually experiencing more negative moods, pain, and feelings of frustration than was once believed. Making this tool even more valuable for treatment adjustment and meeting patients’ needs.
In this case, it means that health care providers are getting an eyeful … of communication.
Make America grave again
Here we go again. Somebody just found something else that the United States is not the best at. To go along with math and science education, infrastructure investment, quality of life …
That’s going to go on for a while, so let’s get to the new stuff. An international group of researchers surveyed end-of-life care in 81 countries and ranked them based on the assessment of 181 experts in those countries. They looked at 13 different factors, including proper management of pain and comfort, having a clean and safe space, being treated kindly, lack of cost barriers to appropriate care, and treatments that address quality of life and don’t just extend life.
… press freedom, industrial production, racial equality, Internet connectivity …
Their report card, published in the Journal of Pain and Symptom Management, gave six countries an A, with Great Britain at the top. The other five were Ireland, Taiwan, Australia, South Korea, and Costa Rica. The lowest grade went to Paraguay in 81st place, with Lebanon, Brazil, Senegal, and Haiti just ahead.
… environmental stewardship, body-mass index, social mobility, COVID safeness …
The United States, getting a firm grasp on mediocrity, ranked 43rd. Here are some countries that did better: North Macedonia (7th), Sri Lanka (16th), Uganda (31st), and Uruguay 33rd). In the United States, “we spend so much money trying to get people to live longer, but we don’t spend enough money in helping people die better,” lead author Eric A. Finkelstein, PhD, said in a written statement.
… economic stability, and soccer; we’re also not the best at dying. Wait, did we already say that?
The face mask that launched a thousand ships
Face masks, clearly, have been a source of social strife during the pandemic. People may not agree on mandates, but a mask can be a pretty-low-maintenance face shield if you don’t feel like putting on make-up or want to cover up some blemishes.
Before the pandemic, people thought that those wearing face masks were less attractive because the masks represented illness or disease, according to Dr. Michael Lewis of Cardiff (Wales) University. Back then, no one really wore masks besides doctors and nurses, so if you saw someone wearing one on the street, you probably wondered what they were trying to hide.
Now, though, the subject of face mask attractiveness has been revisited by Dr. Lewis and his associate, Oliver Hies, who found that face masks now make people more attractive.
“Our study suggests faces are considered most attractive when covered by medical face masks. … At a time when we feel vulnerable, we may find the wearing of medical masks reassuring and so feel more positive towards the wearer,” Dr. Lewis told the Guardian.
He suggested that we’re no longer looking at people wearing a mask as disease riddled, but rather doing their part to protect society. Or maybe we focus more on someone’s eyes when that’s all there is to look at. Or, maybe we wind up making up what the rest of someone’s face looks like to meet our attractiveness criteria.
However you feel about masks, they’re cheaper than plastic surgery. And you can go out wearing a new face every day.
Dark plaque on back of ear
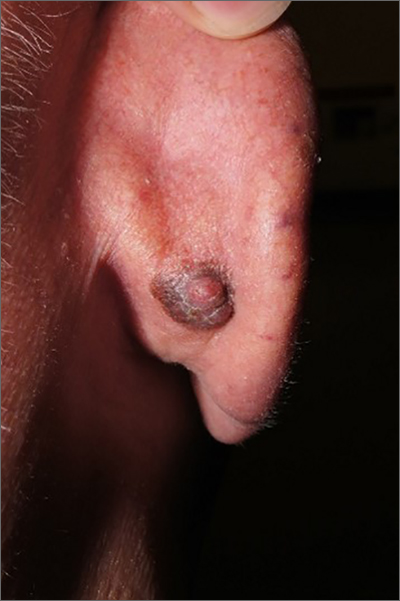
Dermoscopic findings were consistent with a melanocytic lesion and a scoop shave biopsy revealed a 2.7 mm thick nodular melanoma.
Melanoma is the most lethal skin cancer in the United States. The likelihood of metastatic spread to lymph nodes statistically increases beyond a probability of 5% when patients have primary lesions thicker than 0.8 mm.1 Thus, for patients with tumors thicker than 0.8 mm, or some other high-risk features such as high mitotic index, a sentinel lymph node biopsy (SLNB) is recommended. This patient underwent wide local excision and reconstruction of his ear. An SLNB was also performed and the results were negative.
The patient returned for a complete skin exam every 3 months. Ten months after the excision, he presented with episodes of headache and confusion. Magnetic resonance imaging revealed metastasis to the brain; a biopsy confirmed that it was melanoma. Two months later, after attempts at resection of the brain metastasis, the patient died.
This case demonstrates that patients with thick melanoma are at continued risk for recurrence and poor outcomes; they benefit from close surveillance and work-up of unusual symptoms that might suggest metastases. Phase 3 trials are currently underway to consider the use of adjuvant therapy in patients with advanced stage II melanoma who, on average, have worse outcomes than patients with early-stage III disease.2
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. NCCN Guidelines Version 1.2022 Melanoma: Cutaneous. National Comprehensive Cancer Network. December 3, 2021. Accessed January 4, 2022. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
2. Poklepovic AS, Luke JJ. Considering adjuvant therapy for stage II melanoma. Cancer. 2020;126:1166-1174. doi: 10.1002/cncr.32585

Dermoscopic findings were consistent with a melanocytic lesion and a scoop shave biopsy revealed a 2.7 mm thick nodular melanoma.
Melanoma is the most lethal skin cancer in the United States. The likelihood of metastatic spread to lymph nodes statistically increases beyond a probability of 5% when patients have primary lesions thicker than 0.8 mm.1 Thus, for patients with tumors thicker than 0.8 mm, or some other high-risk features such as high mitotic index, a sentinel lymph node biopsy (SLNB) is recommended. This patient underwent wide local excision and reconstruction of his ear. An SLNB was also performed and the results were negative.
The patient returned for a complete skin exam every 3 months. Ten months after the excision, he presented with episodes of headache and confusion. Magnetic resonance imaging revealed metastasis to the brain; a biopsy confirmed that it was melanoma. Two months later, after attempts at resection of the brain metastasis, the patient died.
This case demonstrates that patients with thick melanoma are at continued risk for recurrence and poor outcomes; they benefit from close surveillance and work-up of unusual symptoms that might suggest metastases. Phase 3 trials are currently underway to consider the use of adjuvant therapy in patients with advanced stage II melanoma who, on average, have worse outcomes than patients with early-stage III disease.2
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

Dermoscopic findings were consistent with a melanocytic lesion and a scoop shave biopsy revealed a 2.7 mm thick nodular melanoma.
Melanoma is the most lethal skin cancer in the United States. The likelihood of metastatic spread to lymph nodes statistically increases beyond a probability of 5% when patients have primary lesions thicker than 0.8 mm.1 Thus, for patients with tumors thicker than 0.8 mm, or some other high-risk features such as high mitotic index, a sentinel lymph node biopsy (SLNB) is recommended. This patient underwent wide local excision and reconstruction of his ear. An SLNB was also performed and the results were negative.
The patient returned for a complete skin exam every 3 months. Ten months after the excision, he presented with episodes of headache and confusion. Magnetic resonance imaging revealed metastasis to the brain; a biopsy confirmed that it was melanoma. Two months later, after attempts at resection of the brain metastasis, the patient died.
This case demonstrates that patients with thick melanoma are at continued risk for recurrence and poor outcomes; they benefit from close surveillance and work-up of unusual symptoms that might suggest metastases. Phase 3 trials are currently underway to consider the use of adjuvant therapy in patients with advanced stage II melanoma who, on average, have worse outcomes than patients with early-stage III disease.2
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. NCCN Guidelines Version 1.2022 Melanoma: Cutaneous. National Comprehensive Cancer Network. December 3, 2021. Accessed January 4, 2022. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
2. Poklepovic AS, Luke JJ. Considering adjuvant therapy for stage II melanoma. Cancer. 2020;126:1166-1174. doi: 10.1002/cncr.32585
1. NCCN Guidelines Version 1.2022 Melanoma: Cutaneous. National Comprehensive Cancer Network. December 3, 2021. Accessed January 4, 2022. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
2. Poklepovic AS, Luke JJ. Considering adjuvant therapy for stage II melanoma. Cancer. 2020;126:1166-1174. doi: 10.1002/cncr.32585
Did a switch to a generic antidepressant cause relapse?
Advances in Diabetes and Cardiovascular Care
Real-World Experience With Automated Insulin Pumps
Continuous Blood Glucose Monitoring for T2DM
Statin-Induced Adverse Effects
Long QT and Cardiac Arrest After Pulmonary Edema
And more online
• Clinical Impact of U-500 Insulin Initiation
• Diabetes Self-Management Education
• SGLT2 Inhibitors, T2DM, and Heart Failure
• Alirocumab Use in Statin-Intolerant Veterans
• K Pneumoniae-Induced Aortitis
Real-World Experience With Automated Insulin Pumps
Continuous Blood Glucose Monitoring for T2DM
Statin-Induced Adverse Effects
Long QT and Cardiac Arrest After Pulmonary Edema
And more online
• Clinical Impact of U-500 Insulin Initiation
• Diabetes Self-Management Education
• SGLT2 Inhibitors, T2DM, and Heart Failure
• Alirocumab Use in Statin-Intolerant Veterans
• K Pneumoniae-Induced Aortitis
Real-World Experience With Automated Insulin Pumps
Continuous Blood Glucose Monitoring for T2DM
Statin-Induced Adverse Effects
Long QT and Cardiac Arrest After Pulmonary Edema
And more online
• Clinical Impact of U-500 Insulin Initiation
• Diabetes Self-Management Education
• SGLT2 Inhibitors, T2DM, and Heart Failure
• Alirocumab Use in Statin-Intolerant Veterans
• K Pneumoniae-Induced Aortitis
A Family Physician's Introduction to Lifestyle Medicine
This supplement will teach you about the 6 pillars of Lifestyle Medicine, looks at the future of Lifestyle Medicine, demonstrates the power of Lifestyle Medicine as evidence-based treatment of chronic disease, and describes how you can incorporate Lifestyle Medicine into your practice.
Click here to access this content now
This supplement will teach you about the 6 pillars of Lifestyle Medicine, looks at the future of Lifestyle Medicine, demonstrates the power of Lifestyle Medicine as evidence-based treatment of chronic disease, and describes how you can incorporate Lifestyle Medicine into your practice.
Click here to access this content now
This supplement will teach you about the 6 pillars of Lifestyle Medicine, looks at the future of Lifestyle Medicine, demonstrates the power of Lifestyle Medicine as evidence-based treatment of chronic disease, and describes how you can incorporate Lifestyle Medicine into your practice.
Click here to access this content now
Pandemic weighing on physicians’ happiness outside of work: survey
One of the unexpected consequences of the pandemic is that many people are rethinking their priorities and lifestyles, and physicians are no exception.
Pets, prayer, and partners
The pandemic has taken a toll on physicians outside of work as well as on the job. Eight in 10 physicians (82% of men and 80% of women) said they were “somewhat” or “very” happy outside of work before the pandemic. This is almost exactly the same result as in last year’s survey.
However, when asked how happy they are outside of work currently, only 6 in 10 (59%) reported being “somewhat” or “very” happy. While the pandemic has made life difficult for everyone, health care professionals face particular stresses even outside of work. Wayne M. Sotile, PhD, founder of the Center for Physician Resilience, says he has counseled doctors who witnessed COVID-related suffering and death at work, then came home to a partner who didn’t believe that the pandemic was real.
Still, physicians reported that spending time with people they love and engaging in favorite activities helps them stay happy. “Spending time with pets” and “religious practice/prayer” were frequent “other” responses to the question, “What do you do to maintain happiness and mental health?” Seven in 10 physicians reported having some kind of religious or spiritual beliefs.
The majority of physicians (83%) are either married or living with a partner, with male physicians edging out their female peers (89% vs. 75%). Among married physicians, 8 in 10 physicians reported that their union is “good” or “very good.” The pandemic may have helped in this respect. Dr. Sotile says he’s heard physicians say that they’ve connected more with their families in the past 18 months. Specialists with the highest rates of happy marriages were otolaryngologists and immunologists (both 91%), followed closely by dermatologists, rheumatologists, and nephrologists (all 90%).
Among physicians balancing a medical career and parenthood, female physicians reported feeling conflicted more often than males (48% vs. 29%). Nicole A. Sparks, MD, an ob.gyn. and a health and lifestyle blogger, cites not being there for her kids as a source of stress. She notes that her two young children notice when she’s not there to help with homework, read bedtime stories, or make their dinner. “Mom guilt can definitely set in if I have to miss important events,” she says.
Work-life balance is an important, if elusive, goal for physicians, and not just females. Sixty percent of female doctors and 53% of male doctors said they would be willing to take a cut in pay if it meant more free time and a better work-life balance. Many doctors do manage to get away from work occasionally, with one-fifth of all physicians taking 5 or more weeks of vacation each year.
Seeking a ‘balanced life’
Alexis Polles, MD, medical director for the Professionals Resource Network, points out the importance of taking time for personal health and wellness. “When we work with professionals who have problems with mental health or substance abuse, they often don’t have a balanced life,” she says. “They are usually in a workaholic mindset and disregard their own needs.”
Few physicians seem to prioritize self-care, with a third indicating they “always” or “most of the time” spend enough time on their own health and wellness. But of those who do, males (38%) are more likely than females (27%) to spend enough time on their own health and wellness. Dr. Polles adds that exercising after a shift can help physicians better make the transition from professional to personal life. Though they did not report when they exercised, about a third of physicians reported doing so four or more times per week. Controlling weight is an issue as well, with 49% of male and 55% of female physicians saying they are currently trying to lose weight.
Of physicians who drink alcohol, about a third have three or more drinks per week. (The CDC defines “heavy drinking” as consuming 15 drinks or more per week for men and eight drinks or more per week for women.)
Of those surveyed, 92% say they do not regularly use cannabidiol or cannabis, and a mere 4% of respondents said they would use at least one of these substances if they were to become legal in their state.
A version of this article first appeared on Medscape.com.
One of the unexpected consequences of the pandemic is that many people are rethinking their priorities and lifestyles, and physicians are no exception.
Pets, prayer, and partners
The pandemic has taken a toll on physicians outside of work as well as on the job. Eight in 10 physicians (82% of men and 80% of women) said they were “somewhat” or “very” happy outside of work before the pandemic. This is almost exactly the same result as in last year’s survey.
However, when asked how happy they are outside of work currently, only 6 in 10 (59%) reported being “somewhat” or “very” happy. While the pandemic has made life difficult for everyone, health care professionals face particular stresses even outside of work. Wayne M. Sotile, PhD, founder of the Center for Physician Resilience, says he has counseled doctors who witnessed COVID-related suffering and death at work, then came home to a partner who didn’t believe that the pandemic was real.
Still, physicians reported that spending time with people they love and engaging in favorite activities helps them stay happy. “Spending time with pets” and “religious practice/prayer” were frequent “other” responses to the question, “What do you do to maintain happiness and mental health?” Seven in 10 physicians reported having some kind of religious or spiritual beliefs.
The majority of physicians (83%) are either married or living with a partner, with male physicians edging out their female peers (89% vs. 75%). Among married physicians, 8 in 10 physicians reported that their union is “good” or “very good.” The pandemic may have helped in this respect. Dr. Sotile says he’s heard physicians say that they’ve connected more with their families in the past 18 months. Specialists with the highest rates of happy marriages were otolaryngologists and immunologists (both 91%), followed closely by dermatologists, rheumatologists, and nephrologists (all 90%).
Among physicians balancing a medical career and parenthood, female physicians reported feeling conflicted more often than males (48% vs. 29%). Nicole A. Sparks, MD, an ob.gyn. and a health and lifestyle blogger, cites not being there for her kids as a source of stress. She notes that her two young children notice when she’s not there to help with homework, read bedtime stories, or make their dinner. “Mom guilt can definitely set in if I have to miss important events,” she says.
Work-life balance is an important, if elusive, goal for physicians, and not just females. Sixty percent of female doctors and 53% of male doctors said they would be willing to take a cut in pay if it meant more free time and a better work-life balance. Many doctors do manage to get away from work occasionally, with one-fifth of all physicians taking 5 or more weeks of vacation each year.
Seeking a ‘balanced life’
Alexis Polles, MD, medical director for the Professionals Resource Network, points out the importance of taking time for personal health and wellness. “When we work with professionals who have problems with mental health or substance abuse, they often don’t have a balanced life,” she says. “They are usually in a workaholic mindset and disregard their own needs.”
Few physicians seem to prioritize self-care, with a third indicating they “always” or “most of the time” spend enough time on their own health and wellness. But of those who do, males (38%) are more likely than females (27%) to spend enough time on their own health and wellness. Dr. Polles adds that exercising after a shift can help physicians better make the transition from professional to personal life. Though they did not report when they exercised, about a third of physicians reported doing so four or more times per week. Controlling weight is an issue as well, with 49% of male and 55% of female physicians saying they are currently trying to lose weight.
Of physicians who drink alcohol, about a third have three or more drinks per week. (The CDC defines “heavy drinking” as consuming 15 drinks or more per week for men and eight drinks or more per week for women.)
Of those surveyed, 92% say they do not regularly use cannabidiol or cannabis, and a mere 4% of respondents said they would use at least one of these substances if they were to become legal in their state.
A version of this article first appeared on Medscape.com.
One of the unexpected consequences of the pandemic is that many people are rethinking their priorities and lifestyles, and physicians are no exception.
Pets, prayer, and partners
The pandemic has taken a toll on physicians outside of work as well as on the job. Eight in 10 physicians (82% of men and 80% of women) said they were “somewhat” or “very” happy outside of work before the pandemic. This is almost exactly the same result as in last year’s survey.
However, when asked how happy they are outside of work currently, only 6 in 10 (59%) reported being “somewhat” or “very” happy. While the pandemic has made life difficult for everyone, health care professionals face particular stresses even outside of work. Wayne M. Sotile, PhD, founder of the Center for Physician Resilience, says he has counseled doctors who witnessed COVID-related suffering and death at work, then came home to a partner who didn’t believe that the pandemic was real.
Still, physicians reported that spending time with people they love and engaging in favorite activities helps them stay happy. “Spending time with pets” and “religious practice/prayer” were frequent “other” responses to the question, “What do you do to maintain happiness and mental health?” Seven in 10 physicians reported having some kind of religious or spiritual beliefs.
The majority of physicians (83%) are either married or living with a partner, with male physicians edging out their female peers (89% vs. 75%). Among married physicians, 8 in 10 physicians reported that their union is “good” or “very good.” The pandemic may have helped in this respect. Dr. Sotile says he’s heard physicians say that they’ve connected more with their families in the past 18 months. Specialists with the highest rates of happy marriages were otolaryngologists and immunologists (both 91%), followed closely by dermatologists, rheumatologists, and nephrologists (all 90%).
Among physicians balancing a medical career and parenthood, female physicians reported feeling conflicted more often than males (48% vs. 29%). Nicole A. Sparks, MD, an ob.gyn. and a health and lifestyle blogger, cites not being there for her kids as a source of stress. She notes that her two young children notice when she’s not there to help with homework, read bedtime stories, or make their dinner. “Mom guilt can definitely set in if I have to miss important events,” she says.
Work-life balance is an important, if elusive, goal for physicians, and not just females. Sixty percent of female doctors and 53% of male doctors said they would be willing to take a cut in pay if it meant more free time and a better work-life balance. Many doctors do manage to get away from work occasionally, with one-fifth of all physicians taking 5 or more weeks of vacation each year.
Seeking a ‘balanced life’
Alexis Polles, MD, medical director for the Professionals Resource Network, points out the importance of taking time for personal health and wellness. “When we work with professionals who have problems with mental health or substance abuse, they often don’t have a balanced life,” she says. “They are usually in a workaholic mindset and disregard their own needs.”
Few physicians seem to prioritize self-care, with a third indicating they “always” or “most of the time” spend enough time on their own health and wellness. But of those who do, males (38%) are more likely than females (27%) to spend enough time on their own health and wellness. Dr. Polles adds that exercising after a shift can help physicians better make the transition from professional to personal life. Though they did not report when they exercised, about a third of physicians reported doing so four or more times per week. Controlling weight is an issue as well, with 49% of male and 55% of female physicians saying they are currently trying to lose weight.
Of physicians who drink alcohol, about a third have three or more drinks per week. (The CDC defines “heavy drinking” as consuming 15 drinks or more per week for men and eight drinks or more per week for women.)
Of those surveyed, 92% say they do not regularly use cannabidiol or cannabis, and a mere 4% of respondents said they would use at least one of these substances if they were to become legal in their state.
A version of this article first appeared on Medscape.com.