User login
Antibiotics and COPD: Time to order a C-reactive protein test?
This RCT provided valuable insights as to whether CRP-guided prescribing could safely reduce antibiotic use during acute COPD exacerbations.
ILLUSTRATIVE CASE
A 55-year-old man with a history of chronic obstructive pulmonary disease (COPD) presents to you with increased sputum volume and increased dyspnea, but no fever. You diagnose a COPD exacerbation. Would point-of-care C-reactive protein (CRP) testing be a useful tool to guide antibiotic prescribing?
COPD is a common respiratory condition and one of the leading causes of death in the world.2 COPD requires chronic therapy and frequent treatment for acute exacerbations.3 A systematic review found that exacerbations occur an average of 1.3 times per year for patients with known COPD.4 Antibiotics are often prescribed for COPD exacerbations, but which patients benefit most from antibiotic treatment is unclear and identification often is based on clinical features alone. Additionally, overprescribing of antibiotics can lead to unnecessary adverse effects, drive antibiotic resistance, and be a waste of resources.5
The European Respiratory Society/American Thoracic Society (ERS/ATS) provides a conditional recommendation to consider antibiotics in ambulatory patients with COPD exacerbation based on moderate-quality evidence.6 The 2020 Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend antibiotics for moderately or severely ill patients with a COPD exacerbation who have increased cough and sputum purulence.7 While the ERS/ATS recommendations do not mention CRP, the GOLD guidelines discuss biomarkers as emerging tools in determining antibiotic utility.
Biomarkers such as procalcitonin and CRP are being examined as potential tools to distinguish which patients would benefit from antibiotic treatment in COPD exacerbations. In a 2013 study, CRP levels > 19.6 mg/L in the serum and > 15.2 mg/L in the sputum indicated a bacterial infection, but more research was needed to determine if CRP could help guide antibiotic prescribing.8 In a 2019 randomized trial of 101 patients with COPD exacerbations, researchers compared the GOLD strategy for antibiotic prescribing with a CRP-guided antibiotic strategy (CRP ≥ 50 mg/L) and found no difference in adverse events between study groups.9
This trial focused on point-of-care CRP-guided prescribing of antibiotics for patients with COPD exacerbations in the outpatient setting.
STUDY SUMMARY
Point-of-care CRP testing is noninferior to usual care
This open-label, multicenter, randomized controlled trial at 86 general medical practices in the United Kingdom examined whether the use of point-of-care CRP testing could reduce antibiotic use during acute exacerbations of COPD. Patients (N = 653; 650 needed to provide 81% to 90% power) were ages 40 years and older, had a diagnosis of COPD, and presented for an acute exacerbation of COPD based on the presence of at least 1 Anthonisen criteria (increased dyspnea, increase in sputum volume, and increase in purulent sputum).
Patients were randomized in a 1:1 fashion to receive care guided by point-of-care CRP testing (CRP-guided) or usual care for their COPD exacerbation. Patients in the CRP-guided group received a point-of-care CRP test as part of their assessment at presentation, or at any other appointments for COPD over the following 4 weeks.
The research team provided clinicians with CRP interpretation guidance based on the following CRP values: < 20 mg/L, antibiotics are typically not needed; 20 to 40 mg/L, antibiotics might be beneficial if purulent sputum is present; and > 40 mg/L, antibiotics are usually beneficial. Primary outcomes were patient-reported antibiotic use within 4 weeks and COPD-related health status. Of the patients who received a point-of-care CRP test, the median value was 6 mg/L; 76% had a value < 20 mg/L, 12% had values between 20 and 40 mg/L, and 12% had values > 40 mg/L. In the intention-to-treat analysis, fewer patients in the CRP-guided group reported antibiotic use vs those in the usual-care group (57% vs 77%; adjusted odds ratio [aOR] = 0.31; 95% CI, 0.20-0.47) within 4 weeks. The CRP-guided group also received fewer antibiotics at the initial visit compared to the usual-care group (48% vs 70%; aOR = 0.31; 95% CI, 0.21-0.45).
COPD-related health status was assessed with the Clinical COPD Questionnaire (score range, 0-6; a difference of 0.4 represents minimal clinical importance). At 2 weeks, the adjusted mean difference in the total health status score with the use of CRP was noninferior to usual care and was in favor of the CRP-guided group (mean difference = −0.19 points; two-sided 90% CI, −0.33 to −0.05). There was no evidence of clinically important between-group differences in pneumonia (3% vs 4%; aOR = 0.73; 95% CI, 0.29-1.82) at 6-month follow-up. Rates of hospitalization at 6 months were similar between groups (9.3% vs 8.6%; no P value provided).
Limitations of this trial included patient report of antibiotic use and the lack of a sham test.
WHAT'S NEW
RCT provides evidence to support use of CRP testing
Point-of-care CRP testing can reduce antibiotic prescribing in patients presenting with a COPD exacerbation without affecting symptom improvement or adverse events.
CAVEATS
CRP testing may not be cost effective
CRP testing—especially point-of-care testing—remains expensive in many parts of the United States. A 2015 cost-effectiveness analysis of point-of-care CRP tests for respiratory tract infection in England concluded the cost of the test per patient was not cost effective.10 It is unknown if point-of-care CRP testing would be cost effective in guiding antibiotic prescribing for primary care providers with a focus on COPD exacerbations.
CHALLENGES TO IMPLEMENTATION
Virtual visits and variable access may limit use
CRP-guided antibiotic prescribing may be challenging in some clinical scenarios or clinics with the rise of virtual visits and differential access in primary care clinics to point-of-care CRP tests. JFP
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health. Copyright © 2021. The Family Physicians Inquiries Network. All rights reserved.
- Butler CC, Gillespie D, White P, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med. 2019;381:111-120.
- Lopez AD, Mathers CD, Ezzati M, et al. Global Burden of Disease and Risk Factors. The World Bank; 2006.
- Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741-750.
- Singh J, Palda V, Stanbrook M, et al. Corticosteroid therapy for patients with acute exacerbations of chronic obstructive pulmonary disease: a systematic review. Arch Intern Med. 2002;162:2527-2536.
- Schroeck JL, Ruh CA, Sellick JA, et al. Factors associated with antibiotic misuse in outpatient treatment for upper respiratory tract infections. Antimicrob Agents Chemother. 2015;59:3848-3852.
- Wedzicha JA, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;49:1600791.
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, and Management and Prevention of Chronic Obstructive Pulmonary Disease (2020 report). Accessed May 12, 2021. https://goldcopd.org/gold-reports/
- Peng C, Tian C, Zhang Y, et al. C-reactive protein levels predict bacterial exacerbation in patients with chronic obstructive pulmonary disease. Am J Med Sci. 2013;345:190-194.
- Prins H, Duijkers R, van der Valk P, et al. CRP-guided antibiotic treatment in acute exacerbations of COPD in hospital admissions. Eur Respir J. 2019;53:1802014.
- Hunter R. Cost-effectiveness of point-of-care C-reactive protein tests for respiratory tract infection in primary care in England. Adv Ther. 2015;32:69-85.
This RCT provided valuable insights as to whether CRP-guided prescribing could safely reduce antibiotic use during acute COPD exacerbations.
This RCT provided valuable insights as to whether CRP-guided prescribing could safely reduce antibiotic use during acute COPD exacerbations.
ILLUSTRATIVE CASE
A 55-year-old man with a history of chronic obstructive pulmonary disease (COPD) presents to you with increased sputum volume and increased dyspnea, but no fever. You diagnose a COPD exacerbation. Would point-of-care C-reactive protein (CRP) testing be a useful tool to guide antibiotic prescribing?
COPD is a common respiratory condition and one of the leading causes of death in the world.2 COPD requires chronic therapy and frequent treatment for acute exacerbations.3 A systematic review found that exacerbations occur an average of 1.3 times per year for patients with known COPD.4 Antibiotics are often prescribed for COPD exacerbations, but which patients benefit most from antibiotic treatment is unclear and identification often is based on clinical features alone. Additionally, overprescribing of antibiotics can lead to unnecessary adverse effects, drive antibiotic resistance, and be a waste of resources.5
The European Respiratory Society/American Thoracic Society (ERS/ATS) provides a conditional recommendation to consider antibiotics in ambulatory patients with COPD exacerbation based on moderate-quality evidence.6 The 2020 Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend antibiotics for moderately or severely ill patients with a COPD exacerbation who have increased cough and sputum purulence.7 While the ERS/ATS recommendations do not mention CRP, the GOLD guidelines discuss biomarkers as emerging tools in determining antibiotic utility.
Biomarkers such as procalcitonin and CRP are being examined as potential tools to distinguish which patients would benefit from antibiotic treatment in COPD exacerbations. In a 2013 study, CRP levels > 19.6 mg/L in the serum and > 15.2 mg/L in the sputum indicated a bacterial infection, but more research was needed to determine if CRP could help guide antibiotic prescribing.8 In a 2019 randomized trial of 101 patients with COPD exacerbations, researchers compared the GOLD strategy for antibiotic prescribing with a CRP-guided antibiotic strategy (CRP ≥ 50 mg/L) and found no difference in adverse events between study groups.9
This trial focused on point-of-care CRP-guided prescribing of antibiotics for patients with COPD exacerbations in the outpatient setting.
STUDY SUMMARY
Point-of-care CRP testing is noninferior to usual care
This open-label, multicenter, randomized controlled trial at 86 general medical practices in the United Kingdom examined whether the use of point-of-care CRP testing could reduce antibiotic use during acute exacerbations of COPD. Patients (N = 653; 650 needed to provide 81% to 90% power) were ages 40 years and older, had a diagnosis of COPD, and presented for an acute exacerbation of COPD based on the presence of at least 1 Anthonisen criteria (increased dyspnea, increase in sputum volume, and increase in purulent sputum).
Patients were randomized in a 1:1 fashion to receive care guided by point-of-care CRP testing (CRP-guided) or usual care for their COPD exacerbation. Patients in the CRP-guided group received a point-of-care CRP test as part of their assessment at presentation, or at any other appointments for COPD over the following 4 weeks.
The research team provided clinicians with CRP interpretation guidance based on the following CRP values: < 20 mg/L, antibiotics are typically not needed; 20 to 40 mg/L, antibiotics might be beneficial if purulent sputum is present; and > 40 mg/L, antibiotics are usually beneficial. Primary outcomes were patient-reported antibiotic use within 4 weeks and COPD-related health status. Of the patients who received a point-of-care CRP test, the median value was 6 mg/L; 76% had a value < 20 mg/L, 12% had values between 20 and 40 mg/L, and 12% had values > 40 mg/L. In the intention-to-treat analysis, fewer patients in the CRP-guided group reported antibiotic use vs those in the usual-care group (57% vs 77%; adjusted odds ratio [aOR] = 0.31; 95% CI, 0.20-0.47) within 4 weeks. The CRP-guided group also received fewer antibiotics at the initial visit compared to the usual-care group (48% vs 70%; aOR = 0.31; 95% CI, 0.21-0.45).
COPD-related health status was assessed with the Clinical COPD Questionnaire (score range, 0-6; a difference of 0.4 represents minimal clinical importance). At 2 weeks, the adjusted mean difference in the total health status score with the use of CRP was noninferior to usual care and was in favor of the CRP-guided group (mean difference = −0.19 points; two-sided 90% CI, −0.33 to −0.05). There was no evidence of clinically important between-group differences in pneumonia (3% vs 4%; aOR = 0.73; 95% CI, 0.29-1.82) at 6-month follow-up. Rates of hospitalization at 6 months were similar between groups (9.3% vs 8.6%; no P value provided).
Limitations of this trial included patient report of antibiotic use and the lack of a sham test.
WHAT'S NEW
RCT provides evidence to support use of CRP testing
Point-of-care CRP testing can reduce antibiotic prescribing in patients presenting with a COPD exacerbation without affecting symptom improvement or adverse events.
CAVEATS
CRP testing may not be cost effective
CRP testing—especially point-of-care testing—remains expensive in many parts of the United States. A 2015 cost-effectiveness analysis of point-of-care CRP tests for respiratory tract infection in England concluded the cost of the test per patient was not cost effective.10 It is unknown if point-of-care CRP testing would be cost effective in guiding antibiotic prescribing for primary care providers with a focus on COPD exacerbations.
CHALLENGES TO IMPLEMENTATION
Virtual visits and variable access may limit use
CRP-guided antibiotic prescribing may be challenging in some clinical scenarios or clinics with the rise of virtual visits and differential access in primary care clinics to point-of-care CRP tests. JFP
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health. Copyright © 2021. The Family Physicians Inquiries Network. All rights reserved.
ILLUSTRATIVE CASE
A 55-year-old man with a history of chronic obstructive pulmonary disease (COPD) presents to you with increased sputum volume and increased dyspnea, but no fever. You diagnose a COPD exacerbation. Would point-of-care C-reactive protein (CRP) testing be a useful tool to guide antibiotic prescribing?
COPD is a common respiratory condition and one of the leading causes of death in the world.2 COPD requires chronic therapy and frequent treatment for acute exacerbations.3 A systematic review found that exacerbations occur an average of 1.3 times per year for patients with known COPD.4 Antibiotics are often prescribed for COPD exacerbations, but which patients benefit most from antibiotic treatment is unclear and identification often is based on clinical features alone. Additionally, overprescribing of antibiotics can lead to unnecessary adverse effects, drive antibiotic resistance, and be a waste of resources.5
The European Respiratory Society/American Thoracic Society (ERS/ATS) provides a conditional recommendation to consider antibiotics in ambulatory patients with COPD exacerbation based on moderate-quality evidence.6 The 2020 Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend antibiotics for moderately or severely ill patients with a COPD exacerbation who have increased cough and sputum purulence.7 While the ERS/ATS recommendations do not mention CRP, the GOLD guidelines discuss biomarkers as emerging tools in determining antibiotic utility.
Biomarkers such as procalcitonin and CRP are being examined as potential tools to distinguish which patients would benefit from antibiotic treatment in COPD exacerbations. In a 2013 study, CRP levels > 19.6 mg/L in the serum and > 15.2 mg/L in the sputum indicated a bacterial infection, but more research was needed to determine if CRP could help guide antibiotic prescribing.8 In a 2019 randomized trial of 101 patients with COPD exacerbations, researchers compared the GOLD strategy for antibiotic prescribing with a CRP-guided antibiotic strategy (CRP ≥ 50 mg/L) and found no difference in adverse events between study groups.9
This trial focused on point-of-care CRP-guided prescribing of antibiotics for patients with COPD exacerbations in the outpatient setting.
STUDY SUMMARY
Point-of-care CRP testing is noninferior to usual care
This open-label, multicenter, randomized controlled trial at 86 general medical practices in the United Kingdom examined whether the use of point-of-care CRP testing could reduce antibiotic use during acute exacerbations of COPD. Patients (N = 653; 650 needed to provide 81% to 90% power) were ages 40 years and older, had a diagnosis of COPD, and presented for an acute exacerbation of COPD based on the presence of at least 1 Anthonisen criteria (increased dyspnea, increase in sputum volume, and increase in purulent sputum).
Patients were randomized in a 1:1 fashion to receive care guided by point-of-care CRP testing (CRP-guided) or usual care for their COPD exacerbation. Patients in the CRP-guided group received a point-of-care CRP test as part of their assessment at presentation, or at any other appointments for COPD over the following 4 weeks.
The research team provided clinicians with CRP interpretation guidance based on the following CRP values: < 20 mg/L, antibiotics are typically not needed; 20 to 40 mg/L, antibiotics might be beneficial if purulent sputum is present; and > 40 mg/L, antibiotics are usually beneficial. Primary outcomes were patient-reported antibiotic use within 4 weeks and COPD-related health status. Of the patients who received a point-of-care CRP test, the median value was 6 mg/L; 76% had a value < 20 mg/L, 12% had values between 20 and 40 mg/L, and 12% had values > 40 mg/L. In the intention-to-treat analysis, fewer patients in the CRP-guided group reported antibiotic use vs those in the usual-care group (57% vs 77%; adjusted odds ratio [aOR] = 0.31; 95% CI, 0.20-0.47) within 4 weeks. The CRP-guided group also received fewer antibiotics at the initial visit compared to the usual-care group (48% vs 70%; aOR = 0.31; 95% CI, 0.21-0.45).
COPD-related health status was assessed with the Clinical COPD Questionnaire (score range, 0-6; a difference of 0.4 represents minimal clinical importance). At 2 weeks, the adjusted mean difference in the total health status score with the use of CRP was noninferior to usual care and was in favor of the CRP-guided group (mean difference = −0.19 points; two-sided 90% CI, −0.33 to −0.05). There was no evidence of clinically important between-group differences in pneumonia (3% vs 4%; aOR = 0.73; 95% CI, 0.29-1.82) at 6-month follow-up. Rates of hospitalization at 6 months were similar between groups (9.3% vs 8.6%; no P value provided).
Limitations of this trial included patient report of antibiotic use and the lack of a sham test.
WHAT'S NEW
RCT provides evidence to support use of CRP testing
Point-of-care CRP testing can reduce antibiotic prescribing in patients presenting with a COPD exacerbation without affecting symptom improvement or adverse events.
CAVEATS
CRP testing may not be cost effective
CRP testing—especially point-of-care testing—remains expensive in many parts of the United States. A 2015 cost-effectiveness analysis of point-of-care CRP tests for respiratory tract infection in England concluded the cost of the test per patient was not cost effective.10 It is unknown if point-of-care CRP testing would be cost effective in guiding antibiotic prescribing for primary care providers with a focus on COPD exacerbations.
CHALLENGES TO IMPLEMENTATION
Virtual visits and variable access may limit use
CRP-guided antibiotic prescribing may be challenging in some clinical scenarios or clinics with the rise of virtual visits and differential access in primary care clinics to point-of-care CRP tests. JFP
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health. Copyright © 2021. The Family Physicians Inquiries Network. All rights reserved.
- Butler CC, Gillespie D, White P, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med. 2019;381:111-120.
- Lopez AD, Mathers CD, Ezzati M, et al. Global Burden of Disease and Risk Factors. The World Bank; 2006.
- Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741-750.
- Singh J, Palda V, Stanbrook M, et al. Corticosteroid therapy for patients with acute exacerbations of chronic obstructive pulmonary disease: a systematic review. Arch Intern Med. 2002;162:2527-2536.
- Schroeck JL, Ruh CA, Sellick JA, et al. Factors associated with antibiotic misuse in outpatient treatment for upper respiratory tract infections. Antimicrob Agents Chemother. 2015;59:3848-3852.
- Wedzicha JA, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;49:1600791.
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, and Management and Prevention of Chronic Obstructive Pulmonary Disease (2020 report). Accessed May 12, 2021. https://goldcopd.org/gold-reports/
- Peng C, Tian C, Zhang Y, et al. C-reactive protein levels predict bacterial exacerbation in patients with chronic obstructive pulmonary disease. Am J Med Sci. 2013;345:190-194.
- Prins H, Duijkers R, van der Valk P, et al. CRP-guided antibiotic treatment in acute exacerbations of COPD in hospital admissions. Eur Respir J. 2019;53:1802014.
- Hunter R. Cost-effectiveness of point-of-care C-reactive protein tests for respiratory tract infection in primary care in England. Adv Ther. 2015;32:69-85.
- Butler CC, Gillespie D, White P, et al. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med. 2019;381:111-120.
- Lopez AD, Mathers CD, Ezzati M, et al. Global Burden of Disease and Risk Factors. The World Bank; 2006.
- Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741-750.
- Singh J, Palda V, Stanbrook M, et al. Corticosteroid therapy for patients with acute exacerbations of chronic obstructive pulmonary disease: a systematic review. Arch Intern Med. 2002;162:2527-2536.
- Schroeck JL, Ruh CA, Sellick JA, et al. Factors associated with antibiotic misuse in outpatient treatment for upper respiratory tract infections. Antimicrob Agents Chemother. 2015;59:3848-3852.
- Wedzicha JA, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;49:1600791.
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, and Management and Prevention of Chronic Obstructive Pulmonary Disease (2020 report). Accessed May 12, 2021. https://goldcopd.org/gold-reports/
- Peng C, Tian C, Zhang Y, et al. C-reactive protein levels predict bacterial exacerbation in patients with chronic obstructive pulmonary disease. Am J Med Sci. 2013;345:190-194.
- Prins H, Duijkers R, van der Valk P, et al. CRP-guided antibiotic treatment in acute exacerbations of COPD in hospital admissions. Eur Respir J. 2019;53:1802014.
- Hunter R. Cost-effectiveness of point-of-care C-reactive protein tests for respiratory tract infection in primary care in England. Adv Ther. 2015;32:69-85.
PRACTICE CHANGER
Consider C-reactive protein–guided prescribing of antibiotics in acute chronic obstructive pulmonary disease exacerbations in the outpatient setting, as it results in fewer antibiotic prescriptions without adverse effects.1
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial.1
Butler CC, Gillespie D, White P, et al. C-Reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N Engl J Med. 2019;381:111-120.
To screen or not to screen children for hypertension?
In this issue of JFP, Smith et al recommend following guidelines from the American Academy of Pediatrics to annually screen children for hypertension (see page 220). This recommendation appears to be at odds with the recent US Preventive Services Task Force (USPSTF) statement that concluded there is insufficient evidence for screening children and adolescents for hypertension. But an “I” recommendation from the USPSTF is not the same as a “D” recommendation. “D” means don’t do it, because the evidence indicates that the harms outweigh the benefits. “I” means we don’t have enough evidence to weigh the harms and benefits, so it is up to you and your patients to decide what to do.
So whose recommendations should we follow?
Our decision should be based on a thorough understanding of the evidence, and that evidence is well summarized in the recent USPSTF report.1 The reviewers found no studies that evaluated the benefits and harms of screening children and adolescents for hypertension and no studies evaluating disease outcomes from treating hypertension in these patients.
There is, however, an association between elevated blood pressure in childhood and outcomes such as left ventricular hypertrophy and carotid intimal thickness.2 Some physicians contend that these “disease-oriented outcomes” are sufficient reason to identify and treat hypertension in children and adolescents.3 The USPSTF, however, requires a higher level of evidence that includes patient-oriented outcomes, such as a lower risk of congestive heart failure, renal failure, or death, before recommending treatment. Physicians and patients have to choose what level of evidence is sufficient to take action.
Dr. Smith comments: “As noted in their report, the USPSTF acknowledges that observational studies indicate an association between hypertension in childhood and hypertension in adulthood, but there have been no randomized trials to determine if treating hypertension in children and adolescents reduces risk of cardiovascular events. Although it is a cohort study, not a randomized trial, the ongoing i3C Consortium Outcomes Study4 may provide better information to guide decision-making for children and adolescents with elevated blood pressure.”
What we can all agree on is that, when hypertension is identified in a child or adolescent, it is important to determine if there is a treatable cause of elevated blood pressure such as coarctation of the aorta or renal disease. It is also important to address risk factors for elevated blood pressure and cardiovascular disease, such as obesity, poor dietary habits, and smoking. The treatment is lifestyle modification with diet, exercise, and smoking cessation.
- USPSTF: High blood pressure in children and adolescents: screening. Accessed June 2, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
- Yang L, Magnussen CG, Yang L, et al. Elevated blood pressure in childhood or adolescence and cardiovascular outcomes in adulthood: a systematic review. Hypertension. 2020;75:948–955. doi: 10.1161/hypertensionaha.119.14168
- Falkner B, Lurbe E. The USPSTF call to inaction on blood pressure screening in children and adolescents. Pediatr Nephrol. 2021;36:1327-1329. doi: 10.1007/s00467-021-04926-y
- Sinaiko AR, Jacobs DR Jr, Woo JG, et al. The International Childhood Cardiovascular Cohort (i3C) consortium outcomes study of childhood cardiovascular risk factors and adult cardiovascular morbidity and mortality: Design and recruitment. Contemp Clin Trials. 2018;69:55-64. doi: 10.1016/j.cct.2018.04.009
In this issue of JFP, Smith et al recommend following guidelines from the American Academy of Pediatrics to annually screen children for hypertension (see page 220). This recommendation appears to be at odds with the recent US Preventive Services Task Force (USPSTF) statement that concluded there is insufficient evidence for screening children and adolescents for hypertension. But an “I” recommendation from the USPSTF is not the same as a “D” recommendation. “D” means don’t do it, because the evidence indicates that the harms outweigh the benefits. “I” means we don’t have enough evidence to weigh the harms and benefits, so it is up to you and your patients to decide what to do.
So whose recommendations should we follow?
Our decision should be based on a thorough understanding of the evidence, and that evidence is well summarized in the recent USPSTF report.1 The reviewers found no studies that evaluated the benefits and harms of screening children and adolescents for hypertension and no studies evaluating disease outcomes from treating hypertension in these patients.
There is, however, an association between elevated blood pressure in childhood and outcomes such as left ventricular hypertrophy and carotid intimal thickness.2 Some physicians contend that these “disease-oriented outcomes” are sufficient reason to identify and treat hypertension in children and adolescents.3 The USPSTF, however, requires a higher level of evidence that includes patient-oriented outcomes, such as a lower risk of congestive heart failure, renal failure, or death, before recommending treatment. Physicians and patients have to choose what level of evidence is sufficient to take action.
Dr. Smith comments: “As noted in their report, the USPSTF acknowledges that observational studies indicate an association between hypertension in childhood and hypertension in adulthood, but there have been no randomized trials to determine if treating hypertension in children and adolescents reduces risk of cardiovascular events. Although it is a cohort study, not a randomized trial, the ongoing i3C Consortium Outcomes Study4 may provide better information to guide decision-making for children and adolescents with elevated blood pressure.”
What we can all agree on is that, when hypertension is identified in a child or adolescent, it is important to determine if there is a treatable cause of elevated blood pressure such as coarctation of the aorta or renal disease. It is also important to address risk factors for elevated blood pressure and cardiovascular disease, such as obesity, poor dietary habits, and smoking. The treatment is lifestyle modification with diet, exercise, and smoking cessation.
In this issue of JFP, Smith et al recommend following guidelines from the American Academy of Pediatrics to annually screen children for hypertension (see page 220). This recommendation appears to be at odds with the recent US Preventive Services Task Force (USPSTF) statement that concluded there is insufficient evidence for screening children and adolescents for hypertension. But an “I” recommendation from the USPSTF is not the same as a “D” recommendation. “D” means don’t do it, because the evidence indicates that the harms outweigh the benefits. “I” means we don’t have enough evidence to weigh the harms and benefits, so it is up to you and your patients to decide what to do.
So whose recommendations should we follow?
Our decision should be based on a thorough understanding of the evidence, and that evidence is well summarized in the recent USPSTF report.1 The reviewers found no studies that evaluated the benefits and harms of screening children and adolescents for hypertension and no studies evaluating disease outcomes from treating hypertension in these patients.
There is, however, an association between elevated blood pressure in childhood and outcomes such as left ventricular hypertrophy and carotid intimal thickness.2 Some physicians contend that these “disease-oriented outcomes” are sufficient reason to identify and treat hypertension in children and adolescents.3 The USPSTF, however, requires a higher level of evidence that includes patient-oriented outcomes, such as a lower risk of congestive heart failure, renal failure, or death, before recommending treatment. Physicians and patients have to choose what level of evidence is sufficient to take action.
Dr. Smith comments: “As noted in their report, the USPSTF acknowledges that observational studies indicate an association between hypertension in childhood and hypertension in adulthood, but there have been no randomized trials to determine if treating hypertension in children and adolescents reduces risk of cardiovascular events. Although it is a cohort study, not a randomized trial, the ongoing i3C Consortium Outcomes Study4 may provide better information to guide decision-making for children and adolescents with elevated blood pressure.”
What we can all agree on is that, when hypertension is identified in a child or adolescent, it is important to determine if there is a treatable cause of elevated blood pressure such as coarctation of the aorta or renal disease. It is also important to address risk factors for elevated blood pressure and cardiovascular disease, such as obesity, poor dietary habits, and smoking. The treatment is lifestyle modification with diet, exercise, and smoking cessation.
- USPSTF: High blood pressure in children and adolescents: screening. Accessed June 2, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
- Yang L, Magnussen CG, Yang L, et al. Elevated blood pressure in childhood or adolescence and cardiovascular outcomes in adulthood: a systematic review. Hypertension. 2020;75:948–955. doi: 10.1161/hypertensionaha.119.14168
- Falkner B, Lurbe E. The USPSTF call to inaction on blood pressure screening in children and adolescents. Pediatr Nephrol. 2021;36:1327-1329. doi: 10.1007/s00467-021-04926-y
- Sinaiko AR, Jacobs DR Jr, Woo JG, et al. The International Childhood Cardiovascular Cohort (i3C) consortium outcomes study of childhood cardiovascular risk factors and adult cardiovascular morbidity and mortality: Design and recruitment. Contemp Clin Trials. 2018;69:55-64. doi: 10.1016/j.cct.2018.04.009
- USPSTF: High blood pressure in children and adolescents: screening. Accessed June 2, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
- Yang L, Magnussen CG, Yang L, et al. Elevated blood pressure in childhood or adolescence and cardiovascular outcomes in adulthood: a systematic review. Hypertension. 2020;75:948–955. doi: 10.1161/hypertensionaha.119.14168
- Falkner B, Lurbe E. The USPSTF call to inaction on blood pressure screening in children and adolescents. Pediatr Nephrol. 2021;36:1327-1329. doi: 10.1007/s00467-021-04926-y
- Sinaiko AR, Jacobs DR Jr, Woo JG, et al. The International Childhood Cardiovascular Cohort (i3C) consortium outcomes study of childhood cardiovascular risk factors and adult cardiovascular morbidity and mortality: Design and recruitment. Contemp Clin Trials. 2018;69:55-64. doi: 10.1016/j.cct.2018.04.009
DCIS: Biosignature helps guide postlumpectomy decisions
A biosignature tool helps women avoid unnecessary radiotherapy after undergoing lumpectomy for ductal carcinoma in situ (DCIS) – and also identifies women who need more intense treatment.
The DCISionRT test (PreludeDx) and its response subtype (Rst) biosignature provide personalized risk assessment, explains Frank Vicini, MD, a radiation oncologist at GenesisCare and a member of NRG Oncology, Pontiac, Mich.
He presented data on the test at a poster at the recent American Society of Clinical Oncology Annual Meeting.
They can also identify patients who would likely benefit from radiotherapy, Dr. Vicini reported.
The tool shows promise for identifying those whose cancer is likely to recur despite undergoing postlumpectomy radiotherapy – women who might benefit from intensified or alternate treatment approaches, he added.
The latter finding is particularly provocative because it suggests that the biosignatures “may appropriately identify patients with very radioresistant ductal carcinoma in situ,” Benjamin D. Smith, MD, commented during a poster discussion session at the meeting.
“I think these findings merit validation in translational research models,” said Dr. Smith, a radiation oncologist and professor of radiation oncology and health services research at the University of Texas MD Anderson Cancer Center, Houston.
DCISionRT, Rst, and risk
DCISionRT combines molecular biology innovations with risk-based scores to assess risk for recurrence, which is classified as either low or elevated, according to the test developer, PreludeDx.
Dr. Vicini and colleagues used the test to classify tissue samples from 485 women who were part of previous DCISionRT validation cohorts in Sweden, Australia, and the United States. The patients underwent breast cancer surgery (BCS) with or without radiotherapy between 1996 and 2011.
The Rst biosignature was used to further categorize those in the elevated-risk group as having a good response subtype (good Rst) or a poor response subtype (poor Rst) after BCS plus radiotherapy.
Radiotherapy was associated with significantly reduced recurrence rates among women with elevated risk and a good Rst (the hazard ratios for ipsilateral breast tumor recurrence [IBTR] and invasive breast cancer [IBC] were 0.18 and 0.15, respectively).
No radiotherapy benefit was seen among those with elevated risk and poor Rst.
The investigators also reported that, among patients with a poor Rst, 10-year IBTR and IBC rates were 25% and 16%, respectively, regardless of whether they received radiotherapy. These rates were much higher than the rates among women with good Rst (6.6% and 4.5%; hazard ratio, 3.6 and 4.4, respectively).
No significant difference was seen in 10-year IBTR and IBC rates among patients in the low-risk group, with or without radiotherapy.
Traditional clinicopathologic risk factors, including age younger than 50 years, grade 3 disease, and tumor size greater than 2.5 cm, did not identify poor versus good response subtypes in this cohort, and on multivariable analysis, neither of these factors nor endocrine therapy was significantly associated with IBTR or IBC.
Prospective validation needed
In his discussion, Dr. Smith said that the study provides “important data” that further validate the DCISionRT platform alone for assessing risk among women with DCIS who undergo BCS. But it is the Rst biosignature, which allows clinicians to “predict radioresistance of residual malignant chromogens following lumpectomy plus radiation therapy,” that really stands out, he added.
From the data presented, “it is reasonable to conclude that patients with a poor Rst score treated with lumpectomy and radiation had a much higher risk of in-breast tumor recurrence than one might predict or anticipate based on existing published randomized clinical trial data,” he said.
“In my opinion, it is very important to prospectively validate this finding with other cohorts,” he said. “Moving forward, I think there may come a time where there may be interest in studying radiosensitizing agents for poor-Rst ductal carcinoma in situ that are resistant to standard doses of radiation, and it may be that we consider the Rst as a factor moving forward in selecting patients for BCT versus mastectomy.”
However, because 75% of patients at elevated risk with poor Rst who undergo lumpectomy and radiotherapy do not experience recurrence in the decade following their treatment, it would be “inappropriate and misguided” to start recommending mastectomy for patients at DCISionRT elevated risk who have poor Rst, he said.
The study was funded by PreludeDx. Dr. Vicini reported employment with 21st Century Oncology and financial relationships with ImpediMed, Prelude Therapeutics, and Concure Oncology. Dr. Smith, through his employer, has an equity interest in Oncora Medical through a partnership agreement. He also has an uncompensated relationship with the American Society for Radiation Oncology.
A version of this article first appeared on Medscape.com.
A biosignature tool helps women avoid unnecessary radiotherapy after undergoing lumpectomy for ductal carcinoma in situ (DCIS) – and also identifies women who need more intense treatment.
The DCISionRT test (PreludeDx) and its response subtype (Rst) biosignature provide personalized risk assessment, explains Frank Vicini, MD, a radiation oncologist at GenesisCare and a member of NRG Oncology, Pontiac, Mich.
He presented data on the test at a poster at the recent American Society of Clinical Oncology Annual Meeting.
They can also identify patients who would likely benefit from radiotherapy, Dr. Vicini reported.
The tool shows promise for identifying those whose cancer is likely to recur despite undergoing postlumpectomy radiotherapy – women who might benefit from intensified or alternate treatment approaches, he added.
The latter finding is particularly provocative because it suggests that the biosignatures “may appropriately identify patients with very radioresistant ductal carcinoma in situ,” Benjamin D. Smith, MD, commented during a poster discussion session at the meeting.
“I think these findings merit validation in translational research models,” said Dr. Smith, a radiation oncologist and professor of radiation oncology and health services research at the University of Texas MD Anderson Cancer Center, Houston.
DCISionRT, Rst, and risk
DCISionRT combines molecular biology innovations with risk-based scores to assess risk for recurrence, which is classified as either low or elevated, according to the test developer, PreludeDx.
Dr. Vicini and colleagues used the test to classify tissue samples from 485 women who were part of previous DCISionRT validation cohorts in Sweden, Australia, and the United States. The patients underwent breast cancer surgery (BCS) with or without radiotherapy between 1996 and 2011.
The Rst biosignature was used to further categorize those in the elevated-risk group as having a good response subtype (good Rst) or a poor response subtype (poor Rst) after BCS plus radiotherapy.
Radiotherapy was associated with significantly reduced recurrence rates among women with elevated risk and a good Rst (the hazard ratios for ipsilateral breast tumor recurrence [IBTR] and invasive breast cancer [IBC] were 0.18 and 0.15, respectively).
No radiotherapy benefit was seen among those with elevated risk and poor Rst.
The investigators also reported that, among patients with a poor Rst, 10-year IBTR and IBC rates were 25% and 16%, respectively, regardless of whether they received radiotherapy. These rates were much higher than the rates among women with good Rst (6.6% and 4.5%; hazard ratio, 3.6 and 4.4, respectively).
No significant difference was seen in 10-year IBTR and IBC rates among patients in the low-risk group, with or without radiotherapy.
Traditional clinicopathologic risk factors, including age younger than 50 years, grade 3 disease, and tumor size greater than 2.5 cm, did not identify poor versus good response subtypes in this cohort, and on multivariable analysis, neither of these factors nor endocrine therapy was significantly associated with IBTR or IBC.
Prospective validation needed
In his discussion, Dr. Smith said that the study provides “important data” that further validate the DCISionRT platform alone for assessing risk among women with DCIS who undergo BCS. But it is the Rst biosignature, which allows clinicians to “predict radioresistance of residual malignant chromogens following lumpectomy plus radiation therapy,” that really stands out, he added.
From the data presented, “it is reasonable to conclude that patients with a poor Rst score treated with lumpectomy and radiation had a much higher risk of in-breast tumor recurrence than one might predict or anticipate based on existing published randomized clinical trial data,” he said.
“In my opinion, it is very important to prospectively validate this finding with other cohorts,” he said. “Moving forward, I think there may come a time where there may be interest in studying radiosensitizing agents for poor-Rst ductal carcinoma in situ that are resistant to standard doses of radiation, and it may be that we consider the Rst as a factor moving forward in selecting patients for BCT versus mastectomy.”
However, because 75% of patients at elevated risk with poor Rst who undergo lumpectomy and radiotherapy do not experience recurrence in the decade following their treatment, it would be “inappropriate and misguided” to start recommending mastectomy for patients at DCISionRT elevated risk who have poor Rst, he said.
The study was funded by PreludeDx. Dr. Vicini reported employment with 21st Century Oncology and financial relationships with ImpediMed, Prelude Therapeutics, and Concure Oncology. Dr. Smith, through his employer, has an equity interest in Oncora Medical through a partnership agreement. He also has an uncompensated relationship with the American Society for Radiation Oncology.
A version of this article first appeared on Medscape.com.
A biosignature tool helps women avoid unnecessary radiotherapy after undergoing lumpectomy for ductal carcinoma in situ (DCIS) – and also identifies women who need more intense treatment.
The DCISionRT test (PreludeDx) and its response subtype (Rst) biosignature provide personalized risk assessment, explains Frank Vicini, MD, a radiation oncologist at GenesisCare and a member of NRG Oncology, Pontiac, Mich.
He presented data on the test at a poster at the recent American Society of Clinical Oncology Annual Meeting.
They can also identify patients who would likely benefit from radiotherapy, Dr. Vicini reported.
The tool shows promise for identifying those whose cancer is likely to recur despite undergoing postlumpectomy radiotherapy – women who might benefit from intensified or alternate treatment approaches, he added.
The latter finding is particularly provocative because it suggests that the biosignatures “may appropriately identify patients with very radioresistant ductal carcinoma in situ,” Benjamin D. Smith, MD, commented during a poster discussion session at the meeting.
“I think these findings merit validation in translational research models,” said Dr. Smith, a radiation oncologist and professor of radiation oncology and health services research at the University of Texas MD Anderson Cancer Center, Houston.
DCISionRT, Rst, and risk
DCISionRT combines molecular biology innovations with risk-based scores to assess risk for recurrence, which is classified as either low or elevated, according to the test developer, PreludeDx.
Dr. Vicini and colleagues used the test to classify tissue samples from 485 women who were part of previous DCISionRT validation cohorts in Sweden, Australia, and the United States. The patients underwent breast cancer surgery (BCS) with or without radiotherapy between 1996 and 2011.
The Rst biosignature was used to further categorize those in the elevated-risk group as having a good response subtype (good Rst) or a poor response subtype (poor Rst) after BCS plus radiotherapy.
Radiotherapy was associated with significantly reduced recurrence rates among women with elevated risk and a good Rst (the hazard ratios for ipsilateral breast tumor recurrence [IBTR] and invasive breast cancer [IBC] were 0.18 and 0.15, respectively).
No radiotherapy benefit was seen among those with elevated risk and poor Rst.
The investigators also reported that, among patients with a poor Rst, 10-year IBTR and IBC rates were 25% and 16%, respectively, regardless of whether they received radiotherapy. These rates were much higher than the rates among women with good Rst (6.6% and 4.5%; hazard ratio, 3.6 and 4.4, respectively).
No significant difference was seen in 10-year IBTR and IBC rates among patients in the low-risk group, with or without radiotherapy.
Traditional clinicopathologic risk factors, including age younger than 50 years, grade 3 disease, and tumor size greater than 2.5 cm, did not identify poor versus good response subtypes in this cohort, and on multivariable analysis, neither of these factors nor endocrine therapy was significantly associated with IBTR or IBC.
Prospective validation needed
In his discussion, Dr. Smith said that the study provides “important data” that further validate the DCISionRT platform alone for assessing risk among women with DCIS who undergo BCS. But it is the Rst biosignature, which allows clinicians to “predict radioresistance of residual malignant chromogens following lumpectomy plus radiation therapy,” that really stands out, he added.
From the data presented, “it is reasonable to conclude that patients with a poor Rst score treated with lumpectomy and radiation had a much higher risk of in-breast tumor recurrence than one might predict or anticipate based on existing published randomized clinical trial data,” he said.
“In my opinion, it is very important to prospectively validate this finding with other cohorts,” he said. “Moving forward, I think there may come a time where there may be interest in studying radiosensitizing agents for poor-Rst ductal carcinoma in situ that are resistant to standard doses of radiation, and it may be that we consider the Rst as a factor moving forward in selecting patients for BCT versus mastectomy.”
However, because 75% of patients at elevated risk with poor Rst who undergo lumpectomy and radiotherapy do not experience recurrence in the decade following their treatment, it would be “inappropriate and misguided” to start recommending mastectomy for patients at DCISionRT elevated risk who have poor Rst, he said.
The study was funded by PreludeDx. Dr. Vicini reported employment with 21st Century Oncology and financial relationships with ImpediMed, Prelude Therapeutics, and Concure Oncology. Dr. Smith, through his employer, has an equity interest in Oncora Medical through a partnership agreement. He also has an uncompensated relationship with the American Society for Radiation Oncology.
A version of this article first appeared on Medscape.com.
High rates of work-related trauma, PTSD in intern physicians
Work-related posttraumatic stress disorder is three times higher in interns than the general population, new research shows.
Investigators assessed PTSD in more than 1,100 physicians at the end of their internship year and found that a little over half reported work-related trauma exposure, and of these, 20% screened positive for PTSD.
Overall, 10% of participants screened positive for PTSD by the end of the internship year, compared with a 12-month PTSD prevalence of 3.6% in the general population.
“Work-related trauma exposure and PTSD are common and underdiscussed phenomena among intern physicians,” lead author Mary Vance, MD, assistant professor of psychiatry, Uniformed Services University of the Health Sciences, Bethesda, Md., said in an interview.
“I urge medical educators and policy makers to include this topic in their discussions about physician well-being and to implement effective interventions to mitigate the impact of work-related trauma and PTSD among physician trainees,” she said.
The study was published online June 8 in JAMA Network Open.
Burnout, depression, suicide
“Burnout, depression, and suicide are increasingly recognized as occupational mental health hazards among health care professionals, including physicians,” Dr. Vance said.
“However, in my professional experience as a physician and educator, despite observing anecdotal evidence among my peers and trainees that this is also an issue,” she added.
This gap prompted her “to investigate rates of work-related trauma exposure and PTSD among physicians.”
The researchers sent emails to 4,350 individuals during academic year 2018-2019, 2 months prior to starting internships. Of these, 2,129 agreed to participate and 1,134 (58.6% female, 61.6% non-Hispanic White; mean age, 27.52) completed the study.
Prior to beginning internship, participants completed a baseline survey that assessed demographic characteristics as well as medical education and psychological and psychosocial factors.
Participants completed follow-up surveys sent by email at 3, 6, 9, and 12 months of the internship year. The surveys assessed stressful life events, concern over perceived medical errors in the past 3 months, and number of hours worked over the past week.
At month 12, current PTSD and symptoms of depression and anxiety were also assessed using the Primary Care PTSD Screen for DSM-5, the 9-item Patient Health Questionnaire, and the Generalized Anxiety Disorder 7-item scale, respectively.
Participants were asked to self-report whether they ever had an episode of depression and to complete the Risky Families Questionnaire to assess if they had experienced childhood abuse, neglect, and family conflict. Additionally, they completed an 11-item scale developed specifically for the study regarding recent stressful events.
‘Crucible’ year
A total of 56.4% of respondents reported work-related trauma exposure, and among these, 19.0% screened positive for PTSD. One-tenth (10.8%) of the entire sample screened positive for PTSD by the end of internship year, which is three times higher than the 12-month prevalence of PTSD in the general population (3.6%), the authors noted.
Trauma exposure differed by specialty, ranging from 43.1% in anesthesiology to 72.4% in emergency medicine. Of the respondents in internal medicine, surgery, and medicine/pediatrics, 56.6%, 63.3%, and 71%, respectively, reported work-related trauma exposure.
Work-related PTSD also differed by specialty, ranging from 7.5% in ob.gyn. to 30.0% in pediatrics. Of respondents in internal medicine and family practice, 23.9% and 25.9%, respectively, reported work-related PTSD.
Dr. Vance called the intern year “a crucible, during which newly minted doctors receive intensive on-the-job training at the front lines of patient care [and] work long hours in rapidly shifting environments, often caring for critically ill patients.”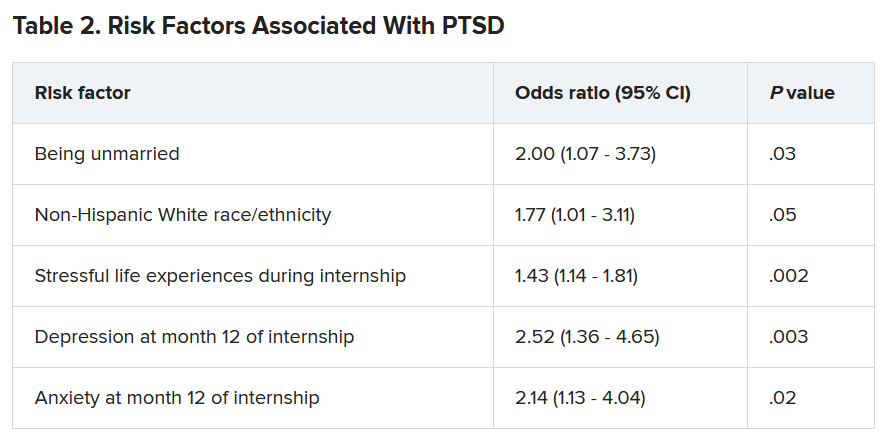
Work-related trauma exposure “is more likely to occur during this high-stress internship year than during the same year in the general population,” she said.
She noted that the “issue of workplace trauma and PTSD among health care workers became even more salient during the height of COVID,” adding that she expects it “to remain a pressure issue for healthcare workers in the post-COVID era.”
Call to action
Commenting on the study David A. Marcus, MD, chair, GME Physician Well-Being Committee, Northwell Health, New Hyde Park, N.Y., noted the study’s “relatively low response rate” is a “significant limitation” of the study.
An additional limitation is the lack of a baseline PTSD assessment, said Dr. Marcus, an assistant professor at Hofstra University, Hempstead, N.Y., who was not involved in the research.
Nevertheless, the “overall prevalence [of work-related PTSD] should serve as a call to action for physician leaders and for leaders in academic medicine,” he said.
Additionally, the study “reminds us that trauma-informed care should be an essential part of mental health support services provided to trainees and to physicians in general,” Dr. Marcus stated.
Also commenting on the study, Lotte N. Dyrbye, MD, professor of medicine and medical education, Mayo Clinic, Rochester, Minn., agreed.
“Organizational strategies should include system-level interventions to reduce the risk of frightening, horrible, or traumatic events from occurring in the workplace in the first place, as well as faculty development efforts to upskill teaching faculty in their ability to support trainees when such events do occur,” she said.
These approaches “should coincide with organizational efforts to support individual trainees by providing adequate time off after traumatic events, ensuring trainees can access affordable mental healthcare, and reducing other barriers to appropriate help-seeking, such as stigma, and efforts to build a culture of well-being,” suggested Dr. Dyrbye, who is codirector of the Mayo Clinic Program on Physician Wellbeing and was not involved in the study.
The study was supported by grants from the Blue Cross Blue Shield Foundation of Michigan and National Institutes of Health. Dr. Vance and coauthors, Dr. Marcus, and Dr. Dyrbye reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Work-related posttraumatic stress disorder is three times higher in interns than the general population, new research shows.
Investigators assessed PTSD in more than 1,100 physicians at the end of their internship year and found that a little over half reported work-related trauma exposure, and of these, 20% screened positive for PTSD.
Overall, 10% of participants screened positive for PTSD by the end of the internship year, compared with a 12-month PTSD prevalence of 3.6% in the general population.
“Work-related trauma exposure and PTSD are common and underdiscussed phenomena among intern physicians,” lead author Mary Vance, MD, assistant professor of psychiatry, Uniformed Services University of the Health Sciences, Bethesda, Md., said in an interview.
“I urge medical educators and policy makers to include this topic in their discussions about physician well-being and to implement effective interventions to mitigate the impact of work-related trauma and PTSD among physician trainees,” she said.
The study was published online June 8 in JAMA Network Open.
Burnout, depression, suicide
“Burnout, depression, and suicide are increasingly recognized as occupational mental health hazards among health care professionals, including physicians,” Dr. Vance said.
“However, in my professional experience as a physician and educator, despite observing anecdotal evidence among my peers and trainees that this is also an issue,” she added.
This gap prompted her “to investigate rates of work-related trauma exposure and PTSD among physicians.”
The researchers sent emails to 4,350 individuals during academic year 2018-2019, 2 months prior to starting internships. Of these, 2,129 agreed to participate and 1,134 (58.6% female, 61.6% non-Hispanic White; mean age, 27.52) completed the study.
Prior to beginning internship, participants completed a baseline survey that assessed demographic characteristics as well as medical education and psychological and psychosocial factors.
Participants completed follow-up surveys sent by email at 3, 6, 9, and 12 months of the internship year. The surveys assessed stressful life events, concern over perceived medical errors in the past 3 months, and number of hours worked over the past week.
At month 12, current PTSD and symptoms of depression and anxiety were also assessed using the Primary Care PTSD Screen for DSM-5, the 9-item Patient Health Questionnaire, and the Generalized Anxiety Disorder 7-item scale, respectively.
Participants were asked to self-report whether they ever had an episode of depression and to complete the Risky Families Questionnaire to assess if they had experienced childhood abuse, neglect, and family conflict. Additionally, they completed an 11-item scale developed specifically for the study regarding recent stressful events.
‘Crucible’ year
A total of 56.4% of respondents reported work-related trauma exposure, and among these, 19.0% screened positive for PTSD. One-tenth (10.8%) of the entire sample screened positive for PTSD by the end of internship year, which is three times higher than the 12-month prevalence of PTSD in the general population (3.6%), the authors noted.
Trauma exposure differed by specialty, ranging from 43.1% in anesthesiology to 72.4% in emergency medicine. Of the respondents in internal medicine, surgery, and medicine/pediatrics, 56.6%, 63.3%, and 71%, respectively, reported work-related trauma exposure.
Work-related PTSD also differed by specialty, ranging from 7.5% in ob.gyn. to 30.0% in pediatrics. Of respondents in internal medicine and family practice, 23.9% and 25.9%, respectively, reported work-related PTSD.
Dr. Vance called the intern year “a crucible, during which newly minted doctors receive intensive on-the-job training at the front lines of patient care [and] work long hours in rapidly shifting environments, often caring for critically ill patients.”
Work-related trauma exposure “is more likely to occur during this high-stress internship year than during the same year in the general population,” she said.
She noted that the “issue of workplace trauma and PTSD among health care workers became even more salient during the height of COVID,” adding that she expects it “to remain a pressure issue for healthcare workers in the post-COVID era.”
Call to action
Commenting on the study David A. Marcus, MD, chair, GME Physician Well-Being Committee, Northwell Health, New Hyde Park, N.Y., noted the study’s “relatively low response rate” is a “significant limitation” of the study.
An additional limitation is the lack of a baseline PTSD assessment, said Dr. Marcus, an assistant professor at Hofstra University, Hempstead, N.Y., who was not involved in the research.
Nevertheless, the “overall prevalence [of work-related PTSD] should serve as a call to action for physician leaders and for leaders in academic medicine,” he said.
Additionally, the study “reminds us that trauma-informed care should be an essential part of mental health support services provided to trainees and to physicians in general,” Dr. Marcus stated.
Also commenting on the study, Lotte N. Dyrbye, MD, professor of medicine and medical education, Mayo Clinic, Rochester, Minn., agreed.
“Organizational strategies should include system-level interventions to reduce the risk of frightening, horrible, or traumatic events from occurring in the workplace in the first place, as well as faculty development efforts to upskill teaching faculty in their ability to support trainees when such events do occur,” she said.
These approaches “should coincide with organizational efforts to support individual trainees by providing adequate time off after traumatic events, ensuring trainees can access affordable mental healthcare, and reducing other barriers to appropriate help-seeking, such as stigma, and efforts to build a culture of well-being,” suggested Dr. Dyrbye, who is codirector of the Mayo Clinic Program on Physician Wellbeing and was not involved in the study.
The study was supported by grants from the Blue Cross Blue Shield Foundation of Michigan and National Institutes of Health. Dr. Vance and coauthors, Dr. Marcus, and Dr. Dyrbye reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Work-related posttraumatic stress disorder is three times higher in interns than the general population, new research shows.
Investigators assessed PTSD in more than 1,100 physicians at the end of their internship year and found that a little over half reported work-related trauma exposure, and of these, 20% screened positive for PTSD.
Overall, 10% of participants screened positive for PTSD by the end of the internship year, compared with a 12-month PTSD prevalence of 3.6% in the general population.
“Work-related trauma exposure and PTSD are common and underdiscussed phenomena among intern physicians,” lead author Mary Vance, MD, assistant professor of psychiatry, Uniformed Services University of the Health Sciences, Bethesda, Md., said in an interview.
“I urge medical educators and policy makers to include this topic in their discussions about physician well-being and to implement effective interventions to mitigate the impact of work-related trauma and PTSD among physician trainees,” she said.
The study was published online June 8 in JAMA Network Open.
Burnout, depression, suicide
“Burnout, depression, and suicide are increasingly recognized as occupational mental health hazards among health care professionals, including physicians,” Dr. Vance said.
“However, in my professional experience as a physician and educator, despite observing anecdotal evidence among my peers and trainees that this is also an issue,” she added.
This gap prompted her “to investigate rates of work-related trauma exposure and PTSD among physicians.”
The researchers sent emails to 4,350 individuals during academic year 2018-2019, 2 months prior to starting internships. Of these, 2,129 agreed to participate and 1,134 (58.6% female, 61.6% non-Hispanic White; mean age, 27.52) completed the study.
Prior to beginning internship, participants completed a baseline survey that assessed demographic characteristics as well as medical education and psychological and psychosocial factors.
Participants completed follow-up surveys sent by email at 3, 6, 9, and 12 months of the internship year. The surveys assessed stressful life events, concern over perceived medical errors in the past 3 months, and number of hours worked over the past week.
At month 12, current PTSD and symptoms of depression and anxiety were also assessed using the Primary Care PTSD Screen for DSM-5, the 9-item Patient Health Questionnaire, and the Generalized Anxiety Disorder 7-item scale, respectively.
Participants were asked to self-report whether they ever had an episode of depression and to complete the Risky Families Questionnaire to assess if they had experienced childhood abuse, neglect, and family conflict. Additionally, they completed an 11-item scale developed specifically for the study regarding recent stressful events.
‘Crucible’ year
A total of 56.4% of respondents reported work-related trauma exposure, and among these, 19.0% screened positive for PTSD. One-tenth (10.8%) of the entire sample screened positive for PTSD by the end of internship year, which is three times higher than the 12-month prevalence of PTSD in the general population (3.6%), the authors noted.
Trauma exposure differed by specialty, ranging from 43.1% in anesthesiology to 72.4% in emergency medicine. Of the respondents in internal medicine, surgery, and medicine/pediatrics, 56.6%, 63.3%, and 71%, respectively, reported work-related trauma exposure.
Work-related PTSD also differed by specialty, ranging from 7.5% in ob.gyn. to 30.0% in pediatrics. Of respondents in internal medicine and family practice, 23.9% and 25.9%, respectively, reported work-related PTSD.
Dr. Vance called the intern year “a crucible, during which newly minted doctors receive intensive on-the-job training at the front lines of patient care [and] work long hours in rapidly shifting environments, often caring for critically ill patients.”
Work-related trauma exposure “is more likely to occur during this high-stress internship year than during the same year in the general population,” she said.
She noted that the “issue of workplace trauma and PTSD among health care workers became even more salient during the height of COVID,” adding that she expects it “to remain a pressure issue for healthcare workers in the post-COVID era.”
Call to action
Commenting on the study David A. Marcus, MD, chair, GME Physician Well-Being Committee, Northwell Health, New Hyde Park, N.Y., noted the study’s “relatively low response rate” is a “significant limitation” of the study.
An additional limitation is the lack of a baseline PTSD assessment, said Dr. Marcus, an assistant professor at Hofstra University, Hempstead, N.Y., who was not involved in the research.
Nevertheless, the “overall prevalence [of work-related PTSD] should serve as a call to action for physician leaders and for leaders in academic medicine,” he said.
Additionally, the study “reminds us that trauma-informed care should be an essential part of mental health support services provided to trainees and to physicians in general,” Dr. Marcus stated.
Also commenting on the study, Lotte N. Dyrbye, MD, professor of medicine and medical education, Mayo Clinic, Rochester, Minn., agreed.
“Organizational strategies should include system-level interventions to reduce the risk of frightening, horrible, or traumatic events from occurring in the workplace in the first place, as well as faculty development efforts to upskill teaching faculty in their ability to support trainees when such events do occur,” she said.
These approaches “should coincide with organizational efforts to support individual trainees by providing adequate time off after traumatic events, ensuring trainees can access affordable mental healthcare, and reducing other barriers to appropriate help-seeking, such as stigma, and efforts to build a culture of well-being,” suggested Dr. Dyrbye, who is codirector of the Mayo Clinic Program on Physician Wellbeing and was not involved in the study.
The study was supported by grants from the Blue Cross Blue Shield Foundation of Michigan and National Institutes of Health. Dr. Vance and coauthors, Dr. Marcus, and Dr. Dyrbye reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ten killer steps to writing a great medical thriller
For many physicians and other professionals, aspirations of crafting a work of fiction are not uncommon — and with good reason. We are, after all, a generally well-disciplined bunch capable of completing complex tasks, and there is certainly no shortage of excitement and drama in medicine and surgery — ample fodder for thrilling stories. Nonetheless, writing a novel is a major commitment, and it requires persistence, patience, and dedicated time, especially for one with a busy medical career.
Getting started is not easy. Writing workshops are helpful, and in my case, I tried to mentor with some of the best. Before writing my novel, I attended workshops for aspiring novelists, given by noted physician authors Tess Gerritsen (Body Double, The Surgeon) and the late Michael Palmer (The Society, The Fifth Vial).
Writers are often advised to “write about what you know.” In my case, I combined my knowledge of medicine and my experience with the thoroughbred racing world to craft a thriller that one reviewer described as “Dick Francis meets Robin Cook.” For those who have never read the Dick Francis series, he was a renowned crime writer whose novels centered on horse racing in England. Having been an avid reader of both authors, that comparison was the ultimate compliment.
So against that backdrop, the novel Shedrow, along with some shared wisdom from a few legendary writers.
1. Start with the big “what if.” Any great story starts with that simple “what if” question. What if a series of high-profile executives in the managed care industry are serially murdered (Michael Palmer’s The Society)? What if a multimillion-dollar stallion dies suddenly under very mysterious circumstances on a supposedly secure farm in Kentucky (Dean DeLuke’s Shedrow)?
2. Put a MacGuffin to work in your story. Popularized by Alfred Hitchcock, the MacGuffin is that essential plot element that drives virtually all characters in the story, although it may be rather vague and meaningless to the story itself. In the iconic movie Pulp Fiction, the MacGuffin is the briefcase — everyone wants it, and we never do find out what’s in it.
3. Pacing is critical. Plot out the timeline of emotional highs and lows in a story. It should look like a rolling pattern of highs and lows that crescendo upward to the ultimate crisis. Take advantage of the fact that following any of those emotional peaks, you probably have the reader’s undivided attention. That would be a good time to provide backstory or fill in needed information for the reader – information that may be critical but perhaps not as exciting as what just transpired.
4. Torture your protagonists. Just when the reader thinks that the hero is finally home free, throw in another obstacle. Readers will empathize with the character and be drawn in by the unexpected hurdle.
5. Be original and surprise your readers. Create twists and turns that are totally unexpected, yet believable. This is easier said than done but will go a long way toward making your novel original, gripping, and unpredictable.
6. As a general rule, consider short sentences and short chapters. This is strictly a personal preference, but who can argue with James Patterson’s short chapters or with Robert Parker’s short and engaging sentences? Sentence length can be varied for effect, too, with shorter sentences serving to heighten action or increase tension.
7. Avoid the passive voice. Your readers want action. This is an important rule in almost any type of writing.
8. Keep descriptions brief. Long, drawn-out descriptions of the way characters look, or even setting descriptions, are easily overdone in a thriller. The thriller genre is very different from literary fiction in this regard. Stephen King advises writers to “just say what they see, then get on with the story.”
9. Sustain the reader’s interest throughout. Assess each chapter ending and determine whether the reader has been given enough reason to want to continue reading. Pose a question, end with a minor cliffhanger, or at least ensure that there is enough accumulated tension in the story.
10. Edit aggressively and cut out the fluff. Ernest Hemingway once confided to F. Scott Fitzgerald, “I write one page of masterpiece to 91 pages of shit. I try to put the shit in the wastebasket.”
Dr. DeLuke is professor emeritus of oral and facial surgery at Virginia Commonwealth University and author of the novel Shedrow.
A version of this article first appeared on Medscape.com.
For many physicians and other professionals, aspirations of crafting a work of fiction are not uncommon — and with good reason. We are, after all, a generally well-disciplined bunch capable of completing complex tasks, and there is certainly no shortage of excitement and drama in medicine and surgery — ample fodder for thrilling stories. Nonetheless, writing a novel is a major commitment, and it requires persistence, patience, and dedicated time, especially for one with a busy medical career.
Getting started is not easy. Writing workshops are helpful, and in my case, I tried to mentor with some of the best. Before writing my novel, I attended workshops for aspiring novelists, given by noted physician authors Tess Gerritsen (Body Double, The Surgeon) and the late Michael Palmer (The Society, The Fifth Vial).
Writers are often advised to “write about what you know.” In my case, I combined my knowledge of medicine and my experience with the thoroughbred racing world to craft a thriller that one reviewer described as “Dick Francis meets Robin Cook.” For those who have never read the Dick Francis series, he was a renowned crime writer whose novels centered on horse racing in England. Having been an avid reader of both authors, that comparison was the ultimate compliment.
So against that backdrop, the novel Shedrow, along with some shared wisdom from a few legendary writers.
1. Start with the big “what if.” Any great story starts with that simple “what if” question. What if a series of high-profile executives in the managed care industry are serially murdered (Michael Palmer’s The Society)? What if a multimillion-dollar stallion dies suddenly under very mysterious circumstances on a supposedly secure farm in Kentucky (Dean DeLuke’s Shedrow)?
2. Put a MacGuffin to work in your story. Popularized by Alfred Hitchcock, the MacGuffin is that essential plot element that drives virtually all characters in the story, although it may be rather vague and meaningless to the story itself. In the iconic movie Pulp Fiction, the MacGuffin is the briefcase — everyone wants it, and we never do find out what’s in it.
3. Pacing is critical. Plot out the timeline of emotional highs and lows in a story. It should look like a rolling pattern of highs and lows that crescendo upward to the ultimate crisis. Take advantage of the fact that following any of those emotional peaks, you probably have the reader’s undivided attention. That would be a good time to provide backstory or fill in needed information for the reader – information that may be critical but perhaps not as exciting as what just transpired.
4. Torture your protagonists. Just when the reader thinks that the hero is finally home free, throw in another obstacle. Readers will empathize with the character and be drawn in by the unexpected hurdle.
5. Be original and surprise your readers. Create twists and turns that are totally unexpected, yet believable. This is easier said than done but will go a long way toward making your novel original, gripping, and unpredictable.
6. As a general rule, consider short sentences and short chapters. This is strictly a personal preference, but who can argue with James Patterson’s short chapters or with Robert Parker’s short and engaging sentences? Sentence length can be varied for effect, too, with shorter sentences serving to heighten action or increase tension.
7. Avoid the passive voice. Your readers want action. This is an important rule in almost any type of writing.
8. Keep descriptions brief. Long, drawn-out descriptions of the way characters look, or even setting descriptions, are easily overdone in a thriller. The thriller genre is very different from literary fiction in this regard. Stephen King advises writers to “just say what they see, then get on with the story.”
9. Sustain the reader’s interest throughout. Assess each chapter ending and determine whether the reader has been given enough reason to want to continue reading. Pose a question, end with a minor cliffhanger, or at least ensure that there is enough accumulated tension in the story.
10. Edit aggressively and cut out the fluff. Ernest Hemingway once confided to F. Scott Fitzgerald, “I write one page of masterpiece to 91 pages of shit. I try to put the shit in the wastebasket.”
Dr. DeLuke is professor emeritus of oral and facial surgery at Virginia Commonwealth University and author of the novel Shedrow.
A version of this article first appeared on Medscape.com.
For many physicians and other professionals, aspirations of crafting a work of fiction are not uncommon — and with good reason. We are, after all, a generally well-disciplined bunch capable of completing complex tasks, and there is certainly no shortage of excitement and drama in medicine and surgery — ample fodder for thrilling stories. Nonetheless, writing a novel is a major commitment, and it requires persistence, patience, and dedicated time, especially for one with a busy medical career.
Getting started is not easy. Writing workshops are helpful, and in my case, I tried to mentor with some of the best. Before writing my novel, I attended workshops for aspiring novelists, given by noted physician authors Tess Gerritsen (Body Double, The Surgeon) and the late Michael Palmer (The Society, The Fifth Vial).
Writers are often advised to “write about what you know.” In my case, I combined my knowledge of medicine and my experience with the thoroughbred racing world to craft a thriller that one reviewer described as “Dick Francis meets Robin Cook.” For those who have never read the Dick Francis series, he was a renowned crime writer whose novels centered on horse racing in England. Having been an avid reader of both authors, that comparison was the ultimate compliment.
So against that backdrop, the novel Shedrow, along with some shared wisdom from a few legendary writers.
1. Start with the big “what if.” Any great story starts with that simple “what if” question. What if a series of high-profile executives in the managed care industry are serially murdered (Michael Palmer’s The Society)? What if a multimillion-dollar stallion dies suddenly under very mysterious circumstances on a supposedly secure farm in Kentucky (Dean DeLuke’s Shedrow)?
2. Put a MacGuffin to work in your story. Popularized by Alfred Hitchcock, the MacGuffin is that essential plot element that drives virtually all characters in the story, although it may be rather vague and meaningless to the story itself. In the iconic movie Pulp Fiction, the MacGuffin is the briefcase — everyone wants it, and we never do find out what’s in it.
3. Pacing is critical. Plot out the timeline of emotional highs and lows in a story. It should look like a rolling pattern of highs and lows that crescendo upward to the ultimate crisis. Take advantage of the fact that following any of those emotional peaks, you probably have the reader’s undivided attention. That would be a good time to provide backstory or fill in needed information for the reader – information that may be critical but perhaps not as exciting as what just transpired.
4. Torture your protagonists. Just when the reader thinks that the hero is finally home free, throw in another obstacle. Readers will empathize with the character and be drawn in by the unexpected hurdle.
5. Be original and surprise your readers. Create twists and turns that are totally unexpected, yet believable. This is easier said than done but will go a long way toward making your novel original, gripping, and unpredictable.
6. As a general rule, consider short sentences and short chapters. This is strictly a personal preference, but who can argue with James Patterson’s short chapters or with Robert Parker’s short and engaging sentences? Sentence length can be varied for effect, too, with shorter sentences serving to heighten action or increase tension.
7. Avoid the passive voice. Your readers want action. This is an important rule in almost any type of writing.
8. Keep descriptions brief. Long, drawn-out descriptions of the way characters look, or even setting descriptions, are easily overdone in a thriller. The thriller genre is very different from literary fiction in this regard. Stephen King advises writers to “just say what they see, then get on with the story.”
9. Sustain the reader’s interest throughout. Assess each chapter ending and determine whether the reader has been given enough reason to want to continue reading. Pose a question, end with a minor cliffhanger, or at least ensure that there is enough accumulated tension in the story.
10. Edit aggressively and cut out the fluff. Ernest Hemingway once confided to F. Scott Fitzgerald, “I write one page of masterpiece to 91 pages of shit. I try to put the shit in the wastebasket.”
Dr. DeLuke is professor emeritus of oral and facial surgery at Virginia Commonwealth University and author of the novel Shedrow.
A version of this article first appeared on Medscape.com.
Treating sleep apnea lowers MI and stroke risk
particularly for patients with moderate to severe OSA and those who are more adherent to CPAP therapy, a new study suggests.
“Most clinical trials on the effect of CPAP on CV diseases to date have focused on secondary CV prevention. This study contributes another piece of evidence about the role of CPAP therapy to prevent CV diseases,” said Diego R. Mazzotti, PhD, an assistant professor at the University of Kansas Medical Center, Kansas City.
“Our study, while observational, suggests that clinical trials focused on understanding how to sustain long-term CPAP adherence in obstructive sleep apnea patients are necessary and could be critical for optimizing comorbidity risk reduction,” Dr. Mazzotti said.
The study was presented at the virtual annual meeting of the Associated Professional Sleep Societies.
Good adherence important
The researchers analyzed the electronic health records of adults referred for a sleep study through the Kaiser Permanente Southern California health system. The sample included 11,145 adults without OSA, 13,898 with OSA who used CPAP, and 20,884 adults with OSA who did not use CPAP. None of them had CV disease at baseline. Median follow-up was 262 days.
The primary outcome was first occurrence of myocardial infarction, stroke, unstable angina, heart failure, or death caused by CV disease.
In adjusted models, adults with moderate to severe OSA (apnea-hypopnea index ≥15) who did not use CPAP were 71% more likely than those without OSA to have a first CV event (hazard ratio, 1.71; 95% CI, 1.11-2.64). However, the risk for a CV event during follow-up was 32% lower among OSA patients with any CPAP use (HR, 0.68; 95% CI, 0.50-0.93; P = .016).
The effect was mostly driven by those who used CPAP for at least 4 hours per night (HR, 0.60; 95% CI, 0.39-0.95). This association was stronger for those with moderate to severe OSA (HR, 0.56; 95% CI, 0.39-0.81).
“This study highlights the importance of long-term management of CPAP therapy in patients with moderate-severe OSA,” Dr. Mazzotti said in an interview.
“It suggests that maintaining good CPAP adherence might be beneficial for cardiovascular health, besides the already established benefits on quality of life, sleepiness, and other cardiometabolic functions,” he said.
Dr. Mazzotti said several mechanisms might explain the association between CPAP use and lower risk for CV events. “CPAP treats OSA by preventing respiratory pauses that occur during sleep, therefore preventing arousals, sleep fragmentation, and decreases in blood oxygen. These improved cardiorespiratory functions can be beneficial to avoid certain molecular changes that are known to contribute to cardiovascular risk, such as oxidative stress and inflammation,” he explained.
“However, specific studies fully understanding these mechanisms are necessary,” Dr. Mazzotti added.
In a comment, Nitun Verma, MD, a spokesperson for the American Academy of Sleep Medicine, said that “the frequent decreases in oxygen levels and fragmented sleep from apnea are associated with cardiovascular disorders. We know this from multiple studies. This, however, was a large study and strengthens the association between improving apnea and reduced serious cardiovascular events.”
Funding for the study was provided by the American Academy of Sleep Medicine Foundation and the American Heart Association. Dr. Mazzotti and Dr. Verma disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
particularly for patients with moderate to severe OSA and those who are more adherent to CPAP therapy, a new study suggests.
“Most clinical trials on the effect of CPAP on CV diseases to date have focused on secondary CV prevention. This study contributes another piece of evidence about the role of CPAP therapy to prevent CV diseases,” said Diego R. Mazzotti, PhD, an assistant professor at the University of Kansas Medical Center, Kansas City.
“Our study, while observational, suggests that clinical trials focused on understanding how to sustain long-term CPAP adherence in obstructive sleep apnea patients are necessary and could be critical for optimizing comorbidity risk reduction,” Dr. Mazzotti said.
The study was presented at the virtual annual meeting of the Associated Professional Sleep Societies.
Good adherence important
The researchers analyzed the electronic health records of adults referred for a sleep study through the Kaiser Permanente Southern California health system. The sample included 11,145 adults without OSA, 13,898 with OSA who used CPAP, and 20,884 adults with OSA who did not use CPAP. None of them had CV disease at baseline. Median follow-up was 262 days.
The primary outcome was first occurrence of myocardial infarction, stroke, unstable angina, heart failure, or death caused by CV disease.
In adjusted models, adults with moderate to severe OSA (apnea-hypopnea index ≥15) who did not use CPAP were 71% more likely than those without OSA to have a first CV event (hazard ratio, 1.71; 95% CI, 1.11-2.64). However, the risk for a CV event during follow-up was 32% lower among OSA patients with any CPAP use (HR, 0.68; 95% CI, 0.50-0.93; P = .016).
The effect was mostly driven by those who used CPAP for at least 4 hours per night (HR, 0.60; 95% CI, 0.39-0.95). This association was stronger for those with moderate to severe OSA (HR, 0.56; 95% CI, 0.39-0.81).
“This study highlights the importance of long-term management of CPAP therapy in patients with moderate-severe OSA,” Dr. Mazzotti said in an interview.
“It suggests that maintaining good CPAP adherence might be beneficial for cardiovascular health, besides the already established benefits on quality of life, sleepiness, and other cardiometabolic functions,” he said.
Dr. Mazzotti said several mechanisms might explain the association between CPAP use and lower risk for CV events. “CPAP treats OSA by preventing respiratory pauses that occur during sleep, therefore preventing arousals, sleep fragmentation, and decreases in blood oxygen. These improved cardiorespiratory functions can be beneficial to avoid certain molecular changes that are known to contribute to cardiovascular risk, such as oxidative stress and inflammation,” he explained.
“However, specific studies fully understanding these mechanisms are necessary,” Dr. Mazzotti added.
In a comment, Nitun Verma, MD, a spokesperson for the American Academy of Sleep Medicine, said that “the frequent decreases in oxygen levels and fragmented sleep from apnea are associated with cardiovascular disorders. We know this from multiple studies. This, however, was a large study and strengthens the association between improving apnea and reduced serious cardiovascular events.”
Funding for the study was provided by the American Academy of Sleep Medicine Foundation and the American Heart Association. Dr. Mazzotti and Dr. Verma disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
particularly for patients with moderate to severe OSA and those who are more adherent to CPAP therapy, a new study suggests.
“Most clinical trials on the effect of CPAP on CV diseases to date have focused on secondary CV prevention. This study contributes another piece of evidence about the role of CPAP therapy to prevent CV diseases,” said Diego R. Mazzotti, PhD, an assistant professor at the University of Kansas Medical Center, Kansas City.
“Our study, while observational, suggests that clinical trials focused on understanding how to sustain long-term CPAP adherence in obstructive sleep apnea patients are necessary and could be critical for optimizing comorbidity risk reduction,” Dr. Mazzotti said.
The study was presented at the virtual annual meeting of the Associated Professional Sleep Societies.
Good adherence important
The researchers analyzed the electronic health records of adults referred for a sleep study through the Kaiser Permanente Southern California health system. The sample included 11,145 adults without OSA, 13,898 with OSA who used CPAP, and 20,884 adults with OSA who did not use CPAP. None of them had CV disease at baseline. Median follow-up was 262 days.
The primary outcome was first occurrence of myocardial infarction, stroke, unstable angina, heart failure, or death caused by CV disease.
In adjusted models, adults with moderate to severe OSA (apnea-hypopnea index ≥15) who did not use CPAP were 71% more likely than those without OSA to have a first CV event (hazard ratio, 1.71; 95% CI, 1.11-2.64). However, the risk for a CV event during follow-up was 32% lower among OSA patients with any CPAP use (HR, 0.68; 95% CI, 0.50-0.93; P = .016).
The effect was mostly driven by those who used CPAP for at least 4 hours per night (HR, 0.60; 95% CI, 0.39-0.95). This association was stronger for those with moderate to severe OSA (HR, 0.56; 95% CI, 0.39-0.81).
“This study highlights the importance of long-term management of CPAP therapy in patients with moderate-severe OSA,” Dr. Mazzotti said in an interview.
“It suggests that maintaining good CPAP adherence might be beneficial for cardiovascular health, besides the already established benefits on quality of life, sleepiness, and other cardiometabolic functions,” he said.
Dr. Mazzotti said several mechanisms might explain the association between CPAP use and lower risk for CV events. “CPAP treats OSA by preventing respiratory pauses that occur during sleep, therefore preventing arousals, sleep fragmentation, and decreases in blood oxygen. These improved cardiorespiratory functions can be beneficial to avoid certain molecular changes that are known to contribute to cardiovascular risk, such as oxidative stress and inflammation,” he explained.
“However, specific studies fully understanding these mechanisms are necessary,” Dr. Mazzotti added.
In a comment, Nitun Verma, MD, a spokesperson for the American Academy of Sleep Medicine, said that “the frequent decreases in oxygen levels and fragmented sleep from apnea are associated with cardiovascular disorders. We know this from multiple studies. This, however, was a large study and strengthens the association between improving apnea and reduced serious cardiovascular events.”
Funding for the study was provided by the American Academy of Sleep Medicine Foundation and the American Heart Association. Dr. Mazzotti and Dr. Verma disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SLEEP 2021
Memory benefit seen with antihypertensives crossing blood-brain barrier
Over a 3-year period, cognitively normal older adults taking BBB-crossing antihypertensives demonstrated superior verbal memory, compared with similar individuals receiving non–BBB-crossing antihypertensives, reported lead author Jean K. Ho, PhD, of the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine, and colleagues.
According to the investigators, the findings add color to a known link between hypertension and neurologic degeneration, and may aid the search for new therapeutic targets.
“Hypertension is a well-established risk factor for cognitive decline and dementia, possibly through its effects on both cerebrovascular disease and Alzheimer’s disease,” Dr. Ho and colleagues wrote in Hypertension. “Studies of antihypertensive treatments have reported possible salutary effects on cognition and cerebrovascular disease, as well as Alzheimer’s disease neuropathology.”
In a previous study, individuals younger than 75 years exposed to antihypertensives had an 8% decreased risk of dementia per year of use, while another trial showed that intensive blood pressure–lowering therapy reduced mild cognitive impairment by 19%.
“Despite these encouraging findings ... larger meta-analytic studies have been hampered by the fact that pharmacokinetic properties are typically not considered in existing studies or routine clinical practice,” wrote Dr. Ho and colleagues. “The present study sought to fill this gap [in that it was] a large and longitudinal meta-analytic study of existing data recoded to assess the effects of BBB-crossing potential in renin-angiotensin system [RAS] treatments among hypertensive adults.”
Methods and results
The meta-analysis included randomized clinical trials, prospective cohort studies, and retrospective observational studies. The researchers assessed data on 12,849 individuals from 14 cohorts that received either BBB-crossing or non–BBB-crossing antihypertensives.
The BBB-crossing properties of RAS treatments were identified by a literature review. Of ACE inhibitors, captopril, fosinopril, lisinopril, perindopril, ramipril, and trandolapril were classified as BBB crossing, and benazepril, enalapril, moexipril, and quinapril were classified as non–BBB-crossing. Of ARBs, telmisartan and candesartan were considered BBB-crossing, and olmesartan, eprosartan, irbesartan, and losartan were tagged as non–BBB-crossing.
Cognition was assessed via the following seven domains: executive function, attention, verbal memory learning, language, mental status, recall, and processing speed.
Compared with individuals taking non–BBB-crossing antihypertensives, those taking BBB-crossing agents had significantly superior verbal memory (recall), with a maximum effect size of 0.07 (P = .03).
According to the investigators, this finding was particularly noteworthy, as the BBB-crossing group had relatively higher vascular risk burden and lower mean education level.
“These differences make it all the more remarkable that the BBB-crossing group displayed better memory ability over time despite these cognitive disadvantages,” the investigators wrote.
Still, not all the findings favored BBB-crossing agents. Individuals in the BBB-crossing group had relatively inferior attention ability, with a minimum effect size of –0.17 (P = .02).
The other cognitive measures were not significantly different between groups.
Clinicians may consider findings after accounting for other factors
Principal investigator Daniel A. Nation, PhD, associate professor of psychological science and a faculty member of the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine, suggested that the small difference in verbal memory between groups could be clinically significant over a longer period of time.
“Although the overall effect size was pretty small, if you look at how long it would take for someone [with dementia] to progress over many years of decline, it would actually end up being a pretty big effect,” Dr. Nation said in an interview. “Small effect sizes could actually end up preventing a lot of cases of dementia,” he added.
The conflicting results in the BBB-crossing group – better verbal memory but worse attention ability – were “surprising,” he noted.
“I sort of didn’t believe it at first,” Dr. Nation said, “because the memory finding is sort of replication – we’d observed the same exact effect on memory in a smaller sample in another study. ... The attention [finding], going another way, was a new thing.”
Dr. Nation suggested that the intergroup differences in attention ability may stem from idiosyncrasies of the tests used to measure that domain, which can be impacted by cardiovascular or brain vascular disease. Or it could be caused by something else entirely, he said, noting that further investigation is needed.
He added that the improvements in verbal memory within the BBB-crossing group could be caused by direct effects on the brain. He pointed out that certain ACE polymorphisms have been linked with Alzheimer’s disease risk, and those same polymorphisms, in animal models, lead to neurodegeneration, with reversal possible through administration of ACE inhibitors.
“It could be that what we’re observing has nothing really to do with blood pressure,” Dr. Nation explained. “This could be a neuronal effect on learning memory systems.”
He went on to suggest that clinicians may consider these findings when selecting antihypertensive agents for their patients, with the caveat that all other prescribing factors have already been taking to account.
“In the event that you’re going to give an ACE inhibitor or an angiotensin receptor blocker anyway, and it ends up being a somewhat arbitrary decision in terms of which specific drug you’re going to give, then perhaps this is a piece of information you would take into account – that one gets in the brain and one doesn’t – in somebody at risk for cognitive decline,” Dr. Nation said.
Exact mechanisms of action unknown
Hélène Girouard, PhD, assistant professor of pharmacology and physiology at the University of Montreal, said in an interview that the findings are “of considerable importance, knowing that brain alterations could begin as much as 30 years before manifestation of dementia.”
Since 2003, Dr. Girouard has been studying the cognitive effects of antihypertensive medications. She noted that previous studies involving rodents “have shown beneficial effects [of BBB-crossing antihypertensive drugs] on cognition independent of their effects on blood pressure.”
The drugs’ exact mechanisms of action, however, remain elusive, according to Dr. Girouard, who offered several possible explanations, including amelioration of BBB disruption, brain inflammation, cerebral blood flow dysregulation, cholinergic dysfunction, and neurologic deficits. “Whether these mechanisms may explain Ho and colleagues’ observations remains to be established,” she added.
Andrea L. Schneider, MD, PhD, assistant professor of neurology at the University of Pennsylvania, Philadelphia, applauded the study, but ultimately suggested that more research is needed to impact clinical decision-making.
“The results of this important and well-done study suggest that further investigation into targeted mechanism-based approaches to selecting hypertension treatment agents, with a specific focus on cognitive outcomes, is warranted,” Dr. Schneider said in an interview. “Before changing clinical practice, further work is necessary to disentangle contributions of medication mechanism, comorbid vascular risk factors, and achieved blood pressure reduction, among others.”
The investigators disclosed support from the National Institutes of Health, the Alzheimer’s Association, the Waksman Foundation of Japan, and others. The interviewees reported no relevant conflicts of interest.
Over a 3-year period, cognitively normal older adults taking BBB-crossing antihypertensives demonstrated superior verbal memory, compared with similar individuals receiving non–BBB-crossing antihypertensives, reported lead author Jean K. Ho, PhD, of the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine, and colleagues.
According to the investigators, the findings add color to a known link between hypertension and neurologic degeneration, and may aid the search for new therapeutic targets.
“Hypertension is a well-established risk factor for cognitive decline and dementia, possibly through its effects on both cerebrovascular disease and Alzheimer’s disease,” Dr. Ho and colleagues wrote in Hypertension. “Studies of antihypertensive treatments have reported possible salutary effects on cognition and cerebrovascular disease, as well as Alzheimer’s disease neuropathology.”
In a previous study, individuals younger than 75 years exposed to antihypertensives had an 8% decreased risk of dementia per year of use, while another trial showed that intensive blood pressure–lowering therapy reduced mild cognitive impairment by 19%.
“Despite these encouraging findings ... larger meta-analytic studies have been hampered by the fact that pharmacokinetic properties are typically not considered in existing studies or routine clinical practice,” wrote Dr. Ho and colleagues. “The present study sought to fill this gap [in that it was] a large and longitudinal meta-analytic study of existing data recoded to assess the effects of BBB-crossing potential in renin-angiotensin system [RAS] treatments among hypertensive adults.”
Methods and results
The meta-analysis included randomized clinical trials, prospective cohort studies, and retrospective observational studies. The researchers assessed data on 12,849 individuals from 14 cohorts that received either BBB-crossing or non–BBB-crossing antihypertensives.
The BBB-crossing properties of RAS treatments were identified by a literature review. Of ACE inhibitors, captopril, fosinopril, lisinopril, perindopril, ramipril, and trandolapril were classified as BBB crossing, and benazepril, enalapril, moexipril, and quinapril were classified as non–BBB-crossing. Of ARBs, telmisartan and candesartan were considered BBB-crossing, and olmesartan, eprosartan, irbesartan, and losartan were tagged as non–BBB-crossing.
Cognition was assessed via the following seven domains: executive function, attention, verbal memory learning, language, mental status, recall, and processing speed.
Compared with individuals taking non–BBB-crossing antihypertensives, those taking BBB-crossing agents had significantly superior verbal memory (recall), with a maximum effect size of 0.07 (P = .03).
According to the investigators, this finding was particularly noteworthy, as the BBB-crossing group had relatively higher vascular risk burden and lower mean education level.
“These differences make it all the more remarkable that the BBB-crossing group displayed better memory ability over time despite these cognitive disadvantages,” the investigators wrote.
Still, not all the findings favored BBB-crossing agents. Individuals in the BBB-crossing group had relatively inferior attention ability, with a minimum effect size of –0.17 (P = .02).
The other cognitive measures were not significantly different between groups.
Clinicians may consider findings after accounting for other factors
Principal investigator Daniel A. Nation, PhD, associate professor of psychological science and a faculty member of the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine, suggested that the small difference in verbal memory between groups could be clinically significant over a longer period of time.
“Although the overall effect size was pretty small, if you look at how long it would take for someone [with dementia] to progress over many years of decline, it would actually end up being a pretty big effect,” Dr. Nation said in an interview. “Small effect sizes could actually end up preventing a lot of cases of dementia,” he added.
The conflicting results in the BBB-crossing group – better verbal memory but worse attention ability – were “surprising,” he noted.
“I sort of didn’t believe it at first,” Dr. Nation said, “because the memory finding is sort of replication – we’d observed the same exact effect on memory in a smaller sample in another study. ... The attention [finding], going another way, was a new thing.”
Dr. Nation suggested that the intergroup differences in attention ability may stem from idiosyncrasies of the tests used to measure that domain, which can be impacted by cardiovascular or brain vascular disease. Or it could be caused by something else entirely, he said, noting that further investigation is needed.
He added that the improvements in verbal memory within the BBB-crossing group could be caused by direct effects on the brain. He pointed out that certain ACE polymorphisms have been linked with Alzheimer’s disease risk, and those same polymorphisms, in animal models, lead to neurodegeneration, with reversal possible through administration of ACE inhibitors.
“It could be that what we’re observing has nothing really to do with blood pressure,” Dr. Nation explained. “This could be a neuronal effect on learning memory systems.”
He went on to suggest that clinicians may consider these findings when selecting antihypertensive agents for their patients, with the caveat that all other prescribing factors have already been taking to account.
“In the event that you’re going to give an ACE inhibitor or an angiotensin receptor blocker anyway, and it ends up being a somewhat arbitrary decision in terms of which specific drug you’re going to give, then perhaps this is a piece of information you would take into account – that one gets in the brain and one doesn’t – in somebody at risk for cognitive decline,” Dr. Nation said.
Exact mechanisms of action unknown
Hélène Girouard, PhD, assistant professor of pharmacology and physiology at the University of Montreal, said in an interview that the findings are “of considerable importance, knowing that brain alterations could begin as much as 30 years before manifestation of dementia.”
Since 2003, Dr. Girouard has been studying the cognitive effects of antihypertensive medications. She noted that previous studies involving rodents “have shown beneficial effects [of BBB-crossing antihypertensive drugs] on cognition independent of their effects on blood pressure.”
The drugs’ exact mechanisms of action, however, remain elusive, according to Dr. Girouard, who offered several possible explanations, including amelioration of BBB disruption, brain inflammation, cerebral blood flow dysregulation, cholinergic dysfunction, and neurologic deficits. “Whether these mechanisms may explain Ho and colleagues’ observations remains to be established,” she added.
Andrea L. Schneider, MD, PhD, assistant professor of neurology at the University of Pennsylvania, Philadelphia, applauded the study, but ultimately suggested that more research is needed to impact clinical decision-making.
“The results of this important and well-done study suggest that further investigation into targeted mechanism-based approaches to selecting hypertension treatment agents, with a specific focus on cognitive outcomes, is warranted,” Dr. Schneider said in an interview. “Before changing clinical practice, further work is necessary to disentangle contributions of medication mechanism, comorbid vascular risk factors, and achieved blood pressure reduction, among others.”
The investigators disclosed support from the National Institutes of Health, the Alzheimer’s Association, the Waksman Foundation of Japan, and others. The interviewees reported no relevant conflicts of interest.
Over a 3-year period, cognitively normal older adults taking BBB-crossing antihypertensives demonstrated superior verbal memory, compared with similar individuals receiving non–BBB-crossing antihypertensives, reported lead author Jean K. Ho, PhD, of the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine, and colleagues.
According to the investigators, the findings add color to a known link between hypertension and neurologic degeneration, and may aid the search for new therapeutic targets.
“Hypertension is a well-established risk factor for cognitive decline and dementia, possibly through its effects on both cerebrovascular disease and Alzheimer’s disease,” Dr. Ho and colleagues wrote in Hypertension. “Studies of antihypertensive treatments have reported possible salutary effects on cognition and cerebrovascular disease, as well as Alzheimer’s disease neuropathology.”
In a previous study, individuals younger than 75 years exposed to antihypertensives had an 8% decreased risk of dementia per year of use, while another trial showed that intensive blood pressure–lowering therapy reduced mild cognitive impairment by 19%.
“Despite these encouraging findings ... larger meta-analytic studies have been hampered by the fact that pharmacokinetic properties are typically not considered in existing studies or routine clinical practice,” wrote Dr. Ho and colleagues. “The present study sought to fill this gap [in that it was] a large and longitudinal meta-analytic study of existing data recoded to assess the effects of BBB-crossing potential in renin-angiotensin system [RAS] treatments among hypertensive adults.”
Methods and results
The meta-analysis included randomized clinical trials, prospective cohort studies, and retrospective observational studies. The researchers assessed data on 12,849 individuals from 14 cohorts that received either BBB-crossing or non–BBB-crossing antihypertensives.
The BBB-crossing properties of RAS treatments were identified by a literature review. Of ACE inhibitors, captopril, fosinopril, lisinopril, perindopril, ramipril, and trandolapril were classified as BBB crossing, and benazepril, enalapril, moexipril, and quinapril were classified as non–BBB-crossing. Of ARBs, telmisartan and candesartan were considered BBB-crossing, and olmesartan, eprosartan, irbesartan, and losartan were tagged as non–BBB-crossing.
Cognition was assessed via the following seven domains: executive function, attention, verbal memory learning, language, mental status, recall, and processing speed.
Compared with individuals taking non–BBB-crossing antihypertensives, those taking BBB-crossing agents had significantly superior verbal memory (recall), with a maximum effect size of 0.07 (P = .03).
According to the investigators, this finding was particularly noteworthy, as the BBB-crossing group had relatively higher vascular risk burden and lower mean education level.
“These differences make it all the more remarkable that the BBB-crossing group displayed better memory ability over time despite these cognitive disadvantages,” the investigators wrote.
Still, not all the findings favored BBB-crossing agents. Individuals in the BBB-crossing group had relatively inferior attention ability, with a minimum effect size of –0.17 (P = .02).
The other cognitive measures were not significantly different between groups.
Clinicians may consider findings after accounting for other factors
Principal investigator Daniel A. Nation, PhD, associate professor of psychological science and a faculty member of the Institute for Memory Impairments and Neurological Disorders at the University of California, Irvine, suggested that the small difference in verbal memory between groups could be clinically significant over a longer period of time.
“Although the overall effect size was pretty small, if you look at how long it would take for someone [with dementia] to progress over many years of decline, it would actually end up being a pretty big effect,” Dr. Nation said in an interview. “Small effect sizes could actually end up preventing a lot of cases of dementia,” he added.
The conflicting results in the BBB-crossing group – better verbal memory but worse attention ability – were “surprising,” he noted.
“I sort of didn’t believe it at first,” Dr. Nation said, “because the memory finding is sort of replication – we’d observed the same exact effect on memory in a smaller sample in another study. ... The attention [finding], going another way, was a new thing.”
Dr. Nation suggested that the intergroup differences in attention ability may stem from idiosyncrasies of the tests used to measure that domain, which can be impacted by cardiovascular or brain vascular disease. Or it could be caused by something else entirely, he said, noting that further investigation is needed.
He added that the improvements in verbal memory within the BBB-crossing group could be caused by direct effects on the brain. He pointed out that certain ACE polymorphisms have been linked with Alzheimer’s disease risk, and those same polymorphisms, in animal models, lead to neurodegeneration, with reversal possible through administration of ACE inhibitors.
“It could be that what we’re observing has nothing really to do with blood pressure,” Dr. Nation explained. “This could be a neuronal effect on learning memory systems.”
He went on to suggest that clinicians may consider these findings when selecting antihypertensive agents for their patients, with the caveat that all other prescribing factors have already been taking to account.
“In the event that you’re going to give an ACE inhibitor or an angiotensin receptor blocker anyway, and it ends up being a somewhat arbitrary decision in terms of which specific drug you’re going to give, then perhaps this is a piece of information you would take into account – that one gets in the brain and one doesn’t – in somebody at risk for cognitive decline,” Dr. Nation said.
Exact mechanisms of action unknown
Hélène Girouard, PhD, assistant professor of pharmacology and physiology at the University of Montreal, said in an interview that the findings are “of considerable importance, knowing that brain alterations could begin as much as 30 years before manifestation of dementia.”
Since 2003, Dr. Girouard has been studying the cognitive effects of antihypertensive medications. She noted that previous studies involving rodents “have shown beneficial effects [of BBB-crossing antihypertensive drugs] on cognition independent of their effects on blood pressure.”
The drugs’ exact mechanisms of action, however, remain elusive, according to Dr. Girouard, who offered several possible explanations, including amelioration of BBB disruption, brain inflammation, cerebral blood flow dysregulation, cholinergic dysfunction, and neurologic deficits. “Whether these mechanisms may explain Ho and colleagues’ observations remains to be established,” she added.
Andrea L. Schneider, MD, PhD, assistant professor of neurology at the University of Pennsylvania, Philadelphia, applauded the study, but ultimately suggested that more research is needed to impact clinical decision-making.
“The results of this important and well-done study suggest that further investigation into targeted mechanism-based approaches to selecting hypertension treatment agents, with a specific focus on cognitive outcomes, is warranted,” Dr. Schneider said in an interview. “Before changing clinical practice, further work is necessary to disentangle contributions of medication mechanism, comorbid vascular risk factors, and achieved blood pressure reduction, among others.”
The investigators disclosed support from the National Institutes of Health, the Alzheimer’s Association, the Waksman Foundation of Japan, and others. The interviewees reported no relevant conflicts of interest.
FROM HYPERTENSION
Potential first-in-class, targeted therapy for myasthenia gravis
(gMG), new research suggests. Results from the phase 3, randomized, placebo-controlled ADAPT trial showed that reduction in disease burden and improvement in strength and quality of life in patients with gMG were consistent across four MG-specific scales for those receiving the novel treatment. In addition, these benefits were observed early and were reproducible and durable, the researchers noted.
Efgartigimod is a “very rapidly acting drug relative to other treatments that may take 4, 6, sometimes 10 months before they start to work, and the side effect profile is much like placebo,” said principal investigator James Howard, Jr., MD, Distinguished Professor of Neuromuscular Disease, department of neurology, University of North Carolina at Chapel Hill.
The ADAPT results are “important for the MG community, and I am hopeful efgartigimod will provide a first-in-class targeted therapy that can be dosed in an individual way for people living with this chronic autoimmune disease,” Dr. Howard added in a news release.
The findings were published online June 17 in Lancet Neurology.
Targeted molecular therapy
The rare and chronic autoimmune neuromuscular disorder of gMG causes debilitating and potentially life-threatening muscle weakness and significantly impaired independence and quality of life. Most patients with gMG have immunoglobulin G (IgG) antibodies, which are most often directed against skeletal muscle nicotinic acetylcholine receptors.
Efgartigimod is an investigational antibody fragment designed to reduce pathogenic IgG antibodies and block the IgG recycling process in patients with gMG.
The novel agent binds to the neonatal Fc receptor (FcRn), which is widely expressed throughout the body and plays a central role in rescuing IgG antibodies from degradation. Blocking FcRn reduces IgG antibody levels.
The ADAPT trial was conducted at 56 neuromuscular academic and community centers in 15 countries in North America, Europe, and Japan. The study included 167 adults with gMG, regardless of acetylcholine receptor antibody status. All had a Myasthenia Gravis Activities of Daily Living (MG-ADL) score of at least 5 (greater than 50% non-ocular) on a background of a stable dose of at least one MG drug.
For 26 weeks, 84 patients were randomly assigned to receive efgartigimod 10 mg/kg and 83 to receive matching placebo. Both treatments were administered as four infusions per cycle at one infusion per week. The process was repeated as needed depending on clinical response no sooner than 8 weeks after initiation of the previous cycle.
ADAPT was designed to allow an individualized treatment approach with an initial treatment cycle followed by a variable number of subsequent treatment cycles, the investigators noted.
Primary endpoint met
The primary efficacy endpoint was number of acetylcholine receptor antibody-positive (AChR-Ab+) patients who achieved a clinically meaningful response on the MG-ADL score. This was defined as at least a 2-point improvement from baseline for 4 or more consecutive weeks. Forty-four (68%) of 65 AChR-Ab+ patients treated with efgartigimod met this endpoint versus 19 (30%) of 64 patients treated with placebo (odds ratio, 4.95; 95% confidence interval, 2.21-11.53; P < .0001).
Many of the patients treated with efgartigimod showed improvement “beyond the clinically meaningful threshold, achieving up to 9-point reductions in MG-ADL,” the investigators reported. In addition, 40% of the efgartigimod group attained an MG-ADL score of 0 or 1 (minimal symptom expression) in cycle 1 versus 11% of the placebo group (P < .0001).
Nearly two-thirds (63%) of AChR-Ab+ patients responded to the first cycle of efgartigimod, and most of these patients (83%) responded to treatment within the first 2 weeks. Among the AChR-Ab+ participants who responded to efgartigimod in cycle 1, the duration of responder status was 6 to 7 weeks in 32% of patients, 8 to 11 weeks in 23% of patients, and 12 weeks or more in 34% of patients.
Safety profile
“Some patients never required retreatment over the 26-week period that they were under observation,” Dr. Howard said. “Patients want to be individuals. They don’t want to be assigned to a regimented therapy, and I think these results show that this therapy can be tailored to the individual patient, rather than simply giving it to them in a cookbook fashion,” he added.
The safety profile of efgartigimod was comparable to placebo. Most adverse events were mild or moderate in severity. The most commonly reported adverse events were headache, nasopharyngitis, nausea, diarrhea, upper respiratory tract infection, and urinary tract infection.
Four (5%) efgartigimod-treated patients had a serious adverse event, which included thrombocytosis, rectal adenocarcinoma, worsening MG, and depression.
The novel agent is currently under review with the U.S. Food and Drug Administration for the treatment of gMG, with a Prescription Drug User Fee Act target action date of Dec. 17. If approved, it would become the first FDA-approved FcRn antagonist.
Expanding therapeutic landscape
In a linked commentary, Shigeaki Suzuki, MD, PhD, department of neurology, Keio University School of Medicine, Tokyo, noted that the therapeutic landscape for patients with MG is “expanding year by year,” with several additional complement inhibitors and FcRn antagonists now in phase 3 testing.
“Biological drugs should be preferentially used as the treatment for patients with refractory myasthenia gravis, although the definition of refractory myasthenia gravis is different depending on the criteria used,” Dr. Suzuki wrote.
He noted that when “cost-effectiveness is not taken into account, targeted molecular therapy might be used widely” in patients with MG.
“Risks of myasthenic exacerbation and crises should be substantially decreased, particularly in patients with refractory myasthenia gravis,” Dr. Suzuki added.
The ADAPT study was supported by argenx. Dr. Howard has reported receiving research support from argenx, Alexion Pharmaceuticals, the Centers for Disease Control and Prevention, the Muscular Dystrophy Association, the National Institutes of Health, Patient-Centered Outcomes Research Institute, and Ra Pharmaceuticals; honoraria from argenx, Alexion, Immunovant, Ra, Regeneron Pharmaceuticals, and Viela Bio; and nonfinancial support from argenx, Alexion, Ra, and Toleranzia. Disclosures for the other investigators are listed in the original article. Dr. Suzuki has reported relationships with Alexion Pharmaceuticals, Japan Blood Products Organization, and Asahi Kasei Medical.
A version of this article first appeared on Medscape.com.
(gMG), new research suggests. Results from the phase 3, randomized, placebo-controlled ADAPT trial showed that reduction in disease burden and improvement in strength and quality of life in patients with gMG were consistent across four MG-specific scales for those receiving the novel treatment. In addition, these benefits were observed early and were reproducible and durable, the researchers noted.
Efgartigimod is a “very rapidly acting drug relative to other treatments that may take 4, 6, sometimes 10 months before they start to work, and the side effect profile is much like placebo,” said principal investigator James Howard, Jr., MD, Distinguished Professor of Neuromuscular Disease, department of neurology, University of North Carolina at Chapel Hill.
The ADAPT results are “important for the MG community, and I am hopeful efgartigimod will provide a first-in-class targeted therapy that can be dosed in an individual way for people living with this chronic autoimmune disease,” Dr. Howard added in a news release.
The findings were published online June 17 in Lancet Neurology.
Targeted molecular therapy
The rare and chronic autoimmune neuromuscular disorder of gMG causes debilitating and potentially life-threatening muscle weakness and significantly impaired independence and quality of life. Most patients with gMG have immunoglobulin G (IgG) antibodies, which are most often directed against skeletal muscle nicotinic acetylcholine receptors.
Efgartigimod is an investigational antibody fragment designed to reduce pathogenic IgG antibodies and block the IgG recycling process in patients with gMG.
The novel agent binds to the neonatal Fc receptor (FcRn), which is widely expressed throughout the body and plays a central role in rescuing IgG antibodies from degradation. Blocking FcRn reduces IgG antibody levels.
The ADAPT trial was conducted at 56 neuromuscular academic and community centers in 15 countries in North America, Europe, and Japan. The study included 167 adults with gMG, regardless of acetylcholine receptor antibody status. All had a Myasthenia Gravis Activities of Daily Living (MG-ADL) score of at least 5 (greater than 50% non-ocular) on a background of a stable dose of at least one MG drug.
For 26 weeks, 84 patients were randomly assigned to receive efgartigimod 10 mg/kg and 83 to receive matching placebo. Both treatments were administered as four infusions per cycle at one infusion per week. The process was repeated as needed depending on clinical response no sooner than 8 weeks after initiation of the previous cycle.
ADAPT was designed to allow an individualized treatment approach with an initial treatment cycle followed by a variable number of subsequent treatment cycles, the investigators noted.
Primary endpoint met
The primary efficacy endpoint was number of acetylcholine receptor antibody-positive (AChR-Ab+) patients who achieved a clinically meaningful response on the MG-ADL score. This was defined as at least a 2-point improvement from baseline for 4 or more consecutive weeks. Forty-four (68%) of 65 AChR-Ab+ patients treated with efgartigimod met this endpoint versus 19 (30%) of 64 patients treated with placebo (odds ratio, 4.95; 95% confidence interval, 2.21-11.53; P < .0001).
Many of the patients treated with efgartigimod showed improvement “beyond the clinically meaningful threshold, achieving up to 9-point reductions in MG-ADL,” the investigators reported. In addition, 40% of the efgartigimod group attained an MG-ADL score of 0 or 1 (minimal symptom expression) in cycle 1 versus 11% of the placebo group (P < .0001).
Nearly two-thirds (63%) of AChR-Ab+ patients responded to the first cycle of efgartigimod, and most of these patients (83%) responded to treatment within the first 2 weeks. Among the AChR-Ab+ participants who responded to efgartigimod in cycle 1, the duration of responder status was 6 to 7 weeks in 32% of patients, 8 to 11 weeks in 23% of patients, and 12 weeks or more in 34% of patients.
Safety profile
“Some patients never required retreatment over the 26-week period that they were under observation,” Dr. Howard said. “Patients want to be individuals. They don’t want to be assigned to a regimented therapy, and I think these results show that this therapy can be tailored to the individual patient, rather than simply giving it to them in a cookbook fashion,” he added.
The safety profile of efgartigimod was comparable to placebo. Most adverse events were mild or moderate in severity. The most commonly reported adverse events were headache, nasopharyngitis, nausea, diarrhea, upper respiratory tract infection, and urinary tract infection.
Four (5%) efgartigimod-treated patients had a serious adverse event, which included thrombocytosis, rectal adenocarcinoma, worsening MG, and depression.
The novel agent is currently under review with the U.S. Food and Drug Administration for the treatment of gMG, with a Prescription Drug User Fee Act target action date of Dec. 17. If approved, it would become the first FDA-approved FcRn antagonist.
Expanding therapeutic landscape
In a linked commentary, Shigeaki Suzuki, MD, PhD, department of neurology, Keio University School of Medicine, Tokyo, noted that the therapeutic landscape for patients with MG is “expanding year by year,” with several additional complement inhibitors and FcRn antagonists now in phase 3 testing.
“Biological drugs should be preferentially used as the treatment for patients with refractory myasthenia gravis, although the definition of refractory myasthenia gravis is different depending on the criteria used,” Dr. Suzuki wrote.
He noted that when “cost-effectiveness is not taken into account, targeted molecular therapy might be used widely” in patients with MG.
“Risks of myasthenic exacerbation and crises should be substantially decreased, particularly in patients with refractory myasthenia gravis,” Dr. Suzuki added.
The ADAPT study was supported by argenx. Dr. Howard has reported receiving research support from argenx, Alexion Pharmaceuticals, the Centers for Disease Control and Prevention, the Muscular Dystrophy Association, the National Institutes of Health, Patient-Centered Outcomes Research Institute, and Ra Pharmaceuticals; honoraria from argenx, Alexion, Immunovant, Ra, Regeneron Pharmaceuticals, and Viela Bio; and nonfinancial support from argenx, Alexion, Ra, and Toleranzia. Disclosures for the other investigators are listed in the original article. Dr. Suzuki has reported relationships with Alexion Pharmaceuticals, Japan Blood Products Organization, and Asahi Kasei Medical.
A version of this article first appeared on Medscape.com.
(gMG), new research suggests. Results from the phase 3, randomized, placebo-controlled ADAPT trial showed that reduction in disease burden and improvement in strength and quality of life in patients with gMG were consistent across four MG-specific scales for those receiving the novel treatment. In addition, these benefits were observed early and were reproducible and durable, the researchers noted.
Efgartigimod is a “very rapidly acting drug relative to other treatments that may take 4, 6, sometimes 10 months before they start to work, and the side effect profile is much like placebo,” said principal investigator James Howard, Jr., MD, Distinguished Professor of Neuromuscular Disease, department of neurology, University of North Carolina at Chapel Hill.
The ADAPT results are “important for the MG community, and I am hopeful efgartigimod will provide a first-in-class targeted therapy that can be dosed in an individual way for people living with this chronic autoimmune disease,” Dr. Howard added in a news release.
The findings were published online June 17 in Lancet Neurology.
Targeted molecular therapy
The rare and chronic autoimmune neuromuscular disorder of gMG causes debilitating and potentially life-threatening muscle weakness and significantly impaired independence and quality of life. Most patients with gMG have immunoglobulin G (IgG) antibodies, which are most often directed against skeletal muscle nicotinic acetylcholine receptors.
Efgartigimod is an investigational antibody fragment designed to reduce pathogenic IgG antibodies and block the IgG recycling process in patients with gMG.
The novel agent binds to the neonatal Fc receptor (FcRn), which is widely expressed throughout the body and plays a central role in rescuing IgG antibodies from degradation. Blocking FcRn reduces IgG antibody levels.
The ADAPT trial was conducted at 56 neuromuscular academic and community centers in 15 countries in North America, Europe, and Japan. The study included 167 adults with gMG, regardless of acetylcholine receptor antibody status. All had a Myasthenia Gravis Activities of Daily Living (MG-ADL) score of at least 5 (greater than 50% non-ocular) on a background of a stable dose of at least one MG drug.
For 26 weeks, 84 patients were randomly assigned to receive efgartigimod 10 mg/kg and 83 to receive matching placebo. Both treatments were administered as four infusions per cycle at one infusion per week. The process was repeated as needed depending on clinical response no sooner than 8 weeks after initiation of the previous cycle.
ADAPT was designed to allow an individualized treatment approach with an initial treatment cycle followed by a variable number of subsequent treatment cycles, the investigators noted.
Primary endpoint met
The primary efficacy endpoint was number of acetylcholine receptor antibody-positive (AChR-Ab+) patients who achieved a clinically meaningful response on the MG-ADL score. This was defined as at least a 2-point improvement from baseline for 4 or more consecutive weeks. Forty-four (68%) of 65 AChR-Ab+ patients treated with efgartigimod met this endpoint versus 19 (30%) of 64 patients treated with placebo (odds ratio, 4.95; 95% confidence interval, 2.21-11.53; P < .0001).
Many of the patients treated with efgartigimod showed improvement “beyond the clinically meaningful threshold, achieving up to 9-point reductions in MG-ADL,” the investigators reported. In addition, 40% of the efgartigimod group attained an MG-ADL score of 0 or 1 (minimal symptom expression) in cycle 1 versus 11% of the placebo group (P < .0001).
Nearly two-thirds (63%) of AChR-Ab+ patients responded to the first cycle of efgartigimod, and most of these patients (83%) responded to treatment within the first 2 weeks. Among the AChR-Ab+ participants who responded to efgartigimod in cycle 1, the duration of responder status was 6 to 7 weeks in 32% of patients, 8 to 11 weeks in 23% of patients, and 12 weeks or more in 34% of patients.
Safety profile
“Some patients never required retreatment over the 26-week period that they were under observation,” Dr. Howard said. “Patients want to be individuals. They don’t want to be assigned to a regimented therapy, and I think these results show that this therapy can be tailored to the individual patient, rather than simply giving it to them in a cookbook fashion,” he added.
The safety profile of efgartigimod was comparable to placebo. Most adverse events were mild or moderate in severity. The most commonly reported adverse events were headache, nasopharyngitis, nausea, diarrhea, upper respiratory tract infection, and urinary tract infection.
Four (5%) efgartigimod-treated patients had a serious adverse event, which included thrombocytosis, rectal adenocarcinoma, worsening MG, and depression.
The novel agent is currently under review with the U.S. Food and Drug Administration for the treatment of gMG, with a Prescription Drug User Fee Act target action date of Dec. 17. If approved, it would become the first FDA-approved FcRn antagonist.
Expanding therapeutic landscape
In a linked commentary, Shigeaki Suzuki, MD, PhD, department of neurology, Keio University School of Medicine, Tokyo, noted that the therapeutic landscape for patients with MG is “expanding year by year,” with several additional complement inhibitors and FcRn antagonists now in phase 3 testing.
“Biological drugs should be preferentially used as the treatment for patients with refractory myasthenia gravis, although the definition of refractory myasthenia gravis is different depending on the criteria used,” Dr. Suzuki wrote.
He noted that when “cost-effectiveness is not taken into account, targeted molecular therapy might be used widely” in patients with MG.
“Risks of myasthenic exacerbation and crises should be substantially decreased, particularly in patients with refractory myasthenia gravis,” Dr. Suzuki added.
The ADAPT study was supported by argenx. Dr. Howard has reported receiving research support from argenx, Alexion Pharmaceuticals, the Centers for Disease Control and Prevention, the Muscular Dystrophy Association, the National Institutes of Health, Patient-Centered Outcomes Research Institute, and Ra Pharmaceuticals; honoraria from argenx, Alexion, Immunovant, Ra, Regeneron Pharmaceuticals, and Viela Bio; and nonfinancial support from argenx, Alexion, Ra, and Toleranzia. Disclosures for the other investigators are listed in the original article. Dr. Suzuki has reported relationships with Alexion Pharmaceuticals, Japan Blood Products Organization, and Asahi Kasei Medical.
A version of this article first appeared on Medscape.com.
From Lancet Neurology
How dreams might prepare you for what’s next
What you experience in your dreams might feel random and disjointed, but that chaos during sleep might serve a function, according to Erin Wamsley, PhD, an associate professor of psychology and neuroscience at Furman University in Greenville, S.C. In fact, evidence uncovered by Dr. Wamsley and associates suggests that
Previous research and anecdotal evidence have shown that dreams use fragments of past experiences, Dr. Wamsley explained. While studying dreams, her team found that the mind is using select fragments of past experiences to prepare for a known upcoming event.
“This is new evidence that dreams reflect a memory-processing function,” said Dr. Wamsley, who presented the work at the virtual annual meeting of the Associated Professional Sleep Societies.
Some high performers already use past experiences to excel in future events. For instance, Michael Phelps, the most decorated Olympic swimmer, with 28 medals, would “mentally rehearse” his swims for up to 2 hours per day, according to his coach, Bob Bowman.
Using sleep to strengthen this process is an exciting prospect that scientists have been eager to figure out, said Allison Brager, PhD, director of human performance at the U.S. Army Warrior Fitness Training Center. Deep REM sleep can lead to improved learning and memory, she said. “So, hypothetically, better dreams mean better sleep, and that equals better performance.”
For their research, Dr. Wamsley’s team hooked 48 students up to a polysomnography machine to measure sleep cycles and how often they were in a deep REM sleep. The students who took part in the study spent the night in a sleep lab.
The students were woken up multiple times during the night and asked to report what they were dreaming about.
In the morning, they were given their reports and asked to identify familiar features or potential sources for particular dreams. More than half the dreams were tied to a memory the students recalled. One-quarter of the dreams were related to specific upcoming events the students reported. And about 40% of the dreams with a future event in them also included memories of past experiences. This was more common the longer the students dreamed, the scientists explained.
And this was also more common later in the night, possibly because the dreamer is closer to waking and the anticipated event is approaching, Dr. Wamsley said.
Studying dreams is a tricky, subjective business and not always taken as seriously as other aspects of sleep and neuroscience because it involves questions of human consciousness itself, said Erik Hoel, PhD, a research assistant professor of neuroscience at Tufts University in Medford, Mass.
In a recent report published in Patterns, he suggested that our weirdest dreams help our brains process our day-to-day experiences in a way that enables deeper learning.
“This type of research is challenged by the method,” Dr. Hoel said.
In the Wamsley study, “waking people up from a deep sleep and asking them to recollect their dream content will only get you part of the experience because it fades so quickly.” That said, the value of connecting what happens as a result could be meaningful, he noted. For example, study participants could be asked whether their future event went as planned and whether they think the outcome was related to how well they “prepared” in their dreams.
Even then, it would still be a subjective analysis. But going in those directions might lead to meaningful new training, Dr. Hoel said.
And training yourself to recall only specific memories right before sleep might prepare your mind in a focused way for certain events, from giving a presentation to having a difficult conversation with someone, or maybe even winning at the Olympics.
A version of this article first appeared on WebMD.com.
What you experience in your dreams might feel random and disjointed, but that chaos during sleep might serve a function, according to Erin Wamsley, PhD, an associate professor of psychology and neuroscience at Furman University in Greenville, S.C. In fact, evidence uncovered by Dr. Wamsley and associates suggests that
Previous research and anecdotal evidence have shown that dreams use fragments of past experiences, Dr. Wamsley explained. While studying dreams, her team found that the mind is using select fragments of past experiences to prepare for a known upcoming event.
“This is new evidence that dreams reflect a memory-processing function,” said Dr. Wamsley, who presented the work at the virtual annual meeting of the Associated Professional Sleep Societies.
Some high performers already use past experiences to excel in future events. For instance, Michael Phelps, the most decorated Olympic swimmer, with 28 medals, would “mentally rehearse” his swims for up to 2 hours per day, according to his coach, Bob Bowman.
Using sleep to strengthen this process is an exciting prospect that scientists have been eager to figure out, said Allison Brager, PhD, director of human performance at the U.S. Army Warrior Fitness Training Center. Deep REM sleep can lead to improved learning and memory, she said. “So, hypothetically, better dreams mean better sleep, and that equals better performance.”
For their research, Dr. Wamsley’s team hooked 48 students up to a polysomnography machine to measure sleep cycles and how often they were in a deep REM sleep. The students who took part in the study spent the night in a sleep lab.
The students were woken up multiple times during the night and asked to report what they were dreaming about.
In the morning, they were given their reports and asked to identify familiar features or potential sources for particular dreams. More than half the dreams were tied to a memory the students recalled. One-quarter of the dreams were related to specific upcoming events the students reported. And about 40% of the dreams with a future event in them also included memories of past experiences. This was more common the longer the students dreamed, the scientists explained.
And this was also more common later in the night, possibly because the dreamer is closer to waking and the anticipated event is approaching, Dr. Wamsley said.
Studying dreams is a tricky, subjective business and not always taken as seriously as other aspects of sleep and neuroscience because it involves questions of human consciousness itself, said Erik Hoel, PhD, a research assistant professor of neuroscience at Tufts University in Medford, Mass.
In a recent report published in Patterns, he suggested that our weirdest dreams help our brains process our day-to-day experiences in a way that enables deeper learning.
“This type of research is challenged by the method,” Dr. Hoel said.
In the Wamsley study, “waking people up from a deep sleep and asking them to recollect their dream content will only get you part of the experience because it fades so quickly.” That said, the value of connecting what happens as a result could be meaningful, he noted. For example, study participants could be asked whether their future event went as planned and whether they think the outcome was related to how well they “prepared” in their dreams.
Even then, it would still be a subjective analysis. But going in those directions might lead to meaningful new training, Dr. Hoel said.
And training yourself to recall only specific memories right before sleep might prepare your mind in a focused way for certain events, from giving a presentation to having a difficult conversation with someone, or maybe even winning at the Olympics.
A version of this article first appeared on WebMD.com.
What you experience in your dreams might feel random and disjointed, but that chaos during sleep might serve a function, according to Erin Wamsley, PhD, an associate professor of psychology and neuroscience at Furman University in Greenville, S.C. In fact, evidence uncovered by Dr. Wamsley and associates suggests that
Previous research and anecdotal evidence have shown that dreams use fragments of past experiences, Dr. Wamsley explained. While studying dreams, her team found that the mind is using select fragments of past experiences to prepare for a known upcoming event.
“This is new evidence that dreams reflect a memory-processing function,” said Dr. Wamsley, who presented the work at the virtual annual meeting of the Associated Professional Sleep Societies.
Some high performers already use past experiences to excel in future events. For instance, Michael Phelps, the most decorated Olympic swimmer, with 28 medals, would “mentally rehearse” his swims for up to 2 hours per day, according to his coach, Bob Bowman.
Using sleep to strengthen this process is an exciting prospect that scientists have been eager to figure out, said Allison Brager, PhD, director of human performance at the U.S. Army Warrior Fitness Training Center. Deep REM sleep can lead to improved learning and memory, she said. “So, hypothetically, better dreams mean better sleep, and that equals better performance.”
For their research, Dr. Wamsley’s team hooked 48 students up to a polysomnography machine to measure sleep cycles and how often they were in a deep REM sleep. The students who took part in the study spent the night in a sleep lab.
The students were woken up multiple times during the night and asked to report what they were dreaming about.
In the morning, they were given their reports and asked to identify familiar features or potential sources for particular dreams. More than half the dreams were tied to a memory the students recalled. One-quarter of the dreams were related to specific upcoming events the students reported. And about 40% of the dreams with a future event in them also included memories of past experiences. This was more common the longer the students dreamed, the scientists explained.
And this was also more common later in the night, possibly because the dreamer is closer to waking and the anticipated event is approaching, Dr. Wamsley said.
Studying dreams is a tricky, subjective business and not always taken as seriously as other aspects of sleep and neuroscience because it involves questions of human consciousness itself, said Erik Hoel, PhD, a research assistant professor of neuroscience at Tufts University in Medford, Mass.
In a recent report published in Patterns, he suggested that our weirdest dreams help our brains process our day-to-day experiences in a way that enables deeper learning.
“This type of research is challenged by the method,” Dr. Hoel said.
In the Wamsley study, “waking people up from a deep sleep and asking them to recollect their dream content will only get you part of the experience because it fades so quickly.” That said, the value of connecting what happens as a result could be meaningful, he noted. For example, study participants could be asked whether their future event went as planned and whether they think the outcome was related to how well they “prepared” in their dreams.
Even then, it would still be a subjective analysis. But going in those directions might lead to meaningful new training, Dr. Hoel said.
And training yourself to recall only specific memories right before sleep might prepare your mind in a focused way for certain events, from giving a presentation to having a difficult conversation with someone, or maybe even winning at the Olympics.
A version of this article first appeared on WebMD.com.
FROM SLEEP 2021
A Veteran Presenting With Shortness of Breath, Cough, and Leukocytosis
Case Presentation: A 62-year-old male presented with shortness of breath and a cough productive of green sputum. He had a history of hyperlipidemia, posttraumatic stress disorder, bipolar disorder, obstructive sleep apnea, and a 50 pack-year history of smoking. His medications included prazosin, melatonin, lithium, and gabapentin. He also had a significant exposure history including asbestos and chemical paints following his leave from the military. At the initial evaluation, laboratory work revealed a leukocytosis with white blood cell (WBC) count 20 k/cm3and otherwise normal transaminases, albumin, and electrolytes. A chest X-ray revealed a new left hilar mass.
►Manisha Apte, MD, Chief Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center (BMC): To work up his new left hilar mass, a computed tomography (CT) of the chest was ordered (Figure 1), which revealed an apical left lower lobe mass extending into the left hilum encasing part of the ascending aorta. Enlarged mediastinal subcentimeter paratracheal and superior mediastinal lymph nodes also were identified and the pattern raised the concern for lymphangitic carcinomatosis. Dr. Fine, what do you make of the CT findings?
► Alan Fine, MD, Section of Pulmonary and Critical Care, VABHS and Professor of Medicine, Boston University School of Medicine: This mass had irregular edges with septal thickening, which may be why there was a concern for lymphangitic spread. There were no clear tissue planes to see if this process was invading the mediastinum. The mass was irregular, a single lesion, and proximal, making it consistent with a lung cancer. In fact, with his history of smoking, asbestos exposure, the numbers 1 to 10 diagnoses were lung cancer. The lack of demarcation of tissue planes supports this. There are some infections, classically actinomycosis, that do cross and invade anatomical barriers.1 But this looked like a primary lung cancer.
► Dr. Apte: The patient was referred to a pulmonologist where an additional history of night sweats and weight loss were noted. Dr. Fine, we have a patient with a newly identified lung mass, and while we have reason to suspect malignancy as you have already noted, there are many other etiologies to consider, including infections (histoplasmosis, cryptococcosis, bacterial abscess), inflammatory processes (sarcoidosis, rheumatoid nodule) and vasculitis (granulomatosis with polyangiitis). What should be the next step taken to make a diagnosis?
►Dr. Fine: For cancer specifically, we would like to both stage and make a diagnosis with one procedure. That’s part of the utility of a positron emission tomography (PET) scan: We can see lymph node involvement and stage the cancer. We must consider the patient’s comorbidities and the location of the lesion (ie, is it amenable to needle biopsy?). In this case, there are enlarged mediastinal lymph nodes, so one could perform a bronchoscopy with endobronchial ultrasound, which is a relatively noninvasive way to sample the lymph nodes to ideally stage and make a diagnosis as safely as possible. If we are considering infection, needle aspiration is not as sensitive.2
► Dr. Apte: The patient underwent a PET CT, which redemonstrated the lung mass with a loss of aortic fat plane suspicious for aortic involvement as well as lymph nodes in levels 7 and 8 that were concerning for malignancy. Subsequent bronchoscopy with biopsy and endobronchial ultrasound did not show evidence of malignancy; washing and brushing from the mass and lymph node specimens did not identify malignant cells. Benign respiratory mucosa with mild chronic inflammation was noted. Dr. Fine, given the nonspecific findings on the PET scan, negative findings on our bronchoscopy, and a negative biopsy, should we be satisfied that we have ruled out cancer?
► Dr. Fine: No, bronchoscopy has its limitations. It’s highly sensitive to the diagnosis of malignancy if you can see an endobronchial lesion, but we did not see one here. You can only go so far with the scope, and it’s not uncommon for us not to be able to make the diagnosis with bronchoscopy. Malignancy is still the most likely diagnosis, and we need to work this up further. I would perform another biopsy.
►Dr. Apte: Four weeks later, the patient presented with continued shortness of breath, fatigue, and fever. A repeat chest CT showed an opacity suggestive of pneumonia. Given the continued concern for cancer a CT-guided needle biopsy was performed and was once again negative for malignancy. The decision was made to pursue a video-assisted thorascopic surgery (VATS). Following the VATS, the patient developed rigors, fever, and tachycardia with new atrial fibrillation. While being evaluated hypercalcemia was identified, with further workup revealing a low parathyroid hormone (PTH) and low PTH-related peptide. Dr. Fine, the presence of hypercalcemia and a new arrythmia raised the possibility of sarcoidosis. Could this be sarcoidosis?
►Dr. Fine: Sarcoidosis is one of the great masqueraders in medicine. There is a type of sarcoidosis called nodular sarcoidosis where you see masslike distribution in the lung, but generally there are multiple masses and so this presentation would be atypical.3 There is also a phenomenon called sarcoidal reactions usually in the presence of cancer. Again, one tends to see multiple tiny lesions in the lung. It is certainly on the differential, but I would consider it to be less likely than cancer. It is also relatively common to develop atrial fibrillation after manipulation from a lung surgery.4 The other possibility I am concerned about is whether the mass is invading the mediastinum and involving the pericardium.
►Dr. Apte: Results from the VATS biopsy once again returned negative for malignancy and instead showed signs of focal micro-abscesses, atypical pneumocytes, and prominent neutrophils. A diagnosis of acute fibrinous organizing pneumonia (AFOP) was offered. Dr. Fine, what is AFOP?
►Dr. Fine: This is the first case of AFOP I had seen and probably the first case many in our department have seen. This is a relatively new entity with limited reported cases in the literature and is a pathological diagnosis originally recorded in autopsies from patients at the National Institutes of Health.5,6 Given the complexity of the lesion, the diagnosis is difficult to make. Most commonly, AFOP is associated with other systemic entities, most commonly hematologic malignancies like lymphomas and leukemias. It has also been associated with vasculitis and certain drugs. The mechanism is poorly understood, and although pneumonia is a part of the term, this just implies there is inflammation of the lung (ie, pneumonitis).
► Dr. Apte: Given the association of AFOP with underlying hematologic malignancies, an emphasis was placed on another finding: the patient’s increasing WBC count. The total WBC count had been 20 k/cm3 at the time of his lung mass discovery but had increased to > 40 k/cm3 with a differential of neutrophils > 80%. Flow cytometry was negative, and his peripheral smear was read as normal. Dr. Gilbert, what might explain this patient’s leukocytosis?
►Gary Gilbert, MD, Section of Hematology and Oncology, VABHS and Associate Professor of Medicine, Harvard Medical School (HMS): This patient had an elevated WBC for 4 months. Initially, the cause was likely lithium as this is known to cause a leukocytosis.7 More recently, the total WBC had increased and there were a couple of other abnormalities: A consistently elevated absolute monocyte count and a markedly elevated mature neutrophil count. These findings are consistent with a leukemoid reaction (ie, a WBC count > 50,000/µL from causes other than leukemia). The question becomes what is this a leukemoid reaction in response to? Once we have excluded a lung malignancy (a well-known common cause of a leukemoid reaction) we must consider a clonal myeloproliferative disorder. This is particularly true because many things that cause a leukemoid reaction (eg, lobar pneumonia) do not cause a persistently elevated neutrophil count. That this patient does have a persistently elevated neutrophil count suggests something abnormal about the neutrophils themselves.
► Dr. Apte: A bone marrow biopsy was performed. Dr. Gilbert, can you comment on this patient’s bone marrow biopsy and whether a myeloproliferative disorder may have played a role in the marked leukocytosis?
► Dr. Gilbert: The bone marrow biopsy was hyperplastic with myeloid predominance and normal maturation in all lineages. A deep sequencing analysis demonstrated the absence of chromosomal abnormalities or genetic mutations that are associated with myeloproliferative disorders. This excludes the possibility of a myeloproliferative disorder.
► Dr. Apte: The patient was started on 60 mg of prednisone daily, which led to marked improvement in his symptoms. He was discharged in stable condition but presented again with abdominal pain. A complete blood count once again showed increased WBC and new thrombocytosis. A CT angiogram (CTA) showed the prior lung mass with new signs of central necrosis. In the abdomen, new splenic and renal infarct were identified, along with signs of multiple arterial thrombi in the abdomen and internal and external iliac vessel wall thickening. These findings were read as concerning for a medium vessel vasculitis. Dr. Kaur, what are some of the imaging findings you would expect to see in vasculitis, and what about this patient’s CT is consistent with a medium vessel vasculitis?
► Maneet Kaur, MD, Section of Rheumatology, VABHS: Vasculitis is inflammation of the vessel wall that can lead to vascular injury and activation of the coagulation cascade. Sometimes these findings can be seen on imaging with evidence of stenosis, microaneurysms, and thrombosis distal to the stenosis. The nomenclature of vasculitis is not simple and has been revised many times. Medium-vessel vasculitis does not just affect the medium vessels (eg, visceral arteries) but can overlap with distal large vessels and smaller cutaneous vessels.
The first thing that comes to mind in this case is polyarteritis nodosa (PAN), an immune complex-mediated medium vessel disease that can involve large and small vessels in muscle, nerve, and skin. It can also present with masses.
►Dr. Apte: To address the arterial thrombi seen on CTA, arterial-brachial indices were obtained and showed bilateral occlusive disease in his distal extremities; findings that could be explained by vasculitis. His VATS biopsy pathology was reviewed for signs of vasculitis. Dr. Huang, can you review these slides for us please?
►Qin Huang, MD, Department of Pathology and Laboratory Medicine, VABHS and Assistant Professor of Pathology, HMS: This patient had a history of smoking, and there are many black pigment-laden macrophages present in the lung tissue. There were areas of hemorrhage and fibrin deposition and an overall picture of organizing pneumonia. At a lower power, you can see neutrophils everywhere, some in the form of micro-abscesses. The arterial walls did not show signs of vasculitis (Figure 2). Based on the clinical information and radiology findings, we suspected an acute infection-related pneumonia or a primary lung malignancy causing obstruction pneumonia. We suggested a rebiopsy of the lung mass to rule out a primary lung malignancy.
► Dr. Apte: Given his CT findings, a serologic rheumatologic workup including antineutrophil cytoplasmic antibody, antinuclear antibody, and rheumatoid factors were sent and returned negative. The location of arterial wall inflammation on imaging made it unamenable for biopsy. The patient began to experience bilateral temporal pain, which raised the concern for a large vessel vasculitis, specifically giant cell arteritis. Bilateral temporal artery biopsies were obtained and were not suggestive of vasculitis. Dr. Kaur, we still do not have any serologic or biopsy confirmation to support a diagnosis of vasculitis. Can we still call this a vasculitis?
►Dr. Kaur: Few things can cause the picture that was seen radiographically. A few noninflammatory causes like fibromuscular dysplasia can cause both large and small vessel stenosis, but the elevations in erythrocyte sedimentation rate and C-reactive protein along with response to steroids makes these diagnoses unlikely. Sometimes we must make a clinical diagnosis for vasculitis based on the clinical picture, and I would feel comfortable treating this patient for vasculitis.
With that said, I remain concerned that this patient also has a malignancy. His WBC increased to > 70 k/cm3 and his calcium to > 13 mg/dL. These findings are hard to explain by vasculitis alone. There are cancer-associated vasculitis, and I suspect this is the explanation here.8 His temporal pain was pointing to large vessel involvement, so he could have an undifferentiated vasculitis.
► Dr. Apte: A decision was made to empirically treat with tocilizumab, an IL-6 receptor antagonist, for an undifferentiated autoimmune disease, in addition to tapering steroids. The patient underwent a second VATS, which again revealed AFOP but no signs of malignancy. Unfortunately, he developed multiple complications over the subsequent weeks and passed away. An autopsy was requested by family members and pathology from his lung mass was reviewed. (Figure 3). Dr. Huang, can you review these slides for us?
► Dr. Huang: The left lung mass at autopsy shows nests, poorly formed clusters, and individuals of malignant neoplastic nonkeratinizing squamous cells embedded in a desmoplastic stroma in the mass center, consistent with poorly differentiated squamous cell carcinoma, and a circumscribed area of residual subacute organizing pneumonia with abscess, granulomatous changes, and early fibrosis at the periphery of this mass.
►Dr. Apte: Based on autopsy findings, the final diagnosis was poorly differentiated squamous cell carcinoma associated with subacute organizing pneumonia and medium vessel vasculitis, which presented with a severe leukocytosis ultimately thought to be a leukemoid reaction from his lung cancer.
1. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197. Published 2014 Jul 5. doi:10.2147/IDR.S39601
2. de Bazelaire C, Coffin A, Cohen-Zarade S, et al. CT-guided biopsies in lung infections in patients with haematological malignancies. Diagn Interv Imaging. 2013;94(2):202-215. doi:10.1016/j.diii.2012.12.008
3. Sweidan AJ, Singh NK, Stein A, Tanios M. Nodular sarcoidosis masquerading as cancer. Clin Med Insights Circ Respir Pulm Med. 2017;11:1179548417703123. Published 2017 Apr 12. doi:10.1177/1179548417703123
4. Bagheri R, Yousefi Y, Rezai R, Azemonfar V, Keshtan FG. Atrial fibrillation after lung surgery: incidence, underlying factors, and predictors. Kardiochir Torakochirurgia Pol. 2019;16(2):53-56. doi:10.5114/kitp.2019.86355
5. Lu J, Yin Q, Zha Y, et al. Acute fibrinous and organizing pneumonia: two case reports and literature review. BMC Pulm Med. 2019;19(1):141. Published 2019 Aug 5. doi:10.1186/s12890-019-0861-3
6. Beasley MB, Franks TJ, Galvin JR, Gochuico B, Travis WD. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med. 2002;126(9):1064-1070. doi:10.5858/2002-126-1064-AFAOP
7. Murphy DL, Goodwin FK, Bunney WE Jr. Leukocytosis during lithium treatment. Am J Psychiatry. 1971;127(11):1559-1561. doi:10.1176/ajp.127.11.1559
8. Fain O, Hamidou M, Cacoub P, et al. Vasculitides associated with malignancies: analysis of sixty patients. Arthritis Rheum. 2007;57(8):1473-1480. doi:10.1002/art.23085
Case Presentation: A 62-year-old male presented with shortness of breath and a cough productive of green sputum. He had a history of hyperlipidemia, posttraumatic stress disorder, bipolar disorder, obstructive sleep apnea, and a 50 pack-year history of smoking. His medications included prazosin, melatonin, lithium, and gabapentin. He also had a significant exposure history including asbestos and chemical paints following his leave from the military. At the initial evaluation, laboratory work revealed a leukocytosis with white blood cell (WBC) count 20 k/cm3and otherwise normal transaminases, albumin, and electrolytes. A chest X-ray revealed a new left hilar mass.
►Manisha Apte, MD, Chief Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center (BMC): To work up his new left hilar mass, a computed tomography (CT) of the chest was ordered (Figure 1), which revealed an apical left lower lobe mass extending into the left hilum encasing part of the ascending aorta. Enlarged mediastinal subcentimeter paratracheal and superior mediastinal lymph nodes also were identified and the pattern raised the concern for lymphangitic carcinomatosis. Dr. Fine, what do you make of the CT findings?
► Alan Fine, MD, Section of Pulmonary and Critical Care, VABHS and Professor of Medicine, Boston University School of Medicine: This mass had irregular edges with septal thickening, which may be why there was a concern for lymphangitic spread. There were no clear tissue planes to see if this process was invading the mediastinum. The mass was irregular, a single lesion, and proximal, making it consistent with a lung cancer. In fact, with his history of smoking, asbestos exposure, the numbers 1 to 10 diagnoses were lung cancer. The lack of demarcation of tissue planes supports this. There are some infections, classically actinomycosis, that do cross and invade anatomical barriers.1 But this looked like a primary lung cancer.
► Dr. Apte: The patient was referred to a pulmonologist where an additional history of night sweats and weight loss were noted. Dr. Fine, we have a patient with a newly identified lung mass, and while we have reason to suspect malignancy as you have already noted, there are many other etiologies to consider, including infections (histoplasmosis, cryptococcosis, bacterial abscess), inflammatory processes (sarcoidosis, rheumatoid nodule) and vasculitis (granulomatosis with polyangiitis). What should be the next step taken to make a diagnosis?
►Dr. Fine: For cancer specifically, we would like to both stage and make a diagnosis with one procedure. That’s part of the utility of a positron emission tomography (PET) scan: We can see lymph node involvement and stage the cancer. We must consider the patient’s comorbidities and the location of the lesion (ie, is it amenable to needle biopsy?). In this case, there are enlarged mediastinal lymph nodes, so one could perform a bronchoscopy with endobronchial ultrasound, which is a relatively noninvasive way to sample the lymph nodes to ideally stage and make a diagnosis as safely as possible. If we are considering infection, needle aspiration is not as sensitive.2
► Dr. Apte: The patient underwent a PET CT, which redemonstrated the lung mass with a loss of aortic fat plane suspicious for aortic involvement as well as lymph nodes in levels 7 and 8 that were concerning for malignancy. Subsequent bronchoscopy with biopsy and endobronchial ultrasound did not show evidence of malignancy; washing and brushing from the mass and lymph node specimens did not identify malignant cells. Benign respiratory mucosa with mild chronic inflammation was noted. Dr. Fine, given the nonspecific findings on the PET scan, negative findings on our bronchoscopy, and a negative biopsy, should we be satisfied that we have ruled out cancer?
► Dr. Fine: No, bronchoscopy has its limitations. It’s highly sensitive to the diagnosis of malignancy if you can see an endobronchial lesion, but we did not see one here. You can only go so far with the scope, and it’s not uncommon for us not to be able to make the diagnosis with bronchoscopy. Malignancy is still the most likely diagnosis, and we need to work this up further. I would perform another biopsy.
►Dr. Apte: Four weeks later, the patient presented with continued shortness of breath, fatigue, and fever. A repeat chest CT showed an opacity suggestive of pneumonia. Given the continued concern for cancer a CT-guided needle biopsy was performed and was once again negative for malignancy. The decision was made to pursue a video-assisted thorascopic surgery (VATS). Following the VATS, the patient developed rigors, fever, and tachycardia with new atrial fibrillation. While being evaluated hypercalcemia was identified, with further workup revealing a low parathyroid hormone (PTH) and low PTH-related peptide. Dr. Fine, the presence of hypercalcemia and a new arrythmia raised the possibility of sarcoidosis. Could this be sarcoidosis?
►Dr. Fine: Sarcoidosis is one of the great masqueraders in medicine. There is a type of sarcoidosis called nodular sarcoidosis where you see masslike distribution in the lung, but generally there are multiple masses and so this presentation would be atypical.3 There is also a phenomenon called sarcoidal reactions usually in the presence of cancer. Again, one tends to see multiple tiny lesions in the lung. It is certainly on the differential, but I would consider it to be less likely than cancer. It is also relatively common to develop atrial fibrillation after manipulation from a lung surgery.4 The other possibility I am concerned about is whether the mass is invading the mediastinum and involving the pericardium.
►Dr. Apte: Results from the VATS biopsy once again returned negative for malignancy and instead showed signs of focal micro-abscesses, atypical pneumocytes, and prominent neutrophils. A diagnosis of acute fibrinous organizing pneumonia (AFOP) was offered. Dr. Fine, what is AFOP?
►Dr. Fine: This is the first case of AFOP I had seen and probably the first case many in our department have seen. This is a relatively new entity with limited reported cases in the literature and is a pathological diagnosis originally recorded in autopsies from patients at the National Institutes of Health.5,6 Given the complexity of the lesion, the diagnosis is difficult to make. Most commonly, AFOP is associated with other systemic entities, most commonly hematologic malignancies like lymphomas and leukemias. It has also been associated with vasculitis and certain drugs. The mechanism is poorly understood, and although pneumonia is a part of the term, this just implies there is inflammation of the lung (ie, pneumonitis).
► Dr. Apte: Given the association of AFOP with underlying hematologic malignancies, an emphasis was placed on another finding: the patient’s increasing WBC count. The total WBC count had been 20 k/cm3 at the time of his lung mass discovery but had increased to > 40 k/cm3 with a differential of neutrophils > 80%. Flow cytometry was negative, and his peripheral smear was read as normal. Dr. Gilbert, what might explain this patient’s leukocytosis?
►Gary Gilbert, MD, Section of Hematology and Oncology, VABHS and Associate Professor of Medicine, Harvard Medical School (HMS): This patient had an elevated WBC for 4 months. Initially, the cause was likely lithium as this is known to cause a leukocytosis.7 More recently, the total WBC had increased and there were a couple of other abnormalities: A consistently elevated absolute monocyte count and a markedly elevated mature neutrophil count. These findings are consistent with a leukemoid reaction (ie, a WBC count > 50,000/µL from causes other than leukemia). The question becomes what is this a leukemoid reaction in response to? Once we have excluded a lung malignancy (a well-known common cause of a leukemoid reaction) we must consider a clonal myeloproliferative disorder. This is particularly true because many things that cause a leukemoid reaction (eg, lobar pneumonia) do not cause a persistently elevated neutrophil count. That this patient does have a persistently elevated neutrophil count suggests something abnormal about the neutrophils themselves.
► Dr. Apte: A bone marrow biopsy was performed. Dr. Gilbert, can you comment on this patient’s bone marrow biopsy and whether a myeloproliferative disorder may have played a role in the marked leukocytosis?
► Dr. Gilbert: The bone marrow biopsy was hyperplastic with myeloid predominance and normal maturation in all lineages. A deep sequencing analysis demonstrated the absence of chromosomal abnormalities or genetic mutations that are associated with myeloproliferative disorders. This excludes the possibility of a myeloproliferative disorder.
► Dr. Apte: The patient was started on 60 mg of prednisone daily, which led to marked improvement in his symptoms. He was discharged in stable condition but presented again with abdominal pain. A complete blood count once again showed increased WBC and new thrombocytosis. A CT angiogram (CTA) showed the prior lung mass with new signs of central necrosis. In the abdomen, new splenic and renal infarct were identified, along with signs of multiple arterial thrombi in the abdomen and internal and external iliac vessel wall thickening. These findings were read as concerning for a medium vessel vasculitis. Dr. Kaur, what are some of the imaging findings you would expect to see in vasculitis, and what about this patient’s CT is consistent with a medium vessel vasculitis?
► Maneet Kaur, MD, Section of Rheumatology, VABHS: Vasculitis is inflammation of the vessel wall that can lead to vascular injury and activation of the coagulation cascade. Sometimes these findings can be seen on imaging with evidence of stenosis, microaneurysms, and thrombosis distal to the stenosis. The nomenclature of vasculitis is not simple and has been revised many times. Medium-vessel vasculitis does not just affect the medium vessels (eg, visceral arteries) but can overlap with distal large vessels and smaller cutaneous vessels.
The first thing that comes to mind in this case is polyarteritis nodosa (PAN), an immune complex-mediated medium vessel disease that can involve large and small vessels in muscle, nerve, and skin. It can also present with masses.
►Dr. Apte: To address the arterial thrombi seen on CTA, arterial-brachial indices were obtained and showed bilateral occlusive disease in his distal extremities; findings that could be explained by vasculitis. His VATS biopsy pathology was reviewed for signs of vasculitis. Dr. Huang, can you review these slides for us please?
►Qin Huang, MD, Department of Pathology and Laboratory Medicine, VABHS and Assistant Professor of Pathology, HMS: This patient had a history of smoking, and there are many black pigment-laden macrophages present in the lung tissue. There were areas of hemorrhage and fibrin deposition and an overall picture of organizing pneumonia. At a lower power, you can see neutrophils everywhere, some in the form of micro-abscesses. The arterial walls did not show signs of vasculitis (Figure 2). Based on the clinical information and radiology findings, we suspected an acute infection-related pneumonia or a primary lung malignancy causing obstruction pneumonia. We suggested a rebiopsy of the lung mass to rule out a primary lung malignancy.
► Dr. Apte: Given his CT findings, a serologic rheumatologic workup including antineutrophil cytoplasmic antibody, antinuclear antibody, and rheumatoid factors were sent and returned negative. The location of arterial wall inflammation on imaging made it unamenable for biopsy. The patient began to experience bilateral temporal pain, which raised the concern for a large vessel vasculitis, specifically giant cell arteritis. Bilateral temporal artery biopsies were obtained and were not suggestive of vasculitis. Dr. Kaur, we still do not have any serologic or biopsy confirmation to support a diagnosis of vasculitis. Can we still call this a vasculitis?
►Dr. Kaur: Few things can cause the picture that was seen radiographically. A few noninflammatory causes like fibromuscular dysplasia can cause both large and small vessel stenosis, but the elevations in erythrocyte sedimentation rate and C-reactive protein along with response to steroids makes these diagnoses unlikely. Sometimes we must make a clinical diagnosis for vasculitis based on the clinical picture, and I would feel comfortable treating this patient for vasculitis.
With that said, I remain concerned that this patient also has a malignancy. His WBC increased to > 70 k/cm3 and his calcium to > 13 mg/dL. These findings are hard to explain by vasculitis alone. There are cancer-associated vasculitis, and I suspect this is the explanation here.8 His temporal pain was pointing to large vessel involvement, so he could have an undifferentiated vasculitis.
► Dr. Apte: A decision was made to empirically treat with tocilizumab, an IL-6 receptor antagonist, for an undifferentiated autoimmune disease, in addition to tapering steroids. The patient underwent a second VATS, which again revealed AFOP but no signs of malignancy. Unfortunately, he developed multiple complications over the subsequent weeks and passed away. An autopsy was requested by family members and pathology from his lung mass was reviewed. (Figure 3). Dr. Huang, can you review these slides for us?
► Dr. Huang: The left lung mass at autopsy shows nests, poorly formed clusters, and individuals of malignant neoplastic nonkeratinizing squamous cells embedded in a desmoplastic stroma in the mass center, consistent with poorly differentiated squamous cell carcinoma, and a circumscribed area of residual subacute organizing pneumonia with abscess, granulomatous changes, and early fibrosis at the periphery of this mass.
►Dr. Apte: Based on autopsy findings, the final diagnosis was poorly differentiated squamous cell carcinoma associated with subacute organizing pneumonia and medium vessel vasculitis, which presented with a severe leukocytosis ultimately thought to be a leukemoid reaction from his lung cancer.
Case Presentation: A 62-year-old male presented with shortness of breath and a cough productive of green sputum. He had a history of hyperlipidemia, posttraumatic stress disorder, bipolar disorder, obstructive sleep apnea, and a 50 pack-year history of smoking. His medications included prazosin, melatonin, lithium, and gabapentin. He also had a significant exposure history including asbestos and chemical paints following his leave from the military. At the initial evaluation, laboratory work revealed a leukocytosis with white blood cell (WBC) count 20 k/cm3and otherwise normal transaminases, albumin, and electrolytes. A chest X-ray revealed a new left hilar mass.
►Manisha Apte, MD, Chief Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center (BMC): To work up his new left hilar mass, a computed tomography (CT) of the chest was ordered (Figure 1), which revealed an apical left lower lobe mass extending into the left hilum encasing part of the ascending aorta. Enlarged mediastinal subcentimeter paratracheal and superior mediastinal lymph nodes also were identified and the pattern raised the concern for lymphangitic carcinomatosis. Dr. Fine, what do you make of the CT findings?
► Alan Fine, MD, Section of Pulmonary and Critical Care, VABHS and Professor of Medicine, Boston University School of Medicine: This mass had irregular edges with septal thickening, which may be why there was a concern for lymphangitic spread. There were no clear tissue planes to see if this process was invading the mediastinum. The mass was irregular, a single lesion, and proximal, making it consistent with a lung cancer. In fact, with his history of smoking, asbestos exposure, the numbers 1 to 10 diagnoses were lung cancer. The lack of demarcation of tissue planes supports this. There are some infections, classically actinomycosis, that do cross and invade anatomical barriers.1 But this looked like a primary lung cancer.
► Dr. Apte: The patient was referred to a pulmonologist where an additional history of night sweats and weight loss were noted. Dr. Fine, we have a patient with a newly identified lung mass, and while we have reason to suspect malignancy as you have already noted, there are many other etiologies to consider, including infections (histoplasmosis, cryptococcosis, bacterial abscess), inflammatory processes (sarcoidosis, rheumatoid nodule) and vasculitis (granulomatosis with polyangiitis). What should be the next step taken to make a diagnosis?
►Dr. Fine: For cancer specifically, we would like to both stage and make a diagnosis with one procedure. That’s part of the utility of a positron emission tomography (PET) scan: We can see lymph node involvement and stage the cancer. We must consider the patient’s comorbidities and the location of the lesion (ie, is it amenable to needle biopsy?). In this case, there are enlarged mediastinal lymph nodes, so one could perform a bronchoscopy with endobronchial ultrasound, which is a relatively noninvasive way to sample the lymph nodes to ideally stage and make a diagnosis as safely as possible. If we are considering infection, needle aspiration is not as sensitive.2
► Dr. Apte: The patient underwent a PET CT, which redemonstrated the lung mass with a loss of aortic fat plane suspicious for aortic involvement as well as lymph nodes in levels 7 and 8 that were concerning for malignancy. Subsequent bronchoscopy with biopsy and endobronchial ultrasound did not show evidence of malignancy; washing and brushing from the mass and lymph node specimens did not identify malignant cells. Benign respiratory mucosa with mild chronic inflammation was noted. Dr. Fine, given the nonspecific findings on the PET scan, negative findings on our bronchoscopy, and a negative biopsy, should we be satisfied that we have ruled out cancer?
► Dr. Fine: No, bronchoscopy has its limitations. It’s highly sensitive to the diagnosis of malignancy if you can see an endobronchial lesion, but we did not see one here. You can only go so far with the scope, and it’s not uncommon for us not to be able to make the diagnosis with bronchoscopy. Malignancy is still the most likely diagnosis, and we need to work this up further. I would perform another biopsy.
►Dr. Apte: Four weeks later, the patient presented with continued shortness of breath, fatigue, and fever. A repeat chest CT showed an opacity suggestive of pneumonia. Given the continued concern for cancer a CT-guided needle biopsy was performed and was once again negative for malignancy. The decision was made to pursue a video-assisted thorascopic surgery (VATS). Following the VATS, the patient developed rigors, fever, and tachycardia with new atrial fibrillation. While being evaluated hypercalcemia was identified, with further workup revealing a low parathyroid hormone (PTH) and low PTH-related peptide. Dr. Fine, the presence of hypercalcemia and a new arrythmia raised the possibility of sarcoidosis. Could this be sarcoidosis?
►Dr. Fine: Sarcoidosis is one of the great masqueraders in medicine. There is a type of sarcoidosis called nodular sarcoidosis where you see masslike distribution in the lung, but generally there are multiple masses and so this presentation would be atypical.3 There is also a phenomenon called sarcoidal reactions usually in the presence of cancer. Again, one tends to see multiple tiny lesions in the lung. It is certainly on the differential, but I would consider it to be less likely than cancer. It is also relatively common to develop atrial fibrillation after manipulation from a lung surgery.4 The other possibility I am concerned about is whether the mass is invading the mediastinum and involving the pericardium.
►Dr. Apte: Results from the VATS biopsy once again returned negative for malignancy and instead showed signs of focal micro-abscesses, atypical pneumocytes, and prominent neutrophils. A diagnosis of acute fibrinous organizing pneumonia (AFOP) was offered. Dr. Fine, what is AFOP?
►Dr. Fine: This is the first case of AFOP I had seen and probably the first case many in our department have seen. This is a relatively new entity with limited reported cases in the literature and is a pathological diagnosis originally recorded in autopsies from patients at the National Institutes of Health.5,6 Given the complexity of the lesion, the diagnosis is difficult to make. Most commonly, AFOP is associated with other systemic entities, most commonly hematologic malignancies like lymphomas and leukemias. It has also been associated with vasculitis and certain drugs. The mechanism is poorly understood, and although pneumonia is a part of the term, this just implies there is inflammation of the lung (ie, pneumonitis).
► Dr. Apte: Given the association of AFOP with underlying hematologic malignancies, an emphasis was placed on another finding: the patient’s increasing WBC count. The total WBC count had been 20 k/cm3 at the time of his lung mass discovery but had increased to > 40 k/cm3 with a differential of neutrophils > 80%. Flow cytometry was negative, and his peripheral smear was read as normal. Dr. Gilbert, what might explain this patient’s leukocytosis?
►Gary Gilbert, MD, Section of Hematology and Oncology, VABHS and Associate Professor of Medicine, Harvard Medical School (HMS): This patient had an elevated WBC for 4 months. Initially, the cause was likely lithium as this is known to cause a leukocytosis.7 More recently, the total WBC had increased and there were a couple of other abnormalities: A consistently elevated absolute monocyte count and a markedly elevated mature neutrophil count. These findings are consistent with a leukemoid reaction (ie, a WBC count > 50,000/µL from causes other than leukemia). The question becomes what is this a leukemoid reaction in response to? Once we have excluded a lung malignancy (a well-known common cause of a leukemoid reaction) we must consider a clonal myeloproliferative disorder. This is particularly true because many things that cause a leukemoid reaction (eg, lobar pneumonia) do not cause a persistently elevated neutrophil count. That this patient does have a persistently elevated neutrophil count suggests something abnormal about the neutrophils themselves.
► Dr. Apte: A bone marrow biopsy was performed. Dr. Gilbert, can you comment on this patient’s bone marrow biopsy and whether a myeloproliferative disorder may have played a role in the marked leukocytosis?
► Dr. Gilbert: The bone marrow biopsy was hyperplastic with myeloid predominance and normal maturation in all lineages. A deep sequencing analysis demonstrated the absence of chromosomal abnormalities or genetic mutations that are associated with myeloproliferative disorders. This excludes the possibility of a myeloproliferative disorder.
► Dr. Apte: The patient was started on 60 mg of prednisone daily, which led to marked improvement in his symptoms. He was discharged in stable condition but presented again with abdominal pain. A complete blood count once again showed increased WBC and new thrombocytosis. A CT angiogram (CTA) showed the prior lung mass with new signs of central necrosis. In the abdomen, new splenic and renal infarct were identified, along with signs of multiple arterial thrombi in the abdomen and internal and external iliac vessel wall thickening. These findings were read as concerning for a medium vessel vasculitis. Dr. Kaur, what are some of the imaging findings you would expect to see in vasculitis, and what about this patient’s CT is consistent with a medium vessel vasculitis?
► Maneet Kaur, MD, Section of Rheumatology, VABHS: Vasculitis is inflammation of the vessel wall that can lead to vascular injury and activation of the coagulation cascade. Sometimes these findings can be seen on imaging with evidence of stenosis, microaneurysms, and thrombosis distal to the stenosis. The nomenclature of vasculitis is not simple and has been revised many times. Medium-vessel vasculitis does not just affect the medium vessels (eg, visceral arteries) but can overlap with distal large vessels and smaller cutaneous vessels.
The first thing that comes to mind in this case is polyarteritis nodosa (PAN), an immune complex-mediated medium vessel disease that can involve large and small vessels in muscle, nerve, and skin. It can also present with masses.
►Dr. Apte: To address the arterial thrombi seen on CTA, arterial-brachial indices were obtained and showed bilateral occlusive disease in his distal extremities; findings that could be explained by vasculitis. His VATS biopsy pathology was reviewed for signs of vasculitis. Dr. Huang, can you review these slides for us please?
►Qin Huang, MD, Department of Pathology and Laboratory Medicine, VABHS and Assistant Professor of Pathology, HMS: This patient had a history of smoking, and there are many black pigment-laden macrophages present in the lung tissue. There were areas of hemorrhage and fibrin deposition and an overall picture of organizing pneumonia. At a lower power, you can see neutrophils everywhere, some in the form of micro-abscesses. The arterial walls did not show signs of vasculitis (Figure 2). Based on the clinical information and radiology findings, we suspected an acute infection-related pneumonia or a primary lung malignancy causing obstruction pneumonia. We suggested a rebiopsy of the lung mass to rule out a primary lung malignancy.
► Dr. Apte: Given his CT findings, a serologic rheumatologic workup including antineutrophil cytoplasmic antibody, antinuclear antibody, and rheumatoid factors were sent and returned negative. The location of arterial wall inflammation on imaging made it unamenable for biopsy. The patient began to experience bilateral temporal pain, which raised the concern for a large vessel vasculitis, specifically giant cell arteritis. Bilateral temporal artery biopsies were obtained and were not suggestive of vasculitis. Dr. Kaur, we still do not have any serologic or biopsy confirmation to support a diagnosis of vasculitis. Can we still call this a vasculitis?
►Dr. Kaur: Few things can cause the picture that was seen radiographically. A few noninflammatory causes like fibromuscular dysplasia can cause both large and small vessel stenosis, but the elevations in erythrocyte sedimentation rate and C-reactive protein along with response to steroids makes these diagnoses unlikely. Sometimes we must make a clinical diagnosis for vasculitis based on the clinical picture, and I would feel comfortable treating this patient for vasculitis.
With that said, I remain concerned that this patient also has a malignancy. His WBC increased to > 70 k/cm3 and his calcium to > 13 mg/dL. These findings are hard to explain by vasculitis alone. There are cancer-associated vasculitis, and I suspect this is the explanation here.8 His temporal pain was pointing to large vessel involvement, so he could have an undifferentiated vasculitis.
► Dr. Apte: A decision was made to empirically treat with tocilizumab, an IL-6 receptor antagonist, for an undifferentiated autoimmune disease, in addition to tapering steroids. The patient underwent a second VATS, which again revealed AFOP but no signs of malignancy. Unfortunately, he developed multiple complications over the subsequent weeks and passed away. An autopsy was requested by family members and pathology from his lung mass was reviewed. (Figure 3). Dr. Huang, can you review these slides for us?
► Dr. Huang: The left lung mass at autopsy shows nests, poorly formed clusters, and individuals of malignant neoplastic nonkeratinizing squamous cells embedded in a desmoplastic stroma in the mass center, consistent with poorly differentiated squamous cell carcinoma, and a circumscribed area of residual subacute organizing pneumonia with abscess, granulomatous changes, and early fibrosis at the periphery of this mass.
►Dr. Apte: Based on autopsy findings, the final diagnosis was poorly differentiated squamous cell carcinoma associated with subacute organizing pneumonia and medium vessel vasculitis, which presented with a severe leukocytosis ultimately thought to be a leukemoid reaction from his lung cancer.
1. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197. Published 2014 Jul 5. doi:10.2147/IDR.S39601
2. de Bazelaire C, Coffin A, Cohen-Zarade S, et al. CT-guided biopsies in lung infections in patients with haematological malignancies. Diagn Interv Imaging. 2013;94(2):202-215. doi:10.1016/j.diii.2012.12.008
3. Sweidan AJ, Singh NK, Stein A, Tanios M. Nodular sarcoidosis masquerading as cancer. Clin Med Insights Circ Respir Pulm Med. 2017;11:1179548417703123. Published 2017 Apr 12. doi:10.1177/1179548417703123
4. Bagheri R, Yousefi Y, Rezai R, Azemonfar V, Keshtan FG. Atrial fibrillation after lung surgery: incidence, underlying factors, and predictors. Kardiochir Torakochirurgia Pol. 2019;16(2):53-56. doi:10.5114/kitp.2019.86355
5. Lu J, Yin Q, Zha Y, et al. Acute fibrinous and organizing pneumonia: two case reports and literature review. BMC Pulm Med. 2019;19(1):141. Published 2019 Aug 5. doi:10.1186/s12890-019-0861-3
6. Beasley MB, Franks TJ, Galvin JR, Gochuico B, Travis WD. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med. 2002;126(9):1064-1070. doi:10.5858/2002-126-1064-AFAOP
7. Murphy DL, Goodwin FK, Bunney WE Jr. Leukocytosis during lithium treatment. Am J Psychiatry. 1971;127(11):1559-1561. doi:10.1176/ajp.127.11.1559
8. Fain O, Hamidou M, Cacoub P, et al. Vasculitides associated with malignancies: analysis of sixty patients. Arthritis Rheum. 2007;57(8):1473-1480. doi:10.1002/art.23085
1. Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197. Published 2014 Jul 5. doi:10.2147/IDR.S39601
2. de Bazelaire C, Coffin A, Cohen-Zarade S, et al. CT-guided biopsies in lung infections in patients with haematological malignancies. Diagn Interv Imaging. 2013;94(2):202-215. doi:10.1016/j.diii.2012.12.008
3. Sweidan AJ, Singh NK, Stein A, Tanios M. Nodular sarcoidosis masquerading as cancer. Clin Med Insights Circ Respir Pulm Med. 2017;11:1179548417703123. Published 2017 Apr 12. doi:10.1177/1179548417703123
4. Bagheri R, Yousefi Y, Rezai R, Azemonfar V, Keshtan FG. Atrial fibrillation after lung surgery: incidence, underlying factors, and predictors. Kardiochir Torakochirurgia Pol. 2019;16(2):53-56. doi:10.5114/kitp.2019.86355
5. Lu J, Yin Q, Zha Y, et al. Acute fibrinous and organizing pneumonia: two case reports and literature review. BMC Pulm Med. 2019;19(1):141. Published 2019 Aug 5. doi:10.1186/s12890-019-0861-3
6. Beasley MB, Franks TJ, Galvin JR, Gochuico B, Travis WD. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med. 2002;126(9):1064-1070. doi:10.5858/2002-126-1064-AFAOP
7. Murphy DL, Goodwin FK, Bunney WE Jr. Leukocytosis during lithium treatment. Am J Psychiatry. 1971;127(11):1559-1561. doi:10.1176/ajp.127.11.1559
8. Fain O, Hamidou M, Cacoub P, et al. Vasculitides associated with malignancies: analysis of sixty patients. Arthritis Rheum. 2007;57(8):1473-1480. doi:10.1002/art.23085