User login
Sleep vs. Netflix, and grape juice BPAP
Sleep vs. Netflix: the eternal struggle
Ladies and gentlemen, welcome to Livin’ on the MDedge World Championship Boxing! Tonight, we bring you a classic match-up in the endless battle for your valuable time.
In the red corner, weighing in at a muscular 8 hours, is the defending champion: a good night’s sleep! And now for the challenger in the blue corner, coming in at a strong “just one more episode, I promise,” it’s binge watching!
Oh, sleep opens the match strong: According to a survey from the American Academy of Sleep Medicine, U.S. adults rank sleep as their second-most important priority, with only family beating it out. My goodness, that is a strong opening offensive.
But wait, binge watching is countering! According to the very same survey, 88% of Americans have admitted that they’d lost sleep because they’d stayed up late to watch extra episodes of a TV show or streaming series, a rate that rises to 95% in people aged 18-44 years. Oh dear, sleep looks like it’s in trouble.
Hang on, what’s binge watching doing? It’s unleashing a quick barrage of attacks: 72% of men aged 18-34 reported delaying sleep for video games, two-thirds of U.S. adults reported losing sleep to read a book, and nearly 60% of adults delayed sleep to watch sports. We feel slightly conflicted about our metaphor choice now.
And with a final haymaker from “guess I’ll watch ‘The Office’ for a sixth time,” binge watching has defeated the defending champion! Be sure to tune in next week, when alcohol takes on common sense. A true fight for the ages there.
Lead us not into temptation
Can anyone resist the temptation of binge watching? Can no one swim against the sleep-depriving, show-streaming current? Is resistance to an “Orange Is the New Black” bender futile?
University of Wyoming researchers say there’s hope. Those who would sleep svelte and sound in a world of streaming services and Krispy Kreme must plan ahead to tame temptation.
Proactive temptation management begins long before those chocolate iced glazed with sprinkles appear at the nurses’ station. Planning your response ahead of time increases the odds that the first episode of “Stranger Things” is also the evening’s last episode.
Using psychology’s human lab mice – undergraduate students – the researchers tested five temptation-proofing self-control strategies.
The first strategy: situation selection. If “Game of Thrones” is on in the den, avoid the room as if it were an unmucked House Lannister horse stall. Second: situation modification. Is your spouse hotboxing GoT on an iPad next to you in the bed? Politely suggest that GoT is even better when viewed on the living room sofa.
The third strategy: distraction. Enjoy the wholesome snap of a Finn Crisp while your coworkers destroy those Krispy Kremes like Daenerys leveling King’s Landing. Fourth: reappraisal. Tell yourself that season 2 of “Ozark” can’t surpass season 1, and will simply swindle you of your precious time. And fifth, the Nancy-Reagan, temptation-resistance classic: response inhibition. When offered the narcotic that is “Breaking Bad,” just say no!
Which temptation strategies worked best?
Planning ahead with one through four led fewer Cowboy State undergrads into temptation.
As for responding in the moment? Well, the Krispy Kremes would’ve never lasted past season 2 of “The Great British Baking Show.”
Stuck between a tongue and a hard place
There once was a 7-year-old boy who loved grape juice. He loved grape juice so much that he didn’t want to waste any after drinking a bottle of the stuff.
To get every last drop, he tried to use his tongue to lick the inside of a grape juice bottle. One particular bottle, however, was evil and had other plans. It grabbed his tongue and wouldn’t let go, even after his mother tried to help him.
She took him to the great healing wizards at Auf der Bult Children’s Hospital in Hannover, Germany – which is quite surprising, because they live in New Jersey. [Just kidding, they’re from Hannover – just checking to see if you’re paying attention.]
When their magic wands didn’t work, doctors at the hospital mildly sedated the boy with midazolam and esketamine and then advanced a 70-mm plastic button cannula between the neck of the bottle and his tongue, hoping to release the presumed vacuum. No such luck.
It was at that point that the greatest of all the wizards, Dr. Christoph Eich, a pediatric anesthesiologist at the hospital, remembered having a similar problem with a particularly villainous bottle of “grape juice” during his magical training days some 20 years earlier.
The solution then, he discovered, was to connect the cannula to a syringe and inject air into the bottle to produce positive pressure and force out the foreign object.
Dr. Eich’s reinvention of BPAP (bottle positive airway pressure) worked on the child, who, once the purple discoloration of his tongue faded after 3 days, was none the worse for wear and lived happily ever after.
We’re just wondering if the good doctor told the child’s mother that the original situation involved a bottle of wine that couldn’t be opened because no one had a corkscrew. Well, maybe she reads the European Journal of Anaesthesiology.
Sleep vs. Netflix: the eternal struggle
Ladies and gentlemen, welcome to Livin’ on the MDedge World Championship Boxing! Tonight, we bring you a classic match-up in the endless battle for your valuable time.
In the red corner, weighing in at a muscular 8 hours, is the defending champion: a good night’s sleep! And now for the challenger in the blue corner, coming in at a strong “just one more episode, I promise,” it’s binge watching!
Oh, sleep opens the match strong: According to a survey from the American Academy of Sleep Medicine, U.S. adults rank sleep as their second-most important priority, with only family beating it out. My goodness, that is a strong opening offensive.
But wait, binge watching is countering! According to the very same survey, 88% of Americans have admitted that they’d lost sleep because they’d stayed up late to watch extra episodes of a TV show or streaming series, a rate that rises to 95% in people aged 18-44 years. Oh dear, sleep looks like it’s in trouble.
Hang on, what’s binge watching doing? It’s unleashing a quick barrage of attacks: 72% of men aged 18-34 reported delaying sleep for video games, two-thirds of U.S. adults reported losing sleep to read a book, and nearly 60% of adults delayed sleep to watch sports. We feel slightly conflicted about our metaphor choice now.
And with a final haymaker from “guess I’ll watch ‘The Office’ for a sixth time,” binge watching has defeated the defending champion! Be sure to tune in next week, when alcohol takes on common sense. A true fight for the ages there.
Lead us not into temptation
Can anyone resist the temptation of binge watching? Can no one swim against the sleep-depriving, show-streaming current? Is resistance to an “Orange Is the New Black” bender futile?
University of Wyoming researchers say there’s hope. Those who would sleep svelte and sound in a world of streaming services and Krispy Kreme must plan ahead to tame temptation.
Proactive temptation management begins long before those chocolate iced glazed with sprinkles appear at the nurses’ station. Planning your response ahead of time increases the odds that the first episode of “Stranger Things” is also the evening’s last episode.
Using psychology’s human lab mice – undergraduate students – the researchers tested five temptation-proofing self-control strategies.
The first strategy: situation selection. If “Game of Thrones” is on in the den, avoid the room as if it were an unmucked House Lannister horse stall. Second: situation modification. Is your spouse hotboxing GoT on an iPad next to you in the bed? Politely suggest that GoT is even better when viewed on the living room sofa.
The third strategy: distraction. Enjoy the wholesome snap of a Finn Crisp while your coworkers destroy those Krispy Kremes like Daenerys leveling King’s Landing. Fourth: reappraisal. Tell yourself that season 2 of “Ozark” can’t surpass season 1, and will simply swindle you of your precious time. And fifth, the Nancy-Reagan, temptation-resistance classic: response inhibition. When offered the narcotic that is “Breaking Bad,” just say no!
Which temptation strategies worked best?
Planning ahead with one through four led fewer Cowboy State undergrads into temptation.
As for responding in the moment? Well, the Krispy Kremes would’ve never lasted past season 2 of “The Great British Baking Show.”
Stuck between a tongue and a hard place
There once was a 7-year-old boy who loved grape juice. He loved grape juice so much that he didn’t want to waste any after drinking a bottle of the stuff.
To get every last drop, he tried to use his tongue to lick the inside of a grape juice bottle. One particular bottle, however, was evil and had other plans. It grabbed his tongue and wouldn’t let go, even after his mother tried to help him.
She took him to the great healing wizards at Auf der Bult Children’s Hospital in Hannover, Germany – which is quite surprising, because they live in New Jersey. [Just kidding, they’re from Hannover – just checking to see if you’re paying attention.]
When their magic wands didn’t work, doctors at the hospital mildly sedated the boy with midazolam and esketamine and then advanced a 70-mm plastic button cannula between the neck of the bottle and his tongue, hoping to release the presumed vacuum. No such luck.
It was at that point that the greatest of all the wizards, Dr. Christoph Eich, a pediatric anesthesiologist at the hospital, remembered having a similar problem with a particularly villainous bottle of “grape juice” during his magical training days some 20 years earlier.
The solution then, he discovered, was to connect the cannula to a syringe and inject air into the bottle to produce positive pressure and force out the foreign object.
Dr. Eich’s reinvention of BPAP (bottle positive airway pressure) worked on the child, who, once the purple discoloration of his tongue faded after 3 days, was none the worse for wear and lived happily ever after.
We’re just wondering if the good doctor told the child’s mother that the original situation involved a bottle of wine that couldn’t be opened because no one had a corkscrew. Well, maybe she reads the European Journal of Anaesthesiology.
Sleep vs. Netflix: the eternal struggle
Ladies and gentlemen, welcome to Livin’ on the MDedge World Championship Boxing! Tonight, we bring you a classic match-up in the endless battle for your valuable time.
In the red corner, weighing in at a muscular 8 hours, is the defending champion: a good night’s sleep! And now for the challenger in the blue corner, coming in at a strong “just one more episode, I promise,” it’s binge watching!
Oh, sleep opens the match strong: According to a survey from the American Academy of Sleep Medicine, U.S. adults rank sleep as their second-most important priority, with only family beating it out. My goodness, that is a strong opening offensive.
But wait, binge watching is countering! According to the very same survey, 88% of Americans have admitted that they’d lost sleep because they’d stayed up late to watch extra episodes of a TV show or streaming series, a rate that rises to 95% in people aged 18-44 years. Oh dear, sleep looks like it’s in trouble.
Hang on, what’s binge watching doing? It’s unleashing a quick barrage of attacks: 72% of men aged 18-34 reported delaying sleep for video games, two-thirds of U.S. adults reported losing sleep to read a book, and nearly 60% of adults delayed sleep to watch sports. We feel slightly conflicted about our metaphor choice now.
And with a final haymaker from “guess I’ll watch ‘The Office’ for a sixth time,” binge watching has defeated the defending champion! Be sure to tune in next week, when alcohol takes on common sense. A true fight for the ages there.
Lead us not into temptation
Can anyone resist the temptation of binge watching? Can no one swim against the sleep-depriving, show-streaming current? Is resistance to an “Orange Is the New Black” bender futile?
University of Wyoming researchers say there’s hope. Those who would sleep svelte and sound in a world of streaming services and Krispy Kreme must plan ahead to tame temptation.
Proactive temptation management begins long before those chocolate iced glazed with sprinkles appear at the nurses’ station. Planning your response ahead of time increases the odds that the first episode of “Stranger Things” is also the evening’s last episode.
Using psychology’s human lab mice – undergraduate students – the researchers tested five temptation-proofing self-control strategies.
The first strategy: situation selection. If “Game of Thrones” is on in the den, avoid the room as if it were an unmucked House Lannister horse stall. Second: situation modification. Is your spouse hotboxing GoT on an iPad next to you in the bed? Politely suggest that GoT is even better when viewed on the living room sofa.
The third strategy: distraction. Enjoy the wholesome snap of a Finn Crisp while your coworkers destroy those Krispy Kremes like Daenerys leveling King’s Landing. Fourth: reappraisal. Tell yourself that season 2 of “Ozark” can’t surpass season 1, and will simply swindle you of your precious time. And fifth, the Nancy-Reagan, temptation-resistance classic: response inhibition. When offered the narcotic that is “Breaking Bad,” just say no!
Which temptation strategies worked best?
Planning ahead with one through four led fewer Cowboy State undergrads into temptation.
As for responding in the moment? Well, the Krispy Kremes would’ve never lasted past season 2 of “The Great British Baking Show.”
Stuck between a tongue and a hard place
There once was a 7-year-old boy who loved grape juice. He loved grape juice so much that he didn’t want to waste any after drinking a bottle of the stuff.
To get every last drop, he tried to use his tongue to lick the inside of a grape juice bottle. One particular bottle, however, was evil and had other plans. It grabbed his tongue and wouldn’t let go, even after his mother tried to help him.
She took him to the great healing wizards at Auf der Bult Children’s Hospital in Hannover, Germany – which is quite surprising, because they live in New Jersey. [Just kidding, they’re from Hannover – just checking to see if you’re paying attention.]
When their magic wands didn’t work, doctors at the hospital mildly sedated the boy with midazolam and esketamine and then advanced a 70-mm plastic button cannula between the neck of the bottle and his tongue, hoping to release the presumed vacuum. No such luck.
It was at that point that the greatest of all the wizards, Dr. Christoph Eich, a pediatric anesthesiologist at the hospital, remembered having a similar problem with a particularly villainous bottle of “grape juice” during his magical training days some 20 years earlier.
The solution then, he discovered, was to connect the cannula to a syringe and inject air into the bottle to produce positive pressure and force out the foreign object.
Dr. Eich’s reinvention of BPAP (bottle positive airway pressure) worked on the child, who, once the purple discoloration of his tongue faded after 3 days, was none the worse for wear and lived happily ever after.
We’re just wondering if the good doctor told the child’s mother that the original situation involved a bottle of wine that couldn’t be opened because no one had a corkscrew. Well, maybe she reads the European Journal of Anaesthesiology.
Melanoma incidence continues to increase, yet mortality stabilizing
LAS VEGAS – The according to data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
At the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, Laura Korb Ferris, MD, PhD, said that SEER data project 96,480 new cases of melanoma in 2019, as well as 7,230 deaths from the disease. In 2016, SEER projected 10,130 deaths from melanoma, “so we’re actually projecting a reduction in melanoma deaths,” said Dr. Ferris, director of clinical trials at the University of Pittsburgh Medical Center’s department of dermatology. She added that the death rate from melanoma in 2016 was 2.17 per 100,000 population, a reduction from 2.69 per 100,000 population in 2011, “so it looks like melanoma mortality may be stable,” or even reduced, despite an increase in melanoma incidence.
A study of SEER data between 1989 and 2009 found that melanoma incidence is increasing across all lesion thicknesses (J Natl Cancer Inst. 2015 Nov 12. doi: 10.1093/jnci/djv294). Specifically, the incidence increased most among thin lesions, but there was a smaller increased incidence of thick melanoma. “This suggests that the overall burden of disease is truly increasing, but it is primarily stemming from an increase in T1/T2 disease,” Dr. Ferris said. “This could be due in part to increased early detection.”
Improvements in melanoma-specific survival, she continued, are likely a combination of improved management of T4 disease, a shift toward detection of thinner T1/T2 melanoma, and increased detection of T1/T2 disease.
The SEER data also showed that the incidence of fatal cases of melanoma has decreased since 1989, but only in thick melanomas. This trend may indicate a modest improvement in the management of T4 tumors. “Optimistically, I think increased detection efforts are improving survival by early detection of thin but ultimately fatal melanomas,” Dr. Ferris said. “Hopefully we are finding disease earlier and we are preventing patients from progressing to these fatal T4 melanomas.”
Disparities in melanoma-specific survival also come into play. Men have poorer survival compared with women, whites have the highest survival, and non-Hispanic whites have a better survival than Hispanic whites, Dr. Ferris said, while lower rates of survival are seen in blacks and nonblack minorities, as well as among those in high poverty and those who are separated/nonmarried. Lesion type also matters. The highest survival is seen in those with superficial spreading melanoma, while lower survival is observed in those with nodular melanoma, and acral lentiginous melanoma.
Early detection of thin nodular melanomas has the potential to significantly impact melanoma mortality, “but we want to keep in mind that the majority of ultimately fatal melanomas are superficial spreading melanomas,” Dr. Ferris said. “That is because they are so much more prevalent. As a dermatologist, I think a lot about screening and early detection. Periodic screening is a good strategy for a slower-growing superficial spreading melanoma, but it’s not necessarily a good strategy for a rapidly growing nodular melanoma. That’s going to require better education and better access to health care.”
Self-detection of melanoma is another strategy to consider. According to Dr. Ferris, results from multiple studies suggest that about 50% of all melanomas are detected by patients, but the ones they find tend to be thicker than the ones that clinicians detect during office visits. “It would be great if we can get that number higher than 50%,” Dr. Ferris said. “If patients really understood what melanoma is, what it looks like, and when they needed to seek medical attention, perhaps we could get that over 50% and see self-detection of thinner melanomas. That’s a very low-cost intervention.”
Targeted screening efforts that stratify by risk factors and by age “makes screening more efficient and more cost-effective,” she added. She cited one analysis, which found that clinicians need to screen 606 people and conduct 25 biopsies in order to find one melanoma. “That’s very resource intensive,” she said. “However, if you only screened people 50 or older or 65 or older, the number needed to screen goes down, and because your pretest probability is higher, your number need to biopsy goes down as well. If you factor in things like a history of atypical nevi or a personal history of melanoma, those patients are at a higher risk of developing melanoma.”
Dr. Ferris closed her presentation by noting that Australia leads other countries in melanoma prevention efforts. There, the combined incidence of skin cancer is higher than the incidence of any other type of cancer. Four decades ago, Australian health officials launched SunSmart, a series of initiatives intended to reduce skin cancer. These include implementation of policies for hat wearing and shade provision in schools and at work, availability of more effective sunscreens, inclusion of sun protection items as a tax-deductible expense for outdoor workers, increased availability since the 1980s of long-sleeved sun protective swimwear, a ban on the use of indoor tanning since 2014, provision of UV forecasts in weather, and a comprehensive program of grants for community shade structures (PLoSMed. 2019 Oct 8;16[10]:e1002932).
“One approach to melanoma prevention won’t fit all,” she concluded. “We need to focus on prevention, public education to improve knowledge and self-detection.”
Dr. Ferris disclosed that she is a consultant to and an investigator for DermTech and Scibase. She is also an investigator for Castle Biosciences.
SDEF and this news organization are owned by the same parent company. Dr. Ferris spoke during a forum on cutaneous malignancies at the meeting.
LAS VEGAS – The according to data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
At the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, Laura Korb Ferris, MD, PhD, said that SEER data project 96,480 new cases of melanoma in 2019, as well as 7,230 deaths from the disease. In 2016, SEER projected 10,130 deaths from melanoma, “so we’re actually projecting a reduction in melanoma deaths,” said Dr. Ferris, director of clinical trials at the University of Pittsburgh Medical Center’s department of dermatology. She added that the death rate from melanoma in 2016 was 2.17 per 100,000 population, a reduction from 2.69 per 100,000 population in 2011, “so it looks like melanoma mortality may be stable,” or even reduced, despite an increase in melanoma incidence.
A study of SEER data between 1989 and 2009 found that melanoma incidence is increasing across all lesion thicknesses (J Natl Cancer Inst. 2015 Nov 12. doi: 10.1093/jnci/djv294). Specifically, the incidence increased most among thin lesions, but there was a smaller increased incidence of thick melanoma. “This suggests that the overall burden of disease is truly increasing, but it is primarily stemming from an increase in T1/T2 disease,” Dr. Ferris said. “This could be due in part to increased early detection.”
Improvements in melanoma-specific survival, she continued, are likely a combination of improved management of T4 disease, a shift toward detection of thinner T1/T2 melanoma, and increased detection of T1/T2 disease.
The SEER data also showed that the incidence of fatal cases of melanoma has decreased since 1989, but only in thick melanomas. This trend may indicate a modest improvement in the management of T4 tumors. “Optimistically, I think increased detection efforts are improving survival by early detection of thin but ultimately fatal melanomas,” Dr. Ferris said. “Hopefully we are finding disease earlier and we are preventing patients from progressing to these fatal T4 melanomas.”
Disparities in melanoma-specific survival also come into play. Men have poorer survival compared with women, whites have the highest survival, and non-Hispanic whites have a better survival than Hispanic whites, Dr. Ferris said, while lower rates of survival are seen in blacks and nonblack minorities, as well as among those in high poverty and those who are separated/nonmarried. Lesion type also matters. The highest survival is seen in those with superficial spreading melanoma, while lower survival is observed in those with nodular melanoma, and acral lentiginous melanoma.
Early detection of thin nodular melanomas has the potential to significantly impact melanoma mortality, “but we want to keep in mind that the majority of ultimately fatal melanomas are superficial spreading melanomas,” Dr. Ferris said. “That is because they are so much more prevalent. As a dermatologist, I think a lot about screening and early detection. Periodic screening is a good strategy for a slower-growing superficial spreading melanoma, but it’s not necessarily a good strategy for a rapidly growing nodular melanoma. That’s going to require better education and better access to health care.”
Self-detection of melanoma is another strategy to consider. According to Dr. Ferris, results from multiple studies suggest that about 50% of all melanomas are detected by patients, but the ones they find tend to be thicker than the ones that clinicians detect during office visits. “It would be great if we can get that number higher than 50%,” Dr. Ferris said. “If patients really understood what melanoma is, what it looks like, and when they needed to seek medical attention, perhaps we could get that over 50% and see self-detection of thinner melanomas. That’s a very low-cost intervention.”
Targeted screening efforts that stratify by risk factors and by age “makes screening more efficient and more cost-effective,” she added. She cited one analysis, which found that clinicians need to screen 606 people and conduct 25 biopsies in order to find one melanoma. “That’s very resource intensive,” she said. “However, if you only screened people 50 or older or 65 or older, the number needed to screen goes down, and because your pretest probability is higher, your number need to biopsy goes down as well. If you factor in things like a history of atypical nevi or a personal history of melanoma, those patients are at a higher risk of developing melanoma.”
Dr. Ferris closed her presentation by noting that Australia leads other countries in melanoma prevention efforts. There, the combined incidence of skin cancer is higher than the incidence of any other type of cancer. Four decades ago, Australian health officials launched SunSmart, a series of initiatives intended to reduce skin cancer. These include implementation of policies for hat wearing and shade provision in schools and at work, availability of more effective sunscreens, inclusion of sun protection items as a tax-deductible expense for outdoor workers, increased availability since the 1980s of long-sleeved sun protective swimwear, a ban on the use of indoor tanning since 2014, provision of UV forecasts in weather, and a comprehensive program of grants for community shade structures (PLoSMed. 2019 Oct 8;16[10]:e1002932).
“One approach to melanoma prevention won’t fit all,” she concluded. “We need to focus on prevention, public education to improve knowledge and self-detection.”
Dr. Ferris disclosed that she is a consultant to and an investigator for DermTech and Scibase. She is also an investigator for Castle Biosciences.
SDEF and this news organization are owned by the same parent company. Dr. Ferris spoke during a forum on cutaneous malignancies at the meeting.
LAS VEGAS – The according to data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
At the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar, Laura Korb Ferris, MD, PhD, said that SEER data project 96,480 new cases of melanoma in 2019, as well as 7,230 deaths from the disease. In 2016, SEER projected 10,130 deaths from melanoma, “so we’re actually projecting a reduction in melanoma deaths,” said Dr. Ferris, director of clinical trials at the University of Pittsburgh Medical Center’s department of dermatology. She added that the death rate from melanoma in 2016 was 2.17 per 100,000 population, a reduction from 2.69 per 100,000 population in 2011, “so it looks like melanoma mortality may be stable,” or even reduced, despite an increase in melanoma incidence.
A study of SEER data between 1989 and 2009 found that melanoma incidence is increasing across all lesion thicknesses (J Natl Cancer Inst. 2015 Nov 12. doi: 10.1093/jnci/djv294). Specifically, the incidence increased most among thin lesions, but there was a smaller increased incidence of thick melanoma. “This suggests that the overall burden of disease is truly increasing, but it is primarily stemming from an increase in T1/T2 disease,” Dr. Ferris said. “This could be due in part to increased early detection.”
Improvements in melanoma-specific survival, she continued, are likely a combination of improved management of T4 disease, a shift toward detection of thinner T1/T2 melanoma, and increased detection of T1/T2 disease.
The SEER data also showed that the incidence of fatal cases of melanoma has decreased since 1989, but only in thick melanomas. This trend may indicate a modest improvement in the management of T4 tumors. “Optimistically, I think increased detection efforts are improving survival by early detection of thin but ultimately fatal melanomas,” Dr. Ferris said. “Hopefully we are finding disease earlier and we are preventing patients from progressing to these fatal T4 melanomas.”
Disparities in melanoma-specific survival also come into play. Men have poorer survival compared with women, whites have the highest survival, and non-Hispanic whites have a better survival than Hispanic whites, Dr. Ferris said, while lower rates of survival are seen in blacks and nonblack minorities, as well as among those in high poverty and those who are separated/nonmarried. Lesion type also matters. The highest survival is seen in those with superficial spreading melanoma, while lower survival is observed in those with nodular melanoma, and acral lentiginous melanoma.
Early detection of thin nodular melanomas has the potential to significantly impact melanoma mortality, “but we want to keep in mind that the majority of ultimately fatal melanomas are superficial spreading melanomas,” Dr. Ferris said. “That is because they are so much more prevalent. As a dermatologist, I think a lot about screening and early detection. Periodic screening is a good strategy for a slower-growing superficial spreading melanoma, but it’s not necessarily a good strategy for a rapidly growing nodular melanoma. That’s going to require better education and better access to health care.”
Self-detection of melanoma is another strategy to consider. According to Dr. Ferris, results from multiple studies suggest that about 50% of all melanomas are detected by patients, but the ones they find tend to be thicker than the ones that clinicians detect during office visits. “It would be great if we can get that number higher than 50%,” Dr. Ferris said. “If patients really understood what melanoma is, what it looks like, and when they needed to seek medical attention, perhaps we could get that over 50% and see self-detection of thinner melanomas. That’s a very low-cost intervention.”
Targeted screening efforts that stratify by risk factors and by age “makes screening more efficient and more cost-effective,” she added. She cited one analysis, which found that clinicians need to screen 606 people and conduct 25 biopsies in order to find one melanoma. “That’s very resource intensive,” she said. “However, if you only screened people 50 or older or 65 or older, the number needed to screen goes down, and because your pretest probability is higher, your number need to biopsy goes down as well. If you factor in things like a history of atypical nevi or a personal history of melanoma, those patients are at a higher risk of developing melanoma.”
Dr. Ferris closed her presentation by noting that Australia leads other countries in melanoma prevention efforts. There, the combined incidence of skin cancer is higher than the incidence of any other type of cancer. Four decades ago, Australian health officials launched SunSmart, a series of initiatives intended to reduce skin cancer. These include implementation of policies for hat wearing and shade provision in schools and at work, availability of more effective sunscreens, inclusion of sun protection items as a tax-deductible expense for outdoor workers, increased availability since the 1980s of long-sleeved sun protective swimwear, a ban on the use of indoor tanning since 2014, provision of UV forecasts in weather, and a comprehensive program of grants for community shade structures (PLoSMed. 2019 Oct 8;16[10]:e1002932).
“One approach to melanoma prevention won’t fit all,” she concluded. “We need to focus on prevention, public education to improve knowledge and self-detection.”
Dr. Ferris disclosed that she is a consultant to and an investigator for DermTech and Scibase. She is also an investigator for Castle Biosciences.
SDEF and this news organization are owned by the same parent company. Dr. Ferris spoke during a forum on cutaneous malignancies at the meeting.
EXPERT ANALYSIS FROM THE SDEF LAS VEGAS DERMATOLOGY SEMINAR
New models predict post-op pain in TKA
two-thirds of the time. Major risk factors include pre-operative pain, sensory testing results, anxiety and anticipated pain.
“The results of this study provide some basis for the identification of patients at risk of PPP after TKA and highlight several modifiable factors that may be targeted by clinicians in an attempt to reduce the risk of developing PPP,” write the authors of the study, which appeared in the British Journal of Anaesthesia.
The authors, led by David Rice, PhD, of Auckland University of Technology, note that moderate to severe levels of PPP affect an estimated 10%-34% of patients at least 3 months after TKA surgery. “PPP adversely affects quality of life, is the most important predictor of patient dissatisfaction after TKA, and is a common reason for undergoing revision surgery.”
The researchers, who launched the study to gain insight into the risk factors that can predict PPP, recruited 300 New Zealand volunteers (average age = 69, 48% female, 92% white, average body mass index [BMI] = 31 kg/m2) to be surveyed before and after TKA surgery. They monitored pain and tracked a long list of possible risk factors including psychological traits (such as anxiety, pain catastrophizing and depression), physical traits (such as gender, BMI), and surgical traits (such as total surgery time).
At 6 months, 21% of 291 patients reported moderate to severe pain, and the percentage fell to 16% in 288 patients at 12 months.
The researchers developed two models that successfully predicted moderate-to-severe PPP.
The 6-month model relied on higher levels of preoperative pain intensity, temporal summation (a statistic that’s based on quantitative sensory testing), trait anxiety (a measure of individual anxiety level), and expected pain. It correctly predicted moderate to severe PPP 66% of the time (area under the curve [AUC] = 0.70, sensitivity = 0.72, specificity = 0.64).
The 12-month model relied on higher levels of all the risk factors except for temporal summation and correctly predicted moderate-to-severe PPP 66% of the time (AUC = 0.66, sensitivity = 0.61, specificity = 0.67).
The researchers noted that other research has linked trait anxiety and expected pain to PPP. In regard to anxiety, “cognitive behavioral interventions in the perioperative period aimed at reducing the threat value of surgery and of postoperative pain, improving patients’ coping strategies, and enhancing self-efficacy might help to reduce the risk of PPP after TKA,” the researchers write. “Furthermore, there is some evidence that anxiolytic medications can diminish perioperative anxiety and reduce APOP [acute postoperative pain] although its effects on PPP are unclear.”
Moving forward, the authors write, “strategies to minimize intraoperative nerve injury, reduce preoperative pain intensity, and address preoperative psychological factors such as expected pain and anxiety may lead to improved outcomes after TKA and should be explored.”
The Australia New Zealand College of Anesthetists and Auckland University of Technology funded the study. The study authors report no relevant disclosures.
SOURCE: Rice D et al. Br J Anaesth 2018;804-12. doi: https://doi.org/10.1016/j.bja.2018.05.070.
two-thirds of the time. Major risk factors include pre-operative pain, sensory testing results, anxiety and anticipated pain.
“The results of this study provide some basis for the identification of patients at risk of PPP after TKA and highlight several modifiable factors that may be targeted by clinicians in an attempt to reduce the risk of developing PPP,” write the authors of the study, which appeared in the British Journal of Anaesthesia.
The authors, led by David Rice, PhD, of Auckland University of Technology, note that moderate to severe levels of PPP affect an estimated 10%-34% of patients at least 3 months after TKA surgery. “PPP adversely affects quality of life, is the most important predictor of patient dissatisfaction after TKA, and is a common reason for undergoing revision surgery.”
The researchers, who launched the study to gain insight into the risk factors that can predict PPP, recruited 300 New Zealand volunteers (average age = 69, 48% female, 92% white, average body mass index [BMI] = 31 kg/m2) to be surveyed before and after TKA surgery. They monitored pain and tracked a long list of possible risk factors including psychological traits (such as anxiety, pain catastrophizing and depression), physical traits (such as gender, BMI), and surgical traits (such as total surgery time).
At 6 months, 21% of 291 patients reported moderate to severe pain, and the percentage fell to 16% in 288 patients at 12 months.
The researchers developed two models that successfully predicted moderate-to-severe PPP.
The 6-month model relied on higher levels of preoperative pain intensity, temporal summation (a statistic that’s based on quantitative sensory testing), trait anxiety (a measure of individual anxiety level), and expected pain. It correctly predicted moderate to severe PPP 66% of the time (area under the curve [AUC] = 0.70, sensitivity = 0.72, specificity = 0.64).
The 12-month model relied on higher levels of all the risk factors except for temporal summation and correctly predicted moderate-to-severe PPP 66% of the time (AUC = 0.66, sensitivity = 0.61, specificity = 0.67).
The researchers noted that other research has linked trait anxiety and expected pain to PPP. In regard to anxiety, “cognitive behavioral interventions in the perioperative period aimed at reducing the threat value of surgery and of postoperative pain, improving patients’ coping strategies, and enhancing self-efficacy might help to reduce the risk of PPP after TKA,” the researchers write. “Furthermore, there is some evidence that anxiolytic medications can diminish perioperative anxiety and reduce APOP [acute postoperative pain] although its effects on PPP are unclear.”
Moving forward, the authors write, “strategies to minimize intraoperative nerve injury, reduce preoperative pain intensity, and address preoperative psychological factors such as expected pain and anxiety may lead to improved outcomes after TKA and should be explored.”
The Australia New Zealand College of Anesthetists and Auckland University of Technology funded the study. The study authors report no relevant disclosures.
SOURCE: Rice D et al. Br J Anaesth 2018;804-12. doi: https://doi.org/10.1016/j.bja.2018.05.070.
two-thirds of the time. Major risk factors include pre-operative pain, sensory testing results, anxiety and anticipated pain.
“The results of this study provide some basis for the identification of patients at risk of PPP after TKA and highlight several modifiable factors that may be targeted by clinicians in an attempt to reduce the risk of developing PPP,” write the authors of the study, which appeared in the British Journal of Anaesthesia.
The authors, led by David Rice, PhD, of Auckland University of Technology, note that moderate to severe levels of PPP affect an estimated 10%-34% of patients at least 3 months after TKA surgery. “PPP adversely affects quality of life, is the most important predictor of patient dissatisfaction after TKA, and is a common reason for undergoing revision surgery.”
The researchers, who launched the study to gain insight into the risk factors that can predict PPP, recruited 300 New Zealand volunteers (average age = 69, 48% female, 92% white, average body mass index [BMI] = 31 kg/m2) to be surveyed before and after TKA surgery. They monitored pain and tracked a long list of possible risk factors including psychological traits (such as anxiety, pain catastrophizing and depression), physical traits (such as gender, BMI), and surgical traits (such as total surgery time).
At 6 months, 21% of 291 patients reported moderate to severe pain, and the percentage fell to 16% in 288 patients at 12 months.
The researchers developed two models that successfully predicted moderate-to-severe PPP.
The 6-month model relied on higher levels of preoperative pain intensity, temporal summation (a statistic that’s based on quantitative sensory testing), trait anxiety (a measure of individual anxiety level), and expected pain. It correctly predicted moderate to severe PPP 66% of the time (area under the curve [AUC] = 0.70, sensitivity = 0.72, specificity = 0.64).
The 12-month model relied on higher levels of all the risk factors except for temporal summation and correctly predicted moderate-to-severe PPP 66% of the time (AUC = 0.66, sensitivity = 0.61, specificity = 0.67).
The researchers noted that other research has linked trait anxiety and expected pain to PPP. In regard to anxiety, “cognitive behavioral interventions in the perioperative period aimed at reducing the threat value of surgery and of postoperative pain, improving patients’ coping strategies, and enhancing self-efficacy might help to reduce the risk of PPP after TKA,” the researchers write. “Furthermore, there is some evidence that anxiolytic medications can diminish perioperative anxiety and reduce APOP [acute postoperative pain] although its effects on PPP are unclear.”
Moving forward, the authors write, “strategies to minimize intraoperative nerve injury, reduce preoperative pain intensity, and address preoperative psychological factors such as expected pain and anxiety may lead to improved outcomes after TKA and should be explored.”
The Australia New Zealand College of Anesthetists and Auckland University of Technology funded the study. The study authors report no relevant disclosures.
SOURCE: Rice D et al. Br J Anaesth 2018;804-12. doi: https://doi.org/10.1016/j.bja.2018.05.070.
FROM BRITISH JOURNAL OF ANESTHESIA
Poll: Clostridium difficile
Choose your answer in the poll below. To check the accuracy of your answer, see PURLs: Do Probiotics Reduce C diff Risk in Hospitalized Patients?
[polldaddy:10452484]
Click on page 2 below to find out what the correct answer is...
The correct answer is a.) 1 to 2
To learn more, see this month's PURLs: Do Probiotics Reduce C diff Risk in Hospitalized Patients?
Choose your answer in the poll below. To check the accuracy of your answer, see PURLs: Do Probiotics Reduce C diff Risk in Hospitalized Patients?
[polldaddy:10452484]
Click on page 2 below to find out what the correct answer is...
The correct answer is a.) 1 to 2
To learn more, see this month's PURLs: Do Probiotics Reduce C diff Risk in Hospitalized Patients?
Choose your answer in the poll below. To check the accuracy of your answer, see PURLs: Do Probiotics Reduce C diff Risk in Hospitalized Patients?
[polldaddy:10452484]
Click on page 2 below to find out what the correct answer is...
The correct answer is a.) 1 to 2
To learn more, see this month's PURLs: Do Probiotics Reduce C diff Risk in Hospitalized Patients?
The Dog Can Stay, but the Rash Must Go
A 50-year-old man presents with a 1-year history of an itchy, bumpy rash on his chest. He denies any history of similar rash and says there have been no “extraordinary changes” in his life that could have triggered this manifestation. Despite consulting various primary care providers, he has been unable to acquire either a definitive diagnosis or effective treatment.
The patient works exclusively in a climate-controlled office. Although there were no changes to laundry detergent, body soap, deodorant, or other products that might have precipitated the rash’s manifestation, he tried alternate products to see what effect they might have. Nothing beneficial came from these experiments. Similarly, the family dogs were temporarily “banished” with no improvement to his condition.
From the outset, the rash and the associated itching have been confined to the patient’s chest. No one else in his family is similarly affected.
The patient is otherwise quite well. He takes no prescription medications and denies any recent foreign travel.
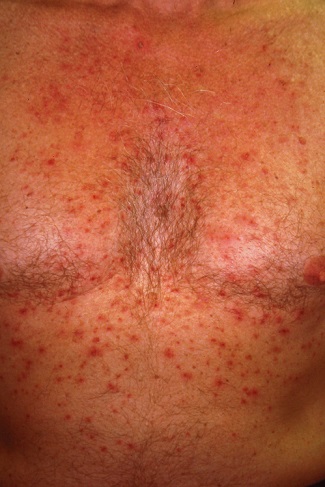
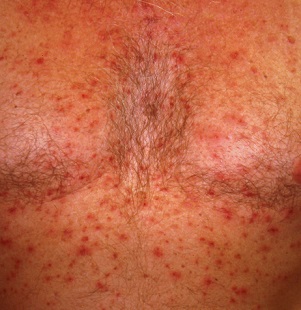
EXAMINATION
The papulovesicular rash is strikingly uniform. The patient’s entire chest is covered with tiny vesicles, many with clear fluid inside. The lesions average 1.2 to 2 mm in width, and nearly all are quite palpable. Each lesion is slightly erythematous but neither warm nor tender on palpation.
Examination of the rest of the patient’s exposed skin reveals no similar lesions. His back, hands, and genitals are notably free of any such lesions.
A shave biopsy is performed, utilizing a saucerization technique, and the specimen is submitted to pathology for routine processing. The report confirms the papulovesicular nature of the lesions—but more significantly, it shows consistent acantholysis (loss of intracellular connections between keratinocytes), along with focal lymphohistiocytic infiltrates.
What’s the diagnosis?
DISCUSSION
This is a classic presentation of Grover disease, also known as transient acantholytic dermatosis (AD). While not rare, it is seen only occasionally in dermatology practices. When it does walk through the door, it is twice as likely to be seen in a male than in a female patient and less commonly seen in those with darker skin.
AD is easy enough to diagnose clinically, without biopsy, particularly in classic cases such as this one. The distribution and morphology of the rash, as well as the gender and age of the patient, are all typical of this idiopathic condition. The biopsy results, besides being consistent with AD, did serve to rule out other items in the differential (eg, bacterial folliculitis, pemphigus, and acne).
Since AD was first described in 1974 by R.W. Grover, MD, much research has been conducted to flesh out the nature of the disease, its potential causes, and possible treatment. One certainty about so-called transient AD is that most cases are far from transient—in fact, they can last for a year or more. Attempts have been made to connect AD with internal disease (eg, occult malignancy) or even mercury exposure, but these theories have not been corroborated.
Consistent treatment success has also been elusive. Most patients achieve decent relief with the use of topical steroid creams, with or without the addition of anti-inflammatory medications (eg, doxycycline). Other options include isotretinoin and psoralen plus ultraviolet A (PUVA) photochemotherapy. Fortunately, most cases eventually clear up.
TAKE-HOME LEARNING POINTS
- Grover disease, also known as transient acantholytic dermatosis (AD), usually manifests with an acute eruption of papulovesicular lesions.
- AD lesions tend to be confined to the chest and are typically pruritic.
- Clinical diagnosis is usually adequate, although biopsy, which will reveal typical findings of acantholysis, may be necessary to rule out other items in the differential.
- Treatment with topical steroids, oral doxycycline, and “tincture of time” usually suffices, but resolution may take a year or more.
A 50-year-old man presents with a 1-year history of an itchy, bumpy rash on his chest. He denies any history of similar rash and says there have been no “extraordinary changes” in his life that could have triggered this manifestation. Despite consulting various primary care providers, he has been unable to acquire either a definitive diagnosis or effective treatment.
The patient works exclusively in a climate-controlled office. Although there were no changes to laundry detergent, body soap, deodorant, or other products that might have precipitated the rash’s manifestation, he tried alternate products to see what effect they might have. Nothing beneficial came from these experiments. Similarly, the family dogs were temporarily “banished” with no improvement to his condition.
From the outset, the rash and the associated itching have been confined to the patient’s chest. No one else in his family is similarly affected.
The patient is otherwise quite well. He takes no prescription medications and denies any recent foreign travel.

EXAMINATION
The papulovesicular rash is strikingly uniform. The patient’s entire chest is covered with tiny vesicles, many with clear fluid inside. The lesions average 1.2 to 2 mm in width, and nearly all are quite palpable. Each lesion is slightly erythematous but neither warm nor tender on palpation.
Examination of the rest of the patient’s exposed skin reveals no similar lesions. His back, hands, and genitals are notably free of any such lesions.
A shave biopsy is performed, utilizing a saucerization technique, and the specimen is submitted to pathology for routine processing. The report confirms the papulovesicular nature of the lesions—but more significantly, it shows consistent acantholysis (loss of intracellular connections between keratinocytes), along with focal lymphohistiocytic infiltrates.
What’s the diagnosis?
DISCUSSION
This is a classic presentation of Grover disease, also known as transient acantholytic dermatosis (AD). While not rare, it is seen only occasionally in dermatology practices. When it does walk through the door, it is twice as likely to be seen in a male than in a female patient and less commonly seen in those with darker skin.
AD is easy enough to diagnose clinically, without biopsy, particularly in classic cases such as this one. The distribution and morphology of the rash, as well as the gender and age of the patient, are all typical of this idiopathic condition. The biopsy results, besides being consistent with AD, did serve to rule out other items in the differential (eg, bacterial folliculitis, pemphigus, and acne).
Since AD was first described in 1974 by R.W. Grover, MD, much research has been conducted to flesh out the nature of the disease, its potential causes, and possible treatment. One certainty about so-called transient AD is that most cases are far from transient—in fact, they can last for a year or more. Attempts have been made to connect AD with internal disease (eg, occult malignancy) or even mercury exposure, but these theories have not been corroborated.
Consistent treatment success has also been elusive. Most patients achieve decent relief with the use of topical steroid creams, with or without the addition of anti-inflammatory medications (eg, doxycycline). Other options include isotretinoin and psoralen plus ultraviolet A (PUVA) photochemotherapy. Fortunately, most cases eventually clear up.
TAKE-HOME LEARNING POINTS
- Grover disease, also known as transient acantholytic dermatosis (AD), usually manifests with an acute eruption of papulovesicular lesions.
- AD lesions tend to be confined to the chest and are typically pruritic.
- Clinical diagnosis is usually adequate, although biopsy, which will reveal typical findings of acantholysis, may be necessary to rule out other items in the differential.
- Treatment with topical steroids, oral doxycycline, and “tincture of time” usually suffices, but resolution may take a year or more.
A 50-year-old man presents with a 1-year history of an itchy, bumpy rash on his chest. He denies any history of similar rash and says there have been no “extraordinary changes” in his life that could have triggered this manifestation. Despite consulting various primary care providers, he has been unable to acquire either a definitive diagnosis or effective treatment.
The patient works exclusively in a climate-controlled office. Although there were no changes to laundry detergent, body soap, deodorant, or other products that might have precipitated the rash’s manifestation, he tried alternate products to see what effect they might have. Nothing beneficial came from these experiments. Similarly, the family dogs were temporarily “banished” with no improvement to his condition.
From the outset, the rash and the associated itching have been confined to the patient’s chest. No one else in his family is similarly affected.
The patient is otherwise quite well. He takes no prescription medications and denies any recent foreign travel.

EXAMINATION
The papulovesicular rash is strikingly uniform. The patient’s entire chest is covered with tiny vesicles, many with clear fluid inside. The lesions average 1.2 to 2 mm in width, and nearly all are quite palpable. Each lesion is slightly erythematous but neither warm nor tender on palpation.
Examination of the rest of the patient’s exposed skin reveals no similar lesions. His back, hands, and genitals are notably free of any such lesions.
A shave biopsy is performed, utilizing a saucerization technique, and the specimen is submitted to pathology for routine processing. The report confirms the papulovesicular nature of the lesions—but more significantly, it shows consistent acantholysis (loss of intracellular connections between keratinocytes), along with focal lymphohistiocytic infiltrates.
What’s the diagnosis?
DISCUSSION
This is a classic presentation of Grover disease, also known as transient acantholytic dermatosis (AD). While not rare, it is seen only occasionally in dermatology practices. When it does walk through the door, it is twice as likely to be seen in a male than in a female patient and less commonly seen in those with darker skin.
AD is easy enough to diagnose clinically, without biopsy, particularly in classic cases such as this one. The distribution and morphology of the rash, as well as the gender and age of the patient, are all typical of this idiopathic condition. The biopsy results, besides being consistent with AD, did serve to rule out other items in the differential (eg, bacterial folliculitis, pemphigus, and acne).
Since AD was first described in 1974 by R.W. Grover, MD, much research has been conducted to flesh out the nature of the disease, its potential causes, and possible treatment. One certainty about so-called transient AD is that most cases are far from transient—in fact, they can last for a year or more. Attempts have been made to connect AD with internal disease (eg, occult malignancy) or even mercury exposure, but these theories have not been corroborated.
Consistent treatment success has also been elusive. Most patients achieve decent relief with the use of topical steroid creams, with or without the addition of anti-inflammatory medications (eg, doxycycline). Other options include isotretinoin and psoralen plus ultraviolet A (PUVA) photochemotherapy. Fortunately, most cases eventually clear up.
TAKE-HOME LEARNING POINTS
- Grover disease, also known as transient acantholytic dermatosis (AD), usually manifests with an acute eruption of papulovesicular lesions.
- AD lesions tend to be confined to the chest and are typically pruritic.
- Clinical diagnosis is usually adequate, although biopsy, which will reveal typical findings of acantholysis, may be necessary to rule out other items in the differential.
- Treatment with topical steroids, oral doxycycline, and “tincture of time” usually suffices, but resolution may take a year or more.
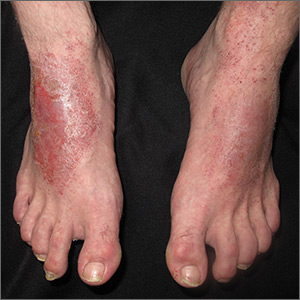
Red patches and thin plaques on feet
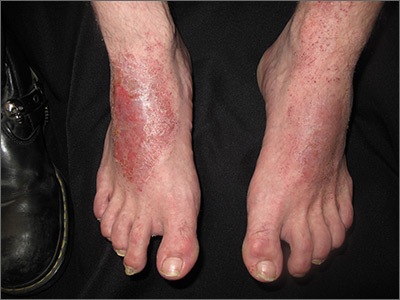
The FP conducted a physical exam and noticed bilateral dorsal foot dermatitis with occasional small vesicles and lichenified papules, which was suggestive of chronic contact or irritant dermatitis. The patient’s favorite pair of boots offered another clue as to the most likely contact allergens. (The boots were leather, and leather is treated with tanning agents and dyes.) A biopsy was not performed but would be expected to show spongiosis with some degree of lichenification (thickening of the dermis)—a sign of the acute on chronic nature of this process. The diagnosis of irritant or allergic contact dermatitis was made empirically.
The differential diagnosis for rashes on the feet can be broad and includes common tinea pedis, pitted keratolysis, stasis dermatitis, psoriasis, eczemas of various types, keratoderma, and contact dermatitis.
Many patients misconstrue that materials they use every day are exempt from becoming allergens. In counseling patients about this, point out that contact allergens often arise from repeated exposure. For example, dentists often develop dental amalgam allergies, hair professionals develop hair dye allergies, and machinists commonly develop cutting oil allergies. These reactions can and do occur years into their use.
The patient was started on topical clobetasol 0.05% ointment bid for 3 weeks, which provided quick relief and cleared his feet of the patches and plaques. He continued to wear his boots until contact allergy patch testing was performed in the office over a series of 3 days. This revealed an allergy to chromium, a common leather tanning agent. The patient was advised to avoid leather products including jackets, car upholstery, and gloves. After he carefully chose different footwear without a leather insole or tongue, the patient required no further therapy and remained clear.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained).

The FP conducted a physical exam and noticed bilateral dorsal foot dermatitis with occasional small vesicles and lichenified papules, which was suggestive of chronic contact or irritant dermatitis. The patient’s favorite pair of boots offered another clue as to the most likely contact allergens. (The boots were leather, and leather is treated with tanning agents and dyes.) A biopsy was not performed but would be expected to show spongiosis with some degree of lichenification (thickening of the dermis)—a sign of the acute on chronic nature of this process. The diagnosis of irritant or allergic contact dermatitis was made empirically.
The differential diagnosis for rashes on the feet can be broad and includes common tinea pedis, pitted keratolysis, stasis dermatitis, psoriasis, eczemas of various types, keratoderma, and contact dermatitis.
Many patients misconstrue that materials they use every day are exempt from becoming allergens. In counseling patients about this, point out that contact allergens often arise from repeated exposure. For example, dentists often develop dental amalgam allergies, hair professionals develop hair dye allergies, and machinists commonly develop cutting oil allergies. These reactions can and do occur years into their use.
The patient was started on topical clobetasol 0.05% ointment bid for 3 weeks, which provided quick relief and cleared his feet of the patches and plaques. He continued to wear his boots until contact allergy patch testing was performed in the office over a series of 3 days. This revealed an allergy to chromium, a common leather tanning agent. The patient was advised to avoid leather products including jackets, car upholstery, and gloves. After he carefully chose different footwear without a leather insole or tongue, the patient required no further therapy and remained clear.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained).

The FP conducted a physical exam and noticed bilateral dorsal foot dermatitis with occasional small vesicles and lichenified papules, which was suggestive of chronic contact or irritant dermatitis. The patient’s favorite pair of boots offered another clue as to the most likely contact allergens. (The boots were leather, and leather is treated with tanning agents and dyes.) A biopsy was not performed but would be expected to show spongiosis with some degree of lichenification (thickening of the dermis)—a sign of the acute on chronic nature of this process. The diagnosis of irritant or allergic contact dermatitis was made empirically.
The differential diagnosis for rashes on the feet can be broad and includes common tinea pedis, pitted keratolysis, stasis dermatitis, psoriasis, eczemas of various types, keratoderma, and contact dermatitis.
Many patients misconstrue that materials they use every day are exempt from becoming allergens. In counseling patients about this, point out that contact allergens often arise from repeated exposure. For example, dentists often develop dental amalgam allergies, hair professionals develop hair dye allergies, and machinists commonly develop cutting oil allergies. These reactions can and do occur years into their use.
The patient was started on topical clobetasol 0.05% ointment bid for 3 weeks, which provided quick relief and cleared his feet of the patches and plaques. He continued to wear his boots until contact allergy patch testing was performed in the office over a series of 3 days. This revealed an allergy to chromium, a common leather tanning agent. The patient was advised to avoid leather products including jackets, car upholstery, and gloves. After he carefully chose different footwear without a leather insole or tongue, the patient required no further therapy and remained clear.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained).
Though metastatic breast cancer survival is improving, rates vary by region
Though survival rates of patients with metastatic breast cancer (MBC) have increased over the last 2 decades, a new study has indicated disparities exist across regions and by variables like age and race.
“It appears from these results that we may be at a crossroads for MBC treatment and survival,” wrote Judith A. Malmgren, PhD, of the University of Washington and her coauthors. The study was published in Cancer. “Access to appropriate, timely, and up‐to‐date diagnosis, care, treatment, and surveillance could turn this fatal disease into a chronic and treatable phenomenon, depending on patient factors, molecular subtype, and insurance capacity to pay for treatment,” they said.
To determine how breast cancer outcomes might vary across regions, the researchers compared breast cancer–specific survival rates (BCSS) from Surveillance, Epidemiology, and End Results-9 (SEER-9) registry data minus a regional subset from the Seattle-Puget Sound (S-PS) region (n = 12,121) to patients from that S-PS region (n = 1,931) and to an individual cohort in that area (n = 261). Five-year BCSS rates were calculated for three time periods: 1990‐1998, 1999‐2004, and 2005‐2011.
All analyzed patients were diagnosed with a first primary, de novo, stage IV breast cancer between the ages of 25 and 84 years from 1990 to 2011. Patients in the SEER-9 group and the S-PS region had a mean age of 61 years, compared with the individual cohort’s mean age of 55 years. Patients in the individual cohort were more likely to reside in a major metropolitan area of over 1 million people, compared with the SEER group and the S-PS region (86% versus 61% and 58%, respectively).
Patients in the SEER-9 group had improved BCSS rates over the study period, from 19% in 1990-1998 (95% confidence interval, 18%-21%; P less than .001) to 26% in 2005-2011 (95% CI, 24%-27%; P less than .001). Patients in the S-PS region saw even greater improvements in BCSS rates, from 21% in 1990-1998 (95% CI, 18%-24%; P less than .001) to 35% in 2005-2011 (95% CI, 32%-39%; P less than .001). But the largest improvement in survival rates came from patients in the individual cohort, who went from 29% in 1990-1998 (95% CI, 18%-37%; P less than .001) to 56% in 2005-2011 (95% CI, 45%-65%; P = .004).
In a proportional hazards model for breast cancer–specific death, reduced hazard in the SEER-9 group was associated with surgery (hazard ratio, 0.58; 95% CI, 0.55-0.61; P less than .001), an age less than 70 (HR, 0.77; 95% CI, 0.73-0.82; P less than .001) and white race (HR, 0.84; 95% CI, 0.79-0.89; P less than .001). Similar associations were seen in the S-PS region with surgery (HR, 0.57; 95% CI, 0.50-0.66; P less than .001) and an age less than 70 (HR, 0.72; 95% CI, 0.62-0.84; P less than .001), but not white race.
The study results “indicate that the stage IV population that is living longer may be benefiting from many of the same therapies used to treat early breast cancer, especially for patients who are able to handle adjuvant chemotherapy treatment and are HR‐positive,” the researchers said. “However, the lag in survival improvement across different population‐based, geographic regions suggests that some groups and regions may benefit unequally from treatment advances as well as timely diagnosis.”
The study was funded by the Kaplan Cancer Research Fund, the Metastatic Breast Cancer Alliance, and the Surveillance, Epidemiology, and End Results Cancer Surveillance System program of the National Cancer Institute. The authors reported no conflicts of interest.
SOURCE: Malmgren JA et al. Cancer. 2019 Oct 22. doi: 10.1002/cncr.32531.
Though survival rates of patients with metastatic breast cancer (MBC) have increased over the last 2 decades, a new study has indicated disparities exist across regions and by variables like age and race.
“It appears from these results that we may be at a crossroads for MBC treatment and survival,” wrote Judith A. Malmgren, PhD, of the University of Washington and her coauthors. The study was published in Cancer. “Access to appropriate, timely, and up‐to‐date diagnosis, care, treatment, and surveillance could turn this fatal disease into a chronic and treatable phenomenon, depending on patient factors, molecular subtype, and insurance capacity to pay for treatment,” they said.
To determine how breast cancer outcomes might vary across regions, the researchers compared breast cancer–specific survival rates (BCSS) from Surveillance, Epidemiology, and End Results-9 (SEER-9) registry data minus a regional subset from the Seattle-Puget Sound (S-PS) region (n = 12,121) to patients from that S-PS region (n = 1,931) and to an individual cohort in that area (n = 261). Five-year BCSS rates were calculated for three time periods: 1990‐1998, 1999‐2004, and 2005‐2011.
All analyzed patients were diagnosed with a first primary, de novo, stage IV breast cancer between the ages of 25 and 84 years from 1990 to 2011. Patients in the SEER-9 group and the S-PS region had a mean age of 61 years, compared with the individual cohort’s mean age of 55 years. Patients in the individual cohort were more likely to reside in a major metropolitan area of over 1 million people, compared with the SEER group and the S-PS region (86% versus 61% and 58%, respectively).
Patients in the SEER-9 group had improved BCSS rates over the study period, from 19% in 1990-1998 (95% confidence interval, 18%-21%; P less than .001) to 26% in 2005-2011 (95% CI, 24%-27%; P less than .001). Patients in the S-PS region saw even greater improvements in BCSS rates, from 21% in 1990-1998 (95% CI, 18%-24%; P less than .001) to 35% in 2005-2011 (95% CI, 32%-39%; P less than .001). But the largest improvement in survival rates came from patients in the individual cohort, who went from 29% in 1990-1998 (95% CI, 18%-37%; P less than .001) to 56% in 2005-2011 (95% CI, 45%-65%; P = .004).
In a proportional hazards model for breast cancer–specific death, reduced hazard in the SEER-9 group was associated with surgery (hazard ratio, 0.58; 95% CI, 0.55-0.61; P less than .001), an age less than 70 (HR, 0.77; 95% CI, 0.73-0.82; P less than .001) and white race (HR, 0.84; 95% CI, 0.79-0.89; P less than .001). Similar associations were seen in the S-PS region with surgery (HR, 0.57; 95% CI, 0.50-0.66; P less than .001) and an age less than 70 (HR, 0.72; 95% CI, 0.62-0.84; P less than .001), but not white race.
The study results “indicate that the stage IV population that is living longer may be benefiting from many of the same therapies used to treat early breast cancer, especially for patients who are able to handle adjuvant chemotherapy treatment and are HR‐positive,” the researchers said. “However, the lag in survival improvement across different population‐based, geographic regions suggests that some groups and regions may benefit unequally from treatment advances as well as timely diagnosis.”
The study was funded by the Kaplan Cancer Research Fund, the Metastatic Breast Cancer Alliance, and the Surveillance, Epidemiology, and End Results Cancer Surveillance System program of the National Cancer Institute. The authors reported no conflicts of interest.
SOURCE: Malmgren JA et al. Cancer. 2019 Oct 22. doi: 10.1002/cncr.32531.
Though survival rates of patients with metastatic breast cancer (MBC) have increased over the last 2 decades, a new study has indicated disparities exist across regions and by variables like age and race.
“It appears from these results that we may be at a crossroads for MBC treatment and survival,” wrote Judith A. Malmgren, PhD, of the University of Washington and her coauthors. The study was published in Cancer. “Access to appropriate, timely, and up‐to‐date diagnosis, care, treatment, and surveillance could turn this fatal disease into a chronic and treatable phenomenon, depending on patient factors, molecular subtype, and insurance capacity to pay for treatment,” they said.
To determine how breast cancer outcomes might vary across regions, the researchers compared breast cancer–specific survival rates (BCSS) from Surveillance, Epidemiology, and End Results-9 (SEER-9) registry data minus a regional subset from the Seattle-Puget Sound (S-PS) region (n = 12,121) to patients from that S-PS region (n = 1,931) and to an individual cohort in that area (n = 261). Five-year BCSS rates were calculated for three time periods: 1990‐1998, 1999‐2004, and 2005‐2011.
All analyzed patients were diagnosed with a first primary, de novo, stage IV breast cancer between the ages of 25 and 84 years from 1990 to 2011. Patients in the SEER-9 group and the S-PS region had a mean age of 61 years, compared with the individual cohort’s mean age of 55 years. Patients in the individual cohort were more likely to reside in a major metropolitan area of over 1 million people, compared with the SEER group and the S-PS region (86% versus 61% and 58%, respectively).
Patients in the SEER-9 group had improved BCSS rates over the study period, from 19% in 1990-1998 (95% confidence interval, 18%-21%; P less than .001) to 26% in 2005-2011 (95% CI, 24%-27%; P less than .001). Patients in the S-PS region saw even greater improvements in BCSS rates, from 21% in 1990-1998 (95% CI, 18%-24%; P less than .001) to 35% in 2005-2011 (95% CI, 32%-39%; P less than .001). But the largest improvement in survival rates came from patients in the individual cohort, who went from 29% in 1990-1998 (95% CI, 18%-37%; P less than .001) to 56% in 2005-2011 (95% CI, 45%-65%; P = .004).
In a proportional hazards model for breast cancer–specific death, reduced hazard in the SEER-9 group was associated with surgery (hazard ratio, 0.58; 95% CI, 0.55-0.61; P less than .001), an age less than 70 (HR, 0.77; 95% CI, 0.73-0.82; P less than .001) and white race (HR, 0.84; 95% CI, 0.79-0.89; P less than .001). Similar associations were seen in the S-PS region with surgery (HR, 0.57; 95% CI, 0.50-0.66; P less than .001) and an age less than 70 (HR, 0.72; 95% CI, 0.62-0.84; P less than .001), but not white race.
The study results “indicate that the stage IV population that is living longer may be benefiting from many of the same therapies used to treat early breast cancer, especially for patients who are able to handle adjuvant chemotherapy treatment and are HR‐positive,” the researchers said. “However, the lag in survival improvement across different population‐based, geographic regions suggests that some groups and regions may benefit unequally from treatment advances as well as timely diagnosis.”
The study was funded by the Kaplan Cancer Research Fund, the Metastatic Breast Cancer Alliance, and the Surveillance, Epidemiology, and End Results Cancer Surveillance System program of the National Cancer Institute. The authors reported no conflicts of interest.
SOURCE: Malmgren JA et al. Cancer. 2019 Oct 22. doi: 10.1002/cncr.32531.
FROM CANCER
Nivolumab benefit for NSCLC persists at 5-year follow-up
BARCELONA – Nivolumab, compared with docetaxel chemotherapy, led to a fivefold improvement in 5-year overall survival among previously treated patients with non–small cell lung cancer (NSCLC), according to a pooled analysis of data from the phase 3 CheckMate 017 and 057 trials.
The 5-year overall survival (OS) rates from the two randomized registrational trials, which established the programmed death-1 (PD-1) inhibitor nivolumab as the standard salvage therapy for NSCLC, were 13.4% vs. 2.6% (median, 11.1 vs. 8.1 months) with nivolumab and docetaxel, respectively, Scott Gettinger, MD, reported at the World Conference on Lung Cancer.
“These are the first randomized trials to report 5-year outcomes for a PD-1 axis inhibitor in patients with previously treated advanced non–small lung cancer,” said Dr. Gettinger, a professor at the Yale Comprehensive Cancer Center, New Haven, Conn. “This is really unprecedented; we wouldn’t expect many patients to be out 5 years in this scenario.”
Notably, the 5-year OS benefit was seen in both trials, he said, explaining that each compared nivolumab and docetaxel, but CheckMate 017 included patients with only squamous NSCLC, and CheckMate 057 included only non–squamous NSCLC patients.
The trials randomized 272 and 582 patients, respectively, and both demonstrated significantly improved 12-month OS with nivolumab – regardless of programmed death-ligand 1 (PD-L1) expression levels. Common eligibility criteria included stage IIIb/IV disease, good performance status (ECOG performance score of 0-1), and 1 prior platinum-based chemotherapy; CheckMate 057 further allowed prior tyrosine kinase inhibitor treatment for known anaplastic lymphoma kinase (ALK) translocation or epidermal growth factor receptor (EGFR) mutation, and allowed prior maintenance therapy. Doses in both trials were 3 mg/kg of nivolumab every 2 weeks or 75 mg/m2 of intravenous docetaxel every 3 weeks until disease progression or unacceptable toxicity.
The pooled data also showed an improvement in progression-free survival (PFS) at 5 years (8% vs. 0%) with nivolumab vs. docetaxel groups.
“Again, we don’t see this in trials – more commonly we see zero patients without progression, and that’s what we saw with the docetaxel arm,” said Dr. Gettinger, who also is the Disease Aligned Research Team Leader, Thoracic Oncology Program, at the cancer center.
The median duration of responses with nivolumab was 19.9 months vs. 5.6 months with docetaxel, and 32.2% of nivolumab responders were still without progression at 5 years, he noted.
A common question in the clinic relates to the prognosis in patients who do well with PD-1 axis inhibitors, which prompted an additional analysis across the two trials, he said, noting that 60%, 78%, and 88% of patients who had not progressed at 2, 3, or 4 years, respectively, also had not progressed at 5 years, and 80%, 93%, and 100%, of patients in those groups were alive at 5 years. In the docetaxel arm, only 4, 1, and 0 patients had PFS at 2, 3, and 4, years, respectively, and none of those patients survived to 5 years, he said.
No new safety signals were seen with long-term follow-up, he added.
“In fact there was only one grade 3 or higher toxicity that was related to treatment in the nivolumab arm, and this was a grade 3 lipase elevation. There was one patient who discontinued nivolumab after 3 years, and this was for a grade 2 rash and eczema that had waxed and waned since starting nivolumab,” he said.
Also of note, 10% of nivolumab-treated patients who were off treatment at 5 years – for variable periods of time – had not progressed and had not received subsequent therapy.
“So we clearly see benefit in our patients long after they finish a course or stop for some reason,” he said.
CheckMate 017 and 057 were funded by Bristol-Myers Squibb. Dr. Gettinger reported advisory board and/or consulting work for, and/or research funding from Bristol-Myers Squibb, Nektar Therapeutics, Genentech/Roche, Iovance, and Takeda/Ariad.
SOURCE: Gettinger S et al. WCLC 2019, Abstract PR04.03.
BARCELONA – Nivolumab, compared with docetaxel chemotherapy, led to a fivefold improvement in 5-year overall survival among previously treated patients with non–small cell lung cancer (NSCLC), according to a pooled analysis of data from the phase 3 CheckMate 017 and 057 trials.
The 5-year overall survival (OS) rates from the two randomized registrational trials, which established the programmed death-1 (PD-1) inhibitor nivolumab as the standard salvage therapy for NSCLC, were 13.4% vs. 2.6% (median, 11.1 vs. 8.1 months) with nivolumab and docetaxel, respectively, Scott Gettinger, MD, reported at the World Conference on Lung Cancer.
“These are the first randomized trials to report 5-year outcomes for a PD-1 axis inhibitor in patients with previously treated advanced non–small lung cancer,” said Dr. Gettinger, a professor at the Yale Comprehensive Cancer Center, New Haven, Conn. “This is really unprecedented; we wouldn’t expect many patients to be out 5 years in this scenario.”
Notably, the 5-year OS benefit was seen in both trials, he said, explaining that each compared nivolumab and docetaxel, but CheckMate 017 included patients with only squamous NSCLC, and CheckMate 057 included only non–squamous NSCLC patients.
The trials randomized 272 and 582 patients, respectively, and both demonstrated significantly improved 12-month OS with nivolumab – regardless of programmed death-ligand 1 (PD-L1) expression levels. Common eligibility criteria included stage IIIb/IV disease, good performance status (ECOG performance score of 0-1), and 1 prior platinum-based chemotherapy; CheckMate 057 further allowed prior tyrosine kinase inhibitor treatment for known anaplastic lymphoma kinase (ALK) translocation or epidermal growth factor receptor (EGFR) mutation, and allowed prior maintenance therapy. Doses in both trials were 3 mg/kg of nivolumab every 2 weeks or 75 mg/m2 of intravenous docetaxel every 3 weeks until disease progression or unacceptable toxicity.
The pooled data also showed an improvement in progression-free survival (PFS) at 5 years (8% vs. 0%) with nivolumab vs. docetaxel groups.
“Again, we don’t see this in trials – more commonly we see zero patients without progression, and that’s what we saw with the docetaxel arm,” said Dr. Gettinger, who also is the Disease Aligned Research Team Leader, Thoracic Oncology Program, at the cancer center.
The median duration of responses with nivolumab was 19.9 months vs. 5.6 months with docetaxel, and 32.2% of nivolumab responders were still without progression at 5 years, he noted.
A common question in the clinic relates to the prognosis in patients who do well with PD-1 axis inhibitors, which prompted an additional analysis across the two trials, he said, noting that 60%, 78%, and 88% of patients who had not progressed at 2, 3, or 4 years, respectively, also had not progressed at 5 years, and 80%, 93%, and 100%, of patients in those groups were alive at 5 years. In the docetaxel arm, only 4, 1, and 0 patients had PFS at 2, 3, and 4, years, respectively, and none of those patients survived to 5 years, he said.
No new safety signals were seen with long-term follow-up, he added.
“In fact there was only one grade 3 or higher toxicity that was related to treatment in the nivolumab arm, and this was a grade 3 lipase elevation. There was one patient who discontinued nivolumab after 3 years, and this was for a grade 2 rash and eczema that had waxed and waned since starting nivolumab,” he said.
Also of note, 10% of nivolumab-treated patients who were off treatment at 5 years – for variable periods of time – had not progressed and had not received subsequent therapy.
“So we clearly see benefit in our patients long after they finish a course or stop for some reason,” he said.
CheckMate 017 and 057 were funded by Bristol-Myers Squibb. Dr. Gettinger reported advisory board and/or consulting work for, and/or research funding from Bristol-Myers Squibb, Nektar Therapeutics, Genentech/Roche, Iovance, and Takeda/Ariad.
SOURCE: Gettinger S et al. WCLC 2019, Abstract PR04.03.
BARCELONA – Nivolumab, compared with docetaxel chemotherapy, led to a fivefold improvement in 5-year overall survival among previously treated patients with non–small cell lung cancer (NSCLC), according to a pooled analysis of data from the phase 3 CheckMate 017 and 057 trials.
The 5-year overall survival (OS) rates from the two randomized registrational trials, which established the programmed death-1 (PD-1) inhibitor nivolumab as the standard salvage therapy for NSCLC, were 13.4% vs. 2.6% (median, 11.1 vs. 8.1 months) with nivolumab and docetaxel, respectively, Scott Gettinger, MD, reported at the World Conference on Lung Cancer.
“These are the first randomized trials to report 5-year outcomes for a PD-1 axis inhibitor in patients with previously treated advanced non–small lung cancer,” said Dr. Gettinger, a professor at the Yale Comprehensive Cancer Center, New Haven, Conn. “This is really unprecedented; we wouldn’t expect many patients to be out 5 years in this scenario.”
Notably, the 5-year OS benefit was seen in both trials, he said, explaining that each compared nivolumab and docetaxel, but CheckMate 017 included patients with only squamous NSCLC, and CheckMate 057 included only non–squamous NSCLC patients.
The trials randomized 272 and 582 patients, respectively, and both demonstrated significantly improved 12-month OS with nivolumab – regardless of programmed death-ligand 1 (PD-L1) expression levels. Common eligibility criteria included stage IIIb/IV disease, good performance status (ECOG performance score of 0-1), and 1 prior platinum-based chemotherapy; CheckMate 057 further allowed prior tyrosine kinase inhibitor treatment for known anaplastic lymphoma kinase (ALK) translocation or epidermal growth factor receptor (EGFR) mutation, and allowed prior maintenance therapy. Doses in both trials were 3 mg/kg of nivolumab every 2 weeks or 75 mg/m2 of intravenous docetaxel every 3 weeks until disease progression or unacceptable toxicity.
The pooled data also showed an improvement in progression-free survival (PFS) at 5 years (8% vs. 0%) with nivolumab vs. docetaxel groups.
“Again, we don’t see this in trials – more commonly we see zero patients without progression, and that’s what we saw with the docetaxel arm,” said Dr. Gettinger, who also is the Disease Aligned Research Team Leader, Thoracic Oncology Program, at the cancer center.
The median duration of responses with nivolumab was 19.9 months vs. 5.6 months with docetaxel, and 32.2% of nivolumab responders were still without progression at 5 years, he noted.
A common question in the clinic relates to the prognosis in patients who do well with PD-1 axis inhibitors, which prompted an additional analysis across the two trials, he said, noting that 60%, 78%, and 88% of patients who had not progressed at 2, 3, or 4 years, respectively, also had not progressed at 5 years, and 80%, 93%, and 100%, of patients in those groups were alive at 5 years. In the docetaxel arm, only 4, 1, and 0 patients had PFS at 2, 3, and 4, years, respectively, and none of those patients survived to 5 years, he said.
No new safety signals were seen with long-term follow-up, he added.
“In fact there was only one grade 3 or higher toxicity that was related to treatment in the nivolumab arm, and this was a grade 3 lipase elevation. There was one patient who discontinued nivolumab after 3 years, and this was for a grade 2 rash and eczema that had waxed and waned since starting nivolumab,” he said.
Also of note, 10% of nivolumab-treated patients who were off treatment at 5 years – for variable periods of time – had not progressed and had not received subsequent therapy.
“So we clearly see benefit in our patients long after they finish a course or stop for some reason,” he said.
CheckMate 017 and 057 were funded by Bristol-Myers Squibb. Dr. Gettinger reported advisory board and/or consulting work for, and/or research funding from Bristol-Myers Squibb, Nektar Therapeutics, Genentech/Roche, Iovance, and Takeda/Ariad.
SOURCE: Gettinger S et al. WCLC 2019, Abstract PR04.03.
REPORTING FROM WCLC 2019
Case-control study IDs several novel risk factors of post-HCT melanoma
(HCT), according to findings from a nested case-control study.
The study included 140 cases of melanoma and 557 controls matched by age at HCT, sex, primary disease, and survival time. The results showed a significantly increased melanoma risk in HCT survivors who received total body irradiation–based myeloablative conditioning, reduced-intensity conditioning with melphalan, or reduced-intensity conditioning with fludarabine, compared with those who received busulfan-based myeloablative conditioning (odds ratios, 1.77, 2.60, and 2.72, respectively), Megan M. Herr, PhD, of the division of cancer epidemiology and genetics at the National Cancer Institute, and the Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and colleagues reported in the Journal of the American Academy of Dermatology.
Melanoma risk also was increased in patients who experienced acute graft-versus-host disease (GVHD) with stage 2 or greater skin involvement (OR, 1.92 vs. those with no acute GVHD), chronic GVHD without skin involvement (OR, 1.91 vs. those with no chronic GVHD), or keratinocytic carcinoma (OR, 2.37), and in those who resided in areas with higher ambient ultraviolet radiation (OR for the highest vs. lowest tertile, 1.64).
The UV radiation finding was more pronounced for melanomas occurring 6 or more years after transplant (OR, 3.04 for highest vs. lowest tertile), whereas ambient UV radiation was not associated with melanomas occurring earlier (ORs, 1.37 for less than 3 years and 0.98 at 3-6 years), the investigators noted.
The findings, based on large-scale and detailed clinical data from the Center for International Blood and Marrow Transplant Research for HCT performed during 1985-2012, show that melanoma after HCT has a multifactorial etiology that includes patient-, transplant-, and posttransplant-related factors, they said, noting that the findings also underscore the importance of “prioritization of high-risk survivors for adherence to prevention and screening recommendations.”
Those recommendations call for routine skin examination and photoprotective precautions – particularly in HCT survivors at the highest risk – but studies of screening behaviors suggest that fewer than two-thirds of HCT survivors adhere to these recommendations, they said, concluding that further research on the cost-effectiveness of melanoma screening is warranted, as is investigation into whether current approaches are associated with melanoma risk.
This work was supported by the intramural research program of the National Cancer Institute, the National Institutes of Health, and the Department of Health & Human Services. The authors reported having no conflicts of interest.
SOURCE: Herr MM et al. J Am Acad Dermatol. 2019 Oct 22. doi: 10.1016/j.jaad.2019.10.034.
(HCT), according to findings from a nested case-control study.
The study included 140 cases of melanoma and 557 controls matched by age at HCT, sex, primary disease, and survival time. The results showed a significantly increased melanoma risk in HCT survivors who received total body irradiation–based myeloablative conditioning, reduced-intensity conditioning with melphalan, or reduced-intensity conditioning with fludarabine, compared with those who received busulfan-based myeloablative conditioning (odds ratios, 1.77, 2.60, and 2.72, respectively), Megan M. Herr, PhD, of the division of cancer epidemiology and genetics at the National Cancer Institute, and the Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and colleagues reported in the Journal of the American Academy of Dermatology.
Melanoma risk also was increased in patients who experienced acute graft-versus-host disease (GVHD) with stage 2 or greater skin involvement (OR, 1.92 vs. those with no acute GVHD), chronic GVHD without skin involvement (OR, 1.91 vs. those with no chronic GVHD), or keratinocytic carcinoma (OR, 2.37), and in those who resided in areas with higher ambient ultraviolet radiation (OR for the highest vs. lowest tertile, 1.64).
The UV radiation finding was more pronounced for melanomas occurring 6 or more years after transplant (OR, 3.04 for highest vs. lowest tertile), whereas ambient UV radiation was not associated with melanomas occurring earlier (ORs, 1.37 for less than 3 years and 0.98 at 3-6 years), the investigators noted.
The findings, based on large-scale and detailed clinical data from the Center for International Blood and Marrow Transplant Research for HCT performed during 1985-2012, show that melanoma after HCT has a multifactorial etiology that includes patient-, transplant-, and posttransplant-related factors, they said, noting that the findings also underscore the importance of “prioritization of high-risk survivors for adherence to prevention and screening recommendations.”
Those recommendations call for routine skin examination and photoprotective precautions – particularly in HCT survivors at the highest risk – but studies of screening behaviors suggest that fewer than two-thirds of HCT survivors adhere to these recommendations, they said, concluding that further research on the cost-effectiveness of melanoma screening is warranted, as is investigation into whether current approaches are associated with melanoma risk.
This work was supported by the intramural research program of the National Cancer Institute, the National Institutes of Health, and the Department of Health & Human Services. The authors reported having no conflicts of interest.
SOURCE: Herr MM et al. J Am Acad Dermatol. 2019 Oct 22. doi: 10.1016/j.jaad.2019.10.034.
(HCT), according to findings from a nested case-control study.
The study included 140 cases of melanoma and 557 controls matched by age at HCT, sex, primary disease, and survival time. The results showed a significantly increased melanoma risk in HCT survivors who received total body irradiation–based myeloablative conditioning, reduced-intensity conditioning with melphalan, or reduced-intensity conditioning with fludarabine, compared with those who received busulfan-based myeloablative conditioning (odds ratios, 1.77, 2.60, and 2.72, respectively), Megan M. Herr, PhD, of the division of cancer epidemiology and genetics at the National Cancer Institute, and the Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and colleagues reported in the Journal of the American Academy of Dermatology.
Melanoma risk also was increased in patients who experienced acute graft-versus-host disease (GVHD) with stage 2 or greater skin involvement (OR, 1.92 vs. those with no acute GVHD), chronic GVHD without skin involvement (OR, 1.91 vs. those with no chronic GVHD), or keratinocytic carcinoma (OR, 2.37), and in those who resided in areas with higher ambient ultraviolet radiation (OR for the highest vs. lowest tertile, 1.64).
The UV radiation finding was more pronounced for melanomas occurring 6 or more years after transplant (OR, 3.04 for highest vs. lowest tertile), whereas ambient UV radiation was not associated with melanomas occurring earlier (ORs, 1.37 for less than 3 years and 0.98 at 3-6 years), the investigators noted.
The findings, based on large-scale and detailed clinical data from the Center for International Blood and Marrow Transplant Research for HCT performed during 1985-2012, show that melanoma after HCT has a multifactorial etiology that includes patient-, transplant-, and posttransplant-related factors, they said, noting that the findings also underscore the importance of “prioritization of high-risk survivors for adherence to prevention and screening recommendations.”
Those recommendations call for routine skin examination and photoprotective precautions – particularly in HCT survivors at the highest risk – but studies of screening behaviors suggest that fewer than two-thirds of HCT survivors adhere to these recommendations, they said, concluding that further research on the cost-effectiveness of melanoma screening is warranted, as is investigation into whether current approaches are associated with melanoma risk.
This work was supported by the intramural research program of the National Cancer Institute, the National Institutes of Health, and the Department of Health & Human Services. The authors reported having no conflicts of interest.
SOURCE: Herr MM et al. J Am Acad Dermatol. 2019 Oct 22. doi: 10.1016/j.jaad.2019.10.034.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Insomnia symptoms increase likelihood of stroke and heart disease
, according to a large cohort study of adults in China. A greater number of insomnia symptoms is associated with increased risk, and this relationship is more evident in younger adults and in adults without hypertension at baseline, researchers reported Nov. 6 in Neurology.
“These results suggest that, if we can target people who are having trouble sleeping with behavioral therapies, it’s possible that we could reduce the number of cases of stroke, heart attack, and other diseases later down the line,” study author Liming Li, MD, professor of epidemiology at Peking University, Beijing, said in a news release.
To clarify the relationships between individual insomnia symptoms, cardiocerebral vascular diseases, and potential effect modifiers, Dr. Li and colleagues analyzed data from the China Kadoorie Biobank Study. For this study, more than 500,000 adults in China aged 30-79 years completed a baseline survey during 2004-2008. The present analysis included data from 487,200 participants who did not have a history of stroke, coronary heart disease, or cancer at baseline.
For the baseline survey, participants answered questions about whether specific insomnia symptoms occurred at least 3 days per week during the past month. The symptoms included difficulty initiating or maintaining sleep (that is, sleep onset latency of 30 minutes or more after going to bed or waking up in the middle of the night); waking too early and being unable to fall back asleep; and trouble functioning during the day because of bad sleep.
The researchers assessed the incidence of cardiocerebral vascular diseases through 2016 by examining disease registries, national health insurance claims databases, and local records. Investigators identified participants with any cardiocerebral vascular disease and assessed the incidence of ischemic heart disease, acute myocardial infarction, hemorrhagic stroke, and ischemic stroke. The researchers followed each participant until the diagnosis of a cardiocerebral vascular disease outcome, death from any cause, loss to follow-up, or Dec. 31, 2016. The researchers used Cox proportional hazard models to estimate hazard ratios for the association between each insomnia symptom and cardiocerebral vascular disease outcomes. They adjusted the models for established and potential confounding factors, including age, income, smoking status, diet, and physical activity.
More than 16% had any insomnia symptom
Of the 487,200 participants, 11.3% had difficulty initiating or maintaining sleep, 10.4% had early morning awakening, and 2.2% had daytime dysfunction attributed to poor sleep. Compared with participants without insomnia symptoms, participants with insomnia symptoms tended to be older and were more likely to be female, not married, and from a rural area. In addition, those with insomnia symptoms were more likely have depression or anxiety symptoms, lower education level, lower household income, and lower body mass index. They also were more likely to have a history of diabetes mellitus. During a median follow-up of 9.6 years, 130,032 cases of cardiocerebral vascular disease occurred, including 40,348 cases of ischemic heart disease and 45,316 cases of stroke.
After adjustment for potential confounders, each insomnia symptom was associated with greater risk of cardiocerebral vascular disease. For difficulty initiating or maintaining sleep, the hazard ratio was 1.09. For early-morning awakening, the HR was 1.07. For daytime dysfunction, the HR was 1.13. Each insomnia symptom was associated with increased risk of ischemic heart disease and ischemic stroke, whereas only difficulty initiating or maintaining sleep was associated with increased risk of acute MI.
In all, 16.4% of participants reported any insomnia symptom; 10% had one symptom, 5.2% had two symptoms, and 1.2% had three symptoms. “Compared with those without any insomnia symptoms, participants with one, two, or three symptoms had a 7%, 10%, or 18% higher risk of total [cardiocerebral vascular disease] incidence, respectively,” the authors wrote. “Our study is the first large-scale cohort study that identified positive dose-response relationships between the number of insomnia symptoms and risks of [cardiocerebral vascular diseases, ischemic heart disease] and stroke incidence.”
Opportunity for intervention
Compared with clinical diagnostic criteria for insomnia, “individual insomnia symptoms are better defined and more feasible to assess with questionnaires in large-scale population studies and clinical practice,” Dr. Li and colleagues wrote. “Moreover, it is reasonable that insomnia symptoms are more modifiable and precisely targetable through behavioral therapies before developing into clinically significant insomnia disorder. Therefore, future clinical trials or community-based intervention studies should be conducted to test whether lifestyle or sleep hygiene interventions for insomnia symptoms can reduce subsequent [cardiocerebral vascular disease] risks.”
The results suggest that efforts aimed at early detection and intervention should include a focus on younger adults and people who do not have high blood pressure, Dr. Li said.
The self-reported insomnia symptoms used in this study have not been fully validated, the investigators noted. The researchers also lacked information about potential confounders, such as shift work and obstructive sleep apnea, that are risk factors for coronary heart disease or stroke and may interfere with insomnia symptoms. In addition, the study did not capture changes in insomnia symptoms over time.
This study was supported by the National Key Research and Development Program of China, the Chinese Ministry of Science and Technology, and the National Natural Science Foundation of China. The China Kadoorie Biobank surveys were supported by grants from the Kadoorie Charitable Foundation and the U.K. Wellcome Trust. The authors had no relevant disclosures.
SOURCE: Zheng B et al. Neurology. 2019 Nov 6. doi: 10.1212/WNL.0000000000008581.
, according to a large cohort study of adults in China. A greater number of insomnia symptoms is associated with increased risk, and this relationship is more evident in younger adults and in adults without hypertension at baseline, researchers reported Nov. 6 in Neurology.
“These results suggest that, if we can target people who are having trouble sleeping with behavioral therapies, it’s possible that we could reduce the number of cases of stroke, heart attack, and other diseases later down the line,” study author Liming Li, MD, professor of epidemiology at Peking University, Beijing, said in a news release.
To clarify the relationships between individual insomnia symptoms, cardiocerebral vascular diseases, and potential effect modifiers, Dr. Li and colleagues analyzed data from the China Kadoorie Biobank Study. For this study, more than 500,000 adults in China aged 30-79 years completed a baseline survey during 2004-2008. The present analysis included data from 487,200 participants who did not have a history of stroke, coronary heart disease, or cancer at baseline.
For the baseline survey, participants answered questions about whether specific insomnia symptoms occurred at least 3 days per week during the past month. The symptoms included difficulty initiating or maintaining sleep (that is, sleep onset latency of 30 minutes or more after going to bed or waking up in the middle of the night); waking too early and being unable to fall back asleep; and trouble functioning during the day because of bad sleep.
The researchers assessed the incidence of cardiocerebral vascular diseases through 2016 by examining disease registries, national health insurance claims databases, and local records. Investigators identified participants with any cardiocerebral vascular disease and assessed the incidence of ischemic heart disease, acute myocardial infarction, hemorrhagic stroke, and ischemic stroke. The researchers followed each participant until the diagnosis of a cardiocerebral vascular disease outcome, death from any cause, loss to follow-up, or Dec. 31, 2016. The researchers used Cox proportional hazard models to estimate hazard ratios for the association between each insomnia symptom and cardiocerebral vascular disease outcomes. They adjusted the models for established and potential confounding factors, including age, income, smoking status, diet, and physical activity.
More than 16% had any insomnia symptom
Of the 487,200 participants, 11.3% had difficulty initiating or maintaining sleep, 10.4% had early morning awakening, and 2.2% had daytime dysfunction attributed to poor sleep. Compared with participants without insomnia symptoms, participants with insomnia symptoms tended to be older and were more likely to be female, not married, and from a rural area. In addition, those with insomnia symptoms were more likely have depression or anxiety symptoms, lower education level, lower household income, and lower body mass index. They also were more likely to have a history of diabetes mellitus. During a median follow-up of 9.6 years, 130,032 cases of cardiocerebral vascular disease occurred, including 40,348 cases of ischemic heart disease and 45,316 cases of stroke.
After adjustment for potential confounders, each insomnia symptom was associated with greater risk of cardiocerebral vascular disease. For difficulty initiating or maintaining sleep, the hazard ratio was 1.09. For early-morning awakening, the HR was 1.07. For daytime dysfunction, the HR was 1.13. Each insomnia symptom was associated with increased risk of ischemic heart disease and ischemic stroke, whereas only difficulty initiating or maintaining sleep was associated with increased risk of acute MI.
In all, 16.4% of participants reported any insomnia symptom; 10% had one symptom, 5.2% had two symptoms, and 1.2% had three symptoms. “Compared with those without any insomnia symptoms, participants with one, two, or three symptoms had a 7%, 10%, or 18% higher risk of total [cardiocerebral vascular disease] incidence, respectively,” the authors wrote. “Our study is the first large-scale cohort study that identified positive dose-response relationships between the number of insomnia symptoms and risks of [cardiocerebral vascular diseases, ischemic heart disease] and stroke incidence.”
Opportunity for intervention
Compared with clinical diagnostic criteria for insomnia, “individual insomnia symptoms are better defined and more feasible to assess with questionnaires in large-scale population studies and clinical practice,” Dr. Li and colleagues wrote. “Moreover, it is reasonable that insomnia symptoms are more modifiable and precisely targetable through behavioral therapies before developing into clinically significant insomnia disorder. Therefore, future clinical trials or community-based intervention studies should be conducted to test whether lifestyle or sleep hygiene interventions for insomnia symptoms can reduce subsequent [cardiocerebral vascular disease] risks.”
The results suggest that efforts aimed at early detection and intervention should include a focus on younger adults and people who do not have high blood pressure, Dr. Li said.
The self-reported insomnia symptoms used in this study have not been fully validated, the investigators noted. The researchers also lacked information about potential confounders, such as shift work and obstructive sleep apnea, that are risk factors for coronary heart disease or stroke and may interfere with insomnia symptoms. In addition, the study did not capture changes in insomnia symptoms over time.
This study was supported by the National Key Research and Development Program of China, the Chinese Ministry of Science and Technology, and the National Natural Science Foundation of China. The China Kadoorie Biobank surveys were supported by grants from the Kadoorie Charitable Foundation and the U.K. Wellcome Trust. The authors had no relevant disclosures.
SOURCE: Zheng B et al. Neurology. 2019 Nov 6. doi: 10.1212/WNL.0000000000008581.
, according to a large cohort study of adults in China. A greater number of insomnia symptoms is associated with increased risk, and this relationship is more evident in younger adults and in adults without hypertension at baseline, researchers reported Nov. 6 in Neurology.
“These results suggest that, if we can target people who are having trouble sleeping with behavioral therapies, it’s possible that we could reduce the number of cases of stroke, heart attack, and other diseases later down the line,” study author Liming Li, MD, professor of epidemiology at Peking University, Beijing, said in a news release.
To clarify the relationships between individual insomnia symptoms, cardiocerebral vascular diseases, and potential effect modifiers, Dr. Li and colleagues analyzed data from the China Kadoorie Biobank Study. For this study, more than 500,000 adults in China aged 30-79 years completed a baseline survey during 2004-2008. The present analysis included data from 487,200 participants who did not have a history of stroke, coronary heart disease, or cancer at baseline.
For the baseline survey, participants answered questions about whether specific insomnia symptoms occurred at least 3 days per week during the past month. The symptoms included difficulty initiating or maintaining sleep (that is, sleep onset latency of 30 minutes or more after going to bed or waking up in the middle of the night); waking too early and being unable to fall back asleep; and trouble functioning during the day because of bad sleep.
The researchers assessed the incidence of cardiocerebral vascular diseases through 2016 by examining disease registries, national health insurance claims databases, and local records. Investigators identified participants with any cardiocerebral vascular disease and assessed the incidence of ischemic heart disease, acute myocardial infarction, hemorrhagic stroke, and ischemic stroke. The researchers followed each participant until the diagnosis of a cardiocerebral vascular disease outcome, death from any cause, loss to follow-up, or Dec. 31, 2016. The researchers used Cox proportional hazard models to estimate hazard ratios for the association between each insomnia symptom and cardiocerebral vascular disease outcomes. They adjusted the models for established and potential confounding factors, including age, income, smoking status, diet, and physical activity.
More than 16% had any insomnia symptom
Of the 487,200 participants, 11.3% had difficulty initiating or maintaining sleep, 10.4% had early morning awakening, and 2.2% had daytime dysfunction attributed to poor sleep. Compared with participants without insomnia symptoms, participants with insomnia symptoms tended to be older and were more likely to be female, not married, and from a rural area. In addition, those with insomnia symptoms were more likely have depression or anxiety symptoms, lower education level, lower household income, and lower body mass index. They also were more likely to have a history of diabetes mellitus. During a median follow-up of 9.6 years, 130,032 cases of cardiocerebral vascular disease occurred, including 40,348 cases of ischemic heart disease and 45,316 cases of stroke.
After adjustment for potential confounders, each insomnia symptom was associated with greater risk of cardiocerebral vascular disease. For difficulty initiating or maintaining sleep, the hazard ratio was 1.09. For early-morning awakening, the HR was 1.07. For daytime dysfunction, the HR was 1.13. Each insomnia symptom was associated with increased risk of ischemic heart disease and ischemic stroke, whereas only difficulty initiating or maintaining sleep was associated with increased risk of acute MI.
In all, 16.4% of participants reported any insomnia symptom; 10% had one symptom, 5.2% had two symptoms, and 1.2% had three symptoms. “Compared with those without any insomnia symptoms, participants with one, two, or three symptoms had a 7%, 10%, or 18% higher risk of total [cardiocerebral vascular disease] incidence, respectively,” the authors wrote. “Our study is the first large-scale cohort study that identified positive dose-response relationships between the number of insomnia symptoms and risks of [cardiocerebral vascular diseases, ischemic heart disease] and stroke incidence.”
Opportunity for intervention
Compared with clinical diagnostic criteria for insomnia, “individual insomnia symptoms are better defined and more feasible to assess with questionnaires in large-scale population studies and clinical practice,” Dr. Li and colleagues wrote. “Moreover, it is reasonable that insomnia symptoms are more modifiable and precisely targetable through behavioral therapies before developing into clinically significant insomnia disorder. Therefore, future clinical trials or community-based intervention studies should be conducted to test whether lifestyle or sleep hygiene interventions for insomnia symptoms can reduce subsequent [cardiocerebral vascular disease] risks.”
The results suggest that efforts aimed at early detection and intervention should include a focus on younger adults and people who do not have high blood pressure, Dr. Li said.
The self-reported insomnia symptoms used in this study have not been fully validated, the investigators noted. The researchers also lacked information about potential confounders, such as shift work and obstructive sleep apnea, that are risk factors for coronary heart disease or stroke and may interfere with insomnia symptoms. In addition, the study did not capture changes in insomnia symptoms over time.
This study was supported by the National Key Research and Development Program of China, the Chinese Ministry of Science and Technology, and the National Natural Science Foundation of China. The China Kadoorie Biobank surveys were supported by grants from the Kadoorie Charitable Foundation and the U.K. Wellcome Trust. The authors had no relevant disclosures.
SOURCE: Zheng B et al. Neurology. 2019 Nov 6. doi: 10.1212/WNL.0000000000008581.
FROM NEUROLOGY
Key clinical point: The presence of insomnia symptoms increases the likelihood of cardiovascular or cerebrovascular disease during approximately 10 years of follow-up.
Major finding: After adjustment for potential confounders, each insomnia symptom was associated with greater risk of cardiocerebral vascular disease. For difficulty initiating or maintaining sleep, the hazard ratio was 1.09. For early-morning awakening, the HR was 1.07. For daytime dysfunction, the HR was 1.13.
Study details: An analysis of data from 487,200 adults in China aged 30-79 years who completed a baseline survey during 2004-2008 and were followed through 2016.
Disclosures: This study was supported by the National Key Research and Development Program of China, the Chinese Ministry of Science and Technology, and the National Natural Science Foundation of China. The China Kadoorie Biobank surveys were supported by grants from the Kadoorie Charitable Foundation and the U.K. Wellcome Trust. The authors had no relevant disclosures.
Source: Zheng B et al. Neurology. 2019 Nov 6. doi: 10.1212/WNL.0000000000008581.