User login
Important lessons about telehealth
Telehealth exploded into the public consciousness this year as a way for clinicians and patients to safely connect during the COVID-19 crisis. While telehealth has been part of care delivery at Providence St. Joseph Health (PSJH) for more than a decade, it transitioned almost overnight from an offering most often focused on serving patients in rural areas to a way for any patient to get the care they need virtually whether in a hospital, outpatient facility, or from the comfort and safety of their own home.
Telehealth growth was fueled by changes in regulation and reimbursement during the public health emergency enabling providers to see new and established patients at home across all payer types. To put this growth into perspective, the large PSJH system averaged a few thousand video visits per month in January and February 2020. As COVID transmission spread and lockdowns began, that number climbed to over 15,000 video visits in March to a height of more than 150,000 video visits in May. As of the end of October 2020, PSJH has conducted more than 1.2 million video visits since the beginning of January, steadily accounting for 20%-25% of total visit volume.
Going virtual with gastroenterology
PSJH gastroenterology providers have been a part of this wave, conducting more than 12,000 video visits so far this year (as documented in our Epic EMR), which has been an entirely new method of care delivery for most of these clinicians. We also have many affiliated, private practice gastroenterology providers who practice in our facilities and transitioned quickly to video for outpatient care. Pre- and postprocedure follow-up visits were some of the most common visit types that went virtual, along with new patient visits to establish care and existing patient visits to check in on the status of a health condition, medication, or other concern. Complementary services for gastroenterology patients were transitioned to video over the past 8 months as well. Care management, nutrition services, online support groups, bariatric care information sessions, behavioral health, and more are now available for patients to access virtually.
Remembering it’s not about the tech
New technologies can be challenging to adopt – especially at a pace as rapid as it was this year. Fortunately for PSJH, we had inpatient and outpatient video platforms already in place and an experienced internal telehealth team to scale them quickly to providers and caregivers across system. But even with those advantages, it was still a huge challenge to transition so many providers and caregivers to video visits in such a short time without change management hurdles and bumps along the way.
Too often, there is an overemphasis placed on the technology. It’s a tool, and some technologies are better than others, and they continue to evolve over time. True success or failure lies in the clinical and operational work flows and how well the providers and care teams engage with and adapt them. We found that the providers and staff members willing to venture outside their comfort zone of “how we’ve always done it” and collaborate on the transition to virtual care had the best results. Openness and flexibility to trying new things and using temporary workarounds if existing functionality didn’t meet the need was key to transitioning quickly. Then, by listening to ideas from and sharing feedback among providers, clinics, and geographies, we were able to identify fixes and optimizations that needed to be made to improve the experience for all.
Selecting a video visit platform
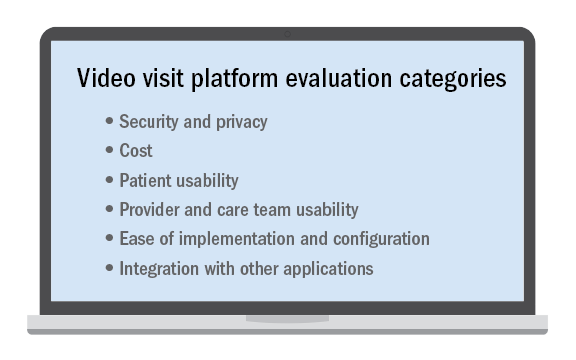
No telehealth platform is perfect and meets every patient, provider, and staff need or request despite what a technology vendor may claim. This is especially true in a large and/or diverse system with many different types of clinical use cases. Determining the “must-have” requirements from among those that may be important or simply nice to have is critical when selecting the video visit platform to use.
It’s not an easy decision and nearly impossible to please everyone. Ensuring that there are clinician, operator, and technical stakeholders all contributing to the requirements and decision-making is essential. While some may prefer a “best-of-breed” solution that does one thing very well, it may have to be paired with a set of other complimentary applications to meet all of the organization’s needs. Alternatively, there may be a platform with an expansive feature set but not all of the features are as strong as desired. Then there are solutions that integrate with your existing applications, which is a compelling option to consider.
Regardless of the tool chosen, best-practice work flows, easy-to-follow documentation, a mix of different training options, and internal technical help that responds quickly is key to implementing it successfully. And once implemented, optimization is an ongoing process to make it easier, faster, and better.
Looking ahead
As we came to the end of 2020, all providers and health systems were paying close attention to the Centers for Medicaid & Medicare Services and state-level regulations and reimbursement changes for 2021 to evaluate the impact on telehealth after the public health emergency and COVID-19 waivers are ended. Advocacy efforts are urging lawmakers to not lose the gains that were made during this time and have enabled millions of patients to access care more easily – changes which we believe they will now expect as an option going forward.
We at Providence believe telehealth’s future is a bright one, especially where value-based/managed care arrangements with payers are in place. In addition to integrating video visits and consults into normal clinical practice, we see further growth in serving patients at home with remote patient monitoring and other home-based programs that leverage connected devices and virtual tools. We also anticipate more providers will acquire licenses in other states to virtually care for patients who lack access to specialty services in their own community, which increases access where it is most needed. After 2020, we hope that telehealth will no longer be a specialized service only some patients can receive but a normal way of delivering care to all.
Ms. Winkelman is the system director of telehealth product development and delivery at Providence St. Joseph Health. Providence is the third-largest nonprofit health system in the United States with 51 hospitals, more than 800 clinics, and a comprehensive range of health and social services across Alaska, California, Montana, New Mexico, Oregon, Texas, and Washington.
Telehealth exploded into the public consciousness this year as a way for clinicians and patients to safely connect during the COVID-19 crisis. While telehealth has been part of care delivery at Providence St. Joseph Health (PSJH) for more than a decade, it transitioned almost overnight from an offering most often focused on serving patients in rural areas to a way for any patient to get the care they need virtually whether in a hospital, outpatient facility, or from the comfort and safety of their own home.
Telehealth growth was fueled by changes in regulation and reimbursement during the public health emergency enabling providers to see new and established patients at home across all payer types. To put this growth into perspective, the large PSJH system averaged a few thousand video visits per month in January and February 2020. As COVID transmission spread and lockdowns began, that number climbed to over 15,000 video visits in March to a height of more than 150,000 video visits in May. As of the end of October 2020, PSJH has conducted more than 1.2 million video visits since the beginning of January, steadily accounting for 20%-25% of total visit volume.
Going virtual with gastroenterology
PSJH gastroenterology providers have been a part of this wave, conducting more than 12,000 video visits so far this year (as documented in our Epic EMR), which has been an entirely new method of care delivery for most of these clinicians. We also have many affiliated, private practice gastroenterology providers who practice in our facilities and transitioned quickly to video for outpatient care. Pre- and postprocedure follow-up visits were some of the most common visit types that went virtual, along with new patient visits to establish care and existing patient visits to check in on the status of a health condition, medication, or other concern. Complementary services for gastroenterology patients were transitioned to video over the past 8 months as well. Care management, nutrition services, online support groups, bariatric care information sessions, behavioral health, and more are now available for patients to access virtually.
Remembering it’s not about the tech
New technologies can be challenging to adopt – especially at a pace as rapid as it was this year. Fortunately for PSJH, we had inpatient and outpatient video platforms already in place and an experienced internal telehealth team to scale them quickly to providers and caregivers across system. But even with those advantages, it was still a huge challenge to transition so many providers and caregivers to video visits in such a short time without change management hurdles and bumps along the way.
Too often, there is an overemphasis placed on the technology. It’s a tool, and some technologies are better than others, and they continue to evolve over time. True success or failure lies in the clinical and operational work flows and how well the providers and care teams engage with and adapt them. We found that the providers and staff members willing to venture outside their comfort zone of “how we’ve always done it” and collaborate on the transition to virtual care had the best results. Openness and flexibility to trying new things and using temporary workarounds if existing functionality didn’t meet the need was key to transitioning quickly. Then, by listening to ideas from and sharing feedback among providers, clinics, and geographies, we were able to identify fixes and optimizations that needed to be made to improve the experience for all.
Selecting a video visit platform

No telehealth platform is perfect and meets every patient, provider, and staff need or request despite what a technology vendor may claim. This is especially true in a large and/or diverse system with many different types of clinical use cases. Determining the “must-have” requirements from among those that may be important or simply nice to have is critical when selecting the video visit platform to use.
It’s not an easy decision and nearly impossible to please everyone. Ensuring that there are clinician, operator, and technical stakeholders all contributing to the requirements and decision-making is essential. While some may prefer a “best-of-breed” solution that does one thing very well, it may have to be paired with a set of other complimentary applications to meet all of the organization’s needs. Alternatively, there may be a platform with an expansive feature set but not all of the features are as strong as desired. Then there are solutions that integrate with your existing applications, which is a compelling option to consider.
Regardless of the tool chosen, best-practice work flows, easy-to-follow documentation, a mix of different training options, and internal technical help that responds quickly is key to implementing it successfully. And once implemented, optimization is an ongoing process to make it easier, faster, and better.
Looking ahead
As we came to the end of 2020, all providers and health systems were paying close attention to the Centers for Medicaid & Medicare Services and state-level regulations and reimbursement changes for 2021 to evaluate the impact on telehealth after the public health emergency and COVID-19 waivers are ended. Advocacy efforts are urging lawmakers to not lose the gains that were made during this time and have enabled millions of patients to access care more easily – changes which we believe they will now expect as an option going forward.
We at Providence believe telehealth’s future is a bright one, especially where value-based/managed care arrangements with payers are in place. In addition to integrating video visits and consults into normal clinical practice, we see further growth in serving patients at home with remote patient monitoring and other home-based programs that leverage connected devices and virtual tools. We also anticipate more providers will acquire licenses in other states to virtually care for patients who lack access to specialty services in their own community, which increases access where it is most needed. After 2020, we hope that telehealth will no longer be a specialized service only some patients can receive but a normal way of delivering care to all.
Ms. Winkelman is the system director of telehealth product development and delivery at Providence St. Joseph Health. Providence is the third-largest nonprofit health system in the United States with 51 hospitals, more than 800 clinics, and a comprehensive range of health and social services across Alaska, California, Montana, New Mexico, Oregon, Texas, and Washington.
Telehealth exploded into the public consciousness this year as a way for clinicians and patients to safely connect during the COVID-19 crisis. While telehealth has been part of care delivery at Providence St. Joseph Health (PSJH) for more than a decade, it transitioned almost overnight from an offering most often focused on serving patients in rural areas to a way for any patient to get the care they need virtually whether in a hospital, outpatient facility, or from the comfort and safety of their own home.
Telehealth growth was fueled by changes in regulation and reimbursement during the public health emergency enabling providers to see new and established patients at home across all payer types. To put this growth into perspective, the large PSJH system averaged a few thousand video visits per month in January and February 2020. As COVID transmission spread and lockdowns began, that number climbed to over 15,000 video visits in March to a height of more than 150,000 video visits in May. As of the end of October 2020, PSJH has conducted more than 1.2 million video visits since the beginning of January, steadily accounting for 20%-25% of total visit volume.
Going virtual with gastroenterology
PSJH gastroenterology providers have been a part of this wave, conducting more than 12,000 video visits so far this year (as documented in our Epic EMR), which has been an entirely new method of care delivery for most of these clinicians. We also have many affiliated, private practice gastroenterology providers who practice in our facilities and transitioned quickly to video for outpatient care. Pre- and postprocedure follow-up visits were some of the most common visit types that went virtual, along with new patient visits to establish care and existing patient visits to check in on the status of a health condition, medication, or other concern. Complementary services for gastroenterology patients were transitioned to video over the past 8 months as well. Care management, nutrition services, online support groups, bariatric care information sessions, behavioral health, and more are now available for patients to access virtually.
Remembering it’s not about the tech
New technologies can be challenging to adopt – especially at a pace as rapid as it was this year. Fortunately for PSJH, we had inpatient and outpatient video platforms already in place and an experienced internal telehealth team to scale them quickly to providers and caregivers across system. But even with those advantages, it was still a huge challenge to transition so many providers and caregivers to video visits in such a short time without change management hurdles and bumps along the way.
Too often, there is an overemphasis placed on the technology. It’s a tool, and some technologies are better than others, and they continue to evolve over time. True success or failure lies in the clinical and operational work flows and how well the providers and care teams engage with and adapt them. We found that the providers and staff members willing to venture outside their comfort zone of “how we’ve always done it” and collaborate on the transition to virtual care had the best results. Openness and flexibility to trying new things and using temporary workarounds if existing functionality didn’t meet the need was key to transitioning quickly. Then, by listening to ideas from and sharing feedback among providers, clinics, and geographies, we were able to identify fixes and optimizations that needed to be made to improve the experience for all.
Selecting a video visit platform

No telehealth platform is perfect and meets every patient, provider, and staff need or request despite what a technology vendor may claim. This is especially true in a large and/or diverse system with many different types of clinical use cases. Determining the “must-have” requirements from among those that may be important or simply nice to have is critical when selecting the video visit platform to use.
It’s not an easy decision and nearly impossible to please everyone. Ensuring that there are clinician, operator, and technical stakeholders all contributing to the requirements and decision-making is essential. While some may prefer a “best-of-breed” solution that does one thing very well, it may have to be paired with a set of other complimentary applications to meet all of the organization’s needs. Alternatively, there may be a platform with an expansive feature set but not all of the features are as strong as desired. Then there are solutions that integrate with your existing applications, which is a compelling option to consider.
Regardless of the tool chosen, best-practice work flows, easy-to-follow documentation, a mix of different training options, and internal technical help that responds quickly is key to implementing it successfully. And once implemented, optimization is an ongoing process to make it easier, faster, and better.
Looking ahead
As we came to the end of 2020, all providers and health systems were paying close attention to the Centers for Medicaid & Medicare Services and state-level regulations and reimbursement changes for 2021 to evaluate the impact on telehealth after the public health emergency and COVID-19 waivers are ended. Advocacy efforts are urging lawmakers to not lose the gains that were made during this time and have enabled millions of patients to access care more easily – changes which we believe they will now expect as an option going forward.
We at Providence believe telehealth’s future is a bright one, especially where value-based/managed care arrangements with payers are in place. In addition to integrating video visits and consults into normal clinical practice, we see further growth in serving patients at home with remote patient monitoring and other home-based programs that leverage connected devices and virtual tools. We also anticipate more providers will acquire licenses in other states to virtually care for patients who lack access to specialty services in their own community, which increases access where it is most needed. After 2020, we hope that telehealth will no longer be a specialized service only some patients can receive but a normal way of delivering care to all.
Ms. Winkelman is the system director of telehealth product development and delivery at Providence St. Joseph Health. Providence is the third-largest nonprofit health system in the United States with 51 hospitals, more than 800 clinics, and a comprehensive range of health and social services across Alaska, California, Montana, New Mexico, Oregon, Texas, and Washington.
How a gift of stock to the AGA Research Foundation can be a win-win
If you own stock that’s increased in value since you purchased it (and you’ve owned it for at least 1 year), you have a unique opportunity for philanthropy. When you donate securities to the AGA Research Foundation, you receive the same income tax savings (if you itemize) that you would if you wrote the AGA Research Foundation a check, but with the added benefit of eliminating capital gains taxes on the transfer, which can be as high as 20%.
Making a gift of securities to support the AGA Research Foundation’s mission to raise funds to support young researchers in gastroenterology and hepatology is as easy as instructing your broker to transfer the shares. Using assets other than cash also allows you more flexibility when planning your gift.
Benefits:
- Receive an income tax deduction for gifts of securities if you itemize.
- Provide relief from capital gains tax with gifts of securities.
- Help fulfill our mission with your contribution.
Take the next step:
The AGA Research Foundation can help clarify and document the steps to donate stock to us. Contact us at [email protected] to make your donation.
If you own stock that’s increased in value since you purchased it (and you’ve owned it for at least 1 year), you have a unique opportunity for philanthropy. When you donate securities to the AGA Research Foundation, you receive the same income tax savings (if you itemize) that you would if you wrote the AGA Research Foundation a check, but with the added benefit of eliminating capital gains taxes on the transfer, which can be as high as 20%.
Making a gift of securities to support the AGA Research Foundation’s mission to raise funds to support young researchers in gastroenterology and hepatology is as easy as instructing your broker to transfer the shares. Using assets other than cash also allows you more flexibility when planning your gift.
Benefits:
- Receive an income tax deduction for gifts of securities if you itemize.
- Provide relief from capital gains tax with gifts of securities.
- Help fulfill our mission with your contribution.
Take the next step:
The AGA Research Foundation can help clarify and document the steps to donate stock to us. Contact us at [email protected] to make your donation.
If you own stock that’s increased in value since you purchased it (and you’ve owned it for at least 1 year), you have a unique opportunity for philanthropy. When you donate securities to the AGA Research Foundation, you receive the same income tax savings (if you itemize) that you would if you wrote the AGA Research Foundation a check, but with the added benefit of eliminating capital gains taxes on the transfer, which can be as high as 20%.
Making a gift of securities to support the AGA Research Foundation’s mission to raise funds to support young researchers in gastroenterology and hepatology is as easy as instructing your broker to transfer the shares. Using assets other than cash also allows you more flexibility when planning your gift.
Benefits:
- Receive an income tax deduction for gifts of securities if you itemize.
- Provide relief from capital gains tax with gifts of securities.
- Help fulfill our mission with your contribution.
Take the next step:
The AGA Research Foundation can help clarify and document the steps to donate stock to us. Contact us at [email protected] to make your donation.
Registration for DDW® 2021 is now open
Join your colleagues in the digestive disease community at the most prestigious meeting for GI professionals. Registration for Digestive Disease Week® (DDW) 2021 is now open. Register on or before March 31 to receive a discounted rate. AGA member trainees, postdoctoral fellows, medical residents and students also receive complimentary registration during this early bird period.
In 2021, DDW moves online as a fully virtual meeting, taking place May 21–23, 2021. While DDW Virtual™ will look a little different, we’re excited by opportunities the new format provides to learn, share, and connect, such as the following:
- Explore today’s most pressing topics and new developments, shared in oral abstract and ePoster presentations.
- Gain the kind of insight that you can’t get out of a textbook, presented in sessions led by top GI and hepatology experts.
- Network and build connections with your colleagues in an engaging, interactive setting.
Learn more and register at ddw.org.
Join your colleagues in the digestive disease community at the most prestigious meeting for GI professionals. Registration for Digestive Disease Week® (DDW) 2021 is now open. Register on or before March 31 to receive a discounted rate. AGA member trainees, postdoctoral fellows, medical residents and students also receive complimentary registration during this early bird period.
In 2021, DDW moves online as a fully virtual meeting, taking place May 21–23, 2021. While DDW Virtual™ will look a little different, we’re excited by opportunities the new format provides to learn, share, and connect, such as the following:
- Explore today’s most pressing topics and new developments, shared in oral abstract and ePoster presentations.
- Gain the kind of insight that you can’t get out of a textbook, presented in sessions led by top GI and hepatology experts.
- Network and build connections with your colleagues in an engaging, interactive setting.
Learn more and register at ddw.org.
Join your colleagues in the digestive disease community at the most prestigious meeting for GI professionals. Registration for Digestive Disease Week® (DDW) 2021 is now open. Register on or before March 31 to receive a discounted rate. AGA member trainees, postdoctoral fellows, medical residents and students also receive complimentary registration during this early bird period.
In 2021, DDW moves online as a fully virtual meeting, taking place May 21–23, 2021. While DDW Virtual™ will look a little different, we’re excited by opportunities the new format provides to learn, share, and connect, such as the following:
- Explore today’s most pressing topics and new developments, shared in oral abstract and ePoster presentations.
- Gain the kind of insight that you can’t get out of a textbook, presented in sessions led by top GI and hepatology experts.
- Network and build connections with your colleagues in an engaging, interactive setting.
Learn more and register at ddw.org.
Meet the 2021 AGA Fellowship inductees
Join the GI community in a round of applause for the 120 members adding the designation “AGAF” in their professional activities. Along with a recognition pin and certificate of acceptance, American Gastroenterological Association President Bishr Omary commends the group in the AGA Community for their superior professional achievements and contributions to the field of gastroenterology. See the full list and join the discussion at https://community.gastro.org.
Join the GI community in a round of applause for the 120 members adding the designation “AGAF” in their professional activities. Along with a recognition pin and certificate of acceptance, American Gastroenterological Association President Bishr Omary commends the group in the AGA Community for their superior professional achievements and contributions to the field of gastroenterology. See the full list and join the discussion at https://community.gastro.org.
Join the GI community in a round of applause for the 120 members adding the designation “AGAF” in their professional activities. Along with a recognition pin and certificate of acceptance, American Gastroenterological Association President Bishr Omary commends the group in the AGA Community for their superior professional achievements and contributions to the field of gastroenterology. See the full list and join the discussion at https://community.gastro.org.
Advocacy in gastroenterology: Advancing health policies for our patients and our profession
Physician advocacy is an important tool for health care professionals to protect patients and the vitality of the profession. Medical associations across the spectrum participate in advocacy because of its value in preserving the beneficial role of physicians in health care policy decision making. This is especially true for specialty physician associations, like the American Gastroenterological Association, which represents more than 9,000 U.S. GI physicians and researchers. Advocacy allows for the voice of GIs and their patients to be heard on Capitol Hill, in the White House, and among various regulatory agencies. When we advocate as a profession, we help ensure good policies gain momentum and halt harmful legislative or regulatory efforts from enactment.
What is physician advocacy?
Physicians are advocating every day for their patients by helping patients make the right decisions about their care. This naturally translates into advocacy at the health policy level. Advocacy is lobbying. While that word may take on a negative meaning for some, it also means being a persuasive communicator, passionate educator, and a leader. National associations, like AGA, often call on members to do just that: educate lawmakers on policies affecting GI, communicate how policies could affect lawmakers’ constituencies back in their respective districts, and lead others to support GI policy agendas.
Physician advocacy works. AGA had its busiest year for policy work, but this was coupled with a large uptick in GI advocacy engagement. The public health emergency placed many burdens on the health care community and our profession. However, through our advocacy work, we also saw many changes, including increased federal research funding for digestive diseases and GI cancers, passage of legislation to remove patients’ barriers to colorectal cancer screening, increased regulatory and reimbursement flexibilities incorporated to ensure physicians could continue to deliver timely care, and creation of federal financial and small business relief programs to support gastroenterology practices.
Physician advocacy in GI is especially critical because specialty care is often viewed as having a smaller voice when compared with those of the larger bodies, such as primary care, surgery, or emergency physicians. As a health care specialty with a known shortage across the United States, we need all the help we can get to inform policy makers of our position on controversial policies. In many cases, non–health care professionals are informing policy makers on how to address issues that impact our profession. Additionally, there is a lack of knowledge about health care complexities and needs among decision makers who are ultimately determining how health care is delivered. As health care experts, we are best suited to educate lawmakers on the true impact of health policies. If we do not engage and educate policy makers, our profession and patients will suffer the consequences.
GI policy priorities for 2021
AGA will continue its advocacy work in 2021 on the following issues and encourage you and your colleagues to get involved:
Administrative burden relief
Utilization management protocols, like prior authorizations and step therapy, continue to increase and force physicians and their staff to spend hours of extra work time each week to process the paperwork. Prior authorizations are especially troublesome because they have increased for upper GI procedures and other common procedures. Step therapy protocols have also increased for IBD patients on biologics or other high-cost therapies, resulting in patients not receiving effective therapies as determined by their physician in a timely manner.
Patient access and protections
Coverage
Coverage for patients includes the following two areas:
COVID-19 relief: The public health emergency has weakened the health care workforce with physician practices and researchers facing financial instability and threatened patient access to specialty care. To support the health care community and to combat the pandemic, the following is necessary: Increased access to personal protective equipment and medical supplies for testing and vaccination distribution and increased rapid tests, testing sites, and health care workers. The public health emergency response also requires a stronger emphasis on health equity given the disproportionate impact it has had on communities of color.
- Preserving Affordable Care Act patient protections: The Supreme Court will rule on the Affordable Care Act, a decision which threatens to dismantle the law, including provisions that require insurers to cover preexisting conditions and preventive services. With patients delaying screenings because of the COVID-19 pandemic and the increased incidence among minority and younger populations, it is imperative that preventative screening services – like colorectal cancer screenings – remain fully covered by payers. Moreover, because of the nature of GI diseases, patients often develop multiple conditions throughout their lifetime. The preexisting conditions protections in the ACA ensure that GI patients can gain the insurance coverage they need to obtain quality treatment.
Choice
Health plans and pharmacy benefit managers are using burdensome practices, such as step therapy, to limit patient access to drugs and biologics. These practices disrupt treatment and restrict individuals with digestive diseases from the medicines that work best for them.
Affordability
High out-of-pocket drug and biologics costs limit access to necessary therapies for people with digestive diseases, such as Crohn’s disease and ulcerative colitis. High out-of-pocket costs contribute to noncompliance, which in turn results in disease progression and complications and increases in overall health care costs.
Research funding
Sustainable long-term funding for federal research is critical to ensure the United States remains a leading contributor to innovative research breakthroughs. Under the current appropriations process in Congress, federal research funding can vary dramatically from year to year. Often enough, research funding for the next fiscal year is delayed by politics in Congress that result in continuing resolutions to fund the government and U.S. research institutions. Unstable funding causes a turbulent environment for investigators and is a deterrent for new investigators entering the field.
Member engagement
GIs need to engage in the policymaking process as there are too many threats and opportunities in today’s policy arena. The effectiveness of AGA’s advocacy work in the federal government is contingent upon members’ engagement in public policy. To increase physician advocacy and AGA member engagement, AGA offers the following avenues for members:
AGA political action committee
Political engagement is a powerful tool physician advocates can use to increase the visibility of GI on Capitol Hill. Political action committees (PACs) help provide access to lawmakers and their staff so that our advocates can educate them on the rationale for supporting our clinical and research priorities. Although PACs do not guarantee successes in Congress, it is important to note that contributions to legislators’ campaigns help them to be run more smoothly and effectively and allow the legislators to continue to serve their constituents. AGA PAC is a bipartisan political arm of AGA and is the only PAC dedicated to gastroenterology. Learn more at gastro.org/AGA-PAC.
Grassroots engagement
Build a relationship with your elected officials and their health policy staff by communicating with them often and offering to serve as a resource to the office on issues related to specialty medicine. AGA makes this easy with its online advocacy action center: gastro.quorum.us. Find out who your lawmakers are and research their background, engage them by email or Twitter on priority policy issues, and share stories with AGA staff about your interactions with congressional offices.
Congressional Advocates Program
This program creates a national grassroots network of engaged gastroenterologists interested in advocating for our profession and patients. Congressional Advocates are mentored and receive year-round advocacy training by AGA leadership and staff. Learn more at gastro.org/advocacy-and-policy/congressional-advocates-program.
Start advocating for gastroenterology
A new session of Congress has just begun, a new administration with a heavy health care agenda was elected into office, and gastroenterology needs your voice more than ever as we advocate for what really matters to us and our patients.
Join your colleagues at AGA’s spring virtual Advocacy Day on April 22, 2021. The event allows AGA members to meet with lawmakers and health policy staff virtually to educate them on the priority issues affecting our profession.
AGA staff makes it easy for you to participate. Webinar trainings, meeting schedules, and talking points will be provided to you ahead of time. For this event, we will speak to lawmakers about increasing federal research funding, addressing regulatory burdens like prior authorizations and step therapy protocols, and ensuring gastroenterologists and investigators have continued support during the COVID-19 pandemic.
For more information, visit gastro.org/aga-advocacy-day or contact AGA’s senior public policy coordinator, Jonathan Sollish, at [email protected].
Physician advocacy is an important tool for health care professionals to protect patients and the vitality of the profession. Medical associations across the spectrum participate in advocacy because of its value in preserving the beneficial role of physicians in health care policy decision making. This is especially true for specialty physician associations, like the American Gastroenterological Association, which represents more than 9,000 U.S. GI physicians and researchers. Advocacy allows for the voice of GIs and their patients to be heard on Capitol Hill, in the White House, and among various regulatory agencies. When we advocate as a profession, we help ensure good policies gain momentum and halt harmful legislative or regulatory efforts from enactment.
What is physician advocacy?
Physicians are advocating every day for their patients by helping patients make the right decisions about their care. This naturally translates into advocacy at the health policy level. Advocacy is lobbying. While that word may take on a negative meaning for some, it also means being a persuasive communicator, passionate educator, and a leader. National associations, like AGA, often call on members to do just that: educate lawmakers on policies affecting GI, communicate how policies could affect lawmakers’ constituencies back in their respective districts, and lead others to support GI policy agendas.
Physician advocacy works. AGA had its busiest year for policy work, but this was coupled with a large uptick in GI advocacy engagement. The public health emergency placed many burdens on the health care community and our profession. However, through our advocacy work, we also saw many changes, including increased federal research funding for digestive diseases and GI cancers, passage of legislation to remove patients’ barriers to colorectal cancer screening, increased regulatory and reimbursement flexibilities incorporated to ensure physicians could continue to deliver timely care, and creation of federal financial and small business relief programs to support gastroenterology practices.
Physician advocacy in GI is especially critical because specialty care is often viewed as having a smaller voice when compared with those of the larger bodies, such as primary care, surgery, or emergency physicians. As a health care specialty with a known shortage across the United States, we need all the help we can get to inform policy makers of our position on controversial policies. In many cases, non–health care professionals are informing policy makers on how to address issues that impact our profession. Additionally, there is a lack of knowledge about health care complexities and needs among decision makers who are ultimately determining how health care is delivered. As health care experts, we are best suited to educate lawmakers on the true impact of health policies. If we do not engage and educate policy makers, our profession and patients will suffer the consequences.
GI policy priorities for 2021
AGA will continue its advocacy work in 2021 on the following issues and encourage you and your colleagues to get involved:
Administrative burden relief
Utilization management protocols, like prior authorizations and step therapy, continue to increase and force physicians and their staff to spend hours of extra work time each week to process the paperwork. Prior authorizations are especially troublesome because they have increased for upper GI procedures and other common procedures. Step therapy protocols have also increased for IBD patients on biologics or other high-cost therapies, resulting in patients not receiving effective therapies as determined by their physician in a timely manner.
Patient access and protections
Coverage
Coverage for patients includes the following two areas:
COVID-19 relief: The public health emergency has weakened the health care workforce with physician practices and researchers facing financial instability and threatened patient access to specialty care. To support the health care community and to combat the pandemic, the following is necessary: Increased access to personal protective equipment and medical supplies for testing and vaccination distribution and increased rapid tests, testing sites, and health care workers. The public health emergency response also requires a stronger emphasis on health equity given the disproportionate impact it has had on communities of color.
- Preserving Affordable Care Act patient protections: The Supreme Court will rule on the Affordable Care Act, a decision which threatens to dismantle the law, including provisions that require insurers to cover preexisting conditions and preventive services. With patients delaying screenings because of the COVID-19 pandemic and the increased incidence among minority and younger populations, it is imperative that preventative screening services – like colorectal cancer screenings – remain fully covered by payers. Moreover, because of the nature of GI diseases, patients often develop multiple conditions throughout their lifetime. The preexisting conditions protections in the ACA ensure that GI patients can gain the insurance coverage they need to obtain quality treatment.
Choice
Health plans and pharmacy benefit managers are using burdensome practices, such as step therapy, to limit patient access to drugs and biologics. These practices disrupt treatment and restrict individuals with digestive diseases from the medicines that work best for them.
Affordability
High out-of-pocket drug and biologics costs limit access to necessary therapies for people with digestive diseases, such as Crohn’s disease and ulcerative colitis. High out-of-pocket costs contribute to noncompliance, which in turn results in disease progression and complications and increases in overall health care costs.
Research funding
Sustainable long-term funding for federal research is critical to ensure the United States remains a leading contributor to innovative research breakthroughs. Under the current appropriations process in Congress, federal research funding can vary dramatically from year to year. Often enough, research funding for the next fiscal year is delayed by politics in Congress that result in continuing resolutions to fund the government and U.S. research institutions. Unstable funding causes a turbulent environment for investigators and is a deterrent for new investigators entering the field.
Member engagement
GIs need to engage in the policymaking process as there are too many threats and opportunities in today’s policy arena. The effectiveness of AGA’s advocacy work in the federal government is contingent upon members’ engagement in public policy. To increase physician advocacy and AGA member engagement, AGA offers the following avenues for members:
AGA political action committee
Political engagement is a powerful tool physician advocates can use to increase the visibility of GI on Capitol Hill. Political action committees (PACs) help provide access to lawmakers and their staff so that our advocates can educate them on the rationale for supporting our clinical and research priorities. Although PACs do not guarantee successes in Congress, it is important to note that contributions to legislators’ campaigns help them to be run more smoothly and effectively and allow the legislators to continue to serve their constituents. AGA PAC is a bipartisan political arm of AGA and is the only PAC dedicated to gastroenterology. Learn more at gastro.org/AGA-PAC.
Grassroots engagement
Build a relationship with your elected officials and their health policy staff by communicating with them often and offering to serve as a resource to the office on issues related to specialty medicine. AGA makes this easy with its online advocacy action center: gastro.quorum.us. Find out who your lawmakers are and research their background, engage them by email or Twitter on priority policy issues, and share stories with AGA staff about your interactions with congressional offices.
Congressional Advocates Program
This program creates a national grassroots network of engaged gastroenterologists interested in advocating for our profession and patients. Congressional Advocates are mentored and receive year-round advocacy training by AGA leadership and staff. Learn more at gastro.org/advocacy-and-policy/congressional-advocates-program.
Start advocating for gastroenterology
A new session of Congress has just begun, a new administration with a heavy health care agenda was elected into office, and gastroenterology needs your voice more than ever as we advocate for what really matters to us and our patients.
Join your colleagues at AGA’s spring virtual Advocacy Day on April 22, 2021. The event allows AGA members to meet with lawmakers and health policy staff virtually to educate them on the priority issues affecting our profession.
AGA staff makes it easy for you to participate. Webinar trainings, meeting schedules, and talking points will be provided to you ahead of time. For this event, we will speak to lawmakers about increasing federal research funding, addressing regulatory burdens like prior authorizations and step therapy protocols, and ensuring gastroenterologists and investigators have continued support during the COVID-19 pandemic.
For more information, visit gastro.org/aga-advocacy-day or contact AGA’s senior public policy coordinator, Jonathan Sollish, at [email protected].
Physician advocacy is an important tool for health care professionals to protect patients and the vitality of the profession. Medical associations across the spectrum participate in advocacy because of its value in preserving the beneficial role of physicians in health care policy decision making. This is especially true for specialty physician associations, like the American Gastroenterological Association, which represents more than 9,000 U.S. GI physicians and researchers. Advocacy allows for the voice of GIs and their patients to be heard on Capitol Hill, in the White House, and among various regulatory agencies. When we advocate as a profession, we help ensure good policies gain momentum and halt harmful legislative or regulatory efforts from enactment.
What is physician advocacy?
Physicians are advocating every day for their patients by helping patients make the right decisions about their care. This naturally translates into advocacy at the health policy level. Advocacy is lobbying. While that word may take on a negative meaning for some, it also means being a persuasive communicator, passionate educator, and a leader. National associations, like AGA, often call on members to do just that: educate lawmakers on policies affecting GI, communicate how policies could affect lawmakers’ constituencies back in their respective districts, and lead others to support GI policy agendas.
Physician advocacy works. AGA had its busiest year for policy work, but this was coupled with a large uptick in GI advocacy engagement. The public health emergency placed many burdens on the health care community and our profession. However, through our advocacy work, we also saw many changes, including increased federal research funding for digestive diseases and GI cancers, passage of legislation to remove patients’ barriers to colorectal cancer screening, increased regulatory and reimbursement flexibilities incorporated to ensure physicians could continue to deliver timely care, and creation of federal financial and small business relief programs to support gastroenterology practices.
Physician advocacy in GI is especially critical because specialty care is often viewed as having a smaller voice when compared with those of the larger bodies, such as primary care, surgery, or emergency physicians. As a health care specialty with a known shortage across the United States, we need all the help we can get to inform policy makers of our position on controversial policies. In many cases, non–health care professionals are informing policy makers on how to address issues that impact our profession. Additionally, there is a lack of knowledge about health care complexities and needs among decision makers who are ultimately determining how health care is delivered. As health care experts, we are best suited to educate lawmakers on the true impact of health policies. If we do not engage and educate policy makers, our profession and patients will suffer the consequences.
GI policy priorities for 2021
AGA will continue its advocacy work in 2021 on the following issues and encourage you and your colleagues to get involved:
Administrative burden relief
Utilization management protocols, like prior authorizations and step therapy, continue to increase and force physicians and their staff to spend hours of extra work time each week to process the paperwork. Prior authorizations are especially troublesome because they have increased for upper GI procedures and other common procedures. Step therapy protocols have also increased for IBD patients on biologics or other high-cost therapies, resulting in patients not receiving effective therapies as determined by their physician in a timely manner.
Patient access and protections
Coverage
Coverage for patients includes the following two areas:
COVID-19 relief: The public health emergency has weakened the health care workforce with physician practices and researchers facing financial instability and threatened patient access to specialty care. To support the health care community and to combat the pandemic, the following is necessary: Increased access to personal protective equipment and medical supplies for testing and vaccination distribution and increased rapid tests, testing sites, and health care workers. The public health emergency response also requires a stronger emphasis on health equity given the disproportionate impact it has had on communities of color.
- Preserving Affordable Care Act patient protections: The Supreme Court will rule on the Affordable Care Act, a decision which threatens to dismantle the law, including provisions that require insurers to cover preexisting conditions and preventive services. With patients delaying screenings because of the COVID-19 pandemic and the increased incidence among minority and younger populations, it is imperative that preventative screening services – like colorectal cancer screenings – remain fully covered by payers. Moreover, because of the nature of GI diseases, patients often develop multiple conditions throughout their lifetime. The preexisting conditions protections in the ACA ensure that GI patients can gain the insurance coverage they need to obtain quality treatment.
Choice
Health plans and pharmacy benefit managers are using burdensome practices, such as step therapy, to limit patient access to drugs and biologics. These practices disrupt treatment and restrict individuals with digestive diseases from the medicines that work best for them.
Affordability
High out-of-pocket drug and biologics costs limit access to necessary therapies for people with digestive diseases, such as Crohn’s disease and ulcerative colitis. High out-of-pocket costs contribute to noncompliance, which in turn results in disease progression and complications and increases in overall health care costs.
Research funding
Sustainable long-term funding for federal research is critical to ensure the United States remains a leading contributor to innovative research breakthroughs. Under the current appropriations process in Congress, federal research funding can vary dramatically from year to year. Often enough, research funding for the next fiscal year is delayed by politics in Congress that result in continuing resolutions to fund the government and U.S. research institutions. Unstable funding causes a turbulent environment for investigators and is a deterrent for new investigators entering the field.
Member engagement
GIs need to engage in the policymaking process as there are too many threats and opportunities in today’s policy arena. The effectiveness of AGA’s advocacy work in the federal government is contingent upon members’ engagement in public policy. To increase physician advocacy and AGA member engagement, AGA offers the following avenues for members:
AGA political action committee
Political engagement is a powerful tool physician advocates can use to increase the visibility of GI on Capitol Hill. Political action committees (PACs) help provide access to lawmakers and their staff so that our advocates can educate them on the rationale for supporting our clinical and research priorities. Although PACs do not guarantee successes in Congress, it is important to note that contributions to legislators’ campaigns help them to be run more smoothly and effectively and allow the legislators to continue to serve their constituents. AGA PAC is a bipartisan political arm of AGA and is the only PAC dedicated to gastroenterology. Learn more at gastro.org/AGA-PAC.
Grassroots engagement
Build a relationship with your elected officials and their health policy staff by communicating with them often and offering to serve as a resource to the office on issues related to specialty medicine. AGA makes this easy with its online advocacy action center: gastro.quorum.us. Find out who your lawmakers are and research their background, engage them by email or Twitter on priority policy issues, and share stories with AGA staff about your interactions with congressional offices.
Congressional Advocates Program
This program creates a national grassroots network of engaged gastroenterologists interested in advocating for our profession and patients. Congressional Advocates are mentored and receive year-round advocacy training by AGA leadership and staff. Learn more at gastro.org/advocacy-and-policy/congressional-advocates-program.
Start advocating for gastroenterology
A new session of Congress has just begun, a new administration with a heavy health care agenda was elected into office, and gastroenterology needs your voice more than ever as we advocate for what really matters to us and our patients.
Join your colleagues at AGA’s spring virtual Advocacy Day on April 22, 2021. The event allows AGA members to meet with lawmakers and health policy staff virtually to educate them on the priority issues affecting our profession.
AGA staff makes it easy for you to participate. Webinar trainings, meeting schedules, and talking points will be provided to you ahead of time. For this event, we will speak to lawmakers about increasing federal research funding, addressing regulatory burdens like prior authorizations and step therapy protocols, and ensuring gastroenterologists and investigators have continued support during the COVID-19 pandemic.
For more information, visit gastro.org/aga-advocacy-day or contact AGA’s senior public policy coordinator, Jonathan Sollish, at [email protected].
PET predicts response to endocrine therapy in ER+ breast cancer
Endocrine therapy is the standard of care for estrogen receptor–positive (ER+) breast cancer, but only about half of women respond. At present, there is no method for identifying the women who are likely – and also unlikely – to respond.
But a new approach looks to be useful. It involves a trial of estrogen followed by imaging that measures the function of estrogen receptors in the cancer cells.
This functional testing of estrogen receptors on breast cancer cells was perfectly accurate in predicting endocrine therapy response in 43 postmenopausal women with advanced ER+ disease, say researchers from Washington University, St. Louis, led by Farrokh Dehdashti, MD.
“There is an unmet clinical need to develop more precise predictive biomarkers. The results of this study are extremely promising,” they conclude.
The study was published online in Nature Communications.
For the study, the women were first infused with a radioactive progestin analog – 21-[18F]fluorofuranylnorprogesterone (FFNP) – that binds progesterone receptors. About 40 minutes later, they had a PET scan to assess its uptake, an indication of progesterone-receptor abundance.
The women were then given three 200-mg doses of estradiol over 24 hours.
The FFNP infusion and PET scan were repeated the next day.
Estradiol will cause cancer cells with functional estrogen receptors to produce more progesterone receptors, so increased uptake of the radioactive analog indicates functional estrogen receptors that will respond to endocrine therapy. If estrogen receptors are not functional, and therefore not amenable to endocrine therapy (ET), estradiol will not upregulate progesterone receptors.
The results proved the theory. FFNP uptake increased more than 6.7% in 28 subjects and a median of 25.4%. All 28 women responded to subsequent ET, including 15 partial responses and 13 women with stable disease at 6 months.
Median survival was not reached after a median follow up of 27.1 months.
Uptake increased no more than 6.7% in 15 subjects and, in fact, fell a median of 0.7% from baseline. None of these women responded to ET. The median survival was 22.6 months.
“We observed 100% agreement between the response to estrogen challenge and the response to hormone therapy. … This method should work for any therapy that depends on a functional estrogen receptor, and it could provide valuable information to oncologists deciding how best to treat their patients,” Dr. Dehdashti said in a press release.
A larger multicenter confirmation trial is in the works.
Oncology needs “to get away from empiric therapies and make therapy more individualized” to save patients from the morbidity and expense of ineffective treatment and wasting time when other options are available, Dr. Dehdashti told this news organization.
“It would be a good thing if we could identify endocrine-resistant patients,” said Charles Shapiro, MD, a professor and director of translational breast cancer research at Mount Sinai Hospital, New York.
However, he wondered “about the exportability to less resource-intensive community settings where most oncology care occurs. This technology, assuming the results are confirmed in a larger study, [needs] a cost-effectiveness analysis” vs. the empiric approach, Dr. Shapiro said in an interview.
The women taking part in this study were a median of 60 years old, and most had metastatic disease. PET imaging extended from the base of the skull to the upper thighs, with data derived from bone, lung, breast, and other tumor sites. ET options included aromatase inhibitors, fulvestrant, and tamoxifen in combination with other agents.
Almost three-quarters of the women had prior systemic treatment, most often a hormone therapy–based regimen. Prior treatment had no effect on FFNP uptake.
There were no adverse events with the radiotracer, but the estradiol made a few women nauseous, among other transient discomforts, the team reported.
The work was funded by the National Cancer Institute and Washington University, St. Louis. Dr. Shapiro and Dr. Dehdashti have disclosed no relevant financial relationships. Several investigators reported consulting fees and/or other ties to a number of companies, including Pfizer, Merck, Avid Radiopharmaceutical, and Radius Health.
A version of this article first appeared on Medscape.com.
Endocrine therapy is the standard of care for estrogen receptor–positive (ER+) breast cancer, but only about half of women respond. At present, there is no method for identifying the women who are likely – and also unlikely – to respond.
But a new approach looks to be useful. It involves a trial of estrogen followed by imaging that measures the function of estrogen receptors in the cancer cells.
This functional testing of estrogen receptors on breast cancer cells was perfectly accurate in predicting endocrine therapy response in 43 postmenopausal women with advanced ER+ disease, say researchers from Washington University, St. Louis, led by Farrokh Dehdashti, MD.
“There is an unmet clinical need to develop more precise predictive biomarkers. The results of this study are extremely promising,” they conclude.
The study was published online in Nature Communications.
For the study, the women were first infused with a radioactive progestin analog – 21-[18F]fluorofuranylnorprogesterone (FFNP) – that binds progesterone receptors. About 40 minutes later, they had a PET scan to assess its uptake, an indication of progesterone-receptor abundance.
The women were then given three 200-mg doses of estradiol over 24 hours.
The FFNP infusion and PET scan were repeated the next day.
Estradiol will cause cancer cells with functional estrogen receptors to produce more progesterone receptors, so increased uptake of the radioactive analog indicates functional estrogen receptors that will respond to endocrine therapy. If estrogen receptors are not functional, and therefore not amenable to endocrine therapy (ET), estradiol will not upregulate progesterone receptors.
The results proved the theory. FFNP uptake increased more than 6.7% in 28 subjects and a median of 25.4%. All 28 women responded to subsequent ET, including 15 partial responses and 13 women with stable disease at 6 months.
Median survival was not reached after a median follow up of 27.1 months.
Uptake increased no more than 6.7% in 15 subjects and, in fact, fell a median of 0.7% from baseline. None of these women responded to ET. The median survival was 22.6 months.
“We observed 100% agreement between the response to estrogen challenge and the response to hormone therapy. … This method should work for any therapy that depends on a functional estrogen receptor, and it could provide valuable information to oncologists deciding how best to treat their patients,” Dr. Dehdashti said in a press release.
A larger multicenter confirmation trial is in the works.
Oncology needs “to get away from empiric therapies and make therapy more individualized” to save patients from the morbidity and expense of ineffective treatment and wasting time when other options are available, Dr. Dehdashti told this news organization.
“It would be a good thing if we could identify endocrine-resistant patients,” said Charles Shapiro, MD, a professor and director of translational breast cancer research at Mount Sinai Hospital, New York.
However, he wondered “about the exportability to less resource-intensive community settings where most oncology care occurs. This technology, assuming the results are confirmed in a larger study, [needs] a cost-effectiveness analysis” vs. the empiric approach, Dr. Shapiro said in an interview.
The women taking part in this study were a median of 60 years old, and most had metastatic disease. PET imaging extended from the base of the skull to the upper thighs, with data derived from bone, lung, breast, and other tumor sites. ET options included aromatase inhibitors, fulvestrant, and tamoxifen in combination with other agents.
Almost three-quarters of the women had prior systemic treatment, most often a hormone therapy–based regimen. Prior treatment had no effect on FFNP uptake.
There were no adverse events with the radiotracer, but the estradiol made a few women nauseous, among other transient discomforts, the team reported.
The work was funded by the National Cancer Institute and Washington University, St. Louis. Dr. Shapiro and Dr. Dehdashti have disclosed no relevant financial relationships. Several investigators reported consulting fees and/or other ties to a number of companies, including Pfizer, Merck, Avid Radiopharmaceutical, and Radius Health.
A version of this article first appeared on Medscape.com.
Endocrine therapy is the standard of care for estrogen receptor–positive (ER+) breast cancer, but only about half of women respond. At present, there is no method for identifying the women who are likely – and also unlikely – to respond.
But a new approach looks to be useful. It involves a trial of estrogen followed by imaging that measures the function of estrogen receptors in the cancer cells.
This functional testing of estrogen receptors on breast cancer cells was perfectly accurate in predicting endocrine therapy response in 43 postmenopausal women with advanced ER+ disease, say researchers from Washington University, St. Louis, led by Farrokh Dehdashti, MD.
“There is an unmet clinical need to develop more precise predictive biomarkers. The results of this study are extremely promising,” they conclude.
The study was published online in Nature Communications.
For the study, the women were first infused with a radioactive progestin analog – 21-[18F]fluorofuranylnorprogesterone (FFNP) – that binds progesterone receptors. About 40 minutes later, they had a PET scan to assess its uptake, an indication of progesterone-receptor abundance.
The women were then given three 200-mg doses of estradiol over 24 hours.
The FFNP infusion and PET scan were repeated the next day.
Estradiol will cause cancer cells with functional estrogen receptors to produce more progesterone receptors, so increased uptake of the radioactive analog indicates functional estrogen receptors that will respond to endocrine therapy. If estrogen receptors are not functional, and therefore not amenable to endocrine therapy (ET), estradiol will not upregulate progesterone receptors.
The results proved the theory. FFNP uptake increased more than 6.7% in 28 subjects and a median of 25.4%. All 28 women responded to subsequent ET, including 15 partial responses and 13 women with stable disease at 6 months.
Median survival was not reached after a median follow up of 27.1 months.
Uptake increased no more than 6.7% in 15 subjects and, in fact, fell a median of 0.7% from baseline. None of these women responded to ET. The median survival was 22.6 months.
“We observed 100% agreement between the response to estrogen challenge and the response to hormone therapy. … This method should work for any therapy that depends on a functional estrogen receptor, and it could provide valuable information to oncologists deciding how best to treat their patients,” Dr. Dehdashti said in a press release.
A larger multicenter confirmation trial is in the works.
Oncology needs “to get away from empiric therapies and make therapy more individualized” to save patients from the morbidity and expense of ineffective treatment and wasting time when other options are available, Dr. Dehdashti told this news organization.
“It would be a good thing if we could identify endocrine-resistant patients,” said Charles Shapiro, MD, a professor and director of translational breast cancer research at Mount Sinai Hospital, New York.
However, he wondered “about the exportability to less resource-intensive community settings where most oncology care occurs. This technology, assuming the results are confirmed in a larger study, [needs] a cost-effectiveness analysis” vs. the empiric approach, Dr. Shapiro said in an interview.
The women taking part in this study were a median of 60 years old, and most had metastatic disease. PET imaging extended from the base of the skull to the upper thighs, with data derived from bone, lung, breast, and other tumor sites. ET options included aromatase inhibitors, fulvestrant, and tamoxifen in combination with other agents.
Almost three-quarters of the women had prior systemic treatment, most often a hormone therapy–based regimen. Prior treatment had no effect on FFNP uptake.
There were no adverse events with the radiotracer, but the estradiol made a few women nauseous, among other transient discomforts, the team reported.
The work was funded by the National Cancer Institute and Washington University, St. Louis. Dr. Shapiro and Dr. Dehdashti have disclosed no relevant financial relationships. Several investigators reported consulting fees and/or other ties to a number of companies, including Pfizer, Merck, Avid Radiopharmaceutical, and Radius Health.
A version of this article first appeared on Medscape.com.
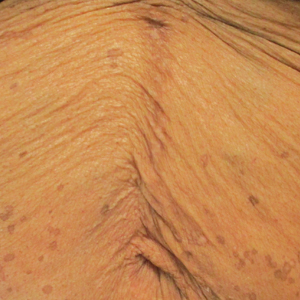
Eruptive Annular Papules on the Trunk of an Organ Transplant Recipient
The Diagnosis: Epidermodysplasia Verruciformis
Histopathologic examination of our patient's biopsy specimen revealed mild acanthosis with prominent hypergranulosis and enlarged keratinocytes with blue-gray cytoplasm (Figure). A diagnosis of acquired epidermodysplasia verruciformis (EV) was rendered. The patient was treated with photodynamic therapy utilizing 5-aminolevulinic acid.
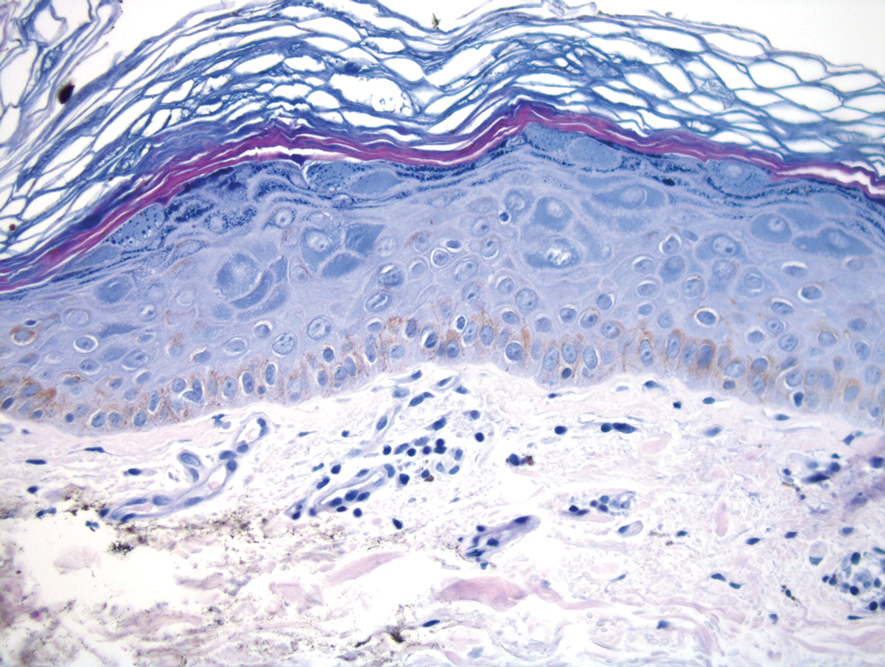
Epidermodysplasia verruciformis is characterized by susceptibility to human papillomavirus (HPV) infections via a defect in cellular immunity. Epidermodysplasia verruciformis was first described as an autosomal-recessive genodermatosis, but it can be acquired in immunosuppressed states with an atypical clinical appearance.1 There are few case reports in skin of color. Acquired EV appears in patients with acquired immunodeficiencies that are susceptible to EV-causing HPVs via a similar mechanism found in inherited EV.2 The most common HPV serotypes involved in EV are HPV-5 and HPV-8. The duration of immunosuppression has been found to be positively correlated with the risk for EV development, with the majority of patients developing lesions after 5 years of immunosuppression.3 There is an approximately 60% risk of malignant transformation of EV lesions into nonmelanoma skin cancer.2 This risk is believed to be lower in patients with darker skin.4
Preventative measures including sun protection and annual surveillance are crucial in EV patients given the high rate of malignant transformation in sun-exposed lesions.5 Treatment options for EV are anecdotal and have variable results, ranging from topicals including 5-fluorouracil and imiquimod to systemic medications including acitretin and interferon.3 Photodynamic therapy can be used for extensive EV. Surgical modalities and other destructive methods also have been tried.6
Epidermodysplasia verruciformis often can be confused with similar dermatoses. Porokeratosis appears as annular pink papules with waferlike peripheral scales. Tinea versicolor is a dermatophyte infection caused by Malassezia furfur and presents as multiple dyspigmented, finely scaling, thin papules and plaques. Subacute cutaneous lupus erythematosus presents as pink, scaly, annular or psoriasiform papules and plaques most commonly on the trunk. Discoid lupus erythematosus presents as pink, hypopigmented or depigmented, atrophic plaques with a peripheral rim of erythema that indicates activity. Secondary syphilis, commonly denoted as the "great mimicker," presents as psoriasiform papules and plaques among other variable morphologies.
- Sa NB, Guerini MB, Barbato MT, et al. Epidermodysplasia verruciformis: clinical presentation with varied forms of lesions. An Bras Dermatol. 2011;86(4 suppl 1):S57-S60.
- Rogers HD, Macgregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60:315-320.
- Henley JK, Hossler EW. Acquired epidermodysplasia verruciformis occurring in a renal transplant recipient. Cutis. 2017;99:E9-E12.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Fox SH, Elston DM. Epidermodysplasia verruciformis and the risk for malignancy. Cutis. 2016;98:E10-E12.
- Shruti S, Siraj F, Singh A, et al. Epidermodysplasia verruciformis: three case reports and a brief review. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:59-61.
The Diagnosis: Epidermodysplasia Verruciformis
Histopathologic examination of our patient's biopsy specimen revealed mild acanthosis with prominent hypergranulosis and enlarged keratinocytes with blue-gray cytoplasm (Figure). A diagnosis of acquired epidermodysplasia verruciformis (EV) was rendered. The patient was treated with photodynamic therapy utilizing 5-aminolevulinic acid.

Epidermodysplasia verruciformis is characterized by susceptibility to human papillomavirus (HPV) infections via a defect in cellular immunity. Epidermodysplasia verruciformis was first described as an autosomal-recessive genodermatosis, but it can be acquired in immunosuppressed states with an atypical clinical appearance.1 There are few case reports in skin of color. Acquired EV appears in patients with acquired immunodeficiencies that are susceptible to EV-causing HPVs via a similar mechanism found in inherited EV.2 The most common HPV serotypes involved in EV are HPV-5 and HPV-8. The duration of immunosuppression has been found to be positively correlated with the risk for EV development, with the majority of patients developing lesions after 5 years of immunosuppression.3 There is an approximately 60% risk of malignant transformation of EV lesions into nonmelanoma skin cancer.2 This risk is believed to be lower in patients with darker skin.4
Preventative measures including sun protection and annual surveillance are crucial in EV patients given the high rate of malignant transformation in sun-exposed lesions.5 Treatment options for EV are anecdotal and have variable results, ranging from topicals including 5-fluorouracil and imiquimod to systemic medications including acitretin and interferon.3 Photodynamic therapy can be used for extensive EV. Surgical modalities and other destructive methods also have been tried.6
Epidermodysplasia verruciformis often can be confused with similar dermatoses. Porokeratosis appears as annular pink papules with waferlike peripheral scales. Tinea versicolor is a dermatophyte infection caused by Malassezia furfur and presents as multiple dyspigmented, finely scaling, thin papules and plaques. Subacute cutaneous lupus erythematosus presents as pink, scaly, annular or psoriasiform papules and plaques most commonly on the trunk. Discoid lupus erythematosus presents as pink, hypopigmented or depigmented, atrophic plaques with a peripheral rim of erythema that indicates activity. Secondary syphilis, commonly denoted as the "great mimicker," presents as psoriasiform papules and plaques among other variable morphologies.
The Diagnosis: Epidermodysplasia Verruciformis
Histopathologic examination of our patient's biopsy specimen revealed mild acanthosis with prominent hypergranulosis and enlarged keratinocytes with blue-gray cytoplasm (Figure). A diagnosis of acquired epidermodysplasia verruciformis (EV) was rendered. The patient was treated with photodynamic therapy utilizing 5-aminolevulinic acid.

Epidermodysplasia verruciformis is characterized by susceptibility to human papillomavirus (HPV) infections via a defect in cellular immunity. Epidermodysplasia verruciformis was first described as an autosomal-recessive genodermatosis, but it can be acquired in immunosuppressed states with an atypical clinical appearance.1 There are few case reports in skin of color. Acquired EV appears in patients with acquired immunodeficiencies that are susceptible to EV-causing HPVs via a similar mechanism found in inherited EV.2 The most common HPV serotypes involved in EV are HPV-5 and HPV-8. The duration of immunosuppression has been found to be positively correlated with the risk for EV development, with the majority of patients developing lesions after 5 years of immunosuppression.3 There is an approximately 60% risk of malignant transformation of EV lesions into nonmelanoma skin cancer.2 This risk is believed to be lower in patients with darker skin.4
Preventative measures including sun protection and annual surveillance are crucial in EV patients given the high rate of malignant transformation in sun-exposed lesions.5 Treatment options for EV are anecdotal and have variable results, ranging from topicals including 5-fluorouracil and imiquimod to systemic medications including acitretin and interferon.3 Photodynamic therapy can be used for extensive EV. Surgical modalities and other destructive methods also have been tried.6
Epidermodysplasia verruciformis often can be confused with similar dermatoses. Porokeratosis appears as annular pink papules with waferlike peripheral scales. Tinea versicolor is a dermatophyte infection caused by Malassezia furfur and presents as multiple dyspigmented, finely scaling, thin papules and plaques. Subacute cutaneous lupus erythematosus presents as pink, scaly, annular or psoriasiform papules and plaques most commonly on the trunk. Discoid lupus erythematosus presents as pink, hypopigmented or depigmented, atrophic plaques with a peripheral rim of erythema that indicates activity. Secondary syphilis, commonly denoted as the "great mimicker," presents as psoriasiform papules and plaques among other variable morphologies.
- Sa NB, Guerini MB, Barbato MT, et al. Epidermodysplasia verruciformis: clinical presentation with varied forms of lesions. An Bras Dermatol. 2011;86(4 suppl 1):S57-S60.
- Rogers HD, Macgregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60:315-320.
- Henley JK, Hossler EW. Acquired epidermodysplasia verruciformis occurring in a renal transplant recipient. Cutis. 2017;99:E9-E12.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Fox SH, Elston DM. Epidermodysplasia verruciformis and the risk for malignancy. Cutis. 2016;98:E10-E12.
- Shruti S, Siraj F, Singh A, et al. Epidermodysplasia verruciformis: three case reports and a brief review. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:59-61.
- Sa NB, Guerini MB, Barbato MT, et al. Epidermodysplasia verruciformis: clinical presentation with varied forms of lesions. An Bras Dermatol. 2011;86(4 suppl 1):S57-S60.
- Rogers HD, Macgregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60:315-320.
- Henley JK, Hossler EW. Acquired epidermodysplasia verruciformis occurring in a renal transplant recipient. Cutis. 2017;99:E9-E12.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Fox SH, Elston DM. Epidermodysplasia verruciformis and the risk for malignancy. Cutis. 2016;98:E10-E12.
- Shruti S, Siraj F, Singh A, et al. Epidermodysplasia verruciformis: three case reports and a brief review. Acta Dermatovenerol Alp Pannonica Adriat. 2017;26:59-61.
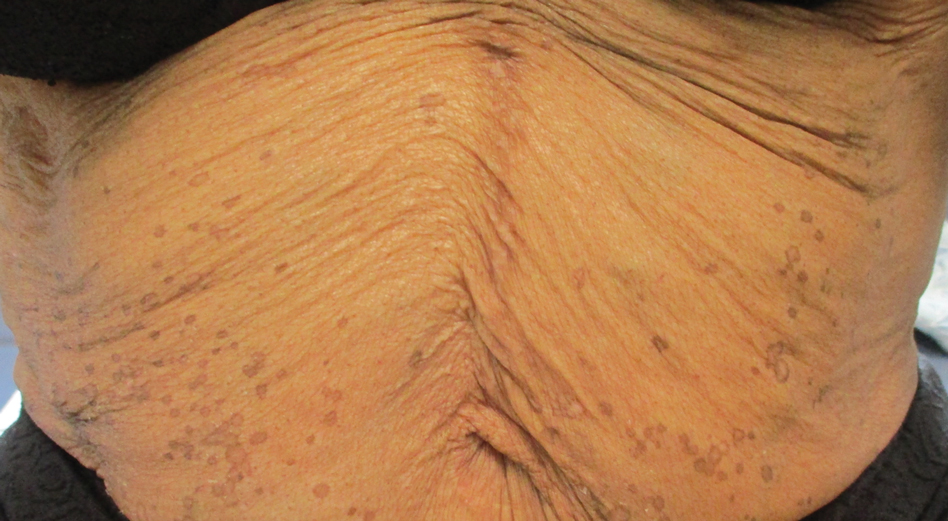
A 50-year-old Black woman with systemic lupus erythematosus and a renal transplant 15 years prior due to lupus nephritis presented with a nonpruritic rash on the abdomen of 1 year’s duration. Her immunosuppressive regimen consisted of tacrolimus, azathioprine, and prednisone. Physical examination revealed numerous monomorphic, annular, hyperpigmented, and thin papules with central clearing present on the abdomen extending to the flanks and groin. The patient denied any family history of similar lesions. A 4-mm punch biopsy of an abdominal lesion was performed.
ASDSA warns of rogue insulin pen use for DIY fillers
.
In the safety warning, issued on Feb. 18, the ASDSA reported that ASDSA members, all board-certified dermatologists, have seen evidence online of young people using so-called “hyaluron pens” to inject hyaluronic acid filler in the epidermal and upper dermal skin.
The pens being used and promoted in social media for do-it-yourself filler injections are medical devices originally developed for insulin injections. “The use of air pressure technology causes these pens to deliver the hyaluronic acid to insert nanoscale molecules of the filler through the skin,” according to the ASDSA statement. Marketing materials state that the pens can be used to create volume and shape in the lips, and to improve the appearance of nasolabial lines, marionette lines, brow lines known as “elevens,” and forehead wrinkles. Claims that the hyaluronic acid only reaches the papillary layer of the dermis, and is therefore safe, do not alleviate the risk of injury in inexperienced hands, the ASDSA statement points out.
“We are concerned about California children falling prey to products that are not appropriate and safe for them to use,” Elan Newland, MD, member of the ASDSA and the California Society for Dermatology and Dermatological Surgery (CalDerm), said in the statement. “The power of social media is very strong, especially for impressionable teenagers. CalDerm supports alerting consumers and regulators of the dangers of these pens,” he said.
“TikTok is proving to be an extremely powerful platform to communicate, entertain, and even educate, which is why many physicians are getting involved and finding success there. Unfortunately, just like the World Wide Web, there is misinformation there and even dangerous lies,” Sandra Lee, MD, who practices in Upland, Calif. (and is also known as “Dr. Pimple Popper”), said in the statement.
“It’s very concerning to see young people posting a How To on injecting their own lips with hyaluronic acid serum using an ‘airgun’ pen, which acts much like a BB gun to push with force the product under the skin,” she added. “So many things can go wrong.”
The ASDSA has contacted the Food and Drug Administration to report these safety concerns. “In addition, the ASDSA is alerting state medical and estheticians’ boards regarding these patient safety concerns and alerting consumers directly about the risks through social media and other education materials,” according to the statement.
.
In the safety warning, issued on Feb. 18, the ASDSA reported that ASDSA members, all board-certified dermatologists, have seen evidence online of young people using so-called “hyaluron pens” to inject hyaluronic acid filler in the epidermal and upper dermal skin.
The pens being used and promoted in social media for do-it-yourself filler injections are medical devices originally developed for insulin injections. “The use of air pressure technology causes these pens to deliver the hyaluronic acid to insert nanoscale molecules of the filler through the skin,” according to the ASDSA statement. Marketing materials state that the pens can be used to create volume and shape in the lips, and to improve the appearance of nasolabial lines, marionette lines, brow lines known as “elevens,” and forehead wrinkles. Claims that the hyaluronic acid only reaches the papillary layer of the dermis, and is therefore safe, do not alleviate the risk of injury in inexperienced hands, the ASDSA statement points out.
“We are concerned about California children falling prey to products that are not appropriate and safe for them to use,” Elan Newland, MD, member of the ASDSA and the California Society for Dermatology and Dermatological Surgery (CalDerm), said in the statement. “The power of social media is very strong, especially for impressionable teenagers. CalDerm supports alerting consumers and regulators of the dangers of these pens,” he said.
“TikTok is proving to be an extremely powerful platform to communicate, entertain, and even educate, which is why many physicians are getting involved and finding success there. Unfortunately, just like the World Wide Web, there is misinformation there and even dangerous lies,” Sandra Lee, MD, who practices in Upland, Calif. (and is also known as “Dr. Pimple Popper”), said in the statement.
“It’s very concerning to see young people posting a How To on injecting their own lips with hyaluronic acid serum using an ‘airgun’ pen, which acts much like a BB gun to push with force the product under the skin,” she added. “So many things can go wrong.”
The ASDSA has contacted the Food and Drug Administration to report these safety concerns. “In addition, the ASDSA is alerting state medical and estheticians’ boards regarding these patient safety concerns and alerting consumers directly about the risks through social media and other education materials,” according to the statement.
.
In the safety warning, issued on Feb. 18, the ASDSA reported that ASDSA members, all board-certified dermatologists, have seen evidence online of young people using so-called “hyaluron pens” to inject hyaluronic acid filler in the epidermal and upper dermal skin.
The pens being used and promoted in social media for do-it-yourself filler injections are medical devices originally developed for insulin injections. “The use of air pressure technology causes these pens to deliver the hyaluronic acid to insert nanoscale molecules of the filler through the skin,” according to the ASDSA statement. Marketing materials state that the pens can be used to create volume and shape in the lips, and to improve the appearance of nasolabial lines, marionette lines, brow lines known as “elevens,” and forehead wrinkles. Claims that the hyaluronic acid only reaches the papillary layer of the dermis, and is therefore safe, do not alleviate the risk of injury in inexperienced hands, the ASDSA statement points out.
“We are concerned about California children falling prey to products that are not appropriate and safe for them to use,” Elan Newland, MD, member of the ASDSA and the California Society for Dermatology and Dermatological Surgery (CalDerm), said in the statement. “The power of social media is very strong, especially for impressionable teenagers. CalDerm supports alerting consumers and regulators of the dangers of these pens,” he said.
“TikTok is proving to be an extremely powerful platform to communicate, entertain, and even educate, which is why many physicians are getting involved and finding success there. Unfortunately, just like the World Wide Web, there is misinformation there and even dangerous lies,” Sandra Lee, MD, who practices in Upland, Calif. (and is also known as “Dr. Pimple Popper”), said in the statement.
“It’s very concerning to see young people posting a How To on injecting their own lips with hyaluronic acid serum using an ‘airgun’ pen, which acts much like a BB gun to push with force the product under the skin,” she added. “So many things can go wrong.”
The ASDSA has contacted the Food and Drug Administration to report these safety concerns. “In addition, the ASDSA is alerting state medical and estheticians’ boards regarding these patient safety concerns and alerting consumers directly about the risks through social media and other education materials,” according to the statement.
Steroid complications in GVHD common, boost costs of care
Steroids are usually the first choice of therapy for the treatment of patients with graft-vs.-host disease (GVHD), but complications from steroid use may carry a high financial cost, investigators caution.
Among 689 patients with a diagnosis of GVHD following a hematopoietic stem cell transplant (HSCT) who received steroids, 685 (97%) had at least one steroid-related complication, resulting in nearly $165,000 in mean health-care costs over 24 months, said Elizabeth J. Bell, PhD, MPH, an epidemiologist at Optum Inc.
“For both acute and chronic GVHD, the standard of care for first-line treatment is systemic steroids. The complications associated with steroid treatment are well known. However, the health-care resources utilized and the costs incurred by these patients are not well-quantified,” she said at the Transplantation & Cellular Therapies Meetings (Abstract 12).
Dr. Bell reported the results of a retrospective database analysis on costs associated with steroid complications in HSCT recipients at the meeting, which was held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
She and colleagues from Optum, Incyte, and the University of Minnesota in Minneapolis looked at data on 689 patients with a diagnosis of GVHD after HSCT who received systemic steroids from July 1, 2010, through Aug. 31, 2019. The data were extracted from the Optum Research database, and included U.S. commercial and Medicare Advantage patients.
They looked at total complications and steroid-associated complications in each of four categories: infections; metabolic or endocrine complications (for example, diabetes, dyslipidemia); gastrointestinal (GI) complications (e.g., peptic ulcer disease); and bone or muscle complications (myopathy, etc).
They estimated costs based on International Classification of Diseases (ICD) codes for any steroid complications during the 24 months after steroid initiation, including those complications that may have been present at the time of GVHD diagnosis.
The median patient age was 55 years, and 60% of the sample were male. The mean Charlson Comorbidity Index score at baseline was 3.
Overall, 22% of patients had only acute GVHD, 21% had only chronic GVHD, and 39% had both acute and chronic disease. The GVHD type was unspecified in the remaining 18%.
The median time from GVHD diagnosis to initiating steroids was 30 days for patients with both acute and chronic disease, as well as those with both presentations. The median time to initiation was 36 days for patients with unspecified GVHD type.
The median cumulative duration of steroid use over 24 months was 62 days for patients with acute GVHD, 208 days for those with chronic GVHD, 166 days for those with both, and 74 days for patients with unspecified GVHD type.
As noted before, complications occurred in 97% of patients, with infections being the most common complications, occurring in 80% of patients, followed by metabolic/endocrine complications in 32%, gastrointestinal in 29%, and bone/muscle complications in 20%.
For the 665 patients who had any steroid-related complication, the mean costs of steroid-associated care in the 24 months after they were started on steroids was $164,787, and the median cost was $50,834.
Health care costs were highest among patients with infections, at a mean of $167,473, and a median of $57,680, followed by bone/muscle conditions ($75,289 and $2,057, respectively), GI conditions ($67,861 and $3,360), and metabolic or endocrine conditions ($47, 101 and $1,164).
In all categories, hospitalizations accounted for the large majority of costs.
Two-thirds (66%) of patients who experienced any steroid-related complication required hospitalization, primarily for infections.
Among all patients with complications, the median cumulative hospital stay over 24 months was 20 days, with bone/muscle complications and infections associated with a median of 19 and 18 days of hospitalization, respectively.
Dr. Bell acknowledged that the study was limited by use of ICD coding to identify steroid complication-related health-care utilization and costs, which can be imprecise, and by the fact that the analysis included only complications resulting in health care use as documented in medical claims. In addition, the investigators noted that they could not control for the possibility that steroids exacerbated conditions that existed at baseline.
“These findings emphasize the need to cautiously evaluate the treatment options for patients with GVHD. Future study with medical records is needed to provide insights on the clinical aspects of the complications (e.g., severity and suspected causality),” Dr. Bell and colleagues concluded in the study’s abstract.
Definitions questioned
An HSCT specialist approached for comment said that the findings of the study made sense, but she had questions regarding the study methodology.
“I would intuitively think that steroid-associated complications are a major cause of health care use in GVHD patients and it’s interesting to see that there is emerging data to support this hypothesis,” HSCT specialist Hélène Schoemans, MD of the University of Leuven, Belgium, said in an interview.
She noted, however, that “it is surprising that the period of steroid initiation was the same for acute and chronic GVHD,” and questioned whether that anomalous finding could be due to the study’s definition of acute and chronic GVHD or to how the period from baseline to steroid initiation was defined.
The questions about the definitions and timing of therapy make it uncertain as to whether the complications reported were caused by steroids or by some other factor, she suggested.
The study was supported by Optum Inc. Dr. Bell is an employee of the company, and a paid consultant of Incyte. Dr. Schoemans has received travel expenses from Celgene, Abbvie, and Incyte; is part of the advisory boards for Incyte; and has received speakers fees from Novartis, Incyte, Jazz Pharmaceuticals, and Takeda.
Steroids are usually the first choice of therapy for the treatment of patients with graft-vs.-host disease (GVHD), but complications from steroid use may carry a high financial cost, investigators caution.
Among 689 patients with a diagnosis of GVHD following a hematopoietic stem cell transplant (HSCT) who received steroids, 685 (97%) had at least one steroid-related complication, resulting in nearly $165,000 in mean health-care costs over 24 months, said Elizabeth J. Bell, PhD, MPH, an epidemiologist at Optum Inc.
“For both acute and chronic GVHD, the standard of care for first-line treatment is systemic steroids. The complications associated with steroid treatment are well known. However, the health-care resources utilized and the costs incurred by these patients are not well-quantified,” she said at the Transplantation & Cellular Therapies Meetings (Abstract 12).
Dr. Bell reported the results of a retrospective database analysis on costs associated with steroid complications in HSCT recipients at the meeting, which was held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
She and colleagues from Optum, Incyte, and the University of Minnesota in Minneapolis looked at data on 689 patients with a diagnosis of GVHD after HSCT who received systemic steroids from July 1, 2010, through Aug. 31, 2019. The data were extracted from the Optum Research database, and included U.S. commercial and Medicare Advantage patients.
They looked at total complications and steroid-associated complications in each of four categories: infections; metabolic or endocrine complications (for example, diabetes, dyslipidemia); gastrointestinal (GI) complications (e.g., peptic ulcer disease); and bone or muscle complications (myopathy, etc).
They estimated costs based on International Classification of Diseases (ICD) codes for any steroid complications during the 24 months after steroid initiation, including those complications that may have been present at the time of GVHD diagnosis.
The median patient age was 55 years, and 60% of the sample were male. The mean Charlson Comorbidity Index score at baseline was 3.
Overall, 22% of patients had only acute GVHD, 21% had only chronic GVHD, and 39% had both acute and chronic disease. The GVHD type was unspecified in the remaining 18%.
The median time from GVHD diagnosis to initiating steroids was 30 days for patients with both acute and chronic disease, as well as those with both presentations. The median time to initiation was 36 days for patients with unspecified GVHD type.
The median cumulative duration of steroid use over 24 months was 62 days for patients with acute GVHD, 208 days for those with chronic GVHD, 166 days for those with both, and 74 days for patients with unspecified GVHD type.
As noted before, complications occurred in 97% of patients, with infections being the most common complications, occurring in 80% of patients, followed by metabolic/endocrine complications in 32%, gastrointestinal in 29%, and bone/muscle complications in 20%.
For the 665 patients who had any steroid-related complication, the mean costs of steroid-associated care in the 24 months after they were started on steroids was $164,787, and the median cost was $50,834.
Health care costs were highest among patients with infections, at a mean of $167,473, and a median of $57,680, followed by bone/muscle conditions ($75,289 and $2,057, respectively), GI conditions ($67,861 and $3,360), and metabolic or endocrine conditions ($47, 101 and $1,164).
In all categories, hospitalizations accounted for the large majority of costs.
Two-thirds (66%) of patients who experienced any steroid-related complication required hospitalization, primarily for infections.
Among all patients with complications, the median cumulative hospital stay over 24 months was 20 days, with bone/muscle complications and infections associated with a median of 19 and 18 days of hospitalization, respectively.
Dr. Bell acknowledged that the study was limited by use of ICD coding to identify steroid complication-related health-care utilization and costs, which can be imprecise, and by the fact that the analysis included only complications resulting in health care use as documented in medical claims. In addition, the investigators noted that they could not control for the possibility that steroids exacerbated conditions that existed at baseline.
“These findings emphasize the need to cautiously evaluate the treatment options for patients with GVHD. Future study with medical records is needed to provide insights on the clinical aspects of the complications (e.g., severity and suspected causality),” Dr. Bell and colleagues concluded in the study’s abstract.
Definitions questioned
An HSCT specialist approached for comment said that the findings of the study made sense, but she had questions regarding the study methodology.
“I would intuitively think that steroid-associated complications are a major cause of health care use in GVHD patients and it’s interesting to see that there is emerging data to support this hypothesis,” HSCT specialist Hélène Schoemans, MD of the University of Leuven, Belgium, said in an interview.
She noted, however, that “it is surprising that the period of steroid initiation was the same for acute and chronic GVHD,” and questioned whether that anomalous finding could be due to the study’s definition of acute and chronic GVHD or to how the period from baseline to steroid initiation was defined.
The questions about the definitions and timing of therapy make it uncertain as to whether the complications reported were caused by steroids or by some other factor, she suggested.
The study was supported by Optum Inc. Dr. Bell is an employee of the company, and a paid consultant of Incyte. Dr. Schoemans has received travel expenses from Celgene, Abbvie, and Incyte; is part of the advisory boards for Incyte; and has received speakers fees from Novartis, Incyte, Jazz Pharmaceuticals, and Takeda.
Steroids are usually the first choice of therapy for the treatment of patients with graft-vs.-host disease (GVHD), but complications from steroid use may carry a high financial cost, investigators caution.
Among 689 patients with a diagnosis of GVHD following a hematopoietic stem cell transplant (HSCT) who received steroids, 685 (97%) had at least one steroid-related complication, resulting in nearly $165,000 in mean health-care costs over 24 months, said Elizabeth J. Bell, PhD, MPH, an epidemiologist at Optum Inc.
“For both acute and chronic GVHD, the standard of care for first-line treatment is systemic steroids. The complications associated with steroid treatment are well known. However, the health-care resources utilized and the costs incurred by these patients are not well-quantified,” she said at the Transplantation & Cellular Therapies Meetings (Abstract 12).
Dr. Bell reported the results of a retrospective database analysis on costs associated with steroid complications in HSCT recipients at the meeting, which was held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
She and colleagues from Optum, Incyte, and the University of Minnesota in Minneapolis looked at data on 689 patients with a diagnosis of GVHD after HSCT who received systemic steroids from July 1, 2010, through Aug. 31, 2019. The data were extracted from the Optum Research database, and included U.S. commercial and Medicare Advantage patients.
They looked at total complications and steroid-associated complications in each of four categories: infections; metabolic or endocrine complications (for example, diabetes, dyslipidemia); gastrointestinal (GI) complications (e.g., peptic ulcer disease); and bone or muscle complications (myopathy, etc).
They estimated costs based on International Classification of Diseases (ICD) codes for any steroid complications during the 24 months after steroid initiation, including those complications that may have been present at the time of GVHD diagnosis.
The median patient age was 55 years, and 60% of the sample were male. The mean Charlson Comorbidity Index score at baseline was 3.
Overall, 22% of patients had only acute GVHD, 21% had only chronic GVHD, and 39% had both acute and chronic disease. The GVHD type was unspecified in the remaining 18%.
The median time from GVHD diagnosis to initiating steroids was 30 days for patients with both acute and chronic disease, as well as those with both presentations. The median time to initiation was 36 days for patients with unspecified GVHD type.
The median cumulative duration of steroid use over 24 months was 62 days for patients with acute GVHD, 208 days for those with chronic GVHD, 166 days for those with both, and 74 days for patients with unspecified GVHD type.
As noted before, complications occurred in 97% of patients, with infections being the most common complications, occurring in 80% of patients, followed by metabolic/endocrine complications in 32%, gastrointestinal in 29%, and bone/muscle complications in 20%.
For the 665 patients who had any steroid-related complication, the mean costs of steroid-associated care in the 24 months after they were started on steroids was $164,787, and the median cost was $50,834.
Health care costs were highest among patients with infections, at a mean of $167,473, and a median of $57,680, followed by bone/muscle conditions ($75,289 and $2,057, respectively), GI conditions ($67,861 and $3,360), and metabolic or endocrine conditions ($47, 101 and $1,164).
In all categories, hospitalizations accounted for the large majority of costs.
Two-thirds (66%) of patients who experienced any steroid-related complication required hospitalization, primarily for infections.
Among all patients with complications, the median cumulative hospital stay over 24 months was 20 days, with bone/muscle complications and infections associated with a median of 19 and 18 days of hospitalization, respectively.
Dr. Bell acknowledged that the study was limited by use of ICD coding to identify steroid complication-related health-care utilization and costs, which can be imprecise, and by the fact that the analysis included only complications resulting in health care use as documented in medical claims. In addition, the investigators noted that they could not control for the possibility that steroids exacerbated conditions that existed at baseline.
“These findings emphasize the need to cautiously evaluate the treatment options for patients with GVHD. Future study with medical records is needed to provide insights on the clinical aspects of the complications (e.g., severity and suspected causality),” Dr. Bell and colleagues concluded in the study’s abstract.
Definitions questioned
An HSCT specialist approached for comment said that the findings of the study made sense, but she had questions regarding the study methodology.
“I would intuitively think that steroid-associated complications are a major cause of health care use in GVHD patients and it’s interesting to see that there is emerging data to support this hypothesis,” HSCT specialist Hélène Schoemans, MD of the University of Leuven, Belgium, said in an interview.
She noted, however, that “it is surprising that the period of steroid initiation was the same for acute and chronic GVHD,” and questioned whether that anomalous finding could be due to the study’s definition of acute and chronic GVHD or to how the period from baseline to steroid initiation was defined.
The questions about the definitions and timing of therapy make it uncertain as to whether the complications reported were caused by steroids or by some other factor, she suggested.
The study was supported by Optum Inc. Dr. Bell is an employee of the company, and a paid consultant of Incyte. Dr. Schoemans has received travel expenses from Celgene, Abbvie, and Incyte; is part of the advisory boards for Incyte; and has received speakers fees from Novartis, Incyte, Jazz Pharmaceuticals, and Takeda.
FROM TCT 2021
Ivabradine knocks down heart rate, symptoms in POTS
The heart failure drug ivabradine (Corlanor) can provide relief from the elevated heart rate and often debilitating symptoms associated with postural orthostatic tachycardia syndrome (POTS), a new study suggests.
Ivabradine significantly lowered standing heart rate, compared with placebo (77.9 vs. 94.2 beats/min; P < .001). The typical surge in heart rate that occurs upon standing in these patients was also blunted, compared with baseline (13.0 vs. 21.4 beats/min; P = .001).
“There are really not a lot of great options for patients with POTS and, mechanistically, ivabradine just make sense because it’s a drug that lowers heart rate very selectively and doesn’t lower blood pressure,” lead study author Pam R. Taub, MD, told this news organization.
Surprisingly, the reduction in heart rate translated into improved physical (P = .008) and social (P = .021) functioning after just 1 month of ivabradine, without any other background POTS medications or a change in nonpharmacologic therapies, she said. “What’s really nice to see is when you tackle a really significant part of the disease, which is the elevated heart rate, just how much better they feel.”
POTS patients are mostly healthy, active young women, who after some inciting event – such as viral infection, trauma, or surgery – experience an increase in heart rate of at least 30 beats/min upon standing accompanied by a range of symptoms, including dizziness, palpitations, brain fog, and fatigue.
A COVID connection?
The study enrolled patients with hyperadrenergic POTS as the predominant subtype, but another group to keep in mind that might benefit is the post-COVID POTS patient, said Dr. Taub, from the University of California, San Diego.
“We’re seeing an incredible number of patients post COVID that meet the criteria for POTS, and a lot of these patients also have COVID fatigue,” she said. “So clinically, myself and many other cardiologists who understand ivabradine have been using it off-label for the COVID patients, as long as they meet the criteria. You don’t want to use it in every COVID patient, but if someone’s predominant complaint is that their heart rate is going up when they’re standing and they’re debilitated by it, this is a drug to consider.”
Anecdotal findings in patients with long-hauler COVID need to be translated into rigorous research protocols, but mechanistically, whether it’s POTS from COVID or from another type of infection – like Lyme disease or some other viral syndrome – it should work the same, Dr. Taub said. “POTS is POTS.”
There are no first-line drugs for POTS, and current class IIb recommendations include midodrine, which increases blood pressure and can make people feel awful, and fludrocortisone, which can cause a lot of weight gain and fluid retention, she observed. Other agents that lower heart rate, like beta-blockers, also lower blood pressure and can aggravate depression and fatigue.
Ivabradine regulates heart rate by specifically blocking the Ifunny channel of the sinoatrial node. It was approved in 2015 in the United States to reduce hospitalizations in patients with systolic heart failure, and it also has a second class IIb recommendation for inappropriate sinus tachycardia.
The present study, reported in the Feb. 23 issue of the Journal of the American College of Cardiology, is the first randomized clinical trial using ivabradine to treat POTS.
A total of 26 patients with POTS were started on ivabradine 5 mg or placebo twice daily for 1 month, then were crossed over to the other treatment for 1 month after a 1-week washout period. Six patients were started on a 2.5-mg twice-daily dose. Doses were adjusted during the study based on the patient’s heart rate response and tolerance. Patients had seven clinic visits in which norepinephrine (NE) levels were measured and head-up tilt testing conducted.
Four patients in the ivabradine arm withdrew because of adverse effects, and one withdrew during crossover.
Among the 22 patients who completed the study, exploratory analyses showed a strong trend for greater reduction in plasma NE upon standing with ivabradine (P = .056). The effect was also more profound in patients with very high baseline standing NE levels (at least 1,000 pg/mL) than in those with lower NE levels (600 to 1,000 pg/mL).
“It makes sense because that means their sympathetic nervous system is more overactive; they have a higher heart rate,” Dr. Taub said. “So it’s a potential clinical tool that people can use in their practice to determine, ‘okay, is this a patient I should be considering ivabradine on?’ ”
Although the present study had only 22 patients, “it should definitely be looked at as a step forward, both in terms of ivabradine specifically and in terms of setting the standard for the types of studies we want to see in our patients,” Satish R. Raj, MD, MSCI, University of Calgary (Alta.), said in an interview.
In a related editorial, however, Dr. Raj and coauthor Robert S. Sheldon, MD, PhD, also from the University of Calgary, point out that the standing heart rate in the placebo phase was only 94 beats/min, “suggesting that these patients may be affected only mildly by their POTS.”
Asked about the point, Dr. Taub said: “I don’t know if I agree with that.” She noted that the diagnosis of POTS was confirmed by tilt-table testing and NE levels and that patients’ symptoms vary from day to day. “The standard deviation was plus or minus 16.8, so there’s variability.”
Both Dr. Raj and Dr. Taub said they expect the results will be included in the next scientific statement for POTS, but in the meantime, it may be a struggle to get the drug covered by insurance.
“The challenge is that this is a very off-label use for this medication, and the medication’s not cheap,” Dr. Raj observed. The price for 60 tablets, which is about a 1-month supply, is $485 on GoodRx.
Another question going forward, he said, is whether ivabradine is superior to beta-blockers, which will be studied in a 20-patient crossover trial sponsored by the University of Calgary that is about to launch. The primary completion date is set for 2024.
The study was supported by a grant from Amgen. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA. Dr. Raj has received a research grant from the Canadian Institutes of Health Research and research grants from Dysautonomia International to address the pathophysiology of POTS. Dr. Sheldon has received a research grant from Dysautonomia International for a clinical trial assessing ivabradine and propranolol for the treatment of POTS.
A version of this article first appeared on Medscape.com.
The heart failure drug ivabradine (Corlanor) can provide relief from the elevated heart rate and often debilitating symptoms associated with postural orthostatic tachycardia syndrome (POTS), a new study suggests.
Ivabradine significantly lowered standing heart rate, compared with placebo (77.9 vs. 94.2 beats/min; P < .001). The typical surge in heart rate that occurs upon standing in these patients was also blunted, compared with baseline (13.0 vs. 21.4 beats/min; P = .001).
“There are really not a lot of great options for patients with POTS and, mechanistically, ivabradine just make sense because it’s a drug that lowers heart rate very selectively and doesn’t lower blood pressure,” lead study author Pam R. Taub, MD, told this news organization.
Surprisingly, the reduction in heart rate translated into improved physical (P = .008) and social (P = .021) functioning after just 1 month of ivabradine, without any other background POTS medications or a change in nonpharmacologic therapies, she said. “What’s really nice to see is when you tackle a really significant part of the disease, which is the elevated heart rate, just how much better they feel.”
POTS patients are mostly healthy, active young women, who after some inciting event – such as viral infection, trauma, or surgery – experience an increase in heart rate of at least 30 beats/min upon standing accompanied by a range of symptoms, including dizziness, palpitations, brain fog, and fatigue.
A COVID connection?
The study enrolled patients with hyperadrenergic POTS as the predominant subtype, but another group to keep in mind that might benefit is the post-COVID POTS patient, said Dr. Taub, from the University of California, San Diego.
“We’re seeing an incredible number of patients post COVID that meet the criteria for POTS, and a lot of these patients also have COVID fatigue,” she said. “So clinically, myself and many other cardiologists who understand ivabradine have been using it off-label for the COVID patients, as long as they meet the criteria. You don’t want to use it in every COVID patient, but if someone’s predominant complaint is that their heart rate is going up when they’re standing and they’re debilitated by it, this is a drug to consider.”
Anecdotal findings in patients with long-hauler COVID need to be translated into rigorous research protocols, but mechanistically, whether it’s POTS from COVID or from another type of infection – like Lyme disease or some other viral syndrome – it should work the same, Dr. Taub said. “POTS is POTS.”
There are no first-line drugs for POTS, and current class IIb recommendations include midodrine, which increases blood pressure and can make people feel awful, and fludrocortisone, which can cause a lot of weight gain and fluid retention, she observed. Other agents that lower heart rate, like beta-blockers, also lower blood pressure and can aggravate depression and fatigue.
Ivabradine regulates heart rate by specifically blocking the Ifunny channel of the sinoatrial node. It was approved in 2015 in the United States to reduce hospitalizations in patients with systolic heart failure, and it also has a second class IIb recommendation for inappropriate sinus tachycardia.
The present study, reported in the Feb. 23 issue of the Journal of the American College of Cardiology, is the first randomized clinical trial using ivabradine to treat POTS.
A total of 26 patients with POTS were started on ivabradine 5 mg or placebo twice daily for 1 month, then were crossed over to the other treatment for 1 month after a 1-week washout period. Six patients were started on a 2.5-mg twice-daily dose. Doses were adjusted during the study based on the patient’s heart rate response and tolerance. Patients had seven clinic visits in which norepinephrine (NE) levels were measured and head-up tilt testing conducted.
Four patients in the ivabradine arm withdrew because of adverse effects, and one withdrew during crossover.
Among the 22 patients who completed the study, exploratory analyses showed a strong trend for greater reduction in plasma NE upon standing with ivabradine (P = .056). The effect was also more profound in patients with very high baseline standing NE levels (at least 1,000 pg/mL) than in those with lower NE levels (600 to 1,000 pg/mL).
“It makes sense because that means their sympathetic nervous system is more overactive; they have a higher heart rate,” Dr. Taub said. “So it’s a potential clinical tool that people can use in their practice to determine, ‘okay, is this a patient I should be considering ivabradine on?’ ”
Although the present study had only 22 patients, “it should definitely be looked at as a step forward, both in terms of ivabradine specifically and in terms of setting the standard for the types of studies we want to see in our patients,” Satish R. Raj, MD, MSCI, University of Calgary (Alta.), said in an interview.
In a related editorial, however, Dr. Raj and coauthor Robert S. Sheldon, MD, PhD, also from the University of Calgary, point out that the standing heart rate in the placebo phase was only 94 beats/min, “suggesting that these patients may be affected only mildly by their POTS.”
Asked about the point, Dr. Taub said: “I don’t know if I agree with that.” She noted that the diagnosis of POTS was confirmed by tilt-table testing and NE levels and that patients’ symptoms vary from day to day. “The standard deviation was plus or minus 16.8, so there’s variability.”
Both Dr. Raj and Dr. Taub said they expect the results will be included in the next scientific statement for POTS, but in the meantime, it may be a struggle to get the drug covered by insurance.
“The challenge is that this is a very off-label use for this medication, and the medication’s not cheap,” Dr. Raj observed. The price for 60 tablets, which is about a 1-month supply, is $485 on GoodRx.
Another question going forward, he said, is whether ivabradine is superior to beta-blockers, which will be studied in a 20-patient crossover trial sponsored by the University of Calgary that is about to launch. The primary completion date is set for 2024.
The study was supported by a grant from Amgen. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA. Dr. Raj has received a research grant from the Canadian Institutes of Health Research and research grants from Dysautonomia International to address the pathophysiology of POTS. Dr. Sheldon has received a research grant from Dysautonomia International for a clinical trial assessing ivabradine and propranolol for the treatment of POTS.
A version of this article first appeared on Medscape.com.
The heart failure drug ivabradine (Corlanor) can provide relief from the elevated heart rate and often debilitating symptoms associated with postural orthostatic tachycardia syndrome (POTS), a new study suggests.
Ivabradine significantly lowered standing heart rate, compared with placebo (77.9 vs. 94.2 beats/min; P < .001). The typical surge in heart rate that occurs upon standing in these patients was also blunted, compared with baseline (13.0 vs. 21.4 beats/min; P = .001).
“There are really not a lot of great options for patients with POTS and, mechanistically, ivabradine just make sense because it’s a drug that lowers heart rate very selectively and doesn’t lower blood pressure,” lead study author Pam R. Taub, MD, told this news organization.
Surprisingly, the reduction in heart rate translated into improved physical (P = .008) and social (P = .021) functioning after just 1 month of ivabradine, without any other background POTS medications or a change in nonpharmacologic therapies, she said. “What’s really nice to see is when you tackle a really significant part of the disease, which is the elevated heart rate, just how much better they feel.”
POTS patients are mostly healthy, active young women, who after some inciting event – such as viral infection, trauma, or surgery – experience an increase in heart rate of at least 30 beats/min upon standing accompanied by a range of symptoms, including dizziness, palpitations, brain fog, and fatigue.
A COVID connection?
The study enrolled patients with hyperadrenergic POTS as the predominant subtype, but another group to keep in mind that might benefit is the post-COVID POTS patient, said Dr. Taub, from the University of California, San Diego.
“We’re seeing an incredible number of patients post COVID that meet the criteria for POTS, and a lot of these patients also have COVID fatigue,” she said. “So clinically, myself and many other cardiologists who understand ivabradine have been using it off-label for the COVID patients, as long as they meet the criteria. You don’t want to use it in every COVID patient, but if someone’s predominant complaint is that their heart rate is going up when they’re standing and they’re debilitated by it, this is a drug to consider.”
Anecdotal findings in patients with long-hauler COVID need to be translated into rigorous research protocols, but mechanistically, whether it’s POTS from COVID or from another type of infection – like Lyme disease or some other viral syndrome – it should work the same, Dr. Taub said. “POTS is POTS.”
There are no first-line drugs for POTS, and current class IIb recommendations include midodrine, which increases blood pressure and can make people feel awful, and fludrocortisone, which can cause a lot of weight gain and fluid retention, she observed. Other agents that lower heart rate, like beta-blockers, also lower blood pressure and can aggravate depression and fatigue.
Ivabradine regulates heart rate by specifically blocking the Ifunny channel of the sinoatrial node. It was approved in 2015 in the United States to reduce hospitalizations in patients with systolic heart failure, and it also has a second class IIb recommendation for inappropriate sinus tachycardia.
The present study, reported in the Feb. 23 issue of the Journal of the American College of Cardiology, is the first randomized clinical trial using ivabradine to treat POTS.
A total of 26 patients with POTS were started on ivabradine 5 mg or placebo twice daily for 1 month, then were crossed over to the other treatment for 1 month after a 1-week washout period. Six patients were started on a 2.5-mg twice-daily dose. Doses were adjusted during the study based on the patient’s heart rate response and tolerance. Patients had seven clinic visits in which norepinephrine (NE) levels were measured and head-up tilt testing conducted.
Four patients in the ivabradine arm withdrew because of adverse effects, and one withdrew during crossover.
Among the 22 patients who completed the study, exploratory analyses showed a strong trend for greater reduction in plasma NE upon standing with ivabradine (P = .056). The effect was also more profound in patients with very high baseline standing NE levels (at least 1,000 pg/mL) than in those with lower NE levels (600 to 1,000 pg/mL).
“It makes sense because that means their sympathetic nervous system is more overactive; they have a higher heart rate,” Dr. Taub said. “So it’s a potential clinical tool that people can use in their practice to determine, ‘okay, is this a patient I should be considering ivabradine on?’ ”
Although the present study had only 22 patients, “it should definitely be looked at as a step forward, both in terms of ivabradine specifically and in terms of setting the standard for the types of studies we want to see in our patients,” Satish R. Raj, MD, MSCI, University of Calgary (Alta.), said in an interview.
In a related editorial, however, Dr. Raj and coauthor Robert S. Sheldon, MD, PhD, also from the University of Calgary, point out that the standing heart rate in the placebo phase was only 94 beats/min, “suggesting that these patients may be affected only mildly by their POTS.”
Asked about the point, Dr. Taub said: “I don’t know if I agree with that.” She noted that the diagnosis of POTS was confirmed by tilt-table testing and NE levels and that patients’ symptoms vary from day to day. “The standard deviation was plus or minus 16.8, so there’s variability.”
Both Dr. Raj and Dr. Taub said they expect the results will be included in the next scientific statement for POTS, but in the meantime, it may be a struggle to get the drug covered by insurance.
“The challenge is that this is a very off-label use for this medication, and the medication’s not cheap,” Dr. Raj observed. The price for 60 tablets, which is about a 1-month supply, is $485 on GoodRx.
Another question going forward, he said, is whether ivabradine is superior to beta-blockers, which will be studied in a 20-patient crossover trial sponsored by the University of Calgary that is about to launch. The primary completion date is set for 2024.
The study was supported by a grant from Amgen. Dr. Taub has served as a consultant for Amgen, Bayer, Esperion, Boehringer Ingelheim, Novo Nordisk, and Sanofi; is a shareholder in Epirium Bio; and has received research grants from the National Institutes of Health, the American Heart Association, and the Department of Homeland Security/FEMA. Dr. Raj has received a research grant from the Canadian Institutes of Health Research and research grants from Dysautonomia International to address the pathophysiology of POTS. Dr. Sheldon has received a research grant from Dysautonomia International for a clinical trial assessing ivabradine and propranolol for the treatment of POTS.
A version of this article first appeared on Medscape.com.