User login
EMA panel endorses two cancer drugs, one sickle cell drug
The drugs are enfortumab vedotin (Padcev, Astellas/Seagen) for urothelial cancer, tepotinib (Tepmetko, Merck) for non–small cell lung cancer (NSCLC), and voxelotor (Oxbryta, Global Blood Therapeutics) for sickle cell hemolytic anemia.
EMA’s Committee for Medicinal Products for Human Use gave the nod for marketing authorization on Dec. 16, 2021, and agency approval generally follows about 6 weeks later. All three products are already approved in the United States.
Enfortumab vedotin for urothelial cancer
The recommendation for Astellas’ antibody-drug conjugate infusion was based on the phase 3 EV-301 trial, which found about a 4-month median overall survival benefit with the antibody-drug conjugate versus investigator-chosen chemotherapy across 608 patients with locally advanced or metastatic urothelial carcinoma previously treated with platinum-based chemotherapy and a programmed death 1 or PD–ligand 1 inhibitor.
The planned European indication is for adults with locally advanced or metastatic disease who met the same criteria – previous platinum-based chemotherapy plus a PD-1 or PD-L1 inhibitor.
The Food and Drug Administration approval came in December 2019, and with the same indication as well as for patients ineligible for cisplatin-containing chemotherapy who have had one or more prior lines of therapy. The U.S. labeling carries a boxed warning of severe and fatal skin reactions including Stevens-Johnson syndrome and toxic epidermal necrolysis.
Tepotinib for NSCLC
The recommendation for Merck’s tepotinib, a once-daily oral MET inhibitor, followed results from the phase 2 VISION study. The study found an investigator-assessed response rate of 56% across 152 patients with advanced or metastatic NSCLC with a confirmed MET exon 14 skipping mutation, regardless of previous therapy.
The planned European indication will be for monotherapy in adults with advanced disease harboring the mutation who require systemic therapy following prior treatment with immunotherapy and/or platinum-based chemotherapy.
The FDA approved the drug in February 2021, and carries the same indication, minus the prior therapy requirement.
Voxelotor for sickle cell disease
Voxelotor is an oral hemoglobin S polymerization inhibitor from Global Blood Therapeutics.
The European approval recommendation was based on a phase 3 trial in 274 patients with sickle cell disease that found a greater than 1 g/dL increase in hemoglobin levels at 24 weeks in 51.1% of patients versus 6.5% randomized to placebo, regardless of whether patients were on concomitant hydroxyurea.
The small molecule binds and stabilizes hemoglobin, preventing the hemoglobin polymerization that causes red blood cells to sickle.
“There is a high unmet need for medicines to treat hemolytic anemia” in sickle cell disease because available treatment options are limited to blood transfusions and allogenic hematopoietic stem cell transplantation,” the EMA explained in a press release announcing the approval recommendation.
The planned European indication is for treating hemolytic anemia in sickle cell disease in patients 12 years or older either alone or in combination with hydroxycarbamide (hydroxyurea).
The FDA approved the agent in November 2019 for the same indication, but can be given to children as young as 4 years old.
A version of this article first appeared on Medscape.com.
The drugs are enfortumab vedotin (Padcev, Astellas/Seagen) for urothelial cancer, tepotinib (Tepmetko, Merck) for non–small cell lung cancer (NSCLC), and voxelotor (Oxbryta, Global Blood Therapeutics) for sickle cell hemolytic anemia.
EMA’s Committee for Medicinal Products for Human Use gave the nod for marketing authorization on Dec. 16, 2021, and agency approval generally follows about 6 weeks later. All three products are already approved in the United States.
Enfortumab vedotin for urothelial cancer
The recommendation for Astellas’ antibody-drug conjugate infusion was based on the phase 3 EV-301 trial, which found about a 4-month median overall survival benefit with the antibody-drug conjugate versus investigator-chosen chemotherapy across 608 patients with locally advanced or metastatic urothelial carcinoma previously treated with platinum-based chemotherapy and a programmed death 1 or PD–ligand 1 inhibitor.
The planned European indication is for adults with locally advanced or metastatic disease who met the same criteria – previous platinum-based chemotherapy plus a PD-1 or PD-L1 inhibitor.
The Food and Drug Administration approval came in December 2019, and with the same indication as well as for patients ineligible for cisplatin-containing chemotherapy who have had one or more prior lines of therapy. The U.S. labeling carries a boxed warning of severe and fatal skin reactions including Stevens-Johnson syndrome and toxic epidermal necrolysis.
Tepotinib for NSCLC
The recommendation for Merck’s tepotinib, a once-daily oral MET inhibitor, followed results from the phase 2 VISION study. The study found an investigator-assessed response rate of 56% across 152 patients with advanced or metastatic NSCLC with a confirmed MET exon 14 skipping mutation, regardless of previous therapy.
The planned European indication will be for monotherapy in adults with advanced disease harboring the mutation who require systemic therapy following prior treatment with immunotherapy and/or platinum-based chemotherapy.
The FDA approved the drug in February 2021, and carries the same indication, minus the prior therapy requirement.
Voxelotor for sickle cell disease
Voxelotor is an oral hemoglobin S polymerization inhibitor from Global Blood Therapeutics.
The European approval recommendation was based on a phase 3 trial in 274 patients with sickle cell disease that found a greater than 1 g/dL increase in hemoglobin levels at 24 weeks in 51.1% of patients versus 6.5% randomized to placebo, regardless of whether patients were on concomitant hydroxyurea.
The small molecule binds and stabilizes hemoglobin, preventing the hemoglobin polymerization that causes red blood cells to sickle.
“There is a high unmet need for medicines to treat hemolytic anemia” in sickle cell disease because available treatment options are limited to blood transfusions and allogenic hematopoietic stem cell transplantation,” the EMA explained in a press release announcing the approval recommendation.
The planned European indication is for treating hemolytic anemia in sickle cell disease in patients 12 years or older either alone or in combination with hydroxycarbamide (hydroxyurea).
The FDA approved the agent in November 2019 for the same indication, but can be given to children as young as 4 years old.
A version of this article first appeared on Medscape.com.
The drugs are enfortumab vedotin (Padcev, Astellas/Seagen) for urothelial cancer, tepotinib (Tepmetko, Merck) for non–small cell lung cancer (NSCLC), and voxelotor (Oxbryta, Global Blood Therapeutics) for sickle cell hemolytic anemia.
EMA’s Committee for Medicinal Products for Human Use gave the nod for marketing authorization on Dec. 16, 2021, and agency approval generally follows about 6 weeks later. All three products are already approved in the United States.
Enfortumab vedotin for urothelial cancer
The recommendation for Astellas’ antibody-drug conjugate infusion was based on the phase 3 EV-301 trial, which found about a 4-month median overall survival benefit with the antibody-drug conjugate versus investigator-chosen chemotherapy across 608 patients with locally advanced or metastatic urothelial carcinoma previously treated with platinum-based chemotherapy and a programmed death 1 or PD–ligand 1 inhibitor.
The planned European indication is for adults with locally advanced or metastatic disease who met the same criteria – previous platinum-based chemotherapy plus a PD-1 or PD-L1 inhibitor.
The Food and Drug Administration approval came in December 2019, and with the same indication as well as for patients ineligible for cisplatin-containing chemotherapy who have had one or more prior lines of therapy. The U.S. labeling carries a boxed warning of severe and fatal skin reactions including Stevens-Johnson syndrome and toxic epidermal necrolysis.
Tepotinib for NSCLC
The recommendation for Merck’s tepotinib, a once-daily oral MET inhibitor, followed results from the phase 2 VISION study. The study found an investigator-assessed response rate of 56% across 152 patients with advanced or metastatic NSCLC with a confirmed MET exon 14 skipping mutation, regardless of previous therapy.
The planned European indication will be for monotherapy in adults with advanced disease harboring the mutation who require systemic therapy following prior treatment with immunotherapy and/or platinum-based chemotherapy.
The FDA approved the drug in February 2021, and carries the same indication, minus the prior therapy requirement.
Voxelotor for sickle cell disease
Voxelotor is an oral hemoglobin S polymerization inhibitor from Global Blood Therapeutics.
The European approval recommendation was based on a phase 3 trial in 274 patients with sickle cell disease that found a greater than 1 g/dL increase in hemoglobin levels at 24 weeks in 51.1% of patients versus 6.5% randomized to placebo, regardless of whether patients were on concomitant hydroxyurea.
The small molecule binds and stabilizes hemoglobin, preventing the hemoglobin polymerization that causes red blood cells to sickle.
“There is a high unmet need for medicines to treat hemolytic anemia” in sickle cell disease because available treatment options are limited to blood transfusions and allogenic hematopoietic stem cell transplantation,” the EMA explained in a press release announcing the approval recommendation.
The planned European indication is for treating hemolytic anemia in sickle cell disease in patients 12 years or older either alone or in combination with hydroxycarbamide (hydroxyurea).
The FDA approved the agent in November 2019 for the same indication, but can be given to children as young as 4 years old.
A version of this article first appeared on Medscape.com.
Too close for comfort: When the psychiatrist is stalked
Dr. A has been treating Ms. W, a graduate student, for depression. Ms. W made subtle comments expressing her interest in pursuing a romantic relationship with her psychiatrist. Dr. A gently redirected her, and she seemed to respond appropriately. However, over the past 2 weeks, Dr. A has seen Ms. W at a local park and at the grocery store. Today, Dr. A is startled to see Ms. W at her weekly yoga class. Dr. A plans to ask her supervisor for advice.
Dr. M is a child psychiatrist who spoke at his local school board meeting in support of masking requirements for students during COVID-19. During the discussion, Dr. M shared that, as a psychiatrist, he does not believe it is especially distressing for students to wear masks, and that doing so is a necessary public health measure. On leaving, other parents shouted, “We know who you are and where you live!” The next day, his integrated clinic started receiving threatening and harassing messages, including threats to kill him or his staff if they take part in vaccinating children against COVID-19.
Because of their work, mental health professionals—like other health care professionals—face an elevated risk of being harassed or stalked. Stalking often includes online harassment and may escalate to serious physical violence. Stalking is criminal behavior by a patient and should not be constructed as a “failure to manage transference.” This article explores basic strategies to reduce the risk of harassment and stalking, describes how to recognize early behaviors, and outlines basic steps health care professionals and their employers can take to respond to stalking and harassing behaviors.
Although this article is intended for psychiatrists, it is important to note that all health professionals have significant risk for experiencing stalking or harassment. This is due in part, but not exclusively, to our clinical work. Estimates of how many health professionals experience stalking vary substantially depending upon the study, and differences in methodologies limit easy comparison or extrapolation. More thorough reviews have reported ranges from 2% to 70% among physicians; psychiatrists and other mental health professionals appear to be at greater risk than those in other specialties and the general population.1-3 Physicians who are active on social media may also be at elevated risk.4 Unexpected communications from patients and their family members—especially those with threatening, harassing, or sexualized tones, or involving contact outside of a work setting—can be distressing. These behaviors represent potential harbingers of more dangerous behavior, including physical assault, sexual assault, or homicide. Despite their elevated risk, many psychiatrists are unaware of how to prevent or respond to stalking or harassment.
Recognizing harassment and stalking
Repeated and unwanted contact or communication, regardless of intent, may constitute stalking. Legal definitions vary by jurisdiction and may not align with subjective experiences or understanding of what constitutes stalking.5 At its essence, stalking is repeated harassing behaviors likely to provoke fear in the targeted person. FOUR is a helpful mnemonic when conceptualizing the attributes of stalking: Fixated, Obsessive, Unwanted, and Repetitive.6Table 1 lists examples of common stalking behaviors. Stalking and harassing behavior may be from a known source (eg, a patient, coworker, or paramour), a masked source (ie, someone known to the target but who conceals or obscures their identity), or from otherwise unknown persons. Behaviors that persist after the person engaging in the behaviors has clearly been informed that they are unwanted or inappropriate are especially concerning. Stalking may escalate to include physical or sexual assault and, in some cases, homicide.

Stalking duration can vary substantially, as can the factors that lead to the cessation of the behavior. Indicators of increased risk for physical violence include unwanted physical presence/following of the target (“approach behaviors”), having a prior violent intimate relationship, property destruction, explicit threats, and having a prior intimate relationship with the target.7
Stalking contact or communication may be unwanted because of the content (eg, sexualized or threatening tone), location (eg, at a professional’s home), or means (eg, through social media). Stalking behaviors are not appropriate in any relationship, including a clinical relationship. They should not be treated as a “failure to manage transference” or in other victim-blaming ways.
There are multiple typologies for stalking behavior. Common motivations for stalking health professionals include resentment or grievance, misjudgment of social boundaries, and delusional fixation, including erotomania.8 Associated psychopathologies vary significantly and, while some may be more amenable to psychiatric treatment than others, psychiatrists should not feel compelled to treat patients who repeatedly violate boundaries, regardless of intent or comorbidity.
Patients are not the exclusive perpetrators of stalking; a recent study found that 4% of physicians surveyed reported current or recent stalking by a current or former intimate partner.9 When a person who is a victim of intimate partner violence is also stalked as part of the abuse, homicide risk increases.10 Workplace homicides of health care professionals are most likely to be committed by a current or former partner or other personal acquaintance, not by a patient.11 Workplace harassment and stalking of health care professionals is especially concerning because this behavior can escalate and endanger coworkers or clients.
Continue to: Risk awareness: Recognize your exposure...
Risk awareness: Recognize your exposure
About 80% of stalking involves some form of technology—often telephone calls but also online or other “cyber” elements.12 One recent survey found the rate of online harassment, including threats of physical and sexual violence, was >20% among physicians who were active on social media.4 Health professionals may be at greater risk of having patients find their personal information simply because patients routinely search online for information about new clinicians. Personal information about a clinician may be readily visible among professional information in search results, or a curious patient may simply scroll further down in the results. For a potential stalker, clicking on a search result linking to a personal social media page may be far easier than finding a home address and going in person—but the action may be just as distressing or risky for the clinician.13 Additionally, items visible in a clinician’s office—or visible in the background of those providing telehealth services from their home—may inadvertently reveal personal information about the clinician, their home, or their family.
Psychiatrists are often in a special position in relation to patients and times of crises. They may be involved in involuntary commitment—or declining an admission when a patient or family wishes it. They may be present at the time of the revelation of a serious diagnosis, abuse, injury, or death. They may be a mandated reporter of child or elder abuse.2 Additionally, physicians may be engaged in discourse on politically charged public health topics.14 These factors may increase their risk of being stalked.
Conducting an online visibility self-assessment can be a useful way to learn what information others can find. Table 2 outlines the steps for completing this exercise. Searching multiple iterations of your current and former names (with and without degrees, titles, and cities) will yield differing results in various search engines. After establishing a baseline of what information is available online, it can be helpful to periodically repeat this exercise, and to set up automated alerts for your name, number(s), email(s), and address(es).

Basic mitigation strategies
In the modern era, being invisible online is impractical and likely impossible—especially for a health care professional. Instead, it may be prudent to limit your public visibility to professional portals (eg, LinkedIn or Doximity) and maximize privacy settings on other platforms. Another basic strategy is to avoid providing personal contact information (your home address, phone number, or personal email) for professional purposes, such as licensing and credentialing, conference submissions, or journal publications. Be aware that driving a visually distinct vehicle—one with vanity plates or distinct bumper stickers, or an exotic sportscar—can make it easier to be recognized and located. A personally recorded voicemail greeting (vs one recorded by, for example, an office manager) may be inappropriately reinforcing for some stalkers.
Workplaces should have an established safety policy that addresses stalking and harassment of employees. Similarly, patients and others should receive clear education on how to contact different staff, including physicians, with consideration of how and when to use electronic health information portals, office numbers, and emails. Workplaces should not disclose staff schedules. For example, a receptionist should say “I’ll have Dr. Diaz return your call when she can” instead of “Dr. Diaz is not in until tomorrow.” Avoid unnecessary location/name signals (eg, a parking spot labeled “Dr. Diaz”). Consider creating alert words or phrases for staff to use to signal they are concerned about their immediate safety—and provide education and training, including drills, to test emergency responses when the words/phrases are used. Leaders and managers should nurture a workplace culture where people are comfortable seeking support if they feel they may be the target of harassment or stalking. Many larger health care organizations have threat management programs, which can play a critical role in preventing, investigating, and responding to stalking of employees. Increasingly, threat management teams are being identified as a best practice in health care settings.15Table 3 summarizes measures to mitigate risk.
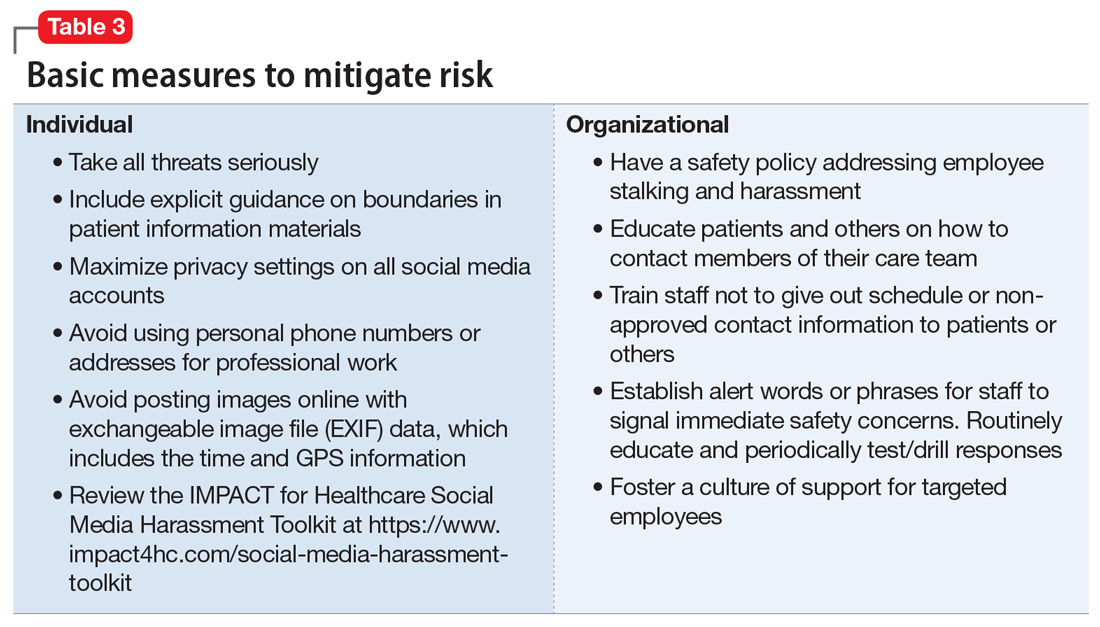
What to do when harassment or stalking occurs
Consulting with subject matter experts is essential. Approach behaviors, stalking patterns, and immediate circumstances vary highly, and so too must responses. A socially inept approach outside of the work setting by a patient may be effectively responded to with a firm explanation of why the behavior was inappropriate and a reiteration of limits. More persistent or serious threats may require taking actions for immediate safety, calling law enforcement or security (who may have the expertise to assist appropriately), or even run/hide/fight measures. Others to notify early on include human resources, supervisors, front desk staff, and coworkers. Although no single measure is always indicated and no single measure will always be effective, consultation with a specialist is always advisable.
Attempting to assess your own risk may be subject to bias and error, even for an experienced forensic psychiatrist. Risk assessment in stalking and harassment cases is complex, nuanced, and beyond the scope of this article; engagement with specialized threat programs or subject matter experts is advisable.15,16 If your medical center or area has police or security officers, engage them early. Risk management, insurers, and legal can also be helpful to consult. Attorneys specializing in harassment, stalking, and domestic violence may be helpful in extreme situations.17Table 417,18 highlights steps to take.
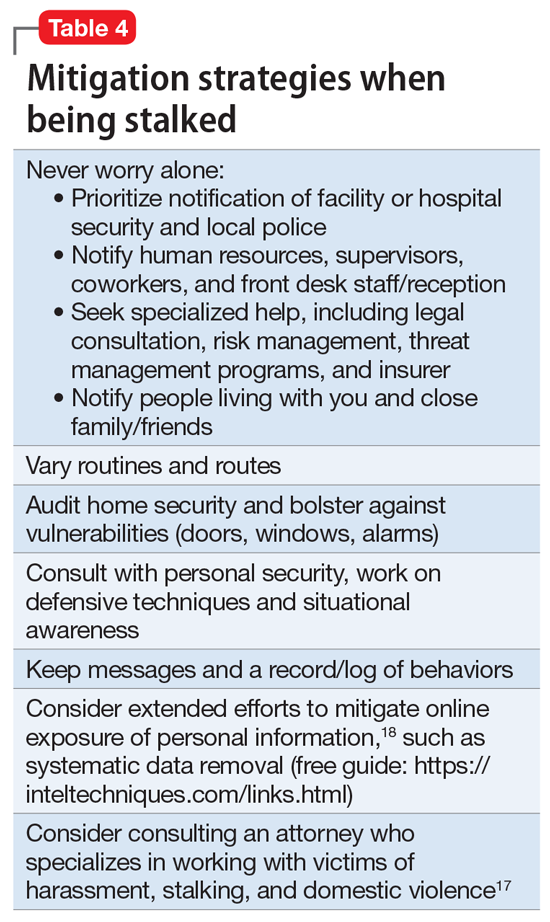
While effective interventions to stop or redirect stalking behavior may vary, some initial considerations include changing established routines (eg, your parking location or daily/weekly patterns such as gym, class, etc.) and letting family and others you live with know what is occurring. Consider implementing and bolstering personal, work, and home security; honing situational awareness skills; and learning advanced situational awareness and self-defense techniques.
Continue to: Clinical documentation and termination of care...
Clinical documentation and termination of care
Repeated and unwanted contact behaviors by a patient may be considered grounds for termination of care by the targeted clinician. Termination may occur through a direct conversation, followed by a mailed letter explaining that the patient’s inappropriate behaviors are the basis for termination. The letter should outline steps for establishing care with another psychiatrist and signing a release to facilitate transfer of records to the next psychiatrist. Ensure that the patient has access to a reasonable supply of medications or refills according to jurisdictional standards for transfer or termination of care.19 While these are common legal standards for termination of care in the United States, clinicians would be well served by appropriate consultation to verify the most appropriate standards for their location.
Documentation of a patient’s behavior should be factual and clear. Under the 21st Century Cures Act, patients often have access to their own electronic records.20 Therefore, clinicians should avoid documenting personal security measures or other information that is not clinically relevant. Communications with legal or risk management should not be documented unless otherwise advised, because such communications may be privileged and may not be clinically relevant.
In some circumstances, continuing to treat a patient who has stalked a member of the current treatment team may be appropriate or necessary. For example, a patient may respond appropriately to redirection after an initial approach behavior and continue to make clinical progress, or may be in a forensic specialty setting with appropriate operational support to continue with treatment.
Ethical dilemmas may arise in underserved areas where there are limited options for psychiatric care and in communicating the reasons for termination to a new clinician. Consultation may help to address these issues. However, as noted before, clinicians should be permitted to discontinue and transfer treatment and should not be compelled to continue to treat a patient who has threatened or harassed them.
Organizational and employer considerations
Victims of stalking have reported that they appreciated explicit support from their supervisor, regular meetings, and measures to reduce potential stalking or violence in the workplace; unsurprisingly, victim blaming and leaving the employee to address the situation on their own were labeled experienced as negative.2 Employers may consider implementing physical security, access controls and panic alarms, and enhancing coworkers’ situational awareness.21 Explicit policies about and attention to reducing workplace violence, including stalking, are always beneficial—and in some settings such policies may be a regulatory requirement.22 Large health care organizations may benefit from developing specialized threat management programs to assist with the evaluation and mitigation of stalking and other workplace violence risks.15,23
Self-care considerations
The impact of stalking can include psychological distress, disruption of work and personal relationships, and false allegations of impropriety. Stalking can make targets feel isolated, violated, and fearful, which makes it challenging to reach out to others for support and safety. It takes time to regain a sense of safety and to find a “new normal,” particularly while experiencing and responding to stalking behavior. Notifying close personal contacts such as family and coworkers about what is occurring (without sharing protected health information) can be helpful for recovery and important for the clinician’s safety. Reaching out for organizational and legal supports is also prudent. It is also important to allow time for, and patience with, a targeted individual’s normal responses, such as decreased work performance, sleep/appetite changes, and hypervigilance, without pathologizing these common stress reactions. Further review of appropriate resources by impacted clinicians is advisable.24-26
1. Nelsen AJ, Johnson RS, Ostermeyer B, et al. The prevalence of physicians who have been stalked: a systematic review. J Am Acad Psychiatry Law. 2015;43(2):177-182.
2. Jutasi C, McEwan TE. Stalking of professionals: a scoping review. Journal of Threat Assessment and Management. 2021;8(3):94-124.
3. Pathé MT, Meloy JR. Commentary: Stalking by patients—psychiatrists’ tales of anger, lust and ignorance. J Am Acad Psychiatry Law. 2013;41(2):200-205.
4. Pendergrast TR, Jain S, Trueger NS, et al. Prevalence of personal attacks and sexual harassment of physicians on social media. JAMA Intern Med. 2021;181(4):550-552.
5. Owens JG. Why definitions matter: stalking victimization in the United States. J Interpers Violence. 2016;31(12):2196-2226.
6. College of Policing. Stalking or harassment. May 2019. Accessed March 8, 2020. https://library.college.police.uk/docs/college-of-policing/Stalking_or_harassment_guidance_200519.pdf
7. McEwan TE, Daffern M, MacKenzie RD, et al. Risk factors for stalking violence, persistence, and recurrence. Journal of Forensic Psychiatry & Psychology. 2017;28(1):3856.
8. Pathé MT, Mullen PE, Purcell R. Patients who stalk doctors: their motives and management. Med J Australia. 2002;176(7):335-338.
9. Reibling ET, Distelberg B, Guptill M, et al. Intimate partner violence experienced by physicians. J Prim Care Community Health. 2020;11:2150132720965077.
10. Matias A, Gonçalves M, Soeiro C, et al. Intimate partner homicide: a meta-analysis of risk factors. Aggression and Violent Behavior. 2019;50:101358.
11. US Bureau of Labor Statistics. Fact sheet. Workplace violence in healthcare, 2018. April 2020. Accessed November 24, 2021. https://www.bls.gov/iif/oshwc/cfoi/workplace-violence-healthcare-2018.htm
12. Truman JL, Morgan RE. Stalking victimization, 2016. Bureau of Justice Statistics, Office of Justice Programs, U.S. Department of Justice. Report No.: NCJ 253526. April 2021. Accessed November 24, 2021. https://bjs.ojp.gov/library/publications/stalking-victimization-2016
13. Reyns BW, Henson B, Fisher BS. Being pursued online: applying cyberlifestyle–routine activities theory to cyberstalking victimization. Criminal Justice and Behavior. 2011;38(11):1149-1169.
14. Stea JN. When promoting knowledge makes you a target. Scientific American Blog Network. March 16, 2020. Accessed November 24, 2021. https://blogs.scientificamerican.com/observations/when-promoting-knowledge-makes-you-a-target/
15. Henkel SJ. Threat assessment strategies to mitigate violence in healthcare. IAHSS Foundation. IAHSS-F RS-19-02. November 2019. Accessed November 24, 2021. https://iahssf.org/assets/IAHSS-Foundation-Threat-Assessment-Strategies-to-Mitigate-Violence-in-Healthcare.pdf
16. McEwan TE. Stalking threat and risk assessment. In: Reid Meloy J, Hoffman J (eds). International Handbook of Threat Assessment. 2nd ed. Oxford University Press; 2021:210-234.
17. Goldberg C. Nobody’s Victim: Fighting Psychos, Stalkers, Pervs, and Trolls. Plume; 2019.
18. Bazzell M. Extreme Privacy: What It Takes to Disappear. 2nd ed. Independently published; 2020.
19. Simon RI, Shuman DW. The doctor-patient relationship. Focus. 2007;5(4):423-431.
20. Department of Health and Human Services. 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program Final Rule (To be codified at 45 CFR 170 and 171). Federal Register. 2020;85(85):25642-25961.
21. Sheridan L, North AC, Scott AJ. Stalking in the workplace. Journal of Threat Assessment and Management. 2019;6(2):61-75.
22. The Joint Commission. Workplace Violence Prevention Standards. R3 Report: Requirement, Rationale, Reference. Issue 30. June 18, 2021. Accessed November 24, 2021. https://www.jointcommission.org/-/media/tjc/documents/standards/r3-reports/wpvp-r3-30_revised_06302021.pdf
23. Terry LP. Threat assessment teams. J Healthc Prot Manage. 2015;31(2):23-35.
24. Pathé M. Surviving Stalking. Cambridge University Press; 2002.
25. Noffsinger S. What stalking victims need to restore their mental and somatic health. Current Psychiatry. 2015;14(6):43-47.
26. Mullen P, Whyte S, McIvor R; Psychiatrists’ Support Service, Royal College of Psychiatry. PSS Information Guide: Stalking. Report No. 11. 2017. Accessed November 24, 2021. https://www.rcpsych.ac.uk/docs/default-source/members/supporting-you/pss/pss-guide-11-stalking.pdf?sfvrsn=2f1c7253_2
Dr. A has been treating Ms. W, a graduate student, for depression. Ms. W made subtle comments expressing her interest in pursuing a romantic relationship with her psychiatrist. Dr. A gently redirected her, and she seemed to respond appropriately. However, over the past 2 weeks, Dr. A has seen Ms. W at a local park and at the grocery store. Today, Dr. A is startled to see Ms. W at her weekly yoga class. Dr. A plans to ask her supervisor for advice.
Dr. M is a child psychiatrist who spoke at his local school board meeting in support of masking requirements for students during COVID-19. During the discussion, Dr. M shared that, as a psychiatrist, he does not believe it is especially distressing for students to wear masks, and that doing so is a necessary public health measure. On leaving, other parents shouted, “We know who you are and where you live!” The next day, his integrated clinic started receiving threatening and harassing messages, including threats to kill him or his staff if they take part in vaccinating children against COVID-19.
Because of their work, mental health professionals—like other health care professionals—face an elevated risk of being harassed or stalked. Stalking often includes online harassment and may escalate to serious physical violence. Stalking is criminal behavior by a patient and should not be constructed as a “failure to manage transference.” This article explores basic strategies to reduce the risk of harassment and stalking, describes how to recognize early behaviors, and outlines basic steps health care professionals and their employers can take to respond to stalking and harassing behaviors.
Although this article is intended for psychiatrists, it is important to note that all health professionals have significant risk for experiencing stalking or harassment. This is due in part, but not exclusively, to our clinical work. Estimates of how many health professionals experience stalking vary substantially depending upon the study, and differences in methodologies limit easy comparison or extrapolation. More thorough reviews have reported ranges from 2% to 70% among physicians; psychiatrists and other mental health professionals appear to be at greater risk than those in other specialties and the general population.1-3 Physicians who are active on social media may also be at elevated risk.4 Unexpected communications from patients and their family members—especially those with threatening, harassing, or sexualized tones, or involving contact outside of a work setting—can be distressing. These behaviors represent potential harbingers of more dangerous behavior, including physical assault, sexual assault, or homicide. Despite their elevated risk, many psychiatrists are unaware of how to prevent or respond to stalking or harassment.
Recognizing harassment and stalking
Repeated and unwanted contact or communication, regardless of intent, may constitute stalking. Legal definitions vary by jurisdiction and may not align with subjective experiences or understanding of what constitutes stalking.5 At its essence, stalking is repeated harassing behaviors likely to provoke fear in the targeted person. FOUR is a helpful mnemonic when conceptualizing the attributes of stalking: Fixated, Obsessive, Unwanted, and Repetitive.6Table 1 lists examples of common stalking behaviors. Stalking and harassing behavior may be from a known source (eg, a patient, coworker, or paramour), a masked source (ie, someone known to the target but who conceals or obscures their identity), or from otherwise unknown persons. Behaviors that persist after the person engaging in the behaviors has clearly been informed that they are unwanted or inappropriate are especially concerning. Stalking may escalate to include physical or sexual assault and, in some cases, homicide.

Stalking duration can vary substantially, as can the factors that lead to the cessation of the behavior. Indicators of increased risk for physical violence include unwanted physical presence/following of the target (“approach behaviors”), having a prior violent intimate relationship, property destruction, explicit threats, and having a prior intimate relationship with the target.7
Stalking contact or communication may be unwanted because of the content (eg, sexualized or threatening tone), location (eg, at a professional’s home), or means (eg, through social media). Stalking behaviors are not appropriate in any relationship, including a clinical relationship. They should not be treated as a “failure to manage transference” or in other victim-blaming ways.
There are multiple typologies for stalking behavior. Common motivations for stalking health professionals include resentment or grievance, misjudgment of social boundaries, and delusional fixation, including erotomania.8 Associated psychopathologies vary significantly and, while some may be more amenable to psychiatric treatment than others, psychiatrists should not feel compelled to treat patients who repeatedly violate boundaries, regardless of intent or comorbidity.
Patients are not the exclusive perpetrators of stalking; a recent study found that 4% of physicians surveyed reported current or recent stalking by a current or former intimate partner.9 When a person who is a victim of intimate partner violence is also stalked as part of the abuse, homicide risk increases.10 Workplace homicides of health care professionals are most likely to be committed by a current or former partner or other personal acquaintance, not by a patient.11 Workplace harassment and stalking of health care professionals is especially concerning because this behavior can escalate and endanger coworkers or clients.
Continue to: Risk awareness: Recognize your exposure...
Risk awareness: Recognize your exposure
About 80% of stalking involves some form of technology—often telephone calls but also online or other “cyber” elements.12 One recent survey found the rate of online harassment, including threats of physical and sexual violence, was >20% among physicians who were active on social media.4 Health professionals may be at greater risk of having patients find their personal information simply because patients routinely search online for information about new clinicians. Personal information about a clinician may be readily visible among professional information in search results, or a curious patient may simply scroll further down in the results. For a potential stalker, clicking on a search result linking to a personal social media page may be far easier than finding a home address and going in person—but the action may be just as distressing or risky for the clinician.13 Additionally, items visible in a clinician’s office—or visible in the background of those providing telehealth services from their home—may inadvertently reveal personal information about the clinician, their home, or their family.
Psychiatrists are often in a special position in relation to patients and times of crises. They may be involved in involuntary commitment—or declining an admission when a patient or family wishes it. They may be present at the time of the revelation of a serious diagnosis, abuse, injury, or death. They may be a mandated reporter of child or elder abuse.2 Additionally, physicians may be engaged in discourse on politically charged public health topics.14 These factors may increase their risk of being stalked.
Conducting an online visibility self-assessment can be a useful way to learn what information others can find. Table 2 outlines the steps for completing this exercise. Searching multiple iterations of your current and former names (with and without degrees, titles, and cities) will yield differing results in various search engines. After establishing a baseline of what information is available online, it can be helpful to periodically repeat this exercise, and to set up automated alerts for your name, number(s), email(s), and address(es).

Basic mitigation strategies
In the modern era, being invisible online is impractical and likely impossible—especially for a health care professional. Instead, it may be prudent to limit your public visibility to professional portals (eg, LinkedIn or Doximity) and maximize privacy settings on other platforms. Another basic strategy is to avoid providing personal contact information (your home address, phone number, or personal email) for professional purposes, such as licensing and credentialing, conference submissions, or journal publications. Be aware that driving a visually distinct vehicle—one with vanity plates or distinct bumper stickers, or an exotic sportscar—can make it easier to be recognized and located. A personally recorded voicemail greeting (vs one recorded by, for example, an office manager) may be inappropriately reinforcing for some stalkers.
Workplaces should have an established safety policy that addresses stalking and harassment of employees. Similarly, patients and others should receive clear education on how to contact different staff, including physicians, with consideration of how and when to use electronic health information portals, office numbers, and emails. Workplaces should not disclose staff schedules. For example, a receptionist should say “I’ll have Dr. Diaz return your call when she can” instead of “Dr. Diaz is not in until tomorrow.” Avoid unnecessary location/name signals (eg, a parking spot labeled “Dr. Diaz”). Consider creating alert words or phrases for staff to use to signal they are concerned about their immediate safety—and provide education and training, including drills, to test emergency responses when the words/phrases are used. Leaders and managers should nurture a workplace culture where people are comfortable seeking support if they feel they may be the target of harassment or stalking. Many larger health care organizations have threat management programs, which can play a critical role in preventing, investigating, and responding to stalking of employees. Increasingly, threat management teams are being identified as a best practice in health care settings.15Table 3 summarizes measures to mitigate risk.

What to do when harassment or stalking occurs
Consulting with subject matter experts is essential. Approach behaviors, stalking patterns, and immediate circumstances vary highly, and so too must responses. A socially inept approach outside of the work setting by a patient may be effectively responded to with a firm explanation of why the behavior was inappropriate and a reiteration of limits. More persistent or serious threats may require taking actions for immediate safety, calling law enforcement or security (who may have the expertise to assist appropriately), or even run/hide/fight measures. Others to notify early on include human resources, supervisors, front desk staff, and coworkers. Although no single measure is always indicated and no single measure will always be effective, consultation with a specialist is always advisable.
Attempting to assess your own risk may be subject to bias and error, even for an experienced forensic psychiatrist. Risk assessment in stalking and harassment cases is complex, nuanced, and beyond the scope of this article; engagement with specialized threat programs or subject matter experts is advisable.15,16 If your medical center or area has police or security officers, engage them early. Risk management, insurers, and legal can also be helpful to consult. Attorneys specializing in harassment, stalking, and domestic violence may be helpful in extreme situations.17Table 417,18 highlights steps to take.

While effective interventions to stop or redirect stalking behavior may vary, some initial considerations include changing established routines (eg, your parking location or daily/weekly patterns such as gym, class, etc.) and letting family and others you live with know what is occurring. Consider implementing and bolstering personal, work, and home security; honing situational awareness skills; and learning advanced situational awareness and self-defense techniques.
Continue to: Clinical documentation and termination of care...
Clinical documentation and termination of care
Repeated and unwanted contact behaviors by a patient may be considered grounds for termination of care by the targeted clinician. Termination may occur through a direct conversation, followed by a mailed letter explaining that the patient’s inappropriate behaviors are the basis for termination. The letter should outline steps for establishing care with another psychiatrist and signing a release to facilitate transfer of records to the next psychiatrist. Ensure that the patient has access to a reasonable supply of medications or refills according to jurisdictional standards for transfer or termination of care.19 While these are common legal standards for termination of care in the United States, clinicians would be well served by appropriate consultation to verify the most appropriate standards for their location.
Documentation of a patient’s behavior should be factual and clear. Under the 21st Century Cures Act, patients often have access to their own electronic records.20 Therefore, clinicians should avoid documenting personal security measures or other information that is not clinically relevant. Communications with legal or risk management should not be documented unless otherwise advised, because such communications may be privileged and may not be clinically relevant.
In some circumstances, continuing to treat a patient who has stalked a member of the current treatment team may be appropriate or necessary. For example, a patient may respond appropriately to redirection after an initial approach behavior and continue to make clinical progress, or may be in a forensic specialty setting with appropriate operational support to continue with treatment.
Ethical dilemmas may arise in underserved areas where there are limited options for psychiatric care and in communicating the reasons for termination to a new clinician. Consultation may help to address these issues. However, as noted before, clinicians should be permitted to discontinue and transfer treatment and should not be compelled to continue to treat a patient who has threatened or harassed them.
Organizational and employer considerations
Victims of stalking have reported that they appreciated explicit support from their supervisor, regular meetings, and measures to reduce potential stalking or violence in the workplace; unsurprisingly, victim blaming and leaving the employee to address the situation on their own were labeled experienced as negative.2 Employers may consider implementing physical security, access controls and panic alarms, and enhancing coworkers’ situational awareness.21 Explicit policies about and attention to reducing workplace violence, including stalking, are always beneficial—and in some settings such policies may be a regulatory requirement.22 Large health care organizations may benefit from developing specialized threat management programs to assist with the evaluation and mitigation of stalking and other workplace violence risks.15,23
Self-care considerations
The impact of stalking can include psychological distress, disruption of work and personal relationships, and false allegations of impropriety. Stalking can make targets feel isolated, violated, and fearful, which makes it challenging to reach out to others for support and safety. It takes time to regain a sense of safety and to find a “new normal,” particularly while experiencing and responding to stalking behavior. Notifying close personal contacts such as family and coworkers about what is occurring (without sharing protected health information) can be helpful for recovery and important for the clinician’s safety. Reaching out for organizational and legal supports is also prudent. It is also important to allow time for, and patience with, a targeted individual’s normal responses, such as decreased work performance, sleep/appetite changes, and hypervigilance, without pathologizing these common stress reactions. Further review of appropriate resources by impacted clinicians is advisable.24-26
Dr. A has been treating Ms. W, a graduate student, for depression. Ms. W made subtle comments expressing her interest in pursuing a romantic relationship with her psychiatrist. Dr. A gently redirected her, and she seemed to respond appropriately. However, over the past 2 weeks, Dr. A has seen Ms. W at a local park and at the grocery store. Today, Dr. A is startled to see Ms. W at her weekly yoga class. Dr. A plans to ask her supervisor for advice.
Dr. M is a child psychiatrist who spoke at his local school board meeting in support of masking requirements for students during COVID-19. During the discussion, Dr. M shared that, as a psychiatrist, he does not believe it is especially distressing for students to wear masks, and that doing so is a necessary public health measure. On leaving, other parents shouted, “We know who you are and where you live!” The next day, his integrated clinic started receiving threatening and harassing messages, including threats to kill him or his staff if they take part in vaccinating children against COVID-19.
Because of their work, mental health professionals—like other health care professionals—face an elevated risk of being harassed or stalked. Stalking often includes online harassment and may escalate to serious physical violence. Stalking is criminal behavior by a patient and should not be constructed as a “failure to manage transference.” This article explores basic strategies to reduce the risk of harassment and stalking, describes how to recognize early behaviors, and outlines basic steps health care professionals and their employers can take to respond to stalking and harassing behaviors.
Although this article is intended for psychiatrists, it is important to note that all health professionals have significant risk for experiencing stalking or harassment. This is due in part, but not exclusively, to our clinical work. Estimates of how many health professionals experience stalking vary substantially depending upon the study, and differences in methodologies limit easy comparison or extrapolation. More thorough reviews have reported ranges from 2% to 70% among physicians; psychiatrists and other mental health professionals appear to be at greater risk than those in other specialties and the general population.1-3 Physicians who are active on social media may also be at elevated risk.4 Unexpected communications from patients and their family members—especially those with threatening, harassing, or sexualized tones, or involving contact outside of a work setting—can be distressing. These behaviors represent potential harbingers of more dangerous behavior, including physical assault, sexual assault, or homicide. Despite their elevated risk, many psychiatrists are unaware of how to prevent or respond to stalking or harassment.
Recognizing harassment and stalking
Repeated and unwanted contact or communication, regardless of intent, may constitute stalking. Legal definitions vary by jurisdiction and may not align with subjective experiences or understanding of what constitutes stalking.5 At its essence, stalking is repeated harassing behaviors likely to provoke fear in the targeted person. FOUR is a helpful mnemonic when conceptualizing the attributes of stalking: Fixated, Obsessive, Unwanted, and Repetitive.6Table 1 lists examples of common stalking behaviors. Stalking and harassing behavior may be from a known source (eg, a patient, coworker, or paramour), a masked source (ie, someone known to the target but who conceals or obscures their identity), or from otherwise unknown persons. Behaviors that persist after the person engaging in the behaviors has clearly been informed that they are unwanted or inappropriate are especially concerning. Stalking may escalate to include physical or sexual assault and, in some cases, homicide.

Stalking duration can vary substantially, as can the factors that lead to the cessation of the behavior. Indicators of increased risk for physical violence include unwanted physical presence/following of the target (“approach behaviors”), having a prior violent intimate relationship, property destruction, explicit threats, and having a prior intimate relationship with the target.7
Stalking contact or communication may be unwanted because of the content (eg, sexualized or threatening tone), location (eg, at a professional’s home), or means (eg, through social media). Stalking behaviors are not appropriate in any relationship, including a clinical relationship. They should not be treated as a “failure to manage transference” or in other victim-blaming ways.
There are multiple typologies for stalking behavior. Common motivations for stalking health professionals include resentment or grievance, misjudgment of social boundaries, and delusional fixation, including erotomania.8 Associated psychopathologies vary significantly and, while some may be more amenable to psychiatric treatment than others, psychiatrists should not feel compelled to treat patients who repeatedly violate boundaries, regardless of intent or comorbidity.
Patients are not the exclusive perpetrators of stalking; a recent study found that 4% of physicians surveyed reported current or recent stalking by a current or former intimate partner.9 When a person who is a victim of intimate partner violence is also stalked as part of the abuse, homicide risk increases.10 Workplace homicides of health care professionals are most likely to be committed by a current or former partner or other personal acquaintance, not by a patient.11 Workplace harassment and stalking of health care professionals is especially concerning because this behavior can escalate and endanger coworkers or clients.
Continue to: Risk awareness: Recognize your exposure...
Risk awareness: Recognize your exposure
About 80% of stalking involves some form of technology—often telephone calls but also online or other “cyber” elements.12 One recent survey found the rate of online harassment, including threats of physical and sexual violence, was >20% among physicians who were active on social media.4 Health professionals may be at greater risk of having patients find their personal information simply because patients routinely search online for information about new clinicians. Personal information about a clinician may be readily visible among professional information in search results, or a curious patient may simply scroll further down in the results. For a potential stalker, clicking on a search result linking to a personal social media page may be far easier than finding a home address and going in person—but the action may be just as distressing or risky for the clinician.13 Additionally, items visible in a clinician’s office—or visible in the background of those providing telehealth services from their home—may inadvertently reveal personal information about the clinician, their home, or their family.
Psychiatrists are often in a special position in relation to patients and times of crises. They may be involved in involuntary commitment—or declining an admission when a patient or family wishes it. They may be present at the time of the revelation of a serious diagnosis, abuse, injury, or death. They may be a mandated reporter of child or elder abuse.2 Additionally, physicians may be engaged in discourse on politically charged public health topics.14 These factors may increase their risk of being stalked.
Conducting an online visibility self-assessment can be a useful way to learn what information others can find. Table 2 outlines the steps for completing this exercise. Searching multiple iterations of your current and former names (with and without degrees, titles, and cities) will yield differing results in various search engines. After establishing a baseline of what information is available online, it can be helpful to periodically repeat this exercise, and to set up automated alerts for your name, number(s), email(s), and address(es).

Basic mitigation strategies
In the modern era, being invisible online is impractical and likely impossible—especially for a health care professional. Instead, it may be prudent to limit your public visibility to professional portals (eg, LinkedIn or Doximity) and maximize privacy settings on other platforms. Another basic strategy is to avoid providing personal contact information (your home address, phone number, or personal email) for professional purposes, such as licensing and credentialing, conference submissions, or journal publications. Be aware that driving a visually distinct vehicle—one with vanity plates or distinct bumper stickers, or an exotic sportscar—can make it easier to be recognized and located. A personally recorded voicemail greeting (vs one recorded by, for example, an office manager) may be inappropriately reinforcing for some stalkers.
Workplaces should have an established safety policy that addresses stalking and harassment of employees. Similarly, patients and others should receive clear education on how to contact different staff, including physicians, with consideration of how and when to use electronic health information portals, office numbers, and emails. Workplaces should not disclose staff schedules. For example, a receptionist should say “I’ll have Dr. Diaz return your call when she can” instead of “Dr. Diaz is not in until tomorrow.” Avoid unnecessary location/name signals (eg, a parking spot labeled “Dr. Diaz”). Consider creating alert words or phrases for staff to use to signal they are concerned about their immediate safety—and provide education and training, including drills, to test emergency responses when the words/phrases are used. Leaders and managers should nurture a workplace culture where people are comfortable seeking support if they feel they may be the target of harassment or stalking. Many larger health care organizations have threat management programs, which can play a critical role in preventing, investigating, and responding to stalking of employees. Increasingly, threat management teams are being identified as a best practice in health care settings.15Table 3 summarizes measures to mitigate risk.

What to do when harassment or stalking occurs
Consulting with subject matter experts is essential. Approach behaviors, stalking patterns, and immediate circumstances vary highly, and so too must responses. A socially inept approach outside of the work setting by a patient may be effectively responded to with a firm explanation of why the behavior was inappropriate and a reiteration of limits. More persistent or serious threats may require taking actions for immediate safety, calling law enforcement or security (who may have the expertise to assist appropriately), or even run/hide/fight measures. Others to notify early on include human resources, supervisors, front desk staff, and coworkers. Although no single measure is always indicated and no single measure will always be effective, consultation with a specialist is always advisable.
Attempting to assess your own risk may be subject to bias and error, even for an experienced forensic psychiatrist. Risk assessment in stalking and harassment cases is complex, nuanced, and beyond the scope of this article; engagement with specialized threat programs or subject matter experts is advisable.15,16 If your medical center or area has police or security officers, engage them early. Risk management, insurers, and legal can also be helpful to consult. Attorneys specializing in harassment, stalking, and domestic violence may be helpful in extreme situations.17Table 417,18 highlights steps to take.

While effective interventions to stop or redirect stalking behavior may vary, some initial considerations include changing established routines (eg, your parking location or daily/weekly patterns such as gym, class, etc.) and letting family and others you live with know what is occurring. Consider implementing and bolstering personal, work, and home security; honing situational awareness skills; and learning advanced situational awareness and self-defense techniques.
Continue to: Clinical documentation and termination of care...
Clinical documentation and termination of care
Repeated and unwanted contact behaviors by a patient may be considered grounds for termination of care by the targeted clinician. Termination may occur through a direct conversation, followed by a mailed letter explaining that the patient’s inappropriate behaviors are the basis for termination. The letter should outline steps for establishing care with another psychiatrist and signing a release to facilitate transfer of records to the next psychiatrist. Ensure that the patient has access to a reasonable supply of medications or refills according to jurisdictional standards for transfer or termination of care.19 While these are common legal standards for termination of care in the United States, clinicians would be well served by appropriate consultation to verify the most appropriate standards for their location.
Documentation of a patient’s behavior should be factual and clear. Under the 21st Century Cures Act, patients often have access to their own electronic records.20 Therefore, clinicians should avoid documenting personal security measures or other information that is not clinically relevant. Communications with legal or risk management should not be documented unless otherwise advised, because such communications may be privileged and may not be clinically relevant.
In some circumstances, continuing to treat a patient who has stalked a member of the current treatment team may be appropriate or necessary. For example, a patient may respond appropriately to redirection after an initial approach behavior and continue to make clinical progress, or may be in a forensic specialty setting with appropriate operational support to continue with treatment.
Ethical dilemmas may arise in underserved areas where there are limited options for psychiatric care and in communicating the reasons for termination to a new clinician. Consultation may help to address these issues. However, as noted before, clinicians should be permitted to discontinue and transfer treatment and should not be compelled to continue to treat a patient who has threatened or harassed them.
Organizational and employer considerations
Victims of stalking have reported that they appreciated explicit support from their supervisor, regular meetings, and measures to reduce potential stalking or violence in the workplace; unsurprisingly, victim blaming and leaving the employee to address the situation on their own were labeled experienced as negative.2 Employers may consider implementing physical security, access controls and panic alarms, and enhancing coworkers’ situational awareness.21 Explicit policies about and attention to reducing workplace violence, including stalking, are always beneficial—and in some settings such policies may be a regulatory requirement.22 Large health care organizations may benefit from developing specialized threat management programs to assist with the evaluation and mitigation of stalking and other workplace violence risks.15,23
Self-care considerations
The impact of stalking can include psychological distress, disruption of work and personal relationships, and false allegations of impropriety. Stalking can make targets feel isolated, violated, and fearful, which makes it challenging to reach out to others for support and safety. It takes time to regain a sense of safety and to find a “new normal,” particularly while experiencing and responding to stalking behavior. Notifying close personal contacts such as family and coworkers about what is occurring (without sharing protected health information) can be helpful for recovery and important for the clinician’s safety. Reaching out for organizational and legal supports is also prudent. It is also important to allow time for, and patience with, a targeted individual’s normal responses, such as decreased work performance, sleep/appetite changes, and hypervigilance, without pathologizing these common stress reactions. Further review of appropriate resources by impacted clinicians is advisable.24-26
1. Nelsen AJ, Johnson RS, Ostermeyer B, et al. The prevalence of physicians who have been stalked: a systematic review. J Am Acad Psychiatry Law. 2015;43(2):177-182.
2. Jutasi C, McEwan TE. Stalking of professionals: a scoping review. Journal of Threat Assessment and Management. 2021;8(3):94-124.
3. Pathé MT, Meloy JR. Commentary: Stalking by patients—psychiatrists’ tales of anger, lust and ignorance. J Am Acad Psychiatry Law. 2013;41(2):200-205.
4. Pendergrast TR, Jain S, Trueger NS, et al. Prevalence of personal attacks and sexual harassment of physicians on social media. JAMA Intern Med. 2021;181(4):550-552.
5. Owens JG. Why definitions matter: stalking victimization in the United States. J Interpers Violence. 2016;31(12):2196-2226.
6. College of Policing. Stalking or harassment. May 2019. Accessed March 8, 2020. https://library.college.police.uk/docs/college-of-policing/Stalking_or_harassment_guidance_200519.pdf
7. McEwan TE, Daffern M, MacKenzie RD, et al. Risk factors for stalking violence, persistence, and recurrence. Journal of Forensic Psychiatry & Psychology. 2017;28(1):3856.
8. Pathé MT, Mullen PE, Purcell R. Patients who stalk doctors: their motives and management. Med J Australia. 2002;176(7):335-338.
9. Reibling ET, Distelberg B, Guptill M, et al. Intimate partner violence experienced by physicians. J Prim Care Community Health. 2020;11:2150132720965077.
10. Matias A, Gonçalves M, Soeiro C, et al. Intimate partner homicide: a meta-analysis of risk factors. Aggression and Violent Behavior. 2019;50:101358.
11. US Bureau of Labor Statistics. Fact sheet. Workplace violence in healthcare, 2018. April 2020. Accessed November 24, 2021. https://www.bls.gov/iif/oshwc/cfoi/workplace-violence-healthcare-2018.htm
12. Truman JL, Morgan RE. Stalking victimization, 2016. Bureau of Justice Statistics, Office of Justice Programs, U.S. Department of Justice. Report No.: NCJ 253526. April 2021. Accessed November 24, 2021. https://bjs.ojp.gov/library/publications/stalking-victimization-2016
13. Reyns BW, Henson B, Fisher BS. Being pursued online: applying cyberlifestyle–routine activities theory to cyberstalking victimization. Criminal Justice and Behavior. 2011;38(11):1149-1169.
14. Stea JN. When promoting knowledge makes you a target. Scientific American Blog Network. March 16, 2020. Accessed November 24, 2021. https://blogs.scientificamerican.com/observations/when-promoting-knowledge-makes-you-a-target/
15. Henkel SJ. Threat assessment strategies to mitigate violence in healthcare. IAHSS Foundation. IAHSS-F RS-19-02. November 2019. Accessed November 24, 2021. https://iahssf.org/assets/IAHSS-Foundation-Threat-Assessment-Strategies-to-Mitigate-Violence-in-Healthcare.pdf
16. McEwan TE. Stalking threat and risk assessment. In: Reid Meloy J, Hoffman J (eds). International Handbook of Threat Assessment. 2nd ed. Oxford University Press; 2021:210-234.
17. Goldberg C. Nobody’s Victim: Fighting Psychos, Stalkers, Pervs, and Trolls. Plume; 2019.
18. Bazzell M. Extreme Privacy: What It Takes to Disappear. 2nd ed. Independently published; 2020.
19. Simon RI, Shuman DW. The doctor-patient relationship. Focus. 2007;5(4):423-431.
20. Department of Health and Human Services. 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program Final Rule (To be codified at 45 CFR 170 and 171). Federal Register. 2020;85(85):25642-25961.
21. Sheridan L, North AC, Scott AJ. Stalking in the workplace. Journal of Threat Assessment and Management. 2019;6(2):61-75.
22. The Joint Commission. Workplace Violence Prevention Standards. R3 Report: Requirement, Rationale, Reference. Issue 30. June 18, 2021. Accessed November 24, 2021. https://www.jointcommission.org/-/media/tjc/documents/standards/r3-reports/wpvp-r3-30_revised_06302021.pdf
23. Terry LP. Threat assessment teams. J Healthc Prot Manage. 2015;31(2):23-35.
24. Pathé M. Surviving Stalking. Cambridge University Press; 2002.
25. Noffsinger S. What stalking victims need to restore their mental and somatic health. Current Psychiatry. 2015;14(6):43-47.
26. Mullen P, Whyte S, McIvor R; Psychiatrists’ Support Service, Royal College of Psychiatry. PSS Information Guide: Stalking. Report No. 11. 2017. Accessed November 24, 2021. https://www.rcpsych.ac.uk/docs/default-source/members/supporting-you/pss/pss-guide-11-stalking.pdf?sfvrsn=2f1c7253_2
1. Nelsen AJ, Johnson RS, Ostermeyer B, et al. The prevalence of physicians who have been stalked: a systematic review. J Am Acad Psychiatry Law. 2015;43(2):177-182.
2. Jutasi C, McEwan TE. Stalking of professionals: a scoping review. Journal of Threat Assessment and Management. 2021;8(3):94-124.
3. Pathé MT, Meloy JR. Commentary: Stalking by patients—psychiatrists’ tales of anger, lust and ignorance. J Am Acad Psychiatry Law. 2013;41(2):200-205.
4. Pendergrast TR, Jain S, Trueger NS, et al. Prevalence of personal attacks and sexual harassment of physicians on social media. JAMA Intern Med. 2021;181(4):550-552.
5. Owens JG. Why definitions matter: stalking victimization in the United States. J Interpers Violence. 2016;31(12):2196-2226.
6. College of Policing. Stalking or harassment. May 2019. Accessed March 8, 2020. https://library.college.police.uk/docs/college-of-policing/Stalking_or_harassment_guidance_200519.pdf
7. McEwan TE, Daffern M, MacKenzie RD, et al. Risk factors for stalking violence, persistence, and recurrence. Journal of Forensic Psychiatry & Psychology. 2017;28(1):3856.
8. Pathé MT, Mullen PE, Purcell R. Patients who stalk doctors: their motives and management. Med J Australia. 2002;176(7):335-338.
9. Reibling ET, Distelberg B, Guptill M, et al. Intimate partner violence experienced by physicians. J Prim Care Community Health. 2020;11:2150132720965077.
10. Matias A, Gonçalves M, Soeiro C, et al. Intimate partner homicide: a meta-analysis of risk factors. Aggression and Violent Behavior. 2019;50:101358.
11. US Bureau of Labor Statistics. Fact sheet. Workplace violence in healthcare, 2018. April 2020. Accessed November 24, 2021. https://www.bls.gov/iif/oshwc/cfoi/workplace-violence-healthcare-2018.htm
12. Truman JL, Morgan RE. Stalking victimization, 2016. Bureau of Justice Statistics, Office of Justice Programs, U.S. Department of Justice. Report No.: NCJ 253526. April 2021. Accessed November 24, 2021. https://bjs.ojp.gov/library/publications/stalking-victimization-2016
13. Reyns BW, Henson B, Fisher BS. Being pursued online: applying cyberlifestyle–routine activities theory to cyberstalking victimization. Criminal Justice and Behavior. 2011;38(11):1149-1169.
14. Stea JN. When promoting knowledge makes you a target. Scientific American Blog Network. March 16, 2020. Accessed November 24, 2021. https://blogs.scientificamerican.com/observations/when-promoting-knowledge-makes-you-a-target/
15. Henkel SJ. Threat assessment strategies to mitigate violence in healthcare. IAHSS Foundation. IAHSS-F RS-19-02. November 2019. Accessed November 24, 2021. https://iahssf.org/assets/IAHSS-Foundation-Threat-Assessment-Strategies-to-Mitigate-Violence-in-Healthcare.pdf
16. McEwan TE. Stalking threat and risk assessment. In: Reid Meloy J, Hoffman J (eds). International Handbook of Threat Assessment. 2nd ed. Oxford University Press; 2021:210-234.
17. Goldberg C. Nobody’s Victim: Fighting Psychos, Stalkers, Pervs, and Trolls. Plume; 2019.
18. Bazzell M. Extreme Privacy: What It Takes to Disappear. 2nd ed. Independently published; 2020.
19. Simon RI, Shuman DW. The doctor-patient relationship. Focus. 2007;5(4):423-431.
20. Department of Health and Human Services. 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program Final Rule (To be codified at 45 CFR 170 and 171). Federal Register. 2020;85(85):25642-25961.
21. Sheridan L, North AC, Scott AJ. Stalking in the workplace. Journal of Threat Assessment and Management. 2019;6(2):61-75.
22. The Joint Commission. Workplace Violence Prevention Standards. R3 Report: Requirement, Rationale, Reference. Issue 30. June 18, 2021. Accessed November 24, 2021. https://www.jointcommission.org/-/media/tjc/documents/standards/r3-reports/wpvp-r3-30_revised_06302021.pdf
23. Terry LP. Threat assessment teams. J Healthc Prot Manage. 2015;31(2):23-35.
24. Pathé M. Surviving Stalking. Cambridge University Press; 2002.
25. Noffsinger S. What stalking victims need to restore their mental and somatic health. Current Psychiatry. 2015;14(6):43-47.
26. Mullen P, Whyte S, McIvor R; Psychiatrists’ Support Service, Royal College of Psychiatry. PSS Information Guide: Stalking. Report No. 11. 2017. Accessed November 24, 2021. https://www.rcpsych.ac.uk/docs/default-source/members/supporting-you/pss/pss-guide-11-stalking.pdf?sfvrsn=2f1c7253_2
Travel/school disruptions as COVID-19 cases grow in 2022
As the United States enters a third year of the COVID-19 pandemic,
The United States is reporting a 7-day average of more than 386,000 cases after several record-breaking days, according to the data tracker by the New York Times. The United States surpassed 585,000 cases on Dec. 30, setting a new record before the New Year’s holiday.
New York, Washington, D.C., and other states along the East Coast are leading the national surge. New York reported more than 85,000 new cases on the last day of 2021, marking the highest 1-day total in the state since the pandemic began.
“As we fight the winter surge, we need to keep the most vulnerable among us in mind – do what you can to keep others in your community safe from COVID-19,” New York Gov. Kathy Hochul said in a statement on Jan. 1, 2022.
“Wear a mask, wash your hands, and take advantage of the best tool we have at our disposal – the vaccine,” she said.
The 2021 winter surge in the United States peaked around Jan. 12, which may suggest that the country has a week or so before the current wave reaches its height and begins to drop, the newspaper reported.
In the meantime, people are dealing with disruptions as they return from holiday travel and begin the new year. Airlines canceled more than 2,700 flights on Jan. 1 and more than 1,900 flights on Jan. 2, bringing the total since Christmas Eve to more than 14,000 canceled flights.
About half of cancellations were connected to wintry weather at key airline hubs in Chicago and Denver, the newspaper reported, as well as ongoing flight crew shortages caused by the Omicron variant.
More disruptions could continue, the Federal Aviation Administration warned, as an increasing number of its air-traffic control employees test positive for COVID-19.
“To maintain safety, traffic volumes at some facilities could be reduced, which might result in delays during busy periods,” an FAA spokesman told The Wall Street Journal.
The current COVID-19 surge will also affect businesses and schools as the new year begins. A growing number of universities are opting to start the next semester with remote instruction.
American University, Duke University, and Michigan State University announced in recent days that they would delay in-person classes to slow the spread of the coronavirus on campus. They will begin classes online on Jan. 10 and return to campus the following week or later.
“I realize that students prefer to be in person, and so do I. But it is important that we do so in a safe manner,” Samuel Stanley Jr., MD, president of Michigan State University, said in a statement on New Year’s Eve.
K-12 school districts are deciding how to adapt as well. Some districts are bringing back mask requirements, and some are ramping up testing. Others are moving to remote learning – and signaling the need for flexibility as the Omicron variant brings new surprises.
“Change has been the only constant in this fight,” Roger Leon, the superintendent for Newark (N.J.) Public Schools, wrote in a note to parents. He announced on Dec. 30, 2021, that students would learn remotely for at least the first 2 weeks of the new year.
This continues “to be a brutal, relentless, and ruthless virus that rears its ugly head at inopportune times,” he said.
A version of this article first appeared on WebMD.com.
As the United States enters a third year of the COVID-19 pandemic,
The United States is reporting a 7-day average of more than 386,000 cases after several record-breaking days, according to the data tracker by the New York Times. The United States surpassed 585,000 cases on Dec. 30, setting a new record before the New Year’s holiday.
New York, Washington, D.C., and other states along the East Coast are leading the national surge. New York reported more than 85,000 new cases on the last day of 2021, marking the highest 1-day total in the state since the pandemic began.
“As we fight the winter surge, we need to keep the most vulnerable among us in mind – do what you can to keep others in your community safe from COVID-19,” New York Gov. Kathy Hochul said in a statement on Jan. 1, 2022.
“Wear a mask, wash your hands, and take advantage of the best tool we have at our disposal – the vaccine,” she said.
The 2021 winter surge in the United States peaked around Jan. 12, which may suggest that the country has a week or so before the current wave reaches its height and begins to drop, the newspaper reported.
In the meantime, people are dealing with disruptions as they return from holiday travel and begin the new year. Airlines canceled more than 2,700 flights on Jan. 1 and more than 1,900 flights on Jan. 2, bringing the total since Christmas Eve to more than 14,000 canceled flights.
About half of cancellations were connected to wintry weather at key airline hubs in Chicago and Denver, the newspaper reported, as well as ongoing flight crew shortages caused by the Omicron variant.
More disruptions could continue, the Federal Aviation Administration warned, as an increasing number of its air-traffic control employees test positive for COVID-19.
“To maintain safety, traffic volumes at some facilities could be reduced, which might result in delays during busy periods,” an FAA spokesman told The Wall Street Journal.
The current COVID-19 surge will also affect businesses and schools as the new year begins. A growing number of universities are opting to start the next semester with remote instruction.
American University, Duke University, and Michigan State University announced in recent days that they would delay in-person classes to slow the spread of the coronavirus on campus. They will begin classes online on Jan. 10 and return to campus the following week or later.
“I realize that students prefer to be in person, and so do I. But it is important that we do so in a safe manner,” Samuel Stanley Jr., MD, president of Michigan State University, said in a statement on New Year’s Eve.
K-12 school districts are deciding how to adapt as well. Some districts are bringing back mask requirements, and some are ramping up testing. Others are moving to remote learning – and signaling the need for flexibility as the Omicron variant brings new surprises.
“Change has been the only constant in this fight,” Roger Leon, the superintendent for Newark (N.J.) Public Schools, wrote in a note to parents. He announced on Dec. 30, 2021, that students would learn remotely for at least the first 2 weeks of the new year.
This continues “to be a brutal, relentless, and ruthless virus that rears its ugly head at inopportune times,” he said.
A version of this article first appeared on WebMD.com.
As the United States enters a third year of the COVID-19 pandemic,
The United States is reporting a 7-day average of more than 386,000 cases after several record-breaking days, according to the data tracker by the New York Times. The United States surpassed 585,000 cases on Dec. 30, setting a new record before the New Year’s holiday.
New York, Washington, D.C., and other states along the East Coast are leading the national surge. New York reported more than 85,000 new cases on the last day of 2021, marking the highest 1-day total in the state since the pandemic began.
“As we fight the winter surge, we need to keep the most vulnerable among us in mind – do what you can to keep others in your community safe from COVID-19,” New York Gov. Kathy Hochul said in a statement on Jan. 1, 2022.
“Wear a mask, wash your hands, and take advantage of the best tool we have at our disposal – the vaccine,” she said.
The 2021 winter surge in the United States peaked around Jan. 12, which may suggest that the country has a week or so before the current wave reaches its height and begins to drop, the newspaper reported.
In the meantime, people are dealing with disruptions as they return from holiday travel and begin the new year. Airlines canceled more than 2,700 flights on Jan. 1 and more than 1,900 flights on Jan. 2, bringing the total since Christmas Eve to more than 14,000 canceled flights.
About half of cancellations were connected to wintry weather at key airline hubs in Chicago and Denver, the newspaper reported, as well as ongoing flight crew shortages caused by the Omicron variant.
More disruptions could continue, the Federal Aviation Administration warned, as an increasing number of its air-traffic control employees test positive for COVID-19.
“To maintain safety, traffic volumes at some facilities could be reduced, which might result in delays during busy periods,” an FAA spokesman told The Wall Street Journal.
The current COVID-19 surge will also affect businesses and schools as the new year begins. A growing number of universities are opting to start the next semester with remote instruction.
American University, Duke University, and Michigan State University announced in recent days that they would delay in-person classes to slow the spread of the coronavirus on campus. They will begin classes online on Jan. 10 and return to campus the following week or later.
“I realize that students prefer to be in person, and so do I. But it is important that we do so in a safe manner,” Samuel Stanley Jr., MD, president of Michigan State University, said in a statement on New Year’s Eve.
K-12 school districts are deciding how to adapt as well. Some districts are bringing back mask requirements, and some are ramping up testing. Others are moving to remote learning – and signaling the need for flexibility as the Omicron variant brings new surprises.
“Change has been the only constant in this fight,” Roger Leon, the superintendent for Newark (N.J.) Public Schools, wrote in a note to parents. He announced on Dec. 30, 2021, that students would learn remotely for at least the first 2 weeks of the new year.
This continues “to be a brutal, relentless, and ruthless virus that rears its ugly head at inopportune times,” he said.
A version of this article first appeared on WebMD.com.
Vedolizumab does not increase risk of C. diff infection in UC
Vedolizumab does not seem to increase the risk of Clostridioides difficile infection (CDI), compared with anti–tumor necrosis factor (TNF) therapies in biologic-naive patients with ulcerative colitis (UC), despite concerns that the gut-selective monoclonal antibody treatment may increase gastrointestinal infections at a greater rate than other biologics in this patient population.
Perturbations of the gut microbiota that occur in IBD predispose patients to CDI. Given that treatment with monoclonal antibody vedolizumab exerts an inhibitory action on lymphocyte trafficking to the intestines, questions have been raised on whether this action could increase the risk of CDI in an already vulnerable population.
In patients with UC, the incidence of CDI typically confers a higher risk of adverse outcomes. Unfortunately, CDI is a common complication associated with inflammatory bowel disease (IBD) that can lead to disease flares, further adding to the physical and psychological burden associated with the condition, according to recent studies.
These concerns, however, may not be warranted in patients with UC, according to findings from a retrospective study presented at the annual Advances in Inflammatory Bowel Diseases conference by Rahul Dalal, MD, a gastroenterology fellow at Brigham and Women’s Hospital in Boston.
In the study, Dr. Dalal and colleagues retrospectively analyzed electronic medical records of adult patients with UC who initiated infliximab, adalimumab, or vedolizumab between June 2014 and December 2020. Patients in this retrospective cohort were followed until there was a documented occurrence of CDI, colectomy, or biologic discontinuation/switch, or until the last recorded gastroenterology encounter.
The researchers analyzed the time from biologic initiation to first CDI, which was characterized by a positive stool for C. difficile toxin or toxigenic C. difficile polymerase chain reaction with CDI-specific antibiotic prescriptions. Additionally, the investigators evaluated rates of CDI-related hospitalization, colectomy, or death within a 30-day period of CDI. The primary analysis compared patients with UC who initiated vedolizumab (n = 195) versus anti-TNF therapy (n = 610).
Compared with those treated with anti-TNF agents, patients who initiated vedolizumab were older and less frequently received systemic corticosteroids or had UC-related hospitalization within 12 months prior to starting biologics.
Over 1,436 patient-years’ worth of follow-up, the investigators observed 43 CDIs. Patients treated with vedolizumab less frequently had CDI (1.0% vs. 6.7%; P =.001) and CDI hospitalization (1.0% vs. 3.8%; P =.042), compared with those treated with anti-TNF therapies. The investigators reported no significant differences in the rates of colectomies or deaths or rates of exposure to antibiotics/corticosteroids during the follow-up period or within 30 days prior to CDI onset.
In the unadjusted Cox model, the researchers reported that vedolizumab featured a lower hazard of CDI, compared with anti-TNF (hazard ratio, 0.17; 95% confidence interval, 0.04-0.71). The multivariable Cox model found no significant difference in hazard of CDI for vedolizumab when compared with anti-TNF therapy (HR, 0.33; 95% CI, 0.05-2.03) or immunomodulator exposure (HR, 1.01; 95% CI, 0.41-2.40). The incidence of CDI prior to biologic initiation was associated with an increased hazard of subsequent CDI (HR, 5.95; 95% CI, 2.93-12.09). In the subgroup of patients who experienced a CDI, approximately 39.5% had CDI before biologic initiation at a median of 227 days preceding the subsequent event.
“Vedolizumab is one of the safest biologics that we have in the clinic,” said Jean-Frederic Colombel, MD, who was asked to comment on the study. Dr. Colombel, who wasn’t involved in the study, is a gastroenterologist and serves as director of the Feinstein IBD Center at Mount Sinai Hospital and professor of medicine (division of gastroenterology) at the Icahn School of Medicine at Mount Sinai, both in New York. “Findings from this study reinforce the safety profile of vedolizumab” despite the potential concerns regarding gastroenterological infection with the agent, he added.
Recurrence worries
Recurrent CDI is an issue in patients with IBD, many of whom are considered at high risk for initial and recurrent infection. During a session on CDI and recurrence at the AIBD meeting, Sahil Khanna, MBBS, of the Mayo Clinic, explained that there are three different treatment guidelines to manage initial CDI in patients with IBD.
Predominantly, these guidelines also suggest human monoclonal antibody bezlotoxumab could be used for prevention of CDI recurrence in patients at high risk of recurrence, including those who had experienced severe CDI. “One can argue that anyone with IBD who has C. difficile can be a severe CDI patient because of the bad outcomes we can see,” he explained.
“We do know that IBD is a state of chronic microbial dysbiosis compared to our patients without IBD who get C. difficile because of antibiotic exposure, and that’s why these patients have a high risk of recurrence, compared with non-IBD patients,” said Dr. Khanna. He noted that the bezlotoxumab studies showed numerically lower CDI recurrence rates compared with other treatments in patients with IBD who were initially treated with the monoclonal antibody, but this difference was not statistically significant. “But again, this agent has been shown to be safe in this patient population.”
Dr. Dalal reported having no relevant conflicts of interest. Dr. Colombel has consulted for Takeda, which markets Entyvio for UC. Dr. Khanna has research grants from Rebiotix, as well as consulting fees from Shire Plc, Premier, Facile Therapeutics, and ProbioTech.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff .
Vedolizumab does not seem to increase the risk of Clostridioides difficile infection (CDI), compared with anti–tumor necrosis factor (TNF) therapies in biologic-naive patients with ulcerative colitis (UC), despite concerns that the gut-selective monoclonal antibody treatment may increase gastrointestinal infections at a greater rate than other biologics in this patient population.
Perturbations of the gut microbiota that occur in IBD predispose patients to CDI. Given that treatment with monoclonal antibody vedolizumab exerts an inhibitory action on lymphocyte trafficking to the intestines, questions have been raised on whether this action could increase the risk of CDI in an already vulnerable population.
In patients with UC, the incidence of CDI typically confers a higher risk of adverse outcomes. Unfortunately, CDI is a common complication associated with inflammatory bowel disease (IBD) that can lead to disease flares, further adding to the physical and psychological burden associated with the condition, according to recent studies.
These concerns, however, may not be warranted in patients with UC, according to findings from a retrospective study presented at the annual Advances in Inflammatory Bowel Diseases conference by Rahul Dalal, MD, a gastroenterology fellow at Brigham and Women’s Hospital in Boston.
In the study, Dr. Dalal and colleagues retrospectively analyzed electronic medical records of adult patients with UC who initiated infliximab, adalimumab, or vedolizumab between June 2014 and December 2020. Patients in this retrospective cohort were followed until there was a documented occurrence of CDI, colectomy, or biologic discontinuation/switch, or until the last recorded gastroenterology encounter.
The researchers analyzed the time from biologic initiation to first CDI, which was characterized by a positive stool for C. difficile toxin or toxigenic C. difficile polymerase chain reaction with CDI-specific antibiotic prescriptions. Additionally, the investigators evaluated rates of CDI-related hospitalization, colectomy, or death within a 30-day period of CDI. The primary analysis compared patients with UC who initiated vedolizumab (n = 195) versus anti-TNF therapy (n = 610).
Compared with those treated with anti-TNF agents, patients who initiated vedolizumab were older and less frequently received systemic corticosteroids or had UC-related hospitalization within 12 months prior to starting biologics.
Over 1,436 patient-years’ worth of follow-up, the investigators observed 43 CDIs. Patients treated with vedolizumab less frequently had CDI (1.0% vs. 6.7%; P =.001) and CDI hospitalization (1.0% vs. 3.8%; P =.042), compared with those treated with anti-TNF therapies. The investigators reported no significant differences in the rates of colectomies or deaths or rates of exposure to antibiotics/corticosteroids during the follow-up period or within 30 days prior to CDI onset.
In the unadjusted Cox model, the researchers reported that vedolizumab featured a lower hazard of CDI, compared with anti-TNF (hazard ratio, 0.17; 95% confidence interval, 0.04-0.71). The multivariable Cox model found no significant difference in hazard of CDI for vedolizumab when compared with anti-TNF therapy (HR, 0.33; 95% CI, 0.05-2.03) or immunomodulator exposure (HR, 1.01; 95% CI, 0.41-2.40). The incidence of CDI prior to biologic initiation was associated with an increased hazard of subsequent CDI (HR, 5.95; 95% CI, 2.93-12.09). In the subgroup of patients who experienced a CDI, approximately 39.5% had CDI before biologic initiation at a median of 227 days preceding the subsequent event.
“Vedolizumab is one of the safest biologics that we have in the clinic,” said Jean-Frederic Colombel, MD, who was asked to comment on the study. Dr. Colombel, who wasn’t involved in the study, is a gastroenterologist and serves as director of the Feinstein IBD Center at Mount Sinai Hospital and professor of medicine (division of gastroenterology) at the Icahn School of Medicine at Mount Sinai, both in New York. “Findings from this study reinforce the safety profile of vedolizumab” despite the potential concerns regarding gastroenterological infection with the agent, he added.
Recurrence worries
Recurrent CDI is an issue in patients with IBD, many of whom are considered at high risk for initial and recurrent infection. During a session on CDI and recurrence at the AIBD meeting, Sahil Khanna, MBBS, of the Mayo Clinic, explained that there are three different treatment guidelines to manage initial CDI in patients with IBD.
Predominantly, these guidelines also suggest human monoclonal antibody bezlotoxumab could be used for prevention of CDI recurrence in patients at high risk of recurrence, including those who had experienced severe CDI. “One can argue that anyone with IBD who has C. difficile can be a severe CDI patient because of the bad outcomes we can see,” he explained.
“We do know that IBD is a state of chronic microbial dysbiosis compared to our patients without IBD who get C. difficile because of antibiotic exposure, and that’s why these patients have a high risk of recurrence, compared with non-IBD patients,” said Dr. Khanna. He noted that the bezlotoxumab studies showed numerically lower CDI recurrence rates compared with other treatments in patients with IBD who were initially treated with the monoclonal antibody, but this difference was not statistically significant. “But again, this agent has been shown to be safe in this patient population.”
Dr. Dalal reported having no relevant conflicts of interest. Dr. Colombel has consulted for Takeda, which markets Entyvio for UC. Dr. Khanna has research grants from Rebiotix, as well as consulting fees from Shire Plc, Premier, Facile Therapeutics, and ProbioTech.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff .
Vedolizumab does not seem to increase the risk of Clostridioides difficile infection (CDI), compared with anti–tumor necrosis factor (TNF) therapies in biologic-naive patients with ulcerative colitis (UC), despite concerns that the gut-selective monoclonal antibody treatment may increase gastrointestinal infections at a greater rate than other biologics in this patient population.
Perturbations of the gut microbiota that occur in IBD predispose patients to CDI. Given that treatment with monoclonal antibody vedolizumab exerts an inhibitory action on lymphocyte trafficking to the intestines, questions have been raised on whether this action could increase the risk of CDI in an already vulnerable population.
In patients with UC, the incidence of CDI typically confers a higher risk of adverse outcomes. Unfortunately, CDI is a common complication associated with inflammatory bowel disease (IBD) that can lead to disease flares, further adding to the physical and psychological burden associated with the condition, according to recent studies.
These concerns, however, may not be warranted in patients with UC, according to findings from a retrospective study presented at the annual Advances in Inflammatory Bowel Diseases conference by Rahul Dalal, MD, a gastroenterology fellow at Brigham and Women’s Hospital in Boston.
In the study, Dr. Dalal and colleagues retrospectively analyzed electronic medical records of adult patients with UC who initiated infliximab, adalimumab, or vedolizumab between June 2014 and December 2020. Patients in this retrospective cohort were followed until there was a documented occurrence of CDI, colectomy, or biologic discontinuation/switch, or until the last recorded gastroenterology encounter.
The researchers analyzed the time from biologic initiation to first CDI, which was characterized by a positive stool for C. difficile toxin or toxigenic C. difficile polymerase chain reaction with CDI-specific antibiotic prescriptions. Additionally, the investigators evaluated rates of CDI-related hospitalization, colectomy, or death within a 30-day period of CDI. The primary analysis compared patients with UC who initiated vedolizumab (n = 195) versus anti-TNF therapy (n = 610).
Compared with those treated with anti-TNF agents, patients who initiated vedolizumab were older and less frequently received systemic corticosteroids or had UC-related hospitalization within 12 months prior to starting biologics.
Over 1,436 patient-years’ worth of follow-up, the investigators observed 43 CDIs. Patients treated with vedolizumab less frequently had CDI (1.0% vs. 6.7%; P =.001) and CDI hospitalization (1.0% vs. 3.8%; P =.042), compared with those treated with anti-TNF therapies. The investigators reported no significant differences in the rates of colectomies or deaths or rates of exposure to antibiotics/corticosteroids during the follow-up period or within 30 days prior to CDI onset.
In the unadjusted Cox model, the researchers reported that vedolizumab featured a lower hazard of CDI, compared with anti-TNF (hazard ratio, 0.17; 95% confidence interval, 0.04-0.71). The multivariable Cox model found no significant difference in hazard of CDI for vedolizumab when compared with anti-TNF therapy (HR, 0.33; 95% CI, 0.05-2.03) or immunomodulator exposure (HR, 1.01; 95% CI, 0.41-2.40). The incidence of CDI prior to biologic initiation was associated with an increased hazard of subsequent CDI (HR, 5.95; 95% CI, 2.93-12.09). In the subgroup of patients who experienced a CDI, approximately 39.5% had CDI before biologic initiation at a median of 227 days preceding the subsequent event.
“Vedolizumab is one of the safest biologics that we have in the clinic,” said Jean-Frederic Colombel, MD, who was asked to comment on the study. Dr. Colombel, who wasn’t involved in the study, is a gastroenterologist and serves as director of the Feinstein IBD Center at Mount Sinai Hospital and professor of medicine (division of gastroenterology) at the Icahn School of Medicine at Mount Sinai, both in New York. “Findings from this study reinforce the safety profile of vedolizumab” despite the potential concerns regarding gastroenterological infection with the agent, he added.
Recurrence worries
Recurrent CDI is an issue in patients with IBD, many of whom are considered at high risk for initial and recurrent infection. During a session on CDI and recurrence at the AIBD meeting, Sahil Khanna, MBBS, of the Mayo Clinic, explained that there are three different treatment guidelines to manage initial CDI in patients with IBD.
Predominantly, these guidelines also suggest human monoclonal antibody bezlotoxumab could be used for prevention of CDI recurrence in patients at high risk of recurrence, including those who had experienced severe CDI. “One can argue that anyone with IBD who has C. difficile can be a severe CDI patient because of the bad outcomes we can see,” he explained.
“We do know that IBD is a state of chronic microbial dysbiosis compared to our patients without IBD who get C. difficile because of antibiotic exposure, and that’s why these patients have a high risk of recurrence, compared with non-IBD patients,” said Dr. Khanna. He noted that the bezlotoxumab studies showed numerically lower CDI recurrence rates compared with other treatments in patients with IBD who were initially treated with the monoclonal antibody, but this difference was not statistically significant. “But again, this agent has been shown to be safe in this patient population.”
Dr. Dalal reported having no relevant conflicts of interest. Dr. Colombel has consulted for Takeda, which markets Entyvio for UC. Dr. Khanna has research grants from Rebiotix, as well as consulting fees from Shire Plc, Premier, Facile Therapeutics, and ProbioTech.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff .
FROM AIBD 2021
Antiretroviral pill better at suppressing HIV in children
Dolutegravir suppresses HIV by inhibiting integrase, an enzyme that the virus needs to replicate.
The pill-based regimen, which researchers described as easier to take than standard treatment, reduced the chances of treatment failure among children aged 3-18 years by about 40%, compared with other treatments. Dolutegravir is already used for the suppression of HIV in adults.
“About 1.8 million children live with HIV but they have had limited treatment options, with medicines that taste unpalatable, that need to be taken twice a day, or that come in large pills that are difficult to swallow” said lead author Anna Turkova, MD, from the MRC clinical trials unit at UCL. “Dolutegravir is given in small tablets usually once a day and the baby pills can be dispersed in water, meaning it’s a lot easier for young children to take. This is important in encouraging uptake of the treatment and adherence to it over many years.
“Sadly, only about half of children living with HIV are currently receiving treatment, and those who are not treated face high risks of impaired immunity and worsening health.”
Study details
The randomized controlled trial, called ODYSSEY, involved more than 700 children from 29 clinical centers located in Africa, Europe, and Asia. The children were given either dolutegravir or standard anti-HIV drugs, and were followed up for at least 2 years.
The study showed that 14% of children receiving dolutegravir experienced treatment failure over 2 years, compared with 22% of those receiving standard treatment. Treatment failure was deemed to occur if measurable virus appeared in the blood or if the child had symptoms of HIV-related illness.
“Our findings provide strong evidence for the global rollout of dolutegravir for children with HIV,” said Diana Gibb, MD, also from the MRC clinical trials unit at UCL, principal investigator of the trial and one of the senior authors of the paper.
“Medical treatments for children often lag woefully behind those of adults because of the separate formulations and studies that are needed,” she added. “With the evidence from ODYSSEY which used simplified dosing of both adult and baby pills, this treatment gap has been reduced and we hope that countries can quickly scale up access to children globally.”
Simplified dosing
“Simplifying the dosing is crucial,” concurred Cissy Kityo Mutuluuza, MD, from the Joint Clinical Research Centre in Uganda, the country enrolling most children in the trial. “Older children being able to take the same tablets as adults immediately opens access to dolutegravir for the majority of children living with HIV. It greatly simplifies procurement for national health systems in low- and middle-income countries, and lowers costs.”
Evidence from adults shows dolutegravir has a high genetic barrier to resistance, meaning viruses are less likely to become resistant to it over time. This was confirmed in the ODYSSEY trial, with much less resistance occurring among children and adolescents on dolutegravir-based treatment. In addition, past studies of the drug have shown that it may be associated with weight gain in adults, but the findings were reassuring for children. Those given dolutegravir gained on average 1 kg more and grew 1 cm higher over the study period, indicating better growth rather than abnormal weight gain.
Early findings from the trial have informed new guidance by the World Health Organization, recommending the use of dolutegravir for children.
The study was sponsored by the Penta Foundation, an international independent research network, and funded by specialist pharmaceutical company ViiV Healthcare.
A version of this article first appeared on Medscape.com.
Dolutegravir suppresses HIV by inhibiting integrase, an enzyme that the virus needs to replicate.
The pill-based regimen, which researchers described as easier to take than standard treatment, reduced the chances of treatment failure among children aged 3-18 years by about 40%, compared with other treatments. Dolutegravir is already used for the suppression of HIV in adults.
“About 1.8 million children live with HIV but they have had limited treatment options, with medicines that taste unpalatable, that need to be taken twice a day, or that come in large pills that are difficult to swallow” said lead author Anna Turkova, MD, from the MRC clinical trials unit at UCL. “Dolutegravir is given in small tablets usually once a day and the baby pills can be dispersed in water, meaning it’s a lot easier for young children to take. This is important in encouraging uptake of the treatment and adherence to it over many years.
“Sadly, only about half of children living with HIV are currently receiving treatment, and those who are not treated face high risks of impaired immunity and worsening health.”
Study details
The randomized controlled trial, called ODYSSEY, involved more than 700 children from 29 clinical centers located in Africa, Europe, and Asia. The children were given either dolutegravir or standard anti-HIV drugs, and were followed up for at least 2 years.
The study showed that 14% of children receiving dolutegravir experienced treatment failure over 2 years, compared with 22% of those receiving standard treatment. Treatment failure was deemed to occur if measurable virus appeared in the blood or if the child had symptoms of HIV-related illness.
“Our findings provide strong evidence for the global rollout of dolutegravir for children with HIV,” said Diana Gibb, MD, also from the MRC clinical trials unit at UCL, principal investigator of the trial and one of the senior authors of the paper.
“Medical treatments for children often lag woefully behind those of adults because of the separate formulations and studies that are needed,” she added. “With the evidence from ODYSSEY which used simplified dosing of both adult and baby pills, this treatment gap has been reduced and we hope that countries can quickly scale up access to children globally.”
Simplified dosing
“Simplifying the dosing is crucial,” concurred Cissy Kityo Mutuluuza, MD, from the Joint Clinical Research Centre in Uganda, the country enrolling most children in the trial. “Older children being able to take the same tablets as adults immediately opens access to dolutegravir for the majority of children living with HIV. It greatly simplifies procurement for national health systems in low- and middle-income countries, and lowers costs.”
Evidence from adults shows dolutegravir has a high genetic barrier to resistance, meaning viruses are less likely to become resistant to it over time. This was confirmed in the ODYSSEY trial, with much less resistance occurring among children and adolescents on dolutegravir-based treatment. In addition, past studies of the drug have shown that it may be associated with weight gain in adults, but the findings were reassuring for children. Those given dolutegravir gained on average 1 kg more and grew 1 cm higher over the study period, indicating better growth rather than abnormal weight gain.
Early findings from the trial have informed new guidance by the World Health Organization, recommending the use of dolutegravir for children.
The study was sponsored by the Penta Foundation, an international independent research network, and funded by specialist pharmaceutical company ViiV Healthcare.
A version of this article first appeared on Medscape.com.
Dolutegravir suppresses HIV by inhibiting integrase, an enzyme that the virus needs to replicate.
The pill-based regimen, which researchers described as easier to take than standard treatment, reduced the chances of treatment failure among children aged 3-18 years by about 40%, compared with other treatments. Dolutegravir is already used for the suppression of HIV in adults.
“About 1.8 million children live with HIV but they have had limited treatment options, with medicines that taste unpalatable, that need to be taken twice a day, or that come in large pills that are difficult to swallow” said lead author Anna Turkova, MD, from the MRC clinical trials unit at UCL. “Dolutegravir is given in small tablets usually once a day and the baby pills can be dispersed in water, meaning it’s a lot easier for young children to take. This is important in encouraging uptake of the treatment and adherence to it over many years.
“Sadly, only about half of children living with HIV are currently receiving treatment, and those who are not treated face high risks of impaired immunity and worsening health.”
Study details
The randomized controlled trial, called ODYSSEY, involved more than 700 children from 29 clinical centers located in Africa, Europe, and Asia. The children were given either dolutegravir or standard anti-HIV drugs, and were followed up for at least 2 years.
The study showed that 14% of children receiving dolutegravir experienced treatment failure over 2 years, compared with 22% of those receiving standard treatment. Treatment failure was deemed to occur if measurable virus appeared in the blood or if the child had symptoms of HIV-related illness.
“Our findings provide strong evidence for the global rollout of dolutegravir for children with HIV,” said Diana Gibb, MD, also from the MRC clinical trials unit at UCL, principal investigator of the trial and one of the senior authors of the paper.
“Medical treatments for children often lag woefully behind those of adults because of the separate formulations and studies that are needed,” she added. “With the evidence from ODYSSEY which used simplified dosing of both adult and baby pills, this treatment gap has been reduced and we hope that countries can quickly scale up access to children globally.”
Simplified dosing
“Simplifying the dosing is crucial,” concurred Cissy Kityo Mutuluuza, MD, from the Joint Clinical Research Centre in Uganda, the country enrolling most children in the trial. “Older children being able to take the same tablets as adults immediately opens access to dolutegravir for the majority of children living with HIV. It greatly simplifies procurement for national health systems in low- and middle-income countries, and lowers costs.”
Evidence from adults shows dolutegravir has a high genetic barrier to resistance, meaning viruses are less likely to become resistant to it over time. This was confirmed in the ODYSSEY trial, with much less resistance occurring among children and adolescents on dolutegravir-based treatment. In addition, past studies of the drug have shown that it may be associated with weight gain in adults, but the findings were reassuring for children. Those given dolutegravir gained on average 1 kg more and grew 1 cm higher over the study period, indicating better growth rather than abnormal weight gain.
Early findings from the trial have informed new guidance by the World Health Organization, recommending the use of dolutegravir for children.
The study was sponsored by the Penta Foundation, an international independent research network, and funded by specialist pharmaceutical company ViiV Healthcare.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Pandemic screen time linked to anxiety, depression in older kids
However, the study doesn’t definitively prove that screen time is harmful, and an expert challenged the conclusions.
Still, the findings highlight the potential harms of excessive screen time, especially in the context of pandemic-era virtual learning. Clinicians “really need to advocate for policies that would be protective for children to reduce their screen time and social isolation and increase their involvement with school, sports, and academic activities,” Catherine S. Birken, MD, a pediatrician at the University of Toronto and study coauthor said in an interview.
The study appeared Dec. 28, 2021, in the journal JAMA Network Open (doi: 10.1001/jamanetworkopen.2021.40875).
Dr. Birken and colleagues launched the study to examine whether heightened levels of screen time during the pandemic disrupted mental health in kids. In particular, they wanted to break down different types of screen time, such as virtual learning, watching television, and playing video games.
“The bulk of the literature is supportive of a strong relationship between screen time and mental health symptoms like anxiety,” Dr. Birken said.
For the study, the researchers surveyed parents to track the screen time of 2,026 children between May 2020 and April 2021.
In a cohort of 532 younger children (average age, 5.9 years; 52% male; 58% of European ancestry), the researchers linked each extra daily hour of TV or use of digital media to worse behavior, as measured by the Strengths and Difficulties Questionnaire: 0.22 in an adjusted model for children aged 2-4;(95% confidence interval, 0.10-0.35; P < .001) and 0.07 in an adjusted model in those aged 4 and older (95% CI, 0.02-0.11; P = .007).
However, the researchers observed no statistically significant links to more anxiety/depression or hyperactivity/inattention in this group of children.
Among 1,494 older kids (mean age, 11.3; 57% male; 58% of European ancestry), researchers linked greater daily use of TV or digital media to higher levels of depression symptoms in a dose-dependent relationship, Dr. Birken said (1 hour: beta, 0.21; 95% CI, –1.28 to 0.78; 2-3 hours: beta, 1.81; 95% CI, 0.29-3.33; 4-5 hours: beta, 2.80; 95% CI, 1.15-4.44; 6-8 hours: beta, 5.16; 95% CI, 3.32-7.01; 9 hours: beta, 5.42; 95% CI, 3.30-7.54; overall P < .001).
“Similarly, higher TV or digital media time per day was associated with higher levels of anxiety symptoms,” the researchers reported. “TV or digital media time per day was also significantly associated with differences in symptoms of irritability, inattention, and hyperactivity/inattention.”
More time spent learning virtually was associated with higher levels of depression and anxiety in both groups of children, according to the researchers. Whether this finding reflects an effect of screens themselves or because the children most exposed to virtual learning may also have been the most exposed to the stressful disruptiveness of the pandemic is unclear.
The researchers also found “insufficient evidence” to link more virtual learning to irritability, inattention and hyperactivity, inattention, and hyperactivity/impulsivity in adjusted models.
Video chatting did not appear to have a protective effect, Dr. Birken said. The researchers also specifically analyzed children with autism and found no link between more screen time and various mental health/conduct problems.
Is it possible that kids with more anxiety, depression, and isolation simply turn to screens because they’re anxious, depressed, and isolated? Dr. Birken said the researchers adjusted the findings to account for previous mental health problems. And she noted that the study linked more pandemic-era virtual learning to more depression/anxiety. It’s “hard to imagine” how more mental health problems would cause more virtual learning.
Bad news or bad stats?
Chris Ferguson, PhD, a professor of psychology at Stetson University. DeLand, Fla., who studies screen time, criticized the study in an interview. “The observed effects are so tiny, it’s impossible to know if they are real or a false-positive artifact common to social science research,” he said. “Ultimately, this study is better evidence about how many scholars are bad at statistics than anything having to do with kids and screens.”
Dr. Ferguson said that the results may be confounded because kids turn to screens to reduce their anxiety. “For the most part, screens were a godsend during COVID-19,” he said. “They helped kids stay inside and gave them something to do while social distancing and allowed them to keep in touch with friends and families. Honestly, what else were we expecting kids to do, stare at the wallpaper?”
Children with depression and anxiety often retreat into screens or books to escape the unpleasantries of real life. “That doesn’t mean the screens or books are the culprits,” he said.
Instead of focusing on screen time, Dr. Ferguson suggested parents consider these factors: “Keeping in mind not every kid is a genius, is your kid doing about as well in school as you’d expect, given their natural ability? Are they getting at least some exercise every day? Are they getting adequate sleep? Are they able to socialize with friends in some context, either in real life or online? Are they happy?”
The study was funded by the Canadian Institutes of Health Research, the Center for Brain & Mental Health at The Hospital for Sick Children, the Ontario Ministry of Health, and the Miner’s Lamp Innovation Fund in Prevention and Early Detection of Severe Mental Illness at the University of Toronto. The study authors reported various financial relationships. Dr. Ferguson reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
However, the study doesn’t definitively prove that screen time is harmful, and an expert challenged the conclusions.
Still, the findings highlight the potential harms of excessive screen time, especially in the context of pandemic-era virtual learning. Clinicians “really need to advocate for policies that would be protective for children to reduce their screen time and social isolation and increase their involvement with school, sports, and academic activities,” Catherine S. Birken, MD, a pediatrician at the University of Toronto and study coauthor said in an interview.
The study appeared Dec. 28, 2021, in the journal JAMA Network Open (doi: 10.1001/jamanetworkopen.2021.40875).
Dr. Birken and colleagues launched the study to examine whether heightened levels of screen time during the pandemic disrupted mental health in kids. In particular, they wanted to break down different types of screen time, such as virtual learning, watching television, and playing video games.
“The bulk of the literature is supportive of a strong relationship between screen time and mental health symptoms like anxiety,” Dr. Birken said.
For the study, the researchers surveyed parents to track the screen time of 2,026 children between May 2020 and April 2021.
In a cohort of 532 younger children (average age, 5.9 years; 52% male; 58% of European ancestry), the researchers linked each extra daily hour of TV or use of digital media to worse behavior, as measured by the Strengths and Difficulties Questionnaire: 0.22 in an adjusted model for children aged 2-4;(95% confidence interval, 0.10-0.35; P < .001) and 0.07 in an adjusted model in those aged 4 and older (95% CI, 0.02-0.11; P = .007).
However, the researchers observed no statistically significant links to more anxiety/depression or hyperactivity/inattention in this group of children.
Among 1,494 older kids (mean age, 11.3; 57% male; 58% of European ancestry), researchers linked greater daily use of TV or digital media to higher levels of depression symptoms in a dose-dependent relationship, Dr. Birken said (1 hour: beta, 0.21; 95% CI, –1.28 to 0.78; 2-3 hours: beta, 1.81; 95% CI, 0.29-3.33; 4-5 hours: beta, 2.80; 95% CI, 1.15-4.44; 6-8 hours: beta, 5.16; 95% CI, 3.32-7.01; 9 hours: beta, 5.42; 95% CI, 3.30-7.54; overall P < .001).
“Similarly, higher TV or digital media time per day was associated with higher levels of anxiety symptoms,” the researchers reported. “TV or digital media time per day was also significantly associated with differences in symptoms of irritability, inattention, and hyperactivity/inattention.”
More time spent learning virtually was associated with higher levels of depression and anxiety in both groups of children, according to the researchers. Whether this finding reflects an effect of screens themselves or because the children most exposed to virtual learning may also have been the most exposed to the stressful disruptiveness of the pandemic is unclear.
The researchers also found “insufficient evidence” to link more virtual learning to irritability, inattention and hyperactivity, inattention, and hyperactivity/impulsivity in adjusted models.
Video chatting did not appear to have a protective effect, Dr. Birken said. The researchers also specifically analyzed children with autism and found no link between more screen time and various mental health/conduct problems.
Is it possible that kids with more anxiety, depression, and isolation simply turn to screens because they’re anxious, depressed, and isolated? Dr. Birken said the researchers adjusted the findings to account for previous mental health problems. And she noted that the study linked more pandemic-era virtual learning to more depression/anxiety. It’s “hard to imagine” how more mental health problems would cause more virtual learning.
Bad news or bad stats?
Chris Ferguson, PhD, a professor of psychology at Stetson University. DeLand, Fla., who studies screen time, criticized the study in an interview. “The observed effects are so tiny, it’s impossible to know if they are real or a false-positive artifact common to social science research,” he said. “Ultimately, this study is better evidence about how many scholars are bad at statistics than anything having to do with kids and screens.”
Dr. Ferguson said that the results may be confounded because kids turn to screens to reduce their anxiety. “For the most part, screens were a godsend during COVID-19,” he said. “They helped kids stay inside and gave them something to do while social distancing and allowed them to keep in touch with friends and families. Honestly, what else were we expecting kids to do, stare at the wallpaper?”
Children with depression and anxiety often retreat into screens or books to escape the unpleasantries of real life. “That doesn’t mean the screens or books are the culprits,” he said.
Instead of focusing on screen time, Dr. Ferguson suggested parents consider these factors: “Keeping in mind not every kid is a genius, is your kid doing about as well in school as you’d expect, given their natural ability? Are they getting at least some exercise every day? Are they getting adequate sleep? Are they able to socialize with friends in some context, either in real life or online? Are they happy?”
The study was funded by the Canadian Institutes of Health Research, the Center for Brain & Mental Health at The Hospital for Sick Children, the Ontario Ministry of Health, and the Miner’s Lamp Innovation Fund in Prevention and Early Detection of Severe Mental Illness at the University of Toronto. The study authors reported various financial relationships. Dr. Ferguson reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
However, the study doesn’t definitively prove that screen time is harmful, and an expert challenged the conclusions.
Still, the findings highlight the potential harms of excessive screen time, especially in the context of pandemic-era virtual learning. Clinicians “really need to advocate for policies that would be protective for children to reduce their screen time and social isolation and increase their involvement with school, sports, and academic activities,” Catherine S. Birken, MD, a pediatrician at the University of Toronto and study coauthor said in an interview.
The study appeared Dec. 28, 2021, in the journal JAMA Network Open (doi: 10.1001/jamanetworkopen.2021.40875).
Dr. Birken and colleagues launched the study to examine whether heightened levels of screen time during the pandemic disrupted mental health in kids. In particular, they wanted to break down different types of screen time, such as virtual learning, watching television, and playing video games.
“The bulk of the literature is supportive of a strong relationship between screen time and mental health symptoms like anxiety,” Dr. Birken said.
For the study, the researchers surveyed parents to track the screen time of 2,026 children between May 2020 and April 2021.
In a cohort of 532 younger children (average age, 5.9 years; 52% male; 58% of European ancestry), the researchers linked each extra daily hour of TV or use of digital media to worse behavior, as measured by the Strengths and Difficulties Questionnaire: 0.22 in an adjusted model for children aged 2-4;(95% confidence interval, 0.10-0.35; P < .001) and 0.07 in an adjusted model in those aged 4 and older (95% CI, 0.02-0.11; P = .007).
However, the researchers observed no statistically significant links to more anxiety/depression or hyperactivity/inattention in this group of children.
Among 1,494 older kids (mean age, 11.3; 57% male; 58% of European ancestry), researchers linked greater daily use of TV or digital media to higher levels of depression symptoms in a dose-dependent relationship, Dr. Birken said (1 hour: beta, 0.21; 95% CI, –1.28 to 0.78; 2-3 hours: beta, 1.81; 95% CI, 0.29-3.33; 4-5 hours: beta, 2.80; 95% CI, 1.15-4.44; 6-8 hours: beta, 5.16; 95% CI, 3.32-7.01; 9 hours: beta, 5.42; 95% CI, 3.30-7.54; overall P < .001).
“Similarly, higher TV or digital media time per day was associated with higher levels of anxiety symptoms,” the researchers reported. “TV or digital media time per day was also significantly associated with differences in symptoms of irritability, inattention, and hyperactivity/inattention.”
More time spent learning virtually was associated with higher levels of depression and anxiety in both groups of children, according to the researchers. Whether this finding reflects an effect of screens themselves or because the children most exposed to virtual learning may also have been the most exposed to the stressful disruptiveness of the pandemic is unclear.
The researchers also found “insufficient evidence” to link more virtual learning to irritability, inattention and hyperactivity, inattention, and hyperactivity/impulsivity in adjusted models.
Video chatting did not appear to have a protective effect, Dr. Birken said. The researchers also specifically analyzed children with autism and found no link between more screen time and various mental health/conduct problems.
Is it possible that kids with more anxiety, depression, and isolation simply turn to screens because they’re anxious, depressed, and isolated? Dr. Birken said the researchers adjusted the findings to account for previous mental health problems. And she noted that the study linked more pandemic-era virtual learning to more depression/anxiety. It’s “hard to imagine” how more mental health problems would cause more virtual learning.
Bad news or bad stats?
Chris Ferguson, PhD, a professor of psychology at Stetson University. DeLand, Fla., who studies screen time, criticized the study in an interview. “The observed effects are so tiny, it’s impossible to know if they are real or a false-positive artifact common to social science research,” he said. “Ultimately, this study is better evidence about how many scholars are bad at statistics than anything having to do with kids and screens.”
Dr. Ferguson said that the results may be confounded because kids turn to screens to reduce their anxiety. “For the most part, screens were a godsend during COVID-19,” he said. “They helped kids stay inside and gave them something to do while social distancing and allowed them to keep in touch with friends and families. Honestly, what else were we expecting kids to do, stare at the wallpaper?”
Children with depression and anxiety often retreat into screens or books to escape the unpleasantries of real life. “That doesn’t mean the screens or books are the culprits,” he said.
Instead of focusing on screen time, Dr. Ferguson suggested parents consider these factors: “Keeping in mind not every kid is a genius, is your kid doing about as well in school as you’d expect, given their natural ability? Are they getting at least some exercise every day? Are they getting adequate sleep? Are they able to socialize with friends in some context, either in real life or online? Are they happy?”
The study was funded by the Canadian Institutes of Health Research, the Center for Brain & Mental Health at The Hospital for Sick Children, the Ontario Ministry of Health, and the Miner’s Lamp Innovation Fund in Prevention and Early Detection of Severe Mental Illness at the University of Toronto. The study authors reported various financial relationships. Dr. Ferguson reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Medicaid implements waivers for some clinical trial coverage
Federal officials will allow some flexibility in meeting new requirements on covering the costs of clinical trials for people enrolled in Medicaid, seeking to accommodate states where legislatures will not meet in time to make needed changes in rules.
Congress in 2020 ordered U.S. states to have their Medicaid programs cover expenses related to participation in certain clinical trials, a move that was hailed by the American Society of Clinical Oncology (ASCO) and other groups as a boost to trials as well as to patients with serious illness who have lower incomes.
The mandate went into effect on Jan. 1, but the Centers for Medicare & Medicaid Services will allow accommodations in terms of implementation time for states that have not yet been able to make needed legislative changes, Daniel Tsai, deputy administrator and director of the Center for Medicaid and CHIP Services, wrote in a Dec. 7 letter. Mr. Tsai’s letter doesn’t mention specific states. The CMS did not immediately respond to a request seeking information on the states expected to apply for waivers.
Medicaid has in recent years been a rare large U.S. insurance program that does not cover the costs of clinical trials. The Affordable Care Act of 2010 mandated this coverage for people in private insurance plans. The federal government in 2000 decided that Medicare would do so.
‘A hidden opportunity’
A perspective article last May in the New England Journal of Medicine referred to the new Medicaid mandate on clinical trials as a “hidden opportunity,” referring to its genesis as an add-on in a massive federal spending package enacted in December 2020.
In the article, Samuel U. Takvorian, MD, MSHP, of the University of Pennsylvania, Philadelphia, and coauthors noted that rates of participation in clinical trials remain low for racial and ethnic minority groups, due in part to the lack of Medicaid coverage.
“For example, non-Hispanic White patients are nearly twice as likely as Black patients and three times as likely as Hispanic patients to enroll in cancer clinical trials – a gap that has widened over time,” Dr. Takvorian and coauthors wrote. “Inequities in enrollment have also manifested during the COVID-19 pandemic, which has disproportionately affected non-White patients, without their commensurate representation in trials of COVID-19 therapeutics.”
In October, researchers from the Arthur G. James Cancer Hospital and Ohio State University, Columbus, published results of a retrospective study of patients with stage I-IV pancreatic cancer that also found inequities in enrollment. Mariam F. Eskander, MD, MPH, and coauthors reported what they found by examining records for 1,127 patients (0.4%) enrolled in clinical trials and 301,340 (99.6%) who did not enroll. They found that enrollment in trials increased over the study period, but not for Black patients or patients on Medicaid.
In an interview, Dr. Eskander said the new Medicaid policy will remove a major obstacle to participation in clinical trials. An oncologist, Dr. Eskander said she is looking forward to being able to help more of her patients get access to experimental medicines and treatments.
But that may not be enough to draw more people with low incomes into these studies, said Dr. Eskander, who is now at Rutgers Cancer Institute of New Jersey in New Brunswick. She urges greater use of patient navigators to help people on Medicaid understand the resources available to them, as well as broad use of Medicaid’s nonemergency medical transportation (NEMT) benefit.
“Some patients will be offered clinical trial enrollment and some will accept, but I really worry about the challenges low-income people face with things like transportation and getting time off work,” she said.
A version of this article first appeared on Medscape.com.
Federal officials will allow some flexibility in meeting new requirements on covering the costs of clinical trials for people enrolled in Medicaid, seeking to accommodate states where legislatures will not meet in time to make needed changes in rules.
Congress in 2020 ordered U.S. states to have their Medicaid programs cover expenses related to participation in certain clinical trials, a move that was hailed by the American Society of Clinical Oncology (ASCO) and other groups as a boost to trials as well as to patients with serious illness who have lower incomes.
The mandate went into effect on Jan. 1, but the Centers for Medicare & Medicaid Services will allow accommodations in terms of implementation time for states that have not yet been able to make needed legislative changes, Daniel Tsai, deputy administrator and director of the Center for Medicaid and CHIP Services, wrote in a Dec. 7 letter. Mr. Tsai’s letter doesn’t mention specific states. The CMS did not immediately respond to a request seeking information on the states expected to apply for waivers.
Medicaid has in recent years been a rare large U.S. insurance program that does not cover the costs of clinical trials. The Affordable Care Act of 2010 mandated this coverage for people in private insurance plans. The federal government in 2000 decided that Medicare would do so.
‘A hidden opportunity’
A perspective article last May in the New England Journal of Medicine referred to the new Medicaid mandate on clinical trials as a “hidden opportunity,” referring to its genesis as an add-on in a massive federal spending package enacted in December 2020.
In the article, Samuel U. Takvorian, MD, MSHP, of the University of Pennsylvania, Philadelphia, and coauthors noted that rates of participation in clinical trials remain low for racial and ethnic minority groups, due in part to the lack of Medicaid coverage.
“For example, non-Hispanic White patients are nearly twice as likely as Black patients and three times as likely as Hispanic patients to enroll in cancer clinical trials – a gap that has widened over time,” Dr. Takvorian and coauthors wrote. “Inequities in enrollment have also manifested during the COVID-19 pandemic, which has disproportionately affected non-White patients, without their commensurate representation in trials of COVID-19 therapeutics.”
In October, researchers from the Arthur G. James Cancer Hospital and Ohio State University, Columbus, published results of a retrospective study of patients with stage I-IV pancreatic cancer that also found inequities in enrollment. Mariam F. Eskander, MD, MPH, and coauthors reported what they found by examining records for 1,127 patients (0.4%) enrolled in clinical trials and 301,340 (99.6%) who did not enroll. They found that enrollment in trials increased over the study period, but not for Black patients or patients on Medicaid.
In an interview, Dr. Eskander said the new Medicaid policy will remove a major obstacle to participation in clinical trials. An oncologist, Dr. Eskander said she is looking forward to being able to help more of her patients get access to experimental medicines and treatments.
But that may not be enough to draw more people with low incomes into these studies, said Dr. Eskander, who is now at Rutgers Cancer Institute of New Jersey in New Brunswick. She urges greater use of patient navigators to help people on Medicaid understand the resources available to them, as well as broad use of Medicaid’s nonemergency medical transportation (NEMT) benefit.
“Some patients will be offered clinical trial enrollment and some will accept, but I really worry about the challenges low-income people face with things like transportation and getting time off work,” she said.
A version of this article first appeared on Medscape.com.
Federal officials will allow some flexibility in meeting new requirements on covering the costs of clinical trials for people enrolled in Medicaid, seeking to accommodate states where legislatures will not meet in time to make needed changes in rules.
Congress in 2020 ordered U.S. states to have their Medicaid programs cover expenses related to participation in certain clinical trials, a move that was hailed by the American Society of Clinical Oncology (ASCO) and other groups as a boost to trials as well as to patients with serious illness who have lower incomes.
The mandate went into effect on Jan. 1, but the Centers for Medicare & Medicaid Services will allow accommodations in terms of implementation time for states that have not yet been able to make needed legislative changes, Daniel Tsai, deputy administrator and director of the Center for Medicaid and CHIP Services, wrote in a Dec. 7 letter. Mr. Tsai’s letter doesn’t mention specific states. The CMS did not immediately respond to a request seeking information on the states expected to apply for waivers.
Medicaid has in recent years been a rare large U.S. insurance program that does not cover the costs of clinical trials. The Affordable Care Act of 2010 mandated this coverage for people in private insurance plans. The federal government in 2000 decided that Medicare would do so.
‘A hidden opportunity’
A perspective article last May in the New England Journal of Medicine referred to the new Medicaid mandate on clinical trials as a “hidden opportunity,” referring to its genesis as an add-on in a massive federal spending package enacted in December 2020.
In the article, Samuel U. Takvorian, MD, MSHP, of the University of Pennsylvania, Philadelphia, and coauthors noted that rates of participation in clinical trials remain low for racial and ethnic minority groups, due in part to the lack of Medicaid coverage.
“For example, non-Hispanic White patients are nearly twice as likely as Black patients and three times as likely as Hispanic patients to enroll in cancer clinical trials – a gap that has widened over time,” Dr. Takvorian and coauthors wrote. “Inequities in enrollment have also manifested during the COVID-19 pandemic, which has disproportionately affected non-White patients, without their commensurate representation in trials of COVID-19 therapeutics.”
In October, researchers from the Arthur G. James Cancer Hospital and Ohio State University, Columbus, published results of a retrospective study of patients with stage I-IV pancreatic cancer that also found inequities in enrollment. Mariam F. Eskander, MD, MPH, and coauthors reported what they found by examining records for 1,127 patients (0.4%) enrolled in clinical trials and 301,340 (99.6%) who did not enroll. They found that enrollment in trials increased over the study period, but not for Black patients or patients on Medicaid.
In an interview, Dr. Eskander said the new Medicaid policy will remove a major obstacle to participation in clinical trials. An oncologist, Dr. Eskander said she is looking forward to being able to help more of her patients get access to experimental medicines and treatments.
But that may not be enough to draw more people with low incomes into these studies, said Dr. Eskander, who is now at Rutgers Cancer Institute of New Jersey in New Brunswick. She urges greater use of patient navigators to help people on Medicaid understand the resources available to them, as well as broad use of Medicaid’s nonemergency medical transportation (NEMT) benefit.
“Some patients will be offered clinical trial enrollment and some will accept, but I really worry about the challenges low-income people face with things like transportation and getting time off work,” she said.
A version of this article first appeared on Medscape.com.
Cardiac device interrogation after death ‘richly informative’
Interrogating the cardiac implantable electronic device (CIED) after death can yield important information about critical device malfunction, premortem abnormalities, and the mechanism and timing of death, a new study suggests.
Postmortem CIED interrogation is “richly informative” in assisting both cardiac and forensic investigations and “should be considered for select patients with CIEDs undergoing autopsy,” say Elizabeth Paratz, MBBS, department of cardiology, Baker Heart and Diabetes Institute, Prahran, Australia, and colleagues.
Their study results were published online in JACC: Clinical Electrophysiology.
Cause of death revealed in half of cases
They reviewed CIED interrogations in 260 deceased individuals undergoing medicolegal investigation of sudden death (162 patients) or unexplained death (98 patients) by the Victorian Institute of Forensic Medicine between 2005 and 2020.
Roughly two-thirds were male (68.8%) and their median age was 72.8 years; 202 patients had pacemakers, 56 had defibrillators, and 2 had loop recorders. The cause of death was cardiac in 79.6% of cases.
Postmortem CIED interrogation was successful in 98.5% cases and directly informed cause of death in 131 cases (50.4%), with fatal ventricular arrhythmias identified in 121 patients.
CIED interrogation assisted in determining the cause of death in 63.6% of cases of sudden death and 28.6% of nonsudden death cases.
In 20 cases (7.7%), CIED interrogation uncovered potential device malfunction. Issues included failure to appropriately treat ventricular arrhythmias in 13 cases; lead issues in 3 cases, including 2 cases resulting in failure to treat ventricular arrhythmias; as well as battery depletion in 6 cases.
In 72 patients (27.7%), the device recorded abnormalities in the 30 days before death. These abnormalities included nonsustained ventricular tachycardia in 26 cases, rapid atrial fibrillation in 17, elective replacement indicator or end-of-life status in 22, intrathoracic impedance alarms or lead issues in 3 each, and therapy delivered in 1 instance.
“In several cases, the absence of an arrhythmia carried medicolegal implications: For example, in eight fatal motor vehicle accident cases, only one patient had a ventricular arrhythmia documented on their CIED,” Dr. Paratz and colleagues report.
And in six cases in which the patient was found dead after a prolonged period, CIED interrogation determined time of death. And in one case, CIED interrogation was the primary means of identifying the patient.
Still, postmortem CIED interrogation remains uncommon, the study team notes.
They point to a 2007 survey of Chicago morticians that found roughly 370 CIEDs were explanted per year prior to cremation, but only 4% of morticians had ever returned a CIED to the manufacturer for analysis.
“Encouraging postmortem interrogation of CIEDs may assist in postmarketing surveillance for critical faults, as well as in providing an electrophysiological appraisal of terminal rhythms and device responses in a variety of physiological scenarios,” the researchers say.
The study had no commercial funding. Dr. Paratz is supported by a National Health and Medical Research Council/National Heart Foundation cofunded Postgraduate Scholarship, Royal Australasian College of Physicians JJ Billings Scholarship, and PSA Insurance Cardiovascular Scholarship. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Interrogating the cardiac implantable electronic device (CIED) after death can yield important information about critical device malfunction, premortem abnormalities, and the mechanism and timing of death, a new study suggests.
Postmortem CIED interrogation is “richly informative” in assisting both cardiac and forensic investigations and “should be considered for select patients with CIEDs undergoing autopsy,” say Elizabeth Paratz, MBBS, department of cardiology, Baker Heart and Diabetes Institute, Prahran, Australia, and colleagues.
Their study results were published online in JACC: Clinical Electrophysiology.
Cause of death revealed in half of cases
They reviewed CIED interrogations in 260 deceased individuals undergoing medicolegal investigation of sudden death (162 patients) or unexplained death (98 patients) by the Victorian Institute of Forensic Medicine between 2005 and 2020.
Roughly two-thirds were male (68.8%) and their median age was 72.8 years; 202 patients had pacemakers, 56 had defibrillators, and 2 had loop recorders. The cause of death was cardiac in 79.6% of cases.
Postmortem CIED interrogation was successful in 98.5% cases and directly informed cause of death in 131 cases (50.4%), with fatal ventricular arrhythmias identified in 121 patients.
CIED interrogation assisted in determining the cause of death in 63.6% of cases of sudden death and 28.6% of nonsudden death cases.
In 20 cases (7.7%), CIED interrogation uncovered potential device malfunction. Issues included failure to appropriately treat ventricular arrhythmias in 13 cases; lead issues in 3 cases, including 2 cases resulting in failure to treat ventricular arrhythmias; as well as battery depletion in 6 cases.
In 72 patients (27.7%), the device recorded abnormalities in the 30 days before death. These abnormalities included nonsustained ventricular tachycardia in 26 cases, rapid atrial fibrillation in 17, elective replacement indicator or end-of-life status in 22, intrathoracic impedance alarms or lead issues in 3 each, and therapy delivered in 1 instance.
“In several cases, the absence of an arrhythmia carried medicolegal implications: For example, in eight fatal motor vehicle accident cases, only one patient had a ventricular arrhythmia documented on their CIED,” Dr. Paratz and colleagues report.
And in six cases in which the patient was found dead after a prolonged period, CIED interrogation determined time of death. And in one case, CIED interrogation was the primary means of identifying the patient.
Still, postmortem CIED interrogation remains uncommon, the study team notes.
They point to a 2007 survey of Chicago morticians that found roughly 370 CIEDs were explanted per year prior to cremation, but only 4% of morticians had ever returned a CIED to the manufacturer for analysis.
“Encouraging postmortem interrogation of CIEDs may assist in postmarketing surveillance for critical faults, as well as in providing an electrophysiological appraisal of terminal rhythms and device responses in a variety of physiological scenarios,” the researchers say.
The study had no commercial funding. Dr. Paratz is supported by a National Health and Medical Research Council/National Heart Foundation cofunded Postgraduate Scholarship, Royal Australasian College of Physicians JJ Billings Scholarship, and PSA Insurance Cardiovascular Scholarship. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Interrogating the cardiac implantable electronic device (CIED) after death can yield important information about critical device malfunction, premortem abnormalities, and the mechanism and timing of death, a new study suggests.
Postmortem CIED interrogation is “richly informative” in assisting both cardiac and forensic investigations and “should be considered for select patients with CIEDs undergoing autopsy,” say Elizabeth Paratz, MBBS, department of cardiology, Baker Heart and Diabetes Institute, Prahran, Australia, and colleagues.
Their study results were published online in JACC: Clinical Electrophysiology.
Cause of death revealed in half of cases
They reviewed CIED interrogations in 260 deceased individuals undergoing medicolegal investigation of sudden death (162 patients) or unexplained death (98 patients) by the Victorian Institute of Forensic Medicine between 2005 and 2020.
Roughly two-thirds were male (68.8%) and their median age was 72.8 years; 202 patients had pacemakers, 56 had defibrillators, and 2 had loop recorders. The cause of death was cardiac in 79.6% of cases.
Postmortem CIED interrogation was successful in 98.5% cases and directly informed cause of death in 131 cases (50.4%), with fatal ventricular arrhythmias identified in 121 patients.
CIED interrogation assisted in determining the cause of death in 63.6% of cases of sudden death and 28.6% of nonsudden death cases.
In 20 cases (7.7%), CIED interrogation uncovered potential device malfunction. Issues included failure to appropriately treat ventricular arrhythmias in 13 cases; lead issues in 3 cases, including 2 cases resulting in failure to treat ventricular arrhythmias; as well as battery depletion in 6 cases.
In 72 patients (27.7%), the device recorded abnormalities in the 30 days before death. These abnormalities included nonsustained ventricular tachycardia in 26 cases, rapid atrial fibrillation in 17, elective replacement indicator or end-of-life status in 22, intrathoracic impedance alarms or lead issues in 3 each, and therapy delivered in 1 instance.
“In several cases, the absence of an arrhythmia carried medicolegal implications: For example, in eight fatal motor vehicle accident cases, only one patient had a ventricular arrhythmia documented on their CIED,” Dr. Paratz and colleagues report.
And in six cases in which the patient was found dead after a prolonged period, CIED interrogation determined time of death. And in one case, CIED interrogation was the primary means of identifying the patient.
Still, postmortem CIED interrogation remains uncommon, the study team notes.
They point to a 2007 survey of Chicago morticians that found roughly 370 CIEDs were explanted per year prior to cremation, but only 4% of morticians had ever returned a CIED to the manufacturer for analysis.
“Encouraging postmortem interrogation of CIEDs may assist in postmarketing surveillance for critical faults, as well as in providing an electrophysiological appraisal of terminal rhythms and device responses in a variety of physiological scenarios,” the researchers say.
The study had no commercial funding. Dr. Paratz is supported by a National Health and Medical Research Council/National Heart Foundation cofunded Postgraduate Scholarship, Royal Australasian College of Physicians JJ Billings Scholarship, and PSA Insurance Cardiovascular Scholarship. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JACC: CLINICAL ELECTROPHYSIOLOGY
Breast cancer treatment worse for incarcerated patients
The study was presented at the 2021 San Antonio Breast Cancer Symposium on Dec. 10 (Abstract P5-14-10).
Examining the records of more than 4,300 patients with breast cancer who were treated between 2014 and 2020 in North Carolina, researchers identified 34 who were either incarcerated at the time of diagnosis or who were diagnosed before they were imprisoned.
They found that neoadjuvant therapy was not given to incarcerated breast cancer patients as compared to 8% of women who were never incarcerated and 20% of women incarcerated later. Incarcerated patients treated with surgery upfront had to wait on average more than 3 weeks longer than other patients for their procedure. Their findings were followed by a recently published study in JAMA Network Open indicating that young people with a history of incarceration were significantly more likely to experience early mortality and that mortality was higher among Black prisoners.
“These findings are concerning for missed treatment opportunities within the carceral system,” wrote researchers who were led by Oluwadamilola “Lola” Fayanju, MD, MPHS, FACS, chief of breast surgery for the University of Pennsylvania Health System, Philadelphia.
Dr. Fayanju told this news organization that she was “not surprised by the finding that there was no neoadjuvant chemotherapy given to patients at all. Even in the practice of care outside of the carceral system it is striking how much variation there is in regards to treatment sequence if it is not approached in an evidence-based way. Many of the social ills that contribute to incarceration also contribute to this variation in care, and it’s not surprising that in women who are experiencing incarceration, there is geometric escalation of disparities with regards to their opportunities for treatment.”
Erica L. Mayer, MD, MPH, a medical oncologist and clinical investigator in the Breast Oncology Center at the Dana-Faber Cancer Institute, Boston, said “this is really interesting and important work showing some worrisome trends. On the one hand, this is a very small experience and such a small sample size is always vulnerable to bias or skew from factors that become more important. However, this is not the first observation that there are disparities of care in incarcerated populations,”said Dr. Mayer, who was not involved in the study. “This is a topic that has been studied in diseases outside of oncology, such as heart disease and diabetes. There is a theme that patients who are incarcerated have a disparity and inequity of care compared to those who are not.”
The current findings “fit in with general themes,” she said. As rates of cancer are expected to grow in the coming years, “understanding how to provide the best possible care in those settings is very important. This is early data but it’s an important signal and is suggesting to us that a greater understanding of health care access for incarcerated individuals is a very important area of study, and hopefully an area for which one could provide interventions that might help to reduce these disparities.”
Dr. Fayanju and associates. set out to determine the disease and treatment characteristics of individuals with breast cancer and a history of incarceration. They focused on women who had a breast cancer diagnosis at the University of North Carolina Hospitals between April 2014 and December 2020. They gathered data on patient demographics, incarceration status, disease characteristics, treatment types, and dates of receipt of treatment, but there were few data available. “It is really striking how little data there is available. This is a very small study and is the best we could glean from a large state-wide dataset,” she said.
Of 4,332 breast cancer cases, 34 (0.8%) were diagnosed while incarcerated (70.6%) or before incarceration (29.4%). Those who were diagnosed during incarceration were significantly more likely to be single (P < .001), use illicit drugs at the time of diagnosis (P = .01), and have a family history of breast cancer (P = .03) as compared with patients who were never incarcerated and those who were diagnosed before incarceration.
The results also showed that patients diagnosed with breast cancer during incarceration were significantly less likely to receive neoadjuvant therapy at 0% versus 8.2% for those who were never incarcerated, and 20% for those who were diagnosed before incarceration (P = .01 for trend).
“Further research is needed to understand the full scope of cancer inequities and identify factors that contribute to them among patients who experience incarceration,” Dr. Fayanju said.
No funding or relevant financial relationships were declared for this featured study.
The study was presented at the 2021 San Antonio Breast Cancer Symposium on Dec. 10 (Abstract P5-14-10).
Examining the records of more than 4,300 patients with breast cancer who were treated between 2014 and 2020 in North Carolina, researchers identified 34 who were either incarcerated at the time of diagnosis or who were diagnosed before they were imprisoned.
They found that neoadjuvant therapy was not given to incarcerated breast cancer patients as compared to 8% of women who were never incarcerated and 20% of women incarcerated later. Incarcerated patients treated with surgery upfront had to wait on average more than 3 weeks longer than other patients for their procedure. Their findings were followed by a recently published study in JAMA Network Open indicating that young people with a history of incarceration were significantly more likely to experience early mortality and that mortality was higher among Black prisoners.
“These findings are concerning for missed treatment opportunities within the carceral system,” wrote researchers who were led by Oluwadamilola “Lola” Fayanju, MD, MPHS, FACS, chief of breast surgery for the University of Pennsylvania Health System, Philadelphia.
Dr. Fayanju told this news organization that she was “not surprised by the finding that there was no neoadjuvant chemotherapy given to patients at all. Even in the practice of care outside of the carceral system it is striking how much variation there is in regards to treatment sequence if it is not approached in an evidence-based way. Many of the social ills that contribute to incarceration also contribute to this variation in care, and it’s not surprising that in women who are experiencing incarceration, there is geometric escalation of disparities with regards to their opportunities for treatment.”
Erica L. Mayer, MD, MPH, a medical oncologist and clinical investigator in the Breast Oncology Center at the Dana-Faber Cancer Institute, Boston, said “this is really interesting and important work showing some worrisome trends. On the one hand, this is a very small experience and such a small sample size is always vulnerable to bias or skew from factors that become more important. However, this is not the first observation that there are disparities of care in incarcerated populations,”said Dr. Mayer, who was not involved in the study. “This is a topic that has been studied in diseases outside of oncology, such as heart disease and diabetes. There is a theme that patients who are incarcerated have a disparity and inequity of care compared to those who are not.”
The current findings “fit in with general themes,” she said. As rates of cancer are expected to grow in the coming years, “understanding how to provide the best possible care in those settings is very important. This is early data but it’s an important signal and is suggesting to us that a greater understanding of health care access for incarcerated individuals is a very important area of study, and hopefully an area for which one could provide interventions that might help to reduce these disparities.”
Dr. Fayanju and associates. set out to determine the disease and treatment characteristics of individuals with breast cancer and a history of incarceration. They focused on women who had a breast cancer diagnosis at the University of North Carolina Hospitals between April 2014 and December 2020. They gathered data on patient demographics, incarceration status, disease characteristics, treatment types, and dates of receipt of treatment, but there were few data available. “It is really striking how little data there is available. This is a very small study and is the best we could glean from a large state-wide dataset,” she said.
Of 4,332 breast cancer cases, 34 (0.8%) were diagnosed while incarcerated (70.6%) or before incarceration (29.4%). Those who were diagnosed during incarceration were significantly more likely to be single (P < .001), use illicit drugs at the time of diagnosis (P = .01), and have a family history of breast cancer (P = .03) as compared with patients who were never incarcerated and those who were diagnosed before incarceration.
The results also showed that patients diagnosed with breast cancer during incarceration were significantly less likely to receive neoadjuvant therapy at 0% versus 8.2% for those who were never incarcerated, and 20% for those who were diagnosed before incarceration (P = .01 for trend).
“Further research is needed to understand the full scope of cancer inequities and identify factors that contribute to them among patients who experience incarceration,” Dr. Fayanju said.
No funding or relevant financial relationships were declared for this featured study.
The study was presented at the 2021 San Antonio Breast Cancer Symposium on Dec. 10 (Abstract P5-14-10).
Examining the records of more than 4,300 patients with breast cancer who were treated between 2014 and 2020 in North Carolina, researchers identified 34 who were either incarcerated at the time of diagnosis or who were diagnosed before they were imprisoned.
They found that neoadjuvant therapy was not given to incarcerated breast cancer patients as compared to 8% of women who were never incarcerated and 20% of women incarcerated later. Incarcerated patients treated with surgery upfront had to wait on average more than 3 weeks longer than other patients for their procedure. Their findings were followed by a recently published study in JAMA Network Open indicating that young people with a history of incarceration were significantly more likely to experience early mortality and that mortality was higher among Black prisoners.
“These findings are concerning for missed treatment opportunities within the carceral system,” wrote researchers who were led by Oluwadamilola “Lola” Fayanju, MD, MPHS, FACS, chief of breast surgery for the University of Pennsylvania Health System, Philadelphia.
Dr. Fayanju told this news organization that she was “not surprised by the finding that there was no neoadjuvant chemotherapy given to patients at all. Even in the practice of care outside of the carceral system it is striking how much variation there is in regards to treatment sequence if it is not approached in an evidence-based way. Many of the social ills that contribute to incarceration also contribute to this variation in care, and it’s not surprising that in women who are experiencing incarceration, there is geometric escalation of disparities with regards to their opportunities for treatment.”
Erica L. Mayer, MD, MPH, a medical oncologist and clinical investigator in the Breast Oncology Center at the Dana-Faber Cancer Institute, Boston, said “this is really interesting and important work showing some worrisome trends. On the one hand, this is a very small experience and such a small sample size is always vulnerable to bias or skew from factors that become more important. However, this is not the first observation that there are disparities of care in incarcerated populations,”said Dr. Mayer, who was not involved in the study. “This is a topic that has been studied in diseases outside of oncology, such as heart disease and diabetes. There is a theme that patients who are incarcerated have a disparity and inequity of care compared to those who are not.”
The current findings “fit in with general themes,” she said. As rates of cancer are expected to grow in the coming years, “understanding how to provide the best possible care in those settings is very important. This is early data but it’s an important signal and is suggesting to us that a greater understanding of health care access for incarcerated individuals is a very important area of study, and hopefully an area for which one could provide interventions that might help to reduce these disparities.”
Dr. Fayanju and associates. set out to determine the disease and treatment characteristics of individuals with breast cancer and a history of incarceration. They focused on women who had a breast cancer diagnosis at the University of North Carolina Hospitals between April 2014 and December 2020. They gathered data on patient demographics, incarceration status, disease characteristics, treatment types, and dates of receipt of treatment, but there were few data available. “It is really striking how little data there is available. This is a very small study and is the best we could glean from a large state-wide dataset,” she said.
Of 4,332 breast cancer cases, 34 (0.8%) were diagnosed while incarcerated (70.6%) or before incarceration (29.4%). Those who were diagnosed during incarceration were significantly more likely to be single (P < .001), use illicit drugs at the time of diagnosis (P = .01), and have a family history of breast cancer (P = .03) as compared with patients who were never incarcerated and those who were diagnosed before incarceration.
The results also showed that patients diagnosed with breast cancer during incarceration were significantly less likely to receive neoadjuvant therapy at 0% versus 8.2% for those who were never incarcerated, and 20% for those who were diagnosed before incarceration (P = .01 for trend).
“Further research is needed to understand the full scope of cancer inequities and identify factors that contribute to them among patients who experience incarceration,” Dr. Fayanju said.
No funding or relevant financial relationships were declared for this featured study.
FROM SABCS 2021
More lots of metformin recalled
The drumbeat of U.S. recalls continues for various lots of extended-release metformin because of contamination with unacceptably high levels of a nitrosamine that pose a cancer risk.
On Dec. 28, 2021, Viona Pharmaceuticals voluntarily recalled 33 lots of metformin hydrochloride extended-release tablets, USP 750 mg to the retail level, as a precautionary measure, because of possible contamination with N-nitrosodimethylamine (NDMA).
Metformin is used as an adjunct to diet and exercise to improve blood glucose control in adults with type 2 diabetes mellitus. Patients who have received impacted lots of metformin are advised to continue taking their medication and contact their physician for advice regarding an alternative treatment
The product can be identified as white to off-white, capsule shaped, uncoated tablets, debossed with “Z,” “C” on one side and “20” on the other side, and come in bottles of 100 tablets, which have been distributed nationwide. The 33 batch numbers are listed in a company statement.
The affected product was manufactured by Cadila Healthcare, Ahmedabad, India, for U.S. distribution by Viona.
In its statement, Viona said: “NDMA is classified as a probable human carcinogen (a substance that could cause cancer) based on results from laboratory tests. NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables.”
This recall is being conducted “with the knowledge of the U.S. Food and Drug Administration,” it added.
Consumers with questions regarding this recall can contact the recall processor Eversana Life Science Services by phone at 1-888-304-5022, option 1; Monday-Friday, 8:00 a.m.–7:00 p.m. CT. Customers with medical-related questions who wish to report an adverse event or quality issues about the products being recalled should contact Viona Pharmaceuticals by phone at 888-304-5011, Monday-Friday, 8:30 p.m.–5:30 p.m., EST.
Latest in a long line of metformin recalls
This is the second time in 2021 that Viona has voluntarily recalled extended-release metformin tablets, 750 mg, because of potential contamination with NDMA. It recalled two lots in June, as reported by this news organization.
And in January 2021, Nostrum Laboratories recalled another lot of metformin extended-release 750-mg tablets, following on from a prior recall in November 2020.
These recalls follows 258 distinct U.S. lot recalls tracked by the FDA during the past 2 years because of unacceptably high NDMA levels in lots of metformin hydrochloride extended-release tablets.
The FDA has issued several statements about NDMA contamination of metformin formulations over the past 2 years, including a review of the methods used to detect NDMA and a summary of the information the agency had collected on excessive levels of NDMA in metformin.
According to the FDA’s 2020 summary, the agency has not yet determined how or why high levels of NDMA turn up so often in multiple batches of metformin hydrochloride extended-release tablets. However, published research attributed the contamination to certain methods of manufacturing metformin tablets.
A version of this article first appeared on Medscape.com.
The drumbeat of U.S. recalls continues for various lots of extended-release metformin because of contamination with unacceptably high levels of a nitrosamine that pose a cancer risk.
On Dec. 28, 2021, Viona Pharmaceuticals voluntarily recalled 33 lots of metformin hydrochloride extended-release tablets, USP 750 mg to the retail level, as a precautionary measure, because of possible contamination with N-nitrosodimethylamine (NDMA).
Metformin is used as an adjunct to diet and exercise to improve blood glucose control in adults with type 2 diabetes mellitus. Patients who have received impacted lots of metformin are advised to continue taking their medication and contact their physician for advice regarding an alternative treatment
The product can be identified as white to off-white, capsule shaped, uncoated tablets, debossed with “Z,” “C” on one side and “20” on the other side, and come in bottles of 100 tablets, which have been distributed nationwide. The 33 batch numbers are listed in a company statement.
The affected product was manufactured by Cadila Healthcare, Ahmedabad, India, for U.S. distribution by Viona.
In its statement, Viona said: “NDMA is classified as a probable human carcinogen (a substance that could cause cancer) based on results from laboratory tests. NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables.”
This recall is being conducted “with the knowledge of the U.S. Food and Drug Administration,” it added.
Consumers with questions regarding this recall can contact the recall processor Eversana Life Science Services by phone at 1-888-304-5022, option 1; Monday-Friday, 8:00 a.m.–7:00 p.m. CT. Customers with medical-related questions who wish to report an adverse event or quality issues about the products being recalled should contact Viona Pharmaceuticals by phone at 888-304-5011, Monday-Friday, 8:30 p.m.–5:30 p.m., EST.
Latest in a long line of metformin recalls
This is the second time in 2021 that Viona has voluntarily recalled extended-release metformin tablets, 750 mg, because of potential contamination with NDMA. It recalled two lots in June, as reported by this news organization.
And in January 2021, Nostrum Laboratories recalled another lot of metformin extended-release 750-mg tablets, following on from a prior recall in November 2020.
These recalls follows 258 distinct U.S. lot recalls tracked by the FDA during the past 2 years because of unacceptably high NDMA levels in lots of metformin hydrochloride extended-release tablets.
The FDA has issued several statements about NDMA contamination of metformin formulations over the past 2 years, including a review of the methods used to detect NDMA and a summary of the information the agency had collected on excessive levels of NDMA in metformin.
According to the FDA’s 2020 summary, the agency has not yet determined how or why high levels of NDMA turn up so often in multiple batches of metformin hydrochloride extended-release tablets. However, published research attributed the contamination to certain methods of manufacturing metformin tablets.
A version of this article first appeared on Medscape.com.
The drumbeat of U.S. recalls continues for various lots of extended-release metformin because of contamination with unacceptably high levels of a nitrosamine that pose a cancer risk.
On Dec. 28, 2021, Viona Pharmaceuticals voluntarily recalled 33 lots of metformin hydrochloride extended-release tablets, USP 750 mg to the retail level, as a precautionary measure, because of possible contamination with N-nitrosodimethylamine (NDMA).
Metformin is used as an adjunct to diet and exercise to improve blood glucose control in adults with type 2 diabetes mellitus. Patients who have received impacted lots of metformin are advised to continue taking their medication and contact their physician for advice regarding an alternative treatment
The product can be identified as white to off-white, capsule shaped, uncoated tablets, debossed with “Z,” “C” on one side and “20” on the other side, and come in bottles of 100 tablets, which have been distributed nationwide. The 33 batch numbers are listed in a company statement.
The affected product was manufactured by Cadila Healthcare, Ahmedabad, India, for U.S. distribution by Viona.
In its statement, Viona said: “NDMA is classified as a probable human carcinogen (a substance that could cause cancer) based on results from laboratory tests. NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables.”
This recall is being conducted “with the knowledge of the U.S. Food and Drug Administration,” it added.
Consumers with questions regarding this recall can contact the recall processor Eversana Life Science Services by phone at 1-888-304-5022, option 1; Monday-Friday, 8:00 a.m.–7:00 p.m. CT. Customers with medical-related questions who wish to report an adverse event or quality issues about the products being recalled should contact Viona Pharmaceuticals by phone at 888-304-5011, Monday-Friday, 8:30 p.m.–5:30 p.m., EST.
Latest in a long line of metformin recalls
This is the second time in 2021 that Viona has voluntarily recalled extended-release metformin tablets, 750 mg, because of potential contamination with NDMA. It recalled two lots in June, as reported by this news organization.
And in January 2021, Nostrum Laboratories recalled another lot of metformin extended-release 750-mg tablets, following on from a prior recall in November 2020.
These recalls follows 258 distinct U.S. lot recalls tracked by the FDA during the past 2 years because of unacceptably high NDMA levels in lots of metformin hydrochloride extended-release tablets.
The FDA has issued several statements about NDMA contamination of metformin formulations over the past 2 years, including a review of the methods used to detect NDMA and a summary of the information the agency had collected on excessive levels of NDMA in metformin.
According to the FDA’s 2020 summary, the agency has not yet determined how or why high levels of NDMA turn up so often in multiple batches of metformin hydrochloride extended-release tablets. However, published research attributed the contamination to certain methods of manufacturing metformin tablets.
A version of this article first appeared on Medscape.com.
