User login
A practical guide to appendicitis evaluation and treatment
CASE
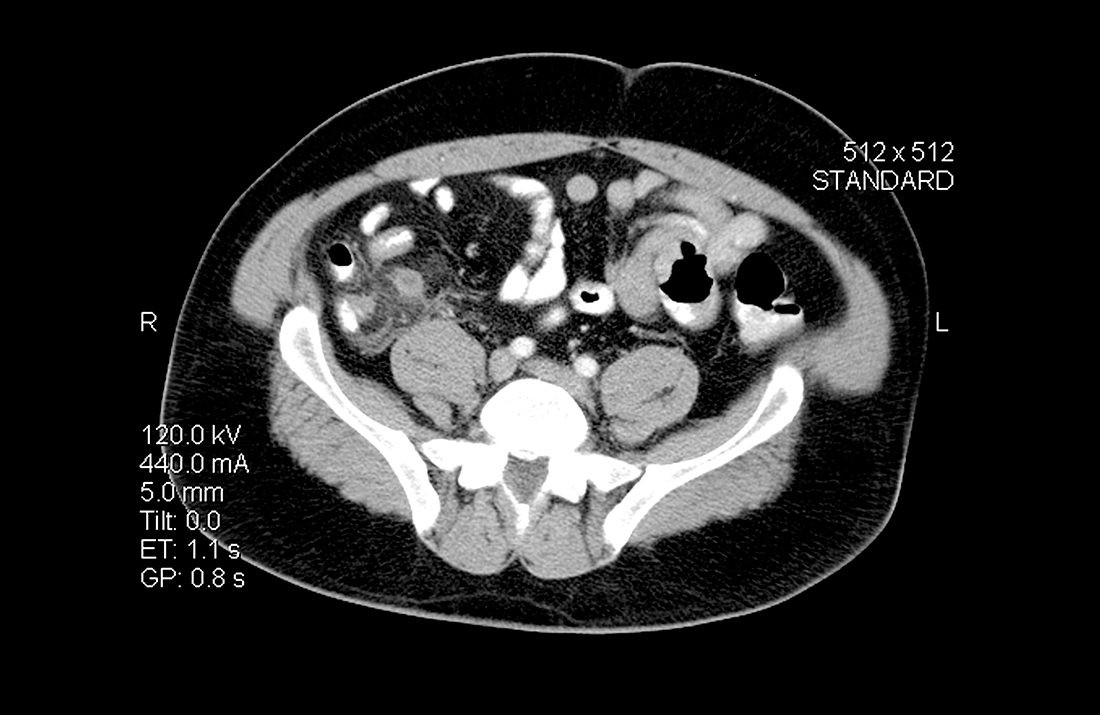
A 35-year-old man with a body mass index of 20 presented to the emergency department after 24 hours of abdominal pain that began in the periumbilical region and then migrated to the right lower quadrant. The pain was exacerbated during ambulation and was intense when the car transporting him to the hospital encountered bumps in the road. After his pain started, he had associated anorexia, followed by nausea and emesis. He reported fever and chills. On examination, his temperature was 100.8 °F (38.2 °C), and palpation of the right and left lower quadrants elicited right lower quadrant pain. Laboratory evaluation revealed a white blood cell (WBC) count of 14,000 cells/mcL with 85% neutrophils, C-reactive protein of 40 mg/L, and a negative urinalysis.
How would you proceed with this patient?
Acute appendicitis is the most common cause of abdominal pain resulting in the need for surgical treatment; lifetime risk of appendicitis is 6% to 7%.1 Appendicitis is caused by intraluminal obstruction in the appendix from enlarged lymphoid tissue or a fecalith. The obstruction leads to elevated intraluminal pressure due to persistent mucus and gas production by bacteria, ultimately leading to ischemia and perforation.1 Additionally, obstruction leads to bacterial overgrowth, most commonly colonic flora such as Escherichia coli, Bacteroides fragilis, Streptococcus viridans, Enterococcus sp., Pseudomonas aeruginosa, and Klebsiella pneumoniaei.1,2
The following review provides a look at how 3 clinical scoring systems compare in the identification of acute appendicitis and details which imaging studies you should order—and when. But first, we’ll quickly detail the relevant physical findings and lab values that point to a diagnosis of acute appendicitis.
Physical findings. The patient typically first experiences vague abdominal pain that then localizes to the right lower quadrant due to peritoneal inflammation. Anorexia and nausea typically follow the abdominal pain. On examination, the patient often appears ill and exhibits abdominal guarding due to peritonitis. Tachycardia and fever are common; however, the absence of either does not exclude appendicitis. Classically, on palpation, the patient will have pain at McBurney’s point (one-third the distance from the anterior iliac spine to the umbilicus). The exact point of maximal tenderness can differ because of the varying anatomy of the appendix (retrocecal, paracolic, pelvic, pre/post ileal, promontoric, or subcecal).1 Right lower quadrant pain, abdominal rigidity, and radiation of periumbilical pain to the right lower quadrant are the most accurate findings in adults to rule in appendicitis.3 For children, physical exam findings have the highest likelihood in predicting appendicitis and include a positive Obturator sign, positive Rovsing sign, or a positive Psoas sign, and absent or decreased bowel sounds.4
Laboratory studies can support a diagnosis of appendicitis but cannot exclude it. Leukocytosis with neutrophil predominance is present in 90% of cases.5 An elevated C-reactive protein level renders the highest diagnostic accuracy.5 Perform a pregnancy test for any woman of child-bearing age, to assist in the diagnosis and guide imaging choices for evaluation. Additional laboratory tests are not needed unless there are concerns about volume depletion.
Clinical scoring systems
Several clinical scoring systems (TABLE6-10) have been validated to aid clinicians in evaluating patients with possible appendicitis, to decrease unnecessary exposure to ionizing radiation from computed tomography (CT) scans, to identify and reassure patients with low likelihoods of appendicitis, and to conduct outpatient follow-up.
Continue to: The Alvarado score
The Alvarado score is the oldest scoring rule, developed in 1986; it entails 8 clinical and laboratory variables.6 Ebell et al altered the proposed cutoff values of the Alvarado score to be low risk (< 4), intermediate risk (4-8), and high risk (≥ 9), effectively improving the sensitivity and specificity rates.7
In a meta-analysis of the Alvarado score that included 42 studies of men, women, and children, the sensitivity for “ruling out” appendicitis with a cutoff of 5 points was 96% for men, 99% for women, and 99% for children.8 The accuracy of a high-risk score (> 7) for “ruling in” appendicitis was less with an overall specificity of 82%.8 The Alvarado score did seem to overestimate appendicitis in women in all score categories.8
The Pediatric Appendicitis Score (PAS) is similar to Alvarado and was prospectively validated in 1170 children in 2002 for more specific guidance in this age group.9 The PAS had excellent specificity in the study; those with a score of ≥ 6 had a high probability of appendicitis. In a study comparing Alvarado with PAS in 311 patients, insignificant differences were noted at a score of ≥ 7 for both tests (sensitivity 86% vs 89%, and specificity 59% vs 50%, respectively).11 No scoring system has been found to be sufficiently accurate for use in children 4 years of age and younger.12
The Appendicitis Inflammatory Response (AIR) Score was prospectively validated in 545 patients representing all age groups.10 Subsequently, in a larger prospective multicenter study of 3878 patients older than 5 years, the original cut points were altered, thereby improving test sensitivity and negative predictive value to 99% for those with low probability (0 to 3), and test specificity to 98% for those with high-probability (9 to 12).13 Compared with the Alvarado Score, the AIR Score has higher specificity for those in the high-probability range, and similar exclusion rates in the low-probability range.14
Caveats with clinical decision scores. These tools are accepted and often used. However, challenges that affect generalizability of study data include differences in patient selection for each study (undifferentiated abdominal pain vs appendicitis), prospective vs retrospective designs, and age and gender variations in the patient populations. Despite the numerous scoring systems developed, none can accurately be used to rule in appendicitis. They are best used to assist in ruling out appendicitis and to aid in deciding for or against imaging.
Continue to: A look at the imaging options
A look at the imaging options
Abdominal CT has sensitivity and specificity rates between 76% and 100% and 83% and 100%, respectively.15,20,21 Ultrasonography has sensitivity and specificity rates of 71% to 94% and 81% to 98%, respectively.15,20,21 Formal US is reliable to confirm appendicitis, but less so to rule out appendicitis. Special considerations for imagining in pregnant patients and children are discussed in a bit.
Timing of surgical consultation
Surgical consultation is paramount once the diagnosis of appendicitis is probable. Imaging is best obtained prior to surgical consultation to streamline evaluation and enhance decision- making. Typically, patients will be categorized as complicated or uncomplicated based on the presence or absence of perforation, a gangrenous appendix, an intra-abdominal abscess (IAA), or purulent peritonitis. Active continuous surgical involvement (co-management or assumption of care) is recommended in all cases of appendicitis, especially if nonoperative management is selected, given that some cases must convert to immediate operative treatment or may be selected for delayed future (interval) appendectomy.22
Management
Uncomplicated appendicitis
Prompt appendectomy has been the gold standard of care for uncomplicated acute appendicitis for 60 years. However, several studies have investigated an antibiotic-based strategy rather than surgical treatment for uncomplicated appendicitis.
Antibiotics vs appendectomy. In 2020, the CODA Collaborative published a randomized trial comparing a 10-day course of antibiotics with appendectomy in patients with uncomplicated appendicitis. In this multicenter study based in the United States, 1552 patients 18 years of age or older were randomized to receive antibiotics or undergo appendectomy (95% performed laparoscopically). The antibiotic treatment consisted of at least 24 hours of IV antibiotics, with or without admission to the hospital. Antibiotic choice was individualized according to guidelines for intra-abdominal infection published by the Infectious Diseases Society of America, with the most common IV medications being ertapenem, cefoxitin, or metronidazole plus one of the following: ceftriaxone, cefazolin, or levofloxacin. For the remaining 10 days, oral metronidazole plus ciprofloxacin or cefdinir were used.22
Continue to: The primary endpoint...
The primary endpoint was the European Quality of Life-5 Dimensions (EQ-5D) questionnaire, with secondary outcomes including appendectomy in the antibiotics group and complications through 90 days. Exclusion criteria included pregnancy, sepsis, peritonitis, recurrent appendicitis, severe phlegmon on imaging, or evidence of neoplasm.22
Antibiotics were noninferior to appendectomy for the 30-day study. However, antibiotics failed in 29%, who then proceeded to appendectomy by 90 days; these patients also accounted for 41% of those with an appendicolith. Overall complications were more common in the antibiotics group than in the appendectomy group (8.1 vs 3.5 per 100 participants; 95% CI, 1.3-3.98). Also more common in the antibiotic group were serious adverse events (4 vs 3 per 100 participants; hazard ratio [HR] = 1.29; 95% CI, 0.67-2.50). The presence of an appendicolith in the antibiotics group increased the conversion risk to appendectomy, as well as adverse events risk.22
The takeaway. Antibiotic treatment is a noninferior method to treat acute uncomplicated appendicitis. However, the informed consent process is important, given the ~30% failure rate. Patient factors such as continued access to care should help inform the decision.
Two main surgical approaches exist for appendectomy: open and minimally invasive. At this time, the minimally invasive options include laparoscopic, single incision laparoscopic surgery (SILS), and robotic appendectomy. A study comparing cost, availability, or complications of these options has not been conducted at this time.
A large Cochrane review of 67 studies examining open vs laparoscopic appendectomy in adults and children completed in 2018 revealed that the laparoscopic approach reduced early postoperative pain intensity and led to a shorter hospital stay, earlier return to work or usual activities, and a decrease in wound infections.23 The odds of IAA occurring with laparoscopic appendectomy increased by 65% compared with an open procedure; however, postoperative bowel obstruction and incisional hernias were less likely to occur.23 Additionally, following laparoscopic surgery, postoperative bowel obstruction and incisional hernias are less likely to occur. The laparoscopic approach is preferred due to overall increased patient satisfaction and a reduction in most, if not all, complications.
Continue to: Complicated appendicitis
Complicated appendicitis
Excluding patients with severe sepsis or purulent peritonitis requiring resuscitation and immediate surgical intervention of intra-abdominal infection, the approach to patients with complicated appendicitis varies between aggressive surgical intervention and nonoperative management.
In a 2007 meta-analysis reviewing nonsurgical treatment of appendiceal abscess/phlegmon, immediate surgery was associated with higher morbidity.24 Within the nonoperative management group 7.2% (CI, 4.0-10.5) required surgical intervention and 19.7% (CI, 11.0-28.3) required abscess drainage. Malignant disease was detected in 1.2% (CI, 0.6-1.7).24 Small subsequent studies concluded different results.25
Ultimately, the 2015 European Association of Endoscopic Surgery guidelines recommend a new systematic review; but with current data, initial nonoperative management is preferred.15 After initial nonoperative treatment, the only benefits from interval appendectomy are identification of an underlying malignancy (6% to 20%) and mitigating the risk of recurrent appendicitis (5% to 44%).15,25-30
Multiple single institutional series found increased neoplasm incidence (9% to 20%) in complicated appendicitis in patients 40 years and older.26-30 Prior to interval appendectomy in patients 45 years and older, ensuring they have an up-to-date screening colonoscopy is important. This is in line with 2021 US Preventive Services Task Force (Grade “B” recommendation), 2018 American Cancer Society (qualified recommendation), and 2021 American College of Gastroenterology (conditional recommendation) guidelines for colorectal cancer screening to start at age 45 in average-risk patients.31 Patients younger than 45 can consider screening through shared decision-making.
Special populations
Pregnant patients
In pregnancy, challenges exist with the presence of traditional signs and symptoms of appendicitis, with the most predictive sign being a WBC count higher than 18,000.32 The American College of Radiology’s (ACR) Appropriateness Criteria recommend US as the imaging modality of choice in pregnancy, with MRI as the best option when US is inconclusive.33 Two meta-analyses demonstrated high sensitivity (91.8%-96.6%) and specificity (95.9%-97.9%) of MRI in diagnosing appendicitis.34,35 CT scan is not the preferred initial imagining modality in pregnancy unless urgent information is needed and other modalities are insufficient or unavailable.36
Continue to: The most common...
The most common nonobstetric surgical intervention during pregnancy is appendectomy, at a rate of 6.3/10,000 person-years, which increases to 9.9/10,000 in the postpartum period.37 Two large population studies demonstrate the rate of appendicitis varies over the course of pregnancy, with the lowest rates in the third trimester,38,39 and a significant rebound lasting for 2 years postpartum.39 Peritonitis, septic shock, pneumonia, postoperative infection, and longer hospital stays occur more frequently in pregnant women than in nonpregnant women with appendicitis.40 Fetal loss is higher in the first trimester.32
In a 14-year review of 63,145 appendicitis cases, an increased risk of fetal loss and maternal death was noted across ages and ethnicities, with the largest risk of maternal death occurring in Hispanics and fetal death in non-Hispanic Blacks.41 In a large study of 1018 adverse events after appendectomy or cholecystectomy, the 3 most common events were preterm delivery (35.4%), preterm labor without preterm delivery (26.4%), and miscarriage (25.7%).42 The surgery itself was not a major risk factor for adverse events. Major risk factors included cervical incompetence (odds ratio [OR] = 24.3), preterm labor in current pregnancy (OR = 18.3), and presence of vulvovaginitis (OR = 5.2).42
Nonoperative management in pregnancy is not recommended; only 1 prospective trial has been done, with 20 patients, showing a 25% failure rate.43 Two meta-analyses published in 2019 highlight the potential increase of fetal loss with laparoscopic approaches to appendectomy.44,45 However, recently published literature demonstrates no significant maternal-fetal morbidity. Current guidelines of the Society of American Gastrointestinal and Endoscopic Surgeons agree that laparoscopy is the operative choice in pregnancy.36
Children
Acute appendicitis is the most common surgical emergency in children.4 Physical exam findings and laboratory results are not classic in this population, obtaining an accurate history can be challenging, and results of clinical scoring systems can be inconclusive.4 Additional serum biomarkers, procalcitonin and calprotectin, are gaining evidence for use in improving scoring systems to refine low-risk groups. Unavailability of timely, reliable biomarker testing in rural practice locations limits definitive recommendations at this time.46 ACR recommends no imaging in a pediatric patient whose risk of having appendicitis is low based on any of several scoring systems.47 For those assessed as having higher risk, US is the recommended initial modality,with CT with IV contrast or MRI without contrast equally recommended if the US is equivocal.47
Despite promising data from trials of nonoperative treatment for adults with appendicitis, no definitive evidence and recommendations are available for children. Two systematic reviews show nonoperative treatment is safe, with an efficacy rate of 76% to 82% at long-term follow-up,48,49 although the success of antibiotic regimens varies. Within the nonoperative treatment group, 16% of patients had appendectomy during the follow-up period, which varied from 8 weeks to 4 years.48 A randomized controlled trial is needed for final guidance.
Continue to: CASE
CASE
The patient had an Alvarado score of 9 (high probability) and an AIR score of 6 (intermediate probability). A CT with IV contrast showed a 9-mm fluid-filled appendix with periappendiceal fluid. During surgical consultation, he was offered laparoscopic appendectomy or nonoperative treatment with antibiotics. He opted for a preoperative dose of piperacillin-tazobactam 3.375 g IV and laparoscopic appendectomy. The patient was discharged home 6 hours after his procedure.
CORRESPONDENCE
Jessica Servey, MD, MHPE, 4301 Jones Bridge Road, Bethesda, MD 20814; [email protected]
1. Prystowsky JB, Pugh CM, Nagle AP. Current problems in surgery. Appendicitis. Curr Probl Surg. 2005;42:688-742.
2. Song DW, Park BK, Suh SW, et al. Bacterial culture and antibiotic susceptibility in patients with acute appendicitis. Int J Colorectal Dis. 2018;33:441-447.
3. Wagner JM, McKinney WP, Carpenter JL. Does this patient have appendicitis? JAMA. 1996;276:1589-1594.
4. Benabbas R, Hanna M, Shah J, et al. Diagnostic accuracy of history, physical examination, laboratory tests, and point-of-care ultrasound for pediatric acute appendicitis in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2017;24:523-551.
5. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg. 2004;91:28-37.
6. Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986;15:557-564.
7. Ebell MH, Shinholser J. What are the most clinically useful cutoffs for the Alvarado and Pediatric Appendicitis Scores? A systematic review. Ann Emerg Med. 2014;64:365-372.e2.
8. Ohle R, O’Reilly F, O’Brien KK, et al. The Alvarado score for predicting acute appendicitis: a systematic review. BMC Med. 2011;9:139.
9. Samuel M. Pediatric appendicitis score. J Pediatr Surg. 2002;37:877-881.
10. Andersson M, Andersson RE. The appendicitis inflammatory response score: a tool for the diagnosis of acute appendicitis that outperforms the Alvarado score. World J Surg. 2008;32:1843-1849.
11. Pogorelić Z, Rak S, Mrklić I, et al. Prospective validation of Alvarado score and Pediatric Appendicitis Score for the diagnosis of acute appendicitis in children. Pediatr Emerg Care. 2015;31:164-168.
12. Rassi R, Muse F, Sánchez-Martínez J, et al. Diagnostic value of clinical prediction scores for acute appendicitis in children younger than 4 years. Eur J Pediatr Surg. 2021. [Online ahead of print]
13. Andersson M, Kolodziej B, Andersson RE. Validation of the Appendicitis Inflammatory Response (AIR) score. World J Surg. 2021;45:2081-2091.
14. Kollár D, McCartan DP, Bourke M, et al. Predicting acute appendicitis? A comparison of the Alvarado score, the Appendicitis Inflammatory Response Score and clinical assessment. World J Surg. 2015;39:104-109.
15. Gorter RR, Eker HH, Gorter-Stam MA, et al. Diagnosis and management of acute appendicitis. EAES consensus development conference 2015. Surg Endosc. 2016;30:4668-4690.
16. Matthew Fields J, Davis J, Alsup C, et al. Accuracy of point-of-care ultrasonography for diagnosing acute appendicitis: a systematic review and meta-analysis. Acad Emerg Med. 2017;24:1124-1136.
17. Sharif S, Skitch S, Vlahaki D, et al. Point-of-care ultrasound to diagnose appendicitis in a Canadian emergency department. CJEM. 2018;20:732-735.
18. Doniger SJ, Kornblith A. Point-of-care ultrasound integrated into a staged diagnostic algorithm for pediatric appendicitis. Pediatr Emerg Care. 2018;34:109-115.
19. Menon N, Kumar S, Keeler B, et al. A systematic review of point-of-care abdominal ultrasound scans performed by general surgeons. Surgeon. 2021. [Online ahead of print]
20. Doria AS, Moineddin R, Kellenberger CJ, et al. US or CT for diagnosis of appendicitis in children and adults? A meta-analysis. Radiology. 2006;241:83-94.
21. van Randen A, Laméris W, van Es HW, et al. A comparison of the accuracy of ultrasound and computed tomography in common diagnoses causing acute abdominal pain. Eur Radiol. 2011;21:1535-1545.
22. Flum DR, Davidson GH, Monsell SE, et al. A randomized trial comparing antibiotics with appendectomy for appendicitis. N Engl J Med. 2020;383:1907-1919.
23. Jaschinski T, Mosch CG, Eikermann M, et al. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2018;11:CD001546.
24. Andersson RE, Petzold MG. Nonsurgical treatment of appendiceal abscess or phlegmon: a systematic review and meta-analysis. Ann Surg. 2007;246:741-748.
25. Deelder JD, Richir MC, Schoorl T, et al. How to treat an appendiceal inflammatory mass: operatively or nonoperatively? J Gastrointest Surg. 2014;18:641-645.
26. Carpenter SG, Chapital AB, Merritt MV, et al. Increased risk of neoplasm in appendicitis treated with interval appendectomy: single-institution experience and literature review. Am Surg. 2012;78:339-343.
27. Hayes D, Reiter S, Hagen E, et al. Is interval appendectomy really needed? A closer look at neoplasm rates in adult patients undergoing interval appendectomy after complicated appendicitis. Surg Endosc. 2021;35:3855-3860.
28. Peltrini R, Cantoni V, Green R, et al. Risk of appendiceal neoplasm after interval appendectomy for complicated appendicitis: a systematic review and meta-analysis. Surgeon. 2021. [Online ahead of print.]
29. Mällinen J, Rautio T, Grönroos J, et al. Risk of appendiceal neoplasm in periappendicular abscess in patients treated with interval appendectomy vs follow-up with magnetic resonance imaging: 1-year outcomes of the peri-appendicitis acuta randomized clinical trial. JAMA Surg. 2019;154:200-207.
30. Son J, Park YJ, Lee SR, et al. Increased risk of neoplasms in adult patients undergoing interval appendectomy. Ann Coloproctol. 2020;36:311-315.
31. Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977.
32. Theilen LH, Mellnick VM, Shanks AL, et al. Acute appendicitis in pregnancy: predictive clinical factors and pregnancy outcomes. Am J Perinatol. 2017;34:523-528.
33. Garcia EM, Camacho MA, Karolyi DR, et al. ACR Appropriateness Criteria right lower quadrant pain-suspected appendicitis. J Am Coll Radiol. 2018;15:S373-s387.
34. Kave M, Parooie F, Salarzaei M. Pregnancy and appendicitis: a systematic review and meta-analysis on the clinical use of MRI in diagnosis of appendicitis in pregnant women. World J Emerg Surg. 2019;14:37.
35. Repplinger MD, Levy JF, Peethumnongsin E, et al. Systematic review and meta-analysis of the accuracy of MRI to diagnose appendicitis in the general population. J Magn Reson Imaging. 2016;43:1346-1354.
36. Pearl JP, Price RR, Tonkin AE, et al. SAGES guidelines for the use of laparoscopy during pregnancy. Surg Endosc. 2017;31:3767-3782.
37. Zingone F, Sultan AA, Humes DJ, et al. Risk of acute appendicitis in and around pregnancy: a population-based cohort study from England. Ann Surg. 2015;261:332-337.
38. Andersson RE, Lambe M. Incidence of appendicitis during pregnancy. Int J Epidemiol. 2001;30:1281-1285.
39. Moltubak E, Landerholm K, Blomberg M, et al. Major variation in the incidence of appendicitis before, during and after pregnancy: a population-based cohort study. World J Surg. 2020;44:2601-2608.
40. Abbasi N, Patenaude V, Abenhaim HA. Management and outcomes of acute appendicitis in pregnancy-population-based study of over 7000 cases. BJOG. 2014;121:1509-1514.
41. Dongarwar D, Taylor J, Ajewole V, et al. Trends in appendicitis among pregnant women, the risk for cardiac arrest, and maternal-fetal mortality. World J Surg. 2020;44:3999-4005.
42. Sachs A, Guglielminotti J, Miller R, et al. Risk factors and risk stratification for adverse obstetrical outcomes after appendectomy or cholecystectomy during pregnancy. JAMA Surg. 2017;152:436-441.
43. Joo JI, Park HC, Kim MJ, et al. Outcomes of antibiotic therapy for uncomplicated appendicitis in pregnancy. Am J Med. 2017;130:1467-1469.
44. Lee SH, Lee JY, Choi YY, Lee JG. Laparoscopic appendectomy versus open appendectomy for suspected appendicitis during pregnancy: a systematic review and updated meta-analysis. BMC Surg. 2019;19:41.
45. Frountzas M, Nikolaou C, Stergios K, et al. Is the laparoscopic approach a safe choice for the management of acute appendicitis in pregnant women? A meta-analysis of observational studies. Ann R Coll Surg Engl. 2019;101:235-248.
46. Di Saverio S, Podda M, De Simone B, et al. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg. 2020;15:27.
47. Koberlein GC, Trout AT, Rigsby CK, et al. ACR Appropriateness Criteria suspected appendicitis-child. J Am Coll Radiol. 2019;16:S252-S263.
48. Maita S, Andersson B, Svensson JF, et al. Nonoperative treatment for nonperforated appendicitis in children: a systematic review and meta-analysis. Pediatr Surg Int. 2020;36:261-269.
49. Georgiou R, Eaton S, Stanton MP, et al. Efficacy and safety of nonoperative treatment for acute appendicitis: a meta-analysis. Pediatrics. 2017;139:e20163003.
CASE
A 35-year-old man with a body mass index of 20 presented to the emergency department after 24 hours of abdominal pain that began in the periumbilical region and then migrated to the right lower quadrant. The pain was exacerbated during ambulation and was intense when the car transporting him to the hospital encountered bumps in the road. After his pain started, he had associated anorexia, followed by nausea and emesis. He reported fever and chills. On examination, his temperature was 100.8 °F (38.2 °C), and palpation of the right and left lower quadrants elicited right lower quadrant pain. Laboratory evaluation revealed a white blood cell (WBC) count of 14,000 cells/mcL with 85% neutrophils, C-reactive protein of 40 mg/L, and a negative urinalysis.
How would you proceed with this patient?
Acute appendicitis is the most common cause of abdominal pain resulting in the need for surgical treatment; lifetime risk of appendicitis is 6% to 7%.1 Appendicitis is caused by intraluminal obstruction in the appendix from enlarged lymphoid tissue or a fecalith. The obstruction leads to elevated intraluminal pressure due to persistent mucus and gas production by bacteria, ultimately leading to ischemia and perforation.1 Additionally, obstruction leads to bacterial overgrowth, most commonly colonic flora such as Escherichia coli, Bacteroides fragilis, Streptococcus viridans, Enterococcus sp., Pseudomonas aeruginosa, and Klebsiella pneumoniaei.1,2
The following review provides a look at how 3 clinical scoring systems compare in the identification of acute appendicitis and details which imaging studies you should order—and when. But first, we’ll quickly detail the relevant physical findings and lab values that point to a diagnosis of acute appendicitis.
Physical findings. The patient typically first experiences vague abdominal pain that then localizes to the right lower quadrant due to peritoneal inflammation. Anorexia and nausea typically follow the abdominal pain. On examination, the patient often appears ill and exhibits abdominal guarding due to peritonitis. Tachycardia and fever are common; however, the absence of either does not exclude appendicitis. Classically, on palpation, the patient will have pain at McBurney’s point (one-third the distance from the anterior iliac spine to the umbilicus). The exact point of maximal tenderness can differ because of the varying anatomy of the appendix (retrocecal, paracolic, pelvic, pre/post ileal, promontoric, or subcecal).1 Right lower quadrant pain, abdominal rigidity, and radiation of periumbilical pain to the right lower quadrant are the most accurate findings in adults to rule in appendicitis.3 For children, physical exam findings have the highest likelihood in predicting appendicitis and include a positive Obturator sign, positive Rovsing sign, or a positive Psoas sign, and absent or decreased bowel sounds.4
Laboratory studies can support a diagnosis of appendicitis but cannot exclude it. Leukocytosis with neutrophil predominance is present in 90% of cases.5 An elevated C-reactive protein level renders the highest diagnostic accuracy.5 Perform a pregnancy test for any woman of child-bearing age, to assist in the diagnosis and guide imaging choices for evaluation. Additional laboratory tests are not needed unless there are concerns about volume depletion.
Clinical scoring systems
Several clinical scoring systems (TABLE6-10) have been validated to aid clinicians in evaluating patients with possible appendicitis, to decrease unnecessary exposure to ionizing radiation from computed tomography (CT) scans, to identify and reassure patients with low likelihoods of appendicitis, and to conduct outpatient follow-up.

Continue to: The Alvarado score
The Alvarado score is the oldest scoring rule, developed in 1986; it entails 8 clinical and laboratory variables.6 Ebell et al altered the proposed cutoff values of the Alvarado score to be low risk (< 4), intermediate risk (4-8), and high risk (≥ 9), effectively improving the sensitivity and specificity rates.7
In a meta-analysis of the Alvarado score that included 42 studies of men, women, and children, the sensitivity for “ruling out” appendicitis with a cutoff of 5 points was 96% for men, 99% for women, and 99% for children.8 The accuracy of a high-risk score (> 7) for “ruling in” appendicitis was less with an overall specificity of 82%.8 The Alvarado score did seem to overestimate appendicitis in women in all score categories.8
The Pediatric Appendicitis Score (PAS) is similar to Alvarado and was prospectively validated in 1170 children in 2002 for more specific guidance in this age group.9 The PAS had excellent specificity in the study; those with a score of ≥ 6 had a high probability of appendicitis. In a study comparing Alvarado with PAS in 311 patients, insignificant differences were noted at a score of ≥ 7 for both tests (sensitivity 86% vs 89%, and specificity 59% vs 50%, respectively).11 No scoring system has been found to be sufficiently accurate for use in children 4 years of age and younger.12
The Appendicitis Inflammatory Response (AIR) Score was prospectively validated in 545 patients representing all age groups.10 Subsequently, in a larger prospective multicenter study of 3878 patients older than 5 years, the original cut points were altered, thereby improving test sensitivity and negative predictive value to 99% for those with low probability (0 to 3), and test specificity to 98% for those with high-probability (9 to 12).13 Compared with the Alvarado Score, the AIR Score has higher specificity for those in the high-probability range, and similar exclusion rates in the low-probability range.14
Caveats with clinical decision scores. These tools are accepted and often used. However, challenges that affect generalizability of study data include differences in patient selection for each study (undifferentiated abdominal pain vs appendicitis), prospective vs retrospective designs, and age and gender variations in the patient populations. Despite the numerous scoring systems developed, none can accurately be used to rule in appendicitis. They are best used to assist in ruling out appendicitis and to aid in deciding for or against imaging.
Continue to: A look at the imaging options
A look at the imaging options
Abdominal CT has sensitivity and specificity rates between 76% and 100% and 83% and 100%, respectively.15,20,21 Ultrasonography has sensitivity and specificity rates of 71% to 94% and 81% to 98%, respectively.15,20,21 Formal US is reliable to confirm appendicitis, but less so to rule out appendicitis. Special considerations for imagining in pregnant patients and children are discussed in a bit.
Timing of surgical consultation
Surgical consultation is paramount once the diagnosis of appendicitis is probable. Imaging is best obtained prior to surgical consultation to streamline evaluation and enhance decision- making. Typically, patients will be categorized as complicated or uncomplicated based on the presence or absence of perforation, a gangrenous appendix, an intra-abdominal abscess (IAA), or purulent peritonitis. Active continuous surgical involvement (co-management or assumption of care) is recommended in all cases of appendicitis, especially if nonoperative management is selected, given that some cases must convert to immediate operative treatment or may be selected for delayed future (interval) appendectomy.22
Management
Uncomplicated appendicitis
Prompt appendectomy has been the gold standard of care for uncomplicated acute appendicitis for 60 years. However, several studies have investigated an antibiotic-based strategy rather than surgical treatment for uncomplicated appendicitis.
Antibiotics vs appendectomy. In 2020, the CODA Collaborative published a randomized trial comparing a 10-day course of antibiotics with appendectomy in patients with uncomplicated appendicitis. In this multicenter study based in the United States, 1552 patients 18 years of age or older were randomized to receive antibiotics or undergo appendectomy (95% performed laparoscopically). The antibiotic treatment consisted of at least 24 hours of IV antibiotics, with or without admission to the hospital. Antibiotic choice was individualized according to guidelines for intra-abdominal infection published by the Infectious Diseases Society of America, with the most common IV medications being ertapenem, cefoxitin, or metronidazole plus one of the following: ceftriaxone, cefazolin, or levofloxacin. For the remaining 10 days, oral metronidazole plus ciprofloxacin or cefdinir were used.22
Continue to: The primary endpoint...
The primary endpoint was the European Quality of Life-5 Dimensions (EQ-5D) questionnaire, with secondary outcomes including appendectomy in the antibiotics group and complications through 90 days. Exclusion criteria included pregnancy, sepsis, peritonitis, recurrent appendicitis, severe phlegmon on imaging, or evidence of neoplasm.22
Antibiotics were noninferior to appendectomy for the 30-day study. However, antibiotics failed in 29%, who then proceeded to appendectomy by 90 days; these patients also accounted for 41% of those with an appendicolith. Overall complications were more common in the antibiotics group than in the appendectomy group (8.1 vs 3.5 per 100 participants; 95% CI, 1.3-3.98). Also more common in the antibiotic group were serious adverse events (4 vs 3 per 100 participants; hazard ratio [HR] = 1.29; 95% CI, 0.67-2.50). The presence of an appendicolith in the antibiotics group increased the conversion risk to appendectomy, as well as adverse events risk.22
The takeaway. Antibiotic treatment is a noninferior method to treat acute uncomplicated appendicitis. However, the informed consent process is important, given the ~30% failure rate. Patient factors such as continued access to care should help inform the decision.
Two main surgical approaches exist for appendectomy: open and minimally invasive. At this time, the minimally invasive options include laparoscopic, single incision laparoscopic surgery (SILS), and robotic appendectomy. A study comparing cost, availability, or complications of these options has not been conducted at this time.
A large Cochrane review of 67 studies examining open vs laparoscopic appendectomy in adults and children completed in 2018 revealed that the laparoscopic approach reduced early postoperative pain intensity and led to a shorter hospital stay, earlier return to work or usual activities, and a decrease in wound infections.23 The odds of IAA occurring with laparoscopic appendectomy increased by 65% compared with an open procedure; however, postoperative bowel obstruction and incisional hernias were less likely to occur.23 Additionally, following laparoscopic surgery, postoperative bowel obstruction and incisional hernias are less likely to occur. The laparoscopic approach is preferred due to overall increased patient satisfaction and a reduction in most, if not all, complications.
Continue to: Complicated appendicitis
Complicated appendicitis
Excluding patients with severe sepsis or purulent peritonitis requiring resuscitation and immediate surgical intervention of intra-abdominal infection, the approach to patients with complicated appendicitis varies between aggressive surgical intervention and nonoperative management.
In a 2007 meta-analysis reviewing nonsurgical treatment of appendiceal abscess/phlegmon, immediate surgery was associated with higher morbidity.24 Within the nonoperative management group 7.2% (CI, 4.0-10.5) required surgical intervention and 19.7% (CI, 11.0-28.3) required abscess drainage. Malignant disease was detected in 1.2% (CI, 0.6-1.7).24 Small subsequent studies concluded different results.25
Ultimately, the 2015 European Association of Endoscopic Surgery guidelines recommend a new systematic review; but with current data, initial nonoperative management is preferred.15 After initial nonoperative treatment, the only benefits from interval appendectomy are identification of an underlying malignancy (6% to 20%) and mitigating the risk of recurrent appendicitis (5% to 44%).15,25-30
Multiple single institutional series found increased neoplasm incidence (9% to 20%) in complicated appendicitis in patients 40 years and older.26-30 Prior to interval appendectomy in patients 45 years and older, ensuring they have an up-to-date screening colonoscopy is important. This is in line with 2021 US Preventive Services Task Force (Grade “B” recommendation), 2018 American Cancer Society (qualified recommendation), and 2021 American College of Gastroenterology (conditional recommendation) guidelines for colorectal cancer screening to start at age 45 in average-risk patients.31 Patients younger than 45 can consider screening through shared decision-making.
Special populations
Pregnant patients
In pregnancy, challenges exist with the presence of traditional signs and symptoms of appendicitis, with the most predictive sign being a WBC count higher than 18,000.32 The American College of Radiology’s (ACR) Appropriateness Criteria recommend US as the imaging modality of choice in pregnancy, with MRI as the best option when US is inconclusive.33 Two meta-analyses demonstrated high sensitivity (91.8%-96.6%) and specificity (95.9%-97.9%) of MRI in diagnosing appendicitis.34,35 CT scan is not the preferred initial imagining modality in pregnancy unless urgent information is needed and other modalities are insufficient or unavailable.36
Continue to: The most common...
The most common nonobstetric surgical intervention during pregnancy is appendectomy, at a rate of 6.3/10,000 person-years, which increases to 9.9/10,000 in the postpartum period.37 Two large population studies demonstrate the rate of appendicitis varies over the course of pregnancy, with the lowest rates in the third trimester,38,39 and a significant rebound lasting for 2 years postpartum.39 Peritonitis, septic shock, pneumonia, postoperative infection, and longer hospital stays occur more frequently in pregnant women than in nonpregnant women with appendicitis.40 Fetal loss is higher in the first trimester.32
In a 14-year review of 63,145 appendicitis cases, an increased risk of fetal loss and maternal death was noted across ages and ethnicities, with the largest risk of maternal death occurring in Hispanics and fetal death in non-Hispanic Blacks.41 In a large study of 1018 adverse events after appendectomy or cholecystectomy, the 3 most common events were preterm delivery (35.4%), preterm labor without preterm delivery (26.4%), and miscarriage (25.7%).42 The surgery itself was not a major risk factor for adverse events. Major risk factors included cervical incompetence (odds ratio [OR] = 24.3), preterm labor in current pregnancy (OR = 18.3), and presence of vulvovaginitis (OR = 5.2).42
Nonoperative management in pregnancy is not recommended; only 1 prospective trial has been done, with 20 patients, showing a 25% failure rate.43 Two meta-analyses published in 2019 highlight the potential increase of fetal loss with laparoscopic approaches to appendectomy.44,45 However, recently published literature demonstrates no significant maternal-fetal morbidity. Current guidelines of the Society of American Gastrointestinal and Endoscopic Surgeons agree that laparoscopy is the operative choice in pregnancy.36
Children
Acute appendicitis is the most common surgical emergency in children.4 Physical exam findings and laboratory results are not classic in this population, obtaining an accurate history can be challenging, and results of clinical scoring systems can be inconclusive.4 Additional serum biomarkers, procalcitonin and calprotectin, are gaining evidence for use in improving scoring systems to refine low-risk groups. Unavailability of timely, reliable biomarker testing in rural practice locations limits definitive recommendations at this time.46 ACR recommends no imaging in a pediatric patient whose risk of having appendicitis is low based on any of several scoring systems.47 For those assessed as having higher risk, US is the recommended initial modality,with CT with IV contrast or MRI without contrast equally recommended if the US is equivocal.47
Despite promising data from trials of nonoperative treatment for adults with appendicitis, no definitive evidence and recommendations are available for children. Two systematic reviews show nonoperative treatment is safe, with an efficacy rate of 76% to 82% at long-term follow-up,48,49 although the success of antibiotic regimens varies. Within the nonoperative treatment group, 16% of patients had appendectomy during the follow-up period, which varied from 8 weeks to 4 years.48 A randomized controlled trial is needed for final guidance.
Continue to: CASE
CASE
The patient had an Alvarado score of 9 (high probability) and an AIR score of 6 (intermediate probability). A CT with IV contrast showed a 9-mm fluid-filled appendix with periappendiceal fluid. During surgical consultation, he was offered laparoscopic appendectomy or nonoperative treatment with antibiotics. He opted for a preoperative dose of piperacillin-tazobactam 3.375 g IV and laparoscopic appendectomy. The patient was discharged home 6 hours after his procedure.
CORRESPONDENCE
Jessica Servey, MD, MHPE, 4301 Jones Bridge Road, Bethesda, MD 20814; [email protected]
CASE
A 35-year-old man with a body mass index of 20 presented to the emergency department after 24 hours of abdominal pain that began in the periumbilical region and then migrated to the right lower quadrant. The pain was exacerbated during ambulation and was intense when the car transporting him to the hospital encountered bumps in the road. After his pain started, he had associated anorexia, followed by nausea and emesis. He reported fever and chills. On examination, his temperature was 100.8 °F (38.2 °C), and palpation of the right and left lower quadrants elicited right lower quadrant pain. Laboratory evaluation revealed a white blood cell (WBC) count of 14,000 cells/mcL with 85% neutrophils, C-reactive protein of 40 mg/L, and a negative urinalysis.
How would you proceed with this patient?
Acute appendicitis is the most common cause of abdominal pain resulting in the need for surgical treatment; lifetime risk of appendicitis is 6% to 7%.1 Appendicitis is caused by intraluminal obstruction in the appendix from enlarged lymphoid tissue or a fecalith. The obstruction leads to elevated intraluminal pressure due to persistent mucus and gas production by bacteria, ultimately leading to ischemia and perforation.1 Additionally, obstruction leads to bacterial overgrowth, most commonly colonic flora such as Escherichia coli, Bacteroides fragilis, Streptococcus viridans, Enterococcus sp., Pseudomonas aeruginosa, and Klebsiella pneumoniaei.1,2
The following review provides a look at how 3 clinical scoring systems compare in the identification of acute appendicitis and details which imaging studies you should order—and when. But first, we’ll quickly detail the relevant physical findings and lab values that point to a diagnosis of acute appendicitis.
Physical findings. The patient typically first experiences vague abdominal pain that then localizes to the right lower quadrant due to peritoneal inflammation. Anorexia and nausea typically follow the abdominal pain. On examination, the patient often appears ill and exhibits abdominal guarding due to peritonitis. Tachycardia and fever are common; however, the absence of either does not exclude appendicitis. Classically, on palpation, the patient will have pain at McBurney’s point (one-third the distance from the anterior iliac spine to the umbilicus). The exact point of maximal tenderness can differ because of the varying anatomy of the appendix (retrocecal, paracolic, pelvic, pre/post ileal, promontoric, or subcecal).1 Right lower quadrant pain, abdominal rigidity, and radiation of periumbilical pain to the right lower quadrant are the most accurate findings in adults to rule in appendicitis.3 For children, physical exam findings have the highest likelihood in predicting appendicitis and include a positive Obturator sign, positive Rovsing sign, or a positive Psoas sign, and absent or decreased bowel sounds.4
Laboratory studies can support a diagnosis of appendicitis but cannot exclude it. Leukocytosis with neutrophil predominance is present in 90% of cases.5 An elevated C-reactive protein level renders the highest diagnostic accuracy.5 Perform a pregnancy test for any woman of child-bearing age, to assist in the diagnosis and guide imaging choices for evaluation. Additional laboratory tests are not needed unless there are concerns about volume depletion.
Clinical scoring systems
Several clinical scoring systems (TABLE6-10) have been validated to aid clinicians in evaluating patients with possible appendicitis, to decrease unnecessary exposure to ionizing radiation from computed tomography (CT) scans, to identify and reassure patients with low likelihoods of appendicitis, and to conduct outpatient follow-up.

Continue to: The Alvarado score
The Alvarado score is the oldest scoring rule, developed in 1986; it entails 8 clinical and laboratory variables.6 Ebell et al altered the proposed cutoff values of the Alvarado score to be low risk (< 4), intermediate risk (4-8), and high risk (≥ 9), effectively improving the sensitivity and specificity rates.7
In a meta-analysis of the Alvarado score that included 42 studies of men, women, and children, the sensitivity for “ruling out” appendicitis with a cutoff of 5 points was 96% for men, 99% for women, and 99% for children.8 The accuracy of a high-risk score (> 7) for “ruling in” appendicitis was less with an overall specificity of 82%.8 The Alvarado score did seem to overestimate appendicitis in women in all score categories.8
The Pediatric Appendicitis Score (PAS) is similar to Alvarado and was prospectively validated in 1170 children in 2002 for more specific guidance in this age group.9 The PAS had excellent specificity in the study; those with a score of ≥ 6 had a high probability of appendicitis. In a study comparing Alvarado with PAS in 311 patients, insignificant differences were noted at a score of ≥ 7 for both tests (sensitivity 86% vs 89%, and specificity 59% vs 50%, respectively).11 No scoring system has been found to be sufficiently accurate for use in children 4 years of age and younger.12
The Appendicitis Inflammatory Response (AIR) Score was prospectively validated in 545 patients representing all age groups.10 Subsequently, in a larger prospective multicenter study of 3878 patients older than 5 years, the original cut points were altered, thereby improving test sensitivity and negative predictive value to 99% for those with low probability (0 to 3), and test specificity to 98% for those with high-probability (9 to 12).13 Compared with the Alvarado Score, the AIR Score has higher specificity for those in the high-probability range, and similar exclusion rates in the low-probability range.14
Caveats with clinical decision scores. These tools are accepted and often used. However, challenges that affect generalizability of study data include differences in patient selection for each study (undifferentiated abdominal pain vs appendicitis), prospective vs retrospective designs, and age and gender variations in the patient populations. Despite the numerous scoring systems developed, none can accurately be used to rule in appendicitis. They are best used to assist in ruling out appendicitis and to aid in deciding for or against imaging.
Continue to: A look at the imaging options
A look at the imaging options
Abdominal CT has sensitivity and specificity rates between 76% and 100% and 83% and 100%, respectively.15,20,21 Ultrasonography has sensitivity and specificity rates of 71% to 94% and 81% to 98%, respectively.15,20,21 Formal US is reliable to confirm appendicitis, but less so to rule out appendicitis. Special considerations for imagining in pregnant patients and children are discussed in a bit.
Timing of surgical consultation
Surgical consultation is paramount once the diagnosis of appendicitis is probable. Imaging is best obtained prior to surgical consultation to streamline evaluation and enhance decision- making. Typically, patients will be categorized as complicated or uncomplicated based on the presence or absence of perforation, a gangrenous appendix, an intra-abdominal abscess (IAA), or purulent peritonitis. Active continuous surgical involvement (co-management or assumption of care) is recommended in all cases of appendicitis, especially if nonoperative management is selected, given that some cases must convert to immediate operative treatment or may be selected for delayed future (interval) appendectomy.22
Management
Uncomplicated appendicitis
Prompt appendectomy has been the gold standard of care for uncomplicated acute appendicitis for 60 years. However, several studies have investigated an antibiotic-based strategy rather than surgical treatment for uncomplicated appendicitis.
Antibiotics vs appendectomy. In 2020, the CODA Collaborative published a randomized trial comparing a 10-day course of antibiotics with appendectomy in patients with uncomplicated appendicitis. In this multicenter study based in the United States, 1552 patients 18 years of age or older were randomized to receive antibiotics or undergo appendectomy (95% performed laparoscopically). The antibiotic treatment consisted of at least 24 hours of IV antibiotics, with or without admission to the hospital. Antibiotic choice was individualized according to guidelines for intra-abdominal infection published by the Infectious Diseases Society of America, with the most common IV medications being ertapenem, cefoxitin, or metronidazole plus one of the following: ceftriaxone, cefazolin, or levofloxacin. For the remaining 10 days, oral metronidazole plus ciprofloxacin or cefdinir were used.22
Continue to: The primary endpoint...
The primary endpoint was the European Quality of Life-5 Dimensions (EQ-5D) questionnaire, with secondary outcomes including appendectomy in the antibiotics group and complications through 90 days. Exclusion criteria included pregnancy, sepsis, peritonitis, recurrent appendicitis, severe phlegmon on imaging, or evidence of neoplasm.22
Antibiotics were noninferior to appendectomy for the 30-day study. However, antibiotics failed in 29%, who then proceeded to appendectomy by 90 days; these patients also accounted for 41% of those with an appendicolith. Overall complications were more common in the antibiotics group than in the appendectomy group (8.1 vs 3.5 per 100 participants; 95% CI, 1.3-3.98). Also more common in the antibiotic group were serious adverse events (4 vs 3 per 100 participants; hazard ratio [HR] = 1.29; 95% CI, 0.67-2.50). The presence of an appendicolith in the antibiotics group increased the conversion risk to appendectomy, as well as adverse events risk.22
The takeaway. Antibiotic treatment is a noninferior method to treat acute uncomplicated appendicitis. However, the informed consent process is important, given the ~30% failure rate. Patient factors such as continued access to care should help inform the decision.
Two main surgical approaches exist for appendectomy: open and minimally invasive. At this time, the minimally invasive options include laparoscopic, single incision laparoscopic surgery (SILS), and robotic appendectomy. A study comparing cost, availability, or complications of these options has not been conducted at this time.
A large Cochrane review of 67 studies examining open vs laparoscopic appendectomy in adults and children completed in 2018 revealed that the laparoscopic approach reduced early postoperative pain intensity and led to a shorter hospital stay, earlier return to work or usual activities, and a decrease in wound infections.23 The odds of IAA occurring with laparoscopic appendectomy increased by 65% compared with an open procedure; however, postoperative bowel obstruction and incisional hernias were less likely to occur.23 Additionally, following laparoscopic surgery, postoperative bowel obstruction and incisional hernias are less likely to occur. The laparoscopic approach is preferred due to overall increased patient satisfaction and a reduction in most, if not all, complications.
Continue to: Complicated appendicitis
Complicated appendicitis
Excluding patients with severe sepsis or purulent peritonitis requiring resuscitation and immediate surgical intervention of intra-abdominal infection, the approach to patients with complicated appendicitis varies between aggressive surgical intervention and nonoperative management.
In a 2007 meta-analysis reviewing nonsurgical treatment of appendiceal abscess/phlegmon, immediate surgery was associated with higher morbidity.24 Within the nonoperative management group 7.2% (CI, 4.0-10.5) required surgical intervention and 19.7% (CI, 11.0-28.3) required abscess drainage. Malignant disease was detected in 1.2% (CI, 0.6-1.7).24 Small subsequent studies concluded different results.25
Ultimately, the 2015 European Association of Endoscopic Surgery guidelines recommend a new systematic review; but with current data, initial nonoperative management is preferred.15 After initial nonoperative treatment, the only benefits from interval appendectomy are identification of an underlying malignancy (6% to 20%) and mitigating the risk of recurrent appendicitis (5% to 44%).15,25-30
Multiple single institutional series found increased neoplasm incidence (9% to 20%) in complicated appendicitis in patients 40 years and older.26-30 Prior to interval appendectomy in patients 45 years and older, ensuring they have an up-to-date screening colonoscopy is important. This is in line with 2021 US Preventive Services Task Force (Grade “B” recommendation), 2018 American Cancer Society (qualified recommendation), and 2021 American College of Gastroenterology (conditional recommendation) guidelines for colorectal cancer screening to start at age 45 in average-risk patients.31 Patients younger than 45 can consider screening through shared decision-making.
Special populations
Pregnant patients
In pregnancy, challenges exist with the presence of traditional signs and symptoms of appendicitis, with the most predictive sign being a WBC count higher than 18,000.32 The American College of Radiology’s (ACR) Appropriateness Criteria recommend US as the imaging modality of choice in pregnancy, with MRI as the best option when US is inconclusive.33 Two meta-analyses demonstrated high sensitivity (91.8%-96.6%) and specificity (95.9%-97.9%) of MRI in diagnosing appendicitis.34,35 CT scan is not the preferred initial imagining modality in pregnancy unless urgent information is needed and other modalities are insufficient or unavailable.36
Continue to: The most common...
The most common nonobstetric surgical intervention during pregnancy is appendectomy, at a rate of 6.3/10,000 person-years, which increases to 9.9/10,000 in the postpartum period.37 Two large population studies demonstrate the rate of appendicitis varies over the course of pregnancy, with the lowest rates in the third trimester,38,39 and a significant rebound lasting for 2 years postpartum.39 Peritonitis, septic shock, pneumonia, postoperative infection, and longer hospital stays occur more frequently in pregnant women than in nonpregnant women with appendicitis.40 Fetal loss is higher in the first trimester.32
In a 14-year review of 63,145 appendicitis cases, an increased risk of fetal loss and maternal death was noted across ages and ethnicities, with the largest risk of maternal death occurring in Hispanics and fetal death in non-Hispanic Blacks.41 In a large study of 1018 adverse events after appendectomy or cholecystectomy, the 3 most common events were preterm delivery (35.4%), preterm labor without preterm delivery (26.4%), and miscarriage (25.7%).42 The surgery itself was not a major risk factor for adverse events. Major risk factors included cervical incompetence (odds ratio [OR] = 24.3), preterm labor in current pregnancy (OR = 18.3), and presence of vulvovaginitis (OR = 5.2).42
Nonoperative management in pregnancy is not recommended; only 1 prospective trial has been done, with 20 patients, showing a 25% failure rate.43 Two meta-analyses published in 2019 highlight the potential increase of fetal loss with laparoscopic approaches to appendectomy.44,45 However, recently published literature demonstrates no significant maternal-fetal morbidity. Current guidelines of the Society of American Gastrointestinal and Endoscopic Surgeons agree that laparoscopy is the operative choice in pregnancy.36
Children
Acute appendicitis is the most common surgical emergency in children.4 Physical exam findings and laboratory results are not classic in this population, obtaining an accurate history can be challenging, and results of clinical scoring systems can be inconclusive.4 Additional serum biomarkers, procalcitonin and calprotectin, are gaining evidence for use in improving scoring systems to refine low-risk groups. Unavailability of timely, reliable biomarker testing in rural practice locations limits definitive recommendations at this time.46 ACR recommends no imaging in a pediatric patient whose risk of having appendicitis is low based on any of several scoring systems.47 For those assessed as having higher risk, US is the recommended initial modality,with CT with IV contrast or MRI without contrast equally recommended if the US is equivocal.47
Despite promising data from trials of nonoperative treatment for adults with appendicitis, no definitive evidence and recommendations are available for children. Two systematic reviews show nonoperative treatment is safe, with an efficacy rate of 76% to 82% at long-term follow-up,48,49 although the success of antibiotic regimens varies. Within the nonoperative treatment group, 16% of patients had appendectomy during the follow-up period, which varied from 8 weeks to 4 years.48 A randomized controlled trial is needed for final guidance.
Continue to: CASE
CASE
The patient had an Alvarado score of 9 (high probability) and an AIR score of 6 (intermediate probability). A CT with IV contrast showed a 9-mm fluid-filled appendix with periappendiceal fluid. During surgical consultation, he was offered laparoscopic appendectomy or nonoperative treatment with antibiotics. He opted for a preoperative dose of piperacillin-tazobactam 3.375 g IV and laparoscopic appendectomy. The patient was discharged home 6 hours after his procedure.
CORRESPONDENCE
Jessica Servey, MD, MHPE, 4301 Jones Bridge Road, Bethesda, MD 20814; [email protected]
1. Prystowsky JB, Pugh CM, Nagle AP. Current problems in surgery. Appendicitis. Curr Probl Surg. 2005;42:688-742.
2. Song DW, Park BK, Suh SW, et al. Bacterial culture and antibiotic susceptibility in patients with acute appendicitis. Int J Colorectal Dis. 2018;33:441-447.
3. Wagner JM, McKinney WP, Carpenter JL. Does this patient have appendicitis? JAMA. 1996;276:1589-1594.
4. Benabbas R, Hanna M, Shah J, et al. Diagnostic accuracy of history, physical examination, laboratory tests, and point-of-care ultrasound for pediatric acute appendicitis in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2017;24:523-551.
5. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg. 2004;91:28-37.
6. Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986;15:557-564.
7. Ebell MH, Shinholser J. What are the most clinically useful cutoffs for the Alvarado and Pediatric Appendicitis Scores? A systematic review. Ann Emerg Med. 2014;64:365-372.e2.
8. Ohle R, O’Reilly F, O’Brien KK, et al. The Alvarado score for predicting acute appendicitis: a systematic review. BMC Med. 2011;9:139.
9. Samuel M. Pediatric appendicitis score. J Pediatr Surg. 2002;37:877-881.
10. Andersson M, Andersson RE. The appendicitis inflammatory response score: a tool for the diagnosis of acute appendicitis that outperforms the Alvarado score. World J Surg. 2008;32:1843-1849.
11. Pogorelić Z, Rak S, Mrklić I, et al. Prospective validation of Alvarado score and Pediatric Appendicitis Score for the diagnosis of acute appendicitis in children. Pediatr Emerg Care. 2015;31:164-168.
12. Rassi R, Muse F, Sánchez-Martínez J, et al. Diagnostic value of clinical prediction scores for acute appendicitis in children younger than 4 years. Eur J Pediatr Surg. 2021. [Online ahead of print]
13. Andersson M, Kolodziej B, Andersson RE. Validation of the Appendicitis Inflammatory Response (AIR) score. World J Surg. 2021;45:2081-2091.
14. Kollár D, McCartan DP, Bourke M, et al. Predicting acute appendicitis? A comparison of the Alvarado score, the Appendicitis Inflammatory Response Score and clinical assessment. World J Surg. 2015;39:104-109.
15. Gorter RR, Eker HH, Gorter-Stam MA, et al. Diagnosis and management of acute appendicitis. EAES consensus development conference 2015. Surg Endosc. 2016;30:4668-4690.
16. Matthew Fields J, Davis J, Alsup C, et al. Accuracy of point-of-care ultrasonography for diagnosing acute appendicitis: a systematic review and meta-analysis. Acad Emerg Med. 2017;24:1124-1136.
17. Sharif S, Skitch S, Vlahaki D, et al. Point-of-care ultrasound to diagnose appendicitis in a Canadian emergency department. CJEM. 2018;20:732-735.
18. Doniger SJ, Kornblith A. Point-of-care ultrasound integrated into a staged diagnostic algorithm for pediatric appendicitis. Pediatr Emerg Care. 2018;34:109-115.
19. Menon N, Kumar S, Keeler B, et al. A systematic review of point-of-care abdominal ultrasound scans performed by general surgeons. Surgeon. 2021. [Online ahead of print]
20. Doria AS, Moineddin R, Kellenberger CJ, et al. US or CT for diagnosis of appendicitis in children and adults? A meta-analysis. Radiology. 2006;241:83-94.
21. van Randen A, Laméris W, van Es HW, et al. A comparison of the accuracy of ultrasound and computed tomography in common diagnoses causing acute abdominal pain. Eur Radiol. 2011;21:1535-1545.
22. Flum DR, Davidson GH, Monsell SE, et al. A randomized trial comparing antibiotics with appendectomy for appendicitis. N Engl J Med. 2020;383:1907-1919.
23. Jaschinski T, Mosch CG, Eikermann M, et al. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2018;11:CD001546.
24. Andersson RE, Petzold MG. Nonsurgical treatment of appendiceal abscess or phlegmon: a systematic review and meta-analysis. Ann Surg. 2007;246:741-748.
25. Deelder JD, Richir MC, Schoorl T, et al. How to treat an appendiceal inflammatory mass: operatively or nonoperatively? J Gastrointest Surg. 2014;18:641-645.
26. Carpenter SG, Chapital AB, Merritt MV, et al. Increased risk of neoplasm in appendicitis treated with interval appendectomy: single-institution experience and literature review. Am Surg. 2012;78:339-343.
27. Hayes D, Reiter S, Hagen E, et al. Is interval appendectomy really needed? A closer look at neoplasm rates in adult patients undergoing interval appendectomy after complicated appendicitis. Surg Endosc. 2021;35:3855-3860.
28. Peltrini R, Cantoni V, Green R, et al. Risk of appendiceal neoplasm after interval appendectomy for complicated appendicitis: a systematic review and meta-analysis. Surgeon. 2021. [Online ahead of print.]
29. Mällinen J, Rautio T, Grönroos J, et al. Risk of appendiceal neoplasm in periappendicular abscess in patients treated with interval appendectomy vs follow-up with magnetic resonance imaging: 1-year outcomes of the peri-appendicitis acuta randomized clinical trial. JAMA Surg. 2019;154:200-207.
30. Son J, Park YJ, Lee SR, et al. Increased risk of neoplasms in adult patients undergoing interval appendectomy. Ann Coloproctol. 2020;36:311-315.
31. Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977.
32. Theilen LH, Mellnick VM, Shanks AL, et al. Acute appendicitis in pregnancy: predictive clinical factors and pregnancy outcomes. Am J Perinatol. 2017;34:523-528.
33. Garcia EM, Camacho MA, Karolyi DR, et al. ACR Appropriateness Criteria right lower quadrant pain-suspected appendicitis. J Am Coll Radiol. 2018;15:S373-s387.
34. Kave M, Parooie F, Salarzaei M. Pregnancy and appendicitis: a systematic review and meta-analysis on the clinical use of MRI in diagnosis of appendicitis in pregnant women. World J Emerg Surg. 2019;14:37.
35. Repplinger MD, Levy JF, Peethumnongsin E, et al. Systematic review and meta-analysis of the accuracy of MRI to diagnose appendicitis in the general population. J Magn Reson Imaging. 2016;43:1346-1354.
36. Pearl JP, Price RR, Tonkin AE, et al. SAGES guidelines for the use of laparoscopy during pregnancy. Surg Endosc. 2017;31:3767-3782.
37. Zingone F, Sultan AA, Humes DJ, et al. Risk of acute appendicitis in and around pregnancy: a population-based cohort study from England. Ann Surg. 2015;261:332-337.
38. Andersson RE, Lambe M. Incidence of appendicitis during pregnancy. Int J Epidemiol. 2001;30:1281-1285.
39. Moltubak E, Landerholm K, Blomberg M, et al. Major variation in the incidence of appendicitis before, during and after pregnancy: a population-based cohort study. World J Surg. 2020;44:2601-2608.
40. Abbasi N, Patenaude V, Abenhaim HA. Management and outcomes of acute appendicitis in pregnancy-population-based study of over 7000 cases. BJOG. 2014;121:1509-1514.
41. Dongarwar D, Taylor J, Ajewole V, et al. Trends in appendicitis among pregnant women, the risk for cardiac arrest, and maternal-fetal mortality. World J Surg. 2020;44:3999-4005.
42. Sachs A, Guglielminotti J, Miller R, et al. Risk factors and risk stratification for adverse obstetrical outcomes after appendectomy or cholecystectomy during pregnancy. JAMA Surg. 2017;152:436-441.
43. Joo JI, Park HC, Kim MJ, et al. Outcomes of antibiotic therapy for uncomplicated appendicitis in pregnancy. Am J Med. 2017;130:1467-1469.
44. Lee SH, Lee JY, Choi YY, Lee JG. Laparoscopic appendectomy versus open appendectomy for suspected appendicitis during pregnancy: a systematic review and updated meta-analysis. BMC Surg. 2019;19:41.
45. Frountzas M, Nikolaou C, Stergios K, et al. Is the laparoscopic approach a safe choice for the management of acute appendicitis in pregnant women? A meta-analysis of observational studies. Ann R Coll Surg Engl. 2019;101:235-248.
46. Di Saverio S, Podda M, De Simone B, et al. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg. 2020;15:27.
47. Koberlein GC, Trout AT, Rigsby CK, et al. ACR Appropriateness Criteria suspected appendicitis-child. J Am Coll Radiol. 2019;16:S252-S263.
48. Maita S, Andersson B, Svensson JF, et al. Nonoperative treatment for nonperforated appendicitis in children: a systematic review and meta-analysis. Pediatr Surg Int. 2020;36:261-269.
49. Georgiou R, Eaton S, Stanton MP, et al. Efficacy and safety of nonoperative treatment for acute appendicitis: a meta-analysis. Pediatrics. 2017;139:e20163003.
1. Prystowsky JB, Pugh CM, Nagle AP. Current problems in surgery. Appendicitis. Curr Probl Surg. 2005;42:688-742.
2. Song DW, Park BK, Suh SW, et al. Bacterial culture and antibiotic susceptibility in patients with acute appendicitis. Int J Colorectal Dis. 2018;33:441-447.
3. Wagner JM, McKinney WP, Carpenter JL. Does this patient have appendicitis? JAMA. 1996;276:1589-1594.
4. Benabbas R, Hanna M, Shah J, et al. Diagnostic accuracy of history, physical examination, laboratory tests, and point-of-care ultrasound for pediatric acute appendicitis in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2017;24:523-551.
5. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg. 2004;91:28-37.
6. Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986;15:557-564.
7. Ebell MH, Shinholser J. What are the most clinically useful cutoffs for the Alvarado and Pediatric Appendicitis Scores? A systematic review. Ann Emerg Med. 2014;64:365-372.e2.
8. Ohle R, O’Reilly F, O’Brien KK, et al. The Alvarado score for predicting acute appendicitis: a systematic review. BMC Med. 2011;9:139.
9. Samuel M. Pediatric appendicitis score. J Pediatr Surg. 2002;37:877-881.
10. Andersson M, Andersson RE. The appendicitis inflammatory response score: a tool for the diagnosis of acute appendicitis that outperforms the Alvarado score. World J Surg. 2008;32:1843-1849.
11. Pogorelić Z, Rak S, Mrklić I, et al. Prospective validation of Alvarado score and Pediatric Appendicitis Score for the diagnosis of acute appendicitis in children. Pediatr Emerg Care. 2015;31:164-168.
12. Rassi R, Muse F, Sánchez-Martínez J, et al. Diagnostic value of clinical prediction scores for acute appendicitis in children younger than 4 years. Eur J Pediatr Surg. 2021. [Online ahead of print]
13. Andersson M, Kolodziej B, Andersson RE. Validation of the Appendicitis Inflammatory Response (AIR) score. World J Surg. 2021;45:2081-2091.
14. Kollár D, McCartan DP, Bourke M, et al. Predicting acute appendicitis? A comparison of the Alvarado score, the Appendicitis Inflammatory Response Score and clinical assessment. World J Surg. 2015;39:104-109.
15. Gorter RR, Eker HH, Gorter-Stam MA, et al. Diagnosis and management of acute appendicitis. EAES consensus development conference 2015. Surg Endosc. 2016;30:4668-4690.
16. Matthew Fields J, Davis J, Alsup C, et al. Accuracy of point-of-care ultrasonography for diagnosing acute appendicitis: a systematic review and meta-analysis. Acad Emerg Med. 2017;24:1124-1136.
17. Sharif S, Skitch S, Vlahaki D, et al. Point-of-care ultrasound to diagnose appendicitis in a Canadian emergency department. CJEM. 2018;20:732-735.
18. Doniger SJ, Kornblith A. Point-of-care ultrasound integrated into a staged diagnostic algorithm for pediatric appendicitis. Pediatr Emerg Care. 2018;34:109-115.
19. Menon N, Kumar S, Keeler B, et al. A systematic review of point-of-care abdominal ultrasound scans performed by general surgeons. Surgeon. 2021. [Online ahead of print]
20. Doria AS, Moineddin R, Kellenberger CJ, et al. US or CT for diagnosis of appendicitis in children and adults? A meta-analysis. Radiology. 2006;241:83-94.
21. van Randen A, Laméris W, van Es HW, et al. A comparison of the accuracy of ultrasound and computed tomography in common diagnoses causing acute abdominal pain. Eur Radiol. 2011;21:1535-1545.
22. Flum DR, Davidson GH, Monsell SE, et al. A randomized trial comparing antibiotics with appendectomy for appendicitis. N Engl J Med. 2020;383:1907-1919.
23. Jaschinski T, Mosch CG, Eikermann M, et al. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2018;11:CD001546.
24. Andersson RE, Petzold MG. Nonsurgical treatment of appendiceal abscess or phlegmon: a systematic review and meta-analysis. Ann Surg. 2007;246:741-748.
25. Deelder JD, Richir MC, Schoorl T, et al. How to treat an appendiceal inflammatory mass: operatively or nonoperatively? J Gastrointest Surg. 2014;18:641-645.
26. Carpenter SG, Chapital AB, Merritt MV, et al. Increased risk of neoplasm in appendicitis treated with interval appendectomy: single-institution experience and literature review. Am Surg. 2012;78:339-343.
27. Hayes D, Reiter S, Hagen E, et al. Is interval appendectomy really needed? A closer look at neoplasm rates in adult patients undergoing interval appendectomy after complicated appendicitis. Surg Endosc. 2021;35:3855-3860.
28. Peltrini R, Cantoni V, Green R, et al. Risk of appendiceal neoplasm after interval appendectomy for complicated appendicitis: a systematic review and meta-analysis. Surgeon. 2021. [Online ahead of print.]
29. Mällinen J, Rautio T, Grönroos J, et al. Risk of appendiceal neoplasm in periappendicular abscess in patients treated with interval appendectomy vs follow-up with magnetic resonance imaging: 1-year outcomes of the peri-appendicitis acuta randomized clinical trial. JAMA Surg. 2019;154:200-207.
30. Son J, Park YJ, Lee SR, et al. Increased risk of neoplasms in adult patients undergoing interval appendectomy. Ann Coloproctol. 2020;36:311-315.
31. Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977.
32. Theilen LH, Mellnick VM, Shanks AL, et al. Acute appendicitis in pregnancy: predictive clinical factors and pregnancy outcomes. Am J Perinatol. 2017;34:523-528.
33. Garcia EM, Camacho MA, Karolyi DR, et al. ACR Appropriateness Criteria right lower quadrant pain-suspected appendicitis. J Am Coll Radiol. 2018;15:S373-s387.
34. Kave M, Parooie F, Salarzaei M. Pregnancy and appendicitis: a systematic review and meta-analysis on the clinical use of MRI in diagnosis of appendicitis in pregnant women. World J Emerg Surg. 2019;14:37.
35. Repplinger MD, Levy JF, Peethumnongsin E, et al. Systematic review and meta-analysis of the accuracy of MRI to diagnose appendicitis in the general population. J Magn Reson Imaging. 2016;43:1346-1354.
36. Pearl JP, Price RR, Tonkin AE, et al. SAGES guidelines for the use of laparoscopy during pregnancy. Surg Endosc. 2017;31:3767-3782.
37. Zingone F, Sultan AA, Humes DJ, et al. Risk of acute appendicitis in and around pregnancy: a population-based cohort study from England. Ann Surg. 2015;261:332-337.
38. Andersson RE, Lambe M. Incidence of appendicitis during pregnancy. Int J Epidemiol. 2001;30:1281-1285.
39. Moltubak E, Landerholm K, Blomberg M, et al. Major variation in the incidence of appendicitis before, during and after pregnancy: a population-based cohort study. World J Surg. 2020;44:2601-2608.
40. Abbasi N, Patenaude V, Abenhaim HA. Management and outcomes of acute appendicitis in pregnancy-population-based study of over 7000 cases. BJOG. 2014;121:1509-1514.
41. Dongarwar D, Taylor J, Ajewole V, et al. Trends in appendicitis among pregnant women, the risk for cardiac arrest, and maternal-fetal mortality. World J Surg. 2020;44:3999-4005.
42. Sachs A, Guglielminotti J, Miller R, et al. Risk factors and risk stratification for adverse obstetrical outcomes after appendectomy or cholecystectomy during pregnancy. JAMA Surg. 2017;152:436-441.
43. Joo JI, Park HC, Kim MJ, et al. Outcomes of antibiotic therapy for uncomplicated appendicitis in pregnancy. Am J Med. 2017;130:1467-1469.
44. Lee SH, Lee JY, Choi YY, Lee JG. Laparoscopic appendectomy versus open appendectomy for suspected appendicitis during pregnancy: a systematic review and updated meta-analysis. BMC Surg. 2019;19:41.
45. Frountzas M, Nikolaou C, Stergios K, et al. Is the laparoscopic approach a safe choice for the management of acute appendicitis in pregnant women? A meta-analysis of observational studies. Ann R Coll Surg Engl. 2019;101:235-248.
46. Di Saverio S, Podda M, De Simone B, et al. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg. 2020;15:27.
47. Koberlein GC, Trout AT, Rigsby CK, et al. ACR Appropriateness Criteria suspected appendicitis-child. J Am Coll Radiol. 2019;16:S252-S263.
48. Maita S, Andersson B, Svensson JF, et al. Nonoperative treatment for nonperforated appendicitis in children: a systematic review and meta-analysis. Pediatr Surg Int. 2020;36:261-269.
49. Georgiou R, Eaton S, Stanton MP, et al. Efficacy and safety of nonoperative treatment for acute appendicitis: a meta-analysis. Pediatrics. 2017;139:e20163003.
PRACTICE RECOMMENDATIONS
› Use the Alvarado Score, Pediatric Appendicitis Score, or Appendicitis Inflammatory Response Score to help rule out appendicitis and thereby reduce unnecessary imaging. A
› Choose ultrasound first as the imaging procedure for children and pregnant women, followed by magnetic resonance imaging if needed, to reduce ionizing radiation in these populations. B
› Consider an antibiotic-based strategy under the care of a surgeon in lieu of immediate surgery for uncomplicated appendicitis. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
DKMS: Small nonprofit to world’s largest stem cell donor registry
When Mechtild Harf was diagnosed with acute leukemia in 1990, physicians told her and her husband Peter that a bone marrow transplant was her best hope for survival. Back then, her native Germany had only 3,000 registered donors, and none was a match.
“My dad just went crazy, you know, to save his wife,” recalled Katharina Harf, who was a young teen at the time of her mother’s diagnosis.
In the course of 1 year, the Harfs recruited more than 68,000 potential bone marrow donors, but their heroic efforts couldn’t save Mechtild.
“She unfortunately didn’t make it. She died because of leukemia,” Katharina said.
Although Mechtild Harf did not survive, her legacy lives on in the bone marrow and stem cell donor recruitment organization DKMS (Deutsche Knochenmarkspenderdatei, or German Bone Marrow Donor Center).
In May of 1991, Peter Harf and Gerhard Ehninger, MD, the hematologist who treated Mechtild, founded DKMS with the mission, as its website states, “to provide as many blood cancer patients as possible with a second chance at life.”
From its German roots, the nonprofit organization has extended its mission to the United States (where it was initially known as Delete Blood Cancer DKMS), Poland, the United Kingdom, Chile, and in 2021, to South Africa.
Three decades after her mother’s death, Katharina Harf serves as Executive Chairwoman of DKMS U.S., based in New York.
World’s largest registry
“DKMS has the largest number of unrelated donors of any organization in the world,” noted Richard E. Champlin, MD, chair of the department of stem cell transplantation and cellular therapy at the University of Texas MD Anderson Cancer Center in Houston.
“In a large fraction of our donor searches, we find matches that are in the DKMS registry,” he said in an interview,
Alexander Schmidt, MD, PhD, global chief medical officer for DKMS, said that approximately 25% of all registered donors worldwide were recruited by his organization, and 39% of all unrelated donor transplants are made with peripheral blood stem cell or bone marrow products, donated by volunteers who are recruited by DKMS.
Since its founding, DKMS has registered 7.1 million potential donors in Germany, who made a total of 80,000 stem cell donations. DKMS U.S., which began operations in 2004, has registered 1.1 million donors and enabled 4,700 donations.
Global partners
DKMS partners with donor centers and recruitment organizations in each country where it operates. In the United States, DKMS works with the National Marrow Donor Program (NMDP) and its “Be The Match” donor registry.
“DKMS donors, both those from DKMS in Germany and those from DKMS in the United States are also listed in the NMDP registry, to make it easier for US search coordinators to accept these donors,” Dr. Schmidt explained in an interview.
The international cooperation and coordination makes it possible for a donor in the UK, for example, to save a life of a patient in Germany, the U.S., Chile, India, or many other parts of the world – anywhere that can be reached in time for a patient in need to receive a stem cell donation.
Pandemic affects donations
But, as with just about every aspect of life, the COVID-19 pandemic has created enormous challenges for recruiters, donor centers, and stem cell transplant centers.
Dr. Schmidt said that decline in donations during the pandemic was less severe than initially feared, with a decrease of just 3.5% in 2020, compared with the prepandemic year of 2019. In contrast, though, the average annual growth rate for donations prior to the pandemic was about 4%.
“Nevertheless, at the beginning of the pandemic in March 2020, for a few days things looked quite terrible, because all the borders were closed and flights were canceled, and about 50% of all stem cell products go abroad, and between 20% and 25% go intercontinental,” Dr. Schmidt said.
However, close cooperation and coordination between donor centers and national health authorities soon resolved the problem and helped insure that the flow of life-saving donations could continue with minimal disruption, he noted.
“I don’t think we had any product that could not be delivered at the end of the day, due to the pandemic,” he told this news organization.
Workforce and clinical problems
Although the flow of donations within and between nations has continued, the COVID-19 pandemic has had profound negative effects on transplant centers, particularly during the wave of infections caused by the Omicron variant, according to a transplant expert.
“With this most recent strain and how transmissible it is, what we’re dealing with is mass workforce shortages,” said Yi-Bin Chen, MD, director of the bone marrow transplant program at Massachusetts General Hospital in Boston.
“On top of a short-staffed hospital, you then take a very transmissible variant and deplete it even more due to the need to quarantine,” he said in an interview.
Both Dr. Champlin and Dr. Chen said that on-again, off-again pandemic travel bans and donor illnesses have necessitated first obtaining products and cryopreserving them before starting the recipient on a conditioning regimen for the transplant.
“The problem is that, while you can preserve peripheral blood stem cells pretty reliably, cryopreserving bone marrow is a bit more difficult,” Dr. Chen said.
In addition, evidence from recent studies comparing stem cell sources suggest that outcomes are less good with cryopreserved products than with fresh products, and with peripheral blood stem cells compared with bone marrow.
“But you’ve got to make do. A transplant with a cryopreserved product is better than no transplant,” Dr. Chen said.
To make things even more frustrating, as the pandemic waxed and waned throughout 2020 and 2021, the recommendations from donor centers seesawed between using fresh or cryopreserved product, making it difficult to plan a transplant for an individual patient.
The Omicron wave has also resulted in a much higher rate of donor dropout than anticipated, making it that much harder to schedule a transplant, Dr. Chen noted.
‘Every patient saved’
The pandemic will eventually subside, however, while the need for stem cell transplantation to treat hematologic malignancies will continue.
DKMS recently launched special aid programs to improve access to stem cell transplants in developing nations by offering financial support, free HLA typing, and other services.
In addition to its core mission of recruiting donors, DKMS is dedicated to improving the quality and efficiency of stem cell transplants. For example, in 2017 scientists in DKMS’ Life Science Lab created an antibody test for donor cytomegalovirus (CMV) infection, using a simple buccal swab rather than a more invasive blood sample. CMV infections can compromise the integrity of stem cell grafts and could be fatal to immunocompromised transplant recipients.
The last word goes to Mechtild Harf’s daughter Katharina.
“My big dream is that every patient will be saved from blood cancer,” she said in a video posted on the DKMS website. “When they get sick, we have a solution for them, whether it’s because they need a donor, with research, building hospitals, providing them with the best medical care we can. I will just keep fighting and keep spreading the word, recruiting donors, raising money – all the things that it takes for us to delete blood cancer.”
“I have to believe that this dream will come true because otherwise, why dream, right?” she said.
Dr. Champlin was the recipient of a Mechtild Harf Science Award and is a member of the board of DKMS U.S. Dr. Schmidt is employed by DKMS. Dr. Chen reported having no relevant disclosures.
When Mechtild Harf was diagnosed with acute leukemia in 1990, physicians told her and her husband Peter that a bone marrow transplant was her best hope for survival. Back then, her native Germany had only 3,000 registered donors, and none was a match.
“My dad just went crazy, you know, to save his wife,” recalled Katharina Harf, who was a young teen at the time of her mother’s diagnosis.
In the course of 1 year, the Harfs recruited more than 68,000 potential bone marrow donors, but their heroic efforts couldn’t save Mechtild.
“She unfortunately didn’t make it. She died because of leukemia,” Katharina said.
Although Mechtild Harf did not survive, her legacy lives on in the bone marrow and stem cell donor recruitment organization DKMS (Deutsche Knochenmarkspenderdatei, or German Bone Marrow Donor Center).
In May of 1991, Peter Harf and Gerhard Ehninger, MD, the hematologist who treated Mechtild, founded DKMS with the mission, as its website states, “to provide as many blood cancer patients as possible with a second chance at life.”
From its German roots, the nonprofit organization has extended its mission to the United States (where it was initially known as Delete Blood Cancer DKMS), Poland, the United Kingdom, Chile, and in 2021, to South Africa.
Three decades after her mother’s death, Katharina Harf serves as Executive Chairwoman of DKMS U.S., based in New York.
World’s largest registry
“DKMS has the largest number of unrelated donors of any organization in the world,” noted Richard E. Champlin, MD, chair of the department of stem cell transplantation and cellular therapy at the University of Texas MD Anderson Cancer Center in Houston.
“In a large fraction of our donor searches, we find matches that are in the DKMS registry,” he said in an interview,
Alexander Schmidt, MD, PhD, global chief medical officer for DKMS, said that approximately 25% of all registered donors worldwide were recruited by his organization, and 39% of all unrelated donor transplants are made with peripheral blood stem cell or bone marrow products, donated by volunteers who are recruited by DKMS.
Since its founding, DKMS has registered 7.1 million potential donors in Germany, who made a total of 80,000 stem cell donations. DKMS U.S., which began operations in 2004, has registered 1.1 million donors and enabled 4,700 donations.
Global partners
DKMS partners with donor centers and recruitment organizations in each country where it operates. In the United States, DKMS works with the National Marrow Donor Program (NMDP) and its “Be The Match” donor registry.
“DKMS donors, both those from DKMS in Germany and those from DKMS in the United States are also listed in the NMDP registry, to make it easier for US search coordinators to accept these donors,” Dr. Schmidt explained in an interview.
The international cooperation and coordination makes it possible for a donor in the UK, for example, to save a life of a patient in Germany, the U.S., Chile, India, or many other parts of the world – anywhere that can be reached in time for a patient in need to receive a stem cell donation.
Pandemic affects donations
But, as with just about every aspect of life, the COVID-19 pandemic has created enormous challenges for recruiters, donor centers, and stem cell transplant centers.
Dr. Schmidt said that decline in donations during the pandemic was less severe than initially feared, with a decrease of just 3.5% in 2020, compared with the prepandemic year of 2019. In contrast, though, the average annual growth rate for donations prior to the pandemic was about 4%.
“Nevertheless, at the beginning of the pandemic in March 2020, for a few days things looked quite terrible, because all the borders were closed and flights were canceled, and about 50% of all stem cell products go abroad, and between 20% and 25% go intercontinental,” Dr. Schmidt said.
However, close cooperation and coordination between donor centers and national health authorities soon resolved the problem and helped insure that the flow of life-saving donations could continue with minimal disruption, he noted.
“I don’t think we had any product that could not be delivered at the end of the day, due to the pandemic,” he told this news organization.
Workforce and clinical problems
Although the flow of donations within and between nations has continued, the COVID-19 pandemic has had profound negative effects on transplant centers, particularly during the wave of infections caused by the Omicron variant, according to a transplant expert.
“With this most recent strain and how transmissible it is, what we’re dealing with is mass workforce shortages,” said Yi-Bin Chen, MD, director of the bone marrow transplant program at Massachusetts General Hospital in Boston.
“On top of a short-staffed hospital, you then take a very transmissible variant and deplete it even more due to the need to quarantine,” he said in an interview.
Both Dr. Champlin and Dr. Chen said that on-again, off-again pandemic travel bans and donor illnesses have necessitated first obtaining products and cryopreserving them before starting the recipient on a conditioning regimen for the transplant.
“The problem is that, while you can preserve peripheral blood stem cells pretty reliably, cryopreserving bone marrow is a bit more difficult,” Dr. Chen said.
In addition, evidence from recent studies comparing stem cell sources suggest that outcomes are less good with cryopreserved products than with fresh products, and with peripheral blood stem cells compared with bone marrow.
“But you’ve got to make do. A transplant with a cryopreserved product is better than no transplant,” Dr. Chen said.
To make things even more frustrating, as the pandemic waxed and waned throughout 2020 and 2021, the recommendations from donor centers seesawed between using fresh or cryopreserved product, making it difficult to plan a transplant for an individual patient.
The Omicron wave has also resulted in a much higher rate of donor dropout than anticipated, making it that much harder to schedule a transplant, Dr. Chen noted.
‘Every patient saved’
The pandemic will eventually subside, however, while the need for stem cell transplantation to treat hematologic malignancies will continue.
DKMS recently launched special aid programs to improve access to stem cell transplants in developing nations by offering financial support, free HLA typing, and other services.
In addition to its core mission of recruiting donors, DKMS is dedicated to improving the quality and efficiency of stem cell transplants. For example, in 2017 scientists in DKMS’ Life Science Lab created an antibody test for donor cytomegalovirus (CMV) infection, using a simple buccal swab rather than a more invasive blood sample. CMV infections can compromise the integrity of stem cell grafts and could be fatal to immunocompromised transplant recipients.
The last word goes to Mechtild Harf’s daughter Katharina.
“My big dream is that every patient will be saved from blood cancer,” she said in a video posted on the DKMS website. “When they get sick, we have a solution for them, whether it’s because they need a donor, with research, building hospitals, providing them with the best medical care we can. I will just keep fighting and keep spreading the word, recruiting donors, raising money – all the things that it takes for us to delete blood cancer.”
“I have to believe that this dream will come true because otherwise, why dream, right?” she said.
Dr. Champlin was the recipient of a Mechtild Harf Science Award and is a member of the board of DKMS U.S. Dr. Schmidt is employed by DKMS. Dr. Chen reported having no relevant disclosures.
When Mechtild Harf was diagnosed with acute leukemia in 1990, physicians told her and her husband Peter that a bone marrow transplant was her best hope for survival. Back then, her native Germany had only 3,000 registered donors, and none was a match.
“My dad just went crazy, you know, to save his wife,” recalled Katharina Harf, who was a young teen at the time of her mother’s diagnosis.
In the course of 1 year, the Harfs recruited more than 68,000 potential bone marrow donors, but their heroic efforts couldn’t save Mechtild.
“She unfortunately didn’t make it. She died because of leukemia,” Katharina said.
Although Mechtild Harf did not survive, her legacy lives on in the bone marrow and stem cell donor recruitment organization DKMS (Deutsche Knochenmarkspenderdatei, or German Bone Marrow Donor Center).
In May of 1991, Peter Harf and Gerhard Ehninger, MD, the hematologist who treated Mechtild, founded DKMS with the mission, as its website states, “to provide as many blood cancer patients as possible with a second chance at life.”
From its German roots, the nonprofit organization has extended its mission to the United States (where it was initially known as Delete Blood Cancer DKMS), Poland, the United Kingdom, Chile, and in 2021, to South Africa.
Three decades after her mother’s death, Katharina Harf serves as Executive Chairwoman of DKMS U.S., based in New York.
World’s largest registry
“DKMS has the largest number of unrelated donors of any organization in the world,” noted Richard E. Champlin, MD, chair of the department of stem cell transplantation and cellular therapy at the University of Texas MD Anderson Cancer Center in Houston.
“In a large fraction of our donor searches, we find matches that are in the DKMS registry,” he said in an interview,
Alexander Schmidt, MD, PhD, global chief medical officer for DKMS, said that approximately 25% of all registered donors worldwide were recruited by his organization, and 39% of all unrelated donor transplants are made with peripheral blood stem cell or bone marrow products, donated by volunteers who are recruited by DKMS.
Since its founding, DKMS has registered 7.1 million potential donors in Germany, who made a total of 80,000 stem cell donations. DKMS U.S., which began operations in 2004, has registered 1.1 million donors and enabled 4,700 donations.
Global partners
DKMS partners with donor centers and recruitment organizations in each country where it operates. In the United States, DKMS works with the National Marrow Donor Program (NMDP) and its “Be The Match” donor registry.
“DKMS donors, both those from DKMS in Germany and those from DKMS in the United States are also listed in the NMDP registry, to make it easier for US search coordinators to accept these donors,” Dr. Schmidt explained in an interview.
The international cooperation and coordination makes it possible for a donor in the UK, for example, to save a life of a patient in Germany, the U.S., Chile, India, or many other parts of the world – anywhere that can be reached in time for a patient in need to receive a stem cell donation.
Pandemic affects donations
But, as with just about every aspect of life, the COVID-19 pandemic has created enormous challenges for recruiters, donor centers, and stem cell transplant centers.
Dr. Schmidt said that decline in donations during the pandemic was less severe than initially feared, with a decrease of just 3.5% in 2020, compared with the prepandemic year of 2019. In contrast, though, the average annual growth rate for donations prior to the pandemic was about 4%.
“Nevertheless, at the beginning of the pandemic in March 2020, for a few days things looked quite terrible, because all the borders were closed and flights were canceled, and about 50% of all stem cell products go abroad, and between 20% and 25% go intercontinental,” Dr. Schmidt said.
However, close cooperation and coordination between donor centers and national health authorities soon resolved the problem and helped insure that the flow of life-saving donations could continue with minimal disruption, he noted.
“I don’t think we had any product that could not be delivered at the end of the day, due to the pandemic,” he told this news organization.
Workforce and clinical problems
Although the flow of donations within and between nations has continued, the COVID-19 pandemic has had profound negative effects on transplant centers, particularly during the wave of infections caused by the Omicron variant, according to a transplant expert.
“With this most recent strain and how transmissible it is, what we’re dealing with is mass workforce shortages,” said Yi-Bin Chen, MD, director of the bone marrow transplant program at Massachusetts General Hospital in Boston.
“On top of a short-staffed hospital, you then take a very transmissible variant and deplete it even more due to the need to quarantine,” he said in an interview.
Both Dr. Champlin and Dr. Chen said that on-again, off-again pandemic travel bans and donor illnesses have necessitated first obtaining products and cryopreserving them before starting the recipient on a conditioning regimen for the transplant.
“The problem is that, while you can preserve peripheral blood stem cells pretty reliably, cryopreserving bone marrow is a bit more difficult,” Dr. Chen said.
In addition, evidence from recent studies comparing stem cell sources suggest that outcomes are less good with cryopreserved products than with fresh products, and with peripheral blood stem cells compared with bone marrow.
“But you’ve got to make do. A transplant with a cryopreserved product is better than no transplant,” Dr. Chen said.
To make things even more frustrating, as the pandemic waxed and waned throughout 2020 and 2021, the recommendations from donor centers seesawed between using fresh or cryopreserved product, making it difficult to plan a transplant for an individual patient.
The Omicron wave has also resulted in a much higher rate of donor dropout than anticipated, making it that much harder to schedule a transplant, Dr. Chen noted.
‘Every patient saved’
The pandemic will eventually subside, however, while the need for stem cell transplantation to treat hematologic malignancies will continue.
DKMS recently launched special aid programs to improve access to stem cell transplants in developing nations by offering financial support, free HLA typing, and other services.
In addition to its core mission of recruiting donors, DKMS is dedicated to improving the quality and efficiency of stem cell transplants. For example, in 2017 scientists in DKMS’ Life Science Lab created an antibody test for donor cytomegalovirus (CMV) infection, using a simple buccal swab rather than a more invasive blood sample. CMV infections can compromise the integrity of stem cell grafts and could be fatal to immunocompromised transplant recipients.
The last word goes to Mechtild Harf’s daughter Katharina.
“My big dream is that every patient will be saved from blood cancer,” she said in a video posted on the DKMS website. “When they get sick, we have a solution for them, whether it’s because they need a donor, with research, building hospitals, providing them with the best medical care we can. I will just keep fighting and keep spreading the word, recruiting donors, raising money – all the things that it takes for us to delete blood cancer.”
“I have to believe that this dream will come true because otherwise, why dream, right?” she said.
Dr. Champlin was the recipient of a Mechtild Harf Science Award and is a member of the board of DKMS U.S. Dr. Schmidt is employed by DKMS. Dr. Chen reported having no relevant disclosures.
Fibroids: Growing management options for a prevalent problem
OBG Manag. 33(12). | doi 10.12788/obgm.0169
See Gastroenterology’s curated ‘Equity in GI’ journal collection
Gastroenterology, an AGA journal, is proud to announce the release of a special collection of articles focused on the intersection of diversity, equity, and inclusion (DEI) within gastroenterology and hepatology. This curated collection, under the guidance of the journal’s new DEI section editor Chyke Doubeni, MBBS, MPH, includes original research, reviews, commentaries, and editorials on matters of health disparities, socioeconomic determinants of health outcomes, and population-based studies on disease incidence among races and ethnicities, among others. New articles are added to the collection as they are published.
View the special collection on Gastroenterology’s website, which is designed to help you quickly and easily look over the latest DEI articles and content of interest. Recent articles include the following:
- “How to incorporate health equity training into GI/hepatology fellowships,” by Jannel Lee-Allen, MD, and Brijen J. Shah, MD.
- “Disparities in preventable mortality from colorectal cancer: Are they the result of structural racism?” by Chyke A. Doubeni, MBBS, MPH; Kevin Selby, MD; and Theodore R. Levin, MD.
- “COVID-19 pediatric patients: GI symptoms, presentations and disparities by race/ethnicity in a large, multicenter U.S. study,” by Yusuf Ashktorab, MD; Anas Brim, MD; Antonio Pizuorno, MD; Vijay Gayam, MD; Sahar Nikdel, MD; and Hassan Brim, PhD.
View all of Gastroenterology’s curated article collections.
Gastroenterology, an AGA journal, is proud to announce the release of a special collection of articles focused on the intersection of diversity, equity, and inclusion (DEI) within gastroenterology and hepatology. This curated collection, under the guidance of the journal’s new DEI section editor Chyke Doubeni, MBBS, MPH, includes original research, reviews, commentaries, and editorials on matters of health disparities, socioeconomic determinants of health outcomes, and population-based studies on disease incidence among races and ethnicities, among others. New articles are added to the collection as they are published.
View the special collection on Gastroenterology’s website, which is designed to help you quickly and easily look over the latest DEI articles and content of interest. Recent articles include the following:
- “How to incorporate health equity training into GI/hepatology fellowships,” by Jannel Lee-Allen, MD, and Brijen J. Shah, MD.
- “Disparities in preventable mortality from colorectal cancer: Are they the result of structural racism?” by Chyke A. Doubeni, MBBS, MPH; Kevin Selby, MD; and Theodore R. Levin, MD.
- “COVID-19 pediatric patients: GI symptoms, presentations and disparities by race/ethnicity in a large, multicenter U.S. study,” by Yusuf Ashktorab, MD; Anas Brim, MD; Antonio Pizuorno, MD; Vijay Gayam, MD; Sahar Nikdel, MD; and Hassan Brim, PhD.
View all of Gastroenterology’s curated article collections.
Gastroenterology, an AGA journal, is proud to announce the release of a special collection of articles focused on the intersection of diversity, equity, and inclusion (DEI) within gastroenterology and hepatology. This curated collection, under the guidance of the journal’s new DEI section editor Chyke Doubeni, MBBS, MPH, includes original research, reviews, commentaries, and editorials on matters of health disparities, socioeconomic determinants of health outcomes, and population-based studies on disease incidence among races and ethnicities, among others. New articles are added to the collection as they are published.
View the special collection on Gastroenterology’s website, which is designed to help you quickly and easily look over the latest DEI articles and content of interest. Recent articles include the following:
- “How to incorporate health equity training into GI/hepatology fellowships,” by Jannel Lee-Allen, MD, and Brijen J. Shah, MD.
- “Disparities in preventable mortality from colorectal cancer: Are they the result of structural racism?” by Chyke A. Doubeni, MBBS, MPH; Kevin Selby, MD; and Theodore R. Levin, MD.
- “COVID-19 pediatric patients: GI symptoms, presentations and disparities by race/ethnicity in a large, multicenter U.S. study,” by Yusuf Ashktorab, MD; Anas Brim, MD; Antonio Pizuorno, MD; Vijay Gayam, MD; Sahar Nikdel, MD; and Hassan Brim, PhD.
View all of Gastroenterology’s curated article collections.
Closer post-ESD surveillance for early GI neoplasia warranted
The new AGA Clinical Practice Update on Surveillance After Pathologically Curative Endoscopic Submucosal Dissection of Early Gastrointestinal Neoplasia in the United States: Commentary offers advice regarding surveillance intervals using endoscopy and other relevant modalities after endoscopic removal of dysplastic lesions and early GI cancers with endoscopic submucosal dissection (ESD) which were deemed pathologically curative.
Main takeaway: Patients with malignant lesions removed by curative ESD possess a higher risk of lymph node metastasis and should be surveilled more closely than those with resection dysplasia not associated with lymphatic spread.
The new AGA Clinical Practice Update on Surveillance After Pathologically Curative Endoscopic Submucosal Dissection of Early Gastrointestinal Neoplasia in the United States: Commentary offers advice regarding surveillance intervals using endoscopy and other relevant modalities after endoscopic removal of dysplastic lesions and early GI cancers with endoscopic submucosal dissection (ESD) which were deemed pathologically curative.
Main takeaway: Patients with malignant lesions removed by curative ESD possess a higher risk of lymph node metastasis and should be surveilled more closely than those with resection dysplasia not associated with lymphatic spread.
The new AGA Clinical Practice Update on Surveillance After Pathologically Curative Endoscopic Submucosal Dissection of Early Gastrointestinal Neoplasia in the United States: Commentary offers advice regarding surveillance intervals using endoscopy and other relevant modalities after endoscopic removal of dysplastic lesions and early GI cancers with endoscopic submucosal dissection (ESD) which were deemed pathologically curative.
Main takeaway: Patients with malignant lesions removed by curative ESD possess a higher risk of lymph node metastasis and should be surveilled more closely than those with resection dysplasia not associated with lymphatic spread.
Busting three myths about planned giving
Gifts to charitable organizations, such as the AGA Research Foundation, in your future plans can ensure that your support for our mission to fund young investigators will continue even after your lifetime. See these three fast facts about planned giving.
- Planned gifts are complicated and confusing. They don’t have to be. There are many types of planned gifts: Most are simple and affordable, like a gift in your will or living trust. You just need to find the one that best meets your needs.
- Wills are only for older adults. Having a plan for the future is important – no matter your age. A will makes your wishes known and provides your loved ones with peace of mind.
- Planned gifts are only for the wealthy. Anyone can make a planned gift. Gifts of all sizes make a difference at the AGA Research Foundation. In fact, you may even be able to make a bigger impact than you thought possible when you make a planned gift.
For 2022, consider including a gift to the AGA Research Foundation in your will. You will help spark future discoveries in GI.
Want to learn more about including a gift to the AGA Research Foundation in your plans? Visit our website at https://gastro.planmylegacy.org or contact us at [email protected].
Gifts to charitable organizations, such as the AGA Research Foundation, in your future plans can ensure that your support for our mission to fund young investigators will continue even after your lifetime. See these three fast facts about planned giving.
- Planned gifts are complicated and confusing. They don’t have to be. There are many types of planned gifts: Most are simple and affordable, like a gift in your will or living trust. You just need to find the one that best meets your needs.
- Wills are only for older adults. Having a plan for the future is important – no matter your age. A will makes your wishes known and provides your loved ones with peace of mind.
- Planned gifts are only for the wealthy. Anyone can make a planned gift. Gifts of all sizes make a difference at the AGA Research Foundation. In fact, you may even be able to make a bigger impact than you thought possible when you make a planned gift.
For 2022, consider including a gift to the AGA Research Foundation in your will. You will help spark future discoveries in GI.
Want to learn more about including a gift to the AGA Research Foundation in your plans? Visit our website at https://gastro.planmylegacy.org or contact us at [email protected].
Gifts to charitable organizations, such as the AGA Research Foundation, in your future plans can ensure that your support for our mission to fund young investigators will continue even after your lifetime. See these three fast facts about planned giving.
- Planned gifts are complicated and confusing. They don’t have to be. There are many types of planned gifts: Most are simple and affordable, like a gift in your will or living trust. You just need to find the one that best meets your needs.
- Wills are only for older adults. Having a plan for the future is important – no matter your age. A will makes your wishes known and provides your loved ones with peace of mind.
- Planned gifts are only for the wealthy. Anyone can make a planned gift. Gifts of all sizes make a difference at the AGA Research Foundation. In fact, you may even be able to make a bigger impact than you thought possible when you make a planned gift.
For 2022, consider including a gift to the AGA Research Foundation in your will. You will help spark future discoveries in GI.
Want to learn more about including a gift to the AGA Research Foundation in your plans? Visit our website at https://gastro.planmylegacy.org or contact us at [email protected].
Migraine Signs and Symptoms
COMMENT & CONTROVERSY
HOW TO CHOOSE THE RIGHT VAGINAL MOISTURIZER OR LUBRICANT FOR YOUR PATIENT
JOHN PENNYCUFF, MD, MSPH, AND CHERYL IGLESIA, MD (JUNE 2021)
Which vaginal products to recommend
We applaud Drs. Pennycuff and Iglesia for providing education on lubricants and vaginal moisturizers in their recent article, and agree that ObGyns, urogynecologists, and primary care providers should be aware of the types of products available. However, the authors underplayed the health risks associated with the use of poor-quality lubricants and moisturizers.
Women often turn to lubricants or vaginal moisturizers because they experience vaginal dryness during intercourse, related to menopause, and from certain medications. Vaginal fluid is primarily composed of exudate from capillaries in the vaginal wall. During sexual arousal, blood flow to the vaginal wall increases, and in turn, this should increase exudate. But chronic inflammation can suppress these increases in vaginal blood flow, preventing adequate vaginal fluid production. One such cause of chronic inflammation is using hyperosmolar lubricants, as this has been shown to negatively affect the vaginal epithelium.1,2 In this way, use of hyperosmolar lubricants can actually worsen symptoms, creating a vicious circle of dryness, lubricant use, and worsening dryness.
In addition, hyperosmolar lubricants have been shown to reduce the epithelial barrier properties of the vaginal epithelium, increasing susceptibility to microbes associated with bacterial vaginosis and to true pathogens, including herpes simplex virus type 2.3 In fact, hyperosmolar lubricants are a serious enough problem that the World Health Organization has weighed in, recommending osmolality of personal lubricants be under 380 mOsm/kg to prevent damage to the vaginal epithelium.4
Appropriately acidic pH is just as critical as osmolality. Using products with a pH higher than 4.5 will reduce amounts of protective lactobacilli and other commensal vaginal bacteria, encouraging growth of opportunistic bacteria and yeast already present. This can lead to bacterial vaginosis, aerobic vaginitis, and candidiasis. Bacterial vaginosis can lead to other serious sequelae such as increased risk in acquisition of HIV infection and preterm birth in pregnancy. Unfortunately, much of the data cited in Drs. Pennycuff and Iglesia’s article were sourced from another study (by Edwards and Panay published in Climacteric in 2016), which measured product pH values with an inappropriately calibrated device; the study’s supplemental information stated that calibration was between 5 and 9, and so any measurement below 5 was invalid and subject to error. For example, the Good Clean Love lubricant is listed as having a pH of 4.7, but its pH is never higher than 4.4.
The products on the market that meet the dual criteria of appropriate pH and isotonicity to vaginal epithelial cells may be less well known to consumers. But this should not be a reason to encourage use of hyperosmolar products whose main selling point is that they are the “leading brand.” Educating women on their choices in personal lubricants should include a full discussion of product ingredients and properties, based upon the available literature to help them select a product that supports the health of their intimate tissues.
Members of the Scientific Advisory Board for the Sexual Health and Wellness Institute: Jill Krapf, MD, MEd, IF; Cathy Chung Hwa Yi, MD; Christine Enzmann, MD, PhD, NMCP; Susan Kellogg-Spadt, PhD, CRNP, IF, CSC, FCST; Betsy Greenleaf, DO, MBA; Elizabeth DuPriest, PhD
References
- Dezzutti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV-1 activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal .pone.0048328.
- Ayehunie S, Wang YY, Landry T, et al. Hyperosmolal vaginal lubricants markedly reduce epithelial barrier properties in a threedimensional vaginal epithelium model. Toxicol Rep. 2017;5:134-140. doi: 10.1016 /j.toxrep.2017.12.011.
- Moench TR, Mumper RJ, Hoen TE, et al. Microbicide excipients can greatly increase susceptibility to genital herpes transmission in the mouse. BMC Infect Dis. 2010;10:331. doi: 10.1186/1471 -2334-10-331.
- Use and procurement of additional lubricants for male and female condoms: WHO/UNFPA /FHI360 Advisory note. World Health Organization, 2012. http://apps.who.int/iris/bitstream /handle/10665/76580/WHO_RHR_12.33_eng .pdf?sequence=1. Accessed December 27, 2021.
Drs. Pennycuff And Iglesia Respond
We thank the members of the scientific advisory board for the Sexual Health and Wellness Institute for their thoughtful and insightful comments to our article. We agree with their comments on the importance of both pH and osmolality for vaginal moisturizers and lubricants. We also agree that selection of an incorrectly formulated product may lead to worsening of vulvovaginal symptoms as well as dysbiosis and all of its sequelae as the letter writers mentioned.
In writing the review article, we attempted to address the role that pH and osmolality play in vaginal moisturizers and lubricants and make clinicians more aware of the importance of these factors in product formulation. Our goal was to help to improve patient counseling. We tried to amass as much of the available literature as we could to act as a resource for practitioners, such as the table included in the article as well as the supplemental table included online. We hoped that by writing this article we would heighten awareness among female health practitioners about vaginal health products and encourage them to consider those products that may be better suited for their patients based on pH and osmolality.
While there remains a paucity of research on vaginal moisturizers and lubricants, there is even less consumer knowledge regarding ingredients and formulations of these products. We wholeheartedly agree with the scientific advisory board that we as health providers need to help educate women on the full spectrum of products available beyond the “leading brands.” Furthermore, we advocate that there be continued research on these products as well as more manufacturer transparency regarding not only the ingredients contained within these products but also the pH and osmolality. Simple steps such as these would ensure that providers could help counsel patients to make informed decisions regarding products for their pelvic health.
Continue to: DISMANTLING RACISM IN YOUR PERSONAL AND PROFESSIONAL SPHERES...
DISMANTLING RACISM IN YOUR PERSONAL AND PROFESSIONAL SPHERES
CASSANDRA CARBERRY, MD, MS; ANNETTA MADSEN, MD; OLIVIA CARDENAS-TROWERS, MD; OLUWATENIOLA BROWN, MD; MOIURI SIDDIQUE, MD; AND BLAIR WASHINGTON, MD, MHA (AUGUST 2021)
Dissenting opinion
“Race is real but it’s not biologic.” “Race is not based on genetic or biologic inheritance.” Am I the only one with a dissenting voice of opinion when it comes to these types of statements?
Scott Peters, MD
Oak Ridge, Tennessee
The Authors Respond
Thank you for your opinion, Dr. Peters. Although it is not completely clear what your question is, it seems that it concerns the validity of the idea that race is a social construct. We will address this question with the assumption that this letter was an effort to invite discussion and increase understanding.
The National Human Genome Research Institute describes race in this way: “Race is a fluid concept used to group people according to various factors, including ancestral background and social identity. Race is also used to group people that share a set of visible characteristics, such as skin color and facial features. Though these visible traits are influenced by genes, the vast majority of genetic variation exists within racial groups and not between them.”1
The understanding that race is a social construct has been upheld by numerous medical organizations. In August 2020, a Joint Statement was published by the American College of Obstetricians and Gynecologists, the American Board of Obstetricians and Gynecologists, and 22 other organizations representing our specialty. This document states: “Recognizing that race is a social construct, not biologically based, is important to understanding that racism, not race, impacts health care, health, and health outcomes.”2
This idea is also endorsed by the AMA, who in November 2020 adopted the following policies3:
- “Recognize that race is a social construct and is distinct from ethnicity, genetic ancestry, or biology
- Support ending the practice of using race as a proxy for biology or genetics in medical education, research, and clinical practice.”
There are numerous sources that further illuminate why race is a social construct. Here are a few:
- https://www.racepowerofanillusion .org/resources/
- https ://www.pewresearch.org /fact-tank/2020/02/25/the-changing -categories-the-u-s-has-used-to -measure-race/
- Roberts D. Fatal Invention: How Science, Politics and Big Business Re-create Race in the Twenty-First Century. The New Press. 2011.
- Yudell M, Roberts D, DeSalle R, et al. Science and society. Taking race out of human genetics. Science. 2016;351(6273):564-5. doi: 10.1126/science.aac4951.
References
- National Human Genome Research Institute. Race. https://www.genome.gov/genetic-glossary /Race. Accessed December 27, 2021.
- The American College of Obstetricians and Gynecologists. Joint Statement: Collective Action Addressing Racism. https://www.acog.org /news/news-articles/2020/08/joint-statementobstetrics-and-gynecology-collective-actionaddressing-racism.
- O’Reilly KB. AMA: Racism is a threat to public health. November 16, 2020. https://www.ama -assn.org/delivering-care/health-equity/ama -racism-threat-public-health. Accessed December 27, 2021.
HOW TO CHOOSE THE RIGHT VAGINAL MOISTURIZER OR LUBRICANT FOR YOUR PATIENT
JOHN PENNYCUFF, MD, MSPH, AND CHERYL IGLESIA, MD (JUNE 2021)
Which vaginal products to recommend
We applaud Drs. Pennycuff and Iglesia for providing education on lubricants and vaginal moisturizers in their recent article, and agree that ObGyns, urogynecologists, and primary care providers should be aware of the types of products available. However, the authors underplayed the health risks associated with the use of poor-quality lubricants and moisturizers.
Women often turn to lubricants or vaginal moisturizers because they experience vaginal dryness during intercourse, related to menopause, and from certain medications. Vaginal fluid is primarily composed of exudate from capillaries in the vaginal wall. During sexual arousal, blood flow to the vaginal wall increases, and in turn, this should increase exudate. But chronic inflammation can suppress these increases in vaginal blood flow, preventing adequate vaginal fluid production. One such cause of chronic inflammation is using hyperosmolar lubricants, as this has been shown to negatively affect the vaginal epithelium.1,2 In this way, use of hyperosmolar lubricants can actually worsen symptoms, creating a vicious circle of dryness, lubricant use, and worsening dryness.
In addition, hyperosmolar lubricants have been shown to reduce the epithelial barrier properties of the vaginal epithelium, increasing susceptibility to microbes associated with bacterial vaginosis and to true pathogens, including herpes simplex virus type 2.3 In fact, hyperosmolar lubricants are a serious enough problem that the World Health Organization has weighed in, recommending osmolality of personal lubricants be under 380 mOsm/kg to prevent damage to the vaginal epithelium.4
Appropriately acidic pH is just as critical as osmolality. Using products with a pH higher than 4.5 will reduce amounts of protective lactobacilli and other commensal vaginal bacteria, encouraging growth of opportunistic bacteria and yeast already present. This can lead to bacterial vaginosis, aerobic vaginitis, and candidiasis. Bacterial vaginosis can lead to other serious sequelae such as increased risk in acquisition of HIV infection and preterm birth in pregnancy. Unfortunately, much of the data cited in Drs. Pennycuff and Iglesia’s article were sourced from another study (by Edwards and Panay published in Climacteric in 2016), which measured product pH values with an inappropriately calibrated device; the study’s supplemental information stated that calibration was between 5 and 9, and so any measurement below 5 was invalid and subject to error. For example, the Good Clean Love lubricant is listed as having a pH of 4.7, but its pH is never higher than 4.4.
The products on the market that meet the dual criteria of appropriate pH and isotonicity to vaginal epithelial cells may be less well known to consumers. But this should not be a reason to encourage use of hyperosmolar products whose main selling point is that they are the “leading brand.” Educating women on their choices in personal lubricants should include a full discussion of product ingredients and properties, based upon the available literature to help them select a product that supports the health of their intimate tissues.
Members of the Scientific Advisory Board for the Sexual Health and Wellness Institute: Jill Krapf, MD, MEd, IF; Cathy Chung Hwa Yi, MD; Christine Enzmann, MD, PhD, NMCP; Susan Kellogg-Spadt, PhD, CRNP, IF, CSC, FCST; Betsy Greenleaf, DO, MBA; Elizabeth DuPriest, PhD
References
- Dezzutti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV-1 activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal .pone.0048328.
- Ayehunie S, Wang YY, Landry T, et al. Hyperosmolal vaginal lubricants markedly reduce epithelial barrier properties in a threedimensional vaginal epithelium model. Toxicol Rep. 2017;5:134-140. doi: 10.1016 /j.toxrep.2017.12.011.
- Moench TR, Mumper RJ, Hoen TE, et al. Microbicide excipients can greatly increase susceptibility to genital herpes transmission in the mouse. BMC Infect Dis. 2010;10:331. doi: 10.1186/1471 -2334-10-331.
- Use and procurement of additional lubricants for male and female condoms: WHO/UNFPA /FHI360 Advisory note. World Health Organization, 2012. http://apps.who.int/iris/bitstream /handle/10665/76580/WHO_RHR_12.33_eng .pdf?sequence=1. Accessed December 27, 2021.
Drs. Pennycuff And Iglesia Respond
We thank the members of the scientific advisory board for the Sexual Health and Wellness Institute for their thoughtful and insightful comments to our article. We agree with their comments on the importance of both pH and osmolality for vaginal moisturizers and lubricants. We also agree that selection of an incorrectly formulated product may lead to worsening of vulvovaginal symptoms as well as dysbiosis and all of its sequelae as the letter writers mentioned.
In writing the review article, we attempted to address the role that pH and osmolality play in vaginal moisturizers and lubricants and make clinicians more aware of the importance of these factors in product formulation. Our goal was to help to improve patient counseling. We tried to amass as much of the available literature as we could to act as a resource for practitioners, such as the table included in the article as well as the supplemental table included online. We hoped that by writing this article we would heighten awareness among female health practitioners about vaginal health products and encourage them to consider those products that may be better suited for their patients based on pH and osmolality.
While there remains a paucity of research on vaginal moisturizers and lubricants, there is even less consumer knowledge regarding ingredients and formulations of these products. We wholeheartedly agree with the scientific advisory board that we as health providers need to help educate women on the full spectrum of products available beyond the “leading brands.” Furthermore, we advocate that there be continued research on these products as well as more manufacturer transparency regarding not only the ingredients contained within these products but also the pH and osmolality. Simple steps such as these would ensure that providers could help counsel patients to make informed decisions regarding products for their pelvic health.
Continue to: DISMANTLING RACISM IN YOUR PERSONAL AND PROFESSIONAL SPHERES...
DISMANTLING RACISM IN YOUR PERSONAL AND PROFESSIONAL SPHERES
CASSANDRA CARBERRY, MD, MS; ANNETTA MADSEN, MD; OLIVIA CARDENAS-TROWERS, MD; OLUWATENIOLA BROWN, MD; MOIURI SIDDIQUE, MD; AND BLAIR WASHINGTON, MD, MHA (AUGUST 2021)
Dissenting opinion
“Race is real but it’s not biologic.” “Race is not based on genetic or biologic inheritance.” Am I the only one with a dissenting voice of opinion when it comes to these types of statements?
Scott Peters, MD
Oak Ridge, Tennessee
The Authors Respond
Thank you for your opinion, Dr. Peters. Although it is not completely clear what your question is, it seems that it concerns the validity of the idea that race is a social construct. We will address this question with the assumption that this letter was an effort to invite discussion and increase understanding.
The National Human Genome Research Institute describes race in this way: “Race is a fluid concept used to group people according to various factors, including ancestral background and social identity. Race is also used to group people that share a set of visible characteristics, such as skin color and facial features. Though these visible traits are influenced by genes, the vast majority of genetic variation exists within racial groups and not between them.”1
The understanding that race is a social construct has been upheld by numerous medical organizations. In August 2020, a Joint Statement was published by the American College of Obstetricians and Gynecologists, the American Board of Obstetricians and Gynecologists, and 22 other organizations representing our specialty. This document states: “Recognizing that race is a social construct, not biologically based, is important to understanding that racism, not race, impacts health care, health, and health outcomes.”2
This idea is also endorsed by the AMA, who in November 2020 adopted the following policies3:
- “Recognize that race is a social construct and is distinct from ethnicity, genetic ancestry, or biology
- Support ending the practice of using race as a proxy for biology or genetics in medical education, research, and clinical practice.”
There are numerous sources that further illuminate why race is a social construct. Here are a few:
- https://www.racepowerofanillusion .org/resources/
- https ://www.pewresearch.org /fact-tank/2020/02/25/the-changing -categories-the-u-s-has-used-to -measure-race/
- Roberts D. Fatal Invention: How Science, Politics and Big Business Re-create Race in the Twenty-First Century. The New Press. 2011.
- Yudell M, Roberts D, DeSalle R, et al. Science and society. Taking race out of human genetics. Science. 2016;351(6273):564-5. doi: 10.1126/science.aac4951.
References
- National Human Genome Research Institute. Race. https://www.genome.gov/genetic-glossary /Race. Accessed December 27, 2021.
- The American College of Obstetricians and Gynecologists. Joint Statement: Collective Action Addressing Racism. https://www.acog.org /news/news-articles/2020/08/joint-statementobstetrics-and-gynecology-collective-actionaddressing-racism.
- O’Reilly KB. AMA: Racism is a threat to public health. November 16, 2020. https://www.ama -assn.org/delivering-care/health-equity/ama -racism-threat-public-health. Accessed December 27, 2021.
HOW TO CHOOSE THE RIGHT VAGINAL MOISTURIZER OR LUBRICANT FOR YOUR PATIENT
JOHN PENNYCUFF, MD, MSPH, AND CHERYL IGLESIA, MD (JUNE 2021)
Which vaginal products to recommend
We applaud Drs. Pennycuff and Iglesia for providing education on lubricants and vaginal moisturizers in their recent article, and agree that ObGyns, urogynecologists, and primary care providers should be aware of the types of products available. However, the authors underplayed the health risks associated with the use of poor-quality lubricants and moisturizers.
Women often turn to lubricants or vaginal moisturizers because they experience vaginal dryness during intercourse, related to menopause, and from certain medications. Vaginal fluid is primarily composed of exudate from capillaries in the vaginal wall. During sexual arousal, blood flow to the vaginal wall increases, and in turn, this should increase exudate. But chronic inflammation can suppress these increases in vaginal blood flow, preventing adequate vaginal fluid production. One such cause of chronic inflammation is using hyperosmolar lubricants, as this has been shown to negatively affect the vaginal epithelium.1,2 In this way, use of hyperosmolar lubricants can actually worsen symptoms, creating a vicious circle of dryness, lubricant use, and worsening dryness.
In addition, hyperosmolar lubricants have been shown to reduce the epithelial barrier properties of the vaginal epithelium, increasing susceptibility to microbes associated with bacterial vaginosis and to true pathogens, including herpes simplex virus type 2.3 In fact, hyperosmolar lubricants are a serious enough problem that the World Health Organization has weighed in, recommending osmolality of personal lubricants be under 380 mOsm/kg to prevent damage to the vaginal epithelium.4
Appropriately acidic pH is just as critical as osmolality. Using products with a pH higher than 4.5 will reduce amounts of protective lactobacilli and other commensal vaginal bacteria, encouraging growth of opportunistic bacteria and yeast already present. This can lead to bacterial vaginosis, aerobic vaginitis, and candidiasis. Bacterial vaginosis can lead to other serious sequelae such as increased risk in acquisition of HIV infection and preterm birth in pregnancy. Unfortunately, much of the data cited in Drs. Pennycuff and Iglesia’s article were sourced from another study (by Edwards and Panay published in Climacteric in 2016), which measured product pH values with an inappropriately calibrated device; the study’s supplemental information stated that calibration was between 5 and 9, and so any measurement below 5 was invalid and subject to error. For example, the Good Clean Love lubricant is listed as having a pH of 4.7, but its pH is never higher than 4.4.
The products on the market that meet the dual criteria of appropriate pH and isotonicity to vaginal epithelial cells may be less well known to consumers. But this should not be a reason to encourage use of hyperosmolar products whose main selling point is that they are the “leading brand.” Educating women on their choices in personal lubricants should include a full discussion of product ingredients and properties, based upon the available literature to help them select a product that supports the health of their intimate tissues.
Members of the Scientific Advisory Board for the Sexual Health and Wellness Institute: Jill Krapf, MD, MEd, IF; Cathy Chung Hwa Yi, MD; Christine Enzmann, MD, PhD, NMCP; Susan Kellogg-Spadt, PhD, CRNP, IF, CSC, FCST; Betsy Greenleaf, DO, MBA; Elizabeth DuPriest, PhD
References
- Dezzutti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV-1 activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal .pone.0048328.
- Ayehunie S, Wang YY, Landry T, et al. Hyperosmolal vaginal lubricants markedly reduce epithelial barrier properties in a threedimensional vaginal epithelium model. Toxicol Rep. 2017;5:134-140. doi: 10.1016 /j.toxrep.2017.12.011.
- Moench TR, Mumper RJ, Hoen TE, et al. Microbicide excipients can greatly increase susceptibility to genital herpes transmission in the mouse. BMC Infect Dis. 2010;10:331. doi: 10.1186/1471 -2334-10-331.
- Use and procurement of additional lubricants for male and female condoms: WHO/UNFPA /FHI360 Advisory note. World Health Organization, 2012. http://apps.who.int/iris/bitstream /handle/10665/76580/WHO_RHR_12.33_eng .pdf?sequence=1. Accessed December 27, 2021.
Drs. Pennycuff And Iglesia Respond
We thank the members of the scientific advisory board for the Sexual Health and Wellness Institute for their thoughtful and insightful comments to our article. We agree with their comments on the importance of both pH and osmolality for vaginal moisturizers and lubricants. We also agree that selection of an incorrectly formulated product may lead to worsening of vulvovaginal symptoms as well as dysbiosis and all of its sequelae as the letter writers mentioned.
In writing the review article, we attempted to address the role that pH and osmolality play in vaginal moisturizers and lubricants and make clinicians more aware of the importance of these factors in product formulation. Our goal was to help to improve patient counseling. We tried to amass as much of the available literature as we could to act as a resource for practitioners, such as the table included in the article as well as the supplemental table included online. We hoped that by writing this article we would heighten awareness among female health practitioners about vaginal health products and encourage them to consider those products that may be better suited for their patients based on pH and osmolality.
While there remains a paucity of research on vaginal moisturizers and lubricants, there is even less consumer knowledge regarding ingredients and formulations of these products. We wholeheartedly agree with the scientific advisory board that we as health providers need to help educate women on the full spectrum of products available beyond the “leading brands.” Furthermore, we advocate that there be continued research on these products as well as more manufacturer transparency regarding not only the ingredients contained within these products but also the pH and osmolality. Simple steps such as these would ensure that providers could help counsel patients to make informed decisions regarding products for their pelvic health.
Continue to: DISMANTLING RACISM IN YOUR PERSONAL AND PROFESSIONAL SPHERES...
DISMANTLING RACISM IN YOUR PERSONAL AND PROFESSIONAL SPHERES
CASSANDRA CARBERRY, MD, MS; ANNETTA MADSEN, MD; OLIVIA CARDENAS-TROWERS, MD; OLUWATENIOLA BROWN, MD; MOIURI SIDDIQUE, MD; AND BLAIR WASHINGTON, MD, MHA (AUGUST 2021)
Dissenting opinion
“Race is real but it’s not biologic.” “Race is not based on genetic or biologic inheritance.” Am I the only one with a dissenting voice of opinion when it comes to these types of statements?
Scott Peters, MD
Oak Ridge, Tennessee
The Authors Respond
Thank you for your opinion, Dr. Peters. Although it is not completely clear what your question is, it seems that it concerns the validity of the idea that race is a social construct. We will address this question with the assumption that this letter was an effort to invite discussion and increase understanding.
The National Human Genome Research Institute describes race in this way: “Race is a fluid concept used to group people according to various factors, including ancestral background and social identity. Race is also used to group people that share a set of visible characteristics, such as skin color and facial features. Though these visible traits are influenced by genes, the vast majority of genetic variation exists within racial groups and not between them.”1
The understanding that race is a social construct has been upheld by numerous medical organizations. In August 2020, a Joint Statement was published by the American College of Obstetricians and Gynecologists, the American Board of Obstetricians and Gynecologists, and 22 other organizations representing our specialty. This document states: “Recognizing that race is a social construct, not biologically based, is important to understanding that racism, not race, impacts health care, health, and health outcomes.”2
This idea is also endorsed by the AMA, who in November 2020 adopted the following policies3:
- “Recognize that race is a social construct and is distinct from ethnicity, genetic ancestry, or biology
- Support ending the practice of using race as a proxy for biology or genetics in medical education, research, and clinical practice.”
There are numerous sources that further illuminate why race is a social construct. Here are a few:
- https://www.racepowerofanillusion .org/resources/
- https ://www.pewresearch.org /fact-tank/2020/02/25/the-changing -categories-the-u-s-has-used-to -measure-race/
- Roberts D. Fatal Invention: How Science, Politics and Big Business Re-create Race in the Twenty-First Century. The New Press. 2011.
- Yudell M, Roberts D, DeSalle R, et al. Science and society. Taking race out of human genetics. Science. 2016;351(6273):564-5. doi: 10.1126/science.aac4951.
References
- National Human Genome Research Institute. Race. https://www.genome.gov/genetic-glossary /Race. Accessed December 27, 2021.
- The American College of Obstetricians and Gynecologists. Joint Statement: Collective Action Addressing Racism. https://www.acog.org /news/news-articles/2020/08/joint-statementobstetrics-and-gynecology-collective-actionaddressing-racism.
- O’Reilly KB. AMA: Racism is a threat to public health. November 16, 2020. https://www.ama -assn.org/delivering-care/health-equity/ama -racism-threat-public-health. Accessed December 27, 2021.
Unraveling plaque changes in CAD With elevated Lp(a)
New research suggests serial coronary CT angiography (CCTA) can provide novel insights into the association between lipoprotein(a) and plaque progression over time in patients with advanced coronary artery disease.
Researchers examined data from 191 individuals with multivessel coronary disease receiving preventive statin (95%) and antiplatelet (100%) therapy in the single-center Scottish DIAMOND trial and compared CCTA at baseline and 12 months available for 160 patients.
As reported in the Journal of the American College of Cardiology, patients with high Lp(a), defined as at least 70 mg/dL, had higher baseline high-density lipoprotein cholesterol and ASSIGN scores than those with low Lp(a) but had comparable coronary artery calcium (CAC) scores and total, calcific, noncalcific, and low-attenuation plaque (LAP) volumes.
At 1 year, however, LAP volume – a marker for necrotic core – increased by 26.2 mm3 in the high-Lp(a) group and decreased by –0.7 mm3 in the low-Lp(a) group (P = .020).
There was no significant difference in change in total, calcific, and noncalcific plaque volumes between groups.
In multivariate linear regression analysis adjusting for body mass index, ASSIGN score, and segment involvement score, LAP volume increased by 10.5% for each 50 mg/dL increment in Lp(a) (P = .034).
“It’s an exciting observation, because we’ve done previous studies where we’ve demonstrated the association of that particular plaque type with future myocardial infarction,” senior author Marc R. Dweck, MD, PhD, University of Amsterdam, told this news organization. “So, you’ve potentially got an explanation for the adverse prognosis associated with high lipoprotein(a) and its link to cardiovascular events and, in particular, myocardial infarction.”
The team’s recent SCOT-HEART analysis found that LAP burden was a stronger predictor of myocardial infarction (MI) than cardiovascular risk scores, stenosis severity, and CAC scoring, with MI risk nearly five-fold higher if LAP was above 4%.
As to why total, calcific, and noncalcific plaque volumes didn’t change significantly on repeat CCTA in the present study, Dr. Dweck said it’s possible that the sample was too small and follow-up too short but also that “total plaque volume is really dominated by the fibrous plaque, which doesn’t appear affected by Lp(a).” Nevertheless, Lp(a)’s effect on low-attenuation plaque was clearly present and supported by the change in fibro-fatty plaque, the next-most unstable plaque type.
At 1 year, fibro-fatty plaque volume was 55.0 mm3 in the high-Lp(a) group versus –25.0 mm3 in the low-Lp(a) group (P = .020).
Lp(a) was associated with fibro-fatty plaque progression in univariate analysis (β = 6.7%; P = .034) and showed a trend in multivariable analysis (β = 6.0%; P = .062).
“This study shows you can track changes in plaque over time and highlight important disease mechanisms and use them to understand the pathology of the disease,” Dr. Dweck said. “I’m very encouraged by this.”
What’s novel in the present study is that “it represents the beginning of our understanding of the role of Lp(a) in plaque progression,” Sotirios Tsimikas, MD, University of California, San Diego, and Jagat Narula, MD, PhD, Icahn School of Medicine at Mount Sinai, New York, say in an accompanying commentary.
They note that prior studies, including the Dallas Heart Study, have struggled to find a strong association between Lp(a) with the extent or progression of CAC, despite elevated Lp(a) and CAC identifying higher-risk patients.
Similarly, a meta-analysis of intravascular ultrasound trials turned up only a 1.2% absolute difference in atheroma volume in patients with elevated Lp(a), and a recent optical coherence tomography study found an association of Lp(a) with thin-cap fibroatheromas but not lipid core.
With just 36 patients with elevated Lp(a), however, the current findings need validation in a larger data set, Dr. Tsimikas and Dr. Narula say.
Although Lp(a) is genetically elevated in about one in five individuals and measurement is recommended in European dyslipidemia guidelines, testing rates are low, in part because the argument has been that there are no Lp(a)-lowering therapies available, Dr. Dweck observed. That may change with the phase 3 cardiovascular outcomes Lp(a)HORIZON trial, which follows strong phase 2 results with the antisense agent AKCEA-APO(a)-LRx and is enrolling patients similar to the current cohort.
“Ultimately it comes down to that fundamental thing, that you need an action once you’ve done the test and then insurers will be happy to pay for it and clinicians will ask for it. That’s why that trial is so important,” Dr. Dweck said.
Dr. Tsimikas and Dr. Narula also point to the eagerly awaited results of that trial, expected in 2025. “A positive trial is likely to lead to additional trials and new drugs that may reinvigorate the use of imaging modalities that could go beyond plaque volume and atherosclerosis to also predict clinically relevant inflammation and atherothrombosis,” they conclude.
Dr. Dweck is supported by the British Heart Foundation and is the recipient of the Sir Jules Thorn Award for Biomedical Research 2015; has received speaker fees from Pfizer and Novartis; and has received consultancy fees from Novartis, Jupiter Bioventures, and Silence Therapeutics. Coauthor disclosures are listed in the paper. Dr. Tsimikas has a dual appointment at the University of California, San Diego, (UCSD) and Ionis Pharmaceuticals; is a coinventor and receives royalties from patents owned by UCSD; and is a cofounder and has an equity interest in Oxitope and its affiliates, Kleanthi Diagnostics, and Covicept Therapeutics. Dr. Narula reports having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research suggests serial coronary CT angiography (CCTA) can provide novel insights into the association between lipoprotein(a) and plaque progression over time in patients with advanced coronary artery disease.
Researchers examined data from 191 individuals with multivessel coronary disease receiving preventive statin (95%) and antiplatelet (100%) therapy in the single-center Scottish DIAMOND trial and compared CCTA at baseline and 12 months available for 160 patients.
As reported in the Journal of the American College of Cardiology, patients with high Lp(a), defined as at least 70 mg/dL, had higher baseline high-density lipoprotein cholesterol and ASSIGN scores than those with low Lp(a) but had comparable coronary artery calcium (CAC) scores and total, calcific, noncalcific, and low-attenuation plaque (LAP) volumes.
At 1 year, however, LAP volume – a marker for necrotic core – increased by 26.2 mm3 in the high-Lp(a) group and decreased by –0.7 mm3 in the low-Lp(a) group (P = .020).
There was no significant difference in change in total, calcific, and noncalcific plaque volumes between groups.
In multivariate linear regression analysis adjusting for body mass index, ASSIGN score, and segment involvement score, LAP volume increased by 10.5% for each 50 mg/dL increment in Lp(a) (P = .034).
“It’s an exciting observation, because we’ve done previous studies where we’ve demonstrated the association of that particular plaque type with future myocardial infarction,” senior author Marc R. Dweck, MD, PhD, University of Amsterdam, told this news organization. “So, you’ve potentially got an explanation for the adverse prognosis associated with high lipoprotein(a) and its link to cardiovascular events and, in particular, myocardial infarction.”
The team’s recent SCOT-HEART analysis found that LAP burden was a stronger predictor of myocardial infarction (MI) than cardiovascular risk scores, stenosis severity, and CAC scoring, with MI risk nearly five-fold higher if LAP was above 4%.
As to why total, calcific, and noncalcific plaque volumes didn’t change significantly on repeat CCTA in the present study, Dr. Dweck said it’s possible that the sample was too small and follow-up too short but also that “total plaque volume is really dominated by the fibrous plaque, which doesn’t appear affected by Lp(a).” Nevertheless, Lp(a)’s effect on low-attenuation plaque was clearly present and supported by the change in fibro-fatty plaque, the next-most unstable plaque type.
At 1 year, fibro-fatty plaque volume was 55.0 mm3 in the high-Lp(a) group versus –25.0 mm3 in the low-Lp(a) group (P = .020).
Lp(a) was associated with fibro-fatty plaque progression in univariate analysis (β = 6.7%; P = .034) and showed a trend in multivariable analysis (β = 6.0%; P = .062).
“This study shows you can track changes in plaque over time and highlight important disease mechanisms and use them to understand the pathology of the disease,” Dr. Dweck said. “I’m very encouraged by this.”
What’s novel in the present study is that “it represents the beginning of our understanding of the role of Lp(a) in plaque progression,” Sotirios Tsimikas, MD, University of California, San Diego, and Jagat Narula, MD, PhD, Icahn School of Medicine at Mount Sinai, New York, say in an accompanying commentary.
They note that prior studies, including the Dallas Heart Study, have struggled to find a strong association between Lp(a) with the extent or progression of CAC, despite elevated Lp(a) and CAC identifying higher-risk patients.
Similarly, a meta-analysis of intravascular ultrasound trials turned up only a 1.2% absolute difference in atheroma volume in patients with elevated Lp(a), and a recent optical coherence tomography study found an association of Lp(a) with thin-cap fibroatheromas but not lipid core.
With just 36 patients with elevated Lp(a), however, the current findings need validation in a larger data set, Dr. Tsimikas and Dr. Narula say.
Although Lp(a) is genetically elevated in about one in five individuals and measurement is recommended in European dyslipidemia guidelines, testing rates are low, in part because the argument has been that there are no Lp(a)-lowering therapies available, Dr. Dweck observed. That may change with the phase 3 cardiovascular outcomes Lp(a)HORIZON trial, which follows strong phase 2 results with the antisense agent AKCEA-APO(a)-LRx and is enrolling patients similar to the current cohort.
“Ultimately it comes down to that fundamental thing, that you need an action once you’ve done the test and then insurers will be happy to pay for it and clinicians will ask for it. That’s why that trial is so important,” Dr. Dweck said.
Dr. Tsimikas and Dr. Narula also point to the eagerly awaited results of that trial, expected in 2025. “A positive trial is likely to lead to additional trials and new drugs that may reinvigorate the use of imaging modalities that could go beyond plaque volume and atherosclerosis to also predict clinically relevant inflammation and atherothrombosis,” they conclude.
Dr. Dweck is supported by the British Heart Foundation and is the recipient of the Sir Jules Thorn Award for Biomedical Research 2015; has received speaker fees from Pfizer and Novartis; and has received consultancy fees from Novartis, Jupiter Bioventures, and Silence Therapeutics. Coauthor disclosures are listed in the paper. Dr. Tsimikas has a dual appointment at the University of California, San Diego, (UCSD) and Ionis Pharmaceuticals; is a coinventor and receives royalties from patents owned by UCSD; and is a cofounder and has an equity interest in Oxitope and its affiliates, Kleanthi Diagnostics, and Covicept Therapeutics. Dr. Narula reports having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research suggests serial coronary CT angiography (CCTA) can provide novel insights into the association between lipoprotein(a) and plaque progression over time in patients with advanced coronary artery disease.
Researchers examined data from 191 individuals with multivessel coronary disease receiving preventive statin (95%) and antiplatelet (100%) therapy in the single-center Scottish DIAMOND trial and compared CCTA at baseline and 12 months available for 160 patients.
As reported in the Journal of the American College of Cardiology, patients with high Lp(a), defined as at least 70 mg/dL, had higher baseline high-density lipoprotein cholesterol and ASSIGN scores than those with low Lp(a) but had comparable coronary artery calcium (CAC) scores and total, calcific, noncalcific, and low-attenuation plaque (LAP) volumes.
At 1 year, however, LAP volume – a marker for necrotic core – increased by 26.2 mm3 in the high-Lp(a) group and decreased by –0.7 mm3 in the low-Lp(a) group (P = .020).
There was no significant difference in change in total, calcific, and noncalcific plaque volumes between groups.
In multivariate linear regression analysis adjusting for body mass index, ASSIGN score, and segment involvement score, LAP volume increased by 10.5% for each 50 mg/dL increment in Lp(a) (P = .034).
“It’s an exciting observation, because we’ve done previous studies where we’ve demonstrated the association of that particular plaque type with future myocardial infarction,” senior author Marc R. Dweck, MD, PhD, University of Amsterdam, told this news organization. “So, you’ve potentially got an explanation for the adverse prognosis associated with high lipoprotein(a) and its link to cardiovascular events and, in particular, myocardial infarction.”
The team’s recent SCOT-HEART analysis found that LAP burden was a stronger predictor of myocardial infarction (MI) than cardiovascular risk scores, stenosis severity, and CAC scoring, with MI risk nearly five-fold higher if LAP was above 4%.
As to why total, calcific, and noncalcific plaque volumes didn’t change significantly on repeat CCTA in the present study, Dr. Dweck said it’s possible that the sample was too small and follow-up too short but also that “total plaque volume is really dominated by the fibrous plaque, which doesn’t appear affected by Lp(a).” Nevertheless, Lp(a)’s effect on low-attenuation plaque was clearly present and supported by the change in fibro-fatty plaque, the next-most unstable plaque type.
At 1 year, fibro-fatty plaque volume was 55.0 mm3 in the high-Lp(a) group versus –25.0 mm3 in the low-Lp(a) group (P = .020).
Lp(a) was associated with fibro-fatty plaque progression in univariate analysis (β = 6.7%; P = .034) and showed a trend in multivariable analysis (β = 6.0%; P = .062).
“This study shows you can track changes in plaque over time and highlight important disease mechanisms and use them to understand the pathology of the disease,” Dr. Dweck said. “I’m very encouraged by this.”
What’s novel in the present study is that “it represents the beginning of our understanding of the role of Lp(a) in plaque progression,” Sotirios Tsimikas, MD, University of California, San Diego, and Jagat Narula, MD, PhD, Icahn School of Medicine at Mount Sinai, New York, say in an accompanying commentary.
They note that prior studies, including the Dallas Heart Study, have struggled to find a strong association between Lp(a) with the extent or progression of CAC, despite elevated Lp(a) and CAC identifying higher-risk patients.
Similarly, a meta-analysis of intravascular ultrasound trials turned up only a 1.2% absolute difference in atheroma volume in patients with elevated Lp(a), and a recent optical coherence tomography study found an association of Lp(a) with thin-cap fibroatheromas but not lipid core.
With just 36 patients with elevated Lp(a), however, the current findings need validation in a larger data set, Dr. Tsimikas and Dr. Narula say.
Although Lp(a) is genetically elevated in about one in five individuals and measurement is recommended in European dyslipidemia guidelines, testing rates are low, in part because the argument has been that there are no Lp(a)-lowering therapies available, Dr. Dweck observed. That may change with the phase 3 cardiovascular outcomes Lp(a)HORIZON trial, which follows strong phase 2 results with the antisense agent AKCEA-APO(a)-LRx and is enrolling patients similar to the current cohort.
“Ultimately it comes down to that fundamental thing, that you need an action once you’ve done the test and then insurers will be happy to pay for it and clinicians will ask for it. That’s why that trial is so important,” Dr. Dweck said.
Dr. Tsimikas and Dr. Narula also point to the eagerly awaited results of that trial, expected in 2025. “A positive trial is likely to lead to additional trials and new drugs that may reinvigorate the use of imaging modalities that could go beyond plaque volume and atherosclerosis to also predict clinically relevant inflammation and atherothrombosis,” they conclude.
Dr. Dweck is supported by the British Heart Foundation and is the recipient of the Sir Jules Thorn Award for Biomedical Research 2015; has received speaker fees from Pfizer and Novartis; and has received consultancy fees from Novartis, Jupiter Bioventures, and Silence Therapeutics. Coauthor disclosures are listed in the paper. Dr. Tsimikas has a dual appointment at the University of California, San Diego, (UCSD) and Ionis Pharmaceuticals; is a coinventor and receives royalties from patents owned by UCSD; and is a cofounder and has an equity interest in Oxitope and its affiliates, Kleanthi Diagnostics, and Covicept Therapeutics. Dr. Narula reports having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
How to identify balance disorders and reduce fall risk
CASE Mr. J, a 75-year-old man, presents to your family practice reporting that he feels increasingly unsteady and slow while walking. He fell twice last year, without resulting injury. He now worries about tripping while walking around the house and relies on his spouse to run errands.
Clearly, Mr. J is experiencing a problem with balance. What management approach should you undertake to prevent him from falling?

Balance disorders are common in older people and drastically hinder quality of life.1-4 Patients often describe imbalance as vague symptoms: dizziness, unsteadiness, faintness, spinning sensations.5,6 Importantly, balance disorders disrupt normal gait and contribute to falls that are a major cause of disability and morbidity in older people. Almost 30% of people older than 65 years report 1 or more falls annually.7 Factors that increase the risk of falls include impaired mobility, previously reported falls, reduced psychological functioning, chronic medical conditions, and polypharmacy.7,8
The cause of any single case of imbalance is often multifactorial, resulting from dysfunction of multiple body systems (TABLE 17-56); in our clinical experience, most patients with imbalance and who are at risk of falls do not have a detectable deficit of the vestibular system. These alterations in function arise in 3 key systems—vision, proprioception, and vestibular function—which signal to, and are incorporated by, the cerebellum to mediate balance. Cognitive and neurologic decline are also factors in imbalance.
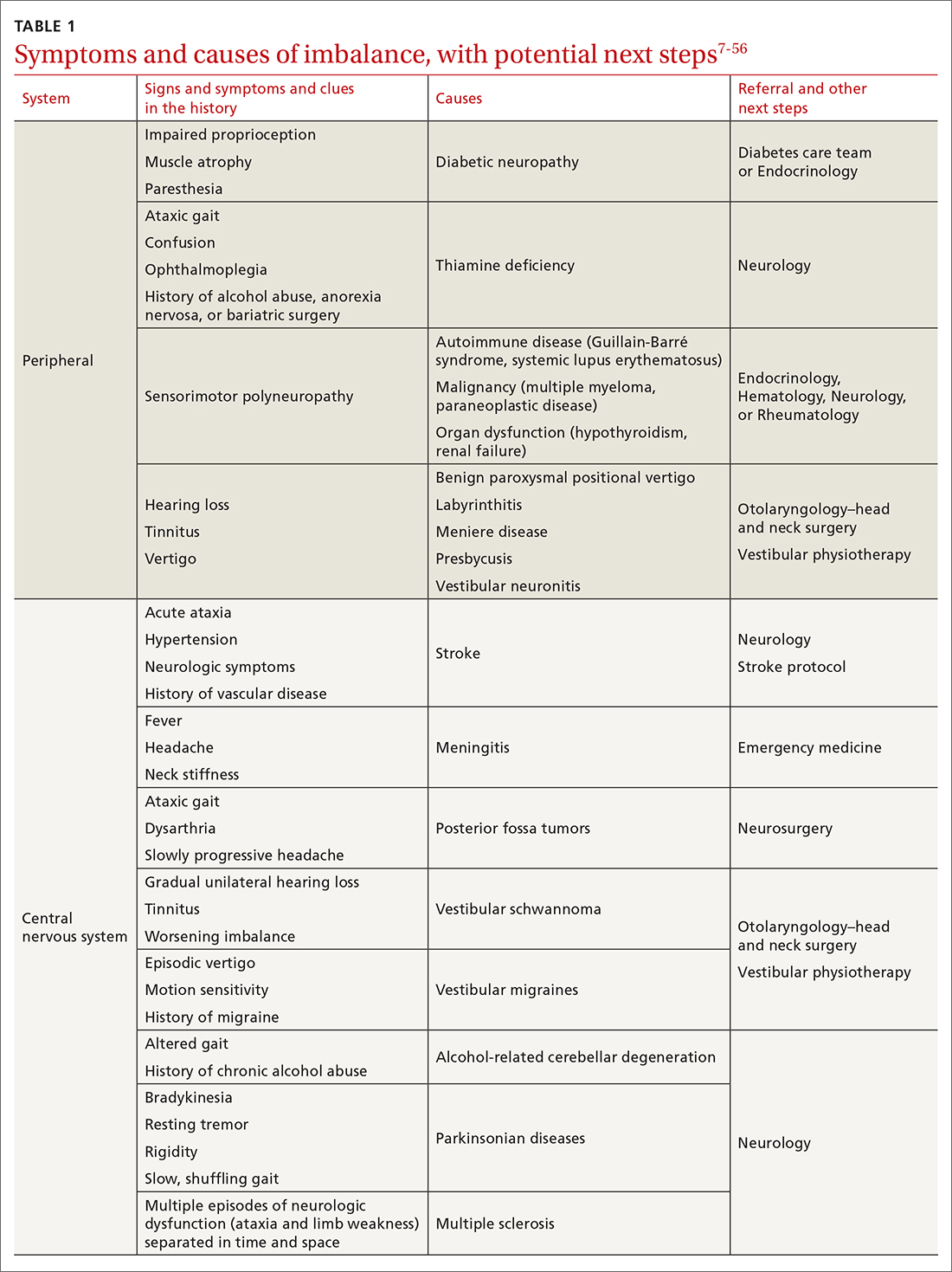
Considering that 20% of falls result in serious injury in older populations, it is important to identify balance disorders and implement preventive strategies to mitigate harmful consequences of falls on patients’ health and independence.7,57 In this article, we answer the question that the case presentation raises about the proper management approach to imbalance in family practice, including assessment of risk and rehabilitation strategies to reduce the risk of falls. Our insights and recommendations are based on our clinical experience and a review of the medical literature from the past 40 years.
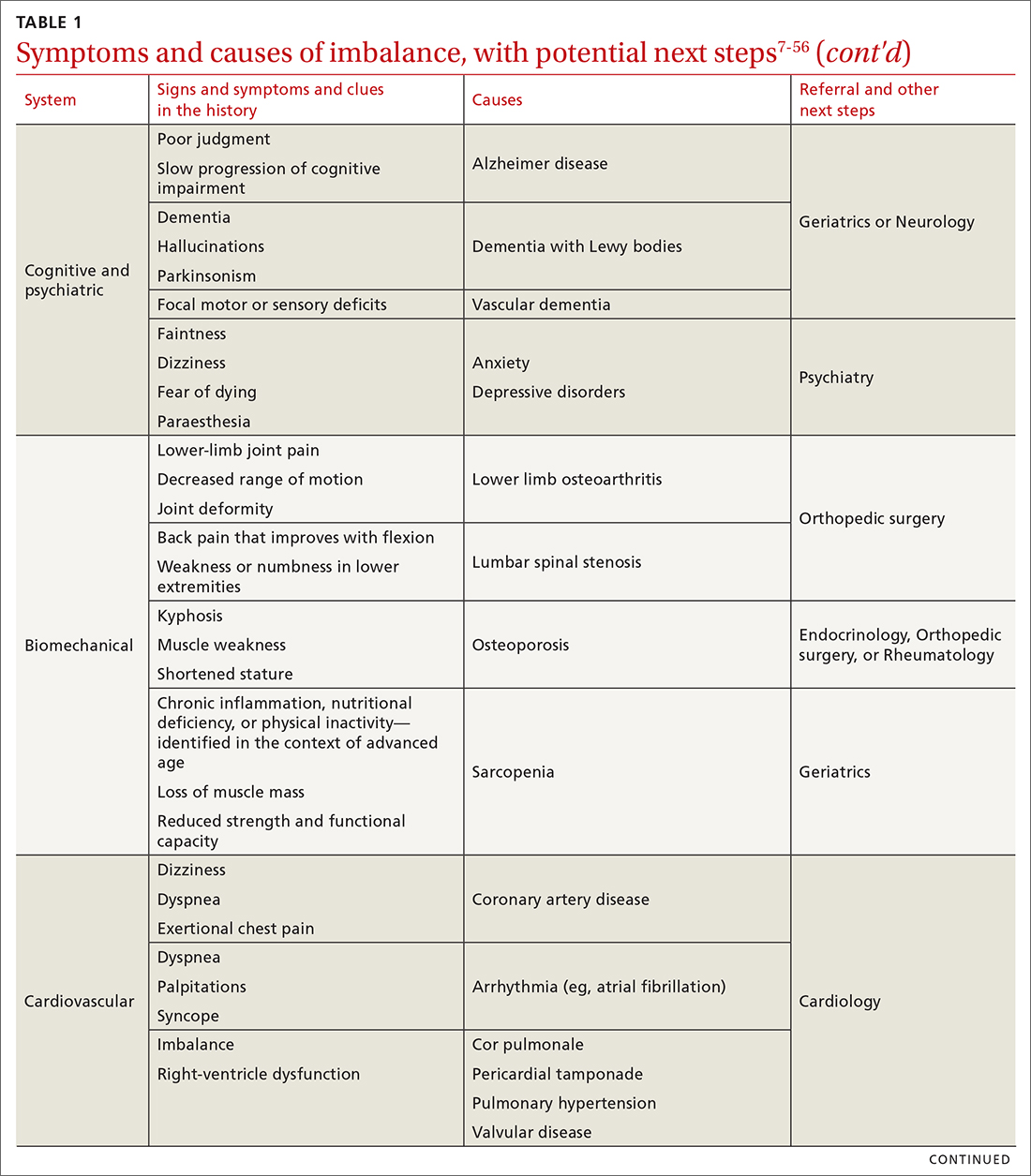
CASE Mr. J has a history of hypertension, age-related hearing loss, and osteoarthritis of the knees; he has not had surgery for the arthritis. His medications are antihypertensives and extra-strength acetaminophen for knee pain.
Making the diagnosis of a balance disorder
History
A thorough clinical history, often including a collateral history from caregivers, narrows the differential diagnosis. Information regarding onset, duration, timing, character, and previous episodes of imbalance is essential. Symptoms of imbalance are often challenging for the patient to describe: They might use terms such as vertigo or dizziness, when, in fact, on further questioning, they are describing balance difficulties. Inquiry into (1) their use of assistive walking devices and (2) development or exacerbation of neurologic, musculoskeletal, auditory, visual, and mood symptoms is necessary. Note the current level of their mobility, episodes of pain or fatigue, previous falls and associated injuries, fear of falling, balance confidence, and sensations that precede falls.58
Continue to: The medical and surgical histories
The medical and surgical histories are key pieces of information. The history of smoking, alcohol habits, and substance use is relevant.
A robust medication history is essential to evaluate a patient’s risk of falling. Polypharmacy—typically, defined as taking 4 or more medications—has been repeatedly associated with a heightened risk of falls.53,59-61 Moreover, a dose-dependent association between polypharmacy and hospitalization following falls has been identified, and demonstrates that taking 10 or more medications greatly increases the risk of hospitalization.59 Studies of polypharmacy cement the importance of inquiring about medication use when assessing imbalance, particularly in older patients.
Physical examination
A focused and detailed physical examination provides insight into systems that should be investigated:
- Obtain vital signs, including orthostatic vitals to test for orthostatic hypotension62; keep in mind that symptoms of orthostatic dizziness can occur without orthostatic hypotension.
- Examine gait, which can distinguish between causes of imbalance (TABLE 2).21,40,63-70
- Perform a cardiac examination.
- Assess visual acuity and visual fields; test for nystagmus and identify any optic-nerve and retinal abnormalities.
- Evaluate lower-limb sensation, proprioception, and motor function.
- Evaluate suspected vestibular dysfunction, including dysfunction with positional testing (the Dix-Hallpike maneuver71). The patient is taken from sitting to supine while the head is rotated 45° to the tested side by the examiner. As the patient moves into a supine position, the neck is extended 30° off the table and held for at least 30 seconds. The maneuver is positive if torsional nystagmus is noted while the head is held rotated during neck extension. The maneuver is negative if the patient reports dizziness, vertigo, unsteadiness, or “pressure in the head.” Torsional nystagmus must be present to confirm a diagnosis of benign paroxysmal positional vertigo.
- If you suspect a central nervous system cause of imbalance, assess the cranial nerves, coordination, strength, and, of course, balance.
CASE
Mr. J’s physical examination showed normal vital signs without significant postural changes in blood pressure. Gait analysis revealed a slowed gait, with reduced range of motion in both knees over the entire gait cycle. Audiometry revealed symmetric moderate sensorineural hearing loss characteristic of presbycusis.
Diagnostic investigations
Consider focused investigations into imbalance based on the history and physical examination. We discourage overly broad testing and imaging; in primary care, cost and limited access to technology can bar robust investigations into causes of imbalance. However, identification of acute pathologies should prompt immediate referral to the emergency department. Furthermore, specific symptoms (TABLE 17-56) should prompt referral to specialists for assessment.
Continue to: In the emergency department...
In the emergency department and academic hospitals, key investigations can identify causes of imbalance:
- Electrocardiography and Holter monitoring test for cardiac arrhythmias.
- Echocardiography identifies structural abnormalities.
- Radiography and computed tomography are useful for detecting musculoskeletal abnormalities.
- Bone densitometry can identify osteoporosis.
- Head and spinal cord magnetic resonance imaging can be used to identify lesions of the central nervous system.
- Computed tomographic angiography of the head and neck is useful for identifying stroke, cerebral atrophy, and stenotic lesions of the carotid and vertebral arteries.
- Nerve conduction studies and levels of serum vitamin B12, hemoglobin A1C, thyroid-stimulating hormone, and random cortisol can uncover causes of peripheral neuropathy.
- Bedside cognitive screening tests can be used to measure cognitive decline.72
- Suspicion of vestibular disease requires audiometry and vestibular testing, including videonystagmography, head impulse testing, and vestibular evoked myogenic potentials.
In many cases of imbalance, no specific underlying correctable cause is discovered.
Management of imbalance
Pharmacotherapy
Targeted pharmacotherapy can be utilized in select clinical scenarios:
- Medical treatment of peripheral neuropathy should target the underlying condition.
- Cognitive behavioral therapy and antidepressants are useful for treating anxiety and depressive disorders.73
- Musculoskeletal pain can be managed with acetaminophen and topical nonsteroidal anti-inflammatory drugs (NSAIDs), using a short course of an oral NSAID when needed.74
- Cardiovascular disease management might include any of several classes of pharmacotherapy, including antiplatelet and lipid-lowering medications, antiarrhythmic drugs, and antihypertensive agents.
- Acute episodes of vertigo due to vestibular neuritis or labyrinthitis can be managed with an antiemetic.46
Surgical treatment
Surgery is infrequently considered for patients with imbalance. Examples of indications include microsurgical resection of vestibular schwannoma, resection of central nervous system tumors, lens replacement surgery for cataract, surgical management of severe spinal fracture, and hip or knee arthroplasty in select patients.
Tools for assessing the risk of falls
Scoring systems called falls risk assessment tools, or FRAT, have been developed to gauge a patient’s risk of falling. The various FRATs differ in specificity and sensitivity for predicting the risk of falls, and are typically designed for specific clinical environments, such as hospital inpatient care or long-term care facilities. Specifically, FRATs attempt to classify risk using sets of risk factors known to be associated with falls.
Continue to: Research abounds into...
Research abounds into the validity of commonly used FRATs across institutions, patient populations, and clinical environments:
The Johns Hopkins FRATa determines risk using metrics such as age, fall history, incontinence, cognition, mobility, and medications75; it is predominantly used for assessment in hospital inpatient units. This tool has been validated repeatedly.76,77
Peninsula Health FRATb stratifies patients in subacute and residential aged-care settings, based on risk factors that include recent falls, medications, psychological status, and cognition.78
FRAT-upc is a web-based tool that generates falls risk using risk factors that users input. This tool has been studied in the context of patients older than 65 years living in the community.79
Although FRATs are reasonably useful for predicting falls, their utility varies by patient population and clinical context. Moreover, it has been suggested that FRATs neglect environmental and personal factors when assessing risk by focusing primarily on bodily factors.80 Implementing a FRAT requires extensive consideration of the target population and should be accompanied by clinical judgment that is grounded in an individual patient’s circumstances.81
Continue to: Preventing falls in primary care
Preventing falls in primary care
An approach to preventing falls includes the development of individualized programs that account for frailty, a syndrome of physiologic decline associated with aging. Because frailty leads to diminished balance and mobility, a patient’s frailty index—determined using the 5 frailty phenotype criteria (exhaustion, weight loss, low physical activity, weakness, slowness)82 or the Canadian Study of Health and Aging Clinical Frailty Scale83—is a useful tool for predicting falls risk and readmission for falls following trauma-related injury. Prevention of falls in communities is critical for reducing mortality and allowing older people to maintain their independence and quality of life.
Exercise. In some areas, exercise and falls prevention programs are accessible to seniors.84 Community exercise programs that focus on balance retraining and muscle strengthening can reduce the risk of falls.73,85 The Choosing Wisely initiative of the ABIM [American Board of Internal Medicine] Foundation recommends that exercise programs be designed around an accurate functional baseline of the patient to avoid underdosed strength training.54
Multifactorial risk assessment in high-risk patients can reduce the rate of falls. Such an assessment includes examination of orthostatic blood pressure, vision and hearing, bone health, gait, activities of daily living, cognition, and environmental hazards, and enables provision of necessary interventions.73,86 Hearing amplification, specifically, correlates with enhanced postural control, slowed cognitive decline, and a reduced likelihood of falls.87-93 The mechanism behind improved balance performance might be reduced cognitive load through supporting a patient’s listening needs.88-90
Pharmacotherapy. Optimizing medications and performing a complete medication review before prescribing new medications is highly recommended to avoid unnecessary polypharmacy7,8,18,53-56 (TABLE 17-56).
Management of comorbidities associated with a higher risk of falls, including arthritis, cancer, stroke, diabetes, depression, kidney disease, chronic obstructive pulmonary disease, cognitive impairment, hypertension, and atrial fibrillation, is essential.94-96
Continue to: Home safety interventions
Home safety interventions, through occupational therapy, are important. These include removing unsafe mats and step-overs and installing nonslip strips on stairs, double-sided tape under mats, and handrails.73-97
Screening for risk of falls. The Centers for Disease Control and Prevention recommends that (1) all patients older than 65 years and (2) any patient presenting with an acute fall undergo screening for their risk of falls.98 When a patient is identified as at risk of falling, you can, when appropriate, assess modifiable risk factors and facilitate interventions.98 This strategy is supported by a 2018 statement from the US Preventive Services Task Force99 that recommends identifying high-risk patients who have:
- a history of falling
- a balance disturbance that causes a deficit of mobility or function
- poor performance on clinical tests, such as the 3-meter Timed Up and Go (TUG) assessment (www.cdc.gov/steadi/pdf/TUG_test-print.pdf).
An increased risk of falls should prompt you to refer the patient to community programs and physiotherapy in accordance with the individual’s personal goals99; a balance and vestibular physiotherapist is ideally positioned to accurately assess and manage patients at risk of falls. Specifically, the Task Force identified exercise programs and multifactorial interventions as being beneficial in preventing falls in high-risk older people.99
Balance assessment and rehabilitation in specialty centers
An individualized rehabilitation program aims to restore safe mobility by testing and addressing specific balance deficits, improving functional balance, and increasing balance confidence. Collaboration with colleagues from physiotherapy and occupational therapy aids in tailoring individualized programs.
Many tests are available to assess balance, determine the risk of falls, and guide rehabilitation:
- The timed 10-meter walk testd and the TUG test are simple assessments that measure functional mobility; both have normalized values for the risk of falls. A TUG time of ≥ 12 seconds suggests a high risk of falls.
- The 30-second chair stande evaluates functional lower-extremity strength in older patients. The test can indicate if lower-extremity strength is contributing to a patient’s imbalance.
- The modified clinical test of sensory interaction in balancef is a static balance test that measures the integrity of sensory inputs. The test can suggest if 1 or more sensory systems are compromised.
- The mini balance evaluation systems testg is similar: It can differentiate balance deficits by underlying system and allows individualization of a rehabilitation program.
- The functional gait assessmenth is a modification of the dynamic gait index that assesses postural stability during everyday dynamic activities, including tasks such as walking with head turns and pivots.
- The Berg Balance Scalei continues to be used extensively to assess balance.
Continue to: The mini balance evaluation systems test...
The mini balance evaluation systems test, functional gait index, and Berg Balance Scale all have normative age-graded values to predict fall risk.
CASE
Mr. J was referred for balance assessment and to a rehabilitation program. He underwent balance physiotherapy, including multifactorial balance assessment, joined a community exercise program, was fitted with hearing aids, and had his home environment optimized by an occupational therapist. (See examples of “home safety interventions” under “Preventing falls in primary care.”)
3 months later. Mr. J says he feels stronger on his feet. His knee pain has eased, and he is more confident walking around his home. He continues to engage in exercise programs and is comfortable running errands with his spouse.
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Division of OtolaryngologyHead and Neck Surgery, Queen’s University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected]
a www.hopkinsmedicine.org/institute_nursing/models_tools/jhfrat_acute%20care%20original_6_22_17.pdf
c www.ncbi.nlm.nih.gov/pmc/articles/PMC4376110/figure/figure14/?report=objectonly
e www.cdc.gov/steadi/pdf/STEADI-Assessment-30Sec-508.pdf
f www.mdapp.co/mctsib-modified-clinical-test-of-sensory-interaction-in-balance-calculator-404/
g www.sralab.org/sites/default/files/2017-07/MiniBEST_revised_final_3_8_13.pdf
1. Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 2011;4:189-201. doi: 10.2165/11591150-000000000-00000
2. TB, ZF, ES, et al. The relationship of balance disorders with falling, the effect of health problems, and social life on postural balance in the elderly living in a district in Turkey. Geriatrics (Basel). 2019;4:37. doi: 10.3390/geriatrics4020037
3. R, Sixt E, Landahl S, et al. Prevalence of dizziness and vertigo in an urban elderly population. J Vestib Res. 2004;14:47-52.
4. Sturnieks DL, St George R, Lord SR. Balance disorders in the elderly. Neurophysiol Clin. 2008;38:467-478. doi: 10.1016/j.neucli.2008.09.001
5. Boult C, Murphy J, Sloane P, et al. The relation of dizziness to functional decline. J Am Geriatr Soc. 1991;39:858-861. doi: 10.1111/j.1532-5415.1991.tb04451.x
6. Lin HW, Bhattacharyya N. Balance disorders in the elderly: epidemiology and functional impact. Laryngoscope. 2012;122:1858-1861. doi: 10.1002/lary.23376
7. Jia H, Lubetkin EI, DeMichele K, et al. Prevalence, risk factors, and burden of disease for falls and balance or walking problems among older adults in the U.S. Prev Med. 2019;126:105737. doi: 10.1016/j.ypmed.2019.05.025
8. Al-Momani M, Al-Momani F, Alghadir AH, et al. Factors related to gait and balance deficits in older adults. Clin Interv Aging. 2016;11:1043-1049. doi: 10.2147/CIA.S112282
9. Agrawal Y, Ward BK, Minor LB. Vestibular dysfunction: prevalence, impact and need for targeted treatment. J Vestib Res. 2013;23:113-117. doi: 10.3233/VES-130498
10. Altinsoy B, Erboy F, Tanriverdi H, et al. Syncope as a presentation of acute pulmonary embolism. Ther Clin Risk Manag. 2016;12:1023-1028. doi: 10.2147/TCRM.S105722
11. Belvederi Murri M, Triolo F, Coni A, et al. Instrumental assessment of balance and gait in depression: a systematic review. Psychiatry Res. 2020;284:112687. doi: 10.1016/j.psychres.2019.112687
12. Bhattacharyya N, Gubbels SP, Schwartz SR, et al. Clinical practice guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg. 2017;156(suppl 3):S1-S47. doi: 10.1177/0194599816689667
13. S, Schwarm S, Grevenrath P, et al. Prevalence, aetiologies and prognosis of the symptom dizziness in primary care - a systematic review. BMC Fam Pract. 2018;19:33. doi: 10.1186/s12875-017-0695-0
14. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467-492. doi: 10.1128/CMR.00070-09
15. Chad DA. Lumbar spinal stenosis. Neurol Clin. 2007;25:407-418. doi: 10.1016/j.ncl.2007.01.003
16. Conrad BP, Shokat MS, Abbasi AZ, et al. Associations of self-report measures with gait, range of motion and proprioception in patients with lumbar spinal stenosis. Gait Posture. 2013;38:987-992. doi: 10.1016/j.gaitpost.2013.05.010
17. de Luna RA, Mihailovic A, Nguyen AM, et al. The association of glaucomatous visual field loss and balance. Transl Vis Sci Technol. 2017;6:8. doi: 10.1167/tvst.6.3.8
18. DiSogra RM. Common aminoglycosides and platinum-based ototoxic drugs: cochlear/vestibular side effects and incidence. Semin Hear. 2019;40:104-107. doi: 10.1055/s-0039-1684040
19. Ebersbach G, Moreau C, Gandor F, et al. Clinical syndromes: parkinsonian gait. Mov Disord. 2013;28:1552-1559. doi: 10.1002/mds.25675
20. Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S-1127S. doi: 10.3945/ajcn.2010.28608A
21. Filli L, Sutter T, Easthope CS, et al. Profiling walking dysfunction in multiple sclerosis: characterisation, classification and progression over time. Sci Rep. 2018;8:4984. doi: 10.1038/s41598-018-22676-0
22. Fritz NE, Kegelmeyer DA, Kloos AD, et al. Motor performance differentiates individuals with Lewy body dementia, Parkinson’s and Alzheimer’s disease. Gait Posture. 2016;50:1-7. doi: 10.1016/j.gaitpost.2016.08.009
23. Furman JM, Jacob RG. A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J Anxiety Disord. 2001;15:9-26. doi: 10.1016/s0887-6185(00)00040-2
24. Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12:706-715. doi: 10.1016/S1474-4422(13)70107-8
25. Gerson LW, Jarjoura D, McCord G. Risk of imbalance in elderly people with impaired hearing or vision. Age Ageing. 1989;18:31-34. doi: 10.1093/ageing/18.1.31
26. Goudakos JK, Markou KD, Franco-Vidal V, et al. Corticosteroids in the treatment of vestibular neuritis: a systematic review and meta-analysis. Otol Neurotol. 2010;31:183-189. doi: 10.1097/MAO.0b013e3181ca843d
27. Green AD, CS, Bastian L, et al. Does this woman have osteoporosis? JAMA. 2004;292:2890-2900. doi: 10.1001/jama.292.23.2890
28. Hallemans A, Ortibus E, Meire F, et al. Low vision affects dynamic stability of gait. Gait Posture. 2010;32:547-551. doi: 10.1016/j.gaitpost.2010.07.018
29. Handelsman JA. Vestibulotoxicity: strategies for clinical diagnosis and rehabilitation. Int J Audiol. 2018;57(suppl 4):S99-S107. doi: 10.1080/14992027.2018.1468092
30. Head VA, Wakerley BR. Guillain-Barré syndrome in general practice: clinical features suggestive of early diagnosis. Br J Gen Pract. 2016;66:218-219. doi: 10.3399/bjgp16X684733
31. Helbostad JL, Vereijken B, Hesseberg K, et al. Altered vision destabilizes gait in older persons. Gait Posture. 2009;30:233-238. doi: 10.1016/j.gaitpost.2009.05.004
32. Hsu W-L, Chen C-Y, Tsauo J-Y, et al. Balance control in elderly people with osteoporosis. J Formos Med Assoc. 2014;113:334-339. doi: 10.1016/j.jfma.2014.02.006
33. Kim H-S, Yun DH, Yoo SD, et al. Balance control and knee osteoarthritis severity. Ann Rehabil Med. 2011;35:701-709. doi: 10.5535/arm.2011.35.5.701
34. Li L, Simonsick EM, Ferrucci L, et al. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2013;38:25-29.
35. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88-100. doi: 10.1212/WNL.0000000000004058
36. Milner KA, Funk M, Richards S, et al. Gender differences in symptom presentation associated with coronary heart disease. Am J Cardiol. 1999;84:396-399. doi: 10.1016/s0002-9149(99)00322-7
37. Paillard T, F, Bru N, et al. The impact of time of day on the gait and balance control of Alzheimer’s patients. Chronobiol Int. 2016;33:161-168. doi: 10.3109/07420528.2015.1124885
38. Paldor I, Chen AS, Kaye AH. Growth rate of vestibular schwannoma. J Clin Neurosci. 2016;32:1-8. doi: 10.1016/j.jocn.2016.05.003
39. Picorelli AMA, Hatton AL, Gane EM, et al. Balance performance in older adults with hip osteoarthritis: a systematic review. Gait Posture. 2018;65:89-99. doi: 10.1016/j.gaitpost.2018.07.001
40. Raccagni C, Nonnekes J, Bloem BR, et al. Gait and postural disorders in parkinsonism: a clinical approach. J Neurol. 2020;267:3169-3176. doi: 10.1007/s00415-019-09382-1
41. Shanmugarajah PD, Hoggard N, Currie S, et al. Alcohol-related cerebellar degeneration: not all down to toxicity? Cerebellum Ataxias. 2016;3:17. doi: 10.1186/s40673-016-0055-1
42. Shih RY, Smirniotopoulos JG. Posterior fossa tumors in adult patients. Neuroimaging Clin N Am. 2016;26:493-510. doi: 10.1016/j.nic.2016.06.003
43. Smith EE. Clinical presentations and epidemiology of vascular dementia. Clin Sci (Lond). 2017;131:1059-1068. doi: 10.1042/CS20160607
44. Streur M, Ratcliffe SJ, Ball J, et al. Symptom clusters in adults with chronic atrial fibrillation. J Cardiovasc Nurs. 2017;32:296-303. doi: 10.1097/JCN.0000000000000344
45. Strupp M, M, JA. Peripheral vestibular disorders: an update. Curr Opin Neurol. 2019;32:165-173. doi: 10.1097/WCO.0000000000000649
46. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
47. Timar B, Timar R, L, et al. The impact of diabetic neuropathy on balance and on the risk of falls in patients with type 2 diabetes mellitus: a cross-sectional study. PLoS One. 2016;11:e0154654. doi: 10.1371/journal.pone.0154654
48. Walls R, Hockberger R, Gausche-Hill M. Peripheral nerve disorders. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 9th ed. Elsevier, Inc; 2018:1307-1320.
49. Watson JC, Dyck PJB. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc. 2015;90:940-951. doi: 10.1016/j.mayocp.2015.05.004
50. Whitfield KC, Bourassa MW, Adamolekun B, et al. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann N Y Acad Sci. 2018;1430:3-43. doi: 10.1111/nyas.13919
51. Wu V, Sykes EA, Beyea MM, et al. Approach to Meniere disease management. Can Fam Physician. 2019;65:463-467.
52. Yew KS, Cheng EM. Diagnosis of acute stroke. Am Fam Physician. 2015;91:528-536.
53. Seppala LJ, van de Glind EMM, Daams JG, et al; . Fall-risk-increasing drugs: a systematic review and meta-analysis: III. Others. J Am Med Dir Assoc. 2018;19:372.e1-372.e8. doi: 10.1016/j.jamda.2017.12.099
54. ABIM Foundation. Choosing wisely. Choosing Wisely website. 2021. Accessed November 11. 2021. www.choosingwisely.org/
55. Berlie HD, Garwood CL. Diabetes medications related to an increased risk of falls and fall-related morbidity in the elderly. Ann Pharmacother. 2010;44:712-717. doi: 10.1345/aph.1M551
56. Hartikainen S, E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62:1172-1181. doi: 10.1093/gerona/62.10.1172
57. Khanuja K, Joki J, Bachmann G, et al. Gait and balance in the aging population: Fall prevention using innovation and technology. Maturitas. 2018;110:51-56. doi: 10.1016/j.maturitas.2018.01.021
58. Salzman B. Gait and balance disorders in older adults. Am Fam Physician. 2010;82:61-68.
59. Zaninotto P, Huang YT, Di Gessa G, et al. Polypharmacy is a risk factor for hospital admission due to a fall: evidence from the English Longitudinal Study of Ageing. BMC Public Health. 2020;20:1804. doi: 10.1186/s12889-020-09920-x
60. Morin L, Calderon A, Welmer AK, et al. Polypharmacy and injurious falls in older adults: a nationwide nested case-control study. Clin Epidemiol. 2019;11:483-493. doi: 10.2147/CLEP.S201614
61. Dhalwani NN, Fahami R, Sathanapally H, et al. Association between polypharmacy and falls in older adults: a longitudinal study from England. BMJ Open. 2017;7:e016358. doi: 10.1136/bmjopen-2017-016358
62. Arnold AC, Raj SR. Orthostatic hypotension: a practical approach to investigation and management. Can J Cardiol. 2017;33:1725-1728. doi: 10.1016/j.cjca.2017.05.007
63. Alexander NB. Differential diagnosis of gait disorders in older adults. Clin Geriatr Med. 1996;12:689-703.
64. Baker JM. Gait disorders. Am J Med. 2018;131:602-607. doi: 10.1016/j.amjmed.2017.11.051
65. Cameron MH, Wagner JM. Gait abnormalities in multiple sclerosis: pathogenesis, evaluation, and advances in treatment. Curr Neurol Neurosci Rep. 2011;11:507-515. doi: 10.1007/s11910-011-0214-y
66. Chen C-L, Chen H-C, Tang SF-T, et al. Gait performance with compensatory adaptations in stroke patients with different degrees of motor recovery. Am J Phys Med Rehabil. 2003;82:925-935. doi: 10.1097/01.PHM.0000098040.13355.B5
67. Marsden J, Harris C. Cerebellar ataxia: pathophysiology and rehabilitation. Clin Rehabil. 2011;25:195-216. doi: 10.1177/0269215510382495
68. Mirek E, Filip M, W, et al. Three-dimensional trunk and lower limbs characteristics during gait in patients with Huntington’s disease. Front Neurosci. 2017;11:566. doi: 10.3389/fnins.2017.00566
69. Paramanandam V, Lizarraga KJ, Soh D, et al. Unusual gait disorders: a phenomenological approach and classification. Expert Rev Neurother. 2019;19:119-132. doi: 10.1080/14737175.2019.1562337
70. Sahyouni R, Goshtasbi K, Mahmoodi A, et al. Chronic subdural hematoma: a historical and clinical perspective. World Neurosurg. 2017;108:948-953. doi: 10.1016/j.wneu.2017.09.064
71. Talmud JD, Coffey R, Edemekong PF. Dix Hallpike maneuver. StatPearls [Internet]. StatPearls Publishing Updated September 5, 2021. Accessed December 6, 2021. www.ncbi.nlm.nih.gov/books/NBK459307/
72. Molnar FJ, Benjamin S, Hawkins SA, et al. One size does not fit all: choosing practical cognitive screening tools for your practice. J Am Geriatr Soc. 2020;68:2207-2213. doi: 10.1111/jgs.16713
73. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012:CD007146. doi: 10.1002/14651858.CD007146.pub3
74. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150. doi: 10.14336/AD.2017.0306
75. Poe SS, Cvach M, Dawson PB, Straus H, Hill EE. The Johns Hopkins Fall Risk Assessment Tool: postimplementation evaluation. J Nurs Care Qual. 2007;22:293-298. doi: 10.1097/01.NCQ.0000290408.74027.39
76. Poe SS, Dawson PB, Cvach M, et al. The Johns Hopkins Fall Risk Assessment Tool: a study of reliability and validity. J Nurs Care Qual. 2018;33:10-19. doi: 10.1097/NCQ.0000000000000301
77. Klinkenberg WD, Potter P. Validity of the Johns Hopkins Fall Risk Assessment Tool for predicting falls on inpatient medicine services. J Nurs Care Qual. 2017;32:108-113. doi: 10.1097/NCQ.0000000000000210
78. Stapleton C, Hough P, Oldmeadow L, et al. Four-item fall risk screening tool for subacute and residential aged care: the first step in fall prevention. Australas J Ageing. 2009;28:139-143. doi: 10.1111/j.1741-6612.2009.00375.x
79. Cattelani L, Palumbo P, Palmerini L, et al. FRAT-up, a Web-based fall-risk assessment tool for elderly people living in the community. J Med Internet Res. 2015;17:e41. doi: 10.2196/jmir.4064
80. De Clercq H, Naudé A, Bornman J. Factors included in adult fall risk assessment tools (FRATs): a systematic review. Ageing Soc. 2020;41:2558-2582. doi: 10.1017/S0144686X2000046X
81. Yap G, Melder A. Accuracy of validated falls risk assessment tools and clinical judgement. Centre for Clinical Effectiveness, Monash Innovation and Quality. Monash Health. February 5, 2020. Accessed November 11, 2021. https://monashhealth.org/wp-content/uploads/2019/01/Rapid-Review_Falls-risk-tools-FINAL.pdf
82. Chittrakul J, Siviroj P, Sungkarat S, et al. Physical frailty and fall risk in community-dwelling older adults: a cross-sectional study. J Aging Res. 2020;2020:3964973. doi: 10.1155/2020/3964973
83. Hatcher VH, Galet C, Lilienthal M, et al. Association of clinical frailty scores with hospital readmission for falls after index admission for trauma-related injury. JAMA Netw Open. 2019;2:e1912409. doi: 10.1001/jamanetworkopen.2019.12409
84. Exercise and fall prevention programs. Government of Ontario Ministry of Health. Updated April 9, 2019. Accessed November 11. 2021. www.ontario.ca/page/exercise-and-falls-prevention-programs
85. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1:CD012424. doi: 10.1002/14651858.CD012424.pub2
86. Hopewell S, Copsey B, Nicolson P, et al. Multifactorial interventions for preventing falls in older people living in the community: a systematic review and meta-analysis of 41 trials and almost 20 000 participants. Br J Sports Med. 2020;54:1340-1350. doi: 10.1136/bjsports-2019-100732
87. Jafari Z, Kolb BE, Mohajerani MH. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. 2019;56:100963. doi: 10.1016/j.arr.2019.100963
88. Griffiths TD, Lad M, Kumar S, et al. How can hearing loss cause dementia? Neuron. 2020;108:401-412. doi: 10.1016/j.neuron.2020.08.003
89. Martini A, Castiglione A, Bovo R, et al. Aging, cognitive load, dementia and hearing loss. Audiol Neurootol. 2014;19(suppl 1):2-5. doi: 10.1159/000371593
90. Vitkovic J, Le C, Lee S-L, et al. The contribution of hearing and hearing loss to balance control. Audiol Neurootol. 2016;21:195-202. doi: 10.1159/000445100
91. Maheu M, Behtani L, Nooristani M, et al. Vestibular function modulates the benefit of hearing aids in people with hearing loss during static postural control. Ear Hear. 2019;40:1418-1424. doi: 10.1097/AUD.0000000000000720
92. Negahban H, Bavarsad Cheshmeh Ali M, Nassadj G. Effect of hearing aids on static balance function in elderly with hearing loss. Gait Posture. 2017;58:126-129. doi: 10.1016/j.gaitpost.2017.07.112
93. Mahmoudi E, Basu T, Langa K, et al. Can hearing aids delay time to diagnosis of dementia, depression, or falls in older adults? J Am Geriatr Soc. 2019;67:2362-2369. doi: 10.1111/jgs.16109
94. Paliwal Y, Slattum PW, Ratliff SM. Chronic health conditions as a risk factor for falls among the community-dwelling US older adults: a zero-inflated regression modeling approach. Biomed Res Int. 2017;2017:5146378. doi: 10.1155/2017/5146378
95. Deandrea S, Lucenteforte E, Bravi F, et al. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21:658-668. doi: 10.1097/EDE.0b013e3181e89905
96. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61. doi: 10.1016/j.maturitas.2013.02.009
97. Stevens M, Holman CD, Bennett N. Preventing falls in older people: impact of an intervention to reduce environmental hazards in the home. J Am Geriatr Soc. 2001;49:1442-1447. doi: 10.1046/j.1532-5415.2001.4911235.x
98. Clinical resources. Centers for Disease Control and Prevention STEADI-Older Adult Fall Prevention website. 2020. Accessed November 12, 2021. www.cdc.gov/steadi/materials.html
99. ; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1696-1704. doi: 10.1001/jama.2018.3097
CASE Mr. J, a 75-year-old man, presents to your family practice reporting that he feels increasingly unsteady and slow while walking. He fell twice last year, without resulting injury. He now worries about tripping while walking around the house and relies on his spouse to run errands.
Clearly, Mr. J is experiencing a problem with balance. What management approach should you undertake to prevent him from falling?

Balance disorders are common in older people and drastically hinder quality of life.1-4 Patients often describe imbalance as vague symptoms: dizziness, unsteadiness, faintness, spinning sensations.5,6 Importantly, balance disorders disrupt normal gait and contribute to falls that are a major cause of disability and morbidity in older people. Almost 30% of people older than 65 years report 1 or more falls annually.7 Factors that increase the risk of falls include impaired mobility, previously reported falls, reduced psychological functioning, chronic medical conditions, and polypharmacy.7,8
The cause of any single case of imbalance is often multifactorial, resulting from dysfunction of multiple body systems (TABLE 17-56); in our clinical experience, most patients with imbalance and who are at risk of falls do not have a detectable deficit of the vestibular system. These alterations in function arise in 3 key systems—vision, proprioception, and vestibular function—which signal to, and are incorporated by, the cerebellum to mediate balance. Cognitive and neurologic decline are also factors in imbalance.

Considering that 20% of falls result in serious injury in older populations, it is important to identify balance disorders and implement preventive strategies to mitigate harmful consequences of falls on patients’ health and independence.7,57 In this article, we answer the question that the case presentation raises about the proper management approach to imbalance in family practice, including assessment of risk and rehabilitation strategies to reduce the risk of falls. Our insights and recommendations are based on our clinical experience and a review of the medical literature from the past 40 years.

CASE Mr. J has a history of hypertension, age-related hearing loss, and osteoarthritis of the knees; he has not had surgery for the arthritis. His medications are antihypertensives and extra-strength acetaminophen for knee pain.

Making the diagnosis of a balance disorder
History
A thorough clinical history, often including a collateral history from caregivers, narrows the differential diagnosis. Information regarding onset, duration, timing, character, and previous episodes of imbalance is essential. Symptoms of imbalance are often challenging for the patient to describe: They might use terms such as vertigo or dizziness, when, in fact, on further questioning, they are describing balance difficulties. Inquiry into (1) their use of assistive walking devices and (2) development or exacerbation of neurologic, musculoskeletal, auditory, visual, and mood symptoms is necessary. Note the current level of their mobility, episodes of pain or fatigue, previous falls and associated injuries, fear of falling, balance confidence, and sensations that precede falls.58
Continue to: The medical and surgical histories
The medical and surgical histories are key pieces of information. The history of smoking, alcohol habits, and substance use is relevant.
A robust medication history is essential to evaluate a patient’s risk of falling. Polypharmacy—typically, defined as taking 4 or more medications—has been repeatedly associated with a heightened risk of falls.53,59-61 Moreover, a dose-dependent association between polypharmacy and hospitalization following falls has been identified, and demonstrates that taking 10 or more medications greatly increases the risk of hospitalization.59 Studies of polypharmacy cement the importance of inquiring about medication use when assessing imbalance, particularly in older patients.
Physical examination
A focused and detailed physical examination provides insight into systems that should be investigated:
- Obtain vital signs, including orthostatic vitals to test for orthostatic hypotension62; keep in mind that symptoms of orthostatic dizziness can occur without orthostatic hypotension.
- Examine gait, which can distinguish between causes of imbalance (TABLE 2).21,40,63-70
- Perform a cardiac examination.
- Assess visual acuity and visual fields; test for nystagmus and identify any optic-nerve and retinal abnormalities.
- Evaluate lower-limb sensation, proprioception, and motor function.
- Evaluate suspected vestibular dysfunction, including dysfunction with positional testing (the Dix-Hallpike maneuver71). The patient is taken from sitting to supine while the head is rotated 45° to the tested side by the examiner. As the patient moves into a supine position, the neck is extended 30° off the table and held for at least 30 seconds. The maneuver is positive if torsional nystagmus is noted while the head is held rotated during neck extension. The maneuver is negative if the patient reports dizziness, vertigo, unsteadiness, or “pressure in the head.” Torsional nystagmus must be present to confirm a diagnosis of benign paroxysmal positional vertigo.
- If you suspect a central nervous system cause of imbalance, assess the cranial nerves, coordination, strength, and, of course, balance.

CASE
Mr. J’s physical examination showed normal vital signs without significant postural changes in blood pressure. Gait analysis revealed a slowed gait, with reduced range of motion in both knees over the entire gait cycle. Audiometry revealed symmetric moderate sensorineural hearing loss characteristic of presbycusis.
Diagnostic investigations
Consider focused investigations into imbalance based on the history and physical examination. We discourage overly broad testing and imaging; in primary care, cost and limited access to technology can bar robust investigations into causes of imbalance. However, identification of acute pathologies should prompt immediate referral to the emergency department. Furthermore, specific symptoms (TABLE 17-56) should prompt referral to specialists for assessment.
Continue to: In the emergency department...
In the emergency department and academic hospitals, key investigations can identify causes of imbalance:
- Electrocardiography and Holter monitoring test for cardiac arrhythmias.
- Echocardiography identifies structural abnormalities.
- Radiography and computed tomography are useful for detecting musculoskeletal abnormalities.
- Bone densitometry can identify osteoporosis.
- Head and spinal cord magnetic resonance imaging can be used to identify lesions of the central nervous system.
- Computed tomographic angiography of the head and neck is useful for identifying stroke, cerebral atrophy, and stenotic lesions of the carotid and vertebral arteries.
- Nerve conduction studies and levels of serum vitamin B12, hemoglobin A1C, thyroid-stimulating hormone, and random cortisol can uncover causes of peripheral neuropathy.
- Bedside cognitive screening tests can be used to measure cognitive decline.72
- Suspicion of vestibular disease requires audiometry and vestibular testing, including videonystagmography, head impulse testing, and vestibular evoked myogenic potentials.
In many cases of imbalance, no specific underlying correctable cause is discovered.
Management of imbalance
Pharmacotherapy
Targeted pharmacotherapy can be utilized in select clinical scenarios:
- Medical treatment of peripheral neuropathy should target the underlying condition.
- Cognitive behavioral therapy and antidepressants are useful for treating anxiety and depressive disorders.73
- Musculoskeletal pain can be managed with acetaminophen and topical nonsteroidal anti-inflammatory drugs (NSAIDs), using a short course of an oral NSAID when needed.74
- Cardiovascular disease management might include any of several classes of pharmacotherapy, including antiplatelet and lipid-lowering medications, antiarrhythmic drugs, and antihypertensive agents.
- Acute episodes of vertigo due to vestibular neuritis or labyrinthitis can be managed with an antiemetic.46
Surgical treatment
Surgery is infrequently considered for patients with imbalance. Examples of indications include microsurgical resection of vestibular schwannoma, resection of central nervous system tumors, lens replacement surgery for cataract, surgical management of severe spinal fracture, and hip or knee arthroplasty in select patients.
Tools for assessing the risk of falls
Scoring systems called falls risk assessment tools, or FRAT, have been developed to gauge a patient’s risk of falling. The various FRATs differ in specificity and sensitivity for predicting the risk of falls, and are typically designed for specific clinical environments, such as hospital inpatient care or long-term care facilities. Specifically, FRATs attempt to classify risk using sets of risk factors known to be associated with falls.
Continue to: Research abounds into...
Research abounds into the validity of commonly used FRATs across institutions, patient populations, and clinical environments:
The Johns Hopkins FRATa determines risk using metrics such as age, fall history, incontinence, cognition, mobility, and medications75; it is predominantly used for assessment in hospital inpatient units. This tool has been validated repeatedly.76,77
Peninsula Health FRATb stratifies patients in subacute and residential aged-care settings, based on risk factors that include recent falls, medications, psychological status, and cognition.78
FRAT-upc is a web-based tool that generates falls risk using risk factors that users input. This tool has been studied in the context of patients older than 65 years living in the community.79
Although FRATs are reasonably useful for predicting falls, their utility varies by patient population and clinical context. Moreover, it has been suggested that FRATs neglect environmental and personal factors when assessing risk by focusing primarily on bodily factors.80 Implementing a FRAT requires extensive consideration of the target population and should be accompanied by clinical judgment that is grounded in an individual patient’s circumstances.81
Continue to: Preventing falls in primary care
Preventing falls in primary care
An approach to preventing falls includes the development of individualized programs that account for frailty, a syndrome of physiologic decline associated with aging. Because frailty leads to diminished balance and mobility, a patient’s frailty index—determined using the 5 frailty phenotype criteria (exhaustion, weight loss, low physical activity, weakness, slowness)82 or the Canadian Study of Health and Aging Clinical Frailty Scale83—is a useful tool for predicting falls risk and readmission for falls following trauma-related injury. Prevention of falls in communities is critical for reducing mortality and allowing older people to maintain their independence and quality of life.
Exercise. In some areas, exercise and falls prevention programs are accessible to seniors.84 Community exercise programs that focus on balance retraining and muscle strengthening can reduce the risk of falls.73,85 The Choosing Wisely initiative of the ABIM [American Board of Internal Medicine] Foundation recommends that exercise programs be designed around an accurate functional baseline of the patient to avoid underdosed strength training.54
Multifactorial risk assessment in high-risk patients can reduce the rate of falls. Such an assessment includes examination of orthostatic blood pressure, vision and hearing, bone health, gait, activities of daily living, cognition, and environmental hazards, and enables provision of necessary interventions.73,86 Hearing amplification, specifically, correlates with enhanced postural control, slowed cognitive decline, and a reduced likelihood of falls.87-93 The mechanism behind improved balance performance might be reduced cognitive load through supporting a patient’s listening needs.88-90
Pharmacotherapy. Optimizing medications and performing a complete medication review before prescribing new medications is highly recommended to avoid unnecessary polypharmacy7,8,18,53-56 (TABLE 17-56).
Management of comorbidities associated with a higher risk of falls, including arthritis, cancer, stroke, diabetes, depression, kidney disease, chronic obstructive pulmonary disease, cognitive impairment, hypertension, and atrial fibrillation, is essential.94-96
Continue to: Home safety interventions
Home safety interventions, through occupational therapy, are important. These include removing unsafe mats and step-overs and installing nonslip strips on stairs, double-sided tape under mats, and handrails.73-97
Screening for risk of falls. The Centers for Disease Control and Prevention recommends that (1) all patients older than 65 years and (2) any patient presenting with an acute fall undergo screening for their risk of falls.98 When a patient is identified as at risk of falling, you can, when appropriate, assess modifiable risk factors and facilitate interventions.98 This strategy is supported by a 2018 statement from the US Preventive Services Task Force99 that recommends identifying high-risk patients who have:
- a history of falling
- a balance disturbance that causes a deficit of mobility or function
- poor performance on clinical tests, such as the 3-meter Timed Up and Go (TUG) assessment (www.cdc.gov/steadi/pdf/TUG_test-print.pdf).
An increased risk of falls should prompt you to refer the patient to community programs and physiotherapy in accordance with the individual’s personal goals99; a balance and vestibular physiotherapist is ideally positioned to accurately assess and manage patients at risk of falls. Specifically, the Task Force identified exercise programs and multifactorial interventions as being beneficial in preventing falls in high-risk older people.99
Balance assessment and rehabilitation in specialty centers
An individualized rehabilitation program aims to restore safe mobility by testing and addressing specific balance deficits, improving functional balance, and increasing balance confidence. Collaboration with colleagues from physiotherapy and occupational therapy aids in tailoring individualized programs.
Many tests are available to assess balance, determine the risk of falls, and guide rehabilitation:
- The timed 10-meter walk testd and the TUG test are simple assessments that measure functional mobility; both have normalized values for the risk of falls. A TUG time of ≥ 12 seconds suggests a high risk of falls.
- The 30-second chair stande evaluates functional lower-extremity strength in older patients. The test can indicate if lower-extremity strength is contributing to a patient’s imbalance.
- The modified clinical test of sensory interaction in balancef is a static balance test that measures the integrity of sensory inputs. The test can suggest if 1 or more sensory systems are compromised.
- The mini balance evaluation systems testg is similar: It can differentiate balance deficits by underlying system and allows individualization of a rehabilitation program.
- The functional gait assessmenth is a modification of the dynamic gait index that assesses postural stability during everyday dynamic activities, including tasks such as walking with head turns and pivots.
- The Berg Balance Scalei continues to be used extensively to assess balance.
Continue to: The mini balance evaluation systems test...
The mini balance evaluation systems test, functional gait index, and Berg Balance Scale all have normative age-graded values to predict fall risk.
CASE
Mr. J was referred for balance assessment and to a rehabilitation program. He underwent balance physiotherapy, including multifactorial balance assessment, joined a community exercise program, was fitted with hearing aids, and had his home environment optimized by an occupational therapist. (See examples of “home safety interventions” under “Preventing falls in primary care.”)
3 months later. Mr. J says he feels stronger on his feet. His knee pain has eased, and he is more confident walking around his home. He continues to engage in exercise programs and is comfortable running errands with his spouse.
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Division of OtolaryngologyHead and Neck Surgery, Queen’s University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected]
a www.hopkinsmedicine.org/institute_nursing/models_tools/jhfrat_acute%20care%20original_6_22_17.pdf
c www.ncbi.nlm.nih.gov/pmc/articles/PMC4376110/figure/figure14/?report=objectonly
e www.cdc.gov/steadi/pdf/STEADI-Assessment-30Sec-508.pdf
f www.mdapp.co/mctsib-modified-clinical-test-of-sensory-interaction-in-balance-calculator-404/
g www.sralab.org/sites/default/files/2017-07/MiniBEST_revised_final_3_8_13.pdf
CASE Mr. J, a 75-year-old man, presents to your family practice reporting that he feels increasingly unsteady and slow while walking. He fell twice last year, without resulting injury. He now worries about tripping while walking around the house and relies on his spouse to run errands.
Clearly, Mr. J is experiencing a problem with balance. What management approach should you undertake to prevent him from falling?

Balance disorders are common in older people and drastically hinder quality of life.1-4 Patients often describe imbalance as vague symptoms: dizziness, unsteadiness, faintness, spinning sensations.5,6 Importantly, balance disorders disrupt normal gait and contribute to falls that are a major cause of disability and morbidity in older people. Almost 30% of people older than 65 years report 1 or more falls annually.7 Factors that increase the risk of falls include impaired mobility, previously reported falls, reduced psychological functioning, chronic medical conditions, and polypharmacy.7,8
The cause of any single case of imbalance is often multifactorial, resulting from dysfunction of multiple body systems (TABLE 17-56); in our clinical experience, most patients with imbalance and who are at risk of falls do not have a detectable deficit of the vestibular system. These alterations in function arise in 3 key systems—vision, proprioception, and vestibular function—which signal to, and are incorporated by, the cerebellum to mediate balance. Cognitive and neurologic decline are also factors in imbalance.

Considering that 20% of falls result in serious injury in older populations, it is important to identify balance disorders and implement preventive strategies to mitigate harmful consequences of falls on patients’ health and independence.7,57 In this article, we answer the question that the case presentation raises about the proper management approach to imbalance in family practice, including assessment of risk and rehabilitation strategies to reduce the risk of falls. Our insights and recommendations are based on our clinical experience and a review of the medical literature from the past 40 years.

CASE Mr. J has a history of hypertension, age-related hearing loss, and osteoarthritis of the knees; he has not had surgery for the arthritis. His medications are antihypertensives and extra-strength acetaminophen for knee pain.

Making the diagnosis of a balance disorder
History
A thorough clinical history, often including a collateral history from caregivers, narrows the differential diagnosis. Information regarding onset, duration, timing, character, and previous episodes of imbalance is essential. Symptoms of imbalance are often challenging for the patient to describe: They might use terms such as vertigo or dizziness, when, in fact, on further questioning, they are describing balance difficulties. Inquiry into (1) their use of assistive walking devices and (2) development or exacerbation of neurologic, musculoskeletal, auditory, visual, and mood symptoms is necessary. Note the current level of their mobility, episodes of pain or fatigue, previous falls and associated injuries, fear of falling, balance confidence, and sensations that precede falls.58
Continue to: The medical and surgical histories
The medical and surgical histories are key pieces of information. The history of smoking, alcohol habits, and substance use is relevant.
A robust medication history is essential to evaluate a patient’s risk of falling. Polypharmacy—typically, defined as taking 4 or more medications—has been repeatedly associated with a heightened risk of falls.53,59-61 Moreover, a dose-dependent association between polypharmacy and hospitalization following falls has been identified, and demonstrates that taking 10 or more medications greatly increases the risk of hospitalization.59 Studies of polypharmacy cement the importance of inquiring about medication use when assessing imbalance, particularly in older patients.
Physical examination
A focused and detailed physical examination provides insight into systems that should be investigated:
- Obtain vital signs, including orthostatic vitals to test for orthostatic hypotension62; keep in mind that symptoms of orthostatic dizziness can occur without orthostatic hypotension.
- Examine gait, which can distinguish between causes of imbalance (TABLE 2).21,40,63-70
- Perform a cardiac examination.
- Assess visual acuity and visual fields; test for nystagmus and identify any optic-nerve and retinal abnormalities.
- Evaluate lower-limb sensation, proprioception, and motor function.
- Evaluate suspected vestibular dysfunction, including dysfunction with positional testing (the Dix-Hallpike maneuver71). The patient is taken from sitting to supine while the head is rotated 45° to the tested side by the examiner. As the patient moves into a supine position, the neck is extended 30° off the table and held for at least 30 seconds. The maneuver is positive if torsional nystagmus is noted while the head is held rotated during neck extension. The maneuver is negative if the patient reports dizziness, vertigo, unsteadiness, or “pressure in the head.” Torsional nystagmus must be present to confirm a diagnosis of benign paroxysmal positional vertigo.
- If you suspect a central nervous system cause of imbalance, assess the cranial nerves, coordination, strength, and, of course, balance.

CASE
Mr. J’s physical examination showed normal vital signs without significant postural changes in blood pressure. Gait analysis revealed a slowed gait, with reduced range of motion in both knees over the entire gait cycle. Audiometry revealed symmetric moderate sensorineural hearing loss characteristic of presbycusis.
Diagnostic investigations
Consider focused investigations into imbalance based on the history and physical examination. We discourage overly broad testing and imaging; in primary care, cost and limited access to technology can bar robust investigations into causes of imbalance. However, identification of acute pathologies should prompt immediate referral to the emergency department. Furthermore, specific symptoms (TABLE 17-56) should prompt referral to specialists for assessment.
Continue to: In the emergency department...
In the emergency department and academic hospitals, key investigations can identify causes of imbalance:
- Electrocardiography and Holter monitoring test for cardiac arrhythmias.
- Echocardiography identifies structural abnormalities.
- Radiography and computed tomography are useful for detecting musculoskeletal abnormalities.
- Bone densitometry can identify osteoporosis.
- Head and spinal cord magnetic resonance imaging can be used to identify lesions of the central nervous system.
- Computed tomographic angiography of the head and neck is useful for identifying stroke, cerebral atrophy, and stenotic lesions of the carotid and vertebral arteries.
- Nerve conduction studies and levels of serum vitamin B12, hemoglobin A1C, thyroid-stimulating hormone, and random cortisol can uncover causes of peripheral neuropathy.
- Bedside cognitive screening tests can be used to measure cognitive decline.72
- Suspicion of vestibular disease requires audiometry and vestibular testing, including videonystagmography, head impulse testing, and vestibular evoked myogenic potentials.
In many cases of imbalance, no specific underlying correctable cause is discovered.
Management of imbalance
Pharmacotherapy
Targeted pharmacotherapy can be utilized in select clinical scenarios:
- Medical treatment of peripheral neuropathy should target the underlying condition.
- Cognitive behavioral therapy and antidepressants are useful for treating anxiety and depressive disorders.73
- Musculoskeletal pain can be managed with acetaminophen and topical nonsteroidal anti-inflammatory drugs (NSAIDs), using a short course of an oral NSAID when needed.74
- Cardiovascular disease management might include any of several classes of pharmacotherapy, including antiplatelet and lipid-lowering medications, antiarrhythmic drugs, and antihypertensive agents.
- Acute episodes of vertigo due to vestibular neuritis or labyrinthitis can be managed with an antiemetic.46
Surgical treatment
Surgery is infrequently considered for patients with imbalance. Examples of indications include microsurgical resection of vestibular schwannoma, resection of central nervous system tumors, lens replacement surgery for cataract, surgical management of severe spinal fracture, and hip or knee arthroplasty in select patients.
Tools for assessing the risk of falls
Scoring systems called falls risk assessment tools, or FRAT, have been developed to gauge a patient’s risk of falling. The various FRATs differ in specificity and sensitivity for predicting the risk of falls, and are typically designed for specific clinical environments, such as hospital inpatient care or long-term care facilities. Specifically, FRATs attempt to classify risk using sets of risk factors known to be associated with falls.
Continue to: Research abounds into...
Research abounds into the validity of commonly used FRATs across institutions, patient populations, and clinical environments:
The Johns Hopkins FRATa determines risk using metrics such as age, fall history, incontinence, cognition, mobility, and medications75; it is predominantly used for assessment in hospital inpatient units. This tool has been validated repeatedly.76,77
Peninsula Health FRATb stratifies patients in subacute and residential aged-care settings, based on risk factors that include recent falls, medications, psychological status, and cognition.78
FRAT-upc is a web-based tool that generates falls risk using risk factors that users input. This tool has been studied in the context of patients older than 65 years living in the community.79
Although FRATs are reasonably useful for predicting falls, their utility varies by patient population and clinical context. Moreover, it has been suggested that FRATs neglect environmental and personal factors when assessing risk by focusing primarily on bodily factors.80 Implementing a FRAT requires extensive consideration of the target population and should be accompanied by clinical judgment that is grounded in an individual patient’s circumstances.81
Continue to: Preventing falls in primary care
Preventing falls in primary care
An approach to preventing falls includes the development of individualized programs that account for frailty, a syndrome of physiologic decline associated with aging. Because frailty leads to diminished balance and mobility, a patient’s frailty index—determined using the 5 frailty phenotype criteria (exhaustion, weight loss, low physical activity, weakness, slowness)82 or the Canadian Study of Health and Aging Clinical Frailty Scale83—is a useful tool for predicting falls risk and readmission for falls following trauma-related injury. Prevention of falls in communities is critical for reducing mortality and allowing older people to maintain their independence and quality of life.
Exercise. In some areas, exercise and falls prevention programs are accessible to seniors.84 Community exercise programs that focus on balance retraining and muscle strengthening can reduce the risk of falls.73,85 The Choosing Wisely initiative of the ABIM [American Board of Internal Medicine] Foundation recommends that exercise programs be designed around an accurate functional baseline of the patient to avoid underdosed strength training.54
Multifactorial risk assessment in high-risk patients can reduce the rate of falls. Such an assessment includes examination of orthostatic blood pressure, vision and hearing, bone health, gait, activities of daily living, cognition, and environmental hazards, and enables provision of necessary interventions.73,86 Hearing amplification, specifically, correlates with enhanced postural control, slowed cognitive decline, and a reduced likelihood of falls.87-93 The mechanism behind improved balance performance might be reduced cognitive load through supporting a patient’s listening needs.88-90
Pharmacotherapy. Optimizing medications and performing a complete medication review before prescribing new medications is highly recommended to avoid unnecessary polypharmacy7,8,18,53-56 (TABLE 17-56).
Management of comorbidities associated with a higher risk of falls, including arthritis, cancer, stroke, diabetes, depression, kidney disease, chronic obstructive pulmonary disease, cognitive impairment, hypertension, and atrial fibrillation, is essential.94-96
Continue to: Home safety interventions
Home safety interventions, through occupational therapy, are important. These include removing unsafe mats and step-overs and installing nonslip strips on stairs, double-sided tape under mats, and handrails.73-97
Screening for risk of falls. The Centers for Disease Control and Prevention recommends that (1) all patients older than 65 years and (2) any patient presenting with an acute fall undergo screening for their risk of falls.98 When a patient is identified as at risk of falling, you can, when appropriate, assess modifiable risk factors and facilitate interventions.98 This strategy is supported by a 2018 statement from the US Preventive Services Task Force99 that recommends identifying high-risk patients who have:
- a history of falling
- a balance disturbance that causes a deficit of mobility or function
- poor performance on clinical tests, such as the 3-meter Timed Up and Go (TUG) assessment (www.cdc.gov/steadi/pdf/TUG_test-print.pdf).
An increased risk of falls should prompt you to refer the patient to community programs and physiotherapy in accordance with the individual’s personal goals99; a balance and vestibular physiotherapist is ideally positioned to accurately assess and manage patients at risk of falls. Specifically, the Task Force identified exercise programs and multifactorial interventions as being beneficial in preventing falls in high-risk older people.99
Balance assessment and rehabilitation in specialty centers
An individualized rehabilitation program aims to restore safe mobility by testing and addressing specific balance deficits, improving functional balance, and increasing balance confidence. Collaboration with colleagues from physiotherapy and occupational therapy aids in tailoring individualized programs.
Many tests are available to assess balance, determine the risk of falls, and guide rehabilitation:
- The timed 10-meter walk testd and the TUG test are simple assessments that measure functional mobility; both have normalized values for the risk of falls. A TUG time of ≥ 12 seconds suggests a high risk of falls.
- The 30-second chair stande evaluates functional lower-extremity strength in older patients. The test can indicate if lower-extremity strength is contributing to a patient’s imbalance.
- The modified clinical test of sensory interaction in balancef is a static balance test that measures the integrity of sensory inputs. The test can suggest if 1 or more sensory systems are compromised.
- The mini balance evaluation systems testg is similar: It can differentiate balance deficits by underlying system and allows individualization of a rehabilitation program.
- The functional gait assessmenth is a modification of the dynamic gait index that assesses postural stability during everyday dynamic activities, including tasks such as walking with head turns and pivots.
- The Berg Balance Scalei continues to be used extensively to assess balance.
Continue to: The mini balance evaluation systems test...
The mini balance evaluation systems test, functional gait index, and Berg Balance Scale all have normative age-graded values to predict fall risk.
CASE
Mr. J was referred for balance assessment and to a rehabilitation program. He underwent balance physiotherapy, including multifactorial balance assessment, joined a community exercise program, was fitted with hearing aids, and had his home environment optimized by an occupational therapist. (See examples of “home safety interventions” under “Preventing falls in primary care.”)
3 months later. Mr. J says he feels stronger on his feet. His knee pain has eased, and he is more confident walking around his home. He continues to engage in exercise programs and is comfortable running errands with his spouse.
CORRESPONDENCE
Jason A. Beyea, MD, PhD, FRCSC, Division of OtolaryngologyHead and Neck Surgery, Queen’s University, 144 Brock Street, Kingston, Ontario, Canada, K7L 5G2; [email protected]
a www.hopkinsmedicine.org/institute_nursing/models_tools/jhfrat_acute%20care%20original_6_22_17.pdf
c www.ncbi.nlm.nih.gov/pmc/articles/PMC4376110/figure/figure14/?report=objectonly
e www.cdc.gov/steadi/pdf/STEADI-Assessment-30Sec-508.pdf
f www.mdapp.co/mctsib-modified-clinical-test-of-sensory-interaction-in-balance-calculator-404/
g www.sralab.org/sites/default/files/2017-07/MiniBEST_revised_final_3_8_13.pdf
1. Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 2011;4:189-201. doi: 10.2165/11591150-000000000-00000
2. TB, ZF, ES, et al. The relationship of balance disorders with falling, the effect of health problems, and social life on postural balance in the elderly living in a district in Turkey. Geriatrics (Basel). 2019;4:37. doi: 10.3390/geriatrics4020037
3. R, Sixt E, Landahl S, et al. Prevalence of dizziness and vertigo in an urban elderly population. J Vestib Res. 2004;14:47-52.
4. Sturnieks DL, St George R, Lord SR. Balance disorders in the elderly. Neurophysiol Clin. 2008;38:467-478. doi: 10.1016/j.neucli.2008.09.001
5. Boult C, Murphy J, Sloane P, et al. The relation of dizziness to functional decline. J Am Geriatr Soc. 1991;39:858-861. doi: 10.1111/j.1532-5415.1991.tb04451.x
6. Lin HW, Bhattacharyya N. Balance disorders in the elderly: epidemiology and functional impact. Laryngoscope. 2012;122:1858-1861. doi: 10.1002/lary.23376
7. Jia H, Lubetkin EI, DeMichele K, et al. Prevalence, risk factors, and burden of disease for falls and balance or walking problems among older adults in the U.S. Prev Med. 2019;126:105737. doi: 10.1016/j.ypmed.2019.05.025
8. Al-Momani M, Al-Momani F, Alghadir AH, et al. Factors related to gait and balance deficits in older adults. Clin Interv Aging. 2016;11:1043-1049. doi: 10.2147/CIA.S112282
9. Agrawal Y, Ward BK, Minor LB. Vestibular dysfunction: prevalence, impact and need for targeted treatment. J Vestib Res. 2013;23:113-117. doi: 10.3233/VES-130498
10. Altinsoy B, Erboy F, Tanriverdi H, et al. Syncope as a presentation of acute pulmonary embolism. Ther Clin Risk Manag. 2016;12:1023-1028. doi: 10.2147/TCRM.S105722
11. Belvederi Murri M, Triolo F, Coni A, et al. Instrumental assessment of balance and gait in depression: a systematic review. Psychiatry Res. 2020;284:112687. doi: 10.1016/j.psychres.2019.112687
12. Bhattacharyya N, Gubbels SP, Schwartz SR, et al. Clinical practice guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg. 2017;156(suppl 3):S1-S47. doi: 10.1177/0194599816689667
13. S, Schwarm S, Grevenrath P, et al. Prevalence, aetiologies and prognosis of the symptom dizziness in primary care - a systematic review. BMC Fam Pract. 2018;19:33. doi: 10.1186/s12875-017-0695-0
14. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467-492. doi: 10.1128/CMR.00070-09
15. Chad DA. Lumbar spinal stenosis. Neurol Clin. 2007;25:407-418. doi: 10.1016/j.ncl.2007.01.003
16. Conrad BP, Shokat MS, Abbasi AZ, et al. Associations of self-report measures with gait, range of motion and proprioception in patients with lumbar spinal stenosis. Gait Posture. 2013;38:987-992. doi: 10.1016/j.gaitpost.2013.05.010
17. de Luna RA, Mihailovic A, Nguyen AM, et al. The association of glaucomatous visual field loss and balance. Transl Vis Sci Technol. 2017;6:8. doi: 10.1167/tvst.6.3.8
18. DiSogra RM. Common aminoglycosides and platinum-based ototoxic drugs: cochlear/vestibular side effects and incidence. Semin Hear. 2019;40:104-107. doi: 10.1055/s-0039-1684040
19. Ebersbach G, Moreau C, Gandor F, et al. Clinical syndromes: parkinsonian gait. Mov Disord. 2013;28:1552-1559. doi: 10.1002/mds.25675
20. Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S-1127S. doi: 10.3945/ajcn.2010.28608A
21. Filli L, Sutter T, Easthope CS, et al. Profiling walking dysfunction in multiple sclerosis: characterisation, classification and progression over time. Sci Rep. 2018;8:4984. doi: 10.1038/s41598-018-22676-0
22. Fritz NE, Kegelmeyer DA, Kloos AD, et al. Motor performance differentiates individuals with Lewy body dementia, Parkinson’s and Alzheimer’s disease. Gait Posture. 2016;50:1-7. doi: 10.1016/j.gaitpost.2016.08.009
23. Furman JM, Jacob RG. A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J Anxiety Disord. 2001;15:9-26. doi: 10.1016/s0887-6185(00)00040-2
24. Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12:706-715. doi: 10.1016/S1474-4422(13)70107-8
25. Gerson LW, Jarjoura D, McCord G. Risk of imbalance in elderly people with impaired hearing or vision. Age Ageing. 1989;18:31-34. doi: 10.1093/ageing/18.1.31
26. Goudakos JK, Markou KD, Franco-Vidal V, et al. Corticosteroids in the treatment of vestibular neuritis: a systematic review and meta-analysis. Otol Neurotol. 2010;31:183-189. doi: 10.1097/MAO.0b013e3181ca843d
27. Green AD, CS, Bastian L, et al. Does this woman have osteoporosis? JAMA. 2004;292:2890-2900. doi: 10.1001/jama.292.23.2890
28. Hallemans A, Ortibus E, Meire F, et al. Low vision affects dynamic stability of gait. Gait Posture. 2010;32:547-551. doi: 10.1016/j.gaitpost.2010.07.018
29. Handelsman JA. Vestibulotoxicity: strategies for clinical diagnosis and rehabilitation. Int J Audiol. 2018;57(suppl 4):S99-S107. doi: 10.1080/14992027.2018.1468092
30. Head VA, Wakerley BR. Guillain-Barré syndrome in general practice: clinical features suggestive of early diagnosis. Br J Gen Pract. 2016;66:218-219. doi: 10.3399/bjgp16X684733
31. Helbostad JL, Vereijken B, Hesseberg K, et al. Altered vision destabilizes gait in older persons. Gait Posture. 2009;30:233-238. doi: 10.1016/j.gaitpost.2009.05.004
32. Hsu W-L, Chen C-Y, Tsauo J-Y, et al. Balance control in elderly people with osteoporosis. J Formos Med Assoc. 2014;113:334-339. doi: 10.1016/j.jfma.2014.02.006
33. Kim H-S, Yun DH, Yoo SD, et al. Balance control and knee osteoarthritis severity. Ann Rehabil Med. 2011;35:701-709. doi: 10.5535/arm.2011.35.5.701
34. Li L, Simonsick EM, Ferrucci L, et al. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2013;38:25-29.
35. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88-100. doi: 10.1212/WNL.0000000000004058
36. Milner KA, Funk M, Richards S, et al. Gender differences in symptom presentation associated with coronary heart disease. Am J Cardiol. 1999;84:396-399. doi: 10.1016/s0002-9149(99)00322-7
37. Paillard T, F, Bru N, et al. The impact of time of day on the gait and balance control of Alzheimer’s patients. Chronobiol Int. 2016;33:161-168. doi: 10.3109/07420528.2015.1124885
38. Paldor I, Chen AS, Kaye AH. Growth rate of vestibular schwannoma. J Clin Neurosci. 2016;32:1-8. doi: 10.1016/j.jocn.2016.05.003
39. Picorelli AMA, Hatton AL, Gane EM, et al. Balance performance in older adults with hip osteoarthritis: a systematic review. Gait Posture. 2018;65:89-99. doi: 10.1016/j.gaitpost.2018.07.001
40. Raccagni C, Nonnekes J, Bloem BR, et al. Gait and postural disorders in parkinsonism: a clinical approach. J Neurol. 2020;267:3169-3176. doi: 10.1007/s00415-019-09382-1
41. Shanmugarajah PD, Hoggard N, Currie S, et al. Alcohol-related cerebellar degeneration: not all down to toxicity? Cerebellum Ataxias. 2016;3:17. doi: 10.1186/s40673-016-0055-1
42. Shih RY, Smirniotopoulos JG. Posterior fossa tumors in adult patients. Neuroimaging Clin N Am. 2016;26:493-510. doi: 10.1016/j.nic.2016.06.003
43. Smith EE. Clinical presentations and epidemiology of vascular dementia. Clin Sci (Lond). 2017;131:1059-1068. doi: 10.1042/CS20160607
44. Streur M, Ratcliffe SJ, Ball J, et al. Symptom clusters in adults with chronic atrial fibrillation. J Cardiovasc Nurs. 2017;32:296-303. doi: 10.1097/JCN.0000000000000344
45. Strupp M, M, JA. Peripheral vestibular disorders: an update. Curr Opin Neurol. 2019;32:165-173. doi: 10.1097/WCO.0000000000000649
46. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
47. Timar B, Timar R, L, et al. The impact of diabetic neuropathy on balance and on the risk of falls in patients with type 2 diabetes mellitus: a cross-sectional study. PLoS One. 2016;11:e0154654. doi: 10.1371/journal.pone.0154654
48. Walls R, Hockberger R, Gausche-Hill M. Peripheral nerve disorders. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 9th ed. Elsevier, Inc; 2018:1307-1320.
49. Watson JC, Dyck PJB. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc. 2015;90:940-951. doi: 10.1016/j.mayocp.2015.05.004
50. Whitfield KC, Bourassa MW, Adamolekun B, et al. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann N Y Acad Sci. 2018;1430:3-43. doi: 10.1111/nyas.13919
51. Wu V, Sykes EA, Beyea MM, et al. Approach to Meniere disease management. Can Fam Physician. 2019;65:463-467.
52. Yew KS, Cheng EM. Diagnosis of acute stroke. Am Fam Physician. 2015;91:528-536.
53. Seppala LJ, van de Glind EMM, Daams JG, et al; . Fall-risk-increasing drugs: a systematic review and meta-analysis: III. Others. J Am Med Dir Assoc. 2018;19:372.e1-372.e8. doi: 10.1016/j.jamda.2017.12.099
54. ABIM Foundation. Choosing wisely. Choosing Wisely website. 2021. Accessed November 11. 2021. www.choosingwisely.org/
55. Berlie HD, Garwood CL. Diabetes medications related to an increased risk of falls and fall-related morbidity in the elderly. Ann Pharmacother. 2010;44:712-717. doi: 10.1345/aph.1M551
56. Hartikainen S, E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62:1172-1181. doi: 10.1093/gerona/62.10.1172
57. Khanuja K, Joki J, Bachmann G, et al. Gait and balance in the aging population: Fall prevention using innovation and technology. Maturitas. 2018;110:51-56. doi: 10.1016/j.maturitas.2018.01.021
58. Salzman B. Gait and balance disorders in older adults. Am Fam Physician. 2010;82:61-68.
59. Zaninotto P, Huang YT, Di Gessa G, et al. Polypharmacy is a risk factor for hospital admission due to a fall: evidence from the English Longitudinal Study of Ageing. BMC Public Health. 2020;20:1804. doi: 10.1186/s12889-020-09920-x
60. Morin L, Calderon A, Welmer AK, et al. Polypharmacy and injurious falls in older adults: a nationwide nested case-control study. Clin Epidemiol. 2019;11:483-493. doi: 10.2147/CLEP.S201614
61. Dhalwani NN, Fahami R, Sathanapally H, et al. Association between polypharmacy and falls in older adults: a longitudinal study from England. BMJ Open. 2017;7:e016358. doi: 10.1136/bmjopen-2017-016358
62. Arnold AC, Raj SR. Orthostatic hypotension: a practical approach to investigation and management. Can J Cardiol. 2017;33:1725-1728. doi: 10.1016/j.cjca.2017.05.007
63. Alexander NB. Differential diagnosis of gait disorders in older adults. Clin Geriatr Med. 1996;12:689-703.
64. Baker JM. Gait disorders. Am J Med. 2018;131:602-607. doi: 10.1016/j.amjmed.2017.11.051
65. Cameron MH, Wagner JM. Gait abnormalities in multiple sclerosis: pathogenesis, evaluation, and advances in treatment. Curr Neurol Neurosci Rep. 2011;11:507-515. doi: 10.1007/s11910-011-0214-y
66. Chen C-L, Chen H-C, Tang SF-T, et al. Gait performance with compensatory adaptations in stroke patients with different degrees of motor recovery. Am J Phys Med Rehabil. 2003;82:925-935. doi: 10.1097/01.PHM.0000098040.13355.B5
67. Marsden J, Harris C. Cerebellar ataxia: pathophysiology and rehabilitation. Clin Rehabil. 2011;25:195-216. doi: 10.1177/0269215510382495
68. Mirek E, Filip M, W, et al. Three-dimensional trunk and lower limbs characteristics during gait in patients with Huntington’s disease. Front Neurosci. 2017;11:566. doi: 10.3389/fnins.2017.00566
69. Paramanandam V, Lizarraga KJ, Soh D, et al. Unusual gait disorders: a phenomenological approach and classification. Expert Rev Neurother. 2019;19:119-132. doi: 10.1080/14737175.2019.1562337
70. Sahyouni R, Goshtasbi K, Mahmoodi A, et al. Chronic subdural hematoma: a historical and clinical perspective. World Neurosurg. 2017;108:948-953. doi: 10.1016/j.wneu.2017.09.064
71. Talmud JD, Coffey R, Edemekong PF. Dix Hallpike maneuver. StatPearls [Internet]. StatPearls Publishing Updated September 5, 2021. Accessed December 6, 2021. www.ncbi.nlm.nih.gov/books/NBK459307/
72. Molnar FJ, Benjamin S, Hawkins SA, et al. One size does not fit all: choosing practical cognitive screening tools for your practice. J Am Geriatr Soc. 2020;68:2207-2213. doi: 10.1111/jgs.16713
73. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012:CD007146. doi: 10.1002/14651858.CD007146.pub3
74. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150. doi: 10.14336/AD.2017.0306
75. Poe SS, Cvach M, Dawson PB, Straus H, Hill EE. The Johns Hopkins Fall Risk Assessment Tool: postimplementation evaluation. J Nurs Care Qual. 2007;22:293-298. doi: 10.1097/01.NCQ.0000290408.74027.39
76. Poe SS, Dawson PB, Cvach M, et al. The Johns Hopkins Fall Risk Assessment Tool: a study of reliability and validity. J Nurs Care Qual. 2018;33:10-19. doi: 10.1097/NCQ.0000000000000301
77. Klinkenberg WD, Potter P. Validity of the Johns Hopkins Fall Risk Assessment Tool for predicting falls on inpatient medicine services. J Nurs Care Qual. 2017;32:108-113. doi: 10.1097/NCQ.0000000000000210
78. Stapleton C, Hough P, Oldmeadow L, et al. Four-item fall risk screening tool for subacute and residential aged care: the first step in fall prevention. Australas J Ageing. 2009;28:139-143. doi: 10.1111/j.1741-6612.2009.00375.x
79. Cattelani L, Palumbo P, Palmerini L, et al. FRAT-up, a Web-based fall-risk assessment tool for elderly people living in the community. J Med Internet Res. 2015;17:e41. doi: 10.2196/jmir.4064
80. De Clercq H, Naudé A, Bornman J. Factors included in adult fall risk assessment tools (FRATs): a systematic review. Ageing Soc. 2020;41:2558-2582. doi: 10.1017/S0144686X2000046X
81. Yap G, Melder A. Accuracy of validated falls risk assessment tools and clinical judgement. Centre for Clinical Effectiveness, Monash Innovation and Quality. Monash Health. February 5, 2020. Accessed November 11, 2021. https://monashhealth.org/wp-content/uploads/2019/01/Rapid-Review_Falls-risk-tools-FINAL.pdf
82. Chittrakul J, Siviroj P, Sungkarat S, et al. Physical frailty and fall risk in community-dwelling older adults: a cross-sectional study. J Aging Res. 2020;2020:3964973. doi: 10.1155/2020/3964973
83. Hatcher VH, Galet C, Lilienthal M, et al. Association of clinical frailty scores with hospital readmission for falls after index admission for trauma-related injury. JAMA Netw Open. 2019;2:e1912409. doi: 10.1001/jamanetworkopen.2019.12409
84. Exercise and fall prevention programs. Government of Ontario Ministry of Health. Updated April 9, 2019. Accessed November 11. 2021. www.ontario.ca/page/exercise-and-falls-prevention-programs
85. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1:CD012424. doi: 10.1002/14651858.CD012424.pub2
86. Hopewell S, Copsey B, Nicolson P, et al. Multifactorial interventions for preventing falls in older people living in the community: a systematic review and meta-analysis of 41 trials and almost 20 000 participants. Br J Sports Med. 2020;54:1340-1350. doi: 10.1136/bjsports-2019-100732
87. Jafari Z, Kolb BE, Mohajerani MH. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. 2019;56:100963. doi: 10.1016/j.arr.2019.100963
88. Griffiths TD, Lad M, Kumar S, et al. How can hearing loss cause dementia? Neuron. 2020;108:401-412. doi: 10.1016/j.neuron.2020.08.003
89. Martini A, Castiglione A, Bovo R, et al. Aging, cognitive load, dementia and hearing loss. Audiol Neurootol. 2014;19(suppl 1):2-5. doi: 10.1159/000371593
90. Vitkovic J, Le C, Lee S-L, et al. The contribution of hearing and hearing loss to balance control. Audiol Neurootol. 2016;21:195-202. doi: 10.1159/000445100
91. Maheu M, Behtani L, Nooristani M, et al. Vestibular function modulates the benefit of hearing aids in people with hearing loss during static postural control. Ear Hear. 2019;40:1418-1424. doi: 10.1097/AUD.0000000000000720
92. Negahban H, Bavarsad Cheshmeh Ali M, Nassadj G. Effect of hearing aids on static balance function in elderly with hearing loss. Gait Posture. 2017;58:126-129. doi: 10.1016/j.gaitpost.2017.07.112
93. Mahmoudi E, Basu T, Langa K, et al. Can hearing aids delay time to diagnosis of dementia, depression, or falls in older adults? J Am Geriatr Soc. 2019;67:2362-2369. doi: 10.1111/jgs.16109
94. Paliwal Y, Slattum PW, Ratliff SM. Chronic health conditions as a risk factor for falls among the community-dwelling US older adults: a zero-inflated regression modeling approach. Biomed Res Int. 2017;2017:5146378. doi: 10.1155/2017/5146378
95. Deandrea S, Lucenteforte E, Bravi F, et al. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21:658-668. doi: 10.1097/EDE.0b013e3181e89905
96. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61. doi: 10.1016/j.maturitas.2013.02.009
97. Stevens M, Holman CD, Bennett N. Preventing falls in older people: impact of an intervention to reduce environmental hazards in the home. J Am Geriatr Soc. 2001;49:1442-1447. doi: 10.1046/j.1532-5415.2001.4911235.x
98. Clinical resources. Centers for Disease Control and Prevention STEADI-Older Adult Fall Prevention website. 2020. Accessed November 12, 2021. www.cdc.gov/steadi/materials.html
99. ; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1696-1704. doi: 10.1001/jama.2018.3097
1. Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 2011;4:189-201. doi: 10.2165/11591150-000000000-00000
2. TB, ZF, ES, et al. The relationship of balance disorders with falling, the effect of health problems, and social life on postural balance in the elderly living in a district in Turkey. Geriatrics (Basel). 2019;4:37. doi: 10.3390/geriatrics4020037
3. R, Sixt E, Landahl S, et al. Prevalence of dizziness and vertigo in an urban elderly population. J Vestib Res. 2004;14:47-52.
4. Sturnieks DL, St George R, Lord SR. Balance disorders in the elderly. Neurophysiol Clin. 2008;38:467-478. doi: 10.1016/j.neucli.2008.09.001
5. Boult C, Murphy J, Sloane P, et al. The relation of dizziness to functional decline. J Am Geriatr Soc. 1991;39:858-861. doi: 10.1111/j.1532-5415.1991.tb04451.x
6. Lin HW, Bhattacharyya N. Balance disorders in the elderly: epidemiology and functional impact. Laryngoscope. 2012;122:1858-1861. doi: 10.1002/lary.23376
7. Jia H, Lubetkin EI, DeMichele K, et al. Prevalence, risk factors, and burden of disease for falls and balance or walking problems among older adults in the U.S. Prev Med. 2019;126:105737. doi: 10.1016/j.ypmed.2019.05.025
8. Al-Momani M, Al-Momani F, Alghadir AH, et al. Factors related to gait and balance deficits in older adults. Clin Interv Aging. 2016;11:1043-1049. doi: 10.2147/CIA.S112282
9. Agrawal Y, Ward BK, Minor LB. Vestibular dysfunction: prevalence, impact and need for targeted treatment. J Vestib Res. 2013;23:113-117. doi: 10.3233/VES-130498
10. Altinsoy B, Erboy F, Tanriverdi H, et al. Syncope as a presentation of acute pulmonary embolism. Ther Clin Risk Manag. 2016;12:1023-1028. doi: 10.2147/TCRM.S105722
11. Belvederi Murri M, Triolo F, Coni A, et al. Instrumental assessment of balance and gait in depression: a systematic review. Psychiatry Res. 2020;284:112687. doi: 10.1016/j.psychres.2019.112687
12. Bhattacharyya N, Gubbels SP, Schwartz SR, et al. Clinical practice guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg. 2017;156(suppl 3):S1-S47. doi: 10.1177/0194599816689667
13. S, Schwarm S, Grevenrath P, et al. Prevalence, aetiologies and prognosis of the symptom dizziness in primary care - a systematic review. BMC Fam Pract. 2018;19:33. doi: 10.1186/s12875-017-0695-0
14. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23:467-492. doi: 10.1128/CMR.00070-09
15. Chad DA. Lumbar spinal stenosis. Neurol Clin. 2007;25:407-418. doi: 10.1016/j.ncl.2007.01.003
16. Conrad BP, Shokat MS, Abbasi AZ, et al. Associations of self-report measures with gait, range of motion and proprioception in patients with lumbar spinal stenosis. Gait Posture. 2013;38:987-992. doi: 10.1016/j.gaitpost.2013.05.010
17. de Luna RA, Mihailovic A, Nguyen AM, et al. The association of glaucomatous visual field loss and balance. Transl Vis Sci Technol. 2017;6:8. doi: 10.1167/tvst.6.3.8
18. DiSogra RM. Common aminoglycosides and platinum-based ototoxic drugs: cochlear/vestibular side effects and incidence. Semin Hear. 2019;40:104-107. doi: 10.1055/s-0039-1684040
19. Ebersbach G, Moreau C, Gandor F, et al. Clinical syndromes: parkinsonian gait. Mov Disord. 2013;28:1552-1559. doi: 10.1002/mds.25675
20. Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S-1127S. doi: 10.3945/ajcn.2010.28608A
21. Filli L, Sutter T, Easthope CS, et al. Profiling walking dysfunction in multiple sclerosis: characterisation, classification and progression over time. Sci Rep. 2018;8:4984. doi: 10.1038/s41598-018-22676-0
22. Fritz NE, Kegelmeyer DA, Kloos AD, et al. Motor performance differentiates individuals with Lewy body dementia, Parkinson’s and Alzheimer’s disease. Gait Posture. 2016;50:1-7. doi: 10.1016/j.gaitpost.2016.08.009
23. Furman JM, Jacob RG. A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J Anxiety Disord. 2001;15:9-26. doi: 10.1016/s0887-6185(00)00040-2
24. Furman JM, Marcus DA, Balaban CD. Vestibular migraine: clinical aspects and pathophysiology. Lancet Neurol. 2013;12:706-715. doi: 10.1016/S1474-4422(13)70107-8
25. Gerson LW, Jarjoura D, McCord G. Risk of imbalance in elderly people with impaired hearing or vision. Age Ageing. 1989;18:31-34. doi: 10.1093/ageing/18.1.31
26. Goudakos JK, Markou KD, Franco-Vidal V, et al. Corticosteroids in the treatment of vestibular neuritis: a systematic review and meta-analysis. Otol Neurotol. 2010;31:183-189. doi: 10.1097/MAO.0b013e3181ca843d
27. Green AD, CS, Bastian L, et al. Does this woman have osteoporosis? JAMA. 2004;292:2890-2900. doi: 10.1001/jama.292.23.2890
28. Hallemans A, Ortibus E, Meire F, et al. Low vision affects dynamic stability of gait. Gait Posture. 2010;32:547-551. doi: 10.1016/j.gaitpost.2010.07.018
29. Handelsman JA. Vestibulotoxicity: strategies for clinical diagnosis and rehabilitation. Int J Audiol. 2018;57(suppl 4):S99-S107. doi: 10.1080/14992027.2018.1468092
30. Head VA, Wakerley BR. Guillain-Barré syndrome in general practice: clinical features suggestive of early diagnosis. Br J Gen Pract. 2016;66:218-219. doi: 10.3399/bjgp16X684733
31. Helbostad JL, Vereijken B, Hesseberg K, et al. Altered vision destabilizes gait in older persons. Gait Posture. 2009;30:233-238. doi: 10.1016/j.gaitpost.2009.05.004
32. Hsu W-L, Chen C-Y, Tsauo J-Y, et al. Balance control in elderly people with osteoporosis. J Formos Med Assoc. 2014;113:334-339. doi: 10.1016/j.jfma.2014.02.006
33. Kim H-S, Yun DH, Yoo SD, et al. Balance control and knee osteoarthritis severity. Ann Rehabil Med. 2011;35:701-709. doi: 10.5535/arm.2011.35.5.701
34. Li L, Simonsick EM, Ferrucci L, et al. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2013;38:25-29.
35. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88-100. doi: 10.1212/WNL.0000000000004058
36. Milner KA, Funk M, Richards S, et al. Gender differences in symptom presentation associated with coronary heart disease. Am J Cardiol. 1999;84:396-399. doi: 10.1016/s0002-9149(99)00322-7
37. Paillard T, F, Bru N, et al. The impact of time of day on the gait and balance control of Alzheimer’s patients. Chronobiol Int. 2016;33:161-168. doi: 10.3109/07420528.2015.1124885
38. Paldor I, Chen AS, Kaye AH. Growth rate of vestibular schwannoma. J Clin Neurosci. 2016;32:1-8. doi: 10.1016/j.jocn.2016.05.003
39. Picorelli AMA, Hatton AL, Gane EM, et al. Balance performance in older adults with hip osteoarthritis: a systematic review. Gait Posture. 2018;65:89-99. doi: 10.1016/j.gaitpost.2018.07.001
40. Raccagni C, Nonnekes J, Bloem BR, et al. Gait and postural disorders in parkinsonism: a clinical approach. J Neurol. 2020;267:3169-3176. doi: 10.1007/s00415-019-09382-1
41. Shanmugarajah PD, Hoggard N, Currie S, et al. Alcohol-related cerebellar degeneration: not all down to toxicity? Cerebellum Ataxias. 2016;3:17. doi: 10.1186/s40673-016-0055-1
42. Shih RY, Smirniotopoulos JG. Posterior fossa tumors in adult patients. Neuroimaging Clin N Am. 2016;26:493-510. doi: 10.1016/j.nic.2016.06.003
43. Smith EE. Clinical presentations and epidemiology of vascular dementia. Clin Sci (Lond). 2017;131:1059-1068. doi: 10.1042/CS20160607
44. Streur M, Ratcliffe SJ, Ball J, et al. Symptom clusters in adults with chronic atrial fibrillation. J Cardiovasc Nurs. 2017;32:296-303. doi: 10.1097/JCN.0000000000000344
45. Strupp M, M, JA. Peripheral vestibular disorders: an update. Curr Opin Neurol. 2019;32:165-173. doi: 10.1097/WCO.0000000000000649
46. Thompson TL, Amedee R. Vertigo: a review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20-26.
47. Timar B, Timar R, L, et al. The impact of diabetic neuropathy on balance and on the risk of falls in patients with type 2 diabetes mellitus: a cross-sectional study. PLoS One. 2016;11:e0154654. doi: 10.1371/journal.pone.0154654
48. Walls R, Hockberger R, Gausche-Hill M. Peripheral nerve disorders. In: Rosen’s Emergency Medicine: Concepts and Clinical Practice. 9th ed. Elsevier, Inc; 2018:1307-1320.
49. Watson JC, Dyck PJB. Peripheral neuropathy: a practical approach to diagnosis and symptom management. Mayo Clin Proc. 2015;90:940-951. doi: 10.1016/j.mayocp.2015.05.004
50. Whitfield KC, Bourassa MW, Adamolekun B, et al. Thiamine deficiency disorders: diagnosis, prevalence, and a roadmap for global control programs. Ann N Y Acad Sci. 2018;1430:3-43. doi: 10.1111/nyas.13919
51. Wu V, Sykes EA, Beyea MM, et al. Approach to Meniere disease management. Can Fam Physician. 2019;65:463-467.
52. Yew KS, Cheng EM. Diagnosis of acute stroke. Am Fam Physician. 2015;91:528-536.
53. Seppala LJ, van de Glind EMM, Daams JG, et al; . Fall-risk-increasing drugs: a systematic review and meta-analysis: III. Others. J Am Med Dir Assoc. 2018;19:372.e1-372.e8. doi: 10.1016/j.jamda.2017.12.099
54. ABIM Foundation. Choosing wisely. Choosing Wisely website. 2021. Accessed November 11. 2021. www.choosingwisely.org/
55. Berlie HD, Garwood CL. Diabetes medications related to an increased risk of falls and fall-related morbidity in the elderly. Ann Pharmacother. 2010;44:712-717. doi: 10.1345/aph.1M551
56. Hartikainen S, E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62:1172-1181. doi: 10.1093/gerona/62.10.1172
57. Khanuja K, Joki J, Bachmann G, et al. Gait and balance in the aging population: Fall prevention using innovation and technology. Maturitas. 2018;110:51-56. doi: 10.1016/j.maturitas.2018.01.021
58. Salzman B. Gait and balance disorders in older adults. Am Fam Physician. 2010;82:61-68.
59. Zaninotto P, Huang YT, Di Gessa G, et al. Polypharmacy is a risk factor for hospital admission due to a fall: evidence from the English Longitudinal Study of Ageing. BMC Public Health. 2020;20:1804. doi: 10.1186/s12889-020-09920-x
60. Morin L, Calderon A, Welmer AK, et al. Polypharmacy and injurious falls in older adults: a nationwide nested case-control study. Clin Epidemiol. 2019;11:483-493. doi: 10.2147/CLEP.S201614
61. Dhalwani NN, Fahami R, Sathanapally H, et al. Association between polypharmacy and falls in older adults: a longitudinal study from England. BMJ Open. 2017;7:e016358. doi: 10.1136/bmjopen-2017-016358
62. Arnold AC, Raj SR. Orthostatic hypotension: a practical approach to investigation and management. Can J Cardiol. 2017;33:1725-1728. doi: 10.1016/j.cjca.2017.05.007
63. Alexander NB. Differential diagnosis of gait disorders in older adults. Clin Geriatr Med. 1996;12:689-703.
64. Baker JM. Gait disorders. Am J Med. 2018;131:602-607. doi: 10.1016/j.amjmed.2017.11.051
65. Cameron MH, Wagner JM. Gait abnormalities in multiple sclerosis: pathogenesis, evaluation, and advances in treatment. Curr Neurol Neurosci Rep. 2011;11:507-515. doi: 10.1007/s11910-011-0214-y
66. Chen C-L, Chen H-C, Tang SF-T, et al. Gait performance with compensatory adaptations in stroke patients with different degrees of motor recovery. Am J Phys Med Rehabil. 2003;82:925-935. doi: 10.1097/01.PHM.0000098040.13355.B5
67. Marsden J, Harris C. Cerebellar ataxia: pathophysiology and rehabilitation. Clin Rehabil. 2011;25:195-216. doi: 10.1177/0269215510382495
68. Mirek E, Filip M, W, et al. Three-dimensional trunk and lower limbs characteristics during gait in patients with Huntington’s disease. Front Neurosci. 2017;11:566. doi: 10.3389/fnins.2017.00566
69. Paramanandam V, Lizarraga KJ, Soh D, et al. Unusual gait disorders: a phenomenological approach and classification. Expert Rev Neurother. 2019;19:119-132. doi: 10.1080/14737175.2019.1562337
70. Sahyouni R, Goshtasbi K, Mahmoodi A, et al. Chronic subdural hematoma: a historical and clinical perspective. World Neurosurg. 2017;108:948-953. doi: 10.1016/j.wneu.2017.09.064
71. Talmud JD, Coffey R, Edemekong PF. Dix Hallpike maneuver. StatPearls [Internet]. StatPearls Publishing Updated September 5, 2021. Accessed December 6, 2021. www.ncbi.nlm.nih.gov/books/NBK459307/
72. Molnar FJ, Benjamin S, Hawkins SA, et al. One size does not fit all: choosing practical cognitive screening tools for your practice. J Am Geriatr Soc. 2020;68:2207-2213. doi: 10.1111/jgs.16713
73. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012:CD007146. doi: 10.1002/14651858.CD007146.pub3
74. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150. doi: 10.14336/AD.2017.0306
75. Poe SS, Cvach M, Dawson PB, Straus H, Hill EE. The Johns Hopkins Fall Risk Assessment Tool: postimplementation evaluation. J Nurs Care Qual. 2007;22:293-298. doi: 10.1097/01.NCQ.0000290408.74027.39
76. Poe SS, Dawson PB, Cvach M, et al. The Johns Hopkins Fall Risk Assessment Tool: a study of reliability and validity. J Nurs Care Qual. 2018;33:10-19. doi: 10.1097/NCQ.0000000000000301
77. Klinkenberg WD, Potter P. Validity of the Johns Hopkins Fall Risk Assessment Tool for predicting falls on inpatient medicine services. J Nurs Care Qual. 2017;32:108-113. doi: 10.1097/NCQ.0000000000000210
78. Stapleton C, Hough P, Oldmeadow L, et al. Four-item fall risk screening tool for subacute and residential aged care: the first step in fall prevention. Australas J Ageing. 2009;28:139-143. doi: 10.1111/j.1741-6612.2009.00375.x
79. Cattelani L, Palumbo P, Palmerini L, et al. FRAT-up, a Web-based fall-risk assessment tool for elderly people living in the community. J Med Internet Res. 2015;17:e41. doi: 10.2196/jmir.4064
80. De Clercq H, Naudé A, Bornman J. Factors included in adult fall risk assessment tools (FRATs): a systematic review. Ageing Soc. 2020;41:2558-2582. doi: 10.1017/S0144686X2000046X
81. Yap G, Melder A. Accuracy of validated falls risk assessment tools and clinical judgement. Centre for Clinical Effectiveness, Monash Innovation and Quality. Monash Health. February 5, 2020. Accessed November 11, 2021. https://monashhealth.org/wp-content/uploads/2019/01/Rapid-Review_Falls-risk-tools-FINAL.pdf
82. Chittrakul J, Siviroj P, Sungkarat S, et al. Physical frailty and fall risk in community-dwelling older adults: a cross-sectional study. J Aging Res. 2020;2020:3964973. doi: 10.1155/2020/3964973
83. Hatcher VH, Galet C, Lilienthal M, et al. Association of clinical frailty scores with hospital readmission for falls after index admission for trauma-related injury. JAMA Netw Open. 2019;2:e1912409. doi: 10.1001/jamanetworkopen.2019.12409
84. Exercise and fall prevention programs. Government of Ontario Ministry of Health. Updated April 9, 2019. Accessed November 11. 2021. www.ontario.ca/page/exercise-and-falls-prevention-programs
85. Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1:CD012424. doi: 10.1002/14651858.CD012424.pub2
86. Hopewell S, Copsey B, Nicolson P, et al. Multifactorial interventions for preventing falls in older people living in the community: a systematic review and meta-analysis of 41 trials and almost 20 000 participants. Br J Sports Med. 2020;54:1340-1350. doi: 10.1136/bjsports-2019-100732
87. Jafari Z, Kolb BE, Mohajerani MH. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. 2019;56:100963. doi: 10.1016/j.arr.2019.100963
88. Griffiths TD, Lad M, Kumar S, et al. How can hearing loss cause dementia? Neuron. 2020;108:401-412. doi: 10.1016/j.neuron.2020.08.003
89. Martini A, Castiglione A, Bovo R, et al. Aging, cognitive load, dementia and hearing loss. Audiol Neurootol. 2014;19(suppl 1):2-5. doi: 10.1159/000371593
90. Vitkovic J, Le C, Lee S-L, et al. The contribution of hearing and hearing loss to balance control. Audiol Neurootol. 2016;21:195-202. doi: 10.1159/000445100
91. Maheu M, Behtani L, Nooristani M, et al. Vestibular function modulates the benefit of hearing aids in people with hearing loss during static postural control. Ear Hear. 2019;40:1418-1424. doi: 10.1097/AUD.0000000000000720
92. Negahban H, Bavarsad Cheshmeh Ali M, Nassadj G. Effect of hearing aids on static balance function in elderly with hearing loss. Gait Posture. 2017;58:126-129. doi: 10.1016/j.gaitpost.2017.07.112
93. Mahmoudi E, Basu T, Langa K, et al. Can hearing aids delay time to diagnosis of dementia, depression, or falls in older adults? J Am Geriatr Soc. 2019;67:2362-2369. doi: 10.1111/jgs.16109
94. Paliwal Y, Slattum PW, Ratliff SM. Chronic health conditions as a risk factor for falls among the community-dwelling US older adults: a zero-inflated regression modeling approach. Biomed Res Int. 2017;2017:5146378. doi: 10.1155/2017/5146378
95. Deandrea S, Lucenteforte E, Bravi F, et al. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21:658-668. doi: 10.1097/EDE.0b013e3181e89905
96. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61. doi: 10.1016/j.maturitas.2013.02.009
97. Stevens M, Holman CD, Bennett N. Preventing falls in older people: impact of an intervention to reduce environmental hazards in the home. J Am Geriatr Soc. 2001;49:1442-1447. doi: 10.1046/j.1532-5415.2001.4911235.x
98. Clinical resources. Centers for Disease Control and Prevention STEADI-Older Adult Fall Prevention website. 2020. Accessed November 12, 2021. www.cdc.gov/steadi/materials.html
99. ; Grossman DC, Curry SJ, Owens DK, et al. Interventions to prevent falls in community-dwelling older adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1696-1704. doi: 10.1001/jama.2018.3097
PRACTICE RECOMMENDATIONS
› Utilize a falls-prevention program for older patients that focuses on balance and functional exercises. A
› Perform a multifactorial assessment of the risk of falls in older patients that includes optimizing medications, managing comorbidities, and addressing environmental hazards. B
› Use a systems-based approach to presentations of imbalance to direct your clinical judgment and highlight the need for referral to specialists for management and rehabilitation. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series