User login
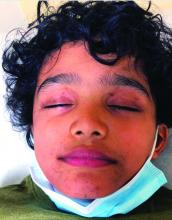
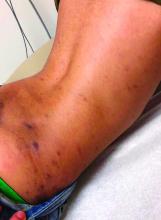
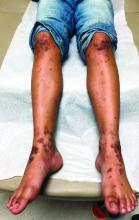
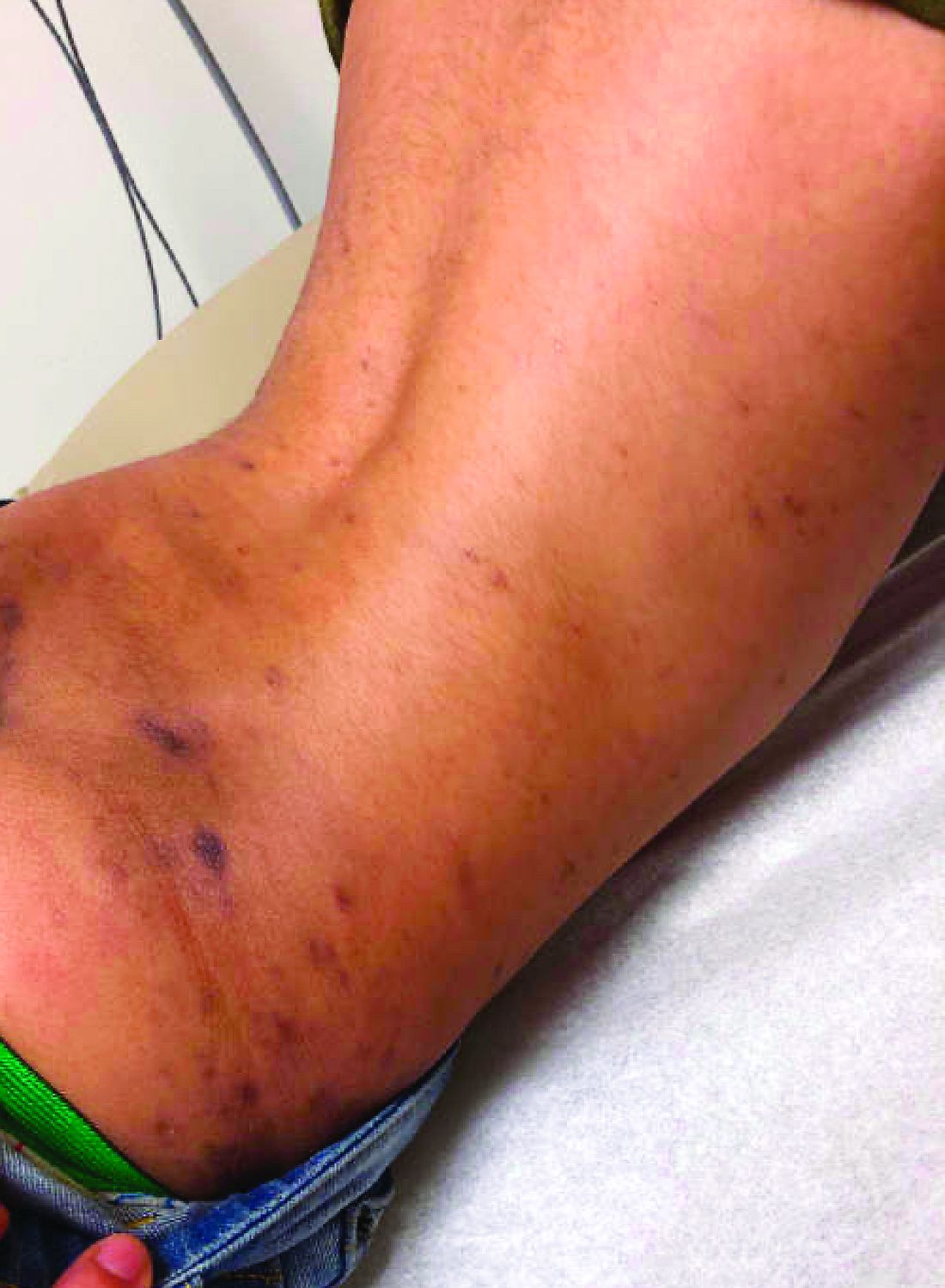
Emerging research shows link between suicidality, ‘high-potency’ cannabis products
Number of suicides positive for marijuana on rise soared among Colorado youth
In the days since recreational sales of marijuana became legal in Colorado in January 2014, concerning trends have emerged among the state’s young cannabis users.
According to a report from the Rocky Mountain High Intensity Drug Trafficking Area, between 2014 and 2017, the number of suicides positive for marijuana increased 250% among those aged 10-19 years (from 4 to 14) and 22% among those aged 20 and older (from 118 to 144). “Other states are seeing something similar, and there is an emerging research showing a relationship between suicidality and the use of marijuana, especially high-potency products that are available in legalized markets,” Paula D. Riggs, MD, reported during an annual psychopharmacology update held by the Nevada Psychiatric Association.
During that same 3-year time span, the proportion of Colorado youth aged 12 years and older who used marijuana in the past month jumped by 45%, which is more than 85% above the national average. “Similarly, among college-age students, we’ve seen an 18% increase in past-month marijuana use, which is 60% above the national average,” said Dr. Riggs, professor and vice chair of psychiatry at the University of Colorado at Denver, Aurora.
Among adolescents, state health officials have observed a 5% increase in the proportion of those who used marijuana in the past month, which is more than 54% above the national average. “But a concerning trend is that we’re seeing an increase in the use of concentrates such as dabs and waxes,” she said. “That’s worrisome in terms of exposure to high-potency products.”
In other findings, 48% of young marijuana users reported going to work high (40% at least once per week), and there has been a 170% increase in youth ED urgent care visits for marijuana-related illnesses such as cannabinoid hyperemesis syndrome or first-episode psychosis. State health officials have also observed a 148% increase in marijuana-related hospitalizations.
According to Dr. Riggs, who also directs the University of Colorado’s division of addiction science, prevention, and treatment, the average marijuana joint in the 1960s contained about 3% tetrahydrocannabinol (THC), a level that crept up to the 4%-6% range in 2002. In today’s postlegalization era, the average joint now contains 13%-23% THC. “What’s concerning is that the concentrates – the dabs, waxes, shatter, and butane hash oils – contain upward of 70%-95% THC,” Dr. Riggs said. “Those are highly potent products that represent about 25% of the market share now. That’s a very big concern because the higher the potency the cannabis product used, the greater the abuse liability and addictive potential.”
The use of high-potency products also doubles the risk of developing generalized anxiety disorder, triples the risk of tobacco dependence, doubles the risk of other illicit substance disorders, and it at least quadruples the risk of developing first-episode psychosis in young people. “So, when you’re taking a cannabis use history, it’s important to ask patients about the potency of the products being used,” she said.
In the 2019 Monitoring the Future survey, 12% of U.S. 8th graders self-reported marijuana use in the past year and 7% in the past month, compared with 29% and 18% of 10th graders, respectively. Self-reported use by 12th graders was even more elevated (36% in the past year and 29% in the past month). “The concern is, this survey doesn’t really capture what’s happening with marijuana concentrates,” Dr. Riggs said.
A survey of Colorado youth conducted by the state’s Department of Public Health and Environment found that the percentage of students who reported using concentrated forms of marijuana has risen steadily in recent years and now stands at roughly 34%. “The use of edibles has also crept up,” said Dr. Riggs, who noted that marijuana dispensaries in Colorado outnumber Starbucks locations and McDonald’s restaurants. “You might not think that’s particularly concerning, except that the use of edibles is even more associated with onset of psychosis than other forms. This is probably because when you eat a marijuana product, you can’t control the exposure or the dose that you’re ingesting. We need to be concerned about these trends.”
European studies report that 30%-50% of new cases of first-onset psychosis are attributed to high-potency cannabis. “There is a dose-response relationship between cannabis and psychosis,” Dr. Riggs said. “That is, the frequency and duration of cannabis use, or the use of high-potency products, and the age of onset, are strongly associated with the risk of first-episode psychosis.
Researchers have known for some time that alterations in the endocannabinoid system are associated with psychosis independent of cannabis exposure. “Dysregulation of that endocannabinoid system occurs in patients at all stages of the psychosis continuum,” she continued. “It also means that the endocannabinoid system is a potential therapeutic target for psychosis.”
According to Dr. Riggs, THC exposure acutely increases dopamine in the ventral striatum and it can produce transient psychotomimetic effects in clinical and nonclinical populations. Genetic differences in the dopaminergic system can also interact with cannabis use to increase the risk of psychosis.
“For example, the COMT (catechol-O-methyltransferase) breaks down catecholamines such as dopamine in the prefrontal cortex,” she explained. “If you have a COMT gene polymorphism, that increases your risk of developing psychosis due to increased levels of dopamine signaling.”
She emphasized the importance of clinicians to understand that the age of cannabis use onset, the duration, frequency, and THC potency is related to the psychosis risk and worse prognosis. The earlier the initiation of marijuana use, the greater potential for first-episode psychosis. “Those who continue using cannabis after a first-episode psychosis have greater severity of psychotic illness and more treatment resistance, and they’re less likely to engage or be compliant with treatment recommendations,” Dr. Riggs said. “So, Because if they resume cannabis use, this can turn into a more chronic psychotic disorder.”
She added that, while insufficient evidence exists to determine whether cannabis plays a causal role in the development of schizophrenia or not, mounting evidence suggests that cannabis use may precipitate earlier onset of schizophrenia in those with other risk factors for the disorder. “There is considerable evidence that cannabis use increases the risk of psychosis in a dose-related manner, especially with an onset before age 16,” Dr. Riggs said. “However, this does not mean that cannabis is safe for young adults. Cannabis-induced psychotic symptoms often develop during young adulthood and may become chronic.”
Dr. Riggs disclosed that she had received grant funding from the National Institute on Drug Abuse. She is also executive director for Encompass, which provides integrated treatment for adolescents and young adults.
Number of suicides positive for marijuana on rise soared among Colorado youth
Number of suicides positive for marijuana on rise soared among Colorado youth
In the days since recreational sales of marijuana became legal in Colorado in January 2014, concerning trends have emerged among the state’s young cannabis users.
According to a report from the Rocky Mountain High Intensity Drug Trafficking Area, between 2014 and 2017, the number of suicides positive for marijuana increased 250% among those aged 10-19 years (from 4 to 14) and 22% among those aged 20 and older (from 118 to 144). “Other states are seeing something similar, and there is an emerging research showing a relationship between suicidality and the use of marijuana, especially high-potency products that are available in legalized markets,” Paula D. Riggs, MD, reported during an annual psychopharmacology update held by the Nevada Psychiatric Association.
During that same 3-year time span, the proportion of Colorado youth aged 12 years and older who used marijuana in the past month jumped by 45%, which is more than 85% above the national average. “Similarly, among college-age students, we’ve seen an 18% increase in past-month marijuana use, which is 60% above the national average,” said Dr. Riggs, professor and vice chair of psychiatry at the University of Colorado at Denver, Aurora.
Among adolescents, state health officials have observed a 5% increase in the proportion of those who used marijuana in the past month, which is more than 54% above the national average. “But a concerning trend is that we’re seeing an increase in the use of concentrates such as dabs and waxes,” she said. “That’s worrisome in terms of exposure to high-potency products.”
In other findings, 48% of young marijuana users reported going to work high (40% at least once per week), and there has been a 170% increase in youth ED urgent care visits for marijuana-related illnesses such as cannabinoid hyperemesis syndrome or first-episode psychosis. State health officials have also observed a 148% increase in marijuana-related hospitalizations.
According to Dr. Riggs, who also directs the University of Colorado’s division of addiction science, prevention, and treatment, the average marijuana joint in the 1960s contained about 3% tetrahydrocannabinol (THC), a level that crept up to the 4%-6% range in 2002. In today’s postlegalization era, the average joint now contains 13%-23% THC. “What’s concerning is that the concentrates – the dabs, waxes, shatter, and butane hash oils – contain upward of 70%-95% THC,” Dr. Riggs said. “Those are highly potent products that represent about 25% of the market share now. That’s a very big concern because the higher the potency the cannabis product used, the greater the abuse liability and addictive potential.”
The use of high-potency products also doubles the risk of developing generalized anxiety disorder, triples the risk of tobacco dependence, doubles the risk of other illicit substance disorders, and it at least quadruples the risk of developing first-episode psychosis in young people. “So, when you’re taking a cannabis use history, it’s important to ask patients about the potency of the products being used,” she said.
In the 2019 Monitoring the Future survey, 12% of U.S. 8th graders self-reported marijuana use in the past year and 7% in the past month, compared with 29% and 18% of 10th graders, respectively. Self-reported use by 12th graders was even more elevated (36% in the past year and 29% in the past month). “The concern is, this survey doesn’t really capture what’s happening with marijuana concentrates,” Dr. Riggs said.
A survey of Colorado youth conducted by the state’s Department of Public Health and Environment found that the percentage of students who reported using concentrated forms of marijuana has risen steadily in recent years and now stands at roughly 34%. “The use of edibles has also crept up,” said Dr. Riggs, who noted that marijuana dispensaries in Colorado outnumber Starbucks locations and McDonald’s restaurants. “You might not think that’s particularly concerning, except that the use of edibles is even more associated with onset of psychosis than other forms. This is probably because when you eat a marijuana product, you can’t control the exposure or the dose that you’re ingesting. We need to be concerned about these trends.”
European studies report that 30%-50% of new cases of first-onset psychosis are attributed to high-potency cannabis. “There is a dose-response relationship between cannabis and psychosis,” Dr. Riggs said. “That is, the frequency and duration of cannabis use, or the use of high-potency products, and the age of onset, are strongly associated with the risk of first-episode psychosis.
Researchers have known for some time that alterations in the endocannabinoid system are associated with psychosis independent of cannabis exposure. “Dysregulation of that endocannabinoid system occurs in patients at all stages of the psychosis continuum,” she continued. “It also means that the endocannabinoid system is a potential therapeutic target for psychosis.”
According to Dr. Riggs, THC exposure acutely increases dopamine in the ventral striatum and it can produce transient psychotomimetic effects in clinical and nonclinical populations. Genetic differences in the dopaminergic system can also interact with cannabis use to increase the risk of psychosis.
“For example, the COMT (catechol-O-methyltransferase) breaks down catecholamines such as dopamine in the prefrontal cortex,” she explained. “If you have a COMT gene polymorphism, that increases your risk of developing psychosis due to increased levels of dopamine signaling.”
She emphasized the importance of clinicians to understand that the age of cannabis use onset, the duration, frequency, and THC potency is related to the psychosis risk and worse prognosis. The earlier the initiation of marijuana use, the greater potential for first-episode psychosis. “Those who continue using cannabis after a first-episode psychosis have greater severity of psychotic illness and more treatment resistance, and they’re less likely to engage or be compliant with treatment recommendations,” Dr. Riggs said. “So, Because if they resume cannabis use, this can turn into a more chronic psychotic disorder.”
She added that, while insufficient evidence exists to determine whether cannabis plays a causal role in the development of schizophrenia or not, mounting evidence suggests that cannabis use may precipitate earlier onset of schizophrenia in those with other risk factors for the disorder. “There is considerable evidence that cannabis use increases the risk of psychosis in a dose-related manner, especially with an onset before age 16,” Dr. Riggs said. “However, this does not mean that cannabis is safe for young adults. Cannabis-induced psychotic symptoms often develop during young adulthood and may become chronic.”
Dr. Riggs disclosed that she had received grant funding from the National Institute on Drug Abuse. She is also executive director for Encompass, which provides integrated treatment for adolescents and young adults.
In the days since recreational sales of marijuana became legal in Colorado in January 2014, concerning trends have emerged among the state’s young cannabis users.
According to a report from the Rocky Mountain High Intensity Drug Trafficking Area, between 2014 and 2017, the number of suicides positive for marijuana increased 250% among those aged 10-19 years (from 4 to 14) and 22% among those aged 20 and older (from 118 to 144). “Other states are seeing something similar, and there is an emerging research showing a relationship between suicidality and the use of marijuana, especially high-potency products that are available in legalized markets,” Paula D. Riggs, MD, reported during an annual psychopharmacology update held by the Nevada Psychiatric Association.
During that same 3-year time span, the proportion of Colorado youth aged 12 years and older who used marijuana in the past month jumped by 45%, which is more than 85% above the national average. “Similarly, among college-age students, we’ve seen an 18% increase in past-month marijuana use, which is 60% above the national average,” said Dr. Riggs, professor and vice chair of psychiatry at the University of Colorado at Denver, Aurora.
Among adolescents, state health officials have observed a 5% increase in the proportion of those who used marijuana in the past month, which is more than 54% above the national average. “But a concerning trend is that we’re seeing an increase in the use of concentrates such as dabs and waxes,” she said. “That’s worrisome in terms of exposure to high-potency products.”
In other findings, 48% of young marijuana users reported going to work high (40% at least once per week), and there has been a 170% increase in youth ED urgent care visits for marijuana-related illnesses such as cannabinoid hyperemesis syndrome or first-episode psychosis. State health officials have also observed a 148% increase in marijuana-related hospitalizations.
According to Dr. Riggs, who also directs the University of Colorado’s division of addiction science, prevention, and treatment, the average marijuana joint in the 1960s contained about 3% tetrahydrocannabinol (THC), a level that crept up to the 4%-6% range in 2002. In today’s postlegalization era, the average joint now contains 13%-23% THC. “What’s concerning is that the concentrates – the dabs, waxes, shatter, and butane hash oils – contain upward of 70%-95% THC,” Dr. Riggs said. “Those are highly potent products that represent about 25% of the market share now. That’s a very big concern because the higher the potency the cannabis product used, the greater the abuse liability and addictive potential.”
The use of high-potency products also doubles the risk of developing generalized anxiety disorder, triples the risk of tobacco dependence, doubles the risk of other illicit substance disorders, and it at least quadruples the risk of developing first-episode psychosis in young people. “So, when you’re taking a cannabis use history, it’s important to ask patients about the potency of the products being used,” she said.
In the 2019 Monitoring the Future survey, 12% of U.S. 8th graders self-reported marijuana use in the past year and 7% in the past month, compared with 29% and 18% of 10th graders, respectively. Self-reported use by 12th graders was even more elevated (36% in the past year and 29% in the past month). “The concern is, this survey doesn’t really capture what’s happening with marijuana concentrates,” Dr. Riggs said.
A survey of Colorado youth conducted by the state’s Department of Public Health and Environment found that the percentage of students who reported using concentrated forms of marijuana has risen steadily in recent years and now stands at roughly 34%. “The use of edibles has also crept up,” said Dr. Riggs, who noted that marijuana dispensaries in Colorado outnumber Starbucks locations and McDonald’s restaurants. “You might not think that’s particularly concerning, except that the use of edibles is even more associated with onset of psychosis than other forms. This is probably because when you eat a marijuana product, you can’t control the exposure or the dose that you’re ingesting. We need to be concerned about these trends.”
European studies report that 30%-50% of new cases of first-onset psychosis are attributed to high-potency cannabis. “There is a dose-response relationship between cannabis and psychosis,” Dr. Riggs said. “That is, the frequency and duration of cannabis use, or the use of high-potency products, and the age of onset, are strongly associated with the risk of first-episode psychosis.
Researchers have known for some time that alterations in the endocannabinoid system are associated with psychosis independent of cannabis exposure. “Dysregulation of that endocannabinoid system occurs in patients at all stages of the psychosis continuum,” she continued. “It also means that the endocannabinoid system is a potential therapeutic target for psychosis.”
According to Dr. Riggs, THC exposure acutely increases dopamine in the ventral striatum and it can produce transient psychotomimetic effects in clinical and nonclinical populations. Genetic differences in the dopaminergic system can also interact with cannabis use to increase the risk of psychosis.
“For example, the COMT (catechol-O-methyltransferase) breaks down catecholamines such as dopamine in the prefrontal cortex,” she explained. “If you have a COMT gene polymorphism, that increases your risk of developing psychosis due to increased levels of dopamine signaling.”
She emphasized the importance of clinicians to understand that the age of cannabis use onset, the duration, frequency, and THC potency is related to the psychosis risk and worse prognosis. The earlier the initiation of marijuana use, the greater potential for first-episode psychosis. “Those who continue using cannabis after a first-episode psychosis have greater severity of psychotic illness and more treatment resistance, and they’re less likely to engage or be compliant with treatment recommendations,” Dr. Riggs said. “So, Because if they resume cannabis use, this can turn into a more chronic psychotic disorder.”
She added that, while insufficient evidence exists to determine whether cannabis plays a causal role in the development of schizophrenia or not, mounting evidence suggests that cannabis use may precipitate earlier onset of schizophrenia in those with other risk factors for the disorder. “There is considerable evidence that cannabis use increases the risk of psychosis in a dose-related manner, especially with an onset before age 16,” Dr. Riggs said. “However, this does not mean that cannabis is safe for young adults. Cannabis-induced psychotic symptoms often develop during young adulthood and may become chronic.”
Dr. Riggs disclosed that she had received grant funding from the National Institute on Drug Abuse. She is also executive director for Encompass, which provides integrated treatment for adolescents and young adults.
FROM NPA 2021
Routine COVID-19 screening unnecessary for cancer outpatients
There were no significant differences in COVID-19 outcomes between cases caught by routine screening and screening based on symptoms/exposure history among cancer outpatients treated at Mayo Clinic facilities, according to a review of 224 cases.
The finding led to a shift away from routine COVID-19 screening to screening based on symptoms and exposures, said lead investigator Zhuoer Xie, MD, a hematology/oncology fellow at Mayo’s Rochester, Minn., campus.
“We are so happy” to see these results and be able to move away from routine screening. It’s burdensome and uncomfortable for patients and expensive to administer, Dr. Xie said at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S06-03).
Also, “our results provide reassurance that cancer care may safely continue during the pandemic with appropriate precautions,” she said.
Like many institutions, Mayo instituted routine COVID-19 screening for cancer outpatients at the start of the pandemic, requiring patients be tested 24 hours before systemic treatment, radiation therapy, or surgery. People on multiday regimens were screened twice a week.
Among 5,452 patients at the Rochester campus and its surrounding satellites, plus Mayo’s facilities in Phoenix and Jacksonville, Fla., routine screening picked up 63 COVID-19 cases (1.2%) from March 18 to July 31, 2020.
The outcomes were compared with 161 COVID-19 cases screened due to symptoms and exposure history. Most of the patients were on cancer surveillance as opposed to active treatment with routine testing.
Overall, 17.5% of cases caught by routine screening (11/63) were hospitalized versus 26.7% of patients screened for risk factors (43/161).
There was one COVID-19-related ICU admission among the 63 routine screening cases (1.6%) and nine ICU admissions (5.6%) among the risk-factor screening group. Three people diagnosed by routine screening (4.8%) died, compared with six deaths in the risk factor screening group (3.7%). The differences were not statistically significant, and there was no difference in treatment delay based on screening method.
The mortality rate was substantially lower than previously reported for COVID-19 among cancer patients, perhaps in part because Mayo facilities were not overwhelmed with cases early in the pandemic, so there was never a shortage of hospital beds and other resources, Dr. Xie said.
“Many of us are glad to see your data. It’s comforting,” said presentation moderator Solange Peters, MD, PhD, head of medical oncology at the Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland.
With proper precautions, “we can firmly encourage patients to come” in for their “cancer treatment without any hesitation,” Dr. Peters said.
“We feel the same way. We tell our patients this might be the safest place for you to be. Everybody is masked; everybody is taking all the precautions,” said Sheena Bhalla, MD, a hematology/oncology fellow as the Icahn School of Medicine at Mount Sinai, New York.
“We are [also] reaching out to patients who have been hesitant” about the COVID-19 vaccine, Dr. Bhalla said, “and trying to get them vaccinated. We are still learning how cancer patients will do with the vaccine, but we think that some protection is better than no protection.”
Currently at Mayo’s main campus in Rochester and its surrounding clinics, COVID-19 screening is based on symptoms, exposure, and factors such as high risk for neutropenic fever.
Mayo’s Arizona and Florida campuses had a surge of cases a few months ago, so routine screening is still used there but only on a monthly basis for people on active treatment.
Consistent with previous reports, older age and lymphopenia increased the risk of COVID-19 hospitalization in Mayo’s study, but comorbidities and active cancer treatment did not.
COVID-19 patients were a median of 62 years old, and 42% were women. Breast, genitourinary, and gastrointestinal tumors were the most common cancers.
Respiratory failure and sepsis were the most common complications among the 54 hospital admissions; eight patients required intubation.
The funding source wasn’t reported. The speakers had no relevant disclosures.
There were no significant differences in COVID-19 outcomes between cases caught by routine screening and screening based on symptoms/exposure history among cancer outpatients treated at Mayo Clinic facilities, according to a review of 224 cases.
The finding led to a shift away from routine COVID-19 screening to screening based on symptoms and exposures, said lead investigator Zhuoer Xie, MD, a hematology/oncology fellow at Mayo’s Rochester, Minn., campus.
“We are so happy” to see these results and be able to move away from routine screening. It’s burdensome and uncomfortable for patients and expensive to administer, Dr. Xie said at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S06-03).
Also, “our results provide reassurance that cancer care may safely continue during the pandemic with appropriate precautions,” she said.
Like many institutions, Mayo instituted routine COVID-19 screening for cancer outpatients at the start of the pandemic, requiring patients be tested 24 hours before systemic treatment, radiation therapy, or surgery. People on multiday regimens were screened twice a week.
Among 5,452 patients at the Rochester campus and its surrounding satellites, plus Mayo’s facilities in Phoenix and Jacksonville, Fla., routine screening picked up 63 COVID-19 cases (1.2%) from March 18 to July 31, 2020.
The outcomes were compared with 161 COVID-19 cases screened due to symptoms and exposure history. Most of the patients were on cancer surveillance as opposed to active treatment with routine testing.
Overall, 17.5% of cases caught by routine screening (11/63) were hospitalized versus 26.7% of patients screened for risk factors (43/161).
There was one COVID-19-related ICU admission among the 63 routine screening cases (1.6%) and nine ICU admissions (5.6%) among the risk-factor screening group. Three people diagnosed by routine screening (4.8%) died, compared with six deaths in the risk factor screening group (3.7%). The differences were not statistically significant, and there was no difference in treatment delay based on screening method.
The mortality rate was substantially lower than previously reported for COVID-19 among cancer patients, perhaps in part because Mayo facilities were not overwhelmed with cases early in the pandemic, so there was never a shortage of hospital beds and other resources, Dr. Xie said.
“Many of us are glad to see your data. It’s comforting,” said presentation moderator Solange Peters, MD, PhD, head of medical oncology at the Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland.
With proper precautions, “we can firmly encourage patients to come” in for their “cancer treatment without any hesitation,” Dr. Peters said.
“We feel the same way. We tell our patients this might be the safest place for you to be. Everybody is masked; everybody is taking all the precautions,” said Sheena Bhalla, MD, a hematology/oncology fellow as the Icahn School of Medicine at Mount Sinai, New York.
“We are [also] reaching out to patients who have been hesitant” about the COVID-19 vaccine, Dr. Bhalla said, “and trying to get them vaccinated. We are still learning how cancer patients will do with the vaccine, but we think that some protection is better than no protection.”
Currently at Mayo’s main campus in Rochester and its surrounding clinics, COVID-19 screening is based on symptoms, exposure, and factors such as high risk for neutropenic fever.
Mayo’s Arizona and Florida campuses had a surge of cases a few months ago, so routine screening is still used there but only on a monthly basis for people on active treatment.
Consistent with previous reports, older age and lymphopenia increased the risk of COVID-19 hospitalization in Mayo’s study, but comorbidities and active cancer treatment did not.
COVID-19 patients were a median of 62 years old, and 42% were women. Breast, genitourinary, and gastrointestinal tumors were the most common cancers.
Respiratory failure and sepsis were the most common complications among the 54 hospital admissions; eight patients required intubation.
The funding source wasn’t reported. The speakers had no relevant disclosures.
There were no significant differences in COVID-19 outcomes between cases caught by routine screening and screening based on symptoms/exposure history among cancer outpatients treated at Mayo Clinic facilities, according to a review of 224 cases.
The finding led to a shift away from routine COVID-19 screening to screening based on symptoms and exposures, said lead investigator Zhuoer Xie, MD, a hematology/oncology fellow at Mayo’s Rochester, Minn., campus.
“We are so happy” to see these results and be able to move away from routine screening. It’s burdensome and uncomfortable for patients and expensive to administer, Dr. Xie said at the AACR Virtual Meeting: COVID-19 and Cancer (Abstract S06-03).
Also, “our results provide reassurance that cancer care may safely continue during the pandemic with appropriate precautions,” she said.
Like many institutions, Mayo instituted routine COVID-19 screening for cancer outpatients at the start of the pandemic, requiring patients be tested 24 hours before systemic treatment, radiation therapy, or surgery. People on multiday regimens were screened twice a week.
Among 5,452 patients at the Rochester campus and its surrounding satellites, plus Mayo’s facilities in Phoenix and Jacksonville, Fla., routine screening picked up 63 COVID-19 cases (1.2%) from March 18 to July 31, 2020.
The outcomes were compared with 161 COVID-19 cases screened due to symptoms and exposure history. Most of the patients were on cancer surveillance as opposed to active treatment with routine testing.
Overall, 17.5% of cases caught by routine screening (11/63) were hospitalized versus 26.7% of patients screened for risk factors (43/161).
There was one COVID-19-related ICU admission among the 63 routine screening cases (1.6%) and nine ICU admissions (5.6%) among the risk-factor screening group. Three people diagnosed by routine screening (4.8%) died, compared with six deaths in the risk factor screening group (3.7%). The differences were not statistically significant, and there was no difference in treatment delay based on screening method.
The mortality rate was substantially lower than previously reported for COVID-19 among cancer patients, perhaps in part because Mayo facilities were not overwhelmed with cases early in the pandemic, so there was never a shortage of hospital beds and other resources, Dr. Xie said.
“Many of us are glad to see your data. It’s comforting,” said presentation moderator Solange Peters, MD, PhD, head of medical oncology at the Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland.
With proper precautions, “we can firmly encourage patients to come” in for their “cancer treatment without any hesitation,” Dr. Peters said.
“We feel the same way. We tell our patients this might be the safest place for you to be. Everybody is masked; everybody is taking all the precautions,” said Sheena Bhalla, MD, a hematology/oncology fellow as the Icahn School of Medicine at Mount Sinai, New York.
“We are [also] reaching out to patients who have been hesitant” about the COVID-19 vaccine, Dr. Bhalla said, “and trying to get them vaccinated. We are still learning how cancer patients will do with the vaccine, but we think that some protection is better than no protection.”
Currently at Mayo’s main campus in Rochester and its surrounding clinics, COVID-19 screening is based on symptoms, exposure, and factors such as high risk for neutropenic fever.
Mayo’s Arizona and Florida campuses had a surge of cases a few months ago, so routine screening is still used there but only on a monthly basis for people on active treatment.
Consistent with previous reports, older age and lymphopenia increased the risk of COVID-19 hospitalization in Mayo’s study, but comorbidities and active cancer treatment did not.
COVID-19 patients were a median of 62 years old, and 42% were women. Breast, genitourinary, and gastrointestinal tumors were the most common cancers.
Respiratory failure and sepsis were the most common complications among the 54 hospital admissions; eight patients required intubation.
The funding source wasn’t reported. The speakers had no relevant disclosures.
FROM AACR: COVID-19 AND CANCER 2021
New steroid dosing regimen for myasthenia gravis
. The trial showed that the conventional slow tapering regimen enabled discontinuation of prednisone earlier than previously reported but the new rapid-tapering regimen enabled an even faster discontinuation.
Noting that although both regimens led to a comparable myasthenia gravis status and prednisone dose at 15 months, the authors stated: “We think that the reduction of the cumulative dose over a year (equivalent to 5 mg/day) is a clinically relevant reduction, since the risk of complications is proportional to the daily or cumulative doses of prednisone.
“Our results warrant testing of a more rapid-tapering regimen in a future trial. In the meantime, our trial provides useful information on how prednisone tapering could be managed in patients with generalized myasthenia gravis treated with azathioprine,” they concluded.
The trial was published online Feb. 8 in JAMA Neurology.
Myasthenia gravis is a disorder of neuromuscular transmission, resulting from autoantibodies to components of the neuromuscular junction, most commonly the acetylcholine receptor. The incidence ranges from 0.3 to 2.8 per 100,000, and it is estimated to affect more than 700,000 people worldwide.
The authors of the current paper, led by Tarek Sharshar, MD, PhD, Groupe Hospitalier Universitaire, Paris, explained that many patients whose symptoms are not controlled by cholinesterase inhibitors are treated with corticosteroids and an immunosuppressant, usually azathioprine. No specific dosing protocol for prednisone has been validated, but it is commonly gradually increased to 0.75 mg/kg on alternate days and reduced progressively when minimal manifestation status (MMS; no symptoms or functional limitations) is reached.
They noted that this regimen leads to high and prolonged corticosteroid treatment – often for several years – with the mean daily prednisone dose exceeding 30 mg/day at 15 months and 20 mg/day at 36 months. As long-term use of corticosteroids is often associated with significant complications, reducing or even discontinuing prednisone treatment without destabilizing myasthenia gravis is therefore a therapeutic goal.
Evaluating dosage regimens
To investigate whether different dosage regimens could help wean patients with generalized myasthenia gravis from corticosteroid therapy without compromising efficacy, the researchers conducted this study in which the current recommended regimen was compared with an approach using higher initial corticosteroid doses followed by rapid tapering.
In the conventional slow-tapering group (control group), prednisone was given on alternate days, starting at a dose of 10 mg then increased by increments of 10 mg every 2 days up to 1.5 mg/kg on alternate days without exceeding 100 mg. This dose was maintained until MMS was reached and then reduced by 10 mg every 2 weeks until a dosage of 40 mg was reached, with subsequent slowing of the taper to 5 mg monthly. If MMS was not maintained, the alternate-day prednisone dose was increased by 10 mg every 2 weeks until MMS was restored, and the tapering resumed 4 weeks later.
In the new rapid-tapering group, oral prednisone was immediately started at 0.75 mg/kg per day, and this was followed by an earlier and rapid decrease once improved myasthenia gravis status was attained. Three different tapering schedules were applied dependent on the improvement status of the patient.
First, If the patient reached MMS at 1 month, the dose of prednisone was reduced by 0.1 mg/kg every 10 days up to 0.45 mg/kg per day, then 0.05 mg/kg every 10 days up to 0.25 mg/kg per day, then in decrements of 1 mg by adjusting the duration of the decrements according to the participant’s weight with the aim of achieving complete cessation of corticosteroid therapy within 18-20 weeks for this third stage of tapering.
Second, if the state of MMS was not reached at 1 month but the participant had improved, a slower tapering was conducted, with the dosage reduced in a similar way to the first instance but with each reduction introduced every 20 days. If the participant reached MMS during this tapering process, the tapering of prednisone was similar to the sequence described in the first group.
Third, if MMS was not reached and the participant had not improved, the initial dose was maintained for the first 3 months; beyond that time, a decrease in the prednisone dose was undertaken as in the second group to a minimum dose of 0.25 mg/kg per day, after which the prednisone dose was not reduced further. If the patient improved, the tapering of prednisone followed the sequence described in the second category.
Reductions in prednisone dose could be accelerated in the case of severe prednisone adverse effects, according to the prescriber’s decision.
In the event of a myasthenia gravis exacerbation, the patient was hospitalized and the dose of prednisone was routinely doubled, or for a more moderate aggravation, the dose was increased to the previous dose recommended in the tapering regimen.
Azathioprine, up to a maximum dose of 3 mg/kg per day, was prescribed for all participants. In all, 117 patients were randomly assigned, and 113 completed the study.
The primary outcome was the proportion of participants having reached MMS without prednisone at 12 months and having not relapsed or taken prednisone between months 12 and 15. This was achieved by significantly more patients in the rapid-tapering group (39% vs. 9%; risk ratio, 3.61; P < .001).
Rapid tapering allowed sparing of a mean of 1,898 mg of prednisone over 1 year (5.3 mg/day) per patient.
The rate of myasthenia gravis exacerbation or worsening did not differ significantly between the two groups, nor did the use of plasmapheresis or IVIG or the doses of azathioprine.
The overall number of serious adverse events did not differ significantly between the two groups (slow tapering, 22% vs. rapid-tapering, 36%; P = .15).
The researchers said it is possible that prednisone tapering would differ with another immunosuppressive agent but as azathioprine is the first-line immunosuppressant usually recommended, these results are relevant for a large proportion of patients.
They said the better outcome of the intervention group could have been related to one or more of four differences in prednisone administration: An immediate high dose versus a slow increase of the prednisone dose; daily versus alternate-day dosing; earlier tapering initiation; and faster tapering. However, the structure of the study did not allow identification of which of these factors was responsible.
“Researching the best prednisone-tapering scheme is not only a major issue for patients with myasthenia gravis but also for other autoimmune or inflammatory diseases, because validated prednisone-tapering regimens are scarce,” the authors said.
The rapid tapering of prednisone therapy appears to be feasible, beneficial, and safe in patients with generalized myasthenia gravis and “warrants testing in other autoimmune diseases,” they added.
Particularly relevant to late-onset disease
Commenting on the study, Raffi Topakian, MD, Klinikum Wels-Grieskirchen, Wels, Austria, said the results showed that in patients with moderate to severe generalized myasthenia gravis requiring high-dose prednisone, azathioprine, a widely used immunosuppressant, may have a quicker steroid-sparing effect than previously thought, and that rapid steroid tapering can be achieved safely, resulting in a reduction of the cumulative steroid dose over a year despite higher initial doses.
Dr. Topakian, who was not involved with the research, pointed out that the median age was advanced (around 56 years), and the benefit of a regimen that leads to a reduction of the cumulative steroid dose over a year may be disproportionately larger for older, sicker patients with many comorbidities who are at considerably higher risk for a prednisone-induced increase in cardiovascular complications, osteoporotic fractures, and gastrointestinal bleeding.
“The study findings are particularly relevant for the management of late-onset myasthenia gravis (when first symptoms start after age 45-50 years), which is being encountered more frequently over the past years,” he said.
“But the holy grail of myasthenia gravis treatment has not been found yet,” Dr. Topakian noted. “Disappointingly, rapid tapering of steroids (compared to slow tapering) resulted in a reduction of the cumulative steroid dose only, but was not associated with better myasthenia gravis functional status or lower doses of steroids at 15 months. To my view, this finding points to the limited immunosuppressive efficacy of azathioprine.”
He added that the study findings should not be extrapolated to patients with mild presentations or to those with muscle-specific kinase myasthenia gravis.
Dr. Sharshar disclosed no relevant financial relationships. Disclosures for the study coauthors appear in the original article.
A version of this article first appeared on Medscape.com.
. The trial showed that the conventional slow tapering regimen enabled discontinuation of prednisone earlier than previously reported but the new rapid-tapering regimen enabled an even faster discontinuation.
Noting that although both regimens led to a comparable myasthenia gravis status and prednisone dose at 15 months, the authors stated: “We think that the reduction of the cumulative dose over a year (equivalent to 5 mg/day) is a clinically relevant reduction, since the risk of complications is proportional to the daily or cumulative doses of prednisone.
“Our results warrant testing of a more rapid-tapering regimen in a future trial. In the meantime, our trial provides useful information on how prednisone tapering could be managed in patients with generalized myasthenia gravis treated with azathioprine,” they concluded.
The trial was published online Feb. 8 in JAMA Neurology.
Myasthenia gravis is a disorder of neuromuscular transmission, resulting from autoantibodies to components of the neuromuscular junction, most commonly the acetylcholine receptor. The incidence ranges from 0.3 to 2.8 per 100,000, and it is estimated to affect more than 700,000 people worldwide.
The authors of the current paper, led by Tarek Sharshar, MD, PhD, Groupe Hospitalier Universitaire, Paris, explained that many patients whose symptoms are not controlled by cholinesterase inhibitors are treated with corticosteroids and an immunosuppressant, usually azathioprine. No specific dosing protocol for prednisone has been validated, but it is commonly gradually increased to 0.75 mg/kg on alternate days and reduced progressively when minimal manifestation status (MMS; no symptoms or functional limitations) is reached.
They noted that this regimen leads to high and prolonged corticosteroid treatment – often for several years – with the mean daily prednisone dose exceeding 30 mg/day at 15 months and 20 mg/day at 36 months. As long-term use of corticosteroids is often associated with significant complications, reducing or even discontinuing prednisone treatment without destabilizing myasthenia gravis is therefore a therapeutic goal.
Evaluating dosage regimens
To investigate whether different dosage regimens could help wean patients with generalized myasthenia gravis from corticosteroid therapy without compromising efficacy, the researchers conducted this study in which the current recommended regimen was compared with an approach using higher initial corticosteroid doses followed by rapid tapering.
In the conventional slow-tapering group (control group), prednisone was given on alternate days, starting at a dose of 10 mg then increased by increments of 10 mg every 2 days up to 1.5 mg/kg on alternate days without exceeding 100 mg. This dose was maintained until MMS was reached and then reduced by 10 mg every 2 weeks until a dosage of 40 mg was reached, with subsequent slowing of the taper to 5 mg monthly. If MMS was not maintained, the alternate-day prednisone dose was increased by 10 mg every 2 weeks until MMS was restored, and the tapering resumed 4 weeks later.
In the new rapid-tapering group, oral prednisone was immediately started at 0.75 mg/kg per day, and this was followed by an earlier and rapid decrease once improved myasthenia gravis status was attained. Three different tapering schedules were applied dependent on the improvement status of the patient.
First, If the patient reached MMS at 1 month, the dose of prednisone was reduced by 0.1 mg/kg every 10 days up to 0.45 mg/kg per day, then 0.05 mg/kg every 10 days up to 0.25 mg/kg per day, then in decrements of 1 mg by adjusting the duration of the decrements according to the participant’s weight with the aim of achieving complete cessation of corticosteroid therapy within 18-20 weeks for this third stage of tapering.
Second, if the state of MMS was not reached at 1 month but the participant had improved, a slower tapering was conducted, with the dosage reduced in a similar way to the first instance but with each reduction introduced every 20 days. If the participant reached MMS during this tapering process, the tapering of prednisone was similar to the sequence described in the first group.
Third, if MMS was not reached and the participant had not improved, the initial dose was maintained for the first 3 months; beyond that time, a decrease in the prednisone dose was undertaken as in the second group to a minimum dose of 0.25 mg/kg per day, after which the prednisone dose was not reduced further. If the patient improved, the tapering of prednisone followed the sequence described in the second category.
Reductions in prednisone dose could be accelerated in the case of severe prednisone adverse effects, according to the prescriber’s decision.
In the event of a myasthenia gravis exacerbation, the patient was hospitalized and the dose of prednisone was routinely doubled, or for a more moderate aggravation, the dose was increased to the previous dose recommended in the tapering regimen.
Azathioprine, up to a maximum dose of 3 mg/kg per day, was prescribed for all participants. In all, 117 patients were randomly assigned, and 113 completed the study.
The primary outcome was the proportion of participants having reached MMS without prednisone at 12 months and having not relapsed or taken prednisone between months 12 and 15. This was achieved by significantly more patients in the rapid-tapering group (39% vs. 9%; risk ratio, 3.61; P < .001).
Rapid tapering allowed sparing of a mean of 1,898 mg of prednisone over 1 year (5.3 mg/day) per patient.
The rate of myasthenia gravis exacerbation or worsening did not differ significantly between the two groups, nor did the use of plasmapheresis or IVIG or the doses of azathioprine.
The overall number of serious adverse events did not differ significantly between the two groups (slow tapering, 22% vs. rapid-tapering, 36%; P = .15).
The researchers said it is possible that prednisone tapering would differ with another immunosuppressive agent but as azathioprine is the first-line immunosuppressant usually recommended, these results are relevant for a large proportion of patients.
They said the better outcome of the intervention group could have been related to one or more of four differences in prednisone administration: An immediate high dose versus a slow increase of the prednisone dose; daily versus alternate-day dosing; earlier tapering initiation; and faster tapering. However, the structure of the study did not allow identification of which of these factors was responsible.
“Researching the best prednisone-tapering scheme is not only a major issue for patients with myasthenia gravis but also for other autoimmune or inflammatory diseases, because validated prednisone-tapering regimens are scarce,” the authors said.
The rapid tapering of prednisone therapy appears to be feasible, beneficial, and safe in patients with generalized myasthenia gravis and “warrants testing in other autoimmune diseases,” they added.
Particularly relevant to late-onset disease
Commenting on the study, Raffi Topakian, MD, Klinikum Wels-Grieskirchen, Wels, Austria, said the results showed that in patients with moderate to severe generalized myasthenia gravis requiring high-dose prednisone, azathioprine, a widely used immunosuppressant, may have a quicker steroid-sparing effect than previously thought, and that rapid steroid tapering can be achieved safely, resulting in a reduction of the cumulative steroid dose over a year despite higher initial doses.
Dr. Topakian, who was not involved with the research, pointed out that the median age was advanced (around 56 years), and the benefit of a regimen that leads to a reduction of the cumulative steroid dose over a year may be disproportionately larger for older, sicker patients with many comorbidities who are at considerably higher risk for a prednisone-induced increase in cardiovascular complications, osteoporotic fractures, and gastrointestinal bleeding.
“The study findings are particularly relevant for the management of late-onset myasthenia gravis (when first symptoms start after age 45-50 years), which is being encountered more frequently over the past years,” he said.
“But the holy grail of myasthenia gravis treatment has not been found yet,” Dr. Topakian noted. “Disappointingly, rapid tapering of steroids (compared to slow tapering) resulted in a reduction of the cumulative steroid dose only, but was not associated with better myasthenia gravis functional status or lower doses of steroids at 15 months. To my view, this finding points to the limited immunosuppressive efficacy of azathioprine.”
He added that the study findings should not be extrapolated to patients with mild presentations or to those with muscle-specific kinase myasthenia gravis.
Dr. Sharshar disclosed no relevant financial relationships. Disclosures for the study coauthors appear in the original article.
A version of this article first appeared on Medscape.com.
. The trial showed that the conventional slow tapering regimen enabled discontinuation of prednisone earlier than previously reported but the new rapid-tapering regimen enabled an even faster discontinuation.
Noting that although both regimens led to a comparable myasthenia gravis status and prednisone dose at 15 months, the authors stated: “We think that the reduction of the cumulative dose over a year (equivalent to 5 mg/day) is a clinically relevant reduction, since the risk of complications is proportional to the daily or cumulative doses of prednisone.
“Our results warrant testing of a more rapid-tapering regimen in a future trial. In the meantime, our trial provides useful information on how prednisone tapering could be managed in patients with generalized myasthenia gravis treated with azathioprine,” they concluded.
The trial was published online Feb. 8 in JAMA Neurology.
Myasthenia gravis is a disorder of neuromuscular transmission, resulting from autoantibodies to components of the neuromuscular junction, most commonly the acetylcholine receptor. The incidence ranges from 0.3 to 2.8 per 100,000, and it is estimated to affect more than 700,000 people worldwide.
The authors of the current paper, led by Tarek Sharshar, MD, PhD, Groupe Hospitalier Universitaire, Paris, explained that many patients whose symptoms are not controlled by cholinesterase inhibitors are treated with corticosteroids and an immunosuppressant, usually azathioprine. No specific dosing protocol for prednisone has been validated, but it is commonly gradually increased to 0.75 mg/kg on alternate days and reduced progressively when minimal manifestation status (MMS; no symptoms or functional limitations) is reached.
They noted that this regimen leads to high and prolonged corticosteroid treatment – often for several years – with the mean daily prednisone dose exceeding 30 mg/day at 15 months and 20 mg/day at 36 months. As long-term use of corticosteroids is often associated with significant complications, reducing or even discontinuing prednisone treatment without destabilizing myasthenia gravis is therefore a therapeutic goal.
Evaluating dosage regimens
To investigate whether different dosage regimens could help wean patients with generalized myasthenia gravis from corticosteroid therapy without compromising efficacy, the researchers conducted this study in which the current recommended regimen was compared with an approach using higher initial corticosteroid doses followed by rapid tapering.
In the conventional slow-tapering group (control group), prednisone was given on alternate days, starting at a dose of 10 mg then increased by increments of 10 mg every 2 days up to 1.5 mg/kg on alternate days without exceeding 100 mg. This dose was maintained until MMS was reached and then reduced by 10 mg every 2 weeks until a dosage of 40 mg was reached, with subsequent slowing of the taper to 5 mg monthly. If MMS was not maintained, the alternate-day prednisone dose was increased by 10 mg every 2 weeks until MMS was restored, and the tapering resumed 4 weeks later.
In the new rapid-tapering group, oral prednisone was immediately started at 0.75 mg/kg per day, and this was followed by an earlier and rapid decrease once improved myasthenia gravis status was attained. Three different tapering schedules were applied dependent on the improvement status of the patient.
First, If the patient reached MMS at 1 month, the dose of prednisone was reduced by 0.1 mg/kg every 10 days up to 0.45 mg/kg per day, then 0.05 mg/kg every 10 days up to 0.25 mg/kg per day, then in decrements of 1 mg by adjusting the duration of the decrements according to the participant’s weight with the aim of achieving complete cessation of corticosteroid therapy within 18-20 weeks for this third stage of tapering.
Second, if the state of MMS was not reached at 1 month but the participant had improved, a slower tapering was conducted, with the dosage reduced in a similar way to the first instance but with each reduction introduced every 20 days. If the participant reached MMS during this tapering process, the tapering of prednisone was similar to the sequence described in the first group.
Third, if MMS was not reached and the participant had not improved, the initial dose was maintained for the first 3 months; beyond that time, a decrease in the prednisone dose was undertaken as in the second group to a minimum dose of 0.25 mg/kg per day, after which the prednisone dose was not reduced further. If the patient improved, the tapering of prednisone followed the sequence described in the second category.
Reductions in prednisone dose could be accelerated in the case of severe prednisone adverse effects, according to the prescriber’s decision.
In the event of a myasthenia gravis exacerbation, the patient was hospitalized and the dose of prednisone was routinely doubled, or for a more moderate aggravation, the dose was increased to the previous dose recommended in the tapering regimen.
Azathioprine, up to a maximum dose of 3 mg/kg per day, was prescribed for all participants. In all, 117 patients were randomly assigned, and 113 completed the study.
The primary outcome was the proportion of participants having reached MMS without prednisone at 12 months and having not relapsed or taken prednisone between months 12 and 15. This was achieved by significantly more patients in the rapid-tapering group (39% vs. 9%; risk ratio, 3.61; P < .001).
Rapid tapering allowed sparing of a mean of 1,898 mg of prednisone over 1 year (5.3 mg/day) per patient.
The rate of myasthenia gravis exacerbation or worsening did not differ significantly between the two groups, nor did the use of plasmapheresis or IVIG or the doses of azathioprine.
The overall number of serious adverse events did not differ significantly between the two groups (slow tapering, 22% vs. rapid-tapering, 36%; P = .15).
The researchers said it is possible that prednisone tapering would differ with another immunosuppressive agent but as azathioprine is the first-line immunosuppressant usually recommended, these results are relevant for a large proportion of patients.
They said the better outcome of the intervention group could have been related to one or more of four differences in prednisone administration: An immediate high dose versus a slow increase of the prednisone dose; daily versus alternate-day dosing; earlier tapering initiation; and faster tapering. However, the structure of the study did not allow identification of which of these factors was responsible.
“Researching the best prednisone-tapering scheme is not only a major issue for patients with myasthenia gravis but also for other autoimmune or inflammatory diseases, because validated prednisone-tapering regimens are scarce,” the authors said.
The rapid tapering of prednisone therapy appears to be feasible, beneficial, and safe in patients with generalized myasthenia gravis and “warrants testing in other autoimmune diseases,” they added.
Particularly relevant to late-onset disease
Commenting on the study, Raffi Topakian, MD, Klinikum Wels-Grieskirchen, Wels, Austria, said the results showed that in patients with moderate to severe generalized myasthenia gravis requiring high-dose prednisone, azathioprine, a widely used immunosuppressant, may have a quicker steroid-sparing effect than previously thought, and that rapid steroid tapering can be achieved safely, resulting in a reduction of the cumulative steroid dose over a year despite higher initial doses.
Dr. Topakian, who was not involved with the research, pointed out that the median age was advanced (around 56 years), and the benefit of a regimen that leads to a reduction of the cumulative steroid dose over a year may be disproportionately larger for older, sicker patients with many comorbidities who are at considerably higher risk for a prednisone-induced increase in cardiovascular complications, osteoporotic fractures, and gastrointestinal bleeding.
“The study findings are particularly relevant for the management of late-onset myasthenia gravis (when first symptoms start after age 45-50 years), which is being encountered more frequently over the past years,” he said.
“But the holy grail of myasthenia gravis treatment has not been found yet,” Dr. Topakian noted. “Disappointingly, rapid tapering of steroids (compared to slow tapering) resulted in a reduction of the cumulative steroid dose only, but was not associated with better myasthenia gravis functional status or lower doses of steroids at 15 months. To my view, this finding points to the limited immunosuppressive efficacy of azathioprine.”
He added that the study findings should not be extrapolated to patients with mild presentations or to those with muscle-specific kinase myasthenia gravis.
Dr. Sharshar disclosed no relevant financial relationships. Disclosures for the study coauthors appear in the original article.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Erenumab improves functional outcomes in patients with episodic migraine
Key clinical point: Treatment with erenumab significantly improved functional outcomes in 3 months in patients with episodic migraine refractory to previous prophylactic therapies.
Major finding: At 12 weeks, erenumab significantly improved “Migraine Physical Function Impact Diary”-“Physical Impairment” and “Everyday Activities” scores compared with placebo (treatment difference [TD], −3.5; P = .003 and TD, −3.9; P less than .001, respectively). A significantly higher proportion of patients had a 5 or more point reduction in the Headache Impact Test score from baseline compared with placebo (odds ratio, 2.4; P = .002).
Study details: Data come from the 12-week, double-blind, multicenter, Liberty study involving 246 episodic migraine patients who did not previously benefit from 2-4 prophylactic treatments. They were randomly assigned to receive either erenumab 140 mg (n = 121) or placebo (n = 125) once every 4 weeks for 12 weeks.
Disclosures: The study was supported by Novartis Pharma AG, Basel, Switzerland. S Wen, P Hours-Zesiger, and J Klatt were employees of, and hold stocks in, Novartis. M Lanteri-Minet, PJ Goadsby, U Reuter, and MD Ferrari reported relationships with various pharmaceutical companies and/or research organizations.
Source:Lanteri-Minet M et al. J Neurol Neurosurg Psychiatry. 2021 Jan 5. doi: 10.1136/jnnp-2020-324396.
Key clinical point: Treatment with erenumab significantly improved functional outcomes in 3 months in patients with episodic migraine refractory to previous prophylactic therapies.
Major finding: At 12 weeks, erenumab significantly improved “Migraine Physical Function Impact Diary”-“Physical Impairment” and “Everyday Activities” scores compared with placebo (treatment difference [TD], −3.5; P = .003 and TD, −3.9; P less than .001, respectively). A significantly higher proportion of patients had a 5 or more point reduction in the Headache Impact Test score from baseline compared with placebo (odds ratio, 2.4; P = .002).
Study details: Data come from the 12-week, double-blind, multicenter, Liberty study involving 246 episodic migraine patients who did not previously benefit from 2-4 prophylactic treatments. They were randomly assigned to receive either erenumab 140 mg (n = 121) or placebo (n = 125) once every 4 weeks for 12 weeks.
Disclosures: The study was supported by Novartis Pharma AG, Basel, Switzerland. S Wen, P Hours-Zesiger, and J Klatt were employees of, and hold stocks in, Novartis. M Lanteri-Minet, PJ Goadsby, U Reuter, and MD Ferrari reported relationships with various pharmaceutical companies and/or research organizations.
Source:Lanteri-Minet M et al. J Neurol Neurosurg Psychiatry. 2021 Jan 5. doi: 10.1136/jnnp-2020-324396.
Key clinical point: Treatment with erenumab significantly improved functional outcomes in 3 months in patients with episodic migraine refractory to previous prophylactic therapies.
Major finding: At 12 weeks, erenumab significantly improved “Migraine Physical Function Impact Diary”-“Physical Impairment” and “Everyday Activities” scores compared with placebo (treatment difference [TD], −3.5; P = .003 and TD, −3.9; P less than .001, respectively). A significantly higher proportion of patients had a 5 or more point reduction in the Headache Impact Test score from baseline compared with placebo (odds ratio, 2.4; P = .002).
Study details: Data come from the 12-week, double-blind, multicenter, Liberty study involving 246 episodic migraine patients who did not previously benefit from 2-4 prophylactic treatments. They were randomly assigned to receive either erenumab 140 mg (n = 121) or placebo (n = 125) once every 4 weeks for 12 weeks.
Disclosures: The study was supported by Novartis Pharma AG, Basel, Switzerland. S Wen, P Hours-Zesiger, and J Klatt were employees of, and hold stocks in, Novartis. M Lanteri-Minet, PJ Goadsby, U Reuter, and MD Ferrari reported relationships with various pharmaceutical companies and/or research organizations.
Source:Lanteri-Minet M et al. J Neurol Neurosurg Psychiatry. 2021 Jan 5. doi: 10.1136/jnnp-2020-324396.
Fewer headache days with erenumab in onabotulinumtoxin A resistant chronic migraine
Key clinical point: Erenumab treatment significantly improved the number of headache days in patients with onabotulinumtoxinA-resistant chronic migraine.
Major finding: Erenumab significantly reduced the number of headache days that limited activities of daily living at 3 months (−6.4 days), 6 months (−6.8 days), and 9 months (−6.5 days; P for all = .001).
Study details: Real-world outcomes were assessed in 98 patients with difficult-to-control chronic migraine and a prior unsatisfactory response to onabotulinumtoxinA.
Disclosures: This study did not receive any funding. Erenumab was provided free of charge by Novartis. S Weatherby attended conferences/received speakers fees from Novartis.
Source:Talbot J et al. J Headache Pain. 2021 Jan 9. doi: 10.1186/s10194-020-01214-2.
Key clinical point: Erenumab treatment significantly improved the number of headache days in patients with onabotulinumtoxinA-resistant chronic migraine.
Major finding: Erenumab significantly reduced the number of headache days that limited activities of daily living at 3 months (−6.4 days), 6 months (−6.8 days), and 9 months (−6.5 days; P for all = .001).
Study details: Real-world outcomes were assessed in 98 patients with difficult-to-control chronic migraine and a prior unsatisfactory response to onabotulinumtoxinA.
Disclosures: This study did not receive any funding. Erenumab was provided free of charge by Novartis. S Weatherby attended conferences/received speakers fees from Novartis.
Source:Talbot J et al. J Headache Pain. 2021 Jan 9. doi: 10.1186/s10194-020-01214-2.
Key clinical point: Erenumab treatment significantly improved the number of headache days in patients with onabotulinumtoxinA-resistant chronic migraine.
Major finding: Erenumab significantly reduced the number of headache days that limited activities of daily living at 3 months (−6.4 days), 6 months (−6.8 days), and 9 months (−6.5 days; P for all = .001).
Study details: Real-world outcomes were assessed in 98 patients with difficult-to-control chronic migraine and a prior unsatisfactory response to onabotulinumtoxinA.
Disclosures: This study did not receive any funding. Erenumab was provided free of charge by Novartis. S Weatherby attended conferences/received speakers fees from Novartis.
Source:Talbot J et al. J Headache Pain. 2021 Jan 9. doi: 10.1186/s10194-020-01214-2.
Migraine linked to more than doubled risk for irritable bowel syndrome
Key clinical point: People with a history of migraine are more than twice as likely as those with no such history to have irritable bowel syndrome (IBS).
Major finding: IBS odds were significantly higher in patients with migraine vs. those without: overall (pooled odds ratio [OR], 2.49; 95% confidence interval [CI], 2.22-2.78); migraine with aura (pooled OR, 3.03; 95% CI, 1.72-5.35); and migraine without aura (pooled OR, 2.20; 95% CI, 1.49-3.25).
Study details: Meta-analysis of 11 studies including 28,336 migraineurs and 1,535,758 nonmigraineurs.
Disclosures: The study did not receive any funding. The authors declared no conflicts of interest.
Source:Wongtrakul W et al. Eur J Gastroenterol Hepatol. 2021 Jan 18. doi: 10.1097/MEG.0000000000002065.
Key clinical point: People with a history of migraine are more than twice as likely as those with no such history to have irritable bowel syndrome (IBS).
Major finding: IBS odds were significantly higher in patients with migraine vs. those without: overall (pooled odds ratio [OR], 2.49; 95% confidence interval [CI], 2.22-2.78); migraine with aura (pooled OR, 3.03; 95% CI, 1.72-5.35); and migraine without aura (pooled OR, 2.20; 95% CI, 1.49-3.25).
Study details: Meta-analysis of 11 studies including 28,336 migraineurs and 1,535,758 nonmigraineurs.
Disclosures: The study did not receive any funding. The authors declared no conflicts of interest.
Source:Wongtrakul W et al. Eur J Gastroenterol Hepatol. 2021 Jan 18. doi: 10.1097/MEG.0000000000002065.
Key clinical point: People with a history of migraine are more than twice as likely as those with no such history to have irritable bowel syndrome (IBS).
Major finding: IBS odds were significantly higher in patients with migraine vs. those without: overall (pooled odds ratio [OR], 2.49; 95% confidence interval [CI], 2.22-2.78); migraine with aura (pooled OR, 3.03; 95% CI, 1.72-5.35); and migraine without aura (pooled OR, 2.20; 95% CI, 1.49-3.25).
Study details: Meta-analysis of 11 studies including 28,336 migraineurs and 1,535,758 nonmigraineurs.
Disclosures: The study did not receive any funding. The authors declared no conflicts of interest.
Source:Wongtrakul W et al. Eur J Gastroenterol Hepatol. 2021 Jan 18. doi: 10.1097/MEG.0000000000002065.
Migraine: Triptan responders are more likely to respond to erenumab
Key clinical point: Patients with migraine who showed a favorable response to at least one triptan had a higher likelihood to respond to erenumab treatment than those not responding to triptans.
Major finding: Triptan responders had higher odds for responding to erenumab treatment than the triptan nonresponders (odds ratio, 3.64; P = .014).
Study details: Findings are from an ancillary study from a real-life observational study involving 140 patients with migraine treated with erenumab for at least 6 months.
Disclosures: The publication fee was unconditionally granted by Novartis Farma S.r.l. The lead author along with some other authors declared no competing interests. Some authors declared financial and nonfinancial relationships with various pharmaceutical sources such as Eli Lilly, Novartis, Allergan, Teva, Abbott, Innovet Italia Srl, Epitech Group, and Lusofarmaco.
Source:Frattale I et al. J Headache Pain. 2021 Jan 6. doi: 10.1186/s10194-020-01213-3.
Key clinical point: Patients with migraine who showed a favorable response to at least one triptan had a higher likelihood to respond to erenumab treatment than those not responding to triptans.
Major finding: Triptan responders had higher odds for responding to erenumab treatment than the triptan nonresponders (odds ratio, 3.64; P = .014).
Study details: Findings are from an ancillary study from a real-life observational study involving 140 patients with migraine treated with erenumab for at least 6 months.
Disclosures: The publication fee was unconditionally granted by Novartis Farma S.r.l. The lead author along with some other authors declared no competing interests. Some authors declared financial and nonfinancial relationships with various pharmaceutical sources such as Eli Lilly, Novartis, Allergan, Teva, Abbott, Innovet Italia Srl, Epitech Group, and Lusofarmaco.
Source:Frattale I et al. J Headache Pain. 2021 Jan 6. doi: 10.1186/s10194-020-01213-3.
Key clinical point: Patients with migraine who showed a favorable response to at least one triptan had a higher likelihood to respond to erenumab treatment than those not responding to triptans.
Major finding: Triptan responders had higher odds for responding to erenumab treatment than the triptan nonresponders (odds ratio, 3.64; P = .014).
Study details: Findings are from an ancillary study from a real-life observational study involving 140 patients with migraine treated with erenumab for at least 6 months.
Disclosures: The publication fee was unconditionally granted by Novartis Farma S.r.l. The lead author along with some other authors declared no competing interests. Some authors declared financial and nonfinancial relationships with various pharmaceutical sources such as Eli Lilly, Novartis, Allergan, Teva, Abbott, Innovet Italia Srl, Epitech Group, and Lusofarmaco.
Source:Frattale I et al. J Headache Pain. 2021 Jan 6. doi: 10.1186/s10194-020-01213-3.
How does an emotionally drained workforce move on post pandemic?
Psychiatric community is facing ‘triple challenges’ tied to COVID
When cases of COVID-19 began to surge in New York City in March 2020, Carol A. Bernstein, MD, did her best to practice psychiatry and carry out administrative tasks from a home office, but by mid-May, she became stir-crazy.
“I just couldn’t stand it, anymore,” Dr. Bernstein said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “I came back to work at least just to see my colleagues, because I felt so disconnected. Normally, in a disaster, people come together – whether it’s responding to an earthquake or a fire or whatever. People come together to provide themselves with support. They hug each other and hold each other’s hands. We could not and cannot do that in this pandemic.”
According to Dr. Bernstein, stress, fear, and uncertainty triggered by the COVID-19 pandemic require special attention to the needs of health care personnel.
“Taking care of yourself and encouraging others to do the same sustains the ability to care for those in need,” said Dr. Bernstein, who is vice chair for faculty development and well-being in the departments of psychiatry and behavioral science and obstetrics and gynecology at Montefiore Medical Center/Albert Einstein College of Medicine, New York. “This includes both meeting practical needs as well as physical and emotional self-care. Everyone is impacted by this, so emotional support needs to be available to everyone. In the psychiatric community, we have triple challenges. We have to take care of our patients, our colleagues, and ourselves. It’s a lot.”
Specific challenges for health care workers include the potential for a surge in care demand and uncertainty about future outbreaks.
“Although we don’t have [personal protective] and respirator shortages at the moment, we’re worried about the vaccine shortages,” she said. Then there’s the fact that patients with comorbid conditions have the highest risk of death and the task of providing supportive care as well as medical care. “Of course, we still have a risk of becoming infected or infecting our families. There is additional psychological stress: fear, grief, frustration, guilt, insomnia, and exhaustion.”
Now, more than a year removed from the start of the pandemic, health care personnel are experiencing compassion fatigue, which she described as the inability to feel compassion for our patients because of our inability to feel compassion for ourselves. “We’re certainly experiencing burnout, although the primary aspect of burnout that we are experiencing is emotional exhaustion,” said Dr. Bernstein, who also is a past president of the American Psychiatric Association.
General risk factors for burnout and distress include sleep deprivation, high levels of work/life conflict, work interrupted by personal concerns, high levels of anger, loneliness, or anxiety, the stress of work relationships/work outcomes, anxiety about competency, difficulty “unplugging” after work, and regular use of alcohol and other drugs. At the same time, she continued, signs of burnout and secondary traumatic stress include sadness, depression, or apathy; feeling easily frustrated; feeling isolated and disconnected from others; excessive worry or fear about something bad happening; feeling like a failure, and feeling tired, exhausted, or overwhelmed.
“Why is this crisis so hard for us docs?” she asked. “Because This can lead to medical errors and unprofessional behavior. There are significant feelings of guilt that ‘I’m not doing enough.’
“This was true for a lot of us in psychiatry who were working virtually early during the pandemic while our medicine colleagues were on the front lines exposing themselves to COVID. Even the people working on the COVID units at the height on the initial surge felt guilty because treatment algorithms were changing almost every day. Fortunately, protocols are more established now, but the sense of not doing enough is pervasive and makes it difficult for us to ask for help.”
Fear of the unknown also posed a challenge to the workforce. “We didn’t know what we were dealing with at first,” she said. “The loss of control and autonomy, which is a major driver of burnout in the best of circumstances, was particularly true here in New York. People were told what to do. They were deployed into new circumstances. We experienced a significant loss of control, both of the virus and of what we were doing, and a widespread sense of isolation and loneliness.”
To cultivate resilience going forward, Dr. Bernstein advocates for the concept of psychological flexibility, which she defined as the ability to stay in contact with the present moment regardless of unpleasant thoughts, feelings, and bodily sensations, while choosing one’s behaviors based on the situation and personal values. “It is understanding that you can feel demoralized and bad one minute and better the next day,” she said. “This is a key concept for being able to continuously adapt under stressful circumstances and to tolerate uncertainty.”
She advises clinicians to identify safe areas and behaviors, and to maximize their ability to care for themselves and their families – including keeping in touch with colleagues and people you care about. “You also want to take advantage of calming skills and the maintenance of natural body rhythms,” she said. “This includes sensible nutrition and getting adequate rest and exercise.”
Dr. Bernstein also emphasized the importance of trying to maintain hope and optimism while not denying risk. “We also have to think about ethics, to provide the best possible care given the circumstances,” she said. “The crisis standards of care are necessarily different. We are not ethically required to offer futile care, but we must tell the truth.”
She pointed out that resilience is sometimes thought of as returning to the way you were before a stressful or life-altering event. “But here we refer to it as using your coping resources, connecting to others, and cultivating your values and purpose in life as you ride through this time of stress,” Dr. Bernstein said. “You are aware of the time it takes to develop and test for treatment and vaccine efficacy, and to then roll out these interventions, so you do know there will be an end to this, hopefully by the summer. While you won’t forget this time, focus on what you can control, your positive relationships, remind yourself of your purpose, and practice gratitude for what you are thankful for in your life. We need to cultivate what is positive and promote the message that emotional health should have the same priority level as physical health. The goal is to flourish.”
Dr. Bernstein reported having no financial disclosures.
Psychiatric community is facing ‘triple challenges’ tied to COVID
Psychiatric community is facing ‘triple challenges’ tied to COVID
When cases of COVID-19 began to surge in New York City in March 2020, Carol A. Bernstein, MD, did her best to practice psychiatry and carry out administrative tasks from a home office, but by mid-May, she became stir-crazy.
“I just couldn’t stand it, anymore,” Dr. Bernstein said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “I came back to work at least just to see my colleagues, because I felt so disconnected. Normally, in a disaster, people come together – whether it’s responding to an earthquake or a fire or whatever. People come together to provide themselves with support. They hug each other and hold each other’s hands. We could not and cannot do that in this pandemic.”
According to Dr. Bernstein, stress, fear, and uncertainty triggered by the COVID-19 pandemic require special attention to the needs of health care personnel.
“Taking care of yourself and encouraging others to do the same sustains the ability to care for those in need,” said Dr. Bernstein, who is vice chair for faculty development and well-being in the departments of psychiatry and behavioral science and obstetrics and gynecology at Montefiore Medical Center/Albert Einstein College of Medicine, New York. “This includes both meeting practical needs as well as physical and emotional self-care. Everyone is impacted by this, so emotional support needs to be available to everyone. In the psychiatric community, we have triple challenges. We have to take care of our patients, our colleagues, and ourselves. It’s a lot.”
Specific challenges for health care workers include the potential for a surge in care demand and uncertainty about future outbreaks.
“Although we don’t have [personal protective] and respirator shortages at the moment, we’re worried about the vaccine shortages,” she said. Then there’s the fact that patients with comorbid conditions have the highest risk of death and the task of providing supportive care as well as medical care. “Of course, we still have a risk of becoming infected or infecting our families. There is additional psychological stress: fear, grief, frustration, guilt, insomnia, and exhaustion.”
Now, more than a year removed from the start of the pandemic, health care personnel are experiencing compassion fatigue, which she described as the inability to feel compassion for our patients because of our inability to feel compassion for ourselves. “We’re certainly experiencing burnout, although the primary aspect of burnout that we are experiencing is emotional exhaustion,” said Dr. Bernstein, who also is a past president of the American Psychiatric Association.
General risk factors for burnout and distress include sleep deprivation, high levels of work/life conflict, work interrupted by personal concerns, high levels of anger, loneliness, or anxiety, the stress of work relationships/work outcomes, anxiety about competency, difficulty “unplugging” after work, and regular use of alcohol and other drugs. At the same time, she continued, signs of burnout and secondary traumatic stress include sadness, depression, or apathy; feeling easily frustrated; feeling isolated and disconnected from others; excessive worry or fear about something bad happening; feeling like a failure, and feeling tired, exhausted, or overwhelmed.
“Why is this crisis so hard for us docs?” she asked. “Because This can lead to medical errors and unprofessional behavior. There are significant feelings of guilt that ‘I’m not doing enough.’
“This was true for a lot of us in psychiatry who were working virtually early during the pandemic while our medicine colleagues were on the front lines exposing themselves to COVID. Even the people working on the COVID units at the height on the initial surge felt guilty because treatment algorithms were changing almost every day. Fortunately, protocols are more established now, but the sense of not doing enough is pervasive and makes it difficult for us to ask for help.”
Fear of the unknown also posed a challenge to the workforce. “We didn’t know what we were dealing with at first,” she said. “The loss of control and autonomy, which is a major driver of burnout in the best of circumstances, was particularly true here in New York. People were told what to do. They were deployed into new circumstances. We experienced a significant loss of control, both of the virus and of what we were doing, and a widespread sense of isolation and loneliness.”
To cultivate resilience going forward, Dr. Bernstein advocates for the concept of psychological flexibility, which she defined as the ability to stay in contact with the present moment regardless of unpleasant thoughts, feelings, and bodily sensations, while choosing one’s behaviors based on the situation and personal values. “It is understanding that you can feel demoralized and bad one minute and better the next day,” she said. “This is a key concept for being able to continuously adapt under stressful circumstances and to tolerate uncertainty.”
She advises clinicians to identify safe areas and behaviors, and to maximize their ability to care for themselves and their families – including keeping in touch with colleagues and people you care about. “You also want to take advantage of calming skills and the maintenance of natural body rhythms,” she said. “This includes sensible nutrition and getting adequate rest and exercise.”
Dr. Bernstein also emphasized the importance of trying to maintain hope and optimism while not denying risk. “We also have to think about ethics, to provide the best possible care given the circumstances,” she said. “The crisis standards of care are necessarily different. We are not ethically required to offer futile care, but we must tell the truth.”
She pointed out that resilience is sometimes thought of as returning to the way you were before a stressful or life-altering event. “But here we refer to it as using your coping resources, connecting to others, and cultivating your values and purpose in life as you ride through this time of stress,” Dr. Bernstein said. “You are aware of the time it takes to develop and test for treatment and vaccine efficacy, and to then roll out these interventions, so you do know there will be an end to this, hopefully by the summer. While you won’t forget this time, focus on what you can control, your positive relationships, remind yourself of your purpose, and practice gratitude for what you are thankful for in your life. We need to cultivate what is positive and promote the message that emotional health should have the same priority level as physical health. The goal is to flourish.”
Dr. Bernstein reported having no financial disclosures.
When cases of COVID-19 began to surge in New York City in March 2020, Carol A. Bernstein, MD, did her best to practice psychiatry and carry out administrative tasks from a home office, but by mid-May, she became stir-crazy.
“I just couldn’t stand it, anymore,” Dr. Bernstein said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “I came back to work at least just to see my colleagues, because I felt so disconnected. Normally, in a disaster, people come together – whether it’s responding to an earthquake or a fire or whatever. People come together to provide themselves with support. They hug each other and hold each other’s hands. We could not and cannot do that in this pandemic.”
According to Dr. Bernstein, stress, fear, and uncertainty triggered by the COVID-19 pandemic require special attention to the needs of health care personnel.
“Taking care of yourself and encouraging others to do the same sustains the ability to care for those in need,” said Dr. Bernstein, who is vice chair for faculty development and well-being in the departments of psychiatry and behavioral science and obstetrics and gynecology at Montefiore Medical Center/Albert Einstein College of Medicine, New York. “This includes both meeting practical needs as well as physical and emotional self-care. Everyone is impacted by this, so emotional support needs to be available to everyone. In the psychiatric community, we have triple challenges. We have to take care of our patients, our colleagues, and ourselves. It’s a lot.”
Specific challenges for health care workers include the potential for a surge in care demand and uncertainty about future outbreaks.
“Although we don’t have [personal protective] and respirator shortages at the moment, we’re worried about the vaccine shortages,” she said. Then there’s the fact that patients with comorbid conditions have the highest risk of death and the task of providing supportive care as well as medical care. “Of course, we still have a risk of becoming infected or infecting our families. There is additional psychological stress: fear, grief, frustration, guilt, insomnia, and exhaustion.”
Now, more than a year removed from the start of the pandemic, health care personnel are experiencing compassion fatigue, which she described as the inability to feel compassion for our patients because of our inability to feel compassion for ourselves. “We’re certainly experiencing burnout, although the primary aspect of burnout that we are experiencing is emotional exhaustion,” said Dr. Bernstein, who also is a past president of the American Psychiatric Association.
General risk factors for burnout and distress include sleep deprivation, high levels of work/life conflict, work interrupted by personal concerns, high levels of anger, loneliness, or anxiety, the stress of work relationships/work outcomes, anxiety about competency, difficulty “unplugging” after work, and regular use of alcohol and other drugs. At the same time, she continued, signs of burnout and secondary traumatic stress include sadness, depression, or apathy; feeling easily frustrated; feeling isolated and disconnected from others; excessive worry or fear about something bad happening; feeling like a failure, and feeling tired, exhausted, or overwhelmed.
“Why is this crisis so hard for us docs?” she asked. “Because This can lead to medical errors and unprofessional behavior. There are significant feelings of guilt that ‘I’m not doing enough.’
“This was true for a lot of us in psychiatry who were working virtually early during the pandemic while our medicine colleagues were on the front lines exposing themselves to COVID. Even the people working on the COVID units at the height on the initial surge felt guilty because treatment algorithms were changing almost every day. Fortunately, protocols are more established now, but the sense of not doing enough is pervasive and makes it difficult for us to ask for help.”
Fear of the unknown also posed a challenge to the workforce. “We didn’t know what we were dealing with at first,” she said. “The loss of control and autonomy, which is a major driver of burnout in the best of circumstances, was particularly true here in New York. People were told what to do. They were deployed into new circumstances. We experienced a significant loss of control, both of the virus and of what we were doing, and a widespread sense of isolation and loneliness.”
To cultivate resilience going forward, Dr. Bernstein advocates for the concept of psychological flexibility, which she defined as the ability to stay in contact with the present moment regardless of unpleasant thoughts, feelings, and bodily sensations, while choosing one’s behaviors based on the situation and personal values. “It is understanding that you can feel demoralized and bad one minute and better the next day,” she said. “This is a key concept for being able to continuously adapt under stressful circumstances and to tolerate uncertainty.”
She advises clinicians to identify safe areas and behaviors, and to maximize their ability to care for themselves and their families – including keeping in touch with colleagues and people you care about. “You also want to take advantage of calming skills and the maintenance of natural body rhythms,” she said. “This includes sensible nutrition and getting adequate rest and exercise.”
Dr. Bernstein also emphasized the importance of trying to maintain hope and optimism while not denying risk. “We also have to think about ethics, to provide the best possible care given the circumstances,” she said. “The crisis standards of care are necessarily different. We are not ethically required to offer futile care, but we must tell the truth.”
She pointed out that resilience is sometimes thought of as returning to the way you were before a stressful or life-altering event. “But here we refer to it as using your coping resources, connecting to others, and cultivating your values and purpose in life as you ride through this time of stress,” Dr. Bernstein said. “You are aware of the time it takes to develop and test for treatment and vaccine efficacy, and to then roll out these interventions, so you do know there will be an end to this, hopefully by the summer. While you won’t forget this time, focus on what you can control, your positive relationships, remind yourself of your purpose, and practice gratitude for what you are thankful for in your life. We need to cultivate what is positive and promote the message that emotional health should have the same priority level as physical health. The goal is to flourish.”
Dr. Bernstein reported having no financial disclosures.
FROM NPA 2021
Quick byte: Curing diabetes
Harvard biologist Doug Melton, PhD, is exploring the use of stem cells to create replacement beta cells that produce insulin, according to Time magazine.
In 2014, he co-founded Semma Therapeutics to develop the technology, which was acquired by Vertex Pharmaceuticals.
“The company has created a small, implantable device that holds millions of replacement beta cells, letting glucose and insulin through but keeping immune cells out. ‘If it works in people as well as it does in animals, it’s possible that people will not be diabetic,’ said Dr. Melton, co-director of the Harvard Stem Cell Institute and an investigator of the Howard Hughes Medical Institute. ‘They will eat and drink and play like those of us who are not.’”
Reference
Steinberg D. 12 innovations that will change health care and medicine in the 2020s. Time. 2019 Oct 25. https://time.com/5710295/top-health-innovations/ Accessed Dec 5, 2019.
Harvard biologist Doug Melton, PhD, is exploring the use of stem cells to create replacement beta cells that produce insulin, according to Time magazine.
In 2014, he co-founded Semma Therapeutics to develop the technology, which was acquired by Vertex Pharmaceuticals.
“The company has created a small, implantable device that holds millions of replacement beta cells, letting glucose and insulin through but keeping immune cells out. ‘If it works in people as well as it does in animals, it’s possible that people will not be diabetic,’ said Dr. Melton, co-director of the Harvard Stem Cell Institute and an investigator of the Howard Hughes Medical Institute. ‘They will eat and drink and play like those of us who are not.’”
Reference
Steinberg D. 12 innovations that will change health care and medicine in the 2020s. Time. 2019 Oct 25. https://time.com/5710295/top-health-innovations/ Accessed Dec 5, 2019.
Harvard biologist Doug Melton, PhD, is exploring the use of stem cells to create replacement beta cells that produce insulin, according to Time magazine.
In 2014, he co-founded Semma Therapeutics to develop the technology, which was acquired by Vertex Pharmaceuticals.
“The company has created a small, implantable device that holds millions of replacement beta cells, letting glucose and insulin through but keeping immune cells out. ‘If it works in people as well as it does in animals, it’s possible that people will not be diabetic,’ said Dr. Melton, co-director of the Harvard Stem Cell Institute and an investigator of the Howard Hughes Medical Institute. ‘They will eat and drink and play like those of us who are not.’”
Reference
Steinberg D. 12 innovations that will change health care and medicine in the 2020s. Time. 2019 Oct 25. https://time.com/5710295/top-health-innovations/ Accessed Dec 5, 2019.
Make the Diagnosis - March 2021
Because of the lack of improvement with topical corticosteroids, a skin biopsy was performed from a lesion on the lower back which showed an epidermis with compact hyperkeratosis and a thickened granular layer. Within the dermis, there was a lichenoid infiltrate of lymphocytes with a prominent interface change and rare dyskeratotic keratinocytes consistent with lichen planus.
Lichen planus is an inflammatory condition of the skin seen mainly in the adult population and is rare in children. This condition affects 0.5%-1% of the population, with maybe a higher prevalence in woman with no racial predilection in the adult or pediatric population. Most patients diagnosed are described to be over 40 years of age, but in children, the mean age for presentation is reported between the ages of 7 and 11.8 years.1 Interestingly, most of the published larger studies of lichen planus in children originate from India. In a U.K. study, about 80% of the cases reported were from children of Indian descent, as is our patient; so it is possible that lichen planus may be more prevalent in India.1 In a study based in the United States, cases were more prevalent in African American children.2
The exact cause of this condition is not known but studies have suggested that activated T cells, particularly CD8+, attack and cause apoptosis of the basal keratinocytes.3 There appears to be an up-regulation of Th1 cytokines such as interferon‐gamma, tumor necrosis factor–alpha, interleukin‐1 alpha, IL‐6, and IL‐8, as well as other apoptosis-related molecules.3
Lichen planus has been associated with other systemic conditions especially liver disease (chronic active hepatitis C and primary biliary cirrhosis). Children and adults may also have coexistence of other autoimmune diseases such as autoimmune polyendocrinopathy, myasthenia gravis, autoimmune thyroid disease, vitiligo, and thymoma. Some reports have also found a higher prevalence of atopic dermatitis in children with lichen planus.4
The lesions are typically described as the four “Ps” for pruritic, polygonal, purpuric flat-topped papules, and plaques. The papules of lichen planus have characteristically dry fine white streaks known as Wickham’s striae. The lesions can occur anywhere on the body, but they tend to occur more commonly on the flexures of the forearms, the wrists, ankles, shins, knees, and the torso. The face is rarely affected. In some patients oral, scalp (lichen planopilaris), nails, and rarely conjunctival, genital, and esophageal involvement can occur.2
In histopathology, the lesions are characterized by a wedge-shaped hypergranulosis, marked hyperkeratosis, and irregular sawtooth-like acanthosis of rete ridges on the epidermis. The dermal-epidermal junction typically shows an interstitial dermatitis. Civatte bodies may also be seen. On direct immunofluorescence, IgM-staining of the cytoid bodies in the dermal papilla or peribasilar areas are suggestive of lichen planus.1
The differential diagnosis of lichen planus includes severe lichenified atopic dermatitis, drug-induced lichen planus, graft-versus-host disease, psoriasis, pityriasis rosea, subacute cutaneous lupus, discoid lupus, secondary syphilis, and lichen simplex chronicus. Interestingly, our patient presented with lesions that were not pruritic and more generalized. Compared with eczema, were flexures are commonly affected, our patient’s lesions were localized to the ankles, wrists, extensor knees, and elbows, and no pruritus was reported. Lichenification of skin lesions occurs as a response to chronic scratching as it occurs in atopic dermatitis and lichen simplex chronicus, was considered in our patient, but the lack of pruritus and the more acute presentation made it unlikely.
Lichen planus is considered a self-limiting disease, so treatment is focused on the control of pruritus and to accelerate resolution. The first-line therapy for classic cutaneous lichen planus is the use of potent or superpotent topical corticosteroids for localized disease on the body and extremities and mild to mid-potency for intertriginous areas and the face. Clinical response should be assessed after 2-3 weeks of treatment. For patients with more generalized or recalcitrant disease like our patient, other treatment modalities like phototherapy (narrow-band UVB), a 4- to 6-week course of oral glucocorticoids, or acitretin may be considered. Our patient recently started narrow-band UVB. Other medications that have been reported beneficial for more severe cases include methotrexate, cyclosporine, griseofulvin, hydroxychloroquine, metronidazole, dapsone, and mycophenolate. Recent studies in the adult population have shown apremilast, a phosphodiesterase inhibitor, to be a promising medication for patients with cutaneous lichen planus, though this medication has not been approved yet for use in the pediatric population.5
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Payette MJ et al. Clin Dermatol. 2015 Nov-Dec;33(6):631-43.
2. Walton KE et al. Pediatr Dermatol. 2010;27:34-8.
3. Lehman JS et al. Int J Dermatol. 2009 Jul;48(7):682-94.
4. Laughter D et al. J Am Acad Dermatol. 2000;43:649-55.
5. Paul J et al. J Am Acad Dermatol. 2013 Feb;68(2):255-61.
Because of the lack of improvement with topical corticosteroids, a skin biopsy was performed from a lesion on the lower back which showed an epidermis with compact hyperkeratosis and a thickened granular layer. Within the dermis, there was a lichenoid infiltrate of lymphocytes with a prominent interface change and rare dyskeratotic keratinocytes consistent with lichen planus.
Lichen planus is an inflammatory condition of the skin seen mainly in the adult population and is rare in children. This condition affects 0.5%-1% of the population, with maybe a higher prevalence in woman with no racial predilection in the adult or pediatric population. Most patients diagnosed are described to be over 40 years of age, but in children, the mean age for presentation is reported between the ages of 7 and 11.8 years.1 Interestingly, most of the published larger studies of lichen planus in children originate from India. In a U.K. study, about 80% of the cases reported were from children of Indian descent, as is our patient; so it is possible that lichen planus may be more prevalent in India.1 In a study based in the United States, cases were more prevalent in African American children.2
The exact cause of this condition is not known but studies have suggested that activated T cells, particularly CD8+, attack and cause apoptosis of the basal keratinocytes.3 There appears to be an up-regulation of Th1 cytokines such as interferon‐gamma, tumor necrosis factor–alpha, interleukin‐1 alpha, IL‐6, and IL‐8, as well as other apoptosis-related molecules.3
Lichen planus has been associated with other systemic conditions especially liver disease (chronic active hepatitis C and primary biliary cirrhosis). Children and adults may also have coexistence of other autoimmune diseases such as autoimmune polyendocrinopathy, myasthenia gravis, autoimmune thyroid disease, vitiligo, and thymoma. Some reports have also found a higher prevalence of atopic dermatitis in children with lichen planus.4
The lesions are typically described as the four “Ps” for pruritic, polygonal, purpuric flat-topped papules, and plaques. The papules of lichen planus have characteristically dry fine white streaks known as Wickham’s striae. The lesions can occur anywhere on the body, but they tend to occur more commonly on the flexures of the forearms, the wrists, ankles, shins, knees, and the torso. The face is rarely affected. In some patients oral, scalp (lichen planopilaris), nails, and rarely conjunctival, genital, and esophageal involvement can occur.2
In histopathology, the lesions are characterized by a wedge-shaped hypergranulosis, marked hyperkeratosis, and irregular sawtooth-like acanthosis of rete ridges on the epidermis. The dermal-epidermal junction typically shows an interstitial dermatitis. Civatte bodies may also be seen. On direct immunofluorescence, IgM-staining of the cytoid bodies in the dermal papilla or peribasilar areas are suggestive of lichen planus.1
The differential diagnosis of lichen planus includes severe lichenified atopic dermatitis, drug-induced lichen planus, graft-versus-host disease, psoriasis, pityriasis rosea, subacute cutaneous lupus, discoid lupus, secondary syphilis, and lichen simplex chronicus. Interestingly, our patient presented with lesions that were not pruritic and more generalized. Compared with eczema, were flexures are commonly affected, our patient’s lesions were localized to the ankles, wrists, extensor knees, and elbows, and no pruritus was reported. Lichenification of skin lesions occurs as a response to chronic scratching as it occurs in atopic dermatitis and lichen simplex chronicus, was considered in our patient, but the lack of pruritus and the more acute presentation made it unlikely.
Lichen planus is considered a self-limiting disease, so treatment is focused on the control of pruritus and to accelerate resolution. The first-line therapy for classic cutaneous lichen planus is the use of potent or superpotent topical corticosteroids for localized disease on the body and extremities and mild to mid-potency for intertriginous areas and the face. Clinical response should be assessed after 2-3 weeks of treatment. For patients with more generalized or recalcitrant disease like our patient, other treatment modalities like phototherapy (narrow-band UVB), a 4- to 6-week course of oral glucocorticoids, or acitretin may be considered. Our patient recently started narrow-band UVB. Other medications that have been reported beneficial for more severe cases include methotrexate, cyclosporine, griseofulvin, hydroxychloroquine, metronidazole, dapsone, and mycophenolate. Recent studies in the adult population have shown apremilast, a phosphodiesterase inhibitor, to be a promising medication for patients with cutaneous lichen planus, though this medication has not been approved yet for use in the pediatric population.5
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Payette MJ et al. Clin Dermatol. 2015 Nov-Dec;33(6):631-43.
2. Walton KE et al. Pediatr Dermatol. 2010;27:34-8.
3. Lehman JS et al. Int J Dermatol. 2009 Jul;48(7):682-94.
4. Laughter D et al. J Am Acad Dermatol. 2000;43:649-55.
5. Paul J et al. J Am Acad Dermatol. 2013 Feb;68(2):255-61.
Because of the lack of improvement with topical corticosteroids, a skin biopsy was performed from a lesion on the lower back which showed an epidermis with compact hyperkeratosis and a thickened granular layer. Within the dermis, there was a lichenoid infiltrate of lymphocytes with a prominent interface change and rare dyskeratotic keratinocytes consistent with lichen planus.
Lichen planus is an inflammatory condition of the skin seen mainly in the adult population and is rare in children. This condition affects 0.5%-1% of the population, with maybe a higher prevalence in woman with no racial predilection in the adult or pediatric population. Most patients diagnosed are described to be over 40 years of age, but in children, the mean age for presentation is reported between the ages of 7 and 11.8 years.1 Interestingly, most of the published larger studies of lichen planus in children originate from India. In a U.K. study, about 80% of the cases reported were from children of Indian descent, as is our patient; so it is possible that lichen planus may be more prevalent in India.1 In a study based in the United States, cases were more prevalent in African American children.2
The exact cause of this condition is not known but studies have suggested that activated T cells, particularly CD8+, attack and cause apoptosis of the basal keratinocytes.3 There appears to be an up-regulation of Th1 cytokines such as interferon‐gamma, tumor necrosis factor–alpha, interleukin‐1 alpha, IL‐6, and IL‐8, as well as other apoptosis-related molecules.3
Lichen planus has been associated with other systemic conditions especially liver disease (chronic active hepatitis C and primary biliary cirrhosis). Children and adults may also have coexistence of other autoimmune diseases such as autoimmune polyendocrinopathy, myasthenia gravis, autoimmune thyroid disease, vitiligo, and thymoma. Some reports have also found a higher prevalence of atopic dermatitis in children with lichen planus.4
The lesions are typically described as the four “Ps” for pruritic, polygonal, purpuric flat-topped papules, and plaques. The papules of lichen planus have characteristically dry fine white streaks known as Wickham’s striae. The lesions can occur anywhere on the body, but they tend to occur more commonly on the flexures of the forearms, the wrists, ankles, shins, knees, and the torso. The face is rarely affected. In some patients oral, scalp (lichen planopilaris), nails, and rarely conjunctival, genital, and esophageal involvement can occur.2
In histopathology, the lesions are characterized by a wedge-shaped hypergranulosis, marked hyperkeratosis, and irregular sawtooth-like acanthosis of rete ridges on the epidermis. The dermal-epidermal junction typically shows an interstitial dermatitis. Civatte bodies may also be seen. On direct immunofluorescence, IgM-staining of the cytoid bodies in the dermal papilla or peribasilar areas are suggestive of lichen planus.1
The differential diagnosis of lichen planus includes severe lichenified atopic dermatitis, drug-induced lichen planus, graft-versus-host disease, psoriasis, pityriasis rosea, subacute cutaneous lupus, discoid lupus, secondary syphilis, and lichen simplex chronicus. Interestingly, our patient presented with lesions that were not pruritic and more generalized. Compared with eczema, were flexures are commonly affected, our patient’s lesions were localized to the ankles, wrists, extensor knees, and elbows, and no pruritus was reported. Lichenification of skin lesions occurs as a response to chronic scratching as it occurs in atopic dermatitis and lichen simplex chronicus, was considered in our patient, but the lack of pruritus and the more acute presentation made it unlikely.
Lichen planus is considered a self-limiting disease, so treatment is focused on the control of pruritus and to accelerate resolution. The first-line therapy for classic cutaneous lichen planus is the use of potent or superpotent topical corticosteroids for localized disease on the body and extremities and mild to mid-potency for intertriginous areas and the face. Clinical response should be assessed after 2-3 weeks of treatment. For patients with more generalized or recalcitrant disease like our patient, other treatment modalities like phototherapy (narrow-band UVB), a 4- to 6-week course of oral glucocorticoids, or acitretin may be considered. Our patient recently started narrow-band UVB. Other medications that have been reported beneficial for more severe cases include methotrexate, cyclosporine, griseofulvin, hydroxychloroquine, metronidazole, dapsone, and mycophenolate. Recent studies in the adult population have shown apremilast, a phosphodiesterase inhibitor, to be a promising medication for patients with cutaneous lichen planus, though this medication has not been approved yet for use in the pediatric population.5
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
1. Payette MJ et al. Clin Dermatol. 2015 Nov-Dec;33(6):631-43.
2. Walton KE et al. Pediatr Dermatol. 2010;27:34-8.
3. Lehman JS et al. Int J Dermatol. 2009 Jul;48(7):682-94.
4. Laughter D et al. J Am Acad Dermatol. 2000;43:649-55.
5. Paul J et al. J Am Acad Dermatol. 2013 Feb;68(2):255-61.
There was no prior personal or family history of atopic dermatitis or psoriasis. He has no other medical conditions and is not taking any medications.
He denied any joint pain, sun sensitivity, mouth sores, or other symptoms. After the initial consultation he was treated with fluocinonide 0.05% ointment for 2 weeks with slight improvement on the lesions.
On physical exam he presented with hyperpigmented and violaceous lichenified papules and plaques on the extremities and the torso. (photos 1 and 2). He also had hyperpigmented violaceous macules on the eyelids and around the mouth (photos 1 and 2).