User login
ADA scientific sessions address the old and the new
Long-awaited twincretin data, a study to inform prescribing in type 2 diabetes, COVID-19 and diabetes, and new guidance for treating type 1 diabetes in adults will be among the hot topics at the annual scientific sessions of the American Diabetes Association.
The meeting, to be held virtually for a second year, will take place June 25-29. As usual, the sessions will cover a wide range of basic, translational, and clinical material pertaining to type 1 and type 2 diabetes, complications, related subjects such as obesity and cardiovascular disease, and health care delivery.
New to this year’s agenda is COVID-19 and the many ways it has affected people with diabetes and health care delivery. And, more than in the past, the meeting will focus on ethnic and racial disparities in the delivery of care to people with diabetes.
And of course, there will be a tribute to another special aspect of 2021: the 100th anniversary of the discovery of insulin.
“I think there will undoubtedly be several things that will come out of this meeting that will change practice, and it will be important for clinicians to be aware of those, whether that’s groundbreaking trials or interpretation of data that will help us understand the interrelation between diabetes and COVID-19, which is still with us,” ADA chief scientific and medical officer Robert A. Gabbay, MD, PhD, said in an interview.
And ADA president of medicine and science Ruth S. Weinstock, MD, PhD, said in an interview: “I think there are many exciting sessions at this year’s meeting. ...I hope that it will help [clinicians] take better care of their patients with diabetes.
”
Will the twincretin tirzepatide live up to the hype?
Between December 2020 and May 2021, Eli Lilly issued a series of four press releases touting positive top-line results from a series of phase 3 studies on its novel agent tirzepatide, dubbed a twincretin for its dual actions as an agonist of the glucose-dependent insulinotropic polypeptide (GIP) and glucagonlike peptide-1 receptors.
Detailed results from those four trials, SURPASS-1, -2, -3, and -5, will be presented in a symposium on Tuesday, June 29. Results from SURPASS-4 will be presented at the annual meeting of the European Association for the Study of Diabetes in September 2021.
According to the company, the drug met its phase 3 primary efficacy endpoints for both hemoglobin A1c reduction and weight loss.
“At least the buzz on it has been good, but now we want to see the real data,” Dr. Gabbay said, noting that “the early data on weight loss in particular were quite good. So then the question would be: Do you go to a GLP-1 [agonist] or a dual agonist? There will be studies to tease that out.”
Regarding tirzepatide, Dr. Weinstock said: “Hopefully, more people with type 2 diabetes could achieve their glycemic goals, and those who would benefit from weight loss could have better weight loss. I haven’t seen the data, but if the addition of GIP can further improve glucose lowering as well as weight loss that would be great.”
How far will GRADE go in answering the second-drug question?
On Monday, June 28, results will be presented from the long-awaited Glycemia Reduction Approaches in Diabetes – A Comparative Effectiveness (GRADE) study.
Launched in 2013, the trial is funded by the National Institutes of Health and several pharmaceutical company partners. Over 5,000 patients diagnosed with type 2 diabetes within the prior 10 years and already taking metformin were randomized to one of four commonly used second-line glucose-lowering agents: glimepiride, sitagliptin, liraglutide, and basal insulin glargine. The aim was to determine which combination produced the best glycemic control with the fewest side effects.
Dr. Weinstock said: “Clinicians now have increasing numbers of medications to choose from when treating hyperglycemia in type 2 diabetes, and a common dilemma is which one to select. The results of GRADE should be informative for people taking care of type 2 diabetes in different populations.”
However, she also pointed out that GRADE does not include a group with a sodium-glucose transporter 2 inhibitor, as the trial was designed prior to the availability of the drug class. Now, SGLT2 inhibitors are widely used and recommended for cardiovascular and kidney benefit as well as glucose lowering.
“I believe the future is really precision medicine where we individualize treatment. So, for someone with heart failure you might choose an SGLT2 inhibitor, but there are plenty of other subpopulations. They are going to be looking at different subpopulations. I think we’re all very interested in seeing what the results are, but it’s not the end of the story. We will still have to individualize therapy and keep in mind their kidney, heart, heart failure status, and other factors,” she said.
Dr. Gabbay pointed out that GRADE is important because it’s one of the few comparative effectiveness trials conducted in diabetes. “I think it will be very rich [data] that will impact practice in a variety of ways. On the one hand, it doesn’t do everything we’d want it to do, but on the other hand, if you think of the number of comparative effectiveness trials in diabetes, there are not a lot ... I think it will be big.”
COVID-19 and diabetes: A lot to discuss
In contrast to the ADA scientific sessions in 2020, which took place too soon after the start of the COVID-19 pandemic to include much material about it, this year’s meeting will address many different aspects of the novel coronavirus.
Sessions will cover minimizing risk in people with diabetes during the pandemic, the latest data on whether COVID-19 triggers diabetes, and if so, by what mechanism, mental health issues related to COVID-19, as well as the management of foot care, pregnancy, and the pediatric population during the pandemic.
On Sunday, June 27, a symposium will be devoted to results of the DARE-19 trial, which explored the effects of the SGLT2 inhibitor dapagliflozin in more than 1200 patients hospitalized with COVID-19. The overall results, presented in May 2021 at the scientific sessions of the American College of Cardiology, showed a nonsignificant trend for benefit in time to organ failure or death compared with placebo. At ADA, separate efficacy and safety results for patients with and without diabetes will be presented.
According to Dr. Weinstock, “We know that in nonhospitalized patients with type 2 diabetes the SGLT2 inhibitors can help preserve kidney function and reduce heart failure. But we also know there can be diabetic ketoacidosis and genital infections and other side effects, so it’s been unclear up till now in type 2 diabetes whether they are safe and effective in people hospitalized in respiratory failure with COVID-19. And, given that people with type 2 diabetes and COVID-19 are more likely to require mechanical ventilation and are at greater risk of mortality, we’re anxious to see what these results are.”
Dr. Gabbay commented that, when the DARE study was initiated in April 2020, there were concerns about whether it was safe. And even now, “we’re still not sure about whether SGLT2 inhibitors should be stopped in hospitalized patients. The recommendations say to stop. I think this will be interesting.”
Also to be addressed in several meeting sessions are related issues the pandemic has brought forth, such as the use of telehealth for routine diabetes management, inpatient use of continuous glucose monitoring, and, of course, health care disparities.
“A lot of important issues related to COVID-19 of great interest will be discussed in a variety of sessions,” Dr. Weinstock said.
Type 1 diabetes in adults: It’s not just a pediatric disease
On Monday, June 28, a draft of the first-ever ADA/EASD consensus report on the management of type 1 diabetes in adults will be presented, with the final version slated for the annual meeting of the EASD in September 2021.
A previous ADA position statement had addressed management of type 1 diabetes across all age groups, but this will be the first to focus on adults. This is important, given that type 1 diabetes was formerly called juvenile diabetes and is still often perceived as a childhood disease. Adults who develop it are commonly misdiagnosed as having type 2 diabetes, Dr. Gabbay noted.
“A big-time issue is recognition of type 1 in adults. We often see patients come in who were misdiagnosed, on metformin, and not given insulin. Often they go for a while and get sicker and sicker.” Or, he said, sometimes they’re prescribed insulin but not the intensive regimens that are required for adequate glycemic control in type 1 diabetes. “They can be suboptimally treated and it can take years to get the right therapy. ... It’s unfortunate that they have to experience that.”
Dr. Weinstock, one of the authors of the statement, said it will cover a range of issues, including care schedules, therapies, psychosocial issues, and social determinants of health. “We tried to be comprehensive in this in terms of glycemic management. It doesn’t include a discussion of complications or their management. It really focuses on diagnosis and glycemic management.”
Dealing with disparities: ADA has taken several steps
A priority of the ADA is addressing disparities in the delivery of health care to people with diabetes, both Dr. Weinstock and Dr. Gabbay stressed. Quite a few sessions at the meeting will touch on various aspects, including sessions on Friday afternoon on “Health Care as a Social Justice Issue in the Diagnosis and Management of Diabetes,” and separate sessions on “Challenges and Successes With Health Inequities and Health Disparities in Diabetes” in adult and pediatric populations.
“For us at ADA, addressing health disparities is extremely important and we have a number of new programs this year to address this very important issue,” Dr. Weinstock said.
In August 2020, the ADA issued a Health Equity Bill of Rights, which includes access to insulin and other medications, affordable health care, and freedom from stigma and discrimination. The Association has also requested applications from researchers studying disparities in diabetes care.
Celebrating 100 years of lifesaving medication
Of course, the ADA will be celebrating the 100th anniversary of the discovery of insulin. A session on Saturday afternoon, entitled, “Insulin at Its 100th Birthday,” will cover the history of the landmark discovery, as well as insulin biosynthesis and mechanisms of action, and “the future of insulin as a therapy.”
Dr. Weinstock noted: “The discovery of insulin was an incredible achievement that, of course, saved the lives of many millions of children and adults. Before insulin became available, children and adults only survived for days or at most a few years after diagnosis. We will commemorate this anniversary.”
The virtual platform: Like last year, only better
Dr. Gabbay said in an interview that the virtual setup will be similar to last year’s in that talks will be prerecorded to ensure there are no technical glitches, but for many, presenters will be available afterward for live question and answers.
This year, though, the chat functionality will be enhanced to allow for discussion during the presentation, separate from the scientific question and answers. And, he noted, the virtual exhibit hall will be “bigger and better.”
Despite these improvements, Dr. Gabbay said, the plan is to go back to an in-person meeting in 2022 in New Orleans.
Dr. Weinstock’s institution receives research grants from Medtronic, Insulet, Lilly, Novo Nordisk, and Boehringer Ingelheim. Dr. Gabbay reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-awaited twincretin data, a study to inform prescribing in type 2 diabetes, COVID-19 and diabetes, and new guidance for treating type 1 diabetes in adults will be among the hot topics at the annual scientific sessions of the American Diabetes Association.
The meeting, to be held virtually for a second year, will take place June 25-29. As usual, the sessions will cover a wide range of basic, translational, and clinical material pertaining to type 1 and type 2 diabetes, complications, related subjects such as obesity and cardiovascular disease, and health care delivery.
New to this year’s agenda is COVID-19 and the many ways it has affected people with diabetes and health care delivery. And, more than in the past, the meeting will focus on ethnic and racial disparities in the delivery of care to people with diabetes.
And of course, there will be a tribute to another special aspect of 2021: the 100th anniversary of the discovery of insulin.
“I think there will undoubtedly be several things that will come out of this meeting that will change practice, and it will be important for clinicians to be aware of those, whether that’s groundbreaking trials or interpretation of data that will help us understand the interrelation between diabetes and COVID-19, which is still with us,” ADA chief scientific and medical officer Robert A. Gabbay, MD, PhD, said in an interview.
And ADA president of medicine and science Ruth S. Weinstock, MD, PhD, said in an interview: “I think there are many exciting sessions at this year’s meeting. ...I hope that it will help [clinicians] take better care of their patients with diabetes.
”
Will the twincretin tirzepatide live up to the hype?
Between December 2020 and May 2021, Eli Lilly issued a series of four press releases touting positive top-line results from a series of phase 3 studies on its novel agent tirzepatide, dubbed a twincretin for its dual actions as an agonist of the glucose-dependent insulinotropic polypeptide (GIP) and glucagonlike peptide-1 receptors.
Detailed results from those four trials, SURPASS-1, -2, -3, and -5, will be presented in a symposium on Tuesday, June 29. Results from SURPASS-4 will be presented at the annual meeting of the European Association for the Study of Diabetes in September 2021.
According to the company, the drug met its phase 3 primary efficacy endpoints for both hemoglobin A1c reduction and weight loss.
“At least the buzz on it has been good, but now we want to see the real data,” Dr. Gabbay said, noting that “the early data on weight loss in particular were quite good. So then the question would be: Do you go to a GLP-1 [agonist] or a dual agonist? There will be studies to tease that out.”
Regarding tirzepatide, Dr. Weinstock said: “Hopefully, more people with type 2 diabetes could achieve their glycemic goals, and those who would benefit from weight loss could have better weight loss. I haven’t seen the data, but if the addition of GIP can further improve glucose lowering as well as weight loss that would be great.”
How far will GRADE go in answering the second-drug question?
On Monday, June 28, results will be presented from the long-awaited Glycemia Reduction Approaches in Diabetes – A Comparative Effectiveness (GRADE) study.
Launched in 2013, the trial is funded by the National Institutes of Health and several pharmaceutical company partners. Over 5,000 patients diagnosed with type 2 diabetes within the prior 10 years and already taking metformin were randomized to one of four commonly used second-line glucose-lowering agents: glimepiride, sitagliptin, liraglutide, and basal insulin glargine. The aim was to determine which combination produced the best glycemic control with the fewest side effects.
Dr. Weinstock said: “Clinicians now have increasing numbers of medications to choose from when treating hyperglycemia in type 2 diabetes, and a common dilemma is which one to select. The results of GRADE should be informative for people taking care of type 2 diabetes in different populations.”
However, she also pointed out that GRADE does not include a group with a sodium-glucose transporter 2 inhibitor, as the trial was designed prior to the availability of the drug class. Now, SGLT2 inhibitors are widely used and recommended for cardiovascular and kidney benefit as well as glucose lowering.
“I believe the future is really precision medicine where we individualize treatment. So, for someone with heart failure you might choose an SGLT2 inhibitor, but there are plenty of other subpopulations. They are going to be looking at different subpopulations. I think we’re all very interested in seeing what the results are, but it’s not the end of the story. We will still have to individualize therapy and keep in mind their kidney, heart, heart failure status, and other factors,” she said.
Dr. Gabbay pointed out that GRADE is important because it’s one of the few comparative effectiveness trials conducted in diabetes. “I think it will be very rich [data] that will impact practice in a variety of ways. On the one hand, it doesn’t do everything we’d want it to do, but on the other hand, if you think of the number of comparative effectiveness trials in diabetes, there are not a lot ... I think it will be big.”
COVID-19 and diabetes: A lot to discuss
In contrast to the ADA scientific sessions in 2020, which took place too soon after the start of the COVID-19 pandemic to include much material about it, this year’s meeting will address many different aspects of the novel coronavirus.
Sessions will cover minimizing risk in people with diabetes during the pandemic, the latest data on whether COVID-19 triggers diabetes, and if so, by what mechanism, mental health issues related to COVID-19, as well as the management of foot care, pregnancy, and the pediatric population during the pandemic.
On Sunday, June 27, a symposium will be devoted to results of the DARE-19 trial, which explored the effects of the SGLT2 inhibitor dapagliflozin in more than 1200 patients hospitalized with COVID-19. The overall results, presented in May 2021 at the scientific sessions of the American College of Cardiology, showed a nonsignificant trend for benefit in time to organ failure or death compared with placebo. At ADA, separate efficacy and safety results for patients with and without diabetes will be presented.
According to Dr. Weinstock, “We know that in nonhospitalized patients with type 2 diabetes the SGLT2 inhibitors can help preserve kidney function and reduce heart failure. But we also know there can be diabetic ketoacidosis and genital infections and other side effects, so it’s been unclear up till now in type 2 diabetes whether they are safe and effective in people hospitalized in respiratory failure with COVID-19. And, given that people with type 2 diabetes and COVID-19 are more likely to require mechanical ventilation and are at greater risk of mortality, we’re anxious to see what these results are.”
Dr. Gabbay commented that, when the DARE study was initiated in April 2020, there were concerns about whether it was safe. And even now, “we’re still not sure about whether SGLT2 inhibitors should be stopped in hospitalized patients. The recommendations say to stop. I think this will be interesting.”
Also to be addressed in several meeting sessions are related issues the pandemic has brought forth, such as the use of telehealth for routine diabetes management, inpatient use of continuous glucose monitoring, and, of course, health care disparities.
“A lot of important issues related to COVID-19 of great interest will be discussed in a variety of sessions,” Dr. Weinstock said.
Type 1 diabetes in adults: It’s not just a pediatric disease
On Monday, June 28, a draft of the first-ever ADA/EASD consensus report on the management of type 1 diabetes in adults will be presented, with the final version slated for the annual meeting of the EASD in September 2021.
A previous ADA position statement had addressed management of type 1 diabetes across all age groups, but this will be the first to focus on adults. This is important, given that type 1 diabetes was formerly called juvenile diabetes and is still often perceived as a childhood disease. Adults who develop it are commonly misdiagnosed as having type 2 diabetes, Dr. Gabbay noted.
“A big-time issue is recognition of type 1 in adults. We often see patients come in who were misdiagnosed, on metformin, and not given insulin. Often they go for a while and get sicker and sicker.” Or, he said, sometimes they’re prescribed insulin but not the intensive regimens that are required for adequate glycemic control in type 1 diabetes. “They can be suboptimally treated and it can take years to get the right therapy. ... It’s unfortunate that they have to experience that.”
Dr. Weinstock, one of the authors of the statement, said it will cover a range of issues, including care schedules, therapies, psychosocial issues, and social determinants of health. “We tried to be comprehensive in this in terms of glycemic management. It doesn’t include a discussion of complications or their management. It really focuses on diagnosis and glycemic management.”
Dealing with disparities: ADA has taken several steps
A priority of the ADA is addressing disparities in the delivery of health care to people with diabetes, both Dr. Weinstock and Dr. Gabbay stressed. Quite a few sessions at the meeting will touch on various aspects, including sessions on Friday afternoon on “Health Care as a Social Justice Issue in the Diagnosis and Management of Diabetes,” and separate sessions on “Challenges and Successes With Health Inequities and Health Disparities in Diabetes” in adult and pediatric populations.
“For us at ADA, addressing health disparities is extremely important and we have a number of new programs this year to address this very important issue,” Dr. Weinstock said.
In August 2020, the ADA issued a Health Equity Bill of Rights, which includes access to insulin and other medications, affordable health care, and freedom from stigma and discrimination. The Association has also requested applications from researchers studying disparities in diabetes care.
Celebrating 100 years of lifesaving medication
Of course, the ADA will be celebrating the 100th anniversary of the discovery of insulin. A session on Saturday afternoon, entitled, “Insulin at Its 100th Birthday,” will cover the history of the landmark discovery, as well as insulin biosynthesis and mechanisms of action, and “the future of insulin as a therapy.”
Dr. Weinstock noted: “The discovery of insulin was an incredible achievement that, of course, saved the lives of many millions of children and adults. Before insulin became available, children and adults only survived for days or at most a few years after diagnosis. We will commemorate this anniversary.”
The virtual platform: Like last year, only better
Dr. Gabbay said in an interview that the virtual setup will be similar to last year’s in that talks will be prerecorded to ensure there are no technical glitches, but for many, presenters will be available afterward for live question and answers.
This year, though, the chat functionality will be enhanced to allow for discussion during the presentation, separate from the scientific question and answers. And, he noted, the virtual exhibit hall will be “bigger and better.”
Despite these improvements, Dr. Gabbay said, the plan is to go back to an in-person meeting in 2022 in New Orleans.
Dr. Weinstock’s institution receives research grants from Medtronic, Insulet, Lilly, Novo Nordisk, and Boehringer Ingelheim. Dr. Gabbay reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-awaited twincretin data, a study to inform prescribing in type 2 diabetes, COVID-19 and diabetes, and new guidance for treating type 1 diabetes in adults will be among the hot topics at the annual scientific sessions of the American Diabetes Association.
The meeting, to be held virtually for a second year, will take place June 25-29. As usual, the sessions will cover a wide range of basic, translational, and clinical material pertaining to type 1 and type 2 diabetes, complications, related subjects such as obesity and cardiovascular disease, and health care delivery.
New to this year’s agenda is COVID-19 and the many ways it has affected people with diabetes and health care delivery. And, more than in the past, the meeting will focus on ethnic and racial disparities in the delivery of care to people with diabetes.
And of course, there will be a tribute to another special aspect of 2021: the 100th anniversary of the discovery of insulin.
“I think there will undoubtedly be several things that will come out of this meeting that will change practice, and it will be important for clinicians to be aware of those, whether that’s groundbreaking trials or interpretation of data that will help us understand the interrelation between diabetes and COVID-19, which is still with us,” ADA chief scientific and medical officer Robert A. Gabbay, MD, PhD, said in an interview.
And ADA president of medicine and science Ruth S. Weinstock, MD, PhD, said in an interview: “I think there are many exciting sessions at this year’s meeting. ...I hope that it will help [clinicians] take better care of their patients with diabetes.
”
Will the twincretin tirzepatide live up to the hype?
Between December 2020 and May 2021, Eli Lilly issued a series of four press releases touting positive top-line results from a series of phase 3 studies on its novel agent tirzepatide, dubbed a twincretin for its dual actions as an agonist of the glucose-dependent insulinotropic polypeptide (GIP) and glucagonlike peptide-1 receptors.
Detailed results from those four trials, SURPASS-1, -2, -3, and -5, will be presented in a symposium on Tuesday, June 29. Results from SURPASS-4 will be presented at the annual meeting of the European Association for the Study of Diabetes in September 2021.
According to the company, the drug met its phase 3 primary efficacy endpoints for both hemoglobin A1c reduction and weight loss.
“At least the buzz on it has been good, but now we want to see the real data,” Dr. Gabbay said, noting that “the early data on weight loss in particular were quite good. So then the question would be: Do you go to a GLP-1 [agonist] or a dual agonist? There will be studies to tease that out.”
Regarding tirzepatide, Dr. Weinstock said: “Hopefully, more people with type 2 diabetes could achieve their glycemic goals, and those who would benefit from weight loss could have better weight loss. I haven’t seen the data, but if the addition of GIP can further improve glucose lowering as well as weight loss that would be great.”
How far will GRADE go in answering the second-drug question?
On Monday, June 28, results will be presented from the long-awaited Glycemia Reduction Approaches in Diabetes – A Comparative Effectiveness (GRADE) study.
Launched in 2013, the trial is funded by the National Institutes of Health and several pharmaceutical company partners. Over 5,000 patients diagnosed with type 2 diabetes within the prior 10 years and already taking metformin were randomized to one of four commonly used second-line glucose-lowering agents: glimepiride, sitagliptin, liraglutide, and basal insulin glargine. The aim was to determine which combination produced the best glycemic control with the fewest side effects.
Dr. Weinstock said: “Clinicians now have increasing numbers of medications to choose from when treating hyperglycemia in type 2 diabetes, and a common dilemma is which one to select. The results of GRADE should be informative for people taking care of type 2 diabetes in different populations.”
However, she also pointed out that GRADE does not include a group with a sodium-glucose transporter 2 inhibitor, as the trial was designed prior to the availability of the drug class. Now, SGLT2 inhibitors are widely used and recommended for cardiovascular and kidney benefit as well as glucose lowering.
“I believe the future is really precision medicine where we individualize treatment. So, for someone with heart failure you might choose an SGLT2 inhibitor, but there are plenty of other subpopulations. They are going to be looking at different subpopulations. I think we’re all very interested in seeing what the results are, but it’s not the end of the story. We will still have to individualize therapy and keep in mind their kidney, heart, heart failure status, and other factors,” she said.
Dr. Gabbay pointed out that GRADE is important because it’s one of the few comparative effectiveness trials conducted in diabetes. “I think it will be very rich [data] that will impact practice in a variety of ways. On the one hand, it doesn’t do everything we’d want it to do, but on the other hand, if you think of the number of comparative effectiveness trials in diabetes, there are not a lot ... I think it will be big.”
COVID-19 and diabetes: A lot to discuss
In contrast to the ADA scientific sessions in 2020, which took place too soon after the start of the COVID-19 pandemic to include much material about it, this year’s meeting will address many different aspects of the novel coronavirus.
Sessions will cover minimizing risk in people with diabetes during the pandemic, the latest data on whether COVID-19 triggers diabetes, and if so, by what mechanism, mental health issues related to COVID-19, as well as the management of foot care, pregnancy, and the pediatric population during the pandemic.
On Sunday, June 27, a symposium will be devoted to results of the DARE-19 trial, which explored the effects of the SGLT2 inhibitor dapagliflozin in more than 1200 patients hospitalized with COVID-19. The overall results, presented in May 2021 at the scientific sessions of the American College of Cardiology, showed a nonsignificant trend for benefit in time to organ failure or death compared with placebo. At ADA, separate efficacy and safety results for patients with and without diabetes will be presented.
According to Dr. Weinstock, “We know that in nonhospitalized patients with type 2 diabetes the SGLT2 inhibitors can help preserve kidney function and reduce heart failure. But we also know there can be diabetic ketoacidosis and genital infections and other side effects, so it’s been unclear up till now in type 2 diabetes whether they are safe and effective in people hospitalized in respiratory failure with COVID-19. And, given that people with type 2 diabetes and COVID-19 are more likely to require mechanical ventilation and are at greater risk of mortality, we’re anxious to see what these results are.”
Dr. Gabbay commented that, when the DARE study was initiated in April 2020, there were concerns about whether it was safe. And even now, “we’re still not sure about whether SGLT2 inhibitors should be stopped in hospitalized patients. The recommendations say to stop. I think this will be interesting.”
Also to be addressed in several meeting sessions are related issues the pandemic has brought forth, such as the use of telehealth for routine diabetes management, inpatient use of continuous glucose monitoring, and, of course, health care disparities.
“A lot of important issues related to COVID-19 of great interest will be discussed in a variety of sessions,” Dr. Weinstock said.
Type 1 diabetes in adults: It’s not just a pediatric disease
On Monday, June 28, a draft of the first-ever ADA/EASD consensus report on the management of type 1 diabetes in adults will be presented, with the final version slated for the annual meeting of the EASD in September 2021.
A previous ADA position statement had addressed management of type 1 diabetes across all age groups, but this will be the first to focus on adults. This is important, given that type 1 diabetes was formerly called juvenile diabetes and is still often perceived as a childhood disease. Adults who develop it are commonly misdiagnosed as having type 2 diabetes, Dr. Gabbay noted.
“A big-time issue is recognition of type 1 in adults. We often see patients come in who were misdiagnosed, on metformin, and not given insulin. Often they go for a while and get sicker and sicker.” Or, he said, sometimes they’re prescribed insulin but not the intensive regimens that are required for adequate glycemic control in type 1 diabetes. “They can be suboptimally treated and it can take years to get the right therapy. ... It’s unfortunate that they have to experience that.”
Dr. Weinstock, one of the authors of the statement, said it will cover a range of issues, including care schedules, therapies, psychosocial issues, and social determinants of health. “We tried to be comprehensive in this in terms of glycemic management. It doesn’t include a discussion of complications or their management. It really focuses on diagnosis and glycemic management.”
Dealing with disparities: ADA has taken several steps
A priority of the ADA is addressing disparities in the delivery of health care to people with diabetes, both Dr. Weinstock and Dr. Gabbay stressed. Quite a few sessions at the meeting will touch on various aspects, including sessions on Friday afternoon on “Health Care as a Social Justice Issue in the Diagnosis and Management of Diabetes,” and separate sessions on “Challenges and Successes With Health Inequities and Health Disparities in Diabetes” in adult and pediatric populations.
“For us at ADA, addressing health disparities is extremely important and we have a number of new programs this year to address this very important issue,” Dr. Weinstock said.
In August 2020, the ADA issued a Health Equity Bill of Rights, which includes access to insulin and other medications, affordable health care, and freedom from stigma and discrimination. The Association has also requested applications from researchers studying disparities in diabetes care.
Celebrating 100 years of lifesaving medication
Of course, the ADA will be celebrating the 100th anniversary of the discovery of insulin. A session on Saturday afternoon, entitled, “Insulin at Its 100th Birthday,” will cover the history of the landmark discovery, as well as insulin biosynthesis and mechanisms of action, and “the future of insulin as a therapy.”
Dr. Weinstock noted: “The discovery of insulin was an incredible achievement that, of course, saved the lives of many millions of children and adults. Before insulin became available, children and adults only survived for days or at most a few years after diagnosis. We will commemorate this anniversary.”
The virtual platform: Like last year, only better
Dr. Gabbay said in an interview that the virtual setup will be similar to last year’s in that talks will be prerecorded to ensure there are no technical glitches, but for many, presenters will be available afterward for live question and answers.
This year, though, the chat functionality will be enhanced to allow for discussion during the presentation, separate from the scientific question and answers. And, he noted, the virtual exhibit hall will be “bigger and better.”
Despite these improvements, Dr. Gabbay said, the plan is to go back to an in-person meeting in 2022 in New Orleans.
Dr. Weinstock’s institution receives research grants from Medtronic, Insulet, Lilly, Novo Nordisk, and Boehringer Ingelheim. Dr. Gabbay reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘Dreck’ to drama: How the media handled, and got handled by, COVID
For well over a year, the COVID-19 pandemic has been the biggest story in the world, costing millions of lives, impacting a presidential election, and quaking economies around the world.
But as vaccination rates increase and restrictions relax across the United States, relief is beginning to mix with reflection. Part of that contemplation means grappling with how the media depicted the crisis – in ways that were helpful, harmful, and somewhere in between.
“This story was so overwhelming, and the amount of journalism done about it was also overwhelming, and it’s going to be a while before we can do any kind of comprehensive overview of how journalism really performed,” said Maryn McKenna, an independent journalist and journalism professor at Emory University, Atlanta, who specializes in public and global health.
Some ‘heroically good’ reporting
The pandemic hit at a time when journalism was under a lot of pressure from external forces – undermined by politics, swimming through a sea of misinformation, and pressed by financial pressure to produce more stories more quickly, said Emily Bell, founding director of the Tow Center for Digital Journalism at Columbia University, New York.
The pandemic drove enormous audiences to news outlets, as people searched for reliable information, and increased the appreciation many people felt for the work of journalists, she said.
“I think there’s been some heroically good reporting and some really empathetic reporting as well,” said Ms. Bell. She cites The New York Times stories honoring the nearly 100,000 people lost to COVID-19 in May 2020 and The Atlantic’s COVID Tracking Project as exceptionally good examples.
Journalism is part of a complex, and evolving, information ecosystem characterized by “traditional” television, radio, and newspapers but also social media, search engine results, niche online news outlets, and clickbait sites.
On the one hand, social media provided a way for physicians, nurses, and scientists to speak directly to the world about their experiences and research. On the other hand, it’s challenging to elevate the really good work of traditional media over all of the bad or unhelpful signals, said Ms. Bell.
But, at the end of the day, much of journalism is a business. There are incentives in the market for tabloids to do sensational coverage and for outlets to push misleading, clickbait headlines, Ms. Bell said.
“Sometimes we’ll criticize journalists for ‘getting it wrong,’ but they might be getting it right in their business model but getting it wrong in terms of what it’s doing for society,” she said.
“We need to do a self-examination, when or if the dust from this ever settles, [on] how much of the past year was viewed as a business opportunity and did that get in the way of informing the public adequately,” Ms. McKenna said.
Digital platforms and journalists also need to reflect on how narratives build on one another, particularly online, said Ms. Bell. If you search for side effects of the Johnson & Johnson vaccine, for example, you will see a list of dozens of headlines that might give you the impression this is a major problem without the context that these effects are exceedingly rare, she notes.
There was also a personnel problem. Shrinking newsrooms over the last decade meant many outlets didn’t have dedicated science and health reporting, or very few staffers, if any. During the pandemic, suddenly general assignment and politics reporters had to be science and health reporters, too.
“You have a hard enough time with these issues if you’re a fairly seasoned science journalist,” said Gary Schwitzer, a former head of the health care news unit for CNN, journalism professor at the University of Minnesota, and founder of the watchdog site HealthNewsReview.org.
And outlets that had the staffing didn’t always put science reporters to full use, Ms. McKenna said. In March and April of 2020, major media outlets should have sent science reporters, not politics reporters, to President Donald Trump’s White House press briefings, which often included incorrect statements about COVID-19 science.
“I just don’t feel that the big outlets understood that that expertise would have made a difference,” she said.
New challenges, old problems
Some of the science journalism done during the pandemic has been some of the best ever seen in this country, said Mr. Schwitzer. But between the peaks of excellence, there is “the daily drumbeat coverage of dreck,” he added.
Many of the issues with this dreck coverage aren’t new or unique to the pandemic. For example, over the last year there have been far too many news stories based solely on weak information sources, like a drug company press release or a not-yet-peer-reviewed preprint article that hasn’t been put into proper context, said Mr. Schwitzer.
A quality science story should always include an independent perspective, he said, but many COVID-19 stories missed that perspective. This isn’t a new issue for science coverage – at Health News Review, Mr. Schwitzer and his colleagues saw stories without appropriate independent sources every day for 15 years.
It’s also challenging to write about uncertainty without over- or underselling what scientists know about a particular phenomenon. “We know that the media in general tends to portray science as more certain than it is,” said Dominique Brossard, PhD, professor and department chair at the University of Wisconsin–Madison and an expert on the intersection between science, media, and policy. This can lead to confusion when the science, and the advice based on that science, changes.
“The public has a really difficult time understanding what uncertainty means within science,” said Todd P. Newman, PhD, assistant professor at the University of Wisconsin–Madison who studies strategic communication within the context of science, technology, and the environment.
“I think the media generally has been good on the subject,” said Paul Offit, MD, director of the Vaccine Education Center, attending physician in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, and a prominent expert voice throughout the pandemic. “I think where they’ve been imperfect is they tend to be a little more dramatic in terms of how we’re doing.”
Dr. Offit isn’t the only expert to point to the drama of COVID-19 coverage. A study published in March 2021 by the National Bureau of Economic Research found 87% of stories by major U.S. media outlets leaned negative in the tone of their COVID-19 reporting, compared with 50% of stories from non-U.S. major outlets and 64% of articles in scientific journals. The negative emphasis persists even around positive developments, like vaccine trials and school re-openings.
John Whyte, MD, chief medical officer for WebMD, said he is very proud of the way WebMD and Medscape ramped up production of video series and other content to give health care providers the most up-to-date guidance on a rapidly evolving medical situation.
“But I think as [we] started to make progress – especially in the last 6 months – the coverage was never balanced enough; any positive news was immediately proceeded by negative,” he said.
“You want to be honest, but you also don’t want to be alarmist – and that’s where I think the challenge is at times in the media,” said Dr. Whyte. “We didn’t put enough optimism in at times, especially in recent months.”
“Any good coverage on vaccines immediately [was] covered by ‘[we] might need boosters in the fall.’ Why can’t [we] have an opportunity to breathe for a little while and see the good news?” he asked.
Variants or scariants?
Negativity and fear shaped much of the coverage around variants and vaccines earlier this year. In February 2021, Zeynep Tufekci, PhD, a sociologist at the University of North Carolina at Chapel Hill school of information and library science, wrote in The Atlantic about how much reporting has not reflected “the truly amazing reality of these vaccines,” and has instead highlighted “a chorus of relentless pessimism.”
This felt especially true earlier in 2021, when lots of coverage repeatedly emphasized what vaccinated people still could not do.
Eric Topol, MD, editor-in-chief of Medscape and executive vice president of Scripps Research in La Jolla, California, said New York Times editors told him earlier in the pandemic that he couldn’t use the word “scariant” in an opinion piece about the media’s overly fearful and sometimes inaccurate reporting around COVID-19 variants because they worried it would seem like the Times was coming after other media outlets.
“A variant is innocent until proven guilty,” said Dr. Topol. Had journalists approached the subject from that point of view, he said we would have seen “much more faithful reporting.”
Dr. Brossard and Dr. Newman worry that focusing on uncommon negative behavior, like people who break social distancing and mask rules by gathering at the beach or the bar, makes those actions seem more common than they actually are.
The evidence suggests that “if you show these kinds of things to people, you encourage them to do the same behavior,” said Dr. Brossard.
There have been other mistakes along the way, too. Early in the pandemic, many outlets pointed viewers to official government sources of information, some of which, like the White House press briefings in March and April of 2020, ended up being some of the most virulent spreaders of misinformation, said Ms. Bell.
Before that, a handful of journalists like Roxanne Khamsi were the few pushing back against the dominant media narrative in early 2020 that the novel coronavirus was less concerning than the seasonal flu.
“Science journalists have always been writing about studies that sometimes contradict each other, and what’s happened is that has only been condensed in time,” said Ms. Khamsi, a health care reporter for outlets like WIRED magazine and The New York Times and a former chief news editor for Nature Medicine.
Politics and misinformation
It’s impossible to talk about media coverage of COVID-19 without touching on politics and misinformation.
Coverage of the pandemic was politicized and polarized from the very beginning, said Sedona Chinn, PhD, an assistant professor at the University of Wisconsin–Madison who researches the prevalence and effects of scientific disagreements in media.
By looking at network news transcripts and articles from national outlets like the Washington Post and The New York Times, Dr. Chinn and her colleagues were able to determine politicization of coverage by counting the mentions of politicians versus scientists in COVID-19 coverage and polarization by looking at how different or similar the language was surrounding mentions of Republicans and Democrats.
If the two parties were working together or on the same page, they reasoned, the language would be similar.
From mid-March through May 2020, Dr. Chinn and fellow researchers found politicians were featured more often than scientists in newspaper coverage and as frequently as scientists in network news coverage. They also found polarized language around Republicans and Democrats, particularly in stories describing duels between the (at the time) Republican national government and Democratic state and local leaders.
It’s possible that polarization in news coverage helped contribute to polarized attitudes around the virus, the authors write in the study, which was published in August 2020 in the journal Science Communication.
The politicization and polarization of the issue is mirrored in our fractured media environment, where people tend to read, listen, and watch outlets that align with their political leanings. If that trusted outlet features misinformation, the people who follow it are more likely to accept that false information as truth, said Matt Motta, PhD, a political scientist at Oklahoma State University whose research includes public opinion and science communication.
This is true across the political spectrum, he said. When it comes to COVID-19, however, right-wing media outlets like Fox News and Breitbart are more likely to promote conspiratorial tropes and misinformation about the pandemic, according to Dr. Motta and his collaborator Dominik Stecula, PhD, a political scientist at Colorado State University who studies the news media environment and its effects on society.
Across the media ecosystem, reporting on the “infodemic” accompanying the pandemic – the rapid spread of misinformation and disinformation about the virus – has been a major challenge. Outlets may not be creating the misinformation, but they are the ones choosing to give it a platform, said Dr. Motta.
By repeating a false idea, even with the goal of debunking it, you can unintentionally cause the information to stick in people’s minds, said Dr. Brossard.
“Just because something is controversial doesn’t mean it’s worth covering,” said Dr. Motta. Using vaccines as an example, he said many reporters and scientists alike assume that if people have all the facts, they’ll land on the side of science.
“That is just fundamentally not how people think about the decision to get vaccinated,” he said. Instead, the choice is wrapped up with cultural factors, religious beliefs, political identity, and more.
The factors and challenges that shaped the media’s coverage of the pandemic aren’t going anywhere. Improving science and medical coverage in the future is a collective project for journalists, scientists, and everyone in between, said Dr. Newman.
“I call on scientists, too, to think really deeply about how they’re communicating – and especially how they’re communicating what they know and don’t know,” he said.
A version of this article first appeared on Medscape.com.
For well over a year, the COVID-19 pandemic has been the biggest story in the world, costing millions of lives, impacting a presidential election, and quaking economies around the world.
But as vaccination rates increase and restrictions relax across the United States, relief is beginning to mix with reflection. Part of that contemplation means grappling with how the media depicted the crisis – in ways that were helpful, harmful, and somewhere in between.
“This story was so overwhelming, and the amount of journalism done about it was also overwhelming, and it’s going to be a while before we can do any kind of comprehensive overview of how journalism really performed,” said Maryn McKenna, an independent journalist and journalism professor at Emory University, Atlanta, who specializes in public and global health.
Some ‘heroically good’ reporting
The pandemic hit at a time when journalism was under a lot of pressure from external forces – undermined by politics, swimming through a sea of misinformation, and pressed by financial pressure to produce more stories more quickly, said Emily Bell, founding director of the Tow Center for Digital Journalism at Columbia University, New York.
The pandemic drove enormous audiences to news outlets, as people searched for reliable information, and increased the appreciation many people felt for the work of journalists, she said.
“I think there’s been some heroically good reporting and some really empathetic reporting as well,” said Ms. Bell. She cites The New York Times stories honoring the nearly 100,000 people lost to COVID-19 in May 2020 and The Atlantic’s COVID Tracking Project as exceptionally good examples.
Journalism is part of a complex, and evolving, information ecosystem characterized by “traditional” television, radio, and newspapers but also social media, search engine results, niche online news outlets, and clickbait sites.
On the one hand, social media provided a way for physicians, nurses, and scientists to speak directly to the world about their experiences and research. On the other hand, it’s challenging to elevate the really good work of traditional media over all of the bad or unhelpful signals, said Ms. Bell.
But, at the end of the day, much of journalism is a business. There are incentives in the market for tabloids to do sensational coverage and for outlets to push misleading, clickbait headlines, Ms. Bell said.
“Sometimes we’ll criticize journalists for ‘getting it wrong,’ but they might be getting it right in their business model but getting it wrong in terms of what it’s doing for society,” she said.
“We need to do a self-examination, when or if the dust from this ever settles, [on] how much of the past year was viewed as a business opportunity and did that get in the way of informing the public adequately,” Ms. McKenna said.
Digital platforms and journalists also need to reflect on how narratives build on one another, particularly online, said Ms. Bell. If you search for side effects of the Johnson & Johnson vaccine, for example, you will see a list of dozens of headlines that might give you the impression this is a major problem without the context that these effects are exceedingly rare, she notes.
There was also a personnel problem. Shrinking newsrooms over the last decade meant many outlets didn’t have dedicated science and health reporting, or very few staffers, if any. During the pandemic, suddenly general assignment and politics reporters had to be science and health reporters, too.
“You have a hard enough time with these issues if you’re a fairly seasoned science journalist,” said Gary Schwitzer, a former head of the health care news unit for CNN, journalism professor at the University of Minnesota, and founder of the watchdog site HealthNewsReview.org.
And outlets that had the staffing didn’t always put science reporters to full use, Ms. McKenna said. In March and April of 2020, major media outlets should have sent science reporters, not politics reporters, to President Donald Trump’s White House press briefings, which often included incorrect statements about COVID-19 science.
“I just don’t feel that the big outlets understood that that expertise would have made a difference,” she said.
New challenges, old problems
Some of the science journalism done during the pandemic has been some of the best ever seen in this country, said Mr. Schwitzer. But between the peaks of excellence, there is “the daily drumbeat coverage of dreck,” he added.
Many of the issues with this dreck coverage aren’t new or unique to the pandemic. For example, over the last year there have been far too many news stories based solely on weak information sources, like a drug company press release or a not-yet-peer-reviewed preprint article that hasn’t been put into proper context, said Mr. Schwitzer.
A quality science story should always include an independent perspective, he said, but many COVID-19 stories missed that perspective. This isn’t a new issue for science coverage – at Health News Review, Mr. Schwitzer and his colleagues saw stories without appropriate independent sources every day for 15 years.
It’s also challenging to write about uncertainty without over- or underselling what scientists know about a particular phenomenon. “We know that the media in general tends to portray science as more certain than it is,” said Dominique Brossard, PhD, professor and department chair at the University of Wisconsin–Madison and an expert on the intersection between science, media, and policy. This can lead to confusion when the science, and the advice based on that science, changes.
“The public has a really difficult time understanding what uncertainty means within science,” said Todd P. Newman, PhD, assistant professor at the University of Wisconsin–Madison who studies strategic communication within the context of science, technology, and the environment.
“I think the media generally has been good on the subject,” said Paul Offit, MD, director of the Vaccine Education Center, attending physician in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, and a prominent expert voice throughout the pandemic. “I think where they’ve been imperfect is they tend to be a little more dramatic in terms of how we’re doing.”
Dr. Offit isn’t the only expert to point to the drama of COVID-19 coverage. A study published in March 2021 by the National Bureau of Economic Research found 87% of stories by major U.S. media outlets leaned negative in the tone of their COVID-19 reporting, compared with 50% of stories from non-U.S. major outlets and 64% of articles in scientific journals. The negative emphasis persists even around positive developments, like vaccine trials and school re-openings.
John Whyte, MD, chief medical officer for WebMD, said he is very proud of the way WebMD and Medscape ramped up production of video series and other content to give health care providers the most up-to-date guidance on a rapidly evolving medical situation.
“But I think as [we] started to make progress – especially in the last 6 months – the coverage was never balanced enough; any positive news was immediately proceeded by negative,” he said.
“You want to be honest, but you also don’t want to be alarmist – and that’s where I think the challenge is at times in the media,” said Dr. Whyte. “We didn’t put enough optimism in at times, especially in recent months.”
“Any good coverage on vaccines immediately [was] covered by ‘[we] might need boosters in the fall.’ Why can’t [we] have an opportunity to breathe for a little while and see the good news?” he asked.
Variants or scariants?
Negativity and fear shaped much of the coverage around variants and vaccines earlier this year. In February 2021, Zeynep Tufekci, PhD, a sociologist at the University of North Carolina at Chapel Hill school of information and library science, wrote in The Atlantic about how much reporting has not reflected “the truly amazing reality of these vaccines,” and has instead highlighted “a chorus of relentless pessimism.”
This felt especially true earlier in 2021, when lots of coverage repeatedly emphasized what vaccinated people still could not do.
Eric Topol, MD, editor-in-chief of Medscape and executive vice president of Scripps Research in La Jolla, California, said New York Times editors told him earlier in the pandemic that he couldn’t use the word “scariant” in an opinion piece about the media’s overly fearful and sometimes inaccurate reporting around COVID-19 variants because they worried it would seem like the Times was coming after other media outlets.
“A variant is innocent until proven guilty,” said Dr. Topol. Had journalists approached the subject from that point of view, he said we would have seen “much more faithful reporting.”
Dr. Brossard and Dr. Newman worry that focusing on uncommon negative behavior, like people who break social distancing and mask rules by gathering at the beach or the bar, makes those actions seem more common than they actually are.
The evidence suggests that “if you show these kinds of things to people, you encourage them to do the same behavior,” said Dr. Brossard.
There have been other mistakes along the way, too. Early in the pandemic, many outlets pointed viewers to official government sources of information, some of which, like the White House press briefings in March and April of 2020, ended up being some of the most virulent spreaders of misinformation, said Ms. Bell.
Before that, a handful of journalists like Roxanne Khamsi were the few pushing back against the dominant media narrative in early 2020 that the novel coronavirus was less concerning than the seasonal flu.
“Science journalists have always been writing about studies that sometimes contradict each other, and what’s happened is that has only been condensed in time,” said Ms. Khamsi, a health care reporter for outlets like WIRED magazine and The New York Times and a former chief news editor for Nature Medicine.
Politics and misinformation
It’s impossible to talk about media coverage of COVID-19 without touching on politics and misinformation.
Coverage of the pandemic was politicized and polarized from the very beginning, said Sedona Chinn, PhD, an assistant professor at the University of Wisconsin–Madison who researches the prevalence and effects of scientific disagreements in media.
By looking at network news transcripts and articles from national outlets like the Washington Post and The New York Times, Dr. Chinn and her colleagues were able to determine politicization of coverage by counting the mentions of politicians versus scientists in COVID-19 coverage and polarization by looking at how different or similar the language was surrounding mentions of Republicans and Democrats.
If the two parties were working together or on the same page, they reasoned, the language would be similar.
From mid-March through May 2020, Dr. Chinn and fellow researchers found politicians were featured more often than scientists in newspaper coverage and as frequently as scientists in network news coverage. They also found polarized language around Republicans and Democrats, particularly in stories describing duels between the (at the time) Republican national government and Democratic state and local leaders.
It’s possible that polarization in news coverage helped contribute to polarized attitudes around the virus, the authors write in the study, which was published in August 2020 in the journal Science Communication.
The politicization and polarization of the issue is mirrored in our fractured media environment, where people tend to read, listen, and watch outlets that align with their political leanings. If that trusted outlet features misinformation, the people who follow it are more likely to accept that false information as truth, said Matt Motta, PhD, a political scientist at Oklahoma State University whose research includes public opinion and science communication.
This is true across the political spectrum, he said. When it comes to COVID-19, however, right-wing media outlets like Fox News and Breitbart are more likely to promote conspiratorial tropes and misinformation about the pandemic, according to Dr. Motta and his collaborator Dominik Stecula, PhD, a political scientist at Colorado State University who studies the news media environment and its effects on society.
Across the media ecosystem, reporting on the “infodemic” accompanying the pandemic – the rapid spread of misinformation and disinformation about the virus – has been a major challenge. Outlets may not be creating the misinformation, but they are the ones choosing to give it a platform, said Dr. Motta.
By repeating a false idea, even with the goal of debunking it, you can unintentionally cause the information to stick in people’s minds, said Dr. Brossard.
“Just because something is controversial doesn’t mean it’s worth covering,” said Dr. Motta. Using vaccines as an example, he said many reporters and scientists alike assume that if people have all the facts, they’ll land on the side of science.
“That is just fundamentally not how people think about the decision to get vaccinated,” he said. Instead, the choice is wrapped up with cultural factors, religious beliefs, political identity, and more.
The factors and challenges that shaped the media’s coverage of the pandemic aren’t going anywhere. Improving science and medical coverage in the future is a collective project for journalists, scientists, and everyone in between, said Dr. Newman.
“I call on scientists, too, to think really deeply about how they’re communicating – and especially how they’re communicating what they know and don’t know,” he said.
A version of this article first appeared on Medscape.com.
For well over a year, the COVID-19 pandemic has been the biggest story in the world, costing millions of lives, impacting a presidential election, and quaking economies around the world.
But as vaccination rates increase and restrictions relax across the United States, relief is beginning to mix with reflection. Part of that contemplation means grappling with how the media depicted the crisis – in ways that were helpful, harmful, and somewhere in between.
“This story was so overwhelming, and the amount of journalism done about it was also overwhelming, and it’s going to be a while before we can do any kind of comprehensive overview of how journalism really performed,” said Maryn McKenna, an independent journalist and journalism professor at Emory University, Atlanta, who specializes in public and global health.
Some ‘heroically good’ reporting
The pandemic hit at a time when journalism was under a lot of pressure from external forces – undermined by politics, swimming through a sea of misinformation, and pressed by financial pressure to produce more stories more quickly, said Emily Bell, founding director of the Tow Center for Digital Journalism at Columbia University, New York.
The pandemic drove enormous audiences to news outlets, as people searched for reliable information, and increased the appreciation many people felt for the work of journalists, she said.
“I think there’s been some heroically good reporting and some really empathetic reporting as well,” said Ms. Bell. She cites The New York Times stories honoring the nearly 100,000 people lost to COVID-19 in May 2020 and The Atlantic’s COVID Tracking Project as exceptionally good examples.
Journalism is part of a complex, and evolving, information ecosystem characterized by “traditional” television, radio, and newspapers but also social media, search engine results, niche online news outlets, and clickbait sites.
On the one hand, social media provided a way for physicians, nurses, and scientists to speak directly to the world about their experiences and research. On the other hand, it’s challenging to elevate the really good work of traditional media over all of the bad or unhelpful signals, said Ms. Bell.
But, at the end of the day, much of journalism is a business. There are incentives in the market for tabloids to do sensational coverage and for outlets to push misleading, clickbait headlines, Ms. Bell said.
“Sometimes we’ll criticize journalists for ‘getting it wrong,’ but they might be getting it right in their business model but getting it wrong in terms of what it’s doing for society,” she said.
“We need to do a self-examination, when or if the dust from this ever settles, [on] how much of the past year was viewed as a business opportunity and did that get in the way of informing the public adequately,” Ms. McKenna said.
Digital platforms and journalists also need to reflect on how narratives build on one another, particularly online, said Ms. Bell. If you search for side effects of the Johnson & Johnson vaccine, for example, you will see a list of dozens of headlines that might give you the impression this is a major problem without the context that these effects are exceedingly rare, she notes.
There was also a personnel problem. Shrinking newsrooms over the last decade meant many outlets didn’t have dedicated science and health reporting, or very few staffers, if any. During the pandemic, suddenly general assignment and politics reporters had to be science and health reporters, too.
“You have a hard enough time with these issues if you’re a fairly seasoned science journalist,” said Gary Schwitzer, a former head of the health care news unit for CNN, journalism professor at the University of Minnesota, and founder of the watchdog site HealthNewsReview.org.
And outlets that had the staffing didn’t always put science reporters to full use, Ms. McKenna said. In March and April of 2020, major media outlets should have sent science reporters, not politics reporters, to President Donald Trump’s White House press briefings, which often included incorrect statements about COVID-19 science.
“I just don’t feel that the big outlets understood that that expertise would have made a difference,” she said.
New challenges, old problems
Some of the science journalism done during the pandemic has been some of the best ever seen in this country, said Mr. Schwitzer. But between the peaks of excellence, there is “the daily drumbeat coverage of dreck,” he added.
Many of the issues with this dreck coverage aren’t new or unique to the pandemic. For example, over the last year there have been far too many news stories based solely on weak information sources, like a drug company press release or a not-yet-peer-reviewed preprint article that hasn’t been put into proper context, said Mr. Schwitzer.
A quality science story should always include an independent perspective, he said, but many COVID-19 stories missed that perspective. This isn’t a new issue for science coverage – at Health News Review, Mr. Schwitzer and his colleagues saw stories without appropriate independent sources every day for 15 years.
It’s also challenging to write about uncertainty without over- or underselling what scientists know about a particular phenomenon. “We know that the media in general tends to portray science as more certain than it is,” said Dominique Brossard, PhD, professor and department chair at the University of Wisconsin–Madison and an expert on the intersection between science, media, and policy. This can lead to confusion when the science, and the advice based on that science, changes.
“The public has a really difficult time understanding what uncertainty means within science,” said Todd P. Newman, PhD, assistant professor at the University of Wisconsin–Madison who studies strategic communication within the context of science, technology, and the environment.
“I think the media generally has been good on the subject,” said Paul Offit, MD, director of the Vaccine Education Center, attending physician in the Division of Infectious Diseases at the Children’s Hospital of Philadelphia, and a prominent expert voice throughout the pandemic. “I think where they’ve been imperfect is they tend to be a little more dramatic in terms of how we’re doing.”
Dr. Offit isn’t the only expert to point to the drama of COVID-19 coverage. A study published in March 2021 by the National Bureau of Economic Research found 87% of stories by major U.S. media outlets leaned negative in the tone of their COVID-19 reporting, compared with 50% of stories from non-U.S. major outlets and 64% of articles in scientific journals. The negative emphasis persists even around positive developments, like vaccine trials and school re-openings.
John Whyte, MD, chief medical officer for WebMD, said he is very proud of the way WebMD and Medscape ramped up production of video series and other content to give health care providers the most up-to-date guidance on a rapidly evolving medical situation.
“But I think as [we] started to make progress – especially in the last 6 months – the coverage was never balanced enough; any positive news was immediately proceeded by negative,” he said.
“You want to be honest, but you also don’t want to be alarmist – and that’s where I think the challenge is at times in the media,” said Dr. Whyte. “We didn’t put enough optimism in at times, especially in recent months.”
“Any good coverage on vaccines immediately [was] covered by ‘[we] might need boosters in the fall.’ Why can’t [we] have an opportunity to breathe for a little while and see the good news?” he asked.
Variants or scariants?
Negativity and fear shaped much of the coverage around variants and vaccines earlier this year. In February 2021, Zeynep Tufekci, PhD, a sociologist at the University of North Carolina at Chapel Hill school of information and library science, wrote in The Atlantic about how much reporting has not reflected “the truly amazing reality of these vaccines,” and has instead highlighted “a chorus of relentless pessimism.”
This felt especially true earlier in 2021, when lots of coverage repeatedly emphasized what vaccinated people still could not do.
Eric Topol, MD, editor-in-chief of Medscape and executive vice president of Scripps Research in La Jolla, California, said New York Times editors told him earlier in the pandemic that he couldn’t use the word “scariant” in an opinion piece about the media’s overly fearful and sometimes inaccurate reporting around COVID-19 variants because they worried it would seem like the Times was coming after other media outlets.
“A variant is innocent until proven guilty,” said Dr. Topol. Had journalists approached the subject from that point of view, he said we would have seen “much more faithful reporting.”
Dr. Brossard and Dr. Newman worry that focusing on uncommon negative behavior, like people who break social distancing and mask rules by gathering at the beach or the bar, makes those actions seem more common than they actually are.
The evidence suggests that “if you show these kinds of things to people, you encourage them to do the same behavior,” said Dr. Brossard.
There have been other mistakes along the way, too. Early in the pandemic, many outlets pointed viewers to official government sources of information, some of which, like the White House press briefings in March and April of 2020, ended up being some of the most virulent spreaders of misinformation, said Ms. Bell.
Before that, a handful of journalists like Roxanne Khamsi were the few pushing back against the dominant media narrative in early 2020 that the novel coronavirus was less concerning than the seasonal flu.
“Science journalists have always been writing about studies that sometimes contradict each other, and what’s happened is that has only been condensed in time,” said Ms. Khamsi, a health care reporter for outlets like WIRED magazine and The New York Times and a former chief news editor for Nature Medicine.
Politics and misinformation
It’s impossible to talk about media coverage of COVID-19 without touching on politics and misinformation.
Coverage of the pandemic was politicized and polarized from the very beginning, said Sedona Chinn, PhD, an assistant professor at the University of Wisconsin–Madison who researches the prevalence and effects of scientific disagreements in media.
By looking at network news transcripts and articles from national outlets like the Washington Post and The New York Times, Dr. Chinn and her colleagues were able to determine politicization of coverage by counting the mentions of politicians versus scientists in COVID-19 coverage and polarization by looking at how different or similar the language was surrounding mentions of Republicans and Democrats.
If the two parties were working together or on the same page, they reasoned, the language would be similar.
From mid-March through May 2020, Dr. Chinn and fellow researchers found politicians were featured more often than scientists in newspaper coverage and as frequently as scientists in network news coverage. They also found polarized language around Republicans and Democrats, particularly in stories describing duels between the (at the time) Republican national government and Democratic state and local leaders.
It’s possible that polarization in news coverage helped contribute to polarized attitudes around the virus, the authors write in the study, which was published in August 2020 in the journal Science Communication.
The politicization and polarization of the issue is mirrored in our fractured media environment, where people tend to read, listen, and watch outlets that align with their political leanings. If that trusted outlet features misinformation, the people who follow it are more likely to accept that false information as truth, said Matt Motta, PhD, a political scientist at Oklahoma State University whose research includes public opinion and science communication.
This is true across the political spectrum, he said. When it comes to COVID-19, however, right-wing media outlets like Fox News and Breitbart are more likely to promote conspiratorial tropes and misinformation about the pandemic, according to Dr. Motta and his collaborator Dominik Stecula, PhD, a political scientist at Colorado State University who studies the news media environment and its effects on society.
Across the media ecosystem, reporting on the “infodemic” accompanying the pandemic – the rapid spread of misinformation and disinformation about the virus – has been a major challenge. Outlets may not be creating the misinformation, but they are the ones choosing to give it a platform, said Dr. Motta.
By repeating a false idea, even with the goal of debunking it, you can unintentionally cause the information to stick in people’s minds, said Dr. Brossard.
“Just because something is controversial doesn’t mean it’s worth covering,” said Dr. Motta. Using vaccines as an example, he said many reporters and scientists alike assume that if people have all the facts, they’ll land on the side of science.
“That is just fundamentally not how people think about the decision to get vaccinated,” he said. Instead, the choice is wrapped up with cultural factors, religious beliefs, political identity, and more.
The factors and challenges that shaped the media’s coverage of the pandemic aren’t going anywhere. Improving science and medical coverage in the future is a collective project for journalists, scientists, and everyone in between, said Dr. Newman.
“I call on scientists, too, to think really deeply about how they’re communicating – and especially how they’re communicating what they know and don’t know,” he said.
A version of this article first appeared on Medscape.com.
Clinicians slow to implement lipid-lowering guidelines: GOULD registry
Among patients with atherosclerotic cardiovascular disease (ASCVD), 2 years after release of treat-to-target guidelines from the American Heart Association and the European Society of Cardiology and European Atherosclerosis Society, most patients with LDL cholesterol higher than 70 mg/dL did not receive intensification of therapy, and two-thirds continued to have LDL levels above that level, according to a prospective registry study.
Both guidelines recommend driving LDL-C levels to 50% or below of baseline levels; results from the Getting to an Improved Understanding of Low-Density Lipoprotein Cholesterol and Dyslipidemia Management (GOULD) registry suggest this is rarely achieved. “Unfortunately it’s not a total surprise, but it’s disappointing,” said Christopher Cannon, MD, the study’s lead author.
“Therapeutic inertia seems to be the rule in clinical practice,” said Jennifer G. Robinson, MD, MPH, who was asked to comment on the study. Dr. Robinson is professor epidemiology and cardiology at the University of Iowa, Iowa City.
“This is yet another disappointing reminder of how we are failing our patients. Lipid lowering is one of the safest, most effective ways to prevent cardiovascular disease, and yet we are falling short. We have the tools in our toolkit to achieve guideline-based lipid lowering goals, but we just aren’t using them,” said Ann Marie Navar, MD, PhD, associate professor of cardiology at the University of Texas, Dallas.
Patients hesitant
Changes in practice following guidelines can often be slow, but in this case may have been complicated by the fact that statins have a reputation for causing side effects, so some patients may be refusing treatment based on what they’ve seen on the Internet. Even though the study looked at all lipid-lowering agents, the misinformation around statins may be spilling over, according to Dr. Cannon. “There’s in general so much misinformation around COVID and every other topic in the world. That makes people question what is real [about] anything,” said Dr. Cannon, a cardiologist at Brigham and Women’s hospital and professor of medicine at Harvard Medical School, both in Boston.
Patient characteristics may partly explain slow uptake. “Clinicians may not think further LDL-C lowering is a high enough priority in terms of potential benefit for a given patient in light of the effort being expended to take care of all their other issues and chronic health problems. If the clinician does bring it up to the patient, there may be barriers in terms of additional medication burden, cost, or acquisition issues,” said Dr. Robinson.
The answer may be better evidence and a more personalized approach. Clinical trials that explore defined patient populations could convince patients of a benefit, and payers to reimburse, according to Dr. Robinson.
Changing guidance
Another complication is that both the guidelines and the field are rapidly changing. The 2013 AHA guidelines did not include a treatment to goal and focused instead on use of high-dose statins. But the 2018 update reversed course after randomized studies demonstrated a benefit to treating to target. The researchers found no increase in the frequency of treating to target after the release of the 2018 guidelines. “Publication and announcement of guidelines doesn’t mean that people are getting treated better. We really have to implement them,” said Dr. Cannon.
On a positive note, the GOULD researchers found high acceptance of the new proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) inhibitors, with over 90% of patients continuing those medications after 2 years. “That’s nice and high. If people do get onto the very intensive lipid-lowing therapies, they tend to stay on them,” said Dr. Cannon.
What’s next
Still, the lack of intensification is concerning, and the findings led to some consternation in Twitter exchanges, said Dr. Cannon. “People posted ‘Well, what do we do now?’ ” Dr. Cannon’s team is addressing the issue with an algorithm-based risk management program with prospective enrollment. They have conducted educational webinars and provided site-specific reports on LDL status among patients at each center compared to others, and hope that information will improve compliance. In 2020, the group published an interim analysis of the first 5,000 enrollees, and Dr. Cannon expects to finish that study by the end of the year.
Dr. Navar agreed that physicians need to do a better job of testing LDL-C levels after treatment to identify patients who require more aggressive therapy. That can be deferred in some primary prevention patients with high LDL-C but normal particle numbers as measured with ApoB. “But in those at high risk for disease and those with established CVD who are not at goal, as long as they don’t have a life-limiting condition, we should always up-titrate therapy. It’s one of the safest, most effective ways to lower cardiovascular risk,” said Dr. Navar.
Key study results
The prospective study included 5,006 patients at 119 centers with a mean age of 68 years. About 40% were women, and 86.1% were White. All had ASCVD and LDL levels of at least 70 mg/dL. After 2 years, 17% had undergone intensification of lipid-lowering therapy (LLT). Among patients with LDL-C levels ≥ 100 mg/dL, 22% underwent LLT intensification, compared with 14% of patients with LDL-C levels of 70-99 mg/dL.
The vast majority, 92%, of patients who underwent LLT via addition of PCSK9 inhibitors were still taking the drug after 2 years.
Three-quarters (3,768) had lipid level measurements at least once during follow-up, and median LDL-C levels dropped from 120 to 95 mg/dL in the ≥100-mg/dL cohort (P < .001), and from 82 to 77 mg/dL in the 70- to 99-mg/dL cohort (P <. 001). There was no significant difference in the median values in the patients on PCSK9 inhibitors.
In all, 21% of the ≥100-mg/dL cohort achieved LDL-C levels <70 mg/dL at 2 years, versus 34% in the 77- to 99-mg/dL cohort and 52% of patients taking PCSK9 inhibitors.
Patients seen at teaching hospitals were more likely to undergo LLT intensification compared to nonteaching hospitals (25% versus 17%; P < .001), as were those where lipid protocols were in place (22% versus 15%; P < .001), and those treated in cardiology (22%) compared to treatment in internal or family medicine (12%; P <.001). The study was published online June 16 in JAMA Cardiology.
Dr. Cannon, Dr. Navar, and Dr. Robinson disclosed ties with Amgen, which funded the study, and other companies.
Among patients with atherosclerotic cardiovascular disease (ASCVD), 2 years after release of treat-to-target guidelines from the American Heart Association and the European Society of Cardiology and European Atherosclerosis Society, most patients with LDL cholesterol higher than 70 mg/dL did not receive intensification of therapy, and two-thirds continued to have LDL levels above that level, according to a prospective registry study.
Both guidelines recommend driving LDL-C levels to 50% or below of baseline levels; results from the Getting to an Improved Understanding of Low-Density Lipoprotein Cholesterol and Dyslipidemia Management (GOULD) registry suggest this is rarely achieved. “Unfortunately it’s not a total surprise, but it’s disappointing,” said Christopher Cannon, MD, the study’s lead author.
“Therapeutic inertia seems to be the rule in clinical practice,” said Jennifer G. Robinson, MD, MPH, who was asked to comment on the study. Dr. Robinson is professor epidemiology and cardiology at the University of Iowa, Iowa City.
“This is yet another disappointing reminder of how we are failing our patients. Lipid lowering is one of the safest, most effective ways to prevent cardiovascular disease, and yet we are falling short. We have the tools in our toolkit to achieve guideline-based lipid lowering goals, but we just aren’t using them,” said Ann Marie Navar, MD, PhD, associate professor of cardiology at the University of Texas, Dallas.
Patients hesitant
Changes in practice following guidelines can often be slow, but in this case may have been complicated by the fact that statins have a reputation for causing side effects, so some patients may be refusing treatment based on what they’ve seen on the Internet. Even though the study looked at all lipid-lowering agents, the misinformation around statins may be spilling over, according to Dr. Cannon. “There’s in general so much misinformation around COVID and every other topic in the world. That makes people question what is real [about] anything,” said Dr. Cannon, a cardiologist at Brigham and Women’s hospital and professor of medicine at Harvard Medical School, both in Boston.
Patient characteristics may partly explain slow uptake. “Clinicians may not think further LDL-C lowering is a high enough priority in terms of potential benefit for a given patient in light of the effort being expended to take care of all their other issues and chronic health problems. If the clinician does bring it up to the patient, there may be barriers in terms of additional medication burden, cost, or acquisition issues,” said Dr. Robinson.
The answer may be better evidence and a more personalized approach. Clinical trials that explore defined patient populations could convince patients of a benefit, and payers to reimburse, according to Dr. Robinson.
Changing guidance
Another complication is that both the guidelines and the field are rapidly changing. The 2013 AHA guidelines did not include a treatment to goal and focused instead on use of high-dose statins. But the 2018 update reversed course after randomized studies demonstrated a benefit to treating to target. The researchers found no increase in the frequency of treating to target after the release of the 2018 guidelines. “Publication and announcement of guidelines doesn’t mean that people are getting treated better. We really have to implement them,” said Dr. Cannon.
On a positive note, the GOULD researchers found high acceptance of the new proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) inhibitors, with over 90% of patients continuing those medications after 2 years. “That’s nice and high. If people do get onto the very intensive lipid-lowing therapies, they tend to stay on them,” said Dr. Cannon.
What’s next
Still, the lack of intensification is concerning, and the findings led to some consternation in Twitter exchanges, said Dr. Cannon. “People posted ‘Well, what do we do now?’ ” Dr. Cannon’s team is addressing the issue with an algorithm-based risk management program with prospective enrollment. They have conducted educational webinars and provided site-specific reports on LDL status among patients at each center compared to others, and hope that information will improve compliance. In 2020, the group published an interim analysis of the first 5,000 enrollees, and Dr. Cannon expects to finish that study by the end of the year.
Dr. Navar agreed that physicians need to do a better job of testing LDL-C levels after treatment to identify patients who require more aggressive therapy. That can be deferred in some primary prevention patients with high LDL-C but normal particle numbers as measured with ApoB. “But in those at high risk for disease and those with established CVD who are not at goal, as long as they don’t have a life-limiting condition, we should always up-titrate therapy. It’s one of the safest, most effective ways to lower cardiovascular risk,” said Dr. Navar.
Key study results
The prospective study included 5,006 patients at 119 centers with a mean age of 68 years. About 40% were women, and 86.1% were White. All had ASCVD and LDL levels of at least 70 mg/dL. After 2 years, 17% had undergone intensification of lipid-lowering therapy (LLT). Among patients with LDL-C levels ≥ 100 mg/dL, 22% underwent LLT intensification, compared with 14% of patients with LDL-C levels of 70-99 mg/dL.
The vast majority, 92%, of patients who underwent LLT via addition of PCSK9 inhibitors were still taking the drug after 2 years.
Three-quarters (3,768) had lipid level measurements at least once during follow-up, and median LDL-C levels dropped from 120 to 95 mg/dL in the ≥100-mg/dL cohort (P < .001), and from 82 to 77 mg/dL in the 70- to 99-mg/dL cohort (P <. 001). There was no significant difference in the median values in the patients on PCSK9 inhibitors.
In all, 21% of the ≥100-mg/dL cohort achieved LDL-C levels <70 mg/dL at 2 years, versus 34% in the 77- to 99-mg/dL cohort and 52% of patients taking PCSK9 inhibitors.
Patients seen at teaching hospitals were more likely to undergo LLT intensification compared to nonteaching hospitals (25% versus 17%; P < .001), as were those where lipid protocols were in place (22% versus 15%; P < .001), and those treated in cardiology (22%) compared to treatment in internal or family medicine (12%; P <.001). The study was published online June 16 in JAMA Cardiology.
Dr. Cannon, Dr. Navar, and Dr. Robinson disclosed ties with Amgen, which funded the study, and other companies.
Among patients with atherosclerotic cardiovascular disease (ASCVD), 2 years after release of treat-to-target guidelines from the American Heart Association and the European Society of Cardiology and European Atherosclerosis Society, most patients with LDL cholesterol higher than 70 mg/dL did not receive intensification of therapy, and two-thirds continued to have LDL levels above that level, according to a prospective registry study.
Both guidelines recommend driving LDL-C levels to 50% or below of baseline levels; results from the Getting to an Improved Understanding of Low-Density Lipoprotein Cholesterol and Dyslipidemia Management (GOULD) registry suggest this is rarely achieved. “Unfortunately it’s not a total surprise, but it’s disappointing,” said Christopher Cannon, MD, the study’s lead author.
“Therapeutic inertia seems to be the rule in clinical practice,” said Jennifer G. Robinson, MD, MPH, who was asked to comment on the study. Dr. Robinson is professor epidemiology and cardiology at the University of Iowa, Iowa City.
“This is yet another disappointing reminder of how we are failing our patients. Lipid lowering is one of the safest, most effective ways to prevent cardiovascular disease, and yet we are falling short. We have the tools in our toolkit to achieve guideline-based lipid lowering goals, but we just aren’t using them,” said Ann Marie Navar, MD, PhD, associate professor of cardiology at the University of Texas, Dallas.
Patients hesitant
Changes in practice following guidelines can often be slow, but in this case may have been complicated by the fact that statins have a reputation for causing side effects, so some patients may be refusing treatment based on what they’ve seen on the Internet. Even though the study looked at all lipid-lowering agents, the misinformation around statins may be spilling over, according to Dr. Cannon. “There’s in general so much misinformation around COVID and every other topic in the world. That makes people question what is real [about] anything,” said Dr. Cannon, a cardiologist at Brigham and Women’s hospital and professor of medicine at Harvard Medical School, both in Boston.
Patient characteristics may partly explain slow uptake. “Clinicians may not think further LDL-C lowering is a high enough priority in terms of potential benefit for a given patient in light of the effort being expended to take care of all their other issues and chronic health problems. If the clinician does bring it up to the patient, there may be barriers in terms of additional medication burden, cost, or acquisition issues,” said Dr. Robinson.
The answer may be better evidence and a more personalized approach. Clinical trials that explore defined patient populations could convince patients of a benefit, and payers to reimburse, according to Dr. Robinson.
Changing guidance
Another complication is that both the guidelines and the field are rapidly changing. The 2013 AHA guidelines did not include a treatment to goal and focused instead on use of high-dose statins. But the 2018 update reversed course after randomized studies demonstrated a benefit to treating to target. The researchers found no increase in the frequency of treating to target after the release of the 2018 guidelines. “Publication and announcement of guidelines doesn’t mean that people are getting treated better. We really have to implement them,” said Dr. Cannon.
On a positive note, the GOULD researchers found high acceptance of the new proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) inhibitors, with over 90% of patients continuing those medications after 2 years. “That’s nice and high. If people do get onto the very intensive lipid-lowing therapies, they tend to stay on them,” said Dr. Cannon.
What’s next
Still, the lack of intensification is concerning, and the findings led to some consternation in Twitter exchanges, said Dr. Cannon. “People posted ‘Well, what do we do now?’ ” Dr. Cannon’s team is addressing the issue with an algorithm-based risk management program with prospective enrollment. They have conducted educational webinars and provided site-specific reports on LDL status among patients at each center compared to others, and hope that information will improve compliance. In 2020, the group published an interim analysis of the first 5,000 enrollees, and Dr. Cannon expects to finish that study by the end of the year.
Dr. Navar agreed that physicians need to do a better job of testing LDL-C levels after treatment to identify patients who require more aggressive therapy. That can be deferred in some primary prevention patients with high LDL-C but normal particle numbers as measured with ApoB. “But in those at high risk for disease and those with established CVD who are not at goal, as long as they don’t have a life-limiting condition, we should always up-titrate therapy. It’s one of the safest, most effective ways to lower cardiovascular risk,” said Dr. Navar.
Key study results
The prospective study included 5,006 patients at 119 centers with a mean age of 68 years. About 40% were women, and 86.1% were White. All had ASCVD and LDL levels of at least 70 mg/dL. After 2 years, 17% had undergone intensification of lipid-lowering therapy (LLT). Among patients with LDL-C levels ≥ 100 mg/dL, 22% underwent LLT intensification, compared with 14% of patients with LDL-C levels of 70-99 mg/dL.
The vast majority, 92%, of patients who underwent LLT via addition of PCSK9 inhibitors were still taking the drug after 2 years.
Three-quarters (3,768) had lipid level measurements at least once during follow-up, and median LDL-C levels dropped from 120 to 95 mg/dL in the ≥100-mg/dL cohort (P < .001), and from 82 to 77 mg/dL in the 70- to 99-mg/dL cohort (P <. 001). There was no significant difference in the median values in the patients on PCSK9 inhibitors.
In all, 21% of the ≥100-mg/dL cohort achieved LDL-C levels <70 mg/dL at 2 years, versus 34% in the 77- to 99-mg/dL cohort and 52% of patients taking PCSK9 inhibitors.
Patients seen at teaching hospitals were more likely to undergo LLT intensification compared to nonteaching hospitals (25% versus 17%; P < .001), as were those where lipid protocols were in place (22% versus 15%; P < .001), and those treated in cardiology (22%) compared to treatment in internal or family medicine (12%; P <.001). The study was published online June 16 in JAMA Cardiology.
Dr. Cannon, Dr. Navar, and Dr. Robinson disclosed ties with Amgen, which funded the study, and other companies.
FROM JAMA CARDIOLOGY
Patient-facing mobile apps: Are ObGyns uniquely positioned to integrate them into practice?
Incorporating mobile apps into patient care programs can add immense value. Mobile apps enable data collection when a patient is beyond the walls of a doctor’s office and equip clinicians with new and relevant patient data. Patient engagement apps also provide a mechanism to “nudge” patients to encourage adherence to their care programs. These additional data and increased patient adherence can enable more personalized care,1 and ultimately can lead to improved outcomes. For example, a meta-analysis of 1,657 patients with diabetes showed a 5% reduction in hemoglobin A1c (HbA1c) values for those who used a diabetes-related app.2 The literature also shows positive results for heart failure, weight management, smoking reduction, and lifestyle improvement.3-5 Given their value, why aren’t patient engagement apps more routinely integrated into patient care programs?
From a software development perspective, mobile apps are fairly easy to create. However, from a user retention standpoint, creating an app with a large, sustainable userbase is challenging.5 User retention is measured in monthly active users. We are familiar with unused apps collecting digital dust on our smartphone home screens. A 2019 study showed that 25% of people typically use an application only once,6 and health care apps are not an exception. There are hundreds of thousands of mobile health apps on the market, but only 7% of these applications have more than 50,000 monthly active users. Further, 62% of digital health apps have less than 1,000 monthly active users.7
Using a new app daily or several times weekly requires new habit formation. Anyone who has tried to incorporate a new routine into their daily life knows how difficult habit formation can be. However, ObGyn patients may be particularly well suited to incorporate ObGyn-related apps into their care, given how many women already use mobile applications to track their menstrual cycles. A recent survey found that across all age groups, 47% of women use a mobile phone app to track their menstrual cycle,8 compared with 8% of US adults who regularly use an app to measure general health metrics.7 This removes one of the largest obstacles of market penetration since the habit of using an app for ObGyn purposes has already been established. As such, it presents an exciting opportunity to capitalize on the userbase already leveraging mobile apps to track their cycles and expand the patient engagement footprint into additional features that can broaden care to create a seamless, holistic patient application for ObGyn patient care.
One can envision a future in which a patient is “prescribed” an ObGyn app during their ObGyn appointment. Within the app, the patient can track their health data, engage with health providers, and gain access to educational materials. The clinician would be able to access data captured in the patient app at an aggregate level to analyze trends over time.
Current, patient-facing ObGyn mobile apps available for download on smartphones are for targeted aspects of ObGyn health. For example, there are separate apps for menstrual cycle tracking, contraception education, and medication adherence tracking. In the future, it would be ideal for clinicians and patients to have access to a single, holistic ObGyn mobile app that supports the end-to-end ObGyn patient journey, one in which clinicians could turn modules on or off given specific patient concerns. The technology for this type of holistic patient engagement platform exists, but unfortunately it is not as simple as downloading a mobile app. Standing up an end-to-end patient engagement platform requires enterprise-wide buy-in, tailoring user workflows, and building out integrations (eg, integrating provider dashboards into the existing electronic health record system). Full-scale solutions are powerful, but they can be expensive and time consuming to stand up. Until there is a more streamlined, outside-the-box ObGyn-tailored solution, there are patientfacing mobile apps available to support your patients for specific concerns.
Continue to: The top 3 recommended menstrual cycle tracking apps...
The top 3 recommended menstrual cycle tracking apps from Moglia and colleagues9 are listed in Dr. Chen’s article, “Top free menstrual cycle tracking apps for your patients.”10 The top 3 recommended contraception education mobile apps from Lunde and colleagues11 are listed in TABLE 1 and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, important special features).12 The top 3 recommended patient medication adherence mobile apps from the study by Santo and colleagues13 are listed in TABLE 2. The apps in the Stoyanov et al14 study were evaluated using the 23-item Mobile App Rating System scale. ●
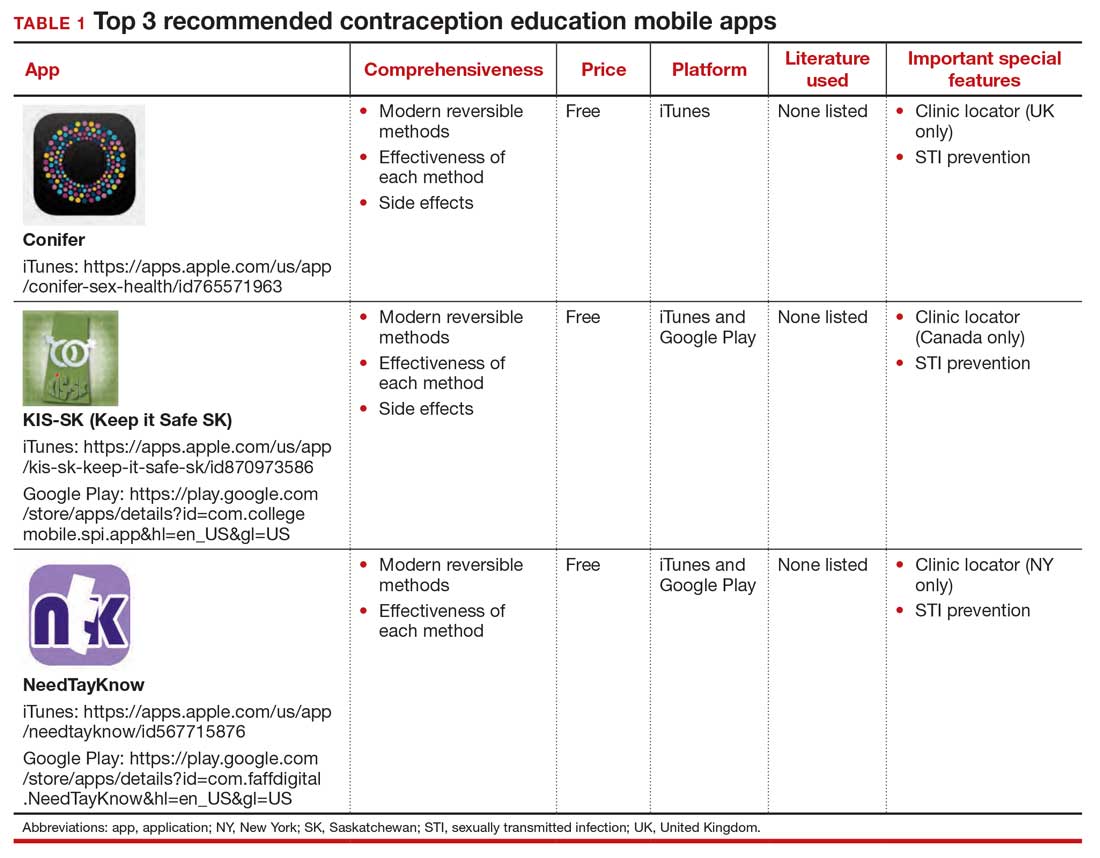
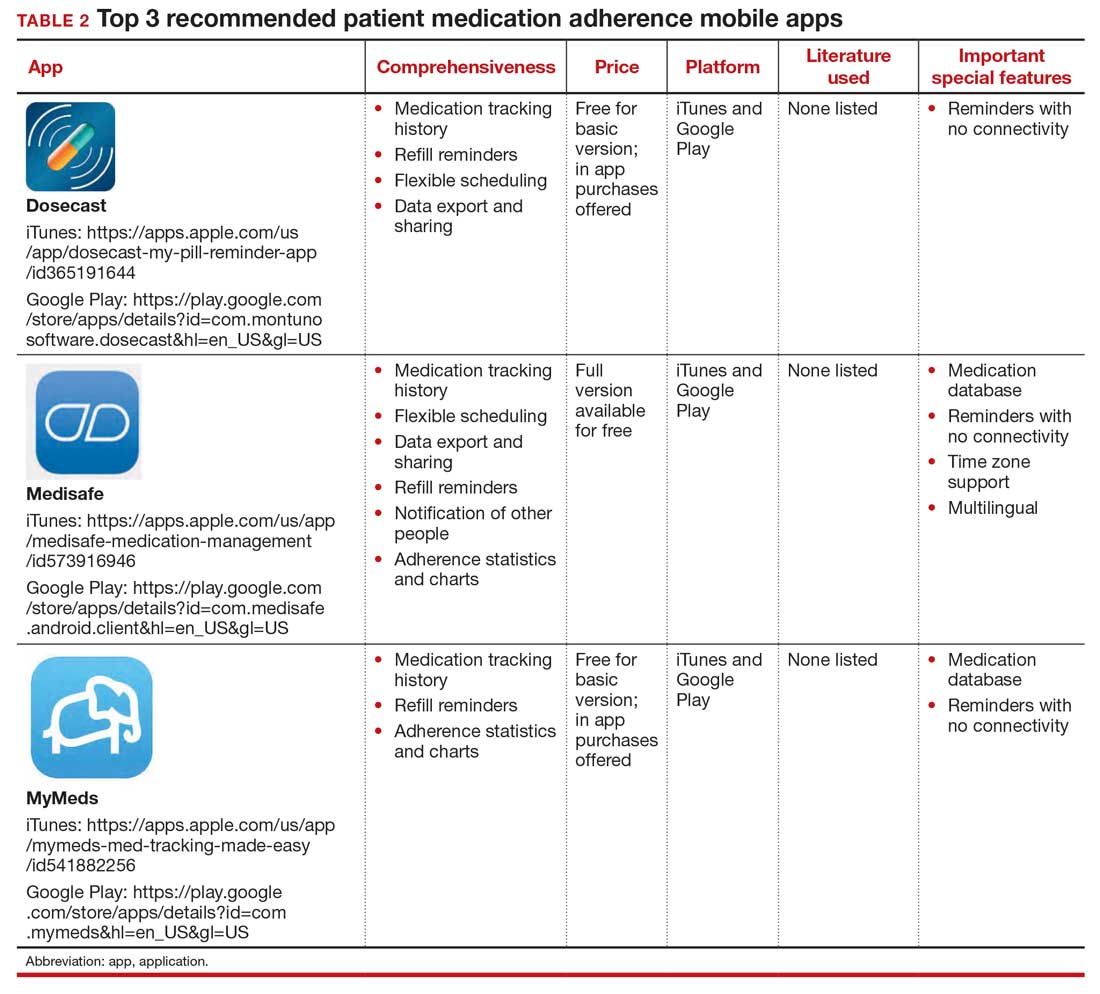
- van Dijk MR, Koster MPH, et al. Healthy preconception nutrition and lifestyle using personalized mobile health coaching is associated with enhanced pregnancy chance. Reprod BioMed Online. 2017;35:453-460. doi:10.1016/j. rbmo.2017.06.014.
- Liang X, Wang Q, Yang X, et al. Effect of mobile phone intervention for diabetes on glycaemic control: a metaanalysis. Diabet Med. 2011;28:455-463. doi:10.1111/j.1464- 5491.2010.03180.x.
- Laing BY, Mangione CM, Tseng C-H, et al. Effectiveness of a smartphone application for weight loss compared with usual care in overweight primary care patients. Ann Intern Med. 2014;161(10 suppl):S5-S12. doi:10.7326/m13-3005.
- Dennison L, Morrison L, Conway G, et al. Opportunities and challenges for smartphone applications in supporting health behavior change: qualitative study. J Med Internet Res. 2013;15:E86. doi:10.2196/jmir.2583.
- Schoeppe S, Alley S, Van Lippevelde W, et al. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: a systematic review. Int J Behav Nutr Phys Act. 2016;13:127. doi:10.1186/s12966- 016-0454-y.
- 25% of users abandon apps after one use. Upland Software website. Accessed May 6, 2021. https://uplandsoftware.com /localytics/resources/blog/25-of-users-abandon-apps-afterone-use/.
- mHealth economics 2017/2018 – connectivity in digital health. Published March 5, 2019. Accessed May 6, 2021. https:// research2guidance.com/product/connectivity-in-digitalhealth/.
- Epstein DA, Lee NB, Kang JH, et al. Examining menstrual tracking to inform the design of personal informatics tools. Proc SIGCHI Conf Hum Factor Comput Syst. 2017;2017:6876- 6888. doi:10.1145/3025453.3025635.
- Moglia M, Nguyen H, Chyjek K, et al. Evaluation of smartphone menstrual cycle tracking applications using an adapted APPLICATIONS scoring system. Obstet Gynecol. 2016;127:1153-1160. doi:10.1097/AOG.0000000000001444.
- Chen KT. Top free menstrual cycle tracking apps for your patients. OBG Manag. 2017;27:44-45.
- Lunde B, Perry R, Sridhar A, et al. An evaluation of contraception education and health promotion applications for patients. Womens Health Issues. 2017;27:29-35. doi:10.1016/j.whi.2016.09.012.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483. doi:10.1097 /AOG.0000000000000842.
- Santo K, Richtering SS, Chalmers J, et al. Mobile phone apps to improve medication adherence: a systematic stepwise process to identify high-quality apps. JMIR Mhealth Uhealth. 2016;4:E132. doi:10.2196/mhealth.6742.
- Stoyanov SR, Hides L, Kavanagh DJ, et al. Mobile app rating scale: a new tool for assessing the quality of health mobile apps. JMIR Mhealth Uhealth. 2015;3:E27. doi:10.2196 /mhealth.3422.
Incorporating mobile apps into patient care programs can add immense value. Mobile apps enable data collection when a patient is beyond the walls of a doctor’s office and equip clinicians with new and relevant patient data. Patient engagement apps also provide a mechanism to “nudge” patients to encourage adherence to their care programs. These additional data and increased patient adherence can enable more personalized care,1 and ultimately can lead to improved outcomes. For example, a meta-analysis of 1,657 patients with diabetes showed a 5% reduction in hemoglobin A1c (HbA1c) values for those who used a diabetes-related app.2 The literature also shows positive results for heart failure, weight management, smoking reduction, and lifestyle improvement.3-5 Given their value, why aren’t patient engagement apps more routinely integrated into patient care programs?
From a software development perspective, mobile apps are fairly easy to create. However, from a user retention standpoint, creating an app with a large, sustainable userbase is challenging.5 User retention is measured in monthly active users. We are familiar with unused apps collecting digital dust on our smartphone home screens. A 2019 study showed that 25% of people typically use an application only once,6 and health care apps are not an exception. There are hundreds of thousands of mobile health apps on the market, but only 7% of these applications have more than 50,000 monthly active users. Further, 62% of digital health apps have less than 1,000 monthly active users.7
Using a new app daily or several times weekly requires new habit formation. Anyone who has tried to incorporate a new routine into their daily life knows how difficult habit formation can be. However, ObGyn patients may be particularly well suited to incorporate ObGyn-related apps into their care, given how many women already use mobile applications to track their menstrual cycles. A recent survey found that across all age groups, 47% of women use a mobile phone app to track their menstrual cycle,8 compared with 8% of US adults who regularly use an app to measure general health metrics.7 This removes one of the largest obstacles of market penetration since the habit of using an app for ObGyn purposes has already been established. As such, it presents an exciting opportunity to capitalize on the userbase already leveraging mobile apps to track their cycles and expand the patient engagement footprint into additional features that can broaden care to create a seamless, holistic patient application for ObGyn patient care.
One can envision a future in which a patient is “prescribed” an ObGyn app during their ObGyn appointment. Within the app, the patient can track their health data, engage with health providers, and gain access to educational materials. The clinician would be able to access data captured in the patient app at an aggregate level to analyze trends over time.
Current, patient-facing ObGyn mobile apps available for download on smartphones are for targeted aspects of ObGyn health. For example, there are separate apps for menstrual cycle tracking, contraception education, and medication adherence tracking. In the future, it would be ideal for clinicians and patients to have access to a single, holistic ObGyn mobile app that supports the end-to-end ObGyn patient journey, one in which clinicians could turn modules on or off given specific patient concerns. The technology for this type of holistic patient engagement platform exists, but unfortunately it is not as simple as downloading a mobile app. Standing up an end-to-end patient engagement platform requires enterprise-wide buy-in, tailoring user workflows, and building out integrations (eg, integrating provider dashboards into the existing electronic health record system). Full-scale solutions are powerful, but they can be expensive and time consuming to stand up. Until there is a more streamlined, outside-the-box ObGyn-tailored solution, there are patientfacing mobile apps available to support your patients for specific concerns.
Continue to: The top 3 recommended menstrual cycle tracking apps...
The top 3 recommended menstrual cycle tracking apps from Moglia and colleagues9 are listed in Dr. Chen’s article, “Top free menstrual cycle tracking apps for your patients.”10 The top 3 recommended contraception education mobile apps from Lunde and colleagues11 are listed in TABLE 1 and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, important special features).12 The top 3 recommended patient medication adherence mobile apps from the study by Santo and colleagues13 are listed in TABLE 2. The apps in the Stoyanov et al14 study were evaluated using the 23-item Mobile App Rating System scale. ●


Incorporating mobile apps into patient care programs can add immense value. Mobile apps enable data collection when a patient is beyond the walls of a doctor’s office and equip clinicians with new and relevant patient data. Patient engagement apps also provide a mechanism to “nudge” patients to encourage adherence to their care programs. These additional data and increased patient adherence can enable more personalized care,1 and ultimately can lead to improved outcomes. For example, a meta-analysis of 1,657 patients with diabetes showed a 5% reduction in hemoglobin A1c (HbA1c) values for those who used a diabetes-related app.2 The literature also shows positive results for heart failure, weight management, smoking reduction, and lifestyle improvement.3-5 Given their value, why aren’t patient engagement apps more routinely integrated into patient care programs?
From a software development perspective, mobile apps are fairly easy to create. However, from a user retention standpoint, creating an app with a large, sustainable userbase is challenging.5 User retention is measured in monthly active users. We are familiar with unused apps collecting digital dust on our smartphone home screens. A 2019 study showed that 25% of people typically use an application only once,6 and health care apps are not an exception. There are hundreds of thousands of mobile health apps on the market, but only 7% of these applications have more than 50,000 monthly active users. Further, 62% of digital health apps have less than 1,000 monthly active users.7
Using a new app daily or several times weekly requires new habit formation. Anyone who has tried to incorporate a new routine into their daily life knows how difficult habit formation can be. However, ObGyn patients may be particularly well suited to incorporate ObGyn-related apps into their care, given how many women already use mobile applications to track their menstrual cycles. A recent survey found that across all age groups, 47% of women use a mobile phone app to track their menstrual cycle,8 compared with 8% of US adults who regularly use an app to measure general health metrics.7 This removes one of the largest obstacles of market penetration since the habit of using an app for ObGyn purposes has already been established. As such, it presents an exciting opportunity to capitalize on the userbase already leveraging mobile apps to track their cycles and expand the patient engagement footprint into additional features that can broaden care to create a seamless, holistic patient application for ObGyn patient care.
One can envision a future in which a patient is “prescribed” an ObGyn app during their ObGyn appointment. Within the app, the patient can track their health data, engage with health providers, and gain access to educational materials. The clinician would be able to access data captured in the patient app at an aggregate level to analyze trends over time.
Current, patient-facing ObGyn mobile apps available for download on smartphones are for targeted aspects of ObGyn health. For example, there are separate apps for menstrual cycle tracking, contraception education, and medication adherence tracking. In the future, it would be ideal for clinicians and patients to have access to a single, holistic ObGyn mobile app that supports the end-to-end ObGyn patient journey, one in which clinicians could turn modules on or off given specific patient concerns. The technology for this type of holistic patient engagement platform exists, but unfortunately it is not as simple as downloading a mobile app. Standing up an end-to-end patient engagement platform requires enterprise-wide buy-in, tailoring user workflows, and building out integrations (eg, integrating provider dashboards into the existing electronic health record system). Full-scale solutions are powerful, but they can be expensive and time consuming to stand up. Until there is a more streamlined, outside-the-box ObGyn-tailored solution, there are patientfacing mobile apps available to support your patients for specific concerns.
Continue to: The top 3 recommended menstrual cycle tracking apps...
The top 3 recommended menstrual cycle tracking apps from Moglia and colleagues9 are listed in Dr. Chen’s article, “Top free menstrual cycle tracking apps for your patients.”10 The top 3 recommended contraception education mobile apps from Lunde and colleagues11 are listed in TABLE 1 and are detailed with a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, important special features).12 The top 3 recommended patient medication adherence mobile apps from the study by Santo and colleagues13 are listed in TABLE 2. The apps in the Stoyanov et al14 study were evaluated using the 23-item Mobile App Rating System scale. ●


- van Dijk MR, Koster MPH, et al. Healthy preconception nutrition and lifestyle using personalized mobile health coaching is associated with enhanced pregnancy chance. Reprod BioMed Online. 2017;35:453-460. doi:10.1016/j. rbmo.2017.06.014.
- Liang X, Wang Q, Yang X, et al. Effect of mobile phone intervention for diabetes on glycaemic control: a metaanalysis. Diabet Med. 2011;28:455-463. doi:10.1111/j.1464- 5491.2010.03180.x.
- Laing BY, Mangione CM, Tseng C-H, et al. Effectiveness of a smartphone application for weight loss compared with usual care in overweight primary care patients. Ann Intern Med. 2014;161(10 suppl):S5-S12. doi:10.7326/m13-3005.
- Dennison L, Morrison L, Conway G, et al. Opportunities and challenges for smartphone applications in supporting health behavior change: qualitative study. J Med Internet Res. 2013;15:E86. doi:10.2196/jmir.2583.
- Schoeppe S, Alley S, Van Lippevelde W, et al. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: a systematic review. Int J Behav Nutr Phys Act. 2016;13:127. doi:10.1186/s12966- 016-0454-y.
- 25% of users abandon apps after one use. Upland Software website. Accessed May 6, 2021. https://uplandsoftware.com /localytics/resources/blog/25-of-users-abandon-apps-afterone-use/.
- mHealth economics 2017/2018 – connectivity in digital health. Published March 5, 2019. Accessed May 6, 2021. https:// research2guidance.com/product/connectivity-in-digitalhealth/.
- Epstein DA, Lee NB, Kang JH, et al. Examining menstrual tracking to inform the design of personal informatics tools. Proc SIGCHI Conf Hum Factor Comput Syst. 2017;2017:6876- 6888. doi:10.1145/3025453.3025635.
- Moglia M, Nguyen H, Chyjek K, et al. Evaluation of smartphone menstrual cycle tracking applications using an adapted APPLICATIONS scoring system. Obstet Gynecol. 2016;127:1153-1160. doi:10.1097/AOG.0000000000001444.
- Chen KT. Top free menstrual cycle tracking apps for your patients. OBG Manag. 2017;27:44-45.
- Lunde B, Perry R, Sridhar A, et al. An evaluation of contraception education and health promotion applications for patients. Womens Health Issues. 2017;27:29-35. doi:10.1016/j.whi.2016.09.012.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483. doi:10.1097 /AOG.0000000000000842.
- Santo K, Richtering SS, Chalmers J, et al. Mobile phone apps to improve medication adherence: a systematic stepwise process to identify high-quality apps. JMIR Mhealth Uhealth. 2016;4:E132. doi:10.2196/mhealth.6742.
- Stoyanov SR, Hides L, Kavanagh DJ, et al. Mobile app rating scale: a new tool for assessing the quality of health mobile apps. JMIR Mhealth Uhealth. 2015;3:E27. doi:10.2196 /mhealth.3422.
- van Dijk MR, Koster MPH, et al. Healthy preconception nutrition and lifestyle using personalized mobile health coaching is associated with enhanced pregnancy chance. Reprod BioMed Online. 2017;35:453-460. doi:10.1016/j. rbmo.2017.06.014.
- Liang X, Wang Q, Yang X, et al. Effect of mobile phone intervention for diabetes on glycaemic control: a metaanalysis. Diabet Med. 2011;28:455-463. doi:10.1111/j.1464- 5491.2010.03180.x.
- Laing BY, Mangione CM, Tseng C-H, et al. Effectiveness of a smartphone application for weight loss compared with usual care in overweight primary care patients. Ann Intern Med. 2014;161(10 suppl):S5-S12. doi:10.7326/m13-3005.
- Dennison L, Morrison L, Conway G, et al. Opportunities and challenges for smartphone applications in supporting health behavior change: qualitative study. J Med Internet Res. 2013;15:E86. doi:10.2196/jmir.2583.
- Schoeppe S, Alley S, Van Lippevelde W, et al. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: a systematic review. Int J Behav Nutr Phys Act. 2016;13:127. doi:10.1186/s12966- 016-0454-y.
- 25% of users abandon apps after one use. Upland Software website. Accessed May 6, 2021. https://uplandsoftware.com /localytics/resources/blog/25-of-users-abandon-apps-afterone-use/.
- mHealth economics 2017/2018 – connectivity in digital health. Published March 5, 2019. Accessed May 6, 2021. https:// research2guidance.com/product/connectivity-in-digitalhealth/.
- Epstein DA, Lee NB, Kang JH, et al. Examining menstrual tracking to inform the design of personal informatics tools. Proc SIGCHI Conf Hum Factor Comput Syst. 2017;2017:6876- 6888. doi:10.1145/3025453.3025635.
- Moglia M, Nguyen H, Chyjek K, et al. Evaluation of smartphone menstrual cycle tracking applications using an adapted APPLICATIONS scoring system. Obstet Gynecol. 2016;127:1153-1160. doi:10.1097/AOG.0000000000001444.
- Chen KT. Top free menstrual cycle tracking apps for your patients. OBG Manag. 2017;27:44-45.
- Lunde B, Perry R, Sridhar A, et al. An evaluation of contraception education and health promotion applications for patients. Womens Health Issues. 2017;27:29-35. doi:10.1016/j.whi.2016.09.012.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483. doi:10.1097 /AOG.0000000000000842.
- Santo K, Richtering SS, Chalmers J, et al. Mobile phone apps to improve medication adherence: a systematic stepwise process to identify high-quality apps. JMIR Mhealth Uhealth. 2016;4:E132. doi:10.2196/mhealth.6742.
- Stoyanov SR, Hides L, Kavanagh DJ, et al. Mobile app rating scale: a new tool for assessing the quality of health mobile apps. JMIR Mhealth Uhealth. 2015;3:E27. doi:10.2196 /mhealth.3422.
COVID-19 vaccinations may be weakened by liver disease
Patients who have received liver transplants or have advanced liver fibrosis may not get adequate protection against COVID-19 from two doses of the Pfizer vaccine, researchers say.
Physicians should test these patients and consider administering a third vaccine dose for those with low levels of antibodies to the SARS-CoV-2 virus, said Rifaat Safadi, MD, director of the Liver Unit in the Institute of Gastroenterology and Liver Diseases at Hadassah Hebrew University Medical Center, Jerusalem.
“If they are not responding, if the serology is going down to zero over the next year, you should revaccinate them or boost them,” Dr. Safadi said in an interview.
The suggestion contradicts the U.S. Food and Drug Administration, which issued a recommendation on May 19 against using antibody tests to check the effectiveness of vaccination against the virus and has not approved a three-dose regimen or booster of any SARS-CoV-2 vaccine.
But Dr. Safadi said he is more convinced by the data, which he presented at the meeting sponsored by the European Association for the Study of the Liver, than by the FDA’s recommendation.
“I’m not sure how scientific this recommendation is,” he said.
Several SARS-CoV-2 vaccines have proven highly effective in clinical trials and real-world studies. But some vaccinated people have sickened and even died from breakthrough infections, despite receiving Pfizer’s and Moderna’s mRNA vaccines, which have produced the most impressive results.
Few researchers have explored whether patients with weakened immune systems achieve the same level of protection as the general population. Fibrosis impairs the immune function of the liver, and people with transplants take immunosuppressant drugs to prevent rejection of the transplant.
New data on vaccine efficacy in the immunocompromised
To address this knowledge gap, Dr. Safadi and his colleagues measured antibodies (immunoglobulins) to the spike protein in the virus (S IgG) in vaccinated people who had liver transplants and in other vaccinated people whose liver fibrosis they had assessed with blood tests and biopsies.
About 32 of 90 people who received liver transplants at Hadassah University Medical Center and had received the Pfizer vaccine had an anti–S IgG less than 19 AU/mL, the researchers’ cutoff for a “good” antibody response to the vaccine.
Five other vaccinated people with liver transplants were diagnosed with COVID-19 after receiving the vaccine, one after the first dose and four after the second dose. Altogether, this added up to a 41.1% failure rate of the vaccine, Dr. Safadi said.
To determine the effectiveness of the vaccine in a larger population, Dr. Safadi and colleagues measured the S IgG levels in 719 employees of Hadassah University Medical Center who had received their second doses of the vaccine at least 7 days before (72% had received it 14 days before).
Of these, 708 (98.5%) had titers greater than 19 AU/mL.
Another eight (1.1%) had titers less than 12 AU/mL, which the researchers defined as a negative response. All eight had suppressed immune systems: two had kidney transplants, one had rheumatoid arthritis, two had lymphoma, two had metabolic syndrome, and one had both multiple sclerosis and metabolic syndrome.
The remaining three (0.4%) had S IgG titers from 12 to 19 AU/mL. Of this group, one person each had hemodialysis and cardiovascular disease, rheumatoid arthritis, cryoglobulinemia, and metabolic syndrome.
Of those patients with S IgG titers greater than 19 AU/mL, Fibrosis-4 (Fib-4) scores were available for 501 (the Fib-4 score is a measure of liver fibrosis based on levels of aspartate aminotransferase and alanine aminotransferase, platelet counts, and age). Those with higher Fib-4 scores had lower mean S IgG levels. Of those with Fib-4 scores less than 1.3, 68.0% had S IgG titers greater than 200 AU/mL, and of those with Fib-4 scores greater than 2, 44.2% had S IgG titers greater than 200 AU/mL. The difference was statistically significant (P = .002).
The researchers found a similar correlation when they measured liver health by biopsy for 140 vaccinated people with nonalcoholic fatty liver disease (NAFLD). They found that lower NAFLD activity scores corresponded to higher S IgG titers. Using FibroScan, they also found a trend toward lower S IgG titers with higher fibrosis kilopascals.
In addition, Dr. Safadi and colleagues noted that older age and male sex correlated with lower S IgG titers.
Differing opinions on value of antibody titers, third doses
The virus is likely to remain a threat for a year or more to come, and antibody tests may give some indication of who is likely to have the longest-lasting protection, Dr. Safadi said.
“I believe that stronger responders will maintain longer, while the weaker responders will maintain shorter and maybe will need a third shot at some time,” he added.
Pauline Vetter, MD, an infectious disease specialist at Geneva University Hospitals, questioned whether antibody titers can be used to estimate an individual’s level of resistance to the virus. There is some evidence that higher titers correlate with better protection, but it’s not clear to what degree, she said.
“There’s no definitive cutoff,” Dr. Vetter said in an interview. “I can’t say if you have more than a titer of 5, then you’re protected or you’re more protected – or if you have less, then you’re not protected.”
Testing to see whether a person has any S IgG antibodies at all following a vaccination might be worthwhile, she said. She noted that people who know that they did not have an antibody response may be more careful.
Dr. Vetter concluded that not enough is yet known to recommend a third dose or booster for people whose immunity is suppressed.
“There might be a benefit in these populations. But the question is, when and in which situations?” she said.
Dr. Vetter and Dr. Safadi have disclosed no relevant financial relationships.
Patients who have received liver transplants or have advanced liver fibrosis may not get adequate protection against COVID-19 from two doses of the Pfizer vaccine, researchers say.
Physicians should test these patients and consider administering a third vaccine dose for those with low levels of antibodies to the SARS-CoV-2 virus, said Rifaat Safadi, MD, director of the Liver Unit in the Institute of Gastroenterology and Liver Diseases at Hadassah Hebrew University Medical Center, Jerusalem.
“If they are not responding, if the serology is going down to zero over the next year, you should revaccinate them or boost them,” Dr. Safadi said in an interview.
The suggestion contradicts the U.S. Food and Drug Administration, which issued a recommendation on May 19 against using antibody tests to check the effectiveness of vaccination against the virus and has not approved a three-dose regimen or booster of any SARS-CoV-2 vaccine.
But Dr. Safadi said he is more convinced by the data, which he presented at the meeting sponsored by the European Association for the Study of the Liver, than by the FDA’s recommendation.
“I’m not sure how scientific this recommendation is,” he said.
Several SARS-CoV-2 vaccines have proven highly effective in clinical trials and real-world studies. But some vaccinated people have sickened and even died from breakthrough infections, despite receiving Pfizer’s and Moderna’s mRNA vaccines, which have produced the most impressive results.
Few researchers have explored whether patients with weakened immune systems achieve the same level of protection as the general population. Fibrosis impairs the immune function of the liver, and people with transplants take immunosuppressant drugs to prevent rejection of the transplant.
New data on vaccine efficacy in the immunocompromised
To address this knowledge gap, Dr. Safadi and his colleagues measured antibodies (immunoglobulins) to the spike protein in the virus (S IgG) in vaccinated people who had liver transplants and in other vaccinated people whose liver fibrosis they had assessed with blood tests and biopsies.
About 32 of 90 people who received liver transplants at Hadassah University Medical Center and had received the Pfizer vaccine had an anti–S IgG less than 19 AU/mL, the researchers’ cutoff for a “good” antibody response to the vaccine.
Five other vaccinated people with liver transplants were diagnosed with COVID-19 after receiving the vaccine, one after the first dose and four after the second dose. Altogether, this added up to a 41.1% failure rate of the vaccine, Dr. Safadi said.
To determine the effectiveness of the vaccine in a larger population, Dr. Safadi and colleagues measured the S IgG levels in 719 employees of Hadassah University Medical Center who had received their second doses of the vaccine at least 7 days before (72% had received it 14 days before).
Of these, 708 (98.5%) had titers greater than 19 AU/mL.
Another eight (1.1%) had titers less than 12 AU/mL, which the researchers defined as a negative response. All eight had suppressed immune systems: two had kidney transplants, one had rheumatoid arthritis, two had lymphoma, two had metabolic syndrome, and one had both multiple sclerosis and metabolic syndrome.
The remaining three (0.4%) had S IgG titers from 12 to 19 AU/mL. Of this group, one person each had hemodialysis and cardiovascular disease, rheumatoid arthritis, cryoglobulinemia, and metabolic syndrome.
Of those patients with S IgG titers greater than 19 AU/mL, Fibrosis-4 (Fib-4) scores were available for 501 (the Fib-4 score is a measure of liver fibrosis based on levels of aspartate aminotransferase and alanine aminotransferase, platelet counts, and age). Those with higher Fib-4 scores had lower mean S IgG levels. Of those with Fib-4 scores less than 1.3, 68.0% had S IgG titers greater than 200 AU/mL, and of those with Fib-4 scores greater than 2, 44.2% had S IgG titers greater than 200 AU/mL. The difference was statistically significant (P = .002).
The researchers found a similar correlation when they measured liver health by biopsy for 140 vaccinated people with nonalcoholic fatty liver disease (NAFLD). They found that lower NAFLD activity scores corresponded to higher S IgG titers. Using FibroScan, they also found a trend toward lower S IgG titers with higher fibrosis kilopascals.
In addition, Dr. Safadi and colleagues noted that older age and male sex correlated with lower S IgG titers.
Differing opinions on value of antibody titers, third doses
The virus is likely to remain a threat for a year or more to come, and antibody tests may give some indication of who is likely to have the longest-lasting protection, Dr. Safadi said.
“I believe that stronger responders will maintain longer, while the weaker responders will maintain shorter and maybe will need a third shot at some time,” he added.
Pauline Vetter, MD, an infectious disease specialist at Geneva University Hospitals, questioned whether antibody titers can be used to estimate an individual’s level of resistance to the virus. There is some evidence that higher titers correlate with better protection, but it’s not clear to what degree, she said.
“There’s no definitive cutoff,” Dr. Vetter said in an interview. “I can’t say if you have more than a titer of 5, then you’re protected or you’re more protected – or if you have less, then you’re not protected.”
Testing to see whether a person has any S IgG antibodies at all following a vaccination might be worthwhile, she said. She noted that people who know that they did not have an antibody response may be more careful.
Dr. Vetter concluded that not enough is yet known to recommend a third dose or booster for people whose immunity is suppressed.
“There might be a benefit in these populations. But the question is, when and in which situations?” she said.
Dr. Vetter and Dr. Safadi have disclosed no relevant financial relationships.
Patients who have received liver transplants or have advanced liver fibrosis may not get adequate protection against COVID-19 from two doses of the Pfizer vaccine, researchers say.
Physicians should test these patients and consider administering a third vaccine dose for those with low levels of antibodies to the SARS-CoV-2 virus, said Rifaat Safadi, MD, director of the Liver Unit in the Institute of Gastroenterology and Liver Diseases at Hadassah Hebrew University Medical Center, Jerusalem.
“If they are not responding, if the serology is going down to zero over the next year, you should revaccinate them or boost them,” Dr. Safadi said in an interview.
The suggestion contradicts the U.S. Food and Drug Administration, which issued a recommendation on May 19 against using antibody tests to check the effectiveness of vaccination against the virus and has not approved a three-dose regimen or booster of any SARS-CoV-2 vaccine.
But Dr. Safadi said he is more convinced by the data, which he presented at the meeting sponsored by the European Association for the Study of the Liver, than by the FDA’s recommendation.
“I’m not sure how scientific this recommendation is,” he said.
Several SARS-CoV-2 vaccines have proven highly effective in clinical trials and real-world studies. But some vaccinated people have sickened and even died from breakthrough infections, despite receiving Pfizer’s and Moderna’s mRNA vaccines, which have produced the most impressive results.
Few researchers have explored whether patients with weakened immune systems achieve the same level of protection as the general population. Fibrosis impairs the immune function of the liver, and people with transplants take immunosuppressant drugs to prevent rejection of the transplant.
New data on vaccine efficacy in the immunocompromised
To address this knowledge gap, Dr. Safadi and his colleagues measured antibodies (immunoglobulins) to the spike protein in the virus (S IgG) in vaccinated people who had liver transplants and in other vaccinated people whose liver fibrosis they had assessed with blood tests and biopsies.
About 32 of 90 people who received liver transplants at Hadassah University Medical Center and had received the Pfizer vaccine had an anti–S IgG less than 19 AU/mL, the researchers’ cutoff for a “good” antibody response to the vaccine.
Five other vaccinated people with liver transplants were diagnosed with COVID-19 after receiving the vaccine, one after the first dose and four after the second dose. Altogether, this added up to a 41.1% failure rate of the vaccine, Dr. Safadi said.
To determine the effectiveness of the vaccine in a larger population, Dr. Safadi and colleagues measured the S IgG levels in 719 employees of Hadassah University Medical Center who had received their second doses of the vaccine at least 7 days before (72% had received it 14 days before).
Of these, 708 (98.5%) had titers greater than 19 AU/mL.
Another eight (1.1%) had titers less than 12 AU/mL, which the researchers defined as a negative response. All eight had suppressed immune systems: two had kidney transplants, one had rheumatoid arthritis, two had lymphoma, two had metabolic syndrome, and one had both multiple sclerosis and metabolic syndrome.
The remaining three (0.4%) had S IgG titers from 12 to 19 AU/mL. Of this group, one person each had hemodialysis and cardiovascular disease, rheumatoid arthritis, cryoglobulinemia, and metabolic syndrome.
Of those patients with S IgG titers greater than 19 AU/mL, Fibrosis-4 (Fib-4) scores were available for 501 (the Fib-4 score is a measure of liver fibrosis based on levels of aspartate aminotransferase and alanine aminotransferase, platelet counts, and age). Those with higher Fib-4 scores had lower mean S IgG levels. Of those with Fib-4 scores less than 1.3, 68.0% had S IgG titers greater than 200 AU/mL, and of those with Fib-4 scores greater than 2, 44.2% had S IgG titers greater than 200 AU/mL. The difference was statistically significant (P = .002).
The researchers found a similar correlation when they measured liver health by biopsy for 140 vaccinated people with nonalcoholic fatty liver disease (NAFLD). They found that lower NAFLD activity scores corresponded to higher S IgG titers. Using FibroScan, they also found a trend toward lower S IgG titers with higher fibrosis kilopascals.
In addition, Dr. Safadi and colleagues noted that older age and male sex correlated with lower S IgG titers.
Differing opinions on value of antibody titers, third doses
The virus is likely to remain a threat for a year or more to come, and antibody tests may give some indication of who is likely to have the longest-lasting protection, Dr. Safadi said.
“I believe that stronger responders will maintain longer, while the weaker responders will maintain shorter and maybe will need a third shot at some time,” he added.
Pauline Vetter, MD, an infectious disease specialist at Geneva University Hospitals, questioned whether antibody titers can be used to estimate an individual’s level of resistance to the virus. There is some evidence that higher titers correlate with better protection, but it’s not clear to what degree, she said.
“There’s no definitive cutoff,” Dr. Vetter said in an interview. “I can’t say if you have more than a titer of 5, then you’re protected or you’re more protected – or if you have less, then you’re not protected.”
Testing to see whether a person has any S IgG antibodies at all following a vaccination might be worthwhile, she said. She noted that people who know that they did not have an antibody response may be more careful.
Dr. Vetter concluded that not enough is yet known to recommend a third dose or booster for people whose immunity is suppressed.
“There might be a benefit in these populations. But the question is, when and in which situations?” she said.
Dr. Vetter and Dr. Safadi have disclosed no relevant financial relationships.
Tofacitinib shows mortality benefit in patients with COVID-19 pneumonia
The Janus kinase inhibitor tofacitinib reduces the risk of both death and respiratory failure in hospitalized adults with COVID-19 pneumonia, a new Brazilian study has found.
“Whether the use of JAK inhibitors is superior or additive to other specific immunomodulatory therapies in patients hospitalized with COVID-19 remains to be determined,” Patrícia O. Guimarães, MD, PhD, of the Hospital Israelita Albert Einstein in São Paulo, and coauthors wrote. The study was published in the New England Journal of Medicine.
The results of previous trials that tested JAK inhibitors as therapies for COVID-19 have been mixed. The second iteration of the Adaptive COVID-19 Treatment Trial (ACTT-2) found that a combination treatment of baricitinib and the Food and Drug Administration–authorized remdesivir was superior to remdesivir alone, but ACTT-4 – which compared baricitinib plus remdesivir with dexamethasone plus remdesivir – was stopped for futility in April 2021.
To assess the efficacy and safety of tofacitinib as a potential treatment for COVID-19, the researchers launched a randomized, double-blind trial made up of 289 patients from 15 sites in Brazil. The Study of Tofacitinib in Hospitalized Patients with COVID-19 Pneumonia (STOP-COVID) split its participants into two groups: one (n = 144) received 10 mg of oral tofacitinib twice daily and the other (n = 145) received placebo. Treatment was to be administered for up to 14 days or until hospital discharge. The participants’ mean age was 56 years, and 34.9% were women.
Over 89% of participants received glucocorticoids during hospitalization, a significant increase, compared with ACTT-2’s 12%. Through 28 days, death or respiratory failure occurred in 18.1% of the tofacitinib group and in 29.0% of the placebo group (risk ratio, 0.63; 95% confidence interval, 0.41-0.97; P = .04). Death from any cause occurred in 2.8% of the tofacitinib group and 5.5% of the placebo group (hazard ratio, 0.49; 95% CI, 0.15-1.63). The median number of days that treatment was administered was 5 in the tofacitinib group and 6 in the placebo group, and the median duration of hospital and ICU stays were similar across groups.
On the eight-level National Institute of Allergy and Infectious Diseases ordinal scale of disease severity, the proportional odds of having a worse score with tofacitinib, compared with placebo, was 0.6 (95% CI, 0.36-1.00) at day 14 and 0.54 (95% CI, 0.27-1.06) at day 28. Adverse events occurred in 26.1% of the tofacitinib group and 22.5% of the placebo group, with serious adverse events occurring in 20 patients (14.1%) on tofacitinib and 17 patients (12%) on placebo. Patients on tofacitinib suffered from events like deep vein thrombosis, acute myocardial infarction, ventricular tachycardia, and myocarditis, each of which affected one person, while one placebo patient each suffered from hemorrhagic stroke and cardiogenic shock. The incidence of serious infection was 3.5% in the tofacitinib group and 4.2% in the placebo group.
Timing may be everything
“There is a lot of interest in repurposing a variety of disease-modifying antirheumatic drugs for the treatment of COVID-19, which includes JAK inhibitors,” Zachary S. Wallace, MD, of the rheumatology unit at Massachusetts General Hospital, Boston, said in an interview. “The ACTT-2 data was compelling; it did suggest perhaps a benefit associated with baricitinib for COVID. This study certainly is more compelling.”
“For many people, there is this hyperinflammatory response in COVID-19 that seems to drive a lot of the morbidity and mortality that we see,” he added. “I think we all hypothesize that some of our treatments may be beneficial there. The challenge that we face is figuring out when the best time is to administer these medicines, and whether they need to be administered as part of a cocktail of therapy.”
Along those lines, Dr. Wallace cited a recent study he coauthored in which rheumatoid arthritis patients who were on JAK inhibitors at baseline had worse COVID-19 severity. But he emphasized that, despite their differing findings, the two studies are not irreconcilable.
“What this might speak to is, the timing of your exposure may be really important,” he said. “At the time of your initial infection, you may need certain aspects of your immune system that a JAK inhibitor may interfere with. But when you initiate a JAK inhibitor, once that phase is complete and you’re in this hyperinflammatory phase, you may have more benefit to target and treat the intense inflammation that we observe in patients who have COVID.”
He also offered up another variable potentially in play: different JAK inhibitors having different targets among the JAK receptors. “It may be that targeting specific JAKs is more beneficial when it comes to treating the hyperinflammatory response of COVID-19.”
The trial was sponsored by Pfizer. Several authors acknowledged potential conflicts of interest, including receiving grants and personal fees from Pfizer and various other pharmaceutical companies.
The Janus kinase inhibitor tofacitinib reduces the risk of both death and respiratory failure in hospitalized adults with COVID-19 pneumonia, a new Brazilian study has found.
“Whether the use of JAK inhibitors is superior or additive to other specific immunomodulatory therapies in patients hospitalized with COVID-19 remains to be determined,” Patrícia O. Guimarães, MD, PhD, of the Hospital Israelita Albert Einstein in São Paulo, and coauthors wrote. The study was published in the New England Journal of Medicine.
The results of previous trials that tested JAK inhibitors as therapies for COVID-19 have been mixed. The second iteration of the Adaptive COVID-19 Treatment Trial (ACTT-2) found that a combination treatment of baricitinib and the Food and Drug Administration–authorized remdesivir was superior to remdesivir alone, but ACTT-4 – which compared baricitinib plus remdesivir with dexamethasone plus remdesivir – was stopped for futility in April 2021.
To assess the efficacy and safety of tofacitinib as a potential treatment for COVID-19, the researchers launched a randomized, double-blind trial made up of 289 patients from 15 sites in Brazil. The Study of Tofacitinib in Hospitalized Patients with COVID-19 Pneumonia (STOP-COVID) split its participants into two groups: one (n = 144) received 10 mg of oral tofacitinib twice daily and the other (n = 145) received placebo. Treatment was to be administered for up to 14 days or until hospital discharge. The participants’ mean age was 56 years, and 34.9% were women.
Over 89% of participants received glucocorticoids during hospitalization, a significant increase, compared with ACTT-2’s 12%. Through 28 days, death or respiratory failure occurred in 18.1% of the tofacitinib group and in 29.0% of the placebo group (risk ratio, 0.63; 95% confidence interval, 0.41-0.97; P = .04). Death from any cause occurred in 2.8% of the tofacitinib group and 5.5% of the placebo group (hazard ratio, 0.49; 95% CI, 0.15-1.63). The median number of days that treatment was administered was 5 in the tofacitinib group and 6 in the placebo group, and the median duration of hospital and ICU stays were similar across groups.
On the eight-level National Institute of Allergy and Infectious Diseases ordinal scale of disease severity, the proportional odds of having a worse score with tofacitinib, compared with placebo, was 0.6 (95% CI, 0.36-1.00) at day 14 and 0.54 (95% CI, 0.27-1.06) at day 28. Adverse events occurred in 26.1% of the tofacitinib group and 22.5% of the placebo group, with serious adverse events occurring in 20 patients (14.1%) on tofacitinib and 17 patients (12%) on placebo. Patients on tofacitinib suffered from events like deep vein thrombosis, acute myocardial infarction, ventricular tachycardia, and myocarditis, each of which affected one person, while one placebo patient each suffered from hemorrhagic stroke and cardiogenic shock. The incidence of serious infection was 3.5% in the tofacitinib group and 4.2% in the placebo group.
Timing may be everything
“There is a lot of interest in repurposing a variety of disease-modifying antirheumatic drugs for the treatment of COVID-19, which includes JAK inhibitors,” Zachary S. Wallace, MD, of the rheumatology unit at Massachusetts General Hospital, Boston, said in an interview. “The ACTT-2 data was compelling; it did suggest perhaps a benefit associated with baricitinib for COVID. This study certainly is more compelling.”
“For many people, there is this hyperinflammatory response in COVID-19 that seems to drive a lot of the morbidity and mortality that we see,” he added. “I think we all hypothesize that some of our treatments may be beneficial there. The challenge that we face is figuring out when the best time is to administer these medicines, and whether they need to be administered as part of a cocktail of therapy.”
Along those lines, Dr. Wallace cited a recent study he coauthored in which rheumatoid arthritis patients who were on JAK inhibitors at baseline had worse COVID-19 severity. But he emphasized that, despite their differing findings, the two studies are not irreconcilable.
“What this might speak to is, the timing of your exposure may be really important,” he said. “At the time of your initial infection, you may need certain aspects of your immune system that a JAK inhibitor may interfere with. But when you initiate a JAK inhibitor, once that phase is complete and you’re in this hyperinflammatory phase, you may have more benefit to target and treat the intense inflammation that we observe in patients who have COVID.”
He also offered up another variable potentially in play: different JAK inhibitors having different targets among the JAK receptors. “It may be that targeting specific JAKs is more beneficial when it comes to treating the hyperinflammatory response of COVID-19.”
The trial was sponsored by Pfizer. Several authors acknowledged potential conflicts of interest, including receiving grants and personal fees from Pfizer and various other pharmaceutical companies.
The Janus kinase inhibitor tofacitinib reduces the risk of both death and respiratory failure in hospitalized adults with COVID-19 pneumonia, a new Brazilian study has found.
“Whether the use of JAK inhibitors is superior or additive to other specific immunomodulatory therapies in patients hospitalized with COVID-19 remains to be determined,” Patrícia O. Guimarães, MD, PhD, of the Hospital Israelita Albert Einstein in São Paulo, and coauthors wrote. The study was published in the New England Journal of Medicine.
The results of previous trials that tested JAK inhibitors as therapies for COVID-19 have been mixed. The second iteration of the Adaptive COVID-19 Treatment Trial (ACTT-2) found that a combination treatment of baricitinib and the Food and Drug Administration–authorized remdesivir was superior to remdesivir alone, but ACTT-4 – which compared baricitinib plus remdesivir with dexamethasone plus remdesivir – was stopped for futility in April 2021.
To assess the efficacy and safety of tofacitinib as a potential treatment for COVID-19, the researchers launched a randomized, double-blind trial made up of 289 patients from 15 sites in Brazil. The Study of Tofacitinib in Hospitalized Patients with COVID-19 Pneumonia (STOP-COVID) split its participants into two groups: one (n = 144) received 10 mg of oral tofacitinib twice daily and the other (n = 145) received placebo. Treatment was to be administered for up to 14 days or until hospital discharge. The participants’ mean age was 56 years, and 34.9% were women.
Over 89% of participants received glucocorticoids during hospitalization, a significant increase, compared with ACTT-2’s 12%. Through 28 days, death or respiratory failure occurred in 18.1% of the tofacitinib group and in 29.0% of the placebo group (risk ratio, 0.63; 95% confidence interval, 0.41-0.97; P = .04). Death from any cause occurred in 2.8% of the tofacitinib group and 5.5% of the placebo group (hazard ratio, 0.49; 95% CI, 0.15-1.63). The median number of days that treatment was administered was 5 in the tofacitinib group and 6 in the placebo group, and the median duration of hospital and ICU stays were similar across groups.
On the eight-level National Institute of Allergy and Infectious Diseases ordinal scale of disease severity, the proportional odds of having a worse score with tofacitinib, compared with placebo, was 0.6 (95% CI, 0.36-1.00) at day 14 and 0.54 (95% CI, 0.27-1.06) at day 28. Adverse events occurred in 26.1% of the tofacitinib group and 22.5% of the placebo group, with serious adverse events occurring in 20 patients (14.1%) on tofacitinib and 17 patients (12%) on placebo. Patients on tofacitinib suffered from events like deep vein thrombosis, acute myocardial infarction, ventricular tachycardia, and myocarditis, each of which affected one person, while one placebo patient each suffered from hemorrhagic stroke and cardiogenic shock. The incidence of serious infection was 3.5% in the tofacitinib group and 4.2% in the placebo group.
Timing may be everything
“There is a lot of interest in repurposing a variety of disease-modifying antirheumatic drugs for the treatment of COVID-19, which includes JAK inhibitors,” Zachary S. Wallace, MD, of the rheumatology unit at Massachusetts General Hospital, Boston, said in an interview. “The ACTT-2 data was compelling; it did suggest perhaps a benefit associated with baricitinib for COVID. This study certainly is more compelling.”
“For many people, there is this hyperinflammatory response in COVID-19 that seems to drive a lot of the morbidity and mortality that we see,” he added. “I think we all hypothesize that some of our treatments may be beneficial there. The challenge that we face is figuring out when the best time is to administer these medicines, and whether they need to be administered as part of a cocktail of therapy.”
Along those lines, Dr. Wallace cited a recent study he coauthored in which rheumatoid arthritis patients who were on JAK inhibitors at baseline had worse COVID-19 severity. But he emphasized that, despite their differing findings, the two studies are not irreconcilable.
“What this might speak to is, the timing of your exposure may be really important,” he said. “At the time of your initial infection, you may need certain aspects of your immune system that a JAK inhibitor may interfere with. But when you initiate a JAK inhibitor, once that phase is complete and you’re in this hyperinflammatory phase, you may have more benefit to target and treat the intense inflammation that we observe in patients who have COVID.”
He also offered up another variable potentially in play: different JAK inhibitors having different targets among the JAK receptors. “It may be that targeting specific JAKs is more beneficial when it comes to treating the hyperinflammatory response of COVID-19.”
The trial was sponsored by Pfizer. Several authors acknowledged potential conflicts of interest, including receiving grants and personal fees from Pfizer and various other pharmaceutical companies.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
No overall statin effect seen on dementia, cognition in ASPREE analysis
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”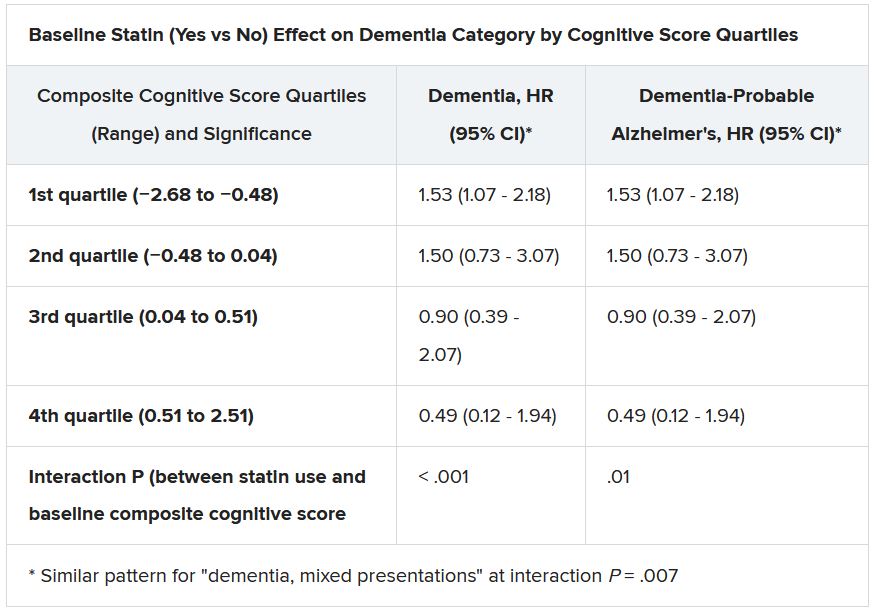
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Novel molecule prolongs half-life of bleeding disorder treatments
A novel therapeutic approach using aptamers – short single strands of DNA or RNA designed to selectively bind to a target – shows promise for treating von Willebrand Disease (VWD), and other congenital bleed disorders such as hemophilia A, investigators say.
In a proof-of-concept study using healthy volunteers, the experimental anti–von Willebrand factor (VWF) molecule BT200 appeared to decrease clearance of VWF and resulted in a twofold increase in endogenous levels of VWF and factor VIII at low doses.
BT200 is currently being explored in a phase 2 trial in patients with hemophilia A and VWD type 2B, Katarina Kovacevic, MPharm, from the Medical University of Vienna, reported at the European Hematology Association annual congress.
“We expect to see a half-life increase of 2 to 4 times of factor VIII products, which will allow us to have a longer time between treatments,” she said in an oral abstract presentation (Abstract S302).
Lab-made nucleotide strings
Aptamers are sometimes call “chemical antibodies” because of their high affinity and high specificity for extracellular targets, Dr. Kovacevic said. Unlike conventional humanized or human-derived antibodies, however, they are nonimmunogenic and are less costly to manufacture.
In a previous study from her center, a different anti-VWF aptamer labeled ARC1779 increased plasma levels of VWF, factor VIII, and platelet counts in patients with VWD type 2B.
However, the drug was inconvenient to use, requiring 72-hour infusions, she noted.
In a study published in Feb. 4, 2021 in Scientific Reports, Dr. Kovacevic and colleagues reported that BT200 blocks VWF and platelet function in patients with ischemic strokes, even in the presence of high levels of VWF in patients with left carotid artery atherosclerotic strokes.
The ability of the molecule to block VWF platelet binding may explain how the anti-VWF agent actually results in higher circulating levels of VWF, which also carries factor VIII, said Veronica H. Flood, MD, a VWD specialist at Children’s Hospital of Wisconsin and associate professor at the Medical College of Wisconsin in Milwaukee.
“It might inhibit clearance of the von Willebrand factor, so it’s almost like this was an incidental side effect,” she said in an interview. “Incidentally, this happens to also give you higher levels of the von Willebrand factor and the factor VIII, and with a longer half-life than anything we currently have, so it’s a super-creative strategy,” she said.
Dr. Flood was not involved in the study.
Long half-life
BT200 is a third-generation peglyated anti-VWF aptamer that has been shown in preclinical studies to have a long half-life in nonhuman primates. It inhibits the A1 domain of VWF to prevent it from binding to platelet glycoprotein 1b (GP1b).
In the randomized, double-blind, placebo-controlled trial reported at the EHA congress, 88 healthy volunteers received single doses of BT200 ranging from 0.l8 mg to 48 mg by subcutaneous injection or intravenous infusion, 8 received multiple doses, 8 were evaluated for possible interactions with desmopressin, and 8 were evaluated for bioavailability of the aptamer.
The investigators observed a dose-related increase in BT200 concentrations, with a mean plasma terminal elimination half life of between 118 and 284 hours (about 5-12 days). There was also a dose-dependent increase in bioavailability of the agents, reaching 90% at the highest dose level.
The ability of BT200 to inhibit the A1 domains of VWF also was dose dependent, with the largest effect seen with doses of 12 mg and higher. The molecule decreased VWF activity and ristocetin-induced platelet aggregation, and prolonged collagen adenosine diphosphate closure time.
At the highest doses, BT200 caused complete inhibition of VWF (P < .001), and volunteers developed clinical signs of mild mucosal bleeding.
But the aptamer also increased in a dose-dependent fashion VWF antigen levels and factor VIII clotting activity more than fourfold (P <.001).
“This resulted in increased thrombogenicity as measured by thrombin generation and enhanced clotting. In the absence of an increase in VWF propeptide levels, this effect is considered due to decreased clearance of VWF,” the investigators wrote in the study abstract.
They noted that they saw a clinically meaningful twofold increase in both VWF and factor VIII at doses lower than 6 mg.
‘Super-exciting strategy’
“This trial identified a novel mechanism of action for BT200: It decreases the clearance of VWF/FVIII, which can be exploited for congenital bleeding disorders. This built a solid foundation for an ongoing basket trial in patients with von Willebrand disease or hemophilia A, which already confirms the expected effect sizes,” Dr. Kovacevic and colleagues wrote.
“I will be interested to see what the clinical side effects are, because there may be some off-target effects, but in reality it is a super-exciting strategy, and there is really a dire need for longer half-life products for these patients,” Dr. Flood said.
The study was sponsored by Band Therapeutics, a division of Guardian Therapeutics. Dr. Kovacevic and Dr. Flood reported having no conflicts of interest to disclose.
A novel therapeutic approach using aptamers – short single strands of DNA or RNA designed to selectively bind to a target – shows promise for treating von Willebrand Disease (VWD), and other congenital bleed disorders such as hemophilia A, investigators say.
In a proof-of-concept study using healthy volunteers, the experimental anti–von Willebrand factor (VWF) molecule BT200 appeared to decrease clearance of VWF and resulted in a twofold increase in endogenous levels of VWF and factor VIII at low doses.
BT200 is currently being explored in a phase 2 trial in patients with hemophilia A and VWD type 2B, Katarina Kovacevic, MPharm, from the Medical University of Vienna, reported at the European Hematology Association annual congress.
“We expect to see a half-life increase of 2 to 4 times of factor VIII products, which will allow us to have a longer time between treatments,” she said in an oral abstract presentation (Abstract S302).
Lab-made nucleotide strings
Aptamers are sometimes call “chemical antibodies” because of their high affinity and high specificity for extracellular targets, Dr. Kovacevic said. Unlike conventional humanized or human-derived antibodies, however, they are nonimmunogenic and are less costly to manufacture.
In a previous study from her center, a different anti-VWF aptamer labeled ARC1779 increased plasma levels of VWF, factor VIII, and platelet counts in patients with VWD type 2B.
However, the drug was inconvenient to use, requiring 72-hour infusions, she noted.
In a study published in Feb. 4, 2021 in Scientific Reports, Dr. Kovacevic and colleagues reported that BT200 blocks VWF and platelet function in patients with ischemic strokes, even in the presence of high levels of VWF in patients with left carotid artery atherosclerotic strokes.
The ability of the molecule to block VWF platelet binding may explain how the anti-VWF agent actually results in higher circulating levels of VWF, which also carries factor VIII, said Veronica H. Flood, MD, a VWD specialist at Children’s Hospital of Wisconsin and associate professor at the Medical College of Wisconsin in Milwaukee.
“It might inhibit clearance of the von Willebrand factor, so it’s almost like this was an incidental side effect,” she said in an interview. “Incidentally, this happens to also give you higher levels of the von Willebrand factor and the factor VIII, and with a longer half-life than anything we currently have, so it’s a super-creative strategy,” she said.
Dr. Flood was not involved in the study.
Long half-life
BT200 is a third-generation peglyated anti-VWF aptamer that has been shown in preclinical studies to have a long half-life in nonhuman primates. It inhibits the A1 domain of VWF to prevent it from binding to platelet glycoprotein 1b (GP1b).
In the randomized, double-blind, placebo-controlled trial reported at the EHA congress, 88 healthy volunteers received single doses of BT200 ranging from 0.l8 mg to 48 mg by subcutaneous injection or intravenous infusion, 8 received multiple doses, 8 were evaluated for possible interactions with desmopressin, and 8 were evaluated for bioavailability of the aptamer.
The investigators observed a dose-related increase in BT200 concentrations, with a mean plasma terminal elimination half life of between 118 and 284 hours (about 5-12 days). There was also a dose-dependent increase in bioavailability of the agents, reaching 90% at the highest dose level.
The ability of BT200 to inhibit the A1 domains of VWF also was dose dependent, with the largest effect seen with doses of 12 mg and higher. The molecule decreased VWF activity and ristocetin-induced platelet aggregation, and prolonged collagen adenosine diphosphate closure time.
At the highest doses, BT200 caused complete inhibition of VWF (P < .001), and volunteers developed clinical signs of mild mucosal bleeding.
But the aptamer also increased in a dose-dependent fashion VWF antigen levels and factor VIII clotting activity more than fourfold (P <.001).
“This resulted in increased thrombogenicity as measured by thrombin generation and enhanced clotting. In the absence of an increase in VWF propeptide levels, this effect is considered due to decreased clearance of VWF,” the investigators wrote in the study abstract.
They noted that they saw a clinically meaningful twofold increase in both VWF and factor VIII at doses lower than 6 mg.
‘Super-exciting strategy’
“This trial identified a novel mechanism of action for BT200: It decreases the clearance of VWF/FVIII, which can be exploited for congenital bleeding disorders. This built a solid foundation for an ongoing basket trial in patients with von Willebrand disease or hemophilia A, which already confirms the expected effect sizes,” Dr. Kovacevic and colleagues wrote.
“I will be interested to see what the clinical side effects are, because there may be some off-target effects, but in reality it is a super-exciting strategy, and there is really a dire need for longer half-life products for these patients,” Dr. Flood said.
The study was sponsored by Band Therapeutics, a division of Guardian Therapeutics. Dr. Kovacevic and Dr. Flood reported having no conflicts of interest to disclose.
A novel therapeutic approach using aptamers – short single strands of DNA or RNA designed to selectively bind to a target – shows promise for treating von Willebrand Disease (VWD), and other congenital bleed disorders such as hemophilia A, investigators say.
In a proof-of-concept study using healthy volunteers, the experimental anti–von Willebrand factor (VWF) molecule BT200 appeared to decrease clearance of VWF and resulted in a twofold increase in endogenous levels of VWF and factor VIII at low doses.
BT200 is currently being explored in a phase 2 trial in patients with hemophilia A and VWD type 2B, Katarina Kovacevic, MPharm, from the Medical University of Vienna, reported at the European Hematology Association annual congress.
“We expect to see a half-life increase of 2 to 4 times of factor VIII products, which will allow us to have a longer time between treatments,” she said in an oral abstract presentation (Abstract S302).
Lab-made nucleotide strings
Aptamers are sometimes call “chemical antibodies” because of their high affinity and high specificity for extracellular targets, Dr. Kovacevic said. Unlike conventional humanized or human-derived antibodies, however, they are nonimmunogenic and are less costly to manufacture.
In a previous study from her center, a different anti-VWF aptamer labeled ARC1779 increased plasma levels of VWF, factor VIII, and platelet counts in patients with VWD type 2B.
However, the drug was inconvenient to use, requiring 72-hour infusions, she noted.
In a study published in Feb. 4, 2021 in Scientific Reports, Dr. Kovacevic and colleagues reported that BT200 blocks VWF and platelet function in patients with ischemic strokes, even in the presence of high levels of VWF in patients with left carotid artery atherosclerotic strokes.
The ability of the molecule to block VWF platelet binding may explain how the anti-VWF agent actually results in higher circulating levels of VWF, which also carries factor VIII, said Veronica H. Flood, MD, a VWD specialist at Children’s Hospital of Wisconsin and associate professor at the Medical College of Wisconsin in Milwaukee.
“It might inhibit clearance of the von Willebrand factor, so it’s almost like this was an incidental side effect,” she said in an interview. “Incidentally, this happens to also give you higher levels of the von Willebrand factor and the factor VIII, and with a longer half-life than anything we currently have, so it’s a super-creative strategy,” she said.
Dr. Flood was not involved in the study.
Long half-life
BT200 is a third-generation peglyated anti-VWF aptamer that has been shown in preclinical studies to have a long half-life in nonhuman primates. It inhibits the A1 domain of VWF to prevent it from binding to platelet glycoprotein 1b (GP1b).
In the randomized, double-blind, placebo-controlled trial reported at the EHA congress, 88 healthy volunteers received single doses of BT200 ranging from 0.l8 mg to 48 mg by subcutaneous injection or intravenous infusion, 8 received multiple doses, 8 were evaluated for possible interactions with desmopressin, and 8 were evaluated for bioavailability of the aptamer.
The investigators observed a dose-related increase in BT200 concentrations, with a mean plasma terminal elimination half life of between 118 and 284 hours (about 5-12 days). There was also a dose-dependent increase in bioavailability of the agents, reaching 90% at the highest dose level.
The ability of BT200 to inhibit the A1 domains of VWF also was dose dependent, with the largest effect seen with doses of 12 mg and higher. The molecule decreased VWF activity and ristocetin-induced platelet aggregation, and prolonged collagen adenosine diphosphate closure time.
At the highest doses, BT200 caused complete inhibition of VWF (P < .001), and volunteers developed clinical signs of mild mucosal bleeding.
But the aptamer also increased in a dose-dependent fashion VWF antigen levels and factor VIII clotting activity more than fourfold (P <.001).
“This resulted in increased thrombogenicity as measured by thrombin generation and enhanced clotting. In the absence of an increase in VWF propeptide levels, this effect is considered due to decreased clearance of VWF,” the investigators wrote in the study abstract.
They noted that they saw a clinically meaningful twofold increase in both VWF and factor VIII at doses lower than 6 mg.
‘Super-exciting strategy’
“This trial identified a novel mechanism of action for BT200: It decreases the clearance of VWF/FVIII, which can be exploited for congenital bleeding disorders. This built a solid foundation for an ongoing basket trial in patients with von Willebrand disease or hemophilia A, which already confirms the expected effect sizes,” Dr. Kovacevic and colleagues wrote.
“I will be interested to see what the clinical side effects are, because there may be some off-target effects, but in reality it is a super-exciting strategy, and there is really a dire need for longer half-life products for these patients,” Dr. Flood said.
The study was sponsored by Band Therapeutics, a division of Guardian Therapeutics. Dr. Kovacevic and Dr. Flood reported having no conflicts of interest to disclose.
FROM EHA 2021
Bathroom blues: Inexpensive dye tracks digestive transit time
When it comes to measuring gut transit time, blue dye could be a cost-effective and simple alternative to other, more burdensome methods.
The approach, which only requires fasting followed by eating dyed food, revealed an association between microbiome composition and transit time in healthy individuals, according to authors led by Francisco Asnicar, PhD, of the University of Trento (Italy). The researchers chose the blue food coloring over carmine red dye partly because of its vegetable origin and because the blue color makes it unlikely the recipient would mistake the coloring in stool as originating from some other food, such as beetroot.
Gut motility is connected to digestion, the immune system, the endocrine system, and gut microbiota, according to the authors. For example, some have suggested that transit time may affect postprandial glycemia and lipemia through a potential effect on nutrient absorption and gut microbiome composition. “[This blue dye’s] use therefore has the potential to provide another piece of the puzzle to advance precision medicine,” the authors wrote.
Validated methods to measure transit time include scintigraphy, wireless motility capsule, radio-opaque markers and breath testing, but they require specialized equipment and staff, participants must make at least one in-person visit, and they can be expensive.
Transit time’s position in research
Those limitations may explain why the effect of transit time has been understudied, though it has gained momentum in recent years, according to Henrik Roager, PhD, who was asked to comment on the study. “I think it has become clear that it is probably one of the most important factors that explain the [microbiota] differences that we see from individual to individual,” said Dr. Roager, of the department of nutrition, exercise, and sports at the University of Copenhagen.
The relationship is complex, since gut microbes may be releasing metabolites that can affect motility, which in turn would affect the microbes. “The speed by which nutrients pass through a fermenter in the lab will have a huge impact on microbial physiology and metabolism. It’s basically the same principle in humans,” added Dr. Roager, who is engaged in research to identify such microbial metabolites.
To better understand those relationships will require epidemiological studies in healthy populations. Blue dye is one approach. Another is sweet corn, which individuals can obtain even more easily. Dye has one advantage in that it’s unlikely to impact transit time, while the quantity of sweet corn eaten could have an effect. “I definitely think that either this or sweet corn would be standard in many studies in the future,” said Dr. Roager.
Epidemiological studies made easier by dye or sweet corn could also reveal how diet interacts with the microbiome by including transit time as a variable. Transit time can vary from day to day, and Dr. Roager believes those variations may be linked to changes in the gut microbiome. With simpler techniques for measuring transit time, “I think we might be able to better identify effects of diets or drugs or lifestyle on the microbiome.”
How the blue dye fared
The researchers analyzed data from 866 twins and unrelated adults from the United States and the United Kingdom who were enrolled in the PREDICT 1 study, which quantified metabolic responses to standardized meals. Participants underwent fasting and then ate two blue muffins, along with a glass of chocolate milk, then logged the first sign of blue coloring in their stool using an app. Participants also answered a questionnaire detailing the frequency and consistency of bowel movements. The researchers also conducted sequencing of stool samples to determine microbiome profile.
There was a strong correlation between stool consistency and frequency, as well as microbial diversity and the composition of the gut microbiome. The dye measurement identified different fast and slow transit time clusters (area under the receiver operating characteristic curve, 0.82), which were associated with the composition of the gut microbiome, including species like Akkermansia muciniphila, Bacteroides species, and Alistipes species (false discovery rate–adjusted P values < .01). Transit times measured with the blue dye was a better predictor of gut function than either stool consistency and stool frequency, suggesting that the dye may be a more useful method for large cohorts of healthy individuals.
Although associations with diet and cardiometabolic factors were more modest, longer transit times appear predictive of greater visceral fat and higher postprandial responses, “which are key measures of health.”
The authors cited some limitations, including the fact that the blue-dye method has not yet been compared with other transit methodologies. However, the gut transit time in this study was found to be strongly correlated with stool consistency and frequency.
“To conclude, our findings indicate that the blue dye method is a novel, inexpensive and scalable method of gut transit assessment providing valuable gut health and metabolic insights,” they wrote. “Its wide use in both research and clinical settings could facilitate the advancement of our understanding of gut function and its determinants, as well as the complex interactions between gut physiology and health outcomes.”
The study authors received funding from a wide range of nonindustry sources. Dr. Roager had no relevant financial disclosures.
When it comes to measuring gut transit time, blue dye could be a cost-effective and simple alternative to other, more burdensome methods.
The approach, which only requires fasting followed by eating dyed food, revealed an association between microbiome composition and transit time in healthy individuals, according to authors led by Francisco Asnicar, PhD, of the University of Trento (Italy). The researchers chose the blue food coloring over carmine red dye partly because of its vegetable origin and because the blue color makes it unlikely the recipient would mistake the coloring in stool as originating from some other food, such as beetroot.
Gut motility is connected to digestion, the immune system, the endocrine system, and gut microbiota, according to the authors. For example, some have suggested that transit time may affect postprandial glycemia and lipemia through a potential effect on nutrient absorption and gut microbiome composition. “[This blue dye’s] use therefore has the potential to provide another piece of the puzzle to advance precision medicine,” the authors wrote.
Validated methods to measure transit time include scintigraphy, wireless motility capsule, radio-opaque markers and breath testing, but they require specialized equipment and staff, participants must make at least one in-person visit, and they can be expensive.
Transit time’s position in research
Those limitations may explain why the effect of transit time has been understudied, though it has gained momentum in recent years, according to Henrik Roager, PhD, who was asked to comment on the study. “I think it has become clear that it is probably one of the most important factors that explain the [microbiota] differences that we see from individual to individual,” said Dr. Roager, of the department of nutrition, exercise, and sports at the University of Copenhagen.
The relationship is complex, since gut microbes may be releasing metabolites that can affect motility, which in turn would affect the microbes. “The speed by which nutrients pass through a fermenter in the lab will have a huge impact on microbial physiology and metabolism. It’s basically the same principle in humans,” added Dr. Roager, who is engaged in research to identify such microbial metabolites.
To better understand those relationships will require epidemiological studies in healthy populations. Blue dye is one approach. Another is sweet corn, which individuals can obtain even more easily. Dye has one advantage in that it’s unlikely to impact transit time, while the quantity of sweet corn eaten could have an effect. “I definitely think that either this or sweet corn would be standard in many studies in the future,” said Dr. Roager.
Epidemiological studies made easier by dye or sweet corn could also reveal how diet interacts with the microbiome by including transit time as a variable. Transit time can vary from day to day, and Dr. Roager believes those variations may be linked to changes in the gut microbiome. With simpler techniques for measuring transit time, “I think we might be able to better identify effects of diets or drugs or lifestyle on the microbiome.”
How the blue dye fared
The researchers analyzed data from 866 twins and unrelated adults from the United States and the United Kingdom who were enrolled in the PREDICT 1 study, which quantified metabolic responses to standardized meals. Participants underwent fasting and then ate two blue muffins, along with a glass of chocolate milk, then logged the first sign of blue coloring in their stool using an app. Participants also answered a questionnaire detailing the frequency and consistency of bowel movements. The researchers also conducted sequencing of stool samples to determine microbiome profile.
There was a strong correlation between stool consistency and frequency, as well as microbial diversity and the composition of the gut microbiome. The dye measurement identified different fast and slow transit time clusters (area under the receiver operating characteristic curve, 0.82), which were associated with the composition of the gut microbiome, including species like Akkermansia muciniphila, Bacteroides species, and Alistipes species (false discovery rate–adjusted P values < .01). Transit times measured with the blue dye was a better predictor of gut function than either stool consistency and stool frequency, suggesting that the dye may be a more useful method for large cohorts of healthy individuals.
Although associations with diet and cardiometabolic factors were more modest, longer transit times appear predictive of greater visceral fat and higher postprandial responses, “which are key measures of health.”
The authors cited some limitations, including the fact that the blue-dye method has not yet been compared with other transit methodologies. However, the gut transit time in this study was found to be strongly correlated with stool consistency and frequency.
“To conclude, our findings indicate that the blue dye method is a novel, inexpensive and scalable method of gut transit assessment providing valuable gut health and metabolic insights,” they wrote. “Its wide use in both research and clinical settings could facilitate the advancement of our understanding of gut function and its determinants, as well as the complex interactions between gut physiology and health outcomes.”
The study authors received funding from a wide range of nonindustry sources. Dr. Roager had no relevant financial disclosures.
When it comes to measuring gut transit time, blue dye could be a cost-effective and simple alternative to other, more burdensome methods.
The approach, which only requires fasting followed by eating dyed food, revealed an association between microbiome composition and transit time in healthy individuals, according to authors led by Francisco Asnicar, PhD, of the University of Trento (Italy). The researchers chose the blue food coloring over carmine red dye partly because of its vegetable origin and because the blue color makes it unlikely the recipient would mistake the coloring in stool as originating from some other food, such as beetroot.
Gut motility is connected to digestion, the immune system, the endocrine system, and gut microbiota, according to the authors. For example, some have suggested that transit time may affect postprandial glycemia and lipemia through a potential effect on nutrient absorption and gut microbiome composition. “[This blue dye’s] use therefore has the potential to provide another piece of the puzzle to advance precision medicine,” the authors wrote.
Validated methods to measure transit time include scintigraphy, wireless motility capsule, radio-opaque markers and breath testing, but they require specialized equipment and staff, participants must make at least one in-person visit, and they can be expensive.
Transit time’s position in research
Those limitations may explain why the effect of transit time has been understudied, though it has gained momentum in recent years, according to Henrik Roager, PhD, who was asked to comment on the study. “I think it has become clear that it is probably one of the most important factors that explain the [microbiota] differences that we see from individual to individual,” said Dr. Roager, of the department of nutrition, exercise, and sports at the University of Copenhagen.
The relationship is complex, since gut microbes may be releasing metabolites that can affect motility, which in turn would affect the microbes. “The speed by which nutrients pass through a fermenter in the lab will have a huge impact on microbial physiology and metabolism. It’s basically the same principle in humans,” added Dr. Roager, who is engaged in research to identify such microbial metabolites.
To better understand those relationships will require epidemiological studies in healthy populations. Blue dye is one approach. Another is sweet corn, which individuals can obtain even more easily. Dye has one advantage in that it’s unlikely to impact transit time, while the quantity of sweet corn eaten could have an effect. “I definitely think that either this or sweet corn would be standard in many studies in the future,” said Dr. Roager.
Epidemiological studies made easier by dye or sweet corn could also reveal how diet interacts with the microbiome by including transit time as a variable. Transit time can vary from day to day, and Dr. Roager believes those variations may be linked to changes in the gut microbiome. With simpler techniques for measuring transit time, “I think we might be able to better identify effects of diets or drugs or lifestyle on the microbiome.”
How the blue dye fared
The researchers analyzed data from 866 twins and unrelated adults from the United States and the United Kingdom who were enrolled in the PREDICT 1 study, which quantified metabolic responses to standardized meals. Participants underwent fasting and then ate two blue muffins, along with a glass of chocolate milk, then logged the first sign of blue coloring in their stool using an app. Participants also answered a questionnaire detailing the frequency and consistency of bowel movements. The researchers also conducted sequencing of stool samples to determine microbiome profile.
There was a strong correlation between stool consistency and frequency, as well as microbial diversity and the composition of the gut microbiome. The dye measurement identified different fast and slow transit time clusters (area under the receiver operating characteristic curve, 0.82), which were associated with the composition of the gut microbiome, including species like Akkermansia muciniphila, Bacteroides species, and Alistipes species (false discovery rate–adjusted P values < .01). Transit times measured with the blue dye was a better predictor of gut function than either stool consistency and stool frequency, suggesting that the dye may be a more useful method for large cohorts of healthy individuals.
Although associations with diet and cardiometabolic factors were more modest, longer transit times appear predictive of greater visceral fat and higher postprandial responses, “which are key measures of health.”
The authors cited some limitations, including the fact that the blue-dye method has not yet been compared with other transit methodologies. However, the gut transit time in this study was found to be strongly correlated with stool consistency and frequency.
“To conclude, our findings indicate that the blue dye method is a novel, inexpensive and scalable method of gut transit assessment providing valuable gut health and metabolic insights,” they wrote. “Its wide use in both research and clinical settings could facilitate the advancement of our understanding of gut function and its determinants, as well as the complex interactions between gut physiology and health outcomes.”
The study authors received funding from a wide range of nonindustry sources. Dr. Roager had no relevant financial disclosures.
FROM GUT
Pigmented Basal Cell Carcinoma With Annular Leukoderma
To the Editor:
Annular leukoderma, or the halo phenomenon, is a circular reaction of hypopigmentation that most commonly is observed alongside congenital nevi, acquired melanocytic nevi, blue nevi, Spitz nevi, vitiligo, and rarely melanoma.1 There is limited literature on the mechanism of the halo phenomenon. Most of the literature proposes a T cell–mediated immune response to antigens, which causes not only surrounding pigment loss but also heralds the regression of central lesions.2 Others have suggested a vascular mechanism, with blood shunted away from the lesions.3 Because guidelines discourage biopsy of typical halo nevi, it becomes important to evaluate lesions for worrisome features such as ulceration or asymmetry, especially in older patients. We present a case of a pigmented basal cell carcinoma (BCC) that exhibited the halo phenomenon. Four other cases have been described in the literature.3-6
A 53-year-old man presented for evaluation of an asymptomatic lesion on the left side of the abdomen of approximately 8 months’ duration. He had no personal or family history of skin cancer. Physical examination revealed a central 1-cm, pink, verrucous papule surrounded by a 2×1.2-cm, depigmented, circular patch on the left side of the inferior abdomen (Figure 1). Upon questioning, the patient produced cell phone photographs of the trunk from 3 years prior, which did not show any lesions present. Full-body skin examination did not reveal any other concerning pigmented lesions. Excisional biopsy was performed due to concern for amelanotic melanoma, and histopathology revealed a superficial and pigmented BCC (Figure 2). Immunohistochemistry with Melan-A was negative for atypical melanocytes, with no uptake in the leukoderma areas.
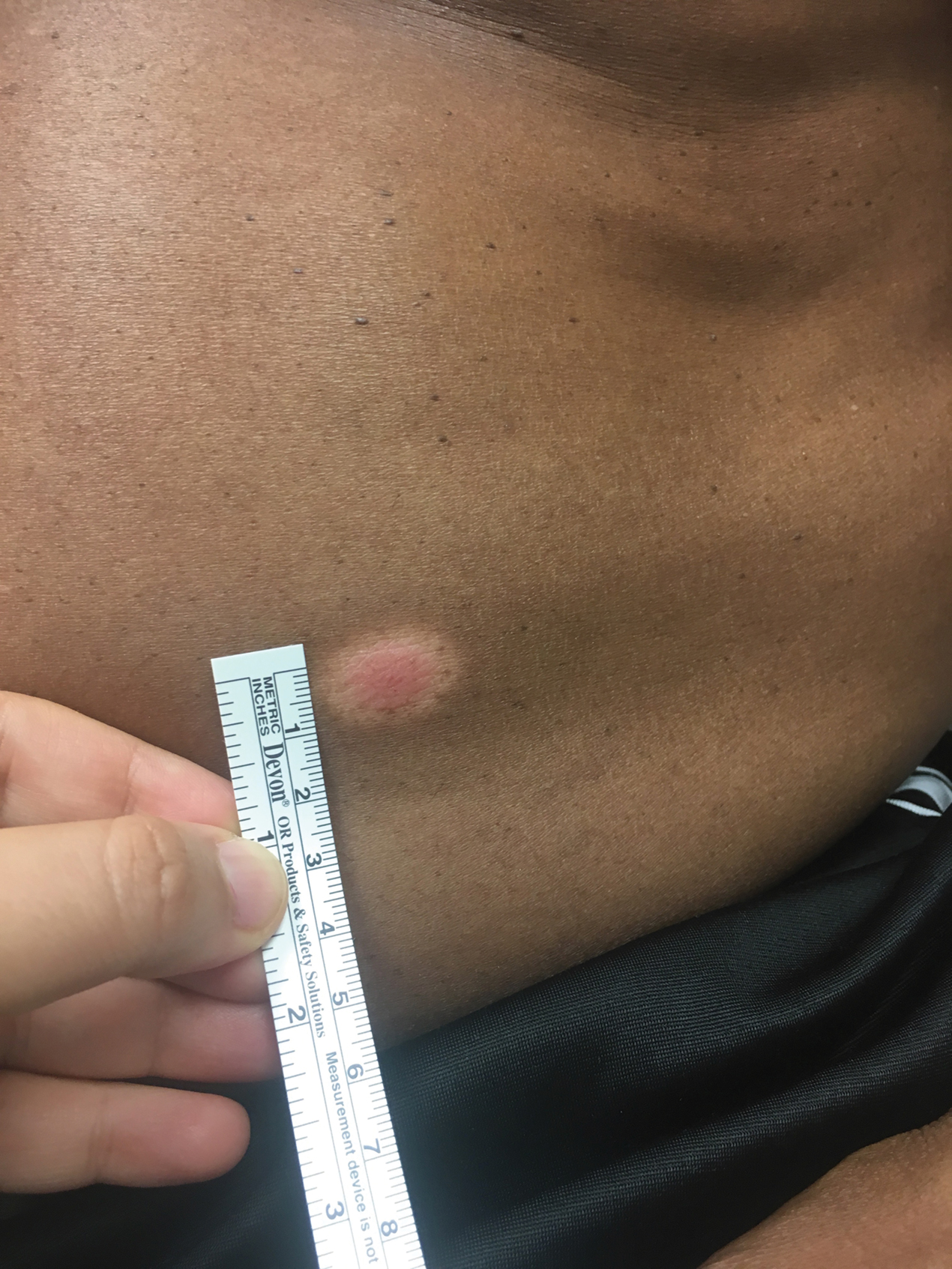
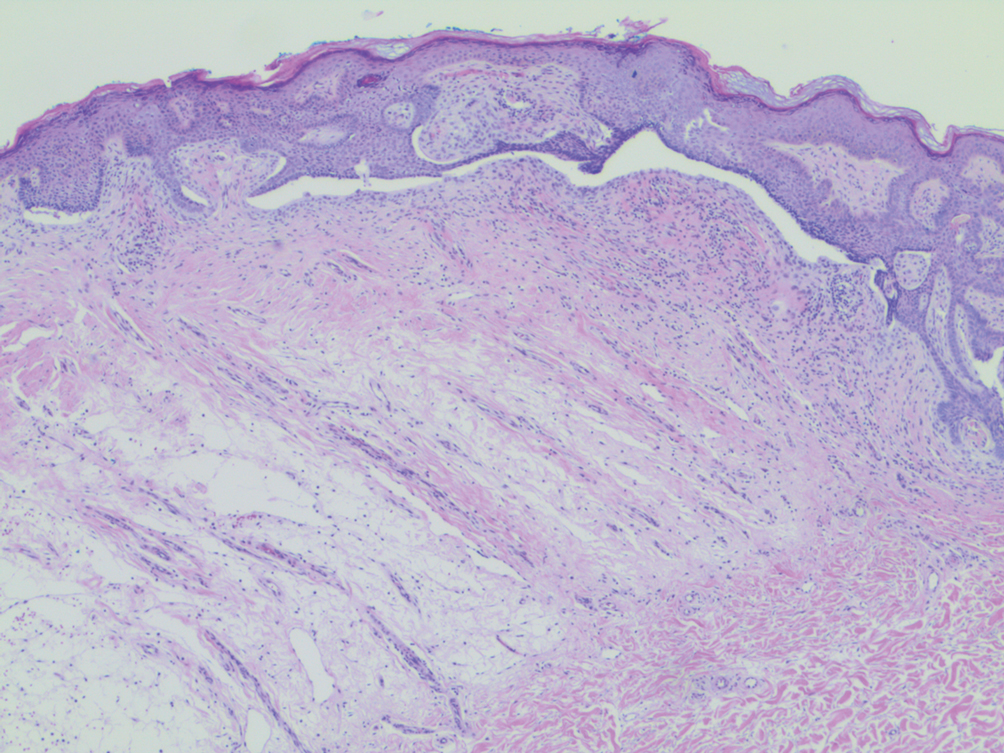
The clinical presentation initially was concerning for amelanotic melanoma. All melanoma subtypes may appear as hypomelanotic lesions, though these most commonly are observed in the desmoplastic or nodular subtypes. Amelanotic melanomas may present as well-defined red or pink macules, plaques, or nodules, with some tumors presenting with light brown pigmentation.7
The differential diagnosis for lesions with the halo phenomenon is large. In adults, the halo phenomenon may be concerning for malignant or regressing melanoma. As an immunogenic tumor, melanoma’s immunogenic melanocytes may incite a cell-mediated immune response to antigens common to neoplastic and normal melanocytes, which can clinically manifest not only as local annular leukoderma but also as distant vitiligo or halo nevi.7 The halo phenomenon more commonly is associated with benign processes such as vitiligo and halo nevi in children. In most children, halo nevi occur as an isolated phenomenon but still warrant a complete skin examination for melanoma and vitiligo. The presence of halo nevi has been associated with distant vitiligo—possibly through shared immunologic mechanisms—especially if patients present with the Koebner phenomenon, multiple halo nevi, or a family history of vitiligo.8 A prospective study also found that the presence of halo nevi was an independent risk factor for the progression of segmental vitiligo to mixed vitiligo.9 Hormones also may play a role in the leukoderma acquisitum centrifugum, or halo, nevi. Halo nevi most commonly affect adolescents and pregnant women. It has been postulated that congenital nevi may be unique in their response to altered estrogen levels, increasing the rate not only of halo nevi but also of melanoma in pregnant women.10
Our patient’s final histologic diagnosis was pigmented BCC, which comprises only 6% of all BCCs.3 The proposed mechanism is that melanocytes colonize the tumor in the surrounding stroma and produce excess melanin. Basal cell carcinoma with halo phenomenon is a rare presentation. As in our case, 2 prior BCC reports also involved patients older than 50 years,3,5 with the 2 other cases describing women in their late twenties and early thirties.4,6 Additionally, 2 of 4 reports described patients with a history of multiple BCCs.3,5
In summary, the seemingly benign halo phenomenon may accompany malignant processes such as nonmelanoma skin cancer. Careful consideration of lesion time course and atypia is imperative for proper clinical suspicion in such cases.
- Mooney MA, Barr RJ, Buxton MG. Halo nevus or halo phenomenon? a study of 142 cases. J Cutan Pathol. 1995;22:342-348.
- Zeff RA, Freitag A, Grin CM, et al. The immune response in halo nevi. J Am Acad Dermatol. 1997;37:620-624.
- Johnson DB, Ceilley RI. Basal cell carcinoma with annular leukoderma mimicking leukoderma acquisitum centrifugum. Arch Dermatol. 1980;116:352-353.
- Basak PY, Meric G, Ciris M. Basal cell carcinoma with halo phenomenon in a young female: significance of dermatoscopy in early diagnosis. Indian J Dermatol. 2015;60:214.
- Pembroke AC, Liddell K. Basal cell epithelioma with a hypopigmented halo. Arch Dermatol. 1981;117:317.
- Rustemeyer J, Günther L, Deichert L. A rare association: basal cell carcinoma in a vitiliginous macula. Oral Maxillofac Surg. 2011;15:175-177.
- Naveh HP, Rao UN, Butterfield LH. Melanoma‐associated leukoderma—immunology in black and white? Pigment Cell Melanoma Res. 2013;26:796-804.
- Zhou H, Wu L-C, Chen M-K, et al. Factors associated with development of vitiligo in patients with halo nevus. Chinese Med J. 2017;130:2703.
- Ezzedine K, Diallo A, Léauté‐Labrèze C, et al. Halo naevi and leukotrichia are strong predictors of the passage to mixed vitiligo in a subgroup of segmental vitiligo. Br J Dermatol. 2012;166:539-544.
- Nading MA, Nanney LB, Ellis DL. Pregnancy and estrogen receptor β expression in a large congenital nevus. Arch Dermatol. 2009;145:691-694.
To the Editor:
Annular leukoderma, or the halo phenomenon, is a circular reaction of hypopigmentation that most commonly is observed alongside congenital nevi, acquired melanocytic nevi, blue nevi, Spitz nevi, vitiligo, and rarely melanoma.1 There is limited literature on the mechanism of the halo phenomenon. Most of the literature proposes a T cell–mediated immune response to antigens, which causes not only surrounding pigment loss but also heralds the regression of central lesions.2 Others have suggested a vascular mechanism, with blood shunted away from the lesions.3 Because guidelines discourage biopsy of typical halo nevi, it becomes important to evaluate lesions for worrisome features such as ulceration or asymmetry, especially in older patients. We present a case of a pigmented basal cell carcinoma (BCC) that exhibited the halo phenomenon. Four other cases have been described in the literature.3-6
A 53-year-old man presented for evaluation of an asymptomatic lesion on the left side of the abdomen of approximately 8 months’ duration. He had no personal or family history of skin cancer. Physical examination revealed a central 1-cm, pink, verrucous papule surrounded by a 2×1.2-cm, depigmented, circular patch on the left side of the inferior abdomen (Figure 1). Upon questioning, the patient produced cell phone photographs of the trunk from 3 years prior, which did not show any lesions present. Full-body skin examination did not reveal any other concerning pigmented lesions. Excisional biopsy was performed due to concern for amelanotic melanoma, and histopathology revealed a superficial and pigmented BCC (Figure 2). Immunohistochemistry with Melan-A was negative for atypical melanocytes, with no uptake in the leukoderma areas.


The clinical presentation initially was concerning for amelanotic melanoma. All melanoma subtypes may appear as hypomelanotic lesions, though these most commonly are observed in the desmoplastic or nodular subtypes. Amelanotic melanomas may present as well-defined red or pink macules, plaques, or nodules, with some tumors presenting with light brown pigmentation.7
The differential diagnosis for lesions with the halo phenomenon is large. In adults, the halo phenomenon may be concerning for malignant or regressing melanoma. As an immunogenic tumor, melanoma’s immunogenic melanocytes may incite a cell-mediated immune response to antigens common to neoplastic and normal melanocytes, which can clinically manifest not only as local annular leukoderma but also as distant vitiligo or halo nevi.7 The halo phenomenon more commonly is associated with benign processes such as vitiligo and halo nevi in children. In most children, halo nevi occur as an isolated phenomenon but still warrant a complete skin examination for melanoma and vitiligo. The presence of halo nevi has been associated with distant vitiligo—possibly through shared immunologic mechanisms—especially if patients present with the Koebner phenomenon, multiple halo nevi, or a family history of vitiligo.8 A prospective study also found that the presence of halo nevi was an independent risk factor for the progression of segmental vitiligo to mixed vitiligo.9 Hormones also may play a role in the leukoderma acquisitum centrifugum, or halo, nevi. Halo nevi most commonly affect adolescents and pregnant women. It has been postulated that congenital nevi may be unique in their response to altered estrogen levels, increasing the rate not only of halo nevi but also of melanoma in pregnant women.10
Our patient’s final histologic diagnosis was pigmented BCC, which comprises only 6% of all BCCs.3 The proposed mechanism is that melanocytes colonize the tumor in the surrounding stroma and produce excess melanin. Basal cell carcinoma with halo phenomenon is a rare presentation. As in our case, 2 prior BCC reports also involved patients older than 50 years,3,5 with the 2 other cases describing women in their late twenties and early thirties.4,6 Additionally, 2 of 4 reports described patients with a history of multiple BCCs.3,5
In summary, the seemingly benign halo phenomenon may accompany malignant processes such as nonmelanoma skin cancer. Careful consideration of lesion time course and atypia is imperative for proper clinical suspicion in such cases.
To the Editor:
Annular leukoderma, or the halo phenomenon, is a circular reaction of hypopigmentation that most commonly is observed alongside congenital nevi, acquired melanocytic nevi, blue nevi, Spitz nevi, vitiligo, and rarely melanoma.1 There is limited literature on the mechanism of the halo phenomenon. Most of the literature proposes a T cell–mediated immune response to antigens, which causes not only surrounding pigment loss but also heralds the regression of central lesions.2 Others have suggested a vascular mechanism, with blood shunted away from the lesions.3 Because guidelines discourage biopsy of typical halo nevi, it becomes important to evaluate lesions for worrisome features such as ulceration or asymmetry, especially in older patients. We present a case of a pigmented basal cell carcinoma (BCC) that exhibited the halo phenomenon. Four other cases have been described in the literature.3-6
A 53-year-old man presented for evaluation of an asymptomatic lesion on the left side of the abdomen of approximately 8 months’ duration. He had no personal or family history of skin cancer. Physical examination revealed a central 1-cm, pink, verrucous papule surrounded by a 2×1.2-cm, depigmented, circular patch on the left side of the inferior abdomen (Figure 1). Upon questioning, the patient produced cell phone photographs of the trunk from 3 years prior, which did not show any lesions present. Full-body skin examination did not reveal any other concerning pigmented lesions. Excisional biopsy was performed due to concern for amelanotic melanoma, and histopathology revealed a superficial and pigmented BCC (Figure 2). Immunohistochemistry with Melan-A was negative for atypical melanocytes, with no uptake in the leukoderma areas.


The clinical presentation initially was concerning for amelanotic melanoma. All melanoma subtypes may appear as hypomelanotic lesions, though these most commonly are observed in the desmoplastic or nodular subtypes. Amelanotic melanomas may present as well-defined red or pink macules, plaques, or nodules, with some tumors presenting with light brown pigmentation.7
The differential diagnosis for lesions with the halo phenomenon is large. In adults, the halo phenomenon may be concerning for malignant or regressing melanoma. As an immunogenic tumor, melanoma’s immunogenic melanocytes may incite a cell-mediated immune response to antigens common to neoplastic and normal melanocytes, which can clinically manifest not only as local annular leukoderma but also as distant vitiligo or halo nevi.7 The halo phenomenon more commonly is associated with benign processes such as vitiligo and halo nevi in children. In most children, halo nevi occur as an isolated phenomenon but still warrant a complete skin examination for melanoma and vitiligo. The presence of halo nevi has been associated with distant vitiligo—possibly through shared immunologic mechanisms—especially if patients present with the Koebner phenomenon, multiple halo nevi, or a family history of vitiligo.8 A prospective study also found that the presence of halo nevi was an independent risk factor for the progression of segmental vitiligo to mixed vitiligo.9 Hormones also may play a role in the leukoderma acquisitum centrifugum, or halo, nevi. Halo nevi most commonly affect adolescents and pregnant women. It has been postulated that congenital nevi may be unique in their response to altered estrogen levels, increasing the rate not only of halo nevi but also of melanoma in pregnant women.10
Our patient’s final histologic diagnosis was pigmented BCC, which comprises only 6% of all BCCs.3 The proposed mechanism is that melanocytes colonize the tumor in the surrounding stroma and produce excess melanin. Basal cell carcinoma with halo phenomenon is a rare presentation. As in our case, 2 prior BCC reports also involved patients older than 50 years,3,5 with the 2 other cases describing women in their late twenties and early thirties.4,6 Additionally, 2 of 4 reports described patients with a history of multiple BCCs.3,5
In summary, the seemingly benign halo phenomenon may accompany malignant processes such as nonmelanoma skin cancer. Careful consideration of lesion time course and atypia is imperative for proper clinical suspicion in such cases.
- Mooney MA, Barr RJ, Buxton MG. Halo nevus or halo phenomenon? a study of 142 cases. J Cutan Pathol. 1995;22:342-348.
- Zeff RA, Freitag A, Grin CM, et al. The immune response in halo nevi. J Am Acad Dermatol. 1997;37:620-624.
- Johnson DB, Ceilley RI. Basal cell carcinoma with annular leukoderma mimicking leukoderma acquisitum centrifugum. Arch Dermatol. 1980;116:352-353.
- Basak PY, Meric G, Ciris M. Basal cell carcinoma with halo phenomenon in a young female: significance of dermatoscopy in early diagnosis. Indian J Dermatol. 2015;60:214.
- Pembroke AC, Liddell K. Basal cell epithelioma with a hypopigmented halo. Arch Dermatol. 1981;117:317.
- Rustemeyer J, Günther L, Deichert L. A rare association: basal cell carcinoma in a vitiliginous macula. Oral Maxillofac Surg. 2011;15:175-177.
- Naveh HP, Rao UN, Butterfield LH. Melanoma‐associated leukoderma—immunology in black and white? Pigment Cell Melanoma Res. 2013;26:796-804.
- Zhou H, Wu L-C, Chen M-K, et al. Factors associated with development of vitiligo in patients with halo nevus. Chinese Med J. 2017;130:2703.
- Ezzedine K, Diallo A, Léauté‐Labrèze C, et al. Halo naevi and leukotrichia are strong predictors of the passage to mixed vitiligo in a subgroup of segmental vitiligo. Br J Dermatol. 2012;166:539-544.
- Nading MA, Nanney LB, Ellis DL. Pregnancy and estrogen receptor β expression in a large congenital nevus. Arch Dermatol. 2009;145:691-694.
- Mooney MA, Barr RJ, Buxton MG. Halo nevus or halo phenomenon? a study of 142 cases. J Cutan Pathol. 1995;22:342-348.
- Zeff RA, Freitag A, Grin CM, et al. The immune response in halo nevi. J Am Acad Dermatol. 1997;37:620-624.
- Johnson DB, Ceilley RI. Basal cell carcinoma with annular leukoderma mimicking leukoderma acquisitum centrifugum. Arch Dermatol. 1980;116:352-353.
- Basak PY, Meric G, Ciris M. Basal cell carcinoma with halo phenomenon in a young female: significance of dermatoscopy in early diagnosis. Indian J Dermatol. 2015;60:214.
- Pembroke AC, Liddell K. Basal cell epithelioma with a hypopigmented halo. Arch Dermatol. 1981;117:317.
- Rustemeyer J, Günther L, Deichert L. A rare association: basal cell carcinoma in a vitiliginous macula. Oral Maxillofac Surg. 2011;15:175-177.
- Naveh HP, Rao UN, Butterfield LH. Melanoma‐associated leukoderma—immunology in black and white? Pigment Cell Melanoma Res. 2013;26:796-804.
- Zhou H, Wu L-C, Chen M-K, et al. Factors associated with development of vitiligo in patients with halo nevus. Chinese Med J. 2017;130:2703.
- Ezzedine K, Diallo A, Léauté‐Labrèze C, et al. Halo naevi and leukotrichia are strong predictors of the passage to mixed vitiligo in a subgroup of segmental vitiligo. Br J Dermatol. 2012;166:539-544.
- Nading MA, Nanney LB, Ellis DL. Pregnancy and estrogen receptor β expression in a large congenital nevus. Arch Dermatol. 2009;145:691-694.
Practice Points
- Annular leukoderma, or the halo phenomenon, is a circular reaction of hypopigmentation that more commonly is associated with benign processes such as halo nevi.
- The halo phenomenon may accompany malignant processes, such as nonmelanoma skin cancer. Careful consideration of lesion time course and atypia is imperative for proper clinical suspicion in such cases.