User login
Tivozanib after sorafenib promising in patients with advanced RCC
For patients with advanced renal cell carcinoma (RCC) progressing after sorafenib treatment, tivozanib was well tolerated and provided promising survival outcomes, investigators in a phase 2 study have reported.
Incidence of adverse events on tivozanib was low, and the safety profile was favorable in comparison with other agents in its class, the investigators said in the European Journal of Cancer.
The findings also help clarify results of a previous randomized phase 3 trial of tivozanib versus sorafenib where the investigators say crossover may have confounded overall survival results to the detriment of the tivozanib arm.
“Collectively, these data provide evidence of the anti-tumor activity of tivozanib and may be used to help frame future studies in recurrent disease,” wrote Ana M. Molina, MD, of Weill Cornell Medicine, New York, and her coauthors.
Tivozanib, recently approved in Europe for untreated RCC, is characterized by highly potent and selective inhibition of the three known vascular endothelial growth factor (VEGF) receptors.
Dr. Molina and her colleagues reported a single-arm crossover study of patients who were previously enrolled in the randomized phase 3 TIVO-1 trial of tivozanib versus sorafenib.
They enrolled a total of 161 patients who were randomized to the sorafenib arm of TIVO-1 and went on to receive tivozanib after disease progression.
Median progression-free survival was 11.0 months and median overall survival was 21.6 months for these crossover patients, Dr. Molina and co-investigators reported.
No patients in the study had a complete response, while 29 (18%) had a partial response and 83 (52%) had stable disease.
“These data compare favorably with other second-line therapies for RCC,” Dr. Molina and co-authors said.
Grade 3 or greater adverse events occurred in 48% of patients, including 24% that were treatment related. The most common grade 3 treatment-related adverse event was hypertension in 11%.
Approximately 4% of patients discontinued tivozanib due to adverse events.
“This study also provided clarity of the TIVO-1 trial, in which patient crossover was thought to have confounded the overall survival results,” Dr. Molina and colleagues said.
In TIVO-1, the primary end point of progression-free survival was improved for tivozanib versus sorafenib (median of 11.9 vs 9.1 months; P = .042), they noted.
However, median overall survival was not statistically different between arms, possibly because 74% of patients randomized to sorafenib were treated with next-line therapy, mainly tivozanib, investigators said.
AVEO Oncology and Astellas Pharma US, Inc. funded the study. Dr. Molina reported receiving honoraria from AVEO, Novartis, and Eisai.
SOURCE: Molina AM, et al. Eur J Cancer. 2018 Mar 13. doi: 10.1016/j.ejca.2018.02.009.
For patients with advanced renal cell carcinoma (RCC) progressing after sorafenib treatment, tivozanib was well tolerated and provided promising survival outcomes, investigators in a phase 2 study have reported.
Incidence of adverse events on tivozanib was low, and the safety profile was favorable in comparison with other agents in its class, the investigators said in the European Journal of Cancer.
The findings also help clarify results of a previous randomized phase 3 trial of tivozanib versus sorafenib where the investigators say crossover may have confounded overall survival results to the detriment of the tivozanib arm.
“Collectively, these data provide evidence of the anti-tumor activity of tivozanib and may be used to help frame future studies in recurrent disease,” wrote Ana M. Molina, MD, of Weill Cornell Medicine, New York, and her coauthors.
Tivozanib, recently approved in Europe for untreated RCC, is characterized by highly potent and selective inhibition of the three known vascular endothelial growth factor (VEGF) receptors.
Dr. Molina and her colleagues reported a single-arm crossover study of patients who were previously enrolled in the randomized phase 3 TIVO-1 trial of tivozanib versus sorafenib.
They enrolled a total of 161 patients who were randomized to the sorafenib arm of TIVO-1 and went on to receive tivozanib after disease progression.
Median progression-free survival was 11.0 months and median overall survival was 21.6 months for these crossover patients, Dr. Molina and co-investigators reported.
No patients in the study had a complete response, while 29 (18%) had a partial response and 83 (52%) had stable disease.
“These data compare favorably with other second-line therapies for RCC,” Dr. Molina and co-authors said.
Grade 3 or greater adverse events occurred in 48% of patients, including 24% that were treatment related. The most common grade 3 treatment-related adverse event was hypertension in 11%.
Approximately 4% of patients discontinued tivozanib due to adverse events.
“This study also provided clarity of the TIVO-1 trial, in which patient crossover was thought to have confounded the overall survival results,” Dr. Molina and colleagues said.
In TIVO-1, the primary end point of progression-free survival was improved for tivozanib versus sorafenib (median of 11.9 vs 9.1 months; P = .042), they noted.
However, median overall survival was not statistically different between arms, possibly because 74% of patients randomized to sorafenib were treated with next-line therapy, mainly tivozanib, investigators said.
AVEO Oncology and Astellas Pharma US, Inc. funded the study. Dr. Molina reported receiving honoraria from AVEO, Novartis, and Eisai.
SOURCE: Molina AM, et al. Eur J Cancer. 2018 Mar 13. doi: 10.1016/j.ejca.2018.02.009.
For patients with advanced renal cell carcinoma (RCC) progressing after sorafenib treatment, tivozanib was well tolerated and provided promising survival outcomes, investigators in a phase 2 study have reported.
Incidence of adverse events on tivozanib was low, and the safety profile was favorable in comparison with other agents in its class, the investigators said in the European Journal of Cancer.
The findings also help clarify results of a previous randomized phase 3 trial of tivozanib versus sorafenib where the investigators say crossover may have confounded overall survival results to the detriment of the tivozanib arm.
“Collectively, these data provide evidence of the anti-tumor activity of tivozanib and may be used to help frame future studies in recurrent disease,” wrote Ana M. Molina, MD, of Weill Cornell Medicine, New York, and her coauthors.
Tivozanib, recently approved in Europe for untreated RCC, is characterized by highly potent and selective inhibition of the three known vascular endothelial growth factor (VEGF) receptors.
Dr. Molina and her colleagues reported a single-arm crossover study of patients who were previously enrolled in the randomized phase 3 TIVO-1 trial of tivozanib versus sorafenib.
They enrolled a total of 161 patients who were randomized to the sorafenib arm of TIVO-1 and went on to receive tivozanib after disease progression.
Median progression-free survival was 11.0 months and median overall survival was 21.6 months for these crossover patients, Dr. Molina and co-investigators reported.
No patients in the study had a complete response, while 29 (18%) had a partial response and 83 (52%) had stable disease.
“These data compare favorably with other second-line therapies for RCC,” Dr. Molina and co-authors said.
Grade 3 or greater adverse events occurred in 48% of patients, including 24% that were treatment related. The most common grade 3 treatment-related adverse event was hypertension in 11%.
Approximately 4% of patients discontinued tivozanib due to adverse events.
“This study also provided clarity of the TIVO-1 trial, in which patient crossover was thought to have confounded the overall survival results,” Dr. Molina and colleagues said.
In TIVO-1, the primary end point of progression-free survival was improved for tivozanib versus sorafenib (median of 11.9 vs 9.1 months; P = .042), they noted.
However, median overall survival was not statistically different between arms, possibly because 74% of patients randomized to sorafenib were treated with next-line therapy, mainly tivozanib, investigators said.
AVEO Oncology and Astellas Pharma US, Inc. funded the study. Dr. Molina reported receiving honoraria from AVEO, Novartis, and Eisai.
SOURCE: Molina AM, et al. Eur J Cancer. 2018 Mar 13. doi: 10.1016/j.ejca.2018.02.009.
FROM THE EUROPEAN JOURNAL OF CANCER
Key clinical point: Tivozanib has potent antitumor activity in patients with advanced renal cell carcinoma (RCC) who previously progressed on sorafenib.
Major finding: Median progression-free survival was 11.0 months, and median overall survival was 21.6 months for patients receiving tivozanib.
Study details: A single-arm, phase 2 crossover study of patients previously randomized to the sorafenib arm of the phase 3 TIVO-1 study.
Disclosures: AVEO Oncology and Astellas Pharma US, Inc. funded the study. Investigators reported potential conflict of interests related to AVEO, Novartis, Eisai, Pfizer, and others.
Source: Molina AM, et al. Eur J Cancer. 2018 Mar 13. doi: 10.1016/j.ejca.2018.02.009.
Unknown primary melanoma looks a lot like known
Stage IV melanoma of unknown primary (MUP) origin, in which a primary tumor has either resolved or remains undiscovered, shares similar outcomes and prognostic factors to melanoma of known primary (MKP) origin, according to a new analysis of the nationwide Surveillance, Epidemiology, and End Results (SEER)-18 registries spanning from 1973 to 2014.
Previous studies of MUP have been single institutional or multi-institutional studies. The current work is the first population-level study.
MUP is uncommon, representing 2.5-5% of melanoma cases. As with MKP, worse survival of MUP patients was tied to age greater than 50 years and not undergoing a surgical procedure. The researchers did find a slight advantage in one-year survival for MUP patients compared to MKP, which could be because many of the MUP patients had experienced an immune response that eliminated the primary tumor.
“You could imagine that if the body attacks the primary tumor and it goes away, you’re set up to fight off the metastatic melanoma better,” said lead study author Jeffrey Scott, MD, a micrographic surgery and dermatologic oncology fellow at University Hospitals Cleveland Medical Center, Case Western Reserve University.
The study appeared online March 23 in the Journal of the American Academy of Dermatology.
The researchers analyzed 322 stage IV MUP cases and 12,796 stage IV MKP cases. The incidence of stage IV MUP increased over time, from 1.52 per 100,000 between 1973 and 1984, to 5.83 per 100,000 from 2005 to 2014. MUP patients were more likely to be recommended for surgery than MKP patients (surgery not recommended for 47.7% of MKP cases, compared to 37.7% of MUP cases), and they had better 1-year survival rates compared to the general U.S. population than did MKP patients (0.54, 95% CI, 0.48-0.60 versus 0.41, 95% CI 0.39-0.42). The improved survival of MUP over MKP remained steady at each measured time point out to 5 years.
However, there was no difference in 5-year disease-specific survival (DSS) in MUP versus MKP (HR, 0.91; 95% CI, 0.79-1.04; P =.16), or in the 5-year DSS Kaplan-Meier curve after adjustment for year of diagnosis, age, sex, race, and surgical treatment (log-rank P = .93).
A multivariate analysis showed increased 5-year DSS among patients who received surgery (HR, 0.41; 95% CI, 0.30-0.56; P less than .001) and decreased 5-year DSS among patients over 50 (HR 3.27, 95% CI, 1.17-9.17; P = .02 for age 50-59).
“The prognostic factors are very similar, so you should treat these patients (with MUP) similarly to patients with melanoma of known primary, the same treatments, the same clinical trials,” Dr. Scott and associates said.
The results also raise the possibility of gaining a better understanding of how immune response affects the course of metastatic melanoma.
“If MUP is due to the fact that your immune system is attacking the primary tumor, then what characteristics of the person would cause that to happen? Are younger patients (exhibiting) a more robust immune response? Could that explain why their prognosis is better? More molecular studies of the actual tumors and the immune characteristics of these patients would help us answer that,” they added.
A resolved primary tumor isn’t the only explanation for MUP, however. It’s also possible that melanocytes are found in unexpected sites, perhaps because they did not complete their migration during development. “They could give rise to melanoma that would present (as MUP). Maybe there never was a skin tumor,” the authors wrote.
The investigators recommend a thorough search for a primary tumor, employing ophthalmologists, gynecologists, and other specialists if necessary. “You could argue that once you have metastatic disease, what’s the point of finding the primary tumor? But it’s important to correctly classify these patients, in terms of what clinical trials and treatments they may be eligible for,” Dr. Scott and associates said.
SOURCE: Scott JF et al. J Am Acad Dermatol. 2018 Mar 23. doi: 10.1016/j.jaad.2018.03.021.
Stage IV melanoma of unknown primary (MUP) origin, in which a primary tumor has either resolved or remains undiscovered, shares similar outcomes and prognostic factors to melanoma of known primary (MKP) origin, according to a new analysis of the nationwide Surveillance, Epidemiology, and End Results (SEER)-18 registries spanning from 1973 to 2014.
Previous studies of MUP have been single institutional or multi-institutional studies. The current work is the first population-level study.
MUP is uncommon, representing 2.5-5% of melanoma cases. As with MKP, worse survival of MUP patients was tied to age greater than 50 years and not undergoing a surgical procedure. The researchers did find a slight advantage in one-year survival for MUP patients compared to MKP, which could be because many of the MUP patients had experienced an immune response that eliminated the primary tumor.
“You could imagine that if the body attacks the primary tumor and it goes away, you’re set up to fight off the metastatic melanoma better,” said lead study author Jeffrey Scott, MD, a micrographic surgery and dermatologic oncology fellow at University Hospitals Cleveland Medical Center, Case Western Reserve University.
The study appeared online March 23 in the Journal of the American Academy of Dermatology.
The researchers analyzed 322 stage IV MUP cases and 12,796 stage IV MKP cases. The incidence of stage IV MUP increased over time, from 1.52 per 100,000 between 1973 and 1984, to 5.83 per 100,000 from 2005 to 2014. MUP patients were more likely to be recommended for surgery than MKP patients (surgery not recommended for 47.7% of MKP cases, compared to 37.7% of MUP cases), and they had better 1-year survival rates compared to the general U.S. population than did MKP patients (0.54, 95% CI, 0.48-0.60 versus 0.41, 95% CI 0.39-0.42). The improved survival of MUP over MKP remained steady at each measured time point out to 5 years.
However, there was no difference in 5-year disease-specific survival (DSS) in MUP versus MKP (HR, 0.91; 95% CI, 0.79-1.04; P =.16), or in the 5-year DSS Kaplan-Meier curve after adjustment for year of diagnosis, age, sex, race, and surgical treatment (log-rank P = .93).
A multivariate analysis showed increased 5-year DSS among patients who received surgery (HR, 0.41; 95% CI, 0.30-0.56; P less than .001) and decreased 5-year DSS among patients over 50 (HR 3.27, 95% CI, 1.17-9.17; P = .02 for age 50-59).
“The prognostic factors are very similar, so you should treat these patients (with MUP) similarly to patients with melanoma of known primary, the same treatments, the same clinical trials,” Dr. Scott and associates said.
The results also raise the possibility of gaining a better understanding of how immune response affects the course of metastatic melanoma.
“If MUP is due to the fact that your immune system is attacking the primary tumor, then what characteristics of the person would cause that to happen? Are younger patients (exhibiting) a more robust immune response? Could that explain why their prognosis is better? More molecular studies of the actual tumors and the immune characteristics of these patients would help us answer that,” they added.
A resolved primary tumor isn’t the only explanation for MUP, however. It’s also possible that melanocytes are found in unexpected sites, perhaps because they did not complete their migration during development. “They could give rise to melanoma that would present (as MUP). Maybe there never was a skin tumor,” the authors wrote.
The investigators recommend a thorough search for a primary tumor, employing ophthalmologists, gynecologists, and other specialists if necessary. “You could argue that once you have metastatic disease, what’s the point of finding the primary tumor? But it’s important to correctly classify these patients, in terms of what clinical trials and treatments they may be eligible for,” Dr. Scott and associates said.
SOURCE: Scott JF et al. J Am Acad Dermatol. 2018 Mar 23. doi: 10.1016/j.jaad.2018.03.021.
Stage IV melanoma of unknown primary (MUP) origin, in which a primary tumor has either resolved or remains undiscovered, shares similar outcomes and prognostic factors to melanoma of known primary (MKP) origin, according to a new analysis of the nationwide Surveillance, Epidemiology, and End Results (SEER)-18 registries spanning from 1973 to 2014.
Previous studies of MUP have been single institutional or multi-institutional studies. The current work is the first population-level study.
MUP is uncommon, representing 2.5-5% of melanoma cases. As with MKP, worse survival of MUP patients was tied to age greater than 50 years and not undergoing a surgical procedure. The researchers did find a slight advantage in one-year survival for MUP patients compared to MKP, which could be because many of the MUP patients had experienced an immune response that eliminated the primary tumor.
“You could imagine that if the body attacks the primary tumor and it goes away, you’re set up to fight off the metastatic melanoma better,” said lead study author Jeffrey Scott, MD, a micrographic surgery and dermatologic oncology fellow at University Hospitals Cleveland Medical Center, Case Western Reserve University.
The study appeared online March 23 in the Journal of the American Academy of Dermatology.
The researchers analyzed 322 stage IV MUP cases and 12,796 stage IV MKP cases. The incidence of stage IV MUP increased over time, from 1.52 per 100,000 between 1973 and 1984, to 5.83 per 100,000 from 2005 to 2014. MUP patients were more likely to be recommended for surgery than MKP patients (surgery not recommended for 47.7% of MKP cases, compared to 37.7% of MUP cases), and they had better 1-year survival rates compared to the general U.S. population than did MKP patients (0.54, 95% CI, 0.48-0.60 versus 0.41, 95% CI 0.39-0.42). The improved survival of MUP over MKP remained steady at each measured time point out to 5 years.
However, there was no difference in 5-year disease-specific survival (DSS) in MUP versus MKP (HR, 0.91; 95% CI, 0.79-1.04; P =.16), or in the 5-year DSS Kaplan-Meier curve after adjustment for year of diagnosis, age, sex, race, and surgical treatment (log-rank P = .93).
A multivariate analysis showed increased 5-year DSS among patients who received surgery (HR, 0.41; 95% CI, 0.30-0.56; P less than .001) and decreased 5-year DSS among patients over 50 (HR 3.27, 95% CI, 1.17-9.17; P = .02 for age 50-59).
“The prognostic factors are very similar, so you should treat these patients (with MUP) similarly to patients with melanoma of known primary, the same treatments, the same clinical trials,” Dr. Scott and associates said.
The results also raise the possibility of gaining a better understanding of how immune response affects the course of metastatic melanoma.
“If MUP is due to the fact that your immune system is attacking the primary tumor, then what characteristics of the person would cause that to happen? Are younger patients (exhibiting) a more robust immune response? Could that explain why their prognosis is better? More molecular studies of the actual tumors and the immune characteristics of these patients would help us answer that,” they added.
A resolved primary tumor isn’t the only explanation for MUP, however. It’s also possible that melanocytes are found in unexpected sites, perhaps because they did not complete their migration during development. “They could give rise to melanoma that would present (as MUP). Maybe there never was a skin tumor,” the authors wrote.
The investigators recommend a thorough search for a primary tumor, employing ophthalmologists, gynecologists, and other specialists if necessary. “You could argue that once you have metastatic disease, what’s the point of finding the primary tumor? But it’s important to correctly classify these patients, in terms of what clinical trials and treatments they may be eligible for,” Dr. Scott and associates said.
SOURCE: Scott JF et al. J Am Acad Dermatol. 2018 Mar 23. doi: 10.1016/j.jaad.2018.03.021.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Unknown primary melanomas should be approached similar to melanomas with known primaries.
Major finding: The 5-year DSS rate was lower in patients age 50-59 (HR, 3.27).
Study details: Retrospective analysis of 322 stage IV MUP cases and 12,796 stage IV MKP.
Disclosures: The study was funded by the Char and Chuck Fowler Family Foundation. Dr. Scott reported no relevant financial relationships.
Source: Scott JF et al. J Am Acad Dermatol. 2018 Mar 23. doi: 10.1016/j.jaad.2018.03.021.
MicroRNAs required for cGVHD development
New research suggests a family of microRNAs, miR-17-92, plays a key role in chronic graft-versus-host disease (cGVHD).
Researchers found miR-17-92 is responsible for the T- and B-cell pathogenicity that causes cGVHD.
The team also discovered that pharmacological inhibition of miR-17 alleviated the symptoms of cGVHD in mice.
Yongxia Wu, PhD, of the Medical University of South Carolina in Charleston, and her colleagues reported these findings in Blood.
The researchers previously found that miR-17-92 regulates CD4 T-cell proliferation and Th1 and Treg differentiation in acute (a) GVHD.
So the team set out to investigate whether miR-17-92 regulates T- and B-cell differentiation and function in the development of cGVHD.
“Chronic GVHD has a different pathophysiology and different target organs than aGVHD,” Dr Wu noted. “It’s been a big challenge to try to find a target for cGVHD therapies because of the more complex immune reaction in cGVHD and the fact that its cellular and molecular mechanisms are not as well understood.”
“We decided to extend our aGVHD study to cGVHD, but there’s no single, well-defined murine model that can reflect all of the clinical manifestations seen in cGVHD patients. So we decided to study 4 different cGVHD models to best understand how miR-17-92 contributes overall, across many clinical presentations.”
The team performed a series of experiments in murine models of cGVHD after allogeneic bone marrow transplant (BMT). This included models of scleroderma that had transitioned from aGVHD to cGVHD, classic cGVHD scleroderma, lung inflammation, and a lupus-like condition.
The experiments revealed shared mechanisms by which miR-17-92 mediates cGVHD progression—namely, by regulating T helper-cell differentiation, B-cell activation, germinal center responses, and autoantibody production.
“The mechanism for how miR-17-92 regulates T and B cells was very consistent,” Dr Wu said. “In other words, we did not find any big differences among the models.”
The researchers also assessed whether pharmacological inhibition of miR-17 or miR-19—“key members in the miR-17-92 cluster”—might be effective in the treatment of cGVHD.
The team tested antagomirs specific for miR-17 or miR-19 in the scleroderma cGVHD model and the lupus-like condition.
Anti-miR-17, but not anti-miR-19, reduced skin damage in the scleroderma model and alleviated proteinuria in the lupus-like condition.
“So we not only found a new mechanism for cGVHD development by demonstrating that miR-17-92 is heavily involved in the T- and B-cell responses that lead to cGVHD, but we also found that blocking miR-17 substantially reduced cGVHD symptoms in mice,” Dr Wu said.
“That’s exciting because it provides strong evidence that this miR may be a good target for controlling cGVHD after allogeneic BMT.”
Now, Dr Wu and her colleagues are investigating how other microRNAs may be involved in regulating T- and B-cell function during allogeneic BMT.
New research suggests a family of microRNAs, miR-17-92, plays a key role in chronic graft-versus-host disease (cGVHD).
Researchers found miR-17-92 is responsible for the T- and B-cell pathogenicity that causes cGVHD.
The team also discovered that pharmacological inhibition of miR-17 alleviated the symptoms of cGVHD in mice.
Yongxia Wu, PhD, of the Medical University of South Carolina in Charleston, and her colleagues reported these findings in Blood.
The researchers previously found that miR-17-92 regulates CD4 T-cell proliferation and Th1 and Treg differentiation in acute (a) GVHD.
So the team set out to investigate whether miR-17-92 regulates T- and B-cell differentiation and function in the development of cGVHD.
“Chronic GVHD has a different pathophysiology and different target organs than aGVHD,” Dr Wu noted. “It’s been a big challenge to try to find a target for cGVHD therapies because of the more complex immune reaction in cGVHD and the fact that its cellular and molecular mechanisms are not as well understood.”
“We decided to extend our aGVHD study to cGVHD, but there’s no single, well-defined murine model that can reflect all of the clinical manifestations seen in cGVHD patients. So we decided to study 4 different cGVHD models to best understand how miR-17-92 contributes overall, across many clinical presentations.”
The team performed a series of experiments in murine models of cGVHD after allogeneic bone marrow transplant (BMT). This included models of scleroderma that had transitioned from aGVHD to cGVHD, classic cGVHD scleroderma, lung inflammation, and a lupus-like condition.
The experiments revealed shared mechanisms by which miR-17-92 mediates cGVHD progression—namely, by regulating T helper-cell differentiation, B-cell activation, germinal center responses, and autoantibody production.
“The mechanism for how miR-17-92 regulates T and B cells was very consistent,” Dr Wu said. “In other words, we did not find any big differences among the models.”
The researchers also assessed whether pharmacological inhibition of miR-17 or miR-19—“key members in the miR-17-92 cluster”—might be effective in the treatment of cGVHD.
The team tested antagomirs specific for miR-17 or miR-19 in the scleroderma cGVHD model and the lupus-like condition.
Anti-miR-17, but not anti-miR-19, reduced skin damage in the scleroderma model and alleviated proteinuria in the lupus-like condition.
“So we not only found a new mechanism for cGVHD development by demonstrating that miR-17-92 is heavily involved in the T- and B-cell responses that lead to cGVHD, but we also found that blocking miR-17 substantially reduced cGVHD symptoms in mice,” Dr Wu said.
“That’s exciting because it provides strong evidence that this miR may be a good target for controlling cGVHD after allogeneic BMT.”
Now, Dr Wu and her colleagues are investigating how other microRNAs may be involved in regulating T- and B-cell function during allogeneic BMT.
New research suggests a family of microRNAs, miR-17-92, plays a key role in chronic graft-versus-host disease (cGVHD).
Researchers found miR-17-92 is responsible for the T- and B-cell pathogenicity that causes cGVHD.
The team also discovered that pharmacological inhibition of miR-17 alleviated the symptoms of cGVHD in mice.
Yongxia Wu, PhD, of the Medical University of South Carolina in Charleston, and her colleagues reported these findings in Blood.
The researchers previously found that miR-17-92 regulates CD4 T-cell proliferation and Th1 and Treg differentiation in acute (a) GVHD.
So the team set out to investigate whether miR-17-92 regulates T- and B-cell differentiation and function in the development of cGVHD.
“Chronic GVHD has a different pathophysiology and different target organs than aGVHD,” Dr Wu noted. “It’s been a big challenge to try to find a target for cGVHD therapies because of the more complex immune reaction in cGVHD and the fact that its cellular and molecular mechanisms are not as well understood.”
“We decided to extend our aGVHD study to cGVHD, but there’s no single, well-defined murine model that can reflect all of the clinical manifestations seen in cGVHD patients. So we decided to study 4 different cGVHD models to best understand how miR-17-92 contributes overall, across many clinical presentations.”
The team performed a series of experiments in murine models of cGVHD after allogeneic bone marrow transplant (BMT). This included models of scleroderma that had transitioned from aGVHD to cGVHD, classic cGVHD scleroderma, lung inflammation, and a lupus-like condition.
The experiments revealed shared mechanisms by which miR-17-92 mediates cGVHD progression—namely, by regulating T helper-cell differentiation, B-cell activation, germinal center responses, and autoantibody production.
“The mechanism for how miR-17-92 regulates T and B cells was very consistent,” Dr Wu said. “In other words, we did not find any big differences among the models.”
The researchers also assessed whether pharmacological inhibition of miR-17 or miR-19—“key members in the miR-17-92 cluster”—might be effective in the treatment of cGVHD.
The team tested antagomirs specific for miR-17 or miR-19 in the scleroderma cGVHD model and the lupus-like condition.
Anti-miR-17, but not anti-miR-19, reduced skin damage in the scleroderma model and alleviated proteinuria in the lupus-like condition.
“So we not only found a new mechanism for cGVHD development by demonstrating that miR-17-92 is heavily involved in the T- and B-cell responses that lead to cGVHD, but we also found that blocking miR-17 substantially reduced cGVHD symptoms in mice,” Dr Wu said.
“That’s exciting because it provides strong evidence that this miR may be a good target for controlling cGVHD after allogeneic BMT.”
Now, Dr Wu and her colleagues are investigating how other microRNAs may be involved in regulating T- and B-cell function during allogeneic BMT.
Melanoma in young children may be biologically distinct from that in teens
Pediatric melanomas appear to be more progressive in adolescents than in young children, based on data from a retrospective study of 32 cases.
Few young children with melanoma die, despite a greater likelihood of thicker tumors, lymph node metastasis, and later diagnosis, which suggests that melanoma in young children may be biologically distinct from melanoma in adolescents, wrote Diana W. Bartenstein, of Harvard University Medical School, Boston, and her colleagues.
Overall, significantly more children than adolescents had spitzoid melanoma (50% vs. 10%, P = .01). In addition, children were more likely than adolescents to present with stage 3 or 4 cancer (58% vs. 25%) and with Clark level IV and V tumors (42% vs. 35%), although these differences were not significant. The median Breslow thickness of lesions was greater in children than in adolescents (3.5 mm vs. 1.5 mm) as was the median mitotic index (5 mitotic figures per mm2 vs. 2 mitotic figures per mm2) and children were more likely than adolescents to have neural invasion, but these differences were not significant either.
During the study period of more than 20 years, none of the children younger than 11 years died, compared with four deaths in adolescents, a statistically significant difference (P = .04). The follow-up for surviving individuals ranged from 9-37 months with a median of 44 months.
The study findings were limited by several factors including the small sample size and difficulty in assessing spitzoid tumors, the researchers noted.
However, “these results support the hypothesis that melanoma in young children may be biologically distinct from melanoma in adults,” they said. “Alternatively, melanoma subtype may drive survival differences between children and adolescents.”
No conflicts of interest were reported. The study was supported by the Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship and the Society for Pediatric Dermatology and Pediatric Dermatology Research Alliance.
SOURCE: Bartenstein DW et al. Pediatr Dermatol. 2018 Mar 23. doi: 10.1111/pde.13454.
Pediatric melanomas appear to be more progressive in adolescents than in young children, based on data from a retrospective study of 32 cases.
Few young children with melanoma die, despite a greater likelihood of thicker tumors, lymph node metastasis, and later diagnosis, which suggests that melanoma in young children may be biologically distinct from melanoma in adolescents, wrote Diana W. Bartenstein, of Harvard University Medical School, Boston, and her colleagues.
Overall, significantly more children than adolescents had spitzoid melanoma (50% vs. 10%, P = .01). In addition, children were more likely than adolescents to present with stage 3 or 4 cancer (58% vs. 25%) and with Clark level IV and V tumors (42% vs. 35%), although these differences were not significant. The median Breslow thickness of lesions was greater in children than in adolescents (3.5 mm vs. 1.5 mm) as was the median mitotic index (5 mitotic figures per mm2 vs. 2 mitotic figures per mm2) and children were more likely than adolescents to have neural invasion, but these differences were not significant either.
During the study period of more than 20 years, none of the children younger than 11 years died, compared with four deaths in adolescents, a statistically significant difference (P = .04). The follow-up for surviving individuals ranged from 9-37 months with a median of 44 months.
The study findings were limited by several factors including the small sample size and difficulty in assessing spitzoid tumors, the researchers noted.
However, “these results support the hypothesis that melanoma in young children may be biologically distinct from melanoma in adults,” they said. “Alternatively, melanoma subtype may drive survival differences between children and adolescents.”
No conflicts of interest were reported. The study was supported by the Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship and the Society for Pediatric Dermatology and Pediatric Dermatology Research Alliance.
SOURCE: Bartenstein DW et al. Pediatr Dermatol. 2018 Mar 23. doi: 10.1111/pde.13454.
Pediatric melanomas appear to be more progressive in adolescents than in young children, based on data from a retrospective study of 32 cases.
Few young children with melanoma die, despite a greater likelihood of thicker tumors, lymph node metastasis, and later diagnosis, which suggests that melanoma in young children may be biologically distinct from melanoma in adolescents, wrote Diana W. Bartenstein, of Harvard University Medical School, Boston, and her colleagues.
Overall, significantly more children than adolescents had spitzoid melanoma (50% vs. 10%, P = .01). In addition, children were more likely than adolescents to present with stage 3 or 4 cancer (58% vs. 25%) and with Clark level IV and V tumors (42% vs. 35%), although these differences were not significant. The median Breslow thickness of lesions was greater in children than in adolescents (3.5 mm vs. 1.5 mm) as was the median mitotic index (5 mitotic figures per mm2 vs. 2 mitotic figures per mm2) and children were more likely than adolescents to have neural invasion, but these differences were not significant either.
During the study period of more than 20 years, none of the children younger than 11 years died, compared with four deaths in adolescents, a statistically significant difference (P = .04). The follow-up for surviving individuals ranged from 9-37 months with a median of 44 months.
The study findings were limited by several factors including the small sample size and difficulty in assessing spitzoid tumors, the researchers noted.
However, “these results support the hypothesis that melanoma in young children may be biologically distinct from melanoma in adults,” they said. “Alternatively, melanoma subtype may drive survival differences between children and adolescents.”
No conflicts of interest were reported. The study was supported by the Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship and the Society for Pediatric Dermatology and Pediatric Dermatology Research Alliance.
SOURCE: Bartenstein DW et al. Pediatr Dermatol. 2018 Mar 23. doi: 10.1111/pde.13454.
FROM PEDIATRIC DERMATOLOGY
Key clinical point:
Major finding: Significantly more children than adolescents had spitzoid melanoma (50% vs. 10%, P = .01).
Study details: A retrospective cohort study of 32 children and adolescents with melanoma.
Disclosures: The study was supported by the Alpha Omega Alpha–Carolyn L. Kuckein Student Research Fellowship and the Society for Pediatric Dermatology and Pediatric Dermatology Research Alliance. No conflicts of interest were reported.
Source: Bartenstein DW et al. Pediatr Dermatol. 2018 Mar 23. doi: 10.1111/pde.13454.
AbbVie, Samsung Bioepis settle suits with delayed U.S. entry for adalimumab biosimilar
A new adalimumab biosimilar will become available in the European Union later this year, but a court settlement will keep Samsung Bioepis’ competitor off U.S. shelves until 2023.
Under the settlement, AbbVie, which manufactures adalimumab (Humira), will grant Bioepis and its partner, Biogen, a nonexclusive license to the intellectual property relating to the antibody. Bioepis’ version, dubbed SB5 (Imraldi), will enter global markets in a staggered fashion, according to an AbbVie press statement. In most countries in the European Union, the license period will begin on Oct. 16, 2018. In the United States, Samsung Bioepis’ license period will begin on June 30, 2023, according to the Abbvie statement.
Biogen and Bioepis hailed the settlement as a victory, but Imraldi won’t be the first Humira biosimilar to break into the U.S. market. Last September, AbbVie settled a similar suit with Amgen, granting patent licenses for the global use and sale of its anti–tumor necrosis factor–alpha antibody, Amgevita/Amjevita. Amgen expects to launch Amgevita in Europe on Oct. 16, 2018, and Amjevita in the United States on Jan. 31, 2023. Samsung Bioepis’ U.S. license date will not be accelerated upon Amgen’s entry.
Ian Henshaw, Biogen’s global head of biosimilars, said the deal further strengthens the company’s European biosimilars reach.
“Biogen is a leader in the emerging field of biosimilars through Samsung Bioepis, our joint venture with Samsung BioLogics,” Mr. Henshaw said in a press statement. “Biogen already markets two biosimilars in Europe and the planned introduction of Imraldi on Oct. 16 could potentially expand patient choice by offering physicians more options to meet the needs of patients while delivering significant savings to healthcare systems.”
AbbVie focused on the settlement as a global recognition of its leadership role in developing the anti-TNF-alpha antibody.
“The Samsung Bioepis settlement reflects the strength and breadth of AbbVie’s intellectual property,” Laura Schumacher, the company’s general counsel, said in the Abbvie statement. “We continue to believe biosimilars will play an important role in our healthcare system, but we also believe it is important to protect our investment in innovation. This agreement accomplishes both objectives.”
Samsung Bioepis will pay royalties to AbbVie for licensing its adalimumab patents once its biosimilar product is launched. As is the case with the prior Amgen resolution, AbbVie will not make any payments to Samsung Bioepis. “All litigation pending between the parties, as well as all litigation with Samsung Bioepis’ European partner, Biogen, will be dismissed. The precise terms of the agreements are confidential,” the Abbvie statement said.
The settlement brings to a closing a flurry of lawsuits Samsung Bioepis filed against AbbVie in 2017.
A new adalimumab biosimilar will become available in the European Union later this year, but a court settlement will keep Samsung Bioepis’ competitor off U.S. shelves until 2023.
Under the settlement, AbbVie, which manufactures adalimumab (Humira), will grant Bioepis and its partner, Biogen, a nonexclusive license to the intellectual property relating to the antibody. Bioepis’ version, dubbed SB5 (Imraldi), will enter global markets in a staggered fashion, according to an AbbVie press statement. In most countries in the European Union, the license period will begin on Oct. 16, 2018. In the United States, Samsung Bioepis’ license period will begin on June 30, 2023, according to the Abbvie statement.
Biogen and Bioepis hailed the settlement as a victory, but Imraldi won’t be the first Humira biosimilar to break into the U.S. market. Last September, AbbVie settled a similar suit with Amgen, granting patent licenses for the global use and sale of its anti–tumor necrosis factor–alpha antibody, Amgevita/Amjevita. Amgen expects to launch Amgevita in Europe on Oct. 16, 2018, and Amjevita in the United States on Jan. 31, 2023. Samsung Bioepis’ U.S. license date will not be accelerated upon Amgen’s entry.
Ian Henshaw, Biogen’s global head of biosimilars, said the deal further strengthens the company’s European biosimilars reach.
“Biogen is a leader in the emerging field of biosimilars through Samsung Bioepis, our joint venture with Samsung BioLogics,” Mr. Henshaw said in a press statement. “Biogen already markets two biosimilars in Europe and the planned introduction of Imraldi on Oct. 16 could potentially expand patient choice by offering physicians more options to meet the needs of patients while delivering significant savings to healthcare systems.”
AbbVie focused on the settlement as a global recognition of its leadership role in developing the anti-TNF-alpha antibody.
“The Samsung Bioepis settlement reflects the strength and breadth of AbbVie’s intellectual property,” Laura Schumacher, the company’s general counsel, said in the Abbvie statement. “We continue to believe biosimilars will play an important role in our healthcare system, but we also believe it is important to protect our investment in innovation. This agreement accomplishes both objectives.”
Samsung Bioepis will pay royalties to AbbVie for licensing its adalimumab patents once its biosimilar product is launched. As is the case with the prior Amgen resolution, AbbVie will not make any payments to Samsung Bioepis. “All litigation pending between the parties, as well as all litigation with Samsung Bioepis’ European partner, Biogen, will be dismissed. The precise terms of the agreements are confidential,” the Abbvie statement said.
The settlement brings to a closing a flurry of lawsuits Samsung Bioepis filed against AbbVie in 2017.
A new adalimumab biosimilar will become available in the European Union later this year, but a court settlement will keep Samsung Bioepis’ competitor off U.S. shelves until 2023.
Under the settlement, AbbVie, which manufactures adalimumab (Humira), will grant Bioepis and its partner, Biogen, a nonexclusive license to the intellectual property relating to the antibody. Bioepis’ version, dubbed SB5 (Imraldi), will enter global markets in a staggered fashion, according to an AbbVie press statement. In most countries in the European Union, the license period will begin on Oct. 16, 2018. In the United States, Samsung Bioepis’ license period will begin on June 30, 2023, according to the Abbvie statement.
Biogen and Bioepis hailed the settlement as a victory, but Imraldi won’t be the first Humira biosimilar to break into the U.S. market. Last September, AbbVie settled a similar suit with Amgen, granting patent licenses for the global use and sale of its anti–tumor necrosis factor–alpha antibody, Amgevita/Amjevita. Amgen expects to launch Amgevita in Europe on Oct. 16, 2018, and Amjevita in the United States on Jan. 31, 2023. Samsung Bioepis’ U.S. license date will not be accelerated upon Amgen’s entry.
Ian Henshaw, Biogen’s global head of biosimilars, said the deal further strengthens the company’s European biosimilars reach.
“Biogen is a leader in the emerging field of biosimilars through Samsung Bioepis, our joint venture with Samsung BioLogics,” Mr. Henshaw said in a press statement. “Biogen already markets two biosimilars in Europe and the planned introduction of Imraldi on Oct. 16 could potentially expand patient choice by offering physicians more options to meet the needs of patients while delivering significant savings to healthcare systems.”
AbbVie focused on the settlement as a global recognition of its leadership role in developing the anti-TNF-alpha antibody.
“The Samsung Bioepis settlement reflects the strength and breadth of AbbVie’s intellectual property,” Laura Schumacher, the company’s general counsel, said in the Abbvie statement. “We continue to believe biosimilars will play an important role in our healthcare system, but we also believe it is important to protect our investment in innovation. This agreement accomplishes both objectives.”
Samsung Bioepis will pay royalties to AbbVie for licensing its adalimumab patents once its biosimilar product is launched. As is the case with the prior Amgen resolution, AbbVie will not make any payments to Samsung Bioepis. “All litigation pending between the parties, as well as all litigation with Samsung Bioepis’ European partner, Biogen, will be dismissed. The precise terms of the agreements are confidential,” the Abbvie statement said.
The settlement brings to a closing a flurry of lawsuits Samsung Bioepis filed against AbbVie in 2017.
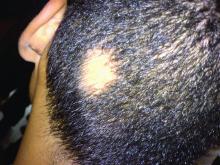
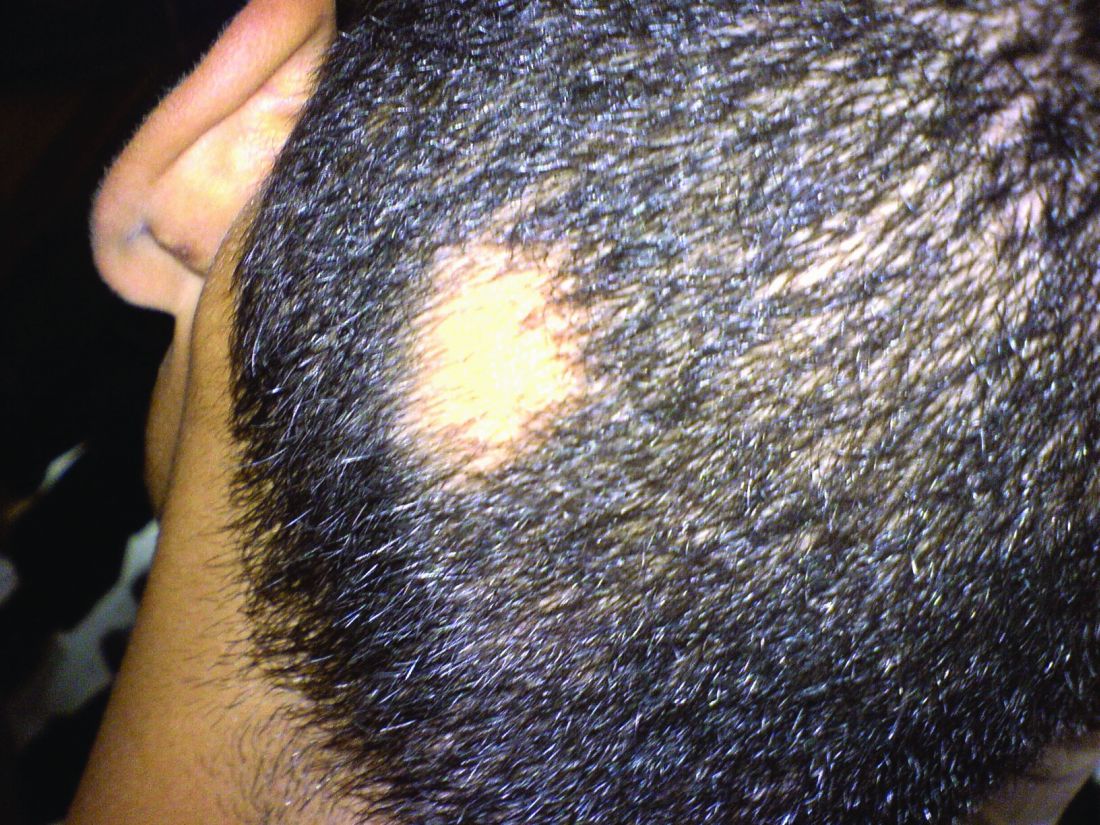
Consider hydroxychloroquine in treating pediatric alopecia areata
according to Duri Yun, MD, of the University of Chicago Medicine, and associates.
In a retrospective review published in Pediatric Dermatology, nine children aged 6-16 years with AA and diverse ethnicities were treated with hydroxychloroquine between July 1, 2013, and July 1, 2015; all had failed multiple previous treatment modalities. In patient 1, hydroxychloroquine therapy was initiated, fine hair regrowth occurred after 5 months of therapy and was maintained, with dosage tapered to 200 mg once daily after 1 year. After 2 years of therapy, hair had nearly completely regrown. Similar results occurred in patient 2, who had nearly complete hair loss within 2 weeks of initiating hydroxychloroquine. Steady regrowth continued to near-complete regrowth after 1 year of treatment, when dosage was tapered to 200 mg once daily.
Four patients (44%) had no evidence of regrowth after 4-6 months of hydroxychloroquine therapy so they discontinued therapy. The most common adverse events while taking hydroxychloroquine were abdominal pain in two patients (22%) and headache in two patients (22%).
“In the context of children with severe AA failing multiple first-line therapies, our findings suggest that there may be a subgroup that benefits from therapy with hydroxychloroquine,” the researchers concluded. “Determining which factors might predict response to various therapies will come from combined efforts to conduct well-controlled clinical trials of treatments for AA.”
SOURCE: Yun D et al., Pediatr Dermatol. 2018 Mar 25. doi: 10.1111/pde.13451.
according to Duri Yun, MD, of the University of Chicago Medicine, and associates.
In a retrospective review published in Pediatric Dermatology, nine children aged 6-16 years with AA and diverse ethnicities were treated with hydroxychloroquine between July 1, 2013, and July 1, 2015; all had failed multiple previous treatment modalities. In patient 1, hydroxychloroquine therapy was initiated, fine hair regrowth occurred after 5 months of therapy and was maintained, with dosage tapered to 200 mg once daily after 1 year. After 2 years of therapy, hair had nearly completely regrown. Similar results occurred in patient 2, who had nearly complete hair loss within 2 weeks of initiating hydroxychloroquine. Steady regrowth continued to near-complete regrowth after 1 year of treatment, when dosage was tapered to 200 mg once daily.
Four patients (44%) had no evidence of regrowth after 4-6 months of hydroxychloroquine therapy so they discontinued therapy. The most common adverse events while taking hydroxychloroquine were abdominal pain in two patients (22%) and headache in two patients (22%).
“In the context of children with severe AA failing multiple first-line therapies, our findings suggest that there may be a subgroup that benefits from therapy with hydroxychloroquine,” the researchers concluded. “Determining which factors might predict response to various therapies will come from combined efforts to conduct well-controlled clinical trials of treatments for AA.”
SOURCE: Yun D et al., Pediatr Dermatol. 2018 Mar 25. doi: 10.1111/pde.13451.
according to Duri Yun, MD, of the University of Chicago Medicine, and associates.
In a retrospective review published in Pediatric Dermatology, nine children aged 6-16 years with AA and diverse ethnicities were treated with hydroxychloroquine between July 1, 2013, and July 1, 2015; all had failed multiple previous treatment modalities. In patient 1, hydroxychloroquine therapy was initiated, fine hair regrowth occurred after 5 months of therapy and was maintained, with dosage tapered to 200 mg once daily after 1 year. After 2 years of therapy, hair had nearly completely regrown. Similar results occurred in patient 2, who had nearly complete hair loss within 2 weeks of initiating hydroxychloroquine. Steady regrowth continued to near-complete regrowth after 1 year of treatment, when dosage was tapered to 200 mg once daily.
Four patients (44%) had no evidence of regrowth after 4-6 months of hydroxychloroquine therapy so they discontinued therapy. The most common adverse events while taking hydroxychloroquine were abdominal pain in two patients (22%) and headache in two patients (22%).
“In the context of children with severe AA failing multiple first-line therapies, our findings suggest that there may be a subgroup that benefits from therapy with hydroxychloroquine,” the researchers concluded. “Determining which factors might predict response to various therapies will come from combined efforts to conduct well-controlled clinical trials of treatments for AA.”
SOURCE: Yun D et al., Pediatr Dermatol. 2018 Mar 25. doi: 10.1111/pde.13451.
FROM PEDIATRIC DERMATOLOGY
Prioritize topical treatments in pregnant women
SAN DIEGO – “Some doctors don’t treat them at all, or they undertreat because they’re afraid of prescribing anything,” said dermatologist Kelly H. Tyler, MD.
But the Food and Drug Administration’s new system of prescription drug labeling for pregnant women offers improved insight for dermatologists, and a variety of alternatives are available to commonly used dermatologic drugs that pose risks, said Dr. Tyler, who has a unique perspective on this issue: She used to practice as an ob.gyn.
In her presentation, she provided the following tips when caring for pregnant patients.
Check the prescription drug labeling for pregnant and lactating women
Prior to 2015, the FDA used a letter ratings to denote the risk of medications during pregnancy. The ratings – A, B, C, D, and X – go from “A” showing no fetal risk in controlled studies to “X” in which a drug is contraindicated because “there is no reason to risk use of drug in pregnancy.”
This rating system is problematic, Dr. Tyler said, since it’s imprecise. She noted that about two-thirds of medications have a risk rating of C, which means “Risk cannot be ruled out; human studies may or may not show risk; potential benefits may justify potential risk.” But, she asked, “How are you supposed to know what to do with that kind of information?”
By June 30, 2015, FDA had retired the letter classifications and replaced this system with the Pregnancy and Lactation Labeling final rule, which requires that labels include a pregnancy section with information on pregnancy exposure registries, in addition to risk summaries, clinical considerations, and available data. There are also sections on lactation, including nursing mothers, and females and males of reproductive potential. (This information appears in the prescription drug labeling sections 8.1-8.3.) Labels for medications approved prior to June 30, 2001 did not need to be updated. Labels for newer medications are required to comply within 3-5 years of the 2015 policy change.
Ask female patients about sexual activity
It’s important to ask all premenopausal female patients whether they’re sexually active and whether they are using birth control, trying to conceive, or are currently pregnant. If they’re pregnant, what trimester are they in?
Keep in mind, she said, that women can be pregnant for quite some time without knowing it. Home pregnancy tests may not show positive results for up to 5 weeks after conception, she said. But the embryonic/organogenesis stage – from 2 to 8 weeks – is the most important period for a pregnant mother avoid drugs that could damage the unborn child and is “when you want to avoid most medications,” she said.
Risks don’t end after a few months, however. Dr. Tyler urged dermatologists to keep in mind that the brain, teeth, and bones remain susceptible to damage from teratogenic medications after the eighth week.
Keep topical medications in mind
When it comes to pregnant women, “for most dermatologic therapies, topical medications will be your No. 1 choice” because of less absorption, Dr. Tyler said.
But there are exceptions, she noted. In acne and rosacea, for example, there’s controversy about the safety of topical retinoids. “I would just urge you to not use those medications in pregnancy because you don’t really need to use them,” Dr. Tyler said. “You have other options that are safer.”
Adapalene and tretinoin are listed as category C (risk cannot be ruled out in pregnancy) and topical tazarotene is category X (contraindicated in pregnancy) because of retinoid-like anomalies in animal studies, she said.
Avoid 3 major contraindicated systemic drugs
Isotretinoin, acitretin, and methotrexate are “absolutely contraindicated” for anyone who is pregnant or could become pregnant, Dr. Tyler said.
Use some systemic drugs with caution
Tetracyclines are category D (positive evidence of risk to human fetus, but benefits may outweigh risks of drug) with the highest risk in the second and third trimesters, Dr. Tyler said. “If for some reason you have a patient who’s on tetracycline, stop before she’s in her second trimester.”
She recommended avoiding the antibiotic erythromycin because of studies hinting at risks when used early in pregnancy. Spironolactone is theoretically risky after the week 8 of pregnancy, she said. “Because of the animal studies, we typically do not use this during pregnancy.”
Psoriasis often improves during pregnancy
Psoriasis improves during pregnancy in about half of women with psoriasis and worsens in about 20%, Dr. Tyler said. During pregnancy, topical treatments are the first-line treatment, said Dr. Tyler, who recommends that treatment should begin with topical steroids, then calcipotriene or tacrolimus if needed (J Am Acad Dermatol. 2013 Apr;68[4]:663-71). Cyclosporine is an option, as is phototherapy, she said. In an interview, she noted that phototherapy (narrowband UVB) would be considered next-line therapy in patients who are past the first 28 days of gestation and have failed topical therapy, given they are taking adequate folic acid supplementation, which is present in prenatal vitamins.
“As for biologics, you have to go with older ones that have more data. When we look at newer medications, we don’t know a lot about them,” she said during the presentation.
It’s okay to prescribe oral steroids and antihistamines
Oral steroids are safe during pregnancy, Dr. Tyler said, but “just be judicious” with moderate doses and short durations.
Antihistamines are also appropriate, she said, but be aware of the potential for neonatal sedation during lactation.
As for antibiotics for bacterial infections, azithromycin, penicillins, and cephalosporins are all category B (no risk to human fetus despite possible animal risk; or no risk in animal studies and human studies not done), as are all topical antibiotics except dapsone, which is category C because of a theoretical risk of neonatal hyperbilirubinemia if used near the time of delivery.
Hydroxychloroquine may be appropriate for connective tissue disease, she said, although steroids may be a better option in some cases.
And topical antifungals are considered safer for fungal diseases than systemic medications. She said she prefers clotrimazole and oxiconazole, both category B.
Finally, Dr. Tyler recommended permethrin (category B) for parasitic infections since it has been used extensively in pregnancy without a sign of risk and is the preferred treatment for scabies. It’s a better option than ivermectin, she said.
Postpone surgery until at least the second trimester
If it’s not possible to delay nonemergent dermatologic surgery until after pregnancy, she recommended performing procedures during the second trimester. Destruction of local lesions, however, is safe without anesthesia.
“In summary,” when treating pregnant patients, Dr. Tyler said, “a conservative approach is always best, topical medications are always first-line for any condition, and certain oral medications are safe.”
Dr. Tyler reported no relevant disclosures.
SAN DIEGO – “Some doctors don’t treat them at all, or they undertreat because they’re afraid of prescribing anything,” said dermatologist Kelly H. Tyler, MD.
But the Food and Drug Administration’s new system of prescription drug labeling for pregnant women offers improved insight for dermatologists, and a variety of alternatives are available to commonly used dermatologic drugs that pose risks, said Dr. Tyler, who has a unique perspective on this issue: She used to practice as an ob.gyn.
In her presentation, she provided the following tips when caring for pregnant patients.
Check the prescription drug labeling for pregnant and lactating women
Prior to 2015, the FDA used a letter ratings to denote the risk of medications during pregnancy. The ratings – A, B, C, D, and X – go from “A” showing no fetal risk in controlled studies to “X” in which a drug is contraindicated because “there is no reason to risk use of drug in pregnancy.”
This rating system is problematic, Dr. Tyler said, since it’s imprecise. She noted that about two-thirds of medications have a risk rating of C, which means “Risk cannot be ruled out; human studies may or may not show risk; potential benefits may justify potential risk.” But, she asked, “How are you supposed to know what to do with that kind of information?”
By June 30, 2015, FDA had retired the letter classifications and replaced this system with the Pregnancy and Lactation Labeling final rule, which requires that labels include a pregnancy section with information on pregnancy exposure registries, in addition to risk summaries, clinical considerations, and available data. There are also sections on lactation, including nursing mothers, and females and males of reproductive potential. (This information appears in the prescription drug labeling sections 8.1-8.3.) Labels for medications approved prior to June 30, 2001 did not need to be updated. Labels for newer medications are required to comply within 3-5 years of the 2015 policy change.
Ask female patients about sexual activity
It’s important to ask all premenopausal female patients whether they’re sexually active and whether they are using birth control, trying to conceive, or are currently pregnant. If they’re pregnant, what trimester are they in?
Keep in mind, she said, that women can be pregnant for quite some time without knowing it. Home pregnancy tests may not show positive results for up to 5 weeks after conception, she said. But the embryonic/organogenesis stage – from 2 to 8 weeks – is the most important period for a pregnant mother avoid drugs that could damage the unborn child and is “when you want to avoid most medications,” she said.
Risks don’t end after a few months, however. Dr. Tyler urged dermatologists to keep in mind that the brain, teeth, and bones remain susceptible to damage from teratogenic medications after the eighth week.
Keep topical medications in mind
When it comes to pregnant women, “for most dermatologic therapies, topical medications will be your No. 1 choice” because of less absorption, Dr. Tyler said.
But there are exceptions, she noted. In acne and rosacea, for example, there’s controversy about the safety of topical retinoids. “I would just urge you to not use those medications in pregnancy because you don’t really need to use them,” Dr. Tyler said. “You have other options that are safer.”
Adapalene and tretinoin are listed as category C (risk cannot be ruled out in pregnancy) and topical tazarotene is category X (contraindicated in pregnancy) because of retinoid-like anomalies in animal studies, she said.
Avoid 3 major contraindicated systemic drugs
Isotretinoin, acitretin, and methotrexate are “absolutely contraindicated” for anyone who is pregnant or could become pregnant, Dr. Tyler said.
Use some systemic drugs with caution
Tetracyclines are category D (positive evidence of risk to human fetus, but benefits may outweigh risks of drug) with the highest risk in the second and third trimesters, Dr. Tyler said. “If for some reason you have a patient who’s on tetracycline, stop before she’s in her second trimester.”
She recommended avoiding the antibiotic erythromycin because of studies hinting at risks when used early in pregnancy. Spironolactone is theoretically risky after the week 8 of pregnancy, she said. “Because of the animal studies, we typically do not use this during pregnancy.”
Psoriasis often improves during pregnancy
Psoriasis improves during pregnancy in about half of women with psoriasis and worsens in about 20%, Dr. Tyler said. During pregnancy, topical treatments are the first-line treatment, said Dr. Tyler, who recommends that treatment should begin with topical steroids, then calcipotriene or tacrolimus if needed (J Am Acad Dermatol. 2013 Apr;68[4]:663-71). Cyclosporine is an option, as is phototherapy, she said. In an interview, she noted that phototherapy (narrowband UVB) would be considered next-line therapy in patients who are past the first 28 days of gestation and have failed topical therapy, given they are taking adequate folic acid supplementation, which is present in prenatal vitamins.
“As for biologics, you have to go with older ones that have more data. When we look at newer medications, we don’t know a lot about them,” she said during the presentation.
It’s okay to prescribe oral steroids and antihistamines
Oral steroids are safe during pregnancy, Dr. Tyler said, but “just be judicious” with moderate doses and short durations.
Antihistamines are also appropriate, she said, but be aware of the potential for neonatal sedation during lactation.
As for antibiotics for bacterial infections, azithromycin, penicillins, and cephalosporins are all category B (no risk to human fetus despite possible animal risk; or no risk in animal studies and human studies not done), as are all topical antibiotics except dapsone, which is category C because of a theoretical risk of neonatal hyperbilirubinemia if used near the time of delivery.
Hydroxychloroquine may be appropriate for connective tissue disease, she said, although steroids may be a better option in some cases.
And topical antifungals are considered safer for fungal diseases than systemic medications. She said she prefers clotrimazole and oxiconazole, both category B.
Finally, Dr. Tyler recommended permethrin (category B) for parasitic infections since it has been used extensively in pregnancy without a sign of risk and is the preferred treatment for scabies. It’s a better option than ivermectin, she said.
Postpone surgery until at least the second trimester
If it’s not possible to delay nonemergent dermatologic surgery until after pregnancy, she recommended performing procedures during the second trimester. Destruction of local lesions, however, is safe without anesthesia.
“In summary,” when treating pregnant patients, Dr. Tyler said, “a conservative approach is always best, topical medications are always first-line for any condition, and certain oral medications are safe.”
Dr. Tyler reported no relevant disclosures.
SAN DIEGO – “Some doctors don’t treat them at all, or they undertreat because they’re afraid of prescribing anything,” said dermatologist Kelly H. Tyler, MD.
But the Food and Drug Administration’s new system of prescription drug labeling for pregnant women offers improved insight for dermatologists, and a variety of alternatives are available to commonly used dermatologic drugs that pose risks, said Dr. Tyler, who has a unique perspective on this issue: She used to practice as an ob.gyn.
In her presentation, she provided the following tips when caring for pregnant patients.
Check the prescription drug labeling for pregnant and lactating women
Prior to 2015, the FDA used a letter ratings to denote the risk of medications during pregnancy. The ratings – A, B, C, D, and X – go from “A” showing no fetal risk in controlled studies to “X” in which a drug is contraindicated because “there is no reason to risk use of drug in pregnancy.”
This rating system is problematic, Dr. Tyler said, since it’s imprecise. She noted that about two-thirds of medications have a risk rating of C, which means “Risk cannot be ruled out; human studies may or may not show risk; potential benefits may justify potential risk.” But, she asked, “How are you supposed to know what to do with that kind of information?”
By June 30, 2015, FDA had retired the letter classifications and replaced this system with the Pregnancy and Lactation Labeling final rule, which requires that labels include a pregnancy section with information on pregnancy exposure registries, in addition to risk summaries, clinical considerations, and available data. There are also sections on lactation, including nursing mothers, and females and males of reproductive potential. (This information appears in the prescription drug labeling sections 8.1-8.3.) Labels for medications approved prior to June 30, 2001 did not need to be updated. Labels for newer medications are required to comply within 3-5 years of the 2015 policy change.
Ask female patients about sexual activity
It’s important to ask all premenopausal female patients whether they’re sexually active and whether they are using birth control, trying to conceive, or are currently pregnant. If they’re pregnant, what trimester are they in?
Keep in mind, she said, that women can be pregnant for quite some time without knowing it. Home pregnancy tests may not show positive results for up to 5 weeks after conception, she said. But the embryonic/organogenesis stage – from 2 to 8 weeks – is the most important period for a pregnant mother avoid drugs that could damage the unborn child and is “when you want to avoid most medications,” she said.
Risks don’t end after a few months, however. Dr. Tyler urged dermatologists to keep in mind that the brain, teeth, and bones remain susceptible to damage from teratogenic medications after the eighth week.
Keep topical medications in mind
When it comes to pregnant women, “for most dermatologic therapies, topical medications will be your No. 1 choice” because of less absorption, Dr. Tyler said.
But there are exceptions, she noted. In acne and rosacea, for example, there’s controversy about the safety of topical retinoids. “I would just urge you to not use those medications in pregnancy because you don’t really need to use them,” Dr. Tyler said. “You have other options that are safer.”
Adapalene and tretinoin are listed as category C (risk cannot be ruled out in pregnancy) and topical tazarotene is category X (contraindicated in pregnancy) because of retinoid-like anomalies in animal studies, she said.
Avoid 3 major contraindicated systemic drugs
Isotretinoin, acitretin, and methotrexate are “absolutely contraindicated” for anyone who is pregnant or could become pregnant, Dr. Tyler said.
Use some systemic drugs with caution
Tetracyclines are category D (positive evidence of risk to human fetus, but benefits may outweigh risks of drug) with the highest risk in the second and third trimesters, Dr. Tyler said. “If for some reason you have a patient who’s on tetracycline, stop before she’s in her second trimester.”
She recommended avoiding the antibiotic erythromycin because of studies hinting at risks when used early in pregnancy. Spironolactone is theoretically risky after the week 8 of pregnancy, she said. “Because of the animal studies, we typically do not use this during pregnancy.”
Psoriasis often improves during pregnancy
Psoriasis improves during pregnancy in about half of women with psoriasis and worsens in about 20%, Dr. Tyler said. During pregnancy, topical treatments are the first-line treatment, said Dr. Tyler, who recommends that treatment should begin with topical steroids, then calcipotriene or tacrolimus if needed (J Am Acad Dermatol. 2013 Apr;68[4]:663-71). Cyclosporine is an option, as is phototherapy, she said. In an interview, she noted that phototherapy (narrowband UVB) would be considered next-line therapy in patients who are past the first 28 days of gestation and have failed topical therapy, given they are taking adequate folic acid supplementation, which is present in prenatal vitamins.
“As for biologics, you have to go with older ones that have more data. When we look at newer medications, we don’t know a lot about them,” she said during the presentation.
It’s okay to prescribe oral steroids and antihistamines
Oral steroids are safe during pregnancy, Dr. Tyler said, but “just be judicious” with moderate doses and short durations.
Antihistamines are also appropriate, she said, but be aware of the potential for neonatal sedation during lactation.
As for antibiotics for bacterial infections, azithromycin, penicillins, and cephalosporins are all category B (no risk to human fetus despite possible animal risk; or no risk in animal studies and human studies not done), as are all topical antibiotics except dapsone, which is category C because of a theoretical risk of neonatal hyperbilirubinemia if used near the time of delivery.
Hydroxychloroquine may be appropriate for connective tissue disease, she said, although steroids may be a better option in some cases.
And topical antifungals are considered safer for fungal diseases than systemic medications. She said she prefers clotrimazole and oxiconazole, both category B.
Finally, Dr. Tyler recommended permethrin (category B) for parasitic infections since it has been used extensively in pregnancy without a sign of risk and is the preferred treatment for scabies. It’s a better option than ivermectin, she said.
Postpone surgery until at least the second trimester
If it’s not possible to delay nonemergent dermatologic surgery until after pregnancy, she recommended performing procedures during the second trimester. Destruction of local lesions, however, is safe without anesthesia.
“In summary,” when treating pregnant patients, Dr. Tyler said, “a conservative approach is always best, topical medications are always first-line for any condition, and certain oral medications are safe.”
Dr. Tyler reported no relevant disclosures.
EXPERT ANALYSIS FROM AAD 18
VIDEO: Cervical cancer laparotomy outperforms minimally invasive surgery
NEW ORLEANS – Use of minimally invasive radical hysterectomy to treat early-stage cervical cancer has grown over the past decade, and in current U.S. practice, roughly half of these cases are done with a minimally-invasive approach, with the rest done by conventional laparotomy. But the first data ever reported from a large, prospective trial that compared the efficacy of both methods for cervical cancer had the unexpected finding that disease-free survival following minimally invasive procedures significantly lagged behind radical hysterectomies done by open laparotomy, Pedro T. Ramirez, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Just after this report came results from a second study that used propensity score–adjusted observational data from the National Cancer Database and found significantly worse overall survival following minimally invasive radical hysterectomy for early-stage cervical cancer, compared with laparotomy, said J. Alejandro Rauh-Hain, MD, a gynecologic oncologist at the University of Texas MD Anderson Cancer Center in Houston.
Both findings were “very surprising,” said Dr. Rauh-Hain in a video interview. “I was pretty sure we’d see no difference” in outcomes between minimally invasive radical hysterectomies and the same surgery either done by laparoscope or robotically assisted.
Prior prospective comparisons of minimally invasive and open surgical methods for other cancer types, including endometrial, gastric, and ovarian, showed no differences in cancer recurrences and survival, which led to widening use of minimally invasive surgery (MIS) for cervical cancer despite no direct evidence supporting equivalence, Dr. Rauh-Hain noted. “We adopted it with no data. It made sense that cervical cancer would be the same as endometrial cancer,” he explained.
The Laparoscopic Approach to Cervical Cancer (LACC) trial ran at 33 centers in 12 countries, including six U.S. centers. The study randomized women during 2008-2017 who had stage 1A1, 1A2, or 1B1 cervical cancer to either MIS or open surgery for a radical hysterectomy. Each participating center had to submit to a trial review committee full case records for 10 patients and unedited surgical videos of two patients who had previously undergone a minimally invasive radical hysterectomy at the center to document local prowess with MIS.
Dr. Ramirez and his colleagues designed LACC to prove the noninferiority of MIS and calculated an expected enrollment of 740 patients based on statistical expectations, but the study stopped early after enrolling 631 patients because of the adverse outcomes identified in the MIS patients, with a median follow-up of 2.5 years instead of the planned follow-up of 4.5 years. The study reached the 4.5-year follow-up in about 39% of patients. Of the 312 patients randomized to undergo laparotomy, 88% actually underwent the surgery; of the 319 patients randomized to MIS, 91% received this surgery, with 16% of the MIS procedures done using robotic assistance.
The study’s primary endpoint was disease-free survival at 4.5 years, which occurred in 86% of the MIS patients and in 96.5% of the laparotomy patients, a difference that failed to meet the study’s prespecified definition of noninferiority for MIS, reported Dr. Ramirez, a professor of gynecologic oncology and director of Minimally Invasive Surgery Research and Education at the MD Anderson Cancer Center. In addition, several secondary analyses of the data all showed starkly superior outcomes in the laparotomy subgroup.
Disease-free survival among all patients regardless of follow-up duration occurred in 98% of laparotomy patients and 92% of MIS patients, which translated into a 3.74 hazard ratio (P = .002) for disease recurrence or death among the MIS patients when compared with laparotomy patients. The all-cause mortality rates were 1% in the laparotomy patients and 6% among the MIS patients, a hazard ratio of 6.00 (P = .004). The risk of local or regional recurrences was more than fourfold higher in the MIS patients. A blinded, central panel adjudicated all recurrences identified during the study.
The LACC results “should be discussed with patients scheduled to undergo radical hysterectomy” for cervical cancer, Dr. Ramirez concluded.
The observational data from the National Cancer Database used in the analysis led by Dr. Rauh-Hain came from 2,221 patients hospitalized and treated with radical hysterectomy and pelvic lymph node dissection at a U.S. center during 2010-2012 for either stage 1A2 or 1B1 cervical cancer. Among these patients, 47.5% underwent MIS, with 79% of those procedures done with robotic assistance, while the other 52.5% underwent open laparotomy, Dr. Rauh-Hain reported. Additional analysis of data from this database by the researchers showed that, although the first report of MIS for radical hysterectomy appeared in 1992, the approach remained largely unused in U.S. practice until 2007, when use of MIS began to sharply rise. By 2010, about a third of radical hysterectomies for cervical cancer involved MIS, and usage increased still further during 2011 and 2012 to produce a nearly 48% rate during the 3-year study period.
The primary endpoint of Dr. Rauh-Hain’s analysis was overall survival following propensity-score matching of the MIS and laparotomy patients using 13 demographic and clinical criteria. The analysis showed 4-year mortality rates of 5.8% among the laparotomy patients and 8.4% among the MIS patients, which calculated to a relatively increased mortality hazard from MIS of 48% (P = .02).
Dr. Rauh-Hain also reported results from an interrupted time series analysis using data from the Surveillance, Epidemiology, and End Results database of the National Cancer Institute. This analysis compared annual 4-year relative survival rates among women undergoing radical hysterectomy for cervical cancer and found that, after survival rates showed a gradual, steady rise during the years culminating in 2006, once MIS began being more widely used in 2007 survival rates began to drop, with a statistically significant annualized decline of 1% through 2010.
Based on the results from both studies, “at MD Anderson we discuss the results with patients,” with the consequence that the percentage of patients treated with laparotomy is now increasing, Dr. Rauh-Hain said. The results from both studies “are concerning,” he explained.
SOURCE: Ramirez PT and Rauh-Hain JA. SGO 2018, Late-Breaking Abstracts 1 and 2.
The findings from these studies appear valid and should be discussed with patients.
The findings raise a major question: Why has minimally invasive surgery (MIS) led to worse survival rates than laparotomy? Several possible explanations can be hypothesized: The uterine manipulator used in MIS led to local spread of cancer cells; MIS involves a learning curve and initial attempts at MIS did not remove enough of the tumor; and MIS led to increased exposure of the peritoneal cavity to the cancer. The findings also raise another question: Why has MIS for cervical cancer performed less well than MIS for cancers from other organs, such as endometrial and prostate?
The overall survival results from the LACC trial calculate out to 4.75 added deaths per year for every 1,000 patients treated with MIS, compared with laparoscopy. But the National Inpatient Sample data suggest that MIS cuts mortality by about two deaths per year per 1,000 patients, compared with laparotomy, and mortality data from a different analysis (Gynecol Oncol. 2012 Oct;127[1]:11-7) suggest that MIS might prevent six deaths annually for every 1,000 patients, compared with laparotomy. Overall, these three sets of findings suggest roughly comparable mortality outcomes from MIS and laparotomy, but with MIS having the bonus of fewer complications and less need for transfusions.
The cautions and concerns raised by the LACC trial and Dr. Rauh-Hain’s analysis of observational data cannot be easily dismissed. We need to figure out why the results from both studies show worse survival and recurrence rates with MIS, and we need to identify whether subgroups of patients exist who might clearly benefit from either the MIS or open-surgery approach.
Shitanshu Uppal, MD , is a gynecologic oncologist at the University of Michigan in Ann Arbor. He made these comments as designated discussant for the two studies. He had no disclosures.
The findings from these studies appear valid and should be discussed with patients.
The findings raise a major question: Why has minimally invasive surgery (MIS) led to worse survival rates than laparotomy? Several possible explanations can be hypothesized: The uterine manipulator used in MIS led to local spread of cancer cells; MIS involves a learning curve and initial attempts at MIS did not remove enough of the tumor; and MIS led to increased exposure of the peritoneal cavity to the cancer. The findings also raise another question: Why has MIS for cervical cancer performed less well than MIS for cancers from other organs, such as endometrial and prostate?
The overall survival results from the LACC trial calculate out to 4.75 added deaths per year for every 1,000 patients treated with MIS, compared with laparoscopy. But the National Inpatient Sample data suggest that MIS cuts mortality by about two deaths per year per 1,000 patients, compared with laparotomy, and mortality data from a different analysis (Gynecol Oncol. 2012 Oct;127[1]:11-7) suggest that MIS might prevent six deaths annually for every 1,000 patients, compared with laparotomy. Overall, these three sets of findings suggest roughly comparable mortality outcomes from MIS and laparotomy, but with MIS having the bonus of fewer complications and less need for transfusions.
The cautions and concerns raised by the LACC trial and Dr. Rauh-Hain’s analysis of observational data cannot be easily dismissed. We need to figure out why the results from both studies show worse survival and recurrence rates with MIS, and we need to identify whether subgroups of patients exist who might clearly benefit from either the MIS or open-surgery approach.
Shitanshu Uppal, MD , is a gynecologic oncologist at the University of Michigan in Ann Arbor. He made these comments as designated discussant for the two studies. He had no disclosures.
The findings from these studies appear valid and should be discussed with patients.
The findings raise a major question: Why has minimally invasive surgery (MIS) led to worse survival rates than laparotomy? Several possible explanations can be hypothesized: The uterine manipulator used in MIS led to local spread of cancer cells; MIS involves a learning curve and initial attempts at MIS did not remove enough of the tumor; and MIS led to increased exposure of the peritoneal cavity to the cancer. The findings also raise another question: Why has MIS for cervical cancer performed less well than MIS for cancers from other organs, such as endometrial and prostate?
The overall survival results from the LACC trial calculate out to 4.75 added deaths per year for every 1,000 patients treated with MIS, compared with laparoscopy. But the National Inpatient Sample data suggest that MIS cuts mortality by about two deaths per year per 1,000 patients, compared with laparotomy, and mortality data from a different analysis (Gynecol Oncol. 2012 Oct;127[1]:11-7) suggest that MIS might prevent six deaths annually for every 1,000 patients, compared with laparotomy. Overall, these three sets of findings suggest roughly comparable mortality outcomes from MIS and laparotomy, but with MIS having the bonus of fewer complications and less need for transfusions.
The cautions and concerns raised by the LACC trial and Dr. Rauh-Hain’s analysis of observational data cannot be easily dismissed. We need to figure out why the results from both studies show worse survival and recurrence rates with MIS, and we need to identify whether subgroups of patients exist who might clearly benefit from either the MIS or open-surgery approach.
Shitanshu Uppal, MD , is a gynecologic oncologist at the University of Michigan in Ann Arbor. He made these comments as designated discussant for the two studies. He had no disclosures.
NEW ORLEANS – Use of minimally invasive radical hysterectomy to treat early-stage cervical cancer has grown over the past decade, and in current U.S. practice, roughly half of these cases are done with a minimally-invasive approach, with the rest done by conventional laparotomy. But the first data ever reported from a large, prospective trial that compared the efficacy of both methods for cervical cancer had the unexpected finding that disease-free survival following minimally invasive procedures significantly lagged behind radical hysterectomies done by open laparotomy, Pedro T. Ramirez, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Just after this report came results from a second study that used propensity score–adjusted observational data from the National Cancer Database and found significantly worse overall survival following minimally invasive radical hysterectomy for early-stage cervical cancer, compared with laparotomy, said J. Alejandro Rauh-Hain, MD, a gynecologic oncologist at the University of Texas MD Anderson Cancer Center in Houston.
Both findings were “very surprising,” said Dr. Rauh-Hain in a video interview. “I was pretty sure we’d see no difference” in outcomes between minimally invasive radical hysterectomies and the same surgery either done by laparoscope or robotically assisted.
Prior prospective comparisons of minimally invasive and open surgical methods for other cancer types, including endometrial, gastric, and ovarian, showed no differences in cancer recurrences and survival, which led to widening use of minimally invasive surgery (MIS) for cervical cancer despite no direct evidence supporting equivalence, Dr. Rauh-Hain noted. “We adopted it with no data. It made sense that cervical cancer would be the same as endometrial cancer,” he explained.
The Laparoscopic Approach to Cervical Cancer (LACC) trial ran at 33 centers in 12 countries, including six U.S. centers. The study randomized women during 2008-2017 who had stage 1A1, 1A2, or 1B1 cervical cancer to either MIS or open surgery for a radical hysterectomy. Each participating center had to submit to a trial review committee full case records for 10 patients and unedited surgical videos of two patients who had previously undergone a minimally invasive radical hysterectomy at the center to document local prowess with MIS.
Dr. Ramirez and his colleagues designed LACC to prove the noninferiority of MIS and calculated an expected enrollment of 740 patients based on statistical expectations, but the study stopped early after enrolling 631 patients because of the adverse outcomes identified in the MIS patients, with a median follow-up of 2.5 years instead of the planned follow-up of 4.5 years. The study reached the 4.5-year follow-up in about 39% of patients. Of the 312 patients randomized to undergo laparotomy, 88% actually underwent the surgery; of the 319 patients randomized to MIS, 91% received this surgery, with 16% of the MIS procedures done using robotic assistance.
The study’s primary endpoint was disease-free survival at 4.5 years, which occurred in 86% of the MIS patients and in 96.5% of the laparotomy patients, a difference that failed to meet the study’s prespecified definition of noninferiority for MIS, reported Dr. Ramirez, a professor of gynecologic oncology and director of Minimally Invasive Surgery Research and Education at the MD Anderson Cancer Center. In addition, several secondary analyses of the data all showed starkly superior outcomes in the laparotomy subgroup.
Disease-free survival among all patients regardless of follow-up duration occurred in 98% of laparotomy patients and 92% of MIS patients, which translated into a 3.74 hazard ratio (P = .002) for disease recurrence or death among the MIS patients when compared with laparotomy patients. The all-cause mortality rates were 1% in the laparotomy patients and 6% among the MIS patients, a hazard ratio of 6.00 (P = .004). The risk of local or regional recurrences was more than fourfold higher in the MIS patients. A blinded, central panel adjudicated all recurrences identified during the study.
The LACC results “should be discussed with patients scheduled to undergo radical hysterectomy” for cervical cancer, Dr. Ramirez concluded.
The observational data from the National Cancer Database used in the analysis led by Dr. Rauh-Hain came from 2,221 patients hospitalized and treated with radical hysterectomy and pelvic lymph node dissection at a U.S. center during 2010-2012 for either stage 1A2 or 1B1 cervical cancer. Among these patients, 47.5% underwent MIS, with 79% of those procedures done with robotic assistance, while the other 52.5% underwent open laparotomy, Dr. Rauh-Hain reported. Additional analysis of data from this database by the researchers showed that, although the first report of MIS for radical hysterectomy appeared in 1992, the approach remained largely unused in U.S. practice until 2007, when use of MIS began to sharply rise. By 2010, about a third of radical hysterectomies for cervical cancer involved MIS, and usage increased still further during 2011 and 2012 to produce a nearly 48% rate during the 3-year study period.
The primary endpoint of Dr. Rauh-Hain’s analysis was overall survival following propensity-score matching of the MIS and laparotomy patients using 13 demographic and clinical criteria. The analysis showed 4-year mortality rates of 5.8% among the laparotomy patients and 8.4% among the MIS patients, which calculated to a relatively increased mortality hazard from MIS of 48% (P = .02).
Dr. Rauh-Hain also reported results from an interrupted time series analysis using data from the Surveillance, Epidemiology, and End Results database of the National Cancer Institute. This analysis compared annual 4-year relative survival rates among women undergoing radical hysterectomy for cervical cancer and found that, after survival rates showed a gradual, steady rise during the years culminating in 2006, once MIS began being more widely used in 2007 survival rates began to drop, with a statistically significant annualized decline of 1% through 2010.
Based on the results from both studies, “at MD Anderson we discuss the results with patients,” with the consequence that the percentage of patients treated with laparotomy is now increasing, Dr. Rauh-Hain said. The results from both studies “are concerning,” he explained.
SOURCE: Ramirez PT and Rauh-Hain JA. SGO 2018, Late-Breaking Abstracts 1 and 2.
NEW ORLEANS – Use of minimally invasive radical hysterectomy to treat early-stage cervical cancer has grown over the past decade, and in current U.S. practice, roughly half of these cases are done with a minimally-invasive approach, with the rest done by conventional laparotomy. But the first data ever reported from a large, prospective trial that compared the efficacy of both methods for cervical cancer had the unexpected finding that disease-free survival following minimally invasive procedures significantly lagged behind radical hysterectomies done by open laparotomy, Pedro T. Ramirez, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Just after this report came results from a second study that used propensity score–adjusted observational data from the National Cancer Database and found significantly worse overall survival following minimally invasive radical hysterectomy for early-stage cervical cancer, compared with laparotomy, said J. Alejandro Rauh-Hain, MD, a gynecologic oncologist at the University of Texas MD Anderson Cancer Center in Houston.
Both findings were “very surprising,” said Dr. Rauh-Hain in a video interview. “I was pretty sure we’d see no difference” in outcomes between minimally invasive radical hysterectomies and the same surgery either done by laparoscope or robotically assisted.
Prior prospective comparisons of minimally invasive and open surgical methods for other cancer types, including endometrial, gastric, and ovarian, showed no differences in cancer recurrences and survival, which led to widening use of minimally invasive surgery (MIS) for cervical cancer despite no direct evidence supporting equivalence, Dr. Rauh-Hain noted. “We adopted it with no data. It made sense that cervical cancer would be the same as endometrial cancer,” he explained.
The Laparoscopic Approach to Cervical Cancer (LACC) trial ran at 33 centers in 12 countries, including six U.S. centers. The study randomized women during 2008-2017 who had stage 1A1, 1A2, or 1B1 cervical cancer to either MIS or open surgery for a radical hysterectomy. Each participating center had to submit to a trial review committee full case records for 10 patients and unedited surgical videos of two patients who had previously undergone a minimally invasive radical hysterectomy at the center to document local prowess with MIS.
Dr. Ramirez and his colleagues designed LACC to prove the noninferiority of MIS and calculated an expected enrollment of 740 patients based on statistical expectations, but the study stopped early after enrolling 631 patients because of the adverse outcomes identified in the MIS patients, with a median follow-up of 2.5 years instead of the planned follow-up of 4.5 years. The study reached the 4.5-year follow-up in about 39% of patients. Of the 312 patients randomized to undergo laparotomy, 88% actually underwent the surgery; of the 319 patients randomized to MIS, 91% received this surgery, with 16% of the MIS procedures done using robotic assistance.
The study’s primary endpoint was disease-free survival at 4.5 years, which occurred in 86% of the MIS patients and in 96.5% of the laparotomy patients, a difference that failed to meet the study’s prespecified definition of noninferiority for MIS, reported Dr. Ramirez, a professor of gynecologic oncology and director of Minimally Invasive Surgery Research and Education at the MD Anderson Cancer Center. In addition, several secondary analyses of the data all showed starkly superior outcomes in the laparotomy subgroup.
Disease-free survival among all patients regardless of follow-up duration occurred in 98% of laparotomy patients and 92% of MIS patients, which translated into a 3.74 hazard ratio (P = .002) for disease recurrence or death among the MIS patients when compared with laparotomy patients. The all-cause mortality rates were 1% in the laparotomy patients and 6% among the MIS patients, a hazard ratio of 6.00 (P = .004). The risk of local or regional recurrences was more than fourfold higher in the MIS patients. A blinded, central panel adjudicated all recurrences identified during the study.
The LACC results “should be discussed with patients scheduled to undergo radical hysterectomy” for cervical cancer, Dr. Ramirez concluded.
The observational data from the National Cancer Database used in the analysis led by Dr. Rauh-Hain came from 2,221 patients hospitalized and treated with radical hysterectomy and pelvic lymph node dissection at a U.S. center during 2010-2012 for either stage 1A2 or 1B1 cervical cancer. Among these patients, 47.5% underwent MIS, with 79% of those procedures done with robotic assistance, while the other 52.5% underwent open laparotomy, Dr. Rauh-Hain reported. Additional analysis of data from this database by the researchers showed that, although the first report of MIS for radical hysterectomy appeared in 1992, the approach remained largely unused in U.S. practice until 2007, when use of MIS began to sharply rise. By 2010, about a third of radical hysterectomies for cervical cancer involved MIS, and usage increased still further during 2011 and 2012 to produce a nearly 48% rate during the 3-year study period.
The primary endpoint of Dr. Rauh-Hain’s analysis was overall survival following propensity-score matching of the MIS and laparotomy patients using 13 demographic and clinical criteria. The analysis showed 4-year mortality rates of 5.8% among the laparotomy patients and 8.4% among the MIS patients, which calculated to a relatively increased mortality hazard from MIS of 48% (P = .02).
Dr. Rauh-Hain also reported results from an interrupted time series analysis using data from the Surveillance, Epidemiology, and End Results database of the National Cancer Institute. This analysis compared annual 4-year relative survival rates among women undergoing radical hysterectomy for cervical cancer and found that, after survival rates showed a gradual, steady rise during the years culminating in 2006, once MIS began being more widely used in 2007 survival rates began to drop, with a statistically significant annualized decline of 1% through 2010.
Based on the results from both studies, “at MD Anderson we discuss the results with patients,” with the consequence that the percentage of patients treated with laparotomy is now increasing, Dr. Rauh-Hain said. The results from both studies “are concerning,” he explained.
SOURCE: Ramirez PT and Rauh-Hain JA. SGO 2018, Late-Breaking Abstracts 1 and 2.
REPORTING FROM SGO 2018
Key clinical point: Laparotomy produced better survival than did minimally invasive surgery for cervical cancer.
Major finding: Disease-free survival after 4.5 years was 96.5% with laparotomy and 86.0% with minimally invasive surgery.
Study details: LACC was a multicenter, randomized trial with 631 patients. The observational study included 2,221 patients from the National Cancer Database during 2010-2012.
Disclosures: Dr. Ramirez and Dr. Rauh-Hain had no disclosures.
Source: Ramirez PT and Rauh-Hain JA. SGO 2018, Late-Breaking Abstracts 1 and 2.
Homework
How do you feel about homework? Do you think your school-age patients are given too much homework? Would they be better off spending their after-school time at home in free play or exploring nonacademic interests? Or, do you feel the school day is too short to adequately cover what a well-educated child needs to know? Doesn’t homework foster good independent work habits and discipline?
Do you have fond memories of doing homework? Are you glad those days of bringing home an hour or 3 of extra work are behind you? Maybe they aren’t behind you. Are you still spending an hour or more getting stuff done at home you didn’t get done in the office?
Primary care pediatrics has never promised its practitioners that they will arrive at home at the end of the workday free of unfinished business. If you have after-hours call responsibilities, there always have been phone calls, decisions to make, and trips to EDs and delivery rooms. Even if you are fortunate enough to not have after-hours call responsibilities, there are certainly evenings when you are nagged by second thoughts and worries about troublesome patients you have seen during the day. Did you make the correct diagnosis or forget to order a critical lab test?
This kind of homework is expected. It’s what you signed up for. But with experience, you learn how to provide better anticipatory guidance that can decrease the number of after-hours calls. You can minimize, but never eliminate, second-guessing by learning to make wiser diagnostic and therapeutic decisions.
However, arriving home with a laptop or notebook loaded with unfinished electronic health records and work-related emails is not what you thought primary care pediatrics was about ... and it didn’t used to be. For the first 35 years of practice, when I saw my last patient, my office work was over. If I wasn’t on call, I could enjoy the entire evening with my family uninterrupted.
But change happens. Coincident with the launch of a new computer system, my workday became an hour longer so that I could complete my electronic office notes before I went home. For some of my colleagues, this unwelcome addition ran more than an hour and a half or 2 hours, and many of them leapt at the practice administrator’s offer to link their home computers with our new office EHR. Buried in what sounded like a good deal to them, I could hear the creaky opening of a Pandora’s box.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
How do you feel about homework? Do you think your school-age patients are given too much homework? Would they be better off spending their after-school time at home in free play or exploring nonacademic interests? Or, do you feel the school day is too short to adequately cover what a well-educated child needs to know? Doesn’t homework foster good independent work habits and discipline?
Do you have fond memories of doing homework? Are you glad those days of bringing home an hour or 3 of extra work are behind you? Maybe they aren’t behind you. Are you still spending an hour or more getting stuff done at home you didn’t get done in the office?
Primary care pediatrics has never promised its practitioners that they will arrive at home at the end of the workday free of unfinished business. If you have after-hours call responsibilities, there always have been phone calls, decisions to make, and trips to EDs and delivery rooms. Even if you are fortunate enough to not have after-hours call responsibilities, there are certainly evenings when you are nagged by second thoughts and worries about troublesome patients you have seen during the day. Did you make the correct diagnosis or forget to order a critical lab test?
This kind of homework is expected. It’s what you signed up for. But with experience, you learn how to provide better anticipatory guidance that can decrease the number of after-hours calls. You can minimize, but never eliminate, second-guessing by learning to make wiser diagnostic and therapeutic decisions.
However, arriving home with a laptop or notebook loaded with unfinished electronic health records and work-related emails is not what you thought primary care pediatrics was about ... and it didn’t used to be. For the first 35 years of practice, when I saw my last patient, my office work was over. If I wasn’t on call, I could enjoy the entire evening with my family uninterrupted.
But change happens. Coincident with the launch of a new computer system, my workday became an hour longer so that I could complete my electronic office notes before I went home. For some of my colleagues, this unwelcome addition ran more than an hour and a half or 2 hours, and many of them leapt at the practice administrator’s offer to link their home computers with our new office EHR. Buried in what sounded like a good deal to them, I could hear the creaky opening of a Pandora’s box.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
How do you feel about homework? Do you think your school-age patients are given too much homework? Would they be better off spending their after-school time at home in free play or exploring nonacademic interests? Or, do you feel the school day is too short to adequately cover what a well-educated child needs to know? Doesn’t homework foster good independent work habits and discipline?
Do you have fond memories of doing homework? Are you glad those days of bringing home an hour or 3 of extra work are behind you? Maybe they aren’t behind you. Are you still spending an hour or more getting stuff done at home you didn’t get done in the office?
Primary care pediatrics has never promised its practitioners that they will arrive at home at the end of the workday free of unfinished business. If you have after-hours call responsibilities, there always have been phone calls, decisions to make, and trips to EDs and delivery rooms. Even if you are fortunate enough to not have after-hours call responsibilities, there are certainly evenings when you are nagged by second thoughts and worries about troublesome patients you have seen during the day. Did you make the correct diagnosis or forget to order a critical lab test?
This kind of homework is expected. It’s what you signed up for. But with experience, you learn how to provide better anticipatory guidance that can decrease the number of after-hours calls. You can minimize, but never eliminate, second-guessing by learning to make wiser diagnostic and therapeutic decisions.
However, arriving home with a laptop or notebook loaded with unfinished electronic health records and work-related emails is not what you thought primary care pediatrics was about ... and it didn’t used to be. For the first 35 years of practice, when I saw my last patient, my office work was over. If I wasn’t on call, I could enjoy the entire evening with my family uninterrupted.
But change happens. Coincident with the launch of a new computer system, my workday became an hour longer so that I could complete my electronic office notes before I went home. For some of my colleagues, this unwelcome addition ran more than an hour and a half or 2 hours, and many of them leapt at the practice administrator’s offer to link their home computers with our new office EHR. Buried in what sounded like a good deal to them, I could hear the creaky opening of a Pandora’s box.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
AHRQ Practice Toolbox: Practice facilitation
This is the eighth in a series of articles from the National Center for Excellence in Primary Care Research (NCEPCR) in the Agency for Healthcare Research and Quality (AHRQ). This series introduces sets of tools and resources designed to help your practice.
Last month’s article discussed AHRQ’s tool and resources for practice transformation, or how to help make your practice ready for advances such as shared decision making, team-based care, and integrating behavioral health and primary care. Use of practice facilitation, an evidence-based approach to quality improvement, can be a key element to helping primary care practices make the necessary changes for transformation. Practice facilitators (also known as practice coaches, quality improvement coaches, or practice enhancement assistants) are specially trained individuals who assist primary care clinicians in research and quality improvement projects. They are distinguished from consultants through specialized training, broad scope of practice, and long-term relationships with an organization, its staff and clinicians, and its patients.
If you are wondering how a practice facilitator can help you, AHRQ offers How a Practice Facilitator Can Support Your Practice. This tip sheet provides ideas and techniques for primary care practices interested in getting started with quality improvement activities and describes the benefits of working with a practice facilitator. The tip sheet is distilled from a more extensive white paper on Engaging Primary Care Practices in Quality Improvement: Strategies for Practice Facilitators, which presents a framework for engaging primary care practices in quality improvement and provides strategies for sustained involvement in the undertaking.
For those with greater interest in implementation of practice facilitation, AHRQ offers the Practice Facilitation Handbook: Training Modules for New Facilitators and Their Trainers. The Handbook is designed to assist in the training of new practice facilitators as they begin to develop the knowledge and skills needed to support meaningful improvement in primary care practices. AHRQ built upon the modules in the Handbook with the Primary Care Practice Facilitation Curriculum. The Curriculum updates the modules in the Handbook and adds an additional twelve modules for a total of 32 modules. More importantly, some of the modules can be used by clinicians and staff as a sort of Quality Improvement 101. Modules on appreciative inquiry, approaches to quality improvement, redesigning work flow, root cause analysis, and measuring clinical performance can be useful to anyone interested in increasing their knowledge and skills for quality improvement.
Mr. McNellis is senior adviser for primary care at AHRQ and Dr. Ganiats is director of the National Center for Excellence in Primary Care Research at AHRQ.
Links from the AHRQ Web site:
How a Practice Facilitator Can Support Your Practice
Engaging Primary Care Practices in Quality Improvement: Strategies for Practice Facilitators
Primary Care Practice Facilitation Curriculum
Practice Facilitation Handbook
A How-To Guide on Developing and Running a Practice Facilitation Program
These and other tools can be found at the NCEPCR Web site.
This is the eighth in a series of articles from the National Center for Excellence in Primary Care Research (NCEPCR) in the Agency for Healthcare Research and Quality (AHRQ). This series introduces sets of tools and resources designed to help your practice.
Last month’s article discussed AHRQ’s tool and resources for practice transformation, or how to help make your practice ready for advances such as shared decision making, team-based care, and integrating behavioral health and primary care. Use of practice facilitation, an evidence-based approach to quality improvement, can be a key element to helping primary care practices make the necessary changes for transformation. Practice facilitators (also known as practice coaches, quality improvement coaches, or practice enhancement assistants) are specially trained individuals who assist primary care clinicians in research and quality improvement projects. They are distinguished from consultants through specialized training, broad scope of practice, and long-term relationships with an organization, its staff and clinicians, and its patients.
If you are wondering how a practice facilitator can help you, AHRQ offers How a Practice Facilitator Can Support Your Practice. This tip sheet provides ideas and techniques for primary care practices interested in getting started with quality improvement activities and describes the benefits of working with a practice facilitator. The tip sheet is distilled from a more extensive white paper on Engaging Primary Care Practices in Quality Improvement: Strategies for Practice Facilitators, which presents a framework for engaging primary care practices in quality improvement and provides strategies for sustained involvement in the undertaking.
For those with greater interest in implementation of practice facilitation, AHRQ offers the Practice Facilitation Handbook: Training Modules for New Facilitators and Their Trainers. The Handbook is designed to assist in the training of new practice facilitators as they begin to develop the knowledge and skills needed to support meaningful improvement in primary care practices. AHRQ built upon the modules in the Handbook with the Primary Care Practice Facilitation Curriculum. The Curriculum updates the modules in the Handbook and adds an additional twelve modules for a total of 32 modules. More importantly, some of the modules can be used by clinicians and staff as a sort of Quality Improvement 101. Modules on appreciative inquiry, approaches to quality improvement, redesigning work flow, root cause analysis, and measuring clinical performance can be useful to anyone interested in increasing their knowledge and skills for quality improvement.
Mr. McNellis is senior adviser for primary care at AHRQ and Dr. Ganiats is director of the National Center for Excellence in Primary Care Research at AHRQ.
Links from the AHRQ Web site:
How a Practice Facilitator Can Support Your Practice
Engaging Primary Care Practices in Quality Improvement: Strategies for Practice Facilitators
Primary Care Practice Facilitation Curriculum
Practice Facilitation Handbook
A How-To Guide on Developing and Running a Practice Facilitation Program
These and other tools can be found at the NCEPCR Web site.
This is the eighth in a series of articles from the National Center for Excellence in Primary Care Research (NCEPCR) in the Agency for Healthcare Research and Quality (AHRQ). This series introduces sets of tools and resources designed to help your practice.
Last month’s article discussed AHRQ’s tool and resources for practice transformation, or how to help make your practice ready for advances such as shared decision making, team-based care, and integrating behavioral health and primary care. Use of practice facilitation, an evidence-based approach to quality improvement, can be a key element to helping primary care practices make the necessary changes for transformation. Practice facilitators (also known as practice coaches, quality improvement coaches, or practice enhancement assistants) are specially trained individuals who assist primary care clinicians in research and quality improvement projects. They are distinguished from consultants through specialized training, broad scope of practice, and long-term relationships with an organization, its staff and clinicians, and its patients.
If you are wondering how a practice facilitator can help you, AHRQ offers How a Practice Facilitator Can Support Your Practice. This tip sheet provides ideas and techniques for primary care practices interested in getting started with quality improvement activities and describes the benefits of working with a practice facilitator. The tip sheet is distilled from a more extensive white paper on Engaging Primary Care Practices in Quality Improvement: Strategies for Practice Facilitators, which presents a framework for engaging primary care practices in quality improvement and provides strategies for sustained involvement in the undertaking.
For those with greater interest in implementation of practice facilitation, AHRQ offers the Practice Facilitation Handbook: Training Modules for New Facilitators and Their Trainers. The Handbook is designed to assist in the training of new practice facilitators as they begin to develop the knowledge and skills needed to support meaningful improvement in primary care practices. AHRQ built upon the modules in the Handbook with the Primary Care Practice Facilitation Curriculum. The Curriculum updates the modules in the Handbook and adds an additional twelve modules for a total of 32 modules. More importantly, some of the modules can be used by clinicians and staff as a sort of Quality Improvement 101. Modules on appreciative inquiry, approaches to quality improvement, redesigning work flow, root cause analysis, and measuring clinical performance can be useful to anyone interested in increasing their knowledge and skills for quality improvement.
Mr. McNellis is senior adviser for primary care at AHRQ and Dr. Ganiats is director of the National Center for Excellence in Primary Care Research at AHRQ.
Links from the AHRQ Web site:
How a Practice Facilitator Can Support Your Practice
Engaging Primary Care Practices in Quality Improvement: Strategies for Practice Facilitators
Primary Care Practice Facilitation Curriculum
Practice Facilitation Handbook
A How-To Guide on Developing and Running a Practice Facilitation Program
These and other tools can be found at the NCEPCR Web site.