User login
Analgesic management in radiation oncology for painful bone metastases
Bone metastases are a common cause of pain in patients with advanced cancer, with about three-quarters of patients with bone metastases experiencing pain as the dominant symptom.1 Inadequately treated cancer pain impairs patient quality of life, and is associated with higher rates of depression, anxiety, and fatigue. Palliative radiotherapy (RT) is effective in alleviating pain from bone metastases.4 Local field external beam radiotherapy can provide some pain relief at the site of treated metastasis in 80%-90% of cases, with complete pain relief in 50%-60% of cases.5,6 However, maximal pain relief from RT is delayed, in some cases taking days to up to multiple weeks to attain.7,8 Therefore, optimal management of bone metastases pain may require the use of analgesics until RT takes adequate effect.
National Comprehensive Cancer Network (NCCN) Guidelines for Adult Cancer Pain (v. 2.2015) recommend that pain intensity rating (PIR; range, 0-10, where 0 denotes no pain and 10, worst pain imaginable) be used to quantify pain for all symptomatic patients. These guidelines also recommend the pain medication regimen be assessed for all symptomatic patients. For patients with moderate or severe pain (PIR of ≥4), NCCN guidelines recommend that analgesic regimen be intervened upon by alteration of the analgesic regimen (initiating, rotating, or titrating analgesic) or consideration of referral to pain/symptom management specialty.
Previous findings have demonstrated inadequate analgesic management for cancer pain,2,9 including within the radiation oncology (RO) clinic, suggesting that patients seen in consultation for palliative RT may experience uncontrolled pain for days to weeks before the onset of relief from RT. Possible reasons for inadequate acute pain intervention in the RO clinic may be provider discomfort with analgesic management and infrequent formal integration of palliative care within RO.10
Limited single-institution data from the few institutions with dedicated palliative RO services have suggested that these services improve the quality of palliative care delivery, as demonstrated by providers perceptions’ of the clinical impact of a dedicated service11 and the implementation of expedited palliative RT delivery for acute cancer pain.12,13 To our knowledge, the impact of a dedicated palliative RO service on analgesic management for cancer pain has not been assessed.
Here, we report how often patients with symptomatic bone metastases had assessments of existing analgesic regimens and interventions at RO consultation at 2 cancer centers. Center 1 had implemented a dedicated palliative RO service in 2011, consisting of rotating attending physicians and residents as well as dedicated palliative care trained nurse practitioners and a fellow, with the service structured around daily rounds,11 whereas Center 2 had not yet implemented a dedicated service. Using data from both centers, we assessed the impact of a palliative RO service on analgesic assessment and management in patients with bone metastases.
Methods
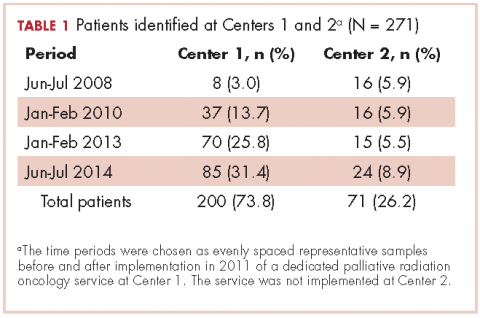
We searched our institutional databases for patients seen in RO consultation for bone metastases using ICD-9 code 198.5, and retrospectively reviewed consultation notes for those patients during June-July 2008, January-February 2010, January-February 2013, and June-July 2014. Those time periods were chosen as evenly spaced representative samples before and after implementation of a dedicated palliative RO service in 2011 at Center 1. Center 2 did not implement a dedicated palliative RO service in these time periods.
Within consultation notes, we recorded the following data from the History of the Present Illness section: symptoms from bone metastases (symptomatic was defined as any pain present); PIR (range, 0-10); and whether or not the preconsultation analgesic regimen was reported for symptomatic patients (including analgesic type, dosing, effectiveness, and adherence).
Documentation of the analgesic regimen in the history section of the notes was considered the proxy for analgesic regimen assessment at time of RO consultation. Analgesics within the Medications list, which were autopopulated in the consultation note by the electronic medical record, were recorded.
Whether or not pain was addressed with initiation or titration of analgesics for patients with a PIR of ≥4 was recorded from the Assessment and Plan portion of the notes, and that metric was considered the proxy for pain intervention. In addition, the case was coded as having had pain intervention if there was documentation of the patient declining recommended analgesic intervention, or the patient had been referred to a symptom management service for intervention (eg, referral to a specialty palliative care clinic), or there was recommendation for the patient to discuss uncontrolled pain with the original prescriber. A PIR of 4 was chosen as the threshold for analgesic intervention because at that level, NCCN guidelines for cancer pain state that the analgesic regimen should be titrated, whereas for a PIR of 3 or less, the guidelines recommend only consideration of titrating the analgesic. Only patients with a documented PIR were included in the pain intervention analysis.
Frequencies of analgesic assessment and analgesic intervention were compared using t tests (Wizard Pro, v1.8.5; Evan Miller, Chicago IL).
Results
A total of 271 patients with RO consultation notes were identified at the 2 centers within the 4 time periods (Table 1).
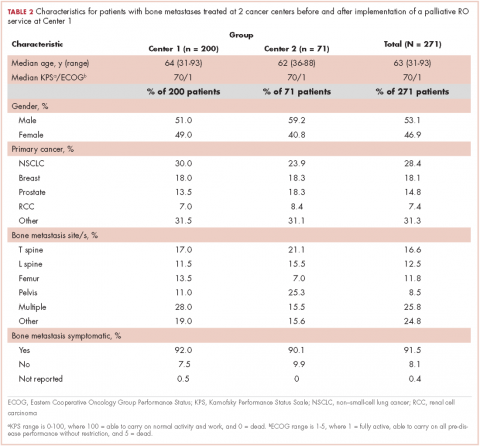
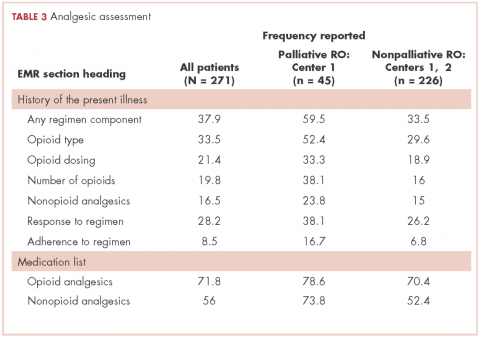
Among symptomatic patients, any component of the preconsultation analgesic regimen (including analgesic type, dosing, pain response, and adherence) was documented for 37.9% of the entire cohort at RO consultation (Table 3). At Centers 1 and 2, the frequencies of analgesic regimen assessment were documented for 41.3% and 28.1%, respectively (P = .06). Among symptomatic patients, 81.5% had an opioid or nonopioid analgesic listed in the Medications section in the electronic medical record at time of consultation.
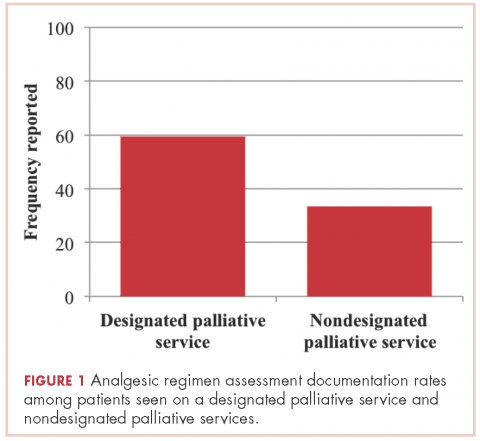
Patients seen on the dedicated palliative RO service at Center 1 had an analgesic assessment documentation rate of 59.5%, whereas the patients not seen on a palliative RO service (ie, patients seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had an assessment documentation rate of 33.5% (P = .002; Figure 1). There was no significant difference between rates of analgesic regimen assessment between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (28.1% vs 35.9%, respectively; P = .27).
In patients seen at Center 1 only, those seen on the palliative RO service had a higher documentation rate of analgesic assessment compared with those seen by other services after implementation of the dedicated service (59.5% vs 38%, respectively; P = .018). Time period (after versus before 2011) was not significantly associated with the rate of documentation of analgesic assessment at either Center 1 (after vs before 2011: 44.4% vs 31%, P = .23) or Center 2 (31.4% vs 24.1%, P = .60).
Among patients with a PIR of ≥4, analgesic intervention was reported for 17.2% of patients within the entire cohort (20.8% at Center 1 and 0% at Center 2, P = .05). Among those with a PIR of ≥4, documentation of analgesic assessment noted in the History of the Present Illness section was associated with increased documentation of an analgesic intervention in the Assessment and Plan section (25% vs 7.3%; odds ratio [OR], 4.22; 95% confidence interval [CI], 1.1-16.0; P = .03).
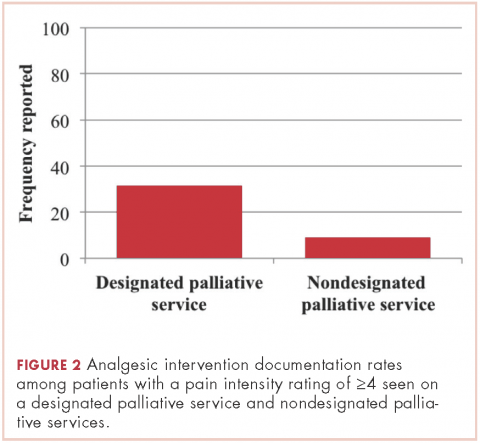
Patients seen on the dedicated palliative RO service at Center 1 had a documented analgesic intervention rate of 31.6%, whereas the patients not seen on a palliative RO service (ie, those seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had a documented analgesic intervention rate of 9.2% (P = .01; Figure 2). There was no statistically significant difference between rates of documentation of an analgesic regimen intervention between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (0% vs 17.2%, respectively; P = .07).
Looking at only patients seen at Center 1, patients with a PIR of ≥4 seen on the dedicated palliative RO service had a nearly significant higher rate of documented analgesic interventions in the time period after implementation of the dedicate service (31.6% if seen on the dedicated service vs 12% if seen on a nondedicated service, P = .06).
Discussion
Multiple studies demonstrate the undertreatment of cancer pain in the outpatient setting.2,9,14,15 At 2 cancer centers, we found that about half of patients who present for consideration of palliative RT for bone metastases had a PIR of ≥4, yet only 17% of them had documentation of analgesic intervention as recommended by NCCN guidelines for cancer pain. Underlying this low rate of appropriate intervention may be the assumption of rapid pain relief by RT. However, RT often does not begin at time of consultation,16 and maximal pain relief may take days to weeks after commencement of RT.17 It is estimated that a quarter of all patients with cancer develop bone metastases during the course of their disease,12 and most of those patients suffer from pain. Thus, inherent delay in pain relief before, during, and after RT results in significant morbidity for the cancer patient population if adequate analgesic management is not provided.
The low rate of appropriate analgesic intervention at the time of RO consultation may also be related to the low incidence of proper analgesic assessment. In our cohort, 80% of symptomatic patients had an opioid or nonopioid analgesic listed in their medications within the electronic medical record at time of consultation, but only 38% had the analgesic regimen and/or its effectiveness described in the History of the Present Illness section of the record. Inattentiveness to analgesic type, dosing, and effectiveness during consultation may result in any inadequacies of the analgesic regimen going unnoticed. Consistent with this notion, we found that the rate of appropriate intervention for patients with a PIR of ≥4 was higher among patients who had analgesic regimen reported in the consultation note. Thus, interventions to implement routine review and documentation of the analgesic regimen, for example within the electronic medical record, may be one way to improve pain management.
Another possible reason for low rates of acute pain management within the RO clinic is low provider confidence in regard to analgesic management. In a recent national survey, 96% of radiation oncologists stated they were at least moderately confident with assessment of pain, yet only 77% were at least moderately confident with titrating opioids, and just 56% were at least moderately confident with rotating opioids.10 Educational interventions that improve providers’ facility with analgesic management may increase the frequency of pain management in the RO clinic.
Patients seen on the dedicated palliative RO service had significantly higher rates of documented analgesic regimen assessment and appropriate intervention during RO consultation, compared with patients seen at Center 2 and those not seen on the dedicated palliative RO service at Center 1. The improvements we observed in analgesic assessment and intervention at Center 1 for patients seen on the palliative RO service are likely owing to involvement of palliative RO and not to secular trends, because there were not similar improvements for patients at Center 1 who were not seen by the palliative RO service and those at Center 2, where there was no service.
At Center 1, the dedicated palliative RO service was created to provide specialized care to patients with metastatic disease undergoing palliative radiation. Within its structure, topics within palliative RO, such as technical aspects of palliative RT, symptom management, and communication are taught and reinforced in a case-based approach. Such palliative care awareness, integration, and education within RO achieved by the palliative RO service likely contribute to the improved rates of analgesic management we found in our study. We do note that rate of analgesic intervention in the palliative RO cohort, though higher than in the nonpalliative RO group, was still low, with only a third of patients receiving proper analgesic management. These findings highlight the importance of continued effort in increasing providers’ awareness of the need to assess pain and raise comfort with analgesic initiation and titration and of having dedicated palliative care clinicians embedded within the RO setting.
Since the data for this study was acquired, Center 2 has implemented a short palliative RO didactic course for residents, which improved their comfort levels in assessing analgesic effectiveness and intervening for uncontrolled pain.18 The impact of this intervention on clinical care will need to be evaluated, but the improved provider comfort levels may translate into better-quality care.
Limitations
An important limitation of this retrospective study is the reliance on the documentation provided in the consultation note for determining frequencies of analgesic regimen assessment and intervention. The actual rates of analgesic management that occurred in clinic may have been higher than reported in the documentation. However, such discrepancy in documentation of analgesic management would also be an area for quality improvement. Inadequate documentation limits the ability for proper follow-up of cancer pain as recommended by a joint guidance statement from the American Society of Clinical Oncology and the American Academy of Hospice and Palliative Medicine.19,20 The results of our study may also partly reflect a positive impact in documentation of analgesic management by a dedicated palliative RO service.
Given the multi-institutional nature of this study, it may be that general practice differences confound the impact of the dedicated palliative RO service at Center 1. However, with excluding Center 2, the dedicated service was still strongly associated with a higher rate of analgesic assessment within Center 1 and was almost significantly associated with appropriate analgesic intervention within Center 1.
We used a PIR of ≥4 as a threshold for appropriate analgesic regimen intervention because it is what is recommended by the NCCN guidelines. However, close attention should be paid to the impact that any amount of pain has on an individual patient. The functional, spiritual, and existential impact of pain is unique to each patient’s experience, and optimal symptom management should take those elements into account.
Conclusion
In conclusion, this study indicates that advanced cancer patient pain assessment and intervention according to NCCN cancer pain management guidelines is not common in the RO setting, and it is an area that should be targeted for quality improvement because of the positive implications for patient well-being. Pain assessment and intervention were greater in the setting of a dedicated structure for palliative care within RO, suggesting that the integration of palliative care within RO is a promising means of improving quality of pain management.
This work was presented at the 2016 ASCO Palliative Care in Oncology Symposium (September 9-10, 2016), where this work received a Conquer Cancer Foundation Merit Award.
1. Amichetti M, Orrù P, Madeddu A, et al. Comparative evaluation of two hypofractionated radiotherapy regimens for painful bone metastases. Tumori. 2004;90(1):91-95.
2. Vuong S, Pulenzas N, DeAngelis C, et al. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support Care Cancer. 2016;24(2):887-892.
3. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1-2):129-134.
4. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Sze WM, ed. Cochrane Database Syst Rev. 2004;(2):CD004721-CD004721.
5. Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44(1):1-18.
6. Kirou-Mauro A, Hird A, Wong J, et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys. 2008;71(4):1208-1212.
7. Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-S164.
8. McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol. 2017 Jul 1;3(7):953-959.
9. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149-4154.
10. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the united states: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
11. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
12. Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
13. de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer. 2009;17(7):757-762.
14. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-1991.
15. Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259-267.
16. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38-43.
17. Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients – current approaches. Breast Care (Basel). 2012;7(2):108-112.
18. Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2017;97(5):884-885.
19. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
20. Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12(9):e828-e838.
Bone metastases are a common cause of pain in patients with advanced cancer, with about three-quarters of patients with bone metastases experiencing pain as the dominant symptom.1 Inadequately treated cancer pain impairs patient quality of life, and is associated with higher rates of depression, anxiety, and fatigue. Palliative radiotherapy (RT) is effective in alleviating pain from bone metastases.4 Local field external beam radiotherapy can provide some pain relief at the site of treated metastasis in 80%-90% of cases, with complete pain relief in 50%-60% of cases.5,6 However, maximal pain relief from RT is delayed, in some cases taking days to up to multiple weeks to attain.7,8 Therefore, optimal management of bone metastases pain may require the use of analgesics until RT takes adequate effect.
National Comprehensive Cancer Network (NCCN) Guidelines for Adult Cancer Pain (v. 2.2015) recommend that pain intensity rating (PIR; range, 0-10, where 0 denotes no pain and 10, worst pain imaginable) be used to quantify pain for all symptomatic patients. These guidelines also recommend the pain medication regimen be assessed for all symptomatic patients. For patients with moderate or severe pain (PIR of ≥4), NCCN guidelines recommend that analgesic regimen be intervened upon by alteration of the analgesic regimen (initiating, rotating, or titrating analgesic) or consideration of referral to pain/symptom management specialty.
Previous findings have demonstrated inadequate analgesic management for cancer pain,2,9 including within the radiation oncology (RO) clinic, suggesting that patients seen in consultation for palliative RT may experience uncontrolled pain for days to weeks before the onset of relief from RT. Possible reasons for inadequate acute pain intervention in the RO clinic may be provider discomfort with analgesic management and infrequent formal integration of palliative care within RO.10
Limited single-institution data from the few institutions with dedicated palliative RO services have suggested that these services improve the quality of palliative care delivery, as demonstrated by providers perceptions’ of the clinical impact of a dedicated service11 and the implementation of expedited palliative RT delivery for acute cancer pain.12,13 To our knowledge, the impact of a dedicated palliative RO service on analgesic management for cancer pain has not been assessed.
Here, we report how often patients with symptomatic bone metastases had assessments of existing analgesic regimens and interventions at RO consultation at 2 cancer centers. Center 1 had implemented a dedicated palliative RO service in 2011, consisting of rotating attending physicians and residents as well as dedicated palliative care trained nurse practitioners and a fellow, with the service structured around daily rounds,11 whereas Center 2 had not yet implemented a dedicated service. Using data from both centers, we assessed the impact of a palliative RO service on analgesic assessment and management in patients with bone metastases.
Methods
We searched our institutional databases for patients seen in RO consultation for bone metastases using ICD-9 code 198.5, and retrospectively reviewed consultation notes for those patients during June-July 2008, January-February 2010, January-February 2013, and June-July 2014. Those time periods were chosen as evenly spaced representative samples before and after implementation of a dedicated palliative RO service in 2011 at Center 1. Center 2 did not implement a dedicated palliative RO service in these time periods.
Within consultation notes, we recorded the following data from the History of the Present Illness section: symptoms from bone metastases (symptomatic was defined as any pain present); PIR (range, 0-10); and whether or not the preconsultation analgesic regimen was reported for symptomatic patients (including analgesic type, dosing, effectiveness, and adherence).
Documentation of the analgesic regimen in the history section of the notes was considered the proxy for analgesic regimen assessment at time of RO consultation. Analgesics within the Medications list, which were autopopulated in the consultation note by the electronic medical record, were recorded.
Whether or not pain was addressed with initiation or titration of analgesics for patients with a PIR of ≥4 was recorded from the Assessment and Plan portion of the notes, and that metric was considered the proxy for pain intervention. In addition, the case was coded as having had pain intervention if there was documentation of the patient declining recommended analgesic intervention, or the patient had been referred to a symptom management service for intervention (eg, referral to a specialty palliative care clinic), or there was recommendation for the patient to discuss uncontrolled pain with the original prescriber. A PIR of 4 was chosen as the threshold for analgesic intervention because at that level, NCCN guidelines for cancer pain state that the analgesic regimen should be titrated, whereas for a PIR of 3 or less, the guidelines recommend only consideration of titrating the analgesic. Only patients with a documented PIR were included in the pain intervention analysis.
Frequencies of analgesic assessment and analgesic intervention were compared using t tests (Wizard Pro, v1.8.5; Evan Miller, Chicago IL).
Results
A total of 271 patients with RO consultation notes were identified at the 2 centers within the 4 time periods (Table 1).
Among symptomatic patients, any component of the preconsultation analgesic regimen (including analgesic type, dosing, pain response, and adherence) was documented for 37.9% of the entire cohort at RO consultation (Table 3). At Centers 1 and 2, the frequencies of analgesic regimen assessment were documented for 41.3% and 28.1%, respectively (P = .06). Among symptomatic patients, 81.5% had an opioid or nonopioid analgesic listed in the Medications section in the electronic medical record at time of consultation.
Patients seen on the dedicated palliative RO service at Center 1 had an analgesic assessment documentation rate of 59.5%, whereas the patients not seen on a palliative RO service (ie, patients seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had an assessment documentation rate of 33.5% (P = .002; Figure 1). There was no significant difference between rates of analgesic regimen assessment between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (28.1% vs 35.9%, respectively; P = .27).
In patients seen at Center 1 only, those seen on the palliative RO service had a higher documentation rate of analgesic assessment compared with those seen by other services after implementation of the dedicated service (59.5% vs 38%, respectively; P = .018). Time period (after versus before 2011) was not significantly associated with the rate of documentation of analgesic assessment at either Center 1 (after vs before 2011: 44.4% vs 31%, P = .23) or Center 2 (31.4% vs 24.1%, P = .60).
Among patients with a PIR of ≥4, analgesic intervention was reported for 17.2% of patients within the entire cohort (20.8% at Center 1 and 0% at Center 2, P = .05). Among those with a PIR of ≥4, documentation of analgesic assessment noted in the History of the Present Illness section was associated with increased documentation of an analgesic intervention in the Assessment and Plan section (25% vs 7.3%; odds ratio [OR], 4.22; 95% confidence interval [CI], 1.1-16.0; P = .03).
Patients seen on the dedicated palliative RO service at Center 1 had a documented analgesic intervention rate of 31.6%, whereas the patients not seen on a palliative RO service (ie, those seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had a documented analgesic intervention rate of 9.2% (P = .01; Figure 2). There was no statistically significant difference between rates of documentation of an analgesic regimen intervention between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (0% vs 17.2%, respectively; P = .07).
Looking at only patients seen at Center 1, patients with a PIR of ≥4 seen on the dedicated palliative RO service had a nearly significant higher rate of documented analgesic interventions in the time period after implementation of the dedicate service (31.6% if seen on the dedicated service vs 12% if seen on a nondedicated service, P = .06).
Discussion
Multiple studies demonstrate the undertreatment of cancer pain in the outpatient setting.2,9,14,15 At 2 cancer centers, we found that about half of patients who present for consideration of palliative RT for bone metastases had a PIR of ≥4, yet only 17% of them had documentation of analgesic intervention as recommended by NCCN guidelines for cancer pain. Underlying this low rate of appropriate intervention may be the assumption of rapid pain relief by RT. However, RT often does not begin at time of consultation,16 and maximal pain relief may take days to weeks after commencement of RT.17 It is estimated that a quarter of all patients with cancer develop bone metastases during the course of their disease,12 and most of those patients suffer from pain. Thus, inherent delay in pain relief before, during, and after RT results in significant morbidity for the cancer patient population if adequate analgesic management is not provided.
The low rate of appropriate analgesic intervention at the time of RO consultation may also be related to the low incidence of proper analgesic assessment. In our cohort, 80% of symptomatic patients had an opioid or nonopioid analgesic listed in their medications within the electronic medical record at time of consultation, but only 38% had the analgesic regimen and/or its effectiveness described in the History of the Present Illness section of the record. Inattentiveness to analgesic type, dosing, and effectiveness during consultation may result in any inadequacies of the analgesic regimen going unnoticed. Consistent with this notion, we found that the rate of appropriate intervention for patients with a PIR of ≥4 was higher among patients who had analgesic regimen reported in the consultation note. Thus, interventions to implement routine review and documentation of the analgesic regimen, for example within the electronic medical record, may be one way to improve pain management.
Another possible reason for low rates of acute pain management within the RO clinic is low provider confidence in regard to analgesic management. In a recent national survey, 96% of radiation oncologists stated they were at least moderately confident with assessment of pain, yet only 77% were at least moderately confident with titrating opioids, and just 56% were at least moderately confident with rotating opioids.10 Educational interventions that improve providers’ facility with analgesic management may increase the frequency of pain management in the RO clinic.
Patients seen on the dedicated palliative RO service had significantly higher rates of documented analgesic regimen assessment and appropriate intervention during RO consultation, compared with patients seen at Center 2 and those not seen on the dedicated palliative RO service at Center 1. The improvements we observed in analgesic assessment and intervention at Center 1 for patients seen on the palliative RO service are likely owing to involvement of palliative RO and not to secular trends, because there were not similar improvements for patients at Center 1 who were not seen by the palliative RO service and those at Center 2, where there was no service.
At Center 1, the dedicated palliative RO service was created to provide specialized care to patients with metastatic disease undergoing palliative radiation. Within its structure, topics within palliative RO, such as technical aspects of palliative RT, symptom management, and communication are taught and reinforced in a case-based approach. Such palliative care awareness, integration, and education within RO achieved by the palliative RO service likely contribute to the improved rates of analgesic management we found in our study. We do note that rate of analgesic intervention in the palliative RO cohort, though higher than in the nonpalliative RO group, was still low, with only a third of patients receiving proper analgesic management. These findings highlight the importance of continued effort in increasing providers’ awareness of the need to assess pain and raise comfort with analgesic initiation and titration and of having dedicated palliative care clinicians embedded within the RO setting.
Since the data for this study was acquired, Center 2 has implemented a short palliative RO didactic course for residents, which improved their comfort levels in assessing analgesic effectiveness and intervening for uncontrolled pain.18 The impact of this intervention on clinical care will need to be evaluated, but the improved provider comfort levels may translate into better-quality care.
Limitations
An important limitation of this retrospective study is the reliance on the documentation provided in the consultation note for determining frequencies of analgesic regimen assessment and intervention. The actual rates of analgesic management that occurred in clinic may have been higher than reported in the documentation. However, such discrepancy in documentation of analgesic management would also be an area for quality improvement. Inadequate documentation limits the ability for proper follow-up of cancer pain as recommended by a joint guidance statement from the American Society of Clinical Oncology and the American Academy of Hospice and Palliative Medicine.19,20 The results of our study may also partly reflect a positive impact in documentation of analgesic management by a dedicated palliative RO service.
Given the multi-institutional nature of this study, it may be that general practice differences confound the impact of the dedicated palliative RO service at Center 1. However, with excluding Center 2, the dedicated service was still strongly associated with a higher rate of analgesic assessment within Center 1 and was almost significantly associated with appropriate analgesic intervention within Center 1.
We used a PIR of ≥4 as a threshold for appropriate analgesic regimen intervention because it is what is recommended by the NCCN guidelines. However, close attention should be paid to the impact that any amount of pain has on an individual patient. The functional, spiritual, and existential impact of pain is unique to each patient’s experience, and optimal symptom management should take those elements into account.
Conclusion
In conclusion, this study indicates that advanced cancer patient pain assessment and intervention according to NCCN cancer pain management guidelines is not common in the RO setting, and it is an area that should be targeted for quality improvement because of the positive implications for patient well-being. Pain assessment and intervention were greater in the setting of a dedicated structure for palliative care within RO, suggesting that the integration of palliative care within RO is a promising means of improving quality of pain management.
This work was presented at the 2016 ASCO Palliative Care in Oncology Symposium (September 9-10, 2016), where this work received a Conquer Cancer Foundation Merit Award.
Bone metastases are a common cause of pain in patients with advanced cancer, with about three-quarters of patients with bone metastases experiencing pain as the dominant symptom.1 Inadequately treated cancer pain impairs patient quality of life, and is associated with higher rates of depression, anxiety, and fatigue. Palliative radiotherapy (RT) is effective in alleviating pain from bone metastases.4 Local field external beam radiotherapy can provide some pain relief at the site of treated metastasis in 80%-90% of cases, with complete pain relief in 50%-60% of cases.5,6 However, maximal pain relief from RT is delayed, in some cases taking days to up to multiple weeks to attain.7,8 Therefore, optimal management of bone metastases pain may require the use of analgesics until RT takes adequate effect.
National Comprehensive Cancer Network (NCCN) Guidelines for Adult Cancer Pain (v. 2.2015) recommend that pain intensity rating (PIR; range, 0-10, where 0 denotes no pain and 10, worst pain imaginable) be used to quantify pain for all symptomatic patients. These guidelines also recommend the pain medication regimen be assessed for all symptomatic patients. For patients with moderate or severe pain (PIR of ≥4), NCCN guidelines recommend that analgesic regimen be intervened upon by alteration of the analgesic regimen (initiating, rotating, or titrating analgesic) or consideration of referral to pain/symptom management specialty.
Previous findings have demonstrated inadequate analgesic management for cancer pain,2,9 including within the radiation oncology (RO) clinic, suggesting that patients seen in consultation for palliative RT may experience uncontrolled pain for days to weeks before the onset of relief from RT. Possible reasons for inadequate acute pain intervention in the RO clinic may be provider discomfort with analgesic management and infrequent formal integration of palliative care within RO.10
Limited single-institution data from the few institutions with dedicated palliative RO services have suggested that these services improve the quality of palliative care delivery, as demonstrated by providers perceptions’ of the clinical impact of a dedicated service11 and the implementation of expedited palliative RT delivery for acute cancer pain.12,13 To our knowledge, the impact of a dedicated palliative RO service on analgesic management for cancer pain has not been assessed.
Here, we report how often patients with symptomatic bone metastases had assessments of existing analgesic regimens and interventions at RO consultation at 2 cancer centers. Center 1 had implemented a dedicated palliative RO service in 2011, consisting of rotating attending physicians and residents as well as dedicated palliative care trained nurse practitioners and a fellow, with the service structured around daily rounds,11 whereas Center 2 had not yet implemented a dedicated service. Using data from both centers, we assessed the impact of a palliative RO service on analgesic assessment and management in patients with bone metastases.
Methods
We searched our institutional databases for patients seen in RO consultation for bone metastases using ICD-9 code 198.5, and retrospectively reviewed consultation notes for those patients during June-July 2008, January-February 2010, January-February 2013, and June-July 2014. Those time periods were chosen as evenly spaced representative samples before and after implementation of a dedicated palliative RO service in 2011 at Center 1. Center 2 did not implement a dedicated palliative RO service in these time periods.
Within consultation notes, we recorded the following data from the History of the Present Illness section: symptoms from bone metastases (symptomatic was defined as any pain present); PIR (range, 0-10); and whether or not the preconsultation analgesic regimen was reported for symptomatic patients (including analgesic type, dosing, effectiveness, and adherence).
Documentation of the analgesic regimen in the history section of the notes was considered the proxy for analgesic regimen assessment at time of RO consultation. Analgesics within the Medications list, which were autopopulated in the consultation note by the electronic medical record, were recorded.
Whether or not pain was addressed with initiation or titration of analgesics for patients with a PIR of ≥4 was recorded from the Assessment and Plan portion of the notes, and that metric was considered the proxy for pain intervention. In addition, the case was coded as having had pain intervention if there was documentation of the patient declining recommended analgesic intervention, or the patient had been referred to a symptom management service for intervention (eg, referral to a specialty palliative care clinic), or there was recommendation for the patient to discuss uncontrolled pain with the original prescriber. A PIR of 4 was chosen as the threshold for analgesic intervention because at that level, NCCN guidelines for cancer pain state that the analgesic regimen should be titrated, whereas for a PIR of 3 or less, the guidelines recommend only consideration of titrating the analgesic. Only patients with a documented PIR were included in the pain intervention analysis.
Frequencies of analgesic assessment and analgesic intervention were compared using t tests (Wizard Pro, v1.8.5; Evan Miller, Chicago IL).
Results
A total of 271 patients with RO consultation notes were identified at the 2 centers within the 4 time periods (Table 1).
Among symptomatic patients, any component of the preconsultation analgesic regimen (including analgesic type, dosing, pain response, and adherence) was documented for 37.9% of the entire cohort at RO consultation (Table 3). At Centers 1 and 2, the frequencies of analgesic regimen assessment were documented for 41.3% and 28.1%, respectively (P = .06). Among symptomatic patients, 81.5% had an opioid or nonopioid analgesic listed in the Medications section in the electronic medical record at time of consultation.
Patients seen on the dedicated palliative RO service at Center 1 had an analgesic assessment documentation rate of 59.5%, whereas the patients not seen on a palliative RO service (ie, patients seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had an assessment documentation rate of 33.5% (P = .002; Figure 1). There was no significant difference between rates of analgesic regimen assessment between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (28.1% vs 35.9%, respectively; P = .27).
In patients seen at Center 1 only, those seen on the palliative RO service had a higher documentation rate of analgesic assessment compared with those seen by other services after implementation of the dedicated service (59.5% vs 38%, respectively; P = .018). Time period (after versus before 2011) was not significantly associated with the rate of documentation of analgesic assessment at either Center 1 (after vs before 2011: 44.4% vs 31%, P = .23) or Center 2 (31.4% vs 24.1%, P = .60).
Among patients with a PIR of ≥4, analgesic intervention was reported for 17.2% of patients within the entire cohort (20.8% at Center 1 and 0% at Center 2, P = .05). Among those with a PIR of ≥4, documentation of analgesic assessment noted in the History of the Present Illness section was associated with increased documentation of an analgesic intervention in the Assessment and Plan section (25% vs 7.3%; odds ratio [OR], 4.22; 95% confidence interval [CI], 1.1-16.0; P = .03).
Patients seen on the dedicated palliative RO service at Center 1 had a documented analgesic intervention rate of 31.6%, whereas the patients not seen on a palliative RO service (ie, those seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had a documented analgesic intervention rate of 9.2% (P = .01; Figure 2). There was no statistically significant difference between rates of documentation of an analgesic regimen intervention between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (0% vs 17.2%, respectively; P = .07).
Looking at only patients seen at Center 1, patients with a PIR of ≥4 seen on the dedicated palliative RO service had a nearly significant higher rate of documented analgesic interventions in the time period after implementation of the dedicate service (31.6% if seen on the dedicated service vs 12% if seen on a nondedicated service, P = .06).
Discussion
Multiple studies demonstrate the undertreatment of cancer pain in the outpatient setting.2,9,14,15 At 2 cancer centers, we found that about half of patients who present for consideration of palliative RT for bone metastases had a PIR of ≥4, yet only 17% of them had documentation of analgesic intervention as recommended by NCCN guidelines for cancer pain. Underlying this low rate of appropriate intervention may be the assumption of rapid pain relief by RT. However, RT often does not begin at time of consultation,16 and maximal pain relief may take days to weeks after commencement of RT.17 It is estimated that a quarter of all patients with cancer develop bone metastases during the course of their disease,12 and most of those patients suffer from pain. Thus, inherent delay in pain relief before, during, and after RT results in significant morbidity for the cancer patient population if adequate analgesic management is not provided.
The low rate of appropriate analgesic intervention at the time of RO consultation may also be related to the low incidence of proper analgesic assessment. In our cohort, 80% of symptomatic patients had an opioid or nonopioid analgesic listed in their medications within the electronic medical record at time of consultation, but only 38% had the analgesic regimen and/or its effectiveness described in the History of the Present Illness section of the record. Inattentiveness to analgesic type, dosing, and effectiveness during consultation may result in any inadequacies of the analgesic regimen going unnoticed. Consistent with this notion, we found that the rate of appropriate intervention for patients with a PIR of ≥4 was higher among patients who had analgesic regimen reported in the consultation note. Thus, interventions to implement routine review and documentation of the analgesic regimen, for example within the electronic medical record, may be one way to improve pain management.
Another possible reason for low rates of acute pain management within the RO clinic is low provider confidence in regard to analgesic management. In a recent national survey, 96% of radiation oncologists stated they were at least moderately confident with assessment of pain, yet only 77% were at least moderately confident with titrating opioids, and just 56% were at least moderately confident with rotating opioids.10 Educational interventions that improve providers’ facility with analgesic management may increase the frequency of pain management in the RO clinic.
Patients seen on the dedicated palliative RO service had significantly higher rates of documented analgesic regimen assessment and appropriate intervention during RO consultation, compared with patients seen at Center 2 and those not seen on the dedicated palliative RO service at Center 1. The improvements we observed in analgesic assessment and intervention at Center 1 for patients seen on the palliative RO service are likely owing to involvement of palliative RO and not to secular trends, because there were not similar improvements for patients at Center 1 who were not seen by the palliative RO service and those at Center 2, where there was no service.
At Center 1, the dedicated palliative RO service was created to provide specialized care to patients with metastatic disease undergoing palliative radiation. Within its structure, topics within palliative RO, such as technical aspects of palliative RT, symptom management, and communication are taught and reinforced in a case-based approach. Such palliative care awareness, integration, and education within RO achieved by the palliative RO service likely contribute to the improved rates of analgesic management we found in our study. We do note that rate of analgesic intervention in the palliative RO cohort, though higher than in the nonpalliative RO group, was still low, with only a third of patients receiving proper analgesic management. These findings highlight the importance of continued effort in increasing providers’ awareness of the need to assess pain and raise comfort with analgesic initiation and titration and of having dedicated palliative care clinicians embedded within the RO setting.
Since the data for this study was acquired, Center 2 has implemented a short palliative RO didactic course for residents, which improved their comfort levels in assessing analgesic effectiveness and intervening for uncontrolled pain.18 The impact of this intervention on clinical care will need to be evaluated, but the improved provider comfort levels may translate into better-quality care.
Limitations
An important limitation of this retrospective study is the reliance on the documentation provided in the consultation note for determining frequencies of analgesic regimen assessment and intervention. The actual rates of analgesic management that occurred in clinic may have been higher than reported in the documentation. However, such discrepancy in documentation of analgesic management would also be an area for quality improvement. Inadequate documentation limits the ability for proper follow-up of cancer pain as recommended by a joint guidance statement from the American Society of Clinical Oncology and the American Academy of Hospice and Palliative Medicine.19,20 The results of our study may also partly reflect a positive impact in documentation of analgesic management by a dedicated palliative RO service.
Given the multi-institutional nature of this study, it may be that general practice differences confound the impact of the dedicated palliative RO service at Center 1. However, with excluding Center 2, the dedicated service was still strongly associated with a higher rate of analgesic assessment within Center 1 and was almost significantly associated with appropriate analgesic intervention within Center 1.
We used a PIR of ≥4 as a threshold for appropriate analgesic regimen intervention because it is what is recommended by the NCCN guidelines. However, close attention should be paid to the impact that any amount of pain has on an individual patient. The functional, spiritual, and existential impact of pain is unique to each patient’s experience, and optimal symptom management should take those elements into account.
Conclusion
In conclusion, this study indicates that advanced cancer patient pain assessment and intervention according to NCCN cancer pain management guidelines is not common in the RO setting, and it is an area that should be targeted for quality improvement because of the positive implications for patient well-being. Pain assessment and intervention were greater in the setting of a dedicated structure for palliative care within RO, suggesting that the integration of palliative care within RO is a promising means of improving quality of pain management.
This work was presented at the 2016 ASCO Palliative Care in Oncology Symposium (September 9-10, 2016), where this work received a Conquer Cancer Foundation Merit Award.
1. Amichetti M, Orrù P, Madeddu A, et al. Comparative evaluation of two hypofractionated radiotherapy regimens for painful bone metastases. Tumori. 2004;90(1):91-95.
2. Vuong S, Pulenzas N, DeAngelis C, et al. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support Care Cancer. 2016;24(2):887-892.
3. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1-2):129-134.
4. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Sze WM, ed. Cochrane Database Syst Rev. 2004;(2):CD004721-CD004721.
5. Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44(1):1-18.
6. Kirou-Mauro A, Hird A, Wong J, et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys. 2008;71(4):1208-1212.
7. Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-S164.
8. McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol. 2017 Jul 1;3(7):953-959.
9. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149-4154.
10. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the united states: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
11. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
12. Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
13. de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer. 2009;17(7):757-762.
14. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-1991.
15. Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259-267.
16. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38-43.
17. Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients – current approaches. Breast Care (Basel). 2012;7(2):108-112.
18. Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2017;97(5):884-885.
19. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
20. Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12(9):e828-e838.
1. Amichetti M, Orrù P, Madeddu A, et al. Comparative evaluation of two hypofractionated radiotherapy regimens for painful bone metastases. Tumori. 2004;90(1):91-95.
2. Vuong S, Pulenzas N, DeAngelis C, et al. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support Care Cancer. 2016;24(2):887-892.
3. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1-2):129-134.
4. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Sze WM, ed. Cochrane Database Syst Rev. 2004;(2):CD004721-CD004721.
5. Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44(1):1-18.
6. Kirou-Mauro A, Hird A, Wong J, et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys. 2008;71(4):1208-1212.
7. Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-S164.
8. McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol. 2017 Jul 1;3(7):953-959.
9. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149-4154.
10. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the united states: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
11. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
12. Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
13. de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer. 2009;17(7):757-762.
14. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-1991.
15. Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259-267.
16. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38-43.
17. Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients – current approaches. Breast Care (Basel). 2012;7(2):108-112.
18. Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2017;97(5):884-885.
19. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
20. Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12(9):e828-e838.
Why iron can worsen malaria infection
Researchers believe they may have discovered why iron can sometimes worsen malaria infection.
By studying mice and samples from malaria patients, the researchers found that extra iron interferes with ferroportin, a protein that prevents a toxic buildup of iron in red blood cells and helps protect these cells against malaria infection.
The team also found a mutant form of ferroportin that occurs in African populations appears to protect against malaria.
These findings, published in Science, may help researchers and healthcare officials develop strategies to prevent and treat malaria.
“Our study helps solve a long-standing mystery,” said study author Tracey Rouault, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland.
“Iron supplements can sometimes worsen malaria infection and, conversely, iron deficiency can be protective in some cases. Our findings reveal that ferroportin—its function, as well as its regulation by iron levels—helps to explain these observations.”
The team found that red blood cells use ferroportin to remove excess iron, which malaria parasites consume as a food source.
In studies of mice, the researchers found the absence of ferroportin in erythroid cells caused iron to accumulate to toxic levels inside red blood cells. This, in turn, stressed the cells and shortened their life span.
In addition, the team found that mice lacking ferroportin had more parasites and worse outcomes when infected with malaria, compared to malaria-infected mice with intact ferroportin.
When they fed mice a high-iron diet, the researchers found that hepcidin regulated ferroportin in erythroid cells. The hormone, which is more abundant in high-iron environments, lowered ferroportin levels on erythroblasts and, subsequently, in red blood cells.
Additionally, hepcidin physically bound to ferroportin, preventing iron removal from the cells.
Next, the researchers sought to determine whether the ferroportin mutation Q248H, which is found in African populations, protects against malaria. This mutation shields ferroportin from hepcidin’s effects.
The team analyzed patient samples from 2 existing malaria studies. In one study, which enrolled children hospitalized for malaria in Zambia, 19.7% of the 66 patients had the Q248H mutation.
Children with the mutation tended to have fewer malarial parasites in their blood and tolerated their fevers for a longer period before coming to the hospital. While the trends were not statistically significant, they raise the possibility that Q248H reduces the iron available in the blood, therefore reducing the malaria parasite’s food source.
In the other study, which enrolled 290 pregnant women in Ghana, 8.6% had the Q248H mutation. Women with the mutation were significantly less likely to have pregnancy-associated malaria, in which parasites accumulate in the placenta and can cause adverse pregnancy and birth outcomes.
“Our findings suggest that Q248H does protect against malaria, possibly explaining why it occurs in people who live in malaria-endemic regions,” said study author De-Liang Zhang, PhD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
“Given the importance of iron metabolism overall, we will continue studying the ferroportin mutation and explore its other potential health effects.”
Researchers believe they may have discovered why iron can sometimes worsen malaria infection.
By studying mice and samples from malaria patients, the researchers found that extra iron interferes with ferroportin, a protein that prevents a toxic buildup of iron in red blood cells and helps protect these cells against malaria infection.
The team also found a mutant form of ferroportin that occurs in African populations appears to protect against malaria.
These findings, published in Science, may help researchers and healthcare officials develop strategies to prevent and treat malaria.
“Our study helps solve a long-standing mystery,” said study author Tracey Rouault, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland.
“Iron supplements can sometimes worsen malaria infection and, conversely, iron deficiency can be protective in some cases. Our findings reveal that ferroportin—its function, as well as its regulation by iron levels—helps to explain these observations.”
The team found that red blood cells use ferroportin to remove excess iron, which malaria parasites consume as a food source.
In studies of mice, the researchers found the absence of ferroportin in erythroid cells caused iron to accumulate to toxic levels inside red blood cells. This, in turn, stressed the cells and shortened their life span.
In addition, the team found that mice lacking ferroportin had more parasites and worse outcomes when infected with malaria, compared to malaria-infected mice with intact ferroportin.
When they fed mice a high-iron diet, the researchers found that hepcidin regulated ferroportin in erythroid cells. The hormone, which is more abundant in high-iron environments, lowered ferroportin levels on erythroblasts and, subsequently, in red blood cells.
Additionally, hepcidin physically bound to ferroportin, preventing iron removal from the cells.
Next, the researchers sought to determine whether the ferroportin mutation Q248H, which is found in African populations, protects against malaria. This mutation shields ferroportin from hepcidin’s effects.
The team analyzed patient samples from 2 existing malaria studies. In one study, which enrolled children hospitalized for malaria in Zambia, 19.7% of the 66 patients had the Q248H mutation.
Children with the mutation tended to have fewer malarial parasites in their blood and tolerated their fevers for a longer period before coming to the hospital. While the trends were not statistically significant, they raise the possibility that Q248H reduces the iron available in the blood, therefore reducing the malaria parasite’s food source.
In the other study, which enrolled 290 pregnant women in Ghana, 8.6% had the Q248H mutation. Women with the mutation were significantly less likely to have pregnancy-associated malaria, in which parasites accumulate in the placenta and can cause adverse pregnancy and birth outcomes.
“Our findings suggest that Q248H does protect against malaria, possibly explaining why it occurs in people who live in malaria-endemic regions,” said study author De-Liang Zhang, PhD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
“Given the importance of iron metabolism overall, we will continue studying the ferroportin mutation and explore its other potential health effects.”
Researchers believe they may have discovered why iron can sometimes worsen malaria infection.
By studying mice and samples from malaria patients, the researchers found that extra iron interferes with ferroportin, a protein that prevents a toxic buildup of iron in red blood cells and helps protect these cells against malaria infection.
The team also found a mutant form of ferroportin that occurs in African populations appears to protect against malaria.
These findings, published in Science, may help researchers and healthcare officials develop strategies to prevent and treat malaria.
“Our study helps solve a long-standing mystery,” said study author Tracey Rouault, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland.
“Iron supplements can sometimes worsen malaria infection and, conversely, iron deficiency can be protective in some cases. Our findings reveal that ferroportin—its function, as well as its regulation by iron levels—helps to explain these observations.”
The team found that red blood cells use ferroportin to remove excess iron, which malaria parasites consume as a food source.
In studies of mice, the researchers found the absence of ferroportin in erythroid cells caused iron to accumulate to toxic levels inside red blood cells. This, in turn, stressed the cells and shortened their life span.
In addition, the team found that mice lacking ferroportin had more parasites and worse outcomes when infected with malaria, compared to malaria-infected mice with intact ferroportin.
When they fed mice a high-iron diet, the researchers found that hepcidin regulated ferroportin in erythroid cells. The hormone, which is more abundant in high-iron environments, lowered ferroportin levels on erythroblasts and, subsequently, in red blood cells.
Additionally, hepcidin physically bound to ferroportin, preventing iron removal from the cells.
Next, the researchers sought to determine whether the ferroportin mutation Q248H, which is found in African populations, protects against malaria. This mutation shields ferroportin from hepcidin’s effects.
The team analyzed patient samples from 2 existing malaria studies. In one study, which enrolled children hospitalized for malaria in Zambia, 19.7% of the 66 patients had the Q248H mutation.
Children with the mutation tended to have fewer malarial parasites in their blood and tolerated their fevers for a longer period before coming to the hospital. While the trends were not statistically significant, they raise the possibility that Q248H reduces the iron available in the blood, therefore reducing the malaria parasite’s food source.
In the other study, which enrolled 290 pregnant women in Ghana, 8.6% had the Q248H mutation. Women with the mutation were significantly less likely to have pregnancy-associated malaria, in which parasites accumulate in the placenta and can cause adverse pregnancy and birth outcomes.
“Our findings suggest that Q248H does protect against malaria, possibly explaining why it occurs in people who live in malaria-endemic regions,” said study author De-Liang Zhang, PhD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
“Given the importance of iron metabolism overall, we will continue studying the ferroportin mutation and explore its other potential health effects.”
Ponatinib bests older TKIs against Ph+ALL
Every generation aspires to be better than its predecessors, and for the third-generation tyrosine kinase inhibitor ponatinib (Iclusig), that might just be true, investigators claim.
A retrospective analysis comparing clinical trial outcomes for patients with newly diagnosed acute lymphoblastic leukemia positive for the Philadelphia chromosome (Ph+ALL) suggests that first-line ponatinib offers modestly better complete molecular response (CMR) rates and 3-year overall survival (OS) than either first-generation TKIs such as imatinib (Gleevec) or second-generation agents such as dasatinib (Sprycel) and nilotinib (Tasigna).
“Although only 1 relevant study of ponatinib combined with chemotherapy in Ph+ALL has been reported and our ability to adjust for baseline patient characteristics was limited, the results suggest that ponatinib combined with chemotherapy might represent a more effective front-line treatment option than chemotherapy combined with an earlier generation TKI for patients with newly diagnosed Ph+ALL, including those who cannot or choose not to undergo [stem cell transplant],” wrote Elias Jabbour, MD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues.
They based their conclusions on a meta-regression analysis of 25 studies looking at first- or second-generation TKIs and one study of ponatinib as frontline therapy for patients with Ph+ALL. They described their results in Clinical Lymphoma, Myeloma & Leukemia.
The investigators created pooled estimates of outcomes from studies of earlier-generation TKIs plus combination chemotherapy using a random-effects meta-analysis method. For the sole ponatinib study – a single-arm trial of combination chemotherapy plus ponatinib – they used a binomial distribution method to calculate 95% confidence intervals (CI). The method essentially estimates the probability of success or failure of a repeated experiment.
They found that 79% of patients in the ponatinib trial achieved a CMR, compared with 34% of patients treated with earlier generation TKIs plus chemotherapy. This translates into an odds ratio (OR) for CMR with ponatinib of 6.09 (P = .034).
Two-year OS rates were 83% with ponatinib versus 58% for all patients treated with other TKIs. Although the OR (3.70) seemed to run in favor of ponatinib, the difference was not statistically significant (P = .062).
For 3-year OS, however, ponatinib was superior to the pooled data for the other TKIs, at 79% versus 50%, translating into an OR of 4.49 (P = .050).
The authors acknowledged that the study was limited by differences in treatment regimens and centers, and a limited ability to adjust for patient characteristics, due to the study’s reliance on only those covariates published across different studies. Head-to-head comparison trials are needed to confirm their results, they noted.
Nonetheless, “[w]e believe that the improved efficacy of ponatinib combined with chemotherapy for newly diagnosed Ph+ALL might prevent the need for allogeneic SCT,” they wrote.
Ariad Pharmaceuticals funded the study. Dr. Jabbour and three coauthors reported research funding from the company; other study authors reported employment or other financial relationships with Ariad or its parent company Takeda.
SOURCE: Jabbour E et al. Clin Lymphoma Myeloma Leuk. 2018 18(4):257-65.
Every generation aspires to be better than its predecessors, and for the third-generation tyrosine kinase inhibitor ponatinib (Iclusig), that might just be true, investigators claim.
A retrospective analysis comparing clinical trial outcomes for patients with newly diagnosed acute lymphoblastic leukemia positive for the Philadelphia chromosome (Ph+ALL) suggests that first-line ponatinib offers modestly better complete molecular response (CMR) rates and 3-year overall survival (OS) than either first-generation TKIs such as imatinib (Gleevec) or second-generation agents such as dasatinib (Sprycel) and nilotinib (Tasigna).
“Although only 1 relevant study of ponatinib combined with chemotherapy in Ph+ALL has been reported and our ability to adjust for baseline patient characteristics was limited, the results suggest that ponatinib combined with chemotherapy might represent a more effective front-line treatment option than chemotherapy combined with an earlier generation TKI for patients with newly diagnosed Ph+ALL, including those who cannot or choose not to undergo [stem cell transplant],” wrote Elias Jabbour, MD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues.
They based their conclusions on a meta-regression analysis of 25 studies looking at first- or second-generation TKIs and one study of ponatinib as frontline therapy for patients with Ph+ALL. They described their results in Clinical Lymphoma, Myeloma & Leukemia.
The investigators created pooled estimates of outcomes from studies of earlier-generation TKIs plus combination chemotherapy using a random-effects meta-analysis method. For the sole ponatinib study – a single-arm trial of combination chemotherapy plus ponatinib – they used a binomial distribution method to calculate 95% confidence intervals (CI). The method essentially estimates the probability of success or failure of a repeated experiment.
They found that 79% of patients in the ponatinib trial achieved a CMR, compared with 34% of patients treated with earlier generation TKIs plus chemotherapy. This translates into an odds ratio (OR) for CMR with ponatinib of 6.09 (P = .034).
Two-year OS rates were 83% with ponatinib versus 58% for all patients treated with other TKIs. Although the OR (3.70) seemed to run in favor of ponatinib, the difference was not statistically significant (P = .062).
For 3-year OS, however, ponatinib was superior to the pooled data for the other TKIs, at 79% versus 50%, translating into an OR of 4.49 (P = .050).
The authors acknowledged that the study was limited by differences in treatment regimens and centers, and a limited ability to adjust for patient characteristics, due to the study’s reliance on only those covariates published across different studies. Head-to-head comparison trials are needed to confirm their results, they noted.
Nonetheless, “[w]e believe that the improved efficacy of ponatinib combined with chemotherapy for newly diagnosed Ph+ALL might prevent the need for allogeneic SCT,” they wrote.
Ariad Pharmaceuticals funded the study. Dr. Jabbour and three coauthors reported research funding from the company; other study authors reported employment or other financial relationships with Ariad or its parent company Takeda.
SOURCE: Jabbour E et al. Clin Lymphoma Myeloma Leuk. 2018 18(4):257-65.
Every generation aspires to be better than its predecessors, and for the third-generation tyrosine kinase inhibitor ponatinib (Iclusig), that might just be true, investigators claim.
A retrospective analysis comparing clinical trial outcomes for patients with newly diagnosed acute lymphoblastic leukemia positive for the Philadelphia chromosome (Ph+ALL) suggests that first-line ponatinib offers modestly better complete molecular response (CMR) rates and 3-year overall survival (OS) than either first-generation TKIs such as imatinib (Gleevec) or second-generation agents such as dasatinib (Sprycel) and nilotinib (Tasigna).
“Although only 1 relevant study of ponatinib combined with chemotherapy in Ph+ALL has been reported and our ability to adjust for baseline patient characteristics was limited, the results suggest that ponatinib combined with chemotherapy might represent a more effective front-line treatment option than chemotherapy combined with an earlier generation TKI for patients with newly diagnosed Ph+ALL, including those who cannot or choose not to undergo [stem cell transplant],” wrote Elias Jabbour, MD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues.
They based their conclusions on a meta-regression analysis of 25 studies looking at first- or second-generation TKIs and one study of ponatinib as frontline therapy for patients with Ph+ALL. They described their results in Clinical Lymphoma, Myeloma & Leukemia.
The investigators created pooled estimates of outcomes from studies of earlier-generation TKIs plus combination chemotherapy using a random-effects meta-analysis method. For the sole ponatinib study – a single-arm trial of combination chemotherapy plus ponatinib – they used a binomial distribution method to calculate 95% confidence intervals (CI). The method essentially estimates the probability of success or failure of a repeated experiment.
They found that 79% of patients in the ponatinib trial achieved a CMR, compared with 34% of patients treated with earlier generation TKIs plus chemotherapy. This translates into an odds ratio (OR) for CMR with ponatinib of 6.09 (P = .034).
Two-year OS rates were 83% with ponatinib versus 58% for all patients treated with other TKIs. Although the OR (3.70) seemed to run in favor of ponatinib, the difference was not statistically significant (P = .062).
For 3-year OS, however, ponatinib was superior to the pooled data for the other TKIs, at 79% versus 50%, translating into an OR of 4.49 (P = .050).
The authors acknowledged that the study was limited by differences in treatment regimens and centers, and a limited ability to adjust for patient characteristics, due to the study’s reliance on only those covariates published across different studies. Head-to-head comparison trials are needed to confirm their results, they noted.
Nonetheless, “[w]e believe that the improved efficacy of ponatinib combined with chemotherapy for newly diagnosed Ph+ALL might prevent the need for allogeneic SCT,” they wrote.
Ariad Pharmaceuticals funded the study. Dr. Jabbour and three coauthors reported research funding from the company; other study authors reported employment or other financial relationships with Ariad or its parent company Takeda.
SOURCE: Jabbour E et al. Clin Lymphoma Myeloma Leuk. 2018 18(4):257-65.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: Complete molecular response rates and 3-year overall survival were better with ponatinib in combination with chemotherapy.
Study details: Meta-regression analysis of 26 studies of TKIs in combination with chemotherapy as first-line therapy for patients with Ph+ALL.
Disclosures: Ariad Pharmaceuticals funded the study. Dr. Jabbour and three coauthors reported research funding from the company; other study authors reported employment or other financial relationships with Ariad or its parent company, Takeda.
Source: Jabbour E et al. Clin Lymphoma Myeloma Leuk. 2018 18(4):257-65.
CtDNA agrees (mostly) with tissue analysis in mCRC
For identifying patients with metastatic colorectal cancer who might benefit from therapy with drugs targeted against the epidermal growth factor receptor (EGFR), analysis of plasma for circulating tumor DNA – aka “liquid biopsy” – is about as capable as and considerably faster than tissue analysis of RAS mutational status, investigators contend.
Among 412 chemotherapy-naive patients with metastatic colorectal cancer (mCRC) for whom both plasma and tissue samples were available, the kappa coefficient (a measure of acceptable concordance) between ctDNA RAS mutation detection and tissue-based analysis was 0.71, just over the minimum 0.7, and the accuracy of the liquid biopsy sampling was 85.2%, reported Pierre Laurent-Puig, MD, PhD, of Sorbonne University in Paris, and his colleagues.
“[E]ven if our results are limited to the techniques used with their analytic sensitivity, we show here for the first time in a prospective study an excellent concordance between plasma and tumor RAS mutation status in metastatic colorectal cancer patients, especially those with liver metastases. These results validate the routine use of plasma RAS analysis in patients with colorectal cancer and liver metastases,” they wrote. The report was published in Annals of Oncology.
The investigators found that ctDNA was more likely to yield inconclusive results in patients without liver metastases. In patients with liver metastases, the accuracy of the liquid biopsy using next-generation sequencing (NGS) alone to detect RAS mutations was 93.5%, and when detection of methylated biomarkers was added in, the accuracy was 97%.
In the AGEO RASANC prospective multicenter study, the investigators prospectively collected plasma samples from patients with mCRC and sent them to a central lab for analysis by NGS with the colon/lung cancer V2 Ampliseq panel and with digital polymerase chain reaction dPCR for genes associated with DNA methylation (WIF1 and NPY).
Matched tissue samples from the same patients were analyzed locally according to routine practice.
As noted before, the kappa coefficient was 0.71, indicating that agreement between the tests was at least 70% better than by chance alone.
In 329 patients who had detectable ctDNA, defined as at least one mutation or one methylated biomarker, the kappa coefficient rose to 89% and the accuracy to 94.8%.
Also as noted, in the 293 patients with liver metastases, the accuracy improved to close to 100% with both NGS and methylated biomarker detection.
“Based solely on the plasma analysis using our prespecified analysis, 14 patients would have received ineffective and potentially deleterious anti-EGFR therapy. All were considered as truly RAS wild-type in their plasma because of the presence of other mutations detected by NGS and/or a positive methylation assay,” the researchers wrote.
The lack of sensitivity of ctDNA in these patients may have been due to the fact that the fraction of mutated alleles in these patients was lower than in patients for whom both plasma and tissue analysis were negative for RAS mutations. All of these patients had early metastases, which could explain why the sensitivity of the plasma assay was lower than in patients with advanced-stage disease, they said.
“On the contrary detection of RAS mutations in plasma but not in tumor cells could exclude some patients from potentially effective anti-EGFR therapy,” they added. They identified eight patients with plasma-positive, tissue-negative discordance, which could be due to errors in tissue sampling due to heterogeneity of mutations within tissues, or to a lack of sensitivity of RAS mutation detection in tissues.
The trial was sponsored by AGEO and supported by a grant from Merck Serono. Dr. Laurent-Puig disclosed grants from AGEO and personal fees from Merck Serono and other companies. Multiple coauthors reported fees from Merck Serono and others.
SOURCE: Laurent-Puig P et al. Ann Oncol. 2018 Feb 9. doi: 10.1093/annonc/mdy061/4846852.
Regarding next steps, several studies have now shown a high correlation between RAS testing in plasma versus tissue, and a better understanding of the technical and clinical characteristics of RAS ctDNA testing has helped in increasing the accuracy to almost 100%. With that, RAS ctDNA testing should be ready for its clinical use.
However, as clinicians, we need to remember that the goal of RAS testing is to select patients that will benefit from anti-EGFR therapy, and thus, a threshold of RAS detection in ctDNA that is clinically meaningful needs to be established in future studies.
In summary, Laurent-Puig et al. confirm the high correlation between tissue and ctDNA RAS testing, but more importantly they help by optimizing the limits for its clinical implementation. ctDNA RAS testing is ready for clinical use, but we now know that it may not be a good tool for a minority of patients with specific clinical characteristics.
Clara Montagut, MD, is with Hospital del Mar Medical Research Institute in Barcelona. Dana Tsui, PhD, and Luis Alberto Diaz Jr., MD, are with Memorial Sloan Kettering Cancer Center in New York. Dr. Montagut is an advisory consultant to Merck Serono and other companies. Dr. Diaz is a founder and shareholder of PapGene and Personal Genome Diagnostics and a consultant for Merck and others. Dr. Tsui is a former consultant of Inivata Ltd, and a contributor to patents on cell-free DNA detection methodologies, and may receive royalties related to the licenses of those patents to Inivata Ltd. Their remarks are adapted from an editorial (Ann Oncol. 2018 Feb 9. doi: 10.1093/annonc/mdy091).
Regarding next steps, several studies have now shown a high correlation between RAS testing in plasma versus tissue, and a better understanding of the technical and clinical characteristics of RAS ctDNA testing has helped in increasing the accuracy to almost 100%. With that, RAS ctDNA testing should be ready for its clinical use.
However, as clinicians, we need to remember that the goal of RAS testing is to select patients that will benefit from anti-EGFR therapy, and thus, a threshold of RAS detection in ctDNA that is clinically meaningful needs to be established in future studies.
In summary, Laurent-Puig et al. confirm the high correlation between tissue and ctDNA RAS testing, but more importantly they help by optimizing the limits for its clinical implementation. ctDNA RAS testing is ready for clinical use, but we now know that it may not be a good tool for a minority of patients with specific clinical characteristics.
Clara Montagut, MD, is with Hospital del Mar Medical Research Institute in Barcelona. Dana Tsui, PhD, and Luis Alberto Diaz Jr., MD, are with Memorial Sloan Kettering Cancer Center in New York. Dr. Montagut is an advisory consultant to Merck Serono and other companies. Dr. Diaz is a founder and shareholder of PapGene and Personal Genome Diagnostics and a consultant for Merck and others. Dr. Tsui is a former consultant of Inivata Ltd, and a contributor to patents on cell-free DNA detection methodologies, and may receive royalties related to the licenses of those patents to Inivata Ltd. Their remarks are adapted from an editorial (Ann Oncol. 2018 Feb 9. doi: 10.1093/annonc/mdy091).
Regarding next steps, several studies have now shown a high correlation between RAS testing in plasma versus tissue, and a better understanding of the technical and clinical characteristics of RAS ctDNA testing has helped in increasing the accuracy to almost 100%. With that, RAS ctDNA testing should be ready for its clinical use.
However, as clinicians, we need to remember that the goal of RAS testing is to select patients that will benefit from anti-EGFR therapy, and thus, a threshold of RAS detection in ctDNA that is clinically meaningful needs to be established in future studies.
In summary, Laurent-Puig et al. confirm the high correlation between tissue and ctDNA RAS testing, but more importantly they help by optimizing the limits for its clinical implementation. ctDNA RAS testing is ready for clinical use, but we now know that it may not be a good tool for a minority of patients with specific clinical characteristics.
Clara Montagut, MD, is with Hospital del Mar Medical Research Institute in Barcelona. Dana Tsui, PhD, and Luis Alberto Diaz Jr., MD, are with Memorial Sloan Kettering Cancer Center in New York. Dr. Montagut is an advisory consultant to Merck Serono and other companies. Dr. Diaz is a founder and shareholder of PapGene and Personal Genome Diagnostics and a consultant for Merck and others. Dr. Tsui is a former consultant of Inivata Ltd, and a contributor to patents on cell-free DNA detection methodologies, and may receive royalties related to the licenses of those patents to Inivata Ltd. Their remarks are adapted from an editorial (Ann Oncol. 2018 Feb 9. doi: 10.1093/annonc/mdy091).
For identifying patients with metastatic colorectal cancer who might benefit from therapy with drugs targeted against the epidermal growth factor receptor (EGFR), analysis of plasma for circulating tumor DNA – aka “liquid biopsy” – is about as capable as and considerably faster than tissue analysis of RAS mutational status, investigators contend.
Among 412 chemotherapy-naive patients with metastatic colorectal cancer (mCRC) for whom both plasma and tissue samples were available, the kappa coefficient (a measure of acceptable concordance) between ctDNA RAS mutation detection and tissue-based analysis was 0.71, just over the minimum 0.7, and the accuracy of the liquid biopsy sampling was 85.2%, reported Pierre Laurent-Puig, MD, PhD, of Sorbonne University in Paris, and his colleagues.
“[E]ven if our results are limited to the techniques used with their analytic sensitivity, we show here for the first time in a prospective study an excellent concordance between plasma and tumor RAS mutation status in metastatic colorectal cancer patients, especially those with liver metastases. These results validate the routine use of plasma RAS analysis in patients with colorectal cancer and liver metastases,” they wrote. The report was published in Annals of Oncology.
The investigators found that ctDNA was more likely to yield inconclusive results in patients without liver metastases. In patients with liver metastases, the accuracy of the liquid biopsy using next-generation sequencing (NGS) alone to detect RAS mutations was 93.5%, and when detection of methylated biomarkers was added in, the accuracy was 97%.
In the AGEO RASANC prospective multicenter study, the investigators prospectively collected plasma samples from patients with mCRC and sent them to a central lab for analysis by NGS with the colon/lung cancer V2 Ampliseq panel and with digital polymerase chain reaction dPCR for genes associated with DNA methylation (WIF1 and NPY).
Matched tissue samples from the same patients were analyzed locally according to routine practice.
As noted before, the kappa coefficient was 0.71, indicating that agreement between the tests was at least 70% better than by chance alone.
In 329 patients who had detectable ctDNA, defined as at least one mutation or one methylated biomarker, the kappa coefficient rose to 89% and the accuracy to 94.8%.
Also as noted, in the 293 patients with liver metastases, the accuracy improved to close to 100% with both NGS and methylated biomarker detection.
“Based solely on the plasma analysis using our prespecified analysis, 14 patients would have received ineffective and potentially deleterious anti-EGFR therapy. All were considered as truly RAS wild-type in their plasma because of the presence of other mutations detected by NGS and/or a positive methylation assay,” the researchers wrote.
The lack of sensitivity of ctDNA in these patients may have been due to the fact that the fraction of mutated alleles in these patients was lower than in patients for whom both plasma and tissue analysis were negative for RAS mutations. All of these patients had early metastases, which could explain why the sensitivity of the plasma assay was lower than in patients with advanced-stage disease, they said.
“On the contrary detection of RAS mutations in plasma but not in tumor cells could exclude some patients from potentially effective anti-EGFR therapy,” they added. They identified eight patients with plasma-positive, tissue-negative discordance, which could be due to errors in tissue sampling due to heterogeneity of mutations within tissues, or to a lack of sensitivity of RAS mutation detection in tissues.
The trial was sponsored by AGEO and supported by a grant from Merck Serono. Dr. Laurent-Puig disclosed grants from AGEO and personal fees from Merck Serono and other companies. Multiple coauthors reported fees from Merck Serono and others.
SOURCE: Laurent-Puig P et al. Ann Oncol. 2018 Feb 9. doi: 10.1093/annonc/mdy061/4846852.
For identifying patients with metastatic colorectal cancer who might benefit from therapy with drugs targeted against the epidermal growth factor receptor (EGFR), analysis of plasma for circulating tumor DNA – aka “liquid biopsy” – is about as capable as and considerably faster than tissue analysis of RAS mutational status, investigators contend.
Among 412 chemotherapy-naive patients with metastatic colorectal cancer (mCRC) for whom both plasma and tissue samples were available, the kappa coefficient (a measure of acceptable concordance) between ctDNA RAS mutation detection and tissue-based analysis was 0.71, just over the minimum 0.7, and the accuracy of the liquid biopsy sampling was 85.2%, reported Pierre Laurent-Puig, MD, PhD, of Sorbonne University in Paris, and his colleagues.
“[E]ven if our results are limited to the techniques used with their analytic sensitivity, we show here for the first time in a prospective study an excellent concordance between plasma and tumor RAS mutation status in metastatic colorectal cancer patients, especially those with liver metastases. These results validate the routine use of plasma RAS analysis in patients with colorectal cancer and liver metastases,” they wrote. The report was published in Annals of Oncology.
The investigators found that ctDNA was more likely to yield inconclusive results in patients without liver metastases. In patients with liver metastases, the accuracy of the liquid biopsy using next-generation sequencing (NGS) alone to detect RAS mutations was 93.5%, and when detection of methylated biomarkers was added in, the accuracy was 97%.
In the AGEO RASANC prospective multicenter study, the investigators prospectively collected plasma samples from patients with mCRC and sent them to a central lab for analysis by NGS with the colon/lung cancer V2 Ampliseq panel and with digital polymerase chain reaction dPCR for genes associated with DNA methylation (WIF1 and NPY).
Matched tissue samples from the same patients were analyzed locally according to routine practice.
As noted before, the kappa coefficient was 0.71, indicating that agreement between the tests was at least 70% better than by chance alone.
In 329 patients who had detectable ctDNA, defined as at least one mutation or one methylated biomarker, the kappa coefficient rose to 89% and the accuracy to 94.8%.
Also as noted, in the 293 patients with liver metastases, the accuracy improved to close to 100% with both NGS and methylated biomarker detection.
“Based solely on the plasma analysis using our prespecified analysis, 14 patients would have received ineffective and potentially deleterious anti-EGFR therapy. All were considered as truly RAS wild-type in their plasma because of the presence of other mutations detected by NGS and/or a positive methylation assay,” the researchers wrote.
The lack of sensitivity of ctDNA in these patients may have been due to the fact that the fraction of mutated alleles in these patients was lower than in patients for whom both plasma and tissue analysis were negative for RAS mutations. All of these patients had early metastases, which could explain why the sensitivity of the plasma assay was lower than in patients with advanced-stage disease, they said.
“On the contrary detection of RAS mutations in plasma but not in tumor cells could exclude some patients from potentially effective anti-EGFR therapy,” they added. They identified eight patients with plasma-positive, tissue-negative discordance, which could be due to errors in tissue sampling due to heterogeneity of mutations within tissues, or to a lack of sensitivity of RAS mutation detection in tissues.
The trial was sponsored by AGEO and supported by a grant from Merck Serono. Dr. Laurent-Puig disclosed grants from AGEO and personal fees from Merck Serono and other companies. Multiple coauthors reported fees from Merck Serono and others.
SOURCE: Laurent-Puig P et al. Ann Oncol. 2018 Feb 9. doi: 10.1093/annonc/mdy061/4846852.
FROM ANNALS OF ONCOLOGY
Key clinical point: Circulating tumor DNA (ctDNA) may offer a more rapid method for accurately determining RAS mutational status.
Major finding: In patients with colorectal cancer with liver metastases, concordance between ctDNA and tissues samples was high.
Study details: Prospective study comparing RAS mutational analysis results with plasma ctDNA and tissue analysis in 412 chemotherapy-naive patients with metastatic colorectal cancer.
Disclosures: The trial was sponsored by AGEO and supported by a grant from Merck Serono. Dr. Laurent-Puig disclosed grants from AGEO and personal fees from Merck Serono and other companies. Multiple coauthors reported fees from Merck Serono and others.
Source: Laurent-Puig P et al. Ann Oncol. 2018 Feb 9. doi: 10.1093/annonc/mdy061/4846852.
No increased complication risk with delaying resection for LARC
CHICAGO – Delaying surgery after neoadjuvant therapy for locally advanced rectal cancer for up to 12 weeks does not seem to impact complication rates compared to surgery at 8 weeks or earlier, findings that run counter to results from a major European clinical trial reported in 2016, investigators reported at the Society of Surgical Oncology Annual Cancer Symposium.
“There’s an increasing trend toward delayed surgery beyond eight to 12 weeks after neoadjuvant therapy (NT) for locally advanced rectal cancer (LARC),” said Campbell Roxburgh, FRCS, PhD, of the University of Glasgow in Scotland. “Although we saw an increase in all complications in patients who had surgery beyond 12 weeks, there were no increases in surgical site complications, grade 3-5 complications, or anastomotic leaks. Before 12 weeks we did not observe increases in any type of complication where surgery was performed prior to or after 8 weeks.”
The study involved 798 patients who had received NT for LARC from June 2009 to March 2014 at Memorial Sloan Kettering Cancer Center in New York. The vast majority – 76% (607) – had rectal resection within 16 weeks of completing NT. Among them, 52% (317) had surgery 5-8 weeks after NT, 38% (229) had surgery at 8-12 weeks post-NT, and 10% (61) had surgery 12-16 weeks after completing NT. Those who had surgery beyond 16 weeks mostly had it deferred because they were undergoing nonoperative management in the case of complete clinical response to treatment or had a comorbidity that prevented earlier surgery, Dr. Roxburgh said.
The complication rate was 42.3% among the patients who had surgery up to 16 weeks after NT, Dr. Roxburgh said. The most common complication was surgical site infection (SSI) in 16.6% (101), followed by a grade 3-5 complication in 10.5% (64) and anastomotic leak in 6.4% (39). Overall complication rates among the two groups that had surgery within 12 weeks were not statistically different from the overall complication rate, Dr. Roxburgh said: 42.5% (138) in the 5- to 8-week group; and 36.7% (84) in the 8- to 12-week group. The 12- to16-week group had a complication rate of 56% (34, P = .022).
Dr. Roxburgh noted that the idea of delaying surgery beyond 8 weeks after NT has been a subject of debate, and that these findings run counter to those reported in the GRECCAR-6 trial (J Clin Oncol. 2016;34:3773-80). That study compared groups that had surgery for rectal cancer at 7 and 11 weeks after neoadjuvant radiochemotherapy and found that those in the 11-week group had higher rates of complications.
Dr. Roxburgh also reported on an analysis of the 12- to 16-week subgroup that found the highest complication rates were among those who had low anterior resection (53% vs. 41% in the 5- to 8-week group and 31% in the 8- to 12-week population), and patients who had a poor treatment response (no T-downstaging, 66% vs. 44% and 33%, respectively). Age, pretreatment and posttreatment TNM stages, surgical approach (open or minimally invasive), and year of treatment did not factor in complication rates in the subgroup analysis, Dr. Roxburgh noted.
The univariate regression analysis determined a trend toward increased rates of all complications in the 12- to 16-week group (P = .081). But the multivariate analysis did not find timing of surgery to be an independent risk factor for all complications, Dr. Roxburgh said. “We believe other factors, including tumor location, the type of NT, operative approach, and treatment response, however, were more important on multivariate analysis,” he said. For example, open surgery had an odds ratio of 1.7 (P = .004).
During the discussion, Dr. Roxburgh was asked what would be the optimal timing for resection after NT in LARC. “I would recommend posttreatment assessment with MRI and proctoscopy between 8 to 12 weeks and in the case of residual tumor or incomplete response to treatment, scheduling surgery at that time,” he said.
Dr. Roxburgh and coauthors reported having no financial disclosures.
SOURCE: Roxburgh C, et al. Society of Surgical Oncology Annual Cancer Symposium Abstract No. 3.
CHICAGO – Delaying surgery after neoadjuvant therapy for locally advanced rectal cancer for up to 12 weeks does not seem to impact complication rates compared to surgery at 8 weeks or earlier, findings that run counter to results from a major European clinical trial reported in 2016, investigators reported at the Society of Surgical Oncology Annual Cancer Symposium.
“There’s an increasing trend toward delayed surgery beyond eight to 12 weeks after neoadjuvant therapy (NT) for locally advanced rectal cancer (LARC),” said Campbell Roxburgh, FRCS, PhD, of the University of Glasgow in Scotland. “Although we saw an increase in all complications in patients who had surgery beyond 12 weeks, there were no increases in surgical site complications, grade 3-5 complications, or anastomotic leaks. Before 12 weeks we did not observe increases in any type of complication where surgery was performed prior to or after 8 weeks.”
The study involved 798 patients who had received NT for LARC from June 2009 to March 2014 at Memorial Sloan Kettering Cancer Center in New York. The vast majority – 76% (607) – had rectal resection within 16 weeks of completing NT. Among them, 52% (317) had surgery 5-8 weeks after NT, 38% (229) had surgery at 8-12 weeks post-NT, and 10% (61) had surgery 12-16 weeks after completing NT. Those who had surgery beyond 16 weeks mostly had it deferred because they were undergoing nonoperative management in the case of complete clinical response to treatment or had a comorbidity that prevented earlier surgery, Dr. Roxburgh said.
The complication rate was 42.3% among the patients who had surgery up to 16 weeks after NT, Dr. Roxburgh said. The most common complication was surgical site infection (SSI) in 16.6% (101), followed by a grade 3-5 complication in 10.5% (64) and anastomotic leak in 6.4% (39). Overall complication rates among the two groups that had surgery within 12 weeks were not statistically different from the overall complication rate, Dr. Roxburgh said: 42.5% (138) in the 5- to 8-week group; and 36.7% (84) in the 8- to 12-week group. The 12- to16-week group had a complication rate of 56% (34, P = .022).
Dr. Roxburgh noted that the idea of delaying surgery beyond 8 weeks after NT has been a subject of debate, and that these findings run counter to those reported in the GRECCAR-6 trial (J Clin Oncol. 2016;34:3773-80). That study compared groups that had surgery for rectal cancer at 7 and 11 weeks after neoadjuvant radiochemotherapy and found that those in the 11-week group had higher rates of complications.
Dr. Roxburgh also reported on an analysis of the 12- to 16-week subgroup that found the highest complication rates were among those who had low anterior resection (53% vs. 41% in the 5- to 8-week group and 31% in the 8- to 12-week population), and patients who had a poor treatment response (no T-downstaging, 66% vs. 44% and 33%, respectively). Age, pretreatment and posttreatment TNM stages, surgical approach (open or minimally invasive), and year of treatment did not factor in complication rates in the subgroup analysis, Dr. Roxburgh noted.
The univariate regression analysis determined a trend toward increased rates of all complications in the 12- to 16-week group (P = .081). But the multivariate analysis did not find timing of surgery to be an independent risk factor for all complications, Dr. Roxburgh said. “We believe other factors, including tumor location, the type of NT, operative approach, and treatment response, however, were more important on multivariate analysis,” he said. For example, open surgery had an odds ratio of 1.7 (P = .004).
During the discussion, Dr. Roxburgh was asked what would be the optimal timing for resection after NT in LARC. “I would recommend posttreatment assessment with MRI and proctoscopy between 8 to 12 weeks and in the case of residual tumor or incomplete response to treatment, scheduling surgery at that time,” he said.
Dr. Roxburgh and coauthors reported having no financial disclosures.
SOURCE: Roxburgh C, et al. Society of Surgical Oncology Annual Cancer Symposium Abstract No. 3.
CHICAGO – Delaying surgery after neoadjuvant therapy for locally advanced rectal cancer for up to 12 weeks does not seem to impact complication rates compared to surgery at 8 weeks or earlier, findings that run counter to results from a major European clinical trial reported in 2016, investigators reported at the Society of Surgical Oncology Annual Cancer Symposium.
“There’s an increasing trend toward delayed surgery beyond eight to 12 weeks after neoadjuvant therapy (NT) for locally advanced rectal cancer (LARC),” said Campbell Roxburgh, FRCS, PhD, of the University of Glasgow in Scotland. “Although we saw an increase in all complications in patients who had surgery beyond 12 weeks, there were no increases in surgical site complications, grade 3-5 complications, or anastomotic leaks. Before 12 weeks we did not observe increases in any type of complication where surgery was performed prior to or after 8 weeks.”
The study involved 798 patients who had received NT for LARC from June 2009 to March 2014 at Memorial Sloan Kettering Cancer Center in New York. The vast majority – 76% (607) – had rectal resection within 16 weeks of completing NT. Among them, 52% (317) had surgery 5-8 weeks after NT, 38% (229) had surgery at 8-12 weeks post-NT, and 10% (61) had surgery 12-16 weeks after completing NT. Those who had surgery beyond 16 weeks mostly had it deferred because they were undergoing nonoperative management in the case of complete clinical response to treatment or had a comorbidity that prevented earlier surgery, Dr. Roxburgh said.
The complication rate was 42.3% among the patients who had surgery up to 16 weeks after NT, Dr. Roxburgh said. The most common complication was surgical site infection (SSI) in 16.6% (101), followed by a grade 3-5 complication in 10.5% (64) and anastomotic leak in 6.4% (39). Overall complication rates among the two groups that had surgery within 12 weeks were not statistically different from the overall complication rate, Dr. Roxburgh said: 42.5% (138) in the 5- to 8-week group; and 36.7% (84) in the 8- to 12-week group. The 12- to16-week group had a complication rate of 56% (34, P = .022).
Dr. Roxburgh noted that the idea of delaying surgery beyond 8 weeks after NT has been a subject of debate, and that these findings run counter to those reported in the GRECCAR-6 trial (J Clin Oncol. 2016;34:3773-80). That study compared groups that had surgery for rectal cancer at 7 and 11 weeks after neoadjuvant radiochemotherapy and found that those in the 11-week group had higher rates of complications.
Dr. Roxburgh also reported on an analysis of the 12- to 16-week subgroup that found the highest complication rates were among those who had low anterior resection (53% vs. 41% in the 5- to 8-week group and 31% in the 8- to 12-week population), and patients who had a poor treatment response (no T-downstaging, 66% vs. 44% and 33%, respectively). Age, pretreatment and posttreatment TNM stages, surgical approach (open or minimally invasive), and year of treatment did not factor in complication rates in the subgroup analysis, Dr. Roxburgh noted.
The univariate regression analysis determined a trend toward increased rates of all complications in the 12- to 16-week group (P = .081). But the multivariate analysis did not find timing of surgery to be an independent risk factor for all complications, Dr. Roxburgh said. “We believe other factors, including tumor location, the type of NT, operative approach, and treatment response, however, were more important on multivariate analysis,” he said. For example, open surgery had an odds ratio of 1.7 (P = .004).
During the discussion, Dr. Roxburgh was asked what would be the optimal timing for resection after NT in LARC. “I would recommend posttreatment assessment with MRI and proctoscopy between 8 to 12 weeks and in the case of residual tumor or incomplete response to treatment, scheduling surgery at that time,” he said.
Dr. Roxburgh and coauthors reported having no financial disclosures.
SOURCE: Roxburgh C, et al. Society of Surgical Oncology Annual Cancer Symposium Abstract No. 3.
REPORTING FROM SSO 2018
Key clinical point: Timing of surgery for rectal cancer within 12 weeks of neoadjuvant therapy does not influence complications.
Major finding: Complication rates in early and later surgery groups were 44% and 38%.
Study details: Institutional cohort of 607 patients who had rectal resection within 16 weeks of completing NT between June 2009 and March 2015.
Disclosure: Dr. Roxburgh and coauthors reported having no financial disclosures.
Source: Roxburgh C, et al. Society of Surgical Oncology Annual Cancer Symposium Abstract No. 3.
Tivozanib after sorafenib promising in patients with advanced RCC
For patients with advanced renal cell carcinoma (RCC) progressing after sorafenib treatment, tivozanib was well tolerated and provided promising survival outcomes, investigators in a phase 2 study have reported.
Incidence of adverse events on tivozanib was low, and the safety profile was favorable in comparison with other agents in its class, the investigators said in the European Journal of Cancer.
The findings also help clarify results of a previous randomized phase 3 trial of tivozanib versus sorafenib where the investigators say crossover may have confounded overall survival results to the detriment of the tivozanib arm.
“Collectively, these data provide evidence of the anti-tumor activity of tivozanib and may be used to help frame future studies in recurrent disease,” wrote Ana M. Molina, MD, of Weill Cornell Medicine, New York, and her coauthors.
Tivozanib, recently approved in Europe for untreated RCC, is characterized by highly potent and selective inhibition of the three known vascular endothelial growth factor (VEGF) receptors.
Dr. Molina and her colleagues reported a single-arm crossover study of patients who were previously enrolled in the randomized phase 3 TIVO-1 trial of tivozanib versus sorafenib.
They enrolled a total of 161 patients who were randomized to the sorafenib arm of TIVO-1 and went on to receive tivozanib after disease progression.
Median progression-free survival was 11.0 months and median overall survival was 21.6 months for these crossover patients, Dr. Molina and co-investigators reported.
No patients in the study had a complete response, while 29 (18%) had a partial response and 83 (52%) had stable disease.
“These data compare favorably with other second-line therapies for RCC,” Dr. Molina and co-authors said.
Grade 3 or greater adverse events occurred in 48% of patients, including 24% that were treatment related. The most common grade 3 treatment-related adverse event was hypertension in 11%.
Approximately 4% of patients discontinued tivozanib due to adverse events.
“This study also provided clarity of the TIVO-1 trial, in which patient crossover was thought to have confounded the overall survival results,” Dr. Molina and colleagues said.
In TIVO-1, the primary end point of progression-free survival was improved for tivozanib versus sorafenib (median of 11.9 vs 9.1 months; P = .042), they noted.
However, median overall survival was not statistically different between arms, possibly because 74% of patients randomized to sorafenib were treated with next-line therapy, mainly tivozanib, investigators said.
AVEO Oncology and Astellas Pharma US, Inc. funded the study. Dr. Molina reported receiving honoraria from AVEO, Novartis, and Eisai.
SOURCE: Molina AM, et al. Eur J Cancer. 2018 Mar 13. doi: 10.1016/j.ejca.2018.02.009.
For patients with advanced renal cell carcinoma (RCC) progressing after sorafenib treatment, tivozanib was well tolerated and provided promising survival outcomes, investigators in a phase 2 study have reported.
Incidence of adverse events on tivozanib was low, and the safety profile was favorable in comparison with other agents in its class, the investigators said in the European Journal of Cancer.
The findings also help clarify results of a previous randomized phase 3 trial of tivozanib versus sorafenib where the investigators say crossover may have confounded overall survival results to the detriment of the tivozanib arm.
“Collectively, these data provide evidence of the anti-tumor activity of tivozanib and may be used to help frame future studies in recurrent disease,” wrote Ana M. Molina, MD, of Weill Cornell Medicine, New York, and her coauthors.
Tivozanib, recently approved in Europe for untreated RCC, is characterized by highly potent and selective inhibition of the three known vascular endothelial growth factor (VEGF) receptors.
Dr. Molina and her colleagues reported a single-arm crossover study of patients who were previously enrolled in the randomized phase 3 TIVO-1 trial of tivozanib versus sorafenib.
They enrolled a total of 161 patients who were randomized to the sorafenib arm of TIVO-1 and went on to receive tivozanib after disease progression.
Median progression-free survival was 11.0 months and median overall survival was 21.6 months for these crossover patients, Dr. Molina and co-investigators reported.
No patients in the study had a complete response, while 29 (18%) had a partial response and 83 (52%) had stable disease.
“These data compare favorably with other second-line therapies for RCC,” Dr. Molina and co-authors said.
Grade 3 or greater adverse events occurred in 48% of patients, including 24% that were treatment related. The most common grade 3 treatment-related adverse event was hypertension in 11%.
Approximately 4% of patients discontinued tivozanib due to adverse events.
“This study also provided clarity of the TIVO-1 trial, in which patient crossover was thought to have confounded the overall survival results,” Dr. Molina and colleagues said.
In TIVO-1, the primary end point of progression-free survival was improved for tivozanib versus sorafenib (median of 11.9 vs 9.1 months; P = .042), they noted.
However, median overall survival was not statistically different between arms, possibly because 74% of patients randomized to sorafenib were treated with next-line therapy, mainly tivozanib, investigators said.
AVEO Oncology and Astellas Pharma US, Inc. funded the study. Dr. Molina reported receiving honoraria from AVEO, Novartis, and Eisai.
SOURCE: Molina AM, et al. Eur J Cancer. 2018 Mar 13. doi: 10.1016/j.ejca.2018.02.009.
For patients with advanced renal cell carcinoma (RCC) progressing after sorafenib treatment, tivozanib was well tolerated and provided promising survival outcomes, investigators in a phase 2 study have reported.
Incidence of adverse events on tivozanib was low, and the safety profile was favorable in comparison with other agents in its class, the investigators said in the European Journal of Cancer.
The findings also help clarify results of a previous randomized phase 3 trial of tivozanib versus sorafenib where the investigators say crossover may have confounded overall survival results to the detriment of the tivozanib arm.
“Collectively, these data provide evidence of the anti-tumor activity of tivozanib and may be used to help frame future studies in recurrent disease,” wrote Ana M. Molina, MD, of Weill Cornell Medicine, New York, and her coauthors.
Tivozanib, recently approved in Europe for untreated RCC, is characterized by highly potent and selective inhibition of the three known vascular endothelial growth factor (VEGF) receptors.
Dr. Molina and her colleagues reported a single-arm crossover study of patients who were previously enrolled in the randomized phase 3 TIVO-1 trial of tivozanib versus sorafenib.
They enrolled a total of 161 patients who were randomized to the sorafenib arm of TIVO-1 and went on to receive tivozanib after disease progression.
Median progression-free survival was 11.0 months and median overall survival was 21.6 months for these crossover patients, Dr. Molina and co-investigators reported.
No patients in the study had a complete response, while 29 (18%) had a partial response and 83 (52%) had stable disease.
“These data compare favorably with other second-line therapies for RCC,” Dr. Molina and co-authors said.
Grade 3 or greater adverse events occurred in 48% of patients, including 24% that were treatment related. The most common grade 3 treatment-related adverse event was hypertension in 11%.
Approximately 4% of patients discontinued tivozanib due to adverse events.
“This study also provided clarity of the TIVO-1 trial, in which patient crossover was thought to have confounded the overall survival results,” Dr. Molina and colleagues said.
In TIVO-1, the primary end point of progression-free survival was improved for tivozanib versus sorafenib (median of 11.9 vs 9.1 months; P = .042), they noted.
However, median overall survival was not statistically different between arms, possibly because 74% of patients randomized to sorafenib were treated with next-line therapy, mainly tivozanib, investigators said.
AVEO Oncology and Astellas Pharma US, Inc. funded the study. Dr. Molina reported receiving honoraria from AVEO, Novartis, and Eisai.
SOURCE: Molina AM, et al. Eur J Cancer. 2018 Mar 13. doi: 10.1016/j.ejca.2018.02.009.
FROM THE EUROPEAN JOURNAL OF CANCER
Key clinical point: Tivozanib has potent antitumor activity in patients with advanced renal cell carcinoma (RCC) who previously progressed on sorafenib.
Major finding: Median progression-free survival was 11.0 months, and median overall survival was 21.6 months for patients receiving tivozanib.
Study details: A single-arm, phase 2 crossover study of patients previously randomized to the sorafenib arm of the phase 3 TIVO-1 study.
Disclosures: AVEO Oncology and Astellas Pharma US, Inc. funded the study. Investigators reported potential conflict of interests related to AVEO, Novartis, Eisai, Pfizer, and others.
Source: Molina AM, et al. Eur J Cancer. 2018 Mar 13. doi: 10.1016/j.ejca.2018.02.009.
Unknown primary melanoma looks a lot like known
Stage IV melanoma of unknown primary (MUP) origin, in which a primary tumor has either resolved or remains undiscovered, shares similar outcomes and prognostic factors to melanoma of known primary (MKP) origin, according to a new analysis of the nationwide Surveillance, Epidemiology, and End Results (SEER)-18 registries spanning from 1973 to 2014.
Previous studies of MUP have been single institutional or multi-institutional studies. The current work is the first population-level study.
MUP is uncommon, representing 2.5-5% of melanoma cases. As with MKP, worse survival of MUP patients was tied to age greater than 50 years and not undergoing a surgical procedure. The researchers did find a slight advantage in one-year survival for MUP patients compared to MKP, which could be because many of the MUP patients had experienced an immune response that eliminated the primary tumor.
“You could imagine that if the body attacks the primary tumor and it goes away, you’re set up to fight off the metastatic melanoma better,” said lead study author Jeffrey Scott, MD, a micrographic surgery and dermatologic oncology fellow at University Hospitals Cleveland Medical Center, Case Western Reserve University.
The study appeared online March 23 in the Journal of the American Academy of Dermatology.
The researchers analyzed 322 stage IV MUP cases and 12,796 stage IV MKP cases. The incidence of stage IV MUP increased over time, from 1.52 per 100,000 between 1973 and 1984, to 5.83 per 100,000 from 2005 to 2014. MUP patients were more likely to be recommended for surgery than MKP patients (surgery not recommended for 47.7% of MKP cases, compared to 37.7% of MUP cases), and they had better 1-year survival rates compared to the general U.S. population than did MKP patients (0.54, 95% CI, 0.48-0.60 versus 0.41, 95% CI 0.39-0.42). The improved survival of MUP over MKP remained steady at each measured time point out to 5 years.
However, there was no difference in 5-year disease-specific survival (DSS) in MUP versus MKP (HR, 0.91; 95% CI, 0.79-1.04; P =.16), or in the 5-year DSS Kaplan-Meier curve after adjustment for year of diagnosis, age, sex, race, and surgical treatment (log-rank P = .93).
A multivariate analysis showed increased 5-year DSS among patients who received surgery (HR, 0.41; 95% CI, 0.30-0.56; P less than .001) and decreased 5-year DSS among patients over 50 (HR 3.27, 95% CI, 1.17-9.17; P = .02 for age 50-59).
“The prognostic factors are very similar, so you should treat these patients (with MUP) similarly to patients with melanoma of known primary, the same treatments, the same clinical trials,” Dr. Scott and associates said.
The results also raise the possibility of gaining a better understanding of how immune response affects the course of metastatic melanoma.
“If MUP is due to the fact that your immune system is attacking the primary tumor, then what characteristics of the person would cause that to happen? Are younger patients (exhibiting) a more robust immune response? Could that explain why their prognosis is better? More molecular studies of the actual tumors and the immune characteristics of these patients would help us answer that,” they added.
A resolved primary tumor isn’t the only explanation for MUP, however. It’s also possible that melanocytes are found in unexpected sites, perhaps because they did not complete their migration during development. “They could give rise to melanoma that would present (as MUP). Maybe there never was a skin tumor,” the authors wrote.
The investigators recommend a thorough search for a primary tumor, employing ophthalmologists, gynecologists, and other specialists if necessary. “You could argue that once you have metastatic disease, what’s the point of finding the primary tumor? But it’s important to correctly classify these patients, in terms of what clinical trials and treatments they may be eligible for,” Dr. Scott and associates said.
SOURCE: Scott JF et al. J Am Acad Dermatol. 2018 Mar 23. doi: 10.1016/j.jaad.2018.03.021.
Stage IV melanoma of unknown primary (MUP) origin, in which a primary tumor has either resolved or remains undiscovered, shares similar outcomes and prognostic factors to melanoma of known primary (MKP) origin, according to a new analysis of the nationwide Surveillance, Epidemiology, and End Results (SEER)-18 registries spanning from 1973 to 2014.
Previous studies of MUP have been single institutional or multi-institutional studies. The current work is the first population-level study.
MUP is uncommon, representing 2.5-5% of melanoma cases. As with MKP, worse survival of MUP patients was tied to age greater than 50 years and not undergoing a surgical procedure. The researchers did find a slight advantage in one-year survival for MUP patients compared to MKP, which could be because many of the MUP patients had experienced an immune response that eliminated the primary tumor.
“You could imagine that if the body attacks the primary tumor and it goes away, you’re set up to fight off the metastatic melanoma better,” said lead study author Jeffrey Scott, MD, a micrographic surgery and dermatologic oncology fellow at University Hospitals Cleveland Medical Center, Case Western Reserve University.
The study appeared online March 23 in the Journal of the American Academy of Dermatology.
The researchers analyzed 322 stage IV MUP cases and 12,796 stage IV MKP cases. The incidence of stage IV MUP increased over time, from 1.52 per 100,000 between 1973 and 1984, to 5.83 per 100,000 from 2005 to 2014. MUP patients were more likely to be recommended for surgery than MKP patients (surgery not recommended for 47.7% of MKP cases, compared to 37.7% of MUP cases), and they had better 1-year survival rates compared to the general U.S. population than did MKP patients (0.54, 95% CI, 0.48-0.60 versus 0.41, 95% CI 0.39-0.42). The improved survival of MUP over MKP remained steady at each measured time point out to 5 years.
However, there was no difference in 5-year disease-specific survival (DSS) in MUP versus MKP (HR, 0.91; 95% CI, 0.79-1.04; P =.16), or in the 5-year DSS Kaplan-Meier curve after adjustment for year of diagnosis, age, sex, race, and surgical treatment (log-rank P = .93).
A multivariate analysis showed increased 5-year DSS among patients who received surgery (HR, 0.41; 95% CI, 0.30-0.56; P less than .001) and decreased 5-year DSS among patients over 50 (HR 3.27, 95% CI, 1.17-9.17; P = .02 for age 50-59).
“The prognostic factors are very similar, so you should treat these patients (with MUP) similarly to patients with melanoma of known primary, the same treatments, the same clinical trials,” Dr. Scott and associates said.
The results also raise the possibility of gaining a better understanding of how immune response affects the course of metastatic melanoma.
“If MUP is due to the fact that your immune system is attacking the primary tumor, then what characteristics of the person would cause that to happen? Are younger patients (exhibiting) a more robust immune response? Could that explain why their prognosis is better? More molecular studies of the actual tumors and the immune characteristics of these patients would help us answer that,” they added.
A resolved primary tumor isn’t the only explanation for MUP, however. It’s also possible that melanocytes are found in unexpected sites, perhaps because they did not complete their migration during development. “They could give rise to melanoma that would present (as MUP). Maybe there never was a skin tumor,” the authors wrote.
The investigators recommend a thorough search for a primary tumor, employing ophthalmologists, gynecologists, and other specialists if necessary. “You could argue that once you have metastatic disease, what’s the point of finding the primary tumor? But it’s important to correctly classify these patients, in terms of what clinical trials and treatments they may be eligible for,” Dr. Scott and associates said.
SOURCE: Scott JF et al. J Am Acad Dermatol. 2018 Mar 23. doi: 10.1016/j.jaad.2018.03.021.
Stage IV melanoma of unknown primary (MUP) origin, in which a primary tumor has either resolved or remains undiscovered, shares similar outcomes and prognostic factors to melanoma of known primary (MKP) origin, according to a new analysis of the nationwide Surveillance, Epidemiology, and End Results (SEER)-18 registries spanning from 1973 to 2014.
Previous studies of MUP have been single institutional or multi-institutional studies. The current work is the first population-level study.
MUP is uncommon, representing 2.5-5% of melanoma cases. As with MKP, worse survival of MUP patients was tied to age greater than 50 years and not undergoing a surgical procedure. The researchers did find a slight advantage in one-year survival for MUP patients compared to MKP, which could be because many of the MUP patients had experienced an immune response that eliminated the primary tumor.
“You could imagine that if the body attacks the primary tumor and it goes away, you’re set up to fight off the metastatic melanoma better,” said lead study author Jeffrey Scott, MD, a micrographic surgery and dermatologic oncology fellow at University Hospitals Cleveland Medical Center, Case Western Reserve University.
The study appeared online March 23 in the Journal of the American Academy of Dermatology.
The researchers analyzed 322 stage IV MUP cases and 12,796 stage IV MKP cases. The incidence of stage IV MUP increased over time, from 1.52 per 100,000 between 1973 and 1984, to 5.83 per 100,000 from 2005 to 2014. MUP patients were more likely to be recommended for surgery than MKP patients (surgery not recommended for 47.7% of MKP cases, compared to 37.7% of MUP cases), and they had better 1-year survival rates compared to the general U.S. population than did MKP patients (0.54, 95% CI, 0.48-0.60 versus 0.41, 95% CI 0.39-0.42). The improved survival of MUP over MKP remained steady at each measured time point out to 5 years.
However, there was no difference in 5-year disease-specific survival (DSS) in MUP versus MKP (HR, 0.91; 95% CI, 0.79-1.04; P =.16), or in the 5-year DSS Kaplan-Meier curve after adjustment for year of diagnosis, age, sex, race, and surgical treatment (log-rank P = .93).
A multivariate analysis showed increased 5-year DSS among patients who received surgery (HR, 0.41; 95% CI, 0.30-0.56; P less than .001) and decreased 5-year DSS among patients over 50 (HR 3.27, 95% CI, 1.17-9.17; P = .02 for age 50-59).
“The prognostic factors are very similar, so you should treat these patients (with MUP) similarly to patients with melanoma of known primary, the same treatments, the same clinical trials,” Dr. Scott and associates said.
The results also raise the possibility of gaining a better understanding of how immune response affects the course of metastatic melanoma.
“If MUP is due to the fact that your immune system is attacking the primary tumor, then what characteristics of the person would cause that to happen? Are younger patients (exhibiting) a more robust immune response? Could that explain why their prognosis is better? More molecular studies of the actual tumors and the immune characteristics of these patients would help us answer that,” they added.
A resolved primary tumor isn’t the only explanation for MUP, however. It’s also possible that melanocytes are found in unexpected sites, perhaps because they did not complete their migration during development. “They could give rise to melanoma that would present (as MUP). Maybe there never was a skin tumor,” the authors wrote.
The investigators recommend a thorough search for a primary tumor, employing ophthalmologists, gynecologists, and other specialists if necessary. “You could argue that once you have metastatic disease, what’s the point of finding the primary tumor? But it’s important to correctly classify these patients, in terms of what clinical trials and treatments they may be eligible for,” Dr. Scott and associates said.
SOURCE: Scott JF et al. J Am Acad Dermatol. 2018 Mar 23. doi: 10.1016/j.jaad.2018.03.021.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Unknown primary melanomas should be approached similar to melanomas with known primaries.
Major finding: The 5-year DSS rate was lower in patients age 50-59 (HR, 3.27).
Study details: Retrospective analysis of 322 stage IV MUP cases and 12,796 stage IV MKP.
Disclosures: The study was funded by the Char and Chuck Fowler Family Foundation. Dr. Scott reported no relevant financial relationships.
Source: Scott JF et al. J Am Acad Dermatol. 2018 Mar 23. doi: 10.1016/j.jaad.2018.03.021.
MicroRNAs required for cGVHD development
New research suggests a family of microRNAs, miR-17-92, plays a key role in chronic graft-versus-host disease (cGVHD).
Researchers found miR-17-92 is responsible for the T- and B-cell pathogenicity that causes cGVHD.
The team also discovered that pharmacological inhibition of miR-17 alleviated the symptoms of cGVHD in mice.
Yongxia Wu, PhD, of the Medical University of South Carolina in Charleston, and her colleagues reported these findings in Blood.
The researchers previously found that miR-17-92 regulates CD4 T-cell proliferation and Th1 and Treg differentiation in acute (a) GVHD.
So the team set out to investigate whether miR-17-92 regulates T- and B-cell differentiation and function in the development of cGVHD.
“Chronic GVHD has a different pathophysiology and different target organs than aGVHD,” Dr Wu noted. “It’s been a big challenge to try to find a target for cGVHD therapies because of the more complex immune reaction in cGVHD and the fact that its cellular and molecular mechanisms are not as well understood.”
“We decided to extend our aGVHD study to cGVHD, but there’s no single, well-defined murine model that can reflect all of the clinical manifestations seen in cGVHD patients. So we decided to study 4 different cGVHD models to best understand how miR-17-92 contributes overall, across many clinical presentations.”
The team performed a series of experiments in murine models of cGVHD after allogeneic bone marrow transplant (BMT). This included models of scleroderma that had transitioned from aGVHD to cGVHD, classic cGVHD scleroderma, lung inflammation, and a lupus-like condition.
The experiments revealed shared mechanisms by which miR-17-92 mediates cGVHD progression—namely, by regulating T helper-cell differentiation, B-cell activation, germinal center responses, and autoantibody production.
“The mechanism for how miR-17-92 regulates T and B cells was very consistent,” Dr Wu said. “In other words, we did not find any big differences among the models.”
The researchers also assessed whether pharmacological inhibition of miR-17 or miR-19—“key members in the miR-17-92 cluster”—might be effective in the treatment of cGVHD.
The team tested antagomirs specific for miR-17 or miR-19 in the scleroderma cGVHD model and the lupus-like condition.
Anti-miR-17, but not anti-miR-19, reduced skin damage in the scleroderma model and alleviated proteinuria in the lupus-like condition.
“So we not only found a new mechanism for cGVHD development by demonstrating that miR-17-92 is heavily involved in the T- and B-cell responses that lead to cGVHD, but we also found that blocking miR-17 substantially reduced cGVHD symptoms in mice,” Dr Wu said.
“That’s exciting because it provides strong evidence that this miR may be a good target for controlling cGVHD after allogeneic BMT.”
Now, Dr Wu and her colleagues are investigating how other microRNAs may be involved in regulating T- and B-cell function during allogeneic BMT.
New research suggests a family of microRNAs, miR-17-92, plays a key role in chronic graft-versus-host disease (cGVHD).
Researchers found miR-17-92 is responsible for the T- and B-cell pathogenicity that causes cGVHD.
The team also discovered that pharmacological inhibition of miR-17 alleviated the symptoms of cGVHD in mice.
Yongxia Wu, PhD, of the Medical University of South Carolina in Charleston, and her colleagues reported these findings in Blood.
The researchers previously found that miR-17-92 regulates CD4 T-cell proliferation and Th1 and Treg differentiation in acute (a) GVHD.
So the team set out to investigate whether miR-17-92 regulates T- and B-cell differentiation and function in the development of cGVHD.
“Chronic GVHD has a different pathophysiology and different target organs than aGVHD,” Dr Wu noted. “It’s been a big challenge to try to find a target for cGVHD therapies because of the more complex immune reaction in cGVHD and the fact that its cellular and molecular mechanisms are not as well understood.”
“We decided to extend our aGVHD study to cGVHD, but there’s no single, well-defined murine model that can reflect all of the clinical manifestations seen in cGVHD patients. So we decided to study 4 different cGVHD models to best understand how miR-17-92 contributes overall, across many clinical presentations.”
The team performed a series of experiments in murine models of cGVHD after allogeneic bone marrow transplant (BMT). This included models of scleroderma that had transitioned from aGVHD to cGVHD, classic cGVHD scleroderma, lung inflammation, and a lupus-like condition.
The experiments revealed shared mechanisms by which miR-17-92 mediates cGVHD progression—namely, by regulating T helper-cell differentiation, B-cell activation, germinal center responses, and autoantibody production.
“The mechanism for how miR-17-92 regulates T and B cells was very consistent,” Dr Wu said. “In other words, we did not find any big differences among the models.”
The researchers also assessed whether pharmacological inhibition of miR-17 or miR-19—“key members in the miR-17-92 cluster”—might be effective in the treatment of cGVHD.
The team tested antagomirs specific for miR-17 or miR-19 in the scleroderma cGVHD model and the lupus-like condition.
Anti-miR-17, but not anti-miR-19, reduced skin damage in the scleroderma model and alleviated proteinuria in the lupus-like condition.
“So we not only found a new mechanism for cGVHD development by demonstrating that miR-17-92 is heavily involved in the T- and B-cell responses that lead to cGVHD, but we also found that blocking miR-17 substantially reduced cGVHD symptoms in mice,” Dr Wu said.
“That’s exciting because it provides strong evidence that this miR may be a good target for controlling cGVHD after allogeneic BMT.”
Now, Dr Wu and her colleagues are investigating how other microRNAs may be involved in regulating T- and B-cell function during allogeneic BMT.
New research suggests a family of microRNAs, miR-17-92, plays a key role in chronic graft-versus-host disease (cGVHD).
Researchers found miR-17-92 is responsible for the T- and B-cell pathogenicity that causes cGVHD.
The team also discovered that pharmacological inhibition of miR-17 alleviated the symptoms of cGVHD in mice.
Yongxia Wu, PhD, of the Medical University of South Carolina in Charleston, and her colleagues reported these findings in Blood.
The researchers previously found that miR-17-92 regulates CD4 T-cell proliferation and Th1 and Treg differentiation in acute (a) GVHD.
So the team set out to investigate whether miR-17-92 regulates T- and B-cell differentiation and function in the development of cGVHD.
“Chronic GVHD has a different pathophysiology and different target organs than aGVHD,” Dr Wu noted. “It’s been a big challenge to try to find a target for cGVHD therapies because of the more complex immune reaction in cGVHD and the fact that its cellular and molecular mechanisms are not as well understood.”
“We decided to extend our aGVHD study to cGVHD, but there’s no single, well-defined murine model that can reflect all of the clinical manifestations seen in cGVHD patients. So we decided to study 4 different cGVHD models to best understand how miR-17-92 contributes overall, across many clinical presentations.”
The team performed a series of experiments in murine models of cGVHD after allogeneic bone marrow transplant (BMT). This included models of scleroderma that had transitioned from aGVHD to cGVHD, classic cGVHD scleroderma, lung inflammation, and a lupus-like condition.
The experiments revealed shared mechanisms by which miR-17-92 mediates cGVHD progression—namely, by regulating T helper-cell differentiation, B-cell activation, germinal center responses, and autoantibody production.
“The mechanism for how miR-17-92 regulates T and B cells was very consistent,” Dr Wu said. “In other words, we did not find any big differences among the models.”
The researchers also assessed whether pharmacological inhibition of miR-17 or miR-19—“key members in the miR-17-92 cluster”—might be effective in the treatment of cGVHD.
The team tested antagomirs specific for miR-17 or miR-19 in the scleroderma cGVHD model and the lupus-like condition.
Anti-miR-17, but not anti-miR-19, reduced skin damage in the scleroderma model and alleviated proteinuria in the lupus-like condition.
“So we not only found a new mechanism for cGVHD development by demonstrating that miR-17-92 is heavily involved in the T- and B-cell responses that lead to cGVHD, but we also found that blocking miR-17 substantially reduced cGVHD symptoms in mice,” Dr Wu said.
“That’s exciting because it provides strong evidence that this miR may be a good target for controlling cGVHD after allogeneic BMT.”
Now, Dr Wu and her colleagues are investigating how other microRNAs may be involved in regulating T- and B-cell function during allogeneic BMT.
Melanoma in young children may be biologically distinct from that in teens
Pediatric melanomas appear to be more progressive in adolescents than in young children, based on data from a retrospective study of 32 cases.
Few young children with melanoma die, despite a greater likelihood of thicker tumors, lymph node metastasis, and later diagnosis, which suggests that melanoma in young children may be biologically distinct from melanoma in adolescents, wrote Diana W. Bartenstein, of Harvard University Medical School, Boston, and her colleagues.
Overall, significantly more children than adolescents had spitzoid melanoma (50% vs. 10%, P = .01). In addition, children were more likely than adolescents to present with stage 3 or 4 cancer (58% vs. 25%) and with Clark level IV and V tumors (42% vs. 35%), although these differences were not significant. The median Breslow thickness of lesions was greater in children than in adolescents (3.5 mm vs. 1.5 mm) as was the median mitotic index (5 mitotic figures per mm2 vs. 2 mitotic figures per mm2) and children were more likely than adolescents to have neural invasion, but these differences were not significant either.
During the study period of more than 20 years, none of the children younger than 11 years died, compared with four deaths in adolescents, a statistically significant difference (P = .04). The follow-up for surviving individuals ranged from 9-37 months with a median of 44 months.
The study findings were limited by several factors including the small sample size and difficulty in assessing spitzoid tumors, the researchers noted.
However, “these results support the hypothesis that melanoma in young children may be biologically distinct from melanoma in adults,” they said. “Alternatively, melanoma subtype may drive survival differences between children and adolescents.”
No conflicts of interest were reported. The study was supported by the Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship and the Society for Pediatric Dermatology and Pediatric Dermatology Research Alliance.
SOURCE: Bartenstein DW et al. Pediatr Dermatol. 2018 Mar 23. doi: 10.1111/pde.13454.
Pediatric melanomas appear to be more progressive in adolescents than in young children, based on data from a retrospective study of 32 cases.
Few young children with melanoma die, despite a greater likelihood of thicker tumors, lymph node metastasis, and later diagnosis, which suggests that melanoma in young children may be biologically distinct from melanoma in adolescents, wrote Diana W. Bartenstein, of Harvard University Medical School, Boston, and her colleagues.
Overall, significantly more children than adolescents had spitzoid melanoma (50% vs. 10%, P = .01). In addition, children were more likely than adolescents to present with stage 3 or 4 cancer (58% vs. 25%) and with Clark level IV and V tumors (42% vs. 35%), although these differences were not significant. The median Breslow thickness of lesions was greater in children than in adolescents (3.5 mm vs. 1.5 mm) as was the median mitotic index (5 mitotic figures per mm2 vs. 2 mitotic figures per mm2) and children were more likely than adolescents to have neural invasion, but these differences were not significant either.
During the study period of more than 20 years, none of the children younger than 11 years died, compared with four deaths in adolescents, a statistically significant difference (P = .04). The follow-up for surviving individuals ranged from 9-37 months with a median of 44 months.
The study findings were limited by several factors including the small sample size and difficulty in assessing spitzoid tumors, the researchers noted.
However, “these results support the hypothesis that melanoma in young children may be biologically distinct from melanoma in adults,” they said. “Alternatively, melanoma subtype may drive survival differences between children and adolescents.”
No conflicts of interest were reported. The study was supported by the Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship and the Society for Pediatric Dermatology and Pediatric Dermatology Research Alliance.
SOURCE: Bartenstein DW et al. Pediatr Dermatol. 2018 Mar 23. doi: 10.1111/pde.13454.
Pediatric melanomas appear to be more progressive in adolescents than in young children, based on data from a retrospective study of 32 cases.
Few young children with melanoma die, despite a greater likelihood of thicker tumors, lymph node metastasis, and later diagnosis, which suggests that melanoma in young children may be biologically distinct from melanoma in adolescents, wrote Diana W. Bartenstein, of Harvard University Medical School, Boston, and her colleagues.
Overall, significantly more children than adolescents had spitzoid melanoma (50% vs. 10%, P = .01). In addition, children were more likely than adolescents to present with stage 3 or 4 cancer (58% vs. 25%) and with Clark level IV and V tumors (42% vs. 35%), although these differences were not significant. The median Breslow thickness of lesions was greater in children than in adolescents (3.5 mm vs. 1.5 mm) as was the median mitotic index (5 mitotic figures per mm2 vs. 2 mitotic figures per mm2) and children were more likely than adolescents to have neural invasion, but these differences were not significant either.
During the study period of more than 20 years, none of the children younger than 11 years died, compared with four deaths in adolescents, a statistically significant difference (P = .04). The follow-up for surviving individuals ranged from 9-37 months with a median of 44 months.
The study findings were limited by several factors including the small sample size and difficulty in assessing spitzoid tumors, the researchers noted.
However, “these results support the hypothesis that melanoma in young children may be biologically distinct from melanoma in adults,” they said. “Alternatively, melanoma subtype may drive survival differences between children and adolescents.”
No conflicts of interest were reported. The study was supported by the Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship and the Society for Pediatric Dermatology and Pediatric Dermatology Research Alliance.
SOURCE: Bartenstein DW et al. Pediatr Dermatol. 2018 Mar 23. doi: 10.1111/pde.13454.
FROM PEDIATRIC DERMATOLOGY
Key clinical point:
Major finding: Significantly more children than adolescents had spitzoid melanoma (50% vs. 10%, P = .01).
Study details: A retrospective cohort study of 32 children and adolescents with melanoma.
Disclosures: The study was supported by the Alpha Omega Alpha–Carolyn L. Kuckein Student Research Fellowship and the Society for Pediatric Dermatology and Pediatric Dermatology Research Alliance. No conflicts of interest were reported.
Source: Bartenstein DW et al. Pediatr Dermatol. 2018 Mar 23. doi: 10.1111/pde.13454.
AbbVie, Samsung Bioepis settle suits with delayed U.S. entry for adalimumab biosimilar
A new adalimumab biosimilar will become available in the European Union later this year, but a court settlement will keep Samsung Bioepis’ competitor off U.S. shelves until 2023.
Under the settlement, AbbVie, which manufactures adalimumab (Humira), will grant Bioepis and its partner, Biogen, a nonexclusive license to the intellectual property relating to the antibody. Bioepis’ version, dubbed SB5 (Imraldi), will enter global markets in a staggered fashion, according to an AbbVie press statement. In most countries in the European Union, the license period will begin on Oct. 16, 2018. In the United States, Samsung Bioepis’ license period will begin on June 30, 2023, according to the Abbvie statement.
Biogen and Bioepis hailed the settlement as a victory, but Imraldi won’t be the first Humira biosimilar to break into the U.S. market. Last September, AbbVie settled a similar suit with Amgen, granting patent licenses for the global use and sale of its anti–tumor necrosis factor–alpha antibody, Amgevita/Amjevita. Amgen expects to launch Amgevita in Europe on Oct. 16, 2018, and Amjevita in the United States on Jan. 31, 2023. Samsung Bioepis’ U.S. license date will not be accelerated upon Amgen’s entry.
Ian Henshaw, Biogen’s global head of biosimilars, said the deal further strengthens the company’s European biosimilars reach.
“Biogen is a leader in the emerging field of biosimilars through Samsung Bioepis, our joint venture with Samsung BioLogics,” Mr. Henshaw said in a press statement. “Biogen already markets two biosimilars in Europe and the planned introduction of Imraldi on Oct. 16 could potentially expand patient choice by offering physicians more options to meet the needs of patients while delivering significant savings to healthcare systems.”
AbbVie focused on the settlement as a global recognition of its leadership role in developing the anti-TNF-alpha antibody.
“The Samsung Bioepis settlement reflects the strength and breadth of AbbVie’s intellectual property,” Laura Schumacher, the company’s general counsel, said in the Abbvie statement. “We continue to believe biosimilars will play an important role in our healthcare system, but we also believe it is important to protect our investment in innovation. This agreement accomplishes both objectives.”
Samsung Bioepis will pay royalties to AbbVie for licensing its adalimumab patents once its biosimilar product is launched. As is the case with the prior Amgen resolution, AbbVie will not make any payments to Samsung Bioepis. “All litigation pending between the parties, as well as all litigation with Samsung Bioepis’ European partner, Biogen, will be dismissed. The precise terms of the agreements are confidential,” the Abbvie statement said.
The settlement brings to a closing a flurry of lawsuits Samsung Bioepis filed against AbbVie in 2017.
A new adalimumab biosimilar will become available in the European Union later this year, but a court settlement will keep Samsung Bioepis’ competitor off U.S. shelves until 2023.
Under the settlement, AbbVie, which manufactures adalimumab (Humira), will grant Bioepis and its partner, Biogen, a nonexclusive license to the intellectual property relating to the antibody. Bioepis’ version, dubbed SB5 (Imraldi), will enter global markets in a staggered fashion, according to an AbbVie press statement. In most countries in the European Union, the license period will begin on Oct. 16, 2018. In the United States, Samsung Bioepis’ license period will begin on June 30, 2023, according to the Abbvie statement.
Biogen and Bioepis hailed the settlement as a victory, but Imraldi won’t be the first Humira biosimilar to break into the U.S. market. Last September, AbbVie settled a similar suit with Amgen, granting patent licenses for the global use and sale of its anti–tumor necrosis factor–alpha antibody, Amgevita/Amjevita. Amgen expects to launch Amgevita in Europe on Oct. 16, 2018, and Amjevita in the United States on Jan. 31, 2023. Samsung Bioepis’ U.S. license date will not be accelerated upon Amgen’s entry.
Ian Henshaw, Biogen’s global head of biosimilars, said the deal further strengthens the company’s European biosimilars reach.
“Biogen is a leader in the emerging field of biosimilars through Samsung Bioepis, our joint venture with Samsung BioLogics,” Mr. Henshaw said in a press statement. “Biogen already markets two biosimilars in Europe and the planned introduction of Imraldi on Oct. 16 could potentially expand patient choice by offering physicians more options to meet the needs of patients while delivering significant savings to healthcare systems.”
AbbVie focused on the settlement as a global recognition of its leadership role in developing the anti-TNF-alpha antibody.
“The Samsung Bioepis settlement reflects the strength and breadth of AbbVie’s intellectual property,” Laura Schumacher, the company’s general counsel, said in the Abbvie statement. “We continue to believe biosimilars will play an important role in our healthcare system, but we also believe it is important to protect our investment in innovation. This agreement accomplishes both objectives.”
Samsung Bioepis will pay royalties to AbbVie for licensing its adalimumab patents once its biosimilar product is launched. As is the case with the prior Amgen resolution, AbbVie will not make any payments to Samsung Bioepis. “All litigation pending between the parties, as well as all litigation with Samsung Bioepis’ European partner, Biogen, will be dismissed. The precise terms of the agreements are confidential,” the Abbvie statement said.
The settlement brings to a closing a flurry of lawsuits Samsung Bioepis filed against AbbVie in 2017.
A new adalimumab biosimilar will become available in the European Union later this year, but a court settlement will keep Samsung Bioepis’ competitor off U.S. shelves until 2023.
Under the settlement, AbbVie, which manufactures adalimumab (Humira), will grant Bioepis and its partner, Biogen, a nonexclusive license to the intellectual property relating to the antibody. Bioepis’ version, dubbed SB5 (Imraldi), will enter global markets in a staggered fashion, according to an AbbVie press statement. In most countries in the European Union, the license period will begin on Oct. 16, 2018. In the United States, Samsung Bioepis’ license period will begin on June 30, 2023, according to the Abbvie statement.
Biogen and Bioepis hailed the settlement as a victory, but Imraldi won’t be the first Humira biosimilar to break into the U.S. market. Last September, AbbVie settled a similar suit with Amgen, granting patent licenses for the global use and sale of its anti–tumor necrosis factor–alpha antibody, Amgevita/Amjevita. Amgen expects to launch Amgevita in Europe on Oct. 16, 2018, and Amjevita in the United States on Jan. 31, 2023. Samsung Bioepis’ U.S. license date will not be accelerated upon Amgen’s entry.
Ian Henshaw, Biogen’s global head of biosimilars, said the deal further strengthens the company’s European biosimilars reach.
“Biogen is a leader in the emerging field of biosimilars through Samsung Bioepis, our joint venture with Samsung BioLogics,” Mr. Henshaw said in a press statement. “Biogen already markets two biosimilars in Europe and the planned introduction of Imraldi on Oct. 16 could potentially expand patient choice by offering physicians more options to meet the needs of patients while delivering significant savings to healthcare systems.”
AbbVie focused on the settlement as a global recognition of its leadership role in developing the anti-TNF-alpha antibody.
“The Samsung Bioepis settlement reflects the strength and breadth of AbbVie’s intellectual property,” Laura Schumacher, the company’s general counsel, said in the Abbvie statement. “We continue to believe biosimilars will play an important role in our healthcare system, but we also believe it is important to protect our investment in innovation. This agreement accomplishes both objectives.”
Samsung Bioepis will pay royalties to AbbVie for licensing its adalimumab patents once its biosimilar product is launched. As is the case with the prior Amgen resolution, AbbVie will not make any payments to Samsung Bioepis. “All litigation pending between the parties, as well as all litigation with Samsung Bioepis’ European partner, Biogen, will be dismissed. The precise terms of the agreements are confidential,” the Abbvie statement said.
The settlement brings to a closing a flurry of lawsuits Samsung Bioepis filed against AbbVie in 2017.