User login
After 48 years, NCI aims to track breast cancer recurrences
Change to SEER eventually planned.
Patients with breast cancer want accurate information on the risk of their cancer recurring once they have completed treatment.
“I would like to know the true stats of how many breast cancers come back no matter what the hell we do for treatment,” comments a typical post on a breast cancer patient bulletin board.
But those statistics have not been available from a robust population-based source.
Now, there is hope that they will – at last – be collected.
A new pilot project at the National Cancer Institute is setting out to collect that information, although the researchers say it is a “long-term goal” that will take a few years.
But it has already been a long time coming. The mother lode of all U.S. cancer data, the NCI’s Surveillance, Epidemiology, and End Results (SEER) Program, started collecting cancer data in 1973.
“When they began to capture cancer data, the focus was primarily on the incidence of cancer, the different types of cancer, and survival,” explained Esmeralda Ramirez-Pena, PhD, MPH, cancer prevention fellow at the NCI.
“Later, SEER expanded to include subgroups of various cancers and different stages at diagnosis,” she added.
But this database has never included information on cancer recurrence.
In a 2017 press statement, the NCI commented: “Collecting recurrence data has been challenging for cancer registries because recurrence can be diagnosed through diverse methods and in a variety of locations.”
New project
The NCI now has a “long-term goal” to implement additional “data elements” into SEER that will allow calculation of breast cancer recurrences, said Dr. Ramirez-Pena.
The Breast Cancer Recurrence Project, a pilot program funded via an NCI–Department of Energy collaboration, “will take a couple of years,” she said.
She presented some details of the new project as a poster at the recent San Antonio Breast Cancer Symposium 2020.
“SEER has added data elements over time,” she said, and this latest move will – at last – include information on breast cancer recurrence.
Why the change now?
“There’s been so much interest [in breast cancer recurrence]. It’s a top cause of cancer death in the United States and globally. The urgent need is evident,” she explained.
Breast cancer advocates have long been calling for SEER to count recurrence, including metastatic recurrence.
Katherine O’Brien, a breast cancer “metser” from Chicago, is credited with especially turning the heat up on the NCI.
In 2015, Ms. O’Brien spearheaded the creation of an online petition on the website change.org, calling on the NCI’s SEER, the Centers for Disease Control and Prevention, and all state cancer registries to start counting all people living with metastatic breast cancer, including those whose early-stage disease progressed. The petition, which is now closed, collected nearly 12,000 signatures.
Tracking recurrences
In the new project, cancer recurrence is defined as a cancer that was treated, reduced to undetectable levels, and later returned either locally, regionally, or distantly.
Tracking recurrence is not a simple matter because posttreatment surveillance to detect it includes clinical exams, biomarker testing, pathologic studies, molecular testing, imaging, and patient-reported symptoms and because recurrence frequency varies by subtype of breast cancer and TNM classification. Additionally, recurrence may depend on age at diagnosis, a variety of risk factors, treatment type, and access to quality of care.
“It’s likely there are many elements that influence recurrence,” said Dr. Ramirez-Pena.
To get a handle on the complexity, the NCI needs to first identify which data are needed to tally recurrence and the frequency at which they are collected, explained Dr. Ramirez-Pena. To do so, she and her coinvestigators conducted a systematic review of phase 3 clinical trials of early-stage breast cancer.
On their own, such trials are not sufficient to provide recurrence estimates at the population level because they lack diversity, represent fewer than 5% of all cancer patients, and the study period may not be long enough to capture recurrences for long-latency breast cancers, such as estrogen receptor–positive malignancies.
Nonetheless, these clinical trials provide a starting place.
The investigators identified 444 early-stage clinical trials. They stratified participants by subtype and tumor characteristics, which will enable analysis of risk-group and treatment-dependent differences in recurrence.
The changing science of breast cancer makes this work a challenge, the investigators said. For example, in clinical trials from the early 1990s through the early 2000s, receptor status and subtyping was not commonly reported, and some treatment endpoints were added during the past few years.
“Our next step will be to extract recurrence rates from these trials so we can eventually provide individualized information about recurrence risk to survivors,” Dr. Ramirez-Pena said, describing the big-picture aims.
The Breast Cancer Recurrence Project is collaborating with external agencies, such as the International Agency for Research on Cancer and Public Health England, in fine-tuning data elements, because “recurrence is not captured well globally either,” said Dr. Ramirez-Pena.
The study was supported by NCI.
A version of this article first appeared on Medscape.com.
Change to SEER eventually planned.
Change to SEER eventually planned.
Patients with breast cancer want accurate information on the risk of their cancer recurring once they have completed treatment.
“I would like to know the true stats of how many breast cancers come back no matter what the hell we do for treatment,” comments a typical post on a breast cancer patient bulletin board.
But those statistics have not been available from a robust population-based source.
Now, there is hope that they will – at last – be collected.
A new pilot project at the National Cancer Institute is setting out to collect that information, although the researchers say it is a “long-term goal” that will take a few years.
But it has already been a long time coming. The mother lode of all U.S. cancer data, the NCI’s Surveillance, Epidemiology, and End Results (SEER) Program, started collecting cancer data in 1973.
“When they began to capture cancer data, the focus was primarily on the incidence of cancer, the different types of cancer, and survival,” explained Esmeralda Ramirez-Pena, PhD, MPH, cancer prevention fellow at the NCI.
“Later, SEER expanded to include subgroups of various cancers and different stages at diagnosis,” she added.
But this database has never included information on cancer recurrence.
In a 2017 press statement, the NCI commented: “Collecting recurrence data has been challenging for cancer registries because recurrence can be diagnosed through diverse methods and in a variety of locations.”
New project
The NCI now has a “long-term goal” to implement additional “data elements” into SEER that will allow calculation of breast cancer recurrences, said Dr. Ramirez-Pena.
The Breast Cancer Recurrence Project, a pilot program funded via an NCI–Department of Energy collaboration, “will take a couple of years,” she said.
She presented some details of the new project as a poster at the recent San Antonio Breast Cancer Symposium 2020.
“SEER has added data elements over time,” she said, and this latest move will – at last – include information on breast cancer recurrence.
Why the change now?
“There’s been so much interest [in breast cancer recurrence]. It’s a top cause of cancer death in the United States and globally. The urgent need is evident,” she explained.
Breast cancer advocates have long been calling for SEER to count recurrence, including metastatic recurrence.
Katherine O’Brien, a breast cancer “metser” from Chicago, is credited with especially turning the heat up on the NCI.
In 2015, Ms. O’Brien spearheaded the creation of an online petition on the website change.org, calling on the NCI’s SEER, the Centers for Disease Control and Prevention, and all state cancer registries to start counting all people living with metastatic breast cancer, including those whose early-stage disease progressed. The petition, which is now closed, collected nearly 12,000 signatures.
Tracking recurrences
In the new project, cancer recurrence is defined as a cancer that was treated, reduced to undetectable levels, and later returned either locally, regionally, or distantly.
Tracking recurrence is not a simple matter because posttreatment surveillance to detect it includes clinical exams, biomarker testing, pathologic studies, molecular testing, imaging, and patient-reported symptoms and because recurrence frequency varies by subtype of breast cancer and TNM classification. Additionally, recurrence may depend on age at diagnosis, a variety of risk factors, treatment type, and access to quality of care.
“It’s likely there are many elements that influence recurrence,” said Dr. Ramirez-Pena.
To get a handle on the complexity, the NCI needs to first identify which data are needed to tally recurrence and the frequency at which they are collected, explained Dr. Ramirez-Pena. To do so, she and her coinvestigators conducted a systematic review of phase 3 clinical trials of early-stage breast cancer.
On their own, such trials are not sufficient to provide recurrence estimates at the population level because they lack diversity, represent fewer than 5% of all cancer patients, and the study period may not be long enough to capture recurrences for long-latency breast cancers, such as estrogen receptor–positive malignancies.
Nonetheless, these clinical trials provide a starting place.
The investigators identified 444 early-stage clinical trials. They stratified participants by subtype and tumor characteristics, which will enable analysis of risk-group and treatment-dependent differences in recurrence.
The changing science of breast cancer makes this work a challenge, the investigators said. For example, in clinical trials from the early 1990s through the early 2000s, receptor status and subtyping was not commonly reported, and some treatment endpoints were added during the past few years.
“Our next step will be to extract recurrence rates from these trials so we can eventually provide individualized information about recurrence risk to survivors,” Dr. Ramirez-Pena said, describing the big-picture aims.
The Breast Cancer Recurrence Project is collaborating with external agencies, such as the International Agency for Research on Cancer and Public Health England, in fine-tuning data elements, because “recurrence is not captured well globally either,” said Dr. Ramirez-Pena.
The study was supported by NCI.
A version of this article first appeared on Medscape.com.
Patients with breast cancer want accurate information on the risk of their cancer recurring once they have completed treatment.
“I would like to know the true stats of how many breast cancers come back no matter what the hell we do for treatment,” comments a typical post on a breast cancer patient bulletin board.
But those statistics have not been available from a robust population-based source.
Now, there is hope that they will – at last – be collected.
A new pilot project at the National Cancer Institute is setting out to collect that information, although the researchers say it is a “long-term goal” that will take a few years.
But it has already been a long time coming. The mother lode of all U.S. cancer data, the NCI’s Surveillance, Epidemiology, and End Results (SEER) Program, started collecting cancer data in 1973.
“When they began to capture cancer data, the focus was primarily on the incidence of cancer, the different types of cancer, and survival,” explained Esmeralda Ramirez-Pena, PhD, MPH, cancer prevention fellow at the NCI.
“Later, SEER expanded to include subgroups of various cancers and different stages at diagnosis,” she added.
But this database has never included information on cancer recurrence.
In a 2017 press statement, the NCI commented: “Collecting recurrence data has been challenging for cancer registries because recurrence can be diagnosed through diverse methods and in a variety of locations.”
New project
The NCI now has a “long-term goal” to implement additional “data elements” into SEER that will allow calculation of breast cancer recurrences, said Dr. Ramirez-Pena.
The Breast Cancer Recurrence Project, a pilot program funded via an NCI–Department of Energy collaboration, “will take a couple of years,” she said.
She presented some details of the new project as a poster at the recent San Antonio Breast Cancer Symposium 2020.
“SEER has added data elements over time,” she said, and this latest move will – at last – include information on breast cancer recurrence.
Why the change now?
“There’s been so much interest [in breast cancer recurrence]. It’s a top cause of cancer death in the United States and globally. The urgent need is evident,” she explained.
Breast cancer advocates have long been calling for SEER to count recurrence, including metastatic recurrence.
Katherine O’Brien, a breast cancer “metser” from Chicago, is credited with especially turning the heat up on the NCI.
In 2015, Ms. O’Brien spearheaded the creation of an online petition on the website change.org, calling on the NCI’s SEER, the Centers for Disease Control and Prevention, and all state cancer registries to start counting all people living with metastatic breast cancer, including those whose early-stage disease progressed. The petition, which is now closed, collected nearly 12,000 signatures.
Tracking recurrences
In the new project, cancer recurrence is defined as a cancer that was treated, reduced to undetectable levels, and later returned either locally, regionally, or distantly.
Tracking recurrence is not a simple matter because posttreatment surveillance to detect it includes clinical exams, biomarker testing, pathologic studies, molecular testing, imaging, and patient-reported symptoms and because recurrence frequency varies by subtype of breast cancer and TNM classification. Additionally, recurrence may depend on age at diagnosis, a variety of risk factors, treatment type, and access to quality of care.
“It’s likely there are many elements that influence recurrence,” said Dr. Ramirez-Pena.
To get a handle on the complexity, the NCI needs to first identify which data are needed to tally recurrence and the frequency at which they are collected, explained Dr. Ramirez-Pena. To do so, she and her coinvestigators conducted a systematic review of phase 3 clinical trials of early-stage breast cancer.
On their own, such trials are not sufficient to provide recurrence estimates at the population level because they lack diversity, represent fewer than 5% of all cancer patients, and the study period may not be long enough to capture recurrences for long-latency breast cancers, such as estrogen receptor–positive malignancies.
Nonetheless, these clinical trials provide a starting place.
The investigators identified 444 early-stage clinical trials. They stratified participants by subtype and tumor characteristics, which will enable analysis of risk-group and treatment-dependent differences in recurrence.
The changing science of breast cancer makes this work a challenge, the investigators said. For example, in clinical trials from the early 1990s through the early 2000s, receptor status and subtyping was not commonly reported, and some treatment endpoints were added during the past few years.
“Our next step will be to extract recurrence rates from these trials so we can eventually provide individualized information about recurrence risk to survivors,” Dr. Ramirez-Pena said, describing the big-picture aims.
The Breast Cancer Recurrence Project is collaborating with external agencies, such as the International Agency for Research on Cancer and Public Health England, in fine-tuning data elements, because “recurrence is not captured well globally either,” said Dr. Ramirez-Pena.
The study was supported by NCI.
A version of this article first appeared on Medscape.com.
CRC risk in young adults: Not as high as previously reported
Implications for CRC screening.
New estimates for the risk of CRC in young adults, which differentiate colorectal adenocarcinoma from other types, are reported in a study published Dec. 15, 2020, in Annals of Internal Medicine.
They are important because this finding has implications for CRC screening, say a trio of experts in an accompanying editorial.
Reports of an increase in the incidence of CRC in younger adults have led to changes in screening for this cancer in the United States. The age for starting CRC screening has been lowered to 45 years (instead of 50 years) in recommendations issued in 2018 by the American Cancer Society, and also more recently in preliminary recommendations from the U.S. Preventive Services Task Force.
However, that 2018 ACS recommendation to lower the starting age to 45 years was based to a large extent on a report of a higher incidence of CRC in younger adults from a 2017 study that used the SEER (Surveillance, Epidemiology, and End Results) database).
But that SEER-based study considered “colorectal cancer” as a homogeneous group defined by topology, the editorialists pointed out.
The new study, the editorialists said, uses that same SEER database but has “disentangled colorectal adenocarcinoma, the target for screening, from other histologic CRC types, including neuroendocrine (carcinoid) tumors, for which screening is not recommended.”
The study authors explained that adenocarcinoma is a target for prevention through screening because it arises from precancerous polyps. Those growths can be detected and removed before cancer develops. That doesn’t apply to carcinoid tumors, which are frequently incidental findings on flexible sigmoidoscopy or colonoscopy.
These carcinoid tumors typically are indolent, with a better prognosis than most other cancer types, the editorialists added. “Most likely, the majority of carcinoid tumors identified by screening represent incidental findings with little health benefit from detection. In fact, many may be characterized as overdiagnosed tumors, which by definition increase the burden and harms of screening without the balance of additional benefit.”
This new analysis showed that 4%-20% of the lesions previously described as CRC were not adenocarcinoma but carcinoid tumors, the editorialists pointed out.
This figure rose even higher in the subgroup of findings pertaining to the rectum, the colonic segment with the largest reported increase in early-onset CRC. Here, up to 34% of lesions (depending on patient age) were carcinoid tumors rather than adenocarcinoma, they noted.
The three editorialists – Michael Bretthauer, MD, PhD, and Mette Kalager, MD, PhD, both of the University of Oslo, and David Weinberg, MD, MSc, of Fox Chase Cancer Center, Philadelphia – call for action based on the new findings.
“The ACS’s 2018 estimate of about 7,000 new CRC cases among persons aged 45-49 years in the United States (the justification for screening) needs to be adjusted downward on the basis of the new evidence,” the trio wrote.
They conclude that “caution is warranted when promoting the benefits of CRC screening for persons younger than 50 years.”
However, the senior author of the new study, Jordan Karlitz, MD, of Tulane University, New Orleans, strongly disagreed.
Contrary to the editorialists, Dr. Karlitz said in an interview that he and his colleagues firmly believe that colorectal cancer screening for average-risk patients should begin at age 45 and that their new research, despite its clarification about carcinoid tumors, provides evidence for that.
“There are a number of other studies that support screening at age 45 as well,” he said. “This [new] finding supports the presence of a large preclinical colorectal cancer case burden in patients in their 40s that is ultimately uncovered with screening initiation at age 50. Many of these cancers could be prevented or diagnosed at an earlier stage with screening at age 45.”
“This is the first study to analyze early-onset colorectal cancer by specific histologic subtype,” Dr. Karlitz also pointed out.
“Although colorectal carcinoids are increasing at a faster rate than adenocarcinomas, adenocarcinomas constitute the overwhelming majority of colorectal cancers in people in their 40s and are also steadily increasing, which has implications for beginning screening at age 45,” he said.
Adenocarcinomas also make up the “overwhelming majority” of colorectal cancers in patients under 50 overall and “are the main driving force behind the increased colorectal cancer burden we are seeing in young patients,” Dr. Karlitz added.
Furthermore, “modeling studies on which the USPSTF screening recommendations were based [which recommended starting at age 45] were confined to adenocarcinoma, thus excluding carcinoids from their analysis,” he said.
Steepest changes in adenocarcinomas in younger groups
In their study, Dr. Karlitz and colleagues assessed the incidence rates of early colorectal cancer, using SEER data from 2000 to 2016, and stratifying the data by histologic subtype (primarily adenocarcinoma and carcinoid tumors), age group (20-29, 30-39, 40-49, and 50-54 years), and subsite.
A total of 123,143 CRC cases were identified in 119,624 patients between the ages of 20-54 years during that time period.
The absolute incidence rates in the younger age groups (20-29 and 30-39 years) were very low, compared with those aged 40-49 and 50-54 years.
The greatest 3-year average annual incident rate changes in adenocarcinoma (2000-2002 vs. 2014-2016) for any age group or subsite were for rectal-only cases in the 20-29 years group (+39%), as well as rectal-only cases in those aged 30-39 years (+39%), and colon-only cases in the age 30-39 group (+20%).
There was also significant increase in rectal-only adenocarcinoma in individuals aged 50-54 years (+10%). A statistically significant increase in the annual percentage change for adenocarcinomas was observed for all age groups, except for colon-only cases in the 20-29 years group (0.7%) and for both colorectal (0.2%) and colon-only cases (–0.1%) in those aged 50-54 years.
Even though the absolute carcinoid tumor incidence rates were lower than for adenocarcinoma in all age groups and subsites, a statistically significant increase was observed in the 3-year average annual incidence rate of combined-site colorectal carcinoid tumors in all age groups from 2000–2002 and 2014–2016. This increase was largely the result of increases in rectal carcinoid tumors, the authors note.
The authors also highlighted the results in the 40- to 49-year age group “because of differing opinions on whether to begin average-risk screening at age 45 or 50 years.”
They reported that rates of rectal and colon adenocarcinoma are increasing “substantially,” whether measured by changes in 3-year average annual incidence rate or by annual percentage changes. The change in average annual incidence rate of colon-only adenocarcinoma for persons aged 40-49 years was 13% (12.21 to 13.85 per 100,000), and that of rectal adenocarcinoma was 16% (7.50 to 8.72 per 100,000). Corresponding annual percentage changes were 0.8% and 1.2%, respectively. “These significant increases in adenocarcinoma incident rates add to the debate over earlier screening at age 45 years,” they commented.
Calls for next steps
The editorialists emphasize restraint when promoting the benefits of colorectal screening for persons younger than 50 years.
They point out that the USPSTF released a provisional update of its CRC screening recommendations about lowering the age to initiate screening to 45 years, as reported by this news organization.
“No new empirical evidence has been found since the USPSTF update in 2016 to inform the effectiveness of screening in persons younger than 50 years,” they write, adding that similar to the American Cancer Society in 2018, the task force has relied exclusively on modeling studies.
This new data from Dr. Karlitz and colleagues “should prompt the modelers to recalculate their estimates of benefits and harms of screening,” they suggested. “Revisiting the model would also allow competing forms of CRC screening to be compared in light of new risk assumptions.
“Previous assumptions that screening tests are equally effective in younger and older patients and that screening adherence will approach 100% may also be reconsidered,” the editorialist commented.
The study authors concluded somewhat differently.
“In conclusion, adenocarcinoma rates increased in many early-onset subgroups but showed no significant increase in others, including colon-only cases in persons aged 20-29 and 50-54 years,” the investigators wrote.
They also observed that “rectal carcinoid tumors are increasing in young patients and may have a substantial impact on overall CRC incident rates.”
Those findings on rectal carcinoid tumors “underscore the importance of assessing histologic CRC subtypes independently,” the researchers said.
This new approach, of which the current study is a first effort, “may lead to a better understanding of the drivers of temporal changes in overall CRC incidence and a more accurate measurement of the outcomes of adenocarcinoma risk reduction efforts, and can guide future research.”
The study had no outside funding. Dr. Karlitz reported personal fees from Exact Sciences, personal fees from Myriad Genetics, and other fees from Gastro Girl and GI OnDEMAND, outside the submitted work. Dr. Bretthauer reports grants from Norwegian Research Council, grants from Norwegian Cancer Society for research in colorectal cancer screening. Dr. Weinberg and Dr. Kalager have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Implications for CRC screening.
Implications for CRC screening.
New estimates for the risk of CRC in young adults, which differentiate colorectal adenocarcinoma from other types, are reported in a study published Dec. 15, 2020, in Annals of Internal Medicine.
They are important because this finding has implications for CRC screening, say a trio of experts in an accompanying editorial.
Reports of an increase in the incidence of CRC in younger adults have led to changes in screening for this cancer in the United States. The age for starting CRC screening has been lowered to 45 years (instead of 50 years) in recommendations issued in 2018 by the American Cancer Society, and also more recently in preliminary recommendations from the U.S. Preventive Services Task Force.
However, that 2018 ACS recommendation to lower the starting age to 45 years was based to a large extent on a report of a higher incidence of CRC in younger adults from a 2017 study that used the SEER (Surveillance, Epidemiology, and End Results) database).
But that SEER-based study considered “colorectal cancer” as a homogeneous group defined by topology, the editorialists pointed out.
The new study, the editorialists said, uses that same SEER database but has “disentangled colorectal adenocarcinoma, the target for screening, from other histologic CRC types, including neuroendocrine (carcinoid) tumors, for which screening is not recommended.”
The study authors explained that adenocarcinoma is a target for prevention through screening because it arises from precancerous polyps. Those growths can be detected and removed before cancer develops. That doesn’t apply to carcinoid tumors, which are frequently incidental findings on flexible sigmoidoscopy or colonoscopy.
These carcinoid tumors typically are indolent, with a better prognosis than most other cancer types, the editorialists added. “Most likely, the majority of carcinoid tumors identified by screening represent incidental findings with little health benefit from detection. In fact, many may be characterized as overdiagnosed tumors, which by definition increase the burden and harms of screening without the balance of additional benefit.”
This new analysis showed that 4%-20% of the lesions previously described as CRC were not adenocarcinoma but carcinoid tumors, the editorialists pointed out.
This figure rose even higher in the subgroup of findings pertaining to the rectum, the colonic segment with the largest reported increase in early-onset CRC. Here, up to 34% of lesions (depending on patient age) were carcinoid tumors rather than adenocarcinoma, they noted.
The three editorialists – Michael Bretthauer, MD, PhD, and Mette Kalager, MD, PhD, both of the University of Oslo, and David Weinberg, MD, MSc, of Fox Chase Cancer Center, Philadelphia – call for action based on the new findings.
“The ACS’s 2018 estimate of about 7,000 new CRC cases among persons aged 45-49 years in the United States (the justification for screening) needs to be adjusted downward on the basis of the new evidence,” the trio wrote.
They conclude that “caution is warranted when promoting the benefits of CRC screening for persons younger than 50 years.”
However, the senior author of the new study, Jordan Karlitz, MD, of Tulane University, New Orleans, strongly disagreed.
Contrary to the editorialists, Dr. Karlitz said in an interview that he and his colleagues firmly believe that colorectal cancer screening for average-risk patients should begin at age 45 and that their new research, despite its clarification about carcinoid tumors, provides evidence for that.
“There are a number of other studies that support screening at age 45 as well,” he said. “This [new] finding supports the presence of a large preclinical colorectal cancer case burden in patients in their 40s that is ultimately uncovered with screening initiation at age 50. Many of these cancers could be prevented or diagnosed at an earlier stage with screening at age 45.”
“This is the first study to analyze early-onset colorectal cancer by specific histologic subtype,” Dr. Karlitz also pointed out.
“Although colorectal carcinoids are increasing at a faster rate than adenocarcinomas, adenocarcinomas constitute the overwhelming majority of colorectal cancers in people in their 40s and are also steadily increasing, which has implications for beginning screening at age 45,” he said.
Adenocarcinomas also make up the “overwhelming majority” of colorectal cancers in patients under 50 overall and “are the main driving force behind the increased colorectal cancer burden we are seeing in young patients,” Dr. Karlitz added.
Furthermore, “modeling studies on which the USPSTF screening recommendations were based [which recommended starting at age 45] were confined to adenocarcinoma, thus excluding carcinoids from their analysis,” he said.
Steepest changes in adenocarcinomas in younger groups
In their study, Dr. Karlitz and colleagues assessed the incidence rates of early colorectal cancer, using SEER data from 2000 to 2016, and stratifying the data by histologic subtype (primarily adenocarcinoma and carcinoid tumors), age group (20-29, 30-39, 40-49, and 50-54 years), and subsite.
A total of 123,143 CRC cases were identified in 119,624 patients between the ages of 20-54 years during that time period.
The absolute incidence rates in the younger age groups (20-29 and 30-39 years) were very low, compared with those aged 40-49 and 50-54 years.
The greatest 3-year average annual incident rate changes in adenocarcinoma (2000-2002 vs. 2014-2016) for any age group or subsite were for rectal-only cases in the 20-29 years group (+39%), as well as rectal-only cases in those aged 30-39 years (+39%), and colon-only cases in the age 30-39 group (+20%).
There was also significant increase in rectal-only adenocarcinoma in individuals aged 50-54 years (+10%). A statistically significant increase in the annual percentage change for adenocarcinomas was observed for all age groups, except for colon-only cases in the 20-29 years group (0.7%) and for both colorectal (0.2%) and colon-only cases (–0.1%) in those aged 50-54 years.
Even though the absolute carcinoid tumor incidence rates were lower than for adenocarcinoma in all age groups and subsites, a statistically significant increase was observed in the 3-year average annual incidence rate of combined-site colorectal carcinoid tumors in all age groups from 2000–2002 and 2014–2016. This increase was largely the result of increases in rectal carcinoid tumors, the authors note.
The authors also highlighted the results in the 40- to 49-year age group “because of differing opinions on whether to begin average-risk screening at age 45 or 50 years.”
They reported that rates of rectal and colon adenocarcinoma are increasing “substantially,” whether measured by changes in 3-year average annual incidence rate or by annual percentage changes. The change in average annual incidence rate of colon-only adenocarcinoma for persons aged 40-49 years was 13% (12.21 to 13.85 per 100,000), and that of rectal adenocarcinoma was 16% (7.50 to 8.72 per 100,000). Corresponding annual percentage changes were 0.8% and 1.2%, respectively. “These significant increases in adenocarcinoma incident rates add to the debate over earlier screening at age 45 years,” they commented.
Calls for next steps
The editorialists emphasize restraint when promoting the benefits of colorectal screening for persons younger than 50 years.
They point out that the USPSTF released a provisional update of its CRC screening recommendations about lowering the age to initiate screening to 45 years, as reported by this news organization.
“No new empirical evidence has been found since the USPSTF update in 2016 to inform the effectiveness of screening in persons younger than 50 years,” they write, adding that similar to the American Cancer Society in 2018, the task force has relied exclusively on modeling studies.
This new data from Dr. Karlitz and colleagues “should prompt the modelers to recalculate their estimates of benefits and harms of screening,” they suggested. “Revisiting the model would also allow competing forms of CRC screening to be compared in light of new risk assumptions.
“Previous assumptions that screening tests are equally effective in younger and older patients and that screening adherence will approach 100% may also be reconsidered,” the editorialist commented.
The study authors concluded somewhat differently.
“In conclusion, adenocarcinoma rates increased in many early-onset subgroups but showed no significant increase in others, including colon-only cases in persons aged 20-29 and 50-54 years,” the investigators wrote.
They also observed that “rectal carcinoid tumors are increasing in young patients and may have a substantial impact on overall CRC incident rates.”
Those findings on rectal carcinoid tumors “underscore the importance of assessing histologic CRC subtypes independently,” the researchers said.
This new approach, of which the current study is a first effort, “may lead to a better understanding of the drivers of temporal changes in overall CRC incidence and a more accurate measurement of the outcomes of adenocarcinoma risk reduction efforts, and can guide future research.”
The study had no outside funding. Dr. Karlitz reported personal fees from Exact Sciences, personal fees from Myriad Genetics, and other fees from Gastro Girl and GI OnDEMAND, outside the submitted work. Dr. Bretthauer reports grants from Norwegian Research Council, grants from Norwegian Cancer Society for research in colorectal cancer screening. Dr. Weinberg and Dr. Kalager have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New estimates for the risk of CRC in young adults, which differentiate colorectal adenocarcinoma from other types, are reported in a study published Dec. 15, 2020, in Annals of Internal Medicine.
They are important because this finding has implications for CRC screening, say a trio of experts in an accompanying editorial.
Reports of an increase in the incidence of CRC in younger adults have led to changes in screening for this cancer in the United States. The age for starting CRC screening has been lowered to 45 years (instead of 50 years) in recommendations issued in 2018 by the American Cancer Society, and also more recently in preliminary recommendations from the U.S. Preventive Services Task Force.
However, that 2018 ACS recommendation to lower the starting age to 45 years was based to a large extent on a report of a higher incidence of CRC in younger adults from a 2017 study that used the SEER (Surveillance, Epidemiology, and End Results) database).
But that SEER-based study considered “colorectal cancer” as a homogeneous group defined by topology, the editorialists pointed out.
The new study, the editorialists said, uses that same SEER database but has “disentangled colorectal adenocarcinoma, the target for screening, from other histologic CRC types, including neuroendocrine (carcinoid) tumors, for which screening is not recommended.”
The study authors explained that adenocarcinoma is a target for prevention through screening because it arises from precancerous polyps. Those growths can be detected and removed before cancer develops. That doesn’t apply to carcinoid tumors, which are frequently incidental findings on flexible sigmoidoscopy or colonoscopy.
These carcinoid tumors typically are indolent, with a better prognosis than most other cancer types, the editorialists added. “Most likely, the majority of carcinoid tumors identified by screening represent incidental findings with little health benefit from detection. In fact, many may be characterized as overdiagnosed tumors, which by definition increase the burden and harms of screening without the balance of additional benefit.”
This new analysis showed that 4%-20% of the lesions previously described as CRC were not adenocarcinoma but carcinoid tumors, the editorialists pointed out.
This figure rose even higher in the subgroup of findings pertaining to the rectum, the colonic segment with the largest reported increase in early-onset CRC. Here, up to 34% of lesions (depending on patient age) were carcinoid tumors rather than adenocarcinoma, they noted.
The three editorialists – Michael Bretthauer, MD, PhD, and Mette Kalager, MD, PhD, both of the University of Oslo, and David Weinberg, MD, MSc, of Fox Chase Cancer Center, Philadelphia – call for action based on the new findings.
“The ACS’s 2018 estimate of about 7,000 new CRC cases among persons aged 45-49 years in the United States (the justification for screening) needs to be adjusted downward on the basis of the new evidence,” the trio wrote.
They conclude that “caution is warranted when promoting the benefits of CRC screening for persons younger than 50 years.”
However, the senior author of the new study, Jordan Karlitz, MD, of Tulane University, New Orleans, strongly disagreed.
Contrary to the editorialists, Dr. Karlitz said in an interview that he and his colleagues firmly believe that colorectal cancer screening for average-risk patients should begin at age 45 and that their new research, despite its clarification about carcinoid tumors, provides evidence for that.
“There are a number of other studies that support screening at age 45 as well,” he said. “This [new] finding supports the presence of a large preclinical colorectal cancer case burden in patients in their 40s that is ultimately uncovered with screening initiation at age 50. Many of these cancers could be prevented or diagnosed at an earlier stage with screening at age 45.”
“This is the first study to analyze early-onset colorectal cancer by specific histologic subtype,” Dr. Karlitz also pointed out.
“Although colorectal carcinoids are increasing at a faster rate than adenocarcinomas, adenocarcinomas constitute the overwhelming majority of colorectal cancers in people in their 40s and are also steadily increasing, which has implications for beginning screening at age 45,” he said.
Adenocarcinomas also make up the “overwhelming majority” of colorectal cancers in patients under 50 overall and “are the main driving force behind the increased colorectal cancer burden we are seeing in young patients,” Dr. Karlitz added.
Furthermore, “modeling studies on which the USPSTF screening recommendations were based [which recommended starting at age 45] were confined to adenocarcinoma, thus excluding carcinoids from their analysis,” he said.
Steepest changes in adenocarcinomas in younger groups
In their study, Dr. Karlitz and colleagues assessed the incidence rates of early colorectal cancer, using SEER data from 2000 to 2016, and stratifying the data by histologic subtype (primarily adenocarcinoma and carcinoid tumors), age group (20-29, 30-39, 40-49, and 50-54 years), and subsite.
A total of 123,143 CRC cases were identified in 119,624 patients between the ages of 20-54 years during that time period.
The absolute incidence rates in the younger age groups (20-29 and 30-39 years) were very low, compared with those aged 40-49 and 50-54 years.
The greatest 3-year average annual incident rate changes in adenocarcinoma (2000-2002 vs. 2014-2016) for any age group or subsite were for rectal-only cases in the 20-29 years group (+39%), as well as rectal-only cases in those aged 30-39 years (+39%), and colon-only cases in the age 30-39 group (+20%).
There was also significant increase in rectal-only adenocarcinoma in individuals aged 50-54 years (+10%). A statistically significant increase in the annual percentage change for adenocarcinomas was observed for all age groups, except for colon-only cases in the 20-29 years group (0.7%) and for both colorectal (0.2%) and colon-only cases (–0.1%) in those aged 50-54 years.
Even though the absolute carcinoid tumor incidence rates were lower than for adenocarcinoma in all age groups and subsites, a statistically significant increase was observed in the 3-year average annual incidence rate of combined-site colorectal carcinoid tumors in all age groups from 2000–2002 and 2014–2016. This increase was largely the result of increases in rectal carcinoid tumors, the authors note.
The authors also highlighted the results in the 40- to 49-year age group “because of differing opinions on whether to begin average-risk screening at age 45 or 50 years.”
They reported that rates of rectal and colon adenocarcinoma are increasing “substantially,” whether measured by changes in 3-year average annual incidence rate or by annual percentage changes. The change in average annual incidence rate of colon-only adenocarcinoma for persons aged 40-49 years was 13% (12.21 to 13.85 per 100,000), and that of rectal adenocarcinoma was 16% (7.50 to 8.72 per 100,000). Corresponding annual percentage changes were 0.8% and 1.2%, respectively. “These significant increases in adenocarcinoma incident rates add to the debate over earlier screening at age 45 years,” they commented.
Calls for next steps
The editorialists emphasize restraint when promoting the benefits of colorectal screening for persons younger than 50 years.
They point out that the USPSTF released a provisional update of its CRC screening recommendations about lowering the age to initiate screening to 45 years, as reported by this news organization.
“No new empirical evidence has been found since the USPSTF update in 2016 to inform the effectiveness of screening in persons younger than 50 years,” they write, adding that similar to the American Cancer Society in 2018, the task force has relied exclusively on modeling studies.
This new data from Dr. Karlitz and colleagues “should prompt the modelers to recalculate their estimates of benefits and harms of screening,” they suggested. “Revisiting the model would also allow competing forms of CRC screening to be compared in light of new risk assumptions.
“Previous assumptions that screening tests are equally effective in younger and older patients and that screening adherence will approach 100% may also be reconsidered,” the editorialist commented.
The study authors concluded somewhat differently.
“In conclusion, adenocarcinoma rates increased in many early-onset subgroups but showed no significant increase in others, including colon-only cases in persons aged 20-29 and 50-54 years,” the investigators wrote.
They also observed that “rectal carcinoid tumors are increasing in young patients and may have a substantial impact on overall CRC incident rates.”
Those findings on rectal carcinoid tumors “underscore the importance of assessing histologic CRC subtypes independently,” the researchers said.
This new approach, of which the current study is a first effort, “may lead to a better understanding of the drivers of temporal changes in overall CRC incidence and a more accurate measurement of the outcomes of adenocarcinoma risk reduction efforts, and can guide future research.”
The study had no outside funding. Dr. Karlitz reported personal fees from Exact Sciences, personal fees from Myriad Genetics, and other fees from Gastro Girl and GI OnDEMAND, outside the submitted work. Dr. Bretthauer reports grants from Norwegian Research Council, grants from Norwegian Cancer Society for research in colorectal cancer screening. Dr. Weinberg and Dr. Kalager have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CRC risk in young adults: Not as high as previously reported
Implications for CRC screening.
New estimates for the risk of CRC in young adults, which differentiate colorectal adenocarcinoma from other types, are reported in a study published Dec. 15, 2020, in Annals of Internal Medicine.
They are important because this finding has implications for CRC screening, say a trio of experts in an accompanying editorial.
Reports of an increase in the incidence of CRC in younger adults have led to changes in screening for this cancer in the United States. The age for starting CRC screening has been lowered to 45 years (instead of 50 years) in recommendations issued in 2018 by the American Cancer Society, and also more recently in preliminary recommendations from the U.S. Preventive Services Task Force.
However, that 2018 ACS recommendation to lower the starting age to 45 years was based to a large extent on a report of a higher incidence of CRC in younger adults from a 2017 study that used the SEER (Surveillance, Epidemiology, and End Results) database).
But that SEER-based study considered “colorectal cancer” as a homogeneous group defined by topology, the editorialists pointed out.
The new study, the editorialists said, uses that same SEER database but has “disentangled colorectal adenocarcinoma, the target for screening, from other histologic CRC types, including neuroendocrine (carcinoid) tumors, for which screening is not recommended.”
The study authors explained that adenocarcinoma is a target for prevention through screening because it arises from precancerous polyps. Those growths can be detected and removed before cancer develops. That doesn’t apply to carcinoid tumors, which are frequently incidental findings on flexible sigmoidoscopy or colonoscopy.
These carcinoid tumors typically are indolent, with a better prognosis than most other cancer types, the editorialists added. “Most likely, the majority of carcinoid tumors identified by screening represent incidental findings with little health benefit from detection. In fact, many may be characterized as overdiagnosed tumors, which by definition increase the burden and harms of screening without the balance of additional benefit.”
This new analysis showed that 4%-20% of the lesions previously described as CRC were not adenocarcinoma but carcinoid tumors, the editorialists pointed out.
This figure rose even higher in the subgroup of findings pertaining to the rectum, the colonic segment with the largest reported increase in early-onset CRC. Here, up to 34% of lesions (depending on patient age) were carcinoid tumors rather than adenocarcinoma, they noted.
The three editorialists – Michael Bretthauer, MD, PhD, and Mette Kalager, MD, PhD, both of the University of Oslo, and David Weinberg, MD, MSc, of Fox Chase Cancer Center, Philadelphia – call for action based on the new findings.
“The ACS’s 2018 estimate of about 7,000 new CRC cases among persons aged 45-49 years in the United States (the justification for screening) needs to be adjusted downward on the basis of the new evidence,” the trio wrote.
They conclude that “caution is warranted when promoting the benefits of CRC screening for persons younger than 50 years.”
However, the senior author of the new study, Jordan Karlitz, MD, of Tulane University, New Orleans, strongly disagreed.
Contrary to the editorialists, Dr. Karlitz said in an interview that he and his colleagues firmly believe that colorectal cancer screening for average-risk patients should begin at age 45 and that their new research, despite its clarification about carcinoid tumors, provides evidence for that.
“There are a number of other studies that support screening at age 45 as well,” he said. “This [new] finding supports the presence of a large preclinical colorectal cancer case burden in patients in their 40s that is ultimately uncovered with screening initiation at age 50. Many of these cancers could be prevented or diagnosed at an earlier stage with screening at age 45.”
“This is the first study to analyze early-onset colorectal cancer by specific histologic subtype,” Dr. Karlitz also pointed out.
“Although colorectal carcinoids are increasing at a faster rate than adenocarcinomas, adenocarcinomas constitute the overwhelming majority of colorectal cancers in people in their 40s and are also steadily increasing, which has implications for beginning screening at age 45,” he said.
Adenocarcinomas also make up the “overwhelming majority” of colorectal cancers in patients under 50 overall and “are the main driving force behind the increased colorectal cancer burden we are seeing in young patients,” Dr. Karlitz added.
Furthermore, “modeling studies on which the USPSTF screening recommendations were based [which recommended starting at age 45] were confined to adenocarcinoma, thus excluding carcinoids from their analysis,” he said.
Steepest changes in adenocarcinomas in younger groups
In their study, Dr. Karlitz and colleagues assessed the incidence rates of early colorectal cancer, using SEER data from 2000 to 2016, and stratifying the data by histologic subtype (primarily adenocarcinoma and carcinoid tumors), age group (20-29, 30-39, 40-49, and 50-54 years), and subsite.
A total of 123,143 CRC cases were identified in 119,624 patients between the ages of 20-54 years during that time period.
The absolute incidence rates in the younger age groups (20-29 and 30-39 years) were very low, compared with those aged 40-49 and 50-54 years.
The greatest 3-year average annual incident rate changes in adenocarcinoma (2000-2002 vs. 2014-2016) for any age group or subsite were for rectal-only cases in the 20-29 years group (+39%), as well as rectal-only cases in those aged 30-39 years (+39%), and colon-only cases in the age 30-39 group (+20%).
There was also significant increase in rectal-only adenocarcinoma in individuals aged 50-54 years (+10%). A statistically significant increase in the annual percentage change for adenocarcinomas was observed for all age groups, except for colon-only cases in the 20-29 years group (0.7%) and for both colorectal (0.2%) and colon-only cases (–0.1%) in those aged 50-54 years.
Even though the absolute carcinoid tumor incidence rates were lower than for adenocarcinoma in all age groups and subsites, a statistically significant increase was observed in the 3-year average annual incidence rate of combined-site colorectal carcinoid tumors in all age groups from 2000–2002 and 2014–2016. This increase was largely the result of increases in rectal carcinoid tumors, the authors note.
The authors also highlighted the results in the 40- to 49-year age group “because of differing opinions on whether to begin average-risk screening at age 45 or 50 years.”
They reported that rates of rectal and colon adenocarcinoma are increasing “substantially,” whether measured by changes in 3-year average annual incidence rate or by annual percentage changes. The change in average annual incidence rate of colon-only adenocarcinoma for persons aged 40-49 years was 13% (12.21 to 13.85 per 100,000), and that of rectal adenocarcinoma was 16% (7.50 to 8.72 per 100,000). Corresponding annual percentage changes were 0.8% and 1.2%, respectively. “These significant increases in adenocarcinoma incident rates add to the debate over earlier screening at age 45 years,” they commented.
Calls for next steps
The editorialists emphasize restraint when promoting the benefits of colorectal screening for persons younger than 50 years.
They point out that the USPSTF released a provisional update of its CRC screening recommendations about lowering the age to initiate screening to 45 years, as reported by this news organization.
“No new empirical evidence has been found since the USPSTF update in 2016 to inform the effectiveness of screening in persons younger than 50 years,” they write, adding that similar to the American Cancer Society in 2018, the task force has relied exclusively on modeling studies.
This new data from Dr. Karlitz and colleagues “should prompt the modelers to recalculate their estimates of benefits and harms of screening,” they suggested. “Revisiting the model would also allow competing forms of CRC screening to be compared in light of new risk assumptions.
“Previous assumptions that screening tests are equally effective in younger and older patients and that screening adherence will approach 100% may also be reconsidered,” the editorialist commented.
The study authors concluded somewhat differently.
“In conclusion, adenocarcinoma rates increased in many early-onset subgroups but showed no significant increase in others, including colon-only cases in persons aged 20-29 and 50-54 years,” the investigators wrote.
They also observed that “rectal carcinoid tumors are increasing in young patients and may have a substantial impact on overall CRC incident rates.”
Those findings on rectal carcinoid tumors “underscore the importance of assessing histologic CRC subtypes independently,” the researchers said.
This new approach, of which the current study is a first effort, “may lead to a better understanding of the drivers of temporal changes in overall CRC incidence and a more accurate measurement of the outcomes of adenocarcinoma risk reduction efforts, and can guide future research.”
The study had no outside funding. Dr. Karlitz reported personal fees from Exact Sciences, personal fees from Myriad Genetics, and other fees from Gastro Girl and GI OnDEMAND, outside the submitted work. Dr. Bretthauer reports grants from Norwegian Research Council, grants from Norwegian Cancer Society for research in colorectal cancer screening. Dr. Weinberg and Dr. Kalager have disclosed no relevant financial relationships.
Help your patients understand colorectal cancer prevention and screening options by sharing AGA’s patient education from the GI Patient Center: www.gastro.org/CRC.
A version of this article first appeared on Medscape.com.
Implications for CRC screening.
Implications for CRC screening.
New estimates for the risk of CRC in young adults, which differentiate colorectal adenocarcinoma from other types, are reported in a study published Dec. 15, 2020, in Annals of Internal Medicine.
They are important because this finding has implications for CRC screening, say a trio of experts in an accompanying editorial.
Reports of an increase in the incidence of CRC in younger adults have led to changes in screening for this cancer in the United States. The age for starting CRC screening has been lowered to 45 years (instead of 50 years) in recommendations issued in 2018 by the American Cancer Society, and also more recently in preliminary recommendations from the U.S. Preventive Services Task Force.
However, that 2018 ACS recommendation to lower the starting age to 45 years was based to a large extent on a report of a higher incidence of CRC in younger adults from a 2017 study that used the SEER (Surveillance, Epidemiology, and End Results) database).
But that SEER-based study considered “colorectal cancer” as a homogeneous group defined by topology, the editorialists pointed out.
The new study, the editorialists said, uses that same SEER database but has “disentangled colorectal adenocarcinoma, the target for screening, from other histologic CRC types, including neuroendocrine (carcinoid) tumors, for which screening is not recommended.”
The study authors explained that adenocarcinoma is a target for prevention through screening because it arises from precancerous polyps. Those growths can be detected and removed before cancer develops. That doesn’t apply to carcinoid tumors, which are frequently incidental findings on flexible sigmoidoscopy or colonoscopy.
These carcinoid tumors typically are indolent, with a better prognosis than most other cancer types, the editorialists added. “Most likely, the majority of carcinoid tumors identified by screening represent incidental findings with little health benefit from detection. In fact, many may be characterized as overdiagnosed tumors, which by definition increase the burden and harms of screening without the balance of additional benefit.”
This new analysis showed that 4%-20% of the lesions previously described as CRC were not adenocarcinoma but carcinoid tumors, the editorialists pointed out.
This figure rose even higher in the subgroup of findings pertaining to the rectum, the colonic segment with the largest reported increase in early-onset CRC. Here, up to 34% of lesions (depending on patient age) were carcinoid tumors rather than adenocarcinoma, they noted.
The three editorialists – Michael Bretthauer, MD, PhD, and Mette Kalager, MD, PhD, both of the University of Oslo, and David Weinberg, MD, MSc, of Fox Chase Cancer Center, Philadelphia – call for action based on the new findings.
“The ACS’s 2018 estimate of about 7,000 new CRC cases among persons aged 45-49 years in the United States (the justification for screening) needs to be adjusted downward on the basis of the new evidence,” the trio wrote.
They conclude that “caution is warranted when promoting the benefits of CRC screening for persons younger than 50 years.”
However, the senior author of the new study, Jordan Karlitz, MD, of Tulane University, New Orleans, strongly disagreed.
Contrary to the editorialists, Dr. Karlitz said in an interview that he and his colleagues firmly believe that colorectal cancer screening for average-risk patients should begin at age 45 and that their new research, despite its clarification about carcinoid tumors, provides evidence for that.
“There are a number of other studies that support screening at age 45 as well,” he said. “This [new] finding supports the presence of a large preclinical colorectal cancer case burden in patients in their 40s that is ultimately uncovered with screening initiation at age 50. Many of these cancers could be prevented or diagnosed at an earlier stage with screening at age 45.”
“This is the first study to analyze early-onset colorectal cancer by specific histologic subtype,” Dr. Karlitz also pointed out.
“Although colorectal carcinoids are increasing at a faster rate than adenocarcinomas, adenocarcinomas constitute the overwhelming majority of colorectal cancers in people in their 40s and are also steadily increasing, which has implications for beginning screening at age 45,” he said.
Adenocarcinomas also make up the “overwhelming majority” of colorectal cancers in patients under 50 overall and “are the main driving force behind the increased colorectal cancer burden we are seeing in young patients,” Dr. Karlitz added.
Furthermore, “modeling studies on which the USPSTF screening recommendations were based [which recommended starting at age 45] were confined to adenocarcinoma, thus excluding carcinoids from their analysis,” he said.
Steepest changes in adenocarcinomas in younger groups
In their study, Dr. Karlitz and colleagues assessed the incidence rates of early colorectal cancer, using SEER data from 2000 to 2016, and stratifying the data by histologic subtype (primarily adenocarcinoma and carcinoid tumors), age group (20-29, 30-39, 40-49, and 50-54 years), and subsite.
A total of 123,143 CRC cases were identified in 119,624 patients between the ages of 20-54 years during that time period.
The absolute incidence rates in the younger age groups (20-29 and 30-39 years) were very low, compared with those aged 40-49 and 50-54 years.
The greatest 3-year average annual incident rate changes in adenocarcinoma (2000-2002 vs. 2014-2016) for any age group or subsite were for rectal-only cases in the 20-29 years group (+39%), as well as rectal-only cases in those aged 30-39 years (+39%), and colon-only cases in the age 30-39 group (+20%).
There was also significant increase in rectal-only adenocarcinoma in individuals aged 50-54 years (+10%). A statistically significant increase in the annual percentage change for adenocarcinomas was observed for all age groups, except for colon-only cases in the 20-29 years group (0.7%) and for both colorectal (0.2%) and colon-only cases (–0.1%) in those aged 50-54 years.
Even though the absolute carcinoid tumor incidence rates were lower than for adenocarcinoma in all age groups and subsites, a statistically significant increase was observed in the 3-year average annual incidence rate of combined-site colorectal carcinoid tumors in all age groups from 2000–2002 and 2014–2016. This increase was largely the result of increases in rectal carcinoid tumors, the authors note.
The authors also highlighted the results in the 40- to 49-year age group “because of differing opinions on whether to begin average-risk screening at age 45 or 50 years.”
They reported that rates of rectal and colon adenocarcinoma are increasing “substantially,” whether measured by changes in 3-year average annual incidence rate or by annual percentage changes. The change in average annual incidence rate of colon-only adenocarcinoma for persons aged 40-49 years was 13% (12.21 to 13.85 per 100,000), and that of rectal adenocarcinoma was 16% (7.50 to 8.72 per 100,000). Corresponding annual percentage changes were 0.8% and 1.2%, respectively. “These significant increases in adenocarcinoma incident rates add to the debate over earlier screening at age 45 years,” they commented.
Calls for next steps
The editorialists emphasize restraint when promoting the benefits of colorectal screening for persons younger than 50 years.
They point out that the USPSTF released a provisional update of its CRC screening recommendations about lowering the age to initiate screening to 45 years, as reported by this news organization.
“No new empirical evidence has been found since the USPSTF update in 2016 to inform the effectiveness of screening in persons younger than 50 years,” they write, adding that similar to the American Cancer Society in 2018, the task force has relied exclusively on modeling studies.
This new data from Dr. Karlitz and colleagues “should prompt the modelers to recalculate their estimates of benefits and harms of screening,” they suggested. “Revisiting the model would also allow competing forms of CRC screening to be compared in light of new risk assumptions.
“Previous assumptions that screening tests are equally effective in younger and older patients and that screening adherence will approach 100% may also be reconsidered,” the editorialist commented.
The study authors concluded somewhat differently.
“In conclusion, adenocarcinoma rates increased in many early-onset subgroups but showed no significant increase in others, including colon-only cases in persons aged 20-29 and 50-54 years,” the investigators wrote.
They also observed that “rectal carcinoid tumors are increasing in young patients and may have a substantial impact on overall CRC incident rates.”
Those findings on rectal carcinoid tumors “underscore the importance of assessing histologic CRC subtypes independently,” the researchers said.
This new approach, of which the current study is a first effort, “may lead to a better understanding of the drivers of temporal changes in overall CRC incidence and a more accurate measurement of the outcomes of adenocarcinoma risk reduction efforts, and can guide future research.”
The study had no outside funding. Dr. Karlitz reported personal fees from Exact Sciences, personal fees from Myriad Genetics, and other fees from Gastro Girl and GI OnDEMAND, outside the submitted work. Dr. Bretthauer reports grants from Norwegian Research Council, grants from Norwegian Cancer Society for research in colorectal cancer screening. Dr. Weinberg and Dr. Kalager have disclosed no relevant financial relationships.
Help your patients understand colorectal cancer prevention and screening options by sharing AGA’s patient education from the GI Patient Center: www.gastro.org/CRC.
A version of this article first appeared on Medscape.com.
New estimates for the risk of CRC in young adults, which differentiate colorectal adenocarcinoma from other types, are reported in a study published Dec. 15, 2020, in Annals of Internal Medicine.
They are important because this finding has implications for CRC screening, say a trio of experts in an accompanying editorial.
Reports of an increase in the incidence of CRC in younger adults have led to changes in screening for this cancer in the United States. The age for starting CRC screening has been lowered to 45 years (instead of 50 years) in recommendations issued in 2018 by the American Cancer Society, and also more recently in preliminary recommendations from the U.S. Preventive Services Task Force.
However, that 2018 ACS recommendation to lower the starting age to 45 years was based to a large extent on a report of a higher incidence of CRC in younger adults from a 2017 study that used the SEER (Surveillance, Epidemiology, and End Results) database).
But that SEER-based study considered “colorectal cancer” as a homogeneous group defined by topology, the editorialists pointed out.
The new study, the editorialists said, uses that same SEER database but has “disentangled colorectal adenocarcinoma, the target for screening, from other histologic CRC types, including neuroendocrine (carcinoid) tumors, for which screening is not recommended.”
The study authors explained that adenocarcinoma is a target for prevention through screening because it arises from precancerous polyps. Those growths can be detected and removed before cancer develops. That doesn’t apply to carcinoid tumors, which are frequently incidental findings on flexible sigmoidoscopy or colonoscopy.
These carcinoid tumors typically are indolent, with a better prognosis than most other cancer types, the editorialists added. “Most likely, the majority of carcinoid tumors identified by screening represent incidental findings with little health benefit from detection. In fact, many may be characterized as overdiagnosed tumors, which by definition increase the burden and harms of screening without the balance of additional benefit.”
This new analysis showed that 4%-20% of the lesions previously described as CRC were not adenocarcinoma but carcinoid tumors, the editorialists pointed out.
This figure rose even higher in the subgroup of findings pertaining to the rectum, the colonic segment with the largest reported increase in early-onset CRC. Here, up to 34% of lesions (depending on patient age) were carcinoid tumors rather than adenocarcinoma, they noted.
The three editorialists – Michael Bretthauer, MD, PhD, and Mette Kalager, MD, PhD, both of the University of Oslo, and David Weinberg, MD, MSc, of Fox Chase Cancer Center, Philadelphia – call for action based on the new findings.
“The ACS’s 2018 estimate of about 7,000 new CRC cases among persons aged 45-49 years in the United States (the justification for screening) needs to be adjusted downward on the basis of the new evidence,” the trio wrote.
They conclude that “caution is warranted when promoting the benefits of CRC screening for persons younger than 50 years.”
However, the senior author of the new study, Jordan Karlitz, MD, of Tulane University, New Orleans, strongly disagreed.
Contrary to the editorialists, Dr. Karlitz said in an interview that he and his colleagues firmly believe that colorectal cancer screening for average-risk patients should begin at age 45 and that their new research, despite its clarification about carcinoid tumors, provides evidence for that.
“There are a number of other studies that support screening at age 45 as well,” he said. “This [new] finding supports the presence of a large preclinical colorectal cancer case burden in patients in their 40s that is ultimately uncovered with screening initiation at age 50. Many of these cancers could be prevented or diagnosed at an earlier stage with screening at age 45.”
“This is the first study to analyze early-onset colorectal cancer by specific histologic subtype,” Dr. Karlitz also pointed out.
“Although colorectal carcinoids are increasing at a faster rate than adenocarcinomas, adenocarcinomas constitute the overwhelming majority of colorectal cancers in people in their 40s and are also steadily increasing, which has implications for beginning screening at age 45,” he said.
Adenocarcinomas also make up the “overwhelming majority” of colorectal cancers in patients under 50 overall and “are the main driving force behind the increased colorectal cancer burden we are seeing in young patients,” Dr. Karlitz added.
Furthermore, “modeling studies on which the USPSTF screening recommendations were based [which recommended starting at age 45] were confined to adenocarcinoma, thus excluding carcinoids from their analysis,” he said.
Steepest changes in adenocarcinomas in younger groups
In their study, Dr. Karlitz and colleagues assessed the incidence rates of early colorectal cancer, using SEER data from 2000 to 2016, and stratifying the data by histologic subtype (primarily adenocarcinoma and carcinoid tumors), age group (20-29, 30-39, 40-49, and 50-54 years), and subsite.
A total of 123,143 CRC cases were identified in 119,624 patients between the ages of 20-54 years during that time period.
The absolute incidence rates in the younger age groups (20-29 and 30-39 years) were very low, compared with those aged 40-49 and 50-54 years.
The greatest 3-year average annual incident rate changes in adenocarcinoma (2000-2002 vs. 2014-2016) for any age group or subsite were for rectal-only cases in the 20-29 years group (+39%), as well as rectal-only cases in those aged 30-39 years (+39%), and colon-only cases in the age 30-39 group (+20%).
There was also significant increase in rectal-only adenocarcinoma in individuals aged 50-54 years (+10%). A statistically significant increase in the annual percentage change for adenocarcinomas was observed for all age groups, except for colon-only cases in the 20-29 years group (0.7%) and for both colorectal (0.2%) and colon-only cases (–0.1%) in those aged 50-54 years.
Even though the absolute carcinoid tumor incidence rates were lower than for adenocarcinoma in all age groups and subsites, a statistically significant increase was observed in the 3-year average annual incidence rate of combined-site colorectal carcinoid tumors in all age groups from 2000–2002 and 2014–2016. This increase was largely the result of increases in rectal carcinoid tumors, the authors note.
The authors also highlighted the results in the 40- to 49-year age group “because of differing opinions on whether to begin average-risk screening at age 45 or 50 years.”
They reported that rates of rectal and colon adenocarcinoma are increasing “substantially,” whether measured by changes in 3-year average annual incidence rate or by annual percentage changes. The change in average annual incidence rate of colon-only adenocarcinoma for persons aged 40-49 years was 13% (12.21 to 13.85 per 100,000), and that of rectal adenocarcinoma was 16% (7.50 to 8.72 per 100,000). Corresponding annual percentage changes were 0.8% and 1.2%, respectively. “These significant increases in adenocarcinoma incident rates add to the debate over earlier screening at age 45 years,” they commented.
Calls for next steps
The editorialists emphasize restraint when promoting the benefits of colorectal screening for persons younger than 50 years.
They point out that the USPSTF released a provisional update of its CRC screening recommendations about lowering the age to initiate screening to 45 years, as reported by this news organization.
“No new empirical evidence has been found since the USPSTF update in 2016 to inform the effectiveness of screening in persons younger than 50 years,” they write, adding that similar to the American Cancer Society in 2018, the task force has relied exclusively on modeling studies.
This new data from Dr. Karlitz and colleagues “should prompt the modelers to recalculate their estimates of benefits and harms of screening,” they suggested. “Revisiting the model would also allow competing forms of CRC screening to be compared in light of new risk assumptions.
“Previous assumptions that screening tests are equally effective in younger and older patients and that screening adherence will approach 100% may also be reconsidered,” the editorialist commented.
The study authors concluded somewhat differently.
“In conclusion, adenocarcinoma rates increased in many early-onset subgroups but showed no significant increase in others, including colon-only cases in persons aged 20-29 and 50-54 years,” the investigators wrote.
They also observed that “rectal carcinoid tumors are increasing in young patients and may have a substantial impact on overall CRC incident rates.”
Those findings on rectal carcinoid tumors “underscore the importance of assessing histologic CRC subtypes independently,” the researchers said.
This new approach, of which the current study is a first effort, “may lead to a better understanding of the drivers of temporal changes in overall CRC incidence and a more accurate measurement of the outcomes of adenocarcinoma risk reduction efforts, and can guide future research.”
The study had no outside funding. Dr. Karlitz reported personal fees from Exact Sciences, personal fees from Myriad Genetics, and other fees from Gastro Girl and GI OnDEMAND, outside the submitted work. Dr. Bretthauer reports grants from Norwegian Research Council, grants from Norwegian Cancer Society for research in colorectal cancer screening. Dr. Weinberg and Dr. Kalager have disclosed no relevant financial relationships.
Help your patients understand colorectal cancer prevention and screening options by sharing AGA’s patient education from the GI Patient Center: www.gastro.org/CRC.
A version of this article first appeared on Medscape.com.
VA Ramps up Vaccinations as COVID-19 Cases Continue to Rise
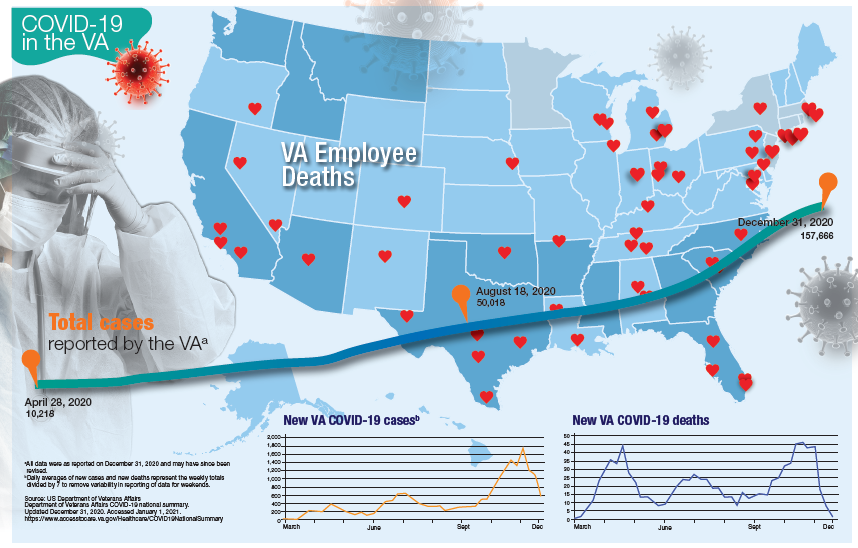
Updated January 12, 2020
More than 181,000 veterans have contracted the COVID-19 virus and 7,385 have died, according to data released by the US Department of Veterans Affairs (VA) on January 12, 2020. The number of cases and deaths have increased sharply since November 2020. The VA also reports that it has administered at least 1 dose of the 2 approved vaccines to 33,875 veterans and 174,724 employees as of January 6.
Currently, the VA reports nearly 19,000 active cases of COVID-19, including 1,270 among VA employees. One hundred five VA employees have died from COVID-19.
Although facilities across the country are facing increased pressure as the number of cases rise, those in Southern California and Texas are reporting significant infection rates. Thirteen facilities have at least 300 active cases, including facilities in Loma Linda (418), Long Beach (381), Greater Los Angeles (361), and San Diego (274), all in California. In Texas, San Antonio (394), Dallas (370), Temple (338), and Houston (328) have all seen large numbers of active cases. Facilities in Columbia, South Carolina (420); Phoenix (407); Atlanta, Georgia (359); Cleveland, Ohio (352); and Orlando, (341) and Gainesville, Florida (340) also have reported significant numbers of cases.
While early on in the pandemic facilities in New York and New Jersey had reported the largest number of deaths, now nearly every facility has reported at least 1 death. Fourteen facilities have reported at least 100 deaths and 53 have reported between 50 and 99 deaths. The 7,385 VA COVID-19 deaths represent 2.0% of the 375,300 deaths reported in the US by Johns Hopkins University. VA has reported 0.8% of the total number of COVID-19 cases.
The VA also reports the demographic breakdown of its COVID-19 cases. Among the active cases, 56.9% are White, 18.3% Black, 9.4% Hispanic, and 1.4% Native American, Alaska Native, or Pacific Islander.

Updated January 12, 2020
More than 181,000 veterans have contracted the COVID-19 virus and 7,385 have died, according to data released by the US Department of Veterans Affairs (VA) on January 12, 2020. The number of cases and deaths have increased sharply since November 2020. The VA also reports that it has administered at least 1 dose of the 2 approved vaccines to 33,875 veterans and 174,724 employees as of January 6.
Currently, the VA reports nearly 19,000 active cases of COVID-19, including 1,270 among VA employees. One hundred five VA employees have died from COVID-19.
Although facilities across the country are facing increased pressure as the number of cases rise, those in Southern California and Texas are reporting significant infection rates. Thirteen facilities have at least 300 active cases, including facilities in Loma Linda (418), Long Beach (381), Greater Los Angeles (361), and San Diego (274), all in California. In Texas, San Antonio (394), Dallas (370), Temple (338), and Houston (328) have all seen large numbers of active cases. Facilities in Columbia, South Carolina (420); Phoenix (407); Atlanta, Georgia (359); Cleveland, Ohio (352); and Orlando, (341) and Gainesville, Florida (340) also have reported significant numbers of cases.
While early on in the pandemic facilities in New York and New Jersey had reported the largest number of deaths, now nearly every facility has reported at least 1 death. Fourteen facilities have reported at least 100 deaths and 53 have reported between 50 and 99 deaths. The 7,385 VA COVID-19 deaths represent 2.0% of the 375,300 deaths reported in the US by Johns Hopkins University. VA has reported 0.8% of the total number of COVID-19 cases.
The VA also reports the demographic breakdown of its COVID-19 cases. Among the active cases, 56.9% are White, 18.3% Black, 9.4% Hispanic, and 1.4% Native American, Alaska Native, or Pacific Islander.

Updated January 12, 2020
More than 181,000 veterans have contracted the COVID-19 virus and 7,385 have died, according to data released by the US Department of Veterans Affairs (VA) on January 12, 2020. The number of cases and deaths have increased sharply since November 2020. The VA also reports that it has administered at least 1 dose of the 2 approved vaccines to 33,875 veterans and 174,724 employees as of January 6.
Currently, the VA reports nearly 19,000 active cases of COVID-19, including 1,270 among VA employees. One hundred five VA employees have died from COVID-19.
Although facilities across the country are facing increased pressure as the number of cases rise, those in Southern California and Texas are reporting significant infection rates. Thirteen facilities have at least 300 active cases, including facilities in Loma Linda (418), Long Beach (381), Greater Los Angeles (361), and San Diego (274), all in California. In Texas, San Antonio (394), Dallas (370), Temple (338), and Houston (328) have all seen large numbers of active cases. Facilities in Columbia, South Carolina (420); Phoenix (407); Atlanta, Georgia (359); Cleveland, Ohio (352); and Orlando, (341) and Gainesville, Florida (340) also have reported significant numbers of cases.
While early on in the pandemic facilities in New York and New Jersey had reported the largest number of deaths, now nearly every facility has reported at least 1 death. Fourteen facilities have reported at least 100 deaths and 53 have reported between 50 and 99 deaths. The 7,385 VA COVID-19 deaths represent 2.0% of the 375,300 deaths reported in the US by Johns Hopkins University. VA has reported 0.8% of the total number of COVID-19 cases.
The VA also reports the demographic breakdown of its COVID-19 cases. Among the active cases, 56.9% are White, 18.3% Black, 9.4% Hispanic, and 1.4% Native American, Alaska Native, or Pacific Islander.
Deucravacitinib offers biologic-like psoriasis efficacy in oral form
and a range of other chronic inflammatory diseases, Bruce E. Strober, MD, PhD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Deucravacitinib solely blocks tyrosine kinase 2 (TYK2) signaling without touching Janus kinase (JAK) 1, 2, or 3. In so doing, it inhibits several cytokines important for inflammation: interleukin-12, IL-13, and interferon-alpha and -beta. Yet it doesn’t affect the numerous pathways mediated by JAKs 1-3, many of which relate to growth and development of cell lineages, including production of erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor, prolactin, growth hormone, and leptin. These deucravacitinib characteristics should translate into fewer off-target side effects than with oral JAK inhibitors.
“The promise of TYK2 inhibition that’s brought to you by deucravacitinib is there will be no laboratory monitoring and the effects will be narrow in blocking inflammation,” said Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and in private practice in Cromwell, Conn.
He highlighted the positive results of a randomized, phase 2, dose-ranging study conducted in 267 patients with moderate or severe plaque psoriasis. Participants had an average baseline Psoriasis Area and Severity Index (PASI) score of 19, with a Dermatology Life Quality Index score of about 12. At the top dose of 12 mg once daily, 75% of patients achieved a PASI 75 response at week 12, and 44% reached a PASI 90, as did 69% and 44%, respectively, who were on deucravacitinib at 3 mg twice daily. Those are collective efficacy numbers similar to adalimumab (Humira) or ustekinumab (Stelara).
Deucravacitinib may provide efficacy “like one of our second-tier biological therapies, yet it will be oral,” Dr. Strober commented.
Importantly, no laboratory abnormalities were detected in this trial. Only mild side effects were documented, most prominently acne, which occurred in dose-dependent fashion in 2% of patients on 3 mg of deucravacitinib twice daily and 4% at 12 mg once daily.
“The treatment of the acne that is elicited by this drug is yet to be fully described, but I’m sure we’ll learn the best approaches, given that acne is in our wheel house,” the dermatologist added.
Bristol-Myers Squibb has announced positive results from the pivotal phase 3 POETYK PSO-1 trial. Deucravacitinib at 6 mg once daily met both of its coprimary efficacy endpoints in the study, which included 666 patients with moderate to severe psoriasis. The TYK 2 inhibitor demonstrated superiority to both placebo and oral apremilast (Otezla) at week 16. The company said the safety profile was consistent with the phase 2 results, and that the full details of the phase 3 trial will be presented next year at a major medical meeting.
In addition, positive phase 2 results were reported for deucravacitinib in the treatment of psoriatic arthritis in a randomized trial presented at the fall 2020 meeting of the American College of Rheumatology. Deucravacitinib is also under study for lupus and inflammatory bowel disease.
Dr. Strober, an active clinical trialist, reported serving as a consultant to more than two dozen pharmaceutical companies, including Bristol-Myers Squibb.
MedscapeLive and this news organization are owned by the same parent company.
and a range of other chronic inflammatory diseases, Bruce E. Strober, MD, PhD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Deucravacitinib solely blocks tyrosine kinase 2 (TYK2) signaling without touching Janus kinase (JAK) 1, 2, or 3. In so doing, it inhibits several cytokines important for inflammation: interleukin-12, IL-13, and interferon-alpha and -beta. Yet it doesn’t affect the numerous pathways mediated by JAKs 1-3, many of which relate to growth and development of cell lineages, including production of erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor, prolactin, growth hormone, and leptin. These deucravacitinib characteristics should translate into fewer off-target side effects than with oral JAK inhibitors.
“The promise of TYK2 inhibition that’s brought to you by deucravacitinib is there will be no laboratory monitoring and the effects will be narrow in blocking inflammation,” said Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and in private practice in Cromwell, Conn.
He highlighted the positive results of a randomized, phase 2, dose-ranging study conducted in 267 patients with moderate or severe plaque psoriasis. Participants had an average baseline Psoriasis Area and Severity Index (PASI) score of 19, with a Dermatology Life Quality Index score of about 12. At the top dose of 12 mg once daily, 75% of patients achieved a PASI 75 response at week 12, and 44% reached a PASI 90, as did 69% and 44%, respectively, who were on deucravacitinib at 3 mg twice daily. Those are collective efficacy numbers similar to adalimumab (Humira) or ustekinumab (Stelara).
Deucravacitinib may provide efficacy “like one of our second-tier biological therapies, yet it will be oral,” Dr. Strober commented.
Importantly, no laboratory abnormalities were detected in this trial. Only mild side effects were documented, most prominently acne, which occurred in dose-dependent fashion in 2% of patients on 3 mg of deucravacitinib twice daily and 4% at 12 mg once daily.
“The treatment of the acne that is elicited by this drug is yet to be fully described, but I’m sure we’ll learn the best approaches, given that acne is in our wheel house,” the dermatologist added.
Bristol-Myers Squibb has announced positive results from the pivotal phase 3 POETYK PSO-1 trial. Deucravacitinib at 6 mg once daily met both of its coprimary efficacy endpoints in the study, which included 666 patients with moderate to severe psoriasis. The TYK 2 inhibitor demonstrated superiority to both placebo and oral apremilast (Otezla) at week 16. The company said the safety profile was consistent with the phase 2 results, and that the full details of the phase 3 trial will be presented next year at a major medical meeting.
In addition, positive phase 2 results were reported for deucravacitinib in the treatment of psoriatic arthritis in a randomized trial presented at the fall 2020 meeting of the American College of Rheumatology. Deucravacitinib is also under study for lupus and inflammatory bowel disease.
Dr. Strober, an active clinical trialist, reported serving as a consultant to more than two dozen pharmaceutical companies, including Bristol-Myers Squibb.
MedscapeLive and this news organization are owned by the same parent company.
and a range of other chronic inflammatory diseases, Bruce E. Strober, MD, PhD, said at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year.
Deucravacitinib solely blocks tyrosine kinase 2 (TYK2) signaling without touching Janus kinase (JAK) 1, 2, or 3. In so doing, it inhibits several cytokines important for inflammation: interleukin-12, IL-13, and interferon-alpha and -beta. Yet it doesn’t affect the numerous pathways mediated by JAKs 1-3, many of which relate to growth and development of cell lineages, including production of erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor, prolactin, growth hormone, and leptin. These deucravacitinib characteristics should translate into fewer off-target side effects than with oral JAK inhibitors.
“The promise of TYK2 inhibition that’s brought to you by deucravacitinib is there will be no laboratory monitoring and the effects will be narrow in blocking inflammation,” said Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and in private practice in Cromwell, Conn.
He highlighted the positive results of a randomized, phase 2, dose-ranging study conducted in 267 patients with moderate or severe plaque psoriasis. Participants had an average baseline Psoriasis Area and Severity Index (PASI) score of 19, with a Dermatology Life Quality Index score of about 12. At the top dose of 12 mg once daily, 75% of patients achieved a PASI 75 response at week 12, and 44% reached a PASI 90, as did 69% and 44%, respectively, who were on deucravacitinib at 3 mg twice daily. Those are collective efficacy numbers similar to adalimumab (Humira) or ustekinumab (Stelara).
Deucravacitinib may provide efficacy “like one of our second-tier biological therapies, yet it will be oral,” Dr. Strober commented.
Importantly, no laboratory abnormalities were detected in this trial. Only mild side effects were documented, most prominently acne, which occurred in dose-dependent fashion in 2% of patients on 3 mg of deucravacitinib twice daily and 4% at 12 mg once daily.
“The treatment of the acne that is elicited by this drug is yet to be fully described, but I’m sure we’ll learn the best approaches, given that acne is in our wheel house,” the dermatologist added.
Bristol-Myers Squibb has announced positive results from the pivotal phase 3 POETYK PSO-1 trial. Deucravacitinib at 6 mg once daily met both of its coprimary efficacy endpoints in the study, which included 666 patients with moderate to severe psoriasis. The TYK 2 inhibitor demonstrated superiority to both placebo and oral apremilast (Otezla) at week 16. The company said the safety profile was consistent with the phase 2 results, and that the full details of the phase 3 trial will be presented next year at a major medical meeting.
In addition, positive phase 2 results were reported for deucravacitinib in the treatment of psoriatic arthritis in a randomized trial presented at the fall 2020 meeting of the American College of Rheumatology. Deucravacitinib is also under study for lupus and inflammatory bowel disease.
Dr. Strober, an active clinical trialist, reported serving as a consultant to more than two dozen pharmaceutical companies, including Bristol-Myers Squibb.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Over half of COVID-19 transmission may occur via asymptomatic people
As COVID-19 cases surge and vaccinations lag, health authorities continue to seek additional ways to mitigate the spread of the novel coronavirus.
Now, a modeling study estimates that more than half of transmissions come from pre-, never-, and asymptomatic individuals, indicating that symptom-based screening will have little effect on spread.
The Centers for Disease Control and Prevention study, published online Jan. 7 in JAMA Network Open, concludes that for optimal control, protective measures such as masking and social distancing should be supplemented with strategic testing of potentially exposed but asymptomatic individuals .
“In the absence of effective and widespread use of therapeutics or vaccines that can shorten or eliminate infectivity, successful control of SARS-CoV-2 cannot rely solely on identifying and isolating symptomatic cases; even if implemented effectively, this strategy would be insufficient,” CDC biologist Michael J. Johansson, PhD, and colleagues warn. “Multiple measures that effectively address transmission risk in the absence of symptoms are imperative to control SARS-CoV-2.”
According to the authors, the effectiveness of some current transmission prevention efforts has been disputed and subject to mixed messaging. Therefore, they decided to model the proportion of COVID-19 infections that are likely the result of individuals who show no symptoms and may be unknowingly infecting others.
“Unfortunately, there continues to be some skepticism about the value of community-wide mitigation efforts for preventing transmission such as masking, distancing, and hand hygiene, particularly for people without symptoms,” corresponding author Jay C. Butler, MD, said in an interview. “So we wanted to have a base assumption about how much transmission occurs from asymptomatic people to underscore the importance of mitigation measures and of creating immunity through vaccine delivery.”
Such a yardstick is especially germane in the context of the new, more transmissible variant. “It really puts [things] in a bigger box and underscores, boldfaces, and italicizes the need to change people’s behaviors and the importance of mitigation,” Dr. Butler said. It also highlights the advisability of targeted strategic testing in congregate settings, schools, and universities, which is already underway.
The analysis
Based on data from several COVID-19 studies from last year, the CDC’s analytical model assumes at baseline that infectiousness peaks at the median point of symptom onset, and that 30% of infected individuals never develop symptoms but are nevertheless 75% as infectious as those who develop overt symptoms.
The investigators then model multiple scenarios of transmission based pre- and never-symptomatic individuals, assuming different incubation and infectious periods, and varying numbers of days from point of infection to symptom onset.
When combined, the models predicts that 59% of all transmission would come from asymptomatic transmission – 35% from presymptomatic individuals and 24% from never-symptomatic individuals.
The findings complement those of an earlier CDC analysis, according to the authors.
The overall proportion of transmission from presymptomatic and never-symptomatic individuals is key to identifying mitigation measures that may be able to control SARS-CoV-2, the authors stated.
For example, they explain, if the infection reproduction number (R) in a particular setting is 2.0, a reduction in transmission of at least 50% is needed in order to reduce R to below 1.0. “Given that in some settings R is likely much greater than 2 and more than half of transmissions may come from individuals who are asymptomatic at the time of transmission, effective control must mitigate transmission risk from people without symptoms,” they wrote.
The authors acknowledge that the study applies a simplistic model to a complex and evolving phenomenon, and that the exact proportions of presymptomatic and never-symptomatic transmission and the incubation periods are not known. They also note symptoms and transmissions appear to vary across different population groups, with older individuals more likely than younger persons to experience symptoms, according to previous studies.
“Assume that everyone is potentially infected”
Other experts agree that expanded testing of asymptomatic individuals is important. “Screening for fever and isolation of symptomatic individuals is a common-sense approach to help prevent spread, but these measures are by no means adequate since it’s been clearly documented that individuals who are either asymptomatic or presymptomatic can still spread the virus,” said Brett Williams, MD, an infectious disease specialist and assistant professor of medicine at Rush University in Chicago.
“As we saw with the White House Rose Garden superspreader outbreak, testing does not reliably exclude infection either because the tested individual has not yet become positive or the test is falsely negative,” Dr. Williams, who was not involved in the CDC study, said in an interview. He further noted that when prevalence is as high as it currently is in the United States, the rate of false negatives will be high because a large proportion of those screened will be unknowingly infected.
At his center, all visitors and staff are screened with a temperature probe on entry, and since the earliest days of the pandemic, universal masking has been required. “Nationally there have been many instances of hospital break room outbreaks because of staff eating lunch together, and these outbreaks also demonstrate the incompleteness of symptomatic isolation,” Dr. Williams said.
For his part, virologist Frank Esper, MD, a pediatric infectious disease specialist at the Cleveland Clinic, said that while it’s been understood for some time that many infected people will not exhibit symptoms, “the question that remains is just how infectious are they?”
Dr. Esper’s takeaway from the modeling study is not so much that we need more screening of possibly exposed but asymptomatic people, but rather testing symptomatic people and tracing their contacts is not enough.
“We need to continue to assume that everyone is potentially infected whether they know it or not. And even though we have ramped up our testing to a much greater capacity than in the first wave, we need to continue to wear masks and socially distance because just identifying people who are sick and isolating or quarantining them is not going to be enough to contain the pandemic.”
And although assumption-based modeling is helpful, it cannot tell us “how many asymptomatic people are actually infected,” said Dr. Esper, who was not involved in the CDC study.
Dr. Esper also pointed out that the study estimates are based on data from early Chinese studies, but the virus has since changed. The new, more transmissible strain in the United States and elsewhere may involve not only more infections but also a longer presymptomatic stage. “So the CDC study may actually undershoot asymptomatic infections,” he said.
He also agreed with the authors that when it comes to infection, not all humans are equal. “Older people tend to be more symptomatic and become symptomatic more quickly so the asymptomatic rate is not the same across board from young people age 20 to older people.”
The bottom line, said David. A. Hirschwerk, MD, an infectious disease specialist at Northwell Health in Manhasset, N.Y., is that these data support the maintenance of protective measures we’ve been taking over the past months. “They support the concept that asymptomatic people are a significant source of transmission and that we need to adhere to mask wearing and social distancing, particularly indoors,” Dr. Hirschwerk, who was not involved in the analysis, said in an interview. “More testing would be better but it has to be fast and it has to be efficient, and there are a lot of challenges to overcome.”
The study was done as part of the CDC’s coronavirus disease 2019 response and was supported solely by federal base and response funding. The authors and commentators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As COVID-19 cases surge and vaccinations lag, health authorities continue to seek additional ways to mitigate the spread of the novel coronavirus.
Now, a modeling study estimates that more than half of transmissions come from pre-, never-, and asymptomatic individuals, indicating that symptom-based screening will have little effect on spread.
The Centers for Disease Control and Prevention study, published online Jan. 7 in JAMA Network Open, concludes that for optimal control, protective measures such as masking and social distancing should be supplemented with strategic testing of potentially exposed but asymptomatic individuals .
“In the absence of effective and widespread use of therapeutics or vaccines that can shorten or eliminate infectivity, successful control of SARS-CoV-2 cannot rely solely on identifying and isolating symptomatic cases; even if implemented effectively, this strategy would be insufficient,” CDC biologist Michael J. Johansson, PhD, and colleagues warn. “Multiple measures that effectively address transmission risk in the absence of symptoms are imperative to control SARS-CoV-2.”
According to the authors, the effectiveness of some current transmission prevention efforts has been disputed and subject to mixed messaging. Therefore, they decided to model the proportion of COVID-19 infections that are likely the result of individuals who show no symptoms and may be unknowingly infecting others.
“Unfortunately, there continues to be some skepticism about the value of community-wide mitigation efforts for preventing transmission such as masking, distancing, and hand hygiene, particularly for people without symptoms,” corresponding author Jay C. Butler, MD, said in an interview. “So we wanted to have a base assumption about how much transmission occurs from asymptomatic people to underscore the importance of mitigation measures and of creating immunity through vaccine delivery.”
Such a yardstick is especially germane in the context of the new, more transmissible variant. “It really puts [things] in a bigger box and underscores, boldfaces, and italicizes the need to change people’s behaviors and the importance of mitigation,” Dr. Butler said. It also highlights the advisability of targeted strategic testing in congregate settings, schools, and universities, which is already underway.
The analysis
Based on data from several COVID-19 studies from last year, the CDC’s analytical model assumes at baseline that infectiousness peaks at the median point of symptom onset, and that 30% of infected individuals never develop symptoms but are nevertheless 75% as infectious as those who develop overt symptoms.
The investigators then model multiple scenarios of transmission based pre- and never-symptomatic individuals, assuming different incubation and infectious periods, and varying numbers of days from point of infection to symptom onset.
When combined, the models predicts that 59% of all transmission would come from asymptomatic transmission – 35% from presymptomatic individuals and 24% from never-symptomatic individuals.
The findings complement those of an earlier CDC analysis, according to the authors.
The overall proportion of transmission from presymptomatic and never-symptomatic individuals is key to identifying mitigation measures that may be able to control SARS-CoV-2, the authors stated.
For example, they explain, if the infection reproduction number (R) in a particular setting is 2.0, a reduction in transmission of at least 50% is needed in order to reduce R to below 1.0. “Given that in some settings R is likely much greater than 2 and more than half of transmissions may come from individuals who are asymptomatic at the time of transmission, effective control must mitigate transmission risk from people without symptoms,” they wrote.
The authors acknowledge that the study applies a simplistic model to a complex and evolving phenomenon, and that the exact proportions of presymptomatic and never-symptomatic transmission and the incubation periods are not known. They also note symptoms and transmissions appear to vary across different population groups, with older individuals more likely than younger persons to experience symptoms, according to previous studies.
“Assume that everyone is potentially infected”
Other experts agree that expanded testing of asymptomatic individuals is important. “Screening for fever and isolation of symptomatic individuals is a common-sense approach to help prevent spread, but these measures are by no means adequate since it’s been clearly documented that individuals who are either asymptomatic or presymptomatic can still spread the virus,” said Brett Williams, MD, an infectious disease specialist and assistant professor of medicine at Rush University in Chicago.
“As we saw with the White House Rose Garden superspreader outbreak, testing does not reliably exclude infection either because the tested individual has not yet become positive or the test is falsely negative,” Dr. Williams, who was not involved in the CDC study, said in an interview. He further noted that when prevalence is as high as it currently is in the United States, the rate of false negatives will be high because a large proportion of those screened will be unknowingly infected.
At his center, all visitors and staff are screened with a temperature probe on entry, and since the earliest days of the pandemic, universal masking has been required. “Nationally there have been many instances of hospital break room outbreaks because of staff eating lunch together, and these outbreaks also demonstrate the incompleteness of symptomatic isolation,” Dr. Williams said.
For his part, virologist Frank Esper, MD, a pediatric infectious disease specialist at the Cleveland Clinic, said that while it’s been understood for some time that many infected people will not exhibit symptoms, “the question that remains is just how infectious are they?”
Dr. Esper’s takeaway from the modeling study is not so much that we need more screening of possibly exposed but asymptomatic people, but rather testing symptomatic people and tracing their contacts is not enough.
“We need to continue to assume that everyone is potentially infected whether they know it or not. And even though we have ramped up our testing to a much greater capacity than in the first wave, we need to continue to wear masks and socially distance because just identifying people who are sick and isolating or quarantining them is not going to be enough to contain the pandemic.”
And although assumption-based modeling is helpful, it cannot tell us “how many asymptomatic people are actually infected,” said Dr. Esper, who was not involved in the CDC study.
Dr. Esper also pointed out that the study estimates are based on data from early Chinese studies, but the virus has since changed. The new, more transmissible strain in the United States and elsewhere may involve not only more infections but also a longer presymptomatic stage. “So the CDC study may actually undershoot asymptomatic infections,” he said.
He also agreed with the authors that when it comes to infection, not all humans are equal. “Older people tend to be more symptomatic and become symptomatic more quickly so the asymptomatic rate is not the same across board from young people age 20 to older people.”
The bottom line, said David. A. Hirschwerk, MD, an infectious disease specialist at Northwell Health in Manhasset, N.Y., is that these data support the maintenance of protective measures we’ve been taking over the past months. “They support the concept that asymptomatic people are a significant source of transmission and that we need to adhere to mask wearing and social distancing, particularly indoors,” Dr. Hirschwerk, who was not involved in the analysis, said in an interview. “More testing would be better but it has to be fast and it has to be efficient, and there are a lot of challenges to overcome.”
The study was done as part of the CDC’s coronavirus disease 2019 response and was supported solely by federal base and response funding. The authors and commentators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As COVID-19 cases surge and vaccinations lag, health authorities continue to seek additional ways to mitigate the spread of the novel coronavirus.
Now, a modeling study estimates that more than half of transmissions come from pre-, never-, and asymptomatic individuals, indicating that symptom-based screening will have little effect on spread.
The Centers for Disease Control and Prevention study, published online Jan. 7 in JAMA Network Open, concludes that for optimal control, protective measures such as masking and social distancing should be supplemented with strategic testing of potentially exposed but asymptomatic individuals .
“In the absence of effective and widespread use of therapeutics or vaccines that can shorten or eliminate infectivity, successful control of SARS-CoV-2 cannot rely solely on identifying and isolating symptomatic cases; even if implemented effectively, this strategy would be insufficient,” CDC biologist Michael J. Johansson, PhD, and colleagues warn. “Multiple measures that effectively address transmission risk in the absence of symptoms are imperative to control SARS-CoV-2.”
According to the authors, the effectiveness of some current transmission prevention efforts has been disputed and subject to mixed messaging. Therefore, they decided to model the proportion of COVID-19 infections that are likely the result of individuals who show no symptoms and may be unknowingly infecting others.
“Unfortunately, there continues to be some skepticism about the value of community-wide mitigation efforts for preventing transmission such as masking, distancing, and hand hygiene, particularly for people without symptoms,” corresponding author Jay C. Butler, MD, said in an interview. “So we wanted to have a base assumption about how much transmission occurs from asymptomatic people to underscore the importance of mitigation measures and of creating immunity through vaccine delivery.”
Such a yardstick is especially germane in the context of the new, more transmissible variant. “It really puts [things] in a bigger box and underscores, boldfaces, and italicizes the need to change people’s behaviors and the importance of mitigation,” Dr. Butler said. It also highlights the advisability of targeted strategic testing in congregate settings, schools, and universities, which is already underway.
The analysis
Based on data from several COVID-19 studies from last year, the CDC’s analytical model assumes at baseline that infectiousness peaks at the median point of symptom onset, and that 30% of infected individuals never develop symptoms but are nevertheless 75% as infectious as those who develop overt symptoms.
The investigators then model multiple scenarios of transmission based pre- and never-symptomatic individuals, assuming different incubation and infectious periods, and varying numbers of days from point of infection to symptom onset.
When combined, the models predicts that 59% of all transmission would come from asymptomatic transmission – 35% from presymptomatic individuals and 24% from never-symptomatic individuals.
The findings complement those of an earlier CDC analysis, according to the authors.
The overall proportion of transmission from presymptomatic and never-symptomatic individuals is key to identifying mitigation measures that may be able to control SARS-CoV-2, the authors stated.
For example, they explain, if the infection reproduction number (R) in a particular setting is 2.0, a reduction in transmission of at least 50% is needed in order to reduce R to below 1.0. “Given that in some settings R is likely much greater than 2 and more than half of transmissions may come from individuals who are asymptomatic at the time of transmission, effective control must mitigate transmission risk from people without symptoms,” they wrote.
The authors acknowledge that the study applies a simplistic model to a complex and evolving phenomenon, and that the exact proportions of presymptomatic and never-symptomatic transmission and the incubation periods are not known. They also note symptoms and transmissions appear to vary across different population groups, with older individuals more likely than younger persons to experience symptoms, according to previous studies.
“Assume that everyone is potentially infected”
Other experts agree that expanded testing of asymptomatic individuals is important. “Screening for fever and isolation of symptomatic individuals is a common-sense approach to help prevent spread, but these measures are by no means adequate since it’s been clearly documented that individuals who are either asymptomatic or presymptomatic can still spread the virus,” said Brett Williams, MD, an infectious disease specialist and assistant professor of medicine at Rush University in Chicago.
“As we saw with the White House Rose Garden superspreader outbreak, testing does not reliably exclude infection either because the tested individual has not yet become positive or the test is falsely negative,” Dr. Williams, who was not involved in the CDC study, said in an interview. He further noted that when prevalence is as high as it currently is in the United States, the rate of false negatives will be high because a large proportion of those screened will be unknowingly infected.
At his center, all visitors and staff are screened with a temperature probe on entry, and since the earliest days of the pandemic, universal masking has been required. “Nationally there have been many instances of hospital break room outbreaks because of staff eating lunch together, and these outbreaks also demonstrate the incompleteness of symptomatic isolation,” Dr. Williams said.
For his part, virologist Frank Esper, MD, a pediatric infectious disease specialist at the Cleveland Clinic, said that while it’s been understood for some time that many infected people will not exhibit symptoms, “the question that remains is just how infectious are they?”
Dr. Esper’s takeaway from the modeling study is not so much that we need more screening of possibly exposed but asymptomatic people, but rather testing symptomatic people and tracing their contacts is not enough.
“We need to continue to assume that everyone is potentially infected whether they know it or not. And even though we have ramped up our testing to a much greater capacity than in the first wave, we need to continue to wear masks and socially distance because just identifying people who are sick and isolating or quarantining them is not going to be enough to contain the pandemic.”
And although assumption-based modeling is helpful, it cannot tell us “how many asymptomatic people are actually infected,” said Dr. Esper, who was not involved in the CDC study.
Dr. Esper also pointed out that the study estimates are based on data from early Chinese studies, but the virus has since changed. The new, more transmissible strain in the United States and elsewhere may involve not only more infections but also a longer presymptomatic stage. “So the CDC study may actually undershoot asymptomatic infections,” he said.
He also agreed with the authors that when it comes to infection, not all humans are equal. “Older people tend to be more symptomatic and become symptomatic more quickly so the asymptomatic rate is not the same across board from young people age 20 to older people.”
The bottom line, said David. A. Hirschwerk, MD, an infectious disease specialist at Northwell Health in Manhasset, N.Y., is that these data support the maintenance of protective measures we’ve been taking over the past months. “They support the concept that asymptomatic people are a significant source of transmission and that we need to adhere to mask wearing and social distancing, particularly indoors,” Dr. Hirschwerk, who was not involved in the analysis, said in an interview. “More testing would be better but it has to be fast and it has to be efficient, and there are a lot of challenges to overcome.”
The study was done as part of the CDC’s coronavirus disease 2019 response and was supported solely by federal base and response funding. The authors and commentators have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Outpatient penicillin allergy testing found safe in pregnancy
Successful outpatient penicillin allergy testing with a low incidence of anaphylaxis during pregnancy demonstrates the feasibility of performing allergy testing in the outpatient setting, reported Nerlyne Desravines, MD, of the University of North Carolina, Chapel Hill, and colleagues.
In a prospective cohort study of 74 pregnant patients with previous self reports of penicillin allergy, Dr. Desravines and colleagues sought to determine the feasibility, acceptability, and safety of performing penicillin allergy testing in an outpatient setting. Patients included in the study were aged 18-55 years with gestational age between 14 and 36 weeks and planned delivery within the University of North Carolina heath care system receiving care between March 2019 and March 2020.
Of the 74 women enrolled to participate, 24 failed to present for testing, including some citing scheduling conflicts or fear of adverse reactions. Only 46 of the remaining 50 successfully completed testing; 4 patients were scheduled for testing but unable to participate because of COVID-19 restrictions.
Insurance status may affect participation in testing
Those who had public insurance were less likely to complete testing; those who completed testing were significantly more likely to be married and carry private insurance.
Fully 52% of the 46 women who completed testing were in the second trimester. The majority (85%) experienced their initial penicillin allergy reaction more than 10 years earlier.
Ultimately, 43 of the 46 women (93%) received a negative test result despite previous self reports of severe allergic reaction. Two of the three confirmed with penicillin allergy failed the 10% oral drug challenge; the other tested positive for penicillin G on intradermal testing. The two women who were found to have severe penicillin allergy experienced coughing, chest tightening, and skin and oropharynx pruritus within 30 minutes after their 10% amoxicillin drug challenge; they also experienced vomiting at 1 and 2 hours post ingestion. Following intramuscular injection of epinephrine, oral cetirizine with periodic vital sign measures, and albuterol updraft in one patient with a history of well controlled asthma, symptom resolution was achieved and both women were discharged without the need for further care.
The systemic reactions observed in just 4% of the study population is lower than normally reported in the general population, suggesting that the study sample size may underestimate the actual prevalence of systemic reactions, the authors noted. “The primary factor in safely conducting allergy testing in pregnancy is an outpatient facility that is appropriately outfitted with trained personnel and medications for possible serious reactions,” they added.
Noteworthy is the allergy testing protocol used by Dr. Desravines and colleagues in this study. Their graded oral drug challenge has not been used in previous studies of outpatient penicillin testing in pregnancy. Two of the three participants with positive test results had penicillin allergy confirmed following reaction to the first step (10% dose) of oral challenge to amoxicillin.
Prevalence of systemic reactions may be higher than expected
The authors cited ease of implementation in an obstetrics or allergy clinic as a strength of the study. One limitation is the observed rate of systemic reaction. The wide confidence interval observed indicates the rates of anaphylaxis may actually be as high as 15%, suggested the authors. The small sample size also limits the safety analysis for rare outcomes such as death.
Patient-reported barriers included time commitment for the testing visit. Rural women or those receiving prenatal care from health departments or community health centers were not able to be enrolled. Only one Spanish-speaking woman participated despite availability of bilingual staff and interpreters.
Such outpatient testing for those at greatest risk offers the opportunity to mitigate emerging drug resistance and should ideally take place preconception or at the time of initial allergic reaction, the authors advised. As emphasized in the latest Committee Opinion issued by the American College of Obstetricians and Gynecologists, obstetricians have a real opportunity to counsel patients preconception and postpartum regarding the benefits of penicillin allergy testing.
In a separate interview, Angela Martin, MD, assistant professor, maternal-fetal medicine, at University of Kansas, Kansas City, noted the large clinical implications of this study given that more than 90% of women undergoing allergy testing following self-reported penicillin allergy had a negative test result. “By performing allergy testing on appropriate candidates, as these authors have done, clinicians can treat infections and implement group B streptococcus prophylaxis with the narrowest spectrum antibiotic. This has potential to combat antibiotic resistance and may protect patients from harms caused by unnecessary broad-spectrum antibiotic use during pregnancy and beyond,” said Dr. Martin.
“It should be mentioned that 2 out of the 46 women tested (4%) had an anaphylactic reaction. This highlights the need to perform allergy testing in a qualified center capable of managing acute anaphylactic reactions should they occur,” she advised.
Dr. Desravines and colleagues, as well as Dr. Martin, had no conflicts of interest and no relevant financial disclosures.
SOURCE: Obstet Gynecol. 2021;137:56-61. doi: 10.1097/AOG.0000000000004213.
Successful outpatient penicillin allergy testing with a low incidence of anaphylaxis during pregnancy demonstrates the feasibility of performing allergy testing in the outpatient setting, reported Nerlyne Desravines, MD, of the University of North Carolina, Chapel Hill, and colleagues.
In a prospective cohort study of 74 pregnant patients with previous self reports of penicillin allergy, Dr. Desravines and colleagues sought to determine the feasibility, acceptability, and safety of performing penicillin allergy testing in an outpatient setting. Patients included in the study were aged 18-55 years with gestational age between 14 and 36 weeks and planned delivery within the University of North Carolina heath care system receiving care between March 2019 and March 2020.
Of the 74 women enrolled to participate, 24 failed to present for testing, including some citing scheduling conflicts or fear of adverse reactions. Only 46 of the remaining 50 successfully completed testing; 4 patients were scheduled for testing but unable to participate because of COVID-19 restrictions.
Insurance status may affect participation in testing
Those who had public insurance were less likely to complete testing; those who completed testing were significantly more likely to be married and carry private insurance.
Fully 52% of the 46 women who completed testing were in the second trimester. The majority (85%) experienced their initial penicillin allergy reaction more than 10 years earlier.
Ultimately, 43 of the 46 women (93%) received a negative test result despite previous self reports of severe allergic reaction. Two of the three confirmed with penicillin allergy failed the 10% oral drug challenge; the other tested positive for penicillin G on intradermal testing. The two women who were found to have severe penicillin allergy experienced coughing, chest tightening, and skin and oropharynx pruritus within 30 minutes after their 10% amoxicillin drug challenge; they also experienced vomiting at 1 and 2 hours post ingestion. Following intramuscular injection of epinephrine, oral cetirizine with periodic vital sign measures, and albuterol updraft in one patient with a history of well controlled asthma, symptom resolution was achieved and both women were discharged without the need for further care.
The systemic reactions observed in just 4% of the study population is lower than normally reported in the general population, suggesting that the study sample size may underestimate the actual prevalence of systemic reactions, the authors noted. “The primary factor in safely conducting allergy testing in pregnancy is an outpatient facility that is appropriately outfitted with trained personnel and medications for possible serious reactions,” they added.
Noteworthy is the allergy testing protocol used by Dr. Desravines and colleagues in this study. Their graded oral drug challenge has not been used in previous studies of outpatient penicillin testing in pregnancy. Two of the three participants with positive test results had penicillin allergy confirmed following reaction to the first step (10% dose) of oral challenge to amoxicillin.
Prevalence of systemic reactions may be higher than expected
The authors cited ease of implementation in an obstetrics or allergy clinic as a strength of the study. One limitation is the observed rate of systemic reaction. The wide confidence interval observed indicates the rates of anaphylaxis may actually be as high as 15%, suggested the authors. The small sample size also limits the safety analysis for rare outcomes such as death.
Patient-reported barriers included time commitment for the testing visit. Rural women or those receiving prenatal care from health departments or community health centers were not able to be enrolled. Only one Spanish-speaking woman participated despite availability of bilingual staff and interpreters.
Such outpatient testing for those at greatest risk offers the opportunity to mitigate emerging drug resistance and should ideally take place preconception or at the time of initial allergic reaction, the authors advised. As emphasized in the latest Committee Opinion issued by the American College of Obstetricians and Gynecologists, obstetricians have a real opportunity to counsel patients preconception and postpartum regarding the benefits of penicillin allergy testing.
In a separate interview, Angela Martin, MD, assistant professor, maternal-fetal medicine, at University of Kansas, Kansas City, noted the large clinical implications of this study given that more than 90% of women undergoing allergy testing following self-reported penicillin allergy had a negative test result. “By performing allergy testing on appropriate candidates, as these authors have done, clinicians can treat infections and implement group B streptococcus prophylaxis with the narrowest spectrum antibiotic. This has potential to combat antibiotic resistance and may protect patients from harms caused by unnecessary broad-spectrum antibiotic use during pregnancy and beyond,” said Dr. Martin.
“It should be mentioned that 2 out of the 46 women tested (4%) had an anaphylactic reaction. This highlights the need to perform allergy testing in a qualified center capable of managing acute anaphylactic reactions should they occur,” she advised.
Dr. Desravines and colleagues, as well as Dr. Martin, had no conflicts of interest and no relevant financial disclosures.
SOURCE: Obstet Gynecol. 2021;137:56-61. doi: 10.1097/AOG.0000000000004213.
Successful outpatient penicillin allergy testing with a low incidence of anaphylaxis during pregnancy demonstrates the feasibility of performing allergy testing in the outpatient setting, reported Nerlyne Desravines, MD, of the University of North Carolina, Chapel Hill, and colleagues.
In a prospective cohort study of 74 pregnant patients with previous self reports of penicillin allergy, Dr. Desravines and colleagues sought to determine the feasibility, acceptability, and safety of performing penicillin allergy testing in an outpatient setting. Patients included in the study were aged 18-55 years with gestational age between 14 and 36 weeks and planned delivery within the University of North Carolina heath care system receiving care between March 2019 and March 2020.
Of the 74 women enrolled to participate, 24 failed to present for testing, including some citing scheduling conflicts or fear of adverse reactions. Only 46 of the remaining 50 successfully completed testing; 4 patients were scheduled for testing but unable to participate because of COVID-19 restrictions.
Insurance status may affect participation in testing
Those who had public insurance were less likely to complete testing; those who completed testing were significantly more likely to be married and carry private insurance.
Fully 52% of the 46 women who completed testing were in the second trimester. The majority (85%) experienced their initial penicillin allergy reaction more than 10 years earlier.
Ultimately, 43 of the 46 women (93%) received a negative test result despite previous self reports of severe allergic reaction. Two of the three confirmed with penicillin allergy failed the 10% oral drug challenge; the other tested positive for penicillin G on intradermal testing. The two women who were found to have severe penicillin allergy experienced coughing, chest tightening, and skin and oropharynx pruritus within 30 minutes after their 10% amoxicillin drug challenge; they also experienced vomiting at 1 and 2 hours post ingestion. Following intramuscular injection of epinephrine, oral cetirizine with periodic vital sign measures, and albuterol updraft in one patient with a history of well controlled asthma, symptom resolution was achieved and both women were discharged without the need for further care.
The systemic reactions observed in just 4% of the study population is lower than normally reported in the general population, suggesting that the study sample size may underestimate the actual prevalence of systemic reactions, the authors noted. “The primary factor in safely conducting allergy testing in pregnancy is an outpatient facility that is appropriately outfitted with trained personnel and medications for possible serious reactions,” they added.
Noteworthy is the allergy testing protocol used by Dr. Desravines and colleagues in this study. Their graded oral drug challenge has not been used in previous studies of outpatient penicillin testing in pregnancy. Two of the three participants with positive test results had penicillin allergy confirmed following reaction to the first step (10% dose) of oral challenge to amoxicillin.
Prevalence of systemic reactions may be higher than expected
The authors cited ease of implementation in an obstetrics or allergy clinic as a strength of the study. One limitation is the observed rate of systemic reaction. The wide confidence interval observed indicates the rates of anaphylaxis may actually be as high as 15%, suggested the authors. The small sample size also limits the safety analysis for rare outcomes such as death.
Patient-reported barriers included time commitment for the testing visit. Rural women or those receiving prenatal care from health departments or community health centers were not able to be enrolled. Only one Spanish-speaking woman participated despite availability of bilingual staff and interpreters.
Such outpatient testing for those at greatest risk offers the opportunity to mitigate emerging drug resistance and should ideally take place preconception or at the time of initial allergic reaction, the authors advised. As emphasized in the latest Committee Opinion issued by the American College of Obstetricians and Gynecologists, obstetricians have a real opportunity to counsel patients preconception and postpartum regarding the benefits of penicillin allergy testing.
In a separate interview, Angela Martin, MD, assistant professor, maternal-fetal medicine, at University of Kansas, Kansas City, noted the large clinical implications of this study given that more than 90% of women undergoing allergy testing following self-reported penicillin allergy had a negative test result. “By performing allergy testing on appropriate candidates, as these authors have done, clinicians can treat infections and implement group B streptococcus prophylaxis with the narrowest spectrum antibiotic. This has potential to combat antibiotic resistance and may protect patients from harms caused by unnecessary broad-spectrum antibiotic use during pregnancy and beyond,” said Dr. Martin.
“It should be mentioned that 2 out of the 46 women tested (4%) had an anaphylactic reaction. This highlights the need to perform allergy testing in a qualified center capable of managing acute anaphylactic reactions should they occur,” she advised.
Dr. Desravines and colleagues, as well as Dr. Martin, had no conflicts of interest and no relevant financial disclosures.
SOURCE: Obstet Gynecol. 2021;137:56-61. doi: 10.1097/AOG.0000000000004213.
FROM OBSTETRICS & GYNECOLOGY
Baseline body surface area may drive optimal baricitinib responses
results from an analysis of phase 3 data showed.
“This proposed clinical tailoring approach for baricitinib 2 mg allows for treatment of patients who are more likely to respond to therapy and rapid decision on discontinuation of treatment for those who are not likely to benefit from baricitinib 2 mg,” Eric L. Simpson, MD, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium.
Baricitinib is an oral, reversible and selective Janus kinase 1/JAK2 inhibitor that is approved in Europe for the treatment of moderate to severe AD in adults who are candidates for systemic therapy. In the United States, it is approved for treating rheumatoid arthritis, and is currently under Food and Drug Administration review in the United States for AD.
For the current analysis, Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, and colleagues set out to identify responders to baricitinib 2 mg using a tailored approach based on baseline BSA affected and early clinical improvement in the phase 3 monotherapy trial BREEZE-AD5. The trial enrolled 440 patients: 147 to placebo, 147 to baricitinib 1 mg once daily, and 146 to baricitinib 2 mg once daily. The primary endpoint was Eczema Area and Severity Index (EASI)–75 at week 16.
“Understanding which patients can benefit most from this treatment was our goal,” Dr. Simpson said. “By tailoring your therapy, you can significantly improve the patient experience, increase the cost-effectiveness of a therapy, and you can ensure that only patients who are likely to benefit are exposed to a drug.”
The researchers used a classification and regression tree algorithm that identified baseline BSA as the strongest predictor of EASI-75 response at week 16. A BSA cutoff of 50% was established as the optimal cutoff for sensitivity and negative predictive value. Results for EASI-75 and Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores of 0 or 1 were confirmed using a BSA of 10%-50% at baseline to predict response, compared with a BSA or greater than 50% at baseline.
Sensitivity analyses revealed that about 90% of patients with an EASI-75 response were in the BSA 10%-50% group. Conversely, among patients with a BSA greater than 50%, the negative predictive value was greater than 90%, “so there’s a 90% chance you’re not going to hit that EASI-75 at week 16 if your BSA is greater than 50%,” Dr. Simpson explained. “The same holds true for vIGA-AD, so that 50% cutoff is important for understanding whether someone is going to respond or not.”
On the EASI-75, 38% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 10% in the BSA greater than 50% group. A similar association was observed on the vIGA-AD, where 32% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 5% in the BSA greater than 50% group.
When stratified by early response assessed at week 4, based on a 4-point improvement or greater on the Itch Numeric Rating Scale, 55% of those patients became EASI-75 responders, compared with 17% who were not. A similar association was observed by early response assessed at week 8.
“Due to the rapid onset of response, clinical assessment of patients after 4-8 weeks of initiation of baricitinib 2 mg treatment provided a positive feedback to patients who are likely to benefit from long-term therapy,” Dr. Simpson said. “This analysis may allow for a precision-medicine approach to therapy in moderate to severe AD.”
The study was supported by Eli Lilly, and was under license from Incyte. Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
results from an analysis of phase 3 data showed.
“This proposed clinical tailoring approach for baricitinib 2 mg allows for treatment of patients who are more likely to respond to therapy and rapid decision on discontinuation of treatment for those who are not likely to benefit from baricitinib 2 mg,” Eric L. Simpson, MD, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium.
Baricitinib is an oral, reversible and selective Janus kinase 1/JAK2 inhibitor that is approved in Europe for the treatment of moderate to severe AD in adults who are candidates for systemic therapy. In the United States, it is approved for treating rheumatoid arthritis, and is currently under Food and Drug Administration review in the United States for AD.
For the current analysis, Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, and colleagues set out to identify responders to baricitinib 2 mg using a tailored approach based on baseline BSA affected and early clinical improvement in the phase 3 monotherapy trial BREEZE-AD5. The trial enrolled 440 patients: 147 to placebo, 147 to baricitinib 1 mg once daily, and 146 to baricitinib 2 mg once daily. The primary endpoint was Eczema Area and Severity Index (EASI)–75 at week 16.
“Understanding which patients can benefit most from this treatment was our goal,” Dr. Simpson said. “By tailoring your therapy, you can significantly improve the patient experience, increase the cost-effectiveness of a therapy, and you can ensure that only patients who are likely to benefit are exposed to a drug.”
The researchers used a classification and regression tree algorithm that identified baseline BSA as the strongest predictor of EASI-75 response at week 16. A BSA cutoff of 50% was established as the optimal cutoff for sensitivity and negative predictive value. Results for EASI-75 and Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores of 0 or 1 were confirmed using a BSA of 10%-50% at baseline to predict response, compared with a BSA or greater than 50% at baseline.
Sensitivity analyses revealed that about 90% of patients with an EASI-75 response were in the BSA 10%-50% group. Conversely, among patients with a BSA greater than 50%, the negative predictive value was greater than 90%, “so there’s a 90% chance you’re not going to hit that EASI-75 at week 16 if your BSA is greater than 50%,” Dr. Simpson explained. “The same holds true for vIGA-AD, so that 50% cutoff is important for understanding whether someone is going to respond or not.”
On the EASI-75, 38% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 10% in the BSA greater than 50% group. A similar association was observed on the vIGA-AD, where 32% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 5% in the BSA greater than 50% group.
When stratified by early response assessed at week 4, based on a 4-point improvement or greater on the Itch Numeric Rating Scale, 55% of those patients became EASI-75 responders, compared with 17% who were not. A similar association was observed by early response assessed at week 8.
“Due to the rapid onset of response, clinical assessment of patients after 4-8 weeks of initiation of baricitinib 2 mg treatment provided a positive feedback to patients who are likely to benefit from long-term therapy,” Dr. Simpson said. “This analysis may allow for a precision-medicine approach to therapy in moderate to severe AD.”
The study was supported by Eli Lilly, and was under license from Incyte. Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
results from an analysis of phase 3 data showed.
“This proposed clinical tailoring approach for baricitinib 2 mg allows for treatment of patients who are more likely to respond to therapy and rapid decision on discontinuation of treatment for those who are not likely to benefit from baricitinib 2 mg,” Eric L. Simpson, MD, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium.
Baricitinib is an oral, reversible and selective Janus kinase 1/JAK2 inhibitor that is approved in Europe for the treatment of moderate to severe AD in adults who are candidates for systemic therapy. In the United States, it is approved for treating rheumatoid arthritis, and is currently under Food and Drug Administration review in the United States for AD.
For the current analysis, Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland, and colleagues set out to identify responders to baricitinib 2 mg using a tailored approach based on baseline BSA affected and early clinical improvement in the phase 3 monotherapy trial BREEZE-AD5. The trial enrolled 440 patients: 147 to placebo, 147 to baricitinib 1 mg once daily, and 146 to baricitinib 2 mg once daily. The primary endpoint was Eczema Area and Severity Index (EASI)–75 at week 16.
“Understanding which patients can benefit most from this treatment was our goal,” Dr. Simpson said. “By tailoring your therapy, you can significantly improve the patient experience, increase the cost-effectiveness of a therapy, and you can ensure that only patients who are likely to benefit are exposed to a drug.”
The researchers used a classification and regression tree algorithm that identified baseline BSA as the strongest predictor of EASI-75 response at week 16. A BSA cutoff of 50% was established as the optimal cutoff for sensitivity and negative predictive value. Results for EASI-75 and Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) scores of 0 or 1 were confirmed using a BSA of 10%-50% at baseline to predict response, compared with a BSA or greater than 50% at baseline.
Sensitivity analyses revealed that about 90% of patients with an EASI-75 response were in the BSA 10%-50% group. Conversely, among patients with a BSA greater than 50%, the negative predictive value was greater than 90%, “so there’s a 90% chance you’re not going to hit that EASI-75 at week 16 if your BSA is greater than 50%,” Dr. Simpson explained. “The same holds true for vIGA-AD, so that 50% cutoff is important for understanding whether someone is going to respond or not.”
On the EASI-75, 38% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 10% in the BSA greater than 50% group. A similar association was observed on the vIGA-AD, where 32% of patients in the BSA 10%-50% group responded to baricitinib at week 16, compared with 5% in the BSA greater than 50% group.
When stratified by early response assessed at week 4, based on a 4-point improvement or greater on the Itch Numeric Rating Scale, 55% of those patients became EASI-75 responders, compared with 17% who were not. A similar association was observed by early response assessed at week 8.
“Due to the rapid onset of response, clinical assessment of patients after 4-8 weeks of initiation of baricitinib 2 mg treatment provided a positive feedback to patients who are likely to benefit from long-term therapy,” Dr. Simpson said. “This analysis may allow for a precision-medicine approach to therapy in moderate to severe AD.”
The study was supported by Eli Lilly, and was under license from Incyte. Dr. Simpson reported serving as an investigator for and consultant to numerous pharmaceutical companies.
FROM REVOLUTIONIZING AD 2020
Avoiding atopic dermatitis triggers easier said than done
“Guidelines on trigger avoidance are written as if it’s easy to do,” Jonathan I. Silverberg, MD, PhD, MPH, said during the Revolutionizing Atopic Dermatitis virtual symposium. “It turns out that trigger avoidance is really complicated.”
He and his colleagues conducted a study of most common triggers for itch based on a prospective dermatology practice–based study of 587 adults with AD . About two-thirds (65%) reported one or more itch trigger in the past week and 36% had three or more itch triggers in the past week. The two most common triggers were stress (35%) and sweat (31%).
“To me, this is provocative, because this is not how I was trained in residency,” said Dr. Silverberg, director of clinical research in the division of dermatology at George Washington University, Washington. “I was trained that it’s all about excess showering, dry air, or cold temperature. Those are important, but the most common triggers are stress and sweat.”
AD triggers are also commonly linked to seasonality. “If you ask patients when their AD is worse, sometimes it’s winter,” he said. “Sometimes it’s spring. Sometimes it’s summer. It turns out that there is a distinct set of triggers that are associated with AD seasonality.” Wintertime worsening of disease is associated with cold temperature and weather change, he continued, while springtime worsening of disease is often linked to weather change and dry air. Common summertime triggers for flares include hot temperature, heat, sweat, weather change, sunlight, humid air, and dry air. “In the fall, the weather change again comes up as a trigger. Humid air does as well.”
In their prospective study, Dr. Silverberg and colleagues found that 90% of those who had at least three itch triggers reported 3 months or less of AD remission in the past year, “meaning that 90% are reporting persistent disease when they have multiple itch triggers,” he said. In addition, 78% reported two or more flares per year and 61% reported that AD is worse during certain seasons.
Potential mitigation strategies for stress include stress management, biofeedback, meditation, relaxation training, and mindfulness. “These don’t necessarily require expensive psychotherapy,” he said. Freely available iPhone apps can be incorporated into daily practice, such as Calm, Relax with Andrew Johnson, Nature Sounds Relax and Sleep, Breathe2Relax, and Headspace.
Many AD patients are sedentary and avoid vigorous physical activity owing to heat and sweat as triggers. Simple solutions include exercising in a cooler temperature environment, “not just using fans,” he said. “Take a quick shower right after working out and consider pre- and/or post treatment with topical medication.”
High temperature and sweating can be problematic at bedtime, he continued. Even if the indoor temperature is 70° F, that might jump to 85° F or 90° F under a thick blanket. “That heat can trigger itch and may cause sweating, which can trigger itch,” said Dr. Silverberg, who has AD and is director of patch testing at George Washington University. Potential solutions include using a lighter blanket, lowering the indoor temperature, and wearing breathable pajamas.
Dryness, another common AD trigger, can be secondary to a combination of low outdoor and/or indoor humidity. “Lower outdoor humidity is a particular problem in the wintertime, because cold air doesn’t hold moisture as well,” he said. “That’s why the air feels much dryer in the wintertime. There’s also a problem of indoor heating and cooling. Sometimes central air systems can lower humidity to the point where it’s bone dry.”
In an effort to determine the impact of specific climatic factors on the U.S. prevalence of AD, Dr. Silverberg and colleagues conducted a study using a merged analysis of the 2007 National Survey of Children’s Health from a representative sample of 91,642 children aged 0-17 years and 2006-2007 measurements from the National Climate Data Center and Weather Service. They found that childhood AD prevalence was increased in geographical areas that use more indoor heat and cooling and had lower outdoor humidity. “So, we see that there’s a direct correlate of this dryness issue that is leading to more AD throughout the U.S.,” he said.
Practical solutions to mitigate the effect of dry air on AD include opening windows to allow entry of moist air, “which can be particularly helpful in residences that are overheated,” he said. “I deal with this a lot in patients who live in dormitories. Use humidifiers to add moisture back into the air. Aim for 40%-50% indoor humidity to avoid mold and dust mites. It’s better to use demineralized water to reduce bacterial growth. This can be helpful for aeroallergies. Of note, there are really no well-done studies that have examined the efficacy of humidifiers in AD, but based on our anecdotal experience, this is a good way to go.”
Cold temperatures and trigger intense itch, even in the setting of high humidity. “For me personally, this is one of my most brutal triggers,” Dr. Silverberg said. “When I’m in a place with extremes of cold, I get a rapid onset of itch, a mix of itch and pain, particularly on the dorsal hands. For solutions, you can encourage patients to avoid extremely low temperatures, to bundle up, and to potentially use hand warmers or other heating devices.”
Clothing can be a trigger as well, especially tight-fitting clothes, hot and nonbreathable clothes, and large-diameter wool, which has been shown to induce itching and irritation. Mitigation strategies include wearing loose-fitting, lightweight, nonirritating fabric. “Traditional cotton and silk fabrics have mixed evidence in improving AD but are generally safe,” he said. “Ultra- or superfine merino wool has been shown to be nonpruritic. There is sparse evidence to support chemically treated/coated clothing for AD, but this may be an emerging area.”
Dr. Silverberg pointed out variability of cultural perspectives and preferences for bathing practices, including temperature, duration, frequency, optimal bathing products, and the use of loofahs and other scrubbing products. “This stems from different perceptions of what it means to be clean, and how dry our skin should feel after a shower,” he said. “Many clinicians and patients were taught that regular bathing is harmful in AD. It turns out that’s not true.”
In a recently published systematic review and meta-analysis of 13 studies, he and his colleagues examined efficacy outcomes of different bathing/showering regimens in AD. All 13 studies showed numerically reduced AD severity with any bathing regimen in at least one time point. Numerical decreases over time were observed for body surface area (BSA), Eczema Area and Severity Index (EASI), and/or SCORAD measures for daily and less than daily bathing, with or without application of emollients or topical corticosteroids. In random effects regression models, taking baths more than or less than seven times per week were not associated with significant differences of Cohen’s D scores for EASI, SCORAD, or BSA. “The take-home message here is, let your AD patients bathe,” Dr. Silverberg said. “Bathing is good. It can be channeled to help the eczema, but it has to be done the right way.”
Patients should be counseled to use nonirritating cleansers and shampoos, avoid excessively long baths/showers, avoid excessively hot baths/showers, avoid excessive rubbing or scrubbing of skin, and to apply emollients and/or topical corticosteroids immediately after the bath/shower.
PROMIS Itch-Triggers is a simple and feasible checklist to screen for the most common itch triggers in AD in clinical practice (patients are asked to check off which of the following have caused their itch in the previous 7 days: cold temperature, hot temperature, heat, sweat, tight clothing, fragrances, boredom, talking about itch, stress, weather change, sunlight, humid air, dry air). “It takes less than 1 minute to complete,” he said. “Additional testing with skin patch and/or prick testing may be warranted to identify allergenic triggers.”
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
“Guidelines on trigger avoidance are written as if it’s easy to do,” Jonathan I. Silverberg, MD, PhD, MPH, said during the Revolutionizing Atopic Dermatitis virtual symposium. “It turns out that trigger avoidance is really complicated.”
He and his colleagues conducted a study of most common triggers for itch based on a prospective dermatology practice–based study of 587 adults with AD . About two-thirds (65%) reported one or more itch trigger in the past week and 36% had three or more itch triggers in the past week. The two most common triggers were stress (35%) and sweat (31%).
“To me, this is provocative, because this is not how I was trained in residency,” said Dr. Silverberg, director of clinical research in the division of dermatology at George Washington University, Washington. “I was trained that it’s all about excess showering, dry air, or cold temperature. Those are important, but the most common triggers are stress and sweat.”
AD triggers are also commonly linked to seasonality. “If you ask patients when their AD is worse, sometimes it’s winter,” he said. “Sometimes it’s spring. Sometimes it’s summer. It turns out that there is a distinct set of triggers that are associated with AD seasonality.” Wintertime worsening of disease is associated with cold temperature and weather change, he continued, while springtime worsening of disease is often linked to weather change and dry air. Common summertime triggers for flares include hot temperature, heat, sweat, weather change, sunlight, humid air, and dry air. “In the fall, the weather change again comes up as a trigger. Humid air does as well.”
In their prospective study, Dr. Silverberg and colleagues found that 90% of those who had at least three itch triggers reported 3 months or less of AD remission in the past year, “meaning that 90% are reporting persistent disease when they have multiple itch triggers,” he said. In addition, 78% reported two or more flares per year and 61% reported that AD is worse during certain seasons.
Potential mitigation strategies for stress include stress management, biofeedback, meditation, relaxation training, and mindfulness. “These don’t necessarily require expensive psychotherapy,” he said. Freely available iPhone apps can be incorporated into daily practice, such as Calm, Relax with Andrew Johnson, Nature Sounds Relax and Sleep, Breathe2Relax, and Headspace.
Many AD patients are sedentary and avoid vigorous physical activity owing to heat and sweat as triggers. Simple solutions include exercising in a cooler temperature environment, “not just using fans,” he said. “Take a quick shower right after working out and consider pre- and/or post treatment with topical medication.”
High temperature and sweating can be problematic at bedtime, he continued. Even if the indoor temperature is 70° F, that might jump to 85° F or 90° F under a thick blanket. “That heat can trigger itch and may cause sweating, which can trigger itch,” said Dr. Silverberg, who has AD and is director of patch testing at George Washington University. Potential solutions include using a lighter blanket, lowering the indoor temperature, and wearing breathable pajamas.
Dryness, another common AD trigger, can be secondary to a combination of low outdoor and/or indoor humidity. “Lower outdoor humidity is a particular problem in the wintertime, because cold air doesn’t hold moisture as well,” he said. “That’s why the air feels much dryer in the wintertime. There’s also a problem of indoor heating and cooling. Sometimes central air systems can lower humidity to the point where it’s bone dry.”
In an effort to determine the impact of specific climatic factors on the U.S. prevalence of AD, Dr. Silverberg and colleagues conducted a study using a merged analysis of the 2007 National Survey of Children’s Health from a representative sample of 91,642 children aged 0-17 years and 2006-2007 measurements from the National Climate Data Center and Weather Service. They found that childhood AD prevalence was increased in geographical areas that use more indoor heat and cooling and had lower outdoor humidity. “So, we see that there’s a direct correlate of this dryness issue that is leading to more AD throughout the U.S.,” he said.
Practical solutions to mitigate the effect of dry air on AD include opening windows to allow entry of moist air, “which can be particularly helpful in residences that are overheated,” he said. “I deal with this a lot in patients who live in dormitories. Use humidifiers to add moisture back into the air. Aim for 40%-50% indoor humidity to avoid mold and dust mites. It’s better to use demineralized water to reduce bacterial growth. This can be helpful for aeroallergies. Of note, there are really no well-done studies that have examined the efficacy of humidifiers in AD, but based on our anecdotal experience, this is a good way to go.”
Cold temperatures and trigger intense itch, even in the setting of high humidity. “For me personally, this is one of my most brutal triggers,” Dr. Silverberg said. “When I’m in a place with extremes of cold, I get a rapid onset of itch, a mix of itch and pain, particularly on the dorsal hands. For solutions, you can encourage patients to avoid extremely low temperatures, to bundle up, and to potentially use hand warmers or other heating devices.”
Clothing can be a trigger as well, especially tight-fitting clothes, hot and nonbreathable clothes, and large-diameter wool, which has been shown to induce itching and irritation. Mitigation strategies include wearing loose-fitting, lightweight, nonirritating fabric. “Traditional cotton and silk fabrics have mixed evidence in improving AD but are generally safe,” he said. “Ultra- or superfine merino wool has been shown to be nonpruritic. There is sparse evidence to support chemically treated/coated clothing for AD, but this may be an emerging area.”
Dr. Silverberg pointed out variability of cultural perspectives and preferences for bathing practices, including temperature, duration, frequency, optimal bathing products, and the use of loofahs and other scrubbing products. “This stems from different perceptions of what it means to be clean, and how dry our skin should feel after a shower,” he said. “Many clinicians and patients were taught that regular bathing is harmful in AD. It turns out that’s not true.”
In a recently published systematic review and meta-analysis of 13 studies, he and his colleagues examined efficacy outcomes of different bathing/showering regimens in AD. All 13 studies showed numerically reduced AD severity with any bathing regimen in at least one time point. Numerical decreases over time were observed for body surface area (BSA), Eczema Area and Severity Index (EASI), and/or SCORAD measures for daily and less than daily bathing, with or without application of emollients or topical corticosteroids. In random effects regression models, taking baths more than or less than seven times per week were not associated with significant differences of Cohen’s D scores for EASI, SCORAD, or BSA. “The take-home message here is, let your AD patients bathe,” Dr. Silverberg said. “Bathing is good. It can be channeled to help the eczema, but it has to be done the right way.”
Patients should be counseled to use nonirritating cleansers and shampoos, avoid excessively long baths/showers, avoid excessively hot baths/showers, avoid excessive rubbing or scrubbing of skin, and to apply emollients and/or topical corticosteroids immediately after the bath/shower.
PROMIS Itch-Triggers is a simple and feasible checklist to screen for the most common itch triggers in AD in clinical practice (patients are asked to check off which of the following have caused their itch in the previous 7 days: cold temperature, hot temperature, heat, sweat, tight clothing, fragrances, boredom, talking about itch, stress, weather change, sunlight, humid air, dry air). “It takes less than 1 minute to complete,” he said. “Additional testing with skin patch and/or prick testing may be warranted to identify allergenic triggers.”
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
“Guidelines on trigger avoidance are written as if it’s easy to do,” Jonathan I. Silverberg, MD, PhD, MPH, said during the Revolutionizing Atopic Dermatitis virtual symposium. “It turns out that trigger avoidance is really complicated.”
He and his colleagues conducted a study of most common triggers for itch based on a prospective dermatology practice–based study of 587 adults with AD . About two-thirds (65%) reported one or more itch trigger in the past week and 36% had three or more itch triggers in the past week. The two most common triggers were stress (35%) and sweat (31%).
“To me, this is provocative, because this is not how I was trained in residency,” said Dr. Silverberg, director of clinical research in the division of dermatology at George Washington University, Washington. “I was trained that it’s all about excess showering, dry air, or cold temperature. Those are important, but the most common triggers are stress and sweat.”
AD triggers are also commonly linked to seasonality. “If you ask patients when their AD is worse, sometimes it’s winter,” he said. “Sometimes it’s spring. Sometimes it’s summer. It turns out that there is a distinct set of triggers that are associated with AD seasonality.” Wintertime worsening of disease is associated with cold temperature and weather change, he continued, while springtime worsening of disease is often linked to weather change and dry air. Common summertime triggers for flares include hot temperature, heat, sweat, weather change, sunlight, humid air, and dry air. “In the fall, the weather change again comes up as a trigger. Humid air does as well.”
In their prospective study, Dr. Silverberg and colleagues found that 90% of those who had at least three itch triggers reported 3 months or less of AD remission in the past year, “meaning that 90% are reporting persistent disease when they have multiple itch triggers,” he said. In addition, 78% reported two or more flares per year and 61% reported that AD is worse during certain seasons.
Potential mitigation strategies for stress include stress management, biofeedback, meditation, relaxation training, and mindfulness. “These don’t necessarily require expensive psychotherapy,” he said. Freely available iPhone apps can be incorporated into daily practice, such as Calm, Relax with Andrew Johnson, Nature Sounds Relax and Sleep, Breathe2Relax, and Headspace.
Many AD patients are sedentary and avoid vigorous physical activity owing to heat and sweat as triggers. Simple solutions include exercising in a cooler temperature environment, “not just using fans,” he said. “Take a quick shower right after working out and consider pre- and/or post treatment with topical medication.”
High temperature and sweating can be problematic at bedtime, he continued. Even if the indoor temperature is 70° F, that might jump to 85° F or 90° F under a thick blanket. “That heat can trigger itch and may cause sweating, which can trigger itch,” said Dr. Silverberg, who has AD and is director of patch testing at George Washington University. Potential solutions include using a lighter blanket, lowering the indoor temperature, and wearing breathable pajamas.
Dryness, another common AD trigger, can be secondary to a combination of low outdoor and/or indoor humidity. “Lower outdoor humidity is a particular problem in the wintertime, because cold air doesn’t hold moisture as well,” he said. “That’s why the air feels much dryer in the wintertime. There’s also a problem of indoor heating and cooling. Sometimes central air systems can lower humidity to the point where it’s bone dry.”
In an effort to determine the impact of specific climatic factors on the U.S. prevalence of AD, Dr. Silverberg and colleagues conducted a study using a merged analysis of the 2007 National Survey of Children’s Health from a representative sample of 91,642 children aged 0-17 years and 2006-2007 measurements from the National Climate Data Center and Weather Service. They found that childhood AD prevalence was increased in geographical areas that use more indoor heat and cooling and had lower outdoor humidity. “So, we see that there’s a direct correlate of this dryness issue that is leading to more AD throughout the U.S.,” he said.
Practical solutions to mitigate the effect of dry air on AD include opening windows to allow entry of moist air, “which can be particularly helpful in residences that are overheated,” he said. “I deal with this a lot in patients who live in dormitories. Use humidifiers to add moisture back into the air. Aim for 40%-50% indoor humidity to avoid mold and dust mites. It’s better to use demineralized water to reduce bacterial growth. This can be helpful for aeroallergies. Of note, there are really no well-done studies that have examined the efficacy of humidifiers in AD, but based on our anecdotal experience, this is a good way to go.”
Cold temperatures and trigger intense itch, even in the setting of high humidity. “For me personally, this is one of my most brutal triggers,” Dr. Silverberg said. “When I’m in a place with extremes of cold, I get a rapid onset of itch, a mix of itch and pain, particularly on the dorsal hands. For solutions, you can encourage patients to avoid extremely low temperatures, to bundle up, and to potentially use hand warmers or other heating devices.”
Clothing can be a trigger as well, especially tight-fitting clothes, hot and nonbreathable clothes, and large-diameter wool, which has been shown to induce itching and irritation. Mitigation strategies include wearing loose-fitting, lightweight, nonirritating fabric. “Traditional cotton and silk fabrics have mixed evidence in improving AD but are generally safe,” he said. “Ultra- or superfine merino wool has been shown to be nonpruritic. There is sparse evidence to support chemically treated/coated clothing for AD, but this may be an emerging area.”
Dr. Silverberg pointed out variability of cultural perspectives and preferences for bathing practices, including temperature, duration, frequency, optimal bathing products, and the use of loofahs and other scrubbing products. “This stems from different perceptions of what it means to be clean, and how dry our skin should feel after a shower,” he said. “Many clinicians and patients were taught that regular bathing is harmful in AD. It turns out that’s not true.”
In a recently published systematic review and meta-analysis of 13 studies, he and his colleagues examined efficacy outcomes of different bathing/showering regimens in AD. All 13 studies showed numerically reduced AD severity with any bathing regimen in at least one time point. Numerical decreases over time were observed for body surface area (BSA), Eczema Area and Severity Index (EASI), and/or SCORAD measures for daily and less than daily bathing, with or without application of emollients or topical corticosteroids. In random effects regression models, taking baths more than or less than seven times per week were not associated with significant differences of Cohen’s D scores for EASI, SCORAD, or BSA. “The take-home message here is, let your AD patients bathe,” Dr. Silverberg said. “Bathing is good. It can be channeled to help the eczema, but it has to be done the right way.”
Patients should be counseled to use nonirritating cleansers and shampoos, avoid excessively long baths/showers, avoid excessively hot baths/showers, avoid excessive rubbing or scrubbing of skin, and to apply emollients and/or topical corticosteroids immediately after the bath/shower.
PROMIS Itch-Triggers is a simple and feasible checklist to screen for the most common itch triggers in AD in clinical practice (patients are asked to check off which of the following have caused their itch in the previous 7 days: cold temperature, hot temperature, heat, sweat, tight clothing, fragrances, boredom, talking about itch, stress, weather change, sunlight, humid air, dry air). “It takes less than 1 minute to complete,” he said. “Additional testing with skin patch and/or prick testing may be warranted to identify allergenic triggers.”
Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
FROM REVOLUTIONIZING AD 2020
Data call for biologics trials in undertreated juvenile arthritis subtype
Children with enthesitis-related arthritis often have a high burden of disease and could benefit from medications currently approved for adults with spondyloarthritis, according to a review published in Arthritis Care & Research.
“Enthesitis-related arthritis (ERA) was the JIA [juvenile idiopathic arthritis] category applied to children with spondyloarthritis (SpA), recognizing enthesitis as a defining characteristic,” wrote Pamela F. Weiss, MD, of Children’s Hospital of Philadelphia, and colleagues.
The ERA criteria include “arthritis plus enthesitis; or arthritis or enthesitis plus at least two of the following: sacroiliac tenderness or inflammatory back pain, HLA-B27 positivity, first-degree relative with HLA-B27–associated disease, acute anterior uveitis, and arthritis in a male older than 6 years,” the review authors noted.
“None of the [Food and Drug Administration]–approved therapies for peripheral SpA or nonradiographic axial SpA” have been studied or approved for use in children with ERA, but data support biologic similarity to SpA in adults; notably, studies of the HLA-B27 allele have identified it as a risk factor for both SpA and ERA, they said.
Common factors in adult and childhood conditions
“The principal commonalities of children with ERA and axial arthritis, and adults with nonradiographic axial SpA, include enthesitis, arthritis, inflammatory back pain, anterior uveitis, HLA-B27 positivity, and family history of HLA-B27–associated disease,” the review authors wrote.
The first-line treatment for both ERA with axial arthritis and nonradiographic axial SpA is NSAIDs, followed by tumor necrosis factor (TNF) inhibitors if needed, they said. However, conventional disease-modifying antirheumatic drugs (cDMARDs) may be used in cases of peripheral disease affecting five or more joints. Studies of treatment response show similarities between ERA in children and SpA in adults, the authors added, with nearly half of adults with axial disease unable to achieve remission and approximately one-third of children with ERA failing to respond to therapy.
Clinical trials could improve options and outcomes for those with ERA who need advanced therapy and such trials should evaluate response of axial and peripheral disease separately, the review authors emphasized. For example, “Eligibility criteria for children with ERA and axial features could include the presence of some of the following disease features: active inflammatory sacroiliitis based on typical MRI changes according to ASAS/OMERACT [Assessment of SpondyloArthritis international Society/Outcome Measures in Rheumatology Clinical Trials] criteria; elevated CRP [C-reactive protein]; and inadequate response or intolerance to NSAIDs,” they noted. “Considering the similarities between adult spondyloarthritis and ERA in terms of etiology, genetics, pathogenesis, and clinical manifestations, it is evident that medications approved for axial or peripheral SpA should be studied in children with ERA involving axial or peripheral joints, respectively, with the intent to achieve labeling for use in children,” they concluded.
New data highlight ERA disease burden
The need for additional therapies for ERA patients gained more support from a recent study in which a majority of children with ERA or juvenile psoriatic arthritis (jPsA) used biologics, but those with sacroiliitis in particular showed a significant disease burden despite high biologic use.
The International Leagues Against Rheumatism criteria include seven categories of juvenile idiopathic arthritis, of which ERA and jPsA are the most common; however, characteristics of these children have not been well described, wrote Dax G. Rumsey, MD, of the University of Alberta, Edmonton, and colleagues.
“Children with ERA are more likely to have a clinical picture with predominantly peripheral arthritis, typically described as an oligoarthritis involving the lower limbs with high risk of axial disease, relative to the other categories of JIA,” and report more intense pain and worse health status, compared with children in other categories, the researchers wrote.
To more completely characterize children with ERA and jPsA, the researchers assessed 522 children with ERA and 380 with jPsA. The children were enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. The findings were published in a brief report in Arthritis Care & Research.
Overall, 69% of the children took at least one biologic, including 72% with ERA and 64% with jPsA. Biologic use was even higher (81%) among the 28% of patients with sacroiliitis (40% of ERA patients and 12% of jPsA patients). Approximately 36% of the patients with sacroiliitis were positive for HLA-B27. In addition, Physician Global Assessment scores and clinical Juvenile Arthritis Disease Activity Score-10 (cJADAS10) scores were significantly higher at the first clinical visit with sacroiliitis, compared with the first visit without, which confirms “the clinical impression that active sacroiliitis significantly impacts children and their families,” the researchers said.
The average age at diagnosis was 10.8 years for ERA and 8.2 years for jPsA, and significantly more ERA patients were male (56% vs. 38%). However, more of the patients with sacroiliitis (54%) were female. More than half of the patients reported polyarticular involvement.
The study findings were limited by several factors, including the classification of ERA or jPsA and the reliance on physician diagnoses, as well as the variation in identifying sacroiliitis, the researchers said. However, the results increase understanding of the pathophysiology of ERA and jPsA to help determine optimal treatment, they concluded.
Data highlight research and treatment gaps
“Recent research demonstrates a large, unmet medical need in the treatment of JIA with 52%-65% of all JIA patients, including those with ERA and jPsA, having been treated with at least one biologic DMARD and 15%-19% having been treated with an FDA-unapproved biologic. In those with ERA or jPsA, 72%-79% of the children had been treated with a biologic DMARD, although no biologic DMARD has ever been FDA approved for these JIA categories,” Daniel J. Lovell, MD, and Hermine I. Brunner, MD, both with Cincinnati Children’s Hospital Medical Center, wrote in an editorial that accompanied the new study. Dr. Lovell and Dr. Brunner also were coauthors of the review article.
The new study supports findings from other recent publications, the editorialists noted. The new results showed “a significant proportion of the JIA population with active sacroiliitis with high disease burden despite very frequent (over 80% of the population) [treatment] with unstudied and unapproved biologic DMARDs,” they said. “These children with sacroiliitis had significantly greater disease burden with higher physician assessment of disease activity, higher parent assessment of disease impact, and higher disease activity as measured by the Juvenile Idiopathic Arthritis Disease Activity Score, compared to the children with ERA or jPsA without sacroiliitis,” they noted.
Previously, “the FDA granted pharmaceutical companies studying new treatments in adult SpA automatic full waivers from doing studies in children for new medications for ‘axial spondyloarthropathies including ankylosing spondylitis’ up until July 2020,” the editorialists said. However, “It is now time now for the pharmaceutical industry to perform FDA-monitored clinical trials of children and adolescents with SpA,” they emphasized. “This will allow for the scientific assessment of proper dosing, efficacy, and safety of the increasing number of new medications that are being licensed by the FDA for the treatment of SpA, such as the anti-TNF, anti–IL[interleukin]-17, and anti–IL-23 biologics, and perhaps JAK [Janus kinase] agents, to address this unmet medical need in these patients with juvenile SpA,” they concluded.
Dr. Weiss disclosed grant support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and financial relationships with Eli Lilly and Pfizer. Dr. Lovell disclosed relationships with companies including Abbott, AbbVie Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Novartis, Pfizer, Takeda, UCB, and Wyeth, as well as serving on the data and safety monitoring board for Forest Research and NIAMS. Dr. Brunner disclosed relationships with companies including Ablynx, AbbVie, AstraZeneca-MedImmune, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, EMD Serono, F. Hoffmann-La Roche, Genzyme, GlaxoSmithKline, Merck, Novartis, R-Pharm, and Sanofi. The study by Dr. Rumsey and colleagues was supported by Amgen. Dr. Rumsey and colleagues had no relevant financial conflicts to disclose.
SOURCES: Weiss PF et al. Arthritis Care Res. 2020 Dec 5. doi: 10.1002/acr.24529; Rumsey DG et al. Arthritis Care Res. 2020 Dec. 16. doi: 10.1002/acr.24537; Lovell DJ and Brunner HI. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24536.
Children with enthesitis-related arthritis often have a high burden of disease and could benefit from medications currently approved for adults with spondyloarthritis, according to a review published in Arthritis Care & Research.
“Enthesitis-related arthritis (ERA) was the JIA [juvenile idiopathic arthritis] category applied to children with spondyloarthritis (SpA), recognizing enthesitis as a defining characteristic,” wrote Pamela F. Weiss, MD, of Children’s Hospital of Philadelphia, and colleagues.
The ERA criteria include “arthritis plus enthesitis; or arthritis or enthesitis plus at least two of the following: sacroiliac tenderness or inflammatory back pain, HLA-B27 positivity, first-degree relative with HLA-B27–associated disease, acute anterior uveitis, and arthritis in a male older than 6 years,” the review authors noted.
“None of the [Food and Drug Administration]–approved therapies for peripheral SpA or nonradiographic axial SpA” have been studied or approved for use in children with ERA, but data support biologic similarity to SpA in adults; notably, studies of the HLA-B27 allele have identified it as a risk factor for both SpA and ERA, they said.
Common factors in adult and childhood conditions
“The principal commonalities of children with ERA and axial arthritis, and adults with nonradiographic axial SpA, include enthesitis, arthritis, inflammatory back pain, anterior uveitis, HLA-B27 positivity, and family history of HLA-B27–associated disease,” the review authors wrote.
The first-line treatment for both ERA with axial arthritis and nonradiographic axial SpA is NSAIDs, followed by tumor necrosis factor (TNF) inhibitors if needed, they said. However, conventional disease-modifying antirheumatic drugs (cDMARDs) may be used in cases of peripheral disease affecting five or more joints. Studies of treatment response show similarities between ERA in children and SpA in adults, the authors added, with nearly half of adults with axial disease unable to achieve remission and approximately one-third of children with ERA failing to respond to therapy.
Clinical trials could improve options and outcomes for those with ERA who need advanced therapy and such trials should evaluate response of axial and peripheral disease separately, the review authors emphasized. For example, “Eligibility criteria for children with ERA and axial features could include the presence of some of the following disease features: active inflammatory sacroiliitis based on typical MRI changes according to ASAS/OMERACT [Assessment of SpondyloArthritis international Society/Outcome Measures in Rheumatology Clinical Trials] criteria; elevated CRP [C-reactive protein]; and inadequate response or intolerance to NSAIDs,” they noted. “Considering the similarities between adult spondyloarthritis and ERA in terms of etiology, genetics, pathogenesis, and clinical manifestations, it is evident that medications approved for axial or peripheral SpA should be studied in children with ERA involving axial or peripheral joints, respectively, with the intent to achieve labeling for use in children,” they concluded.
New data highlight ERA disease burden
The need for additional therapies for ERA patients gained more support from a recent study in which a majority of children with ERA or juvenile psoriatic arthritis (jPsA) used biologics, but those with sacroiliitis in particular showed a significant disease burden despite high biologic use.
The International Leagues Against Rheumatism criteria include seven categories of juvenile idiopathic arthritis, of which ERA and jPsA are the most common; however, characteristics of these children have not been well described, wrote Dax G. Rumsey, MD, of the University of Alberta, Edmonton, and colleagues.
“Children with ERA are more likely to have a clinical picture with predominantly peripheral arthritis, typically described as an oligoarthritis involving the lower limbs with high risk of axial disease, relative to the other categories of JIA,” and report more intense pain and worse health status, compared with children in other categories, the researchers wrote.
To more completely characterize children with ERA and jPsA, the researchers assessed 522 children with ERA and 380 with jPsA. The children were enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. The findings were published in a brief report in Arthritis Care & Research.
Overall, 69% of the children took at least one biologic, including 72% with ERA and 64% with jPsA. Biologic use was even higher (81%) among the 28% of patients with sacroiliitis (40% of ERA patients and 12% of jPsA patients). Approximately 36% of the patients with sacroiliitis were positive for HLA-B27. In addition, Physician Global Assessment scores and clinical Juvenile Arthritis Disease Activity Score-10 (cJADAS10) scores were significantly higher at the first clinical visit with sacroiliitis, compared with the first visit without, which confirms “the clinical impression that active sacroiliitis significantly impacts children and their families,” the researchers said.
The average age at diagnosis was 10.8 years for ERA and 8.2 years for jPsA, and significantly more ERA patients were male (56% vs. 38%). However, more of the patients with sacroiliitis (54%) were female. More than half of the patients reported polyarticular involvement.
The study findings were limited by several factors, including the classification of ERA or jPsA and the reliance on physician diagnoses, as well as the variation in identifying sacroiliitis, the researchers said. However, the results increase understanding of the pathophysiology of ERA and jPsA to help determine optimal treatment, they concluded.
Data highlight research and treatment gaps
“Recent research demonstrates a large, unmet medical need in the treatment of JIA with 52%-65% of all JIA patients, including those with ERA and jPsA, having been treated with at least one biologic DMARD and 15%-19% having been treated with an FDA-unapproved biologic. In those with ERA or jPsA, 72%-79% of the children had been treated with a biologic DMARD, although no biologic DMARD has ever been FDA approved for these JIA categories,” Daniel J. Lovell, MD, and Hermine I. Brunner, MD, both with Cincinnati Children’s Hospital Medical Center, wrote in an editorial that accompanied the new study. Dr. Lovell and Dr. Brunner also were coauthors of the review article.
The new study supports findings from other recent publications, the editorialists noted. The new results showed “a significant proportion of the JIA population with active sacroiliitis with high disease burden despite very frequent (over 80% of the population) [treatment] with unstudied and unapproved biologic DMARDs,” they said. “These children with sacroiliitis had significantly greater disease burden with higher physician assessment of disease activity, higher parent assessment of disease impact, and higher disease activity as measured by the Juvenile Idiopathic Arthritis Disease Activity Score, compared to the children with ERA or jPsA without sacroiliitis,” they noted.
Previously, “the FDA granted pharmaceutical companies studying new treatments in adult SpA automatic full waivers from doing studies in children for new medications for ‘axial spondyloarthropathies including ankylosing spondylitis’ up until July 2020,” the editorialists said. However, “It is now time now for the pharmaceutical industry to perform FDA-monitored clinical trials of children and adolescents with SpA,” they emphasized. “This will allow for the scientific assessment of proper dosing, efficacy, and safety of the increasing number of new medications that are being licensed by the FDA for the treatment of SpA, such as the anti-TNF, anti–IL[interleukin]-17, and anti–IL-23 biologics, and perhaps JAK [Janus kinase] agents, to address this unmet medical need in these patients with juvenile SpA,” they concluded.
Dr. Weiss disclosed grant support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and financial relationships with Eli Lilly and Pfizer. Dr. Lovell disclosed relationships with companies including Abbott, AbbVie Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Novartis, Pfizer, Takeda, UCB, and Wyeth, as well as serving on the data and safety monitoring board for Forest Research and NIAMS. Dr. Brunner disclosed relationships with companies including Ablynx, AbbVie, AstraZeneca-MedImmune, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, EMD Serono, F. Hoffmann-La Roche, Genzyme, GlaxoSmithKline, Merck, Novartis, R-Pharm, and Sanofi. The study by Dr. Rumsey and colleagues was supported by Amgen. Dr. Rumsey and colleagues had no relevant financial conflicts to disclose.
SOURCES: Weiss PF et al. Arthritis Care Res. 2020 Dec 5. doi: 10.1002/acr.24529; Rumsey DG et al. Arthritis Care Res. 2020 Dec. 16. doi: 10.1002/acr.24537; Lovell DJ and Brunner HI. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24536.
Children with enthesitis-related arthritis often have a high burden of disease and could benefit from medications currently approved for adults with spondyloarthritis, according to a review published in Arthritis Care & Research.
“Enthesitis-related arthritis (ERA) was the JIA [juvenile idiopathic arthritis] category applied to children with spondyloarthritis (SpA), recognizing enthesitis as a defining characteristic,” wrote Pamela F. Weiss, MD, of Children’s Hospital of Philadelphia, and colleagues.
The ERA criteria include “arthritis plus enthesitis; or arthritis or enthesitis plus at least two of the following: sacroiliac tenderness or inflammatory back pain, HLA-B27 positivity, first-degree relative with HLA-B27–associated disease, acute anterior uveitis, and arthritis in a male older than 6 years,” the review authors noted.
“None of the [Food and Drug Administration]–approved therapies for peripheral SpA or nonradiographic axial SpA” have been studied or approved for use in children with ERA, but data support biologic similarity to SpA in adults; notably, studies of the HLA-B27 allele have identified it as a risk factor for both SpA and ERA, they said.
Common factors in adult and childhood conditions
“The principal commonalities of children with ERA and axial arthritis, and adults with nonradiographic axial SpA, include enthesitis, arthritis, inflammatory back pain, anterior uveitis, HLA-B27 positivity, and family history of HLA-B27–associated disease,” the review authors wrote.
The first-line treatment for both ERA with axial arthritis and nonradiographic axial SpA is NSAIDs, followed by tumor necrosis factor (TNF) inhibitors if needed, they said. However, conventional disease-modifying antirheumatic drugs (cDMARDs) may be used in cases of peripheral disease affecting five or more joints. Studies of treatment response show similarities between ERA in children and SpA in adults, the authors added, with nearly half of adults with axial disease unable to achieve remission and approximately one-third of children with ERA failing to respond to therapy.
Clinical trials could improve options and outcomes for those with ERA who need advanced therapy and such trials should evaluate response of axial and peripheral disease separately, the review authors emphasized. For example, “Eligibility criteria for children with ERA and axial features could include the presence of some of the following disease features: active inflammatory sacroiliitis based on typical MRI changes according to ASAS/OMERACT [Assessment of SpondyloArthritis international Society/Outcome Measures in Rheumatology Clinical Trials] criteria; elevated CRP [C-reactive protein]; and inadequate response or intolerance to NSAIDs,” they noted. “Considering the similarities between adult spondyloarthritis and ERA in terms of etiology, genetics, pathogenesis, and clinical manifestations, it is evident that medications approved for axial or peripheral SpA should be studied in children with ERA involving axial or peripheral joints, respectively, with the intent to achieve labeling for use in children,” they concluded.
New data highlight ERA disease burden
The need for additional therapies for ERA patients gained more support from a recent study in which a majority of children with ERA or juvenile psoriatic arthritis (jPsA) used biologics, but those with sacroiliitis in particular showed a significant disease burden despite high biologic use.
The International Leagues Against Rheumatism criteria include seven categories of juvenile idiopathic arthritis, of which ERA and jPsA are the most common; however, characteristics of these children have not been well described, wrote Dax G. Rumsey, MD, of the University of Alberta, Edmonton, and colleagues.
“Children with ERA are more likely to have a clinical picture with predominantly peripheral arthritis, typically described as an oligoarthritis involving the lower limbs with high risk of axial disease, relative to the other categories of JIA,” and report more intense pain and worse health status, compared with children in other categories, the researchers wrote.
To more completely characterize children with ERA and jPsA, the researchers assessed 522 children with ERA and 380 with jPsA. The children were enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. The findings were published in a brief report in Arthritis Care & Research.
Overall, 69% of the children took at least one biologic, including 72% with ERA and 64% with jPsA. Biologic use was even higher (81%) among the 28% of patients with sacroiliitis (40% of ERA patients and 12% of jPsA patients). Approximately 36% of the patients with sacroiliitis were positive for HLA-B27. In addition, Physician Global Assessment scores and clinical Juvenile Arthritis Disease Activity Score-10 (cJADAS10) scores were significantly higher at the first clinical visit with sacroiliitis, compared with the first visit without, which confirms “the clinical impression that active sacroiliitis significantly impacts children and their families,” the researchers said.
The average age at diagnosis was 10.8 years for ERA and 8.2 years for jPsA, and significantly more ERA patients were male (56% vs. 38%). However, more of the patients with sacroiliitis (54%) were female. More than half of the patients reported polyarticular involvement.
The study findings were limited by several factors, including the classification of ERA or jPsA and the reliance on physician diagnoses, as well as the variation in identifying sacroiliitis, the researchers said. However, the results increase understanding of the pathophysiology of ERA and jPsA to help determine optimal treatment, they concluded.
Data highlight research and treatment gaps
“Recent research demonstrates a large, unmet medical need in the treatment of JIA with 52%-65% of all JIA patients, including those with ERA and jPsA, having been treated with at least one biologic DMARD and 15%-19% having been treated with an FDA-unapproved biologic. In those with ERA or jPsA, 72%-79% of the children had been treated with a biologic DMARD, although no biologic DMARD has ever been FDA approved for these JIA categories,” Daniel J. Lovell, MD, and Hermine I. Brunner, MD, both with Cincinnati Children’s Hospital Medical Center, wrote in an editorial that accompanied the new study. Dr. Lovell and Dr. Brunner also were coauthors of the review article.
The new study supports findings from other recent publications, the editorialists noted. The new results showed “a significant proportion of the JIA population with active sacroiliitis with high disease burden despite very frequent (over 80% of the population) [treatment] with unstudied and unapproved biologic DMARDs,” they said. “These children with sacroiliitis had significantly greater disease burden with higher physician assessment of disease activity, higher parent assessment of disease impact, and higher disease activity as measured by the Juvenile Idiopathic Arthritis Disease Activity Score, compared to the children with ERA or jPsA without sacroiliitis,” they noted.
Previously, “the FDA granted pharmaceutical companies studying new treatments in adult SpA automatic full waivers from doing studies in children for new medications for ‘axial spondyloarthropathies including ankylosing spondylitis’ up until July 2020,” the editorialists said. However, “It is now time now for the pharmaceutical industry to perform FDA-monitored clinical trials of children and adolescents with SpA,” they emphasized. “This will allow for the scientific assessment of proper dosing, efficacy, and safety of the increasing number of new medications that are being licensed by the FDA for the treatment of SpA, such as the anti-TNF, anti–IL[interleukin]-17, and anti–IL-23 biologics, and perhaps JAK [Janus kinase] agents, to address this unmet medical need in these patients with juvenile SpA,” they concluded.
Dr. Weiss disclosed grant support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and financial relationships with Eli Lilly and Pfizer. Dr. Lovell disclosed relationships with companies including Abbott, AbbVie Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Novartis, Pfizer, Takeda, UCB, and Wyeth, as well as serving on the data and safety monitoring board for Forest Research and NIAMS. Dr. Brunner disclosed relationships with companies including Ablynx, AbbVie, AstraZeneca-MedImmune, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, EMD Serono, F. Hoffmann-La Roche, Genzyme, GlaxoSmithKline, Merck, Novartis, R-Pharm, and Sanofi. The study by Dr. Rumsey and colleagues was supported by Amgen. Dr. Rumsey and colleagues had no relevant financial conflicts to disclose.
SOURCES: Weiss PF et al. Arthritis Care Res. 2020 Dec 5. doi: 10.1002/acr.24529; Rumsey DG et al. Arthritis Care Res. 2020 Dec. 16. doi: 10.1002/acr.24537; Lovell DJ and Brunner HI. Arthritis Care Res. 2020 Dec 16. doi: 10.1002/acr.24536.
FROM ARTHRITIS CARE & RESEARCH












