User login
Virtual Visitation: Exploring the Impact on Patients and Families During COVID-19 and Beyond
From Northwell Health, Lake Success, NY.
Objective: Northwell Health, New York’s largest health care organization, rapidly adopted technology solutions to support patient and family communication during the COVID-19 pandemic.
Methods: This case series outlines the pragmatic, interdisciplinary approach Northwell underwent to rapidly implement patient virtual visitation processes during the peak of the initial crisis.
Results: Implementation of large-scale virtual visitation required leadership, technology, and dedicated, empathetic frontline professionals. Patient and family feedback uncovered varied feelings and perspectives, from confusion to gratitude.
Conclusion: Subsequent efforts to obtain direct patient and family perspectives and insights helped Northwell identify areas of strength and ongoing performance improvement.
Keywords: virtual visitation; COVID-19; technology; communication; patient experience.
The power of human connection has become increasingly apparent throughout the COVID-19 pandemic and subsequent recovery phases. Due to the need for social distancing, people worldwide have turned to virtual means of communication, staying in touch with family, friends, and colleagues via digital technology platforms. On March 18, 2020, the New York State Department of Health (NYSDOH) issued a health advisory, suspending all hospital visitation.1 As a result, hospitals rapidly transformed existing in-person visitation practices to meet large-scale virtual programming needs.
Family members often take on various roles—such as advocate, emotional support person, and postdischarge caregiver—for an ill or injured loved one.2 The Institute for Patient- and Family-Centered Care, a nonprofit organization founded in 1992, has been leading a cultural transformation where families are valued as care partners, as opposed to “visitors.”3 Although widely adopted and well-received in specialized units, such as neonatal intensive care units,4 virtual visitation had not been widely implemented across adult care settings. The NYSDOH guidance therefore required organizational leadership, innovation, flexibility, and systems ingenuity to meet the evolving needs of patients, families, and health care professionals. An overarching goal was ensuring patients and families were afforded opportunities to stay connected throughout hospitalization.
Reflecting the impact of COVID-19 surges, hospital environments became increasingly depersonalized, with health care providers wearing extensive personal protective equipment (PPE) and taking remarkable measures to socially distance and minimize exposure. Patients’ room doors were kept primarily closed, while codes and alerts blared in the halls overhead. The lack of families and visitors became increasingly obvious, aiding feelings of isolation and confinement. With fear of nosocomial transmission, impactful modalities (such as sitting at the bedside) and empathetic, therapeutic touch were no longer taking place.
With those scenarios—common to so many health care systems during the pandemic—as a backdrop, comes our experience. Northwell Health is the largest health care system in New York State, geographically spread throughout New York City’s 5 boroughs, Westchester County, and Long Island. With 23 hospitals, approximately 820 medical practices, and over 72 000 employees, Northwell has cared for more than 100 000 COVID-positive patients to date. This case series outlines a pragmatic approach to implementing virtual visitation during the initial peak and obtaining patient and family perspectives to help inform performance improvement and future programming.
Methods
Implementing virtual visitation
Through swift and focused multidisciplinary collaboration, numerous Northwell teams came together to implement large-scale virtual visitation across the organization during the first wave of the COVID crisis. The initial priority involved securing devices that could support patient-family communication. Prior to COVID, each facility had only a handful of tablets that were used primarily during leadership rounding, so once visitation was restricted, we needed a large quantity of devices within a matter of days. Through diligent work from System Procurement and internal Foundation, Northwell was able to acquire nearly 900 devices, including iPads, PadInMotion tablets, and Samsung tablets.
Typically, the benefits of using wireless tablets within a health care setting include long battery life, powerful data processing, advanced operating systems, large screens, and easy end-user navigation.4 During COVID-19 and its associated isolation precautions, tablets offered a lifeline for effective and socially distant communication. With new devices in hand, the system Office of the Chief Information Officer (OCIO) and site-based Information Technology (IT) teams were engaged. They worked tirelessly to streamline connectivity, download necessary apps, test devices on approved WiFi networks, and troubleshoot issues. Once set up, devices were strategically deployed across all Northwell hospitals and post-acute rehabilitation facilities.
Frontline teams quickly realized that a model similar to mobile proning teams, who focus solely on turning and positioning COVID patients to promote optimal respiratory ventilation,5 was needed to support virtual visitation. During the initial COVID wave, elective surgeries were not permissible, as per the NYSDOH. As a result, large numbers of clinical and nonclinical ambulatory surgery employees were redeployed throughout the organization, with many assigned and dedicated to facilitating newly created virtual visitation processes. These employees were primarily responsible for creating unit-based schedules, coordinating logistics, navigating devices on behalf of patients, being present during video calls, and sanitizing the devices between uses. Finally, if necessary, virtual interpretation services were used to overcome language barriers between staff and patients.
What began as an ad hoc function quickly became a valued and meaningful role. Utilizing triage mentality, virtual visitation was first offered during unit-based rounding protocols to those patients with the highest acuity and need to connect with family. We had no formal script; instead, unit-based leaders and frontline team members had open dialogues with patients and families to gauge their interest in virtual visitation. That included patients with an active end-of-life care plan, critically ill patients within intensive care units, and those soon to be intubated or recently extubated. Utilization also occurred within specialty areas such as labor and delivery, pediatrics, inpatient psychiatry, medical units, and long-term rehab facilities. Frontline teams appreciated the supplementary support so they could prioritize ongoing physical assessments and medical interventions. Donned in PPE, virtual visitation team members often served as physical extensions of the patient’s loved ones—holding their hand, offering prayers, and, at times, bearing witness to a last breath. In reflecting on that time, this role required absolute professionalism, empathy, and compassion.
In summer 2020, although demand for virtual visitation was still at an all-time high when ambulatory surgery was reinstated, redeployed staff returned to their responsibilities. To fill this void without interruption to patients and their families, site leaders quickly pivoted and refined processes and protocols utilizing Patient & Customer Experience and Hospitality department team members. Throughout spring 2021, the NYSDOH offered guidance to open in-person visitation, and the institution’s Clinical Advisory Group has been taking a pragmatic approach to doing that in a measured and safe manner across care settings.
Listening to the ‘voice’ of patients and families
Our institution’s mission is grounded in providing “quality service and patient-centered care.” Honoring those tenets, during the initial COVID wave, the system “Voice of the Customer End User Device Workgroup” was created with system and site-based interdisciplinary representation. Despite challenging and unprecedented times, conscious attention and effort was undertaken to assess the use and impact of virtual devices. One of the major work streams was to capture and examine patient and family thoughts, feedback, and the overall experience as it relates to virtual visitation.
The system Office of Patient & Customer Experience (OPCE), led by Sven Gierlinger, SVP Chief Experience Officer, reached out to our colleagues at Press Ganey to add a custom question to patient experience surveys. Beginning on December 1, 2020, discharged inpatients were asked to rate the “Degree to which you were able to stay connected with your family/caregiver during your stay.” Potential answers include the Likert scale responses of Always, Usually, Sometimes, and Never, with “Always” representing the Top Box score. The OPCE team believes these quantitative insights are important to track and trend, particularly since in-person and virtual visitation remain in constant flux.
In an effort to obtain additional, focused, qualitative feedback, OPCE partnered with our institution’s Digital Patient Experience (dPX) colleagues. The approach consisted of voluntary, semistructured, interview-type conversations with patients and family members who engaged in virtual visitation multiple times while the patient was hospitalized. OPCE contacted site-based Patient Experience leads, also known as Culture Leaders, at 3 hospitals, asking them to identify potential participants. This convenience sample excluded instances where the patient passed away during and/or immediately following hospitalization.
The OPCE team phoned potential interview candidates to make a personalized connection, explain the purpose of the interviews, and schedule them, if interested. For consistency, the same Digital Customer Experience Researcher on the dPX team facilitated all sessions, which were 30-minute, semiscripted interviews conducted virtually via Microsoft Teams. The tone was intentionally conversational so that patients and family members would feel comfortable delving into themes that were most impactful during their experience. After some initial ice breakers, such as “What were some of your feelings about being a patient/having a loved one in the hospital during the early days of the COVID-19 pandemic?” we moved on to some more pragmatic, implementation questions and rating scales. These included questions such as “How did you first learn about the option for virtual visitation? Was it something you inquired about or did someone offer it to you? How was it explained to you?” Patients were also asked, on a scale of 1 (easy) to 5 (difficult), to rate their experience with the technology aspect when connecting with their loved ones. They also provided verbal consent to be recorded and were given a $15 gift card upon completion of the interview.
Transcriptions were generated by uploading the interview recordings to a platform called UserTesting. In addition to these transcriptions, this platform also allowed for a keyword mapping tool that organized high-level themes and adjectives into groupings along a sentiment axis from negative to neutral to positive. Transcripts were then read carefully and annotated by the Digital Customer Experience Researcher, which allowed for strengthening of some of the automated themes as well as the emergence of new, more nuanced themes. These themes were organized into those that we could address with design and/or procedure updates (actionable insights), those that came up most frequently overall (frequency), and those that came up across our 3 interview sessions (commonality).
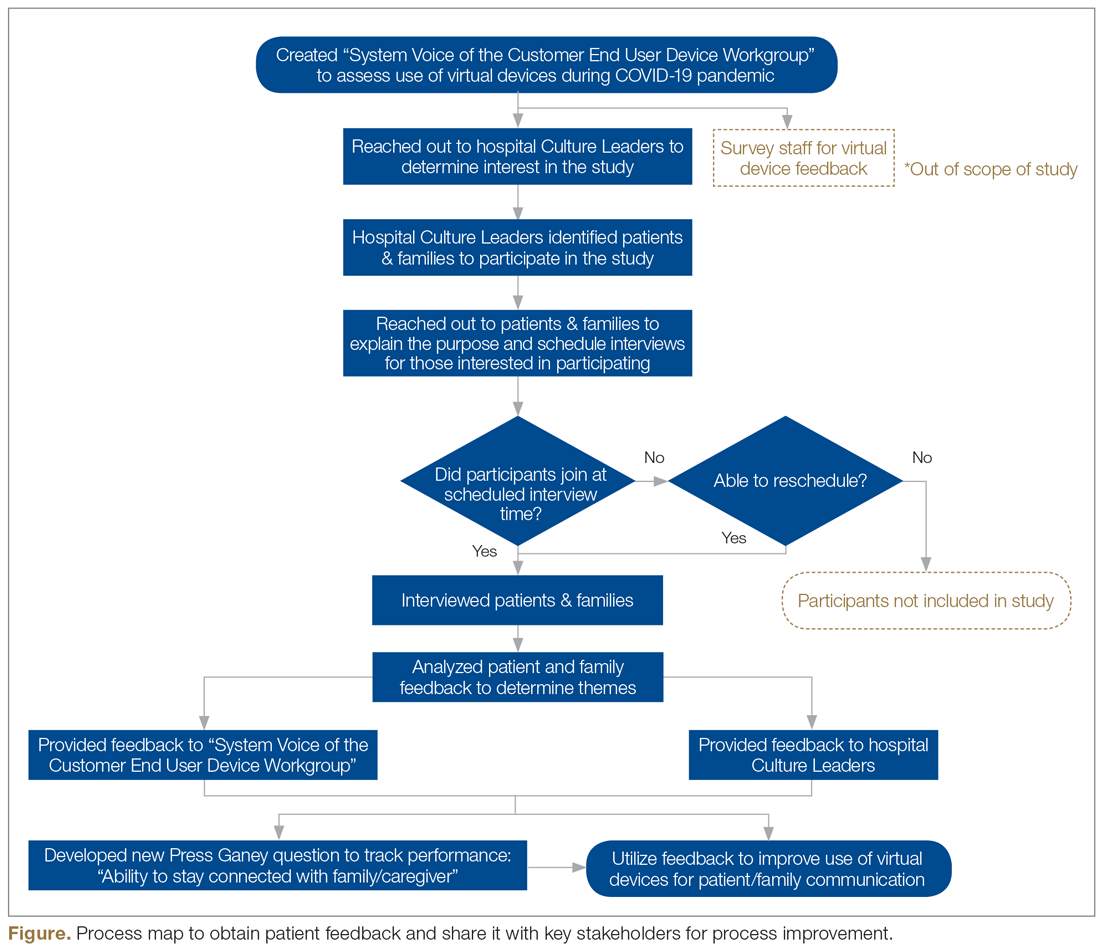
This feedback, along with the responses to the new Press Ganey question, was presented to the system Voice of the Customer End User Device Workgroup. The results led to robust discussion and brainstorming regarding how to improve the process to be more patient-centered. Findings were also shared with our hospital-based Culture Leaders. As many of their local strategic plans focused on patient-family communication, this information was helpful to them in considering plans for expansion and/or sustaining virtual visitation efforts. The process map in the Figure outlines key milestones within this feedback loop.
Outcomes
During the height of the initial COVID-19 crisis, virtual visitation was a new and ever-evolving process. Amidst the chaos, mechanisms to capture the quantity and quality of virtual visits were not in place. Based on informal observation, a majority of patients utilized personal devices to connect with loved ones, and staff even offered their own cellular devices to facilitate timely patient-family communication. The technology primarily used included FaceTime, Zoom, and EZCall, as there was much public awareness and comfort with those platforms.
In the first quarter of 2021, our institution overall performed at a Top Box score of 60.2 for our ability to assist patients with staying connected to their family/caregiver during their inpatient visit. With more than 6700 returned surveys during that time period, our hospitals earned Top Box scores ranging between 48.0 and 75.3. At this time, obtaining a national benchmark ranking is not possible, because the question regarding connectedness is unique to Northwell inpatient settings. As other health care organizations adopt this customized question, further peer-to-peer measurements can be established.
Regarding virtual interviews, 25 patients were initially contacted to determine their interest in participating. Of that sample, 17 patients were engaged over the phone, representing a reach rate of 68%. Overall, 10 interviews were scheduled; 7 patients did not show up, resulting in 3 completed interviews. During follow-up, “no-show” participants either gave no response or stated they had a conflict at their originally scheduled time but were not interested in rescheduling due to personal circumstances. Through such conversations, ongoing health complications were found to be a reoccurring barrier to participation.
Each of the participating patients had experienced being placed on a ventilator. They described their hospitalization as a time of “confusion and despair” in the first days after extubation. After we reviewed interview recordings, a reoccurring theme across all interviews was the feeling of gratitude. Patients expressed deep and heartfelt appreciation for being given the opportunity to connect as a family. One patient described virtual visitation sessions as her “only tether to reality when nothing else made sense.”
Interestingly enough, none of the participants knew that virtual visitation was an option and/or thought to inquire about it before a hospital staff member offered to set up a session. Patients recounted how they were weak and physically unable to connect to the sessions without significant assistance. They reported examples of not having the physical strength to hold up the tablet or needing a staff member to facilitate the conversation because the patient could not speak loudly enough and/or they were having difficulty hearing over background medical equipment noises. Participants also described times when a nurse or social worker would stand and hold the tablet for 20 to 30 minutes at a time, further describing mixed feelings of gratitude, guilt for “taking up their time,” and a desire for more privacy to have those precious conversations.
Discussion
Our institution encountered various barriers when establishing, implementing, and sustaining virtual visitation. The acquisition and bulk purchasing of devices, so that each hospital unit and department had adequate par levels during a high-demand time frame, was an initial challenge. Ensuring appropriate safeguards, software programming, and access to WiFi required ingenuity from IT teams. Leaders sought to advocate for the importance of prioritizing virtual visitation alongside clinical interventions. For team members, education was needed to build awareness, learn how to navigate technology, and troubleshoot, in real-time, issues such as poor connectivity. However, despite these organizational struggles, the hospital’s frontline professionals fully recognized and understood the humanistic value of connecting ill patients with their loved ones. Harnessing their teamwork, empathy, and innovative spirits, they forged through such difficulties to create meaningful interactions.
Although virtual visitation occurred prior to the COVID-19 pandemic, particularly in subspecialty areas such as neonatal intensive care units,6 it was not commonplace in most adult inpatient care settings. However, now that virtual means to communication are widely accepted and preferred, our hospital anticipates these offerings will become a broad patient expectation and, therefore, part of standard hospital care and operations. Health care leaders and interdisciplinary teams must therefore prioritize virtual visitation protocols, efforts, and future programming. It is no longer an exception to the rule, but rather a critical approach when ensuring quality communication between patients, families, and care teams.
We strive to continually improve by including user feedback as part of an interactive design process. For a broader, more permanent installation of virtual visitation, health care organizations must proactively promote this capability as a valued option. Considering health literacy and comfort with technology, functionality, and logistics must be carefully explained to patients and their families. This may require additional staff training so that they are knowledgeable, comfortable with, and able to troubleshoot questions/concerns in real time. There needs to be an adequate number of mobile devices available at a unit or departmental level to meet short-term and long-term demands. Additionally, now that we have emerged from our initial crisis-based mentality, it is time to consider alternatives to alleviate the need for staff assistance, such as mounts to hold devices and enabling voice controls.
Conclusion
As an organization grounded in the spirit of innovation, Northwell has been able to quickly pivot, adopting virtual visitation to address emerging and complex communication needs. Taking a best practice established during a crisis period and engraining it into sustainable organizational culture and operations requires visionary leadership, strong teamwork, and an unbridled commitment to patient and family centeredness. Despite unprecedented challenges, our commitment to listening to the “voice” of patients and families never wavered. Using their insights and feedback as critical components to the decision-making process, there is much work ahead within the realm of virtual visitation.
Acknowledgements: The authors would like to acknowledge the Northwell Health providers, frontline health care professionals, and team members who worked tirelessly to care for its community during initial COVID-19 waves and every day thereafter. Heartfelt gratitude to Northwell’s senior leaders for the visionary leadership; the OCIO and hospital-based IT teams for their swift collaboration; and dedicated Culture Leaders, Patient Experience team members, and redeployed staff for their unbridled passion for caring for patients and families. Special thanks to Agnes Barden, DNP, RN, CPXP, Joseph Narvaez, MBA, and Natalie Bashkin, MBA, from the system Office of Patient & Customer Experience, and Carolyne Burgess, MPH, from the Digital Patient Experience teams, for their participation, leadership, and syngeristic partnerships.
Corresponding Author: Nicole Giammarinaro, MSN, RN, CPXP, Director, Patient & Customer Experience, Northwell Health, 2000 Marcus Ave, Lake Success, NY 11042; [email protected].
Financial disclosures: Sven Gierlinger serves on the Speakers Bureau for Northwell Health and as an Executive Board Member for The Beryl Institute.
1. New York State Department of Health. Health advisory: COVID-19 guidance for hospital operators regarding visitation. March 18, 2020. https://coronavirus.health.ny.gov/system/files/documents/2020/03/covid19-hospital-visitation-guidance-3.18.20.pdf
2. Zhang Y. Family functioning in the context of an adult family member with illness: a concept analysis. J Clin Nurs. 2018;27(15-16):3205-3224. doi:10.1111/jocn.14500
3. Institute for Patient- & Family-Centered Care. Better Together: Partnering with Families. https://www.ipfcc.org/bestpractices/better-together-ny.html
4. Marceglia S, Bonacina S, Zaccaria V, et al. How might the iPad change healthcare? J R Soc Med. 2012;105(6):233-241. doi:10.1258/jrsm.2012.110296
5. Short B, Parekh M, Ryan P, et al. Rapid implementation of a mobile prone team during the COVID-19 pandemic. J Crit Care. 2020;60:230-234. doi:10.1016/j.jcrc.2020.08.020
6. Yeo C, Ho SK, Khong K, Lau Y. Virtual visitation in the neonatal intensive care: experience with the use of internet and telemedicine in a tertiary neonatal unit. Perm J. 2011;15(3):32-36.
From Northwell Health, Lake Success, NY.
Objective: Northwell Health, New York’s largest health care organization, rapidly adopted technology solutions to support patient and family communication during the COVID-19 pandemic.
Methods: This case series outlines the pragmatic, interdisciplinary approach Northwell underwent to rapidly implement patient virtual visitation processes during the peak of the initial crisis.
Results: Implementation of large-scale virtual visitation required leadership, technology, and dedicated, empathetic frontline professionals. Patient and family feedback uncovered varied feelings and perspectives, from confusion to gratitude.
Conclusion: Subsequent efforts to obtain direct patient and family perspectives and insights helped Northwell identify areas of strength and ongoing performance improvement.
Keywords: virtual visitation; COVID-19; technology; communication; patient experience.
The power of human connection has become increasingly apparent throughout the COVID-19 pandemic and subsequent recovery phases. Due to the need for social distancing, people worldwide have turned to virtual means of communication, staying in touch with family, friends, and colleagues via digital technology platforms. On March 18, 2020, the New York State Department of Health (NYSDOH) issued a health advisory, suspending all hospital visitation.1 As a result, hospitals rapidly transformed existing in-person visitation practices to meet large-scale virtual programming needs.
Family members often take on various roles—such as advocate, emotional support person, and postdischarge caregiver—for an ill or injured loved one.2 The Institute for Patient- and Family-Centered Care, a nonprofit organization founded in 1992, has been leading a cultural transformation where families are valued as care partners, as opposed to “visitors.”3 Although widely adopted and well-received in specialized units, such as neonatal intensive care units,4 virtual visitation had not been widely implemented across adult care settings. The NYSDOH guidance therefore required organizational leadership, innovation, flexibility, and systems ingenuity to meet the evolving needs of patients, families, and health care professionals. An overarching goal was ensuring patients and families were afforded opportunities to stay connected throughout hospitalization.
Reflecting the impact of COVID-19 surges, hospital environments became increasingly depersonalized, with health care providers wearing extensive personal protective equipment (PPE) and taking remarkable measures to socially distance and minimize exposure. Patients’ room doors were kept primarily closed, while codes and alerts blared in the halls overhead. The lack of families and visitors became increasingly obvious, aiding feelings of isolation and confinement. With fear of nosocomial transmission, impactful modalities (such as sitting at the bedside) and empathetic, therapeutic touch were no longer taking place.
With those scenarios—common to so many health care systems during the pandemic—as a backdrop, comes our experience. Northwell Health is the largest health care system in New York State, geographically spread throughout New York City’s 5 boroughs, Westchester County, and Long Island. With 23 hospitals, approximately 820 medical practices, and over 72 000 employees, Northwell has cared for more than 100 000 COVID-positive patients to date. This case series outlines a pragmatic approach to implementing virtual visitation during the initial peak and obtaining patient and family perspectives to help inform performance improvement and future programming.
Methods
Implementing virtual visitation
Through swift and focused multidisciplinary collaboration, numerous Northwell teams came together to implement large-scale virtual visitation across the organization during the first wave of the COVID crisis. The initial priority involved securing devices that could support patient-family communication. Prior to COVID, each facility had only a handful of tablets that were used primarily during leadership rounding, so once visitation was restricted, we needed a large quantity of devices within a matter of days. Through diligent work from System Procurement and internal Foundation, Northwell was able to acquire nearly 900 devices, including iPads, PadInMotion tablets, and Samsung tablets.
Typically, the benefits of using wireless tablets within a health care setting include long battery life, powerful data processing, advanced operating systems, large screens, and easy end-user navigation.4 During COVID-19 and its associated isolation precautions, tablets offered a lifeline for effective and socially distant communication. With new devices in hand, the system Office of the Chief Information Officer (OCIO) and site-based Information Technology (IT) teams were engaged. They worked tirelessly to streamline connectivity, download necessary apps, test devices on approved WiFi networks, and troubleshoot issues. Once set up, devices were strategically deployed across all Northwell hospitals and post-acute rehabilitation facilities.
Frontline teams quickly realized that a model similar to mobile proning teams, who focus solely on turning and positioning COVID patients to promote optimal respiratory ventilation,5 was needed to support virtual visitation. During the initial COVID wave, elective surgeries were not permissible, as per the NYSDOH. As a result, large numbers of clinical and nonclinical ambulatory surgery employees were redeployed throughout the organization, with many assigned and dedicated to facilitating newly created virtual visitation processes. These employees were primarily responsible for creating unit-based schedules, coordinating logistics, navigating devices on behalf of patients, being present during video calls, and sanitizing the devices between uses. Finally, if necessary, virtual interpretation services were used to overcome language barriers between staff and patients.
What began as an ad hoc function quickly became a valued and meaningful role. Utilizing triage mentality, virtual visitation was first offered during unit-based rounding protocols to those patients with the highest acuity and need to connect with family. We had no formal script; instead, unit-based leaders and frontline team members had open dialogues with patients and families to gauge their interest in virtual visitation. That included patients with an active end-of-life care plan, critically ill patients within intensive care units, and those soon to be intubated or recently extubated. Utilization also occurred within specialty areas such as labor and delivery, pediatrics, inpatient psychiatry, medical units, and long-term rehab facilities. Frontline teams appreciated the supplementary support so they could prioritize ongoing physical assessments and medical interventions. Donned in PPE, virtual visitation team members often served as physical extensions of the patient’s loved ones—holding their hand, offering prayers, and, at times, bearing witness to a last breath. In reflecting on that time, this role required absolute professionalism, empathy, and compassion.
In summer 2020, although demand for virtual visitation was still at an all-time high when ambulatory surgery was reinstated, redeployed staff returned to their responsibilities. To fill this void without interruption to patients and their families, site leaders quickly pivoted and refined processes and protocols utilizing Patient & Customer Experience and Hospitality department team members. Throughout spring 2021, the NYSDOH offered guidance to open in-person visitation, and the institution’s Clinical Advisory Group has been taking a pragmatic approach to doing that in a measured and safe manner across care settings.
Listening to the ‘voice’ of patients and families
Our institution’s mission is grounded in providing “quality service and patient-centered care.” Honoring those tenets, during the initial COVID wave, the system “Voice of the Customer End User Device Workgroup” was created with system and site-based interdisciplinary representation. Despite challenging and unprecedented times, conscious attention and effort was undertaken to assess the use and impact of virtual devices. One of the major work streams was to capture and examine patient and family thoughts, feedback, and the overall experience as it relates to virtual visitation.
The system Office of Patient & Customer Experience (OPCE), led by Sven Gierlinger, SVP Chief Experience Officer, reached out to our colleagues at Press Ganey to add a custom question to patient experience surveys. Beginning on December 1, 2020, discharged inpatients were asked to rate the “Degree to which you were able to stay connected with your family/caregiver during your stay.” Potential answers include the Likert scale responses of Always, Usually, Sometimes, and Never, with “Always” representing the Top Box score. The OPCE team believes these quantitative insights are important to track and trend, particularly since in-person and virtual visitation remain in constant flux.
In an effort to obtain additional, focused, qualitative feedback, OPCE partnered with our institution’s Digital Patient Experience (dPX) colleagues. The approach consisted of voluntary, semistructured, interview-type conversations with patients and family members who engaged in virtual visitation multiple times while the patient was hospitalized. OPCE contacted site-based Patient Experience leads, also known as Culture Leaders, at 3 hospitals, asking them to identify potential participants. This convenience sample excluded instances where the patient passed away during and/or immediately following hospitalization.
The OPCE team phoned potential interview candidates to make a personalized connection, explain the purpose of the interviews, and schedule them, if interested. For consistency, the same Digital Customer Experience Researcher on the dPX team facilitated all sessions, which were 30-minute, semiscripted interviews conducted virtually via Microsoft Teams. The tone was intentionally conversational so that patients and family members would feel comfortable delving into themes that were most impactful during their experience. After some initial ice breakers, such as “What were some of your feelings about being a patient/having a loved one in the hospital during the early days of the COVID-19 pandemic?” we moved on to some more pragmatic, implementation questions and rating scales. These included questions such as “How did you first learn about the option for virtual visitation? Was it something you inquired about or did someone offer it to you? How was it explained to you?” Patients were also asked, on a scale of 1 (easy) to 5 (difficult), to rate their experience with the technology aspect when connecting with their loved ones. They also provided verbal consent to be recorded and were given a $15 gift card upon completion of the interview.
Transcriptions were generated by uploading the interview recordings to a platform called UserTesting. In addition to these transcriptions, this platform also allowed for a keyword mapping tool that organized high-level themes and adjectives into groupings along a sentiment axis from negative to neutral to positive. Transcripts were then read carefully and annotated by the Digital Customer Experience Researcher, which allowed for strengthening of some of the automated themes as well as the emergence of new, more nuanced themes. These themes were organized into those that we could address with design and/or procedure updates (actionable insights), those that came up most frequently overall (frequency), and those that came up across our 3 interview sessions (commonality).
This feedback, along with the responses to the new Press Ganey question, was presented to the system Voice of the Customer End User Device Workgroup. The results led to robust discussion and brainstorming regarding how to improve the process to be more patient-centered. Findings were also shared with our hospital-based Culture Leaders. As many of their local strategic plans focused on patient-family communication, this information was helpful to them in considering plans for expansion and/or sustaining virtual visitation efforts. The process map in the Figure outlines key milestones within this feedback loop.

Outcomes
During the height of the initial COVID-19 crisis, virtual visitation was a new and ever-evolving process. Amidst the chaos, mechanisms to capture the quantity and quality of virtual visits were not in place. Based on informal observation, a majority of patients utilized personal devices to connect with loved ones, and staff even offered their own cellular devices to facilitate timely patient-family communication. The technology primarily used included FaceTime, Zoom, and EZCall, as there was much public awareness and comfort with those platforms.
In the first quarter of 2021, our institution overall performed at a Top Box score of 60.2 for our ability to assist patients with staying connected to their family/caregiver during their inpatient visit. With more than 6700 returned surveys during that time period, our hospitals earned Top Box scores ranging between 48.0 and 75.3. At this time, obtaining a national benchmark ranking is not possible, because the question regarding connectedness is unique to Northwell inpatient settings. As other health care organizations adopt this customized question, further peer-to-peer measurements can be established.
Regarding virtual interviews, 25 patients were initially contacted to determine their interest in participating. Of that sample, 17 patients were engaged over the phone, representing a reach rate of 68%. Overall, 10 interviews were scheduled; 7 patients did not show up, resulting in 3 completed interviews. During follow-up, “no-show” participants either gave no response or stated they had a conflict at their originally scheduled time but were not interested in rescheduling due to personal circumstances. Through such conversations, ongoing health complications were found to be a reoccurring barrier to participation.
Each of the participating patients had experienced being placed on a ventilator. They described their hospitalization as a time of “confusion and despair” in the first days after extubation. After we reviewed interview recordings, a reoccurring theme across all interviews was the feeling of gratitude. Patients expressed deep and heartfelt appreciation for being given the opportunity to connect as a family. One patient described virtual visitation sessions as her “only tether to reality when nothing else made sense.”
Interestingly enough, none of the participants knew that virtual visitation was an option and/or thought to inquire about it before a hospital staff member offered to set up a session. Patients recounted how they were weak and physically unable to connect to the sessions without significant assistance. They reported examples of not having the physical strength to hold up the tablet or needing a staff member to facilitate the conversation because the patient could not speak loudly enough and/or they were having difficulty hearing over background medical equipment noises. Participants also described times when a nurse or social worker would stand and hold the tablet for 20 to 30 minutes at a time, further describing mixed feelings of gratitude, guilt for “taking up their time,” and a desire for more privacy to have those precious conversations.
Discussion
Our institution encountered various barriers when establishing, implementing, and sustaining virtual visitation. The acquisition and bulk purchasing of devices, so that each hospital unit and department had adequate par levels during a high-demand time frame, was an initial challenge. Ensuring appropriate safeguards, software programming, and access to WiFi required ingenuity from IT teams. Leaders sought to advocate for the importance of prioritizing virtual visitation alongside clinical interventions. For team members, education was needed to build awareness, learn how to navigate technology, and troubleshoot, in real-time, issues such as poor connectivity. However, despite these organizational struggles, the hospital’s frontline professionals fully recognized and understood the humanistic value of connecting ill patients with their loved ones. Harnessing their teamwork, empathy, and innovative spirits, they forged through such difficulties to create meaningful interactions.
Although virtual visitation occurred prior to the COVID-19 pandemic, particularly in subspecialty areas such as neonatal intensive care units,6 it was not commonplace in most adult inpatient care settings. However, now that virtual means to communication are widely accepted and preferred, our hospital anticipates these offerings will become a broad patient expectation and, therefore, part of standard hospital care and operations. Health care leaders and interdisciplinary teams must therefore prioritize virtual visitation protocols, efforts, and future programming. It is no longer an exception to the rule, but rather a critical approach when ensuring quality communication between patients, families, and care teams.
We strive to continually improve by including user feedback as part of an interactive design process. For a broader, more permanent installation of virtual visitation, health care organizations must proactively promote this capability as a valued option. Considering health literacy and comfort with technology, functionality, and logistics must be carefully explained to patients and their families. This may require additional staff training so that they are knowledgeable, comfortable with, and able to troubleshoot questions/concerns in real time. There needs to be an adequate number of mobile devices available at a unit or departmental level to meet short-term and long-term demands. Additionally, now that we have emerged from our initial crisis-based mentality, it is time to consider alternatives to alleviate the need for staff assistance, such as mounts to hold devices and enabling voice controls.
Conclusion
As an organization grounded in the spirit of innovation, Northwell has been able to quickly pivot, adopting virtual visitation to address emerging and complex communication needs. Taking a best practice established during a crisis period and engraining it into sustainable organizational culture and operations requires visionary leadership, strong teamwork, and an unbridled commitment to patient and family centeredness. Despite unprecedented challenges, our commitment to listening to the “voice” of patients and families never wavered. Using their insights and feedback as critical components to the decision-making process, there is much work ahead within the realm of virtual visitation.
Acknowledgements: The authors would like to acknowledge the Northwell Health providers, frontline health care professionals, and team members who worked tirelessly to care for its community during initial COVID-19 waves and every day thereafter. Heartfelt gratitude to Northwell’s senior leaders for the visionary leadership; the OCIO and hospital-based IT teams for their swift collaboration; and dedicated Culture Leaders, Patient Experience team members, and redeployed staff for their unbridled passion for caring for patients and families. Special thanks to Agnes Barden, DNP, RN, CPXP, Joseph Narvaez, MBA, and Natalie Bashkin, MBA, from the system Office of Patient & Customer Experience, and Carolyne Burgess, MPH, from the Digital Patient Experience teams, for their participation, leadership, and syngeristic partnerships.
Corresponding Author: Nicole Giammarinaro, MSN, RN, CPXP, Director, Patient & Customer Experience, Northwell Health, 2000 Marcus Ave, Lake Success, NY 11042; [email protected].
Financial disclosures: Sven Gierlinger serves on the Speakers Bureau for Northwell Health and as an Executive Board Member for The Beryl Institute.
From Northwell Health, Lake Success, NY.
Objective: Northwell Health, New York’s largest health care organization, rapidly adopted technology solutions to support patient and family communication during the COVID-19 pandemic.
Methods: This case series outlines the pragmatic, interdisciplinary approach Northwell underwent to rapidly implement patient virtual visitation processes during the peak of the initial crisis.
Results: Implementation of large-scale virtual visitation required leadership, technology, and dedicated, empathetic frontline professionals. Patient and family feedback uncovered varied feelings and perspectives, from confusion to gratitude.
Conclusion: Subsequent efforts to obtain direct patient and family perspectives and insights helped Northwell identify areas of strength and ongoing performance improvement.
Keywords: virtual visitation; COVID-19; technology; communication; patient experience.
The power of human connection has become increasingly apparent throughout the COVID-19 pandemic and subsequent recovery phases. Due to the need for social distancing, people worldwide have turned to virtual means of communication, staying in touch with family, friends, and colleagues via digital technology platforms. On March 18, 2020, the New York State Department of Health (NYSDOH) issued a health advisory, suspending all hospital visitation.1 As a result, hospitals rapidly transformed existing in-person visitation practices to meet large-scale virtual programming needs.
Family members often take on various roles—such as advocate, emotional support person, and postdischarge caregiver—for an ill or injured loved one.2 The Institute for Patient- and Family-Centered Care, a nonprofit organization founded in 1992, has been leading a cultural transformation where families are valued as care partners, as opposed to “visitors.”3 Although widely adopted and well-received in specialized units, such as neonatal intensive care units,4 virtual visitation had not been widely implemented across adult care settings. The NYSDOH guidance therefore required organizational leadership, innovation, flexibility, and systems ingenuity to meet the evolving needs of patients, families, and health care professionals. An overarching goal was ensuring patients and families were afforded opportunities to stay connected throughout hospitalization.
Reflecting the impact of COVID-19 surges, hospital environments became increasingly depersonalized, with health care providers wearing extensive personal protective equipment (PPE) and taking remarkable measures to socially distance and minimize exposure. Patients’ room doors were kept primarily closed, while codes and alerts blared in the halls overhead. The lack of families and visitors became increasingly obvious, aiding feelings of isolation and confinement. With fear of nosocomial transmission, impactful modalities (such as sitting at the bedside) and empathetic, therapeutic touch were no longer taking place.
With those scenarios—common to so many health care systems during the pandemic—as a backdrop, comes our experience. Northwell Health is the largest health care system in New York State, geographically spread throughout New York City’s 5 boroughs, Westchester County, and Long Island. With 23 hospitals, approximately 820 medical practices, and over 72 000 employees, Northwell has cared for more than 100 000 COVID-positive patients to date. This case series outlines a pragmatic approach to implementing virtual visitation during the initial peak and obtaining patient and family perspectives to help inform performance improvement and future programming.
Methods
Implementing virtual visitation
Through swift and focused multidisciplinary collaboration, numerous Northwell teams came together to implement large-scale virtual visitation across the organization during the first wave of the COVID crisis. The initial priority involved securing devices that could support patient-family communication. Prior to COVID, each facility had only a handful of tablets that were used primarily during leadership rounding, so once visitation was restricted, we needed a large quantity of devices within a matter of days. Through diligent work from System Procurement and internal Foundation, Northwell was able to acquire nearly 900 devices, including iPads, PadInMotion tablets, and Samsung tablets.
Typically, the benefits of using wireless tablets within a health care setting include long battery life, powerful data processing, advanced operating systems, large screens, and easy end-user navigation.4 During COVID-19 and its associated isolation precautions, tablets offered a lifeline for effective and socially distant communication. With new devices in hand, the system Office of the Chief Information Officer (OCIO) and site-based Information Technology (IT) teams were engaged. They worked tirelessly to streamline connectivity, download necessary apps, test devices on approved WiFi networks, and troubleshoot issues. Once set up, devices were strategically deployed across all Northwell hospitals and post-acute rehabilitation facilities.
Frontline teams quickly realized that a model similar to mobile proning teams, who focus solely on turning and positioning COVID patients to promote optimal respiratory ventilation,5 was needed to support virtual visitation. During the initial COVID wave, elective surgeries were not permissible, as per the NYSDOH. As a result, large numbers of clinical and nonclinical ambulatory surgery employees were redeployed throughout the organization, with many assigned and dedicated to facilitating newly created virtual visitation processes. These employees were primarily responsible for creating unit-based schedules, coordinating logistics, navigating devices on behalf of patients, being present during video calls, and sanitizing the devices between uses. Finally, if necessary, virtual interpretation services were used to overcome language barriers between staff and patients.
What began as an ad hoc function quickly became a valued and meaningful role. Utilizing triage mentality, virtual visitation was first offered during unit-based rounding protocols to those patients with the highest acuity and need to connect with family. We had no formal script; instead, unit-based leaders and frontline team members had open dialogues with patients and families to gauge their interest in virtual visitation. That included patients with an active end-of-life care plan, critically ill patients within intensive care units, and those soon to be intubated or recently extubated. Utilization also occurred within specialty areas such as labor and delivery, pediatrics, inpatient psychiatry, medical units, and long-term rehab facilities. Frontline teams appreciated the supplementary support so they could prioritize ongoing physical assessments and medical interventions. Donned in PPE, virtual visitation team members often served as physical extensions of the patient’s loved ones—holding their hand, offering prayers, and, at times, bearing witness to a last breath. In reflecting on that time, this role required absolute professionalism, empathy, and compassion.
In summer 2020, although demand for virtual visitation was still at an all-time high when ambulatory surgery was reinstated, redeployed staff returned to their responsibilities. To fill this void without interruption to patients and their families, site leaders quickly pivoted and refined processes and protocols utilizing Patient & Customer Experience and Hospitality department team members. Throughout spring 2021, the NYSDOH offered guidance to open in-person visitation, and the institution’s Clinical Advisory Group has been taking a pragmatic approach to doing that in a measured and safe manner across care settings.
Listening to the ‘voice’ of patients and families
Our institution’s mission is grounded in providing “quality service and patient-centered care.” Honoring those tenets, during the initial COVID wave, the system “Voice of the Customer End User Device Workgroup” was created with system and site-based interdisciplinary representation. Despite challenging and unprecedented times, conscious attention and effort was undertaken to assess the use and impact of virtual devices. One of the major work streams was to capture and examine patient and family thoughts, feedback, and the overall experience as it relates to virtual visitation.
The system Office of Patient & Customer Experience (OPCE), led by Sven Gierlinger, SVP Chief Experience Officer, reached out to our colleagues at Press Ganey to add a custom question to patient experience surveys. Beginning on December 1, 2020, discharged inpatients were asked to rate the “Degree to which you were able to stay connected with your family/caregiver during your stay.” Potential answers include the Likert scale responses of Always, Usually, Sometimes, and Never, with “Always” representing the Top Box score. The OPCE team believes these quantitative insights are important to track and trend, particularly since in-person and virtual visitation remain in constant flux.
In an effort to obtain additional, focused, qualitative feedback, OPCE partnered with our institution’s Digital Patient Experience (dPX) colleagues. The approach consisted of voluntary, semistructured, interview-type conversations with patients and family members who engaged in virtual visitation multiple times while the patient was hospitalized. OPCE contacted site-based Patient Experience leads, also known as Culture Leaders, at 3 hospitals, asking them to identify potential participants. This convenience sample excluded instances where the patient passed away during and/or immediately following hospitalization.
The OPCE team phoned potential interview candidates to make a personalized connection, explain the purpose of the interviews, and schedule them, if interested. For consistency, the same Digital Customer Experience Researcher on the dPX team facilitated all sessions, which were 30-minute, semiscripted interviews conducted virtually via Microsoft Teams. The tone was intentionally conversational so that patients and family members would feel comfortable delving into themes that were most impactful during their experience. After some initial ice breakers, such as “What were some of your feelings about being a patient/having a loved one in the hospital during the early days of the COVID-19 pandemic?” we moved on to some more pragmatic, implementation questions and rating scales. These included questions such as “How did you first learn about the option for virtual visitation? Was it something you inquired about or did someone offer it to you? How was it explained to you?” Patients were also asked, on a scale of 1 (easy) to 5 (difficult), to rate their experience with the technology aspect when connecting with their loved ones. They also provided verbal consent to be recorded and were given a $15 gift card upon completion of the interview.
Transcriptions were generated by uploading the interview recordings to a platform called UserTesting. In addition to these transcriptions, this platform also allowed for a keyword mapping tool that organized high-level themes and adjectives into groupings along a sentiment axis from negative to neutral to positive. Transcripts were then read carefully and annotated by the Digital Customer Experience Researcher, which allowed for strengthening of some of the automated themes as well as the emergence of new, more nuanced themes. These themes were organized into those that we could address with design and/or procedure updates (actionable insights), those that came up most frequently overall (frequency), and those that came up across our 3 interview sessions (commonality).
This feedback, along with the responses to the new Press Ganey question, was presented to the system Voice of the Customer End User Device Workgroup. The results led to robust discussion and brainstorming regarding how to improve the process to be more patient-centered. Findings were also shared with our hospital-based Culture Leaders. As many of their local strategic plans focused on patient-family communication, this information was helpful to them in considering plans for expansion and/or sustaining virtual visitation efforts. The process map in the Figure outlines key milestones within this feedback loop.

Outcomes
During the height of the initial COVID-19 crisis, virtual visitation was a new and ever-evolving process. Amidst the chaos, mechanisms to capture the quantity and quality of virtual visits were not in place. Based on informal observation, a majority of patients utilized personal devices to connect with loved ones, and staff even offered their own cellular devices to facilitate timely patient-family communication. The technology primarily used included FaceTime, Zoom, and EZCall, as there was much public awareness and comfort with those platforms.
In the first quarter of 2021, our institution overall performed at a Top Box score of 60.2 for our ability to assist patients with staying connected to their family/caregiver during their inpatient visit. With more than 6700 returned surveys during that time period, our hospitals earned Top Box scores ranging between 48.0 and 75.3. At this time, obtaining a national benchmark ranking is not possible, because the question regarding connectedness is unique to Northwell inpatient settings. As other health care organizations adopt this customized question, further peer-to-peer measurements can be established.
Regarding virtual interviews, 25 patients were initially contacted to determine their interest in participating. Of that sample, 17 patients were engaged over the phone, representing a reach rate of 68%. Overall, 10 interviews were scheduled; 7 patients did not show up, resulting in 3 completed interviews. During follow-up, “no-show” participants either gave no response or stated they had a conflict at their originally scheduled time but were not interested in rescheduling due to personal circumstances. Through such conversations, ongoing health complications were found to be a reoccurring barrier to participation.
Each of the participating patients had experienced being placed on a ventilator. They described their hospitalization as a time of “confusion and despair” in the first days after extubation. After we reviewed interview recordings, a reoccurring theme across all interviews was the feeling of gratitude. Patients expressed deep and heartfelt appreciation for being given the opportunity to connect as a family. One patient described virtual visitation sessions as her “only tether to reality when nothing else made sense.”
Interestingly enough, none of the participants knew that virtual visitation was an option and/or thought to inquire about it before a hospital staff member offered to set up a session. Patients recounted how they were weak and physically unable to connect to the sessions without significant assistance. They reported examples of not having the physical strength to hold up the tablet or needing a staff member to facilitate the conversation because the patient could not speak loudly enough and/or they were having difficulty hearing over background medical equipment noises. Participants also described times when a nurse or social worker would stand and hold the tablet for 20 to 30 minutes at a time, further describing mixed feelings of gratitude, guilt for “taking up their time,” and a desire for more privacy to have those precious conversations.
Discussion
Our institution encountered various barriers when establishing, implementing, and sustaining virtual visitation. The acquisition and bulk purchasing of devices, so that each hospital unit and department had adequate par levels during a high-demand time frame, was an initial challenge. Ensuring appropriate safeguards, software programming, and access to WiFi required ingenuity from IT teams. Leaders sought to advocate for the importance of prioritizing virtual visitation alongside clinical interventions. For team members, education was needed to build awareness, learn how to navigate technology, and troubleshoot, in real-time, issues such as poor connectivity. However, despite these organizational struggles, the hospital’s frontline professionals fully recognized and understood the humanistic value of connecting ill patients with their loved ones. Harnessing their teamwork, empathy, and innovative spirits, they forged through such difficulties to create meaningful interactions.
Although virtual visitation occurred prior to the COVID-19 pandemic, particularly in subspecialty areas such as neonatal intensive care units,6 it was not commonplace in most adult inpatient care settings. However, now that virtual means to communication are widely accepted and preferred, our hospital anticipates these offerings will become a broad patient expectation and, therefore, part of standard hospital care and operations. Health care leaders and interdisciplinary teams must therefore prioritize virtual visitation protocols, efforts, and future programming. It is no longer an exception to the rule, but rather a critical approach when ensuring quality communication between patients, families, and care teams.
We strive to continually improve by including user feedback as part of an interactive design process. For a broader, more permanent installation of virtual visitation, health care organizations must proactively promote this capability as a valued option. Considering health literacy and comfort with technology, functionality, and logistics must be carefully explained to patients and their families. This may require additional staff training so that they are knowledgeable, comfortable with, and able to troubleshoot questions/concerns in real time. There needs to be an adequate number of mobile devices available at a unit or departmental level to meet short-term and long-term demands. Additionally, now that we have emerged from our initial crisis-based mentality, it is time to consider alternatives to alleviate the need for staff assistance, such as mounts to hold devices and enabling voice controls.
Conclusion
As an organization grounded in the spirit of innovation, Northwell has been able to quickly pivot, adopting virtual visitation to address emerging and complex communication needs. Taking a best practice established during a crisis period and engraining it into sustainable organizational culture and operations requires visionary leadership, strong teamwork, and an unbridled commitment to patient and family centeredness. Despite unprecedented challenges, our commitment to listening to the “voice” of patients and families never wavered. Using their insights and feedback as critical components to the decision-making process, there is much work ahead within the realm of virtual visitation.
Acknowledgements: The authors would like to acknowledge the Northwell Health providers, frontline health care professionals, and team members who worked tirelessly to care for its community during initial COVID-19 waves and every day thereafter. Heartfelt gratitude to Northwell’s senior leaders for the visionary leadership; the OCIO and hospital-based IT teams for their swift collaboration; and dedicated Culture Leaders, Patient Experience team members, and redeployed staff for their unbridled passion for caring for patients and families. Special thanks to Agnes Barden, DNP, RN, CPXP, Joseph Narvaez, MBA, and Natalie Bashkin, MBA, from the system Office of Patient & Customer Experience, and Carolyne Burgess, MPH, from the Digital Patient Experience teams, for their participation, leadership, and syngeristic partnerships.
Corresponding Author: Nicole Giammarinaro, MSN, RN, CPXP, Director, Patient & Customer Experience, Northwell Health, 2000 Marcus Ave, Lake Success, NY 11042; [email protected].
Financial disclosures: Sven Gierlinger serves on the Speakers Bureau for Northwell Health and as an Executive Board Member for The Beryl Institute.
1. New York State Department of Health. Health advisory: COVID-19 guidance for hospital operators regarding visitation. March 18, 2020. https://coronavirus.health.ny.gov/system/files/documents/2020/03/covid19-hospital-visitation-guidance-3.18.20.pdf
2. Zhang Y. Family functioning in the context of an adult family member with illness: a concept analysis. J Clin Nurs. 2018;27(15-16):3205-3224. doi:10.1111/jocn.14500
3. Institute for Patient- & Family-Centered Care. Better Together: Partnering with Families. https://www.ipfcc.org/bestpractices/better-together-ny.html
4. Marceglia S, Bonacina S, Zaccaria V, et al. How might the iPad change healthcare? J R Soc Med. 2012;105(6):233-241. doi:10.1258/jrsm.2012.110296
5. Short B, Parekh M, Ryan P, et al. Rapid implementation of a mobile prone team during the COVID-19 pandemic. J Crit Care. 2020;60:230-234. doi:10.1016/j.jcrc.2020.08.020
6. Yeo C, Ho SK, Khong K, Lau Y. Virtual visitation in the neonatal intensive care: experience with the use of internet and telemedicine in a tertiary neonatal unit. Perm J. 2011;15(3):32-36.
1. New York State Department of Health. Health advisory: COVID-19 guidance for hospital operators regarding visitation. March 18, 2020. https://coronavirus.health.ny.gov/system/files/documents/2020/03/covid19-hospital-visitation-guidance-3.18.20.pdf
2. Zhang Y. Family functioning in the context of an adult family member with illness: a concept analysis. J Clin Nurs. 2018;27(15-16):3205-3224. doi:10.1111/jocn.14500
3. Institute for Patient- & Family-Centered Care. Better Together: Partnering with Families. https://www.ipfcc.org/bestpractices/better-together-ny.html
4. Marceglia S, Bonacina S, Zaccaria V, et al. How might the iPad change healthcare? J R Soc Med. 2012;105(6):233-241. doi:10.1258/jrsm.2012.110296
5. Short B, Parekh M, Ryan P, et al. Rapid implementation of a mobile prone team during the COVID-19 pandemic. J Crit Care. 2020;60:230-234. doi:10.1016/j.jcrc.2020.08.020
6. Yeo C, Ho SK, Khong K, Lau Y. Virtual visitation in the neonatal intensive care: experience with the use of internet and telemedicine in a tertiary neonatal unit. Perm J. 2011;15(3):32-36.
Improving Physicians’ Bowel Documentation on Geriatric Wards
From Sheffield Teaching Hospitals, Sheffield, UK, S5 7AU.
Objective: Constipation is widely prevalent in older adults and may result in complications such as urinary retention, delirium, and bowel obstruction. Previous studies have indicated that while the nursing staff do well in completing stool charts, doctors monitor them infrequently. This project aimed to improve the documentation of bowel movement by doctors on ward rounds to 85%, by the end of a 3-month period.
Methods: Baseline, postintervention, and sustainability data were collected from inpatient notes on weekdays on a geriatric ward in Northern General Hospital, Sheffield, UK. Posters and stickers of the poo emoji were placed on walls and in inpatient notes, respectively, as a reminder.
Results: Data on bowel activity documentation were collected from 28 patients. The baseline data showed that bowel activity was monitored daily on the ward 60.49% of the time. However, following the interventions, there was a significant increase in documentation, to 86.78%. The sustainability study showed that bowel activity was documented on the ward 56.56% of the time.
Conclusion: This study shows how a strong initial effect on behavioral change can be accomplished through simple interventions such as stickers and posters. As most wards currently still use paper notes, this is a generalizable model that other wards can trial. However, this study also shows the difficulty in maintaining behavioral change over extended periods of time.
Keywords: bowel movement; documentation; obstruction; constipation; geriatrics; incontinence; junior doctor; quality improvement.
Constipation is widely prevalent in the elderly, encountered frequently in both community and hospital medicine.1 Its estimated prevalence in adults over 84 years old is 34% for women and 25% for men, rising to up to 80% for long-term care residents.2
Chronic constipation is generally characterized by unsatisfactory defecation due to infrequent bowel emptying or difficulty with stool passage, which may lead to incomplete evacuation.2-4 Constipation in the elderly, in addition to causing abdominal pain, nausea, and reduced appetite, may result in complications such as fecal incontinence (and overflow diarrhea), urinary retention, delirium, and bowel obstruction, which may in result in life-threatening perforation.5,6 For inpatients on geriatric wards, these consequences may increase morbidity and mortality, while prolonging hospital stays, thereby also increasing exposure to hospital-acquired infections.7 Furthermore, constipation is also associated with impaired health-related quality of life.8
Management includes treating the cause, stopping contributing medications, early mobilization, diet modification, and, if all else fails, prescription laxatives. Therefore, early identification and appropriate treatment of constipation is beneficial in inpatient care, as well as when planning safe and patient-centered discharges.
Given the risks and complications of constipation in the elderly, we, a group of Foundation Year 2 (FY2) doctors in the UK Foundation Programme, decided to explore how doctors can help to recognize this condition early. Regular bowel movement documentation in patient notes on ward rounds is crucial, as it has been shown to reduce constipation-associated complications.5 However, complications from constipation can take significant amounts of time to develop and, therefore, documenting bowel movements on a daily basis is not necessary.
Based on these observations along with targets set out in previous studies,7 our aim was to improve documentation of bowel movement on ward rounds to 85% by March 2020.
Methods
Before the data collection process, a fishbone diagram was designed to identify the potential causes of poor documentation of bowel movement on geriatric wards. There were several aspects that were reviewed, including, for example, patients, health care professionals, organizational policies, procedures, and equipment. It was then decided to focus on raising awareness of the documentation of bowel movement by doctors specifically.
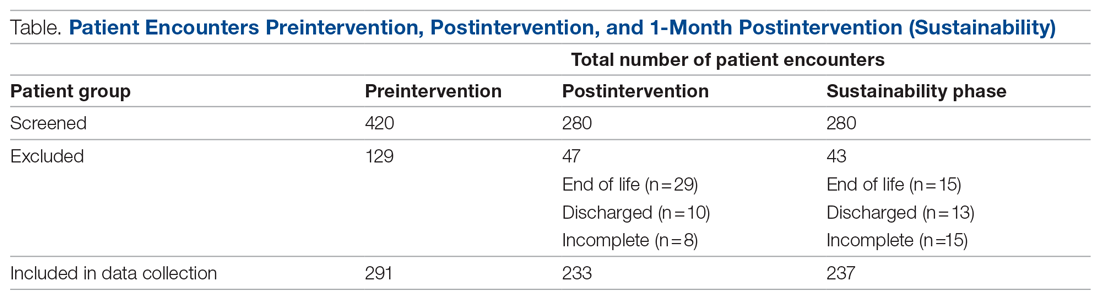
Retrospective data were collected from the inpatient paper notes of 28 patients on Brearley 6, a geriatric ward at the Northern General Hospital within Sheffield Teaching Hospitals (STH), on weekdays over a 3-week period. The baseline data collected included the bed number of the patient, whether or not bowel movement on initial ward round was documented, and whether it was the junior, registrar, or consultant leading the ward round. End-of-life and discharged patients were excluded (Table).
The interventions consisted of posters and stickers. Posters were displayed on Brearley 6, including the doctors’ office, nurses’ station, and around the bays where notes were kept, in order to emphasize their importance. The stickers of the poo emoji were also printed and placed at the front of each set of inpatient paper notes as a reminder for the doctor documenting on the ward round. The interventions were also introduced in the morning board meeting to ensure all staff on Brearley 6 were aware of them.
Data were collected on weekdays over a 3-week period starting 2 weeks after the interventions were put in place (Table). In order to assess that the intervention had been sustained, data were again collected 1 month later over a 2-week period (Table). Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) was used to analyze all data, and control charts were used to assess variability in the data.
Results
The baseline data showed that bowel movement was documented 60.49% of the time by doctors on the initial ward round before intervention, as illustrated in Figure 1. There was no evidence of an out-of-control process in this baseline data set.
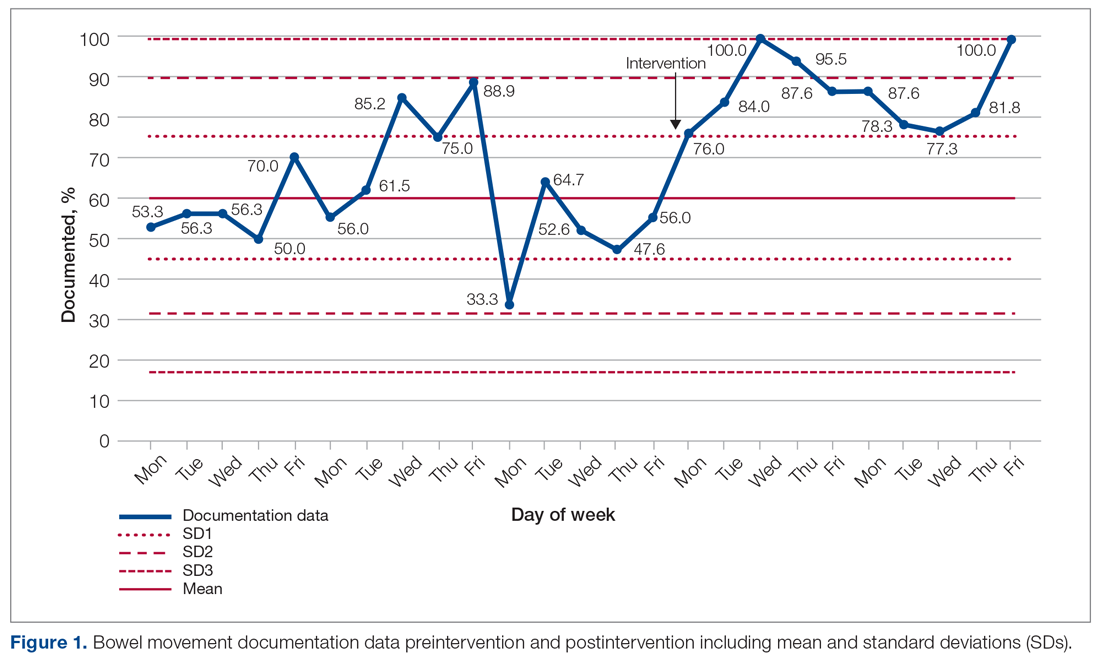
The comparison between the preintervention and postintervention data is illustrated in Figure 1. The postintervention data, which were taken 2 weeks after intervention, showed a significant increase in the documentation of bowel movements, to 86.78%. The figure displays a number of features consistent with an out-of-control process: beyond limits (≥ 1 points beyond control limits), Zone A rule (2 out of 3 consecutive points beyond 2 standard deviations from the mean), Zone B rule (4 out of 5 consecutive points beyond 1 standard deviation from the mean), and Zone C rule (≥ 8 consecutive points on 1 side of the mean). These findings demonstrate a special cause variation in the documentation of bowel movements.
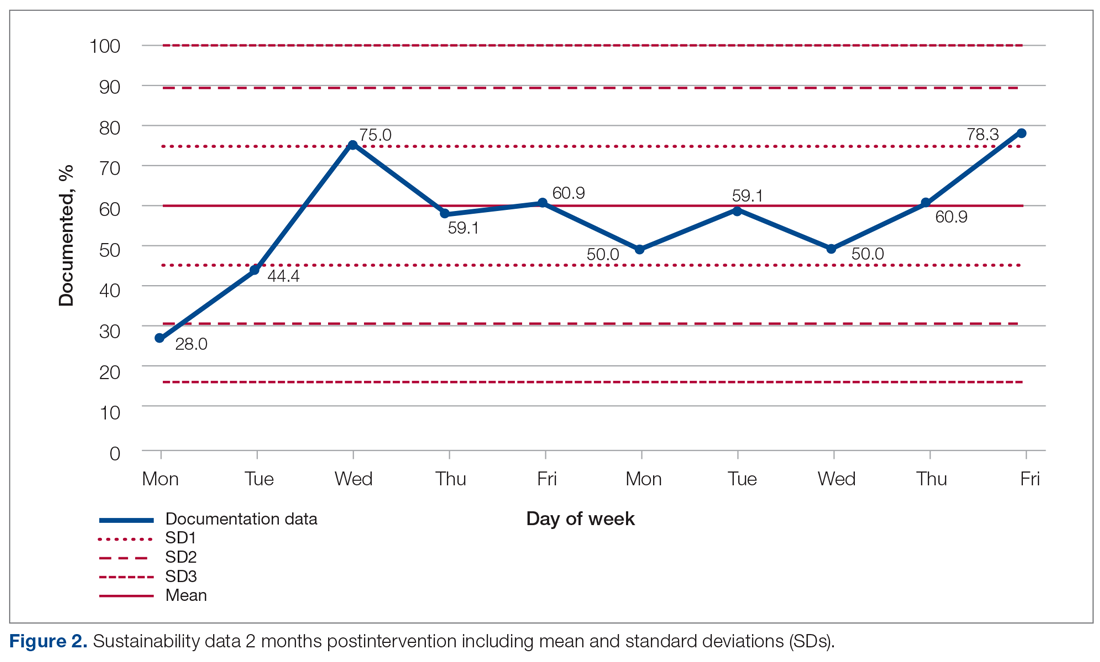
Figure 2 shows the sustainability of the intervention, which averaged 56.56% postintervention nearly 2 months later. The data returned to preintervention variability levels.
Discussion
Our project explored an important issue that was frequently encountered by department clinicians. Our team of FY2 doctors, in general, had little experience with quality improvement. We have developed our understanding and experience through planning, making, and measuring improvement.
It was challenging deciding on how to deal with the problem. A number of ways were considered to improve the paper rounding chart, but the nursing team had already planned to make changes to it. Bowel activity is mainly documented by nursing staff, but there was no specific protocol for recognizing constipation and when to inform the medical team. We decided to focus on doctors’ documentation in patient notes during the ward round, as this is where the decision regarding management of bowels is made, including interventions that could only be done by doctors, such as prescribing laxatives.
Strom et al9 have described a number of successful quality improvement interventions, and we decided to follow the authors’ guidance to implement a reminder system strategy using both posters and stickers to prompt doctors to document bowel activity. Both of these were simple, and the text on the poster was concise. The only cost incurred on the project was from printing the stickers; this totalled £2.99 (US $4.13). Individual stickers for each ward round entry were considered but not used, as it would create an additional task for doctors.
The data initially indicated that the interventions had their desired effect. However, this positive change was unsustainable, most likely suggesting that the novelty of the stickers and posters wore off at some point, leading to junior doctors no longer noticing them. Further Plan-Do-Study-Act cycles should examine the reasons why the change is difficult to sustain and implement new policies that aim to overcome them.
There were a number of limitations to this study. A patient could be discharged before data collection, which was done twice weekly. This could have resulted in missed data during the collection period. In addition, the accuracy of the documentation is dependent on nursing staff correctly recording—as well as the doctors correctly viewing—all sources of information on bowel activity. Observer bias is possible, too, as a steering group member was involved in data collection. Their awareness of the project could cause a positive skew in the data. And, unfortunately, the project came to an abrupt end because of COVID-19 cases on the ward.
We examined the daily documentation of bowel activity, which may not be necessary considering that internationally recognized constipation classifications, such as the Rome III criteria, define constipation as fewer than 3 bowel movements per week.10 However, the data collection sheet did not include patient identifiers, so it was impossible to determine whether bowel activity had been documented 3 or more times per week for each patient. This is important because a clinician may only decide to act if there is no bowel movement activity for 3 or more days.
Because our data were collected on a single geriatric ward, which had an emphasis on Parkinson’s disease, it is unclear whether our findings are generalizable to other clinical areas in STH. However, constipation is common in the elderly, so it is likely to be relevant to other wards, as more than a third of STH hospital beds are occupied by patients aged 75 years and older.11
Conclusion
Overall, our study highlights the fact that monitoring bowel activity is important on a geriatric ward. Recognizing constipation early prevents complications and delays to discharge. As mentioned earlier, our aim was achieved initially but not sustained. Therefore, future development should focus on sustainability. For example, laxative-focused ward rounds have shown to be effective at recognizing and preventing constipation by intervening early.12 Future cycles that we considered included using an electronic reminder on the hospital IT system, as the trust is aiming to introduce electronic documentation. Focus could also be placed on improving documentation in bowel charts by ward staff. This could be achieved by organizing regular educational sessions on the complications of constipation and when to inform the medical team regarding concerns.
Acknowledgments: The authors thank Dr. Jamie Kapur, Sheffield Teaching Hospitals, for his guidance and supervision, as well as our collaborators: Rachel Hallam, Claire Walker, Monisha Chakravorty, and Hamza Khan.
Corresponding author: Alexander P. Noar, BMBCh, BA, 10 Stanhope Gardens, London, N6 5TS; [email protected].
Financial disclosures: None.
1. Forootan M, Bagheri N, Darvishi M. Chronic constipation: A review of literature. Medicine (Baltimore). 2018;97:e10631. doi:10.1097/MD.00000000000.10631
2. Schuster BG, Kosar L, Kamrul R. Constipation in older adults: stepwise approach to keep things moving. Can Fam Physician. 2015;61:152-158.
3. Gray JR. What is chronic constipation? Definition and diagnosis. Can J Gastroenterol. 2011;25 (Suppl B):7B-10B.
4. American Gastroenterological Association, Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211-217. doi:10.1053/j.gastro.2012.10.029
5. Maung TZ, Singh K. Regular monitoring with stool chart prevents constipation, urinary retention and delirium in elderly patients: an audit leading to clinical effectiveness, efficiency and patient centredness. Future Healthc J. 2019;6(Suppl 2):3. doi:10.7861/futurehosp.6-2s-s3
6. Mostafa SM, Bhandari S, Ritchie G, et al. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003;91:815-819. doi:10.1093/bja/aeg275
7. Jackson R, Cheng P, Moreman S, et al. “The constipation conundrum”: Improving recognition of constipation on a gastroenterology ward. BMJ Qual Improv Rep. 2016;5(1):u212167.w3007. doi:10.1136/bmjquality.u212167.w3007
8. Rao S, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171. doi:10.2147/cia.s8100
9. Strom KL. Quality improvement interventions: what works? J Healthc Qual. 2001;23(5):4-24. doi:10.1111/j.1945-1474.2001.tb00368.x
10. De Giorgio R, Ruggeri E, Stanghellini V, et al. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi:10.1186/s12876-015-366-3
11. The Health Foundation. Improving the flow of older people. April 2013. Accessed August 11, 2021. https://www.england.nhs.uk/wp-content/uploads/2013/08/sheff-study.pdf
12. Linton A. Improving management of constipation in an inpatient setting using a care bundle. BMJ Qual Improv Rep. 2014;3(1):u201903.w1002. doi:10.1136/bmjquality.u201903.w1002
From Sheffield Teaching Hospitals, Sheffield, UK, S5 7AU.
Objective: Constipation is widely prevalent in older adults and may result in complications such as urinary retention, delirium, and bowel obstruction. Previous studies have indicated that while the nursing staff do well in completing stool charts, doctors monitor them infrequently. This project aimed to improve the documentation of bowel movement by doctors on ward rounds to 85%, by the end of a 3-month period.
Methods: Baseline, postintervention, and sustainability data were collected from inpatient notes on weekdays on a geriatric ward in Northern General Hospital, Sheffield, UK. Posters and stickers of the poo emoji were placed on walls and in inpatient notes, respectively, as a reminder.
Results: Data on bowel activity documentation were collected from 28 patients. The baseline data showed that bowel activity was monitored daily on the ward 60.49% of the time. However, following the interventions, there was a significant increase in documentation, to 86.78%. The sustainability study showed that bowel activity was documented on the ward 56.56% of the time.
Conclusion: This study shows how a strong initial effect on behavioral change can be accomplished through simple interventions such as stickers and posters. As most wards currently still use paper notes, this is a generalizable model that other wards can trial. However, this study also shows the difficulty in maintaining behavioral change over extended periods of time.
Keywords: bowel movement; documentation; obstruction; constipation; geriatrics; incontinence; junior doctor; quality improvement.
Constipation is widely prevalent in the elderly, encountered frequently in both community and hospital medicine.1 Its estimated prevalence in adults over 84 years old is 34% for women and 25% for men, rising to up to 80% for long-term care residents.2
Chronic constipation is generally characterized by unsatisfactory defecation due to infrequent bowel emptying or difficulty with stool passage, which may lead to incomplete evacuation.2-4 Constipation in the elderly, in addition to causing abdominal pain, nausea, and reduced appetite, may result in complications such as fecal incontinence (and overflow diarrhea), urinary retention, delirium, and bowel obstruction, which may in result in life-threatening perforation.5,6 For inpatients on geriatric wards, these consequences may increase morbidity and mortality, while prolonging hospital stays, thereby also increasing exposure to hospital-acquired infections.7 Furthermore, constipation is also associated with impaired health-related quality of life.8
Management includes treating the cause, stopping contributing medications, early mobilization, diet modification, and, if all else fails, prescription laxatives. Therefore, early identification and appropriate treatment of constipation is beneficial in inpatient care, as well as when planning safe and patient-centered discharges.
Given the risks and complications of constipation in the elderly, we, a group of Foundation Year 2 (FY2) doctors in the UK Foundation Programme, decided to explore how doctors can help to recognize this condition early. Regular bowel movement documentation in patient notes on ward rounds is crucial, as it has been shown to reduce constipation-associated complications.5 However, complications from constipation can take significant amounts of time to develop and, therefore, documenting bowel movements on a daily basis is not necessary.
Based on these observations along with targets set out in previous studies,7 our aim was to improve documentation of bowel movement on ward rounds to 85% by March 2020.
Methods
Before the data collection process, a fishbone diagram was designed to identify the potential causes of poor documentation of bowel movement on geriatric wards. There were several aspects that were reviewed, including, for example, patients, health care professionals, organizational policies, procedures, and equipment. It was then decided to focus on raising awareness of the documentation of bowel movement by doctors specifically.
Retrospective data were collected from the inpatient paper notes of 28 patients on Brearley 6, a geriatric ward at the Northern General Hospital within Sheffield Teaching Hospitals (STH), on weekdays over a 3-week period. The baseline data collected included the bed number of the patient, whether or not bowel movement on initial ward round was documented, and whether it was the junior, registrar, or consultant leading the ward round. End-of-life and discharged patients were excluded (Table).

The interventions consisted of posters and stickers. Posters were displayed on Brearley 6, including the doctors’ office, nurses’ station, and around the bays where notes were kept, in order to emphasize their importance. The stickers of the poo emoji were also printed and placed at the front of each set of inpatient paper notes as a reminder for the doctor documenting on the ward round. The interventions were also introduced in the morning board meeting to ensure all staff on Brearley 6 were aware of them.
Data were collected on weekdays over a 3-week period starting 2 weeks after the interventions were put in place (Table). In order to assess that the intervention had been sustained, data were again collected 1 month later over a 2-week period (Table). Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) was used to analyze all data, and control charts were used to assess variability in the data.
Results
The baseline data showed that bowel movement was documented 60.49% of the time by doctors on the initial ward round before intervention, as illustrated in Figure 1. There was no evidence of an out-of-control process in this baseline data set.

The comparison between the preintervention and postintervention data is illustrated in Figure 1. The postintervention data, which were taken 2 weeks after intervention, showed a significant increase in the documentation of bowel movements, to 86.78%. The figure displays a number of features consistent with an out-of-control process: beyond limits (≥ 1 points beyond control limits), Zone A rule (2 out of 3 consecutive points beyond 2 standard deviations from the mean), Zone B rule (4 out of 5 consecutive points beyond 1 standard deviation from the mean), and Zone C rule (≥ 8 consecutive points on 1 side of the mean). These findings demonstrate a special cause variation in the documentation of bowel movements.
Figure 2 shows the sustainability of the intervention, which averaged 56.56% postintervention nearly 2 months later. The data returned to preintervention variability levels.

Discussion
Our project explored an important issue that was frequently encountered by department clinicians. Our team of FY2 doctors, in general, had little experience with quality improvement. We have developed our understanding and experience through planning, making, and measuring improvement.
It was challenging deciding on how to deal with the problem. A number of ways were considered to improve the paper rounding chart, but the nursing team had already planned to make changes to it. Bowel activity is mainly documented by nursing staff, but there was no specific protocol for recognizing constipation and when to inform the medical team. We decided to focus on doctors’ documentation in patient notes during the ward round, as this is where the decision regarding management of bowels is made, including interventions that could only be done by doctors, such as prescribing laxatives.
Strom et al9 have described a number of successful quality improvement interventions, and we decided to follow the authors’ guidance to implement a reminder system strategy using both posters and stickers to prompt doctors to document bowel activity. Both of these were simple, and the text on the poster was concise. The only cost incurred on the project was from printing the stickers; this totalled £2.99 (US $4.13). Individual stickers for each ward round entry were considered but not used, as it would create an additional task for doctors.
The data initially indicated that the interventions had their desired effect. However, this positive change was unsustainable, most likely suggesting that the novelty of the stickers and posters wore off at some point, leading to junior doctors no longer noticing them. Further Plan-Do-Study-Act cycles should examine the reasons why the change is difficult to sustain and implement new policies that aim to overcome them.
There were a number of limitations to this study. A patient could be discharged before data collection, which was done twice weekly. This could have resulted in missed data during the collection period. In addition, the accuracy of the documentation is dependent on nursing staff correctly recording—as well as the doctors correctly viewing—all sources of information on bowel activity. Observer bias is possible, too, as a steering group member was involved in data collection. Their awareness of the project could cause a positive skew in the data. And, unfortunately, the project came to an abrupt end because of COVID-19 cases on the ward.
We examined the daily documentation of bowel activity, which may not be necessary considering that internationally recognized constipation classifications, such as the Rome III criteria, define constipation as fewer than 3 bowel movements per week.10 However, the data collection sheet did not include patient identifiers, so it was impossible to determine whether bowel activity had been documented 3 or more times per week for each patient. This is important because a clinician may only decide to act if there is no bowel movement activity for 3 or more days.
Because our data were collected on a single geriatric ward, which had an emphasis on Parkinson’s disease, it is unclear whether our findings are generalizable to other clinical areas in STH. However, constipation is common in the elderly, so it is likely to be relevant to other wards, as more than a third of STH hospital beds are occupied by patients aged 75 years and older.11
Conclusion
Overall, our study highlights the fact that monitoring bowel activity is important on a geriatric ward. Recognizing constipation early prevents complications and delays to discharge. As mentioned earlier, our aim was achieved initially but not sustained. Therefore, future development should focus on sustainability. For example, laxative-focused ward rounds have shown to be effective at recognizing and preventing constipation by intervening early.12 Future cycles that we considered included using an electronic reminder on the hospital IT system, as the trust is aiming to introduce electronic documentation. Focus could also be placed on improving documentation in bowel charts by ward staff. This could be achieved by organizing regular educational sessions on the complications of constipation and when to inform the medical team regarding concerns.
Acknowledgments: The authors thank Dr. Jamie Kapur, Sheffield Teaching Hospitals, for his guidance and supervision, as well as our collaborators: Rachel Hallam, Claire Walker, Monisha Chakravorty, and Hamza Khan.
Corresponding author: Alexander P. Noar, BMBCh, BA, 10 Stanhope Gardens, London, N6 5TS; [email protected].
Financial disclosures: None.
From Sheffield Teaching Hospitals, Sheffield, UK, S5 7AU.
Objective: Constipation is widely prevalent in older adults and may result in complications such as urinary retention, delirium, and bowel obstruction. Previous studies have indicated that while the nursing staff do well in completing stool charts, doctors monitor them infrequently. This project aimed to improve the documentation of bowel movement by doctors on ward rounds to 85%, by the end of a 3-month period.
Methods: Baseline, postintervention, and sustainability data were collected from inpatient notes on weekdays on a geriatric ward in Northern General Hospital, Sheffield, UK. Posters and stickers of the poo emoji were placed on walls and in inpatient notes, respectively, as a reminder.
Results: Data on bowel activity documentation were collected from 28 patients. The baseline data showed that bowel activity was monitored daily on the ward 60.49% of the time. However, following the interventions, there was a significant increase in documentation, to 86.78%. The sustainability study showed that bowel activity was documented on the ward 56.56% of the time.
Conclusion: This study shows how a strong initial effect on behavioral change can be accomplished through simple interventions such as stickers and posters. As most wards currently still use paper notes, this is a generalizable model that other wards can trial. However, this study also shows the difficulty in maintaining behavioral change over extended periods of time.
Keywords: bowel movement; documentation; obstruction; constipation; geriatrics; incontinence; junior doctor; quality improvement.
Constipation is widely prevalent in the elderly, encountered frequently in both community and hospital medicine.1 Its estimated prevalence in adults over 84 years old is 34% for women and 25% for men, rising to up to 80% for long-term care residents.2
Chronic constipation is generally characterized by unsatisfactory defecation due to infrequent bowel emptying or difficulty with stool passage, which may lead to incomplete evacuation.2-4 Constipation in the elderly, in addition to causing abdominal pain, nausea, and reduced appetite, may result in complications such as fecal incontinence (and overflow diarrhea), urinary retention, delirium, and bowel obstruction, which may in result in life-threatening perforation.5,6 For inpatients on geriatric wards, these consequences may increase morbidity and mortality, while prolonging hospital stays, thereby also increasing exposure to hospital-acquired infections.7 Furthermore, constipation is also associated with impaired health-related quality of life.8
Management includes treating the cause, stopping contributing medications, early mobilization, diet modification, and, if all else fails, prescription laxatives. Therefore, early identification and appropriate treatment of constipation is beneficial in inpatient care, as well as when planning safe and patient-centered discharges.
Given the risks and complications of constipation in the elderly, we, a group of Foundation Year 2 (FY2) doctors in the UK Foundation Programme, decided to explore how doctors can help to recognize this condition early. Regular bowel movement documentation in patient notes on ward rounds is crucial, as it has been shown to reduce constipation-associated complications.5 However, complications from constipation can take significant amounts of time to develop and, therefore, documenting bowel movements on a daily basis is not necessary.
Based on these observations along with targets set out in previous studies,7 our aim was to improve documentation of bowel movement on ward rounds to 85% by March 2020.
Methods
Before the data collection process, a fishbone diagram was designed to identify the potential causes of poor documentation of bowel movement on geriatric wards. There were several aspects that were reviewed, including, for example, patients, health care professionals, organizational policies, procedures, and equipment. It was then decided to focus on raising awareness of the documentation of bowel movement by doctors specifically.
Retrospective data were collected from the inpatient paper notes of 28 patients on Brearley 6, a geriatric ward at the Northern General Hospital within Sheffield Teaching Hospitals (STH), on weekdays over a 3-week period. The baseline data collected included the bed number of the patient, whether or not bowel movement on initial ward round was documented, and whether it was the junior, registrar, or consultant leading the ward round. End-of-life and discharged patients were excluded (Table).

The interventions consisted of posters and stickers. Posters were displayed on Brearley 6, including the doctors’ office, nurses’ station, and around the bays where notes were kept, in order to emphasize their importance. The stickers of the poo emoji were also printed and placed at the front of each set of inpatient paper notes as a reminder for the doctor documenting on the ward round. The interventions were also introduced in the morning board meeting to ensure all staff on Brearley 6 were aware of them.
Data were collected on weekdays over a 3-week period starting 2 weeks after the interventions were put in place (Table). In order to assess that the intervention had been sustained, data were again collected 1 month later over a 2-week period (Table). Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) was used to analyze all data, and control charts were used to assess variability in the data.
Results
The baseline data showed that bowel movement was documented 60.49% of the time by doctors on the initial ward round before intervention, as illustrated in Figure 1. There was no evidence of an out-of-control process in this baseline data set.

The comparison between the preintervention and postintervention data is illustrated in Figure 1. The postintervention data, which were taken 2 weeks after intervention, showed a significant increase in the documentation of bowel movements, to 86.78%. The figure displays a number of features consistent with an out-of-control process: beyond limits (≥ 1 points beyond control limits), Zone A rule (2 out of 3 consecutive points beyond 2 standard deviations from the mean), Zone B rule (4 out of 5 consecutive points beyond 1 standard deviation from the mean), and Zone C rule (≥ 8 consecutive points on 1 side of the mean). These findings demonstrate a special cause variation in the documentation of bowel movements.
Figure 2 shows the sustainability of the intervention, which averaged 56.56% postintervention nearly 2 months later. The data returned to preintervention variability levels.

Discussion
Our project explored an important issue that was frequently encountered by department clinicians. Our team of FY2 doctors, in general, had little experience with quality improvement. We have developed our understanding and experience through planning, making, and measuring improvement.
It was challenging deciding on how to deal with the problem. A number of ways were considered to improve the paper rounding chart, but the nursing team had already planned to make changes to it. Bowel activity is mainly documented by nursing staff, but there was no specific protocol for recognizing constipation and when to inform the medical team. We decided to focus on doctors’ documentation in patient notes during the ward round, as this is where the decision regarding management of bowels is made, including interventions that could only be done by doctors, such as prescribing laxatives.
Strom et al9 have described a number of successful quality improvement interventions, and we decided to follow the authors’ guidance to implement a reminder system strategy using both posters and stickers to prompt doctors to document bowel activity. Both of these were simple, and the text on the poster was concise. The only cost incurred on the project was from printing the stickers; this totalled £2.99 (US $4.13). Individual stickers for each ward round entry were considered but not used, as it would create an additional task for doctors.
The data initially indicated that the interventions had their desired effect. However, this positive change was unsustainable, most likely suggesting that the novelty of the stickers and posters wore off at some point, leading to junior doctors no longer noticing them. Further Plan-Do-Study-Act cycles should examine the reasons why the change is difficult to sustain and implement new policies that aim to overcome them.
There were a number of limitations to this study. A patient could be discharged before data collection, which was done twice weekly. This could have resulted in missed data during the collection period. In addition, the accuracy of the documentation is dependent on nursing staff correctly recording—as well as the doctors correctly viewing—all sources of information on bowel activity. Observer bias is possible, too, as a steering group member was involved in data collection. Their awareness of the project could cause a positive skew in the data. And, unfortunately, the project came to an abrupt end because of COVID-19 cases on the ward.
We examined the daily documentation of bowel activity, which may not be necessary considering that internationally recognized constipation classifications, such as the Rome III criteria, define constipation as fewer than 3 bowel movements per week.10 However, the data collection sheet did not include patient identifiers, so it was impossible to determine whether bowel activity had been documented 3 or more times per week for each patient. This is important because a clinician may only decide to act if there is no bowel movement activity for 3 or more days.
Because our data were collected on a single geriatric ward, which had an emphasis on Parkinson’s disease, it is unclear whether our findings are generalizable to other clinical areas in STH. However, constipation is common in the elderly, so it is likely to be relevant to other wards, as more than a third of STH hospital beds are occupied by patients aged 75 years and older.11
Conclusion
Overall, our study highlights the fact that monitoring bowel activity is important on a geriatric ward. Recognizing constipation early prevents complications and delays to discharge. As mentioned earlier, our aim was achieved initially but not sustained. Therefore, future development should focus on sustainability. For example, laxative-focused ward rounds have shown to be effective at recognizing and preventing constipation by intervening early.12 Future cycles that we considered included using an electronic reminder on the hospital IT system, as the trust is aiming to introduce electronic documentation. Focus could also be placed on improving documentation in bowel charts by ward staff. This could be achieved by organizing regular educational sessions on the complications of constipation and when to inform the medical team regarding concerns.
Acknowledgments: The authors thank Dr. Jamie Kapur, Sheffield Teaching Hospitals, for his guidance and supervision, as well as our collaborators: Rachel Hallam, Claire Walker, Monisha Chakravorty, and Hamza Khan.
Corresponding author: Alexander P. Noar, BMBCh, BA, 10 Stanhope Gardens, London, N6 5TS; [email protected].
Financial disclosures: None.
1. Forootan M, Bagheri N, Darvishi M. Chronic constipation: A review of literature. Medicine (Baltimore). 2018;97:e10631. doi:10.1097/MD.00000000000.10631
2. Schuster BG, Kosar L, Kamrul R. Constipation in older adults: stepwise approach to keep things moving. Can Fam Physician. 2015;61:152-158.
3. Gray JR. What is chronic constipation? Definition and diagnosis. Can J Gastroenterol. 2011;25 (Suppl B):7B-10B.
4. American Gastroenterological Association, Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211-217. doi:10.1053/j.gastro.2012.10.029
5. Maung TZ, Singh K. Regular monitoring with stool chart prevents constipation, urinary retention and delirium in elderly patients: an audit leading to clinical effectiveness, efficiency and patient centredness. Future Healthc J. 2019;6(Suppl 2):3. doi:10.7861/futurehosp.6-2s-s3
6. Mostafa SM, Bhandari S, Ritchie G, et al. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003;91:815-819. doi:10.1093/bja/aeg275
7. Jackson R, Cheng P, Moreman S, et al. “The constipation conundrum”: Improving recognition of constipation on a gastroenterology ward. BMJ Qual Improv Rep. 2016;5(1):u212167.w3007. doi:10.1136/bmjquality.u212167.w3007
8. Rao S, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171. doi:10.2147/cia.s8100
9. Strom KL. Quality improvement interventions: what works? J Healthc Qual. 2001;23(5):4-24. doi:10.1111/j.1945-1474.2001.tb00368.x
10. De Giorgio R, Ruggeri E, Stanghellini V, et al. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi:10.1186/s12876-015-366-3
11. The Health Foundation. Improving the flow of older people. April 2013. Accessed August 11, 2021. https://www.england.nhs.uk/wp-content/uploads/2013/08/sheff-study.pdf
12. Linton A. Improving management of constipation in an inpatient setting using a care bundle. BMJ Qual Improv Rep. 2014;3(1):u201903.w1002. doi:10.1136/bmjquality.u201903.w1002
1. Forootan M, Bagheri N, Darvishi M. Chronic constipation: A review of literature. Medicine (Baltimore). 2018;97:e10631. doi:10.1097/MD.00000000000.10631
2. Schuster BG, Kosar L, Kamrul R. Constipation in older adults: stepwise approach to keep things moving. Can Fam Physician. 2015;61:152-158.
3. Gray JR. What is chronic constipation? Definition and diagnosis. Can J Gastroenterol. 2011;25 (Suppl B):7B-10B.
4. American Gastroenterological Association, Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211-217. doi:10.1053/j.gastro.2012.10.029
5. Maung TZ, Singh K. Regular monitoring with stool chart prevents constipation, urinary retention and delirium in elderly patients: an audit leading to clinical effectiveness, efficiency and patient centredness. Future Healthc J. 2019;6(Suppl 2):3. doi:10.7861/futurehosp.6-2s-s3
6. Mostafa SM, Bhandari S, Ritchie G, et al. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003;91:815-819. doi:10.1093/bja/aeg275
7. Jackson R, Cheng P, Moreman S, et al. “The constipation conundrum”: Improving recognition of constipation on a gastroenterology ward. BMJ Qual Improv Rep. 2016;5(1):u212167.w3007. doi:10.1136/bmjquality.u212167.w3007
8. Rao S, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163-171. doi:10.2147/cia.s8100
9. Strom KL. Quality improvement interventions: what works? J Healthc Qual. 2001;23(5):4-24. doi:10.1111/j.1945-1474.2001.tb00368.x
10. De Giorgio R, Ruggeri E, Stanghellini V, et al. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi:10.1186/s12876-015-366-3
11. The Health Foundation. Improving the flow of older people. April 2013. Accessed August 11, 2021. https://www.england.nhs.uk/wp-content/uploads/2013/08/sheff-study.pdf
12. Linton A. Improving management of constipation in an inpatient setting using a care bundle. BMJ Qual Improv Rep. 2014;3(1):u201903.w1002. doi:10.1136/bmjquality.u201903.w1002
Feasibility of a Saliva-Based COVID-19 Screening Program in Abu Dhabi Primary Schools
From Health Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates (Dr. Virji and Aisha Al Hamiz), Public Health, Abu Dhabi Public Health Center, Abu Dhabi, United Arab Emirates (Drs. Al Hajeri, Al Shehhi, Al Memari, and Ahlam Al Maskari), College of Medicine and Health Sciences, Khalifa University, Abu Dhabi, United Arab Emirates, Department of Medicine, Sheikh Shakhbout Medical City, Abu Dhabi, United Arab Emirates (Dr. Alhajri), Public Health Research Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates, Oxford University Hospitals NHS Foundation Trust, Oxford, England, and the MRC Epidemiology Unit, University of Cambridge, Cambridge, England (Dr. Ali).
Objective: The pandemic has forced closures of primary schools, resulting in loss of learning time on a global scale. In addition to face coverings, social distancing, and hand hygiene, an efficient testing method is important to mitigate the spread of COVID-19 in schools. We evaluated the feasibility of a saliva-based SARS-CoV-2 polymerase chain reaction testing program among 18 primary schools in the Emirate of Abu Dhabi, United Arab Emirates. Qualitative results show that children 4 to 5 years old had difficulty producing an adequate saliva specimen compared to those 6 to 12 years old.
Methods: A short training video on saliva collection beforehand helps demystify the process for students and parents alike. Informed consent was challenging yet should be done beforehand by school health nurses or other medical professionals to reassure parents and maximize participation.
Results: Telephone interviews with school administrators resulted in an 83% response rate. Overall, 93% of school administrators had a positive experience with saliva testing and felt the program improved the safety of their schools. The ongoing use of saliva testing for SARS-CoV-2 was supported by 73% of respondents.
Conclusion: On-campus saliva testing is a feasible option for primary schools to screen for COVID-19 in their student population to help keep their campuses safe and open for learning.
Keywords: COVID-19; saliva testing; mitigation; primary school.
The COVID-19 pandemic is a leading cause of morbidity and mortality worldwide and continues to exhaust health care resources on a large scale.1 Efficient testing is critical to identify cases early and to help mitigate the deleterious effects of the pandemic.2 Saliva polymerase chain reaction (PCR) nucleic acid amplification testing (NAAT) is more comfortable than nasopharyngeal (NP) NAAT and has been validated as a test for SARS-CoV-2.1 Although children are less susceptible to severe disease, primary schools are considered a vector for transmission and community spread.3 Efficient and scalable methods of routine testing are needed globally to help keep schools open. Saliva testing has proven a useful resource for this population.4,5
Abu Dhabi is the largest Emirate in the United Arab Emirates (UAE), with an estimated population of 2.5 million.6 The first case of COVID-19 was discovered in the UAE on January 29, 2020.7 The UAE has been recognized worldwide for its robust pandemic response. Along with the coordinated and swift application of public health measures, the country has one of the highest COVID-19 testing rates per capita and one of the highest vaccination rates worldwide.8,9 The Abu Dhabi Public Health Center (ADPHC) works alongside the Ministry of Education (MOE) to establish testing, quarantine, and general safety guidelines for primary schools. In December 2020, the ADPHC partnered with a local, accredited diagnostic laboratory to test the feasibility of a saliva-based screening program for COVID-19 directly on school campuses for 18 primary schools in the Emirate.
Saliva-based PCR testing for COVID-19 was approved for use in schools in the UAE on January 24, 2021.10 As part of a greater mitigation strategy to reduce both school-based transmission and, hence, community spread, the ADPHC focused its on-site testing program on children aged 4 to 12 years. The program required collaboration among medical professionals, school administrators and teachers, students, and parents. Our study evaluates the feasibility of implementing a saliva-based COVID-19 screening program directly on primary school campuses involving children as young as 4 years of age.
Methods
The ADPHC, in collaboration with G42 Biogenix Labs, conducted a saliva SARS-CoV-2 NAAT testing program in 18 primary schools in the Emirate. Schools were selected based on outbreak prevalence at the time and focused on “hot spot” areas. The school on-site saliva testing program included children aged 4 to 12 years old in a “bubble” attendance model during the school day. This model involved children being assigned to groups or “pods.” This allowed us to limit a potential outbreak to a single pod, as opposed to risk exposing the entire school, should a single student test positive. The well-established SalivaDirect protocol developed at Yale University was used for testing and included an RNA extraction-free, RT-qPCR method for SARS-CoV-2 detection.11
We conducted a qualitative study involving telephone interviews of school administrators to evaluate their experience with the ADPHC testing program at their schools. In addition, we interviewed the G42 Biogenix Lab providers to understand the logistics that supported on-campus collection of saliva specimens for this age group. We also gathered the attitudes of school children before and after testing. This study was reviewed and approved by the Abu Dhabi Health Research and Technology Committee and the Institutional Review Board (IRB), New York University Abu Dhabi (NYUAD).
Sample and recruitment
The original sample collection of saliva specimens was performed by the ADPHC in collaboration with G42 Biogenix Lab providers on school campuses between December 6 and December 10, 2020. During this time, schools operated in a hybrid teaching model, where learning took place both online and in person. Infection control measures were deployed based on ADPHC standards and guidelines. Nurses utilized appropriate patient protective equipment, frequent hand hygiene, and social distancing during the collection process. Inclusion criteria included asymptomatic students aged 4 to 12 years attending in-person classes on campus. Students with respiratory symptoms who were asked to stay home or those not attending in-person classes were excluded.
Data collection
Data with regard to school children’s attitudes before and after testing were compiled through an online survey sent randomly to participants postintervention. Data from school administrators were collected through video and telephone interviews between April 14 and April 29, 2021. We first interviewed G42 Biogenix Lab providers to obtain previously acquired qualitative and quantitative data, which were collected during the intervention itself. After obtaining this information, we designed a questionnaire and proceeded with a structured interview process for school officials.
We interviewed school principals and administrators to collect their overall experiences with the saliva testing program. Before starting each interview, we established the interviewees preferred language, either English or Arabic. We then introduced the meeting attendees and provided study details, aims, and objectives, and described collaborating entities. We obtained verbal informed consent from a script approved by the NYUAD IRB and then proceeded with the interview, which included 4 questions. The first 3 questions were answered on a 5-point Likert scale model that consisted of 5 answer options: 5 being completely agree, 4 agree, 3 somewhat agree, 2 somewhat disagree, and 1 completely disagree. The fourth question invited open-ended feedback and comments on the following statements:
- I believe the COVID-19 saliva testing program improved the safety for my school campus.
- Our community had an overall positive experience with the COVID saliva testing.
- We would like to continue a saliva-based COVID testing program on our school campus.
- Please provide any additional comments you feel important about the program.
During the interview, we transcribed the answers as the interviewee was answering. We then translated those in Arabic into English and collected the data in 1 Excel spreadsheet. School interviewees and school names were de-identified in the collection and storage process.
Results
A total of 2011 saliva samples were collected from 18 different primary school campuses. Samples were sent the same day to G42 Biogenix Labs in Abu Dhabi for COVID PCR testing. A team consisting of 5 doctors providing general oversight, along with 2 to 6 nurses per site, were able to manage the collection process for all 18 school campuses. Samples were collected between 8
Sample stations were set up in either the school auditorium or gymnasium to ensure appropriate crowd control and ventilation. Teachers and other school staff, including public safety, were able to manage lines and the shuttling of students back and forth from classes to testing stations, which allowed medical staff to focus on sample collection.
Informed consent was obtained by prior electronic communication to parents from school staff, asking them to agree to allow their child to participate in the testing program. Informed consent was identified as a challenge: Getting parents to understand that saliva testing was more comfortable than NP testing, and that the results were only being used to help keep the school safe, took time. School staff are used to obtaining consent from parents for field trips, but this was clearly more challenging for them.
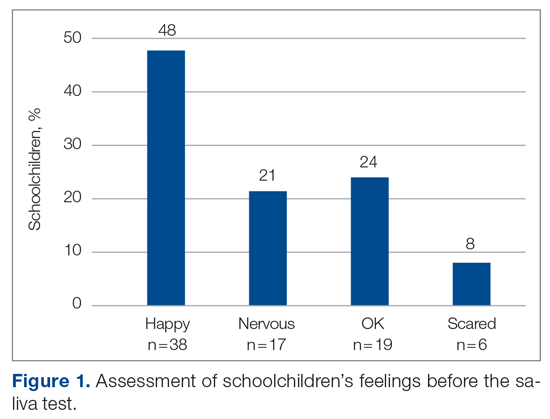
The saliva collection process per child took more time than expected. Children fasted for 45 minutes before saliva collection. We used an active drool technique, which required children to pool saliva in their mouth then express it into a collection tube. Adults can generally do this on command, but we found it took 10 to 12 minutes per child. Saliva production was cued by asking the children to think about food, and by showing them pictures and TV commercials depicting food. Children 4 to 5 years old had more difficulty with the process despite active cueing, while those 6 to 12 years old had an easier time with the process. We collected data on a cohort of 80 children regarding their attitudes pre (Figure 1) and post collection (Figure 2). Children felt happier, less nervous, and less scared after collection than before collection. This trend reassured us that future collections would be easier for students.
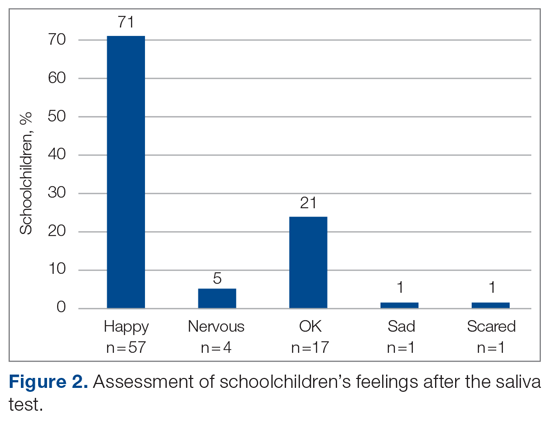
A total of 15 of 18 school principals completed the telephone interview, yielding a response rate of 83%. Overall, 93% of the school principals agreed or completely agreed that the COVID-19 saliva testing program improved school safety; 93% agreed or completely agreed that they had an overall positive experience with the program; and 73% supported the ongoing use of saliva testing in their schools (Table 1). Administrators’ open-ended comments on their experience were positive overall (Table 2).
Discussion
By March 2020, many kindergarten to grade 12 public and private schools suspended in-person classes due to the pandemic and turned to online learning platforms. The negative impact of school closures on academic achievement is projected to be significant.7,12,13 Ensuring schools can stay open and run operations safely will require routine SARS-CoV-2 testing. Our study investigated the feasibility of routine saliva testing on children aged 4 to 12 years on their school campuses. The ADPHC school on-site saliva testing program involved bringing lab providers onto 18 primary school campuses and required cooperation among parents, students, school administrators, and health care professionals.

Children younger than 6 years had difficulty producing an adequate saliva specimen, whereas those 6 to 12 years did so with relative ease when cued by thoughts or pictures of food while waiting in line for collection. Schools considering on-site testing programs should consider the age range of 6 to 12 years as a viable age range for saliva screening. Children should fast for a minimum of 45 minutes prior to saliva collection and should be cued by thoughts of food, food pictures, or food commercials. Setting up a sampling station close to the cafeteria where students can smell meal preparation may also help.14,15 Sampling before breakfast or lunch, when children are potentially at their hungriest, should also be considered.
The greatest challenge was obtaining informed consent from parents who were not yet familiar with the reliability of saliva testing as a tool for SARS-CoV-2 screening or with the saliva collection process as a whole. Informed consent was initially done electronically, lacking direct human interaction to answer parents’ questions. Parents who refused had a follow-up call from the school nurse to further explain the logistics and rationale for saliva screening. Having medical professionals directly answer parents’ questions was helpful. Parents were reassured that the process was painless, confidential, and only to be used for school safety purposes. Despite school administrators being experienced in obtaining consent from parents for field trips, obtaining informed consent for a medical testing procedure is more complicated, and parents aren’t accustomed to providing such consent in a school environment. Schools considering on-site testing should ensure that their school nurse or other health care providers are on the front line obtaining informed consent and allaying parents’ fears.
School staff were able to effectively provide crowd control for testing, and children felt at ease being in a familiar environment. Teachers and public safety officers are well-equipped at managing the shuttling of students to class, to lunch, to physical education, and, finally, to dismissal. They were equally equipped at handling the logistics of students to and from testing, including minimizing crowds and helping students feel at ease during the process. This effective collaboration allowed the lab personnel to focus on sample collection and storage, while school staff managed all other aspects of the children’s safety and care.
Conclusion
Overall, school administrators had a positive experience with the testing program, felt the program improved the safety of their schools, and supported the ongoing use of saliva testing for SARS-CoV-2 on their school campuses. Children aged 6 years and older were able to provide adequate saliva samples, and children felt happier and less nervous after the process, indicating repeatability. Our findings highlight the feasibility of an integrated on-site saliva testing model for primary school campuses. Further research is needed to determine the scalability of such a model and whether the added compliance and safety of on-site testing compensates for the potential loss of learning time that testing during school hours would require.
Corresponding author: Ayaz Virji, MD, New York University Abu Dhabi, PO Box 129188, Abu Dhabi, United Arab Emirates; [email protected].
Financial disclosures: None.
1. Kuehn BM. Despite improvements, COVID-19’s health care disruptions persist. JAMA. 2021;325(23):2335. doi:10.1001/jama.2021.9134
2. National Institute on Aging. Why COVID-19 testing is the key to getting back to normal. September 4, 2020. Accessed September 8, 2021. https://www.nia.nih.gov/news/why-covid-19-testing-key-getting-back-normal
3. Centers for Disease Control and Prevention. Science brief: Transmission of SARS-CoV-2 in K-12 schools. Updated July 9, 2021. Accessed September 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/transmission_k_12_schools.html
4. Butler-Laporte G, Lawandi A, Schiller I, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med. 2021;181(3):353-360. doi:10.1001/jamainternmed.2020.8876
5. Al Suwaidi H, Senok A, Varghese R, et al. Saliva for molecular detection of SARS-CoV-2 in school-age children. Clin Microbiol Infect. 2021;27(9):1330-1335. doi:10.1016/j.cmi.2021.02.009
6. Abu Dhabi. Accessed September 8, 2021. https://u.ae/en/about-the-uae/the-seven-emirates/abu-dhabi
7. Alsuwaidi AR, Al Hosani FI, Al Memari S, et al. Seroprevalence of COVID-19 infection in the Emirate of Abu Dhabi, United Arab Emirates: a population-based cross-sectional study. Int J Epidemiol. 2021;50(4):1077-1090. doi:10.1093/ije/dyab077
8. Al Hosany F, Ganesan S, Al Memari S, et al. Response to COVID-19 pandemic in the UAE: a public health perspective. J Glob Health. 2021;11:03050. doi:10.7189/jogh.11.03050
9. Bremmer I. The best global responses to the COVID-19 pandemic, 1 year later. Time Magazine. Updated February 23, 2021. Accessed September 8, 2021. https://time.com/5851633/best-global-responses-covid-19/
10. Department of Health, Abu Dhabi. Laboratory diagnostic test for COVID-19: update regarding saliva-based testing using RT-PCR test. 2021.
11. Vogels C, Brackney DE, Kalinich CC, et al. SalivaDirect: RNA extraction-free SARS-CoV-2 diagnostics. Protocols.io. Accessed September 8, 2021. https://www.protocols.io/view/salivadirect-rna-extraction-free-sars-cov-2-diagno-bh6jj9cn?version_warning=no
12. Education Endowment Foundation. Impact of school closures on the attainment gap: rapid evidence assessment. June 2020. Accessed September 8, 2021. https://www.researchgate.net/publication/342501263_EEF_2020_-_Impact_of_School_Closures_on_the_Attainment_Gap
13. United Nations. Policy brief: Education during COVID-19 and beyond. Accessed September 8, 2021. https://www.un.org/development/desa/dspd/wp-content/uploads/sites/22/2020/08/sg_policy_brief_covid-19_and_education_august_2020.pdf
14. Schiffman SS, Miletic ID. Effect of taste and smell on secretion rate of salivary IgA in elderly and young persons. J Nutr Health Aging. 1999;3(3):158-164.
15. Lee VM, Linden RW. The effect of odours on stimulated parotid salivary flow in humans. Physiol Behav. 1992;52(6):1121-1125. doi:10.1016/0031-9384(92)90470-m
From Health Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates (Dr. Virji and Aisha Al Hamiz), Public Health, Abu Dhabi Public Health Center, Abu Dhabi, United Arab Emirates (Drs. Al Hajeri, Al Shehhi, Al Memari, and Ahlam Al Maskari), College of Medicine and Health Sciences, Khalifa University, Abu Dhabi, United Arab Emirates, Department of Medicine, Sheikh Shakhbout Medical City, Abu Dhabi, United Arab Emirates (Dr. Alhajri), Public Health Research Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates, Oxford University Hospitals NHS Foundation Trust, Oxford, England, and the MRC Epidemiology Unit, University of Cambridge, Cambridge, England (Dr. Ali).
Objective: The pandemic has forced closures of primary schools, resulting in loss of learning time on a global scale. In addition to face coverings, social distancing, and hand hygiene, an efficient testing method is important to mitigate the spread of COVID-19 in schools. We evaluated the feasibility of a saliva-based SARS-CoV-2 polymerase chain reaction testing program among 18 primary schools in the Emirate of Abu Dhabi, United Arab Emirates. Qualitative results show that children 4 to 5 years old had difficulty producing an adequate saliva specimen compared to those 6 to 12 years old.
Methods: A short training video on saliva collection beforehand helps demystify the process for students and parents alike. Informed consent was challenging yet should be done beforehand by school health nurses or other medical professionals to reassure parents and maximize participation.
Results: Telephone interviews with school administrators resulted in an 83% response rate. Overall, 93% of school administrators had a positive experience with saliva testing and felt the program improved the safety of their schools. The ongoing use of saliva testing for SARS-CoV-2 was supported by 73% of respondents.
Conclusion: On-campus saliva testing is a feasible option for primary schools to screen for COVID-19 in their student population to help keep their campuses safe and open for learning.
Keywords: COVID-19; saliva testing; mitigation; primary school.
The COVID-19 pandemic is a leading cause of morbidity and mortality worldwide and continues to exhaust health care resources on a large scale.1 Efficient testing is critical to identify cases early and to help mitigate the deleterious effects of the pandemic.2 Saliva polymerase chain reaction (PCR) nucleic acid amplification testing (NAAT) is more comfortable than nasopharyngeal (NP) NAAT and has been validated as a test for SARS-CoV-2.1 Although children are less susceptible to severe disease, primary schools are considered a vector for transmission and community spread.3 Efficient and scalable methods of routine testing are needed globally to help keep schools open. Saliva testing has proven a useful resource for this population.4,5
Abu Dhabi is the largest Emirate in the United Arab Emirates (UAE), with an estimated population of 2.5 million.6 The first case of COVID-19 was discovered in the UAE on January 29, 2020.7 The UAE has been recognized worldwide for its robust pandemic response. Along with the coordinated and swift application of public health measures, the country has one of the highest COVID-19 testing rates per capita and one of the highest vaccination rates worldwide.8,9 The Abu Dhabi Public Health Center (ADPHC) works alongside the Ministry of Education (MOE) to establish testing, quarantine, and general safety guidelines for primary schools. In December 2020, the ADPHC partnered with a local, accredited diagnostic laboratory to test the feasibility of a saliva-based screening program for COVID-19 directly on school campuses for 18 primary schools in the Emirate.
Saliva-based PCR testing for COVID-19 was approved for use in schools in the UAE on January 24, 2021.10 As part of a greater mitigation strategy to reduce both school-based transmission and, hence, community spread, the ADPHC focused its on-site testing program on children aged 4 to 12 years. The program required collaboration among medical professionals, school administrators and teachers, students, and parents. Our study evaluates the feasibility of implementing a saliva-based COVID-19 screening program directly on primary school campuses involving children as young as 4 years of age.
Methods
The ADPHC, in collaboration with G42 Biogenix Labs, conducted a saliva SARS-CoV-2 NAAT testing program in 18 primary schools in the Emirate. Schools were selected based on outbreak prevalence at the time and focused on “hot spot” areas. The school on-site saliva testing program included children aged 4 to 12 years old in a “bubble” attendance model during the school day. This model involved children being assigned to groups or “pods.” This allowed us to limit a potential outbreak to a single pod, as opposed to risk exposing the entire school, should a single student test positive. The well-established SalivaDirect protocol developed at Yale University was used for testing and included an RNA extraction-free, RT-qPCR method for SARS-CoV-2 detection.11
We conducted a qualitative study involving telephone interviews of school administrators to evaluate their experience with the ADPHC testing program at their schools. In addition, we interviewed the G42 Biogenix Lab providers to understand the logistics that supported on-campus collection of saliva specimens for this age group. We also gathered the attitudes of school children before and after testing. This study was reviewed and approved by the Abu Dhabi Health Research and Technology Committee and the Institutional Review Board (IRB), New York University Abu Dhabi (NYUAD).
Sample and recruitment
The original sample collection of saliva specimens was performed by the ADPHC in collaboration with G42 Biogenix Lab providers on school campuses between December 6 and December 10, 2020. During this time, schools operated in a hybrid teaching model, where learning took place both online and in person. Infection control measures were deployed based on ADPHC standards and guidelines. Nurses utilized appropriate patient protective equipment, frequent hand hygiene, and social distancing during the collection process. Inclusion criteria included asymptomatic students aged 4 to 12 years attending in-person classes on campus. Students with respiratory symptoms who were asked to stay home or those not attending in-person classes were excluded.
Data collection
Data with regard to school children’s attitudes before and after testing were compiled through an online survey sent randomly to participants postintervention. Data from school administrators were collected through video and telephone interviews between April 14 and April 29, 2021. We first interviewed G42 Biogenix Lab providers to obtain previously acquired qualitative and quantitative data, which were collected during the intervention itself. After obtaining this information, we designed a questionnaire and proceeded with a structured interview process for school officials.
We interviewed school principals and administrators to collect their overall experiences with the saliva testing program. Before starting each interview, we established the interviewees preferred language, either English or Arabic. We then introduced the meeting attendees and provided study details, aims, and objectives, and described collaborating entities. We obtained verbal informed consent from a script approved by the NYUAD IRB and then proceeded with the interview, which included 4 questions. The first 3 questions were answered on a 5-point Likert scale model that consisted of 5 answer options: 5 being completely agree, 4 agree, 3 somewhat agree, 2 somewhat disagree, and 1 completely disagree. The fourth question invited open-ended feedback and comments on the following statements:
- I believe the COVID-19 saliva testing program improved the safety for my school campus.
- Our community had an overall positive experience with the COVID saliva testing.
- We would like to continue a saliva-based COVID testing program on our school campus.
- Please provide any additional comments you feel important about the program.
During the interview, we transcribed the answers as the interviewee was answering. We then translated those in Arabic into English and collected the data in 1 Excel spreadsheet. School interviewees and school names were de-identified in the collection and storage process.
Results
A total of 2011 saliva samples were collected from 18 different primary school campuses. Samples were sent the same day to G42 Biogenix Labs in Abu Dhabi for COVID PCR testing. A team consisting of 5 doctors providing general oversight, along with 2 to 6 nurses per site, were able to manage the collection process for all 18 school campuses. Samples were collected between 8
Sample stations were set up in either the school auditorium or gymnasium to ensure appropriate crowd control and ventilation. Teachers and other school staff, including public safety, were able to manage lines and the shuttling of students back and forth from classes to testing stations, which allowed medical staff to focus on sample collection.
Informed consent was obtained by prior electronic communication to parents from school staff, asking them to agree to allow their child to participate in the testing program. Informed consent was identified as a challenge: Getting parents to understand that saliva testing was more comfortable than NP testing, and that the results were only being used to help keep the school safe, took time. School staff are used to obtaining consent from parents for field trips, but this was clearly more challenging for them.

The saliva collection process per child took more time than expected. Children fasted for 45 minutes before saliva collection. We used an active drool technique, which required children to pool saliva in their mouth then express it into a collection tube. Adults can generally do this on command, but we found it took 10 to 12 minutes per child. Saliva production was cued by asking the children to think about food, and by showing them pictures and TV commercials depicting food. Children 4 to 5 years old had more difficulty with the process despite active cueing, while those 6 to 12 years old had an easier time with the process. We collected data on a cohort of 80 children regarding their attitudes pre (Figure 1) and post collection (Figure 2). Children felt happier, less nervous, and less scared after collection than before collection. This trend reassured us that future collections would be easier for students.

A total of 15 of 18 school principals completed the telephone interview, yielding a response rate of 83%. Overall, 93% of the school principals agreed or completely agreed that the COVID-19 saliva testing program improved school safety; 93% agreed or completely agreed that they had an overall positive experience with the program; and 73% supported the ongoing use of saliva testing in their schools (Table 1). Administrators’ open-ended comments on their experience were positive overall (Table 2).

Discussion
By March 2020, many kindergarten to grade 12 public and private schools suspended in-person classes due to the pandemic and turned to online learning platforms. The negative impact of school closures on academic achievement is projected to be significant.7,12,13 Ensuring schools can stay open and run operations safely will require routine SARS-CoV-2 testing. Our study investigated the feasibility of routine saliva testing on children aged 4 to 12 years on their school campuses. The ADPHC school on-site saliva testing program involved bringing lab providers onto 18 primary school campuses and required cooperation among parents, students, school administrators, and health care professionals.

Children younger than 6 years had difficulty producing an adequate saliva specimen, whereas those 6 to 12 years did so with relative ease when cued by thoughts or pictures of food while waiting in line for collection. Schools considering on-site testing programs should consider the age range of 6 to 12 years as a viable age range for saliva screening. Children should fast for a minimum of 45 minutes prior to saliva collection and should be cued by thoughts of food, food pictures, or food commercials. Setting up a sampling station close to the cafeteria where students can smell meal preparation may also help.14,15 Sampling before breakfast or lunch, when children are potentially at their hungriest, should also be considered.
The greatest challenge was obtaining informed consent from parents who were not yet familiar with the reliability of saliva testing as a tool for SARS-CoV-2 screening or with the saliva collection process as a whole. Informed consent was initially done electronically, lacking direct human interaction to answer parents’ questions. Parents who refused had a follow-up call from the school nurse to further explain the logistics and rationale for saliva screening. Having medical professionals directly answer parents’ questions was helpful. Parents were reassured that the process was painless, confidential, and only to be used for school safety purposes. Despite school administrators being experienced in obtaining consent from parents for field trips, obtaining informed consent for a medical testing procedure is more complicated, and parents aren’t accustomed to providing such consent in a school environment. Schools considering on-site testing should ensure that their school nurse or other health care providers are on the front line obtaining informed consent and allaying parents’ fears.
School staff were able to effectively provide crowd control for testing, and children felt at ease being in a familiar environment. Teachers and public safety officers are well-equipped at managing the shuttling of students to class, to lunch, to physical education, and, finally, to dismissal. They were equally equipped at handling the logistics of students to and from testing, including minimizing crowds and helping students feel at ease during the process. This effective collaboration allowed the lab personnel to focus on sample collection and storage, while school staff managed all other aspects of the children’s safety and care.
Conclusion
Overall, school administrators had a positive experience with the testing program, felt the program improved the safety of their schools, and supported the ongoing use of saliva testing for SARS-CoV-2 on their school campuses. Children aged 6 years and older were able to provide adequate saliva samples, and children felt happier and less nervous after the process, indicating repeatability. Our findings highlight the feasibility of an integrated on-site saliva testing model for primary school campuses. Further research is needed to determine the scalability of such a model and whether the added compliance and safety of on-site testing compensates for the potential loss of learning time that testing during school hours would require.
Corresponding author: Ayaz Virji, MD, New York University Abu Dhabi, PO Box 129188, Abu Dhabi, United Arab Emirates; [email protected].
Financial disclosures: None.
From Health Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates (Dr. Virji and Aisha Al Hamiz), Public Health, Abu Dhabi Public Health Center, Abu Dhabi, United Arab Emirates (Drs. Al Hajeri, Al Shehhi, Al Memari, and Ahlam Al Maskari), College of Medicine and Health Sciences, Khalifa University, Abu Dhabi, United Arab Emirates, Department of Medicine, Sheikh Shakhbout Medical City, Abu Dhabi, United Arab Emirates (Dr. Alhajri), Public Health Research Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates, Oxford University Hospitals NHS Foundation Trust, Oxford, England, and the MRC Epidemiology Unit, University of Cambridge, Cambridge, England (Dr. Ali).
Objective: The pandemic has forced closures of primary schools, resulting in loss of learning time on a global scale. In addition to face coverings, social distancing, and hand hygiene, an efficient testing method is important to mitigate the spread of COVID-19 in schools. We evaluated the feasibility of a saliva-based SARS-CoV-2 polymerase chain reaction testing program among 18 primary schools in the Emirate of Abu Dhabi, United Arab Emirates. Qualitative results show that children 4 to 5 years old had difficulty producing an adequate saliva specimen compared to those 6 to 12 years old.
Methods: A short training video on saliva collection beforehand helps demystify the process for students and parents alike. Informed consent was challenging yet should be done beforehand by school health nurses or other medical professionals to reassure parents and maximize participation.
Results: Telephone interviews with school administrators resulted in an 83% response rate. Overall, 93% of school administrators had a positive experience with saliva testing and felt the program improved the safety of their schools. The ongoing use of saliva testing for SARS-CoV-2 was supported by 73% of respondents.
Conclusion: On-campus saliva testing is a feasible option for primary schools to screen for COVID-19 in their student population to help keep their campuses safe and open for learning.
Keywords: COVID-19; saliva testing; mitigation; primary school.
The COVID-19 pandemic is a leading cause of morbidity and mortality worldwide and continues to exhaust health care resources on a large scale.1 Efficient testing is critical to identify cases early and to help mitigate the deleterious effects of the pandemic.2 Saliva polymerase chain reaction (PCR) nucleic acid amplification testing (NAAT) is more comfortable than nasopharyngeal (NP) NAAT and has been validated as a test for SARS-CoV-2.1 Although children are less susceptible to severe disease, primary schools are considered a vector for transmission and community spread.3 Efficient and scalable methods of routine testing are needed globally to help keep schools open. Saliva testing has proven a useful resource for this population.4,5
Abu Dhabi is the largest Emirate in the United Arab Emirates (UAE), with an estimated population of 2.5 million.6 The first case of COVID-19 was discovered in the UAE on January 29, 2020.7 The UAE has been recognized worldwide for its robust pandemic response. Along with the coordinated and swift application of public health measures, the country has one of the highest COVID-19 testing rates per capita and one of the highest vaccination rates worldwide.8,9 The Abu Dhabi Public Health Center (ADPHC) works alongside the Ministry of Education (MOE) to establish testing, quarantine, and general safety guidelines for primary schools. In December 2020, the ADPHC partnered with a local, accredited diagnostic laboratory to test the feasibility of a saliva-based screening program for COVID-19 directly on school campuses for 18 primary schools in the Emirate.
Saliva-based PCR testing for COVID-19 was approved for use in schools in the UAE on January 24, 2021.10 As part of a greater mitigation strategy to reduce both school-based transmission and, hence, community spread, the ADPHC focused its on-site testing program on children aged 4 to 12 years. The program required collaboration among medical professionals, school administrators and teachers, students, and parents. Our study evaluates the feasibility of implementing a saliva-based COVID-19 screening program directly on primary school campuses involving children as young as 4 years of age.
Methods
The ADPHC, in collaboration with G42 Biogenix Labs, conducted a saliva SARS-CoV-2 NAAT testing program in 18 primary schools in the Emirate. Schools were selected based on outbreak prevalence at the time and focused on “hot spot” areas. The school on-site saliva testing program included children aged 4 to 12 years old in a “bubble” attendance model during the school day. This model involved children being assigned to groups or “pods.” This allowed us to limit a potential outbreak to a single pod, as opposed to risk exposing the entire school, should a single student test positive. The well-established SalivaDirect protocol developed at Yale University was used for testing and included an RNA extraction-free, RT-qPCR method for SARS-CoV-2 detection.11
We conducted a qualitative study involving telephone interviews of school administrators to evaluate their experience with the ADPHC testing program at their schools. In addition, we interviewed the G42 Biogenix Lab providers to understand the logistics that supported on-campus collection of saliva specimens for this age group. We also gathered the attitudes of school children before and after testing. This study was reviewed and approved by the Abu Dhabi Health Research and Technology Committee and the Institutional Review Board (IRB), New York University Abu Dhabi (NYUAD).
Sample and recruitment
The original sample collection of saliva specimens was performed by the ADPHC in collaboration with G42 Biogenix Lab providers on school campuses between December 6 and December 10, 2020. During this time, schools operated in a hybrid teaching model, where learning took place both online and in person. Infection control measures were deployed based on ADPHC standards and guidelines. Nurses utilized appropriate patient protective equipment, frequent hand hygiene, and social distancing during the collection process. Inclusion criteria included asymptomatic students aged 4 to 12 years attending in-person classes on campus. Students with respiratory symptoms who were asked to stay home or those not attending in-person classes were excluded.
Data collection
Data with regard to school children’s attitudes before and after testing were compiled through an online survey sent randomly to participants postintervention. Data from school administrators were collected through video and telephone interviews between April 14 and April 29, 2021. We first interviewed G42 Biogenix Lab providers to obtain previously acquired qualitative and quantitative data, which were collected during the intervention itself. After obtaining this information, we designed a questionnaire and proceeded with a structured interview process for school officials.
We interviewed school principals and administrators to collect their overall experiences with the saliva testing program. Before starting each interview, we established the interviewees preferred language, either English or Arabic. We then introduced the meeting attendees and provided study details, aims, and objectives, and described collaborating entities. We obtained verbal informed consent from a script approved by the NYUAD IRB and then proceeded with the interview, which included 4 questions. The first 3 questions were answered on a 5-point Likert scale model that consisted of 5 answer options: 5 being completely agree, 4 agree, 3 somewhat agree, 2 somewhat disagree, and 1 completely disagree. The fourth question invited open-ended feedback and comments on the following statements:
- I believe the COVID-19 saliva testing program improved the safety for my school campus.
- Our community had an overall positive experience with the COVID saliva testing.
- We would like to continue a saliva-based COVID testing program on our school campus.
- Please provide any additional comments you feel important about the program.
During the interview, we transcribed the answers as the interviewee was answering. We then translated those in Arabic into English and collected the data in 1 Excel spreadsheet. School interviewees and school names were de-identified in the collection and storage process.
Results
A total of 2011 saliva samples were collected from 18 different primary school campuses. Samples were sent the same day to G42 Biogenix Labs in Abu Dhabi for COVID PCR testing. A team consisting of 5 doctors providing general oversight, along with 2 to 6 nurses per site, were able to manage the collection process for all 18 school campuses. Samples were collected between 8
Sample stations were set up in either the school auditorium or gymnasium to ensure appropriate crowd control and ventilation. Teachers and other school staff, including public safety, were able to manage lines and the shuttling of students back and forth from classes to testing stations, which allowed medical staff to focus on sample collection.
Informed consent was obtained by prior electronic communication to parents from school staff, asking them to agree to allow their child to participate in the testing program. Informed consent was identified as a challenge: Getting parents to understand that saliva testing was more comfortable than NP testing, and that the results were only being used to help keep the school safe, took time. School staff are used to obtaining consent from parents for field trips, but this was clearly more challenging for them.

The saliva collection process per child took more time than expected. Children fasted for 45 minutes before saliva collection. We used an active drool technique, which required children to pool saliva in their mouth then express it into a collection tube. Adults can generally do this on command, but we found it took 10 to 12 minutes per child. Saliva production was cued by asking the children to think about food, and by showing them pictures and TV commercials depicting food. Children 4 to 5 years old had more difficulty with the process despite active cueing, while those 6 to 12 years old had an easier time with the process. We collected data on a cohort of 80 children regarding their attitudes pre (Figure 1) and post collection (Figure 2). Children felt happier, less nervous, and less scared after collection than before collection. This trend reassured us that future collections would be easier for students.

A total of 15 of 18 school principals completed the telephone interview, yielding a response rate of 83%. Overall, 93% of the school principals agreed or completely agreed that the COVID-19 saliva testing program improved school safety; 93% agreed or completely agreed that they had an overall positive experience with the program; and 73% supported the ongoing use of saliva testing in their schools (Table 1). Administrators’ open-ended comments on their experience were positive overall (Table 2).

Discussion
By March 2020, many kindergarten to grade 12 public and private schools suspended in-person classes due to the pandemic and turned to online learning platforms. The negative impact of school closures on academic achievement is projected to be significant.7,12,13 Ensuring schools can stay open and run operations safely will require routine SARS-CoV-2 testing. Our study investigated the feasibility of routine saliva testing on children aged 4 to 12 years on their school campuses. The ADPHC school on-site saliva testing program involved bringing lab providers onto 18 primary school campuses and required cooperation among parents, students, school administrators, and health care professionals.

Children younger than 6 years had difficulty producing an adequate saliva specimen, whereas those 6 to 12 years did so with relative ease when cued by thoughts or pictures of food while waiting in line for collection. Schools considering on-site testing programs should consider the age range of 6 to 12 years as a viable age range for saliva screening. Children should fast for a minimum of 45 minutes prior to saliva collection and should be cued by thoughts of food, food pictures, or food commercials. Setting up a sampling station close to the cafeteria where students can smell meal preparation may also help.14,15 Sampling before breakfast or lunch, when children are potentially at their hungriest, should also be considered.
The greatest challenge was obtaining informed consent from parents who were not yet familiar with the reliability of saliva testing as a tool for SARS-CoV-2 screening or with the saliva collection process as a whole. Informed consent was initially done electronically, lacking direct human interaction to answer parents’ questions. Parents who refused had a follow-up call from the school nurse to further explain the logistics and rationale for saliva screening. Having medical professionals directly answer parents’ questions was helpful. Parents were reassured that the process was painless, confidential, and only to be used for school safety purposes. Despite school administrators being experienced in obtaining consent from parents for field trips, obtaining informed consent for a medical testing procedure is more complicated, and parents aren’t accustomed to providing such consent in a school environment. Schools considering on-site testing should ensure that their school nurse or other health care providers are on the front line obtaining informed consent and allaying parents’ fears.
School staff were able to effectively provide crowd control for testing, and children felt at ease being in a familiar environment. Teachers and public safety officers are well-equipped at managing the shuttling of students to class, to lunch, to physical education, and, finally, to dismissal. They were equally equipped at handling the logistics of students to and from testing, including minimizing crowds and helping students feel at ease during the process. This effective collaboration allowed the lab personnel to focus on sample collection and storage, while school staff managed all other aspects of the children’s safety and care.
Conclusion
Overall, school administrators had a positive experience with the testing program, felt the program improved the safety of their schools, and supported the ongoing use of saliva testing for SARS-CoV-2 on their school campuses. Children aged 6 years and older were able to provide adequate saliva samples, and children felt happier and less nervous after the process, indicating repeatability. Our findings highlight the feasibility of an integrated on-site saliva testing model for primary school campuses. Further research is needed to determine the scalability of such a model and whether the added compliance and safety of on-site testing compensates for the potential loss of learning time that testing during school hours would require.
Corresponding author: Ayaz Virji, MD, New York University Abu Dhabi, PO Box 129188, Abu Dhabi, United Arab Emirates; [email protected].
Financial disclosures: None.
1. Kuehn BM. Despite improvements, COVID-19’s health care disruptions persist. JAMA. 2021;325(23):2335. doi:10.1001/jama.2021.9134
2. National Institute on Aging. Why COVID-19 testing is the key to getting back to normal. September 4, 2020. Accessed September 8, 2021. https://www.nia.nih.gov/news/why-covid-19-testing-key-getting-back-normal
3. Centers for Disease Control and Prevention. Science brief: Transmission of SARS-CoV-2 in K-12 schools. Updated July 9, 2021. Accessed September 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/transmission_k_12_schools.html
4. Butler-Laporte G, Lawandi A, Schiller I, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med. 2021;181(3):353-360. doi:10.1001/jamainternmed.2020.8876
5. Al Suwaidi H, Senok A, Varghese R, et al. Saliva for molecular detection of SARS-CoV-2 in school-age children. Clin Microbiol Infect. 2021;27(9):1330-1335. doi:10.1016/j.cmi.2021.02.009
6. Abu Dhabi. Accessed September 8, 2021. https://u.ae/en/about-the-uae/the-seven-emirates/abu-dhabi
7. Alsuwaidi AR, Al Hosani FI, Al Memari S, et al. Seroprevalence of COVID-19 infection in the Emirate of Abu Dhabi, United Arab Emirates: a population-based cross-sectional study. Int J Epidemiol. 2021;50(4):1077-1090. doi:10.1093/ije/dyab077
8. Al Hosany F, Ganesan S, Al Memari S, et al. Response to COVID-19 pandemic in the UAE: a public health perspective. J Glob Health. 2021;11:03050. doi:10.7189/jogh.11.03050
9. Bremmer I. The best global responses to the COVID-19 pandemic, 1 year later. Time Magazine. Updated February 23, 2021. Accessed September 8, 2021. https://time.com/5851633/best-global-responses-covid-19/
10. Department of Health, Abu Dhabi. Laboratory diagnostic test for COVID-19: update regarding saliva-based testing using RT-PCR test. 2021.
11. Vogels C, Brackney DE, Kalinich CC, et al. SalivaDirect: RNA extraction-free SARS-CoV-2 diagnostics. Protocols.io. Accessed September 8, 2021. https://www.protocols.io/view/salivadirect-rna-extraction-free-sars-cov-2-diagno-bh6jj9cn?version_warning=no
12. Education Endowment Foundation. Impact of school closures on the attainment gap: rapid evidence assessment. June 2020. Accessed September 8, 2021. https://www.researchgate.net/publication/342501263_EEF_2020_-_Impact_of_School_Closures_on_the_Attainment_Gap
13. United Nations. Policy brief: Education during COVID-19 and beyond. Accessed September 8, 2021. https://www.un.org/development/desa/dspd/wp-content/uploads/sites/22/2020/08/sg_policy_brief_covid-19_and_education_august_2020.pdf
14. Schiffman SS, Miletic ID. Effect of taste and smell on secretion rate of salivary IgA in elderly and young persons. J Nutr Health Aging. 1999;3(3):158-164.
15. Lee VM, Linden RW. The effect of odours on stimulated parotid salivary flow in humans. Physiol Behav. 1992;52(6):1121-1125. doi:10.1016/0031-9384(92)90470-m
1. Kuehn BM. Despite improvements, COVID-19’s health care disruptions persist. JAMA. 2021;325(23):2335. doi:10.1001/jama.2021.9134
2. National Institute on Aging. Why COVID-19 testing is the key to getting back to normal. September 4, 2020. Accessed September 8, 2021. https://www.nia.nih.gov/news/why-covid-19-testing-key-getting-back-normal
3. Centers for Disease Control and Prevention. Science brief: Transmission of SARS-CoV-2 in K-12 schools. Updated July 9, 2021. Accessed September 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/transmission_k_12_schools.html
4. Butler-Laporte G, Lawandi A, Schiller I, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med. 2021;181(3):353-360. doi:10.1001/jamainternmed.2020.8876
5. Al Suwaidi H, Senok A, Varghese R, et al. Saliva for molecular detection of SARS-CoV-2 in school-age children. Clin Microbiol Infect. 2021;27(9):1330-1335. doi:10.1016/j.cmi.2021.02.009
6. Abu Dhabi. Accessed September 8, 2021. https://u.ae/en/about-the-uae/the-seven-emirates/abu-dhabi
7. Alsuwaidi AR, Al Hosani FI, Al Memari S, et al. Seroprevalence of COVID-19 infection in the Emirate of Abu Dhabi, United Arab Emirates: a population-based cross-sectional study. Int J Epidemiol. 2021;50(4):1077-1090. doi:10.1093/ije/dyab077
8. Al Hosany F, Ganesan S, Al Memari S, et al. Response to COVID-19 pandemic in the UAE: a public health perspective. J Glob Health. 2021;11:03050. doi:10.7189/jogh.11.03050
9. Bremmer I. The best global responses to the COVID-19 pandemic, 1 year later. Time Magazine. Updated February 23, 2021. Accessed September 8, 2021. https://time.com/5851633/best-global-responses-covid-19/
10. Department of Health, Abu Dhabi. Laboratory diagnostic test for COVID-19: update regarding saliva-based testing using RT-PCR test. 2021.
11. Vogels C, Brackney DE, Kalinich CC, et al. SalivaDirect: RNA extraction-free SARS-CoV-2 diagnostics. Protocols.io. Accessed September 8, 2021. https://www.protocols.io/view/salivadirect-rna-extraction-free-sars-cov-2-diagno-bh6jj9cn?version_warning=no
12. Education Endowment Foundation. Impact of school closures on the attainment gap: rapid evidence assessment. June 2020. Accessed September 8, 2021. https://www.researchgate.net/publication/342501263_EEF_2020_-_Impact_of_School_Closures_on_the_Attainment_Gap
13. United Nations. Policy brief: Education during COVID-19 and beyond. Accessed September 8, 2021. https://www.un.org/development/desa/dspd/wp-content/uploads/sites/22/2020/08/sg_policy_brief_covid-19_and_education_august_2020.pdf
14. Schiffman SS, Miletic ID. Effect of taste and smell on secretion rate of salivary IgA in elderly and young persons. J Nutr Health Aging. 1999;3(3):158-164.
15. Lee VM, Linden RW. The effect of odours on stimulated parotid salivary flow in humans. Physiol Behav. 1992;52(6):1121-1125. doi:10.1016/0031-9384(92)90470-m
What I Learned About Change From Practicing During the COVID-19 Surge
While sick at home with a 26-day symptomatic course of COVID-19 in March 2020, I watched the surge unfold in my state and the hospital where I work as an inpatient adult medicine physician. Although the preponderance of my professional life is dedicated to leading teams in implementing delivery system transformation, the hat I wore in that moment involved living through and keeping up with the changes around me. Once I recovered and returned to the arena as a COVID doctor, I adapted to and made changes during constant shifts in how we provided care.
Looking back on those months during the worst of the COVID-19 hospital surge in my region, I reflect on the factors that helped me, as a frontline and shift-work clinician, adapt to and make those changes. In reflecting on the elements that were meaningful to me during the crisis, I recognize a set of change-enabling factors that have broad relevance for those of us who work to improve outcomes for patients and populations.
Confidence engendered by liberating data
In the early days of the surge, there was much uncertainty, and unfortunately, some seriously imperfect messaging. Trust was broken or badly bruised for many frontline clinicians. I share this painful phase not to criticize, but rather reflect on what mattered to me during that crisis of confidence. It was data. Raw, unadjusted, best-available data. Produced and pushed out. Available, trended over time, telling the story of where we are, now. Counts of tests, beds, and ventilators. The consistent, transparent availability of relevant and straightforward data provided an active antidote to a sense of uncertainty during a crisis of confidence.
Personal practice change stimulated by relevance and urgency
For half a decade, I have been encouraging interdisciplinary inpatient teams to identify and actively engage the family and/or care partner as a member of the care team. Despite even the American Association of Retired Persons mobilizing an impressive regulatory approach in 32 states to require that family and/or care partners are included as such, the practice change efforts continued on a slow and steady path. Why? We just didn’t believe it was of urgent, relevant, mission-critical importance to our daily practice to do so. That all changed in March 2020.
Without needing to be told, educated, or incentivized, my first night as a COVID doctor found me calling every single patient’s family upon admission, regardless of what time it was. It was critical to review the diagnosis, transparently discuss the uncertainty regarding the upcoming hours and days, review the potential contingencies, and ask, right there and then, whether intubation is consistent with goals of care. It was that urgent and relevant. Without exception, families were grateful for the effort and candor.
The significance of this practice—undoubtedly adopted by every inpatient provider who has worked a COVID surge—is rooted in decades of academic deliberation on which is the “right” doctor to have these discussions. None of that mattered. Historical opinions changed due to what was urgent and relevant given the situation at hand and the job we had to do. Imagine, for example, what we could do and how we could change if we now consider it urgent and relevant to identify and mobilize enhanced services and supports to patients who experience inequities because we believe it to be mission-critical to the job we show up to do every day.
Change fostered by a creative problem-solving ecosystem
Embracing personal practice change was made easier and implicitly affirmed by the creative problem solving that occurred everywhere. Tents, drive-throughs, and even college field houses were now settings of care. Primary care physicians, cardiologists, and gastrointestinal (GI) and postanesthesia care nurses staffed the COVID floors. Rolling stands held iPads so staff could communicate with patients without entering the room. This creative ecosystem fostered individual practice change. No debates were needed to recognize that standard processes were inadequate. No single role or service of any discipline was singularly asked to change to meet the needs of the moment. Because of this ecosystem of creative, active change, there was a much greater flexibility among individuals, role types, departments, and disciplines to change. This is particularly poignant to me in light of the work I lead to improve care for patients who experience systemic inequities in our health care system. When we ask a single role type or discipline to change, it can be met with resistance; far more success is achieved when we engage an interdisciplinary and interdepartmental approach to change. When surrounded by others making change, it makes us more willing to change, too.
Change catalyzed teamwork
It is so often invoked that health care is a team sport. In practicality, while we may aspire to work as a team, health care delivery is still all too often comprised of a set of individual actors with individualized responsibilities trying to communicate the best they can with each other.
What I experienced during the surge at my hospital was the very best version of teamwork I have ever been a part of in health care: empathetic, mutually interdependent strangers coming together during daily changes in staffing, processes, and resources. I will never forget nights walking into the pediatric floor or day surgery recovery area—now repurposed as a COVID unit—to entirely new faces comprised of GI suite nurses, outpatient doctors, and moonlighting intensivists.
We were all new to each other, all new to working in this setting, and all new to whatever the newest changes of the day brought. I will never forget how we greeted each other and introduced ourselves. We asked each other where we were “from,” and held a genuine appreciation to each other for being there. Imagine how this impacted how we worked together. Looking back on those night shifts, I remember us as a truly interdependent team. I will endeavor to bring that sense of mutual regard and interdependency into my work to foster effective interdisciplinary and cross-continuum teamwork.
Takeaways
As a student and practitioner of delivery system transformation, I am often in conversations about imperfect data, incomplete evidence, and role-specific and organizational resistance to change. As an acute care provider during the COVID-19 hospital surge in my region, the experiences I had as a participant in the COVID-related delivery system change will stay with me as I lead value-based delivery system change. What worked in an infectious disease crisis holds great relevance to our pressing, urgent, relevant work to create a more person-centered, equitable, and value-based delivery system.
I am confident that if those of us seeking to improve outcomes use visible and accessible data to engender confidence, clearly link practice change to the relevant and urgent issue at hand, promote broadly visible creative problem solving to foster an ecosystem of change, and cultivate empathy and mutual interdependence to promote the teamwork we aspire to have, that we will foster meaningful progress in our efforts to improve care for patients and populations.
Corresponding author: Amy Boutwell, MD, MPP, President, Collaborative Healthcare Strategies, Lexington, MA; [email protected].
Financial disclosures: None.
While sick at home with a 26-day symptomatic course of COVID-19 in March 2020, I watched the surge unfold in my state and the hospital where I work as an inpatient adult medicine physician. Although the preponderance of my professional life is dedicated to leading teams in implementing delivery system transformation, the hat I wore in that moment involved living through and keeping up with the changes around me. Once I recovered and returned to the arena as a COVID doctor, I adapted to and made changes during constant shifts in how we provided care.
Looking back on those months during the worst of the COVID-19 hospital surge in my region, I reflect on the factors that helped me, as a frontline and shift-work clinician, adapt to and make those changes. In reflecting on the elements that were meaningful to me during the crisis, I recognize a set of change-enabling factors that have broad relevance for those of us who work to improve outcomes for patients and populations.
Confidence engendered by liberating data
In the early days of the surge, there was much uncertainty, and unfortunately, some seriously imperfect messaging. Trust was broken or badly bruised for many frontline clinicians. I share this painful phase not to criticize, but rather reflect on what mattered to me during that crisis of confidence. It was data. Raw, unadjusted, best-available data. Produced and pushed out. Available, trended over time, telling the story of where we are, now. Counts of tests, beds, and ventilators. The consistent, transparent availability of relevant and straightforward data provided an active antidote to a sense of uncertainty during a crisis of confidence.
Personal practice change stimulated by relevance and urgency
For half a decade, I have been encouraging interdisciplinary inpatient teams to identify and actively engage the family and/or care partner as a member of the care team. Despite even the American Association of Retired Persons mobilizing an impressive regulatory approach in 32 states to require that family and/or care partners are included as such, the practice change efforts continued on a slow and steady path. Why? We just didn’t believe it was of urgent, relevant, mission-critical importance to our daily practice to do so. That all changed in March 2020.
Without needing to be told, educated, or incentivized, my first night as a COVID doctor found me calling every single patient’s family upon admission, regardless of what time it was. It was critical to review the diagnosis, transparently discuss the uncertainty regarding the upcoming hours and days, review the potential contingencies, and ask, right there and then, whether intubation is consistent with goals of care. It was that urgent and relevant. Without exception, families were grateful for the effort and candor.
The significance of this practice—undoubtedly adopted by every inpatient provider who has worked a COVID surge—is rooted in decades of academic deliberation on which is the “right” doctor to have these discussions. None of that mattered. Historical opinions changed due to what was urgent and relevant given the situation at hand and the job we had to do. Imagine, for example, what we could do and how we could change if we now consider it urgent and relevant to identify and mobilize enhanced services and supports to patients who experience inequities because we believe it to be mission-critical to the job we show up to do every day.
Change fostered by a creative problem-solving ecosystem
Embracing personal practice change was made easier and implicitly affirmed by the creative problem solving that occurred everywhere. Tents, drive-throughs, and even college field houses were now settings of care. Primary care physicians, cardiologists, and gastrointestinal (GI) and postanesthesia care nurses staffed the COVID floors. Rolling stands held iPads so staff could communicate with patients without entering the room. This creative ecosystem fostered individual practice change. No debates were needed to recognize that standard processes were inadequate. No single role or service of any discipline was singularly asked to change to meet the needs of the moment. Because of this ecosystem of creative, active change, there was a much greater flexibility among individuals, role types, departments, and disciplines to change. This is particularly poignant to me in light of the work I lead to improve care for patients who experience systemic inequities in our health care system. When we ask a single role type or discipline to change, it can be met with resistance; far more success is achieved when we engage an interdisciplinary and interdepartmental approach to change. When surrounded by others making change, it makes us more willing to change, too.
Change catalyzed teamwork
It is so often invoked that health care is a team sport. In practicality, while we may aspire to work as a team, health care delivery is still all too often comprised of a set of individual actors with individualized responsibilities trying to communicate the best they can with each other.
What I experienced during the surge at my hospital was the very best version of teamwork I have ever been a part of in health care: empathetic, mutually interdependent strangers coming together during daily changes in staffing, processes, and resources. I will never forget nights walking into the pediatric floor or day surgery recovery area—now repurposed as a COVID unit—to entirely new faces comprised of GI suite nurses, outpatient doctors, and moonlighting intensivists.
We were all new to each other, all new to working in this setting, and all new to whatever the newest changes of the day brought. I will never forget how we greeted each other and introduced ourselves. We asked each other where we were “from,” and held a genuine appreciation to each other for being there. Imagine how this impacted how we worked together. Looking back on those night shifts, I remember us as a truly interdependent team. I will endeavor to bring that sense of mutual regard and interdependency into my work to foster effective interdisciplinary and cross-continuum teamwork.
Takeaways
As a student and practitioner of delivery system transformation, I am often in conversations about imperfect data, incomplete evidence, and role-specific and organizational resistance to change. As an acute care provider during the COVID-19 hospital surge in my region, the experiences I had as a participant in the COVID-related delivery system change will stay with me as I lead value-based delivery system change. What worked in an infectious disease crisis holds great relevance to our pressing, urgent, relevant work to create a more person-centered, equitable, and value-based delivery system.
I am confident that if those of us seeking to improve outcomes use visible and accessible data to engender confidence, clearly link practice change to the relevant and urgent issue at hand, promote broadly visible creative problem solving to foster an ecosystem of change, and cultivate empathy and mutual interdependence to promote the teamwork we aspire to have, that we will foster meaningful progress in our efforts to improve care for patients and populations.
Corresponding author: Amy Boutwell, MD, MPP, President, Collaborative Healthcare Strategies, Lexington, MA; [email protected].
Financial disclosures: None.
While sick at home with a 26-day symptomatic course of COVID-19 in March 2020, I watched the surge unfold in my state and the hospital where I work as an inpatient adult medicine physician. Although the preponderance of my professional life is dedicated to leading teams in implementing delivery system transformation, the hat I wore in that moment involved living through and keeping up with the changes around me. Once I recovered and returned to the arena as a COVID doctor, I adapted to and made changes during constant shifts in how we provided care.
Looking back on those months during the worst of the COVID-19 hospital surge in my region, I reflect on the factors that helped me, as a frontline and shift-work clinician, adapt to and make those changes. In reflecting on the elements that were meaningful to me during the crisis, I recognize a set of change-enabling factors that have broad relevance for those of us who work to improve outcomes for patients and populations.
Confidence engendered by liberating data
In the early days of the surge, there was much uncertainty, and unfortunately, some seriously imperfect messaging. Trust was broken or badly bruised for many frontline clinicians. I share this painful phase not to criticize, but rather reflect on what mattered to me during that crisis of confidence. It was data. Raw, unadjusted, best-available data. Produced and pushed out. Available, trended over time, telling the story of where we are, now. Counts of tests, beds, and ventilators. The consistent, transparent availability of relevant and straightforward data provided an active antidote to a sense of uncertainty during a crisis of confidence.
Personal practice change stimulated by relevance and urgency
For half a decade, I have been encouraging interdisciplinary inpatient teams to identify and actively engage the family and/or care partner as a member of the care team. Despite even the American Association of Retired Persons mobilizing an impressive regulatory approach in 32 states to require that family and/or care partners are included as such, the practice change efforts continued on a slow and steady path. Why? We just didn’t believe it was of urgent, relevant, mission-critical importance to our daily practice to do so. That all changed in March 2020.
Without needing to be told, educated, or incentivized, my first night as a COVID doctor found me calling every single patient’s family upon admission, regardless of what time it was. It was critical to review the diagnosis, transparently discuss the uncertainty regarding the upcoming hours and days, review the potential contingencies, and ask, right there and then, whether intubation is consistent with goals of care. It was that urgent and relevant. Without exception, families were grateful for the effort and candor.
The significance of this practice—undoubtedly adopted by every inpatient provider who has worked a COVID surge—is rooted in decades of academic deliberation on which is the “right” doctor to have these discussions. None of that mattered. Historical opinions changed due to what was urgent and relevant given the situation at hand and the job we had to do. Imagine, for example, what we could do and how we could change if we now consider it urgent and relevant to identify and mobilize enhanced services and supports to patients who experience inequities because we believe it to be mission-critical to the job we show up to do every day.
Change fostered by a creative problem-solving ecosystem
Embracing personal practice change was made easier and implicitly affirmed by the creative problem solving that occurred everywhere. Tents, drive-throughs, and even college field houses were now settings of care. Primary care physicians, cardiologists, and gastrointestinal (GI) and postanesthesia care nurses staffed the COVID floors. Rolling stands held iPads so staff could communicate with patients without entering the room. This creative ecosystem fostered individual practice change. No debates were needed to recognize that standard processes were inadequate. No single role or service of any discipline was singularly asked to change to meet the needs of the moment. Because of this ecosystem of creative, active change, there was a much greater flexibility among individuals, role types, departments, and disciplines to change. This is particularly poignant to me in light of the work I lead to improve care for patients who experience systemic inequities in our health care system. When we ask a single role type or discipline to change, it can be met with resistance; far more success is achieved when we engage an interdisciplinary and interdepartmental approach to change. When surrounded by others making change, it makes us more willing to change, too.
Change catalyzed teamwork
It is so often invoked that health care is a team sport. In practicality, while we may aspire to work as a team, health care delivery is still all too often comprised of a set of individual actors with individualized responsibilities trying to communicate the best they can with each other.
What I experienced during the surge at my hospital was the very best version of teamwork I have ever been a part of in health care: empathetic, mutually interdependent strangers coming together during daily changes in staffing, processes, and resources. I will never forget nights walking into the pediatric floor or day surgery recovery area—now repurposed as a COVID unit—to entirely new faces comprised of GI suite nurses, outpatient doctors, and moonlighting intensivists.
We were all new to each other, all new to working in this setting, and all new to whatever the newest changes of the day brought. I will never forget how we greeted each other and introduced ourselves. We asked each other where we were “from,” and held a genuine appreciation to each other for being there. Imagine how this impacted how we worked together. Looking back on those night shifts, I remember us as a truly interdependent team. I will endeavor to bring that sense of mutual regard and interdependency into my work to foster effective interdisciplinary and cross-continuum teamwork.
Takeaways
As a student and practitioner of delivery system transformation, I am often in conversations about imperfect data, incomplete evidence, and role-specific and organizational resistance to change. As an acute care provider during the COVID-19 hospital surge in my region, the experiences I had as a participant in the COVID-related delivery system change will stay with me as I lead value-based delivery system change. What worked in an infectious disease crisis holds great relevance to our pressing, urgent, relevant work to create a more person-centered, equitable, and value-based delivery system.
I am confident that if those of us seeking to improve outcomes use visible and accessible data to engender confidence, clearly link practice change to the relevant and urgent issue at hand, promote broadly visible creative problem solving to foster an ecosystem of change, and cultivate empathy and mutual interdependence to promote the teamwork we aspire to have, that we will foster meaningful progress in our efforts to improve care for patients and populations.
Corresponding author: Amy Boutwell, MD, MPP, President, Collaborative Healthcare Strategies, Lexington, MA; [email protected].
Financial disclosures: None.
Evaluation of a Digital Intervention for Hypertension Management in Primary Care Combining Self-monitoring of Blood Pressure With Guided Self-management
Study Overview
Objective. To evaluate whether a digital intervention comprising self-monitoring of blood pressure (BP) with reminders and predetermined drug changes combined with lifestyle change support resulted in lower systolic BP in people receiving treatment for hypertension that was poorly controlled, and whether this approach was cost effective.
Design. Unmasked randomized controlled trial.
Settings and participants. Eligible participants were identified from clinical codes recorded in the electronic health records of 76 collaborating general practices from the National Institute for Health Research Clinical Research Network, a United Kingdom government agency. The practices sent invitation letters to eligible participants to come to the clinic to establish eligibility, take consent, and collect baseline data via online questionnaires.
Eligible participants were aged 18 years or older with treated hypertension, a mean baseline BP reading of more than 140/90 mm Hg and were taking no more than 3 antihypertensive drugs. Participants also needed to be willing to self-monitor and have access to the internet (with support from a family member if needed). Exclusions included BP greater than 180/110 mm Hg, atrial fibrillation, hypertension not managed by their general practitioner, chronic kidney disease stage 4-5, postural hypotension (> 20 mm Hg systolic drop), an acute cardiovascular event in the previous 3 months, terminal disease, or another condition which in the opinion of their general practitioner made participation inappropriate.
Of the 11 399 invitation letters sent out, 1389 (12%) potential participants responded positively and were screened for eligibility. Those who declined to take part could optionally give their reasons, and responses were gained from 2426 of 10 010 (24%). The mean age of those who gave a reason for declining was 73 years. The most commonly selected reasons for declining were not having access to the internet (982, 41%), not wanting to participate in a research trial (617, 25%) or an internet study (543, 22%), and not wanting to change drugs (535, 22%). Of the 1389 screened, 734 were ineligible, and 33 did not complete baseline measures and randomization. The remaining 622 people who were randomized in a 1:1 ratio to receive the HOME BP intervention (n = 305) or usual care (n = 317).
Intervention vs usual care. The HOME BP intervention for the self-management of high BP consisted of an integrated patient and health care practitioner online digital intervention, BP self-monitoring (using an Omron M3 monitor), health care practitioner directed and supervised titration of antihypertensive drugs, and user-selected lifestyle modifications. Participants were advised via automated email reminders to take 2 morning BP readings for 7 days each month and to enter online each second reading. Mean home BP was calculated, accompanied by feedback of BP results to both patients and professionals with optional evidence-based lifestyle advice (for healthy eating, physical activity, losing weight if appropriate, and salt and alcohol reduction) and motivational support through practice nurses or health care assistances (using the CARE approach – congratulate, ask, reassure, encourage).
Participants allocated to usual care were not provided with self-monitoring equipment or the HOME BP intervention but had online access to the information provided in a patient leaflet for hypertension. This information comprised definitions of hypertension, causes, and brief guidance on treatment, including lifestyle changes and drugs. These participants received routine hypertension care that typically consisted of clinic BP monitoring to titrate drugs, with appointments and drug changes made at the discretion of the general practitioner. Participants were not prevented from self-monitoring, but data on self-monitoring practices were collected at the end of the trial from patients and practitioners.
Measures and analysis. The primary outcome measure was the difference in systolic BP at 12-month follow-up between the intervention and usual care groups (adjusting for baseline BP, practice, BP target levels, and sex). Secondary outcomes included systolic and diastolic BP at 6 and 12 months, weight, modified patient enablement instrument, drug adherence, health-related quality of life, and side effects from the symptoms section of an adjusted illness perceptions questionnaire. At trial, registration participants and general practitioners were asked about their use of self-monitoring in the usual care group.
The primary analysis used general linear modelling to compare systolic BP in the intervention and usual care groups at follow-up, adjusting for baseline BP, practice (as a random effect to take into account clustering), BP target levels, and sex. Analyses were on an intention-to-treat basis and used multiple imputation for missing data. Sensitivity analyses used complete cases and a repeated measures technique. Secondary analyses used similar techniques to assess differences between groups. A within-trial economic analysis estimated cost per unit reduction in systolic BP by using similar adjustments and multiple imputation for missing values. Repeated bootstrapping was used to estimate the probability of the intervention being cost-effective at different levels of willingness to pay per unit reduction in BP.
Main results. The intervention and usual care groups did not differ significantly – participants had a mean age of 66 years and mean baseline clinical BP of 151.6/85.3 mm Hg and 151.7/86.4 mm Hg (usual care and intervention, respectively). Most participants were White British (94%), just more than half were men, and the time since diagnosis averaged around 11 years. The most deprived group (based on the English Index of Multiple Deprivation) accounted for 63/622 (10%), with the least deprived group accounting for 326/622 (52%).
After 1 year, data were available from 552 participants (88.6%) with imputation for the remaining 70 participants (11.4%). Mean BP dropped from 151.7/86.4 to 138.4/80.2 mm Hg in the intervention group and from 151.6/85.3 to 141.8/79.8 mm Hg in the usual care group, giving a mean difference in systolic BP of −3.4 mm Hg (95% CI −6.1 to −0.8 mm Hg) and a mean difference in diastolic BP of −0.5 mm Hg (−1.9 to 0.9 mm Hg). Exploratory subgroup analyses suggested that participants aged 67 years or older had a smaller effect size than those younger than 67. Similarly, while the effect sizes in the standard and diabetes target groups were similar, those older than 80 years with a higher target of 145/85 mm Hg showed little evidence of benefit. Results for other subgroups, including sex, baseline BP, deprivation, and history of self-monitoring, were similar between groups.
Engagement with the digital intervention was high, with 281/305 (92%) participants completing the 2 core training sessions, 268/305 (88%) completing a week of practice BP readings, and 243/305 (80%) completing at least 3 weeks of BP entries. Furthermore, 214/305 (70%) were still monitoring in the last 3 months of participation. However, less than 1/3 of participants chose to register on 1 of the optional lifestyle change modules. In the usual care group, a post-hoc analysis after 12 months showed that 112/234 (47%) patients reported monitoring their own BP at home at least once per month during the trial.
The difference in mean cost per patient was £38 (US $51.30, €41.9; 95% CI £27 to £47), which along with the decrease in systolic BP, gave an incremental cost per mm Hg BP reduction of £11 (£6 to £29). Bootstrapping analysis showed the intervention had high (90%) probability of being cost-effective at willingness to pay above £20 per unit reduction. The probabilities of being cost-effective for the intervention against usual care were 87%, 93%, and 97% at thresholds of £20, £30, and £50, respectively.
Conclusion. The HOME BP digital intervention for the management of hypertension by using self-monitored BP led to better control of systolic BP after 1 year than usual care, with low incremental costs. Implementation in primary care will require integration into clinical workflows and consideration of people who are digitally excluded.
Commentary
Elevated BP, also known as hypertension, is the most important, modifiable risk factor for cardiovascular disease and mortality.1 Clinically significant effects and improvements in mortality can be achieved with relatively small reductions in BP levels. Long-established lifestyle modifications that effectively lower BP include weight loss, reduced sodium intake, increased physical activity, and limited alcohol intake. However, motivating patients to achieve lifestyle modifications is among the most difficult aspects of managing hypertension. Importantly, for individuals taking antihypertensive medication, lifestyle modification is recommended as adjunctive therapy to reduce BP. Given that target blood pressure levels are reached for less than half of adults, novel interventions are needed to improve BP control – in particular, individualized cognitive behavioral interventions are more likely to be effective than standardized, single-component interventions.
Guided self-management for hypertension as part of systematic, planned care offers the potential for improvements in adherence and in turn improved long-term patient outcomes.2 Self-management can encompass a wide range of behaviors in addition to medication titration and monitoring of symptoms, such as individuals’ ability to manage physical, psychosocial and lifestyle behaviors related to their condition.3 Digital interventions leveraging apps, software, and/or technologies in particular have the potential to support people in self-management, allow for remote monitoring, and enable personalized and adaptive strategies for chronic disease management.4-5 An example of a digital intervention in the context of guided self-management for hypertension can be a web-based program delivered by computer or phone that combines health information with decision support to help inform behavior change in patients and remote monitoring of patient status by health professionals. Well-designed digital interventions can effectively change patient health-related behaviors, improve patient knowledge and confidence for self-management of health, and lead to better health outcomes.6-7
This study adds to the literature as a large, randomized controlled trial evaluating the effectiveness of a digital intervention in the field of hypertension and with follow-up for a year. The authors highlight that relatively few studies have been performed that combine self-monitoring with a digitally delivered cointervention, and none has shown a major effect in an adequately powered trial over a year. Results from this study showed that HOME BP, a digital intervention enabling self-management of hypertension, including self-monitoring, titration based on self-monitored BP, lifestyle advice, and behavioral support for patients and health care professionals, resulted in a worthwhile reduction of systolic BP. In addition, this reduction was achieved at modest cost based on the within trial cost effectiveness analysis.
There are many important strengths of this study, especially related to the design and analysis strategy, and some limitations. This study was designed as a randomized controlled trial with a 1 year follow-up period, although participants were unmasked to the group they were randomized to, which may have impacted their behaviors while in the study. As the authors state, the study was not only adequately powered to detect a difference in blood pressure, but also over-recruitment ensured such an effect was not missed. Recruiting from a large number of general practices ensured generalizability in terms of health care professionals. Importantly, while study participants mostly identified as predominantly White and tended to be of higher socioeconomic status, this is representative of the aged population in England and Wales. Nevertheless, generalizability of findings from this study is still limited to the demographic characteristics of the study population. Other strengths included inclusion of intention-to-treat analysis, multiple imputation for missing data, sensitivity analysis, as well as economic analysis and cost effectiveness analysis.
Of note, results from the study are only attributable to the digital interventions used in this study (digital web-based with limited mechanisms of behavior change and engagement built-in) and thus should not be generalized to all digital interventions for managing hypertension. Also, as the authors highlight, the relative importance of the different parts of the digital intervention were unable to be distinguished, although this type of analysis is important in multicomponent interventions to better understand the most effective mechanism impacting change in the primary outcome.
Applications for Clinical Practice
Results of this study demonstrated that among participants being treated with hypertension, those engaged with the HOME BP digital intervention (combining self-monitoring of blood pressure with guided self-management) had better control of systolic BP after 1 year compared to participants receiving usual care. While these findings have important implications in the management of hypertension in health care systems, its integration into clinical workflow, sustainability, long-term clinical effectiveness, and effectiveness among diverse populations is unclear. However, clinicians can still encourage and support the use of evidence-based digital tools for patient self-monitoring of BP and guided-management of lifestyle modifications to lower BP. Additionally, clinicians can proactively propose incorporating evidence-based digital interventions like HOME BP into routine clinical practice guidelines.
Financial disclosures: None.
1. Samadian F, Dalili N, Jamalian A. Lifestyle Modifications to Prevent and Control Hypertension. Iran J Kidney Dis. 2016;10(5):237-263.
2. McLean G, Band R, Saunderson K, et al. Digital interventions to promote self-management in adults with hypertension systematic review and meta-analysis. J Hypertens. 2016;34(4):600-612. doi:10.1097/HJH.0000000000000859
3. Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002 Nov 20;288(19):2469-2475. doi:10.1001/jama.288.19.2469
4. Morton K, Dennison L, May C, et al. Using digital interventions for self-management of chronic physical health conditions: A meta-ethnography review of published studies. Patient Educ Couns. 2017;100(4):616-635. doi:10.1016/j.ped.2016.10.019
5. Kario K. Management of Hypertension in the Digital Era: Small Wearable Monitoring Devices for Remote Blood Pressure Monitoring. Hypertension. 2020;76(3):640-650. doi:10.1161/HYPERTENSIONAHA.120.14742
6. Murray E, Burns J, See TS, et al. Interactive Health Communication Applications for people with chronic disease. Cochrane Database Syst Rev. 2005;(4):CD004274. doi:10.1002/14651858.CD004274.pub4
7. Webb TL, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010;12(1):e4. doi:10.2196/jmir.1376
Study Overview
Objective. To evaluate whether a digital intervention comprising self-monitoring of blood pressure (BP) with reminders and predetermined drug changes combined with lifestyle change support resulted in lower systolic BP in people receiving treatment for hypertension that was poorly controlled, and whether this approach was cost effective.
Design. Unmasked randomized controlled trial.
Settings and participants. Eligible participants were identified from clinical codes recorded in the electronic health records of 76 collaborating general practices from the National Institute for Health Research Clinical Research Network, a United Kingdom government agency. The practices sent invitation letters to eligible participants to come to the clinic to establish eligibility, take consent, and collect baseline data via online questionnaires.
Eligible participants were aged 18 years or older with treated hypertension, a mean baseline BP reading of more than 140/90 mm Hg and were taking no more than 3 antihypertensive drugs. Participants also needed to be willing to self-monitor and have access to the internet (with support from a family member if needed). Exclusions included BP greater than 180/110 mm Hg, atrial fibrillation, hypertension not managed by their general practitioner, chronic kidney disease stage 4-5, postural hypotension (> 20 mm Hg systolic drop), an acute cardiovascular event in the previous 3 months, terminal disease, or another condition which in the opinion of their general practitioner made participation inappropriate.
Of the 11 399 invitation letters sent out, 1389 (12%) potential participants responded positively and were screened for eligibility. Those who declined to take part could optionally give their reasons, and responses were gained from 2426 of 10 010 (24%). The mean age of those who gave a reason for declining was 73 years. The most commonly selected reasons for declining were not having access to the internet (982, 41%), not wanting to participate in a research trial (617, 25%) or an internet study (543, 22%), and not wanting to change drugs (535, 22%). Of the 1389 screened, 734 were ineligible, and 33 did not complete baseline measures and randomization. The remaining 622 people who were randomized in a 1:1 ratio to receive the HOME BP intervention (n = 305) or usual care (n = 317).
Intervention vs usual care. The HOME BP intervention for the self-management of high BP consisted of an integrated patient and health care practitioner online digital intervention, BP self-monitoring (using an Omron M3 monitor), health care practitioner directed and supervised titration of antihypertensive drugs, and user-selected lifestyle modifications. Participants were advised via automated email reminders to take 2 morning BP readings for 7 days each month and to enter online each second reading. Mean home BP was calculated, accompanied by feedback of BP results to both patients and professionals with optional evidence-based lifestyle advice (for healthy eating, physical activity, losing weight if appropriate, and salt and alcohol reduction) and motivational support through practice nurses or health care assistances (using the CARE approach – congratulate, ask, reassure, encourage).
Participants allocated to usual care were not provided with self-monitoring equipment or the HOME BP intervention but had online access to the information provided in a patient leaflet for hypertension. This information comprised definitions of hypertension, causes, and brief guidance on treatment, including lifestyle changes and drugs. These participants received routine hypertension care that typically consisted of clinic BP monitoring to titrate drugs, with appointments and drug changes made at the discretion of the general practitioner. Participants were not prevented from self-monitoring, but data on self-monitoring practices were collected at the end of the trial from patients and practitioners.
Measures and analysis. The primary outcome measure was the difference in systolic BP at 12-month follow-up between the intervention and usual care groups (adjusting for baseline BP, practice, BP target levels, and sex). Secondary outcomes included systolic and diastolic BP at 6 and 12 months, weight, modified patient enablement instrument, drug adherence, health-related quality of life, and side effects from the symptoms section of an adjusted illness perceptions questionnaire. At trial, registration participants and general practitioners were asked about their use of self-monitoring in the usual care group.
The primary analysis used general linear modelling to compare systolic BP in the intervention and usual care groups at follow-up, adjusting for baseline BP, practice (as a random effect to take into account clustering), BP target levels, and sex. Analyses were on an intention-to-treat basis and used multiple imputation for missing data. Sensitivity analyses used complete cases and a repeated measures technique. Secondary analyses used similar techniques to assess differences between groups. A within-trial economic analysis estimated cost per unit reduction in systolic BP by using similar adjustments and multiple imputation for missing values. Repeated bootstrapping was used to estimate the probability of the intervention being cost-effective at different levels of willingness to pay per unit reduction in BP.
Main results. The intervention and usual care groups did not differ significantly – participants had a mean age of 66 years and mean baseline clinical BP of 151.6/85.3 mm Hg and 151.7/86.4 mm Hg (usual care and intervention, respectively). Most participants were White British (94%), just more than half were men, and the time since diagnosis averaged around 11 years. The most deprived group (based on the English Index of Multiple Deprivation) accounted for 63/622 (10%), with the least deprived group accounting for 326/622 (52%).
After 1 year, data were available from 552 participants (88.6%) with imputation for the remaining 70 participants (11.4%). Mean BP dropped from 151.7/86.4 to 138.4/80.2 mm Hg in the intervention group and from 151.6/85.3 to 141.8/79.8 mm Hg in the usual care group, giving a mean difference in systolic BP of −3.4 mm Hg (95% CI −6.1 to −0.8 mm Hg) and a mean difference in diastolic BP of −0.5 mm Hg (−1.9 to 0.9 mm Hg). Exploratory subgroup analyses suggested that participants aged 67 years or older had a smaller effect size than those younger than 67. Similarly, while the effect sizes in the standard and diabetes target groups were similar, those older than 80 years with a higher target of 145/85 mm Hg showed little evidence of benefit. Results for other subgroups, including sex, baseline BP, deprivation, and history of self-monitoring, were similar between groups.
Engagement with the digital intervention was high, with 281/305 (92%) participants completing the 2 core training sessions, 268/305 (88%) completing a week of practice BP readings, and 243/305 (80%) completing at least 3 weeks of BP entries. Furthermore, 214/305 (70%) were still monitoring in the last 3 months of participation. However, less than 1/3 of participants chose to register on 1 of the optional lifestyle change modules. In the usual care group, a post-hoc analysis after 12 months showed that 112/234 (47%) patients reported monitoring their own BP at home at least once per month during the trial.
The difference in mean cost per patient was £38 (US $51.30, €41.9; 95% CI £27 to £47), which along with the decrease in systolic BP, gave an incremental cost per mm Hg BP reduction of £11 (£6 to £29). Bootstrapping analysis showed the intervention had high (90%) probability of being cost-effective at willingness to pay above £20 per unit reduction. The probabilities of being cost-effective for the intervention against usual care were 87%, 93%, and 97% at thresholds of £20, £30, and £50, respectively.
Conclusion. The HOME BP digital intervention for the management of hypertension by using self-monitored BP led to better control of systolic BP after 1 year than usual care, with low incremental costs. Implementation in primary care will require integration into clinical workflows and consideration of people who are digitally excluded.
Commentary
Elevated BP, also known as hypertension, is the most important, modifiable risk factor for cardiovascular disease and mortality.1 Clinically significant effects and improvements in mortality can be achieved with relatively small reductions in BP levels. Long-established lifestyle modifications that effectively lower BP include weight loss, reduced sodium intake, increased physical activity, and limited alcohol intake. However, motivating patients to achieve lifestyle modifications is among the most difficult aspects of managing hypertension. Importantly, for individuals taking antihypertensive medication, lifestyle modification is recommended as adjunctive therapy to reduce BP. Given that target blood pressure levels are reached for less than half of adults, novel interventions are needed to improve BP control – in particular, individualized cognitive behavioral interventions are more likely to be effective than standardized, single-component interventions.
Guided self-management for hypertension as part of systematic, planned care offers the potential for improvements in adherence and in turn improved long-term patient outcomes.2 Self-management can encompass a wide range of behaviors in addition to medication titration and monitoring of symptoms, such as individuals’ ability to manage physical, psychosocial and lifestyle behaviors related to their condition.3 Digital interventions leveraging apps, software, and/or technologies in particular have the potential to support people in self-management, allow for remote monitoring, and enable personalized and adaptive strategies for chronic disease management.4-5 An example of a digital intervention in the context of guided self-management for hypertension can be a web-based program delivered by computer or phone that combines health information with decision support to help inform behavior change in patients and remote monitoring of patient status by health professionals. Well-designed digital interventions can effectively change patient health-related behaviors, improve patient knowledge and confidence for self-management of health, and lead to better health outcomes.6-7
This study adds to the literature as a large, randomized controlled trial evaluating the effectiveness of a digital intervention in the field of hypertension and with follow-up for a year. The authors highlight that relatively few studies have been performed that combine self-monitoring with a digitally delivered cointervention, and none has shown a major effect in an adequately powered trial over a year. Results from this study showed that HOME BP, a digital intervention enabling self-management of hypertension, including self-monitoring, titration based on self-monitored BP, lifestyle advice, and behavioral support for patients and health care professionals, resulted in a worthwhile reduction of systolic BP. In addition, this reduction was achieved at modest cost based on the within trial cost effectiveness analysis.
There are many important strengths of this study, especially related to the design and analysis strategy, and some limitations. This study was designed as a randomized controlled trial with a 1 year follow-up period, although participants were unmasked to the group they were randomized to, which may have impacted their behaviors while in the study. As the authors state, the study was not only adequately powered to detect a difference in blood pressure, but also over-recruitment ensured such an effect was not missed. Recruiting from a large number of general practices ensured generalizability in terms of health care professionals. Importantly, while study participants mostly identified as predominantly White and tended to be of higher socioeconomic status, this is representative of the aged population in England and Wales. Nevertheless, generalizability of findings from this study is still limited to the demographic characteristics of the study population. Other strengths included inclusion of intention-to-treat analysis, multiple imputation for missing data, sensitivity analysis, as well as economic analysis and cost effectiveness analysis.
Of note, results from the study are only attributable to the digital interventions used in this study (digital web-based with limited mechanisms of behavior change and engagement built-in) and thus should not be generalized to all digital interventions for managing hypertension. Also, as the authors highlight, the relative importance of the different parts of the digital intervention were unable to be distinguished, although this type of analysis is important in multicomponent interventions to better understand the most effective mechanism impacting change in the primary outcome.
Applications for Clinical Practice
Results of this study demonstrated that among participants being treated with hypertension, those engaged with the HOME BP digital intervention (combining self-monitoring of blood pressure with guided self-management) had better control of systolic BP after 1 year compared to participants receiving usual care. While these findings have important implications in the management of hypertension in health care systems, its integration into clinical workflow, sustainability, long-term clinical effectiveness, and effectiveness among diverse populations is unclear. However, clinicians can still encourage and support the use of evidence-based digital tools for patient self-monitoring of BP and guided-management of lifestyle modifications to lower BP. Additionally, clinicians can proactively propose incorporating evidence-based digital interventions like HOME BP into routine clinical practice guidelines.
Financial disclosures: None.
Study Overview
Objective. To evaluate whether a digital intervention comprising self-monitoring of blood pressure (BP) with reminders and predetermined drug changes combined with lifestyle change support resulted in lower systolic BP in people receiving treatment for hypertension that was poorly controlled, and whether this approach was cost effective.
Design. Unmasked randomized controlled trial.
Settings and participants. Eligible participants were identified from clinical codes recorded in the electronic health records of 76 collaborating general practices from the National Institute for Health Research Clinical Research Network, a United Kingdom government agency. The practices sent invitation letters to eligible participants to come to the clinic to establish eligibility, take consent, and collect baseline data via online questionnaires.
Eligible participants were aged 18 years or older with treated hypertension, a mean baseline BP reading of more than 140/90 mm Hg and were taking no more than 3 antihypertensive drugs. Participants also needed to be willing to self-monitor and have access to the internet (with support from a family member if needed). Exclusions included BP greater than 180/110 mm Hg, atrial fibrillation, hypertension not managed by their general practitioner, chronic kidney disease stage 4-5, postural hypotension (> 20 mm Hg systolic drop), an acute cardiovascular event in the previous 3 months, terminal disease, or another condition which in the opinion of their general practitioner made participation inappropriate.
Of the 11 399 invitation letters sent out, 1389 (12%) potential participants responded positively and were screened for eligibility. Those who declined to take part could optionally give their reasons, and responses were gained from 2426 of 10 010 (24%). The mean age of those who gave a reason for declining was 73 years. The most commonly selected reasons for declining were not having access to the internet (982, 41%), not wanting to participate in a research trial (617, 25%) or an internet study (543, 22%), and not wanting to change drugs (535, 22%). Of the 1389 screened, 734 were ineligible, and 33 did not complete baseline measures and randomization. The remaining 622 people who were randomized in a 1:1 ratio to receive the HOME BP intervention (n = 305) or usual care (n = 317).
Intervention vs usual care. The HOME BP intervention for the self-management of high BP consisted of an integrated patient and health care practitioner online digital intervention, BP self-monitoring (using an Omron M3 monitor), health care practitioner directed and supervised titration of antihypertensive drugs, and user-selected lifestyle modifications. Participants were advised via automated email reminders to take 2 morning BP readings for 7 days each month and to enter online each second reading. Mean home BP was calculated, accompanied by feedback of BP results to both patients and professionals with optional evidence-based lifestyle advice (for healthy eating, physical activity, losing weight if appropriate, and salt and alcohol reduction) and motivational support through practice nurses or health care assistances (using the CARE approach – congratulate, ask, reassure, encourage).
Participants allocated to usual care were not provided with self-monitoring equipment or the HOME BP intervention but had online access to the information provided in a patient leaflet for hypertension. This information comprised definitions of hypertension, causes, and brief guidance on treatment, including lifestyle changes and drugs. These participants received routine hypertension care that typically consisted of clinic BP monitoring to titrate drugs, with appointments and drug changes made at the discretion of the general practitioner. Participants were not prevented from self-monitoring, but data on self-monitoring practices were collected at the end of the trial from patients and practitioners.
Measures and analysis. The primary outcome measure was the difference in systolic BP at 12-month follow-up between the intervention and usual care groups (adjusting for baseline BP, practice, BP target levels, and sex). Secondary outcomes included systolic and diastolic BP at 6 and 12 months, weight, modified patient enablement instrument, drug adherence, health-related quality of life, and side effects from the symptoms section of an adjusted illness perceptions questionnaire. At trial, registration participants and general practitioners were asked about their use of self-monitoring in the usual care group.
The primary analysis used general linear modelling to compare systolic BP in the intervention and usual care groups at follow-up, adjusting for baseline BP, practice (as a random effect to take into account clustering), BP target levels, and sex. Analyses were on an intention-to-treat basis and used multiple imputation for missing data. Sensitivity analyses used complete cases and a repeated measures technique. Secondary analyses used similar techniques to assess differences between groups. A within-trial economic analysis estimated cost per unit reduction in systolic BP by using similar adjustments and multiple imputation for missing values. Repeated bootstrapping was used to estimate the probability of the intervention being cost-effective at different levels of willingness to pay per unit reduction in BP.
Main results. The intervention and usual care groups did not differ significantly – participants had a mean age of 66 years and mean baseline clinical BP of 151.6/85.3 mm Hg and 151.7/86.4 mm Hg (usual care and intervention, respectively). Most participants were White British (94%), just more than half were men, and the time since diagnosis averaged around 11 years. The most deprived group (based on the English Index of Multiple Deprivation) accounted for 63/622 (10%), with the least deprived group accounting for 326/622 (52%).
After 1 year, data were available from 552 participants (88.6%) with imputation for the remaining 70 participants (11.4%). Mean BP dropped from 151.7/86.4 to 138.4/80.2 mm Hg in the intervention group and from 151.6/85.3 to 141.8/79.8 mm Hg in the usual care group, giving a mean difference in systolic BP of −3.4 mm Hg (95% CI −6.1 to −0.8 mm Hg) and a mean difference in diastolic BP of −0.5 mm Hg (−1.9 to 0.9 mm Hg). Exploratory subgroup analyses suggested that participants aged 67 years or older had a smaller effect size than those younger than 67. Similarly, while the effect sizes in the standard and diabetes target groups were similar, those older than 80 years with a higher target of 145/85 mm Hg showed little evidence of benefit. Results for other subgroups, including sex, baseline BP, deprivation, and history of self-monitoring, were similar between groups.
Engagement with the digital intervention was high, with 281/305 (92%) participants completing the 2 core training sessions, 268/305 (88%) completing a week of practice BP readings, and 243/305 (80%) completing at least 3 weeks of BP entries. Furthermore, 214/305 (70%) were still monitoring in the last 3 months of participation. However, less than 1/3 of participants chose to register on 1 of the optional lifestyle change modules. In the usual care group, a post-hoc analysis after 12 months showed that 112/234 (47%) patients reported monitoring their own BP at home at least once per month during the trial.
The difference in mean cost per patient was £38 (US $51.30, €41.9; 95% CI £27 to £47), which along with the decrease in systolic BP, gave an incremental cost per mm Hg BP reduction of £11 (£6 to £29). Bootstrapping analysis showed the intervention had high (90%) probability of being cost-effective at willingness to pay above £20 per unit reduction. The probabilities of being cost-effective for the intervention against usual care were 87%, 93%, and 97% at thresholds of £20, £30, and £50, respectively.
Conclusion. The HOME BP digital intervention for the management of hypertension by using self-monitored BP led to better control of systolic BP after 1 year than usual care, with low incremental costs. Implementation in primary care will require integration into clinical workflows and consideration of people who are digitally excluded.
Commentary
Elevated BP, also known as hypertension, is the most important, modifiable risk factor for cardiovascular disease and mortality.1 Clinically significant effects and improvements in mortality can be achieved with relatively small reductions in BP levels. Long-established lifestyle modifications that effectively lower BP include weight loss, reduced sodium intake, increased physical activity, and limited alcohol intake. However, motivating patients to achieve lifestyle modifications is among the most difficult aspects of managing hypertension. Importantly, for individuals taking antihypertensive medication, lifestyle modification is recommended as adjunctive therapy to reduce BP. Given that target blood pressure levels are reached for less than half of adults, novel interventions are needed to improve BP control – in particular, individualized cognitive behavioral interventions are more likely to be effective than standardized, single-component interventions.
Guided self-management for hypertension as part of systematic, planned care offers the potential for improvements in adherence and in turn improved long-term patient outcomes.2 Self-management can encompass a wide range of behaviors in addition to medication titration and monitoring of symptoms, such as individuals’ ability to manage physical, psychosocial and lifestyle behaviors related to their condition.3 Digital interventions leveraging apps, software, and/or technologies in particular have the potential to support people in self-management, allow for remote monitoring, and enable personalized and adaptive strategies for chronic disease management.4-5 An example of a digital intervention in the context of guided self-management for hypertension can be a web-based program delivered by computer or phone that combines health information with decision support to help inform behavior change in patients and remote monitoring of patient status by health professionals. Well-designed digital interventions can effectively change patient health-related behaviors, improve patient knowledge and confidence for self-management of health, and lead to better health outcomes.6-7
This study adds to the literature as a large, randomized controlled trial evaluating the effectiveness of a digital intervention in the field of hypertension and with follow-up for a year. The authors highlight that relatively few studies have been performed that combine self-monitoring with a digitally delivered cointervention, and none has shown a major effect in an adequately powered trial over a year. Results from this study showed that HOME BP, a digital intervention enabling self-management of hypertension, including self-monitoring, titration based on self-monitored BP, lifestyle advice, and behavioral support for patients and health care professionals, resulted in a worthwhile reduction of systolic BP. In addition, this reduction was achieved at modest cost based on the within trial cost effectiveness analysis.
There are many important strengths of this study, especially related to the design and analysis strategy, and some limitations. This study was designed as a randomized controlled trial with a 1 year follow-up period, although participants were unmasked to the group they were randomized to, which may have impacted their behaviors while in the study. As the authors state, the study was not only adequately powered to detect a difference in blood pressure, but also over-recruitment ensured such an effect was not missed. Recruiting from a large number of general practices ensured generalizability in terms of health care professionals. Importantly, while study participants mostly identified as predominantly White and tended to be of higher socioeconomic status, this is representative of the aged population in England and Wales. Nevertheless, generalizability of findings from this study is still limited to the demographic characteristics of the study population. Other strengths included inclusion of intention-to-treat analysis, multiple imputation for missing data, sensitivity analysis, as well as economic analysis and cost effectiveness analysis.
Of note, results from the study are only attributable to the digital interventions used in this study (digital web-based with limited mechanisms of behavior change and engagement built-in) and thus should not be generalized to all digital interventions for managing hypertension. Also, as the authors highlight, the relative importance of the different parts of the digital intervention were unable to be distinguished, although this type of analysis is important in multicomponent interventions to better understand the most effective mechanism impacting change in the primary outcome.
Applications for Clinical Practice
Results of this study demonstrated that among participants being treated with hypertension, those engaged with the HOME BP digital intervention (combining self-monitoring of blood pressure with guided self-management) had better control of systolic BP after 1 year compared to participants receiving usual care. While these findings have important implications in the management of hypertension in health care systems, its integration into clinical workflow, sustainability, long-term clinical effectiveness, and effectiveness among diverse populations is unclear. However, clinicians can still encourage and support the use of evidence-based digital tools for patient self-monitoring of BP and guided-management of lifestyle modifications to lower BP. Additionally, clinicians can proactively propose incorporating evidence-based digital interventions like HOME BP into routine clinical practice guidelines.
Financial disclosures: None.
1. Samadian F, Dalili N, Jamalian A. Lifestyle Modifications to Prevent and Control Hypertension. Iran J Kidney Dis. 2016;10(5):237-263.
2. McLean G, Band R, Saunderson K, et al. Digital interventions to promote self-management in adults with hypertension systematic review and meta-analysis. J Hypertens. 2016;34(4):600-612. doi:10.1097/HJH.0000000000000859
3. Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002 Nov 20;288(19):2469-2475. doi:10.1001/jama.288.19.2469
4. Morton K, Dennison L, May C, et al. Using digital interventions for self-management of chronic physical health conditions: A meta-ethnography review of published studies. Patient Educ Couns. 2017;100(4):616-635. doi:10.1016/j.ped.2016.10.019
5. Kario K. Management of Hypertension in the Digital Era: Small Wearable Monitoring Devices for Remote Blood Pressure Monitoring. Hypertension. 2020;76(3):640-650. doi:10.1161/HYPERTENSIONAHA.120.14742
6. Murray E, Burns J, See TS, et al. Interactive Health Communication Applications for people with chronic disease. Cochrane Database Syst Rev. 2005;(4):CD004274. doi:10.1002/14651858.CD004274.pub4
7. Webb TL, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010;12(1):e4. doi:10.2196/jmir.1376
1. Samadian F, Dalili N, Jamalian A. Lifestyle Modifications to Prevent and Control Hypertension. Iran J Kidney Dis. 2016;10(5):237-263.
2. McLean G, Band R, Saunderson K, et al. Digital interventions to promote self-management in adults with hypertension systematic review and meta-analysis. J Hypertens. 2016;34(4):600-612. doi:10.1097/HJH.0000000000000859
3. Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002 Nov 20;288(19):2469-2475. doi:10.1001/jama.288.19.2469
4. Morton K, Dennison L, May C, et al. Using digital interventions for self-management of chronic physical health conditions: A meta-ethnography review of published studies. Patient Educ Couns. 2017;100(4):616-635. doi:10.1016/j.ped.2016.10.019
5. Kario K. Management of Hypertension in the Digital Era: Small Wearable Monitoring Devices for Remote Blood Pressure Monitoring. Hypertension. 2020;76(3):640-650. doi:10.1161/HYPERTENSIONAHA.120.14742
6. Murray E, Burns J, See TS, et al. Interactive Health Communication Applications for people with chronic disease. Cochrane Database Syst Rev. 2005;(4):CD004274. doi:10.1002/14651858.CD004274.pub4
7. Webb TL, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010;12(1):e4. doi:10.2196/jmir.1376
‘Locker room talk’ about death: Time for oncologists to stop
In a recent inpatient service block, I was seeing patients alongside a resident I had gotten to know well. We were consulted on a patient with metastatic head and neck cancer who had not sought care for over a year.
When the patient presented, his voice was raspy and he could not swallow. He had lost 40 pounds. In addition to his locally advanced disease, his lungs were riddled with metastatic lesions.
When we left the room, the resident and I went to speak to the patient’s primary team, and he began to relay our recommendations.
The first words out of his mouth were, “Well, it’s pretty clear he’s going to die.”
The statement took me aback. I wasn’t alarmed by the accuracy of what he had said. The patient was obviously not doing well, and he ended up dying soon after this visit.
It was more the abrupt manner in which the resident had spoken about death. The brusque phrasing felt atypical coming from the otherwise gentle-hearted trainee. He wasn’t referring to a faceless person. We had just seen the man a few minutes ago and heard his personal struggles. I tried to see if anyone else on the team was caught off guard, but everyone was taking notes or continuing to listen, seemingly undeterred.
Oncologists’ ‘locker room talk’
And now, with the COVID pandemic forcing most of our tumor boards to go virtual, I find this locker room talk comes even more readily; phrases like “this patient is going to die” are often passed around flippantly, as if saying so will help ease the tension. During these interactions, my colleagues and I rarely acknowledge the seriousness of what a patient death will do to their family and loved ones – or what losing a patient whom we’ve known for years may do to our own psyche.
This language can even creep into how we speak with patients. We are often taught to offer prognoses coldly, ensuring that patients have a clear sense of how long they have left and to help inform their treatment choices. And yet, this training does not necessarily align with what patients want and need. For instance, in a recent survey of patients with chronic obstructive pulmonary disease, patients consistently rated physicians poorly at discussing prognosis, what dying might be like, as well as spirituality and religion.
But at the same time, these matter-of-fact statements about death probably help protect us. Death is a routine, inevitable part of an oncologist’s life, and over time, oncology training and practice hardens us to it. During medical school, I remember that a patient dying would trigger immediate reflection, sadness, and conversation with our peers. Now, unless I know a patient well, I find myself rarely reflecting on the patient behind the facts. This evolution is natural for an oncologist: If you don’t develop a tough skin about death, you may become overwhelmed with the frequency of it.
The COVID pandemic has amped our hardness toward death into overdrive. Whether we are in the intensive care unit or simply viewing death rates during the most recent COVID Delta wave, many of us cope by disassociating a face from a name.
Making time for reflection
But taking time to reflect can be therapeutic.
I recently referred a patient with metastatic prostate cancer for a phase 1 trial at an outside institution. He was one of the first patients in my genitourinary malignancies clinic when I started as an attending. The patient had progressed through several lines of therapy and was being referred for an investigational phase 1 therapy. We had discussed hospice referral, and the patient was ready for it if this therapy didn’t work out.
I did not see or hear from the man while he was on the trial. A few months later, however, the principal investigator of the trial called me to let me know the patient had progressed through the agent, suffering from significant urinary obstruction, and he was on hospice. “Unfortunately,” the investigator told me, “he’s not going to live much longer.”
When I checked in with the hospice, the patient had died.
I was surprised again at how matter-of-fact the discussion of death had been. But I was even more surprised by my own reaction. Despite the relationship I had formed with the patient, I did not feel much when I heard he had died. I didn’t have time to process the news in the moment. It was time to move on to the next patient.
It was only later, when I called the patient’s family, that I allowed my emotions to flood in. I told his family how grateful I was to know him, how strong he’d been. The patient’s family and I talked about the human, not his passing. It felt good.
Abandoning locker room talk
So how do we change how we talk about death? I don’t think the answer is massive educational programs or passing responsibility for advance care planning onto palliative care specialists. The change needs to be driven by individual oncologists. We can call out discussions of death that make us uncomfortable, gently reminding each other that we’re talking about a human life.
We can learn from our palliative care colleagues; their conversations about death routinely include a patient’s support system and personal stories. Palliative care doctors always refer to the patient by name, which helps humanize the person behind the chart.
We can emphasize a feeling of hope, a sentiment that may also be therapeutic to our patients. Even when a patient is dying, there is always something to be done. We can comfort their family, explaining what brought us to this point and how sorry we are that this is happening. We can provide options for symptom control and help patients manage those symptoms.
And we can allow ourselves to talk about how much a death affects us. We can acknowledge how much it sucks that a patient is going to die, how challenging that will be to his/her family, and how we wish it could have ended differently.
Subtle changes like these will improve our own ability to process and discuss death and will ultimately lead to better relationships with our patients. But it starts with eliminating the “locker room talk” of how we discuss death.
Ravi B. Parikh, MD, MPP, is a medical oncologist and faculty member at the University of Pennsylvania and the Philadelphia VA Medical Center, an adjunct fellow at the Leonard Davis Institute of Health Economics, and senior clinical advisor at the Coalition to Transform Advanced Care (C-TAC). He has served as a director, officer, partner, employee, adviser, consultant, or trustee for GNS Healthcare, Nanology, and Cancer Study Group, and he has received research grant from Embedded Healthcare, Veterans Administration, PCF, National Palliative Care Research Center, and MUSC. A version of this article first appeared on Medscape.com.
In a recent inpatient service block, I was seeing patients alongside a resident I had gotten to know well. We were consulted on a patient with metastatic head and neck cancer who had not sought care for over a year.
When the patient presented, his voice was raspy and he could not swallow. He had lost 40 pounds. In addition to his locally advanced disease, his lungs were riddled with metastatic lesions.
When we left the room, the resident and I went to speak to the patient’s primary team, and he began to relay our recommendations.
The first words out of his mouth were, “Well, it’s pretty clear he’s going to die.”
The statement took me aback. I wasn’t alarmed by the accuracy of what he had said. The patient was obviously not doing well, and he ended up dying soon after this visit.
It was more the abrupt manner in which the resident had spoken about death. The brusque phrasing felt atypical coming from the otherwise gentle-hearted trainee. He wasn’t referring to a faceless person. We had just seen the man a few minutes ago and heard his personal struggles. I tried to see if anyone else on the team was caught off guard, but everyone was taking notes or continuing to listen, seemingly undeterred.
Oncologists’ ‘locker room talk’
And now, with the COVID pandemic forcing most of our tumor boards to go virtual, I find this locker room talk comes even more readily; phrases like “this patient is going to die” are often passed around flippantly, as if saying so will help ease the tension. During these interactions, my colleagues and I rarely acknowledge the seriousness of what a patient death will do to their family and loved ones – or what losing a patient whom we’ve known for years may do to our own psyche.
This language can even creep into how we speak with patients. We are often taught to offer prognoses coldly, ensuring that patients have a clear sense of how long they have left and to help inform their treatment choices. And yet, this training does not necessarily align with what patients want and need. For instance, in a recent survey of patients with chronic obstructive pulmonary disease, patients consistently rated physicians poorly at discussing prognosis, what dying might be like, as well as spirituality and religion.
But at the same time, these matter-of-fact statements about death probably help protect us. Death is a routine, inevitable part of an oncologist’s life, and over time, oncology training and practice hardens us to it. During medical school, I remember that a patient dying would trigger immediate reflection, sadness, and conversation with our peers. Now, unless I know a patient well, I find myself rarely reflecting on the patient behind the facts. This evolution is natural for an oncologist: If you don’t develop a tough skin about death, you may become overwhelmed with the frequency of it.
The COVID pandemic has amped our hardness toward death into overdrive. Whether we are in the intensive care unit or simply viewing death rates during the most recent COVID Delta wave, many of us cope by disassociating a face from a name.
Making time for reflection
But taking time to reflect can be therapeutic.
I recently referred a patient with metastatic prostate cancer for a phase 1 trial at an outside institution. He was one of the first patients in my genitourinary malignancies clinic when I started as an attending. The patient had progressed through several lines of therapy and was being referred for an investigational phase 1 therapy. We had discussed hospice referral, and the patient was ready for it if this therapy didn’t work out.
I did not see or hear from the man while he was on the trial. A few months later, however, the principal investigator of the trial called me to let me know the patient had progressed through the agent, suffering from significant urinary obstruction, and he was on hospice. “Unfortunately,” the investigator told me, “he’s not going to live much longer.”
When I checked in with the hospice, the patient had died.
I was surprised again at how matter-of-fact the discussion of death had been. But I was even more surprised by my own reaction. Despite the relationship I had formed with the patient, I did not feel much when I heard he had died. I didn’t have time to process the news in the moment. It was time to move on to the next patient.
It was only later, when I called the patient’s family, that I allowed my emotions to flood in. I told his family how grateful I was to know him, how strong he’d been. The patient’s family and I talked about the human, not his passing. It felt good.
Abandoning locker room talk
So how do we change how we talk about death? I don’t think the answer is massive educational programs or passing responsibility for advance care planning onto palliative care specialists. The change needs to be driven by individual oncologists. We can call out discussions of death that make us uncomfortable, gently reminding each other that we’re talking about a human life.
We can learn from our palliative care colleagues; their conversations about death routinely include a patient’s support system and personal stories. Palliative care doctors always refer to the patient by name, which helps humanize the person behind the chart.
We can emphasize a feeling of hope, a sentiment that may also be therapeutic to our patients. Even when a patient is dying, there is always something to be done. We can comfort their family, explaining what brought us to this point and how sorry we are that this is happening. We can provide options for symptom control and help patients manage those symptoms.
And we can allow ourselves to talk about how much a death affects us. We can acknowledge how much it sucks that a patient is going to die, how challenging that will be to his/her family, and how we wish it could have ended differently.
Subtle changes like these will improve our own ability to process and discuss death and will ultimately lead to better relationships with our patients. But it starts with eliminating the “locker room talk” of how we discuss death.
Ravi B. Parikh, MD, MPP, is a medical oncologist and faculty member at the University of Pennsylvania and the Philadelphia VA Medical Center, an adjunct fellow at the Leonard Davis Institute of Health Economics, and senior clinical advisor at the Coalition to Transform Advanced Care (C-TAC). He has served as a director, officer, partner, employee, adviser, consultant, or trustee for GNS Healthcare, Nanology, and Cancer Study Group, and he has received research grant from Embedded Healthcare, Veterans Administration, PCF, National Palliative Care Research Center, and MUSC. A version of this article first appeared on Medscape.com.
In a recent inpatient service block, I was seeing patients alongside a resident I had gotten to know well. We were consulted on a patient with metastatic head and neck cancer who had not sought care for over a year.
When the patient presented, his voice was raspy and he could not swallow. He had lost 40 pounds. In addition to his locally advanced disease, his lungs were riddled with metastatic lesions.
When we left the room, the resident and I went to speak to the patient’s primary team, and he began to relay our recommendations.
The first words out of his mouth were, “Well, it’s pretty clear he’s going to die.”
The statement took me aback. I wasn’t alarmed by the accuracy of what he had said. The patient was obviously not doing well, and he ended up dying soon after this visit.
It was more the abrupt manner in which the resident had spoken about death. The brusque phrasing felt atypical coming from the otherwise gentle-hearted trainee. He wasn’t referring to a faceless person. We had just seen the man a few minutes ago and heard his personal struggles. I tried to see if anyone else on the team was caught off guard, but everyone was taking notes or continuing to listen, seemingly undeterred.
Oncologists’ ‘locker room talk’
And now, with the COVID pandemic forcing most of our tumor boards to go virtual, I find this locker room talk comes even more readily; phrases like “this patient is going to die” are often passed around flippantly, as if saying so will help ease the tension. During these interactions, my colleagues and I rarely acknowledge the seriousness of what a patient death will do to their family and loved ones – or what losing a patient whom we’ve known for years may do to our own psyche.
This language can even creep into how we speak with patients. We are often taught to offer prognoses coldly, ensuring that patients have a clear sense of how long they have left and to help inform their treatment choices. And yet, this training does not necessarily align with what patients want and need. For instance, in a recent survey of patients with chronic obstructive pulmonary disease, patients consistently rated physicians poorly at discussing prognosis, what dying might be like, as well as spirituality and religion.
But at the same time, these matter-of-fact statements about death probably help protect us. Death is a routine, inevitable part of an oncologist’s life, and over time, oncology training and practice hardens us to it. During medical school, I remember that a patient dying would trigger immediate reflection, sadness, and conversation with our peers. Now, unless I know a patient well, I find myself rarely reflecting on the patient behind the facts. This evolution is natural for an oncologist: If you don’t develop a tough skin about death, you may become overwhelmed with the frequency of it.
The COVID pandemic has amped our hardness toward death into overdrive. Whether we are in the intensive care unit or simply viewing death rates during the most recent COVID Delta wave, many of us cope by disassociating a face from a name.
Making time for reflection
But taking time to reflect can be therapeutic.
I recently referred a patient with metastatic prostate cancer for a phase 1 trial at an outside institution. He was one of the first patients in my genitourinary malignancies clinic when I started as an attending. The patient had progressed through several lines of therapy and was being referred for an investigational phase 1 therapy. We had discussed hospice referral, and the patient was ready for it if this therapy didn’t work out.
I did not see or hear from the man while he was on the trial. A few months later, however, the principal investigator of the trial called me to let me know the patient had progressed through the agent, suffering from significant urinary obstruction, and he was on hospice. “Unfortunately,” the investigator told me, “he’s not going to live much longer.”
When I checked in with the hospice, the patient had died.
I was surprised again at how matter-of-fact the discussion of death had been. But I was even more surprised by my own reaction. Despite the relationship I had formed with the patient, I did not feel much when I heard he had died. I didn’t have time to process the news in the moment. It was time to move on to the next patient.
It was only later, when I called the patient’s family, that I allowed my emotions to flood in. I told his family how grateful I was to know him, how strong he’d been. The patient’s family and I talked about the human, not his passing. It felt good.
Abandoning locker room talk
So how do we change how we talk about death? I don’t think the answer is massive educational programs or passing responsibility for advance care planning onto palliative care specialists. The change needs to be driven by individual oncologists. We can call out discussions of death that make us uncomfortable, gently reminding each other that we’re talking about a human life.
We can learn from our palliative care colleagues; their conversations about death routinely include a patient’s support system and personal stories. Palliative care doctors always refer to the patient by name, which helps humanize the person behind the chart.
We can emphasize a feeling of hope, a sentiment that may also be therapeutic to our patients. Even when a patient is dying, there is always something to be done. We can comfort their family, explaining what brought us to this point and how sorry we are that this is happening. We can provide options for symptom control and help patients manage those symptoms.
And we can allow ourselves to talk about how much a death affects us. We can acknowledge how much it sucks that a patient is going to die, how challenging that will be to his/her family, and how we wish it could have ended differently.
Subtle changes like these will improve our own ability to process and discuss death and will ultimately lead to better relationships with our patients. But it starts with eliminating the “locker room talk” of how we discuss death.
Ravi B. Parikh, MD, MPP, is a medical oncologist and faculty member at the University of Pennsylvania and the Philadelphia VA Medical Center, an adjunct fellow at the Leonard Davis Institute of Health Economics, and senior clinical advisor at the Coalition to Transform Advanced Care (C-TAC). He has served as a director, officer, partner, employee, adviser, consultant, or trustee for GNS Healthcare, Nanology, and Cancer Study Group, and he has received research grant from Embedded Healthcare, Veterans Administration, PCF, National Palliative Care Research Center, and MUSC. A version of this article first appeared on Medscape.com.
Differences in Care by Race in Older Nursing Home Residents With Dementia
Study Overview
Objective. To examine differences in care, specifically hospitalization towards the end of life, among nursing home residents with dementia who were Black compared with those who were White.
Design. Population based cohort study in the US. The study included all decedents with Alzheimer’s disease or related dementia (ADRD) who resided in a nursing home from 2014 to 2017. Decedents from nursing homes were identified by death within 1 day of an identified nursing home stay or within 8 days of a hospital transfer from nursing home. Data were obtained from Minimum Data Set 3.0 (MDS) which contains clinical data from all Medicaid or Medicare certified nursing homes, and from the Medicare Beneficiary Summary File (MBSF) and Medicare Provider and Analysis and Review (MedPAR) which contains hospitalization events for all Medicare Beneficiaries. These files were linked to identify nursing home residents with ADRD who were hospitalized at the end of life. ADRD diagnosis was identified from the chronic condition list from the MBSF and from MDS diagnosis list.
Setting and participants. The study included 665 033 residents from 14 595 nursing homes who died during the study period. Resident race was categorized as White or Black based on the MBSF. Severe cognitive impairment was identified using the MDS that categorized residents as severe or not using the Brief Interview for Mental Status and the Cognitive Performance Scale. The mean (SD) age of the study population was 86.7 (9.2) years for White residents and 82.6 (11.1) years for Black residents. Of the participants, 68.8% and 61.2% were female for Black and White residents, respectively. Approximately 23.4% of White and 32.5% of Black residents had severe cognitive impairment. For nursing home characteristics, 71.5% of the 14 595 nursing homes represented were for profit; average bedside was 109.5 (57.0) and occupancy rate was on average 81.2% (14.3%).
Main outcome measures. The study outcome measure was any hospitalization within 30 days prior to death. The outcome was selected as an indicator of quality of care because as older adults living with ADRD experience progressive worsening of cognitive symptoms, at the end of life when dementia is severe, advance care planning and communication with health care proxies and surrogates often result in coordinated care that avoids acute hospitalizations, which are often burdensome to both patient and family and may yield poorer quality of life.
Main results. The study found that approximately 29.5% of White decedents and 40.7% of Black decedents were hospitalized towards the end of life. Nursing homes with a higher proportion of Black residents were more likely to have residents hospitalized towards the end of life with 35% of residents hospitalized in the highest quartile (27% Black) compared with 17% hospitalized for nursing homes in the lowest quartile (0% Black).After adjusting for covariates, Black residents were 7.9% more likely to be hospitalized in the last 30 days of life compared with White residents. Blacks with severe cognitive impairment has elevated risk of hospitalization by 4.9% when compared with White residents. After accounting for nursing home facility–level characteristics, nursing homes with a low proportion of Black residents had a 5.2% higher risk of hospitalizations compared with nursing homes with no Black residents, and nursing homes with a higher percentage of Black residents had a 13.3% higher risk of hospitalization compared with nursing homes with no Black residents.
Conclusion. Race is associated with care disparities in older nursing home residents with dementia. This study suggests that hospitalization towards the end of life as a quality of care marker differs across nursing homes, and nursing homes with a higher proportion of Black residents were more likely to be hospitalized. This suggests that these nursing homes may have fewer resources and delivered poorer quality of care, and that disparities in health systems or institutions contribute to differences in quality of care for this vulnerable group.
Commentary
Disparities of health status, health care, and affordability across race and ethnicity have persisted throughout the past 20 years.1 There is further evidence to support systemic differences that can contribute to differences in health outcomes.2 Although changes in health care policy such as the Affordable Care Act have expanded health care coverage, and instituted changes that aims to improve health care quality and reduce disparities, it is clear that factors contributing to disparities in care are structural and perhaps systemic. The latest evidence comes in this study that examines racial disparities in health care quality in one of the most vulnerable populations—older adults with Alzheimer’s disease and dementia. The finding that Black nursing home residents, when compared with White residents, often has higher risk of hospitalization at the end of life, even among those with severe dementia where better coordinated care, clear goals of care and perhaps instituting palliative care would result in lower rate of hospitalization. The disparities were observed across nursing homes as well, where nursing homes with higher proportion of Black residents appear to have lower quality of care.
These findings are consistent with prior work that has examined differences in Black and White population on uptake of palliative care, discussion, and the documentation of advance care planning.3 Factors that may contribute to these differences include mistrust of the health care system among minorities, and not being connected to adequate health care resources. Family members and surrogate health care decision makers may consider receiving more aggressive care as advocating for better health care for their family members.4 These differences may contribute to the differences in hospitalization rates among residents within the same nursing home; however, the differences between nursing homes even after accounting for individual differences may indicate more widespread systemic differences that is associated with race. Policy changes that will address these differences are needed to level these differences so that quality care can be delivered regardless of race.5 For this vulnerable population with a terminal illness, approaches to enhance uptake of palliative approaches and care delivery for dementia patients at terminal stage are needed and understanding and targeting factors that contribute to low uptake of these approaches will enhance end of life care. Understanding the differences in resources and systems of care in nursing homes and perhaps how palliative care is integrated in these settings will be important to address care disparities that occurs across nursing homes.
Applications for Clinical Practice
Clinicians who take care of this population of older adults with advanced dementia should be aware of the potential for racial disparities that may lead to differences in the quality of care. The underlying reasons for these differences could be targeted so that older adults in all racial groups may have equal access to quality care including palliative approaches that avoid aggressive care for terminal illnesses across settings that may yield better care and quality of life. Policy makers and health systems leaders need to consider the current realities with racial disparities that policies need to address these differences so that they may not continue to persist in our systems of care.
Financial disclosures: None.
1. Mahajan S, Caraballo C, Lu Y, et al. Trends in Differences in Health Status and Health Care Access and Affordability by Race and Ethnicity in the United States, 1999-2018. JAMA. 2021;326(7):637-648. doi:10.1001/jama.2021.9907
2. Gill TM, Zang EX, Murphy TE, et al. Association Between Neighborhood Disadvantage and Functional Well-being in Community-Living Older Persons. [published online ahead of print, 2021 Aug 23]. JAMA Intern Med. doi:10.1001/jamainternmed.2021.4260
3. Bazargan M, Bazargan-Hejazi S. Disparities in Palliative and Hospice Care and Completion of Advance Care Planning and Directives Among Non-Hispanic Blacks: A Scoping Review of Recent Literature. Am J Hosp Palliat Care. 2021;38(6):688-718. doi:10.1177/1049909120966585
4. Siler S, Arora K, Doyon K, Fischer SM. Spirituality and the Illness Experience: Perspectives of African American Older Adults. Am J Hosp Palliat Care. 2021;38(6):618-625. doi:10.1177/1049909120988280
5. Council on Ethical and Judicial Affairs. Black-white disparities in health care. JAMA. 1990;263(17):2344-2346. doi:10.1001/jama.1990.03440170066038
Study Overview
Objective. To examine differences in care, specifically hospitalization towards the end of life, among nursing home residents with dementia who were Black compared with those who were White.
Design. Population based cohort study in the US. The study included all decedents with Alzheimer’s disease or related dementia (ADRD) who resided in a nursing home from 2014 to 2017. Decedents from nursing homes were identified by death within 1 day of an identified nursing home stay or within 8 days of a hospital transfer from nursing home. Data were obtained from Minimum Data Set 3.0 (MDS) which contains clinical data from all Medicaid or Medicare certified nursing homes, and from the Medicare Beneficiary Summary File (MBSF) and Medicare Provider and Analysis and Review (MedPAR) which contains hospitalization events for all Medicare Beneficiaries. These files were linked to identify nursing home residents with ADRD who were hospitalized at the end of life. ADRD diagnosis was identified from the chronic condition list from the MBSF and from MDS diagnosis list.
Setting and participants. The study included 665 033 residents from 14 595 nursing homes who died during the study period. Resident race was categorized as White or Black based on the MBSF. Severe cognitive impairment was identified using the MDS that categorized residents as severe or not using the Brief Interview for Mental Status and the Cognitive Performance Scale. The mean (SD) age of the study population was 86.7 (9.2) years for White residents and 82.6 (11.1) years for Black residents. Of the participants, 68.8% and 61.2% were female for Black and White residents, respectively. Approximately 23.4% of White and 32.5% of Black residents had severe cognitive impairment. For nursing home characteristics, 71.5% of the 14 595 nursing homes represented were for profit; average bedside was 109.5 (57.0) and occupancy rate was on average 81.2% (14.3%).
Main outcome measures. The study outcome measure was any hospitalization within 30 days prior to death. The outcome was selected as an indicator of quality of care because as older adults living with ADRD experience progressive worsening of cognitive symptoms, at the end of life when dementia is severe, advance care planning and communication with health care proxies and surrogates often result in coordinated care that avoids acute hospitalizations, which are often burdensome to both patient and family and may yield poorer quality of life.
Main results. The study found that approximately 29.5% of White decedents and 40.7% of Black decedents were hospitalized towards the end of life. Nursing homes with a higher proportion of Black residents were more likely to have residents hospitalized towards the end of life with 35% of residents hospitalized in the highest quartile (27% Black) compared with 17% hospitalized for nursing homes in the lowest quartile (0% Black).After adjusting for covariates, Black residents were 7.9% more likely to be hospitalized in the last 30 days of life compared with White residents. Blacks with severe cognitive impairment has elevated risk of hospitalization by 4.9% when compared with White residents. After accounting for nursing home facility–level characteristics, nursing homes with a low proportion of Black residents had a 5.2% higher risk of hospitalizations compared with nursing homes with no Black residents, and nursing homes with a higher percentage of Black residents had a 13.3% higher risk of hospitalization compared with nursing homes with no Black residents.
Conclusion. Race is associated with care disparities in older nursing home residents with dementia. This study suggests that hospitalization towards the end of life as a quality of care marker differs across nursing homes, and nursing homes with a higher proportion of Black residents were more likely to be hospitalized. This suggests that these nursing homes may have fewer resources and delivered poorer quality of care, and that disparities in health systems or institutions contribute to differences in quality of care for this vulnerable group.
Commentary
Disparities of health status, health care, and affordability across race and ethnicity have persisted throughout the past 20 years.1 There is further evidence to support systemic differences that can contribute to differences in health outcomes.2 Although changes in health care policy such as the Affordable Care Act have expanded health care coverage, and instituted changes that aims to improve health care quality and reduce disparities, it is clear that factors contributing to disparities in care are structural and perhaps systemic. The latest evidence comes in this study that examines racial disparities in health care quality in one of the most vulnerable populations—older adults with Alzheimer’s disease and dementia. The finding that Black nursing home residents, when compared with White residents, often has higher risk of hospitalization at the end of life, even among those with severe dementia where better coordinated care, clear goals of care and perhaps instituting palliative care would result in lower rate of hospitalization. The disparities were observed across nursing homes as well, where nursing homes with higher proportion of Black residents appear to have lower quality of care.
These findings are consistent with prior work that has examined differences in Black and White population on uptake of palliative care, discussion, and the documentation of advance care planning.3 Factors that may contribute to these differences include mistrust of the health care system among minorities, and not being connected to adequate health care resources. Family members and surrogate health care decision makers may consider receiving more aggressive care as advocating for better health care for their family members.4 These differences may contribute to the differences in hospitalization rates among residents within the same nursing home; however, the differences between nursing homes even after accounting for individual differences may indicate more widespread systemic differences that is associated with race. Policy changes that will address these differences are needed to level these differences so that quality care can be delivered regardless of race.5 For this vulnerable population with a terminal illness, approaches to enhance uptake of palliative approaches and care delivery for dementia patients at terminal stage are needed and understanding and targeting factors that contribute to low uptake of these approaches will enhance end of life care. Understanding the differences in resources and systems of care in nursing homes and perhaps how palliative care is integrated in these settings will be important to address care disparities that occurs across nursing homes.
Applications for Clinical Practice
Clinicians who take care of this population of older adults with advanced dementia should be aware of the potential for racial disparities that may lead to differences in the quality of care. The underlying reasons for these differences could be targeted so that older adults in all racial groups may have equal access to quality care including palliative approaches that avoid aggressive care for terminal illnesses across settings that may yield better care and quality of life. Policy makers and health systems leaders need to consider the current realities with racial disparities that policies need to address these differences so that they may not continue to persist in our systems of care.
Financial disclosures: None.
Study Overview
Objective. To examine differences in care, specifically hospitalization towards the end of life, among nursing home residents with dementia who were Black compared with those who were White.
Design. Population based cohort study in the US. The study included all decedents with Alzheimer’s disease or related dementia (ADRD) who resided in a nursing home from 2014 to 2017. Decedents from nursing homes were identified by death within 1 day of an identified nursing home stay or within 8 days of a hospital transfer from nursing home. Data were obtained from Minimum Data Set 3.0 (MDS) which contains clinical data from all Medicaid or Medicare certified nursing homes, and from the Medicare Beneficiary Summary File (MBSF) and Medicare Provider and Analysis and Review (MedPAR) which contains hospitalization events for all Medicare Beneficiaries. These files were linked to identify nursing home residents with ADRD who were hospitalized at the end of life. ADRD diagnosis was identified from the chronic condition list from the MBSF and from MDS diagnosis list.
Setting and participants. The study included 665 033 residents from 14 595 nursing homes who died during the study period. Resident race was categorized as White or Black based on the MBSF. Severe cognitive impairment was identified using the MDS that categorized residents as severe or not using the Brief Interview for Mental Status and the Cognitive Performance Scale. The mean (SD) age of the study population was 86.7 (9.2) years for White residents and 82.6 (11.1) years for Black residents. Of the participants, 68.8% and 61.2% were female for Black and White residents, respectively. Approximately 23.4% of White and 32.5% of Black residents had severe cognitive impairment. For nursing home characteristics, 71.5% of the 14 595 nursing homes represented were for profit; average bedside was 109.5 (57.0) and occupancy rate was on average 81.2% (14.3%).
Main outcome measures. The study outcome measure was any hospitalization within 30 days prior to death. The outcome was selected as an indicator of quality of care because as older adults living with ADRD experience progressive worsening of cognitive symptoms, at the end of life when dementia is severe, advance care planning and communication with health care proxies and surrogates often result in coordinated care that avoids acute hospitalizations, which are often burdensome to both patient and family and may yield poorer quality of life.
Main results. The study found that approximately 29.5% of White decedents and 40.7% of Black decedents were hospitalized towards the end of life. Nursing homes with a higher proportion of Black residents were more likely to have residents hospitalized towards the end of life with 35% of residents hospitalized in the highest quartile (27% Black) compared with 17% hospitalized for nursing homes in the lowest quartile (0% Black).After adjusting for covariates, Black residents were 7.9% more likely to be hospitalized in the last 30 days of life compared with White residents. Blacks with severe cognitive impairment has elevated risk of hospitalization by 4.9% when compared with White residents. After accounting for nursing home facility–level characteristics, nursing homes with a low proportion of Black residents had a 5.2% higher risk of hospitalizations compared with nursing homes with no Black residents, and nursing homes with a higher percentage of Black residents had a 13.3% higher risk of hospitalization compared with nursing homes with no Black residents.
Conclusion. Race is associated with care disparities in older nursing home residents with dementia. This study suggests that hospitalization towards the end of life as a quality of care marker differs across nursing homes, and nursing homes with a higher proportion of Black residents were more likely to be hospitalized. This suggests that these nursing homes may have fewer resources and delivered poorer quality of care, and that disparities in health systems or institutions contribute to differences in quality of care for this vulnerable group.
Commentary
Disparities of health status, health care, and affordability across race and ethnicity have persisted throughout the past 20 years.1 There is further evidence to support systemic differences that can contribute to differences in health outcomes.2 Although changes in health care policy such as the Affordable Care Act have expanded health care coverage, and instituted changes that aims to improve health care quality and reduce disparities, it is clear that factors contributing to disparities in care are structural and perhaps systemic. The latest evidence comes in this study that examines racial disparities in health care quality in one of the most vulnerable populations—older adults with Alzheimer’s disease and dementia. The finding that Black nursing home residents, when compared with White residents, often has higher risk of hospitalization at the end of life, even among those with severe dementia where better coordinated care, clear goals of care and perhaps instituting palliative care would result in lower rate of hospitalization. The disparities were observed across nursing homes as well, where nursing homes with higher proportion of Black residents appear to have lower quality of care.
These findings are consistent with prior work that has examined differences in Black and White population on uptake of palliative care, discussion, and the documentation of advance care planning.3 Factors that may contribute to these differences include mistrust of the health care system among minorities, and not being connected to adequate health care resources. Family members and surrogate health care decision makers may consider receiving more aggressive care as advocating for better health care for their family members.4 These differences may contribute to the differences in hospitalization rates among residents within the same nursing home; however, the differences between nursing homes even after accounting for individual differences may indicate more widespread systemic differences that is associated with race. Policy changes that will address these differences are needed to level these differences so that quality care can be delivered regardless of race.5 For this vulnerable population with a terminal illness, approaches to enhance uptake of palliative approaches and care delivery for dementia patients at terminal stage are needed and understanding and targeting factors that contribute to low uptake of these approaches will enhance end of life care. Understanding the differences in resources and systems of care in nursing homes and perhaps how palliative care is integrated in these settings will be important to address care disparities that occurs across nursing homes.
Applications for Clinical Practice
Clinicians who take care of this population of older adults with advanced dementia should be aware of the potential for racial disparities that may lead to differences in the quality of care. The underlying reasons for these differences could be targeted so that older adults in all racial groups may have equal access to quality care including palliative approaches that avoid aggressive care for terminal illnesses across settings that may yield better care and quality of life. Policy makers and health systems leaders need to consider the current realities with racial disparities that policies need to address these differences so that they may not continue to persist in our systems of care.
Financial disclosures: None.
1. Mahajan S, Caraballo C, Lu Y, et al. Trends in Differences in Health Status and Health Care Access and Affordability by Race and Ethnicity in the United States, 1999-2018. JAMA. 2021;326(7):637-648. doi:10.1001/jama.2021.9907
2. Gill TM, Zang EX, Murphy TE, et al. Association Between Neighborhood Disadvantage and Functional Well-being in Community-Living Older Persons. [published online ahead of print, 2021 Aug 23]. JAMA Intern Med. doi:10.1001/jamainternmed.2021.4260
3. Bazargan M, Bazargan-Hejazi S. Disparities in Palliative and Hospice Care and Completion of Advance Care Planning and Directives Among Non-Hispanic Blacks: A Scoping Review of Recent Literature. Am J Hosp Palliat Care. 2021;38(6):688-718. doi:10.1177/1049909120966585
4. Siler S, Arora K, Doyon K, Fischer SM. Spirituality and the Illness Experience: Perspectives of African American Older Adults. Am J Hosp Palliat Care. 2021;38(6):618-625. doi:10.1177/1049909120988280
5. Council on Ethical and Judicial Affairs. Black-white disparities in health care. JAMA. 1990;263(17):2344-2346. doi:10.1001/jama.1990.03440170066038
1. Mahajan S, Caraballo C, Lu Y, et al. Trends in Differences in Health Status and Health Care Access and Affordability by Race and Ethnicity in the United States, 1999-2018. JAMA. 2021;326(7):637-648. doi:10.1001/jama.2021.9907
2. Gill TM, Zang EX, Murphy TE, et al. Association Between Neighborhood Disadvantage and Functional Well-being in Community-Living Older Persons. [published online ahead of print, 2021 Aug 23]. JAMA Intern Med. doi:10.1001/jamainternmed.2021.4260
3. Bazargan M, Bazargan-Hejazi S. Disparities in Palliative and Hospice Care and Completion of Advance Care Planning and Directives Among Non-Hispanic Blacks: A Scoping Review of Recent Literature. Am J Hosp Palliat Care. 2021;38(6):688-718. doi:10.1177/1049909120966585
4. Siler S, Arora K, Doyon K, Fischer SM. Spirituality and the Illness Experience: Perspectives of African American Older Adults. Am J Hosp Palliat Care. 2021;38(6):618-625. doi:10.1177/1049909120988280
5. Council on Ethical and Judicial Affairs. Black-white disparities in health care. JAMA. 1990;263(17):2344-2346. doi:10.1001/jama.1990.03440170066038
Preoperative Advance Care Planning for Older Adults Undergoing High-Risk Surgery: An Essential but Underutilized Aspect of Clinical Care
Study Overview
Objective. The objectives of this study were to (1) quantify the frequency of preoperative advance care planning (ACP) discussion and documentation for older adults undergoing major surgery in a national sample, and (2) characterize how surgical patients and their family members considered ACP after postoperative complications.
Design. A secondary analysis of data from a multisite randomized clinical trial testing the effects of a question prompt list intervention (a Question Problem List [QPL] brochure with 11 questions) given to patients aged 60 years or older undergoing high-risk surgery on preoperative communication with their surgeons.
Setting and participants. This multisite randomized controlled trial involved 5 study sites that encompassed distinct US geographic areas, including University of Wisconsin Hospital and Clinics (UWHC), Madison; the University of California, San Francisco, Medical Center (UCSF); Oregon Health & Science University (OHSU), Portland; the University Hospital of Rutgers New Jersey Medical School (Rutgers), Newark; and the Brigham and Women’s Hospital (BWH), Boston, Massachusetts. The study enrolled 40 surgeons who routinely performed high-risk oncological or vascular surgery via purposeful sampling; patients aged 60 years or older with at least 1 comorbidity and an oncological or vascular problem that were treatable with high-risk surgery; and 1 invited family member per enrolled patient to participate in open-ended interviews postsurgery. High-risk surgery was defined as an operation that has a 30-day in-hospital mortality rate greater than or equal to 1%. Data were collected from June 1, 2016, to November 30, 2018.
Main outcome measures. The frequency of preoperative discussions and documentation of ACP was determined. For patients who had major surgery, any mention of ACP (ie, mention of advance directive [AD], health care power of attorney, or preference for limitations of life-sustaining treatments) by the surgeon, patient or family member during the audio recorded, transcribed, and coded preoperative consultation was counted. The presence of a written AD in the medical record at the time of the initial consultation, filed between the consultation and the date of surgery, or added postoperatively, was recorded using a standardized abstraction form. Postoperative treatments administered and complications experienced within 6 weeks after surgery were recorded. Open-ended interviews with patients who experienced significant postoperative complications (eg, prolonged hospitalization > 8 days, intensive care unit stay > 3 days) and their family members were conducted 6 weeks after surgery. Information ascertained during interviews focused on treatment decisions, postoperative experiences, and interpersonal relationships among patients, families, and clinicians. Transcripts of these interviews were then subjected to qualitative content analysis.
Main results. A total of 446 patients were enrolled in the primary study. Of these patients, 213 (122 men [57%]; 91 women [43%]; mean [SD] age, 72 [7] years) underwent major surgery. Only 13 (6.1%) of those who had major surgery had any discussion related to ACP in the preoperative consultation. In this cohort, 141 (66%) patients did not have an AD on file before undergoing major surgery. The presence of AD was not associated with age (60-69 years, 26 [31%]; 70-79 years, 31 [33%]; ≥ 80 years, 15 [42%]; P = .55), number of comorbidities (1, 35 [32%]; 2, 18 [33%]; ≥ 3, 19 [40%]; P = .62), or type of procedure (oncological, 53 [32%]; vascular, 19 [42%]; P = .22). Moreover, there was no difference in preoperative communication about ACP or documentation of an AD for patients who were mailed a QPL brochure compared to those who received usual care (intervention, 38 [35%]; usual care, 34 [33%]; P = .77). Rates of AD documentation were associated with individual study sites with BWH and UWHC having higher rates of documentation (20 [50%] and 27 [44%], respectively) compared to OHSU, UCSF, or Rutgers (7 [17%], 17 [35%], and 1 [5%], respectively). Analysis from the interviews indicated that patients and families felt unprepared for serious surgical complications and had varied interpretations of ACP. Patients with complications were enthusiastic about ACP but did not think it was important to discuss their preferences for life-sustaining treatments with their surgeon preoperatively.
Conclusion. Although surgeons and patients report that they believe ACP is important, preoperative discussion of patient preferences rarely occurs. This study found that the frequency of ACP discussions or AD documentations among older patients undergoing high-risk oncologic or vascular surgery was low. Interventions that are aimed to increase rates of preoperative ACP discussions should be implemented to help prepare patients and their families for difficult decisions in the setting of serious surgical complications and could help decrease postoperative conflicts that result from unclear patient care goals.
Commentary
Surgeons and patients approach surgical interventions with optimistic outlooks while simultaneously preparing for unintended adverse outcomes. For patients, preoperative ACP discussions ease the burden on their families and ensure their wishes and care goals are communicated. For surgeons, these discussions inform them how best to support the values of the patient. Therefore, it is unsurprising that preoperative ACP is viewed favorably by both groups. Given the consensus that ACP is important in the care of older adults undergoing high-risk surgery, one would assume that preoperative ACP discussion is a standard of practice among surgeons and their aging patients. However, in a secondary analysis of a randomized control trial testing a patient-mediated intervention to improve preoperative communication, Kalbfell et al1 showed that ACP discussions rarely take place prior to major surgery in older adults. This finding highlights the significant discrepancy between the belief that ACP is important, and the actual rate that it is practiced, in older patients undergoing high-risk surgery. This discordance is highly concerning because it suggests that surgeons who provide care to a very vulnerable subset of older patients may overlook an essential aspect of preoperative care and therefore lack a thorough and thoughtful understanding of the patient’s care goals. In practice, this omission can pose significant challenges associated with the surgeon and family’s decisions to use postoperative life-sustaining interventions or to manage unforeseen complications should a patient become unable to make medical decisions.
The barriers to conducting successful ACP discussions between surgeons and patients are multifactorial. Kalbfell et al1 highlighted several of these barriers, including lack of patient efficacy, physician attitudes, and institutional values in older adults who require major surgeries. The inadequacy of patient efficacy in preoperative ACP is illustrated by findings from the primary, multisite trial of QPL intervention conducted by Schwarze et al. Interestingly, the authors found that patients who did not receive QPL brochure had no ACP discussions, and that QPL implementation did not significantly improve discussion rates despite its intent to encourage these discussions.2 Possible explanations for this lack of engagement might be a lack of health literacy or patient efficacy in the study population. Qualitative data from the current study provided further evidence to support these explanations. For instance, some patients provided limited or incomplete information about their wishes for health care management while others felt it was unnecessary to have ACP discussions unless complications arose.1 However, the latter example counters the purpose of ACP which is to enable patients to make plans about future health care and not reactive to a medical complication or emergency.
Surgeons bear a large responsibility in providing treatments that are consistent with the care goals of the patient. Thus, surgeons play a crucial role in engaging, guiding, and facilitating ACP discussions with patients. This role is even more critical when patients are unable or unwilling to initiate care goal discussions. Physician attitudes towards ACP, therefore, greatly influence the effectiveness of these discussions. In a study of self-administered surveys by vascular, neurologic, and cardiothoracic surgeons, greater than 90% of respondents viewed postoperative life-supporting therapy as necessary, and 54% would decline to operate on patients with an AD limiting life-supporting therapy.3 Moreover, the same study showed that 52% of respondents reported discussing AD before surgery, a figure that exceeded the actual rates at which ACP discussions occur in many other studies. In the current study, Kalbfell et al1 also found that surgeons viewed ACP discussions largely in the context of AD creation and declined to investigate the full scope of patient preferences. These findings, when combined with other studies that indicate an incomplete understanding of ACP in some surgeons, suggest that not all physicians are able or willing to navigate these sometimes lengthy and difficult conversations with patients. This gap in practice provides opportunities for training in surgical specialties that center on optimizing preoperative ACP discussions to meet the care needs of older patients.
Institutional value and culture are important factors that impact physician behavior and the practice of ACP discussion. In the current study, the authors reported that the majority of ACP discussions were held by a minority of surgeons and that different institutions and study sites had vastly different rates of ACP documentation.1 These results are further supported by findings of large variations between physicians and hospitals in ACP reporting in hospitalized frail older adults.4 These variations in practices at different institutions suggest that it is possible to improve rates of preoperative ACP discussion. Reasons for these differences need to be further investigated in order to identify strategies, resources, or trainings required by medical institutions to support surgeons to carry out ACP discussions with patients undergoing high-risk surgeries.
The study conducted by Kalbfell et al1 has several strengths. For example, it included Spanish-speaking patients and the use of a Spanish version of the QPL intervention to account for cultural differences. The study also included multiple surgical specialties and institutions and captured a large and national sample, thus making its findings more generalizable. However, the lack of data on the duration of preoperative consultation visits in patients who completed ACP discussions poses a limitation to this study. This is relevant because surgeon availability to engage in lengthy ACP discussions may be limited due to busy clinical schedules. Additional data on the duration of preoperative visits inclusive of a thoughtfully conducted ACP discussion could help to modify clinical workflow to facilitate its uptake in surgical practices.
Applications for Clinical Practice
The findings from the current study indicate that patients and surgeons agree that preoperative ACP discussions are beneficial to the clinical care of older adults before high-risk surgeries. However, these important conversations do not occur frequently. Surgeons and health care institutions need to identify strategies to initiate, facilitate, and optimize productive preoperative ACP discussions to provide patient-centered care in vulnerable older surgical patients.
Financial disclosures: None.
1. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
2. Schwarze ML, Buffington A, Tucholka JL, et al. Effectiveness of a Question Prompt List Intervention for Older Patients Considering Major Surgery: A Multisite Randomized Clinical Trial. JAMA Surg. 2020;155(1):6-13. doi:10.1001/jamasurg.2019.3778
3. Redmann AJ, Brasel KJ, Alexander CG, Schwarze ML. Use of advance directives for high-risk operations: a national survey of surgeons. Ann Surgery. 2012;255(3):418-423. doi:10.1097/SLA.0b013e31823b6782
4. Hopkins SA, Bentley A, Phillips V, Barclay S. Advance care plans and hospitalized frail older adults: a systematic review. BMJ Support Palliat Care. 2020;10:164-174. doi:10.1136/bmjspcare-2019-002093
Study Overview
Objective. The objectives of this study were to (1) quantify the frequency of preoperative advance care planning (ACP) discussion and documentation for older adults undergoing major surgery in a national sample, and (2) characterize how surgical patients and their family members considered ACP after postoperative complications.
Design. A secondary analysis of data from a multisite randomized clinical trial testing the effects of a question prompt list intervention (a Question Problem List [QPL] brochure with 11 questions) given to patients aged 60 years or older undergoing high-risk surgery on preoperative communication with their surgeons.
Setting and participants. This multisite randomized controlled trial involved 5 study sites that encompassed distinct US geographic areas, including University of Wisconsin Hospital and Clinics (UWHC), Madison; the University of California, San Francisco, Medical Center (UCSF); Oregon Health & Science University (OHSU), Portland; the University Hospital of Rutgers New Jersey Medical School (Rutgers), Newark; and the Brigham and Women’s Hospital (BWH), Boston, Massachusetts. The study enrolled 40 surgeons who routinely performed high-risk oncological or vascular surgery via purposeful sampling; patients aged 60 years or older with at least 1 comorbidity and an oncological or vascular problem that were treatable with high-risk surgery; and 1 invited family member per enrolled patient to participate in open-ended interviews postsurgery. High-risk surgery was defined as an operation that has a 30-day in-hospital mortality rate greater than or equal to 1%. Data were collected from June 1, 2016, to November 30, 2018.
Main outcome measures. The frequency of preoperative discussions and documentation of ACP was determined. For patients who had major surgery, any mention of ACP (ie, mention of advance directive [AD], health care power of attorney, or preference for limitations of life-sustaining treatments) by the surgeon, patient or family member during the audio recorded, transcribed, and coded preoperative consultation was counted. The presence of a written AD in the medical record at the time of the initial consultation, filed between the consultation and the date of surgery, or added postoperatively, was recorded using a standardized abstraction form. Postoperative treatments administered and complications experienced within 6 weeks after surgery were recorded. Open-ended interviews with patients who experienced significant postoperative complications (eg, prolonged hospitalization > 8 days, intensive care unit stay > 3 days) and their family members were conducted 6 weeks after surgery. Information ascertained during interviews focused on treatment decisions, postoperative experiences, and interpersonal relationships among patients, families, and clinicians. Transcripts of these interviews were then subjected to qualitative content analysis.
Main results. A total of 446 patients were enrolled in the primary study. Of these patients, 213 (122 men [57%]; 91 women [43%]; mean [SD] age, 72 [7] years) underwent major surgery. Only 13 (6.1%) of those who had major surgery had any discussion related to ACP in the preoperative consultation. In this cohort, 141 (66%) patients did not have an AD on file before undergoing major surgery. The presence of AD was not associated with age (60-69 years, 26 [31%]; 70-79 years, 31 [33%]; ≥ 80 years, 15 [42%]; P = .55), number of comorbidities (1, 35 [32%]; 2, 18 [33%]; ≥ 3, 19 [40%]; P = .62), or type of procedure (oncological, 53 [32%]; vascular, 19 [42%]; P = .22). Moreover, there was no difference in preoperative communication about ACP or documentation of an AD for patients who were mailed a QPL brochure compared to those who received usual care (intervention, 38 [35%]; usual care, 34 [33%]; P = .77). Rates of AD documentation were associated with individual study sites with BWH and UWHC having higher rates of documentation (20 [50%] and 27 [44%], respectively) compared to OHSU, UCSF, or Rutgers (7 [17%], 17 [35%], and 1 [5%], respectively). Analysis from the interviews indicated that patients and families felt unprepared for serious surgical complications and had varied interpretations of ACP. Patients with complications were enthusiastic about ACP but did not think it was important to discuss their preferences for life-sustaining treatments with their surgeon preoperatively.
Conclusion. Although surgeons and patients report that they believe ACP is important, preoperative discussion of patient preferences rarely occurs. This study found that the frequency of ACP discussions or AD documentations among older patients undergoing high-risk oncologic or vascular surgery was low. Interventions that are aimed to increase rates of preoperative ACP discussions should be implemented to help prepare patients and their families for difficult decisions in the setting of serious surgical complications and could help decrease postoperative conflicts that result from unclear patient care goals.
Commentary
Surgeons and patients approach surgical interventions with optimistic outlooks while simultaneously preparing for unintended adverse outcomes. For patients, preoperative ACP discussions ease the burden on their families and ensure their wishes and care goals are communicated. For surgeons, these discussions inform them how best to support the values of the patient. Therefore, it is unsurprising that preoperative ACP is viewed favorably by both groups. Given the consensus that ACP is important in the care of older adults undergoing high-risk surgery, one would assume that preoperative ACP discussion is a standard of practice among surgeons and their aging patients. However, in a secondary analysis of a randomized control trial testing a patient-mediated intervention to improve preoperative communication, Kalbfell et al1 showed that ACP discussions rarely take place prior to major surgery in older adults. This finding highlights the significant discrepancy between the belief that ACP is important, and the actual rate that it is practiced, in older patients undergoing high-risk surgery. This discordance is highly concerning because it suggests that surgeons who provide care to a very vulnerable subset of older patients may overlook an essential aspect of preoperative care and therefore lack a thorough and thoughtful understanding of the patient’s care goals. In practice, this omission can pose significant challenges associated with the surgeon and family’s decisions to use postoperative life-sustaining interventions or to manage unforeseen complications should a patient become unable to make medical decisions.
The barriers to conducting successful ACP discussions between surgeons and patients are multifactorial. Kalbfell et al1 highlighted several of these barriers, including lack of patient efficacy, physician attitudes, and institutional values in older adults who require major surgeries. The inadequacy of patient efficacy in preoperative ACP is illustrated by findings from the primary, multisite trial of QPL intervention conducted by Schwarze et al. Interestingly, the authors found that patients who did not receive QPL brochure had no ACP discussions, and that QPL implementation did not significantly improve discussion rates despite its intent to encourage these discussions.2 Possible explanations for this lack of engagement might be a lack of health literacy or patient efficacy in the study population. Qualitative data from the current study provided further evidence to support these explanations. For instance, some patients provided limited or incomplete information about their wishes for health care management while others felt it was unnecessary to have ACP discussions unless complications arose.1 However, the latter example counters the purpose of ACP which is to enable patients to make plans about future health care and not reactive to a medical complication or emergency.
Surgeons bear a large responsibility in providing treatments that are consistent with the care goals of the patient. Thus, surgeons play a crucial role in engaging, guiding, and facilitating ACP discussions with patients. This role is even more critical when patients are unable or unwilling to initiate care goal discussions. Physician attitudes towards ACP, therefore, greatly influence the effectiveness of these discussions. In a study of self-administered surveys by vascular, neurologic, and cardiothoracic surgeons, greater than 90% of respondents viewed postoperative life-supporting therapy as necessary, and 54% would decline to operate on patients with an AD limiting life-supporting therapy.3 Moreover, the same study showed that 52% of respondents reported discussing AD before surgery, a figure that exceeded the actual rates at which ACP discussions occur in many other studies. In the current study, Kalbfell et al1 also found that surgeons viewed ACP discussions largely in the context of AD creation and declined to investigate the full scope of patient preferences. These findings, when combined with other studies that indicate an incomplete understanding of ACP in some surgeons, suggest that not all physicians are able or willing to navigate these sometimes lengthy and difficult conversations with patients. This gap in practice provides opportunities for training in surgical specialties that center on optimizing preoperative ACP discussions to meet the care needs of older patients.
Institutional value and culture are important factors that impact physician behavior and the practice of ACP discussion. In the current study, the authors reported that the majority of ACP discussions were held by a minority of surgeons and that different institutions and study sites had vastly different rates of ACP documentation.1 These results are further supported by findings of large variations between physicians and hospitals in ACP reporting in hospitalized frail older adults.4 These variations in practices at different institutions suggest that it is possible to improve rates of preoperative ACP discussion. Reasons for these differences need to be further investigated in order to identify strategies, resources, or trainings required by medical institutions to support surgeons to carry out ACP discussions with patients undergoing high-risk surgeries.
The study conducted by Kalbfell et al1 has several strengths. For example, it included Spanish-speaking patients and the use of a Spanish version of the QPL intervention to account for cultural differences. The study also included multiple surgical specialties and institutions and captured a large and national sample, thus making its findings more generalizable. However, the lack of data on the duration of preoperative consultation visits in patients who completed ACP discussions poses a limitation to this study. This is relevant because surgeon availability to engage in lengthy ACP discussions may be limited due to busy clinical schedules. Additional data on the duration of preoperative visits inclusive of a thoughtfully conducted ACP discussion could help to modify clinical workflow to facilitate its uptake in surgical practices.
Applications for Clinical Practice
The findings from the current study indicate that patients and surgeons agree that preoperative ACP discussions are beneficial to the clinical care of older adults before high-risk surgeries. However, these important conversations do not occur frequently. Surgeons and health care institutions need to identify strategies to initiate, facilitate, and optimize productive preoperative ACP discussions to provide patient-centered care in vulnerable older surgical patients.
Financial disclosures: None.
Study Overview
Objective. The objectives of this study were to (1) quantify the frequency of preoperative advance care planning (ACP) discussion and documentation for older adults undergoing major surgery in a national sample, and (2) characterize how surgical patients and their family members considered ACP after postoperative complications.
Design. A secondary analysis of data from a multisite randomized clinical trial testing the effects of a question prompt list intervention (a Question Problem List [QPL] brochure with 11 questions) given to patients aged 60 years or older undergoing high-risk surgery on preoperative communication with their surgeons.
Setting and participants. This multisite randomized controlled trial involved 5 study sites that encompassed distinct US geographic areas, including University of Wisconsin Hospital and Clinics (UWHC), Madison; the University of California, San Francisco, Medical Center (UCSF); Oregon Health & Science University (OHSU), Portland; the University Hospital of Rutgers New Jersey Medical School (Rutgers), Newark; and the Brigham and Women’s Hospital (BWH), Boston, Massachusetts. The study enrolled 40 surgeons who routinely performed high-risk oncological or vascular surgery via purposeful sampling; patients aged 60 years or older with at least 1 comorbidity and an oncological or vascular problem that were treatable with high-risk surgery; and 1 invited family member per enrolled patient to participate in open-ended interviews postsurgery. High-risk surgery was defined as an operation that has a 30-day in-hospital mortality rate greater than or equal to 1%. Data were collected from June 1, 2016, to November 30, 2018.
Main outcome measures. The frequency of preoperative discussions and documentation of ACP was determined. For patients who had major surgery, any mention of ACP (ie, mention of advance directive [AD], health care power of attorney, or preference for limitations of life-sustaining treatments) by the surgeon, patient or family member during the audio recorded, transcribed, and coded preoperative consultation was counted. The presence of a written AD in the medical record at the time of the initial consultation, filed between the consultation and the date of surgery, or added postoperatively, was recorded using a standardized abstraction form. Postoperative treatments administered and complications experienced within 6 weeks after surgery were recorded. Open-ended interviews with patients who experienced significant postoperative complications (eg, prolonged hospitalization > 8 days, intensive care unit stay > 3 days) and their family members were conducted 6 weeks after surgery. Information ascertained during interviews focused on treatment decisions, postoperative experiences, and interpersonal relationships among patients, families, and clinicians. Transcripts of these interviews were then subjected to qualitative content analysis.
Main results. A total of 446 patients were enrolled in the primary study. Of these patients, 213 (122 men [57%]; 91 women [43%]; mean [SD] age, 72 [7] years) underwent major surgery. Only 13 (6.1%) of those who had major surgery had any discussion related to ACP in the preoperative consultation. In this cohort, 141 (66%) patients did not have an AD on file before undergoing major surgery. The presence of AD was not associated with age (60-69 years, 26 [31%]; 70-79 years, 31 [33%]; ≥ 80 years, 15 [42%]; P = .55), number of comorbidities (1, 35 [32%]; 2, 18 [33%]; ≥ 3, 19 [40%]; P = .62), or type of procedure (oncological, 53 [32%]; vascular, 19 [42%]; P = .22). Moreover, there was no difference in preoperative communication about ACP or documentation of an AD for patients who were mailed a QPL brochure compared to those who received usual care (intervention, 38 [35%]; usual care, 34 [33%]; P = .77). Rates of AD documentation were associated with individual study sites with BWH and UWHC having higher rates of documentation (20 [50%] and 27 [44%], respectively) compared to OHSU, UCSF, or Rutgers (7 [17%], 17 [35%], and 1 [5%], respectively). Analysis from the interviews indicated that patients and families felt unprepared for serious surgical complications and had varied interpretations of ACP. Patients with complications were enthusiastic about ACP but did not think it was important to discuss their preferences for life-sustaining treatments with their surgeon preoperatively.
Conclusion. Although surgeons and patients report that they believe ACP is important, preoperative discussion of patient preferences rarely occurs. This study found that the frequency of ACP discussions or AD documentations among older patients undergoing high-risk oncologic or vascular surgery was low. Interventions that are aimed to increase rates of preoperative ACP discussions should be implemented to help prepare patients and their families for difficult decisions in the setting of serious surgical complications and could help decrease postoperative conflicts that result from unclear patient care goals.
Commentary
Surgeons and patients approach surgical interventions with optimistic outlooks while simultaneously preparing for unintended adverse outcomes. For patients, preoperative ACP discussions ease the burden on their families and ensure their wishes and care goals are communicated. For surgeons, these discussions inform them how best to support the values of the patient. Therefore, it is unsurprising that preoperative ACP is viewed favorably by both groups. Given the consensus that ACP is important in the care of older adults undergoing high-risk surgery, one would assume that preoperative ACP discussion is a standard of practice among surgeons and their aging patients. However, in a secondary analysis of a randomized control trial testing a patient-mediated intervention to improve preoperative communication, Kalbfell et al1 showed that ACP discussions rarely take place prior to major surgery in older adults. This finding highlights the significant discrepancy between the belief that ACP is important, and the actual rate that it is practiced, in older patients undergoing high-risk surgery. This discordance is highly concerning because it suggests that surgeons who provide care to a very vulnerable subset of older patients may overlook an essential aspect of preoperative care and therefore lack a thorough and thoughtful understanding of the patient’s care goals. In practice, this omission can pose significant challenges associated with the surgeon and family’s decisions to use postoperative life-sustaining interventions or to manage unforeseen complications should a patient become unable to make medical decisions.
The barriers to conducting successful ACP discussions between surgeons and patients are multifactorial. Kalbfell et al1 highlighted several of these barriers, including lack of patient efficacy, physician attitudes, and institutional values in older adults who require major surgeries. The inadequacy of patient efficacy in preoperative ACP is illustrated by findings from the primary, multisite trial of QPL intervention conducted by Schwarze et al. Interestingly, the authors found that patients who did not receive QPL brochure had no ACP discussions, and that QPL implementation did not significantly improve discussion rates despite its intent to encourage these discussions.2 Possible explanations for this lack of engagement might be a lack of health literacy or patient efficacy in the study population. Qualitative data from the current study provided further evidence to support these explanations. For instance, some patients provided limited or incomplete information about their wishes for health care management while others felt it was unnecessary to have ACP discussions unless complications arose.1 However, the latter example counters the purpose of ACP which is to enable patients to make plans about future health care and not reactive to a medical complication or emergency.
Surgeons bear a large responsibility in providing treatments that are consistent with the care goals of the patient. Thus, surgeons play a crucial role in engaging, guiding, and facilitating ACP discussions with patients. This role is even more critical when patients are unable or unwilling to initiate care goal discussions. Physician attitudes towards ACP, therefore, greatly influence the effectiveness of these discussions. In a study of self-administered surveys by vascular, neurologic, and cardiothoracic surgeons, greater than 90% of respondents viewed postoperative life-supporting therapy as necessary, and 54% would decline to operate on patients with an AD limiting life-supporting therapy.3 Moreover, the same study showed that 52% of respondents reported discussing AD before surgery, a figure that exceeded the actual rates at which ACP discussions occur in many other studies. In the current study, Kalbfell et al1 also found that surgeons viewed ACP discussions largely in the context of AD creation and declined to investigate the full scope of patient preferences. These findings, when combined with other studies that indicate an incomplete understanding of ACP in some surgeons, suggest that not all physicians are able or willing to navigate these sometimes lengthy and difficult conversations with patients. This gap in practice provides opportunities for training in surgical specialties that center on optimizing preoperative ACP discussions to meet the care needs of older patients.
Institutional value and culture are important factors that impact physician behavior and the practice of ACP discussion. In the current study, the authors reported that the majority of ACP discussions were held by a minority of surgeons and that different institutions and study sites had vastly different rates of ACP documentation.1 These results are further supported by findings of large variations between physicians and hospitals in ACP reporting in hospitalized frail older adults.4 These variations in practices at different institutions suggest that it is possible to improve rates of preoperative ACP discussion. Reasons for these differences need to be further investigated in order to identify strategies, resources, or trainings required by medical institutions to support surgeons to carry out ACP discussions with patients undergoing high-risk surgeries.
The study conducted by Kalbfell et al1 has several strengths. For example, it included Spanish-speaking patients and the use of a Spanish version of the QPL intervention to account for cultural differences. The study also included multiple surgical specialties and institutions and captured a large and national sample, thus making its findings more generalizable. However, the lack of data on the duration of preoperative consultation visits in patients who completed ACP discussions poses a limitation to this study. This is relevant because surgeon availability to engage in lengthy ACP discussions may be limited due to busy clinical schedules. Additional data on the duration of preoperative visits inclusive of a thoughtfully conducted ACP discussion could help to modify clinical workflow to facilitate its uptake in surgical practices.
Applications for Clinical Practice
The findings from the current study indicate that patients and surgeons agree that preoperative ACP discussions are beneficial to the clinical care of older adults before high-risk surgeries. However, these important conversations do not occur frequently. Surgeons and health care institutions need to identify strategies to initiate, facilitate, and optimize productive preoperative ACP discussions to provide patient-centered care in vulnerable older surgical patients.
Financial disclosures: None.
1. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
2. Schwarze ML, Buffington A, Tucholka JL, et al. Effectiveness of a Question Prompt List Intervention for Older Patients Considering Major Surgery: A Multisite Randomized Clinical Trial. JAMA Surg. 2020;155(1):6-13. doi:10.1001/jamasurg.2019.3778
3. Redmann AJ, Brasel KJ, Alexander CG, Schwarze ML. Use of advance directives for high-risk operations: a national survey of surgeons. Ann Surgery. 2012;255(3):418-423. doi:10.1097/SLA.0b013e31823b6782
4. Hopkins SA, Bentley A, Phillips V, Barclay S. Advance care plans and hospitalized frail older adults: a systematic review. BMJ Support Palliat Care. 2020;10:164-174. doi:10.1136/bmjspcare-2019-002093
1. Kalbfell E, Kata A, Buffington AS, et al. Frequency of Preoperative Advance Care Planning for Older Adults Undergoing High-risk Surgery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Surg. 2021;156(7):e211521. doi:10.1001/jamasurg.2021.1521
2. Schwarze ML, Buffington A, Tucholka JL, et al. Effectiveness of a Question Prompt List Intervention for Older Patients Considering Major Surgery: A Multisite Randomized Clinical Trial. JAMA Surg. 2020;155(1):6-13. doi:10.1001/jamasurg.2019.3778
3. Redmann AJ, Brasel KJ, Alexander CG, Schwarze ML. Use of advance directives for high-risk operations: a national survey of surgeons. Ann Surgery. 2012;255(3):418-423. doi:10.1097/SLA.0b013e31823b6782
4. Hopkins SA, Bentley A, Phillips V, Barclay S. Advance care plans and hospitalized frail older adults: a systematic review. BMJ Support Palliat Care. 2020;10:164-174. doi:10.1136/bmjspcare-2019-002093
Should Geriatric Veterans Get Immunotherapy?
Patients in their 90s with cancer tolerated immunotherapy well with few serious adverse effects, and they lived for an average of 1.6 years after treatment, a small new study within the US Department of Veterans Affairs (VA) health system reports.
Only 6.3% of 48 patients who were treated with immune checkpoint inhibitors experienced the most severe types of side effects – grade III/IV events – and a total of 27% had any adverse effects, according to the report, which was presented at the 2021 annual meeting of the Association of VA Hematology/Oncology (AVAHO) being held virtually and inperson in Denver Colorado, September 24 to September 26, 2021.
“Our project should help give confidence to oncologists treating the elderly,” said Andrew Joseph Benefield, MD, a hematology/oncology fellow at Wake Forest Baptist Medical Center, in an interview. “Immunotherapy can be given safely and likely effectively in select individuals over the age of 90 with good performance status.”
Benefield and colleagues launched their study to gain insight into a little-studied area: How does cancer treatment affects nonagenarians? “I think many oncologists have been in a situation where they encounter an individual over the age of 90 years who has a good performance status, and they've wondered if immunotherapy would be helpful and safe, particularly given our knowledge of waning immune strength as people age,” he said.
The researchers retrospectively tracked patients with cancer who were at least 90 years old from 2016 to 2017 and were treated with immune checkpoint inhibitors. Most were fit or fairly fit with Eastern Cooperative Oncology Group (ECOG) physical performance scales of 0 or 1 (n = 26), and nearly all had cancer in stage IV (n = 42). Melanoma was the most common type of cancer (n = 19), followed by non-small-cell lung cancer (n = 15). Patients were treated with an average of 12.2 cycles.
“In general, we saw that treatment was well-tolerated,” Dr. Benefield said. “We also noted that a trend toward better long-term survival outcomes in individuals with very good performance status at the start of treatment. We hope to parse this out more as we add more data to our data-set, as the numbers are still too small for confident direct comparison.”
Dr. Benefield said he has treated a limited number of patients in their 90s who were highly physical fit for their age and “very eager” to be treated. “They wanted to do anything they could to maintain their lifestyle,” he said. “In my experience, aggressive supportive care and close monitoring for developing toxicities has been most helpful.”
The researchers don’t know the causes of death of many of the patients, and it’s not clear how they fared in their final days. Still, Dr. Benefield said, “extending someone's life by more than 1 year with relatively low risk of adverse effects is reasonable.”
Oncologist Melisa Wong, MD, MAS, of the University of California, San Francisco, reviewed the study and said in an interview that it “a valuable description of outcomes for nonagenarians receiving immunotherapy in the VA healthcare system.” As she noted, “many other studies of immunotherapy among older adults focus on patients aged 65 or 70 and older while very few focus on octogenarians or nonagenarians.”
The findings suggest that “it is important to move beyond chronological age and assess patients’ physiologic age through a geriatric assessment,” she said. “Geriatric assessment-derived risk scores have been shown to predict chemotherapy toxicity for older adults and research to develop similar tools for immunotherapy are ongoing.”
However, she cautioned that older patients may become suffer so much from the most common side effect of immunotherapy -- fatigue – that “their independence is at stake.”
“Some of these patient choose to stop immunotherapy because the side effects aren’t worth it anymore,” she said. “The challenge for oncologists is not knowing in advance which patients will fall into each of these categories.”
She added that her geriatric oncology research focuses on improving risk stratification for older adults, such as those who are at least 70 with lung adenocarcinoma.
Oncologist Grant R. Williams, MD, MSPH, director of the Cancer & Aging Program at the University of Alabama at Birmingham, agreed in an interview that comprehensive geriatric assessments are important to guide treatment in the oldest adults. “In addition, it is important to elicit the goals of treatment as well,” he said. “For older adults that are fit or at least pre-frail and desire aggressive treatment, immunotherapy is a very reasonable approach, particularly when patients are closely monitored for side effects.”
No study funding is reported. The authors report no disclosures. Dr. Wong discloses an immediate family member is an employee and stock holder of Genentech. Dr. Williams has no disclosures.
Patients in their 90s with cancer tolerated immunotherapy well with few serious adverse effects, and they lived for an average of 1.6 years after treatment, a small new study within the US Department of Veterans Affairs (VA) health system reports.
Only 6.3% of 48 patients who were treated with immune checkpoint inhibitors experienced the most severe types of side effects – grade III/IV events – and a total of 27% had any adverse effects, according to the report, which was presented at the 2021 annual meeting of the Association of VA Hematology/Oncology (AVAHO) being held virtually and inperson in Denver Colorado, September 24 to September 26, 2021.
“Our project should help give confidence to oncologists treating the elderly,” said Andrew Joseph Benefield, MD, a hematology/oncology fellow at Wake Forest Baptist Medical Center, in an interview. “Immunotherapy can be given safely and likely effectively in select individuals over the age of 90 with good performance status.”
Benefield and colleagues launched their study to gain insight into a little-studied area: How does cancer treatment affects nonagenarians? “I think many oncologists have been in a situation where they encounter an individual over the age of 90 years who has a good performance status, and they've wondered if immunotherapy would be helpful and safe, particularly given our knowledge of waning immune strength as people age,” he said.
The researchers retrospectively tracked patients with cancer who were at least 90 years old from 2016 to 2017 and were treated with immune checkpoint inhibitors. Most were fit or fairly fit with Eastern Cooperative Oncology Group (ECOG) physical performance scales of 0 or 1 (n = 26), and nearly all had cancer in stage IV (n = 42). Melanoma was the most common type of cancer (n = 19), followed by non-small-cell lung cancer (n = 15). Patients were treated with an average of 12.2 cycles.
“In general, we saw that treatment was well-tolerated,” Dr. Benefield said. “We also noted that a trend toward better long-term survival outcomes in individuals with very good performance status at the start of treatment. We hope to parse this out more as we add more data to our data-set, as the numbers are still too small for confident direct comparison.”
Dr. Benefield said he has treated a limited number of patients in their 90s who were highly physical fit for their age and “very eager” to be treated. “They wanted to do anything they could to maintain their lifestyle,” he said. “In my experience, aggressive supportive care and close monitoring for developing toxicities has been most helpful.”
The researchers don’t know the causes of death of many of the patients, and it’s not clear how they fared in their final days. Still, Dr. Benefield said, “extending someone's life by more than 1 year with relatively low risk of adverse effects is reasonable.”
Oncologist Melisa Wong, MD, MAS, of the University of California, San Francisco, reviewed the study and said in an interview that it “a valuable description of outcomes for nonagenarians receiving immunotherapy in the VA healthcare system.” As she noted, “many other studies of immunotherapy among older adults focus on patients aged 65 or 70 and older while very few focus on octogenarians or nonagenarians.”
The findings suggest that “it is important to move beyond chronological age and assess patients’ physiologic age through a geriatric assessment,” she said. “Geriatric assessment-derived risk scores have been shown to predict chemotherapy toxicity for older adults and research to develop similar tools for immunotherapy are ongoing.”
However, she cautioned that older patients may become suffer so much from the most common side effect of immunotherapy -- fatigue – that “their independence is at stake.”
“Some of these patient choose to stop immunotherapy because the side effects aren’t worth it anymore,” she said. “The challenge for oncologists is not knowing in advance which patients will fall into each of these categories.”
She added that her geriatric oncology research focuses on improving risk stratification for older adults, such as those who are at least 70 with lung adenocarcinoma.
Oncologist Grant R. Williams, MD, MSPH, director of the Cancer & Aging Program at the University of Alabama at Birmingham, agreed in an interview that comprehensive geriatric assessments are important to guide treatment in the oldest adults. “In addition, it is important to elicit the goals of treatment as well,” he said. “For older adults that are fit or at least pre-frail and desire aggressive treatment, immunotherapy is a very reasonable approach, particularly when patients are closely monitored for side effects.”
No study funding is reported. The authors report no disclosures. Dr. Wong discloses an immediate family member is an employee and stock holder of Genentech. Dr. Williams has no disclosures.
Patients in their 90s with cancer tolerated immunotherapy well with few serious adverse effects, and they lived for an average of 1.6 years after treatment, a small new study within the US Department of Veterans Affairs (VA) health system reports.
Only 6.3% of 48 patients who were treated with immune checkpoint inhibitors experienced the most severe types of side effects – grade III/IV events – and a total of 27% had any adverse effects, according to the report, which was presented at the 2021 annual meeting of the Association of VA Hematology/Oncology (AVAHO) being held virtually and inperson in Denver Colorado, September 24 to September 26, 2021.
“Our project should help give confidence to oncologists treating the elderly,” said Andrew Joseph Benefield, MD, a hematology/oncology fellow at Wake Forest Baptist Medical Center, in an interview. “Immunotherapy can be given safely and likely effectively in select individuals over the age of 90 with good performance status.”
Benefield and colleagues launched their study to gain insight into a little-studied area: How does cancer treatment affects nonagenarians? “I think many oncologists have been in a situation where they encounter an individual over the age of 90 years who has a good performance status, and they've wondered if immunotherapy would be helpful and safe, particularly given our knowledge of waning immune strength as people age,” he said.
The researchers retrospectively tracked patients with cancer who were at least 90 years old from 2016 to 2017 and were treated with immune checkpoint inhibitors. Most were fit or fairly fit with Eastern Cooperative Oncology Group (ECOG) physical performance scales of 0 or 1 (n = 26), and nearly all had cancer in stage IV (n = 42). Melanoma was the most common type of cancer (n = 19), followed by non-small-cell lung cancer (n = 15). Patients were treated with an average of 12.2 cycles.
“In general, we saw that treatment was well-tolerated,” Dr. Benefield said. “We also noted that a trend toward better long-term survival outcomes in individuals with very good performance status at the start of treatment. We hope to parse this out more as we add more data to our data-set, as the numbers are still too small for confident direct comparison.”
Dr. Benefield said he has treated a limited number of patients in their 90s who were highly physical fit for their age and “very eager” to be treated. “They wanted to do anything they could to maintain their lifestyle,” he said. “In my experience, aggressive supportive care and close monitoring for developing toxicities has been most helpful.”
The researchers don’t know the causes of death of many of the patients, and it’s not clear how they fared in their final days. Still, Dr. Benefield said, “extending someone's life by more than 1 year with relatively low risk of adverse effects is reasonable.”
Oncologist Melisa Wong, MD, MAS, of the University of California, San Francisco, reviewed the study and said in an interview that it “a valuable description of outcomes for nonagenarians receiving immunotherapy in the VA healthcare system.” As she noted, “many other studies of immunotherapy among older adults focus on patients aged 65 or 70 and older while very few focus on octogenarians or nonagenarians.”
The findings suggest that “it is important to move beyond chronological age and assess patients’ physiologic age through a geriatric assessment,” she said. “Geriatric assessment-derived risk scores have been shown to predict chemotherapy toxicity for older adults and research to develop similar tools for immunotherapy are ongoing.”
However, she cautioned that older patients may become suffer so much from the most common side effect of immunotherapy -- fatigue – that “their independence is at stake.”
“Some of these patient choose to stop immunotherapy because the side effects aren’t worth it anymore,” she said. “The challenge for oncologists is not knowing in advance which patients will fall into each of these categories.”
She added that her geriatric oncology research focuses on improving risk stratification for older adults, such as those who are at least 70 with lung adenocarcinoma.
Oncologist Grant R. Williams, MD, MSPH, director of the Cancer & Aging Program at the University of Alabama at Birmingham, agreed in an interview that comprehensive geriatric assessments are important to guide treatment in the oldest adults. “In addition, it is important to elicit the goals of treatment as well,” he said. “For older adults that are fit or at least pre-frail and desire aggressive treatment, immunotherapy is a very reasonable approach, particularly when patients are closely monitored for side effects.”
No study funding is reported. The authors report no disclosures. Dr. Wong discloses an immediate family member is an employee and stock holder of Genentech. Dr. Williams has no disclosures.
One in six HIV PrEP Descovy switches contraindicated
George Froehle, PA, a primary care clinician at CentraCare in rural St. Cloud, Minn., has been prescribing the HIV prevention pill tenofovir disoproxil fumarate plus emtricitabine since it was marketed by the brand name Truvada and the Food and Drug Administration approved it in 2012. But recently, he’s been having conversations with patients about the new HIV prevention pill, tenofovir alafenamide plus emtricitabine (TAF/FTC, Descovy) as well.
“They may have a friend who has heard that Descovy is newer and safer,” Mr. Froehle said. But that’s not necessarily the case, at least according to lab values. A recent study in the journal Open Forum Infectious Diseases suggests that only between 1 in 10 and 1 in 3 switches to the new formulation of HIV pre-exposure prophylaxis (PrEP) are indicated by lab work – and that nearly half of people receiving a prescription for the new version had lab results actually contraindicating the switch.
This, combined with the lower cost of generic Truvada and the steep cost of Descovy, led study coauthor and HIV PrEP prescriber Douglas Krakower, MD, and colleagues to suggest that the generic version should be standard of care for all people on PrEP unless otherwise indicated.
This just “makes good sense,” Dr. Krakower, assistant professor of medicine at Harvard Medical School, Boston, told this news organization.
“It’s important to ultimately allow for patients and providers to have access to all of the PrEP options so they can choose the best option for each person,” he said. “But our data suggest that strategies to optimize the cost-effectiveness of PrEP prescribing, such as formulary interventions and education for patients and providers, could be beneficial – as long as there is an easy mechanism for patients and providers to override restrictions when there are clinical indications.”
Current PrEP guidelines from the Centers for Disease Control and Prevention don’t list a first-line or second-line treatment for PrEP. But recent guidance issued to insurance companies by the Biden administration specifically grants insurers permission to employ stepped formularies and cost sharing.
“Since the branded version of PrEP is not specified in the [U.S. Preventive Services Task Force] recommendation, plans and issuers may cover a generic version of PrEP without cost sharing and impose cost sharing on an equivalent branded version,” the rule, issued July 19, states. “However, plans and insurers must accommodate any individual for whom a particular PrEP medication [generic or brand name] would be medically inappropriate, as determined by the individual’s health care provider, by having a mechanism for waiving the otherwise applicable cost-sharing for the brand or nonpreferred brand version.”
Both drugs have been found to be 99% effective in stopping HIV acquisition in people at risk for it. Descovy is approved specifically for gay and bisexual men, transgender women, and anyone having anal sex. Ongoing studies are looking at the effectiveness of Descovy in people having vaginal sex. Generic Truvada has been approved for all people.
The biomarkers of switching
To be clear, both medicines are exceedingly safe, said lead author and epidemiologist Julia Marcus, PhD, MPH, associate professor at Harvard Medical School. Side effects have been mild and include nausea and diarrhea in the first month. What lab work tells clinicians is the potential for physiologic changes, but those changes don’t necessarily translate to clinical events.
“When I say harmful, I mean potentially harmful,” she said in an interview. “It’s really based on these incremental changes that maybe, in the long run, could be harmful.”
But she added that there are two types of damage from medicines: “There’s potential physiological damage, but there’s also potential financial damage.” While generic Truvada has a list price as low as $30 a bottle, Descovy has a list price of up to $2,000 a month. And the push for PrEP is growing. Recently, the head of the division of HIV/AIDS at the National Institute of Allergy and Infectious Diseases urged providers to get all their “HIV-negative, at-risk patients on PrEP tomorrow,” in light of the latest HIV vaccine failure.
So Dr. Marcus and team looked at data from the 2892 people who started taking PrEP in the year before the FDA approved Descovy in October 2019. Participants accessed PrEP through Fenway Health, a Boston-area health clinic serving a largely gay, lesbian, bisexual, transgender, and otherwise queer population, and the largest PrEP prescriber in New England. They then tracked which participants switched to Descovy and correlated the switches to lab work and CDC guidance for PrEP.
What they found was that just 11.9% of participants, or 343 people, switched to the newer formulation. That’s lower than the 27.2% who switched in nationally available data, which were released at a recent HIV conference. But when Dr. Krakower and colleagues looked at whether their PrEP prescriptions were appropriate based on the patients’ lab work, the findings were mixed.
On the one hand, they showed that 24 of those 343 people who switched to Descovy had creatinine clearance levels or bone mineral density measurements low enough to make the switch a good option. But that’s just 7% of all people who switched. They then ran a secondary model, in which they broadened the criteria for a switch from strictly those lab values to conditions that might indicate borderline kidney function, which could eventually lead to kidney damage. These included diagnoses of hypertension or diabetes, or borderline creatinine levels between 60 and 70 mL/minute.
“Even when we defined clinical indications as generously as we could, we still saw that only a minority had clinical indications for switching,” said “Most of the switching to TAF/FTC was potentially unnecessary, and some of it may have been harmful for people who had cardiovascular risk factors.”
That’s because although Descovy doesn’t affect renal and bone mineral markers, it does affect cholesterol levels and weight. Aftermarket and FDA data revealed a small but noticeable increase in statin use among people taking the new brand-name PrEP pill. When Marcus and colleagues looked for those biomarkers – total cholesterol greater than200, BMI of 30 or more, LDL cholesterol of more than 160 or HDL cholesterol of less than 40 – 14% of switches fit the criteria for contraindications for Descovy. That’s 10 times the rate of potential harm in switching as there was for those who stayed on the generic Truvada and would have been better served on Descovy. That came in at just 1.4%.
“There may be many reasons why patients or providers might choose to switch that we couldn’t document in our study,” she said. For instance, the newer formulation, Descovy, is a significantly smaller pill than the generic is. Or the perception of novelty might drive some switches.
“But I think we need qualitative work to understand how these decisions are being made,” she said in an interview. “It will be important to follow these patients to see what happens in terms of clinical outcomes.”
For his part, Mr. Froehle found the study intriguing. It reflects his own thinking around the value of the newer formulation. He also prescribes for people living with HIV. For them, the benefit of the new formulation of tenofovir present in Descovy has clear clinical relevance. After all, people living with HIV can be on their drug regimens for decades.
But people on PrEP aren’t likely to be on the pills as long, and so the real benefit of the newer, more expensive formulation is less clear. And he added that he’s already getting “pushback” from some insurance companies on the name-brand version, with companies asking for proof via lab values that a person has a history of kidney impairment or bone mineral density loss.
“It doesn’t happen a ton,” he said. “But it’s starting to happen, and normally it kind of builds from there.”
So when a patient comes in and asks specifically for Descovy, he usually will talk to them about it.
“If it’s what the patient wants and insurance covers it and it’s not unsafe for them to be on it, there might not be a reason to not prescribe Descovy,” said Mr. Froehle, who served as a sub-principal investigator for the DISCOVER clinical trial that showed the new PrEP was as effective as Truvada. “But now with Truvada being generic, we will talk about Truvada as being something we start up front because it may have a lower cost and it’s cheaper to the system. Then we can always switch to Descovy as needed.”
This study was funded by the National Institute of Allergy and Infectious Diseases. Dr. Marcus reported receiving fees from Kaiser Permanente Northern California on a research grant from Gilead Sciences. Dr. Krakower reported having conducted research that was funded by Gilead Sciences and Merck, as well as honoraria for medical education content and presentations for Medscape Medical News, MED-IQ, and DKBMed and royalties from work conducted by UpToDate. Mr. Froehle reported receiving fees from Gilead Sciences in connection with a Gilead advisory board.
A version of this article first appeared on Medscape.com.
George Froehle, PA, a primary care clinician at CentraCare in rural St. Cloud, Minn., has been prescribing the HIV prevention pill tenofovir disoproxil fumarate plus emtricitabine since it was marketed by the brand name Truvada and the Food and Drug Administration approved it in 2012. But recently, he’s been having conversations with patients about the new HIV prevention pill, tenofovir alafenamide plus emtricitabine (TAF/FTC, Descovy) as well.
“They may have a friend who has heard that Descovy is newer and safer,” Mr. Froehle said. But that’s not necessarily the case, at least according to lab values. A recent study in the journal Open Forum Infectious Diseases suggests that only between 1 in 10 and 1 in 3 switches to the new formulation of HIV pre-exposure prophylaxis (PrEP) are indicated by lab work – and that nearly half of people receiving a prescription for the new version had lab results actually contraindicating the switch.
This, combined with the lower cost of generic Truvada and the steep cost of Descovy, led study coauthor and HIV PrEP prescriber Douglas Krakower, MD, and colleagues to suggest that the generic version should be standard of care for all people on PrEP unless otherwise indicated.
This just “makes good sense,” Dr. Krakower, assistant professor of medicine at Harvard Medical School, Boston, told this news organization.
“It’s important to ultimately allow for patients and providers to have access to all of the PrEP options so they can choose the best option for each person,” he said. “But our data suggest that strategies to optimize the cost-effectiveness of PrEP prescribing, such as formulary interventions and education for patients and providers, could be beneficial – as long as there is an easy mechanism for patients and providers to override restrictions when there are clinical indications.”
Current PrEP guidelines from the Centers for Disease Control and Prevention don’t list a first-line or second-line treatment for PrEP. But recent guidance issued to insurance companies by the Biden administration specifically grants insurers permission to employ stepped formularies and cost sharing.
“Since the branded version of PrEP is not specified in the [U.S. Preventive Services Task Force] recommendation, plans and issuers may cover a generic version of PrEP without cost sharing and impose cost sharing on an equivalent branded version,” the rule, issued July 19, states. “However, plans and insurers must accommodate any individual for whom a particular PrEP medication [generic or brand name] would be medically inappropriate, as determined by the individual’s health care provider, by having a mechanism for waiving the otherwise applicable cost-sharing for the brand or nonpreferred brand version.”
Both drugs have been found to be 99% effective in stopping HIV acquisition in people at risk for it. Descovy is approved specifically for gay and bisexual men, transgender women, and anyone having anal sex. Ongoing studies are looking at the effectiveness of Descovy in people having vaginal sex. Generic Truvada has been approved for all people.
The biomarkers of switching
To be clear, both medicines are exceedingly safe, said lead author and epidemiologist Julia Marcus, PhD, MPH, associate professor at Harvard Medical School. Side effects have been mild and include nausea and diarrhea in the first month. What lab work tells clinicians is the potential for physiologic changes, but those changes don’t necessarily translate to clinical events.
“When I say harmful, I mean potentially harmful,” she said in an interview. “It’s really based on these incremental changes that maybe, in the long run, could be harmful.”
But she added that there are two types of damage from medicines: “There’s potential physiological damage, but there’s also potential financial damage.” While generic Truvada has a list price as low as $30 a bottle, Descovy has a list price of up to $2,000 a month. And the push for PrEP is growing. Recently, the head of the division of HIV/AIDS at the National Institute of Allergy and Infectious Diseases urged providers to get all their “HIV-negative, at-risk patients on PrEP tomorrow,” in light of the latest HIV vaccine failure.
So Dr. Marcus and team looked at data from the 2892 people who started taking PrEP in the year before the FDA approved Descovy in October 2019. Participants accessed PrEP through Fenway Health, a Boston-area health clinic serving a largely gay, lesbian, bisexual, transgender, and otherwise queer population, and the largest PrEP prescriber in New England. They then tracked which participants switched to Descovy and correlated the switches to lab work and CDC guidance for PrEP.
What they found was that just 11.9% of participants, or 343 people, switched to the newer formulation. That’s lower than the 27.2% who switched in nationally available data, which were released at a recent HIV conference. But when Dr. Krakower and colleagues looked at whether their PrEP prescriptions were appropriate based on the patients’ lab work, the findings were mixed.
On the one hand, they showed that 24 of those 343 people who switched to Descovy had creatinine clearance levels or bone mineral density measurements low enough to make the switch a good option. But that’s just 7% of all people who switched. They then ran a secondary model, in which they broadened the criteria for a switch from strictly those lab values to conditions that might indicate borderline kidney function, which could eventually lead to kidney damage. These included diagnoses of hypertension or diabetes, or borderline creatinine levels between 60 and 70 mL/minute.
“Even when we defined clinical indications as generously as we could, we still saw that only a minority had clinical indications for switching,” said “Most of the switching to TAF/FTC was potentially unnecessary, and some of it may have been harmful for people who had cardiovascular risk factors.”
That’s because although Descovy doesn’t affect renal and bone mineral markers, it does affect cholesterol levels and weight. Aftermarket and FDA data revealed a small but noticeable increase in statin use among people taking the new brand-name PrEP pill. When Marcus and colleagues looked for those biomarkers – total cholesterol greater than200, BMI of 30 or more, LDL cholesterol of more than 160 or HDL cholesterol of less than 40 – 14% of switches fit the criteria for contraindications for Descovy. That’s 10 times the rate of potential harm in switching as there was for those who stayed on the generic Truvada and would have been better served on Descovy. That came in at just 1.4%.
“There may be many reasons why patients or providers might choose to switch that we couldn’t document in our study,” she said. For instance, the newer formulation, Descovy, is a significantly smaller pill than the generic is. Or the perception of novelty might drive some switches.
“But I think we need qualitative work to understand how these decisions are being made,” she said in an interview. “It will be important to follow these patients to see what happens in terms of clinical outcomes.”
For his part, Mr. Froehle found the study intriguing. It reflects his own thinking around the value of the newer formulation. He also prescribes for people living with HIV. For them, the benefit of the new formulation of tenofovir present in Descovy has clear clinical relevance. After all, people living with HIV can be on their drug regimens for decades.
But people on PrEP aren’t likely to be on the pills as long, and so the real benefit of the newer, more expensive formulation is less clear. And he added that he’s already getting “pushback” from some insurance companies on the name-brand version, with companies asking for proof via lab values that a person has a history of kidney impairment or bone mineral density loss.
“It doesn’t happen a ton,” he said. “But it’s starting to happen, and normally it kind of builds from there.”
So when a patient comes in and asks specifically for Descovy, he usually will talk to them about it.
“If it’s what the patient wants and insurance covers it and it’s not unsafe for them to be on it, there might not be a reason to not prescribe Descovy,” said Mr. Froehle, who served as a sub-principal investigator for the DISCOVER clinical trial that showed the new PrEP was as effective as Truvada. “But now with Truvada being generic, we will talk about Truvada as being something we start up front because it may have a lower cost and it’s cheaper to the system. Then we can always switch to Descovy as needed.”
This study was funded by the National Institute of Allergy and Infectious Diseases. Dr. Marcus reported receiving fees from Kaiser Permanente Northern California on a research grant from Gilead Sciences. Dr. Krakower reported having conducted research that was funded by Gilead Sciences and Merck, as well as honoraria for medical education content and presentations for Medscape Medical News, MED-IQ, and DKBMed and royalties from work conducted by UpToDate. Mr. Froehle reported receiving fees from Gilead Sciences in connection with a Gilead advisory board.
A version of this article first appeared on Medscape.com.
George Froehle, PA, a primary care clinician at CentraCare in rural St. Cloud, Minn., has been prescribing the HIV prevention pill tenofovir disoproxil fumarate plus emtricitabine since it was marketed by the brand name Truvada and the Food and Drug Administration approved it in 2012. But recently, he’s been having conversations with patients about the new HIV prevention pill, tenofovir alafenamide plus emtricitabine (TAF/FTC, Descovy) as well.
“They may have a friend who has heard that Descovy is newer and safer,” Mr. Froehle said. But that’s not necessarily the case, at least according to lab values. A recent study in the journal Open Forum Infectious Diseases suggests that only between 1 in 10 and 1 in 3 switches to the new formulation of HIV pre-exposure prophylaxis (PrEP) are indicated by lab work – and that nearly half of people receiving a prescription for the new version had lab results actually contraindicating the switch.
This, combined with the lower cost of generic Truvada and the steep cost of Descovy, led study coauthor and HIV PrEP prescriber Douglas Krakower, MD, and colleagues to suggest that the generic version should be standard of care for all people on PrEP unless otherwise indicated.
This just “makes good sense,” Dr. Krakower, assistant professor of medicine at Harvard Medical School, Boston, told this news organization.
“It’s important to ultimately allow for patients and providers to have access to all of the PrEP options so they can choose the best option for each person,” he said. “But our data suggest that strategies to optimize the cost-effectiveness of PrEP prescribing, such as formulary interventions and education for patients and providers, could be beneficial – as long as there is an easy mechanism for patients and providers to override restrictions when there are clinical indications.”
Current PrEP guidelines from the Centers for Disease Control and Prevention don’t list a first-line or second-line treatment for PrEP. But recent guidance issued to insurance companies by the Biden administration specifically grants insurers permission to employ stepped formularies and cost sharing.
“Since the branded version of PrEP is not specified in the [U.S. Preventive Services Task Force] recommendation, plans and issuers may cover a generic version of PrEP without cost sharing and impose cost sharing on an equivalent branded version,” the rule, issued July 19, states. “However, plans and insurers must accommodate any individual for whom a particular PrEP medication [generic or brand name] would be medically inappropriate, as determined by the individual’s health care provider, by having a mechanism for waiving the otherwise applicable cost-sharing for the brand or nonpreferred brand version.”
Both drugs have been found to be 99% effective in stopping HIV acquisition in people at risk for it. Descovy is approved specifically for gay and bisexual men, transgender women, and anyone having anal sex. Ongoing studies are looking at the effectiveness of Descovy in people having vaginal sex. Generic Truvada has been approved for all people.
The biomarkers of switching
To be clear, both medicines are exceedingly safe, said lead author and epidemiologist Julia Marcus, PhD, MPH, associate professor at Harvard Medical School. Side effects have been mild and include nausea and diarrhea in the first month. What lab work tells clinicians is the potential for physiologic changes, but those changes don’t necessarily translate to clinical events.
“When I say harmful, I mean potentially harmful,” she said in an interview. “It’s really based on these incremental changes that maybe, in the long run, could be harmful.”
But she added that there are two types of damage from medicines: “There’s potential physiological damage, but there’s also potential financial damage.” While generic Truvada has a list price as low as $30 a bottle, Descovy has a list price of up to $2,000 a month. And the push for PrEP is growing. Recently, the head of the division of HIV/AIDS at the National Institute of Allergy and Infectious Diseases urged providers to get all their “HIV-negative, at-risk patients on PrEP tomorrow,” in light of the latest HIV vaccine failure.
So Dr. Marcus and team looked at data from the 2892 people who started taking PrEP in the year before the FDA approved Descovy in October 2019. Participants accessed PrEP through Fenway Health, a Boston-area health clinic serving a largely gay, lesbian, bisexual, transgender, and otherwise queer population, and the largest PrEP prescriber in New England. They then tracked which participants switched to Descovy and correlated the switches to lab work and CDC guidance for PrEP.
What they found was that just 11.9% of participants, or 343 people, switched to the newer formulation. That’s lower than the 27.2% who switched in nationally available data, which were released at a recent HIV conference. But when Dr. Krakower and colleagues looked at whether their PrEP prescriptions were appropriate based on the patients’ lab work, the findings were mixed.
On the one hand, they showed that 24 of those 343 people who switched to Descovy had creatinine clearance levels or bone mineral density measurements low enough to make the switch a good option. But that’s just 7% of all people who switched. They then ran a secondary model, in which they broadened the criteria for a switch from strictly those lab values to conditions that might indicate borderline kidney function, which could eventually lead to kidney damage. These included diagnoses of hypertension or diabetes, or borderline creatinine levels between 60 and 70 mL/minute.
“Even when we defined clinical indications as generously as we could, we still saw that only a minority had clinical indications for switching,” said “Most of the switching to TAF/FTC was potentially unnecessary, and some of it may have been harmful for people who had cardiovascular risk factors.”
That’s because although Descovy doesn’t affect renal and bone mineral markers, it does affect cholesterol levels and weight. Aftermarket and FDA data revealed a small but noticeable increase in statin use among people taking the new brand-name PrEP pill. When Marcus and colleagues looked for those biomarkers – total cholesterol greater than200, BMI of 30 or more, LDL cholesterol of more than 160 or HDL cholesterol of less than 40 – 14% of switches fit the criteria for contraindications for Descovy. That’s 10 times the rate of potential harm in switching as there was for those who stayed on the generic Truvada and would have been better served on Descovy. That came in at just 1.4%.
“There may be many reasons why patients or providers might choose to switch that we couldn’t document in our study,” she said. For instance, the newer formulation, Descovy, is a significantly smaller pill than the generic is. Or the perception of novelty might drive some switches.
“But I think we need qualitative work to understand how these decisions are being made,” she said in an interview. “It will be important to follow these patients to see what happens in terms of clinical outcomes.”
For his part, Mr. Froehle found the study intriguing. It reflects his own thinking around the value of the newer formulation. He also prescribes for people living with HIV. For them, the benefit of the new formulation of tenofovir present in Descovy has clear clinical relevance. After all, people living with HIV can be on their drug regimens for decades.
But people on PrEP aren’t likely to be on the pills as long, and so the real benefit of the newer, more expensive formulation is less clear. And he added that he’s already getting “pushback” from some insurance companies on the name-brand version, with companies asking for proof via lab values that a person has a history of kidney impairment or bone mineral density loss.
“It doesn’t happen a ton,” he said. “But it’s starting to happen, and normally it kind of builds from there.”
So when a patient comes in and asks specifically for Descovy, he usually will talk to them about it.
“If it’s what the patient wants and insurance covers it and it’s not unsafe for them to be on it, there might not be a reason to not prescribe Descovy,” said Mr. Froehle, who served as a sub-principal investigator for the DISCOVER clinical trial that showed the new PrEP was as effective as Truvada. “But now with Truvada being generic, we will talk about Truvada as being something we start up front because it may have a lower cost and it’s cheaper to the system. Then we can always switch to Descovy as needed.”
This study was funded by the National Institute of Allergy and Infectious Diseases. Dr. Marcus reported receiving fees from Kaiser Permanente Northern California on a research grant from Gilead Sciences. Dr. Krakower reported having conducted research that was funded by Gilead Sciences and Merck, as well as honoraria for medical education content and presentations for Medscape Medical News, MED-IQ, and DKBMed and royalties from work conducted by UpToDate. Mr. Froehle reported receiving fees from Gilead Sciences in connection with a Gilead advisory board.
A version of this article first appeared on Medscape.com.