User login
Young BRCA carriers with BC history may safely opt for pregnancy
Key clinical point: Women with germline BRCA1 or BRCA2 pathogenic mutations who had a pregnancy after diagnosis of early breast cancer (BC) reported prognostic outcomes similar to that of women without a pregnancy.
Major finding: The cumulative incidence of pregnancy was 22% at 10 years. The disease-free survival outcomes were comparable between patients with BC who did vs did not become pregnant (adjusted hazard ratio 0.99; P = .90).
Study details: Findings are from a retrospective cohort study including 4732 young women age ≤ 40 years with a history of BC who had germline pathogenic BRCA mutations, of whom 659 women reported ≥1 pregnancy after BC.
Disclosures: The study was partly supported by the Italian Association for Cancer Research and the 2022 Gilead Research Scholars Program in Solid Tumors. The authors declared receiving speaker honoraria, travel grants, research funding, or speaker fees from and having other ties with Gilead and several other sources.
Source: Lambertini M et al. Pregnancy after breast cancer in young BRCA carriers: An international hospital-based cohort study. JAMA. 2023 (Dec 7). doi: 10.1001/jama.2023.25463
Key clinical point: Women with germline BRCA1 or BRCA2 pathogenic mutations who had a pregnancy after diagnosis of early breast cancer (BC) reported prognostic outcomes similar to that of women without a pregnancy.
Major finding: The cumulative incidence of pregnancy was 22% at 10 years. The disease-free survival outcomes were comparable between patients with BC who did vs did not become pregnant (adjusted hazard ratio 0.99; P = .90).
Study details: Findings are from a retrospective cohort study including 4732 young women age ≤ 40 years with a history of BC who had germline pathogenic BRCA mutations, of whom 659 women reported ≥1 pregnancy after BC.
Disclosures: The study was partly supported by the Italian Association for Cancer Research and the 2022 Gilead Research Scholars Program in Solid Tumors. The authors declared receiving speaker honoraria, travel grants, research funding, or speaker fees from and having other ties with Gilead and several other sources.
Source: Lambertini M et al. Pregnancy after breast cancer in young BRCA carriers: An international hospital-based cohort study. JAMA. 2023 (Dec 7). doi: 10.1001/jama.2023.25463
Key clinical point: Women with germline BRCA1 or BRCA2 pathogenic mutations who had a pregnancy after diagnosis of early breast cancer (BC) reported prognostic outcomes similar to that of women without a pregnancy.
Major finding: The cumulative incidence of pregnancy was 22% at 10 years. The disease-free survival outcomes were comparable between patients with BC who did vs did not become pregnant (adjusted hazard ratio 0.99; P = .90).
Study details: Findings are from a retrospective cohort study including 4732 young women age ≤ 40 years with a history of BC who had germline pathogenic BRCA mutations, of whom 659 women reported ≥1 pregnancy after BC.
Disclosures: The study was partly supported by the Italian Association for Cancer Research and the 2022 Gilead Research Scholars Program in Solid Tumors. The authors declared receiving speaker honoraria, travel grants, research funding, or speaker fees from and having other ties with Gilead and several other sources.
Source: Lambertini M et al. Pregnancy after breast cancer in young BRCA carriers: An international hospital-based cohort study. JAMA. 2023 (Dec 7). doi: 10.1001/jama.2023.25463
Sodium deoxycholate and triamcinolone: A good mix?
In September 2023, Goldman et al. published a communication in Dermatologic Surgery describing their use of subcutaneous sodium deoxycholate injection (SDOC), with or without triamcinolone acetonide, for reduction of submental fat..
As they note, “patients experience a variable degree of edema and discomfort following subcutaneous injection,” of SDOC, something that I and others have also observed in our practices.
In their double-blind study of 20 patients with a baseline Clinician-Reported Submental Fat Rating Scale of 2 or 3 out of 4, 5 patients were randomized to receive SDOC as recommended in the label, while 15 received SDOC plus triamcinolone. In the latter group, 2 mL of SDOC was mixed with 0.5 mL of 40 mg/mL of triamcinolone acetate, then administered in up to 50 injections in the submentum spaced 1.0 cm apart at 0.25 mL per injection. Three treatments were administered 1 month apart.
For both groups, volumes between 5 mL and 8 mL per treatment were delivered. There were no significant differences in efficacy 30, 60, and 90 days after the final injection between the two groups. However, at day 180, the group that received only SDOC had a significantly greater reduction in submental fat, which the authors wrote indicated that the addition of triamcinolone “may mildly diminish the fat reduction effects” at that time point.
Subcutaneous SDOC (deoxycholic acid) injections for reduction of submental fullness was approved by the Food and Drug Administration in 2015 for improving the appearance of moderate to severe convexity or fullness associated with submental fat in adults. (I was involved in the clinical trials.) We found that in the trial, for optimal efficacy, most patients require two to four treatments spread at least a month apart, with patients who had larger treatment areas requiring up to six treatments.
While the clinical trial treatments were spaced 4 weeks apart, post approval, we found that patients would sometimes report further efficacy even 2-3 months post injection. Since not everyone wants to go around with edema every month for 2-4 consecutive months, spacing the treatments farther apart allows patients more time to heal and coordinate the recovery appearance around their work and social schedules.
In my practice, very rarely have we seen minimal to moderate prolonged edema, particularly in younger patients, beyond 1 month post injection. Most people have the most noticeable edema — the “bull-frog” appearance — for the first 1-3 days, with some minor fullness that appears to be almost back to baseline at 1 week. In some of these patients with prolonged submental fullness, it looks fuller than it appeared pretreatment even months afterwards.
While rare, like the study authors, I have found intralesional triamcinolone to be helpful at reducing this persistent fullness should it occur. It is likely to be reducing any persistent inflammation or posttreatment fibrosis in these patients.
Unlike the study authors, I do not combine SDOC and triamcinolone injections at the time of treatment. Rather, I consider injecting triamcinolone if submental fullness is greater than at baseline or edema persists after SDOC treatment. It is rare that I’ve had to do this, as most cases self-resolve, but I have used triamcinolone 10 mg/mL, up to 1cc total, injected 6-8 weeks apart one to three times to the affected area and found it to be effective if fullness has persisted beyond 6 months. Liposuction may also be an option, if needed, if fullness/edema persists.
Overall, SDOC is an effective treatment for small pockets of subcutaneous fat. Approved for submental fullness, it is now sometimes used off-label for other parts of the body, such as bra fat, small pockets of the abdomen, and lipomas. While some inflammation after treatment is expected — and desired — to achieve an effective outcome of fat apoptosis, intralesional triamcinolone is an interesting tool to utilize should inflammation or posttreatment fullness persist.
Dr. Wesley practices dermatology in Beverly Hills, California. Write to her at [email protected]. She was an investigator in clinical trials of Kybella.
In September 2023, Goldman et al. published a communication in Dermatologic Surgery describing their use of subcutaneous sodium deoxycholate injection (SDOC), with or without triamcinolone acetonide, for reduction of submental fat..
As they note, “patients experience a variable degree of edema and discomfort following subcutaneous injection,” of SDOC, something that I and others have also observed in our practices.
In their double-blind study of 20 patients with a baseline Clinician-Reported Submental Fat Rating Scale of 2 or 3 out of 4, 5 patients were randomized to receive SDOC as recommended in the label, while 15 received SDOC plus triamcinolone. In the latter group, 2 mL of SDOC was mixed with 0.5 mL of 40 mg/mL of triamcinolone acetate, then administered in up to 50 injections in the submentum spaced 1.0 cm apart at 0.25 mL per injection. Three treatments were administered 1 month apart.
For both groups, volumes between 5 mL and 8 mL per treatment were delivered. There were no significant differences in efficacy 30, 60, and 90 days after the final injection between the two groups. However, at day 180, the group that received only SDOC had a significantly greater reduction in submental fat, which the authors wrote indicated that the addition of triamcinolone “may mildly diminish the fat reduction effects” at that time point.
Subcutaneous SDOC (deoxycholic acid) injections for reduction of submental fullness was approved by the Food and Drug Administration in 2015 for improving the appearance of moderate to severe convexity or fullness associated with submental fat in adults. (I was involved in the clinical trials.) We found that in the trial, for optimal efficacy, most patients require two to four treatments spread at least a month apart, with patients who had larger treatment areas requiring up to six treatments.
While the clinical trial treatments were spaced 4 weeks apart, post approval, we found that patients would sometimes report further efficacy even 2-3 months post injection. Since not everyone wants to go around with edema every month for 2-4 consecutive months, spacing the treatments farther apart allows patients more time to heal and coordinate the recovery appearance around their work and social schedules.
In my practice, very rarely have we seen minimal to moderate prolonged edema, particularly in younger patients, beyond 1 month post injection. Most people have the most noticeable edema — the “bull-frog” appearance — for the first 1-3 days, with some minor fullness that appears to be almost back to baseline at 1 week. In some of these patients with prolonged submental fullness, it looks fuller than it appeared pretreatment even months afterwards.
While rare, like the study authors, I have found intralesional triamcinolone to be helpful at reducing this persistent fullness should it occur. It is likely to be reducing any persistent inflammation or posttreatment fibrosis in these patients.
Unlike the study authors, I do not combine SDOC and triamcinolone injections at the time of treatment. Rather, I consider injecting triamcinolone if submental fullness is greater than at baseline or edema persists after SDOC treatment. It is rare that I’ve had to do this, as most cases self-resolve, but I have used triamcinolone 10 mg/mL, up to 1cc total, injected 6-8 weeks apart one to three times to the affected area and found it to be effective if fullness has persisted beyond 6 months. Liposuction may also be an option, if needed, if fullness/edema persists.
Overall, SDOC is an effective treatment for small pockets of subcutaneous fat. Approved for submental fullness, it is now sometimes used off-label for other parts of the body, such as bra fat, small pockets of the abdomen, and lipomas. While some inflammation after treatment is expected — and desired — to achieve an effective outcome of fat apoptosis, intralesional triamcinolone is an interesting tool to utilize should inflammation or posttreatment fullness persist.
Dr. Wesley practices dermatology in Beverly Hills, California. Write to her at [email protected]. She was an investigator in clinical trials of Kybella.
In September 2023, Goldman et al. published a communication in Dermatologic Surgery describing their use of subcutaneous sodium deoxycholate injection (SDOC), with or without triamcinolone acetonide, for reduction of submental fat..
As they note, “patients experience a variable degree of edema and discomfort following subcutaneous injection,” of SDOC, something that I and others have also observed in our practices.
In their double-blind study of 20 patients with a baseline Clinician-Reported Submental Fat Rating Scale of 2 or 3 out of 4, 5 patients were randomized to receive SDOC as recommended in the label, while 15 received SDOC plus triamcinolone. In the latter group, 2 mL of SDOC was mixed with 0.5 mL of 40 mg/mL of triamcinolone acetate, then administered in up to 50 injections in the submentum spaced 1.0 cm apart at 0.25 mL per injection. Three treatments were administered 1 month apart.
For both groups, volumes between 5 mL and 8 mL per treatment were delivered. There were no significant differences in efficacy 30, 60, and 90 days after the final injection between the two groups. However, at day 180, the group that received only SDOC had a significantly greater reduction in submental fat, which the authors wrote indicated that the addition of triamcinolone “may mildly diminish the fat reduction effects” at that time point.
Subcutaneous SDOC (deoxycholic acid) injections for reduction of submental fullness was approved by the Food and Drug Administration in 2015 for improving the appearance of moderate to severe convexity or fullness associated with submental fat in adults. (I was involved in the clinical trials.) We found that in the trial, for optimal efficacy, most patients require two to four treatments spread at least a month apart, with patients who had larger treatment areas requiring up to six treatments.
While the clinical trial treatments were spaced 4 weeks apart, post approval, we found that patients would sometimes report further efficacy even 2-3 months post injection. Since not everyone wants to go around with edema every month for 2-4 consecutive months, spacing the treatments farther apart allows patients more time to heal and coordinate the recovery appearance around their work and social schedules.
In my practice, very rarely have we seen minimal to moderate prolonged edema, particularly in younger patients, beyond 1 month post injection. Most people have the most noticeable edema — the “bull-frog” appearance — for the first 1-3 days, with some minor fullness that appears to be almost back to baseline at 1 week. In some of these patients with prolonged submental fullness, it looks fuller than it appeared pretreatment even months afterwards.
While rare, like the study authors, I have found intralesional triamcinolone to be helpful at reducing this persistent fullness should it occur. It is likely to be reducing any persistent inflammation or posttreatment fibrosis in these patients.
Unlike the study authors, I do not combine SDOC and triamcinolone injections at the time of treatment. Rather, I consider injecting triamcinolone if submental fullness is greater than at baseline or edema persists after SDOC treatment. It is rare that I’ve had to do this, as most cases self-resolve, but I have used triamcinolone 10 mg/mL, up to 1cc total, injected 6-8 weeks apart one to three times to the affected area and found it to be effective if fullness has persisted beyond 6 months. Liposuction may also be an option, if needed, if fullness/edema persists.
Overall, SDOC is an effective treatment for small pockets of subcutaneous fat. Approved for submental fullness, it is now sometimes used off-label for other parts of the body, such as bra fat, small pockets of the abdomen, and lipomas. While some inflammation after treatment is expected — and desired — to achieve an effective outcome of fat apoptosis, intralesional triamcinolone is an interesting tool to utilize should inflammation or posttreatment fullness persist.
Dr. Wesley practices dermatology in Beverly Hills, California. Write to her at [email protected]. She was an investigator in clinical trials of Kybella.
GLP-1 RAs Associated With Reduced Colorectal Cancer Risk in Patients With Type 2 Diabetes
according to a new analysis.
In particular, GLP-1 RAs were associated with decreased risk compared with other antidiabetic treatments, including insulin, metformin, sodium-glucose cotransporter 2 (SGLT2) inhibitors, sulfonylureas, and thiazolidinediones.
More profound effects were seen in patients with overweight or obesity, “suggesting a potential protective effect against CRC partially mediated by weight loss and other mechanisms related to weight loss,” Lindsey Wang, an undergraduate student at Case Western Reserve University, Cleveland, Ohio, and colleagues wrote in JAMA Oncology.
Testing Treatments
GLP-1 RAs, usually given by injection, are approved by the US Food and Drug Administration to treat type 2 diabetes. They can lower blood sugar levels, improve insulin sensitivity, and help patients manage their weight.
Diabetes, overweight, and obesity are known risk factors for CRC and make prognosis worse. Ms. Wang and colleagues hypothesized that GLP-1 RAs might reduce CRC risk compared with other antidiabetics, including metformin and insulin, which have also been shown to reduce CRC risk.
Using a national database of more than 101 million electronic health records, Ms. Wang and colleagues conducted a population-based study of more than 1.2 million patients who had medical encounters for type 2 diabetes and were subsequently prescribed antidiabetic medications between 2005 and 2019. The patients had no prior antidiabetic medication use nor CRC diagnosis.
The researchers analyzed the effects of GLP-1 RAs on CRC incidence compared with the other prescribed antidiabetic drugs, matching for demographics, adverse socioeconomic determinants of health, preexisting medical conditions, family and personal history of cancers and colonic polyps, lifestyle factors, and procedures such as colonoscopy.
During a 15-year follow-up, GLP-1 RAs were associated with decreased risk for CRC compared with insulin (hazard ratio [HR], 0.56), metformin (HR, 0.75), SGLT2 inhibitors (HR, 0.77), sulfonylureas (HR, 0.82), and thiazolidinediones (HR, 0.82) in the overall study population.
For instance, among 22,572 patients who took insulin, 167 cases of CRC occurred, compared with 94 cases among the matched GLP-1 RA cohort. Among 18,518 patients who took metformin, 153 cases of CRC occurred compared with 96 cases among the matched GLP-1 RA cohort.
GLP-1 RAs also were associated with lower but not statistically significant risk than alpha-glucosidase inhibitors (HR, 0.59) and dipeptidyl-peptidase-4 (DPP-4) inhibitors (HR, 0.93).
In patients with overweight or obesity, GLP-1 RAs were associated with a lower risk for CRC than most of the other antidiabetics, including insulin (HR, 0.5), metformin (HR, 0.58), SGLT2 inhibitors (HR, 0.68), sulfonylureas (HR, 0.63), thiazolidinediones (HR, 0.73), and DPP-4 inhibitors (HR, 0.77).
Consistent findings were observed in women and men.
“Our results clearly demonstrate that GLP-1 RAs are significantly more effective than popular antidiabetic drugs, such as metformin or insulin, at preventing the development of CRC,” said Nathan Berger, MD, co-lead researcher, professor of experimental medicine, and member of the Case Comprehensive Cancer Center.
Targets for Future Research
Study limitations include potential unmeasured or uncontrolled confounders, self-selection, reverse causality, and other biases involved in observational studies, the research team noted.
Further research is warranted to investigate the effects in patients with prior antidiabetic treatments, underlying mechanisms, potential variation in effects among different GLP-1 RAs, and the potential of GLP-1 RAs to reduce the risks for other obesity-associated cancers, the researchers wrote.
“To our knowledge, this is the first indication this popular weight loss and antidiabetic class of drugs reduces incidence of CRC, relative to other antidiabetic agents,” said Rong Xu, PhD, co-lead researcher, professor of medicine, and member of the Case Comprehensive Cancer Center.
The study was supported by the National Cancer Institute Case Comprehensive Cancer Center, American Cancer Society, Landon Foundation-American Association for Cancer Research, National Institutes of Health Director’s New Innovator Award Program, National Institute on Aging, and National Institute on Alcohol Abuse and Alcoholism. Several authors reported grants from the National Institutes of Health during the conduct of the study.
A version of this article appeared on Medscape.com.
according to a new analysis.
In particular, GLP-1 RAs were associated with decreased risk compared with other antidiabetic treatments, including insulin, metformin, sodium-glucose cotransporter 2 (SGLT2) inhibitors, sulfonylureas, and thiazolidinediones.
More profound effects were seen in patients with overweight or obesity, “suggesting a potential protective effect against CRC partially mediated by weight loss and other mechanisms related to weight loss,” Lindsey Wang, an undergraduate student at Case Western Reserve University, Cleveland, Ohio, and colleagues wrote in JAMA Oncology.
Testing Treatments
GLP-1 RAs, usually given by injection, are approved by the US Food and Drug Administration to treat type 2 diabetes. They can lower blood sugar levels, improve insulin sensitivity, and help patients manage their weight.
Diabetes, overweight, and obesity are known risk factors for CRC and make prognosis worse. Ms. Wang and colleagues hypothesized that GLP-1 RAs might reduce CRC risk compared with other antidiabetics, including metformin and insulin, which have also been shown to reduce CRC risk.
Using a national database of more than 101 million electronic health records, Ms. Wang and colleagues conducted a population-based study of more than 1.2 million patients who had medical encounters for type 2 diabetes and were subsequently prescribed antidiabetic medications between 2005 and 2019. The patients had no prior antidiabetic medication use nor CRC diagnosis.
The researchers analyzed the effects of GLP-1 RAs on CRC incidence compared with the other prescribed antidiabetic drugs, matching for demographics, adverse socioeconomic determinants of health, preexisting medical conditions, family and personal history of cancers and colonic polyps, lifestyle factors, and procedures such as colonoscopy.
During a 15-year follow-up, GLP-1 RAs were associated with decreased risk for CRC compared with insulin (hazard ratio [HR], 0.56), metformin (HR, 0.75), SGLT2 inhibitors (HR, 0.77), sulfonylureas (HR, 0.82), and thiazolidinediones (HR, 0.82) in the overall study population.
For instance, among 22,572 patients who took insulin, 167 cases of CRC occurred, compared with 94 cases among the matched GLP-1 RA cohort. Among 18,518 patients who took metformin, 153 cases of CRC occurred compared with 96 cases among the matched GLP-1 RA cohort.
GLP-1 RAs also were associated with lower but not statistically significant risk than alpha-glucosidase inhibitors (HR, 0.59) and dipeptidyl-peptidase-4 (DPP-4) inhibitors (HR, 0.93).
In patients with overweight or obesity, GLP-1 RAs were associated with a lower risk for CRC than most of the other antidiabetics, including insulin (HR, 0.5), metformin (HR, 0.58), SGLT2 inhibitors (HR, 0.68), sulfonylureas (HR, 0.63), thiazolidinediones (HR, 0.73), and DPP-4 inhibitors (HR, 0.77).
Consistent findings were observed in women and men.
“Our results clearly demonstrate that GLP-1 RAs are significantly more effective than popular antidiabetic drugs, such as metformin or insulin, at preventing the development of CRC,” said Nathan Berger, MD, co-lead researcher, professor of experimental medicine, and member of the Case Comprehensive Cancer Center.
Targets for Future Research
Study limitations include potential unmeasured or uncontrolled confounders, self-selection, reverse causality, and other biases involved in observational studies, the research team noted.
Further research is warranted to investigate the effects in patients with prior antidiabetic treatments, underlying mechanisms, potential variation in effects among different GLP-1 RAs, and the potential of GLP-1 RAs to reduce the risks for other obesity-associated cancers, the researchers wrote.
“To our knowledge, this is the first indication this popular weight loss and antidiabetic class of drugs reduces incidence of CRC, relative to other antidiabetic agents,” said Rong Xu, PhD, co-lead researcher, professor of medicine, and member of the Case Comprehensive Cancer Center.
The study was supported by the National Cancer Institute Case Comprehensive Cancer Center, American Cancer Society, Landon Foundation-American Association for Cancer Research, National Institutes of Health Director’s New Innovator Award Program, National Institute on Aging, and National Institute on Alcohol Abuse and Alcoholism. Several authors reported grants from the National Institutes of Health during the conduct of the study.
A version of this article appeared on Medscape.com.
according to a new analysis.
In particular, GLP-1 RAs were associated with decreased risk compared with other antidiabetic treatments, including insulin, metformin, sodium-glucose cotransporter 2 (SGLT2) inhibitors, sulfonylureas, and thiazolidinediones.
More profound effects were seen in patients with overweight or obesity, “suggesting a potential protective effect against CRC partially mediated by weight loss and other mechanisms related to weight loss,” Lindsey Wang, an undergraduate student at Case Western Reserve University, Cleveland, Ohio, and colleagues wrote in JAMA Oncology.
Testing Treatments
GLP-1 RAs, usually given by injection, are approved by the US Food and Drug Administration to treat type 2 diabetes. They can lower blood sugar levels, improve insulin sensitivity, and help patients manage their weight.
Diabetes, overweight, and obesity are known risk factors for CRC and make prognosis worse. Ms. Wang and colleagues hypothesized that GLP-1 RAs might reduce CRC risk compared with other antidiabetics, including metformin and insulin, which have also been shown to reduce CRC risk.
Using a national database of more than 101 million electronic health records, Ms. Wang and colleagues conducted a population-based study of more than 1.2 million patients who had medical encounters for type 2 diabetes and were subsequently prescribed antidiabetic medications between 2005 and 2019. The patients had no prior antidiabetic medication use nor CRC diagnosis.
The researchers analyzed the effects of GLP-1 RAs on CRC incidence compared with the other prescribed antidiabetic drugs, matching for demographics, adverse socioeconomic determinants of health, preexisting medical conditions, family and personal history of cancers and colonic polyps, lifestyle factors, and procedures such as colonoscopy.
During a 15-year follow-up, GLP-1 RAs were associated with decreased risk for CRC compared with insulin (hazard ratio [HR], 0.56), metformin (HR, 0.75), SGLT2 inhibitors (HR, 0.77), sulfonylureas (HR, 0.82), and thiazolidinediones (HR, 0.82) in the overall study population.
For instance, among 22,572 patients who took insulin, 167 cases of CRC occurred, compared with 94 cases among the matched GLP-1 RA cohort. Among 18,518 patients who took metformin, 153 cases of CRC occurred compared with 96 cases among the matched GLP-1 RA cohort.
GLP-1 RAs also were associated with lower but not statistically significant risk than alpha-glucosidase inhibitors (HR, 0.59) and dipeptidyl-peptidase-4 (DPP-4) inhibitors (HR, 0.93).
In patients with overweight or obesity, GLP-1 RAs were associated with a lower risk for CRC than most of the other antidiabetics, including insulin (HR, 0.5), metformin (HR, 0.58), SGLT2 inhibitors (HR, 0.68), sulfonylureas (HR, 0.63), thiazolidinediones (HR, 0.73), and DPP-4 inhibitors (HR, 0.77).
Consistent findings were observed in women and men.
“Our results clearly demonstrate that GLP-1 RAs are significantly more effective than popular antidiabetic drugs, such as metformin or insulin, at preventing the development of CRC,” said Nathan Berger, MD, co-lead researcher, professor of experimental medicine, and member of the Case Comprehensive Cancer Center.
Targets for Future Research
Study limitations include potential unmeasured or uncontrolled confounders, self-selection, reverse causality, and other biases involved in observational studies, the research team noted.
Further research is warranted to investigate the effects in patients with prior antidiabetic treatments, underlying mechanisms, potential variation in effects among different GLP-1 RAs, and the potential of GLP-1 RAs to reduce the risks for other obesity-associated cancers, the researchers wrote.
“To our knowledge, this is the first indication this popular weight loss and antidiabetic class of drugs reduces incidence of CRC, relative to other antidiabetic agents,” said Rong Xu, PhD, co-lead researcher, professor of medicine, and member of the Case Comprehensive Cancer Center.
The study was supported by the National Cancer Institute Case Comprehensive Cancer Center, American Cancer Society, Landon Foundation-American Association for Cancer Research, National Institutes of Health Director’s New Innovator Award Program, National Institute on Aging, and National Institute on Alcohol Abuse and Alcoholism. Several authors reported grants from the National Institutes of Health during the conduct of the study.
A version of this article appeared on Medscape.com.
Sickle Cell CRISPR Gene Therapy May Offer Patients ‘Functional Cure’
One therapy — exagamglogene autotemcel or exa-cel (Casgevy) — is the first to use CRISPR gene-editing technology, and could “provide a one-time functional cure to patients with sickle cell disease,” said Haydar Frangoul, MD, of The Children’s Hospital at TriStar Centennial, Nashville, Tennessee.
Dr. Frangoul, who presented a recent interim analysis on the therapy at the American Society of Hematology (ASH) annual meeting earlier this month, reported that one infusion of exa-cel prompted rapid increases in total hemoglobin levels and almost completely eliminated a common and painful complication of sickle cell disease that can lead to irreversible organ damage, known as vaso-occlusive crisis.
Overall, the gene therapy led to “a rapid, robust, and durable increase in total hemoglobin to normal or near normal levels,” Dr. Frangoul said.
Exa-cel, from Vertex Pharmaceuticals and CRISPR Therapeutics, is a single-dose infusion containing a patient’s modified cells. First, a patient’s stem cells are harvested and then genetically modified to produce fetal hemoglobin.
The development of exa-cel was “grounded in human genetics, which show that fetal hemoglobin can substitute for sickle hemoglobin,” Dr. Frangoul explained. Patients receive these edited cells, which then help restore normal hemoglobin production.
The analysis showed that a one-time infusion of exa-cel following myeloablative conditioning prevented vaso-occlusive crisis in all but one patient with severe sickle cell disease. The therapy also prevented inpatient hospitalizations for vaso-occlusive crisis in all patients and led to sustained improvements in quality of life.
The results are “really striking,” said Sarah H. O’Brien, MD, of Nationwide Children’s Hospital in Columbus, Ohio, who was not involved in the research. “The majority of our admissions on the hematology service are our patients with sickle cell. They’re uncomfortable, they’re in pain, they’re missing school, and they’re missing their activities,” which makes these interim findings quite “impactful.”
To examine the impact of exa-cel on vaso-occlusive crisis, the phase 3 trial included individuals aged 12 to 35 years with severe sickle cell disease and a history of at least two vaso-occlusive crises per year over the past 2 years.
Participants underwent cell CD34+ stem cell collection. These cells then underwent gene editing using CRISPR technology, explained Dr. Frangoul.
At the transplant center, patients received myeloablative conditioning chemotherapy with busulfan for 4 days before receiving an exa-cel infusion.
At the data cutoff in June 2023, 44 patients had been enrolled, of whom 30 were available for efficacy analysis. The mean age at screening was 22.1 years, and almost half (46.7%) were female. Prior to study recruitment, patients had a mean of 3.9 vaso-occlusive crises per year and a mean of 2.7 inpatient hospitalizations per year for severe vaso-occlusive crisis.
All but one patient (96.7%) met the primary endpoint of freedom from severe vaso-occlusive crisis for at least 12 consecutive months. The mean duration of freedom from vaso-occlusive crisis was 22.4 months, ranging from 14.8 months to 45.5 months. Moreover, 28 of the 29 patients who remained crisis-free at 12 months did not have a further vaso-occlusive crisis throughout the rest of the follow-up period.
Dr. Frangoul noted that results were similar for both adults and adolescents.
Exa-cel also led to a significant increase in freedom from inpatient hospitalizations, with 100% of patients achieving that goal, as well as early and sustained increases in both total and fetal hemoglobin levels, suggesting a “long-term meaningful benefit” from the therapy.
All 44 patients experienced adverse events related to myeloablative conditioning with busulfan, but only 29.5% had events linked to exa-cel. The most common adverse events overall were nausea (70.5%), stomatitis (63.6%), vomiting (56.8%), and febrile neutropenia (54.5%).
In a separate poster presented at ASH, Akshay Sharma, MBBS, of St. Jude Children’s Research Hospital in Memphis, Tennessee, Dr. Frangoul, and colleagues reported that exa-cel also led to better health-related quality of life.
Patients showed “substantial improvements” in measures of quality of life, which included physical, emotional, social, and functional well-being as well as pain at a 6-month follow-up through year 2.
Typical outcomes studied in most trials are “emergency room visits and hospitalizations but what people may not appreciate as much is how much these patients are dealing with pain and discomfort at home,” Dr. O’Brien said. These recently reported quality-of-life metrics “are so key and really help us understand the impact” of this new therapy.
Dr. O’Brien noted, however, that “patients may be reluctant to undergo” this therapy because of the impact myeloablative conditioning has on fertility. That is why ongoing research on how stem cell transplants can be delivered “without impacting fertility is very important.”
It is “hard to know,” Dr. O’Brien explained, whether exa-cel will be a one-time treatment in practice, as many of the patients “already have end-organ damage from their disease.”
To that end, Dr. Frangoul noted that patients who complete the current trial can enroll in one that will include 13 years of additional follow-up.
Finally, Dr. O’Brien cautioned, gene therapies such as exa-cel “are only going to apply to a small segment of the population” — patients with the most severe form of the disease. That’s why “it’s important that we still prioritize hydroxyurea [and] multidisciplinary care for patients with sickle cell disease,” she said.
The study was sponsored by Vertex Pharmaceuticals in collaboration with CRISPR Therapeutics. Dr. Frangoul declared relationships with Editas Medicine, Rocket Pharmaceuticals, Jazz Pharmaceuticals, Vertex Pharmaceuticals, CRISPR Therapeutics, Bluebird Bio, and others. Dr. Sharma declared relationships with Vertex Pharmaceuticals, CRISPR Therapeutics, and others. Other authors declare numerous financial relationships.
A version of this article appeared on Medscape.com.
One therapy — exagamglogene autotemcel or exa-cel (Casgevy) — is the first to use CRISPR gene-editing technology, and could “provide a one-time functional cure to patients with sickle cell disease,” said Haydar Frangoul, MD, of The Children’s Hospital at TriStar Centennial, Nashville, Tennessee.
Dr. Frangoul, who presented a recent interim analysis on the therapy at the American Society of Hematology (ASH) annual meeting earlier this month, reported that one infusion of exa-cel prompted rapid increases in total hemoglobin levels and almost completely eliminated a common and painful complication of sickle cell disease that can lead to irreversible organ damage, known as vaso-occlusive crisis.
Overall, the gene therapy led to “a rapid, robust, and durable increase in total hemoglobin to normal or near normal levels,” Dr. Frangoul said.
Exa-cel, from Vertex Pharmaceuticals and CRISPR Therapeutics, is a single-dose infusion containing a patient’s modified cells. First, a patient’s stem cells are harvested and then genetically modified to produce fetal hemoglobin.
The development of exa-cel was “grounded in human genetics, which show that fetal hemoglobin can substitute for sickle hemoglobin,” Dr. Frangoul explained. Patients receive these edited cells, which then help restore normal hemoglobin production.
The analysis showed that a one-time infusion of exa-cel following myeloablative conditioning prevented vaso-occlusive crisis in all but one patient with severe sickle cell disease. The therapy also prevented inpatient hospitalizations for vaso-occlusive crisis in all patients and led to sustained improvements in quality of life.
The results are “really striking,” said Sarah H. O’Brien, MD, of Nationwide Children’s Hospital in Columbus, Ohio, who was not involved in the research. “The majority of our admissions on the hematology service are our patients with sickle cell. They’re uncomfortable, they’re in pain, they’re missing school, and they’re missing their activities,” which makes these interim findings quite “impactful.”
To examine the impact of exa-cel on vaso-occlusive crisis, the phase 3 trial included individuals aged 12 to 35 years with severe sickle cell disease and a history of at least two vaso-occlusive crises per year over the past 2 years.
Participants underwent cell CD34+ stem cell collection. These cells then underwent gene editing using CRISPR technology, explained Dr. Frangoul.
At the transplant center, patients received myeloablative conditioning chemotherapy with busulfan for 4 days before receiving an exa-cel infusion.
At the data cutoff in June 2023, 44 patients had been enrolled, of whom 30 were available for efficacy analysis. The mean age at screening was 22.1 years, and almost half (46.7%) were female. Prior to study recruitment, patients had a mean of 3.9 vaso-occlusive crises per year and a mean of 2.7 inpatient hospitalizations per year for severe vaso-occlusive crisis.
All but one patient (96.7%) met the primary endpoint of freedom from severe vaso-occlusive crisis for at least 12 consecutive months. The mean duration of freedom from vaso-occlusive crisis was 22.4 months, ranging from 14.8 months to 45.5 months. Moreover, 28 of the 29 patients who remained crisis-free at 12 months did not have a further vaso-occlusive crisis throughout the rest of the follow-up period.
Dr. Frangoul noted that results were similar for both adults and adolescents.
Exa-cel also led to a significant increase in freedom from inpatient hospitalizations, with 100% of patients achieving that goal, as well as early and sustained increases in both total and fetal hemoglobin levels, suggesting a “long-term meaningful benefit” from the therapy.
All 44 patients experienced adverse events related to myeloablative conditioning with busulfan, but only 29.5% had events linked to exa-cel. The most common adverse events overall were nausea (70.5%), stomatitis (63.6%), vomiting (56.8%), and febrile neutropenia (54.5%).
In a separate poster presented at ASH, Akshay Sharma, MBBS, of St. Jude Children’s Research Hospital in Memphis, Tennessee, Dr. Frangoul, and colleagues reported that exa-cel also led to better health-related quality of life.
Patients showed “substantial improvements” in measures of quality of life, which included physical, emotional, social, and functional well-being as well as pain at a 6-month follow-up through year 2.
Typical outcomes studied in most trials are “emergency room visits and hospitalizations but what people may not appreciate as much is how much these patients are dealing with pain and discomfort at home,” Dr. O’Brien said. These recently reported quality-of-life metrics “are so key and really help us understand the impact” of this new therapy.
Dr. O’Brien noted, however, that “patients may be reluctant to undergo” this therapy because of the impact myeloablative conditioning has on fertility. That is why ongoing research on how stem cell transplants can be delivered “without impacting fertility is very important.”
It is “hard to know,” Dr. O’Brien explained, whether exa-cel will be a one-time treatment in practice, as many of the patients “already have end-organ damage from their disease.”
To that end, Dr. Frangoul noted that patients who complete the current trial can enroll in one that will include 13 years of additional follow-up.
Finally, Dr. O’Brien cautioned, gene therapies such as exa-cel “are only going to apply to a small segment of the population” — patients with the most severe form of the disease. That’s why “it’s important that we still prioritize hydroxyurea [and] multidisciplinary care for patients with sickle cell disease,” she said.
The study was sponsored by Vertex Pharmaceuticals in collaboration with CRISPR Therapeutics. Dr. Frangoul declared relationships with Editas Medicine, Rocket Pharmaceuticals, Jazz Pharmaceuticals, Vertex Pharmaceuticals, CRISPR Therapeutics, Bluebird Bio, and others. Dr. Sharma declared relationships with Vertex Pharmaceuticals, CRISPR Therapeutics, and others. Other authors declare numerous financial relationships.
A version of this article appeared on Medscape.com.
One therapy — exagamglogene autotemcel or exa-cel (Casgevy) — is the first to use CRISPR gene-editing technology, and could “provide a one-time functional cure to patients with sickle cell disease,” said Haydar Frangoul, MD, of The Children’s Hospital at TriStar Centennial, Nashville, Tennessee.
Dr. Frangoul, who presented a recent interim analysis on the therapy at the American Society of Hematology (ASH) annual meeting earlier this month, reported that one infusion of exa-cel prompted rapid increases in total hemoglobin levels and almost completely eliminated a common and painful complication of sickle cell disease that can lead to irreversible organ damage, known as vaso-occlusive crisis.
Overall, the gene therapy led to “a rapid, robust, and durable increase in total hemoglobin to normal or near normal levels,” Dr. Frangoul said.
Exa-cel, from Vertex Pharmaceuticals and CRISPR Therapeutics, is a single-dose infusion containing a patient’s modified cells. First, a patient’s stem cells are harvested and then genetically modified to produce fetal hemoglobin.
The development of exa-cel was “grounded in human genetics, which show that fetal hemoglobin can substitute for sickle hemoglobin,” Dr. Frangoul explained. Patients receive these edited cells, which then help restore normal hemoglobin production.
The analysis showed that a one-time infusion of exa-cel following myeloablative conditioning prevented vaso-occlusive crisis in all but one patient with severe sickle cell disease. The therapy also prevented inpatient hospitalizations for vaso-occlusive crisis in all patients and led to sustained improvements in quality of life.
The results are “really striking,” said Sarah H. O’Brien, MD, of Nationwide Children’s Hospital in Columbus, Ohio, who was not involved in the research. “The majority of our admissions on the hematology service are our patients with sickle cell. They’re uncomfortable, they’re in pain, they’re missing school, and they’re missing their activities,” which makes these interim findings quite “impactful.”
To examine the impact of exa-cel on vaso-occlusive crisis, the phase 3 trial included individuals aged 12 to 35 years with severe sickle cell disease and a history of at least two vaso-occlusive crises per year over the past 2 years.
Participants underwent cell CD34+ stem cell collection. These cells then underwent gene editing using CRISPR technology, explained Dr. Frangoul.
At the transplant center, patients received myeloablative conditioning chemotherapy with busulfan for 4 days before receiving an exa-cel infusion.
At the data cutoff in June 2023, 44 patients had been enrolled, of whom 30 were available for efficacy analysis. The mean age at screening was 22.1 years, and almost half (46.7%) were female. Prior to study recruitment, patients had a mean of 3.9 vaso-occlusive crises per year and a mean of 2.7 inpatient hospitalizations per year for severe vaso-occlusive crisis.
All but one patient (96.7%) met the primary endpoint of freedom from severe vaso-occlusive crisis for at least 12 consecutive months. The mean duration of freedom from vaso-occlusive crisis was 22.4 months, ranging from 14.8 months to 45.5 months. Moreover, 28 of the 29 patients who remained crisis-free at 12 months did not have a further vaso-occlusive crisis throughout the rest of the follow-up period.
Dr. Frangoul noted that results were similar for both adults and adolescents.
Exa-cel also led to a significant increase in freedom from inpatient hospitalizations, with 100% of patients achieving that goal, as well as early and sustained increases in both total and fetal hemoglobin levels, suggesting a “long-term meaningful benefit” from the therapy.
All 44 patients experienced adverse events related to myeloablative conditioning with busulfan, but only 29.5% had events linked to exa-cel. The most common adverse events overall were nausea (70.5%), stomatitis (63.6%), vomiting (56.8%), and febrile neutropenia (54.5%).
In a separate poster presented at ASH, Akshay Sharma, MBBS, of St. Jude Children’s Research Hospital in Memphis, Tennessee, Dr. Frangoul, and colleagues reported that exa-cel also led to better health-related quality of life.
Patients showed “substantial improvements” in measures of quality of life, which included physical, emotional, social, and functional well-being as well as pain at a 6-month follow-up through year 2.
Typical outcomes studied in most trials are “emergency room visits and hospitalizations but what people may not appreciate as much is how much these patients are dealing with pain and discomfort at home,” Dr. O’Brien said. These recently reported quality-of-life metrics “are so key and really help us understand the impact” of this new therapy.
Dr. O’Brien noted, however, that “patients may be reluctant to undergo” this therapy because of the impact myeloablative conditioning has on fertility. That is why ongoing research on how stem cell transplants can be delivered “without impacting fertility is very important.”
It is “hard to know,” Dr. O’Brien explained, whether exa-cel will be a one-time treatment in practice, as many of the patients “already have end-organ damage from their disease.”
To that end, Dr. Frangoul noted that patients who complete the current trial can enroll in one that will include 13 years of additional follow-up.
Finally, Dr. O’Brien cautioned, gene therapies such as exa-cel “are only going to apply to a small segment of the population” — patients with the most severe form of the disease. That’s why “it’s important that we still prioritize hydroxyurea [and] multidisciplinary care for patients with sickle cell disease,” she said.
The study was sponsored by Vertex Pharmaceuticals in collaboration with CRISPR Therapeutics. Dr. Frangoul declared relationships with Editas Medicine, Rocket Pharmaceuticals, Jazz Pharmaceuticals, Vertex Pharmaceuticals, CRISPR Therapeutics, Bluebird Bio, and others. Dr. Sharma declared relationships with Vertex Pharmaceuticals, CRISPR Therapeutics, and others. Other authors declare numerous financial relationships.
A version of this article appeared on Medscape.com.
FROM ASH 2023
Study Suggests Inappropriate Use of Thyroid Ultrasounds
“The number of thyroid ultrasounds performed in the United States has increased fivefold since 2002. This substantial increase produces a significant strain on healthcare resources and leads to over-detection and overtreatment of benign thyroid nodules and small, indolent cancers with questionable clinical relevance,” wrote Elena Kennedy, MD, then a medical student in the department of surgery at the University of Wisconsin School of Medicine and Public Health, Madison, and colleagues.
The data, published online in Thyroid, come from a retrospective chart analysis of more than 1700 people who underwent dedicated (ie, specifically to look for a nodule) thyroid ultrasounds at a tertiary academic center. The rates of detecting both nodules and biopsy-recommended nodules were highest when the indication was a nodule seen incidentally on other imaging (aka “incidentaloma”) and lowest when the ultrasound was ordered because the patient had either metabolic or compressive symptoms.
And for the most commonly listed indication, a suspected palpable nodule, nearly half of the ultrasounds found no nodule, and only one in five detected a nodule that warranted a biopsy.
The principal investigator of the study David O. Francis, MD, an otolaryngologist at the University of Wisconsin, Madison, said in an interview, “Thyroid cancer has grown in incidence three to four times over the last 30 years without a good explanation for why…It seems to be that we’re detecting smaller and smaller nodules…Why are people being referred for all these ultrasounds? We looked for the upstream factors.”
One clear clinical implication of the new data, Dr. Francis noted, is that “if someone has compressive symptoms including dysphagia, swallowing problems, voice change, or globus sensation, ultrasound should not be the first way to work them up…It would be smarter to have someone evaluate their voice or their swallowing to see if there’s another reason besides the thyroid. The thyroid would have to get pretty big to cause dysphagia or swallowing problems.”
No Current Guidelines Advise When not to Order a Thyroid Ultrasound
Problematically, while there are professional society guidelines for what to do when a thyroid “incidentaloma” is found and other specific situations, there are no overall guidelines addressing when it’s appropriate to order a thyroid ultrasound, Dr. Kennedy, now an otolaryngology resident at the Indiana University of Indianapolis, and colleagues, point out.
According to Dr. Francis, “Ultrasounds are low cost and low risk. Those two factors result in people ordering more tests…The problem with that is we find things, and then we have to figure out what to do with them. That leads to incidentalomas, the surveillance, worry and anxiety, and costs…It’s tricky. We don’t want to discourage people from ever ordering ultrasounds, but there need to be some guidelines around when it’s appropriate to order.”
Asked to comment, Trevor E. Angell, MD, associate medical director of Thyroid Center at Keck School of Medicine of the University of Southern California, Los Angeles, said that the study is “clinically very important.”
Dr. Angell pointed out that the current American Thyroid Association (ATA) guidelines on thyroid nodule management, of which he is an author, recommend ultrasound for a known or suspected nodule. But he added, “there certainly should be a message that obtaining ultrasound for these other reasons are less likely to identify a nodule or anything causative. Whether it’s gastroesophageal reflux or allergic rhinitis or vocal cord dysfunction, an ultrasound isn’t a good test for those either.”
Dr. Angell said that the next ATA thyroid nodule guidelines, expected out in 2024, will address this topic more fully, but he couldn’t provide more specific information because the document is still in development. He did say, however, “Addressing when not to do an ultrasound will be an important consideration in the next guidelines.”
Low Detection Rates for Most Indications
The retrospective observational cohort study included 1739 adults (76% women; mean age, 53 years) who underwent dedicated thyroid ultrasounds between 2017 and 2019. In most cases, the recommendation for biopsy was determined using the American College of Radiology TI-RADS system, based on nodule size and TI-RADS category.
The most common indication for thyroid ultrasound, suspected palpable nodule, accounted for 40% of those performed. Follow-up for an “incidentaloma” was the indication in 28% of patients, and referral for compressive and metabolic symptoms accounted for 13% and 6% of ultrasounds, respectively.
Among all ultrasounds performed, 62% identified a thyroid nodule. Patients referred for incidental findings had the highest percentage of ultrasounds with thyroid nodules present at 94%. By contrast, in those referred for suspected palpable nodule on exam and for compressive symptoms, nodules were identified on 55% and 39% of ultrasounds, respectively. Patients with metabolic symptoms had a nodule identified on ultrasound 43% of the time. Among those referred for high risk factors, 57% had a nodule present.
Overall, only 27% of ultrasounds identified a thyroid nodule that was recommended for a biopsy. Again, those referred because of an incidental imaging finding had the highest percentage (55%), followed by those referred for a suspected palpable nodule (21%), high risk factors (20%), combined indications (16%), metabolic symptoms (10%), and compressive symptoms (6%).
Mean nodule size was largest among the patients referred for incidentalomas (2.4 cm), whereas all the other groups had mean nodule sizes between 1.2 cm and 1.8 cm, a significant difference (P < .05). The median size of nodules among those referred to ultrasound for a suspected palpable nodule was 1.4 cm.
“That’s pretty small. It would have had to be in the front of the thyroid where they could actually touch it. I would argue that the number of clinicians who actually palpated something was smaller. We’ve done several projects looking at how small a nodule a clinician can actually feel in the thyroid gland from the neck. It turns out we’re pretty bad at physical examination of the thyroid. This paper kind of reinforces that,” Dr. Francis said in an interview.
Patients with incidental nodules were over 10 times more likely to have a nodule found on an ultrasound than those referred for a suspected palpable nodule on exam (odds ratio [OR], 10.6). Conversely, those referred for compressive symptoms were half as likely to have an identifiable nodule compared with those referred for physical exam findings (OR, 0.5).
The odds of finding a nodule increased with age, especially for those aged ≥ 65 years compared with those younger than 45 years (OR, 3.6). Women were twice as likely to have a nodule found on thyroid ultrasound (OR, 2.0). Results were similar for the biopsy-recommended nodules, except that there was no difference between sexes (female vs male OR, 1.2).
Dr. Angell called the study “a very robust comprehensive evaluation,” but also noted that the single center source is a limitation. “It would be nice to have those big databases of national healthcare settings, but getting that granular level of information about why something was done is nearly impossible in that context.”
Dr. Kennedy, Dr. Francis, and Dr. Angell have no disclosures.
A version of this article appeared on Medscape.com.
“The number of thyroid ultrasounds performed in the United States has increased fivefold since 2002. This substantial increase produces a significant strain on healthcare resources and leads to over-detection and overtreatment of benign thyroid nodules and small, indolent cancers with questionable clinical relevance,” wrote Elena Kennedy, MD, then a medical student in the department of surgery at the University of Wisconsin School of Medicine and Public Health, Madison, and colleagues.
The data, published online in Thyroid, come from a retrospective chart analysis of more than 1700 people who underwent dedicated (ie, specifically to look for a nodule) thyroid ultrasounds at a tertiary academic center. The rates of detecting both nodules and biopsy-recommended nodules were highest when the indication was a nodule seen incidentally on other imaging (aka “incidentaloma”) and lowest when the ultrasound was ordered because the patient had either metabolic or compressive symptoms.
And for the most commonly listed indication, a suspected palpable nodule, nearly half of the ultrasounds found no nodule, and only one in five detected a nodule that warranted a biopsy.
The principal investigator of the study David O. Francis, MD, an otolaryngologist at the University of Wisconsin, Madison, said in an interview, “Thyroid cancer has grown in incidence three to four times over the last 30 years without a good explanation for why…It seems to be that we’re detecting smaller and smaller nodules…Why are people being referred for all these ultrasounds? We looked for the upstream factors.”
One clear clinical implication of the new data, Dr. Francis noted, is that “if someone has compressive symptoms including dysphagia, swallowing problems, voice change, or globus sensation, ultrasound should not be the first way to work them up…It would be smarter to have someone evaluate their voice or their swallowing to see if there’s another reason besides the thyroid. The thyroid would have to get pretty big to cause dysphagia or swallowing problems.”
No Current Guidelines Advise When not to Order a Thyroid Ultrasound
Problematically, while there are professional society guidelines for what to do when a thyroid “incidentaloma” is found and other specific situations, there are no overall guidelines addressing when it’s appropriate to order a thyroid ultrasound, Dr. Kennedy, now an otolaryngology resident at the Indiana University of Indianapolis, and colleagues, point out.
According to Dr. Francis, “Ultrasounds are low cost and low risk. Those two factors result in people ordering more tests…The problem with that is we find things, and then we have to figure out what to do with them. That leads to incidentalomas, the surveillance, worry and anxiety, and costs…It’s tricky. We don’t want to discourage people from ever ordering ultrasounds, but there need to be some guidelines around when it’s appropriate to order.”
Asked to comment, Trevor E. Angell, MD, associate medical director of Thyroid Center at Keck School of Medicine of the University of Southern California, Los Angeles, said that the study is “clinically very important.”
Dr. Angell pointed out that the current American Thyroid Association (ATA) guidelines on thyroid nodule management, of which he is an author, recommend ultrasound for a known or suspected nodule. But he added, “there certainly should be a message that obtaining ultrasound for these other reasons are less likely to identify a nodule or anything causative. Whether it’s gastroesophageal reflux or allergic rhinitis or vocal cord dysfunction, an ultrasound isn’t a good test for those either.”
Dr. Angell said that the next ATA thyroid nodule guidelines, expected out in 2024, will address this topic more fully, but he couldn’t provide more specific information because the document is still in development. He did say, however, “Addressing when not to do an ultrasound will be an important consideration in the next guidelines.”
Low Detection Rates for Most Indications
The retrospective observational cohort study included 1739 adults (76% women; mean age, 53 years) who underwent dedicated thyroid ultrasounds between 2017 and 2019. In most cases, the recommendation for biopsy was determined using the American College of Radiology TI-RADS system, based on nodule size and TI-RADS category.
The most common indication for thyroid ultrasound, suspected palpable nodule, accounted for 40% of those performed. Follow-up for an “incidentaloma” was the indication in 28% of patients, and referral for compressive and metabolic symptoms accounted for 13% and 6% of ultrasounds, respectively.
Among all ultrasounds performed, 62% identified a thyroid nodule. Patients referred for incidental findings had the highest percentage of ultrasounds with thyroid nodules present at 94%. By contrast, in those referred for suspected palpable nodule on exam and for compressive symptoms, nodules were identified on 55% and 39% of ultrasounds, respectively. Patients with metabolic symptoms had a nodule identified on ultrasound 43% of the time. Among those referred for high risk factors, 57% had a nodule present.
Overall, only 27% of ultrasounds identified a thyroid nodule that was recommended for a biopsy. Again, those referred because of an incidental imaging finding had the highest percentage (55%), followed by those referred for a suspected palpable nodule (21%), high risk factors (20%), combined indications (16%), metabolic symptoms (10%), and compressive symptoms (6%).
Mean nodule size was largest among the patients referred for incidentalomas (2.4 cm), whereas all the other groups had mean nodule sizes between 1.2 cm and 1.8 cm, a significant difference (P < .05). The median size of nodules among those referred to ultrasound for a suspected palpable nodule was 1.4 cm.
“That’s pretty small. It would have had to be in the front of the thyroid where they could actually touch it. I would argue that the number of clinicians who actually palpated something was smaller. We’ve done several projects looking at how small a nodule a clinician can actually feel in the thyroid gland from the neck. It turns out we’re pretty bad at physical examination of the thyroid. This paper kind of reinforces that,” Dr. Francis said in an interview.
Patients with incidental nodules were over 10 times more likely to have a nodule found on an ultrasound than those referred for a suspected palpable nodule on exam (odds ratio [OR], 10.6). Conversely, those referred for compressive symptoms were half as likely to have an identifiable nodule compared with those referred for physical exam findings (OR, 0.5).
The odds of finding a nodule increased with age, especially for those aged ≥ 65 years compared with those younger than 45 years (OR, 3.6). Women were twice as likely to have a nodule found on thyroid ultrasound (OR, 2.0). Results were similar for the biopsy-recommended nodules, except that there was no difference between sexes (female vs male OR, 1.2).
Dr. Angell called the study “a very robust comprehensive evaluation,” but also noted that the single center source is a limitation. “It would be nice to have those big databases of national healthcare settings, but getting that granular level of information about why something was done is nearly impossible in that context.”
Dr. Kennedy, Dr. Francis, and Dr. Angell have no disclosures.
A version of this article appeared on Medscape.com.
“The number of thyroid ultrasounds performed in the United States has increased fivefold since 2002. This substantial increase produces a significant strain on healthcare resources and leads to over-detection and overtreatment of benign thyroid nodules and small, indolent cancers with questionable clinical relevance,” wrote Elena Kennedy, MD, then a medical student in the department of surgery at the University of Wisconsin School of Medicine and Public Health, Madison, and colleagues.
The data, published online in Thyroid, come from a retrospective chart analysis of more than 1700 people who underwent dedicated (ie, specifically to look for a nodule) thyroid ultrasounds at a tertiary academic center. The rates of detecting both nodules and biopsy-recommended nodules were highest when the indication was a nodule seen incidentally on other imaging (aka “incidentaloma”) and lowest when the ultrasound was ordered because the patient had either metabolic or compressive symptoms.
And for the most commonly listed indication, a suspected palpable nodule, nearly half of the ultrasounds found no nodule, and only one in five detected a nodule that warranted a biopsy.
The principal investigator of the study David O. Francis, MD, an otolaryngologist at the University of Wisconsin, Madison, said in an interview, “Thyroid cancer has grown in incidence three to four times over the last 30 years without a good explanation for why…It seems to be that we’re detecting smaller and smaller nodules…Why are people being referred for all these ultrasounds? We looked for the upstream factors.”
One clear clinical implication of the new data, Dr. Francis noted, is that “if someone has compressive symptoms including dysphagia, swallowing problems, voice change, or globus sensation, ultrasound should not be the first way to work them up…It would be smarter to have someone evaluate their voice or their swallowing to see if there’s another reason besides the thyroid. The thyroid would have to get pretty big to cause dysphagia or swallowing problems.”
No Current Guidelines Advise When not to Order a Thyroid Ultrasound
Problematically, while there are professional society guidelines for what to do when a thyroid “incidentaloma” is found and other specific situations, there are no overall guidelines addressing when it’s appropriate to order a thyroid ultrasound, Dr. Kennedy, now an otolaryngology resident at the Indiana University of Indianapolis, and colleagues, point out.
According to Dr. Francis, “Ultrasounds are low cost and low risk. Those two factors result in people ordering more tests…The problem with that is we find things, and then we have to figure out what to do with them. That leads to incidentalomas, the surveillance, worry and anxiety, and costs…It’s tricky. We don’t want to discourage people from ever ordering ultrasounds, but there need to be some guidelines around when it’s appropriate to order.”
Asked to comment, Trevor E. Angell, MD, associate medical director of Thyroid Center at Keck School of Medicine of the University of Southern California, Los Angeles, said that the study is “clinically very important.”
Dr. Angell pointed out that the current American Thyroid Association (ATA) guidelines on thyroid nodule management, of which he is an author, recommend ultrasound for a known or suspected nodule. But he added, “there certainly should be a message that obtaining ultrasound for these other reasons are less likely to identify a nodule or anything causative. Whether it’s gastroesophageal reflux or allergic rhinitis or vocal cord dysfunction, an ultrasound isn’t a good test for those either.”
Dr. Angell said that the next ATA thyroid nodule guidelines, expected out in 2024, will address this topic more fully, but he couldn’t provide more specific information because the document is still in development. He did say, however, “Addressing when not to do an ultrasound will be an important consideration in the next guidelines.”
Low Detection Rates for Most Indications
The retrospective observational cohort study included 1739 adults (76% women; mean age, 53 years) who underwent dedicated thyroid ultrasounds between 2017 and 2019. In most cases, the recommendation for biopsy was determined using the American College of Radiology TI-RADS system, based on nodule size and TI-RADS category.
The most common indication for thyroid ultrasound, suspected palpable nodule, accounted for 40% of those performed. Follow-up for an “incidentaloma” was the indication in 28% of patients, and referral for compressive and metabolic symptoms accounted for 13% and 6% of ultrasounds, respectively.
Among all ultrasounds performed, 62% identified a thyroid nodule. Patients referred for incidental findings had the highest percentage of ultrasounds with thyroid nodules present at 94%. By contrast, in those referred for suspected palpable nodule on exam and for compressive symptoms, nodules were identified on 55% and 39% of ultrasounds, respectively. Patients with metabolic symptoms had a nodule identified on ultrasound 43% of the time. Among those referred for high risk factors, 57% had a nodule present.
Overall, only 27% of ultrasounds identified a thyroid nodule that was recommended for a biopsy. Again, those referred because of an incidental imaging finding had the highest percentage (55%), followed by those referred for a suspected palpable nodule (21%), high risk factors (20%), combined indications (16%), metabolic symptoms (10%), and compressive symptoms (6%).
Mean nodule size was largest among the patients referred for incidentalomas (2.4 cm), whereas all the other groups had mean nodule sizes between 1.2 cm and 1.8 cm, a significant difference (P < .05). The median size of nodules among those referred to ultrasound for a suspected palpable nodule was 1.4 cm.
“That’s pretty small. It would have had to be in the front of the thyroid where they could actually touch it. I would argue that the number of clinicians who actually palpated something was smaller. We’ve done several projects looking at how small a nodule a clinician can actually feel in the thyroid gland from the neck. It turns out we’re pretty bad at physical examination of the thyroid. This paper kind of reinforces that,” Dr. Francis said in an interview.
Patients with incidental nodules were over 10 times more likely to have a nodule found on an ultrasound than those referred for a suspected palpable nodule on exam (odds ratio [OR], 10.6). Conversely, those referred for compressive symptoms were half as likely to have an identifiable nodule compared with those referred for physical exam findings (OR, 0.5).
The odds of finding a nodule increased with age, especially for those aged ≥ 65 years compared with those younger than 45 years (OR, 3.6). Women were twice as likely to have a nodule found on thyroid ultrasound (OR, 2.0). Results were similar for the biopsy-recommended nodules, except that there was no difference between sexes (female vs male OR, 1.2).
Dr. Angell called the study “a very robust comprehensive evaluation,” but also noted that the single center source is a limitation. “It would be nice to have those big databases of national healthcare settings, but getting that granular level of information about why something was done is nearly impossible in that context.”
Dr. Kennedy, Dr. Francis, and Dr. Angell have no disclosures.
A version of this article appeared on Medscape.com.
FROM THYROID
GLP-1s Face Off Against Each Other, Weight-Loss Surgery in New GI Studies
VANCOUVER — Glucagon-like peptide-1 (GLP-1) agonists, like semaglutide, liraglutide, and the newly US Food and Drug Administration–approved tirzepatide, not only are gaining popularity among the public for weight loss but also are the focus of considerable attention from gastroenterology researchers.
how they compare to bariatric surgery for weight loss or prevention of metabolic dysfunction–associated steatotic liver disease, and their potential role to prevent regain after weight-loss surgery.
Head-to-Head Comparison
Tirzepatide 15 mg emerged as superior to other GLP-1 agonists for weight loss, for example, in a network meta-analysis of randomized controlled trials looking into obesity management.
Tirzepatide 15 mg was associated with the most effective mean weight loss at just over 15% when Jena Velji-Ibrahim, MD, and colleagues combined data from 14 studies with 18,714 participants with overweight or obesity but without diabetes.
Next up in order of weight-loss efficacy was tirzepatide 10 mg with 13% mean weight loss, semaglutide 2.4 mg with just over 11% mean weight loss, and tirzepatide 5 mg with almost 10% mean weight loss. The only outlier was dulaglutide 0.75 mg, which was linked to about 8% weight gain.
“While clinical trials have been conducted to assess the weight-loss efficacy of GLP-1 agonists, there has been limited head-to-head comparisons, and the data that has been obtained has been quite inconsistent,” Dr. Velji-Ibrahim said when presenting results at the meeting.
Researchers found little difference in efficacies between tirzepatide 15 mg and 10 mg, suggesting both are a viable option for weight loss, said Dr. Velji-Ibrahim of Prisma Health Greenville Memorial Hospital and University of South Carolina School of Medicine in Greenville.
She also reported similar efficacies between oral semaglutide 50 mg and subcutaneous semaglutide 2.4 mg, “meaning that we have another option for weight management.”
Side effects among the different GLP-1 agonists, and among the same agent at different doses, were not significantly different.
Comparison With Bariatric Surgery for Reducing Major Adverse Cardiovascular Events (MACE)
For many years, bariatric surgeons have pointed to the health benefits of weight-loss surgery in the right candidates, including a reduced risk for adverse cardiovascular events.
The weight loss associated with GLP-1 agonists has likewise shown benefits in reducing MACE. However, it remains unclear if one of these weight-loss strategies is better than the other in reducing these outcomes.
To determine this, researchers compared 118,828 people who had bariatric surgery to another propensity-matched group of 118,828 others prescribed GLP-1 agonists. They included adults with a body mass index (BMI) of 35 or higher in the national TriNetX database.
The multicenter, retrospective study revealed bariatric surgery was superior in reducing the risk for heart failure, MACE, and cerebrovascular disease at 3, 5, 7, and 10 years. At 10 years, for example, bariatric surgery was associated with 31% fewer composite cardiovascular events than the GLP-1 agonists.
“Our results suggest that bariatric surgery is more effective than GLP-1 analogs in preventing adverse cardiovascular events in obese patients,” Ayowumi A. Adekolu, MD, an internal medicine resident at West Virginia School of Medicine in Morgantown, said in audio comments accompanying his ePoster at the meeting. “Although these findings highlight the benefit of bariatric surgery in mitigating adverse cardiovascular events, well-designed prospective studies are necessary to confirm these benefits in this patient population.”
Possible Role in Fatty Liver Disease Prevention
In another large multicenter study from the same institution, Ethan M. Cohen, MD, along with co-author Dr. Adekolu and others, compared the effectiveness of bariatric surgery to GLP-1 agonists for preventing nonalcoholic fatty liver disease (NAFLD). Since the study was conducted, the official name of NAFLD has changed to metabolic dysfunction–associated steatotic liver disease.
Dr. Cohen and colleagues evaluated data from the TriNetX database and included adults with a BMI of 35 or higher. They propensity matched 124,022 people who had sleeve gastrectomy or Roux-en-Y gastric bypass to another 124,022 others prescribed GLP-1 agonists. Again, they looked at outcomes at 3, 5, 7, and 10 years.
They found bariatric surgery superior to GLP-1 agonists for reducing the risk of developing NAFLD. Relative risk reduction was 25% at 3 years, 28% at 5 years, 27% at 7 years, and 26% at 10 years.
Although not to the same extent as surgery in this study, GLP-1–associated weight loss did reduce risks as well.
“An important aspect of this is that for some of these people, bariatric surgery is not even an option,” Dr. Cohen said in an interview, citing as an example those who do not meet the criteria for surgery.
Dr. Cohen and colleagues plan to continue the study with a larger number of participants.
Real-World Weight Regain
In another instance where a surgical procedure trumped GLP-1 agonists, revisional endoscopic sleeve gastroplasty (ESG) offered significantly higher weight loss than GLP-1 agonists among people who regained weight following initial weight-loss surgery, according to a case-control real-world study presented at the meeting.
“Laparoscopic sleeve gastrectomy [LSG] is a frequently performed bariatric surgery worldwide resulting in significant weight loss and improvement in obesity-related comorbidities,” said Firas Bahdi, MD, gastroenterology fellow at the David Geffen School of Medicine at University of California, Los Angeles. “Despite its success, around one third of patients, unfortunately, develop weight regain warranting intervention.”
Dr. Bahdi and colleagues retrospectively studied 68 adults prescribed subcutaneous semaglutide or tirzepatide after LSG, another 20 who had ESG for weight regain after LSG, and 87 controls with intact stomachs who also took GLP-1 agonists for weight loss.
They found that the ESG group experienced a significantly higher percentage of total body weight loss at 3 months than the GLP-1 group (10% vs 4.3%, respectively; P = .0001). Similarly, at the 6-month follow-up, the ESG group experienced 11.5% total body weight loss compared to 6.8% in the GLP-1 group (P = .03).
The GLP-1 after LSG group still fared better than the GLP-1 control group of people who never had surgery. Total body weight loss was 4.3% vs 5.7% at 3 months (P = .02), 6.8% vs 9.2% at 6 months (P = .02), and 9.2% vs 12.7% at 12 months (P = .03).
“In this real-world experience, revisional ESG offers significantly more weight loss than GLP-1 agonists for patients with weight regain, while also avoiding the challenges of medication refills, making it an attractive option,” Dr. Bahdi said.
Future multicenter studies are warranted to confirm these results and explore physiological explanations, he added.
The study received an Outstanding Research Award in the Obesity Category (Trainee).
Dr. Velji-Ibrahim, Dr. Adekolu, Dr. Cohen, and Dr. Bahdi indicated no relevant financial relationships.
A version of this article appeared on Medscape.com.
VANCOUVER — Glucagon-like peptide-1 (GLP-1) agonists, like semaglutide, liraglutide, and the newly US Food and Drug Administration–approved tirzepatide, not only are gaining popularity among the public for weight loss but also are the focus of considerable attention from gastroenterology researchers.
how they compare to bariatric surgery for weight loss or prevention of metabolic dysfunction–associated steatotic liver disease, and their potential role to prevent regain after weight-loss surgery.
Head-to-Head Comparison
Tirzepatide 15 mg emerged as superior to other GLP-1 agonists for weight loss, for example, in a network meta-analysis of randomized controlled trials looking into obesity management.
Tirzepatide 15 mg was associated with the most effective mean weight loss at just over 15% when Jena Velji-Ibrahim, MD, and colleagues combined data from 14 studies with 18,714 participants with overweight or obesity but without diabetes.
Next up in order of weight-loss efficacy was tirzepatide 10 mg with 13% mean weight loss, semaglutide 2.4 mg with just over 11% mean weight loss, and tirzepatide 5 mg with almost 10% mean weight loss. The only outlier was dulaglutide 0.75 mg, which was linked to about 8% weight gain.
“While clinical trials have been conducted to assess the weight-loss efficacy of GLP-1 agonists, there has been limited head-to-head comparisons, and the data that has been obtained has been quite inconsistent,” Dr. Velji-Ibrahim said when presenting results at the meeting.
Researchers found little difference in efficacies between tirzepatide 15 mg and 10 mg, suggesting both are a viable option for weight loss, said Dr. Velji-Ibrahim of Prisma Health Greenville Memorial Hospital and University of South Carolina School of Medicine in Greenville.
She also reported similar efficacies between oral semaglutide 50 mg and subcutaneous semaglutide 2.4 mg, “meaning that we have another option for weight management.”
Side effects among the different GLP-1 agonists, and among the same agent at different doses, were not significantly different.
Comparison With Bariatric Surgery for Reducing Major Adverse Cardiovascular Events (MACE)
For many years, bariatric surgeons have pointed to the health benefits of weight-loss surgery in the right candidates, including a reduced risk for adverse cardiovascular events.
The weight loss associated with GLP-1 agonists has likewise shown benefits in reducing MACE. However, it remains unclear if one of these weight-loss strategies is better than the other in reducing these outcomes.
To determine this, researchers compared 118,828 people who had bariatric surgery to another propensity-matched group of 118,828 others prescribed GLP-1 agonists. They included adults with a body mass index (BMI) of 35 or higher in the national TriNetX database.
The multicenter, retrospective study revealed bariatric surgery was superior in reducing the risk for heart failure, MACE, and cerebrovascular disease at 3, 5, 7, and 10 years. At 10 years, for example, bariatric surgery was associated with 31% fewer composite cardiovascular events than the GLP-1 agonists.
“Our results suggest that bariatric surgery is more effective than GLP-1 analogs in preventing adverse cardiovascular events in obese patients,” Ayowumi A. Adekolu, MD, an internal medicine resident at West Virginia School of Medicine in Morgantown, said in audio comments accompanying his ePoster at the meeting. “Although these findings highlight the benefit of bariatric surgery in mitigating adverse cardiovascular events, well-designed prospective studies are necessary to confirm these benefits in this patient population.”
Possible Role in Fatty Liver Disease Prevention
In another large multicenter study from the same institution, Ethan M. Cohen, MD, along with co-author Dr. Adekolu and others, compared the effectiveness of bariatric surgery to GLP-1 agonists for preventing nonalcoholic fatty liver disease (NAFLD). Since the study was conducted, the official name of NAFLD has changed to metabolic dysfunction–associated steatotic liver disease.
Dr. Cohen and colleagues evaluated data from the TriNetX database and included adults with a BMI of 35 or higher. They propensity matched 124,022 people who had sleeve gastrectomy or Roux-en-Y gastric bypass to another 124,022 others prescribed GLP-1 agonists. Again, they looked at outcomes at 3, 5, 7, and 10 years.
They found bariatric surgery superior to GLP-1 agonists for reducing the risk of developing NAFLD. Relative risk reduction was 25% at 3 years, 28% at 5 years, 27% at 7 years, and 26% at 10 years.
Although not to the same extent as surgery in this study, GLP-1–associated weight loss did reduce risks as well.
“An important aspect of this is that for some of these people, bariatric surgery is not even an option,” Dr. Cohen said in an interview, citing as an example those who do not meet the criteria for surgery.
Dr. Cohen and colleagues plan to continue the study with a larger number of participants.
Real-World Weight Regain
In another instance where a surgical procedure trumped GLP-1 agonists, revisional endoscopic sleeve gastroplasty (ESG) offered significantly higher weight loss than GLP-1 agonists among people who regained weight following initial weight-loss surgery, according to a case-control real-world study presented at the meeting.
“Laparoscopic sleeve gastrectomy [LSG] is a frequently performed bariatric surgery worldwide resulting in significant weight loss and improvement in obesity-related comorbidities,” said Firas Bahdi, MD, gastroenterology fellow at the David Geffen School of Medicine at University of California, Los Angeles. “Despite its success, around one third of patients, unfortunately, develop weight regain warranting intervention.”
Dr. Bahdi and colleagues retrospectively studied 68 adults prescribed subcutaneous semaglutide or tirzepatide after LSG, another 20 who had ESG for weight regain after LSG, and 87 controls with intact stomachs who also took GLP-1 agonists for weight loss.
They found that the ESG group experienced a significantly higher percentage of total body weight loss at 3 months than the GLP-1 group (10% vs 4.3%, respectively; P = .0001). Similarly, at the 6-month follow-up, the ESG group experienced 11.5% total body weight loss compared to 6.8% in the GLP-1 group (P = .03).
The GLP-1 after LSG group still fared better than the GLP-1 control group of people who never had surgery. Total body weight loss was 4.3% vs 5.7% at 3 months (P = .02), 6.8% vs 9.2% at 6 months (P = .02), and 9.2% vs 12.7% at 12 months (P = .03).
“In this real-world experience, revisional ESG offers significantly more weight loss than GLP-1 agonists for patients with weight regain, while also avoiding the challenges of medication refills, making it an attractive option,” Dr. Bahdi said.
Future multicenter studies are warranted to confirm these results and explore physiological explanations, he added.
The study received an Outstanding Research Award in the Obesity Category (Trainee).
Dr. Velji-Ibrahim, Dr. Adekolu, Dr. Cohen, and Dr. Bahdi indicated no relevant financial relationships.
A version of this article appeared on Medscape.com.
VANCOUVER — Glucagon-like peptide-1 (GLP-1) agonists, like semaglutide, liraglutide, and the newly US Food and Drug Administration–approved tirzepatide, not only are gaining popularity among the public for weight loss but also are the focus of considerable attention from gastroenterology researchers.
how they compare to bariatric surgery for weight loss or prevention of metabolic dysfunction–associated steatotic liver disease, and their potential role to prevent regain after weight-loss surgery.
Head-to-Head Comparison
Tirzepatide 15 mg emerged as superior to other GLP-1 agonists for weight loss, for example, in a network meta-analysis of randomized controlled trials looking into obesity management.
Tirzepatide 15 mg was associated with the most effective mean weight loss at just over 15% when Jena Velji-Ibrahim, MD, and colleagues combined data from 14 studies with 18,714 participants with overweight or obesity but without diabetes.
Next up in order of weight-loss efficacy was tirzepatide 10 mg with 13% mean weight loss, semaglutide 2.4 mg with just over 11% mean weight loss, and tirzepatide 5 mg with almost 10% mean weight loss. The only outlier was dulaglutide 0.75 mg, which was linked to about 8% weight gain.
“While clinical trials have been conducted to assess the weight-loss efficacy of GLP-1 agonists, there has been limited head-to-head comparisons, and the data that has been obtained has been quite inconsistent,” Dr. Velji-Ibrahim said when presenting results at the meeting.
Researchers found little difference in efficacies between tirzepatide 15 mg and 10 mg, suggesting both are a viable option for weight loss, said Dr. Velji-Ibrahim of Prisma Health Greenville Memorial Hospital and University of South Carolina School of Medicine in Greenville.
She also reported similar efficacies between oral semaglutide 50 mg and subcutaneous semaglutide 2.4 mg, “meaning that we have another option for weight management.”
Side effects among the different GLP-1 agonists, and among the same agent at different doses, were not significantly different.
Comparison With Bariatric Surgery for Reducing Major Adverse Cardiovascular Events (MACE)
For many years, bariatric surgeons have pointed to the health benefits of weight-loss surgery in the right candidates, including a reduced risk for adverse cardiovascular events.
The weight loss associated with GLP-1 agonists has likewise shown benefits in reducing MACE. However, it remains unclear if one of these weight-loss strategies is better than the other in reducing these outcomes.
To determine this, researchers compared 118,828 people who had bariatric surgery to another propensity-matched group of 118,828 others prescribed GLP-1 agonists. They included adults with a body mass index (BMI) of 35 or higher in the national TriNetX database.
The multicenter, retrospective study revealed bariatric surgery was superior in reducing the risk for heart failure, MACE, and cerebrovascular disease at 3, 5, 7, and 10 years. At 10 years, for example, bariatric surgery was associated with 31% fewer composite cardiovascular events than the GLP-1 agonists.
“Our results suggest that bariatric surgery is more effective than GLP-1 analogs in preventing adverse cardiovascular events in obese patients,” Ayowumi A. Adekolu, MD, an internal medicine resident at West Virginia School of Medicine in Morgantown, said in audio comments accompanying his ePoster at the meeting. “Although these findings highlight the benefit of bariatric surgery in mitigating adverse cardiovascular events, well-designed prospective studies are necessary to confirm these benefits in this patient population.”
Possible Role in Fatty Liver Disease Prevention
In another large multicenter study from the same institution, Ethan M. Cohen, MD, along with co-author Dr. Adekolu and others, compared the effectiveness of bariatric surgery to GLP-1 agonists for preventing nonalcoholic fatty liver disease (NAFLD). Since the study was conducted, the official name of NAFLD has changed to metabolic dysfunction–associated steatotic liver disease.
Dr. Cohen and colleagues evaluated data from the TriNetX database and included adults with a BMI of 35 or higher. They propensity matched 124,022 people who had sleeve gastrectomy or Roux-en-Y gastric bypass to another 124,022 others prescribed GLP-1 agonists. Again, they looked at outcomes at 3, 5, 7, and 10 years.
They found bariatric surgery superior to GLP-1 agonists for reducing the risk of developing NAFLD. Relative risk reduction was 25% at 3 years, 28% at 5 years, 27% at 7 years, and 26% at 10 years.
Although not to the same extent as surgery in this study, GLP-1–associated weight loss did reduce risks as well.
“An important aspect of this is that for some of these people, bariatric surgery is not even an option,” Dr. Cohen said in an interview, citing as an example those who do not meet the criteria for surgery.
Dr. Cohen and colleagues plan to continue the study with a larger number of participants.
Real-World Weight Regain
In another instance where a surgical procedure trumped GLP-1 agonists, revisional endoscopic sleeve gastroplasty (ESG) offered significantly higher weight loss than GLP-1 agonists among people who regained weight following initial weight-loss surgery, according to a case-control real-world study presented at the meeting.
“Laparoscopic sleeve gastrectomy [LSG] is a frequently performed bariatric surgery worldwide resulting in significant weight loss and improvement in obesity-related comorbidities,” said Firas Bahdi, MD, gastroenterology fellow at the David Geffen School of Medicine at University of California, Los Angeles. “Despite its success, around one third of patients, unfortunately, develop weight regain warranting intervention.”
Dr. Bahdi and colleagues retrospectively studied 68 adults prescribed subcutaneous semaglutide or tirzepatide after LSG, another 20 who had ESG for weight regain after LSG, and 87 controls with intact stomachs who also took GLP-1 agonists for weight loss.
They found that the ESG group experienced a significantly higher percentage of total body weight loss at 3 months than the GLP-1 group (10% vs 4.3%, respectively; P = .0001). Similarly, at the 6-month follow-up, the ESG group experienced 11.5% total body weight loss compared to 6.8% in the GLP-1 group (P = .03).
The GLP-1 after LSG group still fared better than the GLP-1 control group of people who never had surgery. Total body weight loss was 4.3% vs 5.7% at 3 months (P = .02), 6.8% vs 9.2% at 6 months (P = .02), and 9.2% vs 12.7% at 12 months (P = .03).
“In this real-world experience, revisional ESG offers significantly more weight loss than GLP-1 agonists for patients with weight regain, while also avoiding the challenges of medication refills, making it an attractive option,” Dr. Bahdi said.
Future multicenter studies are warranted to confirm these results and explore physiological explanations, he added.
The study received an Outstanding Research Award in the Obesity Category (Trainee).
Dr. Velji-Ibrahim, Dr. Adekolu, Dr. Cohen, and Dr. Bahdi indicated no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM ASG 2023
Novel Solutions Needed to Attract Residents to Pediatric Rheumatology
Pediatric rheumatologists are calling a “Code (p)RED” — a pediatric rheumatology educational deficit.
There are too few pediatric rheumatologists to meet patient demand in the United States, and projections suggest that gap will continue to widen. Disappointing match trends also reflect issues with recruitment: Since 2019, only 50%-75% of pediatric rheumatology fellowship positions have been filled each year. For 2024, the subspecialty filled 32 of 52 positions.
Lack of exposure during medical school and residency, financial concerns, and a lengthy, research-focused fellowship are seen as major contributors to the workforce shortage, and novel solutions are needed to close the gap, experts argued in a recent presentation at the annual meeting of the American College of Rheumatology.
“It’s so important now to get ahead of this because what I’m afraid of is in 10-20 years, we’re not going to have a field,” Colleen Correll, MD, MPH, an associate professor in the division of pediatric rheumatology at the University of Minnesota Medical School in Minneapolis, told this news organization.
Growing Demand, Falling Supply
Because the subspecialty was officially recognized by the American Board of Pediatrics in 1991, “it’s always been a small group of providers,” Dr. Correll said. “It’s honestly always been a recognized issue in our field.”
But a 2022 report by the ACR on the pediatric workforce has brought more attention to the issue. Dr. Correll led the study and is the chair of ACR›s Pediatric Rheumatology Committee. According to the report, an estimated 287 pediatric rheumatologists were working as full-time clinicians in 2015, while the estimated demand was 382 providers. By 2030, this projected supply of pediatric rheumatologists fell to 261, while demand rose to 461 full-time providers.
The distribution of pediatric rheumatologists is also an issue. It’s generally thought that there should be at least one pediatric rheumatologist per 100,000 children, Dr. Correll explained. According to ACR estimates, the northeast region had approximately 0.83 pediatric rheumatologists per 100,000 in 2015, while the south central and southwest regions had 0.17 and 0.20 providers per 100,000 children, respectively. Projected estimates for 2030 dipped to 0.04 or lower for the south central, southwest, and southeast regions.
A separate study from the American Board of Pediatrics, also led by Dr. Correll, that is still under review offered more optimistic projections, suggesting that there would be a 75% increase in pediatric rheumatologists from 0.27 per 100,000 children in 2020 to 0.47 per 100,000 children in 2040.
“This does look better than the ACR study, though 0.47 is still a really small number and an inadequate number to treat our children in need,” she said during her presentation at the annual meeting of the American College of Rheumatology.
Lack of Exposure During Medical Education
Few medical schools have pediatric rheumatology built into their curriculum, whether that is a whole course or a single lecture, said Jay Mehta, MD, who directs the pediatric rheumatology fellowship at the Children’s Hospital of Philadelphia. Dr. Mehta, for example, did not know that pediatric rheumatology was a field before entering residency, he said. But residencies can also lack exposure: An estimated one third of residencies do not have a single pediatric rheumatologist on staff, he said.
“Those are places where people aren’t necessarily getting exposure to pediatric rheumatology,” he told this news organization, “and we know that if you’re not exposed to a field, it’s very, very unlikely that you will go into that field.”
The ACR’s Pediatric Rheumatology Residency Program is one way that the organization is working to address this issue. The program sends pediatric residents with an interest in rheumatology to the ACR annual meeting. The Rheumatology Research Foundation also runs a visiting professorship program, where a pediatric rheumatologist conducts a rheumatology education forum at an institution with no pediatric rheumatology program.
“I’ve done it a couple of times,” Dr. Mehta said during his presentation at the annual meeting. “It’s one of the most rewarding things I’ve done.”
Financial Concerns
Additionally, although pediatric rheumatology requires more training, these subspecialists will likely make less than their general pediatric colleagues over their career. According to one study in Pediatrics, a pediatric resident pursuing rheumatology is projected to make $1.2 million dollars less over the course of their career compared with someone who started their career in general pediatrics immediately after residency. (Negative financial returns were also found for all pediatric subspecialities except for cardiology, critical care, and neonatology.)
This lower earning potential is likely a deterrent, especially for those with educational debt. In one analysis published in October, medical students with at least $200,000 in education debt were 43% more likely to go into higher-paying pediatric subspecialities than those with no debt. Nearly three out of four medical graduates have education debt, according to the American Association of Medical Colleges, with a median debt of $200,000.
While the Pediatric Specialty Loan Repayment Program was specifically designed to aid pediatric subspecialists with their educational debt, qualifying for the program is difficult for pediatric rheumatologists, explained Kristen N. Hayward, MD, of Seattle Children’s in Washington. The program provides up to $100,000 in loan forgiveness in exchange for 3 years of practicing in an underserved area; however, the program stipulates that providers must provide full-time (40 hours per week) clinical care. At academic institutions, where most pediatric rheumatologists practice, there is usually a research component to their position, and even if a provider works the equivalent of 40 hours per week in a clinic in addition to their research, they don’t qualify for the program, Dr. Hayward said.
“It’s very difficult to find someone who’s actually only doing clinical work,” she said.
The ACR has worked to combat some of these economic constraints by demonstrating the direct and downstream value of rheumatologic care, Dr. Hayward said. In a recent white paper, it was estimated that including office visits, consultations, lab testing, and radiology services, one full-time equivalent rheumatologist generates $3.5 million in revenue every year and saves health systems more than $2700 per patient per year.
In addition to placing greater value on rheumatologic care, the healthcare system also needs to recognize the current nonbillable hours that pediatric rheumatologists spend taking care of patients, Dr. Hayward noted.
Especially with electronic medical records (EMRs) and online communication with patients, “there is increasingly a lot of patient care that happens outside of clinic and that takes a lot of time,” Dr. Hayward said. For example, she spends between 1 and 2 hours every day in the EMR refilling medications and responding to patient concerns, and “that all is done in my spare time,” she said. “That’s not billed to the patient in anyway.”
Length of Fellowship
The pediatric rheumatology fellowship is a 3-year program — like other pediatric subspecialities — with a research requirement. By comparison, adult rheumatology fellowships are 2 years, and fellows can pursue additional research training if they have a strong interest.
“It sounds like just 1 more year, but I think it’s coming at a really pivotal point in people’s lives, and that 1 year can make a huge difference,” Dr. Hayward explained.
The 2 years of research might also be a deterrent for individuals who know they are only interested in clinical work, she added. About half of pediatric subspecialists only pursue clinical work after graduation, according to a recent report by the National Academies of Sciences, Engineering, and Medicine (NASEM) focused on the future pediatric physician workforce.
Additionally, only 17% of pediatric rheumatologists spend more than half of their time in research, said Fred Rivara, MD, MPH, chair of the NASEM report, in a statement included in Dr. Hayward’s ACR presentation. The report, which recommended strategies to bolster the pediatric workforce, argued that the American Board of Pediatrics should develop alternative training pathways, including 2-year, clinically heavy fellowships.
The ACR workforce team is also exploring alternative training models like competency-based education, Dr. Hayward said. The Education in Pediatrics Across the Continuum project is already using this approach from medical school to pediatric residency. While this type of outcome-based program has not been tried at the fellowship level, «this has been done, it could be done, and I think we could learn from our colleagues about how they have done this successfully,» she noted.
Ultimately, Dr. Hayward emphasized that there needs to be a “sea change” to close the workforce gap — with multiple interventions addressing these individual challenges.
“Unless we all pitch in and find one way that we can all move this issue forward, we are going to be drowning in a sea of Epic inbox messages,” she said, “and never get to see the patients we want to see.”
Dr. Hayward previously owned stock/stock options for AbbVie/Abbott, Cigna/Express Scripts, Merck, and Teva and has received an educational grant from Pfizer. Dr. Correll and Dr. Mehta had no relevant disclosures.
A version of this article appeared on Medscape.com.
Pediatric rheumatologists are calling a “Code (p)RED” — a pediatric rheumatology educational deficit.
There are too few pediatric rheumatologists to meet patient demand in the United States, and projections suggest that gap will continue to widen. Disappointing match trends also reflect issues with recruitment: Since 2019, only 50%-75% of pediatric rheumatology fellowship positions have been filled each year. For 2024, the subspecialty filled 32 of 52 positions.
Lack of exposure during medical school and residency, financial concerns, and a lengthy, research-focused fellowship are seen as major contributors to the workforce shortage, and novel solutions are needed to close the gap, experts argued in a recent presentation at the annual meeting of the American College of Rheumatology.
“It’s so important now to get ahead of this because what I’m afraid of is in 10-20 years, we’re not going to have a field,” Colleen Correll, MD, MPH, an associate professor in the division of pediatric rheumatology at the University of Minnesota Medical School in Minneapolis, told this news organization.
Growing Demand, Falling Supply
Because the subspecialty was officially recognized by the American Board of Pediatrics in 1991, “it’s always been a small group of providers,” Dr. Correll said. “It’s honestly always been a recognized issue in our field.”
But a 2022 report by the ACR on the pediatric workforce has brought more attention to the issue. Dr. Correll led the study and is the chair of ACR›s Pediatric Rheumatology Committee. According to the report, an estimated 287 pediatric rheumatologists were working as full-time clinicians in 2015, while the estimated demand was 382 providers. By 2030, this projected supply of pediatric rheumatologists fell to 261, while demand rose to 461 full-time providers.
The distribution of pediatric rheumatologists is also an issue. It’s generally thought that there should be at least one pediatric rheumatologist per 100,000 children, Dr. Correll explained. According to ACR estimates, the northeast region had approximately 0.83 pediatric rheumatologists per 100,000 in 2015, while the south central and southwest regions had 0.17 and 0.20 providers per 100,000 children, respectively. Projected estimates for 2030 dipped to 0.04 or lower for the south central, southwest, and southeast regions.
A separate study from the American Board of Pediatrics, also led by Dr. Correll, that is still under review offered more optimistic projections, suggesting that there would be a 75% increase in pediatric rheumatologists from 0.27 per 100,000 children in 2020 to 0.47 per 100,000 children in 2040.
“This does look better than the ACR study, though 0.47 is still a really small number and an inadequate number to treat our children in need,” she said during her presentation at the annual meeting of the American College of Rheumatology.
Lack of Exposure During Medical Education
Few medical schools have pediatric rheumatology built into their curriculum, whether that is a whole course or a single lecture, said Jay Mehta, MD, who directs the pediatric rheumatology fellowship at the Children’s Hospital of Philadelphia. Dr. Mehta, for example, did not know that pediatric rheumatology was a field before entering residency, he said. But residencies can also lack exposure: An estimated one third of residencies do not have a single pediatric rheumatologist on staff, he said.
“Those are places where people aren’t necessarily getting exposure to pediatric rheumatology,” he told this news organization, “and we know that if you’re not exposed to a field, it’s very, very unlikely that you will go into that field.”
The ACR’s Pediatric Rheumatology Residency Program is one way that the organization is working to address this issue. The program sends pediatric residents with an interest in rheumatology to the ACR annual meeting. The Rheumatology Research Foundation also runs a visiting professorship program, where a pediatric rheumatologist conducts a rheumatology education forum at an institution with no pediatric rheumatology program.
“I’ve done it a couple of times,” Dr. Mehta said during his presentation at the annual meeting. “It’s one of the most rewarding things I’ve done.”
Financial Concerns
Additionally, although pediatric rheumatology requires more training, these subspecialists will likely make less than their general pediatric colleagues over their career. According to one study in Pediatrics, a pediatric resident pursuing rheumatology is projected to make $1.2 million dollars less over the course of their career compared with someone who started their career in general pediatrics immediately after residency. (Negative financial returns were also found for all pediatric subspecialities except for cardiology, critical care, and neonatology.)
This lower earning potential is likely a deterrent, especially for those with educational debt. In one analysis published in October, medical students with at least $200,000 in education debt were 43% more likely to go into higher-paying pediatric subspecialities than those with no debt. Nearly three out of four medical graduates have education debt, according to the American Association of Medical Colleges, with a median debt of $200,000.
While the Pediatric Specialty Loan Repayment Program was specifically designed to aid pediatric subspecialists with their educational debt, qualifying for the program is difficult for pediatric rheumatologists, explained Kristen N. Hayward, MD, of Seattle Children’s in Washington. The program provides up to $100,000 in loan forgiveness in exchange for 3 years of practicing in an underserved area; however, the program stipulates that providers must provide full-time (40 hours per week) clinical care. At academic institutions, where most pediatric rheumatologists practice, there is usually a research component to their position, and even if a provider works the equivalent of 40 hours per week in a clinic in addition to their research, they don’t qualify for the program, Dr. Hayward said.
“It’s very difficult to find someone who’s actually only doing clinical work,” she said.
The ACR has worked to combat some of these economic constraints by demonstrating the direct and downstream value of rheumatologic care, Dr. Hayward said. In a recent white paper, it was estimated that including office visits, consultations, lab testing, and radiology services, one full-time equivalent rheumatologist generates $3.5 million in revenue every year and saves health systems more than $2700 per patient per year.
In addition to placing greater value on rheumatologic care, the healthcare system also needs to recognize the current nonbillable hours that pediatric rheumatologists spend taking care of patients, Dr. Hayward noted.
Especially with electronic medical records (EMRs) and online communication with patients, “there is increasingly a lot of patient care that happens outside of clinic and that takes a lot of time,” Dr. Hayward said. For example, she spends between 1 and 2 hours every day in the EMR refilling medications and responding to patient concerns, and “that all is done in my spare time,” she said. “That’s not billed to the patient in anyway.”
Length of Fellowship
The pediatric rheumatology fellowship is a 3-year program — like other pediatric subspecialities — with a research requirement. By comparison, adult rheumatology fellowships are 2 years, and fellows can pursue additional research training if they have a strong interest.
“It sounds like just 1 more year, but I think it’s coming at a really pivotal point in people’s lives, and that 1 year can make a huge difference,” Dr. Hayward explained.
The 2 years of research might also be a deterrent for individuals who know they are only interested in clinical work, she added. About half of pediatric subspecialists only pursue clinical work after graduation, according to a recent report by the National Academies of Sciences, Engineering, and Medicine (NASEM) focused on the future pediatric physician workforce.
Additionally, only 17% of pediatric rheumatologists spend more than half of their time in research, said Fred Rivara, MD, MPH, chair of the NASEM report, in a statement included in Dr. Hayward’s ACR presentation. The report, which recommended strategies to bolster the pediatric workforce, argued that the American Board of Pediatrics should develop alternative training pathways, including 2-year, clinically heavy fellowships.
The ACR workforce team is also exploring alternative training models like competency-based education, Dr. Hayward said. The Education in Pediatrics Across the Continuum project is already using this approach from medical school to pediatric residency. While this type of outcome-based program has not been tried at the fellowship level, «this has been done, it could be done, and I think we could learn from our colleagues about how they have done this successfully,» she noted.
Ultimately, Dr. Hayward emphasized that there needs to be a “sea change” to close the workforce gap — with multiple interventions addressing these individual challenges.
“Unless we all pitch in and find one way that we can all move this issue forward, we are going to be drowning in a sea of Epic inbox messages,” she said, “and never get to see the patients we want to see.”
Dr. Hayward previously owned stock/stock options for AbbVie/Abbott, Cigna/Express Scripts, Merck, and Teva and has received an educational grant from Pfizer. Dr. Correll and Dr. Mehta had no relevant disclosures.
A version of this article appeared on Medscape.com.
Pediatric rheumatologists are calling a “Code (p)RED” — a pediatric rheumatology educational deficit.
There are too few pediatric rheumatologists to meet patient demand in the United States, and projections suggest that gap will continue to widen. Disappointing match trends also reflect issues with recruitment: Since 2019, only 50%-75% of pediatric rheumatology fellowship positions have been filled each year. For 2024, the subspecialty filled 32 of 52 positions.
Lack of exposure during medical school and residency, financial concerns, and a lengthy, research-focused fellowship are seen as major contributors to the workforce shortage, and novel solutions are needed to close the gap, experts argued in a recent presentation at the annual meeting of the American College of Rheumatology.
“It’s so important now to get ahead of this because what I’m afraid of is in 10-20 years, we’re not going to have a field,” Colleen Correll, MD, MPH, an associate professor in the division of pediatric rheumatology at the University of Minnesota Medical School in Minneapolis, told this news organization.
Growing Demand, Falling Supply
Because the subspecialty was officially recognized by the American Board of Pediatrics in 1991, “it’s always been a small group of providers,” Dr. Correll said. “It’s honestly always been a recognized issue in our field.”
But a 2022 report by the ACR on the pediatric workforce has brought more attention to the issue. Dr. Correll led the study and is the chair of ACR›s Pediatric Rheumatology Committee. According to the report, an estimated 287 pediatric rheumatologists were working as full-time clinicians in 2015, while the estimated demand was 382 providers. By 2030, this projected supply of pediatric rheumatologists fell to 261, while demand rose to 461 full-time providers.
The distribution of pediatric rheumatologists is also an issue. It’s generally thought that there should be at least one pediatric rheumatologist per 100,000 children, Dr. Correll explained. According to ACR estimates, the northeast region had approximately 0.83 pediatric rheumatologists per 100,000 in 2015, while the south central and southwest regions had 0.17 and 0.20 providers per 100,000 children, respectively. Projected estimates for 2030 dipped to 0.04 or lower for the south central, southwest, and southeast regions.
A separate study from the American Board of Pediatrics, also led by Dr. Correll, that is still under review offered more optimistic projections, suggesting that there would be a 75% increase in pediatric rheumatologists from 0.27 per 100,000 children in 2020 to 0.47 per 100,000 children in 2040.
“This does look better than the ACR study, though 0.47 is still a really small number and an inadequate number to treat our children in need,” she said during her presentation at the annual meeting of the American College of Rheumatology.
Lack of Exposure During Medical Education
Few medical schools have pediatric rheumatology built into their curriculum, whether that is a whole course or a single lecture, said Jay Mehta, MD, who directs the pediatric rheumatology fellowship at the Children’s Hospital of Philadelphia. Dr. Mehta, for example, did not know that pediatric rheumatology was a field before entering residency, he said. But residencies can also lack exposure: An estimated one third of residencies do not have a single pediatric rheumatologist on staff, he said.
“Those are places where people aren’t necessarily getting exposure to pediatric rheumatology,” he told this news organization, “and we know that if you’re not exposed to a field, it’s very, very unlikely that you will go into that field.”
The ACR’s Pediatric Rheumatology Residency Program is one way that the organization is working to address this issue. The program sends pediatric residents with an interest in rheumatology to the ACR annual meeting. The Rheumatology Research Foundation also runs a visiting professorship program, where a pediatric rheumatologist conducts a rheumatology education forum at an institution with no pediatric rheumatology program.
“I’ve done it a couple of times,” Dr. Mehta said during his presentation at the annual meeting. “It’s one of the most rewarding things I’ve done.”
Financial Concerns
Additionally, although pediatric rheumatology requires more training, these subspecialists will likely make less than their general pediatric colleagues over their career. According to one study in Pediatrics, a pediatric resident pursuing rheumatology is projected to make $1.2 million dollars less over the course of their career compared with someone who started their career in general pediatrics immediately after residency. (Negative financial returns were also found for all pediatric subspecialities except for cardiology, critical care, and neonatology.)
This lower earning potential is likely a deterrent, especially for those with educational debt. In one analysis published in October, medical students with at least $200,000 in education debt were 43% more likely to go into higher-paying pediatric subspecialities than those with no debt. Nearly three out of four medical graduates have education debt, according to the American Association of Medical Colleges, with a median debt of $200,000.
While the Pediatric Specialty Loan Repayment Program was specifically designed to aid pediatric subspecialists with their educational debt, qualifying for the program is difficult for pediatric rheumatologists, explained Kristen N. Hayward, MD, of Seattle Children’s in Washington. The program provides up to $100,000 in loan forgiveness in exchange for 3 years of practicing in an underserved area; however, the program stipulates that providers must provide full-time (40 hours per week) clinical care. At academic institutions, where most pediatric rheumatologists practice, there is usually a research component to their position, and even if a provider works the equivalent of 40 hours per week in a clinic in addition to their research, they don’t qualify for the program, Dr. Hayward said.
“It’s very difficult to find someone who’s actually only doing clinical work,” she said.
The ACR has worked to combat some of these economic constraints by demonstrating the direct and downstream value of rheumatologic care, Dr. Hayward said. In a recent white paper, it was estimated that including office visits, consultations, lab testing, and radiology services, one full-time equivalent rheumatologist generates $3.5 million in revenue every year and saves health systems more than $2700 per patient per year.
In addition to placing greater value on rheumatologic care, the healthcare system also needs to recognize the current nonbillable hours that pediatric rheumatologists spend taking care of patients, Dr. Hayward noted.
Especially with electronic medical records (EMRs) and online communication with patients, “there is increasingly a lot of patient care that happens outside of clinic and that takes a lot of time,” Dr. Hayward said. For example, she spends between 1 and 2 hours every day in the EMR refilling medications and responding to patient concerns, and “that all is done in my spare time,” she said. “That’s not billed to the patient in anyway.”
Length of Fellowship
The pediatric rheumatology fellowship is a 3-year program — like other pediatric subspecialities — with a research requirement. By comparison, adult rheumatology fellowships are 2 years, and fellows can pursue additional research training if they have a strong interest.
“It sounds like just 1 more year, but I think it’s coming at a really pivotal point in people’s lives, and that 1 year can make a huge difference,” Dr. Hayward explained.
The 2 years of research might also be a deterrent for individuals who know they are only interested in clinical work, she added. About half of pediatric subspecialists only pursue clinical work after graduation, according to a recent report by the National Academies of Sciences, Engineering, and Medicine (NASEM) focused on the future pediatric physician workforce.
Additionally, only 17% of pediatric rheumatologists spend more than half of their time in research, said Fred Rivara, MD, MPH, chair of the NASEM report, in a statement included in Dr. Hayward’s ACR presentation. The report, which recommended strategies to bolster the pediatric workforce, argued that the American Board of Pediatrics should develop alternative training pathways, including 2-year, clinically heavy fellowships.
The ACR workforce team is also exploring alternative training models like competency-based education, Dr. Hayward said. The Education in Pediatrics Across the Continuum project is already using this approach from medical school to pediatric residency. While this type of outcome-based program has not been tried at the fellowship level, «this has been done, it could be done, and I think we could learn from our colleagues about how they have done this successfully,» she noted.
Ultimately, Dr. Hayward emphasized that there needs to be a “sea change” to close the workforce gap — with multiple interventions addressing these individual challenges.
“Unless we all pitch in and find one way that we can all move this issue forward, we are going to be drowning in a sea of Epic inbox messages,” she said, “and never get to see the patients we want to see.”
Dr. Hayward previously owned stock/stock options for AbbVie/Abbott, Cigna/Express Scripts, Merck, and Teva and has received an educational grant from Pfizer. Dr. Correll and Dr. Mehta had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM ACR 2023
How to Reduce Cardiovascular Morbidity and Mortality in Psoriasis and PsA
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.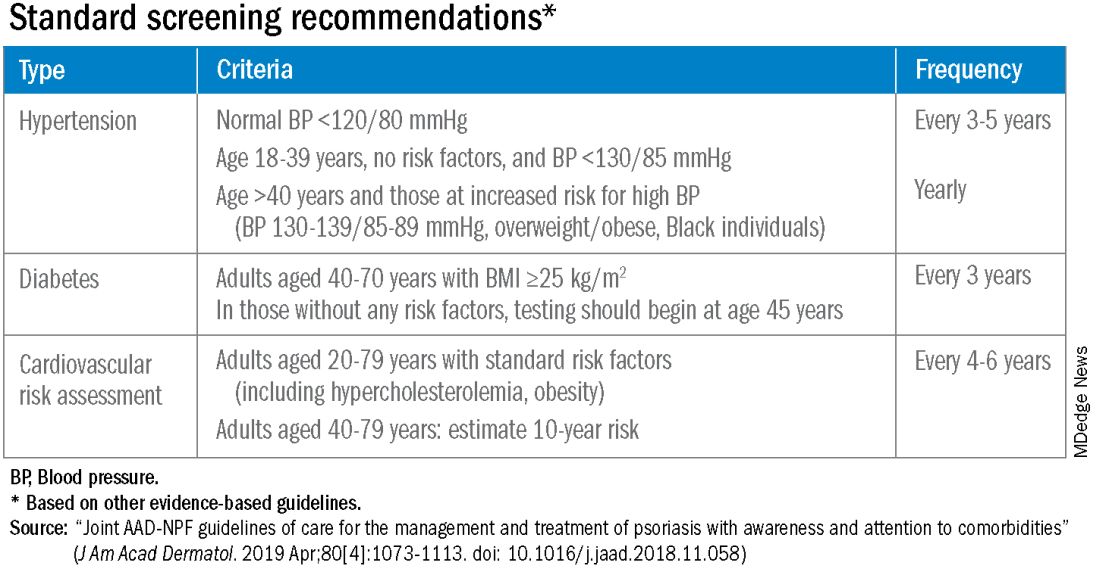
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Cluster of Eye Syphilis Cases Prompts CDC Concern
, according to a report by the Centers for Disease Control and Prevention.
With the incidence of syphilis infection in women increasing in the United States, experts are asking clinicians to be on the lookout for unusual ocular presentations.
“This is the first time such a cluster has been reported in the US,” the International Society for Infectious Diseases posted on ProMED.
Five women in Southwest Michigan who had a common male sex partner developed syphilis infections in their eyes. No new cases have been found related to these five cases after the women and the man received medical care.
If left untreated, the bacterium, Treponema pallidum, can infect the eyes, the ears, and the central nervous system.
The women, identified as non-Hispanic White, were aged 40-60 years and were not infected with HIV. They were diagnosed with early-stage syphilis and all were hospitalized and treated with intravenous penicillin. Routes of sexual exposure among the women included anal (40%), oral (40%), and vaginal (100%), the report states.
The common male sex partner they all met online was found to have early latent syphilis but never developed ocular syphilis.
It is not the eyes that are being exposed. Rather, it is an ocular presentation brought about by a systemic infection carried through the bloodstream after sexual exposure, explains William Nettleton, MD, MPH, medical director of the Kalamazoo and Calhoun public health departments in Michigan and lead author of the report.
“If we screen, identify, and treat syphilis promptly, we can prevent systemic manifestations,” he says.
Clinicians should be aware that the ocular manifestations can come at different stages of syphilis. “For patients you think may have ocular syphilis,” Dr. Nettleton says, “an immediate ophthalmologic evaluation is indicated.”
Symptoms Differed
The five women presented with a variety of symptoms.
Multiple attempts to contact the male partner by telephone and text were made by Michigan Department of Health and Human Services, but he did not respond. Local public health physicians reviewed the man’s electronic health record and discovered that he had sought care at a hospital emergency department in January 2022 for ulcerative penile and anal lesions.
He reported having multiple female sex partners during the previous 12 months but declined to disclose their identities; he reported no male or transgender sexual contact, according to the CDC report. Eventually he agreed to an evaluation, was found to have early latent syphilis, and was treated with penicillin.
Cases of syphilis have been soaring in the United States in recent years, reaching a 70-year high.
A version of this article appeared on Medscape.com.
, according to a report by the Centers for Disease Control and Prevention.
With the incidence of syphilis infection in women increasing in the United States, experts are asking clinicians to be on the lookout for unusual ocular presentations.
“This is the first time such a cluster has been reported in the US,” the International Society for Infectious Diseases posted on ProMED.
Five women in Southwest Michigan who had a common male sex partner developed syphilis infections in their eyes. No new cases have been found related to these five cases after the women and the man received medical care.
If left untreated, the bacterium, Treponema pallidum, can infect the eyes, the ears, and the central nervous system.
The women, identified as non-Hispanic White, were aged 40-60 years and were not infected with HIV. They were diagnosed with early-stage syphilis and all were hospitalized and treated with intravenous penicillin. Routes of sexual exposure among the women included anal (40%), oral (40%), and vaginal (100%), the report states.
The common male sex partner they all met online was found to have early latent syphilis but never developed ocular syphilis.
It is not the eyes that are being exposed. Rather, it is an ocular presentation brought about by a systemic infection carried through the bloodstream after sexual exposure, explains William Nettleton, MD, MPH, medical director of the Kalamazoo and Calhoun public health departments in Michigan and lead author of the report.
“If we screen, identify, and treat syphilis promptly, we can prevent systemic manifestations,” he says.
Clinicians should be aware that the ocular manifestations can come at different stages of syphilis. “For patients you think may have ocular syphilis,” Dr. Nettleton says, “an immediate ophthalmologic evaluation is indicated.”
Symptoms Differed
The five women presented with a variety of symptoms.
Multiple attempts to contact the male partner by telephone and text were made by Michigan Department of Health and Human Services, but he did not respond. Local public health physicians reviewed the man’s electronic health record and discovered that he had sought care at a hospital emergency department in January 2022 for ulcerative penile and anal lesions.
He reported having multiple female sex partners during the previous 12 months but declined to disclose their identities; he reported no male or transgender sexual contact, according to the CDC report. Eventually he agreed to an evaluation, was found to have early latent syphilis, and was treated with penicillin.
Cases of syphilis have been soaring in the United States in recent years, reaching a 70-year high.
A version of this article appeared on Medscape.com.
, according to a report by the Centers for Disease Control and Prevention.
With the incidence of syphilis infection in women increasing in the United States, experts are asking clinicians to be on the lookout for unusual ocular presentations.
“This is the first time such a cluster has been reported in the US,” the International Society for Infectious Diseases posted on ProMED.
Five women in Southwest Michigan who had a common male sex partner developed syphilis infections in their eyes. No new cases have been found related to these five cases after the women and the man received medical care.
If left untreated, the bacterium, Treponema pallidum, can infect the eyes, the ears, and the central nervous system.
The women, identified as non-Hispanic White, were aged 40-60 years and were not infected with HIV. They were diagnosed with early-stage syphilis and all were hospitalized and treated with intravenous penicillin. Routes of sexual exposure among the women included anal (40%), oral (40%), and vaginal (100%), the report states.
The common male sex partner they all met online was found to have early latent syphilis but never developed ocular syphilis.
It is not the eyes that are being exposed. Rather, it is an ocular presentation brought about by a systemic infection carried through the bloodstream after sexual exposure, explains William Nettleton, MD, MPH, medical director of the Kalamazoo and Calhoun public health departments in Michigan and lead author of the report.
“If we screen, identify, and treat syphilis promptly, we can prevent systemic manifestations,” he says.
Clinicians should be aware that the ocular manifestations can come at different stages of syphilis. “For patients you think may have ocular syphilis,” Dr. Nettleton says, “an immediate ophthalmologic evaluation is indicated.”
Symptoms Differed
The five women presented with a variety of symptoms.
Multiple attempts to contact the male partner by telephone and text were made by Michigan Department of Health and Human Services, but he did not respond. Local public health physicians reviewed the man’s electronic health record and discovered that he had sought care at a hospital emergency department in January 2022 for ulcerative penile and anal lesions.
He reported having multiple female sex partners during the previous 12 months but declined to disclose their identities; he reported no male or transgender sexual contact, according to the CDC report. Eventually he agreed to an evaluation, was found to have early latent syphilis, and was treated with penicillin.
Cases of syphilis have been soaring in the United States in recent years, reaching a 70-year high.
A version of this article appeared on Medscape.com.
FROM MMWR
Bilateral Burning Palmoplantar Lesions
The Diagnosis: Lichen Sclerosus
Histopathology revealed a thin epidermis with homogenization of the upper dermal collagen. By contrast, the lower dermis was sclerotic with patchy chronic dermal infiltrate (Figure). Ultimately, the patient’s clinical presentation and histopathologic findings led to a diagnosis of lichen sclerosus (LS).
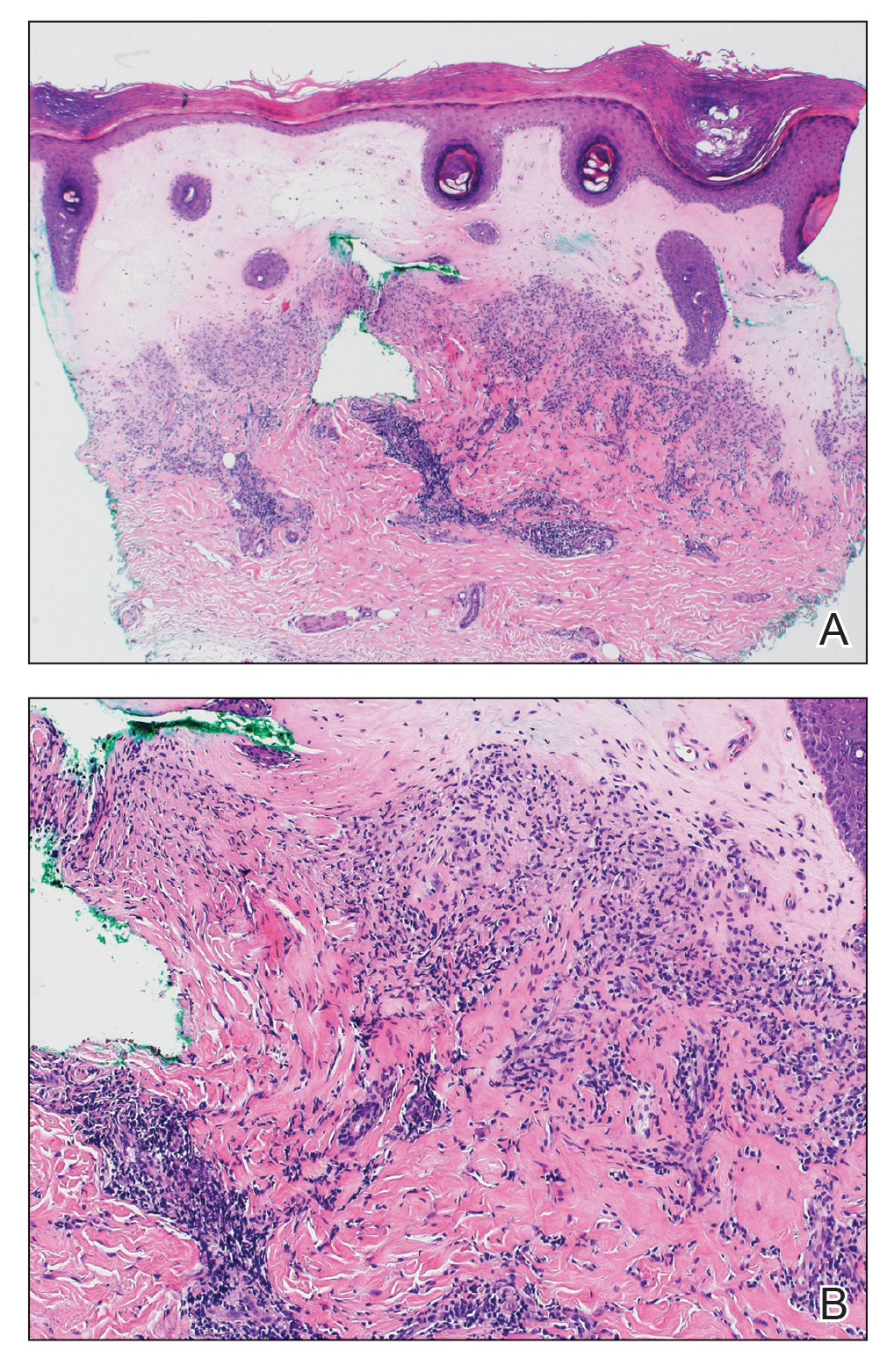
Lichen sclerosus is a rare chronic inflammatory skin condition that typically is characterized by porcelainwhite atrophic plaques on the skin, most often involving the external female genitalia including the vulva and perianal area.1 It is thought to be underdiagnosed and underreported.2 Extragenital manifestations may occur, though some cases are characterized by concomitant genital involvement.3,4 Our patient presented with palmoplantar distribution of plaques without genitalia involvement. Approximately 6% to 10% of patients with extragenital LS do not have genital involvement at the time of diagnosis.3,5 Furthermore, LS involving the palms and soles is exceedingly rare.2 Although extragenital LS may be asymptomatic, patients can experience debilitating pruritus; bullae with hemorrhage and erosion; plaque thickening with repeated excoriations; and painful fissuring, especially if lesions are in areas that are susceptible to friction or tension.3,6 New lesions on previously unaffected skin also may develop secondary to trauma through the Koebner phenomenon.1,6
Histologically, LS is characterized by epidermal hyperkeratosis accompanied by follicular plugging, epidermal atrophy with flattened rete ridges, vacuolization of the basal epidermis, marked edema in the superficial dermis (in early lesions) or homogenized collagen in the upper dermis (in established lesions), and a lymphohistiocytic infiltrate beneath the homogenized collagen. Although the pathogenesis of LS is unclear, purported etiologic factors from studies in genital disease include immune dysfunction, genetic predisposition, infection, and trauma.6 Lichen sclerosus is associated strongly with autoimmune diseases including alopecia areata, vitiligo, autoimmune thyroiditis, diabetes mellitus, and pernicious anemia, indicating its potential multifactorial etiology and linkage to T-lymphocyte dysfunction.1 Early LS lesions often appear as flat-topped and slightly scaly, hypopigmented, white or mildly erythematous, polygonal papules that coalesce to form larger plaques with peripheral erythema. With time, the inflammation subsides, and lesions become porcelain-white with varying degrees of palpable sclerosis, resembling thin paperlike wrinkles indicative of epidermal atrophy.6
The differential diagnosis of LS includes lichen planus (LP), morphea, discoid lupus erythematosus (DLE), and vitiligo.3 Lesions of LP commonly are described as flat-topped, polygonal, pink-purple papules localized mostly along the volar wrists, shins, presacral area, and hands.7 Lichen planus is considered to be more pruritic3 than LS and can be further distinguished by biopsy through identifying a well-formed granular layer and numerous cytoid bodies. Unlike LS, LP is not characterized by basement membrane thickening or epidermal atrophy.8
Skin lesions seen in morphea may resemble the classic atrophic white lesions of extragenital LS; however, it is unclear if the appearance of LS-like lesions with morphea is a simultaneous occurrence of 2 separate disorders or the development of clinical findings resembling LS in lesions of morphea.6 Furthermore, morphea involves deep inflammation and sclerosis of the dermis that may extend into subcutaneous fat without follicular plugging of the epidermis.3,9 In contrast, LS primarily affects the epidermis and dermis with the presence of epidermal follicular plugging.6
Lesions seen in DLE are characterized as well-defined, annular, erythematous patches and plaques followed by follicular hyperkeratosis with adherent scaling. Upon removal of the scale, follicle-sized keratotic spikes (carpet tacks) are present.10 Scaling of lesions and the carpet tack sign were absent in our patient. In addition, DLE typically reveals surrounding pigmentation and scarring over plaques,3 which were not observed in our patient.
Vitiligo commonly is associated with extragenital LS. As with LS, vitiligo can be explained by mechanisms of immune checkpoint inhibitor–induced cytotoxicity as well as perforin and granzyme-B expression.11 Although vitiligo resembles the late hypopigmented lesions of extragenital LS, there are no plaques or surface changes, and a larger, more generalized area of the skin typically is involved.3
- Chamli A, Souissi A. Lichen sclerosus. StatPearls [Internet]. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK538246/
- Gaddis KJ, Huang J, Haun PL. An atrophic and spiny eruption of the palms. JAMA Dermatol. 2018;154:1344-1345. doi:10.1001 /jamadermatol.2018.1265
- Arif T, Fatima R, Sami M. Extragenital lichen sclerosus: a comprehensive review [published online August 11, 2022]. Australas J Dermatol. doi:10.1111/ajd.13890
- Heibel HD, Styles AR, Cockerell CJ. A case of acral lichen sclerosus et atrophicus. JAAD Case Rep. 2020;8:26-27. doi:10.1016/j.jdcr.2020.12.008
- Seyffert J, Bibliowicz N, Harding T, et al. Palmar lichen sclerosus et atrophicus. JAAD Case Rep. 2020;6:697-699. doi:10.1016/j.jdcr.2020.06.005
- Jacobe H. Extragenital lichen sclerosus: clinical features and diagnosis. UpToDate. Updated July 11, 2023. Accessed December 14, 2023. https://www.uptodate.com/contents/extragenital-lichen-sclerosus?search=Lichen%20sclerosus&source =search_result&selectedTitle=2~66&usage_type=default&display_ rank=2
- Goldstein BG, Goldstein AO, Mostow E. Lichen planus. UpToDate. Updated October 25, 2021. Accessed December 14, 2023. https://www.uptodate.com/contents/lichen-planus?search=lichen%20 sclerosus&topicRef=15838&source=see_link
- Tallon B. Lichen sclerosus pathology. DermNet NZ website. Accessed December 5, 2023. https://dermnetnz.org/topics/lichen-sclerosus-pathology
- Jacobe H. Pathogenesis, clinical manifestations, and diagnosis of morphea (localized scleroderma) in adults. UpToDate. Updated November 15, 2021. Accessed December 14, 2023. https://medilib.ir/uptodate/show/13776
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing; 2022. Updated August 28, 2023. Accessed December 14, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493145/
- Veronesi G, Scarfì F, Misciali C, et al. An unusual skin reaction in uveal melanoma during treatment with nivolumab: extragenital lichen sclerosus. Anticancer Drugs. 2019;30:969-972. doi:10.1097/ CAD.0000000000000819
The Diagnosis: Lichen Sclerosus
Histopathology revealed a thin epidermis with homogenization of the upper dermal collagen. By contrast, the lower dermis was sclerotic with patchy chronic dermal infiltrate (Figure). Ultimately, the patient’s clinical presentation and histopathologic findings led to a diagnosis of lichen sclerosus (LS).

Lichen sclerosus is a rare chronic inflammatory skin condition that typically is characterized by porcelainwhite atrophic plaques on the skin, most often involving the external female genitalia including the vulva and perianal area.1 It is thought to be underdiagnosed and underreported.2 Extragenital manifestations may occur, though some cases are characterized by concomitant genital involvement.3,4 Our patient presented with palmoplantar distribution of plaques without genitalia involvement. Approximately 6% to 10% of patients with extragenital LS do not have genital involvement at the time of diagnosis.3,5 Furthermore, LS involving the palms and soles is exceedingly rare.2 Although extragenital LS may be asymptomatic, patients can experience debilitating pruritus; bullae with hemorrhage and erosion; plaque thickening with repeated excoriations; and painful fissuring, especially if lesions are in areas that are susceptible to friction or tension.3,6 New lesions on previously unaffected skin also may develop secondary to trauma through the Koebner phenomenon.1,6
Histologically, LS is characterized by epidermal hyperkeratosis accompanied by follicular plugging, epidermal atrophy with flattened rete ridges, vacuolization of the basal epidermis, marked edema in the superficial dermis (in early lesions) or homogenized collagen in the upper dermis (in established lesions), and a lymphohistiocytic infiltrate beneath the homogenized collagen. Although the pathogenesis of LS is unclear, purported etiologic factors from studies in genital disease include immune dysfunction, genetic predisposition, infection, and trauma.6 Lichen sclerosus is associated strongly with autoimmune diseases including alopecia areata, vitiligo, autoimmune thyroiditis, diabetes mellitus, and pernicious anemia, indicating its potential multifactorial etiology and linkage to T-lymphocyte dysfunction.1 Early LS lesions often appear as flat-topped and slightly scaly, hypopigmented, white or mildly erythematous, polygonal papules that coalesce to form larger plaques with peripheral erythema. With time, the inflammation subsides, and lesions become porcelain-white with varying degrees of palpable sclerosis, resembling thin paperlike wrinkles indicative of epidermal atrophy.6
The differential diagnosis of LS includes lichen planus (LP), morphea, discoid lupus erythematosus (DLE), and vitiligo.3 Lesions of LP commonly are described as flat-topped, polygonal, pink-purple papules localized mostly along the volar wrists, shins, presacral area, and hands.7 Lichen planus is considered to be more pruritic3 than LS and can be further distinguished by biopsy through identifying a well-formed granular layer and numerous cytoid bodies. Unlike LS, LP is not characterized by basement membrane thickening or epidermal atrophy.8
Skin lesions seen in morphea may resemble the classic atrophic white lesions of extragenital LS; however, it is unclear if the appearance of LS-like lesions with morphea is a simultaneous occurrence of 2 separate disorders or the development of clinical findings resembling LS in lesions of morphea.6 Furthermore, morphea involves deep inflammation and sclerosis of the dermis that may extend into subcutaneous fat without follicular plugging of the epidermis.3,9 In contrast, LS primarily affects the epidermis and dermis with the presence of epidermal follicular plugging.6
Lesions seen in DLE are characterized as well-defined, annular, erythematous patches and plaques followed by follicular hyperkeratosis with adherent scaling. Upon removal of the scale, follicle-sized keratotic spikes (carpet tacks) are present.10 Scaling of lesions and the carpet tack sign were absent in our patient. In addition, DLE typically reveals surrounding pigmentation and scarring over plaques,3 which were not observed in our patient.
Vitiligo commonly is associated with extragenital LS. As with LS, vitiligo can be explained by mechanisms of immune checkpoint inhibitor–induced cytotoxicity as well as perforin and granzyme-B expression.11 Although vitiligo resembles the late hypopigmented lesions of extragenital LS, there are no plaques or surface changes, and a larger, more generalized area of the skin typically is involved.3
The Diagnosis: Lichen Sclerosus
Histopathology revealed a thin epidermis with homogenization of the upper dermal collagen. By contrast, the lower dermis was sclerotic with patchy chronic dermal infiltrate (Figure). Ultimately, the patient’s clinical presentation and histopathologic findings led to a diagnosis of lichen sclerosus (LS).

Lichen sclerosus is a rare chronic inflammatory skin condition that typically is characterized by porcelainwhite atrophic plaques on the skin, most often involving the external female genitalia including the vulva and perianal area.1 It is thought to be underdiagnosed and underreported.2 Extragenital manifestations may occur, though some cases are characterized by concomitant genital involvement.3,4 Our patient presented with palmoplantar distribution of plaques without genitalia involvement. Approximately 6% to 10% of patients with extragenital LS do not have genital involvement at the time of diagnosis.3,5 Furthermore, LS involving the palms and soles is exceedingly rare.2 Although extragenital LS may be asymptomatic, patients can experience debilitating pruritus; bullae with hemorrhage and erosion; plaque thickening with repeated excoriations; and painful fissuring, especially if lesions are in areas that are susceptible to friction or tension.3,6 New lesions on previously unaffected skin also may develop secondary to trauma through the Koebner phenomenon.1,6
Histologically, LS is characterized by epidermal hyperkeratosis accompanied by follicular plugging, epidermal atrophy with flattened rete ridges, vacuolization of the basal epidermis, marked edema in the superficial dermis (in early lesions) or homogenized collagen in the upper dermis (in established lesions), and a lymphohistiocytic infiltrate beneath the homogenized collagen. Although the pathogenesis of LS is unclear, purported etiologic factors from studies in genital disease include immune dysfunction, genetic predisposition, infection, and trauma.6 Lichen sclerosus is associated strongly with autoimmune diseases including alopecia areata, vitiligo, autoimmune thyroiditis, diabetes mellitus, and pernicious anemia, indicating its potential multifactorial etiology and linkage to T-lymphocyte dysfunction.1 Early LS lesions often appear as flat-topped and slightly scaly, hypopigmented, white or mildly erythematous, polygonal papules that coalesce to form larger plaques with peripheral erythema. With time, the inflammation subsides, and lesions become porcelain-white with varying degrees of palpable sclerosis, resembling thin paperlike wrinkles indicative of epidermal atrophy.6
The differential diagnosis of LS includes lichen planus (LP), morphea, discoid lupus erythematosus (DLE), and vitiligo.3 Lesions of LP commonly are described as flat-topped, polygonal, pink-purple papules localized mostly along the volar wrists, shins, presacral area, and hands.7 Lichen planus is considered to be more pruritic3 than LS and can be further distinguished by biopsy through identifying a well-formed granular layer and numerous cytoid bodies. Unlike LS, LP is not characterized by basement membrane thickening or epidermal atrophy.8
Skin lesions seen in morphea may resemble the classic atrophic white lesions of extragenital LS; however, it is unclear if the appearance of LS-like lesions with morphea is a simultaneous occurrence of 2 separate disorders or the development of clinical findings resembling LS in lesions of morphea.6 Furthermore, morphea involves deep inflammation and sclerosis of the dermis that may extend into subcutaneous fat without follicular plugging of the epidermis.3,9 In contrast, LS primarily affects the epidermis and dermis with the presence of epidermal follicular plugging.6
Lesions seen in DLE are characterized as well-defined, annular, erythematous patches and plaques followed by follicular hyperkeratosis with adherent scaling. Upon removal of the scale, follicle-sized keratotic spikes (carpet tacks) are present.10 Scaling of lesions and the carpet tack sign were absent in our patient. In addition, DLE typically reveals surrounding pigmentation and scarring over plaques,3 which were not observed in our patient.
Vitiligo commonly is associated with extragenital LS. As with LS, vitiligo can be explained by mechanisms of immune checkpoint inhibitor–induced cytotoxicity as well as perforin and granzyme-B expression.11 Although vitiligo resembles the late hypopigmented lesions of extragenital LS, there are no plaques or surface changes, and a larger, more generalized area of the skin typically is involved.3
- Chamli A, Souissi A. Lichen sclerosus. StatPearls [Internet]. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK538246/
- Gaddis KJ, Huang J, Haun PL. An atrophic and spiny eruption of the palms. JAMA Dermatol. 2018;154:1344-1345. doi:10.1001 /jamadermatol.2018.1265
- Arif T, Fatima R, Sami M. Extragenital lichen sclerosus: a comprehensive review [published online August 11, 2022]. Australas J Dermatol. doi:10.1111/ajd.13890
- Heibel HD, Styles AR, Cockerell CJ. A case of acral lichen sclerosus et atrophicus. JAAD Case Rep. 2020;8:26-27. doi:10.1016/j.jdcr.2020.12.008
- Seyffert J, Bibliowicz N, Harding T, et al. Palmar lichen sclerosus et atrophicus. JAAD Case Rep. 2020;6:697-699. doi:10.1016/j.jdcr.2020.06.005
- Jacobe H. Extragenital lichen sclerosus: clinical features and diagnosis. UpToDate. Updated July 11, 2023. Accessed December 14, 2023. https://www.uptodate.com/contents/extragenital-lichen-sclerosus?search=Lichen%20sclerosus&source =search_result&selectedTitle=2~66&usage_type=default&display_ rank=2
- Goldstein BG, Goldstein AO, Mostow E. Lichen planus. UpToDate. Updated October 25, 2021. Accessed December 14, 2023. https://www.uptodate.com/contents/lichen-planus?search=lichen%20 sclerosus&topicRef=15838&source=see_link
- Tallon B. Lichen sclerosus pathology. DermNet NZ website. Accessed December 5, 2023. https://dermnetnz.org/topics/lichen-sclerosus-pathology
- Jacobe H. Pathogenesis, clinical manifestations, and diagnosis of morphea (localized scleroderma) in adults. UpToDate. Updated November 15, 2021. Accessed December 14, 2023. https://medilib.ir/uptodate/show/13776
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing; 2022. Updated August 28, 2023. Accessed December 14, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493145/
- Veronesi G, Scarfì F, Misciali C, et al. An unusual skin reaction in uveal melanoma during treatment with nivolumab: extragenital lichen sclerosus. Anticancer Drugs. 2019;30:969-972. doi:10.1097/ CAD.0000000000000819
- Chamli A, Souissi A. Lichen sclerosus. StatPearls [Internet]. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK538246/
- Gaddis KJ, Huang J, Haun PL. An atrophic and spiny eruption of the palms. JAMA Dermatol. 2018;154:1344-1345. doi:10.1001 /jamadermatol.2018.1265
- Arif T, Fatima R, Sami M. Extragenital lichen sclerosus: a comprehensive review [published online August 11, 2022]. Australas J Dermatol. doi:10.1111/ajd.13890
- Heibel HD, Styles AR, Cockerell CJ. A case of acral lichen sclerosus et atrophicus. JAAD Case Rep. 2020;8:26-27. doi:10.1016/j.jdcr.2020.12.008
- Seyffert J, Bibliowicz N, Harding T, et al. Palmar lichen sclerosus et atrophicus. JAAD Case Rep. 2020;6:697-699. doi:10.1016/j.jdcr.2020.06.005
- Jacobe H. Extragenital lichen sclerosus: clinical features and diagnosis. UpToDate. Updated July 11, 2023. Accessed December 14, 2023. https://www.uptodate.com/contents/extragenital-lichen-sclerosus?search=Lichen%20sclerosus&source =search_result&selectedTitle=2~66&usage_type=default&display_ rank=2
- Goldstein BG, Goldstein AO, Mostow E. Lichen planus. UpToDate. Updated October 25, 2021. Accessed December 14, 2023. https://www.uptodate.com/contents/lichen-planus?search=lichen%20 sclerosus&topicRef=15838&source=see_link
- Tallon B. Lichen sclerosus pathology. DermNet NZ website. Accessed December 5, 2023. https://dermnetnz.org/topics/lichen-sclerosus-pathology
- Jacobe H. Pathogenesis, clinical manifestations, and diagnosis of morphea (localized scleroderma) in adults. UpToDate. Updated November 15, 2021. Accessed December 14, 2023. https://medilib.ir/uptodate/show/13776
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing; 2022. Updated August 28, 2023. Accessed December 14, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493145/
- Veronesi G, Scarfì F, Misciali C, et al. An unusual skin reaction in uveal melanoma during treatment with nivolumab: extragenital lichen sclerosus. Anticancer Drugs. 2019;30:969-972. doi:10.1097/ CAD.0000000000000819
A 59-year-old woman presented with atrophic, hypopigmented, ivory papules and plaques localized to the central palms and soles of 3 years’ duration. The lesions were associated with burning that was most notable after extended periods of ambulation. The lesions initially were diagnosed as plaque psoriasis by an external dermatology clinic. At the time of presentation to our clinic, treatment with several highpotency topical steroids and biologics approved for plaque psoriasis had failed. Her medical history and concurrent medical workup were notable for type 2 diabetes mellitus, liver dysfunction, thyroid nodules overseen by an endocrinologist, vitamin B12 and vitamin D deficiencies managed with supplementation, and diffuse androgenic alopecia with suspected telogen effluvium. Physical examination revealed no plaque fissuring, pruritus, or scaling. She had no history of radiation therapy or organ transplantation. A punch biopsy of the left palm was performed.