User login
Research on statin for preeclampsia prevention advances
WASHINGTON – with a large National Institutes of Health–funded trial currently recruiting women with a prior history of the disorder with preterm delivery at less than 34 weeks, Maged Costantine, MD, said at the biennial Diabetes in Pregnancy Study Group of North America meeting.
More should be learned about low-dose aspirin, in the meantime, once the outcomes of a global study involving first-trimester initiation are published, said another speaker, Cynthia Gyamfi-Bannerman, MD, MS. Low-dose aspirin currently is recommended for preeclampsia prevention starting between 12 and 28 weeks, optimally before 16 weeks.
The biological plausibility of using pravastatin for preeclampsia prevention stems from the overlapping pathophysiology of preeclampsia with atherosclerotic cardiovascular disease – endothelial dysfunction and inflammation are common key mechanisms – as well as common risk factors, including diabetes and obesity, said Dr. Costantine, director of the division of maternal-fetal medicine at Ohio State University, Columbus, who is chairing the study.
In animal models of preeclampsia, pravastatin has been shown to upregulate placental growth factor, reduce antiangiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt1), and upregulate endothelial nitric oxide synthase. Mice have shown improved vascular reactivity, decreased proteinuria, decreased oxidative stress, and other positive effects, without any detrimental outcomes.
A pilot randomized controlled trial conducted with the Obstetric-Fetal Pharmacology Research Units Network and published in the American Journal of Obstetrics and Gynecology in 2016 assigned 10 women to 10 mg daily pravastatin and 10 women to placebo. The drug reduced maternal cholesterol concentrations but there were no differences in birth weight or umbilical cord cholesterol concentrations between the two groups.
Women in the pravastatin group were less likely to develop preeclampsia (none, compared with four in the placebo group), less likely to have an indicated preterm delivery (one, compared with five in the placebo group), and less likely to have their neonates admitted to the neonatal ICU.
There were no differences in side effects, congenital anomalies, or other adverse events. Dr. Costantine, principal investigator of the pilot study, and his colleagues wrote in the paper that the “favorable risk-benefit analysis justifies continued research with a dose escalation” (Am J Obstet Gynecol. 2016 Jun;214[6]:720.e1-17).
The new multicenter randomized controlled trial is randomizing 1,550 women to either 20 mg pravastatin or placebo starting between 12 weeks 0 days and 16 weeks 6 days. The primary outcome is a composite of preeclampsia, maternal death, or fetal loss. Secondary outcomes include a composite of severe maternal morbidity and various measures representing preeclampsia severity and complications, as well as preterm delivery less than 37 weeks and less than 34 weeks and various fetal/neonatal outcomes.
“In addition, we’ll look at development,” Dr. Costantine said, with offspring assessed at 2 and 5 years of age. The trial is sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute.
In the meantime, he said, the use of pravastatin to ameliorate early-onset preeclampsia is being tested in a small European proof-of-concept trial that has randomized women with early-onset preeclampsia (between 24 and 31 6/7 weeks) to 40 mg pravastatin or placebo. The primary outcome is reduction of antiangiogenic markers. Results are expected in another year or 2, he said.
The aspirin trial referred to by Dr. Gyamfi-Bannerman has been looking at the 81-mg dose of aspirin initiated between 6 0/7 and 13 6/7 weeks in nulliparous women who had no more than two previous pregnancy losses. The key question of the Aspirin Supplementation for Pregnancy Indicated Risk Reduction in Nulliparas (ASPIRIN) trial – conducted in the NICHD Global Network for Women’s and Children’s Health – is whether low-dose aspirin can reduce the rate of preterm birth. Preeclampsia is a secondary outcome (https://clinicaltrials.gov/ct2/show/NCT02409680).
“It may eventually be that the use of baby aspirin is further expanded to reduce the risk of preterm birth,” she said.
Overall, “we need more data on first-trimester use [of low-dose aspirin] and long-term outcomes,” Dr. Gyamfi-Bannerman said. And with respect to preeclampsia prevention specifically, more research is needed looking at risk reduction levels within specific groups of patients.
Since 2014, the U.S. Preventive Services Task Force (USPSTF) has called for low-dose aspirin at 81 mg/day in women who have one or more high-risk factors for preeclampsia (including type 1 or type 2 diabetes mellitus), and consideration of such treatment in patients with several moderate-risk factors. The American College of Obstetricians and Gynecologists’ recommendation varies slightly in that it advises treatment in patients with more than one (versus several) moderate-level risk factors (Obstet Gynecol. 2018;132[1]:e44-52).
Moderate-level risk factors include nulliparity, obesity, family history of preeclampsia, a baseline demographic risk (African-American or low socioeconomic status), and prior poor history (intrauterine growth restriction/small-for-gestational-age, previous poor outcome). “This is just about everyone I see,” Dr. Gyamfi-Bannerman said.
Dr. Gyamfi-Bannerman said she’d “love to see more data on higher doses” of low-dose aspirin – data that compares 81 mg/day with 150 mg/day, for instance.
A study published in 2017 in the New England Journal of Medicine randomized 1,776 women at high risk for preeclampsia to 150 mg/day or placebo and found a significant reduction in preterm preeclampsia (4.3% vs. 1.6%) in the aspirin group. Women in this European trial were deemed to be at high risk, however, based on a first-trimester screening algorithm that incorporated serum markers (maternal serum pregnancy-associated plasma protein A and placental growth factor) and uterine artery Doppler measures (N Engl J Med. 2017 Aug 17;377[7]:613-22).
“So it was a very interesting study, very provocative, but it’s hard to know how it would translate to the U.S. population [given that such screening practices] are not the way most of us are practicing here,” said Dr. Gyamfi-Bannerman, codirector of the Preterm Birth Prevention Center at Columbia University, New York, and professor of obstetrics and gynecology at the university.
The USPSTF based its recommendations on a systematic review that pooled data from 15 high-quality randomized controlled trials, including 13 that reported preeclampsia incidence among women at highest risk of disease. They found a 24% reduction in preeclampsia, but the actual risk reduction depends on the baseline population risk and may be closer to 10%, she said.
In a presentation on gaps in knowledge, Leslie Myatt, PhD, of the department of obstetrics and gynecology at Oregon Health and Science University, Portland, emphasized that preeclampsia is a syndrome with a heterogeneity of presentation and pathophysiology. “We don’t completely understand the pathophysiology,” he said.
Research needs to be “directed at the existence of multiple pathways [and subtypes],” he said, such that future therapies can be targeted and personalized.
Dr. Costantine did not report any disclosures. Dr. Gyamfi-Bannerman reported a Society of Maternal Fetal Medicine/AMAG Pharmaceuticals unrestricted grant and Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Heart, Lung and Blood Institute funding. Dr. Myatt reported that he has no financial or other ties that pose a conflict of interest.
WASHINGTON – with a large National Institutes of Health–funded trial currently recruiting women with a prior history of the disorder with preterm delivery at less than 34 weeks, Maged Costantine, MD, said at the biennial Diabetes in Pregnancy Study Group of North America meeting.
More should be learned about low-dose aspirin, in the meantime, once the outcomes of a global study involving first-trimester initiation are published, said another speaker, Cynthia Gyamfi-Bannerman, MD, MS. Low-dose aspirin currently is recommended for preeclampsia prevention starting between 12 and 28 weeks, optimally before 16 weeks.
The biological plausibility of using pravastatin for preeclampsia prevention stems from the overlapping pathophysiology of preeclampsia with atherosclerotic cardiovascular disease – endothelial dysfunction and inflammation are common key mechanisms – as well as common risk factors, including diabetes and obesity, said Dr. Costantine, director of the division of maternal-fetal medicine at Ohio State University, Columbus, who is chairing the study.
In animal models of preeclampsia, pravastatin has been shown to upregulate placental growth factor, reduce antiangiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt1), and upregulate endothelial nitric oxide synthase. Mice have shown improved vascular reactivity, decreased proteinuria, decreased oxidative stress, and other positive effects, without any detrimental outcomes.
A pilot randomized controlled trial conducted with the Obstetric-Fetal Pharmacology Research Units Network and published in the American Journal of Obstetrics and Gynecology in 2016 assigned 10 women to 10 mg daily pravastatin and 10 women to placebo. The drug reduced maternal cholesterol concentrations but there were no differences in birth weight or umbilical cord cholesterol concentrations between the two groups.
Women in the pravastatin group were less likely to develop preeclampsia (none, compared with four in the placebo group), less likely to have an indicated preterm delivery (one, compared with five in the placebo group), and less likely to have their neonates admitted to the neonatal ICU.
There were no differences in side effects, congenital anomalies, or other adverse events. Dr. Costantine, principal investigator of the pilot study, and his colleagues wrote in the paper that the “favorable risk-benefit analysis justifies continued research with a dose escalation” (Am J Obstet Gynecol. 2016 Jun;214[6]:720.e1-17).
The new multicenter randomized controlled trial is randomizing 1,550 women to either 20 mg pravastatin or placebo starting between 12 weeks 0 days and 16 weeks 6 days. The primary outcome is a composite of preeclampsia, maternal death, or fetal loss. Secondary outcomes include a composite of severe maternal morbidity and various measures representing preeclampsia severity and complications, as well as preterm delivery less than 37 weeks and less than 34 weeks and various fetal/neonatal outcomes.
“In addition, we’ll look at development,” Dr. Costantine said, with offspring assessed at 2 and 5 years of age. The trial is sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute.
In the meantime, he said, the use of pravastatin to ameliorate early-onset preeclampsia is being tested in a small European proof-of-concept trial that has randomized women with early-onset preeclampsia (between 24 and 31 6/7 weeks) to 40 mg pravastatin or placebo. The primary outcome is reduction of antiangiogenic markers. Results are expected in another year or 2, he said.
The aspirin trial referred to by Dr. Gyamfi-Bannerman has been looking at the 81-mg dose of aspirin initiated between 6 0/7 and 13 6/7 weeks in nulliparous women who had no more than two previous pregnancy losses. The key question of the Aspirin Supplementation for Pregnancy Indicated Risk Reduction in Nulliparas (ASPIRIN) trial – conducted in the NICHD Global Network for Women’s and Children’s Health – is whether low-dose aspirin can reduce the rate of preterm birth. Preeclampsia is a secondary outcome (https://clinicaltrials.gov/ct2/show/NCT02409680).
“It may eventually be that the use of baby aspirin is further expanded to reduce the risk of preterm birth,” she said.
Overall, “we need more data on first-trimester use [of low-dose aspirin] and long-term outcomes,” Dr. Gyamfi-Bannerman said. And with respect to preeclampsia prevention specifically, more research is needed looking at risk reduction levels within specific groups of patients.
Since 2014, the U.S. Preventive Services Task Force (USPSTF) has called for low-dose aspirin at 81 mg/day in women who have one or more high-risk factors for preeclampsia (including type 1 or type 2 diabetes mellitus), and consideration of such treatment in patients with several moderate-risk factors. The American College of Obstetricians and Gynecologists’ recommendation varies slightly in that it advises treatment in patients with more than one (versus several) moderate-level risk factors (Obstet Gynecol. 2018;132[1]:e44-52).
Moderate-level risk factors include nulliparity, obesity, family history of preeclampsia, a baseline demographic risk (African-American or low socioeconomic status), and prior poor history (intrauterine growth restriction/small-for-gestational-age, previous poor outcome). “This is just about everyone I see,” Dr. Gyamfi-Bannerman said.
Dr. Gyamfi-Bannerman said she’d “love to see more data on higher doses” of low-dose aspirin – data that compares 81 mg/day with 150 mg/day, for instance.
A study published in 2017 in the New England Journal of Medicine randomized 1,776 women at high risk for preeclampsia to 150 mg/day or placebo and found a significant reduction in preterm preeclampsia (4.3% vs. 1.6%) in the aspirin group. Women in this European trial were deemed to be at high risk, however, based on a first-trimester screening algorithm that incorporated serum markers (maternal serum pregnancy-associated plasma protein A and placental growth factor) and uterine artery Doppler measures (N Engl J Med. 2017 Aug 17;377[7]:613-22).
“So it was a very interesting study, very provocative, but it’s hard to know how it would translate to the U.S. population [given that such screening practices] are not the way most of us are practicing here,” said Dr. Gyamfi-Bannerman, codirector of the Preterm Birth Prevention Center at Columbia University, New York, and professor of obstetrics and gynecology at the university.
The USPSTF based its recommendations on a systematic review that pooled data from 15 high-quality randomized controlled trials, including 13 that reported preeclampsia incidence among women at highest risk of disease. They found a 24% reduction in preeclampsia, but the actual risk reduction depends on the baseline population risk and may be closer to 10%, she said.
In a presentation on gaps in knowledge, Leslie Myatt, PhD, of the department of obstetrics and gynecology at Oregon Health and Science University, Portland, emphasized that preeclampsia is a syndrome with a heterogeneity of presentation and pathophysiology. “We don’t completely understand the pathophysiology,” he said.
Research needs to be “directed at the existence of multiple pathways [and subtypes],” he said, such that future therapies can be targeted and personalized.
Dr. Costantine did not report any disclosures. Dr. Gyamfi-Bannerman reported a Society of Maternal Fetal Medicine/AMAG Pharmaceuticals unrestricted grant and Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Heart, Lung and Blood Institute funding. Dr. Myatt reported that he has no financial or other ties that pose a conflict of interest.
WASHINGTON – with a large National Institutes of Health–funded trial currently recruiting women with a prior history of the disorder with preterm delivery at less than 34 weeks, Maged Costantine, MD, said at the biennial Diabetes in Pregnancy Study Group of North America meeting.
More should be learned about low-dose aspirin, in the meantime, once the outcomes of a global study involving first-trimester initiation are published, said another speaker, Cynthia Gyamfi-Bannerman, MD, MS. Low-dose aspirin currently is recommended for preeclampsia prevention starting between 12 and 28 weeks, optimally before 16 weeks.
The biological plausibility of using pravastatin for preeclampsia prevention stems from the overlapping pathophysiology of preeclampsia with atherosclerotic cardiovascular disease – endothelial dysfunction and inflammation are common key mechanisms – as well as common risk factors, including diabetes and obesity, said Dr. Costantine, director of the division of maternal-fetal medicine at Ohio State University, Columbus, who is chairing the study.
In animal models of preeclampsia, pravastatin has been shown to upregulate placental growth factor, reduce antiangiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt1), and upregulate endothelial nitric oxide synthase. Mice have shown improved vascular reactivity, decreased proteinuria, decreased oxidative stress, and other positive effects, without any detrimental outcomes.
A pilot randomized controlled trial conducted with the Obstetric-Fetal Pharmacology Research Units Network and published in the American Journal of Obstetrics and Gynecology in 2016 assigned 10 women to 10 mg daily pravastatin and 10 women to placebo. The drug reduced maternal cholesterol concentrations but there were no differences in birth weight or umbilical cord cholesterol concentrations between the two groups.
Women in the pravastatin group were less likely to develop preeclampsia (none, compared with four in the placebo group), less likely to have an indicated preterm delivery (one, compared with five in the placebo group), and less likely to have their neonates admitted to the neonatal ICU.
There were no differences in side effects, congenital anomalies, or other adverse events. Dr. Costantine, principal investigator of the pilot study, and his colleagues wrote in the paper that the “favorable risk-benefit analysis justifies continued research with a dose escalation” (Am J Obstet Gynecol. 2016 Jun;214[6]:720.e1-17).
The new multicenter randomized controlled trial is randomizing 1,550 women to either 20 mg pravastatin or placebo starting between 12 weeks 0 days and 16 weeks 6 days. The primary outcome is a composite of preeclampsia, maternal death, or fetal loss. Secondary outcomes include a composite of severe maternal morbidity and various measures representing preeclampsia severity and complications, as well as preterm delivery less than 37 weeks and less than 34 weeks and various fetal/neonatal outcomes.
“In addition, we’ll look at development,” Dr. Costantine said, with offspring assessed at 2 and 5 years of age. The trial is sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute.
In the meantime, he said, the use of pravastatin to ameliorate early-onset preeclampsia is being tested in a small European proof-of-concept trial that has randomized women with early-onset preeclampsia (between 24 and 31 6/7 weeks) to 40 mg pravastatin or placebo. The primary outcome is reduction of antiangiogenic markers. Results are expected in another year or 2, he said.
The aspirin trial referred to by Dr. Gyamfi-Bannerman has been looking at the 81-mg dose of aspirin initiated between 6 0/7 and 13 6/7 weeks in nulliparous women who had no more than two previous pregnancy losses. The key question of the Aspirin Supplementation for Pregnancy Indicated Risk Reduction in Nulliparas (ASPIRIN) trial – conducted in the NICHD Global Network for Women’s and Children’s Health – is whether low-dose aspirin can reduce the rate of preterm birth. Preeclampsia is a secondary outcome (https://clinicaltrials.gov/ct2/show/NCT02409680).
“It may eventually be that the use of baby aspirin is further expanded to reduce the risk of preterm birth,” she said.
Overall, “we need more data on first-trimester use [of low-dose aspirin] and long-term outcomes,” Dr. Gyamfi-Bannerman said. And with respect to preeclampsia prevention specifically, more research is needed looking at risk reduction levels within specific groups of patients.
Since 2014, the U.S. Preventive Services Task Force (USPSTF) has called for low-dose aspirin at 81 mg/day in women who have one or more high-risk factors for preeclampsia (including type 1 or type 2 diabetes mellitus), and consideration of such treatment in patients with several moderate-risk factors. The American College of Obstetricians and Gynecologists’ recommendation varies slightly in that it advises treatment in patients with more than one (versus several) moderate-level risk factors (Obstet Gynecol. 2018;132[1]:e44-52).
Moderate-level risk factors include nulliparity, obesity, family history of preeclampsia, a baseline demographic risk (African-American or low socioeconomic status), and prior poor history (intrauterine growth restriction/small-for-gestational-age, previous poor outcome). “This is just about everyone I see,” Dr. Gyamfi-Bannerman said.
Dr. Gyamfi-Bannerman said she’d “love to see more data on higher doses” of low-dose aspirin – data that compares 81 mg/day with 150 mg/day, for instance.
A study published in 2017 in the New England Journal of Medicine randomized 1,776 women at high risk for preeclampsia to 150 mg/day or placebo and found a significant reduction in preterm preeclampsia (4.3% vs. 1.6%) in the aspirin group. Women in this European trial were deemed to be at high risk, however, based on a first-trimester screening algorithm that incorporated serum markers (maternal serum pregnancy-associated plasma protein A and placental growth factor) and uterine artery Doppler measures (N Engl J Med. 2017 Aug 17;377[7]:613-22).
“So it was a very interesting study, very provocative, but it’s hard to know how it would translate to the U.S. population [given that such screening practices] are not the way most of us are practicing here,” said Dr. Gyamfi-Bannerman, codirector of the Preterm Birth Prevention Center at Columbia University, New York, and professor of obstetrics and gynecology at the university.
The USPSTF based its recommendations on a systematic review that pooled data from 15 high-quality randomized controlled trials, including 13 that reported preeclampsia incidence among women at highest risk of disease. They found a 24% reduction in preeclampsia, but the actual risk reduction depends on the baseline population risk and may be closer to 10%, she said.
In a presentation on gaps in knowledge, Leslie Myatt, PhD, of the department of obstetrics and gynecology at Oregon Health and Science University, Portland, emphasized that preeclampsia is a syndrome with a heterogeneity of presentation and pathophysiology. “We don’t completely understand the pathophysiology,” he said.
Research needs to be “directed at the existence of multiple pathways [and subtypes],” he said, such that future therapies can be targeted and personalized.
Dr. Costantine did not report any disclosures. Dr. Gyamfi-Bannerman reported a Society of Maternal Fetal Medicine/AMAG Pharmaceuticals unrestricted grant and Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Heart, Lung and Blood Institute funding. Dr. Myatt reported that he has no financial or other ties that pose a conflict of interest.
EXPERT ANALYSIS FROM THE DPSG-NA 2019
SimLEARN Musculoskeletal Training for VHA Primary Care Providers and Health Professions Educators
Diseases of the musculoskeletal (MSK) system are common, accounting for some of the most frequent visits to primary care clinics.1-3 In addition, care for patients with chronic MSK diseases represents a substantial economic burden.4-6
In response to this clinical training need, the Veterans Health Administration (VHA) developed a portfolio of educational experiences for VHA health care providers and trainees, including both the Salt Lake City and National MSK “mini-residencies.”17-19 These programs have educated more than 800 individuals. Early observations show a progressive increase in the number of joint injections performed at participant’s VHA clinics as well as a reduction in unnecessary magnetic resonance imaging orders of the knee.20,21 These findings may be interpreted as markers for improved access to care for veterans as well as cost savings for the health care system.
The success of these early initiatives was recognized by the medical leadership of the VHA Simulation Learning, Education and Research Network (SimLEARN), who requested the Mini-Residency course directors to implement a similar educational program at the National Simulation Center in Orlando, Florida. SimLEARN was created to promote best practices in learning and education and provides a high-tech immersive environment for the development and delivery of simulation-based training curricula to facilitate workforce development.22 This article describes the initial experience of the VHA SimLEARN MSK continuing professional development (CPD) training programs, including curriculum design and educational impact on early learners, and how this informed additional CPD needs to continue advancing MSK education and care.
Methods
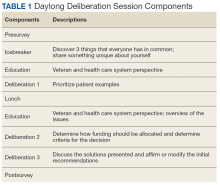
The initial vision was inspired by the national MSK Mini-Residency initiative for PCPs, which involved 13 US Department of Veterans Affairs (VA) medical centers; its development, dissemination, and validity evidence for assessment methods have been previously described.17,18,23 SimLEARN leadership attended a Mini-Residency, observing the educational experience and identifying learning objectives most aligned with national goals. The director and codirector of the MSK Mini-Residency (MJB, AMB) then worked with SimLEARN using its educational platform and train-the-trainer model to create a condensed 2-day course, centered on primary care evaluation and management of shoulder and knee pain. The course also included elements supporting educational leaders in providing similar trainings at their local facility (Table 1).
Curriculum was introduced through didactics and reinforced in hands-on sessions enhanced by peer-teaching, arthrocentesis task trainers, and simulated patient experiences. At the end of day 1, participants engaged in critical reflection, reviewing knowledge and skills they had acquired.
On day 2, each participant was evaluated using an observed structured clinical examination (OSCE) for the shoulder, followed by an observed structured teaching experience (OSTE). Given the complexity of the physical examination and the greater potential for appropriate interpretation of clinical findings to influence best practice care, the shoulder was emphasized for these experiences. Time constraints of a 2-day program based on SimLEARN format requirements prevented including an additional OSCE for the knee. At the conclusion of the course, faculty and participants discussed strategies for bringing this educational experience to learners at their local facilities as well as for avoiding potential barriers to implementation. The course was accredited through the VHA Employee Education System (EES), and participants received 16 hours of CPD credit.
Participants
Opportunity to attend was communicated through national, regional, and local VHA organizational networks. Participants self-registered online through the VHA Talent Management System, the main learning resource for VHA employee education, and registration was open to both PCPs and clinician educators. Class size was limited to 10 to facilitate detailed faculty observation during skill acquisition experiences, simulations, and assessment exercises.
Program Evaluation
A standard process for evaluating and measuring learning objectives was performed through VHA EES. Self-assessment surveys and OSCEs were used to assess the activity.
Self-assessment surveys were administered at the beginning and end of the program. Content was adapted from that used in the national MSK Mini-Residency initiative and revised by experts in survey design.18,24,25 Pre- and postcourse surveys asked participants to rate how important it was for them to be competent in evaluating shoulder and knee pain and in performing related joint injections, as well as to rate their level of confidence in their ability to evaluate and manage these conditions. The survey used 5 construct-specific response options distributed equally on a visual scale. Participants’ learning goals were collected on the precourse survey.
Participants’ competence in performing and interpreting a systematic and thorough physical examination of the shoulder and in suggesting a reasonable plan of management were assessed using a single-station OSCE. This tool, which presented learners with a simulated case depicting rotator cuff pathology, has been described in multiple educational settings, and validity evidence supporting its use has been published.18,19,23 Course faculty conducted the OSCE, one as the simulated patient, the other as the rater. Immediately following the examination, both faculty conducted a debriefing session with each participant. The OSCE was scored using the validated checklist for specific elements of the shoulder exam, followed by a structured sequence of questions exploring participants’ interpretation of findings, diagnostic impressions, and recommendations for initial management. Scores for participants’ differential diagnosis were based on the completeness and specificity of diagnoses given; scores for management plans were based on appropriateness and accuracy of both the primary and secondary approach to treatment or further diagnostic efforts. A global rating (range 1 to 9) was assigned, independent of scores in other domains.
Following the OSCE, participants rotated through a 3-cycle OSTE where they practiced the roles of simulated patient, learner, and educator. Faculty observed each OSTE and led focused debriefing sessions immediately following each rotation to facilitate participants’ critical reflection of their involvement in these elements of the course. This exercise was formative without quantitative assessment of performance.
Statistical Analysis
Pre- and postsurvey data were analyzed using a paired Student t test. Comparisons between multiple variables (eg, OSCE scores by years of experience or level of credentials) were analyzed using analysis of variance. Relationships between variables were analyzed with a Pearson correlation. All statistical analyses were conducted using IBM SPSS, Version 24 (Armonk, NY).
This project was reviewed by the institutional review board of the University of Utah and the Salt Lake City VA and was determined to be exempt from review because the work did not meet the definition of research with human subjects and was considered a quality improvement study.
Results
Twenty-four participants completed the program over 3 course offerings between February and May 2016, and all completed pre- and postcourse self-assessment surveys (Table 2). Self-ratings of the importance of competence in shoulder and knee MSK skills remained high before and after the course, and confidence improved significantly across all learning objectives. Despite the emphasis on the evaluation and management of shoulder pain, participants’ self-confidence still improved significantly with the knee—though these improvements were generally smaller in scale compared with those of the shoulder.
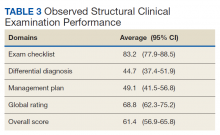
Overall OSCE scores and scores by domain were not found to be statistically different based on either years of experience or by level of credential or specialty (advanced practice registered nurse/physician assistant, PCP, or specialty care physician)(Table 3). However, there was a trend toward higher performance among the specialty care physician group, and a trend toward lower performance among participants with less than 3 years’ experience.
Discussion
Building on the foundation of other successful innovations in MSK education, the first year of the SimLEARN National MSK Training Program demonstrated the feasibility of a 2-day centralized national course as a method to increase participants’ confidence and competence in evaluating and managing MSK problems, and to disseminate a portable curriculum to a range of clinician educators. Although this course focused on developing competence for shoulder skills, including an OSCE on day 2, self-perceived improvements in participants’ ability to evaluate and manage knee pain were observed. Future program refinement and follow-up of participants’ experience and needs may lead to increased time allocated to the knee exam as well as objective measures of competence for knee skills.
In comparing our findings to the work that others have previously described, we looked for reports of CPD programs in 2 contexts: those that focused on acquisition of MSK skills relevant to clinical practice, and those designed as clinician educator or faculty development initiatives. Although there are few reports of MSK-themed CPD experiences designed specifically for nurses and allied health professionals, a recent effort to survey members of these disciplines in the United Kingdom was an important contribution to a systematic needs assessment.26-28 Increased support from leadership, mostly in terms of time allowance and budgetary support, was identified as an important driver to facilitate participation in MSK CPD experiences. Through SimLEARN, the VHA is investing in CPD, providing the MSK Training Programs and other courses at no cost to its employees.
Most published reports on physician education have not evaluated content knowledge or physical examination skills with measures for which validity evidence has been published.19,29,30 One notable exception is the 2000 Canadian Viscosupplementation Injector Preceptor experience, in which Bellamy and colleagues examined patient outcomes in evaluating their program.31
Our experience is congruent with the work of Macedo and colleagues and Sturpe and colleagues, who described the effectiveness and acceptability of an OSTE for faculty development.32,33 These studies emphasize debriefing, a critical element in faculty development identified by Steinert and colleagues in a 2006 best evidence medical education (BEME) review.34 The shoulder OSTE was one of the most well-received elements of our course, and each debrief was critical to facilitating rich discussions between educators and practitioners playing the role of teacher or student during this simulated experience, gaining insight into each other’s perspectives.
This program has several significant strengths: First, this is the most recent step in the development of a portfolio of innovative MSK CPD programs that were envisioned through a systematic process involving projections of cost-effectiveness, local pilot testing, and national expansion.17,18,35 Second, the SimLEARN program uses assessment tools for which validity evidence has been published, made available for reflective critique by educational scholars.19,23 This supports a national consortium of MSK educators, advancing clinical teaching and educational scholarship, and creating opportunities for interprofessional collaboration in congruence with the vision expressed in the 2010 Institute of Medicine report, “Redesigning Continuing Education in the Health Professions,” as well as the 2016 update of the BEME recommendations for faculty development.36,37
Our experience with the SimLEARN National MSK Training Program demonstrates need for 2 distinct courses: (1) the MSK Clinician—serving PCPs seeking to develop their skills in evaluating and managing patients with MSK problems; and (2), the MSK Master Educator—for those with preexisting content expertise who would value the introduction to a national curriculum and connections with other MSK master educators. Both of these are now offered regularly through SimLEARN for VHA and US Department of Defense employees. The MSK Clinician program establishes competence in systematically evaluating and managing shoulder and knee MSK problems in an educational setting and prepares participants for subsequent clinical experiences where they can perform related procedures if desired, under appropriate supervision. The Master Educator program introduces partici pants to the clinician curriculum and provides the opportunity to develop an individualized plan for implementation of an MSK educational program at their home institutions. Participants are selected through a competitive application process, and funding for travel to attend the Master Educator program is provided by SimLEARN for participants who are accepted. Additionally, the Master Educator program serves as a repository for potential future SimLEARN MSK Clinician course faculty.
Limitations
The small number of participants may limit the validity of our conclusions. Although we included an OSCE to measure competence in performing and interpreting the shoulder exam, the durability of these skills is not known. Periodic postcourse OSCEs could help determine this and refresh and preserve accuracy in the performance of specific maneuvers. Second, although this experience was rated highly by participants, we do not know the impact of the program on their daily work or career trajectory. Sustained follow-up of learners, perhaps developed on the model of the Long-Term Career Outcome Study, may increase the value of this experience for future participants.38 This program appealed to a diverse pool of learners, with a broad range of precourse expertise and varied expectations of how course experiences would impact their future work and career development. Some clinical educator attendees came from tertiary care facilities affiliated with academic medical centers, held specialist or subspecialist credentials, and had formal responsibilities as leaders in HPE. Other clinical practitioner participants were solitary PCPs, often in rural or home-based settings; although they may have been eager to apply new knowledge and skills in patient care, they neither anticipated nor desired any role as an educator.
Conclusion
The initial SimLEARN MSK Training Program provides PCPs and clinician educators with rich learning experiences, increasing confidence in addressing MSK problems and competence in performing and interpreting a systematic physical examination of the shoulder. The success of this program has created new opportunities for practitioners seeking to strengthen clinical skills and for leaders in health professions education looking to disseminate similar trainings and connect with a national group of educators.
Acknowledgments
The authors gratefully acknowledge the faculty and staff at the Veterans Health Administration SimLEARN National Simulation Center, the faculty of the Salt Lake City Musculoskeletal Mini-Residency program, the supportive leadership of the George E. Wahlen Salt Lake City Veterans Affairs Medical Center, and the efforts of Danielle Blake for logistical support and data entry.
1. Helmick CG, Felson DT, Lawrence RC, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15-25.
2. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001-2005. Arthritis Care Res (Hoboken). 2010;62(4):460-464.
4. Gupta S, Hawker GA, Laporte A, Croxford R, Coyte PC. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford). 2005;44(12):1531-1537.
5. Gore M, Tai KS, Sadosky A, Leslie D, Stacey BR. Clinical comorbidities, treatment patterns, and direct medical costs of patients with osteoarthritis in usual care: a retrospective claims database analysis. J Med Econ. 2011;14(4):497-507.
6. Rabenda V, Manette C, Lemmens R, Mariani AM, Struvay N, Reginster JY. Direct and indirect costs attributable to osteoarthritis in active subjects. J Rheumatol. 2006;33(6):1152-1158.
7. Day CS, Yeh AC. Evidence of educational inadequacies in region-specific musculoskeletal medicine. Clin Orthop Relat Res. 2008;466(10):2542-2547.
8. Glazier RH, Dalby DM, Badley EM, Hawker GA, Bell MJ, Buchbinder R. Determinants of physician confidence in the primary care management of musculoskeletal disorders. J Rheumatol. 1996;23(2):351-356.
9. Haywood BL, Porter SL, Grana WA. Assessment of musculoskeletal knowledge in primary care residents. Am J Orthop (Belle Mead NJ). 2006;35(6):273-275.
10. Monrad SU, Zeller JL, Craig CL, Diponio LA. Musculoskeletal education in US medical schools: lessons from the past and suggestions for the future. Curr Rev Musculoskelet Med. 2011;4(3):91-98.
11. O’Dunn-Orto A, Hartling L, Campbell S, Oswald AE. Teaching musculoskeletal clinical skills to medical trainees and physicians: a Best Evidence in Medical Education systematic review of strategies and their effectiveness: BEME Guide No. 18. Med Teach. 2012;34(2):93-102.
12. Wilcox T, Oyler J, Harada C, Utset T. Musculoskeletal exam and joint injection training for internal medicine residents. J Gen Intern Med. 2006;21(5):521-523.
13. Petron DJ, Greis PE, Aoki SK, et al. Use of knee magnetic resonance imaging by primary care physicians in patients aged 40 years and older. Sports Health. 2010;2(5):385-390.
14. Roberts TT, Singer N, Hushmendy S, et al. MRI for the evaluation of knee pain: comparison of ordering practices of primary care physicians and orthopaedic surgeons. J Bone Joint Surg Am. 2015;97(9):709-714.
15. Wylie JD, Crim JR, Working ZM, Schmidt RL, Burks RT. Physician provider type influences utilization and diagnostic utility of magnetic resonance imaging of the knee. J Bone Joint Surg Am. 2015;97(1):56-62.
16. Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC; 2013.
17. Battistone MJ, Barker AM, Lawrence P, Grotzke MP, Cannon GW. Mini-residency in musculoskeletal care: an interprofessional, mixed-methods educational initiative for primary care providers. Arthritis Care Res (Hoboken). 2016;68(2):275-279.
18. Battistone MJ, Barker AM, Grotzke MP, Beck JP, Lawrence P, Cannon GW. “Mini-residency” in musculoskeletal care: a national continuing professional development program for primary care providers. J Gen Intern Med. 2016;31(11):1301-1307.
19. Battistone MJ, Barker AM, Grotzke MP, et al. Effectiveness of an interprofessional and multidisciplinary musculoskeletal training program. J Grad Med Educ. 2016;8(3):398-404.
20. Battistone MJ, Barker AM, Lawrence P, Grotzke M, Cannon GW. Two-year impact of a continuing professional education program to train primary care providers to perform arthrocentesis. Presented at: 2017 ACR/ARHP Annual Meeting [Abstract 909]. https://acrabstracts.org/abstract/two-year-impact-of-a-continuing-professional-education-program-to-train-primary-care-providers-to-perform-arthrocentesis. Accessed November 14, 2019.
21. Call MR, Barker AM, Lawrence P, Cannon GW, Battistone MJ. Impact of a musculoskeltal “mini-residency” continuing professional education program on knee mri orders by primary care providers. Presented at: 2015 ACR/ARHP Annual Meeting [Abstract 1011]. https://acrabstracts.org/abstract/impact-of-a-musculoskeletal-aeoemini-residencyae%ef%bf%bd-continuing-professional-education-program-on-knee-mri-orders-by-primary-care-providers. Accessed November 14, 2019.
22. US Department of Veterans Affairs. VHA SimLEARN. https://www.simlearn.va.gov/SIMLEARN/about_us.asp. Updated January 24, 2019. Accessed November 13, 2019.
23. Battistone MJ, Barker AM, Beck JP, Tashjian RZ, Cannon GW. Validity evidence for two objective structured clinical examination stations to evaluate core skills of the shoulder and knee assessment. BMC Med Educ. 2017;17(1):13.
24. Artino AR Jr, La Rochelle JS, Dezee KJ, Gehlbach H. Developing questionnaires for educational research: AMEE Guide No. 87. Med Teach. 2014;36(6):463-474.
25. Gehlbach H, Artino AR Jr. The survey checklist (Manifesto). Acad Med. 2018;93(3):360-366.
26. Haywood H, Pain H, Ryan S, Adams J. The continuing professional development for nurses and allied health professionals working within musculoskeletal services: a national UK survey. Musculoskeletal Care. 2013;11(2):63-70.
27. Haywood H, Pain H, Ryan S, Adams J. Continuing professional development: issues raised by nurses and allied health professionals working in musculoskeletal settings. Musculoskeletal Care. 2013;11(3):136-144.
28. Warburton L. Continuing professional development in musculoskeletal domains. Musculoskeletal Care. 2012;10(3):125-126.
29. Stansfield RB, Diponio L, Craig C, et al. Assessing musculoskeletal examination skills and diagnostic reasoning of 4th year medical students using a novel objective structured clinical exam. BMC Med Educ. 2016;16(1):268.
30. Hose MK, Fontanesi J, Woytowitz M, Jarrin D, Quan A. Competency based clinical shoulder examination training improves physical exam, confidence, and knowledge in common shoulder conditions. J Gen Intern Med. 2017;32(11):1261-1265.
31. Bellamy N, Goldstein LD, Tekanoff RA. Continuing medical education-driven skills acquisition and impact on improved patient outcomes in family practice setting. J Contin Educ Health Prof. 2000;20(1):52-61.
32. Macedo L, Sturpe DA, Haines ST, Layson-Wolf C, Tofade TS, McPherson ML. An objective structured teaching exercise (OSTE) for preceptor development. Curr Pharm Teach Learn. 2015;7(5):627-634.
33. Sturpe DA, Schaivone KA. A primer for objective structured teaching exercises. Am J Pharm Educ. 2014;78(5):104.
34. Steinert Y, Mann K, Centeno A, et al. A systematic review of faculty development initiatives designed to improve teaching effectiveness in medical education: BEME Guide No. 8. Med Teach. 2006;28(6):497-526.
35. Nelson SD, Nelson RE, Cannon GW, et al. Cost-effectiveness of training rural providers to identify and treat patients at risk for fragility fractures. Osteoporos Int. 2014;25(12):2701-2707.
36. Steinert Y, Mann K, Anderson B, et al. A systematic review of faculty development initiatives designed to enhance teaching effectiveness: A 10-year update: BEME Guide No. 40. Med Teach. 2016;38(8):769-786.
37. Institute of Medicine. Redesigning Continuing Education in the Health Professions. Washington, DC: National Academies Press; 2010.
38. Durning SJ, Dong T, LaRochelle JL, et al. The long-term career outcome study: lessons learned and implications for educational practice. Mil Med. 2015;180(suppl 4):164-170.
Diseases of the musculoskeletal (MSK) system are common, accounting for some of the most frequent visits to primary care clinics.1-3 In addition, care for patients with chronic MSK diseases represents a substantial economic burden.4-6
In response to this clinical training need, the Veterans Health Administration (VHA) developed a portfolio of educational experiences for VHA health care providers and trainees, including both the Salt Lake City and National MSK “mini-residencies.”17-19 These programs have educated more than 800 individuals. Early observations show a progressive increase in the number of joint injections performed at participant’s VHA clinics as well as a reduction in unnecessary magnetic resonance imaging orders of the knee.20,21 These findings may be interpreted as markers for improved access to care for veterans as well as cost savings for the health care system.
The success of these early initiatives was recognized by the medical leadership of the VHA Simulation Learning, Education and Research Network (SimLEARN), who requested the Mini-Residency course directors to implement a similar educational program at the National Simulation Center in Orlando, Florida. SimLEARN was created to promote best practices in learning and education and provides a high-tech immersive environment for the development and delivery of simulation-based training curricula to facilitate workforce development.22 This article describes the initial experience of the VHA SimLEARN MSK continuing professional development (CPD) training programs, including curriculum design and educational impact on early learners, and how this informed additional CPD needs to continue advancing MSK education and care.
Methods
The initial vision was inspired by the national MSK Mini-Residency initiative for PCPs, which involved 13 US Department of Veterans Affairs (VA) medical centers; its development, dissemination, and validity evidence for assessment methods have been previously described.17,18,23 SimLEARN leadership attended a Mini-Residency, observing the educational experience and identifying learning objectives most aligned with national goals. The director and codirector of the MSK Mini-Residency (MJB, AMB) then worked with SimLEARN using its educational platform and train-the-trainer model to create a condensed 2-day course, centered on primary care evaluation and management of shoulder and knee pain. The course also included elements supporting educational leaders in providing similar trainings at their local facility (Table 1).
Curriculum was introduced through didactics and reinforced in hands-on sessions enhanced by peer-teaching, arthrocentesis task trainers, and simulated patient experiences. At the end of day 1, participants engaged in critical reflection, reviewing knowledge and skills they had acquired.
On day 2, each participant was evaluated using an observed structured clinical examination (OSCE) for the shoulder, followed by an observed structured teaching experience (OSTE). Given the complexity of the physical examination and the greater potential for appropriate interpretation of clinical findings to influence best practice care, the shoulder was emphasized for these experiences. Time constraints of a 2-day program based on SimLEARN format requirements prevented including an additional OSCE for the knee. At the conclusion of the course, faculty and participants discussed strategies for bringing this educational experience to learners at their local facilities as well as for avoiding potential barriers to implementation. The course was accredited through the VHA Employee Education System (EES), and participants received 16 hours of CPD credit.
Participants
Opportunity to attend was communicated through national, regional, and local VHA organizational networks. Participants self-registered online through the VHA Talent Management System, the main learning resource for VHA employee education, and registration was open to both PCPs and clinician educators. Class size was limited to 10 to facilitate detailed faculty observation during skill acquisition experiences, simulations, and assessment exercises.
Program Evaluation
A standard process for evaluating and measuring learning objectives was performed through VHA EES. Self-assessment surveys and OSCEs were used to assess the activity.
Self-assessment surveys were administered at the beginning and end of the program. Content was adapted from that used in the national MSK Mini-Residency initiative and revised by experts in survey design.18,24,25 Pre- and postcourse surveys asked participants to rate how important it was for them to be competent in evaluating shoulder and knee pain and in performing related joint injections, as well as to rate their level of confidence in their ability to evaluate and manage these conditions. The survey used 5 construct-specific response options distributed equally on a visual scale. Participants’ learning goals were collected on the precourse survey.
Participants’ competence in performing and interpreting a systematic and thorough physical examination of the shoulder and in suggesting a reasonable plan of management were assessed using a single-station OSCE. This tool, which presented learners with a simulated case depicting rotator cuff pathology, has been described in multiple educational settings, and validity evidence supporting its use has been published.18,19,23 Course faculty conducted the OSCE, one as the simulated patient, the other as the rater. Immediately following the examination, both faculty conducted a debriefing session with each participant. The OSCE was scored using the validated checklist for specific elements of the shoulder exam, followed by a structured sequence of questions exploring participants’ interpretation of findings, diagnostic impressions, and recommendations for initial management. Scores for participants’ differential diagnosis were based on the completeness and specificity of diagnoses given; scores for management plans were based on appropriateness and accuracy of both the primary and secondary approach to treatment or further diagnostic efforts. A global rating (range 1 to 9) was assigned, independent of scores in other domains.
Following the OSCE, participants rotated through a 3-cycle OSTE where they practiced the roles of simulated patient, learner, and educator. Faculty observed each OSTE and led focused debriefing sessions immediately following each rotation to facilitate participants’ critical reflection of their involvement in these elements of the course. This exercise was formative without quantitative assessment of performance.
Statistical Analysis
Pre- and postsurvey data were analyzed using a paired Student t test. Comparisons between multiple variables (eg, OSCE scores by years of experience or level of credentials) were analyzed using analysis of variance. Relationships between variables were analyzed with a Pearson correlation. All statistical analyses were conducted using IBM SPSS, Version 24 (Armonk, NY).
This project was reviewed by the institutional review board of the University of Utah and the Salt Lake City VA and was determined to be exempt from review because the work did not meet the definition of research with human subjects and was considered a quality improvement study.
Results
Twenty-four participants completed the program over 3 course offerings between February and May 2016, and all completed pre- and postcourse self-assessment surveys (Table 2). Self-ratings of the importance of competence in shoulder and knee MSK skills remained high before and after the course, and confidence improved significantly across all learning objectives. Despite the emphasis on the evaluation and management of shoulder pain, participants’ self-confidence still improved significantly with the knee—though these improvements were generally smaller in scale compared with those of the shoulder.
Overall OSCE scores and scores by domain were not found to be statistically different based on either years of experience or by level of credential or specialty (advanced practice registered nurse/physician assistant, PCP, or specialty care physician)(Table 3). However, there was a trend toward higher performance among the specialty care physician group, and a trend toward lower performance among participants with less than 3 years’ experience.
Discussion
Building on the foundation of other successful innovations in MSK education, the first year of the SimLEARN National MSK Training Program demonstrated the feasibility of a 2-day centralized national course as a method to increase participants’ confidence and competence in evaluating and managing MSK problems, and to disseminate a portable curriculum to a range of clinician educators. Although this course focused on developing competence for shoulder skills, including an OSCE on day 2, self-perceived improvements in participants’ ability to evaluate and manage knee pain were observed. Future program refinement and follow-up of participants’ experience and needs may lead to increased time allocated to the knee exam as well as objective measures of competence for knee skills.
In comparing our findings to the work that others have previously described, we looked for reports of CPD programs in 2 contexts: those that focused on acquisition of MSK skills relevant to clinical practice, and those designed as clinician educator or faculty development initiatives. Although there are few reports of MSK-themed CPD experiences designed specifically for nurses and allied health professionals, a recent effort to survey members of these disciplines in the United Kingdom was an important contribution to a systematic needs assessment.26-28 Increased support from leadership, mostly in terms of time allowance and budgetary support, was identified as an important driver to facilitate participation in MSK CPD experiences. Through SimLEARN, the VHA is investing in CPD, providing the MSK Training Programs and other courses at no cost to its employees.
Most published reports on physician education have not evaluated content knowledge or physical examination skills with measures for which validity evidence has been published.19,29,30 One notable exception is the 2000 Canadian Viscosupplementation Injector Preceptor experience, in which Bellamy and colleagues examined patient outcomes in evaluating their program.31
Our experience is congruent with the work of Macedo and colleagues and Sturpe and colleagues, who described the effectiveness and acceptability of an OSTE for faculty development.32,33 These studies emphasize debriefing, a critical element in faculty development identified by Steinert and colleagues in a 2006 best evidence medical education (BEME) review.34 The shoulder OSTE was one of the most well-received elements of our course, and each debrief was critical to facilitating rich discussions between educators and practitioners playing the role of teacher or student during this simulated experience, gaining insight into each other’s perspectives.
This program has several significant strengths: First, this is the most recent step in the development of a portfolio of innovative MSK CPD programs that were envisioned through a systematic process involving projections of cost-effectiveness, local pilot testing, and national expansion.17,18,35 Second, the SimLEARN program uses assessment tools for which validity evidence has been published, made available for reflective critique by educational scholars.19,23 This supports a national consortium of MSK educators, advancing clinical teaching and educational scholarship, and creating opportunities for interprofessional collaboration in congruence with the vision expressed in the 2010 Institute of Medicine report, “Redesigning Continuing Education in the Health Professions,” as well as the 2016 update of the BEME recommendations for faculty development.36,37
Our experience with the SimLEARN National MSK Training Program demonstrates need for 2 distinct courses: (1) the MSK Clinician—serving PCPs seeking to develop their skills in evaluating and managing patients with MSK problems; and (2), the MSK Master Educator—for those with preexisting content expertise who would value the introduction to a national curriculum and connections with other MSK master educators. Both of these are now offered regularly through SimLEARN for VHA and US Department of Defense employees. The MSK Clinician program establishes competence in systematically evaluating and managing shoulder and knee MSK problems in an educational setting and prepares participants for subsequent clinical experiences where they can perform related procedures if desired, under appropriate supervision. The Master Educator program introduces partici pants to the clinician curriculum and provides the opportunity to develop an individualized plan for implementation of an MSK educational program at their home institutions. Participants are selected through a competitive application process, and funding for travel to attend the Master Educator program is provided by SimLEARN for participants who are accepted. Additionally, the Master Educator program serves as a repository for potential future SimLEARN MSK Clinician course faculty.
Limitations
The small number of participants may limit the validity of our conclusions. Although we included an OSCE to measure competence in performing and interpreting the shoulder exam, the durability of these skills is not known. Periodic postcourse OSCEs could help determine this and refresh and preserve accuracy in the performance of specific maneuvers. Second, although this experience was rated highly by participants, we do not know the impact of the program on their daily work or career trajectory. Sustained follow-up of learners, perhaps developed on the model of the Long-Term Career Outcome Study, may increase the value of this experience for future participants.38 This program appealed to a diverse pool of learners, with a broad range of precourse expertise and varied expectations of how course experiences would impact their future work and career development. Some clinical educator attendees came from tertiary care facilities affiliated with academic medical centers, held specialist or subspecialist credentials, and had formal responsibilities as leaders in HPE. Other clinical practitioner participants were solitary PCPs, often in rural or home-based settings; although they may have been eager to apply new knowledge and skills in patient care, they neither anticipated nor desired any role as an educator.
Conclusion
The initial SimLEARN MSK Training Program provides PCPs and clinician educators with rich learning experiences, increasing confidence in addressing MSK problems and competence in performing and interpreting a systematic physical examination of the shoulder. The success of this program has created new opportunities for practitioners seeking to strengthen clinical skills and for leaders in health professions education looking to disseminate similar trainings and connect with a national group of educators.
Acknowledgments
The authors gratefully acknowledge the faculty and staff at the Veterans Health Administration SimLEARN National Simulation Center, the faculty of the Salt Lake City Musculoskeletal Mini-Residency program, the supportive leadership of the George E. Wahlen Salt Lake City Veterans Affairs Medical Center, and the efforts of Danielle Blake for logistical support and data entry.
Diseases of the musculoskeletal (MSK) system are common, accounting for some of the most frequent visits to primary care clinics.1-3 In addition, care for patients with chronic MSK diseases represents a substantial economic burden.4-6
In response to this clinical training need, the Veterans Health Administration (VHA) developed a portfolio of educational experiences for VHA health care providers and trainees, including both the Salt Lake City and National MSK “mini-residencies.”17-19 These programs have educated more than 800 individuals. Early observations show a progressive increase in the number of joint injections performed at participant’s VHA clinics as well as a reduction in unnecessary magnetic resonance imaging orders of the knee.20,21 These findings may be interpreted as markers for improved access to care for veterans as well as cost savings for the health care system.
The success of these early initiatives was recognized by the medical leadership of the VHA Simulation Learning, Education and Research Network (SimLEARN), who requested the Mini-Residency course directors to implement a similar educational program at the National Simulation Center in Orlando, Florida. SimLEARN was created to promote best practices in learning and education and provides a high-tech immersive environment for the development and delivery of simulation-based training curricula to facilitate workforce development.22 This article describes the initial experience of the VHA SimLEARN MSK continuing professional development (CPD) training programs, including curriculum design and educational impact on early learners, and how this informed additional CPD needs to continue advancing MSK education and care.
Methods
The initial vision was inspired by the national MSK Mini-Residency initiative for PCPs, which involved 13 US Department of Veterans Affairs (VA) medical centers; its development, dissemination, and validity evidence for assessment methods have been previously described.17,18,23 SimLEARN leadership attended a Mini-Residency, observing the educational experience and identifying learning objectives most aligned with national goals. The director and codirector of the MSK Mini-Residency (MJB, AMB) then worked with SimLEARN using its educational platform and train-the-trainer model to create a condensed 2-day course, centered on primary care evaluation and management of shoulder and knee pain. The course also included elements supporting educational leaders in providing similar trainings at their local facility (Table 1).
Curriculum was introduced through didactics and reinforced in hands-on sessions enhanced by peer-teaching, arthrocentesis task trainers, and simulated patient experiences. At the end of day 1, participants engaged in critical reflection, reviewing knowledge and skills they had acquired.
On day 2, each participant was evaluated using an observed structured clinical examination (OSCE) for the shoulder, followed by an observed structured teaching experience (OSTE). Given the complexity of the physical examination and the greater potential for appropriate interpretation of clinical findings to influence best practice care, the shoulder was emphasized for these experiences. Time constraints of a 2-day program based on SimLEARN format requirements prevented including an additional OSCE for the knee. At the conclusion of the course, faculty and participants discussed strategies for bringing this educational experience to learners at their local facilities as well as for avoiding potential barriers to implementation. The course was accredited through the VHA Employee Education System (EES), and participants received 16 hours of CPD credit.
Participants
Opportunity to attend was communicated through national, regional, and local VHA organizational networks. Participants self-registered online through the VHA Talent Management System, the main learning resource for VHA employee education, and registration was open to both PCPs and clinician educators. Class size was limited to 10 to facilitate detailed faculty observation during skill acquisition experiences, simulations, and assessment exercises.
Program Evaluation
A standard process for evaluating and measuring learning objectives was performed through VHA EES. Self-assessment surveys and OSCEs were used to assess the activity.
Self-assessment surveys were administered at the beginning and end of the program. Content was adapted from that used in the national MSK Mini-Residency initiative and revised by experts in survey design.18,24,25 Pre- and postcourse surveys asked participants to rate how important it was for them to be competent in evaluating shoulder and knee pain and in performing related joint injections, as well as to rate their level of confidence in their ability to evaluate and manage these conditions. The survey used 5 construct-specific response options distributed equally on a visual scale. Participants’ learning goals were collected on the precourse survey.
Participants’ competence in performing and interpreting a systematic and thorough physical examination of the shoulder and in suggesting a reasonable plan of management were assessed using a single-station OSCE. This tool, which presented learners with a simulated case depicting rotator cuff pathology, has been described in multiple educational settings, and validity evidence supporting its use has been published.18,19,23 Course faculty conducted the OSCE, one as the simulated patient, the other as the rater. Immediately following the examination, both faculty conducted a debriefing session with each participant. The OSCE was scored using the validated checklist for specific elements of the shoulder exam, followed by a structured sequence of questions exploring participants’ interpretation of findings, diagnostic impressions, and recommendations for initial management. Scores for participants’ differential diagnosis were based on the completeness and specificity of diagnoses given; scores for management plans were based on appropriateness and accuracy of both the primary and secondary approach to treatment or further diagnostic efforts. A global rating (range 1 to 9) was assigned, independent of scores in other domains.
Following the OSCE, participants rotated through a 3-cycle OSTE where they practiced the roles of simulated patient, learner, and educator. Faculty observed each OSTE and led focused debriefing sessions immediately following each rotation to facilitate participants’ critical reflection of their involvement in these elements of the course. This exercise was formative without quantitative assessment of performance.
Statistical Analysis
Pre- and postsurvey data were analyzed using a paired Student t test. Comparisons between multiple variables (eg, OSCE scores by years of experience or level of credentials) were analyzed using analysis of variance. Relationships between variables were analyzed with a Pearson correlation. All statistical analyses were conducted using IBM SPSS, Version 24 (Armonk, NY).
This project was reviewed by the institutional review board of the University of Utah and the Salt Lake City VA and was determined to be exempt from review because the work did not meet the definition of research with human subjects and was considered a quality improvement study.
Results
Twenty-four participants completed the program over 3 course offerings between February and May 2016, and all completed pre- and postcourse self-assessment surveys (Table 2). Self-ratings of the importance of competence in shoulder and knee MSK skills remained high before and after the course, and confidence improved significantly across all learning objectives. Despite the emphasis on the evaluation and management of shoulder pain, participants’ self-confidence still improved significantly with the knee—though these improvements were generally smaller in scale compared with those of the shoulder.
Overall OSCE scores and scores by domain were not found to be statistically different based on either years of experience or by level of credential or specialty (advanced practice registered nurse/physician assistant, PCP, or specialty care physician)(Table 3). However, there was a trend toward higher performance among the specialty care physician group, and a trend toward lower performance among participants with less than 3 years’ experience.
Discussion
Building on the foundation of other successful innovations in MSK education, the first year of the SimLEARN National MSK Training Program demonstrated the feasibility of a 2-day centralized national course as a method to increase participants’ confidence and competence in evaluating and managing MSK problems, and to disseminate a portable curriculum to a range of clinician educators. Although this course focused on developing competence for shoulder skills, including an OSCE on day 2, self-perceived improvements in participants’ ability to evaluate and manage knee pain were observed. Future program refinement and follow-up of participants’ experience and needs may lead to increased time allocated to the knee exam as well as objective measures of competence for knee skills.
In comparing our findings to the work that others have previously described, we looked for reports of CPD programs in 2 contexts: those that focused on acquisition of MSK skills relevant to clinical practice, and those designed as clinician educator or faculty development initiatives. Although there are few reports of MSK-themed CPD experiences designed specifically for nurses and allied health professionals, a recent effort to survey members of these disciplines in the United Kingdom was an important contribution to a systematic needs assessment.26-28 Increased support from leadership, mostly in terms of time allowance and budgetary support, was identified as an important driver to facilitate participation in MSK CPD experiences. Through SimLEARN, the VHA is investing in CPD, providing the MSK Training Programs and other courses at no cost to its employees.
Most published reports on physician education have not evaluated content knowledge or physical examination skills with measures for which validity evidence has been published.19,29,30 One notable exception is the 2000 Canadian Viscosupplementation Injector Preceptor experience, in which Bellamy and colleagues examined patient outcomes in evaluating their program.31
Our experience is congruent with the work of Macedo and colleagues and Sturpe and colleagues, who described the effectiveness and acceptability of an OSTE for faculty development.32,33 These studies emphasize debriefing, a critical element in faculty development identified by Steinert and colleagues in a 2006 best evidence medical education (BEME) review.34 The shoulder OSTE was one of the most well-received elements of our course, and each debrief was critical to facilitating rich discussions between educators and practitioners playing the role of teacher or student during this simulated experience, gaining insight into each other’s perspectives.
This program has several significant strengths: First, this is the most recent step in the development of a portfolio of innovative MSK CPD programs that were envisioned through a systematic process involving projections of cost-effectiveness, local pilot testing, and national expansion.17,18,35 Second, the SimLEARN program uses assessment tools for which validity evidence has been published, made available for reflective critique by educational scholars.19,23 This supports a national consortium of MSK educators, advancing clinical teaching and educational scholarship, and creating opportunities for interprofessional collaboration in congruence with the vision expressed in the 2010 Institute of Medicine report, “Redesigning Continuing Education in the Health Professions,” as well as the 2016 update of the BEME recommendations for faculty development.36,37
Our experience with the SimLEARN National MSK Training Program demonstrates need for 2 distinct courses: (1) the MSK Clinician—serving PCPs seeking to develop their skills in evaluating and managing patients with MSK problems; and (2), the MSK Master Educator—for those with preexisting content expertise who would value the introduction to a national curriculum and connections with other MSK master educators. Both of these are now offered regularly through SimLEARN for VHA and US Department of Defense employees. The MSK Clinician program establishes competence in systematically evaluating and managing shoulder and knee MSK problems in an educational setting and prepares participants for subsequent clinical experiences where they can perform related procedures if desired, under appropriate supervision. The Master Educator program introduces partici pants to the clinician curriculum and provides the opportunity to develop an individualized plan for implementation of an MSK educational program at their home institutions. Participants are selected through a competitive application process, and funding for travel to attend the Master Educator program is provided by SimLEARN for participants who are accepted. Additionally, the Master Educator program serves as a repository for potential future SimLEARN MSK Clinician course faculty.
Limitations
The small number of participants may limit the validity of our conclusions. Although we included an OSCE to measure competence in performing and interpreting the shoulder exam, the durability of these skills is not known. Periodic postcourse OSCEs could help determine this and refresh and preserve accuracy in the performance of specific maneuvers. Second, although this experience was rated highly by participants, we do not know the impact of the program on their daily work or career trajectory. Sustained follow-up of learners, perhaps developed on the model of the Long-Term Career Outcome Study, may increase the value of this experience for future participants.38 This program appealed to a diverse pool of learners, with a broad range of precourse expertise and varied expectations of how course experiences would impact their future work and career development. Some clinical educator attendees came from tertiary care facilities affiliated with academic medical centers, held specialist or subspecialist credentials, and had formal responsibilities as leaders in HPE. Other clinical practitioner participants were solitary PCPs, often in rural or home-based settings; although they may have been eager to apply new knowledge and skills in patient care, they neither anticipated nor desired any role as an educator.
Conclusion
The initial SimLEARN MSK Training Program provides PCPs and clinician educators with rich learning experiences, increasing confidence in addressing MSK problems and competence in performing and interpreting a systematic physical examination of the shoulder. The success of this program has created new opportunities for practitioners seeking to strengthen clinical skills and for leaders in health professions education looking to disseminate similar trainings and connect with a national group of educators.
Acknowledgments
The authors gratefully acknowledge the faculty and staff at the Veterans Health Administration SimLEARN National Simulation Center, the faculty of the Salt Lake City Musculoskeletal Mini-Residency program, the supportive leadership of the George E. Wahlen Salt Lake City Veterans Affairs Medical Center, and the efforts of Danielle Blake for logistical support and data entry.
1. Helmick CG, Felson DT, Lawrence RC, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15-25.
2. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001-2005. Arthritis Care Res (Hoboken). 2010;62(4):460-464.
4. Gupta S, Hawker GA, Laporte A, Croxford R, Coyte PC. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford). 2005;44(12):1531-1537.
5. Gore M, Tai KS, Sadosky A, Leslie D, Stacey BR. Clinical comorbidities, treatment patterns, and direct medical costs of patients with osteoarthritis in usual care: a retrospective claims database analysis. J Med Econ. 2011;14(4):497-507.
6. Rabenda V, Manette C, Lemmens R, Mariani AM, Struvay N, Reginster JY. Direct and indirect costs attributable to osteoarthritis in active subjects. J Rheumatol. 2006;33(6):1152-1158.
7. Day CS, Yeh AC. Evidence of educational inadequacies in region-specific musculoskeletal medicine. Clin Orthop Relat Res. 2008;466(10):2542-2547.
8. Glazier RH, Dalby DM, Badley EM, Hawker GA, Bell MJ, Buchbinder R. Determinants of physician confidence in the primary care management of musculoskeletal disorders. J Rheumatol. 1996;23(2):351-356.
9. Haywood BL, Porter SL, Grana WA. Assessment of musculoskeletal knowledge in primary care residents. Am J Orthop (Belle Mead NJ). 2006;35(6):273-275.
10. Monrad SU, Zeller JL, Craig CL, Diponio LA. Musculoskeletal education in US medical schools: lessons from the past and suggestions for the future. Curr Rev Musculoskelet Med. 2011;4(3):91-98.
11. O’Dunn-Orto A, Hartling L, Campbell S, Oswald AE. Teaching musculoskeletal clinical skills to medical trainees and physicians: a Best Evidence in Medical Education systematic review of strategies and their effectiveness: BEME Guide No. 18. Med Teach. 2012;34(2):93-102.
12. Wilcox T, Oyler J, Harada C, Utset T. Musculoskeletal exam and joint injection training for internal medicine residents. J Gen Intern Med. 2006;21(5):521-523.
13. Petron DJ, Greis PE, Aoki SK, et al. Use of knee magnetic resonance imaging by primary care physicians in patients aged 40 years and older. Sports Health. 2010;2(5):385-390.
14. Roberts TT, Singer N, Hushmendy S, et al. MRI for the evaluation of knee pain: comparison of ordering practices of primary care physicians and orthopaedic surgeons. J Bone Joint Surg Am. 2015;97(9):709-714.
15. Wylie JD, Crim JR, Working ZM, Schmidt RL, Burks RT. Physician provider type influences utilization and diagnostic utility of magnetic resonance imaging of the knee. J Bone Joint Surg Am. 2015;97(1):56-62.
16. Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC; 2013.
17. Battistone MJ, Barker AM, Lawrence P, Grotzke MP, Cannon GW. Mini-residency in musculoskeletal care: an interprofessional, mixed-methods educational initiative for primary care providers. Arthritis Care Res (Hoboken). 2016;68(2):275-279.
18. Battistone MJ, Barker AM, Grotzke MP, Beck JP, Lawrence P, Cannon GW. “Mini-residency” in musculoskeletal care: a national continuing professional development program for primary care providers. J Gen Intern Med. 2016;31(11):1301-1307.
19. Battistone MJ, Barker AM, Grotzke MP, et al. Effectiveness of an interprofessional and multidisciplinary musculoskeletal training program. J Grad Med Educ. 2016;8(3):398-404.
20. Battistone MJ, Barker AM, Lawrence P, Grotzke M, Cannon GW. Two-year impact of a continuing professional education program to train primary care providers to perform arthrocentesis. Presented at: 2017 ACR/ARHP Annual Meeting [Abstract 909]. https://acrabstracts.org/abstract/two-year-impact-of-a-continuing-professional-education-program-to-train-primary-care-providers-to-perform-arthrocentesis. Accessed November 14, 2019.
21. Call MR, Barker AM, Lawrence P, Cannon GW, Battistone MJ. Impact of a musculoskeltal “mini-residency” continuing professional education program on knee mri orders by primary care providers. Presented at: 2015 ACR/ARHP Annual Meeting [Abstract 1011]. https://acrabstracts.org/abstract/impact-of-a-musculoskeletal-aeoemini-residencyae%ef%bf%bd-continuing-professional-education-program-on-knee-mri-orders-by-primary-care-providers. Accessed November 14, 2019.
22. US Department of Veterans Affairs. VHA SimLEARN. https://www.simlearn.va.gov/SIMLEARN/about_us.asp. Updated January 24, 2019. Accessed November 13, 2019.
23. Battistone MJ, Barker AM, Beck JP, Tashjian RZ, Cannon GW. Validity evidence for two objective structured clinical examination stations to evaluate core skills of the shoulder and knee assessment. BMC Med Educ. 2017;17(1):13.
24. Artino AR Jr, La Rochelle JS, Dezee KJ, Gehlbach H. Developing questionnaires for educational research: AMEE Guide No. 87. Med Teach. 2014;36(6):463-474.
25. Gehlbach H, Artino AR Jr. The survey checklist (Manifesto). Acad Med. 2018;93(3):360-366.
26. Haywood H, Pain H, Ryan S, Adams J. The continuing professional development for nurses and allied health professionals working within musculoskeletal services: a national UK survey. Musculoskeletal Care. 2013;11(2):63-70.
27. Haywood H, Pain H, Ryan S, Adams J. Continuing professional development: issues raised by nurses and allied health professionals working in musculoskeletal settings. Musculoskeletal Care. 2013;11(3):136-144.
28. Warburton L. Continuing professional development in musculoskeletal domains. Musculoskeletal Care. 2012;10(3):125-126.
29. Stansfield RB, Diponio L, Craig C, et al. Assessing musculoskeletal examination skills and diagnostic reasoning of 4th year medical students using a novel objective structured clinical exam. BMC Med Educ. 2016;16(1):268.
30. Hose MK, Fontanesi J, Woytowitz M, Jarrin D, Quan A. Competency based clinical shoulder examination training improves physical exam, confidence, and knowledge in common shoulder conditions. J Gen Intern Med. 2017;32(11):1261-1265.
31. Bellamy N, Goldstein LD, Tekanoff RA. Continuing medical education-driven skills acquisition and impact on improved patient outcomes in family practice setting. J Contin Educ Health Prof. 2000;20(1):52-61.
32. Macedo L, Sturpe DA, Haines ST, Layson-Wolf C, Tofade TS, McPherson ML. An objective structured teaching exercise (OSTE) for preceptor development. Curr Pharm Teach Learn. 2015;7(5):627-634.
33. Sturpe DA, Schaivone KA. A primer for objective structured teaching exercises. Am J Pharm Educ. 2014;78(5):104.
34. Steinert Y, Mann K, Centeno A, et al. A systematic review of faculty development initiatives designed to improve teaching effectiveness in medical education: BEME Guide No. 8. Med Teach. 2006;28(6):497-526.
35. Nelson SD, Nelson RE, Cannon GW, et al. Cost-effectiveness of training rural providers to identify and treat patients at risk for fragility fractures. Osteoporos Int. 2014;25(12):2701-2707.
36. Steinert Y, Mann K, Anderson B, et al. A systematic review of faculty development initiatives designed to enhance teaching effectiveness: A 10-year update: BEME Guide No. 40. Med Teach. 2016;38(8):769-786.
37. Institute of Medicine. Redesigning Continuing Education in the Health Professions. Washington, DC: National Academies Press; 2010.
38. Durning SJ, Dong T, LaRochelle JL, et al. The long-term career outcome study: lessons learned and implications for educational practice. Mil Med. 2015;180(suppl 4):164-170.
1. Helmick CG, Felson DT, Lawrence RC, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15-25.
2. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35.
3. Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001-2005. Arthritis Care Res (Hoboken). 2010;62(4):460-464.
4. Gupta S, Hawker GA, Laporte A, Croxford R, Coyte PC. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford). 2005;44(12):1531-1537.
5. Gore M, Tai KS, Sadosky A, Leslie D, Stacey BR. Clinical comorbidities, treatment patterns, and direct medical costs of patients with osteoarthritis in usual care: a retrospective claims database analysis. J Med Econ. 2011;14(4):497-507.
6. Rabenda V, Manette C, Lemmens R, Mariani AM, Struvay N, Reginster JY. Direct and indirect costs attributable to osteoarthritis in active subjects. J Rheumatol. 2006;33(6):1152-1158.
7. Day CS, Yeh AC. Evidence of educational inadequacies in region-specific musculoskeletal medicine. Clin Orthop Relat Res. 2008;466(10):2542-2547.
8. Glazier RH, Dalby DM, Badley EM, Hawker GA, Bell MJ, Buchbinder R. Determinants of physician confidence in the primary care management of musculoskeletal disorders. J Rheumatol. 1996;23(2):351-356.
9. Haywood BL, Porter SL, Grana WA. Assessment of musculoskeletal knowledge in primary care residents. Am J Orthop (Belle Mead NJ). 2006;35(6):273-275.
10. Monrad SU, Zeller JL, Craig CL, Diponio LA. Musculoskeletal education in US medical schools: lessons from the past and suggestions for the future. Curr Rev Musculoskelet Med. 2011;4(3):91-98.
11. O’Dunn-Orto A, Hartling L, Campbell S, Oswald AE. Teaching musculoskeletal clinical skills to medical trainees and physicians: a Best Evidence in Medical Education systematic review of strategies and their effectiveness: BEME Guide No. 18. Med Teach. 2012;34(2):93-102.
12. Wilcox T, Oyler J, Harada C, Utset T. Musculoskeletal exam and joint injection training for internal medicine residents. J Gen Intern Med. 2006;21(5):521-523.
13. Petron DJ, Greis PE, Aoki SK, et al. Use of knee magnetic resonance imaging by primary care physicians in patients aged 40 years and older. Sports Health. 2010;2(5):385-390.
14. Roberts TT, Singer N, Hushmendy S, et al. MRI for the evaluation of knee pain: comparison of ordering practices of primary care physicians and orthopaedic surgeons. J Bone Joint Surg Am. 2015;97(9):709-714.
15. Wylie JD, Crim JR, Working ZM, Schmidt RL, Burks RT. Physician provider type influences utilization and diagnostic utility of magnetic resonance imaging of the knee. J Bone Joint Surg Am. 2015;97(1):56-62.
16. Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC; 2013.
17. Battistone MJ, Barker AM, Lawrence P, Grotzke MP, Cannon GW. Mini-residency in musculoskeletal care: an interprofessional, mixed-methods educational initiative for primary care providers. Arthritis Care Res (Hoboken). 2016;68(2):275-279.
18. Battistone MJ, Barker AM, Grotzke MP, Beck JP, Lawrence P, Cannon GW. “Mini-residency” in musculoskeletal care: a national continuing professional development program for primary care providers. J Gen Intern Med. 2016;31(11):1301-1307.
19. Battistone MJ, Barker AM, Grotzke MP, et al. Effectiveness of an interprofessional and multidisciplinary musculoskeletal training program. J Grad Med Educ. 2016;8(3):398-404.
20. Battistone MJ, Barker AM, Lawrence P, Grotzke M, Cannon GW. Two-year impact of a continuing professional education program to train primary care providers to perform arthrocentesis. Presented at: 2017 ACR/ARHP Annual Meeting [Abstract 909]. https://acrabstracts.org/abstract/two-year-impact-of-a-continuing-professional-education-program-to-train-primary-care-providers-to-perform-arthrocentesis. Accessed November 14, 2019.
21. Call MR, Barker AM, Lawrence P, Cannon GW, Battistone MJ. Impact of a musculoskeltal “mini-residency” continuing professional education program on knee mri orders by primary care providers. Presented at: 2015 ACR/ARHP Annual Meeting [Abstract 1011]. https://acrabstracts.org/abstract/impact-of-a-musculoskeletal-aeoemini-residencyae%ef%bf%bd-continuing-professional-education-program-on-knee-mri-orders-by-primary-care-providers. Accessed November 14, 2019.
22. US Department of Veterans Affairs. VHA SimLEARN. https://www.simlearn.va.gov/SIMLEARN/about_us.asp. Updated January 24, 2019. Accessed November 13, 2019.
23. Battistone MJ, Barker AM, Beck JP, Tashjian RZ, Cannon GW. Validity evidence for two objective structured clinical examination stations to evaluate core skills of the shoulder and knee assessment. BMC Med Educ. 2017;17(1):13.
24. Artino AR Jr, La Rochelle JS, Dezee KJ, Gehlbach H. Developing questionnaires for educational research: AMEE Guide No. 87. Med Teach. 2014;36(6):463-474.
25. Gehlbach H, Artino AR Jr. The survey checklist (Manifesto). Acad Med. 2018;93(3):360-366.
26. Haywood H, Pain H, Ryan S, Adams J. The continuing professional development for nurses and allied health professionals working within musculoskeletal services: a national UK survey. Musculoskeletal Care. 2013;11(2):63-70.
27. Haywood H, Pain H, Ryan S, Adams J. Continuing professional development: issues raised by nurses and allied health professionals working in musculoskeletal settings. Musculoskeletal Care. 2013;11(3):136-144.
28. Warburton L. Continuing professional development in musculoskeletal domains. Musculoskeletal Care. 2012;10(3):125-126.
29. Stansfield RB, Diponio L, Craig C, et al. Assessing musculoskeletal examination skills and diagnostic reasoning of 4th year medical students using a novel objective structured clinical exam. BMC Med Educ. 2016;16(1):268.
30. Hose MK, Fontanesi J, Woytowitz M, Jarrin D, Quan A. Competency based clinical shoulder examination training improves physical exam, confidence, and knowledge in common shoulder conditions. J Gen Intern Med. 2017;32(11):1261-1265.
31. Bellamy N, Goldstein LD, Tekanoff RA. Continuing medical education-driven skills acquisition and impact on improved patient outcomes in family practice setting. J Contin Educ Health Prof. 2000;20(1):52-61.
32. Macedo L, Sturpe DA, Haines ST, Layson-Wolf C, Tofade TS, McPherson ML. An objective structured teaching exercise (OSTE) for preceptor development. Curr Pharm Teach Learn. 2015;7(5):627-634.
33. Sturpe DA, Schaivone KA. A primer for objective structured teaching exercises. Am J Pharm Educ. 2014;78(5):104.
34. Steinert Y, Mann K, Centeno A, et al. A systematic review of faculty development initiatives designed to improve teaching effectiveness in medical education: BEME Guide No. 8. Med Teach. 2006;28(6):497-526.
35. Nelson SD, Nelson RE, Cannon GW, et al. Cost-effectiveness of training rural providers to identify and treat patients at risk for fragility fractures. Osteoporos Int. 2014;25(12):2701-2707.
36. Steinert Y, Mann K, Anderson B, et al. A systematic review of faculty development initiatives designed to enhance teaching effectiveness: A 10-year update: BEME Guide No. 40. Med Teach. 2016;38(8):769-786.
37. Institute of Medicine. Redesigning Continuing Education in the Health Professions. Washington, DC: National Academies Press; 2010.
38. Durning SJ, Dong T, LaRochelle JL, et al. The long-term career outcome study: lessons learned and implications for educational practice. Mil Med. 2015;180(suppl 4):164-170.
Incidentally Discovered Ochronosis Explaining Decades of Chronic Pain
Alkaptonuria is a rare autosomal recessive disorder uniquely known for causing black, or darkened, urine when left standing due to the renal excretion of excess homogentisic acid (HGA). When this disorder goes undiagnosed, as demonstrated in this case, patients experience its many complications without a unifying explanation. The disorder has 3 clinical stages that occur in a predictable order: clinical silence, clinical ochronosis, and ochronotic arthropathy. These stages lead to multiple musculoskeletal, cardiovascular (CV), and renal complications that can be mitigated with management focused on decreasing homogentisic acid buildup, alleviating symptoms, and close monitoring for these complications.
Case Presentation
A 61-year-old African American male with a medical history of multiple traumatic fractures, right Achilles tendon injury, early-onset multijoint osteoarthritis, chronic low back pain, and recurrent nephrolithiasis presented to the emergency department with sudden onset of sharp left ankle pain while moving furniture. His physical exam revealed a positive Thompson test—lack of foot plantar flexion with calf squeeze—and a subsequent magnetic resonance image (MRI) showed evidence of an acute Achilles tendon rupture.
Given these findings the patient was treated with nonsteroidal anti-inflammatory drugs (NSAIDs) and rest to allow for resolution of swelling and inflammation, followed by elective surgery a month later to repair the ruptured tendon. An operative report following his surgery described “black ends to the area where the Achilles was ruptured…and tendinopathy of the flexor hallucis longus with blackening of the flexor.”
A more in-depth patient history revealed that he underwent multiple invasive and noninvasive interventions for his chronic low back and joint pain with medical management of a prior right Achilles tendon injury. His medical history also included multiple nonspecific diagnoses, such as premature atherosclerosis (diagnosed in his third decade), severe lumbar degenerative disc disease, several tendonopathies and cartilage injuries (Figure 1), pseudogout (following calcium pyrophosphate dehydrate crystals found from a left knee aspirate), and chronic pain syndrome. Along this diagnostic journey, he had several health care providers (HCPs) in rheumatology, orthopedic surgery, pain management, and podiatry who offered a range of symptom management options, including physical therapy, NSAIDs, opioid agonists, tricyclic antidepressants, gabapentin, colchicine, and epidural steroid injections, all of which provided little or no relief of his pain. The patient reported that he told a HCP, “I’ll just live with [the pain].”
At the postsurgery follow-up, the patient reported that he had noticed dark urine and dark spots on his ears in the past. He also recounted that chronic joint pain was common in his family, with both his mother and brother receiving bilateral total knee replacements. Taking into consideration the surgical report and this new history, a urine assessment for HGA was ordered and yielded a diagnosis of alkaptonuria.
He later suffered an acute myocardial infarction leading to an incidental discovery of severe aortic stenosis on echocardiography, requiring coronary stent placements and transcatheter aortic valve replacement, respectively. He reported that with CV interventions and joint replacement surgeries, including bilateral knees and hips, his symptoms and quality of life began to significantly improve. However, he continued to have diffuse chronic joint pain unimproved with any single agent or intervention.
Discussion
Alkaptonuria is a rare autosomal recessive disorder, with a prevalence of about 1 in 100,000 to 250,000, which results from an enzyme error in an essential amino acid metabolism pathway (Figure 2).1 This inheritable gene mutation leads to ineffective homogentisate 1,2-dioxygenase (HGD), an enzyme required to break down HGA—which is a product of phenylalanine and tyrosine metabolism.2 As these patients engage in normal dietary protein intake, which includes essential amino acid phenylalanine, they develop clinically evident manifestations of the buildup and deposition of HGA.
The rarity of alkaptonuria combined with the gradual buildup of HGA makes it difficult to diagnose. A common diagnostic technique is the visualization of discolored cartilage during surgical procedures, especially when discoloration in urine or skin is not immediately evident. A few case reports have noted surgical diagnosis of black or darkening tissue, known as ochronosis, following tendon rupture—a common complication of this disorder.3-5 Additional intervention-related case reports linked to the discovery of ochronosis include aortic valve replacement, lumbar discectomy, and bronchoscopy.6-9 Cases like these illustrate the complex, disabling, and unclear nature of this disorder when not diagnosed early in life.
The patient in this case communicated via e-mail about his tendon repair surgery. “Something very interesting was found during the surgery,” the patient explained. “I was diagnosed with the disease called ochronosis. I don’t know much about this disease but I am beginning to know why some of the things are happening to me and why I am always in constant pain.” This was the first recognized clue toward a diagnosis of alkaptonuria.
Pathophysiology
The pathophysiology of alkaptonuria is based on the extensive deposition of HGA throughout the body. Its progression is based on 3 clinical stages: clinical silence, clinical ochronosis, and ochronotic arthropathy.1 In the first stage the disorder is asymptomatic but includes its most notable feature—the gradual darkening of urine when exposed to air through oxidation of the renally excreted HGA. A similar process occurs in the blood through formed HGA-melanin compounds, which cause discoloration in cartilage.1 This internal metabolic disruption accounts for the disorder’s eventual second stage, clinical ochronosis, usually with an onset in the second or third decade. Prominent features noted on physical examination primarily include discoloration of ear pinnae and eye sclera but can involve the nose, gums, teeth, and hands. The third, final, and symptomatic stage, ochronotic arthropathy, occurs by the patient’s fourth to fifth decade and presents as joint pain, usually starting with the vertebrae and larger joints like hips, knees, and shoulders, that can appear as advanced early osteoarthritis on imaging.
Treatment
This clinical manifestation of alkaptonuria requires that HCPs manage patients with 3 strategies: decrease HGA buildup, alleviate symptoms, and monitor for disorder complications. Decreasing HGA buildup is a difficult aspect of management given the natural physiology of protein intake and metabolism. Three approaches to limit HGA buildup incorporate decreasing protein intake, inhibiting enzyme production of HGA, and increasing HGA excretion. Phenylalanine is an essential amino acid—meaning its levels are dependent on dietary protein intake. Patients should be advised to adhere to a low protein diet, especially phenylalanine and tyrosine, to lessen HGA concentrations.
Manipulating the metabolic pathway of phenylalanine with medication is a second option. An example of this is nitisinone, a US Food and Drug Administration-approved medication for treatment of tyrosinemia. It acts by inhibiting hydroxyphenylpyruvic acid dioxygenase, one of the enzymes that converts tyrosine into HGA, to prevent the buildup of damaging tyrosine byproducts. At low doses it has been effective in decreasing HGA concentrations in alkaptonuria and tyrosinemia.10,11 Due to this mechanism of action, nitisinone directly causes increased tyrosine levels. Therefore, tyrosine toxicity, usually not predicted by tyrosine levels, has been associated with eye-related adverse effects (AEs), including keratopathy, diminished visual acuity, and corneal tissue damage.1,2,10 Incidence of these AEs have not been clearly documented, but routine monitoring should include patient education on ocular symptoms and slit-lamp examinations.12
In addition, case reports have shown that high-dose ascorbic acid (vitamin C) promotes HGA, tyrosine, and phenylalanine excretion in urine, which may slow the progression of alkaptonuria, but clinical effect has not been proven.13 Additionally, high vitamin C intake is considered a risk factor for nephrolithiasis, which must be balanced with the increased risk of stone formation from HGA excretion.14 These dietary and medical options can be considered, especially in the setting of severe symptoms or complications, but the risks must be discussed with patients.
A second and commonly utilized strategy for caring for these patients is symptom management. As demonstrated through this case report, there is no clear medication that adequately addresses the pain caused by HGA deposition. Patients should be referred to a pain specialist to allow for single provider prescribing of pain medications. This patient found most relief and least AEs with tramadol but eventually self-discontinued due to diminishing pain relief. Given the eventual involvement of large joints, these patients will often require further symptom management with joint replacement surgery, usually much earlier than patients who undergo these surgeries for age-related osteoarthritis. The imperative aspect of symptom management is to engage patients in shared decision making with clear expectation setting.
Given the progressive nature of alkaptonuria, providers must monitor and address complications that are a result of this disorder. HGA becomes pathologic by binding to and weakening collagen fibers.5 This gradual buildup leads to degenerative changes in weight-bearing lower vertebrae and large joints that can become severe. Due to HGA’s interaction with collagen fibers, tendon involvement leading to inflammation, calcification, and rupture can result as patients enter the third stage, ochronotic arthropathy, of the disorder (Figure 3).15 Many of these arthropathies will require medical and surgical management and can be urgent in situations like tendon ruptures and meniscal tears. Understanding the pathophysiology of tendinopathies in patients with alkaptonuria also can aid orthopedic surgeons during the postoperative period where patients may be at risk for poor healing.5
A second area of complications includes CV involvement. This patient was diagnosed with premature atherosclerosis and underwent cardiac interventions, including coronary stent placement and valve replacements at age 63 years. This early cardiac involvement was likely due in part to the deposition of HGA and collagen injury in CV tissue leading to damage of the endocardium, aortic intima, heart valves, and coronary arteries.1 HCPs should monitor for these manifestations with regular visits, chest computed tomography, and echocardiographic studies.2
The most classic aspect of this rare disorder is urine darkening due to the renal excretion of HGA and comprises the third area of complications. This process leads to chronically acidic urine—every urinalysis in this patient’s chart displayed the lowest pH measurable—and an increased risk for calcification and precipitation of solutes within the kidney and urinary tract (Figure 4). Both X-ray and ultrasound imaging should be used to identify kidney and prostate stones in the setting of abdominal or genitourinary pain or infection. Patients with diminished renal function may manifest a more severe and rapidly progressing form of alkaptonuria that exacerbates symptoms and complications, but direct damage to the kidneys by HGA is not evident.
Conclusion
Alkaptonuria is a rare autosomal recessive metabolic disorder that has a progressively debilitating pathophysiologic course spanning decades of a patient’s life. Its low prevalence and gradually progressive nature make it a difficult diagnosis to make without clinical suspicion. In patients with early-onset degenerative joint disease, tendinopathy, and cartilage or skin discoloration, congenital metabolic disorders like alkaptonuria should be considered.
As this case shows, suspicion and diagnosis can occur during surgical intervention in which tendon discoloration is directly visualized, especially in patients without prominent skin or cartilage discoloration. Once the diagnosis is made through elevated levels of urine HGA, there are 3 management strategies, including decreasing homogentisic acid buildup, providing symptom management, and monitoring for arthropathic, CV, and genitourinary complications.
1. Aquaron R. Alkaptonuria: a very rare metabolic disorder. Indian J Biochem Biophys. 2013;50(5):339-344.
2. Phornphutkul C, Introne WJ, Perry MB, et al. Natural history of alkaptonuria. N Engl J Med. 2002;347(26):2111-2121.
3. Alajoulin OA, Alsbou MS, Ja’afreh SO, Kalbouneh HM. Spontaneous Achilles tendon rupture in alkaptonuria. Saudi Med J. 2015;36(12):1486-1489.
4. Manoj Kumar RV, Rajasekaran S. Spontaneous tendon ruptures in alkaptonuria. J Bone Joint Surg Br. 2003;85(6):883-886.
5. Tanoglu O, Arican G, Ozmeric A, Alemdaroglu KB, Caydere M. Calcaneal avulsion of an ochronotic Achilles tendon: a case report. J Foot Ankle Surg. 2018;57(1):179-183.
6. Schuuring MJ, Delemarre B, Keyhan-Falsafi AM, van der Bilt IA. Mending a darkened heart: alkaptonuria discovered during aortic valve replacement. Circulation. 2016;133(12):e444-445.
7. Hiroyoshi J, Saito A, Panthee N, et al. Aortic valve replacement for aortic stenosis caused by alkaptonuria. Ann Thorac Surg. 2013;95(3):1076-1079.
8. Parambil JG, Daniels CE, Zehr KJ, Utz JP. Alkaptonuria diagnosed by flexible bronchoscopy. Chest. 2005;128(5):3678-3680.
9. Farzannia A, Shokouhi G, Hadidchi S. Alkaptonuria and lumbar disc herniation. Report of three cases. J Neurosurg. 2003;98(suppl 1):87-89.
10. Introne WJ, Perry MB, Troendle J, et al. A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol Genet Metab. 2011;103(4):307-314.
11. Gissen P, Preece MA, Willshaw HA, McKiernan PJ. Ophthalmic follow-up of patients with tyrosinaemia type I on NTBC. J Inherit Metab Dis. 2003;26(1):13-16.
12. Khedr M, Judd S, Briggs MC, et al. Asymptomatic corneal keratopathy secondary to hypertyrosinaemia following low dose nitisinone and a literature review of tyrosine keratopathy in alkaptonuria. JIMD Rep. 2018;40:31-37.
13. Wolff JA, Barshop B, Nyhan WL, et al. Effects of ascorbic acid in alkaptonuria: alterations in benzoquinone acetic acid and an ontogenic effect in infancy. Pediatr Res. 1989;26(2):140-144.
14. Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004;15(12):3225-3232.
15. Abate M, Salini V, Andia I. Tendons involvement in congenital metabolic disorders. Adv Exp Med Biol. 2016;920:117-122.
Alkaptonuria is a rare autosomal recessive disorder uniquely known for causing black, or darkened, urine when left standing due to the renal excretion of excess homogentisic acid (HGA). When this disorder goes undiagnosed, as demonstrated in this case, patients experience its many complications without a unifying explanation. The disorder has 3 clinical stages that occur in a predictable order: clinical silence, clinical ochronosis, and ochronotic arthropathy. These stages lead to multiple musculoskeletal, cardiovascular (CV), and renal complications that can be mitigated with management focused on decreasing homogentisic acid buildup, alleviating symptoms, and close monitoring for these complications.
Case Presentation
A 61-year-old African American male with a medical history of multiple traumatic fractures, right Achilles tendon injury, early-onset multijoint osteoarthritis, chronic low back pain, and recurrent nephrolithiasis presented to the emergency department with sudden onset of sharp left ankle pain while moving furniture. His physical exam revealed a positive Thompson test—lack of foot plantar flexion with calf squeeze—and a subsequent magnetic resonance image (MRI) showed evidence of an acute Achilles tendon rupture.
Given these findings the patient was treated with nonsteroidal anti-inflammatory drugs (NSAIDs) and rest to allow for resolution of swelling and inflammation, followed by elective surgery a month later to repair the ruptured tendon. An operative report following his surgery described “black ends to the area where the Achilles was ruptured…and tendinopathy of the flexor hallucis longus with blackening of the flexor.”
A more in-depth patient history revealed that he underwent multiple invasive and noninvasive interventions for his chronic low back and joint pain with medical management of a prior right Achilles tendon injury. His medical history also included multiple nonspecific diagnoses, such as premature atherosclerosis (diagnosed in his third decade), severe lumbar degenerative disc disease, several tendonopathies and cartilage injuries (Figure 1), pseudogout (following calcium pyrophosphate dehydrate crystals found from a left knee aspirate), and chronic pain syndrome. Along this diagnostic journey, he had several health care providers (HCPs) in rheumatology, orthopedic surgery, pain management, and podiatry who offered a range of symptom management options, including physical therapy, NSAIDs, opioid agonists, tricyclic antidepressants, gabapentin, colchicine, and epidural steroid injections, all of which provided little or no relief of his pain. The patient reported that he told a HCP, “I’ll just live with [the pain].”
At the postsurgery follow-up, the patient reported that he had noticed dark urine and dark spots on his ears in the past. He also recounted that chronic joint pain was common in his family, with both his mother and brother receiving bilateral total knee replacements. Taking into consideration the surgical report and this new history, a urine assessment for HGA was ordered and yielded a diagnosis of alkaptonuria.
He later suffered an acute myocardial infarction leading to an incidental discovery of severe aortic stenosis on echocardiography, requiring coronary stent placements and transcatheter aortic valve replacement, respectively. He reported that with CV interventions and joint replacement surgeries, including bilateral knees and hips, his symptoms and quality of life began to significantly improve. However, he continued to have diffuse chronic joint pain unimproved with any single agent or intervention.
Discussion
Alkaptonuria is a rare autosomal recessive disorder, with a prevalence of about 1 in 100,000 to 250,000, which results from an enzyme error in an essential amino acid metabolism pathway (Figure 2).1 This inheritable gene mutation leads to ineffective homogentisate 1,2-dioxygenase (HGD), an enzyme required to break down HGA—which is a product of phenylalanine and tyrosine metabolism.2 As these patients engage in normal dietary protein intake, which includes essential amino acid phenylalanine, they develop clinically evident manifestations of the buildup and deposition of HGA.
The rarity of alkaptonuria combined with the gradual buildup of HGA makes it difficult to diagnose. A common diagnostic technique is the visualization of discolored cartilage during surgical procedures, especially when discoloration in urine or skin is not immediately evident. A few case reports have noted surgical diagnosis of black or darkening tissue, known as ochronosis, following tendon rupture—a common complication of this disorder.3-5 Additional intervention-related case reports linked to the discovery of ochronosis include aortic valve replacement, lumbar discectomy, and bronchoscopy.6-9 Cases like these illustrate the complex, disabling, and unclear nature of this disorder when not diagnosed early in life.
The patient in this case communicated via e-mail about his tendon repair surgery. “Something very interesting was found during the surgery,” the patient explained. “I was diagnosed with the disease called ochronosis. I don’t know much about this disease but I am beginning to know why some of the things are happening to me and why I am always in constant pain.” This was the first recognized clue toward a diagnosis of alkaptonuria.
Pathophysiology
The pathophysiology of alkaptonuria is based on the extensive deposition of HGA throughout the body. Its progression is based on 3 clinical stages: clinical silence, clinical ochronosis, and ochronotic arthropathy.1 In the first stage the disorder is asymptomatic but includes its most notable feature—the gradual darkening of urine when exposed to air through oxidation of the renally excreted HGA. A similar process occurs in the blood through formed HGA-melanin compounds, which cause discoloration in cartilage.1 This internal metabolic disruption accounts for the disorder’s eventual second stage, clinical ochronosis, usually with an onset in the second or third decade. Prominent features noted on physical examination primarily include discoloration of ear pinnae and eye sclera but can involve the nose, gums, teeth, and hands. The third, final, and symptomatic stage, ochronotic arthropathy, occurs by the patient’s fourth to fifth decade and presents as joint pain, usually starting with the vertebrae and larger joints like hips, knees, and shoulders, that can appear as advanced early osteoarthritis on imaging.
Treatment
This clinical manifestation of alkaptonuria requires that HCPs manage patients with 3 strategies: decrease HGA buildup, alleviate symptoms, and monitor for disorder complications. Decreasing HGA buildup is a difficult aspect of management given the natural physiology of protein intake and metabolism. Three approaches to limit HGA buildup incorporate decreasing protein intake, inhibiting enzyme production of HGA, and increasing HGA excretion. Phenylalanine is an essential amino acid—meaning its levels are dependent on dietary protein intake. Patients should be advised to adhere to a low protein diet, especially phenylalanine and tyrosine, to lessen HGA concentrations.
Manipulating the metabolic pathway of phenylalanine with medication is a second option. An example of this is nitisinone, a US Food and Drug Administration-approved medication for treatment of tyrosinemia. It acts by inhibiting hydroxyphenylpyruvic acid dioxygenase, one of the enzymes that converts tyrosine into HGA, to prevent the buildup of damaging tyrosine byproducts. At low doses it has been effective in decreasing HGA concentrations in alkaptonuria and tyrosinemia.10,11 Due to this mechanism of action, nitisinone directly causes increased tyrosine levels. Therefore, tyrosine toxicity, usually not predicted by tyrosine levels, has been associated with eye-related adverse effects (AEs), including keratopathy, diminished visual acuity, and corneal tissue damage.1,2,10 Incidence of these AEs have not been clearly documented, but routine monitoring should include patient education on ocular symptoms and slit-lamp examinations.12
In addition, case reports have shown that high-dose ascorbic acid (vitamin C) promotes HGA, tyrosine, and phenylalanine excretion in urine, which may slow the progression of alkaptonuria, but clinical effect has not been proven.13 Additionally, high vitamin C intake is considered a risk factor for nephrolithiasis, which must be balanced with the increased risk of stone formation from HGA excretion.14 These dietary and medical options can be considered, especially in the setting of severe symptoms or complications, but the risks must be discussed with patients.
A second and commonly utilized strategy for caring for these patients is symptom management. As demonstrated through this case report, there is no clear medication that adequately addresses the pain caused by HGA deposition. Patients should be referred to a pain specialist to allow for single provider prescribing of pain medications. This patient found most relief and least AEs with tramadol but eventually self-discontinued due to diminishing pain relief. Given the eventual involvement of large joints, these patients will often require further symptom management with joint replacement surgery, usually much earlier than patients who undergo these surgeries for age-related osteoarthritis. The imperative aspect of symptom management is to engage patients in shared decision making with clear expectation setting.
Given the progressive nature of alkaptonuria, providers must monitor and address complications that are a result of this disorder. HGA becomes pathologic by binding to and weakening collagen fibers.5 This gradual buildup leads to degenerative changes in weight-bearing lower vertebrae and large joints that can become severe. Due to HGA’s interaction with collagen fibers, tendon involvement leading to inflammation, calcification, and rupture can result as patients enter the third stage, ochronotic arthropathy, of the disorder (Figure 3).15 Many of these arthropathies will require medical and surgical management and can be urgent in situations like tendon ruptures and meniscal tears. Understanding the pathophysiology of tendinopathies in patients with alkaptonuria also can aid orthopedic surgeons during the postoperative period where patients may be at risk for poor healing.5
A second area of complications includes CV involvement. This patient was diagnosed with premature atherosclerosis and underwent cardiac interventions, including coronary stent placement and valve replacements at age 63 years. This early cardiac involvement was likely due in part to the deposition of HGA and collagen injury in CV tissue leading to damage of the endocardium, aortic intima, heart valves, and coronary arteries.1 HCPs should monitor for these manifestations with regular visits, chest computed tomography, and echocardiographic studies.2
The most classic aspect of this rare disorder is urine darkening due to the renal excretion of HGA and comprises the third area of complications. This process leads to chronically acidic urine—every urinalysis in this patient’s chart displayed the lowest pH measurable—and an increased risk for calcification and precipitation of solutes within the kidney and urinary tract (Figure 4). Both X-ray and ultrasound imaging should be used to identify kidney and prostate stones in the setting of abdominal or genitourinary pain or infection. Patients with diminished renal function may manifest a more severe and rapidly progressing form of alkaptonuria that exacerbates symptoms and complications, but direct damage to the kidneys by HGA is not evident.
Conclusion
Alkaptonuria is a rare autosomal recessive metabolic disorder that has a progressively debilitating pathophysiologic course spanning decades of a patient’s life. Its low prevalence and gradually progressive nature make it a difficult diagnosis to make without clinical suspicion. In patients with early-onset degenerative joint disease, tendinopathy, and cartilage or skin discoloration, congenital metabolic disorders like alkaptonuria should be considered.
As this case shows, suspicion and diagnosis can occur during surgical intervention in which tendon discoloration is directly visualized, especially in patients without prominent skin or cartilage discoloration. Once the diagnosis is made through elevated levels of urine HGA, there are 3 management strategies, including decreasing homogentisic acid buildup, providing symptom management, and monitoring for arthropathic, CV, and genitourinary complications.
Alkaptonuria is a rare autosomal recessive disorder uniquely known for causing black, or darkened, urine when left standing due to the renal excretion of excess homogentisic acid (HGA). When this disorder goes undiagnosed, as demonstrated in this case, patients experience its many complications without a unifying explanation. The disorder has 3 clinical stages that occur in a predictable order: clinical silence, clinical ochronosis, and ochronotic arthropathy. These stages lead to multiple musculoskeletal, cardiovascular (CV), and renal complications that can be mitigated with management focused on decreasing homogentisic acid buildup, alleviating symptoms, and close monitoring for these complications.
Case Presentation
A 61-year-old African American male with a medical history of multiple traumatic fractures, right Achilles tendon injury, early-onset multijoint osteoarthritis, chronic low back pain, and recurrent nephrolithiasis presented to the emergency department with sudden onset of sharp left ankle pain while moving furniture. His physical exam revealed a positive Thompson test—lack of foot plantar flexion with calf squeeze—and a subsequent magnetic resonance image (MRI) showed evidence of an acute Achilles tendon rupture.
Given these findings the patient was treated with nonsteroidal anti-inflammatory drugs (NSAIDs) and rest to allow for resolution of swelling and inflammation, followed by elective surgery a month later to repair the ruptured tendon. An operative report following his surgery described “black ends to the area where the Achilles was ruptured…and tendinopathy of the flexor hallucis longus with blackening of the flexor.”
A more in-depth patient history revealed that he underwent multiple invasive and noninvasive interventions for his chronic low back and joint pain with medical management of a prior right Achilles tendon injury. His medical history also included multiple nonspecific diagnoses, such as premature atherosclerosis (diagnosed in his third decade), severe lumbar degenerative disc disease, several tendonopathies and cartilage injuries (Figure 1), pseudogout (following calcium pyrophosphate dehydrate crystals found from a left knee aspirate), and chronic pain syndrome. Along this diagnostic journey, he had several health care providers (HCPs) in rheumatology, orthopedic surgery, pain management, and podiatry who offered a range of symptom management options, including physical therapy, NSAIDs, opioid agonists, tricyclic antidepressants, gabapentin, colchicine, and epidural steroid injections, all of which provided little or no relief of his pain. The patient reported that he told a HCP, “I’ll just live with [the pain].”
At the postsurgery follow-up, the patient reported that he had noticed dark urine and dark spots on his ears in the past. He also recounted that chronic joint pain was common in his family, with both his mother and brother receiving bilateral total knee replacements. Taking into consideration the surgical report and this new history, a urine assessment for HGA was ordered and yielded a diagnosis of alkaptonuria.
He later suffered an acute myocardial infarction leading to an incidental discovery of severe aortic stenosis on echocardiography, requiring coronary stent placements and transcatheter aortic valve replacement, respectively. He reported that with CV interventions and joint replacement surgeries, including bilateral knees and hips, his symptoms and quality of life began to significantly improve. However, he continued to have diffuse chronic joint pain unimproved with any single agent or intervention.
Discussion
Alkaptonuria is a rare autosomal recessive disorder, with a prevalence of about 1 in 100,000 to 250,000, which results from an enzyme error in an essential amino acid metabolism pathway (Figure 2).1 This inheritable gene mutation leads to ineffective homogentisate 1,2-dioxygenase (HGD), an enzyme required to break down HGA—which is a product of phenylalanine and tyrosine metabolism.2 As these patients engage in normal dietary protein intake, which includes essential amino acid phenylalanine, they develop clinically evident manifestations of the buildup and deposition of HGA.
The rarity of alkaptonuria combined with the gradual buildup of HGA makes it difficult to diagnose. A common diagnostic technique is the visualization of discolored cartilage during surgical procedures, especially when discoloration in urine or skin is not immediately evident. A few case reports have noted surgical diagnosis of black or darkening tissue, known as ochronosis, following tendon rupture—a common complication of this disorder.3-5 Additional intervention-related case reports linked to the discovery of ochronosis include aortic valve replacement, lumbar discectomy, and bronchoscopy.6-9 Cases like these illustrate the complex, disabling, and unclear nature of this disorder when not diagnosed early in life.
The patient in this case communicated via e-mail about his tendon repair surgery. “Something very interesting was found during the surgery,” the patient explained. “I was diagnosed with the disease called ochronosis. I don’t know much about this disease but I am beginning to know why some of the things are happening to me and why I am always in constant pain.” This was the first recognized clue toward a diagnosis of alkaptonuria.
Pathophysiology
The pathophysiology of alkaptonuria is based on the extensive deposition of HGA throughout the body. Its progression is based on 3 clinical stages: clinical silence, clinical ochronosis, and ochronotic arthropathy.1 In the first stage the disorder is asymptomatic but includes its most notable feature—the gradual darkening of urine when exposed to air through oxidation of the renally excreted HGA. A similar process occurs in the blood through formed HGA-melanin compounds, which cause discoloration in cartilage.1 This internal metabolic disruption accounts for the disorder’s eventual second stage, clinical ochronosis, usually with an onset in the second or third decade. Prominent features noted on physical examination primarily include discoloration of ear pinnae and eye sclera but can involve the nose, gums, teeth, and hands. The third, final, and symptomatic stage, ochronotic arthropathy, occurs by the patient’s fourth to fifth decade and presents as joint pain, usually starting with the vertebrae and larger joints like hips, knees, and shoulders, that can appear as advanced early osteoarthritis on imaging.
Treatment
This clinical manifestation of alkaptonuria requires that HCPs manage patients with 3 strategies: decrease HGA buildup, alleviate symptoms, and monitor for disorder complications. Decreasing HGA buildup is a difficult aspect of management given the natural physiology of protein intake and metabolism. Three approaches to limit HGA buildup incorporate decreasing protein intake, inhibiting enzyme production of HGA, and increasing HGA excretion. Phenylalanine is an essential amino acid—meaning its levels are dependent on dietary protein intake. Patients should be advised to adhere to a low protein diet, especially phenylalanine and tyrosine, to lessen HGA concentrations.
Manipulating the metabolic pathway of phenylalanine with medication is a second option. An example of this is nitisinone, a US Food and Drug Administration-approved medication for treatment of tyrosinemia. It acts by inhibiting hydroxyphenylpyruvic acid dioxygenase, one of the enzymes that converts tyrosine into HGA, to prevent the buildup of damaging tyrosine byproducts. At low doses it has been effective in decreasing HGA concentrations in alkaptonuria and tyrosinemia.10,11 Due to this mechanism of action, nitisinone directly causes increased tyrosine levels. Therefore, tyrosine toxicity, usually not predicted by tyrosine levels, has been associated with eye-related adverse effects (AEs), including keratopathy, diminished visual acuity, and corneal tissue damage.1,2,10 Incidence of these AEs have not been clearly documented, but routine monitoring should include patient education on ocular symptoms and slit-lamp examinations.12
In addition, case reports have shown that high-dose ascorbic acid (vitamin C) promotes HGA, tyrosine, and phenylalanine excretion in urine, which may slow the progression of alkaptonuria, but clinical effect has not been proven.13 Additionally, high vitamin C intake is considered a risk factor for nephrolithiasis, which must be balanced with the increased risk of stone formation from HGA excretion.14 These dietary and medical options can be considered, especially in the setting of severe symptoms or complications, but the risks must be discussed with patients.
A second and commonly utilized strategy for caring for these patients is symptom management. As demonstrated through this case report, there is no clear medication that adequately addresses the pain caused by HGA deposition. Patients should be referred to a pain specialist to allow for single provider prescribing of pain medications. This patient found most relief and least AEs with tramadol but eventually self-discontinued due to diminishing pain relief. Given the eventual involvement of large joints, these patients will often require further symptom management with joint replacement surgery, usually much earlier than patients who undergo these surgeries for age-related osteoarthritis. The imperative aspect of symptom management is to engage patients in shared decision making with clear expectation setting.
Given the progressive nature of alkaptonuria, providers must monitor and address complications that are a result of this disorder. HGA becomes pathologic by binding to and weakening collagen fibers.5 This gradual buildup leads to degenerative changes in weight-bearing lower vertebrae and large joints that can become severe. Due to HGA’s interaction with collagen fibers, tendon involvement leading to inflammation, calcification, and rupture can result as patients enter the third stage, ochronotic arthropathy, of the disorder (Figure 3).15 Many of these arthropathies will require medical and surgical management and can be urgent in situations like tendon ruptures and meniscal tears. Understanding the pathophysiology of tendinopathies in patients with alkaptonuria also can aid orthopedic surgeons during the postoperative period where patients may be at risk for poor healing.5
A second area of complications includes CV involvement. This patient was diagnosed with premature atherosclerosis and underwent cardiac interventions, including coronary stent placement and valve replacements at age 63 years. This early cardiac involvement was likely due in part to the deposition of HGA and collagen injury in CV tissue leading to damage of the endocardium, aortic intima, heart valves, and coronary arteries.1 HCPs should monitor for these manifestations with regular visits, chest computed tomography, and echocardiographic studies.2
The most classic aspect of this rare disorder is urine darkening due to the renal excretion of HGA and comprises the third area of complications. This process leads to chronically acidic urine—every urinalysis in this patient’s chart displayed the lowest pH measurable—and an increased risk for calcification and precipitation of solutes within the kidney and urinary tract (Figure 4). Both X-ray and ultrasound imaging should be used to identify kidney and prostate stones in the setting of abdominal or genitourinary pain or infection. Patients with diminished renal function may manifest a more severe and rapidly progressing form of alkaptonuria that exacerbates symptoms and complications, but direct damage to the kidneys by HGA is not evident.
Conclusion
Alkaptonuria is a rare autosomal recessive metabolic disorder that has a progressively debilitating pathophysiologic course spanning decades of a patient’s life. Its low prevalence and gradually progressive nature make it a difficult diagnosis to make without clinical suspicion. In patients with early-onset degenerative joint disease, tendinopathy, and cartilage or skin discoloration, congenital metabolic disorders like alkaptonuria should be considered.
As this case shows, suspicion and diagnosis can occur during surgical intervention in which tendon discoloration is directly visualized, especially in patients without prominent skin or cartilage discoloration. Once the diagnosis is made through elevated levels of urine HGA, there are 3 management strategies, including decreasing homogentisic acid buildup, providing symptom management, and monitoring for arthropathic, CV, and genitourinary complications.
1. Aquaron R. Alkaptonuria: a very rare metabolic disorder. Indian J Biochem Biophys. 2013;50(5):339-344.
2. Phornphutkul C, Introne WJ, Perry MB, et al. Natural history of alkaptonuria. N Engl J Med. 2002;347(26):2111-2121.
3. Alajoulin OA, Alsbou MS, Ja’afreh SO, Kalbouneh HM. Spontaneous Achilles tendon rupture in alkaptonuria. Saudi Med J. 2015;36(12):1486-1489.
4. Manoj Kumar RV, Rajasekaran S. Spontaneous tendon ruptures in alkaptonuria. J Bone Joint Surg Br. 2003;85(6):883-886.
5. Tanoglu O, Arican G, Ozmeric A, Alemdaroglu KB, Caydere M. Calcaneal avulsion of an ochronotic Achilles tendon: a case report. J Foot Ankle Surg. 2018;57(1):179-183.
6. Schuuring MJ, Delemarre B, Keyhan-Falsafi AM, van der Bilt IA. Mending a darkened heart: alkaptonuria discovered during aortic valve replacement. Circulation. 2016;133(12):e444-445.
7. Hiroyoshi J, Saito A, Panthee N, et al. Aortic valve replacement for aortic stenosis caused by alkaptonuria. Ann Thorac Surg. 2013;95(3):1076-1079.
8. Parambil JG, Daniels CE, Zehr KJ, Utz JP. Alkaptonuria diagnosed by flexible bronchoscopy. Chest. 2005;128(5):3678-3680.
9. Farzannia A, Shokouhi G, Hadidchi S. Alkaptonuria and lumbar disc herniation. Report of three cases. J Neurosurg. 2003;98(suppl 1):87-89.
10. Introne WJ, Perry MB, Troendle J, et al. A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol Genet Metab. 2011;103(4):307-314.
11. Gissen P, Preece MA, Willshaw HA, McKiernan PJ. Ophthalmic follow-up of patients with tyrosinaemia type I on NTBC. J Inherit Metab Dis. 2003;26(1):13-16.
12. Khedr M, Judd S, Briggs MC, et al. Asymptomatic corneal keratopathy secondary to hypertyrosinaemia following low dose nitisinone and a literature review of tyrosine keratopathy in alkaptonuria. JIMD Rep. 2018;40:31-37.
13. Wolff JA, Barshop B, Nyhan WL, et al. Effects of ascorbic acid in alkaptonuria: alterations in benzoquinone acetic acid and an ontogenic effect in infancy. Pediatr Res. 1989;26(2):140-144.
14. Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004;15(12):3225-3232.
15. Abate M, Salini V, Andia I. Tendons involvement in congenital metabolic disorders. Adv Exp Med Biol. 2016;920:117-122.
1. Aquaron R. Alkaptonuria: a very rare metabolic disorder. Indian J Biochem Biophys. 2013;50(5):339-344.
2. Phornphutkul C, Introne WJ, Perry MB, et al. Natural history of alkaptonuria. N Engl J Med. 2002;347(26):2111-2121.
3. Alajoulin OA, Alsbou MS, Ja’afreh SO, Kalbouneh HM. Spontaneous Achilles tendon rupture in alkaptonuria. Saudi Med J. 2015;36(12):1486-1489.
4. Manoj Kumar RV, Rajasekaran S. Spontaneous tendon ruptures in alkaptonuria. J Bone Joint Surg Br. 2003;85(6):883-886.
5. Tanoglu O, Arican G, Ozmeric A, Alemdaroglu KB, Caydere M. Calcaneal avulsion of an ochronotic Achilles tendon: a case report. J Foot Ankle Surg. 2018;57(1):179-183.
6. Schuuring MJ, Delemarre B, Keyhan-Falsafi AM, van der Bilt IA. Mending a darkened heart: alkaptonuria discovered during aortic valve replacement. Circulation. 2016;133(12):e444-445.
7. Hiroyoshi J, Saito A, Panthee N, et al. Aortic valve replacement for aortic stenosis caused by alkaptonuria. Ann Thorac Surg. 2013;95(3):1076-1079.
8. Parambil JG, Daniels CE, Zehr KJ, Utz JP. Alkaptonuria diagnosed by flexible bronchoscopy. Chest. 2005;128(5):3678-3680.
9. Farzannia A, Shokouhi G, Hadidchi S. Alkaptonuria and lumbar disc herniation. Report of three cases. J Neurosurg. 2003;98(suppl 1):87-89.
10. Introne WJ, Perry MB, Troendle J, et al. A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol Genet Metab. 2011;103(4):307-314.
11. Gissen P, Preece MA, Willshaw HA, McKiernan PJ. Ophthalmic follow-up of patients with tyrosinaemia type I on NTBC. J Inherit Metab Dis. 2003;26(1):13-16.
12. Khedr M, Judd S, Briggs MC, et al. Asymptomatic corneal keratopathy secondary to hypertyrosinaemia following low dose nitisinone and a literature review of tyrosine keratopathy in alkaptonuria. JIMD Rep. 2018;40:31-37.
13. Wolff JA, Barshop B, Nyhan WL, et al. Effects of ascorbic acid in alkaptonuria: alterations in benzoquinone acetic acid and an ontogenic effect in infancy. Pediatr Res. 1989;26(2):140-144.
14. Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004;15(12):3225-3232.
15. Abate M, Salini V, Andia I. Tendons involvement in congenital metabolic disorders. Adv Exp Med Biol. 2016;920:117-122.
A Veteran With a Solitary Pulmonary Nodule
Case Presentation. A 69-year-old veteran presented with an intermittent, waxing and waning cough. He had never smoked and had no family history of lung cancer. His primary care physician ordered a chest radiograph, which revealed a nodular opacity within the lingula concerning for a parenchymal nodule. Further characterization with a chest computed tomography (CT) demonstrated a 1.4-cm left upper lobe subpleural nodule with small satellite nodules (Figure 1). Given these imaging findings, the patient was referred to the pulmonary clinic.
►Lauren Kearney, MD, Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center. What is the differential diagnosis of a solitary pulmonary nodule? What characteristics of the nodule do you consider to differentiate these diagnoses?
►Renda Wiener, MD, Pulmonary and Critical Care, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. Pulmonary nodules are well-defined lesions < 3 cm in diameter that are surrounded by lung parenchyma. Although cancer is a possibility (including primary lung cancers, metastatic cancers, or carcinoid tumors), most small nodules do not turn out to be malignant.1 Benign etiologies include infections, benign tumors, vascular malformations, and inflammatory conditions. Infectious causes of nodules are often granulomatous in nature, including fungi, Mycobacterium tuberculosis, and nontuberculous mycobacteria. Benign tumors are most commonly hamartomas, and these may be clearly distinguished based on imaging characteristics. Pulmonary arteriovenous malformations, hematomas, and infarcts may present as nodules as well. Inflammatory causes of nodules are important and relatively common, including granulomatosis with polyangiitis, rheumatoid arthritis, sarcoidosis, amyloidosis, and rounded atelectasis.
To distinguish benign from malignant etiologies, we look for several features of pulmonary nodules on imaging. Larger size, irregular borders, and upper lobe location all increase the likelihood of cancer, whereas solid attenuation and calcification make cancer less likely. One of the most reassuring findings that suggests a benign etiology is lack of growth over a period of surveillance; after 2 years without growth we typically consider a nodule benign.1 And of course, we also consider the patient’s symptoms and risk factors: weight loss, hemoptysis, a history of cigarette smoking or asbestos exposure, or family history of cancer all increase the likelihood of malignancy.
►Dr. Kearney. Given that the differential diagnosis is so broad, how do you think about the next step in evaluating a pulmonary nodule? How do you approach shared decision making with the patient?
►Dr. Wiener. The characteristics of the patient, the nodule, and the circumstances in which the nodule were discovered are all important to consider. Incidental pulmonary nodules are often found on chest imaging. The imaging characteristics of the nodule are important, as are the patient’s risk factors. A similarly appearing nodule can have very different implications if the patient is a never-smoker exposed to endemic fungi, or a long-time smoker enrolled in a lung cancer screening program. Consultation with a pulmonologist is often appropriate.
It’s important to note that we lack high-quality evidence on the optimal strategy to evaluate pulmonary nodules, and there is no single “right answer“ for all patients. For patients with a low risk of malignancy (< 5%-10%)—which comprises the majority of the incidental nodules discovered—we typically favor serial CT surveillance of the nodule over a period of a few years, whereas for patients at high risk of malignancy (> 65%), we favor early surgical resection if the patient is able to tolerate that. For patients with an intermediate risk of malignancy (~5%-65%), we might consider serial CT surveillance, positron emission tomography (PET) scan, or biopsy.1 The American College of Chest Physicians guidelines for pulmonary nodule evaluation recommend discussing with patients the different options and the trade-offs of these options in a shared decision-making process.1
►Dr. Kearney. The patient’s pulmonologist laid out options, including monitoring with serial CT scans, obtaining a PET scan, performing CT-guided needle biopsy, or referring for surgical excision. In this case, the patient elected to undergo CT-guided needle biopsy. Dr. Huang, can you discuss the pathology results?
►Qin Huang, MD, Pathology and Laboratory Medicine, VABHS, and Assistant Professor of Pathology, Harvard Medical School (HMS). The microscopic examination of the needle biopsy of the lung mass revealed rare clusters of atypical cells with crushed cells adjacent to an extensive area of necrosis with scarring. The atypical cells were suspicious for carcinoma. The Gomori methenamine silver (GMS) and periodic acid-Schiff (PAS) stains were negative for common bacterial and fungal microorganisms.
►Dr. Kearney. The tumor board, pulmonologist, and patient decide to move forward with video-assisted excisional biopsy with lymphadenectomy. Dr. Huang, can you interpret the pathology?
►Dr. Huang. Figure 2 showed an hemotoxylin and eosin (H&E)-stained lung resection tissue section with multiple caseating necrotic granulomas. No foreign bodies were identified. There was no evidence of malignancy. The GMS stain revealed a fungal microorganism oval with morphology typical of histoplasma capsulatum (Figure 3).
►Dr. Kearney. What are some of the different ways histoplasmosis can present? Which of these diagnoses fits this patient’s presentation?
►Judy Strymish, MD, Infectious Disease, VABHS, and Assistant Professor of Medicine, HMS. Most patients who inhale histoplasmosis spores develop asymptomatic or self-limited infection that is usually not detected. Patients at risk of symptomatic and clinically relevant disease include those who are immunocompromised, at extremes of ages, or exposed to larger inoculums. Acute pulmonary histoplasmosis can present with cough, shortness of breath, fever, chills, and less commonly, rheumatologic complaints such as erythema nodosum or erythema multiforme. Imaging often shows patchy infiltrates and enlarged mediastinal and hilar lymphadenopathy. Patients can go on to develop subacute or chronic pulmonary disease with focal opacities and mediastinal and hilar lymphadenopathy. Those with chronic disease can have cavitary lesions similar to patients with tuberculosis. Progressive disseminated histoplasmosis can develop in immunocompromised patients and disseminate through the reticuloendothelial system to other organs with the gastrointestinal tract, central nervous system, and adrenal glands.2
Pulmonary nodules are common incidental finding on chest imaging of patients who reside in histoplasmosis endemic regions, and they are often hard to differentiate from malignancies. There are 3 mediastinal manifestations: adenitis, granuloma, and fibrosis. Usually the syndromes are subclinical, but occasionally the nodes cause symptoms by impinging on other structures.2
This patient had a solitary pulmonary nodule with none of the associated features mentioned above. Pathology showed caseating granuloma and confirmed histoplasmosis.
►Dr. Kearney. Given the diagnosis of solitary histoplasmoma, how should this patient be managed?
►Dr. Strymish. The optimal therapy for histoplasmosis depends on the patient’s clinical syndrome. Most infections are self-limited and require no therapy. However, patients who are immunocompromised, exposed to large inoculum, and have progressive disease require antifungal treatment, usually with itraconazole for mild-to-moderate disease and a combination of azole therapy and amphotericin B with extensive disease. Patients with few solitary pulmonary nodules do not benefit from antifungal therapy as the nodule could represent quiescent disease that is unlikely to have clinical impact; in this case, the treatment would be higher risk than the nodule.3
►Dr. Kearney. While the discussion of the diagnosis is interesting, it is also important to acknowledge what the patient went through to arrive at this, an essentially benign diagnosis: 8 months, multiple imaging studies, and 2 invasive diagnostic procedures. Further, the patient had to grapple with the possibility of a diagnosis of cancer. Dr. Wiener, can you talk about the challenges in communicating with patients about pulmonary nodules when cancer is on the differential? What are some of the harms patients face and how can clinicians work to mitigate these harms?
►Dr. Wiener. My colleague Dr. Christopher Slatore of the Portland VA Medical Center and I studied communication about pulmonary nodules in a series of surveys and qualitative studies of patients with pulmonary nodules and the clinicians who take care of them. We found that there seems to be a disconnect between patients’ perceptions of pulmonary nodules and their clinicians, often due to inadequate communication about the nodule. Many clinicians indicated that they do not tell patients about the chance that a nodule may be cancer, because the clinicians know that cancer is unlikely (< 5% of incidentally detected pulmonary nodules turn out to be malignant), and they do not want to alarm patients unnecessarily. However, we found that patients almost immediately wondered about cancer when they learned about their pulmonary nodule, and without hearing explicitly from their clinician that cancer was unlikely, patients tended to overestimate the likelihood of a malignant nodule. Moreover, patients often were not told much about the evaluation plan for the nodule or the rationale for CT surveillance of small nodules instead of biopsy. This uncertainty about the risk of cancer and the plan for evaluating the nodule was difficult for some patients to live with; we found that about one-quarter of patients with a small pulmonary nodule experienced mild-moderate distress during the period of radiographic surveillance. Reassuringly, high-quality patient-clinician communication was associated with lower distress and higher adherence to pulmonary nodule evaluation.4
►Dr. Kearney. The patient was educated about his diagnosis of solitary histoplasmoma. Given that the patient was otherwise well appearing with no complicating factors, he was not treated with antifungal therapy. After an 8-month-long workup, the patient was relieved to receive a diagnosis that excluded cancer and did not require any further treatment. His case provides a good example of how to proceed in the workup of a solitary pulmonary nodule and on the importance of communication and shared decision making with our patients.
1. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e93S-e120S.
2. Azar MM, Hage CA. Clinical perspectives in the diagnosis and management of histoplasmosis. Clin Chest Med. 2017;38(3):403-415.
3. Wheat LJ, Freifeld A, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807-825.
4. Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153(4):1004-1015.
Case Presentation. A 69-year-old veteran presented with an intermittent, waxing and waning cough. He had never smoked and had no family history of lung cancer. His primary care physician ordered a chest radiograph, which revealed a nodular opacity within the lingula concerning for a parenchymal nodule. Further characterization with a chest computed tomography (CT) demonstrated a 1.4-cm left upper lobe subpleural nodule with small satellite nodules (Figure 1). Given these imaging findings, the patient was referred to the pulmonary clinic.
►Lauren Kearney, MD, Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center. What is the differential diagnosis of a solitary pulmonary nodule? What characteristics of the nodule do you consider to differentiate these diagnoses?
►Renda Wiener, MD, Pulmonary and Critical Care, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. Pulmonary nodules are well-defined lesions < 3 cm in diameter that are surrounded by lung parenchyma. Although cancer is a possibility (including primary lung cancers, metastatic cancers, or carcinoid tumors), most small nodules do not turn out to be malignant.1 Benign etiologies include infections, benign tumors, vascular malformations, and inflammatory conditions. Infectious causes of nodules are often granulomatous in nature, including fungi, Mycobacterium tuberculosis, and nontuberculous mycobacteria. Benign tumors are most commonly hamartomas, and these may be clearly distinguished based on imaging characteristics. Pulmonary arteriovenous malformations, hematomas, and infarcts may present as nodules as well. Inflammatory causes of nodules are important and relatively common, including granulomatosis with polyangiitis, rheumatoid arthritis, sarcoidosis, amyloidosis, and rounded atelectasis.
To distinguish benign from malignant etiologies, we look for several features of pulmonary nodules on imaging. Larger size, irregular borders, and upper lobe location all increase the likelihood of cancer, whereas solid attenuation and calcification make cancer less likely. One of the most reassuring findings that suggests a benign etiology is lack of growth over a period of surveillance; after 2 years without growth we typically consider a nodule benign.1 And of course, we also consider the patient’s symptoms and risk factors: weight loss, hemoptysis, a history of cigarette smoking or asbestos exposure, or family history of cancer all increase the likelihood of malignancy.
►Dr. Kearney. Given that the differential diagnosis is so broad, how do you think about the next step in evaluating a pulmonary nodule? How do you approach shared decision making with the patient?
►Dr. Wiener. The characteristics of the patient, the nodule, and the circumstances in which the nodule were discovered are all important to consider. Incidental pulmonary nodules are often found on chest imaging. The imaging characteristics of the nodule are important, as are the patient’s risk factors. A similarly appearing nodule can have very different implications if the patient is a never-smoker exposed to endemic fungi, or a long-time smoker enrolled in a lung cancer screening program. Consultation with a pulmonologist is often appropriate.
It’s important to note that we lack high-quality evidence on the optimal strategy to evaluate pulmonary nodules, and there is no single “right answer“ for all patients. For patients with a low risk of malignancy (< 5%-10%)—which comprises the majority of the incidental nodules discovered—we typically favor serial CT surveillance of the nodule over a period of a few years, whereas for patients at high risk of malignancy (> 65%), we favor early surgical resection if the patient is able to tolerate that. For patients with an intermediate risk of malignancy (~5%-65%), we might consider serial CT surveillance, positron emission tomography (PET) scan, or biopsy.1 The American College of Chest Physicians guidelines for pulmonary nodule evaluation recommend discussing with patients the different options and the trade-offs of these options in a shared decision-making process.1
►Dr. Kearney. The patient’s pulmonologist laid out options, including monitoring with serial CT scans, obtaining a PET scan, performing CT-guided needle biopsy, or referring for surgical excision. In this case, the patient elected to undergo CT-guided needle biopsy. Dr. Huang, can you discuss the pathology results?
►Qin Huang, MD, Pathology and Laboratory Medicine, VABHS, and Assistant Professor of Pathology, Harvard Medical School (HMS). The microscopic examination of the needle biopsy of the lung mass revealed rare clusters of atypical cells with crushed cells adjacent to an extensive area of necrosis with scarring. The atypical cells were suspicious for carcinoma. The Gomori methenamine silver (GMS) and periodic acid-Schiff (PAS) stains were negative for common bacterial and fungal microorganisms.
►Dr. Kearney. The tumor board, pulmonologist, and patient decide to move forward with video-assisted excisional biopsy with lymphadenectomy. Dr. Huang, can you interpret the pathology?
►Dr. Huang. Figure 2 showed an hemotoxylin and eosin (H&E)-stained lung resection tissue section with multiple caseating necrotic granulomas. No foreign bodies were identified. There was no evidence of malignancy. The GMS stain revealed a fungal microorganism oval with morphology typical of histoplasma capsulatum (Figure 3).
►Dr. Kearney. What are some of the different ways histoplasmosis can present? Which of these diagnoses fits this patient’s presentation?
►Judy Strymish, MD, Infectious Disease, VABHS, and Assistant Professor of Medicine, HMS. Most patients who inhale histoplasmosis spores develop asymptomatic or self-limited infection that is usually not detected. Patients at risk of symptomatic and clinically relevant disease include those who are immunocompromised, at extremes of ages, or exposed to larger inoculums. Acute pulmonary histoplasmosis can present with cough, shortness of breath, fever, chills, and less commonly, rheumatologic complaints such as erythema nodosum or erythema multiforme. Imaging often shows patchy infiltrates and enlarged mediastinal and hilar lymphadenopathy. Patients can go on to develop subacute or chronic pulmonary disease with focal opacities and mediastinal and hilar lymphadenopathy. Those with chronic disease can have cavitary lesions similar to patients with tuberculosis. Progressive disseminated histoplasmosis can develop in immunocompromised patients and disseminate through the reticuloendothelial system to other organs with the gastrointestinal tract, central nervous system, and adrenal glands.2
Pulmonary nodules are common incidental finding on chest imaging of patients who reside in histoplasmosis endemic regions, and they are often hard to differentiate from malignancies. There are 3 mediastinal manifestations: adenitis, granuloma, and fibrosis. Usually the syndromes are subclinical, but occasionally the nodes cause symptoms by impinging on other structures.2
This patient had a solitary pulmonary nodule with none of the associated features mentioned above. Pathology showed caseating granuloma and confirmed histoplasmosis.
►Dr. Kearney. Given the diagnosis of solitary histoplasmoma, how should this patient be managed?
►Dr. Strymish. The optimal therapy for histoplasmosis depends on the patient’s clinical syndrome. Most infections are self-limited and require no therapy. However, patients who are immunocompromised, exposed to large inoculum, and have progressive disease require antifungal treatment, usually with itraconazole for mild-to-moderate disease and a combination of azole therapy and amphotericin B with extensive disease. Patients with few solitary pulmonary nodules do not benefit from antifungal therapy as the nodule could represent quiescent disease that is unlikely to have clinical impact; in this case, the treatment would be higher risk than the nodule.3
►Dr. Kearney. While the discussion of the diagnosis is interesting, it is also important to acknowledge what the patient went through to arrive at this, an essentially benign diagnosis: 8 months, multiple imaging studies, and 2 invasive diagnostic procedures. Further, the patient had to grapple with the possibility of a diagnosis of cancer. Dr. Wiener, can you talk about the challenges in communicating with patients about pulmonary nodules when cancer is on the differential? What are some of the harms patients face and how can clinicians work to mitigate these harms?
►Dr. Wiener. My colleague Dr. Christopher Slatore of the Portland VA Medical Center and I studied communication about pulmonary nodules in a series of surveys and qualitative studies of patients with pulmonary nodules and the clinicians who take care of them. We found that there seems to be a disconnect between patients’ perceptions of pulmonary nodules and their clinicians, often due to inadequate communication about the nodule. Many clinicians indicated that they do not tell patients about the chance that a nodule may be cancer, because the clinicians know that cancer is unlikely (< 5% of incidentally detected pulmonary nodules turn out to be malignant), and they do not want to alarm patients unnecessarily. However, we found that patients almost immediately wondered about cancer when they learned about their pulmonary nodule, and without hearing explicitly from their clinician that cancer was unlikely, patients tended to overestimate the likelihood of a malignant nodule. Moreover, patients often were not told much about the evaluation plan for the nodule or the rationale for CT surveillance of small nodules instead of biopsy. This uncertainty about the risk of cancer and the plan for evaluating the nodule was difficult for some patients to live with; we found that about one-quarter of patients with a small pulmonary nodule experienced mild-moderate distress during the period of radiographic surveillance. Reassuringly, high-quality patient-clinician communication was associated with lower distress and higher adherence to pulmonary nodule evaluation.4
►Dr. Kearney. The patient was educated about his diagnosis of solitary histoplasmoma. Given that the patient was otherwise well appearing with no complicating factors, he was not treated with antifungal therapy. After an 8-month-long workup, the patient was relieved to receive a diagnosis that excluded cancer and did not require any further treatment. His case provides a good example of how to proceed in the workup of a solitary pulmonary nodule and on the importance of communication and shared decision making with our patients.
Case Presentation. A 69-year-old veteran presented with an intermittent, waxing and waning cough. He had never smoked and had no family history of lung cancer. His primary care physician ordered a chest radiograph, which revealed a nodular opacity within the lingula concerning for a parenchymal nodule. Further characterization with a chest computed tomography (CT) demonstrated a 1.4-cm left upper lobe subpleural nodule with small satellite nodules (Figure 1). Given these imaging findings, the patient was referred to the pulmonary clinic.
►Lauren Kearney, MD, Medical Resident, VA Boston Healthcare System (VABHS) and Boston Medical Center. What is the differential diagnosis of a solitary pulmonary nodule? What characteristics of the nodule do you consider to differentiate these diagnoses?
►Renda Wiener, MD, Pulmonary and Critical Care, VABHS, and Assistant Professor of Medicine, Boston University School of Medicine. Pulmonary nodules are well-defined lesions < 3 cm in diameter that are surrounded by lung parenchyma. Although cancer is a possibility (including primary lung cancers, metastatic cancers, or carcinoid tumors), most small nodules do not turn out to be malignant.1 Benign etiologies include infections, benign tumors, vascular malformations, and inflammatory conditions. Infectious causes of nodules are often granulomatous in nature, including fungi, Mycobacterium tuberculosis, and nontuberculous mycobacteria. Benign tumors are most commonly hamartomas, and these may be clearly distinguished based on imaging characteristics. Pulmonary arteriovenous malformations, hematomas, and infarcts may present as nodules as well. Inflammatory causes of nodules are important and relatively common, including granulomatosis with polyangiitis, rheumatoid arthritis, sarcoidosis, amyloidosis, and rounded atelectasis.
To distinguish benign from malignant etiologies, we look for several features of pulmonary nodules on imaging. Larger size, irregular borders, and upper lobe location all increase the likelihood of cancer, whereas solid attenuation and calcification make cancer less likely. One of the most reassuring findings that suggests a benign etiology is lack of growth over a period of surveillance; after 2 years without growth we typically consider a nodule benign.1 And of course, we also consider the patient’s symptoms and risk factors: weight loss, hemoptysis, a history of cigarette smoking or asbestos exposure, or family history of cancer all increase the likelihood of malignancy.
►Dr. Kearney. Given that the differential diagnosis is so broad, how do you think about the next step in evaluating a pulmonary nodule? How do you approach shared decision making with the patient?
►Dr. Wiener. The characteristics of the patient, the nodule, and the circumstances in which the nodule were discovered are all important to consider. Incidental pulmonary nodules are often found on chest imaging. The imaging characteristics of the nodule are important, as are the patient’s risk factors. A similarly appearing nodule can have very different implications if the patient is a never-smoker exposed to endemic fungi, or a long-time smoker enrolled in a lung cancer screening program. Consultation with a pulmonologist is often appropriate.
It’s important to note that we lack high-quality evidence on the optimal strategy to evaluate pulmonary nodules, and there is no single “right answer“ for all patients. For patients with a low risk of malignancy (< 5%-10%)—which comprises the majority of the incidental nodules discovered—we typically favor serial CT surveillance of the nodule over a period of a few years, whereas for patients at high risk of malignancy (> 65%), we favor early surgical resection if the patient is able to tolerate that. For patients with an intermediate risk of malignancy (~5%-65%), we might consider serial CT surveillance, positron emission tomography (PET) scan, or biopsy.1 The American College of Chest Physicians guidelines for pulmonary nodule evaluation recommend discussing with patients the different options and the trade-offs of these options in a shared decision-making process.1
►Dr. Kearney. The patient’s pulmonologist laid out options, including monitoring with serial CT scans, obtaining a PET scan, performing CT-guided needle biopsy, or referring for surgical excision. In this case, the patient elected to undergo CT-guided needle biopsy. Dr. Huang, can you discuss the pathology results?
►Qin Huang, MD, Pathology and Laboratory Medicine, VABHS, and Assistant Professor of Pathology, Harvard Medical School (HMS). The microscopic examination of the needle biopsy of the lung mass revealed rare clusters of atypical cells with crushed cells adjacent to an extensive area of necrosis with scarring. The atypical cells were suspicious for carcinoma. The Gomori methenamine silver (GMS) and periodic acid-Schiff (PAS) stains were negative for common bacterial and fungal microorganisms.
►Dr. Kearney. The tumor board, pulmonologist, and patient decide to move forward with video-assisted excisional biopsy with lymphadenectomy. Dr. Huang, can you interpret the pathology?
►Dr. Huang. Figure 2 showed an hemotoxylin and eosin (H&E)-stained lung resection tissue section with multiple caseating necrotic granulomas. No foreign bodies were identified. There was no evidence of malignancy. The GMS stain revealed a fungal microorganism oval with morphology typical of histoplasma capsulatum (Figure 3).
►Dr. Kearney. What are some of the different ways histoplasmosis can present? Which of these diagnoses fits this patient’s presentation?
►Judy Strymish, MD, Infectious Disease, VABHS, and Assistant Professor of Medicine, HMS. Most patients who inhale histoplasmosis spores develop asymptomatic or self-limited infection that is usually not detected. Patients at risk of symptomatic and clinically relevant disease include those who are immunocompromised, at extremes of ages, or exposed to larger inoculums. Acute pulmonary histoplasmosis can present with cough, shortness of breath, fever, chills, and less commonly, rheumatologic complaints such as erythema nodosum or erythema multiforme. Imaging often shows patchy infiltrates and enlarged mediastinal and hilar lymphadenopathy. Patients can go on to develop subacute or chronic pulmonary disease with focal opacities and mediastinal and hilar lymphadenopathy. Those with chronic disease can have cavitary lesions similar to patients with tuberculosis. Progressive disseminated histoplasmosis can develop in immunocompromised patients and disseminate through the reticuloendothelial system to other organs with the gastrointestinal tract, central nervous system, and adrenal glands.2
Pulmonary nodules are common incidental finding on chest imaging of patients who reside in histoplasmosis endemic regions, and they are often hard to differentiate from malignancies. There are 3 mediastinal manifestations: adenitis, granuloma, and fibrosis. Usually the syndromes are subclinical, but occasionally the nodes cause symptoms by impinging on other structures.2
This patient had a solitary pulmonary nodule with none of the associated features mentioned above. Pathology showed caseating granuloma and confirmed histoplasmosis.
►Dr. Kearney. Given the diagnosis of solitary histoplasmoma, how should this patient be managed?
►Dr. Strymish. The optimal therapy for histoplasmosis depends on the patient’s clinical syndrome. Most infections are self-limited and require no therapy. However, patients who are immunocompromised, exposed to large inoculum, and have progressive disease require antifungal treatment, usually with itraconazole for mild-to-moderate disease and a combination of azole therapy and amphotericin B with extensive disease. Patients with few solitary pulmonary nodules do not benefit from antifungal therapy as the nodule could represent quiescent disease that is unlikely to have clinical impact; in this case, the treatment would be higher risk than the nodule.3
►Dr. Kearney. While the discussion of the diagnosis is interesting, it is also important to acknowledge what the patient went through to arrive at this, an essentially benign diagnosis: 8 months, multiple imaging studies, and 2 invasive diagnostic procedures. Further, the patient had to grapple with the possibility of a diagnosis of cancer. Dr. Wiener, can you talk about the challenges in communicating with patients about pulmonary nodules when cancer is on the differential? What are some of the harms patients face and how can clinicians work to mitigate these harms?
►Dr. Wiener. My colleague Dr. Christopher Slatore of the Portland VA Medical Center and I studied communication about pulmonary nodules in a series of surveys and qualitative studies of patients with pulmonary nodules and the clinicians who take care of them. We found that there seems to be a disconnect between patients’ perceptions of pulmonary nodules and their clinicians, often due to inadequate communication about the nodule. Many clinicians indicated that they do not tell patients about the chance that a nodule may be cancer, because the clinicians know that cancer is unlikely (< 5% of incidentally detected pulmonary nodules turn out to be malignant), and they do not want to alarm patients unnecessarily. However, we found that patients almost immediately wondered about cancer when they learned about their pulmonary nodule, and without hearing explicitly from their clinician that cancer was unlikely, patients tended to overestimate the likelihood of a malignant nodule. Moreover, patients often were not told much about the evaluation plan for the nodule or the rationale for CT surveillance of small nodules instead of biopsy. This uncertainty about the risk of cancer and the plan for evaluating the nodule was difficult for some patients to live with; we found that about one-quarter of patients with a small pulmonary nodule experienced mild-moderate distress during the period of radiographic surveillance. Reassuringly, high-quality patient-clinician communication was associated with lower distress and higher adherence to pulmonary nodule evaluation.4
►Dr. Kearney. The patient was educated about his diagnosis of solitary histoplasmoma. Given that the patient was otherwise well appearing with no complicating factors, he was not treated with antifungal therapy. After an 8-month-long workup, the patient was relieved to receive a diagnosis that excluded cancer and did not require any further treatment. His case provides a good example of how to proceed in the workup of a solitary pulmonary nodule and on the importance of communication and shared decision making with our patients.
1. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e93S-e120S.
2. Azar MM, Hage CA. Clinical perspectives in the diagnosis and management of histoplasmosis. Clin Chest Med. 2017;38(3):403-415.
3. Wheat LJ, Freifeld A, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807-825.
4. Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153(4):1004-1015.
1. Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(suppl 5):e93S-e120S.
2. Azar MM, Hage CA. Clinical perspectives in the diagnosis and management of histoplasmosis. Clin Chest Med. 2017;38(3):403-415.
3. Wheat LJ, Freifeld A, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807-825.
4. Slatore CG, Wiener RS. Pulmonary nodules: a small problem for many, severe distress for some, and how to communicate about it. Chest. 2018;153(4):1004-1015.
First-time marathon runners rewind the clock on vascular aging
Persons who trained for a marathon showed improvement in age-related aortic stiffness and reduction in blood pressure in a study of 138 first-time completers of the London Marathon.
Compared with pretraining values, the descending aortas of marathon completers were 9% more distensible at the level of the bifurcation of the pulmonary artery and 16% more distensible at the level of the diaphragm (P = .0009 and .002, respectively). There was no change in distensibility of the ascending aorta.
Additionally, central systolic BP dropped by 4 mm Hg and diastolic BP by 3 mm Hg by the time marathon training was completed.
“Training and completion of a first-time marathon result in beneficial reductions in BP and intrinsic aortic stiffening in healthy participants,” concluded Anish Bhuva, MBBS, and coinvestigators. “These changes are equivalent to approximately a 4-year reduction in vascular age.”
The study points to a role for exercise in the reduction of arterial stiffness, a known aging-related contributor to cardiovascular disease for which there currently is no good pharmacologic option, said Julio Chirinos, MD, Phd, in an accompanying editorial (J Am Coll Cardiol. 2020 Jan 6. doi: 10.1016/j.jacc.2019.11.007). The challenge lies in implementing exercise interventions on a large scale in societies where “there remains an immense paradoxical gap” between the known benefits of physical activity and increasingly sedentary populations, he added, calling for increased implementation research.
Using cardiovascular magnetic resonance to assess aortic distensibility, Dr. Bhuva, of the Institute of Cardiovascular Science, University College London, and colleagues assessed aortic BP and aortic stiffness at two points via the noninvasive imaging method. The first assessment was conducted before the study participants began marathon training; the second was obtained between 1 and 3 weeks after marathon completion, after any acute effects of the marathon had abated.
Anthropometric data, peripheral BP, and aerobic capacity (peak VO2) were also assessed at both study points.
Although the study wasn’t designed to track individual training regimens, first-time London Marathon participants were given a 17-week “Beginner’s Training Plan” by event organizers, and asked to follow the plan while participating in the study. The goal of the beginner’s plan was marathon completion, with a schedule of about three runs weekly increasing in duration and intensity over the training period.
Participants had to be first-time marathon participants and running less than 2 hours per week at enrollment. Only those who completed the marathon were included in the data analysis, though baseline characteristics didn’t differ between completers and those who dropped out.
For 2016, the first study year, only participants aged 18-39 years were included, while in 2017, all ages were included in the study. The final age range was 21-69 years, with a mean age of 37; 51% of participants were female. Those with a history of hypertension or taking antihypertensive medication and those who had other significant medical conditions were excluded.
The differential increase in distensibility along the length of the aorta reflects known differences in tissue composition, agreed the authors and Dr. Chirinos, a cardiologist at the University of Pennsylvania, Philadelphia. In addition to the magnetic resonance–obtained distensibility measurements, the investigators conducted further calculations to adjust for baseline mean central arterial pressure, since arterial stiffness is a function both of intrinsic tissue characteristics and loading conditions.
In youth, aortic distensibility buffers the effect of pulse pressure on both the left ventricle and the peripheral vascular system. As the aorta and other large arteries stiffen predictably with age, isolated systolic hypertension can result. The stiffening “also favors adverse patterns of pulsatile left ventricular overload,” which can lead to left ventricular remodeling and, eventually, heart failure, noted Dr. Chirinos. Reduced aortic pliancy also allows pulse pressure variation to be transmitted downstream “into the microvasculature of target organs (such as the kidney and brain) that require high blood flow and thus operate at low arteriolar resistance,” he added.
The assessment that marathon training reversed aortic age by a median 3.9 years was derived from the baseline cross-sectional data regarding participants’ age and aortic stiffness. The effect size was largest in those older than 37 years and in those with higher baseline systolic BP, and men saw greater benefit by a median 1.4 years. Those with slower running times also saw greater benefit.
Study participants had small but significant reductions in heart rate, body fat percentage, and weight by the postmarathon assessment, but these differences were not associated with changes in aortic stiffness. Aerobic exercise capacity as measured by peak VO2 didn’t change significantly from pre- to post training, but the fact that participants were semirecumbent during exercise testing (to allow concurrent echocardiography) may have affected results.
The real-world design of this study had the strengths of assessing free-living, healthy individuals who participated in a self-directed training plan. Dr. Bhuva and coauthors acknowledged that marathon training may include changes in diet, sleep, and other potentially confounding lifestyle factors, as well as improvement in lipid and glucose metabolism. Further, noted Dr. Chirinos, there was no control group. Also, results from individuals training for an endurance event may have limited generalizability to the general population.
Still, said Dr. Chirinos, the innovative study design took advantage of a large-scale athletic event to see how a realistic training regimen affected healthy individuals. “Perhaps the contemporary marathon can teach us some lessons about exploiting the confluence of interests of the general public, media, industry, scientific community, and government to accomplish worthy goals at the individual and societal levels.”
The study was funded by the British Heart Foundation, Cardiac Risk in the Young, and the Barts Cardiovascular Biomedical Research Centre. Exercise testing equipment and technical support were provided by COSMED. Dr. Bhuva reported receiving funding from the British Heart Foundation. Dr. Chirinos reported having been a consultant or receiving research funding from multiple pharmaceutical companies and Microsoft; he is also an inventor of University of Pennsylvania–held patents for cardiovascular pharmaceutical agents.
SOURCE: Bhuva A et al. J Am Coll Cardiol. 2020 Jan;75(1):60-71.
Persons who trained for a marathon showed improvement in age-related aortic stiffness and reduction in blood pressure in a study of 138 first-time completers of the London Marathon.
Compared with pretraining values, the descending aortas of marathon completers were 9% more distensible at the level of the bifurcation of the pulmonary artery and 16% more distensible at the level of the diaphragm (P = .0009 and .002, respectively). There was no change in distensibility of the ascending aorta.
Additionally, central systolic BP dropped by 4 mm Hg and diastolic BP by 3 mm Hg by the time marathon training was completed.
“Training and completion of a first-time marathon result in beneficial reductions in BP and intrinsic aortic stiffening in healthy participants,” concluded Anish Bhuva, MBBS, and coinvestigators. “These changes are equivalent to approximately a 4-year reduction in vascular age.”
The study points to a role for exercise in the reduction of arterial stiffness, a known aging-related contributor to cardiovascular disease for which there currently is no good pharmacologic option, said Julio Chirinos, MD, Phd, in an accompanying editorial (J Am Coll Cardiol. 2020 Jan 6. doi: 10.1016/j.jacc.2019.11.007). The challenge lies in implementing exercise interventions on a large scale in societies where “there remains an immense paradoxical gap” between the known benefits of physical activity and increasingly sedentary populations, he added, calling for increased implementation research.
Using cardiovascular magnetic resonance to assess aortic distensibility, Dr. Bhuva, of the Institute of Cardiovascular Science, University College London, and colleagues assessed aortic BP and aortic stiffness at two points via the noninvasive imaging method. The first assessment was conducted before the study participants began marathon training; the second was obtained between 1 and 3 weeks after marathon completion, after any acute effects of the marathon had abated.
Anthropometric data, peripheral BP, and aerobic capacity (peak VO2) were also assessed at both study points.
Although the study wasn’t designed to track individual training regimens, first-time London Marathon participants were given a 17-week “Beginner’s Training Plan” by event organizers, and asked to follow the plan while participating in the study. The goal of the beginner’s plan was marathon completion, with a schedule of about three runs weekly increasing in duration and intensity over the training period.
Participants had to be first-time marathon participants and running less than 2 hours per week at enrollment. Only those who completed the marathon were included in the data analysis, though baseline characteristics didn’t differ between completers and those who dropped out.
For 2016, the first study year, only participants aged 18-39 years were included, while in 2017, all ages were included in the study. The final age range was 21-69 years, with a mean age of 37; 51% of participants were female. Those with a history of hypertension or taking antihypertensive medication and those who had other significant medical conditions were excluded.
The differential increase in distensibility along the length of the aorta reflects known differences in tissue composition, agreed the authors and Dr. Chirinos, a cardiologist at the University of Pennsylvania, Philadelphia. In addition to the magnetic resonance–obtained distensibility measurements, the investigators conducted further calculations to adjust for baseline mean central arterial pressure, since arterial stiffness is a function both of intrinsic tissue characteristics and loading conditions.
In youth, aortic distensibility buffers the effect of pulse pressure on both the left ventricle and the peripheral vascular system. As the aorta and other large arteries stiffen predictably with age, isolated systolic hypertension can result. The stiffening “also favors adverse patterns of pulsatile left ventricular overload,” which can lead to left ventricular remodeling and, eventually, heart failure, noted Dr. Chirinos. Reduced aortic pliancy also allows pulse pressure variation to be transmitted downstream “into the microvasculature of target organs (such as the kidney and brain) that require high blood flow and thus operate at low arteriolar resistance,” he added.
The assessment that marathon training reversed aortic age by a median 3.9 years was derived from the baseline cross-sectional data regarding participants’ age and aortic stiffness. The effect size was largest in those older than 37 years and in those with higher baseline systolic BP, and men saw greater benefit by a median 1.4 years. Those with slower running times also saw greater benefit.
Study participants had small but significant reductions in heart rate, body fat percentage, and weight by the postmarathon assessment, but these differences were not associated with changes in aortic stiffness. Aerobic exercise capacity as measured by peak VO2 didn’t change significantly from pre- to post training, but the fact that participants were semirecumbent during exercise testing (to allow concurrent echocardiography) may have affected results.
The real-world design of this study had the strengths of assessing free-living, healthy individuals who participated in a self-directed training plan. Dr. Bhuva and coauthors acknowledged that marathon training may include changes in diet, sleep, and other potentially confounding lifestyle factors, as well as improvement in lipid and glucose metabolism. Further, noted Dr. Chirinos, there was no control group. Also, results from individuals training for an endurance event may have limited generalizability to the general population.
Still, said Dr. Chirinos, the innovative study design took advantage of a large-scale athletic event to see how a realistic training regimen affected healthy individuals. “Perhaps the contemporary marathon can teach us some lessons about exploiting the confluence of interests of the general public, media, industry, scientific community, and government to accomplish worthy goals at the individual and societal levels.”
The study was funded by the British Heart Foundation, Cardiac Risk in the Young, and the Barts Cardiovascular Biomedical Research Centre. Exercise testing equipment and technical support were provided by COSMED. Dr. Bhuva reported receiving funding from the British Heart Foundation. Dr. Chirinos reported having been a consultant or receiving research funding from multiple pharmaceutical companies and Microsoft; he is also an inventor of University of Pennsylvania–held patents for cardiovascular pharmaceutical agents.
SOURCE: Bhuva A et al. J Am Coll Cardiol. 2020 Jan;75(1):60-71.
Persons who trained for a marathon showed improvement in age-related aortic stiffness and reduction in blood pressure in a study of 138 first-time completers of the London Marathon.
Compared with pretraining values, the descending aortas of marathon completers were 9% more distensible at the level of the bifurcation of the pulmonary artery and 16% more distensible at the level of the diaphragm (P = .0009 and .002, respectively). There was no change in distensibility of the ascending aorta.
Additionally, central systolic BP dropped by 4 mm Hg and diastolic BP by 3 mm Hg by the time marathon training was completed.
“Training and completion of a first-time marathon result in beneficial reductions in BP and intrinsic aortic stiffening in healthy participants,” concluded Anish Bhuva, MBBS, and coinvestigators. “These changes are equivalent to approximately a 4-year reduction in vascular age.”
The study points to a role for exercise in the reduction of arterial stiffness, a known aging-related contributor to cardiovascular disease for which there currently is no good pharmacologic option, said Julio Chirinos, MD, Phd, in an accompanying editorial (J Am Coll Cardiol. 2020 Jan 6. doi: 10.1016/j.jacc.2019.11.007). The challenge lies in implementing exercise interventions on a large scale in societies where “there remains an immense paradoxical gap” between the known benefits of physical activity and increasingly sedentary populations, he added, calling for increased implementation research.
Using cardiovascular magnetic resonance to assess aortic distensibility, Dr. Bhuva, of the Institute of Cardiovascular Science, University College London, and colleagues assessed aortic BP and aortic stiffness at two points via the noninvasive imaging method. The first assessment was conducted before the study participants began marathon training; the second was obtained between 1 and 3 weeks after marathon completion, after any acute effects of the marathon had abated.
Anthropometric data, peripheral BP, and aerobic capacity (peak VO2) were also assessed at both study points.
Although the study wasn’t designed to track individual training regimens, first-time London Marathon participants were given a 17-week “Beginner’s Training Plan” by event organizers, and asked to follow the plan while participating in the study. The goal of the beginner’s plan was marathon completion, with a schedule of about three runs weekly increasing in duration and intensity over the training period.
Participants had to be first-time marathon participants and running less than 2 hours per week at enrollment. Only those who completed the marathon were included in the data analysis, though baseline characteristics didn’t differ between completers and those who dropped out.
For 2016, the first study year, only participants aged 18-39 years were included, while in 2017, all ages were included in the study. The final age range was 21-69 years, with a mean age of 37; 51% of participants were female. Those with a history of hypertension or taking antihypertensive medication and those who had other significant medical conditions were excluded.
The differential increase in distensibility along the length of the aorta reflects known differences in tissue composition, agreed the authors and Dr. Chirinos, a cardiologist at the University of Pennsylvania, Philadelphia. In addition to the magnetic resonance–obtained distensibility measurements, the investigators conducted further calculations to adjust for baseline mean central arterial pressure, since arterial stiffness is a function both of intrinsic tissue characteristics and loading conditions.
In youth, aortic distensibility buffers the effect of pulse pressure on both the left ventricle and the peripheral vascular system. As the aorta and other large arteries stiffen predictably with age, isolated systolic hypertension can result. The stiffening “also favors adverse patterns of pulsatile left ventricular overload,” which can lead to left ventricular remodeling and, eventually, heart failure, noted Dr. Chirinos. Reduced aortic pliancy also allows pulse pressure variation to be transmitted downstream “into the microvasculature of target organs (such as the kidney and brain) that require high blood flow and thus operate at low arteriolar resistance,” he added.
The assessment that marathon training reversed aortic age by a median 3.9 years was derived from the baseline cross-sectional data regarding participants’ age and aortic stiffness. The effect size was largest in those older than 37 years and in those with higher baseline systolic BP, and men saw greater benefit by a median 1.4 years. Those with slower running times also saw greater benefit.
Study participants had small but significant reductions in heart rate, body fat percentage, and weight by the postmarathon assessment, but these differences were not associated with changes in aortic stiffness. Aerobic exercise capacity as measured by peak VO2 didn’t change significantly from pre- to post training, but the fact that participants were semirecumbent during exercise testing (to allow concurrent echocardiography) may have affected results.
The real-world design of this study had the strengths of assessing free-living, healthy individuals who participated in a self-directed training plan. Dr. Bhuva and coauthors acknowledged that marathon training may include changes in diet, sleep, and other potentially confounding lifestyle factors, as well as improvement in lipid and glucose metabolism. Further, noted Dr. Chirinos, there was no control group. Also, results from individuals training for an endurance event may have limited generalizability to the general population.
Still, said Dr. Chirinos, the innovative study design took advantage of a large-scale athletic event to see how a realistic training regimen affected healthy individuals. “Perhaps the contemporary marathon can teach us some lessons about exploiting the confluence of interests of the general public, media, industry, scientific community, and government to accomplish worthy goals at the individual and societal levels.”
The study was funded by the British Heart Foundation, Cardiac Risk in the Young, and the Barts Cardiovascular Biomedical Research Centre. Exercise testing equipment and technical support were provided by COSMED. Dr. Bhuva reported receiving funding from the British Heart Foundation. Dr. Chirinos reported having been a consultant or receiving research funding from multiple pharmaceutical companies and Microsoft; he is also an inventor of University of Pennsylvania–held patents for cardiovascular pharmaceutical agents.
SOURCE: Bhuva A et al. J Am Coll Cardiol. 2020 Jan;75(1):60-71.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Using Democratic Deliberation to Engage Veterans in Complex Policy Making for the Veterans Health Administration
Providing high-quality, patient-centered health care is a top priority for the US Department of Veterans Affairs (VA) Veteran Health Administration (VHA), whose core mission is to improve the health and well-being of US veterans. Thus, news of long wait times for medical appointments in the VHA sparked intense national attention and debate and led to changes in senior management and legislative action. 1 On August 8, 2014, President Bara c k Obama signed the Veterans Access, Choice, and Accountability Act of 2014, also known as the Choice Act, which provided an additional $16 billion in emergency spending over 3 years to improve veterans’ access to timely health care. 2 The Choice Act sought to develop an integrated health care network that allowed qualified VHA patients to receive specific health care services in their communities delivered by non-VHA health care providers (HCPs) but paid for by the VHA. The Choice Act also laid out explicit criteria for how to prioritize who would be eligible for VHA-purchased civilian care: (1) veterans who could not get timely appointments at a VHA medical facility within 30 days of referral; or (2) veterans who lived > 40 miles from the closest VHA medical facility.
VHA decision makers seeking to improve care delivery also need to weigh trade-offs between alternative approaches to providing rapid access. For instance, increasing access to non-VHA HCPs may not always decrease wait times and could result in loss of continuity, limited care coordination, limited ability to ensure and enforce high-quality standards at the VHA, and other challenges.3-6 Although the concerns and views of elected representatives, advocacy groups, and health system leaders are important, it is unknown whether these views and preferences align with those of veterans. Arguably, the range of views and concerns of informed veterans whose health is at stake should be particularly prominent in such policy decision making.
To identify the considerations that were most important to veterans regarding VHA policy around decreasing wait times, a study was designed to engage a group of veterans who were eligible for civilian care under the Choice Act. The study took place 1 year after the Choice Act was passed. Veterans were asked to focus on 2 related questions: First, how should funding be used for building VHA capacity (build) vs purchasing civilian care (buy)? Second, under what circumstances should civilian care be prioritized?
The aim of this paper is to describe democratic deliberation (DD), a specific method that engaged veteran patients in complex policy decisions around access to care. DD methods have been used increasingly in health care for developing policy guidance, setting priorities, providing advice on ethical dilemmas, weighing risk-benefit trade-offs, and determining decision-making authority.7-12 For example, DD helped guide national policy for mammography screening for breast cancer in New Zealand.13 The Agency for Healthcare Research and Quality has completed a systematic review and a large, randomized experiment on best practices for carrying out public deliberation.8,13,14 However, despite the potential value of this approach, there has been little use of deliberative methods within the VHA for the explicit purpose of informing veteran health care delivery.
This paper describes the experience engaging veterans by using DD methodology and informing VHA leadership about the results of those deliberations. The specific aims were to understand whether DD is an acceptable approach to use to engage patients in the medical services policy-making process within VHA and whether veterans are able to come to an informed consensus.
Methods
Engaging patients and incorporating their needs and concerns within the policy-making process may improve health system policies and make those policies more patient centered. Such engagement also could be a way to generate creative solutions. However, because health-system decisions often involve making difficult trade-offs, effectively obtaining patient population input on complex care delivery issues can be challenging.
Although surveys can provide intuitive, top-of-mind input from respondents, these opinions are generally not sufficient for resolving complex problems.15 Focus groups and interviews may produce results that are more in-depth than surveys, but these methods tend to elicit settled private preferences rather than opinions about what the community should do.16 DD, on the other hand, is designed to elicit deeply informed public opinions on complex, value-laden topics to develop recommendations and policies for a larger community.17 The goal is to find collective solutions to challenging social problems. DD achieves this by giving participants an opportunity to explore a topic in-depth, question experts, and engage peers in reason-based discussions.18,19 This method has its roots in political science and has been used over several decades to successfully inform policy making on a broad array of topics nationally and internationally—from health research ethics in the US to nuclear and energy policy in Japan.7,16,20,21 DD has been found to promote ownership of public programs and lend legitimacy to policy decisions, political institutions, and democracy itself.18
A single day (8 hours) DD session was convened, following a Citizens Jury model of deliberation, which brings veteran patients together to learn about a topic, ask questions of experts, deliberate with peers, and generate a “citizen’s report” that contains a set of recommendations (Table 1). An overview of the different models of DD and rationale for each can be found elsewhere.8,15
Recruitment Considerations
A purposively selected sample of civilian care-eligible veterans from a midwestern VHA health care system (1 medical center and 3 community-based outpatient clinics [CBOCs]) were invited to the DD session. The targeted number of participants was 30. Female veterans, who comprise only 7% of the local veteran population, were oversampled to account for their potentially different health care needs and to create balance between males and females in the session. Oversampling for other characteristics was not possible due to the relatively small sample size. Based on prior experience,7 it was assumed that 70% of willing participants would attend the session; therefore 34 veterans were invited and 24 attended. Each participant received a $200 incentive in appreciation for their substantial time commitment and to offset transportation costs.
Background Materials
A packet with educational materials (Flesch-Kincaid Grade Level of 10.5) was mailed to participants about 2 weeks before the DD session. Participants were asked to review prior to attending the session. These materials described the session (eg, purpose, organizers, importance) and provided factual information about the Choice Act (eg, eligibility, out-of-pocket costs, travel pay, prescription drug policies).
Session Overview
The session was structured to accomplish the following goals: (1) Elicit participants’ opinions about access to health care and reasons for those opinions; (2) Provide in-depth education about the Choice Act through presentations and discussions with topical experts; and (3) Elicit reasoning and recommendations on both the criteria by which participants prioritize candidates for civilian care and how participants would allocate additional funding to improve access (ie, by building VHA capacity to deliver more timely health care vs purchasing health care from civilian HCPs).
Participants were asked to fill out a survey on arrival in the morning and were assigned to 1 of 3 tables or small groups. Each table had a facilitator who had extensive experience in qualitative data collection methods and guided the dialogue using a scripted protocol that they helped develop and refine. The facilitation materials drew from and used previously published studies.22,23 Each facilitator audio recorded the sessions and took notes. Three experts presented during plenary education sessions. Presentations were designed to provide balanced factual information and included a veteran’s perspective. One presenter was a clinician on the project team, another was a local clinical leader responsible for making decisions about what services to provide via civilian care (buy) vs enhancing the local VHA health system’s ability to provide those services (build), and the third presenter was a veteran who was on the project team.
Education Session 1
The first plenary education session with expert presentations was conducted after each table completed an icebreaker exercise. The project team physician provided a brief history and description of the Choice Act to reinforce educational materials sent to participants prior to the session. The health system clinical leader described his decision process and principles and highlighted constraints placed on him by the Choice Act that were in place at the time of the DD session. He also described existing local and national programs to provide civilian care (eg, local fee-basis non-VHA care programs) and how these programs sought to achieve goals similar to the Choice Act. The veteran presenter focused on the importance of session participants providing candid insight and observations and emphasized that this session was a significant opportunity to “have their voices heard.”
Deliberation 1: What criteria should be used to prioritize patients for receiving civilian care paid for by the VHA? To elicit preferences on the central question of this deliberation, participants were presented with 8 real-world cases that were based on interviews conducted with Choice Act-eligible veterans (Table 2 and eAppendices A
Education Session 2
In the second plenary session, the project team physician provided information about health care access issues, both inside and outside of the VHA, particularly between urban and rural areas. He also discussed factors related to the insufficient capacity to meet growing demand that contributed to the VHA wait-time crisis. The veteran presenter shared reflections on health care access from a veteran’s perspective.
Deliberation 2: How should additional funding be divided between increasing the ability of the VHA to (1) provide care by VHA HCPs; and (2) pay for care from non-VHA civilian HCPs? Participants were presented the patient examples and Choice Act funding scenarios (the buy policy option) and contrasted that with a build policy option. Participants were explicitly encouraged to shift their perspectives from thinking about individual cases to considering policy-level decisions and the broader social good (Table 2).
Ensuring Robust Deliberations
If participants do not adequately grasp the complexities of the topic, a deliberation can fail. To facilitate nuanced reasoning, real-world concrete examples were developed as the starting point of each deliberation based on interviews with actual patients (deliberation 1) and actual policy proposals relevant to the funding allocation decisions within the Choice Act (deliberation 2).
A deliberation also can fail with self-silencing, where participants withhold opinions that differ from those articulated first or by more vocal members of the group.24 To combat self-silencing, highly experienced facilitators were used to ensure sharing from all participants and to support an open-minded, courteous, and reason-based environment for discourse. It was specified that the best solutions are achieved through reason-based and cordial disagreement and that success can be undermined when participants simply agree because it is easier or more comfortable.
A third way a deliberation can fail is if individuals do not adopt a group or system-level perspective. To counter this, facilitators reinforced at multiple points the importance of taking a broader social perspective rather than sharing only one’s personal preferences.
Finally, it is important to assess the quality of the deliberative process itself, to ensure that results are trustworthy.25 To assess the quality of the deliberative process, participants knowledge about key issues pre- and postdeliberation were assessed. Participants also were asked to rate the quality of the facilitators and how well they felt their voice was heard and respected, and facilitators made qualitative assessments about the extent to which participants were engaged in reason-based and collaborative discussion.
Data
Quantitative data were collected via pre- and postsession surveys. The surveys contained items related to knowledge about the Choice Act, expectations for the DD session, beliefs and opinions about the provision of health care for veterans, recommended funding allocations between build vs buy policy options, and general demographics. Qualitative data were collected through detailed notes taken by the 3 facilitators. Each table’s deliberations were audio recorded so that gaps in the notes could be filled.
The 3 facilitators, who were all experienced qualitative researchers, typed their written notes into a template immediately after the session. Two of the 3 facilitators led the analysis of the session notes. Findings within and across the 3 deliberation tables were developed using content and matrix analysis methods.26 Descriptive statistics were generated from survey responses and compared survey items pre- and postsession using paired t tests or χ2 tests for categorical responses.
Results
Thirty-three percent of individuals invited (n = 127) agreed to participate. Those who declined cited conflicts related to distance, transportation, work/school, medical appointments, family commitments, or were not interested. In all, 24 (69%) of the 35 veterans who accepted the invitation attended the deliberation session. Of the 11 who accepted but did not attend, 5 cancelled ahead of time because of conflicts (Figure). Most participants were male (70%), 48% were aged 61 to 75 years, 65% were white, 43% had some college education, 43% reported an annual income of between $25,000 and $40,000, and only 35% reported very good health (eAppendix D).
Deliberation 1
During the deliberation on the prioritization criteria, the concept of “condition severity” emerged as an important criterion for veterans. This criterion captured simultaneous consideration of both clinical necessity and burden on the veteran to obtain care. For example, participants felt that patients with a life-threatening illness should be prioritized for civilian care over patients who need preventative or primary care (clinical necessity) and that elderly patients with substantial difficulty traveling to VHA appointments should be prioritized over patients who can travel more easily (burden). The Choice Act regulations at the time of the DD session did not reflect this nuanced perspective, stipulating only that veterans must live > 40 miles from the nearest VHA medical facility.
One of the 3 groups did not prioritize the patient cases because some members felt that no veteran should be constrained from receiving civilian care if they desired it. Nonetheless, this group did agree with prioritizing the first 2 cases in Table 3. The other groups prioritized all 8 cases in generally similar ways.
Deliberation 2
No clear consensus emerged on the buy vs build question. A representative from each table presented their group’s positions, rationale, and recommendations after deliberations were completed. After hearing the range of positions, the groups then had another opportunity to deliberate based on what they heard from the other tables; no new recommendations or consensus emerged.
Participants who were in favor of allocating more funds toward the build policy offered a range of rationales, saying that it would (1) increase access for rural veterans by building CBOCs and deploying more mobile units that could bring outlets for health care closer to their home communities; (2) provide critical and unique medical expertise to address veteran-specific issues such as prosthetics, traumatic brain injury, posttraumatic stress disorder, spinal cord injury, and shrapnel wounds that are typically not available through civilian providers; (3) give VHA more oversight over the quality and cost of care, which is more challenging to do with civilian providers; and (4) Improve VHA infrastructure by, for example, upgrading technology and attracting the best clinicians and staff to support “our VHA.”
Participants who were in favor of allocating more funds toward the buy policy also offered a range of rationales, saying that it would (1) decrease patient burden by increasing access through community providers, decreasing wait time, and lessening personal cost and travel time; (2) allow more patients to receive civilian care, which was generally seen as beneficial by a few participants because of perceptions that the VHA provides lower quality care due to a shortage of VHA providers, run-down/older facilities, lack of technology, and poorer-quality VHA providers; and (3) provide an opportunity to divest of costly facilities and invest in other innovative approaches. Regarding this last reason, a few participants felt that the VHA is “gouged” when building medical centers that overrun budgets. They also were concerned that investing in facilities tied VHA to specific locations when current locations of veterans may change “25 years from now.”
Survey Results
Twenty-three of the 24 participants completed both pre- and postsession surveys. The majority of participants in the session felt people in the group respected their opinion (96%); felt that the facilitator did not try to influence the group with her own opinions (96%); indicated they understood the information enough to participate as much as they wanted (100%); and were hopeful that their reasoning and recommendations would help inform VHA policy makers (82%).
The surveys also provided an opportunity to examine the extent to which knowledge, attitudes, and opinions changed from before to after the deliberation. Even with the small sample, responses revealed a trend toward improved knowledge about key elements of the Choice Act and its goals. Further, there was a shift in some participants’ opinions about how patients should be prioritized to receive civilian care. For example, before the deliberation participants generally felt that all veterans should be able to receive civilian care, whereas postdeliberation this was not the case. Postdeliberation, most participants felt that primary care should not be a high priority for civilian care but continued to endorse prioritizing civilian care for specialty services like orthopedic or cardiology-related care. Finally, participants moved from more diverse recommendations regarding additional funds allocations, toward consensus after the deliberation around allocating funds to the build policy. Eight participants supported a build policy beforehand, whereas 16 supported this policy afterward.
Discussion
This study explored DD as a method for deeply engaging veterans in complex policy making to guide funding allocation and prioritization decisions related to the Choice Act, decisions that are still very relevant today within the context of the Mission Act and have substantial implications for how health care is delivered in the VHA. The Mission Act passed on June 6, 2018, with the goal of improving access to and the reliability of civilian or community care for eligible veterans.27 Decisions related to appropriating scarce funding to improve access to care is an emotional and value-laden topic that elicited strong and divergent opinions among the participants. Veterans were eager to have their voices heard and had strong expectations that VHA leadership would be briefed about their recommendations. The majority of participants were satisfied with the deliberation process, felt they understood the issues, and felt their opinions were respected. They expressed feelings of comradery and community throughout the process.
In this single deliberation session, the groups did not achieve a single, final consensus regarding how VHA funding should ultimately be allocated between buy and build policy options. Nonetheless, participants provided a rich array of recommendations and rationale for them. Session moderators observed rich, sophisticated, fair, and reason-based discussions on this complex topic. Participants left with a deeper knowledge and appreciation for the complex trade-offs and expressed strong rationales for both sides of the policy debate on build vs buy. In addition, the project yielded results of high interest to VHA policy makers.
This work was presented in multiple venues between 2015 to 2016, and to both local and national VHA leadership, including the local Executive Quality Leadership Boards, the VHA Central Office Committee on the Future State of VA Community Care, the VA Office of Patient Centered Care, and the National Veteran Experience Committee. Through these discussions and others, we saw great interest within the VHA system and high-level leaders to explore ways to include veterans’ voices in the policy-making process. This work was invaluable to our research team (eAppendix E
Many health system decisions regarding what care should be delivered (and how) involve making difficult, value-laden choices in the context of limited resources. DD methods can be used to target and obtain specific viewpoints from diverse populations, such as the informed perspectives of minority and underrepresented populations within the VHA.19 For example, female veterans were oversampled to ensure that the informed preferences of this population was obtained. Thus, DD methods could provide a valuable tool for health systems to elicit in-depth diverse patient input on high-profile policies that will have a substantial impact on the system’s patient population.
Limitations
One potential downside of DD is that, because of the resource-intensive nature of deliberation sessions, they are often conducted with relatively small groups.9 Viewpoints of those within these small samples who are willing to spend an entire day discussing a complex topic may not be representative of the larger patient community. However, the core goal of DD is diversity of opinions rather than representativeness.
A stratified random sampling strategy that oversampled for underrepresented and minority populations was used to help select a diverse group that represents the population on key characteristics and partially addresses concern about representativeness. Efforts to optimize participation rates, including providing monetary incentives, also are helpful and have led to high participation rates in past deliberations.7
Health system communication strategies that promote the importance of becoming involved in DD sessions also may be helpful in improving rates of recruitment. On particularly important topics where health system leaders feel a larger resource investment is justified, conducting larger scale deliberations with many small groups may obtain more generalizable evidence about what individual patients and groups of patients recommend.7 However, due to the inherent limitations of surveys and focus group approaches for obtaining informed views on complex topics, there are no clear systematic alternatives to the DD approach.
Conclusion
DD is an effective method to meaningfully engage patients in deep deliberations to guide complex policy making. Although design of deliberative sessions is resource-intensive, patient engagement efforts, such as those described in this paper, could be an important aspect of a well-functioning learning health system. Further research into alternative, streamlined methods that can also engage veterans more deeply is needed. DD also can be combined with other approaches to broaden and confirm findings, including focus groups, town hall meetings, or surveys.
Although this study did not provide consensus on how the VHA should allocate funds with respect to the Choice Act, it did provide insight into the importance and feasibility of engaging veterans in the policy-making process. As more policies aimed at improving veterans’ access to civilian care are created, such as the most recent Mission Act, policy makers should strongly consider using the DD method of obtaining informed veteran input into future policy decisions.
Acknowledgments
Funding was provided by the US Department of Veterans Affairs Office of Analytics and Business Intelligence (OABI) and the VA Quality Enhancement Research Initiative (QUERI). Dr. Caverly was supported in part by a VA Career Development Award (CDA 16-151). Dr. Krein is supported by a VA Health Services Research and Development Research Career Scientist Award (RCS 11-222). The authors thank the veterans who participated in this work. They also thank Caitlin Reardon and Natalya Wawrin for their assistance in organizing the deliberation session.
1. VA Office of the Inspector General. Veterans Health Administration. Interim report: review of patient wait times, scheduling practices, and alleged patient deaths at the Phoenix Health Care System. https://www.va.gov/oig/pubs/VAOIG-14-02603-178.pdf. Published May 28, 2014. Accessed December 9, 2019.
2. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
3. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
4. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare Part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019; July 22. [Epub ahead of print.]
5. Moyo P, Zhao X, Thorpe CT, et al. Dual receipt of prescription opioids from the Department of Veterans Affairs and Medicare Part D and prescription opioid overdose death among veterans: a nested case-control study. Ann Intern Med. 2019;170(7):433-442.
6. Meyer LJ, Clancy CM. Care fragmentation and prescription opioids. Ann Intern Med. 2019;170(7):497-498.
7. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64(1):223-235.
8. Street J, Duszynski K, Krawczyk S, Braunack-Mayer A. The use of citizens’ juries in health policy decision-making: a systematic review. Soc Sci Med. 2014;109:1-9.
9. Paul C, Nicholls R, Priest P, McGee R. Making policy decisions about population screening for breast cancer: the role of citizens’ deliberation. Health Policy. 2008;85(3):314-320.
10. Martin D, Abelson J, Singer P. Participation in health care priority-setting through the eyes of the participants. J Health Serv Res Pol. 2002;7(4):222-229.
11. Mort M, Finch T. Principles for telemedicine and telecare: the perspective of a citizens’ panel. J Telemed Telecare. 2005;11(suppl 1):66-68.
12. Kass N, Faden R, Fabi RE, et al. Alternative consent models for comparative effectiveness studies: views of patients from two institutions. AJOB Empir Bioeth. 2016;7(2):92-105.
13. Carman KL, Mallery C, Maurer M, et al. Effectiveness of public deliberation methods for gathering input on issues in healthcare: results from a randomized trial. Soc Sci Med. 2015;133:11-20.
14. Carman KL, Maurer M, Mangrum R, et al. Understanding an informed public’s views on the role of evidence in making health care decisions. Health Aff (Millwood). 2016;35(4):566-574.
15. Kim SYH, Wall IF, Stanczyk A, De Vries R. Assessing the public’s views in research ethics controversies: deliberative democracy and bioethics as natural allies, J Empir Res Hum Res Ethics. 2009;4(4):3-16.
16. Gastil J, Levine P, eds. The Deliberative Democracy Handbook: Strategies for Effective Civic Engagement in the Twenty-First Century. San Francisco, CA: Jossey-Bass; 2005.
17. Dryzek JS, Bächtiger A, Chambers S, et al. The crisis of democracy and the science of deliberation. Science. 2019;363(6432):1144-1146.
18. Blacksher E, Diebel A, Forest PG, Goold SD, Abelson J. What is public deliberation? Hastings Cent Rep. 2012;4(2):14-17.
19. Wang G, Gold M, Siegel J, et al. Deliberation: obtaining informed input from a diverse public. J Health Care Poor Underserved. 2015;26(1):223-242.
20. Simon RL, ed. The Blackwell Guide to Social and Political Philosophy. Malden, MA: Wiley-Blackwell; 2002.
21. Stanford University, Center for Deliberative Democracy. Deliberative polling on energy and environmental policy options in Japan. https://cdd.stanford.edu/2012/deliberative-polling-on-energy-and-environmental-policy-options-in-japan. Published August 12, 2012. Accessed December 9, 2019.
22. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64(1):223-235.
23. Carman KL, Maurer M, Mallery C, et al. Community forum deliberative methods demonstration: evaluating effectiveness and eliciting public views on use of evidence. Final report. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/deliberative-methods_research-2013-1.pdf. Published November 2014. Accessed December 9, 2019.
24. Sunstein CR, Hastie R. Wiser: Getting Beyond Groupthink to Make Groups Smarter. Boston, MA: Harvard Business Review Press; 2014.
25. Damschroder LJ, Kim SY. Assessing the quality of democratic deliberation: a case study of public deliberation on the ethics of surrogate consent for research. Soc Sci Med. 2010;70(12):1896-1903.
26. Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. 2nd ed. Thousand Oaks: SAGE Publications, Inc; 1994.
27. US Department of Veterans Affairs. Veteran community care – general information. https://www.va.gov/COMMUNITYCARE/docs/pubfiles/factsheets/VHA-FS_MISSION-Act.pdf. Published September 9 2019. Accessed December 9, 2019.
Providing high-quality, patient-centered health care is a top priority for the US Department of Veterans Affairs (VA) Veteran Health Administration (VHA), whose core mission is to improve the health and well-being of US veterans. Thus, news of long wait times for medical appointments in the VHA sparked intense national attention and debate and led to changes in senior management and legislative action. 1 On August 8, 2014, President Bara c k Obama signed the Veterans Access, Choice, and Accountability Act of 2014, also known as the Choice Act, which provided an additional $16 billion in emergency spending over 3 years to improve veterans’ access to timely health care. 2 The Choice Act sought to develop an integrated health care network that allowed qualified VHA patients to receive specific health care services in their communities delivered by non-VHA health care providers (HCPs) but paid for by the VHA. The Choice Act also laid out explicit criteria for how to prioritize who would be eligible for VHA-purchased civilian care: (1) veterans who could not get timely appointments at a VHA medical facility within 30 days of referral; or (2) veterans who lived > 40 miles from the closest VHA medical facility.
VHA decision makers seeking to improve care delivery also need to weigh trade-offs between alternative approaches to providing rapid access. For instance, increasing access to non-VHA HCPs may not always decrease wait times and could result in loss of continuity, limited care coordination, limited ability to ensure and enforce high-quality standards at the VHA, and other challenges.3-6 Although the concerns and views of elected representatives, advocacy groups, and health system leaders are important, it is unknown whether these views and preferences align with those of veterans. Arguably, the range of views and concerns of informed veterans whose health is at stake should be particularly prominent in such policy decision making.
To identify the considerations that were most important to veterans regarding VHA policy around decreasing wait times, a study was designed to engage a group of veterans who were eligible for civilian care under the Choice Act. The study took place 1 year after the Choice Act was passed. Veterans were asked to focus on 2 related questions: First, how should funding be used for building VHA capacity (build) vs purchasing civilian care (buy)? Second, under what circumstances should civilian care be prioritized?
The aim of this paper is to describe democratic deliberation (DD), a specific method that engaged veteran patients in complex policy decisions around access to care. DD methods have been used increasingly in health care for developing policy guidance, setting priorities, providing advice on ethical dilemmas, weighing risk-benefit trade-offs, and determining decision-making authority.7-12 For example, DD helped guide national policy for mammography screening for breast cancer in New Zealand.13 The Agency for Healthcare Research and Quality has completed a systematic review and a large, randomized experiment on best practices for carrying out public deliberation.8,13,14 However, despite the potential value of this approach, there has been little use of deliberative methods within the VHA for the explicit purpose of informing veteran health care delivery.
This paper describes the experience engaging veterans by using DD methodology and informing VHA leadership about the results of those deliberations. The specific aims were to understand whether DD is an acceptable approach to use to engage patients in the medical services policy-making process within VHA and whether veterans are able to come to an informed consensus.
Methods
Engaging patients and incorporating their needs and concerns within the policy-making process may improve health system policies and make those policies more patient centered. Such engagement also could be a way to generate creative solutions. However, because health-system decisions often involve making difficult trade-offs, effectively obtaining patient population input on complex care delivery issues can be challenging.
Although surveys can provide intuitive, top-of-mind input from respondents, these opinions are generally not sufficient for resolving complex problems.15 Focus groups and interviews may produce results that are more in-depth than surveys, but these methods tend to elicit settled private preferences rather than opinions about what the community should do.16 DD, on the other hand, is designed to elicit deeply informed public opinions on complex, value-laden topics to develop recommendations and policies for a larger community.17 The goal is to find collective solutions to challenging social problems. DD achieves this by giving participants an opportunity to explore a topic in-depth, question experts, and engage peers in reason-based discussions.18,19 This method has its roots in political science and has been used over several decades to successfully inform policy making on a broad array of topics nationally and internationally—from health research ethics in the US to nuclear and energy policy in Japan.7,16,20,21 DD has been found to promote ownership of public programs and lend legitimacy to policy decisions, political institutions, and democracy itself.18
A single day (8 hours) DD session was convened, following a Citizens Jury model of deliberation, which brings veteran patients together to learn about a topic, ask questions of experts, deliberate with peers, and generate a “citizen’s report” that contains a set of recommendations (Table 1). An overview of the different models of DD and rationale for each can be found elsewhere.8,15
Recruitment Considerations
A purposively selected sample of civilian care-eligible veterans from a midwestern VHA health care system (1 medical center and 3 community-based outpatient clinics [CBOCs]) were invited to the DD session. The targeted number of participants was 30. Female veterans, who comprise only 7% of the local veteran population, were oversampled to account for their potentially different health care needs and to create balance between males and females in the session. Oversampling for other characteristics was not possible due to the relatively small sample size. Based on prior experience,7 it was assumed that 70% of willing participants would attend the session; therefore 34 veterans were invited and 24 attended. Each participant received a $200 incentive in appreciation for their substantial time commitment and to offset transportation costs.
Background Materials
A packet with educational materials (Flesch-Kincaid Grade Level of 10.5) was mailed to participants about 2 weeks before the DD session. Participants were asked to review prior to attending the session. These materials described the session (eg, purpose, organizers, importance) and provided factual information about the Choice Act (eg, eligibility, out-of-pocket costs, travel pay, prescription drug policies).
Session Overview
The session was structured to accomplish the following goals: (1) Elicit participants’ opinions about access to health care and reasons for those opinions; (2) Provide in-depth education about the Choice Act through presentations and discussions with topical experts; and (3) Elicit reasoning and recommendations on both the criteria by which participants prioritize candidates for civilian care and how participants would allocate additional funding to improve access (ie, by building VHA capacity to deliver more timely health care vs purchasing health care from civilian HCPs).
Participants were asked to fill out a survey on arrival in the morning and were assigned to 1 of 3 tables or small groups. Each table had a facilitator who had extensive experience in qualitative data collection methods and guided the dialogue using a scripted protocol that they helped develop and refine. The facilitation materials drew from and used previously published studies.22,23 Each facilitator audio recorded the sessions and took notes. Three experts presented during plenary education sessions. Presentations were designed to provide balanced factual information and included a veteran’s perspective. One presenter was a clinician on the project team, another was a local clinical leader responsible for making decisions about what services to provide via civilian care (buy) vs enhancing the local VHA health system’s ability to provide those services (build), and the third presenter was a veteran who was on the project team.
Education Session 1
The first plenary education session with expert presentations was conducted after each table completed an icebreaker exercise. The project team physician provided a brief history and description of the Choice Act to reinforce educational materials sent to participants prior to the session. The health system clinical leader described his decision process and principles and highlighted constraints placed on him by the Choice Act that were in place at the time of the DD session. He also described existing local and national programs to provide civilian care (eg, local fee-basis non-VHA care programs) and how these programs sought to achieve goals similar to the Choice Act. The veteran presenter focused on the importance of session participants providing candid insight and observations and emphasized that this session was a significant opportunity to “have their voices heard.”
Deliberation 1: What criteria should be used to prioritize patients for receiving civilian care paid for by the VHA? To elicit preferences on the central question of this deliberation, participants were presented with 8 real-world cases that were based on interviews conducted with Choice Act-eligible veterans (Table 2 and eAppendices A
Education Session 2
In the second plenary session, the project team physician provided information about health care access issues, both inside and outside of the VHA, particularly between urban and rural areas. He also discussed factors related to the insufficient capacity to meet growing demand that contributed to the VHA wait-time crisis. The veteran presenter shared reflections on health care access from a veteran’s perspective.
Deliberation 2: How should additional funding be divided between increasing the ability of the VHA to (1) provide care by VHA HCPs; and (2) pay for care from non-VHA civilian HCPs? Participants were presented the patient examples and Choice Act funding scenarios (the buy policy option) and contrasted that with a build policy option. Participants were explicitly encouraged to shift their perspectives from thinking about individual cases to considering policy-level decisions and the broader social good (Table 2).
Ensuring Robust Deliberations
If participants do not adequately grasp the complexities of the topic, a deliberation can fail. To facilitate nuanced reasoning, real-world concrete examples were developed as the starting point of each deliberation based on interviews with actual patients (deliberation 1) and actual policy proposals relevant to the funding allocation decisions within the Choice Act (deliberation 2).
A deliberation also can fail with self-silencing, where participants withhold opinions that differ from those articulated first or by more vocal members of the group.24 To combat self-silencing, highly experienced facilitators were used to ensure sharing from all participants and to support an open-minded, courteous, and reason-based environment for discourse. It was specified that the best solutions are achieved through reason-based and cordial disagreement and that success can be undermined when participants simply agree because it is easier or more comfortable.
A third way a deliberation can fail is if individuals do not adopt a group or system-level perspective. To counter this, facilitators reinforced at multiple points the importance of taking a broader social perspective rather than sharing only one’s personal preferences.
Finally, it is important to assess the quality of the deliberative process itself, to ensure that results are trustworthy.25 To assess the quality of the deliberative process, participants knowledge about key issues pre- and postdeliberation were assessed. Participants also were asked to rate the quality of the facilitators and how well they felt their voice was heard and respected, and facilitators made qualitative assessments about the extent to which participants were engaged in reason-based and collaborative discussion.
Data
Quantitative data were collected via pre- and postsession surveys. The surveys contained items related to knowledge about the Choice Act, expectations for the DD session, beliefs and opinions about the provision of health care for veterans, recommended funding allocations between build vs buy policy options, and general demographics. Qualitative data were collected through detailed notes taken by the 3 facilitators. Each table’s deliberations were audio recorded so that gaps in the notes could be filled.
The 3 facilitators, who were all experienced qualitative researchers, typed their written notes into a template immediately after the session. Two of the 3 facilitators led the analysis of the session notes. Findings within and across the 3 deliberation tables were developed using content and matrix analysis methods.26 Descriptive statistics were generated from survey responses and compared survey items pre- and postsession using paired t tests or χ2 tests for categorical responses.
Results
Thirty-three percent of individuals invited (n = 127) agreed to participate. Those who declined cited conflicts related to distance, transportation, work/school, medical appointments, family commitments, or were not interested. In all, 24 (69%) of the 35 veterans who accepted the invitation attended the deliberation session. Of the 11 who accepted but did not attend, 5 cancelled ahead of time because of conflicts (Figure). Most participants were male (70%), 48% were aged 61 to 75 years, 65% were white, 43% had some college education, 43% reported an annual income of between $25,000 and $40,000, and only 35% reported very good health (eAppendix D).
Deliberation 1
During the deliberation on the prioritization criteria, the concept of “condition severity” emerged as an important criterion for veterans. This criterion captured simultaneous consideration of both clinical necessity and burden on the veteran to obtain care. For example, participants felt that patients with a life-threatening illness should be prioritized for civilian care over patients who need preventative or primary care (clinical necessity) and that elderly patients with substantial difficulty traveling to VHA appointments should be prioritized over patients who can travel more easily (burden). The Choice Act regulations at the time of the DD session did not reflect this nuanced perspective, stipulating only that veterans must live > 40 miles from the nearest VHA medical facility.
One of the 3 groups did not prioritize the patient cases because some members felt that no veteran should be constrained from receiving civilian care if they desired it. Nonetheless, this group did agree with prioritizing the first 2 cases in Table 3. The other groups prioritized all 8 cases in generally similar ways.
Deliberation 2
No clear consensus emerged on the buy vs build question. A representative from each table presented their group’s positions, rationale, and recommendations after deliberations were completed. After hearing the range of positions, the groups then had another opportunity to deliberate based on what they heard from the other tables; no new recommendations or consensus emerged.
Participants who were in favor of allocating more funds toward the build policy offered a range of rationales, saying that it would (1) increase access for rural veterans by building CBOCs and deploying more mobile units that could bring outlets for health care closer to their home communities; (2) provide critical and unique medical expertise to address veteran-specific issues such as prosthetics, traumatic brain injury, posttraumatic stress disorder, spinal cord injury, and shrapnel wounds that are typically not available through civilian providers; (3) give VHA more oversight over the quality and cost of care, which is more challenging to do with civilian providers; and (4) Improve VHA infrastructure by, for example, upgrading technology and attracting the best clinicians and staff to support “our VHA.”
Participants who were in favor of allocating more funds toward the buy policy also offered a range of rationales, saying that it would (1) decrease patient burden by increasing access through community providers, decreasing wait time, and lessening personal cost and travel time; (2) allow more patients to receive civilian care, which was generally seen as beneficial by a few participants because of perceptions that the VHA provides lower quality care due to a shortage of VHA providers, run-down/older facilities, lack of technology, and poorer-quality VHA providers; and (3) provide an opportunity to divest of costly facilities and invest in other innovative approaches. Regarding this last reason, a few participants felt that the VHA is “gouged” when building medical centers that overrun budgets. They also were concerned that investing in facilities tied VHA to specific locations when current locations of veterans may change “25 years from now.”
Survey Results
Twenty-three of the 24 participants completed both pre- and postsession surveys. The majority of participants in the session felt people in the group respected their opinion (96%); felt that the facilitator did not try to influence the group with her own opinions (96%); indicated they understood the information enough to participate as much as they wanted (100%); and were hopeful that their reasoning and recommendations would help inform VHA policy makers (82%).
The surveys also provided an opportunity to examine the extent to which knowledge, attitudes, and opinions changed from before to after the deliberation. Even with the small sample, responses revealed a trend toward improved knowledge about key elements of the Choice Act and its goals. Further, there was a shift in some participants’ opinions about how patients should be prioritized to receive civilian care. For example, before the deliberation participants generally felt that all veterans should be able to receive civilian care, whereas postdeliberation this was not the case. Postdeliberation, most participants felt that primary care should not be a high priority for civilian care but continued to endorse prioritizing civilian care for specialty services like orthopedic or cardiology-related care. Finally, participants moved from more diverse recommendations regarding additional funds allocations, toward consensus after the deliberation around allocating funds to the build policy. Eight participants supported a build policy beforehand, whereas 16 supported this policy afterward.
Discussion
This study explored DD as a method for deeply engaging veterans in complex policy making to guide funding allocation and prioritization decisions related to the Choice Act, decisions that are still very relevant today within the context of the Mission Act and have substantial implications for how health care is delivered in the VHA. The Mission Act passed on June 6, 2018, with the goal of improving access to and the reliability of civilian or community care for eligible veterans.27 Decisions related to appropriating scarce funding to improve access to care is an emotional and value-laden topic that elicited strong and divergent opinions among the participants. Veterans were eager to have their voices heard and had strong expectations that VHA leadership would be briefed about their recommendations. The majority of participants were satisfied with the deliberation process, felt they understood the issues, and felt their opinions were respected. They expressed feelings of comradery and community throughout the process.
In this single deliberation session, the groups did not achieve a single, final consensus regarding how VHA funding should ultimately be allocated between buy and build policy options. Nonetheless, participants provided a rich array of recommendations and rationale for them. Session moderators observed rich, sophisticated, fair, and reason-based discussions on this complex topic. Participants left with a deeper knowledge and appreciation for the complex trade-offs and expressed strong rationales for both sides of the policy debate on build vs buy. In addition, the project yielded results of high interest to VHA policy makers.
This work was presented in multiple venues between 2015 to 2016, and to both local and national VHA leadership, including the local Executive Quality Leadership Boards, the VHA Central Office Committee on the Future State of VA Community Care, the VA Office of Patient Centered Care, and the National Veteran Experience Committee. Through these discussions and others, we saw great interest within the VHA system and high-level leaders to explore ways to include veterans’ voices in the policy-making process. This work was invaluable to our research team (eAppendix E
Many health system decisions regarding what care should be delivered (and how) involve making difficult, value-laden choices in the context of limited resources. DD methods can be used to target and obtain specific viewpoints from diverse populations, such as the informed perspectives of minority and underrepresented populations within the VHA.19 For example, female veterans were oversampled to ensure that the informed preferences of this population was obtained. Thus, DD methods could provide a valuable tool for health systems to elicit in-depth diverse patient input on high-profile policies that will have a substantial impact on the system’s patient population.
Limitations
One potential downside of DD is that, because of the resource-intensive nature of deliberation sessions, they are often conducted with relatively small groups.9 Viewpoints of those within these small samples who are willing to spend an entire day discussing a complex topic may not be representative of the larger patient community. However, the core goal of DD is diversity of opinions rather than representativeness.
A stratified random sampling strategy that oversampled for underrepresented and minority populations was used to help select a diverse group that represents the population on key characteristics and partially addresses concern about representativeness. Efforts to optimize participation rates, including providing monetary incentives, also are helpful and have led to high participation rates in past deliberations.7
Health system communication strategies that promote the importance of becoming involved in DD sessions also may be helpful in improving rates of recruitment. On particularly important topics where health system leaders feel a larger resource investment is justified, conducting larger scale deliberations with many small groups may obtain more generalizable evidence about what individual patients and groups of patients recommend.7 However, due to the inherent limitations of surveys and focus group approaches for obtaining informed views on complex topics, there are no clear systematic alternatives to the DD approach.
Conclusion
DD is an effective method to meaningfully engage patients in deep deliberations to guide complex policy making. Although design of deliberative sessions is resource-intensive, patient engagement efforts, such as those described in this paper, could be an important aspect of a well-functioning learning health system. Further research into alternative, streamlined methods that can also engage veterans more deeply is needed. DD also can be combined with other approaches to broaden and confirm findings, including focus groups, town hall meetings, or surveys.
Although this study did not provide consensus on how the VHA should allocate funds with respect to the Choice Act, it did provide insight into the importance and feasibility of engaging veterans in the policy-making process. As more policies aimed at improving veterans’ access to civilian care are created, such as the most recent Mission Act, policy makers should strongly consider using the DD method of obtaining informed veteran input into future policy decisions.
Acknowledgments
Funding was provided by the US Department of Veterans Affairs Office of Analytics and Business Intelligence (OABI) and the VA Quality Enhancement Research Initiative (QUERI). Dr. Caverly was supported in part by a VA Career Development Award (CDA 16-151). Dr. Krein is supported by a VA Health Services Research and Development Research Career Scientist Award (RCS 11-222). The authors thank the veterans who participated in this work. They also thank Caitlin Reardon and Natalya Wawrin for their assistance in organizing the deliberation session.
Providing high-quality, patient-centered health care is a top priority for the US Department of Veterans Affairs (VA) Veteran Health Administration (VHA), whose core mission is to improve the health and well-being of US veterans. Thus, news of long wait times for medical appointments in the VHA sparked intense national attention and debate and led to changes in senior management and legislative action. 1 On August 8, 2014, President Bara c k Obama signed the Veterans Access, Choice, and Accountability Act of 2014, also known as the Choice Act, which provided an additional $16 billion in emergency spending over 3 years to improve veterans’ access to timely health care. 2 The Choice Act sought to develop an integrated health care network that allowed qualified VHA patients to receive specific health care services in their communities delivered by non-VHA health care providers (HCPs) but paid for by the VHA. The Choice Act also laid out explicit criteria for how to prioritize who would be eligible for VHA-purchased civilian care: (1) veterans who could not get timely appointments at a VHA medical facility within 30 days of referral; or (2) veterans who lived > 40 miles from the closest VHA medical facility.
VHA decision makers seeking to improve care delivery also need to weigh trade-offs between alternative approaches to providing rapid access. For instance, increasing access to non-VHA HCPs may not always decrease wait times and could result in loss of continuity, limited care coordination, limited ability to ensure and enforce high-quality standards at the VHA, and other challenges.3-6 Although the concerns and views of elected representatives, advocacy groups, and health system leaders are important, it is unknown whether these views and preferences align with those of veterans. Arguably, the range of views and concerns of informed veterans whose health is at stake should be particularly prominent in such policy decision making.
To identify the considerations that were most important to veterans regarding VHA policy around decreasing wait times, a study was designed to engage a group of veterans who were eligible for civilian care under the Choice Act. The study took place 1 year after the Choice Act was passed. Veterans were asked to focus on 2 related questions: First, how should funding be used for building VHA capacity (build) vs purchasing civilian care (buy)? Second, under what circumstances should civilian care be prioritized?
The aim of this paper is to describe democratic deliberation (DD), a specific method that engaged veteran patients in complex policy decisions around access to care. DD methods have been used increasingly in health care for developing policy guidance, setting priorities, providing advice on ethical dilemmas, weighing risk-benefit trade-offs, and determining decision-making authority.7-12 For example, DD helped guide national policy for mammography screening for breast cancer in New Zealand.13 The Agency for Healthcare Research and Quality has completed a systematic review and a large, randomized experiment on best practices for carrying out public deliberation.8,13,14 However, despite the potential value of this approach, there has been little use of deliberative methods within the VHA for the explicit purpose of informing veteran health care delivery.
This paper describes the experience engaging veterans by using DD methodology and informing VHA leadership about the results of those deliberations. The specific aims were to understand whether DD is an acceptable approach to use to engage patients in the medical services policy-making process within VHA and whether veterans are able to come to an informed consensus.
Methods
Engaging patients and incorporating their needs and concerns within the policy-making process may improve health system policies and make those policies more patient centered. Such engagement also could be a way to generate creative solutions. However, because health-system decisions often involve making difficult trade-offs, effectively obtaining patient population input on complex care delivery issues can be challenging.
Although surveys can provide intuitive, top-of-mind input from respondents, these opinions are generally not sufficient for resolving complex problems.15 Focus groups and interviews may produce results that are more in-depth than surveys, but these methods tend to elicit settled private preferences rather than opinions about what the community should do.16 DD, on the other hand, is designed to elicit deeply informed public opinions on complex, value-laden topics to develop recommendations and policies for a larger community.17 The goal is to find collective solutions to challenging social problems. DD achieves this by giving participants an opportunity to explore a topic in-depth, question experts, and engage peers in reason-based discussions.18,19 This method has its roots in political science and has been used over several decades to successfully inform policy making on a broad array of topics nationally and internationally—from health research ethics in the US to nuclear and energy policy in Japan.7,16,20,21 DD has been found to promote ownership of public programs and lend legitimacy to policy decisions, political institutions, and democracy itself.18
A single day (8 hours) DD session was convened, following a Citizens Jury model of deliberation, which brings veteran patients together to learn about a topic, ask questions of experts, deliberate with peers, and generate a “citizen’s report” that contains a set of recommendations (Table 1). An overview of the different models of DD and rationale for each can be found elsewhere.8,15
Recruitment Considerations
A purposively selected sample of civilian care-eligible veterans from a midwestern VHA health care system (1 medical center and 3 community-based outpatient clinics [CBOCs]) were invited to the DD session. The targeted number of participants was 30. Female veterans, who comprise only 7% of the local veteran population, were oversampled to account for their potentially different health care needs and to create balance between males and females in the session. Oversampling for other characteristics was not possible due to the relatively small sample size. Based on prior experience,7 it was assumed that 70% of willing participants would attend the session; therefore 34 veterans were invited and 24 attended. Each participant received a $200 incentive in appreciation for their substantial time commitment and to offset transportation costs.
Background Materials
A packet with educational materials (Flesch-Kincaid Grade Level of 10.5) was mailed to participants about 2 weeks before the DD session. Participants were asked to review prior to attending the session. These materials described the session (eg, purpose, organizers, importance) and provided factual information about the Choice Act (eg, eligibility, out-of-pocket costs, travel pay, prescription drug policies).
Session Overview
The session was structured to accomplish the following goals: (1) Elicit participants’ opinions about access to health care and reasons for those opinions; (2) Provide in-depth education about the Choice Act through presentations and discussions with topical experts; and (3) Elicit reasoning and recommendations on both the criteria by which participants prioritize candidates for civilian care and how participants would allocate additional funding to improve access (ie, by building VHA capacity to deliver more timely health care vs purchasing health care from civilian HCPs).
Participants were asked to fill out a survey on arrival in the morning and were assigned to 1 of 3 tables or small groups. Each table had a facilitator who had extensive experience in qualitative data collection methods and guided the dialogue using a scripted protocol that they helped develop and refine. The facilitation materials drew from and used previously published studies.22,23 Each facilitator audio recorded the sessions and took notes. Three experts presented during plenary education sessions. Presentations were designed to provide balanced factual information and included a veteran’s perspective. One presenter was a clinician on the project team, another was a local clinical leader responsible for making decisions about what services to provide via civilian care (buy) vs enhancing the local VHA health system’s ability to provide those services (build), and the third presenter was a veteran who was on the project team.
Education Session 1
The first plenary education session with expert presentations was conducted after each table completed an icebreaker exercise. The project team physician provided a brief history and description of the Choice Act to reinforce educational materials sent to participants prior to the session. The health system clinical leader described his decision process and principles and highlighted constraints placed on him by the Choice Act that were in place at the time of the DD session. He also described existing local and national programs to provide civilian care (eg, local fee-basis non-VHA care programs) and how these programs sought to achieve goals similar to the Choice Act. The veteran presenter focused on the importance of session participants providing candid insight and observations and emphasized that this session was a significant opportunity to “have their voices heard.”
Deliberation 1: What criteria should be used to prioritize patients for receiving civilian care paid for by the VHA? To elicit preferences on the central question of this deliberation, participants were presented with 8 real-world cases that were based on interviews conducted with Choice Act-eligible veterans (Table 2 and eAppendices A
Education Session 2
In the second plenary session, the project team physician provided information about health care access issues, both inside and outside of the VHA, particularly between urban and rural areas. He also discussed factors related to the insufficient capacity to meet growing demand that contributed to the VHA wait-time crisis. The veteran presenter shared reflections on health care access from a veteran’s perspective.
Deliberation 2: How should additional funding be divided between increasing the ability of the VHA to (1) provide care by VHA HCPs; and (2) pay for care from non-VHA civilian HCPs? Participants were presented the patient examples and Choice Act funding scenarios (the buy policy option) and contrasted that with a build policy option. Participants were explicitly encouraged to shift their perspectives from thinking about individual cases to considering policy-level decisions and the broader social good (Table 2).
Ensuring Robust Deliberations
If participants do not adequately grasp the complexities of the topic, a deliberation can fail. To facilitate nuanced reasoning, real-world concrete examples were developed as the starting point of each deliberation based on interviews with actual patients (deliberation 1) and actual policy proposals relevant to the funding allocation decisions within the Choice Act (deliberation 2).
A deliberation also can fail with self-silencing, where participants withhold opinions that differ from those articulated first or by more vocal members of the group.24 To combat self-silencing, highly experienced facilitators were used to ensure sharing from all participants and to support an open-minded, courteous, and reason-based environment for discourse. It was specified that the best solutions are achieved through reason-based and cordial disagreement and that success can be undermined when participants simply agree because it is easier or more comfortable.
A third way a deliberation can fail is if individuals do not adopt a group or system-level perspective. To counter this, facilitators reinforced at multiple points the importance of taking a broader social perspective rather than sharing only one’s personal preferences.
Finally, it is important to assess the quality of the deliberative process itself, to ensure that results are trustworthy.25 To assess the quality of the deliberative process, participants knowledge about key issues pre- and postdeliberation were assessed. Participants also were asked to rate the quality of the facilitators and how well they felt their voice was heard and respected, and facilitators made qualitative assessments about the extent to which participants were engaged in reason-based and collaborative discussion.
Data
Quantitative data were collected via pre- and postsession surveys. The surveys contained items related to knowledge about the Choice Act, expectations for the DD session, beliefs and opinions about the provision of health care for veterans, recommended funding allocations between build vs buy policy options, and general demographics. Qualitative data were collected through detailed notes taken by the 3 facilitators. Each table’s deliberations were audio recorded so that gaps in the notes could be filled.
The 3 facilitators, who were all experienced qualitative researchers, typed their written notes into a template immediately after the session. Two of the 3 facilitators led the analysis of the session notes. Findings within and across the 3 deliberation tables were developed using content and matrix analysis methods.26 Descriptive statistics were generated from survey responses and compared survey items pre- and postsession using paired t tests or χ2 tests for categorical responses.
Results
Thirty-three percent of individuals invited (n = 127) agreed to participate. Those who declined cited conflicts related to distance, transportation, work/school, medical appointments, family commitments, or were not interested. In all, 24 (69%) of the 35 veterans who accepted the invitation attended the deliberation session. Of the 11 who accepted but did not attend, 5 cancelled ahead of time because of conflicts (Figure). Most participants were male (70%), 48% were aged 61 to 75 years, 65% were white, 43% had some college education, 43% reported an annual income of between $25,000 and $40,000, and only 35% reported very good health (eAppendix D).
Deliberation 1
During the deliberation on the prioritization criteria, the concept of “condition severity” emerged as an important criterion for veterans. This criterion captured simultaneous consideration of both clinical necessity and burden on the veteran to obtain care. For example, participants felt that patients with a life-threatening illness should be prioritized for civilian care over patients who need preventative or primary care (clinical necessity) and that elderly patients with substantial difficulty traveling to VHA appointments should be prioritized over patients who can travel more easily (burden). The Choice Act regulations at the time of the DD session did not reflect this nuanced perspective, stipulating only that veterans must live > 40 miles from the nearest VHA medical facility.
One of the 3 groups did not prioritize the patient cases because some members felt that no veteran should be constrained from receiving civilian care if they desired it. Nonetheless, this group did agree with prioritizing the first 2 cases in Table 3. The other groups prioritized all 8 cases in generally similar ways.
Deliberation 2
No clear consensus emerged on the buy vs build question. A representative from each table presented their group’s positions, rationale, and recommendations after deliberations were completed. After hearing the range of positions, the groups then had another opportunity to deliberate based on what they heard from the other tables; no new recommendations or consensus emerged.
Participants who were in favor of allocating more funds toward the build policy offered a range of rationales, saying that it would (1) increase access for rural veterans by building CBOCs and deploying more mobile units that could bring outlets for health care closer to their home communities; (2) provide critical and unique medical expertise to address veteran-specific issues such as prosthetics, traumatic brain injury, posttraumatic stress disorder, spinal cord injury, and shrapnel wounds that are typically not available through civilian providers; (3) give VHA more oversight over the quality and cost of care, which is more challenging to do with civilian providers; and (4) Improve VHA infrastructure by, for example, upgrading technology and attracting the best clinicians and staff to support “our VHA.”
Participants who were in favor of allocating more funds toward the buy policy also offered a range of rationales, saying that it would (1) decrease patient burden by increasing access through community providers, decreasing wait time, and lessening personal cost and travel time; (2) allow more patients to receive civilian care, which was generally seen as beneficial by a few participants because of perceptions that the VHA provides lower quality care due to a shortage of VHA providers, run-down/older facilities, lack of technology, and poorer-quality VHA providers; and (3) provide an opportunity to divest of costly facilities and invest in other innovative approaches. Regarding this last reason, a few participants felt that the VHA is “gouged” when building medical centers that overrun budgets. They also were concerned that investing in facilities tied VHA to specific locations when current locations of veterans may change “25 years from now.”
Survey Results
Twenty-three of the 24 participants completed both pre- and postsession surveys. The majority of participants in the session felt people in the group respected their opinion (96%); felt that the facilitator did not try to influence the group with her own opinions (96%); indicated they understood the information enough to participate as much as they wanted (100%); and were hopeful that their reasoning and recommendations would help inform VHA policy makers (82%).
The surveys also provided an opportunity to examine the extent to which knowledge, attitudes, and opinions changed from before to after the deliberation. Even with the small sample, responses revealed a trend toward improved knowledge about key elements of the Choice Act and its goals. Further, there was a shift in some participants’ opinions about how patients should be prioritized to receive civilian care. For example, before the deliberation participants generally felt that all veterans should be able to receive civilian care, whereas postdeliberation this was not the case. Postdeliberation, most participants felt that primary care should not be a high priority for civilian care but continued to endorse prioritizing civilian care for specialty services like orthopedic or cardiology-related care. Finally, participants moved from more diverse recommendations regarding additional funds allocations, toward consensus after the deliberation around allocating funds to the build policy. Eight participants supported a build policy beforehand, whereas 16 supported this policy afterward.
Discussion
This study explored DD as a method for deeply engaging veterans in complex policy making to guide funding allocation and prioritization decisions related to the Choice Act, decisions that are still very relevant today within the context of the Mission Act and have substantial implications for how health care is delivered in the VHA. The Mission Act passed on June 6, 2018, with the goal of improving access to and the reliability of civilian or community care for eligible veterans.27 Decisions related to appropriating scarce funding to improve access to care is an emotional and value-laden topic that elicited strong and divergent opinions among the participants. Veterans were eager to have their voices heard and had strong expectations that VHA leadership would be briefed about their recommendations. The majority of participants were satisfied with the deliberation process, felt they understood the issues, and felt their opinions were respected. They expressed feelings of comradery and community throughout the process.
In this single deliberation session, the groups did not achieve a single, final consensus regarding how VHA funding should ultimately be allocated between buy and build policy options. Nonetheless, participants provided a rich array of recommendations and rationale for them. Session moderators observed rich, sophisticated, fair, and reason-based discussions on this complex topic. Participants left with a deeper knowledge and appreciation for the complex trade-offs and expressed strong rationales for both sides of the policy debate on build vs buy. In addition, the project yielded results of high interest to VHA policy makers.
This work was presented in multiple venues between 2015 to 2016, and to both local and national VHA leadership, including the local Executive Quality Leadership Boards, the VHA Central Office Committee on the Future State of VA Community Care, the VA Office of Patient Centered Care, and the National Veteran Experience Committee. Through these discussions and others, we saw great interest within the VHA system and high-level leaders to explore ways to include veterans’ voices in the policy-making process. This work was invaluable to our research team (eAppendix E
Many health system decisions regarding what care should be delivered (and how) involve making difficult, value-laden choices in the context of limited resources. DD methods can be used to target and obtain specific viewpoints from diverse populations, such as the informed perspectives of minority and underrepresented populations within the VHA.19 For example, female veterans were oversampled to ensure that the informed preferences of this population was obtained. Thus, DD methods could provide a valuable tool for health systems to elicit in-depth diverse patient input on high-profile policies that will have a substantial impact on the system’s patient population.
Limitations
One potential downside of DD is that, because of the resource-intensive nature of deliberation sessions, they are often conducted with relatively small groups.9 Viewpoints of those within these small samples who are willing to spend an entire day discussing a complex topic may not be representative of the larger patient community. However, the core goal of DD is diversity of opinions rather than representativeness.
A stratified random sampling strategy that oversampled for underrepresented and minority populations was used to help select a diverse group that represents the population on key characteristics and partially addresses concern about representativeness. Efforts to optimize participation rates, including providing monetary incentives, also are helpful and have led to high participation rates in past deliberations.7
Health system communication strategies that promote the importance of becoming involved in DD sessions also may be helpful in improving rates of recruitment. On particularly important topics where health system leaders feel a larger resource investment is justified, conducting larger scale deliberations with many small groups may obtain more generalizable evidence about what individual patients and groups of patients recommend.7 However, due to the inherent limitations of surveys and focus group approaches for obtaining informed views on complex topics, there are no clear systematic alternatives to the DD approach.
Conclusion
DD is an effective method to meaningfully engage patients in deep deliberations to guide complex policy making. Although design of deliberative sessions is resource-intensive, patient engagement efforts, such as those described in this paper, could be an important aspect of a well-functioning learning health system. Further research into alternative, streamlined methods that can also engage veterans more deeply is needed. DD also can be combined with other approaches to broaden and confirm findings, including focus groups, town hall meetings, or surveys.
Although this study did not provide consensus on how the VHA should allocate funds with respect to the Choice Act, it did provide insight into the importance and feasibility of engaging veterans in the policy-making process. As more policies aimed at improving veterans’ access to civilian care are created, such as the most recent Mission Act, policy makers should strongly consider using the DD method of obtaining informed veteran input into future policy decisions.
Acknowledgments
Funding was provided by the US Department of Veterans Affairs Office of Analytics and Business Intelligence (OABI) and the VA Quality Enhancement Research Initiative (QUERI). Dr. Caverly was supported in part by a VA Career Development Award (CDA 16-151). Dr. Krein is supported by a VA Health Services Research and Development Research Career Scientist Award (RCS 11-222). The authors thank the veterans who participated in this work. They also thank Caitlin Reardon and Natalya Wawrin for their assistance in organizing the deliberation session.
1. VA Office of the Inspector General. Veterans Health Administration. Interim report: review of patient wait times, scheduling practices, and alleged patient deaths at the Phoenix Health Care System. https://www.va.gov/oig/pubs/VAOIG-14-02603-178.pdf. Published May 28, 2014. Accessed December 9, 2019.
2. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
3. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
4. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare Part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019; July 22. [Epub ahead of print.]
5. Moyo P, Zhao X, Thorpe CT, et al. Dual receipt of prescription opioids from the Department of Veterans Affairs and Medicare Part D and prescription opioid overdose death among veterans: a nested case-control study. Ann Intern Med. 2019;170(7):433-442.
6. Meyer LJ, Clancy CM. Care fragmentation and prescription opioids. Ann Intern Med. 2019;170(7):497-498.
7. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64(1):223-235.
8. Street J, Duszynski K, Krawczyk S, Braunack-Mayer A. The use of citizens’ juries in health policy decision-making: a systematic review. Soc Sci Med. 2014;109:1-9.
9. Paul C, Nicholls R, Priest P, McGee R. Making policy decisions about population screening for breast cancer: the role of citizens’ deliberation. Health Policy. 2008;85(3):314-320.
10. Martin D, Abelson J, Singer P. Participation in health care priority-setting through the eyes of the participants. J Health Serv Res Pol. 2002;7(4):222-229.
11. Mort M, Finch T. Principles for telemedicine and telecare: the perspective of a citizens’ panel. J Telemed Telecare. 2005;11(suppl 1):66-68.
12. Kass N, Faden R, Fabi RE, et al. Alternative consent models for comparative effectiveness studies: views of patients from two institutions. AJOB Empir Bioeth. 2016;7(2):92-105.
13. Carman KL, Mallery C, Maurer M, et al. Effectiveness of public deliberation methods for gathering input on issues in healthcare: results from a randomized trial. Soc Sci Med. 2015;133:11-20.
14. Carman KL, Maurer M, Mangrum R, et al. Understanding an informed public’s views on the role of evidence in making health care decisions. Health Aff (Millwood). 2016;35(4):566-574.
15. Kim SYH, Wall IF, Stanczyk A, De Vries R. Assessing the public’s views in research ethics controversies: deliberative democracy and bioethics as natural allies, J Empir Res Hum Res Ethics. 2009;4(4):3-16.
16. Gastil J, Levine P, eds. The Deliberative Democracy Handbook: Strategies for Effective Civic Engagement in the Twenty-First Century. San Francisco, CA: Jossey-Bass; 2005.
17. Dryzek JS, Bächtiger A, Chambers S, et al. The crisis of democracy and the science of deliberation. Science. 2019;363(6432):1144-1146.
18. Blacksher E, Diebel A, Forest PG, Goold SD, Abelson J. What is public deliberation? Hastings Cent Rep. 2012;4(2):14-17.
19. Wang G, Gold M, Siegel J, et al. Deliberation: obtaining informed input from a diverse public. J Health Care Poor Underserved. 2015;26(1):223-242.
20. Simon RL, ed. The Blackwell Guide to Social and Political Philosophy. Malden, MA: Wiley-Blackwell; 2002.
21. Stanford University, Center for Deliberative Democracy. Deliberative polling on energy and environmental policy options in Japan. https://cdd.stanford.edu/2012/deliberative-polling-on-energy-and-environmental-policy-options-in-japan. Published August 12, 2012. Accessed December 9, 2019.
22. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64(1):223-235.
23. Carman KL, Maurer M, Mallery C, et al. Community forum deliberative methods demonstration: evaluating effectiveness and eliciting public views on use of evidence. Final report. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/deliberative-methods_research-2013-1.pdf. Published November 2014. Accessed December 9, 2019.
24. Sunstein CR, Hastie R. Wiser: Getting Beyond Groupthink to Make Groups Smarter. Boston, MA: Harvard Business Review Press; 2014.
25. Damschroder LJ, Kim SY. Assessing the quality of democratic deliberation: a case study of public deliberation on the ethics of surrogate consent for research. Soc Sci Med. 2010;70(12):1896-1903.
26. Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. 2nd ed. Thousand Oaks: SAGE Publications, Inc; 1994.
27. US Department of Veterans Affairs. Veteran community care – general information. https://www.va.gov/COMMUNITYCARE/docs/pubfiles/factsheets/VHA-FS_MISSION-Act.pdf. Published September 9 2019. Accessed December 9, 2019.
1. VA Office of the Inspector General. Veterans Health Administration. Interim report: review of patient wait times, scheduling practices, and alleged patient deaths at the Phoenix Health Care System. https://www.va.gov/oig/pubs/VAOIG-14-02603-178.pdf. Published May 28, 2014. Accessed December 9, 2019.
2. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
3. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
4. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare Part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019; July 22. [Epub ahead of print.]
5. Moyo P, Zhao X, Thorpe CT, et al. Dual receipt of prescription opioids from the Department of Veterans Affairs and Medicare Part D and prescription opioid overdose death among veterans: a nested case-control study. Ann Intern Med. 2019;170(7):433-442.
6. Meyer LJ, Clancy CM. Care fragmentation and prescription opioids. Ann Intern Med. 2019;170(7):497-498.
7. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64(1):223-235.
8. Street J, Duszynski K, Krawczyk S, Braunack-Mayer A. The use of citizens’ juries in health policy decision-making: a systematic review. Soc Sci Med. 2014;109:1-9.
9. Paul C, Nicholls R, Priest P, McGee R. Making policy decisions about population screening for breast cancer: the role of citizens’ deliberation. Health Policy. 2008;85(3):314-320.
10. Martin D, Abelson J, Singer P. Participation in health care priority-setting through the eyes of the participants. J Health Serv Res Pol. 2002;7(4):222-229.
11. Mort M, Finch T. Principles for telemedicine and telecare: the perspective of a citizens’ panel. J Telemed Telecare. 2005;11(suppl 1):66-68.
12. Kass N, Faden R, Fabi RE, et al. Alternative consent models for comparative effectiveness studies: views of patients from two institutions. AJOB Empir Bioeth. 2016;7(2):92-105.
13. Carman KL, Mallery C, Maurer M, et al. Effectiveness of public deliberation methods for gathering input on issues in healthcare: results from a randomized trial. Soc Sci Med. 2015;133:11-20.
14. Carman KL, Maurer M, Mangrum R, et al. Understanding an informed public’s views on the role of evidence in making health care decisions. Health Aff (Millwood). 2016;35(4):566-574.
15. Kim SYH, Wall IF, Stanczyk A, De Vries R. Assessing the public’s views in research ethics controversies: deliberative democracy and bioethics as natural allies, J Empir Res Hum Res Ethics. 2009;4(4):3-16.
16. Gastil J, Levine P, eds. The Deliberative Democracy Handbook: Strategies for Effective Civic Engagement in the Twenty-First Century. San Francisco, CA: Jossey-Bass; 2005.
17. Dryzek JS, Bächtiger A, Chambers S, et al. The crisis of democracy and the science of deliberation. Science. 2019;363(6432):1144-1146.
18. Blacksher E, Diebel A, Forest PG, Goold SD, Abelson J. What is public deliberation? Hastings Cent Rep. 2012;4(2):14-17.
19. Wang G, Gold M, Siegel J, et al. Deliberation: obtaining informed input from a diverse public. J Health Care Poor Underserved. 2015;26(1):223-242.
20. Simon RL, ed. The Blackwell Guide to Social and Political Philosophy. Malden, MA: Wiley-Blackwell; 2002.
21. Stanford University, Center for Deliberative Democracy. Deliberative polling on energy and environmental policy options in Japan. https://cdd.stanford.edu/2012/deliberative-polling-on-energy-and-environmental-policy-options-in-japan. Published August 12, 2012. Accessed December 9, 2019.
22. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64(1):223-235.
23. Carman KL, Maurer M, Mallery C, et al. Community forum deliberative methods demonstration: evaluating effectiveness and eliciting public views on use of evidence. Final report. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/deliberative-methods_research-2013-1.pdf. Published November 2014. Accessed December 9, 2019.
24. Sunstein CR, Hastie R. Wiser: Getting Beyond Groupthink to Make Groups Smarter. Boston, MA: Harvard Business Review Press; 2014.
25. Damschroder LJ, Kim SY. Assessing the quality of democratic deliberation: a case study of public deliberation on the ethics of surrogate consent for research. Soc Sci Med. 2010;70(12):1896-1903.
26. Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. 2nd ed. Thousand Oaks: SAGE Publications, Inc; 1994.
27. US Department of Veterans Affairs. Veteran community care – general information. https://www.va.gov/COMMUNITYCARE/docs/pubfiles/factsheets/VHA-FS_MISSION-Act.pdf. Published September 9 2019. Accessed December 9, 2019.
Food Insecurity Among Veterans: Resources to Screen and Intervene
Nearly 1 in 8 households—and 1 in 6 households with children—experienced food insecurity in 2017, defined as limited or uncertain availability of nutritionally adequate and safe foods.1 Food insecurity is often even more pronounced among households with individuals with acute or chronic medical conditions.2-6 Moreover, food insecurity is independently associated with a range of adverse health outcomes, including poorer control of diabetes mellitus, hypertension, depression and other major psychiatric disorders, HIV, and chronic lung and kidney disease, as well as poorer overall health status.7-14 Food insecurity also has been associated with increased health care costs and acute care utilization as well as increased probability of delayed or missed care.15-19
The relationship between food insecurity and poor health outcomes is a complex and often cyclic phenomenon (Figure 1). Poor nutritional status is fueled by limited access to healthful foods as well as increased reliance on calorie-dense and nutrient-poor “junk” foods, which are less expensive and often more readily available in low-income neighborhoods.5,20-24 These compensatory dietary patterns place individuals at higher risk for developing cardiometabolic conditions and for poor control of these conditions.5,8,9,12,25,26 Additionally, the physiological and psychological stressors of food insecurity may precipitate depression and anxiety or worsen existing mental health conditions, resulting in feelings of overwhelm and decreased self-management capacity.5,8,27-31 Food insecurity has further been associated with poor sleep, declines in cognitive function, and increased falls, particularly among the frail and elderly.32-34
Individuals experiencing food insecurity often report having to make trade-offs between food and other necessities, such as paying rent or utilities. Additional strategies to stretch limited resources include cost-related underuse of medication and delays in needed medical care.4,17,31,35 In a nationally representative survey among adults with at least 1 chronic medical condition, 1 in 3 reported having to choose between food and medicine; 11% were unable to afford either.3 Furthermore, the inability to reliably adhere to medication regimens that need to be taken with food can result in potentially life-threatening hypoglycemia (as can lack of food regardless of medication use).5,26,36 In addition to the more obvious risks of glucose-lowering medications, such as insulin and long-acting sulfonylureas in patients experiencing food insecurity, many drugs commonly used among nondiabetic adults such as ACE-inhibitors, β blockers, quinolones, and salicylates can also precipitate hypoglycemia, and food insecurity has been associated with experiences of hypoglycemia even among individuals without diabetes mellitus.32,37 In one study the risk for hospital admissions for hypoglycemia among low-income populations increased by 27% at the end of the month when food budgets were more likely to be exhausted.38 Worsening health status and increased emergency department visits and hospitalizations may then result in lost wages and mounting medical bills, contributing to further financial strain and worsening food insecurity.
Prevalence and Importance of Food Insecurity Among US Veterans
Nearly 1.5 million veterans in the US are living below the federal poverty level (FPL).39 An additional 2.4 million veterans are living paycheck to paycheck at < 200% of the FPL.40 Veterans living in poverty are at even higher risk than nonveterans for food insecurity, homelessness, and other material hardship.41
Estimates of food insecurity among veterans vary widely, ranging from 6% to 24%—nearly twice that of the general US population.8,42-45 Higher rates of food insecurity have been reported among certain high-risk subgroups, including veterans who served in Iraq and Afghanistan (27%), female veterans (28%), homeless and formerly homeless veterans (49%), and veterans with serious mental illness (35%).6,32,43,46 Additional risk factors for food insecurity specific to veteran populations include younger age, having recently left active-duty military service, and lower final military paygrade.42,45-47 As in the general population, veteran food insecurity is associated with a range of adverse health outcomes, including poorer overall health status as well as increased probability of delayed or missed care.6,8,32,42-44,46
Even among veterans enrolled in federal food assistance programs, many still struggle to afford nutritionally adequate foods. As one example, in a study of mostly male homeless and formerly homeless veterans, O’Toole and colleagues found that nearly half of those reporting food insecurity were already receiving federal food assistance benefits, and 22% relied on emergency food resources.32 Of households served by Feeding America food pantries and meal programs, 20% have a member who has served in the US military.48
Federal Programs To Address Food Insecurity
There are several important federal food assistance programs designed to help alleviate food insecurity. The Supplemental Nutrition Assistance Program (SNAP, formerly the Food Stamp program) is the largest federal food assistance program and provides low-income Americans with cash benefits to purchase food. SNAP has been shown to substantially reduce food insecurity.7,49 The program also is associated with significant decreases in cost-related medication nonadherence as well as reductions in health care costs and both acute care and nursing home utilization.16,50-54 Although nearly 1.4 million veterans live in SNAP-enrolled households, 59% of eligible veterans are not enrolled.43,55 Closing this SNAP eligibility-enrollment gap, has been a focus of recent efforts to improve long-term food security among veterans. There also are several federal food assistance programs for households with children, including the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) and school meals programs. Among federal nutrition programs for seniors, the Older American’s Act contains designated funding to support nutrition services for older adults, including congregate meal programs in community settings like senior centers, places of worship, and housing communities, and home-delivered meals through programs like Meals on Wheels.56
VHA Response to Food Insecurity
The Veterans Health Administration (VHA) is the country’s largest integrated, federally funded health care system.57 In November 2015, congressional briefings on veteran food insecurity organized by the national non-profit organization MAZON: A Jewish Response to Hunger and hosted with bipartisan support were provided to the US House and Senate. As a result of these briefings, VHA chartered the national Ensuring Veteran Food Security Workgroup with a mandate to partner with governmental and nonprofit agencies to “focus on the issue of food insecurity, the identification of veterans at risk, the needed training of VHA staff and the coordination of resources and initiatives to support the veterans for whom we care.” Building off a pilot in US Department of Veterans Affairs (VA) Homeless Patient Aligned Care Teams (H-PACTs),32 VHA subsequently integrated a single-item food insecurity screening tool into the VA electronic health record (EHR) clinical reminder system (Figure 2). The clinical reminder, which was rolled out across VA medical centers nationally in October 2017, provides an alert to screen all noninstitutionalized veterans for food insecurity. To date, nearly 5 million veterans have been screened. When a veteran endorses food insecurity based on the initial screening question, a prompt appears to offer the veteran a referral to a social worker and/or dietitian. Positive screening results also should be communicated to the patient’s primary care provider. Depending on site-specific clinical flow, the reminders are typically completed in the outpatient setting either by nurses or medical assistants during intake or by providers as part of the clinical visit. However, any member of the health care team can complete the clinical reminder at any time. As of September 2019, approximately 74,000 veterans have been identified as food insecure.58
Addressing Food Insecurity
VHA has been a recognized leader in addressing homelessness and other social determinants of health through its integrated care and PACT delivery models.59-61 The food insecurity clinical reminder was designed to facilitate a tailored, interdisciplinary approach to identify and address food insecurity. Interdisciplinary care team members—including medical assistants, clinicians, social workers, registered dietitians, nurse care managers, occupational or physical therapists, and pharmacists—are uniquely positioned to identify veterans impacted by food insecurity, assess for associated clinical and/or social risk factors, and offer appropriate medical and nutrition interventions and resource referrals.
This interdisciplinary team-based model is essential given the range of potential drivers underlying veteran experiences of food insecurity and subsequent health outcomes. It is critically important for clinicians to review the medication list with veterans screening positive for food insecurity to assess for risk of hypoglycemia and/or cost-related nonadherence, make any necessary adjustments to therapeutic regimens, and assess for additional risk factors associated with food insecurity. Examples of tailored nutrition counseling that clinical dietitians may provide include meal preparation strategies for veterans who only have access to a microwave or hotplate, or recommendations for how veterans on medically restricted diets can best navigate food selection at soup kitchens or food pantries. Resource referrals provided by social workers or other care team members may include both emergency food resources to address immediate shortages (eg, food pantries, soup kitchens, or vouchers for free lunch) as well as resources focused on improving longer term food security (eg, federal food assistance programs or home delivered meal programs). Importantly, although providing a list of food resources may be helpful for some patients, such lists are often insufficient.62,63 Many patients require active assistance with program enrollment either onsite the day of their clinic visit or through connection with a partnering community-based organization that can, in turn, identify appropriate resources and facilitate program enrollment.63,64 Planned follow-up is also crucial to determine whether referrals are successful and to assess for ongoing need. Proposed roles for interdisciplinary care team members in addressing a positive food insecurity screen are outlined in Table 1.
VHA-Community Partnerships
In addition to services offered within VA, public and private sector partnerships can greatly enhance the range of resources available to food insecure veterans. Several VA facilities have developed formal community partnerships, such as the Veterans Pantry Pilot (VPP) program, a national partnership between Feeding America food banks and VA medical centers to establish onsite or mobile food pantries. There are currently 17 active Feeding America VPP sites, with a number of additional sites under development. Several of the VPP sites also include other “wraparound services,” such as SNAP application assistance.65,66
State Veterans Affairs offices67 and Veterans Service Organizations (VSOs)68 also can serve as valuable partners for connecting veterans with needed resources. VSOs offer a range of services, including assistancewith benefit claims, employment and housing assistance, emergency food assistance, and transportation to medical appointments. Some VSOs also have established local affiliations with Meals on Wheels focused on veteran outreach and providing hot meals for low-income, homebound, and disabled veterans.
Additional Resources
Although resources vary by regional setting, several key governmental and community-based food assistance programs are summarized in Table 2. Local community partners and online/phone-based directories, such as United Way’s 2-1-1 can help identify additional local resources. For older adults and individuals with disabilities, local Aging and Disability Resources Centers can provide information and assistance connecting to needed resources.69 Finally, there are a number of online resources available for clinicians interested in learning more about the impact of food insecurity on health and tools to use in the clinical setting (Table 3).
Conclusion
The VA has recognized food insecurity as a critical concern for the well-being of our nation’s veterans. Use of the EHR clinical reminder represents a crucial first step toward increasing provider awareness about veteran food insecurity and improving clinical efforts to address food insecurity once identified. Through the reminder, health care teams can connect veterans to needed resources and create both the individual and population-level data necessary to inform VHA and community efforts to address veteran food insecurity. Clinical reminder data are currently being used for local quality improvement efforts and have established the need nationally for formalized partnerships between VHA Social Work Services and Nutrition and Food Services to connect veterans with food and provide them with strategies to best use available food resources.
Moving forward, the Ensuring Veteran Food Security Workgroup continues to work with agencies and organizations across the country to improve food insecure veterans’ access to needed services. In addition to existing VA partnerships with Feeding America for the VPP, memorandums of understanding are currently underway to formalize partnerships with both the Food Research and Action Center (FRAC) and MAZON. Additional research is needed both to formally validate the current food insecurity clinical reminder screening question and to identify best practices and potential models for how to most effectively use VHA-community partnerships to address the unique needs of the veteran population.
Ensuring the food security of our nation’s veterans is essential to VA’s commitment to providing integrated, veteran-centered, whole person care. Toward that goal, VA health care teams are urged to use the clinical reminder and help connect food insecure veterans with relevant resources both within and outside of the VA health care system.
1. Coleman-Jensen A, Rabbitt MP, Gregory CA, Singh A. Household food security in the United States in 2017. http://www.ers.usda.gov/publications/pub-details/?pubid=90022. Published September 2018. Accessed December 9, 2019.
2. Berkowitz SA, Meigs JB, DeWalt D, et al. Material need insecurities, control of diabetes mellitus, and use of health care resources: results of the Measuring Economic Insecurity in Diabetes study. JAMA Intern Med. 2015;175(2):257-265.
3. Berkowitz SA, Seligman HK, Choudhry NK. Treat or eat: food insecurity, cost-related medication underuse, and unmet needs. Am J Med. 2014;127(4):303-310.e3.
4. Lyles CR, Seligman HK, Parker MM, et al. Financial strain and medication adherence among diabetes patients in an integrated health care delivery system: The Diabetes Study of Northern California (DISTANCE). Health Serv Res. 2016;51(2):610-624.
5. Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363(1):6-9.
6. Narain K, Bean-Mayberry B, Washington DL, Canelo IA, Darling JE, Yano EM. Access to care and health outcomes among women veterans using veterans administration health care: association with food insufficiency. Womens Health Issues. 2018;28(3):267-272.
7. Gundersen C, Ziliak JP. Food insecurity and health outcomes. Health Aff. 2015;34(11):1830-1839.
8. Wang EA, McGinnis KA, Goulet J, et al; Veterans Aging Cohort Study Project Team. Food insecurity and health: data from the Veterans Aging Cohort Study. Public Health Rep. 2015;130(3):261-268.
9. Berkowitz SA, Berkowitz TSZ, Meigs JB, Wexler DJ. Trends in food insecurity for adults with cardiometabolic disease in the United States: 2005-2012. PloS One. 2017;12(6):e0179172.
10. Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140(2):304-310.
11. Berkowitz SA, Baggett TP, Wexler DJ, Huskey KW, Wee CC. Food insecurity and metabolic control among U.S. adults with diabetes. Diabetes Care. 2013;36(10):3093-3099.
12. Seligman HK, Jacobs EA, López A, Tschann J, Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012;35(2):233-238.
13. Banerjee T, Crews DC, Wesson DE, et al; CDC CKD Surveillance Team. Food insecurity, CKD, and subsequent ESRD in US adults. Am J Kidney Dis. 2017;70(1):38-47.
14. Bruening M, Dinour LM, Chavez JBR. Food insecurity and emotional health in the USA: a systematic narrative review of longitudinal research. Public Health Nutr. 2017;20(17):3200-3208.
15. Berkowitz SA, Basu S, Meigs JB, Seligman HK. Food insecurity and health care expenditures in the United States, 2011-2013. Health Serv Res. 2018;53(3):1600-1620.
16. Berkowitz SA, Seligman HK, Basu S. Impact of food insecurity and SNAP participation on healthcare utilization and expenditures. http://www.ukcpr.org/research/discussion-papers. Published 2017. Accessed December 9, 2019.
17. Kushel MB, Gupta R, Gee L, Haas JS. Housing instability and food insecurity as barriers to health care among low-income Americans. J Gen Intern Med. 2006;21(1):71-77.
18. Garcia SP, Haddix A, Barnett K. Incremental health care costs associated with food insecurity and chronic conditions among older adults. Chronic Dis. 2018;15:180058.
19. Berkowitz SA, Seligman HK, Meigs JB, Basu S. Food insecurity, healthcare utilization, and high cost: a longitudinal cohort study. Am J Manag Care. 2018;24(9):399-404.
20. Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. 2009;36(1):74-81.
21. Darmon N, Drewnowski A. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: a systematic review and analysis. Nutr Rev. 2015;73(10):643-660.
22. Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87(5):1107-1117.
23. Drewnowski A. The cost of US foods as related to their nutritive value. Am J Clin Nutr. 2010;92(5):1181-1188.
24. Lucan SC, Maroko AR, Seitchik JL, Yoon DH, Sperry LE, Schechter CB. Unexpected neighborhood sources of food and drink: implications for research and community health. Am J Prev Med. 2018;55(2):e29-e38.
25. Castillo DC, Ramsey NL, Yu SS, Ricks M, Courville AB, Sumner AE. Inconsistent access to food and cardiometabolic disease: the effect of food insecurity. Curr Cardiovasc Risk Rep. 2012;6(3):245-250.
26. Seligman HK, Davis TC, Schillinger D, Wolf MS. Food insecurity is associated with hypoglycemia and poor diabetes self-management in a low-income sample with diabetes. J Health Care Poor Underserved. 2010;21(4):1227-1233.
27. Siefert K, Heflin CM, Corcoran ME, Williams DR. Food insufficiency and physical and mental health in a longitudinal survey of welfare recipients. J Health Soc Behav. 2004;45(2):171-186.
28. Mangurian C, Sreshta N, Seligman H. Food insecurity among adults with severe mental illness. Psychiatr Serv. 2013;64(9):931-932.
29. Melchior M, Caspi A, Howard LM, et al. Mental health context of food insecurity: a representative cohort of families with young children. Pediatrics. 2009;124(4):e564-e572.
30. Brostow DP, Gunzburger E, Abbate LM, Brenner LA, Thomas KS. Mental illness, not obesity status, is associated with food insecurity among the elderly in the health and retirement study. J Nutr Gerontol Geriatr. 2019;38(2):149-172.
31. Higashi RT, Craddock Lee SJ, Pezzia C, Quirk L, Leonard T, Pruitt SL. Family and social context contributes to the interplay of economic insecurity, food insecurity, and health. Ann Anthropol Pract. 2017;41(2):67-77.
32. O’Toole TP, Roberts CB, Johnson EE. Screening for food insecurity in six Veterans Administration clinics for the homeless, June-December 2015. Prev Chronic Dis. 2017;14:160375.
33. Feil DG, Pogach LM. Cognitive impairment is a major risk factor for serious hypoglycaemia; public health intervention is warranted. Evid Based Med. 2014;19(2):77.
34. Frith E, Loprinzi PD. Food insecurity and cognitive function in older adults: Brief report. Clin Nutr. 2018;37(5):1765-1768.
35. Herman D, Afulani P, Coleman-Jensen A, Harrison GG. Food insecurity and cost-related medication underuse among nonelderly adults in a nationally representative sample. Am J Public Health. 2015;105(10):e48-e59.
36. Tseng C-L, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174(2):259-268.
37. Vue MH, Setter SM. Drug-induced glucose alterations part 1: drug-induced hypoglycemia. Diabetes Spectr. 2011;24(3):171-177.
38. Seligman HK, Bolger AF, Guzman D, López A, Bibbins-Domingo K. Exhaustion of food budgets at month’s end and hospital admissions for hypoglycemia. Health Aff (Millwood). 2014;33(1):116-123.
39. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran poverty trends. https://www.va.gov/vetdata/docs/specialreports/veteran_poverty_trends.pdf. Published May 2015. Accessed December 9, 2019.
40. Robbins KG, Ravi A. Veterans living paycheck to paycheck are under threat during budget debates. https://www.americanprogress.org/issues/poverty/news/2017/09/19/439023/veterans-living-paycheck-paycheck-threat-budget-debates. Published September 19, 2017. Accessed December 9, 2019.
41. Wilmoth JM, London AS, Heflin CM. Economic well-being among older-adult households: variation by veteran and disability status. J Gerontol Soc Work. 2015;58(4):399-419.
42. Brostow DP, Gunzburger E, Thomas KS. Food insecurity among veterans: findings from the health and retirement study. J Nutr Health Aging. 2017;21(10):1358-1364.
43. Pooler J, Mian P, Srinivasan M, Miller Z. Veterans and food insecurity. https://www.impaqint.com/sites/default/files/issue-briefs/VeteransFoodInsecurity_IssueBrief_V1.3.pdf. Published November 2018. Accessed December 9, 2019.
44. Schure MB, Katon JG, Wong E, Liu C-F. Food and housing insecurity and health status among U.S. adults with and without prior military service. SSM Popul Health. 2016;29(2):244-248.
45. Miller DP, Larson MJ, Byrne T, DeVoe E. Food insecurity in veteran households: findings from nationally representative data. Public Health Nutr. 2016;19(10):1731-1740.
46. Widome R, Jensen A, Bangerter A, Fu SS. Food insecurity among veterans of the US wars in Iraq and Afghanistan. Public Health Nutr. 2015;18(5):844-849.
47. London AS, Heflin CM. Supplemental Nutrition Assistance Program (SNAP) use among active-duty military personnel, veterans, and reservists. Popul Res Policy Rev. 2015;34(6):805-826.
48. Weinfield NS, Mills G, Borger C, et al. Hunger in America 2014. Natl rep prepared for Feeding America. https://www.feedingamerica.org/research/hunger-in-america. Published 2014. Accessed December 9, 2019.
49. Mabli J, Ohls J, Dragoset L, Castner L, Santos B. Measuring the Effect of Supplemental Nutrition Assistance Program (SNAP) Participation on Food Security. Washington, DC: US Department of Agriculture, Food and Nutrition Service; 2013.
50. Srinivasan M, Pooler JA. Cost-related medication nonadherence for older adults participating in SNAP, 2013–2015. Am J Public Health. 2017;108(2):224-230.
51. Heflin C, Hodges L, Mueser P. Supplemental Nutrition Assistance Progam benefits and emergency room visits for hypoglycaemia. Public Health Nutr. 2017;20(7):1314-1321.
52. Samuel LJ, Szanton SL, Cahill R, et al. Does the Supplemental Nutrition Assistance Program affect hospital utilization among older adults? The case of Maryland. Popul Health Manag. 2018;21(2):88-95.
53. Szanton SL, Samuel LJ, Cahill R, et al. Food assistance is associated with decreased nursing home admissions for Maryland’s dually eligible older adults. BMC Geriatr. 2017;17(1):162.
54. Carlson S, Keith-Jennings B. SNAP is linked with improved nutritional outcomes and lower health care costs. https://www.cbpp.org/research/food-assistance/snap-is-linked-with-improved-nutritional-outcomes-and-lower-health-care. Published January 17, 2018. Accessed December 10, 2019.
55. Keith-Jennings B, Cai L. SNAP helps almost 1.4 million low-income veterans, including thousands in every state. https://www.cbpp.org/research/food-assistance/snap-helps-almost-14-million-low-income-veterans-including-thousands-in. Updated November 8, 2018. Accessed December 10, 2019.
56. US Department of Health and Human Services. Older Americans Act nutrition programs. https://acl.gov/sites/default/files/news%202017-03/OAA-Nutrition_Programs_Fact_Sheet.pdf. Accessed December 10, 2019.
57. US Department of Veterans Affairs. About VHA. https://www.va.gov/health/aboutvha.asp. Accessed December 10, 2019.
58. US Department of Veterans Affairs. VA Corporate Data Warehouse.
59. Yano EM, Bair MJ, Carrasquillo O, Krein SL, Rubenstein LV. Patient aligned care teams (PACT): VA’s journey to implement patient-centered medical homes. J Gen Intern Med. 2014;29(suppl 2):S547-s549.
60. O’Toole TP, Pape L. Innovative efforts to address homelessness among veterans. N C Med J. 2015;76(5):311-314.
61. O’Toole TP, Johnson EE, Aiello R, Kane V, Pape L. Tailoring care to vulnerable populations by incorporating social determinants of health: the Veterans Health Administration’s “Homeless Patient Aligned Care Team” Program. Prev Chronic Dis. 2016;13:150567.
62. Marpadga S, Fernandez A, Leung J, Tang A, Seligman H, Murphy EJ. Challenges and successes with food resource referrals for food-insecure patients with diabetes. Perm J. 2019;23.
63. Stenmark SH, Steiner JF, Marpadga S, Debor M, Underhill K, Seligman H. Lessons learned from implementation of the food insecurity screening and referral program at Kaiser Permanente Colorado. Perm J. 2018;22.
64. Martel ML, Klein LR, Hager KA, Cutts DB. Emergency department experience with novel electronic medical record order for referral to food resources. West J Emerg Med. 2018;19(2):232-237.
65. Going C, Cohen AJ, Bares M, Christensen M. Interdisciplinary approaches to addressing the food insecure veteran. Veterans Health Administration Employee Education System webinar; October 30, 2018.
66. Feeding America Announces New Partnership With U.S. Department Of Veterans Affairs. https://www.prnewswire.com/news-releases/feeding-america-announces-new-partnership-with-us-department-of-veterans-affairs-300481891.html. Published June 29, 2017. Accessed December 10, 2019.
67. US Department of Veterans Affairs. State Veterans Affairs offices. https://www.va.gov/statedva.htm. Updated March 20, 2019. Accessed December 10, 2019.
68. US Department of Veterans Affairs. Directory of veterans service organizations. https://www.va.gov/vso. Updated December 24, 2013. Accessed December 10, 2019.
69. ACL Administration for Community Living. Aging and disability resource centers. https://acl.gov/programs/aging-and-disability-networks/aging-and-disability-resource-centers. Updated December 13, 2017. Accessed December 10, 2019.
70. Nutrition and Obesity Policy Research and Evaluation Network (NOPREN). Clinical screening algorithms. https://nopren.org/resource/download-food-insecurity-screening-and-referral-algorithms-for-adults-patients-living-with-diabetes-and-pediatric-patients. Accessed December 10, 2019.
Nearly 1 in 8 households—and 1 in 6 households with children—experienced food insecurity in 2017, defined as limited or uncertain availability of nutritionally adequate and safe foods.1 Food insecurity is often even more pronounced among households with individuals with acute or chronic medical conditions.2-6 Moreover, food insecurity is independently associated with a range of adverse health outcomes, including poorer control of diabetes mellitus, hypertension, depression and other major psychiatric disorders, HIV, and chronic lung and kidney disease, as well as poorer overall health status.7-14 Food insecurity also has been associated with increased health care costs and acute care utilization as well as increased probability of delayed or missed care.15-19
The relationship between food insecurity and poor health outcomes is a complex and often cyclic phenomenon (Figure 1). Poor nutritional status is fueled by limited access to healthful foods as well as increased reliance on calorie-dense and nutrient-poor “junk” foods, which are less expensive and often more readily available in low-income neighborhoods.5,20-24 These compensatory dietary patterns place individuals at higher risk for developing cardiometabolic conditions and for poor control of these conditions.5,8,9,12,25,26 Additionally, the physiological and psychological stressors of food insecurity may precipitate depression and anxiety or worsen existing mental health conditions, resulting in feelings of overwhelm and decreased self-management capacity.5,8,27-31 Food insecurity has further been associated with poor sleep, declines in cognitive function, and increased falls, particularly among the frail and elderly.32-34
Individuals experiencing food insecurity often report having to make trade-offs between food and other necessities, such as paying rent or utilities. Additional strategies to stretch limited resources include cost-related underuse of medication and delays in needed medical care.4,17,31,35 In a nationally representative survey among adults with at least 1 chronic medical condition, 1 in 3 reported having to choose between food and medicine; 11% were unable to afford either.3 Furthermore, the inability to reliably adhere to medication regimens that need to be taken with food can result in potentially life-threatening hypoglycemia (as can lack of food regardless of medication use).5,26,36 In addition to the more obvious risks of glucose-lowering medications, such as insulin and long-acting sulfonylureas in patients experiencing food insecurity, many drugs commonly used among nondiabetic adults such as ACE-inhibitors, β blockers, quinolones, and salicylates can also precipitate hypoglycemia, and food insecurity has been associated with experiences of hypoglycemia even among individuals without diabetes mellitus.32,37 In one study the risk for hospital admissions for hypoglycemia among low-income populations increased by 27% at the end of the month when food budgets were more likely to be exhausted.38 Worsening health status and increased emergency department visits and hospitalizations may then result in lost wages and mounting medical bills, contributing to further financial strain and worsening food insecurity.
Prevalence and Importance of Food Insecurity Among US Veterans
Nearly 1.5 million veterans in the US are living below the federal poverty level (FPL).39 An additional 2.4 million veterans are living paycheck to paycheck at < 200% of the FPL.40 Veterans living in poverty are at even higher risk than nonveterans for food insecurity, homelessness, and other material hardship.41
Estimates of food insecurity among veterans vary widely, ranging from 6% to 24%—nearly twice that of the general US population.8,42-45 Higher rates of food insecurity have been reported among certain high-risk subgroups, including veterans who served in Iraq and Afghanistan (27%), female veterans (28%), homeless and formerly homeless veterans (49%), and veterans with serious mental illness (35%).6,32,43,46 Additional risk factors for food insecurity specific to veteran populations include younger age, having recently left active-duty military service, and lower final military paygrade.42,45-47 As in the general population, veteran food insecurity is associated with a range of adverse health outcomes, including poorer overall health status as well as increased probability of delayed or missed care.6,8,32,42-44,46
Even among veterans enrolled in federal food assistance programs, many still struggle to afford nutritionally adequate foods. As one example, in a study of mostly male homeless and formerly homeless veterans, O’Toole and colleagues found that nearly half of those reporting food insecurity were already receiving federal food assistance benefits, and 22% relied on emergency food resources.32 Of households served by Feeding America food pantries and meal programs, 20% have a member who has served in the US military.48
Federal Programs To Address Food Insecurity
There are several important federal food assistance programs designed to help alleviate food insecurity. The Supplemental Nutrition Assistance Program (SNAP, formerly the Food Stamp program) is the largest federal food assistance program and provides low-income Americans with cash benefits to purchase food. SNAP has been shown to substantially reduce food insecurity.7,49 The program also is associated with significant decreases in cost-related medication nonadherence as well as reductions in health care costs and both acute care and nursing home utilization.16,50-54 Although nearly 1.4 million veterans live in SNAP-enrolled households, 59% of eligible veterans are not enrolled.43,55 Closing this SNAP eligibility-enrollment gap, has been a focus of recent efforts to improve long-term food security among veterans. There also are several federal food assistance programs for households with children, including the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) and school meals programs. Among federal nutrition programs for seniors, the Older American’s Act contains designated funding to support nutrition services for older adults, including congregate meal programs in community settings like senior centers, places of worship, and housing communities, and home-delivered meals through programs like Meals on Wheels.56
VHA Response to Food Insecurity
The Veterans Health Administration (VHA) is the country’s largest integrated, federally funded health care system.57 In November 2015, congressional briefings on veteran food insecurity organized by the national non-profit organization MAZON: A Jewish Response to Hunger and hosted with bipartisan support were provided to the US House and Senate. As a result of these briefings, VHA chartered the national Ensuring Veteran Food Security Workgroup with a mandate to partner with governmental and nonprofit agencies to “focus on the issue of food insecurity, the identification of veterans at risk, the needed training of VHA staff and the coordination of resources and initiatives to support the veterans for whom we care.” Building off a pilot in US Department of Veterans Affairs (VA) Homeless Patient Aligned Care Teams (H-PACTs),32 VHA subsequently integrated a single-item food insecurity screening tool into the VA electronic health record (EHR) clinical reminder system (Figure 2). The clinical reminder, which was rolled out across VA medical centers nationally in October 2017, provides an alert to screen all noninstitutionalized veterans for food insecurity. To date, nearly 5 million veterans have been screened. When a veteran endorses food insecurity based on the initial screening question, a prompt appears to offer the veteran a referral to a social worker and/or dietitian. Positive screening results also should be communicated to the patient’s primary care provider. Depending on site-specific clinical flow, the reminders are typically completed in the outpatient setting either by nurses or medical assistants during intake or by providers as part of the clinical visit. However, any member of the health care team can complete the clinical reminder at any time. As of September 2019, approximately 74,000 veterans have been identified as food insecure.58
Addressing Food Insecurity
VHA has been a recognized leader in addressing homelessness and other social determinants of health through its integrated care and PACT delivery models.59-61 The food insecurity clinical reminder was designed to facilitate a tailored, interdisciplinary approach to identify and address food insecurity. Interdisciplinary care team members—including medical assistants, clinicians, social workers, registered dietitians, nurse care managers, occupational or physical therapists, and pharmacists—are uniquely positioned to identify veterans impacted by food insecurity, assess for associated clinical and/or social risk factors, and offer appropriate medical and nutrition interventions and resource referrals.
This interdisciplinary team-based model is essential given the range of potential drivers underlying veteran experiences of food insecurity and subsequent health outcomes. It is critically important for clinicians to review the medication list with veterans screening positive for food insecurity to assess for risk of hypoglycemia and/or cost-related nonadherence, make any necessary adjustments to therapeutic regimens, and assess for additional risk factors associated with food insecurity. Examples of tailored nutrition counseling that clinical dietitians may provide include meal preparation strategies for veterans who only have access to a microwave or hotplate, or recommendations for how veterans on medically restricted diets can best navigate food selection at soup kitchens or food pantries. Resource referrals provided by social workers or other care team members may include both emergency food resources to address immediate shortages (eg, food pantries, soup kitchens, or vouchers for free lunch) as well as resources focused on improving longer term food security (eg, federal food assistance programs or home delivered meal programs). Importantly, although providing a list of food resources may be helpful for some patients, such lists are often insufficient.62,63 Many patients require active assistance with program enrollment either onsite the day of their clinic visit or through connection with a partnering community-based organization that can, in turn, identify appropriate resources and facilitate program enrollment.63,64 Planned follow-up is also crucial to determine whether referrals are successful and to assess for ongoing need. Proposed roles for interdisciplinary care team members in addressing a positive food insecurity screen are outlined in Table 1.
VHA-Community Partnerships
In addition to services offered within VA, public and private sector partnerships can greatly enhance the range of resources available to food insecure veterans. Several VA facilities have developed formal community partnerships, such as the Veterans Pantry Pilot (VPP) program, a national partnership between Feeding America food banks and VA medical centers to establish onsite or mobile food pantries. There are currently 17 active Feeding America VPP sites, with a number of additional sites under development. Several of the VPP sites also include other “wraparound services,” such as SNAP application assistance.65,66
State Veterans Affairs offices67 and Veterans Service Organizations (VSOs)68 also can serve as valuable partners for connecting veterans with needed resources. VSOs offer a range of services, including assistancewith benefit claims, employment and housing assistance, emergency food assistance, and transportation to medical appointments. Some VSOs also have established local affiliations with Meals on Wheels focused on veteran outreach and providing hot meals for low-income, homebound, and disabled veterans.
Additional Resources
Although resources vary by regional setting, several key governmental and community-based food assistance programs are summarized in Table 2. Local community partners and online/phone-based directories, such as United Way’s 2-1-1 can help identify additional local resources. For older adults and individuals with disabilities, local Aging and Disability Resources Centers can provide information and assistance connecting to needed resources.69 Finally, there are a number of online resources available for clinicians interested in learning more about the impact of food insecurity on health and tools to use in the clinical setting (Table 3).
Conclusion
The VA has recognized food insecurity as a critical concern for the well-being of our nation’s veterans. Use of the EHR clinical reminder represents a crucial first step toward increasing provider awareness about veteran food insecurity and improving clinical efforts to address food insecurity once identified. Through the reminder, health care teams can connect veterans to needed resources and create both the individual and population-level data necessary to inform VHA and community efforts to address veteran food insecurity. Clinical reminder data are currently being used for local quality improvement efforts and have established the need nationally for formalized partnerships between VHA Social Work Services and Nutrition and Food Services to connect veterans with food and provide them with strategies to best use available food resources.
Moving forward, the Ensuring Veteran Food Security Workgroup continues to work with agencies and organizations across the country to improve food insecure veterans’ access to needed services. In addition to existing VA partnerships with Feeding America for the VPP, memorandums of understanding are currently underway to formalize partnerships with both the Food Research and Action Center (FRAC) and MAZON. Additional research is needed both to formally validate the current food insecurity clinical reminder screening question and to identify best practices and potential models for how to most effectively use VHA-community partnerships to address the unique needs of the veteran population.
Ensuring the food security of our nation’s veterans is essential to VA’s commitment to providing integrated, veteran-centered, whole person care. Toward that goal, VA health care teams are urged to use the clinical reminder and help connect food insecure veterans with relevant resources both within and outside of the VA health care system.
Nearly 1 in 8 households—and 1 in 6 households with children—experienced food insecurity in 2017, defined as limited or uncertain availability of nutritionally adequate and safe foods.1 Food insecurity is often even more pronounced among households with individuals with acute or chronic medical conditions.2-6 Moreover, food insecurity is independently associated with a range of adverse health outcomes, including poorer control of diabetes mellitus, hypertension, depression and other major psychiatric disorders, HIV, and chronic lung and kidney disease, as well as poorer overall health status.7-14 Food insecurity also has been associated with increased health care costs and acute care utilization as well as increased probability of delayed or missed care.15-19
The relationship between food insecurity and poor health outcomes is a complex and often cyclic phenomenon (Figure 1). Poor nutritional status is fueled by limited access to healthful foods as well as increased reliance on calorie-dense and nutrient-poor “junk” foods, which are less expensive and often more readily available in low-income neighborhoods.5,20-24 These compensatory dietary patterns place individuals at higher risk for developing cardiometabolic conditions and for poor control of these conditions.5,8,9,12,25,26 Additionally, the physiological and psychological stressors of food insecurity may precipitate depression and anxiety or worsen existing mental health conditions, resulting in feelings of overwhelm and decreased self-management capacity.5,8,27-31 Food insecurity has further been associated with poor sleep, declines in cognitive function, and increased falls, particularly among the frail and elderly.32-34
Individuals experiencing food insecurity often report having to make trade-offs between food and other necessities, such as paying rent or utilities. Additional strategies to stretch limited resources include cost-related underuse of medication and delays in needed medical care.4,17,31,35 In a nationally representative survey among adults with at least 1 chronic medical condition, 1 in 3 reported having to choose between food and medicine; 11% were unable to afford either.3 Furthermore, the inability to reliably adhere to medication regimens that need to be taken with food can result in potentially life-threatening hypoglycemia (as can lack of food regardless of medication use).5,26,36 In addition to the more obvious risks of glucose-lowering medications, such as insulin and long-acting sulfonylureas in patients experiencing food insecurity, many drugs commonly used among nondiabetic adults such as ACE-inhibitors, β blockers, quinolones, and salicylates can also precipitate hypoglycemia, and food insecurity has been associated with experiences of hypoglycemia even among individuals without diabetes mellitus.32,37 In one study the risk for hospital admissions for hypoglycemia among low-income populations increased by 27% at the end of the month when food budgets were more likely to be exhausted.38 Worsening health status and increased emergency department visits and hospitalizations may then result in lost wages and mounting medical bills, contributing to further financial strain and worsening food insecurity.
Prevalence and Importance of Food Insecurity Among US Veterans
Nearly 1.5 million veterans in the US are living below the federal poverty level (FPL).39 An additional 2.4 million veterans are living paycheck to paycheck at < 200% of the FPL.40 Veterans living in poverty are at even higher risk than nonveterans for food insecurity, homelessness, and other material hardship.41
Estimates of food insecurity among veterans vary widely, ranging from 6% to 24%—nearly twice that of the general US population.8,42-45 Higher rates of food insecurity have been reported among certain high-risk subgroups, including veterans who served in Iraq and Afghanistan (27%), female veterans (28%), homeless and formerly homeless veterans (49%), and veterans with serious mental illness (35%).6,32,43,46 Additional risk factors for food insecurity specific to veteran populations include younger age, having recently left active-duty military service, and lower final military paygrade.42,45-47 As in the general population, veteran food insecurity is associated with a range of adverse health outcomes, including poorer overall health status as well as increased probability of delayed or missed care.6,8,32,42-44,46
Even among veterans enrolled in federal food assistance programs, many still struggle to afford nutritionally adequate foods. As one example, in a study of mostly male homeless and formerly homeless veterans, O’Toole and colleagues found that nearly half of those reporting food insecurity were already receiving federal food assistance benefits, and 22% relied on emergency food resources.32 Of households served by Feeding America food pantries and meal programs, 20% have a member who has served in the US military.48
Federal Programs To Address Food Insecurity
There are several important federal food assistance programs designed to help alleviate food insecurity. The Supplemental Nutrition Assistance Program (SNAP, formerly the Food Stamp program) is the largest federal food assistance program and provides low-income Americans with cash benefits to purchase food. SNAP has been shown to substantially reduce food insecurity.7,49 The program also is associated with significant decreases in cost-related medication nonadherence as well as reductions in health care costs and both acute care and nursing home utilization.16,50-54 Although nearly 1.4 million veterans live in SNAP-enrolled households, 59% of eligible veterans are not enrolled.43,55 Closing this SNAP eligibility-enrollment gap, has been a focus of recent efforts to improve long-term food security among veterans. There also are several federal food assistance programs for households with children, including the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) and school meals programs. Among federal nutrition programs for seniors, the Older American’s Act contains designated funding to support nutrition services for older adults, including congregate meal programs in community settings like senior centers, places of worship, and housing communities, and home-delivered meals through programs like Meals on Wheels.56
VHA Response to Food Insecurity
The Veterans Health Administration (VHA) is the country’s largest integrated, federally funded health care system.57 In November 2015, congressional briefings on veteran food insecurity organized by the national non-profit organization MAZON: A Jewish Response to Hunger and hosted with bipartisan support were provided to the US House and Senate. As a result of these briefings, VHA chartered the national Ensuring Veteran Food Security Workgroup with a mandate to partner with governmental and nonprofit agencies to “focus on the issue of food insecurity, the identification of veterans at risk, the needed training of VHA staff and the coordination of resources and initiatives to support the veterans for whom we care.” Building off a pilot in US Department of Veterans Affairs (VA) Homeless Patient Aligned Care Teams (H-PACTs),32 VHA subsequently integrated a single-item food insecurity screening tool into the VA electronic health record (EHR) clinical reminder system (Figure 2). The clinical reminder, which was rolled out across VA medical centers nationally in October 2017, provides an alert to screen all noninstitutionalized veterans for food insecurity. To date, nearly 5 million veterans have been screened. When a veteran endorses food insecurity based on the initial screening question, a prompt appears to offer the veteran a referral to a social worker and/or dietitian. Positive screening results also should be communicated to the patient’s primary care provider. Depending on site-specific clinical flow, the reminders are typically completed in the outpatient setting either by nurses or medical assistants during intake or by providers as part of the clinical visit. However, any member of the health care team can complete the clinical reminder at any time. As of September 2019, approximately 74,000 veterans have been identified as food insecure.58
Addressing Food Insecurity
VHA has been a recognized leader in addressing homelessness and other social determinants of health through its integrated care and PACT delivery models.59-61 The food insecurity clinical reminder was designed to facilitate a tailored, interdisciplinary approach to identify and address food insecurity. Interdisciplinary care team members—including medical assistants, clinicians, social workers, registered dietitians, nurse care managers, occupational or physical therapists, and pharmacists—are uniquely positioned to identify veterans impacted by food insecurity, assess for associated clinical and/or social risk factors, and offer appropriate medical and nutrition interventions and resource referrals.
This interdisciplinary team-based model is essential given the range of potential drivers underlying veteran experiences of food insecurity and subsequent health outcomes. It is critically important for clinicians to review the medication list with veterans screening positive for food insecurity to assess for risk of hypoglycemia and/or cost-related nonadherence, make any necessary adjustments to therapeutic regimens, and assess for additional risk factors associated with food insecurity. Examples of tailored nutrition counseling that clinical dietitians may provide include meal preparation strategies for veterans who only have access to a microwave or hotplate, or recommendations for how veterans on medically restricted diets can best navigate food selection at soup kitchens or food pantries. Resource referrals provided by social workers or other care team members may include both emergency food resources to address immediate shortages (eg, food pantries, soup kitchens, or vouchers for free lunch) as well as resources focused on improving longer term food security (eg, federal food assistance programs or home delivered meal programs). Importantly, although providing a list of food resources may be helpful for some patients, such lists are often insufficient.62,63 Many patients require active assistance with program enrollment either onsite the day of their clinic visit or through connection with a partnering community-based organization that can, in turn, identify appropriate resources and facilitate program enrollment.63,64 Planned follow-up is also crucial to determine whether referrals are successful and to assess for ongoing need. Proposed roles for interdisciplinary care team members in addressing a positive food insecurity screen are outlined in Table 1.
VHA-Community Partnerships
In addition to services offered within VA, public and private sector partnerships can greatly enhance the range of resources available to food insecure veterans. Several VA facilities have developed formal community partnerships, such as the Veterans Pantry Pilot (VPP) program, a national partnership between Feeding America food banks and VA medical centers to establish onsite or mobile food pantries. There are currently 17 active Feeding America VPP sites, with a number of additional sites under development. Several of the VPP sites also include other “wraparound services,” such as SNAP application assistance.65,66
State Veterans Affairs offices67 and Veterans Service Organizations (VSOs)68 also can serve as valuable partners for connecting veterans with needed resources. VSOs offer a range of services, including assistancewith benefit claims, employment and housing assistance, emergency food assistance, and transportation to medical appointments. Some VSOs also have established local affiliations with Meals on Wheels focused on veteran outreach and providing hot meals for low-income, homebound, and disabled veterans.
Additional Resources
Although resources vary by regional setting, several key governmental and community-based food assistance programs are summarized in Table 2. Local community partners and online/phone-based directories, such as United Way’s 2-1-1 can help identify additional local resources. For older adults and individuals with disabilities, local Aging and Disability Resources Centers can provide information and assistance connecting to needed resources.69 Finally, there are a number of online resources available for clinicians interested in learning more about the impact of food insecurity on health and tools to use in the clinical setting (Table 3).
Conclusion
The VA has recognized food insecurity as a critical concern for the well-being of our nation’s veterans. Use of the EHR clinical reminder represents a crucial first step toward increasing provider awareness about veteran food insecurity and improving clinical efforts to address food insecurity once identified. Through the reminder, health care teams can connect veterans to needed resources and create both the individual and population-level data necessary to inform VHA and community efforts to address veteran food insecurity. Clinical reminder data are currently being used for local quality improvement efforts and have established the need nationally for formalized partnerships between VHA Social Work Services and Nutrition and Food Services to connect veterans with food and provide them with strategies to best use available food resources.
Moving forward, the Ensuring Veteran Food Security Workgroup continues to work with agencies and organizations across the country to improve food insecure veterans’ access to needed services. In addition to existing VA partnerships with Feeding America for the VPP, memorandums of understanding are currently underway to formalize partnerships with both the Food Research and Action Center (FRAC) and MAZON. Additional research is needed both to formally validate the current food insecurity clinical reminder screening question and to identify best practices and potential models for how to most effectively use VHA-community partnerships to address the unique needs of the veteran population.
Ensuring the food security of our nation’s veterans is essential to VA’s commitment to providing integrated, veteran-centered, whole person care. Toward that goal, VA health care teams are urged to use the clinical reminder and help connect food insecure veterans with relevant resources both within and outside of the VA health care system.
1. Coleman-Jensen A, Rabbitt MP, Gregory CA, Singh A. Household food security in the United States in 2017. http://www.ers.usda.gov/publications/pub-details/?pubid=90022. Published September 2018. Accessed December 9, 2019.
2. Berkowitz SA, Meigs JB, DeWalt D, et al. Material need insecurities, control of diabetes mellitus, and use of health care resources: results of the Measuring Economic Insecurity in Diabetes study. JAMA Intern Med. 2015;175(2):257-265.
3. Berkowitz SA, Seligman HK, Choudhry NK. Treat or eat: food insecurity, cost-related medication underuse, and unmet needs. Am J Med. 2014;127(4):303-310.e3.
4. Lyles CR, Seligman HK, Parker MM, et al. Financial strain and medication adherence among diabetes patients in an integrated health care delivery system: The Diabetes Study of Northern California (DISTANCE). Health Serv Res. 2016;51(2):610-624.
5. Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363(1):6-9.
6. Narain K, Bean-Mayberry B, Washington DL, Canelo IA, Darling JE, Yano EM. Access to care and health outcomes among women veterans using veterans administration health care: association with food insufficiency. Womens Health Issues. 2018;28(3):267-272.
7. Gundersen C, Ziliak JP. Food insecurity and health outcomes. Health Aff. 2015;34(11):1830-1839.
8. Wang EA, McGinnis KA, Goulet J, et al; Veterans Aging Cohort Study Project Team. Food insecurity and health: data from the Veterans Aging Cohort Study. Public Health Rep. 2015;130(3):261-268.
9. Berkowitz SA, Berkowitz TSZ, Meigs JB, Wexler DJ. Trends in food insecurity for adults with cardiometabolic disease in the United States: 2005-2012. PloS One. 2017;12(6):e0179172.
10. Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140(2):304-310.
11. Berkowitz SA, Baggett TP, Wexler DJ, Huskey KW, Wee CC. Food insecurity and metabolic control among U.S. adults with diabetes. Diabetes Care. 2013;36(10):3093-3099.
12. Seligman HK, Jacobs EA, López A, Tschann J, Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012;35(2):233-238.
13. Banerjee T, Crews DC, Wesson DE, et al; CDC CKD Surveillance Team. Food insecurity, CKD, and subsequent ESRD in US adults. Am J Kidney Dis. 2017;70(1):38-47.
14. Bruening M, Dinour LM, Chavez JBR. Food insecurity and emotional health in the USA: a systematic narrative review of longitudinal research. Public Health Nutr. 2017;20(17):3200-3208.
15. Berkowitz SA, Basu S, Meigs JB, Seligman HK. Food insecurity and health care expenditures in the United States, 2011-2013. Health Serv Res. 2018;53(3):1600-1620.
16. Berkowitz SA, Seligman HK, Basu S. Impact of food insecurity and SNAP participation on healthcare utilization and expenditures. http://www.ukcpr.org/research/discussion-papers. Published 2017. Accessed December 9, 2019.
17. Kushel MB, Gupta R, Gee L, Haas JS. Housing instability and food insecurity as barriers to health care among low-income Americans. J Gen Intern Med. 2006;21(1):71-77.
18. Garcia SP, Haddix A, Barnett K. Incremental health care costs associated with food insecurity and chronic conditions among older adults. Chronic Dis. 2018;15:180058.
19. Berkowitz SA, Seligman HK, Meigs JB, Basu S. Food insecurity, healthcare utilization, and high cost: a longitudinal cohort study. Am J Manag Care. 2018;24(9):399-404.
20. Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. 2009;36(1):74-81.
21. Darmon N, Drewnowski A. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: a systematic review and analysis. Nutr Rev. 2015;73(10):643-660.
22. Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87(5):1107-1117.
23. Drewnowski A. The cost of US foods as related to their nutritive value. Am J Clin Nutr. 2010;92(5):1181-1188.
24. Lucan SC, Maroko AR, Seitchik JL, Yoon DH, Sperry LE, Schechter CB. Unexpected neighborhood sources of food and drink: implications for research and community health. Am J Prev Med. 2018;55(2):e29-e38.
25. Castillo DC, Ramsey NL, Yu SS, Ricks M, Courville AB, Sumner AE. Inconsistent access to food and cardiometabolic disease: the effect of food insecurity. Curr Cardiovasc Risk Rep. 2012;6(3):245-250.
26. Seligman HK, Davis TC, Schillinger D, Wolf MS. Food insecurity is associated with hypoglycemia and poor diabetes self-management in a low-income sample with diabetes. J Health Care Poor Underserved. 2010;21(4):1227-1233.
27. Siefert K, Heflin CM, Corcoran ME, Williams DR. Food insufficiency and physical and mental health in a longitudinal survey of welfare recipients. J Health Soc Behav. 2004;45(2):171-186.
28. Mangurian C, Sreshta N, Seligman H. Food insecurity among adults with severe mental illness. Psychiatr Serv. 2013;64(9):931-932.
29. Melchior M, Caspi A, Howard LM, et al. Mental health context of food insecurity: a representative cohort of families with young children. Pediatrics. 2009;124(4):e564-e572.
30. Brostow DP, Gunzburger E, Abbate LM, Brenner LA, Thomas KS. Mental illness, not obesity status, is associated with food insecurity among the elderly in the health and retirement study. J Nutr Gerontol Geriatr. 2019;38(2):149-172.
31. Higashi RT, Craddock Lee SJ, Pezzia C, Quirk L, Leonard T, Pruitt SL. Family and social context contributes to the interplay of economic insecurity, food insecurity, and health. Ann Anthropol Pract. 2017;41(2):67-77.
32. O’Toole TP, Roberts CB, Johnson EE. Screening for food insecurity in six Veterans Administration clinics for the homeless, June-December 2015. Prev Chronic Dis. 2017;14:160375.
33. Feil DG, Pogach LM. Cognitive impairment is a major risk factor for serious hypoglycaemia; public health intervention is warranted. Evid Based Med. 2014;19(2):77.
34. Frith E, Loprinzi PD. Food insecurity and cognitive function in older adults: Brief report. Clin Nutr. 2018;37(5):1765-1768.
35. Herman D, Afulani P, Coleman-Jensen A, Harrison GG. Food insecurity and cost-related medication underuse among nonelderly adults in a nationally representative sample. Am J Public Health. 2015;105(10):e48-e59.
36. Tseng C-L, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174(2):259-268.
37. Vue MH, Setter SM. Drug-induced glucose alterations part 1: drug-induced hypoglycemia. Diabetes Spectr. 2011;24(3):171-177.
38. Seligman HK, Bolger AF, Guzman D, López A, Bibbins-Domingo K. Exhaustion of food budgets at month’s end and hospital admissions for hypoglycemia. Health Aff (Millwood). 2014;33(1):116-123.
39. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran poverty trends. https://www.va.gov/vetdata/docs/specialreports/veteran_poverty_trends.pdf. Published May 2015. Accessed December 9, 2019.
40. Robbins KG, Ravi A. Veterans living paycheck to paycheck are under threat during budget debates. https://www.americanprogress.org/issues/poverty/news/2017/09/19/439023/veterans-living-paycheck-paycheck-threat-budget-debates. Published September 19, 2017. Accessed December 9, 2019.
41. Wilmoth JM, London AS, Heflin CM. Economic well-being among older-adult households: variation by veteran and disability status. J Gerontol Soc Work. 2015;58(4):399-419.
42. Brostow DP, Gunzburger E, Thomas KS. Food insecurity among veterans: findings from the health and retirement study. J Nutr Health Aging. 2017;21(10):1358-1364.
43. Pooler J, Mian P, Srinivasan M, Miller Z. Veterans and food insecurity. https://www.impaqint.com/sites/default/files/issue-briefs/VeteransFoodInsecurity_IssueBrief_V1.3.pdf. Published November 2018. Accessed December 9, 2019.
44. Schure MB, Katon JG, Wong E, Liu C-F. Food and housing insecurity and health status among U.S. adults with and without prior military service. SSM Popul Health. 2016;29(2):244-248.
45. Miller DP, Larson MJ, Byrne T, DeVoe E. Food insecurity in veteran households: findings from nationally representative data. Public Health Nutr. 2016;19(10):1731-1740.
46. Widome R, Jensen A, Bangerter A, Fu SS. Food insecurity among veterans of the US wars in Iraq and Afghanistan. Public Health Nutr. 2015;18(5):844-849.
47. London AS, Heflin CM. Supplemental Nutrition Assistance Program (SNAP) use among active-duty military personnel, veterans, and reservists. Popul Res Policy Rev. 2015;34(6):805-826.
48. Weinfield NS, Mills G, Borger C, et al. Hunger in America 2014. Natl rep prepared for Feeding America. https://www.feedingamerica.org/research/hunger-in-america. Published 2014. Accessed December 9, 2019.
49. Mabli J, Ohls J, Dragoset L, Castner L, Santos B. Measuring the Effect of Supplemental Nutrition Assistance Program (SNAP) Participation on Food Security. Washington, DC: US Department of Agriculture, Food and Nutrition Service; 2013.
50. Srinivasan M, Pooler JA. Cost-related medication nonadherence for older adults participating in SNAP, 2013–2015. Am J Public Health. 2017;108(2):224-230.
51. Heflin C, Hodges L, Mueser P. Supplemental Nutrition Assistance Progam benefits and emergency room visits for hypoglycaemia. Public Health Nutr. 2017;20(7):1314-1321.
52. Samuel LJ, Szanton SL, Cahill R, et al. Does the Supplemental Nutrition Assistance Program affect hospital utilization among older adults? The case of Maryland. Popul Health Manag. 2018;21(2):88-95.
53. Szanton SL, Samuel LJ, Cahill R, et al. Food assistance is associated with decreased nursing home admissions for Maryland’s dually eligible older adults. BMC Geriatr. 2017;17(1):162.
54. Carlson S, Keith-Jennings B. SNAP is linked with improved nutritional outcomes and lower health care costs. https://www.cbpp.org/research/food-assistance/snap-is-linked-with-improved-nutritional-outcomes-and-lower-health-care. Published January 17, 2018. Accessed December 10, 2019.
55. Keith-Jennings B, Cai L. SNAP helps almost 1.4 million low-income veterans, including thousands in every state. https://www.cbpp.org/research/food-assistance/snap-helps-almost-14-million-low-income-veterans-including-thousands-in. Updated November 8, 2018. Accessed December 10, 2019.
56. US Department of Health and Human Services. Older Americans Act nutrition programs. https://acl.gov/sites/default/files/news%202017-03/OAA-Nutrition_Programs_Fact_Sheet.pdf. Accessed December 10, 2019.
57. US Department of Veterans Affairs. About VHA. https://www.va.gov/health/aboutvha.asp. Accessed December 10, 2019.
58. US Department of Veterans Affairs. VA Corporate Data Warehouse.
59. Yano EM, Bair MJ, Carrasquillo O, Krein SL, Rubenstein LV. Patient aligned care teams (PACT): VA’s journey to implement patient-centered medical homes. J Gen Intern Med. 2014;29(suppl 2):S547-s549.
60. O’Toole TP, Pape L. Innovative efforts to address homelessness among veterans. N C Med J. 2015;76(5):311-314.
61. O’Toole TP, Johnson EE, Aiello R, Kane V, Pape L. Tailoring care to vulnerable populations by incorporating social determinants of health: the Veterans Health Administration’s “Homeless Patient Aligned Care Team” Program. Prev Chronic Dis. 2016;13:150567.
62. Marpadga S, Fernandez A, Leung J, Tang A, Seligman H, Murphy EJ. Challenges and successes with food resource referrals for food-insecure patients with diabetes. Perm J. 2019;23.
63. Stenmark SH, Steiner JF, Marpadga S, Debor M, Underhill K, Seligman H. Lessons learned from implementation of the food insecurity screening and referral program at Kaiser Permanente Colorado. Perm J. 2018;22.
64. Martel ML, Klein LR, Hager KA, Cutts DB. Emergency department experience with novel electronic medical record order for referral to food resources. West J Emerg Med. 2018;19(2):232-237.
65. Going C, Cohen AJ, Bares M, Christensen M. Interdisciplinary approaches to addressing the food insecure veteran. Veterans Health Administration Employee Education System webinar; October 30, 2018.
66. Feeding America Announces New Partnership With U.S. Department Of Veterans Affairs. https://www.prnewswire.com/news-releases/feeding-america-announces-new-partnership-with-us-department-of-veterans-affairs-300481891.html. Published June 29, 2017. Accessed December 10, 2019.
67. US Department of Veterans Affairs. State Veterans Affairs offices. https://www.va.gov/statedva.htm. Updated March 20, 2019. Accessed December 10, 2019.
68. US Department of Veterans Affairs. Directory of veterans service organizations. https://www.va.gov/vso. Updated December 24, 2013. Accessed December 10, 2019.
69. ACL Administration for Community Living. Aging and disability resource centers. https://acl.gov/programs/aging-and-disability-networks/aging-and-disability-resource-centers. Updated December 13, 2017. Accessed December 10, 2019.
70. Nutrition and Obesity Policy Research and Evaluation Network (NOPREN). Clinical screening algorithms. https://nopren.org/resource/download-food-insecurity-screening-and-referral-algorithms-for-adults-patients-living-with-diabetes-and-pediatric-patients. Accessed December 10, 2019.
1. Coleman-Jensen A, Rabbitt MP, Gregory CA, Singh A. Household food security in the United States in 2017. http://www.ers.usda.gov/publications/pub-details/?pubid=90022. Published September 2018. Accessed December 9, 2019.
2. Berkowitz SA, Meigs JB, DeWalt D, et al. Material need insecurities, control of diabetes mellitus, and use of health care resources: results of the Measuring Economic Insecurity in Diabetes study. JAMA Intern Med. 2015;175(2):257-265.
3. Berkowitz SA, Seligman HK, Choudhry NK. Treat or eat: food insecurity, cost-related medication underuse, and unmet needs. Am J Med. 2014;127(4):303-310.e3.
4. Lyles CR, Seligman HK, Parker MM, et al. Financial strain and medication adherence among diabetes patients in an integrated health care delivery system: The Diabetes Study of Northern California (DISTANCE). Health Serv Res. 2016;51(2):610-624.
5. Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363(1):6-9.
6. Narain K, Bean-Mayberry B, Washington DL, Canelo IA, Darling JE, Yano EM. Access to care and health outcomes among women veterans using veterans administration health care: association with food insufficiency. Womens Health Issues. 2018;28(3):267-272.
7. Gundersen C, Ziliak JP. Food insecurity and health outcomes. Health Aff. 2015;34(11):1830-1839.
8. Wang EA, McGinnis KA, Goulet J, et al; Veterans Aging Cohort Study Project Team. Food insecurity and health: data from the Veterans Aging Cohort Study. Public Health Rep. 2015;130(3):261-268.
9. Berkowitz SA, Berkowitz TSZ, Meigs JB, Wexler DJ. Trends in food insecurity for adults with cardiometabolic disease in the United States: 2005-2012. PloS One. 2017;12(6):e0179172.
10. Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140(2):304-310.
11. Berkowitz SA, Baggett TP, Wexler DJ, Huskey KW, Wee CC. Food insecurity and metabolic control among U.S. adults with diabetes. Diabetes Care. 2013;36(10):3093-3099.
12. Seligman HK, Jacobs EA, López A, Tschann J, Fernandez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012;35(2):233-238.
13. Banerjee T, Crews DC, Wesson DE, et al; CDC CKD Surveillance Team. Food insecurity, CKD, and subsequent ESRD in US adults. Am J Kidney Dis. 2017;70(1):38-47.
14. Bruening M, Dinour LM, Chavez JBR. Food insecurity and emotional health in the USA: a systematic narrative review of longitudinal research. Public Health Nutr. 2017;20(17):3200-3208.
15. Berkowitz SA, Basu S, Meigs JB, Seligman HK. Food insecurity and health care expenditures in the United States, 2011-2013. Health Serv Res. 2018;53(3):1600-1620.
16. Berkowitz SA, Seligman HK, Basu S. Impact of food insecurity and SNAP participation on healthcare utilization and expenditures. http://www.ukcpr.org/research/discussion-papers. Published 2017. Accessed December 9, 2019.
17. Kushel MB, Gupta R, Gee L, Haas JS. Housing instability and food insecurity as barriers to health care among low-income Americans. J Gen Intern Med. 2006;21(1):71-77.
18. Garcia SP, Haddix A, Barnett K. Incremental health care costs associated with food insecurity and chronic conditions among older adults. Chronic Dis. 2018;15:180058.
19. Berkowitz SA, Seligman HK, Meigs JB, Basu S. Food insecurity, healthcare utilization, and high cost: a longitudinal cohort study. Am J Manag Care. 2018;24(9):399-404.
20. Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. 2009;36(1):74-81.
21. Darmon N, Drewnowski A. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: a systematic review and analysis. Nutr Rev. 2015;73(10):643-660.
22. Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87(5):1107-1117.
23. Drewnowski A. The cost of US foods as related to their nutritive value. Am J Clin Nutr. 2010;92(5):1181-1188.
24. Lucan SC, Maroko AR, Seitchik JL, Yoon DH, Sperry LE, Schechter CB. Unexpected neighborhood sources of food and drink: implications for research and community health. Am J Prev Med. 2018;55(2):e29-e38.
25. Castillo DC, Ramsey NL, Yu SS, Ricks M, Courville AB, Sumner AE. Inconsistent access to food and cardiometabolic disease: the effect of food insecurity. Curr Cardiovasc Risk Rep. 2012;6(3):245-250.
26. Seligman HK, Davis TC, Schillinger D, Wolf MS. Food insecurity is associated with hypoglycemia and poor diabetes self-management in a low-income sample with diabetes. J Health Care Poor Underserved. 2010;21(4):1227-1233.
27. Siefert K, Heflin CM, Corcoran ME, Williams DR. Food insufficiency and physical and mental health in a longitudinal survey of welfare recipients. J Health Soc Behav. 2004;45(2):171-186.
28. Mangurian C, Sreshta N, Seligman H. Food insecurity among adults with severe mental illness. Psychiatr Serv. 2013;64(9):931-932.
29. Melchior M, Caspi A, Howard LM, et al. Mental health context of food insecurity: a representative cohort of families with young children. Pediatrics. 2009;124(4):e564-e572.
30. Brostow DP, Gunzburger E, Abbate LM, Brenner LA, Thomas KS. Mental illness, not obesity status, is associated with food insecurity among the elderly in the health and retirement study. J Nutr Gerontol Geriatr. 2019;38(2):149-172.
31. Higashi RT, Craddock Lee SJ, Pezzia C, Quirk L, Leonard T, Pruitt SL. Family and social context contributes to the interplay of economic insecurity, food insecurity, and health. Ann Anthropol Pract. 2017;41(2):67-77.
32. O’Toole TP, Roberts CB, Johnson EE. Screening for food insecurity in six Veterans Administration clinics for the homeless, June-December 2015. Prev Chronic Dis. 2017;14:160375.
33. Feil DG, Pogach LM. Cognitive impairment is a major risk factor for serious hypoglycaemia; public health intervention is warranted. Evid Based Med. 2014;19(2):77.
34. Frith E, Loprinzi PD. Food insecurity and cognitive function in older adults: Brief report. Clin Nutr. 2018;37(5):1765-1768.
35. Herman D, Afulani P, Coleman-Jensen A, Harrison GG. Food insecurity and cost-related medication underuse among nonelderly adults in a nationally representative sample. Am J Public Health. 2015;105(10):e48-e59.
36. Tseng C-L, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med. 2014;174(2):259-268.
37. Vue MH, Setter SM. Drug-induced glucose alterations part 1: drug-induced hypoglycemia. Diabetes Spectr. 2011;24(3):171-177.
38. Seligman HK, Bolger AF, Guzman D, López A, Bibbins-Domingo K. Exhaustion of food budgets at month’s end and hospital admissions for hypoglycemia. Health Aff (Millwood). 2014;33(1):116-123.
39. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran poverty trends. https://www.va.gov/vetdata/docs/specialreports/veteran_poverty_trends.pdf. Published May 2015. Accessed December 9, 2019.
40. Robbins KG, Ravi A. Veterans living paycheck to paycheck are under threat during budget debates. https://www.americanprogress.org/issues/poverty/news/2017/09/19/439023/veterans-living-paycheck-paycheck-threat-budget-debates. Published September 19, 2017. Accessed December 9, 2019.
41. Wilmoth JM, London AS, Heflin CM. Economic well-being among older-adult households: variation by veteran and disability status. J Gerontol Soc Work. 2015;58(4):399-419.
42. Brostow DP, Gunzburger E, Thomas KS. Food insecurity among veterans: findings from the health and retirement study. J Nutr Health Aging. 2017;21(10):1358-1364.
43. Pooler J, Mian P, Srinivasan M, Miller Z. Veterans and food insecurity. https://www.impaqint.com/sites/default/files/issue-briefs/VeteransFoodInsecurity_IssueBrief_V1.3.pdf. Published November 2018. Accessed December 9, 2019.
44. Schure MB, Katon JG, Wong E, Liu C-F. Food and housing insecurity and health status among U.S. adults with and without prior military service. SSM Popul Health. 2016;29(2):244-248.
45. Miller DP, Larson MJ, Byrne T, DeVoe E. Food insecurity in veteran households: findings from nationally representative data. Public Health Nutr. 2016;19(10):1731-1740.
46. Widome R, Jensen A, Bangerter A, Fu SS. Food insecurity among veterans of the US wars in Iraq and Afghanistan. Public Health Nutr. 2015;18(5):844-849.
47. London AS, Heflin CM. Supplemental Nutrition Assistance Program (SNAP) use among active-duty military personnel, veterans, and reservists. Popul Res Policy Rev. 2015;34(6):805-826.
48. Weinfield NS, Mills G, Borger C, et al. Hunger in America 2014. Natl rep prepared for Feeding America. https://www.feedingamerica.org/research/hunger-in-america. Published 2014. Accessed December 9, 2019.
49. Mabli J, Ohls J, Dragoset L, Castner L, Santos B. Measuring the Effect of Supplemental Nutrition Assistance Program (SNAP) Participation on Food Security. Washington, DC: US Department of Agriculture, Food and Nutrition Service; 2013.
50. Srinivasan M, Pooler JA. Cost-related medication nonadherence for older adults participating in SNAP, 2013–2015. Am J Public Health. 2017;108(2):224-230.
51. Heflin C, Hodges L, Mueser P. Supplemental Nutrition Assistance Progam benefits and emergency room visits for hypoglycaemia. Public Health Nutr. 2017;20(7):1314-1321.
52. Samuel LJ, Szanton SL, Cahill R, et al. Does the Supplemental Nutrition Assistance Program affect hospital utilization among older adults? The case of Maryland. Popul Health Manag. 2018;21(2):88-95.
53. Szanton SL, Samuel LJ, Cahill R, et al. Food assistance is associated with decreased nursing home admissions for Maryland’s dually eligible older adults. BMC Geriatr. 2017;17(1):162.
54. Carlson S, Keith-Jennings B. SNAP is linked with improved nutritional outcomes and lower health care costs. https://www.cbpp.org/research/food-assistance/snap-is-linked-with-improved-nutritional-outcomes-and-lower-health-care. Published January 17, 2018. Accessed December 10, 2019.
55. Keith-Jennings B, Cai L. SNAP helps almost 1.4 million low-income veterans, including thousands in every state. https://www.cbpp.org/research/food-assistance/snap-helps-almost-14-million-low-income-veterans-including-thousands-in. Updated November 8, 2018. Accessed December 10, 2019.
56. US Department of Health and Human Services. Older Americans Act nutrition programs. https://acl.gov/sites/default/files/news%202017-03/OAA-Nutrition_Programs_Fact_Sheet.pdf. Accessed December 10, 2019.
57. US Department of Veterans Affairs. About VHA. https://www.va.gov/health/aboutvha.asp. Accessed December 10, 2019.
58. US Department of Veterans Affairs. VA Corporate Data Warehouse.
59. Yano EM, Bair MJ, Carrasquillo O, Krein SL, Rubenstein LV. Patient aligned care teams (PACT): VA’s journey to implement patient-centered medical homes. J Gen Intern Med. 2014;29(suppl 2):S547-s549.
60. O’Toole TP, Pape L. Innovative efforts to address homelessness among veterans. N C Med J. 2015;76(5):311-314.
61. O’Toole TP, Johnson EE, Aiello R, Kane V, Pape L. Tailoring care to vulnerable populations by incorporating social determinants of health: the Veterans Health Administration’s “Homeless Patient Aligned Care Team” Program. Prev Chronic Dis. 2016;13:150567.
62. Marpadga S, Fernandez A, Leung J, Tang A, Seligman H, Murphy EJ. Challenges and successes with food resource referrals for food-insecure patients with diabetes. Perm J. 2019;23.
63. Stenmark SH, Steiner JF, Marpadga S, Debor M, Underhill K, Seligman H. Lessons learned from implementation of the food insecurity screening and referral program at Kaiser Permanente Colorado. Perm J. 2018;22.
64. Martel ML, Klein LR, Hager KA, Cutts DB. Emergency department experience with novel electronic medical record order for referral to food resources. West J Emerg Med. 2018;19(2):232-237.
65. Going C, Cohen AJ, Bares M, Christensen M. Interdisciplinary approaches to addressing the food insecure veteran. Veterans Health Administration Employee Education System webinar; October 30, 2018.
66. Feeding America Announces New Partnership With U.S. Department Of Veterans Affairs. https://www.prnewswire.com/news-releases/feeding-america-announces-new-partnership-with-us-department-of-veterans-affairs-300481891.html. Published June 29, 2017. Accessed December 10, 2019.
67. US Department of Veterans Affairs. State Veterans Affairs offices. https://www.va.gov/statedva.htm. Updated March 20, 2019. Accessed December 10, 2019.
68. US Department of Veterans Affairs. Directory of veterans service organizations. https://www.va.gov/vso. Updated December 24, 2013. Accessed December 10, 2019.
69. ACL Administration for Community Living. Aging and disability resource centers. https://acl.gov/programs/aging-and-disability-networks/aging-and-disability-resource-centers. Updated December 13, 2017. Accessed December 10, 2019.
70. Nutrition and Obesity Policy Research and Evaluation Network (NOPREN). Clinical screening algorithms. https://nopren.org/resource/download-food-insecurity-screening-and-referral-algorithms-for-adults-patients-living-with-diabetes-and-pediatric-patients. Accessed December 10, 2019.
Remote-Onset Alopecia Areata Attributed to Ipilimumab
Cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) is a key co-stimulatory receptor expressed on activated T cells that negatively regulates T-cell activation.1-3 It exerts its effects in part by the prevention of IL-2 transcription and inhibition of cell-cycle progression.4 Cytotoxic T-lymphocyte–associated antigen 4 also is expressed by a subset of CD25+CD4+ regulatory T cells (Tregs), where it plays a role in immune tolerance.5 Blockade has demonstrated antitumor activity as well as immune activation, and CTLA-4 dysregulation has been implicated in autoimmune diseases such as alopecia areata (AA).6
Ipilimumab is a fully humanized monoclonal antibody against CTLA-4 and one of a growing class of immune checkpoint inhibitor therapies for metastatic melanoma. Phase 2 and 3 clinical trials have shown an improved survival effect of ipilimumab in patients with advanced melanoma,7-10 with 3-year survival rates ranging from 20.8% to 46.5%.10,11 The US Food and Drug Administration approved ipilimumab in 2011 for treatment of unresectable or metastatic melanoma.12 The most common toxicities of ipilimumab are immune-related adverse effects (irAEs), which represent loss of tolerance to self-antigens.13 Immune-related adverse effects occur in 64.2% of patients,14 with severe or life-threatening irAEs in 17.8% of patients.14 Rates of irAEs appear dose dependent but consistent across increased doses.15 Cutaneous irAEs occur in more than 47% of patients16 and commonly manifest as pruritus with or without a diffuse morbilliform rash,10,17 though less common skin reactions, including vitiligo, vasculitis, and Stevens-Johnson syndrome/toxic epidermal necrolysis, have been documented.9,18
Generalized AA and its more widespread variant, alopecia universalis, have been reported as adverse effects of ipilimumab monotherapy in 2 prior cases in the English-language literature (Table).17,19 Alopecia areata also has been attributed to combination immune checkpoint inhibitor therapy.20,21 We report a case of AA attributable to ipilimumab monotherapy that was localized exclusively to the scalp and remote in onset following treatment.
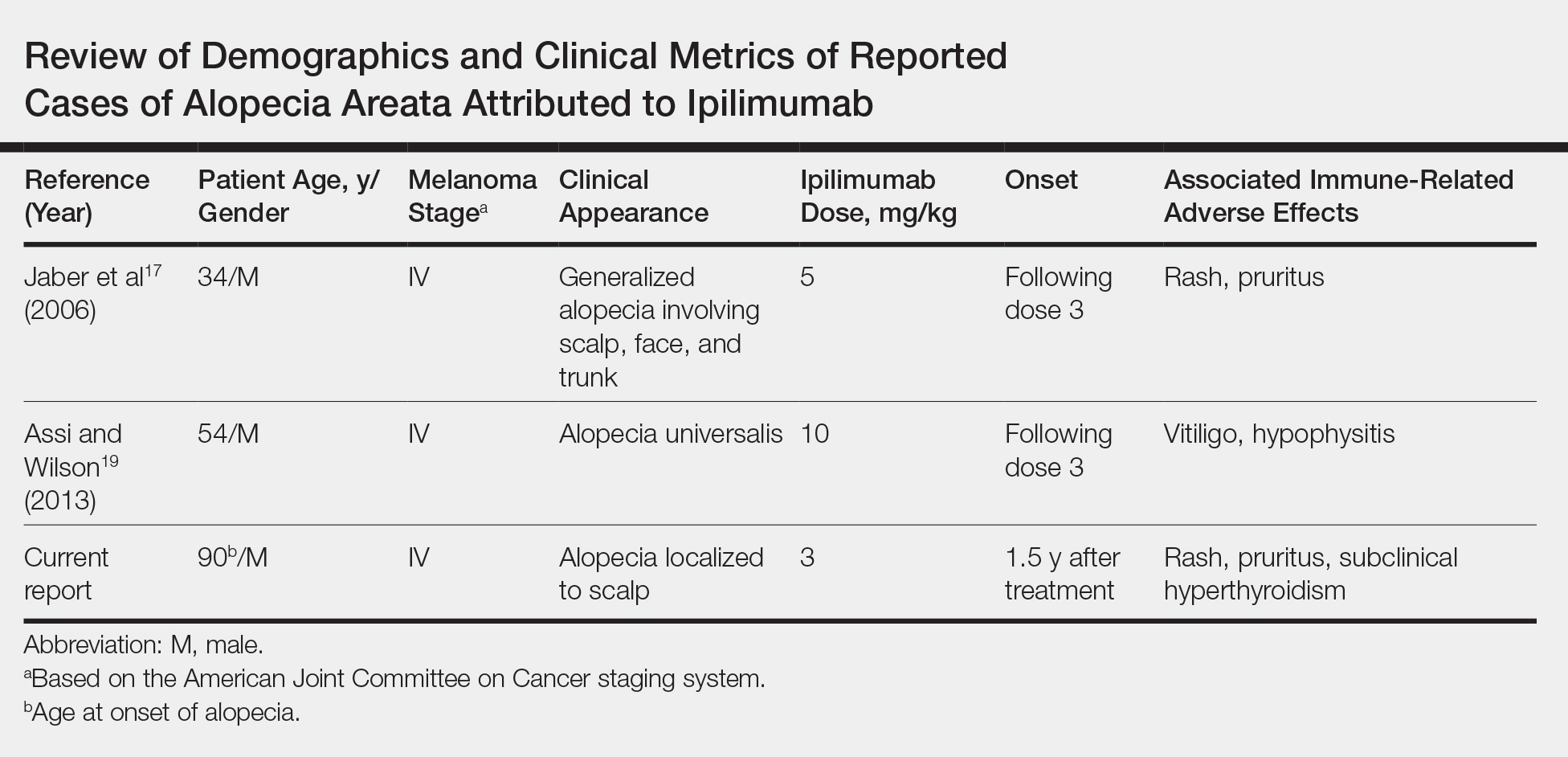
Case Report
An 88-year-old man with pT3bpN3 nodular melanoma of the back demonstrated multiple lung metastases by positron emission tomography–computed tomography. Lactate dehydrogenase was within reference range, and his Eastern Cooperative Oncology Group performance status was 0 (fully active). One month later, he was started on ipilimumab 3 mg/kg intravenous infusion every 3 weeks for a total of 4 doses. At approximately week 6, his course was complicated by mild fatigue, a faintly erythematous morbilliform rash, and mild pruritus, with laboratory evidence of subclinical hyperthyroidism. Follow-up positron emission tomography–computed tomography at the conclusion of treatment demonstrated complete regression of previously noted hypermetabolic foci. His symptoms and subclinical hyperthyroidism resolved several months later.
Seventeen months after completion of ipilimumab therapy (at age 90 years), the patient’s barber noted new-onset hair loss on the right occipital scalp. Physical examination demonstrated a well-circumscribed patch of nonscarring alopecia (approximately 6 cm) that was clinically consistent with AA (Figure). There were no associated symptoms or other involved areas of hair loss. He denied any personal or family history of AA. The patient’s melanoma has remained in remission to date.
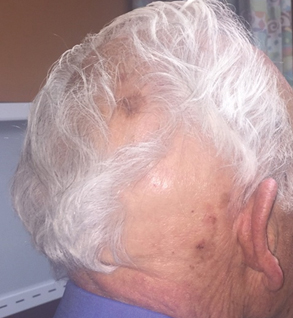
Comment
This case is unique in that AA was localized to a single circumscribed patch on the scalp and occurred nearly 1.5 years after treatment with ipilimumab, which may indicate a robust blockade of CTLA-4 given the remote development of autoimmunity in the setting of persistent remission of melanoma. Although the appearance of AA may be coincidental, onset at 90 years of age would be unusual. The mean age of onset of AA has been reported between 25.2 and 36.3 years,22,23 and its incidence in men older than 60 years is only 6.4 per 100,000 person-years.24
Although AA is a rare irAE of CTLA-4 blockade, the disease has been increasingly linked to CTLA-4 dysregulation in both animal models and humans.6,25,26 A genome-wide association study of 1054 patients with AA and 3278 controls implicated several genes controlling activation and proliferation of Tregs, including CTLA-4.27 More specifically, single-nucleotide polymorphisms of the CTLA-4 gene were found to be associated with AA in a study of 1196 unrelated patients and 1280 controls,28 and Megiorni et al
Given the role of CTLA-4 dysregulation in the pathogenesis of AA, the very low rates of AA in ipilimumab are somewhat surprising, which may represent a reporting bias. Alternatively, there may be sufficient Treg activity to prevent high rates of AA at a lower ipilimumab dose of 3 mg/kg but insufficient activity to prevent development of other irAEs. With US Food and Drug Administration approval of ipilimumab at a higher dose of 10 mg/kg for use as adjuvant therapy for stage III melanomas,12 less common cutaneous irAEs such as AA may be seen with increased frequency. Clinicians planning ipilimumab therapy should discuss this side effect and other potential irAEs with their patients before initiation of treatment.
- Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267-270.
- Scalapino KJ, Daikh DI. CTLA-4: a key regulatory point in the control of autoimmune disease. Immunol Rev. 2008;223:143-155.
- Buchbinder E, Hodi FS. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J Clin Invest. 2015;125:3377-3383.
- Brunner MC, Chambers CA, Chan FK, et al. CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813-5820.
- Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303-310.
- Carroll JM, McElwee KJ, E King L, et al. Gene array profiling and immunomodulation studies define a cell-mediated immune response underlying the pathogenesis of alopecia areata in a mouse model and humans. J Invest Dermatol. 2002;119:392-402.
- Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591-5598.
- O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712-1717.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522-530.
- Yervoy (ipilimumab)[package insert]. Princeton, NJ: Bristol-Myers Squibb; 2019.
- Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864-872.
- Ibrahim RA, Berman DM, DePril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma [abstract]. J Clin Oncol. 2011;29(suppl):8583.
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155-164.
- Kähler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9:277-286.
- Jaber SH, Cowen EW, Haworth LR, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol. 2006;142:166-172.
- Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8:E537545.
- Assi H, Wilson KS. Immune toxicities and long remission duration after ipilimumab therapy for metastatic melanoma: two illustrative cases. Curr Oncol. 2013;20:E165-E169.
- Zarbo A, Belum VR, Sibaud V, et al. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. Br J Dermatol. 2017;176:1649-1652.
- Lakhmiri M, Cavelier-Balloy B, Lacoste C, et al. Nivolumab-induced alopecia areata: a reversible factor of good prognosis? JAAD Case Rep. 2018;4:761-765.
- Tan E, Tay YK, Goh CL, et al. The pattern and profile of alopecia areata in Singapore–a study of 219 Asians. Int J Dermatol. 2002;41:748-753.
- Goh C, Finkel M, Christos PJ, et al. Profile of 513 patients with alopecia areata: associations of disease subtypes with atopy, autoimmune disease and positive family history. J Eur Acad Dermatol Venereol. 2006;20:1055-1060.
- Mirzoyev SA, Schrum AG, Davis MD, et al. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Zöller M, McElwee KJ, Engel P, et al. Transient CD44 variant isoform expression and reduction in CD4(+)/CD25(+) regulatory T cells in C3H/HeJ mice with alopecia areata. J Invest Dermatol. 2002;118:983-992.
- Zöller M, McElwee KJ, Vitacolonna M, et al. The progressive state, in contrast to the stable or regressive state of alopecia areata, is reflected in peripheral blood mononuclear cells. Exp Dermatol. 2004;13:435-444.
- Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113-117.
- John KK, Brockschmidt FF, Redler S, et al. Genetic variants in CTLA4 are strongly associated with alopecia areata. J Invest Dermatol. 2011;131:1169-1172.
- Megiorni F, Mora B, Maxia C, et al. Cytotoxic T-lymphocyte antigen 4 (CTLA4) +49AG and CT60 gene polymorphisms in alopecia areata: a case-control association study in the Italian population. Arch Dermatol Res. 2013;305:665-670
Cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) is a key co-stimulatory receptor expressed on activated T cells that negatively regulates T-cell activation.1-3 It exerts its effects in part by the prevention of IL-2 transcription and inhibition of cell-cycle progression.4 Cytotoxic T-lymphocyte–associated antigen 4 also is expressed by a subset of CD25+CD4+ regulatory T cells (Tregs), where it plays a role in immune tolerance.5 Blockade has demonstrated antitumor activity as well as immune activation, and CTLA-4 dysregulation has been implicated in autoimmune diseases such as alopecia areata (AA).6
Ipilimumab is a fully humanized monoclonal antibody against CTLA-4 and one of a growing class of immune checkpoint inhibitor therapies for metastatic melanoma. Phase 2 and 3 clinical trials have shown an improved survival effect of ipilimumab in patients with advanced melanoma,7-10 with 3-year survival rates ranging from 20.8% to 46.5%.10,11 The US Food and Drug Administration approved ipilimumab in 2011 for treatment of unresectable or metastatic melanoma.12 The most common toxicities of ipilimumab are immune-related adverse effects (irAEs), which represent loss of tolerance to self-antigens.13 Immune-related adverse effects occur in 64.2% of patients,14 with severe or life-threatening irAEs in 17.8% of patients.14 Rates of irAEs appear dose dependent but consistent across increased doses.15 Cutaneous irAEs occur in more than 47% of patients16 and commonly manifest as pruritus with or without a diffuse morbilliform rash,10,17 though less common skin reactions, including vitiligo, vasculitis, and Stevens-Johnson syndrome/toxic epidermal necrolysis, have been documented.9,18
Generalized AA and its more widespread variant, alopecia universalis, have been reported as adverse effects of ipilimumab monotherapy in 2 prior cases in the English-language literature (Table).17,19 Alopecia areata also has been attributed to combination immune checkpoint inhibitor therapy.20,21 We report a case of AA attributable to ipilimumab monotherapy that was localized exclusively to the scalp and remote in onset following treatment.

Case Report
An 88-year-old man with pT3bpN3 nodular melanoma of the back demonstrated multiple lung metastases by positron emission tomography–computed tomography. Lactate dehydrogenase was within reference range, and his Eastern Cooperative Oncology Group performance status was 0 (fully active). One month later, he was started on ipilimumab 3 mg/kg intravenous infusion every 3 weeks for a total of 4 doses. At approximately week 6, his course was complicated by mild fatigue, a faintly erythematous morbilliform rash, and mild pruritus, with laboratory evidence of subclinical hyperthyroidism. Follow-up positron emission tomography–computed tomography at the conclusion of treatment demonstrated complete regression of previously noted hypermetabolic foci. His symptoms and subclinical hyperthyroidism resolved several months later.
Seventeen months after completion of ipilimumab therapy (at age 90 years), the patient’s barber noted new-onset hair loss on the right occipital scalp. Physical examination demonstrated a well-circumscribed patch of nonscarring alopecia (approximately 6 cm) that was clinically consistent with AA (Figure). There were no associated symptoms or other involved areas of hair loss. He denied any personal or family history of AA. The patient’s melanoma has remained in remission to date.

Comment
This case is unique in that AA was localized to a single circumscribed patch on the scalp and occurred nearly 1.5 years after treatment with ipilimumab, which may indicate a robust blockade of CTLA-4 given the remote development of autoimmunity in the setting of persistent remission of melanoma. Although the appearance of AA may be coincidental, onset at 90 years of age would be unusual. The mean age of onset of AA has been reported between 25.2 and 36.3 years,22,23 and its incidence in men older than 60 years is only 6.4 per 100,000 person-years.24
Although AA is a rare irAE of CTLA-4 blockade, the disease has been increasingly linked to CTLA-4 dysregulation in both animal models and humans.6,25,26 A genome-wide association study of 1054 patients with AA and 3278 controls implicated several genes controlling activation and proliferation of Tregs, including CTLA-4.27 More specifically, single-nucleotide polymorphisms of the CTLA-4 gene were found to be associated with AA in a study of 1196 unrelated patients and 1280 controls,28 and Megiorni et al
Given the role of CTLA-4 dysregulation in the pathogenesis of AA, the very low rates of AA in ipilimumab are somewhat surprising, which may represent a reporting bias. Alternatively, there may be sufficient Treg activity to prevent high rates of AA at a lower ipilimumab dose of 3 mg/kg but insufficient activity to prevent development of other irAEs. With US Food and Drug Administration approval of ipilimumab at a higher dose of 10 mg/kg for use as adjuvant therapy for stage III melanomas,12 less common cutaneous irAEs such as AA may be seen with increased frequency. Clinicians planning ipilimumab therapy should discuss this side effect and other potential irAEs with their patients before initiation of treatment.
Cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) is a key co-stimulatory receptor expressed on activated T cells that negatively regulates T-cell activation.1-3 It exerts its effects in part by the prevention of IL-2 transcription and inhibition of cell-cycle progression.4 Cytotoxic T-lymphocyte–associated antigen 4 also is expressed by a subset of CD25+CD4+ regulatory T cells (Tregs), where it plays a role in immune tolerance.5 Blockade has demonstrated antitumor activity as well as immune activation, and CTLA-4 dysregulation has been implicated in autoimmune diseases such as alopecia areata (AA).6
Ipilimumab is a fully humanized monoclonal antibody against CTLA-4 and one of a growing class of immune checkpoint inhibitor therapies for metastatic melanoma. Phase 2 and 3 clinical trials have shown an improved survival effect of ipilimumab in patients with advanced melanoma,7-10 with 3-year survival rates ranging from 20.8% to 46.5%.10,11 The US Food and Drug Administration approved ipilimumab in 2011 for treatment of unresectable or metastatic melanoma.12 The most common toxicities of ipilimumab are immune-related adverse effects (irAEs), which represent loss of tolerance to self-antigens.13 Immune-related adverse effects occur in 64.2% of patients,14 with severe or life-threatening irAEs in 17.8% of patients.14 Rates of irAEs appear dose dependent but consistent across increased doses.15 Cutaneous irAEs occur in more than 47% of patients16 and commonly manifest as pruritus with or without a diffuse morbilliform rash,10,17 though less common skin reactions, including vitiligo, vasculitis, and Stevens-Johnson syndrome/toxic epidermal necrolysis, have been documented.9,18
Generalized AA and its more widespread variant, alopecia universalis, have been reported as adverse effects of ipilimumab monotherapy in 2 prior cases in the English-language literature (Table).17,19 Alopecia areata also has been attributed to combination immune checkpoint inhibitor therapy.20,21 We report a case of AA attributable to ipilimumab monotherapy that was localized exclusively to the scalp and remote in onset following treatment.

Case Report
An 88-year-old man with pT3bpN3 nodular melanoma of the back demonstrated multiple lung metastases by positron emission tomography–computed tomography. Lactate dehydrogenase was within reference range, and his Eastern Cooperative Oncology Group performance status was 0 (fully active). One month later, he was started on ipilimumab 3 mg/kg intravenous infusion every 3 weeks for a total of 4 doses. At approximately week 6, his course was complicated by mild fatigue, a faintly erythematous morbilliform rash, and mild pruritus, with laboratory evidence of subclinical hyperthyroidism. Follow-up positron emission tomography–computed tomography at the conclusion of treatment demonstrated complete regression of previously noted hypermetabolic foci. His symptoms and subclinical hyperthyroidism resolved several months later.
Seventeen months after completion of ipilimumab therapy (at age 90 years), the patient’s barber noted new-onset hair loss on the right occipital scalp. Physical examination demonstrated a well-circumscribed patch of nonscarring alopecia (approximately 6 cm) that was clinically consistent with AA (Figure). There were no associated symptoms or other involved areas of hair loss. He denied any personal or family history of AA. The patient’s melanoma has remained in remission to date.

Comment
This case is unique in that AA was localized to a single circumscribed patch on the scalp and occurred nearly 1.5 years after treatment with ipilimumab, which may indicate a robust blockade of CTLA-4 given the remote development of autoimmunity in the setting of persistent remission of melanoma. Although the appearance of AA may be coincidental, onset at 90 years of age would be unusual. The mean age of onset of AA has been reported between 25.2 and 36.3 years,22,23 and its incidence in men older than 60 years is only 6.4 per 100,000 person-years.24
Although AA is a rare irAE of CTLA-4 blockade, the disease has been increasingly linked to CTLA-4 dysregulation in both animal models and humans.6,25,26 A genome-wide association study of 1054 patients with AA and 3278 controls implicated several genes controlling activation and proliferation of Tregs, including CTLA-4.27 More specifically, single-nucleotide polymorphisms of the CTLA-4 gene were found to be associated with AA in a study of 1196 unrelated patients and 1280 controls,28 and Megiorni et al
Given the role of CTLA-4 dysregulation in the pathogenesis of AA, the very low rates of AA in ipilimumab are somewhat surprising, which may represent a reporting bias. Alternatively, there may be sufficient Treg activity to prevent high rates of AA at a lower ipilimumab dose of 3 mg/kg but insufficient activity to prevent development of other irAEs. With US Food and Drug Administration approval of ipilimumab at a higher dose of 10 mg/kg for use as adjuvant therapy for stage III melanomas,12 less common cutaneous irAEs such as AA may be seen with increased frequency. Clinicians planning ipilimumab therapy should discuss this side effect and other potential irAEs with their patients before initiation of treatment.
- Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267-270.
- Scalapino KJ, Daikh DI. CTLA-4: a key regulatory point in the control of autoimmune disease. Immunol Rev. 2008;223:143-155.
- Buchbinder E, Hodi FS. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J Clin Invest. 2015;125:3377-3383.
- Brunner MC, Chambers CA, Chan FK, et al. CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813-5820.
- Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303-310.
- Carroll JM, McElwee KJ, E King L, et al. Gene array profiling and immunomodulation studies define a cell-mediated immune response underlying the pathogenesis of alopecia areata in a mouse model and humans. J Invest Dermatol. 2002;119:392-402.
- Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591-5598.
- O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712-1717.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522-530.
- Yervoy (ipilimumab)[package insert]. Princeton, NJ: Bristol-Myers Squibb; 2019.
- Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864-872.
- Ibrahim RA, Berman DM, DePril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma [abstract]. J Clin Oncol. 2011;29(suppl):8583.
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155-164.
- Kähler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9:277-286.
- Jaber SH, Cowen EW, Haworth LR, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol. 2006;142:166-172.
- Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8:E537545.
- Assi H, Wilson KS. Immune toxicities and long remission duration after ipilimumab therapy for metastatic melanoma: two illustrative cases. Curr Oncol. 2013;20:E165-E169.
- Zarbo A, Belum VR, Sibaud V, et al. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. Br J Dermatol. 2017;176:1649-1652.
- Lakhmiri M, Cavelier-Balloy B, Lacoste C, et al. Nivolumab-induced alopecia areata: a reversible factor of good prognosis? JAAD Case Rep. 2018;4:761-765.
- Tan E, Tay YK, Goh CL, et al. The pattern and profile of alopecia areata in Singapore–a study of 219 Asians. Int J Dermatol. 2002;41:748-753.
- Goh C, Finkel M, Christos PJ, et al. Profile of 513 patients with alopecia areata: associations of disease subtypes with atopy, autoimmune disease and positive family history. J Eur Acad Dermatol Venereol. 2006;20:1055-1060.
- Mirzoyev SA, Schrum AG, Davis MD, et al. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Zöller M, McElwee KJ, Engel P, et al. Transient CD44 variant isoform expression and reduction in CD4(+)/CD25(+) regulatory T cells in C3H/HeJ mice with alopecia areata. J Invest Dermatol. 2002;118:983-992.
- Zöller M, McElwee KJ, Vitacolonna M, et al. The progressive state, in contrast to the stable or regressive state of alopecia areata, is reflected in peripheral blood mononuclear cells. Exp Dermatol. 2004;13:435-444.
- Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113-117.
- John KK, Brockschmidt FF, Redler S, et al. Genetic variants in CTLA4 are strongly associated with alopecia areata. J Invest Dermatol. 2011;131:1169-1172.
- Megiorni F, Mora B, Maxia C, et al. Cytotoxic T-lymphocyte antigen 4 (CTLA4) +49AG and CT60 gene polymorphisms in alopecia areata: a case-control association study in the Italian population. Arch Dermatol Res. 2013;305:665-670
- Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267-270.
- Scalapino KJ, Daikh DI. CTLA-4: a key regulatory point in the control of autoimmune disease. Immunol Rev. 2008;223:143-155.
- Buchbinder E, Hodi FS. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J Clin Invest. 2015;125:3377-3383.
- Brunner MC, Chambers CA, Chan FK, et al. CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813-5820.
- Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303-310.
- Carroll JM, McElwee KJ, E King L, et al. Gene array profiling and immunomodulation studies define a cell-mediated immune response underlying the pathogenesis of alopecia areata in a mouse model and humans. J Invest Dermatol. 2002;119:392-402.
- Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591-5598.
- O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712-1717.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517-2526.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522-530.
- Yervoy (ipilimumab)[package insert]. Princeton, NJ: Bristol-Myers Squibb; 2019.
- Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12:864-872.
- Ibrahim RA, Berman DM, DePril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma [abstract]. J Clin Oncol. 2011;29(suppl):8583.
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155-164.
- Kähler KC, Hauschild A. Treatment and side effect management of CTLA-4 antibody therapy in metastatic melanoma. J Dtsch Dermatol Ges. 2011;9:277-286.
- Jaber SH, Cowen EW, Haworth LR, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol. 2006;142:166-172.
- Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8:E537545.
- Assi H, Wilson KS. Immune toxicities and long remission duration after ipilimumab therapy for metastatic melanoma: two illustrative cases. Curr Oncol. 2013;20:E165-E169.
- Zarbo A, Belum VR, Sibaud V, et al. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. Br J Dermatol. 2017;176:1649-1652.
- Lakhmiri M, Cavelier-Balloy B, Lacoste C, et al. Nivolumab-induced alopecia areata: a reversible factor of good prognosis? JAAD Case Rep. 2018;4:761-765.
- Tan E, Tay YK, Goh CL, et al. The pattern and profile of alopecia areata in Singapore–a study of 219 Asians. Int J Dermatol. 2002;41:748-753.
- Goh C, Finkel M, Christos PJ, et al. Profile of 513 patients with alopecia areata: associations of disease subtypes with atopy, autoimmune disease and positive family history. J Eur Acad Dermatol Venereol. 2006;20:1055-1060.
- Mirzoyev SA, Schrum AG, Davis MD, et al. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Zöller M, McElwee KJ, Engel P, et al. Transient CD44 variant isoform expression and reduction in CD4(+)/CD25(+) regulatory T cells in C3H/HeJ mice with alopecia areata. J Invest Dermatol. 2002;118:983-992.
- Zöller M, McElwee KJ, Vitacolonna M, et al. The progressive state, in contrast to the stable or regressive state of alopecia areata, is reflected in peripheral blood mononuclear cells. Exp Dermatol. 2004;13:435-444.
- Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113-117.
- John KK, Brockschmidt FF, Redler S, et al. Genetic variants in CTLA4 are strongly associated with alopecia areata. J Invest Dermatol. 2011;131:1169-1172.
- Megiorni F, Mora B, Maxia C, et al. Cytotoxic T-lymphocyte antigen 4 (CTLA4) +49AG and CT60 gene polymorphisms in alopecia areata: a case-control association study in the Italian population. Arch Dermatol Res. 2013;305:665-670
Practice Points
- Cutaneous immune-related adverse effects (irAEs) are among the most common adverse effects of ipilimumab, a fully humanized monoclonal antibody directed against cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) used to treat advanced-stage melanoma.
- Alopecia areata is a rarely reported irAE, but its connection to CTLA-4 dysregulation may mean that clinicians see an increased incidence at higher ipilimumab doses.
Military Health Care at a Crossroads
The certainty that federal health care will be different, and the equal uncertainty about when and how the systems will evolve, were major topics at the recent AMSUS annual meeting. The Veterans Health Administration (VHA) and Military Health System (MHS) are in the midst of major transformations, although they are at very different points in the process and the final outcomes are yet unknown. This editorial, written at the end of 2019, will review some of the highlights of a discussion that is sure to continue in 2020 and beyond.
Almost everyone in the VA and many of the public can pinpoint the exact place (and time) the VHA’s upheaval began: Phoenix, Arizona, in 2014. “The attack on our system,” as VHA Executive in Charge Richard A. Stone, MD, described it at AMSUS, happened because “we were just too slow a bureaucracy,” he explained.1 “We can debate how many veterans died while waiting for care, but the answer is that 1 was too many and it had to be fixed. We had to become a more agile organization.”
The US Department of Veterans Affairs (VA) response to the media firestorm and congressional outrage was uncharacteristically swift and sweeping. Both the VA Secretary and Deputy Under Secretary of Health were removed, as were many others in leadership at Phoenix and elsewhere. The VA faced an existential crisis as many loud voices called for dismantling the entire system in the wake of its perceived inability or unwillingness to care for those it was legally mandated to serve.2 The Veterans’ Access to Care through Choice, Accountability, and Transparency Act of 2014 and its successor the VA Mission Act of 2018 dramatically expanded veterans’ access to covered health care from non-VA health care providers (HCPs).
Debate continues in the veteran community and the wider society about whether this expansion constitutes an abandonment of a health care system dedicated to veterans and their unique health problems or a commitment to deliver the most efficient and high-quality care to veterans that can be obtained.3-5 Many see this as a crossroads for the VA. Still, even if the VA will continue to exist, the question remains: in what form?
The increased use of private sector HCPs has wrought significant and long-lasting modifications to the traditional VA organization. In fiscal year (FY) 2017, the VA paid for care that non-VA HCPs provided for 24% of patients.6 Veterans with higher service-connected disability ratings and aged > 65 years were more likely to rely on the VA for care than were less disabled and younger patients.6 The Mission Act is expected to increase the VA expenditures by nearly $19 billion between FY 2019 and FY 2023, with the bulk of the patients still going to the VHA for their care.6 Stakeholders from unions to politicians are concerned that every dollar spent on community care is one less they can spend in VA institutions. It is unclear to what degree this concern will be actualized, as smaller hospitals and those in rural areas have always had contact with the private sector to obtain the specialty care veterans needed that the VA could not provide.
Compounding these trends is the VA’s ongoing staffing challenges. To meet the demand and eliminate wait times between September 2014 and September 2018, the VHA grew its workforce by > 40,000 individuals, a 13% growth rate. In FY 2019 alone, the VHA hired 28,000 new employees. And yet despite the rapid growth, a lower than average turnover rate, and relatively high employee satisfaction measures (at least when compared with those of other federal employees), the VHA still has 43,000 vacancies.7,8
Which brings us to the very different set of challenges facing the Defense Health Agency (DHA). In an era of ballooning military budgets the DHA is being asked to “transform the MHS into an integrated readiness and health system, eliminate redundancies, and create a common high-quality experience for our beneficiaries.”9 The seeds of change were tucked into the National Defense Authorization Act (NDAA) of 2017, and their ramifications are only now becoming apparent. Among the most consequential of these changes are transfer of the management of hundreds of MHS hospitals and clinics from the medical services of the Army, Navy, and Air Force to the DHA.
“If we don’t shape our future, others will step in and do it for us,” Tom McCaffrey, Assistant Secretary of Defense for Health Affairs explained at AMSUS.10 In October 2019, DoD transitioned the first group of facilities to the DHA, and the remainder will change management by the end of 2022. In the next step of the process, facilities will be combined—along with TRICARE providers—in 21 geographically based “markets” to streamline management and avoid “redundancies.”
Lost in the bland language, though, is the scale of the contemplated changes. Although the exact shape of the changes have not been finalized, up to 18,000 MHS health care providers—civilian or uniformed—may be eliminated as DHA relies more heavily on TRICARE providers.11 Not even the future of the Uniformed Service University for the Health Sciences and its leadership training and health care research are guaranteed.12 The ominous possibility that the nation could lose its only military medical school has raised alarm among medical educators. They fear that the country may sacrifice its ability to train physicians with the highly skilled specialities needed on the battlefield and the familiarity with military culture that enables doctors in uniform to relate to the problems of active-duty families and retired service members.12VHA and MHS colleagues are undergoing a similar organizational transition with all the trepidation and expectation that accompanies the turning of an enormous ship in stormy seas. In the midst of these major institutional transformations, VHA and MHS need to band together if the unique specialty of military and VA medicine is to survive. Unless these unprecedented changes can establish a new spirit of solidarity to 2 often separate partners in one mission to care for those who serve, we may well be asking in the next few years, “Where have all the federal practitioners gone?”
1. Stone R. Plenary session. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
2. Lane C. Why don’t we just abolish the VA? Washington Post. April 22, 2015. https://www.washingtonpost.com/opinions/caring-for-veterans-is-our-national-responsibility/2015/04/22/ae61eb88-e929-11e4-aae1-d642717d8afa_story.html. Accessed December 18, 2019.
3. Lemle RB. Choice Program expansion jeopardizes high-quality VHA mental health services. Fed Pract. 2018;35(3):18-24.
4. Shulkin D. Implications for Veterans’ health care: the danger becomes clearer. JAMA Intern Med. 2019;10.1001/jamainternmed.2019.2996. [Published online ahead of print, 2019 Jul 22.]
5. Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a blueprint for helping veterans make the most of new choices. J Gen Intern Med. 2019;10.1007/s11606-019-05404-w. [Published online ahead of print, 2019 Oct 24.]
6. Statement of Merideth Randles, FSA, MAAA Principal and Consulting Actuary, Milliman, Inc. For Presentation Before the Senate Committee on Veterans’ Affairs. VA Mission Act: Implementing the Veterans Community Care Program. https://www.veterans.senate.gov/imo/media/doc/04.10.19%20Milliman%20Testimony.pdf. Submitted April 10, 2019. Accessed December 18, 2019.
7. Sitterly DR. Statement of Daniel R. Sitterly, Assistant Secretary, Office of Human Resources and Administration/Operations Security, and Preparedness, on behalf of U.S. Department of Veterans Affairs Before the House Committee on Veterans Affairs, September 18, 2019. https://docs.house.gov/meetings/VR/VR00/20190918/109925/HHRG-116-VR00-Wstate-SitterlyD-20190918.pdf. Published September 18, 2019. Accessed December 22, 2019.
8. US Office of Personnel Management, FedScope. Federal workforce data. https://www.fedscope.opm.gov. Accessed December 22, 2019.
9. US Department of Defense. Defense Health Program Fiscal Year (FY) 2020 President’s Budget Operation and Maintenance Introductory Statement. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2020/budget_justification/pdfs/09_Defense_Health_Program/Vol_I_Sec_1_PBA-19_Introductory_Statement_DHP_PB20.pdf. Accessed December 23, 2019.
10. McCaffery T. MHS vision. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
11. Sternberg S. Military Health System in the crosshairs. https://www.usnews.com/news/health-news/articles/2019-12-11/military-health-system-in-the-crosshairs. Published December 11, 2019. Accessed December 23, 2019.
12. Novak D. Officials warn Pentagon cuts could force closing of Bethesda military medical university. https://cnsmaryland.org/2019/11/20/officials-warn-pentagon-cuts-could-force-closing-of-bethesda-military-medical-university. Published November 20, 2019. Accessed December 23, 2019.
The certainty that federal health care will be different, and the equal uncertainty about when and how the systems will evolve, were major topics at the recent AMSUS annual meeting. The Veterans Health Administration (VHA) and Military Health System (MHS) are in the midst of major transformations, although they are at very different points in the process and the final outcomes are yet unknown. This editorial, written at the end of 2019, will review some of the highlights of a discussion that is sure to continue in 2020 and beyond.
Almost everyone in the VA and many of the public can pinpoint the exact place (and time) the VHA’s upheaval began: Phoenix, Arizona, in 2014. “The attack on our system,” as VHA Executive in Charge Richard A. Stone, MD, described it at AMSUS, happened because “we were just too slow a bureaucracy,” he explained.1 “We can debate how many veterans died while waiting for care, but the answer is that 1 was too many and it had to be fixed. We had to become a more agile organization.”
The US Department of Veterans Affairs (VA) response to the media firestorm and congressional outrage was uncharacteristically swift and sweeping. Both the VA Secretary and Deputy Under Secretary of Health were removed, as were many others in leadership at Phoenix and elsewhere. The VA faced an existential crisis as many loud voices called for dismantling the entire system in the wake of its perceived inability or unwillingness to care for those it was legally mandated to serve.2 The Veterans’ Access to Care through Choice, Accountability, and Transparency Act of 2014 and its successor the VA Mission Act of 2018 dramatically expanded veterans’ access to covered health care from non-VA health care providers (HCPs).
Debate continues in the veteran community and the wider society about whether this expansion constitutes an abandonment of a health care system dedicated to veterans and their unique health problems or a commitment to deliver the most efficient and high-quality care to veterans that can be obtained.3-5 Many see this as a crossroads for the VA. Still, even if the VA will continue to exist, the question remains: in what form?
The increased use of private sector HCPs has wrought significant and long-lasting modifications to the traditional VA organization. In fiscal year (FY) 2017, the VA paid for care that non-VA HCPs provided for 24% of patients.6 Veterans with higher service-connected disability ratings and aged > 65 years were more likely to rely on the VA for care than were less disabled and younger patients.6 The Mission Act is expected to increase the VA expenditures by nearly $19 billion between FY 2019 and FY 2023, with the bulk of the patients still going to the VHA for their care.6 Stakeholders from unions to politicians are concerned that every dollar spent on community care is one less they can spend in VA institutions. It is unclear to what degree this concern will be actualized, as smaller hospitals and those in rural areas have always had contact with the private sector to obtain the specialty care veterans needed that the VA could not provide.
Compounding these trends is the VA’s ongoing staffing challenges. To meet the demand and eliminate wait times between September 2014 and September 2018, the VHA grew its workforce by > 40,000 individuals, a 13% growth rate. In FY 2019 alone, the VHA hired 28,000 new employees. And yet despite the rapid growth, a lower than average turnover rate, and relatively high employee satisfaction measures (at least when compared with those of other federal employees), the VHA still has 43,000 vacancies.7,8
Which brings us to the very different set of challenges facing the Defense Health Agency (DHA). In an era of ballooning military budgets the DHA is being asked to “transform the MHS into an integrated readiness and health system, eliminate redundancies, and create a common high-quality experience for our beneficiaries.”9 The seeds of change were tucked into the National Defense Authorization Act (NDAA) of 2017, and their ramifications are only now becoming apparent. Among the most consequential of these changes are transfer of the management of hundreds of MHS hospitals and clinics from the medical services of the Army, Navy, and Air Force to the DHA.
“If we don’t shape our future, others will step in and do it for us,” Tom McCaffrey, Assistant Secretary of Defense for Health Affairs explained at AMSUS.10 In October 2019, DoD transitioned the first group of facilities to the DHA, and the remainder will change management by the end of 2022. In the next step of the process, facilities will be combined—along with TRICARE providers—in 21 geographically based “markets” to streamline management and avoid “redundancies.”
Lost in the bland language, though, is the scale of the contemplated changes. Although the exact shape of the changes have not been finalized, up to 18,000 MHS health care providers—civilian or uniformed—may be eliminated as DHA relies more heavily on TRICARE providers.11 Not even the future of the Uniformed Service University for the Health Sciences and its leadership training and health care research are guaranteed.12 The ominous possibility that the nation could lose its only military medical school has raised alarm among medical educators. They fear that the country may sacrifice its ability to train physicians with the highly skilled specialities needed on the battlefield and the familiarity with military culture that enables doctors in uniform to relate to the problems of active-duty families and retired service members.12VHA and MHS colleagues are undergoing a similar organizational transition with all the trepidation and expectation that accompanies the turning of an enormous ship in stormy seas. In the midst of these major institutional transformations, VHA and MHS need to band together if the unique specialty of military and VA medicine is to survive. Unless these unprecedented changes can establish a new spirit of solidarity to 2 often separate partners in one mission to care for those who serve, we may well be asking in the next few years, “Where have all the federal practitioners gone?”
The certainty that federal health care will be different, and the equal uncertainty about when and how the systems will evolve, were major topics at the recent AMSUS annual meeting. The Veterans Health Administration (VHA) and Military Health System (MHS) are in the midst of major transformations, although they are at very different points in the process and the final outcomes are yet unknown. This editorial, written at the end of 2019, will review some of the highlights of a discussion that is sure to continue in 2020 and beyond.
Almost everyone in the VA and many of the public can pinpoint the exact place (and time) the VHA’s upheaval began: Phoenix, Arizona, in 2014. “The attack on our system,” as VHA Executive in Charge Richard A. Stone, MD, described it at AMSUS, happened because “we were just too slow a bureaucracy,” he explained.1 “We can debate how many veterans died while waiting for care, but the answer is that 1 was too many and it had to be fixed. We had to become a more agile organization.”
The US Department of Veterans Affairs (VA) response to the media firestorm and congressional outrage was uncharacteristically swift and sweeping. Both the VA Secretary and Deputy Under Secretary of Health were removed, as were many others in leadership at Phoenix and elsewhere. The VA faced an existential crisis as many loud voices called for dismantling the entire system in the wake of its perceived inability or unwillingness to care for those it was legally mandated to serve.2 The Veterans’ Access to Care through Choice, Accountability, and Transparency Act of 2014 and its successor the VA Mission Act of 2018 dramatically expanded veterans’ access to covered health care from non-VA health care providers (HCPs).
Debate continues in the veteran community and the wider society about whether this expansion constitutes an abandonment of a health care system dedicated to veterans and their unique health problems or a commitment to deliver the most efficient and high-quality care to veterans that can be obtained.3-5 Many see this as a crossroads for the VA. Still, even if the VA will continue to exist, the question remains: in what form?
The increased use of private sector HCPs has wrought significant and long-lasting modifications to the traditional VA organization. In fiscal year (FY) 2017, the VA paid for care that non-VA HCPs provided for 24% of patients.6 Veterans with higher service-connected disability ratings and aged > 65 years were more likely to rely on the VA for care than were less disabled and younger patients.6 The Mission Act is expected to increase the VA expenditures by nearly $19 billion between FY 2019 and FY 2023, with the bulk of the patients still going to the VHA for their care.6 Stakeholders from unions to politicians are concerned that every dollar spent on community care is one less they can spend in VA institutions. It is unclear to what degree this concern will be actualized, as smaller hospitals and those in rural areas have always had contact with the private sector to obtain the specialty care veterans needed that the VA could not provide.
Compounding these trends is the VA’s ongoing staffing challenges. To meet the demand and eliminate wait times between September 2014 and September 2018, the VHA grew its workforce by > 40,000 individuals, a 13% growth rate. In FY 2019 alone, the VHA hired 28,000 new employees. And yet despite the rapid growth, a lower than average turnover rate, and relatively high employee satisfaction measures (at least when compared with those of other federal employees), the VHA still has 43,000 vacancies.7,8
Which brings us to the very different set of challenges facing the Defense Health Agency (DHA). In an era of ballooning military budgets the DHA is being asked to “transform the MHS into an integrated readiness and health system, eliminate redundancies, and create a common high-quality experience for our beneficiaries.”9 The seeds of change were tucked into the National Defense Authorization Act (NDAA) of 2017, and their ramifications are only now becoming apparent. Among the most consequential of these changes are transfer of the management of hundreds of MHS hospitals and clinics from the medical services of the Army, Navy, and Air Force to the DHA.
“If we don’t shape our future, others will step in and do it for us,” Tom McCaffrey, Assistant Secretary of Defense for Health Affairs explained at AMSUS.10 In October 2019, DoD transitioned the first group of facilities to the DHA, and the remainder will change management by the end of 2022. In the next step of the process, facilities will be combined—along with TRICARE providers—in 21 geographically based “markets” to streamline management and avoid “redundancies.”
Lost in the bland language, though, is the scale of the contemplated changes. Although the exact shape of the changes have not been finalized, up to 18,000 MHS health care providers—civilian or uniformed—may be eliminated as DHA relies more heavily on TRICARE providers.11 Not even the future of the Uniformed Service University for the Health Sciences and its leadership training and health care research are guaranteed.12 The ominous possibility that the nation could lose its only military medical school has raised alarm among medical educators. They fear that the country may sacrifice its ability to train physicians with the highly skilled specialities needed on the battlefield and the familiarity with military culture that enables doctors in uniform to relate to the problems of active-duty families and retired service members.12VHA and MHS colleagues are undergoing a similar organizational transition with all the trepidation and expectation that accompanies the turning of an enormous ship in stormy seas. In the midst of these major institutional transformations, VHA and MHS need to band together if the unique specialty of military and VA medicine is to survive. Unless these unprecedented changes can establish a new spirit of solidarity to 2 often separate partners in one mission to care for those who serve, we may well be asking in the next few years, “Where have all the federal practitioners gone?”
1. Stone R. Plenary session. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
2. Lane C. Why don’t we just abolish the VA? Washington Post. April 22, 2015. https://www.washingtonpost.com/opinions/caring-for-veterans-is-our-national-responsibility/2015/04/22/ae61eb88-e929-11e4-aae1-d642717d8afa_story.html. Accessed December 18, 2019.
3. Lemle RB. Choice Program expansion jeopardizes high-quality VHA mental health services. Fed Pract. 2018;35(3):18-24.
4. Shulkin D. Implications for Veterans’ health care: the danger becomes clearer. JAMA Intern Med. 2019;10.1001/jamainternmed.2019.2996. [Published online ahead of print, 2019 Jul 22.]
5. Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a blueprint for helping veterans make the most of new choices. J Gen Intern Med. 2019;10.1007/s11606-019-05404-w. [Published online ahead of print, 2019 Oct 24.]
6. Statement of Merideth Randles, FSA, MAAA Principal and Consulting Actuary, Milliman, Inc. For Presentation Before the Senate Committee on Veterans’ Affairs. VA Mission Act: Implementing the Veterans Community Care Program. https://www.veterans.senate.gov/imo/media/doc/04.10.19%20Milliman%20Testimony.pdf. Submitted April 10, 2019. Accessed December 18, 2019.
7. Sitterly DR. Statement of Daniel R. Sitterly, Assistant Secretary, Office of Human Resources and Administration/Operations Security, and Preparedness, on behalf of U.S. Department of Veterans Affairs Before the House Committee on Veterans Affairs, September 18, 2019. https://docs.house.gov/meetings/VR/VR00/20190918/109925/HHRG-116-VR00-Wstate-SitterlyD-20190918.pdf. Published September 18, 2019. Accessed December 22, 2019.
8. US Office of Personnel Management, FedScope. Federal workforce data. https://www.fedscope.opm.gov. Accessed December 22, 2019.
9. US Department of Defense. Defense Health Program Fiscal Year (FY) 2020 President’s Budget Operation and Maintenance Introductory Statement. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2020/budget_justification/pdfs/09_Defense_Health_Program/Vol_I_Sec_1_PBA-19_Introductory_Statement_DHP_PB20.pdf. Accessed December 23, 2019.
10. McCaffery T. MHS vision. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
11. Sternberg S. Military Health System in the crosshairs. https://www.usnews.com/news/health-news/articles/2019-12-11/military-health-system-in-the-crosshairs. Published December 11, 2019. Accessed December 23, 2019.
12. Novak D. Officials warn Pentagon cuts could force closing of Bethesda military medical university. https://cnsmaryland.org/2019/11/20/officials-warn-pentagon-cuts-could-force-closing-of-bethesda-military-medical-university. Published November 20, 2019. Accessed December 23, 2019.
1. Stone R. Plenary session. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
2. Lane C. Why don’t we just abolish the VA? Washington Post. April 22, 2015. https://www.washingtonpost.com/opinions/caring-for-veterans-is-our-national-responsibility/2015/04/22/ae61eb88-e929-11e4-aae1-d642717d8afa_story.html. Accessed December 18, 2019.
3. Lemle RB. Choice Program expansion jeopardizes high-quality VHA mental health services. Fed Pract. 2018;35(3):18-24.
4. Shulkin D. Implications for Veterans’ health care: the danger becomes clearer. JAMA Intern Med. 2019;10.1001/jamainternmed.2019.2996. [Published online ahead of print, 2019 Jul 22.]
5. Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a blueprint for helping veterans make the most of new choices. J Gen Intern Med. 2019;10.1007/s11606-019-05404-w. [Published online ahead of print, 2019 Oct 24.]
6. Statement of Merideth Randles, FSA, MAAA Principal and Consulting Actuary, Milliman, Inc. For Presentation Before the Senate Committee on Veterans’ Affairs. VA Mission Act: Implementing the Veterans Community Care Program. https://www.veterans.senate.gov/imo/media/doc/04.10.19%20Milliman%20Testimony.pdf. Submitted April 10, 2019. Accessed December 18, 2019.
7. Sitterly DR. Statement of Daniel R. Sitterly, Assistant Secretary, Office of Human Resources and Administration/Operations Security, and Preparedness, on behalf of U.S. Department of Veterans Affairs Before the House Committee on Veterans Affairs, September 18, 2019. https://docs.house.gov/meetings/VR/VR00/20190918/109925/HHRG-116-VR00-Wstate-SitterlyD-20190918.pdf. Published September 18, 2019. Accessed December 22, 2019.
8. US Office of Personnel Management, FedScope. Federal workforce data. https://www.fedscope.opm.gov. Accessed December 22, 2019.
9. US Department of Defense. Defense Health Program Fiscal Year (FY) 2020 President’s Budget Operation and Maintenance Introductory Statement. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2020/budget_justification/pdfs/09_Defense_Health_Program/Vol_I_Sec_1_PBA-19_Introductory_Statement_DHP_PB20.pdf. Accessed December 23, 2019.
10. McCaffery T. MHS vision. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
11. Sternberg S. Military Health System in the crosshairs. https://www.usnews.com/news/health-news/articles/2019-12-11/military-health-system-in-the-crosshairs. Published December 11, 2019. Accessed December 23, 2019.
12. Novak D. Officials warn Pentagon cuts could force closing of Bethesda military medical university. https://cnsmaryland.org/2019/11/20/officials-warn-pentagon-cuts-could-force-closing-of-bethesda-military-medical-university. Published November 20, 2019. Accessed December 23, 2019.
Endoscopy in a do-not-resuscitate patient: Practical and ethical considerations
Editor’s Note: I am very excited to introduce a section to The New Gastroenterologist that will address topics in clinical medical ethics we frequently face as gastroenterologists. There are several inherent ethical issues in gastroenterology that are not often explicitly discussed, such as periprocedural code status, informed consent, transplantation, performance of endoscopy in the critically ill, and nutrition support in the setting of end of life care. Often the most difficult decisions we make as clinicians are fraught with ethical implications which can be daunting and difficult to navigate. The goal of this section is to address these issues in a case-based format to offer some guidance to young gastroenterologists grappling with similar scenarios.
This month’s issue features the inaugural piece for this series, written by Dr. Lauren Feld (University of Washington), which discusses a clinical scenario in which a patient with a preexisting do-not-resuscitate (DNR) order is about to undergo endoscopy. The article provides a systematic approach to periprocedural code status and highlights existing guidelines that are generally not well known among gastroenterologists.
Vijaya L. Rao, MD
Editor in Chief
An 89-year old female with history of heart failure with reduced ejection fraction, chronic obstructive pulmonary disease, and dementia is admitted to the intensive care unit (ICU) with melena and acute post-hemorrhagic anemia. The family member designated as the patient’s power of attorney (POA) agrees that her code status upon admission will be do-not-resuscitate and do-not-intubate (DNR/DNI) without plan for invasive procedures. However, she has continued overt bleeding with concomitant hemodynamic instability. The POA and ICU team are now asking for urgent endoscopic evaluation, but do not agree to temporary code reversal for the duration of the procedure.
This vignette highlights an important distinction between a patient’s goals of care and the code status. While these two terms are often erroneously used interchangeably, “code status” refers to a patient’s wishes in the event of cardiopulmonary arrest, while “goals of care” refers to a more comprehensive understanding of what care fits within a patient’s values. Patients or their families may still desire interventions such as procedures, but not wish to have a resuscitation attempt in the event of cardiopulmonary arrest. This leads to the commonly encountered clinical scenario in which a patient planning to undergo endoscopy has an active DNR order.
Frequently, DNR orders are temporarily rescinded prior to invasive procedures. There are several reasons this occurs. First, patients or decision makers may decide that the improved rates of survival in intraprocedural arrests changes their risk-benefit assessment about resuscitation procedures. Secondly, proceduralists may feel an ethical duty to resuscitate a patient if the cause of the arrest is considered iatrogenic and potentially reversible. In addition, proceduralists may worry about legal or professional risk if a patient suffers cardiopulmonary arrest during a procedure and an attempt at resuscitation does not occur.
While this is a frequently encountered clinical scenario, there is wide variation in clinical practice. This variation led to the creation of guidelines set forth by the American Society of Anesthesiologists in 1993 and subsequently adopted by the American College of Surgeons. These guidelines recommend a discussion between the physician and the patient prior to the procedure, utilizing shared decision-making around three options: 1) a full attempt at resuscitation; 2) a limited attempt at resuscitation defined with regard to specific procedures; and 3) a limited attempt at resuscitation defined with regard to the patient’s goals and values.
However, these guidelines are both not well known and frequently not applied amongst clinicians and ancillary staff. Patients are frequently told that they must reverse their DNR order to full code prior to undergoing endoscopy. Dissemination of a systematic approach to a patient with a DNR order who requires endoscopy is important to ensure patients have autonomy over their medical decision-making, while also ensuring that health care professionals feel comfortable with their decisions.
The first step when encountering this scenario is to ensure that the procedure is indicated in this particular patient. While guidelines and algorithms have a substantial role in deciding the appropriate work-up for a presenting complaint such as a presumed upper gastrointestinal bleed, the art of medicine lies in the role of the physicians to decide if an invasive procedure is indicated in their specific patients. This decision should be based on the patients’ presenting clinical scenario, their overall comorbidities, their values, and their goals of care.
As the medical complexity of the patient increases, the risks of the procedure increase and it is ultimately up to the endoscopist to frame the informed consent conversation such that the patient and family understand the potential risks and benefits in their specific case.
With a patient who has a desire to avoid aggressive resuscitation attempts, the physician, patient, and family should weigh the risks and benefits of the procedure, and carefully examine if the indication is sufficient. For the patient outlined in the case, her dementia limits her decision-making capacity, and the clinical team is working with a surrogate decision-maker, her POA, to understand the patient’s wishes and goals. Her POA reports upon admission that invasive procedures may not be in line with her previously expressed values or in her best interest. However, with the development of an acute decompensation due to a presumed GI bleed, a potentially reversible cause, the POA requests an endoscopy to attempt to intervene. Occasionally, a patient with clear goals of care can have a change in these goals when a decompensation occurs. The gastroenterologist should assess if this represents a true desire for invasive procedures, or if this is a response to pressure from other members of the clinical team or family, or if palliative needs are not being met. In this patient, her POA desires an endoscopy because her likely upper GI bleed may be contributing to an acute decompensation, but does not wish for other aggressive measures if she should suffer cardiopulmonary arrest. Although upper endoscopy is a generally safe and well-tolerated procedure, this patient’s cardiopulmonary comorbidities increase the risk of the procedure; therefore, the gastroenterology team should proceed with a candid, detailed discussion of risks, benefits, and alternatives with the patient’s POA.
If the decision is made to proceed with endoscopy, the next step is to address the patient’s code status surrounding the procedure. This conversation should focus on three key goals: 1) allow the physician to gain understanding of the patient or surrogate’s perspectives on goals of care; 2) provide the patient or surrogate with an understanding of the risks and potential outcomes of the procedure, as well as resuscitation options; and 3) ultimately arrive at a mutual consensus regarding the patient’s periprocedural code status. Plans for postprocedural care should also be discussed.
While gastroenterology societies do not have specific guidelines surrounding this situation, there are several steps clinicians can take to ensure patient safety and autonomy are preserved:
- Physicians should avoid one-size-fits-all policies, such as the expectation that patients routinely return to full code for procedures.
- The patient and/or decision-makers should have a discussion regarding the risks during the procedure and potential reversibility of these risks.
- The patient should be presented with the option to either reverse to full code, refuse specific resuscitative measures such as defibrillation or intubation, or be allowed to explain his or her own views on goals of care and allow the procedural team to use their clinical judgment should an emergency arise.
- Physicians should be specific regarding the duration of the code status change. For example, in a patient who has reversed the code status to allow a full resuscitation attempt, the team and patient should discuss how long the patient will remain intubated after the procedure.
- This discussion should be documented carefully in the chart to assist with dissemination amongst the medical team.
This process will ensure that clear guidelines are defined such that everyone, including the patient’s potential decision makers, understand to what they are agreeing.
While physicians and care teams are primarily concerned with providing high-quality and individualized care to patients, it is true that concerns surrounding medicolegal risk are present. Careful informed consent and informed refusal conversations will reduce risk. Indeed, in a patient who has a DNR order, physicians are more likely to be at risk performing resuscitation efforts than withholding them. Communication between patients, families, and physicians remains the foundation for a trusting relationship and decreased litigation risk.
For this patient, engaging her POA in an honest and thorough discussion about her goals of care, as well as the risks of both performing and not performing the upper endoscopy are critical to her care. If her POA wishes to proceed with the procedure and have her remain DNR during the procedure, this should be documented and adhered to. Ultimately, the best outcome for this patient will occur with an individualized risk-benefit assessment and open, frequent communication among the care team and her POA.
Dr. Feld is a gastroenterology and hepatology fellow in the department of gastroenterology and hepatology, University of Washington, Seattle. She has no conflicts of interest.
Editor’s Note: I am very excited to introduce a section to The New Gastroenterologist that will address topics in clinical medical ethics we frequently face as gastroenterologists. There are several inherent ethical issues in gastroenterology that are not often explicitly discussed, such as periprocedural code status, informed consent, transplantation, performance of endoscopy in the critically ill, and nutrition support in the setting of end of life care. Often the most difficult decisions we make as clinicians are fraught with ethical implications which can be daunting and difficult to navigate. The goal of this section is to address these issues in a case-based format to offer some guidance to young gastroenterologists grappling with similar scenarios.
This month’s issue features the inaugural piece for this series, written by Dr. Lauren Feld (University of Washington), which discusses a clinical scenario in which a patient with a preexisting do-not-resuscitate (DNR) order is about to undergo endoscopy. The article provides a systematic approach to periprocedural code status and highlights existing guidelines that are generally not well known among gastroenterologists.
Vijaya L. Rao, MD
Editor in Chief
An 89-year old female with history of heart failure with reduced ejection fraction, chronic obstructive pulmonary disease, and dementia is admitted to the intensive care unit (ICU) with melena and acute post-hemorrhagic anemia. The family member designated as the patient’s power of attorney (POA) agrees that her code status upon admission will be do-not-resuscitate and do-not-intubate (DNR/DNI) without plan for invasive procedures. However, she has continued overt bleeding with concomitant hemodynamic instability. The POA and ICU team are now asking for urgent endoscopic evaluation, but do not agree to temporary code reversal for the duration of the procedure.
This vignette highlights an important distinction between a patient’s goals of care and the code status. While these two terms are often erroneously used interchangeably, “code status” refers to a patient’s wishes in the event of cardiopulmonary arrest, while “goals of care” refers to a more comprehensive understanding of what care fits within a patient’s values. Patients or their families may still desire interventions such as procedures, but not wish to have a resuscitation attempt in the event of cardiopulmonary arrest. This leads to the commonly encountered clinical scenario in which a patient planning to undergo endoscopy has an active DNR order.
Frequently, DNR orders are temporarily rescinded prior to invasive procedures. There are several reasons this occurs. First, patients or decision makers may decide that the improved rates of survival in intraprocedural arrests changes their risk-benefit assessment about resuscitation procedures. Secondly, proceduralists may feel an ethical duty to resuscitate a patient if the cause of the arrest is considered iatrogenic and potentially reversible. In addition, proceduralists may worry about legal or professional risk if a patient suffers cardiopulmonary arrest during a procedure and an attempt at resuscitation does not occur.
While this is a frequently encountered clinical scenario, there is wide variation in clinical practice. This variation led to the creation of guidelines set forth by the American Society of Anesthesiologists in 1993 and subsequently adopted by the American College of Surgeons. These guidelines recommend a discussion between the physician and the patient prior to the procedure, utilizing shared decision-making around three options: 1) a full attempt at resuscitation; 2) a limited attempt at resuscitation defined with regard to specific procedures; and 3) a limited attempt at resuscitation defined with regard to the patient’s goals and values.
However, these guidelines are both not well known and frequently not applied amongst clinicians and ancillary staff. Patients are frequently told that they must reverse their DNR order to full code prior to undergoing endoscopy. Dissemination of a systematic approach to a patient with a DNR order who requires endoscopy is important to ensure patients have autonomy over their medical decision-making, while also ensuring that health care professionals feel comfortable with their decisions.
The first step when encountering this scenario is to ensure that the procedure is indicated in this particular patient. While guidelines and algorithms have a substantial role in deciding the appropriate work-up for a presenting complaint such as a presumed upper gastrointestinal bleed, the art of medicine lies in the role of the physicians to decide if an invasive procedure is indicated in their specific patients. This decision should be based on the patients’ presenting clinical scenario, their overall comorbidities, their values, and their goals of care.
As the medical complexity of the patient increases, the risks of the procedure increase and it is ultimately up to the endoscopist to frame the informed consent conversation such that the patient and family understand the potential risks and benefits in their specific case.
With a patient who has a desire to avoid aggressive resuscitation attempts, the physician, patient, and family should weigh the risks and benefits of the procedure, and carefully examine if the indication is sufficient. For the patient outlined in the case, her dementia limits her decision-making capacity, and the clinical team is working with a surrogate decision-maker, her POA, to understand the patient’s wishes and goals. Her POA reports upon admission that invasive procedures may not be in line with her previously expressed values or in her best interest. However, with the development of an acute decompensation due to a presumed GI bleed, a potentially reversible cause, the POA requests an endoscopy to attempt to intervene. Occasionally, a patient with clear goals of care can have a change in these goals when a decompensation occurs. The gastroenterologist should assess if this represents a true desire for invasive procedures, or if this is a response to pressure from other members of the clinical team or family, or if palliative needs are not being met. In this patient, her POA desires an endoscopy because her likely upper GI bleed may be contributing to an acute decompensation, but does not wish for other aggressive measures if she should suffer cardiopulmonary arrest. Although upper endoscopy is a generally safe and well-tolerated procedure, this patient’s cardiopulmonary comorbidities increase the risk of the procedure; therefore, the gastroenterology team should proceed with a candid, detailed discussion of risks, benefits, and alternatives with the patient’s POA.
If the decision is made to proceed with endoscopy, the next step is to address the patient’s code status surrounding the procedure. This conversation should focus on three key goals: 1) allow the physician to gain understanding of the patient or surrogate’s perspectives on goals of care; 2) provide the patient or surrogate with an understanding of the risks and potential outcomes of the procedure, as well as resuscitation options; and 3) ultimately arrive at a mutual consensus regarding the patient’s periprocedural code status. Plans for postprocedural care should also be discussed.
While gastroenterology societies do not have specific guidelines surrounding this situation, there are several steps clinicians can take to ensure patient safety and autonomy are preserved:
- Physicians should avoid one-size-fits-all policies, such as the expectation that patients routinely return to full code for procedures.
- The patient and/or decision-makers should have a discussion regarding the risks during the procedure and potential reversibility of these risks.
- The patient should be presented with the option to either reverse to full code, refuse specific resuscitative measures such as defibrillation or intubation, or be allowed to explain his or her own views on goals of care and allow the procedural team to use their clinical judgment should an emergency arise.
- Physicians should be specific regarding the duration of the code status change. For example, in a patient who has reversed the code status to allow a full resuscitation attempt, the team and patient should discuss how long the patient will remain intubated after the procedure.
- This discussion should be documented carefully in the chart to assist with dissemination amongst the medical team.
This process will ensure that clear guidelines are defined such that everyone, including the patient’s potential decision makers, understand to what they are agreeing.
While physicians and care teams are primarily concerned with providing high-quality and individualized care to patients, it is true that concerns surrounding medicolegal risk are present. Careful informed consent and informed refusal conversations will reduce risk. Indeed, in a patient who has a DNR order, physicians are more likely to be at risk performing resuscitation efforts than withholding them. Communication between patients, families, and physicians remains the foundation for a trusting relationship and decreased litigation risk.
For this patient, engaging her POA in an honest and thorough discussion about her goals of care, as well as the risks of both performing and not performing the upper endoscopy are critical to her care. If her POA wishes to proceed with the procedure and have her remain DNR during the procedure, this should be documented and adhered to. Ultimately, the best outcome for this patient will occur with an individualized risk-benefit assessment and open, frequent communication among the care team and her POA.
Dr. Feld is a gastroenterology and hepatology fellow in the department of gastroenterology and hepatology, University of Washington, Seattle. She has no conflicts of interest.
Editor’s Note: I am very excited to introduce a section to The New Gastroenterologist that will address topics in clinical medical ethics we frequently face as gastroenterologists. There are several inherent ethical issues in gastroenterology that are not often explicitly discussed, such as periprocedural code status, informed consent, transplantation, performance of endoscopy in the critically ill, and nutrition support in the setting of end of life care. Often the most difficult decisions we make as clinicians are fraught with ethical implications which can be daunting and difficult to navigate. The goal of this section is to address these issues in a case-based format to offer some guidance to young gastroenterologists grappling with similar scenarios.
This month’s issue features the inaugural piece for this series, written by Dr. Lauren Feld (University of Washington), which discusses a clinical scenario in which a patient with a preexisting do-not-resuscitate (DNR) order is about to undergo endoscopy. The article provides a systematic approach to periprocedural code status and highlights existing guidelines that are generally not well known among gastroenterologists.
Vijaya L. Rao, MD
Editor in Chief
An 89-year old female with history of heart failure with reduced ejection fraction, chronic obstructive pulmonary disease, and dementia is admitted to the intensive care unit (ICU) with melena and acute post-hemorrhagic anemia. The family member designated as the patient’s power of attorney (POA) agrees that her code status upon admission will be do-not-resuscitate and do-not-intubate (DNR/DNI) without plan for invasive procedures. However, she has continued overt bleeding with concomitant hemodynamic instability. The POA and ICU team are now asking for urgent endoscopic evaluation, but do not agree to temporary code reversal for the duration of the procedure.
This vignette highlights an important distinction between a patient’s goals of care and the code status. While these two terms are often erroneously used interchangeably, “code status” refers to a patient’s wishes in the event of cardiopulmonary arrest, while “goals of care” refers to a more comprehensive understanding of what care fits within a patient’s values. Patients or their families may still desire interventions such as procedures, but not wish to have a resuscitation attempt in the event of cardiopulmonary arrest. This leads to the commonly encountered clinical scenario in which a patient planning to undergo endoscopy has an active DNR order.
Frequently, DNR orders are temporarily rescinded prior to invasive procedures. There are several reasons this occurs. First, patients or decision makers may decide that the improved rates of survival in intraprocedural arrests changes their risk-benefit assessment about resuscitation procedures. Secondly, proceduralists may feel an ethical duty to resuscitate a patient if the cause of the arrest is considered iatrogenic and potentially reversible. In addition, proceduralists may worry about legal or professional risk if a patient suffers cardiopulmonary arrest during a procedure and an attempt at resuscitation does not occur.
While this is a frequently encountered clinical scenario, there is wide variation in clinical practice. This variation led to the creation of guidelines set forth by the American Society of Anesthesiologists in 1993 and subsequently adopted by the American College of Surgeons. These guidelines recommend a discussion between the physician and the patient prior to the procedure, utilizing shared decision-making around three options: 1) a full attempt at resuscitation; 2) a limited attempt at resuscitation defined with regard to specific procedures; and 3) a limited attempt at resuscitation defined with regard to the patient’s goals and values.
However, these guidelines are both not well known and frequently not applied amongst clinicians and ancillary staff. Patients are frequently told that they must reverse their DNR order to full code prior to undergoing endoscopy. Dissemination of a systematic approach to a patient with a DNR order who requires endoscopy is important to ensure patients have autonomy over their medical decision-making, while also ensuring that health care professionals feel comfortable with their decisions.
The first step when encountering this scenario is to ensure that the procedure is indicated in this particular patient. While guidelines and algorithms have a substantial role in deciding the appropriate work-up for a presenting complaint such as a presumed upper gastrointestinal bleed, the art of medicine lies in the role of the physicians to decide if an invasive procedure is indicated in their specific patients. This decision should be based on the patients’ presenting clinical scenario, their overall comorbidities, their values, and their goals of care.
As the medical complexity of the patient increases, the risks of the procedure increase and it is ultimately up to the endoscopist to frame the informed consent conversation such that the patient and family understand the potential risks and benefits in their specific case.
With a patient who has a desire to avoid aggressive resuscitation attempts, the physician, patient, and family should weigh the risks and benefits of the procedure, and carefully examine if the indication is sufficient. For the patient outlined in the case, her dementia limits her decision-making capacity, and the clinical team is working with a surrogate decision-maker, her POA, to understand the patient’s wishes and goals. Her POA reports upon admission that invasive procedures may not be in line with her previously expressed values or in her best interest. However, with the development of an acute decompensation due to a presumed GI bleed, a potentially reversible cause, the POA requests an endoscopy to attempt to intervene. Occasionally, a patient with clear goals of care can have a change in these goals when a decompensation occurs. The gastroenterologist should assess if this represents a true desire for invasive procedures, or if this is a response to pressure from other members of the clinical team or family, or if palliative needs are not being met. In this patient, her POA desires an endoscopy because her likely upper GI bleed may be contributing to an acute decompensation, but does not wish for other aggressive measures if she should suffer cardiopulmonary arrest. Although upper endoscopy is a generally safe and well-tolerated procedure, this patient’s cardiopulmonary comorbidities increase the risk of the procedure; therefore, the gastroenterology team should proceed with a candid, detailed discussion of risks, benefits, and alternatives with the patient’s POA.
If the decision is made to proceed with endoscopy, the next step is to address the patient’s code status surrounding the procedure. This conversation should focus on three key goals: 1) allow the physician to gain understanding of the patient or surrogate’s perspectives on goals of care; 2) provide the patient or surrogate with an understanding of the risks and potential outcomes of the procedure, as well as resuscitation options; and 3) ultimately arrive at a mutual consensus regarding the patient’s periprocedural code status. Plans for postprocedural care should also be discussed.
While gastroenterology societies do not have specific guidelines surrounding this situation, there are several steps clinicians can take to ensure patient safety and autonomy are preserved:
- Physicians should avoid one-size-fits-all policies, such as the expectation that patients routinely return to full code for procedures.
- The patient and/or decision-makers should have a discussion regarding the risks during the procedure and potential reversibility of these risks.
- The patient should be presented with the option to either reverse to full code, refuse specific resuscitative measures such as defibrillation or intubation, or be allowed to explain his or her own views on goals of care and allow the procedural team to use their clinical judgment should an emergency arise.
- Physicians should be specific regarding the duration of the code status change. For example, in a patient who has reversed the code status to allow a full resuscitation attempt, the team and patient should discuss how long the patient will remain intubated after the procedure.
- This discussion should be documented carefully in the chart to assist with dissemination amongst the medical team.
This process will ensure that clear guidelines are defined such that everyone, including the patient’s potential decision makers, understand to what they are agreeing.
While physicians and care teams are primarily concerned with providing high-quality and individualized care to patients, it is true that concerns surrounding medicolegal risk are present. Careful informed consent and informed refusal conversations will reduce risk. Indeed, in a patient who has a DNR order, physicians are more likely to be at risk performing resuscitation efforts than withholding them. Communication between patients, families, and physicians remains the foundation for a trusting relationship and decreased litigation risk.
For this patient, engaging her POA in an honest and thorough discussion about her goals of care, as well as the risks of both performing and not performing the upper endoscopy are critical to her care. If her POA wishes to proceed with the procedure and have her remain DNR during the procedure, this should be documented and adhered to. Ultimately, the best outcome for this patient will occur with an individualized risk-benefit assessment and open, frequent communication among the care team and her POA.
Dr. Feld is a gastroenterology and hepatology fellow in the department of gastroenterology and hepatology, University of Washington, Seattle. She has no conflicts of interest.